
INTERNATIONAL PROGRAMME ON CHEMICAL SAFETY
ENVIRONMENTAL HEALTH CRITERIA 143
METHYL ETHYL KETONE
This report contains the collective views of an international group of
experts and does not necessarily represent the decisions or the stated
policy of the United Nations Environment Programme, the International
Labour Organisation, or the World Health Organization.
Published under the joint sponsorship of
the United Nations Environment Programme,
the International Labour Organisation,
and the World Health Organization
First draft prepared by Dr R.B. Williams,
United States Environmental Protection Agency
World Health Orgnization
Geneva, 1993
The International Programme on Chemical Safety (IPCS) is a
joint venture of the United Nations Environment Programme, the
International Labour Organisation, and the World Health
Organization. The main objective of the IPCS is to carry out and
disseminate evaluations of the effects of chemicals on human health
and the quality of the environment. Supporting activities include
the development of epidemiological, experimental laboratory, and
risk-assessment methods that could produce internationally
comparable results, and the development of manpower in the field of
toxicology. Other activities carried out by the IPCS include the
development of know-how for coping with chemical accidents,
coordination of laboratory testing and epidemiological studies, and
promotion of research on the mechanisms of the biological action of
chemicals.
WHO Library Cataloguing in Publication Data
Methyl ethyl ketone.
(Environmental health criteria ; 143)
1.Butanones - adverse effects 2.Butanones - toxicity
3.Occupational exposure I.Series
ISBN 92 4 157143 8 (NLM Classification: QV 633)
ISSN 0250-863X
The World Health Organization welcomes requests for permission
to reproduce or translate its publications, in part or in full.
Applications and enquiries should be addressed to the Office of
Publications, World Health Organization, Geneva, Switzerland, which
will be glad to provide the latest information on any changes made
to the text, plans for new editions, and reprints and translations
already available.
(c) World Health Organization 1993
Publications of the World Health Organization enjoy copyright
protection in accordance with the provisions of Protocol 2 of the
Universal Copyright Convention. All rights reserved.
The designations employed and the presentation of the material
in this publication do not imply the expression of any opinion
whatsoever on the part of the Secretariat of the World Health
Organization concerning the legal status of any country, territory,
city or area or of its authorities, or concerning the delimitation
of its frontiers or boundaries.
The mention of specific companies or of certain manufacturers'
products does not imply that they are endorsed or recommended by the
World Health Organization in preference to others of a similar
nature that are not mentioned. Errors and omissions excepted, the
names of proprietary products are distinguished by initial capital
letters.
CONTENTS
ENVIRONMENTAL HEALTH CRITERIA FOR METHYL ETHYL KETONE
1. SUMMARY
1.1. Properties and analytical methods
1.2. Sources of exposure and uses
1.2.1. Production and other sources
1.2.2. Uses and loss to the environment
1.3. Environmental transport and distribution
1.4. Environmental levels and human exposure
1.5. Kinetics and metabolism
1.6. Effects on experimental species
1.7. Effects on humans
1.7.1. MEK alone
1.7.2. MEK in solvent mixtures
1.8. Enhancement of the toxicity of other solvents
2. IDENTITY, PHYSICAL AND CHEMICAL PROPERTIES AND ANALYTICAL METHODS
2.1. Identity
2.2. Chemical and physical properties
2.3. Conversion factors
2.4. Sampling and analytical methods
2.4.1. General considerations
2.4.2. Air
2.4.3. Water
2.4.4. Solids
2.4.5. Biological materials
3. SOURCES OF HUMAN AND ENVIRONMENTAL EXPOSURE
3.1. Natural occurrence
3.2. Production levels, processes and uses
3.2.1. World production
3.2.2. Production processes
3.2.3. Other sources
3.2.4. Uses
3.3. Release into the environment
4. ENVIRONMENTAL TRANSPORT, DISTRIBUTION AND TRANSFORMATION
4.1. Transport in the environment
4.2. Bioaccumulation and biodegradation
5. ENVIRONMENTAL LEVELS AND HUMAN EXPOSURE
5.1. Environmental levels
5.1.1. Air
5.1.2. Water
5.1.3. Foodstuffs
5.2. General population exposure
5.3. Occupational exposure
5.4. Peri-occupational exposure
6. KINETICS AND METABOLISM
6.1. Absorption
6.1.1. Percutaneous absorption
6.1.2. Inhalation absorption
6.1.3. Ingestion absorption
6.1.4. Intraperitoneal absorption
6.2. Distribution
6.3. Metabolic transformation
6.3.1. Animal studies
6.3.2. Human studies
6.4. Elimination and excretion
6.5. Turnover
6.6. Metabolic interactions
6.7. Mechanisms of action
7. EFFECTS ON LABORATORY MAMMALS AND IN VITRO TEST SYSTEMS
7.1. Acute exposure
7.1.1. Lethal doses
7.1.2. Non-lethal doses
7.1.3. Skin and eye irritation
7.2. Repeated exposures
7.3. Neurotoxicity
7.3.1. Behavioural testing
7.3.2. Histopathology
7.4. Developmental toxicity
7.5. Mutagenicity and related end-points
7.6. Carcinogenicity
8. EFFECTS ON HUMANS
8.1. General population exposure
8.2. Effects of short-term exposure
8.3. Skin irritation and sensitization
8.4. Occupational exposure
8.4.1. MEK alone
8.4.2. MEK in solvent mixtures
8.5. Carcinogenicity
9. EFFECTS ON OTHER ORGANISMS IN THE LABORATORY AND FIELD
9.1. Microorganisms
9.2. Aquatic organisms
9.3. Terrestrial organisms
9.3.1. Animals
9.3.2. Plants
10. ENHANCEMENT OF THE TOXICITY OF OTHER SOLVENTS BY MEK
10.1. Hexacarbon neuropathy
10.1.1. Introduction
10.1.2. Animal studies
10.1.3. Human studies
10.1.3.1 Solvent abuse
10.1.3.2 Occupational exposure
10.2. Haloalkane solvents
10.2.1. Studies in animals
10.2.2. Potentiation of haloalkane toxicity in humans
11. EVALUATION OF HUMAN HEALTH RISKS AND EFFECTS ON THE ENVIRONMENT
11.1. Human health risks
11.1.1. Non-occupational exposure
11.1.2. Occupational exposure
11.1.3. Relevant animals studies
11.2. Effects on the environment
12. RECOMMENDATIONS FOR THE PROTECTION OF HUMAN HEALTH AND THE
ENVIRONMENT
12.1. Human heath protection
12.2. Environmental protection
13. FURTHER RESEARCH
REFERENCES
APPENDIX 1. Conversion factors for various solvents
RESUME
RESUMEN
WHO TASK GROUP ON ENVIRONMENTAL HEALTH CRITERIA FOR METHYL ETHYL
KETONE
Members
Professor E.A. Bababunmi, Postgraduate Institute for Medical Research
and Training, College of Medicine, Ibadan, Nigeria
Dr P.E.T. Douben, Department of Ecotoxicology, Institute for Forestry
and Nature Research, Arnhem, The Netherlands
Professor C.L. Galli, Toxicology Laboratory, Institute of
Pharmacological Sciences, University of Milan, Milan, Italy
(Chairman)
Dr R.F. Hertel, Fraunhofer Institute of Toxicology and Aerosol
Research, Hanover, Germany
Dr H.P.A. Illing, Head of Toxicology, Health and Safety Executive,
Bootle, United Kingdom
Professor A. Massoud, Department of Community, Environmental &
Occupational Health, Faculty of Medicine, Ain Shams University,
Abbassia, Egypt (Joint Rapporteur)
Dr K. Morimoto, Division of Chem-Bio Informatics, National Institute
of Hygienic Sciences, Setagaya-ku, Tokyo, Japan
Dr V. Riihimäki, Institute of Occupational Health, Helsinki, Finland
Dr E. de Souza Nascimento, University of Sao Paulo, Sao Paulo, Brazil
Dr H. Tilson, Neurotoxicology Division, Health Effects Research
Laboratory, US Environmental Protection Agency, Research Triangle
Park, USA
Dr R.B. Williams, Office of Research and Development, US Environmental
Protection Agency, Washington D.C., USA (Joint Rapporteur)
Secretariat
Dr P.G. Jenkins, International Programme on Chemical Safety, World
Health Organization, Geneva, Switzerland
Dr E. Smith, International Programme on Chemical Safety, World Health
Organization, Geneva, Switzerland
NOTE TO READERS OF THE CRITERIA MONOGRAPHS
Every effort has been made to present information in the criteria
monographs as accurately as possible without unduly delaying their
publication. In the interest of all users of the Environmental Health
Criteria monographs, readers are kindly requested to communicate any
errors that may have occurred to the Director of the International
Programme on Chemical Safety, World Health Organization, Geneva,
Switzerland, in order that they may be included in corrigenda.
* * *
A detailed data profile and a legal file can be obtained from the
International Register of Potentially Toxic Chemicals, Palais des
Nations, 1211 Geneva 10, Switzerland (Telephone No. 7988400 or
7985850).
ENVIRONMENTAL HEALTH CRITERIA FOR METHYL ETHYL KETONE
A WHO Task Group on Environmental Health Criteria for Methyl
Ethyl Ketone (MEK) met at the World Health Organization, Geneva, from
9 to 13 September 1991. Dr E. Smith welcomed the participants on
behalf of Dr M. Mercier, Director, IPCS, and on behalf of the heads of
the three IPCS cooperating organizations (UNEP/ILO/WHO). The Task
Group reviewed and revised the draft monograph and made an evaluation
of the risks for human health and the environment from exposure to
MEK.
The first draft of this monograph was prepared by Dr R.B.
Williams, Office of Research and Development, US Environmental
Protection Agency. Dr E. Smith and Dr P.G. Jenkins, both members of
the IPCS Central Unit, were responsible for the scientific content and
technical editing, respectively.
The efforts of all who helped in the preparation and finalization
of the monograph are gratefully acknowledged.
ABBREVIATIONS
ALT alanine transferase
BEI biological exposure index
DCB dichlorobenzene
DMA dimethylamine
DMF dimethylformamide
DNPH 2,4-dinitrophenyl hydrazine
EBK ethyl n-butyl ketone
ECD electron-capture detection
FID flame ionization detection
FT-IR Fourier transform infrared
GC gas chromatography
GLDH glutamate dehydrogenase
GPT glutamic-pyruvic transaminase
GST glutathione-S-transferase
2,5-HD 2,5-hexanedione
2,5-Hpdn 2,5-heptanedione
HPLC high-performance liquid chromatography
HS headspace
IR infrared
LC50 median lethal concentration
LDQ lowest detectable quantity
MAC maximum allowable concentration
MBK methyl n-butyl ketone
MEK methyl ethyl ketone
MIBK methyl isobutyl ketone
MS mass spectrometry
NADPH reduced nicotinamide adenine dinucleotide phosphate
OCT ornithine carbamyl transferase
PID photo-ionization detection
SRT simple reaction time
STEL short-term exposure limit
TLV threshold limit value
TWA time-weighted average
UV ultraviolet
1. SUMMARY
1.1 Properties and analytical methods
Methyl ethyl ketone (MEK) is a clear, colourless, volatile,
highly flammable liquid with an acetone-like odour. It is stable under
ordinary conditions but can form peroxides on prolonged storage; these
may be explosive. MEK can also form explosive mixtures with air. It is
very soluble in water, miscible with many organic solvents, and forms
azeotropes with water and many organic liquids. In the atmosphere MEK
produces free radicals, which may lead to the formation of
photochemical smog.
Several analytical methods exist for the measurement of MEK at
environmental levels in air, water, biological samples, waste and
other materials. In the more sensitive methods, MEK is trapped and
concentrated either on a solid sorbant or as a derivative of
2,4-dinitrophenylhydrazine (DNPH). Absorbed MEK and other volatile
organic compounds are desorbed, separated by gas chromatography and
measured with a mass spectrometer or flame ionization detector.
Derivatized MEK is separated from related compounds by high
performance liquid chromatography and measured by ultraviolet
absorption. In media such as solid waste and biological materials, MEK
must first be separated from the substrate by methods such as solvent
extraction or steam distillation. High concentrations of MEK in air
can be monitored continuously by infrared absorption. Detection limits
are 3 µg/m3 in air, 0.05 µg/litre in drinking-water, 1.0 µg/litre in
other types of water, 20 µg/litre in whole blood and 100 µg/litre in
urine.
1.2 Sources of exposure and uses
1.2.1 Production and other sources
Recent values for annual industrial manufacture (in thousands of
tonnes) are: USA, 212 to 305; western Europe, 215; Japan, 139. In
addition to its manufacture, sources of MEK in the environment are
exhaust from jet and internal combustion engines, and industrial
activities such as gasification of coal. It is found in substantial
amounts in tobacco smoke. In the USA, production of MEK by engines is
no more than 1% of its deliberate manufacture. In smog episodes,
photochemical production of MEK and other carbonyls from free radicals
can be far greater than direct anthropogenic emission. MEK is produced
biologically and has been identified as a product of microbial
metabolism. It has also been detected in a wide diversity of natural
products including higher plants, insect pheromones, animal tissues,
and human blood, urine and exhaled air. It is probably a minor product
of normal mammalian metabolism.
1.2.2 Uses and loss to the environment
The major use of MEK, application of protective coatings and
adhesives, reflects its excellent characteristics as a solvent. It
also is used as a chemical intermediate, as a solvent in magnetic tape
production and the dewaxing of lubricating oil, and in food
processing. In addition to industrial applications, it is a common
ingredient in consumer products such as varnishes and glues. In most
applications MEK is a component of a mixture of organic solvents.
Losses to the environment are mainly to the air and result principally
from solvent evaporation from coated surfaces. MEK is released into
water as a component of the waste from its manufacture and from a
variety of industrial operations. It has been detected in natural
waters and could have originated from microbial activities and from
atmospheric input, as well as from anthropogenic pollution.
1.3 Environmental transport and distribution
MEK is highly mobile in the natural environment and subject to
rapid turnover. It is very soluble in water and evaporates readily
into the atmosphere. In air MEK is subject to rapid photochemical
decomposition and is also synthesized by photochemical processes. In
water containing free halogens or hypohalites, it reacts to form a
haloform that is more toxic than the original compound. MEK is
distributed by both air and water, but does not accumulate in any
individual compartment, and does not persist long where there is
microbial activity. It is rapidly metabolized by microbes and mammals.
There is no evidence of bioaccumulation. MEK occurs naturally in some
clover species and is produced by fungi to concentrations that affect
the germination of some plants.
1.4 Environmental levels and human exposure
General population exposure to low levels of MEK is widespread.
In minimally polluted air, the concentration is less than 3 µg/m3
(< 1 ppb), but a level of 131 µg/m3 (44.5 ppb) has been measured
under conditions of heavy air pollution. Away from industrial areas
where MEK is manufactured or used, major sources may be vehicle
exhaust and photochemical reactions in the atmosphere. Cigarettes and
other tobacco products that are burned contribute to individual
exposure (20 cigarettes contain up to 1.6 mg). Volatilization of MEK
from building materials and consumer products can pollute indoor air
to levels far above adjacent outdoor air. MEK concentrations in
exposed natural waters are rarely above 100 µg/litre (100 ppb) and are
usually below a detectable level. Trace amounts of MEK, however, have
been detected widely in drinking-water (approximately 2 µg/litre) and
presumably originated as solvent leached from cemented joints of
plastic pipe. Although MEK is a normal component of many foods,
concentrations are low and food consumption cannot be considered a
significant source of population exposure. Average daily per capita
intake in the USA via foodstuffs is estimated to be 1.6 mg, most
coming from white bread, tomatoes and Cheddar cheese. In addition to
MEK present naturally, foods may contain MEK from cheese ripening,
aging of poultry meat, cooking or food processing, or by absorption
from plastic packaging materials.
Industrial exposure to moderate levels of MEK is widespread.
However, in some regions workers in small factories (e.g., shoe
factories, printing plants and painting operations) are exposed to
much higher concentrations due to inadequate ventilation. In these
factories, exposure is usually to a mixture of solvents including
n-hexane.
1.5 Kinetics and metabolism
Absorption of MEK is rapid via dermal contact, inhalation,
ingestion and intraperitoneal injection. It is rapidly transferred
into the blood and thence to other tissues. The solubility of MEK
appears similar for all tissues. The clearance of MEK and its
metabolites in mammals is essentially complete in 24 h. It is
metabolized in the liver where it is mainly oxidized to
3-hydroxy-2-butanone and subsequently reduced to 2,3-butanediol. A
small portion may be reduced to 2-butanol, but 2-butanol is rapidly
oxidized back to MEK. The bulk of MEK taken into the mammalian body
enters the general metabolism and/or is eliminated as simple compounds
such as carbon dioxide and water. Excretion of MEK and its
recognizable metabolites is mainly through the lungs, although small
amounts are excreted via the kidneys.
MEK increases microsomal cytochrome P-450 enzyme activities. This
enhancement of enzymatic activity and thus of the body's potential for
metabolic transformation may well be the mechanism by which MEK
potentiates the toxicity of haloalkane and aliphatic hexacarbon
solvents.
1.6 Effects on experimental species
MEK has low to moderate acute, short-term and chronic toxicity
for mammals. LD50 values for adult mice and rats are 2 to 6 g/kg
body weight, death occurring within 1 to 14 days following a single
oral dose. Average vapour concentrations producing lethality in rats
following a single exposure are around 29 400 mg/m3 (10 000 ppm),
although guinea-pigs survived a 4-h exposure to this concentration.
The lowest acute oral dose modifying body structure is 1 g/kg body
weight, which damaged kidney tubules in the rat. Inhalation by rats of
74 mg/m3 (25 ppm) for 6 h produced measurable changes in behaviour
which persisted for several days. Repeated exposure of rats to 14 750
mg/m3 (5000 ppm) (6 h/day, 5 days/week) produced no lethality, had
only minor effects on growth and structure, and there were no
neuropathological changes. There was no evidence that MEK produced
neuropathological changes in chickens, cats or mice exposed to 3975
mg/m3 (1500 ppm) for periods of up to 12 weeks. Transient effects on
behaviour or neurophysiology were detected following repeated exposure
of rats and baboons to concentrations as low as 295-590 mg/m3 (100
to 200 ppm).
There is evidence for a low level of fetotoxicity without any
maternal toxicity at 8825 mg/m3 (3000 ppm), but no evidence for
embryotoxic or teratogenic effects at lower exposure levels. Repeated
exposure of pregnant rats to 8825 mg/m3 induced in their offspring
a small but significant increase in skeletal abnormalities of types
that occurred at low incidences among the unexposed population.
Although examined in a number of conventional mutagenicity test
systems, the only evidence of mutagenicity was provided by a study on
aneuploidy in the yeast Saccharomyces cerevisiae.
MEK is not acutely toxic to fish or aquatic invertebrates and
LC50 values range from 1382 to 8890 mg/litre.
MEK has an inhibiting effect on the germination of several plant
species, even at levels occurring naturally. The growth of aquatic
algae is inhibited.
Compared with natural background levels, relatively high
concentrations of MEK have been used for fumigation under experimental
conditions. It is moderately effective as a fumigant against the
Caribbean fruit fly and is a very effective attractant for tsetse
flies. Levels of MEK up to 20 mg/litre retard biodegradation but do
not stop the process entirely. At levels of up to 100 mg/litre, MEK is
biostatic to a variety of bacteria. At higher concentrations (1000
mg/litre or more) inhibition of the growth of bacteria and protozoa
occurs.
1.7 Effects on humans
1.7.1 MEK alone
Exposure to 590 mg/m3 (200 ppm) had no significant effect in a
variety of behavioural and psychological tests. Short-term exposure to
MEK alone does not appear to be a significant hazard, either
occupationally or for the general public. Experimental exposure to a
concentration of 794 mg/m3 (270 ppm) for 4 h/day had little or no
effect on behaviour, and a 5-min contact with liquid MEK produced no
more than a temporary whitening of the skin. There is only one
non-occupational report of acute toxicity to MEK. This resulted from
accidental ingestion and appeared to produce no lasting harm. There is
no evidence that occupational MEK exposure has resulted in death.
There have been two reports of chronic occupational poisoning and one
questionable report of acute occupational poisoning. In one of the
chronic cases, exposure to 880-1770 mg/m3 (300-600 ppm) resulted in
dermatoses, numbness of fingers and arms, and various symptoms such as
headache, dizziness, gastrointestinal upset, and loss of appetite and
weight. This paucity of incidents of reputed poisoning by MEK alone
reflects both the low toxicity of MEK and the fact that it is most
commonly used not on its own but as a component of solvent mixtures.
1.7.2 MEK in solvent mixtures
Exposure to solvent mixtures containing MEK has been associated
with some reduction in nerve conduction velocity, memory and motor
alterations, dermatoses and vomiting. In one longitudinal study,
consecutive measurements of simple reaction time showed an improvement
in performance in parallel with decreasing concentrations of MEK to
one tenth the original values (which were up to 4000 mg/m3 for
certain routine tasks).
1.8 Enhancement of the toxicity of other
solvents
MEK potentiates the neurotoxicity of hexacarbon compounds
( n-hexane, methyl- n-butylketone and 2,5-hexanedione) and the liver
and kidney toxicity of haloalkane (carbon tetrachloride and
trichloromethane) solvents.
The potentiation of the neurotoxic effects of hexacarbons has
been demonstrated for all three hexacarbons in animals. The peripheral
neuropathies observed in humans followed changes in the formulations
of solvents to which they had been exposed, either voluntarily or
occupationally. The mechanism by which this potentiation occurs is
unclear.
Evidence for potentiation of the liver and kidney toxicity of
haloalkanes comes from animal studies. MEK probably activates the
haloalkane metabolism to tissue-damaging species as a result of
induction of the relevant oxidation enzymes.
2. IDENTITY, PHYSICAL AND CHEMICAL PROPERTIES AND ANALYTICAL METHODS
2.1 Identity
H H O H
| | || |
Chemical structure: H - C - C - C - C - H
| | |
H H H
Chemical formula: C4H8O
Synonyms: Butanone, 2-butanone, butane-2-one,
ethyl methyl ketone, MEK, MEETCO,
methyl acetone, methylpropanone
CAS registry number: 78-93-3
RTECS registry number: EL 6475000
UN registry number: 1193
EC registry number: 606-002-00-3
Relative molecular mass: 72.10
2.2 Chemical and physical properties
Methyl ethyl ketone (MEK) is an important synthetic organic
chemical. The physical properties of MEK are summarized in Table 1. It
is a highly flammable, volatile, clear, colourless liquid that is
stable under ordinary conditions. The vapour forms explosive mixtures
with air over a range of approximately 2% to 12% (vol./vol.). The
odour is acetone-like and variously described as sharp, fresh or
sweet. The odour threshold appears to be around 5.9 mg/m3 (2 ppm)
although a range between 0.74 and 147.5 mg/m3 has been reported
(Ruth, 1986). MEK is moderately soluble in water; the solubility
decreases with increasing temperature. It is miscible with organic
solvents such as alcohol, ether and benzene, and forms azeotropes with
water and many organic liquids.
The value in Table 1 for log Po/w (logarithm of the octanol/
water partition ratio) of 0.26 is taken from Verschuren (1983).
Banergee & Howard (1988) quoted a slightly higher value of 0.29. Other
partition values for MEK (at 37 °C) are: water/air = 254; blood/air =
202; olive oil/air = 263; olive oil/water = 1.0; and olive oil/blood
= 1.3 (Sato & Nakajima, 1979). Perbellini et al. (1984), however,
determined partition values for saline solution/air and olive oil/air
of 193 and 191 respectively.
Table 1. Physical properties of MEK
Reference
Appearance colourless liquid
Relative molecular mass 72.10 Papa & Sherman (1978)
Specific gravity (liquid density)
(at 20 °/4 °C)a 0.805 Krasavage et al. (1982)
Vapour density (air = 1.00) 2.41 Verschuren (1983)
Vapour pressure at 20 °C (torr) 77.5 Weast (1986)
Boiling point (°C) 79.6 Weast (1986)
Melting point (°C) -86 Weast (1986)
Water solubility at 20 °C (g/litre) 275 Windholz (1983)
Refractive index 1.3788 Krasavage et al. (1982)
Flash point (closed cup) (°C) -6 Papa & Sherman (1978)
Log Po/w 0.26 Verschuren (1983)
0.29 Banergee & Howard (1988)
Saturation concentration in
air (g/m3 at 20 °C) 301 Krasavage et al. (1982)
a Specific gravity at 20 °C relative to the density of water at 4 °C
The physical and chemical properties of MEK are determined
largely by its carbonyl group. MEK engages in reactions typical of
saturated aliphatic ketones. These include condensations with amines,
aldehydes and many other organic compounds, hydrolysis (catalysed with
acid or base), oxidation via concentrated oxidizing acids or acidic
peroxides, and reduction with hydrogen and metal catalysts. None of
these reactions is likely to be important in nature. On the other
hand, MEK and other methyl ketones will react with halogens and
hypohalides in aqueous solution to form a carboxylic acid and a
haloform. The reaction provides a specific test for methyl ketones,
and may produce chloroform in chlorinated water supplies contaminated
with methyl ketones. MEK and other ketones are photochemically
reactive when excited by wavelengths occurring in the atmosphere and
produce free radicals which lead to the formation of photochemical
smog (Grosjean et al., 1983).
2.3 Conversion factors
1 ppm = 2.95 mg/m3; 1 mg/m3 = 0.34 ppm
(at 25 °C and 101.3 kPa)
2.4 Sampling and analytical methods
2.4.1 General considerations
Analytical methods for MEK depend on the matrix. They are
summarized in Table 2.
Where MEK is present in a substantial concentration and is known
to be the only or the dominant organic contaminant, simplified
methodology is feasible. The occupational atmospheric exposure limits,
currently in the range 295-590 mg/m3 (100-200 ppm), permit
monitoring in the workplace with less sensitive procedures.
The precise determination of MEK when present in the environment
at low concentrations is a complex task because of the wide variety of
other organic compounds that may be present and the many possibilities
for error, interference and contamination. MEK, other ketones and
other interfering substances are so prevalent in laboratory and
industrial air that care must be taken in all determinations to
minimize the possibility of contamination of samples, equipment and
reagents. Care must be taken to avoid contamination in sampling since,
for example, easily unnoticed sources like PVC (polyvinyl chloride)
glue in collection equipment may leach a significant amount of MEK
into water samples (Kent et al., 1985).
Table 2. Some analytical techniques for determining MEK concentrations in environmental media and biological materialsa
Methods Detection Comments Reference
limits
Air
Trapping in solid sorbant tube (Tenax(R)); 200 µg/m3 working range is 0.2-100 mg/m3; analysis can Brown & Purnell
thermal desorption: separation-detection; be automated (1979)
GC-FID
Trapping in DNPH; separation: HPLC 3-6 µg/m3 general method for aldehydes and ketones in Riggin (1984)
(reverse phase); detection: UV air; some isomeric aldehydes and ketones are
absorption not well separated
Trapping in solid sorbant tube 0.15 mg per working range is 50-1500 mg/m3; acetone and US NIOSH (1984a)
(Ambersorb XE-347(R)); desorption: sample isopropanol interfere
CS2; separation-detection: GC-FID
Absorption of specific IR wavelengths 3 mg/m3 can measure several different pollutant Persson et al.
from CO2 laser; automated, computer- vapours simultaneously and continuously (1984)
controlled system
Trapping in DNPH; colour matching 300 mg/m3 working range is 300-1200 mg/m3; other Smith & Wood
against standards aldehydes and ketones interfere; method (1972)
requires no specialized equipment
Water
Separation from water sample by heated 0.05-1.0 water samples must be preserved from bacterial Pellizzari et al.
gas purge; trapping on Tenax(R); thermal µg/litreb action with methylene chloride, and free chlorine (1985)
desorption; separation-detection: GC/MS removed with thiosulfate; achieving lower limit
of detection requires concentration by
distillation; no interference reported
Table 2 (contd.)
Methods Detection Comments Reference
limits
Concentration on zeolite (ZSM-5); 2 µg/litre developed for drinking-water analysis; no Ogawa & Fritz
elution with acetonitrile; derivatization interference reported (1985)
with DNPH; separation-detection: HPLC/UV
Direct injection of aqueous sample; 40 µg/litre developed for industrial waste-water analysis; Middleditch et al.
separation-detection: GC/FID no interference reported (1987)
Solids
Solvent extraction with tetraethylene 0.5-5 µg/g tetraglyme must be purified and stabilized to Gurka et al.
glycoldimethyl ether (tetraglyme); purge (wet weight) prevent peroxide formation; no interference (1984)
and trap; separation-detection: GC/MS reported
Heated purge of sample/water slurry or 10 µg/kg method developed for volatile organic compounds Fisk (1986)
of methanol extract of sample; trap; (wet weight) at concentrations of < 1 mg/kg; no interference
desorption; separation-detection: GC/MS reported
Biological Materials
Mixture with dextrose and heating; 20 µg/litre method developed for simultaneous determination US NIOSH (1984b)
HS analysis; GC/FID of MEK, toluene and ethenol in blood; recovery
rates 90-98%
Incubation in sealed vial; HS 100 µg/litre method developed for blood; uses 200-µl sample; Ramsey & Flanagan
analysis; separation-detection: applicable to urine and tissue; no interference (1982)
GC/FID and ECD reported
Concentration by reverse-phase 100-150 method developed for MEK and its metabolites Kezic & Monster
extraction column; separation- µg/litre in urine; no interference reported (1988)
detection: GC/FID
Table 2 (contd.)
Methods Detection Comments Reference
limits
Derivatized with o-nitrophenylhydrazine 100 µg/litre method developed for the determination of MEK Van Doorn et al.
and reacted with cyclohexane; in human urine (1989)
centrifuge separation; reversed-
phase HPLC; UV (254 nm)
Steam distillation of slurry; HS 20 µg/litre method developed for cheese, but probably Lin & Jeon (1985)
analysis; separation-detection: widely applicable; no interference reported
GC/FID
Homogenization; HS analysis; 6 mg/litre method developed for the identification of Deveaux & Huvenne
GC/FT-IR solvents of abuse in biological fluids (1987)
a Abbreviations used in the table
DNPH 2,4-dinitrophenylhydrazine
ECD electron-capture detector
FID flame ionization detector
FT Fourier transformed
GC gas chromatograph
HPLC high performance liquid chromatograph
HS headspace
IR infrared
MS mass spectrometer
UV ultraviolet
b 0.05 µg/litre for drinking-water; 1.0 µg/litre for all other types of water
The general procedure for analysis of MEK is summarized below:
a) collect the sample, and if necessary, chemically stabilize it;
b) separate MEK (and other volatile organic compounds) from the
substrate;
c) trap and concentrate MEK (plus other organic compounds);
d) recover the trapped material;
e) separate MEK and other organic compounds;
f) detect and identify MEK;
g) determine the quantity recovered;
h) calculate the concentration present in the sample.
In actual practice the procedure may be simplified by combining or
omitting certain steps, or it may contain an additional step, i.e. the
preparation of 2,4-dinitrophenylhydrazine (DNPH) derivatives of MEK
and other aldehydes and ketones. The formation of DNPH derivatives
quantitatively captures both aldehydes and ketones, and facilitates
their subsequent separation with either gas or liquid chromatography.
The preparation of DNPH derivatives also forms the basis for a
simplified, non-specific method for roughly measuring high levels of
ketones and aldehydes without the use of sophisticated laboratory
equipment (Smith & Wood, 1972). The use of other derivatives, such as
imines via phenylmethylamine (Hoshika et al., 1976), azines via
3-methyl-2-benzothiazolone (Chiavari et al., 1987), and
O-(2,3,4,5,6-pentafluorobenzyl) oximes via
pentafluorophenylhydrazine and pentafluorobenzyloxyamine (Kobayashi et
al., 1980) has also been proposed.
2.4.2 Air
A general methodology for determining MEK in air consists of
trapping and concentrating MEK and other volatile organic compounds in
sampling devices containing an absorbent material, charcoal
(carbopack) or an artificial resin (Tenax GC(R), Ambersorb XE(R),
Amberlites XAD(R)), followed by desorption and analysis.
MEK decomposes when absorbed on charcoal and sample loss may
occur after a few days (Elskamp & Schultz, 1983; Levin & Carleborg,
1987). Ambersorb XE(R) showed good capacity, and decomposition was
insignificant (Levin & Carleborg, 1987). Kenny & Stratton (1989)
evaluated various mixtures to find a solvent that would provide
optimum desorption efficiency. For samples of MEK collected on
charcoal tubes, a mixture of carbon disulfide with 10% amyl alcohol
was found to be an effective desorption solvent. The substitution of
hexyl for amyl alcohol gave comparable recovery but slower GC/FID
analysis. Both thermal desorption and solvent desorption have been
used to release the MEK from the trapping column.
Collectors may be passive and dependent on diffusion or a packed
tube through which a known volume of air is drawn. Passive collectors,
often in the form of badges, avoid the need for specialized sampling
equipment and are convenient for monitoring individual exposure.
However, the results of several studies suggest that passive
(diffusive) collectors not only show significant individual and brand
variability but also variability in their speed of uptake of different
solvent vapours (Hickey & Bishop, 1981; Feigley & Chastain, 1982), and
thus may require calibration against a more quantitative method. The
trapped organic compounds are desorbed either thermally by application
of heat or microwave radiation, or by solution in carbon disulfide,
and are separated with gas chromatography. A wide diversity of columns
and packings have been found satisfactory for this separation.
Using gas chromatography with a flame ionization detector, an
overall precision (Sr) of 0.069 with a limit of detection of 0.004
mg/sample was achieved (US NIOSH, 1984a).
Methodology for analysing air samples recommended by the United
States Environmental Protection Agency (US EPA) can detect MEK and
most other mono-functional aldehydes and ketones at the 3-6 µg/m3
(1-2 ppb) level (Riggin, 1984). Air is drawn through a mixture of
isooctane and an acidified solution of 2,4-dinitrophenylhydrazine
(DNPH), which reacts chemically with MEK. DNPH derivatives of
aldehydes and ketones are extracted from the aqueous layer, separated
with high performance liquid chromatography (HPLC) and detected by
ultraviolet absorption.
MEK vapour can also be detected and measured directly and
instantaneously by absorption of infrared light. The method (detection
limit, 3 mg/m3) appears suitable for use in the workplace where only
a limited number of solvent vapours are present (Persson et al., 1984)
but may not reliably detect MEK in the presence of a diverse mixture
of organic vapours, due to overlapping of infrared absorption peaks
(Puskar et al., 1986). Ying & Levine (1989) used Fourier
transform-infrared spectrometry (FT-IR) to determine the concentration
of MEK in mixtures of vapours in ambient air and obtained a detection
limit of 1 mg/m3. Surface acoustic wave devices have been tested
experimentally for the detection of MEK and other vapours
(Rose-Pehrsson et al., 1988) and show promise for the development of
electronic devices that can continuously monitor and analyse vapour
mixtures at concentrations likely to be present in the work
environment.
2.4.3 Water
Water samples containing high levels of MEK (e.g., industrial
waste water) can be analysed by direct injection of the sample into a
gas chromatograph; the detection limit is 40 µg/litre (Middleditch et
al., 1987). Samples with low levels of MEK (e.g., drinking-water)
require some form of concentration such as distillation (Pellizzari et
al., 1985) or adsorption on a hydrophobic zeolite (Ogawa & Fritz,
1985). GC/MS analysis gives a detection limit of 0.05 µg/litre
(Pellizzari et al., 1985) whereas HPLC with UV detection has a
detection limit of 2 µg/litre (Ogawa & Fritz, 1985).
2.4.4 Solids
Analysis of solid and semisolid materials such as industrial
wastes for MEK presents special difficulties in terms of both sampling
and analysis. The sample must be representative and of adequate size,
since substrates such as waste tend to be very non-homogeneous and MEK
must be completely removed from both solid and liquid components. One
method accomplishes this by extracting the sample with an appropriate
solvent (tetraglyme) and purging MEK and other volatile organics from
the tetraglyme with an inert gas (Gurka et al., 1984). Another method
(Fisk, 1986) either directly purges MEK and other organic compounds
from a water/solid material slurry held at an elevated temperature or
purges a methanol extract of the solid material at an elevated
temperature.
2.4.5 Biological materials
Biological materials offer the same analytical problems as solid
waste: MEK must be completely removed from both solid and liquid
components of the sample. This can be accomplished by headspace
analysis (Ramsey & Flanagan, 1982; US NIOSH, 1984b), steam
distillation of a sample slurry followed by headspace analysis
(Bassette & Ward, 1975; Lin & Jeon, 1985), derivation with
o-nitrophenylhydrazine (Van Doorn et al., 1989) and, in the case of
an entirely liquid substrate, separation and concentration by
reverse-phase extraction (Kezic & Monster, 1988).
For MEK in blood the United States National Institute of
Occupational Safety and Health method (US NIOSH, 1984b), using GC/FID,
has a detection limit of 20 µg/litre and the Ramsey & Flanagan (1982)
method has a detection limit of 100 µg/litre. The latter method can
also be used for the analysis of MEK in urine with the same limit of
detection. Other methods for analysis in urine are those of Kezic &
Monster (1988), using GC/FID, and Van Doorn et al. (1989) using
HPLC/UV; both methods have limits of detection of 100 µg/litre.
3. SOURCES OF HUMAN AND ENVIRONMENTAL EXPOSURE
3.1 Natural occurrence
MEK occurs naturally at low concentrations. It has been
identified in cigarette smoke (Osborne et al., 1956; Hoshika et al.,
1976; Higgins et al., 1983). It also has been reported in chicken
breast muscle (Grey & Shrimpton, 1967), weed residues (Bradow &
Connick, 1988), southern pea seeds (Fisher et al., 1979), insect
pheromones (Cammaerts et al., 1978; Attygalle et al., 1983), juniper
leaves (Khasanov et al., 1982); marine macroalgae (seaweeds) (Whelan
et al., 1982) and as a product of microbial metabolism (Patel et al.,
1982; Mohren & Juttner, 1983; Zechman et al., 1986), including
cultures isolated from fresh water and soil (Hou et al., 1983; Patel
et al., 1983). Berseem clover, hairy vetch and crimson clover emitted
volatile compounds including MEK (Bradow & Connick, 1990). Six
amaranth species emit MEK which has been shown to cause significant
inhibition of tomato and onion seed germination (Connick et al.,
1989). Some bacteria (e.g., thermophilic obligate methane-oxidizing
bacteria) can oxidise 2-butanol to produce MEK (Imai et al., 1986).
Studies have shown that MEK is a normal component of flavour and odour
in a wide range of foods, especially cheese and other fermented
products (Zakhari et al., 1977), often as a result of bacterial
activity (Lin & Jeon, 1985). Seven types of fish contain MEK, although
reported levels were low relative to other compounds (Sakakibara et
al., 1990). MEK has been detected in coyote urine (Schultz et al.,
1988), in the urine of non-occupationally exposed humans (Tsao &
Pfeiffer, 1957; Mabuchi, 1969), in human blood (Mabuchi, 1969) and in
exhaled air (Conkle et al., 1975). The MEK in exhaled air may have
been derived from food, but the observations of Poli et al. (1985) and
other researchers (see section 6) strongly suggest that MEK and
similar carbonyl compounds are minor products of normal mammalian
metabolism.
3.2 Production levels, processes and uses
3.2.1 World production
Although MEK is an important industrial chemical, world
production figures are not available. Annual production in the USA,
reported by the US International Trade Commission, ranged from 212 to
305 thousand tonnes over the period 1980-1987 and averaged 258
thousand tonnes (USITC, 1981-1988). Current (1987) annual capacity and
production values for western Europe are 308 and 215 thousand tonnes,
respectively (Chemical Business Newsbase, 1988). Japanese annual
capacity and production figures in 1986 were 180 and 139 thousand
tonnes, respectively (Chemical Business Newsbase, 1987). Argentinean
annual capacity was 15 thousand tonnes in 1985 (Chemical Business
Newsbase, 1986). A production plant opened in Brazil in 1991 but
information on capacity and production is not available (personal
communication from E. de S. Nascimento).
3.2.2 Production processes
MEK is produced mainly by dehydrogenation of sec-butyl alcohol
(Liepins et al., 1977; SRI International, 1985, 1988). In the USA, one
process uses sec-butyl alcohol vapour at 400 to 550 °C oxidized with
a zinc oxide catalyst. Reaction gases are condensed and the condensate
fractionated in a distillation column. The yield of MEK is 85 to 90%
(Lowenheim & Moran, 1975). Any uncondensed reaction gases are scrubbed
with water or a non-aqueous solvent and the waste stream from the
scrubber, which contains MEK and reaction by-products, is either
recycled or discarded (Liepins et al., 1977). In Europe, sec-butyl
alcohol is dehydrogenated over Rainey nickel or copper chromite
catalyst at 150 °C (Papa & Sherman, 1978)
MEK is also produced by the oxidation of n-butane, either as
the main product or as a by-product in the manufacture of acetic acid
(Liepins et al., 1977; Papa & Sherman, 1978). Liquid butane reacts
with compressed air in the presence of a transition metal acetate
catalyst, normally cobalt acetate, and the reaction product phase is
separated. The hydrocarbon-rich phase is recycled to the reactor and
the aqueous phase with MEK is withdrawn and purified. MEK and other
organic compounds with low boiling points are separated from acetic
acid by distillation. Reaction conditions determine whether MEK or
acetic acid is the principal product (Lowenheim & Moran, 1975). Butane
oxidation accounted for about 13% of the 1987 MEK production capacity
in the USA (SRI International, 1988) but for none of the 1984
production capacity in western Europe (SRI International, 1985). Other
methods exist for the commercial manufacture of MEK (Papa & Sherman,
1978), but there is no evidence that any of these alternatives are of
current importance.
3.2.3 Other sources
In addition to manufacture by the chemical industry, MEK and
other carbonyls are incidentally produced as components of exhaust
from jet (Miyamoto, 1986) and internal combustion engines (Seizinger
& Dimitriades, 1972; Creech et al., 1982; Hampton et al., 1982) and
from industrial activities such as retort distillation of oil shale
(Hawthorne et al., 1985) and gasification of coal (Pellizzari et al.,
1979). MEK comprises about 0.05% of the hydrocarbon exhaust gases of
motor vehicles, and in 1987 the vehicle emission of MEK in the USA was
estimated to be 1909 tonnes (Somers, 1989). Thus its anthropogenic
production by vehicles plus an additional amount by stationary engines
was no more than 0.1% of the industrial production in the USA.
Grosjean et al. (1983) concluded, however, that during smog episodes
in the Los Angeles basin much of the ambient level of MEK was produced
photochemically.
3.2.4 Uses
The major uses of MEK reflect its excellent characteristics as a
solvent (Table 3). Its high solvency for gums, resins and many
synthetic polymers permits formulations with high solid content and
low viscosity. It is also inert to metal, evaporates rapidly, and is
relatively low in toxicity compared with solvents like benzene which
MEK replaced (Zakhari et al., 1977; Basu et al., 1981).
Table 3. Major uses of MEK in the USAa
End use %
Solvent - protective coatings 65
Solvent - adhesives 15
Solvent - magnetic tape production 8
Lubricating oil dewaxing 5
Chemical intermediate 4
Miscellaneous 3
a From: Manville Chemical Products Corp. (1988)
The largest single use of MEK is as a solvent for vinyl plastic
used in coatings and moulded articles. Other important uses are as a
solvent for lacquers and for cellulose nitrate, cellulose acetate,
acrylics, and adhesive coatings. Its properties as a selective solvent
make it ideal for dewaxing lubricating oils. MEK is also used for
degreasing metals, in the manufacture of magnetic tapes, inks and
smokeless powder, and as a chemical intermediate in the production of
methyl ethyl ketoxime, MEK peroxide, methyl isopropyl ketone and many
other compounds.
In addition to industrial uses, MEK is an ingredient in a variety
of consumer products such as lacquers, varnishes, spray paints, paint
removers, sealers and glues (Zakhari et al., 1977). In both consumer
products and industrial applications, MEK is frequently only one of
several components in a mixture of organic solvents.
MEK is also used as an extraction solvent in the processing of
foodstuffs and food ingredients, e.g., in fractionation of fats and
oils, decaffeination of tea and coffee, and extraction of flavourings.
3.3 Release into the environment
Releases of MEK are mainly into the atmosphere (Reilly, 1988).
These can result from: spillage; venting of gases and fugitive
emissions during manufacture, transfer and use; solvent evaporation
from coated surfaces; loss from landfills and waste dumps; and engine
exhaust (Basu et al., 1981; LaRegina & Bozzelli, 1986). Relatively
little MEK is lost during manufacture when the process is enclosed.
The average annual release from four manufacturing plants in the USA
was estimated to be 82 tonnes per site, equal to a total of 328 tonnes
or about 0.1% of their annual production (Reilly, 1988).
The bulk of MEK eventually evaporates to the atmosphere, since
the major use of MEK is as a solvent for coatings and adhesives. In
industry, some of the MEK evaporated from surface coatings or lost
during cooking and thinning of resin is removed from the ventilation
exhaust by absorption on charcoal filters or by incineration of the
exhaust stream. The latter method can reduce emission by up to 97%
(Gadomski et al., 1974), and removal is accomplished in a single step
without generating a residue for subsequent disposal (DiGiacomo,
1973).
The waste stream from MEK production contains acetic acid and a
variety of alcohols, aldehydes, ketones and other organic compounds.
It is likely that butane and other organic compounds are discharged
into the atmosphere from the reaction section, but no specific
information is available (Liepins et al., 1977).
MEK is released from other industrial operations involving its
use, and from activities such as retort distillation of oil shale and
gasification of coal (Pellizzari et al., 1979; Hawthorne et al.,
1985).
It has been detected in drinking-water (Ogawa & Fritz, 1985), in
well water (Jacot, 1983), in ground water (Botta et al., 1984) and in
leachate from a hazardous waste site (Jacot, 1983). MEK occurs in
water often as a result of natural processes (section 3.1).
Atmospheric input and direct anthropogenic pollution contribute
significantly to elevated levels (Grosjean & Wright, 1983).
4. ENVIRONMENTAL TRANSPORT, DISTRIBUTION AND TRANSFORMATION
4.1 Transport in the environment
MEK appears to be highly mobile in the natural environment (Lande
et al., 1976). It is water soluble (Windholz, 1983) and evaporates
rapidly in air. The generally low values for MEK in outdoor air
probably stem mainly from its rapid removal by photodecompo-sition.
Scavenging by aqueous droplets and dry deposition, which also
represent potential routes of loss from the atmosphere, are balanced
to an unknown extent by evaporation of MEK from water and soil. There
is no specific information on partitioning of MEK in the environment.
Although Basu et al. (1981) estimated from its physical properties
that MEK will "exhibit low sediment-water and soil-water partitioning
and be susceptible to substantial leaching from soils to which it is
not extensively chemically bound", there is no information on chemical
binding of MEK to sediment particles. As mentioned above, MEK has,
however, been detected in ground water and the leachate from hazardous
waste sites (section 3.3).
4.2 Bioaccumulation and biodegradation
On the basis of its octanol/water partition and water solubility,
bioconcentration factors (BCF) of approximately 1 and 0.5,
respectively, have been calculated for MEK (US EPA, 1985b). In view of
its high water solubility, ecosystem modelling (Metcalf et al., 1973;
Chiou et al., 1977) indicates that it is unlikely that MEK will
accumulate in food webs. It is absorbed and metabolized by organisms
present in the environment, e.g., in waste water (Dore et al., 1975;
Bridie et al., 1979a) and in soil (Perry, 1968). It is rapidly
metabolized by mammals (Di Vincenzo et al., 1976, 1978; Dietz et al.,
1981; Miyasaka et al., 1982) and by many microbes (Gerhold & Malaney,
1966; Dojlido, 1977; Urano & Kato, 1986). MEK is nearly completely
degradable at concentrations up to 800 mg/litre on the basis of
biochemical oxygen demand (BOD), and the rate of degradation decreases
with increasing concentration of MEK. Using activated sludge there was
complete degradation of MEK in 8 days at a concentration of 200
mg/litre (200 ppm) and in 9 days at a concentration of 400 mg/litre
(400 ppm) (Dojlido, 1979). At a concentration of 20 mg/litre in river
water containing preadapted microbes, MEK was completely degraded in
2.5 days (Dojlido, 1977). Delfino & Miles (1985) reported a slower
rate of decomposition in aerobic ground water; 1 mg/litre was fully
degraded in 14 days. However, a bacterial species (Alcaligenes
faecalis) found in sewage sludge metabolized MEK slowly if at all
(Marion & Malaney, 1963). The data on mammals and microbes suggest
that MEK is rapidly absorbed and metabolized by most living organisms
(Basu et al., 1981).
MEK in air is rapidly decomposed by photochemical processes,
mainly through oxidation by hydroxyl free radicals as well as some
decomposition by direct photolysis (Levy, 1973; Laity et al., 1973;
Dilling et al., 1976; Grosjean, 1982; Seinfeld, 1989). Basu et al.
(1981) estimated a half-life of 5.4 h for photochemical decomposition
in urban atmospheres. They further concluded that the lower
concentration of photochemically produced oxidants in rural air will
lead to a substantially lower rate of photochemical decomposition in
these areas. The concentration of MEK and other carbonyls is higher in
urban air (Grosjean & Wright, 1983; Snider & Dawson, 1985). Greater
anthropogenic emissions and photochemical synthesis of carbonyls from
free radicals (Grosjean et al., 1983) may overwhelm the more rapid
photochemical decomposition in urban atmospheres. Scavenging by
aqueous droplets and dry deposition may also be important processes in
the removal of atmospheric MEK (Grosjean & Wright, 1983).
MEK (and other saturated aliphatic carbonyls) is not chemically
reactive under conditions found in most natural waters and in general
will not degrade rapidly from physical causes once deposited in water
(US EPA, 1985b). The exception is water containing free halogens (such
as chlorine) or hypohalides. MEK reacts with these to form a haloform
and propionic acid (Basu et al., 1981). This can be a cause for
concern in chlorinated waste water and water supplies, since the
chloroform thus produced is more toxic than the original MEK (US EPA,
1985a).
5. ENVIRONMENTAL LEVELS AND HUMAN EXPOSURE
5.1 Environmental levels
5.1.1 Air
Although MEK is widely present in the natural environment,
concentrations are always low even under conditions of pollution
(Table 4). In minimally polluted outdoor air, the level is less than
3 µg/m3 (1 ppb), but 131 µg/m3 (44.5 ppb) has been measured under
conditions of heavy air pollution in the Los Angeles basin.
Volatilization of MEK from building materials and consumer products
can pollute indoor air to levels above adjacent outside air. In a
study of 15 Italian urban homes, De Bortoli et al. (1985, 1986)
reported 8 µg/m3 as a mean indoor air value and 38 µg/m3 as a
maximum value. Maximum and average values for MEK in outdoor air
adjacent to these homes were 12 and 3.8 µg/m3 respectively. Shah &
Singh (1988) reported four observations of MEK in indoor air in the
USA; the median and mean values were 21 and 27 µg/m3 (7.1 and 9.2
ppb), respectively. In a confined and tightly sealed space, however,
MEK concentrations can be much higher. Liebich et al. (1975) measured
1.9 to 4.4 mg/m3 (665 to 1505 ppb) in Space Lab IV.
Human activities, other than the deliberate manufacture and use
of MEK, may in some circumstances contribute significantly to
environmental levels. MEK is a minor component, < 2.95 mg/m3 (1.0
ppm), of gasoline engine exhaust and also has been detected in the
exhaust from diesel engines and jet aircraft. The US Environmental
Protection Agency estimated that 1909 tonnes of MEK was emitted in
motor vehicle exhaust in the USA in 1987 ("Mobile source estimates for
methyl ethyl ketone"; personal communication by J.H. Somers, 1989). In
addition, Grosjean et al. (1983) concluded that synthesis of MEK and
other carbonyls from hydrocarbons in vehicle exhaust by photochemical
reactions in the atmosphere may greatly exceed their direct production
by motor vehicles. Thus, away from industrial areas where MEK is
manufactured or used, it is likely that motor vehicles are an
important and possibly major source of atmospheric pollution by MEK.
Smoking cigarettes and other tobacco products contributes slightly to
individual exposure. Although the concentration of MEK in cigarette
smoke (Table 4) may exceed recommended levels of permissible
occupational exposure (Table 5) by several a Assuming a respiratory
volume of 20 m3 per day times, the total amount of MEK generated by
smoking a single cigarette is about 1/74th of the acceptable human
daily chronic intake in the USA (15.43 mg/day) (US EPA, 1986). MEK
also has been detected in the gases from structural (building) fires
(Lowry et al., 1981).
5.1.2 Water
MEK concentrations in exposed natural waters are less than 0.1
mg/litre (0.1 ppm) and are usually below the level of detection. Ewing
et al. (l977) analysed 204 samples from rivers with industrialized
basins; only one sample contained MEK (0.023 mg/litre). Jungclaus et
al. (1978) measured significant levels of MEK in waste water from a
chemical plant but could not detect it in either water or sediment of
the brackish Delaware River receiving this waste. Despite its rapid
disappearance from water, trace amounts of MEK have been detected
widely in drinking-water (US EPA, 1985b). A potential source is
solvent leached from the cemented joints of plastic pipe (Wang &
Bricker, 1979; Boettner et al., 1981). A single unexpectedly high
value of 0.47 mg/litre (0.47 ppm) in mist from the landward edge of
the Los Angeles basin probably resulted from scavenging of heavily
polluted air (Grosjean & Wright, 1983). Data on MEK in sediment (US
EPA, 1985b) were based on four samples and are difficult to interpret.
Sawhney & Kozloski (1984) studied organic pollution of leachates from
municipal landfill sites in Connecticut, USA. MEK concentrations
ranging between 4.8 and 8.2 mg/litre were measured over a two-year
period at one site. This high value may have resulted not only from a
substantial input but also from reduced microbial activity and no
evaporative loss to the air.
Environmental concentrations in a number of media are shown in
Table 4.
Table 4. Concentrations of MEK in the environment
Source Concentration Reference
Air (rural)
South-western USA 1.77 µg/m3 Snider & Dawson (1985)
(0.6 ppb)
Air (urban)
South-western USA, Tucson 7.1 µg/m3 Snider & Dawson (1985)
(2.4 ppb)
USA, Los Angeles basin 0-131.3 µg/m3 Grosjean et al. (1983)
(0-44.5 ppb)
Sweden, traffic areas 7.7-94 µg/m3 Jonsson et al. (1985)
(2.6-32 ppb)
Italy < 2.1-12.1 µg/m3 De Bortoli et al. (1985)
(< 0.7-4.1 ppb)
Japan (air pollution) 12.7 µg/m3 Anonymous (1978)
(4.3 ppb)
Air (indoor)
Italy (homes) < 2.1-38.1 µg/m3 De Bortoli et al. (1985)
(< 0.7-12.9 ppb)
USA (homes) detected in 3 out of Jarke et al. (1981)
87 samples
Space Lab IV 1.96-4.44 mg/m3 Liebich et al. (1975)
(0.665-1.505 ppm)
Water
Sea water (Gulf Stream) < 0.022 mg/litre Corwin (1969)
Sea water (Mediterranean) < 0.008 mg/litre Corwin (1969)
Mist (California, USA) < 0.47 mg/litre Grosjean & Wright (1983)
Drinking-water (USA) < 0.0016 mg/litre Ogawa & Fritz (1985)
Ground water (hazardous 4.8-8.2 mg/litre Sawhney & Kozloski (1984)
waste landfill sites, USA)
Table 4 (contd.)
Source Concentration Reference
Rivers (industrialized 0.023 mg/litrea Ewing et al. (1977)
areas, USA)
Waste water (oil well, USA) 1.5 mg/litre Sauer (1981)
Waste water (Chemical plant, 8-20 mg/litre Jungclaus et al. (1978)
USA)
Waste water (plant, Poland) > 100 mg/litre Dojlido (1977)
Sediment
USA 0.050-23 mg/kg US EPA (1985b)
Anthropogenic sources
Automobile exhaust < 0.3-2.95 mg/m3 Seizinger & Dimitriades
(0.1-1.0 ppm) (1972)
Cigarette smoke 80-207 µg/cigarette Higgins et al. (1983)
a MEK was detected in only one of 204 samples.
Table 5. Levels of estimated daily MEK intake from different
sources/routes of exposure
Type/route of exposure Daily intake
Foodstuffs 1590 µg
Drinking-water (2 litres) 3.2 µg
Ambient aira
outdoor, rural 36 µg
outdoor, urban < 2620 µg
indoor < 760 µg
Tobacco smoking (20 cigarettes) < 1620 µg
a Assuming a respiratory volume of 20 m3 per day
5.1.3 Foodstuffs
MEK is produced in small amounts by animals, higher plants, algae
and microbes, and is a widespread, although generally minor, component
of taste and odour in foods (Zakhari et al., 1977). It has been
identified in some foodstuffs and beverages. Using the DNPH method
with column or paper chromatography, it has been identified (but not
quantified) in white bread (Ng et al., 1960), tomatoes (Schormüller &
Grosch, 1964), cooked turkey meat (but not in raw meat and with more
MEK in roasted than in boiled meat, the level increasing with roasting
time) (Hrdlicka & Kuca, 1965), and in egg white (Sato et al., 1968).
Using gas chromatography, traces of MEK were found in fresh chicken
meat (pectoral muscle) with a marked increase in samples kept for 4
days at room temperature. It was not found in caecal gas from living
chickens but was detected in the gas 18 and 24 h after death (Grey &
Shrimpton, 1967). MEK has also been detected in cottonseed oil
(Dornseifer et al., 1965), honey (Cremer & Riedmann, 1964), coffee
(Gianturco et. al., 1966), roast barley (Shimizu et al., 1969), and in
the mushroom Agaricus bisporus (Staüble & Rast, 1971). By means of
GC/MS, Wong et al. (1967) detected MEK in codfish and Kahn et al.
(1968) reported its presence in a low-boiling distillation fraction of
Canadian whisky.
In a study of compounds related to milk flavour, Wong & Patton
(1962) determined MEK concentrations using the DNPH method with column
separation and paper chromatography. The concentrations in two samples
of untreated milk were 0.77 and 0.79 mg/litre and in two samples of
cream were 0.154 and 0.177 mg/litre. Gordon & Morgan (1972) examined
the influence of volatile compounds in milk on "feed" flavour and
reported MEK concentrations of 0.25-0.35 mg/litre in moderately "feed"
flavoured milk and 0.50-1.0 mg/litre in strongly flavoured milk, with
a highest concentration detection of 1.4 mg/litre. They concluded that
MEK is one of the compounds responsible for producing the unpleasant
"feed" flavour in milk. Using the DNPH method and paper
chromatography, Harvey & Walker (1960) detected MEK in New Zealand
cheddar cheese one day after manufacture. The concentration increased
during ripening, reaching 0.9 mg/kg at 40 weeks, and was related to
the development of typical Cheddar cheese flavour. In another study of
the chemical nature of USA Cheddar cheese flavour, Day et al. (1960)
analysed the volatile flavour fraction of cheeses over 1 year old
using DNPH and column partition chromatography, and reported
approximate MEK concentrations of 12.5 mg/kg. Keen et al. (1974)
postulated that the formation of MEK in New Zealand Cheddar cheese,
for which levels as high as 19 mg/kg had been reported, occurred in
steps carried out by different microbial species including
Streptococcus cremaris, Pediococcus cerevisiae, Lactobacillus
plantarum and Lactobacillus brevis. They considered that MEK was
an important flavour constituent in the cheese.
In another investigation of monocarbonyl compounds as flavour
components, Mookherjee et al. (1965) measured MEK in fresh and stale
(8 weeks old) potato chips with the DNPH method and liquid-liquid
chromatography. In fresh potato chips the concentration of MEK was 1.8
µmoles/kg, and this increased to 2.2 µmoles/kg in stale chips.
Amylomaize starches are heat treated in the production of films
and fibres to concentrate the amylose. Bryce & Greenwood (1963) used
gas chromatography to measure pyrolysis products (including MEK) of
potato starch, potato amylose and amylopectin, maltose, isomaltose and
glucose. MEK was not detected in untreated starch nor in starch
pyrolysed in vacuo for 20 min at 200 and 220 °C. With increasing
temperatures the concentration of MEK increased; at 230, 250, 300, 350
and 400 °C the MEK concentrations were 10, 15, 50, 65 and 70 moles x
107/g starch, respectively.
Small quantities (up to 2 ng/1.5 g bean) were found in soybeans
(Clycine max) and winged beans (Psophocarpus tetragonolobus) by
means of dynamic headspace GC/MS (Del Rosario et al., 1984).
However, the reported concentrations of MEK in foods are low and
food consumption is not considered a significant source of population
exposure.
5.2 General population exposure
Stofberg & Grundschober (1984) calculated the consumption ratio
between the quantity of a flavouring material consumed as an
ingredient of basic and traditional foods and the quantity of that
same flavouring material consumed as a component of added flavourings
by a certain population. If the consumption ratio is more than 1, this
substance is consumed predominantly as an ingredient of traditional
foods. For MEK, this ratio may be up to 411. The annual consumption in
the USA of MEK via apple juice is 85 kg, white bread 70 132 kg, butter
34 kg, carrot 154 kg, Cheddar cheese 30 139 kg, Swiss cheese 198 kg,
fish 81 kg, potato chips 31 kg, tomato 31 878 kg, and yoghurt 1104 kg.
Assuming a population of 230 millions, the estimated average daily
intake in the USA amounts to 1.59 mg/kg foodstuff. Some information on
the MEK contents of various foodstuffs is given in Table 6. The
European Economic Community regulates the level of MEK in certain
foodstuffs; these are given in Table 7.
In addition to the MEK that is naturally present, foods may also
contain MEK absorbed from plastic packaging materials. This can be
derived from solvent left in the plastic during manufacture
(Kontominas & Voudouris, 1982) or represent one of the many organic
compounds produced during extrusion (Fernandes et al., 1986). It can
also be produced by irradiation of polyethylene film during the
sterilization of packaged foods with an electron beam (Azuma et al.,
1983). Although the presence of MEK and associated organic compounds
from packaging materials may affect the flavour of foods, it probably
does not represent a significant source of population exposure.
Table 6. MEK concentrations of certain foodstuffs
Source Concentration Reference
Bean seeds (raw) 0.5 mg/kg Del Rosario et al. (1984)
Bean seeds (heated at 0.7-2.0 mg/kg Del Rosario et al. (1984)
190 °C)
Pea seeds 0.074-0.39 mg/kg Fisher et al. (1979)
Milk ("feed"-flavoured) 0.25-14 mg/litre Gordon & Morgan (1972)
Bread 3.06 mg/kg Sosulski & Mahmoud (1979)
Cheddar cheese 12.5 mg/kg Day et al. (1960)
19 mg/kg Bills et al. (1966)
Table 7. Food uses of MEK permitted in the European Economic Community
Conditions of use Maximum residue limits in the extracted
foodstuff, food or food-contact material
In manufactured or regenerated 0.6 mg/dm2 on the side in contact with
cellulose film that comes into foodstuffs
contact with fooda
Fractionation of fats/oilsb 5 mg/kg in the fat/oil
Decaffeination of, or removal of 20 mg/kg in the coffee or tea
irritants and bitterings from, (as granules, powder, leaves,
coffee and teab etc.)
Preparation of flavourings 1 mg/kg in the foodstuff
from natural flavouring materialsb
a Council Directive 83/229/EEC
b Council Directive 88/344/EEC
Higgins et al. (1983) analysed the gas phase organic compounds in
cigarette smoke. In cigarettes with high tar content (7-45 mg per
cigarette), the MEK level was 63-131 µg/cigarette, whereas in ultralow
tar delivery cigarettes (advertised value < 0.01-0.2 mg per
cigarette), it was 0.93-4 µg MEK/cigarette.
Levels of estimated daily MEK intake from different sources/
routes of exposure are given in Table 5.
5.3 Occupational exposure
Information on measured levels of occupational exposure is
summarized in Table 8. Some national occupational exposure limits for
MEK in workplace air are shown in Table 9. In a study of an electronic
parts plant in the USA, Lee & Parkinson (1982) reported that workers
were exposed to mixtures of solvent vapours containing MEK. Inoue et
al. (1983), in a nationwide survey of Japanese factories, found that
MEK was widely used as a component of solvent mixtures. A study by
Falla (1987) of 19 British plants manufacturing or applying surface
coatings reported that none had MEK concentrations in excess of 295
mg/m3 (100 ppm). The highest TWA value, 723 mg/m3 (245 ppm),
reported by Lee & Murphy (1982) represented a worker who entered the
highly polluted vinyl dip room (502-1785 mg/m3; 170-605 ppm) only
occasionally, but without always donning his respirator hood. A
co-worker stationed in the vinyl dip room who wore his respirator hood
constantly had TWA exposures of 307-617 mg/m3 (104-209 ppm). De Rosa
et al. (1985) examined 504 work stations in 81 Italian plants (shoe
factories, painting operations and printing plants) and found that the
TLV for MEK (590 mg/m3, 200 ppm) was rarely exceeded.
5.4 Peri-occupational exposure
Many small industries in the Netherlands are located in inner
city areas. The influence of such industries on the quality of indoor
air in adjacent houses was studied by Verhoeff et al. (1987), who
monitored the indoor air of a car-body repair shop, an offset printing
office and surrounding houses for organic solvents, including MEK.
Monitoring was carried out for one week, and the individual exposure
of workers and residents was investigated by biological monitoring of
the exhaled breath with additional personal air sampling of the
workers. Concentrations in both the factories were lower than 29.5
mg/m3, i.e. 5% of the Dutch MAC values (590 mg/m3). In the
personal air samples of the employees, MEK was at or below the
detection level. In the house located directly over the car-body
repair shop, the average concentrations of MEK were about 50% those in
the shop, while two floors above, MEK was detected only once.
Table 8. Occupational exposure to MEK via air
Activity Country Sampling Concentration Reference
details mg/m3 (ppm)
Printing and printing Japan personnel (62) 0-265 (0-90) Miyasaka et al. (1982)
machine manufacturing
Shoe factory Italy not given 0-300 (0-102) Brugnone et al. (1983)
Shoe factories, painting Italy personnel (1 h) 0-1110 (0-376)a De Rosa et al. (1985)
operations, printing plants (504)
Miscellaneous factories Italy personnel (4 h) (65) 10-953 (3.4-323) Ghittori et al. (1987)
Sheet metal shop Sweden area (continuous) approx. 3-44 Persson et al. (1984)
(approx. 1-15)
Organic chemical waste USA area (9) < 0.06 (< 0.02) Decker et al. (1983)
incinerator (vicinity)
personnel (7) < 0.06-189 (< 0.02-64)
(exposed workers)
Radio components USA personnel (range 0-38 (0-13) Lee & Parkinson (1982)
manufacturing of jobs)
Solvent recycling plant USA < LDQ-166 (< LDQ-38)b Kupferschmid & Perkins (1986)
Plastic items factory USA no details 0.07-0.16 Ahrenholz & Egilman (1983)
(0.024-0.054)
Surface coatings United area (59) < 295 (100) Falla (1987)
factories Kingdom
Table 8 (contd)
Activity Country Sampling Concentration Reference
details mg/m3 (ppm)
Lubricating oil USA personnel (38) 0.09-80 (> 0.029-27) Emmel et al. (1983)
refineries
Miscellaneous factories USA personnel (179) 0-0.59 (0-0.2) Whitehead et al. (1984)
with spray application (non-exposed
of glue and paint workers)
1.18-6.2 (0.4-2.1)
(exposed workers)
Aircraft maintenance USA personnel (9) 0-65 (0-22)c Thoburn & Gunter (1982)
Athletic equipment USA personnel (12) 307-723 Lee & Murphy (1982)
factory (104-245 TWA)d
(vinyl dip room)
0.89-534 (0.3-181)
TWA (elsewhere)
a MEK was found in 85/504 samples
b LDQ = lowest detectable quantity
c MEK was detected in only one of nine samples
d 8-h time-weighted average
Table 9. Some national occupational exposure limits for MEK in air
Country Exposure limit Category of limitb Reference
mg/m3 (ppm)
Argentina 590 (200) TWA IRPTC (1987, 1991)
885 (300) STEL
Brazil 460 (155) for 48 h/week IRPTC (1987, 1991)
Germany 590 (200) TWA IRPTC (1987, 1991)
1180 (400) 30 min. STEL
(average value,
4 x per shift)
Hungary 200 (68) TWA (8 h) IRPTC (1987, 1991)
1000 (2950) STEL (30 min)
Italy 590 (200) TWA Notified by country
Japan 590 (200) TWA IRPTC (1987, 1991)
Netherlands 590 (200) TWA a
Sweden 150 (50) TWA IRPTC (1987, 1991)
300 (100) STEL (15 min)
United Kingdom 590 (200) TWA (8 h), OES Notified by country
885 (300) STEL (10 min), OES
USA (NIOSH/OSHA) 590 (200) TWA (10 h) US NIOSH (1990)
885 (300) STEL (15 min)
8850 (3000) IDLH
USA (ACGIH) 590 (200) TLV (TWA) ACGIH (1991)
885 (300) STEL
2 mg/litre BEI
urine; end of
shift
Yugoslavia 295 (100) Notified by county
TWA and TLV are, with one exception, in the range of 295-590 mg/m3 (100-200 ppm). The
latter is stated to be the highest concentration which can be tolerated by humans without
discomfort (ACGIH, 1986). In addition a short-term exposure limit of 885-1180 mg/m3
(300-400 ppm) has been established by some nations.
a Dutch Expert Committee for Occupational Standards (1991)
b Abbreviations: BEI = biological exposure index (ACGIH); IDLH = concentration
immediately dangerous to life or health (US NIOSH); OES = occupational exposure
standard (UK); STEL = short-term exposure limit; TLV = threshold limit value
(ACGIH); TWA = time weighted average
6. KINETICS AND METABOLISM
6.1 Absorption
6.1.1 Percutaneous absorption
Percutaneous absorption of MEK appears to be rapid (Munies &
Wurster (1965) and Wurster & Munies (1965) reported that MEK was
present in the exhaled air of human subjects within 2.5-3.0 min after
it was applied to normal skin of the forearm, and the concentration of
MEK in exhaled air reached a plateau in 2-3 h. The rate of absorption
was controlled mainly by the moisture content of the skin. With dry
skin, absorption was slow, and it took 4-5 h for the concentration of
MEK in expired air to attain a plateau. With moist skin, absorption
was very rapid initially. MEK was detected in expired air in
measurable concentrations within 30 seconds after an application of
MEK to the forearm, and a maximum concentration in expired air,
averaging four times the plateau level for normal and dry skin, was
achieved in 10-15 min. The concentration of MEK in expired air, and
thus its absorption, subsequently declined to a plateau level somewhat
above that for normal and dry skin because the MEK partially
desiccated the moist skin with which it was in contact. Munies &
Wurster (1965) concluded that the rapid percutaneous absorption of MEK
reflected its olive oil-water partition coefficient, which is close to
unity. Their data have been used to calculate minimum rates of
percutaneous penetration of 0.46 µgÊcm-2Êmin-1 for dry or normal
skin and 0.59 µgÊcm-2Êmin-1 for moist skin (JRB Associates, Inc.,
1980). These rates are minimal because they are based solely on
exhalation from the lungs and ignore all other excretion processes for
MEK.
As the elimination of MEK via inhalation constitutes only 5 to
10% of the total loss (Cushny, 1910), these rates should be multiplied
by factors of 10-20. This gives a percutaneous absorption of 5-10
µgÊcm-2Êmin-1, which is identical to the value measured for methyl
isobutyl ketone by DiVincenzo et al. (1978).
6.1.2 Inhalation absorption
The absorption of MEK via the lungs was examined by Perbellini et
al. (1984) in a study of workers exposed in industrial workplaces. The
MEK concentration in alveolar and expired air correlated significantly
with the environmental concentration, and averaged 30% of the latter.
In more recent studies (Liira et al., 1988a, 1988b), values for
pulmonary absorption ranging from 41.1% to 55.8% were obtained. Liira
et al. (1988b) suggested that differences in their values may have
reflected variations in breathing technique during the collection of
samples rather than actual changes in uptake. Deeper inhalation
increased the alveolar volume relative to the dead space (the
non-alveolar volume of the respiratory system), and thus increased the
apparent absorption (personal communication by J. Liira, 1989). When
the alveolar retention of MEK (about 70%) measured by Perbellini et
al. (1984/1985) is transformed to overall pulmonary retention, their
observations and those of Liira et al. (1988a,b) are in agreement,
showing that about 50% of inhaled MEK is taken up.
Results of studies by Liira et al. (1988a,b, 1990a,b) indicated
a rapid transfer of MEK vapour into the blood stream. Perbellini et
al. (1984, 1985), reported that the concentrations of MEK in the blood
and urine were significantly correlated with the environmental
concentration, indicating rapid transfer to the blood and thence to
other tissues. In human volunteer subjects, exercise during exposure
markedly increased the MEK level in blood in comparison with sedentary
behaviour (Liira et al., 1988b), indicating that the blood MEK level
also depended on the rate of uptake. Ghittori et al. (1987) and
Miyasaka et al. (1982) also found significant correlations between
environmental levels of MEK and amounts excreted in the urine of
exposed workers. The concentration of MEK in urine rose from
essentially zero to 70% of its maximum value during the first 2 h of
an 8-h shift (Miyasaka et al., 1982).
Ong et al. (1991) studied biological monitoring of occupational
exposure to MEK in 67 healthy male workers employed in plastic bag or
video-tape production. Their ages ranged between 18 and 52 years with
an average working experience of 8.6 years. For the majority of the
workers, atmospheric MEK concentrations were in the range 30-885
mg/m3 (10-300 ppm). MEK concentrations in urine, blood and exhaled
air were measured once weekly at the end of a shift. About 10% of
absorbed MEK was eliminated in the exhaled air. Following exposure to
590 mg/m3 (200 ppm), the urinary concentration was 5.1 µmol/litre or
4.11 mg/g creatinine. The correlation coefficients (tau) between
atmospheric MEK concentration and end-of-shift urine, blood and
exhaled air were 0.89, 0.85 and 0.79, respectively. The authors also
reported good correlation between blood and urinary MEK (tau = 0.86)
and between blood and exhaled air concentrations (tau = 0.8) but poor
correlation between exhaled air and urinary MEK concentrations.
6.1.3 Ingestion absorption
In male rats given a large MEK dose (1505 or 1690 mg/kg) in
water, blood concentrations reached maxima of 0.95 and 0.94 mg/ml 4 h
after ingestion and subsequently declined sharply, indicating
protracted absorption of this dose from the gastro-intestinal tract
(Traiger & Bruckner, 1976; Dietz & Traiger, 1979; Dietz et al., 1981).
6.1.4 Intraperitoneal absorption
The results of DiVincenzo et al. (1976) and Zakhari et al.
(1977), who used intraperitoneal injections of MEK in research on
metabolism and toxicity, suggest that absorption from the peritoneal
cavity is rapid.
6.2 Distribution
The distribution of MEK in human tissues was examined by
Perbellini et al. (1984) in two solvent-exposed workers who died
suddenly of heart attacks at the workplace. The results of this study
(Table 10) indicate that the solubility of MEK is similar for all
tissues. Brugnone (1985) calculated the uptake and distribution of MEK
from the lungs. With a blood/air partition coefficient of 202, MEK can
reach equilibrium concentration in a compartment in about 3 min.
Distribution volumes were 6.0 for vessel-rich tissues, 39.6 for muscle
and 12.8 for fat. Biological half-lives for the same tissues were 0.8,
21.8 and 23.3 min, respectively. The results of in vitro
measurements at 37 °C of human tissue-gas partition coefficients,
obtained by exposing samples of blood and tissue to a known
concentration of MEK (Fiserova-Bergerova & Diaz, 1986), differed from
the observations of Perbellini et al. (1984). The partition
coefficients ranged from 96 to 162, but did not exceed 111, with the
exceptions of whole blood (125), blood plasma (133) and fat (162).
Other blood-gas partition coefficients measurements for MEK are 202
(Sato & Nakajima, 1979) and 215 (Pezzagno et al., 1983). Traiger &
Bruckner (1976) and DiVincenzo & Krasavage (1974) provided evidence
that MEK can enter the liver of rats and guinea-pigs, and Dowty et al.
(1976) reported that it can cross the placenta and enter the human
fetus.
6.3 Metabolic transformation
MEK has been reported to be a metabolic end product of natural
gas (methane 88%, ethane 5%, propane 5%, isobutane 2% with traces of
tert-butyl mercaptan and methyl acrylate) inhaled for 2 h by ICR
mice (Tsukamoto et al., 1985a). In an in vivo study of the
metabolism of propane, n-butane and iso-butane inhaled for 1 h by
ICR mice, it was found that n-butane gave rise to sec-butanol and
MEK (Tsukamoto et al., 1985b). In vitro studies showed that mouse
liver microsomal preparations metabolized n-butane to sec-butanol,
the precursor of MEK (Tsukamoto et al., 1985b). Inhalation by male
Sprague-Dawley rats of sec-butanol at concentrations of 5900 mg/m3
(2000 ppm) for 3 days or 1475 mg/m3 (500 ppm) for 5 days caused
marked enzyme induction of cytochrome P-450 in liver and kidney but
other butanol isomers did not have this effect (Aarstad et al., 1985,
1986). The authors concluded that the mechanism of induction was via
the sec-butanol metabolite MEK.
Table 10. Solubility (partition coefficient) of MEK in human tissuesa
Tissues Tissue/air Tissue/blood
Blood 183 1.00
Kidney 197 1.08
Liver 180 0.98
Brain 168 0.92
Fat 161 0.88
Muscle 212 1.16
Heart 254 1.39
Lung 147 0.80
a From: Perbellini et al. (1984)
6.3.1 Animal studies
Traiger & Bruckner (1976) showed that the toxic effects of MEK
and 2-butanol were essentially identical in rats, and that 2-butanol
was rapidly oxidized to MEK. DiVincenzo et al. (1976) identified the
metabolites of MEK in guinea-pigs as 2-butanol, 3-hydroxy-2-butanone
and 2,3-butanediol. They hypothesized that the metabolism followed
both oxidative and reductive pathways, with the latter leading to the
production of 2-butanol. The former, employing microsomal omega-1
oxidization, oxidized MEK to 3-hydroxy-2-butanone, which was
subsequently reduced to 2,3 butanediol. Further research utilizing
rats (Dietz & Traiger, 1979; Dietz et al., 1981) clarified the
pathways of rat MEK metabolism and permitted a calculation of rate
constants for the elimination of MEK and its metabolites from the
blood as well as for the metabolic transformations (Fig. 1). The body
was divided into two compartments: (a) the liver, where metabolic
transformations took place; and (b) the blood, which was the site of
sampling. Experimental data and equations derived from this data
indicated that the major metabolic pathway is butanol -> MEK ->
3-hydroxy-2-butanone -> 2,3-butanediol, with small or non-existent
reverse flows. Dietz et al. (1981) estimated that an oral dose of
2-butanol or MEK resulted, on a molar basis, in the same blood
level-time curve for 2,3-butanediol as 28-30% of this amount given as
an intravenous dose of 2,3-butanediol. Their data indicated that
transformations of 2-butanol to MEK and of 3-hydroxy-2-butanone to
2,3-butanediol are rapid and that transformation of MEK to
3-hydroxy-2-butanone is much slower. It is likely that 2-butanol, like
ethanol (Mezey, 1976), inhibits oxidative pathways of drug metabolism
and thus inhibits the hydroxylation step leading to
3-hydroxy-2-butanone. This possibility is supported by the observation
of Dietz et al. (1981) that 2-butanol, at concentrations similar to
those achieved in the blood of rats in the above experiment,
significantly inhibited N-dealkylation of aminopyrine by rat liver
microsomes in vitro .
In rats exposed by inhalation to 1760 mg MEK/m3 (600 ppm),
there were only marginal effects on microsomal cytochrome P-450
activities (Liira et al., 1991). However, a daily dose of 1.4 ml MEK
per kg for 3 days increased the amounts of ethanol- and
phenobarbital-inducible cytochromes P-450 (P-450 IIEI and P-450 IIB)
(Raunio et al., 1990). In a study on male Sprague-Dawley rats,
pretreatment with MEK elevated total microsomal cytochrome P-450 and
NADPH-dependent cytochrome-c-reductase, the rates of oxidation of
N-nitrosodimethylamine, benzphetamine and pentoxyresorufin, and also
the levels of immunoreactive protein for both P-450 isozymes (Brady et
al., 1989). In a study of hepatotoxicity in rats, Brondeau et al.
(1989) found that MEK increased liver cytochrome P-450 content
(33-86%) and glutathione-S-transferase (GST) activity (42-64%) but had
no effect on serum glutamate dehydrogenase (GLDH) activity. Robertson
et al. (1989) studied the effects on hepatic cytochrome P-450
activities of repeated daily doses of 1.87 ml/kg given by gavage to
male Fischer-344 rats. The activity of 7-ethoxy
coumarin- O-deethylase was increased by up to 500% after 1 to 7 days
of MEK treatment, but there was practically no change in
benzphetamine- N-demethylase activity. In another study in male
Sprague-Dawley rats, administration of MEK by gavage caused an
increase in hepatic acetanilide hydroxylase and a marginal increase in
aminopyrine- N-demethylase activities (Traiger et al., 1989).
6.3.2 Human studies
MEK has been identified as a minor but normal constituent of
urine (Tsao & Pfeiffer, 1957), serum and urine of diabetics (Mabuchi,
1969), and expired air (Conkle et al., 1975). Its production in the
body has been attributed to isoleucine catabolism (Tsao & Pfeiffer,
1957; Przyrembel et al., 1979). Although Smith (1981) mentioned MEK as
a product of autoxidation of cholesterol, no evidence was offered that
this process occurred in vivo . The studies of Perbellini et al.
(1984) and Liira et al. (1988a,b) indicate that the same metabolites
are produced and excreted in humans as in experimental animals. Liira
(personal communication by J. Liira, 1989) further indicated that in
an inhalation exposure to 590 mg MEK/m3 (200 ppm) the calculated
areas under the curves of blood solvent concentration versus time
(AUC) for MEK and 2,3-butanediol were equal, which suggests that MEK
is almost completely transformed to 2,3-butanediol. The bulk of MEK
absorbed thus enters the general metabolism and is transformed to
simple compounds like carbon dioxide and water.
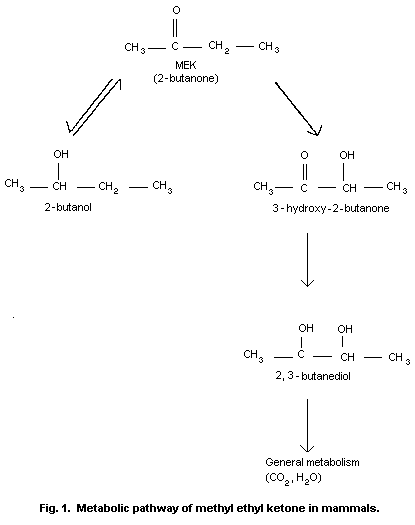 6.4 Elimination and excretion
MEK and its metabolites are excreted by the lungs and kidneys.
Liira et al. (1988a) reported that only 3% of the calculated absorbed
dose during a 4-h exposure to 590 mg MEK/m3 (200 ppm) was secreted
unchanged in the exhaled air of volunteers after the exposure. The
fractional elimination of unchanged substance however depends on the
efficiency of metabolic clearance. Since metabolic saturation for MEK
in humans begins at relatively low levels (about 100 ppm) of exposure
(Liira et al., 1990a), proportionally greater amounts of MEK would be
expected to be excreted via the lungs (and kidneys) at high exposure
levels.
Relatively little of the absorbed MEK is excreted unchanged via
the kidneys; a study of occupationally exposed workers revealed that
it is less than 0.1% of the alveolar uptake (Miyasaka et al., 1982).
In a similar study of workers occupationally exposed to a mixture of
solvents, the excretion of MEK and a major recognizable metabolite,
3-hydroxy-2-butanone, was 0.1% of alveolar uptake (Perbellini et al.,
1984). The concentrations of both MEK and 3-hydroxy-2-butanone in
urine were significantly correlated with the environmental level of
MEK. Other metabolites of MEK, 2-butanol or 2,3-butanediol, which
DiVincenzo et al. (1976) identified in the serum of guinea-pigs, were
not detected in the urine of the exposed workers. Liira et al.
(1988a), however, reported that human excretion of 2,3-butanediol was
individually variable but averaged 2% of the absorbed MEK. The urinary
excretion of 2-butanol, a minor metabolite of MEK, was examined by
Kamil et al. (1953), who found that clearance of 2-butanol
administered by gavage in rabbits was about 14% of the administered
dose and in the form of a glucuronide.
Since MEK and 2,3-butanediol disappear from blood and urine and
there is no evidence for accumulation elsewhere in the body, the above
data suggest that the bulk of MEK absorbed by mammals enters the
general metabolism and is eliminated from the body as simple compounds
like carbon dioxide and water whose source is not readily
identifiable. The specific pathways by which MEK is metabolized have
not been identified. There also is no information on loss of MEK in
faeces.
6.5 Turnover
Both animal and human data indicate a rapid turnover of MEK. In
guinea-pigs receiving an intraperitoneal dose of 450 mg MEK per kg,
the half-life of MEK in blood serum was 4´ h and the clearance time
for MEK in serum was 12 h. For the metabolites 2-butanol,
3-hydroxy-2-butanone and 2,3-butanediol, the clearance time in serum
was 11 h (DiVincenzo et al., 1976). In rats given a 2.2-ml/kg oral
dose of 2-butanol, the butanol was largely cleared from the blood in
15 h and the 2-MEK derived from the butanol was cleared in 24 h
(Traiger & Bruckner, 1976). In a study by Dietz & Traiger (1979) on
rats given an oral dose of 2-butanone of 2.1 mg/kg, there was a
half-life of 3.6 h for MEK in blood if the rate of loss was assumed to
be constant between the two times of measurement (4 h and 18 h) after
dosing. Data from a study of Dietz et al. (1981) on rats receiving
oral doses of 2-butanol or MEK also indicate a half-life of about 4 h
for MEK. These authors reported that the clearance rate for
3-hydroxy-2-butanone and 2,3-butanediol was independent of dose for
the two doses used (0.4 and 0.8 g/kg) and that the half-lives for
these metabolites of MEK were 47 min and 3.45 h, respectively. Liira
et al. (1988a) reported a steady increase in blood concentrations
during 4-h exposures of human volunteer subjects to 590 mg/m3 (200
ppm) and observed a rapid elimination of MEK, with half-lives of 30
min during the first post-exposure hour and 81 min thereafter. An
inhalation study with two volunteer subjects exposed to MEK for 4 h at
concentrations of 74, 590 and 1180 mg/m3 (25, 200 and 400 ppm)
indicated that the kinetics of MEK were dose dependent at higher
exposure concentrations, i.e. much higher levels of MEK in blood were
reached relative to inhaled concentrations, and the post-exposure
elimination of MEK in blood was slower (zero-order kinetics).
Simulated exposure to MEK for 8 h suggests that saturation kinetics
are reached at about 295 mg/m3 (100 ppm) at rest and 148 mg/m3 (50
ppm) during light exercise (Liira et al., 1990a).
6.6 Metabolic interactions
Ingestion of ethanol (0.8 g/kg) combined with an inhalation
exposure to MEK (590 mg/m3, 200 ppm) inhibited the oxidative
metabolism of MEK and led to a marked increase in the blood
concentration of MEK (Liira et al., 1990b). There was a concurrent,
even more pronounced elevation of the blood 2-butanol concentration;
the most likely explanation is competitive inhibition by ethanol of
the oxidation of 2-butanol back to MEK. Ethanol also appeared to
interact with the further biotransformation of 2,3-butanediol as the
urinary excretion of the metabolite was increased. Co-exposure with
xylene, however, had no effect on the human metabolism or rate of
elimination of MEK (Liira et al., 1988b).
6.7 Mechanisms of action
There is very limited information on the mechanisms of toxic
action of MEK. Relatively high inhaled concentrations 1475-29 500
mg/m3 (500-10 000 ppm) caused pulmonary vasoconstriction and
hypertension in cats and dogs (Zakhari et al., 1977). From the
toxicological point of view, interactions leading to the potentiation
of effects, particularly neurotoxicity, by other intrinsically toxic
substances constitute the main hazard of MEK. The mechanisms
underlying these interactions are incompletely known (see section 10).
7. EFFECTS ON LABORATORY MAMMALS AND IN VITRO TEST SYSTEMS
7.1 Acute exposure
7.1.1 Lethal doses
Data on the acute toxicity of MEK to experimental animals are
summarized in Table 11. Oral toxicity is low with LD50 values
ranging from 2 to 6 g/kg for adult mice and rats. Intraperitoneal
LD50 values are lower (approximately 1.5 g/kg for 24 h and 0.6 g/kg
for 14 days). A larger dermal LD50 value for rabbits, i.e. > 8 g/kg
(contact time: 24 h; observation time: 14 days), may reflect slower
and less complete absorption via the skin, although it may also
reflect species differences in sensitivity to MEK. The lethal dose of
MEK given to rats in the only study using dosing by pulmonary
aspiration (Panson & Winek, 1980) was 0.8 g/kg. This is well below the
intraperitoneal 24-h LD50 for adult rats but similar to the 14-day
value (Lundberg et al., 1986). It cannot be excluded that the high and
rapid lethality of this aspirated dose to adult rats (5/6 deaths in
< 24 h, with 4/6 reported as dying "instantly") may reflect serious
damage to the lungs.
Studies examining the effects of acute inhalation of MEK are not
entirely comparable since they used not only different species but
also different concentrations of MEK, exposure times, and periods over
which survival was measured. For mice the LC50 (45-min exposure) was
about 200 000 mg/m3. The lowest concentration lethal to all rats
exposed by inhalation for 8 h was 47 200 mg/m3 and the lowest
concentration producing lethality in a 4-h exposure was 5900 mg/m3
(2000 ppm). Guinea-pigs survived exposure to 29 500 mg/m3 for 4 to
4.7 h and showed no abnormal signs at 9735 mg/m3. A concentration of
97 350 mg/m3 was lethal to all exposed guinea-pigs in 3.3 to 4.2 h,
whereas a slightly lower concentration, 73 750 mg/m3, although
ultimately lethal to all animals, permitted some guinea-pigs to
survive a 5.4-h exposure (Patty et al., 1935; Specht et al., 1940).
7.1.2 Non-lethal doses
Non-lethal acute doses of MEK produced a number of measurable
changes in experimental animals (Table 11). An oral dose of 1.5 g/kg
to rats resulted in a 63% increase in liver triglycerides after 16 to
23 h, but did not alter liver histology or increase either of two
enzymes, serum glutamic-pyruvic transaminase (alanine transferase
(ALT)) and hepatic glucose-6-phosphatase (Traiger & Bruckner, 1976).
These results suggest that this dose caused metabolic disturbances to
the liver of rats. Much smaller acute intraperitoneal doses (0.049 to
0.194 g/kg) to rats appeared not to damage the liver. A single oral
dose (15 mmol/kg) of MEK did not affect the hepatobiliary function of
rats over an observation period of 10 to 96 h (Hewitt et al., 1986).
A graded series of single doses of MEK to guinea-pigs revealed high
sensitivity to small changes in dosage. The low dose (0.75 g/kg)
appeared to produce no liver damage, whereas 1.5 g/kg produced slight
liver damage and 2.0 g/kg produced major liver damage. These results
also suggest that guinea-pigs and rats may be equally sensitive to MEK
in terms of liver damage even though guinea-pigs survive acute
exposure to higher concentrations of MEK vapour than do rats
(DiVincenzo & Krasavage, 1974).
Consistent increases in the frequency of food-reinforced lever
pressing by rats were detected at MEK exposures as low as 74 mg/m3
(25 ppm) for 2 h (Garcia et al., 1978). Vestibulo-ocular effects were
detected during continuous intravenous administration of MEK for 1 h
via the caudal vein at a dosage as low as 0.005 g/kg per min (Tham et
al., 1984). This dosage is roughly equivalent to inhalation of 2700
mg/m3 (900 ppm). Glowa & Dews (1987) reported that exposure of mice
to 885 mg/m3 (300 ppm) for 30 min did not significantly alter
schedule-controlled responses, whereas concentration-related
suppression of response occurred at consecutive increasing
concentrations (for 30 min) ranging from 2950 to 29 500 mg/m3 (1000
to 10 000 ppm) (see section 7.3.1).
It can be concluded from observations of their behaviour that
respiratory irritation occurred in guinea-pigs exposed to 29 500
mg/m3 or more within 2 min (Patty et al., 1935; Specht et al.,
1940). In survivors, post-exposure recovery from this effect was
rapid. Rats exposed for 8 h/day to 29 500 mg/m3 showed severe
irritation of the upper respiratory tract after a "few days"
(Altenkirch et al., 1979).
7.1.3 Skin and eye irritation
In skin irritation studies, a small dose (8 mg) applied to
clipped skin and covered by an impervious plastic film for 24 h (which
was followed by a 14-day observation period) produced only minor
irritation in male New Zealand albino rabbits (Smyth et al., 1962). A
dose of 400 mg applied to the clipped dorsal skin of restrained albino
rabbits in a gauze patch produced mild to moderate irritation in some
cases (Weil & Scala, 1971). Data from this latter study, however, were
highly variable and may reflect the fact that its purpose was
intercomparison of laboratories rather than the effects of MEK on test
animals. Neat MEK (0.1 ml) applied to the clipped skin of the flanks
of guinea-pigs and rabbits daily for 10 days and left uncovered caused
erythema and oedema after 24-72 h. These effects were more marked in
rabbits (Wahlberg, 1984a).
Table 11. Acute toxicity of MEK for mammalsa
Species Number and sex Exposure concentration Exposure Study Effects Reference
(strain) and range duration duration
Oral studies
Rat (Sprague- both sexes, 6-12 not stated one dose LD50 (7 days) < 0.8 g/kg b.w. Kimura et al.
Dawley) new animals/group 2.5 (2.0-3.1) g/kg b.w. (1971)
born 14 day 6 male/group 2.9 (2.3-3.5) g/kg b.w.
young adult 2.7 (2.1-3.5) g/kg b.w.
older adult
Rat (Carworth- 5 female/group not stated one dose 14 days LD50 (14 days) Smyth et al.
Wistar) 5.52 (4.50-6.80) g/kgb (1962)
Rat (Sprague- 5 male/group 1.505 g/kg b.w. one dose 24 h after 16-23 h, no effect on Traiger &
Dawley) liver histology, serum glutamic Bruckner (1976)
pyruvic transaminase, or
hepatic glucose-6-phosphatase;
63% increase in liver
triglycerides
Rat (Fischer- 32 male/group 0.072-1.082 g/kg b.w. one dose 24 h no evidence of liver Brown & Hewitt
344) dysfunction; slight damage at (1984)
highest dose to kidney proximal
tubules
Mouse (CF1) 10 male/group in 2.0-5.1 g/kg b.w. one dose 24 h LD50 (24 h) Zakhari et al.
each of 6 groups 3.14 ± 0.67 g/kg b.w. (1977)
Inhalation studies
Mouse (Swiss 10 male/group four levels over the 4 h decreased duration of De Ceaurriz et
OF1) range 4726-7192 mg/m3 immobility in escape - al. (1983)
(1602-2438 ppm) directed swimming test
Table 11 (contd)
Species Number and sex Exposure concentration Exposure Study Effects Reference
(strain) and range duration duration
Mouse (CD1) 12 males per five concentrations over continuous 2´ h increasing dose-related Glowa & Dews
concentration the range 885-29 500 (30 min per behavioural effects starting (1987)
mg/m3 (300-10 000 ppm) concentration) at 2950 mg/m3 (1000 ppm)
Mouse (CF1) 6 groups of 147 500-294 000 mg/m3 45 min LC50 (45 min) 205 000 ± 32 500 Zakhari et al.
10 males (50 000-100 000 ppm) mg/m3 (69 500 ± 11 000 ppm) (1977)
Mouse (white) both sexes, 6 303 850 mg/m3 until death average survival 43 min La Belle &
animals/group (103 000 ppm) Brieger (1955)
Rat (Sprague- 6 animals, sex 74-2360 mg/m3 2-6 h 2-6 h behavioural change (increased Garcia et al.
Dawley) unspecified (25-800 ppm) frequency of lever pressing) (1978)
which persisted for < 2 to
> 11 days
Rat (albino) 8 males/group 23 158-59 590 mg/m3 4 h 14 days LC50 (4 h) 34 500 mg/m3 La Belle &
(7850-20 200 ppm) (11 700 ppm) Brieger (1955)
Rat (Wistar) 5 males/group 23 600 mg/m3 8 h 14 days lethal to 3/6 within 14 days Smyth et al.
(8000 ppm) (1962)
Rat (Wistar) data not 47 200 mg/m3 8 h 14 days lethal to 6/6 within 14 days Smyth et al.
supplied (16 000 ppm) (1962)
Guinea-pig 6 unspecified approx. 9735 mg/m3 90-810 min 4-8 days no abnormal signs Patty et al.
sex/group; (3300 ppm) (1935)
3 groups
Guinea-pig 6 unspecified approx. 29 500 mg/m3 90-810 min 4-8 days 90 min: no injury; 280 min: Patty et al.
sex/group; (10 000 ppm) unconsciousness and slight (1935)
3 groups injury, no deaths; 810 min:
unconsciousness and moderate
injury, no deaths
Table 11 (contd)
Species Number and sex Exposure concentration Exposure Study Effects Reference
(strain) and range duration duration
Guinea-pig 10 females 73 750 mg/m3 325 min < 2 days all animals died during or Specht et al.
(25 000 ppm) post exposure (1940)
Guinea-pig 6 unspecified approx. 97 350 mg/m3 30-250 min 4-8 days 30 min: slight injury; 90 min: Patty et al.
sex/group; (33 000 ppm) unconsciousness and moderate (1935)
3 groups injury, no deaths; lethal to
all animals in 200-250 min
Guinea-pig 6 unspecified approx. 303 850 mg/m3 10-55 min 4-8 days 10 min: slight injury; 30 min: Patty et al.
sex/group; (103 000 ppm) unconsciousness and moderate (1935)
3 groups injury, no deaths, lethal to
all animals in 45-55 min
Intravenous study
Rat (Sprague- 18 females several infusion rates 60 min 60 min threshold limit for vestibulo- Tham et al.
Dawley) including 5 mg/kg b.w. ocular disturbance (depression (1984)
per min of nystagmus following
accelerated rotation) 5 mg/kg
b.w. per min; MEK concentration
in blood, 101 mg/litre
Pulmonary aspiration study
Rat (Sprague- 3 males and 800 mg/kg b.w. one dose survivors lethal 5/6 in < 24 h; 4/6 died Panson & Winek
Dawley) 3 females sacrificed instantly; produced pulmonary (1980)
at approx. haemorrhage
24 h
Intraperitoneal studies
Mouse (CF1) 10 male/groups; 0.5-2.0 g/kg b.w. one dose 24 h LD50 (24 h) Zakhari et al.
5 groups 1.66 ± 0.74 g/kg b.w. (1977)
Table 11 (contd)
Species Number and sex Exposure concentration Exposure Study Effects Reference
(strain) and range duration duration
Rat (Sprague- 6 female/group; not stated one dose 24 h LD50 (24 h) 1.554 Lundberg et
Dawley) number of groups (1.229-1.966) g/kg b.w. al. (1986)
unstated
Rat (Sprague- 6 female/group; not stated one dose 14 days LD50 (14 day) 0.607 Lundberg et
Dawley) number of groups (0.486-0.748) g/kg b.w. al. (1986)
unstated
Rat (Sprague- 6 female/group; 0.049-0.194 g/kg b.w. one dose 18 h no significant elevation of Lundberg et
Dawley) 3 groups serum sorbital dehydrogenase al. (1986)
Guinea-pig 4 male/group 0.75-2.0 g/kg one dose 24 h 0.75 g/kg b.w.: no elevation DiVincenzo &
in serum OCTc level, no Krasavage
obvious lipid deposition in (1974)
hepatocytes, no deaths;
1.5 g/kg b.w.: elevated serum
OCT level, lipid deposition in
hepatocytes, no deaths;
2.0 g/kg b.w.: elevation
in serum OCT level, lipid
deposition in hepatocytes,
lethal to 1/4 animals
Dermal studies
Rabbit (Albino, 4 males amounts not stated; 24 h 14 days LD50 (14 days) > 8 g/kg Smyth et al.
New Zealand) kept in contact with (1962)
clipped flank skin
under plastic film
Rabbit (Albino, 5 males 8 mg on clipped one dose 24 h minor irritation Smyth et al.
New Zealand) uncovered skin (1962)
Table 11 (contd)
Species Number and sex Exposure concentration Exposure Study Effects Reference
(strain) and range duration duration
Rat (Albino) 8 males 0.4 mg on 2.5 cm2 24 h 72 h variable results: no irritation Weil & Scala
lightly covered pad to moderate irritation 1 day (1971)
on clipped back skin and 3 days after applicationd
Rabbit (Albino) 6 animals intact 0.5 ml to clipped 24 h 72 h slight erythema subsiding by Hazleton
skin, 6 animals abdominal skin with 72 h (1963a)
abraded skin, semi-occlusive
sex unspecified cover
Ocular studies
Rabbit (Albino) 6 of unspecified 0.1 ml to one eye of 30 sec 14 days severe irritation, including Hazleton
sex each rabbit corneal damage and scleral (1963b)
haemorrhage in 2/6 rabbits
Rabbit (Albino) 5 male per not stated 3 min solution of 130 g/litre water Larson et al.
concentration produced significant oedema in (1955)
eyelid in 1 h
Rabbit (Albino, not specified 4 mg/eyee 1 min 18-24 h severe chemical burn Smyth et al.
New Zealand) (1962)
Rabbit (Albino) 6 males 8 mg to one eye of 20 sec 7 days variable results; no irritation Weil & Scala
each rabbit to moderate irritation 1 day, 3 (1971)
days and 7 days after
applicationd
Rabbit (Albino, 6 of unspecified 8 mg to one eye of described 7-10 days slight swelling, redness and MB Research
New Zealand) sex each rabbit as brief discharge from eye for 4 to Lab. Inc.
10 days (1979)
Table 11 (contd)
a Values from the literature were recalculated as necessary to yield dosages in terms of ppm, g or g/kg
b 95% confidence limits
c OCT = ornithine carbamyl transferase
d Purpose of study was intercomparison of testing laboratories that evaluated MEK and other substances by purportedly identical methods
e Not clear whether one eye or both eyes were dosed
The results of studies on eye irritation in rabbits are
inconsistent. Smyth et al. (1962) reported that 0.005 ml (4 mg)
created a severe chemical burn in the rabbit eye, whereas a study by
MB Research Lab. Inc. (1979) reported less severe irritant effects
from the larger dose of 0.1 ml (80 mg). Data from a study by Weil &
Scala (1971) were also inconsistent but indicated that a dose of 0.1
ml (80 mg) produced minimal to moderate eye irritation. Undiluted MEK
was used in all three studies but Smyth et al. (1962) used the grading
system for eye injury described in Carpenter & Smyth (1946), whereas
MB Research Lab., Inc. (1979) and Weil & Scala (1971) used the Draize
scoring system (Draize et al., 1944). In all studies irritation
disappeared or was markedly reduced by 7 days after treatment. Opaque
corneas were apparent following exposure of guinea-pigs to 295 000 mg
MEK/m3 (100 000 ppm) for 30 min; recovery from this effect was
complete in 4-8 days (Patty et al., 1935).
7.2 Repeated exposures
Data on repeated exposure of mammals to MEK are summarized in
Table 12. None of the concentrations tested, not even the highest (17
700 mg/m3 (6000 ppm) 8 h/day for up to 7 weeks) was clearly lethal
or even significantly harmful. The death of experimental animals
(rats) at this highest dose was not associated with neurological signs
and appeared to result exclusively from bronchopneumonia (Altenkirch
et al., 1978, 1979). These authors did not comment on possible
connections between bronchopneumonia and exposure to 17 700 mg/m3.
Female rats exposed to 14 750 mg/m3 (5000 ppm) 6 h/day, 5 days per
week, for 90 days showed only slightly increased liver weight,
slightly decreased brain and spleen weights, and slightly altered
blood chemistry in comparison with controls. Male rats receiving this
exposure showed only a slightly increased liver weight. At lower
concentrations of MEK (3688 and 7375 mg/m3 (1250 and 2500 ppm))
there was only slightly increased liver weight for female rats and no
significant differences for males in comparison with controls
(Cavender et al., 1983). In another subchronic inhalation study
(Toxigenics, 1981), male and female rats were exposed to MEK
concentrations of 3700, 7430 and 14 870 mg/m3 (1254, 2518 and 5041
ppm) 6 h/day, 5 days/week, for 90 days. No significant effects on food
consumption, eyes or nervous system were observed. In addition, no
MEK-induced morphological changes in the central or peripheral nervous
system were detected. Lower levels of exposure resulted in few
measurable effects. Inhalation of 2242 and 2360 mg/m3 (760 and 800
ppm) 6 h/day, 5 days/week, for 4 weeks by rats caused some enlargement
of the liver and slightly modified the in vitro metabolism of liver
microsomes (Nilsen & Toftgard, 1980; Toftgard et al., 1981). Ten
intraperitoneal injections of 0.034 g/kg over 2 weeks produced no
effect on the kidney (Bernard et al., 1989). However, exposure of rats
to 590 mg/m3 (200 ppm) 12 h/day for 24 weeks transiently decreased
nerve conduction velocity after 4 weeks (Takeuchi et al., 1983).
Geller et al. (1978) reported that exposure of baboons to 295 mg/m3
(100 ppm) for 7 days increased the response time in a delayed "match
to sample" task. However, this effect was transient and disappeared
during the course of repeated exposure. Following intermittent
exposure of rats to 3319 g/m3 for up to 5 months there was no
morphological evidence of peripheral neurotoxicity (Saida et al.,
1976). It is possible that the transient nature of the neurological
and behavioural changes induced by MEK exposure may be due to
behavioural and/or physiological adaptations. The latter may reflect
more rapid metabolism of MEK with prolonged exposure.
Short-term dermal exposure to small amounts of MEK resulted, at
most, in mild local irritation. Two topical applications of an
unstated amount to the ears of various strains of mice (Swiss, Balb/c,
CBA, C5681/6, DBA/2, B6D2F1) produced no significant swelling or other
signs (Descotes, 1988). In rabbits and guinea-pigs, a dose of 0.08 g
applied to the skin, without covering, once a day for 10 days to the
same site resulted in a slight to moderate increase in skinfold
thickness, whereas a much smaller dose (8 mg) applied to the same site
in rabbits 3 times a day for 3 days resulted in barely detectable
irritation (Wahlberg, 1984a). Open application of MEK to the shaved
flanks of guinea-pigs for 3 days produced slight erythema, epidermal
thickening and dermal cell infiltration (Anderson et al., 1986). In
the cat, injection of 150 mg MEK (99.98% pure) per kg, twice a day, 5
days/week, for up to 8.5 months, into subcutaneous tissue produced
abscesses, skin ulceration and generalized weakness but no evidence of
damage to the nervous system (Spencer & Schaumburg, 1976). In the one
long-term dermal study of MEK (Horton et al., 1965), 8 mg dissolved in
water was applied by dropper or brush to clipped skin of mice twice a
week for a year. Few details were given of the results because this
was the control for a study on carcinogenesis of the skin in which a
MEK/water solution was the solvent for compounds under test. Horton et
al. (1965) stated that the control mice did not develop skin tumours,
and they did not mention any adverse effects from this prolonged
application of MEK.
Table 12. Repeated exposure of mammals to MEKa
Route Species (strain), Exposure and range Results Reference
number and sex
Inhalation rat (Wistar), 590 ± 118b mg/m3 (200 ± 40b transient differences in nerve conduction claimed Takeuchi et al.
8 males ppm) 12 h/day for 24 weeks after 4 weeks, but no significant differences (1983)
Inhalation rat (Albino), 693 ± 77b mg/m3 (235 ± 26b no significant gross or microscopic La Belle &
25, sex ppm) 7 h/day, 5 days/week pathological changesc Brieger (1955)
unspecified for 12 weeks
Inhalation rat (Wistar), 885 mg/m3 (range 867-894) no significant change in leucocyte or serum Li et al. (1986)
7 females (300 ppm (range 294-303)) alkaline phosphatase activity
8 h/day for 7 days
Inhalation rat (Sprague- 3322 and 7723 mg/m3 (1026 at 7723 mg/m3 minor effects on dams, decreased Schwetz et al.
Dawley), and 2618 ppm) 7 h/day food consumption and weight gain, and increased (1974)
pregnant, 25 per for 10 days (gestation water consumption; no effects on dams at 1180 mg/m3
concentration days 6-15)
Inhalation rat (Sprague- 2242 and 2360 mg/m3 (760 and significant enlargement of liver; no effect on Nilsen &
Dawley), 4 800 ppm), 6 h/day, 5 days total liver microsomal concentration of cytochrome Toftgard (1980);
male/concentration per week for 4 weeks P-450; in vitro liver microsomal metabolism of Toftgard et al.
biphenyl unaffected, but slight effects on the (1981)
metabolism of benzo[a]pyrene, 4-androstene-3,17-
dione, and 5alpha-androstane-3alpha,17ß-diol
Inhalation rat (Fischer- 3688, 7375, 14 750 mg/m3 females at 14 750 mg/m3 increased in liver weight, Cavender et al.
344), 15 males, (1250, 2500, 5000 ppm) smaller braind and spleene weight, slightly altered (1983)
15 females/ 6 h/day, 5 days/week for blood chemistryf; females at 3688, 7375 mg/m3
concentration 90 days nonsignificant increase in liver weight; males at
14 750 mg/m3 increased in liver weight. No pathological
lesions, including peripheral nerves, attributed to
MEK exposure. No effect on reproductive tissues
(testis, epididymis, seminal vesicle, vagina, cervix,
uterus, oviduct, ovary)
Table 12 (contd)
Route Species (strain), Exposure and range Results Reference
number and sex
Inhalation rat (Fischer- 3699, 7430, 14 870 mg/m3 elevated group mean body weights at 3699 and 7430 Toxigenics
344), 15 males, (1254, 2518, 5041 ppm) mg/m3; highest concentration: decreased group mean (1981)
15 females/ 6 h/day, 5 days/week for body weight, increased liver weight, liver/body
concentration 90 days weight ratio and liver/brain weight ratio, increased
mean corpuscular haemoglobin; highest concentration
(males): increased kidney/body weight ratio, decreased
spleen and brain weights; highest concentration
(females): decreased brain/body weight ratio, increased
kidney/brain weight ratio; no significant effects at
any concentration on food consumption, eyes, nervous
system, and no morphological changes in central or
peripheral nervous systems
Inhalation rat (Sprague- 3319 mg/m3 (1125 ppm) 24 h no morphological effects on peripheral nerves Saida et al.
Dawley), 36 of per day for 16 days to 5 (1976)
unspecified sex months
Inhalation rat (Wistar), 29 500 mg/m3 (10 000 ppm) for severe irritation of upper respiratory tract; slight Altenkirch et
5 males a "few days"; 17 700 mg/m3 loss of weight; death during seventh week from al. (1978,
(6000 ppm) ± 15% for 8 h/day, bronchopneumonia; no neurological signs 1979)
7 days/week for 15 weeks
Inhalation guinea-pig, 693 ± 77b mg/m3 (235 ± 26b no significant effects La Belle &
15 of unspecified ppm) 7 h/day, 5 days/week for Brieger (1955)
sex 12 weeks
Inhalation baboon, 295 mg/m3 (100 ppm) for 7 days no impairment of discrimination in a behavioural Geller et al.
4 males test, slight increase in response time early in (1978)
experiment, but not at end of experiment
Table 12 (contd)
Route Species (strain), Exposure and range Results Reference
number and sex
Intra- rat (Sprague- 0.034 g/kg, 5 days/week for no effect on kidney function Bernard et al.
peritoneal Dawley), 2 weeks (1989)
female, number
unspecified
Subcutaneous cat, 6 of 0.15 g/kg twice/day, 5 days abscesses, skin ulceration and generalized weakness Spencer &
unspecified sex per week for up to 8.5 months in some animals; no damage to nervous Schaumburg
system structure or function (1976)
Dermal mouse (Swiss), unstated amount applied twice no significant swelling of treated ear Descotes (1988)
12 of unspecified to one ear
sex
Dermal mouse 8 mg (50 mg of 17% solution) no papilloma evident after 1 year Horton et al.
(C3H/He) applied to clipped skin twice (1965)
10-25 males per week for 1 year
Dermal guinea-pig, 3 80 mg rubbed into skin at slight to moderate increase in skinfold thickness Wahlberg (1984a)
and rabbit, 4, same site once daily for at end of 10 days
of unspecified 10 days
sex
Dermal guinea-pig 80 mg applied to 1.0 cm2 on no reaction until day 2; at end of experiment, Anderson et al.
10 females shaved flank 3 times daily slight redness and increase in dermal inflammatory (1986)
for 3 days cell count
a Values from the literature recalculated as ppm, mg or g/kg
b Standard deviation of the mean
c Depressed growth (average weight 70% of controls at end of experiment) may be due to causes other than MEK exposure; animals showed signs
of "vitamin deficiency" (no details given) and infection in latter part of experiment.
d Significantly different at the < 0.01 level from 0, 3688 and 7375 mg/m3 (0, 1250, and 2500 ppm) female group values
e Significantly different at the < 0.05 level from 0, 3688 and 7375 mg/m3 (0, 1250, and 2500 ppm) female group values
f Females in the 14 750 mg/m3 (5000 ppm) group had a small but significant elevation in serum potassium, alkaline phosphatase and glucose
levels, and a significant reduction in serum glutamic-pyruvic transaminase level.
7.3 Neurotoxicity
7.3.1 Behavioural testing
Neurotoxicity studies have been carried out on MEK, usually as
part of studies on the neurotoxicity of methyl isobutyl ketone (MIBK)
and MIBK/MEK mixtures.
An increase in response rate (lever pressing) was reported in a
group of six adult Sprague-Dawley rats (sex unspecified) exposed to
MEK at various concentrations between 74 and 2360 mg/m3 (25 and 800
ppm) for 2 h at approximately weekly intervals. An increase in
response rate also occurred in a group of four rats exposed to 74
mg/m3 (25 ppm) for 6 h at 2-day intervals (Garcia et al., 1978).
Geller et al. (1978) examined behavioural effects (match to
sample (MTS) test) in baboons exposed to MEK by inhalation. Four young
male baboons (2 years old) were exposed continuously to MEK at a
concentration of 295 mg/m3 (100 ppm) for 7 days. There were no
effects on performance of the test in terms of the ability to
discriminate visual stimuli but reaction time increased. However, in
two of the baboons, response times returned to pre-exposure control
values by day 7.
Tham et al. (1984) examined the vestibulo-oculomotor reflex (VOR)
during intravenous infusion of MEK into the caudal veins of 18 female
Sprague-Dawley rats. The threshold for depression of the VOR was an
infusion rate of 70 µmol/kg (0.005 g/kg per min) for 1 h (total dose
30 mg) and the associated arterial blood level was 1.4 mmol/litre.
General depression of the central nervous system followed depression
of the VOR.
Glowa & Dews (1987) exposed continuously by inhalation a group of
12 adult male white mice (Charles River CD1) to concentrations of MEK
that were increased at 30 min intervals until the mice failed to
respond to a visual stimulus. The concentrations, in ascending order
for each 30 min, were 885, 2950, 8850, 16 520 and 29 500 mg/m3 (300,
1000, 3000, 5600 and 10 000 ppm) with a total exposure time of 2´ h.
Mice responded to a visual stimulus and the response rate was used as
an indicator. There was no effect at a concentration of 885 mg/m3,
a slight decrease in response rate at 2950 mg/m3 and a 75% decrease
at 8850 mg/m3. Most mice ceased to respond at 16 520 mg/m3 and all
failed to respond at 29 500 mg/m3. The response rate returned to the
control value 30 min after exposure ended. The EC50 (concentration
decreasing response rate by 50%) was calculated to be 8528 (SD = 2033)
mg/m3 (2891 (SD = 689) ppm). An EC10 was calculated and
dose-response estimates were derived. The concentrations of MEK
producing a 10% decrease in response rate in 0.1%, 1% and 19% of a
population were 50, 195 and 885 mg/m3 (17, 66 and 300 ppm),
respectively.
Couri et al. (1977) exposed continuously by inhalation young male
Wistar rats to 2210 mg MEK/m3 (750 ppm) for either 7 or 28 days. In
those exposed for 7 days there was a significant reduction in
hexobarbital sleep times. In the group exposed for 28 days the
reduction in sleep times was less marked.
7.3.2 Histopathology
In chickens, cats, rats and mice exposed by inhalation to 3975
mg/m3 (1500 ppm) for periods of up to 12 weeks, there was no
evidence of neuropathy and no histopathological changes were reported
(Couri et al., 1974).
Saida et al. (1976) exposed groups of 36 Sprague-Dawley rats (sex
unspecified) continuously to MEK at a concentration of 3319 mg/m3
(1125 ppm) for periods of 16, 25, 35 and 55 days. Additional studies
were carried out with up to 5 months of exposure. There were no
abnormal clinical observations in any group. At the end of the
exposure period, rats were sacrificed and the sciatic nerve and foot
muscle excised. Spinal cord and dorsal root ganglion specimens were
taken from the same rats. Quantitative histology
(neurofilaments/µm2; frequency of inpouching of myelin sheath,
denuded axons/mm2) showed no abnormality in rats exposed for up to
5 months.
