
INTERNATIONAL PROGRAMME ON CHEMICAL SAFETY
ENVIRONMENTAL HEALTH CRITERIA 131
DIETHYLHEXYL PHTHALATE
This report contains the collective views of an international group of
experts and does not necessarily represent the decisions or the stated
policy of the United Nations Environment Programme, the International
Labour Organisation, or the World Health Organization.
Published under the joint sponsorship of
the United Nations Environment Programme,
the International Labour Organisation,
and the World Health Organization
First draft prepared by Dr P. Lundberg, Dr. J. Hogberg,
and Dr P. Garberg, National Institute of Occupational
Health, Sweden, Dr I. Lundberg, Karolinska
Hospital, Sweden, and Dr S. Dobson and Mr. P. Howe,
Institute of Terrestrial Ecology, United Kingdom
World Health Orgnization
Geneva, 1992
The International Programme on Chemical Safety (IPCS) is a
joint venture of the United Nations Environment Programme, the
International Labour Organisation, and the World Health
Organization. The main objective of the IPCS is to carry out and
disseminate evaluations of the effects of chemicals on human health
and the quality of the environment. Supporting activities include
the development of epidemiological, experimental laboratory, and
risk-assessment methods that could produce internationally
comparable results, and the development of manpower in the field of
toxicology. Other activities carried out by the IPCS include the
development of know-how for coping with chemical accidents,
coordination of laboratory testing and epidemiological studies, and
promotion of research on the mechanisms of the biological action of
chemicals.
WHO Library Cataloguing in Publication Data
Diethylhexyl phthalate.
(Environmental health criteria ; 131)
1.Diethylhexyl phthalate - adverse effects 2.Diethylhexyl
phthalate - toxicity 3.Environmental exposure I.Series
ISBN 92 4 157131 4 (NLM Classification: QV 612)
ISSN 0250-863X
The World Health Organization welcomes requests for permission
to reproduce or translate its publications, in part or in full.
Applications and enquiries should be addressed to the Office of
Publications, World Health Organization, Geneva, Switzerland, which
will be glad to provide the latest information on any changes made
to the text, plans for new editions, and reprints and translations
already available.
(c) World Health Organization 1992
Publications of the World Health Organization enjoy copyright
protection in accordance with the provisions of Protocol 2 of the
Universal Copyright Convention. All rights reserved.
The designations employed and the presentation of the material
in this publication do not imply the expression of any opinion
whatsoever on the part of the Secretariat of the World Health
Organization concerning the legal status of any country, territory,
city or area or of its authorities, or concerning the delimitation
of its frontiers or boundaries.
The mention of specific companies or of certain manufacturers'
products does not imply that they are endorsed or recommended by the
World Health Organization in preference to others of a similar
nature that are not mentioned. Errors and omissions excepted, the
names of proprietary products are distinguished by initial capital
letters.
CONTENTS
ENVIRONMENTAL HEALTH CRITERIA FOR DIETHYLHEXYL PHTHALATE
1. SUMMARY
1.1 Identity, physical and chemical properties, and
analytical methods
1.2 Sources of human and environmental exposure
1.3 Environmental transport, distribution, and
transformation
1.4 Environmental levels and human exposure
1.5 Kinetics and metabolism
1.6 Effects on laboratory mammals and in vitro test
systems
1.7 Effects on humans
1.8 Effects on other organisms in the laboratory
and field
1.9 Evaluation
2. IDENTITY, PHYSICAL AND CHEMICAL PROPERTIES,
AND ANALYTICAL METHODS
2.1 Identity
2.2 Physical and chemical properties
2.3 Conversion factors
2.4 Analytical methods
3. SOURCES OF HUMAN AND ENVIRONMENTAL EXPOSURE
3.1 Natural occurrence
3.2 Anthropogenic sources
3.2.1 Production levels
3.2.2 Uses
3.2.3 Disposal of plasticized products
4. ENVIRONMENTAL TRANSPORT, DISTRIBUTION,
AND TRANSFORMATION
4.1 Environmental transport and distribution
4.1.1 Transport in air
4.1.2 Transport in soil and sediment
4.1.3 Transport in water
4.1.4 Transport between media
4.2 Biotransformation
4.2.1 Abiotic degradation
4.2.2 Biodegradation
4.2.2.1 Aerobic degradation
4.2.2.2 Anaerobic degradation
4.2.3 Bioaccumulation
4.2.3.1 Model ecosystems
4.2.3.2 Aquatic invertebrates
4.2.3.3 Fish
4.2.3.4 Amphibians
4.2.3.5 Plants
4.2.3.6 Birds
5. ENVIRONMENTAL LEVELS AND EXPOSURE
5.1 Environmental levels
5.1.1 Air
5.1.2 Precipitation
5.1.3 Water
5.1.4 Sediment
5.1.5 Soil
5.1.6 Food
5.1.7 Aquatic organisms
5.1.8 Terrestrial organisms
5.2 General population exposure
5.3 Occupational exposure during manufacture,
formulation or use
6. KINETICS AND METABOLISM IN LABORATORY ANIMALS AND HUMANS
6.1 Absorption
6.1.1 Inhalation
6.1.2 Dermal
6.1.3 Oral
6.1.4 Intraperitoneal
6.2 Distribution
6.3 Metabolism
6.4 Elimination and excretion
6.5 Retention and turnover
6.5.1 Half-life and body burden
6.5.2 Indicator media
7. EFFECTS ON LABORATORY MAMMALS AND IN VITRO TEST SYSTEMS
7.1 Single exposure
7.2 Short-term exposure
7.3 Long-term exposure
7.4 Skin and eye irritation; sensitization
7.5 Reproduction, embryotoxicity, and teratogenicity
7.5.1 Reproduction
7.5.2 Embryotoxicity and teratogenicity
7.6 Mutagenicity and related end-points
7.6.1 Mutation
7.6.1.1 Bacteria
7.6.1.2 Fungi
7.6.1.3 Mammalian cells
7.6.1.4 Drosophila
7.6.2 DNA damage
7.6.3 DNA binding
7.6.4 Chromosomal effects
7.6.5 Cell transformation
7.6.6 In vivo effects
7.7 Carcinogenicity
7.8 Special studies
7.9 Mechanisms of hepatotoxicity
8. EFFECTS ON HUMANS
8.1 General population exposure
8.2 Occupational exposure
9. EFFECTS ON OTHER ORGANISMS IN THE LABORATORY AND FIELD
9.1 Toxicity to microorganisms
9.2 Toxicity to aquatic organisms
9.2.1 Invertebrates
9.2.2 Fish
9.2.3 Amphibians
9.3 Toxicity to terrestrial organisms
9.3.1 Plants
9.3.2 Earthworms
9.3.3 Insects
9.3.4 Birds
10. EVALUATION OF HUMAN HEALTH RISKS AND EFFECTS ON THE ENVIRONMENT
10.1 Evaluation of human health risks
10.1.1 Exposure levels
10.1.2 Toxic effects
10.1.3 Conclusion
10.2 Evaluation of effects on the environment
10.2.1 Exposure levels
10.2.2 Toxic effects
10.2.3 Conclusion
11. RECOMMENDATIONS FOR PROTECTION OF HUMAN HEALTH AND THE
ENVIRONMENT
12. FURTHER RESEARCH
13. PREVIOUS EVALUATIONS BY INTERNATIONAL BODIES
REFERENCES
RESUME
RESUMEN
WHO TASK GROUP ON ENVIRONMENTAL HEALTH
CRITERIA FOR DIETHYLHEXYL PHTHALATE
Members
Dr D. Anderson, British Industrial Biological Research Association,
Carshalton, Surrey, United Kingdom
Dr R. Cattley, Department of Experimental Pathology and Toxicology,
Chemical Industry Institute of Toxicology, Research Triangle Park,
North Carolina, USA
Dr U. Chantharaksri, Department of Pharmacology, Mahidol University,
Bangkok, Thailand
Dr S.D. Gangolli, British Industrial Biological Research Association,
Carshalton, Surrey, United Kingdom
Dr J. Högberg, Department of Toxicology, National Institute of
Occupational Health, Solna, Sweden
Mr P. Howe, Institute of Terrestrial Ecology, Monks Wood Experimental
Station, Abbots Ripton, Huntingdon, United Kingdom
Dr F. Matsumura, Toxic Substances Program, Department of Environmental
Toxicology, University of California, Davis, California, USA
(Chairman)
Dr S. Oishi, Department of Toxicology, Metropolitan Research
Laboratory of Public Health, Tokyo, Japan
Dr C.-N. Ong, Department of Community, Occupational and Family
Medicine, National University of Singapore, Singapore (Joint
Rapporteur)
Professor G. Pliss, Laboratory for Chemical Carcinogenic Agents, N.N.
Petrov Research Institute of Oncology, Leningrad, USSR
Professor Y.-L. Wang, Department of Occupational Health, School of
Public Health, Shanghai Medical University, Shanghai, China
Mr G. Welter, Federal Environmental Protection Agency, Berlin, Germany
Representatives of other intergovernmental organizations
Dr M. De Smedt, Commission of the European Communities, Luxembourg
Representatives of non-governmental organizations
Dr C. Elcombe, European Chemical Industry Ecology and Toxicology
Centre, Brussels, Belgium
Dr B. Lake, Conseil Européen des Fédérations de l'Industrie chimique
(CEFIC), Brussels, Belgium
Secretariat
Dr B.-H. Chen, International Programme on Chemical Safety, World
Health Organization, Geneva, Switzerland (Secretary)
Dr P. Lundberg, Department of Toxicology, National Institute of
Occupational Health, Solna, Sweden (Joint Rapporteur)
Dr D. McGregor, International Agency for Research on Cancer, World
Health Organization, Lyon, France
NOTE TO READERS OF THE CRITERIA MONOGRAPHS
Every effort has been made to present information in the criteria
monographs as accurately as possible without unduly delaying their
publication. In the interest of all users of the environmental health
criteria monographs, readers are kindly requested to communicate any
errors that may have occurred to the Manager of the International
Programme on Chemical Safety, World Health Organization, Geneva,
Switzerland, in order that they may be included in corrigenda.
* * *
A detailed data profile and a legal file can be obtained from the
International Register of Potentially Toxic Chemicals, Palais des
Nations, 1211 Geneva 10, Switzerland (Telephone No. 7988400 or
7985850).
ENVIRONMENTAL HEALTH CRITERIA FOR
DIETHYLHEXYL PHTHALATE
A WHO Task Group on Environmental Health Criteria for Diethylhexyl
Phthalate (DEHP) met at the British Industrial Biological Research
Association (BIBRA), Carshalton, Surrey, United Kingdom, from 3 to 7
June 1991. Dr S.D. Gangolli opened the meeting on behalf of BIBRA.
Dr B.-H. Chen, IPCS, welcomed the participants on behalf of the
Manager, IPCS, and the three IPCS cooperating organizations
(UNEP/ILO/WHO). The Task Group reviewed and revised the draft
criteria monograph and made an evaluation of the risks for human
health and the environment from exposure to DEHP.
The first draft of this monograph was prepared by Dr P. Lundberg,
Dr J. Högberg, and Dr P. Garberg of the National Institute of
Occupational Health, Sweden, Dr I. Lundberg of Karolinska Hospital,
Sweden, and Dr S. Dobson and Mr P. Howe of the Institute of
Terrestrial Ecology, Monks Wood Experimental Station, United Kingdom.
The second draft was prepared by Dr P. Lundberg incorporating comments
received following the circulation of the first draft to the IPCS
Contact Points for Environmental Health Criteria monographs.
Particularly valuable comments on the draft were made by the European
Chemical Industry Ecology and Toxicology Centre (ECETOC), the
International Agency for Research on Cancer (IARC), the Toxicology
Division, Exxon Biomedical Sciences, and the Conseil European des
Federations de L'industrie Chimique (CEFIC).
Dr B.-H. Chen and Dr P.G. Jenkins, both members of the IPCS Central
Unit, were responsible for the overall scientific content and
technical editing, respectively.
The efforts of all who helped in the preparation and finalization
of the document are gratefully acknowledged.
* * *
Financial support for this Task Group was provided by the United
Kingdom Department of Health as part of its contributions to the IPCS.
ABBREVIATIONS
DEHP diethylhexyl phthalate
DBP di- n-butyl phthalate
DiBP di-iso-butyl phthalate
ECETOC European Chemical Industry Ecology and Toxicology
Centre
ECMO extracorporeal membrane oxidation
HPLC high-performance liquid chromatography
MEHP monoethylhexyl phthalate
NDMA N-dimethylnitrosamine
NOEL no-observed-effect level
PVC polyvinyl chloride
SHE Syrian hamster embryo
SPF specific pathogen free
UDS unscheduled DNA synthesis
US ATSDR US Agency for Toxic Substances and Disease Registry
US FDA US Food and Drug Administration
1. SUMMARY
1.1 Identity, physical and chemical properties, and analytical
methods
Di(2-ethylhexyl)phthalate (DEHP) is a benzenedicarboxylic acid
ester which at room temperature is a colourless to yellow oily liquid.
Its solubility in water is low (0.3-0.4 mg/litre), and is even lower
in salt water. It is miscible with most common organic solvents. The
volatility of DEHP is relatively low (8.6 x 10-4 Pa).
Many sampling and analytical methods have been developed for the
determination of DEHP in different media. Sensitive methods, such as
gas chromatography, high-performance liquid chromatography, and mass
spectrometry are being used increasingly. Analysis of low
concentrations of DEHP is complicated by contamination from plastic
equipment during sampling and analysis.
1.2 Sources of human and environmental exposure
Almost all the DEHP present in the environment arises from
anthropogenic sources rather than from natural ones.
The worldwide production of DEHP has been increasing during
recent decades and at present amounts to about 1 x 106 tonnes per
year. One third of the total production is in the USA and one third in
Europe.
DEHP is the most widely used plasticizer (comprising 50% of all
phthalate ester plasticizers) that softens resins. It may account for
40% (w/w) or more of the plastic. DEHP is used for making the
polyvinyl chloride (PVC) utilized in building, construction and
packaging, and for medical device components. Smaller amounts are used
in industrial paints and as a dielectric fluid in condensers.
Discarded plasticized products may be disposed of either by
incineration or via dumping in a landfill site. During incineration at
a low temperature, a large percentage of the DEHP may be lost to the
atmosphere. The environmental fate of DEHP in landfill sites has not
been well studied and no definite conclusions can be reached.
1.3 Environmental transport, distribution, and transformation
Transport in the air is the major route by which phthalates enter
the environment. From the atmosphere DEHP either falls or is washed
out via rainfall.
DEHP has a high octanol-water partition coefficient, so the
equilibrium between water and an organic-rich soil or sediment is in
favour of the soil or sediment. It is readily adsorbed by organic soil
particles.
Although the solubility of DEHP in water is low, the amount
present in surface water may be higher due to adsorption onto organic
particles and interaction with dissolved organic matter. It is
adsorbed particularly by small particles, and adsorption is enhanced
in salt water.
Atmospheric photodegradation of DEHP is rapid, but its chemical
hydrolysis in the environment is practically non-existent.
Aerobic degradation has been found to be carried out by several
soil microorganisms. However, the microbial degradation of DEHP in the
environment has been reported to be slow. The biodegradation pathway
begins with hydrolysis to the mono-ester, which is then converted to
phthalic acid. The ring-opening degradation to pyruvate and succinate
and then to CO2 and H2O is similar to the metabolic pathway of
benzoic acid. The aerobic degradation is temperature dependent. Below
10 °C little degradation takes place. At higher temperatures
biodegradation proceeds in the upper layer of the soil, but it is
virtually non-existent deeper down where conditions are anaerobic.
Anaerobic degradation, if it exists, is very much slower than aerobic
degradation.
DEHP is highly lipophilic and moderately persistent. The degree
of bioaccumulation depends on the capability of an organism to
metabolize DEHP. It has been shown to accumulate to a high degree in
a variety of aquatic invertebrates, fish, and amphibians.
When DEHP was applied to plant leaves, there was little loss over
a 15-day period. Uptake by plants from soil or sewage sludge was found
to be low.
1.4 Environmental levels and human exposure
DEHP exists widely in the environment and is found in most
samples, including air, precipitation, water, sediment, soil, and
biota. Levels are generally highest in industrialized areas.
DEHP concentrations of up to 300 ng/m3 have been found in urban
and polluted air. Levels of between 0.5 and 5 ng/m3 have been
reported in the air of oceanic areas, and the rainfall in these areas
contained up to about 200 ng/litre. Precipitation samples from an area
close to a plasticizer production plant indicated that the rate of dry
deposition was 0.7 to 4.7 µg/m2 per day.
In rivers and lakes the concentration of DEHP has been found to
be up to 4 µg/litre, highest levels being associated with industrial
effluent discharge points. The concentration in the sea is less than
1 µg/litre, highest levels being in estuaries.
Due to its hydrophobic character, DEHP is readily absorbed to
soil, sediment, and particulate matter. River sediment levels of up to
70 mg/kg (dry weight) have been reported, and these have reached 1480
mg/kg (dry weight) near discharge points.
The concentration of DEHP in biota varies from less than 1 to
7000 µg/kg. It has been found in various types of food, such as fish,
shellfish, eggs, and cheese. The estimated average exposure was around
300 µg/person per day in the USA in 1974 and 20 µg/person per day in
the United Kingdom in 1986.
Blood transfusions and other medical treatment using plastic
devices may lead to involuntary human exposure to DEHP. Levels from
13.4 to 91.5 mg/kg (dry weight) in lung tissue have been detected in
patients.
The few data available indicate that workplace concentrations of
DEHP are usually below 1 mg/m3.
1.5 Kinetics and metabolism
Available data on oral administration indicate that DEHP is
hydrolysed in the gut by pancreatic lipase. The metabolites formed,
i.e. mono(2-ethylhexyl)phthalate (MEHP) and 2-ethyl-hexanol, are
rapidly absorbed. When 14C-labelled DEHP (2.9 mg/kg) was given
orally to rats, more than 50% was recovered in the urine or bile. The
bioavailability of an oral dose of DEHP seems to be higher in young
rats than in older ones.
When administered orally, DEHP is extensively hydrolysed in the
gut in certain animals, e.g., rats, and is mainly distributed as
monoethylhexyl phthalate (MEHP). However, hydrolysis occurs to a much
lesser extent in primates and humans. MEHP binds to plasma proteins.
The liver seems to be the major organ for the metabolism of MEHP and
2-ethylhexanol. Several further metabolites have been identified,
omega- and omega-1-oxidation being the major metabolic pathways. One
or several of the products of omega-oxidation may be further
metabolized by ß-oxidation. Non-linear kinetics have been observed for
the omega-oxidation. DEHP metabolism shows considerable species
differences; e.g., the omega-oxidation pathway is less extensive in
humans than in rats.
Almost 100% of an oral dose of DEHP (2.9 mg/kg) was recovered in
rat faeces and urine after a week. Bile and urine are the major
excretory pathways. In a human study, 15-25% of an oral dose
(0.45 mg/kg) of DEHP was excreted as MEHP, and oxidized metabolites
constituted a major portion of the excretion products.
1.6 Effects on laboratory mammals and in vitro test systems
The oral LD50 for DEHP is about 25-34 g/kg, depending on the
species, but the value for MEHP is lower. In feeding studies on rats
and mice, DEHP dosages greater than 3 g/kg per day caused deaths
within 90 days, and a level of 0.4 g/kg per day reduced weight gain
within a few days. In other studies, 6.3-12.5 g/kg diet caused a body
weight reduction.
Hepatomegaly and increased relative kidney weights have been
observed in treated animals in long-term studies. In one study, there
were also hypertrophic cells in the anterior pituitary.
Several studies have shown testicular atrophy, evident within a
few days, related to DEHP administration (dietary levels of 10-20 g
DEHP/kg). Younger rats seem to be more susceptible than older ones,
and rats and mice seem to be more sensitive than marmosets and
hamsters. Reversibility of the atrophy has been observed. MEHP has
toxic effects on Sertoli cells in vitro. DEHP, as well as MEHP,
shows teratogenic properties. Malformations were observed at dietary
levels of 0.5-2 g/kg in mice, and embryotoxic effects were observed at
dietary levels greater than 10 g/kg.
Tests for mutagenicity and related end-points have been negative
in most studies. DEHP may induce cellular transformation, and it has
been shown to be carcinogenic at doses of 6 and 12 g DEHP/kg diet in
rats and 3 and 6 g/kg diet in mice. There was a dose-related increase
in hepatocellular tumours in both sexes of both species. The induction
of hepatic peroxisome proliferation and cell replication is strongly
associated with the liver carci-nogenic effect of certain non-
genotoxic carcinogens including DEHP. However, marked differences have
been observed among animal species with respect to DEHP-induced
peroxisome proliferation.
In contrast to rat hepatocytes, DEHP metabolites do not produce
peroxisome proliferation in cultured human hepatocytes.
1.7 Effects on humans
Only very limited information is available on the effects of DEHP
on humans. Mild gastric disturbances, but no other deleterious
effects, were reported for two subjects given 5 or 10 g DEHP.
1.8 Effects on other organisms in the laboratory and field
Most studies have yielded nominal LC50 values in acute toxicity
tests that are in excess of 10 mg/litre, values which give a low
toxicity rating for DEHP. However, these levels exceed the DEHP water
solubility (0.3-0.4 mg/litre). One study suggested greater sensitivity
of the water flea Daphnia pulex, with a nominal 48-h LC50 of 0.133
mg/litre. The only acute test with measured DEHP concentrations was on
the fathead minnow and revealed a 96-h LC50 of > 0.33 mg/litre. In
prolonged studies, the no-observed-effect level (NOEL) for Daphnia
magna was 72 µg/litre. For adult fish a NOEL of > 62 µg/litre was
determined. An exposure of 14 µg/litre, from 12 days prior to
hatching, caused a significant increase in trout fry mortality. DEHP
concentrations of between 3.7 and 11 µg/litre led to a reduction in
the vertebral collagen of fish.
The survival of zebra fish fry is adversely affected by DEHP
concentrations of 50 mg/kg food. Sediment concentrations of 25 mg/kg
(w/w) significantly reduced microbial activity and the number of
tadpoles hatching.
The acute toxicity of DEHP to algae, plants, earthworms, and
birds is low.
1.9 Evaluation
DEHP causes reproductive and hepatocarcinogenic effects in rats
and mice.
Testicular atrophy is the main reproductive effect in rats and
mice, and young animals are more susceptible than older ones to this
effect. The induction of hepatic peroxisome proliferation and cell
replication are strongly associated with the liver carcinogenic effect
of certain non-genotoxic carcinogens including DEHP. However, marked
differences have been observed among animal species with respect to
DEHP-induced peroxisome proliferation. Currently there is not
sufficient evidence to suggest that DEHP is a potential human
carcinogen.
There is no documented information that DEHP presents any hazard,
based on acute exposure to fish and daphnids. However, a reduction of
microbial activity in sediment at environmental levels of DEHP was
reported. A comparison between environmental levels and the
concentrations that produce effects in prolonged studies, especially
early life-stage tests on fish and amphibians, indicates that a hazard
for the environment, particularly via water and sediment, cannot be
excluded. Adverse effects on organisms are likely in areas with highly
contaminated water and sediments which are near to point emission
sources.
Although few relevant studies have been reported, the acute
toxicity of DEHP to algae, plants, earthworms, and birds appears to be
low.
2. IDENTITY, PHYSICAL AND CHEMICAL PROPERTIES, AND ANALYTICAL
METHODS
2.1 Identity
Common name: di(2-ethylhexyl) phthalate
Structural formula: 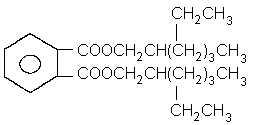 Empirical formula: C24H38O4
Abbreviation: DEHP
Relative molecular
mass: 390.57
Common synonyms:
1,2-benzenedicarboxylic acid bis(2-ethylhexyl) ester (CAS name);
phthalic acid bis(2-ethylhexyl) ester (IUPAC name); BEHP;
1,2-benzenedicarboxylic acid bis(ethylhexyl) ester;
bis(2-ethylhexyl) 1,2-benzenedicarboxylate; bis(2-ethyl-hexyl)
ester of phthalic acid; bis(2-ethylhexyl) phthalate;
di(2-ethylhexyl) ortho-phthalate; di(ethylhexyl) phthalate;
dioctyl phthalate; DOP; ethylhexyl phthalate; 2-ethylhexyl
phthalate; octyl phthalate; di- sec-octyl phthalate; phthalic
acid dioctyl ester
Common trade names:
Bisoflex 81; Bisoflex DOP; Compound 889; DAF 68; Ergoplast FDO;
Eviplast 80; Eviplast 81; Fleximel; Flexol DOP; Goodrite GP 264;
Hatcol DOP; Kodaflex DOP; Mollan O; Nuoplaz DOP; Octoil;
Palatinol AH; Platinol DOP; Pittsburgh; PX-138; Reomol DOP;
Reomol D 79P; Sicol 150; Staflex DOP; Truflex DOP; Vestinol AH;
Vinicizer 80; Witcizer 312 (IARC, 1982; NIOSH, 1985b)
CAS registry
number: 117-81-7
RTECS number: TI 035000
Di(2-ethylhexyl) phthalate (DEHP) is available in a variety of
technical grades. In the USA typical product specifications are:
minimal ester content, 99.0-99.6%; maximal moisture content, 0.1%;
acidity (as acetic acid or phthalic acid), 0.007-0.01%; specific
gravity, 0.980-0.985 (25 °C/25 °C); refractive index, 1.4850-1.4870
(23 °C); and minimal flash-point, 216 °C (IARC, 1982).
In western Europe, DEHP is available with the following
specifications: maximal acid value, 0.06; maximal weight loss on
heating at 140 °C for 3 h, 1%; and saponification value, 284-290 mg
KOH/g (IARC, 1982).
In Japan, DEHP must fulfill the following specifications: maximal
acid value, 0.05; maximal weight loss on heating at 125 °C for 3 h,
0.1%; and specific gravity, 0.983-0.989 (20 °C/20 °C) (IARC, 1982).
2.2 Physical and chemical properties
DEHP is a colourless to yellow, oily liquid at room temperature
and normal atmospheric pressure. The melting point is -46 °C (pour
point) and the boiling point is 370 °C (at atmospheric pressure, 101.3
kPa; 236 °C at 1.33 kPa, and 231 °C at 0.67 kPa). The flash point is
425 °C (open cup) (Clayton & Clayton, 1981; IARC, 1982; SAX, 1984). At
20 °C, the density is 0.98 g/ml (Fishbein & Albro, 1972) and the
vapour pressure 8.6 x 10-4 Pa (Howard et. al., 1985). The log n-
octanol-water partition coefficient is 3-5.
The solubility of uncolloidal DEHP in water is low (45 µg/litre
at 20 °C) (Leyder & Boulanger, 1983). However, DEHP may form colloidal
dispersions which lead to higher values for solubility (Klöpfer et
al., 1982). Values of 285 µg/litre (Hollifield, 1979), 340 µg/litre
(Howard et al., 1985), and 360 µg/litre (Defoe et al., 1990) have been
determined at 20-25 °C. These higher values are probably more
realistic in the environment. Howard et al. (1985) determined a value
of 160 µg/litre at 25 °C in salt water.
DEHP is miscible with most common organic solvents and is more
soluble in blood than water. It is lipophilic and the distribution
ratio in dichloromethane-Krebs bicarbonate buffer has been measured to
be 1130 (Krauskopf, 1973; Clayton & Clayton, 1981; IARC, 1982; Sax,
1984; Weast et al., 1984; Sittig, 1985).
2.3 Conversion factors
1 ppm = 15.87 mg/m3
1 mg/m3 = 0.063 ppm
2.4 Analytical methods
Methods used for the analysis of di(2-ethylhexyl) phthalate in
many types of samples are summarized in Table 1.
Analysis of samples with low concentrations of DEHP is
complicated by the risk of contamination from plastic equipment.
Table 1. Methods for the analysis of di(2-ethylhexyl) phthalate
Sample Sample Assay Limit of
matrix preparation procedure detection Reference
Air collect on cellulose GC/FID range: NIOSH (1977)
ester membrane filter; 2.03-10.9 NIOSH (1985a)
extract disulfide) mg/m3 for
a 32-litre
sample at
23 °C
Air collect with impinger GC/ECD not given Thomas (1973)
(ethylene glycol); GC/MS
extract (hexane)
Marine air trap on glass-fibre filters GC/ECD 0.5 ng/m3 Giam et al.
with foam plugs; Soxhlet (1980)
extract (petroleum ether);
concentrate extracts; clean-up
on deactivated Florisil
columns
Air-borne Soxhlet extract (methanol); GC/MS not given Karasek et al.
particulate concentrate; centrifuge (1978)
matter
River water extract (hexane); filter HPLC/UV 2 ng at Mori (1976)
(normal and 224 nm
reversed-phase
adsorption
chromatography
and gel
chromatography
Table 1 (contd).
Sample Sample Assay Limit of
matrix preparation procedure detection Reference
River water extract (chloroform); thin-layer 50 µg/litre Kataeva (1988)
concentrate extract; dry by chromatography
sodium sulfate treatment;
evaporate; dissolve residue
(chloroform)
Industrial add hydrochloride acid; GC/MC (EI not given Sheldon &
and municipal extract (dichloromethane); and CI modes); Hites (1979)
waste water clean-up by liquid with SIM
chromatographic fractionation
River freeze-dry; homogenize; HPLC/UV 10 ng Schwartz et al.
sediment extract (hexane; acetone; (233 nm) (1979)
methanol); evaporate;
dissolve (hexane); filter
Human serum centrifuge; extract (chloroform: GC/FID 50 µg/litre Lewis et al.
methanol); evaporate; dissolve GC/MS (1977)
(ethyl acetate); treat with
alumina; decant; rinse;
filter; evaporate; dissolve
residue (hexane-containing
butyl benzyl phthalate as an
internal standard)
Human plasma separation on Celite 545; GC/FID 50 ng Piechocki &
extract (diethyl ether); Purdy (1973)
evaporate; dissolve (carbon
disulfide)
Table 1 (contd).
Sample Sample Assay Limit of
matrix preparation procedure detection Reference
Stored blood; lyophilize; suspend; filter; GC/FID not given Contreras et
whole blood wash residue; mix with distilled al. (1974)
water; centrifuge; add silicic
acid to chloroform phase;
mix; centrifuge; decant;
evaporate; dissolve; centrifuge
Human and grind wet tissue samples GC/FID 0.3 µg/g Chen et al.
animal in saline; extract homogenate (wet tissue) (1979b)
tissue and or urine; dilute with 15 ng/ml Chen et al.
urine chloroform:methanol (urine) (1979a)
Intravenous add hydrochloric acid; GC/ECD 4 µg/litre Arbin &
solutions extract (dichloromethane); Östelius
redissolve (1980)
Organic evaporate; dissolve GC/FID not given Ishida et al.
solvents (diethyl ether) (1980)
Solid immerse in chloroform: GC/FID not given Ishida et al.
reagents methanol; filter; rinse; (1980)
evaporate
Aluminium cut into small pieces; GC/FID not given Ishida et al.
foil; rubber immerse in chloroform: (1980)
tubing, etc. methanol; extract
Abbreviations: GC = gas chromatography; FID = flame-ionization detection; ECD =
electron capture detection; MS = mass spectrometry; HPLC = high-
performance liquid chromatography; UV = ultraviolet spectroscopy;
EI = electron impact; CI = chemical ionization; SIM = selected ion
monitoring.
3. SOURCES OF HUMAN AND ENVIRONMENTAL EXPOSURE
It seems likely that by far the major part of the phthalate
esters present in the environment arises from human activity and not
from natural sources. Some dialkyl esters may be found in coals, crude
oil, and shales while others may have plant origins, but most
originate either directly or indirectly from industrial processes.
Phthalic acid has been reported to be formed during the bacterial
metabolism of phenanthrene.
3.1 Natural occurrence
Phthalates have been reported in a wide variety of substances
(oil, soil, plants, and animals) and over a wide geographical area.
Most occurrences have anthropogenic origins but some could be of
natural origin. The nature of the origin is further complicated by the
fact that sampling techniques often lead to contamination of samples
via contamination from plastic bags or bottles. Mathur (1974a)
critically reviewed this question and concluded that the possibility
of the phthalic acid esters found in biological and geochemical
samples being of biosynthetic origin cannot be ruled out. Both Mathur
(1974a) and Peakall (1975) cite reports where phthalates were detected
yet no anthropogenic source could be found. Studies by Manandhar et
al. (1979) and Pare et al. (1981) also revealed residues of phthalates
in biological samples where the source seemed to be natural. Peterson
& Freeman (1984) suggested that some of the phthalates found in older
samples (from the 1920s and 1930s) of sediment cores from Chesapeake
bay, USA, may have been of natural origin.
An ECETOC (European Chemical Industry Ecology and Toxicology
Centre) task force concluded that, although knowledge of naturally
produced phthalates is limited or uncertain, it is unlikely that this
contribution is of significance except, possibly, in very localized
areas (ECETOC, 1985).
3.2 Anthropogenic sources
3.2.1 Production levels
About 2.7 x 106 tonnes of total phthalates are produced
annually, of which the non-plasticizer (dimethyl and diethyl)
phthalates represent a very small fraction. Of the plasticizer
phthalates, DEHP accounts for well over 50% of the tonnage, the
contribution of the remaining compounds ranging from about 1% to 10%
each (ECETOC, 1985).
The production of DEHP has been increasing since it was first
used commercially in 1949. During the period 1950-1954, the production
in the USA was 106 x 103 tonnes, and by the period 1965-1969 the
level had risen to 650 x 103 tonnes (Peakall, 1975).
The estimated world consumption of DEHP in 1984 was 1.09 x 106
tonnes (SRI, 1985).
3.2.2 Uses
Phthalate acid esters are the most widely used plasticizers for
the production of polyvinyl chloride (PVC) products (with DEHP as the
plasticizer). Phthalates are used for the insulation of wires and
cables, in floor tiles, weatherstripping, upholstery, garden hose,
swimming pool liners, footwear and clothing. They are also used in
food wrapping and containers, although in some countries this use is
prohibited by law. They also have non-plasticizer uses, e.g., as
pesticide carriers.
DEHP has been widely used since 1949. An important property is
that it softens resins without reacting with them chemically. This has
led to about 95% of DEHP production being directed towards plasticizer
use, particularly in PVC products such as tubing and medical device
components. It is also used as a plasticizer in cellulose ester
plastics and synthetic elastomers. The DEHP content of these products
generally ranges from 20 to 40%, but for some uses it is up to 55%.
The most important non-plasticizer use of DEHP is as a dielectric
fluid in capacitors.
3.2.3 Disposal of plasticized products
Most discarded plasticized products are disposed of either by
incineration or via dumping in a tip/landfill site. When incinerated
at high temperature the combustion of phthalates is nearly complete.
However, if combustion is uncontrolled and occurs at a low
temperature, a large percentage of the phthalates may be lost to the
atmosphere. After dumping in a landfill site, phthalates may leach
into the aquatic environment, but because of their high affinity for
organic soil particles and their low water solubility this is not
likely to be a major route into the environment. Indiscriminate
dumping is more likely to lead to volatilization of phthalates to the
atmosphere rather than leaching to the aquatic environment (ECETOC,
1985).
4. ENVIRONMENTAL TRANSPORT, DISTRIBUTION, AND TRANSFORMATION
4.1 Environmental transport and distribution
The release of phthalates to the environment may occur as
follows:
a) during production and distribution;
b) during the manufacture of plasticized products;
c) during the use of plasticized product;
d) after disposal.
It was concluded by ECETOC (1985) that most of the phthalates
entering the environment are likely to do so by volatilization to the
atmosphere, only a minor part (perhaps 10%) entering the aquatic
environment by leaching.
The estimated worldwide emissions of DEHP are given in Table 2.
However, as reported by ECETOC (1985), the loss of DEHP from modern
production plants is negligible.
4.1.1 Transport in air
ECETOC (1985) suggested that transport in air is the major route
by which phthalates enter the environment. DEHP is volatilised to the
atmosphere and then either falls as dry deposition or is "washed out"
via rainfall.
DEHP has been measured in air samples from remote sites at
Enewetak Atoll in the North Pacific Ocean (Atlas & Giam, 1981), the
North Atlantic (Giam et al., 1978), the Gulf of Mexico (Giam et al.,
1978, 1980), and Sweden (Thurén & Larsson, 1990).
4.1.2 Transport in soil and sediment
DEHP has a high n-octanol-water partition coefficient, so the
equilibrium between water and an organic-rich soil or sediment will be
very much in favour of the soil or sediment. Absorbance of DEHP by
soil or sediment is also enhanced by van de Waals type bonding with
natural soil minerals, promoted by the presence of benzene rings and
carbonyl groups, and also by the low solubility of DEHP (ECETOC,
1985).
From results with other organic substances, Wams (1987) estimated
that 90% of DEHP is readily adsorbed by organic soil particles.
As can be seen from section 5.1.4, the sediment or hydrosoil
tends to act as a sink for DEHP.
Table 2. Estimated worldwide emission of DEHP, based on an estimated
total annual production of 4 x 106 tonnesa
Phase Emission (tonnes/year) Route
Production up to 40 000 waste water
Distribution 2000 sewage systems
Production of PVC 32 000 air and water
During use of plastics 14 000 air
6000 water
After disposal:
to landfill sites up to 200 000 percolating water
to waste incinerators ? air
uncontrolled burning ? air
a Adapted from Wams (1987). The values are higher than those from
other sources (ECETOC, 1985)
4.1.3 Transport in water
The solubility of DEHP in water is low (0.3 mg/litre at 25 °C).
However, the amount present in surface water may be higher than the
actual solubility as a result of adsorption onto organic particles
(Taylor et al., 1981) and interactions with dissolved organic matter
of high relative molecular mass, such as humic and fulvic acid
(Matsuda & Schnitzer, 1971).
DEHP has been found to adsorb to suspended particulate matter
fairly rapidly, in less than 2 to 3 h, especially to small particles
(Al-Omran & Preston, 1987). This adsorption was more rapid in salt
water than in fresh water. Taylor et al. (1981) reported that between
one-half and two-thirds of the DEHP in Mississippi River water is
associated with particulate matter.
By extrapolating laboratory data on the volatilization of DEHP
from water under defined conditions, Klopffer et al. (1982) obtained
a half-life in water of 146 days, although on purely theoretical
grounds a value of only 25 days was calculated.
Using the Exposure Analysis Modeling System (EXAMS), Wolfe et al.
(1980a) calculated that at equilibrium the loss of DEHP via
volatilization from a model river, a pond, an eutrophic lake, and an
oligotrophic lake would be 0%, 2.8%, 2.2% and 2.3%, respectively.
4.1.4 Transport between media
Eisenreich et al. (1981) estimated that the total annual
deposition of DEHP from air into the Great Lakes, North America,
varied from 3.7 tonnes (Lake Ontario) to 16 tonnes (Lake Superior).
DEHP has a high n-octanol/water partition coefficient. This
means that biota living in phthalate-containing water would be
expected to have a higher phthalate level than the water itself (see
section 4.2.3). However, many organisms are able to metabolize DEHP,
and the concentrations found may be lower than those expected on the
sole basis of partition coefficient.
