
INTERNATIONAL PROGRAMME ON CHEMICAL SAFETY
ENVIRONMENTAL HEALTH CRITERIA 158
AMITROLE
This report contains the collective views of an international group of
experts and does not necessarily represent the decisions or the stated
policy of the United Nations Environment Programme, the International
Labour Organisation, or the World Health Organization.
Published under the joint sponsorship of
the United Nations Environment Programme,
the International Labour Organisation,
and the World Health Organization
First draft prepared by Dr P.J. Abbott,
Department of Health, Housing and
Community Services, Canberra, Australia
World Health Orgnization
Geneva, 1994
The International Programme on Chemical Safety (IPCS) is a
joint venture of the United Nations Environment Programme, the
International Labour Organisation, and the World Health
Organization. The main objective of the IPCS is to carry out and
disseminate evaluations of the effects of chemicals on human health
and the quality of the environment. Supporting activities include
the development of epidemiological, experimental laboratory, and
risk-assessment methods that could produce internationally
comparable results, and the development of manpower in the field of
toxicology. Other activities carried out by the IPCS include the
development of know-how for coping with chemical accidents,
coordination of laboratory testing and epidemiological studies, and
promotion of research on the mechanisms of the biological action of
chemicals.
WHO Library Cataloguing in Publication Data
Amitrole.
(Environmental health criteria ; 158)
1.Amitrole - standards 2.Environmental exposure
3.Herbicides I.Series
ISBN 92 4 157158 6 (NLM Classification: WA 240)
ISSN 0250-863X
The World Health Organization welcomes requests for permission
to reproduce or translate its publications, in part or in full.
Applications and enquiries should be addressed to the Office of
Publications, World Health Organization, Geneva, Switzerland, which
will be glad to provide the latest information on any changes made
to the text, plans for new editions, and reprints and translations
already available.
(c) World Health Organization 1994
Publications of the World Health Organization enjoy copyright
protection in accordance with the provisions of Protocol 2 of the
Universal Copyright Convention. All rights reserved.
The designations employed and the presentation of the material
in this publication do not imply the expression of any opinion
whatsoever on the part of the Secretariat of the World Health
Organization concerning the legal status of any country, territory,
city or area or of its authorities, or concerning the delimitation
of its frontiers or boundaries.
The mention of specific companies or of certain manufacturers'
products does not imply that they are endorsed or recommended by the
World Health Organization in preference to others of a similar
nature that are not mentioned. Errors and omissions excepted, the
names of proprietary products are distinguished by initial capital
letters.
CONTENTS
1. SUMMARY
1.1. Identity, physical and chemical properties, and analytical
methods
1.2. Sources of human and environmental exposure
1.3. Environmental transport, distribution and transformation
1.4. Environmental levels and human exposure
1.5. Kinetics and metabolism in laboratory animals and humans
1.6. Effects on experimental animals and in vitro systems
1.7. Effects on humans
1.8. Effects on other organisms in the laboratory and field
2. IDENTITY, PHYSICAL AND CHEMICAL PROPERTIES AND ANALYTICAL
METHODS
2.1. Identity
2.2. Physical and chemical properties
2.3. Conversion factors
2.4. Analytical methods
2.4.1. Plants
2.4.2. Soil
2.4.3. Water
2.4.4. Formulations
2.4.5. Air
2.4.6. Urine
3. SOURCES OF HUMAN AND ENVIRONMENTAL EXPOSURE
3.1. Natural occurrence
3.2. Anthropogenic sources
3.2.1. Production levels and processes
3.2.2. Uses
4. ENVIRONMENTAL TRANSPORT, DISTRIBUTION AND TRANSFORMATION
4.1. Transport and distribution between media
4.1.1. Air
4.1.2. Water
4.1.3. Soil
4.1.3.1 Adsorption
4.1.4. Vegetation and wildlife
4.1.5. Entry into food chain
4.2. Biotransformation
4.2.1. Biodegradation and abiotic degradation
4.2.1.1 Plants
4.2.1.2 Soils
4.2.3. Bioaccumulation
4.3. Ultimate fate following use
5. ENVIRONMENTAL LEVELS AND HUMAN EXPOSURE
5.1. Environmental levels
5.1.1. Air
5.1.2. Water
5.1.3. Soil
5.2. General population exposure
5.2.1. Environmental sources
5.2.2. Food
5.3. Occupational exposure during manufacture, formulation
or use
6. KINETICS AND METABOLISM IN LABORATORY ANIMALS AND HUMANS
6.1. Absorption, distribution and excretion
6.1.1. Mouse
6.1.2. Rat
6.1.3. Human
6.2. Metabolic transformation
7. EFFECTS ON EXPERIMENTAL ANIMALS IN VITRO TEST SYSTEMS
7.1. Single exposure
7.1.1. Oral
7.1.2. Other routes
7.2. Short-term exposure
7.2.1. Oral
7.2.1.1 Dietary
7.2.1.2 Drinking-water
7.2.1.3 Intubation
7.2.2. Inhalational
7.2.3. Intraperitoneal
7.3. Long-term exposure
7.3.1. Oral
7.3.1.1 Mouse
7.3.1.2 Rat
7.3.1.3 Other species
7.3.2. Other routes
7.4. Skin and eye irritation; skin sensitisation
7.5. Reproduction, embryotoxicity and teratogenicity
7.5.1. Reproduction
7.5.2. Embryotoxicity and teratology
7.6. Mutagenicity and related end-points
7.6.1. DNA damage and repair
7.6.2. Mutation
7.6.3. Chromosome damage
7.6.4. Cell transformation
7.6.5. Other end-points
7.7. Carcinogenicity
7.7.1. Mouse
7.7.2. Rats
7.7.3. Other species
7.7.4. Carcinogenicity of amitrole in combination
with other agents
7.8. Other special studies
7.8.1. Cataractogenic activity in rabbits
7.8.2. Biochemical effects
7.9. Mechanisms of toxicity - mode of action
8. EFFECTS ON HUMANS
8.1. General population exposure
8.2. Occupational exposure
9. EFFECTS ON OTHER ORGANISMS IN THE LABORATORY AND FIELD
9.1. Laboratory experiments
9.1.1. Microorganisms
9.1.2. Aquatic organisms
9.1.2.1 Plants
9.1.2.2 Invertebrates
9.1.2.3 Vertebrates
9.1.3. Terrestrial organisms
9.1.3.1 Plants
9.1.3.2 Invertebrates
9.1.3.3 Birds
9.2. Field observations
9.2.1. Terrestrial organisms
9.2.1.1 Plants
9.2.1.2 Invertebrates
10. EVALUATION OF HUMAN HEALTH RISKS AND EFFECTS ON THE
ENVIRONMENT
10.1. Evaluation of human health risks
10.2. Evaluation of effects on the environment
11. CONCLUSIONS AND RECOMMENDATIONS FOR PROTECTION OF HUMAN HEALTH
AND THE ENVIRONMENT
11.1. Conclusions
11.2. Recommendations for protection of human health and the
environment
12. FURTHER RESEARCH
13. PREVIOUS EVALUATIONS BY NATIONAL AND INTERNATIONAL BODIES
REFERENCES
RESUME
RESUMEN
WHO TASK GROUP FOR ENVIRONMENTAL HEALTH
CRITERIA FOR AMITROLE
Members
Dr P.J. Abbott, Chemicals Safety Unit, Department of Health,
Housing and Community Services, Canberra, Australia
(Rapporteur)
Professor J.F. Borzelleca, School of Basic Health Sciences,
Department of Pharmacology, Richmond, Virginia, USAa
Professor V. Burgat-Sacaze, Ecole Nationale Vétérinaire, Toulouse,
France
Dr E.M. den Tonkellar, Toxicology Advisory Centre, National
Institute of Public Health and Environmental Protection,
Bilthoven, The Netherlands
Dr S. Dobson, Institute of Terrestrial Ecology, Monks Wood
Station, Abbots Ripton, Huntingdon, Cambridgeshire, United
Kingdom
Dr R. Fuchs, Department of Toxicology, Institute for Medical
Research and Occupational Health, University of Zagreb, Zagreb,
Croatia
Dr D. Kanungo, Division of Medical Toxicology, Central
Insecticides Laboratory, Department of Agriculture and
Cooperation, Directorate of Plant Protection, Quarantine and
Storage, Faridabad, Haryana, India
Professor M. Kessabi-Mimoun, Institut Agronomique et Vétérinaire
Hassan II, Rabat, Morocco
Professor M. Lotti, Università di Padova, Istituto di Medicina del
Lavoro, Padua, Italy (Chairman)
Professor A. Rico, Ecole Nationale Vétérinaire, Toulouse, France
(Vice-Chairman)
Observers
Mr C. Chelle, CFPI, Gennevilliers, France (GIFAP Representative)
Dr L. Diesing, Bayer AG, Institute of Toxicology and Agriculture,
Wuppertal, Germany (GIFAP Representative)
a Invited but unable to attend
Dr B. Krauskopf, Bayer AG, Leverkusen-Bayerwerk, Germany (GIFAP
Representative)
Dr Rouaud, Agrochemicals Division, CFPI, Gennevilliers, France
(GIFAP Representative)
Secretariat
Dr D. McGregor, Unit of Carcinogen Identification and Evaluation,
International Agency for Research on Cancer (IARC), Lyon,
France
Dr R. Plestina, International Programme on Chemical Safety, World
Health Organization, Geneva, Switzerland (Secretary)
NOTE TO READERS OF THE CRITERIA MONOGRAPHS
Every effort has been made to present information in the
criteria monographs as accurately as possible without unduly
delaying their publication. In the interest of all users of the
Environmental Health Criteria monographs, readers are kindly
requested to communicate any errors that may have occurred to the
Director of the International Programme on Chemical Safety, World
Health Organization, Geneva, Switzerland, in order that they may be
included in corrigenda.
* * *
A detailed data profile and a legal file can be obtained from
the International Register of Potentially Toxic Chemicals, Case
postale 356, 1219 Châtelaine, Geneva, Switzerland (Telephone No.
9799111).
* * *
This publication was made possible by grant number 5 U01
ES02617-14 from the National Institute of Environmental Health
Sciences, National Institutes of Health, USA.
ENVIRONMENTAL HEALTH CRITERIA FOR AMITROLE
A WHO Task Group on Environmental Health Criteria for Amitrole
met at the Ecole Nationale Vétérinaire, Toulouse, France, from 18 to
22 May 1993, the meeting being sponsored by the Direction générale
de la Santé, Ministère des Affaires sociales, de la Santé et de la
Ville, Paris. Professor A. Rico welcomed the participants on behalf
of the host institute. Dr R. Plestina, IPCS, opened the meeting and
welcomed the participants on behalf of Dr M. Mercier, Director of
the IPCS, and the three IPCS cooperating organizations
(UNEP/ILO/WHO).
The first draft was prepared by Dr P.J. Abbott, Department of
Health, Housing and Community Services, Canberra, Australia.
Extensive scientific comments were received following circulation of
the first draft to the IPCS contact points for Environmental Health
Criteria monographs and these comments were incorporated into the
second draft by the Secretariat. The Group reviewed and revised the
draft document and made an evaluation of the risks for human health
from exposure to amitrole.
Professor M. Lotti deserves special thanks for skilfully
chairing the meeting and for assistance to the Secretariat in
finalizing the monograph. Special thanks are also due to Professor
A. Rico for his technical support and exceptional hospitality.
Thanks are also due to Mrs A. Rico and the staff of the Ecole
Nationale Vétérinaire responsible for administrative aspects of the
meeting.
The fact that Bayer AG and Union Carbide made available to IPCS
and the Task Group proprietary toxicological information on their
products is gratefully acknowledged. This allowed the Task Group to
make its evaluation on a more complete data base.
Dr R. Plestina and Dr P.G. Jenkins, both members of the IPCS
Central Unit, were responsible for the overall scientific content
and technical editing, respectively, of this monograph. The efforts
of all who helped in the preparation and finalization of the
monograph are gratefully acknowledged.
ABBREVIATIONS
3-ATAL 3-(3-amino- s-triazole-1-yl)-2-aminopropionic acid
ACGIH American Conference of Governmental and Industrial
Hygienists
ADI acceptable daily intake
DAB 4-dimethylaminobenzene
DES diethylstilbestrol
DHPN N-bis(2-hydroxypropyl) nitrosamine
DIT diiodotyrosine
EC emulsifiable concentrate
GSH-Px glutathione peroxidase
HPLC high performance liquid chromatography
IC50 median immobilization concentration
MIT monoiodotyrosine
MTD maximum tolerated dose
NBU N-nitrosobutylurea
NOAEL no-observed-adverse-effect-level
NOEC no-observed-effect concentration
NOEL no-observed-effect level
OECD Organisation for Economic Co-operation and Development
PBI protein-bound iodine
PHS prostaglandin-H-synthetase
T3 L-triiodothyronine
T4 L-thyroxine
TC thin layer chromatography
TLV threshold limit value
TSH thyreostimulating hormone
TWA time-weighted average
WP wettable powder
1. SUMMARY
1.1 Identity, physical and chemical properties, and analytical
methods
Amitrole (3-amino-1,2,4-triazole) is a colourless, crystalline
powder. It is thermally stable and has a melting point of
156-159 °C. It is readily soluble in water and ethanol and only
sparingly soluble in organic solvents such as hexane and toluene.
Chemically, amitrole behaves as a typical aromatic amine as well as
an s-triazole. A wide range of analytical methods are available
for detection and quantification of amitrole in plants, soil, water,
air and urine.
1.2 Sources of human and environmental exposure
Amitrole does not occur naturally. It is manufactured by the
condensation of formic acid with aminoguanidine bicarbonate in an
inert solvent at 100-200 °C. Amitrole is used as a herbicide with a
wide spectrum of activity and appears to act by inhibiting the
formation of chlorophyll. It is commonly used around orchard trees,
on fallow land, along roadsides and railway lines, or for pond weed
control.
1.3 Environmental transport, distribution and
transformation
Owing to its low vapour pressure, amitrole does not enter the
atmosphere. It is readily soluble in water with a photodegradation
half-life in distilled water of more than one year.
Photo-degradation does occur in the presence of the photosensitizer
humic acid potassium salt, reducing the half-life to 7.5 h.
Amitrole is adsorbed to soil particles and organic matter by
proton association. The binding is reversible and not strong, even
in favourable acid conditions. Measured n-octanol/water partition
coefficient values classify amitrole as "highly mobile" in soils of
pH > 5 and "medium to highly mobile" at lower pH. There is
considerable variation in leaching of the parent compound through
experimental soil columns. Generally, movement is most readily seen
in sand; increasing the organic matter content reduces mobility.
Degradation in soils is usually fairly rapid but variable with
soil type and temperature. Bacteria capable of degrading amitrole
have been isolated. The herbicide can act as sole nitrogen source,
but not also as sole carbon source, for the bacteria. Microbial
degradation is probably the major route of amitrole breakdown;
little or no breakdown has been recorded in studies with sterilized
soil. However, abiotic mechanisms, including the action of free
radicals, have also been proposed as a means of degradation.
Laboratory studies have indicated degradation to CO2 with a
half-life of between 2 and 30 days. A single field study suggests
that the degradation may take longer at lower temperatures and
different soil moisture levels; the half-life was about 100 days in
a test clay.
Although the parent compound leaches through some soils,
degradation products are tightly bound to soil. Since amitrole is
degraded rapidly in soil, the high leaching potential of the
herbicide does not seem to be realized in practice. Occasional
damage to trees reported during the early usage of amitrole has not
been a regular feature of its use.
When applied to vegetation, amitrole is absorbed through the
foliage and can be translocated throughout the plant. It is also
absorbed through roots and transported in the xylem to shoot tips
within a few days.
High water solubility, a very low octanol-water partition
coefficient and non-persistence in animals means that there is no
possibility for bioaccumulation of amitrole or transport through
food chains.
1.4 Environmental levels and human exposure
Particulates containing amitrole may be released from
production plants; atmospheric levels of 0 to 100 mg/m3 have been
measured close to one plant.
The use of amitrole in waterways and watersheds has led to
transitory water concentrations of up to 150 µg/litre.
Concentrations fall rapidly to non-detectable (<2 µg/litre) levels
in running water within 2 h. Application to ponds gave an initial
water concentration of 1.3 mg/litre falling to 80 µg/litre after 27
weeks. Close to a production plant, river concentrations ranged from
0.5 to 2 mg/litre.
No residues of amitrole have been detected in food following
recommended use. Spraying of ground cover around fruit trees did not
lead to residues in apples. Wild growing fruit in the vicinity of
control areas can develop residues.
There have been no reports of amitrole in drinking-water.
1.5 Kinetics and metabolism in laboratory animals and humans
Following oral administration, amitrole is readily absorbed
from the gastrointestinal tract of mammals. It is rapidly excreted
from the body, mainly as the parent compound. The main route of
excretion in humans and laboratory animals is via the urine, and the
majority of excretion takes place during the first 24 h. Metabolic
transformation in mammals produces two minor metabolites detectable
in the urine of experimental animals. When an amitrole aerosol is
inhaled, a similar rapid excretion via the urine takes place.
1.6 Effects on experimental animals and in vitro test
systems
Amitrole had low acute toxicity when tested in several species
and by various routes of administration (LD50 values were always
higher than 2500 mg/kg body weight). It was found to affect the
thyroid after single, short-term and long-term exposures. Amitrole
is goitrogenic; it causes thyroid hypertrophy and hyperplasia,
depletion of colloid and increased vascularity. In long-term
experiments these changes precede the development of thyroid
neoplasia in rats.
The carcinogenic effect of amitrole on the thyroid is thought
to be related to the continuous stimulation of the gland by
increased thyroid stimulating hormone (TSH) levels, which are caused
by the interference of amitrole with thyroid hormone synthesis.
Equivocal results have been reported in some studies on the
genotoxic potential of amitrole. In carcinogenicity testing in rats,
amitrole did not induce tumours in organs other than the thyroid.
However, high doses of amitrole caused liver tumours in mice.
Several criteria have been used to assess the early effects of
amitrole on the thyroid. The lowest no-observed-adverse-effect level
(NAOEL) derived from these studies was 2 mg/kg in the diet of rats
and was assessed on the basis of thyroid hyperplasia.
1.7 Effects on humans
A single case of contact dermatitis due to amitrole has been
reported. Amitrole did not cause toxic effects when ingested at a
dose of 20 mg/kg. In a controlled experiment, 100 mg was found to
inhibit iodine uptake by the thyroid at 24 h. Weed control operators
exposed dermally to approximately 340 mg amitrole per day for 10
days exhibited no changes in thyroid function.
1.8 Effects on other organisms in the laboratory and field
Several studies on the growth of cyanobacteria (blue-green
algae) have shown no effect of amitrole at concentrations at or
below 4 mg/litre. No consistent adverse effects on nitrogen fixation
have been reported. Bacteria from soil were unaffected by
concentrations of 20 mg/litre medium in the case of nitrogen-fixing
Rhizobium and 150 mg/kg in the case of cellulolytic bacteria.
There were no effects on nitrification or soil respiration at 100 mg
a.i./kg dry soil, 5 times the maximum recommended application rate.
Reduced nodulation in sub-clover was reported at concentrations of
up to 20 mg/litre.
Various unicellular algae have been tested for
growth-inhibiting effects. At 0.2 - 0.5 mg amitrole/litre, the
growth inhibition of Selenastrum was the most sensitive reported
effect.
Most aquatic invertebrates show high tolerance to technical
amitrole: LC50 values were > 10 mg/litre for all organisms other
than the water flea Daphnia magna, where the acute 48-h EC50
(immobilization) was 1.5 mg/litre. Fish and amphibian larvae are
also tolerant to amitrole with LC50 values above 40 mg/litre.
Longer-term studies indicated that young rainbow trout survive an
amitrole concentration of 25 mg/litre for 21 days.
Two earthworm species (Eisenia foetida and Allolobophora
caliginosa) were unaffected by amitrole (SP50) at 1000 mg/kg
soil and Amitrole-T at 100 mg/kg soil, respectively. Carabid beetles
were unaffected after direct spraying with amitrole at rates
equivalent to 30 kg/ha. Effects on nematodes only occurred at high
concentrations of amitrole (the LC50 was 184 mg/kg).
Amitrole was reported to be non-hazardous to bees in field
trials. Amitrole has low toxicity to birds, all reported dietary
LC50 values being above 5000 mg/kg per diet. Acute oral dosing
killed no mallard ducks at 2000 mg/kg body weight.
2. IDENTITY, PHYSICAL AND CHEMICAL PROPERTIES, AND ANALYTICAL
METHODS
2.1 Identity
Common name: Amitrole
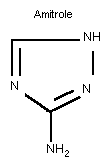 Chemical formula: C2H4N4
Relative molecular mass: 84.08
CAS chemical names: 1 H-1,2,4-triazol-3-amine (9C1)
3-amino- s-triazole (8CI)
IUPAC names: 1 H-1,2,4-triazol-3-ylamine
3-amino-1 H-1,2,4-triazole
3-amino- s-triazole
CAS registry number: 61-82-5
RTECS registry number: XZ3850000
Common synonyms: aminotriazole; 2-aminotriazole;
3-aminotriazole; 3-amino-1,2,4-
triazole; 2-amino-1,3,4-triazole;
3-amino-1H-1,2,4-triazole; AT;
3AT; ATA; 3,A-T; ATZ; AT-90;
triazolamine; 1,2,4-triazol-3-amine;
5-amino-1H-1,2,4-triazole.
Common trade names: Amerol; Aminotriazole Weedkiller
90; Aminotriazol Spritzpulver;
Amitril; Amitril T.L.; Amitrol;
Amitrol 90; Amitrol Plus;
Amitrol-T; Amizine; Amizol;
Amizol DP; Amizol F; AT Liquid;
Azaplant; Azolan; Azole; Azaplant
Kombi; Campaprim A1544; Cytrol;
Cytrole; Destraclol; Diurol. 5030;
Domatol; Domatol 88; Elmasil;
Emisol; Emisol 50; Emosol F; ENT
25445; Exit; Fenamine; Fenavar;
Fyrbar; Kleer-Lot; Lancer;
Nu-Zinole-AA; Orga 414; Preceed;
Radoxone TL; Ramizol; Sapherb;
Solution Concentree T271; Ustinex;
Vorox; Vorox AA; Vorox AS;
Weedar ADS; Weedar AT; Weedazin;
Weedazin Arginit; Weedazol;
Weedazol GP2; Weedazol Super;
Weedex Granulat; Weedoclor; X-All
Liquid.
Technical grade amitrole contains a minimum of 95% active
ingredient and is formulated as a solution of 250 g/litre in water,
usually with an equimolar concentration of ammonium thiocyanate, or
as a 400 g/kg wettable powder, usually in combination with other
herbicides.
The major impurities are 3-(N-formylamino)-1,2,4-triazole,
4 H-1,2,4-triazole-3,4-diamine, and
4 H-1,2,4-triazole-3,5-diamine.
2.2 Physical and chemical properties
Some of the physical and chemical properties of amitrole are
shown in Table 1.
Amitrole is readily soluble in water, methanol, ethanol and
chloroform, sparingly soluble in ethyl acetate, and insoluble in
hydrocarbons, acetone and ether. It forms salts with most acids or
bases and is a powerful chelating agent. It is corrosive to
aluminium, copper and iron. Chemically, amitrole behaves as an
s-triazole and also as an aromatic amine, and hence will diazotize
and couple several dyes.
2.3 Conversion factors:
1 mg/kg = 3.43 mg/m3 1 mg/m3 = 0.29 mg/kg
Table 1. Some physical and chemical properties of amitrole
Physical state crystalline
Colour colourless
Taste bitter
Odour none
Thermal stability stable at 20 °Ca
Hydrolytic stability (pH 4-9; 90 °C) stableb
Melting point 157-159 °Cc
Water solubility (25 °C) 280 g/litrec
Water solubility (53 °C) 500 g/litred
Ethanol solubility (75 °C) 260 g/litred
Solubility in n-hexane (20 °C) < 0.1 g/litred
Solubility in dichloromethane (20 °C) 0.1-1 g/litred
Solubility in 2-propane 20-50 g/litred
Solubility in toluene (20 °C) <0.1 g/litred
Vapour pressure (20 °C) 55 nPac
Octanol/water partition coefficient (21 °C)
(log Pow) -0.969e
a Klusacek & Krasemann (1986)
b Krohn (1982)
c Worthing & Hance (1991)
d Personal communication from Bayer AG to the IPCS (1993)
e Hazleton Laboratories, USA Report HLA-6001-187
2.4 Analytical methods
2.4.1 Plants
Early methods for the detection of amitrole by paper
chromatography or for its quantitative determination by
spectrophotometry involved extraction by ethanol or water,
diazotization of the 3-amino group and, finally, coupling with
either phenol in 20% HCl (Aldrich & McLane, 1957),
N-(1-naphthyl)ethylenediamine dihydrochloride (Storherr & Burke,
1961), H-acid (8-amino-1-naphthol-3,6-disulfonic acid, monosodium
salt) (Racusen, 1958; Herrett & Linck, 1961; Agrawal & Margoliash,
1970) or chromotropic acid (Green & Feinstein, 1957). This technique
has been used for residue analysis in plants (Aldrich & McLane,
1957; Herrett & Linck, 1961), and vegetable crops (Storherr & Burke,
1961). The detection limit was found by Aldrich & McLane (1957) to
be approximately 0.1 µg/spot. The method outlined by Storherr &
Burke (1961) is sensitive to 0.025 mg/kg. Recovery was described by
Herrett & Linck (1961) to be close to 100%. Storherr & Onley (1962)
found that dry-packed cellulose column chromatography was preferable
to paper chromatography for separation of amitrole from some crops.
Several gas chromatographic methods have been developed to
determine amitrole residues in plants (Jarczyk, 1982a, 1985; Jarczyk
& Möllhoff, 1988). The principle of all these methods is similar.
After extraction with an ethanol-water mixture, acetylation with
acetic anhydride (conversion of amitrole to the monoacetyl
derivative) and a clean-up step by gel chromatography, the residue
is dissolved in acetone or ethanol and determined by a gas
chromatograph equipped with a nitrogen-phosphorus detector.
Weber (1988) developed a method for the determination of
amitrole in plant material by high performance liquid chromatography
(HPLC). Amitrole was extracted with an acetone-water mixture and the
water phase was extracted with dichloromethane to remove lipophilic
compounds. After a further clean-up step with column chromatography
on a cation exchange resin and on aluminium oxide, the residues were
determined by HPLC with ion pairing reagent and electrochemical
detection. In plants the detection limit was 0.01 mg/kg and the
recovery was between 91 and 99% in the range 0.01-1.0 mg/kg.
The Codex Committee on Pesticide Residues has recommended the
methods of Lokke (1980) and Van der Poll et al. (1988).
The method of Lokke (1980) uses ion-pair HPLC, which in
potatoes or fodder beets had a limit of detection between 0.005 and
0.01 mg/kg.
The method of Van der Poll et al. (1988) is capable of
determining amitrole in plant tissues and sandy soils by capillary
gas chromatography with an alkali flame ionization detector. Samples
are extracted with ethanol, absorbed on resin and desorbed with
ammonia. After acetylation with acetic anhydride and clean-up with a
SEP-PAK silica cartridge, the residue is determined by gas
chromatography (GC). The limit of detection is 0.02 mg/kg and
average recoveries are 76-81% in the range from 0.05 to 0.2 mg/kg.
2.4.2 Soil
An early method for the determination of residues in soil was
developed by Sund (1956) which involved extraction with water
followed by colour reaction with nitroprusside in alkaline
solutions.
Groves & Chough (1971) developed an improved procedure for the
extraction of amitrole from soil using concentrated ammonium
hydroxide and glycol (1:4). Pribyl et al. (1978) investigated the
extraction of amitrole from soils and its identification and
quantitation by photometry and thin layer chromatography (TLC). The
limit of detection was 0.05 mg/kg. They proposed analysis by TLC
after reaction with 5-dimethylaminonaphthalene-1-sulfonyl chloride
(dansylation), in preference to HPLC. Lokke (1980) suggested that
HPLC separation could be used if preceded by clean-up on a polyamide
column. Both the GC method (Jarczyk, 1985; Jarczyk & Möllhoff, 1988)
and the HPLC method (Weber, 1988) described in section 2.4.1 for
plants are also suitable for the determination of amitrole residues
in soil.
2.4.3 Water
Marston et al. (1968) and Demint et al. (1970) have used cation
ion-exchange column chromatography to extract amitrole from
contaminated creek and canal waters. This is followed by
diazotization and coupling as described by Storherr & Burke (1961).
Alary et al. (1984) have modified these methods to achieve a
spectrophotometric determination of amitrole in waste water in the
vicinity of production plants in the presence of interfering amino
compounds.
A more recent capillary gas-liquid chromatographic method for
determining amitrole in ground water and drinking-water, using an
alkali flame ionisation detector, has been described, the reported
limit of detection being 0.1 µg/litre (Van der Poll et al., 1988).
Legrand et al. (1991) formed a nitroso derivative of amitrole
concentrated from surface and ground waters prior to HPLC analysis.
The nitroso derivative showed an absorption maximum in the near UV
spectrum. Aqueous solutions of amitrole in the range of
0.25-0.50 µg/litre were measurable, and the recoveries were 70 ± 8%
(n = 11). The limit of determination was 0.1 µg/litre.
Pachinger et al. (1992) developed an HPLC analytical method
with amperometric detection for the determination of amitrole
without derivatization in drinking-water and ground water. Detection
limits were 1 mg/litre for directly injected samples and
0.1 µg/litre following an evaporation step to concentrate the
samples. Recoveries were close to 100%.
Both the GC method (Jarczyk, 1985; Jarczyk & Möllhoff, 1988)
and the HPLC method (Weber, 1988) described in section 2.4.1 for
plants are also suitable for the determination of amitrole residues
in water.
An immunochemical approach to the detection of amitrole has
been recently described by Jung et al. (1991). Development of this
rapid and sensitive method is likely to lead to a very effective
method for detecting amitrole in waterways.
2.4.4 Formulations
Ashworth et al. (1980) described a potentiometric precipitation
titration method using silver nitrate and silver/silver chloride or
silver/mercurous sulfate electrode. This method can be used for the
determination of amitrole in its formulations or in the presence of
triazines, substituted urea herbicides or plant growth regulators
such as bromacil and ammonium thiocyanate. Another method for the
determination of amitrole in its formulations has been described by
Gentry et al. (1984). This involves dissolving or extracting the
sample with dimethylformamide, acidifying by adding 0.5 N HCl and
back-titrating the excess acid with 0.5 N sodium hydroxide.
Jacques (1984) has described a simple GC method for the
detection and quantification of amitrole in technical and formulated
products.
A TLC method for the routine identification of amitrole in
pesticide mixtures has been developed by Ebing (1972).
2.4.5 Air
Alary et al. (1984) have described a method for the analysis of
air samples collected on glass-fibre filters followed by
diazotization and coupling to produce a colour reaction. The
detection limit was not reported.
2.4.6 Urine
In order to assay amitrole in urine samples, Geldmacher-von
Mallinckrodt & Schmidt (1970) separated the amitrole by paper
chromatography using phenol saturated with water, or a mixture of
n-butanol:water (15:1) and propionic acid:water (7:6), and
identified amitrole by spraying with a solution of
p-dimethyl-aminobenzaldehyde in acetic acid or hydrochloric acid.
In a more recent paper by Archer (1984), a proposed method for
biological monitoring of urine samples used HPLC separation with a
visible light detector following diazotization and coupling. The
detection limit was 200 µg/litre.
3. SOURCES OF HUMAN AND ENVIRONMENTAL EXPOSURE
3.1 Natural occurrence
Amitrole does not occur naturally.
3.2 Anthropogenic sources
3.2.1 Production levels and processes
The synthesis of amitrole was first reported by Thiele &
Manchot in 1898 and involved the reaction of aminoguanidine with
formic acid (Carter, 1975). The current industrial production
process, described by Allen & Bell (1946), involves the same
reaction, in which an aminoguanidine salt is heated to 100-120 °C
with formic acid in an inert solvent (Carter, 1975; Sittig, 1985).
Amitrole is currently manufactured or formulated in several
countries. Its use has declined, particularly in the USA. However,
in spite of some recent replacements, amitrole remains a widely used
herbicide.
3.2.2 Uses
Amitrole is primarily used as a post-emergent non-selective
herbicide and has a very wide spectrum of activity against annual
and perennial broad leaf and grass type weeds. Its primary mode of
action is unknown but a prominent feature is its inhibition of the
formation of chlorophyll, and weeds initially change colour to
white, brown or red, and subsequently die (Carter, 1975). This
herbicidal activity is enhanced by the addition of ammonium
thiocyanate as a synergist. Amitrole can be used alone as a
concentrated solution in water or as a wettable powder in
combination with other herbicides. It is primarily used as a
herbicide and as a brush killer. It is also used as a non-selective
pre-emergent herbicide on fallow land before planting kale, maize,
oilseed rape, potatoes and wheat, and in other non-crop situations
(Worthing & Hance, 1991). It is also used along roadsides and
railway lines to control weeds. Approved uses of amitrole on soil
are either for non-crop land prior to sowing, or for inter-row weed
control in tree and vine crops, where contact with food plants is
avoided. Amitrole is also used for the control of pond weeds and is
an especially effective herbicide in the control of water hyacinth
(Eichhornia crassipes).
Amitrole has also been used as a cotton defoliant in some
countries (Hassall, 1969).
4. ENVIRONMENTAL TRANSPORT, DISTRIBUTION AND TRANSFORMATION
4.1 Transport and distribution between media
4.1.1 Air
The very low vapour pressure of amitrole (Table 1) means that
it will not enter the atmosphere.
4.1.2 Water
Amitrole is readily soluble in water (280 g/litre at 25 °C) and
has a half-life of more than one year at 22 °C and pH 4-9 (Worthing
& Hance, 1991). Although no direct photolysis occurred in doubly
distilled water, the photodegradation rate increased in the presence
of humic acid, potassium salt (100 mg/litre), a natural
"photosensitizer", resulting in a half life of 7.5 h (Jensen-Korte
et al., 1987).
4.1.3 Soil
4.1.3.1 Adsorption
Amitrole is adsorbed to soil particles and organic matter by
proton association. The adsorbed aminotriazolium cation will enter
into cationic exchange reactions (Nearpass, 1969). Binding is
strongly pH dependent, and the cation is adsorbed to a greater
extent in acid conditions. The aminotriazolium cation is bound more
strongly than sodium but is displaced by calcium ions. The binding
is reversible and not strong, even in favourable acid conditions.
The binding capacity of soils at pH 5 or more is limited (Nearpass,
1970). Anderson & Hellpointner (1989) determined the Koc values
for amitrole in four soils i.e. silty clay, sandy loam, sand and
silt, to be 112, 30, 20 and 52, respectively. Adsorption increased
at lower pHs; adjustment of pH to a constant 4.5 resulted in Koc
values ranging from 77 to 356. The authors classified amitrole as
highly mobile in the soils at their equilibrium pH values of 5.6 to
7.4 and medium to highly mobile with the pH adjusted from 4.2 to
4.5.
There is considerable variation in the literature, both old and
recent, in reported adsorption and leachability of amitrole. Sund
(1956) described adsorption to soil as strong. He demonstrated that
amitrole could be efficiently removed from aqueous solution with a
resin cation exchanger and argued that soil would also bind the
compound efficiently. A correlation between the base exchange
capacity of soil and binding of amitrole was postulated. This agrees
with the theoretical and experimental work of Nearpass (1969, 1970),
although the latter author does not support the strength of
adsorption proposed by Sund (1956). Day et al. (1961) investigated
leaching of amitrole through 400-g, 4-cm diameter columns of three
different soils from a citrus growing area of California following
occasional reports of damage to trees after application of high
rates of amitrole for the control of perennial weeds. Amitrole moved
readily with the leaching water for all soil types (two sandy loams
and one silt loam) and most readily through quartz sand. Zandvoort
et al. (1981) supported this conclusion, suggesting that the high
water solubility of amitrole could result in leaching from sandy
soils. Weller (1987) investigated leaching of amitrole through
27-cm, 5-cm diameter columns of two soils, a sandy "standard soil
2.1" and a second soil with substantially higher organic content.
Immediately after incorporation of the 14C-amitrole to give 2 mg
on the surface area of the column, leaching with deionized water
began, 393 ml being pumped onto the soil column over 2 days. The
leachate was collected in two fractions: 175-191 ml and 185-200 ml.
Duplicate experiments showed 24 and 31% of the initial radioactivity
in the leachate (entirely in fraction II) in the sandy soil, with
11%, 16% and 46%, respectively, remaining in the upper, middle and
lower third of the soil column. The second soil leached markedly
less of the added radioactivity (1.4 and 1.8% for the duplicate
columns); this also appeared in fraction II. The radioactivity in
the leachate was unchanged amitrole.
Since amitrole is degraded rapidly in soil (section 4.2.1), the
high potential of amitrole to leach through sandy soils does not
seem to be realized in practice. The occasional damage to trees
reported in the study by Day et al. (1961) has not been a regular
feature of the use of amitrole. Degradation products of amitrole do
not leach significantly through soil (section 4.2.1).
4.1.4 Vegetation and wildlife
When applied directly to vegetation as a herbicide, amitrole is
absorbed through the foliage and can be translocated throughout the
plant. Translocation occurs in the photosynthetic stream and is
dependent on light. When applied to soil, amitrole can be adsorbed
through the roots and transported in the xylem, within a few days,
to the tips of the shoots (Carter, 1975).
4.1.5 Entry into food chain
Amitrole is not to be used on food crops and therefore food
residues should not occur. Grazing animals could consume amitrole as
surface residues on vegetation after application or as residues
within the plant. Amitrole is not persistent in animals and would
not be expected to pass through the food chain.
4.2 Biotransformation
4.2.1 Biodegradation and abiotic degradation
4.2.1.1 Plants
Racusen (1958) reported the first comprehensive studies of
amitrole metabolism in plants. Two major metabolites were isolated,
neither of which were as phytotoxic as amitrole. These results were
supported by studies by Carter & Naylor (1960). One metabolite was
identified as the product of the reaction of amitrole with serine,
namely, 3-(3-amino- s-triazole-1-yl)-2-aminopropionic acid
(3-ATAL). Formation of 3-ATAL is considered to represent
detoxification since ammonium thiocyanate, which synergizes the
action of amitrole, inhibits the formation of 3-ATAL (Smith et al.
1969). Other products of amitrole metabolism in plants have not been
identified. Fang et al. (1967) found that metabolism of amitrole in
leaves was exponential, with half-lives in sugar beet, corn and bean
leaves being 18.7, 28.0 and 23.2 h, respectively. A review of the
degradation of amitrole in plants has been presented by Carter
(1975).
The soluble metabolites of [3,5-14C]-amitrole in apples were
examined by Schneider et al. (1992) following soil application.
Significant proportions of the radioactivity were found as bound
residues, but 69-90% were extractable with acetonitrile. In addition
to 3-ATAL, 3-(1,2,4-triazole-1-yl)-2-aminopropionic acid
(3-aminotriazolylalanine) was also identified, in both the free form
and as conjugates. This was the major metabolite in apple cell
cultures treated with amitrole (Stock et al., 1991).
4.2.1.2 Soils
There is general agreement that degradation of amitrole in soil
is usually fairly rapid and variable with soil type and temperature.
However, there is no clear consensus on the relative roles of biotic
and abiotic processes in the breakdown of the compound.
Day et al. (1961) measured amitrole colorimetrically in 55
different soils of 5 main types from California and estimated the
depletion after 2 weeks of incubation. The results were very
variable, 26 soils having no measurable amitrole after 2 weeks, 6
soils showing traces and the remaining 23 soils having higher
quantities, in some cases comparable to initial levels. Four soils
had more than half of the original amitrole after the 2-week
incubation. It was not possible to correlate depletion of amitrole
to soil type. The authors classified the soils according to general
type and ranked them in terms of "heaviness"; the four soils
retaining most amitrole ranked 7, 23, 30 and 54 in the list. There
was a geographical correlation with reported incidents of non-target
effects of the herbicide. Some specific characteristic of a variety
of soils from a single location had led to movement of the herbicide
and its retention longer than in apparently comparable soils
elsewhere. Decomposition rates in steam-sterilized soils were much
lower than in unsterilized soils, which led the authors to conclude
that breakdown was principally due to microorganisms. Decomposition
was optimal at temperatures between 20 and 30 °C and at medium to
high soil moisture content. Breakdown was not well correlated with
soil classification, texture, base-exchanged capacity or adsorption
capacity for amitrole. Differences in microbial populations were
cited as the most likely explanation for the variation.
Kaufman et al. (1968) also found that sterilization of soil
reduced the breakdown of amitrole. Within 20 days, 69% of the
radioactivity of 14C-labelled amitrole was released as 14CO2
in unsterilized soil. Soil treated with sodium azide or ethylene
oxide released 46% and 35%, respectively, whilst autoclaved soil
released only 25%. Reinoculation of soil with microorganisms
isolated from the original soil failed to restore the capacity to
degrade amitrole. Amending the soil with other organic compounds
reduced amitrole degradation. The authors concluded that degradation
of amitrole was largely a chemical process and that microbial action
was indirect. Free radicals (such as HO.) were proposed as agents
for oxidation of the amitrole nucleus. Plimmer et al. (1967) studied
the degradation of amitrole by free-radical generating systems. They
demonstrated that riboflavin (and light) or an ascorbate-copper
reagent (Fenton's reagent) promotes oxidation of amitrole, resulting
in ring cleavage, loss of CO2 and production of urea, cyanamide
and possibly molecular nitrogen. Riepma (1962) observed a lag-phase
which he considered typical of microbial breakdown. Carter (1975)
concluded that "whatever the mechanism, triazole ring opening occurs
rapidly in soils and the resulting products ... should be readily
metabolized by soil microorganisms".
Campacci et al. (1977) reported the isolation of bacteria
capable of degrading amitrole, strengthening the argument for
microbial involvement. Only one of three media tested succeeded in
growing organisms that could degrade amitrole. Of 36 isolates from
this culture, 10 were found to be capable of degrading amitrole.
Nine of these were gram-positive rods (Bacillus spp. and
Corynebacterium spp.) and one was identified as a Pseudomonas
sp. The growth of these bacteria was roughly proportional to
amitrole concentration up to 256 mg/litre. The organisms could
degrade amitrole as sole nitrogen source but not also as sole carbon
source; the medium was enriched with sucrose. This explained
previous failures to isolate organisms capable of degrading the
herbicide.
Various studies have quantified the degradation of amitrole in
soil. Scholz (1988) observed the release of 48% of applied
radioactivity (14C-amitrole) as 14CO2 after 5 days. In
degradation studies in the laboratory, half-lives of between 2.4 and
9.6 days were observed in different soils. DT90 (the time required
for degradation of 90% of the amitrole) values were in the range of
13 to 22 days (LUFA, 1977; Jarczyk, 1982b,c,d). Hawkins et al.
(1982b) measured 70-80% degradation to CO2 in standard soil and
40-50% in English clay soil within 28 days. There was no release of
14CO2 from autoclaved soil. Hawkins et al. (1982a) measured
decomposition in the same English clay in the field. Here 53% of the
applied radioactivity remained after 112 days. The slower rate of
breakdown in the field was ascribed to the temperature and soil
moisture content. Schneider et al. (1992) suggested that amitrole
can be deaminated in soil to give triazole.
A study by Weller (1987) examined the leaching of "aged"
residues of amitrole. Soils, with 14C-amitrole incorporated as
described in section 4.1.3.1, were incubated for 30 and 92 days, in
duplicate experiments, and then used in leaching tests as for the
initial soils. For both the "standard soil 2.1" and the second soil,
between 50 and 73% of initial radioactivity was lost as 14CO2
during incubation. Of the remaining radioactive material, negligible
amounts leached through the soil column in tests after 30 and 92
days. Almost all of the activity remained in the upper third of the
soil column. After 30 days 4% or less of the activity was unchanged
amitrole. The breakdown products of amitrole (not characterized
except for traces of urea) were tightly bound to the soil and were
not leachable or easily extractable.
4.2.3 Bioaccumulation
Flow-through studies on fish using 14C-amitrole indicated
that the bioaccumulation of amitrole in bluegill sunfish (Lepomis
macrochirus) and in channel catfish (Ictalurus punctatus),
exposed to 1 mg/litre, was only slight after 21 days of exposure
(approximately 1.7-3.0 times the amitrole concentration in the
water). When the fish were returned to untreated water, the amitrole
concentration in their organs fell rapidly (Iwan et al., 1978).
Bioaccumulation of amitrole by aquatic organisms would not be
expected because of its high water solubility and very low
octanol-water partition coefficient (Table 1).
4.3 Ultimate fate following use
MacCarthy & Djebbar (1986) described a method using chemically
modified peat to decontaminate eluant from chemical production
plants before it enters major waterways. When converted to a
granular product suitable for column chromatography, the peat can
act as an efficient ion-exchange material for the removal of
amitrole and other basic chemicals.
Amitrole is resistant to hydrolysis and the action of oxidizing
agents. Burning the compound with polyethylene is reported to result
in > 99% decomposition (Sittig, 1985).
5. ENVIRONMENTAL LEVELS AND HUMAN EXPOSURE
5.1 Environmental levels
5.1.1 Air
Amitrole-containing particles are released from the stack of
production plants during dry crushing and, to a lesser extent,
bagging operations. Atmospheric levels in the range of 0 to
100 µg/m3 were measured in the vicinity of such a plant (Alary
et al., 1984). Severe chlorosis and defoliation was noted following
atmospheric fallout in the vicinity of the plant.
5.1.2 Water
Grzenda et al. (1966) studied the persistence of amitrole and
three other herbicides in pond water following an aquatic weed
control programme. The initial level on day one of 1.34 mg/kg
decreased gradually to 1.03 mg/kg on day 11, 0.73 mg/kg on day 25,
0.49 mg/kg at 9.5 weeks and 0.08 mg/kg at 27 weeks.
In a study by Marston et al. (1968) in which 100 acres of a
watershed in Oregon was sprayed, the levels of amitrole in water
samples were measured on the downstream edge of the sprayed area. A
maximum concentration of 155 µg/litre was found 30 min after
application began, but this decreased to 26 µg/litre by the end of
the application. No amitrole could be detected 6 days after
spraying. The detection limit was 2 µg/litre.
Demint et al. (1970) measured the amitrole concentration in two
flowing water canals following treatment of a single ditchbank of
each canal with amitrole at the normal treatment rate. Samples taken
at stations up to 7.2 km downstream indicated rapid decreases in
amitrole levels following passage of the initial amitrole-bearing
water, the levels having declined to 1 µg/litre within 2 h. In a
preliminary environmental survey conducted in 1984 in Japan,
amitrole was not detected (detection limit 4 µg/litre) in any of 24
water samples nor was it detected in any of the 24 bottom sediments,
the detection limit being 5-20 µg/kg (Environment Agency Japan,
1987).
Alary et al. (1984) measured the level of amitrole in water
samples collected in a river downstream from the discharge of an
aeration pond in the vicinity of a production plant. The levels were
in the range of 0.5 to 2 mg/litre while the concentration in the
water of the aeration pond was in the range of 50 to 200 mg/litre.
Legrand et al. (1991) tried to detect 38 compounds including
amitrole in different areas of France (13 sampling points) with a
detection limit of 0.1 µg/litre, but no amitrole was found.
5.1.3 Soil
As discussed in chapter 4, amitrole, when applied to soil, is
readily degraded or adsorbed to the soil particles.
5.2 General population exposure
5.2.1 Environmental sources
No exposure would be expected from environmental sources.
5.2.2 Food
Amitrole is not to be used on food crops, and food residues
should therefore not occur. Using a limit of determination of
0.05 mg/kg, amitrole was not detectable in a wide range of food
crops (Duggan et al., 1966, 1967; Corneliussen, 1969, 1970). This
was confirmed by several studies (Bayer AG, 1993a,b).
Experimental studies in West Virginia, USA, indicated that
residues of amitrole on whole apples could not be detected 3 months
after ground cover application, but could be detected when either
fruit or foliage or both were directly treated with amitrole
(Schubert, 1964). The analyses were conducted using the method of
Storherr & Burke (1961) with a detection level of 0.025 mg/kg.
Similarly, residue trials conducted in Tasmania and New South
Wales on apples did not reveal amitrole at a detection limit of
0.01 mg/kg following ground cover application (Moore, 1968, 1969,
1970). A slight modification of the method of Storherr & Burke
(1961) was used.
In one study, residues of amitrole were found in blackberries
growing very near a railway line that was sprayed by amitrole in the
normal way by the railway authorities. Thirteen days after spraying
at a dose 3,5 kg a.i./ha, blackberries were picked close to the
railway at two different locations. The mean residues found at the
two locations were 0.67 (0.2-1.4) mg/kg and 2.0 (0.1-3.8) mg/kg. The
places where the blackberries were picked was prohibited to the
general public. This study shows that spraying of amitrole on
blackberries results in considerable residues (Dornseiffen &
Verwaal, 1988).
5.3 Occupational exposure during manufacture, formulation or use
The potential for toxicity via the dermal or inhalational
routes appears to be low. A threshold limit value (TLV) of
0.2 mg/m3, as an 8-h time-weighted average (TWA), has been set for
amitrole by the American Conference of Governmental & Industrial
Hygienists (ACGIH, 1991-1992). Amitrole is a mild skin and eye
irritant.
6. KINETICS AND METABOLISM IN LABORATORY ANIMALS AND HUMANS
6.1 Absorption, distribution and excretion
6.1.1 Mouse
The distribution of [5-14C]-radiolabelled amitrole has been
examined in non-pregnant C57BL strain female mice (Tjalve, 1975) and
in the fetuses of pregnant NMRI strain mice (Tjalve, 1974). In each
case, the mice received amitrole (5 µCi) either intravenously or
orally, and the distribution of radioactivity was determined by
whole-body autoradiography of the adult or fetus at intervals of
5 min to 5 days after administration. In the non-pregnant animals,
further analysis of the distribution of radioactivity was performed
by microautoradiography of the spleen and thymus, and by subcellular
fractionation of the liver, spleen and thymus. The highest
radioactivity was found in tissues with rapid cell turnover, e.g.,
bone marrow, spleen, thymus and gastrointestinal mucosa. Only a
moderate level of radioactivity was found in the thyroid. The level
of radioactivity in the tissues was the same whether the treatment
was intravenous or oral. In all cases, there was a significant
decrease over the 5-day period. Microautoradiography indicated
amitrole was located mostly in the cytoplasm. 14C-labelled
amitrole crossed the placental barrier and could be detected in
fetal tissues 4 and 8 h after administration to the dams by
intravenous injection or gavage.
Following intravenous administration of 14C-amitrole
(3.4 mg/kg body weight), adult ICR mice were killed at given
intervals (5 min, 30 min, 8 h and 24 h) and submitted to whole-body
autoradiography and microautoradiography. The liver had the highest
accumulation of radioactivity and two distribution patterns were
observed: a homogenous distribution up to 8 h following injection,
and a subsequent heterogenous one. Liver sections were extracted
with trichloroacetic acid and methanol, but considerable amounts of
radioactivity remained non-extractable. A microauto-radiography of
the liver 8 h after 14C-amitrole injection revealed that the
radioactivity was localized in the centrolobular areas (Fujii
et al., 1984).
6.1.2 Rat
Kinetic studies on amitrole in rats were performed by Fang
et al. (1964). Groups of Wistar rats were administered 1 mg
14C-amitrole by gavage and the distribution of radioactivity was
analysed at various time intervals between 30 min and 6 days. High
levels of radioactivity (70-95% of the administered radioactivity)
were found in the urine during the first 24 h, but only low levels
in the faeces, indicating rapid and almost complete absorption from
the gastrointestinal tract followed by rapid excretion. Tissue
levels were very low after 3 days, and significant amounts were
found only in the liver. In a second experiment (Fang et al., 1966),
14C-amitrole was administered at various dose levels (1-200 mg/kg
body weight). The average total radioactivity found in urine and
faeces (as a percentage of the administered dose) was comparable for
all the doses applied. The difference in average half-time for
clearing of radioactivity from various organs was considered to be
insignificant between dosages of 1 and 200 mg/kg. The fate of two
unidentified plant metabolites of amitrole, i.e. 14C-metabolite 1
and 14C-metabolite 3 (isolated from bean plants), was also
examined by Fang et al. (1966). Radioactivity from metabolite-1 was
excreted rapidly in the urine in the first 48 h and identified as
unchanged metabolite-1. Elimination of metabolite-3 was mainly in
the faeces. In a study by Grunow et al. (1975), 14C-amitrole was
administered to rats by gavage at a dose level of 50 mg/kg, and the
urine and faeces were examined over 3 days. The major route of
excretion of radioactivity was the urine, the majority of the
radioactivity being excreted in the first 24 h.
Two groups of five male and five female Sprague-Dawley rats
weighing 200-250 g were exposed (either nose only or whole body) to
atmospheres of 5-14C-amitrole (radiochemical purity > 97%) in
water aerosols at concentrations in air of 49.2 µg/litre
(2.6 µCi/litre) or 25.8 µg/litre (1.4 µCi/litre), respectively, for
1 h, and then observed for 120 h (MacDonald & Pullinger, 1976). The
particle size distribution of the aerosols was not reported. The
calculated elimination half-life of radioactivity was approximately
21 h for both exposures; approximately 75% of the radioactivity was
eliminated in the urine within 12 h.
6.1.3 Human
Urinary excretion of unchanged amitrole has been reported in a
woman who ingested approximately 20 mg/kg of the herbicide
(Geldmacher-von Mallinckrodt & Schmidt, 1970).
6.2 Metabolic transformation
The limited data available indicates that little metabolic
transformation of amitrole occurs in mammalian species. In the
mouse, tissue residues were identified by TLC as mainly unchanged
amitrole (84% of the detected radioactivity) when measured 8 h after
exposure (Tjalve, 1975). Similarly, paper chromatographic analysis
of rat liver residues following oral administration revealed
unchanged amitrole plus one unidentified metabolite (Fang et al.,
1964). In the urine of rats, the majority of the radioactivity was
also unchanged amitrole; one unidentified metabolite was isolated
which represented approximately 20% of the total radioactivity. The
liver was the site of the unidentified metabolite-1 formation and
the rate of elimination of this metabolite from liver and kidney was
much slower (Fang et al., 1964).
In a more extensive analysis of urinary metabolites in the rat
by Grunow et al. (1975), the major part of the radioactivity
identified by paper chromatography corresponded to unchanged
amitrole. Two urinary metabolites were identified as
3-amino-5-mercapto-1,2,4-triazole and
3-amino-1,2,4-triazolyl-(5)-mercapturic acid, which together
amounted to approximately 6% of the administered dose.
In a metabolic study (Turner & Gilbert, 1976), which was
supplementary to the inhalation exposure experiment and is described
in section 6.1.2 (MacDonald & Pullinger, 1976), it was found that
approximately 60% of the urinary radioactivity chromatographed on
silica gel 60 TLC in methanol: 880 ammonia (100: 1.5, s/s) as
amitrole, 15-20% remained at the origin and 5-8% migrated faster
than amitrole. Treatment with ß-glucuronidase had no effect upon
this TLC distribution.
7. EFFECTS ON EXPERIMENTAL ANIMALS AND IN VITRO TEST SYSTEMS
7.1 Single exposure
7.1.1 Oral
The acute oral toxicity data for amitrole when administered as
an aqueous suspension are presented in Table 2.
Table 2. Acute oral toxicity of amitrole
Species LD50 (mg/kg Reference
body weight)a
Rat > 4080 (m and f) Gaines et al. (1973)
> 4200 Seidenberg & Gee
24 600 (m) Bagdon et al.(1956)
> 10 000 Hecht (1954)
> 2500 Kimmerle (1968)
> 5000 (m) Thyssen (1974)
> 5000 (m) Heimann (1982)
Mouse 11 000 Hapke (1967)
14 700 (m) Fogleman (1954)
Cat > 5000 (m and f) Bagdon et al. (1956)
a m = males; f = females
In cats and dogs the general signs of toxicity were dyspnoea,
ataxia, and diarrhoea with vomiting. Coma and death appeared to be
associated with profound respiratory depression. Gastro-intestinal
irritation and haemorrhage were the only treatment-related findings.