Cavender et al. (1983) reported no neurological abnormalities in
Fischer-344 rats in a 90-day inhalation study on MEK alone. Groups of
15 male and 15 female rats were exposed 6 h/day, 5 days/week, for 90
days to MEK concentrations of 3688, 7375 and 14 750 mg/m3 (1150,
2500 and 5000 ppm). All rats were observed twice daily for clinical
signs. At the end of the exposure period, the eyes of each animal were
examined by ophthalmoscopy, and neurological function (posture, gait,
tone and symmetry of facial muscles, and pupillary, palpebral,
extensor-thrust and cross-extensor thrust reflexes) was evaluated. No
abnormalities were found. Necropsy, including histopathology of the
sciatic and tibial nerves, was carried out on all rats, with special
neuropathological studies on the medulla, sciatic and tibial nerves in
5 male and 5 female rats from each group. There were no changes
attributable to MEK.
7.4 Developmental toxicity
Schwetz et al. (1974) exposed 44 pregnant rats from days 6-15 of
gestation (sperm = day 0) to two concentrations of MEK vapour, i.e.
nominally 2950 mg/m3 in 23 rats and 8850 mg/m3 in 21 rats (1000
and 3000 ppm), for 7 h/day; 43 rats were air-exposed as controls. The
average values for measured concentrations in this study were 3322 and
7723 mg/m3 (1126 and 2618 ppm), respectively. There was no evidence
of maternal toxicity. Fetal weight and crown-rump length were
significantly decreased at 3322 mg/m3, but not at 7723 mg/m3. At
3322 mg/m3 there was also a significant increase, compared to the
controls, in the number of litters with fetuses showing skeletal
anomalies, and at 7723 mg/m3 a significantly increased incidence of
litters with sternebral and soft tissue anomalies was reported. Four
grossly malformed fetuses were found (two with brachygnathia and two
acaudate with imperforate anus), all in different litters in the 7723
mg/m3 group. These malformations had not been observed previously in
more than 400 control litters of this strain. Fetal body dimensions,
however, were not significantly different from controls. When the data
were analysed on a per litter basis, there was evidence of a
teratogenic effect.
However, the incidence of major malformations in these studies
was sufficiently low that evidence for teratogenic effects was
considered questionable, and the studies were repeated (John et al.,
1980; Deacon et al., 1981). The methodology was identical except for
the inclusion of an additional level of exposure, nominally 1180
mg/m3 (400 ppm). Average measured MEK concentrations during the
experiment were 1215, 2956 and 8865 mg/m3 (412, 1002 and 3005 ppm).
The only evidence for maternal effects was decreased weight gain and
increased water consumption (no figures given) by dams exposed to 8865
mg/m3. None of the dosages had significant effects on the incidence
of pregnancy, incidence of resorption, average number of implantations
and live fetuses per dam, fetal weight and length, or incidence of
external or soft tissue abnormalities. There were statistically
significant differences in the incidences of some skeletal anomalies
occurring in the 8875 mg/m3 group compared to the controls. There
were increased incidences of lumbar ribs and delayed ossification of
the cervical centra, but a decreased incidence of delayed ossification
of the skull. Since these skeletal abnormalities occurred at low
incidences among the population from which the experimental animals
were drawn, the results of this study were interpreted as indicating
a low level of fetotoxicity and no evidence for embryotoxic or
teratogenic effects for MEK at exposure levels up to 8865 mg/m3.
In a further study (Mast et al., 1989; Schwetz et al., 1991),
groups of 10 virgin female Swiss CD1 mice and 33 plug-positive (day 0)
females were exposed by inhalation on gestation days 6-15 to mean
concentrations of 1174 ± 27, 2980 ± 83 and 8909 ± 233 mg/m3 (398 ±
9, 1010 ± 28 and 3020 ± 79 ppm). There was no evidence of maternal
toxicity, although there was a slight, treatment-related increase in
liver/body weight ratios that was significant at the highest dose
level. Mild fetal toxicity was evident at this maternal dose level as
a reduction in mean fetal body weight, statistically significant for
males. There was no increase in the incidence of intrauterine death,
but there was an increased dose-related incidence of misaligned
sternebrae, statistically significant at the highest dose level. There
were no significant increases in the incidence of malformations,
although there were several malformations in one litter (cleft palate,
fused ribs, missing vertebrae, syndactyly) in treated groups but not
in the control group nor in contemporary control data. On the basis of
this study it was concluded that the no-observed-adverse-effect level
(NOAEL) was 2978 mg/m3 (1010 ppm) and the
lowest-observed-adverse-effect level (LOAEL) was 8096 mg/m3 (3020
ppm).
7.5 Mutagenicity and related end-points
Short-term genotoxicity tests in vitro and in vivo are
summarized in Table 13. Although MEK has given negative results in
most conventional assays, Zimmermann et al. (1985) found that MEK and
certain other polar aprotic solvents were strong inducers of
aneuploidy in the yeast. The induction of aneuploidy by MEK was
markedly potentiated by coexposure to ethyl acetate (Mayer & Goin,
1988) or with nocodazol (methyl [5- (2-thienyl-carbonyl)-1
H-benzimidazol-2-yl]-carbamate) (Mayer & Goin, 1987).
O'Donoghue et al. (1988) conducted mutagenicity studies on MEK.
The test systems comprised the Salmonella/microsome (Ames) assay, the
L5178/TK+/- mouse lymphoma (M/L) assay, the BALB/3T3 cell
transformation (CT) assay, unscheduled DNA synthesis (UDS) and the
micronucleus test. MEK was not found to be genotoxic in these assays.
Other studies of MEK utilizing cultures of mammalian cells as
test systems also yielded little or no evidence of mutagenicity and
related effects. Perocco et al. (1983) tested MEK and other important
industrial chemicals at concentrations of 10-2 to 10-4 mol/litre
(0.72 to 0.0072 g MEK/litre) with an in vitro system utilizing
cultures of human lymphocytes to determine toxicity and ability to
inhibit DNA synthesis. Cultures were grown both with and without S9
mix derived from phenobarbital-induced rat liver. MEK at the
concentrations tested showed no evidence of cytotoxic or genotoxic
action. Chen et al. (1984) examined the effects of MEK on metabolic
cooperation between 6-thioguanine-resistant and
6-thioguanine-sensitive Chinese hamster lung fibroblast V79 cells and
obtained equivocal results. Holmberg & Malmfors (1974) also found some
evidence of MEK cytotoxicity to ascites tumour cells cultured with the
solvent for up to 5 h. Although there was no significant increase in
irreversibly injured cells at MEK concentrations of 0.05 and 0.1
g/litre, there was a moderate increase in damaged cells at 0.1
g/litre. An ultrastructural study (Veronesi, 1984) utilizing a medium
containing relatively high concentrations of MEK (0.3 g/litre)
produced axoplasmic granularities in a few cultures. The relationship
of this effect to possible MEK-induced neurotoxicity in vivo is not
clear.
7.6 Carcinogenicity
No long-term carcinogenicity studies have been reported.
Table 13. Short-term genotoxicity tests on MEK
Method Concentration Experimental conditions; comments Results References
In vitro studies
Bacterial assays 3 µmol/plate Salmonella typhimurium TA98, TA100, TA1535, - Florin et al. (1980)
TA1537 with and without S-9
10 mg/plate S. typhimurium TA98, TA100, TA1535, - Nestmann et al. (1980)
TA1537 with and without S-9
approx. S. typhimurium TA104: maximum non-toxic - Marnett et al. (1985)
1 mg/plate dose > 3 µmol
0.05-32 µl/plate S. typhimurium TA98, TA100, TA1535, - O'Donoghue et al. (1988)
TA1537, TA1538 with and without S-9
4 mg/plate Escherichia coli WP2 and WP2 uvrA - Brooks et al. (1988)
Mitotic gene 5 mg/ml Saccharomyces cerevisiae (JD1) - Brooks et al. (1988)
conversion assay
Induction of mitotic 3.54% S. cerevisiae (D61.M) + Zimmermann et al. (1985)
aneuploidy
0.50-1.96% S. cerevisiae (D61.M) + Mayer & Goin (1987)
Chromosome assay 1 mg/ml rat liver RL4 cells - Brooks et al. (1988)
Cell transformation 9-18 µl/ml BALB/3T3 - O'Donoghue et al. (1988)
UDS test 0.1-5.0 µl/ml primary rat (Sprague-Dawley) hepatocyte - O'Donoghue et al. (1988)
Table 13 (contd)
Method Concentration Experimental conditions; comments Results References
In vivo studies
Micronucleus test 1.9 ml/kg CD1 mice (male and female), - O'Donoghue et al. (1988)
intraperitoneal time: 12, 24, 48 h
411 mg/kg Chinese hamster, time: 12, 24, 48, 72 h - Basler (1986)
intraperitoneal
8. EFFECTS ON HUMANS
8.1 General population exposure
The only record of non-occupational acute toxicity from MEK was
a case of accidental self-poisoning (Kopelman & Kalfayan, 1983). A
47-year old woman inadvertently ingested an unknown amount of MEK and
was found unconscious, hyperventilating, and suffering from severe
metabolic acidosis. Her plasma concentration of MEK was 950 mg/litre.
She responded promptly to an infusion of sodium hydrogen carbonate and
was discharged from the hospital after a week. The metabolic effects
of MEK ingestion by humans are not well characterized and it is
uncertain that the acidosis was produced by MEK.
8.2 Effects of short-term exposure
There are limited data on behavioural and other effects on humans
of short-term exposure to MEK. Nakaaki (1974) found that exposure to
266-797 mg/m3 (90-270 ppm) for up to 4 h per day caused his subjects
to underestimate times of 5 to 30 seconds. Dick et al. (1984, 1988,
1989), on the other hand, found that a 4-h exposure of human subjects
to 590 mg/m3 (200 ppm) had no significant effect in a variety of
behavioural tests. These included psychomotor, visual vigilance, dual
task, sensorimotor and psychological tests. Solvent mixtures of 295 mg
MEK/m3 (100 ppm) and 186 mg toluene/m3 (50 ppm), and of 295 mg
MEK/m3 (100 ppm) and 298 mg acetone/m3 (125 ppm) similarly had no
significant effect on the results of these behavioural tests.
8.3 Skin irritation and sensitization
MEK (0.1 ml) rubbed into volar forearm skin daily for 18 days and
left uncovered did not produce persistent erythema or swelling
(Wahlberg, 1984a). A 5-min contact with 1.5 ml of analytical grade MEK
confined to a 20-mm circle on the forearm produced a temporary
whitening of the skin, but no visible erythema, alteration in
cutaneous blood flow or other indication of irritation to the skin
(Wahlberg, 1984b).
A male painter developed dermatitis 18 months after commencing
spray painting using an epoxy-polyamide paint (Varigos & Nurse, 1986).
A patch test with "a small amount" of MEK applied to areas of skin
3 cm in diameter on each forearm caused these areas of skin to turn
bright red within 10 min. The spots were itchy, but there was no
induration or oedema. The reaction reached its maximum after 15 min
and then gradually faded. The test was repeated after 2 days, and gave
the same results. Application of the same grade of MEK to normal
forearm skin of five male volunteers produced no reaction.
8.4 Occupational exposure
8.4.1 MEK alone
There is no record that MEK toxicity has ever caused death or a
large scale industrial accident, and only one acute occupational
poisoning has been ascribed to MEK. An 18-year-old seaman with good
vision and no previous eye problems was exposed to MEK vapour of
unknown concentration while stripping paint, and promptly noted
headache, mild vertigo and blurred vision (Berg, 1971). The diagnosis
was retrobulbar neuritis. He was given vitamin B complex and steroid
therapy, and his vision returned to normal in 36 h. However, blood
analysis exhibited a positive methanol content (no value reported)
according to the criteria given by Kaye (1961). Thus a potentiation of
the effects due to combined exposure to MEK and methanol cannot be
excluded.
Data for occupational poisoning ascribed to chronic exposure to
MEK in the absence of other solvents are equally limited. Long-term
exposure of 51 Italian workers to MEK produced indications of
neurotoxicity with slightly, but not significantly, reduced nerve
conduction velocities and various other symptoms such as headache,
loss of appetite and weight, gastrointestinal upset, dizziness,
dermatitis and muscular hypotrophy, but no clinically recognizable
neuropathy (Freddi et al., 1982). There has been a brief report of
chronic exposure of American workers, in a factory producing coated
fabric, to 885-1770 mg MEK/m3 (300-600 ppm) in the apparent absence
of other solvents (Smith & Mayers, 1944). Workers complained of
dermatoses and numbness of fingers and arms.
8.4.2 MEK in solvent mixtures
It was reported that MEK was commonly present as part of solvent
mixtures containing hexane, and that the TLV for hexane (148 mg/m3,
50 ppm) was exceeded in 89 of the work stations (mainly in shoe
factories) (De Rosa et al., 1985). MEK potentiates the toxicity of
hexane and these authors concluded that in these shoe factories the
risk of neurotoxicity was extremely high. Observations by Tangredi et
al. (1981), Brugnone et al. (1981) and Cresci et al. (1985) supported
the widespread nature of this health problem in Italian industry,
especially shoe factories. Arques Espi & Quintanilla Almagro (1981)
also found that mixtures of solvent vapours including MEK and hexane
posed an excessive risk in 95 of 114 work stations examined in a study
of Spanish shoe factories. In a nationwide survey of Japanese
factories, Inoue et al. (1983) found that MEK is widely used as a
component of solvent mixtures. In a study of electronic parts plants
in the USA (Lee & Parkinson, 1982), workers were found to be exposed
to mixtures of solvent vapours containing MEK and as many as nine
other components, although not hexane or other solvents whose toxic
action MEK is known to potentiate. Observations on Finnish car
painters (Husman, 1980; Husman & Karli, 1980) suggested that long-term
exposure to complex solvent mixtures whose components individually and
jointly are far below the legal concentration limits may produce
significant adverse effects. Noma et al. (1988) similarly suggested
that complex mixtures of volatile organic compounds, rather than a
high concentration of any single compound, may be responsible for
unhealthy air in buildings.
Descriptions of the effects of occupational exposures to mixtures
of solvent vapours not necessarily potentiated by MEK are summarized
in Table 14. There are only two cases of acute occupationally related
toxicity from such mixtures, and only a limited number of adequately
documented cases of adverse effects from chronic occupational exposure
which did not involve potentiation of hexacarbon toxicity. The only
acute cases involved two young women waterproofing seams of raincoats
with resins dissolved in acetone or MEK (Smith & Mayers, 1944).
Post-exposure measurements revealed workplace concentrations of
785-1178 mg/m3 (330-495 ppm) for acetone and 1174-1655 mg/m3
(398-561 ppm) for MEK. The total solvent concentration was estimated
to be 1000 ppm. Both women fainted at work and subsequently displayed
a number of temporary neurological and other symptoms.
However, the majority of these studies are difficult to interpret
because they lack either a quantitative description of the solvent
vapours in the work environment, or the description is based on
post-exposure analyses that may not be typical of working conditions
during most of the exposure. In these studies it is also impossible to
make any certain assessment of the role(s) played by individual
components. Mixed solvent exposures have been associated with
alteration in nerve conduction velocity (Viader et al., 1975; Dyro,
1978; Triebig et al., 1983), memory and motor alterations (Binaschi et
al., 1976), and dermatoses and vomiting (Lee & Murphy, 1982). Fagius
& Grönqvist (1978) reported neurological effects in 3 out of 42
workers exposed in a steelworks to organic solvents. However, the
actual role of MEK in these effects is not clear.
Table 14. Effects of occupational exposure to mixtures of solvent fumes containing MEK
Concentrations of MEK and other No. of workers Exposure Effects Defects in study Reference
components exposed duration
Acute
MEK 1174-1655 mg/m3 (389-561 ppm), 2 few hours eyes watering, gastric measurements of work Smith &
acetone 785-1178 mg/m3 (330-495 ppm) distress, fainting, environment limited Mayers
convulsions, twitching, to post exposure (1944)
headache, spinal pressure
Chronic
MEK 561-885 mg/m3 (190-300 ppm), 13 1 year or fatigue, frequent headache, measurements of work Lee &
toluene 139-177 mg/m3 (37-60 ppm), more dizziness, incoordination, environment limited Murphy
MIBK 37-41 mg/m3 (9-14 ppm) skin rashes, vomiting to post exposure (1982)
MEK 30 mg/m3 (10 ppm), toluene 2 2 and 6.5 paraesthesis in extremities, measurements of work Dyro
94-488 mg/m3 (25-130 ppm) years reduced motor nerve conduction environment limited (1978)
velocity, weakness in to post exposure
individuals exposed for long
periods, improvement following
cessation of exposure
MEK 62-51 mg/m3 (21-180 ppm), acetone 1 1 year altered consciousness and measurements of work Dyro
86-595 mg/m3 (36-250 ppm) EEG, reduced motor nerve environment limited (1978)
conduction velocity to post exposure
MEK 142 mg/m3 (48 ppm), isobutanol 8 not stated headache, dizziness, fatigue, measurements of work Binaschi
67 mg/m3 (23 ppm), MIBK 16 mg/m3 (4 ppm), depression, significant environment limited et al.
toluene 180 mg/m3 (48 ppm), butyl acetate reduction in short-term to post exposure; (1976)
43 mg/m3 (9 ppm), xylene 347 mg/m3 memory and hand steadiness period of exposure
(80 ppm) not indicated
Table 14 (contd)
Concentrations of MEK and other No. of workers Exposure Effects Defects in study Reference
components exposed duration
MEK 115 (avg), 2655 (max) mg/m3, n-butanol 9 8-35 significant changes in measurements of work Denkhaus
67 (avg), 1200 (max) mg/m3, isobutanol years several sub-populations of environment limited et al.
172 (avg), 3000 (max) mg/m3; 2-butoxy- peripheral blood lymphocytes: to post exposure (1986)
ethanol 25 (avg), 350 (max) mg/m3, decrease in certain type of
2-ethoxyethanol 5 (avg), 53 (max) mg/m3, T-cells and helper cells,
2-methoxyethanol 6 (avg), 150 (max) mg/m3, increase in natural killer
toluene 86 (avg), 750 (max) mg/m3, cells and B-lymphocytes
m-xylene 19 (avg), 220 (max) mg/m3,
MBK 2 (avg), 27 (max) mg/m3
MEK 9-124 mg/m3 (3-42 ppm), 5 > 10 years no significant differences measurements of work Lundberg
xylene 0-6111 mg/m3 (0-1408 ppm), in serum activities of liver environment limited &
toluene 0-1260 mg/m3 (0-336 ppm), enzymes between exposed to post exposure Hakansson
isobutanol 0-1045 mg/m3 (0-345 ppm), workers and controls (1985)
n-butanol 0-1548 mg/m3 (0-511 ppm),
ethanol 0-1094 mg/m3 (0-582 ppm),
ethyl acetate 0-2095 mg/m3 (0-582 ppm),
n-butyl acetate 0-1691 mg/m3 (0-356 ppm),
methyl acetate 3-181 mg/m3 (1-60 ppm),
white spirit 2-30 mg/m3 (1-17 ppm),
methylene chloride 10-2460 mg/m3 (3-707 ppm),
isopropanol 5-260 mg/m3 (2-106 ppm),
exposure to 2-8 solvents plus MEK
MEK < 9-401 mg/m3 (< 3-136 ppm), 66 average significant reduction in measurements of work Triebig
xylene < 4-82 mg/m3 (< 1-19 ppm), 5 years sensory conduction in environment limited et al.
toluene 11-551 mg/m3 (3-147 ppm), comparison with controls, no to post exposure (1983)
ethyl acetate < 11-302 mg/m3 (< 3-84 ppm) suggestion of neuropathy,
trichloroethane 11-601 mg/m3 (2-110 ppm) change in conduction velocity
correlated with exposure
Table 14 (contd)
Concentrations of MEK and other No. of workers Exposure Effects Defects in study Reference
components exposed duration
MEK 15-620 mg/m3 (5-210 ppm), acetone 17 not stated no health problems associated measurements of work Cohen &
< 119-495 mg/m3 (< 50-208 ppm), xylene with exposure to MEK environment limited Maier
< 22 mg/m3 (< 5 ppm), toluene < 19-34 to post exposure (1974)
mg/m3 (< 5-9 ppm), petroleum naphtha
(concentration unknown)
MEK mainly < 443, max. 5115 mg/m3 42 0.5-8 1 likely and 2 suspected measurements of work Fagius &
(mainly < 150, max. 1734 ppm), trichloroethylene years cases of polyneuropathy, environment limited Gronqvist
mainly < 161 mg/m3 (< 30 ppm); slight loss of sensitivity to post exposure (1978)
at low levels butanol, butyl acetate, butyl to vibration correlated
diglycol, cyclohexanol, diacetal, ethyl with level of solvent ex-
glycol acetate, ethanol, isoforone, posure during preceding
methylene chloride, MIBK, toluene, 6 months
xylene, "solvesso 100 and 150"a
MEK 0-74 mg/m3 (avg, 3 mg/m3) (0-25 1006b average overall death rate below none Wen et al.
(avg. 1) ppm), toluene 4 mg/m3 (1 ppm); 21.6 years expected level, excess of (1985)
at very low levels benzene, "hexane", deaths from cancer associ-
MIBK, xylene ated with lubricating oil,
not MEK
a hydrocarbon solvent mixtures
b workers from lube oil and dewaxing plant
In a study on a group of 9 parquet-flooring workers (age, 25-58
years; exposure time, 8-35 years), Denkhaus et al. (1986) noted
significant changes in several subpopulations of peripheral blood
lymphocytes, which could constitute an early indication of a
haematological or immunological effect. Benzene was not detected in
the ambient air and no investigation was made to determine which
components of the solvent mixtures produced the observed changes in
lymphocyte populations. Anshelm Olson et al. (1981) studied the simple
reaction time (SRT) performance in a group of 42 workers (age, 18 to
52 years; employment, 0.5 to 8.1 years) from a plastic coating line of
a steel factory (the same group had previously been studied by Fagius
& Grönqvist, 1978). The study was longitudinal and covered a period of
27 months during which SRT was measured three times. Originally, the
workers had been exposed to significant concentrations of MEK (up to
4000-5000 mg/m3 in certain regular tasks) and to much lower levels
of other solvents. Five months after the completion of major
improvements in the work environment which reduced the levels of MEK
to about 20 mg/m3 (maximum of about 400 mg/m3), a second SRT
measurement was made and a third measurement was performed 15 months
later. The workers' performance on the SRT test improved over the
three measurements. Moreover, on the first occasion SRT was correlated
to the degree of exposure. The authors concluded that the workers'
central nervous functioning had been adversely affected by solvent
exposure.
Mutti et al. (1982a) carried out a study of exposure to organic
solvents in an Italian shoe factory. The exposed group consisted of 95
workers (24 males, 71 females) with an age range of 16-62 years (mean,
30.9 ± 11.7 years), and the exposure duration ranged from 1 to 25
years (mean, 9.1 ± 8 years). The approximate mean air concentrations
in the breathing zone, over a 2-year period, for a number of solvents
were: MEK, 115 mg/m3 (39 ppm); n-hexane, 317 mg/m3 (90 ppm);
cyclohexane, 315 mg/m3 (92 ppm); and ethyl acetate, 205 mg/m3
(57 ppm). The exposed workers complained of sleepiness, dizziness,
weakness, paraesthesia and hypo-aesthesia. Other neurological
symptoms, such as headache, muscular cramps, neurasthenic syndrome and
sleep disturbances, were found more often in exposed workers, but the
differences in incidence between the exposed and reference group were
not statistically significant.
Among exposed workers the mean motor nerve conduction velocity
was significantly reduced in the median and peroneal nerves but not in
the ulnar nerve. The amplitude of the motor action potential (MAP) was
significantly reduced in all nerves and its duration was increased in
the ulnar nerve. There were no significant effects on the distal
latency. The number of abnormal action potentials observed in the
median and peroneal nerves of exposed workers was significantly
increased. There was a correlation between the reduction in motor
conduction velocity and exposure.
In a follow-up study, electrophysiological measurements including
somatosensory evoked potentials (SEPS) were recorded from a group of
15 female shoe factory workers aged 19-53 years (mean age, 26.6 ± 11.4
years) with a solvent exposure duration of 2-8 years (mean, 4.5 ± 2.3
years) (Mutti et al., 1982b). The mean air concentrations for various
solvents in the breathing zone of the workers were: MEK, 177 mg/m3
(60 ppm); n-hexane, 690 mg/m3 (196 ppm); cyclohexane, 585 mg/m3
(170 ppm); and ethyl acetate, 360 mg/m3 (100 ppm).
Electrophysiological measurements in peripheral nerves showed
significant reductions in maximal motor and distal sensory nerve
conduction velocities in the median and ulnar nerves and reduced
maximal motor nerve conduction velocity in the peroneal nerve. The
latency of the sensory peak action potential was significantly
increased in the median and ulnar nerves. The amplitude of all
peripheral nerve action potentials was slightly reduced but this was
not statistically significant. There were also changes in the SEPs
with significant increase in the latency of some early component
peaks. The amplitude of some of the early peaks was significantly
reduced. The neurotoxicity was attributed primarily to n-hexane.
8.5 Carcinogenicity
In a historical prospective mortality study of 446 male workers
in two MEK dewaxing plants, with an average follow up of 13.9 years,
the observed deaths (46) were below the expected (55.51). There was a
slight deficiency of deaths from neoplasms (13 observed; 14.26
expected) but there was a significant increase of deaths from tumours
of the buccal cavity and pharynx (2 observed; 0.13 expected). However,
there were significantly fewer deaths from lung cancer (1 observed;
6.02 expected). In view of the small numbers, it was concluded that
there was no clear evidence of cancer hazard in these workers
(Alderson & Rattan, 1980).
9. EFFECTS ON OTHER ORGANISMS IN THE LABORATORY AND FIELD
9.1 Microorganisms
The effects of MEK on microorganisms have been studied in several
species important to freshwater aquatic systems. As shown in Table 15,
growth inhibition generally occurs at levels ranging from 120 mg/litre
for the cyanobacterium (blue-green alga) Microcystis aeruginosa to
4300 mg/litre for the green alga Scenedesmus quadricauda.
A number of bacterial species have also been tested, the effect
levels ranging from 10 to 5050 mg/litre. Kulshrestha & Marth (1974a-f)
conducted a series of studies to determine if MEK and other volatile
compounds associated with the flavour of raw or mildly heated milk are
able to inhibit the growth of certain pathogenic bacteria and other
bacteria important in the manufacture of fermented dairy products.
Nutrient broth laced with the organisms and MEK at levels of 1, 10,
100 and 1000 mg/litre was plated after 5, 8, 11 and 14 h of incubation
in an air-tight vessel. Results are shown in Table 15. MEK was
considered bacteriostatic to Escherichia coli, Salmonella
typhimurium, Staphylococcus aureus, Leuconostoc citrovorum and
Streptococcus thermophilus at levels as low as 10-100 mg/litre.
However, Walton et al. (1989) reported that MEK at concentrations up
to 1 g/kg dry weight of soil had little effect on the respiration of
microorganisms in moist soil. Volskay & Grady (1988) found that MEK at
1 g/litre depressed respiration of sewage sludge organisms by only
11%. Ingram (1977) noticed changes in the fatty acid and phospholipid
composition of the cell membranes of E. coli when cultured with a
sublethal (0.218 mol/litre) concentration of MEK.
Tests in fungi demonstrated that MEK has a very slight
stimulating effect on the germination of uredospores in two species of
rust (French, 1961; French et al., 1977). Growth of a mixture of other
fungal species was inhibited by about 50% by 6.4 mg MEK/g seed when
the fungi were cultured on moist wheat seed (Nandi & Fries, 1976).
Growth of a mixture of other fungal species on moist wheat seed
at 30 °C was inhibited by 50% following exposure for 5 days to
approximately 6.4 mg MEK/g seed (Nandi & Fries, 1976). Germination of
the wheat seeds was also reduced but there was no statistical
evaluation of these data.
Table 15. Effects of methyl ethyl ketone on microorganisms
Organism Species, strain Concentration Effect Reference
(mg/litre)
Prokaryotes
Cyanobacterium
(Blue-green alga Microcystis aeruginosa 120 inhibition of cell multiplication Bringmann & Kuhn (1978)
Bacteria Pseudomonas putida 1150 inhibition of cell multiplication Bringmann & Kuhn (1977a)
Photobacterium phosphoreum 5050 50% reduction in light output Curtis et al. (1982)
Escherichia coli, ML30 10 slight but not consistently Kulshrestha & Marth (1974a)
significant inhibition of growth
Escherichia coli, ML30 100 significant inhibition after 5 h Kulshrestha & Marth (1974a)
incubation
Escherichia coli, ML30 1000 10% reduction in growth Kulshrestha & Marth (1974a)
Escherichia coli, B15 72.1 65% growth inhibition after 1 h; Egyud (1967)
30% after 2 h
Salmonella typhimurium 1 & 10 slight but not consistently Kulshrestha & Marth (1974b)
significant inhibition of growth
Salmonella typhimurium 100 some inhibition of growth Kulshrestha & Marth (1974b)
Salmonella typhimurium 1000 significant inhibition of growth Kulshrestha & Marth (1974b)
Staphylococcus aureus, 100 1 no significant inhibition of growth Kulshrestha & Marth (1974c)
Staphylococcus aureus, 100 10 some inhibition of growth Kulshrestha & Marth (1974c)
Staphylococcus aureus, 100 100 & 1000 significant inhibition of growth Kulshrestha & Marth (1974c)
Table 15 (contd)
Organism Species, strain Concentration Effect Reference
(mg/litre)
Streptococcus lactis 1 no significant inhibition of growth Kulshrestha & Marth (1974d)
Streptococcus lactis 10 significant inhibition at early Kulshrestha & Marth (1974d)
stages of incubation
Streptococcus lactis 100 & 1000 significant inhibition of growth Kulshrestha & Marth (1974d)
Leuconostoc citrovorum 1 no significant inhibition of growth Kulshrestha & Marth (1974e)
Leuconostoc citrovorum 10 some inhibition of growth Kulshrestha & Marth (1974e)
Leuconostoc citrovorum 100 & 1000 significant inhibition of growth Kulshrestha & Marth (1974e)
Streptococcus thermophilus, 1 no significant inhibition of growth Kulshrestha & Marth (1974f)
ST4
Streptococcus thermophilus, 10 growth inhibition at later stages of Kulshrestha & Marth (1974f)
ST4 incubation
Streptococcus thermophilus, 100 & 1000 significant inhibition of growth Kulshrestha & Marth (1974f)
ST4
Eukaryotes
Fungi Puccinia graminis, var. tritica, not given slight stimulation to germination French (1961)
spores (wheat stem rust)
Uromyces phaseoli, (Reben), 1000 very slight stimulation to French et al. (1977)
Wint., spores (bean rust) germination
Puccinia helianthi, Schw. chr., 50 very slight stimulation to French (1984)
spores (sunflower rust) germination
Table 15 (contd)
Organism Species, strain Concentration Effect Reference
(mg/litre)
Uromyces vignae, Barcl., 500 no effect on germination French (1984)
spores (cowpea rust)
various fungi found on wheat 6.4a slight inhibition of fungal growth Nandi & Fries (1976)
seeds: Septoria nerdorum, Fusarium
nivale, Aspergillus glaucus,
Aspergillus candidus, Penicillium
sp., Alternaria, sp.
Green algae Chlorella sp. 806 no effect on chlorophyll content Dojlido (1979)
after 7 days exposure
Scenedesmus quadricauda 4300 slightly inhibited cell Bringmann & Kuhn (1978)
multiplication
Protozoa Entosiphon sulcatum 190 slightly inhibited cell Bringmann (1978)
multiplication
a mg/g seed.
9.2 Aquatic organisms
MEK has been tested on numerous species of freshwater and marine
vertebrates and invertebrates in short-term tests. The results
indicate that MEK is generally of low toxicity to aquatic animals,
with median lethal (LD50) levels ranging from 1382 to 8890 mg/litre
(Table 16). Almost all of the acute tests were conducted under static
conditions, in open vessels, with nominal measurements of MEK
concentration, all of which may underestimate the true toxicity level
of MEK. No chronic studies at low concentrations have been conducted.
Curtis et al. (1982) related the effect of MEK on the
bioluminescence of a bacterium to the 96-h LC50 in fathead minnows
in an attempt to find an inexpensive yet accurate substitute for
lethality tests. The relationship for ketones (r2 = 0.81, n = 7) is
described by the equation:
log LC50 = 0.74 (log EC50 + 0.79)
where the EC50 (5 min) is the concentration causing a 50% reduction
in light output.
This relationship predicts a 96-h LC50 for fathead minnows of
3388 mg/litre, which agrees well with the measured value (Veith et
al., 1983) of 3200 mg/litre. Similarly, LeBlanc (1984) found a highly
significant correlation (P < 0.01) between the LC50 values
reported for warm-water and cold-water fish, saltwater and freshwater
fish, marine and freshwater invertebrates, and marine and freshwater
algae. There was a remarkable similarity among acute toxicity values
across three trophic levels. Data in Tables 15 and 16 lend further
support to this conclusion.
9.3 Terrestrial organisms
9.3.1 Animals
The effect of MEK on the alarm behaviour of social insects has
been studied. It was considered inactive in producing alarm behaviour
in the ant Iridomyrmex preinosus (Blum et al., 1966), the harvester
ant Pogonomyrmex badius (Blum et al., 1971) and the honeybee Apis
Mellifera (Boch & Shearer, 1971). Alarm behaviour was measured by
the number of insects that were attracted to the chemical and was
indexed relative to the activity of natural pheromones, including
2-heptanone.
Table 16. Effects of methyl ethyl ketone on aquatic organisms
Species Concentration Effects and comments pH Temperature Hardness Reference
(mg/litre) (°C)
Crustacea
Artemia salina 1950 24-h LC50, bottles not not given not given not given Price et al. (1974)
(brine shrimp) sealed and may have lost
MEK during experiment
Daphnia magna (water flea) < 180 no discernible effect 7.21 21.0-23.0 38 mg/litre Union Carbide Corp.
CaCO3 (1980a)
Daphnia magna (water flea) < 520 24-h LC50 8 ± 0.2 not given 173 ± 13 mg/l Le Blanc (1980)
< 520 48-h LC50
< 70 no discernible effect
Daphnia magna (water flea) 1382 48-h LC50 7.21 21.0-23.0 38 mg/litre Union Carbide Corp.
(918-3349)a CaCO3 (1980a)
Daphnia magna (water flea) 2500 24-h LC0 7.6-7.7 20-22 16 ° (German) Bringmann & Kuhn (1977b)
Daphnia magna (water flea) 8890 24-h LC50 7.6-7.7 20-22 16 ° (German) Bringmann & Kuhn (1977b)
Daphnia magna (water flea) 10 000 24-h LC100 7.6-7.7 20-22 16 ° (German) Bringmann & Kuhn (1977b)
Fish
Leucissus idus melanotus 4400b LC0, period not mentioned not given not given not given Juhnke & Luedemann
(golden orfe) 4800b (1978)
Leucissus idus melanotus 4600b LC50 not given not given not given Juhnke & Luedemann
(golden orfe) 4880b (1978)
Table 16 (contd)
Species Concentration Effects and comments pH Temperature Hardness Reference
(mg/litre) (°C)
Leucissus idus melanotus 4800b LC100 not given not given not given Juhnke & Luedemann
(golden orfe) 5040b (1978)
Lebistes reticulatus 2000 disturbed behaviour not given 20 ± 1 27.5 mg/litre Dojlido (1979)
(guppy) CaCl2
Lebistes reticulatus 5700 24-h LC50, open not given 20 ± 1 27.5 mg/litre Dojlido (1979)
(guppy) containers may have lost CaCl2
MEK during test
Pimephales promelas 3200 96-h LC50 7.5 25 ± 1 42.2 mg/litre Veith et al. (1983)
(fathead minnow) CaCO3
Lepomis macrochirus < 1000 no discernible effect 7.93 21 240 mg/litre Union Carbide Corp.
(bluegill sunfish) CaCO3 (1980b)
Lepomis macrochirus 4467 96-h LC50 7.93 21 240 mg/litre Union Carbide Corp.
(bluegill sunfish) CaCO3 (1980b)
Gambusia affinis 5600 96-h LC50 7.8-8.3 room not given Wallen et al. (1957)
(mosquito fish) temperaturec
Cyprinodon variegatus 400 no discernible effect not given 25-31 not given Heitmuller et al.
(sheepshead minnow) (1981)
Carassius auratus > 5000 24-h LC50 7.0 20 ± 1 100 mg/litre Bridie et al. (1979b)
(goldfish)
a 95% confidence limits
b Values are from different laboratories
c No specific value given
MEK was found to be moderately effective as a fumigant against
the Caribbean fruit fly, Anastrepha suspensa (Davis et al., 1977).
Treatments of 790 mg/m3 (286 ppm) for 2 h or 316 mg/m3 (107 ppm)
for 7 h destroyed 100% of the larvae in naturally infected guavas,
whereas exposure to 221 mg/m3 (75 ppm) for 3 h destroyed 92% of the
larvae. Kwan & Gatehouse (1978) applied between 0.31 and 0.37 mg MEK
topically to the dorsal thorax of tsetse flies (Glossina morsitans
morsitans) weighing 17-19 mg. One day after treatment, the MEK had
a significant effect on the activity of males but not females. The
mortality in MEK-treated insects was marginally but consistently
higher than in the untreated controls. No apparent inhibitory or other
effect of MEK was noted with respect to feeding or mating. Vale et al.
(1988) found MEK to be a very effective attractant for tsetse flies
and used it in a successful control effort in which the flies were
attracted to insecticide-coated netting. Uspenskii & Repkina (1974)
reported that an unspecified dose of MEK caused an increase in the
physiological age of the tick Ixodes perculcatus, an effect that
increased the insect's sensitivity to DDT.
9.3.2 Plants
MEK has an effect on the germination of seeds of several plant
species. Nandi & Fries (1976) observed that the germination of wheat
seeds was inhibited when the seeds were treated with 6.4 mg MEK/g
seed. At this level, 10% of the experimental seeds germinated versus
60% of the control seeds. Germination of lettuce was inhibited 50% by
MEK at 12.5 (± 4) mmol/litre (equivalent to 900 ± 288 mg/litre)
dissolved in agar (Reynolds, 1977). Schulz et al. (1981) reported,
however, that a mixture of acetone and MEK had no inhibitory effect on
the growth of rye grass at concentrations up to 1 g/litre.
10. ENHANCEMENT OF THE TOXICITY OF OTHER SOLVENTS BY MEK
The principal toxic effects noted with MEK exposure stem from its
ability to potentiate the known toxicities of other solvents (Table
17). Two such interactions are described in detail below.
10.1 Hexacarbon neuropathy
10.1.1 Introduction
MEK interacts with hexacarbon compounds and potentiates their
neurotoxicity (WHO, 1991). Potentially MEK co-exposure could affect
the metabolism of the hexacarbon compounds or the toxic process by
which the hexacarbons induce the neuropathy. The critical metabolic
pathway for hexacarbon induced neuropathy is outlined in Fig. 2, and
the scheme by which the peripheral nerve axonal degradation is thought
to occur is given in Fig. 3. The metabolic pathway involves hepatic
microsomal oxidation to 2,5-hexanedione, the proximate neurotoxicant,
which is thought to induce cross-linking of neurofilaments, blockage
of transport at the node of Ranvier, and swelling from accumulated
neurofilaments proximal to the nodes and axonal degeneration distal to
the nodes.
10.1.2 Animal studies
The phenomenon of potentiation of hexacarbon neurotoxicity by MEK
has been firmly established by in vivo studies mainly on rats
(Table 17) and also has been demonstrated in tissue culture (Veronesi
et al., 1984). In every study in which the dose of n-hexane, methyl
butyl ketone (MBK), or 2,5-hexanedione (2,5-HD) was large enough and
sustained for a sufficient period, clinical signs of neural
degeneration were produced. These signs were made more severe by
co-exposure to MEK. In addition, the period prior to the onset of
symptoms was frequently shortened by co-exposure to MEK. Minimum
sustained continuous exposure concentrations that induced neuropathy
in these experiments were 295/1408 and 590/1056 mg/m3 (100/400 and
200/300 ppm) MEK/ n-hexane mixtures. An intermittent exposure (8
h/day) of rats to 2950/31 680 mg/m3 (1000/9000 ppm) MEK/ n-hexane
mixture produced severe neuropathy, whereas similar exposure to a
lower concentration, i.e. 590/1760 mg/m3 (200/500 ppm)
MEK/ n-hexane mixture yielded no evidence of hexacarbon
neurotoxicity. Intermittent exposure (8 h/day, 5 days/week) to
5900/820 mg/m3 (2000/200 ppm) MEK/MBK mixture produced some neural
degeneration and mild clinical signs. Oral dosing of rats once a day,
5 days/week, with a mixture of MEK/2,5-HD (0.159/0.253 g/kg) produced
marked clinical signs (Ralston et al., 1985). Even with doses of MBK
too low to produce significant neuropathy, studies generally indicated
that co-exposure with MEK induced changes compatible with enhanced
toxicity, such as reduced velocity of nerve conduction, elevated
hepatic microsomal enzyme activity, or reduced clearance of 2,5-HD
from the blood or the body. The only study reporting no evidence of
potentiation (Spencer & Schaumburg, 1976) compared MBK at 0.150 g/kg
with one tenth this concentration of MBK, 0.015 g/kg, in combination
with MEK. Since there were no control data on the effects of 0.015
g/kg of MBK alone and the dose of MEK was very low, it is difficult to
interpret the meaning of this study. Concentrations of MEK in
inhalation studies did not exceed 3319 mg/m3 (1125 ppm), and
continuous exposure to this concentration was demonstrated by Saida et
al. (1976) not to produce neuropathological effects. Thus it is
considered unlikely that MEK itself produces neuropathy.
The property of potentiation of hexacarbon neurotoxicity is not
unique to MEK, but is shared at least by methyl n-propyl ketone,
methyl n-amyl ketone and methyl n-hexyl ketone, none of which
appear intrinsically neurotoxic (Misumi & Nagano, 1985).
The mechanism by which MEK potentiates hexacarbon neurotoxicity
is not well understood, although potential metabolic interactions have
been examined. In rats, simultaneous inhalation of n-hexane and MEK
resulted in lower levels of 2,5-HD in vivo in urine (Iwata et al.,
1984; Shibata et al., 1990a) and initially lower but later somewhat
elevated levels of MBK and 2,5-HD in vivo in serum (Shibata et al.,
1990b). Both Iwata et al. (1984) and Shibata et al. (1990b) concluded
that potentiation of n-hexane neurotoxicity by MEK could not be
explained solely by increased 2,5-HD formation. Pretreatment of rats
with MEK (1.87 ml/kg, 4 daily doses) prior to inhalation of n-hexane
resulted in higher levels of 2,5-HD in several tissues, including
blood (Robertson et al. (1989). Abdel Rahman et al. (1976) reported
that an 8-h simultaneous exposure of rats to MEK and MBK did not
result in measurable levels of MBK or of 2,5-HD in blood, whereas a
6-day continuous co-exposure resulted in substantially raised levels
of MBK and 2,5-HD. Continuous co-exposure for 23 days, however,
resulted in further elevation of MBK in blood, but no measurable level
of 2,5-HD. Simultaneous administration of 2,5-HD and MEK, either as a
single dose or as six repeated daily doses in rats, resulted in a
greater total area under the curve for 2,5-HD in blood compared with
administration of 2,5-HD alone (Ralston et al., 1985).
In guinea-pigs, increased levels of 2-hexanol and 2,5-HD were
found following intraperitoneal administration of a MEK/MBK mixture
compared with intraperitoneal administration of MBK alone (Couri et
al., 1978).
MEK administration has also been shown to induce a number of in
vivo and in vitro parameters of hepatic oxidative metabolism
(Couri et al., 1977; Wagner et al., 1983; Misume & Nagano, 1985;
Raunio et al., 1990).
Table 17. Interaction of MEK with other solvents and their metabolitesa
Route of administration, Dose and/or Exposure Effects/results Reference
species (strain), concentration
number and sex
n-Hexane
Inhalation, rat, 820 mg/m3 (200 ppm) MBK; 8 h/day, muscular weakness lasted longer after exposure to Duckett et al.
9 sex unspecified 5900/820 mg/m3 5 days/week, MEK/MBK than after MBK alone (1974)
MBK, 8 sex unspecified (2000/200 ppm) MEK/MBK 6 weeks
MEK/MBK
Inhalation, rat 1760 mg/m3 (500 ppm) 8 h/day, no significant enhancement of neuropathological Iwata et al.
(Wistar), 6 males hexane; 1475/1760 mg/m3 7 days/week, damage evident in peripheral nerve (1984)
per groupb (500/500 ppm) MEK/hexane 33 weeks electrophysiological function; 2,5-hexanedione
and other hexane metabolites in urine reduced
with co-exposure
Inhalation, rat 923 mg/m3 (225 ppm) MBK; 24 h/day, MEK enhanced MBK-induced peripheral neurotoxicity Saida et al.
(Sprague-Dawley), 3319/923 mg/m3 (1125/225 16-66 days (1976)
12/group, sex ppm) MEK/MBK
unspecified
Inhalation, rat 923 mg/m3 (225 ppm) MBK; 24 h/day, hexobarbital sleeping time significantly reduced in Couri et al.
(Wistar), 5 males 2213/923 mg/m3 (750/225 7 or 28 days group exposed continuously for 7 days to MEK/MBK (1977)
per group ppm) MEK/MBK (and in 2213 mg MEK/m3 (750 ppm) controls), but not
in MBK group; interpreted as evidence for elevated
microsomal enzyme activity in MEK/MBK and MEK
groups
Table 17 (contd)
Route
Route of administration, Dose and/or Exposure Effects/results Reference
species (strain), concentration
number and sex
Inhalation, rat 1760, 2464 mg/m3 (500, 700 approx. experiments yielded similar results: potentiation of Altenkirch et
(Wistar), 5 males ppm) hexane; 295/1408, 24 h/day, n-hexane neurotoxicity in MEK/hexane groups as al. (1982a)
per group 590/1056, 590/1760 mg/m3 7 days/week, shown by shortened period before onset of clinical
(100/400, 200/300, 200/500 9 weeks signs (weakness and paresis); hypersalination
ppm) MEK/hexane in MEK/hexane groups only; neural degeneration
in all solvent-treated groups (no mention of
differences among these groups)
Inhalation, rat 1760, 2464 mg/m3 (500, 700 8 h/day, every co-exposure to MEK/hexane resulted in earlier and Schnoy et al.
(Wistar), 5 groups ppm) hexane; 295/1408, day for 1-89 more pronounced degeneration of pulmonary (1982);
of 2-5 malesc 590/1056, 590/1760 mg/m3 days nerves than exposure to hexane alone; there were no Schmidt et al.
(100/400, 200/300, 200/500 consistent differences in degeneration of alveolar (1984)
ppm) MEK/hexane epithelium between the hexane and MEK/hexane
groups
Inhalation, rat 1640, 923 mg/m3 (400, 225 24 h/day, 2,5-HD detected in blood after 6 days but not after Abdel-Rahman
(Wistar), unspecified ppm) MBK; 2213/923 mg/m3 6 or 23 days 23 days, and there were elevated MBK blood levels et al. (1976)
number/group, male (750/225 ppm) MEK/MBK in group exposed to MEK/MBK; severe neuropathy in
group exposed to MEK/MBK for 23 days
Inhalation, rat 2464 mg/m3 (700 ppm) 8 h/day, no evidence of potentiation of hexane neurotoxicity: Altenkirch et
(Wistar), 5 males hexane; 590/1760 mg/m3 7 days/week, no abnormal clinical signs or elevated al. (1982a)
per group (200/500 ppm) MEK/hexane 40 weeks neuropathology in solvent-treated groups
Inhalation, rat 3520 mg/m3 (1000 ppm) 8 hd 2,5-hexanedione in urine markedly less after co- Iwata et al.
(Wistar), 5 males hexane; 2950/3520 mg/m3 exposure with MEK than after exposure to hexane (1983)
per group (1000/1000 ppm) MEK/hexane alone
Table 17 (contd)
Route
Route of administration, Dose and/or Exposure Effects/results Reference
species (strain), concentration
number and sex
Inhalation, rat Experiment 1: 35 200 mg/m3 8 h/day, experiments yielded similar results; acceleration Altenkirch et
(Wistar), 5 males (10 000 ppm) hexane; 3245/ 7 days/week, of n-hexane neurotoxicity in MEK/hexane group as al. (1978,
per group 31 330 mg/m3 (1100/8900 15-19 weeks shown by more severe clinical signs (gait 1979)
ppm) MEK/hexane disturbances, weakness and paresis), greater
degeneration of peripheral nerves and shortened
5 males/group Experiment 2: 35 200 mg/m3 period before onset of signs and neural degeneration;
(10 000 ppm) hexane; 2950/ hyper-salivation in MEK/hexane groups
31 680 mg/m3 (1000/9000
ppm) MEK/hexane
12 males/group Experiment 3: 35 200 mg/m3
(10 000 ppm) hexane; 2950/
31 680 mg/m3 (1000/9000
ppm) MEK/hexane
Inhalation, rat 35 200 mg/m3 (10 000 ppm) 4 h or 8 h, MEK did not enhance degeneration of intrapulmonary Schnoy et al.
(Wistar), 7 groups hexane; 2950/31 680 mg/m3 8 h/day for nerves; enhanced degeneration of alveolar epithelium (1982);
of 2-3 males (1000/9000 ppm) 2-14 days in the MEK/hexane group after 14 days exposure in Schmidt et al.
MEK/hexanee comparison with the hexane group (1984)
Inhalation, rat 352 mg/m3 (100 ppm) hexane; 12 h/day, marked impairment of nerve conduction velocities in Takeuchi et
(Wistar), 8 males 590/352 mg/m3 (200/100 7 days/week, MEK/hexane group; no evidence of significant al. (1983)
per group ppm) MEK/hexane 24 weeks potentiation of morphological effects of n-hexane
Inhalation, mouse 615 mg/m3 (150 ppm) MBK; 24 h/day, hexobarbital sleeping time significantly lower in Couri et al.
(Swiss), 5/group 2950/615 mg/m3 (1000/150 7 days MEK/MBK group than in MBK group (or in MEK 2950 (1978)
sex unspecified ppm) MEK/MBK mg/m3 (1000 ppm) controls); interpreted as evidence
for elevated microsomal enzyme activity in
MEK/MBK group
Table 17 (contd)
Route
Route of administration, Dose and/or Exposure Effects/results Reference
species (strain), concentration
number and sex
Subcutaneous injection, 288 mg/kg b.w. MBK; 1/day, 5 days significantly enhanced neurotoxicity (slower motor Misumi &
rat (Donryu), 288/288 mg/kg b.w. per week, 20 fibre conduction velocity and increased motor distal Nagano (1985)
8 males/group MEK/MBK weeks latency of tail nerve) and more pronounced weakness
in rats receiving MEK/MBK than MBK alone
Subcutaneous injection, 150 mg/kg b.w. MBK; 5 days/week peripheral neuropathological changes and paresis Spencer &
cat, 9 (sex 135/15 mg/kg b.w. for up to 8.5 with MBK; possible nerve degeneration in animals Schaumburg
unspecified) MBK, MEK/MBK months treated with MEK/MBK (1976)
4 (sex unspecified)
MEK/MBK
Other ketones
Subcutaneous injection, 150 mg/kg b.w. MIBK; 2/day, 5 days no evidence of enhanced MIBK-induced Spencer &
cat, 4, sex 135/15 mg/kg b.w. per week, up neuropathology Schaumburg
unspecified, MIBK, MEK/MIBK to 8.5 months (1976)
6, sex unspecified,
MEK/MIBK
Inhalation, rat 3220 mg/m3 (700 ppm) EBK; 16-20 h/day, exposure to MEK/EBK at 2065 and 4130 mg MEK/m3 O'Donoghue et
(Charles River), 15 207/3220, 2065/3200, 4 days produced a 2.5-fold increase in serum 2,5-Hpdn over al. (1984)
males/group for 4130/3200 mg/m3 (70/700, that produced by EBK alone; no 2,5-HD detected in
EBK and controls; 700/700, 1400/700 ppm) serum
5 males/group MEK/EBK
for MEK/EBK
Oral, rat (Charles 0.25 to 4 g/kg b.w. EBK; 1/day, 5 days 2 and 4 g EBK/kg b.w. fatal with or without MEK; O'Donoghue et
River), 4 males in 1.5, 0.75/0.25-4 g/kg b.w. per week, 14 greater neurological damage and dysfunction, and al. (1984)
control group MEK/EBK weeks around 1.5-fold increase in 2,5-Hpdn and 2,5-HD
with 1.5/1.0 g/kg b.w. MEK/EBK than with 1.0 g
EBK/kg b.w.; no neurotoxicity evident in doses
of EBK below 1.0 g/kg b.w.
Table 17 (contd)
Route
Route of administration, Dose and/or Exposure Effects/results Reference
species (strain), concentration
number and sex
Oral, rat (Fischer- 253 mg/kg b.w. 2,5-HD; 1/day, 5 days marked neurological dysfunction consistently Ralston et al.
344), 5 males/group 159/253 mg/kg b.w. per week, ca. appeared weeks earlier in rats receiving (1985)
MEK/2,5-HDf 13 weeks MEK/2,5-HD
Oral, rat (Fischer- 253 mg/kg b.w. 2,5-HD; 1/day, 1 MEK did not alter gastric absorption of 2,5-HD; Ralston et al.
344), 5 males/group 159/253 mg/kg b.w. or 7 days clearance of 2,5-HD from blood slower from rats (1985)
MEK/2,5-HD receiving MEK/2,5-HD
Oral, rat (Fischer- 253 mg/kg b.w. 2,5-HD; 1/day, 5 days most of radiolabelled 2,5-HD bound to protein; Ralston et al.
344), 5 males/group 159/253 mg/kg b.w. per week, 1, greater binding to protein with MEK/2,5-HD (1985)
MEK/2,5-HD 2 or 3 weeks after 1 week, but reverse true after 2 and 3 weeks
Halogenated alkanes
Oral, intraperitoneal, 1.505 g/kg b.w. MEK; 1 oral dose potentiation of CCl4 hepatotoxicity by MEK as Traiger &
rat (Sprague- 0.16 g/kg b.w. CCl4 MEK followed shown by greater fatty vacuolation and necrosis Bruckner
Dawley), 3-5 males/ 16 h later of liver, increased hepatic triglyceride and (1976)
experimental group, with 1 ip GPT, and decreased hepatic glucose-6-phosphatase
5-20 males/control dose CCl4
group
Oral, intraperitoneal, 1.691 g/kg b.w. MEK; 1 oral dose potentiation of CCl4 hepatotoxicity by MEK as Dietz & Traiger
rat (Sprague- 0.16 g/kg b.w. CCl4 MEK followed shown by increased hepatic triglyceride and (1979)
Dawley), males at 16 h later GPT
least 5/group with 1 ip
dose CCl4
Oral, intraperitoneal, 1.082 g/kg b.w. MEK; 1 oral dose potentiation of CHCl3 hepatotoxicity by both Hewitt et al.
rat (Sprague- 0.797, 1.196 g/kg b.w. MEK followed doses of MEK as shown by increased GPT and OCT (1983)
Dawley), 5 or 6 CHCl3 18 h later with
males/groupg 1 ip dose CHCl3
Table 17 (contd)
Route of administration, Dose and/or Exposure Effects/results Reference
species (strain), concentration
number and sex
Oral, intraperitoneal, 0.072 to 1.082 g/kg b.w. 1 oral dose potentiation of CHCl3 renal and hepatic toxicity Brown & Hewitt
neal, rat (Fischer- MEK; 0.797 g/kg b.w. MEK followed by MEK at all dosages as shown by histological and (1984)
344), 6 males per CHCl3 18 h later with several biochemical criteria; potentiation greatest
experimental 1 ip dose at 0.361 to 0.721 g/kg b.w. MEK, and slightly
group, 32 males CHCl3 reduced at 1.082 g/kg b.w.h
in control group
Oral, rat (Sprague- 1.082 g/kg b.w. MEK; 1 dose MEK MEK followed by CHCl3 did not induce cholestasis, Hewitt et al.
Dawley), 6 males 0.797 to 1.594 g/kg b.w. followed 10 but MEK given 10 to 24 h prior to CHCl3 potentiated (1986)
per group CHCl3 to 96 h later its ability to increase plasma bilirubin
with 1 dose
CHCl3
Oral, rat (Sprague- 1.082 g/kg b.w. MEK; 1 dose MEK MEK followed by CHCl3 10 to 48 h later potentiated Hewitt et al.
Dawley), 6 males 0.797 g/kg b.w. CHCl3 followed 10 hepatotoxicity as indicated by elevated ALT and (1987)
to 96 h later OCT levels
by 1 dose
CHCl3
a Values from the literature have been recalculated as ppm or g/kg body weight.
b Iwata et al. (1984) states that 24 rats were divided into four groups, but does not specify that groups were of equal numbers.
c Animals were apparently co-exposed with those described in Altenkirch et al. (1982a).
d Description of exposure not entirely clear but a subsequent paper (Iwata et al., 1984) refers to this as a single exposure.
e Animals co-exposed with those described in Altenkirch et al. (1978).
f MEK and 2,5-HD doses were 0.317 and 0.506 g/kg, respectively, for first 8 days of experiment.
g 5 males at the lower dose and 6 at the higher; 11 males in the control group
h Measurements were made 24 h after the oral dose of CHC13 or CCl4.
Abbreviations: GPT = plasma glutamic-pyruvic transaminase; OCT = plasma ornithine carbamyl transferase; ALT = plasma alanine aminotransferase;
b.w. = body weight; ip = intraperitoneal; 2,5-HD = 2,5-hexanedione; 2,5-Hpdn = 2,5-heptanedione; CCl4 = carbon tetrachloride; CHCl3 = chloroform
6.4 Elimination and excretion
MEK and its metabolites are excreted by the lungs and kidneys.
Liira et al. (1988a) reported that only 3% of the calculated absorbed
dose during a 4-h exposure to 590 mg MEK/m3 (200 ppm) was secreted
unchanged in the exhaled air of volunteers after the exposure. The
fractional elimination of unchanged substance however depends on the
efficiency of metabolic clearance. Since metabolic saturation for MEK
in humans begins at relatively low levels (about 100 ppm) of exposure
(Liira et al., 1990a), proportionally greater amounts of MEK would be
expected to be excreted via the lungs (and kidneys) at high exposure
levels.
Relatively little of the absorbed MEK is excreted unchanged via
the kidneys; a study of occupationally exposed workers revealed that
it is less than 0.1% of the alveolar uptake (Miyasaka et al., 1982).
In a similar study of workers occupationally exposed to a mixture of
solvents, the excretion of MEK and a major recognizable metabolite,
3-hydroxy-2-butanone, was 0.1% of alveolar uptake (Perbellini et al.,
1984). The concentrations of both MEK and 3-hydroxy-2-butanone in
urine were significantly correlated with the environmental level of
MEK. Other metabolites of MEK, 2-butanol or 2,3-butanediol, which
DiVincenzo et al. (1976) identified in the serum of guinea-pigs, were
not detected in the urine of the exposed workers. Liira et al.
(1988a), however, reported that human excretion of 2,3-butanediol was
individually variable but averaged 2% of the absorbed MEK. The urinary
excretion of 2-butanol, a minor metabolite of MEK, was examined by
Kamil et al. (1953), who found that clearance of 2-butanol
administered by gavage in rabbits was about 14% of the administered
dose and in the form of a glucuronide.
Since MEK and 2,3-butanediol disappear from blood and urine and
there is no evidence for accumulation elsewhere in the body, the above
data suggest that the bulk of MEK absorbed by mammals enters the
general metabolism and is eliminated from the body as simple compounds
like carbon dioxide and water whose source is not readily
identifiable. The specific pathways by which MEK is metabolized have
not been identified. There also is no information on loss of MEK in
faeces.
6.5 Turnover
Both animal and human data indicate a rapid turnover of MEK. In
guinea-pigs receiving an intraperitoneal dose of 450 mg MEK per kg,
the half-life of MEK in blood serum was 4´ h and the clearance time
for MEK in serum was 12 h. For the metabolites 2-butanol,
3-hydroxy-2-butanone and 2,3-butanediol, the clearance time in serum
was 11 h (DiVincenzo et al., 1976). In rats given a 2.2-ml/kg oral
dose of 2-butanol, the butanol was largely cleared from the blood in
15 h and the 2-MEK derived from the butanol was cleared in 24 h
(Traiger & Bruckner, 1976). In a study by Dietz & Traiger (1979) on
rats given an oral dose of 2-butanone of 2.1 mg/kg, there was a
half-life of 3.6 h for MEK in blood if the rate of loss was assumed to
be constant between the two times of measurement (4 h and 18 h) after
dosing. Data from a study of Dietz et al. (1981) on rats receiving
oral doses of 2-butanol or MEK also indicate a half-life of about 4 h
for MEK. These authors reported that the clearance rate for
3-hydroxy-2-butanone and 2,3-butanediol was independent of dose for
the two doses used (0.4 and 0.8 g/kg) and that the half-lives for
these metabolites of MEK were 47 min and 3.45 h, respectively. Liira
et al. (1988a) reported a steady increase in blood concentrations
during 4-h exposures of human volunteer subjects to 590 mg/m3 (200
ppm) and observed a rapid elimination of MEK, with half-lives of 30
min during the first post-exposure hour and 81 min thereafter. An
inhalation study with two volunteer subjects exposed to MEK for 4 h at
concentrations of 74, 590 and 1180 mg/m3 (25, 200 and 400 ppm)
indicated that the kinetics of MEK were dose dependent at higher
exposure concentrations, i.e. much higher levels of MEK in blood were
reached relative to inhaled concentrations, and the post-exposure
elimination of MEK in blood was slower (zero-order kinetics).
Simulated exposure to MEK for 8 h suggests that saturation kinetics
are reached at about 295 mg/m3 (100 ppm) at rest and 148 mg/m3 (50
ppm) during light exercise (Liira et al., 1990a).
6.6 Metabolic interactions
Ingestion of ethanol (0.8 g/kg) combined with an inhalation
exposure to MEK (590 mg/m3, 200 ppm) inhibited the oxidative
metabolism of MEK and led to a marked increase in the blood
concentration of MEK (Liira et al., 1990b). There was a concurrent,
even more pronounced elevation of the blood 2-butanol concentration;
the most likely explanation is competitive inhibition by ethanol of
the oxidation of 2-butanol back to MEK. Ethanol also appeared to
interact with the further biotransformation of 2,3-butanediol as the
urinary excretion of the metabolite was increased. Co-exposure with
xylene, however, had no effect on the human metabolism or rate of
elimination of MEK (Liira et al., 1988b).
6.7 Mechanisms of action
There is very limited information on the mechanisms of toxic
action of MEK. Relatively high inhaled concentrations 1475-29 500
mg/m3 (500-10 000 ppm) caused pulmonary vasoconstriction and
hypertension in cats and dogs (Zakhari et al., 1977). From the
toxicological point of view, interactions leading to the potentiation
of effects, particularly neurotoxicity, by other intrinsically toxic
substances constitute the main hazard of MEK. The mechanisms
underlying these interactions are incompletely known (see section 10).
7. EFFECTS ON LABORATORY MAMMALS AND IN VITRO TEST SYSTEMS
7.1 Acute exposure
7.1.1 Lethal doses
Data on the acute toxicity of MEK to experimental animals are
summarized in Table 11. Oral toxicity is low with LD50 values
ranging from 2 to 6 g/kg for adult mice and rats. Intraperitoneal
LD50 values are lower (approximately 1.5 g/kg for 24 h and 0.6 g/kg
for 14 days). A larger dermal LD50 value for rabbits, i.e. > 8 g/kg
(contact time: 24 h; observation time: 14 days), may reflect slower
and less complete absorption via the skin, although it may also
reflect species differences in sensitivity to MEK. The lethal dose of
MEK given to rats in the only study using dosing by pulmonary
aspiration (Panson & Winek, 1980) was 0.8 g/kg. This is well below the
intraperitoneal 24-h LD50 for adult rats but similar to the 14-day
value (Lundberg et al., 1986). It cannot be excluded that the high and
rapid lethality of this aspirated dose to adult rats (5/6 deaths in
< 24 h, with 4/6 reported as dying "instantly") may reflect serious
damage to the lungs.
Studies examining the effects of acute inhalation of MEK are not
entirely comparable since they used not only different species but
also different concentrations of MEK, exposure times, and periods over
which survival was measured. For mice the LC50 (45-min exposure) was
about 200 000 mg/m3. The lowest concentration lethal to all rats
exposed by inhalation for 8 h was 47 200 mg/m3 and the lowest
concentration producing lethality in a 4-h exposure was 5900 mg/m3
(2000 ppm). Guinea-pigs survived exposure to 29 500 mg/m3 for 4 to
4.7 h and showed no abnormal signs at 9735 mg/m3. A concentration of
97 350 mg/m3 was lethal to all exposed guinea-pigs in 3.3 to 4.2 h,
whereas a slightly lower concentration, 73 750 mg/m3, although
ultimately lethal to all animals, permitted some guinea-pigs to
survive a 5.4-h exposure (Patty et al., 1935; Specht et al., 1940).
7.1.2 Non-lethal doses
Non-lethal acute doses of MEK produced a number of measurable
changes in experimental animals (Table 11). An oral dose of 1.5 g/kg
to rats resulted in a 63% increase in liver triglycerides after 16 to
23 h, but did not alter liver histology or increase either of two
enzymes, serum glutamic-pyruvic transaminase (alanine transferase
(ALT)) and hepatic glucose-6-phosphatase (Traiger & Bruckner, 1976).
These results suggest that this dose caused metabolic disturbances to
the liver of rats. Much smaller acute intraperitoneal doses (0.049 to
0.194 g/kg) to rats appeared not to damage the liver. A single oral
dose (15 mmol/kg) of MEK did not affect the hepatobiliary function of
rats over an observation period of 10 to 96 h (Hewitt et al., 1986).
A graded series of single doses of MEK to guinea-pigs revealed high
sensitivity to small changes in dosage. The low dose (0.75 g/kg)
appeared to produce no liver damage, whereas 1.5 g/kg produced slight
liver damage and 2.0 g/kg produced major liver damage. These results
also suggest that guinea-pigs and rats may be equally sensitive to MEK
in terms of liver damage even though guinea-pigs survive acute
exposure to higher concentrations of MEK vapour than do rats
(DiVincenzo & Krasavage, 1974).
Consistent increases in the frequency of food-reinforced lever
pressing by rats were detected at MEK exposures as low as 74 mg/m3
(25 ppm) for 2 h (Garcia et al., 1978). Vestibulo-ocular effects were
detected during continuous intravenous administration of MEK for 1 h
via the caudal vein at a dosage as low as 0.005 g/kg per min (Tham et
al., 1984). This dosage is roughly equivalent to inhalation of 2700
mg/m3 (900 ppm). Glowa & Dews (1987) reported that exposure of mice
to 885 mg/m3 (300 ppm) for 30 min did not significantly alter
schedule-controlled responses, whereas concentration-related
suppression of response occurred at consecutive increasing
concentrations (for 30 min) ranging from 2950 to 29 500 mg/m3 (1000
to 10 000 ppm) (see section 7.3.1).
It can be concluded from observations of their behaviour that
respiratory irritation occurred in guinea-pigs exposed to 29 500
mg/m3 or more within 2 min (Patty et al., 1935; Specht et al.,
1940). In survivors, post-exposure recovery from this effect was
rapid. Rats exposed for 8 h/day to 29 500 mg/m3 showed severe
irritation of the upper respiratory tract after a "few days"
(Altenkirch et al., 1979).
7.1.3 Skin and eye irritation
In skin irritation studies, a small dose (8 mg) applied to
clipped skin and covered by an impervious plastic film for 24 h (which
was followed by a 14-day observation period) produced only minor
irritation in male New Zealand albino rabbits (Smyth et al., 1962). A
dose of 400 mg applied to the clipped dorsal skin of restrained albino
rabbits in a gauze patch produced mild to moderate irritation in some
cases (Weil & Scala, 1971). Data from this latter study, however, were
highly variable and may reflect the fact that its purpose was
intercomparison of laboratories rather than the effects of MEK on test
animals. Neat MEK (0.1 ml) applied to the clipped skin of the flanks
of guinea-pigs and rabbits daily for 10 days and left uncovered caused
erythema and oedema after 24-72 h. These effects were more marked in
rabbits (Wahlberg, 1984a).
Table 11. Acute toxicity of MEK for mammalsa
Species Number and sex Exposure concentration Exposure Study Effects Reference
(strain) and range duration duration
Oral studies
Rat (Sprague- both sexes, 6-12 not stated one dose LD50 (7 days) < 0.8 g/kg b.w. Kimura et al.
Dawley) new animals/group 2.5 (2.0-3.1) g/kg b.w. (1971)
born 14 day 6 male/group 2.9 (2.3-3.5) g/kg b.w.
young adult 2.7 (2.1-3.5) g/kg b.w.
older adult
Rat (Carworth- 5 female/group not stated one dose 14 days LD50 (14 days) Smyth et al.
Wistar) 5.52 (4.50-6.80) g/kgb (1962)
Rat (Sprague- 5 male/group 1.505 g/kg b.w. one dose 24 h after 16-23 h, no effect on Traiger &
Dawley) liver histology, serum glutamic Bruckner (1976)
pyruvic transaminase, or
hepatic glucose-6-phosphatase;
63% increase in liver
triglycerides
Rat (Fischer- 32 male/group 0.072-1.082 g/kg b.w. one dose 24 h no evidence of liver Brown & Hewitt
344) dysfunction; slight damage at (1984)
highest dose to kidney proximal
tubules
Mouse (CF1) 10 male/group in 2.0-5.1 g/kg b.w. one dose 24 h LD50 (24 h) Zakhari et al.
each of 6 groups 3.14 ± 0.67 g/kg b.w. (1977)
Inhalation studies
Mouse (Swiss 10 male/group four levels over the 4 h decreased duration of De Ceaurriz et
OF1) range 4726-7192 mg/m3 immobility in escape - al. (1983)
(1602-2438 ppm) directed swimming test
Table 11 (contd)
Species Number and sex Exposure concentration Exposure Study Effects Reference
(strain) and range duration duration
Mouse (CD1) 12 males per five concentrations over continuous 2´ h increasing dose-related Glowa & Dews
concentration the range 885-29 500 (30 min per behavioural effects starting (1987)
mg/m3 (300-10 000 ppm) concentration) at 2950 mg/m3 (1000 ppm)
Mouse (CF1) 6 groups of 147 500-294 000 mg/m3 45 min LC50 (45 min) 205 000 ± 32 500 Zakhari et al.
10 males (50 000-100 000 ppm) mg/m3 (69 500 ± 11 000 ppm) (1977)
Mouse (white) both sexes, 6 303 850 mg/m3 until death average survival 43 min La Belle &
animals/group (103 000 ppm) Brieger (1955)
Rat (Sprague- 6 animals, sex 74-2360 mg/m3 2-6 h 2-6 h behavioural change (increased Garcia et al.
Dawley) unspecified (25-800 ppm) frequency of lever pressing) (1978)
which persisted for < 2 to
> 11 days
Rat (albino) 8 males/group 23 158-59 590 mg/m3 4 h 14 days LC50 (4 h) 34 500 mg/m3 La Belle &
(7850-20 200 ppm) (11 700 ppm) Brieger (1955)
Rat (Wistar) 5 males/group 23 600 mg/m3 8 h 14 days lethal to 3/6 within 14 days Smyth et al.
(8000 ppm) (1962)
Rat (Wistar) data not 47 200 mg/m3 8 h 14 days lethal to 6/6 within 14 days Smyth et al.
supplied (16 000 ppm) (1962)
Guinea-pig 6 unspecified approx. 9735 mg/m3 90-810 min 4-8 days no abnormal signs Patty et al.
sex/group; (3300 ppm) (1935)
3 groups
Guinea-pig 6 unspecified approx. 29 500 mg/m3 90-810 min 4-8 days 90 min: no injury; 280 min: Patty et al.
sex/group; (10 000 ppm) unconsciousness and slight (1935)
3 groups injury, no deaths; 810 min:
unconsciousness and moderate
injury, no deaths
Table 11 (contd)
Species Number and sex Exposure concentration Exposure Study Effects Reference
(strain) and range duration duration
Guinea-pig 10 females 73 750 mg/m3 325 min < 2 days all animals died during or Specht et al.
(25 000 ppm) post exposure (1940)
Guinea-pig 6 unspecified approx. 97 350 mg/m3 30-250 min 4-8 days 30 min: slight injury; 90 min: Patty et al.
sex/group; (33 000 ppm) unconsciousness and moderate (1935)
3 groups injury, no deaths; lethal to
all animals in 200-250 min
Guinea-pig 6 unspecified approx. 303 850 mg/m3 10-55 min 4-8 days 10 min: slight injury; 30 min: Patty et al.
sex/group; (103 000 ppm) unconsciousness and moderate (1935)
3 groups injury, no deaths, lethal to
all animals in 45-55 min
Intravenous study
Rat (Sprague- 18 females several infusion rates 60 min 60 min threshold limit for vestibulo- Tham et al.
Dawley) including 5 mg/kg b.w. ocular disturbance (depression (1984)
per min of nystagmus following
accelerated rotation) 5 mg/kg
b.w. per min; MEK concentration
in blood, 101 mg/litre
Pulmonary aspiration study
Rat (Sprague- 3 males and 800 mg/kg b.w. one dose survivors lethal 5/6 in < 24 h; 4/6 died Panson & Winek
Dawley) 3 females sacrificed instantly; produced pulmonary (1980)
at approx. haemorrhage
24 h
Intraperitoneal studies
Mouse (CF1) 10 male/groups; 0.5-2.0 g/kg b.w. one dose 24 h LD50 (24 h) Zakhari et al.
5 groups 1.66 ± 0.74 g/kg b.w. (1977)
Table 11 (contd)
Species Number and sex Exposure concentration Exposure Study Effects Reference
(strain) and range duration duration
Rat (Sprague- 6 female/group; not stated one dose 24 h LD50 (24 h) 1.554 Lundberg et
Dawley) number of groups (1.229-1.966) g/kg b.w. al. (1986)
unstated
Rat (Sprague- 6 female/group; not stated one dose 14 days LD50 (14 day) 0.607 Lundberg et
Dawley) number of groups (0.486-0.748) g/kg b.w. al. (1986)
unstated
Rat (Sprague- 6 female/group; 0.049-0.194 g/kg b.w. one dose 18 h no significant elevation of Lundberg et
Dawley) 3 groups serum sorbital dehydrogenase al. (1986)
Guinea-pig 4 male/group 0.75-2.0 g/kg one dose 24 h 0.75 g/kg b.w.: no elevation DiVincenzo &
in serum OCTc level, no Krasavage
obvious lipid deposition in (1974)
hepatocytes, no deaths;
1.5 g/kg b.w.: elevated serum
OCT level, lipid deposition in
hepatocytes, no deaths;
2.0 g/kg b.w.: elevation
in serum OCT level, lipid
deposition in hepatocytes,
lethal to 1/4 animals
Dermal studies
Rabbit (Albino, 4 males amounts not stated; 24 h 14 days LD50 (14 days) > 8 g/kg Smyth et al.
New Zealand) kept in contact with (1962)
clipped flank skin
under plastic film
Rabbit (Albino, 5 males 8 mg on clipped one dose 24 h minor irritation Smyth et al.
New Zealand) uncovered skin (1962)
Table 11 (contd)
Species Number and sex Exposure concentration Exposure Study Effects Reference
(strain) and range duration duration
Rat (Albino) 8 males 0.4 mg on 2.5 cm2 24 h 72 h variable results: no irritation Weil & Scala
lightly covered pad to moderate irritation 1 day (1971)
on clipped back skin and 3 days after applicationd
Rabbit (Albino) 6 animals intact 0.5 ml to clipped 24 h 72 h slight erythema subsiding by Hazleton
skin, 6 animals abdominal skin with 72 h (1963a)
abraded skin, semi-occlusive
sex unspecified cover
Ocular studies
Rabbit (Albino) 6 of unspecified 0.1 ml to one eye of 30 sec 14 days severe irritation, including Hazleton
sex each rabbit corneal damage and scleral (1963b)
haemorrhage in 2/6 rabbits
Rabbit (Albino) 5 male per not stated 3 min solution of 130 g/litre water Larson et al.
concentration produced significant oedema in (1955)
eyelid in 1 h
Rabbit (Albino, not specified 4 mg/eyee 1 min 18-24 h severe chemical burn Smyth et al.
New Zealand) (1962)
Rabbit (Albino) 6 males 8 mg to one eye of 20 sec 7 days variable results; no irritation Weil & Scala
each rabbit to moderate irritation 1 day, 3 (1971)
days and 7 days after
applicationd
Rabbit (Albino, 6 of unspecified 8 mg to one eye of described 7-10 days slight swelling, redness and MB Research
New Zealand) sex each rabbit as brief discharge from eye for 4 to Lab. Inc.
10 days (1979)
Table 11 (contd)
a Values from the literature were recalculated as necessary to yield dosages in terms of ppm, g or g/kg
b 95% confidence limits
c OCT = ornithine carbamyl transferase
d Purpose of study was intercomparison of testing laboratories that evaluated MEK and other substances by purportedly identical methods
e Not clear whether one eye or both eyes were dosed
The results of studies on eye irritation in rabbits are
inconsistent. Smyth et al. (1962) reported that 0.005 ml (4 mg)
created a severe chemical burn in the rabbit eye, whereas a study by
MB Research Lab. Inc. (1979) reported less severe irritant effects
from the larger dose of 0.1 ml (80 mg). Data from a study by Weil &
Scala (1971) were also inconsistent but indicated that a dose of 0.1
ml (80 mg) produced minimal to moderate eye irritation. Undiluted MEK
was used in all three studies but Smyth et al. (1962) used the grading
system for eye injury described in Carpenter & Smyth (1946), whereas
MB Research Lab., Inc. (1979) and Weil & Scala (1971) used the Draize
scoring system (Draize et al., 1944). In all studies irritation
disappeared or was markedly reduced by 7 days after treatment. Opaque
corneas were apparent following exposure of guinea-pigs to 295 000 mg
MEK/m3 (100 000 ppm) for 30 min; recovery from this effect was
complete in 4-8 days (Patty et al., 1935).
7.2 Repeated exposures
Data on repeated exposure of mammals to MEK are summarized in
Table 12. None of the concentrations tested, not even the highest (17
700 mg/m3 (6000 ppm) 8 h/day for up to 7 weeks) was clearly lethal
or even significantly harmful. The death of experimental animals
(rats) at this highest dose was not associated with neurological signs
and appeared to result exclusively from bronchopneumonia (Altenkirch
et al., 1978, 1979). These authors did not comment on possible
connections between bronchopneumonia and exposure to 17 700 mg/m3.
Female rats exposed to 14 750 mg/m3 (5000 ppm) 6 h/day, 5 days per
week, for 90 days showed only slightly increased liver weight,
slightly decreased brain and spleen weights, and slightly altered
blood chemistry in comparison with controls. Male rats receiving this
exposure showed only a slightly increased liver weight. At lower
concentrations of MEK (3688 and 7375 mg/m3 (1250 and 2500 ppm))
there was only slightly increased liver weight for female rats and no
significant differences for males in comparison with controls
(Cavender et al., 1983). In another subchronic inhalation study
(Toxigenics, 1981), male and female rats were exposed to MEK
concentrations of 3700, 7430 and 14 870 mg/m3 (1254, 2518 and 5041
ppm) 6 h/day, 5 days/week, for 90 days. No significant effects on food
consumption, eyes or nervous system were observed. In addition, no
MEK-induced morphological changes in the central or peripheral nervous
system were detected. Lower levels of exposure resulted in few
measurable effects. Inhalation of 2242 and 2360 mg/m3 (760 and 800
ppm) 6 h/day, 5 days/week, for 4 weeks by rats caused some enlargement
of the liver and slightly modified the in vitro metabolism of liver
microsomes (Nilsen & Toftgard, 1980; Toftgard et al., 1981). Ten
intraperitoneal injections of 0.034 g/kg over 2 weeks produced no
effect on the kidney (Bernard et al., 1989). However, exposure of rats
to 590 mg/m3 (200 ppm) 12 h/day for 24 weeks transiently decreased
nerve conduction velocity after 4 weeks (Takeuchi et al., 1983).
Geller et al. (1978) reported that exposure of baboons to 295 mg/m3
(100 ppm) for 7 days increased the response time in a delayed "match
to sample" task. However, this effect was transient and disappeared
during the course of repeated exposure. Following intermittent
exposure of rats to 3319 g/m3 for up to 5 months there was no
morphological evidence of peripheral neurotoxicity (Saida et al.,
1976). It is possible that the transient nature of the neurological
and behavioural changes induced by MEK exposure may be due to
behavioural and/or physiological adaptations. The latter may reflect
more rapid metabolism of MEK with prolonged exposure.
Short-term dermal exposure to small amounts of MEK resulted, at
most, in mild local irritation. Two topical applications of an
unstated amount to the ears of various strains of mice (Swiss, Balb/c,
CBA, C5681/6, DBA/2, B6D2F1) produced no significant swelling or other
signs (Descotes, 1988). In rabbits and guinea-pigs, a dose of 0.08 g
applied to the skin, without covering, once a day for 10 days to the
same site resulted in a slight to moderate increase in skinfold
thickness, whereas a much smaller dose (8 mg) applied to the same site
in rabbits 3 times a day for 3 days resulted in barely detectable
irritation (Wahlberg, 1984a). Open application of MEK to the shaved
flanks of guinea-pigs for 3 days produced slight erythema, epidermal
thickening and dermal cell infiltration (Anderson et al., 1986). In
the cat, injection of 150 mg MEK (99.98% pure) per kg, twice a day, 5
days/week, for up to 8.5 months, into subcutaneous tissue produced
abscesses, skin ulceration and generalized weakness but no evidence of
damage to the nervous system (Spencer & Schaumburg, 1976). In the one
long-term dermal study of MEK (Horton et al., 1965), 8 mg dissolved in
water was applied by dropper or brush to clipped skin of mice twice a
week for a year. Few details were given of the results because this
was the control for a study on carcinogenesis of the skin in which a
MEK/water solution was the solvent for compounds under test. Horton et
al. (1965) stated that the control mice did not develop skin tumours,
and they did not mention any adverse effects from this prolonged
application of MEK.
Table 12. Repeated exposure of mammals to MEKa
Route Species (strain), Exposure and range Results Reference
number and sex
Inhalation rat (Wistar), 590 ± 118b mg/m3 (200 ± 40b transient differences in nerve conduction claimed Takeuchi et al.
8 males ppm) 12 h/day for 24 weeks after 4 weeks, but no significant differences (1983)
Inhalation rat (Albino), 693 ± 77b mg/m3 (235 ± 26b no significant gross or microscopic La Belle &
25, sex ppm) 7 h/day, 5 days/week pathological changesc Brieger (1955)
unspecified for 12 weeks
Inhalation rat (Wistar), 885 mg/m3 (range 867-894) no significant change in leucocyte or serum Li et al. (1986)
7 females (300 ppm (range 294-303)) alkaline phosphatase activity
8 h/day for 7 days
Inhalation rat (Sprague- 3322 and 7723 mg/m3 (1026 at 7723 mg/m3 minor effects on dams, decreased Schwetz et al.
Dawley), and 2618 ppm) 7 h/day food consumption and weight gain, and increased (1974)
pregnant, 25 per for 10 days (gestation water consumption; no effects on dams at 1180 mg/m3
concentration days 6-15)
Inhalation rat (Sprague- 2242 and 2360 mg/m3 (760 and significant enlargement of liver; no effect on Nilsen &
Dawley), 4 800 ppm), 6 h/day, 5 days total liver microsomal concentration of cytochrome Toftgard (1980);
male/concentration per week for 4 weeks P-450; in vitro liver microsomal metabolism of Toftgard et al.
biphenyl unaffected, but slight effects on the (1981)
metabolism of benzo[a]pyrene, 4-androstene-3,17-
dione, and 5alpha-androstane-3alpha,17ß-diol
Inhalation rat (Fischer- 3688, 7375, 14 750 mg/m3 females at 14 750 mg/m3 increased in liver weight, Cavender et al.
344), 15 males, (1250, 2500, 5000 ppm) smaller braind and spleene weight, slightly altered (1983)
15 females/ 6 h/day, 5 days/week for blood chemistryf; females at 3688, 7375 mg/m3
concentration 90 days nonsignificant increase in liver weight; males at
14 750 mg/m3 increased in liver weight. No pathological
lesions, including peripheral nerves, attributed to
MEK exposure. No effect on reproductive tissues
(testis, epididymis, seminal vesicle, vagina, cervix,
uterus, oviduct, ovary)
Table 12 (contd)
Route Species (strain), Exposure and range Results Reference
number and sex
Inhalation rat (Fischer- 3699, 7430, 14 870 mg/m3 elevated group mean body weights at 3699 and 7430 Toxigenics
344), 15 males, (1254, 2518, 5041 ppm) mg/m3; highest concentration: decreased group mean (1981)
15 females/ 6 h/day, 5 days/week for body weight, increased liver weight, liver/body
concentration 90 days weight ratio and liver/brain weight ratio, increased
mean corpuscular haemoglobin; highest concentration
(males): increased kidney/body weight ratio, decreased
spleen and brain weights; highest concentration
(females): decreased brain/body weight ratio, increased
kidney/brain weight ratio; no significant effects at
any concentration on food consumption, eyes, nervous
system, and no morphological changes in central or
peripheral nervous systems
Inhalation rat (Sprague- 3319 mg/m3 (1125 ppm) 24 h no morphological effects on peripheral nerves Saida et al.
Dawley), 36 of per day for 16 days to 5 (1976)
unspecified sex months
Inhalation rat (Wistar), 29 500 mg/m3 (10 000 ppm) for severe irritation of upper respiratory tract; slight Altenkirch et
5 males a "few days"; 17 700 mg/m3 loss of weight; death during seventh week from al. (1978,
(6000 ppm) ± 15% for 8 h/day, bronchopneumonia; no neurological signs 1979)
7 days/week for 15 weeks
Inhalation guinea-pig, 693 ± 77b mg/m3 (235 ± 26b no significant effects La Belle &
15 of unspecified ppm) 7 h/day, 5 days/week for Brieger (1955)
sex 12 weeks
Inhalation baboon, 295 mg/m3 (100 ppm) for 7 days no impairment of discrimination in a behavioural Geller et al.
4 males test, slight increase in response time early in (1978)
experiment, but not at end of experiment
Table 12 (contd)
Route Species (strain), Exposure and range Results Reference
number and sex
Intra- rat (Sprague- 0.034 g/kg, 5 days/week for no effect on kidney function Bernard et al.
peritoneal Dawley), 2 weeks (1989)
female, number
unspecified
Subcutaneous cat, 6 of 0.15 g/kg twice/day, 5 days abscesses, skin ulceration and generalized weakness Spencer &
unspecified sex per week for up to 8.5 months in some animals; no damage to nervous Schaumburg
system structure or function (1976)
Dermal mouse (Swiss), unstated amount applied twice no significant swelling of treated ear Descotes (1988)
12 of unspecified to one ear
sex
Dermal mouse 8 mg (50 mg of 17% solution) no papilloma evident after 1 year Horton et al.
(C3H/He) applied to clipped skin twice (1965)
10-25 males per week for 1 year
Dermal guinea-pig, 3 80 mg rubbed into skin at slight to moderate increase in skinfold thickness Wahlberg (1984a)
and rabbit, 4, same site once daily for at end of 10 days
of unspecified 10 days
sex
Dermal guinea-pig 80 mg applied to 1.0 cm2 on no reaction until day 2; at end of experiment, Anderson et al.
10 females shaved flank 3 times daily slight redness and increase in dermal inflammatory (1986)
for 3 days cell count
a Values from the literature recalculated as ppm, mg or g/kg
b Standard deviation of the mean
c Depressed growth (average weight 70% of controls at end of experiment) may be due to causes other than MEK exposure; animals showed signs
of "vitamin deficiency" (no details given) and infection in latter part of experiment.
d Significantly different at the < 0.01 level from 0, 3688 and 7375 mg/m3 (0, 1250, and 2500 ppm) female group values
e Significantly different at the < 0.05 level from 0, 3688 and 7375 mg/m3 (0, 1250, and 2500 ppm) female group values
f Females in the 14 750 mg/m3 (5000 ppm) group had a small but significant elevation in serum potassium, alkaline phosphatase and glucose
levels, and a significant reduction in serum glutamic-pyruvic transaminase level.
7.3 Neurotoxicity
7.3.1 Behavioural testing
Neurotoxicity studies have been carried out on MEK, usually as
part of studies on the neurotoxicity of methyl isobutyl ketone (MIBK)
and MIBK/MEK mixtures.
An increase in response rate (lever pressing) was reported in a
group of six adult Sprague-Dawley rats (sex unspecified) exposed to
MEK at various concentrations between 74 and 2360 mg/m3 (25 and 800
ppm) for 2 h at approximately weekly intervals. An increase in
response rate also occurred in a group of four rats exposed to 74
mg/m3 (25 ppm) for 6 h at 2-day intervals (Garcia et al., 1978).
Geller et al. (1978) examined behavioural effects (match to
sample (MTS) test) in baboons exposed to MEK by inhalation. Four young
male baboons (2 years old) were exposed continuously to MEK at a
concentration of 295 mg/m3 (100 ppm) for 7 days. There were no
effects on performance of the test in terms of the ability to
discriminate visual stimuli but reaction time increased. However, in
two of the baboons, response times returned to pre-exposure control
values by day 7.
Tham et al. (1984) examined the vestibulo-oculomotor reflex (VOR)
during intravenous infusion of MEK into the caudal veins of 18 female
Sprague-Dawley rats. The threshold for depression of the VOR was an
infusion rate of 70 µmol/kg (0.005 g/kg per min) for 1 h (total dose
30 mg) and the associated arterial blood level was 1.4 mmol/litre.
General depression of the central nervous system followed depression
of the VOR.
Glowa & Dews (1987) exposed continuously by inhalation a group of
12 adult male white mice (Charles River CD1) to concentrations of MEK
that were increased at 30 min intervals until the mice failed to
respond to a visual stimulus. The concentrations, in ascending order
for each 30 min, were 885, 2950, 8850, 16 520 and 29 500 mg/m3 (300,
1000, 3000, 5600 and 10 000 ppm) with a total exposure time of 2´ h.
Mice responded to a visual stimulus and the response rate was used as
an indicator. There was no effect at a concentration of 885 mg/m3,
a slight decrease in response rate at 2950 mg/m3 and a 75% decrease
at 8850 mg/m3. Most mice ceased to respond at 16 520 mg/m3 and all
failed to respond at 29 500 mg/m3. The response rate returned to the
control value 30 min after exposure ended. The EC50 (concentration
decreasing response rate by 50%) was calculated to be 8528 (SD = 2033)
mg/m3 (2891 (SD = 689) ppm). An EC10 was calculated and
dose-response estimates were derived. The concentrations of MEK
producing a 10% decrease in response rate in 0.1%, 1% and 19% of a
population were 50, 195 and 885 mg/m3 (17, 66 and 300 ppm),
respectively.
Couri et al. (1977) exposed continuously by inhalation young male
Wistar rats to 2210 mg MEK/m3 (750 ppm) for either 7 or 28 days. In
those exposed for 7 days there was a significant reduction in
hexobarbital sleep times. In the group exposed for 28 days the
reduction in sleep times was less marked.
7.3.2 Histopathology
In chickens, cats, rats and mice exposed by inhalation to 3975
mg/m3 (1500 ppm) for periods of up to 12 weeks, there was no
evidence of neuropathy and no histopathological changes were reported
(Couri et al., 1974).
Saida et al. (1976) exposed groups of 36 Sprague-Dawley rats (sex
unspecified) continuously to MEK at a concentration of 3319 mg/m3
(1125 ppm) for periods of 16, 25, 35 and 55 days. Additional studies
were carried out with up to 5 months of exposure. There were no
abnormal clinical observations in any group. At the end of the
exposure period, rats were sacrificed and the sciatic nerve and foot
muscle excised. Spinal cord and dorsal root ganglion specimens were
taken from the same rats. Quantitative histology
(neurofilaments/µm2; frequency of inpouching of myelin sheath,
denuded axons/mm2) showed no abnormality in rats exposed for up to
5 months.
Cavender et al. (1983) reported no neurological abnormalities in
Fischer-344 rats in a 90-day inhalation study on MEK alone. Groups of
15 male and 15 female rats were exposed 6 h/day, 5 days/week, for 90
days to MEK concentrations of 3688, 7375 and 14 750 mg/m3 (1150,
2500 and 5000 ppm). All rats were observed twice daily for clinical
signs. At the end of the exposure period, the eyes of each animal were
examined by ophthalmoscopy, and neurological function (posture, gait,
tone and symmetry of facial muscles, and pupillary, palpebral,
extensor-thrust and cross-extensor thrust reflexes) was evaluated. No
abnormalities were found. Necropsy, including histopathology of the
sciatic and tibial nerves, was carried out on all rats, with special
neuropathological studies on the medulla, sciatic and tibial nerves in
5 male and 5 female rats from each group. There were no changes
attributable to MEK.
7.4 Developmental toxicity
Schwetz et al. (1974) exposed 44 pregnant rats from days 6-15 of
gestation (sperm = day 0) to two concentrations of MEK vapour, i.e.
nominally 2950 mg/m3 in 23 rats and 8850 mg/m3 in 21 rats (1000
and 3000 ppm), for 7 h/day; 43 rats were air-exposed as controls. The
average values for measured concentrations in this study were 3322 and
7723 mg/m3 (1126 and 2618 ppm), respectively. There was no evidence
of maternal toxicity. Fetal weight and crown-rump length were
significantly decreased at 3322 mg/m3, but not at 7723 mg/m3. At
3322 mg/m3 there was also a significant increase, compared to the
controls, in the number of litters with fetuses showing skeletal
anomalies, and at 7723 mg/m3 a significantly increased incidence of
litters with sternebral and soft tissue anomalies was reported. Four
grossly malformed fetuses were found (two with brachygnathia and two
acaudate with imperforate anus), all in different litters in the 7723
mg/m3 group. These malformations had not been observed previously in
more than 400 control litters of this strain. Fetal body dimensions,
however, were not significantly different from controls. When the data
were analysed on a per litter basis, there was evidence of a
teratogenic effect.
However, the incidence of major malformations in these studies
was sufficiently low that evidence for teratogenic effects was
considered questionable, and the studies were repeated (John et al.,
1980; Deacon et al., 1981). The methodology was identical except for
the inclusion of an additional level of exposure, nominally 1180
mg/m3 (400 ppm). Average measured MEK concentrations during the
experiment were 1215, 2956 and 8865 mg/m3 (412, 1002 and 3005 ppm).
The only evidence for maternal effects was decreased weight gain and
increased water consumption (no figures given) by dams exposed to 8865
mg/m3. None of the dosages had significant effects on the incidence
of pregnancy, incidence of resorption, average number of implantations
and live fetuses per dam, fetal weight and length, or incidence of
external or soft tissue abnormalities. There were statistically
significant differences in the incidences of some skeletal anomalies
occurring in the 8875 mg/m3 group compared to the controls. There
were increased incidences of lumbar ribs and delayed ossification of
the cervical centra, but a decreased incidence of delayed ossification
of the skull. Since these skeletal abnormalities occurred at low
incidences among the population from which the experimental animals
were drawn, the results of this study were interpreted as indicating
a low level of fetotoxicity and no evidence for embryotoxic or
teratogenic effects for MEK at exposure levels up to 8865 mg/m3.
In a further study (Mast et al., 1989; Schwetz et al., 1991),
groups of 10 virgin female Swiss CD1 mice and 33 plug-positive (day 0)
females were exposed by inhalation on gestation days 6-15 to mean
concentrations of 1174 ± 27, 2980 ± 83 and 8909 ± 233 mg/m3 (398 ±
9, 1010 ± 28 and 3020 ± 79 ppm). There was no evidence of maternal
toxicity, although there was a slight, treatment-related increase in
liver/body weight ratios that was significant at the highest dose
level. Mild fetal toxicity was evident at this maternal dose level as
a reduction in mean fetal body weight, statistically significant for
males. There was no increase in the incidence of intrauterine death,
but there was an increased dose-related incidence of misaligned
sternebrae, statistically significant at the highest dose level. There
were no significant increases in the incidence of malformations,
although there were several malformations in one litter (cleft palate,
fused ribs, missing vertebrae, syndactyly) in treated groups but not
in the control group nor in contemporary control data. On the basis of
this study it was concluded that the no-observed-adverse-effect level
(NOAEL) was 2978 mg/m3 (1010 ppm) and the
lowest-observed-adverse-effect level (LOAEL) was 8096 mg/m3 (3020
ppm).
7.5 Mutagenicity and related end-points
Short-term genotoxicity tests in vitro and in vivo are
summarized in Table 13. Although MEK has given negative results in
most conventional assays, Zimmermann et al. (1985) found that MEK and
certain other polar aprotic solvents were strong inducers of
aneuploidy in the yeast. The induction of aneuploidy by MEK was
markedly potentiated by coexposure to ethyl acetate (Mayer & Goin,
1988) or with nocodazol (methyl [5- (2-thienyl-carbonyl)-1
H-benzimidazol-2-yl]-carbamate) (Mayer & Goin, 1987).
O'Donoghue et al. (1988) conducted mutagenicity studies on MEK.
The test systems comprised the Salmonella/microsome (Ames) assay, the
L5178/TK+/- mouse lymphoma (M/L) assay, the BALB/3T3 cell
transformation (CT) assay, unscheduled DNA synthesis (UDS) and the
micronucleus test. MEK was not found to be genotoxic in these assays.
Other studies of MEK utilizing cultures of mammalian cells as
test systems also yielded little or no evidence of mutagenicity and
related effects. Perocco et al. (1983) tested MEK and other important
industrial chemicals at concentrations of 10-2 to 10-4 mol/litre
(0.72 to 0.0072 g MEK/litre) with an in vitro system utilizing
cultures of human lymphocytes to determine toxicity and ability to
inhibit DNA synthesis. Cultures were grown both with and without S9
mix derived from phenobarbital-induced rat liver. MEK at the
concentrations tested showed no evidence of cytotoxic or genotoxic
action. Chen et al. (1984) examined the effects of MEK on metabolic
cooperation between 6-thioguanine-resistant and
6-thioguanine-sensitive Chinese hamster lung fibroblast V79 cells and
obtained equivocal results. Holmberg & Malmfors (1974) also found some
evidence of MEK cytotoxicity to ascites tumour cells cultured with the
solvent for up to 5 h. Although there was no significant increase in
irreversibly injured cells at MEK concentrations of 0.05 and 0.1
g/litre, there was a moderate increase in damaged cells at 0.1
g/litre. An ultrastructural study (Veronesi, 1984) utilizing a medium
containing relatively high concentrations of MEK (0.3 g/litre)
produced axoplasmic granularities in a few cultures. The relationship
of this effect to possible MEK-induced neurotoxicity in vivo is not
clear.
7.6 Carcinogenicity
No long-term carcinogenicity studies have been reported.
Table 13. Short-term genotoxicity tests on MEK
Method Concentration Experimental conditions; comments Results References
In vitro studies
Bacterial assays 3 µmol/plate Salmonella typhimurium TA98, TA100, TA1535, - Florin et al. (1980)
TA1537 with and without S-9
10 mg/plate S. typhimurium TA98, TA100, TA1535, - Nestmann et al. (1980)
TA1537 with and without S-9
approx. S. typhimurium TA104: maximum non-toxic - Marnett et al. (1985)
1 mg/plate dose > 3 µmol
0.05-32 µl/plate S. typhimurium TA98, TA100, TA1535, - O'Donoghue et al. (1988)
TA1537, TA1538 with and without S-9
4 mg/plate Escherichia coli WP2 and WP2 uvrA - Brooks et al. (1988)
Mitotic gene 5 mg/ml Saccharomyces cerevisiae (JD1) - Brooks et al. (1988)
conversion assay
Induction of mitotic 3.54% S. cerevisiae (D61.M) + Zimmermann et al. (1985)
aneuploidy
0.50-1.96% S. cerevisiae (D61.M) + Mayer & Goin (1987)
Chromosome assay 1 mg/ml rat liver RL4 cells - Brooks et al. (1988)
Cell transformation 9-18 µl/ml BALB/3T3 - O'Donoghue et al. (1988)
UDS test 0.1-5.0 µl/ml primary rat (Sprague-Dawley) hepatocyte - O'Donoghue et al. (1988)
Table 13 (contd)
Method Concentration Experimental conditions; comments Results References
In vivo studies
Micronucleus test 1.9 ml/kg CD1 mice (male and female), - O'Donoghue et al. (1988)
intraperitoneal time: 12, 24, 48 h
411 mg/kg Chinese hamster, time: 12, 24, 48, 72 h - Basler (1986)
intraperitoneal
8. EFFECTS ON HUMANS
8.1 General population exposure
The only record of non-occupational acute toxicity from MEK was
a case of accidental self-poisoning (Kopelman & Kalfayan, 1983). A
47-year old woman inadvertently ingested an unknown amount of MEK and
was found unconscious, hyperventilating, and suffering from severe
metabolic acidosis. Her plasma concentration of MEK was 950 mg/litre.
She responded promptly to an infusion of sodium hydrogen carbonate and
was discharged from the hospital after a week. The metabolic effects
of MEK ingestion by humans are not well characterized and it is
uncertain that the acidosis was produced by MEK.
8.2 Effects of short-term exposure
There are limited data on behavioural and other effects on humans
of short-term exposure to MEK. Nakaaki (1974) found that exposure to
266-797 mg/m3 (90-270 ppm) for up to 4 h per day caused his subjects
to underestimate times of 5 to 30 seconds. Dick et al. (1984, 1988,
1989), on the other hand, found that a 4-h exposure of human subjects
to 590 mg/m3 (200 ppm) had no significant effect in a variety of
behavioural tests. These included psychomotor, visual vigilance, dual
task, sensorimotor and psychological tests. Solvent mixtures of 295 mg
MEK/m3 (100 ppm) and 186 mg toluene/m3 (50 ppm), and of 295 mg
MEK/m3 (100 ppm) and 298 mg acetone/m3 (125 ppm) similarly had no
significant effect on the results of these behavioural tests.
8.3 Skin irritation and sensitization
MEK (0.1 ml) rubbed into volar forearm skin daily for 18 days and
left uncovered did not produce persistent erythema or swelling
(Wahlberg, 1984a). A 5-min contact with 1.5 ml of analytical grade MEK
confined to a 20-mm circle on the forearm produced a temporary
whitening of the skin, but no visible erythema, alteration in
cutaneous blood flow or other indication of irritation to the skin
(Wahlberg, 1984b).
A male painter developed dermatitis 18 months after commencing
spray painting using an epoxy-polyamide paint (Varigos & Nurse, 1986).
A patch test with "a small amount" of MEK applied to areas of skin
3 cm in diameter on each forearm caused these areas of skin to turn
bright red within 10 min. The spots were itchy, but there was no
induration or oedema. The reaction reached its maximum after 15 min
and then gradually faded. The test was repeated after 2 days, and gave
the same results. Application of the same grade of MEK to normal
forearm skin of five male volunteers produced no reaction.
8.4 Occupational exposure
8.4.1 MEK alone
There is no record that MEK toxicity has ever caused death or a
large scale industrial accident, and only one acute occupational
poisoning has been ascribed to MEK. An 18-year-old seaman with good
vision and no previous eye problems was exposed to MEK vapour of
unknown concentration while stripping paint, and promptly noted
headache, mild vertigo and blurred vision (Berg, 1971). The diagnosis
was retrobulbar neuritis. He was given vitamin B complex and steroid
therapy, and his vision returned to normal in 36 h. However, blood
analysis exhibited a positive methanol content (no value reported)
according to the criteria given by Kaye (1961). Thus a potentiation of
the effects due to combined exposure to MEK and methanol cannot be
excluded.
Data for occupational poisoning ascribed to chronic exposure to
MEK in the absence of other solvents are equally limited. Long-term
exposure of 51 Italian workers to MEK produced indications of
neurotoxicity with slightly, but not significantly, reduced nerve
conduction velocities and various other symptoms such as headache,
loss of appetite and weight, gastrointestinal upset, dizziness,
dermatitis and muscular hypotrophy, but no clinically recognizable
neuropathy (Freddi et al., 1982). There has been a brief report of
chronic exposure of American workers, in a factory producing coated
fabric, to 885-1770 mg MEK/m3 (300-600 ppm) in the apparent absence
of other solvents (Smith & Mayers, 1944). Workers complained of
dermatoses and numbness of fingers and arms.
8.4.2 MEK in solvent mixtures
It was reported that MEK was commonly present as part of solvent
mixtures containing hexane, and that the TLV for hexane (148 mg/m3,
50 ppm) was exceeded in 89 of the work stations (mainly in shoe
factories) (De Rosa et al., 1985). MEK potentiates the toxicity of
hexane and these authors concluded that in these shoe factories the
risk of neurotoxicity was extremely high. Observations by Tangredi et
al. (1981), Brugnone et al. (1981) and Cresci et al. (1985) supported
the widespread nature of this health problem in Italian industry,
especially shoe factories. Arques Espi & Quintanilla Almagro (1981)
also found that mixtures of solvent vapours including MEK and hexane
posed an excessive risk in 95 of 114 work stations examined in a study
of Spanish shoe factories. In a nationwide survey of Japanese
factories, Inoue et al. (1983) found that MEK is widely used as a
component of solvent mixtures. In a study of electronic parts plants
in the USA (Lee & Parkinson, 1982), workers were found to be exposed
to mixtures of solvent vapours containing MEK and as many as nine
other components, although not hexane or other solvents whose toxic
action MEK is known to potentiate. Observations on Finnish car
painters (Husman, 1980; Husman & Karli, 1980) suggested that long-term
exposure to complex solvent mixtures whose components individually and
jointly are far below the legal concentration limits may produce
significant adverse effects. Noma et al. (1988) similarly suggested
that complex mixtures of volatile organic compounds, rather than a
high concentration of any single compound, may be responsible for
unhealthy air in buildings.
Descriptions of the effects of occupational exposures to mixtures
of solvent vapours not necessarily potentiated by MEK are summarized
in Table 14. There are only two cases of acute occupationally related
toxicity from such mixtures, and only a limited number of adequately
documented cases of adverse effects from chronic occupational exposure
which did not involve potentiation of hexacarbon toxicity. The only
acute cases involved two young women waterproofing seams of raincoats
with resins dissolved in acetone or MEK (Smith & Mayers, 1944).
Post-exposure measurements revealed workplace concentrations of
785-1178 mg/m3 (330-495 ppm) for acetone and 1174-1655 mg/m3
(398-561 ppm) for MEK. The total solvent concentration was estimated
to be 1000 ppm. Both women fainted at work and subsequently displayed
a number of temporary neurological and other symptoms.
However, the majority of these studies are difficult to interpret
because they lack either a quantitative description of the solvent
vapours in the work environment, or the description is based on
post-exposure analyses that may not be typical of working conditions
during most of the exposure. In these studies it is also impossible to
make any certain assessment of the role(s) played by individual
components. Mixed solvent exposures have been associated with
alteration in nerve conduction velocity (Viader et al., 1975; Dyro,
1978; Triebig et al., 1983), memory and motor alterations (Binaschi et
al., 1976), and dermatoses and vomiting (Lee & Murphy, 1982). Fagius
& Grönqvist (1978) reported neurological effects in 3 out of 42
workers exposed in a steelworks to organic solvents. However, the
actual role of MEK in these effects is not clear.
Table 14. Effects of occupational exposure to mixtures of solvent fumes containing MEK
Concentrations of MEK and other No. of workers Exposure Effects Defects in study Reference
components exposed duration
Acute
MEK 1174-1655 mg/m3 (389-561 ppm), 2 few hours eyes watering, gastric measurements of work Smith &
acetone 785-1178 mg/m3 (330-495 ppm) distress, fainting, environment limited Mayers
convulsions, twitching, to post exposure (1944)
headache, spinal pressure
Chronic
MEK 561-885 mg/m3 (190-300 ppm), 13 1 year or fatigue, frequent headache, measurements of work Lee &
toluene 139-177 mg/m3 (37-60 ppm), more dizziness, incoordination, environment limited Murphy
MIBK 37-41 mg/m3 (9-14 ppm) skin rashes, vomiting to post exposure (1982)
MEK 30 mg/m3 (10 ppm), toluene 2 2 and 6.5 paraesthesis in extremities, measurements of work Dyro
94-488 mg/m3 (25-130 ppm) years reduced motor nerve conduction environment limited (1978)
velocity, weakness in to post exposure
individuals exposed for long
periods, improvement following
cessation of exposure
MEK 62-51 mg/m3 (21-180 ppm), acetone 1 1 year altered consciousness and measurements of work Dyro
86-595 mg/m3 (36-250 ppm) EEG, reduced motor nerve environment limited (1978)
conduction velocity to post exposure
MEK 142 mg/m3 (48 ppm), isobutanol 8 not stated headache, dizziness, fatigue, measurements of work Binaschi
67 mg/m3 (23 ppm), MIBK 16 mg/m3 (4 ppm), depression, significant environment limited et al.
toluene 180 mg/m3 (48 ppm), butyl acetate reduction in short-term to post exposure; (1976)
43 mg/m3 (9 ppm), xylene 347 mg/m3 memory and hand steadiness period of exposure
(80 ppm) not indicated
Table 14 (contd)
Concentrations of MEK and other No. of workers Exposure Effects Defects in study Reference
components exposed duration
MEK 115 (avg), 2655 (max) mg/m3, n-butanol 9 8-35 significant changes in measurements of work Denkhaus
67 (avg), 1200 (max) mg/m3, isobutanol years several sub-populations of environment limited et al.
172 (avg), 3000 (max) mg/m3; 2-butoxy- peripheral blood lymphocytes: to post exposure (1986)
ethanol 25 (avg), 350 (max) mg/m3, decrease in certain type of
2-ethoxyethanol 5 (avg), 53 (max) mg/m3, T-cells and helper cells,
2-methoxyethanol 6 (avg), 150 (max) mg/m3, increase in natural killer
toluene 86 (avg), 750 (max) mg/m3, cells and B-lymphocytes
m-xylene 19 (avg), 220 (max) mg/m3,
MBK 2 (avg), 27 (max) mg/m3
MEK 9-124 mg/m3 (3-42 ppm), 5 > 10 years no significant differences measurements of work Lundberg
xylene 0-6111 mg/m3 (0-1408 ppm), in serum activities of liver environment limited &
toluene 0-1260 mg/m3 (0-336 ppm), enzymes between exposed to post exposure Hakansson
isobutanol 0-1045 mg/m3 (0-345 ppm), workers and controls (1985)
n-butanol 0-1548 mg/m3 (0-511 ppm),
ethanol 0-1094 mg/m3 (0-582 ppm),
ethyl acetate 0-2095 mg/m3 (0-582 ppm),
n-butyl acetate 0-1691 mg/m3 (0-356 ppm),
methyl acetate 3-181 mg/m3 (1-60 ppm),
white spirit 2-30 mg/m3 (1-17 ppm),
methylene chloride 10-2460 mg/m3 (3-707 ppm),
isopropanol 5-260 mg/m3 (2-106 ppm),
exposure to 2-8 solvents plus MEK
MEK < 9-401 mg/m3 (< 3-136 ppm), 66 average significant reduction in measurements of work Triebig
xylene < 4-82 mg/m3 (< 1-19 ppm), 5 years sensory conduction in environment limited et al.
toluene 11-551 mg/m3 (3-147 ppm), comparison with controls, no to post exposure (1983)
ethyl acetate < 11-302 mg/m3 (< 3-84 ppm) suggestion of neuropathy,
trichloroethane 11-601 mg/m3 (2-110 ppm) change in conduction velocity
correlated with exposure
Table 14 (contd)
Concentrations of MEK and other No. of workers Exposure Effects Defects in study Reference
components exposed duration
MEK 15-620 mg/m3 (5-210 ppm), acetone 17 not stated no health problems associated measurements of work Cohen &
< 119-495 mg/m3 (< 50-208 ppm), xylene with exposure to MEK environment limited Maier
< 22 mg/m3 (< 5 ppm), toluene < 19-34 to post exposure (1974)
mg/m3 (< 5-9 ppm), petroleum naphtha
(concentration unknown)
MEK mainly < 443, max. 5115 mg/m3 42 0.5-8 1 likely and 2 suspected measurements of work Fagius &
(mainly < 150, max. 1734 ppm), trichloroethylene years cases of polyneuropathy, environment limited Gronqvist
mainly < 161 mg/m3 (< 30 ppm); slight loss of sensitivity to post exposure (1978)
at low levels butanol, butyl acetate, butyl to vibration correlated
diglycol, cyclohexanol, diacetal, ethyl with level of solvent ex-
glycol acetate, ethanol, isoforone, posure during preceding
methylene chloride, MIBK, toluene, 6 months
xylene, "solvesso 100 and 150"a
MEK 0-74 mg/m3 (avg, 3 mg/m3) (0-25 1006b average overall death rate below none Wen et al.
(avg. 1) ppm), toluene 4 mg/m3 (1 ppm); 21.6 years expected level, excess of (1985)
at very low levels benzene, "hexane", deaths from cancer associ-
MIBK, xylene ated with lubricating oil,
not MEK
a hydrocarbon solvent mixtures
b workers from lube oil and dewaxing plant
In a study on a group of 9 parquet-flooring workers (age, 25-58
years; exposure time, 8-35 years), Denkhaus et al. (1986) noted
significant changes in several subpopulations of peripheral blood
lymphocytes, which could constitute an early indication of a
haematological or immunological effect. Benzene was not detected in
the ambient air and no investigation was made to determine which
components of the solvent mixtures produced the observed changes in
lymphocyte populations. Anshelm Olson et al. (1981) studied the simple
reaction time (SRT) performance in a group of 42 workers (age, 18 to
52 years; employment, 0.5 to 8.1 years) from a plastic coating line of
a steel factory (the same group had previously been studied by Fagius
& Grönqvist, 1978). The study was longitudinal and covered a period of
27 months during which SRT was measured three times. Originally, the
workers had been exposed to significant concentrations of MEK (up to
4000-5000 mg/m3 in certain regular tasks) and to much lower levels
of other solvents. Five months after the completion of major
improvements in the work environment which reduced the levels of MEK
to about 20 mg/m3 (maximum of about 400 mg/m3), a second SRT
measurement was made and a third measurement was performed 15 months
later. The workers' performance on the SRT test improved over the
three measurements. Moreover, on the first occasion SRT was correlated
to the degree of exposure. The authors concluded that the workers'
central nervous functioning had been adversely affected by solvent
exposure.
Mutti et al. (1982a) carried out a study of exposure to organic
solvents in an Italian shoe factory. The exposed group consisted of 95
workers (24 males, 71 females) with an age range of 16-62 years (mean,
30.9 ± 11.7 years), and the exposure duration ranged from 1 to 25
years (mean, 9.1 ± 8 years). The approximate mean air concentrations
in the breathing zone, over a 2-year period, for a number of solvents
were: MEK, 115 mg/m3 (39 ppm); n-hexane, 317 mg/m3 (90 ppm);
cyclohexane, 315 mg/m3 (92 ppm); and ethyl acetate, 205 mg/m3
(57 ppm). The exposed workers complained of sleepiness, dizziness,
weakness, paraesthesia and hypo-aesthesia. Other neurological
symptoms, such as headache, muscular cramps, neurasthenic syndrome and
sleep disturbances, were found more often in exposed workers, but the
differences in incidence between the exposed and reference group were
not statistically significant.
Among exposed workers the mean motor nerve conduction velocity
was significantly reduced in the median and peroneal nerves but not in
the ulnar nerve. The amplitude of the motor action potential (MAP) was
significantly reduced in all nerves and its duration was increased in
the ulnar nerve. There were no significant effects on the distal
latency. The number of abnormal action potentials observed in the
median and peroneal nerves of exposed workers was significantly
increased. There was a correlation between the reduction in motor
conduction velocity and exposure.
In a follow-up study, electrophysiological measurements including
somatosensory evoked potentials (SEPS) were recorded from a group of
15 female shoe factory workers aged 19-53 years (mean age, 26.6 ± 11.4
years) with a solvent exposure duration of 2-8 years (mean, 4.5 ± 2.3
years) (Mutti et al., 1982b). The mean air concentrations for various
solvents in the breathing zone of the workers were: MEK, 177 mg/m3
(60 ppm); n-hexane, 690 mg/m3 (196 ppm); cyclohexane, 585 mg/m3
(170 ppm); and ethyl acetate, 360 mg/m3 (100 ppm).
Electrophysiological measurements in peripheral nerves showed
significant reductions in maximal motor and distal sensory nerve
conduction velocities in the median and ulnar nerves and reduced
maximal motor nerve conduction velocity in the peroneal nerve. The
latency of the sensory peak action potential was significantly
increased in the median and ulnar nerves. The amplitude of all
peripheral nerve action potentials was slightly reduced but this was
not statistically significant. There were also changes in the SEPs
with significant increase in the latency of some early component
peaks. The amplitude of some of the early peaks was significantly
reduced. The neurotoxicity was attributed primarily to n-hexane.
8.5 Carcinogenicity
In a historical prospective mortality study of 446 male workers
in two MEK dewaxing plants, with an average follow up of 13.9 years,
the observed deaths (46) were below the expected (55.51). There was a
slight deficiency of deaths from neoplasms (13 observed; 14.26
expected) but there was a significant increase of deaths from tumours
of the buccal cavity and pharynx (2 observed; 0.13 expected). However,
there were significantly fewer deaths from lung cancer (1 observed;
6.02 expected). In view of the small numbers, it was concluded that
there was no clear evidence of cancer hazard in these workers
(Alderson & Rattan, 1980).
9. EFFECTS ON OTHER ORGANISMS IN THE LABORATORY AND FIELD
9.1 Microorganisms
The effects of MEK on microorganisms have been studied in several
species important to freshwater aquatic systems. As shown in Table 15,
growth inhibition generally occurs at levels ranging from 120 mg/litre
for the cyanobacterium (blue-green alga) Microcystis aeruginosa to
4300 mg/litre for the green alga Scenedesmus quadricauda.