During the 33-day period of a model ecosystem study, the
concentration of 14C in the aquatic phase reached a peak of 31
µg/litre at the fifth day after treatment and had declined to 7.7
µg/litre by the end of the experiment. This decline was stated to be
the result of the uptake of DEHP and its degradation products by the
organisms in the model ecosystem (Metcalf et al., 1973).
Lokke & Bro-Rasmussen (1981) treated the leaves of Sinapsis alba
with a mixture of di-iso-butyl phthalate (DiBP), di- n-butyl
phthalate (DBP), and DEHP at a rate of 2.5 µg/cm2. Only very small
amounts of DEHP evaporated from the leaves during the 15-day
experiment, compared with DiBP (71%) and DBP (43%).
4.2 Biotransformation
4.2.1 Abiotic degradation
As a result of atmospheric photodegradation, the atmospheric
half-life of DEHP is less than one day (ECETOC, 1985).
Chemical hydrolysis of DEHP is practically non-existent, the
half-life being > 100 years in water at pH 8 and 30 °C (Wolfe et al.,
1980b).
4.2.2 Biodegradation
Aerobic degradation has been found from several micro-organisms
in soil, sludge, sediment, and water. Anaerobic degradation is very
much slower, or possibly even non-existent.
The first step in the metabolic pathway for the biodegradation of
DEHP is the hydrolysis of the diester to the monoester by esterases
with low substrate specificity (Kurane et al., 1980; Taylor et al.,
1981). The monoester is then converted into phthalic acid (Engelhardt
et al., 1975). The ring-opening degradation to pyruvate and succinate
and then to CO2 and H2O is similar to the metabolism of benzoic
acid. According to Kurane et al. (1984), this is probably why the
biodegradation of phthalic esters is so widespread. It appears that
mixed populations of microorganisms are the most successful at
completely degrading DEHP (Engelhardt et al., 1975; Kurane et al.,
1979). When pure cultures of bacteria, selectively isolated in the
laboratory, are used for the biodegra-dation of phthalates,
accumulation of the breakdown products tends to occur (Keyser et al.,
1976).
4.2.2.1 Aerobic degradation
Aerobic degradation of DEHP has been found with several
microorganisms, including bacteria and fungi. Overall, it appears that
phthalates with short alkyl chains undergo rapid degradation, whereas
those with longer chains, such as DEHP, are only 40-90% degraded after
10-35 days (ECETOC, 1985).
Graham (1973) reported that laboratory-scale activated sludge
processes degraded 91% of introduced DEHP within 38 h. Saeger & Tucker
(1973) demonstrated that all phthalates tested underwent complete
aerobic degradation in activated sludge and river water.
Aerobic degradation of DEHP depends on temperature. Mathur
(1974b) incubated a loam soil with DEHP at 4, 10, 22-25, and 32 °C,
and soil respiration rates were measured after 14 weeks. Increased
rates of respiration, showing that microbial degradation was taking
place, were found at all temperatures. However, at 4 and 10 °C results
indicated that only marginal degradation was taking place.
Johnson & Lulves (1975) incubated freshwater hydrosoil containing
14C-DEHP (1 mg/litre) under aerobic conditions, and after 14 days,
50% of the DEHP had been degraded. This was a much slower rate of
degradation than that found with DBP, where 98% was degraded within 5
days. Johnson et al. (1984) studied the biodegradation of phthalic
acid esters in freshwater sediment and found that the length and
configuration of the alkyl phthalate diester significantly affected
the primary biodegradation rate. After a 14-day incubation in aerobic
sediment at 22 °C, less than 2% of the branched-chain alkyl phthalate
DEHP had been degraded whereas over the same period the linear alkyl
DBP showed 85% degradation. DEHP degradation was significantly greater
at very high concentrations (10 mg/litre) and at temperatures above 22
°C. Neither inorganic nitrogen nor phosphorus influenced the
degradation of DEHP. Engelhardt et al. (1977) found that the fungus
Penicillium lilacinum degraded approximately half of the initial
amount of DEHP within 30 days, yielding the corresponding monoester,
a second metabolite, which is hydroxylated in the alcohol moiety, and
at least four minor metabolites. The bacterium Pseudomonas
acidovorans completely degraded DEHP at a medium concentration of
5000 mg/kg within 72 h (Kurane et al., 1977).
Saeger & Tucker (1976) found that 60% of the DEHP had undergone
primary biodegradation within 5 weeks in Mississippi River water.
Rapid primary degradation was found when DEHP was added to activated
sludge at the rate of 5 mg/24 h. Depending on the source of the
sludge, between 70% and 78% was degraded. To monitor whether complete
biodegradation was being achieved, the authors measured CO2
evolution. Within the 14 days of incubation DEHP had essentially been
completely degraded to CO2 and water under the conditions of this
test. Taylor et al. (1981) showed the presence of significant
populations of taxonomically distinct bacteria that grew on a range of
phthalic acid esters, including DEHP, in the water and sediments of
the Mississippi River region.
Sugatt et al. (1984) used an acclimated shake-flask
CO2-evolution test to study the biodegradation of DEHP and reported
an initial breakdown of the parent compound of > 99% within the 28
days. The authors calculated a half-life of 5.25 days for the primary
biodegradation of DEHP.
In surface waters, DEHP is strongly adsorbed to organic particles
(Taylor et al., 1981), which tends to reduce degradation (Baughman et
al., 1980).
In the upper layer of soil, biodegradation of phthalates proceeds
as in surface water, but deeper down, where conditions are anaerobic,
it is virtually nonexistent (Engelhardt & Wallnofer, 1978). Shanker et
al. (1985) incubated garden soil containing DEHP at a concentration of
500 mg/kg. Within 20 days, 75% of the DEHP had been degraded and,
after 30 days, more than 90%. Again the rate of degradation was much
slower than that found for either di- n-methyl or di- n-butyl
phthalate. No degradation was detectable when sterilized soil was
used.
4.2.2.2 Anaerobic degradation
Johnson & Lulves (1975) found DEHP to be completely resistant to
microbial attack under anaerobic conditions. After 30 days, there was
no significant loss of 14C-DEHP activity in freshwater hydrosoils
overlaid with nitrogen.
Shanker et al. (1985) reported that degradation of DEHP was much
slower in anaerobic soil, flooded with sterile water to reduce the
oxygen tension. After a 30-day incubation, 33% of the DEHP had been
degraded, compared with more than 90% in the case of aerobic soil.
O'Connor et al. (1989) studied the biodegradation of DEHP under
anaerobic conditions in a medium containing municipal digester sludge
over a period of 140 days. DEHP, which was the only carbon source, was
added at a rate of 20, 100, and 200 mg/litre, and 100%, 69%, and 54%
of the DEHP was degraded at the three respective concentrations.
However, complete biodegradation to carbon dioxide and methane was
minimal.
Ziogou et al. (1989) studied the behaviour of DEHP (0.5, 1, and
10 mg/litre) during batch anaerobic digestion of sludge over a 32-day
period. No degradation of DEHP was observed during this period.
4.2.3 Bioaccumulation
DEHP is highly lipophilic, the log n-octanol-water partition
coefficient being 3 to 5, and it is moderately persistent. The
accumulation of DEHP is also influenced by the capability of an
organism to metabolize it. Melancon (1979) reviewed the metabolism of
phthalates in aquatic organisms. Bioconcentration factors for DEHP in
a variety of aquatic organisms are given in Table 3.
Table 3. Bioaccumulation of DEHP in aquatic organisms
Organism Stat/ Exposure Exposure Bioconcentration Reference
flowa period concentration factorc
(µg/litre)
Freshwater organisms
Canadian pondweed stat 24 h 10 274.8d Metcalf et al. (1973)
(Elodea canadensis) stat 12 h 10 000 133.8d Metcalf et al. (1973)
Snail stat 48 h 10 857.5d Metcalf et al. (1973)
( Physa sp) stat 6 h 10 000e 402d Metcalf et al. (1973)
Scud flowb 7 day 0.1 13 600 Sanders et al. (1973)
(Gammarus pseudolimnaeus) flow 7 day 0.1 3900 Mayer & Sanders (1973)
Water flea flowb 7 day 0.3 5200d Sanders et al. (1973)
(Daphnia magna) flow 7 day 0.3 420 Mayer & Sanders (1973)
stat 1 h 10 421d Metcalf et al. (1973)
stat 12 h 10 000e 133.8d Metcalf et al. (1973)
Sowbug flowb 21 day 62.3 250d Sanders et al. (1973)
(Asellus brevicaudus)
Mosquito (larvae) stat 12 h 10 1320.2d Metcalf et al. (1973)
(Culex pipiens stat 24 h 10 000e 1187.3d Metcalf et al. (1973)
quinquefasciatus) stat 24 h 10 20.3d Metcalf et al. (1973)
stat 48 h 10 000e 434.6d Metcalf et al. (1973)
Midge larvae (3rd instar) flowb 7 day 0.2 408 Streufert et al. (1980)
(Chironomus plumosus) flowb 7 day 0.3 3100d Sanders et al. (1973)
flow 7 day 0.3 350d Mayer & Sanders (1973)
Mayfly flowb 7 day 0.1 2300d Sanders et al. (1973)
(Hexagenia bilineata) flow 7 day 0.1 575d Mayer & Sanders (1973)
Table 3 (contd).
Organism Stat/ Exposure Exposure Bioconcentration Reference
flowa period concentration factorc
(µg/litre)
Mosquito fish stat 48 h 100 265.3d Metcalf et al. (1973)
(Gambusia affinis) stat 12 h 10 000e 129.4d Metcalf et al. (1973)
Fathead minnow flow 14 day 1.9 458d Mayer & Sanders (1973)
(Pimephales promelas) flow 56 day 1.9 886d Mehrle & Mayer (1976)
Marine organisms
Eastern oyster (muscle) stat 24 h 100 11.2 Wofford et al. (1981)
(Crassostrea virginica) stat 24 h 500 6.9 Wofford et al. (1981)
Brown shrimp stat 24 h 100 10.2 Wofford et al. (1981)
(Penaeus aztecus) stat 24 h 500 16.6 Wofford et al. (1981)
Sheepshead minnow stat 24 h 100 10.7 Wofford et al. (1981)
(Cyprinodon variegatus) stat 24 h 500 13.5 Wofford et al. (1981)
a Stat = static conditions (water unchanged for the duration of the test); flow = flow-through conditions
(DEHP concentration in water continuously maintained, unless stated otherwise)
b Intermittent flow-through conditions
c Bioconcentration factor = concentration of DEHP in organism divided by concentration of DEHP in water
d Bioconcentration factor calculated using a radioactive isotope (values represent parent compound plus
radiolabelled products)
e DEHP applied directly to water
4.2.3.1 Model ecosystems
Metcalf et al. (1973) studied the uptake of 14C-labelled DEHP
from water by aquatic organisms in a model ecosystem containing algae
(Oedogonium), snails ( Physa sp.), mosquito larvae (Culex pipiens
quinquefasciatus), and fish ( Gambusia sp). The mosquito larvae
showed the highest concentration factor and the fish the lowest.
Labelled DEHP was added to Sorghum plants and at the end of the 33-
day experiment the water contained 0.34 µg DEHP per litre, the algae
18.32 mg/kg (53 890 x), the snails 7.3 mg/kg (21 480 x), the mosquito
larvae 36.61 mg/kg (10 7670 x), and the fish 0.044 mg/kg (130 x).
Sodergren (1982) exposed fish, aquatic invertebrates, and plants
to 14C-labelled DEHP at a concentration of 1.4 mg/litre for 27 days
under static conditions. After 5 days, 1/50 of the added amount of
DEHP was still present in the water, and at the end of the experiment
62% was recovered from the various surfaces (glass walls, sediment and
surface microlayer). All organisms accumulated DEHP. The amphipod
Gammarus pulex, larvae of trichopterans, and the snail Planorbis
corneus accumulated the DEHP to the highest degree, the
concentration factors ranging from 17 000 to 24 000. The submerged
plants, Mentha aquatica and Chara chara, also showed uptake and
storage of large amounts (concentration factor of 18 000). However,
the fish (stickleback, Pungitius pungitius, and minnow, Phoxinus
phoxinus) did not accumulate 14C-DEHP to any great extent
(concentration factors of 300 or less). Large accumulations of DEHP
occurred in organisms living and/or feeding at interfaces.
4.2.3.2 Aquatic invertebrates
Brown & Thompson (1982a) exposed Daphnia magna to nominal
14C-labelled DEHP concentrations of 3.2, 10, 32, and 100 µg/litre
for 21 days and obtained bioconcentration factors of 166, 140, 261,
and 268 at the four respective concentrations.
When Brown & Thompson (1982b) exposed mussels (Mytilus edulis)
to labelled DEHP at concentrations of 4.1 and 42.1 µg per litre, in
both cases equilibrium was reached within 14 days with a concentration
factor of 2500. Exposure ceased on day 28 but the mussels were
monitored for a further 14 days. The half-life for loss of DEHP over
this period was calculated to be 3.5 days.
Laughlin et al. (1978) exposed grass shrimp, during larval
development, to DEHP concentrations of up to 1 mg/litre for 28 days.
DEHP was not detectable in shrimp tissues at or above a level of 2
mg/kg.
When Streufert et al. (1980) exposed midge larvae (Chironomus
plumosus) to a radioactively labelled DEHP concentration of 0.2
µg/litre, the larvae accumulated DEHP to 292 times the concentration
in water within 2 days. DEHP levels in the midge larvae reached a
plateau after 7 days at a bioconcentration factor of 408. Some of the
larvae were transferred to clean water after 4 days, by which time
they had accumulated 56 µg DEHP/kg, and the half-life for loss was
calculated to be 3.4 days.
After 9 weeks of exposure to sediment containing approximately
600 mg/kg, dragonfly larvae had taken up 14.7 mg DEHP per kg. This was
significantly more than control larvae, which contained 2.9 mg/kg
(Woin & Larsson 1987).
Hobson et al. (1984) fed penaeid shrimps on a diet containing
between 40 and 50 000 mg DEHP/kg for 14 days at a rate of 40 g/kg body
weight per day. Whole body residues ranged from 0.249 to 18.3 mg/kg in
a dose-related manner.
4.2.3.3 Fish
Macek et al. (1979) exposed bluegill sunfish (Lepomis
macrochirus) to 14C-DEHP, both via food at a concentration of 2.8
mg/kg and via water at 5.6 µg/litre, for up to 35 days. The steady-
state body burden of 14C-DEHP after exposure via food and water was
0.73 mg/kg and via water alone was 0.64 mg/kg. The authors concluded
that the uptake of DEHP via the aquatic food chain was statistically
indistinguishable from that due to aqueous exposure. The time required
for the fish to eliminate 50% of the residue burden during depuration
in uncontaminated water was < 3 days.
In a study by Karara & Hayton (1989), sheepshead minnows
(Cyprinodon variegatus) were exposed to a 14C-DEHP concentrations
of 60 ng/litre at temperatures ranging from 10 °C to 35 °C for a
period of between 72 h and 160 h. The amount of DEHP accumulated after
72 h was 6 times greater at 35 °C than at 10 °C, and the
bioconcentration factors increased exponentially with temperature from
45 at 10 °C to 6510 at 35 °C. Metabolic clearance also increased as a
function of temperature, the maximum value being reached at a
temperature of between 29 °C and 35 °C.
Tarr et al. (1990) exposed three sizes (2.9 g, 61 g, and 440 g)
of rainbow trout (Oncorhynchus mykiss) to 14C-DEHP at 20 ng/ml
under static conditions for up to 96 h at 12 °C. The body-weight-
associated changes in the pharmacokinetic parameters caused the
bioconcentration factor to decline from 51.5 to 1.6 as body weight
increased.
When Mehrle & Mayer (1976) exposed rainbow trout (Salmo
gairdneri) eggs (12 days prior to hatching to 24 days post-hatching)
to 14C-labelled DEHP at concentrations of 5, 14, and 54 µg/litre,
the bioconcentration factors were 78, 113, and 42, respectively.
Mayer (1976) exposed fathead minnows (Pimephales promelas) to
DEHP concentrations ranging from 1.9 to 62 µg/litre for 56 days under
flow-through conditions. As the exposure concentration increased,
concentration factors, measured after 56 days, decreased from 569 to
91. Equilibrium was attained after 28 days at the lowest dose and
after 56 days at the highest dose. After exposure the fish were placed
in uncontaminated water for 28 days, and the half-lives for loss
ranged from 6.2 days (at 2.5 µg/litre) to 18.3 days (at 62 µg/litre).
4.2.3.4 Amphibians
Larsson & Thuren (1987) exposed moorfrog eggs to sediment DEHP
concentrations ranging from 10 to 800 mg/kg (fresh weight of
sediment). The eggs hatched after about 3 weeks and the tadpoles were
analysed after 60 days. The DEHP was released from the sediment to the
overlying water, and the losses to the water increased linearly with
increasing levels in the sediment (from 0.89 to 187.4 µg/litre). DEHP
accumulated in the tadpoles at concentrations ranging from 0.28 to
246.8 mg/kg wet weight, and the accumulation increased with increasing
DEHP concentration, both in sediment and water.
4.2.3.5 Plants
Lokke & Bro-Rasmussen (1981) applied DEHP as a mixture that also
contained DiBP and DBP at a concentration of 2.5 µg/cm2 to the
leaves of Sinapis alba. The residue level of DEHP on the leaves
immediately after application was 2.7 µg/cm2. After 15 days, DEHP
levels had decreased to 0.8 µg/cm2, but when the growth of the plant
was taken into account, no significant loss of DEHP over this period
was found. Lokke & Rasmussen (1983) also found little loss of DEHP
over a 15-day period when they applied it (as a mixture with DBP) to
Achillea at a concentration of 3.5 µg/cm2 or to Sinapis at 3.1
µg/cm2. Residues ranged from 120 to 155 µg/plant, and approximately
80% of the DEHP accumulated was on the surface of the leaf.
When Schmitzer et al. (1988) grew barley from seed in soil
containing 1 or 3.33 mg 14C-DEHP/kg dry soil for 7 days, only 0.61%
and 1.25%, respectively, of the applied 14C was taken up by the
plants. Aranda et al. (1989) also found low accumulation of DEHP by
plants grown in sewage sludge containing DEHP. Lettuce (Lactuca
sativa), carrot (Daucus carota), chile pepper (Capiscum annuum),
and tall fescue (Festuca arundinaca) were all grown in sludge
containing 14C-DEHP levels of between 2.57 and 14.07 mg/kg.
Bioconcentration factors ranged from 0.06 to 0.53 (based on initial
soil concentration and plant dry weight).
4.2.3.6 Birds
Belisle et al. (1975) fed mallard ducks (Anas platyrhynchos) on
a diet containing 10 mg DEHP/kg for a period of 5 months. No DEHP was
detected in fat tissue, but 0.1 and 0.15 mg/kg (wet weight) were found
in breast muscle and lung tissue, respectively.
O'Shea & Stafford (1980) exposed starlings (Sturnus vulgaris)
to a dietary DEHP concentration of 25 or 250 mg/kg for 30 days. One
of eight birds fed 25 mg/kg contained detectable residues (1.6 mg/kg)
after 30 days exposure, and five of eight birds fed 250 mg/kg
contained an average of 1.8 mg/kg. The same proportion of birds fed
the higher dose still had detectable residues (an average of 1.3
mg/kg) 14 days after dosing had finished.
When Ishida et al. (1982) fed hens on a diet containing 5 or 10
g/kg for up to 230 days, DEHP was detected in all tissue monitored
except the brain. Residues ranged from non-detectable to 42.5 mg/kg
for most tissues. However, adipose tissue (192.7 to 899.6 mg/kg) and
feathers (513.1 to 1165.2 mg/kg) accumulated the highest
concentrations. A similar pattern of uptake was observed in hens fed
2 g/kg for 25 days, although the amount of DEHP accumulated was much
lower. At this dose level no accumulation had occurred in tissues
other than liver and feather within 5 days.
5. ENVIRONMENTAL LEVELS AND EXPOSURE
5.1 Environmental levels
DEHP exists widely in the environment and is found in most
samples, including air, precipitation, water, sediment, soil, and
biota. Residues have also been detected in food and in humans.
In many cases it is not clear whether the phthalate measured in
samples is naturally occurring or is exogenous. However, there seem to
be clear indications that high levels of DEHP are anthropogenic in
origin.
5.1.1 Air
The levels of DEHP in air have been monitored in the North
Atlantic, the Gulf of Mexico, and on Enewetak Atoll in the North
Pacific and found to range from not detectable to 4.1 ng/m3 (Giam et
al., 1978; Giam et al., 1980; Atlas & Giam 1981). Similar levels
(between 0.5 and 5 ng/m3) have been found in the Great Lakes
ecosystem (Eisenreich et al., 1981) and in the Swedish atmosphere
(Thurén & Larsson, 1990). The DEHP content of these samples was at
least an order of magnitude lower than those found in urban areas such
as New York city, where levels of up to 28.6 ng/m3 have been found
(Bove et al., 1978). Based on the analysis of snow samples, Lokke &
Rasmussen (1983) calculated DEHP concentrations in air of 22 ng/m3
at Lyngby, Denmark. Levels of 29-132 ng/m3 have been found in
Antwerp, Belgium (Cautreels et al., 1977), 126 ng/m3 in polluted air
in Belgium (Cautreels & Van Cauwenberghe, 1978), 300 ng/m3 in
polluted air in Canada (Thomas, 1973), and 38-790 ng/m3 in Japan in
1985 (Environment Agency of Japan, 1989).
5.1.2 Precipitation
Atlas & Giam (1981) measured levels of DEHP in rainfall at
Enewetak Atoll, North Pacific, of 5.3-213 ng/litre (mean, 55
ng/litre). Eisenreich et al. (1981) reported between 4 and 10 ng/litre
in precipitation falling on the Great Lakes ecosystem and Thurén &
Larsson (1990) a level of 55 ng/litre in Sweden. Goto (1979) found a
range of mean rainwater concentrations of 0.65 to 3.16 µg/litre in
various Japanese cities.
Lokke & Rasmussen (1983) analysed snow sampled near a plasticizer
production plant 14 days after a snowfall. Levels of DEHP ranged from
0.7 to 4.7 µg/m2 per day over this period, the highest levels being
within 150 m of the plant and the lowest levels at least 600 m away.
5.1.3 Water
Levels of DEHP found in water are summarized in Table 4.
Table 4. Concentrations of DEHP in water
Location Country Year Concentration Reference
µg/litrea
Marine
Northern Atlantic 0.0001-0.006 Giam et al. (1978)
Gulf of Mexico 0.006-0.316 Giam et al. (1978)
Estuaries Germany ND-0.3 Weber & Ernst (1983)
Nueces Estuary,
Texas USA 1980 0.2-0.77 Ray et al. (1983b)
Estuaries United Kingdom 1981 0.058-0.078 Waldock (1983)
Freshwater
Various rivers Japan ND-3.1 Kodama et al. (1975)
Various cities Japan 1974 0.1-2.19 Goto (1979)
River Meuse Netherlands 1983 < 0.1-3.5 Wams (1987)
River Rhine Netherlands 1983 ND-1.2 Wams (1987)
River Rhine Netherlands 1982 ND-4.0 Wams (1987)
a ND = not detectable
Thuren (1986) analysed water samples from the Rivers Ronnebyan
and Svartan, Sweden, and found DEHP concentrations ranging from 0.32
to 3.1 µg/litre and from 0.39 to 1.98 µg/litre, respectively. The
highest concentrations were associated with industrial effluent
discharge points.
Few samples of ground water have been analysed for DEHP. Wams
(1987) reported that contaminated ground water in the Netherlands
contained between 20 and 45 µg/litre, while Rao et al. (1985) found
DEHP levels of up to 170 µg/litre in New York state ground water.
In a non-industrialised estuary in the United Kingdom, Waldock
(1983) measured DEHP levels of 58 to 78 ng/litre. Ray et al. (1983b)
found levels of DEHP ranging from 210 to 770 ng/litre in a Nueces
estuary in Texas, USA, while Weber & Ernst (1983) found DEHP levels of
up to 300 ng/litre in German estuaries. DEHP levels of up to 316
ng/litre have been found in the Gulf of Mexico, but levels in the
North Atlantic were much lower (Giam et al., 1978). In Japan, river
and marine levels in 1982 ranged from 0.1 to 0.8 µg/litre (Environment
Agency of Japan, 1989).
Ritsema et al. (1989) analysed samples from Lake Yssel,
Netherlands, and found DEHP levels of < 0.1 to 0.3 µg/litre in water
and levels of 12-25 mg/kg in suspended particulate matter. The authors
concluded that the probable source of DEHP was the River Yssel.
Preston & Al-Omran (1986) sampled water and suspended
particulates from the Mersey estuary, United Kingdom, in 1985, and
reported DEHP concentrations ranging from 83 to 335 ng/litre in water
and from 182 to 1700 µg/kg in particulate matter. However, in 1986,
levels were 125-693 ng/litre in water and 280-640 µg/kg in particulate
matter (Preston & Al-Omran, 1989).
5.1.4 Sediment
DEHP levels in sediment are summarized in Table 5.
Being lipophilic DEHP tends to be adsorbed onto sediment, which
acts as a sink. Sediment samples from various Dutch rivers have been
found to contain between 1 and 70 mg/kg (Schwartz et al., 1979; Wams,
1987). Taylor et al. (1981) analysed sediment samples from the
Mississippi River and found similar levels. Sediment levels of DEHP
in the Chester River Maryland, USA, were found to be less than 45
µg/kg dry weight, but, in a tributary of this river, sediment levels
were up to 4.8 mg/kg about 2 km downstream from a phthalate ester
plant outfall. The Chester River flows into Chesapeake Bay, which
contained sediment DEHP levels of 110 µg/kg (Peterson & Freeman 1984).
In Sweden, Thuren (1986) found sediment DEHP levels ranging from 1.2
to 628 mg/kg (dry weight) in the River Ronnebyan and 0.15 to 1480
mg/kg in the River Svarten. As was found with water samples (see
section 5.1.3), in both rivers the highest residues of DEHP were near
to industrial effluent discharge points.
Giam et al. (1978) analysed sediment from the Mississippi delta
and reported mean DEHP levels of 69 µg/kg. Sediment samples from
Nueces Estuary, Texas, USA, unlike the water samples, reflected local
inputs of pollutants. The highest levels (up to 16 mg/kg) were
associated with industrial areas, whereas other areas of the estuary
contained levels ranging from 40 to 330 µg/kg. Much lower levels of
DEHP were found on the Gulf of Mexico coast and in the open sea (mean
levels of 6.6 and 2 µg/kg, respectively) (Giam et al., 1978).
Table 5. Concentrations of DEHP in sediment
Location Country Year Concentration Reference
(µg/kg)a
Gulf of Mexico < 0.1-248 ns Giam et al. (1978)
Nueces Estuary, Texas USA 1980 40-16 000 dw Ray et al. (1983b)
Portland, Maine USA 1980 60-7800 dw Ray et al. (1983a)
Chester River, Maryland USA 1978 20-4800 dw Peterson & Freeman (1984)
River Mississippi USA 1981 140 dw Taylor et al. (1981)
River Meuse Netherlands 1977 1000-17 000 dw Schwartz et al. (1979)
River Ijssel Netherlands 1977 2500-52 500 dw Schwartz et al. (1979)
River Rhine Netherlands 1978 4000-36 000 ns Wams (1987)
River Rhine Netherlands 1977 6500-70 500 dw Schwartz et al. (1979)
Various cities Japan 1974 80-1360 dw Goto (1979)
Crouch Estuary, Essex United Kingdom 1981 11.2-26.2 ww Waldock (1983)
River Usk United Kingdom 1974 30 000 dw Eglinton et al. (1975)
a dw = dry weight; ww = wet weight; ns = not stated whether concentrations refer to dry or wet weight
In Japan, the levels of sediments in rivers and seas in 1982
ranged from 9 to 35 000 µg/kg dry weight (Environment Agency of Japan,
1989).
5.1.5 Soil
Contaminated soil analysed in the Netherlands was found to
contain up to 1.5 mg DEHP/kg (Wams 1987), while residues of DEHP in
soil collected in the vicinity of a DEHP manufacturing plant contained
up to 0.5 mg/kg (Persson et al., 1978).
Fatoki & Vernon (1990) reported a DEHP concentration of 1.9
µg/litre in treated sewage effluent from the Manchester area, United
Kingdom, and stated that such a level was consistent with the
industrial activities of the city.
5.1.6 Food
DEHP has been found in many samples of fish and shellfish (see
Table 6). It has also been detected in milk (Cerbulis & Ard, 1967),
bovine pineal gland (Taborsky, 1967), bovine heart muscle (Nazir et
al., 1971), and chicken eggs (Ishida et al., 1982). Perkins (1967)
isolated a substance similar to DEHP from corn oil. The US ATSDR
(1988) quoted an US FDA survey of various foods in 1974 which showed
that DEHP levels in most foods were less than 1 mg/kg; the foods
surveyed included margarine, cheese, meat, cereal, eggs, milk, white
bread, canned corn, corn meal, and baked beans.
Ishida et al. (1981) collected and analysed chicken eggs
available in Japanese markets. DEHP levels in egg white ranged from
0.05 to 0.4 mg/kg, but no DEHP was detected in the egg yolk.
In a study by Antonyuk (1975), DEHP migration from PVC materials
into foodstuffs was noted following 7 days of contact. Levels of 4-16
mg DEHP/kg were detected in cheese, sausage, meat, flour, and rice
while after 30 days levels of 30-150 mg/kg were found in sunflower
oil. The permissible level of DEHP migration into foodstuffs was
considered to be 2.0 mg/kg. Zitko (1972) detected DEHP concentrations
in hatchery-reared juvenile Atlantic salmon of 13 to 16 mg/kg lipid;
fish food contained 8 to 9 mg/kg lipid. When Williams (1973) analysed
fish available to the Canadian consumer, only 6 out of 21 samples
contained measurable amounts of DEHP. Levels of 0.1 and up to 0.16
mg/kg were found in unprocessed eels and in processed canned tuna
fish, respectively.
Table 6. Concentrations of DEHP in biota
Organisms Location Country Concentration Reference
(µg/kg)a
Mollusc (digestive gland) Crouch Estuary, Essex United Kingdom 9.2-214 ww Waldock (1983)
Dragonfly naiads Iowa (industrial) USA 200 Mayer et al. (1972)
Commercial fish food North America 2000-7000 Mayer et al. (1972)
Channel catfish Mississippi & Arkansas USA 3200 Mayer et al. (1972)
(industrial)
Channel catfish Iowa (industrial) USA 400 Mayer et al. (1972)
Various fish species Japan 70-450 Kodama & Takai (1974)
Various fish species Japan < 50-1800 ww Kamata et al. (1978)
Various fish species various cities Japan 50-720 Goto (1979)
Mainly fish Gulf of Mexico < 1-135 Giam et al. (1978)
Various fish species (liver) Tees Bay United Kingdom 43-85.9 ww Waldock (1983)
Various fish species (muscle) Tees Bay United Kingdom 13-51.3 ww Waldock (1983)
Walleye Lake Superior, Ontario Canada 800 Mayer et al. (1972)
Tadpoles Iowa (industrial) USA 300 Mayer et al. (1972)
Common seal pup (blubber) 10 600 lw Zitko (1972)
a ww = wet weight; lw = lipid weight
5.1.7 Aquatic organisms
Ray et al. (1983a) found DEHP levels of up to 490 µg/kg in
sandworms (Neanthes virens) and up to 170 µg/kg in clams collected
from Portland, Maine, USA, but these levels did not seem to reflect
the sediment levels and local pollutant sources.
Musial & Uthe (1980) collected fish of various species from the
Gulf of St Lawrence, Canada, and analysed them for DEHP in lipid
extracts. They reported levels of up to 6.5 mg/kg on a wet weight
basis (51.3 mg/kg on fat weight basis) in mackerel muscle and up to
7.2 mg/kg (47.1 mg/kg fat weight) in herring muscle. Lower levels of
0.37 mg/kg (wet weight) were found in eels, and in both plaice and
redfish concentrations were less than 0.001 mg/kg.
Persson et al. (1978) collected aquatic organisms from the
vicinity of a DEHP factory in Finland. Invertebrates contained up to
0.1 mg DEHP/kg, and levels of 1.1 mg/kg in roach muscle and 2.3 mg/kg
in pike liver were measured. Thuren (1986) analysed biota near to an
industrial discharge point and reported levels of up to 5.3 mg/kg
(fresh weight) in Odonata sp and up to 14.4 mg/kg in Asellus
aquaticus.
In Japan, DEHP levels in various species of fish in rivers and
seas ranged from 0.01 to 19 mg/kg wet weight in 1974 (Environment
Agency of Japan, 1989).
5.1.8 Terrestrial organisms
Persson et al. (1978) analysed soil arthropods collected near a
DEHP factory in Finland and found residues of 2.8 mg/kg.
5.2 General population exposure
Few data are available on general population exposure.
Based on an analysis of DEHP levels in various foods, the average
exposure in the USA has been estimated to be around 0.3 mg/person per
day and the maximum 2 mg/person per day (see section 5.1.6).
In a survey of plasticizer levels in food-contact material and
food, the United Kingdom Ministry of Agriculture, Fisheries and Food
(MAFF, 1987) stated that DEHP has very limited use in food-contact
material, and the maximum daily intake from food sources has been
estimated to be less than 20 µg/person per day.
DEHP found in human tissues may be derived from medical devices,
since it was recognised by Trimble et al. (1966) and by Guess &
Haberman (1968) that DEHP is leached from certain medical devices.
Samples of soft PVC fluid bags containing normal saline and glucose
(50 mg/ml) were shaken for 24 h and analysed for plastic additives
(Smistad et al., 1989). The PVC plastic materials contained DEHP,
epoxidized vegetable oils, and stearates as the main additives. The
same components were found in the solutions. MEHP was also detected,
but only in the solutions.
Marcel & Noel (1970) reported the presence of phthalate esters in
human plasma that had been stored in plastic blood bags.
Jaeger & Rubin (1972) found that DEHP was extracted from PVC
plastic blood bags by human blood at the rate of 2.5 mg/litre per day
at 4 °C. The DEHP was found in both lipid-containing and lipid-free
fractions of plasma, whereas the red cells contained only minor
amounts. Seven out of twelve samples of lung tissue, taken at autopsy
from patients who had received transfusions of stored blood, contained
DEHP at concentrations of 13.4-91.5 mg/kg (dry weight). Rubin & Nair
(1972) reported that DEHP had also been found in the tissues and urine
of patients who had not received blood transfusions, but no details
were given. Mes et al. (1974) analysed human adipose tissue in Canada
and found the levels of DEHP in most samples to range from 0.3 to 1.0
mg/kg.
Schneider et al. (1989) estimated that the highest exposure to
DEHP associated with medical devices resulted from extracorporeal
membrane oxygenation (ECMO), which could result in an exposure of 14
mg/kg per day. In an infant receiving ECMO for 14 and 24 days, the
DEHP serum levels were 26.8 and 33.5 mg/litre, respectively. In
another infant, ECMO for 6 days resulted in liver, heart, and
testicular concentrations of 3.5, 1.0, and 0.4 mg/kg, respectively.
Newborn infants given exchange transfusions may have plasma
levels of about 10 mg/litre (Sjöberg et al., 1985b).
5.3 Occupational exposure during manufacture, formulation or use
Few data on occupational exposure to DEHP have been reported.
In a phthalate manufacturing plant in the USA producing DEHP from
phthalic anhydride and alcohols, Liss et al. (1985) measured, in the
case of six heavily exposed workers, 8-h TWA workplace air
concentrations of DEHP ranging from 0.02 to 4.1 mg/m3. The exposure
level for 44 other workers in the same plant was below the detection
limit (10 µg/sample).
In an Italian factory producing n-butyl phthalate, isobutyl
phthalate, and DEHP, Gilioli et al. (1978) measured total phthalate
exposure concentrations of between 1 and 60 mg/m3, the average being
5 mg/m3.
Nielsen et al. (1985) measured total phthalic acid esters in air
in a PVC-processing plant in Sweden where diisodecyl phthalate, DEHP,
and some butylbenzylphthalate were used. Concentrations of between
0.01 and 2.0 mg/m3 were recorded in 96 2-h personal samples from 54
workers.
Total phthalates concentrations in air of between 1.7 and 66
mg/m3 were recorded in a PVC-processing plant in the USSR using
mainly dibutyl phthalate and higher alkyl phthalates but also some
DEHP and other phthalates (Milkov et al., 1973). Stankevich & Zarembo
(1978) measured DEHP levels of 1.5 to 40 mg/litre in the blood of
workers manufacturing PVC in the USSR.
DEHP concentrations in air of between 0.09 and 0.16 mg/m3 were
recorded in a German factory for phthalate production (Thiess et al.,
1978a). In 9 PVC-processing plants in Finland, the mean concentration
of DEHP ranged from less than 0.02 mg/m3 to 0.5 mg/m3 and the
highest single value was 1.1 mg/m3 (Vainiotalo & Pfäffli, 1990).
6. KINETICS AND METABOLISM IN LABORATORY ANIMALS AND HUMANS
6.1 Absorption
6.1.1 Inhalation
There is no quantitative information available on the pulmonary
route of absorption, although aerosols of DEHP are readily formed
(Albro & Lavenhar, 1989).
6.1.2 Dermal
After a single application of 14C-labelled DEHP (61.5 mg/kg) to
the back of F-344 rats, clipped one hour before treatment, urine and
faeces were collected every 24 h for 7 days. The amount of 14C
excreted was taken as an index of the percutaneous absorption, and
about 5% of the dose given was excreted (Elsisi et al., 1989).
6.1.3 Oral
Studies with 14C-labelled DEHP indicated that at least 50% of
the radioactivity of a single dose (2.9 mg/kg) was absorbed in the rat
intestine since 42% and 14% were excreted in urine and bile,
respectively, after 7 days (Daniel & Bratt, 1974). The same authors
also found that DEHP was rapidly hydrolysed by pancreatic lipase,
suggesting that DEHP is hydrolysed in the gut before absorption. This
was supported by the fact that no unmetabolized DEHP was found in
liver after the administration of low oral doses (< 0.4 g/kg),
although at higher doses (> 0.5 g/kg) DEHP was detected (Albro et
al., 1982). At an oral dose level of 2 g/kg, the bioavailability of
DEHP in rats, as measured in blood by HPLC, was 14%, whereas at an
intraperitoneal dose level of 4 g/kg only 5% was recovered, again
indicating a role for hydrolysis of DEHP in the gut (Pollack et al.,
1985b). Using an inhibitor of mucosal esterases ( S,S,S-tributyl
phosphorothionate), White et al. (1980) observed a marked inhibition
in the uptake of DEHP by the gut. Studies involving oral
administration of MEHP indicated that this metabolite is well
absorbed. After radiolabelled MEHP or DEHP was given to rats, the
radioactivity recovered in plasma from MEHP was 16 times more than
that from DEHP (Teirlynck & Belpaire, 1985).
Oral administration of DEHP (1 g/kg) to young rats leads to a
larger area under the plasma concentration-time curve (measured with
gas chromatography) for MEHP (twice that of DEHP) than in older rats
(Sjöberg et al., 1985a). This indicates either a more rapid hydrolysis
of DEHP or a more efficient absorption of MEHP in young rats.