The toxicity of a mixture of amitrole and ammonium thiocyanate
(1:1), referred to as Amitrol-T, appeared to be slightly higher than
that of amitrole itself, but was still very low. LD50 values
obtained following oral administration in rats were 3500 mg/kg and
10.5 ml/kg of the commercial product (DeProspo & Fogleman, 1973;
Field, 1979).
The possibility that amitrole might form a Schiff's base with
glucose was investigated by Shaffer et al. (1956). An
amitrole-glucose adduct was prepared and administered orally to rats
and mice (10 mg/kg), intraperitoneally to mice (10 mg/kg), and
intravenously to mice (1.6 mg/kg). There were no deaths or signs of
toxicity following treatment.
7.1.2 Other routes
The acute toxicity of amitrole by other routes of
administration is very low, as shown in Table 3.
Table 3. Acute, dermal, intraperitoneal and intravenous toxicity
of amitrole
Species Route LD50 (mg/kg bw) Reference
Rat dermal > 2500 (m and f) Gaines et al. (1973)
intraperitoneal > 4000 (m) Shaffer et al. (1956)
Mouse intravenous > 1600 (m) Shaffer et al. (1956)
intraperitoneal > 10 000 Shaffer et al. (1956)
intraperitoneal 5470 (m) Nomiyama et al. (1965)
subcutaneous 5540 (m) Nomiyama et al. (1965)
Rabbit dermal > 10 000 Elsea (1954)
Dog intravenous > 1800 (m) Fogleman (1954)
Cat intravenous > 1750 (m and f) Shaffer et al. (1956)
a m = males; f = females
Amitrole applied in water formulations to the unabraded skin of
rabbits for 24 h caused a very mild and reversible erythema (Elsea,
1954). Intraperitoneal administration in mice and rats and
intravenous administration in either mice, dogs or cats produced no
signs of toxicity (Fogleman, 1954).
No toxicity was observed in rats after inhalation of amitrole
following either head-only (approximately 50 µg/litre) or whole-body
(approximately 25 µg/litre) exposure for a period of one hour
(MacDonald & Pullinger, 1976).
7.2 Short-term exposure
7.2.1 Oral
7.2.1.1 Dietary
When groups of Carworth Farm male and female rats (five per
group) were administered amitrole in the diet at dose levels of 0,
100, 1000 or 10 000 mg/kg for 63 days, reduced body weight gain for
both males and females was observed at the two highest dose levels,
this being accompanied by reduced food consumption. There were no
deaths or clinical signs of toxicity. Histo-pathological examination
of the liver, kidney, portions of the small intestine, spleen, and
testes revealed increased vacuolization of the liver cells around
the central vein and steatosis at the two highest dose levels
(Fogleman, 1954).
Mayberry (1968) studied the effects on the thyroid of a dietary
level of 1000 mg amitrole/kg in rats during 83 days and compared
this to the effects of other anti-thyroid chemicals,
propylthiouracil (1000 mg/kg) and potassium chlorate (1000 mg/kg).
At various time intervals, starting with 3 days, the relative
thyroid weight and total iodine content of the thyroid were
measured. An increase in thyroid weight and a decrease of total
iodine were observed within the amitrole group; this was already
observable after 3 days and becoming more pronounced during the
course of the experiment. The effects of propylthiouracil were
comparable, but, in the case of potassium chlorate, the weight
increase was less pronounced and the iodine content was lower than
with amitrole. In another experiment, uptake and release of
radioactive iodine was measured after a single 131I injection to a
control group of rats and to a group simultaneously receiving 10 mg
amitrole subcutaneously. Animals were killed after 1, 2, 3, 4, 5 or
6 days. The t´ for 131I in the thyroid was 4.9 and 1.3 days for
control and amitrole-treated animals, respectively. Separation by
paper chromatography of 131I-containing thyroid fractions showed
that levels of monoiodotyrosine (MIT) were increased, diiodotyrosine
(DIT) were constant and T3 and T4 were markedly reduced. The
author concluded that amitrole not only interferes with
organification of iodine but also inhibits the coupling of
iodotyrosines to form iodothyronines (Mayberry, 1968).
The effect of amitrole on thyroid hormones was studied by
giving groups of male Sprague-Dawley rats (20 per dose level)
amitrole (94.6% pure) in the diet at dose levels of 0, 30, 100 or
300 mg/kg during 28 days, followed by a recovery period of 28 days
(Babish, 1977). The assessment of thyroid function was performed by
measuring T3 and T4 in blood by a radioimmuno-assay. On days 3, 7,
14, 21 and 28 of the treatment period and on days 19, 21 and 28 of
the post-treatment period, blood samples were collected from two
animals which were then killed for autopsy. There were no adverse
effects on the general health of the rats during the treatment or
post-treatment period. Consumption of 100 or 300 mg amitrole/kg diet
significantly depressed body weights during the 28-day treatment
period. The mean weekly body weights in the rats given 100 mg/kg
returned to control values by the third week of the post-treatment
period, while the mean weights of animals in the highest-dose group
did not return to control values during the post-treatment period.
The depression of body weights correlated with decreased food
consumption.
Serum T3 levels were significantly depressed by day 7 at
300 mg/kg (about 50%) and by day 14 at 100 mg/kg/diet (about 40%).
An inexplicable return to control values was seen 21 days into the
treatment period, followed by continued depression of T3 values on
day 28 of the treatment period. The depression of T3 appeared to
be dose related after 4 weeks of treatment. All treatments exhibited
essentially normal T3 levels by day 19 of the post-treatment
period. T4 levels followed exactly the same pattern as those of
T3. However, the T3/T4 ratio (which fluctuated between 12 and
18 in control rats) increased as the dose increased, being highest
at 300 mg/kg after 14 days of treatment.
From this study it may be concluded that amitrole, at levels of
100 mg/kg diet or more, rapidly suppressed T3 and T4 hormone
levels and maintained the depressed levels during the treatment
period. Both T3 and T4 levels returned to control values within
three weeks following withdrawal of amitrole from the diet. The
no-observed-adverse-effect level (NOAEL) in this study was
30 mg/kg/diet (Babish, 1977).
Fregly (1968) investigated the dose-response relationship
between amitrole administered in the diet and a variety of clinical
parameters in order to establish the minimal dose with an effect on
thyroid activity. Groups of male Spruce Farm strain rats (10 per
dose level) were administered amitrole in the diet at dose levels of
0, 2, 10 and 50 mg/kg diet for 13 weeks. Body weight gain, food
consumption, haematocrit, haemoglobin concentration and rate of
oxygen consumption were unaffected by the treatment. Mean body
temperature was slightly increased but only at 50 mg/kg. During week
12, uptake of radioactive iodine was measured at various times
between 22-53 h after intraperitoneal injection of 131I. A
slightly lower uptake was found at the highest dose level. At the
end of the study, radioactivity in the thyroid gland, excised 24 h
after intraperitoneal injection, was slightly decreased in all
groups. At the end of the study, the protein-bound iodine (PBI) in
blood, measured as an indicator for the concentration of thyroid
hormones, was decreased at all dose levels. The values were
51 µg/litre (control) and 37, 38 and 33 µg/litre for the 0, 2, 10
and 50 mg/kg groups, respectively. In a second experiment the PBI
levels were not affected by treatment with amitrole at 0.25 and
0.5 mg/kg diet. Values were 32 µg/litre in controls and 39 and
45 µg/litre, respectively, in treated groups. It should be noted,
however, that PBI control values measured in the second experiment
were much lower than those measured in the first experiment. This
implies that there was no biologically significant effect on PBI
since all values were within the same range. The thyroid weight was
increased significantly only in the 50-mg/kg group. The number of
blood vessels/thyroid section, which is a very sensitive measure of
histopathological changes in the thyroid, was increased at 10 and
50 mg/kg. It can be concluded that 2 mg/kg diet was the NOAEL in
this study.
Several short-term studies were carried out by Den Tonkelaar &
Kroes (1974) in order to establish a no-observed-effect level on
thyroid function tests. In all experiments the uptake of 131I by
the thyroid was measured in an in vivo test, 6, 24 and 48 h after
the intraperitoneal administration of 0.6 µc 131I per animal. In
addition, thyroid weight and PBI were measured and the thyroid was
studied histopathologically.
In the first experiment, four groups of eight female Wistar
rats received, respectively, 0, 2, 20 and 200 mg amitrole/kg in the
diet for 6 weeks. After 5 days and 6 weeks the uptake of 131I was
measured. On both occasions a significantly increased uptake was
found in the 200-mg/kg group 6 h after injection, which decreased
fairly rapidly after 24 and 48 h. At that time the radioactivity was
lower than that of the controls. The thyroid weight was increased in
the 200-mg/kg group and histopathologically goitre was found only in
this group.
In the second experiment, eight female animals per group
received, respectively, 0, 20, 50 and 200 mg/kg diet for 6 weeks,
and similar effects were found in the 200-mg/kg group to those
observed in the first experiment. In addition, a significant
decrease in PBI was observed at the end of the experiment compared
with the control value. At 50 mg/kg, a statistically increased
uptake was found 6 h after injection of 131I. However, in this
case the radioactivity in the thyroid remained higher than that in
the controls after 24 and 48 h. Histopathologically only a very
slight activation was found. However, the 200-mg/kg group showed
strong activation and goitre.
In the third experiment 0, 20, 50 and 200 mg/kg diet were given
to 10 female animals per group during 13 weeks. The uptake of 131I
by the thyroid was significantly increased at 200 and 50 mg/kg after
6 and 12 weeks. The difference between the groups was that at
50 mg/kg the radioactivity in the thyroid remained high after 24 and
48 h, whereas with 200 mg/kg a very high uptake was found 6 h after
injection of 131I but this was followed by a rapid decrease, with
still lower values than the controls after 48 h. At 200 mg/kg the
PBI was decreased and the thyroid/body weight ratio increased by a
factor of 6. At 50 mg/kg only a slightly increased relative thyroid
weight was found. Histologically, a strong activation and goitre
were found at 200 mg/kg, and a slight activation at 50 mg/kg. In
this experiment, a tendency to a higher uptake of 131I was found
in the 20-mg/kg group.
The above-mentioned experiments were carried out with a
relatively low iodine content in the diet (about 0.2-0.3 mg/kg
diet). In the fourth experiment, a diet containing 2 mg iodine/kg
was used. In this experiment, eight female rats per group received,
respectively, 0, 20, 50, 200 and 500 mg amitrole/kg in the diet for
6 weeks to see whether iodine could protect against the antithyroid
action of amitrole. At 500 mg/kg, a small increase in iodine uptake
was found 5 h after 131I injection, but thereafter there was a
very rapid decrease. At 200 mg/kg, the uptake was much higher and
the same type of decrease was found as in the other experiments,
whereas at 50 mg/kg a significantly increased thyroid radioactivity
was found at all times. PBI was decreased at 200 and 500 mg/kg only.
Histopathologically, goitre and strongly activated thyroids were
found at the two highest dose levels. Some activation was found in
the 50-mg/kg group and a very slight activation was also found in
the 20-mg/kg group. It can be concluded that measurement of 131I
uptake at different time points is a sensitive method for the
effects of amitrole on the thyroid. At 20 mg/kg only slight effects
were found on uptake and thyroid histopathology. The NOAEL was
2 mg/kg diet, equivalent to 0.1 mg/kg body weight.
7.2.1.2 Drinking-water
When groups of male albino rats (10 per dose level) were
administered amitrole in the drinking-water at dose levels of 0, 50,
250 or 1250 mg/litre for 106 days, there was a dose-related decrease
in body weight gain in all treated groups with a corresponding
reduction in food and water intake. There were no clinical signs of
toxicity. At the end of the study, histopathological examination was
performed on the thyroid, hypophysis, liver, kidney, spleen,
stomach, small intestine, large intestine, bladder, testis, adrenal
and lung. The major gross pathological finding was an increase in
size and vascularity of the thyroid. At the high dose level, colloid
was absent in large and medium size thyroid follicles. High-dose
animals also displayed liver steatosis (Bagdon et al., 1956).
The time-course for development of goitre in rats was examined
by Strum & Karnovsky (1971). Sprague-Dawley rats were administered
amitrole in the drinking-water (2.5 mg/ml), and the thyroid of each
animal was examined by light microscopy at various periods from 3
days to 6 months. Each rat drank approximately 30 ml water per day.
After 3 days, the thyroid size was unchanged although cellular
changes were apparent. By one week, the thyroid was twice its normal
size with marked structural changes. These changes continued to
progress with prolonged administration of amitrole. Goitre formation
was accompanied by increased vascularity and decreased colloid
content in the follicular cells. Electron microscopy revealed
pronounced dilation of the endoplasmic reticulum of thyroid cells.
Thyroid peroxidase activity progressively decreased with
administration of amitrole.
The effects of amitrole on thyroid histology were examined in
seven groups of five female Wistar rats (weighing about 200 g),
which were given amitrole in their drinking-water (2.5 mg/ml) and
killed after 1, 2, 3, 10, 30, 50 or 100 days (Tsuda et al., 1973).
Water consumption was not reported. After 1 and 2 days of exposure
the only change noted was a slight enlargement of some endoplasmic
cisternae of the follicular cells. After 3 days the thyroid gland
was slightly enlarged, follicular colloid was slightly reduced and
in some follicular cells the cisternae were clearly dilated and
stained more lightly for peroxidase activity than did normal cells.
By 10 days the glands had doubled in size, the follicular epithelium
consisted of low, columnar cells, and colloid had been severely
depleted. Nuclei had become located basally and slightly elongated
microvilli projected into the lumen. Peroxidase activity was no
longer detected in the endoplasmic reticulum cisternae, but in
portions of perinuclear cisternae. These changes had progressed in
the 30-day samples, so that the glands were now several times their
normal size. In addition, fibrous thickening of both stroma and
capsule was prominent and cisternal peroxidase activity was absent.
Administration for 50 days resulted in increased irregularity in
follicular size, more prominent papillary growth of the follicular
epithelium and greatly diminished peroxidase activity throughout the
cells.
Histopathological changes induced by amitrole in the liver of
mice were investigated by Reitze & Seitz (1985). Groups of male
albino mice were exposed to amitrole in the drinking-water at dose
levels of 0.5%, 1.0% or 2% for 30 days (water consumption not
reported). Light microscopy revealed dose-related hypertrophy of
hepatocytes, increased pyknotic nucleoli, and increased vacuoles in
the cytoplasm. Electron microscopy revealed also a dose-related
proliferation of smooth endoplasmic reticulum.
7.2.1.3 Intubation
No data available.
7.2.2 Inhalational
Groups of Fischer-344 rats (15 of each sex per dose level) were
exposed to an atmosphere containing amitrole (94.6% pure) at
concentrations of 0, 0.1, 0.32, 0.99 or 4.05 mg/litre (nominal
concentrations adjusted for non-nebulized material) for 5 h/day, 5
times per week, for 4 weeks (particle size not provided). There were
no adverse effects on behaviour, and no body weight changes were
noted. T4 levels were significantly depressed by the 27th day at
the two highest dose levels. T3 levels were significantly
depressed by 14 days at all but the lowest dose level. Pathological
changes were confined to the thyroid, and hyperplasia was noted at
all but the lowest dose level (Cox & Re, 1978).
7.2.3 Intraperitoneal
Alexander (1959a) investigated the uptake of 131I by the
thyroid gland in Sprague-Dawley rats following intraperitoneal
injection of approximately 5 or 250 mg/kg body weight. At both dose
levels thyroid 131I uptake was inhibited, whereas catalase
activity was decreased by about 50% at the highest dose level only.
When 328 White Leghorn 3-day old chicks were injected with
amitrole (500 or 1000 mg/kg day), 5 days per week for 5 weeks,
increases in the relative thyroid-to-body weight ratio was observed
in all birds from day 10 onward. In addition, two groups of chickens
were injected the same doses of amitrole but for 17 consecutive
days. At the cessation of amitrole treatment, an increase in the
relative thyroid-to-body weight ratio was observed until day 13;
this was followed by a decrease and then a stabilization, which
occurred between days 17 and 41. However, the ratio never attained
the levels observed in control animals. Histopathological
examination of the thyroid gland revealed epithelial hyperplasia,
hyperaemia, obliteration of the follicular lumina and disappearance
of the colloid. In birds that were injected with amitrole only for
17 days, the thyroid histology returned to normal two weeks after
treatment (Wishe et al., 1979).
7.3 Long-term exposure
7.3.1 Oral
7.3.1.1 Mouse
Reversible thyroid hyperplasia has been reported during
long-term feeding studies with amitrole at levels of 1000 mg/kg diet
in both C3H and C57BL mice (Feinstein et al., 1978a). The
acatalasemic C3H mice survived longer on the amitrole diet than did
their normal catalasemic counterparts (mean survival times in weeks,
both sexes combined, were 35 ± 10, n = 141, and 26 ± 10, n = 146,
respectively, P < 0.001). Similar differences were observed with
C57BL/6 mice, although group sizes were much smaller (57 ± 5, n =
12, and 42 ± 7, n = 10, respectively). All mice given the treated
diet had a reduced body weight gain compared with mice given the
normal diet. In those mice for which the amitrole diet was withdrawn
at 12 weeks, the thyroid weight reduced in size gradually but the
gland was still enlarged after 60 weeks. A larger proportion of the
acatalasemic C3H mice developed liver tumours, as compared with
normal catalasemic C3H mice. Out of 87 mice in the acatalsemic
group, 21 developed liver tumours that were detected earlier
(beginning at 35 weeks) compared with the normal catalase mice (6/85
beginning at week 50) (Feinstein et al., 1978b).
In a life-time study in NMRI mice (dose levels 0, 1, 10,
100 mg/kg diet), the appearance, behaviour, food intakes, body
weights and survival times of the treated mice did not differ from
those of the controls. The frequency of pituitary hyperaemia was
slightly elevated in the high-dose group; no treatment-related
histological lesions were otherwise found. The frequency of types
and distribution of tumours in the control and treated groups were
similar. The thyroid weights were elevated in male high-dose group
mice at all dose levels and were up to three times the weights in
the control group. The percentage of iodine accumulation and the
iodine level in the thyroid were elevated in the male mice of the
100-mg/kg group. The sum of PBI in the male mice was elevated nine
months after study initiation, but was depressed at later test
dates. Comparable results were observed in the female high-dose
group mice. However, the deviations from control group values were
generally smaller than in the males, and were not significant in
most cases (Weber & Patrick, 1978; Steinhoff & Boehme, 1979b).
7.3.1.2 Rat
The long-term effects of oral administration of amitrole in
rats have been described in two detailed reports by Keller (1959)
and Johnson et al. (1981). The details and results of these studies
are given below.
In the study by Keller (1959), groups of Carworth Farm Wistar
rats (35 of each sex per dose level) were administered amitrole in
the diet at dose levels of 0, 10, 50 or 100 mg/kg diet for two
years. After 13 and 68 weeks, 5 and 3 animals of each sex and dose
level, respectively, were killed for organ weight measurement and
histopathological examination. A separate group received 500 mg/kg
diet for 19 weeks, followed by the control diet for 7 weeks, and
then were killed. In this group, body weight gain and food
consumption were markedly reduced during the amitrole
administration. Animals in all groups, including the controls,
suffered from apparent respiratory infection and 67 of them died,
but there was no relationship with the treatment. Body weight gain
was reduced at 100 mg/kg in male animals during the first 13 weeks
of the study. After 68 and 104 weeks of treatment, relative thyroid
weight was increased at 100 mg/kg (not measured after 13 weeks).
Histopathological examination after 13 weeks showed hyperplasia and
hypertrophy of the thyroid at 500 mg/kg; these effects were found to
be reversible. Histopathological changes in the thyroid were also
seen at 100 mg/kg and in one animal at 50 mg/kg. At 68 weeks three
animals given 50 mg/kg showed definitive evidence of hyperplasia,
while all animals given 100 mg/kg displayed hyperplasia and
hyperfunctioning of the thyroid. At 104 weeks tumours were found
(see section 7.7.2). Based on thyroid hyperplasia, the NOAEL was
10 mg/kg diet (equivalent to 0.5 mg/kg body weight).
In a chronic toxicity study by Johnson et al. (1981), groups of
Fischer-344 rats (75 of each sex per dose level) were administered
amitrole. Group A were the controls. Group B rats were fed 5 mg
amitrole/kg in their diet during weeks 1-39 and then 100 mg/kg
during weeks 40-115 (for males) or 40-119 (for females). Rats in
groups C, D and E received amitrole in their diet at pulsed
intervals (alternate 4-week periods) of 1, 3 and 10 mg/kg,
respectively, during weeks 1-39 and 20, 60 and 100 mg/kg,
respectively, during weeks 40-115 (for males) or 40-199 (for
females). There were no treatment-related clinical signs of toxicity
or changes in body weight or food consumption. There were no
consistent effects on serum T3 and T4 levels. Thyroid weight was
increased in both males and females in groups B and E after 60 weeks
and at termination. There were no treatment-related pathological
changes up to 36 weeks (when only the lower dose levels were
administered). Follicular epithelial hyperplasia in the thyroid was
noted in groups B, D and E and to a much lesser extent in group C.
An increased incidence in thyroid tumours was observed in male and
female rats of groups B and E and in the male animals of group D.
There was no significant difference in tumour incidence between
groups B and E. It should be noted that this study was poorly
reported.
When amitrole was administered to groups of Wistar rats (75 of
each sex) at concentrations in the feed of 0, 1, 10 or 100 mg/kg, no
effect on body weight gain or food intake was observed but a slight
decrease in survival time was found at 100 mg/kg. Thyroid weight was
increased at 100 mg/kg as was uptake of 131I by the thyroid,
measured 24 h after oral administration of 131I. For this
measurement, five animals of each sex per group were killed at 3, 6,
12 and 24 months. PBI, measured as the ratio between radioactivity
in plasma protein and total plasma, was not affected. At the highest
dose level, elevated incidences of haemorrhage and hyperaemia of the
pituitary gland, as well as a very high rate of cystically dilated
thyroid follicles, were seen. The tumour incidence is given in
section 7.7.2. The NOAEL was 10 mg/kg diet, equivalent to 0.57
(males) or 0.85 (females) mg/kg body weight (Weber & Patschke 1978;
Steinhoff & Boehme, 1979a).
Authors who have studied the time-course of the response of the
thyroid to amitrole treatment (e.g., Strum & Karnovsky, 1971; Tsuda
et al., 1973; Wynford-Thomas et al., 1983) have shown that, after a
short lag phase of a few days, there is a rapid rise in TSH that is
paralleled by thyroid hypertrophy and hyperplasia. These effects
peak and plateau after 3-4 months and thereafter remain relatively
stable despite further exposure. A number of studies have shown that
the goitrogenic action of amitrole is reversible on cessation of
exposure (Jukes & Shaffer, 1960).
7.3.1.3 Other species
Other species in which long-term amitrole treatment has been
studied are the hamster and dog.
In a carcinogenicity study on hamsters (Steinhoff & Boehme,
1978; Steinhoff et al., 1983; see section 7.7), there were no
pathological changes at dose levels of up to 100 mg/kg diet. In a
one-year study in dogs, amitrole was given in capsules at dose
levels of 0, 0.25, 1.25, 2.50 and 12.5 mg/kg body weight per day,
6 days/week. There were no clinical signs of toxicity or
pharmacological effects. Haematological, biochemical and urinalysis
parameters were comparable to those of control dogs and were within
normal limits. The dogs fed 12.5 mg/kg per day had a pale pancreas.
Histopathological examination of all dogs did not reveal any
treatment-related effects. The thyroid, in particular, was normal at
all dose levels (Weir, 1958; Hodge et al., 1966).
7.3.2 Other routes
In a chronic 104-week study, 25 male and 25 female rats
(Charles River strain) were exposed (head-nose only) to an amitrole
aerosol (the purity of the amitrole used was not specified) for one
hour each week. An aqueous 0.24% (w/v) solution of amitrole was used
to generate the aerosol. The mean analytical concentration in the
inhalation chamber was 2 mg aerosol per litre of air; based on dry
amitrole, the level was 5 µg/litre air. A control group was exposed
to a water aerosol. No differences between the control group animals
and those exposed to the test substance were found in the mortality,
appearance, behaviour or body weight development. No
treatment-related changes were observed at necropsy. No differences
in the thyroid or liver weights, or in the incidence of tumours,
existed between the two groups of animals (Grapenthien, 1972).
In an inhalation study involving intermittent treatment, groups
of 75 Fischer rats per dose and of each sex were exposed to aerosols
at nominal amitrole levels of 0, 50, or 500 µg/litre air (the purity
of the amitrole used was not specified). The actual amitrole
concentrations in the low-dose group varied between 15.8 and
32.2 µg/litre air on different days of exposure, and the levels
measured in the high-dose group ranged between 97.9 and
376.4 µg/litre air. The animals were exposed for 5 h per day on 5
days per week. The treatment phases during weeks 1-13, 40-52 and
78-90 were interrupted by treatment-free intervals. Interim
necropsies of five animals per dose group and of each sex were
performed after 3, 9 and 18 months, and the study was concluded
after 24 months. A total of 28 rats died in week 51 due to a defect
in the air conditioning system, which led to an increase in the room
temperature. Treatment of the high-dose group was thereupon
concluded, and the surviving animals were necropsied.
The food intake and body weight gain were decreased in the
high-dose group, and the rate of mortality was elevated.
Decreases in the T3 (significant) and T4 (non-significant)
levels were only observed in the high-dose group, this being
assessed in the 13th week of the study. However, values in the
amitrole-treated animals were greater than, or equal to, those of
control rats at all other test dates (weeks 39, 52, 78, 91 and 104).
Epithelial hyperplasia of the thyroid follicles was observed in both
dose groups at the end of the first treatment interval (week 13).
This observation was no longer made after a treatment-free interval
of 24 weeks, but the thyroid weights relative to those in the
control animals were elevated in both dose groups. Follicular
epithelial hyperplasia was again present in most of the animals of
both treatment groups at the end of the second treatment phase (week
51). This observation was still made after a treatment-free interval
of 26 weeks, which indicates that complete reversion no longer
occurred at this time at an amitrole level of 50 µg/litre air.
Neoplasms of the thyroid (adenomas and adenocarcinomas) were found
in addition to hyperplasia at terminal necropsy (Becci, 1983).
Twenty-five male and 25 female rats (Charles River strain) were
dermally exposed to an 0.239% aqueous solution of amitrole (the
purity of the amitrole used was not specified) once weekly for 30
min over a period of 23 months (total of 100 exposures). The
treatment volume was 1 ml/kg body weight, and about 20% of the body
area was treated. The dermal exposure to amitrole thus amounted to
2.39 mg/kg body weight per week. The treatment did not cause skin
damage. No differences between the control group and animals exposed
to the test substance were found with respect to mortality,
appearance, behaviour or body weight development. No
treatment-related alterations were determined at necropsy. No
differences in the thyroid or liver weights, or in the incidence of
tumours, were found between the two groups of animals (Rausina,
1972).
7.4 Skin and eye irritation; skin sensitisation
The potential for dermal irritation by amitrole was examined in
rabbits over a 24-h period following a single application of between
10 and 100 mg/kg body weight (Elsea, 1954). Mild erythema was
observed at the high-dose level only. By 48 h, the skin appeared
normal. The potential for eye irritation by amitrole was examined in
rabbits following application of 3 mg into the conjunctival sac of
the left eye (Elsea, 1954). Observations were made at 1, 4, and 24 h
and at daily intervals for 6 days. Mild irritation was observed at
4 h in all animals, but the majority of animals had recovered by
24 h.
Amitrole was tested for possible dermal sensitization potential
in guinea-pigs using the Magnusson-Kligman maximization test with
Freund's adjuvant. The concentrations employed were 2.5% for
intracutaneous induction, 25% for topical induction, and 12% for the
first and second challenges. Evidence for moderate skin-sensitizing
potential in amitrole was found after both challenges (Mihail,
1984).
No skin-sensitizing effect was observed in a Klecak open
epicutaneous test involving treatment of three groups of animals
with 3%, 10% or 30% amitrole formulations in the induction phase
(Mihail, 1985).
7.5 Reproduction, embryotoxicity and teratogenicity
7.5.1 Reproduction
In a preliminary one-generation reproduction study by Gaines
et al. (1973), groups of 10 male and 10 female rats were fed
amitrole in the diet at concentrations of 0, 500 or 1000 mg/kg for
55 days before pair-mating. The offspring were weaned at 21 days.
Complete autopsies were performed on the parents after a total
exposure of 107-110 days. Ten weaning rats from each dose groups
were killed. Mean body weight gain was reduced at all dose levels.
The average number of pups per litter was significantly reduced at
all dose levels, as were the number surviving to weaning. The body
weight of pups at weaning was also reduced. Relative kidney, spleen
and liver weights were also reduced in parents following treatment,
while thyroid hyperplasia was noted in all treated animals.
In a subsequent multi-generation study by Gaines et al. (1973),
groups of 10 male and 10 female rats were fed amitrole at dietary
levels of 0, 25 or 100 mg/kg for 61 and 173 days before pair-mating
to produce the F1A and the F1B generations, respectively. The
thymus and spleen were examined in weanling rats in the F1A
generation. There was no treatment-related effect on body weight
gain in the FO animals. Hyperplasia of the thyroid was observed in
all animals at the highest dose level but not at 25 mg/kg.
Reproduction parameters were normal at these dose levels. A slight
decrease in body weight gain was noted at 25 and 100 mg/kg in pups
of the F1A and F1B generation. Pathological examination revealed
a slight but significant decrease in liver weight at 25 and
100 mg/kg (male pups) and at 100 mg/kg (female pups). The thymus and
spleen sizes were normal and no histopathological changes could be
detected. F2A generation rats showed a decrease in the number of
litters at 100 mg/kg but there were no other changes, such as
survival to weaning and mean body weight at weaning.
7.5.2 Embryotoxicity and teratology
Teratology studies have been performed in rats, mice and
chickens.
In a study by Gaines et al. (1973), three groups of pregnant
rats were administered amitrole by gavage at dose levels of 0, 20 or
100 mg/kg body weight per day on days 7 to 15 of gestation, and the
animals were allowed to litter and to wean. There was no evidence of
gross abnormalities among the pups.
In a further teratology study on rats by Machemer (1977b),
groups of 20 presumed pregnant rats (strain FB30, Long-Evans) were
administered amitrole by gavage at dose levels of 0, 100, 300 or
1000 mg/kg body weight per day on days 6 to 15 of gestation, and
fetuses were examined on day 20 of gestation. There were no deaths
or signs of toxicity at any dose level. Body weight gain was not
affected by treatment. There were no treatment-related effects on
the resorption rate, fetal weight, number of live fetuses, placental
weight or sex ratio. There was no treatment-related increase in
gross, skeletal or visceral malformation.
In a study by Tjalve (1974), pregnant mice were administered
amitrole at 500, 1000, 2500 and 5000 mg/litre in the drinking-water
on days 6-18 of pregnancy. There was a marked decrease (22-28%) in
body weight gain in the dams and pronounced retardation in the
development in their fetuses at dose levels > 1000 mg/litre. At
the highest dose level used, maternal toxicity was associated with
an increase in the rate of resorption. No teratogenic effects were
observed at any dose level.
Teratogenicity in chickens was investigated by injecting the
yolk sac of eggs with amitrole at dose levels of between 0.5 and
40 mg at 0, 24, 48 and 96 h of incubation (Landauer et al., 1971).
Dose-dependent abnormalities of the beak were found to be present in
chickens following the administration of 20-40 mg amitrole at 24 and
48 h. When injected after 96 h of incubation, beak abnormalities
could be found at dose levels of 10, 20 and 40 mg at a rate of 20,
48, and 60%, respectively. No effects were seen at dose levels up to
and including 2 mg/egg.
7.6 Mutagenicity and related end-points
A referenced summary of the test results with amitrole is given
in Table 4. The important features of these data are described
below.
7.6.1 DNA damage and repair
The possibility of DNA damage being induced by amitrole has
been investigated frequently and in a number of different ways. In
bacteria, the results have been negative, except in one experiment
with the rec assay, in which exogenous metabolic activation was
provided by "liver" preparations from a mollusc and a fish. Among
assays which could be evaluated, a DNA repair assay in yeast gave a
positive result, as did a repair assay in mammalian cells.
7.6.2 Mutation
One study in a single laboratory with Escherichia coli and
Salmonella typhimurium strains gave significant responses (Venitt
& Crofton-Sleigh, 1981). Amitrole did not induce joint mutations in
histidine-requiring mutants of S. typhimurium (Andersen et al.,
(1972). An equivocal response was obtained in another bacterial
mutation assay, but many other in vitro assays gave negative
results. A significant result was obtained in a mouse peritoneal
host-mediated assay with S. typhimurium (Simmon et al., 1979). No
mutation induction has been observed in yeast or fungi. In
Drosophila melanogaster, a significant response was obtained with
a wing-spot test in a single study, but not with several sex-linked
recessive assays. Mutations were not induced in mouse lymphoma
cells, but hprt locus and Na+/K+ ATPase locus mutations were
induced in Syrian hamster embryo cells (Tsutsui et al., 1984). These
latter results may hold particular significance in view of other
properties of these cells described in sections 7.6.4 and 7.6.5.
7.6.3 Chromosome damage
In yeast, there is conflicting evidence for recombinogenic
activity (intragenic and mitotic recombination), while numerical
chromosomal aberrations were induced in three assays. Structural
chromosomal damage was not induced by amitrole in cultured mammalian
cells, but the frequency of sister-chromatid exchanges was increased
in a single study. No effects of amitrole were observed in mice
subjected to bone marrow micronucleus tests or male dominant lethal
tests.
7.6.4 Cell transformation
Assays for anchorage-independent growth and cell transformation
in several systems consistently gave positive results.
Table 4. Summary of mutagenicity and related end-point studies on amitrole
Test Organism Result LED or HIDe Reference
-S9h +S9i
Microorganisms
Prophage induction E. coli 58-161 enVA, lambda n.t. - 10 000 µg/ml Thomson (1981)
prophage
Prophage induction E. coli GY5027, GY4015 n.t. - 2000 µg/plate Mamber et al. (1984)
Rec assay Bacillus subtilis H17 rec+, M45 rec- - n.t. µg/plate Shirasu et al. (1976)
Rec assay Bacillus subtilis H17 rec+, M45 rec- - +a 1000 µg/plate Kada (1981)
Rec assay E. coli JC 2921, 9238, 8471, 5519, - - 500 µg/ml Ichinotsubo et al. (1981)
7623, 7689
Rec assay E. coli WP2, WP100 n.t. - 4000 µg/ml Mamber et al. (1983)
Pol assay E. coli pol A1, pol A+ - n.t. µg/plate Bamford et al. (1976)
Pol assay E. coli WP3110, p3478 - - 333 µg/plate Rosenkranz et al. (1981)
Differential killing E. coli WP2, WP67, CM 871 - - 1000 µg/ml Tweats (1981)
Reverse mutation S. typhimurium TA1535, TA1537, TA1538 - - 100 µg/plate Brusick (1975)
Reverse mutation S. typhimurium TA1535, TA1538, - - 1000 µg/plate Prince (1977)
TA98, TA100
Reverse mutation S. typhimurium TA1535, TA1536, - - 2000 µg/plate Carere et al. (1978)
TA1537, TA1538
Table 4 (contd).
Test Organism Result LED or HIDe Reference
-S9h +S9i
Reverse mutation S. typhimurium TA1535, TA1538 - - 250 µg/plate Rosenkranz & Poirier
(1979)
Forward mutation S. typhimurium TM 677 - - 100 µg/ml Skopek et al. (1981)
Reverse mutation S. typhimurium TA1535, TA1537, - - 12 500 µg/plate Herbold (1980)
TA98, TA100
Reverse mutation S. typhimurium TA1535, TA1537, - - 2000 µg/plate Brooks & Dean (1981)
TA1538, TA98, TA100, TA92
Reverse mutation S. typhimurium TA1537, TA98, TA100 - - 5000 µg/plate MacDonald (1981)
Reverse mutation S. typhimurium TA1535, TA1537, TA1538, - - 10 000 µg/plate Richold & Jones (1981)
TA98, TA100
Reverse mutation S. typhimurium TA1535, TA1537, TA1538, - - 2000 µg/plate Rowland & Severn (1981)
TA98, TA100
Reverse mutation S. typhimurium TA1535, TA1537, TA1538, n.t. - 2500 µg/plate Trueman (1981)
TA98, TA100
Reverse mutation S. typhimurium TA98, TA100 n.d. + f Venitt & Crofton-Sleigh
(1981)
Reverse mutation S. typhimurium TA98, TA100 ± ± 500 µg/ml Hubbard et al. (1981)
Reverse mutation S. typhimurium TA1535, TA1537, TA98 - - 1000 µg/ml Gatehouse (1981)
Reverse mutation S. typhimurium TA1535, TA1537, TA1538, - - 5000 µg/plate Moriya et al. (1983)
TA98, TA100
Table 4 (contd).
Test Organism Result LED or HIDe Reference
-S9h +S9i
Reverse mutation S. typhimurium TA1535, TA1537, TA1538, - - 333 µg/plate Dunkel et al. (1984)b
TA988, TA100
Reverse mutation E. coli WP2, WP2uvrA nd + f Venitt & Crofton-Sleigh
(1981)
Reverse mutation E. coli WP2uvrA - - 500 µg/plate Gatehouse (1981)
Reverse mutation E. coli WP2uvrA - - 5000 µg/plate Moriya et al. (1983)
Reverse mutation E. coli WP2uvrA - - 333 µg/plate Dunkel et al. (1984)b
Forward mutation E. coli CHY832 + - 2500 µg/ml Hayes et al. (1984)
Forward mutation Streptomyces coelicolor A3(2) ± n.t. µg/plate Carere et al. (1978)
Host mediated S. typhimurium TA1950 in NMRI mouse - n.t. 2900 µmol/kg Braun et al. (1977)
reverse mutation
Host mediated S. typhimurium TA1530, TA1535, TA1538 + n.t. 1585 mg/kg i.p. Simmon et al. (1979)
reverse mutation in Swiss-Webster mouse
DNA repair Saccharomyces cerevisiae 197/2d + - 100 µg/ml Sharp & Parry (1981b)
rad 3, rad 18, rad 52, trp 2
Reverse mutation S. cerevisiae D4 - - 100 µg/plate Brusick (1975)
Reverse mutation S. cerevisiae XV 185-14C - - 889 µg/ml Mehta & von Borstel (1981)
Table 4 (contd).
Test Organism Result LED or HIDe Reference
-S9h +S9i
Forward mutation Aspergillus nidulans 35 - n.t. 2000 µg/ml Bignami et al. (1977)
Mitotic S. cerevisiae T1, T2 - - 1000 µg/ml Kassinova et al. (1981)
recombination
Mitotic gene S. cerivisiae D7 - - 12 500 µg/ml Zimmerman & Scheel
conversion (1981)
Mitotic gene S. cerivisiae JD1 + - 300 µg/ml Sharp & Parry (1981a)
conversion
Nondisjunction A. nidulan P ± ± 2000 µg/plate Bignami et al. (1977)
Mitotic A. nidulans P ± ± 2000 µg/plate Bignami et al. (1977)
recombination
Nondisjunction A. nidulans P + n.t. 400 µg/ml Morpurgo et al. (1979)
Nondisjunction A. nidulans strain P1 - n.t. 120 mM Crebelli et al. (1986)
Forward mutation A. nidulans strain 35 - n.t. 72 mM Crebelli et al. (1986)
Mitotic A. nidulans P1 - n.t. 120 mM Crebelli et al. (1986)
recombination
Mitotic gene S. cerevisiae D4 - - 333 µg/ml Jagannath et al. (1981)
conversion
Mitotic S. cerevisiae RS112 - n.t. 60 000 µg/ml Schiestl et al. (1989)
recombination
Table 4 (contd).
Test Organism Result LED or HIDe Reference
-S9h +S9i
Mitotic S. cerevisiae T1 and T2 - - 1000 µg/ml Kassinova et al. (1981)
recombination
Insectsg
Nondisjunction Drosophila melanogaster females - 10 mg/kg diet Laamanen et al. (1976)
Sex linked recessive D. melanogaster males - 10 mg/kg diet Laamanen et al. (1976)
lethal
Sex linked recessive D. melanogaster males - 20 000 mg/kg diet Vogel et al. (1981)
lethal
Sex linked recessive D. melanogaster - 10 mg/kg diet Sorsa & Gripenberg (1976)
lethal
Sex linked recessive D. melanogaster males - 48 000 mg/kg feed Mason et al. (1992)
lethal
Sex linked recessive D. melanogaster males - 12 500 mg/kg Mason et al. (1992)
lethal injection
Somatic mutation D. melanogaster + 1mM Tripathy et al. (1990)
(wing spot test)
Cultured mammalian cells
Unscheduled DNA Hela cells - + 100 µg/ml Martin & McDermid (1981)
synthesis
Table 4 (contd).
Test Organism Result LED or HIDe Reference
-S9h +S9i
Mutation, tk locus Mouse lymphoma L5178Y cells - - 5000 µg/ml McGregor et al. (1987)
Mutation, hprt locus Syrian hamster embryo cells + n.t. 0.3 µg/ml Tsutsui et al. (1984)
Mutation, Syrian hamster embryo cells + n.t. 0.3 µg/ml Tsutsui et al. (1984)
Na+/K+ATPase locus
Sister chromatid Chinese hamster ovary cells - + 0.1 µg/ml Perry & Thomson (1981)
exchange
Chromasomal Human lymphocytes - n.t. 10 000 µg/ml Meretoja et al. (1976)
aberrations
Anchorage BHK-21 cells n.t. + 25 µg/ml Styles (1979)
independent growth
Anchorage BHK-21 cells n.t. + 250 µg/ml Styles (1981)
independent growth
Anchorage BHK-21 cells - - 4000 µg/ml Daniel & Dehnel (1981)
independent growth
Cell transformation BALBc/3T3 cells c n.t. 2500 µg/ml Brusick & Weir (1976)
Cell transformation Syrian hamster embryo cells ± n.t. 50 µg/ml Inoue et al. (1981)
Cell transformation Syrian hamster embryo cells + n.t. 10 µg/ml Dunkel et al. (1981)
Cell transformation Syrian hamster embryo cells + n.t. 0.3 µg/ml Tsutsui et al. (1984)
Table 4 (contd).
Test Organism Result LED or HIDe Reference
-Sy9h +S9i
Cell transformation Rat embryo cells/Rauscher-MULV infected + n.t. 100 µg/ml Dunkel et al. (1981)
Cell transformation Mouse embryo C3H 10T ´ fibroblasts d n.t. 125 µg/ml Dunkel et al. (1988)
Mammals in vivog
Bone marrow CD-1 mouse - 500 mg/kg per day Tsuchimoto & Matter (1981)
micronucleus x2 i.p.
Bone marrow NMRI mouse - 10 000 mg/kg Herbold (1982)
micronucleus per day x1
Sperm morphology (CBA x BALBc)F, mouse - 500 mg/kg per day Topham (1980)
x5 i.p.
Dominant lethal NMRI mouse, male - 1000 mg/kg x1 Machemer (1977a)
Dominant lethal Ha(ICR) mouse, male - 100 mg/kg diet x49 Knickerbocker & Stevens
(1978)
a Liver S9 from Japanese clam and Yellowtail fish
b Results from four laboratories presented
c Positive in one of three tests
d Positive in one of two laboratories
e LED = lowest effective dose (for results scored as positive); HID = highest ineffective dose (for results scored as negative)
f Results given as slope of linear regression
g In in vivo experiments no testing with exogenous metabolic activation
h Without exogenous activation
i With exogenous activation
n.t. not tested
n.d. tested, but data not shown
+ positive response
± weak positive response
- no response
7.6.5 Other end-points
Amitrole had no effect upon testicular DNA synthesis (Seiler,
1977) and did not increase the frequency of morphologically abnormal
sperm in mice.
Prostaglandin-H-synthetase (PHS) and lactoperoxidase catalyse
the binding of 14C-amitrole to protein and transfer RNA in vitro,
and protein binding occurs in the presence of rat and pig thyroid
peroxidase (Krauss & Eling, 1987). These authors suggested that PHS
activity in Syrian hamster embryo cells may be important for the
mutagenic and transforming potential of amitrole seen with these
cells. It is known, for example, that PHS is involved in the
peroxidative metabolism of diethylstilbestrol in these cells and
that structure-activity studies suggest that this type of metabolism
is important for its biological effects (Degen et al., 1983).
7.7 Carcinogenicity
7.7.1 Mouse
Groups of 50 male and 50 female C3H/Anf mice, 2-4 months old,
were injected subcutaneously on a single occasion with 0.02 ml of a
suspension of 50 mg amitrole per ml trioctanoin, or dosed once on a
shaved area of the dorsal skin each week of the experiment with
0.2 ml acetone, 0.2 ml acetone containing 0.1 mg amitrole or 0.2 ml
of a methanol:acetone (35:65) mixture containing 10 mg amitrole
(Hodge et al., 1966). The mice were observed until death. The skin
alone was examined for tumours, but none were found in any group,
including the controls.
In a study by Innes et al. (1969), which investigated the
carcinogenicity of some 120 chemicals, amitrole was used as a
positive control. Groups of (C57BL/6 x C3H/Anf)F1 mice (18 of each
sex) and (C57BL/6 x AKR) F1 mice (18 of each sex) were given
amitrole by gavage at a dose level of 1000 mg/kg per day on days
7-28 of age and in the diet at a concentration of 2192 mg/kg from
day 28 until the end of the experiment. This was planned to be week
80, but all of the mice in the amitrole-treated groups had died by
weeks 53-60. Thyroid tumours were reported to have occurred in
nearly all of the treated mice (64/72). Liver tumours were observed
in 34/36 (C57BL/6 x C3H/Anf)F1 mice and in 33/36 (C57BL/6 x
AKR)F1 treated mice. In pooled control groups, 8/166 (C57BL/6 x
C3H/Anf)F1 mice and 6/172 (C57BL/6 x AKR)F1 mice had liver
tumours.
Feinstein et al. (1978b) fed normal and acatalasemic female C3H
mice a diet containing 10 000 mg/kg amitrole for up to one year (see
section 7.7.4 for details). Unspecified liver tumours were observed
in all of the 33 mice. No untreated control group was included.
A carcinogenicity study in mice was reported by Steinhoff &
Boehme (1979b) and Steinhoff et al. (1983). Groups of NMRI mice (75
of each sex per dose level) were administered amitrole (97% pure) in
the diet at concentrations of 0, 1, 10 or 100 mg/kg throughout their
lifetime. There was no treatment-related effect on appearance, body
weight or food consumption. Histological examination of tissues of
the major organs did not reveal any treatment-related effects apart
from a slight increase in the number of hyperaemias in the
pituitary: 3 in controls, 5 at 1 mg/kg, 2 at 10 mg/kg and 16 at
100 mg/kg. There was no increase in the incidence of
treatment-related tumours.
Three groups of B6C3F1 mice were fed a diet containing 500 mg
amitrole/kg: Group 1, from day 12 of gestation until delivery; Group
2, from delivery until weaning; and Group 3, from weaning until 90
weeks (Vesselinovitch, 1983). Although not specifically stated,
information on other chemicals tested and described in this report
(benzidine, safrole, ethylenethiourea and diethylnitrosamine)
implied that the mice exposed in utero (Group 1) and during
lactation (Group 2) were also observed until 90 weeks. The incidence
of hepatocellular adenomas and carcinomas, respectively, were: Group
1 males, 4/74 and 2/74; Group 2 males, 6/45 and 4/45; Group 3, 15/55
and 11/55; Group 1 females, 0/83 and 0/83; Group 2 females, 0/55 and
0/55; Group 3 females, 5/49 and 4/49. The incidence of these tumours
in untreated B6C3F1 mice (which may or may not have been
concurrent controls) at 90 weeks were: males 1/98 and 0/98; females
0/96 and 0/96; other organs were not examined. It was concluded that
there was no effect of amitrole treatment in Group 1 or in females
of Group 2, but that marginal increases occurred in males of Group 2
and in males and females of Group 3.
The comparative susceptibilities of three mouse strains to the
development of preneoplastic hepatic lesions were examined following
amitrole administration in the drinking-water at 10 000 mg/litre
(Mori et al., 1985). The strains were DS, ICR (Crj: CD-1) and NOD,
which was derived from ICR and found to develop spontaneous
insulitis followed by diabetes. There were indications that the NOD
mice may have carried immunological abnormalities. In each of two
experiments, three groups of female mice were administered amitrole
in the drinking-water for 3 months (experiment 1) or 6 months
(experiment 2), after which they were killed and their livers
examined. The proportions of mice with hyperplastic nodules were: in
experiment 1, 15/19 NOD, 3/5 DS and 0/5 ICR; and, in experiment 2,
19/19 NOD, 18/18 DS and 17/19 ICR. A single hepatocellular carcinoma
developed in an NOD mouse after six months of treatment.
7.7.2 Rats
In the study by Keller (1959) (see section 7.3.1.2), thyroid
tumours were present in 1/10 animals of the 10-mg/kg group, 2/15 at
50 mg/kg and 15/27 at 100 mg/kg at the end of the study. No tumours
were detected in five control group animals but one rat exhibited
early stages of an adenoma. One thyroid gland from the 50-mg/kg
group and four from the 100-mg/kg group exhibited lesions
interpreted as adenocarcinomas by several pathologists and benign
neoplasms by others. This study has also been described by Jukes &
Shaffer (1960).
The thyroid carcinogenicity of amitrole in the rat was further
demonstrated in a study by Doniach (1974) where groups of Hooded
Lister strain rats were administered amitrole in the drinking-water
at a concentration of 1000 mg/litre for 18-20 months. All
amitrole-treated animals (20) developed follicular adenomas and 2/20
animals also developed follicular carcinomas. In studies designed to
investigate the mechanism of thyroid carcinogenesis, Tsuda et al.
(1976, 1978) also demonstrated thyroid tumours in rats after
administration of amitrole in the drinking-water at 2500 mg/litre.
In the study of Steinhoff & Boehme (1979a) described in section
7.3.1.2, both benign and malignant tumours of the thyroid were found
at 100 mg/kg diet. In the pituitary gland there was also an increase
in the incidence of benign tumours at 100 mg/kg. This study was also
described in a report by Steinhoff et al (1983).
In the study by Johnson et al. (1981) described in section
7.3.1.2, follicular thyroid neoplasms were observed in Fischer rats
in the groups that received amitrole in pulsed doses (alternate 4
week periods) of 60 and 200 mg/kg diet and in the group that
received continuous doses of 100 mg/kg diet for 115-119 weeks.
The histological development of thyroid tumours in rats
following amitrole treatment has been studied by Wynford-Thomas
et al. (1983). Groups of 10 male Wistar rats were administered
amitrole in the drinking-water at a concentration of 0.1%, and five
animals were killed at 7 months and five animals at 1 year. After 7
months, 3 tumours were found in 1 of the 5 animals. After 1 year, 16
microtumours were found in 4 of the 5 animals. Most of the tumours
were well-defined adenomas. Their most striking feature was their
vascularity, with the follicles being surrounded by greatly enlarged
vascular spaces lined by endothelium and dilated capillaries.
7.7.3 Other species
The carcinogenicity of amitrole in hamsters was investigated in
a lifetime dietary study by Steinhoff & Boehme (1978). Groups of
golden hamsters (76 of each sex per dose level) were administered
amitrole (97.0% pure) in the diet at concentrations of 0, 1, 10 or
100 mg/kg throughout their lifetime. There was no treatment-related
change in appearance. Body weight gain was decreased at 100 mg/kg
from day 400 onward, and mortality was significantly increased in
the 100-mg/kg groups. Severe amyloidosis of the kidneys was the
major cause of death and was observed in all groups. There was no
evidence of amitrole-related histopathological changes nor of a
treatment-related carcinogenic effect. This study was also described
in a report by Steinhoff et al. (1983).
7.7.4 Carcinogenicity of amitrole in combination with
other agents
Two groups of 30 male albino rats, 2-3 months of age, were fed
0.06% 4-dimethylaminoazobenzene (DAB) in the diet, and one group
also received intraperitoneal injections of amitrole (purity
unspecified; 1000 mg/kg body weight) every second day as a 10%
solution in water. The surviving 16 DAB-treated and 19
DAB-plus-amitrole-treated rats were killed at 21 weeks. The
incidence of liver tumours was 12/16 in the group receiving DAB
alone and 4/19 in the group that received DAB plus amitrole (P <
0.01). The liver carcinomas produced by DAB alone were mostly
hepato-cellular carcinomas, whereas those in the group treated with
DAB plus amitrole were hepatocellular carcinomas and
cholangiocarcinomas (Hoshino, 1960).
Groups of 12 six-week-old male Wistar rats were given the
following treatments: Group 1 received four weekly subcutaneous
injections of N-bis(2-hydroxypropyl) nitrosamine (DHPN) (700 mg/kg
body weight) followed by a diet containing amitrole (purity
unspecified; 2000 mg/kg diet) for an additional 12 weeks; Group 2
received four weekly injections of DHPN only; Group 3 was fed only a
diet containing 2000 mg amitrole/kg beginning at week 4 for 12
weeks; Group 4 received eight weekly injections of DHPN followed by
a diet containing 2000 mg amitrole/kg for 12 weeks; Group 5 received
eight weekly injections of DHPN only; Group 6 was fed a diet
containing 2000 mg amitrole/kg beginning at week 8; and Group 7 was
fed a standard diet and served as untreated controls. All animals
were killed after 20 weeks. No thyroid tumours were found in rats in
Groups 2, 3, 6 or 7, but a significantly increased incidence (P <
0.05) of thyroid tumours compared with that in controls was observed
in rats in Group 1 (9/11), 4 (12/12) and 5 (7/12). Hyperplasia of
the thyroid was not observed in Groups 2 and 7, but occurred in 27%
of Group 1, 25% of Group 3 and 100% of Groups 4, 5 and 6 (Hiasa
et al., 1982).
Seventy-five male Wistar-Furth rats were castrated at 40 days
of age and divided into six groups: Group 1 (five rats) received no
treatment and served as controls; Group 2 (ten rats) was given
amitrole (purity unspecified; 1500 mg/litre) in the drinking-water,
starting at 47 days after castration; Group 3 (ten rats) received
subcutaneous implantation of a pellet on the back containing 5 mg
diethylstilbestrol (DES) plus 45 mg cholesterol, which was replaced
every two months; Group 4 (11 rats) received implantations of DES
pellets every two months plus amitrole in the drinking-water; Group
5 (20 rats) received implantation of the DES pellet followed by
administration of 5 mg N-nitrosobutylurea (NBU) per day in the
drinking-water for 30 days, starting at 50-55 days of age; and Group
6 (19 rats) received implantations of the DES pellet followed by
administration of NBU and, subsequently (seven days after NBU
treatment), amitrole in the drinking-water. Moribund or dead rats
were completely autopsied. All survivors were killed 12 months after
the initial NBU treatment. Rats in Groups 3 and 5 developed numerous
hepatocellular carcinomas and neoplastic nodules (4/9 and 15/17
animals, respectively) and pituitary tumours (7/9 and 12/17,
respectively). Addition of amitrole to these regimens (Groups 4 and
6, respectively) had no effect on the incidence of pituitary tumours
(8/11 and 10/14) but slightly (Group 4; 2/11) and significantly
(Group 6; 3/14) reduced the incidence of hepatocellular carcinomas
and neoplastic nodules. No liver tumour were found in controls
(Group 1) or in rats receiving amitrole only (Group 2) (Sumi et al.,
1985).