A number of bacterial species have also been tested, the effect
levels ranging from 10 to 5050 mg/litre. Kulshrestha & Marth (1974a-f)
conducted a series of studies to determine if MEK and other volatile
compounds associated with the flavour of raw or mildly heated milk are
able to inhibit the growth of certain pathogenic bacteria and other
bacteria important in the manufacture of fermented dairy products.
Nutrient broth laced with the organisms and MEK at levels of 1, 10,
100 and 1000 mg/litre was plated after 5, 8, 11 and 14 h of incubation
in an air-tight vessel. Results are shown in Table 15. MEK was
considered bacteriostatic to Escherichia coli, Salmonella
typhimurium, Staphylococcus aureus, Leuconostoc citrovorum and
Streptococcus thermophilus at levels as low as 10-100 mg/litre.
However, Walton et al. (1989) reported that MEK at concentrations up
to 1 g/kg dry weight of soil had little effect on the respiration of
microorganisms in moist soil. Volskay & Grady (1988) found that MEK at
1 g/litre depressed respiration of sewage sludge organisms by only
11%. Ingram (1977) noticed changes in the fatty acid and phospholipid
composition of the cell membranes of E. coli when cultured with a
sublethal (0.218 mol/litre) concentration of MEK.
Tests in fungi demonstrated that MEK has a very slight
stimulating effect on the germination of uredospores in two species of
rust (French, 1961; French et al., 1977). Growth of a mixture of other
fungal species was inhibited by about 50% by 6.4 mg MEK/g seed when
the fungi were cultured on moist wheat seed (Nandi & Fries, 1976).
Growth of a mixture of other fungal species on moist wheat seed
at 30 °C was inhibited by 50% following exposure for 5 days to
approximately 6.4 mg MEK/g seed (Nandi & Fries, 1976). Germination of
the wheat seeds was also reduced but there was no statistical
evaluation of these data.
Table 15. Effects of methyl ethyl ketone on microorganisms
Organism Species, strain Concentration Effect Reference
(mg/litre)
Prokaryotes
Cyanobacterium
(Blue-green alga Microcystis aeruginosa 120 inhibition of cell multiplication Bringmann & Kuhn (1978)
Bacteria Pseudomonas putida 1150 inhibition of cell multiplication Bringmann & Kuhn (1977a)
Photobacterium phosphoreum 5050 50% reduction in light output Curtis et al. (1982)
Escherichia coli, ML30 10 slight but not consistently Kulshrestha & Marth (1974a)
significant inhibition of growth
Escherichia coli, ML30 100 significant inhibition after 5 h Kulshrestha & Marth (1974a)
incubation
Escherichia coli, ML30 1000 10% reduction in growth Kulshrestha & Marth (1974a)
Escherichia coli, B15 72.1 65% growth inhibition after 1 h; Egyud (1967)
30% after 2 h
Salmonella typhimurium 1 & 10 slight but not consistently Kulshrestha & Marth (1974b)
significant inhibition of growth
Salmonella typhimurium 100 some inhibition of growth Kulshrestha & Marth (1974b)
Salmonella typhimurium 1000 significant inhibition of growth Kulshrestha & Marth (1974b)
Staphylococcus aureus, 100 1 no significant inhibition of growth Kulshrestha & Marth (1974c)
Staphylococcus aureus, 100 10 some inhibition of growth Kulshrestha & Marth (1974c)
Staphylococcus aureus, 100 100 & 1000 significant inhibition of growth Kulshrestha & Marth (1974c)
Table 15 (contd)
Organism Species, strain Concentration Effect Reference
(mg/litre)
Streptococcus lactis 1 no significant inhibition of growth Kulshrestha & Marth (1974d)
Streptococcus lactis 10 significant inhibition at early Kulshrestha & Marth (1974d)
stages of incubation
Streptococcus lactis 100 & 1000 significant inhibition of growth Kulshrestha & Marth (1974d)
Leuconostoc citrovorum 1 no significant inhibition of growth Kulshrestha & Marth (1974e)
Leuconostoc citrovorum 10 some inhibition of growth Kulshrestha & Marth (1974e)
Leuconostoc citrovorum 100 & 1000 significant inhibition of growth Kulshrestha & Marth (1974e)
Streptococcus thermophilus, 1 no significant inhibition of growth Kulshrestha & Marth (1974f)
ST4
Streptococcus thermophilus, 10 growth inhibition at later stages of Kulshrestha & Marth (1974f)
ST4 incubation
Streptococcus thermophilus, 100 & 1000 significant inhibition of growth Kulshrestha & Marth (1974f)
ST4
Eukaryotes
Fungi Puccinia graminis, var. tritica, not given slight stimulation to germination French (1961)
spores (wheat stem rust)
Uromyces phaseoli, (Reben), 1000 very slight stimulation to French et al. (1977)
Wint., spores (bean rust) germination
Puccinia helianthi, Schw. chr., 50 very slight stimulation to French (1984)
spores (sunflower rust) germination
Table 15 (contd)
Organism Species, strain Concentration Effect Reference
(mg/litre)
Uromyces vignae, Barcl., 500 no effect on germination French (1984)
spores (cowpea rust)
various fungi found on wheat 6.4a slight inhibition of fungal growth Nandi & Fries (1976)
seeds: Septoria nerdorum, Fusarium
nivale, Aspergillus glaucus,
Aspergillus candidus, Penicillium
sp., Alternaria, sp.
Green algae Chlorella sp. 806 no effect on chlorophyll content Dojlido (1979)
after 7 days exposure
Scenedesmus quadricauda 4300 slightly inhibited cell Bringmann & Kuhn (1978)
multiplication
Protozoa Entosiphon sulcatum 190 slightly inhibited cell Bringmann (1978)
multiplication
a mg/g seed.
9.2 Aquatic organisms
MEK has been tested on numerous species of freshwater and marine
vertebrates and invertebrates in short-term tests. The results
indicate that MEK is generally of low toxicity to aquatic animals,
with median lethal (LD50) levels ranging from 1382 to 8890 mg/litre
(Table 16). Almost all of the acute tests were conducted under static
conditions, in open vessels, with nominal measurements of MEK
concentration, all of which may underestimate the true toxicity level
of MEK. No chronic studies at low concentrations have been conducted.
Curtis et al. (1982) related the effect of MEK on the
bioluminescence of a bacterium to the 96-h LC50 in fathead minnows
in an attempt to find an inexpensive yet accurate substitute for
lethality tests. The relationship for ketones (r2 = 0.81, n = 7) is
described by the equation:
log LC50 = 0.74 (log EC50 + 0.79)
where the EC50 (5 min) is the concentration causing a 50% reduction
in light output.
This relationship predicts a 96-h LC50 for fathead minnows of
3388 mg/litre, which agrees well with the measured value (Veith et
al., 1983) of 3200 mg/litre. Similarly, LeBlanc (1984) found a highly
significant correlation (P < 0.01) between the LC50 values
reported for warm-water and cold-water fish, saltwater and freshwater
fish, marine and freshwater invertebrates, and marine and freshwater
algae. There was a remarkable similarity among acute toxicity values
across three trophic levels. Data in Tables 15 and 16 lend further
support to this conclusion.
9.3 Terrestrial organisms
9.3.1 Animals
The effect of MEK on the alarm behaviour of social insects has
been studied. It was considered inactive in producing alarm behaviour
in the ant Iridomyrmex preinosus (Blum et al., 1966), the harvester
ant Pogonomyrmex badius (Blum et al., 1971) and the honeybee Apis
Mellifera (Boch & Shearer, 1971). Alarm behaviour was measured by
the number of insects that were attracted to the chemical and was
indexed relative to the activity of natural pheromones, including
2-heptanone.
Table 16. Effects of methyl ethyl ketone on aquatic organisms
Species Concentration Effects and comments pH Temperature Hardness Reference
(mg/litre) (°C)
Crustacea
Artemia salina 1950 24-h LC50, bottles not not given not given not given Price et al. (1974)
(brine shrimp) sealed and may have lost
MEK during experiment
Daphnia magna (water flea) < 180 no discernible effect 7.21 21.0-23.0 38 mg/litre Union Carbide Corp.
CaCO3 (1980a)
Daphnia magna (water flea) < 520 24-h LC50 8 ± 0.2 not given 173 ± 13 mg/l Le Blanc (1980)
< 520 48-h LC50
< 70 no discernible effect
Daphnia magna (water flea) 1382 48-h LC50 7.21 21.0-23.0 38 mg/litre Union Carbide Corp.
(918-3349)a CaCO3 (1980a)
Daphnia magna (water flea) 2500 24-h LC0 7.6-7.7 20-22 16 ° (German) Bringmann & Kuhn (1977b)
Daphnia magna (water flea) 8890 24-h LC50 7.6-7.7 20-22 16 ° (German) Bringmann & Kuhn (1977b)
Daphnia magna (water flea) 10 000 24-h LC100 7.6-7.7 20-22 16 ° (German) Bringmann & Kuhn (1977b)
Fish
Leucissus idus melanotus 4400b LC0, period not mentioned not given not given not given Juhnke & Luedemann
(golden orfe) 4800b (1978)
Leucissus idus melanotus 4600b LC50 not given not given not given Juhnke & Luedemann
(golden orfe) 4880b (1978)
Table 16 (contd)
Species Concentration Effects and comments pH Temperature Hardness Reference
(mg/litre) (°C)
Leucissus idus melanotus 4800b LC100 not given not given not given Juhnke & Luedemann
(golden orfe) 5040b (1978)
Lebistes reticulatus 2000 disturbed behaviour not given 20 ± 1 27.5 mg/litre Dojlido (1979)
(guppy) CaCl2
Lebistes reticulatus 5700 24-h LC50, open not given 20 ± 1 27.5 mg/litre Dojlido (1979)
(guppy) containers may have lost CaCl2
MEK during test
Pimephales promelas 3200 96-h LC50 7.5 25 ± 1 42.2 mg/litre Veith et al. (1983)
(fathead minnow) CaCO3
Lepomis macrochirus < 1000 no discernible effect 7.93 21 240 mg/litre Union Carbide Corp.
(bluegill sunfish) CaCO3 (1980b)
Lepomis macrochirus 4467 96-h LC50 7.93 21 240 mg/litre Union Carbide Corp.
(bluegill sunfish) CaCO3 (1980b)
Gambusia affinis 5600 96-h LC50 7.8-8.3 room not given Wallen et al. (1957)
(mosquito fish) temperaturec
Cyprinodon variegatus 400 no discernible effect not given 25-31 not given Heitmuller et al.
(sheepshead minnow) (1981)
Carassius auratus > 5000 24-h LC50 7.0 20 ± 1 100 mg/litre Bridie et al. (1979b)
(goldfish)
a 95% confidence limits
b Values are from different laboratories
c No specific value given
MEK was found to be moderately effective as a fumigant against
the Caribbean fruit fly, Anastrepha suspensa (Davis et al., 1977).
Treatments of 790 mg/m3 (286 ppm) for 2 h or 316 mg/m3 (107 ppm)
for 7 h destroyed 100% of the larvae in naturally infected guavas,
whereas exposure to 221 mg/m3 (75 ppm) for 3 h destroyed 92% of the
larvae. Kwan & Gatehouse (1978) applied between 0.31 and 0.37 mg MEK
topically to the dorsal thorax of tsetse flies (Glossina morsitans
morsitans) weighing 17-19 mg. One day after treatment, the MEK had
a significant effect on the activity of males but not females. The
mortality in MEK-treated insects was marginally but consistently
higher than in the untreated controls. No apparent inhibitory or other
effect of MEK was noted with respect to feeding or mating. Vale et al.
(1988) found MEK to be a very effective attractant for tsetse flies
and used it in a successful control effort in which the flies were
attracted to insecticide-coated netting. Uspenskii & Repkina (1974)
reported that an unspecified dose of MEK caused an increase in the
physiological age of the tick Ixodes perculcatus, an effect that
increased the insect's sensitivity to DDT.
9.3.2 Plants
MEK has an effect on the germination of seeds of several plant
species. Nandi & Fries (1976) observed that the germination of wheat
seeds was inhibited when the seeds were treated with 6.4 mg MEK/g
seed. At this level, 10% of the experimental seeds germinated versus
60% of the control seeds. Germination of lettuce was inhibited 50% by
MEK at 12.5 (± 4) mmol/litre (equivalent to 900 ± 288 mg/litre)
dissolved in agar (Reynolds, 1977). Schulz et al. (1981) reported,
however, that a mixture of acetone and MEK had no inhibitory effect on
the growth of rye grass at concentrations up to 1 g/litre.
10. ENHANCEMENT OF THE TOXICITY OF OTHER SOLVENTS BY MEK
The principal toxic effects noted with MEK exposure stem from its
ability to potentiate the known toxicities of other solvents (Table
17). Two such interactions are described in detail below.
10.1 Hexacarbon neuropathy
10.1.1 Introduction
MEK interacts with hexacarbon compounds and potentiates their
neurotoxicity (WHO, 1991). Potentially MEK co-exposure could affect
the metabolism of the hexacarbon compounds or the toxic process by
which the hexacarbons induce the neuropathy. The critical metabolic
pathway for hexacarbon induced neuropathy is outlined in Fig. 2, and
the scheme by which the peripheral nerve axonal degradation is thought
to occur is given in Fig. 3. The metabolic pathway involves hepatic
microsomal oxidation to 2,5-hexanedione, the proximate neurotoxicant,
which is thought to induce cross-linking of neurofilaments, blockage
of transport at the node of Ranvier, and swelling from accumulated
neurofilaments proximal to the nodes and axonal degeneration distal to
the nodes.
10.1.2 Animal studies
The phenomenon of potentiation of hexacarbon neurotoxicity by MEK
has been firmly established by in vivo studies mainly on rats
(Table 17) and also has been demonstrated in tissue culture (Veronesi
et al., 1984). In every study in which the dose of n-hexane, methyl
butyl ketone (MBK), or 2,5-hexanedione (2,5-HD) was large enough and
sustained for a sufficient period, clinical signs of neural
degeneration were produced. These signs were made more severe by
co-exposure to MEK. In addition, the period prior to the onset of
symptoms was frequently shortened by co-exposure to MEK. Minimum
sustained continuous exposure concentrations that induced neuropathy
in these experiments were 295/1408 and 590/1056 mg/m3 (100/400 and
200/300 ppm) MEK/ n-hexane mixtures. An intermittent exposure (8
h/day) of rats to 2950/31 680 mg/m3 (1000/9000 ppm) MEK/ n-hexane
mixture produced severe neuropathy, whereas similar exposure to a
lower concentration, i.e. 590/1760 mg/m3 (200/500 ppm)
MEK/ n-hexane mixture yielded no evidence of hexacarbon
neurotoxicity. Intermittent exposure (8 h/day, 5 days/week) to
5900/820 mg/m3 (2000/200 ppm) MEK/MBK mixture produced some neural
degeneration and mild clinical signs. Oral dosing of rats once a day,
5 days/week, with a mixture of MEK/2,5-HD (0.159/0.253 g/kg) produced
marked clinical signs (Ralston et al., 1985). Even with doses of MBK
too low to produce significant neuropathy, studies generally indicated
that co-exposure with MEK induced changes compatible with enhanced
toxicity, such as reduced velocity of nerve conduction, elevated
hepatic microsomal enzyme activity, or reduced clearance of 2,5-HD
from the blood or the body. The only study reporting no evidence of
potentiation (Spencer & Schaumburg, 1976) compared MBK at 0.150 g/kg
with one tenth this concentration of MBK, 0.015 g/kg, in combination
with MEK. Since there were no control data on the effects of 0.015
g/kg of MBK alone and the dose of MEK was very low, it is difficult to
interpret the meaning of this study. Concentrations of MEK in
inhalation studies did not exceed 3319 mg/m3 (1125 ppm), and
continuous exposure to this concentration was demonstrated by Saida et
al. (1976) not to produce neuropathological effects. Thus it is
considered unlikely that MEK itself produces neuropathy.
The property of potentiation of hexacarbon neurotoxicity is not
unique to MEK, but is shared at least by methyl n-propyl ketone,
methyl n-amyl ketone and methyl n-hexyl ketone, none of which
appear intrinsically neurotoxic (Misumi & Nagano, 1985).
The mechanism by which MEK potentiates hexacarbon neurotoxicity
is not well understood, although potential metabolic interactions have
been examined. In rats, simultaneous inhalation of n-hexane and MEK
resulted in lower levels of 2,5-HD in vivo in urine (Iwata et al.,
1984; Shibata et al., 1990a) and initially lower but later somewhat
elevated levels of MBK and 2,5-HD in vivo in serum (Shibata et al.,
1990b). Both Iwata et al. (1984) and Shibata et al. (1990b) concluded
that potentiation of n-hexane neurotoxicity by MEK could not be
explained solely by increased 2,5-HD formation. Pretreatment of rats
with MEK (1.87 ml/kg, 4 daily doses) prior to inhalation of n-hexane
resulted in higher levels of 2,5-HD in several tissues, including
blood (Robertson et al. (1989). Abdel Rahman et al. (1976) reported
that an 8-h simultaneous exposure of rats to MEK and MBK did not
result in measurable levels of MBK or of 2,5-HD in blood, whereas a
6-day continuous co-exposure resulted in substantially raised levels
of MBK and 2,5-HD. Continuous co-exposure for 23 days, however,
resulted in further elevation of MBK in blood, but no measurable level
of 2,5-HD. Simultaneous administration of 2,5-HD and MEK, either as a
single dose or as six repeated daily doses in rats, resulted in a
greater total area under the curve for 2,5-HD in blood compared with
administration of 2,5-HD alone (Ralston et al., 1985).
In guinea-pigs, increased levels of 2-hexanol and 2,5-HD were
found following intraperitoneal administration of a MEK/MBK mixture
compared with intraperitoneal administration of MBK alone (Couri et
al., 1978).
MEK administration has also been shown to induce a number of in
vivo and in vitro parameters of hepatic oxidative metabolism
(Couri et al., 1977; Wagner et al., 1983; Misume & Nagano, 1985;
Raunio et al., 1990).
Table 17. Interaction of MEK with other solvents and their metabolitesa
Route of administration, Dose and/or Exposure Effects/results Reference
species (strain), concentration
number and sex
n-Hexane
Inhalation, rat, 820 mg/m3 (200 ppm) MBK; 8 h/day, muscular weakness lasted longer after exposure to Duckett et al.
9 sex unspecified 5900/820 mg/m3 5 days/week, MEK/MBK than after MBK alone (1974)
MBK, 8 sex unspecified (2000/200 ppm) MEK/MBK 6 weeks
MEK/MBK
Inhalation, rat 1760 mg/m3 (500 ppm) 8 h/day, no significant enhancement of neuropathological Iwata et al.
(Wistar), 6 males hexane; 1475/1760 mg/m3 7 days/week, damage evident in peripheral nerve (1984)
per groupb (500/500 ppm) MEK/hexane 33 weeks electrophysiological function; 2,5-hexanedione
and other hexane metabolites in urine reduced
with co-exposure
Inhalation, rat 923 mg/m3 (225 ppm) MBK; 24 h/day, MEK enhanced MBK-induced peripheral neurotoxicity Saida et al.
(Sprague-Dawley), 3319/923 mg/m3 (1125/225 16-66 days (1976)
12/group, sex ppm) MEK/MBK
unspecified
Inhalation, rat 923 mg/m3 (225 ppm) MBK; 24 h/day, hexobarbital sleeping time significantly reduced in Couri et al.
(Wistar), 5 males 2213/923 mg/m3 (750/225 7 or 28 days group exposed continuously for 7 days to MEK/MBK (1977)
per group ppm) MEK/MBK (and in 2213 mg MEK/m3 (750 ppm) controls), but not
in MBK group; interpreted as evidence for elevated
microsomal enzyme activity in MEK/MBK and MEK
groups
Table 17 (contd)
Route
Route of administration, Dose and/or Exposure Effects/results Reference
species (strain), concentration
number and sex
Inhalation, rat 1760, 2464 mg/m3 (500, 700 approx. experiments yielded similar results: potentiation of Altenkirch et
(Wistar), 5 males ppm) hexane; 295/1408, 24 h/day, n-hexane neurotoxicity in MEK/hexane groups as al. (1982a)
per group 590/1056, 590/1760 mg/m3 7 days/week, shown by shortened period before onset of clinical
(100/400, 200/300, 200/500 9 weeks signs (weakness and paresis); hypersalination
ppm) MEK/hexane in MEK/hexane groups only; neural degeneration
in all solvent-treated groups (no mention of
differences among these groups)
Inhalation, rat 1760, 2464 mg/m3 (500, 700 8 h/day, every co-exposure to MEK/hexane resulted in earlier and Schnoy et al.
(Wistar), 5 groups ppm) hexane; 295/1408, day for 1-89 more pronounced degeneration of pulmonary (1982);
of 2-5 malesc 590/1056, 590/1760 mg/m3 days nerves than exposure to hexane alone; there were no Schmidt et al.
(100/400, 200/300, 200/500 consistent differences in degeneration of alveolar (1984)
ppm) MEK/hexane epithelium between the hexane and MEK/hexane
groups
Inhalation, rat 1640, 923 mg/m3 (400, 225 24 h/day, 2,5-HD detected in blood after 6 days but not after Abdel-Rahman
(Wistar), unspecified ppm) MBK; 2213/923 mg/m3 6 or 23 days 23 days, and there were elevated MBK blood levels et al. (1976)
number/group, male (750/225 ppm) MEK/MBK in group exposed to MEK/MBK; severe neuropathy in
group exposed to MEK/MBK for 23 days
Inhalation, rat 2464 mg/m3 (700 ppm) 8 h/day, no evidence of potentiation of hexane neurotoxicity: Altenkirch et
(Wistar), 5 males hexane; 590/1760 mg/m3 7 days/week, no abnormal clinical signs or elevated al. (1982a)
per group (200/500 ppm) MEK/hexane 40 weeks neuropathology in solvent-treated groups
Inhalation, rat 3520 mg/m3 (1000 ppm) 8 hd 2,5-hexanedione in urine markedly less after co- Iwata et al.
(Wistar), 5 males hexane; 2950/3520 mg/m3 exposure with MEK than after exposure to hexane (1983)
per group (1000/1000 ppm) MEK/hexane alone
Table 17 (contd)
Route
Route of administration, Dose and/or Exposure Effects/results Reference
species (strain), concentration
number and sex
Inhalation, rat Experiment 1: 35 200 mg/m3 8 h/day, experiments yielded similar results; acceleration Altenkirch et
(Wistar), 5 males (10 000 ppm) hexane; 3245/ 7 days/week, of n-hexane neurotoxicity in MEK/hexane group as al. (1978,
per group 31 330 mg/m3 (1100/8900 15-19 weeks shown by more severe clinical signs (gait 1979)
ppm) MEK/hexane disturbances, weakness and paresis), greater
degeneration of peripheral nerves and shortened
5 males/group Experiment 2: 35 200 mg/m3 period before onset of signs and neural degeneration;
(10 000 ppm) hexane; 2950/ hyper-salivation in MEK/hexane groups
31 680 mg/m3 (1000/9000
ppm) MEK/hexane
12 males/group Experiment 3: 35 200 mg/m3
(10 000 ppm) hexane; 2950/
31 680 mg/m3 (1000/9000
ppm) MEK/hexane
Inhalation, rat 35 200 mg/m3 (10 000 ppm) 4 h or 8 h, MEK did not enhance degeneration of intrapulmonary Schnoy et al.
(Wistar), 7 groups hexane; 2950/31 680 mg/m3 8 h/day for nerves; enhanced degeneration of alveolar epithelium (1982);
of 2-3 males (1000/9000 ppm) 2-14 days in the MEK/hexane group after 14 days exposure in Schmidt et al.
MEK/hexanee comparison with the hexane group (1984)
Inhalation, rat 352 mg/m3 (100 ppm) hexane; 12 h/day, marked impairment of nerve conduction velocities in Takeuchi et
(Wistar), 8 males 590/352 mg/m3 (200/100 7 days/week, MEK/hexane group; no evidence of significant al. (1983)
per group ppm) MEK/hexane 24 weeks potentiation of morphological effects of n-hexane
Inhalation, mouse 615 mg/m3 (150 ppm) MBK; 24 h/day, hexobarbital sleeping time significantly lower in Couri et al.
(Swiss), 5/group 2950/615 mg/m3 (1000/150 7 days MEK/MBK group than in MBK group (or in MEK 2950 (1978)
sex unspecified ppm) MEK/MBK mg/m3 (1000 ppm) controls); interpreted as evidence
for elevated microsomal enzyme activity in
MEK/MBK group
Table 17 (contd)
Route
Route of administration, Dose and/or Exposure Effects/results Reference
species (strain), concentration
number and sex
Subcutaneous injection, 288 mg/kg b.w. MBK; 1/day, 5 days significantly enhanced neurotoxicity (slower motor Misumi &
rat (Donryu), 288/288 mg/kg b.w. per week, 20 fibre conduction velocity and increased motor distal Nagano (1985)
8 males/group MEK/MBK weeks latency of tail nerve) and more pronounced weakness
in rats receiving MEK/MBK than MBK alone
Subcutaneous injection, 150 mg/kg b.w. MBK; 5 days/week peripheral neuropathological changes and paresis Spencer &
cat, 9 (sex 135/15 mg/kg b.w. for up to 8.5 with MBK; possible nerve degeneration in animals Schaumburg
unspecified) MBK, MEK/MBK months treated with MEK/MBK (1976)
4 (sex unspecified)
MEK/MBK
Other ketones
Subcutaneous injection, 150 mg/kg b.w. MIBK; 2/day, 5 days no evidence of enhanced MIBK-induced Spencer &
cat, 4, sex 135/15 mg/kg b.w. per week, up neuropathology Schaumburg
unspecified, MIBK, MEK/MIBK to 8.5 months (1976)
6, sex unspecified,
MEK/MIBK
Inhalation, rat 3220 mg/m3 (700 ppm) EBK; 16-20 h/day, exposure to MEK/EBK at 2065 and 4130 mg MEK/m3 O'Donoghue et
(Charles River), 15 207/3220, 2065/3200, 4 days produced a 2.5-fold increase in serum 2,5-Hpdn over al. (1984)
males/group for 4130/3200 mg/m3 (70/700, that produced by EBK alone; no 2,5-HD detected in
EBK and controls; 700/700, 1400/700 ppm) serum
5 males/group MEK/EBK
for MEK/EBK
Oral, rat (Charles 0.25 to 4 g/kg b.w. EBK; 1/day, 5 days 2 and 4 g EBK/kg b.w. fatal with or without MEK; O'Donoghue et
River), 4 males in 1.5, 0.75/0.25-4 g/kg b.w. per week, 14 greater neurological damage and dysfunction, and al. (1984)
control group MEK/EBK weeks around 1.5-fold increase in 2,5-Hpdn and 2,5-HD
with 1.5/1.0 g/kg b.w. MEK/EBK than with 1.0 g
EBK/kg b.w.; no neurotoxicity evident in doses
of EBK below 1.0 g/kg b.w.
Table 17 (contd)
Route
Route of administration, Dose and/or Exposure Effects/results Reference
species (strain), concentration
number and sex
Oral, rat (Fischer- 253 mg/kg b.w. 2,5-HD; 1/day, 5 days marked neurological dysfunction consistently Ralston et al.
344), 5 males/group 159/253 mg/kg b.w. per week, ca. appeared weeks earlier in rats receiving (1985)
MEK/2,5-HDf 13 weeks MEK/2,5-HD
Oral, rat (Fischer- 253 mg/kg b.w. 2,5-HD; 1/day, 1 MEK did not alter gastric absorption of 2,5-HD; Ralston et al.
344), 5 males/group 159/253 mg/kg b.w. or 7 days clearance of 2,5-HD from blood slower from rats (1985)
MEK/2,5-HD receiving MEK/2,5-HD
Oral, rat (Fischer- 253 mg/kg b.w. 2,5-HD; 1/day, 5 days most of radiolabelled 2,5-HD bound to protein; Ralston et al.
344), 5 males/group 159/253 mg/kg b.w. per week, 1, greater binding to protein with MEK/2,5-HD (1985)
MEK/2,5-HD 2 or 3 weeks after 1 week, but reverse true after 2 and 3 weeks
Halogenated alkanes
Oral, intraperitoneal, 1.505 g/kg b.w. MEK; 1 oral dose potentiation of CCl4 hepatotoxicity by MEK as Traiger &
rat (Sprague- 0.16 g/kg b.w. CCl4 MEK followed shown by greater fatty vacuolation and necrosis Bruckner
Dawley), 3-5 males/ 16 h later of liver, increased hepatic triglyceride and (1976)
experimental group, with 1 ip GPT, and decreased hepatic glucose-6-phosphatase
5-20 males/control dose CCl4
group
Oral, intraperitoneal, 1.691 g/kg b.w. MEK; 1 oral dose potentiation of CCl4 hepatotoxicity by MEK as Dietz & Traiger
rat (Sprague- 0.16 g/kg b.w. CCl4 MEK followed shown by increased hepatic triglyceride and (1979)
Dawley), males at 16 h later GPT
least 5/group with 1 ip
dose CCl4
Oral, intraperitoneal, 1.082 g/kg b.w. MEK; 1 oral dose potentiation of CHCl3 hepatotoxicity by both Hewitt et al.
rat (Sprague- 0.797, 1.196 g/kg b.w. MEK followed doses of MEK as shown by increased GPT and OCT (1983)
Dawley), 5 or 6 CHCl3 18 h later with
males/groupg 1 ip dose CHCl3
Table 17 (contd)
Route of administration, Dose and/or Exposure Effects/results Reference
species (strain), concentration
number and sex
Oral, intraperitoneal, 0.072 to 1.082 g/kg b.w. 1 oral dose potentiation of CHCl3 renal and hepatic toxicity Brown & Hewitt
neal, rat (Fischer- MEK; 0.797 g/kg b.w. MEK followed by MEK at all dosages as shown by histological and (1984)
344), 6 males per CHCl3 18 h later with several biochemical criteria; potentiation greatest
experimental 1 ip dose at 0.361 to 0.721 g/kg b.w. MEK, and slightly
group, 32 males CHCl3 reduced at 1.082 g/kg b.w.h
in control group
Oral, rat (Sprague- 1.082 g/kg b.w. MEK; 1 dose MEK MEK followed by CHCl3 did not induce cholestasis, Hewitt et al.
Dawley), 6 males 0.797 to 1.594 g/kg b.w. followed 10 but MEK given 10 to 24 h prior to CHCl3 potentiated (1986)
per group CHCl3 to 96 h later its ability to increase plasma bilirubin
with 1 dose
CHCl3
Oral, rat (Sprague- 1.082 g/kg b.w. MEK; 1 dose MEK MEK followed by CHCl3 10 to 48 h later potentiated Hewitt et al.
Dawley), 6 males 0.797 g/kg b.w. CHCl3 followed 10 hepatotoxicity as indicated by elevated ALT and (1987)
to 96 h later OCT levels
by 1 dose
CHCl3
a Values from the literature have been recalculated as ppm or g/kg body weight.
b Iwata et al. (1984) states that 24 rats were divided into four groups, but does not specify that groups were of equal numbers.
c Animals were apparently co-exposed with those described in Altenkirch et al. (1982a).
d Description of exposure not entirely clear but a subsequent paper (Iwata et al., 1984) refers to this as a single exposure.
e Animals co-exposed with those described in Altenkirch et al. (1978).
f MEK and 2,5-HD doses were 0.317 and 0.506 g/kg, respectively, for first 8 days of experiment.
g 5 males at the lower dose and 6 at the higher; 11 males in the control group
h Measurements were made 24 h after the oral dose of CHC13 or CCl4.
Abbreviations: GPT = plasma glutamic-pyruvic transaminase; OCT = plasma ornithine carbamyl transferase; ALT = plasma alanine aminotransferase;
b.w. = body weight; ip = intraperitoneal; 2,5-HD = 2,5-hexanedione; 2,5-Hpdn = 2,5-heptanedione; CCl4 = carbon tetrachloride; CHCl3 = chloroform
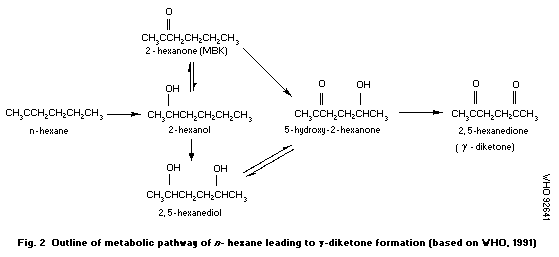
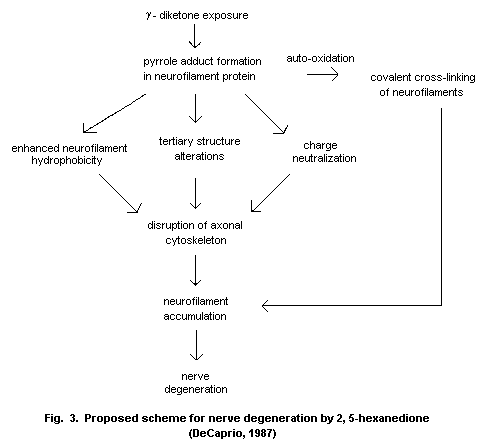 10.1.3 Human studies
10.1.3.1 Solvent abuse
Solvent abuse, the deliberate inhalation of solvent vapours for
their euphoric effects, has been reported in many countries and has
involved the use of lacquer thinners, glues and other readily
available commercial items. Prockop et al. (1974) suggested that there
were several hundred habitual "huffers", i.e. solvent abusers, in
Tampa, Florida, USA, and Altenkirch et al. (1978) reported about 2000
in Berlin, Germany, in 1974. Outbreaks of polyneuropathy among Berlin
huffers, which involved n-hexane toxicity potentiated by MEK,
provided a major stimulus for research on the health effects of MEK
and its interactions with hexacarbon solvents.
Chronic huffers in Berlin inhaled fumes from about a half litre
of liquid per day, either poured into a plastic bag or over rags
(Altenkirch et al., 1977, 1982b). Inhalation sessions extended for as
long as 10 or 12 h, and exposure periods of 5 to 7 years were not
unusual. Prior to the end of 1975 no major damage to health resulting
from chronic solvent abuse had been observed. At that time there
appeared abruptly a number of cases of polyneuropathy among chronic
huffers. All these were young males, mainly between 16 and 21 years of
age. The initial symptom was paraesthesia of the toes accompanied in
some cases by weakness of the legs. The paraesthesia ascended rapidly
from distal to proximal and in 2 to 3 weeks affected the entire legs.
This was followed rapidly by paraesthesia of the arms which also
ascended from distal to proximal. Extensor muscles were always
affected first and most severely. There also was severe muscle atrophy
and a "glove and stocking" type sensory impairment of the hands and
feet. In all cases there also was excessive sweating of the hands and
feet, and in some cases discoloration and reduced skin temperature of
these areas. In addition there was loss of weight and damage to the
teeth. Head, neck and trunk muscles remained undisturbed, although in
severe cases there was paresis of the phrenic nerve and reduced
pulmonary function. The degree of impairment ranged from moderate
crippling, which permitted walking with assistance, to complete
tetraplegia. Symptoms continued to develop for 6 to 10 weeks after
cessation of solvent abuse. Remission was slow, took up to a year,
developed from proximal to distal, and was incomplete in those most
severely affected. Neurophysiological and histological findings were
similar to those reported in experimental animals (section 7). Motor
and sensory nerve conduction velocities were reduced in proportion to
the degree of paresis. There were lesions of the axons, paranodal axon
swellings, clumping of the nerve filaments and demyelination. The
outbreak of neuropathy came a few months after a change in the
formulation of a solvent from one composed of n-hexane, benzene
fraction, ethyl acetate and toluene to a similar mixture with less
(16%) n-hexane and the addition of 11% MEK. This was interpreted as
evidence that even long exposure to the substantial amount of
n-hexane (31%) in the original formulation did not result in
neuropathy, and that neuropathy developed only after the toxicity of
n-hexane was potentiated by simultaneous exposure to MEK.
The seven cases of polyneuropathy reported from Tampa, Florida,
USA, apparently resulted from a small amount of n-hexane (0.5%)
potentiated by other components in the solvent mixture (Prockop et
al., 1974; Spencer et al., 1980). Two similar cases of polyneuropathy
were reported from Japan (Goto et al., 1974), these were produced by
chronic sniffing of a glue that contained 25% n-hexane and 20% MEK.
What is puzzling in view of the absence of neuropathy in Berlin prior
to the addition of MEK to a solvent mixture containing n-hexane is
that Goto et al. (1974) also reported two cases of neuropathy
resulting from chronic sniffing of glue solvent containing only
n-hexane and toluene. However, Oh & Kim (1976) reported a case of
polyneuropathy produced by chronic abuse of mixtures containing MEK,
methyl isobutyl ketone (MIBK) and many other solvents, but apparently
not n-hexane or MBK. There was, however, no analysis of the solvent
mixtures. There was no experimental evidence to suggest that any of
the solvents known to be present in the mixtures alone, or MEK and
MIBK together, could produce this type of neuropathy. An alternative
explanation is that the MIBK contained MBK as an impurity.
10.1.3.2 Occupational exposure
Although there have been many cases of occupationally related
poisoning by exposure to neurotoxic hexacarbon solvents (Spencer et
al., 1980), poisonings in which neurotoxicity has been associated with
concurrent MEK exposure are limited. In occupational health studies
concentrating on n-hexane neurotoxicity, the study populations were
exposed to several other solvents, including MEK (WHO, 1991). One of
the most thoroughly investigated cases occurred in 1973 in a fabric
factory in Ohio, USA (Allen et al., 1974). In 1973, an employee at the
factory was found to have a severe sensorimotor neuropathy. Other
co-workers at the plant were also found to have similar symptoms,
which initiated a search for a causative agent in the workplace. Of
the 1157 workers examined, 86 manifested signs and symptoms indicative
of peripheral neuropathy, including parathesiae in arms and legs and
weakness in the hands and legs. Subsequent investigation found that in
the year prior to the confirmation of the first case, MBK had been
substituted for MIBK as a co-solvent with MEK. Exposure occurred by
skin contact and by inhalation. Measured concentrations of MEK in
certain areas of greatest exposure were 251-2251 mg/m3 (85-763 ppm),
while concentration for MBK ranged from 9 to 640 mg/m3 (2.3-156
ppm). The introduction of MBK into the factory was associated with the
observed neurotoxicity. Spencer et al. (1980) pointed out that several
animal studies showed that concurrent exposure to MEK accelerated
MBK-induced neurotoxicity.
10.2 Haloalkane solvents
10.2.1 Studies in animals
Carbon tetrachloride (CCl4), chloroform (CHCl3) and related
haloalkane solvents are liver and kidney poisons as well as central
nervous depressants (Gosselin et al., 1984). It has long been known
that the hepatotoxic action of CCl4 is potentiated by ethanol, and
more recently that the hepatic and/or renal toxicity of CCl4,
CHCl3, trichloroethylene, 1,1,2-trichloroethane and related
compounds is potentiated by n-hexane, ethanol, isopropanol, acetone,
MEK, MBK, 2,5-HD, and other ketones or chemicals that are metabolized
to ketones (Hewitt et al., 1980). Even an increase of naturally
occurring ketones in the body via diabetes can precipitate
potentiation. Decreasing the transformation of isopropanol to acetone
by the administration of an inhibitor of alcohol dehydrogenase,
pyrazole, has reduced potentiation of haloalkane toxicity. Although
the phenomenon is referred to as haloalkane toxicity, experimental
work in general appears largely or entirely limited to chlorinated
compounds, and specific studies on MEK are limited to interactions
with CCl4 and CHCl3.
The effects of MEK and CCl4 or CHCl3 on rats are summarized
in Table 17. At the doses used, MEK and the haloalkanes separately
produced mild liver and kidney injury at most. When exposure to MEK
was followed within 10 to 48 h by a haloalkane, there was severe
injury to the liver, with marked and abrupt replacement of normal
hepatic cells by necrotic and fatty, vacuolated tissue, an increase in
hepatic triglyceride, and, presumably, a corresponding decrease in
normal liver function. Hepatic enzymes were released into the blood by
breakdown of liver tissue, resulting in elevated levels of plasma
glutamic-pyruvic transaminase, plasma ornithine carbamyltransferase,
and plasma alanine aminotransferase. There was also an increase in
plasma bilirubin, although this was not accompanied by a decrease in
bile secretion as was the case with some other ketones (Hewitt et al.,
1986). A dose of MEK as small as 0.072 g/kg potentiated the effects of
CHCl3 given 18 h later, and 1.505 g/kg MEK potentiated the effects
of CCl4. Lower doses were not studied.
The mechanism of potentiation is not fully understood but
increased bioactivation of haloalkanes is believed to play a central
part in the potentiation effect. CCl4 is metabolized in vivo with
homolytic cleavage of the carbon-chlorine bond to produce highly
reactive free radicals that exert their toxic effects a) by binding
covalently to proteins and other elements, and b) via lipid
peroxidation (Anders, 1988). It is likely that the toxic effects of
CHCl3 also are produced in part by this mechanism (Hewitt et al.,
1980, 1987). The toxicity of CCl4 is enhanced by pretreatment with
various agents such as phenobarbital, ethanol, isopropanol, 2-butanol
and the ketone metabolites of the last two compounds (acetone and MEK)
(Cornish & Adefuin, 1967; Traiger & Plaa, 1972; Traiger & Bruckner,
1976; Gosselin et al., 1984). Recent studies have shown that the
ethanol-inducible cytochrome P-450 isozyme (P-450IIE1) plays an
important role in haloalkane metabolism (Johansson &
Ingelman-Sundberg, 1985). It is a high affinity enzyme and operates at
a low concentration range for various substrates (Nakajima et al.,
1990). CCl4 hepatotoxicity in rats has been found to be potentiated
by induction of the P-50IIE1 isozyme with ethanol and the injury was
most marked in the perivenous liver cells where the expression of
induction was the highest (Lindros et al., 1990). In addition to
ethanol, P-450IIE1 is known to be induced by acetone and MEK (Ko et
al., 1987; Raunio, et al., 1990; Albano et al., 1991). However, while
a relatively large oral dose of MEK (1.4 ml for 3 days) to rats
increased the amount of ethanol- and phenobarbital-inducible
cytochromes P-450 (P-450IIE1 and P-450IIB, respectively) (Raunio et
al., 1990), inhalation exposure of rats to 1770 mg MEK/m3 (600 ppm),
10 h/day for 7 days, caused only marginal effects on microsomal
cytochrome P-450 activities in the liver (Liira et al., 1991).
10.2.2 Potentiation of haloalkane toxicity in humans
There are no reports of MEK potentiation of haloalkane renal and
hepatic toxicity in humans.
11. EVALUATION OF HUMAN HEALTH RISKS AND EFFECTS ON THE ENVIRONMENT
11.1 Human health risks
11.1.1 Non-occupational exposure
Low level non-occupational exposure to MEK is widespread from a
variety of natural and anthropogenic sources, since MEK is a normal,
though minor, mammalian metabolite. External sources include food,
water and air. In the USA, the average daily per capita intake of MEK
from food is estimated to be 1.6 mg. In addition to MEK present
naturally, foods may contain MEK that has been added in food
processing or absorbed from plastic packaging materials. MEK is rarely
detected in exposed natural waters where it may originate from
microbial activity and atmospheric and anthropogenic inputs, but is
frequently detected at low concentrations in drinking-water where it
presumably is leached from cemented joints of plastic pipes. Leaching
from landfill hazardous waste dumps is another potential source of
groundwater, and hence drinking-water, contamination. Measured
concentrations in food and water are so low, however, that it is
unlikely that either of these represent a significant source of
exposure.
In minimally polluted outdoor air, the MEK concentration is less
than 3 µg/m3 (1.0 ppb) but has been measured at 134 µg/m3 (44.5 ppb)
under conditions of dense smog. Away from industrial areas where
MEK is manufactured or used, major sources may be vehicle exhaust and
photochemical reactions in the atmosphere. In smog episodes,
photochemical production of MEK may greatly exceed direct
anthropogenic emission. Volatilization of MEK from building materials
and consumer products can pollute indoor air to levels far above
outdoor air, and a concentration as high as 48 µg/m3 (12.9 ppb) was
measured in an Italian home. MEK also is present in tobacco smoke
(e.g., 80-207 µg/cigarette).
For the general population, daily MEK intake is estimated to
range between 1.6 and 4.2 mg, depending on the location site (rural or
urban), with an additional 1.6 mg in the case of smokers. There is no
evidence of any adverse effects on the general population from
exposure to MEK. Data from experimental animal studies show that toxic
effects occur at dose levels that are 3 orders of magnitude higher
than the estimated daily intake. Non-occupational poisoning from MEK
alone is limited to a single case, which resulted in no lasting
injury.
MEK is, however, known to potentiate the toxicity of two classes
of organic solvents, unbranched aliphatic hexacarbons and haloalkanes,
and chronic exposure to consumer products containing MEK and
n-hexane have produced outbreaks of polyneuropathy among individuals
deliberately inhaling fumes from these mixtures for their euphoric
effects. Co-exposure to MEK and either hexacarbons or haloalkanes via
the abuse of consumer products remains a potential public health
hazard. Injuries from such poisoning can be severe, permanently
disabling and even fatal.
11.1.2 Occupational exposure
MEK is an important industrial chemical which is used mainly as
a component of solvent mixtures for application of a wide variety of
coatings and adhesives. Moderate occupational exposure via air is
widespread because losses to the environment result mainly from
solvent evaporation from coated surfaces, and MEK is not viewed as an
especially dangerous substance. Most national limits for occupational
exposure are set at 590 mg/m3 (200 ppm), with a higher short-term
exposure level of 885 mg/m3 (300 ppm), and these limits appear
acceptable. On site measurements, however, indicate that workers may
be chronically exposed to still higher MEK concentrations in small
factories such as shoe factories, printing plants and painting
operations, due to inadequate ventilation. Lesser amounts of MEK are
lost to the air with concurrent worker exposure during manufacture,
shipping, repackaging and preparation of coatings and adhesives.
Industrial exposure from contact with liquid MEK does not appear an
important problem.
Chronic co-exposure to MEK and either unbranched aliphatic
hexacarbon or haloalkane solvents represents a significant potential
occupational hazard. Serious toxic effects could occur. Although there
are no records of industrial accidents involving MEK potentiation of
haloalkane toxicity, MEK potentiation of hexacarbon neurotoxicity may
have caused at least one major industrial accident in which an
outbreak of polyneuropathy followed introduction of MEK into a solvent
mixture. Thus MEK, in the mixed solvent atmosphere of many industrial
activities, can present a toxic hazard.
11.1.3 Relevant animal studies
Acute MEK toxicity has been shown in animal studies to be low by
the oral and inhalation route. The lowest oral dose modifying body
structure (damage of kidney tubules) was 1 g/kg body weight in rats.
Ten intraperitoneal injections of 34 mg/kg body weight over a 2-week
period produced transient injection site irritations but no effect on
the kidney. In a 90-day inhalation study, female rats exposed to 14.75
g/m3 for 6 h/day, 5 days per week, showed only slightly increased
liver weight, slightly decreased brain and spleen weight, and slightly
altered blood chemistry in comparison with controls; male rats showed
only a slightly increased liver weight. A transient decrease in nerve
conduction velocity was found following exposure to 590 mg/m3 (12
h/day for 24 weeks). The transient nature of neurological and
behavioural changes induced by MEK may be due to adaptation or more
rapid metabolism of MEK. Short-term dermal exposure to small amounts
of MEK results in mild local irritation, at most. Results of studies
of eye irritation are inconsistent, possibly due to different scoring
techniques; 4 mg created severe chemical burns in the eye in one study
whereas in other studies less severe signs were reported following a
dose of 80 mg.
An inhalation study provided evidence for low level fetotoxicity
in the absence of maternal toxicity at 8825 mg/m3. Thus MEK may be
a low grade teratogen in rats. There is a lack of data on other
aspects of reproduction in animals, and no relevant data have been
reported for humans.
MEK has given negative results in most conventional mutagenicity
assays. There is evidence of aneuploidy in yeast but this may not be
relevant to humans or other mammals.
11.2 Effects on the environment
MEK occurs naturally at low concentrations in air, water and
soil. It is highly mobile in the natural environment and is not
accumulated in any individual compartment. MEK is rapidly synthesized
and destroyed by photochemical processes in the atmosphere. There is
no specific information on either partitioning of MEK in any
environmental compartment or on chemical binding to sediment
particles.
MEK is synthesized biologically and is rapidly metabolized by
bacteria (even at high concentrations), mammals and probably many
other organisms. Levels produced by fungi can cause inhibition of
plant germination. Observations on microorganisms, higher plants,
invertebrates, fish and mammals suggest a low level of toxicity.
Environmental levels of MEK appear to be too low to cause any damage
except in the immediate vicinity of highly polluted sites. Effects on
the aquatic environment are likely to appear at levels between 1 and
10 mg/litre. The potentiation of solvent toxicity by MEK appears
environmentally irrelevant, although substantial information is
lacking. Overall, MEK does not represent a significant threat to the
environment.
12. RECOMMENDATIONS FOR THE PROTECTION OF HUMAN HEALTH AND THE
ENVIRONMENT
12.1 Human health protection
MEK on its own appears a relatively safe organic solvent, but its
use in combination with other solvents, in particular haloalkanes or
unbranched aliphatic hexacarbons, should be avoided. Industries should
be strongly encouraged to take all necessary precautions to ensure
that workers are not exposed to both MEK and solvents whose toxicity
is potentiated by MEK.
12.2 Environmental protection
MEK is unlikely to present a hazard to the environment except in
cases of major spills or discharges.
FURTHER RESEARCH
a) Further research should be undertaken to clarify the precise
mechanisms by which MEK potentiates the toxicity of haloalkanes
and hexacarbons.
b) Epidemiological studies are needed to determine exposure-response
relationships regarding MEK-induced potentiation of hexacarbon
and haloalkane toxicity.
c) Radiolabelled balance studies should be conducted to determine
accurately the routes and rates of excretion of MEK and its
metabolites. The results of such studies would be particularly
useful for improving methods of biological monitoring.
d) Comprehensive studies of reproductive and developmental toxicity
should be undertaken in representative rodent and non-rodent
species.
e) The binding capacity of soils and sediments for MEK should be
assessed.
REFERENCES
Aarstad K, Zahlsen K, & Nilsen OG (1985) Inhalation of butanols:
Changes in the cytochrome P-450 enzyme system. Arch Toxicol, 8(Suppl):
418-421.
Aarstad K, Zahlsen K, & Nilsen OG (1986) [Effects of inhalation of
different butanol isomers.] Faerg Lack Scand, 32: 69-74 (in Norwegian
with English summary).
Abdel-Rahman MS, Hetland LB, & Couri D (1976) Toxicity and metabolism
of methyl n-butyl ketone. Am Ind Hyg Assoc J, 37: 95-102.
ACGIH (1986) Documentation of the threshold limit values and
biological exposure indices, 5th ed. Cincinnati, Ohio, American
Conference of Governmental Industrial Hygienists, p 395.
ACGIH (1991) Threshold limit values and biological exposure indices
for 1988-1989. Cincinnati, Ohio, American Conference of Governmental
Industrial Hygienists.
Ahrenholz SH & Egilman DS (1983) Health hazard evaluation: Rubbermaid
Incorporated, Wooster, Ohio. Cincinnati, Ohio, National Institute of
Occupational Safety and Health, 33 pp (HETA 3-1340).
Albano E, Tomasi A, Persson J-O, Terelius Y, Goria-Gatti L,
Ingelman-Sundberg M, & Diazani MU (1991) Role of ethanol-inducible
cytochrome P-450 (P450IIE1) in catalysing the free radical activation
of aliphatic alcohols. Biochem Pharmacol, 41: 1895-1902.
Alderson MR & Rattan NS (1980) Mortality of workers on an isopropyl
alcohol plant and two MEK dewaxing plants. Br J Ind Med, 37: 85-89.
Allen N, Mendell JR, Billmaier D, & Fontaine RE (1974) An outbreak of
a previously undescribed toxic polyneuropathy due to industrial
solvent. Trans Am Neurol Assoc, 99: 74-79.
Altenkirch H, Mager J, Stoltenburg G, & Helmbrecht J (1977) Toxic
polyneuropathies after sniffing a glue thinner. J Neurol, 214:
137-152.
Altenkirch H, Stoltenburg G, & Wagner HM (1978) Experimental studies
on hydrocarbon neuropathies induced by methyl-ethyl-ketone (MEK). J
Neurol, 219: 159-170.
Altenkirch H, Stoltenburg-Didinger G, & Wagner HM (1979) Experimental
data on the neurotoxicity of methyl-ethyl-ketone (MEK).
Experientia(Basel), 35: 503-504.
Altenkirch H, Wagner HM, Stoltenburg G, & Spencer PS (1982a) Nervous
system responses of rats to subchronic inhalation of n-hexane and
n-hexane + methyl-ethyl-ketone mixtures. J Neurol Sci, 57: 209-219.
Altenkirch H, Wagner HM, Stoltenburg-Didinger G, & Steppat R (1982b)
Potentiation of hexacarbon-neurotoxicity by methyl-ethyl-ketone (MEK)
and other substances: Clinical and experimental aspects. Neurobehav
Toxicol Teratol, 4: 623-627.
Anders MW (1988) Bioactivation mechanisms and hepatocellular damage.
In: Arias IM, Jakoby WB, Popper H, Schachter D, & Schafritz DA ed. The
liver: Biology and pathology. New York, Raven Press Ltd, pp 389-400.
Anderson C, Sundberg K, & Groth O (1986) Animal model for assessment
of skin irritancy. Contact Dermatitis, 15: 143-151.
ANONYMOUS (1978) Atmospheric hydrocarbons in air during photochemical
episodes. Kanagawa-Ken Taike Osen Chosa Kenkyu Hokoku, 20: 86-90.
Anshelm Olson B, Gamberale F, & Grönqvist B (1981) Reaction time
changes among steel workers exposed to solvent vapours: A longitudinal
study. Int Arch Occup Environ Health, 48: 211-218.
Arques Espi E & Quintanilla Almagro T (1981) [Study of gluing stations
in 50 shoe plants: Simplified method of risk evaluation and normal
work technique.] In: [Proceedings of the National Congress of
Occupational Medicine, Hygiene and Safety: 9th meeting, 1980.] Madrid,
Spain, Occupational Hygiene and Safety Service, vol 2, pp 551-561 (in
Spanish).
Attygalle AB, Cammaerts MC, & Morgan ED (1983) Dufour gland secretions
of Myrmica rugulosa and Myrmica schencki workers. J Insect
Physiol, 29: 27-32.
Azuma K, Hirata T, Tsunoda H, Ishitani T, & Tanaka Y (1983)
Identification of the volatiles from low density polyethylene film
irradiated with an electron beam. Agric Biol Chem, 47: 855-860.
Banergee S & Howard PH (1988) Improved estimation of solubility and
partitioning through correction of UNIFAC-derived activity
coefficients. Environ Sci Technol, 22: 839-841.
Basler A (1986) Aneuploidy-inducing chemicals in yeast evaluated by
the micronucleus test. Mutat Res, 174: 11-13.
Bassette R & Ward G (1975) Measuring parts per billion of volatile
materials in milk. J Dairy Sci, 58: 428-429.
Basu P, Carpenter J, Nelson H, Perry W, Shochet A, Sun C, & Taylor D
(1981) Toxic Substances Control Act (TSCA), Section 4 - Human exposure
assessment: methyl ethyl ketone. McLean, Virginia, JRB Associates,
pp 1/1-R/1 (EPA Contract No. 68-01-4839).
Berg EF (1971) Retrobulbar neuritis. Ann Ophthalmol, 3: 1351, 1353.
Bernard AM, De Russis R, Normand J-C, & Lauwerys RR (1989) Evaluation
of the subacute nephrotoxicity of cyclohexane and other industrial
solvents in the female Sprague-Dawley rat. Toxicol Lett, 45: 271-280.
Bills DD, Willits RE, & Day EA (1966) Determination of some neutral
volatile constituents in ten commercial Cheddar cheeses. J Dairy Sci,
49: 681-685.
Binaschi S, Gazzaniga G, & Crovato E (1976) Behavioral toxicology in
the evaluation of the effects of solvent mixtures. Adverse Eff Environ
Chem Psychotropic Drugs, 2: 91-98.
Blum M, Warter S, & Traynham J (1966) Chemical releasers of social
behavior - VI. The relation of structure to activity of ketones as
releasers of alarm for Iridomyrex pruinosus (Roger). J Insect
Physiol, 12: 419-427.
Blum M, Warter S, & Traynham J (1971) Alarm pheromones: utilization in
evaluation of olfactory theories. J Insect Physiol, 17: 2351-2361.
Boch R & Shearer D (1971) Chemical releasers of alarm behavior in the
honey-bee, Apis mellifera. J Insect Physiol, 17: 2277-2285.
Boettner EA, Ball GL, Hollingsworth Z, & Aquino R (1981) Organic and
organotin compounds leached from PVC and CPVC pipe. Ann Arbor,
Michigan, US Environmental Protection Agency, 116 pp
(EPA-600/1-81-062).
Botta D, Castellani Pirri L, & Mantica E (1984) Ground water pollution
by organic solvents and their microbial degradation products. In:
Analysis of organic micropollutants in water. Brussels, Commission of
the European Communities, pp 261-275 (Report EUR 8518).
Bradow JM & Connick WJ (1988) Volatile methyl ketone seed-germination
inhibitors from Amaranthus palmeri S Wats. residues. J Chem Ecol,
14: 1617-1631.
Bradow JM & Connick WJ (1990) Volatile seed germination inhibitors
from plant residues. J Chem Ecol, 16: 645-666.
Brady JF, Li D, Ishizaki H, Lee M, Ning SM, Xiao F, & Yang CS (1989)
Induction of cytochromes P450 IIE1 and P450IIB1 by secondary ketones
and the role of P450IIE1 in chloroform metabolism. Toxicol Appl
Pharmacol, 100: 342-349.
Bridie AL, Winter M, & Wolff CJM (1979a) BOD and COD of some
petrochemicals. Water Res, 13(7): 627-630.
Bridie AL, Wolff CJM, & Winter M (1979b) The acute toxicity of some
petrochemicals to goldfish. Water Res, 13: 623.
Bringmann G (1978) [Determination of the biological toxicity of water
pollutants on Protozoa.] Z Wasser Abwasser Forsch, 11: 210-215 (in
German).
Bringmann G & Kuhn R (1977a) [Toxicity threshold for water pollutants
in the cell multiplication test with respect to bacteria (Pseudomonas
putida) and green algae (Scenedesmus quadricauda).] Z Wasser
Abwasser Forsch, 10: 87-98 (in German).
Bringmann G & Kuhn R (1977b) [The effects of water pollutants on
Daphnia magna.] Z Wasser Abwasser Forsch, 10: 161-166 (in German).
Bringmann G & Kuhn R (1978) Testing of substances for their toxicity
threshold: Model organisms Microcystis (Diplocystis) aeruginosa and
Scenedesmus quadricauda. Mitt Int Ver Limnol, 21: 275-284.
Brondeau MT, Ban M, Bonnet P, Guenier JP, & De Ceaurriz J (1989)
Acetone compared to other ketones in modifying the hepatotoxicity of
inhaled 1,2-dichlorobenzene in rats and mice. Toxicol Lett, 49: 69-78.
Brooks TM, Meyer AL, & Hutson DH (1988) The genetic toxicology of some
hydrocarbon and oxygenated solvents. Mutagenesis, 3: 227-232.
Brown EM & Hewitt WR (1984) Dose-response relationships in
ketone-induced potentiation of chloroform hepato- and nephrotoxicity.
Toxicol Appl Pharmacol, 76: 437-453.
Brown RH & Purnell CJ (1979) Collection and analysis of trace organic
vapour pollutants in ambient atmospheres. The performance of a
Tenax-GC absorbent tube. J Chromatogr, 178: 79-90.
Brugnone F (1985) Uptake of solvents from the lungs. Br J Ind Med,
42: 569.
Brugnone F, Perbellini L, Gaffuri E, & Costa G (1981) [Occupational
pollution by shoe factory solvents.] Ann Ist Super Sanità, 17: 531-534
(in Italian).
Brugnone F, Perbellini L, Apostoli P, Caretta D, & Cocheo V (1983)
Environmental and behavioural monitoring of occupational methyl ethyl
ketone exposure. Dev Sci Pract Toxicol, 11: 571-574.
Bryce DJ & Greenwood CT (1963) The thermal degradation of starch. Part
III. The formation of decomposition products from starch and related
materials at temperatures between 175 and 400 °C. Stärke, 15(19):
359-363.
Cammaerts MC, Inwood MR, Morgan ED, Parry K, & Tyler RC (1978)
Comparative study of the pheromones emitted by workers of the ants
Myrmica rubra and Myrmica scabrinodis. J Insect Physiol, 24:
207-214.
Carpenter CP & Smyth HF (1946) Chemical burns of the rabbit cornea. Am
J Ophthamol, 29: 1363-1372.
Cavender FL, Casey HW, Salem H, Swenberg JA, & Gralla EJ (1983) A
90-day vapor inhalation toxicity study of methyl ethyl ketone. Fundam
Appl Toxicol, 3: 264-270.
Chemical Business Newsbase (1986) #537712 [Organic chemicals
production in Argentina 1985.] Ind Quim, 281: 36-37 (in Spanish).
Chemical Business Newsbase (1987) #584370 The chemical industry of
Japan, 1986: B-B fraction derivatives. Japan Chem Ann, 1987/1988: 41.
Chemical Business Newsbase (1988) #600843 Methyl ethyl ketone makers
need Pampa for recovery. Chem Mark Rep, 234(16): 9, 18-19.
Chen T-H, Kavanagh TJ, Chang CC, & Trosko JE (1984) Inhibition of
metabolic cooperation in Chinese hamster V79 cells by various organic
solvents and simple compounds. Cell Biol Toxicol, 1: 155-171.
Chiavari G, Facchini MC, & Fuzzi S (1987) Behavior of
3-methyl-2-benzothiazolone azines of carbonyl compounds in
high-performance liquid chromatography. J Chromatogr, 387: 459-466.
Chiou CT, Freed VH, Siddigi RH, & Mckeon K (1977) Partition
coefficients and bioaccumulation of selected organic chemicals.
Environ Sci Technol, 11: 475-478.
Clayton GD & Clayton FE (1981) Patty's industrial hygiene and
toxicology. New York, John Wiley & Sons, Wiley Interscience, 3 vols.
Cohen SR & Maier AA (1974) Occupational health case report - No. 2,
toluene diisocyanate. J Occup Med, 16: 114-118.
Conkle JP, Camp BJ, & Welch BE (1975) Trace composition of human
respiratory gas. Arch Environ Health, 30: 290-295.
Connick WJ, Bradow JM, & Legendre MG (1989) Identification and
bioactivity of volatile allelochemicals from amaranth residues.
J Agric Food Chem, 37: 792-796.
Cornish HH & Adefuin J (1967) Potentiation of carbon tetrachloride
toxicity by aliphatic alcohols. Arch Environ Health, 14: 447-449.
Corwin JF (1969) Volatile oxygen-containing organic compounds in sea
water: Determination. Bull Mar Sci, 19: 504-509.
Couri D, Hetland LB, O'Neill JJ, Ganansia M-F, Jackson DB, Gardier RW,
Marks BH, Weiss H, Mendell JR, Saida K, Allen N, & Chrisman CL (1974)
Comments on a plastics industry neurotoxicity in relationship to
methylbutyl ketone. In: Proceedings of the 5th Annual Conference on
Environmental Toxicology, Fairborn, Ohio, 24-26 September 1974. Wright
Patterson Air Force Base, Ohio, Aerospace Medical Research Laboratory,
pp 109-120 (AMRL Technical Report No. 74-125).
Couri D, Hetland LB, Abdel-Rahman MS, & Weiss H (1977) The influence
of inhaled ketone solvent vapors on hepatic microsomal
biotransformation activities. Toxicol Appl Pharmacol, 41: 285-289.
Couri D, Abdel-Rahman MS, & Hetland LB (1978) Biotransformation of
n-hexane and methyl n-butyl ketone in guinea-pigs and mice. Am Ind Hyg
Assoc J, 39: 295-300.
Creech G, Johnson RT, & Stoffer JO (1982) A comparison of three
different high-performance liquid chromatography systems for the
determination of aldehydes and ketones in diesel exhaust. Part I.
J Chromatogr Sci, 20: 67-72.
Cresci A, La Rosa F, Pannelli F, Orpianesi C, Saltamacchia G, Spinaci
G, & Pierini M (1985) [Solvent exposure and work sites in eleven shoe
and leather factories.] Nuovi Ann Ig Microbiol, 36: 61-76 (in
Italian).
Curtis C, Lima A, Lozano SJ, & Veith GD (1982) Evaluation of a
bacterial bioluminescence bioassay as a method for predicting acute
toxicity of organic chemicals to fish. In: Pearson JG, Foster RB, &
Bishop WE ed. Aquatic toxicology and hazard assessment: Fifth
Conference. Philadelphia, Pennsylvania, American Society for Testing
and Materials, pp 170-178 (ASTM STP 766).
Cushny AR (1910) On the exhalation of drugs by the lungs. J Physiol,
40: 17-27.
Davis PL, Munroe KA, & Selhime AG (1977) Laboratory bioassay of
volatile naturally occurring compounds against the Caribbean fruit
fly. Citrus Ind, July: 24-26.
Day EA, Bassette R, & Keeney M (1960) Identification of volatile
carbonyl compounds from Cheddar cheese. J Dairy Sci, 43: 463-474.
Deacon MM, Pilny MD, John JA, Schwetz BA, Murray FJ, Yakel HO, & Kuna
RA (1981) Embryo- and fetotoxicity of inhaled methyl ethyl ketone in
rats. Toxicol Appl Pharmacol, 59: 620-622.
De Bortoli M, Knoppel H, Pecchio E, Peil A, Rogora L, Schauenburg H,
Schlitt H, & Vissers H (1985) Measurements of indoor air quality and
comparison with ambient air: A study on 15 homes in northern Italy.
Ispra, Italy, Commission of the European Communities, 57 pp.
De Bortoli M, Knoppel H, Pecchio E, Peil A, Rogora L, Schauenburg H,
Schlitt H, & Vissers H (1986) Concentrations of selected organic
pollutants in indoor and outdoor air in northern Italy. Environ Int,
12: 343-350.
Decaprio AP (1987) n-Hexane neurotoxicity: A mechanism involving
pyrrole adduct formation in axonal cytoskeletal protein.
Neurotoxicology, 8: 199-210.
De Ceaurriz J, Desilies JP, Bonet P, Marignac B, Muller J, & Guenier
JP (1983) Concentration-dependent behavioral changes in mice
following short-term inhalation exposure to various industrial
solvents. Toxicol Appl Pharmacol, 67: 383-389.
Decker DW, Clark CS, Elia VJ, Kominsky JR, & Trapp JH (1983) Worker
exposure to organic vapors at a liquid chemical waste incinerator. Am
Ind Hyg Assoc J, 44: 296-300.
Delfino JJ & Miles CJ (1985) Aerobic and anaerobic degradation of
organic contaminants in Florida groundwater. Soil Crop Sci Soc Florida
Proc, 44: 9-14.
Del Rosario R, De Lumen BO, Habu T, Flath RA, Mon TR, & Teranishi R
(1984) Comparison of headspace volatiles from winged beans and
soybeans. J Agric Food Chem, 32: 1011-1015.
Denkhaus W, Von Steldern D, Botzenhardt U, & Konietzko H (1986)
Lymphocyte subpopulations in solvent-exposed workers. Int Arch Occup
Environ Health, 57: 109-115.
De Rosa E, Bartolucci GB, Brighenti F, Gori GP, Sigon M, & Toffolo D
(1985) The industrial use of solvents and risk of neurotoxicity. Ann
Occup Hyg, 29(1): 391-397.
Descotes J (1988) Identification of contact allergens: The mouse ear
sensitization assay. J Toxicol Cutan Ocular Toxicol, 7: 263-272.
Deveaux M & Huvenne JP (1987) Identification of solvents of abuse
using gas chromatography/Fourier transform infrared spectrometry after
headspace sampling. Chromatographia, 23: 626-630.
Dick RB, Setzer JV, Wait R, Hayden MB, Taylor BJ, Tolos B, &
Putz-Anderson V (1984) Effects of acute exposure of toluene and
methyl ethyl ketone on psychomotor performance. Int Arch Occup Environ
Health, 54: 91-109.
Dick RB, Brown WD, Setzer JV, Taylor BJ, & Shukla R (1988) Effects of
short duration exposures to acetone and methyl ethyl ketone. Toxicol
Lett, 43: 31-49.
Dick RB, Setzer JV, Taylor BJ, & Shukla R (1989) Neurobehavioral
effects of short duration exposures to acetone and methyl ethyl
ketone. Br J Ind Med, 46: 111-121.
Dietz FK & Traiger GJ (1979) Potentiation of CCl4 of hepatotoxicity
in rats by a metabolite of 2-butanone: 2,3-butanediol. Toxicology,
14: 209-215.
Dietz FK, Rodriguez-Giaxola M, Traiger GJ, Stella VJ, & Himmelstein KJ
(1981) Pharmacokinetics of 2-butanol and its metabolites in the rat.
J Pharmacokinet Biopharm, 9: 553-576.
Digiacomo JD (1973) New approaches for the design of afterburners for
varnish cookers. J Air Pollut Control Assoc, 23: 287-290.
Dilling WL, Bredeweg DJ, & Tefertiller NB (1976) Organic
photochemistry. XIII. Simulated atmospheric photodecomposition rates
of methylene chloride, 1,1,1-trichloro-ethane, tetrachloroethylene,
and other compounds. Environ Sci Technol, 10: 351-356.
Divincenzo GD & Krasavage WJ (1974) Serum ornithine carbamyl
transferase as a liver response test for exposure to organic solvents.
Am Ind Hyg Assoc J, 35: 21-29.
Divincenzo GD, Kaplan CJ, & Dedinas J (1976) Characterization of the
metabolites of methyl-N-butyl ketone, methyl iso-butyl ketone, and
methyl ethyl ketone in guinea-pig serum and their clearance. Toxicol
Appl Pharmacol, 36: 511-522.
Divincenzo GD, Hamilton ML, Kaplan CJ, Krasavage WJ, & O'Donoghue JL
(1978) Studies on the respiratory uptake and excretion and the skin
absorption of methyl-n-butyl ketone in humans and dogs. Toxicol Appl
Pharmacol, 44: 593-604.
Dojlido JR (1977) Testing of biodegradability and toxicity of organic
compounds in industrial wastewaters. In: Polish/US Symposium on
Wastewater Treatment and Sludge Disposal, Cincinnati, Ohio, 10-12
February 1976. Cincinnati, Ohio, US Environmental Protection Agency,
vol II, pp 112-131 (EPA 600/9-76-021; NTIS PB-261422).
Dojlido JR (1979) Investigations of biodegradability and toxicity of
organic compounds. Washington, DC, US Environmental Protection Agency,
99 pp (EPA-600/2-79-163).
Dore M, Brunet N, & Legube B (1975) Participation of various organic
compounds in the evaluation of global pollution criteria. Trib
Cebedeau, 28: 3-11.
Dornseifer TP, Kim SC, Keith ES, & Powers JJ (1965) Effect of moisture
level on volatile carbonyls in cottonseed oil heated to 210 °C. J Am
Oil Chem Soc, 42: 1073-1075.
Dowty BJ, Laseter JL, & Storer J (1976) The transplacental migration
and accumulation in blood of volatile organic constituents. Pediatr
Res, 10: 696-701.
Draize JH, Woodard G, & Calvery HO (1944) Methods for the study of
irritation and toxicity of substances applied topically to the skin
and mucous membranes. J Pharmacol Exp Ther, 82: 377-389.
Duckett S, Williams N, & Francis S (1974) Peripheral neuropathy
associated with inhalation of methyl-n-butyl ketone.
Experientia(Basel), 30: 1283-1284.
Dutch Expert Committee for Occupational Standards (1991) Health-based
recommended occupational exposure limit for methyl ethyl ketone.
Voorburg, Directorate General of Labour of the Ministry of Social
Affairs and Employment, Dutch Expert Committee for Occupational
Standards, 45 pp (Report RA 16/90).
Dyro FM (1978) Methyl ethyl ketone polyneuropathy in shoe factory
workers. Clin Toxicol, 13: 371-376.
Egyud LG (1967) Studies on cell division: the effect of aldehydes,
ketones and a-keto-aldehydes on the proliferation of Escherichia
coli. Curr Mod Biol, 1: 14-20.
Elskamp CJ & Schultz GR (1983) An alternative sampling and analytical
method for 2- butanone. Am Ind Hyg Assoc J, 44: 201-204.
Emmel TE, Lee BB, & Simonson AB (1983) Control technology assessment
of petroleum refinery operations. In depth site visit report: Congo
Refinery, Farmer's Valley Refinery. Cincinnati, Ohio, National
Institute of Occupational Safety and Health, 60 pp (Contract No.
210-81-7102).
Ewing BB, Chian ESK, Cook JK, Evans CA, Hopke PK, & Perkins EG (1977)
Monitoring to detect previously unrecognized pollutants in surface
waters. Appendix: Organic analysis data. Washington, DC, US
Environmental Protection Agency, p 75 (EPA- 560/6-77-015).
Fagius J & Grönqvist B (1978) Function of peripheral nerves and signs
of polyneuropathy in solvent-exposed workers at Swedish steelworks.
Acta Neurol Scand, 57: 305-316.
Falla NAR (1987) Solving air pollution problems in the surface
coatings industry. Polym Paint Colour J, 98: 103-104, 106, 108, 110.
Feigley CE & Chastain JB (1982) An experimental comparison of three
diffusion samplers exposed to concentration profiles of organic
vapors. Am Ind Hyg Assoc J, 43: 227-234.
Fernandes MH, Gilbert SG, Paik SW, & Stier EF (1986) Study of the
degradation products formed during extrusion lamination of an ionomer.
J Food Sci, 51: 722-725.
Fiserova-Bergerova V & Diaz ML (1986) Determination and prediction of
tissue-gas partition coefficients. Int Arch Occup Environ Health,
58: 75-87.
Fisher GS, Legendre MG, Lovgren NV, Schuller WH, & Wells JA (1979)
Volatile constituents of southernpea seed [ Vigna unguiculata (L.)
Walp.] J Agric Food Chem, 27: 7-11.
Fisk JF (1986) Volatile organic analytical methods - general
description and quality control considerations. In: Perket CL ed.
Quality control in remedial site investigation: Fifth volume -
Hazardous and industrial solid waste testing. Philadelphia,
Pennsylvania, American Society for Testing and Materials, 12 pp (ASTM
STP 925).
Florin I, Rutberg L, Curvall M, & Enzell CR (1980) Screening of
tobacco smoke constituents for mutagenicity using the Ames' test.
Toxicology, 18: 219-232.
Freddi A, Paci A, Vittori O, De Ciantis R, & Ottaviano PF (1982)
[Clinical and electromyographic study of workers exposed to methyl
ethyl ketone vapor.] Ann Med Perugia, 73: 111-136 (in Italian).
French RC (1961) Stimulation of uredospore germination in wheat stem
rust by terpenes and related compounds. Bot Gaz, 122: 194-198.
French RC (1984) Stimulation of uredospore germination of Puccinia
helianthi and Uromyces vignae by aromatic nitriles and related
flavorlike compounds. J Agric Food Chem, 32: 556-561.
French RC, Graham CL, Gale AW, & Long RK (1977) Structural and
exposure time requirements for chemical stimulation of germination of
uredospores of Uromyces phaseoli. J Agric Food Chem, 25: 84-88.
Gadomski RR, Gimborne AV, & Green WJ (1974) An evaluation of emissions
and control technologies for the metal decorating process. J Air
Pollut Control Assoc, 24: 579-585.
Garcia CR, Geller I, & Kaplan HL (1978) Effects of ketones on
lever-pressing behavior of rats. Proc West Pharmacol Soc, 21: 433-438.
Geller I, Martinez RL, Hartmann RJ, & Kaplan HL (1978) Effects of
ketones on a match to sample task in the baboon. Proc West Pharmacol
Soc, 21: 439-442.
Gerhold RM & Malaney GW (1966) Structural determinants in the
oxidation of aliphatic compounds by activated sludge. J Water Pollut
Control Fed, 38: 562-579.
Ghittori S, Imbriani M, Pezzagno G, & Capodaglio E (1987) The urinary
concentration of solvents as a biological indicator of exposure:
Proposal for the biological equivalent exposure limit for nine
solvents. Am Ind Hyg Assoc J, 48: 786-790.
Gianturco MA, Giammarino S, & Friedel P (1966) Volatile constituents
of coffee-V. Nature (Lond), 210(5043): 1358.
Glowa JR & Dews PB (1987) Behavioral toxicology of volatile organic
solvents. IV. Comparisons of the rate-decreasing effects of acetone,
ethyl acetate, methyl ethyl ketone, toluene, and carbon disulfide on
schedule-controlled behavior of mice. J Am Coll Toxicol, 6: 461-469.
Gordon DT & Morgan ME (1972) Principal volatile compounds in feed
flavored milk. J Dairy Sci, 55: 905-912.
Gosselin RE, Smith RP, & Hodge HC (1984) Clinical toxicology of
commercial products. Baltimore, Maryland, Williams and Wilkins, pp
III/101-III/107.
Goto I, Matsumura M, Inoue N, Murai Y, Shida K, Santa T, & Kuroiwa Y
(1974) Toxic polyneuropathy due to glue sniffing. J Neurol Neurosurg
Psychiatry, 37: 848-853.
Grey TC & Shrimpton DH (1967) Volatile components of raw chicken
breast muscle. Br Poult Sci, 8: 23-33.
Grosjean D (1982) Formaldehyde and other carbonyls in Los Angeles
ambient air. Environ Sci Technol, 16: 254-262.
Grosjean D & Wright B (1983) Carbonyls in urban fog, ice, cloudwater
and rainwater. Atmos Environ, 17: 2093-2096.
Grosjean D, Swanson RD, & Ellis C (1983) Carbonyls in Los Angeles air:
Contribution of direct emissions and photochemistry. Sci Total
Environ, 29: 65-85.
Gurka DF, Warner JS, Silvon LE, Bishop TA, & Mckown MM (1984) Interim
method for determination of volatile organic compounds in hazardous
wastes. J Assoc Off Anal Chem, 67: 776-782.
Hampton CV, Pierson WR, Harvey TM, Updegrove WS, & Marano RS (1982)
Hydrocarbon gases emitted from vehicles on the road. 1. A qualitative
gas chromatography/mass spectrometry survey. Environ Sci Technol, 16:
287-298.
Harvey RJ & Walker JRL (1960) Some volatile compounds in New Zealand
Cheddar cheese and their possible significance in flavour formation.
III Time of first appearance of volatile carbonyl compounds during
ripening. J Dairy Res, 27: 335-340.
Hawthorne SB, Sievers RE, & Barkley RM (1985) Organic emissions from
shale oil wastewaters and their implications for air quality. Environ
Sci Technol, 19: 992-997.
Hazleton (1963a) Primary skin irritation-rabbits. Falls Church,
Virginia, Hazleton Laboratories Inc. (Laboratory Report No. 63 MRL
003).
Hazleton (1963b) Acute eye irritation-rabbits. Falls Church, Virginia,
Hazleton Laboratories Inc. (Laboratory Report No. 63 MRL 004).
Heitmuller PT, Hollister TA, & Parrish PR (1981) Acute toxicity of 54
industrial chemicals to sheepshead minnows (Cyprinodon variegatus).
Bull Environ Contam Toxicol, 27: 596-604.
Hewitt WR, Miyajima H, Cote MG, & Plaa GL (1980) Modification of
haloalkane- induced hepatotoxicity by exogenous ketones and metabolic
ketosis. Fed Proc, 39: 3118-3123.
Hewitt WR, Brown EM, & Plaa GL (1983) Relationship between the carbon
skeleton length of ketonic solvents and potentiation of
chloroform-induced hepatotoxicity in rats. Toxicol Lett, 16: 297-304.
Hewitt LA, Ayotte P, & Plaa GL (1986) Modifications in rat
hepatobiliary function following treatment with acetone, 2-butanone,
2-hexanone, mirex, or chlordecone and subsequently exposed to
chloroform. Toxicol Appl Pharmacol, 83: 465-473.
Hewitt LA, Valiquette C, & Plaa GL (1987) The role of
biotransformation-detoxication in acetone-, 2-butanone-, and
2-hexanone-potentiated chloroform-induced hepatotoxicity. Can J
Physiol Pharmacol, 65: 2313-2318.
Hickey JLS & Bishop CC (1981) Field comparison of charcoal tubes and
passive vapor monitors with mixed organic vapors. Am Ind Hyg Assoc J,
42: 264-267.
Higgins CE, Griest WH, & Olerich G (1983) Application of Tenax
trapping to analysis of gas phase organic compounds in ultra-low tar
cigarette smoke. J Assoc Off Anal Chem, 66: 1074-1083.
Holmberg I & Malmfors T (1974) The cytotoxicity of some organic
solvents. Environ Res, 7: 183-192.
Horton AW, Bingham EL, Burton MJG, & Tye R (1965) Carcinogenesis of
the skin. III. The contribution of elemental sulfur and of organic
sulfur compounds. Cancer Res, 25: 1759-1763.
Hoshika Y, Kozima I, Koike K, & Yosimoto K (1976) [Gas chromatographic
analysis of lower aliphatic carbonyl compounds using the imine
formation reaction.] Aichi-ken Kogai Chosa Senta Shoho, 4: 108-113 (in
Japanese).
Hou CT, Patel R, Laskin AI, Barnabe N, & Barist I (1983) Production of
methyl ketones from secondary alcohols by cell suspensions of C2 to
C4 n-alkane-grown bacteria. Appl Environ Microbiol, 46: 178-184.
Hrdlicka J & Kuca J (1965) The changes of carbonyl compounds in the
heat-processing of meat: 2, Turkey meat. Poult Sci, 44: 27-31.
Husman K (1980) Symptoms of car painters with long-term exposure to a
mixture of organic solvents. Scand J Work Environ Health, 6: 19-32.
Husman K & Karli P (1980) Clinical neurological findings among car
painters exposed to a mixture of organic solvents. Scand J Work
Environ Health, 6: 33-39.
Imai T, Takigawa H, Nakagawa S, Shen GJ, Kodama T, & Minoda Y (1986)
Microbial oxidation of hydrocarbons and related compounds by
whole-cell suspensions of the methane-oxidizing bacterium H-2. Appl
Environ Microbiol, 52: 1403-1406.
Ingram L (1977) Changes in lipid composition of Escherichia coli
resulting from growth with organic solvents and with food additives.
Appl Environ Microbiol, 33: 1233-1236.
Inoue T, Takeuchi Y, Hisanaga N, Ono Y, Iwata M, Ogata M, Saito K,
Sakurai H, Hara I, Matsushita T, & Ikeda M (1983) A nationwide survey
on organic solvent components in various solvent products: Part 1.
Homogeneous products such as thinners, degreasers and reagents. Ind
Health, 21: 175-183.
IRPTC (1987) Methyl ethyl ketone. In: IRPTC legal file 1986. Geneva,
International Register of Potentially Toxic Chemicals, United Nations
Environment Programme, vol I, pp 11/238-11/240.
IRPTC (1991) IRPTC legal file. Geneva, International Register of
Potentially Toxic Chemicals, United Nations Environment Programme,
pp 17/1-17/5.
Iwata M, Takeuchi Y, Hisanaga N, & Ono Y (1983) Changes of n-hexane
metabolites in urine of rats exposed to various concentrations of
n-hexane and to its mixture with toluene or MEK. Int Arch Occup
Environ Health, 53: 1-8.
Iwata M, Takeuchi Y, Hisanaga N, & Ono Y (1984) Changes of n-hexane
neurotoxicity and its urinary metabolites by long-term co-exposure
with MEK or toluene. Int Arch Occup Environ Health, 54: 273-281.
Jacot BJ (1983) OVA field screening at hazardous waste sites. In:
National Conference on Management of Uncontrolled Hazardous Waste
Sites, Washington, 31 October-2 November 1983. Silver Spring,
Maryland, Hazardous Materials Control Research Institute, pp 76-78.
Jarke FH, Dravnieks A, & Gordon SM (1981) Organic contaminants in
indoor air and their relation to outdoor contaminants. Am Soc Heat
Refrig Air-Cond Eng Trans, 87: 153-166.
Johansson I & Ingelman-Sundberg M (1985) Carbon tetrachloride-induced
lipid peroxidation dependent on an ethanol-inducible form of rabbit
liver microsomal cytochrome P-450. FEBS Lett, 183: 265-269.
John JA, Pilny MK, Kuna RA, Deacon MM, & Yakel HO (1980) Teratogenic
evaluation of methyl ethyl ketone in the rat. Teratology, 21(3): 47A.
Jonsson A, Persson KA, & Grigoriadis V (1985) Measurements of
low-molecular-weight oxygenated, aromatic, and chlorinated
hydrocarbons in ambient air and in vehicle emissions. Environ Int,
11: 383-392.
JRB Associates Inc (1980) Support document, chapter IV. Health effects
of methyl ethyl ketone. Washington, DC, US Environmental Protection
Agency, 58 pp (Contract No. 68-01-4839, Task No. 11, Project No.
2-800-08-107-04).
Juhnke I & Luedemann D (1978) [Results of the investigation of 300
chemical compounds for acute toxicity with the Golden Orfe test.]
Z Wasser Abwasser Forsch, 11: 161-164 (in German).
Jungclaus GA, Lopez-Avila V, & Hites RA (1978) Organic compounds in an
industrial wastewater: A case study of their environmental impact.
Environ Sci Technol, 12: 88-96.
Kahn JH, Laroe EG, & Conner HA (1968) Whiskey composition:
identification of components by single-pass gas chromatography - mass
spectrometry. J Food Sci, 33: 395-400.
Kamil IA, Smith JN, & Williams RT (1953) Studies in detoxification.
46. The metabolism of aliphatic alcohols. The glucuronic acid
conjugation of acyclic aliphatic alcohols. Biochem J, 53: 129-135.
Kaye S (1961) Emergency toxicology. Springfield, Illinois, Charles C.
Thomas, pp 214-216.
Keen AR, Walker NJ, & Peberdy MF (1974) The formation of 2-butanone
and 2-butanol in Cheddar cheese. J Dairy Res, 41: 249-257.
Kenny J & Stratton G (1989) An improved desorption solvent for organic
solvent mixtures. Am Ind Hyg Assoc J, 50: 431-434.
Kezic S & Monster AC (1988) Determination of methyl ethyl ketone and
its metabolites in urine using capillary gas chromatography.
J Chromatogr, 428: 275-280.
Khasanov AA, Isidorov VA, Zenkevich IG, & Ioffee BV (1982) [Gas
chromatography/ mass spectrometry study of the composition of volatile
emissions from Juniperus serawechanica Kom.] Dokl Akad Nauk Uzb SSR,
12: 29-31 (in Russian).
Kimura ET, Ebert DM, & Dodge PW (1971) Acute toxicity and limits of
solvent residue for sixteen organic solvents. Toxicol Appl Pharmacol,
19: 699-704.
Ko I-Y, Park SS, Song BJ, Patten CH, Tan Y, Hah YC, Yang CS, & Gelboin
HV (1987) Monoclonal antibodies to ethanol-induced rat liver
cytochrome P-450 that metabolizes aniline and nitrosamines. Cancer
Res, 47: 3101-3109.
Kobayashi K, Tanaka M, & Kawai S (1980) Gas chromatography
determination of low-molecular-weight carbonyl compounds in aqueous
solution as their O-(2,3,4,5,6,-penta-fluorobenzyl) oximes.
J Chromatogr, 187: 413-417.
Kontominas MG & Voudouris E (1982) The determination of residual
solvents in plastics packaging materials in relation to off odors
developed in packaged bakery products. Chem Chron, 11: 215-223.
Kopelman PG & Kalfayan PY (1983) Severe metabolic acidosis after
ingestion of butanone. Br Med J, 286: 21-22.
Krasavage WJ, O'Donoghue JL, & Divincenzo GD (1982) Ketones. In:
Clayton GD & Clayton FE ed. Patty's industrial hygiene and toxicology,
3rd ed. New York, John Wiley and Sons, Wiley Interscience,
pp 4709-4800.
Kulshrestha DC & Marth EH (1974a) Inhibition of bacteria by some
volatile and non-volatile compounds associated with milk. I.
Escherichia coli. J Milk Food Technol, 37: 510-516.
Kulshrestha DC & Marth EH (1974b) Inhibition of bacteria by some
volatile and non-volatile compounds associated with milk. II.
Salmonella typhimurium. J Milk Food Technol, 37: 539-544.
Kulshrestha DC & Marth EH (1974c) Inhibition of bacteria by some
volatile and non-volatile compounds associated with milk. III.
Staphylococcus aureus. J Milk Food Technol, 37: 545-550.
Kulshrestha DC & Marth EH (1974d) Inhibition of bacteria by some
volatile and non-volatile compounds associated with milk. IV.
Streptococcus lactis. J Milk Food Technol, 37: 593-599.
Kulshrestha DC & Marth EH (1974e) Inhibition of bacteria by some
volatile and non-volatile compounds associated with milk. V.
Leuconostoc citrovorum. J Milk Food Technol, 37: 600-606.
Kulshrestha DC & Marth EH (1974f) Inhibition of bacteria by some
volatile and non-volatile compounds associated with milk. VI.
Streptococcus thermophilus. J Milk Food Technol, 37: 606-611.
Kupferschmid LL & Perkins JL (1986) Organic solvent recycling plant
exposure levels. Appl Ind Hyg, 1: 122-124.
Kwan W & Gatehouse A (1978) The effects of low doses of three
insecticides on activity, feeding, mating, reproductive performance
and survival in Glossina morsitans mortistans (Glossinidae). Entomol
Exp Appl, 23: 201-221.
La Belle CW & Brieger H (1955) The vapor toxicity of a composite
solvent and its principal compounds. Arch Ind Health, 12: 623-627.
Laity JL, Burstain IG, & Appel BR (1973) Photochemical smog and the
atmospheric reactions of solvents. In: Tess RW ed. Solvents, theory
and practice. Washington, DC, American Chemical Society, vol 7,
pp 96-112 (Advances in Chemistry Series 124).
Lande SS, Durkin PR, Christopher DH, Howard PH, & Saxena J (1976)
Investigation of selected potential environmental contaminants:
Ketonic solvents. Washington, DC, US Environmental Protection Agency,
330 pp (EPA-560/2-76-003).
Laregina J & Bozzelli JW (1986) Volatile organic compounds at
hazardous waste sites and a sanitary landfill in New Jersey. Environ
Prog, 5: 18-27.
Larson PS, Finnegan JK, & Haag HB (1955) Observations on the effect of
chemical configuration on the edema-producing potency of acids,
aldehydes, ketones and alcohols. J Pharmacol, 116: 119-122.
Leblanc G (1980) Acute toxicity of priority pollutants to water flea
(Daphnia magna). Bull Environ Contam Toxicol, 24: 684-691.
Leblanc G (1984) Interspecies relationships in acute toxicity of
chemicals to aquatic organisms. Environ Toxicol Chem, 3: 47-60.
Lee S & Murphy D (1982) Health hazard evaluation: Medalist-Gladiator
Athletic Products Company, Leesburg, Florida. Cincinnati, Ohio,
National Institute of Occupational Safety and Health, 15 pp (HETA
82-030-1184).
Lee S & Parkinson D (1982) Health hazard evaluation: Rola-Esmark
Company; Dubois, Pennsylvania. Cincinnati, Ohio, National Institute of
Occupational Safety and Health, 26 pp (HETA 80-168-1204).
Levin JO & Carleborg L (1987) Evaluation of solid sorbents for
sampling ketones in work-room air. Ann Occup Hyg, 31: 31-38.
Levy A (1973) The photochemical smog reactivity of organic solvents.
In: Tess RW ed. Solvents, theory and practice. Washington, DC,
American Chemical Society, vol 6, pp 71-93 (Advances in Chemistry
Series 124).
Li G-L, Yin S-N, Watanabe T, Nakatsuka H, Kasahara M, Abe H, & Ikeda
M (1986) Benzene-specific increase in leucocyte alkaline phosphatase
activity in rats exposed to vapors of various organic solvents. J
Toxicol Environ Health, 19: 581-589.
Liebich HM, Bertsch W, Zlatkis A, & Schneider HJ (1975) Volatile
organic components in the Skylab 4 spacecraft atmosphere. Aviat Space
Environ Med, 46: 1002-1007.
Liepins R, Mixon F, Hudak C, & Parsons TB (1977) Industrial process
profiles for environmental use: Chapter 6. The industrial organic
chemicals industry. Research Triangle Park, North Carolina, US
Environmental Protection Agency, pp 164-165, 560-561 (EPA-600/12).
Liira J, Riihimäki V, Engstrom K, & Pfaffli P (1988a) Coexposure of
man to m-xylene and methyl ethyl ketone, kinetics and metabolism.
Scand J Work Environ Health, 14: 322-327.
Liira J, Riihimäki V, & Pfaffli P (1988b) Kinetics of methyl ethyl
ketone in man: absorption, distribution and elimination in inhalation
exposure. Int Arch Occup Environ Health, 60: 195-200.
Liira J, Johanson G, & Riihimäki V (1990a) Dose-dependent kinetics of
inhaled methylethylketone in man. Toxicol Lett, 50: 195-201.
Liira J, Riihimäki V, & Engstrom K (1990b) The effects of ethanol on
the kinetics of methyl ethyl ketone in man. Br J Ind Med, 47: 325-330.
Liira J, Elovaara E, Raunio H, Riihimäki V, & Engstrom K (1991)
Metabolic interaction and disposition of methyl ethyl ketone and
m-xylene in rats at single and repeated inhalation exposures.
Xenobiotica, 21: 53-63.
Lin JCC & Jeon IJ (1985) Headspace gas sampling/GC method for the
quantitative analysis of volatile compounds in cheese. J Food Sci, 50:
843-844, 846.
Lindros KO, Cai Y, & Penttila KE (1990) Role of ethanol-inducible
cytochrome P-450IIE1 in carbon tetrachloride-induced damage to
centrilobular hepatocytes from ethanol-treated rats. Hepatology,
12: 1092-1097. Lowenheim FA & Moran MK (1975) Industrial chemicals, 4th
ed. New York, Chichester, Brisbane, Toronto, John Wiley and Sons,
pp 11-12, 539-542.
Lundberg I & Hakansson M (1985) Normal serum activities of liver
enzymes in Swedish paint industry workers with heavy exposure to
organic solvents. Br J Ind Med, 42: 596-600.
Lundberg I, Ekdahl M, Kronevi T, Vitauts L, & Lundberg S (1986)
Relative hepatotoxicity of some industrial solvents after
intraperitoneal injection or inhalation exposure in rats. Environ Res,
40: 411-420.
Mabuchi H (1969) [Urinary and serum volatile substances in diabetics.]
Nippon Naika Gakkai Zasshi, 58: 731-743 (in Japanese).
Manville Chemical Products Corporation (1988) Chemical products
synopsis, Asbury Park, New Jersey, Manville Chemical Products
Corporation, 1 p.
Marion CV & Malaney GW (1963) The oxidation of aliphatic compounds by
Alcalignes faecalis. J Water Pollut Control Fed, 35: 1269-1281.
Marnett LJ, Hurd HK, Hollstein MC, Levin DE, Esterbauer H, & Ames BN
(1985) Naturally occurring carbonyl compounds are mutagens in
Salmonella tester strain TA104. Mutat Res, 148: 25-34.
Mast TJ, Dill JA, Evanoff JJ, Rommereim RL, Weigel RJ, & Westerberg RB
(1989) Inhalation developmental toxicology studies: teratology study
of methyl ethyl ketone in mice. Richland, Washington, Pacific Norwest
Laboratory (Report No. NIH-YOI-ES-70153).
Mayer VW & Goin CJ (1987) Effects of chemical combinations on the
induction of aneuploidy in Saccharomyces cerevisiae. Mutat Res,
187: 21-30.
Mayer VW & Goin CJ (1988) Investigations of aneuploidy-inducing
chemical combinations in Saccharomyces cerevisiae. Mutat Res,
201: 413-421.
MB Research Laboratories, Inc. (1979) Test for eye irritation in
rabbits. Spinnerstown, Pennsylvania, MB Research Laboratories, Inc.,
5 pp (Unpublished report No. MB 79-3962).
Metcalf RL, Lu P, & Kapoor IP (1973) Environmental distribution and
metabolic fate of key industrial pollutants and pesticides in a model
ecosystem. Urbana, Illinois, University of Illinois, Water Research
Center (Report No. 69, Project B-050 Ill).
Mezey E (1976) Ethanol metabolism and ethanol-drug interactions.
Biochem Pharmacol, 25: 869-875.
Middleditch BS, Sung NJ, Zlatkis A, & Settembre G (1987) Trace
analysis of volatile polar organics by direct aqueous injection gas
chromatography. Chromatographia, 23: 273-278.
Misumi J & Nagano M (1985) Experimental study on the enhancement of
the neurotoxicity of methyl n-butyl ketone by non-neurotoxic aliphatic
monoketones. Br J Ind Med, 42: 155-161.
Miyasaka M, Kumai M, Koizumi A, Watanabe T, Kurasako K, Sato K, &
Ikeda M (1982) Biological monitoring of occupational exposure to
methyl ethyl ketone by means of urinalysis for methyl ethyl ketone
itself. Int Arch Occup Environ Health, 50: 131-137.
Mohren S & Juttner F (1983) Odorous compounds of different strains of
Anabaena and Nostoc (Cyanobacteria). Water Sci Technol, 15: 221-228.
Mookherjee RE, Deck RE, & Chang SS (1965) Relationship between
monocarbonyl compounds and flavor of potato chips. J Agric Food Chem,
13(2): 131-134.
Munies R & Wurster DE (1965) Investigation of some factors influencing
percutaneous absorption--III. Absorption of methyl ethyl ketone.
J Pharm Sci, 54: 1281-1284.
Murphy DC (1984) Acute illness among workers connected to solvent
exposure. Occup Health Saf, May: 36-38.
Mutti A, Cavatorta A, Lucertini S, Arfini G, Falzoi M, & Franchini I
(1982a) Neurophysiological changes in workers exposed to organic
solvents in a shoe factory. Scand J Work Environ Health, 8(Suppl 1):
136-141.
Mutti A, Ferri F, Lommi G, Lotta S, Lucertini S, & Franchini I (1982b)
n-Hexane induced changes in nerve conduction velocities and
somatosensory evoked potentials. Int Arch Environ Health, 51: 45-54.
Nakaaki K (1974) [An experimental study on the effect of exposure to
organic solvent vapor in human subjects.] J Sci Labour 50: 89-96 (in
Japanese).
Nakajima T, Wang R-S, Murayama N, & Sato A (1990) Three forms of
trichloro-ethylene-metabolizing enzymes in rat liver induced by
ethanol, phenobarbital, and 3-methylcholanthrene. Toxicol Appl
Pharmacol, 102: 546-552.
Nandi B & Fries N (1976) Volatile aldehydes, ketones, esters and
terpenoids as preservatives against storage fungi in wheat.
Z Pflanzenkr Pflanzenschutz, 83: 284-294.
Nestmann ER, Lee EGH, Matula TI, Douglas GR, & Mueller JG (1980)
Mutagenicity of constituents identified in pulp and paper mill
effluents using the Salmonella/mammalian-microsome assay. Mutat Res,
79: 203-212.
Ng H, Reed DJ, & Pence JW (1960) Identification of carbonyl compounds
in an ethanol extract of fresh white bread. Cereal Chem, 37: 638-645.
Nilsen OG & Toftgard R (1980) The influence of organic solvents on
cytochrome P-450- mediated metabolism of biphenyl and benzo(a)pyrene.
In: Coon MJ, Conney AH, Estabrook RW, Gelboin HV, Gillete JR, &
O'Brien PJ ed. Microsomes, drug oxidations, and chemical
carcinogenesis. New York, Academic Press, vol II, pp 1235-1238.
Noma E, Berglund B, Berglund U, Johansson I, & Baird JC (1988) Joint
representation of physical locations and volatile organic compounds in
indoor air from a healthy and a sick building. Atmos Environ,
22: 451-460.
Nye D (1975) Laboratory model ecosystem studies of the degradation and
fate of radiolabelled tri-, tetra-, and pentachlorobiphenyl compared
with DDE. Arch Environ Contam Toxicol, 3: 151-165.
O'Donoghue JL, Krasavage WJ, Divincenzo GD, & Katz GV (1984) Further
studies on ketone neurotoxicity and interactions. Toxicol Appl
Pharmacol, 72: 201-209.
O'Donoghue JL, Haworth SR, Curren RD, Kirby PE, Lawlor T, Moran EJ,
Phillips RD, Putnam DL, Rogers-Back AM, Slesinski RS, & Thilagar A
(1988) Mutagenicity studies on ketone solvents: methyl ethyl ketone,
methyl isobutyl ketone, and isophorone. Mutat Res, 206: 149-161.
Ogawa I & Fritz JS (1985) Determination of low concentrations of
low-molecular-weight aldehydes and ketones in aqueous samples.
J Chromatogr, 329: 81-89.
Oh SJ & Kim JM (1976) Giant axonal swelling in "hufferis" neuropathy.
Arch Neurol, 33: 583-586.
Ong CN, Sia GL, Ong HY, Phoon WH, & Tan KT (1991) Biological
monitoring of occupational exposure to methyl ethyl ketone. Int Arch
Occup Environ Health, 63: 319-324.
Osborne JS, Adamek S, & Hobbs ME (1956) Some components of gas phase
of cigarette smoke. Anal Chem, 28: 211-215.
Panson RD & Winek CL (1980) Aspiration toxicity of ketones. Clin
Toxicol, 17: 271-317.
Papa AJ & Sherman PD (1978) Ketones. In: Kirk RE & Othmer DF ed.
Encyclopedia of chemical technology, 3rd ed. New York, John Wiley and
Sons, Wiley-Interscience, vol 13, pp 894-912, 934-941.
Patel RN, Hou CT, & Laskin AI (1982) Oxidation of gaseous hydrocarbons
and related compounds by methanotrophic organisms. Dev Ind Microbiol,
23: 187-205.
Patel RN, Hou CT, Laskin AI, Felix A, & Derelanka P (1983) Oxidation
of alkanes by organisms grown on C2-C4 alkanes. J Appl Biochem,
5: 107-120.
Patty FA, Schrenk HH, & Yant WP (1935) Acute response of guinea pigs
to vapors of some new commercial organic compounds. VIII. Butanone. US
Public Health Rep, 50: 1217-1228.
Pellizzari ED, Castillo NP, Willis S, Smith D, & Bursey JT (1979)
Identification of organic components in energy related processes. In:
Van Hall CE ed. Measurement of organic pollutants in water and
wastewater: Proceedings of a symposium, Denver, Colorado, 19-20 June
1978. Philadelphia, Pennsylvania, American Society for Testing and
Materials, pp 256-274 (ASTM STP 686).
Pellizzari ED, Sheldon LS, Bursey JT, Michael LC, & Zweidinger RA
(1985) Master analytical scheme for organic compounds in water.
Athens, Georgia, US Environmental Protection Agency, pp 46-54, 108-127
(EPA/600/4-84-010A).
Perbellini L, Brugnone F, Mozzo P, Cocheo V, & Caretta D (1984) Methyl
ethyl ketone exposure in industrial workers. Uptake and kinetics. Int
Arch Occup Environ Health, 54: 73-81.
Perbellini L, Bartolucci G, Brugnone F, & De Rosa E (1985)
[2,5-Hexanedione in biological monitoring of occupational exposure to
n-hexane.] Med Lav, 76: 35-43 (in Italian).
Perocco P, Bolognesi S, & Alberghini W (1983) Toxic activity of
seventeen industrial solvents and halogenated compounds on human
lymphocytes cultured in vitro . Toxicol Lett, 16: 69-75.
Perry JJ (1968) Substrate specificity in hydrocarbon utilizing
micro-organisms. Antonie Van Leeuwenhoek, 34: 27-36.
Persson U, Lundqvist S, Marthinsson B, & Eng ST (1984)
Computer-automated carbon dioxide laser long-path absorption system
for air quality monitoring in the working environment. Appl Opt,
23: 998-1002.
Pezzagno G, Ghottori S, Imbriani M, & Capodaglio E (1983) [Measurement
of the solubility coefficients of gases and vapors in blood. II.
Widely used industrial solvents.] G Ital Med Lav, 5: 49-63 (in
Italian).
Poli G, Dianzani MU, Cheeseman KH, Slater TF, Lang J, & Esterbauer H
(1985) Separation and characterization of the aldehydic products of
lipid peroxidation stimulated by carbon tetrachloride or ADP-iron in
isolated rat hepatocytes and rat liver microsomal suspensions. Biochem
J, 227: 629-638.
Price K, Waggy G, & Conway R (1974) Brine shrimp bioassay and seawater
BOD of petrochemicals. J Water Pollut Control Fed, 46: 63-76.
Prockop LD, Alt M, & Tison J (1974) Huffer's neuropathy. J Am Med
Assoc, 229: 1083-1084.
Przyrembel H, Bremer HJ, Duran M, Bruinvis L, Ketting D, Wadman SK,
Baumgartner R, Irle U, & Bachmann C (1979) Propionyl-CoA carboxylase
deficiency with overflow of metabolites of isoleucine catabolism at
all levels. Eur J Pediatr, 130: 1-14.
Puskar MA, Levine SP, & Lowery SR (1986) Computerized infrared
spectral identification of compounds frequently found at hazardous
waste sites. Anal Chem, 58: 1156-1162.
Ralston WH, Hilderbrand RL, Uddin DE, Andersen ME, & Gardier RW (1985)
Potentiation of 2,5-hexanedione neurotoxicity by methyl ethyl ketone.
Toxicol Appl Pharmacol, 81: 319-327.
Ramsey JD & Flanagan RJ (1982) Detection and identification of
volatile organic compounds in blood by headspace gas chromatography as
an aid to the diagnosis of solvent abuse. J Chromatogr, 240: 423-444.
Raunio H, Liira J, Elovaara E, Riihimäki V, & Pelkonin O (1990)
Cytochrome P450 isozyme induction by methyl ethyl ketone and n-xylene
in rat liver. Toxicol Appl Pharmacol, 103: 175-179.
Reilly B (1988) Engineering assessment: Methyl ethyl ketone (MEK) and
methyl isobutyl ketone (MIBK) environmental releases. Washington, DC,
US Environmental Protection Agency, 20 pp (Unpublished document).
Reynolds T (1977) Comparative effects of aliphatic compounds on
inhibition of lettuce fruit germination. Am Bot 41: 637-648.
Riggin RM (1984) Compendium of methods for the determination of toxic
organic compounds in ambient air. Method T05. Research Triangle Park,
North Carolina, US Environmental Protection Agency, pp 1-22
(EPA-600/4-84-041).
Robertson P, White EL, & Bus JS (1989) Effects of methyl ethyl ketone
pretreatment on hepatic mixed function oxidose activity and on in
vivo metabolism of n-hexane. Xenobiotica, 19: 721-729.
Rose-Pehrsson SL, Grate JW, Ballentine DS, & Jurs PC (1988) Detection
of hazardous vapors including mixtures using pattern recognition
analysis of responses from surface acoustic wave devices. Anal Chem,
60: 2801-2811.
Ruth JH (1986) Odor thresholds and irritation levels of several
chemical substances: A review. Am Ind Hyg Assoc J, 47: 142-151.
Saida K, Mendell JR, & Weiss HS (1976) Peripheral nerve changes
induced by methyl n-butyl ketone and potentiation by methyl ethyl
ketone. J Neuropathol Exp Neurol, 35: 207-225.
Sakakibara H, Ide Y, Yanai T, Yajima J, & Hayashi K (1990) Volatile
flavour compounds of some kinds of dried and smoked fish. Agric Biol
Chem, 54: 9-16.
Sato A & Nakajima T (1979) Partition coefficients of some aromatic
hydrocarbons and ketones in water, blood and oil. Br J Ind Med,
36: 231-234.
Sato Y, Watanabe K, & Tanaka Y (1968) Chemical studies on smelling
compounds in hen's egg: Part I, Volatile carbonyl and basic compounds
in egg white. Agric Biol Chem, 32(4): 405-411.
Sauer TC (1981) Volatile liquid hydrocarbon characterization of
underwater hydrovents and formation waters from offshore production
operations. Environ Sci Tech, 15: 917-923.
Sawhney BL & Kozloski RP (1984) Organic pollutants in leachates from
landfill sites. J Environ Qual, 13: 349-352.
Schmidt R, Schnoy N, Altenkirch H, & Wagner HM (1984) Ultrastructural
alteration of intrapulmonary nerves after exposure to organic
solvents. Respiration, 46: 362-369.
Schnoy N, Schmidt R, Altenkirch H, & Wagner HM (1982) Ultrastructural
alteration of the alveolar epithelium after exposure to organic
solvents. Respiration, 43: 221-231.
Schörmuller J & Grosch W (1964) Aromatics of foods. II Occurrence of
additional carbonyl compounds in the tomato. Z Lebensmittel Unters
Forsch, 126: 38-49.
Schultz TH, Flath RA, Stern DJ, Mon TR, Teranishi R, Kruse SM, Butler
B, & Howard WE (1988) Coyote estrous urine volatiles. J Chem Ecol,
14: 701-712.
Schulz F, Mueller P, & Kramer D (1981) [Model experiments on the
phytotoxic effect of organic constituents in wastewater from lignite
refining in soil treatment.] Arch Acker-Pflanzenbau Bodenkd,
25: 745-753 (in German)
Schwetz BA, Leong BKJ, & Gehring PJ (1974) Embryo- and fetotoxicity of
inhaled carbon tetrachloride, 1,1-dichloroethane and methyl ethyl
ketone in rats. Toxicol Appl Pharmacol, 28: 452-464.
Schwetz BA, Mast TJ, Weigel RJ, Dill JA, & Morrissey RE (1991)
Developmental toxicity of inhaled methyl ethyl ketone in Swiss mice.
Fundam Appl Toxicol, 16: 742-748.
Seinfeld JH (1989) Urban air pollution: State of the science. Science,
243: 745-752.
Seizinger DE & Dimitriades B (1972) Oxygenates in exhaust from simple
hydrocarbon fuels. J Air Pollut Control Assoc, 22: 47-51.
Shah JJ & Singh HB (1988) Distribution of volatile organic chemicals
in outdoor and indoor air. Environ Sci Technol, 22: 1381-1388.
Shibata E, Huang J, Ono Y, Hisanaga N, Iwata M, Saito I, & Takeuchi Y
(1990a) Changes in urinary n-hexane metabolites by co-exposure to
various concentrations of methyl ethyl ketone and fixed n-hexane
levels. Arch Toxicol, 64: 165-168.
Shibata E, Huang J, Hisanaga Y, Ono Y, Saito I, & Takeuchi Y (1990b)
Effects of MEK on kinetics of n-hexane metabolites in serum. Arch
Toxicol, 64: 247-250.
Shimizu Y, Matsuto S, Ito T, & Okada I (1969) Studies on the flavor of
roast barley (Mugi-Cha): Part III. Separation and identification of
volatile mono-carbonyl compounds. Nippon Nogei Kagaku Kaishi,
43(4): 217-223 (in Japanese with English summary).
Smith LL (1981) Cholesterol autoxidation. New York, London, Plenum
Press, 674 pp.
Smith AR & Mayers MR (1944) Study of poisoning and fire hazards of
butanone and acetone. NY State Dept Labor Ind Bull, April: 174-176.
Smith AF & Wood R (1972) A field test for the determination of some
ketone vapors in air. Analyst, 97: 363-371.
Smyth HF, Carpenter CP, Weil CS, Pozzani UC, & Striegel JA (1962)
Range-finding toxicity data: list VI. Am Ind Hyg Assoc J, 23: 95-107.
Snider JR & Dawson GA (1985) Tropospheric light alcohols, carbonyls,
and acetonitrile: concentrations in the southwestern United States
and Henry's law data. J Geophys Res D Atmos, 90: 3797-3805.
Sosulski F & Mahmoud RM (1979) Effects of protein supplements on
carbonyl compounds and flavor in bread. Cereal Chem, 56: 533-536.
Specht H, Miller JW, Valaer PJ, & Sayers RR (1940) Acute response of
guinea-pigs to the inhalation of ketone vapors. Bull Natl Inst Health,
176: 1-66.
Spencer PS & Schaumburg HH (1976) Feline nervous system response to
chronic intoxication with commercial grades of methyl n-butyl ketone,
methyl isobutyl ketone, and methyl ethyl ketone. Toxicol Appl
Pharmacol, 37: 301-311.
Spencer PS, Schaumburg HH, Sabri MI, & Veronesi B (1980) The enlarging
view of hexacarbon neurotoxicity. CRC Crit Rev Toxicol, 7: 279-356.
SRI International (1985) Directory of chemical producers, Western
Europe, 8th ed. Menlo Park, California, SRI International, p 1457;
suppl II, p 55.
SRI International (1988) Directory of chemical producers, United
States. Menlo Park, California, SRI International, p 780.
Staüble EJ & Rast D (1971) [Volatile material in Agaricus biosporus.]
Experientia(Basel), 27: 886-888 (in German).
Stofberg J & Grundschober F (1984) Consumption ratio and food
predominance of flavoring materials--second cumulative series. Perfum
Flavorist, 9: 53-56, 58-59, 62, 65-72, 76-83.
Takeuchi Y, Ono Y, Hisanaga N, Iwata M, Aoyama M, Kitoh J, & Sugiura
Y (1983) An experimental study of the combined effects of n-hexane and
methyl ethyl ketone. Br J Ind Med, 40: 199-203.
Tangredi G, Carbone U, Rossi L, & Galdi A (1981) [Environmental
conditions in a paint factory and effects on employee health.] Riv Med
Lav Ig Ind, 5(Oct.-Dec.): 325-337 (in Italian).
Tham R, Bunnfors I, Eriksson B, Larsby B, Lindgrem S, & Odkvist LM
(1984) Vestibulo-ocular disturbances in rats exposed to organic
solvents. Acta Pharmacol Toxicol, 54: 58-63.
Thoburn T & Gunter B (1982) Health hazard evaluation; Davis Monthan
Air Force Base, Tucson, Arizona. Cincinnati, Ohio, National Institute
of Occupational Safety and Health, 23 pp (HETA 81-257-1115).
Toftgard R, Nilsen OG, & Gustafsson J-A (1981) Changes in rat liver
microsomal cytochrome P-450 and enzymatic activities after the
inhalation of n-hexane, xylene, methyl ethyl ketone and
methylchloroform for four weeks. Scand J Work Environ Health, 7:
31-37.
Toxigenics (1981) 90-day vapour inhalation toxicity study of methyl
ethyl ketone in albino rats. Decatur, Illinois, Toxigenics Inc. (Study
420-0305).
Traiger GJ & Bruckner JV (1976) The participation of 2-butanone in
2-butanol-induced potentiation of carbon tetrachloride hepatotoxicity.
J Pharmacol Exp Ther, 196: 493-499.
Traiger GJ & Plaa GL (1972) Relationship of alcohol metabolism to the
potentiation of CCl4 hepatotoxicity induced by aliphatic alcohols.
J Pharmacol Exp Ther, 183: 481-488.
Traiger GJ, Bruckner JV, Jiang WD, Dietz FK, & Cooke PH (1989) Effect
of 2-butanol and 2-butanone on rat hepatic ultrastructure and drug
metabolizing enzyme activity. J Toxicol Environ Health, 28: 235-248.
Triebig G, Bestler W, Baumeister P, & Valentin H (1983)
[Investigations on neurotoxicity of chemical substances at the
workplace. IV. Determination of the motor and sensory nerve conduction
velocity in persons occupationally exposed to a mixture of organic
solvents.] Int Arch Occup Environ Health 52: 139-150 (in German).
Tsao MU & Pfeiffer EI (1957) Isolation and identification of a new
ketone body in normal human urine. Proc Soc Exp Biol Med, 94: 628-629.
Tsukamoto S, Chiba S, Ishikawa T, & Shimaura M (1985a) Experimental
study on the metabolism of volatile hydrocarbons by inhalation of
natural gas. Nihon Univ J Med, 27: 33-38.
Tsukamoto S, Chiba S, Muto T, Ishakawa T, & Shimamura M (1985b) Study
on the metabolism of volatile hydrocarbons in mice--propane, n-butane,
and isobutane. J Toxicol Sci, 10: 323-332.
Union Carbide Corp. (1980a) The acute toxicity of MRD-80-2 to the
bluegill sunfish Lepomis macrochirus Rafinesque. Tarrytown, New York,
Union Carbide Corp., Environmental Services, 8 pp.
Union Carbide Corp. (1980b) The acute toxicity of MRD-80-2 to the
water flea Daphnia magna Straus. Tarrytown, New York, Union Carbide
Corp., Environmental Services, 6 pp.
US EPA (1985a) Health assessment document for chloroform. Research
Triangle Park, North Carolina, US Environmental Protection Agency,
pp 3-40-3-42 (EPA/600/8-84/004F).
US EPA (1985b) Health and environmental effects profile for methyl
ethyl ketone. Cincinnati, Ohio, US Environmental Protection Agency,
71 pp (EPA/600/X-85/363).
US EPA (1986) Health effects assessment for methyl ethyl ketone.
Cincinnati, Ohio, US Environmental Protection Agency, 17 pp
(EPA/540/1-86/003).
US NIOSH (1984a) Analytical method 2500: 2-butanone. Cincinnati, Ohio,
National Institute of Occupational Safety and Health, 3 pp.
US NIOSH (1984b) Analytical method 8002: (1)2-butanone, (2) ethanol
and (3) toluene in blood. Cincinnati, Ohio, National Institute for
Occupational Safety and Health, 4 pp.
US NIOSH (1990) Pocket guide to chemical hazards. Cincinnati, Ohio,
National Institute of Occupational Safety and Health, 48 pp (DHEW
(NIOSH) Publication No. 90-117).