Cynomolgus monkeys hydrolyse DEHP in the gut less efficiently
than rats or mice (Astill, 1989). Rhodes et al. (1986) also noted that
there is less absorption from the gastrointestinal tract in marmosets
than in rodents.
6.1.4 Intraperitoneal
The systemic availability of DEHP was only 5% when a dose of 4
g/kg was given intraperitoneally to rats. Relatively small amounts of
MEHP were recovered in the blood in this study (Pollack et al.,
1985b).
6.2 Distribution
Intravenously administered DEHP is rapidly eliminated from blood.
This was demonstrated in experiments where radioactive DEHP was
injected into male CFN rats and blood levels were determined by thin-
layer chromatography (Schulz & Rubin, 1973). At a low dose level (0.1
mg/kg), there was an initial phase with a half-time of 4.5 min and a
second phase with a half-time of 22 min. At a higher dose level (200
mg/kg) the initial phase had a half-time of 9 min. This indicated that
DEHP was taken up in a tissue compartment by a saturable process
(Schulz & Rubin, 1973). Radioactivity from DEHP was rapidly
distributed to the liver, lungs, and spleen when administered
intravenously (Schulz & Rubin, 1973; Daniel & Bratt, 1974).
Orally administered DEHP is mainly distributed as MEHP in rats
(Pollack et al., 1985b; Teirlynck & Belpaire, 1985). Unmetabolized
DEHP was recovered in the liver only after large oral doses
(> 0.5 g/kg) were given, indicating a threshold phenomenon in the
absorption and distribution (Albro et al., 1982; Agarwal, 1986). The
distribution kinetics of MEHP have been analysed by Pollack et al.
(1985b) and by Teirlynck & Belpaire (1985). Pollack et al. (1985b)
found that the peak concentration of MEHP in blood was reached 15 min
after oral or intraperitoneal administration of MEHP. The half-time of
MEHP in blood or plasma in the rat is shorter than that of DEHP
(Pollack et al., 1985b; Teirlynck & Belpaire, 1985). The in vitro
plasma protein binding of MEHP in the rat reaches approximately 98%
(Sjöberg et al., 1985a).
A phenomenon known as "shock-lung" has been reported to occur
following intravenous administration of DEHP to rats and other
species. Two hours after an emulsion of DEHP was given, between 13%
and 48.6% of DEHP-radiolabelled material was found in the lungs of
rats, as compared to 26.3-38.2% in the liver (Daniel & Bratt, 1974).
This phenomenon may be relevant to human exposure via intravenous
administration from bags and tubing containing DEHP.
The DEHP plasma level in newborn infants given exchange
transfusions may reach about 10 mg/litre (Sjöberg et al., 1985b).
This level is about twice as high as those found in leukaemia patients
receiving platelet concentrations and about five times as high as
levels found in haemodialysed patients. After treatment, this level
falls rapidly to about 3 mg/litre within 2 h, and then there is a
further drop with a half-time of about 10-12 h (Sjöberg et al.,
1985b).
6.3 Metabolism
DEHP is hydrolysed in vitro by pancreatic lipase to MEHP
(Daniel & Bratt, 1974), indicating that this metabolism would occur
mainly in the gut lumen. In rats about 80% of an oral dose of DEHP
undergoes mono-deesterification (Pollack et al., 1985b), while intra-
arterially administered DEHP is only slowly converted to MEHP (Pollack
et al., 1985b). Studies on the hydrolysis of DEHP in homogenates from
different organs (Table 7) indicate a very high activity in pancreatic
juice and a comparatively low activity in liver (Albro & Thomas, 1973;
Daniel & Bratt, 1974).
At relatively high doses of DEHP (2 g/kg body weight per day),
administered by gavage for up to 14 days, approximately 25-40% of the
dose in rats and 50-75% of the dose in marmosets was excreted in the
faeces. This implies that incomplete intestinal hydrolysis and
absorption may occur (Rhodes et al., 1986).
MEHP may in turn be metabolized in the gut wall (Pollack et al.,
1985b) or in other organs. Rat liver cell cultures have been shown to
convert MEHP to several metabolites, as occurs in the intact rat
(Albro et al., 1973; Lhuguenot et al., 1985). Cultures of testicular
cells were, however, apparently not able to metabolize MEHP beyond
slight hydrolysis to phthalic acid within 18-24 h (Albro et al.,
1989).
The metabolic pathways for MEHP are shown in Fig. 1. The omega-
and omega-1-carbon oxidation products constitute more than 85% of the
metabolites (Albro et al., 1973; Mitchell et al., 1985a; Lhuguenot et
al., 1985). The ethyl side chain may also be oxidized (Lhuguenot et
al., 1985). It has been suggested that omega-oxidation leads to a
metabolite that is further degraded by ß-oxidation in the peroxisomes
(Albro et al., 1973; Lhuguenot et al., 1985). Non-linear dose-
dependency has been reported for these pathways in the rat; the
predominance of omega-oxidation over omega-1-oxidation was increased
by high doses of MEHP (Lhuguenot et al., 1985).
The administration of 2-ethyl-(1-14C)-hexyl-labelled DEHP led
to a low level of radioactivity being recovered from purified rat
liver DNA (Albro et al., 1982). In a more recent study (Lutz, 1986),
the administration of 14C-carboxylate-labelled DEHP resulted in no
measurable radioactivity in DNA, whereas radioactivity was clearly
measurable after the administration of DEHP that was 14C- or
3H-labelled in the alcohol moiety.
Table 7. DEHP hydrolase activity of various tissue lipasesa
DEHP hydrolase activity
Enzyme preparation
units/mg protein units/g tissue
Liver acid lipase, homogenate 0.34 101
Liver alkaline lipase, homogenate 0.14 43
Liver "lysosomal" concentrate (acacia)b 8.80 -
Liver microsome + supernatant fraction, pH 8.2 1.22 -
Kidney acid lipase, homogenate 0.15 45
Lung homogenate (cholate)b 0.072 15
Lung homogenate (acacia)b 0.10 21
Mucosal homogenate, pH 7.4 0.86 83
Muscosal homogenate, pH 9.0 0.43 41
Pancreas homogenate 54.9 34 400
Adipose "monoglyceride lipase" 0.021 0.53
Adipose "hormone-sensitive lipase" 0.14 0.82
Purified cholesteryl esterase 0 -
a From: Albro & Thomas (1973)
b 14C-labelled DEHP supplied as dispersion in either sodium cholate or
gum acacia
Empirical formula: C24H38O4
Abbreviation: DEHP
Relative molecular
mass: 390.57
Common synonyms:
1,2-benzenedicarboxylic acid bis(2-ethylhexyl) ester (CAS name);
phthalic acid bis(2-ethylhexyl) ester (IUPAC name); BEHP;
1,2-benzenedicarboxylic acid bis(ethylhexyl) ester;
bis(2-ethylhexyl) 1,2-benzenedicarboxylate; bis(2-ethyl-hexyl)
ester of phthalic acid; bis(2-ethylhexyl) phthalate;
di(2-ethylhexyl) ortho-phthalate; di(ethylhexyl) phthalate;
dioctyl phthalate; DOP; ethylhexyl phthalate; 2-ethylhexyl
phthalate; octyl phthalate; di- sec-octyl phthalate; phthalic
acid dioctyl ester
Common trade names:
Bisoflex 81; Bisoflex DOP; Compound 889; DAF 68; Ergoplast FDO;
Eviplast 80; Eviplast 81; Fleximel; Flexol DOP; Goodrite GP 264;
Hatcol DOP; Kodaflex DOP; Mollan O; Nuoplaz DOP; Octoil;
Palatinol AH; Platinol DOP; Pittsburgh; PX-138; Reomol DOP;
Reomol D 79P; Sicol 150; Staflex DOP; Truflex DOP; Vestinol AH;
Vinicizer 80; Witcizer 312 (IARC, 1982; NIOSH, 1985b)
CAS registry
number: 117-81-7
RTECS number: TI 035000
Di(2-ethylhexyl) phthalate (DEHP) is available in a variety of
technical grades. In the USA typical product specifications are:
minimal ester content, 99.0-99.6%; maximal moisture content, 0.1%;
acidity (as acetic acid or phthalic acid), 0.007-0.01%; specific
gravity, 0.980-0.985 (25 °C/25 °C); refractive index, 1.4850-1.4870
(23 °C); and minimal flash-point, 216 °C (IARC, 1982).
In western Europe, DEHP is available with the following
specifications: maximal acid value, 0.06; maximal weight loss on
heating at 140 °C for 3 h, 1%; and saponification value, 284-290 mg
KOH/g (IARC, 1982).
In Japan, DEHP must fulfill the following specifications: maximal
acid value, 0.05; maximal weight loss on heating at 125 °C for 3 h,
0.1%; and specific gravity, 0.983-0.989 (20 °C/20 °C) (IARC, 1982).
2.2 Physical and chemical properties
DEHP is a colourless to yellow, oily liquid at room temperature
and normal atmospheric pressure. The melting point is -46 °C (pour
point) and the boiling point is 370 °C (at atmospheric pressure, 101.3
kPa; 236 °C at 1.33 kPa, and 231 °C at 0.67 kPa). The flash point is
425 °C (open cup) (Clayton & Clayton, 1981; IARC, 1982; SAX, 1984). At
20 °C, the density is 0.98 g/ml (Fishbein & Albro, 1972) and the
vapour pressure 8.6 x 10-4 Pa (Howard et. al., 1985). The log n-
octanol-water partition coefficient is 3-5.
The solubility of uncolloidal DEHP in water is low (45 µg/litre
at 20 °C) (Leyder & Boulanger, 1983). However, DEHP may form colloidal
dispersions which lead to higher values for solubility (Klöpfer et
al., 1982). Values of 285 µg/litre (Hollifield, 1979), 340 µg/litre
(Howard et al., 1985), and 360 µg/litre (Defoe et al., 1990) have been
determined at 20-25 °C. These higher values are probably more
realistic in the environment. Howard et al. (1985) determined a value
of 160 µg/litre at 25 °C in salt water.
DEHP is miscible with most common organic solvents and is more
soluble in blood than water. It is lipophilic and the distribution
ratio in dichloromethane-Krebs bicarbonate buffer has been measured to
be 1130 (Krauskopf, 1973; Clayton & Clayton, 1981; IARC, 1982; Sax,
1984; Weast et al., 1984; Sittig, 1985).
2.3 Conversion factors
1 ppm = 15.87 mg/m3
1 mg/m3 = 0.063 ppm
2.4 Analytical methods
Methods used for the analysis of di(2-ethylhexyl) phthalate in
many types of samples are summarized in Table 1.
Analysis of samples with low concentrations of DEHP is
complicated by the risk of contamination from plastic equipment.
Table 1. Methods for the analysis of di(2-ethylhexyl) phthalate
Sample Sample Assay Limit of
matrix preparation procedure detection Reference
Air collect on cellulose GC/FID range: NIOSH (1977)
ester membrane filter; 2.03-10.9 NIOSH (1985a)
extract disulfide) mg/m3 for
a 32-litre
sample at
23 °C
Air collect with impinger GC/ECD not given Thomas (1973)
(ethylene glycol); GC/MS
extract (hexane)
Marine air trap on glass-fibre filters GC/ECD 0.5 ng/m3 Giam et al.
with foam plugs; Soxhlet (1980)
extract (petroleum ether);
concentrate extracts; clean-up
on deactivated Florisil
columns
Air-borne Soxhlet extract (methanol); GC/MS not given Karasek et al.
particulate concentrate; centrifuge (1978)
matter
River water extract (hexane); filter HPLC/UV 2 ng at Mori (1976)
(normal and 224 nm
reversed-phase
adsorption
chromatography
and gel
chromatography
Table 1 (contd).
Sample Sample Assay Limit of
matrix preparation procedure detection Reference
River water extract (chloroform); thin-layer 50 µg/litre Kataeva (1988)
concentrate extract; dry by chromatography
sodium sulfate treatment;
evaporate; dissolve residue
(chloroform)
Industrial add hydrochloride acid; GC/MC (EI not given Sheldon &
and municipal extract (dichloromethane); and CI modes); Hites (1979)
waste water clean-up by liquid with SIM
chromatographic fractionation
River freeze-dry; homogenize; HPLC/UV 10 ng Schwartz et al.
sediment extract (hexane; acetone; (233 nm) (1979)
methanol); evaporate;
dissolve (hexane); filter
Human serum centrifuge; extract (chloroform: GC/FID 50 µg/litre Lewis et al.
methanol); evaporate; dissolve GC/MS (1977)
(ethyl acetate); treat with
alumina; decant; rinse;
filter; evaporate; dissolve
residue (hexane-containing
butyl benzyl phthalate as an
internal standard)
Human plasma separation on Celite 545; GC/FID 50 ng Piechocki &
extract (diethyl ether); Purdy (1973)
evaporate; dissolve (carbon
disulfide)
Table 1 (contd).
Sample Sample Assay Limit of
matrix preparation procedure detection Reference
Stored blood; lyophilize; suspend; filter; GC/FID not given Contreras et
whole blood wash residue; mix with distilled al. (1974)
water; centrifuge; add silicic
acid to chloroform phase;
mix; centrifuge; decant;
evaporate; dissolve; centrifuge
Human and grind wet tissue samples GC/FID 0.3 µg/g Chen et al.
animal in saline; extract homogenate (wet tissue) (1979b)
tissue and or urine; dilute with 15 ng/ml Chen et al.
urine chloroform:methanol (urine) (1979a)
Intravenous add hydrochloric acid; GC/ECD 4 µg/litre Arbin &
solutions extract (dichloromethane); Östelius
redissolve (1980)
Organic evaporate; dissolve GC/FID not given Ishida et al.
solvents (diethyl ether) (1980)
Solid immerse in chloroform: GC/FID not given Ishida et al.
reagents methanol; filter; rinse; (1980)
evaporate
Aluminium cut into small pieces; GC/FID not given Ishida et al.
foil; rubber immerse in chloroform: (1980)
tubing, etc. methanol; extract
Abbreviations: GC = gas chromatography; FID = flame-ionization detection; ECD =
electron capture detection; MS = mass spectrometry; HPLC = high-
performance liquid chromatography; UV = ultraviolet spectroscopy;
EI = electron impact; CI = chemical ionization; SIM = selected ion
monitoring.
3. SOURCES OF HUMAN AND ENVIRONMENTAL EXPOSURE
It seems likely that by far the major part of the phthalate
esters present in the environment arises from human activity and not
from natural sources. Some dialkyl esters may be found in coals, crude
oil, and shales while others may have plant origins, but most
originate either directly or indirectly from industrial processes.
Phthalic acid has been reported to be formed during the bacterial
metabolism of phenanthrene.
3.1 Natural occurrence
Phthalates have been reported in a wide variety of substances
(oil, soil, plants, and animals) and over a wide geographical area.
Most occurrences have anthropogenic origins but some could be of
natural origin. The nature of the origin is further complicated by the
fact that sampling techniques often lead to contamination of samples
via contamination from plastic bags or bottles. Mathur (1974a)
critically reviewed this question and concluded that the possibility
of the phthalic acid esters found in biological and geochemical
samples being of biosynthetic origin cannot be ruled out. Both Mathur
(1974a) and Peakall (1975) cite reports where phthalates were detected
yet no anthropogenic source could be found. Studies by Manandhar et
al. (1979) and Pare et al. (1981) also revealed residues of phthalates
in biological samples where the source seemed to be natural. Peterson
& Freeman (1984) suggested that some of the phthalates found in older
samples (from the 1920s and 1930s) of sediment cores from Chesapeake
bay, USA, may have been of natural origin.
An ECETOC (European Chemical Industry Ecology and Toxicology
Centre) task force concluded that, although knowledge of naturally
produced phthalates is limited or uncertain, it is unlikely that this
contribution is of significance except, possibly, in very localized
areas (ECETOC, 1985).
3.2 Anthropogenic sources
3.2.1 Production levels
About 2.7 x 106 tonnes of total phthalates are produced
annually, of which the non-plasticizer (dimethyl and diethyl)
phthalates represent a very small fraction. Of the plasticizer
phthalates, DEHP accounts for well over 50% of the tonnage, the
contribution of the remaining compounds ranging from about 1% to 10%
each (ECETOC, 1985).
The production of DEHP has been increasing since it was first
used commercially in 1949. During the period 1950-1954, the production
in the USA was 106 x 103 tonnes, and by the period 1965-1969 the
level had risen to 650 x 103 tonnes (Peakall, 1975).
The estimated world consumption of DEHP in 1984 was 1.09 x 106
tonnes (SRI, 1985).
3.2.2 Uses
Phthalate acid esters are the most widely used plasticizers for
the production of polyvinyl chloride (PVC) products (with DEHP as the
plasticizer). Phthalates are used for the insulation of wires and
cables, in floor tiles, weatherstripping, upholstery, garden hose,
swimming pool liners, footwear and clothing. They are also used in
food wrapping and containers, although in some countries this use is
prohibited by law. They also have non-plasticizer uses, e.g., as
pesticide carriers.
DEHP has been widely used since 1949. An important property is
that it softens resins without reacting with them chemically. This has
led to about 95% of DEHP production being directed towards plasticizer
use, particularly in PVC products such as tubing and medical device
components. It is also used as a plasticizer in cellulose ester
plastics and synthetic elastomers. The DEHP content of these products
generally ranges from 20 to 40%, but for some uses it is up to 55%.
The most important non-plasticizer use of DEHP is as a dielectric
fluid in capacitors.
3.2.3 Disposal of plasticized products
Most discarded plasticized products are disposed of either by
incineration or via dumping in a tip/landfill site. When incinerated
at high temperature the combustion of phthalates is nearly complete.
However, if combustion is uncontrolled and occurs at a low
temperature, a large percentage of the phthalates may be lost to the
atmosphere. After dumping in a landfill site, phthalates may leach
into the aquatic environment, but because of their high affinity for
organic soil particles and their low water solubility this is not
likely to be a major route into the environment. Indiscriminate
dumping is more likely to lead to volatilization of phthalates to the
atmosphere rather than leaching to the aquatic environment (ECETOC,
1985).
4. ENVIRONMENTAL TRANSPORT, DISTRIBUTION, AND TRANSFORMATION
4.1 Environmental transport and distribution
The release of phthalates to the environment may occur as
follows:
a) during production and distribution;
b) during the manufacture of plasticized products;
c) during the use of plasticized product;
d) after disposal.
It was concluded by ECETOC (1985) that most of the phthalates
entering the environment are likely to do so by volatilization to the
atmosphere, only a minor part (perhaps 10%) entering the aquatic
environment by leaching.
The estimated worldwide emissions of DEHP are given in Table 2.
However, as reported by ECETOC (1985), the loss of DEHP from modern
production plants is negligible.
4.1.1 Transport in air
ECETOC (1985) suggested that transport in air is the major route
by which phthalates enter the environment. DEHP is volatilised to the
atmosphere and then either falls as dry deposition or is "washed out"
via rainfall.
DEHP has been measured in air samples from remote sites at
Enewetak Atoll in the North Pacific Ocean (Atlas & Giam, 1981), the
North Atlantic (Giam et al., 1978), the Gulf of Mexico (Giam et al.,
1978, 1980), and Sweden (Thurén & Larsson, 1990).
4.1.2 Transport in soil and sediment
DEHP has a high n-octanol-water partition coefficient, so the
equilibrium between water and an organic-rich soil or sediment will be
very much in favour of the soil or sediment. Absorbance of DEHP by
soil or sediment is also enhanced by van de Waals type bonding with
natural soil minerals, promoted by the presence of benzene rings and
carbonyl groups, and also by the low solubility of DEHP (ECETOC,
1985).
From results with other organic substances, Wams (1987) estimated
that 90% of DEHP is readily adsorbed by organic soil particles.
As can be seen from section 5.1.4, the sediment or hydrosoil
tends to act as a sink for DEHP.
Table 2. Estimated worldwide emission of DEHP, based on an estimated
total annual production of 4 x 106 tonnesa
Phase Emission (tonnes/year) Route
Production up to 40 000 waste water
Distribution 2000 sewage systems
Production of PVC 32 000 air and water
During use of plastics 14 000 air
6000 water
After disposal:
to landfill sites up to 200 000 percolating water
to waste incinerators ? air
uncontrolled burning ? air
a Adapted from Wams (1987). The values are higher than those from
other sources (ECETOC, 1985)
4.1.3 Transport in water
The solubility of DEHP in water is low (0.3 mg/litre at 25 °C).
However, the amount present in surface water may be higher than the
actual solubility as a result of adsorption onto organic particles
(Taylor et al., 1981) and interactions with dissolved organic matter
of high relative molecular mass, such as humic and fulvic acid
(Matsuda & Schnitzer, 1971).
DEHP has been found to adsorb to suspended particulate matter
fairly rapidly, in less than 2 to 3 h, especially to small particles
(Al-Omran & Preston, 1987). This adsorption was more rapid in salt
water than in fresh water. Taylor et al. (1981) reported that between
one-half and two-thirds of the DEHP in Mississippi River water is
associated with particulate matter.
By extrapolating laboratory data on the volatilization of DEHP
from water under defined conditions, Klopffer et al. (1982) obtained
a half-life in water of 146 days, although on purely theoretical
grounds a value of only 25 days was calculated.
Using the Exposure Analysis Modeling System (EXAMS), Wolfe et al.
(1980a) calculated that at equilibrium the loss of DEHP via
volatilization from a model river, a pond, an eutrophic lake, and an
oligotrophic lake would be 0%, 2.8%, 2.2% and 2.3%, respectively.
4.1.4 Transport between media
Eisenreich et al. (1981) estimated that the total annual
deposition of DEHP from air into the Great Lakes, North America,
varied from 3.7 tonnes (Lake Ontario) to 16 tonnes (Lake Superior).
DEHP has a high n-octanol/water partition coefficient. This
means that biota living in phthalate-containing water would be
expected to have a higher phthalate level than the water itself (see
section 4.2.3). However, many organisms are able to metabolize DEHP,
and the concentrations found may be lower than those expected on the
sole basis of partition coefficient.
During the 33-day period of a model ecosystem study, the
concentration of 14C in the aquatic phase reached a peak of 31
µg/litre at the fifth day after treatment and had declined to 7.7
µg/litre by the end of the experiment. This decline was stated to be
the result of the uptake of DEHP and its degradation products by the
organisms in the model ecosystem (Metcalf et al., 1973).
Lokke & Bro-Rasmussen (1981) treated the leaves of Sinapsis alba
with a mixture of di-iso-butyl phthalate (DiBP), di- n-butyl
phthalate (DBP), and DEHP at a rate of 2.5 µg/cm2. Only very small
amounts of DEHP evaporated from the leaves during the 15-day
experiment, compared with DiBP (71%) and DBP (43%).
4.2 Biotransformation
4.2.1 Abiotic degradation
As a result of atmospheric photodegradation, the atmospheric
half-life of DEHP is less than one day (ECETOC, 1985).
Chemical hydrolysis of DEHP is practically non-existent, the
half-life being > 100 years in water at pH 8 and 30 °C (Wolfe et al.,
1980b).
4.2.2 Biodegradation
Aerobic degradation has been found from several micro-organisms
in soil, sludge, sediment, and water. Anaerobic degradation is very
much slower, or possibly even non-existent.
The first step in the metabolic pathway for the biodegradation of
DEHP is the hydrolysis of the diester to the monoester by esterases
with low substrate specificity (Kurane et al., 1980; Taylor et al.,
1981). The monoester is then converted into phthalic acid (Engelhardt
et al., 1975). The ring-opening degradation to pyruvate and succinate
and then to CO2 and H2O is similar to the metabolism of benzoic
acid. According to Kurane et al. (1984), this is probably why the
biodegradation of phthalic esters is so widespread. It appears that
mixed populations of microorganisms are the most successful at
completely degrading DEHP (Engelhardt et al., 1975; Kurane et al.,
1979). When pure cultures of bacteria, selectively isolated in the
laboratory, are used for the biodegra-dation of phthalates,
accumulation of the breakdown products tends to occur (Keyser et al.,
1976).
4.2.2.1 Aerobic degradation
Aerobic degradation of DEHP has been found with several
microorganisms, including bacteria and fungi. Overall, it appears that
phthalates with short alkyl chains undergo rapid degradation, whereas
those with longer chains, such as DEHP, are only 40-90% degraded after
10-35 days (ECETOC, 1985).
Graham (1973) reported that laboratory-scale activated sludge
processes degraded 91% of introduced DEHP within 38 h. Saeger & Tucker
(1973) demonstrated that all phthalates tested underwent complete
aerobic degradation in activated sludge and river water.
Aerobic degradation of DEHP depends on temperature. Mathur
(1974b) incubated a loam soil with DEHP at 4, 10, 22-25, and 32 °C,
and soil respiration rates were measured after 14 weeks. Increased
rates of respiration, showing that microbial degradation was taking
place, were found at all temperatures. However, at 4 and 10 °C results
indicated that only marginal degradation was taking place.
Johnson & Lulves (1975) incubated freshwater hydrosoil containing
14C-DEHP (1 mg/litre) under aerobic conditions, and after 14 days,
50% of the DEHP had been degraded. This was a much slower rate of
degradation than that found with DBP, where 98% was degraded within 5
days. Johnson et al. (1984) studied the biodegradation of phthalic
acid esters in freshwater sediment and found that the length and
configuration of the alkyl phthalate diester significantly affected
the primary biodegradation rate. After a 14-day incubation in aerobic
sediment at 22 °C, less than 2% of the branched-chain alkyl phthalate
DEHP had been degraded whereas over the same period the linear alkyl
DBP showed 85% degradation. DEHP degradation was significantly greater
at very high concentrations (10 mg/litre) and at temperatures above 22
°C. Neither inorganic nitrogen nor phosphorus influenced the
degradation of DEHP. Engelhardt et al. (1977) found that the fungus
Penicillium lilacinum degraded approximately half of the initial
amount of DEHP within 30 days, yielding the corresponding monoester,
a second metabolite, which is hydroxylated in the alcohol moiety, and
at least four minor metabolites. The bacterium Pseudomonas
acidovorans completely degraded DEHP at a medium concentration of
5000 mg/kg within 72 h (Kurane et al., 1977).
Saeger & Tucker (1976) found that 60% of the DEHP had undergone
primary biodegradation within 5 weeks in Mississippi River water.
Rapid primary degradation was found when DEHP was added to activated
sludge at the rate of 5 mg/24 h. Depending on the source of the
sludge, between 70% and 78% was degraded. To monitor whether complete
biodegradation was being achieved, the authors measured CO2
evolution. Within the 14 days of incubation DEHP had essentially been
completely degraded to CO2 and water under the conditions of this
test. Taylor et al. (1981) showed the presence of significant
populations of taxonomically distinct bacteria that grew on a range of
phthalic acid esters, including DEHP, in the water and sediments of
the Mississippi River region.
Sugatt et al. (1984) used an acclimated shake-flask
CO2-evolution test to study the biodegradation of DEHP and reported
an initial breakdown of the parent compound of > 99% within the 28
days. The authors calculated a half-life of 5.25 days for the primary
biodegradation of DEHP.
In surface waters, DEHP is strongly adsorbed to organic particles
(Taylor et al., 1981), which tends to reduce degradation (Baughman et
al., 1980).
In the upper layer of soil, biodegradation of phthalates proceeds
as in surface water, but deeper down, where conditions are anaerobic,
it is virtually nonexistent (Engelhardt & Wallnofer, 1978). Shanker et
al. (1985) incubated garden soil containing DEHP at a concentration of
500 mg/kg. Within 20 days, 75% of the DEHP had been degraded and,
after 30 days, more than 90%. Again the rate of degradation was much
slower than that found for either di- n-methyl or di- n-butyl
phthalate. No degradation was detectable when sterilized soil was
used.
4.2.2.2 Anaerobic degradation
Johnson & Lulves (1975) found DEHP to be completely resistant to
microbial attack under anaerobic conditions. After 30 days, there was
no significant loss of 14C-DEHP activity in freshwater hydrosoils
overlaid with nitrogen.
Shanker et al. (1985) reported that degradation of DEHP was much
slower in anaerobic soil, flooded with sterile water to reduce the
oxygen tension. After a 30-day incubation, 33% of the DEHP had been
degraded, compared with more than 90% in the case of aerobic soil.
O'Connor et al. (1989) studied the biodegradation of DEHP under
anaerobic conditions in a medium containing municipal digester sludge
over a period of 140 days. DEHP, which was the only carbon source, was
added at a rate of 20, 100, and 200 mg/litre, and 100%, 69%, and 54%
of the DEHP was degraded at the three respective concentrations.
However, complete biodegradation to carbon dioxide and methane was
minimal.
Ziogou et al. (1989) studied the behaviour of DEHP (0.5, 1, and
10 mg/litre) during batch anaerobic digestion of sludge over a 32-day
period. No degradation of DEHP was observed during this period.
4.2.3 Bioaccumulation
DEHP is highly lipophilic, the log n-octanol-water partition
coefficient being 3 to 5, and it is moderately persistent. The
accumulation of DEHP is also influenced by the capability of an
organism to metabolize it. Melancon (1979) reviewed the metabolism of
phthalates in aquatic organisms. Bioconcentration factors for DEHP in
a variety of aquatic organisms are given in Table 3.
Table 3. Bioaccumulation of DEHP in aquatic organisms
Organism Stat/ Exposure Exposure Bioconcentration Reference
flowa period concentration factorc
(µg/litre)
Freshwater organisms
Canadian pondweed stat 24 h 10 274.8d Metcalf et al. (1973)
(Elodea canadensis) stat 12 h 10 000 133.8d Metcalf et al. (1973)
Snail stat 48 h 10 857.5d Metcalf et al. (1973)
( Physa sp) stat 6 h 10 000e 402d Metcalf et al. (1973)
Scud flowb 7 day 0.1 13 600 Sanders et al. (1973)
(Gammarus pseudolimnaeus) flow 7 day 0.1 3900 Mayer & Sanders (1973)
Water flea flowb 7 day 0.3 5200d Sanders et al. (1973)
(Daphnia magna) flow 7 day 0.3 420 Mayer & Sanders (1973)
stat 1 h 10 421d Metcalf et al. (1973)
stat 12 h 10 000e 133.8d Metcalf et al. (1973)
Sowbug flowb 21 day 62.3 250d Sanders et al. (1973)
(Asellus brevicaudus)
Mosquito (larvae) stat 12 h 10 1320.2d Metcalf et al. (1973)
(Culex pipiens stat 24 h 10 000e 1187.3d Metcalf et al. (1973)
quinquefasciatus) stat 24 h 10 20.3d Metcalf et al. (1973)
stat 48 h 10 000e 434.6d Metcalf et al. (1973)
Midge larvae (3rd instar) flowb 7 day 0.2 408 Streufert et al. (1980)
(Chironomus plumosus) flowb 7 day 0.3 3100d Sanders et al. (1973)
flow 7 day 0.3 350d Mayer & Sanders (1973)
Mayfly flowb 7 day 0.1 2300d Sanders et al. (1973)
(Hexagenia bilineata) flow 7 day 0.1 575d Mayer & Sanders (1973)
Table 3 (contd).
Organism Stat/ Exposure Exposure Bioconcentration Reference
flowa period concentration factorc
(µg/litre)
Mosquito fish stat 48 h 100 265.3d Metcalf et al. (1973)
(Gambusia affinis) stat 12 h 10 000e 129.4d Metcalf et al. (1973)
Fathead minnow flow 14 day 1.9 458d Mayer & Sanders (1973)
(Pimephales promelas) flow 56 day 1.9 886d Mehrle & Mayer (1976)
Marine organisms
Eastern oyster (muscle) stat 24 h 100 11.2 Wofford et al. (1981)
(Crassostrea virginica) stat 24 h 500 6.9 Wofford et al. (1981)
Brown shrimp stat 24 h 100 10.2 Wofford et al. (1981)
(Penaeus aztecus) stat 24 h 500 16.6 Wofford et al. (1981)
Sheepshead minnow stat 24 h 100 10.7 Wofford et al. (1981)
(Cyprinodon variegatus) stat 24 h 500 13.5 Wofford et al. (1981)
a Stat = static conditions (water unchanged for the duration of the test); flow = flow-through conditions
(DEHP concentration in water continuously maintained, unless stated otherwise)
b Intermittent flow-through conditions
c Bioconcentration factor = concentration of DEHP in organism divided by concentration of DEHP in water
d Bioconcentration factor calculated using a radioactive isotope (values represent parent compound plus
radiolabelled products)
e DEHP applied directly to water
4.2.3.1 Model ecosystems
Metcalf et al. (1973) studied the uptake of 14C-labelled DEHP
from water by aquatic organisms in a model ecosystem containing algae
(Oedogonium), snails ( Physa sp.), mosquito larvae (Culex pipiens
quinquefasciatus), and fish ( Gambusia sp). The mosquito larvae
showed the highest concentration factor and the fish the lowest.
Labelled DEHP was added to Sorghum plants and at the end of the 33-
day experiment the water contained 0.34 µg DEHP per litre, the algae
18.32 mg/kg (53 890 x), the snails 7.3 mg/kg (21 480 x), the mosquito
larvae 36.61 mg/kg (10 7670 x), and the fish 0.044 mg/kg (130 x).
Sodergren (1982) exposed fish, aquatic invertebrates, and plants
to 14C-labelled DEHP at a concentration of 1.4 mg/litre for 27 days
under static conditions. After 5 days, 1/50 of the added amount of
DEHP was still present in the water, and at the end of the experiment
62% was recovered from the various surfaces (glass walls, sediment and
surface microlayer). All organisms accumulated DEHP. The amphipod
Gammarus pulex, larvae of trichopterans, and the snail Planorbis
corneus accumulated the DEHP to the highest degree, the
concentration factors ranging from 17 000 to 24 000. The submerged
plants, Mentha aquatica and Chara chara, also showed uptake and
storage of large amounts (concentration factor of 18 000). However,
the fish (stickleback, Pungitius pungitius, and minnow, Phoxinus
phoxinus) did not accumulate 14C-DEHP to any great extent
(concentration factors of 300 or less). Large accumulations of DEHP
occurred in organisms living and/or feeding at interfaces.
4.2.3.2 Aquatic invertebrates
Brown & Thompson (1982a) exposed Daphnia magna to nominal
14C-labelled DEHP concentrations of 3.2, 10, 32, and 100 µg/litre
for 21 days and obtained bioconcentration factors of 166, 140, 261,
and 268 at the four respective concentrations.
When Brown & Thompson (1982b) exposed mussels (Mytilus edulis)
to labelled DEHP at concentrations of 4.1 and 42.1 µg per litre, in
both cases equilibrium was reached within 14 days with a concentration
factor of 2500. Exposure ceased on day 28 but the mussels were
monitored for a further 14 days. The half-life for loss of DEHP over
this period was calculated to be 3.5 days.
Laughlin et al. (1978) exposed grass shrimp, during larval
development, to DEHP concentrations of up to 1 mg/litre for 28 days.
DEHP was not detectable in shrimp tissues at or above a level of 2
mg/kg.
When Streufert et al. (1980) exposed midge larvae (Chironomus
plumosus) to a radioactively labelled DEHP concentration of 0.2
µg/litre, the larvae accumulated DEHP to 292 times the concentration
in water within 2 days. DEHP levels in the midge larvae reached a
plateau after 7 days at a bioconcentration factor of 408. Some of the
larvae were transferred to clean water after 4 days, by which time
they had accumulated 56 µg DEHP/kg, and the half-life for loss was
calculated to be 3.4 days.
After 9 weeks of exposure to sediment containing approximately
600 mg/kg, dragonfly larvae had taken up 14.7 mg DEHP per kg. This was
significantly more than control larvae, which contained 2.9 mg/kg
(Woin & Larsson 1987).
Hobson et al. (1984) fed penaeid shrimps on a diet containing
between 40 and 50 000 mg DEHP/kg for 14 days at a rate of 40 g/kg body
weight per day. Whole body residues ranged from 0.249 to 18.3 mg/kg in
a dose-related manner.
4.2.3.3 Fish
Macek et al. (1979) exposed bluegill sunfish (Lepomis
macrochirus) to 14C-DEHP, both via food at a concentration of 2.8
mg/kg and via water at 5.6 µg/litre, for up to 35 days. The steady-
state body burden of 14C-DEHP after exposure via food and water was
0.73 mg/kg and via water alone was 0.64 mg/kg. The authors concluded
that the uptake of DEHP via the aquatic food chain was statistically
indistinguishable from that due to aqueous exposure. The time required
for the fish to eliminate 50% of the residue burden during depuration
in uncontaminated water was < 3 days.
In a study by Karara & Hayton (1989), sheepshead minnows
(Cyprinodon variegatus) were exposed to a 14C-DEHP concentrations
of 60 ng/litre at temperatures ranging from 10 °C to 35 °C for a
period of between 72 h and 160 h. The amount of DEHP accumulated after
72 h was 6 times greater at 35 °C than at 10 °C, and the
bioconcentration factors increased exponentially with temperature from
45 at 10 °C to 6510 at 35 °C. Metabolic clearance also increased as a
function of temperature, the maximum value being reached at a
temperature of between 29 °C and 35 °C.
Tarr et al. (1990) exposed three sizes (2.9 g, 61 g, and 440 g)
of rainbow trout (Oncorhynchus mykiss) to 14C-DEHP at 20 ng/ml
under static conditions for up to 96 h at 12 °C. The body-weight-
associated changes in the pharmacokinetic parameters caused the
bioconcentration factor to decline from 51.5 to 1.6 as body weight
increased.
When Mehrle & Mayer (1976) exposed rainbow trout (Salmo
gairdneri) eggs (12 days prior to hatching to 24 days post-hatching)
to 14C-labelled DEHP at concentrations of 5, 14, and 54 µg/litre,
the bioconcentration factors were 78, 113, and 42, respectively.
Mayer (1976) exposed fathead minnows (Pimephales promelas) to
DEHP concentrations ranging from 1.9 to 62 µg/litre for 56 days under
flow-through conditions. As the exposure concentration increased,
concentration factors, measured after 56 days, decreased from 569 to
91. Equilibrium was attained after 28 days at the lowest dose and
after 56 days at the highest dose. After exposure the fish were placed
in uncontaminated water for 28 days, and the half-lives for loss
ranged from 6.2 days (at 2.5 µg/litre) to 18.3 days (at 62 µg/litre).
4.2.3.4 Amphibians
Larsson & Thuren (1987) exposed moorfrog eggs to sediment DEHP
concentrations ranging from 10 to 800 mg/kg (fresh weight of
sediment). The eggs hatched after about 3 weeks and the tadpoles were
analysed after 60 days. The DEHP was released from the sediment to the
overlying water, and the losses to the water increased linearly with
increasing levels in the sediment (from 0.89 to 187.4 µg/litre). DEHP
accumulated in the tadpoles at concentrations ranging from 0.28 to
246.8 mg/kg wet weight, and the accumulation increased with increasing
DEHP concentration, both in sediment and water.
4.2.3.5 Plants
Lokke & Bro-Rasmussen (1981) applied DEHP as a mixture that also
contained DiBP and DBP at a concentration of 2.5 µg/cm2 to the
leaves of Sinapis alba. The residue level of DEHP on the leaves
immediately after application was 2.7 µg/cm2. After 15 days, DEHP
levels had decreased to 0.8 µg/cm2, but when the growth of the plant
was taken into account, no significant loss of DEHP over this period
was found. Lokke & Rasmussen (1983) also found little loss of DEHP
over a 15-day period when they applied it (as a mixture with DBP) to
Achillea at a concentration of 3.5 µg/cm2 or to Sinapis at 3.1
µg/cm2. Residues ranged from 120 to 155 µg/plant, and approximately
80% of the DEHP accumulated was on the surface of the leaf.