In experiments with acatalasemic and normal C3H mice, groups of
42-47 females were treated at weaning with neutron irradiation (80
rads at 15 rads/min) or fed a diet containing 10 000 mg amitrole/kg
or both (Feinstein et al., 1978b). In order to extend survival, the
amitrole diet was offered during the first 4 weeks of a 5-week
cycle, which was repeated continuously. The experiment was
terminated one year after weaning. Of the mice killed at the end of
the experiment, unspecified liver tumours were found in 2/37 of the
neutrons group, 29/29 of the neutrons + amitrole group and 33/33 of
the amitrole alone group. In contrast, there were significant
reductions in the proportions of mice with Harderian gland or
ovarian tumours in the amitrole-treated groups: Harderian gland
tumours - 12/37 of the neutrons group, 0/29 of the neutrons +
amitrole group and 0/33 of the amitrole alone group. It was also
demonstrated that, when these mice were infected with the murine
mammary tumour virus, almost 100% eventually developed mammary
tumours. If acatalasemic, virus-infected mice were kept on a diet
containing 10 000 mg amitrole/kg from weaning for 12 weeks, then the
time of initial appearance of mammary tumours in 50% of the mice was
delayed from 35 weeks in 29 control diet mice to 50 weeks in 28
amitrole-treated mice.
7.8 Other special studies
7.8.1 Cataractogenic activity in rabbits
The cataractogenic activity of amitrole administered in the
diet or drinking-water to rabbits was investigated by Bhuyan et al.
(1973). A group of four rabbits was fed on a diet containing 0.2%
amitrole while four litter-mates were maintained as a control. Body
weight gain was significantly lower than in controls from 13 weeks
to 25 weeks. All treated animals developed typical bilateral
lenticular changes in the form of cataracts. Initial changes to the
eyes were seen during 2-4 weeks with widening of lens sutures,
individualization of lens fibres and posterior cortical vacuoles.
The opacity progressed towards other regions during 4-8 weeks.
Complete involvement of the posterior cortical, post-equatorial,
equatorial, and anterior cortical regions occurred after a period of
10-16 weeks. After approximately 20 weeks, there was no further
advancement in lenticular opacity.
A separate group of 24 rabbits was kept on continuous 0.2%
amitrole in the drinking-water, while 15 rabbits were kept as
controls. The body weight of the treated animals was significantly
lower at 13 weeks. Of the 24 treated rabbits, 22 developed typical
bilateral lenticular changes in the form of cataracts. The time
course of the changes was as described for the dietary treatment.
7.8.2 Biochemical effects
The effect of amitrole on the import of catalase into
peroxisomes of cultured fibroblasts derived from patients with
peroxisome biogenesis disease has been investigated. The catalase
import is inhibited by prior binding of amitrole to catalase, which
appears to retard unfolding of the protein (Middelkoop et al.,
1991).
Brain catalase activity has been shown to be inhibited in vivo
in rats in a dose-related manner (3 doses) by amitrole in saline
solution administered intraperitoneally. Inhibition occurred within
3-6 h after administration. The activity recovered completely 24 h
following administration of the lowest dose (0.0625 g/kg), while a
period of more than 48 h was needed for larger doses. The pattern of
inhibition and recovery was identical in animals from all dose
groups. The presence of amitrole in the brain was confirmed over the
time period of the observed inhibition of brain catalase (Aragon
et al., 1991b).
It has been proposed that ethanol can be oxidized in the brain
via the peroxidatic activity of catalase and that centrally formed
acetaldehyde may mediate several of the psychopharmacological
actions of ethanol. The study by Aragon et al. (1991a) was designed
to investigate the role of brain catalase in the mediation of
ethanol-induced narcosis, hypothermia and lethality in rats. Rats
were pretreated with the catalase inhibitor amitrole (0.25, 0.5 and
1.0 g/kg i.p.) or with saline, and 5 h later, animals in each
pretreatment group received intraperitoneal injections of ethanol
(3 or 4 g/kg). Ethanol-induced narcosis was significantly attenuated
in amitrole-pretreated rats compared to the saline control group. In
addition, amitrole pretreatment significantly reduced the lethal
effect of ethanol. However, the body temperature of
amitrole-pretreated ethanol-injected animals was significantly
reduced as compared to the saline-ethanol animals. Blood ethanol
determinations revealed that amitrole did interfere with ethanol
metabolism. Amitrole significantly inhibited brain catalase activity
at all doses used in this study and the results indicate a role for
brain catalase in ethanol effects. In a separate study (Tampier &
Quintanilla, 1991), rats were intraperitoneally treated with
amitrole (1 g/kg) as a single injection or as seven injections on
consecutive days. The animals were then exposed to ethanol
(2.76 g/kg) intraperitoneally or by gavage as a single or repeated
exposure, respectively. The effect of amitrole on hypothermia,
narcosis induced by ethanol, and the acquisition of tolerance to the
ethanol hypothermic and narcotic effect was studied. It was found
that amitrole blocked the catalase activity of brain (68%) and liver
(96%) when injected intraperitoneally one hour before exposure to
ethanol. When the rats were treated daily with amitrole, a lower
degree of blocking was obtained in the brain (37%) but not in the
liver (97%).
The effect of amitrole on brain catalase activity was studied
in male Long-Evans rats maintained on voluntary consumption of
ethanol (Aragon & Amit, 1992). It was shown that intraperitoneal
injection of amitrole (62.5-1000 mg/kg body weight) resulted in
dose-dependent reduction in ethanol intake. Consumption of water was
not affected by any dose of amitrole, suggesting specific effects of
amitrole on ethanol intake. Analysis of brain catalase activity
demonstrated a direct inhibitory effect of amitrole on brain
catalase activity.
Amitrole is known to cause irreversible inhibition of catalase
(Margoliash et al., 1960), which is thought to regulate the
intercellular peroxide concentration.
Metodiewa & Dunford (1991) investigated the reaction of
amitrole with mammalian haem enzymes, represented by
lactoper-oxidase and bovine liver catalase. The study indicated that
amitrole is a substrate for lactoperoxidase compounds I, II and III
but does not convert catalase compound I to II under conditions
favouring peroxidase activity of enzyme. This work supports the
hypothesis that amitrole reacts with peroxidase compounds I and II.
This reaction appears to be a one-electron oxidation of amitrole.
The observed multi-step base reduction of lactoper-oxidase III by
amitrole has potential physiological relevance since it could help
maintain the peroxidatic cycle of the enzyme (Metodiewa & Dunford,
1991).
The ability of amitrole to affect different
porphyrin-containing compounds suggests a possible interference with
porphyrin synthesis or inhibition of the activity of these
porphyrin-containing compounds. After intraperitoneal administration
of amitrole to DBA mice, it was found that the level of hepatic
delta-aminolevulinic acid dehydratase activity was reduced within 3
to 4 h. This study demonstrated that amitrole is capable of causing
a simultaneous decrease in activity of both hepatic
delta-aminolevulinic acid dehydratase and catalase as well (Tschudy
& Collins, 1957).
Baron & Tephly (1969), however, found that in vitro amitrole
is not a potent inhibitor of (delta)-aminolevulinic acid
dehydratase, and that the degree of inhibition depends on the
concentration of the enzyme. In the same study it was found that
amitrole produces a decrease in the hepatic microsomal cytochrome
P-450 level of nearly 50% approximately 16 h after an
intraperitoneal injection of amitrole (3 g/kg) to the rats.
Cytochrome b5 levels were not affected by this treatment. The
induction of the N-demethylation of ethylmorphine caused by
phenobarbital was inhibited by amitrole. Treatment of rats with
amitrole alone resulted in a decrease of 40% in ethylmorphine
demethylation.
The effects of amitrole on GSH-Px (glutathione peroxidase)
activity and prostaglandin 3 biosynthesis have been investigated
in vitro. Incubation of amitrole (0.002 mol/litre) with rat
erythrocytes was followed by 88% reduction in GSH-Px activity.
Moreover, platelet prostaglandin synthesis was blocked and aorta
prostacyclin-like activity was inhibited (Doni & Piva, 1983).
Buchmuller-Rouiller et al. (1992) demonstrated that amitrole
inhibits macrophage leishmanicidal activity and nitrite secretion
through a process involving inhibition of NO synthetase activity.
The resemblance between the tautomeric form of amitrole and the
guanidino group of L-arginine, the natural substrate for NO
synthetase, might be responsible for this inhibition
(Buchmuller-Rouiller et al., 1992).
Amitrole is reported to inhibit fatty acid synthesis in
isolated rat hepatocytes, an effect at least partially mediated at
the level of acetyl-CoA carboxylase. Cholesterol synthesis is more
markedly inhibited by amitrole (Beynen et al., 1981). However, the
half-maximal inhibition of fatty acid synthesis is approximately
20 mmol/litre and for cholesterolgenesis is approximately
5 mmol/litre. Consequently, these effects are unlikely to be
important in vivo.
Amitrole treatment affects the synthesis not only of
cholesterol but also of primary bile acids in vivo. Thus, amitrole
treatment activated the biosynthesis of chenodeoxycholic acid from
exogenous cholesterol (1.3 fold) but did not affect that of colic
acid. Aminotriazole hardly affected the synthesis of
chenodeoxy-cholic acid through endogenous cholesterol (from
mevalonate), but decreased to 41.2% that of colic acid (Hashimoto
et al., 1992).
7.9 Mechanisms of toxicity - mode of action
Amitrole has been shown to be goitrogenic in several species
including rats, mice, hamsters and hens (Wishe et al., 1979;
Steinhoff et al., 1983). As in the case of certain other goitrogenic
substances, amitrole causes thyroid cancer in rats after prolonged
exposure (section 7.7). A mechanism for these carcinogens in animals
has been proposed which involves the hormonal imbalance caused by
antithyroid effects (Hill et al., 1989). Thyroid hormonal changes
have been produced by low iodine diet, sub-total thyroidectomy,
splenic transplantation of thyroid tissue and by the transplantation
of pituitary tumours that secrete thyroid stimulating hormone (TSH).
In all these cases the incidence of thyroid cancer increases. The
explanation is that excessive hypothalamus-mediated TSH simulation
of the thyroid gland, induced by either hypothyroidism (chemically
or surgically induced) or TSH-secreting tumours, is the cause of
thyroid hypertrophy and hyperplasia. The continuing stimulation by
TSH might eventually lead to thyroid neoplasia. However, the precise
mechanism triggering the transformation from hyperplasia to
neoplasia is still not understood.
Amitrole is a potent anti-thyroid agent, although the precise
sequence of events leading to the anti-thyroid effect has not been
clarified. Single doses of amitrole were shown to reduce thyroid
uptake of iodine in several species including rats (Alexander,
1959a) and humans (Astwood, 1960). However, in another study (Den
Tonkelaar & Kroes, 1974), when amitrole was given in the diet for
several days, different results on iodine uptake were observed. In
fact, iodine uptake was found to be increased 6 h after iodine
injection, and then decreased below the control values 24 and 48 h
after injection. At a lower dose level of amitrole, the
concentration of radioactive iodine in the thyroid remained slightly
above the control values.
Most of the iodine in the thyroid during amitrole exposure is
not bound to protein, reflecting a failure in its utilization
(Alexander, 1959a). Amitrole has been found to be an inhibitor of
thyroid iodide peroxidase (Alexander, 1959b; Darr & Fridovich, 1986)
both in vitro and in vivo. Histochemical studies confirmed the
reduction of thyroid peroxidase activity during amitrole treatment
(Strum & Karnovsky, 1971). Thyroid iodide peroxidase is thought to
catalyse both thyroglobulin iodination and tyrosine
(3-monoiodotyrosine and 3,5-diiodotyrosine) coupling, which leads to
the formation of the thyroid hormones 3,3,3',5'-tetraiodo-thyronine
(T4) and 3,5,3' triiodothyronine (T3). The influence of amitrole
on these peroxidase-catalysed reactions was also demonstrated by the
relative depletion of T4 in the thyroid of rats treated with
amitrole, compared with the levels of tyrosines (Mayberry, 1968).
These results suggest that amitrole affects both reactions catalysed
by thyroid peroxidase. However, the nature of the mechanism involved
in this reduction of peroxidase activity remains unclear.
In conclusion, amitrole has an antithyroid effect and several
mechanisms have been shown to be involved. Whether the toxic result
is due to all these mechanisms or to various combinations is not
clear. For instance, doses of amitrole to rats that cause a
reduction of thyroid peroxidase activity were found to be greater
than those that inhibit thyroid iodine uptake (Alexander, 1959a).
Consequences of these antithyroid effects have been observed in
rats treated with relatively high doses of amitrole. T3 and T4
levels in serum drop dramatically and in parallel with an increase
in TSH levels. When T3 and T4 are below the limit of detection,
the corresponding rise in TSH is about 10-fold (Wynford-Thomas
et al., 1982a,b). The spacial-temporal relationships between such
hormonal changes and the morphological ones induced by amitrole have
been studied by Wynford-Thomas et al. (1982a,b). Animals were
maintained on the goitrogen amitrole (0.1% in drinking-water) and
killed at various intervals. Serum T3 and T4 rapidly fell to
undetectable levels within two weeks. The level of serum TSH rose to
a stable maximum after four weeks. The T:S iodine ratio (iodine in
thyroid and serum) followed a similar pattern. Thyroid weight and
follicular cell number increased rapidly for the first few weeks but
the growth rate declined progressively, falling almost to zero after
80 days. Mitotic activity rose dramatically after 7 days but then
declined almost to normal after 80 days, consistent with the
observed change in cell number. The authors concluded that there is
a dissociation between the functional and proliferative activity of
the thyroid follicular cells during prolonged stimulation by a
sustained elevation of TSH and suggested the existence of specific
growth regulatory mechanisms which limit the mitotic response. They
further speculated that, whatever this control mechanism may be, it
would be reasonable to assume that its failure represents the first
step in the pathogenesis of neoplasia. The progression towards
thyroid malignancy can be halted by administration of thyroid
hormones or surgical hypophysectomy, procedures aimed at countering
the source of TSH stimulation. However, the extent to which
progression can be halted is unknown.
Although the mechanism involved in the triggering of neoplastic
initiation after hypertrophic and hyperplastic stimulation is
unknown, it remains to be ascertained whether other mechanisms of
carcinogenicity can be initiated by amitrole and, therefore, whether
the rise in TSH must be considered a factor promoting tumour
development. Thyroid tumours induced by X-rays are thought to be the
summation of TSH-induced hyperplasia with neoplastic transformation
initiated by radiation (Doniach, 1974). The addition of thyroxine to
the diet of irradiated rats markedly reduced radiation-induced
tumour production, indicating that TSH may have a facilitative role
in thyroid cancer development after low-dose radiation in
non-suppressed animals.
Mutagenicity studies with amitrole gave equivocal results in
some tests and indicate that this compound might have a relatively
weak genotoxic effect. The genotoxicity of amitrole is not
correlatable with functional action on peroxidase. However, possible
relationships between the anti-thyroid and genotoxic effects of
amitrole when causing thyroid cancer have been not clarified.
Conflicting data have been reported as to whether human
conditions that are related to high TSH predispose to thyroid
cancer. Three recent case control studies in the USA reviewed by
Hill et al. (1989) consistently showed that thyroid cancer is
strongly related to pre-existing goitre or thyroid nodules. However,
of two studies which analysed the association between hypothyroidism
and thyroid cancer, neither showed such a relationship (McTiernan
et al., 1984; Ron et al., 1987).
In conclusion, the role of TSH in thyroid carcinogenesis in
humans (if any) seems different from that played in experimental
thyroid cancer in animals.
8. EFFECTS ON HUMANS
8.1 General population exposure
Astwood (1960) reported in a brief communication that a single
oral dose of 100 mg amitrole inhibited radio-iodine uptake by the
thyroid of both normal and thyrotoxic subjects for 24 h. However, a
dose of 10 mg was said to have had a slight effect on iodine uptake.
The author suggested that amitrole could be used therapeutically in
the treatment of hyperthyroidism.
The intentional ingestion of a commercial mixture of amitrole
and diuron, at a dose equivalent to 20 mg amitrole/kg, was reported
to have caused no symptoms of poisoning in a female subject. Within
a few hours, the compound appeared in the urine at a concentration
of 1000 mg/litre. Metabolites could not be detected in the urine
(Geldmacher-von Mallinckrodt & Schmidt, 1970).
During a patch test conducted with human volunteers, amitrole
exerted no primary dermal irritant effect after an exposure period
of 4-8 h; a slight irritant effect was observed in three out of six
volunteers after 24 h (Hecht, 1954).
8.2 Occupational exposure
English et al. (1986) reported a case study of a 41-year-old
weed control operator with a 6-month history of dermatitis involving
his face, hands, back, thighs and feet. Patch testing with 1%
amitrole showed a strong positive vesicular reaction at 2 and 4
days, indicative of allergic contact dermatitis.
Balkisson et al. (1992) reported the case of a 74-year-old
previously healthy ex-smoker, who sprayed an amitrole formulation
(containing 19% aminotriazole, 17% ammonium thiocyanate, < 1%
sodium diethylsulfosuccinate and < 1% ethylene oxide), in a
strong-head wind without any protective clothing for 2 h using
500 ml of the formulated material in 10 litre of water. He developed
a dry non-productive cough after 6-8 h, for which he reported for
treatment. On investigation, diffuse, asymmetric, severe alveolar
damage in the lungs was noticed which was reversed after a high dose
of corticosteroid.
A report by Baugher et al. (1982) presented the results of a
study conducted on five men involved in spraying amitrole over a
period of 10 working days on utility rights-of-way in West Virginia.
The amitrole (at a concentration of approximately 500 g of active
ingredient per 100 litres of water), was applied using handheld
hydraulic spray guns. Medical monitoring, particularly of the
thyroid, was carried out both pre- and post-spraying. Thyroid gland
palpation and neck measurements were undertaken 19 days prior to
exposure and 14 days after the last exposure. Thyroid function tests
were performed at 19, 11 and 4 days pre-exposure and 0, 7 and 14
days after the last exposure. All subjects were within the normal
range for thyroid function and no differences were found in any
comparisons of thyroid function. It was estimated that dermal
exposure over the 10-day period was approximately 340 mg per day for
each man.
Epidemiological studies conducted on agricultural workers
(Barthel, 1981) and railway workers (Axelson & Sundell, 1974;
Axelson et al., 1980) exposed to a variety of pesticides showed a
small increase in the number of tumours, particularly of the lung.
Amitrole was one of the pesticides to which these workers were
exposed.
In a study by Miksche (1982), mild cases of dermatitis on the
face due to contact with amitrole occurred yearly in one or two
production workers. The dermatitis was of a primary irritant type
rather than the sensitivity type. No other adverse effects occurred.
The thyroid function in five employees who had been engaged in
production and packaging of amitrole for periods of between 3 and 16
years was checked. Thyroid scintigrams and determinations of the
T3 and T4 levels yielded no evidence of thyroid dysfunction
(Miksche, 1983).
9. EFFECTS ON OTHER ORGANISMS IN THE LABORATORY AND FIELD
9.1 Laboratory experiments
9.1.1 Microorganisms
The effect of amitrole on the growth and pigmentation of the
cyanobacteria (blue-green alga) Anacystis nidulans was studied by
Kumar (1963). Growth inhibition occurred at concentrations above
40 mg/litre. In cultures 5-14 days old, the amounts of carotenoids,
chlorophyll and phycocyanin were decreased by amitrole
concentrations of 20-40 mg/litre, but normal pigmentation was
restored upon withdrawal of amitrole. DaSilva et al. (1975) examined
the effect of amitrole (20 mg/litre) on the nitrogen-fixing capacity
of eight different cyanobacteria and observed partial inhibition,
followed by recovery, in all cases.
Salama et al. (1985) cultured two species of nitrogen-fixing
cyanobacteria, Anabaena flos aquae and Phormidium fragile, from
Nile drains in amitrole solutions (0.4, 1.2, 3.6 and 10.8 mg/litre).
Only the highest concentration markedly reduced the growth of the
cyanobacteria; the growth of Phormidium was slightly stimulated at
the two lowest concentrations. There was little effect on
chlorophyll content, although a slight stimulation occurred in
Anabaena at up to 1.2 mg/litre. There was a slight (statistically
significant) stimulation of nitrogen uptake by Phormidium, but
only at the highest amitrole concentration. In Anabaena there was
an increase in nitrogen fixation at all amitrole concentrations.
When the growth of Rhizobium trifolii TA1 in nutrient broth
was examined in the presence of amitrole (20 mg/litre) no inhibition
was detected. Nodulation of Trifolium subterranium L., however,
following inoculation with R. trifolii, decreased linearly as the
herbicide concentration increased from 0 to 20 mg/litre. Even when
R. trifolii was grown in the presence of amitrole and washed
before inoculation, there was decreased nodulation of the sub-clover
(Eberbach & Douglas, 1989).
The effect of amitrole on cellulose decomposition by
cellulolytic fungi and cellulolytic bacteria in soil was
investigated by Grossbard & Wingfield (1978). Amitrole incorporated
into soil at 500 mg/kg greatly reduced the decomposition of buried
calico. This effect was not, however, observed at 150 mg/kg. In pure
cultures of the test fungi, amitrole was less toxic than paraquat or
glyphosate. Amitrole did not affect the number of cellulolytic
bacteria.
Blumenstock (1989) incorporated Aminotriazol SP (Bayer) into
two soils, a loamy sand and a silt, at rates of 40 mg formulation/kg
(equiv. 20 mg a.i./kg) and at 200 mg formulation/kg dry soil. This
corresponded to the maximum recommended application rate and to a
rate five times higher. The amitrole formulation slightly reduced
nitrification, but this effect disappeared within 90 days. In the
loamy sand, there was a small increase in soil nitrate formation.
Using the same product and the same concentrations in soil, Anderson
(1989) showed no effect of the formulation on soil respiration at
20 mg a.i./kg dry soil. At 100 mg a.i./kg, there was a slight
reduction in microbial respiration in both soils.
The effect of amitrole on photosynthetic microorganisms has
been investigated by Aaronson (1960) and Aaronson & Sher (1960). The
multiplication of Euglena gracilis var. bacillaris was strongly
inhibited by amitrole (150-300 mg/litre). Wolf (1962) noted that the
growth of Chlorella pyrenoidosa was inhibited by approximately 50%
at a dose level of 5 mg/litre. Another microorganism, Ochromonas
danica, was also particularly sensitive, strong bleaching occurring
at 30 mg/litre (Aaronson & Sher, 1960).
When Cain & Cain (1983) cultured the green alga Chlamydomonas
moewusii in amitrole (technical) at concentrations up to
6.7 mg/litre, there was no observed effect on vegetative growth or
on zygospore germination.
Altenburger et al. (1990) cultured the green alga Chlorella
fusca with amitrole and glufosinate-ammonium both separately and
in combination. The two herbicides together showed less toxicity to
the alga than either alone when estimated in terms of cell volume
growth or cell reproduction.
Adams & Dobbs (1984) examined the toxicity of amitrole to the
unicellular alga Selenastrum capricornutum using two different
methods. The highest concentration causing no statistically
significant difference from controls was found to be
0.2-0.5 mg/litre and 5 mg/litre, respectively, in the two tests. The
different results were thought to be due to different growth media
composition.
A growth test on the green alga Scenedesmus subspicatus was
conducted by Anderson (1984). The EC50 for growth, based on cell
counts, was reported to be 2.3 mg a.i./litre. The no-observed-effect
concentration (NOEC) was < 1.0 mg a.i./litre of nutrient solution.
An algal growth inhibition test (OECD 201) was performed by
Knacker & Reifenberg (1989) using Aminotriazol SP50
(500 mg/litre formulation) and the alga Scenedesmus subspicatus
exposed to nominal concentrations of between 8.96 and 350 mg/litre.
An NOEC of 8.96 mg/litre, based on growth (area under growth curves)
and growth rate, was reported.
9.1.2 Aquatic organisms
9.1.2.1 Plants
Amitrole did not affect the secretion of extracellular products
by the macrophyte Porphyra yezoensis or inhibit its photosynthetic
activity in the concentration range 12.5-200 mg/litre (Yoshida
et al., 1986).
The angiosperm Spirodela is particularly sensitive to
amitrole, with strong bleaching at 100 mg/litre (Aaronson & Sher,
1960).
9.1.2.2 Invertebrates
The median tolerance limit of amitrole to some aquatic
invertebrates is shown in Table 5.
In a study of the toxicity of herbicides to the common
water-flea, Daphnia magna, the median immobilization concentration
(IC50) in a 26-h acute static test at 21 °C was 23 mg amitrole/kg
(Crosby & Tucker, 1966).
The EC50 (immobilization) for a 48-h acute toxicity study on
Daphnia magna, conducted under OECD guidelines, was reported by
Heimbach (1983) to be 1.54 (0.38-3.07) mg/litre.
In a semi-static reproduction test on Daphnia magna (OECD
202) performed by Ritter (1989), the NOEC for production of young
was 0.2 mg/litre. At 1.0 mg/litre, amitrole significantly reduced
the number of young produced (by 12.5%). Measured concentrations of
amitrole ranged from 78 to 115% of the nominal concentrations.
Bogers (1989) conducted a comparable test and reported a NOEC of
between 0.32 and 1.0 mg/litre. As in the study by Ritter (1989),
effects were observed at 1.0 mg/litre.
Schultz & Kennedy (1976) suggested that Daphnia pulex is more
sensitive to amitrole-induced toxicity than Daphnia magna and
examined the toxicity of amitrole (10 mg/litre) to Daphnia pulex.
Evidence of altered cell structure was noted within 24 h in the
mitochondria of the muscle fibre, together with general tissue
swelling and dissociation of membranes.
Table 5. Toxicity of amitrole to aquatic invertebrates
Species Test Exposure TLm Reference
materiala period (median
(hours) tolerance
limit,
mg/litre)
Sigara substrata WP 48 >1000 Nishiuchi (1981)
Micronecta sedula WP 48 >1000 Nishiuchi (1981)
Cloeon dipterum WP 48 >40 Nishiuchi (1981)
Orthetrum albistylum Technical 48 >40 Nishiuchi (1981)
speciosum larvae
Sympetrum frequens Technical 48 >40 Nishiuchi (1981)
larvae
Water flea Technical 3 >40 Yoshida &
(Daphnia pulex) Nishimura (1972)
Moina macrocopa Technical 3 >40 Yoshida &
Nishimura (1972)
Crayfish Technical 72 >40 Yoshida &
(Procambarus clarkii) Nishimura (1972)
Scud Technical 96 >10 Mayer & Ellersieck
(Gammarus fasciatus) (1986)
Copepod Technical 96 22.1 Robertson &
(Cyclops vernalis) (18.5-27.7) Bunting (1976)
a wp = wettable powder
9.1.2.3 Vertebrates
The median tolerance limit of amitrole to some aquatic
vertebrates is shown in Table 6.
Table 6. Toxicity of amitrole to aquatic vertebrates
Species Test Exposure TLm Reference
material period (median
(hours) tolerance
limit,
mg/litre)
Common carp Technical 48 >40 Yoshida &
(Cyprinus carpio) Nishimura (1972)
Goldfish Technical 48 >40 Yoshida &
(Carassius auratus) Nishimura (1972)
Golden orfe Technical 48 >40 Yoshida &
(Oryzias latipes) Nishimura (1972)
Misgurnus Technical 48 >40 Yoshida &
anguillicaudatus Nishimura (1972)
Japanese toad tadpole wettable 48 >1000 Yoshida &
(Bufo bufo japonicus) powder Nishimura (1972)
Coho salmon Amitrole-T 96 70 Lorz et al. (1978)
(Oncorhynchus kisutch)
Guppy Technical 96 >12 500 Niehuss & Börner
(Poecilia reticulatus) (1971)
Golden orfe Technical 96 >6000 Bayer AG (1979)
(Oryzias latipes)
Channel catfish Technical 96 >160 Mayer & Ellersieck
(Ictalurus punctatus) (1986)
Fathead minnow Technical 96 >100 Mayer & Ellersieck
(Pimphales promelas) (1986)
Mugil Technical 96 >68 Tag el-Din et al.
(Mugil cephalus) (1981)
A semi-static 21-day test (OECD 204) was conducted on the
rainbow trout, Oncorhynchus mykiss (weight 1.5 ± 0.6 g), at a
single technical amitrole concentration of 100 mg/litre. There were
no deaths or toxic signs in the test fish, and body weight and
length were not different from controls. The authors, therefore,
reported 100 mg/litre as the NOEC (Grau, 1989).
Amitrole was without effect on the development of the teleost
embryo Fundulus heteroclitus at exposure concentrations of 0.01 to
10.0 mg/litre (Crawford & Guarino, 1985).
A 96-h LC50 of 3.0 mg/litre for amitrole as Weedazol TL Plus
was reported for 1-2 week old tadpoles of the Australian frog
Adelotus brevis. The thermal tolerance of tadpoles was reduced
significantly after 96 h of exposure to 1 mg/litre (Johnson, 1976).
Hiltibran (1967) exposed fry of bluegill sunfish, green sunfish
and lake chub sucker fish to amitrole at 50 mg/litre for 8 days and
reported no deaths. Young bluegill survived a 12-day exposure to
25 mg/litre, although some deaths occurred at higher concentrations.
Lorz et al. (1978) exposed yearling Coho salmon (Oncorhynchus
kisutch) to Amitrole-T at concentrations of between 0.25 and
200 mg/litre. The 96-h LC50 in fresh water was calculated to be
70 mg/litre. Transfer of the young salmon to sea water with
comparable concentrations of amitrole increased the toxic effect of
the compound; 56% of fish survived 336 h in sea water containing
25 mg amitrole/litre and 12.5% survived 50 mg/litre. According to
the authors, deaths occurred within 24-48 h. Smoltification, the
adaptation of young salmon to sea water, is under thyroid control.
The goitrogenic properties of amitrole may explain the effect.
9.1.3 Terrestrial organisms
9.1.3.1 Plants
The herbicidal mode of action of amitrole at the molecular
level has not been satisfactorily elucidated. The early work of Sund
(1956) and Wolf (1962) has shown that the effect of amitrole can be
significantly reduced by simultaneous application of purines,
suggesting that amitrole is an inhibitor of purine biosynthesis and
interferes with the development of chloroplasts. While pigment
bleaching is one of the most striking effects of amitrole toxicity,
it is not clear that it is the primary site of action. Amitrole has
been shown to inhibit the activity of the enzymes phytoene
desaturase, lycopene cyclase, imidazole glycerol phosphate
dehydratase and catalase. Imidazole glycerol phosphate dehydratase
has been extensively studied due to its role in the histidine
biosynthetic pathway. Heim & Larrinua (1989) have examined the mode
of action of amitrole in Arabidopsis thaliana seedlings. They
concluded that root elongation is more sensitive to amitrole than
pigmentation, and this may be indicative of the primary block caused
by amitrole.
9.1.3.2 Invertebrates
The mortality and growth of the juvenile earthworm,
Allolobophora caliginosa (Oligochaeta:Lumbricidae), were measured
in soil containing Amitrole-T (1, 10 and 100 mg amitrole/kg plus
ammonium thiocyanate). There was no effect on worm growth or
mortality (Martin, 1982).
Red earthworms of the species Eisenia foetida were exposed to
Aminotriazol SP 50 in artificial soil at 100, 316 and 1000 mg
formulation/kg soil for 14 days (OECD 207) by Heimbach (1990b).
There were no reported effects; the NOEC was, therefore, 1000 mg
formulation/kg, equivalent to 488 mg a.i./kg.
Carabid beetles (Poecilus cupreus) were kept in dishes of
fine sand and sprayed with aminotriazole directly at a rate
equivalent to 30 kg/ha. Pupae of the housefly Musca domestica were
provided as food. No deaths were recorded and no toxic signs
observed; the beetles ate comparable numbers of pupae in both test
and control dishes (Heimbach, 1990a).
Amitrole, at a concentration of 184 mg/kg, reduced the survival
of the nematode Acrobeloides buetschlii by 50% and produced almost
complete mortality at 600 mg/kg. At an intermediate concentration
(300 mg/kg), malformed larvae were common (Frey, 1979).
Mothes-Wagner et al. (1990) studied the effect of amitrole on
the host-plant interaction between the spider mite Tetranychus
urticae and its host bean plant, Phaseolus vulgaris L.,
following applications of 0.1 g/m2 or 0.2 g/m2 per cultivation
box. Structural alterations to the protein-synthesizing apparatus of
the midgut and salivary gland cells of the spider mite were noted,
and these led to a decrease in reproduction rate.
9.1.3.3 Birds
Amitrole has low toxicity for birds (Table 7). There were no
reported signs of toxicity in the young birds of three species fed
diets supplemented with amitrole at a concentration of 5000 mg/kg.
Signs of toxicity, following a single oral dose of 2000 mg/kg body
weight in mallard ducks, included ataxia, weakness and slight
wing-drop during the first three days following dosing. The birds
subsequently recovered.
Table 7. Short-term toxicity of dietary amitrole and Amitrole-T in birdsa
Species Age LD50 LC50
(mg/kg body weight) (mg/kg diet)
Mallard duck 3-4 months >2000
(Anas platyrhynchos) 10 days >5000
Japanese quail 12 days >5000
(Coturnix coturnix
japonica)
Ring necked pheasant 10 days >5000
(Phasianus colchicus)
a Data from Hill et al. (1975), Hudson et al. (1984), Hill & Camardese
(1986); LD50 from acute oral dose; LC50 from dietary exposure for
5 days followed by 3 further days of observation.
9.2 Field observations
9.2.1 Terrestrial organisms
9.2.1.1 Plants
The effect of amitrole on the levels of epicuticular wax,
cuticle, penetration of water, cuticular transpiration and wax
biosynthesis was studied by Reddy et al. (1987) in some semi-arid
scrub. Over a 6-day period, at amitrole concentrations of 1000 and
5000 mg/litre, there was a significant reduction in the deposition
of epicuticular wax and the cuticle content of leaves, which
resulted in increased cuticular transpiration and penetration of
water. Amitrole induced a quantitative alteration in wax
biosynthesis and an accelerated water loss, ultimately leading to
plant death.
9.2.1.2 Invertebrates
Amitrole was non-hazardous to honey-bees when sprayed at an
application rate of 40 kg/ha (Herfs, 1975).
Amitrole, applied at a rate of 5.6 kg/ha to bentgrass
(Agrostis tenuis), reduced the number of nematodes (Anguina
agrostis) in the bentgrass by 49.9% (Courtney et al., 1962).
10. EVALUATION OF HUMAN HEALTH RISKS AND EFFECTS ON THE ENVIRONMENT
10.1 Evaluation of human health risks
General population exposure to amitrole is expected to be
minimal, given that it does not persist in the environment and
residues should not occur in food crops. Levels in drinking-water
supplies would be expected to be extremely low.
Occupational exposure for weed control operators occurs via the
dermal and inhalation routes. However, animal experiments and human
data indicate that dermal absorption of amitrole is low.
Inhalational exposure would be minimized by the use of appropriate
breathing apparatus.
In both animals and humans there is a rapid excretion of
unchanged amitrole following systemic exposure. Amitrole does not
have a teratogenic effect and does not affect reproduction.
The main effect of amitrole in subchronic and chronic studies
on rats is on the thyroid. Amitrole inhibits the production of
thyroid hormones T3 and T4, thereby stimulating the pituitary
gland to produce more thyroid stimulating hormone (TSH), which in
turn activates the thyroid. Consequently, thyroid weight increases
and the thyroid becomes hyperplastic and hypertrophic. These effects
are reversible upon cessation of exposure, even though the extent of
this reversal is undefined. Thyroid tumours occur only upon chronic
exposure at relatively high dose levels in animals already affected
by thyroid changes. The mechanism of neoplastic transformation is
not understood. However, from all available studies it is clear that
hyperplasia always precedes neoplasia because no tumours are found
when the thyroid is not affected. Mutagenicity studies are either
negative or conflicting, and therefore the evidence for amitrole
genotoxicity is equivocal. On this basis, and from Table 8, it can
be concluded that 2 mg/kg diet (equivalent to 0.1 mg/kg body weight
per day) is a no-observed-effect level, based on thyroid hyperplasia
and iodine uptake, and this value can be used to establish a safe
dose for humans.
In carcinogenicity studies on rats, amitrole did not induce
tumours in organs other than the thyroid. However, in some mouse
studies that used high dose levels, liver tumours were also found.
Because the mouse is very sensitive to the induction of liver
tumours and the dosages are far above any potential exposure of man,
this is considered of little consequence for the human risk
evaluation.
Table 8. Summary of rat dietary studies
Concentration Lowest effect level (mg/kg diet)a No-
range and observed- Reference
duration of Body weight Thyroid Thyroid Thyroid Thyroid Uptake of adverse-
study gain weight hyperplasia tumours hormones 131I effect level
(PBI/T3/T4)
30-300 mg/kg 100 - - - 100 - 30 mg/kg Babish (1977)
(28 days)
2-50 mg/kg > 50 50 10 - >50 50 2mg/kg Fregly (1968)
(13 weeks)
2-500 mg/kg - 50/200 50 (20)b - 200 50 (20)b 2mg/kg Den Tonkelaar &
(6-13 weeks) Kroes (1974)
10-500 mg/kg 100 100 50 50 - - 10 mg/kg Keller (1959)
(2 years)
1-100 mg/kg > 100 100 100 100 > 100 100 10 mg/kg Steinhoff & Boehme
(2 years) (1979a)
a indicates not relevant or not measured
b values in parentheses are for very marginal effects
Under normal circumstances of occupational exposure, it is
unlikely that amitrole induces thyroid effects in humans.
Finally, it should be noted that the role of TSH in thyroid
carcinogenesis in humans seems different from that played in
experimental thyroid cancer in rats. This is based on the absence of
a correlation between hypothyroidism and thyroid cancer in human
epidemiological studies.
10.2 Evaluation of effects on the environment
Amitrole has high potential mobility in soil. The rapid
degradation of amitrole in soil and its retention in most soils by
adsorption makes this potential very unlikely to be realized in most
situations. The few reports of effects on non-target vegetation
support this view.
Use of amitrole at maximum recommended application rates to
control terrestrial weeds would lead to soil residues of up to 20 mg
a.i./kg dry soil. The soil invertebrates tested have not been
adversely affected at substantially higher concentrations. Effects
on soil microorganisms would also not occur. Amitrole presents no
hazard to birds.
Overspraying of static water bodies during the control of
terrestrial weeds would lead to maximum initial water concentrations
substantially below reported NOEC values for aquatic organisms. The
use of amitrole to control aquatic weeds has been reported to lead
to water concentrations of about 1 mg/litre, which persist for some
time. This would not affect fish but could be expected to adversely
affect water fleas (the NOEC is 0.2 mg/litre for reproductive
effects).
11. CONCLUSIONS AND RECOMMENDATIONS FOR PROTECTION OF HUMAN HEALTH
AND THE ENVIRONMENT
11.1 Conclusions
Amitrole does not present a significant risk to human health
when manufactured and used according to good handling procedure.
Current restrictions on its use in most countries, particularly its
restriction to non-crop use, will ensure minimum human exposure.
Amitrole is relatively rapidly degraded in the environment and
there is no evidence of bioaccumulation. The available data does not
indicate there are significant effects on the environment. Where
effects occur, these appear to be transient.
11.2 Recommendations for protection of human health and the
environment
Annual monitoring of thyroid function is recommended for
workers regularly involved with amitrole, both at the formulation or
application stages.
Epidemiological studies should be continued on workers exposed
to amitrole.
Patterns of use of amitrole should continue to avoid the risk
of contamination of food crops and water supplies, and limits for
residues in food and water should be maintained at low levels, i.e.
below 0.02 mg/kg in raw agricultural commodities of plant origin
(this level is at or around the limit of determination).
12. FURTHER RESEARCH
Further research data on amitrole are needed in the following
areas:
a) studies on the relationship between the extent of occupational
exposure and urinary excretion of amitrole in relation to
thyroid function;
b) studies aimed at understanding species differences in the
effects produced on the thyroid and liver;
c) in vitro and in vivo studies on the effects on specific
enzyme systems that may be involved in amitrole toxicity;
d) genotoxicity tests in vivo (other than chromosome
aberration).
13. PREVIOUS EVALUATIONS BY NATIONAL AND INTERNATIONAL
BODIES
Amitrole was considered by the International Agency for
Research on Cancer (IARC) in 1974 and 1986. In 1974, IARC evaluated
amitrole and, on the basis of animal carcinogenicity data and
epidemiological data, indicated that amitrole may be carcinogenic to
humans. However, the findings were regarded as inconclusive.
In a further evaluation in 1986 by IARC, it was concluded that
there was sufficient evidence for carcinogenicity of amitrole in
experimental animals, and that there was inadequate evidence for
carcinogenicity of amitrole in humans (2b) (IARC, 1986, 1987).
Amitrole was discussed at the 1974 and 1977 FAO/WHO Joint
Meetings on Pesticide Residues (JMPR). At the 1974 meeting, a
conditional acceptable daily intake (ADI) for amitrole of
0-0.00003 mg/kg body weight was established (FAO/WHO, 1975). At the
1977 meeting, further studies on the effects of amitrole were
considered, and the conditional ADI for amitrole was confirmed
(FAO/WHO, 1978)a. The 17th Codex Alimentarius Commission meeting
recommended a maximum residue limit at or about the limit of
determination by the best available analytical method.
The European Community established MRLs for amitrole on all
food crops at a limit of determination of 0.05 mg/kg.
The US Environmental Protection Agency proposed termination of
a special review of amitrole based on the Agency's determination
that the benefits of use outweighed the risks (USEPA, 1992).
a In September 1993, i.e. 4 months after the Task Group met, JMPR
reviewed amitrole and established a temporary ADI of
0-0.0005 mg/kg body weight. The temporary ADI will be valid
until 1997, when amitrole will again be reviewed by JMPR.
REFERENCES
Aaronson S (1960) Mode of action of 3-amino-1,2,4-triazole of
photosynthetic microorganisms. J Protozool, 7: 289-294.
Aaronson S & Sher S (1960) Effect of aminotriazole and streptomycin
on multiplication and pigment production of photosynthetic
microorganisms. J Protozool, 7: 156-158.
ACGIH (1991-1992) Threshold limit values for chemical substances and
physical agents and biological exposure indices for 1991-1992.
Cincinnati, Ohio, American Conference of Governmental and Industrial
Hygienists, p 12.
Adams N & Dobbs AJ (1984) A comparison of results from two test
methods for assessing the toxicity of aminotriazole to Selenastrum
capricornutum. Chemosphere, 13: 965-971.
Agrawal BBL & Margoliash E (1970) A spectrophotometric method for
the determination of aminotriazole and other aromatic amines. Anal
Biochem, 34: 505-516.
Alary J, Bourbon P, Escrieut C, & Vandaele J (1984)
Spectrophotometric determination of guanazole and aminotriazole in
waters from an aminotriazole production plant. Environ Technol Lett,
6: 93-100.
Aldrich FD & McLane SR Jr (1957) A paper chromatographic method for
the detection of 3-amino-1,2,4-triazole in plant tissues. Plant
Physiol, 32: 153-154.
Alexander NM (1959a) Antithyroid action of 3-amino-1,2,4-triazole. J
Biol Chem, 234(1): 148-150.
Alexander NM (1959b) Iodide peroxidase in rat thyroid and salivary
glands and its inhibition by anti-thyroid compounds. Fed Proc, 18:
1530-1533.
Allen CF & Bell A (1946) 3-Amino-1,2,4-triazole. Org Synth, 26:
11-12.
Altenburger R, Bodeker W, Faust M, & Horst Grimme L (1990)
Evaluation of the isobologram method for the assessment of mixtures
of chemicals, combination effect studies with pesticides in algal
biotests. Ecotoxicol Environ Saf, 20: 98-114.
Andersen KJ, Leighty EG, & Takahashi MT (1972) Evaluation of
herbicides for possible mutagenic properties. J Agric Food Chem,
20(3): 649-656.
Anderson JPE (1984) Influence of amitrole on the growth of green
algae (Scenedesmus subspicatus) in nutrient solution. Leverkusen,
Bayer AG (Unpublished report No. 6984).
Anderson JPE (1989) Influence of the commercial product Aminotriazol
Bayer on the soil respiration after amendment with glucose.
Leverkusen, Bayer AG (Unpublished report No. AJO/64189).
Anderson C & Hellpointner E (1989) Adsorption/desorption of amitrole
in soils. Leverkusen, Bayer AG (Unpublished report No. PF 3218).
Aragon CMG & Amit Z (1992) The effect of 3-amino-1,2,4-triazole on
voluntary ethanol consumption: Evidence for brain catalase
involvement in the mechanism of action. Neuropharmacology, 31(7):
709-712.
Aragon CMG, Spivak K, & Amit Z (1991a) Effect of
3-amino-1,2,4-triazole on ethanol-induced narcosis, lethality and
hypothermia in rats. Phamacol Biochem Behav, 39: 55-59.
Aragon CMG, Rogan F, & Amit Z (1991b) Dose- and time-dependent
effect of an acute 3-amino-1,2,4-triazole injection on rat brain
catalase activity. Biochem Pharmacol, 42: 699-702.
Archer AW (1984) Determination of 3-amino-1,2,4-triazole (amitrole)
in urine by ion-pair high-performance liquid chromatography. J
Chromatogr, 303: 267-271.
Ashworth R de B, Henriet J, Lovett JF, & Martijn A (1980) Amitrole.
In: CIPAC handbook. Volume 1A & 1B (Addendum to CIPAC 1): Analysis
of technical and formulated pesticides. Harpenden, UK, Collaborative
International Pesticide Analytical Council, pp 1720-1725.
Astwood EB (1960) Cranberries, turnips, and goitre. J Am Med Assoc,
172(12): 1319-1320.
Axelson O & Sundell L (1974) Herbicide exposure, mortality and
tumour incidence. An epidemiological investigation on Swedish
railroad workers. Scand J Work Environ Health, 11(1): 21-28.
Axelson O, Sundell L, Andersson K, Edling C, Hogstedt C, & Kling H
(1980) Herbicide exposure and tumour mortality. An updated
epidemiologic investigation on Swedish railroad workers. Scand J
Work Environ Health, 6(1): 73-79.
Babish JG (1977) Triiodothyronine (T3) and thyroxine (T4) levels in
male rats consuming amitrole (3-amino,1-2-4-triazole) in their diets
for a four week recovery period. Waverly, New York, Food and Drug
Research Laboratories, Inc. (Unpublished report No. 5539).
Bagdon RE, Shaffer CB, Vidone LB, & Golz HH (1956) Aminotriazole -
Acute and subacute toxicity. Wayne, New Jersey, American Cyanamid
Company (Unpublished report No. 56-29, submitted to WHO by Bayer
AG).
Balkisson R, Murray D, & Hoffstein V (1992) Alveolar damage due to
inhalation of amitrole-containing herbicide. Chest, 101: 1174-1175.
Bamford D, Sorsa M, Gripenberg U, Laamanen I, & Meretoja T (1976)
Mutagenicity and toxicity of amitrole. III. Microbial tests. Mutat
Res, 40(3): 197-202.
Baron J & Tephly TR (1969 Effect of 3-amino-1,2,4-triazole on the
stimulation of hepatic microsomal heme synthesis and induction of
hepatic microsomal oxidases produced by phenobarbital. Mol
Pharmacol, 5: 10-20.
Barthel E (1981) Increased risk of lung cancer in pesticide-exposed
male agricultural workers. J Toxicol Environ Health, 8: 1027-1040.
Baugher DG, Bookbinder MG, & Blundell KR (1982) Final Report -
Medical monitoring and assessment of exposure of workers applying
amitrole to utility rights-of-way. Rockville, Maryland, Dynamac
Corporation, Enviro Control Division (Unpublished report).
Bayer AG (1979) Aminotriazole - Toxicity to fish. Leverkusen, Bayer
AG (Unpublished report No. FO-227).
Bayer AG (1993a) Residue trials on amitrole in apples. Leverkusen,
Bayer AG (Unpublished reports Nos 0625/79, 0626/79, 0627/79,
0637/79, 0726/90, 0627/90).
Bayer AG (1993b) Residue trials on amitrole in grapes. Leverkusen,
Bayer AG (Unpublished reports Nos 0634/70, 0635/79, 0636/79,
0606/83, 0607/83, 0601/85, 0602/85, 0603/85, 0604/85, 0605/85,
0724/90, 0725/90, 0252/91, 0253/91, 254/91).
Becci P (1983) Evaluation of the chronic inhalation toxicity and
carcinogenicity of amitrole in rats. Waverly, New York, Food and
Drug Research Laboratories, Inc. (Unpublished report No. 5821,
submitted to WHO by Bayer AG).
Beynen AC, Buechler KF, Van der Molen AJ, & Geelen MJH (1981)
Inhibition of lipogenesis in isolated hepatocytes by
3-amino-1,2,4-triazole. Toxicology, 22: 171-178.
Bhuyan KC, Bhuyan DK, & Katzin HM (1973) Amizole-induced cataract
and inhibition of lens catalase in rabbit. Ophthalmol Res, 5:
236-247.
Bignami M, Aulicino F, Velcich A, Carere A, & Morpurgo G (1977)
Mutagenic and recombinogenic action of pesticides in Aspergillus
nidulans. Mutat Res, 46(6): 395-402.
Blumenstock I (1989) Influence of the commercial product
Aminotriazole Bayer on nitrification in soils. Leverkusen, Bayer AG
(Unpublished report No. BSI/64289).
Bogers M (1989) Influence of aminotriazol SP50 on the reproduction
of Daphnia magna. 's-Hertogenbosch, The Netherlands, RCC Notox
B.V. (Unpublished report No. 011339).
Braun R, Schoneich J, & Ziebarth D (1977) in vivo formation of
N-nitroso compounds and detection of their mutagenic activity in the
host-mediated assay. Cancer Res, 37: 4572-4579.
Brooks TM & Dean BJ (1981) Mutagenic activity of 42 coded compounds
in the salmonella/microsome assay with preincubation. In: de Serres
FJ & Ashby J ed. Evaluation of short-term tests for carcinogens.
Report of the international collaborative program. New York,
Amsterdam, Oxford, Elsevier/North-Holland, pp 261-270 (Progress in
Mutation Research, Volume 1).
Brusick D (1975) Mutagenic evaluation of compound,
3-amino-1,2,4-triazole (99.4%) and 3-amino-1,2,4-triazole : ammonium
thiocyanate (1:1). Kensington, Maryland, Litton Bionetics, Inc.
(Unpublished report No. 2547).
Brusick D & Weir RJ (1976) Mutagenicity evaluation of
3-amino-1,2,4-triazole. in vitro cellular transformation in
Balb/3T3 cells. Kensington, Maryland, Litton Bionetics, Inc.
(Unpublished report No. 2549).
Buchmuller-Rouiller Y, Schneider P, Betz-Corradin S, Smith J, &
Mauel J (1992) 3-Amino-1,2,4-triazole inhibits macrophage NO
synthase. Biochem Biophys Res Commun, 183: 150-155.
Cain JR & Cain RK (1983) The effects of selected herbicides on
zygospore germination and growth of Chlamydomonas moewush
(Chlorophyceae Volvocales). J Phycol, 19: 301-305.
Campacci EF, New PB, & Tchan YT (1977) Isolation of
amitrole-degrading bacteria. Nature (Lond), 266: 164-165.
Carere A, Ortali VA, Cardamone G, Torracca AM, & Raschetti R (1978)
Microbiological mutagenicity studies of pesticides in vitro. Mutat
Res, 57(3): 277-286.
Carter MC (1975) Amitrole. In: Kearey PC & Kaufman DD ed.
Herbicides. New York, Basel, Marcel Dekker Inc., vol I, pp 377-398.
Carter MC & Naylor AW (1960) Metabolism of
3-amino-1,2,4-triazole-5-C14 in plants. Bot Gaz, 122: 138-143.
Corneliussen PE (1969) Residues in food and feed: Pesticide residues
in total diet samples (IV). Pestic Monit J, 2(4): 140-152.
Corneliussen PE (1970) Residues in food and feed: Pesticide residues
in total diet samples (V). Pestic Monit J, 4(3): 89-105.
Courtney WD, Peabody DV, & Austenson HM (1962) Effect of herbicides
on nematodes in bentgrass. Plant Dis Rep, 46(4): 256-257.
Cox GE & Re TA (1978) Inhalation toxicity study with
3-amino-1,2,4-triazole in adult Fischer 334 rats. Waverly, New York,
Food and Drug Research Laboratories, Inc. (Unpublished report No.
5492).
Crawford RB & Guarino AM (1985) Effect of environmental toxicants on
development of a teleost embryo. J Environ Pathol Toxicol Oncol,
6(2): 185-194.
Crebelli R, Bellincampi D, Conti G, Conti L, Morpurgo G, & Carere A
(1986) A comparative study on selected chemical carcinogens for
chromosome malsegregation, mitotic crossing-over and forward
mutation induction in Aspergillus nidulans. Mutat Res, 172:
139-149.
Crosby DG & Tucker RK (1966) Toxicity of aquatic herbicides to
Daphnia magna. Science, 154: 289-290.
Daniel MR & Dehnel JM (1981) Cell transformation test with baby
hamster kidney cells. In: de Serres FJ & Ashby J ed. Evaluation of
short-term tests for carcinogens. Report of the international
collaborative program. New York, Amsterdam, Oxford,
Elsevier/North-Holland, pp 626-637 (Progress in Mutation Research,
Volume 1).
Darr D & Fridovich I (1986) Irreversible inactivation of catalase by
3-amino-1,2,4-triazole. Biochem Pharmacol, 35(20): 3642.
Dasilva EJ, Henriksson LE, & Henriksson E (1975) Effects of
pesticides on blue-green algae and nitrogen-fixation. Arch Environ
Contam Toxicol, 3(2): 193-204.
Day BE, Jordan LS, & Hendrixon RT (1961) The decomposition of
amitrole in California soils. Weeds, 9: 443-456.
Degen GH, Wong A, Eling TE, Barrett JC, & McLachlan JA (1983)
Involvement of prostaglandin synthetase in peroxidative metabolism
of diethylstilbestrol in Syrian hamster embryo fibroblast cell
cultures. Cancer Res, 43: 992-996.
Demint RJ, Frank PA, & Comes RD (1970) Amitrole residues and rate of
dissipation in irrigation water. Weed Sci, 18(4): 439-442.
Den Tonkelaar EM & Kroes R (1974) [Toxicity of thyroid function
after subacute and semichronic administration of aminotriazole.]
Bilthoven, National Institute of Public Health (Unpublished report
No. 164/74 TOX) (in Dutch).
Deprospo JR & Fogleman RW (1973) Amitrol-T: Acute oral toxicity in
rats. Princeton, New Jersey, Affiliated Medical Research, Inc.
(Unpublished report No. 121-1921-33).
Doni MG & Piva E (1983) Glutathione peroxidase blockage inhibits
prostaglandin biosynthesis in rat platelets and aorta. Haemostasis,
13: 240-243.
Doniach I (1974) Carcinogenic effect of 100, 250 and 500 rad x-rays
on the rat thyroid gland. Br J Cancer, 30(6): 487-495.
Dornseiffen JW & Kerwaal W (1988) Amitrole residues in blackberries.
Amsterdam, Ford Inspection Service (Unpublished report).
Duggan RE, Barry HC, & Johnson LY (1966) Pesticide residues in
total-diet samples. Science, 151: 101-104.
Duggan RE, Barry HC, & Johnson LY (1967) Residues in food and feed.
Pesticide residues in total diet samples (II). Pestic Monit J, 1(2):
2-12.
Dunkel VC, Pienta RJ, Sivak A, & Traul KA (1981) Comparative
neoplastic transformation responses of Balb/3T3 cells, Syrian
hamster embryo cells, and Rauscher murine leukaemia virus infected
Fischer 344 rat embryo cells to chemical carcinogens. J Natl Cancer
Inst, 67: 1303-1315.
Dunkel VC, Zeiger E, Brusick D, McCoy E, McGregor D, Mortelmans K,
Rosenkranz HS, & Simmon VF (1984) Reproducibility of microbial
mutagenicity assays. 1. Tests with Salmonella typhimurium and
Escheria coli using standardized protocol. Environ Mutagen, 6:
1-254.
Dunkel VC, Schechtman LM, Tu AS, Sivak A, Lubet RA, & Cameron TP
(1988) Interlaboratory evaluation of the C3H/10T1/2 cell
transformation assay. Environ Mol Mutagen, 12: 21-31.