Urano K & Kato Z (1986) Evaluation of biodegradation ranks of priority
organic compounds. J Hazard Mater, 13: 147-159.
USITC (1981) Synthetic organic chemicals: United States production and
sales, 1980. Washington, DC, United States International Trade
Commission, p 263 (USITC Publication No. 1183).
USITC (1982) Synthetic organic chemicals: United States production and
sales, 1981. Washington, DC, United States International Trade
Commission, p 243 (USITC Publication No. 1292).
USITC (1983) Synthetic organic chemicals: United States production and
sales, 1982. Washington, DC, United States International Trade
Commission, p 259 (USITC Publication No. 1422).
USITC (1984) Synthetic organic chemicals: United States production and
sales, 1983. Washington, DC, United States International Trade
Commission, p 257 (USITC Publication No. 1588).
USITC (1985) Synthetic organic chemicals: United States production and
sales, 1984. Washington, DC, United States International Trade
Commission, p 256 (USITC Publication No. 1745).
USITC (1986) Synthetic organic chemicals: United States production and
sales, 1985. Washington, DC, United States International Trade
Commission, p 266 (USITC Publication 1892).
USITC (1987) Synthetic organic chemicals: United States production and
sales, 1986. Washington, DC, United States International Trade
Commission, p 210 (USITC Publication No. 2009).
USITC (1988) Synthetic organic chemicals: United States production and
sales, 1987. Washington, DC, United States International Trade
Commission, p 15-5 (USITC Publication No. 2118).
Uspenskii IV & Repkina L (1974) [Physiological age and sensitivity to
DDT of natural populations of Ixodes persulcatus.] Parazitologiya,
8: 3-11 (in Russian).
Vale GA, Lovemore DF, Flint S, & Cockbill GF (1988) Odour-baited
targets to control tsetse flies, Glossina spp. (Diptera:
Glossinidae) in Zimbabwe. Bull Entomol Res, 78: 31-49.
Van Doorn JE, De Cock J, Kezic S, & Monster AC (1989) Determination of
methyl ethyl ketone in human urine after derivatization with
o-nitrophenylhydrazine, using solid-phase extraction and
reversed-phase liquid chromatography and ultraviolet detection.
J Chromatogr, 489: 419-424.
Varigos GA & Nurse DS (1986) A case of contact urticaria caused by
methyl ethyl ketone. Contact Dermatitis, 15(4): 249-260.
Veith GD, Call DJ, & Brooke LT (1983) Structure-toxicity relationships
for the fathead minnow, Pimephales promelas: Narcotic industrial
chemicals. Can J Fish Aquat Sci, 40: 743-748.
Verhoeff AP, Wilders MMW, Monster AC, & Van Wijnen JH (1987) Organic
solvents in the indoor air of two small factories and surrounding
houses. Int Arch Occup Environ Health, 59: 153-163.
Veronesi B (1984) An ultrastructural study of methyl ethyl ketone's
effect on cultured nerve tissues. Neurotoxicology, 5: 31-44.
Veronesi B, Lington AW, & Spencer PS (1984) A tissue culture model of
methyl ethyl ketone's potentiation of n-hexane neurotoxicity.
Neurotoxicology, 5: 43-52.
Verschuren K (1983) Handbook of environmental data on organic
chemicals. New York, Van Nostrand Reinhold Company, pp 850-852.
Viader F, Lechevalier B, & Morin P (1975) Polynéurite toxique chez un
travailleur du plastique. Rôle possible du méthyl éthyl-cétone. Nouv
Presse Méd, 4: 1813-1814.
Volskay VT & Grady CPL (1988) Toxicity of selected RCRA compounds to
activated sludge microorganisms. J Water Pollut Control Fed,
60: 1850-1856.
Von Cremer E & Riedmann M (1964) Identification of
gas-chromatographically separated aromatic materials in honey.
Z Naturforsch B19: 76-77.
Wahlberg JE (1984a) Edema-inducing effects of solvents following
topical administration. Dermatosen, 3: 91-94.
Wahlberg JE (1984b) Erythema-inducing effects of solvents following
epicutaneous administration to man - studied by laser Doppler
flowmetry. Scand J Work Environ Health, 10: 159-162.
Wallen I, Greer W, & Lasater R (1957) Toxicity to Gambusia affinis
of certain pure chemicals in turbid waters. Sewage Ind Wastes,
29: 265-711.
Walton BT, Anderson TA, Hendricks MS, & Talmage SS (1989)
Physicochemical properties as predictors of organic chemical effects
on soil microbial respiration. Environ Toxicol Chem, 8: 53-63.
Wang TC & Bricker JL (1979) 2-butanone and tetrahydrofuran
contamination in the water supply. Bull Environ Contam Toxicol,
23: 620-623.
Weast RC ed. (1986) CRC handbook of chemistry and physics, 67th ed.
Boca Raton, Florida, CRC Press, p C-170.
Weil CS & Scala RA (1971) Study of intra- and interlaboratory
variability in the results of rabbit eye and skin irritation tests.
Toxicol Appl Pharmacol, 19: 276-360.
Wen CP, Tsai SP, Weiss NS, Gibson RL, Wong O, & Mcclellan WA (1985)
Long-term mortality study of oil refinery workers. IV. Exposure to the
lubricating-dewaxing process. J Natl Cancer Inst, 74: 11-18.
Whelan JK, Tarafa ME, & Hunt JM (1982) Volatile C1-C8 organic
compounds in macroalgae. Nature(Lond), 299: 50-52.
Whitehead LW, Ball GL, Fine LJ, & Langolf GD (1984) Solvent vapor
exposures in booth spray painting and spray gluing, and associated
operations. Am Ind Hyg Assoc J, 45: 767-772.
Windholz M ed. (1983) The Merck index, 10th ed. Rahway, New Jersey,
Merck & Co., p 870.
Wong NP & Patton S (1962) Identification of some volatile compounds
related to the flavor of milk and cream. J Dairy Sci, 45: 724-728.
Wong NP, Damico JN, & Salwin H (1967) Decomposition and filth in
foods: Investigation of volatile compounds in cod fish by gas
chromatography and mass spectrometry. J Assoc Off Anal Chem,
50(1): 8-15.
WHO (1991) Environmental Health Criteria 122: n-Hexane. Geneva, World
Health Organization, 164 pp.
Wurster DE & Munies R (1965) Factors influencing percutaneous
absorption II, absorption of methyl ethyl ketone. J Pharm Sci,
54: 554-556.
Ying L & Levine SP (1989) Fourier transform infrared least-squares
methods for the quantitative analysis of multicomponent mixtures of
airborne vapours of industrial hygiene concern. Anal Chem,
61: 677-683.
Zakhari S, Leibowitz M, Levy P, & Aviado DM (1977) Isopropanol and
ketones in the environment. Cleveland, Ohio, CRC Press, pp 57-89.
Zechman JM, Aldinger S, & Labows JN (1986) Characterization of
pathogenic bacteria by automated headspace concentration-gas
chromatography. J Chromatogr, 377: 49-57.
Zimmermann FK, Mayer VW, Scheel I, & Resnick MA (1985) Acetone, methyl
ethyl ketone, ethyl acetate, acetonitrile and other polar aprotic
solvents are strong inducers of aneuploidy in Saccharomyces
cerevisiae. Mutat Res, 149: 339-351.
APPENDIX 1
Conversion factors for various solvents (ppm --> mg/m3)
acetone 2.38
2-butanol 3.03
n-butanol 3.03
2-butoxyethanol 4.83
butyl acetate 4.75
cyclohexane 3.44
DBP 11.38
DCB 6.01
ethanol 1.88
2-ethoxyethanol 3.68
ethyl acetate 3.6
ethyl butyl ketone 4.6
n-hexane 3.52
isobutanol 3.03
isopropanol 2.45
2-methoxyethanol 3.11
methyl acetate 3.6
methyl butyl ketone 4.1
methyl ethyl ketone 2.95
methyl isobutyl ketone 4.1
methylene chloride 3.48
toluene 3.75
trichloroethane 5.46
trichloroethylene 5.38
white spirit 1.75
xylene 4.34
From: Clayton & Clayton (1981) and Weast (1986)
RESUME
1. Propriétés et méthodes d'analyse
La méthyléthylcétone est un liquide limpide, incolore, volatil et
très inflammable dont l'odeur rappelle celle de l'acétone. Elle est
stable dans les conditions ordinaires, mais peut donner naissance à
des peroxydes explosifs en cas de stockage prolongé. Elle peut aussi
former des mélanges explosifs avec l'air. Elle est très soluble dans
l'eau, miscible à de nombreux solvants organiques et forme des
azéotropes avec l'eau et beaucoup de liquides organiques. Dans
l'atmosphère, elle produit des radicaux libres qui peuvent favoriser
la formation d'un smog photochimique.
On dispose de plusieurs méthodes analytiques pour mesurer les
concentrations de méthyléthylcétone dans l'air, l'eau, les
échantillons biologiques, les effluents et d'autres milieux. Dans les
méthodes les plus sensibles, la méthyléthylcétone est piégée et
concentrée soit sur un sorbant solide, soit sous forme de dérivé de la
2,4-dinitrophénylhydrazine (DNPH). La méthyléthylcétone absorbée et
les autres composés organiques volatils sont désorbés, séparés par
chromatographie gazeuse et dosés à l'aide d'un spectromètre de masse
ou d'un détecteur à ionisation de flammes. Le produit de dérivation de
la méthyléthylcétone est séparé des substances apparentées par
chromatographie liquide à haute performance et mesuré par
spectrophotométrie ultraviolette. Dans des milieux tels que les
déchets solides ou les substances biologiques, la méthyléthylcétone
doit d'abord être séparée du substrat, par exemple par extraction à
l'aide d'un solvant ou par distillation à la vapeur. Les
concentrations élevées de méthyléthylcétone dans l'air peuvent être
mesurées de façon continue par absorption infrarouge. Les limites de
détection sont de 3 µg/m3 dans l'air, 0,05 µg/litre dans l'eau de
boisson, 1,0 µg/litre dans les autres types d'eau, 20 µg/litre dans le
sang et 100 µg/litre dans l'urine.
2. Sources d'exposition et usages
2.1 Production et autres sources
Selon des statistiques récentes, la production annuelle (en
milliers de tonnes) est la suivante: Etats-Unis d'Amérique, 212 à 305;
Europe de l'Ouest, 215; Japon, 139. D'autres sources de contamination
de l'environnement par la méthyléthylcétone sont les gaz d'échappement
des réacteurs et des moteurs à combustion interne ainsi que certaines
activités industrielles comme la gazéification du charbon. Elle est
également présente en quantités notables dans la fumée de tabac. Aux
Etats-Unis d'Amérique, la quantité de méthyléthylcétone émise par les
moteurs représente plus de 1% de la quantité fabriquée volontairement.
En cas de smog, la production photochimique de méthyléthylcétone et
d'autres carbonyles à partir de radicaux libres peut être bien
supérieure aux émissions résultant des activités humaines. La
méthyléthylcétone peut aussi avoir une origine biologique et elle a
été identifiée parmi les produits du métabolisme microbien. Elle a
également été détectée dans divers produits naturels, notamment des
végétaux supérieurs, des phéromones d'insectes, des tissus animaux,
ainsi que chez l'homme dans le sang, l'urine et l'air expiré. Elle
constitue probablement un produit mineur du métabolisme normal des
mammifères.
2.2 Usages et pertes dans l'environnement
La méthyléthylcétone est un excellent solvant, ce qui explique
que sa principale application soit la fabrication de revêtements
protecteurs et d'adhésifs. Elle est également utilisée comme
intermédiaire chimique, comme solvant dans la fabrication des rubans
magnétiques, pour l'élimination des cires dans les huiles de graissage
et dans l'industrie alimentaire. Elle entre également dans la
composition de nombreux produits d'usage courant comme les vernis et
les colles. Dans la plupart de ces applications, la méthyléthylcétone
est mélangée à d'autres solvants organiques. La libération dans
l'environnement résulte principalement de l'évaporation des solvants
à partir des surfaces enduites et concerne essentiellement
l'atmosphère. La libération de méthyléthylcétone dans l'eau est la
conséquence de sa présence dans les effluents provenant de sa
fabrication et de diverses opérations industrielles. Elle a été
détectée dans des eaux naturelles dans lesquelles sa présence pourrait
s'expliquer par une activité microbienne, l'absorption à partir de
l'atmosphère ou une pollution anthropogène.
3. Transport et distribution dans l'environnement
La méthyléthylcétone est extrêmement mobile dans l'environnement
naturel où elle se renouvelle rapidement. Elle est très soluble dans
l'eau et s'évapore facilement. Dans l'atmosphère, elle subit une
décomposition photochimique rapide, mais elle est également
synthétisée par des processus photochimiques. Elle réagit avec les
halogènes libres ou les hypochlorites et leurs homologues halogénés
présents dans l'eau pour former un dérivé halogéné plus toxique que la
molécule initiale. La méthyléthyl-cétone est transportée par l'air et
par l'eau, mais elle ne s'accumule dans aucun compartiment et elle ne
persiste pas longtemps là où il existe une activité microbienne. Elle
est rapidement métabolisée par les microbes et les mammifères. Le
phénomène de bioaccumulation n'a jamais été mis en évidence. La
méthyléthylcétone existe naturellement dans certaines espèces de
trèfle et elle est produite par des champignons à des concentrations
qui peuvent affecter la germination de certaines plantes.
4. Concentration dans l'environnement et exposition humaine
La population générale est fréquemment exposée à de faibles doses
de méthyléthylcétone. Lorsque la pollution atmosphérique est très
faible, la concentration est inférieure à 3 µg/m3 (< 1 ppb), mais
on a mesuré jusqu'à 131 µg/m3 (44,5 ppb) en atmosphère très polluée.
En dehors des zones industrielles de fabrication ou d'utilisation de
la méthyléthylcétone, les gaz d'échappement des véhicules automobiles
et les réactions photochimiques dans l'atmosphère peuvent être les
principales sources de contamination. Les cigarettes et les autres
formes de tabac à fumer contribuent à l'exposition individuelle (20
cigarettes en contiennent jusqu'à 1,6 mg). La volatilisation de la
méthyléthylcétone présente dans les matériaux de construction et les
produits de consommation peut entraîner une pollution de l'air des
locaux bien supérieure à celle de l'air extérieur. Les concentrations
dans les eaux naturelles dépassent rarement 100 µg/litre (100 ppb) et
sont généralement inférieures au seuil de détection. Toutefois, on a
souvent trouvé des traces de méthyléthylcétone dans l'eau de boisson
(environ 2 µg/litre). Il est probable que les solvants entrant dans la
composition des joints des tuyauteries de plastique sont à l'origine
de cette pollution. Bien que la méthyléthylcétone soit un constituant
normal de nombreux aliments, les concentrations sont faibles et
l'alimentation ne peut être considérée comme une source importante
d'exposition. Aux Etats-Unis d'Amérique, la quantité moyenne ingérée
quotidiennement avec les aliments, principalement le pain blanc, les
tomates et le fromage Cheddar, est estimée à 1,6 mg par personne. La
méthyléthylcétone peut être présente naturellement dans les aliments,
mais elle peut aussi se former lors de l'affinage du fromage, de
l'entreposage de la viande de volaille, de la cuisson ou de la
transformation des aliments, ou être absorbée à partir des emballages
en matière plastique.
L'exposition industrielle à des concentrations modérées de
méthyléthylcétone est fréquente. Toutefois, dans certaines régions, le
personnel travaillant dans de petites entreprises (fabriques de
chaussures, imprimeries, ateliers de peinture) peut être exposé à des
concentrations beaucoup plus élevées en raison d'une ventilation
insuffisante. Ces travailleurs sont généralement exposés à un mélange
de solvants, notamment au n-hexane.
5. Cinétique et métabolisme
La méthyléthylcétone est rapidement absorbée par contact cutané,
inhalation, ingestion et injection intrapéritonéale. Elle passe très
vite dans le sang, et de là dans les autres tissus. Il semble que sa
solubilité soit à peu près la même dans tous les tissus. L'élimination
de la méthyléthylcétone et de ses métabolites est pratiquement totale
chez les mammifères au bout de 24 heures. Elle est métabolisée dans le
foie où la plus grande partie est oxydée en 3-hydroxy-2-butanone avant
d'être réduite en 2,3-butanediol. Une petite partie peut être réduite
en 2-butanol, mais celui-ci est rapidement oxydé pour redonner la
molécule initiale. Chez les mammifères, la majeure partie de la
méthyléthylcétone ingérée entre dans le cycle métabolique général
et/ou est éliminée sous forme de molécules simples comme le dioxyde de
carbone et l'eau. L'excrétion de la méthyléthylcétone et de ses
métabolites caractéristiques se fait principalement par les poumons,
bien que de petites quantités soient éliminées par les reins.
La méthyléthylcétone augmente l'activité enzymatique du
cytochrome P-450 microsomal. Il est possible que ce renforcement de
l'activité enzymatique, et par conséquent du potentiel de
transformation métabolique de l'organisme, explique pourquoi la
méthyléthylcétone potentialise la toxicité des solvants du groupe des
alcanes halogénés et des hydrocarbures aliphatiques à six atomes de
carbone.
6. Effets sur les animaux d'expérience
La méthyléthylcétone présente une toxicité faible à modérée pour
les mammifères, qu'il s'agisse de toxicité aiguë, à court terme ou
chronique. Chez la souris et le rat, la LD50 est de 2 à 6 g/kg de
poids corporel, la mort survenant 1 à 14 jours après l'ingestion d'une
dose unique. La dose moyenne entraînant la mort après une exposition
unique aux vapeurs de méthyléthylcétone est d'environ 29 400 mg/m3
(10 000 ppm), bien que des cobayes aient survécu à une exposition de
4 heures à cette concentration. Dans des essais d'intoxication aiguë
par voie orale, la dose la plus faible ayant entraîné une modification
de structure des organes a été de 1 g/kg de poids corporel chez le
rat. Cette dose a provoqué des lésions des tubules du rein.
L'inhalation par des rats d'air contenant 74 mg/m3 (25 ppm) pendant
6 heures a provoqué des changements de comportement mesurables qui ont
persisté pendant plusieurs jours. Une exposition répétée à 14 750
mg/m3 (5000 ppm) (6 h/jour, 5 jours/semaine) n'a provoqué la mort
d'aucun animal. On n'a observé qu'un effet mineur sur la croissance et
la structure et il n'y a eu aucune modification neuropathologique. Des
poulets, des chats ou des souris exposés à 3975 mg/m3 (1500 ppm)
pendant des périodes allant jusqu'à 12 semaines n'ont présenté aucun
signe de changement neuropathologique. Des effets transitoires sur le
comportement ou la neurophysiologie ont été détectés chez des rats et
des babouins à la suite d'expositions répétées à des concentrations ne
dépassant pas 295 à 590 mg/m3 (100 à 200 ppm).
Une faible foetotoxicité a été observée à 8825 mg/m3 (3000
ppm), mais elle ne s'accompagnait d'aucune toxicité maternelle; aucun
effet embryotoxique ou tératogène n'a été constaté à des
concentrations inférieures à cette valeur. Après avoir exposé de façon
répétée des rattes en gestation à une concentration de 8825 mg/m3,
on a observé dans leur progéniture une augmentation légère mais
significative de certains types d'anomalie du squelette rarement
observés chez les animaux non exposés.
Plusieurs épreuves classiques de mutagénicité ont été pratiquées,
mais la seule qui ait donné un résultat positif a été une étude
d'hétéroploïdie sur la levure Saccharomyces cerevisiae.
La méthyléthylcétone ne présente pas de toxicité aiguë pour les
poissons ou les invertébrés aquatiques, la CL50 se situant entre
1382 et 8890 mg/litre.
Elle a un effet inhibiteur sur la germination de plusieurs
plantes, même à des concentrations que l'on peut rencontrer dans la
nature. La croissance des algues aquatiques est également inhibée.
Des concentrations relativement élevées de méthyléthylcétone,
comparées aux concentrations naturelles, ont été utilisées dans des
expériences de fumigation. Cette substance s'est révélée un fumigant
modérément efficace contre la mouche des fruits des Caraïbes. D'autre
part, elle a un effet attractif très net sur la mouche tsé-tsé. Des
concentrations allant jusqu'à 20 mg/litre retardent le processus de
biodégradation mais ne l'arrêtent pas complètement. Jusqu'à 100
mg/litre, la méthyléthylcétone est bactériostatique pour différentes
bactéries. A des concentrations plus élevées (1000 mg/litre et
au-delà) elle inhibe la croissance des bactéries et des protozoaires.
7. Effets sur l'homme
7.1 Méthyléthylcétone seule
Aucun effet notable n'a été observé lors de tests psychologiques
et de comportement après exposition à 590 mg/m3 (200 ppm). Une
exposition de courte durée à la méthyléthylcétone seule ne semble pas
présenter de risques importants, que ce soit pour les professionnels
ou pour le public en général. L'exposition, dans des conditions
expérimentales, à une concentration de 794 mg/m3 (270 ppm), à raison
de 4 heures par jour, a eu un effet à peu près nul sur le comportement
et un contact de 5 minutes avec la substance liquide n'a provoqué
qu'une décoloration temporaire de la peau. On n'a signalé qu'un seul
cas d'intoxication aiguë par la méthyléthylcétone en dehors de tout
contexte professionnel. Il s'agit d'un cas d'ingestion accidentelle
qui ne semble pas avoir laissé de séquelles. On n'a jamais signalé de
cas d'exposition professionnelle ayant entraîné la mort. Il existe
deux rapports faisant état d'intoxication professionnelle chronique et
un rapport d'intoxication professionnelle aiguë, mais ce dernier est
sujet à caution. Dans un des cas d'intoxication chronique,
l'exposition à 880-1770 mg/m3 (300-600 ppm) a provoqué des
dermatoses, un engourdissement des doigts et des bras et divers
symptômes, parmi lesquels des céphalées, des étourdissements, des
troubles gastro-intestinaux et une perte d'appétit et de poids. Le
faible nombre d'intoxications attribuées à la méthyléthylcétone seule
tient à la fois à la faible toxicité de cette substance et au fait
qu'elle est rarement utilisée seule, mais plutôt en mélange avec
d'autres solvants.
7.2 Méthyléthylcétone dans les mélanges de solvants
L'exposition à des mélanges de solvants contenant de la
méthyléthylcétone a été associée à une réduction de la vitesse de
conduction nerveuse, à des troubles de la mémoire, à des troubles
moteurs, à des dermatoses et à des vomissements. Dans une étude
longitudinale, des mesures consécutives du temps de réaction simple
ont montré une amélioration des performances parallèlement à une
diminution de la concentration de méthyléthylcétone jusqu'à un dixième
de la valeur initiale (qui pouvait atteindre 4000 mg/m3 pour
certaines tâches de routine).
8. Renforcement de la toxicité des autres solvants
La méthyléthylcétone potentialise la neurotoxicité des solvants
à six atomes de carbone ( n-hexane, méthyl- n-butylcétone et
2,5-hexanedione) ainsi que la toxicité hépatique et rénale des
solvants de la famille des alcanes halogénés (tétrachlorure de carbone
et trichlorométhane).
La potentialisation des effets neurotoxiques des composés à six
atomes de carbone a été démontrée chez l'animal pour les trois
substances citées ci-dessus. Les neuropathies périphériques observées
chez l'homme se sont produites à la suite de changements dans la
formulation des solvants auxquels les sujets avaient été exposés, soit
volontairement, soit en raison de leur activité professionnelle. Le
mécanisme de cette potentialisation n'a pas été élucidé.
La potentialisation de la toxicité hépatique et rénale des
alcanes halogénés a été mise en évidence dans des études chez
l'animal. La méthyléthylcétone active probablement la métabolisation
des haloalcanes en substances toxiques pour les tissus en induisant la
production des enzymes oxydantes responsables de cette transformation.
RESUMEN
1. Propiedades y métodos analíticos
La metiletilcetona (MEC) es un líquido transparente, incoloro,
volátil, muy inflamable, de olor parecido a la acetona. Es estable en
condiciones normales pero puede formar peróxidos si se almacena
durante mucho tiempo; esos peróxidos pueden ser explosivos. La MEC
también puede formar mezclas explosivas con el aire. Es muy soluble en
agua, miscible con muchos disolventes orgánicos, y forma mezclas
azeotrópicas con el agua y con numerosos líquidos orgánicos. En la
atmósfera, la MEC produce radicales libres que pueden llevar a la
formación de nieblas fotoquímicas.
Existen varios métodos analíticos para medir los niveles
ambientales de MEC en el aire, el agua, las muestras biológicas, los
desechos y otros materiales. Con los métodos más sensibles, la MEC se
separa y se concentra ya sea en un sorbente sólido o como derivado de
la 2,4-dinitrofenilhidrazina (DNFH). La MEC y otros compuestos
orgánicos volátiles absorbidos son desorbidos, separados mediante
cromatografía de gases y medidos con un espectrómetro de masas o un
detector de ionización de llama. La MEC derivada se separa de los
compuestos afines mediante cromatografía de líquidos de alto
rendimiento y se mide mediante absorción ultravioleta. En medios como
desechos sólidos y material biológico, la MEC debe separarse en primer
lugar del sustrato con métodos como la extracción por solventes o la
destilación de vapores. Las concentraciones elevadas de MEC en el aire
pueden controlarse de modo continuo mediante absorción infrarroja. Los
límites de detección son 3 µg/m3 en el aire, 0,05 µg/litro en el
agua potable, 1,0 µg/litro en otros tipos de agua, 20 µg/litro en
sangre total y 100 µg/litro en orina.
2. Fuentes de exposición y usos
2.1 Producción y otras fuentes
Las cifras más recientes de fabricación industrial anual (en
miles de toneladas) son: EEUU, 212 a 305; Europa occidental, 215;
Japón, 139. Además de su fabricación, las fuentes de MEC en el medio
ambiente son los gases de escape de motores de reactores y de
combustión interna, y las actividades industriales como la
gasificación del carbón. Se encuentra en cantidades importantes en el
humo de tabaco. En los Estados Unidos, la producción de MEC en motores
no supera el 1% de su fabricación deliberada. En los episodios de
nieblas, la producción fotoquímica de MEC y otros carbonilos a partir
de radicales libres puede ser mucho mayor que la emisión antropogénica
directa. La MEC se produce biológicamente y se ha identificado como
producto del metabolismo microbiano. Se ha detectado asimismo en gran
diversidad de productos naturales, entre ellos los vegetales
superiores, las feromonas de insectos, en tejidos animales y en el
hombre, en sangre, orina y aire exhalado. Probablemente es un producto
secundario del metabolismo normal en el mamífero.
2.2 Usos y pérdidas al medio ambiente
El uso principal de la MEC, la aplicación de revestimientos
protectores y adhesivos, refleja sus excelentes características como
disolvente. También se utiliza como intermediario químico, como
disolvente en la producción de cintas magnéticas y para eliminar la
cera del aceite lubricante, así como en la manipulación de alimentos.
Además de las aplicaciones industriales, figura como ingrediente común
en productos de consumo como barnices y pegamentos. En la mayoría de
las aplicaciones, la MEC es componente de una mezcla de disolventes
orgánicos. Las pérdidas al medio ambiente son principalmente al aire
y se deben sobre todo a la evaporación de disolventes a partir de las
superficies revestidas. Se libera al agua como componentes de los
desechos de su fabricación y a partir de diversas operaciones
industriales. Se ha detectado en aguas naturales, procedente
probablemente de la actividad microbiana y del aporte atmosférico, así
como de la contaminación antropogénica.
3. Transporte y distribución en el medio ambiente
La MEC es sumamente móvil en el medio ambiente natural y está
sometida a una transformación rápida. Es muy soluble en el agua y se
evapora fácilmente a la atmósfera. En el aire, la MEC sufre una rápida
descomposición fotoquímica y es también sintetizada por procesos
fotoquímicos. En agua que contiene halógenos libres o hipohalitos,
reacciona para formar un haloformo más tóxico que el compuesto
original. La MEC se distribuye tanto por el aire como por el agua,
pero no se acumula en ningún compartimento aislado, ni persiste mucho
tiempo donde existe actividad microbiana. Se metaboliza rápidamente en
los microbios y los mamíferos. No hay pruebas de bioacumulación. La
MEC aparece naturalmente en algunas especies de trébol y es producida
por hongos en concentraciones que afectan a la germinación de algunas
plantas.
4. Niveles ambientales y exposición humana
La exposición de la población general a bajos niveles de MEC es
muy extensa. En aire poco contaminado, la concentración es inferior a
3 µg/m3 (< 1 ppmm), pero en condiciones de fuerte contaminación
atmosférica se ha medido un nivel de 131 µg/m3 (44,5 ppmm). Lejos de
las zonas industriales donde se fabrica o se usa la MEC, las
principales fuentes pueden ser los escapes de vehículos y las
reacciones fotoquímicas en la atmósfera. Los cigarrillos y otros
productos del tabaco que se someten a combustión contribuyen a la
exposición individual (20 cigarrillos contienen hasta 1,6 mg). La
volatilización de la MEC de materiales de construcción y productos de
consumo pueden contaminar el aire de interiores hasta niveles muy
superiores a los del aire libre adyacente. Las concentraciones de MEC
en aguas naturales expuestas rara vez se encuentran por encima de los
100 µg/litro (100 ppmm) y suelen encontrarse por debajo del nivel de
detección. No obstante, se han detectado cantidades muy reducidas de
MEC en el agua potable (aproximadamente 2 µg/litro) que probablemente
proceden de disolventes lixiviados a partir del material de las juntas
de las tuberías de plástico. Aunque la MEC es un componente normal de
muchos alimentos, las concentraciones son bajas y el consumo de
alimentos no puede considerarse una fuente significativa de exposición
para la población. La ingesta media diaria por habitante de los
Estados Unidos con los alimentos se calcula en 1,6 mg, en su mayor
parte a partir del pan blanco, los tomates y el queso tipo Cheddar.
Además de la MEC presente en el medio natural, puede producirse en la
maduración de los quesos, el envejecimiento de la carne de ave, la
cocción o la manipulación de alimentos, o por absorción a partir de
los envases de plástico.
La exposición industrial a niveles moderados de MEC está muy
extendida. No obstante, en algunas regiones los trabajadores de
fábricas pequeñas (por ejemplo, fábricas de calzado, imprentas y
fábricas de pinturas) están expuestos a concentraciones mucho más
elevadas por una ventilación insuficiente. En esas fábricas, la
exposición se da por lo general a una mezcla de disolventes entre los
que figura el n-hexano.
5. Cinética y metabolismo
La absorción de MEC es rápida por contacto cutáneo, inhalación,
ingestión e inyección intraperitoneal. Pasa rápidamente a la sangre y
de ella a otros tejidos. La solubilidad de la MEC parece similar en
todos los tejidos. La eliminación de la MEC y sus metabolitos en
mamíferos se completa en su mayor parte en 24 horas. Se metaboliza en
el hígado, donde se oxida a 3-hidroxi-2-butanona y a continuación se
reduce a 2,3-butanodiol. Una pequeña porción puede reducirse a
2-butanol, pero éste se oxida rápidamente para dar de nuevo MEC. La
mayor parte de la MEC que ingresa al organismo de mamíferos pasa al
metabolismo general y/o se elimina en forma de compuestos simples,
como dióxido de carbono y agua. La excreción de MEC y sus metabolitos
reconocibles se hace principalmente por los pulmones, aunque pequeñas
cantidades se eliminan por el riñón.
La MEC aumenta la actividad enzimática del citocromo P-450 en los
microsomas. Este aumento de la actividad enzimática y con ello del
potencial del organismo para la transformación metabólica puede ser el
mecanismo por el que la MEC potencia la toxicidad de los disolventes
a base de haloalcanos y hexacarbonos alifáticos.
6. Efectos en los animales de experimentación
La MEC tiene toxicidad aguda, a corto plazo y crónica de baja a
moderada en los mamíferos. Los valores de la DL50 en ratones y ratas
adultos son 2-6 g/kg de peso corporal; la muerte sobreviene en los
días 1 a 14 después de una sola dosis por vía oral. Las
concentraciones medias de vapor que producen letalidad en las ratas
tras una sola exposición giran en torno a los 29 400 mg/m3 (10 000
ppm), aunque los cobayos sobrevivieron a una exposición de 4 horas a
esta concentración. La dosis oral aguda más baja en modificar la
estructura corporal fue de 1 g/kg de peso corporal, que produjo
lesiones en los túbulos renales de la rata. La inhalación de 74
mg/m3 (25 ppm) durante 6 horas produjo en la rata cambios de
comportamiento medibles que persistieron durante varios días. La
exposición repetida de ratas a 14 750 mg/m3 (5000 ppm) (6 h/día, 5
días/semana) no produjo letalidad, tuvo sólo ligeros efectos en el
crecimiento y la estructura, y no se observaron cambios
neuropatológicos. No hubo pruebas de que la MEC produjera cambios
neuropatológicos en pollos, gatos o ratones expuestos a 3975 mg/m3
(1500 ppm) durante periodos de hasta 12 semanas. Tras la exposición
repetida de ratas y babuinos a concentraciones tan bajas como 295-590
mg/m3 (100 a 200 ppm) se observaron efectos transitorios en el
comportamiento o la neurofisiología.
Se ha observado un nivel bajo de fetotoxicidad sin toxicidad
materna a 8825 mg/m3 (3000 ppm), pero no hay pruebas de efectos
embriotóxicos o teratogénicos a niveles más bajos de exposición. La
exposición repetida de ratas preñadas a 8825 mg/m3 indujo en sus
crías un aumento pequeño pero significativo de ciertos tipos de
anomalías esqueléticas cuya incidencia entre la población no expuesta
es baja.
Aunque se examinó en varios sistemas de ensayo de mutagenicidad
convencionales, la única prueba de mutagenicidad se observó en un
estudio sobre aneuploidia en la levadura Saccharomyces cerevisiae.
La MEC no presenta toxicidad aguda para los peces ni los
invertebrados acuáticos; los valores de la CL50 varían desde 1382
hasta 8890 mg/litro.
La MEC inhibe la germinación de varias especies vegetales,
incluso con niveles que se dan en la naturaleza. Inhibe el crecimiento
de algas acuáticas.
En comparación con los niveles de base naturales, se han
utilizado concentraciones relativamente elevadas de MEC para fumigar
en condiciones experimentales. Es moderadamente eficaz como fumigante
contra la mosca caribeña de la fruta y atrae con gran eficacia a la
mosca tse-tse. Con niveles de MEC de hasta 20 mg/litro se retrasa la
biodegradación pero no se detiene el proceso por completo. Con niveles
de hasta 100 mg/litro, la MEC es bacteriostatica para algunas
bacterias. Con concentraciones más altas (1000 mg/litro o más) se
inhibe el crecimiento de bacterias y protozoarias.
7. Efectos en el ser humano
7.1 MEC por sí sola
La exposición a 590 mg/m3 (200 ppm) no tuvo efectos de
importancia en varios ensayos comportamentales y psicológicos. La
exposición a corto plazo a MEC por sí sola no parece constituir un
riesgo de importancia, ni ocupacional ni para el público en general.
La exposición experimental a una concentración de 794 mg/m3 (270
ppm) durante 4 h/día tuvo escaso o ningún efecto en el comportamiento,
y un contacto de 5 minutos con MEC líquida no produjo más que un
blanqueamiento temporal de la piel. Sólo hay un informe no ocupacional
de toxicidad aguda a la MEC. Se debió a una ingestión accidental y no
pareció producir lesiones duraderas. No hay pruebas de que la
exposición ocupacional a la MEC haya originado ningún caso de muerte.
Se han notificado dos casos de envenenamiento ocupacional crónico y
uno dudoso de envenenamiento ocupacional agudo. En uno de los casos
crónicos, la exposición a 880-1770 mg/m3 (300-600 ppm) dió lugar a
dermatosis, endormecimiento de los dedos y los brazos, y diversos
síntomas como dolor de cabeza, mareos, trastornos gastrointes-tinales
y pérdida de apetito y de peso. Esta escasez de incidentes de
envenamiento por MEC por sí sola refleja tanto su baja toxicidad como
el hecho de que se usa más comunmente no por sí sola sino como
componente de mezclas de disolventes.
7.2 La MEC en mezclas de disolventes
La exposición a mezclas de disolventes con MEC se ha asociado a
cierta reducción en la velocidad de conducción nerviosa, la memoria y
alteraciones motoras, dermatosis y vómitos. En un estudio
longitudinal, las medidas consecutivas de tiempo de reacción simple
demostraron que mejoraba el rendimiento en paralelo al ir disminuyer
de las con concentraciones de MEC hasta un décimo de los valores
originales (que fueron de hasta 4000 mg/m3 para ciertas tareas
rutinarias).
8. Potenciación de la toxicidad de otros disolventes
La MEC potencia la neurotoxicidad de compuestos hexacarbonados
( n-hexano, metil- n-butilcetona y 2,5-hexanodiona) y la toxicidad
hepatica y renal de los disolventes a base de haloalcanos
(tetracloruro de carbono y triclorometano).
La potenciación de los efectos neurotóxicos de los hexacarbonos
se ha demostrado con los tres hexacarbonos en el animal. Las
neuropatías periféricas observadas en humanos siguieron a cambios en
la formulacion de disolventes a los que habían estado expuestos, ya
sea voluntariamente o en el trabajo. El mecanismo por el que se
produce esta potenciación no está claro.
Las pruebas de la potenciación de la toxicidad hepática y renal
de los haloalcanos proceden de estudios animales. La MEC activa
probablemente el metabolismo de los haloalcanos de las especies que
dañan los tejidos como resultado de la inducción de las enzimas
oxidativas pertinentes.
10.1.3 Human studies
10.1.3.1 Solvent abuse
Solvent abuse, the deliberate inhalation of solvent vapours for
their euphoric effects, has been reported in many countries and has
involved the use of lacquer thinners, glues and other readily
available commercial items. Prockop et al. (1974) suggested that there
were several hundred habitual "huffers", i.e. solvent abusers, in
Tampa, Florida, USA, and Altenkirch et al. (1978) reported about 2000
in Berlin, Germany, in 1974. Outbreaks of polyneuropathy among Berlin
huffers, which involved n-hexane toxicity potentiated by MEK,
provided a major stimulus for research on the health effects of MEK
and its interactions with hexacarbon solvents.
Chronic huffers in Berlin inhaled fumes from about a half litre
of liquid per day, either poured into a plastic bag or over rags
(Altenkirch et al., 1977, 1982b). Inhalation sessions extended for as
long as 10 or 12 h, and exposure periods of 5 to 7 years were not
unusual. Prior to the end of 1975 no major damage to health resulting
from chronic solvent abuse had been observed. At that time there
appeared abruptly a number of cases of polyneuropathy among chronic
huffers. All these were young males, mainly between 16 and 21 years of
age. The initial symptom was paraesthesia of the toes accompanied in
some cases by weakness of the legs. The paraesthesia ascended rapidly
from distal to proximal and in 2 to 3 weeks affected the entire legs.
This was followed rapidly by paraesthesia of the arms which also
ascended from distal to proximal. Extensor muscles were always
affected first and most severely. There also was severe muscle atrophy
and a "glove and stocking" type sensory impairment of the hands and
feet. In all cases there also was excessive sweating of the hands and
feet, and in some cases discoloration and reduced skin temperature of
these areas. In addition there was loss of weight and damage to the
teeth. Head, neck and trunk muscles remained undisturbed, although in
severe cases there was paresis of the phrenic nerve and reduced
pulmonary function. The degree of impairment ranged from moderate
crippling, which permitted walking with assistance, to complete
tetraplegia. Symptoms continued to develop for 6 to 10 weeks after
cessation of solvent abuse. Remission was slow, took up to a year,
developed from proximal to distal, and was incomplete in those most
severely affected. Neurophysiological and histological findings were
similar to those reported in experimental animals (section 7). Motor
and sensory nerve conduction velocities were reduced in proportion to
the degree of paresis. There were lesions of the axons, paranodal axon
swellings, clumping of the nerve filaments and demyelination. The
outbreak of neuropathy came a few months after a change in the
formulation of a solvent from one composed of n-hexane, benzene
fraction, ethyl acetate and toluene to a similar mixture with less
(16%) n-hexane and the addition of 11% MEK. This was interpreted as
evidence that even long exposure to the substantial amount of
n-hexane (31%) in the original formulation did not result in
neuropathy, and that neuropathy developed only after the toxicity of
n-hexane was potentiated by simultaneous exposure to MEK.
The seven cases of polyneuropathy reported from Tampa, Florida,
USA, apparently resulted from a small amount of n-hexane (0.5%)
potentiated by other components in the solvent mixture (Prockop et
al., 1974; Spencer et al., 1980). Two similar cases of polyneuropathy
were reported from Japan (Goto et al., 1974), these were produced by
chronic sniffing of a glue that contained 25% n-hexane and 20% MEK.
What is puzzling in view of the absence of neuropathy in Berlin prior
to the addition of MEK to a solvent mixture containing n-hexane is
that Goto et al. (1974) also reported two cases of neuropathy
resulting from chronic sniffing of glue solvent containing only
n-hexane and toluene. However, Oh & Kim (1976) reported a case of
polyneuropathy produced by chronic abuse of mixtures containing MEK,
methyl isobutyl ketone (MIBK) and many other solvents, but apparently
not n-hexane or MBK. There was, however, no analysis of the solvent
mixtures. There was no experimental evidence to suggest that any of
the solvents known to be present in the mixtures alone, or MEK and
MIBK together, could produce this type of neuropathy. An alternative
explanation is that the MIBK contained MBK as an impurity.
10.1.3.2 Occupational exposure
Although there have been many cases of occupationally related
poisoning by exposure to neurotoxic hexacarbon solvents (Spencer et
al., 1980), poisonings in which neurotoxicity has been associated with
concurrent MEK exposure are limited. In occupational health studies
concentrating on n-hexane neurotoxicity, the study populations were
exposed to several other solvents, including MEK (WHO, 1991). One of
the most thoroughly investigated cases occurred in 1973 in a fabric
factory in Ohio, USA (Allen et al., 1974). In 1973, an employee at the
factory was found to have a severe sensorimotor neuropathy. Other
co-workers at the plant were also found to have similar symptoms,
which initiated a search for a causative agent in the workplace. Of
the 1157 workers examined, 86 manifested signs and symptoms indicative
of peripheral neuropathy, including parathesiae in arms and legs and
weakness in the hands and legs. Subsequent investigation found that in
the year prior to the confirmation of the first case, MBK had been
substituted for MIBK as a co-solvent with MEK. Exposure occurred by
skin contact and by inhalation. Measured concentrations of MEK in
certain areas of greatest exposure were 251-2251 mg/m3 (85-763 ppm),
while concentration for MBK ranged from 9 to 640 mg/m3 (2.3-156
ppm). The introduction of MBK into the factory was associated with the
observed neurotoxicity. Spencer et al. (1980) pointed out that several
animal studies showed that concurrent exposure to MEK accelerated
MBK-induced neurotoxicity.
10.2 Haloalkane solvents
10.2.1 Studies in animals
Carbon tetrachloride (CCl4), chloroform (CHCl3) and related
haloalkane solvents are liver and kidney poisons as well as central
nervous depressants (Gosselin et al., 1984). It has long been known
that the hepatotoxic action of CCl4 is potentiated by ethanol, and
more recently that the hepatic and/or renal toxicity of CCl4,
CHCl3, trichloroethylene, 1,1,2-trichloroethane and related
compounds is potentiated by n-hexane, ethanol, isopropanol, acetone,
MEK, MBK, 2,5-HD, and other ketones or chemicals that are metabolized
to ketones (Hewitt et al., 1980). Even an increase of naturally
occurring ketones in the body via diabetes can precipitate
potentiation. Decreasing the transformation of isopropanol to acetone
by the administration of an inhibitor of alcohol dehydrogenase,
pyrazole, has reduced potentiation of haloalkane toxicity. Although
the phenomenon is referred to as haloalkane toxicity, experimental
work in general appears largely or entirely limited to chlorinated
compounds, and specific studies on MEK are limited to interactions
with CCl4 and CHCl3.
The effects of MEK and CCl4 or CHCl3 on rats are summarized
in Table 17. At the doses used, MEK and the haloalkanes separately
produced mild liver and kidney injury at most. When exposure to MEK
was followed within 10 to 48 h by a haloalkane, there was severe
injury to the liver, with marked and abrupt replacement of normal
hepatic cells by necrotic and fatty, vacuolated tissue, an increase in
hepatic triglyceride, and, presumably, a corresponding decrease in
normal liver function. Hepatic enzymes were released into the blood by
breakdown of liver tissue, resulting in elevated levels of plasma
glutamic-pyruvic transaminase, plasma ornithine carbamyltransferase,
and plasma alanine aminotransferase. There was also an increase in
plasma bilirubin, although this was not accompanied by a decrease in
bile secretion as was the case with some other ketones (Hewitt et al.,
1986). A dose of MEK as small as 0.072 g/kg potentiated the effects of
CHCl3 given 18 h later, and 1.505 g/kg MEK potentiated the effects
of CCl4. Lower doses were not studied.
The mechanism of potentiation is not fully understood but
increased bioactivation of haloalkanes is believed to play a central
part in the potentiation effect. CCl4 is metabolized in vivo with
homolytic cleavage of the carbon-chlorine bond to produce highly
reactive free radicals that exert their toxic effects a) by binding
covalently to proteins and other elements, and b) via lipid
peroxidation (Anders, 1988). It is likely that the toxic effects of
CHCl3 also are produced in part by this mechanism (Hewitt et al.,
1980, 1987). The toxicity of CCl4 is enhanced by pretreatment with
various agents such as phenobarbital, ethanol, isopropanol, 2-butanol
and the ketone metabolites of the last two compounds (acetone and MEK)
(Cornish & Adefuin, 1967; Traiger & Plaa, 1972; Traiger & Bruckner,
1976; Gosselin et al., 1984). Recent studies have shown that the
ethanol-inducible cytochrome P-450 isozyme (P-450IIE1) plays an
important role in haloalkane metabolism (Johansson &
Ingelman-Sundberg, 1985). It is a high affinity enzyme and operates at
a low concentration range for various substrates (Nakajima et al.,
1990). CCl4 hepatotoxicity in rats has been found to be potentiated
by induction of the P-50IIE1 isozyme with ethanol and the injury was
most marked in the perivenous liver cells where the expression of
induction was the highest (Lindros et al., 1990). In addition to
ethanol, P-450IIE1 is known to be induced by acetone and MEK (Ko et
al., 1987; Raunio, et al., 1990; Albano et al., 1991). However, while
a relatively large oral dose of MEK (1.4 ml for 3 days) to rats
increased the amount of ethanol- and phenobarbital-inducible
cytochromes P-450 (P-450IIE1 and P-450IIB, respectively) (Raunio et
al., 1990), inhalation exposure of rats to 1770 mg MEK/m3 (600 ppm),
10 h/day for 7 days, caused only marginal effects on microsomal
cytochrome P-450 activities in the liver (Liira et al., 1991).
10.2.2 Potentiation of haloalkane toxicity in humans
There are no reports of MEK potentiation of haloalkane renal and
hepatic toxicity in humans.
11. EVALUATION OF HUMAN HEALTH RISKS AND EFFECTS ON THE ENVIRONMENT
11.1 Human health risks
11.1.1 Non-occupational exposure
Low level non-occupational exposure to MEK is widespread from a
variety of natural and anthropogenic sources, since MEK is a normal,
though minor, mammalian metabolite. External sources include food,
water and air. In the USA, the average daily per capita intake of MEK
from food is estimated to be 1.6 mg. In addition to MEK present
naturally, foods may contain MEK that has been added in food
processing or absorbed from plastic packaging materials. MEK is rarely
detected in exposed natural waters where it may originate from
microbial activity and atmospheric and anthropogenic inputs, but is
frequently detected at low concentrations in drinking-water where it
presumably is leached from cemented joints of plastic pipes. Leaching
from landfill hazardous waste dumps is another potential source of
groundwater, and hence drinking-water, contamination. Measured
concentrations in food and water are so low, however, that it is
unlikely that either of these represent a significant source of
exposure.
In minimally polluted outdoor air, the MEK concentration is less
than 3 µg/m3 (1.0 ppb) but has been measured at 134 µg/m3 (44.5 ppb)
under conditions of dense smog. Away from industrial areas where
MEK is manufactured or used, major sources may be vehicle exhaust and
photochemical reactions in the atmosphere. In smog episodes,
photochemical production of MEK may greatly exceed direct
anthropogenic emission. Volatilization of MEK from building materials
and consumer products can pollute indoor air to levels far above
outdoor air, and a concentration as high as 48 µg/m3 (12.9 ppb) was
measured in an Italian home. MEK also is present in tobacco smoke
(e.g., 80-207 µg/cigarette).
For the general population, daily MEK intake is estimated to
range between 1.6 and 4.2 mg, depending on the location site (rural or
urban), with an additional 1.6 mg in the case of smokers. There is no
evidence of any adverse effects on the general population from
exposure to MEK. Data from experimental animal studies show that toxic
effects occur at dose levels that are 3 orders of magnitude higher
than the estimated daily intake. Non-occupational poisoning from MEK
alone is limited to a single case, which resulted in no lasting
injury.
MEK is, however, known to potentiate the toxicity of two classes
of organic solvents, unbranched aliphatic hexacarbons and haloalkanes,
and chronic exposure to consumer products containing MEK and
n-hexane have produced outbreaks of polyneuropathy among individuals
deliberately inhaling fumes from these mixtures for their euphoric
effects. Co-exposure to MEK and either hexacarbons or haloalkanes via
the abuse of consumer products remains a potential public health
hazard. Injuries from such poisoning can be severe, permanently
disabling and even fatal.
11.1.2 Occupational exposure
MEK is an important industrial chemical which is used mainly as
a component of solvent mixtures for application of a wide variety of
coatings and adhesives. Moderate occupational exposure via air is
widespread because losses to the environment result mainly from
solvent evaporation from coated surfaces, and MEK is not viewed as an
especially dangerous substance. Most national limits for occupational
exposure are set at 590 mg/m3 (200 ppm), with a higher short-term
exposure level of 885 mg/m3 (300 ppm), and these limits appear
acceptable. On site measurements, however, indicate that workers may
be chronically exposed to still higher MEK concentrations in small
factories such as shoe factories, printing plants and painting
operations, due to inadequate ventilation. Lesser amounts of MEK are
lost to the air with concurrent worker exposure during manufacture,
shipping, repackaging and preparation of coatings and adhesives.
Industrial exposure from contact with liquid MEK does not appear an
important problem.
Chronic co-exposure to MEK and either unbranched aliphatic
hexacarbon or haloalkane solvents represents a significant potential
occupational hazard. Serious toxic effects could occur. Although there
are no records of industrial accidents involving MEK potentiation of
haloalkane toxicity, MEK potentiation of hexacarbon neurotoxicity may
have caused at least one major industrial accident in which an
outbreak of polyneuropathy followed introduction of MEK into a solvent
mixture. Thus MEK, in the mixed solvent atmosphere of many industrial
activities, can present a toxic hazard.
11.1.3 Relevant animal studies
Acute MEK toxicity has been shown in animal studies to be low by
the oral and inhalation route. The lowest oral dose modifying body
structure (damage of kidney tubules) was 1 g/kg body weight in rats.
Ten intraperitoneal injections of 34 mg/kg body weight over a 2-week
period produced transient injection site irritations but no effect on
the kidney. In a 90-day inhalation study, female rats exposed to 14.75
g/m3 for 6 h/day, 5 days per week, showed only slightly increased
liver weight, slightly decreased brain and spleen weight, and slightly
altered blood chemistry in comparison with controls; male rats showed
only a slightly increased liver weight. A transient decrease in nerve
conduction velocity was found following exposure to 590 mg/m3 (12
h/day for 24 weeks). The transient nature of neurological and
behavioural changes induced by MEK may be due to adaptation or more
rapid metabolism of MEK. Short-term dermal exposure to small amounts
of MEK results in mild local irritation, at most. Results of studies
of eye irritation are inconsistent, possibly due to different scoring
techniques; 4 mg created severe chemical burns in the eye in one study
whereas in other studies less severe signs were reported following a
dose of 80 mg.
An inhalation study provided evidence for low level fetotoxicity
in the absence of maternal toxicity at 8825 mg/m3. Thus MEK may be
a low grade teratogen in rats. There is a lack of data on other
aspects of reproduction in animals, and no relevant data have been
reported for humans.
MEK has given negative results in most conventional mutagenicity
assays. There is evidence of aneuploidy in yeast but this may not be
relevant to humans or other mammals.
11.2 Effects on the environment
MEK occurs naturally at low concentrations in air, water and
soil. It is highly mobile in the natural environment and is not
accumulated in any individual compartment. MEK is rapidly synthesized
and destroyed by photochemical processes in the atmosphere. There is
no specific information on either partitioning of MEK in any
environmental compartment or on chemical binding to sediment
particles.
MEK is synthesized biologically and is rapidly metabolized by
bacteria (even at high concentrations), mammals and probably many
other organisms. Levels produced by fungi can cause inhibition of
plant germination. Observations on microorganisms, higher plants,
invertebrates, fish and mammals suggest a low level of toxicity.
Environmental levels of MEK appear to be too low to cause any damage
except in the immediate vicinity of highly polluted sites. Effects on
the aquatic environment are likely to appear at levels between 1 and
10 mg/litre. The potentiation of solvent toxicity by MEK appears
environmentally irrelevant, although substantial information is
lacking. Overall, MEK does not represent a significant threat to the
environment.
12. RECOMMENDATIONS FOR THE PROTECTION OF HUMAN HEALTH AND THE
ENVIRONMENT
12.1 Human health protection
MEK on its own appears a relatively safe organic solvent, but its
use in combination with other solvents, in particular haloalkanes or
unbranched aliphatic hexacarbons, should be avoided. Industries should
be strongly encouraged to take all necessary precautions to ensure
that workers are not exposed to both MEK and solvents whose toxicity
is potentiated by MEK.
12.2 Environmental protection
MEK is unlikely to present a hazard to the environment except in
cases of major spills or discharges.
FURTHER RESEARCH
a) Further research should be undertaken to clarify the precise
mechanisms by which MEK potentiates the toxicity of haloalkanes
and hexacarbons.
b) Epidemiological studies are needed to determine exposure-response
relationships regarding MEK-induced potentiation of hexacarbon
and haloalkane toxicity.
c) Radiolabelled balance studies should be conducted to determine
accurately the routes and rates of excretion of MEK and its
metabolites. The results of such studies would be particularly
useful for improving methods of biological monitoring.
d) Comprehensive studies of reproductive and developmental toxicity
should be undertaken in representative rodent and non-rodent
species.
e) The binding capacity of soils and sediments for MEK should be
assessed.
REFERENCES
Aarstad K, Zahlsen K, & Nilsen OG (1985) Inhalation of butanols:
Changes in the cytochrome P-450 enzyme system. Arch Toxicol, 8(Suppl):
418-421.
Aarstad K, Zahlsen K, & Nilsen OG (1986) [Effects of inhalation of
different butanol isomers.] Faerg Lack Scand, 32: 69-74 (in Norwegian
with English summary).
Abdel-Rahman MS, Hetland LB, & Couri D (1976) Toxicity and metabolism
of methyl n-butyl ketone. Am Ind Hyg Assoc J, 37: 95-102.
ACGIH (1986) Documentation of the threshold limit values and
biological exposure indices, 5th ed. Cincinnati, Ohio, American
Conference of Governmental Industrial Hygienists, p 395.
ACGIH (1991) Threshold limit values and biological exposure indices
for 1988-1989. Cincinnati, Ohio, American Conference of Governmental
Industrial Hygienists.
Ahrenholz SH & Egilman DS (1983) Health hazard evaluation: Rubbermaid
Incorporated, Wooster, Ohio. Cincinnati, Ohio, National Institute of
Occupational Safety and Health, 33 pp (HETA 3-1340).
Albano E, Tomasi A, Persson J-O, Terelius Y, Goria-Gatti L,
Ingelman-Sundberg M, & Diazani MU (1991) Role of ethanol-inducible
cytochrome P-450 (P450IIE1) in catalysing the free radical activation
of aliphatic alcohols. Biochem Pharmacol, 41: 1895-1902.
Alderson MR & Rattan NS (1980) Mortality of workers on an isopropyl
alcohol plant and two MEK dewaxing plants. Br J Ind Med, 37: 85-89.
Allen N, Mendell JR, Billmaier D, & Fontaine RE (1974) An outbreak of
a previously undescribed toxic polyneuropathy due to industrial
solvent. Trans Am Neurol Assoc, 99: 74-79.
Altenkirch H, Mager J, Stoltenburg G, & Helmbrecht J (1977) Toxic
polyneuropathies after sniffing a glue thinner. J Neurol, 214:
137-152.
Altenkirch H, Stoltenburg G, & Wagner HM (1978) Experimental studies
on hydrocarbon neuropathies induced by methyl-ethyl-ketone (MEK). J
Neurol, 219: 159-170.
Altenkirch H, Stoltenburg-Didinger G, & Wagner HM (1979) Experimental
data on the neurotoxicity of methyl-ethyl-ketone (MEK).
Experientia(Basel), 35: 503-504.
Altenkirch H, Wagner HM, Stoltenburg G, & Spencer PS (1982a) Nervous
system responses of rats to subchronic inhalation of n-hexane and
n-hexane + methyl-ethyl-ketone mixtures. J Neurol Sci, 57: 209-219.
Altenkirch H, Wagner HM, Stoltenburg-Didinger G, & Steppat R (1982b)
Potentiation of hexacarbon-neurotoxicity by methyl-ethyl-ketone (MEK)
and other substances: Clinical and experimental aspects. Neurobehav
Toxicol Teratol, 4: 623-627.
Anders MW (1988) Bioactivation mechanisms and hepatocellular damage.
In: Arias IM, Jakoby WB, Popper H, Schachter D, & Schafritz DA ed. The
liver: Biology and pathology. New York, Raven Press Ltd, pp 389-400.
Anderson C, Sundberg K, & Groth O (1986) Animal model for assessment
of skin irritancy. Contact Dermatitis, 15: 143-151.
ANONYMOUS (1978) Atmospheric hydrocarbons in air during photochemical
episodes. Kanagawa-Ken Taike Osen Chosa Kenkyu Hokoku, 20: 86-90.
Anshelm Olson B, Gamberale F, & Grönqvist B (1981) Reaction time
changes among steel workers exposed to solvent vapours: A longitudinal
study. Int Arch Occup Environ Health, 48: 211-218.
Arques Espi E & Quintanilla Almagro T (1981) [Study of gluing stations
in 50 shoe plants: Simplified method of risk evaluation and normal
work technique.] In: [Proceedings of the National Congress of
Occupational Medicine, Hygiene and Safety: 9th meeting, 1980.] Madrid,
Spain, Occupational Hygiene and Safety Service, vol 2, pp 551-561 (in
Spanish).
Attygalle AB, Cammaerts MC, & Morgan ED (1983) Dufour gland secretions
of Myrmica rugulosa and Myrmica schencki workers. J Insect
Physiol, 29: 27-32.
Azuma K, Hirata T, Tsunoda H, Ishitani T, & Tanaka Y (1983)
Identification of the volatiles from low density polyethylene film
irradiated with an electron beam. Agric Biol Chem, 47: 855-860.
Banergee S & Howard PH (1988) Improved estimation of solubility and
partitioning through correction of UNIFAC-derived activity
coefficients. Environ Sci Technol, 22: 839-841.
Basler A (1986) Aneuploidy-inducing chemicals in yeast evaluated by
the micronucleus test. Mutat Res, 174: 11-13.
Bassette R & Ward G (1975) Measuring parts per billion of volatile
materials in milk. J Dairy Sci, 58: 428-429.
Basu P, Carpenter J, Nelson H, Perry W, Shochet A, Sun C, & Taylor D
(1981) Toxic Substances Control Act (TSCA), Section 4 - Human exposure
assessment: methyl ethyl ketone. McLean, Virginia, JRB Associates,
pp 1/1-R/1 (EPA Contract No. 68-01-4839).
Berg EF (1971) Retrobulbar neuritis. Ann Ophthalmol, 3: 1351, 1353.
Bernard AM, De Russis R, Normand J-C, & Lauwerys RR (1989) Evaluation
of the subacute nephrotoxicity of cyclohexane and other industrial
solvents in the female Sprague-Dawley rat. Toxicol Lett, 45: 271-280.
Bills DD, Willits RE, & Day EA (1966) Determination of some neutral
volatile constituents in ten commercial Cheddar cheeses. J Dairy Sci,
49: 681-685.
Binaschi S, Gazzaniga G, & Crovato E (1976) Behavioral toxicology in
the evaluation of the effects of solvent mixtures. Adverse Eff Environ
Chem Psychotropic Drugs, 2: 91-98.
Blum M, Warter S, & Traynham J (1966) Chemical releasers of social
behavior - VI. The relation of structure to activity of ketones as
releasers of alarm for Iridomyrex pruinosus (Roger). J Insect
Physiol, 12: 419-427.
Blum M, Warter S, & Traynham J (1971) Alarm pheromones: utilization in
evaluation of olfactory theories. J Insect Physiol, 17: 2351-2361.
Boch R & Shearer D (1971) Chemical releasers of alarm behavior in the
honey-bee, Apis mellifera. J Insect Physiol, 17: 2277-2285.
Boettner EA, Ball GL, Hollingsworth Z, & Aquino R (1981) Organic and
organotin compounds leached from PVC and CPVC pipe. Ann Arbor,
Michigan, US Environmental Protection Agency, 116 pp
(EPA-600/1-81-062).
Botta D, Castellani Pirri L, & Mantica E (1984) Ground water pollution
by organic solvents and their microbial degradation products. In:
Analysis of organic micropollutants in water. Brussels, Commission of
the European Communities, pp 261-275 (Report EUR 8518).
Bradow JM & Connick WJ (1988) Volatile methyl ketone seed-germination
inhibitors from Amaranthus palmeri S Wats. residues. J Chem Ecol,
14: 1617-1631.
Bradow JM & Connick WJ (1990) Volatile seed germination inhibitors
from plant residues. J Chem Ecol, 16: 645-666.
Brady JF, Li D, Ishizaki H, Lee M, Ning SM, Xiao F, & Yang CS (1989)
Induction of cytochromes P450 IIE1 and P450IIB1 by secondary ketones
and the role of P450IIE1 in chloroform metabolism. Toxicol Appl
Pharmacol, 100: 342-349.
Bridie AL, Winter M, & Wolff CJM (1979a) BOD and COD of some
petrochemicals. Water Res, 13(7): 627-630.
Bridie AL, Wolff CJM, & Winter M (1979b) The acute toxicity of some
petrochemicals to goldfish. Water Res, 13: 623.
Bringmann G (1978) [Determination of the biological toxicity of water
pollutants on Protozoa.] Z Wasser Abwasser Forsch, 11: 210-215 (in
German).
Bringmann G & Kuhn R (1977a) [Toxicity threshold for water pollutants
in the cell multiplication test with respect to bacteria (Pseudomonas
putida) and green algae (Scenedesmus quadricauda).] Z Wasser
Abwasser Forsch, 10: 87-98 (in German).
Bringmann G & Kuhn R (1977b) [The effects of water pollutants on
Daphnia magna.] Z Wasser Abwasser Forsch, 10: 161-166 (in German).
Bringmann G & Kuhn R (1978) Testing of substances for their toxicity
threshold: Model organisms Microcystis (Diplocystis) aeruginosa and
Scenedesmus quadricauda. Mitt Int Ver Limnol, 21: 275-284.
Brondeau MT, Ban M, Bonnet P, Guenier JP, & De Ceaurriz J (1989)
Acetone compared to other ketones in modifying the hepatotoxicity of
inhaled 1,2-dichlorobenzene in rats and mice. Toxicol Lett, 49: 69-78.
Brooks TM, Meyer AL, & Hutson DH (1988) The genetic toxicology of some
hydrocarbon and oxygenated solvents. Mutagenesis, 3: 227-232.
Brown EM & Hewitt WR (1984) Dose-response relationships in
ketone-induced potentiation of chloroform hepato- and nephrotoxicity.
Toxicol Appl Pharmacol, 76: 437-453.
Brown RH & Purnell CJ (1979) Collection and analysis of trace organic
vapour pollutants in ambient atmospheres. The performance of a
Tenax-GC absorbent tube. J Chromatogr, 178: 79-90.
Brugnone F (1985) Uptake of solvents from the lungs. Br J Ind Med,
42: 569.
Brugnone F, Perbellini L, Gaffuri E, & Costa G (1981) [Occupational
pollution by shoe factory solvents.] Ann Ist Super Sanità, 17: 531-534
(in Italian).
Brugnone F, Perbellini L, Apostoli P, Caretta D, & Cocheo V (1983)
Environmental and behavioural monitoring of occupational methyl ethyl
ketone exposure. Dev Sci Pract Toxicol, 11: 571-574.
Bryce DJ & Greenwood CT (1963) The thermal degradation of starch. Part
III. The formation of decomposition products from starch and related
materials at temperatures between 175 and 400 °C. Stärke, 15(19):
359-363.
Cammaerts MC, Inwood MR, Morgan ED, Parry K, & Tyler RC (1978)
Comparative study of the pheromones emitted by workers of the ants
Myrmica rubra and Myrmica scabrinodis. J Insect Physiol, 24:
207-214.
Carpenter CP & Smyth HF (1946) Chemical burns of the rabbit cornea. Am
J Ophthamol, 29: 1363-1372.
Cavender FL, Casey HW, Salem H, Swenberg JA, & Gralla EJ (1983) A
90-day vapor inhalation toxicity study of methyl ethyl ketone. Fundam
Appl Toxicol, 3: 264-270.
Chemical Business Newsbase (1986) #537712 [Organic chemicals
production in Argentina 1985.] Ind Quim, 281: 36-37 (in Spanish).
Chemical Business Newsbase (1987) #584370 The chemical industry of
Japan, 1986: B-B fraction derivatives. Japan Chem Ann, 1987/1988: 41.
Chemical Business Newsbase (1988) #600843 Methyl ethyl ketone makers
need Pampa for recovery. Chem Mark Rep, 234(16): 9, 18-19.
Chen T-H, Kavanagh TJ, Chang CC, & Trosko JE (1984) Inhibition of
metabolic cooperation in Chinese hamster V79 cells by various organic
solvents and simple compounds. Cell Biol Toxicol, 1: 155-171.
Chiavari G, Facchini MC, & Fuzzi S (1987) Behavior of
3-methyl-2-benzothiazolone azines of carbonyl compounds in
high-performance liquid chromatography. J Chromatogr, 387: 459-466.
Chiou CT, Freed VH, Siddigi RH, & Mckeon K (1977) Partition
coefficients and bioaccumulation of selected organic chemicals.
Environ Sci Technol, 11: 475-478.
Clayton GD & Clayton FE (1981) Patty's industrial hygiene and
toxicology. New York, John Wiley & Sons, Wiley Interscience, 3 vols.
Cohen SR & Maier AA (1974) Occupational health case report - No. 2,
toluene diisocyanate. J Occup Med, 16: 114-118.
Conkle JP, Camp BJ, & Welch BE (1975) Trace composition of human
respiratory gas. Arch Environ Health, 30: 290-295.
Connick WJ, Bradow JM, & Legendre MG (1989) Identification and
bioactivity of volatile allelochemicals from amaranth residues.
J Agric Food Chem, 37: 792-796.
Cornish HH & Adefuin J (1967) Potentiation of carbon tetrachloride
toxicity by aliphatic alcohols. Arch Environ Health, 14: 447-449.
Corwin JF (1969) Volatile oxygen-containing organic compounds in sea
water: Determination. Bull Mar Sci, 19: 504-509.
Couri D, Hetland LB, O'Neill JJ, Ganansia M-F, Jackson DB, Gardier RW,
Marks BH, Weiss H, Mendell JR, Saida K, Allen N, & Chrisman CL (1974)
Comments on a plastics industry neurotoxicity in relationship to
methylbutyl ketone. In: Proceedings of the 5th Annual Conference on
Environmental Toxicology, Fairborn, Ohio, 24-26 September 1974. Wright
Patterson Air Force Base, Ohio, Aerospace Medical Research Laboratory,
pp 109-120 (AMRL Technical Report No. 74-125).
Couri D, Hetland LB, Abdel-Rahman MS, & Weiss H (1977) The influence
of inhaled ketone solvent vapors on hepatic microsomal
biotransformation activities. Toxicol Appl Pharmacol, 41: 285-289.
Couri D, Abdel-Rahman MS, & Hetland LB (1978) Biotransformation of
n-hexane and methyl n-butyl ketone in guinea-pigs and mice. Am Ind Hyg
Assoc J, 39: 295-300.
Creech G, Johnson RT, & Stoffer JO (1982) A comparison of three
different high-performance liquid chromatography systems for the
determination of aldehydes and ketones in diesel exhaust. Part I.
J Chromatogr Sci, 20: 67-72.
Cresci A, La Rosa F, Pannelli F, Orpianesi C, Saltamacchia G, Spinaci
G, & Pierini M (1985) [Solvent exposure and work sites in eleven shoe
and leather factories.] Nuovi Ann Ig Microbiol, 36: 61-76 (in
Italian).
Curtis C, Lima A, Lozano SJ, & Veith GD (1982) Evaluation of a
bacterial bioluminescence bioassay as a method for predicting acute
toxicity of organic chemicals to fish. In: Pearson JG, Foster RB, &
Bishop WE ed. Aquatic toxicology and hazard assessment: Fifth
Conference. Philadelphia, Pennsylvania, American Society for Testing
and Materials, pp 170-178 (ASTM STP 766).
Cushny AR (1910) On the exhalation of drugs by the lungs. J Physiol,
40: 17-27.
Davis PL, Munroe KA, & Selhime AG (1977) Laboratory bioassay of
volatile naturally occurring compounds against the Caribbean fruit
fly. Citrus Ind, July: 24-26.
Day EA, Bassette R, & Keeney M (1960) Identification of volatile
carbonyl compounds from Cheddar cheese. J Dairy Sci, 43: 463-474.
Deacon MM, Pilny MD, John JA, Schwetz BA, Murray FJ, Yakel HO, & Kuna
RA (1981) Embryo- and fetotoxicity of inhaled methyl ethyl ketone in
rats. Toxicol Appl Pharmacol, 59: 620-622.
De Bortoli M, Knoppel H, Pecchio E, Peil A, Rogora L, Schauenburg H,
Schlitt H, & Vissers H (1985) Measurements of indoor air quality and
comparison with ambient air: A study on 15 homes in northern Italy.
Ispra, Italy, Commission of the European Communities, 57 pp.
De Bortoli M, Knoppel H, Pecchio E, Peil A, Rogora L, Schauenburg H,
Schlitt H, & Vissers H (1986) Concentrations of selected organic
pollutants in indoor and outdoor air in northern Italy. Environ Int,
12: 343-350.
Decaprio AP (1987) n-Hexane neurotoxicity: A mechanism involving
pyrrole adduct formation in axonal cytoskeletal protein.
Neurotoxicology, 8: 199-210.
De Ceaurriz J, Desilies JP, Bonet P, Marignac B, Muller J, & Guenier
JP (1983) Concentration-dependent behavioral changes in mice
following short-term inhalation exposure to various industrial
solvents. Toxicol Appl Pharmacol, 67: 383-389.
Decker DW, Clark CS, Elia VJ, Kominsky JR, & Trapp JH (1983) Worker
exposure to organic vapors at a liquid chemical waste incinerator. Am
Ind Hyg Assoc J, 44: 296-300.
Delfino JJ & Miles CJ (1985) Aerobic and anaerobic degradation of
organic contaminants in Florida groundwater. Soil Crop Sci Soc Florida
Proc, 44: 9-14.
Del Rosario R, De Lumen BO, Habu T, Flath RA, Mon TR, & Teranishi R
(1984) Comparison of headspace volatiles from winged beans and
soybeans. J Agric Food Chem, 32: 1011-1015.
Denkhaus W, Von Steldern D, Botzenhardt U, & Konietzko H (1986)
Lymphocyte subpopulations in solvent-exposed workers. Int Arch Occup
Environ Health, 57: 109-115.
De Rosa E, Bartolucci GB, Brighenti F, Gori GP, Sigon M, & Toffolo D
(1985) The industrial use of solvents and risk of neurotoxicity. Ann
Occup Hyg, 29(1): 391-397.
Descotes J (1988) Identification of contact allergens: The mouse ear
sensitization assay. J Toxicol Cutan Ocular Toxicol, 7: 263-272.
Deveaux M & Huvenne JP (1987) Identification of solvents of abuse
using gas chromatography/Fourier transform infrared spectrometry after
headspace sampling. Chromatographia, 23: 626-630.
Dick RB, Setzer JV, Wait R, Hayden MB, Taylor BJ, Tolos B, &
Putz-Anderson V (1984) Effects of acute exposure of toluene and
methyl ethyl ketone on psychomotor performance. Int Arch Occup Environ
Health, 54: 91-109.
Dick RB, Brown WD, Setzer JV, Taylor BJ, & Shukla R (1988) Effects of
short duration exposures to acetone and methyl ethyl ketone. Toxicol
Lett, 43: 31-49.
Dick RB, Setzer JV, Taylor BJ, & Shukla R (1989) Neurobehavioral
effects of short duration exposures to acetone and methyl ethyl
ketone. Br J Ind Med, 46: 111-121.
Dietz FK & Traiger GJ (1979) Potentiation of CCl4 of hepatotoxicity
in rats by a metabolite of 2-butanone: 2,3-butanediol. Toxicology,
14: 209-215.
Dietz FK, Rodriguez-Giaxola M, Traiger GJ, Stella VJ, & Himmelstein KJ
(1981) Pharmacokinetics of 2-butanol and its metabolites in the rat.
J Pharmacokinet Biopharm, 9: 553-576.
Digiacomo JD (1973) New approaches for the design of afterburners for
varnish cookers. J Air Pollut Control Assoc, 23: 287-290.
Dilling WL, Bredeweg DJ, & Tefertiller NB (1976) Organic
photochemistry. XIII. Simulated atmospheric photodecomposition rates
of methylene chloride, 1,1,1-trichloro-ethane, tetrachloroethylene,
and other compounds. Environ Sci Technol, 10: 351-356.
Divincenzo GD & Krasavage WJ (1974) Serum ornithine carbamyl
transferase as a liver response test for exposure to organic solvents.
Am Ind Hyg Assoc J, 35: 21-29.
Divincenzo GD, Kaplan CJ, & Dedinas J (1976) Characterization of the
metabolites of methyl-N-butyl ketone, methyl iso-butyl ketone, and
methyl ethyl ketone in guinea-pig serum and their clearance. Toxicol
Appl Pharmacol, 36: 511-522.
Divincenzo GD, Hamilton ML, Kaplan CJ, Krasavage WJ, & O'Donoghue JL
(1978) Studies on the respiratory uptake and excretion and the skin
absorption of methyl-n-butyl ketone in humans and dogs. Toxicol Appl
Pharmacol, 44: 593-604.
Dojlido JR (1977) Testing of biodegradability and toxicity of organic
compounds in industrial wastewaters. In: Polish/US Symposium on
Wastewater Treatment and Sludge Disposal, Cincinnati, Ohio, 10-12
February 1976. Cincinnati, Ohio, US Environmental Protection Agency,
vol II, pp 112-131 (EPA 600/9-76-021; NTIS PB-261422).
Dojlido JR (1979) Investigations of biodegradability and toxicity of
organic compounds. Washington, DC, US Environmental Protection Agency,
99 pp (EPA-600/2-79-163).
Dore M, Brunet N, & Legube B (1975) Participation of various organic
compounds in the evaluation of global pollution criteria. Trib
Cebedeau, 28: 3-11.
Dornseifer TP, Kim SC, Keith ES, & Powers JJ (1965) Effect of moisture
level on volatile carbonyls in cottonseed oil heated to 210 °C. J Am
Oil Chem Soc, 42: 1073-1075.
Dowty BJ, Laseter JL, & Storer J (1976) The transplacental migration
and accumulation in blood of volatile organic constituents. Pediatr
Res, 10: 696-701.
Draize JH, Woodard G, & Calvery HO (1944) Methods for the study of
irritation and toxicity of substances applied topically to the skin
and mucous membranes. J Pharmacol Exp Ther, 82: 377-389.
Duckett S, Williams N, & Francis S (1974) Peripheral neuropathy
associated with inhalation of methyl-n-butyl ketone.
Experientia(Basel), 30: 1283-1284.
Dutch Expert Committee for Occupational Standards (1991) Health-based
recommended occupational exposure limit for methyl ethyl ketone.
Voorburg, Directorate General of Labour of the Ministry of Social
Affairs and Employment, Dutch Expert Committee for Occupational
Standards, 45 pp (Report RA 16/90).
Dyro FM (1978) Methyl ethyl ketone polyneuropathy in shoe factory
workers. Clin Toxicol, 13: 371-376.
Egyud LG (1967) Studies on cell division: the effect of aldehydes,
ketones and a-keto-aldehydes on the proliferation of Escherichia
coli. Curr Mod Biol, 1: 14-20.
Elskamp CJ & Schultz GR (1983) An alternative sampling and analytical
method for 2- butanone. Am Ind Hyg Assoc J, 44: 201-204.
Emmel TE, Lee BB, & Simonson AB (1983) Control technology assessment
of petroleum refinery operations. In depth site visit report: Congo
Refinery, Farmer's Valley Refinery. Cincinnati, Ohio, National
Institute of Occupational Safety and Health, 60 pp (Contract No.
210-81-7102).
Ewing BB, Chian ESK, Cook JK, Evans CA, Hopke PK, & Perkins EG (1977)
Monitoring to detect previously unrecognized pollutants in surface
waters. Appendix: Organic analysis data. Washington, DC, US
Environmental Protection Agency, p 75 (EPA- 560/6-77-015).
Fagius J & Grönqvist B (1978) Function of peripheral nerves and signs
of polyneuropathy in solvent-exposed workers at Swedish steelworks.
Acta Neurol Scand, 57: 305-316.
Falla NAR (1987) Solving air pollution problems in the surface
coatings industry. Polym Paint Colour J, 98: 103-104, 106, 108, 110.
Feigley CE & Chastain JB (1982) An experimental comparison of three
diffusion samplers exposed to concentration profiles of organic
vapors. Am Ind Hyg Assoc J, 43: 227-234.
Fernandes MH, Gilbert SG, Paik SW, & Stier EF (1986) Study of the
degradation products formed during extrusion lamination of an ionomer.
J Food Sci, 51: 722-725.
Fiserova-Bergerova V & Diaz ML (1986) Determination and prediction of
tissue-gas partition coefficients. Int Arch Occup Environ Health,
58: 75-87.
Fisher GS, Legendre MG, Lovgren NV, Schuller WH, & Wells JA (1979)
Volatile constituents of southernpea seed [ Vigna unguiculata (L.)
Walp.] J Agric Food Chem, 27: 7-11.
Fisk JF (1986) Volatile organic analytical methods - general
description and quality control considerations. In: Perket CL ed.
Quality control in remedial site investigation: Fifth volume -
Hazardous and industrial solid waste testing. Philadelphia,
Pennsylvania, American Society for Testing and Materials, 12 pp (ASTM
STP 925).
Florin I, Rutberg L, Curvall M, & Enzell CR (1980) Screening of
tobacco smoke constituents for mutagenicity using the Ames' test.
Toxicology, 18: 219-232.
Freddi A, Paci A, Vittori O, De Ciantis R, & Ottaviano PF (1982)
[Clinical and electromyographic study of workers exposed to methyl
ethyl ketone vapor.] Ann Med Perugia, 73: 111-136 (in Italian).
French RC (1961) Stimulation of uredospore germination in wheat stem
rust by terpenes and related compounds. Bot Gaz, 122: 194-198.
French RC (1984) Stimulation of uredospore germination of Puccinia
helianthi and Uromyces vignae by aromatic nitriles and related
flavorlike compounds. J Agric Food Chem, 32: 556-561.
French RC, Graham CL, Gale AW, & Long RK (1977) Structural and
exposure time requirements for chemical stimulation of germination of
uredospores of Uromyces phaseoli. J Agric Food Chem, 25: 84-88.
Gadomski RR, Gimborne AV, & Green WJ (1974) An evaluation of emissions
and control technologies for the metal decorating process. J Air
Pollut Control Assoc, 24: 579-585.
Garcia CR, Geller I, & Kaplan HL (1978) Effects of ketones on
lever-pressing behavior of rats. Proc West Pharmacol Soc, 21: 433-438.
Geller I, Martinez RL, Hartmann RJ, & Kaplan HL (1978) Effects of
ketones on a match to sample task in the baboon. Proc West Pharmacol
Soc, 21: 439-442.
Gerhold RM & Malaney GW (1966) Structural determinants in the
oxidation of aliphatic compounds by activated sludge. J Water Pollut
Control Fed, 38: 562-579.
Ghittori S, Imbriani M, Pezzagno G, & Capodaglio E (1987) The urinary
concentration of solvents as a biological indicator of exposure:
Proposal for the biological equivalent exposure limit for nine
solvents. Am Ind Hyg Assoc J, 48: 786-790.
Gianturco MA, Giammarino S, & Friedel P (1966) Volatile constituents
of coffee-V. Nature (Lond), 210(5043): 1358.
Glowa JR & Dews PB (1987) Behavioral toxicology of volatile organic
solvents. IV. Comparisons of the rate-decreasing effects of acetone,
ethyl acetate, methyl ethyl ketone, toluene, and carbon disulfide on
schedule-controlled behavior of mice. J Am Coll Toxicol, 6: 461-469.
Gordon DT & Morgan ME (1972) Principal volatile compounds in feed
flavored milk. J Dairy Sci, 55: 905-912.
Gosselin RE, Smith RP, & Hodge HC (1984) Clinical toxicology of
commercial products. Baltimore, Maryland, Williams and Wilkins, pp
III/101-III/107.
Goto I, Matsumura M, Inoue N, Murai Y, Shida K, Santa T, & Kuroiwa Y
(1974) Toxic polyneuropathy due to glue sniffing. J Neurol Neurosurg
Psychiatry, 37: 848-853.
Grey TC & Shrimpton DH (1967) Volatile components of raw chicken
breast muscle. Br Poult Sci, 8: 23-33.
Grosjean D (1982) Formaldehyde and other carbonyls in Los Angeles
ambient air. Environ Sci Technol, 16: 254-262.
Grosjean D & Wright B (1983) Carbonyls in urban fog, ice, cloudwater
and rainwater. Atmos Environ, 17: 2093-2096.
Grosjean D, Swanson RD, & Ellis C (1983) Carbonyls in Los Angeles air:
Contribution of direct emissions and photochemistry. Sci Total
Environ, 29: 65-85.
Gurka DF, Warner JS, Silvon LE, Bishop TA, & Mckown MM (1984) Interim
method for determination of volatile organic compounds in hazardous
wastes. J Assoc Off Anal Chem, 67: 776-782.
Hampton CV, Pierson WR, Harvey TM, Updegrove WS, & Marano RS (1982)
Hydrocarbon gases emitted from vehicles on the road. 1. A qualitative
gas chromatography/mass spectrometry survey. Environ Sci Technol, 16:
287-298.
Harvey RJ & Walker JRL (1960) Some volatile compounds in New Zealand
Cheddar cheese and their possible significance in flavour formation.
III Time of first appearance of volatile carbonyl compounds during
ripening. J Dairy Res, 27: 335-340.
Hawthorne SB, Sievers RE, & Barkley RM (1985) Organic emissions from
shale oil wastewaters and their implications for air quality. Environ
Sci Technol, 19: 992-997.
Hazleton (1963a) Primary skin irritation-rabbits. Falls Church,
Virginia, Hazleton Laboratories Inc. (Laboratory Report No. 63 MRL
003).
Hazleton (1963b) Acute eye irritation-rabbits. Falls Church, Virginia,
Hazleton Laboratories Inc. (Laboratory Report No. 63 MRL 004).
Heitmuller PT, Hollister TA, & Parrish PR (1981) Acute toxicity of 54
industrial chemicals to sheepshead minnows (Cyprinodon variegatus).
Bull Environ Contam Toxicol, 27: 596-604.
Hewitt WR, Miyajima H, Cote MG, & Plaa GL (1980) Modification of
haloalkane- induced hepatotoxicity by exogenous ketones and metabolic
ketosis. Fed Proc, 39: 3118-3123.
Hewitt WR, Brown EM, & Plaa GL (1983) Relationship between the carbon
skeleton length of ketonic solvents and potentiation of
chloroform-induced hepatotoxicity in rats. Toxicol Lett, 16: 297-304.
Hewitt LA, Ayotte P, & Plaa GL (1986) Modifications in rat
hepatobiliary function following treatment with acetone, 2-butanone,
2-hexanone, mirex, or chlordecone and subsequently exposed to
chloroform. Toxicol Appl Pharmacol, 83: 465-473.
Hewitt LA, Valiquette C, & Plaa GL (1987) The role of
biotransformation-detoxication in acetone-, 2-butanone-, and
2-hexanone-potentiated chloroform-induced hepatotoxicity. Can J
Physiol Pharmacol, 65: 2313-2318.
Hickey JLS & Bishop CC (1981) Field comparison of charcoal tubes and
passive vapor monitors with mixed organic vapors. Am Ind Hyg Assoc J,
42: 264-267.
Higgins CE, Griest WH, & Olerich G (1983) Application of Tenax
trapping to analysis of gas phase organic compounds in ultra-low tar
cigarette smoke. J Assoc Off Anal Chem, 66: 1074-1083.
Holmberg I & Malmfors T (1974) The cytotoxicity of some organic
solvents. Environ Res, 7: 183-192.
Horton AW, Bingham EL, Burton MJG, & Tye R (1965) Carcinogenesis of
the skin. III. The contribution of elemental sulfur and of organic
sulfur compounds. Cancer Res, 25: 1759-1763.
Hoshika Y, Kozima I, Koike K, & Yosimoto K (1976) [Gas chromatographic
analysis of lower aliphatic carbonyl compounds using the imine
formation reaction.] Aichi-ken Kogai Chosa Senta Shoho, 4: 108-113 (in
Japanese).
Hou CT, Patel R, Laskin AI, Barnabe N, & Barist I (1983) Production of
methyl ketones from secondary alcohols by cell suspensions of C2 to
C4 n-alkane-grown bacteria. Appl Environ Microbiol, 46: 178-184.
Hrdlicka J & Kuca J (1965) The changes of carbonyl compounds in the
heat-processing of meat: 2, Turkey meat. Poult Sci, 44: 27-31.
Husman K (1980) Symptoms of car painters with long-term exposure to a
mixture of organic solvents. Scand J Work Environ Health, 6: 19-32.
Husman K & Karli P (1980) Clinical neurological findings among car
painters exposed to a mixture of organic solvents. Scand J Work
Environ Health, 6: 33-39.
Imai T, Takigawa H, Nakagawa S, Shen GJ, Kodama T, & Minoda Y (1986)
Microbial oxidation of hydrocarbons and related compounds by
whole-cell suspensions of the methane-oxidizing bacterium H-2. Appl
Environ Microbiol, 52: 1403-1406.
Ingram L (1977) Changes in lipid composition of Escherichia coli
resulting from growth with organic solvents and with food additives.
Appl Environ Microbiol, 33: 1233-1236.
Inoue T, Takeuchi Y, Hisanaga N, Ono Y, Iwata M, Ogata M, Saito K,
Sakurai H, Hara I, Matsushita T, & Ikeda M (1983) A nationwide survey
on organic solvent components in various solvent products: Part 1.
Homogeneous products such as thinners, degreasers and reagents. Ind
Health, 21: 175-183.
IRPTC (1987) Methyl ethyl ketone. In: IRPTC legal file 1986. Geneva,
International Register of Potentially Toxic Chemicals, United Nations
Environment Programme, vol I, pp 11/238-11/240.
IRPTC (1991) IRPTC legal file. Geneva, International Register of
Potentially Toxic Chemicals, United Nations Environment Programme,
pp 17/1-17/5.
Iwata M, Takeuchi Y, Hisanaga N, & Ono Y (1983) Changes of n-hexane
metabolites in urine of rats exposed to various concentrations of
n-hexane and to its mixture with toluene or MEK. Int Arch Occup
Environ Health, 53: 1-8.
Iwata M, Takeuchi Y, Hisanaga N, & Ono Y (1984) Changes of n-hexane
neurotoxicity and its urinary metabolites by long-term co-exposure
with MEK or toluene. Int Arch Occup Environ Health, 54: 273-281.
Jacot BJ (1983) OVA field screening at hazardous waste sites. In:
National Conference on Management of Uncontrolled Hazardous Waste
Sites, Washington, 31 October-2 November 1983. Silver Spring,
Maryland, Hazardous Materials Control Research Institute, pp 76-78.
Jarke FH, Dravnieks A, & Gordon SM (1981) Organic contaminants in
indoor air and their relation to outdoor contaminants. Am Soc Heat
Refrig Air-Cond Eng Trans, 87: 153-166.
Johansson I & Ingelman-Sundberg M (1985) Carbon tetrachloride-induced
lipid peroxidation dependent on an ethanol-inducible form of rabbit
liver microsomal cytochrome P-450. FEBS Lett, 183: 265-269.
John JA, Pilny MK, Kuna RA, Deacon MM, & Yakel HO (1980) Teratogenic
evaluation of methyl ethyl ketone in the rat. Teratology, 21(3): 47A.
Jonsson A, Persson KA, & Grigoriadis V (1985) Measurements of
low-molecular-weight oxygenated, aromatic, and chlorinated
hydrocarbons in ambient air and in vehicle emissions. Environ Int,
11: 383-392.
JRB Associates Inc (1980) Support document, chapter IV. Health effects
of methyl ethyl ketone. Washington, DC, US Environmental Protection
Agency, 58 pp (Contract No. 68-01-4839, Task No. 11, Project No.
2-800-08-107-04).
Juhnke I & Luedemann D (1978) [Results of the investigation of 300
chemical compounds for acute toxicity with the Golden Orfe test.]
Z Wasser Abwasser Forsch, 11: 161-164 (in German).
Jungclaus GA, Lopez-Avila V, & Hites RA (1978) Organic compounds in an
industrial wastewater: A case study of their environmental impact.
Environ Sci Technol, 12: 88-96.
Kahn JH, Laroe EG, & Conner HA (1968) Whiskey composition:
identification of components by single-pass gas chromatography - mass
spectrometry. J Food Sci, 33: 395-400.
Kamil IA, Smith JN, & Williams RT (1953) Studies in detoxification.
46. The metabolism of aliphatic alcohols. The glucuronic acid
conjugation of acyclic aliphatic alcohols. Biochem J, 53: 129-135.
Kaye S (1961) Emergency toxicology. Springfield, Illinois, Charles C.
Thomas, pp 214-216.
Keen AR, Walker NJ, & Peberdy MF (1974) The formation of 2-butanone
and 2-butanol in Cheddar cheese. J Dairy Res, 41: 249-257.
Kenny J & Stratton G (1989) An improved desorption solvent for organic
solvent mixtures. Am Ind Hyg Assoc J, 50: 431-434.
Kezic S & Monster AC (1988) Determination of methyl ethyl ketone and
its metabolites in urine using capillary gas chromatography.
J Chromatogr, 428: 275-280.
Khasanov AA, Isidorov VA, Zenkevich IG, & Ioffee BV (1982) [Gas
chromatography/ mass spectrometry study of the composition of volatile
emissions from Juniperus serawechanica Kom.] Dokl Akad Nauk Uzb SSR,
12: 29-31 (in Russian).
Kimura ET, Ebert DM, & Dodge PW (1971) Acute toxicity and limits of
solvent residue for sixteen organic solvents. Toxicol Appl Pharmacol,
19: 699-704.
Ko I-Y, Park SS, Song BJ, Patten CH, Tan Y, Hah YC, Yang CS, & Gelboin
HV (1987) Monoclonal antibodies to ethanol-induced rat liver
cytochrome P-450 that metabolizes aniline and nitrosamines. Cancer
Res, 47: 3101-3109.
Kobayashi K, Tanaka M, & Kawai S (1980) Gas chromatography
determination of low-molecular-weight carbonyl compounds in aqueous
solution as their O-(2,3,4,5,6,-penta-fluorobenzyl) oximes.
J Chromatogr, 187: 413-417.
Kontominas MG & Voudouris E (1982) The determination of residual
solvents in plastics packaging materials in relation to off odors
developed in packaged bakery products. Chem Chron, 11: 215-223.
Kopelman PG & Kalfayan PY (1983) Severe metabolic acidosis after
ingestion of butanone. Br Med J, 286: 21-22.
Krasavage WJ, O'Donoghue JL, & Divincenzo GD (1982) Ketones. In:
Clayton GD & Clayton FE ed. Patty's industrial hygiene and toxicology,
3rd ed. New York, John Wiley and Sons, Wiley Interscience,
pp 4709-4800.
Kulshrestha DC & Marth EH (1974a) Inhibition of bacteria by some
volatile and non-volatile compounds associated with milk. I.
Escherichia coli. J Milk Food Technol, 37: 510-516.
Kulshrestha DC & Marth EH (1974b) Inhibition of bacteria by some
volatile and non-volatile compounds associated with milk. II.
Salmonella typhimurium. J Milk Food Technol, 37: 539-544.
Kulshrestha DC & Marth EH (1974c) Inhibition of bacteria by some
volatile and non-volatile compounds associated with milk. III.
Staphylococcus aureus. J Milk Food Technol, 37: 545-550.
Kulshrestha DC & Marth EH (1974d) Inhibition of bacteria by some
volatile and non-volatile compounds associated with milk. IV.
Streptococcus lactis. J Milk Food Technol, 37: 593-599.
Kulshrestha DC & Marth EH (1974e) Inhibition of bacteria by some
volatile and non-volatile compounds associated with milk. V.
Leuconostoc citrovorum. J Milk Food Technol, 37: 600-606.
Kulshrestha DC & Marth EH (1974f) Inhibition of bacteria by some
volatile and non-volatile compounds associated with milk. VI.
Streptococcus thermophilus. J Milk Food Technol, 37: 606-611.
Kupferschmid LL & Perkins JL (1986) Organic solvent recycling plant
exposure levels. Appl Ind Hyg, 1: 122-124.
Kwan W & Gatehouse A (1978) The effects of low doses of three
insecticides on activity, feeding, mating, reproductive performance
and survival in Glossina morsitans mortistans (Glossinidae). Entomol
Exp Appl, 23: 201-221.
La Belle CW & Brieger H (1955) The vapor toxicity of a composite
solvent and its principal compounds. Arch Ind Health, 12: 623-627.
Laity JL, Burstain IG, & Appel BR (1973) Photochemical smog and the
atmospheric reactions of solvents. In: Tess RW ed. Solvents, theory
and practice. Washington, DC, American Chemical Society, vol 7,
pp 96-112 (Advances in Chemistry Series 124).
Lande SS, Durkin PR, Christopher DH, Howard PH, & Saxena J (1976)
Investigation of selected potential environmental contaminants:
Ketonic solvents. Washington, DC, US Environmental Protection Agency,
330 pp (EPA-560/2-76-003).
Laregina J & Bozzelli JW (1986) Volatile organic compounds at
hazardous waste sites and a sanitary landfill in New Jersey. Environ
Prog, 5: 18-27.
Larson PS, Finnegan JK, & Haag HB (1955) Observations on the effect of
chemical configuration on the edema-producing potency of acids,
aldehydes, ketones and alcohols. J Pharmacol, 116: 119-122.
Leblanc G (1980) Acute toxicity of priority pollutants to water flea
(Daphnia magna). Bull Environ Contam Toxicol, 24: 684-691.
Leblanc G (1984) Interspecies relationships in acute toxicity of
chemicals to aquatic organisms. Environ Toxicol Chem, 3: 47-60.
Lee S & Murphy D (1982) Health hazard evaluation: Medalist-Gladiator
Athletic Products Company, Leesburg, Florida. Cincinnati, Ohio,
National Institute of Occupational Safety and Health, 15 pp (HETA
82-030-1184).
Lee S & Parkinson D (1982) Health hazard evaluation: Rola-Esmark
Company; Dubois, Pennsylvania. Cincinnati, Ohio, National Institute of
Occupational Safety and Health, 26 pp (HETA 80-168-1204).
Levin JO & Carleborg L (1987) Evaluation of solid sorbents for
sampling ketones in work-room air. Ann Occup Hyg, 31: 31-38.
Levy A (1973) The photochemical smog reactivity of organic solvents.
In: Tess RW ed. Solvents, theory and practice. Washington, DC,
American Chemical Society, vol 6, pp 71-93 (Advances in Chemistry
Series 124).
Li G-L, Yin S-N, Watanabe T, Nakatsuka H, Kasahara M, Abe H, & Ikeda
M (1986) Benzene-specific increase in leucocyte alkaline phosphatase
activity in rats exposed to vapors of various organic solvents. J
Toxicol Environ Health, 19: 581-589.
Liebich HM, Bertsch W, Zlatkis A, & Schneider HJ (1975) Volatile
organic components in the Skylab 4 spacecraft atmosphere. Aviat Space
Environ Med, 46: 1002-1007.
Liepins R, Mixon F, Hudak C, & Parsons TB (1977) Industrial process
profiles for environmental use: Chapter 6. The industrial organic
chemicals industry. Research Triangle Park, North Carolina, US
Environmental Protection Agency, pp 164-165, 560-561 (EPA-600/12).
Liira J, Riihimäki V, Engstrom K, & Pfaffli P (1988a) Coexposure of
man to m-xylene and methyl ethyl ketone, kinetics and metabolism.
Scand J Work Environ Health, 14: 322-327.
Liira J, Riihimäki V, & Pfaffli P (1988b) Kinetics of methyl ethyl
ketone in man: absorption, distribution and elimination in inhalation
exposure. Int Arch Occup Environ Health, 60: 195-200.
Liira J, Johanson G, & Riihimäki V (1990a) Dose-dependent kinetics of
inhaled methylethylketone in man. Toxicol Lett, 50: 195-201.
Liira J, Riihimäki V, & Engstrom K (1990b) The effects of ethanol on
the kinetics of methyl ethyl ketone in man. Br J Ind Med, 47: 325-330.
Liira J, Elovaara E, Raunio H, Riihimäki V, & Engstrom K (1991)
Metabolic interaction and disposition of methyl ethyl ketone and
m-xylene in rats at single and repeated inhalation exposures.
Xenobiotica, 21: 53-63.
Lin JCC & Jeon IJ (1985) Headspace gas sampling/GC method for the
quantitative analysis of volatile compounds in cheese. J Food Sci, 50:
843-844, 846.
Lindros KO, Cai Y, & Penttila KE (1990) Role of ethanol-inducible
cytochrome P-450IIE1 in carbon tetrachloride-induced damage to
centrilobular hepatocytes from ethanol-treated rats. Hepatology,
12: 1092-1097. Lowenheim FA & Moran MK (1975) Industrial chemicals, 4th
ed. New York, Chichester, Brisbane, Toronto, John Wiley and Sons,
pp 11-12, 539-542.
Lundberg I & Hakansson M (1985) Normal serum activities of liver
enzymes in Swedish paint industry workers with heavy exposure to
organic solvents. Br J Ind Med, 42: 596-600.
Lundberg I, Ekdahl M, Kronevi T, Vitauts L, & Lundberg S (1986)
Relative hepatotoxicity of some industrial solvents after
intraperitoneal injection or inhalation exposure in rats. Environ Res,
40: 411-420.
Mabuchi H (1969) [Urinary and serum volatile substances in diabetics.]
Nippon Naika Gakkai Zasshi, 58: 731-743 (in Japanese).
Manville Chemical Products Corporation (1988) Chemical products
synopsis, Asbury Park, New Jersey, Manville Chemical Products
Corporation, 1 p.
Marion CV & Malaney GW (1963) The oxidation of aliphatic compounds by
Alcalignes faecalis. J Water Pollut Control Fed, 35: 1269-1281.
Marnett LJ, Hurd HK, Hollstein MC, Levin DE, Esterbauer H, & Ames BN
(1985) Naturally occurring carbonyl compounds are mutagens in
Salmonella tester strain TA104. Mutat Res, 148: 25-34.
Mast TJ, Dill JA, Evanoff JJ, Rommereim RL, Weigel RJ, & Westerberg RB
(1989) Inhalation developmental toxicology studies: teratology study
of methyl ethyl ketone in mice. Richland, Washington, Pacific Norwest
Laboratory (Report No. NIH-YOI-ES-70153).
Mayer VW & Goin CJ (1987) Effects of chemical combinations on the
induction of aneuploidy in Saccharomyces cerevisiae. Mutat Res,
187: 21-30.
Mayer VW & Goin CJ (1988) Investigations of aneuploidy-inducing
chemical combinations in Saccharomyces cerevisiae. Mutat Res,
201: 413-421.
MB Research Laboratories, Inc. (1979) Test for eye irritation in
rabbits. Spinnerstown, Pennsylvania, MB Research Laboratories, Inc.,
5 pp (Unpublished report No. MB 79-3962).
Metcalf RL, Lu P, & Kapoor IP (1973) Environmental distribution and
metabolic fate of key industrial pollutants and pesticides in a model
ecosystem. Urbana, Illinois, University of Illinois, Water Research
Center (Report No. 69, Project B-050 Ill).
Mezey E (1976) Ethanol metabolism and ethanol-drug interactions.
Biochem Pharmacol, 25: 869-875.
Middleditch BS, Sung NJ, Zlatkis A, & Settembre G (1987) Trace
analysis of volatile polar organics by direct aqueous injection gas
chromatography. Chromatographia, 23: 273-278.
Misumi J & Nagano M (1985) Experimental study on the enhancement of
the neurotoxicity of methyl n-butyl ketone by non-neurotoxic aliphatic
monoketones. Br J Ind Med, 42: 155-161.
Miyasaka M, Kumai M, Koizumi A, Watanabe T, Kurasako K, Sato K, &
Ikeda M (1982) Biological monitoring of occupational exposure to
methyl ethyl ketone by means of urinalysis for methyl ethyl ketone
itself. Int Arch Occup Environ Health, 50: 131-137.
Mohren S & Juttner F (1983) Odorous compounds of different strains of
Anabaena and Nostoc (Cyanobacteria). Water Sci Technol, 15: 221-228.
Mookherjee RE, Deck RE, & Chang SS (1965) Relationship between
monocarbonyl compounds and flavor of potato chips. J Agric Food Chem,
13(2): 131-134.
Munies R & Wurster DE (1965) Investigation of some factors influencing
percutaneous absorption--III. Absorption of methyl ethyl ketone.
J Pharm Sci, 54: 1281-1284.
Murphy DC (1984) Acute illness among workers connected to solvent
exposure. Occup Health Saf, May: 36-38.
Mutti A, Cavatorta A, Lucertini S, Arfini G, Falzoi M, & Franchini I
(1982a) Neurophysiological changes in workers exposed to organic
solvents in a shoe factory. Scand J Work Environ Health, 8(Suppl 1):
136-141.
Mutti A, Ferri F, Lommi G, Lotta S, Lucertini S, & Franchini I (1982b)
n-Hexane induced changes in nerve conduction velocities and
somatosensory evoked potentials. Int Arch Environ Health, 51: 45-54.
Nakaaki K (1974) [An experimental study on the effect of exposure to
organic solvent vapor in human subjects.] J Sci Labour 50: 89-96 (in
Japanese).
Nakajima T, Wang R-S, Murayama N, & Sato A (1990) Three forms of
trichloro-ethylene-metabolizing enzymes in rat liver induced by
ethanol, phenobarbital, and 3-methylcholanthrene. Toxicol Appl
Pharmacol, 102: 546-552.
Nandi B & Fries N (1976) Volatile aldehydes, ketones, esters and
terpenoids as preservatives against storage fungi in wheat.
Z Pflanzenkr Pflanzenschutz, 83: 284-294.
Nestmann ER, Lee EGH, Matula TI, Douglas GR, & Mueller JG (1980)
Mutagenicity of constituents identified in pulp and paper mill
effluents using the Salmonella/mammalian-microsome assay. Mutat Res,
79: 203-212.
Ng H, Reed DJ, & Pence JW (1960) Identification of carbonyl compounds
in an ethanol extract of fresh white bread. Cereal Chem, 37: 638-645.
Nilsen OG & Toftgard R (1980) The influence of organic solvents on
cytochrome P-450- mediated metabolism of biphenyl and benzo(a)pyrene.
In: Coon MJ, Conney AH, Estabrook RW, Gelboin HV, Gillete JR, &
O'Brien PJ ed. Microsomes, drug oxidations, and chemical
carcinogenesis. New York, Academic Press, vol II, pp 1235-1238.
Noma E, Berglund B, Berglund U, Johansson I, & Baird JC (1988) Joint
representation of physical locations and volatile organic compounds in
indoor air from a healthy and a sick building. Atmos Environ,
22: 451-460.
Nye D (1975) Laboratory model ecosystem studies of the degradation and
fate of radiolabelled tri-, tetra-, and pentachlorobiphenyl compared
with DDE. Arch Environ Contam Toxicol, 3: 151-165.
O'Donoghue JL, Krasavage WJ, Divincenzo GD, & Katz GV (1984) Further
studies on ketone neurotoxicity and interactions. Toxicol Appl
Pharmacol, 72: 201-209.
O'Donoghue JL, Haworth SR, Curren RD, Kirby PE, Lawlor T, Moran EJ,
Phillips RD, Putnam DL, Rogers-Back AM, Slesinski RS, & Thilagar A
(1988) Mutagenicity studies on ketone solvents: methyl ethyl ketone,
methyl isobutyl ketone, and isophorone. Mutat Res, 206: 149-161.
Ogawa I & Fritz JS (1985) Determination of low concentrations of
low-molecular-weight aldehydes and ketones in aqueous samples.
J Chromatogr, 329: 81-89.
Oh SJ & Kim JM (1976) Giant axonal swelling in "hufferis" neuropathy.
Arch Neurol, 33: 583-586.
Ong CN, Sia GL, Ong HY, Phoon WH, & Tan KT (1991) Biological
monitoring of occupational exposure to methyl ethyl ketone. Int Arch
Occup Environ Health, 63: 319-324.
Osborne JS, Adamek S, & Hobbs ME (1956) Some components of gas phase
of cigarette smoke. Anal Chem, 28: 211-215.
Panson RD & Winek CL (1980) Aspiration toxicity of ketones. Clin
Toxicol, 17: 271-317.
Papa AJ & Sherman PD (1978) Ketones. In: Kirk RE & Othmer DF ed.
Encyclopedia of chemical technology, 3rd ed. New York, John Wiley and
Sons, Wiley-Interscience, vol 13, pp 894-912, 934-941.
Patel RN, Hou CT, & Laskin AI (1982) Oxidation of gaseous hydrocarbons
and related compounds by methanotrophic organisms. Dev Ind Microbiol,
23: 187-205.
Patel RN, Hou CT, Laskin AI, Felix A, & Derelanka P (1983) Oxidation
of alkanes by organisms grown on C2-C4 alkanes. J Appl Biochem,
5: 107-120.
Patty FA, Schrenk HH, & Yant WP (1935) Acute response of guinea pigs
to vapors of some new commercial organic compounds. VIII. Butanone. US
Public Health Rep, 50: 1217-1228.
Pellizzari ED, Castillo NP, Willis S, Smith D, & Bursey JT (1979)
Identification of organic components in energy related processes. In:
Van Hall CE ed. Measurement of organic pollutants in water and
wastewater: Proceedings of a symposium, Denver, Colorado, 19-20 June
1978. Philadelphia, Pennsylvania, American Society for Testing and
Materials, pp 256-274 (ASTM STP 686).
Pellizzari ED, Sheldon LS, Bursey JT, Michael LC, & Zweidinger RA
(1985) Master analytical scheme for organic compounds in water.
Athens, Georgia, US Environmental Protection Agency, pp 46-54, 108-127
(EPA/600/4-84-010A).
Perbellini L, Brugnone F, Mozzo P, Cocheo V, & Caretta D (1984) Methyl
ethyl ketone exposure in industrial workers. Uptake and kinetics. Int
Arch Occup Environ Health, 54: 73-81.
Perbellini L, Bartolucci G, Brugnone F, & De Rosa E (1985)
[2,5-Hexanedione in biological monitoring of occupational exposure to
n-hexane.] Med Lav, 76: 35-43 (in Italian).
Perocco P, Bolognesi S, & Alberghini W (1983) Toxic activity of
seventeen industrial solvents and halogenated compounds on human
lymphocytes cultured in vitro . Toxicol Lett, 16: 69-75.
Perry JJ (1968) Substrate specificity in hydrocarbon utilizing
micro-organisms. Antonie Van Leeuwenhoek, 34: 27-36.
Persson U, Lundqvist S, Marthinsson B, & Eng ST (1984)
Computer-automated carbon dioxide laser long-path absorption system
for air quality monitoring in the working environment. Appl Opt,
23: 998-1002.
Pezzagno G, Ghottori S, Imbriani M, & Capodaglio E (1983) [Measurement
of the solubility coefficients of gases and vapors in blood. II.
Widely used industrial solvents.] G Ital Med Lav, 5: 49-63 (in
Italian).
Poli G, Dianzani MU, Cheeseman KH, Slater TF, Lang J, & Esterbauer H
(1985) Separation and characterization of the aldehydic products of
lipid peroxidation stimulated by carbon tetrachloride or ADP-iron in
isolated rat hepatocytes and rat liver microsomal suspensions. Biochem
J, 227: 629-638.
Price K, Waggy G, & Conway R (1974) Brine shrimp bioassay and seawater
BOD of petrochemicals. J Water Pollut Control Fed, 46: 63-76.
Prockop LD, Alt M, & Tison J (1974) Huffer's neuropathy. J Am Med
Assoc, 229: 1083-1084.
Przyrembel H, Bremer HJ, Duran M, Bruinvis L, Ketting D, Wadman SK,
Baumgartner R, Irle U, & Bachmann C (1979) Propionyl-CoA carboxylase
deficiency with overflow of metabolites of isoleucine catabolism at
all levels. Eur J Pediatr, 130: 1-14.
Puskar MA, Levine SP, & Lowery SR (1986) Computerized infrared
spectral identification of compounds frequently found at hazardous
waste sites. Anal Chem, 58: 1156-1162.
Ralston WH, Hilderbrand RL, Uddin DE, Andersen ME, & Gardier RW (1985)
Potentiation of 2,5-hexanedione neurotoxicity by methyl ethyl ketone.
Toxicol Appl Pharmacol, 81: 319-327.
Ramsey JD & Flanagan RJ (1982) Detection and identification of
volatile organic compounds in blood by headspace gas chromatography as
an aid to the diagnosis of solvent abuse. J Chromatogr, 240: 423-444.
Raunio H, Liira J, Elovaara E, Riihimäki V, & Pelkonin O (1990)
Cytochrome P450 isozyme induction by methyl ethyl ketone and n-xylene
in rat liver. Toxicol Appl Pharmacol, 103: 175-179.
Reilly B (1988) Engineering assessment: Methyl ethyl ketone (MEK) and
methyl isobutyl ketone (MIBK) environmental releases. Washington, DC,
US Environmental Protection Agency, 20 pp (Unpublished document).
Reynolds T (1977) Comparative effects of aliphatic compounds on
inhibition of lettuce fruit germination. Am Bot 41: 637-648.
Riggin RM (1984) Compendium of methods for the determination of toxic
organic compounds in ambient air. Method T05. Research Triangle Park,
North Carolina, US Environmental Protection Agency, pp 1-22
(EPA-600/4-84-041).
Robertson P, White EL, & Bus JS (1989) Effects of methyl ethyl ketone
pretreatment on hepatic mixed function oxidose activity and on in
vivo metabolism of n-hexane. Xenobiotica, 19: 721-729.
Rose-Pehrsson SL, Grate JW, Ballentine DS, & Jurs PC (1988) Detection
of hazardous vapors including mixtures using pattern recognition
analysis of responses from surface acoustic wave devices. Anal Chem,
60: 2801-2811.
Ruth JH (1986) Odor thresholds and irritation levels of several
chemical substances: A review. Am Ind Hyg Assoc J, 47: 142-151.
Saida K, Mendell JR, & Weiss HS (1976) Peripheral nerve changes
induced by methyl n-butyl ketone and potentiation by methyl ethyl
ketone. J Neuropathol Exp Neurol, 35: 207-225.
Sakakibara H, Ide Y, Yanai T, Yajima J, & Hayashi K (1990) Volatile
flavour compounds of some kinds of dried and smoked fish. Agric Biol
Chem, 54: 9-16.
Sato A & Nakajima T (1979) Partition coefficients of some aromatic
hydrocarbons and ketones in water, blood and oil. Br J Ind Med,
36: 231-234.
Sato Y, Watanabe K, & Tanaka Y (1968) Chemical studies on smelling
compounds in hen's egg: Part I, Volatile carbonyl and basic compounds
in egg white. Agric Biol Chem, 32(4): 405-411.
Sauer TC (1981) Volatile liquid hydrocarbon characterization of
underwater hydrovents and formation waters from offshore production
operations. Environ Sci Tech, 15: 917-923.
Sawhney BL & Kozloski RP (1984) Organic pollutants in leachates from
landfill sites. J Environ Qual, 13: 349-352.
Schmidt R, Schnoy N, Altenkirch H, & Wagner HM (1984) Ultrastructural
alteration of intrapulmonary nerves after exposure to organic
solvents. Respiration, 46: 362-369.
Schnoy N, Schmidt R, Altenkirch H, & Wagner HM (1982) Ultrastructural
alteration of the alveolar epithelium after exposure to organic
solvents. Respiration, 43: 221-231.
Schörmuller J & Grosch W (1964) Aromatics of foods. II Occurrence of
additional carbonyl compounds in the tomato. Z Lebensmittel Unters
Forsch, 126: 38-49.
Schultz TH, Flath RA, Stern DJ, Mon TR, Teranishi R, Kruse SM, Butler
B, & Howard WE (1988) Coyote estrous urine volatiles. J Chem Ecol,
14: 701-712.
Schulz F, Mueller P, & Kramer D (1981) [Model experiments on the
phytotoxic effect of organic constituents in wastewater from lignite
refining in soil treatment.] Arch Acker-Pflanzenbau Bodenkd,
25: 745-753 (in German)
Schwetz BA, Leong BKJ, & Gehring PJ (1974) Embryo- and fetotoxicity of
inhaled carbon tetrachloride, 1,1-dichloroethane and methyl ethyl
ketone in rats. Toxicol Appl Pharmacol, 28: 452-464.
Schwetz BA, Mast TJ, Weigel RJ, Dill JA, & Morrissey RE (1991)
Developmental toxicity of inhaled methyl ethyl ketone in Swiss mice.
Fundam Appl Toxicol, 16: 742-748.
Seinfeld JH (1989) Urban air pollution: State of the science. Science,
243: 745-752.
Seizinger DE & Dimitriades B (1972) Oxygenates in exhaust from simple
hydrocarbon fuels. J Air Pollut Control Assoc, 22: 47-51.
Shah JJ & Singh HB (1988) Distribution of volatile organic chemicals
in outdoor and indoor air. Environ Sci Technol, 22: 1381-1388.
Shibata E, Huang J, Ono Y, Hisanaga N, Iwata M, Saito I, & Takeuchi Y
(1990a) Changes in urinary n-hexane metabolites by co-exposure to
various concentrations of methyl ethyl ketone and fixed n-hexane
levels. Arch Toxicol, 64: 165-168.
Shibata E, Huang J, Hisanaga Y, Ono Y, Saito I, & Takeuchi Y (1990b)
Effects of MEK on kinetics of n-hexane metabolites in serum. Arch
Toxicol, 64: 247-250.
Shimizu Y, Matsuto S, Ito T, & Okada I (1969) Studies on the flavor of
roast barley (Mugi-Cha): Part III. Separation and identification of
volatile mono-carbonyl compounds. Nippon Nogei Kagaku Kaishi,
43(4): 217-223 (in Japanese with English summary).
Smith LL (1981) Cholesterol autoxidation. New York, London, Plenum
Press, 674 pp.
Smith AR & Mayers MR (1944) Study of poisoning and fire hazards of
butanone and acetone. NY State Dept Labor Ind Bull, April: 174-176.
Smith AF & Wood R (1972) A field test for the determination of some
ketone vapors in air. Analyst, 97: 363-371.
Smyth HF, Carpenter CP, Weil CS, Pozzani UC, & Striegel JA (1962)
Range-finding toxicity data: list VI. Am Ind Hyg Assoc J, 23: 95-107.
Snider JR & Dawson GA (1985) Tropospheric light alcohols, carbonyls,
and acetonitrile: concentrations in the southwestern United States
and Henry's law data. J Geophys Res D Atmos, 90: 3797-3805.
Sosulski F & Mahmoud RM (1979) Effects of protein supplements on
carbonyl compounds and flavor in bread. Cereal Chem, 56: 533-536.
Specht H, Miller JW, Valaer PJ, & Sayers RR (1940) Acute response of
guinea-pigs to the inhalation of ketone vapors. Bull Natl Inst Health,
176: 1-66.
Spencer PS & Schaumburg HH (1976) Feline nervous system response to
chronic intoxication with commercial grades of methyl n-butyl ketone,
methyl isobutyl ketone, and methyl ethyl ketone. Toxicol Appl
Pharmacol, 37: 301-311.
Spencer PS, Schaumburg HH, Sabri MI, & Veronesi B (1980) The enlarging
view of hexacarbon neurotoxicity. CRC Crit Rev Toxicol, 7: 279-356.
SRI International (1985) Directory of chemical producers, Western
Europe, 8th ed. Menlo Park, California, SRI International, p 1457;
suppl II, p 55.
SRI International (1988) Directory of chemical producers, United
States. Menlo Park, California, SRI International, p 780.
Staüble EJ & Rast D (1971) [Volatile material in Agaricus biosporus.]
Experientia(Basel), 27: 886-888 (in German).
Stofberg J & Grundschober F (1984) Consumption ratio and food
predominance of flavoring materials--second cumulative series. Perfum
Flavorist, 9: 53-56, 58-59, 62, 65-72, 76-83.