When Schmitzer et al. (1988) grew barley from seed in soil
containing 1 or 3.33 mg 14C-DEHP/kg dry soil for 7 days, only 0.61%
and 1.25%, respectively, of the applied 14C was taken up by the
plants. Aranda et al. (1989) also found low accumulation of DEHP by
plants grown in sewage sludge containing DEHP. Lettuce (Lactuca
sativa), carrot (Daucus carota), chile pepper (Capiscum annuum),
and tall fescue (Festuca arundinaca) were all grown in sludge
containing 14C-DEHP levels of between 2.57 and 14.07 mg/kg.
Bioconcentration factors ranged from 0.06 to 0.53 (based on initial
soil concentration and plant dry weight).
4.2.3.6 Birds
Belisle et al. (1975) fed mallard ducks (Anas platyrhynchos) on
a diet containing 10 mg DEHP/kg for a period of 5 months. No DEHP was
detected in fat tissue, but 0.1 and 0.15 mg/kg (wet weight) were found
in breast muscle and lung tissue, respectively.
O'Shea & Stafford (1980) exposed starlings (Sturnus vulgaris)
to a dietary DEHP concentration of 25 or 250 mg/kg for 30 days. One
of eight birds fed 25 mg/kg contained detectable residues (1.6 mg/kg)
after 30 days exposure, and five of eight birds fed 250 mg/kg
contained an average of 1.8 mg/kg. The same proportion of birds fed
the higher dose still had detectable residues (an average of 1.3
mg/kg) 14 days after dosing had finished.
When Ishida et al. (1982) fed hens on a diet containing 5 or 10
g/kg for up to 230 days, DEHP was detected in all tissue monitored
except the brain. Residues ranged from non-detectable to 42.5 mg/kg
for most tissues. However, adipose tissue (192.7 to 899.6 mg/kg) and
feathers (513.1 to 1165.2 mg/kg) accumulated the highest
concentrations. A similar pattern of uptake was observed in hens fed
2 g/kg for 25 days, although the amount of DEHP accumulated was much
lower. At this dose level no accumulation had occurred in tissues
other than liver and feather within 5 days.
5. ENVIRONMENTAL LEVELS AND EXPOSURE
5.1 Environmental levels
DEHP exists widely in the environment and is found in most
samples, including air, precipitation, water, sediment, soil, and
biota. Residues have also been detected in food and in humans.
In many cases it is not clear whether the phthalate measured in
samples is naturally occurring or is exogenous. However, there seem to
be clear indications that high levels of DEHP are anthropogenic in
origin.
5.1.1 Air
The levels of DEHP in air have been monitored in the North
Atlantic, the Gulf of Mexico, and on Enewetak Atoll in the North
Pacific and found to range from not detectable to 4.1 ng/m3 (Giam et
al., 1978; Giam et al., 1980; Atlas & Giam 1981). Similar levels
(between 0.5 and 5 ng/m3) have been found in the Great Lakes
ecosystem (Eisenreich et al., 1981) and in the Swedish atmosphere
(Thurén & Larsson, 1990). The DEHP content of these samples was at
least an order of magnitude lower than those found in urban areas such
as New York city, where levels of up to 28.6 ng/m3 have been found
(Bove et al., 1978). Based on the analysis of snow samples, Lokke &
Rasmussen (1983) calculated DEHP concentrations in air of 22 ng/m3
at Lyngby, Denmark. Levels of 29-132 ng/m3 have been found in
Antwerp, Belgium (Cautreels et al., 1977), 126 ng/m3 in polluted air
in Belgium (Cautreels & Van Cauwenberghe, 1978), 300 ng/m3 in
polluted air in Canada (Thomas, 1973), and 38-790 ng/m3 in Japan in
1985 (Environment Agency of Japan, 1989).
5.1.2 Precipitation
Atlas & Giam (1981) measured levels of DEHP in rainfall at
Enewetak Atoll, North Pacific, of 5.3-213 ng/litre (mean, 55
ng/litre). Eisenreich et al. (1981) reported between 4 and 10 ng/litre
in precipitation falling on the Great Lakes ecosystem and Thurén &
Larsson (1990) a level of 55 ng/litre in Sweden. Goto (1979) found a
range of mean rainwater concentrations of 0.65 to 3.16 µg/litre in
various Japanese cities.
Lokke & Rasmussen (1983) analysed snow sampled near a plasticizer
production plant 14 days after a snowfall. Levels of DEHP ranged from
0.7 to 4.7 µg/m2 per day over this period, the highest levels being
within 150 m of the plant and the lowest levels at least 600 m away.
5.1.3 Water
Levels of DEHP found in water are summarized in Table 4.
Table 4. Concentrations of DEHP in water
Location Country Year Concentration Reference
µg/litrea
Marine
Northern Atlantic 0.0001-0.006 Giam et al. (1978)
Gulf of Mexico 0.006-0.316 Giam et al. (1978)
Estuaries Germany ND-0.3 Weber & Ernst (1983)
Nueces Estuary,
Texas USA 1980 0.2-0.77 Ray et al. (1983b)
Estuaries United Kingdom 1981 0.058-0.078 Waldock (1983)
Freshwater
Various rivers Japan ND-3.1 Kodama et al. (1975)
Various cities Japan 1974 0.1-2.19 Goto (1979)
River Meuse Netherlands 1983 < 0.1-3.5 Wams (1987)
River Rhine Netherlands 1983 ND-1.2 Wams (1987)
River Rhine Netherlands 1982 ND-4.0 Wams (1987)
a ND = not detectable
Thuren (1986) analysed water samples from the Rivers Ronnebyan
and Svartan, Sweden, and found DEHP concentrations ranging from 0.32
to 3.1 µg/litre and from 0.39 to 1.98 µg/litre, respectively. The
highest concentrations were associated with industrial effluent
discharge points.
Few samples of ground water have been analysed for DEHP. Wams
(1987) reported that contaminated ground water in the Netherlands
contained between 20 and 45 µg/litre, while Rao et al. (1985) found
DEHP levels of up to 170 µg/litre in New York state ground water.
In a non-industrialised estuary in the United Kingdom, Waldock
(1983) measured DEHP levels of 58 to 78 ng/litre. Ray et al. (1983b)
found levels of DEHP ranging from 210 to 770 ng/litre in a Nueces
estuary in Texas, USA, while Weber & Ernst (1983) found DEHP levels of
up to 300 ng/litre in German estuaries. DEHP levels of up to 316
ng/litre have been found in the Gulf of Mexico, but levels in the
North Atlantic were much lower (Giam et al., 1978). In Japan, river
and marine levels in 1982 ranged from 0.1 to 0.8 µg/litre (Environment
Agency of Japan, 1989).
Ritsema et al. (1989) analysed samples from Lake Yssel,
Netherlands, and found DEHP levels of < 0.1 to 0.3 µg/litre in water
and levels of 12-25 mg/kg in suspended particulate matter. The authors
concluded that the probable source of DEHP was the River Yssel.
Preston & Al-Omran (1986) sampled water and suspended
particulates from the Mersey estuary, United Kingdom, in 1985, and
reported DEHP concentrations ranging from 83 to 335 ng/litre in water
and from 182 to 1700 µg/kg in particulate matter. However, in 1986,
levels were 125-693 ng/litre in water and 280-640 µg/kg in particulate
matter (Preston & Al-Omran, 1989).
5.1.4 Sediment
DEHP levels in sediment are summarized in Table 5.
Being lipophilic DEHP tends to be adsorbed onto sediment, which
acts as a sink. Sediment samples from various Dutch rivers have been
found to contain between 1 and 70 mg/kg (Schwartz et al., 1979; Wams,
1987). Taylor et al. (1981) analysed sediment samples from the
Mississippi River and found similar levels. Sediment levels of DEHP
in the Chester River Maryland, USA, were found to be less than 45
µg/kg dry weight, but, in a tributary of this river, sediment levels
were up to 4.8 mg/kg about 2 km downstream from a phthalate ester
plant outfall. The Chester River flows into Chesapeake Bay, which
contained sediment DEHP levels of 110 µg/kg (Peterson & Freeman 1984).
In Sweden, Thuren (1986) found sediment DEHP levels ranging from 1.2
to 628 mg/kg (dry weight) in the River Ronnebyan and 0.15 to 1480
mg/kg in the River Svarten. As was found with water samples (see
section 5.1.3), in both rivers the highest residues of DEHP were near
to industrial effluent discharge points.
Giam et al. (1978) analysed sediment from the Mississippi delta
and reported mean DEHP levels of 69 µg/kg. Sediment samples from
Nueces Estuary, Texas, USA, unlike the water samples, reflected local
inputs of pollutants. The highest levels (up to 16 mg/kg) were
associated with industrial areas, whereas other areas of the estuary
contained levels ranging from 40 to 330 µg/kg. Much lower levels of
DEHP were found on the Gulf of Mexico coast and in the open sea (mean
levels of 6.6 and 2 µg/kg, respectively) (Giam et al., 1978).
Table 5. Concentrations of DEHP in sediment
Location Country Year Concentration Reference
(µg/kg)a
Gulf of Mexico < 0.1-248 ns Giam et al. (1978)
Nueces Estuary, Texas USA 1980 40-16 000 dw Ray et al. (1983b)
Portland, Maine USA 1980 60-7800 dw Ray et al. (1983a)
Chester River, Maryland USA 1978 20-4800 dw Peterson & Freeman (1984)
River Mississippi USA 1981 140 dw Taylor et al. (1981)
River Meuse Netherlands 1977 1000-17 000 dw Schwartz et al. (1979)
River Ijssel Netherlands 1977 2500-52 500 dw Schwartz et al. (1979)
River Rhine Netherlands 1978 4000-36 000 ns Wams (1987)
River Rhine Netherlands 1977 6500-70 500 dw Schwartz et al. (1979)
Various cities Japan 1974 80-1360 dw Goto (1979)
Crouch Estuary, Essex United Kingdom 1981 11.2-26.2 ww Waldock (1983)
River Usk United Kingdom 1974 30 000 dw Eglinton et al. (1975)
a dw = dry weight; ww = wet weight; ns = not stated whether concentrations refer to dry or wet weight
In Japan, the levels of sediments in rivers and seas in 1982
ranged from 9 to 35 000 µg/kg dry weight (Environment Agency of Japan,
1989).
5.1.5 Soil
Contaminated soil analysed in the Netherlands was found to
contain up to 1.5 mg DEHP/kg (Wams 1987), while residues of DEHP in
soil collected in the vicinity of a DEHP manufacturing plant contained
up to 0.5 mg/kg (Persson et al., 1978).
Fatoki & Vernon (1990) reported a DEHP concentration of 1.9
µg/litre in treated sewage effluent from the Manchester area, United
Kingdom, and stated that such a level was consistent with the
industrial activities of the city.
5.1.6 Food
DEHP has been found in many samples of fish and shellfish (see
Table 6). It has also been detected in milk (Cerbulis & Ard, 1967),
bovine pineal gland (Taborsky, 1967), bovine heart muscle (Nazir et
al., 1971), and chicken eggs (Ishida et al., 1982). Perkins (1967)
isolated a substance similar to DEHP from corn oil. The US ATSDR
(1988) quoted an US FDA survey of various foods in 1974 which showed
that DEHP levels in most foods were less than 1 mg/kg; the foods
surveyed included margarine, cheese, meat, cereal, eggs, milk, white
bread, canned corn, corn meal, and baked beans.
Ishida et al. (1981) collected and analysed chicken eggs
available in Japanese markets. DEHP levels in egg white ranged from
0.05 to 0.4 mg/kg, but no DEHP was detected in the egg yolk.
In a study by Antonyuk (1975), DEHP migration from PVC materials
into foodstuffs was noted following 7 days of contact. Levels of 4-16
mg DEHP/kg were detected in cheese, sausage, meat, flour, and rice
while after 30 days levels of 30-150 mg/kg were found in sunflower
oil. The permissible level of DEHP migration into foodstuffs was
considered to be 2.0 mg/kg. Zitko (1972) detected DEHP concentrations
in hatchery-reared juvenile Atlantic salmon of 13 to 16 mg/kg lipid;
fish food contained 8 to 9 mg/kg lipid. When Williams (1973) analysed
fish available to the Canadian consumer, only 6 out of 21 samples
contained measurable amounts of DEHP. Levels of 0.1 and up to 0.16
mg/kg were found in unprocessed eels and in processed canned tuna
fish, respectively.
Table 6. Concentrations of DEHP in biota
Organisms Location Country Concentration Reference
(µg/kg)a
Mollusc (digestive gland) Crouch Estuary, Essex United Kingdom 9.2-214 ww Waldock (1983)
Dragonfly naiads Iowa (industrial) USA 200 Mayer et al. (1972)
Commercial fish food North America 2000-7000 Mayer et al. (1972)
Channel catfish Mississippi & Arkansas USA 3200 Mayer et al. (1972)
(industrial)
Channel catfish Iowa (industrial) USA 400 Mayer et al. (1972)
Various fish species Japan 70-450 Kodama & Takai (1974)
Various fish species Japan < 50-1800 ww Kamata et al. (1978)
Various fish species various cities Japan 50-720 Goto (1979)
Mainly fish Gulf of Mexico < 1-135 Giam et al. (1978)
Various fish species (liver) Tees Bay United Kingdom 43-85.9 ww Waldock (1983)
Various fish species (muscle) Tees Bay United Kingdom 13-51.3 ww Waldock (1983)
Walleye Lake Superior, Ontario Canada 800 Mayer et al. (1972)
Tadpoles Iowa (industrial) USA 300 Mayer et al. (1972)
Common seal pup (blubber) 10 600 lw Zitko (1972)
a ww = wet weight; lw = lipid weight
5.1.7 Aquatic organisms
Ray et al. (1983a) found DEHP levels of up to 490 µg/kg in
sandworms (Neanthes virens) and up to 170 µg/kg in clams collected
from Portland, Maine, USA, but these levels did not seem to reflect
the sediment levels and local pollutant sources.
Musial & Uthe (1980) collected fish of various species from the
Gulf of St Lawrence, Canada, and analysed them for DEHP in lipid
extracts. They reported levels of up to 6.5 mg/kg on a wet weight
basis (51.3 mg/kg on fat weight basis) in mackerel muscle and up to
7.2 mg/kg (47.1 mg/kg fat weight) in herring muscle. Lower levels of
0.37 mg/kg (wet weight) were found in eels, and in both plaice and
redfish concentrations were less than 0.001 mg/kg.
Persson et al. (1978) collected aquatic organisms from the
vicinity of a DEHP factory in Finland. Invertebrates contained up to
0.1 mg DEHP/kg, and levels of 1.1 mg/kg in roach muscle and 2.3 mg/kg
in pike liver were measured. Thuren (1986) analysed biota near to an
industrial discharge point and reported levels of up to 5.3 mg/kg
(fresh weight) in Odonata sp and up to 14.4 mg/kg in Asellus
aquaticus.
In Japan, DEHP levels in various species of fish in rivers and
seas ranged from 0.01 to 19 mg/kg wet weight in 1974 (Environment
Agency of Japan, 1989).
5.1.8 Terrestrial organisms
Persson et al. (1978) analysed soil arthropods collected near a
DEHP factory in Finland and found residues of 2.8 mg/kg.
5.2 General population exposure
Few data are available on general population exposure.
Based on an analysis of DEHP levels in various foods, the average
exposure in the USA has been estimated to be around 0.3 mg/person per
day and the maximum 2 mg/person per day (see section 5.1.6).
In a survey of plasticizer levels in food-contact material and
food, the United Kingdom Ministry of Agriculture, Fisheries and Food
(MAFF, 1987) stated that DEHP has very limited use in food-contact
material, and the maximum daily intake from food sources has been
estimated to be less than 20 µg/person per day.
DEHP found in human tissues may be derived from medical devices,
since it was recognised by Trimble et al. (1966) and by Guess &
Haberman (1968) that DEHP is leached from certain medical devices.
Samples of soft PVC fluid bags containing normal saline and glucose
(50 mg/ml) were shaken for 24 h and analysed for plastic additives
(Smistad et al., 1989). The PVC plastic materials contained DEHP,
epoxidized vegetable oils, and stearates as the main additives. The
same components were found in the solutions. MEHP was also detected,
but only in the solutions.
Marcel & Noel (1970) reported the presence of phthalate esters in
human plasma that had been stored in plastic blood bags.
Jaeger & Rubin (1972) found that DEHP was extracted from PVC
plastic blood bags by human blood at the rate of 2.5 mg/litre per day
at 4 °C. The DEHP was found in both lipid-containing and lipid-free
fractions of plasma, whereas the red cells contained only minor
amounts. Seven out of twelve samples of lung tissue, taken at autopsy
from patients who had received transfusions of stored blood, contained
DEHP at concentrations of 13.4-91.5 mg/kg (dry weight). Rubin & Nair
(1972) reported that DEHP had also been found in the tissues and urine
of patients who had not received blood transfusions, but no details
were given. Mes et al. (1974) analysed human adipose tissue in Canada
and found the levels of DEHP in most samples to range from 0.3 to 1.0
mg/kg.
Schneider et al. (1989) estimated that the highest exposure to
DEHP associated with medical devices resulted from extracorporeal
membrane oxygenation (ECMO), which could result in an exposure of 14
mg/kg per day. In an infant receiving ECMO for 14 and 24 days, the
DEHP serum levels were 26.8 and 33.5 mg/litre, respectively. In
another infant, ECMO for 6 days resulted in liver, heart, and
testicular concentrations of 3.5, 1.0, and 0.4 mg/kg, respectively.
Newborn infants given exchange transfusions may have plasma
levels of about 10 mg/litre (Sjöberg et al., 1985b).
5.3 Occupational exposure during manufacture, formulation or use
Few data on occupational exposure to DEHP have been reported.
In a phthalate manufacturing plant in the USA producing DEHP from
phthalic anhydride and alcohols, Liss et al. (1985) measured, in the
case of six heavily exposed workers, 8-h TWA workplace air
concentrations of DEHP ranging from 0.02 to 4.1 mg/m3. The exposure
level for 44 other workers in the same plant was below the detection
limit (10 µg/sample).
In an Italian factory producing n-butyl phthalate, isobutyl
phthalate, and DEHP, Gilioli et al. (1978) measured total phthalate
exposure concentrations of between 1 and 60 mg/m3, the average being
5 mg/m3.
Nielsen et al. (1985) measured total phthalic acid esters in air
in a PVC-processing plant in Sweden where diisodecyl phthalate, DEHP,
and some butylbenzylphthalate were used. Concentrations of between
0.01 and 2.0 mg/m3 were recorded in 96 2-h personal samples from 54
workers.
Total phthalates concentrations in air of between 1.7 and 66
mg/m3 were recorded in a PVC-processing plant in the USSR using
mainly dibutyl phthalate and higher alkyl phthalates but also some
DEHP and other phthalates (Milkov et al., 1973). Stankevich & Zarembo
(1978) measured DEHP levels of 1.5 to 40 mg/litre in the blood of
workers manufacturing PVC in the USSR.
DEHP concentrations in air of between 0.09 and 0.16 mg/m3 were
recorded in a German factory for phthalate production (Thiess et al.,
1978a). In 9 PVC-processing plants in Finland, the mean concentration
of DEHP ranged from less than 0.02 mg/m3 to 0.5 mg/m3 and the
highest single value was 1.1 mg/m3 (Vainiotalo & Pfäffli, 1990).
6. KINETICS AND METABOLISM IN LABORATORY ANIMALS AND HUMANS
6.1 Absorption
6.1.1 Inhalation
There is no quantitative information available on the pulmonary
route of absorption, although aerosols of DEHP are readily formed
(Albro & Lavenhar, 1989).
6.1.2 Dermal
After a single application of 14C-labelled DEHP (61.5 mg/kg) to
the back of F-344 rats, clipped one hour before treatment, urine and
faeces were collected every 24 h for 7 days. The amount of 14C
excreted was taken as an index of the percutaneous absorption, and
about 5% of the dose given was excreted (Elsisi et al., 1989).
6.1.3 Oral
Studies with 14C-labelled DEHP indicated that at least 50% of
the radioactivity of a single dose (2.9 mg/kg) was absorbed in the rat
intestine since 42% and 14% were excreted in urine and bile,
respectively, after 7 days (Daniel & Bratt, 1974). The same authors
also found that DEHP was rapidly hydrolysed by pancreatic lipase,
suggesting that DEHP is hydrolysed in the gut before absorption. This
was supported by the fact that no unmetabolized DEHP was found in
liver after the administration of low oral doses (< 0.4 g/kg),
although at higher doses (> 0.5 g/kg) DEHP was detected (Albro et
al., 1982). At an oral dose level of 2 g/kg, the bioavailability of
DEHP in rats, as measured in blood by HPLC, was 14%, whereas at an
intraperitoneal dose level of 4 g/kg only 5% was recovered, again
indicating a role for hydrolysis of DEHP in the gut (Pollack et al.,
1985b). Using an inhibitor of mucosal esterases ( S,S,S-tributyl
phosphorothionate), White et al. (1980) observed a marked inhibition
in the uptake of DEHP by the gut. Studies involving oral
administration of MEHP indicated that this metabolite is well
absorbed. After radiolabelled MEHP or DEHP was given to rats, the
radioactivity recovered in plasma from MEHP was 16 times more than
that from DEHP (Teirlynck & Belpaire, 1985).
Oral administration of DEHP (1 g/kg) to young rats leads to a
larger area under the plasma concentration-time curve (measured with
gas chromatography) for MEHP (twice that of DEHP) than in older rats
(Sjöberg et al., 1985a). This indicates either a more rapid hydrolysis
of DEHP or a more efficient absorption of MEHP in young rats.
Cynomolgus monkeys hydrolyse DEHP in the gut less efficiently
than rats or mice (Astill, 1989). Rhodes et al. (1986) also noted that
there is less absorption from the gastrointestinal tract in marmosets
than in rodents.
6.1.4 Intraperitoneal
The systemic availability of DEHP was only 5% when a dose of 4
g/kg was given intraperitoneally to rats. Relatively small amounts of
MEHP were recovered in the blood in this study (Pollack et al.,
1985b).
6.2 Distribution
Intravenously administered DEHP is rapidly eliminated from blood.
This was demonstrated in experiments where radioactive DEHP was
injected into male CFN rats and blood levels were determined by thin-
layer chromatography (Schulz & Rubin, 1973). At a low dose level (0.1
mg/kg), there was an initial phase with a half-time of 4.5 min and a
second phase with a half-time of 22 min. At a higher dose level (200
mg/kg) the initial phase had a half-time of 9 min. This indicated that
DEHP was taken up in a tissue compartment by a saturable process
(Schulz & Rubin, 1973). Radioactivity from DEHP was rapidly
distributed to the liver, lungs, and spleen when administered
intravenously (Schulz & Rubin, 1973; Daniel & Bratt, 1974).
Orally administered DEHP is mainly distributed as MEHP in rats
(Pollack et al., 1985b; Teirlynck & Belpaire, 1985). Unmetabolized
DEHP was recovered in the liver only after large oral doses
(> 0.5 g/kg) were given, indicating a threshold phenomenon in the
absorption and distribution (Albro et al., 1982; Agarwal, 1986). The
distribution kinetics of MEHP have been analysed by Pollack et al.
(1985b) and by Teirlynck & Belpaire (1985). Pollack et al. (1985b)
found that the peak concentration of MEHP in blood was reached 15 min
after oral or intraperitoneal administration of MEHP. The half-time of
MEHP in blood or plasma in the rat is shorter than that of DEHP
(Pollack et al., 1985b; Teirlynck & Belpaire, 1985). The in vitro
plasma protein binding of MEHP in the rat reaches approximately 98%
(Sjöberg et al., 1985a).
A phenomenon known as "shock-lung" has been reported to occur
following intravenous administration of DEHP to rats and other
species. Two hours after an emulsion of DEHP was given, between 13%
and 48.6% of DEHP-radiolabelled material was found in the lungs of
rats, as compared to 26.3-38.2% in the liver (Daniel & Bratt, 1974).
This phenomenon may be relevant to human exposure via intravenous
administration from bags and tubing containing DEHP.
The DEHP plasma level in newborn infants given exchange
transfusions may reach about 10 mg/litre (Sjöberg et al., 1985b).
This level is about twice as high as those found in leukaemia patients
receiving platelet concentrations and about five times as high as
levels found in haemodialysed patients. After treatment, this level
falls rapidly to about 3 mg/litre within 2 h, and then there is a
further drop with a half-time of about 10-12 h (Sjöberg et al.,
1985b).
6.3 Metabolism
DEHP is hydrolysed in vitro by pancreatic lipase to MEHP
(Daniel & Bratt, 1974), indicating that this metabolism would occur
mainly in the gut lumen. In rats about 80% of an oral dose of DEHP
undergoes mono-deesterification (Pollack et al., 1985b), while intra-
arterially administered DEHP is only slowly converted to MEHP (Pollack
et al., 1985b). Studies on the hydrolysis of DEHP in homogenates from
different organs (Table 7) indicate a very high activity in pancreatic
juice and a comparatively low activity in liver (Albro & Thomas, 1973;
Daniel & Bratt, 1974).
At relatively high doses of DEHP (2 g/kg body weight per day),
administered by gavage for up to 14 days, approximately 25-40% of the
dose in rats and 50-75% of the dose in marmosets was excreted in the
faeces. This implies that incomplete intestinal hydrolysis and
absorption may occur (Rhodes et al., 1986).
MEHP may in turn be metabolized in the gut wall (Pollack et al.,
1985b) or in other organs. Rat liver cell cultures have been shown to
convert MEHP to several metabolites, as occurs in the intact rat
(Albro et al., 1973; Lhuguenot et al., 1985). Cultures of testicular
cells were, however, apparently not able to metabolize MEHP beyond
slight hydrolysis to phthalic acid within 18-24 h (Albro et al.,
1989).
The metabolic pathways for MEHP are shown in Fig. 1. The omega-
and omega-1-carbon oxidation products constitute more than 85% of the
metabolites (Albro et al., 1973; Mitchell et al., 1985a; Lhuguenot et
al., 1985). The ethyl side chain may also be oxidized (Lhuguenot et
al., 1985). It has been suggested that omega-oxidation leads to a
metabolite that is further degraded by ß-oxidation in the peroxisomes
(Albro et al., 1973; Lhuguenot et al., 1985). Non-linear dose-
dependency has been reported for these pathways in the rat; the
predominance of omega-oxidation over omega-1-oxidation was increased
by high doses of MEHP (Lhuguenot et al., 1985).
The administration of 2-ethyl-(1-14C)-hexyl-labelled DEHP led
to a low level of radioactivity being recovered from purified rat
liver DNA (Albro et al., 1982). In a more recent study (Lutz, 1986),
the administration of 14C-carboxylate-labelled DEHP resulted in no
measurable radioactivity in DNA, whereas radioactivity was clearly
measurable after the administration of DEHP that was 14C- or
3H-labelled in the alcohol moiety.
Table 7. DEHP hydrolase activity of various tissue lipasesa
DEHP hydrolase activity
Enzyme preparation
units/mg protein units/g tissue
Liver acid lipase, homogenate 0.34 101
Liver alkaline lipase, homogenate 0.14 43
Liver "lysosomal" concentrate (acacia)b 8.80 -
Liver microsome + supernatant fraction, pH 8.2 1.22 -
Kidney acid lipase, homogenate 0.15 45
Lung homogenate (cholate)b 0.072 15
Lung homogenate (acacia)b 0.10 21
Mucosal homogenate, pH 7.4 0.86 83
Muscosal homogenate, pH 9.0 0.43 41
Pancreas homogenate 54.9 34 400
Adipose "monoglyceride lipase" 0.021 0.53
Adipose "hormone-sensitive lipase" 0.14 0.82
Purified cholesteryl esterase 0 -
a From: Albro & Thomas (1973)
b 14C-labelled DEHP supplied as dispersion in either sodium cholate or
gum acacia
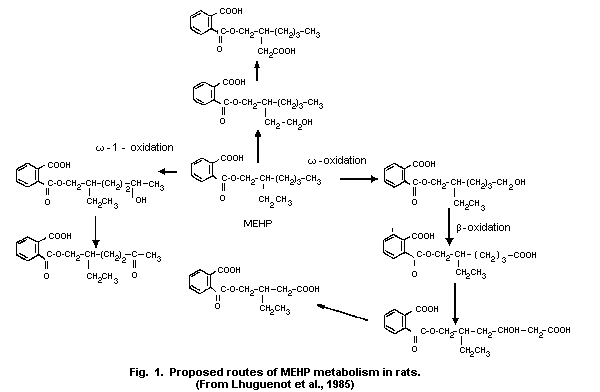 There are marked species differences in the metabolism of DEHP.
Thus omega-oxidation seems to play a dominante role in the rat and
guinea-pig (Albro et al., 1982; Lhuguenot & Elcombe, 1984; Lhuguenot
et al., 1985), but to be a minor pathway in the mouse, hamster, green
monkey, cynomolgus monkey, and marmoset (Albro et al., 1982; Lhguenot
& Elcombe, 1984). In guinea-pigs there are few omega-1 metabolites of
MEHP (Albro et al., 1982). This may have toxicological significance
because certain omega-1-oxidation metabolites have been identified as
active agents in peroxisomal proliferation in rat hepatocytes
(Mitchell et al., 1985a).
No conjugated metabolites were detected in the urine of
DEHP-treated rats, but a minor portion was conjugated in the urine of
hamsters (Albro et al., 1982; Lhuguenot & Elcombe, 1984). A major
portion of glucuronide conjugates was found in the urine of the
marmoset, mouse, guinea-pig, and green monkey, and in human urine
(Albro et al., 1982). Albro (1986) reported that glucuronidation of
DEHP metabolites was insignificant in rats. Studies in primates,
including the African green monkey (Albro et al., 1981), marmoset
(Rhodes et al., 1986), cynomolgus monkey (Short et al., 1987; Astill,
1989), and man (Schmid & Schlatter, 1985), demonstrated that
conjugation of DEHP can occur at the carboxylic acid moiety following
a single ester hydrolysis.
The level of unmetabolized MEHP excreted in urine also varies
considerably between species; it is low in the rat and hamster, but
high in the mouse, guinea-pig, green monkey, and man (Albro et al.,
1982).
Repeated oral administration of DEHP or MEHP at high doses (500
mg/kg) to rats leads to a change in the metabolic profile; there is an
increase in omega-oxidized metabolites and a decrease in
omega-1-oxidized metabolites (Lhuguenot et al., 1985). In rats given
2% DEHP in the diet for one week, a 4-fold increase in peroxisomal ß-
oxidation was found. ß-Oxidation of fatty acids induced by DEHP
appears to occur via mitochondrial and peroxisomal pathways that are
similar to normal pathways (Ganning et al., 1989). Drug-metabolizing
enzyme activities have been studied after DEHP administration, and in
some cases changes were observed (Walseth et al., 1982; Agarwal et
al., 1982; Gollamudi et al., 1985; Pollack et al., 1989).
The same metabolites as those found in rat urine can be detected
in human urine. One study on intravenously injected DEHP (Albro et
al., 1982) and one on orally administered DEHP (Schmid & Schlatter,
1985) indicated that humans metabolize DEHP by omega- and
omega-1-oxidation as well as by oxidation of the ethyl side chain.
However, the omega-oxidation-pathway seems to be a minor pathway in
man (Albro et al., 1982; Schmid & Schlatter, 1985). More than half of
the metabolites recovered in human urine are conjugated metabolites
(Albro et al., 1982; Schmid & Schlatter, 1985).
Time-averaged concentrations of DEHP, MEHP, and phthalic acid in
the blood of patients undergoing maintenance haemodialysis were 1.9,
1.3, and 5.2 mg/litre, respectively (Pollack et al., 1985a). Such
patients are considered to be at risk of potential DEHP toxicity
through prolonged contact with medical plastic products that contain
DEHP. The relatively high circulating level of phthalic acid may
indicate an altered metabolism of DEHP in uraemic patients (Pollack et
al., 1985a).
The levels of DEHP and MEHP in plasma have been studied in
newborn infants given blood exchange transfusions. In one case the
MEHP half-life was the same as for DEHP (about 12 h), indicating that
the hydrolysis of DEHP was the rate-limiting metabolic step. However,
in other children the half-time of MEHP was longer than that of DEHP
(Sjöberg et al., 1985b).
6.4 Elimination and excretion
Radioactivity from intravenously injected 14C-labelled DEHP is
mainly recovered in urine and faeces after 24 h (Schulz & Rubin,
1973), indicating that urine and bile are major excretory pathways.
When a low dose level (0.1 mg/kg) was given to rats, 50-60% of
injected radioactivity was recovered in urine and faeces after 24 h,
whereas at a high dose level (200 mg/kg) less than 50% was recovered
(Schulz & Rubin, 1973). Seven days after an oral dose (2.9 mg/kg) of
DEHP was given to rats, 42% of the radioactivity was recovered in the
urine and 57% in the faeces (Daniel & Bratt, 1974). Biliary excretion
was also measured in these experiments, and it was found that 14% of
the radioactivity was recovered in bile after 4 days (Daniel & Bratt,
1974). The almost 100% recovery reported by Daniel & Bratt (1974) has
been confirmed by Teirlynck & Belpaire (1985). Oral administration of
MEHP (50-500 mg/kg) gave a higher urinary recovery than orally
administered DEHP (50-500 mg/kg) as measured after 24 h (Lhuguenot et
al., 1985).
After the oral administration of non-radioactive DEHP (0.45
mg/kg) to human volunteers, it was found that 15-25% was excreted in
urine as MEHP or oxidized metabolites within 2-3 days (Schmid &
Schlatter, 1985).
In the rat no unmetabolized DEHP is excreted in the urine, but
small amounts are found in mouse or green monkey urine (Albro et al.,
1982). Major amounts of MEHP are excreted in mouse, guinea-pig, green
monkey, and human urine (Albro et al., 1982). However, oxidized
metabolites, either free or conjugated, constitute a major portion of
excretion products in rat, mouse, hamster, green monkey, and human
urine (Albro et al., 1982).
Changes in excretion pathways have been observed after prolonged
administration of DEHP. After oral dosing of rats without
pre-treatment with DEHP, the faecal excretion pathway dominated, while
in rats fed with DEHP for 7 days the urinary pathway dominated (Daniel
& Bratt, 1974).
6.5 Retention and turnover
6.5.1 Half-life and body burden
After the intravenous administration of radiolabelled DEHP, at
least two elimination phases of radioactivity, with short half-lives
(4.5-9 and 22 min, respectively), were observed in rat blood (Schulz
& Rubin, 1973). After 7 weeks of oral administration, the elimination
phase in the liver was considerably slower, the half-life being 3-5
days (Daniel & Bratt, 1974). No accumulation of DEHP or MEHP was
observed when the dosage was 2.8 g/kg per day for 7 days (Teirlynck &
Belpaire, 1985), nor was there any in a long-term (5-7 weeks) feeding
study at a dose level of 1 or 5 g/kg diet (corresponding to a daily
dose of about 50 and 250 mg/kg body weight) (Daniel & Bratt, 1974).
6.5.2 Indicator media
Analysis of the total amount of urinary metabolites, measured as
derivatized phthalic acid, indicate a weak positive correlation
between occupational exposure to phthalate and the presence of
metabolites in the urine (Nielsen et al., 1985; Liss et al., 1985). In
the study by Nielsen et al. (1985), workers were exposed mainly to
DEHP and diisodecyl phthalate, and the urinary level of phthalate
ester metabolites rose from the background level (17 µmol/litre) to
23-25 µmol/litre. In the study by Liss et al. (1985), workers were
exposed to DEHP and phthalic anhydride. Urinary phthalate
concentrations in exposed workers more than doubled after a workshift
and levels up to 44 µmol/litre were recorded. The authors concluded
that phthalic anhydride influenced the urinary level more than DEHP.
Phthalic acid is not a specific marker for DEHP exposure.
7. EFFECTS ON LABORATORY MAMMALS AND IN VITRO TEST SYSTEMS
7.1 Single exposure
Numerous LD50 values have been reported for DEHP. Oral LD50
values generally exceed 25 g/kg in rats and 30 g/kg in mice
(Stankevich et al., 1984; NIOSH, 1985b; Woodward et al., 1986); in the
rabbit it is 33.9 g/kg (Shaffer et al., 1945) and in the guinea-pig
26.3 g/kg (Krauskopf, 1973). Dermal LD50 values for guinea-pigs and
rabbits of 10 g/kg and 25 g/kg, respectively, have been reported
(NIOSH, 1985b).
The LD50 values after intraperitoneal administration were
30.7 g/kg in rats (Shaffer et al., 1945) and 14-75 g/kg in mice
(Lawrence et al., 1975; Woodward et al., 1986). LD50 values in the
range of 200-250 mg/kg were reported for the rat after intravenous
administration of DEHP solubilized with a nonionic detergent (Schmidt
et al., 1975; Rubin & Chang, 1978).
The main symptom of DEHP toxicity after single oral or
intraperitoneal dosing is diarrhoea (Hodge, 1943). An intraperitoneal
dose of 500 mg/kg in rats decreased spontaneous running activity,
thereby indicating behavioural changes (Rubin & Jaeger, 1973). After
intravenous dosing, lung lesions including oedema, haemorrhage, and
infiltrations of polymorphonuclear leucocytes were observed in rats at
doses as low as 50 mg/kg (Schulz et al., 1975). The etiology of the
lung lesions is unknown. It has, however, been suggested that some
changes could be due to the release of lysosomal enzymes from alveolar
macrophages, which was found to occur in vitro in rabbit alveolar
macrophages cultured with DEHP (Bally et al., 1980).
Rabbits treated intravenously with 350 mg DEHP/kg showed a
decrease in blood pressure and an increase in breathing rate. No
deaths occurred after doses up to 650 mg/kg were administered (Calley
et al., 1966).
The monoester, MEHP, may be more toxic than the diester but data
are very limited. In a short note by Villeneuve et al. (1978), the
oral LD50 of MEHP was reported to be 1.34 g/kg in female rats and
1.8 g/kg in males.
7.2 Short-term exposure
Doses of 3.4 g/kg body weight per day given by gavage (in olive
oil) for periods of up to 90 days caused the death of 15 out 20 rats
(Nikonorow et al., 1973). However, no deaths were reported among rats
fed 3% DEHP in the diet (1.9 g/kg body weight) for 90 days (Shaffer et
al., 1945) or in a rat study of the US National Toxicology Program
after dietary dosing (< 50 g/kg) for 14 days (NTP, 1982).
Oral administration of DEHP at a rate of > 0.4 g/kg body
weight per day resulted in a weight gain decrease in rats within a few
days (Nikonorow et al., 1973). In a 17-week feeding study where rats
were given 2, 10 or 20 g DEHP/kg diet, a decreased body weight was
observed (Gray et al., 1977). Reduction in body weight was also
observed in rats given dietary levels of 12.5 or 25 g/kg for 13 weeks.
Dosages of 1.6-6.3 g/kg resulted in either slight elevations of body
weight or no effect (NTP, 1982).