Eberbach PL & Douglas LA (1989) Herbicide effects of the growth and
nodulation potential of Rhizobium trifolii with Triolium
subterraneum L. Plant Soil, 119: 15-23. Ebing W (1972) [Routine
method for identification of pesticide residues of triazine,
carbamate, urea and uracil type compounds by thin layer
chromatography.] J Chromatogr, 65: 533-545 (in German).
Elsea JR (1954) Aminotriazole: Acute dermal application, acute eye
application. Falls Church, Virginia, Hazleton Laboratories
(Unpublished report).
English JSC, Rycroft RJG, & Coleman CD (1986) Allergic dermatitis
from aminotriazole. Contact Dermatitis, 14: 255-256.
Environment Agency Japan (1987) Chemicals in the environment. Report
on environmental survey and wildlife monitoring of chemicals in F.Y.
1984 and 1985. Tokyo, Environment Agency Japan, Department of
Environmental Health, Office of Health Studies.
Fang SC, George M, & Yu TC (1964) Metabolism of
3-amino-1,2,4-triazole-5-14C by rats. J Agric Food Chem, 12:
219-223.
Fang SC, Khanna S, & Rao AV (1966) Further study on the metabolism
of labelled 3-amino-1,2,4-triazole (ATA) and its plant metabolites
in rats. J Agric Food Chem, 14: 262-265.
Fang SC, Fallin E, & Khanna S (1967) The metabolism of labelled
amitrole in plants. Weeds, 15: 343-346.
FAO/WHO (1975) Amitrole. In: 1974 Evaluations of some pesticide
residues in food. Geneva. World Health Organization, pp 3-49 (WHO
Pesticide Residues Series, No. 4).
FAO/WHO (1978) Amitrole. In: Pesticides residues in food - 1977.
Evaluations. Rome, Food and Agriculture Organization of the United
Nations, pp 11-14 (FAO Plant Production and Protection Paper 10
Sup.).
Feinstein RN, Fry RJN, & Staffeldt EF (1978a) Comparative effects of
aminotriazole on normal and acatalasemic mice. J Environ Pathol
Toxicol, 1(6): 779-790.
Feinstein RN, Fry RJM, & Staffeldt EF (1978b) Carcinogenic and
antitumour effects of aminotriazole on acatalasemic and normal
catalase mice. J Natl Cancer Inst, 60(5): 1113-1116.
Field WE (1979) Oral LD50 in rats. Clarks Summit, Pennsylvania,
CDC Research, Inc. (Unpublished report No. CDC-AM-048-79).
Fogleman RW (1954) Amizole (3-amino-1,2,4-triazole) 96.1%: Acute
oral administration in mice and dogs; acute intravenous
administration in dogs; pharmacodynamics in dogs; subacute feeding
in rats. Falls Church, Virginia, Hazleton Laboratories (Unpublished
report).
Fregly MJ (1968) Effect of aminotriazole on thyroid function in the
rat. Toxicol Appl Pharmacol, 13: 271-286.
Frey F (1979) Effects of the herbicide aminotriazole on the
nematode, Acrobeloides buetsclii in culture. Nematologica, 25:
146-147.
Fujii T, Miyazaki H, & Hashimoto M (1984) Autoradiographic and
biochemical studies of drug distribution in the liver III. [14C]
Aminotriazole. Eur J Drug Metab Pharmacokinet, 9: 257-265.
Gaines TB, Kimbrough RD, & Linder RE (1973) The toxicity of amitrole
in the rat. Toxicol Appl Pharmacol, 26: 118-129.
Gatehouse D (1981) Mutagenic activity of 42 coded compounds in the
"Microtiter" fluctuation test. In: de Serres FJ & Ashby J ed.
Evaluation of short-term tests for carcinogens. Report of the
international collaborative program. New York, Amsterdam, Oxford,
Elsevier/North-Holland, pp 376-386 (Progress in Mutation Research,
Volume 1).
Geldmacher-Von Mallinckrodt M & Schmidt HP (1970) [Toxicity and
metabolism of aminotriazole in man.] Arch Toxikol, 27: 13-18 (in
German).
Gentry GM, Jackson ER, Jensen TL, Jung PD, Launer JE, & Torma L ed.
(1984) Pesticide formulations. Amitrole in pesticide formulations:
Titrimetric methods. Final action. In: Official methods of analysis
of the Association of Official Analytical Chemists, 14th ed.
Arlington, Virginia, Association of Official Analytical Chemists,
Inc., p 146.
Grapenthien JR (1972) Two year chronic aerosol inhalation toxicity
study with amitrole 3-AT in albino rats. Northbrook, Illinois,
Industrial Bio-Test Laboratories, Inc. (Unpublished report No.
N7640, submitted to WHO by Bayer AG).
Grau R (1989) Toxicity of aminotriazol techn. to rainbow trout
(Salmo gairdneri) with prolonged exposure (21 days). Leverkusen,
Bayer AG (Unpublished report No. FF-242).
Green FO & Feinstein RN (1957) Quantitative estimation of
3-amino-1,2,4-triazole. Anal Chem, 29: 1658-1660.
Grossbard E & Wingfield GI (1978) Effect of paraquat, aminotriazole,
and glyphosate on cellulose decomposition. Weed Res, 18: 347-353.
Groves K & Chough KS (1971) Extraction of 3-amino-1,2,4-triazole
(amitrole) and 2,6-dichloro-4-nitroaniline (DCNA) from soils. J
Agric Food Chem, 19: 840-841.
Grunow W, Altmann HJ, & Boehme Chr (1975) [Metabolism of
3-amino-1,2,4-triazole in rats.] Arch Toxicol, 34(4): 315-324 (in
German).
Grzenda AR, Nicholson HP, & Cox WS (1966) Persistence of four
herbicides in pond water. J Am Water Works Assoc, 58: 326-332.
Hapke HJ (1967) [Toxicity of aminotriazole for domestic animals.]
Zent.bl Vet.med, 14: 469-486 (in German).
Hashimoto F, Sugimoto C, & Hayashi H (1992) Effects of aminotriazole
treatment on biosyntheses of primary bile acids in vivo. Chem
Pharm Bull, 40: 795-798.
Hassall KA (1969) World crop protection. Volume 2: Pesticides.
London, Iliffe Books Ltd, pp 233-235.
Hawkins DR, Kirkpatrick D, Finn CM, & Conway B (1982) The
biodegradation of 14C-aminotriazole in soil (field studies).
Huntingdon, Huntingdon Research Centre (Unpublished report No. BAY
124/702 054).
Hawkins DR, Kirkpatrick D, Finn CM, & Conway B (1982b) The
biodegradation of 14C-aminotriazole in soil (Laboratory studies).
Huntingdon, Huntingdon Research Centre (Unpublished report No. BAY
123/81 538).
Hayes S, Fordon A, Sadowski I, & Hayes C (1984) RK bacterial test
for independently measuring chemical toxicity and mutagenicity:
Short-term forward selection assay. Mutat Res, 130: 97-106.
Hecht P (1954) [Aminotriazole.] Leverkusen, Bayer AG (Unpublished
report) (in German).
Heim DR & Larrinua IM (1989) Primary site of action of amitrole in
Arabidopsis thaliana involves inhibition of root elongation but
not of histidine or pigment biosynthesis. Plant Physiol, 91:
1226-1231.
Heimann KG (1982) [Aminotriazole.] Leverkusen, Bayer AG (Unpublished
technical report) (in German).
Heimbach F (1983) Acute toxicity of amitrole (tech.) to water fleas.
Leverkusen, Bayer AG (Unpublished report No. HB/DM 22).
Heimbach F (1990a) Toxicity of aminotriazole (SP50) to carabid
beetles (Poecilus cupreus). Leverkusen, Bayer AG (Unpublished
report No. HBF/CA19).
Heimbach F (1990b) Toxicity of aminotriazol to earthworms.
Leverkusen, Bayer AG (Unpublished report No. HBF/RG 118).
Herbold B (1980) Amitrole: Salmonella/microsome test for detection
of point-mutagenic effects. Leverkusen, Bayer AG, Institute of
Toxicology (Unpublished report No. 9339).
Herbold B (1982) Amitrole: Aminotriazole active ingredient.
Micronucleus test in the mouse to evaluate for mutagenic effect.
Leverkusen, Bayer AG (Unpublished report No. 11139).
Herfs W (1975) Identification of crop protection product can be
designated as non-hazardous to honey bees - aminotriazole.
Brunswick, Germany, Federal Biological Institute for Agriculture and
Forestry, Division of Crop Protection Products and Application
Technology
Herrett RA & Linck AJ (1961) Quantitative determination of
3-amino-1,2,4-triazole. J Agric Food Chem, 9(6): 466-467.
Hiasa Y, Ohshima M, Kitahori Y, Yuasa T, Fujita T, & Iwata C (1982)
Promoting effects of 3-amino-1,2,4-triazole on the development of
thyroid tumours in rats treated with N-bis
(2-hydroxypropyl)nitrosamine. Carcinogenesis, 3(4): 381-384. Hill EF
& Camardese MB (1986) Lethal dietary toxicities of environmental
contaminants and pesticides to Coturnix. Washington, DC, US
Department of the Interior, Fish and Wildlife Service (Fish and
Wildlife Technical Report No. 2).
Hill EF, Heath RG, Spann JW, & Williams JD (1975) Lethal dietary
toxicities of environmental pollutants to birds. Washington, DC, US
Department of the Interior, Fish and Wildlife Service (Special
Scientific Report - Wildlife No. 191).
Hill RN, Erdreich LS, Paynter OE, Roberts AA, Rosenthal SL, &
Wilkinson CF (1989) (Review) Thyroid follicular cell carcinogenesis.
Fundam Appl Toxicol, 12: 629-697.
Hiltibran RC (1967) Effects of some herbicides on fertilized fish
eggs and fry. Trans Am Fish Soc, 96(4): 414-416.
Hodge HC, Maynard EA, Downs WL, Ashton JK, & Salerno LL (1966) Tests
on mice for evaluating carcinogenicity. Toxicol Appl Pharmacol, 9:
583-596.
Hoshino M (1960) Effect of 3-amino-1,2,4-triazole on the
experimental production of liver cancer. Nature (Lond), 186(4719):
174-179.
Hubbard SA, Green MHL, Bridges BA, Wain AJ, & Bridges JW (1981)
Fluctuation test with S9 and hepatocyte activation. In: de Serres FJ
& Ashby J ed. Evaluation of short-term tests for carcinogens. Report
of the international collaborative program. New York, Amsterdam,
Oxford, Elsevier/North-Holland, pp 361-370 (Progress in Mutation
Research, Volume 1).
Hudson RH, Tucker RK, & Haegele MA (1984) Handbook of toxicity of
pesticides to wildlife, 2nd ed. Washington, DC, US Department of the
Interior, Fish and Wildlife Service (Resource Publication 153).
IARC (1974) Amitrole. In: Some antithyroid and related substances,
nitrofurans and industrial chemicals. Lyon, International Agency for
Research on Cancer, pp 31-43 (IARC Monographs on the Evaluation of
the Carcinogenic Risk of Chemicals to Man, Volume 7).
IARC (1986) Amitrole. In: Some halogenated hydrocarbons and
pesticide exposures. Lyon, International Agency for Research on
Cancer, pp 293-317 (IARC Monographs on the Evaluation of the
Carcinogenic Risk of Chemicals to Humans, Volume 41).
IARC (1987) Amitrole. In: Overall evaluations of carcinogenicity: An
updating of IARC monographs, volumes 1 to 42. Lyon, International
Agency for Research on Cancer, pp 92-93 (IARC Monographs on the
Evaluation of the Carcinogenic Risk of Chemicals to Humans,
Supplement 7).
Ichinotsubo D, Mower H, & Mandel M (1981a) Testing of a series of
paired compounds (carcinogen and non-carcinogenic structural analog)
by DNA repair-deficient E. coli strains. In: de Serres FJ & Ashby
J ed. Evaluation of short-term tests for carcinogens. Report of the
international collaborative program. New York, Amsterdam, Oxford,
Elsevier/North-Holland, pp 195-198 (Progress in Mutation Research,
Volume 1).
Innes JR, Ulland BM, Valerio MG, Petrucelli L, Fishbein L, Hart ER,
& Palotta AJ (1969) Bioassay of pesticides and industrial chemicals
for tumorigenicity in mice: A preliminary note. J Natl Cancer Inst,
42(6): 1101-1114.
Inoue K, Katoh Y, & Takayama S (1981) In vitro transformation of
hamster embryo cells by 3-(N-salicyloyl)amino-1,2,4-triazole.
Toxicol Lett, 7: 211-215.
Iwan GR, Vilkas AG, & Hutchinson C (1978) 14C-Amitrole in Bluegill
Sunfish, Lepomis Macrochirus: bioconcentration study. Tarry Town,
New York, Union Carbide Corporation, Environmental Services
(Unpublished report).
Jacques A (1984) 3-Amino- s-triazole (amitrole) (Update). Anal
Methods Pestic Plant Growth Regul, XIII: 191-195.
Jagannath DR, Vultaggio DM, & Brusick DJ (1981) Genetic activity of
42 coded compounds in the mitotic gene conversion assay using
Saccharomyces cerevisiae strain D4. In: Dr Serres FJ & Ashby J ed.
Evaluation of short term tests for carcinogens. Report of the
international collaborative program. New York, Amsterdam, Oxford,
Elsevier/North-Holland, pp 456-467 (Progress in Mutation Research,
Volume 1).
Jarczyk HJ (1982a) [Behaviour of active ingredients of pesticides in
the soil (Amitrole).] Leverkusen, Bayer AG (Unpublished report No.
RR0600/81) (in German).
Jarczyk HJ (1982b) [Behaviour of active ingredients of pesticides in
the soil (Amitrole).] Leverkusen, Bayer AG (Unpublished report No.
RR0601/81) (in German).
Jarczyk HJ (1982c) [Behaviour of active ingredients of pesticides in
the soil (Amitrole).] Leverkusen, Bayer AG (Unpublished report No.
RR0602/81) (in German).
Jarczyk HJ (1985) Method for the gas-chromatographic determination
of residues of 3-amino-1,2,4-triazole in apples, pears, cherries,
grapes, soil and water, using an N-specific detector. Leverkusen,
Bayer AG (Unpublished report No. RA-988/296B).
Jarczyk HJ & Mollhoff E (1988) Method for the gaschromatographic
determination of amino-1,2,4-triazole residues in plant, soil and
water using a nitrogen specific detector. Leverkusen, Bayer AG
(Unpublished report No. RA-785/88).
Jensen-Korte U, Anderson C, & Spiteller M (1987) Photodegradation of
pesticides in the presence of humic substances. Sci Total Environ,
62: 335-340.
Johnson CR (1976) Herbicide toxicity in some Australian anurans and
the effect of subacute dosages on temperature tolerance. Zool J Linn
Soc, 59: 79-83.
Johnson WD, Becci PJ, & Parent RA (1981) Lifetime feeding study of
amitrole in Fischer 344 rats. Waverly, New York, Food and Drug
Research Laboratories, Inc. (Unpublished report No. 5651).
Jukes TH & Shaffer CB (1960) Antithyroid effects of aminotriazole.
Science, 132: 296-297.
Jung F, Szekacs A, Li Q, & Hammock BD (1991) Immunochemical approach
to the detection of aminotriazole using selective amino group
protection by chromophores. J Agric Food Chem, 39: 129-136.
Kada T (1981) The DNA-damaging activity of 42 coded compounds in the
rec-assay. In: de Serres FJ & Ashby J ed. Evaluation of short-term
tests for carcinogens. Report of the international collaborative
program. New York, Amsterdam, Oxford, Elsevier/North-Holland, pp
171-182 (Progress in Mutation Research, Volume 1).
Kassinova GV, Kovaltsova SV, Marfin SV, & Zakharov IA (1981)
Activity of 40 coded compounds in differential inhibition and
mitotic crossing-over assays in yeast. In: de Serres FJ & Ashby J
ed. Evaluation of short term tests for carcinogens. Report of the
international collaborative program. New York, Amsterdam, Oxford,
Elsevier/North-Holland, pp 434-455 (Progress in Mutation Research,
Volume 1).
Kaufman DD, Plimmer JR, Kearney PC, Blake J, & Guardia FS (1968)
Chemical versus microbial decomposition of amitrole in soil. Weed
Sci, 16(2): 266-272.
Keller JG (1959) Aminotriazole (3-amino-1,2,4-triazole): Final
report of 2-year feeding study in rats. Falls Church, Virginia,
Hazleton Laboratories (Unpublished report).
Kimmerle G (1968) [Aminotriazole.] Leverkusen, Bayer AG (Unpublished
report) (in German).
Klusacek H & Krasemann R (1986) Thermal stability of the
agrochemical active ingredient. Leverkusen, Bayer AG (Unpublished
report No. 85/10472TA).
Knacker Th & Reifenberg P (1989) A study of the toxicity to algae of
aminotriazol SP50. Frankfurt am Main, Battelle Europe (Unpublished
report No. BEEA 148901 ALG7).
Knickerbocker M & Stevens KR (1978) A study to determine the
potential of amitrole to induce dominant lethal mutations in Ha
(ICR) mice. Waverly, New York, Food and Drug Research Laboratories,
Inc. (Unpublished report No. 5502).
Krauss RS & Eling TE (1987) Macromolecular binding of the thyroid
carcinogen 3-amino-1,2,4-triazole (amitrole) catalyzed by
prostaglandin H synthetase, lactoperoxidase and thyroid peroxidase.
Carcinogenesis, 8: 659-664.
Krohn A (1982) Fate/behaviour of crop protection products in water:
(hydrolytic stability/volatility from water). Leverkusen, Bayer AG
(Unpublished report No. M2067).
Kumar HD (1963) Inhibition of growth and pigment production of a
blue-green alga by 3-amino-1,2,4-triazole. Indian J Plant Physiol,
6: 150-155.
Laamanen I, Sorsa M, Bamford D, Gripenberg U, & Meretoja T (1976)
Mutagenicity and toxicity of amitrole. I. Drosophila tests. Mutat
Res, 40(3): 185-190.
Landauer W, Salam N, & Sopher D (1971) The herbicide
3-amino-1,2,4-triazole (amitrole) as teratogen. Environ Res, 4:
539-543.
Legrand MF, Costentin E, & Bruchet A (1991) Occurrence of 38
pesticides in various French surface and ground waters. Environ
Technol, 12: 985-996.
Lokke H (1980) Determination of amitrole by ion-pair
high-performance liquid chromatography. J Chromatogr, 200: 234-237.
LUFA (1977) [Behaviour of active ingredients of pesticides in the
soil (Amitrole).] Speyer am Rhein, Agriculture Monitoring and
Research Institute (Unpublished report No. M7750).
MacCarthy P & Djebbar KE (1986) Removal of paraquat, diquat and
amitrole from aqueous solution by chemically modified peat. J
Environ Qual, 15: 103-107.
MacDonald DJ (1981) Salmonella/microsome tests on 42 coded
chemicals. In: de Serres FJ & Ashby J ed. Evaluation of short-term
tests for carcinogens. Report of the international collaborative
program. New York, Amsterdam, Oxford, Elsevier/North-Holland, pp
285-297 (Progress in Mutation Research, Volume 1).
MacDonald CM & Pullinger DH (1976) A pharmakinetic study to compare
"head only" and "whole body" inhalation methods of 14C-amitrole
exposure. Harrogate, UK, Hazleton Laboratories Europe Ltd
(Unpublished report No. 291/1).
McGregor DB, Martin R, Cattanach P, Edwards I, McBride D, & Caspary
WJ (1987) Responses of the L5178Y tk+/tk-mouse Lymphoma cell
forward mutation assay to coded chemicals. Environ Mutagen, 9:
143-160.
Machemer L (1977a) Amitrole. Dominant lethal test on the male mouse
to evaluate for mutagenic effects. Leverkusen, Bayer AG (Unpublished
report No.6679).
Machemer L (1977b) Amitrole. Study for embryotoxic and teratogenic
effects after oral administration to the rat. Leverkusen, Bayer AG
(Unpublished report No. 6634).
McTiernan AM, Weiss NS, & Dalling JR (1984), Incidence of thyroid
cancer in women in relation to previous exposure to radiation
therapy and history of thyroid disease. J Natl Cancer Inst, 73:
575-581.
Mamber SW, Bryson V, & Katz SE (1983) The Escherichia coli
WP2/WP100 rec assay for detection of potential carcinogens. Mutat
Res, 119(2): 135-144.
Mamber SW, Bryson V, & Katz SE (1984) Evaluation of the Escheria
coli K12 induce test for detection of potential chemical
carcinogens. Mutat Res, 130: 141-151.
Margoliash E, Novogradsky AR, & Schejter A (1960) Irreversible
reaction of 3-amino-1,2,4-triazole and related inhibitors with the
protein of catalase. Biochem J, 74: 339-348.
Marston RB, Schults DW, Shiroyama T, & Snyder LV (1968) Pesticides
in water. Amitrole concentrations in creek waters downstream from an
aerially sprayed watershed sub-basin. Pestic Monit J, 2(3): 123-128.
Martin NA (1982) The effects of herbicides used on asparagus on the
growth rate of the earthworm, Allolobophora Caliginosa. In:
Proceedings of the 35th New Zealand Weed and Pest Control
Conference, pp 328-329.
Martin CN & McDermid AC (1981) Testing of 42 coded compounds for
their ability to induce unscheduled DNA repair synthesis in HeLa
cells. In: de Serres FJ & Ashby J ed. Evaluation of short-term tests
for carcinogens. Report of the international collaborative program.
New York, Amsterdam, Oxford, Elsevier/North-Holland, pp 533-537
(Progress in Mutation Research, Volume 1).
Mason, JM, Valencia R, & Zimmering S (1992) Chemical mutagenesis
testing in Drosophila. VIII. Reexamination of equivocal results.
Environ. Mol Mutagen, 19: 227-234.
Mayberry WE (1968) Antithyroid effects of 3-amino-1,2,4-triazole.
Proc Soc Exp Biol Med, 129: 551-556.
Mayer FL & Ellersieck MR (1986) Manual of acute toxicity:
Interpretation and data base for 410 chemicals and 66 species of
freshwater animals. Washington, DC, US Department of the Interior,
Fish and Wildlife Service (Resource Publication 160).
Mehta RD & Von Borstel RC (1981) Mutagenic activity of 42 encoded
compounds in the haploid yeast reversion assay, strain XV185 -14C,
In: de Serres FJ & Ashby J ed. Evaluation of short-term tests for
carcinogens. Report of the international collaborative program. New
York, Amsterdam, Oxford, Elsevier/North-Holland, pp 414-423
(Progress in Mutation Research, Volume 1).
Meretoja T, Gripenberg U, Bamford D, Laamanen I, & Sorsa M (1976)
Mutagenicity and toxicity of amitrole. II. Human lymphocyte culture
tests. Mutat Res, 40: 191-196.
Metodiewa D & Dunford HB (1991) 3-Aminotriazole is a substrate for
lactoperoxidase but not for catalase. Biochem Biophys Res Commun,
180: 585-590.
Middelkoop E, Strijland A, & Tager JM (1991) Does aminotriazole
inhibit import of catalase into peroxisomes by retarding unfolding?
FEBS Lett, 279: 79-82.
Mihail F (1984) Aminotriazole - Study for skin sensitization effect
on guinea pigs. Leverkusen, Bayer AG, Institute of Toxicology
(Unpublished report No. 12607).
Mihail F (1985) Aminotriazole - Study for skin-sensitising effect on
guinea pigs in the open epicutaneous test. Leverkusen, Bayer AG,
Institute of Toxicology (Unpublished report No. 13775).
Miksche LW (1982) BBA request - Effects on man. Leverkusen, Bayer
AG, Medical Department (Unpublished report).
Miksche LW (1983) Production of amitrole - Medical monitoring.
Leverkusen, Bayer AG, Medical Department (Unpublished report).
Moore B (1968) Amitrole and simazine residues in Tasmanian apples
treated with Domatol 44. Sydney, Ciba-Geigy Australia Ltd
(Unpublished report No. 68/8/290).
Moore B (1969) Amitrole and simazine residue trials at Orange, NSW,
1967-1968. Sydney, Ciba-Geigy Australia Ltd (Unpublished report No.
69/2/294).
Moore B (1970) Amitrole residue trial, Orange, 1968-69. Sydney,
Ciba-Geigy Australia Ltd (Unpublished report No. 70/1/309).
Mori S, Takeuchi Y, Toyama M, Makino S, Ohhara T, Tochino Y, &
Hayashi Y (1985) Amitrole: Strain differences in morphological
response of the liver following subchronic administration to mice.
Toxicol Lett, 29: 145-152.
Moriya M, Ohta T, Watanabe K, Miyazawa T, Kato K, & Shirasu Y (1983)
Further mutagenicity studies on pesticides in bacterial reversion
assay systems. Mutat Res, 116(3-4): 185-216.
Morpurgo G, Bellincampi D, Gualandi G, Baldinelli L, & Crescenzi OS
(1979) Analysis of mitotic nondisjunction with Aspergillus
nidulans. Environ Health Perspect, 31: 81-95.
Mothes-Wagner U, Reitz HK, & Seitz KA (1990) Environmental actions
of agrochemicals. 1. Side-effects of the herbicide 3-amino-1,2,4-
triazole on a laboratory acarine/host-plant interaction
(Tetranychus uticae/Phaseolus vulgaris) as revealed by electron
microscopy. Environ Appl Acarol, 8: 27-40.
Nearpass DC (1969) Exchange adsorption of 3-amino-1,2,4-triazole by
an organic soil. Proc Soil Sci Soc Am, 33: 524-528.
Nearpass DC (1970) Exchange adsorption of 3-amino-1,2,4-triazole by
montmorillonite. Soil Sci, 109(2): 77-84.
Niehuss M & Borner H (1971) [Investigations into the effects of
herbicides on fish.] Nachr.bl Dtsch Pflanzenschutzd, 8: 113-117 (in
German).
Nishiuchi Y (1981) Toxicity of pesticides to some aquatic organisms.
Toxicity of pesticides to some aquatic insects. Seitai Kagaku, 4:
31-46.
Nomiyama K, Minai M, & Kita H (1965) Median lethal dose of
3-amino-1,2,4-triazole. Bull Tokyo Med Dent Univ, 12(1): 55-57.
Pachinger A, Eisner E, Begutter H, & Klus H (1992) A simple method
for the determination of amitrole in drinking and ground water.
Fresenius J Anal Chem, 342: 413-415.
Perry PE & Thomson JE (1981) Evaluation of the sister chromatid
exchange method in mammalian cells as a screening system for
carcinogens. In: de Serres FJ & Ashby J ed. Evaluation of short-term
tests for carcinogens. Report of the international collaborative
program. New York, Amsterdam, Oxford, Elsevier/North-Holland, pp
560-569 (Progress in Mutation Research, Volume 1).
Plimmer JR, Kearney PC, Kaufman DD, & Guardia FS (1967) Amitrole
decomposition by free radical-generating system and by soils. J
Agric Food Chem, 15(6): 996-999.
Pribyl J, Herzel F, & Schmidt G (1978) [Contribution to the
determination of aminotriazole residues.] Fresenius Z Anal Chem,
289: 81-85 (in German).
Prince HN (1977) Microbial mutagen assays with amitrol
(3-amino-1,2,4-triazole). Fairfield, New Jersey, Gibraltar
Biological Laboratories (Unpublished report No. 5634, submitted to
WHO by Bayer AG).
Racusen D (1958) The metabolism and translocation of 3-aminotriazole
in plants. Arch Biochem Biophys, 74(1): 106-113.
Rausina G (1972) Two year chronic dermal toxicity study with
amitrole 3-AT on albino rats. Northbrook, Illinois, Industrial
Bio-Test Laboratories, Inc. (Unpublished report No. A7639, submitted
to WHO by Bayer AG).
Reddy KR, Rao JV, & Das VSR (1987) Effect of monosodium methane
arsonate and aminotriazole on biosynthesis of epicuticular wax in
some semiarid shrubs. Indian J Exp Biol, 25: 562-566.
Reitze HK & Seitz KA (1985) Light and electron microscopical changes
in the liver of mice following treatment with aminotriazole. Exp
Pathol, 27: 17-31.
Richold M & Jones E (1981) Mutagenic activity of 42 coded compounds
in the salmonella/microsome assay. In: de Serres FJ & Ashby J ed.
Evaluation of short-term tests for carcinogens. Report of the
international collaborative program. New York, Amsterdam, Oxford,
Elsevier/North-Holland, pp 314-332 (Progress in Mutation Research,
Volume 1).
Riepma P (1962) Preliminary observations on the breakdown of
3-amino-1,2,4-triazole in plants. Weed Res, 2: 41-50.
Ritter A (1989) Influence of amitrole technical on the reproduction
of Daphnia magna. Itingen, Switzerland, RCC Umweltchemie AG
(Unpublished report No. 235192).
Robertson EB & Bunting DL (1976) The acute toxicity of four
herbicides to 0-4 hour Nauplii of Cyclops vernalis Fisher
(Copepoda, Cyclopoida). Bull Environ Contam Toxicol, 16(6): 682-688.
Ron E, Kleinerman RA, Boice JD, Livolsi VA, Flannery JT, & Fraumeni
JF (1987) A population-based case-control study of thyroid cancer. J
Natl Cancer Inst, 79: 1-12.
Rosenkranz HS & Poirier LA (1979) Evaluation of the mutagenicity and
DNA-modifying activity of carcinogens and non-carcinogens in
microbial systems. J Natl Cancer Inst, 62: 873-892.
Rosenkranz HS, Hyman J, & Leifer Z (1981) DNA polymerase deficient
assay. In: de Serres FJ & Ashby J ed. Evaluation of short-term tests
for carcinogens. Report of the international collaborative program.
New York, Amsterdam, Oxford, Elsevier/North-Holland, pp 210-218
(Progress in Mutation Research, Volume 1).
Rowland I & Severn B (1981) Mutagenic of carcinogens and
noncarcinogens in the salmonella/microsome test. In: de Serres FJ &
Ashby J ed. Evaluation of short-term tests for carcinogens. Report
of the international collaborative program. New York, Amsterdam,
Oxford, Elsevier/North-Holland, pp 323-332 (Progress in Mutation
Research, Volume 1).
Salama AM, Kobbia IA, Heikal NZ, & Shabana EF (1985) The influence
of the herbicide amitrole on growth and dynamics of carbohydrate and
nitrogenous compounds in two blue-green algae from the Nile drains.
Arch Hydrobiol, 105(1): 117-127.
Schneider B, Stock M, Schneider G, Schutte HR, Schreiber K, Brauner
A, & Kaussmann EU (1992) Metabolism of amitrole in apple. I. Soluble
metabolites from mature fruits. Z Nat.forsch, 47c: 120-125.
Schiestl RH, Geitz RD, Mehta RD, & Hasting PJ (1989) Carcinogens
induce intrachromosomal recombination in yeast. Carcinogenesis,
10(8): 1445-1455.
Scholz K (1988) Metabolism of amitrole in soil under aerobic
conditions. Leverkusen, Bayer AG (Unpublished report No. M5977).
Schubert OE (1964) Residues of amitrole in apple fruit following
ground cover, fruit and leaf applications. Proc West Va Acad Sci,
43: 29-35.
Schultz TW & Kennedy JR (1976) Cytotoxicity effects of the herbicide
3-amino-1,2,4-triazole on Daphnia pulex (Crustacea:Cladocera).
Biol Bull, 151: 370-385.
Seidenberg IL & Gee AH (1953) Report on acute toxicity of
aminotriazole as established by oral administration to rats. New
York, Snell Laboratories, Inc. (Unpublished report).
Seiler JP (1977) Inhibition of testicular DNA synthesis by chemical
mutagens and carcinogens. Preliminary results in the validation of
an novel short-term test. Mutat Res, 46: 305-310.
Shaffer CB, Bagdon RE, Vidone LB, & Golz HH (1956) Aminotriazole:
Acute and subacute toxicity. Wayne, New Jersey, American Cyanamid
Company, Central Medical Department, Industrial Toxicology Section
(Unpublished Report No. 56/29).
Sharp DC & Parry JM (1981a) Use of repair deficient strains of yeast
to assay the activity of 40 coded compounds. In: de Serres FJ &
Ashby J ed. Evaluation of short-term tests for carcinogens. Report
of the international collaborative program. New York, Amsterdam,
Oxford, Elsevier/North-Holland, pp 502-516 (Progress in Mutation
Research, Volume 1).
Sharp DC & Parry JM (1981b) Induction of mitotic gene conversion by
41 coded compounds using the yeast culture JDI. In: de Serres FJ &
Ashby J ed. Evaluation of short-term tests for carcinogens. Report
of the international collaborative program. New York, Amsterdam,
Oxford, Elsevier/North-Holland, pp 491-501 (Progress in Mutation
Research, Volume 1).
Shirasu Y, Moriya M, Kato T, Furviiashi A, & Kadu T (1976)
Mutagenicity screening of pesticides in the microbial system. Mutat
Res, 40: 19-30.
Simmon VF, Rosenkranz HS, Zeiger E, & Poirier LA (1979) Mutagenic
activity of chemical carcinogens and related compounds in the
intraperitoneal host-mediated assay. J Natl Cancer Inst, 62(4):
911-918.
Sittig M (1985) Handbook of toxic and hazardous chemicals and
carcinogens, 2nd ed. Park Ridge, New Jersey, Noyes Publications, pp
69-70.
Skopek TR, Andon BM, Kaden DA, & Thilly WG (1981) Mutagenic activity
of 42 coded compounds using 8-azagunine resistance as a genetic
marker in Salmonella typhimurium. In: de Serres FJ & Ashby J ed.
Evaluation of short-term tests for carcinogens. Report of the
international collaborative program. New York, Amsterdam, Oxford,
Elsevier/North-Holland, pp 371-375 (Progress in Mutation Research,
Volume 1).
Smith LW, Bayer DE, & Foy CL (1969) Metabolism of amitrole in
excised leaves of Canada thistle ecotypes and beans. Weed Sci, 16:
523-537.
Sorsa M & Gripenberg U (1976) Organization of a mutagenicity test
system combining instructive purposes: Testing for mutagenic effects
of the herbicide "amitrole". Mutat Res, 38(4): 132-133.
Steinhoff D & Boehme K (1978) Aminotriazole (amitrole).
Carcinogenicity study on orally dosed golden hamsters. Leverkusen,
Bayer AG (Unpublished proprietary report No. 7521).
Steinhoff D & Boehme K (1979a) Aminotriazole (amitrole).
Cancerogenesis test with oral administration to rats. Leverkusen,
Bayer AG (Unpublished proprietary report No. 8450).
Steinhoff D & Boehme K (1979b) Aminotriazole (amitrole).
Carcinogenesis study with oral administration to mice. Leverkusen,
Bayer AG (Unpublished report No. 8490).
Steinhoff D, Weber H, Mohr U, & Boehme K (1983) Evaluation of
amitrole (aminotriazole) for potential carcinogenicity in orally
dosed rats, mice and golden hamsters. Toxicol Appl Pharmacol, 69:
161-169.
Stock M, Bohm H, Schneider B, Schutte H-R, Schriiber K, Brauner A, &
Koster J (1991) Metabolism of amitrole in cell suspension cultures
of apples. Herbsttag Ges Biol Chem, 372: 763-764.
Storherr RW & Burke J (1961) Determination of 3-amino-1,2,4-triazole
in crops. J Assoc Off Anal Chem, 44(2): 196-199.
Storherr RW & Onley J (1962) Cleanup and separation of
3-amino-1,2,4-triazole and metabolites from vegetable crops. J Assoc
Off Anal Chem, 45(2): 382-387.
Strum JM & Karnovsky MJ (1971) Aminotriazole goiter. Fine structure
and localization of thyroid peroxidase activity. Lab Invest, 24(1):
1-12.
Styles JA (1979) Studies on the detection of carcinogens using a
mammalian cell transformation assay with liver homogenate
activation. In: Norpoth K & Garner RC ed. Short-term mutagenicity
test systems for detecting carcinogens. Proceedings of the
International Symposium on Short-Term Mutagenicity Test Systems,
Dortmund, 15-17 November 1978. New York, Berlin, Heidelberg,
Springer Verlag, pp 226-238.
Styles JA (1981) Activity of 42 coded compounds in the BHK-21 cell
transformation test. In: de Serres FJ & Ashby J ed. Evaluation of
short-term tests for carcinogens. Report of the international
collaborative program. New York, Amsterdam, Oxford,
Elsevier/North-Holland, pp 638-646 (Progress in Mutation Research,
Volume 1).
Sumi C, Yokoro K, & Matsushima R (1985) Inhibition by
3-amino-1 H-1,2,4-triazole of hepatic tumorigenesis induced by
diethylstilbestrol alone or combined with N-nitrobutylurea in WF
rats. J Natl Cancer Inst, 74: 1329-1334.
Sund KA (1956) Residual activity of 3-amino-1,2,4-triazole in soils.
J Agric Food Chem, 4(1): 57-60.
Tag El-Din A, Abbas MM, Aly HA, Tantawy G, & Askar A (1981) Acute
toxicities to Mugil cephalus fry caused by some herbicides and new
pyrethroids. Meded Fac Landbouwwet Rijksuniv Gent, 46(1): 387-391.
Tampier L & Quintanilla ME (1990) Effect of 3-amino-1,2,4-triazole
on the hypothermic effect of ethanol and on ethanol tolerance
development. Alcohol, 8: 279-282.
Thomson JA (1981) Mutagenic activity of 42 coded compounds in the
lambda induction assay. In: de Serres FJ & Ashby J ed. Evaluation of
short-term tests for carcinogens. Report of the international
collaborative program. New York, Amsterdam, Oxford,
Elsevier/North-Holland, pp 224-235 (Progress in Mutation Research,
Volume 1).
Thyssen J (1974) [Aminotriazole Pt. 143 - Determination of acute
toxicity.] Leverkusen, Bayer AG (Unpublished report) (in German).
Tjalve H (1974) Fetal uptake and embryogenetic effects of
aminotriazole in mice. Arch Toxicol, 33(1): 41-48.
Tjalve H (1975) The distribution of labelled aminotriazole in mice.
Toxicology, 3(1): 49-67.
Topham JC (1980) Do induced sperm-head abnormalities in mice
specifically identify mammalian mutagens rather than carcinogens?
Mutat Res, 74: 379-387.
Tripathy NK, Wugler FE, & Frei H (1990) Genetic toxicity of six
carcinogens and six non-carcinogens in the Drosphilia wing spot
test. Mutat Res, 242: 169-180.
Trueman RW (1981) Activity of 42 coded compounds in the Salmonella
reverse mutation test. In: de Serres FJ & Ashby J ed. Evaluation of
short-term tests for carcinogens. Report of the international
collaborative program. New York, Amsterdam, Oxford,
Elsevier/North-Holland, pp 343-350 (Progress in Mutation Research,
Volume 1).
Tschudy DP & Collins A (1957) Effect of 3-amino-1,2,4-triazole on
delta-aminolevulinic acid dehydrase activity. Science, 126: 168.
Tsuchimoto T & Matter BE (1981) Activity of coded compounds in the
micronucleus test. In: de Serres FJ & Ashby J ed. Evaluation of
short-term tests for carcinogens. Report of the international
collaborative program. New York, Amsterdam, Oxford,
Elsevier/North-Holland, pp 705-711 (Progress in Mutation Research,
Volume 1).
Tsuda H, Takahashi M, Fukushima S, & Endo Y (1973) Fine structure
and localization of peroxidase activity in aminotriazole goiter.
Nagoya Med J, 18(3): 183-189.
Tsuda H, Hananouchi M, Tatematsu M, Hirose M, Hirao K, Takahashi M,
& Ito N (1976) Tumorigenic effect of 3-amino-1H-1,2,4-triazole on
rat thyroid. J Natl Cancer Inst, 57(4): 861-864.
Tsuda H, Takahashi M, Murasaki G, Ogiso T, & Tatematsu M (1978)
Effect of 3-amino-1H-1,2,4-triazole or low iodine diet on rat
thyroid carcinogenesis induced by ethylenethiourea. Nagoya Med J,
23: 83-92.
Tsutsui T, Maizumi H, & Barret JC (1984) Amitrole-induced cell
transformation and gene mutations in Syrian hamster embryo cells in
culture. Mutat Res, 140: 205-207.
Turner DM & Gilbert CM (1976) Evidence of metabolism of 14C
amitrole in the rat after inhalation administration. Harrogate, UK,
Hazleton Laboratories Europe Ltd (Unpublished report No. 291/1a).
Tweats DI (1981) Activity of 42 coded compounds in a differential
killing test using Escheria coli strains, WP2, WP67 (uvrA polA),
and CM871 (uvrA lexA recA). In: de Serres FJ & Ashby J ed.
Evaluation of short-term tests for carcinogens. Report of the
international collaborative program. New York, Amsterdam, Oxford,
Elsevier/North-Holland, pp 199-209 (Progress in Mutation Research,
Volume 1).
US EPA (1992) EPA notice setting forth preliminary determination
proposing termination of special review for amitrole. Washington,
DC, Bureau of National Affairs, Inc.
Van der Poll JM, Vink M, & Quirijns JK (1988) Capillary gas
chromatographic determination of amitrole in water with alkali flame
ionization detection. Chromatographia, 25(6): 511-513.
Venitt S & Crofton-Sleigh S (1981), Mutagenicity of 42 coded
compounds in a bacterial assay using Escheria coli and Salmonella
typhimurium. In: de Serres FJ & Ashby J ed. Evaluation of
short-term tests for carcinogens. Report of the international
collaborative program. New York, Amsterdam, Oxford,
Elsevier/North-Holland, pp 351-360 (Progress in Mutation Research,
Volume 1).
Vesselinovitch SD (1983) Perinatal hepatocarcinogenesis. Biol Res
Pregnancy Perinatol, 4(1): 22-25.
Vogel E, Blijleven WGH, Kortselius MJH, & Zijlstra JA (1981)
Mutagenic activity of 17 coded compounds in the sex-linked recessive
lethal test in Drosophila melanogaster. In: de Serres FJ & Ashby J
ed. Evaluation of short-term tests for carcinogens. Report of the
international collaborative program. New York, Amsterdam, Oxford,
Elsevier/North-Holland, pp 660-665 (Progress in Mutation Research,
Volume 1).
Weber E (1988) Method for the determination of amitrole residues in
plant material, soil and water. Leverkusen, Bayer AG (Unpublished
report No. RA-905/88).
Weber H & Patschke K (1978) Effect of long-term administration of
aminotriazole on thyroid function of male and female rats, mice and
hamsters. Leverkusen, Bayer AG (Unpublished report No. 7690).
Weir R (1958) 3-Amino-1,2,4-triazole: Chronic oral administration -
Beagle dogs - 52 weeks. Final report. Falls Church, Virginia,
Hazleton Laboratories (Unpublished report).
Weller H (1987) Leaching characteristics of soil-aged amitrole.
Leverkusen, Bayer AG (Unpublished report No. HM736).
Wishe HI, Rolle-Getz GK, & Goldsmith ED (1979) The effects of
aminotriazole (ATZ) on the thyroid gland and the development of the
white Leghorn chick. Growth, 43(4): 238-251.
Wolf FT (1962) Growth inhibition of Chorella induced by
3-amino-1,2,4-triazole and its reversal by purines. Nature (Lond),
193: 901-902.
Worthing CR & Hance RJ ed. (1991) The pesticide manual, 9th ed.
Farnham, Surrey, The British Crop Protection Council. pp 25-26.
Wynford-Thomas D, Stringer BMJ, & Williams ED (1982a)
Desensitisation of rat thyroid to the growth-stimulating action of
TSH during prolonged goitrogen administration: Persistence of
refractoriness following withdrawal of stimulation. Acta Endocrinol,
101: 562.
Wynford-Thomas D, Stringer BMJ, & Williams ED (1982b)
Goitrogen-induced thyroid growth in the rat: A quantitative
morphometric study. J Endocrinol, 94: 131.
Wynford-Thomas D, Stringer BMJ, Gomez Morales M, & Williams ED
(1983) Vascular changes in early TSH-induced thyroid tumours in the
rat. Br J Cancer, 47(6): 861-865.
Yoshida T, Maruyama T, Kojima HI, Allanpichay I, & Mori S (1986)
Evaluation of the effect of chemicals on aquatic ecosystem by
observing the photosynthetic activity of a macrophyte, Porphyra
yezoensis. Aquat Toxicol, 9: 207-214.
Yoshida T & Nishimura Y (1972) Toxicity of pesticides to some water
organisms. Bull Agric Chem Insp Stn, 12: 122-128.
Zandvoort FK, Braber JM, & Pannebakker EG (1981) The disappearance
of aminotriazole from some railway beds in the Netherlands. Meded
Fac Landbouwwet Rijksuniv Gent, 46(1).
Zimmerman FK & Scheel I (1981) Induction of mitotic gene conversion
in strain D7 of Saccharomyces cervisiae by 42 coded compounds. In:
de Serres FJ & Ashby J ed. Evaluation of short-term tests for
carcinogens. Report of the international collaborative program. New
York, Amsterdam, Oxford, Elsevier/North-Holland, pp 481-490
(Progress in Mutation Research, Volume 1).
1. RESUME
1.1 Identité, propriétés physiques et chimiques et méthodes
d'analyse
L'amitrole, ou 3-amino-1,2,4-triazole, se présente sous la
forme d'une poudre cristalline incolore. Il est stable à la chaleur
et son point de fusion est de 156-159 °C. Il est facilement soluble
dans l'eau et l'éthanol mais seulement légèrement soluble dans les
solvants organiques tels que l'hexane et le toluène. Chimiquement,
l'amitrole se comporte à la fois comme une amine aromatique typique
et comme un s-triazole. On dispose de méthodes d'analyse variées
pour la recherche et le dosage de l'amitrole dans les plantes, le
sol, l'eau, l'air et l'urine.
1.2 Sources d'exposition humaine et environnementale
L'amitrole n'existe pas à l'état naturel. On le prépare par
condensation de l'acide formique avec le bicarbonate
d'aminoguanidine dans un solvant inerte à 100-200 °C. C'est un
herbicide à large spectre dont l'activité s'explique par son action
inhibitrice sur la formation de la chlorophylle. On l'utilise en
application tout autour des arbres fruitiers, sur les friches, sur
l'accotement des routes et des voies ferrées ou encore pour le
désherbage des étangs.
1.3 Transport, distribution et transformation dans l'environnement
En raison de sa faible tension de vapeur, l'amitrole ne pénètre
pas dans l'atmosphère. Il est facilement soluble dans l'eau avec un
temps de demi-photodécomposition dans l'eau distillée qui dépasse un
an. En présence du sel de potassium de l'acide humique, qui agit
comme photosensibilisateur, le temps de demi-photodécomposition est
ramené à 7,5 heures.
L'amitrole est adsorbé aux particules du sol et aux matières
organiques par association protonique. Cette liaison est réversible
et labile, même dans des conditions d'acidité favorables. La mesure
du coefficient de partage n-octanol/eau permet de classer
l'amitrole comme "extrêmement mobile" dans les sols de pH > 5 et
"moyennement à très fortement mobile" dans les sols de plus faible
pH. Le lessivage du composé initial peut varier dans de très fortes
proportions ainsi qu'en témoigne l'expérimentation sur des colonnes
de terre. En général c'est dans le sable que le mouvement se voit le
plus facilement; la mobilité décroît à mesure qu'augmente la teneur
en matières organiques.
La décomposition dans le sol est en général assez rapide mais
elle dépend du type de sol et de la température. On a isolé des
bactéries capables de dégrader l'amitrole. Cet herbicide peut tenir
lieu d'unique source d'azote mais pas d'unique source de carbone
pour les bactéries. La dégradation microbienne est probablement la
principale voie de décomposition de l'amitrole; en effet les études
portant sur des sols stérilisés ont montré que la décomposition
était pratiquement inexistante. Toutefois, on a invoqué la
possibilité d'une dégradation par des voies abiotiques et notamment
sous l'action de radicaux libres. Des études en laboratoire ont
montré que le temps de demi-décomposition de l'amitrole était de 2 à
30 jours, le produit final étant le CO2. Une seule étude en
situation réelle incite à penser que cette dégradation pourrait être
plus longue à températures plus basses et pour différents taux
d'humidité du sol; sur argile, la demi-vie s'est révélée être
d'environ 100 jours.
Bien que le composé initial percole à travers certains sols,
ses produits de décomposition restent fermement fixés aux particules
du sol. Etant donné que l'amitrole se décompose rapidement dans le
sol, la forte tendance au lessivage de cet herbicide ne semble pas
entrer en ligne de compte dans la pratique. Les dégâts occasionnés
aux arbres, dont on avait parfois fait état au début de
l'utilisation de cet herbicide, ne paraissent pas être
caractéristiques du produit.
Une fois appliqué à la végétation, l'amitrole est absorbé par
le feuillage et peut transmigrer dans la plante. Il est également
absorbé par les racines et transporté dans le xylème jusqu'aux
pousses des extrémités en l'espace de quelques jours.
Une forte solubilité dans l'eau, un coefficient de partage
octanol-eau très faible et l'absence de persistance chez l'animal
sont autant de facteurs qui indiquent qu'il n'existe pour l'amitrole
aucune possibilité de bioaccumulation ou de transport le long de la
chaîne alimentaire.
1.4 Concentrations dans l'environnement et exposition
humaine
Les ateliers de production de l'amitrole peuvent libérer dans
l'atmosphère des particules qui en contiennent; c'est ainsi que l'on
a trouvé, à proximité d'un de ces ateliers, des concentrations dans
l'atmosphère allant de 0 à 100 mg/m3.
L'épandage d'amitrole sur les voies d'eau et les bassins
versants peut conduire à des concentrations atteignant passagèrement
150 µg/litre. Ces concentrations tombent rapidement à des niveaux
indécelables (< 2 µg/litre) dans les eaux courantes en l'espace de
2 heures. L'épandage sur des étangs a produit une concentration
initiale de 1,3 mg/litre d'eau, qui tombait à 80 µg/litre au bout de
27 semaines. A proximité d'une unité de production, les
concentrations dans les cours d'eau allaient de 0,5 à 2 mg/litre.
Lorsqu'on l'utilise conformément aux recommandations, il ne
semble pas que l'amitrole laisse subsister des résidus dans les
produits alimentaires. Le traitement du sol dans des plantations de
pommiers n'a pas conduit à la présence de résidus dans les pommes.
Cependant on peut trouver des résidus dans les fruits sauvages qui
poussent à proximité des zones traitées.
On n'a pas signalé la présence d'amitrole dans l'eau de
boisson.
1.5 Cinétique et métabolisme chez les animaux de laboratoire et
l'homme
Après administration par voie orale, l'amitrole est rapidement
absorbé chez les mammifères au niveau des voies digestives. Il est
rapidement excrété, essentiellement sous forme inchangée. Sa
principale voie d'excrétion chez l'homme et les animaux de
laboratoire est la voie urinaire, la majorité du produit étant
éliminée dans les 24 heures. La métabolisation du produit dans
l'organisme des mammifères produit deux métabolites que l'on
retrouve en petites quantités dans l'urine des animaux d'expérience.
Inhalé sous forme d'aérosol, il est de même rapidement excrété dans
les urines.
1.6 Effets sur les animaux d'expérience et les systèmes d'épreuve
in vitro
L'expérimentation sur plusieurs espèces, par diverses voies
d'administration, montre que l'amitrole n'a qu'une faible toxicité
aiguë (les valeurs de la DL50 sont toujours supérieures à
2500 mg/kg de poids corporel). L'exposition à l'amitrole entraîne
une atteinte thyroïdienne, qu'elle soit unique, brève ou prolongée.
L'amitrole a un effet goitrogène; il entraîne une hypertrophie et
une hyperplasie de la thyroïde avec déplétion colloïdale et
accroissement de la vascularisation. L'expérimentation à long terme
chez le rat montre que ces anomalies au niveau de la thyroïde
précèdent la survenue de cancers.
On pense que l'effet cancérogène de l'amitrole sur la thyroïde
est lié à une stimulation permanente de cette glande par
l'accroissement du taux d'hormone thyréotrope (TSH), imputable à la
perturbation de la synthèse de l'hormone thyroïdienne par ce
composé.
Certaines études relatives à l'activité génotoxique de
l'amitrole ont donné des résultats ambigus. Des études de
cancérogénicité chez le rat ont montré que l'action tumorigène se
limitait à la thyroïde. Cependant, des tumeurs hépatiques ont été
observées chez des souris soumises à des doses élevées d'amitrole.
Les effets précoces de l'amitrole sur la thyroïde ont été
évalués en fonction d'un certain nombre de critères. La dose
minimale sans effets nocifs observables serait, d'après ces études,
de 2 mg/kg de nourriture chez le rat; elle a été évaluée sur la base
de l'hyperplasie thyroïdienne.
1.7 Effets sur l'homme
Un seul cas de dermatite de contact due à l'amitrole a été
signalé. Ingéré à la dose de 20 mg/kg, l'amitrole n'a pas provoqué
d'effets toxiques. Lors d'une étude contrôlée, on a constaté qu'une
dose de 100 mg avait, sur 24 heures, une action inhibitrice sur la
fixation de l'iode par la thyroïde. Des employés chargés du
désherbage, et qui subissaient une exposition cutanée à 340 mg
d'amitrole environ pendant dix jours, n'ont présenté aucune
altération de leur fonction thyroïdienne.
1.8 Effets sur les autres êtres vivant au laboratoire et dans leur
milieu naturel
Plusieurs études portant sur la croissance des cyanobactéries
(algues bleues) ont montré que l'amitrole n'exerçait aucun effet à
des concentrations inférieures ou égales à 4 mg/litre. On n'a pas
fait état non plus d'effets indésirables systématiques sur la
fixation d'azote. Aucun effet indésirable n'a été constaté sur des
bactéries terricoles à des concentrations de 20 mg/litre de milieu
dans le cas de Rhizobium fixatrice d'azote et de 250 mg/kg dans le
cas des bactéries cellulolytiques. Aucun effet n'a été constaté sur
la nitrification ou la respiration du sol à des concentrations de
100 mg de matière active par kg de sol sec, soit 5 fois la dose
d'emploi maximale recommandée. A des doses allant jusqu'à
20 mg/litre, on a observé que le trèfle souterrain formait moins de
nodosités.
On a étudié l'effet inhibiteur de l'amitrole sur la croissance
chez un certain nombre d'algues unicellulaires. A des doses de 0,2 à
0,5 mg d'amitrole par litre, c'est chez Selenastrum que cet effet
était le plus sensible.
La plupart des invertébrés aquatiques présentent une forte
tolérance à l'amitrole technique: les valeurs de la CL50 se sont
révélées supérieures à 10 mg/litre pour les organismes étudiés sauf
la daphnie (Daphnia magna) pour laquelle la CE50 aiguë à
48 heures (immobilisation) était de 1,5 mg/litre. La tolérance à
l'amitrole des larves de poissons et d'amphibiens est également
élevée, les valeurs de la CL50 dépassant 40 mg/litre. Des études à
long terme ont montré que de jeunes truites arc-en-ciel pouvaient
survivre pendant 21 jours à une concentration d'amitrole de
25 mg/litre.
Deux espèces de lombric (Eisenia foetida et Allolobophora
caliginosa) soumises respectivement à des doses d'amitrole
(SP50) de 1000 mg/kg de terre et de 100 mg(amitrole T)/kg de
terre, n'en n'ont nullement souffert. Des coléoptères (carabes) ont
subi sans dommage un traitement direct par l'amitrole à des doses
correspondant à 30 kg/hectare. Sur les nématodes, on n'a observé
d'effets qu'à des concentrations élevées (la CL50 était de
184 mg/kg). Des essais sur le terrain ont également montré que
l'amitrole était sans danger pour les abeilles.
L'amitrole est peu toxique pour les oiseaux, toutes les valeurs
de CL50 par voie alimentaire étant supérieures à 5000 mg/kg de
nourriture. L'administration de 2000 mg d'amitrole par kg de poids
corporel à des colverts n'a provoqué aucune mortalité.