Takeuchi Y, Ono Y, Hisanaga N, Iwata M, Aoyama M, Kitoh J, & Sugiura
Y (1983) An experimental study of the combined effects of n-hexane and
methyl ethyl ketone. Br J Ind Med, 40: 199-203.
Tangredi G, Carbone U, Rossi L, & Galdi A (1981) [Environmental
conditions in a paint factory and effects on employee health.] Riv Med
Lav Ig Ind, 5(Oct.-Dec.): 325-337 (in Italian).
Tham R, Bunnfors I, Eriksson B, Larsby B, Lindgrem S, & Odkvist LM
(1984) Vestibulo-ocular disturbances in rats exposed to organic
solvents. Acta Pharmacol Toxicol, 54: 58-63.
Thoburn T & Gunter B (1982) Health hazard evaluation; Davis Monthan
Air Force Base, Tucson, Arizona. Cincinnati, Ohio, National Institute
of Occupational Safety and Health, 23 pp (HETA 81-257-1115).
Toftgard R, Nilsen OG, & Gustafsson J-A (1981) Changes in rat liver
microsomal cytochrome P-450 and enzymatic activities after the
inhalation of n-hexane, xylene, methyl ethyl ketone and
methylchloroform for four weeks. Scand J Work Environ Health, 7:
31-37.
Toxigenics (1981) 90-day vapour inhalation toxicity study of methyl
ethyl ketone in albino rats. Decatur, Illinois, Toxigenics Inc. (Study
420-0305).
Traiger GJ & Bruckner JV (1976) The participation of 2-butanone in
2-butanol-induced potentiation of carbon tetrachloride hepatotoxicity.
J Pharmacol Exp Ther, 196: 493-499.
Traiger GJ & Plaa GL (1972) Relationship of alcohol metabolism to the
potentiation of CCl4 hepatotoxicity induced by aliphatic alcohols.
J Pharmacol Exp Ther, 183: 481-488.
Traiger GJ, Bruckner JV, Jiang WD, Dietz FK, & Cooke PH (1989) Effect
of 2-butanol and 2-butanone on rat hepatic ultrastructure and drug
metabolizing enzyme activity. J Toxicol Environ Health, 28: 235-248.
Triebig G, Bestler W, Baumeister P, & Valentin H (1983)
[Investigations on neurotoxicity of chemical substances at the
workplace. IV. Determination of the motor and sensory nerve conduction
velocity in persons occupationally exposed to a mixture of organic
solvents.] Int Arch Occup Environ Health 52: 139-150 (in German).
Tsao MU & Pfeiffer EI (1957) Isolation and identification of a new
ketone body in normal human urine. Proc Soc Exp Biol Med, 94: 628-629.
Tsukamoto S, Chiba S, Ishikawa T, & Shimaura M (1985a) Experimental
study on the metabolism of volatile hydrocarbons by inhalation of
natural gas. Nihon Univ J Med, 27: 33-38.
Tsukamoto S, Chiba S, Muto T, Ishakawa T, & Shimamura M (1985b) Study
on the metabolism of volatile hydrocarbons in mice--propane, n-butane,
and isobutane. J Toxicol Sci, 10: 323-332.
Union Carbide Corp. (1980a) The acute toxicity of MRD-80-2 to the
bluegill sunfish Lepomis macrochirus Rafinesque. Tarrytown, New York,
Union Carbide Corp., Environmental Services, 8 pp.
Union Carbide Corp. (1980b) The acute toxicity of MRD-80-2 to the
water flea Daphnia magna Straus. Tarrytown, New York, Union Carbide
Corp., Environmental Services, 6 pp.
US EPA (1985a) Health assessment document for chloroform. Research
Triangle Park, North Carolina, US Environmental Protection Agency,
pp 3-40-3-42 (EPA/600/8-84/004F).
US EPA (1985b) Health and environmental effects profile for methyl
ethyl ketone. Cincinnati, Ohio, US Environmental Protection Agency,
71 pp (EPA/600/X-85/363).
US EPA (1986) Health effects assessment for methyl ethyl ketone.
Cincinnati, Ohio, US Environmental Protection Agency, 17 pp
(EPA/540/1-86/003).
US NIOSH (1984a) Analytical method 2500: 2-butanone. Cincinnati, Ohio,
National Institute of Occupational Safety and Health, 3 pp.
US NIOSH (1984b) Analytical method 8002: (1)2-butanone, (2) ethanol
and (3) toluene in blood. Cincinnati, Ohio, National Institute for
Occupational Safety and Health, 4 pp.
US NIOSH (1990) Pocket guide to chemical hazards. Cincinnati, Ohio,
National Institute of Occupational Safety and Health, 48 pp (DHEW
(NIOSH) Publication No. 90-117).
Urano K & Kato Z (1986) Evaluation of biodegradation ranks of priority
organic compounds. J Hazard Mater, 13: 147-159.
USITC (1981) Synthetic organic chemicals: United States production and
sales, 1980. Washington, DC, United States International Trade
Commission, p 263 (USITC Publication No. 1183).
USITC (1982) Synthetic organic chemicals: United States production and
sales, 1981. Washington, DC, United States International Trade
Commission, p 243 (USITC Publication No. 1292).
USITC (1983) Synthetic organic chemicals: United States production and
sales, 1982. Washington, DC, United States International Trade
Commission, p 259 (USITC Publication No. 1422).
USITC (1984) Synthetic organic chemicals: United States production and
sales, 1983. Washington, DC, United States International Trade
Commission, p 257 (USITC Publication No. 1588).
USITC (1985) Synthetic organic chemicals: United States production and
sales, 1984. Washington, DC, United States International Trade
Commission, p 256 (USITC Publication No. 1745).
USITC (1986) Synthetic organic chemicals: United States production and
sales, 1985. Washington, DC, United States International Trade
Commission, p 266 (USITC Publication 1892).
USITC (1987) Synthetic organic chemicals: United States production and
sales, 1986. Washington, DC, United States International Trade
Commission, p 210 (USITC Publication No. 2009).
USITC (1988) Synthetic organic chemicals: United States production and
sales, 1987. Washington, DC, United States International Trade
Commission, p 15-5 (USITC Publication No. 2118).
Uspenskii IV & Repkina L (1974) [Physiological age and sensitivity to
DDT of natural populations of Ixodes persulcatus.] Parazitologiya,
8: 3-11 (in Russian).
Vale GA, Lovemore DF, Flint S, & Cockbill GF (1988) Odour-baited
targets to control tsetse flies, Glossina spp. (Diptera:
Glossinidae) in Zimbabwe. Bull Entomol Res, 78: 31-49.
Van Doorn JE, De Cock J, Kezic S, & Monster AC (1989) Determination of
methyl ethyl ketone in human urine after derivatization with
o-nitrophenylhydrazine, using solid-phase extraction and
reversed-phase liquid chromatography and ultraviolet detection.
J Chromatogr, 489: 419-424.
Varigos GA & Nurse DS (1986) A case of contact urticaria caused by
methyl ethyl ketone. Contact Dermatitis, 15(4): 249-260.
Veith GD, Call DJ, & Brooke LT (1983) Structure-toxicity relationships
for the fathead minnow, Pimephales promelas: Narcotic industrial
chemicals. Can J Fish Aquat Sci, 40: 743-748.
Verhoeff AP, Wilders MMW, Monster AC, & Van Wijnen JH (1987) Organic
solvents in the indoor air of two small factories and surrounding
houses. Int Arch Occup Environ Health, 59: 153-163.
Veronesi B (1984) An ultrastructural study of methyl ethyl ketone's
effect on cultured nerve tissues. Neurotoxicology, 5: 31-44.
Veronesi B, Lington AW, & Spencer PS (1984) A tissue culture model of
methyl ethyl ketone's potentiation of n-hexane neurotoxicity.
Neurotoxicology, 5: 43-52.
Verschuren K (1983) Handbook of environmental data on organic
chemicals. New York, Van Nostrand Reinhold Company, pp 850-852.
Viader F, Lechevalier B, & Morin P (1975) Polynéurite toxique chez un
travailleur du plastique. Rôle possible du méthyl éthyl-cétone. Nouv
Presse Méd, 4: 1813-1814.
Volskay VT & Grady CPL (1988) Toxicity of selected RCRA compounds to
activated sludge microorganisms. J Water Pollut Control Fed,
60: 1850-1856.
Von Cremer E & Riedmann M (1964) Identification of
gas-chromatographically separated aromatic materials in honey.
Z Naturforsch B19: 76-77.
Wahlberg JE (1984a) Edema-inducing effects of solvents following
topical administration. Dermatosen, 3: 91-94.
Wahlberg JE (1984b) Erythema-inducing effects of solvents following
epicutaneous administration to man - studied by laser Doppler
flowmetry. Scand J Work Environ Health, 10: 159-162.
Wallen I, Greer W, & Lasater R (1957) Toxicity to Gambusia affinis
of certain pure chemicals in turbid waters. Sewage Ind Wastes,
29: 265-711.
Walton BT, Anderson TA, Hendricks MS, & Talmage SS (1989)
Physicochemical properties as predictors of organic chemical effects
on soil microbial respiration. Environ Toxicol Chem, 8: 53-63.
Wang TC & Bricker JL (1979) 2-butanone and tetrahydrofuran
contamination in the water supply. Bull Environ Contam Toxicol,
23: 620-623.
Weast RC ed. (1986) CRC handbook of chemistry and physics, 67th ed.
Boca Raton, Florida, CRC Press, p C-170.
Weil CS & Scala RA (1971) Study of intra- and interlaboratory
variability in the results of rabbit eye and skin irritation tests.
Toxicol Appl Pharmacol, 19: 276-360.
Wen CP, Tsai SP, Weiss NS, Gibson RL, Wong O, & Mcclellan WA (1985)
Long-term mortality study of oil refinery workers. IV. Exposure to the
lubricating-dewaxing process. J Natl Cancer Inst, 74: 11-18.
Whelan JK, Tarafa ME, & Hunt JM (1982) Volatile C1-C8 organic
compounds in macroalgae. Nature(Lond), 299: 50-52.
Whitehead LW, Ball GL, Fine LJ, & Langolf GD (1984) Solvent vapor
exposures in booth spray painting and spray gluing, and associated
operations. Am Ind Hyg Assoc J, 45: 767-772.
Windholz M ed. (1983) The Merck index, 10th ed. Rahway, New Jersey,
Merck & Co., p 870.
Wong NP & Patton S (1962) Identification of some volatile compounds
related to the flavor of milk and cream. J Dairy Sci, 45: 724-728.
Wong NP, Damico JN, & Salwin H (1967) Decomposition and filth in
foods: Investigation of volatile compounds in cod fish by gas
chromatography and mass spectrometry. J Assoc Off Anal Chem,
50(1): 8-15.
WHO (1991) Environmental Health Criteria 122: n-Hexane. Geneva, World
Health Organization, 164 pp.
Wurster DE & Munies R (1965) Factors influencing percutaneous
absorption II, absorption of methyl ethyl ketone. J Pharm Sci,
54: 554-556.
Ying L & Levine SP (1989) Fourier transform infrared least-squares
methods for the quantitative analysis of multicomponent mixtures of
airborne vapours of industrial hygiene concern. Anal Chem,
61: 677-683.
Zakhari S, Leibowitz M, Levy P, & Aviado DM (1977) Isopropanol and
ketones in the environment. Cleveland, Ohio, CRC Press, pp 57-89.
Zechman JM, Aldinger S, & Labows JN (1986) Characterization of
pathogenic bacteria by automated headspace concentration-gas
chromatography. J Chromatogr, 377: 49-57.
Zimmermann FK, Mayer VW, Scheel I, & Resnick MA (1985) Acetone, methyl
ethyl ketone, ethyl acetate, acetonitrile and other polar aprotic
solvents are strong inducers of aneuploidy in Saccharomyces
cerevisiae. Mutat Res, 149: 339-351.
APPENDIX 1
Conversion factors for various solvents (ppm --> mg/m3)
acetone 2.38
2-butanol 3.03
n-butanol 3.03
2-butoxyethanol 4.83
butyl acetate 4.75
cyclohexane 3.44
DBP 11.38
DCB 6.01
ethanol 1.88
2-ethoxyethanol 3.68
ethyl acetate 3.6
ethyl butyl ketone 4.6
n-hexane 3.52
isobutanol 3.03
isopropanol 2.45
2-methoxyethanol 3.11
methyl acetate 3.6
methyl butyl ketone 4.1
methyl ethyl ketone 2.95
methyl isobutyl ketone 4.1
methylene chloride 3.48
toluene 3.75
trichloroethane 5.46
trichloroethylene 5.38
white spirit 1.75
xylene 4.34
From: Clayton & Clayton (1981) and Weast (1986)
RESUME
1. Propriétés et méthodes d'analyse
La méthyléthylcétone est un liquide limpide, incolore, volatil et
très inflammable dont l'odeur rappelle celle de l'acétone. Elle est
stable dans les conditions ordinaires, mais peut donner naissance à
des peroxydes explosifs en cas de stockage prolongé. Elle peut aussi
former des mélanges explosifs avec l'air. Elle est très soluble dans
l'eau, miscible à de nombreux solvants organiques et forme des
azéotropes avec l'eau et beaucoup de liquides organiques. Dans
l'atmosphère, elle produit des radicaux libres qui peuvent favoriser
la formation d'un smog photochimique.
On dispose de plusieurs méthodes analytiques pour mesurer les
concentrations de méthyléthylcétone dans l'air, l'eau, les
échantillons biologiques, les effluents et d'autres milieux. Dans les
méthodes les plus sensibles, la méthyléthylcétone est piégée et
concentrée soit sur un sorbant solide, soit sous forme de dérivé de la
2,4-dinitrophénylhydrazine (DNPH). La méthyléthylcétone absorbée et
les autres composés organiques volatils sont désorbés, séparés par
chromatographie gazeuse et dosés à l'aide d'un spectromètre de masse
ou d'un détecteur à ionisation de flammes. Le produit de dérivation de
la méthyléthylcétone est séparé des substances apparentées par
chromatographie liquide à haute performance et mesuré par
spectrophotométrie ultraviolette. Dans des milieux tels que les
déchets solides ou les substances biologiques, la méthyléthylcétone
doit d'abord être séparée du substrat, par exemple par extraction à
l'aide d'un solvant ou par distillation à la vapeur. Les
concentrations élevées de méthyléthylcétone dans l'air peuvent être
mesurées de façon continue par absorption infrarouge. Les limites de
détection sont de 3 µg/m3 dans l'air, 0,05 µg/litre dans l'eau de
boisson, 1,0 µg/litre dans les autres types d'eau, 20 µg/litre dans le
sang et 100 µg/litre dans l'urine.
2. Sources d'exposition et usages
2.1 Production et autres sources
Selon des statistiques récentes, la production annuelle (en
milliers de tonnes) est la suivante: Etats-Unis d'Amérique, 212 à 305;
Europe de l'Ouest, 215; Japon, 139. D'autres sources de contamination
de l'environnement par la méthyléthylcétone sont les gaz d'échappement
des réacteurs et des moteurs à combustion interne ainsi que certaines
activités industrielles comme la gazéification du charbon. Elle est
également présente en quantités notables dans la fumée de tabac. Aux
Etats-Unis d'Amérique, la quantité de méthyléthylcétone émise par les
moteurs représente plus de 1% de la quantité fabriquée volontairement.
En cas de smog, la production photochimique de méthyléthylcétone et
d'autres carbonyles à partir de radicaux libres peut être bien
supérieure aux émissions résultant des activités humaines. La
méthyléthylcétone peut aussi avoir une origine biologique et elle a
été identifiée parmi les produits du métabolisme microbien. Elle a
également été détectée dans divers produits naturels, notamment des
végétaux supérieurs, des phéromones d'insectes, des tissus animaux,
ainsi que chez l'homme dans le sang, l'urine et l'air expiré. Elle
constitue probablement un produit mineur du métabolisme normal des
mammifères.
2.2 Usages et pertes dans l'environnement
La méthyléthylcétone est un excellent solvant, ce qui explique
que sa principale application soit la fabrication de revêtements
protecteurs et d'adhésifs. Elle est également utilisée comme
intermédiaire chimique, comme solvant dans la fabrication des rubans
magnétiques, pour l'élimination des cires dans les huiles de graissage
et dans l'industrie alimentaire. Elle entre également dans la
composition de nombreux produits d'usage courant comme les vernis et
les colles. Dans la plupart de ces applications, la méthyléthylcétone
est mélangée à d'autres solvants organiques. La libération dans
l'environnement résulte principalement de l'évaporation des solvants
à partir des surfaces enduites et concerne essentiellement
l'atmosphère. La libération de méthyléthylcétone dans l'eau est la
conséquence de sa présence dans les effluents provenant de sa
fabrication et de diverses opérations industrielles. Elle a été
détectée dans des eaux naturelles dans lesquelles sa présence pourrait
s'expliquer par une activité microbienne, l'absorption à partir de
l'atmosphère ou une pollution anthropogène.
3. Transport et distribution dans l'environnement
La méthyléthylcétone est extrêmement mobile dans l'environnement
naturel où elle se renouvelle rapidement. Elle est très soluble dans
l'eau et s'évapore facilement. Dans l'atmosphère, elle subit une
décomposition photochimique rapide, mais elle est également
synthétisée par des processus photochimiques. Elle réagit avec les
halogènes libres ou les hypochlorites et leurs homologues halogénés
présents dans l'eau pour former un dérivé halogéné plus toxique que la
molécule initiale. La méthyléthyl-cétone est transportée par l'air et
par l'eau, mais elle ne s'accumule dans aucun compartiment et elle ne
persiste pas longtemps là où il existe une activité microbienne. Elle
est rapidement métabolisée par les microbes et les mammifères. Le
phénomène de bioaccumulation n'a jamais été mis en évidence. La
méthyléthylcétone existe naturellement dans certaines espèces de
trèfle et elle est produite par des champignons à des concentrations
qui peuvent affecter la germination de certaines plantes.
4. Concentration dans l'environnement et exposition humaine
La population générale est fréquemment exposée à de faibles doses
de méthyléthylcétone. Lorsque la pollution atmosphérique est très
faible, la concentration est inférieure à 3 µg/m3 (< 1 ppb), mais
on a mesuré jusqu'à 131 µg/m3 (44,5 ppb) en atmosphère très polluée.
En dehors des zones industrielles de fabrication ou d'utilisation de
la méthyléthylcétone, les gaz d'échappement des véhicules automobiles
et les réactions photochimiques dans l'atmosphère peuvent être les
principales sources de contamination. Les cigarettes et les autres
formes de tabac à fumer contribuent à l'exposition individuelle (20
cigarettes en contiennent jusqu'à 1,6 mg). La volatilisation de la
méthyléthylcétone présente dans les matériaux de construction et les
produits de consommation peut entraîner une pollution de l'air des
locaux bien supérieure à celle de l'air extérieur. Les concentrations
dans les eaux naturelles dépassent rarement 100 µg/litre (100 ppb) et
sont généralement inférieures au seuil de détection. Toutefois, on a
souvent trouvé des traces de méthyléthylcétone dans l'eau de boisson
(environ 2 µg/litre). Il est probable que les solvants entrant dans la
composition des joints des tuyauteries de plastique sont à l'origine
de cette pollution. Bien que la méthyléthylcétone soit un constituant
normal de nombreux aliments, les concentrations sont faibles et
l'alimentation ne peut être considérée comme une source importante
d'exposition. Aux Etats-Unis d'Amérique, la quantité moyenne ingérée
quotidiennement avec les aliments, principalement le pain blanc, les
tomates et le fromage Cheddar, est estimée à 1,6 mg par personne. La
méthyléthylcétone peut être présente naturellement dans les aliments,
mais elle peut aussi se former lors de l'affinage du fromage, de
l'entreposage de la viande de volaille, de la cuisson ou de la
transformation des aliments, ou être absorbée à partir des emballages
en matière plastique.
L'exposition industrielle à des concentrations modérées de
méthyléthylcétone est fréquente. Toutefois, dans certaines régions, le
personnel travaillant dans de petites entreprises (fabriques de
chaussures, imprimeries, ateliers de peinture) peut être exposé à des
concentrations beaucoup plus élevées en raison d'une ventilation
insuffisante. Ces travailleurs sont généralement exposés à un mélange
de solvants, notamment au n-hexane.
5. Cinétique et métabolisme
La méthyléthylcétone est rapidement absorbée par contact cutané,
inhalation, ingestion et injection intrapéritonéale. Elle passe très
vite dans le sang, et de là dans les autres tissus. Il semble que sa
solubilité soit à peu près la même dans tous les tissus. L'élimination
de la méthyléthylcétone et de ses métabolites est pratiquement totale
chez les mammifères au bout de 24 heures. Elle est métabolisée dans le
foie où la plus grande partie est oxydée en 3-hydroxy-2-butanone avant
d'être réduite en 2,3-butanediol. Une petite partie peut être réduite
en 2-butanol, mais celui-ci est rapidement oxydé pour redonner la
molécule initiale. Chez les mammifères, la majeure partie de la
méthyléthylcétone ingérée entre dans le cycle métabolique général
et/ou est éliminée sous forme de molécules simples comme le dioxyde de
carbone et l'eau. L'excrétion de la méthyléthylcétone et de ses
métabolites caractéristiques se fait principalement par les poumons,
bien que de petites quantités soient éliminées par les reins.
La méthyléthylcétone augmente l'activité enzymatique du
cytochrome P-450 microsomal. Il est possible que ce renforcement de
l'activité enzymatique, et par conséquent du potentiel de
transformation métabolique de l'organisme, explique pourquoi la
méthyléthylcétone potentialise la toxicité des solvants du groupe des
alcanes halogénés et des hydrocarbures aliphatiques à six atomes de
carbone.
6. Effets sur les animaux d'expérience
La méthyléthylcétone présente une toxicité faible à modérée pour
les mammifères, qu'il s'agisse de toxicité aiguë, à court terme ou
chronique. Chez la souris et le rat, la LD50 est de 2 à 6 g/kg de
poids corporel, la mort survenant 1 à 14 jours après l'ingestion d'une
dose unique. La dose moyenne entraînant la mort après une exposition
unique aux vapeurs de méthyléthylcétone est d'environ 29 400 mg/m3
(10 000 ppm), bien que des cobayes aient survécu à une exposition de
4 heures à cette concentration. Dans des essais d'intoxication aiguë
par voie orale, la dose la plus faible ayant entraîné une modification
de structure des organes a été de 1 g/kg de poids corporel chez le
rat. Cette dose a provoqué des lésions des tubules du rein.
L'inhalation par des rats d'air contenant 74 mg/m3 (25 ppm) pendant
6 heures a provoqué des changements de comportement mesurables qui ont
persisté pendant plusieurs jours. Une exposition répétée à 14 750
mg/m3 (5000 ppm) (6 h/jour, 5 jours/semaine) n'a provoqué la mort
d'aucun animal. On n'a observé qu'un effet mineur sur la croissance et
la structure et il n'y a eu aucune modification neuropathologique. Des
poulets, des chats ou des souris exposés à 3975 mg/m3 (1500 ppm)
pendant des périodes allant jusqu'à 12 semaines n'ont présenté aucun
signe de changement neuropathologique. Des effets transitoires sur le
comportement ou la neurophysiologie ont été détectés chez des rats et
des babouins à la suite d'expositions répétées à des concentrations ne
dépassant pas 295 à 590 mg/m3 (100 à 200 ppm).
Une faible foetotoxicité a été observée à 8825 mg/m3 (3000
ppm), mais elle ne s'accompagnait d'aucune toxicité maternelle; aucun
effet embryotoxique ou tératogène n'a été constaté à des
concentrations inférieures à cette valeur. Après avoir exposé de façon
répétée des rattes en gestation à une concentration de 8825 mg/m3,
on a observé dans leur progéniture une augmentation légère mais
significative de certains types d'anomalie du squelette rarement
observés chez les animaux non exposés.
Plusieurs épreuves classiques de mutagénicité ont été pratiquées,
mais la seule qui ait donné un résultat positif a été une étude
d'hétéroploïdie sur la levure Saccharomyces cerevisiae.
La méthyléthylcétone ne présente pas de toxicité aiguë pour les
poissons ou les invertébrés aquatiques, la CL50 se situant entre
1382 et 8890 mg/litre.
Elle a un effet inhibiteur sur la germination de plusieurs
plantes, même à des concentrations que l'on peut rencontrer dans la
nature. La croissance des algues aquatiques est également inhibée.
Des concentrations relativement élevées de méthyléthylcétone,
comparées aux concentrations naturelles, ont été utilisées dans des
expériences de fumigation. Cette substance s'est révélée un fumigant
modérément efficace contre la mouche des fruits des Caraïbes. D'autre
part, elle a un effet attractif très net sur la mouche tsé-tsé. Des
concentrations allant jusqu'à 20 mg/litre retardent le processus de
biodégradation mais ne l'arrêtent pas complètement. Jusqu'à 100
mg/litre, la méthyléthylcétone est bactériostatique pour différentes
bactéries. A des concentrations plus élevées (1000 mg/litre et
au-delà) elle inhibe la croissance des bactéries et des protozoaires.
7. Effets sur l'homme
7.1 Méthyléthylcétone seule
Aucun effet notable n'a été observé lors de tests psychologiques
et de comportement après exposition à 590 mg/m3 (200 ppm). Une
exposition de courte durée à la méthyléthylcétone seule ne semble pas
présenter de risques importants, que ce soit pour les professionnels
ou pour le public en général. L'exposition, dans des conditions
expérimentales, à une concentration de 794 mg/m3 (270 ppm), à raison
de 4 heures par jour, a eu un effet à peu près nul sur le comportement
et un contact de 5 minutes avec la substance liquide n'a provoqué
qu'une décoloration temporaire de la peau. On n'a signalé qu'un seul
cas d'intoxication aiguë par la méthyléthylcétone en dehors de tout
contexte professionnel. Il s'agit d'un cas d'ingestion accidentelle
qui ne semble pas avoir laissé de séquelles. On n'a jamais signalé de
cas d'exposition professionnelle ayant entraîné la mort. Il existe
deux rapports faisant état d'intoxication professionnelle chronique et
un rapport d'intoxication professionnelle aiguë, mais ce dernier est
sujet à caution. Dans un des cas d'intoxication chronique,
l'exposition à 880-1770 mg/m3 (300-600 ppm) a provoqué des
dermatoses, un engourdissement des doigts et des bras et divers
symptômes, parmi lesquels des céphalées, des étourdissements, des
troubles gastro-intestinaux et une perte d'appétit et de poids. Le
faible nombre d'intoxications attribuées à la méthyléthylcétone seule
tient à la fois à la faible toxicité de cette substance et au fait
qu'elle est rarement utilisée seule, mais plutôt en mélange avec
d'autres solvants.
7.2 Méthyléthylcétone dans les mélanges de solvants
L'exposition à des mélanges de solvants contenant de la
méthyléthylcétone a été associée à une réduction de la vitesse de
conduction nerveuse, à des troubles de la mémoire, à des troubles
moteurs, à des dermatoses et à des vomissements. Dans une étude
longitudinale, des mesures consécutives du temps de réaction simple
ont montré une amélioration des performances parallèlement à une
diminution de la concentration de méthyléthylcétone jusqu'à un dixième
de la valeur initiale (qui pouvait atteindre 4000 mg/m3 pour
certaines tâches de routine).
8. Renforcement de la toxicité des autres solvants
La méthyléthylcétone potentialise la neurotoxicité des solvants
à six atomes de carbone ( n-hexane, méthyl- n-butylcétone et
2,5-hexanedione) ainsi que la toxicité hépatique et rénale des
solvants de la famille des alcanes halogénés (tétrachlorure de carbone
et trichlorométhane).
La potentialisation des effets neurotoxiques des composés à six
atomes de carbone a été démontrée chez l'animal pour les trois
substances citées ci-dessus. Les neuropathies périphériques observées
chez l'homme se sont produites à la suite de changements dans la
formulation des solvants auxquels les sujets avaient été exposés, soit
volontairement, soit en raison de leur activité professionnelle. Le
mécanisme de cette potentialisation n'a pas été élucidé.
La potentialisation de la toxicité hépatique et rénale des
alcanes halogénés a été mise en évidence dans des études chez
l'animal. La méthyléthylcétone active probablement la métabolisation
des haloalcanes en substances toxiques pour les tissus en induisant la
production des enzymes oxydantes responsables de cette transformation.
RESUMEN
1. Propiedades y métodos analíticos
La metiletilcetona (MEC) es un líquido transparente, incoloro,
volátil, muy inflamable, de olor parecido a la acetona. Es estable en
condiciones normales pero puede formar peróxidos si se almacena
durante mucho tiempo; esos peróxidos pueden ser explosivos. La MEC
también puede formar mezclas explosivas con el aire. Es muy soluble en
agua, miscible con muchos disolventes orgánicos, y forma mezclas
azeotrópicas con el agua y con numerosos líquidos orgánicos. En la
atmósfera, la MEC produce radicales libres que pueden llevar a la
formación de nieblas fotoquímicas.
Existen varios métodos analíticos para medir los niveles
ambientales de MEC en el aire, el agua, las muestras biológicas, los
desechos y otros materiales. Con los métodos más sensibles, la MEC se
separa y se concentra ya sea en un sorbente sólido o como derivado de
la 2,4-dinitrofenilhidrazina (DNFH). La MEC y otros compuestos
orgánicos volátiles absorbidos son desorbidos, separados mediante
cromatografía de gases y medidos con un espectrómetro de masas o un
detector de ionización de llama. La MEC derivada se separa de los
compuestos afines mediante cromatografía de líquidos de alto
rendimiento y se mide mediante absorción ultravioleta. En medios como
desechos sólidos y material biológico, la MEC debe separarse en primer
lugar del sustrato con métodos como la extracción por solventes o la
destilación de vapores. Las concentraciones elevadas de MEC en el aire
pueden controlarse de modo continuo mediante absorción infrarroja. Los
límites de detección son 3 µg/m3 en el aire, 0,05 µg/litro en el
agua potable, 1,0 µg/litro en otros tipos de agua, 20 µg/litro en
sangre total y 100 µg/litro en orina.
2. Fuentes de exposición y usos
2.1 Producción y otras fuentes
Las cifras más recientes de fabricación industrial anual (en
miles de toneladas) son: EEUU, 212 a 305; Europa occidental, 215;
Japón, 139. Además de su fabricación, las fuentes de MEC en el medio
ambiente son los gases de escape de motores de reactores y de
combustión interna, y las actividades industriales como la
gasificación del carbón. Se encuentra en cantidades importantes en el
humo de tabaco. En los Estados Unidos, la producción de MEC en motores
no supera el 1% de su fabricación deliberada. En los episodios de
nieblas, la producción fotoquímica de MEC y otros carbonilos a partir
de radicales libres puede ser mucho mayor que la emisión antropogénica
directa. La MEC se produce biológicamente y se ha identificado como
producto del metabolismo microbiano. Se ha detectado asimismo en gran
diversidad de productos naturales, entre ellos los vegetales
superiores, las feromonas de insectos, en tejidos animales y en el
hombre, en sangre, orina y aire exhalado. Probablemente es un producto
secundario del metabolismo normal en el mamífero.
2.2 Usos y pérdidas al medio ambiente
El uso principal de la MEC, la aplicación de revestimientos
protectores y adhesivos, refleja sus excelentes características como
disolvente. También se utiliza como intermediario químico, como
disolvente en la producción de cintas magnéticas y para eliminar la
cera del aceite lubricante, así como en la manipulación de alimentos.
Además de las aplicaciones industriales, figura como ingrediente común
en productos de consumo como barnices y pegamentos. En la mayoría de
las aplicaciones, la MEC es componente de una mezcla de disolventes
orgánicos. Las pérdidas al medio ambiente son principalmente al aire
y se deben sobre todo a la evaporación de disolventes a partir de las
superficies revestidas. Se libera al agua como componentes de los
desechos de su fabricación y a partir de diversas operaciones
industriales. Se ha detectado en aguas naturales, procedente
probablemente de la actividad microbiana y del aporte atmosférico, así
como de la contaminación antropogénica.
3. Transporte y distribución en el medio ambiente
La MEC es sumamente móvil en el medio ambiente natural y está
sometida a una transformación rápida. Es muy soluble en el agua y se
evapora fácilmente a la atmósfera. En el aire, la MEC sufre una rápida
descomposición fotoquímica y es también sintetizada por procesos
fotoquímicos. En agua que contiene halógenos libres o hipohalitos,
reacciona para formar un haloformo más tóxico que el compuesto
original. La MEC se distribuye tanto por el aire como por el agua,
pero no se acumula en ningún compartimento aislado, ni persiste mucho
tiempo donde existe actividad microbiana. Se metaboliza rápidamente en
los microbios y los mamíferos. No hay pruebas de bioacumulación. La
MEC aparece naturalmente en algunas especies de trébol y es producida
por hongos en concentraciones que afectan a la germinación de algunas
plantas.
4. Niveles ambientales y exposición humana
La exposición de la población general a bajos niveles de MEC es
muy extensa. En aire poco contaminado, la concentración es inferior a
3 µg/m3 (< 1 ppmm), pero en condiciones de fuerte contaminación
atmosférica se ha medido un nivel de 131 µg/m3 (44,5 ppmm). Lejos de
las zonas industriales donde se fabrica o se usa la MEC, las
principales fuentes pueden ser los escapes de vehículos y las
reacciones fotoquímicas en la atmósfera. Los cigarrillos y otros
productos del tabaco que se someten a combustión contribuyen a la
exposición individual (20 cigarrillos contienen hasta 1,6 mg). La
volatilización de la MEC de materiales de construcción y productos de
consumo pueden contaminar el aire de interiores hasta niveles muy
superiores a los del aire libre adyacente. Las concentraciones de MEC
en aguas naturales expuestas rara vez se encuentran por encima de los
100 µg/litro (100 ppmm) y suelen encontrarse por debajo del nivel de
detección. No obstante, se han detectado cantidades muy reducidas de
MEC en el agua potable (aproximadamente 2 µg/litro) que probablemente
proceden de disolventes lixiviados a partir del material de las juntas
de las tuberías de plástico. Aunque la MEC es un componente normal de
muchos alimentos, las concentraciones son bajas y el consumo de
alimentos no puede considerarse una fuente significativa de exposición
para la población. La ingesta media diaria por habitante de los
Estados Unidos con los alimentos se calcula en 1,6 mg, en su mayor
parte a partir del pan blanco, los tomates y el queso tipo Cheddar.
Además de la MEC presente en el medio natural, puede producirse en la
maduración de los quesos, el envejecimiento de la carne de ave, la
cocción o la manipulación de alimentos, o por absorción a partir de
los envases de plástico.
La exposición industrial a niveles moderados de MEC está muy
extendida. No obstante, en algunas regiones los trabajadores de
fábricas pequeñas (por ejemplo, fábricas de calzado, imprentas y
fábricas de pinturas) están expuestos a concentraciones mucho más
elevadas por una ventilación insuficiente. En esas fábricas, la
exposición se da por lo general a una mezcla de disolventes entre los
que figura el n-hexano.
5. Cinética y metabolismo
La absorción de MEC es rápida por contacto cutáneo, inhalación,
ingestión e inyección intraperitoneal. Pasa rápidamente a la sangre y
de ella a otros tejidos. La solubilidad de la MEC parece similar en
todos los tejidos. La eliminación de la MEC y sus metabolitos en
mamíferos se completa en su mayor parte en 24 horas. Se metaboliza en
el hígado, donde se oxida a 3-hidroxi-2-butanona y a continuación se
reduce a 2,3-butanodiol. Una pequeña porción puede reducirse a
2-butanol, pero éste se oxida rápidamente para dar de nuevo MEC. La
mayor parte de la MEC que ingresa al organismo de mamíferos pasa al
metabolismo general y/o se elimina en forma de compuestos simples,
como dióxido de carbono y agua. La excreción de MEC y sus metabolitos
reconocibles se hace principalmente por los pulmones, aunque pequeñas
cantidades se eliminan por el riñón.
La MEC aumenta la actividad enzimática del citocromo P-450 en los
microsomas. Este aumento de la actividad enzimática y con ello del
potencial del organismo para la transformación metabólica puede ser el
mecanismo por el que la MEC potencia la toxicidad de los disolventes
a base de haloalcanos y hexacarbonos alifáticos.
6. Efectos en los animales de experimentación
La MEC tiene toxicidad aguda, a corto plazo y crónica de baja a
moderada en los mamíferos. Los valores de la DL50 en ratones y ratas
adultos son 2-6 g/kg de peso corporal; la muerte sobreviene en los
días 1 a 14 después de una sola dosis por vía oral. Las
concentraciones medias de vapor que producen letalidad en las ratas
tras una sola exposición giran en torno a los 29 400 mg/m3 (10 000
ppm), aunque los cobayos sobrevivieron a una exposición de 4 horas a
esta concentración. La dosis oral aguda más baja en modificar la
estructura corporal fue de 1 g/kg de peso corporal, que produjo
lesiones en los túbulos renales de la rata. La inhalación de 74
mg/m3 (25 ppm) durante 6 horas produjo en la rata cambios de
comportamiento medibles que persistieron durante varios días. La
exposición repetida de ratas a 14 750 mg/m3 (5000 ppm) (6 h/día, 5
días/semana) no produjo letalidad, tuvo sólo ligeros efectos en el
crecimiento y la estructura, y no se observaron cambios
neuropatológicos. No hubo pruebas de que la MEC produjera cambios
neuropatológicos en pollos, gatos o ratones expuestos a 3975 mg/m3
(1500 ppm) durante periodos de hasta 12 semanas. Tras la exposición
repetida de ratas y babuinos a concentraciones tan bajas como 295-590
mg/m3 (100 a 200 ppm) se observaron efectos transitorios en el
comportamiento o la neurofisiología.
Se ha observado un nivel bajo de fetotoxicidad sin toxicidad
materna a 8825 mg/m3 (3000 ppm), pero no hay pruebas de efectos
embriotóxicos o teratogénicos a niveles más bajos de exposición. La
exposición repetida de ratas preñadas a 8825 mg/m3 indujo en sus
crías un aumento pequeño pero significativo de ciertos tipos de
anomalías esqueléticas cuya incidencia entre la población no expuesta
es baja.
Aunque se examinó en varios sistemas de ensayo de mutagenicidad
convencionales, la única prueba de mutagenicidad se observó en un
estudio sobre aneuploidia en la levadura Saccharomyces cerevisiae.
La MEC no presenta toxicidad aguda para los peces ni los
invertebrados acuáticos; los valores de la CL50 varían desde 1382
hasta 8890 mg/litro.
La MEC inhibe la germinación de varias especies vegetales,
incluso con niveles que se dan en la naturaleza. Inhibe el crecimiento
de algas acuáticas.
En comparación con los niveles de base naturales, se han
utilizado concentraciones relativamente elevadas de MEC para fumigar
en condiciones experimentales. Es moderadamente eficaz como fumigante
contra la mosca caribeña de la fruta y atrae con gran eficacia a la
mosca tse-tse. Con niveles de MEC de hasta 20 mg/litro se retrasa la
biodegradación pero no se detiene el proceso por completo. Con niveles
de hasta 100 mg/litro, la MEC es bacteriostatica para algunas
bacterias. Con concentraciones más altas (1000 mg/litro o más) se
inhibe el crecimiento de bacterias y protozoarias.
7. Efectos en el ser humano
7.1 MEC por sí sola
La exposición a 590 mg/m3 (200 ppm) no tuvo efectos de
importancia en varios ensayos comportamentales y psicológicos. La
exposición a corto plazo a MEC por sí sola no parece constituir un
riesgo de importancia, ni ocupacional ni para el público en general.
La exposición experimental a una concentración de 794 mg/m3 (270
ppm) durante 4 h/día tuvo escaso o ningún efecto en el comportamiento,
y un contacto de 5 minutos con MEC líquida no produjo más que un
blanqueamiento temporal de la piel. Sólo hay un informe no ocupacional
de toxicidad aguda a la MEC. Se debió a una ingestión accidental y no
pareció producir lesiones duraderas. No hay pruebas de que la
exposición ocupacional a la MEC haya originado ningún caso de muerte.
Se han notificado dos casos de envenenamiento ocupacional crónico y
uno dudoso de envenenamiento ocupacional agudo. En uno de los casos
crónicos, la exposición a 880-1770 mg/m3 (300-600 ppm) dió lugar a
dermatosis, endormecimiento de los dedos y los brazos, y diversos
síntomas como dolor de cabeza, mareos, trastornos gastrointes-tinales
y pérdida de apetito y de peso. Esta escasez de incidentes de
envenamiento por MEC por sí sola refleja tanto su baja toxicidad como
el hecho de que se usa más comunmente no por sí sola sino como
componente de mezclas de disolventes.
7.2 La MEC en mezclas de disolventes
La exposición a mezclas de disolventes con MEC se ha asociado a
cierta reducción en la velocidad de conducción nerviosa, la memoria y
alteraciones motoras, dermatosis y vómitos. En un estudio
longitudinal, las medidas consecutivas de tiempo de reacción simple
demostraron que mejoraba el rendimiento en paralelo al ir disminuyer
de las con concentraciones de MEC hasta un décimo de los valores
originales (que fueron de hasta 4000 mg/m3 para ciertas tareas
rutinarias).
8. Potenciación de la toxicidad de otros disolventes
La MEC potencia la neurotoxicidad de compuestos hexacarbonados
( n-hexano, metil- n-butilcetona y 2,5-hexanodiona) y la toxicidad
hepatica y renal de los disolventes a base de haloalcanos
(tetracloruro de carbono y triclorometano).
La potenciación de los efectos neurotóxicos de los hexacarbonos
se ha demostrado con los tres hexacarbonos en el animal. Las
neuropatías periféricas observadas en humanos siguieron a cambios en
la formulacion de disolventes a los que habían estado expuestos, ya
sea voluntariamente o en el trabajo. El mecanismo por el que se
produce esta potenciación no está claro.
Las pruebas de la potenciación de la toxicidad hepática y renal
de los haloalcanos proceden de estudios animales. La MEC activa
probablemente el metabolismo de los haloalcanos de las especies que
dañan los tejidos como resultado de la inducción de las enzimas
oxidativas pertinentes.
 6.4 Elimination and excretion
MEK and its metabolites are excreted by the lungs and kidneys.
Liira et al. (1988a) reported that only 3% of the calculated absorbed
dose during a 4-h exposure to 590 mg MEK/m3 (200 ppm) was secreted
unchanged in the exhaled air of volunteers after the exposure. The
fractional elimination of unchanged substance however depends on the
efficiency of metabolic clearance. Since metabolic saturation for MEK
in humans begins at relatively low levels (about 100 ppm) of exposure
(Liira et al., 1990a), proportionally greater amounts of MEK would be
expected to be excreted via the lungs (and kidneys) at high exposure
levels.
Relatively little of the absorbed MEK is excreted unchanged via
the kidneys; a study of occupationally exposed workers revealed that
it is less than 0.1% of the alveolar uptake (Miyasaka et al., 1982).
In a similar study of workers occupationally exposed to a mixture of
solvents, the excretion of MEK and a major recognizable metabolite,
3-hydroxy-2-butanone, was 0.1% of alveolar uptake (Perbellini et al.,
1984). The concentrations of both MEK and 3-hydroxy-2-butanone in
urine were significantly correlated with the environmental level of
MEK. Other metabolites of MEK, 2-butanol or 2,3-butanediol, which
DiVincenzo et al. (1976) identified in the serum of guinea-pigs, were
not detected in the urine of the exposed workers. Liira et al.
(1988a), however, reported that human excretion of 2,3-butanediol was
individually variable but averaged 2% of the absorbed MEK. The urinary
excretion of 2-butanol, a minor metabolite of MEK, was examined by
Kamil et al. (1953), who found that clearance of 2-butanol
administered by gavage in rabbits was about 14% of the administered
dose and in the form of a glucuronide.
Since MEK and 2,3-butanediol disappear from blood and urine and
there is no evidence for accumulation elsewhere in the body, the above
data suggest that the bulk of MEK absorbed by mammals enters the
general metabolism and is eliminated from the body as simple compounds
like carbon dioxide and water whose source is not readily
identifiable. The specific pathways by which MEK is metabolized have
not been identified. There also is no information on loss of MEK in
faeces.
6.5 Turnover
Both animal and human data indicate a rapid turnover of MEK. In
guinea-pigs receiving an intraperitoneal dose of 450 mg MEK per kg,
the half-life of MEK in blood serum was 4´ h and the clearance time
for MEK in serum was 12 h. For the metabolites 2-butanol,
3-hydroxy-2-butanone and 2,3-butanediol, the clearance time in serum
was 11 h (DiVincenzo et al., 1976). In rats given a 2.2-ml/kg oral
dose of 2-butanol, the butanol was largely cleared from the blood in
15 h and the 2-MEK derived from the butanol was cleared in 24 h
(Traiger & Bruckner, 1976). In a study by Dietz & Traiger (1979) on
rats given an oral dose of 2-butanone of 2.1 mg/kg, there was a
half-life of 3.6 h for MEK in blood if the rate of loss was assumed to
be constant between the two times of measurement (4 h and 18 h) after
dosing. Data from a study of Dietz et al. (1981) on rats receiving
oral doses of 2-butanol or MEK also indicate a half-life of about 4 h
for MEK. These authors reported that the clearance rate for
3-hydroxy-2-butanone and 2,3-butanediol was independent of dose for
the two doses used (0.4 and 0.8 g/kg) and that the half-lives for
these metabolites of MEK were 47 min and 3.45 h, respectively. Liira
et al. (1988a) reported a steady increase in blood concentrations
during 4-h exposures of human volunteer subjects to 590 mg/m3 (200
ppm) and observed a rapid elimination of MEK, with half-lives of 30
min during the first post-exposure hour and 81 min thereafter. An
inhalation study with two volunteer subjects exposed to MEK for 4 h at
concentrations of 74, 590 and 1180 mg/m3 (25, 200 and 400 ppm)
indicated that the kinetics of MEK were dose dependent at higher
exposure concentrations, i.e. much higher levels of MEK in blood were
reached relative to inhaled concentrations, and the post-exposure
elimination of MEK in blood was slower (zero-order kinetics).
Simulated exposure to MEK for 8 h suggests that saturation kinetics
are reached at about 295 mg/m3 (100 ppm) at rest and 148 mg/m3 (50
ppm) during light exercise (Liira et al., 1990a).
6.6 Metabolic interactions
Ingestion of ethanol (0.8 g/kg) combined with an inhalation
exposure to MEK (590 mg/m3, 200 ppm) inhibited the oxidative
metabolism of MEK and led to a marked increase in the blood
concentration of MEK (Liira et al., 1990b). There was a concurrent,
even more pronounced elevation of the blood 2-butanol concentration;
the most likely explanation is competitive inhibition by ethanol of
the oxidation of 2-butanol back to MEK. Ethanol also appeared to
interact with the further biotransformation of 2,3-butanediol as the
urinary excretion of the metabolite was increased. Co-exposure with
xylene, however, had no effect on the human metabolism or rate of
elimination of MEK (Liira et al., 1988b).
6.7 Mechanisms of action
There is very limited information on the mechanisms of toxic
action of MEK. Relatively high inhaled concentrations 1475-29 500
mg/m3 (500-10 000 ppm) caused pulmonary vasoconstriction and
hypertension in cats and dogs (Zakhari et al., 1977). From the
toxicological point of view, interactions leading to the potentiation
of effects, particularly neurotoxicity, by other intrinsically toxic
substances constitute the main hazard of MEK. The mechanisms
underlying these interactions are incompletely known (see section 10).
7. EFFECTS ON LABORATORY MAMMALS AND IN VITRO TEST SYSTEMS
7.1 Acute exposure
7.1.1 Lethal doses
Data on the acute toxicity of MEK to experimental animals are
summarized in Table 11. Oral toxicity is low with LD50 values
ranging from 2 to 6 g/kg for adult mice and rats. Intraperitoneal
LD50 values are lower (approximately 1.5 g/kg for 24 h and 0.6 g/kg
for 14 days). A larger dermal LD50 value for rabbits, i.e. > 8 g/kg
(contact time: 24 h; observation time: 14 days), may reflect slower
and less complete absorption via the skin, although it may also
reflect species differences in sensitivity to MEK. The lethal dose of
MEK given to rats in the only study using dosing by pulmonary
aspiration (Panson & Winek, 1980) was 0.8 g/kg. This is well below the
intraperitoneal 24-h LD50 for adult rats but similar to the 14-day
value (Lundberg et al., 1986). It cannot be excluded that the high and
rapid lethality of this aspirated dose to adult rats (5/6 deaths in
< 24 h, with 4/6 reported as dying "instantly") may reflect serious
damage to the lungs.
Studies examining the effects of acute inhalation of MEK are not
entirely comparable since they used not only different species but
also different concentrations of MEK, exposure times, and periods over
which survival was measured. For mice the LC50 (45-min exposure) was
about 200 000 mg/m3. The lowest concentration lethal to all rats
exposed by inhalation for 8 h was 47 200 mg/m3 and the lowest
concentration producing lethality in a 4-h exposure was 5900 mg/m3
(2000 ppm). Guinea-pigs survived exposure to 29 500 mg/m3 for 4 to
4.7 h and showed no abnormal signs at 9735 mg/m3. A concentration of
97 350 mg/m3 was lethal to all exposed guinea-pigs in 3.3 to 4.2 h,
whereas a slightly lower concentration, 73 750 mg/m3, although
ultimately lethal to all animals, permitted some guinea-pigs to
survive a 5.4-h exposure (Patty et al., 1935; Specht et al., 1940).
7.1.2 Non-lethal doses
Non-lethal acute doses of MEK produced a number of measurable
changes in experimental animals (Table 11). An oral dose of 1.5 g/kg
to rats resulted in a 63% increase in liver triglycerides after 16 to
23 h, but did not alter liver histology or increase either of two
enzymes, serum glutamic-pyruvic transaminase (alanine transferase
(ALT)) and hepatic glucose-6-phosphatase (Traiger & Bruckner, 1976).
These results suggest that this dose caused metabolic disturbances to
the liver of rats. Much smaller acute intraperitoneal doses (0.049 to
0.194 g/kg) to rats appeared not to damage the liver. A single oral
dose (15 mmol/kg) of MEK did not affect the hepatobiliary function of
rats over an observation period of 10 to 96 h (Hewitt et al., 1986).
A graded series of single doses of MEK to guinea-pigs revealed high
sensitivity to small changes in dosage. The low dose (0.75 g/kg)
appeared to produce no liver damage, whereas 1.5 g/kg produced slight
liver damage and 2.0 g/kg produced major liver damage. These results
also suggest that guinea-pigs and rats may be equally sensitive to MEK
in terms of liver damage even though guinea-pigs survive acute
exposure to higher concentrations of MEK vapour than do rats
(DiVincenzo & Krasavage, 1974).
Consistent increases in the frequency of food-reinforced lever
pressing by rats were detected at MEK exposures as low as 74 mg/m3
(25 ppm) for 2 h (Garcia et al., 1978). Vestibulo-ocular effects were
detected during continuous intravenous administration of MEK for 1 h
via the caudal vein at a dosage as low as 0.005 g/kg per min (Tham et
al., 1984). This dosage is roughly equivalent to inhalation of 2700
mg/m3 (900 ppm). Glowa & Dews (1987) reported that exposure of mice
to 885 mg/m3 (300 ppm) for 30 min did not significantly alter
schedule-controlled responses, whereas concentration-related
suppression of response occurred at consecutive increasing
concentrations (for 30 min) ranging from 2950 to 29 500 mg/m3 (1000
to 10 000 ppm) (see section 7.3.1).
It can be concluded from observations of their behaviour that
respiratory irritation occurred in guinea-pigs exposed to 29 500
mg/m3 or more within 2 min (Patty et al., 1935; Specht et al.,
1940). In survivors, post-exposure recovery from this effect was
rapid. Rats exposed for 8 h/day to 29 500 mg/m3 showed severe
irritation of the upper respiratory tract after a "few days"
(Altenkirch et al., 1979).
7.1.3 Skin and eye irritation
In skin irritation studies, a small dose (8 mg) applied to
clipped skin and covered by an impervious plastic film for 24 h (which
was followed by a 14-day observation period) produced only minor
irritation in male New Zealand albino rabbits (Smyth et al., 1962). A
dose of 400 mg applied to the clipped dorsal skin of restrained albino
rabbits in a gauze patch produced mild to moderate irritation in some
cases (Weil & Scala, 1971). Data from this latter study, however, were
highly variable and may reflect the fact that its purpose was
intercomparison of laboratories rather than the effects of MEK on test
animals. Neat MEK (0.1 ml) applied to the clipped skin of the flanks
of guinea-pigs and rabbits daily for 10 days and left uncovered caused
erythema and oedema after 24-72 h. These effects were more marked in
rabbits (Wahlberg, 1984a).
Table 11. Acute toxicity of MEK for mammalsa
Species Number and sex Exposure concentration Exposure Study Effects Reference
(strain) and range duration duration
Oral studies
Rat (Sprague- both sexes, 6-12 not stated one dose LD50 (7 days) < 0.8 g/kg b.w. Kimura et al.
Dawley) new animals/group 2.5 (2.0-3.1) g/kg b.w. (1971)
born 14 day 6 male/group 2.9 (2.3-3.5) g/kg b.w.
young adult 2.7 (2.1-3.5) g/kg b.w.
older adult
Rat (Carworth- 5 female/group not stated one dose 14 days LD50 (14 days) Smyth et al.
Wistar) 5.52 (4.50-6.80) g/kgb (1962)
Rat (Sprague- 5 male/group 1.505 g/kg b.w. one dose 24 h after 16-23 h, no effect on Traiger &
Dawley) liver histology, serum glutamic Bruckner (1976)
pyruvic transaminase, or
hepatic glucose-6-phosphatase;
63% increase in liver
triglycerides
Rat (Fischer- 32 male/group 0.072-1.082 g/kg b.w. one dose 24 h no evidence of liver Brown & Hewitt
344) dysfunction; slight damage at (1984)
highest dose to kidney proximal
tubules
Mouse (CF1) 10 male/group in 2.0-5.1 g/kg b.w. one dose 24 h LD50 (24 h) Zakhari et al.
each of 6 groups 3.14 ± 0.67 g/kg b.w. (1977)
Inhalation studies
Mouse (Swiss 10 male/group four levels over the 4 h decreased duration of De Ceaurriz et
OF1) range 4726-7192 mg/m3 immobility in escape - al. (1983)
(1602-2438 ppm) directed swimming test
Table 11 (contd)
Species Number and sex Exposure concentration Exposure Study Effects Reference
(strain) and range duration duration
Mouse (CD1) 12 males per five concentrations over continuous 2´ h increasing dose-related Glowa & Dews
concentration the range 885-29 500 (30 min per behavioural effects starting (1987)
mg/m3 (300-10 000 ppm) concentration) at 2950 mg/m3 (1000 ppm)
Mouse (CF1) 6 groups of 147 500-294 000 mg/m3 45 min LC50 (45 min) 205 000 ± 32 500 Zakhari et al.
10 males (50 000-100 000 ppm) mg/m3 (69 500 ± 11 000 ppm) (1977)
Mouse (white) both sexes, 6 303 850 mg/m3 until death average survival 43 min La Belle &
animals/group (103 000 ppm) Brieger (1955)
Rat (Sprague- 6 animals, sex 74-2360 mg/m3 2-6 h 2-6 h behavioural change (increased Garcia et al.
Dawley) unspecified (25-800 ppm) frequency of lever pressing) (1978)
which persisted for < 2 to
> 11 days
Rat (albino) 8 males/group 23 158-59 590 mg/m3 4 h 14 days LC50 (4 h) 34 500 mg/m3 La Belle &
(7850-20 200 ppm) (11 700 ppm) Brieger (1955)
Rat (Wistar) 5 males/group 23 600 mg/m3 8 h 14 days lethal to 3/6 within 14 days Smyth et al.
(8000 ppm) (1962)
Rat (Wistar) data not 47 200 mg/m3 8 h 14 days lethal to 6/6 within 14 days Smyth et al.
supplied (16 000 ppm) (1962)
Guinea-pig 6 unspecified approx. 9735 mg/m3 90-810 min 4-8 days no abnormal signs Patty et al.
sex/group; (3300 ppm) (1935)
3 groups
Guinea-pig 6 unspecified approx. 29 500 mg/m3 90-810 min 4-8 days 90 min: no injury; 280 min: Patty et al.
sex/group; (10 000 ppm) unconsciousness and slight (1935)
3 groups injury, no deaths; 810 min:
unconsciousness and moderate
injury, no deaths
Table 11 (contd)
Species Number and sex Exposure concentration Exposure Study Effects Reference
(strain) and range duration duration
Guinea-pig 10 females 73 750 mg/m3 325 min < 2 days all animals died during or Specht et al.
(25 000 ppm) post exposure (1940)
Guinea-pig 6 unspecified approx. 97 350 mg/m3 30-250 min 4-8 days 30 min: slight injury; 90 min: Patty et al.
sex/group; (33 000 ppm) unconsciousness and moderate (1935)
3 groups injury, no deaths; lethal to
all animals in 200-250 min
Guinea-pig 6 unspecified approx. 303 850 mg/m3 10-55 min 4-8 days 10 min: slight injury; 30 min: Patty et al.
sex/group; (103 000 ppm) unconsciousness and moderate (1935)
3 groups injury, no deaths, lethal to
all animals in 45-55 min
Intravenous study
Rat (Sprague- 18 females several infusion rates 60 min 60 min threshold limit for vestibulo- Tham et al.
Dawley) including 5 mg/kg b.w. ocular disturbance (depression (1984)
per min of nystagmus following
accelerated rotation) 5 mg/kg
b.w. per min; MEK concentration
in blood, 101 mg/litre
Pulmonary aspiration study
Rat (Sprague- 3 males and 800 mg/kg b.w. one dose survivors lethal 5/6 in < 24 h; 4/6 died Panson & Winek
Dawley) 3 females sacrificed instantly; produced pulmonary (1980)
at approx. haemorrhage
24 h
Intraperitoneal studies
Mouse (CF1) 10 male/groups; 0.5-2.0 g/kg b.w. one dose 24 h LD50 (24 h) Zakhari et al.
5 groups 1.66 ± 0.74 g/kg b.w. (1977)
Table 11 (contd)
Species Number and sex Exposure concentration Exposure Study Effects Reference
(strain) and range duration duration
Rat (Sprague- 6 female/group; not stated one dose 24 h LD50 (24 h) 1.554 Lundberg et
Dawley) number of groups (1.229-1.966) g/kg b.w. al. (1986)
unstated
Rat (Sprague- 6 female/group; not stated one dose 14 days LD50 (14 day) 0.607 Lundberg et
Dawley) number of groups (0.486-0.748) g/kg b.w. al. (1986)
unstated
Rat (Sprague- 6 female/group; 0.049-0.194 g/kg b.w. one dose 18 h no significant elevation of Lundberg et
Dawley) 3 groups serum sorbital dehydrogenase al. (1986)
Guinea-pig 4 male/group 0.75-2.0 g/kg one dose 24 h 0.75 g/kg b.w.: no elevation DiVincenzo &
in serum OCTc level, no Krasavage
obvious lipid deposition in (1974)
hepatocytes, no deaths;
1.5 g/kg b.w.: elevated serum
OCT level, lipid deposition in
hepatocytes, no deaths;
2.0 g/kg b.w.: elevation
in serum OCT level, lipid
deposition in hepatocytes,
lethal to 1/4 animals
Dermal studies
Rabbit (Albino, 4 males amounts not stated; 24 h 14 days LD50 (14 days) > 8 g/kg Smyth et al.
New Zealand) kept in contact with (1962)
clipped flank skin
under plastic film
Rabbit (Albino, 5 males 8 mg on clipped one dose 24 h minor irritation Smyth et al.
New Zealand) uncovered skin (1962)
Table 11 (contd)
Species Number and sex Exposure concentration Exposure Study Effects Reference
(strain) and range duration duration
Rat (Albino) 8 males 0.4 mg on 2.5 cm2 24 h 72 h variable results: no irritation Weil & Scala
lightly covered pad to moderate irritation 1 day (1971)
on clipped back skin and 3 days after applicationd
Rabbit (Albino) 6 animals intact 0.5 ml to clipped 24 h 72 h slight erythema subsiding by Hazleton
skin, 6 animals abdominal skin with 72 h (1963a)
abraded skin, semi-occlusive
sex unspecified cover
Ocular studies
Rabbit (Albino) 6 of unspecified 0.1 ml to one eye of 30 sec 14 days severe irritation, including Hazleton
sex each rabbit corneal damage and scleral (1963b)
haemorrhage in 2/6 rabbits
Rabbit (Albino) 5 male per not stated 3 min solution of 130 g/litre water Larson et al.
concentration produced significant oedema in (1955)
eyelid in 1 h
Rabbit (Albino, not specified 4 mg/eyee 1 min 18-24 h severe chemical burn Smyth et al.
New Zealand) (1962)
Rabbit (Albino) 6 males 8 mg to one eye of 20 sec 7 days variable results; no irritation Weil & Scala
each rabbit to moderate irritation 1 day, 3 (1971)
days and 7 days after
applicationd
Rabbit (Albino, 6 of unspecified 8 mg to one eye of described 7-10 days slight swelling, redness and MB Research
New Zealand) sex each rabbit as brief discharge from eye for 4 to Lab. Inc.
10 days (1979)
Table 11 (contd)
a Values from the literature were recalculated as necessary to yield dosages in terms of ppm, g or g/kg
b 95% confidence limits
c OCT = ornithine carbamyl transferase
d Purpose of study was intercomparison of testing laboratories that evaluated MEK and other substances by purportedly identical methods
e Not clear whether one eye or both eyes were dosed
The results of studies on eye irritation in rabbits are
inconsistent. Smyth et al. (1962) reported that 0.005 ml (4 mg)
created a severe chemical burn in the rabbit eye, whereas a study by
MB Research Lab. Inc. (1979) reported less severe irritant effects
from the larger dose of 0.1 ml (80 mg). Data from a study by Weil &
Scala (1971) were also inconsistent but indicated that a dose of 0.1
ml (80 mg) produced minimal to moderate eye irritation. Undiluted MEK
was used in all three studies but Smyth et al. (1962) used the grading
system for eye injury described in Carpenter & Smyth (1946), whereas
MB Research Lab., Inc. (1979) and Weil & Scala (1971) used the Draize
scoring system (Draize et al., 1944). In all studies irritation
disappeared or was markedly reduced by 7 days after treatment. Opaque
corneas were apparent following exposure of guinea-pigs to 295 000 mg
MEK/m3 (100 000 ppm) for 30 min; recovery from this effect was
complete in 4-8 days (Patty et al., 1935).
7.2 Repeated exposures
Data on repeated exposure of mammals to MEK are summarized in
Table 12. None of the concentrations tested, not even the highest (17
700 mg/m3 (6000 ppm) 8 h/day for up to 7 weeks) was clearly lethal
or even significantly harmful. The death of experimental animals
(rats) at this highest dose was not associated with neurological signs
and appeared to result exclusively from bronchopneumonia (Altenkirch
et al., 1978, 1979). These authors did not comment on possible
connections between bronchopneumonia and exposure to 17 700 mg/m3.
Female rats exposed to 14 750 mg/m3 (5000 ppm) 6 h/day, 5 days per
week, for 90 days showed only slightly increased liver weight,
slightly decreased brain and spleen weights, and slightly altered
blood chemistry in comparison with controls. Male rats receiving this
exposure showed only a slightly increased liver weight. At lower
concentrations of MEK (3688 and 7375 mg/m3 (1250 and 2500 ppm))
there was only slightly increased liver weight for female rats and no
significant differences for males in comparison with controls
(Cavender et al., 1983). In another subchronic inhalation study
(Toxigenics, 1981), male and female rats were exposed to MEK
concentrations of 3700, 7430 and 14 870 mg/m3 (1254, 2518 and 5041
ppm) 6 h/day, 5 days/week, for 90 days. No significant effects on food
consumption, eyes or nervous system were observed. In addition, no
MEK-induced morphological changes in the central or peripheral nervous
system were detected. Lower levels of exposure resulted in few
measurable effects. Inhalation of 2242 and 2360 mg/m3 (760 and 800
ppm) 6 h/day, 5 days/week, for 4 weeks by rats caused some enlargement
of the liver and slightly modified the in vitro metabolism of liver
microsomes (Nilsen & Toftgard, 1980; Toftgard et al., 1981). Ten
intraperitoneal injections of 0.034 g/kg over 2 weeks produced no
effect on the kidney (Bernard et al., 1989). However, exposure of rats
to 590 mg/m3 (200 ppm) 12 h/day for 24 weeks transiently decreased
nerve conduction velocity after 4 weeks (Takeuchi et al., 1983).
Geller et al. (1978) reported that exposure of baboons to 295 mg/m3
(100 ppm) for 7 days increased the response time in a delayed "match
to sample" task. However, this effect was transient and disappeared
during the course of repeated exposure. Following intermittent
exposure of rats to 3319 g/m3 for up to 5 months there was no
morphological evidence of peripheral neurotoxicity (Saida et al.,
1976). It is possible that the transient nature of the neurological
and behavioural changes induced by MEK exposure may be due to
behavioural and/or physiological adaptations. The latter may reflect
more rapid metabolism of MEK with prolonged exposure.
Short-term dermal exposure to small amounts of MEK resulted, at
most, in mild local irritation. Two topical applications of an
unstated amount to the ears of various strains of mice (Swiss, Balb/c,
CBA, C5681/6, DBA/2, B6D2F1) produced no significant swelling or other
signs (Descotes, 1988). In rabbits and guinea-pigs, a dose of 0.08 g
applied to the skin, without covering, once a day for 10 days to the
same site resulted in a slight to moderate increase in skinfold
thickness, whereas a much smaller dose (8 mg) applied to the same site
in rabbits 3 times a day for 3 days resulted in barely detectable
irritation (Wahlberg, 1984a). Open application of MEK to the shaved
flanks of guinea-pigs for 3 days produced slight erythema, epidermal
thickening and dermal cell infiltration (Anderson et al., 1986). In
the cat, injection of 150 mg MEK (99.98% pure) per kg, twice a day, 5
days/week, for up to 8.5 months, into subcutaneous tissue produced
abscesses, skin ulceration and generalized weakness but no evidence of
damage to the nervous system (Spencer & Schaumburg, 1976). In the one
long-term dermal study of MEK (Horton et al., 1965), 8 mg dissolved in
water was applied by dropper or brush to clipped skin of mice twice a
week for a year. Few details were given of the results because this
was the control for a study on carcinogenesis of the skin in which a
MEK/water solution was the solvent for compounds under test. Horton et
al. (1965) stated that the control mice did not develop skin tumours,
and they did not mention any adverse effects from this prolonged
application of MEK.
Table 12. Repeated exposure of mammals to MEKa
Route Species (strain), Exposure and range Results Reference
number and sex
Inhalation rat (Wistar), 590 ± 118b mg/m3 (200 ± 40b transient differences in nerve conduction claimed Takeuchi et al.
8 males ppm) 12 h/day for 24 weeks after 4 weeks, but no significant differences (1983)
Inhalation rat (Albino), 693 ± 77b mg/m3 (235 ± 26b no significant gross or microscopic La Belle &
25, sex ppm) 7 h/day, 5 days/week pathological changesc Brieger (1955)
unspecified for 12 weeks
Inhalation rat (Wistar), 885 mg/m3 (range 867-894) no significant change in leucocyte or serum Li et al. (1986)
7 females (300 ppm (range 294-303)) alkaline phosphatase activity
8 h/day for 7 days
Inhalation rat (Sprague- 3322 and 7723 mg/m3 (1026 at 7723 mg/m3 minor effects on dams, decreased Schwetz et al.
Dawley), and 2618 ppm) 7 h/day food consumption and weight gain, and increased (1974)
pregnant, 25 per for 10 days (gestation water consumption; no effects on dams at 1180 mg/m3
concentration days 6-15)
Inhalation rat (Sprague- 2242 and 2360 mg/m3 (760 and significant enlargement of liver; no effect on Nilsen &
Dawley), 4 800 ppm), 6 h/day, 5 days total liver microsomal concentration of cytochrome Toftgard (1980);
male/concentration per week for 4 weeks P-450; in vitro liver microsomal metabolism of Toftgard et al.
biphenyl unaffected, but slight effects on the (1981)
metabolism of benzo[a]pyrene, 4-androstene-3,17-
dione, and 5alpha-androstane-3alpha,17ß-diol
Inhalation rat (Fischer- 3688, 7375, 14 750 mg/m3 females at 14 750 mg/m3 increased in liver weight, Cavender et al.
344), 15 males, (1250, 2500, 5000 ppm) smaller braind and spleene weight, slightly altered (1983)
15 females/ 6 h/day, 5 days/week for blood chemistryf; females at 3688, 7375 mg/m3
concentration 90 days nonsignificant increase in liver weight; males at
14 750 mg/m3 increased in liver weight. No pathological
lesions, including peripheral nerves, attributed to
MEK exposure. No effect on reproductive tissues
(testis, epididymis, seminal vesicle, vagina, cervix,
uterus, oviduct, ovary)
Table 12 (contd)
Route Species (strain), Exposure and range Results Reference
number and sex
Inhalation rat (Fischer- 3699, 7430, 14 870 mg/m3 elevated group mean body weights at 3699 and 7430 Toxigenics
344), 15 males, (1254, 2518, 5041 ppm) mg/m3; highest concentration: decreased group mean (1981)
15 females/ 6 h/day, 5 days/week for body weight, increased liver weight, liver/body
concentration 90 days weight ratio and liver/brain weight ratio, increased
mean corpuscular haemoglobin; highest concentration
(males): increased kidney/body weight ratio, decreased
spleen and brain weights; highest concentration
(females): decreased brain/body weight ratio, increased
kidney/brain weight ratio; no significant effects at
any concentration on food consumption, eyes, nervous
system, and no morphological changes in central or
peripheral nervous systems
Inhalation rat (Sprague- 3319 mg/m3 (1125 ppm) 24 h no morphological effects on peripheral nerves Saida et al.
Dawley), 36 of per day for 16 days to 5 (1976)
unspecified sex months
Inhalation rat (Wistar), 29 500 mg/m3 (10 000 ppm) for severe irritation of upper respiratory tract; slight Altenkirch et
5 males a "few days"; 17 700 mg/m3 loss of weight; death during seventh week from al. (1978,
(6000 ppm) ± 15% for 8 h/day, bronchopneumonia; no neurological signs 1979)
7 days/week for 15 weeks
Inhalation guinea-pig, 693 ± 77b mg/m3 (235 ± 26b no significant effects La Belle &
15 of unspecified ppm) 7 h/day, 5 days/week for Brieger (1955)
sex 12 weeks
Inhalation baboon, 295 mg/m3 (100 ppm) for 7 days no impairment of discrimination in a behavioural Geller et al.
4 males test, slight increase in response time early in (1978)
experiment, but not at end of experiment
Table 12 (contd)
Route Species (strain), Exposure and range Results Reference
number and sex
Intra- rat (Sprague- 0.034 g/kg, 5 days/week for no effect on kidney function Bernard et al.
peritoneal Dawley), 2 weeks (1989)
female, number
unspecified
Subcutaneous cat, 6 of 0.15 g/kg twice/day, 5 days abscesses, skin ulceration and generalized weakness Spencer &
unspecified sex per week for up to 8.5 months in some animals; no damage to nervous Schaumburg
system structure or function (1976)
Dermal mouse (Swiss), unstated amount applied twice no significant swelling of treated ear Descotes (1988)
12 of unspecified to one ear
sex
Dermal mouse 8 mg (50 mg of 17% solution) no papilloma evident after 1 year Horton et al.
(C3H/He) applied to clipped skin twice (1965)
10-25 males per week for 1 year
Dermal guinea-pig, 3 80 mg rubbed into skin at slight to moderate increase in skinfold thickness Wahlberg (1984a)
and rabbit, 4, same site once daily for at end of 10 days
of unspecified 10 days
sex
Dermal guinea-pig 80 mg applied to 1.0 cm2 on no reaction until day 2; at end of experiment, Anderson et al.
10 females shaved flank 3 times daily slight redness and increase in dermal inflammatory (1986)
for 3 days cell count
a Values from the literature recalculated as ppm, mg or g/kg
b Standard deviation of the mean
c Depressed growth (average weight 70% of controls at end of experiment) may be due to causes other than MEK exposure; animals showed signs
of "vitamin deficiency" (no details given) and infection in latter part of experiment.
d Significantly different at the < 0.01 level from 0, 3688 and 7375 mg/m3 (0, 1250, and 2500 ppm) female group values
e Significantly different at the < 0.05 level from 0, 3688 and 7375 mg/m3 (0, 1250, and 2500 ppm) female group values
f Females in the 14 750 mg/m3 (5000 ppm) group had a small but significant elevation in serum potassium, alkaline phosphatase and glucose
levels, and a significant reduction in serum glutamic-pyruvic transaminase level.
7.3 Neurotoxicity
7.3.1 Behavioural testing
Neurotoxicity studies have been carried out on MEK, usually as
part of studies on the neurotoxicity of methyl isobutyl ketone (MIBK)
and MIBK/MEK mixtures.
An increase in response rate (lever pressing) was reported in a
group of six adult Sprague-Dawley rats (sex unspecified) exposed to
MEK at various concentrations between 74 and 2360 mg/m3 (25 and 800
ppm) for 2 h at approximately weekly intervals. An increase in
response rate also occurred in a group of four rats exposed to 74
mg/m3 (25 ppm) for 6 h at 2-day intervals (Garcia et al., 1978).
Geller et al. (1978) examined behavioural effects (match to
sample (MTS) test) in baboons exposed to MEK by inhalation. Four young
male baboons (2 years old) were exposed continuously to MEK at a
concentration of 295 mg/m3 (100 ppm) for 7 days. There were no
effects on performance of the test in terms of the ability to
discriminate visual stimuli but reaction time increased. However, in
two of the baboons, response times returned to pre-exposure control
values by day 7.
Tham et al. (1984) examined the vestibulo-oculomotor reflex (VOR)
during intravenous infusion of MEK into the caudal veins of 18 female
Sprague-Dawley rats. The threshold for depression of the VOR was an
infusion rate of 70 µmol/kg (0.005 g/kg per min) for 1 h (total dose
30 mg) and the associated arterial blood level was 1.4 mmol/litre.
General depression of the central nervous system followed depression
of the VOR.
Glowa & Dews (1987) exposed continuously by inhalation a group of
12 adult male white mice (Charles River CD1) to concentrations of MEK
that were increased at 30 min intervals until the mice failed to
respond to a visual stimulus. The concentrations, in ascending order
for each 30 min, were 885, 2950, 8850, 16 520 and 29 500 mg/m3 (300,
1000, 3000, 5600 and 10 000 ppm) with a total exposure time of 2´ h.
Mice responded to a visual stimulus and the response rate was used as
an indicator. There was no effect at a concentration of 885 mg/m3,
a slight decrease in response rate at 2950 mg/m3 and a 75% decrease
at 8850 mg/m3. Most mice ceased to respond at 16 520 mg/m3 and all
failed to respond at 29 500 mg/m3. The response rate returned to the
control value 30 min after exposure ended. The EC50 (concentration
decreasing response rate by 50%) was calculated to be 8528 (SD = 2033)
mg/m3 (2891 (SD = 689) ppm). An EC10 was calculated and
dose-response estimates were derived. The concentrations of MEK
producing a 10% decrease in response rate in 0.1%, 1% and 19% of a
population were 50, 195 and 885 mg/m3 (17, 66 and 300 ppm),
respectively.
Couri et al. (1977) exposed continuously by inhalation young male
Wistar rats to 2210 mg MEK/m3 (750 ppm) for either 7 or 28 days. In
those exposed for 7 days there was a significant reduction in
hexobarbital sleep times. In the group exposed for 28 days the
reduction in sleep times was less marked.
7.3.2 Histopathology
In chickens, cats, rats and mice exposed by inhalation to 3975
mg/m3 (1500 ppm) for periods of up to 12 weeks, there was no
evidence of neuropathy and no histopathological changes were reported
(Couri et al., 1974).
Saida et al. (1976) exposed groups of 36 Sprague-Dawley rats (sex
unspecified) continuously to MEK at a concentration of 3319 mg/m3
(1125 ppm) for periods of 16, 25, 35 and 55 days. Additional studies
were carried out with up to 5 months of exposure. There were no
abnormal clinical observations in any group. At the end of the
exposure period, rats were sacrificed and the sciatic nerve and foot
muscle excised. Spinal cord and dorsal root ganglion specimens were
taken from the same rats. Quantitative histology
(neurofilaments/µm2; frequency of inpouching of myelin sheath,
denuded axons/mm2) showed no abnormality in rats exposed for up to
5 months.
Cavender et al. (1983) reported no neurological abnormalities in
Fischer-344 rats in a 90-day inhalation study on MEK alone. Groups of
15 male and 15 female rats were exposed 6 h/day, 5 days/week, for 90
days to MEK concentrations of 3688, 7375 and 14 750 mg/m3 (1150,
2500 and 5000 ppm). All rats were observed twice daily for clinical
signs. At the end of the exposure period, the eyes of each animal were
examined by ophthalmoscopy, and neurological function (posture, gait,
tone and symmetry of facial muscles, and pupillary, palpebral,
extensor-thrust and cross-extensor thrust reflexes) was evaluated. No
abnormalities were found. Necropsy, including histopathology of the
sciatic and tibial nerves, was carried out on all rats, with special
neuropathological studies on the medulla, sciatic and tibial nerves in
5 male and 5 female rats from each group. There were no changes
attributable to MEK.
7.4 Developmental toxicity
Schwetz et al. (1974) exposed 44 pregnant rats from days 6-15 of
gestation (sperm = day 0) to two concentrations of MEK vapour, i.e.
nominally 2950 mg/m3 in 23 rats and 8850 mg/m3 in 21 rats (1000
and 3000 ppm), for 7 h/day; 43 rats were air-exposed as controls. The
average values for measured concentrations in this study were 3322 and
7723 mg/m3 (1126 and 2618 ppm), respectively. There was no evidence
of maternal toxicity. Fetal weight and crown-rump length were
significantly decreased at 3322 mg/m3, but not at 7723 mg/m3. At
3322 mg/m3 there was also a significant increase, compared to the
controls, in the number of litters with fetuses showing skeletal
anomalies, and at 7723 mg/m3 a significantly increased incidence of
litters with sternebral and soft tissue anomalies was reported. Four
grossly malformed fetuses were found (two with brachygnathia and two
acaudate with imperforate anus), all in different litters in the 7723
mg/m3 group. These malformations had not been observed previously in
more than 400 control litters of this strain. Fetal body dimensions,
however, were not significantly different from controls. When the data
were analysed on a per litter basis, there was evidence of a
teratogenic effect.
However, the incidence of major malformations in these studies
was sufficiently low that evidence for teratogenic effects was
considered questionable, and the studies were repeated (John et al.,
1980; Deacon et al., 1981). The methodology was identical except for
the inclusion of an additional level of exposure, nominally 1180
mg/m3 (400 ppm). Average measured MEK concentrations during the
experiment were 1215, 2956 and 8865 mg/m3 (412, 1002 and 3005 ppm).
The only evidence for maternal effects was decreased weight gain and
increased water consumption (no figures given) by dams exposed to 8865
mg/m3. None of the dosages had significant effects on the incidence
of pregnancy, incidence of resorption, average number of implantations
and live fetuses per dam, fetal weight and length, or incidence of
external or soft tissue abnormalities. There were statistically
significant differences in the incidences of some skeletal anomalies
occurring in the 8875 mg/m3 group compared to the controls. There
were increased incidences of lumbar ribs and delayed ossification of
the cervical centra, but a decreased incidence of delayed ossification
of the skull. Since these skeletal abnormalities occurred at low
incidences among the population from which the experimental animals
were drawn, the results of this study were interpreted as indicating
a low level of fetotoxicity and no evidence for embryotoxic or
teratogenic effects for MEK at exposure levels up to 8865 mg/m3.
In a further study (Mast et al., 1989; Schwetz et al., 1991),
groups of 10 virgin female Swiss CD1 mice and 33 plug-positive (day 0)
females were exposed by inhalation on gestation days 6-15 to mean
concentrations of 1174 ± 27, 2980 ± 83 and 8909 ± 233 mg/m3 (398 ±
9, 1010 ± 28 and 3020 ± 79 ppm). There was no evidence of maternal
toxicity, although there was a slight, treatment-related increase in
liver/body weight ratios that was significant at the highest dose
level. Mild fetal toxicity was evident at this maternal dose level as
a reduction in mean fetal body weight, statistically significant for
males. There was no increase in the incidence of intrauterine death,
but there was an increased dose-related incidence of misaligned
sternebrae, statistically significant at the highest dose level. There
were no significant increases in the incidence of malformations,
although there were several malformations in one litter (cleft palate,
fused ribs, missing vertebrae, syndactyly) in treated groups but not
in the control group nor in contemporary control data. On the basis of
this study it was concluded that the no-observed-adverse-effect level
(NOAEL) was 2978 mg/m3 (1010 ppm) and the
lowest-observed-adverse-effect level (LOAEL) was 8096 mg/m3 (3020
ppm).
7.5 Mutagenicity and related end-points
Short-term genotoxicity tests in vitro and in vivo are
summarized in Table 13. Although MEK has given negative results in
most conventional assays, Zimmermann et al. (1985) found that MEK and
certain other polar aprotic solvents were strong inducers of
aneuploidy in the yeast. The induction of aneuploidy by MEK was
markedly potentiated by coexposure to ethyl acetate (Mayer & Goin,
1988) or with nocodazol (methyl [5- (2-thienyl-carbonyl)-1
H-benzimidazol-2-yl]-carbamate) (Mayer & Goin, 1987).
O'Donoghue et al. (1988) conducted mutagenicity studies on MEK.
The test systems comprised the Salmonella/microsome (Ames) assay, the
L5178/TK+/- mouse lymphoma (M/L) assay, the BALB/3T3 cell
transformation (CT) assay, unscheduled DNA synthesis (UDS) and the
micronucleus test. MEK was not found to be genotoxic in these assays.
Other studies of MEK utilizing cultures of mammalian cells as
test systems also yielded little or no evidence of mutagenicity and
related effects. Perocco et al. (1983) tested MEK and other important
industrial chemicals at concentrations of 10-2 to 10-4 mol/litre
(0.72 to 0.0072 g MEK/litre) with an in vitro system utilizing
cultures of human lymphocytes to determine toxicity and ability to
inhibit DNA synthesis. Cultures were grown both with and without S9
mix derived from phenobarbital-induced rat liver. MEK at the
concentrations tested showed no evidence of cytotoxic or genotoxic
action. Chen et al. (1984) examined the effects of MEK on metabolic
cooperation between 6-thioguanine-resistant and
6-thioguanine-sensitive Chinese hamster lung fibroblast V79 cells and
obtained equivocal results. Holmberg & Malmfors (1974) also found some
evidence of MEK cytotoxicity to ascites tumour cells cultured with the
solvent for up to 5 h. Although there was no significant increase in
irreversibly injured cells at MEK concentrations of 0.05 and 0.1
g/litre, there was a moderate increase in damaged cells at 0.1
g/litre. An ultrastructural study (Veronesi, 1984) utilizing a medium
containing relatively high concentrations of MEK (0.3 g/litre)
produced axoplasmic granularities in a few cultures. The relationship
of this effect to possible MEK-induced neurotoxicity in vivo is not
clear.
7.6 Carcinogenicity
No long-term carcinogenicity studies have been reported.
Table 13. Short-term genotoxicity tests on MEK
Method Concentration Experimental conditions; comments Results References
In vitro studies
Bacterial assays 3 µmol/plate Salmonella typhimurium TA98, TA100, TA1535, - Florin et al. (1980)
TA1537 with and without S-9
10 mg/plate S. typhimurium TA98, TA100, TA1535, - Nestmann et al. (1980)
TA1537 with and without S-9
approx. S. typhimurium TA104: maximum non-toxic - Marnett et al. (1985)
1 mg/plate dose > 3 µmol
0.05-32 µl/plate S. typhimurium TA98, TA100, TA1535, - O'Donoghue et al. (1988)
TA1537, TA1538 with and without S-9
4 mg/plate Escherichia coli WP2 and WP2 uvrA - Brooks et al. (1988)
Mitotic gene 5 mg/ml Saccharomyces cerevisiae (JD1) - Brooks et al. (1988)
conversion assay
Induction of mitotic 3.54% S. cerevisiae (D61.M) + Zimmermann et al. (1985)
aneuploidy
0.50-1.96% S. cerevisiae (D61.M) + Mayer & Goin (1987)
Chromosome assay 1 mg/ml rat liver RL4 cells - Brooks et al. (1988)
Cell transformation 9-18 µl/ml BALB/3T3 - O'Donoghue et al. (1988)
UDS test 0.1-5.0 µl/ml primary rat (Sprague-Dawley) hepatocyte - O'Donoghue et al. (1988)
Table 13 (contd)
Method Concentration Experimental conditions; comments Results References
In vivo studies
Micronucleus test 1.9 ml/kg CD1 mice (male and female), - O'Donoghue et al. (1988)
intraperitoneal time: 12, 24, 48 h
411 mg/kg Chinese hamster, time: 12, 24, 48, 72 h - Basler (1986)
intraperitoneal
8. EFFECTS ON HUMANS
8.1 General population exposure
The only record of non-occupational acute toxicity from MEK was
a case of accidental self-poisoning (Kopelman & Kalfayan, 1983). A
47-year old woman inadvertently ingested an unknown amount of MEK and
was found unconscious, hyperventilating, and suffering from severe
metabolic acidosis. Her plasma concentration of MEK was 950 mg/litre.
She responded promptly to an infusion of sodium hydrogen carbonate and
was discharged from the hospital after a week. The metabolic effects
of MEK ingestion by humans are not well characterized and it is
uncertain that the acidosis was produced by MEK.
8.2 Effects of short-term exposure
There are limited data on behavioural and other effects on humans
of short-term exposure to MEK. Nakaaki (1974) found that exposure to
266-797 mg/m3 (90-270 ppm) for up to 4 h per day caused his subjects
to underestimate times of 5 to 30 seconds. Dick et al. (1984, 1988,
1989), on the other hand, found that a 4-h exposure of human subjects
to 590 mg/m3 (200 ppm) had no significant effect in a variety of
behavioural tests. These included psychomotor, visual vigilance, dual
task, sensorimotor and psychological tests. Solvent mixtures of 295 mg
MEK/m3 (100 ppm) and 186 mg toluene/m3 (50 ppm), and of 295 mg
MEK/m3 (100 ppm) and 298 mg acetone/m3 (125 ppm) similarly had no
significant effect on the results of these behavioural tests.
8.3 Skin irritation and sensitization
MEK (0.1 ml) rubbed into volar forearm skin daily for 18 days and
left uncovered did not produce persistent erythema or swelling
(Wahlberg, 1984a). A 5-min contact with 1.5 ml of analytical grade MEK
confined to a 20-mm circle on the forearm produced a temporary
whitening of the skin, but no visible erythema, alteration in
cutaneous blood flow or other indication of irritation to the skin
(Wahlberg, 1984b).
A male painter developed dermatitis 18 months after commencing
spray painting using an epoxy-polyamide paint (Varigos & Nurse, 1986).
A patch test with "a small amount" of MEK applied to areas of skin
3 cm in diameter on each forearm caused these areas of skin to turn
bright red within 10 min. The spots were itchy, but there was no
induration or oedema. The reaction reached its maximum after 15 min
and then gradually faded. The test was repeated after 2 days, and gave
the same results. Application of the same grade of MEK to normal
forearm skin of five male volunteers produced no reaction.
8.4 Occupational exposure
8.4.1 MEK alone
There is no record that MEK toxicity has ever caused death or a
large scale industrial accident, and only one acute occupational
poisoning has been ascribed to MEK. An 18-year-old seaman with good
vision and no previous eye problems was exposed to MEK vapour of
unknown concentration while stripping paint, and promptly noted
headache, mild vertigo and blurred vision (Berg, 1971). The diagnosis
was retrobulbar neuritis. He was given vitamin B complex and steroid
therapy, and his vision returned to normal in 36 h. However, blood
analysis exhibited a positive methanol content (no value reported)
according to the criteria given by Kaye (1961). Thus a potentiation of
the effects due to combined exposure to MEK and methanol cannot be
excluded.
Data for occupational poisoning ascribed to chronic exposure to
MEK in the absence of other solvents are equally limited. Long-term
exposure of 51 Italian workers to MEK produced indications of
neurotoxicity with slightly, but not significantly, reduced nerve
conduction velocities and various other symptoms such as headache,
loss of appetite and weight, gastrointestinal upset, dizziness,
dermatitis and muscular hypotrophy, but no clinically recognizable
neuropathy (Freddi et al., 1982). There has been a brief report of
chronic exposure of American workers, in a factory producing coated
fabric, to 885-1770 mg MEK/m3 (300-600 ppm) in the apparent absence
of other solvents (Smith & Mayers, 1944). Workers complained of
dermatoses and numbness of fingers and arms.
8.4.2 MEK in solvent mixtures
It was reported that MEK was commonly present as part of solvent
mixtures containing hexane, and that the TLV for hexane (148 mg/m3,
50 ppm) was exceeded in 89 of the work stations (mainly in shoe
factories) (De Rosa et al., 1985). MEK potentiates the toxicity of
hexane and these authors concluded that in these shoe factories the
risk of neurotoxicity was extremely high. Observations by Tangredi et
al. (1981), Brugnone et al. (1981) and Cresci et al. (1985) supported
the widespread nature of this health problem in Italian industry,
especially shoe factories. Arques Espi & Quintanilla Almagro (1981)
also found that mixtures of solvent vapours including MEK and hexane
posed an excessive risk in 95 of 114 work stations examined in a study
of Spanish shoe factories. In a nationwide survey of Japanese
factories, Inoue et al. (1983) found that MEK is widely used as a
component of solvent mixtures. In a study of electronic parts plants
in the USA (Lee & Parkinson, 1982), workers were found to be exposed
to mixtures of solvent vapours containing MEK and as many as nine
other components, although not hexane or other solvents whose toxic
action MEK is known to potentiate. Observations on Finnish car
painters (Husman, 1980; Husman & Karli, 1980) suggested that long-term
exposure to complex solvent mixtures whose components individually and
jointly are far below the legal concentration limits may produce
significant adverse effects. Noma et al. (1988) similarly suggested
that complex mixtures of volatile organic compounds, rather than a
high concentration of any single compound, may be responsible for
unhealthy air in buildings.
Descriptions of the effects of occupational exposures to mixtures
of solvent vapours not necessarily potentiated by MEK are summarized
in Table 14. There are only two cases of acute occupationally related
toxicity from such mixtures, and only a limited number of adequately
documented cases of adverse effects from chronic occupational exposure
which did not involve potentiation of hexacarbon toxicity. The only
acute cases involved two young women waterproofing seams of raincoats
with resins dissolved in acetone or MEK (Smith & Mayers, 1944).
Post-exposure measurements revealed workplace concentrations of
785-1178 mg/m3 (330-495 ppm) for acetone and 1174-1655 mg/m3
(398-561 ppm) for MEK. The total solvent concentration was estimated
to be 1000 ppm. Both women fainted at work and subsequently displayed
a number of temporary neurological and other symptoms.
However, the majority of these studies are difficult to interpret
because they lack either a quantitative description of the solvent
vapours in the work environment, or the description is based on
post-exposure analyses that may not be typical of working conditions
during most of the exposure. In these studies it is also impossible to
make any certain assessment of the role(s) played by individual
components. Mixed solvent exposures have been associated with
alteration in nerve conduction velocity (Viader et al., 1975; Dyro,
1978; Triebig et al., 1983), memory and motor alterations (Binaschi et
al., 1976), and dermatoses and vomiting (Lee & Murphy, 1982). Fagius
& Grönqvist (1978) reported neurological effects in 3 out of 42
workers exposed in a steelworks to organic solvents. However, the
actual role of MEK in these effects is not clear.
Table 14. Effects of occupational exposure to mixtures of solvent fumes containing MEK
Concentrations of MEK and other No. of workers Exposure Effects Defects in study Reference
components exposed duration
Acute
MEK 1174-1655 mg/m3 (389-561 ppm), 2 few hours eyes watering, gastric measurements of work Smith &
acetone 785-1178 mg/m3 (330-495 ppm) distress, fainting, environment limited Mayers
convulsions, twitching, to post exposure (1944)
headache, spinal pressure
Chronic
MEK 561-885 mg/m3 (190-300 ppm), 13 1 year or fatigue, frequent headache, measurements of work Lee &
toluene 139-177 mg/m3 (37-60 ppm), more dizziness, incoordination, environment limited Murphy
MIBK 37-41 mg/m3 (9-14 ppm) skin rashes, vomiting to post exposure (1982)
MEK 30 mg/m3 (10 ppm), toluene 2 2 and 6.5 paraesthesis in extremities, measurements of work Dyro
94-488 mg/m3 (25-130 ppm) years reduced motor nerve conduction environment limited (1978)
velocity, weakness in to post exposure
individuals exposed for long
periods, improvement following
cessation of exposure
MEK 62-51 mg/m3 (21-180 ppm), acetone 1 1 year altered consciousness and measurements of work Dyro
86-595 mg/m3 (36-250 ppm) EEG, reduced motor nerve environment limited (1978)
conduction velocity to post exposure
MEK 142 mg/m3 (48 ppm), isobutanol 8 not stated headache, dizziness, fatigue, measurements of work Binaschi
67 mg/m3 (23 ppm), MIBK 16 mg/m3 (4 ppm), depression, significant environment limited et al.
toluene 180 mg/m3 (48 ppm), butyl acetate reduction in short-term to post exposure; (1976)
43 mg/m3 (9 ppm), xylene 347 mg/m3 memory and hand steadiness period of exposure
(80 ppm) not indicated
Table 14 (contd)
Concentrations of MEK and other No. of workers Exposure Effects Defects in study Reference
components exposed duration
MEK 115 (avg), 2655 (max) mg/m3, n-butanol 9 8-35 significant changes in measurements of work Denkhaus
67 (avg), 1200 (max) mg/m3, isobutanol years several sub-populations of environment limited et al.
172 (avg), 3000 (max) mg/m3; 2-butoxy- peripheral blood lymphocytes: to post exposure (1986)
ethanol 25 (avg), 350 (max) mg/m3, decrease in certain type of
2-ethoxyethanol 5 (avg), 53 (max) mg/m3, T-cells and helper cells,
2-methoxyethanol 6 (avg), 150 (max) mg/m3, increase in natural killer
toluene 86 (avg), 750 (max) mg/m3, cells and B-lymphocytes
m-xylene 19 (avg), 220 (max) mg/m3,
MBK 2 (avg), 27 (max) mg/m3
MEK 9-124 mg/m3 (3-42 ppm), 5 > 10 years no significant differences measurements of work Lundberg
xylene 0-6111 mg/m3 (0-1408 ppm), in serum activities of liver environment limited &
toluene 0-1260 mg/m3 (0-336 ppm), enzymes between exposed to post exposure Hakansson
isobutanol 0-1045 mg/m3 (0-345 ppm), workers and controls (1985)
n-butanol 0-1548 mg/m3 (0-511 ppm),
ethanol 0-1094 mg/m3 (0-582 ppm),
ethyl acetate 0-2095 mg/m3 (0-582 ppm),
n-butyl acetate 0-1691 mg/m3 (0-356 ppm),
methyl acetate 3-181 mg/m3 (1-60 ppm),
white spirit 2-30 mg/m3 (1-17 ppm),
methylene chloride 10-2460 mg/m3 (3-707 ppm),
isopropanol 5-260 mg/m3 (2-106 ppm),
exposure to 2-8 solvents plus MEK
MEK < 9-401 mg/m3 (< 3-136 ppm), 66 average significant reduction in measurements of work Triebig
xylene < 4-82 mg/m3 (< 1-19 ppm), 5 years sensory conduction in environment limited et al.
toluene 11-551 mg/m3 (3-147 ppm), comparison with controls, no to post exposure (1983)
ethyl acetate < 11-302 mg/m3 (< 3-84 ppm) suggestion of neuropathy,
trichloroethane 11-601 mg/m3 (2-110 ppm) change in conduction velocity
correlated with exposure
Table 14 (contd)
Concentrations of MEK and other No. of workers Exposure Effects Defects in study Reference
components exposed duration
MEK 15-620 mg/m3 (5-210 ppm), acetone 17 not stated no health problems associated measurements of work Cohen &
< 119-495 mg/m3 (< 50-208 ppm), xylene with exposure to MEK environment limited Maier
< 22 mg/m3 (< 5 ppm), toluene < 19-34 to post exposure (1974)
mg/m3 (< 5-9 ppm), petroleum naphtha
(concentration unknown)
MEK mainly < 443, max. 5115 mg/m3 42 0.5-8 1 likely and 2 suspected measurements of work Fagius &
(mainly < 150, max. 1734 ppm), trichloroethylene years cases of polyneuropathy, environment limited Gronqvist
mainly < 161 mg/m3 (< 30 ppm); slight loss of sensitivity to post exposure (1978)
at low levels butanol, butyl acetate, butyl to vibration correlated
diglycol, cyclohexanol, diacetal, ethyl with level of solvent ex-
glycol acetate, ethanol, isoforone, posure during preceding
methylene chloride, MIBK, toluene, 6 months
xylene, "solvesso 100 and 150"a
MEK 0-74 mg/m3 (avg, 3 mg/m3) (0-25 1006b average overall death rate below none Wen et al.
(avg. 1) ppm), toluene 4 mg/m3 (1 ppm); 21.6 years expected level, excess of (1985)
at very low levels benzene, "hexane", deaths from cancer associ-
MIBK, xylene ated with lubricating oil,
not MEK
a hydrocarbon solvent mixtures
b workers from lube oil and dewaxing plant
In a study on a group of 9 parquet-flooring workers (age, 25-58
years; exposure time, 8-35 years), Denkhaus et al. (1986) noted
significant changes in several subpopulations of peripheral blood
lymphocytes, which could constitute an early indication of a
haematological or immunological effect. Benzene was not detected in
the ambient air and no investigation was made to determine which
components of the solvent mixtures produced the observed changes in
lymphocyte populations. Anshelm Olson et al. (1981) studied the simple
reaction time (SRT) performance in a group of 42 workers (age, 18 to
52 years; employment, 0.5 to 8.1 years) from a plastic coating line of
a steel factory (the same group had previously been studied by Fagius
& Grönqvist, 1978). The study was longitudinal and covered a period of
27 months during which SRT was measured three times. Originally, the
workers had been exposed to significant concentrations of MEK (up to
4000-5000 mg/m3 in certain regular tasks) and to much lower levels
of other solvents. Five months after the completion of major
improvements in the work environment which reduced the levels of MEK
to about 20 mg/m3 (maximum of about 400 mg/m3), a second SRT
measurement was made and a third measurement was performed 15 months
later. The workers' performance on the SRT test improved over the
three measurements. Moreover, on the first occasion SRT was correlated
to the degree of exposure. The authors concluded that the workers'
central nervous functioning had been adversely affected by solvent
exposure.
Mutti et al. (1982a) carried out a study of exposure to organic
solvents in an Italian shoe factory. The exposed group consisted of 95
workers (24 males, 71 females) with an age range of 16-62 years (mean,
30.9 ± 11.7 years), and the exposure duration ranged from 1 to 25
years (mean, 9.1 ± 8 years). The approximate mean air concentrations
in the breathing zone, over a 2-year period, for a number of solvents
were: MEK, 115 mg/m3 (39 ppm); n-hexane, 317 mg/m3 (90 ppm);
cyclohexane, 315 mg/m3 (92 ppm); and ethyl acetate, 205 mg/m3
(57 ppm). The exposed workers complained of sleepiness, dizziness,
weakness, paraesthesia and hypo-aesthesia. Other neurological
symptoms, such as headache, muscular cramps, neurasthenic syndrome and
sleep disturbances, were found more often in exposed workers, but the
differences in incidence between the exposed and reference group were
not statistically significant.
Among exposed workers the mean motor nerve conduction velocity
was significantly reduced in the median and peroneal nerves but not in
the ulnar nerve. The amplitude of the motor action potential (MAP) was
significantly reduced in all nerves and its duration was increased in
the ulnar nerve. There were no significant effects on the distal
latency. The number of abnormal action potentials observed in the
median and peroneal nerves of exposed workers was significantly
increased. There was a correlation between the reduction in motor
conduction velocity and exposure.
In a follow-up study, electrophysiological measurements including
somatosensory evoked potentials (SEPS) were recorded from a group of
15 female shoe factory workers aged 19-53 years (mean age, 26.6 ± 11.4
years) with a solvent exposure duration of 2-8 years (mean, 4.5 ± 2.3
years) (Mutti et al., 1982b). The mean air concentrations for various
solvents in the breathing zone of the workers were: MEK, 177 mg/m3
(60 ppm); n-hexane, 690 mg/m3 (196 ppm); cyclohexane, 585 mg/m3
(170 ppm); and ethyl acetate, 360 mg/m3 (100 ppm).
Electrophysiological measurements in peripheral nerves showed
significant reductions in maximal motor and distal sensory nerve
conduction velocities in the median and ulnar nerves and reduced
maximal motor nerve conduction velocity in the peroneal nerve. The
latency of the sensory peak action potential was significantly
increased in the median and ulnar nerves. The amplitude of all
peripheral nerve action potentials was slightly reduced but this was
not statistically significant. There were also changes in the SEPs
with significant increase in the latency of some early component
peaks. The amplitude of some of the early peaks was significantly
reduced. The neurotoxicity was attributed primarily to n-hexane.
8.5 Carcinogenicity
In a historical prospective mortality study of 446 male workers
in two MEK dewaxing plants, with an average follow up of 13.9 years,
the observed deaths (46) were below the expected (55.51). There was a
slight deficiency of deaths from neoplasms (13 observed; 14.26
expected) but there was a significant increase of deaths from tumours
of the buccal cavity and pharynx (2 observed; 0.13 expected). However,
there were significantly fewer deaths from lung cancer (1 observed;
6.02 expected). In view of the small numbers, it was concluded that
there was no clear evidence of cancer hazard in these workers
(Alderson & Rattan, 1980).
9. EFFECTS ON OTHER ORGANISMS IN THE LABORATORY AND FIELD
9.1 Microorganisms
The effects of MEK on microorganisms have been studied in several
species important to freshwater aquatic systems. As shown in Table 15,
growth inhibition generally occurs at levels ranging from 120 mg/litre
for the cyanobacterium (blue-green alga) Microcystis aeruginosa to
4300 mg/litre for the green alga Scenedesmus quadricauda.
A number of bacterial species have also been tested, the effect
levels ranging from 10 to 5050 mg/litre. Kulshrestha & Marth (1974a-f)
conducted a series of studies to determine if MEK and other volatile
compounds associated with the flavour of raw or mildly heated milk are
able to inhibit the growth of certain pathogenic bacteria and other
bacteria important in the manufacture of fermented dairy products.
Nutrient broth laced with the organisms and MEK at levels of 1, 10,
100 and 1000 mg/litre was plated after 5, 8, 11 and 14 h of incubation
in an air-tight vessel. Results are shown in Table 15. MEK was
considered bacteriostatic to Escherichia coli, Salmonella
typhimurium, Staphylococcus aureus, Leuconostoc citrovorum and
Streptococcus thermophilus at levels as low as 10-100 mg/litre.
However, Walton et al. (1989) reported that MEK at concentrations up
to 1 g/kg dry weight of soil had little effect on the respiration of
microorganisms in moist soil. Volskay & Grady (1988) found that MEK at
1 g/litre depressed respiration of sewage sludge organisms by only
11%. Ingram (1977) noticed changes in the fatty acid and phospholipid
composition of the cell membranes of E. coli when cultured with a
sublethal (0.218 mol/litre) concentration of MEK.
Tests in fungi demonstrated that MEK has a very slight
stimulating effect on the germination of uredospores in two species of
rust (French, 1961; French et al., 1977). Growth of a mixture of other
fungal species was inhibited by about 50% by 6.4 mg MEK/g seed when
the fungi were cultured on moist wheat seed (Nandi & Fries, 1976).
Growth of a mixture of other fungal species on moist wheat seed
at 30 °C was inhibited by 50% following exposure for 5 days to
approximately 6.4 mg MEK/g seed (Nandi & Fries, 1976). Germination of
the wheat seeds was also reduced but there was no statistical
evaluation of these data.
Table 15. Effects of methyl ethyl ketone on microorganisms
Organism Species, strain Concentration Effect Reference
(mg/litre)
Prokaryotes
Cyanobacterium
(Blue-green alga Microcystis aeruginosa 120 inhibition of cell multiplication Bringmann & Kuhn (1978)
Bacteria Pseudomonas putida 1150 inhibition of cell multiplication Bringmann & Kuhn (1977a)
Photobacterium phosphoreum 5050 50% reduction in light output Curtis et al. (1982)
Escherichia coli, ML30 10 slight but not consistently Kulshrestha & Marth (1974a)
significant inhibition of growth
Escherichia coli, ML30 100 significant inhibition after 5 h Kulshrestha & Marth (1974a)
incubation
Escherichia coli, ML30 1000 10% reduction in growth Kulshrestha & Marth (1974a)
Escherichia coli, B15 72.1 65% growth inhibition after 1 h; Egyud (1967)
30% after 2 h
Salmonella typhimurium 1 & 10 slight but not consistently Kulshrestha & Marth (1974b)
significant inhibition of growth
Salmonella typhimurium 100 some inhibition of growth Kulshrestha & Marth (1974b)
Salmonella typhimurium 1000 significant inhibition of growth Kulshrestha & Marth (1974b)
Staphylococcus aureus, 100 1 no significant inhibition of growth Kulshrestha & Marth (1974c)
Staphylococcus aureus, 100 10 some inhibition of growth Kulshrestha & Marth (1974c)
Staphylococcus aureus, 100 100 & 1000 significant inhibition of growth Kulshrestha & Marth (1974c)
Table 15 (contd)
Organism Species, strain Concentration Effect Reference
(mg/litre)
Streptococcus lactis 1 no significant inhibition of growth Kulshrestha & Marth (1974d)
Streptococcus lactis 10 significant inhibition at early Kulshrestha & Marth (1974d)
stages of incubation
Streptococcus lactis 100 & 1000 significant inhibition of growth Kulshrestha & Marth (1974d)
Leuconostoc citrovorum 1 no significant inhibition of growth Kulshrestha & Marth (1974e)
Leuconostoc citrovorum 10 some inhibition of growth Kulshrestha & Marth (1974e)
Leuconostoc citrovorum 100 & 1000 significant inhibition of growth Kulshrestha & Marth (1974e)
Streptococcus thermophilus, 1 no significant inhibition of growth Kulshrestha & Marth (1974f)
ST4
Streptococcus thermophilus, 10 growth inhibition at later stages of Kulshrestha & Marth (1974f)
ST4 incubation
Streptococcus thermophilus, 100 & 1000 significant inhibition of growth Kulshrestha & Marth (1974f)
ST4
Eukaryotes
Fungi Puccinia graminis, var. tritica, not given slight stimulation to germination French (1961)
spores (wheat stem rust)
Uromyces phaseoli, (Reben), 1000 very slight stimulation to French et al. (1977)
Wint., spores (bean rust) germination
Puccinia helianthi, Schw. chr., 50 very slight stimulation to French (1984)
spores (sunflower rust) germination
Table 15 (contd)
Organism Species, strain Concentration Effect Reference
(mg/litre)
Uromyces vignae, Barcl., 500 no effect on germination French (1984)
spores (cowpea rust)
various fungi found on wheat 6.4a slight inhibition of fungal growth Nandi & Fries (1976)
seeds: Septoria nerdorum, Fusarium
nivale, Aspergillus glaucus,
Aspergillus candidus, Penicillium
sp., Alternaria, sp.
Green algae Chlorella sp. 806 no effect on chlorophyll content Dojlido (1979)
after 7 days exposure
Scenedesmus quadricauda 4300 slightly inhibited cell Bringmann & Kuhn (1978)
multiplication
Protozoa Entosiphon sulcatum 190 slightly inhibited cell Bringmann (1978)
multiplication
a mg/g seed.
9.2 Aquatic organisms
MEK has been tested on numerous species of freshwater and marine
vertebrates and invertebrates in short-term tests. The results
indicate that MEK is generally of low toxicity to aquatic animals,
with median lethal (LD50) levels ranging from 1382 to 8890 mg/litre
(Table 16). Almost all of the acute tests were conducted under static
conditions, in open vessels, with nominal measurements of MEK
concentration, all of which may underestimate the true toxicity level
of MEK. No chronic studies at low concentrations have been conducted.
Curtis et al. (1982) related the effect of MEK on the
bioluminescence of a bacterium to the 96-h LC50 in fathead minnows
in an attempt to find an inexpensive yet accurate substitute for
lethality tests. The relationship for ketones (r2 = 0.81, n = 7) is
described by the equation:
log LC50 = 0.74 (log EC50 + 0.79)
where the EC50 (5 min) is the concentration causing a 50% reduction
in light output.
This relationship predicts a 96-h LC50 for fathead minnows of
3388 mg/litre, which agrees well with the measured value (Veith et
al., 1983) of 3200 mg/litre. Similarly, LeBlanc (1984) found a highly
significant correlation (P < 0.01) between the LC50 values
reported for warm-water and cold-water fish, saltwater and freshwater
fish, marine and freshwater invertebrates, and marine and freshwater
algae. There was a remarkable similarity among acute toxicity values
across three trophic levels. Data in Tables 15 and 16 lend further
support to this conclusion.
9.3 Terrestrial organisms
9.3.1 Animals
The effect of MEK on the alarm behaviour of social insects has
been studied. It was considered inactive in producing alarm behaviour
in the ant Iridomyrmex preinosus (Blum et al., 1966), the harvester
ant Pogonomyrmex badius (Blum et al., 1971) and the honeybee Apis
Mellifera (Boch & Shearer, 1971). Alarm behaviour was measured by
the number of insects that were attracted to the chemical and was
indexed relative to the activity of natural pheromones, including
2-heptanone.
Table 16. Effects of methyl ethyl ketone on aquatic organisms
Species Concentration Effects and comments pH Temperature Hardness Reference
(mg/litre) (°C)
Crustacea
Artemia salina 1950 24-h LC50, bottles not not given not given not given Price et al. (1974)
(brine shrimp) sealed and may have lost
MEK during experiment
Daphnia magna (water flea) < 180 no discernible effect 7.21 21.0-23.0 38 mg/litre Union Carbide Corp.
CaCO3 (1980a)
Daphnia magna (water flea) < 520 24-h LC50 8 ± 0.2 not given 173 ± 13 mg/l Le Blanc (1980)
< 520 48-h LC50
< 70 no discernible effect
Daphnia magna (water flea) 1382 48-h LC50 7.21 21.0-23.0 38 mg/litre Union Carbide Corp.
(918-3349)a CaCO3 (1980a)
Daphnia magna (water flea) 2500 24-h LC0 7.6-7.7 20-22 16 ° (German) Bringmann & Kuhn (1977b)
Daphnia magna (water flea) 8890 24-h LC50 7.6-7.7 20-22 16 ° (German) Bringmann & Kuhn (1977b)
Daphnia magna (water flea) 10 000 24-h LC100 7.6-7.7 20-22 16 ° (German) Bringmann & Kuhn (1977b)
Fish
Leucissus idus melanotus 4400b LC0, period not mentioned not given not given not given Juhnke & Luedemann
(golden orfe) 4800b (1978)
Leucissus idus melanotus 4600b LC50 not given not given not given Juhnke & Luedemann
(golden orfe) 4880b (1978)
Table 16 (contd)
Species Concentration Effects and comments pH Temperature Hardness Reference
(mg/litre) (°C)
Leucissus idus melanotus 4800b LC100 not given not given not given Juhnke & Luedemann
(golden orfe) 5040b (1978)
Lebistes reticulatus 2000 disturbed behaviour not given 20 ± 1 27.5 mg/litre Dojlido (1979)
(guppy) CaCl2
Lebistes reticulatus 5700 24-h LC50, open not given 20 ± 1 27.5 mg/litre Dojlido (1979)
(guppy) containers may have lost CaCl2
MEK during test
Pimephales promelas 3200 96-h LC50 7.5 25 ± 1 42.2 mg/litre Veith et al. (1983)
(fathead minnow) CaCO3
Lepomis macrochirus < 1000 no discernible effect 7.93 21 240 mg/litre Union Carbide Corp.
(bluegill sunfish) CaCO3 (1980b)
Lepomis macrochirus 4467 96-h LC50 7.93 21 240 mg/litre Union Carbide Corp.
(bluegill sunfish) CaCO3 (1980b)
Gambusia affinis 5600 96-h LC50 7.8-8.3 room not given Wallen et al. (1957)
(mosquito fish) temperaturec
Cyprinodon variegatus 400 no discernible effect not given 25-31 not given Heitmuller et al.
(sheepshead minnow) (1981)
Carassius auratus > 5000 24-h LC50 7.0 20 ± 1 100 mg/litre Bridie et al. (1979b)
(goldfish)
a 95% confidence limits
b Values are from different laboratories
c No specific value given
MEK was found to be moderately effective as a fumigant against
the Caribbean fruit fly, Anastrepha suspensa (Davis et al., 1977).
Treatments of 790 mg/m3 (286 ppm) for 2 h or 316 mg/m3 (107 ppm)
for 7 h destroyed 100% of the larvae in naturally infected guavas,
whereas exposure to 221 mg/m3 (75 ppm) for 3 h destroyed 92% of the
larvae. Kwan & Gatehouse (1978) applied between 0.31 and 0.37 mg MEK
topically to the dorsal thorax of tsetse flies (Glossina morsitans
morsitans) weighing 17-19 mg. One day after treatment, the MEK had
a significant effect on the activity of males but not females. The
mortality in MEK-treated insects was marginally but consistently
higher than in the untreated controls. No apparent inhibitory or other
effect of MEK was noted with respect to feeding or mating. Vale et al.
(1988) found MEK to be a very effective attractant for tsetse flies
and used it in a successful control effort in which the flies were
attracted to insecticide-coated netting. Uspenskii & Repkina (1974)
reported that an unspecified dose of MEK caused an increase in the
physiological age of the tick Ixodes perculcatus, an effect that
increased the insect's sensitivity to DDT.
9.3.2 Plants
MEK has an effect on the germination of seeds of several plant
species. Nandi & Fries (1976) observed that the germination of wheat
seeds was inhibited when the seeds were treated with 6.4 mg MEK/g
seed. At this level, 10% of the experimental seeds germinated versus
60% of the control seeds. Germination of lettuce was inhibited 50% by
MEK at 12.5 (± 4) mmol/litre (equivalent to 900 ± 288 mg/litre)
dissolved in agar (Reynolds, 1977). Schulz et al. (1981) reported,
however, that a mixture of acetone and MEK had no inhibitory effect on
the growth of rye grass at concentrations up to 1 g/litre.
10. ENHANCEMENT OF THE TOXICITY OF OTHER SOLVENTS BY MEK
The principal toxic effects noted with MEK exposure stem from its
ability to potentiate the known toxicities of other solvents (Table
17). Two such interactions are described in detail below.
10.1 Hexacarbon neuropathy
10.1.1 Introduction
MEK interacts with hexacarbon compounds and potentiates their
neurotoxicity (WHO, 1991). Potentially MEK co-exposure could affect
the metabolism of the hexacarbon compounds or the toxic process by
which the hexacarbons induce the neuropathy. The critical metabolic
pathway for hexacarbon induced neuropathy is outlined in Fig. 2, and
the scheme by which the peripheral nerve axonal degradation is thought
to occur is given in Fig. 3. The metabolic pathway involves hepatic
microsomal oxidation to 2,5-hexanedione, the proximate neurotoxicant,
which is thought to induce cross-linking of neurofilaments, blockage
of transport at the node of Ranvier, and swelling from accumulated
neurofilaments proximal to the nodes and axonal degeneration distal to
the nodes.
10.1.2 Animal studies
The phenomenon of potentiation of hexacarbon neurotoxicity by MEK
has been firmly established by in vivo studies mainly on rats
(Table 17) and also has been demonstrated in tissue culture (Veronesi
et al., 1984). In every study in which the dose of n-hexane, methyl
butyl ketone (MBK), or 2,5-hexanedione (2,5-HD) was large enough and
sustained for a sufficient period, clinical signs of neural
degeneration were produced. These signs were made more severe by
co-exposure to MEK. In addition, the period prior to the onset of
symptoms was frequently shortened by co-exposure to MEK. Minimum
sustained continuous exposure concentrations that induced neuropathy
in these experiments were 295/1408 and 590/1056 mg/m3 (100/400 and
200/300 ppm) MEK/ n-hexane mixtures. An intermittent exposure (8
h/day) of rats to 2950/31 680 mg/m3 (1000/9000 ppm) MEK/ n-hexane
mixture produced severe neuropathy, whereas similar exposure to a
lower concentration, i.e. 590/1760 mg/m3 (200/500 ppm)
MEK/ n-hexane mixture yielded no evidence of hexacarbon
neurotoxicity. Intermittent exposure (8 h/day, 5 days/week) to
5900/820 mg/m3 (2000/200 ppm) MEK/MBK mixture produced some neural
degeneration and mild clinical signs. Oral dosing of rats once a day,
5 days/week, with a mixture of MEK/2,5-HD (0.159/0.253 g/kg) produced
marked clinical signs (Ralston et al., 1985). Even with doses of MBK
too low to produce significant neuropathy, studies generally indicated
that co-exposure with MEK induced changes compatible with enhanced
toxicity, such as reduced velocity of nerve conduction, elevated
hepatic microsomal enzyme activity, or reduced clearance of 2,5-HD
from the blood or the body. The only study reporting no evidence of
potentiation (Spencer & Schaumburg, 1976) compared MBK at 0.150 g/kg
with one tenth this concentration of MBK, 0.015 g/kg, in combination
with MEK. Since there were no control data on the effects of 0.015
g/kg of MBK alone and the dose of MEK was very low, it is difficult to
interpret the meaning of this study. Concentrations of MEK in
inhalation studies did not exceed 3319 mg/m3 (1125 ppm), and
continuous exposure to this concentration was demonstrated by Saida et
al. (1976) not to produce neuropathological effects. Thus it is
considered unlikely that MEK itself produces neuropathy.