MEHP given at a level of 6.4 g/kg diet caused reduction in the
body weight gain of rats (Chu et al., 1981). No effects on body weight
occurred at dietary levels of 0.625 g/kg given for 3 months, but a
significant decrease in blood glucose was observed.
Reductions in haemoglobin, packed cell volume and erythrocyte
numbers were observed in rats given 10 or 20 g DEHP/kg diet for 17
weeks, but not when they were given 2 g/kg for the same period (Gray
et al., 1977).
Cystic kidneys and centrilobular necrosis were noted in one
strain of mice (ddY) fed 2.5 or 25 g DEHP/kg for 2 weeks, but not in
another strain (B6C3F1), even with a higher exposure level and a
longer exposure period (Woodward et al., 1986).
DEHP administered intravenously at a rate of 25-500 mg/kg per day
for 2-4 weeks to beagle dogs resulted in pulmonary haemorrhage and
inflammatory response similar in appearance to the "shock-lung" effect
(Woodward et al., 1986).
In an inhalation study, Wistar rats were exposed in a head-nose
inhalation system to DEHP aerosols of respirable particle size.
Exposure duration was 6 h/day, 5 days/week for 4 weeks at target
concentrations of 0, 0.01, 0.05, and 1.0 mg/litre. A statistically
significant increase in relative lung weights was found in the males
given the highest dosage, and this was accompanied by foam cell
proliferation and thickening of the alveolar septa (Klimisch et al.,
1991).
Another inhalation study has been reported, but this is
inadequate for assessment (Timofievskaja et al., 1980).
A discussion of the effects of DEHP on the liver is given in
section 7.9.
When rats were treated with DEHP by intraperitoneal injection
(Walseth et al., 1982) or by repeated oral dosing (Agarwal et al.,
1982), an increase in cytochrome P-450 levels was observed. The
increase in hepatic microsomal oxidation appears to be primarily due
to the deesterification products of DEHP, i.e. MEHP and
2-ethoxyhexanol, in long-term exposure, whereas DEHP and its two
metabolites may inhibit microsomal oxidation after acute exposure to
DEHP (Pollack et al., 1989). However, in vitro rat liver microsomal
cytochrome P-450 levels were not affected by DEHP (Gollamudi et al.,
1985).
Liver mitochondrial enzymes and mitochondrial morphology have
been reported to be influenced by DEHP administration (Ohyama, 1977;
Shindo et al., 1978). Recent results suggest that the in vitro
effects of DEHP (> 20 µmol/litre) on mitochondrial functions are
mainly related to the action on membrane lipids surrounding the
adenine nucleotide translocator, which reduces the rate of adenine
nucleotide exchange across the mitochondrial membrane (Kora et al.,
1989).
In male rats given DEHP in the diet, the urinary excretion of
zinc was enhanced and the testicular level of zinc decreased (Gray et
al., 1982; Oishi, 1985) (section 7.5). These changes in zinc
homeostasis could be due to altered levels of metallothionein. In mice
fed 6 or 12 g DEHP/kg diet for 24 weeks, hepatic levels of
metallothionein were increased up to 11-fold (Waalkes & Ward, 1989).
Several studies on rats have shown that DEHP given in the diet
(5-20 g/kg) decreases plasma triglyceride and cholesterol levels
(Yanagita et al., 1978; Sakurai et al., 1978; Bell et al., 1978a; Bell
et al., 1978b; Bell et al., 1979; Yanagita et al., 1979; Curstedt &
Sjövall, 1983). DEHP inhibits the biosynthesis of cholesterol, an
effect which is accompanied by phospholipidosis, and the same effects
have been observed with MEHP (Oishi & Hiraga, 1982).
7.3 Long-term exposure
In a 24-month study by Harris et al. (1956), three groups of
Wistar rats each comprising 43 males and 43 females were fed diets
containing 0, 1, and 5 g DEHP/kg, interim kills being made at 3, 6,
and 12 months. At the end of the study, only two control, four
low-dose, and seven high-dose animals were alive. During the first
year the body weights of the high-dose group were slightly reduced,
but by the second year the body weights of all groups were similar.
During the first 6 months an increase in relative liver and kidney
weights was seen in DEHP-treated animals but later they were similar
in control and treated animals. After 3 months of treatment, one out
of eight rats in the low-dose group was found to have mild renal
tubular atrophy. After 6, 12, and 24 months of treatment, no
compound-related pathological changes were evident. Because of high
mortality due to disease, this study is difficult to validate.
In another 24-month study (Carpenter et al., 1953), groups of
Sherman rats consisting of 32 males and 32 females were given diets
containing 0, 0.4, 1.3 or 4 g DEHP/kg. Owing to reduced life
expectancy due to disease and the small numbers of animals used, the
study was inadequate for assessing the chronic toxicity of DEHP.
In a 12-month study by Nikonorow et al. (1973), a group of 20
male and 20 female Wistar rats was given a diet containing 3.5 g
DEHP/kg, and a control group received the diet without DEHP. The only
gross or micropathological change noted in exposed animals at necropsy
was hepatomegaly. During the study, however, about 30% of the animals
died due to congestion of the small intestine and loss of the gastric
and/or intestinal mucosa, which was complicated by purulent pneumonia
and endometritis.
Crocker et al. (1988) described the renal effects of DEHP given
by gavage to young male rats at a dosage of 2.14 mg/kg body weight
three times per week for up to 12 months. A 50% reduction in
creatinine clearance and an increase in the severity of renal cyst
formation was observed. This lesion was consistent with spontaneous
nephropathy commonly observed in old rats; exposure may cause an onset
in younger rats. Furthermore, DEHP fed at 6 and 12 g/kg diet for two
years did not produce renal lesions in male and female F-344 rats
(NTP, 1982).
In a 2-year study (NTP, 1982; Kluwe et al., 1982), groups of
F-344 rats were given dietary levels of 0, 6, and 12 g DEHP/kg.
Decreased body weight in exposed groups was noted from week 30 until
the cessation of exposure. In addition to neoplastic effects (section
7.7) and testicular atrophy (section 7.5), a compound-related
hypertrophy of cells in the male anterior pituitary was noted in the
high-dose group. In both exposed groups an increased incidence of
clear changes in liver cells was observed. This study also
investigated B6C3F1 mice exposed to 3 or 6 g DEHP/kg diet. A
dose-related decrease of body weight was observed in female mice.
There was no increased incidence of non-neoplastic lesions except for
seminiferous tubular degeneration in the testes of male mice (section
7.5.1).
In a study on groups of male and female guinea-pigs fed diets
containing 0, 0.4 or 1.3 g DEHP/kg for 12 months (Carpenter et al.,
1953), the body weight of the low-dose animals was statistically
higher than that of controls, and liver weight relative to body weight
of dosed females slightly increased. No other exposure-related lesions
were found.
A study on groups of male ferrets fed diets containing 0 and 10
g DEHP/kg for 14 months revealed a reduction in body weight and an
increase in relative liver weight, but no evidence of peroxisome
proliferation (Lake et al., 1976).
7.4 Skin and eye irritation; sensitization
DEHP has been shown to be a weak irritant to mammalian skin when
administered topically or intradermally (0.2 ml of an emulsion of
100 g/litre) (Calley et al., 1966; Woodward et al., 1986).
In a study by Lawrence et al. (1975), no irritation occurred when
undiluted DEHP was instilled into the eye of rabbits.
No data are available on the potential for DEHP to induce skin
sensitization in animals.
7.5 Reproduction, embryotoxicity, and teratogenicity
7.5.1 Reproduction
The effects of DEHP on male reproductive organs have been studied
extensively. The majority of the studies have been carried out on rats
or mice given DEHP in the diet.
Seminiferous tubular atrophy, comprising a loss of spermatids and
spermatocytes, occurred when 4-week-old Wistar rats were given 2800 mg
DEHP/kg by oral intubation for 10 days (Gray & Butterworth, 1980). In
similarly treated 10-week-old rats, about 50% of the tubules were
atrophic and the remainder unaffected. However, no testicular damage
was detected in treated 15-week-old rats. When 20 g DEHP/kg was given
in the diet (approximately 1200 mg DEHP/kg per day) to 4-week-old
rats, the lesions produced were reversible whether treatment stopped
before or continued until after the control rats had reached sexual
maturity.
In rats given 10 or 20 g DEHP/kg diet, the testis atrophy was
dose dependent after approximately 2 weeks of feeding. This atrophy
was accompanied by pituitary changes, i.e. enlargement and
vacuolization of the basophils of the pars distalis, corresponding to
the formation of the so-called castration cells seen after gonadectomy
(Gray et al., 1977). In a subsequent study, there was a reduction in
testicular and prostatic zinc levels concomitant with increased
urinary excretion of zinc (Gray et al., 1982).
In a rat study by Oishi & Hiraga (1980a), the serum testoster-one
levels in rats fed 20 g DEHP/kg diet were reduced by approximately
50%. The total amount per testis decreased, but the concentration rose
to 150% of the original value because of the testicular atrophy.
Simultaneous administration of testosterone or zinc had no protective
effect on the atrophy but did prevent the weight reduction of the sex
organs such as the epididymis (Gray & Butterworth, 1980; Oishi &
Hiraga, 1983). In a further study, it was found that a low-zinc diet
aggravated the DEHP-induced testicular atrophy (Agarwal et al.,
1986a).
DEHP given to 7 young (5 weeks old) Wistar rats in the diet
(20 g/kg) for one week decreased the testicular weight significantly
(P < 0.05), compared to controls, and also the testicular zinc
concentration (Oishi, 1984). In a study by Oishi (1985), groups of 20
male rats were given DEHP (2.0 g/kg per day) by gavage for 14 days.
Ten rats were then killed and the remaining ten were kept for 45 days
on a DEHP-free diet. The histopathological changes of the testes seen
on day 15 were marked shrinkage of the seminiferous tubules, a
germinal epithelium consisting only of Sertoli cells, and very few
spermatogonia. After 45 days the percentage of spermatogenic tubules
had increased from 0 (at day 15) to 12.8%, indicating a limited
reversibility of the testicular atrophy.
Similar degeneration of the seminiferous tubules was observed
when 13-week-old Wistar rats were orally given 2 g DEHP/kg body weight
for 7 consecutive days (Saxena et al., 1985) .
In a dietary study, DEHP was administered to F-344 rats at 6 and
12 g/kg for 103 weeks (NTP, 1982). Degeneration of the seminiferous
tubules was observed only at the higher dose.
The testicular changes induced by oral DEHP administration appear
to be age dependent. A daily dosage of 2.8 g DEHP/kg body weight given
for 10 days caused seminiferous tubular atrophy in 4-week-old Wistar
rats, but this effect was less severe in 10-week-old rats and absent
in 15-week-old rats (Gray & Butterworth, 1980). Sjöberg et al. (1986)
showed that DEHP fed at a level of 1.7 g/kg diet caused reductions in
testis weight to 20%, 55%, and 92% of the control values in
Sprague-Dawley rats at 25, 40, and 60 days of age, respectively. This
was apparently not due to differences in pharmacokinetic parameters,
since the plasma levels of the metabolite MEHP were identical in the
25- and 40-day-old rats (Sjöberg et al., 1982).
Rats given DEHP intraperitoneally at a dosage of 100 mg/kg per
day for 5 days did not develop testicular atrophy (Curto et al.,
1982). However, this lack of response was probably a consequence of
the dose used, rather than the dose route, and there was a 30%
reduction in testicular zinc. Intraperitoneal administration of higher
doses (1-25 g/kg per day for 5 days) to Wistar rats decreased serum
testosterone levels (Oishi & Hiraga, 1979).
Some degenerated primary spermatocytes and altered Sertoli cells
were observed in Sprague-Dawley rats given 3-h intravenous infusions
of an emulsion at a rate of 1 ml/h, which corresponded to a daily dose
of 500 mg DEHP/kg (Sjöberg et al., 1985c). The infusions were given
every other day on six occasions. The emulsion contained DEHP,
fractionated egg yolk phosphatides, glycerol, and water. No effects
were observed when emulsions corresponding to 0, 5 or 50 mg DEHP/kg
were given.
In a study by Curto & Thomas (1982), groups of sexually mature
Swiss-Webster mice were given intraperitoneal injections of 50 or 100
mg DEHP/kg either daily for 5 days or alternate daily for 20 days (10
injections). The animals were killed 24 h after the last injection. No
significant alterations in testicular weight or zinc levels occurred.
Young rats were not more susceptible to testicular damage than
older ones following intravenous infusion of DEHP. It has been
suggested that the age-related difference observed in some studies may
be due to the fact that the rate of gastrointestinal absorption of the
DEHP-derived metabolite MEHP is higher in younger animals than in
older ones (Sjöberg et al., 1986).
In a NTP-sponsored study (Melnick et al., 1987), CD-1 mice given
3 g DEHP/kg diet showed significantly diminished testis and epididymis
weights compared to controls. In addition, the sperm concentration in
the cauda epididymis was reduced and the percentage of abnormal sperm
in the cauda was significantly higher in the treated mice than in the
controls.
A high oral dose (4.2 g/kg) of DEHP gave minimal tubular atrophy
in hamsters but did not produce any effects on urinary zinc excretion,
testicular zinc levels or testicular weights (Gray et al., 1982).
The effects of the monoester MEHP have not been as well studied
as those of DEHP. An oral dosage of 1 g MEHP/kg per day for 5 days
produced a significant decrease in rat testis weight and extensive
testicular atrophy (Gray et al., 1982). On the other hand, rats given
intraperitoneal doses of up to 100 mg MEHP/kg daily for 5 days showed
no abnormal histology (Curto et al., 1982) and an intraperitoneal dose
of 50 mg/kg given on alternate days for 20 days produced only a
reduction in prostatic zinc levels (Curto & Thomas, 1982).
Mice fed diets containing 20 g MEHP/kg for 1 week revealed
markedly reduced testicular zinc and testosterone levels, but there
were no reductions in testicular weight (Oishi & Hiraga, 1980b).
Hamsters given MEHP (1 g/kg per day) for 9 days showed more severe
testicular effects than those given DEHP at a level of 4.2 g/kg per
day for the same period (Gray et al., 1982). More recent studies
(Albro et al., 1989) indicate that testicular atrophy resulting from
DEHP exposure is most probably due to the formation of MEHP.
These studies indicate that the rat is the species most
susceptible to DEHP-induced testicular atrophy. The mechanism of
phthalate-induced testicular damage is not fully understood.
Testicular zinc depletion has been suggested to be a primary event
(Foster et al., 1982, 1983). However, Gray et al. (1982) showed that
an effect on zinc level is not always associated with testicular
atrophy in all species tested. Zinc is essential for normal testicular
function and its depletion is known to lead to testicular atrophy
(Barney et al., 1969). Inhibition of dehydrogenase enzymes, e.g.,
those controlling the biosynthesis of testosterone, leads to reduced
testosterone levels. DEHP administration has been shown to reduce the
level of serum testosterone in the rat (Oishi & Hiraga, 1979; Oishi &
Hiraga, 1980a) as well as in the mouse (Gray et al., 1982), although
in the mouse no testicular atrophy was observed. Administration of
testosterone or zinc did not prevent the testicular damage induced by
DEHP (Gray & Butterworth, 1980; Oishi & Hiraga, 1983).
Co-administration of DEHP and testosterone, on the other hand,
apparently enhanced the testicular damage caused by DEHP (Oishi,
1989a). In a similar study (Oishi, 1989b), luteinizing
hormone-releasing hormone (LRH) significantly decreased testis weight,
sulfhydryl content, and lactate dehydrogenase when given together with
DEHP, whereas LRH or DEHP alone had no effects.
Similar effects were observed with exogenously added follicle
stimulating hormone (FSH) in an in vitro study. Primary testicular
cell cultures pretreated with MEHP showed a dose-related reduction in
FSH-stimulated cyclic adenosine monophosphate (cAMP) production (Lloyd
& Foster, 1988). These results suggest that MEHP produces a
perturbation at the level of the FSH membrane receptor, causing an
inhibition of FSH action.
In vitro studies have indicated that the Sertoli cell is the
target cell. Mixed cultures of Sertoli and germ cells prepared from
rat testes were exposed to DEHP or MEHP (10-7-10-4 mol/litre)
(Gray & Beamand, 1984; Gray & Gangolli, 1986). DEHP had no effect, but
MEHP caused a dose-dependent increase in the rate of germ cell
detachment from Sertoli cells, accompanied by changes in Sertoli cell
morphology. More recent data indicate that Sertoli cell mitochondria
are a target for MEHP (Chapin et al., 1988). However, it has also
been shown that testicular mitochondrial respiratory functions are
decreased in rats given 2 g DEHP/kg body weight by gavage (Oishi,
1990).
In a study by Melnick et al. (1987), CD-1 mice were given 0, 0.1,
1 or 3 g DEHP/kg diet during a 7-day pre-mating period and a
subsequent 98-day cohabitation period. There was complete suppression
of fertility in the 3-g/kg group and a significant reduction in
fertility in the 1-g/kg group, compared to controls, but no effect on
fertility at 0.1 g/kg.
In a fertility study designed to investigate earlier significant
results (Singh et al., 1972), groups of male ICR mice were dosed
subcutaneously with undiluted DEHP at levels of 1, 2, 5, and 10 ml/kg
(equivalent to 0.99, 1.97, 4.93, and 9.86 g/kg) on days 1, 5, and 10
of the study (Agarwal et al., 1985a). They were subsequently mated
with virgin females on days 2, 6, 11, 16 and 21, and then at weekly
intervals until week 8. There were reductions in the incidence of
pregnancies in several groups, but these were dose related only at the
first mating (i.e. on day 2 of the experiment). Examination of
pregnant mice on day 13 of gestation showed that there were
pre-implantation losses corresponding to a DEHP dose level of 10 ml/kg
and a mating on day 6. There were consistent increases in early fetal
deaths relating to matings at day 21, week 4, and week 5. In a similar
study, male and female mice were treated subcutaneously with 1-100 ml
DEHP/kg, and this led to a reduction in testicular but not ovarian
weight. The ovaries exhibited histological injury at lower doses of
DEHP than the testes. Unlike the situation in the testes, there was no
significant dose-related increase in histopathological changes in the
ovaries (Agarwal et al., 1989).
In an investigation of the effects of phthalates on female
reproductive organs, three doses of 4.93 g DEHP/kg were given
intraperitoneally at 5-day intervals to female rats (Seth et al.,
1976). No histopathological changes in the ovaries were seen 22 days
after the first injection, but reductions in the activities of some
enzymes were noted.
The administration of DEHP (1000 mg/kg intraperitoneal or 2000
mg/kg oral) to marmosets daily for 14 days did not lead to testicular
atrophy (Rhodes et al., 1986).
Hence, it appears that rats and guinea-pigs are sensitive to
DEHP-induced testicular atrophy, while mice are fairly resistant and
hamsters and marmosets are highly resistant. The fact that at least
some of the effects associated with this atrophy can be produced
in vitro argues against a hormonally mediated indirect effect. The
earliest effects are seen in Sertoli cells and are described as
vacuolation (Albro, 1987).
7.5.2 Embryotoxicity and teratogenicity
In a study by Nikonorow et al. (1973) on female Wistar rats given
0.34 or 1.7 g DEHP/kg by gavage during the first 21 days of gestation,
the only untoward effect was a reduction in fetal body weight. When
DEHP (0, 5, 10, 15 or 20 g/kg) was administered orally to Fischer-344
rats on gestational days 0 to 20, maternal toxicity and reduced fetal
body weight per litter were observed at the three highest dose levels.
The number of fetuses per litter was unaffected by the treatment (Tyl
et al., 1988).
Intraperitoneal injections of 4.93 or 9.86 g DEHP/kg on days 5,
10, and 15 of gestation resulted in an increase in the number of
resorptions and reduced fetal weight in Sprague-Dawley rats (Singh et
al., 1972). In the highest-dose group, gross abnormalities, such as
twisted hind legs and anophthalmia, were noted but no skeletal defects
were observed.
Rat plasma soluble extracts of two PVC plastics containing DEHP
were administered intravenously to groups of pregnant Sprague-Dawley
rats daily from the 6th to the 15th day of gestation, and the animals
were killed at day 20 (Lewandowski et al., 1980). The daily doses of
DEHP were equivalent to 1.3 mg/kg and 5.2 mg/kg. No significant
teratogenic or embryotoxic effects were noted.
Groups of ICR mice were given DEHP in the diet at levels of 0.5
to 10 g/kg for the first 18 days of gestation and were then sacrificed
(Shiota et al., 1980; Shiota & Nishimura, 1982). The food intake was
an average of 7 g/day. At the 4 g/kg and 10 g/kg dose levels, no live
fetuses were found. At 2 g/kg, 40% of the fetuses had malformations
including exencephaly, spina bifida, and malformed tail. Delayed
ossification was seen in about 15% of the fetuses at 1 g/kg and
2 g/kg.
In a study by Tyl et al. (1988), DEHP was administered in the
diet to CD-1 mice on the first 17 days of gestation at levels of 0,
0.25, 0.5, 1, and 1.5 g/kg. At the two highest dose levels maternal
toxicity, increased resorptions and late fetal deaths, decreased
numbers of live fetuses, and reduced fetal body weight per litter were
observed. The number and percentage of malformed fetuses per litter
were elevated at the three highest dose levels.
A single oral administration of DEHP (0.1 ml/kg) on day 7 of
gestation to ddY-strain SPF (specific pathogen free) mice decreased
the number and the body weights of live fetuses (Tomita et al.,
1982b). In a study by Yagi et al. (1980), DEHP was given orally to SPF
mice on days 6, 7, 8, 9 or 10 of gestation. When 5.0 or 10.0 ml/kg was
given on day 7 there were no live fetuses, whereas 2.5 ml/kg
administered on the same day resulted in 14% live embryos and 1.0
ml/kg gave 40% live embryos. The percentages of live embryos when 10.0
ml/kg was given on day 8, 9 or 10 of gestation were 18, 92, and 95%,
respectively. Gross and skeletal abnormalities occurred in fetuses
given 2.5 and 7.5 ml/kg on day 7 or 8. The abnormalities included
exencephaly, open eyelid, and club-foot.
In a study by Shiota & Mima (1985), groups of ICR mice were given
DEHP by stomach intubation on days 7, 8, and 9 of gestation. The DEHP
doses (in olive oil) were 250, 500, 1000, and 2000 mg/kg, and the mice
were sacrificed on day 18. In the two highest dose groups, the numbers
of resorptions and malformed fetuses were significantly increased.
Fetal weights were significantly depressed. The most frequent
malformations were anencephaly and exencephaly. When doses of up to
8000 mg/kg were given by intraperitoneal injections on days 7,8, and
9 of gestation, no effects were noted. DEHP is highly embryotoxic and
terato-genic in mice when given orally but not when given
intraperitoneally.
The monoester MEHP, at oral doses of 225, 450, and 900 mg/kg per
day, produced significant signs of maternal toxicity when given to
pregnant Wistar rats on days 6-15 of gestation (Ruddick et al., 1981).
In the highest dose group a 73% mortality was observed in the dams.
There was a dose-related decrease in the number of litters and in the
litter weights of live pups.
Oral dosing with 0.1 or 1.0 g MEHP/kg on day 7 of gestation led
to increased incidence of early embryonic deaths in SPF mice (ddY
strain), but dosing on day 8 or 9 had less effect (Yagi et al., 1980;
Tomita et al., 1986). The fetuses had reduced body weight, and there
was a higher incidence of gross abnormalities, compared to controls,
when the higher dose level was given on day 8 or 9. The mice dosed on
day 8 produced fetuses with a high incidence of skeletal effects.
Intravenous injections of MEHP (11.38 mg/kg) to rabbits on days
6-17 of gestation gave a high incidence of resorptions (Thomas et al.,
1979). The incidence of fetal anomalies was similar to that in
controls.
When Nikonorow et al. (1973) administered DEHP (0.34 or 1.7 g/kg
per day) to female Wistar rats by gavage for 3 months prior to mating,
there was an increase in the number of resorptions but no effects on
fetal weights or the incidence of skeletal anomalies.
In utero administration of DEHP (1000 mg/kg body weight) to
rats daily from days 6 to 15 of gestation resulted in retardation of
fetal growth and an increase in fetal liver weight. There were
significant quantities of DEHP and decreased mitochondrial enzyme
activities in the fetal liver. These results indicate an effect on
fetal liver cell bioenergetics (Srivastava et al., 1989).
The mechanism of DEHP or MEHP teratogenicity is not known.
Teratogenic activity could result from zinc deficiency, which is known
to produce such effects (Swenerton & Hurley, 1971).
7.6 Mutagenicity and related end-points
The possible genotoxic effect of DEHP has been thoroughly
investigated in several different short-term tests. The effects of the
major metabolites of DEHP, i.e. MEHP and 2-ethylhexanol, as well as
phthalic acid and phthalic anhydride, have also been studied.
7.6.1 Mutation
Studies on the possible mutagenic effect of DEHP have been
performed in bacteria, fungi, and in cultured mammalian cells.
Drosophila melanogaster has also been used and results from a few
in vivo studies on mice have been reported.
7.6.1.1 Bacteria
Many studies have been performed using a variety of strains of
Salmonella typhimurium and DEHP doses of up to 10 mg/plate.
Incubations both with and without exogenous activation systems have
been performed. S9 mix from rats induced by Aroclor 1254 has
frequently been used, but other species and other inducers have also
been used to produce metabolic activation systems. With one exception
(Tomita et al., 1982a) these test results have all been negative
(Kirby et al., 1983; Yoshikawa et al., 1983; Zeiger et al., 1985;
Agarwal et al., 1985b), and in a IPCS collaborative study (Ashby et
al., 1985) all five laboratories reported negative results. Bacteria
other than S. typhimurium have also been used; negative results were
obtained with E. coli WP2 at doses of up to 2 mg per plate (Yoshikawa
et al., 1983).
The major metabolites of DEHP have also been tested for mutagenic
activity in bacteria. Concentrations of up to 3.333 mg per plate for
MEHP and phthalic anhydride (Zeiger et al., 1985) and 2 mg/plate for
2-ethylhexanol and phthalic acid (Agarwal et al., 1985b) have yielded
negative results in strains of Salmonella (see also Kirby et al.,
1983; Yoshikawa et al., 1983). However, Tomita et al. (1982a) reported
a significant increase in TA100 revertants following exposure to
either DEHP or MEHP (both with and without S9). It should be noted,
however, that MEHP mutagenicity is only demonstrable within a narrow
range of concentration, because it has a sterilizing effect at high
concentration and shows no mutagenic activity at low concentration.
Tomita et al. (1982a) also detected dose-dependent (0.4 and 0.5 mg
MEHP/plate) DNA damage in a B. subtilis Rec assay, while DEHP,
phthalic acid, and 2-ethylhexanol were all negative. In this study
MEHP also gave a positive result in E. coli WP2 b/r.
Negative results were obtained when pooled urine from rats,
treated with 2000 mg DEHP/kg per day for 15 days, was tested for
genotoxic activity. A direct plating procedure was used with
S. typhimurium strains TA98, TA100, TA1535, TA1537, and TA1538, both
with and without S9 and ß-glucuronidase/aryl sulfatase as the
activation system. When 2-ethylhexanol was tested according to the
same protocol, the result was also negative (Divincenzo et al., 1985).
7.6.1.2 Fungi
The induction of mutations by DEHP has been studied in various
fungal species. In the IPCS collaborative study on in vitro assay
systems (Ashby et al., 1985), DEHP was considered to be negative in
six out of seven assays. Positive results were obtained with
Saccharomyces cerevisiae, both with and without S9 activation, at
lowest effective concentrations of 1541 mg/litre and 3081 mg/litre,
respectively. However, other laboratories using different strains of
S. cerevisiae or Schizosaccharomyces pombe reported negative
results at a maximum tested concentration of 5000 mg/litre.
7.6.1.3 Mammalian cells
Mouse lymphoma cells (L5178Y), Chinese hamster V79 cells, and
human lymphoblasts have been used to study the mutagenic effect of
DEHP in cultured mammalian cells. Several investigators obtained
negative results, but a few positive results have also been reported.
In the IPCS collaborative study (Ashby et al., 1985), only one
out of ten investigators reported a positive response. Mouse lymphoma
cells were exposed to DEHP without S9, and two concentrations (7.5 and
20 mg/litre) gave positive results. In a separate study (Kirby et al.,
1983), where MEHP, 2-ethylhexanol, and DEHP were tested in the mouse
lymphoma cell assay, all three substances were found to be
non-mutagenic. The concentrations used were 0.016-1.0 ml/litre
(without S9) and 0.067-5.0 ml/litre (with S9) for DEHP, and 0.013-1.0
ml/litre for MEHP and 2-ethylhexanol.
7.6.1.4 Drosophila
DEHP has also been tested for mutagenicity in Drosophila
melanogaster using the sex-linked recessive lethal (SLRL) test and
various somatic recombination and mutation assays. SLRLs were not
induced by DEHP (20 µg/g) administered by injection (Yoon et al.,
1985). Negative results for DEHP were also reported in the SLRL
mutation assay on Drosophila melanogaster larvae (Zimmering et al.,
1989).
In the IPCS study (Ashby et al., 1985), DEHP gave a positive
response in the unstable eye mosaic test at a dose of 6.1 g/litre, in
two separate experiments, but neither lower nor higher concentrations
produced any response. No activity was seen in the wing spot test with
a single dose of 6.1 g/litre. It was concluded that DEHP exhibits
marginally positive mutagenicity (Ashby et al., 1985).
7.6.2 DNA damage
Various end-points, such as unscheduled DNA synthesis (UDS) and
single strand breaks, have been used to detect DNA damage induced by
DEHP in a variety of mammalian test systems. In the IPCS study (Ashby
et al., 1985), negative results were obtained when single strand
breaks were measured, either by alkaline elution in hepatocytes (up to
3.907 g/litre) or alkaline sucrose sedimentation in Chinese hamster
ovary (CHO) cells (up to 39 g/litre). UDS in either isolated
hepatocytes or cultured HeLa cells was investigated by four different
laboratories. One investigator detected a positive response using
isolated hepatocytes, but, since this result was only statistically
significant at one dose and not dose related, the consensus was that
DEHP does not cause UDS.
In a study by Butterworth et al. (1984), DEHP did not induce DNA
repair in primary rat hepatocytes. Similarly, neither DEHP nor MEHP
induced any DNA repair in primary human hepatocytes from three
different subjects. In this study, concentrations as high as 0.01 mol
DEHP/litre and 5 x 10-4 mol MEHP/litre were used and exposure
continued for 18 h. No induction of DNA repair or increased alkaline
elution of DNA was seen in hepatocytes from either female or male
F-344 rats treated with DEHP in vivo. UDS was not induced in male
rats treated with 500 mg DEHP/kg by gavage 2, 12, 24 or 48 h before
sacrifice. Furthermore, treatment of male rats with 150 mg/kg per day
by gavage for 14 days and treatment of female rats with dietary DEHP
(12 g/kg) for 30 days resulted in peroxisome proliferation, but no UDS
was induced.
Similar in vivo/in vitro results in studies of UDS in
hepatocytes were reported by Kornbrust et al. (1984). No UDS was
observed in primary rat hepatocytes exposed in vitro to 10-5 to
10-2 mol DEHP/litre or in vivo by a single gavage dose of 5 g/kg
2, 15 or 24 h prior to the isolation of hepatocytes. A dietary
concentration of 20 g DEHP/kg led to a marked proliferation of
peroxisomes after 4 weeks. Neither this treatment nor the additional
administration of a single gavage dose of 5 g/kg (15 h before
sacrifice) to animals fed the 20-g/kg diet for either 8 weeks or for
4 weeks with or without pretreatment with 3-amino-1,2,4-triazole (to
inhibit endogenous catalase activity) induced any detectable DNA
repair in hepatocytes. Indeed, the administration of DEHP and some
other proliferators has been shown to increase the levels of
8-hydroxy-deoxyguanosine in rat liver DNA (Takagi et al., 1990).
7.6.3 DNA binding
In an in vivo study by Albro et al. (1982), radioactivity from
carbonyl-labelled DEHP did not associate with purified protein, RNA or
DNA from rat liver. Label from 2-ethyl-(1-14C)-hexyl-labelled DEHP
or MEHP appeared to associate strongly with purified DNA, but this was
not the case with label from free 14C-labelled 2-ethylhexanol.
According to Albro (1987), although there is incorporation into normal
nucleosides, there is no evidence of alkylation.
In a similar study (von Däniken et al., 1984; Lutz, 1986), DEHP
radiolabelled in different positions was administered orally to female
F-344 rats with or without pretreatment with unlabelled DEHP (10 g/kg
diet) for 4 weeks. The administration of 14C-carboxylate-labelled
DEHP resulted in no measurable DNA radioactivity, whereas
radioactivity was clearly measurable after the administration of DEHP,
14C- or 3H-labelled in the alcohol moiety, or of
2-ethyl(1-14C)hexanol. HPLC analysis showed that the normal
nucleosides had incorporated radiolabel, but fractions expected to
contain carcinogen-modified nucleoside adducts did not contain any
radioactivity.
DNA isolated from the livers of male F-344 rats administered 2000
mg DEHP/kg daily by gavage for 3 days was analysed for possible
carcinogen-DNA adducts by the 32P-postlabelling technique (Gupta et
al., 1985). No adducts were detected in the DNA, which also was the
case when DNA from hepatocytes exposed to 10-3 mol DEHP/litre
in vitro for 4 h was analysed.
7.6.4 Chromosomal effects
Chromosomal effects of DEHP have mainly been studied in vitro,
although some studies on the induction of micronuclei in the
peripheral blood erythrocytes of mice have been published.
DEHP did not induce any increase in the level of sister-chromatid
exchange (SCE) in Chinese hamster ovary (CHO) cells, treated for 1 h,
either with or without S9, at levels of up to 0.01 mol/litre (Douglas
et al., 1986). On the other hand, MEHP has been reported to induce SCE
in V79 cells treated with 25 or 50 mg/litre for 24 h or 1500 mg/litre
for 3 h (Tomita et al., 1982a). This metabolite also induced
chromosomal aberrations in CHO cells and RL4 cells (from rat liver),
but only at cytotoxic concentrations in the CHO cells (1.0 and 1.3
mmol/litre, with or without S9). MEHP was less toxic to RL4 cells
and concentrations between 2.0 and 5.0 mmol/litre gave a dose-related
increase in chromosomal aberrations (Phillips et al., 1986). According
to the authors, observations on changes in CHO cell structure and
permeability and on the haemolytic effects of phthalate monoesters
suggest that MEHP cytotoxicity may be due primarily to an action on
cell membranes.
The induction of aneuploidy by DEHP was investigated both in
mammalian cells and in fungi in the IPCS study (Ashby et al., 1985).
The mammalian assays gave positive responses at 50 mg/litre in a
fibroblast cell line from Chinese hamster liver and at levels of
between 25 and 50 mg/litre in Chinese hamster primary liver cells. Two
out of four studies using fungi were also positive and the consensus
was that DEHP is capable of inducing aneuploidy in vitro in both
fungi and mammalian cells.
In a study by Ahmed et al. (1990), male Wistar rats were fed for
alternate 7-day periods with a diet containing 20 g DEHP/kg and a
control diet. The rats were examined after 3 days on the DEHP diet or
after 7 days on the control diet. An analysis of nuclear size gave
results consistent with an increase in tetraploid hepatocytes after
treatment with DEHP, which was reversed when the rats returned to the
control diet.
7.6.5 Cell transformation
DEHP-induced cellular transformation has been studied in several
different experimental systems. In a test programme (Astill et al.,
1986), the BALB/3T3 cell transformation assay was used both with and
without rat primary hepatocytes. Both DEHP (0.875-1 µl/litre) and the
two metabolites MEHP and 2-ethylhexanol gave negative results. On the
other hand, the majority of transformation tests in the IPCS study
(Ashby et al., 1985) were positive for DEHP. Negative results were
obtained with BALB/c-3T3 cells, while a study measuring the
enhancement of viral transformation of Syrian hamster embryo (SHE)
cells was considered to be inconclusive. Positive responses were
obtained by four other investigators using SHE cells at 1-300 mg/litre
(two different laboratories), embryonic mouse fibroblasts at 1000
mg/litre with S9 and 10 mg/litre without S9, or retrovirus-infected
Fischer rat embryo cells at 2000 mg/litre (the highest dose tested).
In a separate study (Tomita et al., 1982a), both DEHP (7.5 and 15
g/kg) and MEHP (375 and 750 mg/kg) induced morphological
transformation, as well as chromosomal aberrations, in SHE cells after
transplacental administration.
In a study by Diwan et al. (1985), anchorage-independent growth
of JB6 mouse epidermal cells was enhanced by DEHP at concentrations of
500 to 20 000 ppm/ml (the Task Group noted that the unit used in
Diwan's paper is ppm/ml, but it should read ppm or mg/litre). Ward et
al. (1986) used the same model system and reported that both DEHP
(1.3-51 x 10-6 mol/litre) and MEHP (2-5 x 10-8 mol/litre) were
effective, while 2-ethylhexanol (4-77 x 10-7 mol/litre) was without
effect. The morphological transformation in the SHE cell system
induced by DEHP, MEHP, and other hepatic peroxisome proliferators
seemed not to be correlated with increased peroxisomal ß-oxidation,
increased production of oxidative radicals or peroxisome proliferation
(Mikalsen et al., 1990a; Mikalsen et al., 1990b; Mikalsen et al.,
1990c).
A few studies on DEHP-induced inhibition of metabolic
cooperation, which may be indicative of the promoting potential of a
substance, have been reported. Metabolic cooperation in Chinese
hamster V79 cells was not inhibited by DEHP at non-cytotoxic
concentrations, i.e. 3 x 10-4 mol/litre (0.12 mg/litre) or less
(Kornbrust et al., 1984). In the IPCS study (Ashby et al., 1985), one
investigator reported inhibition in V79 cells at non-cytotoxic
concentrations of DEHP (25-200 mg/litre in two separate experiments
and 5-25 mg/litre in another), while another investigator, using V79
cells in a microassay method, detected a slight (but non-significant)
increased inhibition at doses of between 10-5 and 2 x 10-4
mol/litre (3.9-78 mg/litre).
In a study by Malcolm & Mills (1989) on V79 cells, DEHP inhibited
intercellular communication (gap junctions) at non-cytotoxic
concentrations (10-30 mg/litre).
7.6.6 In vivo effects
Putman et al. (1982) dosed male Fischer-344 rats orally for 5
days with DEHP (5.0, 1.7, and 0.5 g/kg per day), MEHP (0.14, 0.05, and
0.01 g/kg per day), and 2-ethylhexyl (0.21, 0.07, and 0.02 g/kg per
day), and bone marrow metaphase cells were examined. No significant
increases were observed in gap breaks or structural rearrangements. In
addition, the mitotic index was unaffected by treatment.
A micronucleus assay on mouse (B6C3F1) peripheral blood
erythrocytes (intraperitoneal doses of 0.6, 3.0, and 6.0 g/kg per day
for 5 days), sampled at 0, 2, and 4 weeks after the last treatment,
yielded negative results (Douglas et al., 1986). A sperm morphology
assay in B6C3F1 mice and Sprague-Dawley rats at the same dose levels
also gave negative results (Douglas et al., 1986). However, Agarwal
et al. (1986b) reported increases in sperm morphology changes in adult
F-344 rats given 20 g DEHP/kg for 60 consecutive days.