1. RESUMEN
1.1 Identidad, propiedades físicas y químicas y métodos analíticos
El amitrol (3-amino-1,2,4-triazol) es un polvo incoloro
cristalino. Es termoestable y tiene un punto de fusión de
156-159 °C. Es fácilmente soluble en agua y etanol y poco soluble en
disolventes orgánicos tales como el hexano y el tolueno.
Químicamente, el amitrol se comporta como una amina aromática típica
y como un s-triazol. Se dispone de una amplia variedad de métodos
analíticos para la detección y cuantificación del amitrol en las
plantas, el suelo, el agua, el aire y la orina.
1.2 Fuentes de exposición humana y ambiental
El amitrol no se halla presente en la naturaleza. Se fabrica
por condensación del ácido fórmico con bicarbonato de aminoguanidina
en un solvente inerte a 100-200 °C. El amitrol se utiliza como
herbicida con un amplio espectro de actividad y parece actuar
inhibiendo la formación de clorofila. Se aplica corrientemente en
torno de los árboles de los huertos, en terrenos en barbecho, en los
bordes de los caminos o para combatir malas hierbas en los
estanques.
1.3 Transporte, distribución y transformación en el medio ambiente
Debido a su baja presión de vapor, el amitrol no penetra en la
atmósfera. Es fácilmente soluble en agua y tiene un período de
semifotodegradación en agua destilada de más de un año. La
fotodegradación ocurre en presencia de la sal potásica del ácido
húmico, que es fotosensibilizadora y la semivida se reduce a
7,5 horas.
El amitrol se adsorbe en partículas del suelo y materia
orgánica por asociación protónica. El enlace es reversible y no es
fuerte, incluso en condiciones ácidas favorables. Conforme a los
valores hallados del coeficiente de reparto n-octanol/agua, el
amitrol se clasifica como "muy móvil" en suelos con un pH > 5 y "de
moderadamente a muy móvil" con pH más bajos. La lixiviación del
compuesto original en columnas experimentales de suelo es
considerablemente variable. En general, el movimiento se ve más
fácilmente en arena; si el contenido de materia orgánica aumenta, la
movilidad se reduce.
La degradación en el suelo suele ser bastante rápida, pero
variable según el tipo de suelo y la temperatura. Se han aislado
bacterias capaces de degradar el amitrol. El herbicida puede actuar
como única fuente de nitrógeno de la bacteria, pero no como su única
fuente de carbono. La degradación microbiana probablemente sea la
principal vía de descomposición del amitrol; en estudios realizados
con suelo esterilizado se ha registrado poca o ninguna
descomposición. Sin embargo, también se ha propuesto una degradación
por mecanismos abióticos, inclusive por la acción de radicales
libres. Los estudios de laboratorio han mostrado una degradación en
CO2 con una semivida de 2 a 30 días. Un estudio único sobre el
terreno sugiere que la degradación tal vez sea más lenta a
temperaturas más bajas y con diferentes niveles de humedad del
suelo; en una prueba con arcilla, la semivida resultó de unos 100
días.
Aunque el compuesto madre se lixivia a través de algunos
suelos, los productos de la degradación quedan estrechamente ligados
al suelo. Dado que el amitrol se degrada rápidamente en el suelo, el
elevado potencial de lixiviación del herbicida no parece darse en la
práctica. Los daños ocasionales a los árboles notificados durante la
aplicación inicial del amitrol no han resultado ser una
característica regular de su empleo.
El amitrol aplicado a la vegetación se absorbe a través de las
hojas y puede translocarse a toda la planta. También se absorbe a
través de las raíces y se transporta en el xilema, llegando en pocos
días hasta los brotes.
La gran solubilidad en el agua, el coeficiente muy bajo de
repartición octanol-agua y la no permanencia en animales significan
que no hay posibilidades de bioacumulación del amitrol ni transporte
del mismo a través de las cadenas alimentarias.
1.4 Niveles ambientales y exposición humana
Las plantas productoras tal vez liberen material particulado
que contenga amitrol; en las proximidades de una planta se han
detectado niveles atmosféricos de 0 a 100 mg/m3.
Tras la utilización de amitrol en cursos de agua y cuencas
hidrográficas se han registrado concentraciones transitorias en el
agua de hasta 150 µg/litros. La concentración disminuye rápidamente
hasta niveles no detectables (<2 µg/litro) en el agua corriente en
dos horas. Tras la aplicación en estanques se registró una
concentración inicial en el agua de 1,3 mg/litro, que bajó a
80 µg/litro a las 27 semanas. En las proximidades de una planta de
producción, las concentraciones fluviales oscilaban entre 0,5 y
2 mg/litro.
En los alimentos no se han detectado residuos de amitrol si se
ha utilizado conforme a las recomendaciones. La vaporización en la
superficie del suelo alrededor de manzanos no generó residuos en las
manzanas. Las frutas silvestres que crecen en las proximidades de
las zonas de control pueden contener residuos.
No se ha notificado la presencia de amitrol en el agua potable.
1.5 Cinética y metabolismo en animales de laboratorio y en el
hombre
Tras su administración por vía oral, el amitrol se absorbe
fácilmente a través del tracto gastrointestinal de los mamíferos. El
cuerpo lo excreta con rapidez, principalmente en la forma del
compuesto padre. La principal vía de excreción en el ser humano y en
animales de laboratorio es la orina, y la mayor parte de la
excreción se efectúa en el transcurso de las primeras 24 horas. La
transformación metabólica en los mamíferos produce dos metabolitos
menores, detectables en la orina de los animales de experimentación.
Cuando el amitrol en aerosol se inhala, la excreción es igualmente
rápida por orina.
1.6 Efectos en animales de experimentación y en sistemas de prueba
in vitro
El amitrol ha manifestado una toxicidad aguda baja en pruebas
realizadas con varias especies y diversas vías de administración
(los valores de la DL50 fueron siempre superiores a 2500 mg/kg de
peso corporal). Se observó que afectaba la tiroides después de
exposición es única, de corto plazo y de largo plazo. El amitrol es
bócigeno; causa hipertrofia e hiperplasia tiroidea, deplección del
coloide y aumento de la vascularización. En experimentos de largo
plazo dichos cambios preceden el desarrollo de neoplasia de la
tiroides en ratas.
Se cree que el efecto carcinógeno del amitrol en la tiroides
está relacionado con la estimulación continua de la glándula por un
aumento de la hormona tirotrópica (TSH), ocasionado por la
interferencia del amitrol en la síntesis de la hormona tiroidea. Se
han comunicado resultados equívocos de varios estudios sobre el
potencial genotóxico del amitrol. En pruebas de carcinogenicidad en
ratas, el amitrol no indujo tumores en órganos diferentes de la
tiroides. Sin embargo, en dosis elevadas, el amitrol fue causa de
tumores hepáticos en ratones.
Se han utilizado varios criterios para evaluar los efectos
tempranos del amitrol en la tiroides. El más bajo nivel sin efectos
adversos observados derivado de esos estudios fue de 2 mg/kg en la
alimentación de ratas y se evaluó en base a la hiperplasia tiroidea.
1.7 Efectos en el hombre
Se ha comunicado un solo caso de dermatitis por contacto con el
amitrol. El amitrol no tiene efectos tóxicos si se ingiere una dosis
de 20 mg/kg. En un experimento controlado se llegó a la conclusión
de que 100 mg inhibían la absorción de iodo por la tiroides a las 24
horas. No se observaron cambios en la función tiroidea de personas
que trabajaban en el control de malas hierbas y que estuvieron
expuestas por vía dérmica a unos 340 mg de amitrol por día durante
10 días.
1.8 Efectos en otros organismos en el laboratorio y en
el medio ambiente
En varios estudios sobre el desarrollo de cianobacterias (algas
cianofíceas) no se ha observado que el amitrol tenga efecto en
concentraciones iguales o inferiores a 4 mg/litro. No se han
comunicado efectos adversos constantes en la fijación de nitrógeno.
En cuanto a las bacterias del suelo, las Rhizobium fijadoras de
nitrógeno no se vieron afectadas por concentraciones de 20 mg/litro
de medio y las celulolíticas no se vieron afectadas por 150 mg/kg.
No se registraron efectos en la nitrificación ni en la respiración
del suelo con 100 mg de ingrediente activo por kg de suelo seco, es
decir, con el quíntuple de la aplicación máxima recomendada. Se
notificó una nodulación reducida en el trébol subterráneo con
concentraciones de hasta 20 mg/litro.
Se han hecho ensayos con diversas algas unicelulares para
determinar los efectos inhibidores del crecimiento. Con 0,2 a 0,5 mg
de amitrol/litro, la inhibición del crecimiento de Selenastrum fue
el efecto más notable comunicado.
La mayor parte de los invertebrados acuáticos muestran una
tolerancia elevada al amitrol técnico: los valores de la CL50 eran
> 10 mg/litro en todos los organismos diferentes de la pulga
acuática Daphnia magna, en cuyo caso la CE50 aguda
(inmovilización) fue de 1,5 mg/litro. Los peces y las larvas de
anfibios también mostraron tolerancia al amitrol con CL50
superiores a 40 mg/litro. Estudios de más largo plazo indicaron que
la trucha arco iris joven sobrevive 21 días en un medio con una
concentración de 25 mg de amitrol por litro.
Dos especies de gusanos (Eisenia foetida y Allolobophora
caliginosa) no se vieron afectadas por el amitrol (SP50),
1000 mg/kg de suelo y por Amitrol-T, 100 mg/kg de suelo,
respectivamente. Los escarabajos carábidos no resultaron afectados
después de la vaporización directa de amitrol a razón de 30 kg/ha.
En los nematodos se verificaron efectos solamente con
concentraciones elevadas de amitrol (la CL50 fue de 184 mg/kg).
Como resultado de ensayos sobre el terreno se ha comunicado que el
amitrol no es peligroso para las abejas.
El amitrol es poco tóxico para las aves y en todos los casos se
comunicaron CL50 superiores a 5000 mg/kg de alimento. La
administración aguda de una dosis de 2000 mg/kg de peso corporal no
causó la muerte de ningún pato silvestre.
Chemical formula: C2H4N4
Relative molecular mass: 84.08
CAS chemical names: 1 H-1,2,4-triazol-3-amine (9C1)
3-amino- s-triazole (8CI)
IUPAC names: 1 H-1,2,4-triazol-3-ylamine
3-amino-1 H-1,2,4-triazole
3-amino- s-triazole
CAS registry number: 61-82-5
RTECS registry number: XZ3850000
Common synonyms: aminotriazole; 2-aminotriazole;
3-aminotriazole; 3-amino-1,2,4-
triazole; 2-amino-1,3,4-triazole;
3-amino-1H-1,2,4-triazole; AT;
3AT; ATA; 3,A-T; ATZ; AT-90;
triazolamine; 1,2,4-triazol-3-amine;
5-amino-1H-1,2,4-triazole.
Common trade names: Amerol; Aminotriazole Weedkiller
90; Aminotriazol Spritzpulver;
Amitril; Amitril T.L.; Amitrol;
Amitrol 90; Amitrol Plus;
Amitrol-T; Amizine; Amizol;
Amizol DP; Amizol F; AT Liquid;
Azaplant; Azolan; Azole; Azaplant
Kombi; Campaprim A1544; Cytrol;
Cytrole; Destraclol; Diurol. 5030;
Domatol; Domatol 88; Elmasil;
Emisol; Emisol 50; Emosol F; ENT
25445; Exit; Fenamine; Fenavar;
Fyrbar; Kleer-Lot; Lancer;
Nu-Zinole-AA; Orga 414; Preceed;
Radoxone TL; Ramizol; Sapherb;
Solution Concentree T271; Ustinex;
Vorox; Vorox AA; Vorox AS;
Weedar ADS; Weedar AT; Weedazin;
Weedazin Arginit; Weedazol;
Weedazol GP2; Weedazol Super;
Weedex Granulat; Weedoclor; X-All
Liquid.
Technical grade amitrole contains a minimum of 95% active
ingredient and is formulated as a solution of 250 g/litre in water,
usually with an equimolar concentration of ammonium thiocyanate, or
as a 400 g/kg wettable powder, usually in combination with other
herbicides.
The major impurities are 3-(N-formylamino)-1,2,4-triazole,
4 H-1,2,4-triazole-3,4-diamine, and
4 H-1,2,4-triazole-3,5-diamine.
2.2 Physical and chemical properties
Some of the physical and chemical properties of amitrole are
shown in Table 1.
Amitrole is readily soluble in water, methanol, ethanol and
chloroform, sparingly soluble in ethyl acetate, and insoluble in
hydrocarbons, acetone and ether. It forms salts with most acids or
bases and is a powerful chelating agent. It is corrosive to
aluminium, copper and iron. Chemically, amitrole behaves as an
s-triazole and also as an aromatic amine, and hence will diazotize
and couple several dyes.
2.3 Conversion factors:
1 mg/kg = 3.43 mg/m3 1 mg/m3 = 0.29 mg/kg
Table 1. Some physical and chemical properties of amitrole
Physical state crystalline
Colour colourless
Taste bitter
Odour none
Thermal stability stable at 20 °Ca
Hydrolytic stability (pH 4-9; 90 °C) stableb
Melting point 157-159 °Cc
Water solubility (25 °C) 280 g/litrec
Water solubility (53 °C) 500 g/litred
Ethanol solubility (75 °C) 260 g/litred
Solubility in n-hexane (20 °C) < 0.1 g/litred
Solubility in dichloromethane (20 °C) 0.1-1 g/litred
Solubility in 2-propane 20-50 g/litred
Solubility in toluene (20 °C) <0.1 g/litred
Vapour pressure (20 °C) 55 nPac
Octanol/water partition coefficient (21 °C)
(log Pow) -0.969e
a Klusacek & Krasemann (1986)
b Krohn (1982)
c Worthing & Hance (1991)
d Personal communication from Bayer AG to the IPCS (1993)
e Hazleton Laboratories, USA Report HLA-6001-187
2.4 Analytical methods
2.4.1 Plants
Early methods for the detection of amitrole by paper
chromatography or for its quantitative determination by
spectrophotometry involved extraction by ethanol or water,
diazotization of the 3-amino group and, finally, coupling with
either phenol in 20% HCl (Aldrich & McLane, 1957),
N-(1-naphthyl)ethylenediamine dihydrochloride (Storherr & Burke,
1961), H-acid (8-amino-1-naphthol-3,6-disulfonic acid, monosodium
salt) (Racusen, 1958; Herrett & Linck, 1961; Agrawal & Margoliash,
1970) or chromotropic acid (Green & Feinstein, 1957). This technique
has been used for residue analysis in plants (Aldrich & McLane,
1957; Herrett & Linck, 1961), and vegetable crops (Storherr & Burke,
1961). The detection limit was found by Aldrich & McLane (1957) to
be approximately 0.1 µg/spot. The method outlined by Storherr &
Burke (1961) is sensitive to 0.025 mg/kg. Recovery was described by
Herrett & Linck (1961) to be close to 100%. Storherr & Onley (1962)
found that dry-packed cellulose column chromatography was preferable
to paper chromatography for separation of amitrole from some crops.
Several gas chromatographic methods have been developed to
determine amitrole residues in plants (Jarczyk, 1982a, 1985; Jarczyk
& Möllhoff, 1988). The principle of all these methods is similar.
After extraction with an ethanol-water mixture, acetylation with
acetic anhydride (conversion of amitrole to the monoacetyl
derivative) and a clean-up step by gel chromatography, the residue
is dissolved in acetone or ethanol and determined by a gas
chromatograph equipped with a nitrogen-phosphorus detector.
Weber (1988) developed a method for the determination of
amitrole in plant material by high performance liquid chromatography
(HPLC). Amitrole was extracted with an acetone-water mixture and the
water phase was extracted with dichloromethane to remove lipophilic
compounds. After a further clean-up step with column chromatography
on a cation exchange resin and on aluminium oxide, the residues were
determined by HPLC with ion pairing reagent and electrochemical
detection. In plants the detection limit was 0.01 mg/kg and the
recovery was between 91 and 99% in the range 0.01-1.0 mg/kg.
The Codex Committee on Pesticide Residues has recommended the
methods of Lokke (1980) and Van der Poll et al. (1988).
The method of Lokke (1980) uses ion-pair HPLC, which in
potatoes or fodder beets had a limit of detection between 0.005 and
0.01 mg/kg.
The method of Van der Poll et al. (1988) is capable of
determining amitrole in plant tissues and sandy soils by capillary
gas chromatography with an alkali flame ionization detector. Samples
are extracted with ethanol, absorbed on resin and desorbed with
ammonia. After acetylation with acetic anhydride and clean-up with a
SEP-PAK silica cartridge, the residue is determined by gas
chromatography (GC). The limit of detection is 0.02 mg/kg and
average recoveries are 76-81% in the range from 0.05 to 0.2 mg/kg.
2.4.2 Soil
An early method for the determination of residues in soil was
developed by Sund (1956) which involved extraction with water
followed by colour reaction with nitroprusside in alkaline
solutions.
Groves & Chough (1971) developed an improved procedure for the
extraction of amitrole from soil using concentrated ammonium
hydroxide and glycol (1:4). Pribyl et al. (1978) investigated the
extraction of amitrole from soils and its identification and
quantitation by photometry and thin layer chromatography (TLC). The
limit of detection was 0.05 mg/kg. They proposed analysis by TLC
after reaction with 5-dimethylaminonaphthalene-1-sulfonyl chloride
(dansylation), in preference to HPLC. Lokke (1980) suggested that
HPLC separation could be used if preceded by clean-up on a polyamide
column. Both the GC method (Jarczyk, 1985; Jarczyk & Möllhoff, 1988)
and the HPLC method (Weber, 1988) described in section 2.4.1 for
plants are also suitable for the determination of amitrole residues
in soil.
2.4.3 Water
Marston et al. (1968) and Demint et al. (1970) have used cation
ion-exchange column chromatography to extract amitrole from
contaminated creek and canal waters. This is followed by
diazotization and coupling as described by Storherr & Burke (1961).
Alary et al. (1984) have modified these methods to achieve a
spectrophotometric determination of amitrole in waste water in the
vicinity of production plants in the presence of interfering amino
compounds.
A more recent capillary gas-liquid chromatographic method for
determining amitrole in ground water and drinking-water, using an
alkali flame ionisation detector, has been described, the reported
limit of detection being 0.1 µg/litre (Van der Poll et al., 1988).
Legrand et al. (1991) formed a nitroso derivative of amitrole
concentrated from surface and ground waters prior to HPLC analysis.
The nitroso derivative showed an absorption maximum in the near UV
spectrum. Aqueous solutions of amitrole in the range of
0.25-0.50 µg/litre were measurable, and the recoveries were 70 ± 8%
(n = 11). The limit of determination was 0.1 µg/litre.
Pachinger et al. (1992) developed an HPLC analytical method
with amperometric detection for the determination of amitrole
without derivatization in drinking-water and ground water. Detection
limits were 1 mg/litre for directly injected samples and
0.1 µg/litre following an evaporation step to concentrate the
samples. Recoveries were close to 100%.
Both the GC method (Jarczyk, 1985; Jarczyk & Möllhoff, 1988)
and the HPLC method (Weber, 1988) described in section 2.4.1 for
plants are also suitable for the determination of amitrole residues
in water.
An immunochemical approach to the detection of amitrole has
been recently described by Jung et al. (1991). Development of this
rapid and sensitive method is likely to lead to a very effective
method for detecting amitrole in waterways.
2.4.4 Formulations
Ashworth et al. (1980) described a potentiometric precipitation
titration method using silver nitrate and silver/silver chloride or
silver/mercurous sulfate electrode. This method can be used for the
determination of amitrole in its formulations or in the presence of
triazines, substituted urea herbicides or plant growth regulators
such as bromacil and ammonium thiocyanate. Another method for the
determination of amitrole in its formulations has been described by
Gentry et al. (1984). This involves dissolving or extracting the
sample with dimethylformamide, acidifying by adding 0.5 N HCl and
back-titrating the excess acid with 0.5 N sodium hydroxide.
Jacques (1984) has described a simple GC method for the
detection and quantification of amitrole in technical and formulated
products.
A TLC method for the routine identification of amitrole in
pesticide mixtures has been developed by Ebing (1972).
2.4.5 Air
Alary et al. (1984) have described a method for the analysis of
air samples collected on glass-fibre filters followed by
diazotization and coupling to produce a colour reaction. The
detection limit was not reported.
2.4.6 Urine
In order to assay amitrole in urine samples, Geldmacher-von
Mallinckrodt & Schmidt (1970) separated the amitrole by paper
chromatography using phenol saturated with water, or a mixture of
n-butanol:water (15:1) and propionic acid:water (7:6), and
identified amitrole by spraying with a solution of
p-dimethyl-aminobenzaldehyde in acetic acid or hydrochloric acid.
In a more recent paper by Archer (1984), a proposed method for
biological monitoring of urine samples used HPLC separation with a
visible light detector following diazotization and coupling. The
detection limit was 200 µg/litre.
3. SOURCES OF HUMAN AND ENVIRONMENTAL EXPOSURE
3.1 Natural occurrence
Amitrole does not occur naturally.
3.2 Anthropogenic sources
3.2.1 Production levels and processes
The synthesis of amitrole was first reported by Thiele &
Manchot in 1898 and involved the reaction of aminoguanidine with
formic acid (Carter, 1975). The current industrial production
process, described by Allen & Bell (1946), involves the same
reaction, in which an aminoguanidine salt is heated to 100-120 °C
with formic acid in an inert solvent (Carter, 1975; Sittig, 1985).
Amitrole is currently manufactured or formulated in several
countries. Its use has declined, particularly in the USA. However,
in spite of some recent replacements, amitrole remains a widely used
herbicide.
3.2.2 Uses
Amitrole is primarily used as a post-emergent non-selective
herbicide and has a very wide spectrum of activity against annual
and perennial broad leaf and grass type weeds. Its primary mode of
action is unknown but a prominent feature is its inhibition of the
formation of chlorophyll, and weeds initially change colour to
white, brown or red, and subsequently die (Carter, 1975). This
herbicidal activity is enhanced by the addition of ammonium
thiocyanate as a synergist. Amitrole can be used alone as a
concentrated solution in water or as a wettable powder in
combination with other herbicides. It is primarily used as a
herbicide and as a brush killer. It is also used as a non-selective
pre-emergent herbicide on fallow land before planting kale, maize,
oilseed rape, potatoes and wheat, and in other non-crop situations
(Worthing & Hance, 1991). It is also used along roadsides and
railway lines to control weeds. Approved uses of amitrole on soil
are either for non-crop land prior to sowing, or for inter-row weed
control in tree and vine crops, where contact with food plants is
avoided. Amitrole is also used for the control of pond weeds and is
an especially effective herbicide in the control of water hyacinth
(Eichhornia crassipes).
Amitrole has also been used as a cotton defoliant in some
countries (Hassall, 1969).
4. ENVIRONMENTAL TRANSPORT, DISTRIBUTION AND TRANSFORMATION
4.1 Transport and distribution between media
4.1.1 Air
The very low vapour pressure of amitrole (Table 1) means that
it will not enter the atmosphere.
4.1.2 Water
Amitrole is readily soluble in water (280 g/litre at 25 °C) and
has a half-life of more than one year at 22 °C and pH 4-9 (Worthing
& Hance, 1991). Although no direct photolysis occurred in doubly
distilled water, the photodegradation rate increased in the presence
of humic acid, potassium salt (100 mg/litre), a natural
"photosensitizer", resulting in a half life of 7.5 h (Jensen-Korte
et al., 1987).
4.1.3 Soil
4.1.3.1 Adsorption
Amitrole is adsorbed to soil particles and organic matter by
proton association. The adsorbed aminotriazolium cation will enter
into cationic exchange reactions (Nearpass, 1969). Binding is
strongly pH dependent, and the cation is adsorbed to a greater
extent in acid conditions. The aminotriazolium cation is bound more
strongly than sodium but is displaced by calcium ions. The binding
is reversible and not strong, even in favourable acid conditions.
The binding capacity of soils at pH 5 or more is limited (Nearpass,
1970). Anderson & Hellpointner (1989) determined the Koc values
for amitrole in four soils i.e. silty clay, sandy loam, sand and
silt, to be 112, 30, 20 and 52, respectively. Adsorption increased
at lower pHs; adjustment of pH to a constant 4.5 resulted in Koc
values ranging from 77 to 356. The authors classified amitrole as
highly mobile in the soils at their equilibrium pH values of 5.6 to
7.4 and medium to highly mobile with the pH adjusted from 4.2 to
4.5.
There is considerable variation in the literature, both old and
recent, in reported adsorption and leachability of amitrole. Sund
(1956) described adsorption to soil as strong. He demonstrated that
amitrole could be efficiently removed from aqueous solution with a
resin cation exchanger and argued that soil would also bind the
compound efficiently. A correlation between the base exchange
capacity of soil and binding of amitrole was postulated. This agrees
with the theoretical and experimental work of Nearpass (1969, 1970),
although the latter author does not support the strength of
adsorption proposed by Sund (1956). Day et al. (1961) investigated
leaching of amitrole through 400-g, 4-cm diameter columns of three
different soils from a citrus growing area of California following
occasional reports of damage to trees after application of high
rates of amitrole for the control of perennial weeds. Amitrole moved
readily with the leaching water for all soil types (two sandy loams
and one silt loam) and most readily through quartz sand. Zandvoort
et al. (1981) supported this conclusion, suggesting that the high
water solubility of amitrole could result in leaching from sandy
soils. Weller (1987) investigated leaching of amitrole through
27-cm, 5-cm diameter columns of two soils, a sandy "standard soil
2.1" and a second soil with substantially higher organic content.
Immediately after incorporation of the 14C-amitrole to give 2 mg
on the surface area of the column, leaching with deionized water
began, 393 ml being pumped onto the soil column over 2 days. The
leachate was collected in two fractions: 175-191 ml and 185-200 ml.
Duplicate experiments showed 24 and 31% of the initial radioactivity
in the leachate (entirely in fraction II) in the sandy soil, with
11%, 16% and 46%, respectively, remaining in the upper, middle and
lower third of the soil column. The second soil leached markedly
less of the added radioactivity (1.4 and 1.8% for the duplicate
columns); this also appeared in fraction II. The radioactivity in
the leachate was unchanged amitrole.
Since amitrole is degraded rapidly in soil (section 4.2.1), the
high potential of amitrole to leach through sandy soils does not
seem to be realized in practice. The occasional damage to trees
reported in the study by Day et al. (1961) has not been a regular
feature of the use of amitrole. Degradation products of amitrole do
not leach significantly through soil (section 4.2.1).
4.1.4 Vegetation and wildlife
When applied directly to vegetation as a herbicide, amitrole is
absorbed through the foliage and can be translocated throughout the
plant. Translocation occurs in the photosynthetic stream and is
dependent on light. When applied to soil, amitrole can be adsorbed
through the roots and transported in the xylem, within a few days,
to the tips of the shoots (Carter, 1975).
4.1.5 Entry into food chain
Amitrole is not to be used on food crops and therefore food
residues should not occur. Grazing animals could consume amitrole as
surface residues on vegetation after application or as residues
within the plant. Amitrole is not persistent in animals and would
not be expected to pass through the food chain.
4.2 Biotransformation
4.2.1 Biodegradation and abiotic degradation
4.2.1.1 Plants
Racusen (1958) reported the first comprehensive studies of
amitrole metabolism in plants. Two major metabolites were isolated,
neither of which were as phytotoxic as amitrole. These results were
supported by studies by Carter & Naylor (1960). One metabolite was
identified as the product of the reaction of amitrole with serine,
namely, 3-(3-amino- s-triazole-1-yl)-2-aminopropionic acid
(3-ATAL). Formation of 3-ATAL is considered to represent
detoxification since ammonium thiocyanate, which synergizes the
action of amitrole, inhibits the formation of 3-ATAL (Smith et al.
1969). Other products of amitrole metabolism in plants have not been
identified. Fang et al. (1967) found that metabolism of amitrole in
leaves was exponential, with half-lives in sugar beet, corn and bean
leaves being 18.7, 28.0 and 23.2 h, respectively. A review of the
degradation of amitrole in plants has been presented by Carter
(1975).
The soluble metabolites of [3,5-14C]-amitrole in apples were
examined by Schneider et al. (1992) following soil application.
Significant proportions of the radioactivity were found as bound
residues, but 69-90% were extractable with acetonitrile. In addition
to 3-ATAL, 3-(1,2,4-triazole-1-yl)-2-aminopropionic acid
(3-aminotriazolylalanine) was also identified, in both the free form
and as conjugates. This was the major metabolite in apple cell
cultures treated with amitrole (Stock et al., 1991).
4.2.1.2 Soils
There is general agreement that degradation of amitrole in soil
is usually fairly rapid and variable with soil type and temperature.
However, there is no clear consensus on the relative roles of biotic
and abiotic processes in the breakdown of the compound.
Day et al. (1961) measured amitrole colorimetrically in 55
different soils of 5 main types from California and estimated the
depletion after 2 weeks of incubation. The results were very
variable, 26 soils having no measurable amitrole after 2 weeks, 6
soils showing traces and the remaining 23 soils having higher
quantities, in some cases comparable to initial levels. Four soils
had more than half of the original amitrole after the 2-week
incubation. It was not possible to correlate depletion of amitrole
to soil type. The authors classified the soils according to general
type and ranked them in terms of "heaviness"; the four soils
retaining most amitrole ranked 7, 23, 30 and 54 in the list. There
was a geographical correlation with reported incidents of non-target
effects of the herbicide. Some specific characteristic of a variety
of soils from a single location had led to movement of the herbicide
and its retention longer than in apparently comparable soils
elsewhere. Decomposition rates in steam-sterilized soils were much
lower than in unsterilized soils, which led the authors to conclude
that breakdown was principally due to microorganisms. Decomposition
was optimal at temperatures between 20 and 30 °C and at medium to
high soil moisture content. Breakdown was not well correlated with
soil classification, texture, base-exchanged capacity or adsorption
capacity for amitrole. Differences in microbial populations were
cited as the most likely explanation for the variation.
Kaufman et al. (1968) also found that sterilization of soil
reduced the breakdown of amitrole. Within 20 days, 69% of the
radioactivity of 14C-labelled amitrole was released as 14CO2
in unsterilized soil. Soil treated with sodium azide or ethylene
oxide released 46% and 35%, respectively, whilst autoclaved soil
released only 25%. Reinoculation of soil with microorganisms
isolated from the original soil failed to restore the capacity to
degrade amitrole. Amending the soil with other organic compounds
reduced amitrole degradation. The authors concluded that degradation
of amitrole was largely a chemical process and that microbial action
was indirect. Free radicals (such as HO.) were proposed as agents
for oxidation of the amitrole nucleus. Plimmer et al. (1967) studied
the degradation of amitrole by free-radical generating systems. They
demonstrated that riboflavin (and light) or an ascorbate-copper
reagent (Fenton's reagent) promotes oxidation of amitrole, resulting
in ring cleavage, loss of CO2 and production of urea, cyanamide
and possibly molecular nitrogen. Riepma (1962) observed a lag-phase
which he considered typical of microbial breakdown. Carter (1975)
concluded that "whatever the mechanism, triazole ring opening occurs
rapidly in soils and the resulting products ... should be readily
metabolized by soil microorganisms".
Campacci et al. (1977) reported the isolation of bacteria
capable of degrading amitrole, strengthening the argument for
microbial involvement. Only one of three media tested succeeded in
growing organisms that could degrade amitrole. Of 36 isolates from
this culture, 10 were found to be capable of degrading amitrole.
Nine of these were gram-positive rods (Bacillus spp. and
Corynebacterium spp.) and one was identified as a Pseudomonas
sp. The growth of these bacteria was roughly proportional to
amitrole concentration up to 256 mg/litre. The organisms could
degrade amitrole as sole nitrogen source but not also as sole carbon
source; the medium was enriched with sucrose. This explained
previous failures to isolate organisms capable of degrading the
herbicide.
Various studies have quantified the degradation of amitrole in
soil. Scholz (1988) observed the release of 48% of applied
radioactivity (14C-amitrole) as 14CO2 after 5 days. In
degradation studies in the laboratory, half-lives of between 2.4 and
9.6 days were observed in different soils. DT90 (the time required
for degradation of 90% of the amitrole) values were in the range of
13 to 22 days (LUFA, 1977; Jarczyk, 1982b,c,d). Hawkins et al.
(1982b) measured 70-80% degradation to CO2 in standard soil and
40-50% in English clay soil within 28 days. There was no release of
14CO2 from autoclaved soil. Hawkins et al. (1982a) measured
decomposition in the same English clay in the field. Here 53% of the
applied radioactivity remained after 112 days. The slower rate of
breakdown in the field was ascribed to the temperature and soil
moisture content. Schneider et al. (1992) suggested that amitrole
can be deaminated in soil to give triazole.
A study by Weller (1987) examined the leaching of "aged"
residues of amitrole. Soils, with 14C-amitrole incorporated as
described in section 4.1.3.1, were incubated for 30 and 92 days, in
duplicate experiments, and then used in leaching tests as for the
initial soils. For both the "standard soil 2.1" and the second soil,
between 50 and 73% of initial radioactivity was lost as 14CO2
during incubation. Of the remaining radioactive material, negligible
amounts leached through the soil column in tests after 30 and 92
days. Almost all of the activity remained in the upper third of the
soil column. After 30 days 4% or less of the activity was unchanged
amitrole. The breakdown products of amitrole (not characterized
except for traces of urea) were tightly bound to the soil and were
not leachable or easily extractable.
4.2.3 Bioaccumulation
Flow-through studies on fish using 14C-amitrole indicated
that the bioaccumulation of amitrole in bluegill sunfish (Lepomis
macrochirus) and in channel catfish (Ictalurus punctatus),
exposed to 1 mg/litre, was only slight after 21 days of exposure
(approximately 1.7-3.0 times the amitrole concentration in the
water). When the fish were returned to untreated water, the amitrole
concentration in their organs fell rapidly (Iwan et al., 1978).
Bioaccumulation of amitrole by aquatic organisms would not be
expected because of its high water solubility and very low
octanol-water partition coefficient (Table 1).
4.3 Ultimate fate following use
MacCarthy & Djebbar (1986) described a method using chemically
modified peat to decontaminate eluant from chemical production
plants before it enters major waterways. When converted to a
granular product suitable for column chromatography, the peat can
act as an efficient ion-exchange material for the removal of
amitrole and other basic chemicals.
Amitrole is resistant to hydrolysis and the action of oxidizing
agents. Burning the compound with polyethylene is reported to result
in > 99% decomposition (Sittig, 1985).
5. ENVIRONMENTAL LEVELS AND HUMAN EXPOSURE
5.1 Environmental levels
5.1.1 Air
Amitrole-containing particles are released from the stack of
production plants during dry crushing and, to a lesser extent,
bagging operations. Atmospheric levels in the range of 0 to
100 µg/m3 were measured in the vicinity of such a plant (Alary
et al., 1984). Severe chlorosis and defoliation was noted following
atmospheric fallout in the vicinity of the plant.
5.1.2 Water
Grzenda et al. (1966) studied the persistence of amitrole and
three other herbicides in pond water following an aquatic weed
control programme. The initial level on day one of 1.34 mg/kg
decreased gradually to 1.03 mg/kg on day 11, 0.73 mg/kg on day 25,
0.49 mg/kg at 9.5 weeks and 0.08 mg/kg at 27 weeks.
In a study by Marston et al. (1968) in which 100 acres of a
watershed in Oregon was sprayed, the levels of amitrole in water
samples were measured on the downstream edge of the sprayed area. A
maximum concentration of 155 µg/litre was found 30 min after
application began, but this decreased to 26 µg/litre by the end of
the application. No amitrole could be detected 6 days after
spraying. The detection limit was 2 µg/litre.
Demint et al. (1970) measured the amitrole concentration in two
flowing water canals following treatment of a single ditchbank of
each canal with amitrole at the normal treatment rate. Samples taken
at stations up to 7.2 km downstream indicated rapid decreases in
amitrole levels following passage of the initial amitrole-bearing
water, the levels having declined to 1 µg/litre within 2 h. In a
preliminary environmental survey conducted in 1984 in Japan,
amitrole was not detected (detection limit 4 µg/litre) in any of 24
water samples nor was it detected in any of the 24 bottom sediments,
the detection limit being 5-20 µg/kg (Environment Agency Japan,
1987).
Alary et al. (1984) measured the level of amitrole in water
samples collected in a river downstream from the discharge of an
aeration pond in the vicinity of a production plant. The levels were
in the range of 0.5 to 2 mg/litre while the concentration in the
water of the aeration pond was in the range of 50 to 200 mg/litre.
Legrand et al. (1991) tried to detect 38 compounds including
amitrole in different areas of France (13 sampling points) with a
detection limit of 0.1 µg/litre, but no amitrole was found.
5.1.3 Soil
As discussed in chapter 4, amitrole, when applied to soil, is
readily degraded or adsorbed to the soil particles.
5.2 General population exposure
5.2.1 Environmental sources
No exposure would be expected from environmental sources.
5.2.2 Food
Amitrole is not to be used on food crops, and food residues
should therefore not occur. Using a limit of determination of
0.05 mg/kg, amitrole was not detectable in a wide range of food
crops (Duggan et al., 1966, 1967; Corneliussen, 1969, 1970). This
was confirmed by several studies (Bayer AG, 1993a,b).
Experimental studies in West Virginia, USA, indicated that
residues of amitrole on whole apples could not be detected 3 months
after ground cover application, but could be detected when either
fruit or foliage or both were directly treated with amitrole
(Schubert, 1964). The analyses were conducted using the method of
Storherr & Burke (1961) with a detection level of 0.025 mg/kg.
Similarly, residue trials conducted in Tasmania and New South
Wales on apples did not reveal amitrole at a detection limit of
0.01 mg/kg following ground cover application (Moore, 1968, 1969,
1970). A slight modification of the method of Storherr & Burke
(1961) was used.
In one study, residues of amitrole were found in blackberries
growing very near a railway line that was sprayed by amitrole in the
normal way by the railway authorities. Thirteen days after spraying
at a dose 3,5 kg a.i./ha, blackberries were picked close to the
railway at two different locations. The mean residues found at the
two locations were 0.67 (0.2-1.4) mg/kg and 2.0 (0.1-3.8) mg/kg. The
places where the blackberries were picked was prohibited to the
general public. This study shows that spraying of amitrole on
blackberries results in considerable residues (Dornseiffen &
Verwaal, 1988).
5.3 Occupational exposure during manufacture, formulation or use
The potential for toxicity via the dermal or inhalational
routes appears to be low. A threshold limit value (TLV) of
0.2 mg/m3, as an 8-h time-weighted average (TWA), has been set for
amitrole by the American Conference of Governmental & Industrial
Hygienists (ACGIH, 1991-1992). Amitrole is a mild skin and eye
irritant.
6. KINETICS AND METABOLISM IN LABORATORY ANIMALS AND HUMANS
6.1 Absorption, distribution and excretion
6.1.1 Mouse
The distribution of [5-14C]-radiolabelled amitrole has been
examined in non-pregnant C57BL strain female mice (Tjalve, 1975) and
in the fetuses of pregnant NMRI strain mice (Tjalve, 1974). In each
case, the mice received amitrole (5 µCi) either intravenously or
orally, and the distribution of radioactivity was determined by
whole-body autoradiography of the adult or fetus at intervals of
5 min to 5 days after administration. In the non-pregnant animals,
further analysis of the distribution of radioactivity was performed
by microautoradiography of the spleen and thymus, and by subcellular
fractionation of the liver, spleen and thymus. The highest
radioactivity was found in tissues with rapid cell turnover, e.g.,
bone marrow, spleen, thymus and gastrointestinal mucosa. Only a
moderate level of radioactivity was found in the thyroid. The level
of radioactivity in the tissues was the same whether the treatment
was intravenous or oral. In all cases, there was a significant
decrease over the 5-day period. Microautoradiography indicated
amitrole was located mostly in the cytoplasm. 14C-labelled
amitrole crossed the placental barrier and could be detected in
fetal tissues 4 and 8 h after administration to the dams by
intravenous injection or gavage.
Following intravenous administration of 14C-amitrole
(3.4 mg/kg body weight), adult ICR mice were killed at given
intervals (5 min, 30 min, 8 h and 24 h) and submitted to whole-body
autoradiography and microautoradiography. The liver had the highest
accumulation of radioactivity and two distribution patterns were
observed: a homogenous distribution up to 8 h following injection,
and a subsequent heterogenous one. Liver sections were extracted
with trichloroacetic acid and methanol, but considerable amounts of
radioactivity remained non-extractable. A microauto-radiography of
the liver 8 h after 14C-amitrole injection revealed that the
radioactivity was localized in the centrolobular areas (Fujii
et al., 1984).
6.1.2 Rat
Kinetic studies on amitrole in rats were performed by Fang
et al. (1964). Groups of Wistar rats were administered 1 mg
14C-amitrole by gavage and the distribution of radioactivity was
analysed at various time intervals between 30 min and 6 days. High
levels of radioactivity (70-95% of the administered radioactivity)
were found in the urine during the first 24 h, but only low levels
in the faeces, indicating rapid and almost complete absorption from
the gastrointestinal tract followed by rapid excretion. Tissue
levels were very low after 3 days, and significant amounts were
found only in the liver. In a second experiment (Fang et al., 1966),
14C-amitrole was administered at various dose levels (1-200 mg/kg
body weight). The average total radioactivity found in urine and
faeces (as a percentage of the administered dose) was comparable for
all the doses applied. The difference in average half-time for
clearing of radioactivity from various organs was considered to be
insignificant between dosages of 1 and 200 mg/kg. The fate of two
unidentified plant metabolites of amitrole, i.e. 14C-metabolite 1
and 14C-metabolite 3 (isolated from bean plants), was also
examined by Fang et al. (1966). Radioactivity from metabolite-1 was
excreted rapidly in the urine in the first 48 h and identified as
unchanged metabolite-1. Elimination of metabolite-3 was mainly in
the faeces. In a study by Grunow et al. (1975), 14C-amitrole was
administered to rats by gavage at a dose level of 50 mg/kg, and the
urine and faeces were examined over 3 days. The major route of
excretion of radioactivity was the urine, the majority of the
radioactivity being excreted in the first 24 h.
Two groups of five male and five female Sprague-Dawley rats
weighing 200-250 g were exposed (either nose only or whole body) to
atmospheres of 5-14C-amitrole (radiochemical purity > 97%) in
water aerosols at concentrations in air of 49.2 µg/litre
(2.6 µCi/litre) or 25.8 µg/litre (1.4 µCi/litre), respectively, for
1 h, and then observed for 120 h (MacDonald & Pullinger, 1976). The
particle size distribution of the aerosols was not reported. The
calculated elimination half-life of radioactivity was approximately
21 h for both exposures; approximately 75% of the radioactivity was
eliminated in the urine within 12 h.
6.1.3 Human
Urinary excretion of unchanged amitrole has been reported in a
woman who ingested approximately 20 mg/kg of the herbicide
(Geldmacher-von Mallinckrodt & Schmidt, 1970).
6.2 Metabolic transformation
The limited data available indicates that little metabolic
transformation of amitrole occurs in mammalian species. In the
mouse, tissue residues were identified by TLC as mainly unchanged
amitrole (84% of the detected radioactivity) when measured 8 h after
exposure (Tjalve, 1975). Similarly, paper chromatographic analysis
of rat liver residues following oral administration revealed
unchanged amitrole plus one unidentified metabolite (Fang et al.,
1964). In the urine of rats, the majority of the radioactivity was
also unchanged amitrole; one unidentified metabolite was isolated
which represented approximately 20% of the total radioactivity. The
liver was the site of the unidentified metabolite-1 formation and
the rate of elimination of this metabolite from liver and kidney was
much slower (Fang et al., 1964).
In a more extensive analysis of urinary metabolites in the rat
by Grunow et al. (1975), the major part of the radioactivity
identified by paper chromatography corresponded to unchanged
amitrole. Two urinary metabolites were identified as
3-amino-5-mercapto-1,2,4-triazole and
3-amino-1,2,4-triazolyl-(5)-mercapturic acid, which together
amounted to approximately 6% of the administered dose.
In a metabolic study (Turner & Gilbert, 1976), which was
supplementary to the inhalation exposure experiment and is described
in section 6.1.2 (MacDonald & Pullinger, 1976), it was found that
approximately 60% of the urinary radioactivity chromatographed on
silica gel 60 TLC in methanol: 880 ammonia (100: 1.5, s/s) as
amitrole, 15-20% remained at the origin and 5-8% migrated faster
than amitrole. Treatment with ß-glucuronidase had no effect upon
this TLC distribution.
7. EFFECTS ON EXPERIMENTAL ANIMALS AND IN VITRO TEST SYSTEMS
7.1 Single exposure
7.1.1 Oral
The acute oral toxicity data for amitrole when administered as
an aqueous suspension are presented in Table 2.
Table 2. Acute oral toxicity of amitrole
Species LD50 (mg/kg Reference
body weight)a
Rat > 4080 (m and f) Gaines et al. (1973)
> 4200 Seidenberg & Gee
24 600 (m) Bagdon et al.(1956)
> 10 000 Hecht (1954)
> 2500 Kimmerle (1968)
> 5000 (m) Thyssen (1974)
> 5000 (m) Heimann (1982)
Mouse 11 000 Hapke (1967)
14 700 (m) Fogleman (1954)
Cat > 5000 (m and f) Bagdon et al. (1956)
a m = males; f = females
In cats and dogs the general signs of toxicity were dyspnoea,
ataxia, and diarrhoea with vomiting. Coma and death appeared to be
associated with profound respiratory depression. Gastro-intestinal
irritation and haemorrhage were the only treatment-related findings.
The toxicity of a mixture of amitrole and ammonium thiocyanate
(1:1), referred to as Amitrol-T, appeared to be slightly higher than
that of amitrole itself, but was still very low. LD50 values
obtained following oral administration in rats were 3500 mg/kg and
10.5 ml/kg of the commercial product (DeProspo & Fogleman, 1973;
Field, 1979).
The possibility that amitrole might form a Schiff's base with
glucose was investigated by Shaffer et al. (1956). An
amitrole-glucose adduct was prepared and administered orally to rats
and mice (10 mg/kg), intraperitoneally to mice (10 mg/kg), and
intravenously to mice (1.6 mg/kg). There were no deaths or signs of
toxicity following treatment.
7.1.2 Other routes
The acute toxicity of amitrole by other routes of
administration is very low, as shown in Table 3.
Table 3. Acute, dermal, intraperitoneal and intravenous toxicity
of amitrole
Species Route LD50 (mg/kg bw) Reference
Rat dermal > 2500 (m and f) Gaines et al. (1973)
intraperitoneal > 4000 (m) Shaffer et al. (1956)
Mouse intravenous > 1600 (m) Shaffer et al. (1956)
intraperitoneal > 10 000 Shaffer et al. (1956)
intraperitoneal 5470 (m) Nomiyama et al. (1965)
subcutaneous 5540 (m) Nomiyama et al. (1965)
Rabbit dermal > 10 000 Elsea (1954)
Dog intravenous > 1800 (m) Fogleman (1954)
Cat intravenous > 1750 (m and f) Shaffer et al. (1956)
a m = males; f = females
Amitrole applied in water formulations to the unabraded skin of
rabbits for 24 h caused a very mild and reversible erythema (Elsea,
1954). Intraperitoneal administration in mice and rats and
intravenous administration in either mice, dogs or cats produced no
signs of toxicity (Fogleman, 1954).
No toxicity was observed in rats after inhalation of amitrole
following either head-only (approximately 50 µg/litre) or whole-body
(approximately 25 µg/litre) exposure for a period of one hour
(MacDonald & Pullinger, 1976).
7.2 Short-term exposure
7.2.1 Oral
7.2.1.1 Dietary
When groups of Carworth Farm male and female rats (five per
group) were administered amitrole in the diet at dose levels of 0,
100, 1000 or 10 000 mg/kg for 63 days, reduced body weight gain for
both males and females was observed at the two highest dose levels,
this being accompanied by reduced food consumption. There were no
deaths or clinical signs of toxicity. Histo-pathological examination
of the liver, kidney, portions of the small intestine, spleen, and
testes revealed increased vacuolization of the liver cells around
the central vein and steatosis at the two highest dose levels
(Fogleman, 1954).
Mayberry (1968) studied the effects on the thyroid of a dietary
level of 1000 mg amitrole/kg in rats during 83 days and compared
this to the effects of other anti-thyroid chemicals,
propylthiouracil (1000 mg/kg) and potassium chlorate (1000 mg/kg).
At various time intervals, starting with 3 days, the relative
thyroid weight and total iodine content of the thyroid were
measured. An increase in thyroid weight and a decrease of total
iodine were observed within the amitrole group; this was already
observable after 3 days and becoming more pronounced during the
course of the experiment. The effects of propylthiouracil were
comparable, but, in the case of potassium chlorate, the weight
increase was less pronounced and the iodine content was lower than
with amitrole. In another experiment, uptake and release of
radioactive iodine was measured after a single 131I injection to a
control group of rats and to a group simultaneously receiving 10 mg
amitrole subcutaneously. Animals were killed after 1, 2, 3, 4, 5 or
6 days. The t´ for 131I in the thyroid was 4.9 and 1.3 days for
control and amitrole-treated animals, respectively. Separation by
paper chromatography of 131I-containing thyroid fractions showed
that levels of monoiodotyrosine (MIT) were increased, diiodotyrosine
(DIT) were constant and T3 and T4 were markedly reduced. The
author concluded that amitrole not only interferes with
organification of iodine but also inhibits the coupling of
iodotyrosines to form iodothyronines (Mayberry, 1968).
The effect of amitrole on thyroid hormones was studied by
giving groups of male Sprague-Dawley rats (20 per dose level)
amitrole (94.6% pure) in the diet at dose levels of 0, 30, 100 or
300 mg/kg during 28 days, followed by a recovery period of 28 days
(Babish, 1977). The assessment of thyroid function was performed by
measuring T3 and T4 in blood by a radioimmuno-assay. On days 3, 7,
14, 21 and 28 of the treatment period and on days 19, 21 and 28 of
the post-treatment period, blood samples were collected from two
animals which were then killed for autopsy. There were no adverse
effects on the general health of the rats during the treatment or
post-treatment period. Consumption of 100 or 300 mg amitrole/kg diet
significantly depressed body weights during the 28-day treatment
period. The mean weekly body weights in the rats given 100 mg/kg
returned to control values by the third week of the post-treatment
period, while the mean weights of animals in the highest-dose group
did not return to control values during the post-treatment period.
The depression of body weights correlated with decreased food
consumption.
Serum T3 levels were significantly depressed by day 7 at
300 mg/kg (about 50%) and by day 14 at 100 mg/kg/diet (about 40%).
An inexplicable return to control values was seen 21 days into the
treatment period, followed by continued depression of T3 values on
day 28 of the treatment period. The depression of T3 appeared to
be dose related after 4 weeks of treatment. All treatments exhibited
essentially normal T3 levels by day 19 of the post-treatment
period. T4 levels followed exactly the same pattern as those of
T3. However, the T3/T4 ratio (which fluctuated between 12 and
18 in control rats) increased as the dose increased, being highest
at 300 mg/kg after 14 days of treatment.
From this study it may be concluded that amitrole, at levels of
100 mg/kg diet or more, rapidly suppressed T3 and T4 hormone
levels and maintained the depressed levels during the treatment
period. Both T3 and T4 levels returned to control values within
three weeks following withdrawal of amitrole from the diet. The
no-observed-adverse-effect level (NOAEL) in this study was
30 mg/kg/diet (Babish, 1977).
Fregly (1968) investigated the dose-response relationship
between amitrole administered in the diet and a variety of clinical
parameters in order to establish the minimal dose with an effect on
thyroid activity. Groups of male Spruce Farm strain rats (10 per
dose level) were administered amitrole in the diet at dose levels of
0, 2, 10 and 50 mg/kg diet for 13 weeks. Body weight gain, food
consumption, haematocrit, haemoglobin concentration and rate of
oxygen consumption were unaffected by the treatment. Mean body
temperature was slightly increased but only at 50 mg/kg. During week
12, uptake of radioactive iodine was measured at various times
between 22-53 h after intraperitoneal injection of 131I. A
slightly lower uptake was found at the highest dose level. At the
end of the study, radioactivity in the thyroid gland, excised 24 h
after intraperitoneal injection, was slightly decreased in all
groups. At the end of the study, the protein-bound iodine (PBI) in
blood, measured as an indicator for the concentration of thyroid
hormones, was decreased at all dose levels. The values were
51 µg/litre (control) and 37, 38 and 33 µg/litre for the 0, 2, 10
and 50 mg/kg groups, respectively. In a second experiment the PBI
levels were not affected by treatment with amitrole at 0.25 and
0.5 mg/kg diet. Values were 32 µg/litre in controls and 39 and
45 µg/litre, respectively, in treated groups. It should be noted,
however, that PBI control values measured in the second experiment
were much lower than those measured in the first experiment. This
implies that there was no biologically significant effect on PBI
since all values were within the same range. The thyroid weight was
increased significantly only in the 50-mg/kg group. The number of
blood vessels/thyroid section, which is a very sensitive measure of
histopathological changes in the thyroid, was increased at 10 and
50 mg/kg. It can be concluded that 2 mg/kg diet was the NOAEL in
this study.
Several short-term studies were carried out by Den Tonkelaar &
Kroes (1974) in order to establish a no-observed-effect level on
thyroid function tests. In all experiments the uptake of 131I by
the thyroid was measured in an in vivo test, 6, 24 and 48 h after
the intraperitoneal administration of 0.6 µc 131I per animal. In
addition, thyroid weight and PBI were measured and the thyroid was
studied histopathologically.
In the first experiment, four groups of eight female Wistar
rats received, respectively, 0, 2, 20 and 200 mg amitrole/kg in the
diet for 6 weeks. After 5 days and 6 weeks the uptake of 131I was
measured. On both occasions a significantly increased uptake was
found in the 200-mg/kg group 6 h after injection, which decreased
fairly rapidly after 24 and 48 h. At that time the radioactivity was
lower than that of the controls. The thyroid weight was increased in
the 200-mg/kg group and histopathologically goitre was found only in
this group.
In the second experiment, eight female animals per group
received, respectively, 0, 20, 50 and 200 mg/kg diet for 6 weeks,
and similar effects were found in the 200-mg/kg group to those
observed in the first experiment. In addition, a significant
decrease in PBI was observed at the end of the experiment compared
with the control value. At 50 mg/kg, a statistically increased
uptake was found 6 h after injection of 131I. However, in this
case the radioactivity in the thyroid remained higher than that in
the controls after 24 and 48 h. Histopathologically only a very
slight activation was found. However, the 200-mg/kg group showed
strong activation and goitre.
In the third experiment 0, 20, 50 and 200 mg/kg diet were given
to 10 female animals per group during 13 weeks. The uptake of 131I
by the thyroid was significantly increased at 200 and 50 mg/kg after
6 and 12 weeks. The difference between the groups was that at
50 mg/kg the radioactivity in the thyroid remained high after 24 and
48 h, whereas with 200 mg/kg a very high uptake was found 6 h after
injection of 131I but this was followed by a rapid decrease, with
still lower values than the controls after 48 h. At 200 mg/kg the
PBI was decreased and the thyroid/body weight ratio increased by a
factor of 6. At 50 mg/kg only a slightly increased relative thyroid
weight was found. Histologically, a strong activation and goitre
were found at 200 mg/kg, and a slight activation at 50 mg/kg. In
this experiment, a tendency to a higher uptake of 131I was found
in the 20-mg/kg group.
The above-mentioned experiments were carried out with a
relatively low iodine content in the diet (about 0.2-0.3 mg/kg
diet). In the fourth experiment, a diet containing 2 mg iodine/kg
was used. In this experiment, eight female rats per group received,
respectively, 0, 20, 50, 200 and 500 mg amitrole/kg in the diet for
6 weeks to see whether iodine could protect against the antithyroid
action of amitrole. At 500 mg/kg, a small increase in iodine uptake
was found 5 h after 131I injection, but thereafter there was a
very rapid decrease. At 200 mg/kg, the uptake was much higher and
the same type of decrease was found as in the other experiments,
whereas at 50 mg/kg a significantly increased thyroid radioactivity
was found at all times. PBI was decreased at 200 and 500 mg/kg only.