The property of potentiation of hexacarbon neurotoxicity is not
unique to MEK, but is shared at least by methyl n-propyl ketone,
methyl n-amyl ketone and methyl n-hexyl ketone, none of which
appear intrinsically neurotoxic (Misumi & Nagano, 1985).
The mechanism by which MEK potentiates hexacarbon neurotoxicity
is not well understood, although potential metabolic interactions have
been examined. In rats, simultaneous inhalation of n-hexane and MEK
resulted in lower levels of 2,5-HD in vivo in urine (Iwata et al.,
1984; Shibata et al., 1990a) and initially lower but later somewhat
elevated levels of MBK and 2,5-HD in vivo in serum (Shibata et al.,
1990b). Both Iwata et al. (1984) and Shibata et al. (1990b) concluded
that potentiation of n-hexane neurotoxicity by MEK could not be
explained solely by increased 2,5-HD formation. Pretreatment of rats
with MEK (1.87 ml/kg, 4 daily doses) prior to inhalation of n-hexane
resulted in higher levels of 2,5-HD in several tissues, including
blood (Robertson et al. (1989). Abdel Rahman et al. (1976) reported
that an 8-h simultaneous exposure of rats to MEK and MBK did not
result in measurable levels of MBK or of 2,5-HD in blood, whereas a
6-day continuous co-exposure resulted in substantially raised levels
of MBK and 2,5-HD. Continuous co-exposure for 23 days, however,
resulted in further elevation of MBK in blood, but no measurable level
of 2,5-HD. Simultaneous administration of 2,5-HD and MEK, either as a
single dose or as six repeated daily doses in rats, resulted in a
greater total area under the curve for 2,5-HD in blood compared with
administration of 2,5-HD alone (Ralston et al., 1985).
In guinea-pigs, increased levels of 2-hexanol and 2,5-HD were
found following intraperitoneal administration of a MEK/MBK mixture
compared with intraperitoneal administration of MBK alone (Couri et
al., 1978).
MEK administration has also been shown to induce a number of in
vivo and in vitro parameters of hepatic oxidative metabolism
(Couri et al., 1977; Wagner et al., 1983; Misume & Nagano, 1985;
Raunio et al., 1990).
Table 17. Interaction of MEK with other solvents and their metabolitesa
Route of administration, Dose and/or Exposure Effects/results Reference
species (strain), concentration
number and sex
n-Hexane
Inhalation, rat, 820 mg/m3 (200 ppm) MBK; 8 h/day, muscular weakness lasted longer after exposure to Duckett et al.
9 sex unspecified 5900/820 mg/m3 5 days/week, MEK/MBK than after MBK alone (1974)
MBK, 8 sex unspecified (2000/200 ppm) MEK/MBK 6 weeks
MEK/MBK
Inhalation, rat 1760 mg/m3 (500 ppm) 8 h/day, no significant enhancement of neuropathological Iwata et al.
(Wistar), 6 males hexane; 1475/1760 mg/m3 7 days/week, damage evident in peripheral nerve (1984)
per groupb (500/500 ppm) MEK/hexane 33 weeks electrophysiological function; 2,5-hexanedione
and other hexane metabolites in urine reduced
with co-exposure
Inhalation, rat 923 mg/m3 (225 ppm) MBK; 24 h/day, MEK enhanced MBK-induced peripheral neurotoxicity Saida et al.
(Sprague-Dawley), 3319/923 mg/m3 (1125/225 16-66 days (1976)
12/group, sex ppm) MEK/MBK
unspecified
Inhalation, rat 923 mg/m3 (225 ppm) MBK; 24 h/day, hexobarbital sleeping time significantly reduced in Couri et al.
(Wistar), 5 males 2213/923 mg/m3 (750/225 7 or 28 days group exposed continuously for 7 days to MEK/MBK (1977)
per group ppm) MEK/MBK (and in 2213 mg MEK/m3 (750 ppm) controls), but not
in MBK group; interpreted as evidence for elevated
microsomal enzyme activity in MEK/MBK and MEK
groups
Table 17 (contd)
Route
Route of administration, Dose and/or Exposure Effects/results Reference
species (strain), concentration
number and sex
Inhalation, rat 1760, 2464 mg/m3 (500, 700 approx. experiments yielded similar results: potentiation of Altenkirch et
(Wistar), 5 males ppm) hexane; 295/1408, 24 h/day, n-hexane neurotoxicity in MEK/hexane groups as al. (1982a)
per group 590/1056, 590/1760 mg/m3 7 days/week, shown by shortened period before onset of clinical
(100/400, 200/300, 200/500 9 weeks signs (weakness and paresis); hypersalination
ppm) MEK/hexane in MEK/hexane groups only; neural degeneration
in all solvent-treated groups (no mention of
differences among these groups)
Inhalation, rat 1760, 2464 mg/m3 (500, 700 8 h/day, every co-exposure to MEK/hexane resulted in earlier and Schnoy et al.
(Wistar), 5 groups ppm) hexane; 295/1408, day for 1-89 more pronounced degeneration of pulmonary (1982);
of 2-5 malesc 590/1056, 590/1760 mg/m3 days nerves than exposure to hexane alone; there were no Schmidt et al.
(100/400, 200/300, 200/500 consistent differences in degeneration of alveolar (1984)
ppm) MEK/hexane epithelium between the hexane and MEK/hexane
groups
Inhalation, rat 1640, 923 mg/m3 (400, 225 24 h/day, 2,5-HD detected in blood after 6 days but not after Abdel-Rahman
(Wistar), unspecified ppm) MBK; 2213/923 mg/m3 6 or 23 days 23 days, and there were elevated MBK blood levels et al. (1976)
number/group, male (750/225 ppm) MEK/MBK in group exposed to MEK/MBK; severe neuropathy in
group exposed to MEK/MBK for 23 days
Inhalation, rat 2464 mg/m3 (700 ppm) 8 h/day, no evidence of potentiation of hexane neurotoxicity: Altenkirch et
(Wistar), 5 males hexane; 590/1760 mg/m3 7 days/week, no abnormal clinical signs or elevated al. (1982a)
per group (200/500 ppm) MEK/hexane 40 weeks neuropathology in solvent-treated groups
Inhalation, rat 3520 mg/m3 (1000 ppm) 8 hd 2,5-hexanedione in urine markedly less after co- Iwata et al.
(Wistar), 5 males hexane; 2950/3520 mg/m3 exposure with MEK than after exposure to hexane (1983)
per group (1000/1000 ppm) MEK/hexane alone
Table 17 (contd)
Route
Route of administration, Dose and/or Exposure Effects/results Reference
species (strain), concentration
number and sex
Inhalation, rat Experiment 1: 35 200 mg/m3 8 h/day, experiments yielded similar results; acceleration Altenkirch et
(Wistar), 5 males (10 000 ppm) hexane; 3245/ 7 days/week, of n-hexane neurotoxicity in MEK/hexane group as al. (1978,
per group 31 330 mg/m3 (1100/8900 15-19 weeks shown by more severe clinical signs (gait 1979)
ppm) MEK/hexane disturbances, weakness and paresis), greater
degeneration of peripheral nerves and shortened
5 males/group Experiment 2: 35 200 mg/m3 period before onset of signs and neural degeneration;
(10 000 ppm) hexane; 2950/ hyper-salivation in MEK/hexane groups
31 680 mg/m3 (1000/9000
ppm) MEK/hexane
12 males/group Experiment 3: 35 200 mg/m3
(10 000 ppm) hexane; 2950/
31 680 mg/m3 (1000/9000
ppm) MEK/hexane
Inhalation, rat 35 200 mg/m3 (10 000 ppm) 4 h or 8 h, MEK did not enhance degeneration of intrapulmonary Schnoy et al.
(Wistar), 7 groups hexane; 2950/31 680 mg/m3 8 h/day for nerves; enhanced degeneration of alveolar epithelium (1982);
of 2-3 males (1000/9000 ppm) 2-14 days in the MEK/hexane group after 14 days exposure in Schmidt et al.
MEK/hexanee comparison with the hexane group (1984)
Inhalation, rat 352 mg/m3 (100 ppm) hexane; 12 h/day, marked impairment of nerve conduction velocities in Takeuchi et
(Wistar), 8 males 590/352 mg/m3 (200/100 7 days/week, MEK/hexane group; no evidence of significant al. (1983)
per group ppm) MEK/hexane 24 weeks potentiation of morphological effects of n-hexane
Inhalation, mouse 615 mg/m3 (150 ppm) MBK; 24 h/day, hexobarbital sleeping time significantly lower in Couri et al.
(Swiss), 5/group 2950/615 mg/m3 (1000/150 7 days MEK/MBK group than in MBK group (or in MEK 2950 (1978)
sex unspecified ppm) MEK/MBK mg/m3 (1000 ppm) controls); interpreted as evidence
for elevated microsomal enzyme activity in
MEK/MBK group
Table 17 (contd)
Route
Route of administration, Dose and/or Exposure Effects/results Reference
species (strain), concentration
number and sex
Subcutaneous injection, 288 mg/kg b.w. MBK; 1/day, 5 days significantly enhanced neurotoxicity (slower motor Misumi &
rat (Donryu), 288/288 mg/kg b.w. per week, 20 fibre conduction velocity and increased motor distal Nagano (1985)
8 males/group MEK/MBK weeks latency of tail nerve) and more pronounced weakness
in rats receiving MEK/MBK than MBK alone
Subcutaneous injection, 150 mg/kg b.w. MBK; 5 days/week peripheral neuropathological changes and paresis Spencer &
cat, 9 (sex 135/15 mg/kg b.w. for up to 8.5 with MBK; possible nerve degeneration in animals Schaumburg
unspecified) MBK, MEK/MBK months treated with MEK/MBK (1976)
4 (sex unspecified)
MEK/MBK
Other ketones
Subcutaneous injection, 150 mg/kg b.w. MIBK; 2/day, 5 days no evidence of enhanced MIBK-induced Spencer &
cat, 4, sex 135/15 mg/kg b.w. per week, up neuropathology Schaumburg
unspecified, MIBK, MEK/MIBK to 8.5 months (1976)
6, sex unspecified,
MEK/MIBK
Inhalation, rat 3220 mg/m3 (700 ppm) EBK; 16-20 h/day, exposure to MEK/EBK at 2065 and 4130 mg MEK/m3 O'Donoghue et
(Charles River), 15 207/3220, 2065/3200, 4 days produced a 2.5-fold increase in serum 2,5-Hpdn over al. (1984)
males/group for 4130/3200 mg/m3 (70/700, that produced by EBK alone; no 2,5-HD detected in
EBK and controls; 700/700, 1400/700 ppm) serum
5 males/group MEK/EBK
for MEK/EBK
Oral, rat (Charles 0.25 to 4 g/kg b.w. EBK; 1/day, 5 days 2 and 4 g EBK/kg b.w. fatal with or without MEK; O'Donoghue et
River), 4 males in 1.5, 0.75/0.25-4 g/kg b.w. per week, 14 greater neurological damage and dysfunction, and al. (1984)
control group MEK/EBK weeks around 1.5-fold increase in 2,5-Hpdn and 2,5-HD
with 1.5/1.0 g/kg b.w. MEK/EBK than with 1.0 g
EBK/kg b.w.; no neurotoxicity evident in doses
of EBK below 1.0 g/kg b.w.
Table 17 (contd)
Route
Route of administration, Dose and/or Exposure Effects/results Reference
species (strain), concentration
number and sex
Oral, rat (Fischer- 253 mg/kg b.w. 2,5-HD; 1/day, 5 days marked neurological dysfunction consistently Ralston et al.
344), 5 males/group 159/253 mg/kg b.w. per week, ca. appeared weeks earlier in rats receiving (1985)
MEK/2,5-HDf 13 weeks MEK/2,5-HD
Oral, rat (Fischer- 253 mg/kg b.w. 2,5-HD; 1/day, 1 MEK did not alter gastric absorption of 2,5-HD; Ralston et al.
344), 5 males/group 159/253 mg/kg b.w. or 7 days clearance of 2,5-HD from blood slower from rats (1985)
MEK/2,5-HD receiving MEK/2,5-HD
Oral, rat (Fischer- 253 mg/kg b.w. 2,5-HD; 1/day, 5 days most of radiolabelled 2,5-HD bound to protein; Ralston et al.
344), 5 males/group 159/253 mg/kg b.w. per week, 1, greater binding to protein with MEK/2,5-HD (1985)
MEK/2,5-HD 2 or 3 weeks after 1 week, but reverse true after 2 and 3 weeks
Halogenated alkanes
Oral, intraperitoneal, 1.505 g/kg b.w. MEK; 1 oral dose potentiation of CCl4 hepatotoxicity by MEK as Traiger &
rat (Sprague- 0.16 g/kg b.w. CCl4 MEK followed shown by greater fatty vacuolation and necrosis Bruckner
Dawley), 3-5 males/ 16 h later of liver, increased hepatic triglyceride and (1976)
experimental group, with 1 ip GPT, and decreased hepatic glucose-6-phosphatase
5-20 males/control dose CCl4
group
Oral, intraperitoneal, 1.691 g/kg b.w. MEK; 1 oral dose potentiation of CCl4 hepatotoxicity by MEK as Dietz & Traiger
rat (Sprague- 0.16 g/kg b.w. CCl4 MEK followed shown by increased hepatic triglyceride and (1979)
Dawley), males at 16 h later GPT
least 5/group with 1 ip
dose CCl4
Oral, intraperitoneal, 1.082 g/kg b.w. MEK; 1 oral dose potentiation of CHCl3 hepatotoxicity by both Hewitt et al.
rat (Sprague- 0.797, 1.196 g/kg b.w. MEK followed doses of MEK as shown by increased GPT and OCT (1983)
Dawley), 5 or 6 CHCl3 18 h later with
males/groupg 1 ip dose CHCl3
Table 17 (contd)
Route of administration, Dose and/or Exposure Effects/results Reference
species (strain), concentration
number and sex
Oral, intraperitoneal, 0.072 to 1.082 g/kg b.w. 1 oral dose potentiation of CHCl3 renal and hepatic toxicity Brown & Hewitt
neal, rat (Fischer- MEK; 0.797 g/kg b.w. MEK followed by MEK at all dosages as shown by histological and (1984)
344), 6 males per CHCl3 18 h later with several biochemical criteria; potentiation greatest
experimental 1 ip dose at 0.361 to 0.721 g/kg b.w. MEK, and slightly
group, 32 males CHCl3 reduced at 1.082 g/kg b.w.h
in control group
Oral, rat (Sprague- 1.082 g/kg b.w. MEK; 1 dose MEK MEK followed by CHCl3 did not induce cholestasis, Hewitt et al.
Dawley), 6 males 0.797 to 1.594 g/kg b.w. followed 10 but MEK given 10 to 24 h prior to CHCl3 potentiated (1986)
per group CHCl3 to 96 h later its ability to increase plasma bilirubin
with 1 dose
CHCl3
Oral, rat (Sprague- 1.082 g/kg b.w. MEK; 1 dose MEK MEK followed by CHCl3 10 to 48 h later potentiated Hewitt et al.
Dawley), 6 males 0.797 g/kg b.w. CHCl3 followed 10 hepatotoxicity as indicated by elevated ALT and (1987)
to 96 h later OCT levels
by 1 dose
CHCl3
a Values from the literature have been recalculated as ppm or g/kg body weight.
b Iwata et al. (1984) states that 24 rats were divided into four groups, but does not specify that groups were of equal numbers.
c Animals were apparently co-exposed with those described in Altenkirch et al. (1982a).
d Description of exposure not entirely clear but a subsequent paper (Iwata et al., 1984) refers to this as a single exposure.
e Animals co-exposed with those described in Altenkirch et al. (1978).
f MEK and 2,5-HD doses were 0.317 and 0.506 g/kg, respectively, for first 8 days of experiment.
g 5 males at the lower dose and 6 at the higher; 11 males in the control group
h Measurements were made 24 h after the oral dose of CHC13 or CCl4.
Abbreviations: GPT = plasma glutamic-pyruvic transaminase; OCT = plasma ornithine carbamyl transferase; ALT = plasma alanine aminotransferase;
b.w. = body weight; ip = intraperitoneal; 2,5-HD = 2,5-hexanedione; 2,5-Hpdn = 2,5-heptanedione; CCl4 = carbon tetrachloride; CHCl3 = chloroform
6.4 Elimination and excretion
MEK and its metabolites are excreted by the lungs and kidneys.
Liira et al. (1988a) reported that only 3% of the calculated absorbed
dose during a 4-h exposure to 590 mg MEK/m3 (200 ppm) was secreted
unchanged in the exhaled air of volunteers after the exposure. The
fractional elimination of unchanged substance however depends on the
efficiency of metabolic clearance. Since metabolic saturation for MEK
in humans begins at relatively low levels (about 100 ppm) of exposure
(Liira et al., 1990a), proportionally greater amounts of MEK would be
expected to be excreted via the lungs (and kidneys) at high exposure
levels.
Relatively little of the absorbed MEK is excreted unchanged via
the kidneys; a study of occupationally exposed workers revealed that
it is less than 0.1% of the alveolar uptake (Miyasaka et al., 1982).
In a similar study of workers occupationally exposed to a mixture of
solvents, the excretion of MEK and a major recognizable metabolite,
3-hydroxy-2-butanone, was 0.1% of alveolar uptake (Perbellini et al.,
1984). The concentrations of both MEK and 3-hydroxy-2-butanone in
urine were significantly correlated with the environmental level of
MEK. Other metabolites of MEK, 2-butanol or 2,3-butanediol, which
DiVincenzo et al. (1976) identified in the serum of guinea-pigs, were
not detected in the urine of the exposed workers. Liira et al.
(1988a), however, reported that human excretion of 2,3-butanediol was
individually variable but averaged 2% of the absorbed MEK. The urinary
excretion of 2-butanol, a minor metabolite of MEK, was examined by
Kamil et al. (1953), who found that clearance of 2-butanol
administered by gavage in rabbits was about 14% of the administered
dose and in the form of a glucuronide.
Since MEK and 2,3-butanediol disappear from blood and urine and
there is no evidence for accumulation elsewhere in the body, the above
data suggest that the bulk of MEK absorbed by mammals enters the
general metabolism and is eliminated from the body as simple compounds
like carbon dioxide and water whose source is not readily
identifiable. The specific pathways by which MEK is metabolized have
not been identified. There also is no information on loss of MEK in
faeces.
6.5 Turnover
Both animal and human data indicate a rapid turnover of MEK. In
guinea-pigs receiving an intraperitoneal dose of 450 mg MEK per kg,
the half-life of MEK in blood serum was 4´ h and the clearance time
for MEK in serum was 12 h. For the metabolites 2-butanol,
3-hydroxy-2-butanone and 2,3-butanediol, the clearance time in serum
was 11 h (DiVincenzo et al., 1976). In rats given a 2.2-ml/kg oral
dose of 2-butanol, the butanol was largely cleared from the blood in
15 h and the 2-MEK derived from the butanol was cleared in 24 h
(Traiger & Bruckner, 1976). In a study by Dietz & Traiger (1979) on
rats given an oral dose of 2-butanone of 2.1 mg/kg, there was a
half-life of 3.6 h for MEK in blood if the rate of loss was assumed to
be constant between the two times of measurement (4 h and 18 h) after
dosing. Data from a study of Dietz et al. (1981) on rats receiving
oral doses of 2-butanol or MEK also indicate a half-life of about 4 h
for MEK. These authors reported that the clearance rate for
3-hydroxy-2-butanone and 2,3-butanediol was independent of dose for
the two doses used (0.4 and 0.8 g/kg) and that the half-lives for
these metabolites of MEK were 47 min and 3.45 h, respectively. Liira
et al. (1988a) reported a steady increase in blood concentrations
during 4-h exposures of human volunteer subjects to 590 mg/m3 (200
ppm) and observed a rapid elimination of MEK, with half-lives of 30
min during the first post-exposure hour and 81 min thereafter. An
inhalation study with two volunteer subjects exposed to MEK for 4 h at
concentrations of 74, 590 and 1180 mg/m3 (25, 200 and 400 ppm)
indicated that the kinetics of MEK were dose dependent at higher
exposure concentrations, i.e. much higher levels of MEK in blood were
reached relative to inhaled concentrations, and the post-exposure
elimination of MEK in blood was slower (zero-order kinetics).
Simulated exposure to MEK for 8 h suggests that saturation kinetics
are reached at about 295 mg/m3 (100 ppm) at rest and 148 mg/m3 (50
ppm) during light exercise (Liira et al., 1990a).
6.6 Metabolic interactions
Ingestion of ethanol (0.8 g/kg) combined with an inhalation
exposure to MEK (590 mg/m3, 200 ppm) inhibited the oxidative
metabolism of MEK and led to a marked increase in the blood
concentration of MEK (Liira et al., 1990b). There was a concurrent,
even more pronounced elevation of the blood 2-butanol concentration;
the most likely explanation is competitive inhibition by ethanol of
the oxidation of 2-butanol back to MEK. Ethanol also appeared to
interact with the further biotransformation of 2,3-butanediol as the
urinary excretion of the metabolite was increased. Co-exposure with
xylene, however, had no effect on the human metabolism or rate of
elimination of MEK (Liira et al., 1988b).
6.7 Mechanisms of action
There is very limited information on the mechanisms of toxic
action of MEK. Relatively high inhaled concentrations 1475-29 500
mg/m3 (500-10 000 ppm) caused pulmonary vasoconstriction and
hypertension in cats and dogs (Zakhari et al., 1977). From the
toxicological point of view, interactions leading to the potentiation
of effects, particularly neurotoxicity, by other intrinsically toxic
substances constitute the main hazard of MEK. The mechanisms
underlying these interactions are incompletely known (see section 10).
7. EFFECTS ON LABORATORY MAMMALS AND IN VITRO TEST SYSTEMS
7.1 Acute exposure
7.1.1 Lethal doses
Data on the acute toxicity of MEK to experimental animals are
summarized in Table 11. Oral toxicity is low with LD50 values
ranging from 2 to 6 g/kg for adult mice and rats. Intraperitoneal
LD50 values are lower (approximately 1.5 g/kg for 24 h and 0.6 g/kg
for 14 days). A larger dermal LD50 value for rabbits, i.e. > 8 g/kg
(contact time: 24 h; observation time: 14 days), may reflect slower
and less complete absorption via the skin, although it may also
reflect species differences in sensitivity to MEK. The lethal dose of
MEK given to rats in the only study using dosing by pulmonary
aspiration (Panson & Winek, 1980) was 0.8 g/kg. This is well below the
intraperitoneal 24-h LD50 for adult rats but similar to the 14-day
value (Lundberg et al., 1986). It cannot be excluded that the high and
rapid lethality of this aspirated dose to adult rats (5/6 deaths in
< 24 h, with 4/6 reported as dying "instantly") may reflect serious
damage to the lungs.
Studies examining the effects of acute inhalation of MEK are not
entirely comparable since they used not only different species but
also different concentrations of MEK, exposure times, and periods over
which survival was measured. For mice the LC50 (45-min exposure) was
about 200 000 mg/m3. The lowest concentration lethal to all rats
exposed by inhalation for 8 h was 47 200 mg/m3 and the lowest
concentration producing lethality in a 4-h exposure was 5900 mg/m3
(2000 ppm). Guinea-pigs survived exposure to 29 500 mg/m3 for 4 to
4.7 h and showed no abnormal signs at 9735 mg/m3. A concentration of
97 350 mg/m3 was lethal to all exposed guinea-pigs in 3.3 to 4.2 h,
whereas a slightly lower concentration, 73 750 mg/m3, although
ultimately lethal to all animals, permitted some guinea-pigs to
survive a 5.4-h exposure (Patty et al., 1935; Specht et al., 1940).
7.1.2 Non-lethal doses
Non-lethal acute doses of MEK produced a number of measurable
changes in experimental animals (Table 11). An oral dose of 1.5 g/kg
to rats resulted in a 63% increase in liver triglycerides after 16 to
23 h, but did not alter liver histology or increase either of two
enzymes, serum glutamic-pyruvic transaminase (alanine transferase
(ALT)) and hepatic glucose-6-phosphatase (Traiger & Bruckner, 1976).
These results suggest that this dose caused metabolic disturbances to
the liver of rats. Much smaller acute intraperitoneal doses (0.049 to
0.194 g/kg) to rats appeared not to damage the liver. A single oral
dose (15 mmol/kg) of MEK did not affect the hepatobiliary function of
rats over an observation period of 10 to 96 h (Hewitt et al., 1986).
A graded series of single doses of MEK to guinea-pigs revealed high
sensitivity to small changes in dosage. The low dose (0.75 g/kg)
appeared to produce no liver damage, whereas 1.5 g/kg produced slight
liver damage and 2.0 g/kg produced major liver damage. These results
also suggest that guinea-pigs and rats may be equally sensitive to MEK
in terms of liver damage even though guinea-pigs survive acute
exposure to higher concentrations of MEK vapour than do rats
(DiVincenzo & Krasavage, 1974).
Consistent increases in the frequency of food-reinforced lever
pressing by rats were detected at MEK exposures as low as 74 mg/m3
(25 ppm) for 2 h (Garcia et al., 1978). Vestibulo-ocular effects were
detected during continuous intravenous administration of MEK for 1 h
via the caudal vein at a dosage as low as 0.005 g/kg per min (Tham et
al., 1984). This dosage is roughly equivalent to inhalation of 2700
mg/m3 (900 ppm). Glowa & Dews (1987) reported that exposure of mice
to 885 mg/m3 (300 ppm) for 30 min did not significantly alter
schedule-controlled responses, whereas concentration-related
suppression of response occurred at consecutive increasing
concentrations (for 30 min) ranging from 2950 to 29 500 mg/m3 (1000
to 10 000 ppm) (see section 7.3.1).
It can be concluded from observations of their behaviour that
respiratory irritation occurred in guinea-pigs exposed to 29 500
mg/m3 or more within 2 min (Patty et al., 1935; Specht et al.,
1940). In survivors, post-exposure recovery from this effect was
rapid. Rats exposed for 8 h/day to 29 500 mg/m3 showed severe
irritation of the upper respiratory tract after a "few days"
(Altenkirch et al., 1979).
7.1.3 Skin and eye irritation
In skin irritation studies, a small dose (8 mg) applied to
clipped skin and covered by an impervious plastic film for 24 h (which
was followed by a 14-day observation period) produced only minor
irritation in male New Zealand albino rabbits (Smyth et al., 1962). A
dose of 400 mg applied to the clipped dorsal skin of restrained albino
rabbits in a gauze patch produced mild to moderate irritation in some
cases (Weil & Scala, 1971). Data from this latter study, however, were
highly variable and may reflect the fact that its purpose was
intercomparison of laboratories rather than the effects of MEK on test
animals. Neat MEK (0.1 ml) applied to the clipped skin of the flanks
of guinea-pigs and rabbits daily for 10 days and left uncovered caused
erythema and oedema after 24-72 h. These effects were more marked in
rabbits (Wahlberg, 1984a).
Table 11. Acute toxicity of MEK for mammalsa
Species Number and sex Exposure concentration Exposure Study Effects Reference
(strain) and range duration duration
Oral studies
Rat (Sprague- both sexes, 6-12 not stated one dose LD50 (7 days) < 0.8 g/kg b.w. Kimura et al.
Dawley) new animals/group 2.5 (2.0-3.1) g/kg b.w. (1971)
born 14 day 6 male/group 2.9 (2.3-3.5) g/kg b.w.
young adult 2.7 (2.1-3.5) g/kg b.w.
older adult
Rat (Carworth- 5 female/group not stated one dose 14 days LD50 (14 days) Smyth et al.
Wistar) 5.52 (4.50-6.80) g/kgb (1962)
Rat (Sprague- 5 male/group 1.505 g/kg b.w. one dose 24 h after 16-23 h, no effect on Traiger &
Dawley) liver histology, serum glutamic Bruckner (1976)
pyruvic transaminase, or
hepatic glucose-6-phosphatase;
63% increase in liver
triglycerides
Rat (Fischer- 32 male/group 0.072-1.082 g/kg b.w. one dose 24 h no evidence of liver Brown & Hewitt
344) dysfunction; slight damage at (1984)
highest dose to kidney proximal
tubules
Mouse (CF1) 10 male/group in 2.0-5.1 g/kg b.w. one dose 24 h LD50 (24 h) Zakhari et al.
each of 6 groups 3.14 ± 0.67 g/kg b.w. (1977)
Inhalation studies
Mouse (Swiss 10 male/group four levels over the 4 h decreased duration of De Ceaurriz et
OF1) range 4726-7192 mg/m3 immobility in escape - al. (1983)
(1602-2438 ppm) directed swimming test
Table 11 (contd)
Species Number and sex Exposure concentration Exposure Study Effects Reference
(strain) and range duration duration
Mouse (CD1) 12 males per five concentrations over continuous 2´ h increasing dose-related Glowa & Dews
concentration the range 885-29 500 (30 min per behavioural effects starting (1987)
mg/m3 (300-10 000 ppm) concentration) at 2950 mg/m3 (1000 ppm)
Mouse (CF1) 6 groups of 147 500-294 000 mg/m3 45 min LC50 (45 min) 205 000 ± 32 500 Zakhari et al.
10 males (50 000-100 000 ppm) mg/m3 (69 500 ± 11 000 ppm) (1977)
Mouse (white) both sexes, 6 303 850 mg/m3 until death average survival 43 min La Belle &
animals/group (103 000 ppm) Brieger (1955)
Rat (Sprague- 6 animals, sex 74-2360 mg/m3 2-6 h 2-6 h behavioural change (increased Garcia et al.
Dawley) unspecified (25-800 ppm) frequency of lever pressing) (1978)
which persisted for < 2 to
> 11 days
Rat (albino) 8 males/group 23 158-59 590 mg/m3 4 h 14 days LC50 (4 h) 34 500 mg/m3 La Belle &
(7850-20 200 ppm) (11 700 ppm) Brieger (1955)
Rat (Wistar) 5 males/group 23 600 mg/m3 8 h 14 days lethal to 3/6 within 14 days Smyth et al.
(8000 ppm) (1962)
Rat (Wistar) data not 47 200 mg/m3 8 h 14 days lethal to 6/6 within 14 days Smyth et al.
supplied (16 000 ppm) (1962)
Guinea-pig 6 unspecified approx. 9735 mg/m3 90-810 min 4-8 days no abnormal signs Patty et al.
sex/group; (3300 ppm) (1935)
3 groups
Guinea-pig 6 unspecified approx. 29 500 mg/m3 90-810 min 4-8 days 90 min: no injury; 280 min: Patty et al.
sex/group; (10 000 ppm) unconsciousness and slight (1935)
3 groups injury, no deaths; 810 min:
unconsciousness and moderate
injury, no deaths
Table 11 (contd)
Species Number and sex Exposure concentration Exposure Study Effects Reference
(strain) and range duration duration
Guinea-pig 10 females 73 750 mg/m3 325 min < 2 days all animals died during or Specht et al.
(25 000 ppm) post exposure (1940)
Guinea-pig 6 unspecified approx. 97 350 mg/m3 30-250 min 4-8 days 30 min: slight injury; 90 min: Patty et al.
sex/group; (33 000 ppm) unconsciousness and moderate (1935)
3 groups injury, no deaths; lethal to
all animals in 200-250 min
Guinea-pig 6 unspecified approx. 303 850 mg/m3 10-55 min 4-8 days 10 min: slight injury; 30 min: Patty et al.
sex/group; (103 000 ppm) unconsciousness and moderate (1935)
3 groups injury, no deaths, lethal to
all animals in 45-55 min
Intravenous study
Rat (Sprague- 18 females several infusion rates 60 min 60 min threshold limit for vestibulo- Tham et al.
Dawley) including 5 mg/kg b.w. ocular disturbance (depression (1984)
per min of nystagmus following
accelerated rotation) 5 mg/kg
b.w. per min; MEK concentration
in blood, 101 mg/litre
Pulmonary aspiration study
Rat (Sprague- 3 males and 800 mg/kg b.w. one dose survivors lethal 5/6 in < 24 h; 4/6 died Panson & Winek
Dawley) 3 females sacrificed instantly; produced pulmonary (1980)
at approx. haemorrhage
24 h
Intraperitoneal studies
Mouse (CF1) 10 male/groups; 0.5-2.0 g/kg b.w. one dose 24 h LD50 (24 h) Zakhari et al.
5 groups 1.66 ± 0.74 g/kg b.w. (1977)
Table 11 (contd)
Species Number and sex Exposure concentration Exposure Study Effects Reference
(strain) and range duration duration
Rat (Sprague- 6 female/group; not stated one dose 24 h LD50 (24 h) 1.554 Lundberg et
Dawley) number of groups (1.229-1.966) g/kg b.w. al. (1986)
unstated
Rat (Sprague- 6 female/group; not stated one dose 14 days LD50 (14 day) 0.607 Lundberg et
Dawley) number of groups (0.486-0.748) g/kg b.w. al. (1986)
unstated
Rat (Sprague- 6 female/group; 0.049-0.194 g/kg b.w. one dose 18 h no significant elevation of Lundberg et
Dawley) 3 groups serum sorbital dehydrogenase al. (1986)
Guinea-pig 4 male/group 0.75-2.0 g/kg one dose 24 h 0.75 g/kg b.w.: no elevation DiVincenzo &
in serum OCTc level, no Krasavage
obvious lipid deposition in (1974)
hepatocytes, no deaths;
1.5 g/kg b.w.: elevated serum
OCT level, lipid deposition in
hepatocytes, no deaths;
2.0 g/kg b.w.: elevation
in serum OCT level, lipid
deposition in hepatocytes,
lethal to 1/4 animals
Dermal studies
Rabbit (Albino, 4 males amounts not stated; 24 h 14 days LD50 (14 days) > 8 g/kg Smyth et al.
New Zealand) kept in contact with (1962)
clipped flank skin
under plastic film
Rabbit (Albino, 5 males 8 mg on clipped one dose 24 h minor irritation Smyth et al.
New Zealand) uncovered skin (1962)
Table 11 (contd)
Species Number and sex Exposure concentration Exposure Study Effects Reference
(strain) and range duration duration
Rat (Albino) 8 males 0.4 mg on 2.5 cm2 24 h 72 h variable results: no irritation Weil & Scala
lightly covered pad to moderate irritation 1 day (1971)
on clipped back skin and 3 days after applicationd
Rabbit (Albino) 6 animals intact 0.5 ml to clipped 24 h 72 h slight erythema subsiding by Hazleton
skin, 6 animals abdominal skin with 72 h (1963a)
abraded skin, semi-occlusive
sex unspecified cover
Ocular studies
Rabbit (Albino) 6 of unspecified 0.1 ml to one eye of 30 sec 14 days severe irritation, including Hazleton
sex each rabbit corneal damage and scleral (1963b)
haemorrhage in 2/6 rabbits
Rabbit (Albino) 5 male per not stated 3 min solution of 130 g/litre water Larson et al.
concentration produced significant oedema in (1955)
eyelid in 1 h
Rabbit (Albino, not specified 4 mg/eyee 1 min 18-24 h severe chemical burn Smyth et al.
New Zealand) (1962)
Rabbit (Albino) 6 males 8 mg to one eye of 20 sec 7 days variable results; no irritation Weil & Scala
each rabbit to moderate irritation 1 day, 3 (1971)
days and 7 days after
applicationd
Rabbit (Albino, 6 of unspecified 8 mg to one eye of described 7-10 days slight swelling, redness and MB Research
New Zealand) sex each rabbit as brief discharge from eye for 4 to Lab. Inc.
10 days (1979)
Table 11 (contd)
a Values from the literature were recalculated as necessary to yield dosages in terms of ppm, g or g/kg
b 95% confidence limits
c OCT = ornithine carbamyl transferase
d Purpose of study was intercomparison of testing laboratories that evaluated MEK and other substances by purportedly identical methods
e Not clear whether one eye or both eyes were dosed
The results of studies on eye irritation in rabbits are
inconsistent. Smyth et al. (1962) reported that 0.005 ml (4 mg)
created a severe chemical burn in the rabbit eye, whereas a study by
MB Research Lab. Inc. (1979) reported less severe irritant effects
from the larger dose of 0.1 ml (80 mg). Data from a study by Weil &
Scala (1971) were also inconsistent but indicated that a dose of 0.1
ml (80 mg) produced minimal to moderate eye irritation. Undiluted MEK
was used in all three studies but Smyth et al. (1962) used the grading
system for eye injury described in Carpenter & Smyth (1946), whereas
MB Research Lab., Inc. (1979) and Weil & Scala (1971) used the Draize
scoring system (Draize et al., 1944). In all studies irritation
disappeared or was markedly reduced by 7 days after treatment. Opaque
corneas were apparent following exposure of guinea-pigs to 295 000 mg
MEK/m3 (100 000 ppm) for 30 min; recovery from this effect was
complete in 4-8 days (Patty et al., 1935).
7.2 Repeated exposures
Data on repeated exposure of mammals to MEK are summarized in
Table 12. None of the concentrations tested, not even the highest (17
700 mg/m3 (6000 ppm) 8 h/day for up to 7 weeks) was clearly lethal
or even significantly harmful. The death of experimental animals
(rats) at this highest dose was not associated with neurological signs
and appeared to result exclusively from bronchopneumonia (Altenkirch
et al., 1978, 1979). These authors did not comment on possible
connections between bronchopneumonia and exposure to 17 700 mg/m3.
Female rats exposed to 14 750 mg/m3 (5000 ppm) 6 h/day, 5 days per
week, for 90 days showed only slightly increased liver weight,
slightly decreased brain and spleen weights, and slightly altered
blood chemistry in comparison with controls. Male rats receiving this
exposure showed only a slightly increased liver weight. At lower
concentrations of MEK (3688 and 7375 mg/m3 (1250 and 2500 ppm))
there was only slightly increased liver weight for female rats and no
significant differences for males in comparison with controls
(Cavender et al., 1983). In another subchronic inhalation study
(Toxigenics, 1981), male and female rats were exposed to MEK
concentrations of 3700, 7430 and 14 870 mg/m3 (1254, 2518 and 5041
ppm) 6 h/day, 5 days/week, for 90 days. No significant effects on food
consumption, eyes or nervous system were observed. In addition, no
MEK-induced morphological changes in the central or peripheral nervous
system were detected. Lower levels of exposure resulted in few
measurable effects. Inhalation of 2242 and 2360 mg/m3 (760 and 800
ppm) 6 h/day, 5 days/week, for 4 weeks by rats caused some enlargement
of the liver and slightly modified the in vitro metabolism of liver
microsomes (Nilsen & Toftgard, 1980; Toftgard et al., 1981). Ten
intraperitoneal injections of 0.034 g/kg over 2 weeks produced no
effect on the kidney (Bernard et al., 1989). However, exposure of rats
to 590 mg/m3 (200 ppm) 12 h/day for 24 weeks transiently decreased
nerve conduction velocity after 4 weeks (Takeuchi et al., 1983).
Geller et al. (1978) reported that exposure of baboons to 295 mg/m3
(100 ppm) for 7 days increased the response time in a delayed "match
to sample" task. However, this effect was transient and disappeared
during the course of repeated exposure. Following intermittent
exposure of rats to 3319 g/m3 for up to 5 months there was no
morphological evidence of peripheral neurotoxicity (Saida et al.,
1976). It is possible that the transient nature of the neurological
and behavioural changes induced by MEK exposure may be due to
behavioural and/or physiological adaptations. The latter may reflect
more rapid metabolism of MEK with prolonged exposure.
Short-term dermal exposure to small amounts of MEK resulted, at
most, in mild local irritation. Two topical applications of an
unstated amount to the ears of various strains of mice (Swiss, Balb/c,
CBA, C5681/6, DBA/2, B6D2F1) produced no significant swelling or other
signs (Descotes, 1988). In rabbits and guinea-pigs, a dose of 0.08 g
applied to the skin, without covering, once a day for 10 days to the
same site resulted in a slight to moderate increase in skinfold
thickness, whereas a much smaller dose (8 mg) applied to the same site
in rabbits 3 times a day for 3 days resulted in barely detectable
irritation (Wahlberg, 1984a). Open application of MEK to the shaved
flanks of guinea-pigs for 3 days produced slight erythema, epidermal
thickening and dermal cell infiltration (Anderson et al., 1986). In
the cat, injection of 150 mg MEK (99.98% pure) per kg, twice a day, 5
days/week, for up to 8.5 months, into subcutaneous tissue produced
abscesses, skin ulceration and generalized weakness but no evidence of
damage to the nervous system (Spencer & Schaumburg, 1976). In the one
long-term dermal study of MEK (Horton et al., 1965), 8 mg dissolved in
water was applied by dropper or brush to clipped skin of mice twice a
week for a year. Few details were given of the results because this
was the control for a study on carcinogenesis of the skin in which a
MEK/water solution was the solvent for compounds under test. Horton et
al. (1965) stated that the control mice did not develop skin tumours,
and they did not mention any adverse effects from this prolonged
application of MEK.
Table 12. Repeated exposure of mammals to MEKa
Route Species (strain), Exposure and range Results Reference
number and sex
Inhalation rat (Wistar), 590 ± 118b mg/m3 (200 ± 40b transient differences in nerve conduction claimed Takeuchi et al.
8 males ppm) 12 h/day for 24 weeks after 4 weeks, but no significant differences (1983)
Inhalation rat (Albino), 693 ± 77b mg/m3 (235 ± 26b no significant gross or microscopic La Belle &
25, sex ppm) 7 h/day, 5 days/week pathological changesc Brieger (1955)
unspecified for 12 weeks
Inhalation rat (Wistar), 885 mg/m3 (range 867-894) no significant change in leucocyte or serum Li et al. (1986)
7 females (300 ppm (range 294-303)) alkaline phosphatase activity
8 h/day for 7 days
Inhalation rat (Sprague- 3322 and 7723 mg/m3 (1026 at 7723 mg/m3 minor effects on dams, decreased Schwetz et al.
Dawley), and 2618 ppm) 7 h/day food consumption and weight gain, and increased (1974)
pregnant, 25 per for 10 days (gestation water consumption; no effects on dams at 1180 mg/m3
concentration days 6-15)
Inhalation rat (Sprague- 2242 and 2360 mg/m3 (760 and significant enlargement of liver; no effect on Nilsen &
Dawley), 4 800 ppm), 6 h/day, 5 days total liver microsomal concentration of cytochrome Toftgard (1980);
male/concentration per week for 4 weeks P-450; in vitro liver microsomal metabolism of Toftgard et al.
biphenyl unaffected, but slight effects on the (1981)
metabolism of benzo[a]pyrene, 4-androstene-3,17-
dione, and 5alpha-androstane-3alpha,17ß-diol
Inhalation rat (Fischer- 3688, 7375, 14 750 mg/m3 females at 14 750 mg/m3 increased in liver weight, Cavender et al.
344), 15 males, (1250, 2500, 5000 ppm) smaller braind and spleene weight, slightly altered (1983)
15 females/ 6 h/day, 5 days/week for blood chemistryf; females at 3688, 7375 mg/m3
concentration 90 days nonsignificant increase in liver weight; males at
14 750 mg/m3 increased in liver weight. No pathological
lesions, including peripheral nerves, attributed to
MEK exposure. No effect on reproductive tissues
(testis, epididymis, seminal vesicle, vagina, cervix,
uterus, oviduct, ovary)
Table 12 (contd)
Route Species (strain), Exposure and range Results Reference
number and sex
Inhalation rat (Fischer- 3699, 7430, 14 870 mg/m3 elevated group mean body weights at 3699 and 7430 Toxigenics
344), 15 males, (1254, 2518, 5041 ppm) mg/m3; highest concentration: decreased group mean (1981)
15 females/ 6 h/day, 5 days/week for body weight, increased liver weight, liver/body
concentration 90 days weight ratio and liver/brain weight ratio, increased
mean corpuscular haemoglobin; highest concentration
(males): increased kidney/body weight ratio, decreased
spleen and brain weights; highest concentration
(females): decreased brain/body weight ratio, increased
kidney/brain weight ratio; no significant effects at
any concentration on food consumption, eyes, nervous
system, and no morphological changes in central or
peripheral nervous systems
Inhalation rat (Sprague- 3319 mg/m3 (1125 ppm) 24 h no morphological effects on peripheral nerves Saida et al.
Dawley), 36 of per day for 16 days to 5 (1976)
unspecified sex months
Inhalation rat (Wistar), 29 500 mg/m3 (10 000 ppm) for severe irritation of upper respiratory tract; slight Altenkirch et
5 males a "few days"; 17 700 mg/m3 loss of weight; death during seventh week from al. (1978,
(6000 ppm) ± 15% for 8 h/day, bronchopneumonia; no neurological signs 1979)
7 days/week for 15 weeks
Inhalation guinea-pig, 693 ± 77b mg/m3 (235 ± 26b no significant effects La Belle &
15 of unspecified ppm) 7 h/day, 5 days/week for Brieger (1955)
sex 12 weeks
Inhalation baboon, 295 mg/m3 (100 ppm) for 7 days no impairment of discrimination in a behavioural Geller et al.
4 males test, slight increase in response time early in (1978)
experiment, but not at end of experiment
Table 12 (contd)
Route Species (strain), Exposure and range Results Reference
number and sex
Intra- rat (Sprague- 0.034 g/kg, 5 days/week for no effect on kidney function Bernard et al.
peritoneal Dawley), 2 weeks (1989)
female, number
unspecified
Subcutaneous cat, 6 of 0.15 g/kg twice/day, 5 days abscesses, skin ulceration and generalized weakness Spencer &
unspecified sex per week for up to 8.5 months in some animals; no damage to nervous Schaumburg
system structure or function (1976)
Dermal mouse (Swiss), unstated amount applied twice no significant swelling of treated ear Descotes (1988)
12 of unspecified to one ear
sex
Dermal mouse 8 mg (50 mg of 17% solution) no papilloma evident after 1 year Horton et al.
(C3H/He) applied to clipped skin twice (1965)
10-25 males per week for 1 year
Dermal guinea-pig, 3 80 mg rubbed into skin at slight to moderate increase in skinfold thickness Wahlberg (1984a)
and rabbit, 4, same site once daily for at end of 10 days
of unspecified 10 days
sex
Dermal guinea-pig 80 mg applied to 1.0 cm2 on no reaction until day 2; at end of experiment, Anderson et al.
10 females shaved flank 3 times daily slight redness and increase in dermal inflammatory (1986)
for 3 days cell count
a Values from the literature recalculated as ppm, mg or g/kg
b Standard deviation of the mean
c Depressed growth (average weight 70% of controls at end of experiment) may be due to causes other than MEK exposure; animals showed signs
of "vitamin deficiency" (no details given) and infection in latter part of experiment.
d Significantly different at the < 0.01 level from 0, 3688 and 7375 mg/m3 (0, 1250, and 2500 ppm) female group values
e Significantly different at the < 0.05 level from 0, 3688 and 7375 mg/m3 (0, 1250, and 2500 ppm) female group values
f Females in the 14 750 mg/m3 (5000 ppm) group had a small but significant elevation in serum potassium, alkaline phosphatase and glucose
levels, and a significant reduction in serum glutamic-pyruvic transaminase level.
7.3 Neurotoxicity
7.3.1 Behavioural testing
Neurotoxicity studies have been carried out on MEK, usually as
part of studies on the neurotoxicity of methyl isobutyl ketone (MIBK)
and MIBK/MEK mixtures.
An increase in response rate (lever pressing) was reported in a
group of six adult Sprague-Dawley rats (sex unspecified) exposed to
MEK at various concentrations between 74 and 2360 mg/m3 (25 and 800
ppm) for 2 h at approximately weekly intervals. An increase in
response rate also occurred in a group of four rats exposed to 74
mg/m3 (25 ppm) for 6 h at 2-day intervals (Garcia et al., 1978).
Geller et al. (1978) examined behavioural effects (match to
sample (MTS) test) in baboons exposed to MEK by inhalation. Four young
male baboons (2 years old) were exposed continuously to MEK at a
concentration of 295 mg/m3 (100 ppm) for 7 days. There were no
effects on performance of the test in terms of the ability to
discriminate visual stimuli but reaction time increased. However, in
two of the baboons, response times returned to pre-exposure control
values by day 7.
Tham et al. (1984) examined the vestibulo-oculomotor reflex (VOR)
during intravenous infusion of MEK into the caudal veins of 18 female
Sprague-Dawley rats. The threshold for depression of the VOR was an
infusion rate of 70 µmol/kg (0.005 g/kg per min) for 1 h (total dose
30 mg) and the associated arterial blood level was 1.4 mmol/litre.
General depression of the central nervous system followed depression
of the VOR.
Glowa & Dews (1987) exposed continuously by inhalation a group of
12 adult male white mice (Charles River CD1) to concentrations of MEK
that were increased at 30 min intervals until the mice failed to
respond to a visual stimulus. The concentrations, in ascending order
for each 30 min, were 885, 2950, 8850, 16 520 and 29 500 mg/m3 (300,
1000, 3000, 5600 and 10 000 ppm) with a total exposure time of 2´ h.
Mice responded to a visual stimulus and the response rate was used as
an indicator. There was no effect at a concentration of 885 mg/m3,
a slight decrease in response rate at 2950 mg/m3 and a 75% decrease
at 8850 mg/m3. Most mice ceased to respond at 16 520 mg/m3 and all
failed to respond at 29 500 mg/m3. The response rate returned to the
control value 30 min after exposure ended. The EC50 (concentration
decreasing response rate by 50%) was calculated to be 8528 (SD = 2033)
mg/m3 (2891 (SD = 689) ppm). An EC10 was calculated and
dose-response estimates were derived. The concentrations of MEK
producing a 10% decrease in response rate in 0.1%, 1% and 19% of a
population were 50, 195 and 885 mg/m3 (17, 66 and 300 ppm),
respectively.
Couri et al. (1977) exposed continuously by inhalation young male
Wistar rats to 2210 mg MEK/m3 (750 ppm) for either 7 or 28 days. In
those exposed for 7 days there was a significant reduction in
hexobarbital sleep times. In the group exposed for 28 days the
reduction in sleep times was less marked.
7.3.2 Histopathology
In chickens, cats, rats and mice exposed by inhalation to 3975
mg/m3 (1500 ppm) for periods of up to 12 weeks, there was no
evidence of neuropathy and no histopathological changes were reported
(Couri et al., 1974).
Saida et al. (1976) exposed groups of 36 Sprague-Dawley rats (sex
unspecified) continuously to MEK at a concentration of 3319 mg/m3
(1125 ppm) for periods of 16, 25, 35 and 55 days. Additional studies
were carried out with up to 5 months of exposure. There were no
abnormal clinical observations in any group. At the end of the
exposure period, rats were sacrificed and the sciatic nerve and foot
muscle excised. Spinal cord and dorsal root ganglion specimens were
taken from the same rats. Quantitative histology
(neurofilaments/µm2; frequency of inpouching of myelin sheath,
denuded axons/mm2) showed no abnormality in rats exposed for up to
5 months.
Cavender et al. (1983) reported no neurological abnormalities in
Fischer-344 rats in a 90-day inhalation study on MEK alone. Groups of
15 male and 15 female rats were exposed 6 h/day, 5 days/week, for 90
days to MEK concentrations of 3688, 7375 and 14 750 mg/m3 (1150,
2500 and 5000 ppm). All rats were observed twice daily for clinical
signs. At the end of the exposure period, the eyes of each animal were
examined by ophthalmoscopy, and neurological function (posture, gait,
tone and symmetry of facial muscles, and pupillary, palpebral,
extensor-thrust and cross-extensor thrust reflexes) was evaluated. No
abnormalities were found. Necropsy, including histopathology of the
sciatic and tibial nerves, was carried out on all rats, with special
neuropathological studies on the medulla, sciatic and tibial nerves in
5 male and 5 female rats from each group. There were no changes
attributable to MEK.
7.4 Developmental toxicity
Schwetz et al. (1974) exposed 44 pregnant rats from days 6-15 of
gestation (sperm = day 0) to two concentrations of MEK vapour, i.e.
nominally 2950 mg/m3 in 23 rats and 8850 mg/m3 in 21 rats (1000
and 3000 ppm), for 7 h/day; 43 rats were air-exposed as controls. The
average values for measured concentrations in this study were 3322 and
7723 mg/m3 (1126 and 2618 ppm), respectively. There was no evidence
of maternal toxicity. Fetal weight and crown-rump length were
significantly decreased at 3322 mg/m3, but not at 7723 mg/m3. At
3322 mg/m3 there was also a significant increase, compared to the
controls, in the number of litters with fetuses showing skeletal
anomalies, and at 7723 mg/m3 a significantly increased incidence of
litters with sternebral and soft tissue anomalies was reported. Four
grossly malformed fetuses were found (two with brachygnathia and two
acaudate with imperforate anus), all in different litters in the 7723
mg/m3 group. These malformations had not been observed previously in
more than 400 control litters of this strain. Fetal body dimensions,
however, were not significantly different from controls. When the data
were analysed on a per litter basis, there was evidence of a
teratogenic effect.
However, the incidence of major malformations in these studies
was sufficiently low that evidence for teratogenic effects was
considered questionable, and the studies were repeated (John et al.,
1980; Deacon et al., 1981). The methodology was identical except for
the inclusion of an additional level of exposure, nominally 1180
mg/m3 (400 ppm). Average measured MEK concentrations during the
experiment were 1215, 2956 and 8865 mg/m3 (412, 1002 and 3005 ppm).
The only evidence for maternal effects was decreased weight gain and
increased water consumption (no figures given) by dams exposed to 8865
mg/m3. None of the dosages had significant effects on the incidence
of pregnancy, incidence of resorption, average number of implantations
and live fetuses per dam, fetal weight and length, or incidence of
external or soft tissue abnormalities. There were statistically
significant differences in the incidences of some skeletal anomalies
occurring in the 8875 mg/m3 group compared to the controls. There
were increased incidences of lumbar ribs and delayed ossification of
the cervical centra, but a decreased incidence of delayed ossification
of the skull. Since these skeletal abnormalities occurred at low
incidences among the population from which the experimental animals
were drawn, the results of this study were interpreted as indicating
a low level of fetotoxicity and no evidence for embryotoxic or
teratogenic effects for MEK at exposure levels up to 8865 mg/m3.
In a further study (Mast et al., 1989; Schwetz et al., 1991),
groups of 10 virgin female Swiss CD1 mice and 33 plug-positive (day 0)
females were exposed by inhalation on gestation days 6-15 to mean
concentrations of 1174 ± 27, 2980 ± 83 and 8909 ± 233 mg/m3 (398 ±
9, 1010 ± 28 and 3020 ± 79 ppm). There was no evidence of maternal
toxicity, although there was a slight, treatment-related increase in
liver/body weight ratios that was significant at the highest dose
level. Mild fetal toxicity was evident at this maternal dose level as
a reduction in mean fetal body weight, statistically significant for
males. There was no increase in the incidence of intrauterine death,
but there was an increased dose-related incidence of misaligned
sternebrae, statistically significant at the highest dose level. There
were no significant increases in the incidence of malformations,
although there were several malformations in one litter (cleft palate,
fused ribs, missing vertebrae, syndactyly) in treated groups but not
in the control group nor in contemporary control data. On the basis of
this study it was concluded that the no-observed-adverse-effect level
(NOAEL) was 2978 mg/m3 (1010 ppm) and the
lowest-observed-adverse-effect level (LOAEL) was 8096 mg/m3 (3020
ppm).
7.5 Mutagenicity and related end-points
Short-term genotoxicity tests in vitro and in vivo are
summarized in Table 13. Although MEK has given negative results in
most conventional assays, Zimmermann et al. (1985) found that MEK and
certain other polar aprotic solvents were strong inducers of
aneuploidy in the yeast. The induction of aneuploidy by MEK was
markedly potentiated by coexposure to ethyl acetate (Mayer & Goin,
1988) or with nocodazol (methyl [5- (2-thienyl-carbonyl)-1
H-benzimidazol-2-yl]-carbamate) (Mayer & Goin, 1987).
O'Donoghue et al. (1988) conducted mutagenicity studies on MEK.
The test systems comprised the Salmonella/microsome (Ames) assay, the
L5178/TK+/- mouse lymphoma (M/L) assay, the BALB/3T3 cell
transformation (CT) assay, unscheduled DNA synthesis (UDS) and the
micronucleus test. MEK was not found to be genotoxic in these assays.
Other studies of MEK utilizing cultures of mammalian cells as
test systems also yielded little or no evidence of mutagenicity and
related effects. Perocco et al. (1983) tested MEK and other important
industrial chemicals at concentrations of 10-2 to 10-4 mol/litre
(0.72 to 0.0072 g MEK/litre) with an in vitro system utilizing
cultures of human lymphocytes to determine toxicity and ability to
inhibit DNA synthesis. Cultures were grown both with and without S9
mix derived from phenobarbital-induced rat liver. MEK at the
concentrations tested showed no evidence of cytotoxic or genotoxic
action. Chen et al. (1984) examined the effects of MEK on metabolic
cooperation between 6-thioguanine-resistant and
6-thioguanine-sensitive Chinese hamster lung fibroblast V79 cells and
obtained equivocal results. Holmberg & Malmfors (1974) also found some
evidence of MEK cytotoxicity to ascites tumour cells cultured with the
solvent for up to 5 h. Although there was no significant increase in
irreversibly injured cells at MEK concentrations of 0.05 and 0.1
g/litre, there was a moderate increase in damaged cells at 0.1
g/litre. An ultrastructural study (Veronesi, 1984) utilizing a medium
containing relatively high concentrations of MEK (0.3 g/litre)
produced axoplasmic granularities in a few cultures. The relationship
of this effect to possible MEK-induced neurotoxicity in vivo is not
clear.
7.6 Carcinogenicity
No long-term carcinogenicity studies have been reported.
Table 13. Short-term genotoxicity tests on MEK
Method Concentration Experimental conditions; comments Results References
In vitro studies
Bacterial assays 3 µmol/plate Salmonella typhimurium TA98, TA100, TA1535, - Florin et al. (1980)
TA1537 with and without S-9
10 mg/plate S. typhimurium TA98, TA100, TA1535, - Nestmann et al. (1980)
TA1537 with and without S-9
approx. S. typhimurium TA104: maximum non-toxic - Marnett et al. (1985)
1 mg/plate dose > 3 µmol
0.05-32 µl/plate S. typhimurium TA98, TA100, TA1535, - O'Donoghue et al. (1988)
TA1537, TA1538 with and without S-9
4 mg/plate Escherichia coli WP2 and WP2 uvrA - Brooks et al. (1988)
Mitotic gene 5 mg/ml Saccharomyces cerevisiae (JD1) - Brooks et al. (1988)
conversion assay
Induction of mitotic 3.54% S. cerevisiae (D61.M) + Zimmermann et al. (1985)
aneuploidy
0.50-1.96% S. cerevisiae (D61.M) + Mayer & Goin (1987)
Chromosome assay 1 mg/ml rat liver RL4 cells - Brooks et al. (1988)
Cell transformation 9-18 µl/ml BALB/3T3 - O'Donoghue et al. (1988)
UDS test 0.1-5.0 µl/ml primary rat (Sprague-Dawley) hepatocyte - O'Donoghue et al. (1988)
Table 13 (contd)
Method Concentration Experimental conditions; comments Results References
In vivo studies
Micronucleus test 1.9 ml/kg CD1 mice (male and female), - O'Donoghue et al. (1988)
intraperitoneal time: 12, 24, 48 h
411 mg/kg Chinese hamster, time: 12, 24, 48, 72 h - Basler (1986)
intraperitoneal
8. EFFECTS ON HUMANS
8.1 General population exposure
The only record of non-occupational acute toxicity from MEK was
a case of accidental self-poisoning (Kopelman & Kalfayan, 1983). A
47-year old woman inadvertently ingested an unknown amount of MEK and
was found unconscious, hyperventilating, and suffering from severe
metabolic acidosis. Her plasma concentration of MEK was 950 mg/litre.
She responded promptly to an infusion of sodium hydrogen carbonate and
was discharged from the hospital after a week. The metabolic effects
of MEK ingestion by humans are not well characterized and it is
uncertain that the acidosis was produced by MEK.
8.2 Effects of short-term exposure
There are limited data on behavioural and other effects on humans
of short-term exposure to MEK. Nakaaki (1974) found that exposure to
266-797 mg/m3 (90-270 ppm) for up to 4 h per day caused his subjects
to underestimate times of 5 to 30 seconds. Dick et al. (1984, 1988,
1989), on the other hand, found that a 4-h exposure of human subjects
to 590 mg/m3 (200 ppm) had no significant effect in a variety of
behavioural tests. These included psychomotor, visual vigilance, dual
task, sensorimotor and psychological tests. Solvent mixtures of 295 mg
MEK/m3 (100 ppm) and 186 mg toluene/m3 (50 ppm), and of 295 mg
MEK/m3 (100 ppm) and 298 mg acetone/m3 (125 ppm) similarly had no
significant effect on the results of these behavioural tests.
8.3 Skin irritation and sensitization
MEK (0.1 ml) rubbed into volar forearm skin daily for 18 days and
left uncovered did not produce persistent erythema or swelling
(Wahlberg, 1984a). A 5-min contact with 1.5 ml of analytical grade MEK
confined to a 20-mm circle on the forearm produced a temporary
whitening of the skin, but no visible erythema, alteration in
cutaneous blood flow or other indication of irritation to the skin
(Wahlberg, 1984b).
A male painter developed dermatitis 18 months after commencing
spray painting using an epoxy-polyamide paint (Varigos & Nurse, 1986).
A patch test with "a small amount" of MEK applied to areas of skin
3 cm in diameter on each forearm caused these areas of skin to turn
bright red within 10 min. The spots were itchy, but there was no
induration or oedema. The reaction reached its maximum after 15 min
and then gradually faded. The test was repeated after 2 days, and gave
the same results. Application of the same grade of MEK to normal
forearm skin of five male volunteers produced no reaction.
8.4 Occupational exposure
8.4.1 MEK alone
There is no record that MEK toxicity has ever caused death or a
large scale industrial accident, and only one acute occupational
poisoning has been ascribed to MEK. An 18-year-old seaman with good
vision and no previous eye problems was exposed to MEK vapour of
unknown concentration while stripping paint, and promptly noted
headache, mild vertigo and blurred vision (Berg, 1971). The diagnosis
was retrobulbar neuritis. He was given vitamin B complex and steroid
therapy, and his vision returned to normal in 36 h. However, blood
analysis exhibited a positive methanol content (no value reported)
according to the criteria given by Kaye (1961). Thus a potentiation of
the effects due to combined exposure to MEK and methanol cannot be
excluded.
Data for occupational poisoning ascribed to chronic exposure to
MEK in the absence of other solvents are equally limited. Long-term
exposure of 51 Italian workers to MEK produced indications of
neurotoxicity with slightly, but not significantly, reduced nerve
conduction velocities and various other symptoms such as headache,
loss of appetite and weight, gastrointestinal upset, dizziness,
dermatitis and muscular hypotrophy, but no clinically recognizable
neuropathy (Freddi et al., 1982). There has been a brief report of
chronic exposure of American workers, in a factory producing coated
fabric, to 885-1770 mg MEK/m3 (300-600 ppm) in the apparent absence
of other solvents (Smith & Mayers, 1944). Workers complained of
dermatoses and numbness of fingers and arms.
8.4.2 MEK in solvent mixtures
It was reported that MEK was commonly present as part of solvent
mixtures containing hexane, and that the TLV for hexane (148 mg/m3,
50 ppm) was exceeded in 89 of the work stations (mainly in shoe
factories) (De Rosa et al., 1985). MEK potentiates the toxicity of
hexane and these authors concluded that in these shoe factories the
risk of neurotoxicity was extremely high. Observations by Tangredi et
al. (1981), Brugnone et al. (1981) and Cresci et al. (1985) supported
the widespread nature of this health problem in Italian industry,
especially shoe factories. Arques Espi & Quintanilla Almagro (1981)
also found that mixtures of solvent vapours including MEK and hexane
posed an excessive risk in 95 of 114 work stations examined in a study
of Spanish shoe factories. In a nationwide survey of Japanese
factories, Inoue et al. (1983) found that MEK is widely used as a
component of solvent mixtures. In a study of electronic parts plants
in the USA (Lee & Parkinson, 1982), workers were found to be exposed
to mixtures of solvent vapours containing MEK and as many as nine
other components, although not hexane or other solvents whose toxic
action MEK is known to potentiate. Observations on Finnish car
painters (Husman, 1980; Husman & Karli, 1980) suggested that long-term
exposure to complex solvent mixtures whose components individually and
jointly are far below the legal concentration limits may produce
significant adverse effects. Noma et al. (1988) similarly suggested
that complex mixtures of volatile organic compounds, rather than a
high concentration of any single compound, may be responsible for
unhealthy air in buildings.
Descriptions of the effects of occupational exposures to mixtures
of solvent vapours not necessarily potentiated by MEK are summarized
in Table 14. There are only two cases of acute occupationally related
toxicity from such mixtures, and only a limited number of adequately
documented cases of adverse effects from chronic occupational exposure
which did not involve potentiation of hexacarbon toxicity. The only
acute cases involved two young women waterproofing seams of raincoats
with resins dissolved in acetone or MEK (Smith & Mayers, 1944).
Post-exposure measurements revealed workplace concentrations of
785-1178 mg/m3 (330-495 ppm) for acetone and 1174-1655 mg/m3
(398-561 ppm) for MEK. The total solvent concentration was estimated
to be 1000 ppm. Both women fainted at work and subsequently displayed
a number of temporary neurological and other symptoms.
However, the majority of these studies are difficult to interpret
because they lack either a quantitative description of the solvent
vapours in the work environment, or the description is based on
post-exposure analyses that may not be typical of working conditions
during most of the exposure. In these studies it is also impossible to
make any certain assessment of the role(s) played by individual
components. Mixed solvent exposures have been associated with
alteration in nerve conduction velocity (Viader et al., 1975; Dyro,
1978; Triebig et al., 1983), memory and motor alterations (Binaschi et
al., 1976), and dermatoses and vomiting (Lee & Murphy, 1982). Fagius
& Grönqvist (1978) reported neurological effects in 3 out of 42
workers exposed in a steelworks to organic solvents. However, the
actual role of MEK in these effects is not clear.
Table 14. Effects of occupational exposure to mixtures of solvent fumes containing MEK
Concentrations of MEK and other No. of workers Exposure Effects Defects in study Reference
components exposed duration
Acute
MEK 1174-1655 mg/m3 (389-561 ppm), 2 few hours eyes watering, gastric measurements of work Smith &
acetone 785-1178 mg/m3 (330-495 ppm) distress, fainting, environment limited Mayers
convulsions, twitching, to post exposure (1944)
headache, spinal pressure
Chronic
MEK 561-885 mg/m3 (190-300 ppm), 13 1 year or fatigue, frequent headache, measurements of work Lee &
toluene 139-177 mg/m3 (37-60 ppm), more dizziness, incoordination, environment limited Murphy
MIBK 37-41 mg/m3 (9-14 ppm) skin rashes, vomiting to post exposure (1982)
MEK 30 mg/m3 (10 ppm), toluene 2 2 and 6.5 paraesthesis in extremities, measurements of work Dyro
94-488 mg/m3 (25-130 ppm) years reduced motor nerve conduction environment limited (1978)
velocity, weakness in to post exposure
individuals exposed for long
periods, improvement following
cessation of exposure
MEK 62-51 mg/m3 (21-180 ppm), acetone 1 1 year altered consciousness and measurements of work Dyro
86-595 mg/m3 (36-250 ppm) EEG, reduced motor nerve environment limited (1978)
conduction velocity to post exposure
MEK 142 mg/m3 (48 ppm), isobutanol 8 not stated headache, dizziness, fatigue, measurements of work Binaschi
67 mg/m3 (23 ppm), MIBK 16 mg/m3 (4 ppm), depression, significant environment limited et al.
toluene 180 mg/m3 (48 ppm), butyl acetate reduction in short-term to post exposure; (1976)
43 mg/m3 (9 ppm), xylene 347 mg/m3 memory and hand steadiness period of exposure
(80 ppm) not indicated
Table 14 (contd)
Concentrations of MEK and other No. of workers Exposure Effects Defects in study Reference
components exposed duration
MEK 115 (avg), 2655 (max) mg/m3, n-butanol 9 8-35 significant changes in measurements of work Denkhaus
67 (avg), 1200 (max) mg/m3, isobutanol years several sub-populations of environment limited et al.
172 (avg), 3000 (max) mg/m3; 2-butoxy- peripheral blood lymphocytes: to post exposure (1986)
ethanol 25 (avg), 350 (max) mg/m3, decrease in certain type of
2-ethoxyethanol 5 (avg), 53 (max) mg/m3, T-cells and helper cells,
2-methoxyethanol 6 (avg), 150 (max) mg/m3, increase in natural killer
toluene 86 (avg), 750 (max) mg/m3, cells and B-lymphocytes
m-xylene 19 (avg), 220 (max) mg/m3,
MBK 2 (avg), 27 (max) mg/m3
MEK 9-124 mg/m3 (3-42 ppm), 5 > 10 years no significant differences measurements of work Lundberg
xylene 0-6111 mg/m3 (0-1408 ppm), in serum activities of liver environment limited &
toluene 0-1260 mg/m3 (0-336 ppm), enzymes between exposed to post exposure Hakansson
isobutanol 0-1045 mg/m3 (0-345 ppm), workers and controls (1985)
n-butanol 0-1548 mg/m3 (0-511 ppm),
ethanol 0-1094 mg/m3 (0-582 ppm),
ethyl acetate 0-2095 mg/m3 (0-582 ppm),
n-butyl acetate 0-1691 mg/m3 (0-356 ppm),
methyl acetate 3-181 mg/m3 (1-60 ppm),
white spirit 2-30 mg/m3 (1-17 ppm),
methylene chloride 10-2460 mg/m3 (3-707 ppm),
isopropanol 5-260 mg/m3 (2-106 ppm),
exposure to 2-8 solvents plus MEK
MEK < 9-401 mg/m3 (< 3-136 ppm), 66 average significant reduction in measurements of work Triebig
xylene < 4-82 mg/m3 (< 1-19 ppm), 5 years sensory conduction in environment limited et al.
toluene 11-551 mg/m3 (3-147 ppm), comparison with controls, no to post exposure (1983)
ethyl acetate < 11-302 mg/m3 (< 3-84 ppm) suggestion of neuropathy,
trichloroethane 11-601 mg/m3 (2-110 ppm) change in conduction velocity
correlated with exposure
Table 14 (contd)
Concentrations of MEK and other No. of workers Exposure Effects Defects in study Reference
components exposed duration
MEK 15-620 mg/m3 (5-210 ppm), acetone 17 not stated no health problems associated measurements of work Cohen &
< 119-495 mg/m3 (< 50-208 ppm), xylene with exposure to MEK environment limited Maier
< 22 mg/m3 (< 5 ppm), toluene < 19-34 to post exposure (1974)
mg/m3 (< 5-9 ppm), petroleum naphtha
(concentration unknown)
MEK mainly < 443, max. 5115 mg/m3 42 0.5-8 1 likely and 2 suspected measurements of work Fagius &
(mainly < 150, max. 1734 ppm), trichloroethylene years cases of polyneuropathy, environment limited Gronqvist
mainly < 161 mg/m3 (< 30 ppm); slight loss of sensitivity to post exposure (1978)
at low levels butanol, butyl acetate, butyl to vibration correlated
diglycol, cyclohexanol, diacetal, ethyl with level of solvent ex-
glycol acetate, ethanol, isoforone, posure during preceding
methylene chloride, MIBK, toluene, 6 months
xylene, "solvesso 100 and 150"a
MEK 0-74 mg/m3 (avg, 3 mg/m3) (0-25 1006b average overall death rate below none Wen et al.
(avg. 1) ppm), toluene 4 mg/m3 (1 ppm); 21.6 years expected level, excess of (1985)
at very low levels benzene, "hexane", deaths from cancer associ-
MIBK, xylene ated with lubricating oil,
not MEK
a hydrocarbon solvent mixtures
b workers from lube oil and dewaxing plant
In a study on a group of 9 parquet-flooring workers (age, 25-58
years; exposure time, 8-35 years), Denkhaus et al. (1986) noted
significant changes in several subpopulations of peripheral blood
lymphocytes, which could constitute an early indication of a
haematological or immunological effect. Benzene was not detected in
the ambient air and no investigation was made to determine which
components of the solvent mixtures produced the observed changes in
lymphocyte populations. Anshelm Olson et al. (1981) studied the simple
reaction time (SRT) performance in a group of 42 workers (age, 18 to
52 years; employment, 0.5 to 8.1 years) from a plastic coating line of
a steel factory (the same group had previously been studied by Fagius
& Grönqvist, 1978). The study was longitudinal and covered a period of
27 months during which SRT was measured three times. Originally, the
workers had been exposed to significant concentrations of MEK (up to
4000-5000 mg/m3 in certain regular tasks) and to much lower levels
of other solvents. Five months after the completion of major
improvements in the work environment which reduced the levels of MEK
to about 20 mg/m3 (maximum of about 400 mg/m3), a second SRT
measurement was made and a third measurement was performed 15 months
later. The workers' performance on the SRT test improved over the
three measurements. Moreover, on the first occasion SRT was correlated
to the degree of exposure. The authors concluded that the workers'
central nervous functioning had been adversely affected by solvent
exposure.
Mutti et al. (1982a) carried out a study of exposure to organic
solvents in an Italian shoe factory. The exposed group consisted of 95
workers (24 males, 71 females) with an age range of 16-62 years (mean,
30.9 ± 11.7 years), and the exposure duration ranged from 1 to 25
years (mean, 9.1 ± 8 years). The approximate mean air concentrations
in the breathing zone, over a 2-year period, for a number of solvents
were: MEK, 115 mg/m3 (39 ppm); n-hexane, 317 mg/m3 (90 ppm);
cyclohexane, 315 mg/m3 (92 ppm); and ethyl acetate, 205 mg/m3
(57 ppm). The exposed workers complained of sleepiness, dizziness,
weakness, paraesthesia and hypo-aesthesia. Other neurological
symptoms, such as headache, muscular cramps, neurasthenic syndrome and
sleep disturbances, were found more often in exposed workers, but the
differences in incidence between the exposed and reference group were
not statistically significant.
Among exposed workers the mean motor nerve conduction velocity
was significantly reduced in the median and peroneal nerves but not in
the ulnar nerve. The amplitude of the motor action potential (MAP) was
significantly reduced in all nerves and its duration was increased in
the ulnar nerve. There were no significant effects on the distal
latency. The number of abnormal action potentials observed in the
median and peroneal nerves of exposed workers was significantly
increased. There was a correlation between the reduction in motor
conduction velocity and exposure.
In a follow-up study, electrophysiological measurements including
somatosensory evoked potentials (SEPS) were recorded from a group of
15 female shoe factory workers aged 19-53 years (mean age, 26.6 ± 11.4
years) with a solvent exposure duration of 2-8 years (mean, 4.5 ± 2.3
years) (Mutti et al., 1982b). The mean air concentrations for various
solvents in the breathing zone of the workers were: MEK, 177 mg/m3
(60 ppm); n-hexane, 690 mg/m3 (196 ppm); cyclohexane, 585 mg/m3
(170 ppm); and ethyl acetate, 360 mg/m3 (100 ppm).
Electrophysiological measurements in peripheral nerves showed
significant reductions in maximal motor and distal sensory nerve
conduction velocities in the median and ulnar nerves and reduced
maximal motor nerve conduction velocity in the peroneal nerve. The
latency of the sensory peak action potential was significantly
increased in the median and ulnar nerves. The amplitude of all
peripheral nerve action potentials was slightly reduced but this was
not statistically significant. There were also changes in the SEPs
with significant increase in the latency of some early component
peaks. The amplitude of some of the early peaks was significantly
reduced. The neurotoxicity was attributed primarily to n-hexane.
8.5 Carcinogenicity
In a historical prospective mortality study of 446 male workers
in two MEK dewaxing plants, with an average follow up of 13.9 years,
the observed deaths (46) were below the expected (55.51). There was a
slight deficiency of deaths from neoplasms (13 observed; 14.26
expected) but there was a significant increase of deaths from tumours
of the buccal cavity and pharynx (2 observed; 0.13 expected). However,
there were significantly fewer deaths from lung cancer (1 observed;
6.02 expected). In view of the small numbers, it was concluded that
there was no clear evidence of cancer hazard in these workers
(Alderson & Rattan, 1980).
9. EFFECTS ON OTHER ORGANISMS IN THE LABORATORY AND FIELD
9.1 Microorganisms
The effects of MEK on microorganisms have been studied in several
species important to freshwater aquatic systems. As shown in Table 15,
growth inhibition generally occurs at levels ranging from 120 mg/litre
for the cyanobacterium (blue-green alga) Microcystis aeruginosa to
4300 mg/litre for the green alga Scenedesmus quadricauda.
A number of bacterial species have also been tested, the effect
levels ranging from 10 to 5050 mg/litre. Kulshrestha & Marth (1974a-f)
conducted a series of studies to determine if MEK and other volatile
compounds associated with the flavour of raw or mildly heated milk are
able to inhibit the growth of certain pathogenic bacteria and other
bacteria important in the manufacture of fermented dairy products.
Nutrient broth laced with the organisms and MEK at levels of 1, 10,
100 and 1000 mg/litre was plated after 5, 8, 11 and 14 h of incubation
in an air-tight vessel. Results are shown in Table 15. MEK was
considered bacteriostatic to Escherichia coli, Salmonella
typhimurium, Staphylococcus aureus, Leuconostoc citrovorum and
Streptococcus thermophilus at levels as low as 10-100 mg/litre.
However, Walton et al. (1989) reported that MEK at concentrations up
to 1 g/kg dry weight of soil had little effect on the respiration of
microorganisms in moist soil. Volskay & Grady (1988) found that MEK at
1 g/litre depressed respiration of sewage sludge organisms by only
11%. Ingram (1977) noticed changes in the fatty acid and phospholipid
composition of the cell membranes of E. coli when cultured with a
sublethal (0.218 mol/litre) concentration of MEK.
Tests in fungi demonstrated that MEK has a very slight
stimulating effect on the germination of uredospores in two species of
rust (French, 1961; French et al., 1977). Growth of a mixture of other
fungal species was inhibited by about 50% by 6.4 mg MEK/g seed when
the fungi were cultured on moist wheat seed (Nandi & Fries, 1976).
Growth of a mixture of other fungal species on moist wheat seed
at 30 °C was inhibited by 50% following exposure for 5 days to
approximately 6.4 mg MEK/g seed (Nandi & Fries, 1976). Germination of
the wheat seeds was also reduced but there was no statistical
evaluation of these data.
Table 15. Effects of methyl ethyl ketone on microorganisms
Organism Species, strain Concentration Effect Reference
(mg/litre)
Prokaryotes
Cyanobacterium
(Blue-green alga Microcystis aeruginosa 120 inhibition of cell multiplication Bringmann & Kuhn (1978)
Bacteria Pseudomonas putida 1150 inhibition of cell multiplication Bringmann & Kuhn (1977a)
Photobacterium phosphoreum 5050 50% reduction in light output Curtis et al. (1982)
Escherichia coli, ML30 10 slight but not consistently Kulshrestha & Marth (1974a)
significant inhibition of growth
Escherichia coli, ML30 100 significant inhibition after 5 h Kulshrestha & Marth (1974a)
incubation
Escherichia coli, ML30 1000 10% reduction in growth Kulshrestha & Marth (1974a)
Escherichia coli, B15 72.1 65% growth inhibition after 1 h; Egyud (1967)
30% after 2 h
Salmonella typhimurium 1 & 10 slight but not consistently Kulshrestha & Marth (1974b)
significant inhibition of growth
Salmonella typhimurium 100 some inhibition of growth Kulshrestha & Marth (1974b)
Salmonella typhimurium 1000 significant inhibition of growth Kulshrestha & Marth (1974b)
Staphylococcus aureus, 100 1 no significant inhibition of growth Kulshrestha & Marth (1974c)
Staphylococcus aureus, 100 10 some inhibition of growth Kulshrestha & Marth (1974c)
Staphylococcus aureus, 100 100 & 1000 significant inhibition of growth Kulshrestha & Marth (1974c)
Table 15 (contd)
Organism Species, strain Concentration Effect Reference
(mg/litre)
Streptococcus lactis 1 no significant inhibition of growth Kulshrestha & Marth (1974d)
Streptococcus lactis 10 significant inhibition at early Kulshrestha & Marth (1974d)
stages of incubation
Streptococcus lactis 100 & 1000 significant inhibition of growth Kulshrestha & Marth (1974d)
Leuconostoc citrovorum 1 no significant inhibition of growth Kulshrestha & Marth (1974e)
Leuconostoc citrovorum 10 some inhibition of growth Kulshrestha & Marth (1974e)
Leuconostoc citrovorum 100 & 1000 significant inhibition of growth Kulshrestha & Marth (1974e)
Streptococcus thermophilus, 1 no significant inhibition of growth Kulshrestha & Marth (1974f)
ST4
Streptococcus thermophilus, 10 growth inhibition at later stages of Kulshrestha & Marth (1974f)
ST4 incubation
Streptococcus thermophilus, 100 & 1000 significant inhibition of growth Kulshrestha & Marth (1974f)
ST4
Eukaryotes
Fungi Puccinia graminis, var. tritica, not given slight stimulation to germination French (1961)
spores (wheat stem rust)
Uromyces phaseoli, (Reben), 1000 very slight stimulation to French et al. (1977)
Wint., spores (bean rust) germination
Puccinia helianthi, Schw. chr., 50 very slight stimulation to French (1984)
spores (sunflower rust) germination
Table 15 (contd)
Organism Species, strain Concentration Effect Reference
(mg/litre)
Uromyces vignae, Barcl., 500 no effect on germination French (1984)
spores (cowpea rust)
various fungi found on wheat 6.4a slight inhibition of fungal growth Nandi & Fries (1976)
seeds: Septoria nerdorum, Fusarium
nivale, Aspergillus glaucus,
Aspergillus candidus, Penicillium
sp., Alternaria, sp.
Green algae Chlorella sp. 806 no effect on chlorophyll content Dojlido (1979)
after 7 days exposure
Scenedesmus quadricauda 4300 slightly inhibited cell Bringmann & Kuhn (1978)
multiplication
Protozoa Entosiphon sulcatum 190 slightly inhibited cell Bringmann (1978)
multiplication
a mg/g seed.
9.2 Aquatic organisms
MEK has been tested on numerous species of freshwater and marine
vertebrates and invertebrates in short-term tests. The results
indicate that MEK is generally of low toxicity to aquatic animals,
with median lethal (LD50) levels ranging from 1382 to 8890 mg/litre
(Table 16). Almost all of the acute tests were conducted under static
conditions, in open vessels, with nominal measurements of MEK
concentration, all of which may underestimate the true toxicity level
of MEK. No chronic studies at low concentrations have been conducted.
Curtis et al. (1982) related the effect of MEK on the
bioluminescence of a bacterium to the 96-h LC50 in fathead minnows
in an attempt to find an inexpensive yet accurate substitute for
lethality tests. The relationship for ketones (r2 = 0.81, n = 7) is
described by the equation:
log LC50 = 0.74 (log EC50 + 0.79)
where the EC50 (5 min) is the concentration causing a 50% reduction
in light output.
This relationship predicts a 96-h LC50 for fathead minnows of
3388 mg/litre, which agrees well with the measured value (Veith et
al., 1983) of 3200 mg/litre. Similarly, LeBlanc (1984) found a highly
significant correlation (P < 0.01) between the LC50 values
reported for warm-water and cold-water fish, saltwater and freshwater
fish, marine and freshwater invertebrates, and marine and freshwater
algae. There was a remarkable similarity among acute toxicity values
across three trophic levels. Data in Tables 15 and 16 lend further
support to this conclusion.
9.3 Terrestrial organisms
9.3.1 Animals
The effect of MEK on the alarm behaviour of social insects has
been studied. It was considered inactive in producing alarm behaviour
in the ant Iridomyrmex preinosus (Blum et al., 1966), the harvester
ant Pogonomyrmex badius (Blum et al., 1971) and the honeybee Apis
Mellifera (Boch & Shearer, 1971). Alarm behaviour was measured by
the number of insects that were attracted to the chemical and was
indexed relative to the activity of natural pheromones, including
2-heptanone.
Table 16. Effects of methyl ethyl ketone on aquatic organisms
Species Concentration Effects and comments pH Temperature Hardness Reference
(mg/litre) (°C)
Crustacea
Artemia salina 1950 24-h LC50, bottles not not given not given not given Price et al. (1974)
(brine shrimp) sealed and may have lost
MEK during experiment
Daphnia magna (water flea) < 180 no discernible effect 7.21 21.0-23.0 38 mg/litre Union Carbide Corp.
CaCO3 (1980a)
Daphnia magna (water flea) < 520 24-h LC50 8 ± 0.2 not given 173 ± 13 mg/l Le Blanc (1980)
< 520 48-h LC50
< 70 no discernible effect
Daphnia magna (water flea) 1382 48-h LC50 7.21 21.0-23.0 38 mg/litre Union Carbide Corp.
(918-3349)a CaCO3 (1980a)
Daphnia magna (water flea) 2500 24-h LC0 7.6-7.7 20-22 16 ° (German) Bringmann & Kuhn (1977b)
Daphnia magna (water flea) 8890 24-h LC50 7.6-7.7 20-22 16 ° (German) Bringmann & Kuhn (1977b)
Daphnia magna (water flea) 10 000 24-h LC100 7.6-7.7 20-22 16 ° (German) Bringmann & Kuhn (1977b)
Fish
Leucissus idus melanotus 4400b LC0, period not mentioned not given not given not given Juhnke & Luedemann
(golden orfe) 4800b (1978)
Leucissus idus melanotus 4600b LC50 not given not given not given Juhnke & Luedemann
(golden orfe) 4880b (1978)
Table 16 (contd)
Species Concentration Effects and comments pH Temperature Hardness Reference
(mg/litre) (°C)
Leucissus idus melanotus 4800b LC100 not given not given not given Juhnke & Luedemann
(golden orfe) 5040b (1978)
Lebistes reticulatus 2000 disturbed behaviour not given 20 ± 1 27.5 mg/litre Dojlido (1979)
(guppy) CaCl2
Lebistes reticulatus 5700 24-h LC50, open not given 20 ± 1 27.5 mg/litre Dojlido (1979)
(guppy) containers may have lost CaCl2
MEK during test
Pimephales promelas 3200 96-h LC50 7.5 25 ± 1 42.2 mg/litre Veith et al. (1983)
(fathead minnow) CaCO3
Lepomis macrochirus < 1000 no discernible effect 7.93 21 240 mg/litre Union Carbide Corp.
(bluegill sunfish) CaCO3 (1980b)
Lepomis macrochirus 4467 96-h LC50 7.93 21 240 mg/litre Union Carbide Corp.
(bluegill sunfish) CaCO3 (1980b)
Gambusia affinis 5600 96-h LC50 7.8-8.3 room not given Wallen et al. (1957)
(mosquito fish) temperaturec
Cyprinodon variegatus 400 no discernible effect not given 25-31 not given Heitmuller et al.
(sheepshead minnow) (1981)
Carassius auratus > 5000 24-h LC50 7.0 20 ± 1 100 mg/litre Bridie et al. (1979b)
(goldfish)
a 95% confidence limits
b Values are from different laboratories
c No specific value given
MEK was found to be moderately effective as a fumigant against
the Caribbean fruit fly, Anastrepha suspensa (Davis et al., 1977).
Treatments of 790 mg/m3 (286 ppm) for 2 h or 316 mg/m3 (107 ppm)
for 7 h destroyed 100% of the larvae in naturally infected guavas,
whereas exposure to 221 mg/m3 (75 ppm) for 3 h destroyed 92% of the
larvae. Kwan & Gatehouse (1978) applied between 0.31 and 0.37 mg MEK
topically to the dorsal thorax of tsetse flies (Glossina morsitans
morsitans) weighing 17-19 mg. One day after treatment, the MEK had
a significant effect on the activity of males but not females. The
mortality in MEK-treated insects was marginally but consistently
higher than in the untreated controls. No apparent inhibitory or other
effect of MEK was noted with respect to feeding or mating. Vale et al.
(1988) found MEK to be a very effective attractant for tsetse flies
and used it in a successful control effort in which the flies were
attracted to insecticide-coated netting. Uspenskii & Repkina (1974)
reported that an unspecified dose of MEK caused an increase in the
physiological age of the tick Ixodes perculcatus, an effect that
increased the insect's sensitivity to DDT.
9.3.2 Plants
MEK has an effect on the germination of seeds of several plant
species. Nandi & Fries (1976) observed that the germination of wheat
seeds was inhibited when the seeds were treated with 6.4 mg MEK/g
seed. At this level, 10% of the experimental seeds germinated versus
60% of the control seeds. Germination of lettuce was inhibited 50% by
MEK at 12.5 (± 4) mmol/litre (equivalent to 900 ± 288 mg/litre)
dissolved in agar (Reynolds, 1977). Schulz et al. (1981) reported,
however, that a mixture of acetone and MEK had no inhibitory effect on
the growth of rye grass at concentrations up to 1 g/litre.
10. ENHANCEMENT OF THE TOXICITY OF OTHER SOLVENTS BY MEK
The principal toxic effects noted with MEK exposure stem from its
ability to potentiate the known toxicities of other solvents (Table
17). Two such interactions are described in detail below.
10.1 Hexacarbon neuropathy
10.1.1 Introduction
MEK interacts with hexacarbon compounds and potentiates their
neurotoxicity (WHO, 1991). Potentially MEK co-exposure could affect
the metabolism of the hexacarbon compounds or the toxic process by
which the hexacarbons induce the neuropathy. The critical metabolic
pathway for hexacarbon induced neuropathy is outlined in Fig. 2, and
the scheme by which the peripheral nerve axonal degradation is thought
to occur is given in Fig. 3. The metabolic pathway involves hepatic
microsomal oxidation to 2,5-hexanedione, the proximate neurotoxicant,
which is thought to induce cross-linking of neurofilaments, blockage
of transport at the node of Ranvier, and swelling from accumulated
neurofilaments proximal to the nodes and axonal degeneration distal to
the nodes.
10.1.2 Animal studies
The phenomenon of potentiation of hexacarbon neurotoxicity by MEK
has been firmly established by in vivo studies mainly on rats
(Table 17) and also has been demonstrated in tissue culture (Veronesi
et al., 1984). In every study in which the dose of n-hexane, methyl
butyl ketone (MBK), or 2,5-hexanedione (2,5-HD) was large enough and
sustained for a sufficient period, clinical signs of neural
degeneration were produced. These signs were made more severe by
co-exposure to MEK. In addition, the period prior to the onset of
symptoms was frequently shortened by co-exposure to MEK. Minimum
sustained continuous exposure concentrations that induced neuropathy
in these experiments were 295/1408 and 590/1056 mg/m3 (100/400 and
200/300 ppm) MEK/ n-hexane mixtures. An intermittent exposure (8
h/day) of rats to 2950/31 680 mg/m3 (1000/9000 ppm) MEK/ n-hexane
mixture produced severe neuropathy, whereas similar exposure to a
lower concentration, i.e. 590/1760 mg/m3 (200/500 ppm)
MEK/ n-hexane mixture yielded no evidence of hexacarbon
neurotoxicity. Intermittent exposure (8 h/day, 5 days/week) to
5900/820 mg/m3 (2000/200 ppm) MEK/MBK mixture produced some neural
degeneration and mild clinical signs. Oral dosing of rats once a day,
5 days/week, with a mixture of MEK/2,5-HD (0.159/0.253 g/kg) produced
marked clinical signs (Ralston et al., 1985). Even with doses of MBK
too low to produce significant neuropathy, studies generally indicated
that co-exposure with MEK induced changes compatible with enhanced
toxicity, such as reduced velocity of nerve conduction, elevated
hepatic microsomal enzyme activity, or reduced clearance of 2,5-HD
from the blood or the body. The only study reporting no evidence of
potentiation (Spencer & Schaumburg, 1976) compared MBK at 0.150 g/kg
with one tenth this concentration of MBK, 0.015 g/kg, in combination
with MEK. Since there were no control data on the effects of 0.015
g/kg of MBK alone and the dose of MEK was very low, it is difficult to
interpret the meaning of this study. Concentrations of MEK in
inhalation studies did not exceed 3319 mg/m3 (1125 ppm), and
continuous exposure to this concentration was demonstrated by Saida et
al. (1976) not to produce neuropathological effects. Thus it is
considered unlikely that MEK itself produces neuropathy.
The property of potentiation of hexacarbon neurotoxicity is not
unique to MEK, but is shared at least by methyl n-propyl ketone,
methyl n-amyl ketone and methyl n-hexyl ketone, none of which
appear intrinsically neurotoxic (Misumi & Nagano, 1985).
The mechanism by which MEK potentiates hexacarbon neurotoxicity
is not well understood, although potential metabolic interactions have
been examined. In rats, simultaneous inhalation of n-hexane and MEK
resulted in lower levels of 2,5-HD in vivo in urine (Iwata et al.,
1984; Shibata et al., 1990a) and initially lower but later somewhat
elevated levels of MBK and 2,5-HD in vivo in serum (Shibata et al.,
1990b). Both Iwata et al. (1984) and Shibata et al. (1990b) concluded
that potentiation of n-hexane neurotoxicity by MEK could not be
explained solely by increased 2,5-HD formation. Pretreatment of rats
with MEK (1.87 ml/kg, 4 daily doses) prior to inhalation of n-hexane
resulted in higher levels of 2,5-HD in several tissues, including
blood (Robertson et al. (1989). Abdel Rahman et al. (1976) reported
that an 8-h simultaneous exposure of rats to MEK and MBK did not
result in measurable levels of MBK or of 2,5-HD in blood, whereas a
6-day continuous co-exposure resulted in substantially raised levels
of MBK and 2,5-HD. Continuous co-exposure for 23 days, however,
resulted in further elevation of MBK in blood, but no measurable level
of 2,5-HD. Simultaneous administration of 2,5-HD and MEK, either as a
single dose or as six repeated daily doses in rats, resulted in a
greater total area under the curve for 2,5-HD in blood compared with
administration of 2,5-HD alone (Ralston et al., 1985).
In guinea-pigs, increased levels of 2-hexanol and 2,5-HD were
found following intraperitoneal administration of a MEK/MBK mixture
compared with intraperitoneal administration of MBK alone (Couri et
al., 1978).
MEK administration has also been shown to induce a number of in
vivo and in vitro parameters of hepatic oxidative metabolism
(Couri et al., 1977; Wagner et al., 1983; Misume & Nagano, 1985;
Raunio et al., 1990).
Table 17. Interaction of MEK with other solvents and their metabolitesa
Route of administration, Dose and/or Exposure Effects/results Reference
species (strain), concentration
number and sex
n-Hexane
Inhalation, rat, 820 mg/m3 (200 ppm) MBK; 8 h/day, muscular weakness lasted longer after exposure to Duckett et al.
9 sex unspecified 5900/820 mg/m3 5 days/week, MEK/MBK than after MBK alone (1974)
MBK, 8 sex unspecified (2000/200 ppm) MEK/MBK 6 weeks
MEK/MBK
Inhalation, rat 1760 mg/m3 (500 ppm) 8 h/day, no significant enhancement of neuropathological Iwata et al.
(Wistar), 6 males hexane; 1475/1760 mg/m3 7 days/week, damage evident in peripheral nerve (1984)
per groupb (500/500 ppm) MEK/hexane 33 weeks electrophysiological function; 2,5-hexanedione
and other hexane metabolites in urine reduced
with co-exposure
Inhalation, rat 923 mg/m3 (225 ppm) MBK; 24 h/day, MEK enhanced MBK-induced peripheral neurotoxicity Saida et al.
(Sprague-Dawley), 3319/923 mg/m3 (1125/225 16-66 days (1976)
12/group, sex ppm) MEK/MBK
unspecified
Inhalation, rat 923 mg/m3 (225 ppm) MBK; 24 h/day, hexobarbital sleeping time significantly reduced in Couri et al.
(Wistar), 5 males 2213/923 mg/m3 (750/225 7 or 28 days group exposed continuously for 7 days to MEK/MBK (1977)
per group ppm) MEK/MBK (and in 2213 mg MEK/m3 (750 ppm) controls), but not
in MBK group; interpreted as evidence for elevated
microsomal enzyme activity in MEK/MBK and MEK
groups
Table 17 (contd)
Route
Route of administration, Dose and/or Exposure Effects/results Reference
species (strain), concentration
number and sex
Inhalation, rat 1760, 2464 mg/m3 (500, 700 approx. experiments yielded similar results: potentiation of Altenkirch et
(Wistar), 5 males ppm) hexane; 295/1408, 24 h/day, n-hexane neurotoxicity in MEK/hexane groups as al. (1982a)
per group 590/1056, 590/1760 mg/m3 7 days/week, shown by shortened period before onset of clinical
(100/400, 200/300, 200/500 9 weeks signs (weakness and paresis); hypersalination
ppm) MEK/hexane in MEK/hexane groups only; neural degeneration
in all solvent-treated groups (no mention of
differences among these groups)
Inhalation, rat 1760, 2464 mg/m3 (500, 700 8 h/day, every co-exposure to MEK/hexane resulted in earlier and Schnoy et al.
(Wistar), 5 groups ppm) hexane; 295/1408, day for 1-89 more pronounced degeneration of pulmonary (1982);
of 2-5 malesc 590/1056, 590/1760 mg/m3 days nerves than exposure to hexane alone; there were no Schmidt et al.
(100/400, 200/300, 200/500 consistent differences in degeneration of alveolar (1984)
ppm) MEK/hexane epithelium between the hexane and MEK/hexane
groups
Inhalation, rat 1640, 923 mg/m3 (400, 225 24 h/day, 2,5-HD detected in blood after 6 days but not after Abdel-Rahman
(Wistar), unspecified ppm) MBK; 2213/923 mg/m3 6 or 23 days 23 days, and there were elevated MBK blood levels et al. (1976)
number/group, male (750/225 ppm) MEK/MBK in group exposed to MEK/MBK; severe neuropathy in
group exposed to MEK/MBK for 23 days
Inhalation, rat 2464 mg/m3 (700 ppm) 8 h/day, no evidence of potentiation of hexane neurotoxicity: Altenkirch et
(Wistar), 5 males hexane; 590/1760 mg/m3 7 days/week, no abnormal clinical signs or elevated al. (1982a)
per group (200/500 ppm) MEK/hexane 40 weeks neuropathology in solvent-treated groups
Inhalation, rat 3520 mg/m3 (1000 ppm) 8 hd 2,5-hexanedione in urine markedly less after co- Iwata et al.
(Wistar), 5 males hexane; 2950/3520 mg/m3 exposure with MEK than after exposure to hexane (1983)
per group (1000/1000 ppm) MEK/hexane alone
Table 17 (contd)
Route
Route of administration, Dose and/or Exposure Effects/results Reference
species (strain), concentration
number and sex
Inhalation, rat Experiment 1: 35 200 mg/m3 8 h/day, experiments yielded similar results; acceleration Altenkirch et
(Wistar), 5 males (10 000 ppm) hexane; 3245/ 7 days/week, of n-hexane neurotoxicity in MEK/hexane group as al. (1978,
per group 31 330 mg/m3 (1100/8900 15-19 weeks shown by more severe clinical signs (gait 1979)
ppm) MEK/hexane disturbances, weakness and paresis), greater
degeneration of peripheral nerves and shortened
5 males/group Experiment 2: 35 200 mg/m3 period before onset of signs and neural degeneration;
(10 000 ppm) hexane; 2950/ hyper-salivation in MEK/hexane groups
31 680 mg/m3 (1000/9000
ppm) MEK/hexane
12 males/group Experiment 3: 35 200 mg/m3
(10 000 ppm) hexane; 2950/
31 680 mg/m3 (1000/9000
ppm) MEK/hexane
Inhalation, rat 35 200 mg/m3 (10 000 ppm) 4 h or 8 h, MEK did not enhance degeneration of intrapulmonary Schnoy et al.
(Wistar), 7 groups hexane; 2950/31 680 mg/m3 8 h/day for nerves; enhanced degeneration of alveolar epithelium (1982);
of 2-3 males (1000/9000 ppm) 2-14 days in the MEK/hexane group after 14 days exposure in Schmidt et al.
MEK/hexanee comparison with the hexane group (1984)
Inhalation, rat 352 mg/m3 (100 ppm) hexane; 12 h/day, marked impairment of nerve conduction velocities in Takeuchi et
(Wistar), 8 males 590/352 mg/m3 (200/100 7 days/week, MEK/hexane group; no evidence of significant al. (1983)
per group ppm) MEK/hexane 24 weeks potentiation of morphological effects of n-hexane
Inhalation, mouse 615 mg/m3 (150 ppm) MBK; 24 h/day, hexobarbital sleeping time significantly lower in Couri et al.
(Swiss), 5/group 2950/615 mg/m3 (1000/150 7 days MEK/MBK group than in MBK group (or in MEK 2950 (1978)
sex unspecified ppm) MEK/MBK mg/m3 (1000 ppm) controls); interpreted as evidence
for elevated microsomal enzyme activity in
MEK/MBK group
Table 17 (contd)
Route
Route of administration, Dose and/or Exposure Effects/results Reference
species (strain), concentration
number and sex
Subcutaneous injection, 288 mg/kg b.w. MBK; 1/day, 5 days significantly enhanced neurotoxicity (slower motor Misumi &
rat (Donryu), 288/288 mg/kg b.w. per week, 20 fibre conduction velocity and increased motor distal Nagano (1985)
8 males/group MEK/MBK weeks latency of tail nerve) and more pronounced weakness
in rats receiving MEK/MBK than MBK alone
Subcutaneous injection, 150 mg/kg b.w. MBK; 5 days/week peripheral neuropathological changes and paresis Spencer &
cat, 9 (sex 135/15 mg/kg b.w. for up to 8.5 with MBK; possible nerve degeneration in animals Schaumburg
unspecified) MBK, MEK/MBK months treated with MEK/MBK (1976)
4 (sex unspecified)
MEK/MBK
Other ketones
Subcutaneous injection, 150 mg/kg b.w. MIBK; 2/day, 5 days no evidence of enhanced MIBK-induced Spencer &
cat, 4, sex 135/15 mg/kg b.w. per week, up neuropathology Schaumburg
unspecified, MIBK, MEK/MIBK to 8.5 months (1976)
6, sex unspecified,
MEK/MIBK
Inhalation, rat 3220 mg/m3 (700 ppm) EBK; 16-20 h/day, exposure to MEK/EBK at 2065 and 4130 mg MEK/m3 O'Donoghue et
(Charles River), 15 207/3220, 2065/3200, 4 days produced a 2.5-fold increase in serum 2,5-Hpdn over al. (1984)
males/group for 4130/3200 mg/m3 (70/700, that produced by EBK alone; no 2,5-HD detected in
EBK and controls; 700/700, 1400/700 ppm) serum
5 males/group MEK/EBK
for MEK/EBK
Oral, rat (Charles 0.25 to 4 g/kg b.w. EBK; 1/day, 5 days 2 and 4 g EBK/kg b.w. fatal with or without MEK; O'Donoghue et
River), 4 males in 1.5, 0.75/0.25-4 g/kg b.w. per week, 14 greater neurological damage and dysfunction, and al. (1984)
control group MEK/EBK weeks around 1.5-fold increase in 2,5-Hpdn and 2,5-HD
with 1.5/1.0 g/kg b.w. MEK/EBK than with 1.0 g
EBK/kg b.w.; no neurotoxicity evident in doses
of EBK below 1.0 g/kg b.w.
Table 17 (contd)
Route
Route of administration, Dose and/or Exposure Effects/results Reference
species (strain), concentration
number and sex
Oral, rat (Fischer- 253 mg/kg b.w. 2,5-HD; 1/day, 5 days marked neurological dysfunction consistently Ralston et al.
344), 5 males/group 159/253 mg/kg b.w. per week, ca. appeared weeks earlier in rats receiving (1985)
MEK/2,5-HDf 13 weeks MEK/2,5-HD
Oral, rat (Fischer- 253 mg/kg b.w. 2,5-HD; 1/day, 1 MEK did not alter gastric absorption of 2,5-HD; Ralston et al.
344), 5 males/group 159/253 mg/kg b.w. or 7 days clearance of 2,5-HD from blood slower from rats (1985)
MEK/2,5-HD receiving MEK/2,5-HD
Oral, rat (Fischer- 253 mg/kg b.w. 2,5-HD; 1/day, 5 days most of radiolabelled 2,5-HD bound to protein; Ralston et al.
344), 5 males/group 159/253 mg/kg b.w. per week, 1, greater binding to protein with MEK/2,5-HD (1985)
MEK/2,5-HD 2 or 3 weeks after 1 week, but reverse true after 2 and 3 weeks
Halogenated alkanes
Oral, intraperitoneal, 1.505 g/kg b.w. MEK; 1 oral dose potentiation of CCl4 hepatotoxicity by MEK as Traiger &
rat (Sprague- 0.16 g/kg b.w. CCl4 MEK followed shown by greater fatty vacuolation and necrosis Bruckner
Dawley), 3-5 males/ 16 h later of liver, increased hepatic triglyceride and (1976)
experimental group, with 1 ip GPT, and decreased hepatic glucose-6-phosphatase
5-20 males/control dose CCl4
group
Oral, intraperitoneal, 1.691 g/kg b.w. MEK; 1 oral dose potentiation of CCl4 hepatotoxicity by MEK as Dietz & Traiger
rat (Sprague- 0.16 g/kg b.w. CCl4 MEK followed shown by increased hepatic triglyceride and (1979)
Dawley), males at 16 h later GPT
least 5/group with 1 ip
dose CCl4
Oral, intraperitoneal, 1.082 g/kg b.w. MEK; 1 oral dose potentiation of CHCl3 hepatotoxicity by both Hewitt et al.
rat (Sprague- 0.797, 1.196 g/kg b.w. MEK followed doses of MEK as shown by increased GPT and OCT (1983)
Dawley), 5 or 6 CHCl3 18 h later with
males/groupg 1 ip dose CHCl3
Table 17 (contd)
Route of administration, Dose and/or Exposure Effects/results Reference
species (strain), concentration
number and sex
Oral, intraperitoneal, 0.072 to 1.082 g/kg b.w. 1 oral dose potentiation of CHCl3 renal and hepatic toxicity Brown & Hewitt
neal, rat (Fischer- MEK; 0.797 g/kg b.w. MEK followed by MEK at all dosages as shown by histological and (1984)
344), 6 males per CHCl3 18 h later with several biochemical criteria; potentiation greatest
experimental 1 ip dose at 0.361 to 0.721 g/kg b.w. MEK, and slightly
group, 32 males CHCl3 reduced at 1.082 g/kg b.w.h
in control group
Oral, rat (Sprague- 1.082 g/kg b.w. MEK; 1 dose MEK MEK followed by CHCl3 did not induce cholestasis, Hewitt et al.
Dawley), 6 males 0.797 to 1.594 g/kg b.w. followed 10 but MEK given 10 to 24 h prior to CHCl3 potentiated (1986)
per group CHCl3 to 96 h later its ability to increase plasma bilirubin
with 1 dose
CHCl3
Oral, rat (Sprague- 1.082 g/kg b.w. MEK; 1 dose MEK MEK followed by CHCl3 10 to 48 h later potentiated Hewitt et al.
Dawley), 6 males 0.797 g/kg b.w. CHCl3 followed 10 hepatotoxicity as indicated by elevated ALT and (1987)
to 96 h later OCT levels
by 1 dose
CHCl3
a Values from the literature have been recalculated as ppm or g/kg body weight.
b Iwata et al. (1984) states that 24 rats were divided into four groups, but does not specify that groups were of equal numbers.
c Animals were apparently co-exposed with those described in Altenkirch et al. (1982a).
d Description of exposure not entirely clear but a subsequent paper (Iwata et al., 1984) refers to this as a single exposure.
e Animals co-exposed with those described in Altenkirch et al. (1978).
f MEK and 2,5-HD doses were 0.317 and 0.506 g/kg, respectively, for first 8 days of experiment.
g 5 males at the lower dose and 6 at the higher; 11 males in the control group
h Measurements were made 24 h after the oral dose of CHC13 or CCl4.
Abbreviations: GPT = plasma glutamic-pyruvic transaminase; OCT = plasma ornithine carbamyl transferase; ALT = plasma alanine aminotransferase;
b.w. = body weight; ip = intraperitoneal; 2,5-HD = 2,5-hexanedione; 2,5-Hpdn = 2,5-heptanedione; CCl4 = carbon tetrachloride; CHCl3 = chloroform

 10.1.3 Human studies
10.1.3.1 Solvent abuse
Solvent abuse, the deliberate inhalation of solvent vapours for
their euphoric effects, has been reported in many countries and has
involved the use of lacquer thinners, glues and other readily
available commercial items. Prockop et al. (1974) suggested that there
were several hundred habitual "huffers", i.e. solvent abusers, in
Tampa, Florida, USA, and Altenkirch et al. (1978) reported about 2000
in Berlin, Germany, in 1974. Outbreaks of polyneuropathy among Berlin
huffers, which involved n-hexane toxicity potentiated by MEK,
provided a major stimulus for research on the health effects of MEK
and its interactions with hexacarbon solvents.
Chronic huffers in Berlin inhaled fumes from about a half litre
of liquid per day, either poured into a plastic bag or over rags
(Altenkirch et al., 1977, 1982b). Inhalation sessions extended for as
long as 10 or 12 h, and exposure periods of 5 to 7 years were not
unusual. Prior to the end of 1975 no major damage to health resulting
from chronic solvent abuse had been observed. At that time there
appeared abruptly a number of cases of polyneuropathy among chronic
huffers. All these were young males, mainly between 16 and 21 years of
age. The initial symptom was paraesthesia of the toes accompanied in
some cases by weakness of the legs. The paraesthesia ascended rapidly
from distal to proximal and in 2 to 3 weeks affected the entire legs.
This was followed rapidly by paraesthesia of the arms which also
ascended from distal to proximal. Extensor muscles were always
affected first and most severely. There also was severe muscle atrophy
and a "glove and stocking" type sensory impairment of the hands and
feet. In all cases there also was excessive sweating of the hands and
feet, and in some cases discoloration and reduced skin temperature of
these areas. In addition there was loss of weight and damage to the
teeth. Head, neck and trunk muscles remained undisturbed, although in
severe cases there was paresis of the phrenic nerve and reduced
pulmonary function. The degree of impairment ranged from moderate
crippling, which permitted walking with assistance, to complete
tetraplegia. Symptoms continued to develop for 6 to 10 weeks after
cessation of solvent abuse. Remission was slow, took up to a year,
developed from proximal to distal, and was incomplete in those most
severely affected. Neurophysiological and histological findings were
similar to those reported in experimental animals (section 7). Motor
and sensory nerve conduction velocities were reduced in proportion to
the degree of paresis. There were lesions of the axons, paranodal axon
swellings, clumping of the nerve filaments and demyelination. The
outbreak of neuropathy came a few months after a change in the
formulation of a solvent from one composed of n-hexane, benzene
fraction, ethyl acetate and toluene to a similar mixture with less
(16%) n-hexane and the addition of 11% MEK. This was interpreted as
evidence that even long exposure to the substantial amount of
n-hexane (31%) in the original formulation did not result in
neuropathy, and that neuropathy developed only after the toxicity of
n-hexane was potentiated by simultaneous exposure to MEK.
The seven cases of polyneuropathy reported from Tampa, Florida,
USA, apparently resulted from a small amount of n-hexane (0.5%)
potentiated by other components in the solvent mixture (Prockop et
al., 1974; Spencer et al., 1980). Two similar cases of polyneuropathy
were reported from Japan (Goto et al., 1974), these were produced by
chronic sniffing of a glue that contained 25% n-hexane and 20% MEK.
What is puzzling in view of the absence of neuropathy in Berlin prior
to the addition of MEK to a solvent mixture containing n-hexane is
that Goto et al. (1974) also reported two cases of neuropathy
resulting from chronic sniffing of glue solvent containing only
n-hexane and toluene. However, Oh & Kim (1976) reported a case of
polyneuropathy produced by chronic abuse of mixtures containing MEK,
methyl isobutyl ketone (MIBK) and many other solvents, but apparently
not n-hexane or MBK. There was, however, no analysis of the solvent
mixtures. There was no experimental evidence to suggest that any of
the solvents known to be present in the mixtures alone, or MEK and
MIBK together, could produce this type of neuropathy. An alternative
explanation is that the MIBK contained MBK as an impurity.
10.1.3.2 Occupational exposure
Although there have been many cases of occupationally related
poisoning by exposure to neurotoxic hexacarbon solvents (Spencer et
al., 1980), poisonings in which neurotoxicity has been associated with
concurrent MEK exposure are limited. In occupational health studies
concentrating on n-hexane neurotoxicity, the study populations were
exposed to several other solvents, including MEK (WHO, 1991). One of
the most thoroughly investigated cases occurred in 1973 in a fabric
factory in Ohio, USA (Allen et al., 1974). In 1973, an employee at the
factory was found to have a severe sensorimotor neuropathy. Other
co-workers at the plant were also found to have similar symptoms,
which initiated a search for a causative agent in the workplace. Of
the 1157 workers examined, 86 manifested signs and symptoms indicative
of peripheral neuropathy, including parathesiae in arms and legs and
weakness in the hands and legs. Subsequent investigation found that in
the year prior to the confirmation of the first case, MBK had been
substituted for MIBK as a co-solvent with MEK. Exposure occurred by
skin contact and by inhalation. Measured concentrations of MEK in
certain areas of greatest exposure were 251-2251 mg/m3 (85-763 ppm),
while concentration for MBK ranged from 9 to 640 mg/m3 (2.3-156
ppm). The introduction of MBK into the factory was associated with the
observed neurotoxicity. Spencer et al. (1980) pointed out that several
animal studies showed that concurrent exposure to MEK accelerated
MBK-induced neurotoxicity.
10.2 Haloalkane solvents
10.2.1 Studies in animals
Carbon tetrachloride (CCl4), chloroform (CHCl3) and related
haloalkane solvents are liver and kidney poisons as well as central
nervous depressants (Gosselin et al., 1984). It has long been known
that the hepatotoxic action of CCl4 is potentiated by ethanol, and
more recently that the hepatic and/or renal toxicity of CCl4,
CHCl3, trichloroethylene, 1,1,2-trichloroethane and related
compounds is potentiated by n-hexane, ethanol, isopropanol, acetone,
MEK, MBK, 2,5-HD, and other ketones or chemicals that are metabolized
to ketones (Hewitt et al., 1980). Even an increase of naturally
occurring ketones in the body via diabetes can precipitate
potentiation. Decreasing the transformation of isopropanol to acetone
by the administration of an inhibitor of alcohol dehydrogenase,
pyrazole, has reduced potentiation of haloalkane toxicity. Although
the phenomenon is referred to as haloalkane toxicity, experimental
work in general appears largely or entirely limited to chlorinated
compounds, and specific studies on MEK are limited to interactions
with CCl4 and CHCl3.
The effects of MEK and CCl4 or CHCl3 on rats are summarized
in Table 17. At the doses used, MEK and the haloalkanes separately
produced mild liver and kidney injury at most. When exposure to MEK
was followed within 10 to 48 h by a haloalkane, there was severe
injury to the liver, with marked and abrupt replacement of normal
hepatic cells by necrotic and fatty, vacuolated tissue, an increase in
hepatic triglyceride, and, presumably, a corresponding decrease in
normal liver function. Hepatic enzymes were released into the blood by
breakdown of liver tissue, resulting in elevated levels of plasma
glutamic-pyruvic transaminase, plasma ornithine carbamyltransferase,
and plasma alanine aminotransferase. There was also an increase in
plasma bilirubin, although this was not accompanied by a decrease in
bile secretion as was the case with some other ketones (Hewitt et al.,
1986). A dose of MEK as small as 0.072 g/kg potentiated the effects of
CHCl3 given 18 h later, and 1.505 g/kg MEK potentiated the effects
of CCl4. Lower doses were not studied.
The mechanism of potentiation is not fully understood but
increased bioactivation of haloalkanes is believed to play a central
part in the potentiation effect. CCl4 is metabolized in vivo with
homolytic cleavage of the carbon-chlorine bond to produce highly
reactive free radicals that exert their toxic effects a) by binding
covalently to proteins and other elements, and b) via lipid
peroxidation (Anders, 1988). It is likely that the toxic effects of
CHCl3 also are produced in part by this mechanism (Hewitt et al.,
1980, 1987). The toxicity of CCl4 is enhanced by pretreatment with
various agents such as phenobarbital, ethanol, isopropanol, 2-butanol
and the ketone metabolites of the last two compounds (acetone and MEK)
(Cornish & Adefuin, 1967; Traiger & Plaa, 1972; Traiger & Bruckner,
1976; Gosselin et al., 1984). Recent studies have shown that the
ethanol-inducible cytochrome P-450 isozyme (P-450IIE1) plays an
important role in haloalkane metabolism (Johansson &
Ingelman-Sundberg, 1985). It is a high affinity enzyme and operates at
a low concentration range for various substrates (Nakajima et al.,
1990). CCl4 hepatotoxicity in rats has been found to be potentiated
by induction of the P-50IIE1 isozyme with ethanol and the injury was
most marked in the perivenous liver cells where the expression of
induction was the highest (Lindros et al., 1990). In addition to
ethanol, P-450IIE1 is known to be induced by acetone and MEK (Ko et
al., 1987; Raunio, et al., 1990; Albano et al., 1991). However, while
a relatively large oral dose of MEK (1.4 ml for 3 days) to rats
increased the amount of ethanol- and phenobarbital-inducible
cytochromes P-450 (P-450IIE1 and P-450IIB, respectively) (Raunio et
al., 1990), inhalation exposure of rats to 1770 mg MEK/m3 (600 ppm),
10 h/day for 7 days, caused only marginal effects on microsomal
cytochrome P-450 activities in the liver (Liira et al., 1991).