Two studies have yielded negative results in dominant lethal
tests (Hamano et al., 1979; Rushbrook et al., 1982). Hamano et al.
(1979) administered MEHP and DEHP orally, and the observation period
was six weeks. Rushbrook et al. (1982) dosed ICR/SRM mice orally for
5 days with DEHP (2465, 4930, and 9860 mg/kg per day), MEHP (50, 100,
and 200 mg/kg per day) or 2-ethylhexanol (250, 500, and 1000 mg/kg per
day). Each male was mated weekly with virgin females for 8 consecutive
weeks, and females were evaluated for pregnancy, live fetuses, and
early and late fetal deaths. All data were within the normal ranges.
DEHP and its major metabolites have also been tested for their
potential to induce micronuclei. Exposure to 0.6, 3.0 or 6.0 g DEHP/kg
per day for 5 days over a 4-week period did not induce micronuclei in
the peripheral blood erythrocytes of B6C3F1 male mice (Douglas et
al., 1986). Negative results were also obtained in another mouse
micronucleus test after either a single or daily doses of 5 g DEHP/kg.
In this study MEHP and 2-ethylhexanol also gave negative results
(Astill et al., 1986).
7.7 Carcinogenicity
In a carcinogenicity study (NTP, 1982; Kluwe et al., 1982),
groups of 50 male and 50 female Fischer-344 rats were fed diets
containing 6 or 12 g DEHP/kg diet, and 50 male and 50 female B6C3F1
mice were fed diets containing 3 or 6 g DEHP/kg for 103 consecutive
weeks. Concurrent controls (50 of each sex and species) were fed a
diet without the addition of DEHP. Food and water were supplied
ad libitum. All animals were given the control diet for 1-2 weeks
after 103 weeks of treatment and were then killed and examined both
grossly and microscopically. The administered concentrations of DEHP
were estimated to be equal to, or one half of, the maximum tolerated
doses. Under the conditions studied, DEHP caused an increased
incidence of hepatocellular carcinomas in female rats and male and
female mice, and an increased incidence of hepatocellular carcinomas
and neoplastic nodules in female rats (Table 8). Twenty of the 57
hepatocellular carcinomas in the DEHP-treated mice (sexes and doses
combined) had metastasized to the lung. The figure of nine
hepatocellular carcinomas in control male mice is considered to be
within the normal range (NTP, 1982).
The reported decreased incidence of tumours of the thyroid,
pituitary, and testis could be related to an increased endocrine
activity of the pituitary gland (NTP, 1982).
The carcinogenicity of DEHP in rats has been confirmed in two
further studies. Rao et al. (1990) found a 78.5% incidence in a group
of 14 male Fischer-344 rats fed a diet containing 20 g DEHP/kg for up
to 108 weeks, whereas the incidence in the 10 controls was 10%. Popp
et al. (1987) found either hepatocellular carcinomas or neoplastic
nodules in 6 out of 20 animals after female Fischer-344 rats were
exposed for 2 years to a diet containing 12 g/kg.
Table 8. Carcinogenic effects of DEHP on the livera
Control Low dose High dose P valueb
Hepatocellular carcinoma
Male rats 1/50 1/49 5/49 < 0.05
Female rats 0/50 2/49 8/50 < 0.005
Male mice 9/50 14/48 19/50 < 0.05
Female mice 0/50 7/50 17/50 < 0.0001
Neoplastic nodules
Male rats 2/50 5/49 7/49 n.s.
Female rats 0/50 4/49 5/50 < 0.05
Hepatocellular adenoma
Male mice 6/50 11/48 10/50 n.s
Female mice 1/50 5/50 1/50 n.s
a From: NTP (1982)
b Probability level in Codiran-Armitage test for linear trend when
P < 0.05; otherwise not significant (n.s.)
Two other long-term studies on DEHP have been performed by
Carpenter et al. (1953) and Harris et al. (1956), but, due to the
small numbers of animals used, the studies are inadequate to assess
the carcinogenic potential.
DEHP has been investigated in two life-time studies on Syrian
hamsters (Schmezer et al., 1988). In one study, groups of 25 male and
25 female 6-week-old hamsters were assigned to each of six groups:
untreated control; 3 g DEHP/kg body weight given intraperitoneally
once every week for 18 weeks; the same dose once every two weeks for
18 weeks; the same dose once every four weeks for 32 weeks; the same
dose once every four weeks for 32 weeks plus N-dimethylnitrosamine
(NDMA) at 1.67 mg/kg body weight given orally once per week; and the
same dose of NDMA without DEHP treatment. NDMA increased the tumour
rate for malignant liver tumours, mainly haemangioendotheliomas.
Co-administration of DEHP with NDMA neither increased nor decreased
the tumour rates. No significant differences of tumour rates were
observed in groups treated with DEHP alone compared with controls.
Schmezer et al. (1988) also investigated the life-time, whole-
body exposure of Syrian hamsters to DEHP vapour alone in air or in
combination with orally administered NDMA. The low doses of DEHP
(15 µg/m3, resulting in a total exposure of about 7.5 µg/kg body
weight) in this study render it inadequate for the assessment of the
long-term effects of DEHP alone. In combination with NDMA, this low
level of DEHP was associated with highly significant (P < 0.001)
decreases in hepatic haemangioendothe-lioma and fibrosarcomas combined
in males and females combined. This unexpected result should be
further investigated to evaluate its significance.
7.8 Special studies
Since DEHP lacks genotoxic activity in most test systems, it has
been suggested that the carcinogenic effect is exerted during the
promotion phase of hepatocarcinogenicity. DEHP has therefore been
tested in several initiation/promotion experiments in rats and mice
where the end-point has been the number and/or volume of altered liver
cell foci. As expected, DEHP lacked initiating activity in these
studies (Ward et al., 1986; Popp et al., 1987).
DEHP appears to be a tumour promotor in mouse liver. In male
B6C3F1 mice given an intraperitoneal initiating dose of
diethylnitrosamine (80 mg/kg), DEHP at levels of 6 and 12 g/kg diet
caused accelerated growth of foci and increased incidence of
hepatocellular adenomas (Ward et al., 1983; Ward et al., 1986).
However, in a 6-month study, DEHP at a level of 12 g/kg diet did not
appear to promote altered foci in male F-344 rats given an
intraperitoneal initiating dose of diethylnitrosamine of 150 mg/kg
(Popp et al., 1987). In other promotion studies DEHP appeared to
accelerate the regression or inhibit the appearance of some kinds of
foci in rats (DeAngelo & Garrett, 1983; DeAngelo et al., 1984).
However, other studies have shown that peroxisome proliferators can
promote the development of altered foci and tumours in rat liver.
These results are somewhat different from those of other liver tumour
promotors with respect to the histological phenotype of the foci and
the relatively low frequency of the foci (Cattley & Popp, 1989). Thus,
further studies are required to demonstrate that DEHP can promote
altered foci in rat liver.
7.9 Mechanisms of hepatotoxicity
Together with a number of structurally diverse chemicals,
including certain hypolipidaemic drugs, herbicides, and chlorinated
solvents, DEHP has been shown to produce hepatic peroxisome
proliferation in rats and mice (Reddy & Lalwani, 1983; Lock et al.,
1989). This proliferation is accompanied by liver enlargement,
stimulation of replicative DNA synthesis and cell division, and the
induction of peroxisomal and microsomal fatty acid oxidizing enzyme
activities (Reddy & Lalwani, 1983; Marsman et al., 1988; Lock et al.,
1989). The increase in microsomal fatty acid oxidation is due to the
induction of a cytochrome P-450 IVA1 (Lock et al., 1989). Peroxisome
proliferation and induction of peroxisomal and microsomal fatty acid
oxidizing enzyme activities can be observed in primary hepatocyte
in vitro cultures, as well as in the intact animal (Elcombe &
Mitchell, 1986; Lake et al., 1986). Both in vivo and in vitro
studies with rat hepatocytes have demonstrated wide compound potency
differences (Reddy et al., 1986; Barber et al., 1987; Lake et al.,
1988). Compared to some other compounds, DEHP is not considered to be
a particularly potent peroxisome proliferator in rodent liver (Reddy
et al., 1986; Barber et al., 1987). Possible mechanisms of peroxisome
proliferation include a perturbation of hepatic lipid metabolism
and/or the presence of a receptor protein (Lalwani et al., 1987; Lock
et al., 1989; Issemann & Green, 1990).
Certain peroxisome proliferators, including DEHP, have been shown
to increase the incidence of liver tumours in rats and mice (Reddy &
Lalwani, 1983; Reddy et al., 1986; Butterworth et al., 1987).
Generally, there is a good correlation between the potency of a
compound to produce peroxisome proliferation in rat and mouse liver
and its hepatocarcinogenic properties (Reddy et al., 1986). However,
the magnitude of peroxisome induction does not necessarily always
correlate with the carcinogenic response when different compounds are
compared (Marsman et al., 1988). Since the peroxisome proliferators
known to date are non-genotoxic carcinogens, Reddy and coworkers
(Reddy & Lalwani, 1983; Rao & Reddy, 1987; Reddy & Rao, 1989) have
suggested that liver tumour formation arises from a sustained
oxidative stress to the hepatocytes due to an imbalance in the
production and degradation of hydrogen peroxide (H2O2). DEHP
administration results in increased peroxisomal production of H2O2
in hepatocytes (Tomaszewski et al., 1986). There is an imbalance in
H2O2 production and degradation due to the fact that catalase is
induced to a much lesser extent than peroxisomal ß-oxidation enzymes.
In addition, the level of cytosolic glutathione peroxidase is reduced
on prolonged exposure to DEHP (Lake et al., 1987; Marsman et al.,
1988; Conway et al., 1989b; Tamura et al., 1990). Treatment with
peroxisome proliferators also results in a reduction of superoxide
dismutase and glutathione S-transferase activities (Ciriolo et al.,
1982; Goel et al., 1986; Lake et al., 1987). It has been suggested
that the increased level of H2O2 in hepatocytes, either directly or
via other reactive oxygen species (e.g., hydroxyl radical), damages
intracellular membranes and/or DNA (Reddy & Rao, 1989). Indeed chronic
administration of DEHP and other peroxisome proliferators results in
increased lipid peroxidation and lipofuscin deposition in rat
hepatocytes (Mitchell et al., 1985b; Goel et al., 1986; Cattley et
al., 1987; Lake et al., 1987; Reddy & Rao, 1989; Conway et al.,
1989b). Oxygen radicals may damage DNA (Bridges, 1985), leading to
increased levels of 8-hydroxydeoxy-guanosine and other modified bases
in DNA (Reddy & Rao, 1989). Indeed, the administration of DEHP and
some other peroxisome proliferators has been shown to increase levels
of 8-hydroxyde-oxyguanosine in rat liver DNA (Kasai et al., 1989;
Takagi et al., 1989, 1990, 1991). Apart from a role in peroxisome
proliferation, leading to oxidative stress, recent studies on DEHP
have demonstrated a role in the stimulation of replicative DNA
synthesis in the hepatocarcinogenicity of peroxisome proliferators
(Butterworth et al., 1987; Smith-Oliver & Butterworth, 1987; Marsman
et al., 1988). Increased cellular division may result in spontaneous
mutational events or promotional effects (Bridges, 1985; Butterworth
et al., 1987), including the promotion of spontaneously initiated
cells found in the livers of aging rodents (Schulte-Hermann et al.,
1989). A comparative study of the hepatic effects of Wy-14643
([4-chloro-6 (2,3-xylidino) 2-pyrimidinylthio]acetic acid), a potent
peroxisome proliferator, and DEHP indicated that, although both
compounds produced a similar induction of peroxisome proliferation at
the dose levels administered, only Wy-14643 produced liver tumours
within one year (Marsman et al., 1988; Conway et al., 1989b). However,
analysis of these data demonstrates that Wy-14643 produces both a more
marked increase in lipofuscin deposition (presumably reflecting
oxidative stress) and a sustained stimulation of replicative DNA
synthesis. These data suggest a role for both oxidative stress and
increased cell replication in the hepatocarcinogenicity of DEHP and
other peroxisome proliferators.
The mechanism of hepatocarcinogenicity induced by peroxisome
proliferators could include both enhanced cell replication and DNA
damage induced by oxygen radicals. Thus, at dose levels that do not
produce either significant peroxisome proliferation or cell
replication, it is unlikely that DEHP or other peroxisome
proliferators would elicit hepatic tumour formation. In this context
data on threshold values for liver effects in sensitive species (e.g.,
the rat and mouse), together with an assessment of species differences
in response (see below), should be considered. The value for the
subchronic liver effects of DEHP (e.g., peroxisome proliferation and
DNA synthesis) in the rat appears to be around 50 mg/kg per day (Lake
et al., 1984, 1991; Mitchell et al., 1985b; Tomaszewski et al., 1986).
Furthermore, a two-year study of DEHP in female rats demonstrated no
effects at 0.3 g/kg diet, a value which corresponds well to that noted
above (Cattley et al., 1987; Popp et al., 1987).
Many studies have shown dramatic species differences in response
to peroxisome proliferators including DEHP (ECETOC, 1992). For
example, DEHP threshold values for peroxisome proliferation of 25 and
250 mg/kg, respectively, were found in rats and hamsters after 14 days
of daily gastric intubation (Lake et al., 1984). Several other studies
showing species differences in the response to DEHP have been
reported. One experiment demonstrated a large increase in hepatic
peroxisomes after 14 days of oral DEHP administration (2000 mg/kg per
day) to rats, but the same treatment of marmosets resulted in no
peroxisome proliferation (Rhodes et al., 1986). A limited study in
cynomolgus monkeys also revealed no evidence of peroxisome
proliferation due to DEHP (Short et al., 1987). In addition, DEHP did
not elicit peroxisome proliferation in guinea-pigs (Osumi & Hashimoto,
1978; Mitchell et al., 1985a). Some studies have indicated that
intrinsic species differences in hepatocellular sensitivity exist. For
example, MEHP and MEHP metabolite VI (mono(2-ethyl-5-oxohexyl)
phthalate), which is a proximate peroxisome proliferator in the rat,
were good peroxisome proliferators in cultured rat hepatocytes, but
had little or no effect in guinea-pig, marmoset or human hepatocytes
(Mitchell et al., 1985a; Elcombe & Mitchell, 1986). More recently,
Bichet et al. (1990) and Butterworth et al. (1989) have also
demonstrated the inability of MEHP to elicit peroxisome proliferation
in human hepatocyte cultures. Studies using a variety of other
peroxisome proliferators (e.g., clofibric acid, beclobric acid,
methylclofenapate, trichloroacetic acid, ciprofibrate, and
benzbromarone) have also failed to demonstrate peroxisome
proliferation in cultured human hepatocytes, in spite of a good
response in rat hepatocytes (Elcombe, 1985; Allen et al., 1987;
Elcombe & Styles, 1989; Butterworth et al., 1989; Bichet et al., 1990;
Blaauboer et al., 1990).
Species differences in cytochrome P-450 IVA1 induction and
stimulation of replicative DNA synthesis have been less well studied.
However, MEHP and metabolite VI did not induce P-450 IVA1-mediated
lauric acid hydroxylation in vivo in marmosets (Rhodes et al., 1986)
or in cultured marmoset or human hepato-cytes (Elcombe & Mitchell,
1986).
In conclusion, it appears that the livers of rats and mice are
exquisitely sensitive to peroxisome proliferators, including DEHP,
while those of guinea-pigs, monkeys, and humans show minimal or no
response (ECETOC, 1992).
8. EFFECTS ON HUMANS
8.1 General population exposure
Two adults who swallowed 5 or 10 g DEHP experienced no untoward
effects apart from mild gastric disturbances and moderate diarrhoea
with 10 g DEHP (Shaffer et al., 1945). Three cases of nonspecific
hepatitis were described among 27 haemodialysis patients with terminal
renal failure. The PVC blood tubing used released DEHP at a
concentration of 10-20 mg/litre of perfusate. The symptoms and signs
of hepatitis disappeared rapidly when the use of tubing that did not
contain DEHP was resumed (Neergaard et al., 1971).
In three pre-term infants artificially ventilated with PVC
respiratory tubes, unusual lung disorders (opacification of the lung)
were observed during the fourth week of life. It was assumed by the
authors that these lung disorders were causually related to the
exposure to DEHP released from the respiratory tubes (Roth et al.,
1988).
8.2 Occupational exposure
There are very few data on the effects of occupational exposure
specifically to DEHP.
Two studies reported symptoms and signs of polyneuropathy among
47 out of 147 workers at a PVC-processing plant in the USSR and 12 out
of 23 workers at a plant for phthalate production in Italy. The
workers were exposed to a mixture of phthalates and DEHP was a minor
constituent, at least in the USSR plant. Furthermore, tricresyl
phosphate (a neurotoxin) was a component of the incombustible
materials produced in 10-20% of machines assigned to various workers
(Milkov et al., 1973). In the Italian study there was no corresponding
unexposed control group and the authors concluded that no definite
conclusion could be drawn from the study because of the limited number
of workers examined (Gilioli et al., 1978). The total phthalate air
concentrations recorded varied between 1.7 and 66 mg/m3 in the USSR
and 1 and 60 mg/m3 in Italy (Milkov et al., 1973; Gilioli et al.,
1978).
In a study involving a Swedish PVC-processing factory, peripheral
nervous system symptoms and signs were investigated among 54 male
workers exposed mainly to DEHP, diisodecylphthalate, and some
butylbenzylphthalate. The workers were divided into three groups of
approximately equal size and with mean phthalate exposures of 0.1,
0.2, and 0.7 mg/m3. Some workers displayed various peripheral
nervous system symptoms and signs, but these were not related to the
level of exposure. None of the workers reported symptoms indicating
work-related obstructive lung disease. Conventional lung function
tests also showed no association with exposure (Nielsen et al., 1985).
However, several biochemical parameters showed significant
associations with exposure. There was a slight decrease in the
haemoglobin level with longevity of employment as well as with
exposure in the last year. The serum ý-1-antitrypsin level increased
slightly with length of employment and the serum immunoglobulin A
level rose with rising exposure during the last year (Nielsen et al.,
1985).
One case of occupational asthma due to DEHP was reported in
worker at a PVC-processing plant (Brunetti & Moscato, 1984).
Diagnosis was made by exposing the patient to DEHP vapour in an
inhalation chamber; this evoked a dual asthmatic reaction. The action
was inhibited by prior administration of sodium chromoglycate.
A study of blood lipids, serum activities of liver enzymes, and
routine haematological tests was carried out among workers at a German
plant for DEHP production. The results were negative and uninformative
due to very low exposure levels (below 0.16 mg/m3) and the lack of
a control group (Thiess et al., 1978b). Thiess & Flieg (1978)
investigated the frequency of chromosome aberrations in 10 workers who
were employed from 10 to 30 years in this DEHP production plant. The
exposure levels were very low (0.09-0.16 mg/m3), and there was no
increase in chromosome aberrations compared to the control group. A
mortality study of 221 workers exposed to DEHP in the same plant was
also conducted. Eight deaths occurred in the cohort compared with
expected values of 15.9 and 17.0 from the city and county data,
respectively (Thiess et al., 1978a).
9. EFFECTS ON OTHER ORGANISMS IN THE LABORATORY AND FIELD
9.1 Toxicity to microorganisms
Bringmann & Kühn (1980) calculated toxic thresholds from cell
multiplication inhibition tests and found thresholds of > 400
mg/litre for the bacterium Pseudomonas putida, 10 mg/litre for the
alga Scenedesmus quadricaudaia, and 19 mg/litre for the protozoan
Entosiphon sulcatum.
Mathur (1974b) reported that DEHP added to soil (3 g/kg)
inhibited respiration, whereas addition of the same amount of DEHP to
soil preincubated for 14 weeks with DEHP or dioctyl phthalate had no
effect on respiration rate. The experiments were conducted at 22 °C
since no degradation of DEHP occurred at 4 °C or 10 °C. In an
intermittent flow-through hydrosoil microcosm, DEHP (at 1 and 100
mg/litre) produced no significant effect on the numbers or
physiological activity of the microflora monitored, nor on any of the
overall activities that were studied (Mutz & Jones 1977).
Larsson et al. (1986) studied the effect of DEHP on the microbial
activity in sediments. Sediment cores were taken, together with the
overlying water, from a eutrophic lake and the sediment was spiked
with between 25 and 400 mg DEHP/kg at a level of 5 mm below the
surface. Uncontaminated sediment samples had a significantly higher
oxygen uptake than sediment containing DEHP. Microbial activity, as
estimated from decreased oxygen saturation in the soil column,
correlated inversely with increasing levels of DEHP in the sediment.
9.2 Toxicity to aquatic organisms
Many of the toxicity values given in this section are above the
water solubility of DEHP, which ranges from 0.3 to 0.4 mg/litre (see
section 2.2). DEHP adsorbs strongly to sediment, food, dissolved
organic carbon, and even the testing vessels. Many of the toxicity
tests are also based on nominal concentrations. Therefore, the actual
exposure of the organisms is very difficult to determine and care must
be taken when interpreting the results.
9.2.1 Invertebrates
Acute toxicity to aquatic invertebrates is summarized in Table 9.
Table 9. Toxicity of DEHP to aquatic invertebratesa
Organism Age Temperature Hardnessb pH Duration LC50 Reference
(° C) (h) (mg/litre)d
Water flea < 24 h 17 48 0.133 Passino & Smith (1987)
(Daphnia pulex)
Water flea < 24 h 21-23 173 7.4-9.4 24 > 68 Leblanc (1980)
(Daphnia magna) < 24 h 21-23 173 7.4-9.4 48 11 Leblanc (1980)
Crayfish 96 > 10 Mayer & Sanders (1973)
(Orconectes nais)
Harpacticoid adult 20-22 7c 7.8 96 > 300 Linden et al. (1979)
(Nitocra spinipes)
Scud 96 > 32 Sanders et al. (1973)
(Gammarus pseudolimnaeus)
Midge larvae 21-23 270 7.4 48 > 18 Streufert et al. (1980)
(Chironomus plumosus)
a All experiments were performed using static conditions (water unchanged for duration of test)
b Hardness is expressed as mg calcium carbonate per litre. The tests were performed in fresh water
except where stated otherwise
c Salinity (%)
d Nominal values
Brown & Thompson (1982a) found no mortality of Daphnia magna
over an exposure period of 48 h and at DEHP dose levels up to 320
µg/litre. However, at levels of 180 µg/litre or more, the daphnids
were seen to float in the surface layer. The authors suggested that
this observation was connected with the solubility of DEHP (which
tends to precipitate out at > 180 µg/litre, possibly onto the
daphnids, thereby causing them to float). Stephenson (1983) found no
mortality in Gammarus pulex exposed for up to 120 h to a DEHP
concentration (400 µg/litre) in excess of its solubility.
Laughlin et al. (1978) exposed grass shrimps (Palaemonetes
pugio) to DEHP concentrations of up to 1 mg/litre (well above the
solubility limit of the ester) and found no significant difference in
mortality between the control and treated shrimps during the 28-day
exposure period. DEHP had no significant effect on the development
rate of larvae from hatching to moult. No apparent effects were noted
when mussels (Mytilus edulis) were exposed to 50 µg/litre for 28
days (Brown & Thompson, 1982b).
Hobson et al. (1984) found no significant mortality of penaeid
shrimps (Penaeus vannamei) fed a diet containing up to 50 g DEHP/kg
for 14 days, this representing 4% of the body weight per day.
Histological examination revealed no changes and no significant dose-
related effects were observed on moulting.
Sanders et al. (1973) and Mayer & Sanders (1973) exposed Daphnia
magna for a complete life cycle (21 days) to 3, 10 or 30 µg
DEHP/litre in an intermittent-flow system. Reproduction was
significantly inhibited at all concentrations (60, 70, and 80%
inhibition at the three dose levels, respectively). In contrast, Brown
& Thompson (1982a) found no effect on the reproduction of Daphnia
magna at concentrations of up to 100 µg/litre and suggested that the
difference in the numbers of young born to the controls in the two
studies, i.e. 11 offspring per parent (Mayer & Sanders, 1973) compared
with 170 per parent (Brown & Thompson, 1982a), could account for these
conflicting results.
When Knowles et al. (1987) exposed Daphnia magna to DEHP
concentrations of between 12 and 811 µg/litre for up to 21 days under
flow-through conditions (the reproduction rate was approximately 200
offspring per adult), survival was not affected at levels of < 158
µg/litre. At 811 µg/litre, however, survival was significantly reduced
after both 7 and 21 days. The mean number of young per surviving adult
was also reduced at this dose level. The levels of major biochemical
components such as protein, RNA, DNA, and glycogen were all
significantly reduced after 7 days at 811 µg/litre, but all except
glycogen were unaffected after 21 days. The authors concluded that the
maximum acceptable toxicant concentration (MATC), based on survival
and reproduction, was between 158 and 811 µg/litre. A no-observed-
effect level (NOEL) of 72 µg/litre was identified both by DNA content
and RNA/DNA ratio at day 7 and by surfacing of Daphnia at day 0.
Thuren & Woin (1991) exposed the freshwater amphipod Gammarus
pulex to DEHP at concentrations of 100 or 500 µg/litre for 10 days
under flow-through conditions. There was a 5 day pre- and post-
exposure period. The overall locomotor activity of G. pulex was
significantly decreased at the higher exposure level, and the effect
persisted throughout the post-exposure period. No significant effects
were observed at the lower dose level.
Flow-through chronic toxicity tests in both sand and hydrosoil
showed that DEHP concentrations as high as 360 µg/litre (sand) and 240
µg/litre (hydrosoil) had no significant effects on the growth or
development of midge larvae (Chironomus plumosus) over a 35-day
period. The continuous exposure of first-generation midge eggs in sand
substrate to mean DEHP concentrations of between 140 and 360 µg/litre
had no significant effect on hatchability (Streufert et al., 1980).
Woin & Larsson (1987) found that dragonfly larvae ( Aeshna sp.)
caught significantly fewer prey (Chaborus larvae) per attempt when
exposed to sediment DEHP concentrations of approximately 600 mg/kg for
between 3 and 9 weeks.
9.2.2 Fish
The acute toxicity to fish is summarized in Table 10 and the
toxicity to the embryo-larval stages of fish is shown in Table 11.
DEHP caused no deaths among one-year-old rainbow trout (Salmo
gairdneri) exposed to between 1 and 1000 mg/litre for 48 h (Silvo,
1974) or juvenile Atlantic salmon (Salmo salar) exposed to 100
mg/litre for 96 h (Zitko, 1972).
Mehrle & Mayer (1976) reported no effects on growth or survival
of adult fathead minnows (Pimephales promelas) exposed to between 1
and 62 µg/litre for 56 days. They also exposed rainbow trout eggs to
DEHP levels of 5, 14, and 54 µg/litre for 12 days prior to hatching,
but there was no significant effect on hatchability. The resulting fry
were continuously exposed to DEHP for a further 90 days. The two
highest concentrations caused a significant, but not dose related,
increase in the mortality of sac fry within 5 days of hatching. After
yolk absorption (at 24 days), DEHP caused no significant mortality or
effects on growth and development for the remainder of the exposure
period.
Table 10. Toxicity (96-h LC50 values) of DEHP to fish
Organism Size Stat/ Temperature Hardnessb pH LC50 Reference
flowa (° C) (mg/litre)c
Bluegill sunfish > 10 Mayer & Sanders (1973)
(Lepomis macrochirus) 0.32-1.2 g stat 21-23 32-48 6.7-7.8 > 770 Buccafusco et al. (1981)
Fathead minnow > 10 Mayer & Sanders (1973)
(Pimephales promelas) 0.055-0.25 g 24-26 44-46 7-8 > 0.33d Defoe et al. (1990)
Channel catfish > 10 Mayer & Sanders (1973)
(Ictalurus punctatus)
Rainbow trout fingerling stat 14-16 540 Hrudey et al. (1976)
(Oncorhynchus mykiss)
> 10 Mayer & Sanders (1973)
a Stat = static conditions (water unchanged for duration of test)
b Hardness is expressed as mg calcium carbonate per litre. The tests were performed in fresh water
except where stated otherwise
c Nominal values unless stated otherwise
d Measured value
Table 11. Toxicity of DEHP to the embryo-larval stages of fisha
Organism Stat/flowb Hardnessc pH LC50 (mg/litre) 95% confidence limits
Channel catfish stat 90-115 7.6-8.1 0.69 0.55-0.86
(Ictalurus punctatus)
Redear sunfish stat 90-115 7.6-8.1 6.18 4.65-8.04
(Lepomis microlophus)
Largemouth bass flow 45-55 7.5-8.0 42.1 28.8-77.8
(Micropterus salmoides)
flow 190-225 7.5-8.0 32.9 19.8-58.9
Rainbow trout flow 45-55 7.5-8.0 139.5 123.2-165.2
(Oncorhynchus mykiss)
flow 190-225 7.5-8.0 149.2 125.8-203.8
a From: Birge et al. (1978). Exposure was initiated 2 to 6 h after spawning (except in the case of rainbow trout where exposure
was initiated 15 min after fertilisation) and continued until 4 days after hatching. The hatching times were 22 days for rainbow
trout (13.5-14.3 °C), 3 days for catfish (29-31 °C), and 3-4 days for bass and sunfish (20-24 °C).
b Stat = static conditions, but water renewed every 12 h; flow = flow-through conditions (DEHP concentration in water continuously
maintained)
c Hardness is expressed as mg calcium carbonate per litre. The tests were performed in fresh water except where stated otherwise.
DeFoe et al. (1990) exposed rainbow trout (Oncorhynchus mykiss)
and Japanese medaka (Oryzias latipes) to DEHP using concentrations
of up to 0.502 mg/litre for a 90-day trout embryo-larval test and
0.554 mg/litre for a 168-day larval test on medaka. No significant
adverse effects were observed on trout hatchability, survival or
growth, but there was a significant reduction in the growth of
Japanese medaka.
Mayer & Sanders (1973) studied the effects of DEHP on the
reproduction of zebra fish (Brachydanio rerio) and guppies
(Lebistes reticulatus). During the 90-day dietary exposure, zebra
fish were fed on 50 or 100 mg DEHP/kg food, and guppies were fed 100
mg/kg. Although the treated zebra fish spawned more frequently than
the control fish, the latter produced more eggs per spawn. Fry
survival was significantly reduced by DEHP. Treated guppies produced
fewer fry per adult and had an 8% incidence of abortions, whereas
there were no abortions in the control group (no statistics were
given).
In a study of fish exposed to DEHP, Mayer et al. (1977) found
reduced vertebral collagen levels after 150 days at a DEHP
concentration of 3.7 µg/litre in adult brook trout (Salvelinus
fontinalis), after 127 days at 11 µg/litre in fathead minnow, and
after 90 days at 5 µg/litre in rainbow trout. However, there was no
effect on fish growth. Pfuderer & Francis (1975) found that DEHP had
no effect on the heart rate of goldfish when the fish were exposed to
200 mg/litre for 10 min.
9.2.3 Amphibians
The acute toxicity of DEHP to tadpoles of Fowler's toad and the
leopard frog is given in Table 12.
When Larsson & Thuren (1987) exposed eggs of the moorfrog (Rana
arvalis) to sediment concentrations of between 10 and 800 mg DEHP/kg
(fresh weight), the number of tadpoles hatching decreased with
increasing exposure concentration. The percentage hatch was 90% for
the controls eggs, 50% at 150 mg DEHP/kg, and < 30% at > 400 mg/kg.
The lowest DEHP concentration that caused a significant decrease in
the number of tadpoles hatching was 25 mg/kg. After hatching, the
survival of tadpoles was unaffected. There were no delays in hatching
and no abnormalities in the developing tadpoles exposed to the various
DEHP concentrations.
Table 12. Toxicity of DEHP to frog and toad tadpolesa
Organism LC50 (mg/litre) 95% confidence limits
Fowler's toad 3.88 3.08-4.84
(Bufo fowleri)
Leopard frog 4.44 3.65-5.37
(Rana pipiens)
a From: Birge et al. (1978). Exposure occurred under static
conditions, but water was renewed every 12 h. It was initiated 2
to 6 h after spawning and continued until 4 days after hatching.
The hatching times varied between 3 and 4 days, and so the
exposure period varied between 7 and 8 days. The temperature was
20-24 °C, hardness 90-115 mg CaCO3/litre, and pH 7.6-8.1.
Wams (1987) quoted an unpublished Dutch report which stated that
an exposure of 2 mg DEHP/litre caused a reduction in the growth rate
of clawed toad (Xenopus laevis) larvae.
9.3 Toxicity to terrestrial organisms
9.3.1 Plants
When Herring & Bering (1988) grew spinach and pea plants from
seed for 14 to 16 days in soil containing 100 mg DEHP/kg, there was no
effect on plant growth, as measured by height. The effect of DEHP on
seed germination was observed by placing seeds in petri dishes
containing a solution of 100 mg/litre. A reduction of 40% to 50% in
the number of seeds germinating was found.
Lokke & Rasmussen (1983) reported that DEHP had no visible effect
on Sinapis alba, Brassica napusor or Achillea millefolium when
sprayed in the field at concentrations of up to 87.5 kg/ha. According
to Stanley & Tapp (1982), DEHP at a level of 1000 mg/kg soil had no
effect on the growth of rape (Brassica rapa) and only a slight
effect on that of oats (Avena sativa).
9.3.2 Earthworms
In a series of contact toxicity tests, red earthworms (Eisenia
foetida) were exposed to DEHP via filter paper in glass vials. An
LD50 could not be calculated because DEHP was not toxic even at the
highest dose of 25 mg/cm2 (Neuhauser et al., 1986).
9.3.3 Insects
Al-Badry & Knowles (1980) found DEHP to be non-toxic to female
houseflies (Musca domestica) when applied topically or by injection
at a concentration of 20 µg/fly (equivalent to 1000 mg/kg). When it
was topically applied simultaneously with various organophosphates, an
antagonistic interaction was apparent; mortality of 85% to 95% was
reduced to less than 10% by the DEHP. However, when DEHP was topically
applied 30 min before exposure to an organophosphate concentration
that caused 10% to 30% mortality, the resulting interaction was
synergistic with mortality rising to over 60% and in most cases to
over 80%.
9.3.4 Birds
Hill et al. (1975) found no mortality among 10-day-old ring-
necked pheasants or mallard fed up to 5000 mg DEHP/kg for 5 days
followed by 3 days on a normal diet.
When Wood & Bitman (1984) fed broiler hens a diet containing 2000
mg DEHP/kg (226 mg DEHP/hen per day) for 4 weeks, egg production and
body weights were significantly decreased. Ishida et al. (1982)
reported the cessation of egg production and an abnormality of the
ovaries in laying hens fed 5 or 10 g DEHP/kg diet for up to 230 days.
In a study by O'Shea & Stafford (1980), starlings (Sturnus
vulgaris) were fed on a diet containing 25 or 250 mg DEHP/kg for a
30-day period. At both dose levels, the treated birds gained
significantly more body weight than controls during the exposure
period, but at the lower dose level food consumption was significantly
reduced compared to controls.
When Peakall (1974) fed ring doves (Streptopelia risoria) on a
diet containing 10 mg/kg, there were no effects on the eggshell
thickness, ashed egg weight, rate of water loss, surface area or
permeability of the eggs laid.
10. EVALUATION OF HUMAN HEALTH RISKS AND EFFECTS ON THE ENVIRONMENT
10.1 Evaluation of human health risks
10.1.1 Exposure levels
DEHP concentrations of up to 300 ng/m3 have been measured in
urban air, but usually the levels in ambient air are well below 100
ng/m3. Data on occupational exposure levels are limited.
Concentrations of up to 4.1 mg/m3 have been reported but they are
usually below 1 mg/m3. Total phthalate exposure levels have been
reported to be between 0.1 and 60 mg/m3. Clinical treatment,
including transfusion, haemodialysis, extracorporal circulation, and
artificial respiration, may lead to DEHP exposure.
The exposure to DEHP from drinking-water and food is low.
10.1.2 Toxic effects
Dose-dependent kinetics of DEHP or its metabolites and species
differences in the metabolism have been confirmed in several studies.
In animals some inhalation studies have been performed. However,
no consistent findings are reported.
The oral and intraperitoneal LD50 values exceed 25 g/kg,
indicating that DEHP has low acute toxicity.
In rats and mice, DEHP administration produces hepatic
hyperplasia and hypertrophy, which is characterized by peroxisome
proliferation. The threshold dose level for hepatic peroxisome
proliferation in the rat is approximately 50 mg/kg per day. High DEHP
dose levels (12 g DEHP/kg diet in rats; 6 g DEHP/kg diet in mice) in
a feeding study resulted in an increased incidence of hepatic tumours
in rats and mice.
Marked species differences in DEHP-induced hepatomegaly and
peroxisome proliferation exist. Rats and mice are very responsive,
Syrian hamsters less responsive, and guinea-pigs, marmosets, and
cynomolgus monkeys non-responsive. Studies with primary hepatocyte
cultures have shown an excellent in vitro/in vivo correlation
concerning responsiveness to DEHP/DEHP metabolites. Several studies
have demonstrated the non-responsive nature of cultured human
hepatocytes to DEHP metabolites and other peroxisome proliferators.
The induction of peroxisome proliferation and cell replication is
strongly associated with the development of hepatic tumours in rats
and mice. Thus, the available data suggest that species which do not
respond to the hepatic effects of DEHP are unlikely to be susceptible
to the development of hepatic tumours.
Testicular atrophy is one of the most consistent effects of DEHP
in in vivo studies on a variety of experimental animal species. Such
effects have been observed in rats fed 6 g DEHP/kg diet and mice fed
3 g/kg diet, and they are more pronounced in young animals. Hamsters
and marmosets appear to be more resistant to the testicular effects of
DEHP.
When both male and female CD-1 mice were treated with 1 g DEHP/kg
diet, a significant reduction in fertility was observed.
A dietary level of 20 g DEHP/kg throughout gestation produced an
increased incidence of resorptions in rats but no malformations. In
the mouse, however, 1 g/kg throughout pregnancy increased the
incidence of embryolethality and abnormalities. Sensitivity was
greatest on days 7-9 of gestation. The dose level (0.5 g/kg) that
induced fetotoxicity in mice did not induce maternal toxicity. Results
from several different genotoxicity tests indicate that DEHP and its
major metabolites do not exhibit any direct genotoxic effect in either
bacteria or in vitro mammalian cells. This has been confirmed in
in vivo binding studies, which indicated that DEHP and its
metabolites do not interact covalently with DNA. However, it has been
established that DEHP has the potential of inducing aneuploidy in
fungi as well as in in vitro mammalian cells. There are no
consistent results from dominant lethal studies.
Few data on the human health effects of DEHP exposure have been
reported. There have been a few studies on workers exposed to
phthalate mixtures, but no consistent health effects that could be
directly related to DEHP have been reported.
10.1.3 Conclusion
DEHP causes reproductive and hepatocarcinogenic effects in rats
and mice.
Testicular atrophy is the main reproductive effect in rats and
mice, and young animals are more susceptible than older ones to this
effect. The induction of hepatic peroxisome proliferation and cell
replication are strongly associated with the liver carcinogenic effect
of certain non-genotoxic carcinogens including DEHP. However, marked
differences have been observed among animal species with respect to
DEHP-induced peroxisome proliferation. Currently there is not
sufficient evidence to suggest that DEHP is a potential human
carcinogen.