Histopathologically, goitre and strongly activated thyroids were
found at the two highest dose levels. Some activation was found in
the 50-mg/kg group and a very slight activation was also found in
the 20-mg/kg group. It can be concluded that measurement of 131I
uptake at different time points is a sensitive method for the
effects of amitrole on the thyroid. At 20 mg/kg only slight effects
were found on uptake and thyroid histopathology. The NOAEL was
2 mg/kg diet, equivalent to 0.1 mg/kg body weight.
7.2.1.2 Drinking-water
When groups of male albino rats (10 per dose level) were
administered amitrole in the drinking-water at dose levels of 0, 50,
250 or 1250 mg/litre for 106 days, there was a dose-related decrease
in body weight gain in all treated groups with a corresponding
reduction in food and water intake. There were no clinical signs of
toxicity. At the end of the study, histopathological examination was
performed on the thyroid, hypophysis, liver, kidney, spleen,
stomach, small intestine, large intestine, bladder, testis, adrenal
and lung. The major gross pathological finding was an increase in
size and vascularity of the thyroid. At the high dose level, colloid
was absent in large and medium size thyroid follicles. High-dose
animals also displayed liver steatosis (Bagdon et al., 1956).
The time-course for development of goitre in rats was examined
by Strum & Karnovsky (1971). Sprague-Dawley rats were administered
amitrole in the drinking-water (2.5 mg/ml), and the thyroid of each
animal was examined by light microscopy at various periods from 3
days to 6 months. Each rat drank approximately 30 ml water per day.
After 3 days, the thyroid size was unchanged although cellular
changes were apparent. By one week, the thyroid was twice its normal
size with marked structural changes. These changes continued to
progress with prolonged administration of amitrole. Goitre formation
was accompanied by increased vascularity and decreased colloid
content in the follicular cells. Electron microscopy revealed
pronounced dilation of the endoplasmic reticulum of thyroid cells.
Thyroid peroxidase activity progressively decreased with
administration of amitrole.
The effects of amitrole on thyroid histology were examined in
seven groups of five female Wistar rats (weighing about 200 g),
which were given amitrole in their drinking-water (2.5 mg/ml) and
killed after 1, 2, 3, 10, 30, 50 or 100 days (Tsuda et al., 1973).
Water consumption was not reported. After 1 and 2 days of exposure
the only change noted was a slight enlargement of some endoplasmic
cisternae of the follicular cells. After 3 days the thyroid gland
was slightly enlarged, follicular colloid was slightly reduced and
in some follicular cells the cisternae were clearly dilated and
stained more lightly for peroxidase activity than did normal cells.
By 10 days the glands had doubled in size, the follicular epithelium
consisted of low, columnar cells, and colloid had been severely
depleted. Nuclei had become located basally and slightly elongated
microvilli projected into the lumen. Peroxidase activity was no
longer detected in the endoplasmic reticulum cisternae, but in
portions of perinuclear cisternae. These changes had progressed in
the 30-day samples, so that the glands were now several times their
normal size. In addition, fibrous thickening of both stroma and
capsule was prominent and cisternal peroxidase activity was absent.
Administration for 50 days resulted in increased irregularity in
follicular size, more prominent papillary growth of the follicular
epithelium and greatly diminished peroxidase activity throughout the
cells.
Histopathological changes induced by amitrole in the liver of
mice were investigated by Reitze & Seitz (1985). Groups of male
albino mice were exposed to amitrole in the drinking-water at dose
levels of 0.5%, 1.0% or 2% for 30 days (water consumption not
reported). Light microscopy revealed dose-related hypertrophy of
hepatocytes, increased pyknotic nucleoli, and increased vacuoles in
the cytoplasm. Electron microscopy revealed also a dose-related
proliferation of smooth endoplasmic reticulum.
7.2.1.3 Intubation
No data available.
7.2.2 Inhalational
Groups of Fischer-344 rats (15 of each sex per dose level) were
exposed to an atmosphere containing amitrole (94.6% pure) at
concentrations of 0, 0.1, 0.32, 0.99 or 4.05 mg/litre (nominal
concentrations adjusted for non-nebulized material) for 5 h/day, 5
times per week, for 4 weeks (particle size not provided). There were
no adverse effects on behaviour, and no body weight changes were
noted. T4 levels were significantly depressed by the 27th day at
the two highest dose levels. T3 levels were significantly
depressed by 14 days at all but the lowest dose level. Pathological
changes were confined to the thyroid, and hyperplasia was noted at
all but the lowest dose level (Cox & Re, 1978).
7.2.3 Intraperitoneal
Alexander (1959a) investigated the uptake of 131I by the
thyroid gland in Sprague-Dawley rats following intraperitoneal
injection of approximately 5 or 250 mg/kg body weight. At both dose
levels thyroid 131I uptake was inhibited, whereas catalase
activity was decreased by about 50% at the highest dose level only.
When 328 White Leghorn 3-day old chicks were injected with
amitrole (500 or 1000 mg/kg day), 5 days per week for 5 weeks,
increases in the relative thyroid-to-body weight ratio was observed
in all birds from day 10 onward. In addition, two groups of chickens
were injected the same doses of amitrole but for 17 consecutive
days. At the cessation of amitrole treatment, an increase in the
relative thyroid-to-body weight ratio was observed until day 13;
this was followed by a decrease and then a stabilization, which
occurred between days 17 and 41. However, the ratio never attained
the levels observed in control animals. Histopathological
examination of the thyroid gland revealed epithelial hyperplasia,
hyperaemia, obliteration of the follicular lumina and disappearance
of the colloid. In birds that were injected with amitrole only for
17 days, the thyroid histology returned to normal two weeks after
treatment (Wishe et al., 1979).
7.3 Long-term exposure
7.3.1 Oral
7.3.1.1 Mouse
Reversible thyroid hyperplasia has been reported during
long-term feeding studies with amitrole at levels of 1000 mg/kg diet
in both C3H and C57BL mice (Feinstein et al., 1978a). The
acatalasemic C3H mice survived longer on the amitrole diet than did
their normal catalasemic counterparts (mean survival times in weeks,
both sexes combined, were 35 ± 10, n = 141, and 26 ± 10, n = 146,
respectively, P < 0.001). Similar differences were observed with
C57BL/6 mice, although group sizes were much smaller (57 ± 5, n =
12, and 42 ± 7, n = 10, respectively). All mice given the treated
diet had a reduced body weight gain compared with mice given the
normal diet. In those mice for which the amitrole diet was withdrawn
at 12 weeks, the thyroid weight reduced in size gradually but the
gland was still enlarged after 60 weeks. A larger proportion of the
acatalasemic C3H mice developed liver tumours, as compared with
normal catalasemic C3H mice. Out of 87 mice in the acatalsemic
group, 21 developed liver tumours that were detected earlier
(beginning at 35 weeks) compared with the normal catalase mice (6/85
beginning at week 50) (Feinstein et al., 1978b).
In a life-time study in NMRI mice (dose levels 0, 1, 10,
100 mg/kg diet), the appearance, behaviour, food intakes, body
weights and survival times of the treated mice did not differ from
those of the controls. The frequency of pituitary hyperaemia was
slightly elevated in the high-dose group; no treatment-related
histological lesions were otherwise found. The frequency of types
and distribution of tumours in the control and treated groups were
similar. The thyroid weights were elevated in male high-dose group
mice at all dose levels and were up to three times the weights in
the control group. The percentage of iodine accumulation and the
iodine level in the thyroid were elevated in the male mice of the
100-mg/kg group. The sum of PBI in the male mice was elevated nine
months after study initiation, but was depressed at later test
dates. Comparable results were observed in the female high-dose
group mice. However, the deviations from control group values were
generally smaller than in the males, and were not significant in
most cases (Weber & Patrick, 1978; Steinhoff & Boehme, 1979b).
7.3.1.2 Rat
The long-term effects of oral administration of amitrole in
rats have been described in two detailed reports by Keller (1959)
and Johnson et al. (1981). The details and results of these studies
are given below.
In the study by Keller (1959), groups of Carworth Farm Wistar
rats (35 of each sex per dose level) were administered amitrole in
the diet at dose levels of 0, 10, 50 or 100 mg/kg diet for two
years. After 13 and 68 weeks, 5 and 3 animals of each sex and dose
level, respectively, were killed for organ weight measurement and
histopathological examination. A separate group received 500 mg/kg
diet for 19 weeks, followed by the control diet for 7 weeks, and
then were killed. In this group, body weight gain and food
consumption were markedly reduced during the amitrole
administration. Animals in all groups, including the controls,
suffered from apparent respiratory infection and 67 of them died,
but there was no relationship with the treatment. Body weight gain
was reduced at 100 mg/kg in male animals during the first 13 weeks
of the study. After 68 and 104 weeks of treatment, relative thyroid
weight was increased at 100 mg/kg (not measured after 13 weeks).
Histopathological examination after 13 weeks showed hyperplasia and
hypertrophy of the thyroid at 500 mg/kg; these effects were found to
be reversible. Histopathological changes in the thyroid were also
seen at 100 mg/kg and in one animal at 50 mg/kg. At 68 weeks three
animals given 50 mg/kg showed definitive evidence of hyperplasia,
while all animals given 100 mg/kg displayed hyperplasia and
hyperfunctioning of the thyroid. At 104 weeks tumours were found
(see section 7.7.2). Based on thyroid hyperplasia, the NOAEL was
10 mg/kg diet (equivalent to 0.5 mg/kg body weight).
In a chronic toxicity study by Johnson et al. (1981), groups of
Fischer-344 rats (75 of each sex per dose level) were administered
amitrole. Group A were the controls. Group B rats were fed 5 mg
amitrole/kg in their diet during weeks 1-39 and then 100 mg/kg
during weeks 40-115 (for males) or 40-119 (for females). Rats in
groups C, D and E received amitrole in their diet at pulsed
intervals (alternate 4-week periods) of 1, 3 and 10 mg/kg,
respectively, during weeks 1-39 and 20, 60 and 100 mg/kg,
respectively, during weeks 40-115 (for males) or 40-199 (for
females). There were no treatment-related clinical signs of toxicity
or changes in body weight or food consumption. There were no
consistent effects on serum T3 and T4 levels. Thyroid weight was
increased in both males and females in groups B and E after 60 weeks
and at termination. There were no treatment-related pathological
changes up to 36 weeks (when only the lower dose levels were
administered). Follicular epithelial hyperplasia in the thyroid was
noted in groups B, D and E and to a much lesser extent in group C.
An increased incidence in thyroid tumours was observed in male and
female rats of groups B and E and in the male animals of group D.
There was no significant difference in tumour incidence between
groups B and E. It should be noted that this study was poorly
reported.
When amitrole was administered to groups of Wistar rats (75 of
each sex) at concentrations in the feed of 0, 1, 10 or 100 mg/kg, no
effect on body weight gain or food intake was observed but a slight
decrease in survival time was found at 100 mg/kg. Thyroid weight was
increased at 100 mg/kg as was uptake of 131I by the thyroid,
measured 24 h after oral administration of 131I. For this
measurement, five animals of each sex per group were killed at 3, 6,
12 and 24 months. PBI, measured as the ratio between radioactivity
in plasma protein and total plasma, was not affected. At the highest
dose level, elevated incidences of haemorrhage and hyperaemia of the
pituitary gland, as well as a very high rate of cystically dilated
thyroid follicles, were seen. The tumour incidence is given in
section 7.7.2. The NOAEL was 10 mg/kg diet, equivalent to 0.57
(males) or 0.85 (females) mg/kg body weight (Weber & Patschke 1978;
Steinhoff & Boehme, 1979a).
Authors who have studied the time-course of the response of the
thyroid to amitrole treatment (e.g., Strum & Karnovsky, 1971; Tsuda
et al., 1973; Wynford-Thomas et al., 1983) have shown that, after a
short lag phase of a few days, there is a rapid rise in TSH that is
paralleled by thyroid hypertrophy and hyperplasia. These effects
peak and plateau after 3-4 months and thereafter remain relatively
stable despite further exposure. A number of studies have shown that
the goitrogenic action of amitrole is reversible on cessation of
exposure (Jukes & Shaffer, 1960).
7.3.1.3 Other species
Other species in which long-term amitrole treatment has been
studied are the hamster and dog.
In a carcinogenicity study on hamsters (Steinhoff & Boehme,
1978; Steinhoff et al., 1983; see section 7.7), there were no
pathological changes at dose levels of up to 100 mg/kg diet. In a
one-year study in dogs, amitrole was given in capsules at dose
levels of 0, 0.25, 1.25, 2.50 and 12.5 mg/kg body weight per day,
6 days/week. There were no clinical signs of toxicity or
pharmacological effects. Haematological, biochemical and urinalysis
parameters were comparable to those of control dogs and were within
normal limits. The dogs fed 12.5 mg/kg per day had a pale pancreas.
Histopathological examination of all dogs did not reveal any
treatment-related effects. The thyroid, in particular, was normal at
all dose levels (Weir, 1958; Hodge et al., 1966).
7.3.2 Other routes
In a chronic 104-week study, 25 male and 25 female rats
(Charles River strain) were exposed (head-nose only) to an amitrole
aerosol (the purity of the amitrole used was not specified) for one
hour each week. An aqueous 0.24% (w/v) solution of amitrole was used
to generate the aerosol. The mean analytical concentration in the
inhalation chamber was 2 mg aerosol per litre of air; based on dry
amitrole, the level was 5 µg/litre air. A control group was exposed
to a water aerosol. No differences between the control group animals
and those exposed to the test substance were found in the mortality,
appearance, behaviour or body weight development. No
treatment-related changes were observed at necropsy. No differences
in the thyroid or liver weights, or in the incidence of tumours,
existed between the two groups of animals (Grapenthien, 1972).
In an inhalation study involving intermittent treatment, groups
of 75 Fischer rats per dose and of each sex were exposed to aerosols
at nominal amitrole levels of 0, 50, or 500 µg/litre air (the purity
of the amitrole used was not specified). The actual amitrole
concentrations in the low-dose group varied between 15.8 and
32.2 µg/litre air on different days of exposure, and the levels
measured in the high-dose group ranged between 97.9 and
376.4 µg/litre air. The animals were exposed for 5 h per day on 5
days per week. The treatment phases during weeks 1-13, 40-52 and
78-90 were interrupted by treatment-free intervals. Interim
necropsies of five animals per dose group and of each sex were
performed after 3, 9 and 18 months, and the study was concluded
after 24 months. A total of 28 rats died in week 51 due to a defect
in the air conditioning system, which led to an increase in the room
temperature. Treatment of the high-dose group was thereupon
concluded, and the surviving animals were necropsied.
The food intake and body weight gain were decreased in the
high-dose group, and the rate of mortality was elevated.
Decreases in the T3 (significant) and T4 (non-significant)
levels were only observed in the high-dose group, this being
assessed in the 13th week of the study. However, values in the
amitrole-treated animals were greater than, or equal to, those of
control rats at all other test dates (weeks 39, 52, 78, 91 and 104).
Epithelial hyperplasia of the thyroid follicles was observed in both
dose groups at the end of the first treatment interval (week 13).
This observation was no longer made after a treatment-free interval
of 24 weeks, but the thyroid weights relative to those in the
control animals were elevated in both dose groups. Follicular
epithelial hyperplasia was again present in most of the animals of
both treatment groups at the end of the second treatment phase (week
51). This observation was still made after a treatment-free interval
of 26 weeks, which indicates that complete reversion no longer
occurred at this time at an amitrole level of 50 µg/litre air.
Neoplasms of the thyroid (adenomas and adenocarcinomas) were found
in addition to hyperplasia at terminal necropsy (Becci, 1983).
Twenty-five male and 25 female rats (Charles River strain) were
dermally exposed to an 0.239% aqueous solution of amitrole (the
purity of the amitrole used was not specified) once weekly for 30
min over a period of 23 months (total of 100 exposures). The
treatment volume was 1 ml/kg body weight, and about 20% of the body
area was treated. The dermal exposure to amitrole thus amounted to
2.39 mg/kg body weight per week. The treatment did not cause skin
damage. No differences between the control group and animals exposed
to the test substance were found with respect to mortality,
appearance, behaviour or body weight development. No
treatment-related alterations were determined at necropsy. No
differences in the thyroid or liver weights, or in the incidence of
tumours, were found between the two groups of animals (Rausina,
1972).
7.4 Skin and eye irritation; skin sensitisation
The potential for dermal irritation by amitrole was examined in
rabbits over a 24-h period following a single application of between
10 and 100 mg/kg body weight (Elsea, 1954). Mild erythema was
observed at the high-dose level only. By 48 h, the skin appeared
normal. The potential for eye irritation by amitrole was examined in
rabbits following application of 3 mg into the conjunctival sac of
the left eye (Elsea, 1954). Observations were made at 1, 4, and 24 h
and at daily intervals for 6 days. Mild irritation was observed at
4 h in all animals, but the majority of animals had recovered by
24 h.
Amitrole was tested for possible dermal sensitization potential
in guinea-pigs using the Magnusson-Kligman maximization test with
Freund's adjuvant. The concentrations employed were 2.5% for
intracutaneous induction, 25% for topical induction, and 12% for the
first and second challenges. Evidence for moderate skin-sensitizing
potential in amitrole was found after both challenges (Mihail,
1984).
No skin-sensitizing effect was observed in a Klecak open
epicutaneous test involving treatment of three groups of animals
with 3%, 10% or 30% amitrole formulations in the induction phase
(Mihail, 1985).
7.5 Reproduction, embryotoxicity and teratogenicity
7.5.1 Reproduction
In a preliminary one-generation reproduction study by Gaines
et al. (1973), groups of 10 male and 10 female rats were fed
amitrole in the diet at concentrations of 0, 500 or 1000 mg/kg for
55 days before pair-mating. The offspring were weaned at 21 days.
Complete autopsies were performed on the parents after a total
exposure of 107-110 days. Ten weaning rats from each dose groups
were killed. Mean body weight gain was reduced at all dose levels.
The average number of pups per litter was significantly reduced at
all dose levels, as were the number surviving to weaning. The body
weight of pups at weaning was also reduced. Relative kidney, spleen
and liver weights were also reduced in parents following treatment,
while thyroid hyperplasia was noted in all treated animals.
In a subsequent multi-generation study by Gaines et al. (1973),
groups of 10 male and 10 female rats were fed amitrole at dietary
levels of 0, 25 or 100 mg/kg for 61 and 173 days before pair-mating
to produce the F1A and the F1B generations, respectively. The
thymus and spleen were examined in weanling rats in the F1A
generation. There was no treatment-related effect on body weight
gain in the FO animals. Hyperplasia of the thyroid was observed in
all animals at the highest dose level but not at 25 mg/kg.
Reproduction parameters were normal at these dose levels. A slight
decrease in body weight gain was noted at 25 and 100 mg/kg in pups
of the F1A and F1B generation. Pathological examination revealed
a slight but significant decrease in liver weight at 25 and
100 mg/kg (male pups) and at 100 mg/kg (female pups). The thymus and
spleen sizes were normal and no histopathological changes could be
detected. F2A generation rats showed a decrease in the number of
litters at 100 mg/kg but there were no other changes, such as
survival to weaning and mean body weight at weaning.
7.5.2 Embryotoxicity and teratology
Teratology studies have been performed in rats, mice and
chickens.
In a study by Gaines et al. (1973), three groups of pregnant
rats were administered amitrole by gavage at dose levels of 0, 20 or
100 mg/kg body weight per day on days 7 to 15 of gestation, and the
animals were allowed to litter and to wean. There was no evidence of
gross abnormalities among the pups.
In a further teratology study on rats by Machemer (1977b),
groups of 20 presumed pregnant rats (strain FB30, Long-Evans) were
administered amitrole by gavage at dose levels of 0, 100, 300 or
1000 mg/kg body weight per day on days 6 to 15 of gestation, and
fetuses were examined on day 20 of gestation. There were no deaths
or signs of toxicity at any dose level. Body weight gain was not
affected by treatment. There were no treatment-related effects on
the resorption rate, fetal weight, number of live fetuses, placental
weight or sex ratio. There was no treatment-related increase in
gross, skeletal or visceral malformation.
In a study by Tjalve (1974), pregnant mice were administered
amitrole at 500, 1000, 2500 and 5000 mg/litre in the drinking-water
on days 6-18 of pregnancy. There was a marked decrease (22-28%) in
body weight gain in the dams and pronounced retardation in the
development in their fetuses at dose levels > 1000 mg/litre. At
the highest dose level used, maternal toxicity was associated with
an increase in the rate of resorption. No teratogenic effects were
observed at any dose level.
Teratogenicity in chickens was investigated by injecting the
yolk sac of eggs with amitrole at dose levels of between 0.5 and
40 mg at 0, 24, 48 and 96 h of incubation (Landauer et al., 1971).
Dose-dependent abnormalities of the beak were found to be present in
chickens following the administration of 20-40 mg amitrole at 24 and
48 h. When injected after 96 h of incubation, beak abnormalities
could be found at dose levels of 10, 20 and 40 mg at a rate of 20,
48, and 60%, respectively. No effects were seen at dose levels up to
and including 2 mg/egg.
7.6 Mutagenicity and related end-points
A referenced summary of the test results with amitrole is given
in Table 4. The important features of these data are described
below.
7.6.1 DNA damage and repair
The possibility of DNA damage being induced by amitrole has
been investigated frequently and in a number of different ways. In
bacteria, the results have been negative, except in one experiment
with the rec assay, in which exogenous metabolic activation was
provided by "liver" preparations from a mollusc and a fish. Among
assays which could be evaluated, a DNA repair assay in yeast gave a
positive result, as did a repair assay in mammalian cells.
7.6.2 Mutation
One study in a single laboratory with Escherichia coli and
Salmonella typhimurium strains gave significant responses (Venitt
& Crofton-Sleigh, 1981). Amitrole did not induce joint mutations in
histidine-requiring mutants of S. typhimurium (Andersen et al.,
(1972). An equivocal response was obtained in another bacterial
mutation assay, but many other in vitro assays gave negative
results. A significant result was obtained in a mouse peritoneal
host-mediated assay with S. typhimurium (Simmon et al., 1979). No
mutation induction has been observed in yeast or fungi. In
Drosophila melanogaster, a significant response was obtained with
a wing-spot test in a single study, but not with several sex-linked
recessive assays. Mutations were not induced in mouse lymphoma
cells, but hprt locus and Na+/K+ ATPase locus mutations were
induced in Syrian hamster embryo cells (Tsutsui et al., 1984). These
latter results may hold particular significance in view of other
properties of these cells described in sections 7.6.4 and 7.6.5.
7.6.3 Chromosome damage
In yeast, there is conflicting evidence for recombinogenic
activity (intragenic and mitotic recombination), while numerical
chromosomal aberrations were induced in three assays. Structural
chromosomal damage was not induced by amitrole in cultured mammalian
cells, but the frequency of sister-chromatid exchanges was increased
in a single study. No effects of amitrole were observed in mice
subjected to bone marrow micronucleus tests or male dominant lethal
tests.
7.6.4 Cell transformation
Assays for anchorage-independent growth and cell transformation
in several systems consistently gave positive results.
Table 4. Summary of mutagenicity and related end-point studies on amitrole
Test Organism Result LED or HIDe Reference
-S9h +S9i
Microorganisms
Prophage induction E. coli 58-161 enVA, lambda n.t. - 10 000 µg/ml Thomson (1981)
prophage
Prophage induction E. coli GY5027, GY4015 n.t. - 2000 µg/plate Mamber et al. (1984)
Rec assay Bacillus subtilis H17 rec+, M45 rec- - n.t. µg/plate Shirasu et al. (1976)
Rec assay Bacillus subtilis H17 rec+, M45 rec- - +a 1000 µg/plate Kada (1981)
Rec assay E. coli JC 2921, 9238, 8471, 5519, - - 500 µg/ml Ichinotsubo et al. (1981)
7623, 7689
Rec assay E. coli WP2, WP100 n.t. - 4000 µg/ml Mamber et al. (1983)
Pol assay E. coli pol A1, pol A+ - n.t. µg/plate Bamford et al. (1976)
Pol assay E. coli WP3110, p3478 - - 333 µg/plate Rosenkranz et al. (1981)
Differential killing E. coli WP2, WP67, CM 871 - - 1000 µg/ml Tweats (1981)
Reverse mutation S. typhimurium TA1535, TA1537, TA1538 - - 100 µg/plate Brusick (1975)
Reverse mutation S. typhimurium TA1535, TA1538, - - 1000 µg/plate Prince (1977)
TA98, TA100
Reverse mutation S. typhimurium TA1535, TA1536, - - 2000 µg/plate Carere et al. (1978)
TA1537, TA1538
Table 4 (contd).
Test Organism Result LED or HIDe Reference
-S9h +S9i
Reverse mutation S. typhimurium TA1535, TA1538 - - 250 µg/plate Rosenkranz & Poirier
(1979)
Forward mutation S. typhimurium TM 677 - - 100 µg/ml Skopek et al. (1981)
Reverse mutation S. typhimurium TA1535, TA1537, - - 12 500 µg/plate Herbold (1980)
TA98, TA100
Reverse mutation S. typhimurium TA1535, TA1537, - - 2000 µg/plate Brooks & Dean (1981)
TA1538, TA98, TA100, TA92
Reverse mutation S. typhimurium TA1537, TA98, TA100 - - 5000 µg/plate MacDonald (1981)
Reverse mutation S. typhimurium TA1535, TA1537, TA1538, - - 10 000 µg/plate Richold & Jones (1981)
TA98, TA100
Reverse mutation S. typhimurium TA1535, TA1537, TA1538, - - 2000 µg/plate Rowland & Severn (1981)
TA98, TA100
Reverse mutation S. typhimurium TA1535, TA1537, TA1538, n.t. - 2500 µg/plate Trueman (1981)
TA98, TA100
Reverse mutation S. typhimurium TA98, TA100 n.d. + f Venitt & Crofton-Sleigh
(1981)
Reverse mutation S. typhimurium TA98, TA100 ± ± 500 µg/ml Hubbard et al. (1981)
Reverse mutation S. typhimurium TA1535, TA1537, TA98 - - 1000 µg/ml Gatehouse (1981)
Reverse mutation S. typhimurium TA1535, TA1537, TA1538, - - 5000 µg/plate Moriya et al. (1983)
TA98, TA100
Table 4 (contd).
Test Organism Result LED or HIDe Reference
-S9h +S9i
Reverse mutation S. typhimurium TA1535, TA1537, TA1538, - - 333 µg/plate Dunkel et al. (1984)b
TA988, TA100
Reverse mutation E. coli WP2, WP2uvrA nd + f Venitt & Crofton-Sleigh
(1981)
Reverse mutation E. coli WP2uvrA - - 500 µg/plate Gatehouse (1981)
Reverse mutation E. coli WP2uvrA - - 5000 µg/plate Moriya et al. (1983)
Reverse mutation E. coli WP2uvrA - - 333 µg/plate Dunkel et al. (1984)b
Forward mutation E. coli CHY832 + - 2500 µg/ml Hayes et al. (1984)
Forward mutation Streptomyces coelicolor A3(2) ± n.t. µg/plate Carere et al. (1978)
Host mediated S. typhimurium TA1950 in NMRI mouse - n.t. 2900 µmol/kg Braun et al. (1977)
reverse mutation
Host mediated S. typhimurium TA1530, TA1535, TA1538 + n.t. 1585 mg/kg i.p. Simmon et al. (1979)
reverse mutation in Swiss-Webster mouse
DNA repair Saccharomyces cerevisiae 197/2d + - 100 µg/ml Sharp & Parry (1981b)
rad 3, rad 18, rad 52, trp 2
Reverse mutation S. cerevisiae D4 - - 100 µg/plate Brusick (1975)
Reverse mutation S. cerevisiae XV 185-14C - - 889 µg/ml Mehta & von Borstel (1981)
Table 4 (contd).
Test Organism Result LED or HIDe Reference
-S9h +S9i
Forward mutation Aspergillus nidulans 35 - n.t. 2000 µg/ml Bignami et al. (1977)
Mitotic S. cerevisiae T1, T2 - - 1000 µg/ml Kassinova et al. (1981)
recombination
Mitotic gene S. cerivisiae D7 - - 12 500 µg/ml Zimmerman & Scheel
conversion (1981)
Mitotic gene S. cerivisiae JD1 + - 300 µg/ml Sharp & Parry (1981a)
conversion
Nondisjunction A. nidulan P ± ± 2000 µg/plate Bignami et al. (1977)
Mitotic A. nidulans P ± ± 2000 µg/plate Bignami et al. (1977)
recombination
Nondisjunction A. nidulans P + n.t. 400 µg/ml Morpurgo et al. (1979)
Nondisjunction A. nidulans strain P1 - n.t. 120 mM Crebelli et al. (1986)
Forward mutation A. nidulans strain 35 - n.t. 72 mM Crebelli et al. (1986)
Mitotic A. nidulans P1 - n.t. 120 mM Crebelli et al. (1986)
recombination
Mitotic gene S. cerevisiae D4 - - 333 µg/ml Jagannath et al. (1981)
conversion
Mitotic S. cerevisiae RS112 - n.t. 60 000 µg/ml Schiestl et al. (1989)
recombination
Table 4 (contd).
Test Organism Result LED or HIDe Reference
-S9h +S9i
Mitotic S. cerevisiae T1 and T2 - - 1000 µg/ml Kassinova et al. (1981)
recombination
Insectsg
Nondisjunction Drosophila melanogaster females - 10 mg/kg diet Laamanen et al. (1976)
Sex linked recessive D. melanogaster males - 10 mg/kg diet Laamanen et al. (1976)
lethal
Sex linked recessive D. melanogaster males - 20 000 mg/kg diet Vogel et al. (1981)
lethal
Sex linked recessive D. melanogaster - 10 mg/kg diet Sorsa & Gripenberg (1976)
lethal
Sex linked recessive D. melanogaster males - 48 000 mg/kg feed Mason et al. (1992)
lethal
Sex linked recessive D. melanogaster males - 12 500 mg/kg Mason et al. (1992)
lethal injection
Somatic mutation D. melanogaster + 1mM Tripathy et al. (1990)
(wing spot test)
Cultured mammalian cells
Unscheduled DNA Hela cells - + 100 µg/ml Martin & McDermid (1981)
synthesis
Table 4 (contd).
Test Organism Result LED or HIDe Reference
-S9h +S9i
Mutation, tk locus Mouse lymphoma L5178Y cells - - 5000 µg/ml McGregor et al. (1987)
Mutation, hprt locus Syrian hamster embryo cells + n.t. 0.3 µg/ml Tsutsui et al. (1984)
Mutation, Syrian hamster embryo cells + n.t. 0.3 µg/ml Tsutsui et al. (1984)
Na+/K+ATPase locus
Sister chromatid Chinese hamster ovary cells - + 0.1 µg/ml Perry & Thomson (1981)
exchange
Chromasomal Human lymphocytes - n.t. 10 000 µg/ml Meretoja et al. (1976)
aberrations
Anchorage BHK-21 cells n.t. + 25 µg/ml Styles (1979)
independent growth
Anchorage BHK-21 cells n.t. + 250 µg/ml Styles (1981)
independent growth
Anchorage BHK-21 cells - - 4000 µg/ml Daniel & Dehnel (1981)
independent growth
Cell transformation BALBc/3T3 cells c n.t. 2500 µg/ml Brusick & Weir (1976)
Cell transformation Syrian hamster embryo cells ± n.t. 50 µg/ml Inoue et al. (1981)
Cell transformation Syrian hamster embryo cells + n.t. 10 µg/ml Dunkel et al. (1981)
Cell transformation Syrian hamster embryo cells + n.t. 0.3 µg/ml Tsutsui et al. (1984)
Table 4 (contd).
Test Organism Result LED or HIDe Reference
-Sy9h +S9i
Cell transformation Rat embryo cells/Rauscher-MULV infected + n.t. 100 µg/ml Dunkel et al. (1981)
Cell transformation Mouse embryo C3H 10T ´ fibroblasts d n.t. 125 µg/ml Dunkel et al. (1988)
Mammals in vivog
Bone marrow CD-1 mouse - 500 mg/kg per day Tsuchimoto & Matter (1981)
micronucleus x2 i.p.
Bone marrow NMRI mouse - 10 000 mg/kg Herbold (1982)
micronucleus per day x1
Sperm morphology (CBA x BALBc)F, mouse - 500 mg/kg per day Topham (1980)
x5 i.p.
Dominant lethal NMRI mouse, male - 1000 mg/kg x1 Machemer (1977a)
Dominant lethal Ha(ICR) mouse, male - 100 mg/kg diet x49 Knickerbocker & Stevens
(1978)
a Liver S9 from Japanese clam and Yellowtail fish
b Results from four laboratories presented
c Positive in one of three tests
d Positive in one of two laboratories
e LED = lowest effective dose (for results scored as positive); HID = highest ineffective dose (for results scored as negative)
f Results given as slope of linear regression
g In in vivo experiments no testing with exogenous metabolic activation
h Without exogenous activation
i With exogenous activation
n.t. not tested
n.d. tested, but data not shown
+ positive response
± weak positive response
- no response
7.6.5 Other end-points
Amitrole had no effect upon testicular DNA synthesis (Seiler,
1977) and did not increase the frequency of morphologically abnormal
sperm in mice.
Prostaglandin-H-synthetase (PHS) and lactoperoxidase catalyse
the binding of 14C-amitrole to protein and transfer RNA in vitro,
and protein binding occurs in the presence of rat and pig thyroid
peroxidase (Krauss & Eling, 1987). These authors suggested that PHS
activity in Syrian hamster embryo cells may be important for the
mutagenic and transforming potential of amitrole seen with these
cells. It is known, for example, that PHS is involved in the
peroxidative metabolism of diethylstilbestrol in these cells and
that structure-activity studies suggest that this type of metabolism
is important for its biological effects (Degen et al., 1983).
7.7 Carcinogenicity
7.7.1 Mouse
Groups of 50 male and 50 female C3H/Anf mice, 2-4 months old,
were injected subcutaneously on a single occasion with 0.02 ml of a
suspension of 50 mg amitrole per ml trioctanoin, or dosed once on a
shaved area of the dorsal skin each week of the experiment with
0.2 ml acetone, 0.2 ml acetone containing 0.1 mg amitrole or 0.2 ml
of a methanol:acetone (35:65) mixture containing 10 mg amitrole
(Hodge et al., 1966). The mice were observed until death. The skin
alone was examined for tumours, but none were found in any group,
including the controls.
In a study by Innes et al. (1969), which investigated the
carcinogenicity of some 120 chemicals, amitrole was used as a
positive control. Groups of (C57BL/6 x C3H/Anf)F1 mice (18 of each
sex) and (C57BL/6 x AKR) F1 mice (18 of each sex) were given
amitrole by gavage at a dose level of 1000 mg/kg per day on days
7-28 of age and in the diet at a concentration of 2192 mg/kg from
day 28 until the end of the experiment. This was planned to be week
80, but all of the mice in the amitrole-treated groups had died by
weeks 53-60. Thyroid tumours were reported to have occurred in
nearly all of the treated mice (64/72). Liver tumours were observed
in 34/36 (C57BL/6 x C3H/Anf)F1 mice and in 33/36 (C57BL/6 x
AKR)F1 treated mice. In pooled control groups, 8/166 (C57BL/6 x
C3H/Anf)F1 mice and 6/172 (C57BL/6 x AKR)F1 mice had liver
tumours.
Feinstein et al. (1978b) fed normal and acatalasemic female C3H
mice a diet containing 10 000 mg/kg amitrole for up to one year (see
section 7.7.4 for details). Unspecified liver tumours were observed
in all of the 33 mice. No untreated control group was included.
A carcinogenicity study in mice was reported by Steinhoff &
Boehme (1979b) and Steinhoff et al. (1983). Groups of NMRI mice (75
of each sex per dose level) were administered amitrole (97% pure) in
the diet at concentrations of 0, 1, 10 or 100 mg/kg throughout their
lifetime. There was no treatment-related effect on appearance, body
weight or food consumption. Histological examination of tissues of
the major organs did not reveal any treatment-related effects apart
from a slight increase in the number of hyperaemias in the
pituitary: 3 in controls, 5 at 1 mg/kg, 2 at 10 mg/kg and 16 at
100 mg/kg. There was no increase in the incidence of
treatment-related tumours.
Three groups of B6C3F1 mice were fed a diet containing 500 mg
amitrole/kg: Group 1, from day 12 of gestation until delivery; Group
2, from delivery until weaning; and Group 3, from weaning until 90
weeks (Vesselinovitch, 1983). Although not specifically stated,
information on other chemicals tested and described in this report
(benzidine, safrole, ethylenethiourea and diethylnitrosamine)
implied that the mice exposed in utero (Group 1) and during
lactation (Group 2) were also observed until 90 weeks. The incidence
of hepatocellular adenomas and carcinomas, respectively, were: Group
1 males, 4/74 and 2/74; Group 2 males, 6/45 and 4/45; Group 3, 15/55
and 11/55; Group 1 females, 0/83 and 0/83; Group 2 females, 0/55 and
0/55; Group 3 females, 5/49 and 4/49. The incidence of these tumours
in untreated B6C3F1 mice (which may or may not have been
concurrent controls) at 90 weeks were: males 1/98 and 0/98; females
0/96 and 0/96; other organs were not examined. It was concluded that
there was no effect of amitrole treatment in Group 1 or in females
of Group 2, but that marginal increases occurred in males of Group 2
and in males and females of Group 3.
The comparative susceptibilities of three mouse strains to the
development of preneoplastic hepatic lesions were examined following
amitrole administration in the drinking-water at 10 000 mg/litre
(Mori et al., 1985). The strains were DS, ICR (Crj: CD-1) and NOD,
which was derived from ICR and found to develop spontaneous
insulitis followed by diabetes. There were indications that the NOD
mice may have carried immunological abnormalities. In each of two
experiments, three groups of female mice were administered amitrole
in the drinking-water for 3 months (experiment 1) or 6 months
(experiment 2), after which they were killed and their livers
examined. The proportions of mice with hyperplastic nodules were: in
experiment 1, 15/19 NOD, 3/5 DS and 0/5 ICR; and, in experiment 2,
19/19 NOD, 18/18 DS and 17/19 ICR. A single hepatocellular carcinoma
developed in an NOD mouse after six months of treatment.
7.7.2 Rats
In the study by Keller (1959) (see section 7.3.1.2), thyroid
tumours were present in 1/10 animals of the 10-mg/kg group, 2/15 at
50 mg/kg and 15/27 at 100 mg/kg at the end of the study. No tumours
were detected in five control group animals but one rat exhibited
early stages of an adenoma. One thyroid gland from the 50-mg/kg
group and four from the 100-mg/kg group exhibited lesions
interpreted as adenocarcinomas by several pathologists and benign
neoplasms by others. This study has also been described by Jukes &
Shaffer (1960).
The thyroid carcinogenicity of amitrole in the rat was further
demonstrated in a study by Doniach (1974) where groups of Hooded
Lister strain rats were administered amitrole in the drinking-water
at a concentration of 1000 mg/litre for 18-20 months. All
amitrole-treated animals (20) developed follicular adenomas and 2/20
animals also developed follicular carcinomas. In studies designed to
investigate the mechanism of thyroid carcinogenesis, Tsuda et al.
(1976, 1978) also demonstrated thyroid tumours in rats after
administration of amitrole in the drinking-water at 2500 mg/litre.
In the study of Steinhoff & Boehme (1979a) described in section
7.3.1.2, both benign and malignant tumours of the thyroid were found
at 100 mg/kg diet. In the pituitary gland there was also an increase
in the incidence of benign tumours at 100 mg/kg. This study was also
described in a report by Steinhoff et al (1983).
In the study by Johnson et al. (1981) described in section
7.3.1.2, follicular thyroid neoplasms were observed in Fischer rats
in the groups that received amitrole in pulsed doses (alternate 4
week periods) of 60 and 200 mg/kg diet and in the group that
received continuous doses of 100 mg/kg diet for 115-119 weeks.
The histological development of thyroid tumours in rats
following amitrole treatment has been studied by Wynford-Thomas
et al. (1983). Groups of 10 male Wistar rats were administered
amitrole in the drinking-water at a concentration of 0.1%, and five
animals were killed at 7 months and five animals at 1 year. After 7
months, 3 tumours were found in 1 of the 5 animals. After 1 year, 16
microtumours were found in 4 of the 5 animals. Most of the tumours
were well-defined adenomas. Their most striking feature was their
vascularity, with the follicles being surrounded by greatly enlarged
vascular spaces lined by endothelium and dilated capillaries.
7.7.3 Other species
The carcinogenicity of amitrole in hamsters was investigated in
a lifetime dietary study by Steinhoff & Boehme (1978). Groups of
golden hamsters (76 of each sex per dose level) were administered
amitrole (97.0% pure) in the diet at concentrations of 0, 1, 10 or
100 mg/kg throughout their lifetime. There was no treatment-related
change in appearance. Body weight gain was decreased at 100 mg/kg
from day 400 onward, and mortality was significantly increased in
the 100-mg/kg groups. Severe amyloidosis of the kidneys was the
major cause of death and was observed in all groups. There was no
evidence of amitrole-related histopathological changes nor of a
treatment-related carcinogenic effect. This study was also described
in a report by Steinhoff et al. (1983).
7.7.4 Carcinogenicity of amitrole in combination with
other agents
Two groups of 30 male albino rats, 2-3 months of age, were fed
0.06% 4-dimethylaminoazobenzene (DAB) in the diet, and one group
also received intraperitoneal injections of amitrole (purity
unspecified; 1000 mg/kg body weight) every second day as a 10%
solution in water. The surviving 16 DAB-treated and 19
DAB-plus-amitrole-treated rats were killed at 21 weeks. The
incidence of liver tumours was 12/16 in the group receiving DAB
alone and 4/19 in the group that received DAB plus amitrole (P <
0.01). The liver carcinomas produced by DAB alone were mostly
hepato-cellular carcinomas, whereas those in the group treated with
DAB plus amitrole were hepatocellular carcinomas and
cholangiocarcinomas (Hoshino, 1960).
Groups of 12 six-week-old male Wistar rats were given the
following treatments: Group 1 received four weekly subcutaneous
injections of N-bis(2-hydroxypropyl) nitrosamine (DHPN) (700 mg/kg
body weight) followed by a diet containing amitrole (purity
unspecified; 2000 mg/kg diet) for an additional 12 weeks; Group 2
received four weekly injections of DHPN only; Group 3 was fed only a
diet containing 2000 mg amitrole/kg beginning at week 4 for 12
weeks; Group 4 received eight weekly injections of DHPN followed by
a diet containing 2000 mg amitrole/kg for 12 weeks; Group 5 received
eight weekly injections of DHPN only; Group 6 was fed a diet
containing 2000 mg amitrole/kg beginning at week 8; and Group 7 was
fed a standard diet and served as untreated controls. All animals
were killed after 20 weeks. No thyroid tumours were found in rats in
Groups 2, 3, 6 or 7, but a significantly increased incidence (P <
0.05) of thyroid tumours compared with that in controls was observed
in rats in Group 1 (9/11), 4 (12/12) and 5 (7/12). Hyperplasia of
the thyroid was not observed in Groups 2 and 7, but occurred in 27%
of Group 1, 25% of Group 3 and 100% of Groups 4, 5 and 6 (Hiasa
et al., 1982).
Seventy-five male Wistar-Furth rats were castrated at 40 days
of age and divided into six groups: Group 1 (five rats) received no
treatment and served as controls; Group 2 (ten rats) was given
amitrole (purity unspecified; 1500 mg/litre) in the drinking-water,
starting at 47 days after castration; Group 3 (ten rats) received
subcutaneous implantation of a pellet on the back containing 5 mg
diethylstilbestrol (DES) plus 45 mg cholesterol, which was replaced
every two months; Group 4 (11 rats) received implantations of DES
pellets every two months plus amitrole in the drinking-water; Group
5 (20 rats) received implantation of the DES pellet followed by
administration of 5 mg N-nitrosobutylurea (NBU) per day in the
drinking-water for 30 days, starting at 50-55 days of age; and Group
6 (19 rats) received implantations of the DES pellet followed by
administration of NBU and, subsequently (seven days after NBU
treatment), amitrole in the drinking-water. Moribund or dead rats
were completely autopsied. All survivors were killed 12 months after
the initial NBU treatment. Rats in Groups 3 and 5 developed numerous
hepatocellular carcinomas and neoplastic nodules (4/9 and 15/17
animals, respectively) and pituitary tumours (7/9 and 12/17,
respectively). Addition of amitrole to these regimens (Groups 4 and
6, respectively) had no effect on the incidence of pituitary tumours
(8/11 and 10/14) but slightly (Group 4; 2/11) and significantly
(Group 6; 3/14) reduced the incidence of hepatocellular carcinomas
and neoplastic nodules. No liver tumour were found in controls
(Group 1) or in rats receiving amitrole only (Group 2) (Sumi et al.,
1985).
In experiments with acatalasemic and normal C3H mice, groups of
42-47 females were treated at weaning with neutron irradiation (80
rads at 15 rads/min) or fed a diet containing 10 000 mg amitrole/kg
or both (Feinstein et al., 1978b). In order to extend survival, the
amitrole diet was offered during the first 4 weeks of a 5-week
cycle, which was repeated continuously. The experiment was
terminated one year after weaning. Of the mice killed at the end of
the experiment, unspecified liver tumours were found in 2/37 of the
neutrons group, 29/29 of the neutrons + amitrole group and 33/33 of
the amitrole alone group. In contrast, there were significant
reductions in the proportions of mice with Harderian gland or
ovarian tumours in the amitrole-treated groups: Harderian gland
tumours - 12/37 of the neutrons group, 0/29 of the neutrons +
amitrole group and 0/33 of the amitrole alone group. It was also
demonstrated that, when these mice were infected with the murine
mammary tumour virus, almost 100% eventually developed mammary
tumours. If acatalasemic, virus-infected mice were kept on a diet
containing 10 000 mg amitrole/kg from weaning for 12 weeks, then the
time of initial appearance of mammary tumours in 50% of the mice was
delayed from 35 weeks in 29 control diet mice to 50 weeks in 28
amitrole-treated mice.
7.8 Other special studies
7.8.1 Cataractogenic activity in rabbits
The cataractogenic activity of amitrole administered in the
diet or drinking-water to rabbits was investigated by Bhuyan et al.
(1973). A group of four rabbits was fed on a diet containing 0.2%
amitrole while four litter-mates were maintained as a control. Body
weight gain was significantly lower than in controls from 13 weeks
to 25 weeks. All treated animals developed typical bilateral
lenticular changes in the form of cataracts. Initial changes to the
eyes were seen during 2-4 weeks with widening of lens sutures,
individualization of lens fibres and posterior cortical vacuoles.
The opacity progressed towards other regions during 4-8 weeks.
Complete involvement of the posterior cortical, post-equatorial,
equatorial, and anterior cortical regions occurred after a period of
10-16 weeks. After approximately 20 weeks, there was no further
advancement in lenticular opacity.
A separate group of 24 rabbits was kept on continuous 0.2%
amitrole in the drinking-water, while 15 rabbits were kept as
controls. The body weight of the treated animals was significantly
lower at 13 weeks. Of the 24 treated rabbits, 22 developed typical
bilateral lenticular changes in the form of cataracts. The time
course of the changes was as described for the dietary treatment.
7.8.2 Biochemical effects
The effect of amitrole on the import of catalase into
peroxisomes of cultured fibroblasts derived from patients with
peroxisome biogenesis disease has been investigated. The catalase
import is inhibited by prior binding of amitrole to catalase, which
appears to retard unfolding of the protein (Middelkoop et al.,
1991).
Brain catalase activity has been shown to be inhibited in vivo
in rats in a dose-related manner (3 doses) by amitrole in saline
solution administered intraperitoneally. Inhibition occurred within
3-6 h after administration. The activity recovered completely 24 h
following administration of the lowest dose (0.0625 g/kg), while a
period of more than 48 h was needed for larger doses. The pattern of
inhibition and recovery was identical in animals from all dose
groups. The presence of amitrole in the brain was confirmed over the
time period of the observed inhibition of brain catalase (Aragon
et al., 1991b).
It has been proposed that ethanol can be oxidized in the brain
via the peroxidatic activity of catalase and that centrally formed
acetaldehyde may mediate several of the psychopharmacological
actions of ethanol. The study by Aragon et al. (1991a) was designed
to investigate the role of brain catalase in the mediation of
ethanol-induced narcosis, hypothermia and lethality in rats. Rats
were pretreated with the catalase inhibitor amitrole (0.25, 0.5 and
1.0 g/kg i.p.) or with saline, and 5 h later, animals in each
pretreatment group received intraperitoneal injections of ethanol
(3 or 4 g/kg). Ethanol-induced narcosis was significantly attenuated
in amitrole-pretreated rats compared to the saline control group. In
addition, amitrole pretreatment significantly reduced the lethal
effect of ethanol. However, the body temperature of
amitrole-pretreated ethanol-injected animals was significantly
reduced as compared to the saline-ethanol animals. Blood ethanol
determinations revealed that amitrole did interfere with ethanol
metabolism. Amitrole significantly inhibited brain catalase activity
at all doses used in this study and the results indicate a role for
brain catalase in ethanol effects. In a separate study (Tampier &
Quintanilla, 1991), rats were intraperitoneally treated with
amitrole (1 g/kg) as a single injection or as seven injections on
consecutive days. The animals were then exposed to ethanol
(2.76 g/kg) intraperitoneally or by gavage as a single or repeated
exposure, respectively. The effect of amitrole on hypothermia,
narcosis induced by ethanol, and the acquisition of tolerance to the
ethanol hypothermic and narcotic effect was studied. It was found
that amitrole blocked the catalase activity of brain (68%) and liver
(96%) when injected intraperitoneally one hour before exposure to
ethanol. When the rats were treated daily with amitrole, a lower
degree of blocking was obtained in the brain (37%) but not in the
liver (97%).
The effect of amitrole on brain catalase activity was studied
in male Long-Evans rats maintained on voluntary consumption of
ethanol (Aragon & Amit, 1992). It was shown that intraperitoneal
injection of amitrole (62.5-1000 mg/kg body weight) resulted in
dose-dependent reduction in ethanol intake. Consumption of water was
not affected by any dose of amitrole, suggesting specific effects of
amitrole on ethanol intake. Analysis of brain catalase activity
demonstrated a direct inhibitory effect of amitrole on brain
catalase activity.
Amitrole is known to cause irreversible inhibition of catalase
(Margoliash et al., 1960), which is thought to regulate the
intercellular peroxide concentration.
Metodiewa & Dunford (1991) investigated the reaction of
amitrole with mammalian haem enzymes, represented by
lactoper-oxidase and bovine liver catalase. The study indicated that
amitrole is a substrate for lactoperoxidase compounds I, II and III
but does not convert catalase compound I to II under conditions
favouring peroxidase activity of enzyme. This work supports the
hypothesis that amitrole reacts with peroxidase compounds I and II.
This reaction appears to be a one-electron oxidation of amitrole.
The observed multi-step base reduction of lactoper-oxidase III by
amitrole has potential physiological relevance since it could help
maintain the peroxidatic cycle of the enzyme (Metodiewa & Dunford,
1991).
The ability of amitrole to affect different
porphyrin-containing compounds suggests a possible interference with
porphyrin synthesis or inhibition of the activity of these
porphyrin-containing compounds. After intraperitoneal administration
of amitrole to DBA mice, it was found that the level of hepatic
delta-aminolevulinic acid dehydratase activity was reduced within 3
to 4 h. This study demonstrated that amitrole is capable of causing
a simultaneous decrease in activity of both hepatic
delta-aminolevulinic acid dehydratase and catalase as well (Tschudy
& Collins, 1957).
Baron & Tephly (1969), however, found that in vitro amitrole
is not a potent inhibitor of (delta)-aminolevulinic acid
dehydratase, and that the degree of inhibition depends on the
concentration of the enzyme. In the same study it was found that
amitrole produces a decrease in the hepatic microsomal cytochrome
P-450 level of nearly 50% approximately 16 h after an
intraperitoneal injection of amitrole (3 g/kg) to the rats.
Cytochrome b5 levels were not affected by this treatment. The
induction of the N-demethylation of ethylmorphine caused by
phenobarbital was inhibited by amitrole. Treatment of rats with
amitrole alone resulted in a decrease of 40% in ethylmorphine
demethylation.
The effects of amitrole on GSH-Px (glutathione peroxidase)
activity and prostaglandin 3 biosynthesis have been investigated
in vitro. Incubation of amitrole (0.002 mol/litre) with rat
erythrocytes was followed by 88% reduction in GSH-Px activity.
Moreover, platelet prostaglandin synthesis was blocked and aorta
prostacyclin-like activity was inhibited (Doni & Piva, 1983).
Buchmuller-Rouiller et al. (1992) demonstrated that amitrole
inhibits macrophage leishmanicidal activity and nitrite secretion
through a process involving inhibition of NO synthetase activity.
The resemblance between the tautomeric form of amitrole and the
guanidino group of L-arginine, the natural substrate for NO
synthetase, might be responsible for this inhibition
(Buchmuller-Rouiller et al., 1992).
Amitrole is reported to inhibit fatty acid synthesis in
isolated rat hepatocytes, an effect at least partially mediated at
the level of acetyl-CoA carboxylase. Cholesterol synthesis is more
markedly inhibited by amitrole (Beynen et al., 1981). However, the
half-maximal inhibition of fatty acid synthesis is approximately
20 mmol/litre and for cholesterolgenesis is approximately
5 mmol/litre. Consequently, these effects are unlikely to be
important in vivo.
Amitrole treatment affects the synthesis not only of
cholesterol but also of primary bile acids in vivo. Thus, amitrole
treatment activated the biosynthesis of chenodeoxycholic acid from
exogenous cholesterol (1.3 fold) but did not affect that of colic
acid. Aminotriazole hardly affected the synthesis of
chenodeoxy-cholic acid through endogenous cholesterol (from
mevalonate), but decreased to 41.2% that of colic acid (Hashimoto
et al., 1992).
7.9 Mechanisms of toxicity - mode of action
Amitrole has been shown to be goitrogenic in several species
including rats, mice, hamsters and hens (Wishe et al., 1979;
Steinhoff et al., 1983). As in the case of certain other goitrogenic
substances, amitrole causes thyroid cancer in rats after prolonged
exposure (section 7.7). A mechanism for these carcinogens in animals
has been proposed which involves the hormonal imbalance caused by
antithyroid effects (Hill et al., 1989). Thyroid hormonal changes
have been produced by low iodine diet, sub-total thyroidectomy,
splenic transplantation of thyroid tissue and by the transplantation
of pituitary tumours that secrete thyroid stimulating hormone (TSH).
In all these cases the incidence of thyroid cancer increases. The
explanation is that excessive hypothalamus-mediated TSH simulation
of the thyroid gland, induced by either hypothyroidism (chemically
or surgically induced) or TSH-secreting tumours, is the cause of
thyroid hypertrophy and hyperplasia. The continuing stimulation by
TSH might eventually lead to thyroid neoplasia. However, the precise
mechanism triggering the transformation from hyperplasia to
neoplasia is still not understood.
Amitrole is a potent anti-thyroid agent, although the precise
sequence of events leading to the anti-thyroid effect has not been
clarified. Single doses of amitrole were shown to reduce thyroid
uptake of iodine in several species including rats (Alexander,
1959a) and humans (Astwood, 1960). However, in another study (Den
Tonkelaar & Kroes, 1974), when amitrole was given in the diet for
several days, different results on iodine uptake were observed. In
fact, iodine uptake was found to be increased 6 h after iodine
injection, and then decreased below the control values 24 and 48 h
after injection. At a lower dose level of amitrole, the
concentration of radioactive iodine in the thyroid remained slightly
above the control values.