10.2.2 Potentiation of haloalkane toxicity in humans
There are no reports of MEK potentiation of haloalkane renal and
hepatic toxicity in humans.
11. EVALUATION OF HUMAN HEALTH RISKS AND EFFECTS ON THE ENVIRONMENT
11.1 Human health risks
11.1.1 Non-occupational exposure
Low level non-occupational exposure to MEK is widespread from a
variety of natural and anthropogenic sources, since MEK is a normal,
though minor, mammalian metabolite. External sources include food,
water and air. In the USA, the average daily per capita intake of MEK
from food is estimated to be 1.6 mg. In addition to MEK present
naturally, foods may contain MEK that has been added in food
processing or absorbed from plastic packaging materials. MEK is rarely
detected in exposed natural waters where it may originate from
microbial activity and atmospheric and anthropogenic inputs, but is
frequently detected at low concentrations in drinking-water where it
presumably is leached from cemented joints of plastic pipes. Leaching
from landfill hazardous waste dumps is another potential source of
groundwater, and hence drinking-water, contamination. Measured
concentrations in food and water are so low, however, that it is
unlikely that either of these represent a significant source of
exposure.
In minimally polluted outdoor air, the MEK concentration is less
than 3 µg/m3 (1.0 ppb) but has been measured at 134 µg/m3 (44.5 ppb)
under conditions of dense smog. Away from industrial areas where
MEK is manufactured or used, major sources may be vehicle exhaust and
photochemical reactions in the atmosphere. In smog episodes,
photochemical production of MEK may greatly exceed direct
anthropogenic emission. Volatilization of MEK from building materials
and consumer products can pollute indoor air to levels far above
outdoor air, and a concentration as high as 48 µg/m3 (12.9 ppb) was
measured in an Italian home. MEK also is present in tobacco smoke
(e.g., 80-207 µg/cigarette).
For the general population, daily MEK intake is estimated to
range between 1.6 and 4.2 mg, depending on the location site (rural or
urban), with an additional 1.6 mg in the case of smokers. There is no
evidence of any adverse effects on the general population from
exposure to MEK. Data from experimental animal studies show that toxic
effects occur at dose levels that are 3 orders of magnitude higher
than the estimated daily intake. Non-occupational poisoning from MEK
alone is limited to a single case, which resulted in no lasting
injury.
MEK is, however, known to potentiate the toxicity of two classes
of organic solvents, unbranched aliphatic hexacarbons and haloalkanes,
and chronic exposure to consumer products containing MEK and
n-hexane have produced outbreaks of polyneuropathy among individuals
deliberately inhaling fumes from these mixtures for their euphoric
effects. Co-exposure to MEK and either hexacarbons or haloalkanes via
the abuse of consumer products remains a potential public health
hazard. Injuries from such poisoning can be severe, permanently
disabling and even fatal.
11.1.2 Occupational exposure
MEK is an important industrial chemical which is used mainly as
a component of solvent mixtures for application of a wide variety of
coatings and adhesives. Moderate occupational exposure via air is
widespread because losses to the environment result mainly from
solvent evaporation from coated surfaces, and MEK is not viewed as an
especially dangerous substance. Most national limits for occupational
exposure are set at 590 mg/m3 (200 ppm), with a higher short-term
exposure level of 885 mg/m3 (300 ppm), and these limits appear
acceptable. On site measurements, however, indicate that workers may
be chronically exposed to still higher MEK concentrations in small
factories such as shoe factories, printing plants and painting
operations, due to inadequate ventilation. Lesser amounts of MEK are
lost to the air with concurrent worker exposure during manufacture,
shipping, repackaging and preparation of coatings and adhesives.
Industrial exposure from contact with liquid MEK does not appear an
important problem.
Chronic co-exposure to MEK and either unbranched aliphatic
hexacarbon or haloalkane solvents represents a significant potential
occupational hazard. Serious toxic effects could occur. Although there
are no records of industrial accidents involving MEK potentiation of
haloalkane toxicity, MEK potentiation of hexacarbon neurotoxicity may
have caused at least one major industrial accident in which an
outbreak of polyneuropathy followed introduction of MEK into a solvent
mixture. Thus MEK, in the mixed solvent atmosphere of many industrial
activities, can present a toxic hazard.
11.1.3 Relevant animal studies
Acute MEK toxicity has been shown in animal studies to be low by
the oral and inhalation route. The lowest oral dose modifying body
structure (damage of kidney tubules) was 1 g/kg body weight in rats.
Ten intraperitoneal injections of 34 mg/kg body weight over a 2-week
period produced transient injection site irritations but no effect on
the kidney. In a 90-day inhalation study, female rats exposed to 14.75
g/m3 for 6 h/day, 5 days per week, showed only slightly increased
liver weight, slightly decreased brain and spleen weight, and slightly
altered blood chemistry in comparison with controls; male rats showed
only a slightly increased liver weight. A transient decrease in nerve
conduction velocity was found following exposure to 590 mg/m3 (12
h/day for 24 weeks). The transient nature of neurological and
behavioural changes induced by MEK may be due to adaptation or more
rapid metabolism of MEK. Short-term dermal exposure to small amounts
of MEK results in mild local irritation, at most. Results of studies
of eye irritation are inconsistent, possibly due to different scoring
techniques; 4 mg created severe chemical burns in the eye in one study
whereas in other studies less severe signs were reported following a
dose of 80 mg.
An inhalation study provided evidence for low level fetotoxicity
in the absence of maternal toxicity at 8825 mg/m3. Thus MEK may be
a low grade teratogen in rats. There is a lack of data on other
aspects of reproduction in animals, and no relevant data have been
reported for humans.
MEK has given negative results in most conventional mutagenicity
assays. There is evidence of aneuploidy in yeast but this may not be
relevant to humans or other mammals.
11.2 Effects on the environment
MEK occurs naturally at low concentrations in air, water and
soil. It is highly mobile in the natural environment and is not
accumulated in any individual compartment. MEK is rapidly synthesized
and destroyed by photochemical processes in the atmosphere. There is
no specific information on either partitioning of MEK in any
environmental compartment or on chemical binding to sediment
particles.
MEK is synthesized biologically and is rapidly metabolized by
bacteria (even at high concentrations), mammals and probably many
other organisms. Levels produced by fungi can cause inhibition of
plant germination. Observations on microorganisms, higher plants,
invertebrates, fish and mammals suggest a low level of toxicity.
Environmental levels of MEK appear to be too low to cause any damage
except in the immediate vicinity of highly polluted sites. Effects on
the aquatic environment are likely to appear at levels between 1 and
10 mg/litre. The potentiation of solvent toxicity by MEK appears
environmentally irrelevant, although substantial information is
lacking. Overall, MEK does not represent a significant threat to the
environment.
12. RECOMMENDATIONS FOR THE PROTECTION OF HUMAN HEALTH AND THE
ENVIRONMENT
12.1 Human health protection
MEK on its own appears a relatively safe organic solvent, but its
use in combination with other solvents, in particular haloalkanes or
unbranched aliphatic hexacarbons, should be avoided. Industries should
be strongly encouraged to take all necessary precautions to ensure
that workers are not exposed to both MEK and solvents whose toxicity
is potentiated by MEK.
12.2 Environmental protection
MEK is unlikely to present a hazard to the environment except in
cases of major spills or discharges.
FURTHER RESEARCH
a) Further research should be undertaken to clarify the precise
mechanisms by which MEK potentiates the toxicity of haloalkanes
and hexacarbons.
b) Epidemiological studies are needed to determine exposure-response
relationships regarding MEK-induced potentiation of hexacarbon
and haloalkane toxicity.
c) Radiolabelled balance studies should be conducted to determine
accurately the routes and rates of excretion of MEK and its
metabolites. The results of such studies would be particularly
useful for improving methods of biological monitoring.
d) Comprehensive studies of reproductive and developmental toxicity
should be undertaken in representative rodent and non-rodent
species.
e) The binding capacity of soils and sediments for MEK should be
assessed.
REFERENCES
Aarstad K, Zahlsen K, & Nilsen OG (1985) Inhalation of butanols:
Changes in the cytochrome P-450 enzyme system. Arch Toxicol, 8(Suppl):
418-421.
Aarstad K, Zahlsen K, & Nilsen OG (1986) [Effects of inhalation of
different butanol isomers.] Faerg Lack Scand, 32: 69-74 (in Norwegian
with English summary).
Abdel-Rahman MS, Hetland LB, & Couri D (1976) Toxicity and metabolism
of methyl n-butyl ketone. Am Ind Hyg Assoc J, 37: 95-102.
ACGIH (1986) Documentation of the threshold limit values and
biological exposure indices, 5th ed. Cincinnati, Ohio, American
Conference of Governmental Industrial Hygienists, p 395.
ACGIH (1991) Threshold limit values and biological exposure indices
for 1988-1989. Cincinnati, Ohio, American Conference of Governmental
Industrial Hygienists.
Ahrenholz SH & Egilman DS (1983) Health hazard evaluation: Rubbermaid
Incorporated, Wooster, Ohio. Cincinnati, Ohio, National Institute of
Occupational Safety and Health, 33 pp (HETA 3-1340).
Albano E, Tomasi A, Persson J-O, Terelius Y, Goria-Gatti L,
Ingelman-Sundberg M, & Diazani MU (1991) Role of ethanol-inducible
cytochrome P-450 (P450IIE1) in catalysing the free radical activation
of aliphatic alcohols. Biochem Pharmacol, 41: 1895-1902.
Alderson MR & Rattan NS (1980) Mortality of workers on an isopropyl
alcohol plant and two MEK dewaxing plants. Br J Ind Med, 37: 85-89.
Allen N, Mendell JR, Billmaier D, & Fontaine RE (1974) An outbreak of
a previously undescribed toxic polyneuropathy due to industrial
solvent. Trans Am Neurol Assoc, 99: 74-79.
Altenkirch H, Mager J, Stoltenburg G, & Helmbrecht J (1977) Toxic
polyneuropathies after sniffing a glue thinner. J Neurol, 214:
137-152.
Altenkirch H, Stoltenburg G, & Wagner HM (1978) Experimental studies
on hydrocarbon neuropathies induced by methyl-ethyl-ketone (MEK). J
Neurol, 219: 159-170.
Altenkirch H, Stoltenburg-Didinger G, & Wagner HM (1979) Experimental
data on the neurotoxicity of methyl-ethyl-ketone (MEK).
Experientia(Basel), 35: 503-504.
Altenkirch H, Wagner HM, Stoltenburg G, & Spencer PS (1982a) Nervous
system responses of rats to subchronic inhalation of n-hexane and
n-hexane + methyl-ethyl-ketone mixtures. J Neurol Sci, 57: 209-219.
Altenkirch H, Wagner HM, Stoltenburg-Didinger G, & Steppat R (1982b)
Potentiation of hexacarbon-neurotoxicity by methyl-ethyl-ketone (MEK)
and other substances: Clinical and experimental aspects. Neurobehav
Toxicol Teratol, 4: 623-627.
Anders MW (1988) Bioactivation mechanisms and hepatocellular damage.
In: Arias IM, Jakoby WB, Popper H, Schachter D, & Schafritz DA ed. The
liver: Biology and pathology. New York, Raven Press Ltd, pp 389-400.
Anderson C, Sundberg K, & Groth O (1986) Animal model for assessment
of skin irritancy. Contact Dermatitis, 15: 143-151.
ANONYMOUS (1978) Atmospheric hydrocarbons in air during photochemical
episodes. Kanagawa-Ken Taike Osen Chosa Kenkyu Hokoku, 20: 86-90.
Anshelm Olson B, Gamberale F, & Grönqvist B (1981) Reaction time
changes among steel workers exposed to solvent vapours: A longitudinal
study. Int Arch Occup Environ Health, 48: 211-218.
Arques Espi E & Quintanilla Almagro T (1981) [Study of gluing stations
in 50 shoe plants: Simplified method of risk evaluation and normal
work technique.] In: [Proceedings of the National Congress of
Occupational Medicine, Hygiene and Safety: 9th meeting, 1980.] Madrid,
Spain, Occupational Hygiene and Safety Service, vol 2, pp 551-561 (in
Spanish).
Attygalle AB, Cammaerts MC, & Morgan ED (1983) Dufour gland secretions
of Myrmica rugulosa and Myrmica schencki workers. J Insect
Physiol, 29: 27-32.
Azuma K, Hirata T, Tsunoda H, Ishitani T, & Tanaka Y (1983)
Identification of the volatiles from low density polyethylene film
irradiated with an electron beam. Agric Biol Chem, 47: 855-860.
Banergee S & Howard PH (1988) Improved estimation of solubility and
partitioning through correction of UNIFAC-derived activity
coefficients. Environ Sci Technol, 22: 839-841.
Basler A (1986) Aneuploidy-inducing chemicals in yeast evaluated by
the micronucleus test. Mutat Res, 174: 11-13.
Bassette R & Ward G (1975) Measuring parts per billion of volatile
materials in milk. J Dairy Sci, 58: 428-429.
Basu P, Carpenter J, Nelson H, Perry W, Shochet A, Sun C, & Taylor D
(1981) Toxic Substances Control Act (TSCA), Section 4 - Human exposure
assessment: methyl ethyl ketone. McLean, Virginia, JRB Associates,
pp 1/1-R/1 (EPA Contract No. 68-01-4839).
Berg EF (1971) Retrobulbar neuritis. Ann Ophthalmol, 3: 1351, 1353.
Bernard AM, De Russis R, Normand J-C, & Lauwerys RR (1989) Evaluation
of the subacute nephrotoxicity of cyclohexane and other industrial
solvents in the female Sprague-Dawley rat. Toxicol Lett, 45: 271-280.
Bills DD, Willits RE, & Day EA (1966) Determination of some neutral
volatile constituents in ten commercial Cheddar cheeses. J Dairy Sci,
49: 681-685.
Binaschi S, Gazzaniga G, & Crovato E (1976) Behavioral toxicology in
the evaluation of the effects of solvent mixtures. Adverse Eff Environ
Chem Psychotropic Drugs, 2: 91-98.
Blum M, Warter S, & Traynham J (1966) Chemical releasers of social
behavior - VI. The relation of structure to activity of ketones as
releasers of alarm for Iridomyrex pruinosus (Roger). J Insect
Physiol, 12: 419-427.
Blum M, Warter S, & Traynham J (1971) Alarm pheromones: utilization in
evaluation of olfactory theories. J Insect Physiol, 17: 2351-2361.
Boch R & Shearer D (1971) Chemical releasers of alarm behavior in the
honey-bee, Apis mellifera. J Insect Physiol, 17: 2277-2285.
Boettner EA, Ball GL, Hollingsworth Z, & Aquino R (1981) Organic and
organotin compounds leached from PVC and CPVC pipe. Ann Arbor,
Michigan, US Environmental Protection Agency, 116 pp
(EPA-600/1-81-062).
Botta D, Castellani Pirri L, & Mantica E (1984) Ground water pollution
by organic solvents and their microbial degradation products. In:
Analysis of organic micropollutants in water. Brussels, Commission of
the European Communities, pp 261-275 (Report EUR 8518).
Bradow JM & Connick WJ (1988) Volatile methyl ketone seed-germination
inhibitors from Amaranthus palmeri S Wats. residues. J Chem Ecol,
14: 1617-1631.
Bradow JM & Connick WJ (1990) Volatile seed germination inhibitors
from plant residues. J Chem Ecol, 16: 645-666.
Brady JF, Li D, Ishizaki H, Lee M, Ning SM, Xiao F, & Yang CS (1989)
Induction of cytochromes P450 IIE1 and P450IIB1 by secondary ketones
and the role of P450IIE1 in chloroform metabolism. Toxicol Appl
Pharmacol, 100: 342-349.
Bridie AL, Winter M, & Wolff CJM (1979a) BOD and COD of some
petrochemicals. Water Res, 13(7): 627-630.
Bridie AL, Wolff CJM, & Winter M (1979b) The acute toxicity of some
petrochemicals to goldfish. Water Res, 13: 623.
Bringmann G (1978) [Determination of the biological toxicity of water
pollutants on Protozoa.] Z Wasser Abwasser Forsch, 11: 210-215 (in
German).
Bringmann G & Kuhn R (1977a) [Toxicity threshold for water pollutants
in the cell multiplication test with respect to bacteria (Pseudomonas
putida) and green algae (Scenedesmus quadricauda).] Z Wasser
Abwasser Forsch, 10: 87-98 (in German).
Bringmann G & Kuhn R (1977b) [The effects of water pollutants on
Daphnia magna.] Z Wasser Abwasser Forsch, 10: 161-166 (in German).
Bringmann G & Kuhn R (1978) Testing of substances for their toxicity
threshold: Model organisms Microcystis (Diplocystis) aeruginosa and
Scenedesmus quadricauda. Mitt Int Ver Limnol, 21: 275-284.
Brondeau MT, Ban M, Bonnet P, Guenier JP, & De Ceaurriz J (1989)
Acetone compared to other ketones in modifying the hepatotoxicity of
inhaled 1,2-dichlorobenzene in rats and mice. Toxicol Lett, 49: 69-78.
Brooks TM, Meyer AL, & Hutson DH (1988) The genetic toxicology of some
hydrocarbon and oxygenated solvents. Mutagenesis, 3: 227-232.
Brown EM & Hewitt WR (1984) Dose-response relationships in
ketone-induced potentiation of chloroform hepato- and nephrotoxicity.
Toxicol Appl Pharmacol, 76: 437-453.
Brown RH & Purnell CJ (1979) Collection and analysis of trace organic
vapour pollutants in ambient atmospheres. The performance of a
Tenax-GC absorbent tube. J Chromatogr, 178: 79-90.
Brugnone F (1985) Uptake of solvents from the lungs. Br J Ind Med,
42: 569.
Brugnone F, Perbellini L, Gaffuri E, & Costa G (1981) [Occupational
pollution by shoe factory solvents.] Ann Ist Super Sanità, 17: 531-534
(in Italian).
Brugnone F, Perbellini L, Apostoli P, Caretta D, & Cocheo V (1983)
Environmental and behavioural monitoring of occupational methyl ethyl
ketone exposure. Dev Sci Pract Toxicol, 11: 571-574.
Bryce DJ & Greenwood CT (1963) The thermal degradation of starch. Part
III. The formation of decomposition products from starch and related
materials at temperatures between 175 and 400 °C. Stärke, 15(19):
359-363.
Cammaerts MC, Inwood MR, Morgan ED, Parry K, & Tyler RC (1978)
Comparative study of the pheromones emitted by workers of the ants
Myrmica rubra and Myrmica scabrinodis. J Insect Physiol, 24:
207-214.
Carpenter CP & Smyth HF (1946) Chemical burns of the rabbit cornea. Am
J Ophthamol, 29: 1363-1372.
Cavender FL, Casey HW, Salem H, Swenberg JA, & Gralla EJ (1983) A
90-day vapor inhalation toxicity study of methyl ethyl ketone. Fundam
Appl Toxicol, 3: 264-270.
Chemical Business Newsbase (1986) #537712 [Organic chemicals
production in Argentina 1985.] Ind Quim, 281: 36-37 (in Spanish).
Chemical Business Newsbase (1987) #584370 The chemical industry of
Japan, 1986: B-B fraction derivatives. Japan Chem Ann, 1987/1988: 41.
Chemical Business Newsbase (1988) #600843 Methyl ethyl ketone makers
need Pampa for recovery. Chem Mark Rep, 234(16): 9, 18-19.
Chen T-H, Kavanagh TJ, Chang CC, & Trosko JE (1984) Inhibition of
metabolic cooperation in Chinese hamster V79 cells by various organic
solvents and simple compounds. Cell Biol Toxicol, 1: 155-171.
Chiavari G, Facchini MC, & Fuzzi S (1987) Behavior of
3-methyl-2-benzothiazolone azines of carbonyl compounds in
high-performance liquid chromatography. J Chromatogr, 387: 459-466.
Chiou CT, Freed VH, Siddigi RH, & Mckeon K (1977) Partition
coefficients and bioaccumulation of selected organic chemicals.
Environ Sci Technol, 11: 475-478.
Clayton GD & Clayton FE (1981) Patty's industrial hygiene and
toxicology. New York, John Wiley & Sons, Wiley Interscience, 3 vols.
Cohen SR & Maier AA (1974) Occupational health case report - No. 2,
toluene diisocyanate. J Occup Med, 16: 114-118.
Conkle JP, Camp BJ, & Welch BE (1975) Trace composition of human
respiratory gas. Arch Environ Health, 30: 290-295.
Connick WJ, Bradow JM, & Legendre MG (1989) Identification and
bioactivity of volatile allelochemicals from amaranth residues.
J Agric Food Chem, 37: 792-796.
Cornish HH & Adefuin J (1967) Potentiation of carbon tetrachloride
toxicity by aliphatic alcohols. Arch Environ Health, 14: 447-449.
Corwin JF (1969) Volatile oxygen-containing organic compounds in sea
water: Determination. Bull Mar Sci, 19: 504-509.
Couri D, Hetland LB, O'Neill JJ, Ganansia M-F, Jackson DB, Gardier RW,
Marks BH, Weiss H, Mendell JR, Saida K, Allen N, & Chrisman CL (1974)
Comments on a plastics industry neurotoxicity in relationship to
methylbutyl ketone. In: Proceedings of the 5th Annual Conference on
Environmental Toxicology, Fairborn, Ohio, 24-26 September 1974. Wright
Patterson Air Force Base, Ohio, Aerospace Medical Research Laboratory,
pp 109-120 (AMRL Technical Report No. 74-125).
Couri D, Hetland LB, Abdel-Rahman MS, & Weiss H (1977) The influence
of inhaled ketone solvent vapors on hepatic microsomal
biotransformation activities. Toxicol Appl Pharmacol, 41: 285-289.
Couri D, Abdel-Rahman MS, & Hetland LB (1978) Biotransformation of
n-hexane and methyl n-butyl ketone in guinea-pigs and mice. Am Ind Hyg
Assoc J, 39: 295-300.
Creech G, Johnson RT, & Stoffer JO (1982) A comparison of three
different high-performance liquid chromatography systems for the
determination of aldehydes and ketones in diesel exhaust. Part I.
J Chromatogr Sci, 20: 67-72.
Cresci A, La Rosa F, Pannelli F, Orpianesi C, Saltamacchia G, Spinaci
G, & Pierini M (1985) [Solvent exposure and work sites in eleven shoe
and leather factories.] Nuovi Ann Ig Microbiol, 36: 61-76 (in
Italian).
Curtis C, Lima A, Lozano SJ, & Veith GD (1982) Evaluation of a
bacterial bioluminescence bioassay as a method for predicting acute
toxicity of organic chemicals to fish. In: Pearson JG, Foster RB, &
Bishop WE ed. Aquatic toxicology and hazard assessment: Fifth
Conference. Philadelphia, Pennsylvania, American Society for Testing
and Materials, pp 170-178 (ASTM STP 766).
Cushny AR (1910) On the exhalation of drugs by the lungs. J Physiol,
40: 17-27.
Davis PL, Munroe KA, & Selhime AG (1977) Laboratory bioassay of
volatile naturally occurring compounds against the Caribbean fruit
fly. Citrus Ind, July: 24-26.
Day EA, Bassette R, & Keeney M (1960) Identification of volatile
carbonyl compounds from Cheddar cheese. J Dairy Sci, 43: 463-474.
Deacon MM, Pilny MD, John JA, Schwetz BA, Murray FJ, Yakel HO, & Kuna
RA (1981) Embryo- and fetotoxicity of inhaled methyl ethyl ketone in
rats. Toxicol Appl Pharmacol, 59: 620-622.
De Bortoli M, Knoppel H, Pecchio E, Peil A, Rogora L, Schauenburg H,
Schlitt H, & Vissers H (1985) Measurements of indoor air quality and
comparison with ambient air: A study on 15 homes in northern Italy.
Ispra, Italy, Commission of the European Communities, 57 pp.
De Bortoli M, Knoppel H, Pecchio E, Peil A, Rogora L, Schauenburg H,
Schlitt H, & Vissers H (1986) Concentrations of selected organic
pollutants in indoor and outdoor air in northern Italy. Environ Int,
12: 343-350.
Decaprio AP (1987) n-Hexane neurotoxicity: A mechanism involving
pyrrole adduct formation in axonal cytoskeletal protein.
Neurotoxicology, 8: 199-210.
De Ceaurriz J, Desilies JP, Bonet P, Marignac B, Muller J, & Guenier
JP (1983) Concentration-dependent behavioral changes in mice
following short-term inhalation exposure to various industrial
solvents. Toxicol Appl Pharmacol, 67: 383-389.
Decker DW, Clark CS, Elia VJ, Kominsky JR, & Trapp JH (1983) Worker
exposure to organic vapors at a liquid chemical waste incinerator. Am
Ind Hyg Assoc J, 44: 296-300.
Delfino JJ & Miles CJ (1985) Aerobic and anaerobic degradation of
organic contaminants in Florida groundwater. Soil Crop Sci Soc Florida
Proc, 44: 9-14.
Del Rosario R, De Lumen BO, Habu T, Flath RA, Mon TR, & Teranishi R
(1984) Comparison of headspace volatiles from winged beans and
soybeans. J Agric Food Chem, 32: 1011-1015.
Denkhaus W, Von Steldern D, Botzenhardt U, & Konietzko H (1986)
Lymphocyte subpopulations in solvent-exposed workers. Int Arch Occup
Environ Health, 57: 109-115.
De Rosa E, Bartolucci GB, Brighenti F, Gori GP, Sigon M, & Toffolo D
(1985) The industrial use of solvents and risk of neurotoxicity. Ann
Occup Hyg, 29(1): 391-397.
Descotes J (1988) Identification of contact allergens: The mouse ear
sensitization assay. J Toxicol Cutan Ocular Toxicol, 7: 263-272.
Deveaux M & Huvenne JP (1987) Identification of solvents of abuse
using gas chromatography/Fourier transform infrared spectrometry after
headspace sampling. Chromatographia, 23: 626-630.
Dick RB, Setzer JV, Wait R, Hayden MB, Taylor BJ, Tolos B, &
Putz-Anderson V (1984) Effects of acute exposure of toluene and
methyl ethyl ketone on psychomotor performance. Int Arch Occup Environ
Health, 54: 91-109.
Dick RB, Brown WD, Setzer JV, Taylor BJ, & Shukla R (1988) Effects of
short duration exposures to acetone and methyl ethyl ketone. Toxicol
Lett, 43: 31-49.
Dick RB, Setzer JV, Taylor BJ, & Shukla R (1989) Neurobehavioral
effects of short duration exposures to acetone and methyl ethyl
ketone. Br J Ind Med, 46: 111-121.
Dietz FK & Traiger GJ (1979) Potentiation of CCl4 of hepatotoxicity
in rats by a metabolite of 2-butanone: 2,3-butanediol. Toxicology,
14: 209-215.
Dietz FK, Rodriguez-Giaxola M, Traiger GJ, Stella VJ, & Himmelstein KJ
(1981) Pharmacokinetics of 2-butanol and its metabolites in the rat.
J Pharmacokinet Biopharm, 9: 553-576.
Digiacomo JD (1973) New approaches for the design of afterburners for
varnish cookers. J Air Pollut Control Assoc, 23: 287-290.
Dilling WL, Bredeweg DJ, & Tefertiller NB (1976) Organic
photochemistry. XIII. Simulated atmospheric photodecomposition rates
of methylene chloride, 1,1,1-trichloro-ethane, tetrachloroethylene,
and other compounds. Environ Sci Technol, 10: 351-356.
Divincenzo GD & Krasavage WJ (1974) Serum ornithine carbamyl
transferase as a liver response test for exposure to organic solvents.
Am Ind Hyg Assoc J, 35: 21-29.
Divincenzo GD, Kaplan CJ, & Dedinas J (1976) Characterization of the
metabolites of methyl-N-butyl ketone, methyl iso-butyl ketone, and
methyl ethyl ketone in guinea-pig serum and their clearance. Toxicol
Appl Pharmacol, 36: 511-522.
Divincenzo GD, Hamilton ML, Kaplan CJ, Krasavage WJ, & O'Donoghue JL
(1978) Studies on the respiratory uptake and excretion and the skin
absorption of methyl-n-butyl ketone in humans and dogs. Toxicol Appl
Pharmacol, 44: 593-604.
Dojlido JR (1977) Testing of biodegradability and toxicity of organic
compounds in industrial wastewaters. In: Polish/US Symposium on
Wastewater Treatment and Sludge Disposal, Cincinnati, Ohio, 10-12
February 1976. Cincinnati, Ohio, US Environmental Protection Agency,
vol II, pp 112-131 (EPA 600/9-76-021; NTIS PB-261422).
Dojlido JR (1979) Investigations of biodegradability and toxicity of
organic compounds. Washington, DC, US Environmental Protection Agency,
99 pp (EPA-600/2-79-163).
Dore M, Brunet N, & Legube B (1975) Participation of various organic
compounds in the evaluation of global pollution criteria. Trib
Cebedeau, 28: 3-11.
Dornseifer TP, Kim SC, Keith ES, & Powers JJ (1965) Effect of moisture
level on volatile carbonyls in cottonseed oil heated to 210 °C. J Am
Oil Chem Soc, 42: 1073-1075.
Dowty BJ, Laseter JL, & Storer J (1976) The transplacental migration
and accumulation in blood of volatile organic constituents. Pediatr
Res, 10: 696-701.
Draize JH, Woodard G, & Calvery HO (1944) Methods for the study of
irritation and toxicity of substances applied topically to the skin
and mucous membranes. J Pharmacol Exp Ther, 82: 377-389.
Duckett S, Williams N, & Francis S (1974) Peripheral neuropathy
associated with inhalation of methyl-n-butyl ketone.
Experientia(Basel), 30: 1283-1284.
Dutch Expert Committee for Occupational Standards (1991) Health-based
recommended occupational exposure limit for methyl ethyl ketone.
Voorburg, Directorate General of Labour of the Ministry of Social
Affairs and Employment, Dutch Expert Committee for Occupational
Standards, 45 pp (Report RA 16/90).
Dyro FM (1978) Methyl ethyl ketone polyneuropathy in shoe factory
workers. Clin Toxicol, 13: 371-376.
Egyud LG (1967) Studies on cell division: the effect of aldehydes,
ketones and a-keto-aldehydes on the proliferation of Escherichia
coli. Curr Mod Biol, 1: 14-20.
Elskamp CJ & Schultz GR (1983) An alternative sampling and analytical
method for 2- butanone. Am Ind Hyg Assoc J, 44: 201-204.
Emmel TE, Lee BB, & Simonson AB (1983) Control technology assessment
of petroleum refinery operations. In depth site visit report: Congo
Refinery, Farmer's Valley Refinery. Cincinnati, Ohio, National
Institute of Occupational Safety and Health, 60 pp (Contract No.
210-81-7102).
Ewing BB, Chian ESK, Cook JK, Evans CA, Hopke PK, & Perkins EG (1977)
Monitoring to detect previously unrecognized pollutants in surface
waters. Appendix: Organic analysis data. Washington, DC, US
Environmental Protection Agency, p 75 (EPA- 560/6-77-015).
Fagius J & Grönqvist B (1978) Function of peripheral nerves and signs
of polyneuropathy in solvent-exposed workers at Swedish steelworks.
Acta Neurol Scand, 57: 305-316.
Falla NAR (1987) Solving air pollution problems in the surface
coatings industry. Polym Paint Colour J, 98: 103-104, 106, 108, 110.
Feigley CE & Chastain JB (1982) An experimental comparison of three
diffusion samplers exposed to concentration profiles of organic
vapors. Am Ind Hyg Assoc J, 43: 227-234.
Fernandes MH, Gilbert SG, Paik SW, & Stier EF (1986) Study of the
degradation products formed during extrusion lamination of an ionomer.
J Food Sci, 51: 722-725.
Fiserova-Bergerova V & Diaz ML (1986) Determination and prediction of
tissue-gas partition coefficients. Int Arch Occup Environ Health,
58: 75-87.
Fisher GS, Legendre MG, Lovgren NV, Schuller WH, & Wells JA (1979)
Volatile constituents of southernpea seed [ Vigna unguiculata (L.)
Walp.] J Agric Food Chem, 27: 7-11.
Fisk JF (1986) Volatile organic analytical methods - general
description and quality control considerations. In: Perket CL ed.
Quality control in remedial site investigation: Fifth volume -
Hazardous and industrial solid waste testing. Philadelphia,
Pennsylvania, American Society for Testing and Materials, 12 pp (ASTM
STP 925).
Florin I, Rutberg L, Curvall M, & Enzell CR (1980) Screening of
tobacco smoke constituents for mutagenicity using the Ames' test.
Toxicology, 18: 219-232.
Freddi A, Paci A, Vittori O, De Ciantis R, & Ottaviano PF (1982)
[Clinical and electromyographic study of workers exposed to methyl
ethyl ketone vapor.] Ann Med Perugia, 73: 111-136 (in Italian).
French RC (1961) Stimulation of uredospore germination in wheat stem
rust by terpenes and related compounds. Bot Gaz, 122: 194-198.
French RC (1984) Stimulation of uredospore germination of Puccinia
helianthi and Uromyces vignae by aromatic nitriles and related
flavorlike compounds. J Agric Food Chem, 32: 556-561.
French RC, Graham CL, Gale AW, & Long RK (1977) Structural and
exposure time requirements for chemical stimulation of germination of
uredospores of Uromyces phaseoli. J Agric Food Chem, 25: 84-88.
Gadomski RR, Gimborne AV, & Green WJ (1974) An evaluation of emissions
and control technologies for the metal decorating process. J Air
Pollut Control Assoc, 24: 579-585.
Garcia CR, Geller I, & Kaplan HL (1978) Effects of ketones on
lever-pressing behavior of rats. Proc West Pharmacol Soc, 21: 433-438.
Geller I, Martinez RL, Hartmann RJ, & Kaplan HL (1978) Effects of
ketones on a match to sample task in the baboon. Proc West Pharmacol
Soc, 21: 439-442.
Gerhold RM & Malaney GW (1966) Structural determinants in the
oxidation of aliphatic compounds by activated sludge. J Water Pollut
Control Fed, 38: 562-579.
Ghittori S, Imbriani M, Pezzagno G, & Capodaglio E (1987) The urinary
concentration of solvents as a biological indicator of exposure:
Proposal for the biological equivalent exposure limit for nine
solvents. Am Ind Hyg Assoc J, 48: 786-790.
Gianturco MA, Giammarino S, & Friedel P (1966) Volatile constituents
of coffee-V. Nature (Lond), 210(5043): 1358.
Glowa JR & Dews PB (1987) Behavioral toxicology of volatile organic
solvents. IV. Comparisons of the rate-decreasing effects of acetone,
ethyl acetate, methyl ethyl ketone, toluene, and carbon disulfide on
schedule-controlled behavior of mice. J Am Coll Toxicol, 6: 461-469.
Gordon DT & Morgan ME (1972) Principal volatile compounds in feed
flavored milk. J Dairy Sci, 55: 905-912.
Gosselin RE, Smith RP, & Hodge HC (1984) Clinical toxicology of
commercial products. Baltimore, Maryland, Williams and Wilkins, pp
III/101-III/107.
Goto I, Matsumura M, Inoue N, Murai Y, Shida K, Santa T, & Kuroiwa Y
(1974) Toxic polyneuropathy due to glue sniffing. J Neurol Neurosurg
Psychiatry, 37: 848-853.
Grey TC & Shrimpton DH (1967) Volatile components of raw chicken
breast muscle. Br Poult Sci, 8: 23-33.
Grosjean D (1982) Formaldehyde and other carbonyls in Los Angeles
ambient air. Environ Sci Technol, 16: 254-262.
Grosjean D & Wright B (1983) Carbonyls in urban fog, ice, cloudwater
and rainwater. Atmos Environ, 17: 2093-2096.
Grosjean D, Swanson RD, & Ellis C (1983) Carbonyls in Los Angeles air:
Contribution of direct emissions and photochemistry. Sci Total
Environ, 29: 65-85.
Gurka DF, Warner JS, Silvon LE, Bishop TA, & Mckown MM (1984) Interim
method for determination of volatile organic compounds in hazardous
wastes. J Assoc Off Anal Chem, 67: 776-782.
Hampton CV, Pierson WR, Harvey TM, Updegrove WS, & Marano RS (1982)
Hydrocarbon gases emitted from vehicles on the road. 1. A qualitative
gas chromatography/mass spectrometry survey. Environ Sci Technol, 16:
287-298.
Harvey RJ & Walker JRL (1960) Some volatile compounds in New Zealand
Cheddar cheese and their possible significance in flavour formation.
III Time of first appearance of volatile carbonyl compounds during
ripening. J Dairy Res, 27: 335-340.
Hawthorne SB, Sievers RE, & Barkley RM (1985) Organic emissions from
shale oil wastewaters and their implications for air quality. Environ
Sci Technol, 19: 992-997.
Hazleton (1963a) Primary skin irritation-rabbits. Falls Church,
Virginia, Hazleton Laboratories Inc. (Laboratory Report No. 63 MRL
003).
Hazleton (1963b) Acute eye irritation-rabbits. Falls Church, Virginia,
Hazleton Laboratories Inc. (Laboratory Report No. 63 MRL 004).
Heitmuller PT, Hollister TA, & Parrish PR (1981) Acute toxicity of 54
industrial chemicals to sheepshead minnows (Cyprinodon variegatus).
Bull Environ Contam Toxicol, 27: 596-604.
Hewitt WR, Miyajima H, Cote MG, & Plaa GL (1980) Modification of
haloalkane- induced hepatotoxicity by exogenous ketones and metabolic
ketosis. Fed Proc, 39: 3118-3123.
Hewitt WR, Brown EM, & Plaa GL (1983) Relationship between the carbon
skeleton length of ketonic solvents and potentiation of
chloroform-induced hepatotoxicity in rats. Toxicol Lett, 16: 297-304.
Hewitt LA, Ayotte P, & Plaa GL (1986) Modifications in rat
hepatobiliary function following treatment with acetone, 2-butanone,
2-hexanone, mirex, or chlordecone and subsequently exposed to
chloroform. Toxicol Appl Pharmacol, 83: 465-473.
Hewitt LA, Valiquette C, & Plaa GL (1987) The role of
biotransformation-detoxication in acetone-, 2-butanone-, and
2-hexanone-potentiated chloroform-induced hepatotoxicity. Can J
Physiol Pharmacol, 65: 2313-2318.
Hickey JLS & Bishop CC (1981) Field comparison of charcoal tubes and
passive vapor monitors with mixed organic vapors. Am Ind Hyg Assoc J,
42: 264-267.
Higgins CE, Griest WH, & Olerich G (1983) Application of Tenax
trapping to analysis of gas phase organic compounds in ultra-low tar
cigarette smoke. J Assoc Off Anal Chem, 66: 1074-1083.
Holmberg I & Malmfors T (1974) The cytotoxicity of some organic
solvents. Environ Res, 7: 183-192.
Horton AW, Bingham EL, Burton MJG, & Tye R (1965) Carcinogenesis of
the skin. III. The contribution of elemental sulfur and of organic
sulfur compounds. Cancer Res, 25: 1759-1763.
Hoshika Y, Kozima I, Koike K, & Yosimoto K (1976) [Gas chromatographic
analysis of lower aliphatic carbonyl compounds using the imine
formation reaction.] Aichi-ken Kogai Chosa Senta Shoho, 4: 108-113 (in
Japanese).
Hou CT, Patel R, Laskin AI, Barnabe N, & Barist I (1983) Production of
methyl ketones from secondary alcohols by cell suspensions of C2 to
C4 n-alkane-grown bacteria. Appl Environ Microbiol, 46: 178-184.
Hrdlicka J & Kuca J (1965) The changes of carbonyl compounds in the
heat-processing of meat: 2, Turkey meat. Poult Sci, 44: 27-31.
Husman K (1980) Symptoms of car painters with long-term exposure to a
mixture of organic solvents. Scand J Work Environ Health, 6: 19-32.
Husman K & Karli P (1980) Clinical neurological findings among car
painters exposed to a mixture of organic solvents. Scand J Work
Environ Health, 6: 33-39.
Imai T, Takigawa H, Nakagawa S, Shen GJ, Kodama T, & Minoda Y (1986)
Microbial oxidation of hydrocarbons and related compounds by
whole-cell suspensions of the methane-oxidizing bacterium H-2. Appl
Environ Microbiol, 52: 1403-1406.
Ingram L (1977) Changes in lipid composition of Escherichia coli
resulting from growth with organic solvents and with food additives.
Appl Environ Microbiol, 33: 1233-1236.
Inoue T, Takeuchi Y, Hisanaga N, Ono Y, Iwata M, Ogata M, Saito K,
Sakurai H, Hara I, Matsushita T, & Ikeda M (1983) A nationwide survey
on organic solvent components in various solvent products: Part 1.
Homogeneous products such as thinners, degreasers and reagents. Ind
Health, 21: 175-183.
IRPTC (1987) Methyl ethyl ketone. In: IRPTC legal file 1986. Geneva,
International Register of Potentially Toxic Chemicals, United Nations
Environment Programme, vol I, pp 11/238-11/240.
IRPTC (1991) IRPTC legal file. Geneva, International Register of
Potentially Toxic Chemicals, United Nations Environment Programme,
pp 17/1-17/5.
Iwata M, Takeuchi Y, Hisanaga N, & Ono Y (1983) Changes of n-hexane
metabolites in urine of rats exposed to various concentrations of
n-hexane and to its mixture with toluene or MEK. Int Arch Occup
Environ Health, 53: 1-8.
Iwata M, Takeuchi Y, Hisanaga N, & Ono Y (1984) Changes of n-hexane
neurotoxicity and its urinary metabolites by long-term co-exposure
with MEK or toluene. Int Arch Occup Environ Health, 54: 273-281.
Jacot BJ (1983) OVA field screening at hazardous waste sites. In:
National Conference on Management of Uncontrolled Hazardous Waste
Sites, Washington, 31 October-2 November 1983. Silver Spring,
Maryland, Hazardous Materials Control Research Institute, pp 76-78.
Jarke FH, Dravnieks A, & Gordon SM (1981) Organic contaminants in
indoor air and their relation to outdoor contaminants. Am Soc Heat
Refrig Air-Cond Eng Trans, 87: 153-166.
Johansson I & Ingelman-Sundberg M (1985) Carbon tetrachloride-induced
lipid peroxidation dependent on an ethanol-inducible form of rabbit
liver microsomal cytochrome P-450. FEBS Lett, 183: 265-269.
John JA, Pilny MK, Kuna RA, Deacon MM, & Yakel HO (1980) Teratogenic
evaluation of methyl ethyl ketone in the rat. Teratology, 21(3): 47A.
Jonsson A, Persson KA, & Grigoriadis V (1985) Measurements of
low-molecular-weight oxygenated, aromatic, and chlorinated
hydrocarbons in ambient air and in vehicle emissions. Environ Int,
11: 383-392.
JRB Associates Inc (1980) Support document, chapter IV. Health effects
of methyl ethyl ketone. Washington, DC, US Environmental Protection
Agency, 58 pp (Contract No. 68-01-4839, Task No. 11, Project No.
2-800-08-107-04).
Juhnke I & Luedemann D (1978) [Results of the investigation of 300
chemical compounds for acute toxicity with the Golden Orfe test.]
Z Wasser Abwasser Forsch, 11: 161-164 (in German).
Jungclaus GA, Lopez-Avila V, & Hites RA (1978) Organic compounds in an
industrial wastewater: A case study of their environmental impact.
Environ Sci Technol, 12: 88-96.
Kahn JH, Laroe EG, & Conner HA (1968) Whiskey composition:
identification of components by single-pass gas chromatography - mass
spectrometry. J Food Sci, 33: 395-400.
Kamil IA, Smith JN, & Williams RT (1953) Studies in detoxification.
46. The metabolism of aliphatic alcohols. The glucuronic acid
conjugation of acyclic aliphatic alcohols. Biochem J, 53: 129-135.
Kaye S (1961) Emergency toxicology. Springfield, Illinois, Charles C.
Thomas, pp 214-216.
Keen AR, Walker NJ, & Peberdy MF (1974) The formation of 2-butanone
and 2-butanol in Cheddar cheese. J Dairy Res, 41: 249-257.
Kenny J & Stratton G (1989) An improved desorption solvent for organic
solvent mixtures. Am Ind Hyg Assoc J, 50: 431-434.
Kezic S & Monster AC (1988) Determination of methyl ethyl ketone and
its metabolites in urine using capillary gas chromatography.
J Chromatogr, 428: 275-280.
Khasanov AA, Isidorov VA, Zenkevich IG, & Ioffee BV (1982) [Gas
chromatography/ mass spectrometry study of the composition of volatile
emissions from Juniperus serawechanica Kom.] Dokl Akad Nauk Uzb SSR,
12: 29-31 (in Russian).
Kimura ET, Ebert DM, & Dodge PW (1971) Acute toxicity and limits of
solvent residue for sixteen organic solvents. Toxicol Appl Pharmacol,
19: 699-704.
Ko I-Y, Park SS, Song BJ, Patten CH, Tan Y, Hah YC, Yang CS, & Gelboin
HV (1987) Monoclonal antibodies to ethanol-induced rat liver
cytochrome P-450 that metabolizes aniline and nitrosamines. Cancer
Res, 47: 3101-3109.
Kobayashi K, Tanaka M, & Kawai S (1980) Gas chromatography
determination of low-molecular-weight carbonyl compounds in aqueous
solution as their O-(2,3,4,5,6,-penta-fluorobenzyl) oximes.
J Chromatogr, 187: 413-417.
Kontominas MG & Voudouris E (1982) The determination of residual
solvents in plastics packaging materials in relation to off odors
developed in packaged bakery products. Chem Chron, 11: 215-223.
Kopelman PG & Kalfayan PY (1983) Severe metabolic acidosis after
ingestion of butanone. Br Med J, 286: 21-22.
Krasavage WJ, O'Donoghue JL, & Divincenzo GD (1982) Ketones. In:
Clayton GD & Clayton FE ed. Patty's industrial hygiene and toxicology,
3rd ed. New York, John Wiley and Sons, Wiley Interscience,
pp 4709-4800.
Kulshrestha DC & Marth EH (1974a) Inhibition of bacteria by some
volatile and non-volatile compounds associated with milk. I.
Escherichia coli. J Milk Food Technol, 37: 510-516.
Kulshrestha DC & Marth EH (1974b) Inhibition of bacteria by some
volatile and non-volatile compounds associated with milk. II.
Salmonella typhimurium. J Milk Food Technol, 37: 539-544.
Kulshrestha DC & Marth EH (1974c) Inhibition of bacteria by some
volatile and non-volatile compounds associated with milk. III.
Staphylococcus aureus. J Milk Food Technol, 37: 545-550.
Kulshrestha DC & Marth EH (1974d) Inhibition of bacteria by some
volatile and non-volatile compounds associated with milk. IV.
Streptococcus lactis. J Milk Food Technol, 37: 593-599.
Kulshrestha DC & Marth EH (1974e) Inhibition of bacteria by some
volatile and non-volatile compounds associated with milk. V.
Leuconostoc citrovorum. J Milk Food Technol, 37: 600-606.
Kulshrestha DC & Marth EH (1974f) Inhibition of bacteria by some
volatile and non-volatile compounds associated with milk. VI.
Streptococcus thermophilus. J Milk Food Technol, 37: 606-611.
Kupferschmid LL & Perkins JL (1986) Organic solvent recycling plant
exposure levels. Appl Ind Hyg, 1: 122-124.
Kwan W & Gatehouse A (1978) The effects of low doses of three
insecticides on activity, feeding, mating, reproductive performance
and survival in Glossina morsitans mortistans (Glossinidae). Entomol
Exp Appl, 23: 201-221.
La Belle CW & Brieger H (1955) The vapor toxicity of a composite
solvent and its principal compounds. Arch Ind Health, 12: 623-627.
Laity JL, Burstain IG, & Appel BR (1973) Photochemical smog and the
atmospheric reactions of solvents. In: Tess RW ed. Solvents, theory
and practice. Washington, DC, American Chemical Society, vol 7,
pp 96-112 (Advances in Chemistry Series 124).
Lande SS, Durkin PR, Christopher DH, Howard PH, & Saxena J (1976)
Investigation of selected potential environmental contaminants:
Ketonic solvents. Washington, DC, US Environmental Protection Agency,
330 pp (EPA-560/2-76-003).
Laregina J & Bozzelli JW (1986) Volatile organic compounds at
hazardous waste sites and a sanitary landfill in New Jersey. Environ
Prog, 5: 18-27.
Larson PS, Finnegan JK, & Haag HB (1955) Observations on the effect of
chemical configuration on the edema-producing potency of acids,
aldehydes, ketones and alcohols. J Pharmacol, 116: 119-122.
Leblanc G (1980) Acute toxicity of priority pollutants to water flea
(Daphnia magna). Bull Environ Contam Toxicol, 24: 684-691.
Leblanc G (1984) Interspecies relationships in acute toxicity of
chemicals to aquatic organisms. Environ Toxicol Chem, 3: 47-60.
Lee S & Murphy D (1982) Health hazard evaluation: Medalist-Gladiator
Athletic Products Company, Leesburg, Florida. Cincinnati, Ohio,
National Institute of Occupational Safety and Health, 15 pp (HETA
82-030-1184).
Lee S & Parkinson D (1982) Health hazard evaluation: Rola-Esmark
Company; Dubois, Pennsylvania. Cincinnati, Ohio, National Institute of
Occupational Safety and Health, 26 pp (HETA 80-168-1204).
Levin JO & Carleborg L (1987) Evaluation of solid sorbents for
sampling ketones in work-room air. Ann Occup Hyg, 31: 31-38.
Levy A (1973) The photochemical smog reactivity of organic solvents.
In: Tess RW ed. Solvents, theory and practice. Washington, DC,
American Chemical Society, vol 6, pp 71-93 (Advances in Chemistry
Series 124).
Li G-L, Yin S-N, Watanabe T, Nakatsuka H, Kasahara M, Abe H, & Ikeda
M (1986) Benzene-specific increase in leucocyte alkaline phosphatase
activity in rats exposed to vapors of various organic solvents. J
Toxicol Environ Health, 19: 581-589.
Liebich HM, Bertsch W, Zlatkis A, & Schneider HJ (1975) Volatile
organic components in the Skylab 4 spacecraft atmosphere. Aviat Space
Environ Med, 46: 1002-1007.
Liepins R, Mixon F, Hudak C, & Parsons TB (1977) Industrial process
profiles for environmental use: Chapter 6. The industrial organic
chemicals industry. Research Triangle Park, North Carolina, US
Environmental Protection Agency, pp 164-165, 560-561 (EPA-600/12).
Liira J, Riihimäki V, Engstrom K, & Pfaffli P (1988a) Coexposure of
man to m-xylene and methyl ethyl ketone, kinetics and metabolism.
Scand J Work Environ Health, 14: 322-327.
Liira J, Riihimäki V, & Pfaffli P (1988b) Kinetics of methyl ethyl
ketone in man: absorption, distribution and elimination in inhalation
exposure. Int Arch Occup Environ Health, 60: 195-200.
Liira J, Johanson G, & Riihimäki V (1990a) Dose-dependent kinetics of
inhaled methylethylketone in man. Toxicol Lett, 50: 195-201.
Liira J, Riihimäki V, & Engstrom K (1990b) The effects of ethanol on
the kinetics of methyl ethyl ketone in man. Br J Ind Med, 47: 325-330.
Liira J, Elovaara E, Raunio H, Riihimäki V, & Engstrom K (1991)
Metabolic interaction and disposition of methyl ethyl ketone and
m-xylene in rats at single and repeated inhalation exposures.
Xenobiotica, 21: 53-63.
Lin JCC & Jeon IJ (1985) Headspace gas sampling/GC method for the
quantitative analysis of volatile compounds in cheese. J Food Sci, 50:
843-844, 846.
Lindros KO, Cai Y, & Penttila KE (1990) Role of ethanol-inducible
cytochrome P-450IIE1 in carbon tetrachloride-induced damage to
centrilobular hepatocytes from ethanol-treated rats. Hepatology,
12: 1092-1097. Lowenheim FA & Moran MK (1975) Industrial chemicals, 4th
ed. New York, Chichester, Brisbane, Toronto, John Wiley and Sons,
pp 11-12, 539-542.
Lundberg I & Hakansson M (1985) Normal serum activities of liver
enzymes in Swedish paint industry workers with heavy exposure to
organic solvents. Br J Ind Med, 42: 596-600.
Lundberg I, Ekdahl M, Kronevi T, Vitauts L, & Lundberg S (1986)
Relative hepatotoxicity of some industrial solvents after
intraperitoneal injection or inhalation exposure in rats. Environ Res,
40: 411-420.
Mabuchi H (1969) [Urinary and serum volatile substances in diabetics.]
Nippon Naika Gakkai Zasshi, 58: 731-743 (in Japanese).
Manville Chemical Products Corporation (1988) Chemical products
synopsis, Asbury Park, New Jersey, Manville Chemical Products
Corporation, 1 p.
Marion CV & Malaney GW (1963) The oxidation of aliphatic compounds by
Alcalignes faecalis. J Water Pollut Control Fed, 35: 1269-1281.
Marnett LJ, Hurd HK, Hollstein MC, Levin DE, Esterbauer H, & Ames BN
(1985) Naturally occurring carbonyl compounds are mutagens in
Salmonella tester strain TA104. Mutat Res, 148: 25-34.
Mast TJ, Dill JA, Evanoff JJ, Rommereim RL, Weigel RJ, & Westerberg RB
(1989) Inhalation developmental toxicology studies: teratology study
of methyl ethyl ketone in mice. Richland, Washington, Pacific Norwest
Laboratory (Report No. NIH-YOI-ES-70153).
Mayer VW & Goin CJ (1987) Effects of chemical combinations on the
induction of aneuploidy in Saccharomyces cerevisiae. Mutat Res,
187: 21-30.
Mayer VW & Goin CJ (1988) Investigations of aneuploidy-inducing
chemical combinations in Saccharomyces cerevisiae. Mutat Res,
201: 413-421.
MB Research Laboratories, Inc. (1979) Test for eye irritation in
rabbits. Spinnerstown, Pennsylvania, MB Research Laboratories, Inc.,
5 pp (Unpublished report No. MB 79-3962).
Metcalf RL, Lu P, & Kapoor IP (1973) Environmental distribution and
metabolic fate of key industrial pollutants and pesticides in a model
ecosystem. Urbana, Illinois, University of Illinois, Water Research
Center (Report No. 69, Project B-050 Ill).
Mezey E (1976) Ethanol metabolism and ethanol-drug interactions.
Biochem Pharmacol, 25: 869-875.
Middleditch BS, Sung NJ, Zlatkis A, & Settembre G (1987) Trace
analysis of volatile polar organics by direct aqueous injection gas
chromatography. Chromatographia, 23: 273-278.
Misumi J & Nagano M (1985) Experimental study on the enhancement of
the neurotoxicity of methyl n-butyl ketone by non-neurotoxic aliphatic
monoketones. Br J Ind Med, 42: 155-161.
Miyasaka M, Kumai M, Koizumi A, Watanabe T, Kurasako K, Sato K, &
Ikeda M (1982) Biological monitoring of occupational exposure to
methyl ethyl ketone by means of urinalysis for methyl ethyl ketone
itself. Int Arch Occup Environ Health, 50: 131-137.
Mohren S & Juttner F (1983) Odorous compounds of different strains of
Anabaena and Nostoc (Cyanobacteria). Water Sci Technol, 15: 221-228.
Mookherjee RE, Deck RE, & Chang SS (1965) Relationship between
monocarbonyl compounds and flavor of potato chips. J Agric Food Chem,
13(2): 131-134.
Munies R & Wurster DE (1965) Investigation of some factors influencing
percutaneous absorption--III. Absorption of methyl ethyl ketone.
J Pharm Sci, 54: 1281-1284.
Murphy DC (1984) Acute illness among workers connected to solvent
exposure. Occup Health Saf, May: 36-38.
Mutti A, Cavatorta A, Lucertini S, Arfini G, Falzoi M, & Franchini I
(1982a) Neurophysiological changes in workers exposed to organic
solvents in a shoe factory. Scand J Work Environ Health, 8(Suppl 1):
136-141.
Mutti A, Ferri F, Lommi G, Lotta S, Lucertini S, & Franchini I (1982b)
n-Hexane induced changes in nerve conduction velocities and
somatosensory evoked potentials. Int Arch Environ Health, 51: 45-54.
Nakaaki K (1974) [An experimental study on the effect of exposure to
organic solvent vapor in human subjects.] J Sci Labour 50: 89-96 (in
Japanese).
Nakajima T, Wang R-S, Murayama N, & Sato A (1990) Three forms of
trichloro-ethylene-metabolizing enzymes in rat liver induced by
ethanol, phenobarbital, and 3-methylcholanthrene. Toxicol Appl
Pharmacol, 102: 546-552.
Nandi B & Fries N (1976) Volatile aldehydes, ketones, esters and
terpenoids as preservatives against storage fungi in wheat.
Z Pflanzenkr Pflanzenschutz, 83: 284-294.
Nestmann ER, Lee EGH, Matula TI, Douglas GR, & Mueller JG (1980)
Mutagenicity of constituents identified in pulp and paper mill
effluents using the Salmonella/mammalian-microsome assay. Mutat Res,
79: 203-212.
Ng H, Reed DJ, & Pence JW (1960) Identification of carbonyl compounds
in an ethanol extract of fresh white bread. Cereal Chem, 37: 638-645.
Nilsen OG & Toftgard R (1980) The influence of organic solvents on
cytochrome P-450- mediated metabolism of biphenyl and benzo(a)pyrene.
In: Coon MJ, Conney AH, Estabrook RW, Gelboin HV, Gillete JR, &
O'Brien PJ ed. Microsomes, drug oxidations, and chemical
carcinogenesis. New York, Academic Press, vol II, pp 1235-1238.
Noma E, Berglund B, Berglund U, Johansson I, & Baird JC (1988) Joint
representation of physical locations and volatile organic compounds in
indoor air from a healthy and a sick building. Atmos Environ,
22: 451-460.
Nye D (1975) Laboratory model ecosystem studies of the degradation and
fate of radiolabelled tri-, tetra-, and pentachlorobiphenyl compared
with DDE. Arch Environ Contam Toxicol, 3: 151-165.
O'Donoghue JL, Krasavage WJ, Divincenzo GD, & Katz GV (1984) Further
studies on ketone neurotoxicity and interactions. Toxicol Appl
Pharmacol, 72: 201-209.
O'Donoghue JL, Haworth SR, Curren RD, Kirby PE, Lawlor T, Moran EJ,
Phillips RD, Putnam DL, Rogers-Back AM, Slesinski RS, & Thilagar A
(1988) Mutagenicity studies on ketone solvents: methyl ethyl ketone,
methyl isobutyl ketone, and isophorone. Mutat Res, 206: 149-161.
Ogawa I & Fritz JS (1985) Determination of low concentrations of
low-molecular-weight aldehydes and ketones in aqueous samples.
J Chromatogr, 329: 81-89.
Oh SJ & Kim JM (1976) Giant axonal swelling in "hufferis" neuropathy.
Arch Neurol, 33: 583-586.
Ong CN, Sia GL, Ong HY, Phoon WH, & Tan KT (1991) Biological
monitoring of occupational exposure to methyl ethyl ketone. Int Arch
Occup Environ Health, 63: 319-324.
Osborne JS, Adamek S, & Hobbs ME (1956) Some components of gas phase
of cigarette smoke. Anal Chem, 28: 211-215.
Panson RD & Winek CL (1980) Aspiration toxicity of ketones. Clin
Toxicol, 17: 271-317.
Papa AJ & Sherman PD (1978) Ketones. In: Kirk RE & Othmer DF ed.
Encyclopedia of chemical technology, 3rd ed. New York, John Wiley and
Sons, Wiley-Interscience, vol 13, pp 894-912, 934-941.
Patel RN, Hou CT, & Laskin AI (1982) Oxidation of gaseous hydrocarbons
and related compounds by methanotrophic organisms. Dev Ind Microbiol,
23: 187-205.
Patel RN, Hou CT, Laskin AI, Felix A, & Derelanka P (1983) Oxidation
of alkanes by organisms grown on C2-C4 alkanes. J Appl Biochem,
5: 107-120.
Patty FA, Schrenk HH, & Yant WP (1935) Acute response of guinea pigs
to vapors of some new commercial organic compounds. VIII. Butanone. US
Public Health Rep, 50: 1217-1228.
Pellizzari ED, Castillo NP, Willis S, Smith D, & Bursey JT (1979)
Identification of organic components in energy related processes. In:
Van Hall CE ed. Measurement of organic pollutants in water and
wastewater: Proceedings of a symposium, Denver, Colorado, 19-20 June
1978. Philadelphia, Pennsylvania, American Society for Testing and
Materials, pp 256-274 (ASTM STP 686).
Pellizzari ED, Sheldon LS, Bursey JT, Michael LC, & Zweidinger RA
(1985) Master analytical scheme for organic compounds in water.
Athens, Georgia, US Environmental Protection Agency, pp 46-54, 108-127
(EPA/600/4-84-010A).
Perbellini L, Brugnone F, Mozzo P, Cocheo V, & Caretta D (1984) Methyl
ethyl ketone exposure in industrial workers. Uptake and kinetics. Int
Arch Occup Environ Health, 54: 73-81.
Perbellini L, Bartolucci G, Brugnone F, & De Rosa E (1985)
[2,5-Hexanedione in biological monitoring of occupational exposure to
n-hexane.] Med Lav, 76: 35-43 (in Italian).
Perocco P, Bolognesi S, & Alberghini W (1983) Toxic activity of
seventeen industrial solvents and halogenated compounds on human
lymphocytes cultured in vitro . Toxicol Lett, 16: 69-75.
Perry JJ (1968) Substrate specificity in hydrocarbon utilizing
micro-organisms. Antonie Van Leeuwenhoek, 34: 27-36.
Persson U, Lundqvist S, Marthinsson B, & Eng ST (1984)
Computer-automated carbon dioxide laser long-path absorption system
for air quality monitoring in the working environment. Appl Opt,
23: 998-1002.
Pezzagno G, Ghottori S, Imbriani M, & Capodaglio E (1983) [Measurement
of the solubility coefficients of gases and vapors in blood. II.
Widely used industrial solvents.] G Ital Med Lav, 5: 49-63 (in
Italian).
Poli G, Dianzani MU, Cheeseman KH, Slater TF, Lang J, & Esterbauer H
(1985) Separation and characterization of the aldehydic products of
lipid peroxidation stimulated by carbon tetrachloride or ADP-iron in
isolated rat hepatocytes and rat liver microsomal suspensions. Biochem
J, 227: 629-638.
Price K, Waggy G, & Conway R (1974) Brine shrimp bioassay and seawater
BOD of petrochemicals. J Water Pollut Control Fed, 46: 63-76.
Prockop LD, Alt M, & Tison J (1974) Huffer's neuropathy. J Am Med
Assoc, 229: 1083-1084.
Przyrembel H, Bremer HJ, Duran M, Bruinvis L, Ketting D, Wadman SK,
Baumgartner R, Irle U, & Bachmann C (1979) Propionyl-CoA carboxylase
deficiency with overflow of metabolites of isoleucine catabolism at
all levels. Eur J Pediatr, 130: 1-14.
Puskar MA, Levine SP, & Lowery SR (1986) Computerized infrared
spectral identification of compounds frequently found at hazardous
waste sites. Anal Chem, 58: 1156-1162.
Ralston WH, Hilderbrand RL, Uddin DE, Andersen ME, & Gardier RW (1985)
Potentiation of 2,5-hexanedione neurotoxicity by methyl ethyl ketone.
Toxicol Appl Pharmacol, 81: 319-327.
Ramsey JD & Flanagan RJ (1982) Detection and identification of
volatile organic compounds in blood by headspace gas chromatography as
an aid to the diagnosis of solvent abuse. J Chromatogr, 240: 423-444.
Raunio H, Liira J, Elovaara E, Riihimäki V, & Pelkonin O (1990)
Cytochrome P450 isozyme induction by methyl ethyl ketone and n-xylene
in rat liver. Toxicol Appl Pharmacol, 103: 175-179.
Reilly B (1988) Engineering assessment: Methyl ethyl ketone (MEK) and
methyl isobutyl ketone (MIBK) environmental releases. Washington, DC,
US Environmental Protection Agency, 20 pp (Unpublished document).
Reynolds T (1977) Comparative effects of aliphatic compounds on
inhibition of lettuce fruit germination. Am Bot 41: 637-648.
Riggin RM (1984) Compendium of methods for the determination of toxic
organic compounds in ambient air. Method T05. Research Triangle Park,
North Carolina, US Environmental Protection Agency, pp 1-22
(EPA-600/4-84-041).
Robertson P, White EL, & Bus JS (1989) Effects of methyl ethyl ketone
pretreatment on hepatic mixed function oxidose activity and on in
vivo metabolism of n-hexane. Xenobiotica, 19: 721-729.
Rose-Pehrsson SL, Grate JW, Ballentine DS, & Jurs PC (1988) Detection
of hazardous vapors including mixtures using pattern recognition
analysis of responses from surface acoustic wave devices. Anal Chem,
60: 2801-2811.
Ruth JH (1986) Odor thresholds and irritation levels of several
chemical substances: A review. Am Ind Hyg Assoc J, 47: 142-151.
Saida K, Mendell JR, & Weiss HS (1976) Peripheral nerve changes
induced by methyl n-butyl ketone and potentiation by methyl ethyl
ketone. J Neuropathol Exp Neurol, 35: 207-225.
Sakakibara H, Ide Y, Yanai T, Yajima J, & Hayashi K (1990) Volatile
flavour compounds of some kinds of dried and smoked fish. Agric Biol
Chem, 54: 9-16.
Sato A & Nakajima T (1979) Partition coefficients of some aromatic
hydrocarbons and ketones in water, blood and oil. Br J Ind Med,
36: 231-234.
Sato Y, Watanabe K, & Tanaka Y (1968) Chemical studies on smelling
compounds in hen's egg: Part I, Volatile carbonyl and basic compounds
in egg white. Agric Biol Chem, 32(4): 405-411.
Sauer TC (1981) Volatile liquid hydrocarbon characterization of
underwater hydrovents and formation waters from offshore production
operations. Environ Sci Tech, 15: 917-923.
Sawhney BL & Kozloski RP (1984) Organic pollutants in leachates from
landfill sites. J Environ Qual, 13: 349-352.
Schmidt R, Schnoy N, Altenkirch H, & Wagner HM (1984) Ultrastructural
alteration of intrapulmonary nerves after exposure to organic
solvents. Respiration, 46: 362-369.
Schnoy N, Schmidt R, Altenkirch H, & Wagner HM (1982) Ultrastructural
alteration of the alveolar epithelium after exposure to organic
solvents. Respiration, 43: 221-231.
Schörmuller J & Grosch W (1964) Aromatics of foods. II Occurrence of
additional carbonyl compounds in the tomato. Z Lebensmittel Unters
Forsch, 126: 38-49.
Schultz TH, Flath RA, Stern DJ, Mon TR, Teranishi R, Kruse SM, Butler
B, & Howard WE (1988) Coyote estrous urine volatiles. J Chem Ecol,
14: 701-712.
Schulz F, Mueller P, & Kramer D (1981) [Model experiments on the
phytotoxic effect of organic constituents in wastewater from lignite
refining in soil treatment.] Arch Acker-Pflanzenbau Bodenkd,
25: 745-753 (in German)
Schwetz BA, Leong BKJ, & Gehring PJ (1974) Embryo- and fetotoxicity of
inhaled carbon tetrachloride, 1,1-dichloroethane and methyl ethyl
ketone in rats. Toxicol Appl Pharmacol, 28: 452-464.
Schwetz BA, Mast TJ, Weigel RJ, Dill JA, & Morrissey RE (1991)
Developmental toxicity of inhaled methyl ethyl ketone in Swiss mice.
Fundam Appl Toxicol, 16: 742-748.
Seinfeld JH (1989) Urban air pollution: State of the science. Science,
243: 745-752.
Seizinger DE & Dimitriades B (1972) Oxygenates in exhaust from simple
hydrocarbon fuels. J Air Pollut Control Assoc, 22: 47-51.
Shah JJ & Singh HB (1988) Distribution of volatile organic chemicals
in outdoor and indoor air. Environ Sci Technol, 22: 1381-1388.
Shibata E, Huang J, Ono Y, Hisanaga N, Iwata M, Saito I, & Takeuchi Y
(1990a) Changes in urinary n-hexane metabolites by co-exposure to
various concentrations of methyl ethyl ketone and fixed n-hexane
levels. Arch Toxicol, 64: 165-168.
Shibata E, Huang J, Hisanaga Y, Ono Y, Saito I, & Takeuchi Y (1990b)
Effects of MEK on kinetics of n-hexane metabolites in serum. Arch
Toxicol, 64: 247-250.
Shimizu Y, Matsuto S, Ito T, & Okada I (1969) Studies on the flavor of
roast barley (Mugi-Cha): Part III. Separation and identification of
volatile mono-carbonyl compounds. Nippon Nogei Kagaku Kaishi,
43(4): 217-223 (in Japanese with English summary).
Smith LL (1981) Cholesterol autoxidation. New York, London, Plenum
Press, 674 pp.
Smith AR & Mayers MR (1944) Study of poisoning and fire hazards of
butanone and acetone. NY State Dept Labor Ind Bull, April: 174-176.
Smith AF & Wood R (1972) A field test for the determination of some
ketone vapors in air. Analyst, 97: 363-371.
Smyth HF, Carpenter CP, Weil CS, Pozzani UC, & Striegel JA (1962)
Range-finding toxicity data: list VI. Am Ind Hyg Assoc J, 23: 95-107.
Snider JR & Dawson GA (1985) Tropospheric light alcohols, carbonyls,
and acetonitrile: concentrations in the southwestern United States
and Henry's law data. J Geophys Res D Atmos, 90: 3797-3805.
Sosulski F & Mahmoud RM (1979) Effects of protein supplements on
carbonyl compounds and flavor in bread. Cereal Chem, 56: 533-536.
Specht H, Miller JW, Valaer PJ, & Sayers RR (1940) Acute response of
guinea-pigs to the inhalation of ketone vapors. Bull Natl Inst Health,
176: 1-66.
Spencer PS & Schaumburg HH (1976) Feline nervous system response to
chronic intoxication with commercial grades of methyl n-butyl ketone,
methyl isobutyl ketone, and methyl ethyl ketone. Toxicol Appl
Pharmacol, 37: 301-311.
Spencer PS, Schaumburg HH, Sabri MI, & Veronesi B (1980) The enlarging
view of hexacarbon neurotoxicity. CRC Crit Rev Toxicol, 7: 279-356.
SRI International (1985) Directory of chemical producers, Western
Europe, 8th ed. Menlo Park, California, SRI International, p 1457;
suppl II, p 55.
SRI International (1988) Directory of chemical producers, United
States. Menlo Park, California, SRI International, p 780.
Staüble EJ & Rast D (1971) [Volatile material in Agaricus biosporus.]
Experientia(Basel), 27: 886-888 (in German).
Stofberg J & Grundschober F (1984) Consumption ratio and food
predominance of flavoring materials--second cumulative series. Perfum
Flavorist, 9: 53-56, 58-59, 62, 65-72, 76-83.
Takeuchi Y, Ono Y, Hisanaga N, Iwata M, Aoyama M, Kitoh J, & Sugiura
Y (1983) An experimental study of the combined effects of n-hexane and
methyl ethyl ketone. Br J Ind Med, 40: 199-203.
Tangredi G, Carbone U, Rossi L, & Galdi A (1981) [Environmental
conditions in a paint factory and effects on employee health.] Riv Med
Lav Ig Ind, 5(Oct.-Dec.): 325-337 (in Italian).
Tham R, Bunnfors I, Eriksson B, Larsby B, Lindgrem S, & Odkvist LM
(1984) Vestibulo-ocular disturbances in rats exposed to organic
solvents. Acta Pharmacol Toxicol, 54: 58-63.
Thoburn T & Gunter B (1982) Health hazard evaluation; Davis Monthan
Air Force Base, Tucson, Arizona. Cincinnati, Ohio, National Institute
of Occupational Safety and Health, 23 pp (HETA 81-257-1115).
Toftgard R, Nilsen OG, & Gustafsson J-A (1981) Changes in rat liver
microsomal cytochrome P-450 and enzymatic activities after the
inhalation of n-hexane, xylene, methyl ethyl ketone and
methylchloroform for four weeks. Scand J Work Environ Health, 7:
31-37.
Toxigenics (1981) 90-day vapour inhalation toxicity study of methyl
ethyl ketone in albino rats. Decatur, Illinois, Toxigenics Inc. (Study
420-0305).
Traiger GJ & Bruckner JV (1976) The participation of 2-butanone in
2-butanol-induced potentiation of carbon tetrachloride hepatotoxicity.
J Pharmacol Exp Ther, 196: 493-499.
Traiger GJ & Plaa GL (1972) Relationship of alcohol metabolism to the
potentiation of CCl4 hepatotoxicity induced by aliphatic alcohols.
J Pharmacol Exp Ther, 183: 481-488.
Traiger GJ, Bruckner JV, Jiang WD, Dietz FK, & Cooke PH (1989) Effect
of 2-butanol and 2-butanone on rat hepatic ultrastructure and drug
metabolizing enzyme activity. J Toxicol Environ Health, 28: 235-248.
Triebig G, Bestler W, Baumeister P, & Valentin H (1983)
[Investigations on neurotoxicity of chemical substances at the
workplace. IV. Determination of the motor and sensory nerve conduction
velocity in persons occupationally exposed to a mixture of organic
solvents.] Int Arch Occup Environ Health 52: 139-150 (in German).
Tsao MU & Pfeiffer EI (1957) Isolation and identification of a new
ketone body in normal human urine. Proc Soc Exp Biol Med, 94: 628-629.
Tsukamoto S, Chiba S, Ishikawa T, & Shimaura M (1985a) Experimental
study on the metabolism of volatile hydrocarbons by inhalation of
natural gas. Nihon Univ J Med, 27: 33-38.
Tsukamoto S, Chiba S, Muto T, Ishakawa T, & Shimamura M (1985b) Study
on the metabolism of volatile hydrocarbons in mice--propane, n-butane,
and isobutane. J Toxicol Sci, 10: 323-332.
Union Carbide Corp. (1980a) The acute toxicity of MRD-80-2 to the
bluegill sunfish Lepomis macrochirus Rafinesque. Tarrytown, New York,
Union Carbide Corp., Environmental Services, 8 pp.
Union Carbide Corp. (1980b) The acute toxicity of MRD-80-2 to the
water flea Daphnia magna Straus. Tarrytown, New York, Union Carbide
Corp., Environmental Services, 6 pp.
US EPA (1985a) Health assessment document for chloroform. Research
Triangle Park, North Carolina, US Environmental Protection Agency,
pp 3-40-3-42 (EPA/600/8-84/004F).
US EPA (1985b) Health and environmental effects profile for methyl
ethyl ketone. Cincinnati, Ohio, US Environmental Protection Agency,
71 pp (EPA/600/X-85/363).
US EPA (1986) Health effects assessment for methyl ethyl ketone.
Cincinnati, Ohio, US Environmental Protection Agency, 17 pp
(EPA/540/1-86/003).
US NIOSH (1984a) Analytical method 2500: 2-butanone. Cincinnati, Ohio,
National Institute of Occupational Safety and Health, 3 pp.
US NIOSH (1984b) Analytical method 8002: (1)2-butanone, (2) ethanol
and (3) toluene in blood. Cincinnati, Ohio, National Institute for
Occupational Safety and Health, 4 pp.
US NIOSH (1990) Pocket guide to chemical hazards. Cincinnati, Ohio,
National Institute of Occupational Safety and Health, 48 pp (DHEW
(NIOSH) Publication No. 90-117).
Urano K & Kato Z (1986) Evaluation of biodegradation ranks of priority
organic compounds. J Hazard Mater, 13: 147-159.
USITC (1981) Synthetic organic chemicals: United States production and
sales, 1980. Washington, DC, United States International Trade
Commission, p 263 (USITC Publication No. 1183).
USITC (1982) Synthetic organic chemicals: United States production and
sales, 1981. Washington, DC, United States International Trade
Commission, p 243 (USITC Publication No. 1292).
USITC (1983) Synthetic organic chemicals: United States production and
sales, 1982. Washington, DC, United States International Trade
Commission, p 259 (USITC Publication No. 1422).
USITC (1984) Synthetic organic chemicals: United States production and
sales, 1983. Washington, DC, United States International Trade
Commission, p 257 (USITC Publication No. 1588).
USITC (1985) Synthetic organic chemicals: United States production and
sales, 1984. Washington, DC, United States International Trade
Commission, p 256 (USITC Publication No. 1745).
USITC (1986) Synthetic organic chemicals: United States production and
sales, 1985. Washington, DC, United States International Trade
Commission, p 266 (USITC Publication 1892).
USITC (1987) Synthetic organic chemicals: United States production and
sales, 1986. Washington, DC, United States International Trade
Commission, p 210 (USITC Publication No. 2009).
USITC (1988) Synthetic organic chemicals: United States production and
sales, 1987. Washington, DC, United States International Trade
Commission, p 15-5 (USITC Publication No. 2118).
Uspenskii IV & Repkina L (1974) [Physiological age and sensitivity to
DDT of natural populations of Ixodes persulcatus.] Parazitologiya,
8: 3-11 (in Russian).
Vale GA, Lovemore DF, Flint S, & Cockbill GF (1988) Odour-baited
targets to control tsetse flies, Glossina spp. (Diptera:
Glossinidae) in Zimbabwe. Bull Entomol Res, 78: 31-49.
Van Doorn JE, De Cock J, Kezic S, & Monster AC (1989) Determination of
methyl ethyl ketone in human urine after derivatization with
o-nitrophenylhydrazine, using solid-phase extraction and
reversed-phase liquid chromatography and ultraviolet detection.
J Chromatogr, 489: 419-424.
Varigos GA & Nurse DS (1986) A case of contact urticaria caused by
methyl ethyl ketone. Contact Dermatitis, 15(4): 249-260.
Veith GD, Call DJ, & Brooke LT (1983) Structure-toxicity relationships
for the fathead minnow, Pimephales promelas: Narcotic industrial
chemicals. Can J Fish Aquat Sci, 40: 743-748.
Verhoeff AP, Wilders MMW, Monster AC, & Van Wijnen JH (1987) Organic
solvents in the indoor air of two small factories and surrounding
houses. Int Arch Occup Environ Health, 59: 153-163.
Veronesi B (1984) An ultrastructural study of methyl ethyl ketone's
effect on cultured nerve tissues. Neurotoxicology, 5: 31-44.
Veronesi B, Lington AW, & Spencer PS (1984) A tissue culture model of
methyl ethyl ketone's potentiation of n-hexane neurotoxicity.
Neurotoxicology, 5: 43-52.
Verschuren K (1983) Handbook of environmental data on organic
chemicals. New York, Van Nostrand Reinhold Company, pp 850-852.
Viader F, Lechevalier B, & Morin P (1975) Polynéurite toxique chez un
travailleur du plastique. Rôle possible du méthyl éthyl-cétone. Nouv
Presse Méd, 4: 1813-1814.
Volskay VT & Grady CPL (1988) Toxicity of selected RCRA compounds to
activated sludge microorganisms. J Water Pollut Control Fed,
60: 1850-1856.
Von Cremer E & Riedmann M (1964) Identification of
gas-chromatographically separated aromatic materials in honey.
Z Naturforsch B19: 76-77.
Wahlberg JE (1984a) Edema-inducing effects of solvents following
topical administration. Dermatosen, 3: 91-94.
Wahlberg JE (1984b) Erythema-inducing effects of solvents following
epicutaneous administration to man - studied by laser Doppler
flowmetry. Scand J Work Environ Health, 10: 159-162.
Wallen I, Greer W, & Lasater R (1957) Toxicity to Gambusia affinis
of certain pure chemicals in turbid waters. Sewage Ind Wastes,
29: 265-711.
Walton BT, Anderson TA, Hendricks MS, & Talmage SS (1989)
Physicochemical properties as predictors of organic chemical effects
on soil microbial respiration. Environ Toxicol Chem, 8: 53-63.
Wang TC & Bricker JL (1979) 2-butanone and tetrahydrofuran
contamination in the water supply. Bull Environ Contam Toxicol,
23: 620-623.
Weast RC ed. (1986) CRC handbook of chemistry and physics, 67th ed.
Boca Raton, Florida, CRC Press, p C-170.
Weil CS & Scala RA (1971) Study of intra- and interlaboratory
variability in the results of rabbit eye and skin irritation tests.
Toxicol Appl Pharmacol, 19: 276-360.
Wen CP, Tsai SP, Weiss NS, Gibson RL, Wong O, & Mcclellan WA (1985)
Long-term mortality study of oil refinery workers. IV. Exposure to the
lubricating-dewaxing process. J Natl Cancer Inst, 74: 11-18.
Whelan JK, Tarafa ME, & Hunt JM (1982) Volatile C1-C8 organic
compounds in macroalgae. Nature(Lond), 299: 50-52.
Whitehead LW, Ball GL, Fine LJ, & Langolf GD (1984) Solvent vapor
exposures in booth spray painting and spray gluing, and associated
operations. Am Ind Hyg Assoc J, 45: 767-772.
Windholz M ed. (1983) The Merck index, 10th ed. Rahway, New Jersey,
Merck & Co., p 870.
Wong NP & Patton S (1962) Identification of some volatile compounds
related to the flavor of milk and cream. J Dairy Sci, 45: 724-728.
Wong NP, Damico JN, & Salwin H (1967) Decomposition and filth in
foods: Investigation of volatile compounds in cod fish by gas
chromatography and mass spectrometry. J Assoc Off Anal Chem,
50(1): 8-15.
WHO (1991) Environmental Health Criteria 122: n-Hexane. Geneva, World
Health Organization, 164 pp.
Wurster DE & Munies R (1965) Factors influencing percutaneous
absorption II, absorption of methyl ethyl ketone. J Pharm Sci,
54: 554-556.
Ying L & Levine SP (1989) Fourier transform infrared least-squares
methods for the quantitative analysis of multicomponent mixtures of
airborne vapours of industrial hygiene concern. Anal Chem,
61: 677-683.
Zakhari S, Leibowitz M, Levy P, & Aviado DM (1977) Isopropanol and
ketones in the environment. Cleveland, Ohio, CRC Press, pp 57-89.
Zechman JM, Aldinger S, & Labows JN (1986) Characterization of
pathogenic bacteria by automated headspace concentration-gas
chromatography. J Chromatogr, 377: 49-57.
Zimmermann FK, Mayer VW, Scheel I, & Resnick MA (1985) Acetone, methyl
ethyl ketone, ethyl acetate, acetonitrile and other polar aprotic
solvents are strong inducers of aneuploidy in Saccharomyces
cerevisiae. Mutat Res, 149: 339-351.
APPENDIX 1
Conversion factors for various solvents (ppm --> mg/m3)
acetone 2.38
2-butanol 3.03
n-butanol 3.03
2-butoxyethanol 4.83
butyl acetate 4.75
cyclohexane 3.44
DBP 11.38
DCB 6.01
ethanol 1.88
2-ethoxyethanol 3.68
ethyl acetate 3.6
ethyl butyl ketone 4.6
n-hexane 3.52
isobutanol 3.03
isopropanol 2.45
2-methoxyethanol 3.11
methyl acetate 3.6
methyl butyl ketone 4.1
methyl ethyl ketone 2.95
methyl isobutyl ketone 4.1
methylene chloride 3.48
toluene 3.75
trichloroethane 5.46
trichloroethylene 5.38
white spirit 1.75
xylene 4.34
From: Clayton & Clayton (1981) and Weast (1986)
RESUME
1. Propriétés et méthodes d'analyse
La méthyléthylcétone est un liquide limpide, incolore, volatil et
très inflammable dont l'odeur rappelle celle de l'acétone. Elle est
stable dans les conditions ordinaires, mais peut donner naissance à
des peroxydes explosifs en cas de stockage prolongé. Elle peut aussi
former des mélanges explosifs avec l'air. Elle est très soluble dans
l'eau, miscible à de nombreux solvants organiques et forme des
azéotropes avec l'eau et beaucoup de liquides organiques. Dans
l'atmosphère, elle produit des radicaux libres qui peuvent favoriser
la formation d'un smog photochimique.
On dispose de plusieurs méthodes analytiques pour mesurer les
concentrations de méthyléthylcétone dans l'air, l'eau, les
échantillons biologiques, les effluents et d'autres milieux. Dans les
méthodes les plus sensibles, la méthyléthylcétone est piégée et
concentrée soit sur un sorbant solide, soit sous forme de dérivé de la
2,4-dinitrophénylhydrazine (DNPH). La méthyléthylcétone absorbée et
les autres composés organiques volatils sont désorbés, séparés par
chromatographie gazeuse et dosés à l'aide d'un spectromètre de masse
ou d'un détecteur à ionisation de flammes. Le produit de dérivation de
la méthyléthylcétone est séparé des substances apparentées par
chromatographie liquide à haute performance et mesuré par
spectrophotométrie ultraviolette. Dans des milieux tels que les
déchets solides ou les substances biologiques, la méthyléthylcétone
doit d'abord être séparée du substrat, par exemple par extraction à
l'aide d'un solvant ou par distillation à la vapeur. Les
concentrations élevées de méthyléthylcétone dans l'air peuvent être
mesurées de façon continue par absorption infrarouge. Les limites de
détection sont de 3 µg/m3 dans l'air, 0,05 µg/litre dans l'eau de
boisson, 1,0 µg/litre dans les autres types d'eau, 20 µg/litre dans le
sang et 100 µg/litre dans l'urine.
2. Sources d'exposition et usages
2.1 Production et autres sources
Selon des statistiques récentes, la production annuelle (en
milliers de tonnes) est la suivante: Etats-Unis d'Amérique, 212 à 305;
Europe de l'Ouest, 215; Japon, 139. D'autres sources de contamination
de l'environnement par la méthyléthylcétone sont les gaz d'échappement
des réacteurs et des moteurs à combustion interne ainsi que certaines
activités industrielles comme la gazéification du charbon. Elle est
également présente en quantités notables dans la fumée de tabac. Aux
Etats-Unis d'Amérique, la quantité de méthyléthylcétone émise par les
moteurs représente plus de 1% de la quantité fabriquée volontairement.
En cas de smog, la production photochimique de méthyléthylcétone et
d'autres carbonyles à partir de radicaux libres peut être bien
supérieure aux émissions résultant des activités humaines. La
méthyléthylcétone peut aussi avoir une origine biologique et elle a
été identifiée parmi les produits du métabolisme microbien. Elle a
également été détectée dans divers produits naturels, notamment des
végétaux supérieurs, des phéromones d'insectes, des tissus animaux,
ainsi que chez l'homme dans le sang, l'urine et l'air expiré. Elle
constitue probablement un produit mineur du métabolisme normal des
mammifères.
2.2 Usages et pertes dans l'environnement
La méthyléthylcétone est un excellent solvant, ce qui explique
que sa principale application soit la fabrication de revêtements
protecteurs et d'adhésifs. Elle est également utilisée comme
intermédiaire chimique, comme solvant dans la fabrication des rubans
magnétiques, pour l'élimination des cires dans les huiles de graissage
et dans l'industrie alimentaire. Elle entre également dans la
composition de nombreux produits d'usage courant comme les vernis et
les colles. Dans la plupart de ces applications, la méthyléthylcétone
est mélangée à d'autres solvants organiques. La libération dans
l'environnement résulte principalement de l'évaporation des solvants
à partir des surfaces enduites et concerne essentiellement
l'atmosphère. La libération de méthyléthylcétone dans l'eau est la
conséquence de sa présence dans les effluents provenant de sa
fabrication et de diverses opérations industrielles. Elle a été
détectée dans des eaux naturelles dans lesquelles sa présence pourrait
s'expliquer par une activité microbienne, l'absorption à partir de
l'atmosphère ou une pollution anthropogène.
3. Transport et distribution dans l'environnement
La méthyléthylcétone est extrêmement mobile dans l'environnement
naturel où elle se renouvelle rapidement. Elle est très soluble dans
l'eau et s'évapore facilement. Dans l'atmosphère, elle subit une
décomposition photochimique rapide, mais elle est également
synthétisée par des processus photochimiques. Elle réagit avec les
halogènes libres ou les hypochlorites et leurs homologues halogénés
présents dans l'eau pour former un dérivé halogéné plus toxique que la
molécule initiale. La méthyléthyl-cétone est transportée par l'air et
par l'eau, mais elle ne s'accumule dans aucun compartiment et elle ne
persiste pas longtemps là où il existe une activité microbienne. Elle
est rapidement métabolisée par les microbes et les mammifères. Le
phénomène de bioaccumulation n'a jamais été mis en évidence. La
méthyléthylcétone existe naturellement dans certaines espèces de
trèfle et elle est produite par des champignons à des concentrations
qui peuvent affecter la germination de certaines plantes.
4. Concentration dans l'environnement et exposition humaine
La population générale est fréquemment exposée à de faibles doses
de méthyléthylcétone. Lorsque la pollution atmosphérique est très
faible, la concentration est inférieure à 3 µg/m3 (< 1 ppb), mais
on a mesuré jusqu'à 131 µg/m3 (44,5 ppb) en atmosphère très polluée.
En dehors des zones industrielles de fabrication ou d'utilisation de
la méthyléthylcétone, les gaz d'échappement des véhicules automobiles
et les réactions photochimiques dans l'atmosphère peuvent être les
principales sources de contamination. Les cigarettes et les autres
formes de tabac à fumer contribuent à l'exposition individuelle (20
cigarettes en contiennent jusqu'à 1,6 mg). La volatilisation de la
méthyléthylcétone présente dans les matériaux de construction et les
produits de consommation peut entraîner une pollution de l'air des
locaux bien supérieure à celle de l'air extérieur. Les concentrations
dans les eaux naturelles dépassent rarement 100 µg/litre (100 ppb) et
sont généralement inférieures au seuil de détection. Toutefois, on a
souvent trouvé des traces de méthyléthylcétone dans l'eau de boisson
(environ 2 µg/litre). Il est probable que les solvants entrant dans la
composition des joints des tuyauteries de plastique sont à l'origine
de cette pollution. Bien que la méthyléthylcétone soit un constituant
normal de nombreux aliments, les concentrations sont faibles et
l'alimentation ne peut être considérée comme une source importante
d'exposition. Aux Etats-Unis d'Amérique, la quantité moyenne ingérée
quotidiennement avec les aliments, principalement le pain blanc, les
tomates et le fromage Cheddar, est estimée à 1,6 mg par personne. La
méthyléthylcétone peut être présente naturellement dans les aliments,
mais elle peut aussi se former lors de l'affinage du fromage, de
l'entreposage de la viande de volaille, de la cuisson ou de la
transformation des aliments, ou être absorbée à partir des emballages
en matière plastique.
L'exposition industrielle à des concentrations modérées de
méthyléthylcétone est fréquente. Toutefois, dans certaines régions, le
personnel travaillant dans de petites entreprises (fabriques de
chaussures, imprimeries, ateliers de peinture) peut être exposé à des
concentrations beaucoup plus élevées en raison d'une ventilation
insuffisante. Ces travailleurs sont généralement exposés à un mélange
de solvants, notamment au n-hexane.
5. Cinétique et métabolisme
La méthyléthylcétone est rapidement absorbée par contact cutané,
inhalation, ingestion et injection intrapéritonéale. Elle passe très
vite dans le sang, et de là dans les autres tissus. Il semble que sa
solubilité soit à peu près la même dans tous les tissus. L'élimination
de la méthyléthylcétone et de ses métabolites est pratiquement totale
chez les mammifères au bout de 24 heures. Elle est métabolisée dans le
foie où la plus grande partie est oxydée en 3-hydroxy-2-butanone avant
d'être réduite en 2,3-butanediol. Une petite partie peut être réduite
en 2-butanol, mais celui-ci est rapidement oxydé pour redonner la
molécule initiale. Chez les mammifères, la majeure partie de la
méthyléthylcétone ingérée entre dans le cycle métabolique général
et/ou est éliminée sous forme de molécules simples comme le dioxyde de
carbone et l'eau. L'excrétion de la méthyléthylcétone et de ses
métabolites caractéristiques se fait principalement par les poumons,
bien que de petites quantités soient éliminées par les reins.
La méthyléthylcétone augmente l'activité enzymatique du
cytochrome P-450 microsomal. Il est possible que ce renforcement de
l'activité enzymatique, et par conséquent du potentiel de
transformation métabolique de l'organisme, explique pourquoi la
méthyléthylcétone potentialise la toxicité des solvants du groupe des
alcanes halogénés et des hydrocarbures aliphatiques à six atomes de
carbone.
6. Effets sur les animaux d'expérience
La méthyléthylcétone présente une toxicité faible à modérée pour
les mammifères, qu'il s'agisse de toxicité aiguë, à court terme ou
chronique. Chez la souris et le rat, la LD50 est de 2 à 6 g/kg de
poids corporel, la mort survenant 1 à 14 jours après l'ingestion d'une
dose unique. La dose moyenne entraînant la mort après une exposition
unique aux vapeurs de méthyléthylcétone est d'environ 29 400 mg/m3
(10 000 ppm), bien que des cobayes aient survécu à une exposition de
4 heures à cette concentration. Dans des essais d'intoxication aiguë
par voie orale, la dose la plus faible ayant entraîné une modification
de structure des organes a été de 1 g/kg de poids corporel chez le
rat. Cette dose a provoqué des lésions des tubules du rein.
L'inhalation par des rats d'air contenant 74 mg/m3 (25 ppm) pendant
6 heures a provoqué des changements de comportement mesurables qui ont
persisté pendant plusieurs jours. Une exposition répétée à 14 750
mg/m3 (5000 ppm) (6 h/jour, 5 jours/semaine) n'a provoqué la mort
d'aucun animal. On n'a observé qu'un effet mineur sur la croissance et
la structure et il n'y a eu aucune modification neuropathologique. Des
poulets, des chats ou des souris exposés à 3975 mg/m3 (1500 ppm)
pendant des périodes allant jusqu'à 12 semaines n'ont présenté aucun
signe de changement neuropathologique. Des effets transitoires sur le
comportement ou la neurophysiologie ont été détectés chez des rats et
des babouins à la suite d'expositions répétées à des concentrations ne
dépassant pas 295 à 590 mg/m3 (100 à 200 ppm).
Une faible foetotoxicité a été observée à 8825 mg/m3 (3000
ppm), mais elle ne s'accompagnait d'aucune toxicité maternelle; aucun
effet embryotoxique ou tératogène n'a été constaté à des
concentrations inférieures à cette valeur. Après avoir exposé de façon
répétée des rattes en gestation à une concentration de 8825 mg/m3,
on a observé dans leur progéniture une augmentation légère mais
significative de certains types d'anomalie du squelette rarement
observés chez les animaux non exposés.
Plusieurs épreuves classiques de mutagénicité ont été pratiquées,
mais la seule qui ait donné un résultat positif a été une étude
d'hétéroploïdie sur la levure Saccharomyces cerevisiae.
La méthyléthylcétone ne présente pas de toxicité aiguë pour les
poissons ou les invertébrés aquatiques, la CL50 se situant entre
1382 et 8890 mg/litre.
Elle a un effet inhibiteur sur la germination de plusieurs
plantes, même à des concentrations que l'on peut rencontrer dans la
nature. La croissance des algues aquatiques est également inhibée.
Des concentrations relativement élevées de méthyléthylcétone,
comparées aux concentrations naturelles, ont été utilisées dans des
expériences de fumigation. Cette substance s'est révélée un fumigant
modérément efficace contre la mouche des fruits des Caraïbes. D'autre
part, elle a un effet attractif très net sur la mouche tsé-tsé. Des
concentrations allant jusqu'à 20 mg/litre retardent le processus de
biodégradation mais ne l'arrêtent pas complètement. Jusqu'à 100
mg/litre, la méthyléthylcétone est bactériostatique pour différentes
bactéries. A des concentrations plus élevées (1000 mg/litre et
au-delà) elle inhibe la croissance des bactéries et des protozoaires.
7. Effets sur l'homme
7.1 Méthyléthylcétone seule
Aucun effet notable n'a été observé lors de tests psychologiques
et de comportement après exposition à 590 mg/m3 (200 ppm). Une
exposition de courte durée à la méthyléthylcétone seule ne semble pas
présenter de risques importants, que ce soit pour les professionnels
ou pour le public en général. L'exposition, dans des conditions
expérimentales, à une concentration de 794 mg/m3 (270 ppm), à raison
de 4 heures par jour, a eu un effet à peu près nul sur le comportement
et un contact de 5 minutes avec la substance liquide n'a provoqué
qu'une décoloration temporaire de la peau. On n'a signalé qu'un seul
cas d'intoxication aiguë par la méthyléthylcétone en dehors de tout
contexte professionnel. Il s'agit d'un cas d'ingestion accidentelle
qui ne semble pas avoir laissé de séquelles. On n'a jamais signalé de
cas d'exposition professionnelle ayant entraîné la mort. Il existe
deux rapports faisant état d'intoxication professionnelle chronique et
un rapport d'intoxication professionnelle aiguë, mais ce dernier est
sujet à caution. Dans un des cas d'intoxication chronique,
l'exposition à 880-1770 mg/m3 (300-600 ppm) a provoqué des
dermatoses, un engourdissement des doigts et des bras et divers
symptômes, parmi lesquels des céphalées, des étourdissements, des
troubles gastro-intestinaux et une perte d'appétit et de poids. Le
faible nombre d'intoxications attribuées à la méthyléthylcétone seule
tient à la fois à la faible toxicité de cette substance et au fait
qu'elle est rarement utilisée seule, mais plutôt en mélange avec
d'autres solvants.
7.2 Méthyléthylcétone dans les mélanges de solvants
L'exposition à des mélanges de solvants contenant de la
méthyléthylcétone a été associée à une réduction de la vitesse de
conduction nerveuse, à des troubles de la mémoire, à des troubles
moteurs, à des dermatoses et à des vomissements. Dans une étude
longitudinale, des mesures consécutives du temps de réaction simple
ont montré une amélioration des performances parallèlement à une
diminution de la concentration de méthyléthylcétone jusqu'à un dixième
de la valeur initiale (qui pouvait atteindre 4000 mg/m3 pour
certaines tâches de routine).
8. Renforcement de la toxicité des autres solvants
La méthyléthylcétone potentialise la neurotoxicité des solvants
à six atomes de carbone ( n-hexane, méthyl- n-butylcétone et
2,5-hexanedione) ainsi que la toxicité hépatique et rénale des
solvants de la famille des alcanes halogénés (tétrachlorure de carbone
et trichlorométhane).
La potentialisation des effets neurotoxiques des composés à six
atomes de carbone a été démontrée chez l'animal pour les trois
substances citées ci-dessus. Les neuropathies périphériques observées
chez l'homme se sont produites à la suite de changements dans la
formulation des solvants auxquels les sujets avaient été exposés, soit
volontairement, soit en raison de leur activité professionnelle. Le
mécanisme de cette potentialisation n'a pas été élucidé.
La potentialisation de la toxicité hépatique et rénale des
alcanes halogénés a été mise en évidence dans des études chez
l'animal. La méthyléthylcétone active probablement la métabolisation
des haloalcanes en substances toxiques pour les tissus en induisant la
production des enzymes oxydantes responsables de cette transformation.
RESUMEN
1. Propiedades y métodos analíticos
La metiletilcetona (MEC) es un líquido transparente, incoloro,
volátil, muy inflamable, de olor parecido a la acetona. Es estable en
condiciones normales pero puede formar peróxidos si se almacena
durante mucho tiempo; esos peróxidos pueden ser explosivos. La MEC
también puede formar mezclas explosivas con el aire. Es muy soluble en
agua, miscible con muchos disolventes orgánicos, y forma mezclas
azeotrópicas con el agua y con numerosos líquidos orgánicos. En la
atmósfera, la MEC produce radicales libres que pueden llevar a la
formación de nieblas fotoquímicas.
Existen varios métodos analíticos para medir los niveles
ambientales de MEC en el aire, el agua, las muestras biológicas, los
desechos y otros materiales. Con los métodos más sensibles, la MEC se
separa y se concentra ya sea en un sorbente sólido o como derivado de
la 2,4-dinitrofenilhidrazina (DNFH). La MEC y otros compuestos
orgánicos volátiles absorbidos son desorbidos, separados mediante
cromatografía de gases y medidos con un espectrómetro de masas o un
detector de ionización de llama. La MEC derivada se separa de los
compuestos afines mediante cromatografía de líquidos de alto
rendimiento y se mide mediante absorción ultravioleta. En medios como
desechos sólidos y material biológico, la MEC debe separarse en primer
lugar del sustrato con métodos como la extracción por solventes o la
destilación de vapores. Las concentraciones elevadas de MEC en el aire
pueden controlarse de modo continuo mediante absorción infrarroja. Los
límites de detección son 3 µg/m3 en el aire, 0,05 µg/litro en el
agua potable, 1,0 µg/litro en otros tipos de agua, 20 µg/litro en
sangre total y 100 µg/litro en orina.
2. Fuentes de exposición y usos
2.1 Producción y otras fuentes
Las cifras más recientes de fabricación industrial anual (en
miles de toneladas) son: EEUU, 212 a 305; Europa occidental, 215;
Japón, 139. Además de su fabricación, las fuentes de MEC en el medio
ambiente son los gases de escape de motores de reactores y de
combustión interna, y las actividades industriales como la
gasificación del carbón. Se encuentra en cantidades importantes en el
humo de tabaco. En los Estados Unidos, la producción de MEC en motores
no supera el 1% de su fabricación deliberada. En los episodios de
nieblas, la producción fotoquímica de MEC y otros carbonilos a partir
de radicales libres puede ser mucho mayor que la emisión antropogénica
directa. La MEC se produce biológicamente y se ha identificado como
producto del metabolismo microbiano. Se ha detectado asimismo en gran
diversidad de productos naturales, entre ellos los vegetales
superiores, las feromonas de insectos, en tejidos animales y en el
hombre, en sangre, orina y aire exhalado. Probablemente es un producto
secundario del metabolismo normal en el mamífero.
2.2 Usos y pérdidas al medio ambiente
El uso principal de la MEC, la aplicación de revestimientos
protectores y adhesivos, refleja sus excelentes características como
disolvente. También se utiliza como intermediario químico, como
disolvente en la producción de cintas magnéticas y para eliminar la
cera del aceite lubricante, así como en la manipulación de alimentos.
Además de las aplicaciones industriales, figura como ingrediente común
en productos de consumo como barnices y pegamentos. En la mayoría de
las aplicaciones, la MEC es componente de una mezcla de disolventes
orgánicos. Las pérdidas al medio ambiente son principalmente al aire
y se deben sobre todo a la evaporación de disolventes a partir de las
superficies revestidas. Se libera al agua como componentes de los
desechos de su fabricación y a partir de diversas operaciones
industriales. Se ha detectado en aguas naturales, procedente
probablemente de la actividad microbiana y del aporte atmosférico, así
como de la contaminación antropogénica.
3. Transporte y distribución en el medio ambiente
La MEC es sumamente móvil en el medio ambiente natural y está
sometida a una transformación rápida. Es muy soluble en el agua y se
evapora fácilmente a la atmósfera. En el aire, la MEC sufre una rápida
descomposición fotoquímica y es también sintetizada por procesos
fotoquímicos. En agua que contiene halógenos libres o hipohalitos,
reacciona para formar un haloformo más tóxico que el compuesto
original. La MEC se distribuye tanto por el aire como por el agua,
pero no se acumula en ningún compartimento aislado, ni persiste mucho
tiempo donde existe actividad microbiana. Se metaboliza rápidamente en
los microbios y los mamíferos. No hay pruebas de bioacumulación. La
MEC aparece naturalmente en algunas especies de trébol y es producida
por hongos en concentraciones que afectan a la germinación de algunas
plantas.
4. Niveles ambientales y exposición humana
La exposición de la población general a bajos niveles de MEC es
muy extensa. En aire poco contaminado, la concentración es inferior a
3 µg/m3 (< 1 ppmm), pero en condiciones de fuerte contaminación
atmosférica se ha medido un nivel de 131 µg/m3 (44,5 ppmm). Lejos de
las zonas industriales donde se fabrica o se usa la MEC, las
principales fuentes pueden ser los escapes de vehículos y las
reacciones fotoquímicas en la atmósfera. Los cigarrillos y otros
productos del tabaco que se someten a combustión contribuyen a la
exposición individual (20 cigarrillos contienen hasta 1,6 mg). La
volatilización de la MEC de materiales de construcción y productos de
consumo pueden contaminar el aire de interiores hasta niveles muy
superiores a los del aire libre adyacente. Las concentraciones de MEC
en aguas naturales expuestas rara vez se encuentran por encima de los
100 µg/litro (100 ppmm) y suelen encontrarse por debajo del nivel de
detección. No obstante, se han detectado cantidades muy reducidas de
MEC en el agua potable (aproximadamente 2 µg/litro) que probablemente
proceden de disolventes lixiviados a partir del material de las juntas
de las tuberías de plástico. Aunque la MEC es un componente normal de
muchos alimentos, las concentraciones son bajas y el consumo de
alimentos no puede considerarse una fuente significativa de exposición
para la población. La ingesta media diaria por habitante de los
Estados Unidos con los alimentos se calcula en 1,6 mg, en su mayor
parte a partir del pan blanco, los tomates y el queso tipo Cheddar.
Además de la MEC presente en el medio natural, puede producirse en la
maduración de los quesos, el envejecimiento de la carne de ave, la
cocción o la manipulación de alimentos, o por absorción a partir de
los envases de plástico.
La exposición industrial a niveles moderados de MEC está muy
extendida. No obstante, en algunas regiones los trabajadores de
fábricas pequeñas (por ejemplo, fábricas de calzado, imprentas y
fábricas de pinturas) están expuestos a concentraciones mucho más
elevadas por una ventilación insuficiente. En esas fábricas, la
exposición se da por lo general a una mezcla de disolventes entre los
que figura el n-hexano.
5. Cinética y metabolismo
La absorción de MEC es rápida por contacto cutáneo, inhalación,
ingestión e inyección intraperitoneal. Pasa rápidamente a la sangre y
de ella a otros tejidos. La solubilidad de la MEC parece similar en
todos los tejidos. La eliminación de la MEC y sus metabolitos en
mamíferos se completa en su mayor parte en 24 horas. Se metaboliza en
el hígado, donde se oxida a 3-hidroxi-2-butanona y a continuación se
reduce a 2,3-butanodiol. Una pequeña porción puede reducirse a
2-butanol, pero éste se oxida rápidamente para dar de nuevo MEC. La
mayor parte de la MEC que ingresa al organismo de mamíferos pasa al
metabolismo general y/o se elimina en forma de compuestos simples,
como dióxido de carbono y agua. La excreción de MEC y sus metabolitos
reconocibles se hace principalmente por los pulmones, aunque pequeñas
cantidades se eliminan por el riñón.
La MEC aumenta la actividad enzimática del citocromo P-450 en los
microsomas. Este aumento de la actividad enzimática y con ello del
potencial del organismo para la transformación metabólica puede ser el
mecanismo por el que la MEC potencia la toxicidad de los disolventes
a base de haloalcanos y hexacarbonos alifáticos.
6. Efectos en los animales de experimentación
La MEC tiene toxicidad aguda, a corto plazo y crónica de baja a
moderada en los mamíferos. Los valores de la DL50 en ratones y ratas
adultos son 2-6 g/kg de peso corporal; la muerte sobreviene en los
días 1 a 14 después de una sola dosis por vía oral. Las
concentraciones medias de vapor que producen letalidad en las ratas
tras una sola exposición giran en torno a los 29 400 mg/m3 (10 000
ppm), aunque los cobayos sobrevivieron a una exposición de 4 horas a
esta concentración. La dosis oral aguda más baja en modificar la
estructura corporal fue de 1 g/kg de peso corporal, que produjo
lesiones en los túbulos renales de la rata. La inhalación de 74
mg/m3 (25 ppm) durante 6 horas produjo en la rata cambios de
comportamiento medibles que persistieron durante varios días. La
exposición repetida de ratas a 14 750 mg/m3 (5000 ppm) (6 h/día, 5
días/semana) no produjo letalidad, tuvo sólo ligeros efectos en el
crecimiento y la estructura, y no se observaron cambios
neuropatológicos. No hubo pruebas de que la MEC produjera cambios
neuropatológicos en pollos, gatos o ratones expuestos a 3975 mg/m3
(1500 ppm) durante periodos de hasta 12 semanas. Tras la exposición
repetida de ratas y babuinos a concentraciones tan bajas como 295-590
mg/m3 (100 a 200 ppm) se observaron efectos transitorios en el
comportamiento o la neurofisiología.
Se ha observado un nivel bajo de fetotoxicidad sin toxicidad
materna a 8825 mg/m3 (3000 ppm), pero no hay pruebas de efectos
embriotóxicos o teratogénicos a niveles más bajos de exposición. La
exposición repetida de ratas preñadas a 8825 mg/m3 indujo en sus
crías un aumento pequeño pero significativo de ciertos tipos de
anomalías esqueléticas cuya incidencia entre la población no expuesta
es baja.
Aunque se examinó en varios sistemas de ensayo de mutagenicidad
convencionales, la única prueba de mutagenicidad se observó en un
estudio sobre aneuploidia en la levadura Saccharomyces cerevisiae.
La MEC no presenta toxicidad aguda para los peces ni los
invertebrados acuáticos; los valores de la CL50 varían desde 1382
hasta 8890 mg/litro.
La MEC inhibe la germinación de varias especies vegetales,
incluso con niveles que se dan en la naturaleza. Inhibe el crecimiento
de algas acuáticas.
En comparación con los niveles de base naturales, se han
utilizado concentraciones relativamente elevadas de MEC para fumigar
en condiciones experimentales. Es moderadamente eficaz como fumigante
contra la mosca caribeña de la fruta y atrae con gran eficacia a la
mosca tse-tse. Con niveles de MEC de hasta 20 mg/litro se retrasa la
biodegradación pero no se detiene el proceso por completo. Con niveles
de hasta 100 mg/litro, la MEC es bacteriostatica para algunas
bacterias. Con concentraciones más altas (1000 mg/litro o más) se
inhibe el crecimiento de bacterias y protozoarias.
7. Efectos en el ser humano
7.1 MEC por sí sola
La exposición a 590 mg/m3 (200 ppm) no tuvo efectos de
importancia en varios ensayos comportamentales y psicológicos. La
exposición a corto plazo a MEC por sí sola no parece constituir un
riesgo de importancia, ni ocupacional ni para el público en general.
La exposición experimental a una concentración de 794 mg/m3 (270
ppm) durante 4 h/día tuvo escaso o ningún efecto en el comportamiento,
y un contacto de 5 minutos con MEC líquida no produjo más que un
blanqueamiento temporal de la piel. Sólo hay un informe no ocupacional
de toxicidad aguda a la MEC. Se debió a una ingestión accidental y no
pareció producir lesiones duraderas. No hay pruebas de que la
exposición ocupacional a la MEC haya originado ningún caso de muerte.
Se han notificado dos casos de envenenamiento ocupacional crónico y
uno dudoso de envenenamiento ocupacional agudo. En uno de los casos
crónicos, la exposición a 880-1770 mg/m3 (300-600 ppm) dió lugar a
dermatosis, endormecimiento de los dedos y los brazos, y diversos
síntomas como dolor de cabeza, mareos, trastornos gastrointes-tinales
y pérdida de apetito y de peso. Esta escasez de incidentes de
envenamiento por MEC por sí sola refleja tanto su baja toxicidad como
el hecho de que se usa más comunmente no por sí sola sino como
componente de mezclas de disolventes.
7.2 La MEC en mezclas de disolventes
La exposición a mezclas de disolventes con MEC se ha asociado a
cierta reducción en la velocidad de conducción nerviosa, la memoria y
alteraciones motoras, dermatosis y vómitos. En un estudio
longitudinal, las medidas consecutivas de tiempo de reacción simple
demostraron que mejoraba el rendimiento en paralelo al ir disminuyer
de las con concentraciones de MEC hasta un décimo de los valores
originales (que fueron de hasta 4000 mg/m3 para ciertas tareas
rutinarias).
8. Potenciación de la toxicidad de otros disolventes
La MEC potencia la neurotoxicidad de compuestos hexacarbonados
( n-hexano, metil- n-butilcetona y 2,5-hexanodiona) y la toxicidad
hepatica y renal de los disolventes a base de haloalcanos
(tetracloruro de carbono y triclorometano).
La potenciación de los efectos neurotóxicos de los hexacarbonos
se ha demostrado con los tres hexacarbonos en el animal. Las
neuropatías periféricas observadas en humanos siguieron a cambios en
la formulacion de disolventes a los que habían estado expuestos, ya
sea voluntariamente o en el trabajo. El mecanismo por el que se
produce esta potenciación no está claro.
Las pruebas de la potenciación de la toxicidad hepática y renal
de los haloalcanos proceden de estudios animales. La MEC activa
probablemente el metabolismo de los haloalcanos de las especies que
dañan los tejidos como resultado de la inducción de las enzimas
oxidativas pertinentes.
10.1.3 Human studies
10.1.3.1 Solvent abuse
Solvent abuse, the deliberate inhalation of solvent vapours for
their euphoric effects, has been reported in many countries and has
involved the use of lacquer thinners, glues and other readily
available commercial items. Prockop et al. (1974) suggested that there
were several hundred habitual "huffers", i.e. solvent abusers, in
Tampa, Florida, USA, and Altenkirch et al. (1978) reported about 2000
in Berlin, Germany, in 1974. Outbreaks of polyneuropathy among Berlin
huffers, which involved n-hexane toxicity potentiated by MEK,
provided a major stimulus for research on the health effects of MEK
and its interactions with hexacarbon solvents.
Chronic huffers in Berlin inhaled fumes from about a half litre
of liquid per day, either poured into a plastic bag or over rags
(Altenkirch et al., 1977, 1982b). Inhalation sessions extended for as
long as 10 or 12 h, and exposure periods of 5 to 7 years were not
unusual. Prior to the end of 1975 no major damage to health resulting
from chronic solvent abuse had been observed. At that time there
appeared abruptly a number of cases of polyneuropathy among chronic
huffers. All these were young males, mainly between 16 and 21 years of
age. The initial symptom was paraesthesia of the toes accompanied in
some cases by weakness of the legs. The paraesthesia ascended rapidly
from distal to proximal and in 2 to 3 weeks affected the entire legs.
This was followed rapidly by paraesthesia of the arms which also
ascended from distal to proximal. Extensor muscles were always
affected first and most severely. There also was severe muscle atrophy
and a "glove and stocking" type sensory impairment of the hands and
feet. In all cases there also was excessive sweating of the hands and
feet, and in some cases discoloration and reduced skin temperature of
these areas. In addition there was loss of weight and damage to the
teeth. Head, neck and trunk muscles remained undisturbed, although in
severe cases there was paresis of the phrenic nerve and reduced
pulmonary function. The degree of impairment ranged from moderate
crippling, which permitted walking with assistance, to complete
tetraplegia. Symptoms continued to develop for 6 to 10 weeks after
cessation of solvent abuse. Remission was slow, took up to a year,
developed from proximal to distal, and was incomplete in those most
severely affected. Neurophysiological and histological findings were
similar to those reported in experimental animals (section 7). Motor
and sensory nerve conduction velocities were reduced in proportion to
the degree of paresis. There were lesions of the axons, paranodal axon
swellings, clumping of the nerve filaments and demyelination. The
outbreak of neuropathy came a few months after a change in the
formulation of a solvent from one composed of n-hexane, benzene
fraction, ethyl acetate and toluene to a similar mixture with less
(16%) n-hexane and the addition of 11% MEK. This was interpreted as
evidence that even long exposure to the substantial amount of
n-hexane (31%) in the original formulation did not result in
neuropathy, and that neuropathy developed only after the toxicity of
n-hexane was potentiated by simultaneous exposure to MEK.
The seven cases of polyneuropathy reported from Tampa, Florida,
USA, apparently resulted from a small amount of n-hexane (0.5%)
potentiated by other components in the solvent mixture (Prockop et
al., 1974; Spencer et al., 1980). Two similar cases of polyneuropathy
were reported from Japan (Goto et al., 1974), these were produced by
chronic sniffing of a glue that contained 25% n-hexane and 20% MEK.
What is puzzling in view of the absence of neuropathy in Berlin prior
to the addition of MEK to a solvent mixture containing n-hexane is
that Goto et al. (1974) also reported two cases of neuropathy
resulting from chronic sniffing of glue solvent containing only
n-hexane and toluene. However, Oh & Kim (1976) reported a case of
polyneuropathy produced by chronic abuse of mixtures containing MEK,
methyl isobutyl ketone (MIBK) and many other solvents, but apparently
not n-hexane or MBK. There was, however, no analysis of the solvent
mixtures. There was no experimental evidence to suggest that any of
the solvents known to be present in the mixtures alone, or MEK and
MIBK together, could produce this type of neuropathy. An alternative
explanation is that the MIBK contained MBK as an impurity.