10.2 Evaluation of effects on the environment
10.2.1 Exposure levels
DEHP exists widely in the environment, being released during
production, processing, usage, and disposal. Transport in the air is
the major route by which it enters the environment. It can be
deposited by dry or wet deposition and has been found to leach into
ground water.
DEHP is readily photodegraded in the atmosphere. In the
laboratory, aerobic biodegradation has been shown to occur under
certain circumstances. However, in the environment aerobic
biodegradation seems to be slow and anaerobic degradation is even
slower.
Based on a measured log Pow of about 5 and measured
bioaccumulation factors for biota and sediment, it can be seen that
DEHP is highly bioaccumulative.
In rivers and lakes, DEHP concentrations of up to 4 µg/litre have
been measured.
Due to its hydrophobic nature, DEHP adsorbs readily to soil,
sediment, and particulate matter. Levels of up to 70 mg/kg dry weight
have been found in river sediments, but near to a discharge point
levels of up to 1480 mg/kg dry weight have been reported.
10.2.2 Toxic effects
Most acute toxicity studies on aquatic organisms show DEHP to be
of low toxicity. However, one study suggested a greater sensitivity
for the water flea (Daphnia pulex), the nominal 48-h LC50 being
133 µg/litre.
In a 21-day study on Daphnia magna, the NOEL for biochemical
and behavioural effects was 72 µg/litre. A significant increase in
mortality was recorded in trout fry exposed to 14 µg DEHP/litre from
12 days prior to hatching. At concentrations of 3.7 to 11 µg/litre,
reductions in vertebral collagen were reported in three species of
fish exposed for 150 days.
Zebra fish fry survival and guppy reproduction were adversely
affected at DEHP concentrations in food of 50 and 100 mg/kg,
respectively, during a 90-day exposure period.
In two separate studies, DEHP concentrations of 25 mg/kg
(wet weight) of sediment significantly reduced microbial activity and
the number of frog tadpoles hatching.
Available studies indicate that the acute toxicity of DEHP to
algae, plants, earthworms, and birds is low. There are no data
relating to effects upon wild mammals.
10.2.3 Conclusion
There is no documented information that DEHP presents any hazard,
based on acute exposure to fish and daphnids. However, a reduction of
microbial activity in sediment at environmental levels of DEHP was
reported. A comparison between environmental levels and the
concentrations that produce effects in prolonged studies, especially
early life-stage tests on fish and amphibians, indicates that a hazard
for the environment, particularly via water and sediment, cannot be
excluded. Adverse effects on organisms are likely in areas with highly
contaminated water and sediments which are near to point emission
sources.
Although few relevant studies have been reported, the acute
toxicity of DEHP to algae, plants, earthworms, and birds appears to be
low.
11. RECOMMENDATIONS FOR PROTECTION OF HUMAN HEALTH AND THE
ENVIRONMENT
a) Current disposal practises should be improved.
b) Measures must be undertaken to reduce the release of DEHP to the
environment.
c) Medical devices and products that contribute to the body burden
of DEHP must be scrutinized to reduce exposure to DEHP via the
intravenous route.
12. FURTHER RESEARCH
More research is needed on the effects of DEHP on the ecosystem
of sediments and on the subject of DEHP leaching, particularly from
landfill to ground water.
Since DEHP occurs in the air, including workplace air, further
inhalation studies are needed.
Epidemiological surveys on exposed populations should be
encouraged. Further studies are needed on the mechanism of peroxisome-
proliferator-induced hepatocarcinogenicity, with emphasis on human
health effects.
13. PREVIOUS EVALUATIONS BY INTERNATIONAL BODIES
DEHP was one of the substances discussed by a working group of
the International Agency for Research on Cancer in 1981 (IARC, 1982).
The evaluation by the group was that there was sufficient evidence for
the carcinogenicity of DEHP in mice and rats, but that no adequate
epidemiological study was available. The working group concluded that
DEHP is possibly carcinogenic to humans and placed it in group 2B
(IARC, 1987).
DEHP was evaluated at the 28th meeting of the Joint FAO/WHO
Expert Committee on Food Additives. The recommendation was to reduce
human exposure to DEHP from food-contact materials to the lowest level
technologically attainable (WHO/FAO, 1984a,b). During the 33rd meeting
of the Joint FAO/WHO Expert Committee on Food Additives, DEHP was
again evaluated and the recommendation was the same: DEHP in food
should be reduced to the lowest level attainable. The Committee
considered that this might be achieved by using alternative
plasticizers or alternatives to plastic material containing DEHP
(WHO/FAO, 1989a,b).
REFERENCES
AGARWAL, D.K. (1986) Letters to the editor. Toxicol. appl. Pharmacol.,
82: 383-387.
AGARWAL, D.K., AGARWAL, S., & SETH, P.K. (1982) Effect of di-(2-
ethylhexyl) phthalate on drug metabolism, lipid peroxidation, and
sulfhydryl content of rat liver. Drug Metab. Disp., 10: 77-80.
AGARWAL, D.K., LAWRENCE, W.H., & AUTIAN, J. (1985a) Antifertility and
mutagenic effects in mice from parenteral administration of di-2-
ethylhexyl phthalate (DEHP). J. Toxicol. environ. Health, 16: 71-84.
AGARWAL, D.K., LAWRENCE, W.H., NUNEZ, L.J., & AUTIAN, J. (1985b)
Mutagenicity evaluation of phthalic acid esters and metabolites in
Salmonella typhimurium cultures. J. Toxicol. environ. Health, 16:
61-69.
AGARWAL, D.K., EUSTIS, S., LAMB J.C., IV, JAMESON, C.W., & KLUWE, W.M.
(1986a) Influence of dietary zinc on di(2-ethylhexyl)phthalate-induced
testicular atrophy and zinc depletion in adult rats. Toxicol. appl.
Pharmacol., 84: 12-24.
AGARWAL, D.K., EUSTIS, S., LAMB J.C., IV, REEL, J.R., & KLUWE, W.M.
(1986b) Effects of di(2-ethylhexyl)phthalate on the gonadal patho-
physiology, sperm morphology, and reproductive performance of male
rats. Environ. Health Perspect., 65: 343-350.
AGARWAL, D.K., LAWRENCE, W.H., TURNER, J.E., & AUTIAN, J. (1989)
Effects of parenteral di(2-ethylhexyl)phthalate (DEHP) on gonadal
biochemistry, pathology and reproductive performance of mice. J.
Toxicol. environ. Health, 26: 39-59.
AHMED, R.S., PRICE, S.C., GRASSO, P., & HINTON, R.H. (1990) Hepatic
nuclear and cytoplasmic effects following intermittent feeding of rats
with di(2-ethylhexyl)phthalate. Food chem. Toxicol., 28: 427-434.
AL-BADRY, M. & KNOWLES, C.O. (1980) Phthalate-organophosphate
interactions: Toxicity, penetration, and metabolism studies with house
flies. Arch. environ. Contam. Toxicol., 9: 147-161.
ALBRO, P.W. (1986) Absorption, metabolism, and excretion of di(2-
ethylhexyl) phthalate by rats and mice. Environ. Health Perspect., 65:
293-298.
ALBRO, P.W. (1987) The biochemical toxicology of di-(2-ethylhexyl) and
related phthalates: testicular atrophy and hepatocarcinogenesis. In:
Hodgson, E., Bend, J.R., & Philpot, R.M., ed. Reviews in biochemical
toxicology, Amsterdam, Oxford, New York, Elsevier Science Publishers,
Vol. 8, pp. 73-119.
ALBRO, P.W. & LAVENHAR, S.R. (1989) Metabolism of di(2-ethylhexyl)
phthalate. Drug Metabol. Rev., 21: 13-34.
ALBRO, P.W. & THOMAS, R.O. (1973) Enzymatic hydrolysis of di(2-
ethylhexyl) phthalate by lipases. Biochim. Biophys Actaw, 360:
380-390.
ALBRO, P.W., THOMAS, R., & FISHBEIN, L. (1973) Metabolism of
diethylhexyl phthalate by rats. Isolation and characterization of the
urinary metabolites. J. Chromatogr., 76: 321-330.
ALBRO, P.W., HAAS, J.R., PECK, C.C., ODOM, D.G., CORBETT, J.T.,
BAILEY, F.J., BLATT, H.E., & BARRETT, B.B. (1981) Identification of
the metabolites of di-(2-ethyl-hexyl) phthalate in urine from the
African green monkey, Drug Met. Disp., 9: 223-225.
ALBRO, P.W., CORBETT, J.T., SCHROEDER, J.L., JORDAN, S., & MATTHEWS,
H.B. (1982) Pharmacokinetics, interactions with macromolecules and
species differences in metabolism of DEHP. Environ. Health Perspect.,
45: 19-25.
ALBRO, P.W., CHAPIN, R.E., CORBETT, J.T., SCHROEDER, J., & PHELPS,
J.L. (1989) Mono-2-ethylhexylphthalate, a metabolite of di-(2-
ethylhexyl)phthalate, causally linked to testicular atrophy in rats.
Toxicol. appl. Pharmacol., 100: 193-200.
ALLEN, K.L., GREEN, C.E., & TYSON, C.A. (1987) Comparative studies of
peroxisomal enzyme induction in hepatocytes from rat, cynomolgus
monkey and human by hypolipidemic drugs. Toxicologist, 7: 63.
AL-OMRAN, L.A. & PRESTON, M.R. (1987) The interactions of phthalate
esters with suspended particulate material in fresh and marine waters.
Environ. Pollut., 46: 177-186.
ANTONYUK, O.K. (1975) Toxicology of complex ethers of phthalic acid (A
review of literature), Hyg. Labour occup. Dis., 1: 32-35 (in Russian).
ARANDA, J.M., O'CONNOR, G.A., & EICEMAN, G.A. (1989) Effects of sewage
sludge on di-(2-ethylhexyl) phthalate uptake by plants J. environ.
Qual., 18: 45-50.
ARBIN, A. & ÖSTELIUS, J. (1980) Determination by electron-capture gas
chromatography of mono- and di(2-ethylhexyl) phthalate in intravenous
solutions stored in polyvinyl chloride bags. J. Chromatogr., 193:
405-412.
ASHBY, J., DE SERRES, F.J., DRAPER, M., ISHIDATE, M., Jr, MARGOLIN,
B.H., MATTER, B.E., & SHELBY, M.D., ed. (1985) Evaluation of short-
term tests for carcinogens. Report of the International Programme on
Chemical Safety's collaborative study on in vitro assays, Amsterdam,
Oxford, New York, Elsevier Science Publishers, 752 pp (Progress in
Mutation Research, Vol. 5).
ASTILL, B. (1989) Metabolism of DEHP: Effects of prefeeding and dose
variation, and comparative studies in rodents and the cynomolgus
monkey (CMA studies). Drug Metab. Rev., 21: 35-53.
ASTILL, B., BARBER, E., LINGTON, A., MORAN, E., MULHOLLAND, A.,
ROBINSON, E., & SCHEIDER, B. (1986) Chemical industry voluntary test
program for phthalate esters: Health effect studies. Environ. Health
Perspect., 65: 329-336.
ATLAS, E. & GIAM, C.S. (1981) Global transport of organic pollutants:
Ambient concentrations in the remote marine atmosphere. Science, 211:
163-165.
ATSDR (1988) Toxicological profile for di(2-ethylhexyl) phthalate,
Atlanta, Georgia, Agency for Toxic Substances and Disease Registry, US
Public Health Service (Draft for public comment).
BALLY, M.B., OPHEIM, D.J., & SHERTZER, H.G. (1980) Di-(2-ethylhexyl)
phthalate enhances the release of lysosomal enzymes from alveolar
macrophages during phagocytosis. Toxicology, 18: 49-60.
BARBER, E.D., ASTILL, B.D., MORAN, E.J., SCHNEIDER, B.F., GRAY,
T.J.B., LAKE, B.G., & EVANS, J.G. (1987) Peroxisome induction studies
on seven phthalate esters. Toxicol. ind. Health, 3: 7-24.
BARNEY, G.H., ORGEBIN-CRIST, M.C., & MACAPINLAC, M.P. (1969) Genesis
of esophageal parakeratosis and histologic changes in the testes of
the zinc-deficient rat and their reversal by zinc repletion. J. Nutr.,
95: 526-534.
BAUGHMAN, G.L., PARIS, D.F., & STEEN, W.C. (1980) Quantitative
expression of biotransformation. In: Maki, A.W., Dickson, K.L., &
Cairns, J., ed. Biotransformation and fate of chemicals in the
environment, Washington, DC, American Society for Microbiology, pp.
105-111.
BELISLE, A.A., REICHEL, W.L., & SPANN, J.W. (1975) Analysis of tissues
of mallard ducks fed two phthalate esters. Bull. environ. Contam.
Toxicol., 13: 129-132.
BELL, F.P., PATT, C.S., BRUNDAGE, B., GILLIES, P.J., & PHILIPS, W.A.
(1978a) Studies on lipid biosynthesis and cholesterol content of liver
and serum lipoproteins in rats fed various phthalate esters. Lipids,
13: 66-74.
BELL, F.P., PATT, C.S., & GILLIES, P.J. (1978b) Effect of phthalate
esters on serum cholesterol and lipid biosynthesis in liver, testes,
and epididymal fat in the rat and rabbit. Lipids, 13: 673-678.
BELL, F.P., WANG, S., MENDOZA, A.R., & NISHIZAWA, E.E. (1979) Platelet
function and platelet lipid biosynthesis in rats and rabbits fed the
plasticiser DEHP. Bull. environ. Contam. Toxicol., 23: 306-310.
BICHET, N., CAHARD, D., FABRE, G., REMANDET, B., GOUY, D., & CANO,
J.-P. (1990) Toxicological studies on a benzofuran derivative. III.
Comparison of peroxisome proliferation in rat and human hepatocytes in
primary culture. Toxicol. appl. Pharmacol., 106: 509-517.
BIRGE, W.J., BLACK, J.A., & WESTERMAN, A.G. (1978) Effects of
polychlorinated biphenyl compounds and proposed PCB-replacement
products on embryo-larval stages of fish and amphibians, Washington,
DC, US Department of the Interior, 33 pp (Research Report No. 118).
BLAAUBOER, B.J., VAN HOLSTEIJN, C.W.M., BLEUMINK, R., MENNES, W.C.,
VAN PELT, F.N.A.M., YAP, S.H., VAN PELT, J.F., VAN IERSEL, A.A.J.,
TIMMERMANN, A., & SCHMID, B.P. (1990) The effect of beclobric acid and
clofibric acid on peroxisomal beta-oxidation and peroxisome
proliferation in primary cultures of rat, monkey and human
hepatocytes. Biochem. Pharmacol., 40: 521-528.
BOVE, J.L., DALVEN, P., & KUKREJA, V.P. (1978) Airborne di-butyl and
di(2-ethylhexyl)-phthalate at three New York city air sampling
stations. Int. J. environ. anal. Chem., 5: 189-194.
BRIDGES, J.W. (1985) Frontiers in biochemical toxicology. Trends
pharmacol. Sci., 6 (suppl.): 11-15.
BRINGMANN, G. & KÜHN, R. (1980) Comparison of the toxicity thresholds
of water pollutants to bacteria, algae, and protozoa in the cell
multiplication inhibition test. Water Res., 14: 231-241.
BROWN, D. & THOMPSON, R.S. (1982a) Phthalates and the aquatic
environment: Part I. The effect of di-2-ethylhexyl phthalate (DEHP)
and di-isodecyl phthalate (DIDP) on the reproduction of Daphnia magna
and observations on their bioaccumulation. Chemosphere, 11: 417-426.
BROWN, D. & THOMPSON, R.S. (1982b) Phthalates and the aquatic
environment: Part II. The bioconcentration and depuration of di-2-
ethylhexyl phthalate (DEHP) and di-isodecyl phthalate (DIDP) in
mussels (Mytilus edulis). Chemosphere, 11: 427-435.
BRUNETTI, G. & MOSCATO, G. (1984) [Bronchial asthma due to
occupational exposure to dioctyl phthalate.] Med. Lav., 75: 120-124
(in Italian).
BUCCAFUSCO, R.J., ELLS, S.J., & LEBLANC, G.A. (1981) Acute toxicity of
priority pollutants to bluegill (Lepomis macrochirus). Bull. environ.
Contam. Toxicol., 26: 446-452.
BUTTERWORTH, B.E., BERMUDEZ, E., SMITH-OLIVER, T., EARLE, L., CATTLEY,
R., MARTIN, J., POPP, J.A., STROM, S., JIRTLE, R., & MICHALOPOULOS, G.
(1984) Lack of genotoxic activity of di(2-ethylhexyl) phthalate (DEHP)
in rat and human hepatocytes. Carcinogenesis, 5: 1329-1335.
BUTTERWORTH, B.E., LOURY, D.J., SMITH-OLIVER, T., & CATTLEY, R.C.
(1987) The potential role of chemically induced hyperplasia in the
carcinogenic activity of the hypolipidemic carcinogens. Toxicol. ind.
Health, 3: 129-148.
BUTTERWORTH, B.E., SMITH-OLIVER, T., EARLE, L., LOURY, D.J., WHITE,
R.D., DOOLITTLE, D.J., WORKING, P.K., CATTLEY, R.C., JIRTLE, R.,
MICHALOPOULOS, G., & STROM, S. (1989) Use of primary cultures of human
hepatocytes in toxicology studies. Cancer Res., 49: 1075-1084.
CALLEY, D., AUTIAN, J., & GUESS, W.L. (1966) Toxicology of a series of
phthalate esters. J. pharm. Sci., 55: 158-162.
CARPENTER, C.P., WEIL, C.S., & SMYTH, H.F. (1953) Chronic oral
toxicity of di(2-ethylhexyl) phthalate for rats, guinea-pigs, and
dogs. Arch. ind. Hyg., 8: 219-226.
CATTLEY, R.C. & POPP, J.A. (1989) Differences between the promoting
activities of the peroxisome proliferator WY14,643 and phenobarbital
in rat liver. Cancer Res., 49: 3246-3251.
CATTLEY, R.C., CONWAY, J.G., & POPP, J.A. (1987) Association of
persistent peroxisome proliferation and oxidative injury with
hepatocarcinogenicity in female F-344 rats fed di(2-
ethylhexyl)phthalate for 2 years. Cancer Lett., 38: 15-27.
CAUTREELS, W. & VAN CAUWENBERGHE, K. (1978) Experiments on the
distribution of organic pollutants between airborne particulate matter
and the corresponding gas phase. Atmos. Environ., 12: 1133-1141.
CAUTREELS, W., VAN CAUWENBERGHE, K., & GUZMAN, L.A. (1977) Comparison
between the organic fraction of suspended matter at a background and
an urban station. Sci. total Environ., 8: 79-88.
CERBULIS, J. & ARD, J.S. (1967) Method for isolation and detection of
dioctyl phthalate from milk lipids. J. Assoc. Off. Anal. Chem., 50:
646-650.
CHAPIN, R.E., GRAY, T.J.B., PHELPS, J.L., & DUTTON, S.L. (1988) The
effects of mono-(2-ethylhexyl)-phthalate on rat sertoli cell-enriched
primary cultures. Toxicol. appl. Pharmacol., 92: 467-479.
CHEN, W.S., KERKAY, J., PEARSON, K.H., PAGANINI, E.P., & NAKAMOTO, S.
(1979a) Determination of urinary bis(2-ethylhexyl) phthalate levels in
non-uremic subjects by gas chromatography. Anal. Lett., 12: 1501-1515.
CHEN, W.S., KERKAY, J., PEARSON, K.H., PAGANINI, E.P., & NAKAMOTO, S.
(1979b) Tissue bis(2-ethylhexyl) phthalate levels in uremic subjects.
Anal. Lett., 12: 1517-1535.
CHU, I., SECOURS, V.E., MARINO, I.A., VILLENEUVE, D.C., & VALLI, V.E.
(1981) Sub-acute and sub-chronic toxicity of mono-2-ethylhexyl
phthalate in the rat. Arch. environ. Contam. Toxicol., 10: 271-280.
CIRIOLO, M.R., MAVELLI, I., ROTILIO, G., BORZATTA, V., CRISTOFARI, M.,
& STANZANI, L. (1982) Decrease of superoxide dismutase and glutathione
peroxidase in liver of rats treated with hypolipidemic drugs. FEBS
Lett., 144: 264-268.
CLAYTON, G.D. & CLAYTON, F.E. (1981) Patty's industrial hygiene and
toxicology, 3rd ed., New York, Wiley-Interscience, pp. 2342-2353.
CONTRERAS, T.J., SHELBLEY, R.H., & VALERI, C.R. (1974) Accumulation of
di-2-ethylhexyl phthalate (DEHP) in whole blood, platelet
concentrates, and platelet-poor plasma. Transfusion, 14: 34-46.
CONWAY, J.G., CATTLEY, R.C., POPP, J.A., & BUTTERWORTH, B.E. (1989a)
Possible mechanisms in hepatocarcinogenesis by the peroxisome
proliferator di(2-ethylhexyl)phthalate. Drug Metab. Rev., 21: 65-102.
CONWAY, J.G., TOMASZEWSKI, K.E., OLSON, M.J., CATTLEY, R.C., MARSMAN,
D.S., & POPP, J.A. (1989b) Relationship of oxidative damage to the
hepatocarcinogenicity of the peroxisome proliferators di(2-ethylhexyl)
phthalate and Wy-14, 643. Carcinogenesis, 10: 513-519.
CROCKER, J.F.S., SAFE, S.H., & ACOTT, P. (1988) Effects of chronic
phthalate exposure on the kidney. J. Toxicol. environ. Health, 23:
433-444.
CURSTEDT, T. & SJÖVALL, J. (1983) Individual molecular species of
phosphatidylcholines and phosphatidylinositols in livers of rats fed
bis(2-ethylhexyl) phthalate. Med. Biol., 61: 219-222.
CURTO, K.A. & THOMAS, J.A. (1982) Comparative effects of diethylhexyl
phthalate or monoethylhexyl phthalate on male mouse and rat
reproductive organs. Toxicol. appl. Pharmacol., 62: 121-125.
CURTO, K.A., MCCAFFERTY, R.E., DONOVAN, M.P., & THOMAS, J.A. (1982)
Further studies on the effects of the phthalate acid esters (PAE) on
rat male reproductive organs. Toxicologist, 2: 71.
DANIEL, J.W. & BRATT, H. (1974) The absorption, metabolism and tissue
distribution of di(2-ethylhexyl) phthalate in rats. Toxicology, 2:
51-65.
DEANGELO, A.B. & GARRETT, C.T. (1983) Inhibition of development of
preneoplastic lesions in the livers of rats fed a weakly carcinogenic
environmental contaminant. Cancer Lett., 20: 199-205.
DEANGELO, A.B., GARRETT, C.T., & QUERAL, A.E. (1984) Inhibition of
phenobarbital and dietary choline deficiency promoted preneoplastic
lesions in rat liver by environmental contaminant di(2-ethylhexyl)
phthalate. Cancer Lett., 23: 323-330.
DEFOE, D.L., HOLCOMBE, G.W., HAMMERMEISTER, D.E., & BIESINGER, K.E.
(1990) Solubility and toxicity of eight phthalate esters to four
aquatic organisms.Environ. Toxicol. Chem. 9: 623-636.
DIVINCENZO, G.D., HAMILTON, M.L., MUELLER, K.R., DONISH, W.H., &
BARBER, E.D. (1985) Bacterial mutagenicity testing of urine from rats
dosed with 2-ethylhexanol derived plasticizers. Toxicology, 34:
247-259.
DIWAN, B.A., WARD, J.M., RICE, J.M., COLBURN, N.H., & SPANGLER, E.F.
(1985) Tumor-promoting effects of di(2-ethylhexyl) phthalate in JB6
mouse epidermal cells and mouse skin. Carcinogenesis, 6: 343-347.
DOUGLAS, R.G., HUGENHOLTZ, A.P., & BLAKEY, D.H. (1986) Genetic
toxicology of phthalate esters: mutagenic and other genotoxic effects.
Environ. Health Perspect., 65: 255-262.
ECETOC (1985) An assessment of the occurrence and effects of dialkyl
ortho-phthalates in the environment, Brussels, European Chemical
Industry Ecology and Toxicology Centre, 63 pp (Technical Report No.
19).
ECETOC (1992) Hepatic peroxisome proliferation, Brussels, European
Chemical Industry Ecology and Toxicology Centre, (Monograph No. 17).
EGLINTON, G., SIMONEIT, B.R.T., & ZORO, J.A. (1975) The recognition of
organic pollutants in aquatic sediments. Proc. R. Soc. Lond., B189:
415-442.
EISENREICH, S.J., LOONEY, B.B., & THORNTON, J.D. (1981) Airborne
organic contaminants in the Great Lakes ecosystem. Environ. Sci.
Technol., 15: 30-38.
ELCOMBE, C.R. (1985) Species differences in carcinogenicity and
peroxisome proliferation due to trichloroethylene: A biochemical human
hazard assessment.Arch. Toxicol., Suppl. 8: 6-17.
ELCOMBE, C.R. & MITCHELL, A.M. (1986) Peroxisome proliferation due to
di(2-ethylhexyl)phthalate (DEHP): Species differences and possible
mechanisms. Environ. Health Perspect., 70: 211-219.
ELCOMBE, C.R. & STYLES J.A. (1989) Species differences in peroxisome
proliferation. Toxicologist, 9: 63.
ELSISI, A.E., CARTER, D.E., & SIPES, I.G. (1989) Dermal absorption of
phthalate diesters in rats. Fundam. appl. Toxicol., 12: 70-77.
ENGELHARDT, G. & WALLNOFER, P.R. (1978) Metabolism of di- and mono-N-
butyl phthalate by soil bacteria. Appl. environ. Microbiol., 35:
243-246.
ENGELHARDT, G., WALLNOFER, P.R., & HUTZINGER, O. (1975) The microbial
metabolism of di-n-butyl phthalate and related dialkyl phthalates.
Bull. environ. Contam. Toxicol., 13: 342-347.
ENGELHARDT, G., TILLMANNS, G., WALLNOFER, P.R., & HUTZINGER, O. (1977)
Biodegradation of di-iso-butyl phthalate and related dialkyl
phthalates by Penicillium lilacinum. Chemosphere, 6: 347-354.
ENVIRONMENT AGENCY OF JAPAN (1989) [Chemicals in the environment],
Tokyo, Environment Agency of Japan, p. 418 (in Japanese).
FATOKI, O.S. & VERNON, F. (1990) Phthalate esters in rivers of the
greater Manchester area, U.K. Sci. total Environ., 95: 221-232.
FISHBEIN, L. & ALBRO, P.W. (1972) Chromatographic and biological
aspects of the phthalate esters. J. Chromatogr., 70: 365-412.
FOSTER, P.M.D., LAKE, B.G., COOK, M.W., THOMAS, L.V., & GANGOLLI, S.D.
(1982) Structure-activity requirements for the induction of testicular
atrophy by butyl phthalates in immature rats: effects on testicular
zinc content. Adv. exp. Med. Biol., 136A: 445-452.
FOSTER, P.M.D., THOMAS, L.V., COOK, M.W., & WALTERS, D.G. (1983)
Effect of di-n-pentyl phthalate treatment on testicular steroidogenic
enzymes and cytochrome P-450 in the rat. Toxicol. Lett., 15: 265-271.
GANNING, A.E., OLLSON, M.J., PETERSON, E., & DALLNER., G. (1989) Fatty
acid oxidation in hepatic peroxisomes and mitochondria after treatment
of rats with di(2-ethylhexyl) phthalate. Pharmacol. Toxicol., 65:
265-268.
GIAM, C.S., CHAN, H.S., NEFF, G.S., & ATLAS, E.L. (1978) Phthalate
ester plasticizers: A new class of marine pollutant. Science, 199:
419-421.
GIAM, C.S., ATLAS, E., CHAN, H.S., & NEFF, G.S. (1980) Phthalate
esters, PCB and DDT residues in the Gulf of Mexico atmosphere. Atmos.
Environ., 14: 65-69.
GILIOLI, R., BULGHERONI, C., TERRANA, T., FILIPPINI, G., MASSETTO, N.,
& BOERI, R. (1978) [A neurological, electromyographic and
electroneurographic study in subjects working at the production of
phthalate plasticizers.] Med. Lav., 69: 620-631 (in Italian).
GOEL, S.K., LALWANI, N.D., & REDDY, J.K. (1986) Peroxisome
proliferation and lipid peroxidation in rat liver. Cancer Res., 46:
1324-1330.
GOLLAMUDI, R., LAWRENCE, W.H., RAO, R.H., & AUTIAN, J. (1985) Effects
of phthalic acid esters on drug metabolizing enzymes of rat liver. J.
appl. Toxicol., 5: 368-371.
GOTO, M. (1979) Monitoring environmental materials and specimen
banking - State-of-the-art of Japanese experience and knowledge. In:
Luepke, N.-P., ed. Monitoring environmental materials and specimen
banking. Proceedings of an International Workshop, Berlin (West),
23-28 October, 1978, pp. 271-288.
GRAHAM, P.R. (1973) Phthalate ester plasticizers - Why and how they
are used. Environ. Health Perspect., 3: 3-12.
GRAY, T.J.B. & BEAMAND, J.A. (1984) Effect of some phthalate esters
and other testicular toxins on primary cultures of testicular cells.
Food chem. Toxicol., 22: 123-131. GRAY, T.J.B. & BUTTERWORTH, K.R.
(1980) Testicular atrophy produced by phthalate esters. Arch.
Toxicol., Suppl. 4: 452-455.
GRAY, T.J.B. & GANGOLLI, S.D. (1986) Aspects of the testicular
toxicity of phthalate esters. Environ. Health Perspect., 65: 229-235.
GRAY, T.J.B., BUTTERWORTH, K.R., GAUNT, I.F., GRASSO, P., & GANGOLLI,
S.D. (1977) Short-term toxicity study of di-(2-ethylhexyl) phthalate
in rats. Food Cosmet. Toxicol., 15: 389-399.
GRAY, T.J.B., ROWLAND, I.R., FOSTER, P.M.D., & GANGOLLI, S.D. (1982)
Species differences in the testicular toxicity of phthalate esters.
Toxicol. Lett., 11: 141-147.
GUESS, W.L. & HABERMAN, S. (1968) Toxicity profiles of vinyl and
polyolefinic plastics and their additives. J. biomed. Mater. Res., 2:
313-335.
GUPTA, R.C., GOEL, S.K., EARLEY, K., SINGH, B., & REDDY, J.K. (1985)
32P-Postlabeling analysis of peroxisome proliferator-DNA adduct
formation in rat liver in vivo and hepatocytes in vitro.
Carcinogenesis, 6: 933-936.
HAMANO, Y., INOUE, K., ODA, Y., YAMAMOTO, H., & KUNITA, N. (1979)
[Studies on the toxicity of phthalic acid esters. Part 2. Dominant
lethal tests for DEHP and MEHP in mice, Osaka, Osaka Public Health and
Sanitation Research Centre, pp. 1-4 (Food Hygiene Series No. 10) (in
Japanese).
HARRIS, R.S., HODGE, H.C., MAYNARD, E.A., & BLANCHET, H.J., Jr (1956)
Chronic oral toxicity of 2-ethylhexyl phthalate in rats and dogs. Am.
Med. Assoc. Arch. ind. Health, 13: 259-264.
HERRING, R. & BERING, C.L. (1988) Effects of phthalate esters on plant
seedlings and reversal by a soil microorganism. Bull. environ. Contam.
Toxicol., 40: 626-632.
HILL, E.F., HEATH, R.G., SPANN, J.W., & WILLIAMS, J.D. (1975) Lethal
dietary toxicities of environmental pollutants to birds, Washington,
DC, US Fish and Wildlife Service, 61 pp (Special Scientific Report
Wildlife No. 191).
HOBSON, J.F., CARTER, D.E., & LIGHTNER, D.V. (1984) Toxicity of a
phthalate ester in the diet of a penaied shrimp. J. Toxicol. environ.
Health, 13: 959-968.
HODGE, H.C. (1943) Acute toxicity for rats and mice of di-2-ethylhexyl
phthalate with a note upon the mechanism. Proc. Soc. Exp. Biol. Med.,
53: 20-23.
HOLLIFIELD, H.C. (1979) Rapid nephelometric estimate of water
solubility of highly insoluble organic chemicals of environmental
interest. Bull. environ. Contam. Toxicol., 23: 579-586.
HOWARD, P.H., BANERJEE, S., & ROBILLARD, K.H. (1985) Measurement of
water solubilities, octanol/water partition coefficients and vapour
pressures of commercial phthalate esters. Environ. Toxicol. Chem., 4:
653-661.
HRUDEY, S.E., SERGY, G.A., & THACKERAY, T. (1976) Toxicity of oil
sands plant wastewaters and associated organic contaminants. In:
Proceedings of the 11th Canadian Symposium on Water Pollution
Research, Ottawa, Water Pollution Research Canada, pp. 34-45.
IARC (1982) Di(2-ethylhexyl) phthalate. In: Some industrial chemicals
and dyestuffs, Lyon, International Agency for Research on Cancer, pp.
269-294 (IARC Monographs on the Evaluation of the Carcinogenic Risks
of Chemicals to Humans, Vol. 29).
IARC (1987) Overall evaluations of carcinogenicity: An updating of
IARC monographs volumes 1 to 42, Lyon, International Agency for
Research on Cancer, 440 pp (IARC Monographs on the Evaluation of
Carcinogenic Risks to Humans, Supplement 7).
ISHIDA, M., SUYAMA, K., & ADACHI, S. (1980) Background contamination
by phthalates commonly encountered in the chromatographic analysis of
lipid samples. J. Chromatogr., 189: 421-424.
ISHIDA, M., SUYAMA, K., & ADACHI, S. (1981) Occurrence of dibutyl and
di-2-ethylhexyl phthalate in chicken eggs. J. agric. food Chem., 29:
72-74.
ISHIDA, M., SUYAMA, K., ADACHI, S., & HOSHINO, T. (1982) Distribution
of orally administered diethylhexyl phthalate in laying hens. Poult.
Sci., 61: 262-267.
ISSEMANN, I. & GREEN, S. (1990) Activation of a member of the steroid
hormone receptor superfamily by peroxisome proliferators.
Nature(Lond.): 347: 645-650.
JAEGER, R.J. & RUBIN, R.J. (1972) Migration of a phthalate ester
plasticizer from polyvinyl chloride blood bags into stored human blood
and its localization in human tissues. New Engl. J. Med., 287:
1114-1118.
JOHNSON, B.T. & LULVES, W. (1975) Biodegradation of di-butyl phthalate
and di-2-ethylhexyl phthalate in freshwater hydrosoil. J. Fish Res.
Board Can., 32: 333-339.
JOHNSON, B.T., HEITKAMP, M.A., & JONES, J.R. (1984) Environmental and
chemical factors influencing the biodegradation of phthalic acid
esters in freshwater sediments. Environ. Pollut., B8: 101-118.
KAMATA, I., TSUTSUI, G., TAKANA, J., & SHIRAI, T. (1978) [Phthalic
acid esters in fish in the Uji river.] Kyoto-fu Eisei Kogai Kenkyusho
Nempo, 22: 114 (in Japanese).
KARARA, A.H. & HAYTON, W.L. (1989) A pharmacokinetic analysis of the
effect of temperature on the accumulation of di-2-ethylhexyl phthalate
(DEHP) in sheepshead minnow. Aquat. Toxicol., 15: 27-36.
KARASEK, F.W., DENNEY, D.W., CHAN, K.W., & CLEMENT, R.E. (1978)
Analysis of complex organic mixtures on airborne particulate matter.
Anal. Chem., 50: 82-87.
KASAI, H., OKADA, Y., NISHIMURA, S., RAO, M.S., & REDDY, J.K. (1989)
Formation of 8-hydroxydeoxyguanosine in liver DNA of rats following
long-term exposure to a peroxisome proliferator.Cancer Res., 49:
2603-2605.
KATAEVA, S.W. (1988) [The method for determination of dioctyl
phthalates in water and model media imitating foodstuffs.] Gig. i
Sanit., 1: 52-53 (in Russian).
KEYSER, P., PUJAR, G.P., EATON, R.W., & RIBBONS, D.W. (1976)
Biodegradation of the phthalates and their esters by bacteria.
Environ. Health Perspect., 18: 159-166.
KIRBY, P.E., PIZZARELLO, R.F., LAWLOR, T.E., HAWORTH, S.E., & HODGSON,
J.R. (1983) Evaluation of di(2-ethylhexyl) phthalate and its major
metabolites in the Ames test and L5178Y mouse lymphoma mutagenicity
assay. Environ. Mutagen., 5: 657-663.
KLIMISCH, H.-J., HELLWIG, J., KAUFMANN, W., & JÄCKH, R. (1991) Di-(2-
ethylhexyl)phthalate (DEHP): Investigation of inhalation toxicity in
rats after repeated exposure (28 d). Hum. exp. Toxicol., 3: 68.
KLOPFFER, W., KAUFMANN, G., RIPPEN, G., & POREMSKI, H.J. (1982) A
laboratory method for testing the volatility from aqueous solution:
First results and comparison with theory. Ecotoxicol. environ. Saf.,
6: 545.
KLUWE, W., HASEMAN, J.K., DOUGLAS, J.F., & HUFF, J.E. (1982) The
carcinogenicity of dietary di(2-ethylhexyl) phthalate (DEHP) in Fisher
344 rats and B6C3F1 mice. J. Toxicol. environ. Health, 10: 797-815.
KNOWLES, C.O., MCKEE, M.J., & PALAWSKI, D.U. (1987) Chronic effects of
di-2-ethylhexyl phthalate on biochemical composition, survival and
reproduction of Daphnia magna. Environ. Toxicol. Chem., 6: 201-208.
KODAMA, T. & TAKAI, Y. (1974) [Studies on the determination of
phthalic acid esters, IV. Analysis of phthalic acid ester in fish
meat.] Kogai To Taisaku, 10: 850-852 (in Japanese).
KODAMA, T., KOBAYASKI, K., EBA, H., OGAWA, T., TAKAI, Y., NAITO, N.,
OKADA, N., & SAWANO, Y. (1975) [Studies on the determination of
phthalic acid esters: VI. Investigations in Aichi Prefecture.] Kogai
To Taisaku, 11: 341-350 (in Japanese).
KORA, S., SADO, M., KOLKE, H., & TERADA, H. (1989) Protective effect
of the plasticizer di(2-ethylhexyl) phthalate against damage of the
mitochondrial membrane induced by calcium: possible participation of
the adenine nucleotide translocator. Biochim. Biophys. Acta, 985:
286-292.
KORNBRUST, D.J., BARFKNECHT, T.R., INGRAM, P., & SHELBURNE, J.D.
(1984) Effect of di(2-ethylhexyl)phthalate on DNA repair and lipid
peroxidation in rat hepatocytes and on metabolic cooperation in
Chinese hamster V-79 cells. J. Toxicol. environ. Health, 13: 99-116.
KRAUSKOPF, L.G. (1973) Studies on the toxicity of phthalates via
ingestion. Environ. Health Perspect., 3: 61-72.