Most of the iodine in the thyroid during amitrole exposure is
not bound to protein, reflecting a failure in its utilization
(Alexander, 1959a). Amitrole has been found to be an inhibitor of
thyroid iodide peroxidase (Alexander, 1959b; Darr & Fridovich, 1986)
both in vitro and in vivo. Histochemical studies confirmed the
reduction of thyroid peroxidase activity during amitrole treatment
(Strum & Karnovsky, 1971). Thyroid iodide peroxidase is thought to
catalyse both thyroglobulin iodination and tyrosine
(3-monoiodotyrosine and 3,5-diiodotyrosine) coupling, which leads to
the formation of the thyroid hormones 3,3,3',5'-tetraiodo-thyronine
(T4) and 3,5,3' triiodothyronine (T3). The influence of amitrole
on these peroxidase-catalysed reactions was also demonstrated by the
relative depletion of T4 in the thyroid of rats treated with
amitrole, compared with the levels of tyrosines (Mayberry, 1968).
These results suggest that amitrole affects both reactions catalysed
by thyroid peroxidase. However, the nature of the mechanism involved
in this reduction of peroxidase activity remains unclear.
In conclusion, amitrole has an antithyroid effect and several
mechanisms have been shown to be involved. Whether the toxic result
is due to all these mechanisms or to various combinations is not
clear. For instance, doses of amitrole to rats that cause a
reduction of thyroid peroxidase activity were found to be greater
than those that inhibit thyroid iodine uptake (Alexander, 1959a).
Consequences of these antithyroid effects have been observed in
rats treated with relatively high doses of amitrole. T3 and T4
levels in serum drop dramatically and in parallel with an increase
in TSH levels. When T3 and T4 are below the limit of detection,
the corresponding rise in TSH is about 10-fold (Wynford-Thomas
et al., 1982a,b). The spacial-temporal relationships between such
hormonal changes and the morphological ones induced by amitrole have
been studied by Wynford-Thomas et al. (1982a,b). Animals were
maintained on the goitrogen amitrole (0.1% in drinking-water) and
killed at various intervals. Serum T3 and T4 rapidly fell to
undetectable levels within two weeks. The level of serum TSH rose to
a stable maximum after four weeks. The T:S iodine ratio (iodine in
thyroid and serum) followed a similar pattern. Thyroid weight and
follicular cell number increased rapidly for the first few weeks but
the growth rate declined progressively, falling almost to zero after
80 days. Mitotic activity rose dramatically after 7 days but then
declined almost to normal after 80 days, consistent with the
observed change in cell number. The authors concluded that there is
a dissociation between the functional and proliferative activity of
the thyroid follicular cells during prolonged stimulation by a
sustained elevation of TSH and suggested the existence of specific
growth regulatory mechanisms which limit the mitotic response. They
further speculated that, whatever this control mechanism may be, it
would be reasonable to assume that its failure represents the first
step in the pathogenesis of neoplasia. The progression towards
thyroid malignancy can be halted by administration of thyroid
hormones or surgical hypophysectomy, procedures aimed at countering
the source of TSH stimulation. However, the extent to which
progression can be halted is unknown.
Although the mechanism involved in the triggering of neoplastic
initiation after hypertrophic and hyperplastic stimulation is
unknown, it remains to be ascertained whether other mechanisms of
carcinogenicity can be initiated by amitrole and, therefore, whether
the rise in TSH must be considered a factor promoting tumour
development. Thyroid tumours induced by X-rays are thought to be the
summation of TSH-induced hyperplasia with neoplastic transformation
initiated by radiation (Doniach, 1974). The addition of thyroxine to
the diet of irradiated rats markedly reduced radiation-induced
tumour production, indicating that TSH may have a facilitative role
in thyroid cancer development after low-dose radiation in
non-suppressed animals.
Mutagenicity studies with amitrole gave equivocal results in
some tests and indicate that this compound might have a relatively
weak genotoxic effect. The genotoxicity of amitrole is not
correlatable with functional action on peroxidase. However, possible
relationships between the anti-thyroid and genotoxic effects of
amitrole when causing thyroid cancer have been not clarified.
Conflicting data have been reported as to whether human
conditions that are related to high TSH predispose to thyroid
cancer. Three recent case control studies in the USA reviewed by
Hill et al. (1989) consistently showed that thyroid cancer is
strongly related to pre-existing goitre or thyroid nodules. However,
of two studies which analysed the association between hypothyroidism
and thyroid cancer, neither showed such a relationship (McTiernan
et al., 1984; Ron et al., 1987).
In conclusion, the role of TSH in thyroid carcinogenesis in
humans (if any) seems different from that played in experimental
thyroid cancer in animals.
8. EFFECTS ON HUMANS
8.1 General population exposure
Astwood (1960) reported in a brief communication that a single
oral dose of 100 mg amitrole inhibited radio-iodine uptake by the
thyroid of both normal and thyrotoxic subjects for 24 h. However, a
dose of 10 mg was said to have had a slight effect on iodine uptake.
The author suggested that amitrole could be used therapeutically in
the treatment of hyperthyroidism.
The intentional ingestion of a commercial mixture of amitrole
and diuron, at a dose equivalent to 20 mg amitrole/kg, was reported
to have caused no symptoms of poisoning in a female subject. Within
a few hours, the compound appeared in the urine at a concentration
of 1000 mg/litre. Metabolites could not be detected in the urine
(Geldmacher-von Mallinckrodt & Schmidt, 1970).
During a patch test conducted with human volunteers, amitrole
exerted no primary dermal irritant effect after an exposure period
of 4-8 h; a slight irritant effect was observed in three out of six
volunteers after 24 h (Hecht, 1954).
8.2 Occupational exposure
English et al. (1986) reported a case study of a 41-year-old
weed control operator with a 6-month history of dermatitis involving
his face, hands, back, thighs and feet. Patch testing with 1%
amitrole showed a strong positive vesicular reaction at 2 and 4
days, indicative of allergic contact dermatitis.
Balkisson et al. (1992) reported the case of a 74-year-old
previously healthy ex-smoker, who sprayed an amitrole formulation
(containing 19% aminotriazole, 17% ammonium thiocyanate, < 1%
sodium diethylsulfosuccinate and < 1% ethylene oxide), in a
strong-head wind without any protective clothing for 2 h using
500 ml of the formulated material in 10 litre of water. He developed
a dry non-productive cough after 6-8 h, for which he reported for
treatment. On investigation, diffuse, asymmetric, severe alveolar
damage in the lungs was noticed which was reversed after a high dose
of corticosteroid.
A report by Baugher et al. (1982) presented the results of a
study conducted on five men involved in spraying amitrole over a
period of 10 working days on utility rights-of-way in West Virginia.
The amitrole (at a concentration of approximately 500 g of active
ingredient per 100 litres of water), was applied using handheld
hydraulic spray guns. Medical monitoring, particularly of the
thyroid, was carried out both pre- and post-spraying. Thyroid gland
palpation and neck measurements were undertaken 19 days prior to
exposure and 14 days after the last exposure. Thyroid function tests
were performed at 19, 11 and 4 days pre-exposure and 0, 7 and 14
days after the last exposure. All subjects were within the normal
range for thyroid function and no differences were found in any
comparisons of thyroid function. It was estimated that dermal
exposure over the 10-day period was approximately 340 mg per day for
each man.
Epidemiological studies conducted on agricultural workers
(Barthel, 1981) and railway workers (Axelson & Sundell, 1974;
Axelson et al., 1980) exposed to a variety of pesticides showed a
small increase in the number of tumours, particularly of the lung.
Amitrole was one of the pesticides to which these workers were
exposed.
In a study by Miksche (1982), mild cases of dermatitis on the
face due to contact with amitrole occurred yearly in one or two
production workers. The dermatitis was of a primary irritant type
rather than the sensitivity type. No other adverse effects occurred.
The thyroid function in five employees who had been engaged in
production and packaging of amitrole for periods of between 3 and 16
years was checked. Thyroid scintigrams and determinations of the
T3 and T4 levels yielded no evidence of thyroid dysfunction
(Miksche, 1983).
9. EFFECTS ON OTHER ORGANISMS IN THE LABORATORY AND FIELD
9.1 Laboratory experiments
9.1.1 Microorganisms
The effect of amitrole on the growth and pigmentation of the
cyanobacteria (blue-green alga) Anacystis nidulans was studied by
Kumar (1963). Growth inhibition occurred at concentrations above
40 mg/litre. In cultures 5-14 days old, the amounts of carotenoids,
chlorophyll and phycocyanin were decreased by amitrole
concentrations of 20-40 mg/litre, but normal pigmentation was
restored upon withdrawal of amitrole. DaSilva et al. (1975) examined
the effect of amitrole (20 mg/litre) on the nitrogen-fixing capacity
of eight different cyanobacteria and observed partial inhibition,
followed by recovery, in all cases.
Salama et al. (1985) cultured two species of nitrogen-fixing
cyanobacteria, Anabaena flos aquae and Phormidium fragile, from
Nile drains in amitrole solutions (0.4, 1.2, 3.6 and 10.8 mg/litre).
Only the highest concentration markedly reduced the growth of the
cyanobacteria; the growth of Phormidium was slightly stimulated at
the two lowest concentrations. There was little effect on
chlorophyll content, although a slight stimulation occurred in
Anabaena at up to 1.2 mg/litre. There was a slight (statistically
significant) stimulation of nitrogen uptake by Phormidium, but
only at the highest amitrole concentration. In Anabaena there was
an increase in nitrogen fixation at all amitrole concentrations.
When the growth of Rhizobium trifolii TA1 in nutrient broth
was examined in the presence of amitrole (20 mg/litre) no inhibition
was detected. Nodulation of Trifolium subterranium L., however,
following inoculation with R. trifolii, decreased linearly as the
herbicide concentration increased from 0 to 20 mg/litre. Even when
R. trifolii was grown in the presence of amitrole and washed
before inoculation, there was decreased nodulation of the sub-clover
(Eberbach & Douglas, 1989).
The effect of amitrole on cellulose decomposition by
cellulolytic fungi and cellulolytic bacteria in soil was
investigated by Grossbard & Wingfield (1978). Amitrole incorporated
into soil at 500 mg/kg greatly reduced the decomposition of buried
calico. This effect was not, however, observed at 150 mg/kg. In pure
cultures of the test fungi, amitrole was less toxic than paraquat or
glyphosate. Amitrole did not affect the number of cellulolytic
bacteria.
Blumenstock (1989) incorporated Aminotriazol SP (Bayer) into
two soils, a loamy sand and a silt, at rates of 40 mg formulation/kg
(equiv. 20 mg a.i./kg) and at 200 mg formulation/kg dry soil. This
corresponded to the maximum recommended application rate and to a
rate five times higher. The amitrole formulation slightly reduced
nitrification, but this effect disappeared within 90 days. In the
loamy sand, there was a small increase in soil nitrate formation.
Using the same product and the same concentrations in soil, Anderson
(1989) showed no effect of the formulation on soil respiration at
20 mg a.i./kg dry soil. At 100 mg a.i./kg, there was a slight
reduction in microbial respiration in both soils.
The effect of amitrole on photosynthetic microorganisms has
been investigated by Aaronson (1960) and Aaronson & Sher (1960). The
multiplication of Euglena gracilis var. bacillaris was strongly
inhibited by amitrole (150-300 mg/litre). Wolf (1962) noted that the
growth of Chlorella pyrenoidosa was inhibited by approximately 50%
at a dose level of 5 mg/litre. Another microorganism, Ochromonas
danica, was also particularly sensitive, strong bleaching occurring
at 30 mg/litre (Aaronson & Sher, 1960).
When Cain & Cain (1983) cultured the green alga Chlamydomonas
moewusii in amitrole (technical) at concentrations up to
6.7 mg/litre, there was no observed effect on vegetative growth or
on zygospore germination.
Altenburger et al. (1990) cultured the green alga Chlorella
fusca with amitrole and glufosinate-ammonium both separately and
in combination. The two herbicides together showed less toxicity to
the alga than either alone when estimated in terms of cell volume
growth or cell reproduction.
Adams & Dobbs (1984) examined the toxicity of amitrole to the
unicellular alga Selenastrum capricornutum using two different
methods. The highest concentration causing no statistically
significant difference from controls was found to be
0.2-0.5 mg/litre and 5 mg/litre, respectively, in the two tests. The
different results were thought to be due to different growth media
composition.
A growth test on the green alga Scenedesmus subspicatus was
conducted by Anderson (1984). The EC50 for growth, based on cell
counts, was reported to be 2.3 mg a.i./litre. The no-observed-effect
concentration (NOEC) was < 1.0 mg a.i./litre of nutrient solution.
An algal growth inhibition test (OECD 201) was performed by
Knacker & Reifenberg (1989) using Aminotriazol SP50
(500 mg/litre formulation) and the alga Scenedesmus subspicatus
exposed to nominal concentrations of between 8.96 and 350 mg/litre.
An NOEC of 8.96 mg/litre, based on growth (area under growth curves)
and growth rate, was reported.
9.1.2 Aquatic organisms
9.1.2.1 Plants
Amitrole did not affect the secretion of extracellular products
by the macrophyte Porphyra yezoensis or inhibit its photosynthetic
activity in the concentration range 12.5-200 mg/litre (Yoshida
et al., 1986).
The angiosperm Spirodela is particularly sensitive to
amitrole, with strong bleaching at 100 mg/litre (Aaronson & Sher,
1960).
9.1.2.2 Invertebrates
The median tolerance limit of amitrole to some aquatic
invertebrates is shown in Table 5.
In a study of the toxicity of herbicides to the common
water-flea, Daphnia magna, the median immobilization concentration
(IC50) in a 26-h acute static test at 21 °C was 23 mg amitrole/kg
(Crosby & Tucker, 1966).
The EC50 (immobilization) for a 48-h acute toxicity study on
Daphnia magna, conducted under OECD guidelines, was reported by
Heimbach (1983) to be 1.54 (0.38-3.07) mg/litre.
In a semi-static reproduction test on Daphnia magna (OECD
202) performed by Ritter (1989), the NOEC for production of young
was 0.2 mg/litre. At 1.0 mg/litre, amitrole significantly reduced
the number of young produced (by 12.5%). Measured concentrations of
amitrole ranged from 78 to 115% of the nominal concentrations.
Bogers (1989) conducted a comparable test and reported a NOEC of
between 0.32 and 1.0 mg/litre. As in the study by Ritter (1989),
effects were observed at 1.0 mg/litre.
Schultz & Kennedy (1976) suggested that Daphnia pulex is more
sensitive to amitrole-induced toxicity than Daphnia magna and
examined the toxicity of amitrole (10 mg/litre) to Daphnia pulex.
Evidence of altered cell structure was noted within 24 h in the
mitochondria of the muscle fibre, together with general tissue
swelling and dissociation of membranes.
Table 5. Toxicity of amitrole to aquatic invertebrates
Species Test Exposure TLm Reference
materiala period (median
(hours) tolerance
limit,
mg/litre)
Sigara substrata WP 48 >1000 Nishiuchi (1981)
Micronecta sedula WP 48 >1000 Nishiuchi (1981)
Cloeon dipterum WP 48 >40 Nishiuchi (1981)
Orthetrum albistylum Technical 48 >40 Nishiuchi (1981)
speciosum larvae
Sympetrum frequens Technical 48 >40 Nishiuchi (1981)
larvae
Water flea Technical 3 >40 Yoshida &
(Daphnia pulex) Nishimura (1972)
Moina macrocopa Technical 3 >40 Yoshida &
Nishimura (1972)
Crayfish Technical 72 >40 Yoshida &
(Procambarus clarkii) Nishimura (1972)
Scud Technical 96 >10 Mayer & Ellersieck
(Gammarus fasciatus) (1986)
Copepod Technical 96 22.1 Robertson &
(Cyclops vernalis) (18.5-27.7) Bunting (1976)
a wp = wettable powder
9.1.2.3 Vertebrates
The median tolerance limit of amitrole to some aquatic
vertebrates is shown in Table 6.
Table 6. Toxicity of amitrole to aquatic vertebrates
Species Test Exposure TLm Reference
material period (median
(hours) tolerance
limit,
mg/litre)
Common carp Technical 48 >40 Yoshida &
(Cyprinus carpio) Nishimura (1972)
Goldfish Technical 48 >40 Yoshida &
(Carassius auratus) Nishimura (1972)
Golden orfe Technical 48 >40 Yoshida &
(Oryzias latipes) Nishimura (1972)
Misgurnus Technical 48 >40 Yoshida &
anguillicaudatus Nishimura (1972)
Japanese toad tadpole wettable 48 >1000 Yoshida &
(Bufo bufo japonicus) powder Nishimura (1972)
Coho salmon Amitrole-T 96 70 Lorz et al. (1978)
(Oncorhynchus kisutch)
Guppy Technical 96 >12 500 Niehuss & Börner
(Poecilia reticulatus) (1971)
Golden orfe Technical 96 >6000 Bayer AG (1979)
(Oryzias latipes)
Channel catfish Technical 96 >160 Mayer & Ellersieck
(Ictalurus punctatus) (1986)
Fathead minnow Technical 96 >100 Mayer & Ellersieck
(Pimphales promelas) (1986)
Mugil Technical 96 >68 Tag el-Din et al.
(Mugil cephalus) (1981)
A semi-static 21-day test (OECD 204) was conducted on the
rainbow trout, Oncorhynchus mykiss (weight 1.5 ± 0.6 g), at a
single technical amitrole concentration of 100 mg/litre. There were
no deaths or toxic signs in the test fish, and body weight and
length were not different from controls. The authors, therefore,
reported 100 mg/litre as the NOEC (Grau, 1989).
Amitrole was without effect on the development of the teleost
embryo Fundulus heteroclitus at exposure concentrations of 0.01 to
10.0 mg/litre (Crawford & Guarino, 1985).
A 96-h LC50 of 3.0 mg/litre for amitrole as Weedazol TL Plus
was reported for 1-2 week old tadpoles of the Australian frog
Adelotus brevis. The thermal tolerance of tadpoles was reduced
significantly after 96 h of exposure to 1 mg/litre (Johnson, 1976).
Hiltibran (1967) exposed fry of bluegill sunfish, green sunfish
and lake chub sucker fish to amitrole at 50 mg/litre for 8 days and
reported no deaths. Young bluegill survived a 12-day exposure to
25 mg/litre, although some deaths occurred at higher concentrations.
Lorz et al. (1978) exposed yearling Coho salmon (Oncorhynchus
kisutch) to Amitrole-T at concentrations of between 0.25 and
200 mg/litre. The 96-h LC50 in fresh water was calculated to be
70 mg/litre. Transfer of the young salmon to sea water with
comparable concentrations of amitrole increased the toxic effect of
the compound; 56% of fish survived 336 h in sea water containing
25 mg amitrole/litre and 12.5% survived 50 mg/litre. According to
the authors, deaths occurred within 24-48 h. Smoltification, the
adaptation of young salmon to sea water, is under thyroid control.
The goitrogenic properties of amitrole may explain the effect.
9.1.3 Terrestrial organisms
9.1.3.1 Plants
The herbicidal mode of action of amitrole at the molecular
level has not been satisfactorily elucidated. The early work of Sund
(1956) and Wolf (1962) has shown that the effect of amitrole can be
significantly reduced by simultaneous application of purines,
suggesting that amitrole is an inhibitor of purine biosynthesis and
interferes with the development of chloroplasts. While pigment
bleaching is one of the most striking effects of amitrole toxicity,
it is not clear that it is the primary site of action. Amitrole has
been shown to inhibit the activity of the enzymes phytoene
desaturase, lycopene cyclase, imidazole glycerol phosphate
dehydratase and catalase. Imidazole glycerol phosphate dehydratase
has been extensively studied due to its role in the histidine
biosynthetic pathway. Heim & Larrinua (1989) have examined the mode
of action of amitrole in Arabidopsis thaliana seedlings. They
concluded that root elongation is more sensitive to amitrole than
pigmentation, and this may be indicative of the primary block caused
by amitrole.
9.1.3.2 Invertebrates
The mortality and growth of the juvenile earthworm,
Allolobophora caliginosa (Oligochaeta:Lumbricidae), were measured
in soil containing Amitrole-T (1, 10 and 100 mg amitrole/kg plus
ammonium thiocyanate). There was no effect on worm growth or
mortality (Martin, 1982).
Red earthworms of the species Eisenia foetida were exposed to
Aminotriazol SP 50 in artificial soil at 100, 316 and 1000 mg
formulation/kg soil for 14 days (OECD 207) by Heimbach (1990b).
There were no reported effects; the NOEC was, therefore, 1000 mg
formulation/kg, equivalent to 488 mg a.i./kg.
Carabid beetles (Poecilus cupreus) were kept in dishes of
fine sand and sprayed with aminotriazole directly at a rate
equivalent to 30 kg/ha. Pupae of the housefly Musca domestica were
provided as food. No deaths were recorded and no toxic signs
observed; the beetles ate comparable numbers of pupae in both test
and control dishes (Heimbach, 1990a).
Amitrole, at a concentration of 184 mg/kg, reduced the survival
of the nematode Acrobeloides buetschlii by 50% and produced almost
complete mortality at 600 mg/kg. At an intermediate concentration
(300 mg/kg), malformed larvae were common (Frey, 1979).
Mothes-Wagner et al. (1990) studied the effect of amitrole on
the host-plant interaction between the spider mite Tetranychus
urticae and its host bean plant, Phaseolus vulgaris L.,
following applications of 0.1 g/m2 or 0.2 g/m2 per cultivation
box. Structural alterations to the protein-synthesizing apparatus of
the midgut and salivary gland cells of the spider mite were noted,
and these led to a decrease in reproduction rate.
9.1.3.3 Birds
Amitrole has low toxicity for birds (Table 7). There were no
reported signs of toxicity in the young birds of three species fed
diets supplemented with amitrole at a concentration of 5000 mg/kg.
Signs of toxicity, following a single oral dose of 2000 mg/kg body
weight in mallard ducks, included ataxia, weakness and slight
wing-drop during the first three days following dosing. The birds
subsequently recovered.
Table 7. Short-term toxicity of dietary amitrole and Amitrole-T in birdsa
Species Age LD50 LC50
(mg/kg body weight) (mg/kg diet)
Mallard duck 3-4 months >2000
(Anas platyrhynchos) 10 days >5000
Japanese quail 12 days >5000
(Coturnix coturnix
japonica)
Ring necked pheasant 10 days >5000
(Phasianus colchicus)
a Data from Hill et al. (1975), Hudson et al. (1984), Hill & Camardese
(1986); LD50 from acute oral dose; LC50 from dietary exposure for
5 days followed by 3 further days of observation.
9.2 Field observations
9.2.1 Terrestrial organisms
9.2.1.1 Plants
The effect of amitrole on the levels of epicuticular wax,
cuticle, penetration of water, cuticular transpiration and wax
biosynthesis was studied by Reddy et al. (1987) in some semi-arid
scrub. Over a 6-day period, at amitrole concentrations of 1000 and
5000 mg/litre, there was a significant reduction in the deposition
of epicuticular wax and the cuticle content of leaves, which
resulted in increased cuticular transpiration and penetration of
water. Amitrole induced a quantitative alteration in wax
biosynthesis and an accelerated water loss, ultimately leading to
plant death.
9.2.1.2 Invertebrates
Amitrole was non-hazardous to honey-bees when sprayed at an
application rate of 40 kg/ha (Herfs, 1975).
Amitrole, applied at a rate of 5.6 kg/ha to bentgrass
(Agrostis tenuis), reduced the number of nematodes (Anguina
agrostis) in the bentgrass by 49.9% (Courtney et al., 1962).
10. EVALUATION OF HUMAN HEALTH RISKS AND EFFECTS ON THE ENVIRONMENT
10.1 Evaluation of human health risks
General population exposure to amitrole is expected to be
minimal, given that it does not persist in the environment and
residues should not occur in food crops. Levels in drinking-water
supplies would be expected to be extremely low.
Occupational exposure for weed control operators occurs via the
dermal and inhalation routes. However, animal experiments and human
data indicate that dermal absorption of amitrole is low.
Inhalational exposure would be minimized by the use of appropriate
breathing apparatus.
In both animals and humans there is a rapid excretion of
unchanged amitrole following systemic exposure. Amitrole does not
have a teratogenic effect and does not affect reproduction.
The main effect of amitrole in subchronic and chronic studies
on rats is on the thyroid. Amitrole inhibits the production of
thyroid hormones T3 and T4, thereby stimulating the pituitary
gland to produce more thyroid stimulating hormone (TSH), which in
turn activates the thyroid. Consequently, thyroid weight increases
and the thyroid becomes hyperplastic and hypertrophic. These effects
are reversible upon cessation of exposure, even though the extent of
this reversal is undefined. Thyroid tumours occur only upon chronic
exposure at relatively high dose levels in animals already affected
by thyroid changes. The mechanism of neoplastic transformation is
not understood. However, from all available studies it is clear that
hyperplasia always precedes neoplasia because no tumours are found
when the thyroid is not affected. Mutagenicity studies are either
negative or conflicting, and therefore the evidence for amitrole
genotoxicity is equivocal. On this basis, and from Table 8, it can
be concluded that 2 mg/kg diet (equivalent to 0.1 mg/kg body weight
per day) is a no-observed-effect level, based on thyroid hyperplasia
and iodine uptake, and this value can be used to establish a safe
dose for humans.
In carcinogenicity studies on rats, amitrole did not induce
tumours in organs other than the thyroid. However, in some mouse
studies that used high dose levels, liver tumours were also found.
Because the mouse is very sensitive to the induction of liver
tumours and the dosages are far above any potential exposure of man,
this is considered of little consequence for the human risk
evaluation.
Table 8. Summary of rat dietary studies
Concentration Lowest effect level (mg/kg diet)a No-
range and observed- Reference
duration of Body weight Thyroid Thyroid Thyroid Thyroid Uptake of adverse-
study gain weight hyperplasia tumours hormones 131I effect level
(PBI/T3/T4)
30-300 mg/kg 100 - - - 100 - 30 mg/kg Babish (1977)
(28 days)
2-50 mg/kg > 50 50 10 - >50 50 2mg/kg Fregly (1968)
(13 weeks)
2-500 mg/kg - 50/200 50 (20)b - 200 50 (20)b 2mg/kg Den Tonkelaar &
(6-13 weeks) Kroes (1974)
10-500 mg/kg 100 100 50 50 - - 10 mg/kg Keller (1959)
(2 years)
1-100 mg/kg > 100 100 100 100 > 100 100 10 mg/kg Steinhoff & Boehme
(2 years) (1979a)
a indicates not relevant or not measured
b values in parentheses are for very marginal effects
Under normal circumstances of occupational exposure, it is
unlikely that amitrole induces thyroid effects in humans.
Finally, it should be noted that the role of TSH in thyroid
carcinogenesis in humans seems different from that played in
experimental thyroid cancer in rats. This is based on the absence of
a correlation between hypothyroidism and thyroid cancer in human
epidemiological studies.
10.2 Evaluation of effects on the environment
Amitrole has high potential mobility in soil. The rapid
degradation of amitrole in soil and its retention in most soils by
adsorption makes this potential very unlikely to be realized in most
situations. The few reports of effects on non-target vegetation
support this view.
Use of amitrole at maximum recommended application rates to
control terrestrial weeds would lead to soil residues of up to 20 mg
a.i./kg dry soil. The soil invertebrates tested have not been
adversely affected at substantially higher concentrations. Effects
on soil microorganisms would also not occur. Amitrole presents no
hazard to birds.
Overspraying of static water bodies during the control of
terrestrial weeds would lead to maximum initial water concentrations
substantially below reported NOEC values for aquatic organisms. The
use of amitrole to control aquatic weeds has been reported to lead
to water concentrations of about 1 mg/litre, which persist for some
time. This would not affect fish but could be expected to adversely
affect water fleas (the NOEC is 0.2 mg/litre for reproductive
effects).
11. CONCLUSIONS AND RECOMMENDATIONS FOR PROTECTION OF HUMAN HEALTH
AND THE ENVIRONMENT
11.1 Conclusions
Amitrole does not present a significant risk to human health
when manufactured and used according to good handling procedure.
Current restrictions on its use in most countries, particularly its
restriction to non-crop use, will ensure minimum human exposure.
Amitrole is relatively rapidly degraded in the environment and
there is no evidence of bioaccumulation. The available data does not
indicate there are significant effects on the environment. Where
effects occur, these appear to be transient.
11.2 Recommendations for protection of human health and the
environment
Annual monitoring of thyroid function is recommended for
workers regularly involved with amitrole, both at the formulation or
application stages.
Epidemiological studies should be continued on workers exposed
to amitrole.
Patterns of use of amitrole should continue to avoid the risk
of contamination of food crops and water supplies, and limits for
residues in food and water should be maintained at low levels, i.e.
below 0.02 mg/kg in raw agricultural commodities of plant origin
(this level is at or around the limit of determination).
12. FURTHER RESEARCH
Further research data on amitrole are needed in the following
areas:
a) studies on the relationship between the extent of occupational
exposure and urinary excretion of amitrole in relation to
thyroid function;
b) studies aimed at understanding species differences in the
effects produced on the thyroid and liver;
c) in vitro and in vivo studies on the effects on specific
enzyme systems that may be involved in amitrole toxicity;
d) genotoxicity tests in vivo (other than chromosome
aberration).
13. PREVIOUS EVALUATIONS BY NATIONAL AND INTERNATIONAL
BODIES
Amitrole was considered by the International Agency for
Research on Cancer (IARC) in 1974 and 1986. In 1974, IARC evaluated
amitrole and, on the basis of animal carcinogenicity data and
epidemiological data, indicated that amitrole may be carcinogenic to
humans. However, the findings were regarded as inconclusive.
In a further evaluation in 1986 by IARC, it was concluded that
there was sufficient evidence for carcinogenicity of amitrole in
experimental animals, and that there was inadequate evidence for
carcinogenicity of amitrole in humans (2b) (IARC, 1986, 1987).
Amitrole was discussed at the 1974 and 1977 FAO/WHO Joint
Meetings on Pesticide Residues (JMPR). At the 1974 meeting, a
conditional acceptable daily intake (ADI) for amitrole of
0-0.00003 mg/kg body weight was established (FAO/WHO, 1975). At the
1977 meeting, further studies on the effects of amitrole were
considered, and the conditional ADI for amitrole was confirmed
(FAO/WHO, 1978)a. The 17th Codex Alimentarius Commission meeting
recommended a maximum residue limit at or about the limit of
determination by the best available analytical method.
The European Community established MRLs for amitrole on all
food crops at a limit of determination of 0.05 mg/kg.
The US Environmental Protection Agency proposed termination of
a special review of amitrole based on the Agency's determination
that the benefits of use outweighed the risks (USEPA, 1992).
a In September 1993, i.e. 4 months after the Task Group met, JMPR
reviewed amitrole and established a temporary ADI of
0-0.0005 mg/kg body weight. The temporary ADI will be valid
until 1997, when amitrole will again be reviewed by JMPR.
REFERENCES
Aaronson S (1960) Mode of action of 3-amino-1,2,4-triazole of
photosynthetic microorganisms. J Protozool, 7: 289-294.
Aaronson S & Sher S (1960) Effect of aminotriazole and streptomycin
on multiplication and pigment production of photosynthetic
microorganisms. J Protozool, 7: 156-158.
ACGIH (1991-1992) Threshold limit values for chemical substances and
physical agents and biological exposure indices for 1991-1992.
Cincinnati, Ohio, American Conference of Governmental and Industrial
Hygienists, p 12.
Adams N & Dobbs AJ (1984) A comparison of results from two test
methods for assessing the toxicity of aminotriazole to Selenastrum
capricornutum. Chemosphere, 13: 965-971.
Agrawal BBL & Margoliash E (1970) A spectrophotometric method for
the determination of aminotriazole and other aromatic amines. Anal
Biochem, 34: 505-516.
Alary J, Bourbon P, Escrieut C, & Vandaele J (1984)
Spectrophotometric determination of guanazole and aminotriazole in
waters from an aminotriazole production plant. Environ Technol Lett,
6: 93-100.
Aldrich FD & McLane SR Jr (1957) A paper chromatographic method for
the detection of 3-amino-1,2,4-triazole in plant tissues. Plant
Physiol, 32: 153-154.
Alexander NM (1959a) Antithyroid action of 3-amino-1,2,4-triazole. J
Biol Chem, 234(1): 148-150.
Alexander NM (1959b) Iodide peroxidase in rat thyroid and salivary
glands and its inhibition by anti-thyroid compounds. Fed Proc, 18:
1530-1533.
Allen CF & Bell A (1946) 3-Amino-1,2,4-triazole. Org Synth, 26:
11-12.
Altenburger R, Bodeker W, Faust M, & Horst Grimme L (1990)
Evaluation of the isobologram method for the assessment of mixtures
of chemicals, combination effect studies with pesticides in algal
biotests. Ecotoxicol Environ Saf, 20: 98-114.
Andersen KJ, Leighty EG, & Takahashi MT (1972) Evaluation of
herbicides for possible mutagenic properties. J Agric Food Chem,
20(3): 649-656.
Anderson JPE (1984) Influence of amitrole on the growth of green
algae (Scenedesmus subspicatus) in nutrient solution. Leverkusen,
Bayer AG (Unpublished report No. 6984).
Anderson JPE (1989) Influence of the commercial product Aminotriazol
Bayer on the soil respiration after amendment with glucose.
Leverkusen, Bayer AG (Unpublished report No. AJO/64189).
Anderson C & Hellpointner E (1989) Adsorption/desorption of amitrole
in soils. Leverkusen, Bayer AG (Unpublished report No. PF 3218).
Aragon CMG & Amit Z (1992) The effect of 3-amino-1,2,4-triazole on
voluntary ethanol consumption: Evidence for brain catalase
involvement in the mechanism of action. Neuropharmacology, 31(7):
709-712.
Aragon CMG, Spivak K, & Amit Z (1991a) Effect of
3-amino-1,2,4-triazole on ethanol-induced narcosis, lethality and
hypothermia in rats. Phamacol Biochem Behav, 39: 55-59.
Aragon CMG, Rogan F, & Amit Z (1991b) Dose- and time-dependent
effect of an acute 3-amino-1,2,4-triazole injection on rat brain
catalase activity. Biochem Pharmacol, 42: 699-702.
Archer AW (1984) Determination of 3-amino-1,2,4-triazole (amitrole)
in urine by ion-pair high-performance liquid chromatography. J
Chromatogr, 303: 267-271.
Ashworth R de B, Henriet J, Lovett JF, & Martijn A (1980) Amitrole.
In: CIPAC handbook. Volume 1A & 1B (Addendum to CIPAC 1): Analysis
of technical and formulated pesticides. Harpenden, UK, Collaborative
International Pesticide Analytical Council, pp 1720-1725.
Astwood EB (1960) Cranberries, turnips, and goitre. J Am Med Assoc,
172(12): 1319-1320.
Axelson O & Sundell L (1974) Herbicide exposure, mortality and
tumour incidence. An epidemiological investigation on Swedish
railroad workers. Scand J Work Environ Health, 11(1): 21-28.
Axelson O, Sundell L, Andersson K, Edling C, Hogstedt C, & Kling H
(1980) Herbicide exposure and tumour mortality. An updated
epidemiologic investigation on Swedish railroad workers. Scand J
Work Environ Health, 6(1): 73-79.
Babish JG (1977) Triiodothyronine (T3) and thyroxine (T4) levels in
male rats consuming amitrole (3-amino,1-2-4-triazole) in their diets
for a four week recovery period. Waverly, New York, Food and Drug
Research Laboratories, Inc. (Unpublished report No. 5539).
Bagdon RE, Shaffer CB, Vidone LB, & Golz HH (1956) Aminotriazole -
Acute and subacute toxicity. Wayne, New Jersey, American Cyanamid
Company (Unpublished report No. 56-29, submitted to WHO by Bayer
AG).
Balkisson R, Murray D, & Hoffstein V (1992) Alveolar damage due to
inhalation of amitrole-containing herbicide. Chest, 101: 1174-1175.
Bamford D, Sorsa M, Gripenberg U, Laamanen I, & Meretoja T (1976)
Mutagenicity and toxicity of amitrole. III. Microbial tests. Mutat
Res, 40(3): 197-202.
Baron J & Tephly TR (1969 Effect of 3-amino-1,2,4-triazole on the
stimulation of hepatic microsomal heme synthesis and induction of
hepatic microsomal oxidases produced by phenobarbital. Mol
Pharmacol, 5: 10-20.
Barthel E (1981) Increased risk of lung cancer in pesticide-exposed
male agricultural workers. J Toxicol Environ Health, 8: 1027-1040.
Baugher DG, Bookbinder MG, & Blundell KR (1982) Final Report -
Medical monitoring and assessment of exposure of workers applying
amitrole to utility rights-of-way. Rockville, Maryland, Dynamac
Corporation, Enviro Control Division (Unpublished report).
Bayer AG (1979) Aminotriazole - Toxicity to fish. Leverkusen, Bayer
AG (Unpublished report No. FO-227).
Bayer AG (1993a) Residue trials on amitrole in apples. Leverkusen,
Bayer AG (Unpublished reports Nos 0625/79, 0626/79, 0627/79,
0637/79, 0726/90, 0627/90).
Bayer AG (1993b) Residue trials on amitrole in grapes. Leverkusen,
Bayer AG (Unpublished reports Nos 0634/70, 0635/79, 0636/79,
0606/83, 0607/83, 0601/85, 0602/85, 0603/85, 0604/85, 0605/85,
0724/90, 0725/90, 0252/91, 0253/91, 254/91).
Becci P (1983) Evaluation of the chronic inhalation toxicity and
carcinogenicity of amitrole in rats. Waverly, New York, Food and
Drug Research Laboratories, Inc. (Unpublished report No. 5821,
submitted to WHO by Bayer AG).
Beynen AC, Buechler KF, Van der Molen AJ, & Geelen MJH (1981)
Inhibition of lipogenesis in isolated hepatocytes by
3-amino-1,2,4-triazole. Toxicology, 22: 171-178.
Bhuyan KC, Bhuyan DK, & Katzin HM (1973) Amizole-induced cataract
and inhibition of lens catalase in rabbit. Ophthalmol Res, 5:
236-247.
Bignami M, Aulicino F, Velcich A, Carere A, & Morpurgo G (1977)
Mutagenic and recombinogenic action of pesticides in Aspergillus
nidulans. Mutat Res, 46(6): 395-402.
Blumenstock I (1989) Influence of the commercial product
Aminotriazole Bayer on nitrification in soils. Leverkusen, Bayer AG
(Unpublished report No. BSI/64289).
Bogers M (1989) Influence of aminotriazol SP50 on the reproduction
of Daphnia magna. 's-Hertogenbosch, The Netherlands, RCC Notox
B.V. (Unpublished report No. 011339).
Braun R, Schoneich J, & Ziebarth D (1977) in vivo formation of
N-nitroso compounds and detection of their mutagenic activity in the
host-mediated assay. Cancer Res, 37: 4572-4579.
Brooks TM & Dean BJ (1981) Mutagenic activity of 42 coded compounds
in the salmonella/microsome assay with preincubation. In: de Serres
FJ & Ashby J ed. Evaluation of short-term tests for carcinogens.
Report of the international collaborative program. New York,
Amsterdam, Oxford, Elsevier/North-Holland, pp 261-270 (Progress in
Mutation Research, Volume 1).
Brusick D (1975) Mutagenic evaluation of compound,
3-amino-1,2,4-triazole (99.4%) and 3-amino-1,2,4-triazole : ammonium
thiocyanate (1:1). Kensington, Maryland, Litton Bionetics, Inc.
(Unpublished report No. 2547).
Brusick D & Weir RJ (1976) Mutagenicity evaluation of
3-amino-1,2,4-triazole. in vitro cellular transformation in
Balb/3T3 cells. Kensington, Maryland, Litton Bionetics, Inc.
(Unpublished report No. 2549).
Buchmuller-Rouiller Y, Schneider P, Betz-Corradin S, Smith J, &
Mauel J (1992) 3-Amino-1,2,4-triazole inhibits macrophage NO
synthase. Biochem Biophys Res Commun, 183: 150-155.
Cain JR & Cain RK (1983) The effects of selected herbicides on
zygospore germination and growth of Chlamydomonas moewush
(Chlorophyceae Volvocales). J Phycol, 19: 301-305.
Campacci EF, New PB, & Tchan YT (1977) Isolation of
amitrole-degrading bacteria. Nature (Lond), 266: 164-165.
Carere A, Ortali VA, Cardamone G, Torracca AM, & Raschetti R (1978)
Microbiological mutagenicity studies of pesticides in vitro. Mutat
Res, 57(3): 277-286.
Carter MC (1975) Amitrole. In: Kearey PC & Kaufman DD ed.
Herbicides. New York, Basel, Marcel Dekker Inc., vol I, pp 377-398.
Carter MC & Naylor AW (1960) Metabolism of
3-amino-1,2,4-triazole-5-C14 in plants. Bot Gaz, 122: 138-143.
Corneliussen PE (1969) Residues in food and feed: Pesticide residues
in total diet samples (IV). Pestic Monit J, 2(4): 140-152.
Corneliussen PE (1970) Residues in food and feed: Pesticide residues
in total diet samples (V). Pestic Monit J, 4(3): 89-105.
Courtney WD, Peabody DV, & Austenson HM (1962) Effect of herbicides
on nematodes in bentgrass. Plant Dis Rep, 46(4): 256-257.
Cox GE & Re TA (1978) Inhalation toxicity study with
3-amino-1,2,4-triazole in adult Fischer 334 rats. Waverly, New York,
Food and Drug Research Laboratories, Inc. (Unpublished report No.
5492).
Crawford RB & Guarino AM (1985) Effect of environmental toxicants on
development of a teleost embryo. J Environ Pathol Toxicol Oncol,
6(2): 185-194.
Crebelli R, Bellincampi D, Conti G, Conti L, Morpurgo G, & Carere A
(1986) A comparative study on selected chemical carcinogens for
chromosome malsegregation, mitotic crossing-over and forward
mutation induction in Aspergillus nidulans. Mutat Res, 172:
139-149.
Crosby DG & Tucker RK (1966) Toxicity of aquatic herbicides to
Daphnia magna. Science, 154: 289-290.
Daniel MR & Dehnel JM (1981) Cell transformation test with baby
hamster kidney cells. In: de Serres FJ & Ashby J ed. Evaluation of
short-term tests for carcinogens. Report of the international
collaborative program. New York, Amsterdam, Oxford,
Elsevier/North-Holland, pp 626-637 (Progress in Mutation Research,
Volume 1).
Darr D & Fridovich I (1986) Irreversible inactivation of catalase by
3-amino-1,2,4-triazole. Biochem Pharmacol, 35(20): 3642.
Dasilva EJ, Henriksson LE, & Henriksson E (1975) Effects of
pesticides on blue-green algae and nitrogen-fixation. Arch Environ
Contam Toxicol, 3(2): 193-204.
Day BE, Jordan LS, & Hendrixon RT (1961) The decomposition of
amitrole in California soils. Weeds, 9: 443-456.
Degen GH, Wong A, Eling TE, Barrett JC, & McLachlan JA (1983)
Involvement of prostaglandin synthetase in peroxidative metabolism
of diethylstilbestrol in Syrian hamster embryo fibroblast cell
cultures. Cancer Res, 43: 992-996.
Demint RJ, Frank PA, & Comes RD (1970) Amitrole residues and rate of
dissipation in irrigation water. Weed Sci, 18(4): 439-442.
Den Tonkelaar EM & Kroes R (1974) [Toxicity of thyroid function
after subacute and semichronic administration of aminotriazole.]
Bilthoven, National Institute of Public Health (Unpublished report
No. 164/74 TOX) (in Dutch).
Deprospo JR & Fogleman RW (1973) Amitrol-T: Acute oral toxicity in
rats. Princeton, New Jersey, Affiliated Medical Research, Inc.
(Unpublished report No. 121-1921-33).
Doni MG & Piva E (1983) Glutathione peroxidase blockage inhibits
prostaglandin biosynthesis in rat platelets and aorta. Haemostasis,
13: 240-243.
Doniach I (1974) Carcinogenic effect of 100, 250 and 500 rad x-rays
on the rat thyroid gland. Br J Cancer, 30(6): 487-495.
Dornseiffen JW & Kerwaal W (1988) Amitrole residues in blackberries.
Amsterdam, Ford Inspection Service (Unpublished report).
Duggan RE, Barry HC, & Johnson LY (1966) Pesticide residues in
total-diet samples. Science, 151: 101-104.
Duggan RE, Barry HC, & Johnson LY (1967) Residues in food and feed.
Pesticide residues in total diet samples (II). Pestic Monit J, 1(2):
2-12.
Dunkel VC, Pienta RJ, Sivak A, & Traul KA (1981) Comparative
neoplastic transformation responses of Balb/3T3 cells, Syrian
hamster embryo cells, and Rauscher murine leukaemia virus infected
Fischer 344 rat embryo cells to chemical carcinogens. J Natl Cancer
Inst, 67: 1303-1315.
Dunkel VC, Zeiger E, Brusick D, McCoy E, McGregor D, Mortelmans K,
Rosenkranz HS, & Simmon VF (1984) Reproducibility of microbial
mutagenicity assays. 1. Tests with Salmonella typhimurium and
Escheria coli using standardized protocol. Environ Mutagen, 6:
1-254.
Dunkel VC, Schechtman LM, Tu AS, Sivak A, Lubet RA, & Cameron TP
(1988) Interlaboratory evaluation of the C3H/10T1/2 cell
transformation assay. Environ Mol Mutagen, 12: 21-31.
Eberbach PL & Douglas LA (1989) Herbicide effects of the growth and
nodulation potential of Rhizobium trifolii with Triolium
subterraneum L. Plant Soil, 119: 15-23. Ebing W (1972) [Routine
method for identification of pesticide residues of triazine,
carbamate, urea and uracil type compounds by thin layer
chromatography.] J Chromatogr, 65: 533-545 (in German).
Elsea JR (1954) Aminotriazole: Acute dermal application, acute eye
application. Falls Church, Virginia, Hazleton Laboratories
(Unpublished report).
English JSC, Rycroft RJG, & Coleman CD (1986) Allergic dermatitis
from aminotriazole. Contact Dermatitis, 14: 255-256.
Environment Agency Japan (1987) Chemicals in the environment. Report
on environmental survey and wildlife monitoring of chemicals in F.Y.
1984 and 1985. Tokyo, Environment Agency Japan, Department of
Environmental Health, Office of Health Studies.
Fang SC, George M, & Yu TC (1964) Metabolism of
3-amino-1,2,4-triazole-5-14C by rats. J Agric Food Chem, 12:
219-223.
Fang SC, Khanna S, & Rao AV (1966) Further study on the metabolism
of labelled 3-amino-1,2,4-triazole (ATA) and its plant metabolites
in rats. J Agric Food Chem, 14: 262-265.
Fang SC, Fallin E, & Khanna S (1967) The metabolism of labelled
amitrole in plants. Weeds, 15: 343-346.
FAO/WHO (1975) Amitrole. In: 1974 Evaluations of some pesticide
residues in food. Geneva. World Health Organization, pp 3-49 (WHO
Pesticide Residues Series, No. 4).
FAO/WHO (1978) Amitrole. In: Pesticides residues in food - 1977.
Evaluations. Rome, Food and Agriculture Organization of the United
Nations, pp 11-14 (FAO Plant Production and Protection Paper 10
Sup.).
Feinstein RN, Fry RJN, & Staffeldt EF (1978a) Comparative effects of
aminotriazole on normal and acatalasemic mice. J Environ Pathol
Toxicol, 1(6): 779-790.
Feinstein RN, Fry RJM, & Staffeldt EF (1978b) Carcinogenic and
antitumour effects of aminotriazole on acatalasemic and normal
catalase mice. J Natl Cancer Inst, 60(5): 1113-1116.
Field WE (1979) Oral LD50 in rats. Clarks Summit, Pennsylvania,
CDC Research, Inc. (Unpublished report No. CDC-AM-048-79).
Fogleman RW (1954) Amizole (3-amino-1,2,4-triazole) 96.1%: Acute
oral administration in mice and dogs; acute intravenous
administration in dogs; pharmacodynamics in dogs; subacute feeding
in rats. Falls Church, Virginia, Hazleton Laboratories (Unpublished
report).
Fregly MJ (1968) Effect of aminotriazole on thyroid function in the
rat. Toxicol Appl Pharmacol, 13: 271-286.
Frey F (1979) Effects of the herbicide aminotriazole on the
nematode, Acrobeloides buetsclii in culture. Nematologica, 25:
146-147.
Fujii T, Miyazaki H, & Hashimoto M (1984) Autoradiographic and
biochemical studies of drug distribution in the liver III. [14C]
Aminotriazole. Eur J Drug Metab Pharmacokinet, 9: 257-265.
Gaines TB, Kimbrough RD, & Linder RE (1973) The toxicity of amitrole
in the rat. Toxicol Appl Pharmacol, 26: 118-129.
Gatehouse D (1981) Mutagenic activity of 42 coded compounds in the
"Microtiter" fluctuation test. In: de Serres FJ & Ashby J ed.
Evaluation of short-term tests for carcinogens. Report of the
international collaborative program. New York, Amsterdam, Oxford,
Elsevier/North-Holland, pp 376-386 (Progress in Mutation Research,
Volume 1).
Geldmacher-Von Mallinckrodt M & Schmidt HP (1970) [Toxicity and
metabolism of aminotriazole in man.] Arch Toxikol, 27: 13-18 (in
German).
Gentry GM, Jackson ER, Jensen TL, Jung PD, Launer JE, & Torma L ed.
(1984) Pesticide formulations. Amitrole in pesticide formulations:
Titrimetric methods. Final action. In: Official methods of analysis
of the Association of Official Analytical Chemists, 14th ed.
Arlington, Virginia, Association of Official Analytical Chemists,
Inc., p 146.
Grapenthien JR (1972) Two year chronic aerosol inhalation toxicity
study with amitrole 3-AT in albino rats. Northbrook, Illinois,
Industrial Bio-Test Laboratories, Inc. (Unpublished report No.
N7640, submitted to WHO by Bayer AG).
Grau R (1989) Toxicity of aminotriazol techn. to rainbow trout
(Salmo gairdneri) with prolonged exposure (21 days). Leverkusen,
Bayer AG (Unpublished report No. FF-242).
Green FO & Feinstein RN (1957) Quantitative estimation of
3-amino-1,2,4-triazole. Anal Chem, 29: 1658-1660.
Grossbard E & Wingfield GI (1978) Effect of paraquat, aminotriazole,
and glyphosate on cellulose decomposition. Weed Res, 18: 347-353.
Groves K & Chough KS (1971) Extraction of 3-amino-1,2,4-triazole
(amitrole) and 2,6-dichloro-4-nitroaniline (DCNA) from soils. J
Agric Food Chem, 19: 840-841.
Grunow W, Altmann HJ, & Boehme Chr (1975) [Metabolism of
3-amino-1,2,4-triazole in rats.] Arch Toxicol, 34(4): 315-324 (in
German).
Grzenda AR, Nicholson HP, & Cox WS (1966) Persistence of four
herbicides in pond water. J Am Water Works Assoc, 58: 326-332.
Hapke HJ (1967) [Toxicity of aminotriazole for domestic animals.]
Zent.bl Vet.med, 14: 469-486 (in German).
Hashimoto F, Sugimoto C, & Hayashi H (1992) Effects of aminotriazole
treatment on biosyntheses of primary bile acids in vivo. Chem
Pharm Bull, 40: 795-798.
Hassall KA (1969) World crop protection. Volume 2: Pesticides.
London, Iliffe Books Ltd, pp 233-235.
Hawkins DR, Kirkpatrick D, Finn CM, & Conway B (1982) The
biodegradation of 14C-aminotriazole in soil (field studies).
Huntingdon, Huntingdon Research Centre (Unpublished report No. BAY
124/702 054).
Hawkins DR, Kirkpatrick D, Finn CM, & Conway B (1982b) The
biodegradation of 14C-aminotriazole in soil (Laboratory studies).
Huntingdon, Huntingdon Research Centre (Unpublished report No. BAY
123/81 538).
Hayes S, Fordon A, Sadowski I, & Hayes C (1984) RK bacterial test
for independently measuring chemical toxicity and mutagenicity:
Short-term forward selection assay. Mutat Res, 130: 97-106.
Hecht P (1954) [Aminotriazole.] Leverkusen, Bayer AG (Unpublished
report) (in German).
Heim DR & Larrinua IM (1989) Primary site of action of amitrole in
Arabidopsis thaliana involves inhibition of root elongation but
not of histidine or pigment biosynthesis. Plant Physiol, 91:
1226-1231.
Heimann KG (1982) [Aminotriazole.] Leverkusen, Bayer AG (Unpublished
technical report) (in German).
Heimbach F (1983) Acute toxicity of amitrole (tech.) to water fleas.
Leverkusen, Bayer AG (Unpublished report No. HB/DM 22).
Heimbach F (1990a) Toxicity of aminotriazole (SP50) to carabid
beetles (Poecilus cupreus). Leverkusen, Bayer AG (Unpublished
report No. HBF/CA19).
Heimbach F (1990b) Toxicity of aminotriazol to earthworms.
Leverkusen, Bayer AG (Unpublished report No. HBF/RG 118).
Herbold B (1980) Amitrole: Salmonella/microsome test for detection
of point-mutagenic effects. Leverkusen, Bayer AG, Institute of
Toxicology (Unpublished report No. 9339).
Herbold B (1982) Amitrole: Aminotriazole active ingredient.
Micronucleus test in the mouse to evaluate for mutagenic effect.
Leverkusen, Bayer AG (Unpublished report No. 11139).
Herfs W (1975) Identification of crop protection product can be
designated as non-hazardous to honey bees - aminotriazole.
Brunswick, Germany, Federal Biological Institute for Agriculture and
Forestry, Division of Crop Protection Products and Application
Technology
Herrett RA & Linck AJ (1961) Quantitative determination of
3-amino-1,2,4-triazole. J Agric Food Chem, 9(6): 466-467.
Hiasa Y, Ohshima M, Kitahori Y, Yuasa T, Fujita T, & Iwata C (1982)
Promoting effects of 3-amino-1,2,4-triazole on the development of
thyroid tumours in rats treated with N-bis
(2-hydroxypropyl)nitrosamine. Carcinogenesis, 3(4): 381-384. Hill EF
& Camardese MB (1986) Lethal dietary toxicities of environmental
contaminants and pesticides to Coturnix. Washington, DC, US
Department of the Interior, Fish and Wildlife Service (Fish and
Wildlife Technical Report No. 2).
Hill EF, Heath RG, Spann JW, & Williams JD (1975) Lethal dietary
toxicities of environmental pollutants to birds. Washington, DC, US
Department of the Interior, Fish and Wildlife Service (Special
Scientific Report - Wildlife No. 191).
Hill RN, Erdreich LS, Paynter OE, Roberts AA, Rosenthal SL, &
Wilkinson CF (1989) (Review) Thyroid follicular cell carcinogenesis.
Fundam Appl Toxicol, 12: 629-697.
Hiltibran RC (1967) Effects of some herbicides on fertilized fish
eggs and fry. Trans Am Fish Soc, 96(4): 414-416.
Hodge HC, Maynard EA, Downs WL, Ashton JK, & Salerno LL (1966) Tests
on mice for evaluating carcinogenicity. Toxicol Appl Pharmacol, 9:
583-596.
Hoshino M (1960) Effect of 3-amino-1,2,4-triazole on the
experimental production of liver cancer. Nature (Lond), 186(4719):
174-179.
Hubbard SA, Green MHL, Bridges BA, Wain AJ, & Bridges JW (1981)
Fluctuation test with S9 and hepatocyte activation. In: de Serres FJ
& Ashby J ed. Evaluation of short-term tests for carcinogens. Report
of the international collaborative program. New York, Amsterdam,
Oxford, Elsevier/North-Holland, pp 361-370 (Progress in Mutation
Research, Volume 1).
Hudson RH, Tucker RK, & Haegele MA (1984) Handbook of toxicity of
pesticides to wildlife, 2nd ed. Washington, DC, US Department of the
Interior, Fish and Wildlife Service (Resource Publication 153).