10.1.3.2 Occupational exposure
Although there have been many cases of occupationally related
poisoning by exposure to neurotoxic hexacarbon solvents (Spencer et
al., 1980), poisonings in which neurotoxicity has been associated with
concurrent MEK exposure are limited. In occupational health studies
concentrating on n-hexane neurotoxicity, the study populations were
exposed to several other solvents, including MEK (WHO, 1991). One of
the most thoroughly investigated cases occurred in 1973 in a fabric
factory in Ohio, USA (Allen et al., 1974). In 1973, an employee at the
factory was found to have a severe sensorimotor neuropathy. Other
co-workers at the plant were also found to have similar symptoms,
which initiated a search for a causative agent in the workplace. Of
the 1157 workers examined, 86 manifested signs and symptoms indicative
of peripheral neuropathy, including parathesiae in arms and legs and
weakness in the hands and legs. Subsequent investigation found that in
the year prior to the confirmation of the first case, MBK had been
substituted for MIBK as a co-solvent with MEK. Exposure occurred by
skin contact and by inhalation. Measured concentrations of MEK in
certain areas of greatest exposure were 251-2251 mg/m3 (85-763 ppm),
while concentration for MBK ranged from 9 to 640 mg/m3 (2.3-156
ppm). The introduction of MBK into the factory was associated with the
observed neurotoxicity. Spencer et al. (1980) pointed out that several
animal studies showed that concurrent exposure to MEK accelerated
MBK-induced neurotoxicity.
10.2 Haloalkane solvents
10.2.1 Studies in animals
Carbon tetrachloride (CCl4), chloroform (CHCl3) and related
haloalkane solvents are liver and kidney poisons as well as central
nervous depressants (Gosselin et al., 1984). It has long been known
that the hepatotoxic action of CCl4 is potentiated by ethanol, and
more recently that the hepatic and/or renal toxicity of CCl4,
CHCl3, trichloroethylene, 1,1,2-trichloroethane and related
compounds is potentiated by n-hexane, ethanol, isopropanol, acetone,
MEK, MBK, 2,5-HD, and other ketones or chemicals that are metabolized
to ketones (Hewitt et al., 1980). Even an increase of naturally
occurring ketones in the body via diabetes can precipitate
potentiation. Decreasing the transformation of isopropanol to acetone
by the administration of an inhibitor of alcohol dehydrogenase,
pyrazole, has reduced potentiation of haloalkane toxicity. Although
the phenomenon is referred to as haloalkane toxicity, experimental
work in general appears largely or entirely limited to chlorinated
compounds, and specific studies on MEK are limited to interactions
with CCl4 and CHCl3.
The effects of MEK and CCl4 or CHCl3 on rats are summarized
in Table 17. At the doses used, MEK and the haloalkanes separately
produced mild liver and kidney injury at most. When exposure to MEK
was followed within 10 to 48 h by a haloalkane, there was severe
injury to the liver, with marked and abrupt replacement of normal
hepatic cells by necrotic and fatty, vacuolated tissue, an increase in
hepatic triglyceride, and, presumably, a corresponding decrease in
normal liver function. Hepatic enzymes were released into the blood by
breakdown of liver tissue, resulting in elevated levels of plasma
glutamic-pyruvic transaminase, plasma ornithine carbamyltransferase,
and plasma alanine aminotransferase. There was also an increase in
plasma bilirubin, although this was not accompanied by a decrease in
bile secretion as was the case with some other ketones (Hewitt et al.,
1986). A dose of MEK as small as 0.072 g/kg potentiated the effects of
CHCl3 given 18 h later, and 1.505 g/kg MEK potentiated the effects
of CCl4. Lower doses were not studied.
The mechanism of potentiation is not fully understood but
increased bioactivation of haloalkanes is believed to play a central
part in the potentiation effect. CCl4 is metabolized in vivo with
homolytic cleavage of the carbon-chlorine bond to produce highly
reactive free radicals that exert their toxic effects a) by binding
covalently to proteins and other elements, and b) via lipid
peroxidation (Anders, 1988). It is likely that the toxic effects of
CHCl3 also are produced in part by this mechanism (Hewitt et al.,
1980, 1987). The toxicity of CCl4 is enhanced by pretreatment with
various agents such as phenobarbital, ethanol, isopropanol, 2-butanol
and the ketone metabolites of the last two compounds (acetone and MEK)
(Cornish & Adefuin, 1967; Traiger & Plaa, 1972; Traiger & Bruckner,
1976; Gosselin et al., 1984). Recent studies have shown that the
ethanol-inducible cytochrome P-450 isozyme (P-450IIE1) plays an
important role in haloalkane metabolism (Johansson &
Ingelman-Sundberg, 1985). It is a high affinity enzyme and operates at
a low concentration range for various substrates (Nakajima et al.,
1990). CCl4 hepatotoxicity in rats has been found to be potentiated
by induction of the P-50IIE1 isozyme with ethanol and the injury was
most marked in the perivenous liver cells where the expression of
induction was the highest (Lindros et al., 1990). In addition to
ethanol, P-450IIE1 is known to be induced by acetone and MEK (Ko et
al., 1987; Raunio, et al., 1990; Albano et al., 1991). However, while
a relatively large oral dose of MEK (1.4 ml for 3 days) to rats
increased the amount of ethanol- and phenobarbital-inducible
cytochromes P-450 (P-450IIE1 and P-450IIB, respectively) (Raunio et
al., 1990), inhalation exposure of rats to 1770 mg MEK/m3 (600 ppm),
10 h/day for 7 days, caused only marginal effects on microsomal
cytochrome P-450 activities in the liver (Liira et al., 1991).
10.2.2 Potentiation of haloalkane toxicity in humans
There are no reports of MEK potentiation of haloalkane renal and
hepatic toxicity in humans.
11. EVALUATION OF HUMAN HEALTH RISKS AND EFFECTS ON THE ENVIRONMENT
11.1 Human health risks
11.1.1 Non-occupational exposure
Low level non-occupational exposure to MEK is widespread from a
variety of natural and anthropogenic sources, since MEK is a normal,
though minor, mammalian metabolite. External sources include food,
water and air. In the USA, the average daily per capita intake of MEK
from food is estimated to be 1.6 mg. In addition to MEK present
naturally, foods may contain MEK that has been added in food
processing or absorbed from plastic packaging materials. MEK is rarely
detected in exposed natural waters where it may originate from
microbial activity and atmospheric and anthropogenic inputs, but is
frequently detected at low concentrations in drinking-water where it
presumably is leached from cemented joints of plastic pipes. Leaching
from landfill hazardous waste dumps is another potential source of
groundwater, and hence drinking-water, contamination. Measured
concentrations in food and water are so low, however, that it is
unlikely that either of these represent a significant source of
exposure.
In minimally polluted outdoor air, the MEK concentration is less
than 3 µg/m3 (1.0 ppb) but has been measured at 134 µg/m3 (44.5 ppb)
under conditions of dense smog. Away from industrial areas where
MEK is manufactured or used, major sources may be vehicle exhaust and
photochemical reactions in the atmosphere. In smog episodes,
photochemical production of MEK may greatly exceed direct
anthropogenic emission. Volatilization of MEK from building materials
and consumer products can pollute indoor air to levels far above
outdoor air, and a concentration as high as 48 µg/m3 (12.9 ppb) was
measured in an Italian home. MEK also is present in tobacco smoke
(e.g., 80-207 µg/cigarette).
For the general population, daily MEK intake is estimated to
range between 1.6 and 4.2 mg, depending on the location site (rural or
urban), with an additional 1.6 mg in the case of smokers. There is no
evidence of any adverse effects on the general population from
exposure to MEK. Data from experimental animal studies show that toxic
effects occur at dose levels that are 3 orders of magnitude higher
than the estimated daily intake. Non-occupational poisoning from MEK
alone is limited to a single case, which resulted in no lasting
injury.
MEK is, however, known to potentiate the toxicity of two classes
of organic solvents, unbranched aliphatic hexacarbons and haloalkanes,
and chronic exposure to consumer products containing MEK and
n-hexane have produced outbreaks of polyneuropathy among individuals
deliberately inhaling fumes from these mixtures for their euphoric
effects. Co-exposure to MEK and either hexacarbons or haloalkanes via
the abuse of consumer products remains a potential public health
hazard. Injuries from such poisoning can be severe, permanently
disabling and even fatal.
11.1.2 Occupational exposure
MEK is an important industrial chemical which is used mainly as
a component of solvent mixtures for application of a wide variety of
coatings and adhesives. Moderate occupational exposure via air is
widespread because losses to the environment result mainly from
solvent evaporation from coated surfaces, and MEK is not viewed as an
especially dangerous substance. Most national limits for occupational
exposure are set at 590 mg/m3 (200 ppm), with a higher short-term
exposure level of 885 mg/m3 (300 ppm), and these limits appear
acceptable. On site measurements, however, indicate that workers may
be chronically exposed to still higher MEK concentrations in small
factories such as shoe factories, printing plants and painting
operations, due to inadequate ventilation. Lesser amounts of MEK are
lost to the air with concurrent worker exposure during manufacture,
shipping, repackaging and preparation of coatings and adhesives.
Industrial exposure from contact with liquid MEK does not appear an
important problem.
Chronic co-exposure to MEK and either unbranched aliphatic
hexacarbon or haloalkane solvents represents a significant potential
occupational hazard. Serious toxic effects could occur. Although there
are no records of industrial accidents involving MEK potentiation of
haloalkane toxicity, MEK potentiation of hexacarbon neurotoxicity may
have caused at least one major industrial accident in which an
outbreak of polyneuropathy followed introduction of MEK into a solvent
mixture. Thus MEK, in the mixed solvent atmosphere of many industrial
activities, can present a toxic hazard.
11.1.3 Relevant animal studies
Acute MEK toxicity has been shown in animal studies to be low by
the oral and inhalation route. The lowest oral dose modifying body
structure (damage of kidney tubules) was 1 g/kg body weight in rats.
Ten intraperitoneal injections of 34 mg/kg body weight over a 2-week
period produced transient injection site irritations but no effect on
the kidney. In a 90-day inhalation study, female rats exposed to 14.75
g/m3 for 6 h/day, 5 days per week, showed only slightly increased
liver weight, slightly decreased brain and spleen weight, and slightly
altered blood chemistry in comparison with controls; male rats showed
only a slightly increased liver weight. A transient decrease in nerve
conduction velocity was found following exposure to 590 mg/m3 (12
h/day for 24 weeks). The transient nature of neurological and
behavioural changes induced by MEK may be due to adaptation or more
rapid metabolism of MEK. Short-term dermal exposure to small amounts
of MEK results in mild local irritation, at most. Results of studies
of eye irritation are inconsistent, possibly due to different scoring
techniques; 4 mg created severe chemical burns in the eye in one study
whereas in other studies less severe signs were reported following a
dose of 80 mg.
An inhalation study provided evidence for low level fetotoxicity
in the absence of maternal toxicity at 8825 mg/m3. Thus MEK may be
a low grade teratogen in rats. There is a lack of data on other
aspects of reproduction in animals, and no relevant data have been
reported for humans.
MEK has given negative results in most conventional mutagenicity
assays. There is evidence of aneuploidy in yeast but this may not be
relevant to humans or other mammals.
11.2 Effects on the environment
MEK occurs naturally at low concentrations in air, water and
soil. It is highly mobile in the natural environment and is not
accumulated in any individual compartment. MEK is rapidly synthesized
and destroyed by photochemical processes in the atmosphere. There is
no specific information on either partitioning of MEK in any
environmental compartment or on chemical binding to sediment
particles.
MEK is synthesized biologically and is rapidly metabolized by
bacteria (even at high concentrations), mammals and probably many
other organisms. Levels produced by fungi can cause inhibition of
plant germination. Observations on microorganisms, higher plants,
invertebrates, fish and mammals suggest a low level of toxicity.
Environmental levels of MEK appear to be too low to cause any damage
except in the immediate vicinity of highly polluted sites. Effects on
the aquatic environment are likely to appear at levels between 1 and
10 mg/litre. The potentiation of solvent toxicity by MEK appears
environmentally irrelevant, although substantial information is
lacking. Overall, MEK does not represent a significant threat to the
environment.
12. RECOMMENDATIONS FOR THE PROTECTION OF HUMAN HEALTH AND THE
ENVIRONMENT
12.1 Human health protection
MEK on its own appears a relatively safe organic solvent, but its
use in combination with other solvents, in particular haloalkanes or
unbranched aliphatic hexacarbons, should be avoided. Industries should
be strongly encouraged to take all necessary precautions to ensure
that workers are not exposed to both MEK and solvents whose toxicity
is potentiated by MEK.
12.2 Environmental protection
MEK is unlikely to present a hazard to the environment except in
cases of major spills or discharges.
FURTHER RESEARCH
a) Further research should be undertaken to clarify the precise
mechanisms by which MEK potentiates the toxicity of haloalkanes
and hexacarbons.
b) Epidemiological studies are needed to determine exposure-response
relationships regarding MEK-induced potentiation of hexacarbon
and haloalkane toxicity.
c) Radiolabelled balance studies should be conducted to determine
accurately the routes and rates of excretion of MEK and its
metabolites. The results of such studies would be particularly
useful for improving methods of biological monitoring.
d) Comprehensive studies of reproductive and developmental toxicity
should be undertaken in representative rodent and non-rodent
species.
e) The binding capacity of soils and sediments for MEK should be
assessed.
REFERENCES
Aarstad K, Zahlsen K, & Nilsen OG (1985) Inhalation of butanols:
Changes in the cytochrome P-450 enzyme system. Arch Toxicol, 8(Suppl):
418-421.
Aarstad K, Zahlsen K, & Nilsen OG (1986) [Effects of inhalation of
different butanol isomers.] Faerg Lack Scand, 32: 69-74 (in Norwegian
with English summary).
Abdel-Rahman MS, Hetland LB, & Couri D (1976) Toxicity and metabolism
of methyl n-butyl ketone. Am Ind Hyg Assoc J, 37: 95-102.
ACGIH (1986) Documentation of the threshold limit values and
biological exposure indices, 5th ed. Cincinnati, Ohio, American
Conference of Governmental Industrial Hygienists, p 395.
ACGIH (1991) Threshold limit values and biological exposure indices
for 1988-1989. Cincinnati, Ohio, American Conference of Governmental
Industrial Hygienists.
Ahrenholz SH & Egilman DS (1983) Health hazard evaluation: Rubbermaid
Incorporated, Wooster, Ohio. Cincinnati, Ohio, National Institute of
Occupational Safety and Health, 33 pp (HETA 3-1340).
Albano E, Tomasi A, Persson J-O, Terelius Y, Goria-Gatti L,
Ingelman-Sundberg M, & Diazani MU (1991) Role of ethanol-inducible
cytochrome P-450 (P450IIE1) in catalysing the free radical activation
of aliphatic alcohols. Biochem Pharmacol, 41: 1895-1902.
Alderson MR & Rattan NS (1980) Mortality of workers on an isopropyl
alcohol plant and two MEK dewaxing plants. Br J Ind Med, 37: 85-89.
Allen N, Mendell JR, Billmaier D, & Fontaine RE (1974) An outbreak of
a previously undescribed toxic polyneuropathy due to industrial
solvent. Trans Am Neurol Assoc, 99: 74-79.
Altenkirch H, Mager J, Stoltenburg G, & Helmbrecht J (1977) Toxic
polyneuropathies after sniffing a glue thinner. J Neurol, 214:
137-152.
Altenkirch H, Stoltenburg G, & Wagner HM (1978) Experimental studies
on hydrocarbon neuropathies induced by methyl-ethyl-ketone (MEK). J
Neurol, 219: 159-170.
Altenkirch H, Stoltenburg-Didinger G, & Wagner HM (1979) Experimental
data on the neurotoxicity of methyl-ethyl-ketone (MEK).
Experientia(Basel), 35: 503-504.
Altenkirch H, Wagner HM, Stoltenburg G, & Spencer PS (1982a) Nervous
system responses of rats to subchronic inhalation of n-hexane and
n-hexane + methyl-ethyl-ketone mixtures. J Neurol Sci, 57: 209-219.
Altenkirch H, Wagner HM, Stoltenburg-Didinger G, & Steppat R (1982b)
Potentiation of hexacarbon-neurotoxicity by methyl-ethyl-ketone (MEK)
and other substances: Clinical and experimental aspects. Neurobehav
Toxicol Teratol, 4: 623-627.
Anders MW (1988) Bioactivation mechanisms and hepatocellular damage.
In: Arias IM, Jakoby WB, Popper H, Schachter D, & Schafritz DA ed. The
liver: Biology and pathology. New York, Raven Press Ltd, pp 389-400.
Anderson C, Sundberg K, & Groth O (1986) Animal model for assessment
of skin irritancy. Contact Dermatitis, 15: 143-151.
ANONYMOUS (1978) Atmospheric hydrocarbons in air during photochemical
episodes. Kanagawa-Ken Taike Osen Chosa Kenkyu Hokoku, 20: 86-90.
Anshelm Olson B, Gamberale F, & Grönqvist B (1981) Reaction time
changes among steel workers exposed to solvent vapours: A longitudinal
study. Int Arch Occup Environ Health, 48: 211-218.
Arques Espi E & Quintanilla Almagro T (1981) [Study of gluing stations
in 50 shoe plants: Simplified method of risk evaluation and normal
work technique.] In: [Proceedings of the National Congress of
Occupational Medicine, Hygiene and Safety: 9th meeting, 1980.] Madrid,
Spain, Occupational Hygiene and Safety Service, vol 2, pp 551-561 (in
Spanish).
Attygalle AB, Cammaerts MC, & Morgan ED (1983) Dufour gland secretions
of Myrmica rugulosa and Myrmica schencki workers. J Insect
Physiol, 29: 27-32.
Azuma K, Hirata T, Tsunoda H, Ishitani T, & Tanaka Y (1983)
Identification of the volatiles from low density polyethylene film
irradiated with an electron beam. Agric Biol Chem, 47: 855-860.
Banergee S & Howard PH (1988) Improved estimation of solubility and
partitioning through correction of UNIFAC-derived activity
coefficients. Environ Sci Technol, 22: 839-841.
Basler A (1986) Aneuploidy-inducing chemicals in yeast evaluated by
the micronucleus test. Mutat Res, 174: 11-13.
Bassette R & Ward G (1975) Measuring parts per billion of volatile
materials in milk. J Dairy Sci, 58: 428-429.
Basu P, Carpenter J, Nelson H, Perry W, Shochet A, Sun C, & Taylor D
(1981) Toxic Substances Control Act (TSCA), Section 4 - Human exposure
assessment: methyl ethyl ketone. McLean, Virginia, JRB Associates,
pp 1/1-R/1 (EPA Contract No. 68-01-4839).
Berg EF (1971) Retrobulbar neuritis. Ann Ophthalmol, 3: 1351, 1353.
Bernard AM, De Russis R, Normand J-C, & Lauwerys RR (1989) Evaluation
of the subacute nephrotoxicity of cyclohexane and other industrial
solvents in the female Sprague-Dawley rat. Toxicol Lett, 45: 271-280.
Bills DD, Willits RE, & Day EA (1966) Determination of some neutral
volatile constituents in ten commercial Cheddar cheeses. J Dairy Sci,
49: 681-685.
Binaschi S, Gazzaniga G, & Crovato E (1976) Behavioral toxicology in
the evaluation of the effects of solvent mixtures. Adverse Eff Environ
Chem Psychotropic Drugs, 2: 91-98.
Blum M, Warter S, & Traynham J (1966) Chemical releasers of social
behavior - VI. The relation of structure to activity of ketones as
releasers of alarm for Iridomyrex pruinosus (Roger). J Insect
Physiol, 12: 419-427.
Blum M, Warter S, & Traynham J (1971) Alarm pheromones: utilization in
evaluation of olfactory theories. J Insect Physiol, 17: 2351-2361.
Boch R & Shearer D (1971) Chemical releasers of alarm behavior in the
honey-bee, Apis mellifera. J Insect Physiol, 17: 2277-2285.
Boettner EA, Ball GL, Hollingsworth Z, & Aquino R (1981) Organic and
organotin compounds leached from PVC and CPVC pipe. Ann Arbor,
Michigan, US Environmental Protection Agency, 116 pp
(EPA-600/1-81-062).
Botta D, Castellani Pirri L, & Mantica E (1984) Ground water pollution
by organic solvents and their microbial degradation products. In:
Analysis of organic micropollutants in water. Brussels, Commission of
the European Communities, pp 261-275 (Report EUR 8518).
Bradow JM & Connick WJ (1988) Volatile methyl ketone seed-germination
inhibitors from Amaranthus palmeri S Wats. residues. J Chem Ecol,
14: 1617-1631.
Bradow JM & Connick WJ (1990) Volatile seed germination inhibitors
from plant residues. J Chem Ecol, 16: 645-666.
Brady JF, Li D, Ishizaki H, Lee M, Ning SM, Xiao F, & Yang CS (1989)
Induction of cytochromes P450 IIE1 and P450IIB1 by secondary ketones
and the role of P450IIE1 in chloroform metabolism. Toxicol Appl
Pharmacol, 100: 342-349.
Bridie AL, Winter M, & Wolff CJM (1979a) BOD and COD of some
petrochemicals. Water Res, 13(7): 627-630.
Bridie AL, Wolff CJM, & Winter M (1979b) The acute toxicity of some
petrochemicals to goldfish. Water Res, 13: 623.
Bringmann G (1978) [Determination of the biological toxicity of water
pollutants on Protozoa.] Z Wasser Abwasser Forsch, 11: 210-215 (in
German).
Bringmann G & Kuhn R (1977a) [Toxicity threshold for water pollutants
in the cell multiplication test with respect to bacteria (Pseudomonas
putida) and green algae (Scenedesmus quadricauda).] Z Wasser
Abwasser Forsch, 10: 87-98 (in German).
Bringmann G & Kuhn R (1977b) [The effects of water pollutants on
Daphnia magna.] Z Wasser Abwasser Forsch, 10: 161-166 (in German).
Bringmann G & Kuhn R (1978) Testing of substances for their toxicity
threshold: Model organisms Microcystis (Diplocystis) aeruginosa and
Scenedesmus quadricauda. Mitt Int Ver Limnol, 21: 275-284.
Brondeau MT, Ban M, Bonnet P, Guenier JP, & De Ceaurriz J (1989)
Acetone compared to other ketones in modifying the hepatotoxicity of
inhaled 1,2-dichlorobenzene in rats and mice. Toxicol Lett, 49: 69-78.
Brooks TM, Meyer AL, & Hutson DH (1988) The genetic toxicology of some
hydrocarbon and oxygenated solvents. Mutagenesis, 3: 227-232.
Brown EM & Hewitt WR (1984) Dose-response relationships in
ketone-induced potentiation of chloroform hepato- and nephrotoxicity.
Toxicol Appl Pharmacol, 76: 437-453.
Brown RH & Purnell CJ (1979) Collection and analysis of trace organic
vapour pollutants in ambient atmospheres. The performance of a
Tenax-GC absorbent tube. J Chromatogr, 178: 79-90.
Brugnone F (1985) Uptake of solvents from the lungs. Br J Ind Med,
42: 569.
Brugnone F, Perbellini L, Gaffuri E, & Costa G (1981) [Occupational
pollution by shoe factory solvents.] Ann Ist Super Sanità, 17: 531-534
(in Italian).
Brugnone F, Perbellini L, Apostoli P, Caretta D, & Cocheo V (1983)
Environmental and behavioural monitoring of occupational methyl ethyl
ketone exposure. Dev Sci Pract Toxicol, 11: 571-574.
Bryce DJ & Greenwood CT (1963) The thermal degradation of starch. Part
III. The formation of decomposition products from starch and related
materials at temperatures between 175 and 400 °C. Stärke, 15(19):
359-363.
Cammaerts MC, Inwood MR, Morgan ED, Parry K, & Tyler RC (1978)
Comparative study of the pheromones emitted by workers of the ants
Myrmica rubra and Myrmica scabrinodis. J Insect Physiol, 24:
207-214.
Carpenter CP & Smyth HF (1946) Chemical burns of the rabbit cornea. Am
J Ophthamol, 29: 1363-1372.
Cavender FL, Casey HW, Salem H, Swenberg JA, & Gralla EJ (1983) A
90-day vapor inhalation toxicity study of methyl ethyl ketone. Fundam
Appl Toxicol, 3: 264-270.
Chemical Business Newsbase (1986) #537712 [Organic chemicals
production in Argentina 1985.] Ind Quim, 281: 36-37 (in Spanish).
Chemical Business Newsbase (1987) #584370 The chemical industry of
Japan, 1986: B-B fraction derivatives. Japan Chem Ann, 1987/1988: 41.
Chemical Business Newsbase (1988) #600843 Methyl ethyl ketone makers
need Pampa for recovery. Chem Mark Rep, 234(16): 9, 18-19.
Chen T-H, Kavanagh TJ, Chang CC, & Trosko JE (1984) Inhibition of
metabolic cooperation in Chinese hamster V79 cells by various organic
solvents and simple compounds. Cell Biol Toxicol, 1: 155-171.
Chiavari G, Facchini MC, & Fuzzi S (1987) Behavior of
3-methyl-2-benzothiazolone azines of carbonyl compounds in
high-performance liquid chromatography. J Chromatogr, 387: 459-466.
Chiou CT, Freed VH, Siddigi RH, & Mckeon K (1977) Partition
coefficients and bioaccumulation of selected organic chemicals.
Environ Sci Technol, 11: 475-478.
Clayton GD & Clayton FE (1981) Patty's industrial hygiene and
toxicology. New York, John Wiley & Sons, Wiley Interscience, 3 vols.
Cohen SR & Maier AA (1974) Occupational health case report - No. 2,
toluene diisocyanate. J Occup Med, 16: 114-118.
Conkle JP, Camp BJ, & Welch BE (1975) Trace composition of human
respiratory gas. Arch Environ Health, 30: 290-295.
Connick WJ, Bradow JM, & Legendre MG (1989) Identification and
bioactivity of volatile allelochemicals from amaranth residues.
J Agric Food Chem, 37: 792-796.
Cornish HH & Adefuin J (1967) Potentiation of carbon tetrachloride
toxicity by aliphatic alcohols. Arch Environ Health, 14: 447-449.
Corwin JF (1969) Volatile oxygen-containing organic compounds in sea
water: Determination. Bull Mar Sci, 19: 504-509.
Couri D, Hetland LB, O'Neill JJ, Ganansia M-F, Jackson DB, Gardier RW,
Marks BH, Weiss H, Mendell JR, Saida K, Allen N, & Chrisman CL (1974)
Comments on a plastics industry neurotoxicity in relationship to
methylbutyl ketone. In: Proceedings of the 5th Annual Conference on
Environmental Toxicology, Fairborn, Ohio, 24-26 September 1974. Wright
Patterson Air Force Base, Ohio, Aerospace Medical Research Laboratory,
pp 109-120 (AMRL Technical Report No. 74-125).
Couri D, Hetland LB, Abdel-Rahman MS, & Weiss H (1977) The influence
of inhaled ketone solvent vapors on hepatic microsomal
biotransformation activities. Toxicol Appl Pharmacol, 41: 285-289.
Couri D, Abdel-Rahman MS, & Hetland LB (1978) Biotransformation of
n-hexane and methyl n-butyl ketone in guinea-pigs and mice. Am Ind Hyg
Assoc J, 39: 295-300.
Creech G, Johnson RT, & Stoffer JO (1982) A comparison of three
different high-performance liquid chromatography systems for the
determination of aldehydes and ketones in diesel exhaust. Part I.
J Chromatogr Sci, 20: 67-72.
Cresci A, La Rosa F, Pannelli F, Orpianesi C, Saltamacchia G, Spinaci
G, & Pierini M (1985) [Solvent exposure and work sites in eleven shoe
and leather factories.] Nuovi Ann Ig Microbiol, 36: 61-76 (in
Italian).
Curtis C, Lima A, Lozano SJ, & Veith GD (1982) Evaluation of a
bacterial bioluminescence bioassay as a method for predicting acute
toxicity of organic chemicals to fish. In: Pearson JG, Foster RB, &
Bishop WE ed. Aquatic toxicology and hazard assessment: Fifth
Conference. Philadelphia, Pennsylvania, American Society for Testing
and Materials, pp 170-178 (ASTM STP 766).
Cushny AR (1910) On the exhalation of drugs by the lungs. J Physiol,
40: 17-27.
Davis PL, Munroe KA, & Selhime AG (1977) Laboratory bioassay of
volatile naturally occurring compounds against the Caribbean fruit
fly. Citrus Ind, July: 24-26.
Day EA, Bassette R, & Keeney M (1960) Identification of volatile
carbonyl compounds from Cheddar cheese. J Dairy Sci, 43: 463-474.
Deacon MM, Pilny MD, John JA, Schwetz BA, Murray FJ, Yakel HO, & Kuna
RA (1981) Embryo- and fetotoxicity of inhaled methyl ethyl ketone in
rats. Toxicol Appl Pharmacol, 59: 620-622.
De Bortoli M, Knoppel H, Pecchio E, Peil A, Rogora L, Schauenburg H,
Schlitt H, & Vissers H (1985) Measurements of indoor air quality and
comparison with ambient air: A study on 15 homes in northern Italy.
Ispra, Italy, Commission of the European Communities, 57 pp.
De Bortoli M, Knoppel H, Pecchio E, Peil A, Rogora L, Schauenburg H,
Schlitt H, & Vissers H (1986) Concentrations of selected organic
pollutants in indoor and outdoor air in northern Italy. Environ Int,
12: 343-350.
Decaprio AP (1987) n-Hexane neurotoxicity: A mechanism involving
pyrrole adduct formation in axonal cytoskeletal protein.
Neurotoxicology, 8: 199-210.
De Ceaurriz J, Desilies JP, Bonet P, Marignac B, Muller J, & Guenier
JP (1983) Concentration-dependent behavioral changes in mice
following short-term inhalation exposure to various industrial
solvents. Toxicol Appl Pharmacol, 67: 383-389.
Decker DW, Clark CS, Elia VJ, Kominsky JR, & Trapp JH (1983) Worker
exposure to organic vapors at a liquid chemical waste incinerator. Am
Ind Hyg Assoc J, 44: 296-300.
Delfino JJ & Miles CJ (1985) Aerobic and anaerobic degradation of
organic contaminants in Florida groundwater. Soil Crop Sci Soc Florida
Proc, 44: 9-14.
Del Rosario R, De Lumen BO, Habu T, Flath RA, Mon TR, & Teranishi R
(1984) Comparison of headspace volatiles from winged beans and
soybeans. J Agric Food Chem, 32: 1011-1015.
Denkhaus W, Von Steldern D, Botzenhardt U, & Konietzko H (1986)
Lymphocyte subpopulations in solvent-exposed workers. Int Arch Occup
Environ Health, 57: 109-115.
De Rosa E, Bartolucci GB, Brighenti F, Gori GP, Sigon M, & Toffolo D
(1985) The industrial use of solvents and risk of neurotoxicity. Ann
Occup Hyg, 29(1): 391-397.
Descotes J (1988) Identification of contact allergens: The mouse ear
sensitization assay. J Toxicol Cutan Ocular Toxicol, 7: 263-272.
Deveaux M & Huvenne JP (1987) Identification of solvents of abuse
using gas chromatography/Fourier transform infrared spectrometry after
headspace sampling. Chromatographia, 23: 626-630.
Dick RB, Setzer JV, Wait R, Hayden MB, Taylor BJ, Tolos B, &
Putz-Anderson V (1984) Effects of acute exposure of toluene and
methyl ethyl ketone on psychomotor performance. Int Arch Occup Environ
Health, 54: 91-109.
Dick RB, Brown WD, Setzer JV, Taylor BJ, & Shukla R (1988) Effects of
short duration exposures to acetone and methyl ethyl ketone. Toxicol
Lett, 43: 31-49.
Dick RB, Setzer JV, Taylor BJ, & Shukla R (1989) Neurobehavioral
effects of short duration exposures to acetone and methyl ethyl
ketone. Br J Ind Med, 46: 111-121.
Dietz FK & Traiger GJ (1979) Potentiation of CCl4 of hepatotoxicity
in rats by a metabolite of 2-butanone: 2,3-butanediol. Toxicology,
14: 209-215.
Dietz FK, Rodriguez-Giaxola M, Traiger GJ, Stella VJ, & Himmelstein KJ
(1981) Pharmacokinetics of 2-butanol and its metabolites in the rat.
J Pharmacokinet Biopharm, 9: 553-576.
Digiacomo JD (1973) New approaches for the design of afterburners for
varnish cookers. J Air Pollut Control Assoc, 23: 287-290.
Dilling WL, Bredeweg DJ, & Tefertiller NB (1976) Organic
photochemistry. XIII. Simulated atmospheric photodecomposition rates
of methylene chloride, 1,1,1-trichloro-ethane, tetrachloroethylene,
and other compounds. Environ Sci Technol, 10: 351-356.
Divincenzo GD & Krasavage WJ (1974) Serum ornithine carbamyl
transferase as a liver response test for exposure to organic solvents.
Am Ind Hyg Assoc J, 35: 21-29.
Divincenzo GD, Kaplan CJ, & Dedinas J (1976) Characterization of the
metabolites of methyl-N-butyl ketone, methyl iso-butyl ketone, and
methyl ethyl ketone in guinea-pig serum and their clearance. Toxicol
Appl Pharmacol, 36: 511-522.
Divincenzo GD, Hamilton ML, Kaplan CJ, Krasavage WJ, & O'Donoghue JL
(1978) Studies on the respiratory uptake and excretion and the skin
absorption of methyl-n-butyl ketone in humans and dogs. Toxicol Appl
Pharmacol, 44: 593-604.
Dojlido JR (1977) Testing of biodegradability and toxicity of organic
compounds in industrial wastewaters. In: Polish/US Symposium on
Wastewater Treatment and Sludge Disposal, Cincinnati, Ohio, 10-12
February 1976. Cincinnati, Ohio, US Environmental Protection Agency,
vol II, pp 112-131 (EPA 600/9-76-021; NTIS PB-261422).
Dojlido JR (1979) Investigations of biodegradability and toxicity of
organic compounds. Washington, DC, US Environmental Protection Agency,
99 pp (EPA-600/2-79-163).
Dore M, Brunet N, & Legube B (1975) Participation of various organic
compounds in the evaluation of global pollution criteria. Trib
Cebedeau, 28: 3-11.
Dornseifer TP, Kim SC, Keith ES, & Powers JJ (1965) Effect of moisture
level on volatile carbonyls in cottonseed oil heated to 210 °C. J Am
Oil Chem Soc, 42: 1073-1075.
Dowty BJ, Laseter JL, & Storer J (1976) The transplacental migration
and accumulation in blood of volatile organic constituents. Pediatr
Res, 10: 696-701.
Draize JH, Woodard G, & Calvery HO (1944) Methods for the study of
irritation and toxicity of substances applied topically to the skin
and mucous membranes. J Pharmacol Exp Ther, 82: 377-389.
Duckett S, Williams N, & Francis S (1974) Peripheral neuropathy
associated with inhalation of methyl-n-butyl ketone.
Experientia(Basel), 30: 1283-1284.
Dutch Expert Committee for Occupational Standards (1991) Health-based
recommended occupational exposure limit for methyl ethyl ketone.
Voorburg, Directorate General of Labour of the Ministry of Social
Affairs and Employment, Dutch Expert Committee for Occupational
Standards, 45 pp (Report RA 16/90).
Dyro FM (1978) Methyl ethyl ketone polyneuropathy in shoe factory
workers. Clin Toxicol, 13: 371-376.
Egyud LG (1967) Studies on cell division: the effect of aldehydes,
ketones and a-keto-aldehydes on the proliferation of Escherichia
coli. Curr Mod Biol, 1: 14-20.
Elskamp CJ & Schultz GR (1983) An alternative sampling and analytical
method for 2- butanone. Am Ind Hyg Assoc J, 44: 201-204.
Emmel TE, Lee BB, & Simonson AB (1983) Control technology assessment
of petroleum refinery operations. In depth site visit report: Congo
Refinery, Farmer's Valley Refinery. Cincinnati, Ohio, National
Institute of Occupational Safety and Health, 60 pp (Contract No.
210-81-7102).
Ewing BB, Chian ESK, Cook JK, Evans CA, Hopke PK, & Perkins EG (1977)
Monitoring to detect previously unrecognized pollutants in surface
waters. Appendix: Organic analysis data. Washington, DC, US
Environmental Protection Agency, p 75 (EPA- 560/6-77-015).
Fagius J & Grönqvist B (1978) Function of peripheral nerves and signs
of polyneuropathy in solvent-exposed workers at Swedish steelworks.
Acta Neurol Scand, 57: 305-316.
Falla NAR (1987) Solving air pollution problems in the surface
coatings industry. Polym Paint Colour J, 98: 103-104, 106, 108, 110.
Feigley CE & Chastain JB (1982) An experimental comparison of three
diffusion samplers exposed to concentration profiles of organic
vapors. Am Ind Hyg Assoc J, 43: 227-234.
Fernandes MH, Gilbert SG, Paik SW, & Stier EF (1986) Study of the
degradation products formed during extrusion lamination of an ionomer.
J Food Sci, 51: 722-725.
Fiserova-Bergerova V & Diaz ML (1986) Determination and prediction of
tissue-gas partition coefficients. Int Arch Occup Environ Health,
58: 75-87.
Fisher GS, Legendre MG, Lovgren NV, Schuller WH, & Wells JA (1979)
Volatile constituents of southernpea seed [ Vigna unguiculata (L.)
Walp.] J Agric Food Chem, 27: 7-11.
Fisk JF (1986) Volatile organic analytical methods - general
description and quality control considerations. In: Perket CL ed.
Quality control in remedial site investigation: Fifth volume -
Hazardous and industrial solid waste testing. Philadelphia,
Pennsylvania, American Society for Testing and Materials, 12 pp (ASTM
STP 925).
Florin I, Rutberg L, Curvall M, & Enzell CR (1980) Screening of
tobacco smoke constituents for mutagenicity using the Ames' test.
Toxicology, 18: 219-232.
Freddi A, Paci A, Vittori O, De Ciantis R, & Ottaviano PF (1982)
[Clinical and electromyographic study of workers exposed to methyl
ethyl ketone vapor.] Ann Med Perugia, 73: 111-136 (in Italian).
French RC (1961) Stimulation of uredospore germination in wheat stem
rust by terpenes and related compounds. Bot Gaz, 122: 194-198.
French RC (1984) Stimulation of uredospore germination of Puccinia
helianthi and Uromyces vignae by aromatic nitriles and related
flavorlike compounds. J Agric Food Chem, 32: 556-561.
French RC, Graham CL, Gale AW, & Long RK (1977) Structural and
exposure time requirements for chemical stimulation of germination of
uredospores of Uromyces phaseoli. J Agric Food Chem, 25: 84-88.
Gadomski RR, Gimborne AV, & Green WJ (1974) An evaluation of emissions
and control technologies for the metal decorating process. J Air
Pollut Control Assoc, 24: 579-585.
Garcia CR, Geller I, & Kaplan HL (1978) Effects of ketones on
lever-pressing behavior of rats. Proc West Pharmacol Soc, 21: 433-438.
Geller I, Martinez RL, Hartmann RJ, & Kaplan HL (1978) Effects of
ketones on a match to sample task in the baboon. Proc West Pharmacol
Soc, 21: 439-442.
Gerhold RM & Malaney GW (1966) Structural determinants in the
oxidation of aliphatic compounds by activated sludge. J Water Pollut
Control Fed, 38: 562-579.
Ghittori S, Imbriani M, Pezzagno G, & Capodaglio E (1987) The urinary
concentration of solvents as a biological indicator of exposure:
Proposal for the biological equivalent exposure limit for nine
solvents. Am Ind Hyg Assoc J, 48: 786-790.
Gianturco MA, Giammarino S, & Friedel P (1966) Volatile constituents
of coffee-V. Nature (Lond), 210(5043): 1358.
Glowa JR & Dews PB (1987) Behavioral toxicology of volatile organic
solvents. IV. Comparisons of the rate-decreasing effects of acetone,
ethyl acetate, methyl ethyl ketone, toluene, and carbon disulfide on
schedule-controlled behavior of mice. J Am Coll Toxicol, 6: 461-469.
Gordon DT & Morgan ME (1972) Principal volatile compounds in feed
flavored milk. J Dairy Sci, 55: 905-912.
Gosselin RE, Smith RP, & Hodge HC (1984) Clinical toxicology of
commercial products. Baltimore, Maryland, Williams and Wilkins, pp
III/101-III/107.
Goto I, Matsumura M, Inoue N, Murai Y, Shida K, Santa T, & Kuroiwa Y
(1974) Toxic polyneuropathy due to glue sniffing. J Neurol Neurosurg
Psychiatry, 37: 848-853.
Grey TC & Shrimpton DH (1967) Volatile components of raw chicken
breast muscle. Br Poult Sci, 8: 23-33.
Grosjean D (1982) Formaldehyde and other carbonyls in Los Angeles
ambient air. Environ Sci Technol, 16: 254-262.
Grosjean D & Wright B (1983) Carbonyls in urban fog, ice, cloudwater
and rainwater. Atmos Environ, 17: 2093-2096.
Grosjean D, Swanson RD, & Ellis C (1983) Carbonyls in Los Angeles air:
Contribution of direct emissions and photochemistry. Sci Total
Environ, 29: 65-85.
Gurka DF, Warner JS, Silvon LE, Bishop TA, & Mckown MM (1984) Interim
method for determination of volatile organic compounds in hazardous
wastes. J Assoc Off Anal Chem, 67: 776-782.
Hampton CV, Pierson WR, Harvey TM, Updegrove WS, & Marano RS (1982)
Hydrocarbon gases emitted from vehicles on the road. 1. A qualitative
gas chromatography/mass spectrometry survey. Environ Sci Technol, 16:
287-298.
Harvey RJ & Walker JRL (1960) Some volatile compounds in New Zealand
Cheddar cheese and their possible significance in flavour formation.
III Time of first appearance of volatile carbonyl compounds during
ripening. J Dairy Res, 27: 335-340.
Hawthorne SB, Sievers RE, & Barkley RM (1985) Organic emissions from
shale oil wastewaters and their implications for air quality. Environ
Sci Technol, 19: 992-997.
Hazleton (1963a) Primary skin irritation-rabbits. Falls Church,
Virginia, Hazleton Laboratories Inc. (Laboratory Report No. 63 MRL
003).
Hazleton (1963b) Acute eye irritation-rabbits. Falls Church, Virginia,
Hazleton Laboratories Inc. (Laboratory Report No. 63 MRL 004).
Heitmuller PT, Hollister TA, & Parrish PR (1981) Acute toxicity of 54
industrial chemicals to sheepshead minnows (Cyprinodon variegatus).
Bull Environ Contam Toxicol, 27: 596-604.
Hewitt WR, Miyajima H, Cote MG, & Plaa GL (1980) Modification of
haloalkane- induced hepatotoxicity by exogenous ketones and metabolic
ketosis. Fed Proc, 39: 3118-3123.
Hewitt WR, Brown EM, & Plaa GL (1983) Relationship between the carbon
skeleton length of ketonic solvents and potentiation of
chloroform-induced hepatotoxicity in rats. Toxicol Lett, 16: 297-304.
Hewitt LA, Ayotte P, & Plaa GL (1986) Modifications in rat
hepatobiliary function following treatment with acetone, 2-butanone,
2-hexanone, mirex, or chlordecone and subsequently exposed to
chloroform. Toxicol Appl Pharmacol, 83: 465-473.
Hewitt LA, Valiquette C, & Plaa GL (1987) The role of
biotransformation-detoxication in acetone-, 2-butanone-, and
2-hexanone-potentiated chloroform-induced hepatotoxicity. Can J
Physiol Pharmacol, 65: 2313-2318.
Hickey JLS & Bishop CC (1981) Field comparison of charcoal tubes and
passive vapor monitors with mixed organic vapors. Am Ind Hyg Assoc J,
42: 264-267.
Higgins CE, Griest WH, & Olerich G (1983) Application of Tenax
trapping to analysis of gas phase organic compounds in ultra-low tar
cigarette smoke. J Assoc Off Anal Chem, 66: 1074-1083.
Holmberg I & Malmfors T (1974) The cytotoxicity of some organic
solvents. Environ Res, 7: 183-192.
Horton AW, Bingham EL, Burton MJG, & Tye R (1965) Carcinogenesis of
the skin. III. The contribution of elemental sulfur and of organic
sulfur compounds. Cancer Res, 25: 1759-1763.
Hoshika Y, Kozima I, Koike K, & Yosimoto K (1976) [Gas chromatographic
analysis of lower aliphatic carbonyl compounds using the imine
formation reaction.] Aichi-ken Kogai Chosa Senta Shoho, 4: 108-113 (in
Japanese).
Hou CT, Patel R, Laskin AI, Barnabe N, & Barist I (1983) Production of
methyl ketones from secondary alcohols by cell suspensions of C2 to
C4 n-alkane-grown bacteria. Appl Environ Microbiol, 46: 178-184.
Hrdlicka J & Kuca J (1965) The changes of carbonyl compounds in the
heat-processing of meat: 2, Turkey meat. Poult Sci, 44: 27-31.
Husman K (1980) Symptoms of car painters with long-term exposure to a
mixture of organic solvents. Scand J Work Environ Health, 6: 19-32.
Husman K & Karli P (1980) Clinical neurological findings among car
painters exposed to a mixture of organic solvents. Scand J Work
Environ Health, 6: 33-39.
Imai T, Takigawa H, Nakagawa S, Shen GJ, Kodama T, & Minoda Y (1986)
Microbial oxidation of hydrocarbons and related compounds by
whole-cell suspensions of the methane-oxidizing bacterium H-2. Appl
Environ Microbiol, 52: 1403-1406.
Ingram L (1977) Changes in lipid composition of Escherichia coli
resulting from growth with organic solvents and with food additives.
Appl Environ Microbiol, 33: 1233-1236.
Inoue T, Takeuchi Y, Hisanaga N, Ono Y, Iwata M, Ogata M, Saito K,
Sakurai H, Hara I, Matsushita T, & Ikeda M (1983) A nationwide survey
on organic solvent components in various solvent products: Part 1.
Homogeneous products such as thinners, degreasers and reagents. Ind
Health, 21: 175-183.
IRPTC (1987) Methyl ethyl ketone. In: IRPTC legal file 1986. Geneva,
International Register of Potentially Toxic Chemicals, United Nations
Environment Programme, vol I, pp 11/238-11/240.
IRPTC (1991) IRPTC legal file. Geneva, International Register of
Potentially Toxic Chemicals, United Nations Environment Programme,
pp 17/1-17/5.
Iwata M, Takeuchi Y, Hisanaga N, & Ono Y (1983) Changes of n-hexane
metabolites in urine of rats exposed to various concentrations of
n-hexane and to its mixture with toluene or MEK. Int Arch Occup
Environ Health, 53: 1-8.
Iwata M, Takeuchi Y, Hisanaga N, & Ono Y (1984) Changes of n-hexane
neurotoxicity and its urinary metabolites by long-term co-exposure
with MEK or toluene. Int Arch Occup Environ Health, 54: 273-281.
Jacot BJ (1983) OVA field screening at hazardous waste sites. In:
National Conference on Management of Uncontrolled Hazardous Waste
Sites, Washington, 31 October-2 November 1983. Silver Spring,
Maryland, Hazardous Materials Control Research Institute, pp 76-78.
Jarke FH, Dravnieks A, & Gordon SM (1981) Organic contaminants in
indoor air and their relation to outdoor contaminants. Am Soc Heat
Refrig Air-Cond Eng Trans, 87: 153-166.
Johansson I & Ingelman-Sundberg M (1985) Carbon tetrachloride-induced
lipid peroxidation dependent on an ethanol-inducible form of rabbit
liver microsomal cytochrome P-450. FEBS Lett, 183: 265-269.
John JA, Pilny MK, Kuna RA, Deacon MM, & Yakel HO (1980) Teratogenic
evaluation of methyl ethyl ketone in the rat. Teratology, 21(3): 47A.
Jonsson A, Persson KA, & Grigoriadis V (1985) Measurements of
low-molecular-weight oxygenated, aromatic, and chlorinated
hydrocarbons in ambient air and in vehicle emissions. Environ Int,
11: 383-392.
JRB Associates Inc (1980) Support document, chapter IV. Health effects
of methyl ethyl ketone. Washington, DC, US Environmental Protection
Agency, 58 pp (Contract No. 68-01-4839, Task No. 11, Project No.
2-800-08-107-04).
Juhnke I & Luedemann D (1978) [Results of the investigation of 300
chemical compounds for acute toxicity with the Golden Orfe test.]
Z Wasser Abwasser Forsch, 11: 161-164 (in German).
Jungclaus GA, Lopez-Avila V, & Hites RA (1978) Organic compounds in an
industrial wastewater: A case study of their environmental impact.
Environ Sci Technol, 12: 88-96.
Kahn JH, Laroe EG, & Conner HA (1968) Whiskey composition:
identification of components by single-pass gas chromatography - mass
spectrometry. J Food Sci, 33: 395-400.
Kamil IA, Smith JN, & Williams RT (1953) Studies in detoxification.
46. The metabolism of aliphatic alcohols. The glucuronic acid
conjugation of acyclic aliphatic alcohols. Biochem J, 53: 129-135.
Kaye S (1961) Emergency toxicology. Springfield, Illinois, Charles C.
Thomas, pp 214-216.
Keen AR, Walker NJ, & Peberdy MF (1974) The formation of 2-butanone
and 2-butanol in Cheddar cheese. J Dairy Res, 41: 249-257.
Kenny J & Stratton G (1989) An improved desorption solvent for organic
solvent mixtures. Am Ind Hyg Assoc J, 50: 431-434.
Kezic S & Monster AC (1988) Determination of methyl ethyl ketone and
its metabolites in urine using capillary gas chromatography.
J Chromatogr, 428: 275-280.
Khasanov AA, Isidorov VA, Zenkevich IG, & Ioffee BV (1982) [Gas
chromatography/ mass spectrometry study of the composition of volatile
emissions from Juniperus serawechanica Kom.] Dokl Akad Nauk Uzb SSR,
12: 29-31 (in Russian).
Kimura ET, Ebert DM, & Dodge PW (1971) Acute toxicity and limits of
solvent residue for sixteen organic solvents. Toxicol Appl Pharmacol,
19: 699-704.
Ko I-Y, Park SS, Song BJ, Patten CH, Tan Y, Hah YC, Yang CS, & Gelboin
HV (1987) Monoclonal antibodies to ethanol-induced rat liver
cytochrome P-450 that metabolizes aniline and nitrosamines. Cancer
Res, 47: 3101-3109.
Kobayashi K, Tanaka M, & Kawai S (1980) Gas chromatography
determination of low-molecular-weight carbonyl compounds in aqueous
solution as their O-(2,3,4,5,6,-penta-fluorobenzyl) oximes.
J Chromatogr, 187: 413-417.
Kontominas MG & Voudouris E (1982) The determination of residual
solvents in plastics packaging materials in relation to off odors
developed in packaged bakery products. Chem Chron, 11: 215-223.
Kopelman PG & Kalfayan PY (1983) Severe metabolic acidosis after
ingestion of butanone. Br Med J, 286: 21-22.
Krasavage WJ, O'Donoghue JL, & Divincenzo GD (1982) Ketones. In:
Clayton GD & Clayton FE ed. Patty's industrial hygiene and toxicology,
3rd ed. New York, John Wiley and Sons, Wiley Interscience,
pp 4709-4800.
Kulshrestha DC & Marth EH (1974a) Inhibition of bacteria by some
volatile and non-volatile compounds associated with milk. I.
Escherichia coli. J Milk Food Technol, 37: 510-516.
Kulshrestha DC & Marth EH (1974b) Inhibition of bacteria by some
volatile and non-volatile compounds associated with milk. II.
Salmonella typhimurium. J Milk Food Technol, 37: 539-544.
Kulshrestha DC & Marth EH (1974c) Inhibition of bacteria by some
volatile and non-volatile compounds associated with milk. III.
Staphylococcus aureus. J Milk Food Technol, 37: 545-550.
Kulshrestha DC & Marth EH (1974d) Inhibition of bacteria by some
volatile and non-volatile compounds associated with milk. IV.
Streptococcus lactis. J Milk Food Technol, 37: 593-599.
Kulshrestha DC & Marth EH (1974e) Inhibition of bacteria by some
volatile and non-volatile compounds associated with milk. V.
Leuconostoc citrovorum. J Milk Food Technol, 37: 600-606.
Kulshrestha DC & Marth EH (1974f) Inhibition of bacteria by some
volatile and non-volatile compounds associated with milk. VI.
Streptococcus thermophilus. J Milk Food Technol, 37: 606-611.
Kupferschmid LL & Perkins JL (1986) Organic solvent recycling plant
exposure levels. Appl Ind Hyg, 1: 122-124.
Kwan W & Gatehouse A (1978) The effects of low doses of three
insecticides on activity, feeding, mating, reproductive performance
and survival in Glossina morsitans mortistans (Glossinidae). Entomol
Exp Appl, 23: 201-221.
La Belle CW & Brieger H (1955) The vapor toxicity of a composite
solvent and its principal compounds. Arch Ind Health, 12: 623-627.
Laity JL, Burstain IG, & Appel BR (1973) Photochemical smog and the
atmospheric reactions of solvents. In: Tess RW ed. Solvents, theory
and practice. Washington, DC, American Chemical Society, vol 7,
pp 96-112 (Advances in Chemistry Series 124).
Lande SS, Durkin PR, Christopher DH, Howard PH, & Saxena J (1976)
Investigation of selected potential environmental contaminants:
Ketonic solvents. Washington, DC, US Environmental Protection Agency,
330 pp (EPA-560/2-76-003).
Laregina J & Bozzelli JW (1986) Volatile organic compounds at
hazardous waste sites and a sanitary landfill in New Jersey. Environ
Prog, 5: 18-27.
Larson PS, Finnegan JK, & Haag HB (1955) Observations on the effect of
chemical configuration on the edema-producing potency of acids,
aldehydes, ketones and alcohols. J Pharmacol, 116: 119-122.
Leblanc G (1980) Acute toxicity of priority pollutants to water flea
(Daphnia magna). Bull Environ Contam Toxicol, 24: 684-691.
Leblanc G (1984) Interspecies relationships in acute toxicity of
chemicals to aquatic organisms. Environ Toxicol Chem, 3: 47-60.
Lee S & Murphy D (1982) Health hazard evaluation: Medalist-Gladiator
Athletic Products Company, Leesburg, Florida. Cincinnati, Ohio,
National Institute of Occupational Safety and Health, 15 pp (HETA
82-030-1184).
Lee S & Parkinson D (1982) Health hazard evaluation: Rola-Esmark
Company; Dubois, Pennsylvania. Cincinnati, Ohio, National Institute of
Occupational Safety and Health, 26 pp (HETA 80-168-1204).
Levin JO & Carleborg L (1987) Evaluation of solid sorbents for
sampling ketones in work-room air. Ann Occup Hyg, 31: 31-38.
Levy A (1973) The photochemical smog reactivity of organic solvents.
In: Tess RW ed. Solvents, theory and practice. Washington, DC,
American Chemical Society, vol 6, pp 71-93 (Advances in Chemistry
Series 124).
Li G-L, Yin S-N, Watanabe T, Nakatsuka H, Kasahara M, Abe H, & Ikeda
M (1986) Benzene-specific increase in leucocyte alkaline phosphatase
activity in rats exposed to vapors of various organic solvents. J
Toxicol Environ Health, 19: 581-589.
Liebich HM, Bertsch W, Zlatkis A, & Schneider HJ (1975) Volatile
organic components in the Skylab 4 spacecraft atmosphere. Aviat Space
Environ Med, 46: 1002-1007.
Liepins R, Mixon F, Hudak C, & Parsons TB (1977) Industrial process
profiles for environmental use: Chapter 6. The industrial organic
chemicals industry. Research Triangle Park, North Carolina, US
Environmental Protection Agency, pp 164-165, 560-561 (EPA-600/12).
Liira J, Riihimäki V, Engstrom K, & Pfaffli P (1988a) Coexposure of
man to m-xylene and methyl ethyl ketone, kinetics and metabolism.
Scand J Work Environ Health, 14: 322-327.
Liira J, Riihimäki V, & Pfaffli P (1988b) Kinetics of methyl ethyl
ketone in man: absorption, distribution and elimination in inhalation
exposure. Int Arch Occup Environ Health, 60: 195-200.
Liira J, Johanson G, & Riihimäki V (1990a) Dose-dependent kinetics of
inhaled methylethylketone in man. Toxicol Lett, 50: 195-201.
Liira J, Riihimäki V, & Engstrom K (1990b) The effects of ethanol on
the kinetics of methyl ethyl ketone in man. Br J Ind Med, 47: 325-330.
Liira J, Elovaara E, Raunio H, Riihimäki V, & Engstrom K (1991)
Metabolic interaction and disposition of methyl ethyl ketone and
m-xylene in rats at single and repeated inhalation exposures.
Xenobiotica, 21: 53-63.
Lin JCC & Jeon IJ (1985) Headspace gas sampling/GC method for the
quantitative analysis of volatile compounds in cheese. J Food Sci, 50:
843-844, 846.
Lindros KO, Cai Y, & Penttila KE (1990) Role of ethanol-inducible
cytochrome P-450IIE1 in carbon tetrachloride-induced damage to
centrilobular hepatocytes from ethanol-treated rats. Hepatology,
12: 1092-1097. Lowenheim FA & Moran MK (1975) Industrial chemicals, 4th
ed. New York, Chichester, Brisbane, Toronto, John Wiley and Sons,
pp 11-12, 539-542.
Lundberg I & Hakansson M (1985) Normal serum activities of liver
enzymes in Swedish paint industry workers with heavy exposure to
organic solvents. Br J Ind Med, 42: 596-600.
Lundberg I, Ekdahl M, Kronevi T, Vitauts L, & Lundberg S (1986)
Relative hepatotoxicity of some industrial solvents after
intraperitoneal injection or inhalation exposure in rats. Environ Res,
40: 411-420.
Mabuchi H (1969) [Urinary and serum volatile substances in diabetics.]
Nippon Naika Gakkai Zasshi, 58: 731-743 (in Japanese).
Manville Chemical Products Corporation (1988) Chemical products
synopsis, Asbury Park, New Jersey, Manville Chemical Products
Corporation, 1 p.
Marion CV & Malaney GW (1963) The oxidation of aliphatic compounds by
Alcalignes faecalis. J Water Pollut Control Fed, 35: 1269-1281.
Marnett LJ, Hurd HK, Hollstein MC, Levin DE, Esterbauer H, & Ames BN
(1985) Naturally occurring carbonyl compounds are mutagens in
Salmonella tester strain TA104. Mutat Res, 148: 25-34.
Mast TJ, Dill JA, Evanoff JJ, Rommereim RL, Weigel RJ, & Westerberg RB
(1989) Inhalation developmental toxicology studies: teratology study
of methyl ethyl ketone in mice. Richland, Washington, Pacific Norwest
Laboratory (Report No. NIH-YOI-ES-70153).
Mayer VW & Goin CJ (1987) Effects of chemical combinations on the
induction of aneuploidy in Saccharomyces cerevisiae. Mutat Res,
187: 21-30.
Mayer VW & Goin CJ (1988) Investigations of aneuploidy-inducing
chemical combinations in Saccharomyces cerevisiae. Mutat Res,
201: 413-421.
MB Research Laboratories, Inc. (1979) Test for eye irritation in
rabbits. Spinnerstown, Pennsylvania, MB Research Laboratories, Inc.,
5 pp (Unpublished report No. MB 79-3962).
Metcalf RL, Lu P, & Kapoor IP (1973) Environmental distribution and
metabolic fate of key industrial pollutants and pesticides in a model
ecosystem. Urbana, Illinois, University of Illinois, Water Research
Center (Report No. 69, Project B-050 Ill).
Mezey E (1976) Ethanol metabolism and ethanol-drug interactions.
Biochem Pharmacol, 25: 869-875.
Middleditch BS, Sung NJ, Zlatkis A, & Settembre G (1987) Trace
analysis of volatile polar organics by direct aqueous injection gas
chromatography. Chromatographia, 23: 273-278.
Misumi J & Nagano M (1985) Experimental study on the enhancement of
the neurotoxicity of methyl n-butyl ketone by non-neurotoxic aliphatic
monoketones. Br J Ind Med, 42: 155-161.
Miyasaka M, Kumai M, Koizumi A, Watanabe T, Kurasako K, Sato K, &
Ikeda M (1982) Biological monitoring of occupational exposure to
methyl ethyl ketone by means of urinalysis for methyl ethyl ketone
itself. Int Arch Occup Environ Health, 50: 131-137.
Mohren S & Juttner F (1983) Odorous compounds of different strains of
Anabaena and Nostoc (Cyanobacteria). Water Sci Technol, 15: 221-228.
Mookherjee RE, Deck RE, & Chang SS (1965) Relationship between
monocarbonyl compounds and flavor of potato chips. J Agric Food Chem,
13(2): 131-134.
Munies R & Wurster DE (1965) Investigation of some factors influencing
percutaneous absorption--III. Absorption of methyl ethyl ketone.
J Pharm Sci, 54: 1281-1284.
Murphy DC (1984) Acute illness among workers connected to solvent
exposure. Occup Health Saf, May: 36-38.
Mutti A, Cavatorta A, Lucertini S, Arfini G, Falzoi M, & Franchini I
(1982a) Neurophysiological changes in workers exposed to organic
solvents in a shoe factory. Scand J Work Environ Health, 8(Suppl 1):
136-141.
Mutti A, Ferri F, Lommi G, Lotta S, Lucertini S, & Franchini I (1982b)
n-Hexane induced changes in nerve conduction velocities and
somatosensory evoked potentials. Int Arch Environ Health, 51: 45-54.
Nakaaki K (1974) [An experimental study on the effect of exposure to
organic solvent vapor in human subjects.] J Sci Labour 50: 89-96 (in
Japanese).
Nakajima T, Wang R-S, Murayama N, & Sato A (1990) Three forms of
trichloro-ethylene-metabolizing enzymes in rat liver induced by
ethanol, phenobarbital, and 3-methylcholanthrene. Toxicol Appl
Pharmacol, 102: 546-552.
Nandi B & Fries N (1976) Volatile aldehydes, ketones, esters and
terpenoids as preservatives against storage fungi in wheat.
Z Pflanzenkr Pflanzenschutz, 83: 284-294.
Nestmann ER, Lee EGH, Matula TI, Douglas GR, & Mueller JG (1980)
Mutagenicity of constituents identified in pulp and paper mill
effluents using the Salmonella/mammalian-microsome assay. Mutat Res,
79: 203-212.
Ng H, Reed DJ, & Pence JW (1960) Identification of carbonyl compounds
in an ethanol extract of fresh white bread. Cereal Chem, 37: 638-645.
Nilsen OG & Toftgard R (1980) The influence of organic solvents on
cytochrome P-450- mediated metabolism of biphenyl and benzo(a)pyrene.
In: Coon MJ, Conney AH, Estabrook RW, Gelboin HV, Gillete JR, &
O'Brien PJ ed. Microsomes, drug oxidations, and chemical
carcinogenesis. New York, Academic Press, vol II, pp 1235-1238.
Noma E, Berglund B, Berglund U, Johansson I, & Baird JC (1988) Joint
representation of physical locations and volatile organic compounds in
indoor air from a healthy and a sick building. Atmos Environ,
22: 451-460.
Nye D (1975) Laboratory model ecosystem studies of the degradation and
fate of radiolabelled tri-, tetra-, and pentachlorobiphenyl compared
with DDE. Arch Environ Contam Toxicol, 3: 151-165.
O'Donoghue JL, Krasavage WJ, Divincenzo GD, & Katz GV (1984) Further
studies on ketone neurotoxicity and interactions. Toxicol Appl
Pharmacol, 72: 201-209.
O'Donoghue JL, Haworth SR, Curren RD, Kirby PE, Lawlor T, Moran EJ,
Phillips RD, Putnam DL, Rogers-Back AM, Slesinski RS, & Thilagar A
(1988) Mutagenicity studies on ketone solvents: methyl ethyl ketone,
methyl isobutyl ketone, and isophorone. Mutat Res, 206: 149-161.
Ogawa I & Fritz JS (1985) Determination of low concentrations of
low-molecular-weight aldehydes and ketones in aqueous samples.
J Chromatogr, 329: 81-89.
Oh SJ & Kim JM (1976) Giant axonal swelling in "hufferis" neuropathy.
Arch Neurol, 33: 583-586.
Ong CN, Sia GL, Ong HY, Phoon WH, & Tan KT (1991) Biological
monitoring of occupational exposure to methyl ethyl ketone. Int Arch
Occup Environ Health, 63: 319-324.
Osborne JS, Adamek S, & Hobbs ME (1956) Some components of gas phase
of cigarette smoke. Anal Chem, 28: 211-215.
Panson RD & Winek CL (1980) Aspiration toxicity of ketones. Clin
Toxicol, 17: 271-317.
Papa AJ & Sherman PD (1978) Ketones. In: Kirk RE & Othmer DF ed.
Encyclopedia of chemical technology, 3rd ed. New York, John Wiley and
Sons, Wiley-Interscience, vol 13, pp 894-912, 934-941.
Patel RN, Hou CT, & Laskin AI (1982) Oxidation of gaseous hydrocarbons
and related compounds by methanotrophic organisms. Dev Ind Microbiol,
23: 187-205.
Patel RN, Hou CT, Laskin AI, Felix A, & Derelanka P (1983) Oxidation
of alkanes by organisms grown on C2-C4 alkanes. J Appl Biochem,
5: 107-120.
Patty FA, Schrenk HH, & Yant WP (1935) Acute response of guinea pigs
to vapors of some new commercial organic compounds. VIII. Butanone. US
Public Health Rep, 50: 1217-1228.
Pellizzari ED, Castillo NP, Willis S, Smith D, & Bursey JT (1979)
Identification of organic components in energy related processes. In:
Van Hall CE ed. Measurement of organic pollutants in water and
wastewater: Proceedings of a symposium, Denver, Colorado, 19-20 June
1978. Philadelphia, Pennsylvania, American Society for Testing and
Materials, pp 256-274 (ASTM STP 686).
Pellizzari ED, Sheldon LS, Bursey JT, Michael LC, & Zweidinger RA
(1985) Master analytical scheme for organic compounds in water.
Athens, Georgia, US Environmental Protection Agency, pp 46-54, 108-127
(EPA/600/4-84-010A).
Perbellini L, Brugnone F, Mozzo P, Cocheo V, & Caretta D (1984) Methyl
ethyl ketone exposure in industrial workers. Uptake and kinetics. Int
Arch Occup Environ Health, 54: 73-81.
Perbellini L, Bartolucci G, Brugnone F, & De Rosa E (1985)
[2,5-Hexanedione in biological monitoring of occupational exposure to
n-hexane.] Med Lav, 76: 35-43 (in Italian).
Perocco P, Bolognesi S, & Alberghini W (1983) Toxic activity of
seventeen industrial solvents and halogenated compounds on human
lymphocytes cultured in vitro . Toxicol Lett, 16: 69-75.
Perry JJ (1968) Substrate specificity in hydrocarbon utilizing
micro-organisms. Antonie Van Leeuwenhoek, 34: 27-36.
Persson U, Lundqvist S, Marthinsson B, & Eng ST (1984)
Computer-automated carbon dioxide laser long-path absorption system
for air quality monitoring in the working environment. Appl Opt,
23: 998-1002.
Pezzagno G, Ghottori S, Imbriani M, & Capodaglio E (1983) [Measurement
of the solubility coefficients of gases and vapors in blood. II.
Widely used industrial solvents.] G Ital Med Lav, 5: 49-63 (in
Italian).
Poli G, Dianzani MU, Cheeseman KH, Slater TF, Lang J, & Esterbauer H
(1985) Separation and characterization of the aldehydic products of
lipid peroxidation stimulated by carbon tetrachloride or ADP-iron in
isolated rat hepatocytes and rat liver microsomal suspensions. Biochem
J, 227: 629-638.
Price K, Waggy G, & Conway R (1974) Brine shrimp bioassay and seawater
BOD of petrochemicals. J Water Pollut Control Fed, 46: 63-76.
Prockop LD, Alt M, & Tison J (1974) Huffer's neuropathy. J Am Med
Assoc, 229: 1083-1084.
Przyrembel H, Bremer HJ, Duran M, Bruinvis L, Ketting D, Wadman SK,
Baumgartner R, Irle U, & Bachmann C (1979) Propionyl-CoA carboxylase
deficiency with overflow of metabolites of isoleucine catabolism at
all levels. Eur J Pediatr, 130: 1-14.
Puskar MA, Levine SP, & Lowery SR (1986) Computerized infrared
spectral identification of compounds frequently found at hazardous
waste sites. Anal Chem, 58: 1156-1162.
Ralston WH, Hilderbrand RL, Uddin DE, Andersen ME, & Gardier RW (1985)
Potentiation of 2,5-hexanedione neurotoxicity by methyl ethyl ketone.
Toxicol Appl Pharmacol, 81: 319-327.
Ramsey JD & Flanagan RJ (1982) Detection and identification of
volatile organic compounds in blood by headspace gas chromatography as
an aid to the diagnosis of solvent abuse. J Chromatogr, 240: 423-444.
Raunio H, Liira J, Elovaara E, Riihimäki V, & Pelkonin O (1990)
Cytochrome P450 isozyme induction by methyl ethyl ketone and n-xylene
in rat liver. Toxicol Appl Pharmacol, 103: 175-179.
Reilly B (1988) Engineering assessment: Methyl ethyl ketone (MEK) and
methyl isobutyl ketone (MIBK) environmental releases. Washington, DC,
US Environmental Protection Agency, 20 pp (Unpublished document).
Reynolds T (1977) Comparative effects of aliphatic compounds on
inhibition of lettuce fruit germination. Am Bot 41: 637-648.
Riggin RM (1984) Compendium of methods for the determination of toxic
organic compounds in ambient air. Method T05. Research Triangle Park,
North Carolina, US Environmental Protection Agency, pp 1-22
(EPA-600/4-84-041).
Robertson P, White EL, & Bus JS (1989) Effects of methyl ethyl ketone
pretreatment on hepatic mixed function oxidose activity and on in
vivo metabolism of n-hexane. Xenobiotica, 19: 721-729.
Rose-Pehrsson SL, Grate JW, Ballentine DS, & Jurs PC (1988) Detection
of hazardous vapors including mixtures using pattern recognition
analysis of responses from surface acoustic wave devices. Anal Chem,
60: 2801-2811.
Ruth JH (1986) Odor thresholds and irritation levels of several
chemical substances: A review. Am Ind Hyg Assoc J, 47: 142-151.
Saida K, Mendell JR, & Weiss HS (1976) Peripheral nerve changes
induced by methyl n-butyl ketone and potentiation by methyl ethyl
ketone. J Neuropathol Exp Neurol, 35: 207-225.
Sakakibara H, Ide Y, Yanai T, Yajima J, & Hayashi K (1990) Volatile
flavour compounds of some kinds of dried and smoked fish. Agric Biol
Chem, 54: 9-16.
Sato A & Nakajima T (1979) Partition coefficients of some aromatic
hydrocarbons and ketones in water, blood and oil. Br J Ind Med,
36: 231-234.
Sato Y, Watanabe K, & Tanaka Y (1968) Chemical studies on smelling
compounds in hen's egg: Part I, Volatile carbonyl and basic compounds
in egg white. Agric Biol Chem, 32(4): 405-411.
Sauer TC (1981) Volatile liquid hydrocarbon characterization of
underwater hydrovents and formation waters from offshore production
operations. Environ Sci Tech, 15: 917-923.
Sawhney BL & Kozloski RP (1984) Organic pollutants in leachates from
landfill sites. J Environ Qual, 13: 349-352.
Schmidt R, Schnoy N, Altenkirch H, & Wagner HM (1984) Ultrastructural
alteration of intrapulmonary nerves after exposure to organic
solvents. Respiration, 46: 362-369.
Schnoy N, Schmidt R, Altenkirch H, & Wagner HM (1982) Ultrastructural
alteration of the alveolar epithelium after exposure to organic
solvents. Respiration, 43: 221-231.
Schörmuller J & Grosch W (1964) Aromatics of foods. II Occurrence of
additional carbonyl compounds in the tomato. Z Lebensmittel Unters
Forsch, 126: 38-49.
Schultz TH, Flath RA, Stern DJ, Mon TR, Teranishi R, Kruse SM, Butler
B, & Howard WE (1988) Coyote estrous urine volatiles. J Chem Ecol,
14: 701-712.
Schulz F, Mueller P, & Kramer D (1981) [Model experiments on the
phytotoxic effect of organic constituents in wastewater from lignite
refining in soil treatment.] Arch Acker-Pflanzenbau Bodenkd,
25: 745-753 (in German)
Schwetz BA, Leong BKJ, & Gehring PJ (1974) Embryo- and fetotoxicity of
inhaled carbon tetrachloride, 1,1-dichloroethane and methyl ethyl
ketone in rats. Toxicol Appl Pharmacol, 28: 452-464.
Schwetz BA, Mast TJ, Weigel RJ, Dill JA, & Morrissey RE (1991)
Developmental toxicity of inhaled methyl ethyl ketone in Swiss mice.
Fundam Appl Toxicol, 16: 742-748.
Seinfeld JH (1989) Urban air pollution: State of the science. Science,
243: 745-752.
Seizinger DE & Dimitriades B (1972) Oxygenates in exhaust from simple
hydrocarbon fuels. J Air Pollut Control Assoc, 22: 47-51.
Shah JJ & Singh HB (1988) Distribution of volatile organic chemicals
in outdoor and indoor air. Environ Sci Technol, 22: 1381-1388.
Shibata E, Huang J, Ono Y, Hisanaga N, Iwata M, Saito I, & Takeuchi Y
(1990a) Changes in urinary n-hexane metabolites by co-exposure to
various concentrations of methyl ethyl ketone and fixed n-hexane
levels. Arch Toxicol, 64: 165-168.
Shibata E, Huang J, Hisanaga Y, Ono Y, Saito I, & Takeuchi Y (1990b)
Effects of MEK on kinetics of n-hexane metabolites in serum. Arch
Toxicol, 64: 247-250.
Shimizu Y, Matsuto S, Ito T, & Okada I (1969) Studies on the flavor of
roast barley (Mugi-Cha): Part III. Separation and identification of
volatile mono-carbonyl compounds. Nippon Nogei Kagaku Kaishi,
43(4): 217-223 (in Japanese with English summary).
Smith LL (1981) Cholesterol autoxidation. New York, London, Plenum
Press, 674 pp.
Smith AR & Mayers MR (1944) Study of poisoning and fire hazards of
butanone and acetone. NY State Dept Labor Ind Bull, April: 174-176.
Smith AF & Wood R (1972) A field test for the determination of some
ketone vapors in air. Analyst, 97: 363-371.
Smyth HF, Carpenter CP, Weil CS, Pozzani UC, & Striegel JA (1962)
Range-finding toxicity data: list VI. Am Ind Hyg Assoc J, 23: 95-107.
Snider JR & Dawson GA (1985) Tropospheric light alcohols, carbonyls,
and acetonitrile: concentrations in the southwestern United States
and Henry's law data. J Geophys Res D Atmos, 90: 3797-3805.
Sosulski F & Mahmoud RM (1979) Effects of protein supplements on
carbonyl compounds and flavor in bread. Cereal Chem, 56: 533-536.
Specht H, Miller JW, Valaer PJ, & Sayers RR (1940) Acute response of
guinea-pigs to the inhalation of ketone vapors. Bull Natl Inst Health,
176: 1-66.
Spencer PS & Schaumburg HH (1976) Feline nervous system response to
chronic intoxication with commercial grades of methyl n-butyl ketone,
methyl isobutyl ketone, and methyl ethyl ketone. Toxicol Appl
Pharmacol, 37: 301-311.
Spencer PS, Schaumburg HH, Sabri MI, & Veronesi B (1980) The enlarging
view of hexacarbon neurotoxicity. CRC Crit Rev Toxicol, 7: 279-356.
SRI International (1985) Directory of chemical producers, Western
Europe, 8th ed. Menlo Park, California, SRI International, p 1457;
suppl II, p 55.
SRI International (1988) Directory of chemical producers, United
States. Menlo Park, California, SRI International, p 780.
Staüble EJ & Rast D (1971) [Volatile material in Agaricus biosporus.]
Experientia(Basel), 27: 886-888 (in German).
Stofberg J & Grundschober F (1984) Consumption ratio and food
predominance of flavoring materials--second cumulative series. Perfum
Flavorist, 9: 53-56, 58-59, 62, 65-72, 76-83.
Takeuchi Y, Ono Y, Hisanaga N, Iwata M, Aoyama M, Kitoh J, & Sugiura
Y (1983) An experimental study of the combined effects of n-hexane and
methyl ethyl ketone. Br J Ind Med, 40: 199-203.
Tangredi G, Carbone U, Rossi L, & Galdi A (1981) [Environmental
conditions in a paint factory and effects on employee health.] Riv Med
Lav Ig Ind, 5(Oct.-Dec.): 325-337 (in Italian).
Tham R, Bunnfors I, Eriksson B, Larsby B, Lindgrem S, & Odkvist LM
(1984) Vestibulo-ocular disturbances in rats exposed to organic
solvents. Acta Pharmacol Toxicol, 54: 58-63.
Thoburn T & Gunter B (1982) Health hazard evaluation; Davis Monthan
Air Force Base, Tucson, Arizona. Cincinnati, Ohio, National Institute
of Occupational Safety and Health, 23 pp (HETA 81-257-1115).
Toftgard R, Nilsen OG, & Gustafsson J-A (1981) Changes in rat liver
microsomal cytochrome P-450 and enzymatic activities after the
inhalation of n-hexane, xylene, methyl ethyl ketone and
methylchloroform for four weeks. Scand J Work Environ Health, 7:
31-37.
Toxigenics (1981) 90-day vapour inhalation toxicity study of methyl
ethyl ketone in albino rats. Decatur, Illinois, Toxigenics Inc. (Study
420-0305).
Traiger GJ & Bruckner JV (1976) The participation of 2-butanone in
2-butanol-induced potentiation of carbon tetrachloride hepatotoxicity.
J Pharmacol Exp Ther, 196: 493-499.
Traiger GJ & Plaa GL (1972) Relationship of alcohol metabolism to the
potentiation of CCl4 hepatotoxicity induced by aliphatic alcohols.
J Pharmacol Exp Ther, 183: 481-488.
Traiger GJ, Bruckner JV, Jiang WD, Dietz FK, & Cooke PH (1989) Effect
of 2-butanol and 2-butanone on rat hepatic ultrastructure and drug
metabolizing enzyme activity. J Toxicol Environ Health, 28: 235-248.
Triebig G, Bestler W, Baumeister P, & Valentin H (1983)
[Investigations on neurotoxicity of chemical substances at the
workplace. IV. Determination of the motor and sensory nerve conduction
velocity in persons occupationally exposed to a mixture of organic
solvents.] Int Arch Occup Environ Health 52: 139-150 (in German).
Tsao MU & Pfeiffer EI (1957) Isolation and identification of a new
ketone body in normal human urine. Proc Soc Exp Biol Med, 94: 628-629.
Tsukamoto S, Chiba S, Ishikawa T, & Shimaura M (1985a) Experimental
study on the metabolism of volatile hydrocarbons by inhalation of
natural gas. Nihon Univ J Med, 27: 33-38.
Tsukamoto S, Chiba S, Muto T, Ishakawa T, & Shimamura M (1985b) Study
on the metabolism of volatile hydrocarbons in mice--propane, n-butane,
and isobutane. J Toxicol Sci, 10: 323-332.
Union Carbide Corp. (1980a) The acute toxicity of MRD-80-2 to the
bluegill sunfish Lepomis macrochirus Rafinesque. Tarrytown, New York,
Union Carbide Corp., Environmental Services, 8 pp.
Union Carbide Corp. (1980b) The acute toxicity of MRD-80-2 to the
water flea Daphnia magna Straus. Tarrytown, New York, Union Carbide
Corp., Environmental Services, 6 pp.
US EPA (1985a) Health assessment document for chloroform. Research
Triangle Park, North Carolina, US Environmental Protection Agency,
pp 3-40-3-42 (EPA/600/8-84/004F).
US EPA (1985b) Health and environmental effects profile for methyl
ethyl ketone. Cincinnati, Ohio, US Environmental Protection Agency,
71 pp (EPA/600/X-85/363).
US EPA (1986) Health effects assessment for methyl ethyl ketone.
Cincinnati, Ohio, US Environmental Protection Agency, 17 pp
(EPA/540/1-86/003).
US NIOSH (1984a) Analytical method 2500: 2-butanone. Cincinnati, Ohio,
National Institute of Occupational Safety and Health, 3 pp.
US NIOSH (1984b) Analytical method 8002: (1)2-butanone, (2) ethanol
and (3) toluene in blood. Cincinnati, Ohio, National Institute for
Occupational Safety and Health, 4 pp.
US NIOSH (1990) Pocket guide to chemical hazards. Cincinnati, Ohio,
National Institute of Occupational Safety and Health, 48 pp (DHEW
(NIOSH) Publication No. 90-117).
Urano K & Kato Z (1986) Evaluation of biodegradation ranks of priority
organic compounds. J Hazard Mater, 13: 147-159.
USITC (1981) Synthetic organic chemicals: United States production and
sales, 1980. Washington, DC, United States International Trade
Commission, p 263 (USITC Publication No. 1183).
USITC (1982) Synthetic organic chemicals: United States production and
sales, 1981. Washington, DC, United States International Trade
Commission, p 243 (USITC Publication No. 1292).
USITC (1983) Synthetic organic chemicals: United States production and
sales, 1982. Washington, DC, United States International Trade
Commission, p 259 (USITC Publication No. 1422).
USITC (1984) Synthetic organic chemicals: United States production and
sales, 1983. Washington, DC, United States International Trade
Commission, p 257 (USITC Publication No. 1588).
USITC (1985) Synthetic organic chemicals: United States production and
sales, 1984. Washington, DC, United States International Trade
Commission, p 256 (USITC Publication No. 1745).
USITC (1986) Synthetic organic chemicals: United States production and
sales, 1985. Washington, DC, United States International Trade
Commission, p 266 (USITC Publication 1892).
USITC (1987) Synthetic organic chemicals: United States production and
sales, 1986. Washington, DC, United States International Trade
Commission, p 210 (USITC Publication No. 2009).
USITC (1988) Synthetic organic chemicals: United States production and
sales, 1987. Washington, DC, United States International Trade
Commission, p 15-5 (USITC Publication No. 2118).
Uspenskii IV & Repkina L (1974) [Physiological age and sensitivity to
DDT of natural populations of Ixodes persulcatus.] Parazitologiya,
8: 3-11 (in Russian).
Vale GA, Lovemore DF, Flint S, & Cockbill GF (1988) Odour-baited
targets to control tsetse flies, Glossina spp. (Diptera:
Glossinidae) in Zimbabwe. Bull Entomol Res, 78: 31-49.
Van Doorn JE, De Cock J, Kezic S, & Monster AC (1989) Determination of
methyl ethyl ketone in human urine after derivatization with
o-nitrophenylhydrazine, using solid-phase extraction and
reversed-phase liquid chromatography and ultraviolet detection.
J Chromatogr, 489: 419-424.
Varigos GA & Nurse DS (1986) A case of contact urticaria caused by
methyl ethyl ketone. Contact Dermatitis, 15(4): 249-260.
Veith GD, Call DJ, & Brooke LT (1983) Structure-toxicity relationships
for the fathead minnow, Pimephales promelas: Narcotic industrial
chemicals. Can J Fish Aquat Sci, 40: 743-748.
Verhoeff AP, Wilders MMW, Monster AC, & Van Wijnen JH (1987) Organic
solvents in the indoor air of two small factories and surrounding
houses. Int Arch Occup Environ Health, 59: 153-163.
Veronesi B (1984) An ultrastructural study of methyl ethyl ketone's
effect on cultured nerve tissues. Neurotoxicology, 5: 31-44.
Veronesi B, Lington AW, & Spencer PS (1984) A tissue culture model of
methyl ethyl ketone's potentiation of n-hexane neurotoxicity.
Neurotoxicology, 5: 43-52.
Verschuren K (1983) Handbook of environmental data on organic
chemicals. New York, Van Nostrand Reinhold Company, pp 850-852.
Viader F, Lechevalier B, & Morin P (1975) Polynéurite toxique chez un
travailleur du plastique. Rôle possible du méthyl éthyl-cétone. Nouv
Presse Méd, 4: 1813-1814.
Volskay VT & Grady CPL (1988) Toxicity of selected RCRA compounds to
activated sludge microorganisms. J Water Pollut Control Fed,
60: 1850-1856.
Von Cremer E & Riedmann M (1964) Identification of
gas-chromatographically separated aromatic materials in honey.
Z Naturforsch B19: 76-77.
Wahlberg JE (1984a) Edema-inducing effects of solvents following
topical administration. Dermatosen, 3: 91-94.
Wahlberg JE (1984b) Erythema-inducing effects of solvents following
epicutaneous administration to man - studied by laser Doppler
flowmetry. Scand J Work Environ Health, 10: 159-162.
Wallen I, Greer W, & Lasater R (1957) Toxicity to Gambusia affinis
of certain pure chemicals in turbid waters. Sewage Ind Wastes,
29: 265-711.
Walton BT, Anderson TA, Hendricks MS, & Talmage SS (1989)
Physicochemical properties as predictors of organic chemical effects
on soil microbial respiration. Environ Toxicol Chem, 8: 53-63.
Wang TC & Bricker JL (1979) 2-butanone and tetrahydrofuran
contamination in the water supply. Bull Environ Contam Toxicol,
23: 620-623.
Weast RC ed. (1986) CRC handbook of chemistry and physics, 67th ed.
Boca Raton, Florida, CRC Press, p C-170.
Weil CS & Scala RA (1971) Study of intra- and interlaboratory
variability in the results of rabbit eye and skin irritation tests.
Toxicol Appl Pharmacol, 19: 276-360.
Wen CP, Tsai SP, Weiss NS, Gibson RL, Wong O, & Mcclellan WA (1985)
Long-term mortality study of oil refinery workers. IV. Exposure to the
lubricating-dewaxing process. J Natl Cancer Inst, 74: 11-18.
Whelan JK, Tarafa ME, & Hunt JM (1982) Volatile C1-C8 organic
compounds in macroalgae. Nature(Lond), 299: 50-52.
Whitehead LW, Ball GL, Fine LJ, & Langolf GD (1984) Solvent vapor
exposures in booth spray painting and spray gluing, and associated
operations. Am Ind Hyg Assoc J, 45: 767-772.
Windholz M ed. (1983) The Merck index, 10th ed. Rahway, New Jersey,
Merck & Co., p 870.
Wong NP & Patton S (1962) Identification of some volatile compounds
related to the flavor of milk and cream. J Dairy Sci, 45: 724-728.
Wong NP, Damico JN, & Salwin H (1967) Decomposition and filth in
foods: Investigation of volatile compounds in cod fish by gas
chromatography and mass spectrometry. J Assoc Off Anal Chem,
50(1): 8-15.
WHO (1991) Environmental Health Criteria 122: n-Hexane. Geneva, World
Health Organization, 164 pp.
Wurster DE & Munies R (1965) Factors influencing percutaneous
absorption II, absorption of methyl ethyl ketone. J Pharm Sci,
54: 554-556.
Ying L & Levine SP (1989) Fourier transform infrared least-squares
methods for the quantitative analysis of multicomponent mixtures of
airborne vapours of industrial hygiene concern. Anal Chem,
61: 677-683.
Zakhari S, Leibowitz M, Levy P, & Aviado DM (1977) Isopropanol and
ketones in the environment. Cleveland, Ohio, CRC Press, pp 57-89.
Zechman JM, Aldinger S, & Labows JN (1986) Characterization of
pathogenic bacteria by automated headspace concentration-gas
chromatography. J Chromatogr, 377: 49-57.
Zimmermann FK, Mayer VW, Scheel I, & Resnick MA (1985) Acetone, methyl
ethyl ketone, ethyl acetate, acetonitrile and other polar aprotic
solvents are strong inducers of aneuploidy in Saccharomyces
cerevisiae. Mutat Res, 149: 339-351.
APPENDIX 1
Conversion factors for various solvents (ppm --> mg/m3)
acetone 2.38
2-butanol 3.03
n-butanol 3.03
2-butoxyethanol 4.83
butyl acetate 4.75
cyclohexane 3.44
DBP 11.38
DCB 6.01
ethanol 1.88
2-ethoxyethanol 3.68
ethyl acetate 3.6
ethyl butyl ketone 4.6
n-hexane 3.52
isobutanol 3.03
isopropanol 2.45
2-methoxyethanol 3.11
methyl acetate 3.6
methyl butyl ketone 4.1
methyl ethyl ketone 2.95
methyl isobutyl ketone 4.1
methylene chloride 3.48
toluene 3.75
trichloroethane 5.46
trichloroethylene 5.38
white spirit 1.75
xylene 4.34
From: Clayton & Clayton (1981) and Weast (1986)
RESUME
1. Propriétés et méthodes d'analyse
La méthyléthylcétone est un liquide limpide, incolore, volatil et
très inflammable dont l'odeur rappelle celle de l'acétone. Elle est
stable dans les conditions ordinaires, mais peut donner naissance à
des peroxydes explosifs en cas de stockage prolongé. Elle peut aussi
former des mélanges explosifs avec l'air. Elle est très soluble dans
l'eau, miscible à de nombreux solvants organiques et forme des
azéotropes avec l'eau et beaucoup de liquides organiques. Dans
l'atmosphère, elle produit des radicaux libres qui peuvent favoriser
la formation d'un smog photochimique.
On dispose de plusieurs méthodes analytiques pour mesurer les
concentrations de méthyléthylcétone dans l'air, l'eau, les
échantillons biologiques, les effluents et d'autres milieux. Dans les
méthodes les plus sensibles, la méthyléthylcétone est piégée et
concentrée soit sur un sorbant solide, soit sous forme de dérivé de la
2,4-dinitrophénylhydrazine (DNPH). La méthyléthylcétone absorbée et
les autres composés organiques volatils sont désorbés, séparés par
chromatographie gazeuse et dosés à l'aide d'un spectromètre de masse
ou d'un détecteur à ionisation de flammes. Le produit de dérivation de
la méthyléthylcétone est séparé des substances apparentées par
chromatographie liquide à haute performance et mesuré par
spectrophotométrie ultraviolette. Dans des milieux tels que les
déchets solides ou les substances biologiques, la méthyléthylcétone
doit d'abord être séparée du substrat, par exemple par extraction à
l'aide d'un solvant ou par distillation à la vapeur. Les
concentrations élevées de méthyléthylcétone dans l'air peuvent être
mesurées de façon continue par absorption infrarouge. Les limites de
détection sont de 3 µg/m3 dans l'air, 0,05 µg/litre dans l'eau de
boisson, 1,0 µg/litre dans les autres types d'eau, 20 µg/litre dans le
sang et 100 µg/litre dans l'urine.
2. Sources d'exposition et usages
2.1 Production et autres sources
Selon des statistiques récentes, la production annuelle (en
milliers de tonnes) est la suivante: Etats-Unis d'Amérique, 212 à 305;
Europe de l'Ouest, 215; Japon, 139. D'autres sources de contamination
de l'environnement par la méthyléthylcétone sont les gaz d'échappement
des réacteurs et des moteurs à combustion interne ainsi que certaines
activités industrielles comme la gazéification du charbon. Elle est
également présente en quantités notables dans la fumée de tabac. Aux
Etats-Unis d'Amérique, la quantité de méthyléthylcétone émise par les
moteurs représente plus de 1% de la quantité fabriquée volontairement.
En cas de smog, la production photochimique de méthyléthylcétone et
d'autres carbonyles à partir de radicaux libres peut être bien
supérieure aux émissions résultant des activités humaines. La
méthyléthylcétone peut aussi avoir une origine biologique et elle a
été identifiée parmi les produits du métabolisme microbien. Elle a
également été détectée dans divers produits naturels, notamment des
végétaux supérieurs, des phéromones d'insectes, des tissus animaux,
ainsi que chez l'homme dans le sang, l'urine et l'air expiré. Elle
constitue probablement un produit mineur du métabolisme normal des
mammifères.
2.2 Usages et pertes dans l'environnement
La méthyléthylcétone est un excellent solvant, ce qui explique
que sa principale application soit la fabrication de revêtements
protecteurs et d'adhésifs. Elle est également utilisée comme
intermédiaire chimique, comme solvant dans la fabrication des rubans
magnétiques, pour l'élimination des cires dans les huiles de graissage
et dans l'industrie alimentaire. Elle entre également dans la
composition de nombreux produits d'usage courant comme les vernis et
les colles. Dans la plupart de ces applications, la méthyléthylcétone
est mélangée à d'autres solvants organiques. La libération dans
l'environnement résulte principalement de l'évaporation des solvants
à partir des surfaces enduites et concerne essentiellement
l'atmosphère. La libération de méthyléthylcétone dans l'eau est la
conséquence de sa présence dans les effluents provenant de sa
fabrication et de diverses opérations industrielles. Elle a été
détectée dans des eaux naturelles dans lesquelles sa présence pourrait
s'expliquer par une activité microbienne, l'absorption à partir de
l'atmosphère ou une pollution anthropogène.
3. Transport et distribution dans l'environnement
La méthyléthylcétone est extrêmement mobile dans l'environnement
naturel où elle se renouvelle rapidement. Elle est très soluble dans
l'eau et s'évapore facilement. Dans l'atmosphère, elle subit une
décomposition photochimique rapide, mais elle est également
synthétisée par des processus photochimiques. Elle réagit avec les
halogènes libres ou les hypochlorites et leurs homologues halogénés
présents dans l'eau pour former un dérivé halogéné plus toxique que la
molécule initiale. La méthyléthyl-cétone est transportée par l'air et
par l'eau, mais elle ne s'accumule dans aucun compartiment et elle ne
persiste pas longtemps là où il existe une activité microbienne. Elle
est rapidement métabolisée par les microbes et les mammifères. Le
phénomène de bioaccumulation n'a jamais été mis en évidence. La
méthyléthylcétone existe naturellement dans certaines espèces de
trèfle et elle est produite par des champignons à des concentrations
qui peuvent affecter la germination de certaines plantes.
4. Concentration dans l'environnement et exposition humaine
La population générale est fréquemment exposée à de faibles doses
de méthyléthylcétone. Lorsque la pollution atmosphérique est très
faible, la concentration est inférieure à 3 µg/m3 (< 1 ppb), mais
on a mesuré jusqu'à 131 µg/m3 (44,5 ppb) en atmosphère très polluée.
En dehors des zones industrielles de fabrication ou d'utilisation de
la méthyléthylcétone, les gaz d'échappement des véhicules automobiles
et les réactions photochimiques dans l'atmosphère peuvent être les
principales sources de contamination. Les cigarettes et les autres
formes de tabac à fumer contribuent à l'exposition individuelle (20
cigarettes en contiennent jusqu'à 1,6 mg). La volatilisation de la
méthyléthylcétone présente dans les matériaux de construction et les
produits de consommation peut entraîner une pollution de l'air des
locaux bien supérieure à celle de l'air extérieur. Les concentrations
dans les eaux naturelles dépassent rarement 100 µg/litre (100 ppb) et
sont généralement inférieures au seuil de détection. Toutefois, on a
souvent trouvé des traces de méthyléthylcétone dans l'eau de boisson
(environ 2 µg/litre). Il est probable que les solvants entrant dans la
composition des joints des tuyauteries de plastique sont à l'origine
de cette pollution. Bien que la méthyléthylcétone soit un constituant
normal de nombreux aliments, les concentrations sont faibles et
l'alimentation ne peut être considérée comme une source importante
d'exposition. Aux Etats-Unis d'Amérique, la quantité moyenne ingérée
quotidiennement avec les aliments, principalement le pain blanc, les
tomates et le fromage Cheddar, est estimée à 1,6 mg par personne. La
méthyléthylcétone peut être présente naturellement dans les aliments,
mais elle peut aussi se former lors de l'affinage du fromage, de
l'entreposage de la viande de volaille, de la cuisson ou de la
transformation des aliments, ou être absorbée à partir des emballages
en matière plastique.
L'exposition industrielle à des concentrations modérées de
méthyléthylcétone est fréquente. Toutefois, dans certaines régions, le
personnel travaillant dans de petites entreprises (fabriques de
chaussures, imprimeries, ateliers de peinture) peut être exposé à des
concentrations beaucoup plus élevées en raison d'une ventilation
insuffisante. Ces travailleurs sont généralement exposés à un mélange
de solvants, notamment au n-hexane.
5. Cinétique et métabolisme
La méthyléthylcétone est rapidement absorbée par contact cutané,
inhalation, ingestion et injection intrapéritonéale. Elle passe très
vite dans le sang, et de là dans les autres tissus. Il semble que sa
solubilité soit à peu près la même dans tous les tissus. L'élimination
de la méthyléthylcétone et de ses métabolites est pratiquement totale
chez les mammifères au bout de 24 heures. Elle est métabolisée dans le
foie où la plus grande partie est oxydée en 3-hydroxy-2-butanone avant
d'être réduite en 2,3-butanediol. Une petite partie peut être réduite
en 2-butanol, mais celui-ci est rapidement oxydé pour redonner la
molécule initiale. Chez les mammifères, la majeure partie de la
méthyléthylcétone ingérée entre dans le cycle métabolique général
et/ou est éliminée sous forme de molécules simples comme le dioxyde de
carbone et l'eau. L'excrétion de la méthyléthylcétone et de ses
métabolites caractéristiques se fait principalement par les poumons,
bien que de petites quantités soient éliminées par les reins.
La méthyléthylcétone augmente l'activité enzymatique du
cytochrome P-450 microsomal. Il est possible que ce renforcement de
l'activité enzymatique, et par conséquent du potentiel de
transformation métabolique de l'organisme, explique pourquoi la
méthyléthylcétone potentialise la toxicité des solvants du groupe des
alcanes halogénés et des hydrocarbures aliphatiques à six atomes de
carbone.
6. Effets sur les animaux d'expérience
La méthyléthylcétone présente une toxicité faible à modérée pour
les mammifères, qu'il s'agisse de toxicité aiguë, à court terme ou
chronique. Chez la souris et le rat, la LD50 est de 2 à 6 g/kg de
poids corporel, la mort survenant 1 à 14 jours après l'ingestion d'une
dose unique. La dose moyenne entraînant la mort après une exposition
unique aux vapeurs de méthyléthylcétone est d'environ 29 400 mg/m3
(10 000 ppm), bien que des cobayes aient survécu à une exposition de
4 heures à cette concentration. Dans des essais d'intoxication aiguë
par voie orale, la dose la plus faible ayant entraîné une modification
de structure des organes a été de 1 g/kg de poids corporel chez le
rat. Cette dose a provoqué des lésions des tubules du rein.
L'inhalation par des rats d'air contenant 74 mg/m3 (25 ppm) pendant
6 heures a provoqué des changements de comportement mesurables qui ont
persisté pendant plusieurs jours. Une exposition répétée à 14 750
mg/m3 (5000 ppm) (6 h/jour, 5 jours/semaine) n'a provoqué la mort
d'aucun animal. On n'a observé qu'un effet mineur sur la croissance et
la structure et il n'y a eu aucune modification neuropathologique. Des
poulets, des chats ou des souris exposés à 3975 mg/m3 (1500 ppm)
pendant des périodes allant jusqu'à 12 semaines n'ont présenté aucun
signe de changement neuropathologique. Des effets transitoires sur le
comportement ou la neurophysiologie ont été détectés chez des rats et
des babouins à la suite d'expositions répétées à des concentrations ne
dépassant pas 295 à 590 mg/m3 (100 à 200 ppm).
Une faible foetotoxicité a été observée à 8825 mg/m3 (3000
ppm), mais elle ne s'accompagnait d'aucune toxicité maternelle; aucun
effet embryotoxique ou tératogène n'a été constaté à des
concentrations inférieures à cette valeur. Après avoir exposé de façon
répétée des rattes en gestation à une concentration de 8825 mg/m3,
on a observé dans leur progéniture une augmentation légère mais
significative de certains types d'anomalie du squelette rarement
observés chez les animaux non exposés.
Plusieurs épreuves classiques de mutagénicité ont été pratiquées,
mais la seule qui ait donné un résultat positif a été une étude
d'hétéroploïdie sur la levure Saccharomyces cerevisiae.
La méthyléthylcétone ne présente pas de toxicité aiguë pour les
poissons ou les invertébrés aquatiques, la CL50 se situant entre
1382 et 8890 mg/litre.
Elle a un effet inhibiteur sur la germination de plusieurs
plantes, même à des concentrations que l'on peut rencontrer dans la
nature. La croissance des algues aquatiques est également inhibée.
Des concentrations relativement élevées de méthyléthylcétone,
comparées aux concentrations naturelles, ont été utilisées dans des
expériences de fumigation. Cette substance s'est révélée un fumigant
modérément efficace contre la mouche des fruits des Caraïbes. D'autre
part, elle a un effet attractif très net sur la mouche tsé-tsé. Des
concentrations allant jusqu'à 20 mg/litre retardent le processus de
biodégradation mais ne l'arrêtent pas complètement. Jusqu'à 100
mg/litre, la méthyléthylcétone est bactériostatique pour différentes
bactéries. A des concentrations plus élevées (1000 mg/litre et
au-delà) elle inhibe la croissance des bactéries et des protozoaires.
7. Effets sur l'homme
7.1 Méthyléthylcétone seule
Aucun effet notable n'a été observé lors de tests psychologiques
et de comportement après exposition à 590 mg/m3 (200 ppm). Une
exposition de courte durée à la méthyléthylcétone seule ne semble pas
présenter de risques importants, que ce soit pour les professionnels
ou pour le public en général. L'exposition, dans des conditions
expérimentales, à une concentration de 794 mg/m3 (270 ppm), à raison
de 4 heures par jour, a eu un effet à peu près nul sur le comportement
et un contact de 5 minutes avec la substance liquide n'a provoqué
qu'une décoloration temporaire de la peau. On n'a signalé qu'un seul
cas d'intoxication aiguë par la méthyléthylcétone en dehors de tout
contexte professionnel. Il s'agit d'un cas d'ingestion accidentelle
qui ne semble pas avoir laissé de séquelles. On n'a jamais signalé de
cas d'exposition professionnelle ayant entraîné la mort. Il existe
deux rapports faisant état d'intoxication professionnelle chronique et
un rapport d'intoxication professionnelle aiguë, mais ce dernier est
sujet à caution. Dans un des cas d'intoxication chronique,
l'exposition à 880-1770 mg/m3 (300-600 ppm) a provoqué des
dermatoses, un engourdissement des doigts et des bras et divers
symptômes, parmi lesquels des céphalées, des étourdissements, des
troubles gastro-intestinaux et une perte d'appétit et de poids. Le
faible nombre d'intoxications attribuées à la méthyléthylcétone seule
tient à la fois à la faible toxicité de cette substance et au fait
qu'elle est rarement utilisée seule, mais plutôt en mélange avec
d'autres solvants.
7.2 Méthyléthylcétone dans les mélanges de solvants
L'exposition à des mélanges de solvants contenant de la
méthyléthylcétone a été associée à une réduction de la vitesse de
conduction nerveuse, à des troubles de la mémoire, à des troubles
moteurs, à des dermatoses et à des vomissements. Dans une étude
longitudinale, des mesures consécutives du temps de réaction simple
ont montré une amélioration des performances parallèlement à une
diminution de la concentration de méthyléthylcétone jusqu'à un dixième
de la valeur initiale (qui pouvait atteindre 4000 mg/m3 pour
certaines tâches de routine).
8. Renforcement de la toxicité des autres solvants
La méthyléthylcétone potentialise la neurotoxicité des solvants
à six atomes de carbone ( n-hexane, méthyl- n-butylcétone et
2,5-hexanedione) ainsi que la toxicité hépatique et rénale des
solvants de la famille des alcanes halogénés (tétrachlorure de carbone
et trichlorométhane).
La potentialisation des effets neurotoxiques des composés à six
atomes de carbone a été démontrée chez l'animal pour les trois
substances citées ci-dessus. Les neuropathies périphériques observées
chez l'homme se sont produites à la suite de changements dans la
formulation des solvants auxquels les sujets avaient été exposés, soit
volontairement, soit en raison de leur activité professionnelle. Le
mécanisme de cette potentialisation n'a pas été élucidé.
La potentialisation de la toxicité hépatique et rénale des
alcanes halogénés a été mise en évidence dans des études chez
l'animal. La méthyléthylcétone active probablement la métabolisation
des haloalcanes en substances toxiques pour les tissus en induisant la
production des enzymes oxydantes responsables de cette transformation.
RESUMEN
1. Propiedades y métodos analíticos
La metiletilcetona (MEC) es un líquido transparente, incoloro,
volátil, muy inflamable, de olor parecido a la acetona. Es estable en
condiciones normales pero puede formar peróxidos si se almacena
durante mucho tiempo; esos peróxidos pueden ser explosivos. La MEC
también puede formar mezclas explosivas con el aire. Es muy soluble en
agua, miscible con muchos disolventes orgánicos, y forma mezclas
azeotrópicas con el agua y con numerosos líquidos orgánicos. En la
atmósfera, la MEC produce radicales libres que pueden llevar a la
formación de nieblas fotoquímicas.
Existen varios métodos analíticos para medir los niveles
ambientales de MEC en el aire, el agua, las muestras biológicas, los
desechos y otros materiales. Con los métodos más sensibles, la MEC se
separa y se concentra ya sea en un sorbente sólido o como derivado de
la 2,4-dinitrofenilhidrazina (DNFH). La MEC y otros compuestos
orgánicos volátiles absorbidos son desorbidos, separados mediante
cromatografía de gases y medidos con un espectrómetro de masas o un
detector de ionización de llama. La MEC derivada se separa de los
compuestos afines mediante cromatografía de líquidos de alto
rendimiento y se mide mediante absorción ultravioleta. En medios como
desechos sólidos y material biológico, la MEC debe separarse en primer
lugar del sustrato con métodos como la extracción por solventes o la
destilación de vapores. Las concentraciones elevadas de MEC en el aire
pueden controlarse de modo continuo mediante absorción infrarroja. Los
límites de detección son 3 µg/m3 en el aire, 0,05 µg/litro en el
agua potable, 1,0 µg/litro en otros tipos de agua, 20 µg/litro en
sangre total y 100 µg/litro en orina.
2. Fuentes de exposición y usos
2.1 Producción y otras fuentes
Las cifras más recientes de fabricación industrial anual (en
miles de toneladas) son: EEUU, 212 a 305; Europa occidental, 215;
Japón, 139. Además de su fabricación, las fuentes de MEC en el medio
ambiente son los gases de escape de motores de reactores y de
combustión interna, y las actividades industriales como la
gasificación del carbón. Se encuentra en cantidades importantes en el
humo de tabaco. En los Estados Unidos, la producción de MEC en motores
no supera el 1% de su fabricación deliberada. En los episodios de
nieblas, la producción fotoquímica de MEC y otros carbonilos a partir
de radicales libres puede ser mucho mayor que la emisión antropogénica
directa. La MEC se produce biológicamente y se ha identificado como
producto del metabolismo microbiano. Se ha detectado asimismo en gran
diversidad de productos naturales, entre ellos los vegetales
superiores, las feromonas de insectos, en tejidos animales y en el
hombre, en sangre, orina y aire exhalado. Probablemente es un producto
secundario del metabolismo normal en el mamífero.
2.2 Usos y pérdidas al medio ambiente
El uso principal de la MEC, la aplicación de revestimientos
protectores y adhesivos, refleja sus excelentes características como
disolvente. También se utiliza como intermediario químico, como
disolvente en la producción de cintas magnéticas y para eliminar la
cera del aceite lubricante, así como en la manipulación de alimentos.
Además de las aplicaciones industriales, figura como ingrediente común
en productos de consumo como barnices y pegamentos. En la mayoría de
las aplicaciones, la MEC es componente de una mezcla de disolventes
orgánicos. Las pérdidas al medio ambiente son principalmente al aire
y se deben sobre todo a la evaporación de disolventes a partir de las
superficies revestidas. Se libera al agua como componentes de los
desechos de su fabricación y a partir de diversas operaciones
industriales. Se ha detectado en aguas naturales, procedente
probablemente de la actividad microbiana y del aporte atmosférico, así
como de la contaminación antropogénica.
3. Transporte y distribución en el medio ambiente
La MEC es sumamente móvil en el medio ambiente natural y está
sometida a una transformación rápida. Es muy soluble en el agua y se
evapora fácilmente a la atmósfera. En el aire, la MEC sufre una rápida
descomposición fotoquímica y es también sintetizada por procesos
fotoquímicos. En agua que contiene halógenos libres o hipohalitos,
reacciona para formar un haloformo más tóxico que el compuesto
original. La MEC se distribuye tanto por el aire como por el agua,
pero no se acumula en ningún compartimento aislado, ni persiste mucho
tiempo donde existe actividad microbiana. Se metaboliza rápidamente en
los microbios y los mamíferos. No hay pruebas de bioacumulación. La
MEC aparece naturalmente en algunas especies de trébol y es producida
por hongos en concentraciones que afectan a la germinación de algunas
plantas.
4. Niveles ambientales y exposición humana
La exposición de la población general a bajos niveles de MEC es
muy extensa. En aire poco contaminado, la concentración es inferior a
3 µg/m3 (< 1 ppmm), pero en condiciones de fuerte contaminación
atmosférica se ha medido un nivel de 131 µg/m3 (44,5 ppmm). Lejos de
las zonas industriales donde se fabrica o se usa la MEC, las
principales fuentes pueden ser los escapes de vehículos y las
reacciones fotoquímicas en la atmósfera. Los cigarrillos y otros
productos del tabaco que se someten a combustión contribuyen a la
exposición individual (20 cigarrillos contienen hasta 1,6 mg). La
volatilización de la MEC de materiales de construcción y productos de
consumo pueden contaminar el aire de interiores hasta niveles muy
superiores a los del aire libre adyacente. Las concentraciones de MEC
en aguas naturales expuestas rara vez se encuentran por encima de los
100 µg/litro (100 ppmm) y suelen encontrarse por debajo del nivel de
detección. No obstante, se han detectado cantidades muy reducidas de
MEC en el agua potable (aproximadamente 2 µg/litro) que probablemente
proceden de disolventes lixiviados a partir del material de las juntas
de las tuberías de plástico. Aunque la MEC es un componente normal de
muchos alimentos, las concentraciones son bajas y el consumo de
alimentos no puede considerarse una fuente significativa de exposición
para la población. La ingesta media diaria por habitante de los
Estados Unidos con los alimentos se calcula en 1,6 mg, en su mayor
parte a partir del pan blanco, los tomates y el queso tipo Cheddar.
Además de la MEC presente en el medio natural, puede producirse en la
maduración de los quesos, el envejecimiento de la carne de ave, la
cocción o la manipulación de alimentos, o por absorción a partir de
los envases de plástico.
La exposición industrial a niveles moderados de MEC está muy
extendida. No obstante, en algunas regiones los trabajadores de
fábricas pequeñas (por ejemplo, fábricas de calzado, imprentas y
fábricas de pinturas) están expuestos a concentraciones mucho más
elevadas por una ventilación insuficiente. En esas fábricas, la
exposición se da por lo general a una mezcla de disolventes entre los
que figura el n-hexano.
5. Cinética y metabolismo
La absorción de MEC es rápida por contacto cutáneo, inhalación,
ingestión e inyección intraperitoneal. Pasa rápidamente a la sangre y
de ella a otros tejidos. La solubilidad de la MEC parece similar en
todos los tejidos. La eliminación de la MEC y sus metabolitos en
mamíferos se completa en su mayor parte en 24 horas. Se metaboliza en
el hígado, donde se oxida a 3-hidroxi-2-butanona y a continuación se
reduce a 2,3-butanodiol. Una pequeña porción puede reducirse a
2-butanol, pero éste se oxida rápidamente para dar de nuevo MEC. La
mayor parte de la MEC que ingresa al organismo de mamíferos pasa al
metabolismo general y/o se elimina en forma de compuestos simples,
como dióxido de carbono y agua. La excreción de MEC y sus metabolitos
reconocibles se hace principalmente por los pulmones, aunque pequeñas
cantidades se eliminan por el riñón.
La MEC aumenta la actividad enzimática del citocromo P-450 en los
microsomas. Este aumento de la actividad enzimática y con ello del
potencial del organismo para la transformación metabólica puede ser el
mecanismo por el que la MEC potencia la toxicidad de los disolventes
a base de haloalcanos y hexacarbonos alifáticos.
6. Efectos en los animales de experimentación
La MEC tiene toxicidad aguda, a corto plazo y crónica de baja a
moderada en los mamíferos. Los valores de la DL50 en ratones y ratas
adultos son 2-6 g/kg de peso corporal; la muerte sobreviene en los
días 1 a 14 después de una sola dosis por vía oral. Las
concentraciones medias de vapor que producen letalidad en las ratas
tras una sola exposición giran en torno a los 29 400 mg/m3 (10 000
ppm), aunque los cobayos sobrevivieron a una exposición de 4 horas a
esta concentración. La dosis oral aguda más baja en modificar la
estructura corporal fue de 1 g/kg de peso corporal, que produjo
lesiones en los túbulos renales de la rata. La inhalación de 74
mg/m3 (25 ppm) durante 6 horas produjo en la rata cambios de
comportamiento medibles que persistieron durante varios días. La
exposición repetida de ratas a 14 750 mg/m3 (5000 ppm) (6 h/día, 5
días/semana) no produjo letalidad, tuvo sólo ligeros efectos en el
crecimiento y la estructura, y no se observaron cambios
neuropatológicos. No hubo pruebas de que la MEC produjera cambios
neuropatológicos en pollos, gatos o ratones expuestos a 3975 mg/m3
(1500 ppm) durante periodos de hasta 12 semanas. Tras la exposición
repetida de ratas y babuinos a concentraciones tan bajas como 295-590
mg/m3 (100 a 200 ppm) se observaron efectos transitorios en el
comportamiento o la neurofisiología.
Se ha observado un nivel bajo de fetotoxicidad sin toxicidad
materna a 8825 mg/m3 (3000 ppm), pero no hay pruebas de efectos
embriotóxicos o teratogénicos a niveles más bajos de exposición. La
exposición repetida de ratas preñadas a 8825 mg/m3 indujo en sus
crías un aumento pequeño pero significativo de ciertos tipos de
anomalías esqueléticas cuya incidencia entre la población no expuesta
es baja.
Aunque se examinó en varios sistemas de ensayo de mutagenicidad
convencionales, la única prueba de mutagenicidad se observó en un
estudio sobre aneuploidia en la levadura Saccharomyces cerevisiae.
La MEC no presenta toxicidad aguda para los peces ni los
invertebrados acuáticos; los valores de la CL50 varían desde 1382
hasta 8890 mg/litro.
La MEC inhibe la germinación de varias especies vegetales,
incluso con niveles que se dan en la naturaleza. Inhibe el crecimiento
de algas acuáticas.
En comparación con los niveles de base naturales, se han
utilizado concentraciones relativamente elevadas de MEC para fumigar
en condiciones experimentales. Es moderadamente eficaz como fumigante
contra la mosca caribeña de la fruta y atrae con gran eficacia a la
mosca tse-tse. Con niveles de MEC de hasta 20 mg/litro se retrasa la
biodegradación pero no se detiene el proceso por completo. Con niveles
de hasta 100 mg/litro, la MEC es bacteriostatica para algunas
bacterias. Con concentraciones más altas (1000 mg/litro o más) se
inhibe el crecimiento de bacterias y protozoarias.
7. Efectos en el ser humano
7.1 MEC por sí sola
La exposición a 590 mg/m3 (200 ppm) no tuvo efectos de
importancia en varios ensayos comportamentales y psicológicos. La
exposición a corto plazo a MEC por sí sola no parece constituir un
riesgo de importancia, ni ocupacional ni para el público en general.
La exposición experimental a una concentración de 794 mg/m3 (270
ppm) durante 4 h/día tuvo escaso o ningún efecto en el comportamiento,
y un contacto de 5 minutos con MEC líquida no produjo más que un
blanqueamiento temporal de la piel. Sólo hay un informe no ocupacional
de toxicidad aguda a la MEC. Se debió a una ingestión accidental y no
pareció producir lesiones duraderas. No hay pruebas de que la
exposición ocupacional a la MEC haya originado ningún caso de muerte.
Se han notificado dos casos de envenenamiento ocupacional crónico y
uno dudoso de envenenamiento ocupacional agudo. En uno de los casos
crónicos, la exposición a 880-1770 mg/m3 (300-600 ppm) dió lugar a
dermatosis, endormecimiento de los dedos y los brazos, y diversos
síntomas como dolor de cabeza, mareos, trastornos gastrointes-tinales
y pérdida de apetito y de peso. Esta escasez de incidentes de
envenamiento por MEC por sí sola refleja tanto su baja toxicidad como
el hecho de que se usa más comunmente no por sí sola sino como
componente de mezclas de disolventes.
7.2 La MEC en mezclas de disolventes
La exposición a mezclas de disolventes con MEC se ha asociado a
cierta reducción en la velocidad de conducción nerviosa, la memoria y
alteraciones motoras, dermatosis y vómitos. En un estudio
longitudinal, las medidas consecutivas de tiempo de reacción simple
demostraron que mejoraba el rendimiento en paralelo al ir disminuyer
de las con concentraciones de MEC hasta un décimo de los valores
originales (que fueron de hasta 4000 mg/m3 para ciertas tareas
rutinarias).
8. Potenciación de la toxicidad de otros disolventes
La MEC potencia la neurotoxicidad de compuestos hexacarbonados
( n-hexano, metil- n-butilcetona y 2,5-hexanodiona) y la toxicidad
hepatica y renal de los disolventes a base de haloalcanos
(tetracloruro de carbono y triclorometano).
La potenciación de los efectos neurotóxicos de los hexacarbonos
se ha demostrado con los tres hexacarbonos en el animal. Las
neuropatías periféricas observadas en humanos siguieron a cambios en
la formulacion de disolventes a los que habían estado expuestos, ya
sea voluntariamente o en el trabajo. El mecanismo por el que se
produce esta potenciación no está claro.
Las pruebas de la potenciación de la toxicidad hepática y renal
de los haloalcanos proceden de estudios animales. La MEC activa
probablemente el metabolismo de los haloalcanos de las especies que
dañan los tejidos como resultado de la inducción de las enzimas
oxidativas pertinentes.