IARC (1974) Amitrole. In: Some antithyroid and related substances,
nitrofurans and industrial chemicals. Lyon, International Agency for
Research on Cancer, pp 31-43 (IARC Monographs on the Evaluation of
the Carcinogenic Risk of Chemicals to Man, Volume 7).
IARC (1986) Amitrole. In: Some halogenated hydrocarbons and
pesticide exposures. Lyon, International Agency for Research on
Cancer, pp 293-317 (IARC Monographs on the Evaluation of the
Carcinogenic Risk of Chemicals to Humans, Volume 41).
IARC (1987) Amitrole. In: Overall evaluations of carcinogenicity: An
updating of IARC monographs, volumes 1 to 42. Lyon, International
Agency for Research on Cancer, pp 92-93 (IARC Monographs on the
Evaluation of the Carcinogenic Risk of Chemicals to Humans,
Supplement 7).
Ichinotsubo D, Mower H, & Mandel M (1981a) Testing of a series of
paired compounds (carcinogen and non-carcinogenic structural analog)
by DNA repair-deficient E. coli strains. In: de Serres FJ & Ashby
J ed. Evaluation of short-term tests for carcinogens. Report of the
international collaborative program. New York, Amsterdam, Oxford,
Elsevier/North-Holland, pp 195-198 (Progress in Mutation Research,
Volume 1).
Innes JR, Ulland BM, Valerio MG, Petrucelli L, Fishbein L, Hart ER,
& Palotta AJ (1969) Bioassay of pesticides and industrial chemicals
for tumorigenicity in mice: A preliminary note. J Natl Cancer Inst,
42(6): 1101-1114.
Inoue K, Katoh Y, & Takayama S (1981) In vitro transformation of
hamster embryo cells by 3-(N-salicyloyl)amino-1,2,4-triazole.
Toxicol Lett, 7: 211-215.
Iwan GR, Vilkas AG, & Hutchinson C (1978) 14C-Amitrole in Bluegill
Sunfish, Lepomis Macrochirus: bioconcentration study. Tarry Town,
New York, Union Carbide Corporation, Environmental Services
(Unpublished report).
Jacques A (1984) 3-Amino- s-triazole (amitrole) (Update). Anal
Methods Pestic Plant Growth Regul, XIII: 191-195.
Jagannath DR, Vultaggio DM, & Brusick DJ (1981) Genetic activity of
42 coded compounds in the mitotic gene conversion assay using
Saccharomyces cerevisiae strain D4. In: Dr Serres FJ & Ashby J ed.
Evaluation of short term tests for carcinogens. Report of the
international collaborative program. New York, Amsterdam, Oxford,
Elsevier/North-Holland, pp 456-467 (Progress in Mutation Research,
Volume 1).
Jarczyk HJ (1982a) [Behaviour of active ingredients of pesticides in
the soil (Amitrole).] Leverkusen, Bayer AG (Unpublished report No.
RR0600/81) (in German).
Jarczyk HJ (1982b) [Behaviour of active ingredients of pesticides in
the soil (Amitrole).] Leverkusen, Bayer AG (Unpublished report No.
RR0601/81) (in German).
Jarczyk HJ (1982c) [Behaviour of active ingredients of pesticides in
the soil (Amitrole).] Leverkusen, Bayer AG (Unpublished report No.
RR0602/81) (in German).
Jarczyk HJ (1985) Method for the gas-chromatographic determination
of residues of 3-amino-1,2,4-triazole in apples, pears, cherries,
grapes, soil and water, using an N-specific detector. Leverkusen,
Bayer AG (Unpublished report No. RA-988/296B).
Jarczyk HJ & Mollhoff E (1988) Method for the gaschromatographic
determination of amino-1,2,4-triazole residues in plant, soil and
water using a nitrogen specific detector. Leverkusen, Bayer AG
(Unpublished report No. RA-785/88).
Jensen-Korte U, Anderson C, & Spiteller M (1987) Photodegradation of
pesticides in the presence of humic substances. Sci Total Environ,
62: 335-340.
Johnson CR (1976) Herbicide toxicity in some Australian anurans and
the effect of subacute dosages on temperature tolerance. Zool J Linn
Soc, 59: 79-83.
Johnson WD, Becci PJ, & Parent RA (1981) Lifetime feeding study of
amitrole in Fischer 344 rats. Waverly, New York, Food and Drug
Research Laboratories, Inc. (Unpublished report No. 5651).
Jukes TH & Shaffer CB (1960) Antithyroid effects of aminotriazole.
Science, 132: 296-297.
Jung F, Szekacs A, Li Q, & Hammock BD (1991) Immunochemical approach
to the detection of aminotriazole using selective amino group
protection by chromophores. J Agric Food Chem, 39: 129-136.
Kada T (1981) The DNA-damaging activity of 42 coded compounds in the
rec-assay. In: de Serres FJ & Ashby J ed. Evaluation of short-term
tests for carcinogens. Report of the international collaborative
program. New York, Amsterdam, Oxford, Elsevier/North-Holland, pp
171-182 (Progress in Mutation Research, Volume 1).
Kassinova GV, Kovaltsova SV, Marfin SV, & Zakharov IA (1981)
Activity of 40 coded compounds in differential inhibition and
mitotic crossing-over assays in yeast. In: de Serres FJ & Ashby J
ed. Evaluation of short term tests for carcinogens. Report of the
international collaborative program. New York, Amsterdam, Oxford,
Elsevier/North-Holland, pp 434-455 (Progress in Mutation Research,
Volume 1).
Kaufman DD, Plimmer JR, Kearney PC, Blake J, & Guardia FS (1968)
Chemical versus microbial decomposition of amitrole in soil. Weed
Sci, 16(2): 266-272.
Keller JG (1959) Aminotriazole (3-amino-1,2,4-triazole): Final
report of 2-year feeding study in rats. Falls Church, Virginia,
Hazleton Laboratories (Unpublished report).
Kimmerle G (1968) [Aminotriazole.] Leverkusen, Bayer AG (Unpublished
report) (in German).
Klusacek H & Krasemann R (1986) Thermal stability of the
agrochemical active ingredient. Leverkusen, Bayer AG (Unpublished
report No. 85/10472TA).
Knacker Th & Reifenberg P (1989) A study of the toxicity to algae of
aminotriazol SP50. Frankfurt am Main, Battelle Europe (Unpublished
report No. BEEA 148901 ALG7).
Knickerbocker M & Stevens KR (1978) A study to determine the
potential of amitrole to induce dominant lethal mutations in Ha
(ICR) mice. Waverly, New York, Food and Drug Research Laboratories,
Inc. (Unpublished report No. 5502).
Krauss RS & Eling TE (1987) Macromolecular binding of the thyroid
carcinogen 3-amino-1,2,4-triazole (amitrole) catalyzed by
prostaglandin H synthetase, lactoperoxidase and thyroid peroxidase.
Carcinogenesis, 8: 659-664.
Krohn A (1982) Fate/behaviour of crop protection products in water:
(hydrolytic stability/volatility from water). Leverkusen, Bayer AG
(Unpublished report No. M2067).
Kumar HD (1963) Inhibition of growth and pigment production of a
blue-green alga by 3-amino-1,2,4-triazole. Indian J Plant Physiol,
6: 150-155.
Laamanen I, Sorsa M, Bamford D, Gripenberg U, & Meretoja T (1976)
Mutagenicity and toxicity of amitrole. I. Drosophila tests. Mutat
Res, 40(3): 185-190.
Landauer W, Salam N, & Sopher D (1971) The herbicide
3-amino-1,2,4-triazole (amitrole) as teratogen. Environ Res, 4:
539-543.
Legrand MF, Costentin E, & Bruchet A (1991) Occurrence of 38
pesticides in various French surface and ground waters. Environ
Technol, 12: 985-996.
Lokke H (1980) Determination of amitrole by ion-pair
high-performance liquid chromatography. J Chromatogr, 200: 234-237.
LUFA (1977) [Behaviour of active ingredients of pesticides in the
soil (Amitrole).] Speyer am Rhein, Agriculture Monitoring and
Research Institute (Unpublished report No. M7750).
MacCarthy P & Djebbar KE (1986) Removal of paraquat, diquat and
amitrole from aqueous solution by chemically modified peat. J
Environ Qual, 15: 103-107.
MacDonald DJ (1981) Salmonella/microsome tests on 42 coded
chemicals. In: de Serres FJ & Ashby J ed. Evaluation of short-term
tests for carcinogens. Report of the international collaborative
program. New York, Amsterdam, Oxford, Elsevier/North-Holland, pp
285-297 (Progress in Mutation Research, Volume 1).
MacDonald CM & Pullinger DH (1976) A pharmakinetic study to compare
"head only" and "whole body" inhalation methods of 14C-amitrole
exposure. Harrogate, UK, Hazleton Laboratories Europe Ltd
(Unpublished report No. 291/1).
McGregor DB, Martin R, Cattanach P, Edwards I, McBride D, & Caspary
WJ (1987) Responses of the L5178Y tk+/tk-mouse Lymphoma cell
forward mutation assay to coded chemicals. Environ Mutagen, 9:
143-160.
Machemer L (1977a) Amitrole. Dominant lethal test on the male mouse
to evaluate for mutagenic effects. Leverkusen, Bayer AG (Unpublished
report No.6679).
Machemer L (1977b) Amitrole. Study for embryotoxic and teratogenic
effects after oral administration to the rat. Leverkusen, Bayer AG
(Unpublished report No. 6634).
McTiernan AM, Weiss NS, & Dalling JR (1984), Incidence of thyroid
cancer in women in relation to previous exposure to radiation
therapy and history of thyroid disease. J Natl Cancer Inst, 73:
575-581.
Mamber SW, Bryson V, & Katz SE (1983) The Escherichia coli
WP2/WP100 rec assay for detection of potential carcinogens. Mutat
Res, 119(2): 135-144.
Mamber SW, Bryson V, & Katz SE (1984) Evaluation of the Escheria
coli K12 induce test for detection of potential chemical
carcinogens. Mutat Res, 130: 141-151.
Margoliash E, Novogradsky AR, & Schejter A (1960) Irreversible
reaction of 3-amino-1,2,4-triazole and related inhibitors with the
protein of catalase. Biochem J, 74: 339-348.
Marston RB, Schults DW, Shiroyama T, & Snyder LV (1968) Pesticides
in water. Amitrole concentrations in creek waters downstream from an
aerially sprayed watershed sub-basin. Pestic Monit J, 2(3): 123-128.
Martin NA (1982) The effects of herbicides used on asparagus on the
growth rate of the earthworm, Allolobophora Caliginosa. In:
Proceedings of the 35th New Zealand Weed and Pest Control
Conference, pp 328-329.
Martin CN & McDermid AC (1981) Testing of 42 coded compounds for
their ability to induce unscheduled DNA repair synthesis in HeLa
cells. In: de Serres FJ & Ashby J ed. Evaluation of short-term tests
for carcinogens. Report of the international collaborative program.
New York, Amsterdam, Oxford, Elsevier/North-Holland, pp 533-537
(Progress in Mutation Research, Volume 1).
Mason, JM, Valencia R, & Zimmering S (1992) Chemical mutagenesis
testing in Drosophila. VIII. Reexamination of equivocal results.
Environ. Mol Mutagen, 19: 227-234.
Mayberry WE (1968) Antithyroid effects of 3-amino-1,2,4-triazole.
Proc Soc Exp Biol Med, 129: 551-556.
Mayer FL & Ellersieck MR (1986) Manual of acute toxicity:
Interpretation and data base for 410 chemicals and 66 species of
freshwater animals. Washington, DC, US Department of the Interior,
Fish and Wildlife Service (Resource Publication 160).
Mehta RD & Von Borstel RC (1981) Mutagenic activity of 42 encoded
compounds in the haploid yeast reversion assay, strain XV185 -14C,
In: de Serres FJ & Ashby J ed. Evaluation of short-term tests for
carcinogens. Report of the international collaborative program. New
York, Amsterdam, Oxford, Elsevier/North-Holland, pp 414-423
(Progress in Mutation Research, Volume 1).
Meretoja T, Gripenberg U, Bamford D, Laamanen I, & Sorsa M (1976)
Mutagenicity and toxicity of amitrole. II. Human lymphocyte culture
tests. Mutat Res, 40: 191-196.
Metodiewa D & Dunford HB (1991) 3-Aminotriazole is a substrate for
lactoperoxidase but not for catalase. Biochem Biophys Res Commun,
180: 585-590.
Middelkoop E, Strijland A, & Tager JM (1991) Does aminotriazole
inhibit import of catalase into peroxisomes by retarding unfolding?
FEBS Lett, 279: 79-82.
Mihail F (1984) Aminotriazole - Study for skin sensitization effect
on guinea pigs. Leverkusen, Bayer AG, Institute of Toxicology
(Unpublished report No. 12607).
Mihail F (1985) Aminotriazole - Study for skin-sensitising effect on
guinea pigs in the open epicutaneous test. Leverkusen, Bayer AG,
Institute of Toxicology (Unpublished report No. 13775).
Miksche LW (1982) BBA request - Effects on man. Leverkusen, Bayer
AG, Medical Department (Unpublished report).
Miksche LW (1983) Production of amitrole - Medical monitoring.
Leverkusen, Bayer AG, Medical Department (Unpublished report).
Moore B (1968) Amitrole and simazine residues in Tasmanian apples
treated with Domatol 44. Sydney, Ciba-Geigy Australia Ltd
(Unpublished report No. 68/8/290).
Moore B (1969) Amitrole and simazine residue trials at Orange, NSW,
1967-1968. Sydney, Ciba-Geigy Australia Ltd (Unpublished report No.
69/2/294).
Moore B (1970) Amitrole residue trial, Orange, 1968-69. Sydney,
Ciba-Geigy Australia Ltd (Unpublished report No. 70/1/309).
Mori S, Takeuchi Y, Toyama M, Makino S, Ohhara T, Tochino Y, &
Hayashi Y (1985) Amitrole: Strain differences in morphological
response of the liver following subchronic administration to mice.
Toxicol Lett, 29: 145-152.
Moriya M, Ohta T, Watanabe K, Miyazawa T, Kato K, & Shirasu Y (1983)
Further mutagenicity studies on pesticides in bacterial reversion
assay systems. Mutat Res, 116(3-4): 185-216.
Morpurgo G, Bellincampi D, Gualandi G, Baldinelli L, & Crescenzi OS
(1979) Analysis of mitotic nondisjunction with Aspergillus
nidulans. Environ Health Perspect, 31: 81-95.
Mothes-Wagner U, Reitz HK, & Seitz KA (1990) Environmental actions
of agrochemicals. 1. Side-effects of the herbicide 3-amino-1,2,4-
triazole on a laboratory acarine/host-plant interaction
(Tetranychus uticae/Phaseolus vulgaris) as revealed by electron
microscopy. Environ Appl Acarol, 8: 27-40.
Nearpass DC (1969) Exchange adsorption of 3-amino-1,2,4-triazole by
an organic soil. Proc Soil Sci Soc Am, 33: 524-528.
Nearpass DC (1970) Exchange adsorption of 3-amino-1,2,4-triazole by
montmorillonite. Soil Sci, 109(2): 77-84.
Niehuss M & Borner H (1971) [Investigations into the effects of
herbicides on fish.] Nachr.bl Dtsch Pflanzenschutzd, 8: 113-117 (in
German).
Nishiuchi Y (1981) Toxicity of pesticides to some aquatic organisms.
Toxicity of pesticides to some aquatic insects. Seitai Kagaku, 4:
31-46.
Nomiyama K, Minai M, & Kita H (1965) Median lethal dose of
3-amino-1,2,4-triazole. Bull Tokyo Med Dent Univ, 12(1): 55-57.
Pachinger A, Eisner E, Begutter H, & Klus H (1992) A simple method
for the determination of amitrole in drinking and ground water.
Fresenius J Anal Chem, 342: 413-415.
Perry PE & Thomson JE (1981) Evaluation of the sister chromatid
exchange method in mammalian cells as a screening system for
carcinogens. In: de Serres FJ & Ashby J ed. Evaluation of short-term
tests for carcinogens. Report of the international collaborative
program. New York, Amsterdam, Oxford, Elsevier/North-Holland, pp
560-569 (Progress in Mutation Research, Volume 1).
Plimmer JR, Kearney PC, Kaufman DD, & Guardia FS (1967) Amitrole
decomposition by free radical-generating system and by soils. J
Agric Food Chem, 15(6): 996-999.
Pribyl J, Herzel F, & Schmidt G (1978) [Contribution to the
determination of aminotriazole residues.] Fresenius Z Anal Chem,
289: 81-85 (in German).
Prince HN (1977) Microbial mutagen assays with amitrol
(3-amino-1,2,4-triazole). Fairfield, New Jersey, Gibraltar
Biological Laboratories (Unpublished report No. 5634, submitted to
WHO by Bayer AG).
Racusen D (1958) The metabolism and translocation of 3-aminotriazole
in plants. Arch Biochem Biophys, 74(1): 106-113.
Rausina G (1972) Two year chronic dermal toxicity study with
amitrole 3-AT on albino rats. Northbrook, Illinois, Industrial
Bio-Test Laboratories, Inc. (Unpublished report No. A7639, submitted
to WHO by Bayer AG).
Reddy KR, Rao JV, & Das VSR (1987) Effect of monosodium methane
arsonate and aminotriazole on biosynthesis of epicuticular wax in
some semiarid shrubs. Indian J Exp Biol, 25: 562-566.
Reitze HK & Seitz KA (1985) Light and electron microscopical changes
in the liver of mice following treatment with aminotriazole. Exp
Pathol, 27: 17-31.
Richold M & Jones E (1981) Mutagenic activity of 42 coded compounds
in the salmonella/microsome assay. In: de Serres FJ & Ashby J ed.
Evaluation of short-term tests for carcinogens. Report of the
international collaborative program. New York, Amsterdam, Oxford,
Elsevier/North-Holland, pp 314-332 (Progress in Mutation Research,
Volume 1).
Riepma P (1962) Preliminary observations on the breakdown of
3-amino-1,2,4-triazole in plants. Weed Res, 2: 41-50.
Ritter A (1989) Influence of amitrole technical on the reproduction
of Daphnia magna. Itingen, Switzerland, RCC Umweltchemie AG
(Unpublished report No. 235192).
Robertson EB & Bunting DL (1976) The acute toxicity of four
herbicides to 0-4 hour Nauplii of Cyclops vernalis Fisher
(Copepoda, Cyclopoida). Bull Environ Contam Toxicol, 16(6): 682-688.
Ron E, Kleinerman RA, Boice JD, Livolsi VA, Flannery JT, & Fraumeni
JF (1987) A population-based case-control study of thyroid cancer. J
Natl Cancer Inst, 79: 1-12.
Rosenkranz HS & Poirier LA (1979) Evaluation of the mutagenicity and
DNA-modifying activity of carcinogens and non-carcinogens in
microbial systems. J Natl Cancer Inst, 62: 873-892.
Rosenkranz HS, Hyman J, & Leifer Z (1981) DNA polymerase deficient
assay. In: de Serres FJ & Ashby J ed. Evaluation of short-term tests
for carcinogens. Report of the international collaborative program.
New York, Amsterdam, Oxford, Elsevier/North-Holland, pp 210-218
(Progress in Mutation Research, Volume 1).
Rowland I & Severn B (1981) Mutagenic of carcinogens and
noncarcinogens in the salmonella/microsome test. In: de Serres FJ &
Ashby J ed. Evaluation of short-term tests for carcinogens. Report
of the international collaborative program. New York, Amsterdam,
Oxford, Elsevier/North-Holland, pp 323-332 (Progress in Mutation
Research, Volume 1).
Salama AM, Kobbia IA, Heikal NZ, & Shabana EF (1985) The influence
of the herbicide amitrole on growth and dynamics of carbohydrate and
nitrogenous compounds in two blue-green algae from the Nile drains.
Arch Hydrobiol, 105(1): 117-127.
Schneider B, Stock M, Schneider G, Schutte HR, Schreiber K, Brauner
A, & Kaussmann EU (1992) Metabolism of amitrole in apple. I. Soluble
metabolites from mature fruits. Z Nat.forsch, 47c: 120-125.
Schiestl RH, Geitz RD, Mehta RD, & Hasting PJ (1989) Carcinogens
induce intrachromosomal recombination in yeast. Carcinogenesis,
10(8): 1445-1455.
Scholz K (1988) Metabolism of amitrole in soil under aerobic
conditions. Leverkusen, Bayer AG (Unpublished report No. M5977).
Schubert OE (1964) Residues of amitrole in apple fruit following
ground cover, fruit and leaf applications. Proc West Va Acad Sci,
43: 29-35.
Schultz TW & Kennedy JR (1976) Cytotoxicity effects of the herbicide
3-amino-1,2,4-triazole on Daphnia pulex (Crustacea:Cladocera).
Biol Bull, 151: 370-385.
Seidenberg IL & Gee AH (1953) Report on acute toxicity of
aminotriazole as established by oral administration to rats. New
York, Snell Laboratories, Inc. (Unpublished report).
Seiler JP (1977) Inhibition of testicular DNA synthesis by chemical
mutagens and carcinogens. Preliminary results in the validation of
an novel short-term test. Mutat Res, 46: 305-310.
Shaffer CB, Bagdon RE, Vidone LB, & Golz HH (1956) Aminotriazole:
Acute and subacute toxicity. Wayne, New Jersey, American Cyanamid
Company, Central Medical Department, Industrial Toxicology Section
(Unpublished Report No. 56/29).
Sharp DC & Parry JM (1981a) Use of repair deficient strains of yeast
to assay the activity of 40 coded compounds. In: de Serres FJ &
Ashby J ed. Evaluation of short-term tests for carcinogens. Report
of the international collaborative program. New York, Amsterdam,
Oxford, Elsevier/North-Holland, pp 502-516 (Progress in Mutation
Research, Volume 1).
Sharp DC & Parry JM (1981b) Induction of mitotic gene conversion by
41 coded compounds using the yeast culture JDI. In: de Serres FJ &
Ashby J ed. Evaluation of short-term tests for carcinogens. Report
of the international collaborative program. New York, Amsterdam,
Oxford, Elsevier/North-Holland, pp 491-501 (Progress in Mutation
Research, Volume 1).
Shirasu Y, Moriya M, Kato T, Furviiashi A, & Kadu T (1976)
Mutagenicity screening of pesticides in the microbial system. Mutat
Res, 40: 19-30.
Simmon VF, Rosenkranz HS, Zeiger E, & Poirier LA (1979) Mutagenic
activity of chemical carcinogens and related compounds in the
intraperitoneal host-mediated assay. J Natl Cancer Inst, 62(4):
911-918.
Sittig M (1985) Handbook of toxic and hazardous chemicals and
carcinogens, 2nd ed. Park Ridge, New Jersey, Noyes Publications, pp
69-70.
Skopek TR, Andon BM, Kaden DA, & Thilly WG (1981) Mutagenic activity
of 42 coded compounds using 8-azagunine resistance as a genetic
marker in Salmonella typhimurium. In: de Serres FJ & Ashby J ed.
Evaluation of short-term tests for carcinogens. Report of the
international collaborative program. New York, Amsterdam, Oxford,
Elsevier/North-Holland, pp 371-375 (Progress in Mutation Research,
Volume 1).
Smith LW, Bayer DE, & Foy CL (1969) Metabolism of amitrole in
excised leaves of Canada thistle ecotypes and beans. Weed Sci, 16:
523-537.
Sorsa M & Gripenberg U (1976) Organization of a mutagenicity test
system combining instructive purposes: Testing for mutagenic effects
of the herbicide "amitrole". Mutat Res, 38(4): 132-133.
Steinhoff D & Boehme K (1978) Aminotriazole (amitrole).
Carcinogenicity study on orally dosed golden hamsters. Leverkusen,
Bayer AG (Unpublished proprietary report No. 7521).
Steinhoff D & Boehme K (1979a) Aminotriazole (amitrole).
Cancerogenesis test with oral administration to rats. Leverkusen,
Bayer AG (Unpublished proprietary report No. 8450).
Steinhoff D & Boehme K (1979b) Aminotriazole (amitrole).
Carcinogenesis study with oral administration to mice. Leverkusen,
Bayer AG (Unpublished report No. 8490).
Steinhoff D, Weber H, Mohr U, & Boehme K (1983) Evaluation of
amitrole (aminotriazole) for potential carcinogenicity in orally
dosed rats, mice and golden hamsters. Toxicol Appl Pharmacol, 69:
161-169.
Stock M, Bohm H, Schneider B, Schutte H-R, Schriiber K, Brauner A, &
Koster J (1991) Metabolism of amitrole in cell suspension cultures
of apples. Herbsttag Ges Biol Chem, 372: 763-764.
Storherr RW & Burke J (1961) Determination of 3-amino-1,2,4-triazole
in crops. J Assoc Off Anal Chem, 44(2): 196-199.
Storherr RW & Onley J (1962) Cleanup and separation of
3-amino-1,2,4-triazole and metabolites from vegetable crops. J Assoc
Off Anal Chem, 45(2): 382-387.
Strum JM & Karnovsky MJ (1971) Aminotriazole goiter. Fine structure
and localization of thyroid peroxidase activity. Lab Invest, 24(1):
1-12.
Styles JA (1979) Studies on the detection of carcinogens using a
mammalian cell transformation assay with liver homogenate
activation. In: Norpoth K & Garner RC ed. Short-term mutagenicity
test systems for detecting carcinogens. Proceedings of the
International Symposium on Short-Term Mutagenicity Test Systems,
Dortmund, 15-17 November 1978. New York, Berlin, Heidelberg,
Springer Verlag, pp 226-238.
Styles JA (1981) Activity of 42 coded compounds in the BHK-21 cell
transformation test. In: de Serres FJ & Ashby J ed. Evaluation of
short-term tests for carcinogens. Report of the international
collaborative program. New York, Amsterdam, Oxford,
Elsevier/North-Holland, pp 638-646 (Progress in Mutation Research,
Volume 1).
Sumi C, Yokoro K, & Matsushima R (1985) Inhibition by
3-amino-1 H-1,2,4-triazole of hepatic tumorigenesis induced by
diethylstilbestrol alone or combined with N-nitrobutylurea in WF
rats. J Natl Cancer Inst, 74: 1329-1334.
Sund KA (1956) Residual activity of 3-amino-1,2,4-triazole in soils.
J Agric Food Chem, 4(1): 57-60.
Tag El-Din A, Abbas MM, Aly HA, Tantawy G, & Askar A (1981) Acute
toxicities to Mugil cephalus fry caused by some herbicides and new
pyrethroids. Meded Fac Landbouwwet Rijksuniv Gent, 46(1): 387-391.
Tampier L & Quintanilla ME (1990) Effect of 3-amino-1,2,4-triazole
on the hypothermic effect of ethanol and on ethanol tolerance
development. Alcohol, 8: 279-282.
Thomson JA (1981) Mutagenic activity of 42 coded compounds in the
lambda induction assay. In: de Serres FJ & Ashby J ed. Evaluation of
short-term tests for carcinogens. Report of the international
collaborative program. New York, Amsterdam, Oxford,
Elsevier/North-Holland, pp 224-235 (Progress in Mutation Research,
Volume 1).
Thyssen J (1974) [Aminotriazole Pt. 143 - Determination of acute
toxicity.] Leverkusen, Bayer AG (Unpublished report) (in German).
Tjalve H (1974) Fetal uptake and embryogenetic effects of
aminotriazole in mice. Arch Toxicol, 33(1): 41-48.
Tjalve H (1975) The distribution of labelled aminotriazole in mice.
Toxicology, 3(1): 49-67.
Topham JC (1980) Do induced sperm-head abnormalities in mice
specifically identify mammalian mutagens rather than carcinogens?
Mutat Res, 74: 379-387.
Tripathy NK, Wugler FE, & Frei H (1990) Genetic toxicity of six
carcinogens and six non-carcinogens in the Drosphilia wing spot
test. Mutat Res, 242: 169-180.
Trueman RW (1981) Activity of 42 coded compounds in the Salmonella
reverse mutation test. In: de Serres FJ & Ashby J ed. Evaluation of
short-term tests for carcinogens. Report of the international
collaborative program. New York, Amsterdam, Oxford,
Elsevier/North-Holland, pp 343-350 (Progress in Mutation Research,
Volume 1).
Tschudy DP & Collins A (1957) Effect of 3-amino-1,2,4-triazole on
delta-aminolevulinic acid dehydrase activity. Science, 126: 168.
Tsuchimoto T & Matter BE (1981) Activity of coded compounds in the
micronucleus test. In: de Serres FJ & Ashby J ed. Evaluation of
short-term tests for carcinogens. Report of the international
collaborative program. New York, Amsterdam, Oxford,
Elsevier/North-Holland, pp 705-711 (Progress in Mutation Research,
Volume 1).
Tsuda H, Takahashi M, Fukushima S, & Endo Y (1973) Fine structure
and localization of peroxidase activity in aminotriazole goiter.
Nagoya Med J, 18(3): 183-189.
Tsuda H, Hananouchi M, Tatematsu M, Hirose M, Hirao K, Takahashi M,
& Ito N (1976) Tumorigenic effect of 3-amino-1H-1,2,4-triazole on
rat thyroid. J Natl Cancer Inst, 57(4): 861-864.
Tsuda H, Takahashi M, Murasaki G, Ogiso T, & Tatematsu M (1978)
Effect of 3-amino-1H-1,2,4-triazole or low iodine diet on rat
thyroid carcinogenesis induced by ethylenethiourea. Nagoya Med J,
23: 83-92.
Tsutsui T, Maizumi H, & Barret JC (1984) Amitrole-induced cell
transformation and gene mutations in Syrian hamster embryo cells in
culture. Mutat Res, 140: 205-207.
Turner DM & Gilbert CM (1976) Evidence of metabolism of 14C
amitrole in the rat after inhalation administration. Harrogate, UK,
Hazleton Laboratories Europe Ltd (Unpublished report No. 291/1a).
Tweats DI (1981) Activity of 42 coded compounds in a differential
killing test using Escheria coli strains, WP2, WP67 (uvrA polA),
and CM871 (uvrA lexA recA). In: de Serres FJ & Ashby J ed.
Evaluation of short-term tests for carcinogens. Report of the
international collaborative program. New York, Amsterdam, Oxford,
Elsevier/North-Holland, pp 199-209 (Progress in Mutation Research,
Volume 1).
US EPA (1992) EPA notice setting forth preliminary determination
proposing termination of special review for amitrole. Washington,
DC, Bureau of National Affairs, Inc.
Van der Poll JM, Vink M, & Quirijns JK (1988) Capillary gas
chromatographic determination of amitrole in water with alkali flame
ionization detection. Chromatographia, 25(6): 511-513.
Venitt S & Crofton-Sleigh S (1981), Mutagenicity of 42 coded
compounds in a bacterial assay using Escheria coli and Salmonella
typhimurium. In: de Serres FJ & Ashby J ed. Evaluation of
short-term tests for carcinogens. Report of the international
collaborative program. New York, Amsterdam, Oxford,
Elsevier/North-Holland, pp 351-360 (Progress in Mutation Research,
Volume 1).
Vesselinovitch SD (1983) Perinatal hepatocarcinogenesis. Biol Res
Pregnancy Perinatol, 4(1): 22-25.
Vogel E, Blijleven WGH, Kortselius MJH, & Zijlstra JA (1981)
Mutagenic activity of 17 coded compounds in the sex-linked recessive
lethal test in Drosophila melanogaster. In: de Serres FJ & Ashby J
ed. Evaluation of short-term tests for carcinogens. Report of the
international collaborative program. New York, Amsterdam, Oxford,
Elsevier/North-Holland, pp 660-665 (Progress in Mutation Research,
Volume 1).
Weber E (1988) Method for the determination of amitrole residues in
plant material, soil and water. Leverkusen, Bayer AG (Unpublished
report No. RA-905/88).
Weber H & Patschke K (1978) Effect of long-term administration of
aminotriazole on thyroid function of male and female rats, mice and
hamsters. Leverkusen, Bayer AG (Unpublished report No. 7690).
Weir R (1958) 3-Amino-1,2,4-triazole: Chronic oral administration -
Beagle dogs - 52 weeks. Final report. Falls Church, Virginia,
Hazleton Laboratories (Unpublished report).
Weller H (1987) Leaching characteristics of soil-aged amitrole.
Leverkusen, Bayer AG (Unpublished report No. HM736).
Wishe HI, Rolle-Getz GK, & Goldsmith ED (1979) The effects of
aminotriazole (ATZ) on the thyroid gland and the development of the
white Leghorn chick. Growth, 43(4): 238-251.
Wolf FT (1962) Growth inhibition of Chorella induced by
3-amino-1,2,4-triazole and its reversal by purines. Nature (Lond),
193: 901-902.
Worthing CR & Hance RJ ed. (1991) The pesticide manual, 9th ed.
Farnham, Surrey, The British Crop Protection Council. pp 25-26.
Wynford-Thomas D, Stringer BMJ, & Williams ED (1982a)
Desensitisation of rat thyroid to the growth-stimulating action of
TSH during prolonged goitrogen administration: Persistence of
refractoriness following withdrawal of stimulation. Acta Endocrinol,
101: 562.
Wynford-Thomas D, Stringer BMJ, & Williams ED (1982b)
Goitrogen-induced thyroid growth in the rat: A quantitative
morphometric study. J Endocrinol, 94: 131.
Wynford-Thomas D, Stringer BMJ, Gomez Morales M, & Williams ED
(1983) Vascular changes in early TSH-induced thyroid tumours in the
rat. Br J Cancer, 47(6): 861-865.
Yoshida T, Maruyama T, Kojima HI, Allanpichay I, & Mori S (1986)
Evaluation of the effect of chemicals on aquatic ecosystem by
observing the photosynthetic activity of a macrophyte, Porphyra
yezoensis. Aquat Toxicol, 9: 207-214.
Yoshida T & Nishimura Y (1972) Toxicity of pesticides to some water
organisms. Bull Agric Chem Insp Stn, 12: 122-128.
Zandvoort FK, Braber JM, & Pannebakker EG (1981) The disappearance
of aminotriazole from some railway beds in the Netherlands. Meded
Fac Landbouwwet Rijksuniv Gent, 46(1).
Zimmerman FK & Scheel I (1981) Induction of mitotic gene conversion
in strain D7 of Saccharomyces cervisiae by 42 coded compounds. In:
de Serres FJ & Ashby J ed. Evaluation of short-term tests for
carcinogens. Report of the international collaborative program. New
York, Amsterdam, Oxford, Elsevier/North-Holland, pp 481-490
(Progress in Mutation Research, Volume 1).
1. RESUME
1.1 Identité, propriétés physiques et chimiques et méthodes
d'analyse
L'amitrole, ou 3-amino-1,2,4-triazole, se présente sous la
forme d'une poudre cristalline incolore. Il est stable à la chaleur
et son point de fusion est de 156-159 °C. Il est facilement soluble
dans l'eau et l'éthanol mais seulement légèrement soluble dans les
solvants organiques tels que l'hexane et le toluène. Chimiquement,
l'amitrole se comporte à la fois comme une amine aromatique typique
et comme un s-triazole. On dispose de méthodes d'analyse variées
pour la recherche et le dosage de l'amitrole dans les plantes, le
sol, l'eau, l'air et l'urine.
1.2 Sources d'exposition humaine et environnementale
L'amitrole n'existe pas à l'état naturel. On le prépare par
condensation de l'acide formique avec le bicarbonate
d'aminoguanidine dans un solvant inerte à 100-200 °C. C'est un
herbicide à large spectre dont l'activité s'explique par son action
inhibitrice sur la formation de la chlorophylle. On l'utilise en
application tout autour des arbres fruitiers, sur les friches, sur
l'accotement des routes et des voies ferrées ou encore pour le
désherbage des étangs.
1.3 Transport, distribution et transformation dans l'environnement
En raison de sa faible tension de vapeur, l'amitrole ne pénètre
pas dans l'atmosphère. Il est facilement soluble dans l'eau avec un
temps de demi-photodécomposition dans l'eau distillée qui dépasse un
an. En présence du sel de potassium de l'acide humique, qui agit
comme photosensibilisateur, le temps de demi-photodécomposition est
ramené à 7,5 heures.
L'amitrole est adsorbé aux particules du sol et aux matières
organiques par association protonique. Cette liaison est réversible
et labile, même dans des conditions d'acidité favorables. La mesure
du coefficient de partage n-octanol/eau permet de classer
l'amitrole comme "extrêmement mobile" dans les sols de pH > 5 et
"moyennement à très fortement mobile" dans les sols de plus faible
pH. Le lessivage du composé initial peut varier dans de très fortes
proportions ainsi qu'en témoigne l'expérimentation sur des colonnes
de terre. En général c'est dans le sable que le mouvement se voit le
plus facilement; la mobilité décroît à mesure qu'augmente la teneur
en matières organiques.
La décomposition dans le sol est en général assez rapide mais
elle dépend du type de sol et de la température. On a isolé des
bactéries capables de dégrader l'amitrole. Cet herbicide peut tenir
lieu d'unique source d'azote mais pas d'unique source de carbone
pour les bactéries. La dégradation microbienne est probablement la
principale voie de décomposition de l'amitrole; en effet les études
portant sur des sols stérilisés ont montré que la décomposition
était pratiquement inexistante. Toutefois, on a invoqué la
possibilité d'une dégradation par des voies abiotiques et notamment
sous l'action de radicaux libres. Des études en laboratoire ont
montré que le temps de demi-décomposition de l'amitrole était de 2 à
30 jours, le produit final étant le CO2. Une seule étude en
situation réelle incite à penser que cette dégradation pourrait être
plus longue à températures plus basses et pour différents taux
d'humidité du sol; sur argile, la demi-vie s'est révélée être
d'environ 100 jours.
Bien que le composé initial percole à travers certains sols,
ses produits de décomposition restent fermement fixés aux particules
du sol. Etant donné que l'amitrole se décompose rapidement dans le
sol, la forte tendance au lessivage de cet herbicide ne semble pas
entrer en ligne de compte dans la pratique. Les dégâts occasionnés
aux arbres, dont on avait parfois fait état au début de
l'utilisation de cet herbicide, ne paraissent pas être
caractéristiques du produit.
Une fois appliqué à la végétation, l'amitrole est absorbé par
le feuillage et peut transmigrer dans la plante. Il est également
absorbé par les racines et transporté dans le xylème jusqu'aux
pousses des extrémités en l'espace de quelques jours.
Une forte solubilité dans l'eau, un coefficient de partage
octanol-eau très faible et l'absence de persistance chez l'animal
sont autant de facteurs qui indiquent qu'il n'existe pour l'amitrole
aucune possibilité de bioaccumulation ou de transport le long de la
chaîne alimentaire.
1.4 Concentrations dans l'environnement et exposition
humaine
Les ateliers de production de l'amitrole peuvent libérer dans
l'atmosphère des particules qui en contiennent; c'est ainsi que l'on
a trouvé, à proximité d'un de ces ateliers, des concentrations dans
l'atmosphère allant de 0 à 100 mg/m3.
L'épandage d'amitrole sur les voies d'eau et les bassins
versants peut conduire à des concentrations atteignant passagèrement
150 µg/litre. Ces concentrations tombent rapidement à des niveaux
indécelables (< 2 µg/litre) dans les eaux courantes en l'espace de
2 heures. L'épandage sur des étangs a produit une concentration
initiale de 1,3 mg/litre d'eau, qui tombait à 80 µg/litre au bout de
27 semaines. A proximité d'une unité de production, les
concentrations dans les cours d'eau allaient de 0,5 à 2 mg/litre.
Lorsqu'on l'utilise conformément aux recommandations, il ne
semble pas que l'amitrole laisse subsister des résidus dans les
produits alimentaires. Le traitement du sol dans des plantations de
pommiers n'a pas conduit à la présence de résidus dans les pommes.
Cependant on peut trouver des résidus dans les fruits sauvages qui
poussent à proximité des zones traitées.
On n'a pas signalé la présence d'amitrole dans l'eau de
boisson.
1.5 Cinétique et métabolisme chez les animaux de laboratoire et
l'homme
Après administration par voie orale, l'amitrole est rapidement
absorbé chez les mammifères au niveau des voies digestives. Il est
rapidement excrété, essentiellement sous forme inchangée. Sa
principale voie d'excrétion chez l'homme et les animaux de
laboratoire est la voie urinaire, la majorité du produit étant
éliminée dans les 24 heures. La métabolisation du produit dans
l'organisme des mammifères produit deux métabolites que l'on
retrouve en petites quantités dans l'urine des animaux d'expérience.
Inhalé sous forme d'aérosol, il est de même rapidement excrété dans
les urines.
1.6 Effets sur les animaux d'expérience et les systèmes d'épreuve
in vitro
L'expérimentation sur plusieurs espèces, par diverses voies
d'administration, montre que l'amitrole n'a qu'une faible toxicité
aiguë (les valeurs de la DL50 sont toujours supérieures à
2500 mg/kg de poids corporel). L'exposition à l'amitrole entraîne
une atteinte thyroïdienne, qu'elle soit unique, brève ou prolongée.
L'amitrole a un effet goitrogène; il entraîne une hypertrophie et
une hyperplasie de la thyroïde avec déplétion colloïdale et
accroissement de la vascularisation. L'expérimentation à long terme
chez le rat montre que ces anomalies au niveau de la thyroïde
précèdent la survenue de cancers.
On pense que l'effet cancérogène de l'amitrole sur la thyroïde
est lié à une stimulation permanente de cette glande par
l'accroissement du taux d'hormone thyréotrope (TSH), imputable à la
perturbation de la synthèse de l'hormone thyroïdienne par ce
composé.
Certaines études relatives à l'activité génotoxique de
l'amitrole ont donné des résultats ambigus. Des études de
cancérogénicité chez le rat ont montré que l'action tumorigène se
limitait à la thyroïde. Cependant, des tumeurs hépatiques ont été
observées chez des souris soumises à des doses élevées d'amitrole.
Les effets précoces de l'amitrole sur la thyroïde ont été
évalués en fonction d'un certain nombre de critères. La dose
minimale sans effets nocifs observables serait, d'après ces études,
de 2 mg/kg de nourriture chez le rat; elle a été évaluée sur la base
de l'hyperplasie thyroïdienne.
1.7 Effets sur l'homme
Un seul cas de dermatite de contact due à l'amitrole a été
signalé. Ingéré à la dose de 20 mg/kg, l'amitrole n'a pas provoqué
d'effets toxiques. Lors d'une étude contrôlée, on a constaté qu'une
dose de 100 mg avait, sur 24 heures, une action inhibitrice sur la
fixation de l'iode par la thyroïde. Des employés chargés du
désherbage, et qui subissaient une exposition cutanée à 340 mg
d'amitrole environ pendant dix jours, n'ont présenté aucune
altération de leur fonction thyroïdienne.
1.8 Effets sur les autres êtres vivant au laboratoire et dans leur
milieu naturel
Plusieurs études portant sur la croissance des cyanobactéries
(algues bleues) ont montré que l'amitrole n'exerçait aucun effet à
des concentrations inférieures ou égales à 4 mg/litre. On n'a pas
fait état non plus d'effets indésirables systématiques sur la
fixation d'azote. Aucun effet indésirable n'a été constaté sur des
bactéries terricoles à des concentrations de 20 mg/litre de milieu
dans le cas de Rhizobium fixatrice d'azote et de 250 mg/kg dans le
cas des bactéries cellulolytiques. Aucun effet n'a été constaté sur
la nitrification ou la respiration du sol à des concentrations de
100 mg de matière active par kg de sol sec, soit 5 fois la dose
d'emploi maximale recommandée. A des doses allant jusqu'à
20 mg/litre, on a observé que le trèfle souterrain formait moins de
nodosités.
On a étudié l'effet inhibiteur de l'amitrole sur la croissance
chez un certain nombre d'algues unicellulaires. A des doses de 0,2 à
0,5 mg d'amitrole par litre, c'est chez Selenastrum que cet effet
était le plus sensible.
La plupart des invertébrés aquatiques présentent une forte
tolérance à l'amitrole technique: les valeurs de la CL50 se sont
révélées supérieures à 10 mg/litre pour les organismes étudiés sauf
la daphnie (Daphnia magna) pour laquelle la CE50 aiguë à
48 heures (immobilisation) était de 1,5 mg/litre. La tolérance à
l'amitrole des larves de poissons et d'amphibiens est également
élevée, les valeurs de la CL50 dépassant 40 mg/litre. Des études à
long terme ont montré que de jeunes truites arc-en-ciel pouvaient
survivre pendant 21 jours à une concentration d'amitrole de
25 mg/litre.
Deux espèces de lombric (Eisenia foetida et Allolobophora
caliginosa) soumises respectivement à des doses d'amitrole
(SP50) de 1000 mg/kg de terre et de 100 mg(amitrole T)/kg de
terre, n'en n'ont nullement souffert. Des coléoptères (carabes) ont
subi sans dommage un traitement direct par l'amitrole à des doses
correspondant à 30 kg/hectare. Sur les nématodes, on n'a observé
d'effets qu'à des concentrations élevées (la CL50 était de
184 mg/kg). Des essais sur le terrain ont également montré que
l'amitrole était sans danger pour les abeilles.
L'amitrole est peu toxique pour les oiseaux, toutes les valeurs
de CL50 par voie alimentaire étant supérieures à 5000 mg/kg de
nourriture. L'administration de 2000 mg d'amitrole par kg de poids
corporel à des colverts n'a provoqué aucune mortalité.
1. RESUMEN
1.1 Identidad, propiedades físicas y químicas y métodos analíticos
El amitrol (3-amino-1,2,4-triazol) es un polvo incoloro
cristalino. Es termoestable y tiene un punto de fusión de
156-159 °C. Es fácilmente soluble en agua y etanol y poco soluble en
disolventes orgánicos tales como el hexano y el tolueno.
Químicamente, el amitrol se comporta como una amina aromática típica
y como un s-triazol. Se dispone de una amplia variedad de métodos
analíticos para la detección y cuantificación del amitrol en las
plantas, el suelo, el agua, el aire y la orina.
1.2 Fuentes de exposición humana y ambiental
El amitrol no se halla presente en la naturaleza. Se fabrica
por condensación del ácido fórmico con bicarbonato de aminoguanidina
en un solvente inerte a 100-200 °C. El amitrol se utiliza como
herbicida con un amplio espectro de actividad y parece actuar
inhibiendo la formación de clorofila. Se aplica corrientemente en
torno de los árboles de los huertos, en terrenos en barbecho, en los
bordes de los caminos o para combatir malas hierbas en los
estanques.
1.3 Transporte, distribución y transformación en el medio ambiente
Debido a su baja presión de vapor, el amitrol no penetra en la
atmósfera. Es fácilmente soluble en agua y tiene un período de
semifotodegradación en agua destilada de más de un año. La
fotodegradación ocurre en presencia de la sal potásica del ácido
húmico, que es fotosensibilizadora y la semivida se reduce a
7,5 horas.
El amitrol se adsorbe en partículas del suelo y materia
orgánica por asociación protónica. El enlace es reversible y no es
fuerte, incluso en condiciones ácidas favorables. Conforme a los
valores hallados del coeficiente de reparto n-octanol/agua, el
amitrol se clasifica como "muy móvil" en suelos con un pH > 5 y "de
moderadamente a muy móvil" con pH más bajos. La lixiviación del
compuesto original en columnas experimentales de suelo es
considerablemente variable. En general, el movimiento se ve más
fácilmente en arena; si el contenido de materia orgánica aumenta, la
movilidad se reduce.
La degradación en el suelo suele ser bastante rápida, pero
variable según el tipo de suelo y la temperatura. Se han aislado
bacterias capaces de degradar el amitrol. El herbicida puede actuar
como única fuente de nitrógeno de la bacteria, pero no como su única
fuente de carbono. La degradación microbiana probablemente sea la
principal vía de descomposición del amitrol; en estudios realizados
con suelo esterilizado se ha registrado poca o ninguna
descomposición. Sin embargo, también se ha propuesto una degradación
por mecanismos abióticos, inclusive por la acción de radicales
libres. Los estudios de laboratorio han mostrado una degradación en
CO2 con una semivida de 2 a 30 días. Un estudio único sobre el
terreno sugiere que la degradación tal vez sea más lenta a
temperaturas más bajas y con diferentes niveles de humedad del
suelo; en una prueba con arcilla, la semivida resultó de unos 100
días.
Aunque el compuesto madre se lixivia a través de algunos
suelos, los productos de la degradación quedan estrechamente ligados
al suelo. Dado que el amitrol se degrada rápidamente en el suelo, el
elevado potencial de lixiviación del herbicida no parece darse en la
práctica. Los daños ocasionales a los árboles notificados durante la
aplicación inicial del amitrol no han resultado ser una
característica regular de su empleo.
El amitrol aplicado a la vegetación se absorbe a través de las
hojas y puede translocarse a toda la planta. También se absorbe a
través de las raíces y se transporta en el xilema, llegando en pocos
días hasta los brotes.
La gran solubilidad en el agua, el coeficiente muy bajo de
repartición octanol-agua y la no permanencia en animales significan
que no hay posibilidades de bioacumulación del amitrol ni transporte
del mismo a través de las cadenas alimentarias.
1.4 Niveles ambientales y exposición humana
Las plantas productoras tal vez liberen material particulado
que contenga amitrol; en las proximidades de una planta se han
detectado niveles atmosféricos de 0 a 100 mg/m3.
Tras la utilización de amitrol en cursos de agua y cuencas
hidrográficas se han registrado concentraciones transitorias en el
agua de hasta 150 µg/litros. La concentración disminuye rápidamente
hasta niveles no detectables (<2 µg/litro) en el agua corriente en
dos horas. Tras la aplicación en estanques se registró una
concentración inicial en el agua de 1,3 mg/litro, que bajó a
80 µg/litro a las 27 semanas. En las proximidades de una planta de
producción, las concentraciones fluviales oscilaban entre 0,5 y
2 mg/litro.
En los alimentos no se han detectado residuos de amitrol si se
ha utilizado conforme a las recomendaciones. La vaporización en la
superficie del suelo alrededor de manzanos no generó residuos en las
manzanas. Las frutas silvestres que crecen en las proximidades de
las zonas de control pueden contener residuos.
No se ha notificado la presencia de amitrol en el agua potable.
1.5 Cinética y metabolismo en animales de laboratorio y en el
hombre
Tras su administración por vía oral, el amitrol se absorbe
fácilmente a través del tracto gastrointestinal de los mamíferos. El
cuerpo lo excreta con rapidez, principalmente en la forma del
compuesto padre. La principal vía de excreción en el ser humano y en
animales de laboratorio es la orina, y la mayor parte de la
excreción se efectúa en el transcurso de las primeras 24 horas. La
transformación metabólica en los mamíferos produce dos metabolitos
menores, detectables en la orina de los animales de experimentación.
Cuando el amitrol en aerosol se inhala, la excreción es igualmente
rápida por orina.
1.6 Efectos en animales de experimentación y en sistemas de prueba
in vitro
El amitrol ha manifestado una toxicidad aguda baja en pruebas
realizadas con varias especies y diversas vías de administración
(los valores de la DL50 fueron siempre superiores a 2500 mg/kg de
peso corporal). Se observó que afectaba la tiroides después de
exposición es única, de corto plazo y de largo plazo. El amitrol es
bócigeno; causa hipertrofia e hiperplasia tiroidea, deplección del
coloide y aumento de la vascularización. En experimentos de largo
plazo dichos cambios preceden el desarrollo de neoplasia de la
tiroides en ratas.
Se cree que el efecto carcinógeno del amitrol en la tiroides
está relacionado con la estimulación continua de la glándula por un
aumento de la hormona tirotrópica (TSH), ocasionado por la
interferencia del amitrol en la síntesis de la hormona tiroidea. Se
han comunicado resultados equívocos de varios estudios sobre el
potencial genotóxico del amitrol. En pruebas de carcinogenicidad en
ratas, el amitrol no indujo tumores en órganos diferentes de la
tiroides. Sin embargo, en dosis elevadas, el amitrol fue causa de
tumores hepáticos en ratones.
Se han utilizado varios criterios para evaluar los efectos
tempranos del amitrol en la tiroides. El más bajo nivel sin efectos
adversos observados derivado de esos estudios fue de 2 mg/kg en la
alimentación de ratas y se evaluó en base a la hiperplasia tiroidea.
1.7 Efectos en el hombre
Se ha comunicado un solo caso de dermatitis por contacto con el
amitrol. El amitrol no tiene efectos tóxicos si se ingiere una dosis
de 20 mg/kg. En un experimento controlado se llegó a la conclusión
de que 100 mg inhibían la absorción de iodo por la tiroides a las 24
horas. No se observaron cambios en la función tiroidea de personas
que trabajaban en el control de malas hierbas y que estuvieron
expuestas por vía dérmica a unos 340 mg de amitrol por día durante
10 días.
1.8 Efectos en otros organismos en el laboratorio y en
el medio ambiente
En varios estudios sobre el desarrollo de cianobacterias (algas
cianofíceas) no se ha observado que el amitrol tenga efecto en
concentraciones iguales o inferiores a 4 mg/litro. No se han
comunicado efectos adversos constantes en la fijación de nitrógeno.
En cuanto a las bacterias del suelo, las Rhizobium fijadoras de
nitrógeno no se vieron afectadas por concentraciones de 20 mg/litro
de medio y las celulolíticas no se vieron afectadas por 150 mg/kg.
No se registraron efectos en la nitrificación ni en la respiración
del suelo con 100 mg de ingrediente activo por kg de suelo seco, es
decir, con el quíntuple de la aplicación máxima recomendada. Se
notificó una nodulación reducida en el trébol subterráneo con
concentraciones de hasta 20 mg/litro.
Se han hecho ensayos con diversas algas unicelulares para
determinar los efectos inhibidores del crecimiento. Con 0,2 a 0,5 mg
de amitrol/litro, la inhibición del crecimiento de Selenastrum fue
el efecto más notable comunicado.
La mayor parte de los invertebrados acuáticos muestran una
tolerancia elevada al amitrol técnico: los valores de la CL50 eran
> 10 mg/litro en todos los organismos diferentes de la pulga
acuática Daphnia magna, en cuyo caso la CE50 aguda
(inmovilización) fue de 1,5 mg/litro. Los peces y las larvas de
anfibios también mostraron tolerancia al amitrol con CL50
superiores a 40 mg/litro. Estudios de más largo plazo indicaron que
la trucha arco iris joven sobrevive 21 días en un medio con una
concentración de 25 mg de amitrol por litro.
Dos especies de gusanos (Eisenia foetida y Allolobophora
caliginosa) no se vieron afectadas por el amitrol (SP50),
1000 mg/kg de suelo y por Amitrol-T, 100 mg/kg de suelo,
respectivamente. Los escarabajos carábidos no resultaron afectados
después de la vaporización directa de amitrol a razón de 30 kg/ha.
En los nematodos se verificaron efectos solamente con
concentraciones elevadas de amitrol (la CL50 fue de 184 mg/kg).
Como resultado de ensayos sobre el terreno se ha comunicado que el
amitrol no es peligroso para las abejas.
El amitrol es poco tóxico para las aves y en todos los casos se
comunicaron CL50 superiores a 5000 mg/kg de alimento. La
administración aguda de una dosis de 2000 mg/kg de peso corporal no
causó la muerte de ningún pato silvestre.
 Chemical formula: C2H4N4
Relative molecular mass: 84.08
CAS chemical names: 1 H-1,2,4-triazol-3-amine (9C1)
3-amino- s-triazole (8CI)
IUPAC names: 1 H-1,2,4-triazol-3-ylamine
3-amino-1 H-1,2,4-triazole
3-amino- s-triazole
CAS registry number:
Chemical formula: C2H4N4
Relative molecular mass: 84.08
CAS chemical names: 1 H-1,2,4-triazol-3-amine (9C1)
3-amino- s-triazole (8CI)
IUPAC names: 1 H-1,2,4-triazol-3-ylamine
3-amino-1 H-1,2,4-triazole
3-amino- s-triazole
CAS registry number: 