KURANE, R., SUZUKI, T., & TAKAHARA, Y. (1977) Isolation of
microorganisms growing on phthalate esters and degradation of
phthalate esters by Pseudomonas acidovorans 256-1. Agric. biol.
Chem., 41: 2119-2123.
KURANE, R., SUZUKI, T., & TAKAHARA, Y. (1978) Removal of phthalate
esters in soil columns inoculated with micro-organisms. Agric. biol.
Chem., 42: 1469-1478.
KURANE, R., SUZUKI, T., & TAKAHARA, Y. (1979) Microbial population and
identification of phthalate ester-utilising micro-organisms in
activated sludge with micro-organisms. Agric. biol. Chem., 43:
907-917.
KURANE, R., SUZUKI, T., & TAKAHARA, Y. (1980) Induction of enzymes
involved in phthalate esters metabolism in Nocardia erythropolis and
enzymatic hydrolysis of phthalate esters by commercial lipases. Agric.
biol. Chem., 44: 529-536.
KURANE, R., SUZUKI, T., TAKAHARA, Y., & FUKUOKA, S. (1984)
Protocatechuate 3,4-dioxygenase from Nocardia erythropolis. Agric.
biol. Chem., 48: 2105-2111.
LAKE, B.G., BRANTOM, P.G., GANGOLLI, S.H., BUTTERWORTH, K.R., &
GRASSO, P. (1976) Studies on the effects of orally administered di(2-
ethylhexyl)phthalate in the ferret. Toxicology, 6: 341-356.
LAKE, B.G., GRAY, T.J.B., FOSTER, J.R., STUBBERFIELD, C.R., &
GANGOLLI, S.D. (1984) Comparative studies on di-(2-ethylhexyl)
phthalate-induced hepatic peroxisome proliferation in the rat and
hamster. Toxicol. appl. Pharmacol., 72: 46-60.
LAKE, B.G., GRAY, T.J.B., & GANGOLLI, S.D. (1986) Hepatic effects of
phthalate esters and related compounds - in vivo and in vitro
correlations. Environ. Health Perspect., 67: 283-290.
LAKE, B.G., KOZLEN, S.L., EVANS, J.G., GRAY, T.J.B., YOUNG, P.J., &
GANGOLLI, S.D. (1987) Effect of prolonged administration of clofibric
acid and di-(2-ethylhexyl) phthalate on hepatic enzyme activities and
lipid peroxidation in the rat. Toxicology, 44: 213-228.
LAKE, B.G., LEWIS, D.F.V. & GRAY, T.J.B. (1988) Structure-activity
relationships for hepatic peroxisome proliferation. Arch. Toxicol.,
Suppl. 12: 217-224.
LAKE, B.G., COOK, W.M., WORRELL, N.R., CUNNINGHAME, M.E., EVANS, J.G.,
PRICE, R.J., YOUNG, P.J., & CARPANINI, F.M.B. (1991) Dose-response
relationships for induction of hepatic peroxisome proliferation and
testicular atrophy by phthalate esters in the rat. Hum. exp. Toxicol.,
10(1): 67.
LALWANI, N.D., ALVARES, K., REDDY, M.K., REDDY, M.N., PARIKH, I., &
REDDY, J.K. (1987) Peroxisome proliferator-binding protein:
Identification and partial characterization of nafenopin-, clofibric
acid-, and clofibrate-binding proteins from rat liver. Proc. Natl
Acad. Sci. (USA), 84: 5242-5246.
LARSSON, P. & THUREN, A. (1987) Di-2-ethylhexylphthalate inhibits the
hatching of frog eggs and is bioaccumulated by tadpoles. Environ.
Toxicol. Chem., 6: 417-422.
LARSSON, P., THUREN, A., & GAHNSTROM, G. (1986) Phthalate esters
inhibit microbial activity in aquatic sediments. Environ. Pollut., 42:
223-231.
LAUGHLIN, R.B., NEFF, J.M., HRUNG, Y.C., GOODWIN, T.C., & GIAM, C.S.
(1978) The effects of three phthalate esters on the larval development
of the grass shrimp Palaemonetes pugio (Holthuis). Water Air Soil
Pollut., 9: 323-336.
LAWRENCE, W.H., MALIK, M., TURNER, J.E., SINGH, A.R., & AUTIAN, J.
(1975) A toxicological investigation of some acute, short-term, and
chronic effects of administering di-2-ethylhexyl phthalate (DEHP) and
other phthalate esters. Environ. Res., 9: 1-11.
LEBLANC, G.A. (1980) Acute toxicity of priority pollutants to water
flea (Daphnia magna). Bull. environ. Contam. Toxicol., 24: 684-691.
LEWANDOWSKI, M., FERNANDES, J., & CHEN, T.S. (1980) Assessment of the
teratogenic potential of plasma-soluble extracts of diethylhexyl
phthalate plasticized polyvinyl chloride plastics in rats. Toxicol.
appl. Pharmacol., 54: 141-147.
LEWIS, L.M., FLECHTNER, T.W., KERKAY, J., PEARSON, K.H., CHEN, W.T.,
POPOWNIAK, K.L., & NAKAMOTO, S. (1977) Determination of plasticizer
levels in serum of hemodialysis patients. Trans. Am. Soc. Artif.
Intern. Organs, 23: 566-572.
LEYDER, F. & BOULANGER, P. (1983) Ultraviolet absorption, aqueous
solubility, and octanol-water partition for several phthalates. Bull.
environ. Contam. Toxicol., 30: 152-157.
LHUGUENOT, J.C. & ELCOMBE, C.R. (1984) Variation selon l'espèce des
phénomènes de conjugasion lors du métabolisme mono-(éthyl-2-hexyl)
phthalate (MEHP). Sci. Aliments, 4: 227-232.
LHUGUENOT, J.C., MITCHELL, A.M., MILNER, G., LOCK, E.A., & ELCOMBE,
C.R. (1985) The metabolism of di(2-ethylhexyl)phthalate (DEHP) and
mono-(2-ethylhexyl) phthalate (MEHP) in rats: In vivo and in vitro
dose and time dependency of metabolism. Toxicol. appl. Pharmacol., 80:
11-22.
LINDEN, E., BENGTSSON, B.-E., SVANBERG, O., & SUNDSTROM, G. (1979) The
acute toxicity of 78 chemicals and pesticide formulations against two
brackish water organisms, the bleak (Alburnus alburnus) and the
harpacticoid (Nitocra spinipes). Chemosphere, 8: 843-851.
LISS, G.M., ALBRO, P.W., HARTLE, R.W., & STRINGER, W.T. (1985) Urine
phthalate determinations as an index of occupational exposure to
phthalic anhydride and di(2-ethylhexyl) phthalate. Scand. J. Work
Environ. Health, 11: 381-387.
LLOYD, S.C. & FOSTER, P.M.D. (1988) Effect of mono-(2-ethylhexyl)
phthalate on follicle-stimulating hormone responsiveness of cultured
rat Sertoli cells. Toxicol. appl. Pharmacol., 95: 484-489.
LOCK, E.A., MITCHELL, A.M.R., & ELCOMBE, C.R. (1989) Biochemical
mechanisms of induction of hepatic peroxisome proliferation. Annu.
Rev. Pharmacol. Toxicol., 29: 145-168.
LOKKE, H. & BRO-RASMUSSEN, F. (1981) Studies of mobility of di-iso-
butyl phthalate (DiBP), di-n-butyl phthalate (DBP), and di-(2-
ethylhexyl) phthalate (DEHP) by plant foliage treatment in a closed
terrestrial simulation chamber. Chemosphere, 10: 1223-1235.
LOKKE, H. & RASMUSSEN, L. (1983) Phytotoxicological effects of di-(2-
ethylhexyl)-phthalate and n-butyl-phthalate on higher plants in
laboratory and field experiments. Environ. Pollut., 32: 179-199.
LUTZ, W.K. (1986) Investigation of the potential for binding of di(2-
ethylhexyl) phthalate (DEHP) to rat liver DNA in vivo. Environ.
Health Perspect., 65: 267-269.
MACEK, K.J., PETROCELLI, S.R., & SLEIGHT, B.H., III (1979)
Considerations in assessing the potential for, and significance of,
biomagnification of chemical residues in aquatic food chains. In:
Aquatic toxicology, Philadelphia, American Society for Testing and
Materials, pp. 251-268 (ASTM STP 667).
MAFF (1987) Survey of plasticiser levels in food contact materials and
in foods, London, Ministry of Agriculture, Fisheries and Food (Food
Surveillance Paper No. 21).
MALCOLM, A.R. & MILLS, L.J. (1989) Inhibition of gap-junctional
intercellular communication between Chinese hamster lung fibroblasts
by di(2-ethylhexyl) phthalate (DEHP) and trisodium nitrilotriacetate
monohydrate (NTA). Cell Biol. Toxicol., 5: 145-153.
MANANDHAR, M.D., SHOEB, A., KAPIL, R.S., & POPLI, S.P. (1979) Studies
in medicinal plants. Part V, Di-2-n-propyl pentyl phthalate and
reticulene from Cryptocaryo amyggdalina Nees. Indian J. Chem., 178:
411-412.
MARCEL, Y.L. & NOEL, S.P. (1970) Contamination of blood stored in
plastic packs. Lancet, 1: 35-36.
MARSMAN, D.S., CATTLEY, R.C., CONWAY, J.G., & POPP, J.A. (1988)
Relationships of hepatic peroxisome proliferation and replicative DNA
synthesis to the hepatocarcinogenicity of the peroxisome proliferators
di(2-ethylhexyl) phthalate and [4-chloro-6-(2,3-xylidino)-2-
pyrimidinylthio]acetic acid (Wy-14,643) in rats. Cancer Res., 48:
6739-6744.
MATHUR, S.P. (1974a) Phthalate esters in the environment: Pollutants
or natural products. J. environ. Qual., 3: 189.
MATHUR, S.P. (1974b) Respirometric evidence of the utilization in soil
of di-octyl and di-2-ethylhexyl phthalate plasticizers. J. environ.
Qual., 3: 207-209.
MATSUDA, K. & SCHNITZER, M. (1971) Reactions between fulvic acid, a
soil humic material, and dialkyl phthalates. Bull. environ. Contam.
Toxicol., 6: 200-204.
MAYER, F.L. (1976) Residue dynamics of di-2-ethylhexyl phthalate in
fathead minnows (Pimephales promelas). J. Fish Res. Board Can., 33:
2610-2613.
MAYER, F.L. & SANDERS, H.O. (1973) Toxicology of phthalic acid esters
in aquatic organisms. Environ. Health Perspect., 3: 153-157.
MAYER, F.L., STALLING, D.L., & JOHNSON, J.L. (1972) Phthalate esters
as environmental contaminants. Nature (Lond.), 238: 411-413.
MAYER, F.L., MEHRLE, P.M., & SCHOETTGER, R.A. (1977) Collagen
metabolism in fish exposed to organic chemicals. In: Symposium on
Recent Advances in Fish Toxicology, Washington, DC, US Environmental
Protection Agency, p. 31 (EPA 600/3-77-085).
MEHRLE, P.M. & MAYER, F.L. (1976) Di-2-ethylhexyl phthalate: Residue
dynamics and biological effects in rainbow trout and fathead minnow.
Trace Subst. environ. Health, 10: 519-524.
MELANCON, M.J. (1979) Metabolism of phthalate esters in aquatic
species. In: Pesticides and xenobiotic metabolism in aquatic
organisms, Washington, DC, American Chemical Society, pp. 77-94 (ACS
Symposium Series No. 99).
MELNICK, R.L., MORRISSEY, R.E., & TOMASZEWSKI, K.E. (1987) Studies by
the National Toxicology Program on di(2-ethylhexyl) phthalate.
Toxicol. ind. Health, 3: 99-118.
MES, J., COFFIN, D.E., & CAMPBELL, D.S. (1974) Di-n-butyl and di-2-
ethylhexyl phthalate in human adipose tissue. Bull. environ. Contam.
Toxicol., 12: 721-725.
METCALF, R.L., BOOTH, G.M., SCHUTH, C.K., HANSEN, D.J., & LU, P.-Y.
(1973) Uptake and fate of di-2-ethylhexyl phthalate in aquatic
organisms and in a model ecosystem. Environ. Health Perspect., 4:
27-34.
MIKALSEN, S.O., HOLEN, I., & SANNER, T. (1990a) Morphological
transformation and catalase activity of Syrian hamster embryo cells
treated with hepatic peroxisome proliferators, TPA and nickel
sulphate. Cell Biol. Toxicol., 6: 1-13.
MIKALSEN, S.O., KAALHUS, O., REITH, A., & SANNER, T. (1990b) Role of
catalase and oxidative stress in hepatic peroxisome proliferator-
induced morphological transformation of Syrian hamster embryo cell.
Int. J. Cancer, 46: 950-957.
MIKALSEN, S.O., REUTER, B., & SANNER, T. (1990c) Effects of hepatic
peroxisome proliferators and 12-O-tetradecanoyl phorbol-13-acetate on
catalase and other enzyme activities of embryonic cells in vitro.
Biochem. Pharmacol., 39: 527-535.
MILKOV, L.E., ALDYREVA, M.V., POPOVA, T.B., LOPUKHOVA, K.A.,
MAKARENKO, Y.L., MALYAR, L.M., & SHAKHOVA, T.K. (1973) Health status
of workers exposed to phthalate plasticizers in the manufacture of
artificial leather and films based on PVC resins. Environ. Health
Perspect., 3: 175-178.
MITCHELL, A.M., LHUGUENOT, J.-C., BRIDGES, J.W., & ELCOMBE, C.R.
(1985a) Identification of the proximate peroxisome proliferator(s)
derived from di(2-ethylhexyl) phthalate. Toxicol. appl. Pharmacol.,
80: 23-32.
MITCHELL, F.E., PRICE, S.C., HINTON, R.H., GRASSO, P., & BRIDGES, J.W.
(1985b) Time and dose-response study of the effects on rats of the
plasticizer di(2-ethylhexyl) phthalate. Toxicol. appl. Pharmacol., 81:
371-392.
MORI, S. (1976) Identification and determination of phthalate esters
in river water by high-performance liquid chromatography. J.
Chromatogr., 129: 53-60.
MUSIAL, C.J. & UTHE, J.F. (1980) Studies on phthalate esters in
Western Atlantic finfish. Report to International Council for the
Exploration of the Sea (ICES), Copenhagen, International Council for
the Exploration of the Sea, pp. 1-6 (Report E:11).
MUTZ, R.C. & JONES, J.R. (1977) The effects of phthalate esters on
geochemical cycles in freshwater hydrosoil. Trans. Missouri Acad.
Sci., 10-11: 296.
AZIR, D.J., ALCARAZ, A.P., BIERL, B.A., BEROZA, M., & NAIR, P.P.
(1971) Isolation, identification, and specific localization of di-2-
ethylhexyl phthalate in bovine heart muscle mitochondria.
Biochemistry, 10: 4228-4232.
NEERGAARD, J., NIELSEN, B., FAURBY, V., CHRISTENSEN, D.H., & NIELSEN,
O.F. (1971) Plasticizers in P.V.C. and the occurrence of hepatitis in
a haemodialysis unit. Scand. J. Urol. Nephrol., 5: 141-145.
NEUHAUSER, E.F., LOER, R.C., & MALECKI, M.R. (1986) Contact and
artificial soil tests using earthworms to evaluate the impact of
wastes in soil. In: Petros, J.K., Jr, Lacy, W.J., & Conway, R.A., ed.
Hazardous and industrial solid waste testing: Fourth symposium,
Philadelphia, American Society for Testing and Materials, pp. 192-203
(ASTM STP 866).
NIELSEN, J., ÅKESSON, B., & SKERFVING, S. (1985) Phthalate ester
exposure - air levels and health of workers processing
polyvinylchloride. Am. Ind. Hyg. Assoc. J., 46: 643-647.
NIKONOROW, M., MAZUR, H., & PIEKACZ, H. (1973) Effect of orally
administered plasticizers and polyvinyl chloride stabilizers in the
rat. Toxicol. appl. Pharmacol., 26: 253-259.
NIOSH (1977) Manual of analytical methods, 2nd ed., Cincinnati, Ohio,
National Institute for Occupational Safety and Health, Vol. 2, pp.
S40/1-S40/9.
NIOSH (1985a) Manual of analytical methods, 3rd ed., Cincinnati, Ohio,
National Institute for Occupational Safety and Health, Vol. 1, pp.
5020/1-5020/4.
NIOSH (1985b) Registry of toxic effects of chemical substances:
1983-84 Supplement, Cincinnati, Ohio, National Institute for
Occupational Safety and Health, Vol. II, p. 1442.
NTP (1982) Technical report on the carcinogenicity bioassay of di(2-
ethylhexyl) phthalate, Research Triangle Park, NC, National Toxicology
Programme (DHHS Publ. No. (NIH) 82-1773).
O'CONNOR, O.A., RIVERA, M.D., & YOUNG, L.Y. (1989) Toxicity and
biodegradation of phthalic acid esters under methanogenic conditions.
Environ. Toxicol. Chem., 8: 569-576.
OHYAMA, T. (1977) Effects of phthalate esters on glucose-6-phosphate
dehydrogenase and other enzymes in vitro. Toxicol. appl. Pharmacol.,
40: 355-364.
OISHI, S. (1984) Testicular atrophy of rats induced by di-2-ethylhexyl
phthalate: effects of vitamin A and zinc concentrations in the testis,
liver and serum. Toxicol. Lett., 20: 75-78.
OISHI, S. (1985) Reversibility of testicular atrophy induced by di(2-
ethylhexyl) phthalate in rats. Environ. Res., 36: 160-169.
OISHI, S. (1989a) Effects of co-administration of di(2-ethylhexyl)
phthalate and testosterone on several parameters in the testis and
pharmacokinetics of its mono-deesterified metabolite. Arch. Toxicol.,
63: 289-295.
OISHI, S. (1989b) Enhancing effects of luteinizing hormone-releasing
hormone on testicular damage induced by di(2-ethylhexyl) phthalate in
rats. Toxicol. Lett., 47: 271-277.
OISHI, S. (1990) Effects of phthalic acid esters on testicular
mitochondrial functions in the rat. Appl. Toxicol., 64: 143-147.
OISHI, S. & HIRAGA, K. (1979) Effect of phthalic acid esters on
gonadal function in male rats. Bull. environ. Contam. Toxicol., 21:
65-67.
OISHI, S. & HIRAGA, K. (1980a) Testicular atrophy induced by phthalic
esters: effect on testosterone and zinc concentrations. Toxicol. appl.
Pharmacol., 53: 35-41.
OISHI, S. & HIRAGA, K. (1980b) Effect of phthalic acid monoesters on
mouse testes. Toxicol. Lett., 6: 239-242.
OISHI, S. & HIRAGA, K. (1982) Effects of monoesters of o-phthalic acid
on serum lipid composition in rats. Toxicol. Lett., 14: 79-84.
OISHI, S. & HIRAGA, K. (1983) Testicular atrophy induced by di-2-
ethylhexyl phthalate: effect of zinc supplement. Toxicol. appl.
Pharmacol., 70: 43-48.
O'SHEA, T.J. & STAFFORD, C.J. (1980) Phthalate plasticizers:
Accumulation and effects on weight and food consumption in captive
starlings. Bull. environ. Contam. Toxicol., 25: 345-352.
OSUMI, T. & HASHIMOTO, T. (1978) Enhancement of fatty acyl-CoA
oxidizing activity in rat liver peroxisomes by di-(2-
ethylhexyl)phthalate. J. Biochem., 83: 1361-1365.
PARE, J.R.J., BELANGER, J., & JANKOWSKI, K. (1981) Asymmetric
phthalate from Sansevieria trifasciata. J. nat. Prod., 44: 490-492.
PASSINO, D.R.M. & SMITH, S.B. (1987) Acute bioassays and hazard
evaluation of representative contaminants detected in Great Lakes
fish. Environ. Toxicol. Chem., 6: 901-907.
PEAKALL, D.B. (1974) Effects of di-n-butyl and di-2-ethylhexyl
phthalate on the eggs of ring doves. Bull. environ. Contam. Toxicol.,
12: 698-702.
PEAKALL, D.B. (1975) Phthalate esters: Occurrence and biological
effects. Res. Rev., 54: 1-40.
PERERA, M.I.R., KATYAL, S.L., & SHINOZUKA, H. (1986) Suppression of
choline-deficient diet-induced hepatocyte membrane lipid peroxidation
in rats by the peroxisome proliferators 4-chloro-6-(2,3-xylidino)-2-
pyrimidinylthio(N-ß-hydroxyethyl)acetamide and di(2-
ethylhexyl)phthalate. Cancer Res., 46: 3304-3308.
PERKINS, E.G. (1967) Characterization of the nonvolatile compounds
formed during the thermal oxidation of corn oil. II. Phthalate esters.
J. Am. Oil Chem. Soc., 44: 197-199.
PERSSON, P.-E., PENTTINEN, H., & NUORTEVA, P. (1978) DEHP in the
vicinity of an industrial area in Finland. Environ. Pollut., 16:
163-166.
PETERSON, J.C. & FREEMAN, D.H. (1984) Variations of phthalate ester
concentrations in sediments from the Chester river, Maryland. Int. J.
environ. anal. Chem., 18: 237-252.
PFUDERER, P. & FRANCIS, A.A. (1975) Phthalate esters: Heartrate
depressors in the goldfish. Bull. environ. Contam. Toxicol., 13:
275-279.
PHILLIPS, B.J., ANDERSON, D., & GANGOLLI, S.D. (1986) Studies on the
genetic effects of phthalic acid esters on cells in culture. Environ.
Health Perspect., 65: 263-266.
PIECHOCKI, J.T. & PURDY, W.C. (1973) Determination of di-(2-
ethylhexyl)-phthalate (DEHP) in human plasma. Clin. Chim. Acta, 48:
385-391.
POLLACK, G.M., BUCHANAN, J.F., SLAUGHTER, R.L., KOHLI, R.K., & SHEN,
D.D. (1985a) Circulating concentrations of di(2-ethylhexyl) phthalate
and its de-esterified phthalic acid products following plasticizer
exposure in patients receiving hemodialysis. Toxicol. appl.
Pharmacol., 79: 257-267.
POLLACK, G.M., LI, R.C.K., ERMER, J.C., & SHEN, D.D. (1985b) Effects
of route of administration and repetitive dosing on the disposition
kinetics of di(2-ethylhexyl) phthalate and its mono-de-esterified
metabolite in rats. Toxicol. appl. Pharmacol., 79: 246-256.
POLLACK, G.M., SHEN, D.D., & DORR, M.B. (1989) Contribution of
metabolites to the route- and time-dependent hepatic effects of di-(2-
ethylhexyl)phthalate in the rat. J. Pharmacol. exp. Ther., 248:
176-181.
POPP, J.A., GARVEY, L.K., & CATTLEY, R.C. (1987) In vivo studies on
the mechanism of di(2-ethylhexyl) phthalate carcinogenesis. Toxicol.
ind. Health, 3: 151-163.
PRESTON, M.R. & AL-OMRAN, L.A. (1986) Dissolved and particulate
phthalate esters in the river Mersey estuary. Mar. Pollut. Bull., 17:
548-553.
PRESTON, M.R. & AL-OMRAN, L.A. (1989) Phthalate ester speciation in
estuarine water, suspended particulates and sediments. Environ.
Pollut., 62: 183-193.
PUTMAN, D.L., MOORE, W.A., SCHECHTMAN, L.M., & HODGSON, J.R. (1982)
Cytogenetic evaluation of di-(2-ethylhexyl)phthalate and its major
metabolites in Fischer 344 rats. Environ. Mutagen., 4: 387.
RAO, M.S. & REDDY, J.K. (1987) Peroxisome proliferation and
hepatocellular hepatocarcinogenesis. Carcinogenesis, 8: 631-636.
RAO, M.S., YELDANDI, A.V.P., & SUBBARAO, V. (1990) Quantitative
analysis of hepatocellular lesions induced by di(2-
ethylhexyl)phthalate in F344 rats. J. Toxicol. environ. Health, 30:
85-89.
RAO, P.S.C., HORNSBY, A.G., & JESSUP, R.E. (1985) Indices for ranking
the potential for pesticide contamination of groundwater. Soil Crop
Sci. Soc. Proc., 44: 1-8.
RAY, L.E., MURRAY, H.E., & GIAM, C.S. (1983a) Organic pollutants in
marine samples from Portland, Maine. Chemosphere, 12: 1031-1038.
RAY, L.E., MURRAY, H.E., & GIAM, C.S. (1983b) Analysis of water and
sediment from the Nueces estuary/Corpus Christi bay (Texas) for
selected organic pollutants. Chemosphere, 12: 1039-1045.
REDDY, J.K. & LALWANI, N.D. (1983) Carcinogenesis by hepatic
peroxisome proliferators: evaluation of the risk of hypolipidemic
drugs and industrial plasticizers to humans. CRC crit. Rev. Toxicol.,
12: 1-58.
REDDY, J.K. & RAO, M.S. (1989) Oxidative DNA damage caused by
persistent peroxisome proliferation: its role in hepatocarcinogenesis.
Mutat. Res., 214: 63-68.
REDDY, J.K., REDDY, M.K., USMAN, M.I., LALWANI, N.D., & RAO, M.S.
(1986) Comparison of hepatic peroxisome proliferative effect and its
implication for hepatocarcinogenicity of phthalate esters, di(2-
ethylhexyl)phthalate, and di(2-ethylhexyl) adipate with a
hypolipidemic drug. Environ. Health Perspect., 65: 317-327.
RHODES, C., ORTON, T.C., PRATT, I.S., BATTEN, P.L., BRATT, H.,
JACKSON, S.J., & ELCOMBE, C.R. (1986) Comparative pharmacokinetics and
subacute toxicity of di(2-ethylhexyl)phthalate (DEHP) in rats and
marmosets: extrapolation of effects in rodents to man. Environ. Health
Perspect., 65: 299-308.
RITSEMA, R., COFINO, W.P., FRINTROP, P.C.M., & BRINKMAN, V.A.T. (1989)
Trace-level analysis of phthalate esters in surface water and
suspended particulate matter by means of capillary gas chromatography
with electron-capture and mass-selective detection. Chemosphere, 18:
2161-2175.
ROTH, B., HERKENRATH, P., LEHMANN, H.J., OHLES, H.D., HÖMIG, H.J.,
BENZ-BOHM, G., KREUDER, J., & YOUNOSSI-HARTENSTEIN, A. (1988) Di-(2-
ethylhexyl)phthalate as plasticizer in PVC respiratory tubing systems:
indications of hazardous effects on pulmonary function in mechanically
ventilated, preterm infants. Eur. J. Pediatr., 147: 41-46.
RUBIN, R.J. & CHANG, J.F.C. (1978) Effect of the intravenous
administration of the solubilized plasticizer di(2-ethylhexyl)
phthalate on the lung and on survival of transfused rats. Toxicol.
appl. Pharmacol., 45: 230.
RUBIN, R.J. & JAEGER, R.J. (1973) Some pharmacologic and toxicologic
effects of di-2-ethylhexyl phthalate (DEHP) and other plasticizers.
Environ. Health Perspect., 3: 53-59.
RUBIN, R.J. & NAIR, P.P. (1972) Plasticizers in human tissues. New
Engl. J. Med., 288: 915-916.
RUDDICK, J.A., VILLENEUVE, D.C., CHU, I., NESTMANN, E., & MILES, D.
(1981) An assessment of the teratogenicity in the rat and mutagenicity
in Salmonella of mono-2-ethylhexyl phthalate. Bull. environ. Contam.
Toxicol., 27: 181-186.
RUSHBROOK, C.L., JORGENSON, T.A., & HODGSON, J.R. (1982) Dominant
lethal study of di-(2-ethylhexyl)phthalate and its major metabolites
in ICR/SIM mice. Environ. Mutagen., 4: 387.
SAEGER, V.W. & TUCKER, E.S. (1973) Biodegradation of phthalate esters.
In: Flexible vinyls and human safety: An objective analysis. Presented
at the Regional Technical Conference of the Society of Plastics
Engineers, Palisades Section, Kiamesha Lake, New York, 20-22 March,
1973, pp. 105-133.
SAEGER, V.W. & TUCKER, E.S. (1976) Biodegradation of phthalic acid
esters in river water and activated sludge. Appl. environ. Microbiol.,
31: 29-34.
SAKURAI, T., MIYAZAWA, S., & HASHIMOTO, T. (1978) Effect of di(2-
ethylhexyl) phthalate administration on carbohydrate and fatty acid
metabolism in rat liver. J. Biochem., 83: 313-320.
SANDERS, H.O., MAYER, F.L., & WALSH, D.F. (1973) Toxicity, residue
dynamics, and reproductive effects of phthalate esters in aquatic
invertebrates. Environ. Res., 6: 84-90.
SAX, N.I. (1984) Dangerous properties of industrial materials, 6th
ed., New York, Van Nostrand Reinhold Company.
SAXENA, D.K., SRIVASTAVA, S.P., CHANDRA, S.V., & SETH, P.K. (1985)
Testicular effects of di(2-ethylhexyl) phthalate (DEHP): histochemical
and histopathological alterations. Ind. Health, 23: 191-198.
SCHMEZER, P., POOL, B.L., KLEIN, R.G., KOMITOWSKI, D., & SCHMÄHL, D.,
(1988) Various short-term assays and two long-term studies with the
plasticizer di(2-ethylhexyl) phthalate in the Syrian hamster.
Carcinogenesis, 9: 37-43.
SCHMID, P. & SCHLATTER, C. (1985) Excretion and metabolism of di(2-
ethyl-hexyl) phthalate in man. Xenobiotica, 15: 251-256.
SCHMIDT, J.G., GARVIN, P.J., & LEESTMA, J.E. (1975) Effect of vehicle
on the response to intravenous di-(2-ethylhexyl) phthalate (DEHP) in
rats. Toxicol. appl. Pharmacol., 33: 169.
SCHMITZER, J.L., SCHEUNERT, I., & KORTE, F. (1988) Fate of bis(2-
ethylhexyl) [14C] phthalate in laboratory and outdoor soil-plant
systems. J. agric. food Chem., 36: 210-215.
SCHNEIDER, B., SCHENA, J., TROUG, R., JACOBSON, M., & KEVY, S. (1989)
Exposure to di(2-ethylhexyl)phthalate in infants receiving
extracorporeal membrane oxygenation. New Engl. J. Med., 320: 1563.
SCHULTE-HERMANN, R., KRAUPP-GRASL, B., BURSCH, W., GERBRACHT, U., &
TIMMERMANN-TROSIENER, I. (1989) Effects of non-genotoxic hepato-
carcinogens phenobarbital and nafenopin on phenotype and growth of
different populations of altered foci in rat liver. Toxicol. Pathol.,
17: 642-650.
SCHULZ, C.O. (1989) Assessing human health risks from exposure to
di(2-ethylhexyl)phthalate (DEHP) and related phthalates: scientific
issues. Drug Metab. Rev., 21: 111-120.
SCHULZ, C.O. & RUBIN, R.J. (1973) Distribution, metabolism, and
excretion of di-2-ethylhexyl phthalate in the rat. Environ. Health
Perspect., 3: 123-129.
SCHULZ, C.O., RUBIN, R.J., & HUTCHINS, G.M. (1975) Acute lung toxicity
and sudden death in rats following the intravenous administration of
the plasticizer, di(2-ethylhexyl)-phthalate, solubilized with Tween
surfactants. Toxicol. appl. Pharmacol., 33: 514-525.
SCHWARTZ, H.E., ANZION, C.J.M., VAN VLIET, H.P.M., COPIUS PEEREBOOMS,
J.W., & BRINMAN, U.A.T. (1979) Analysis of phthalate esters in
sediments from Dutch rivers by means of high performance liquid
chromatography. Intern. J. environ. anal. Chem., 6: 133-144.
SETH, P.K., SRIVASTAVA, S.P., AGARWAL, D.K., & CHANDRA, S.V. (1976)
Effect of di-2-ethylhexyl phthalate (DEHP) on rat gonads. Environ.
Res., 12: 131-138.
SHAFFER, C.B., CARPENTER, C.P., & SMYTH, H.F., Jr (1945) Acute and
subacute toxicity of di(2-ethylhexyl) phthalate with note upon its
metabolism. J. ind. Hyg. Toxicol., 27: 130-135.
SHANKER, R., RAMAKRISHNA, C., & SETH, P.K. (1985) Degradation of some
phthalic acid esters in soil. Environ. Pollut., 39: 1-7.
SHELDON, L.S. & HITES, R.A. (1979) Sources and movement of organic
chemicals in the Delaware river. Environ. Sci. Technol., 13: 574-579.
SHINDO, Y., OSUMI, T., & HASHIMOTO, T. (1978) Effects of
administration of di(2-ethylhexyl) phthalate on rat liver
mitochondria. Biochem. Pharmacol., 27: 2683-2688.
SHIOTA, K. & MIMA, S. (1985) Assessment of the teratogenicity of di(2-
ethylhexyl) phthalate and mono(2-ethylhexyl) phthalate in mice. Arch.
Toxicol., 56: 263-266.
SHIOTA, K. & NISHIMURA, H. (1982) Teratogenicity of di(2-ethylhexyl)
phthalate (DEHP) and di-n-butyl phthalate (DBP) in mice. Environ.
Health Perspect., 45: 65-70. SHIOTA, K., CHOU, M.J., & NISHIMURA, H.
(1980) Embryotoxic effects of di-2-ethylhexyl phthalate (DEHP) and di-
n-butyl phthalate (DBP) in mice. Environ. Res., 22: 245-253.
SHORT, R.D., ROBINSON, E.C., LINGTON, A.W., & CHIN, A.E. (1987)
Metabolic and peroxisome proliferation studies with di(2-ethylhexyl)
phthalate in rats and monkeys. Toxicol. ind. Health, 3: 185-195.
SHUKLA, R.R., ALBRO, P.W., CORBETT, J.T., & SCHROEDER, J.L. (1989) In
vivo studies of the inhibition of protein kinase C from rat brain by
di-(2-ethylhexyl) phthalate. Chem.-biol. Interact., 69: 73-85.
SILVO, O.E.J. (1974) [Preliminary studies on the acute toxicity of
dioctylphthalate (DOP) to rainbow trout ( Salmo gairdneri Richardson)
and its effect upon phytoplankton and oxygen content of the water.]
Suomen Kalatalous, 47: 19-25 (in Finnish).
SINGH, A.R., LAWRENCE, W.H., & AUTIAN, J. (1972) Teratogenicity of
phthalate esters in rats. J. pharm. Sci., 61: 51-55.
SITTIG, M. (1985) Handbook of toxic and hazardous chemicals and
carcinogens, 2nd ed., Park Ridge, New Jersey, Noyes Publications.
SJÖBERG, P., PLÖEN, L., BONDESSON, U., & LINDQUIST, N.G. (1982)
Testicular toxicity of di-(2-ethylhexyl) phthalate in rats of
different ages. Teratology, 26: 20A.
SJÖBERG, P., BONDESSON, U., KJELLEN, L., LINDQUIST, N.-G., MONTIN, G.,
& PLÖEN, L. (1985a) Kinetics of di-(2-ethylhexyl) phthalate in
immature and mature rats and effect on testis. Acta pharmacol.
toxicol., 56: 30-37.
SJÖBERG, P., BONDESSON, U., SEDIN, G., & GUSTAFSSON, J. (1985b)
Dispositions of di- and mono-(2-ethylhexyl) phthalate in newborn
infants subjected to exchange transfusions. Eur. J. clin. Invest., 15:
430-436.
SJÖBERG, P., LINDQUIST, N.G., MONTIN, G., & PLÖEN, L. (1985c) Effect
of repeated intravenous infusions of the plasticizer di-(2-ethylhexyl)
phthalate in young male rats. Arch. Toxicol., 58: 78-83.
SJÖBERG, P., LINDQUIST, N.G., & PLÖEN, L. (1986) Age-dependent
response of the rat testes to di(2-ethylhexyl) phthalate. Environ.
Health Perspect., 65: 237-242.
SMISTAD, G., WAALER, T., & ROKSVAAG, P.O. (1989) Migration of plastic
additives from soft polyvinyl chloride bags into normal saline and
glucose infusions. Acta pharm. Nord., 1: 287-290.
SMITH-OLIVER, T. & BUTTERWORTH, B.E. (1987) Correlation of the
carcinogenic potential of di(2-ethylhexyl)phthalate (DEHP) with
induced hyperplasia rather than with genotoxic activity. Mutat. Res.,
188: 21-28.
SODERGREN, A. (1982) Significance of interfaces in the distribution
and metabolism of di-2-ethylhexyl phthalate in an aquatic laboratory
model ecosystem. Environ. Pollut., 27: 263-274.
SRI (1985) Chemical economics handbook, Menlo Park, California, SRI
International.
SRIVASTAVA, S., AWASTHI, V.K., SRIVASTAVA, S.P., & SETH, P.K. (1989)
Biochemical alterations in rat fetal liver following in utero exposure
to di(2-ethylhexyl)phthalate (DEHP). Indian J. exp. Biol., 27:
885-888.
STANKEVICH, V.V. & ZAREMBO, O.K. (1978) [Toxicological and hygienic
characteristics of PVC materials plasticizers.] In: [Modern problems
of hygiene of PVC application], Moscow, VNIIMI, pp. 10-16 (Series
Gigiena No. 4) (in Russian).
STANKEVICH, V.V., ZAREMBO, O.K., GUMENNOY, V.S., & LEUITSKAYA, V.N.
(1984) [Present state of the regulation program relating to vinyl
chloride monomers and other additives used as polyvinyl chloride
packing material (A review)], Moscow, VNIIMI, pp. 26-36 (Series
Gigiena) (in Russian).
STANLEY, R.D. & TAPP, J.F. (1982) An assessment of ecological test
methods. VIII. The effect of nine chemicals on the growth of Avena
saliva and Brassica rapa, United Kingdom, ICI Brixham Laboratory
(Internal Report No. BL/A/2164).
STEPHENSON, R.R. (1983) Effects of water hardness, water temperature,
and size of the test organism on the susceptibility of the freshwater
shrimp, Gammarus pulex (L.), to toxicants. Bull. environ. Contam.
Toxicol., 31: 459-466.
STREUFERT, J.M., JONES, J.R., & SANDERS, H.O. (1980) Toxicity and
biological effects of phthalate esters on midges (Chironomus
plumosus). Trans. Missouri Acad. Sci., 14: 33-40.
SUGATT, R.H., O'GRADY, D.P., BANERJEE, S., HOWARD, P.H., & GLEDMILL,
W.E. (1984) Shake flask biodegradation of 14 commercial phthalate
esters. Appl. environ. Microbiol., 47: 601-606.
SWENERTON, H. & HURLEY, L.S. (1971) Teratogenic effects of a chelating
agent and their prevention by zinc. Science, 173: 62-63.
TABORSKY, R.G. (1967) Isolation studies on a lipoidal portion of the
bovine pineal gland. J. agric. food Chem., 15: 1073-1076.
TAKAGI, A., SAI, K., UMEMURA, T., KUROKAWA, Y., & KASAI, H. (1989)
Production of 8-hydroxydeoxyguanosine in rat liver DNA by oral
administration of peroxisome proliferators. Mutat. Res., 216: 379.
TAKAGI, A., SAI, K., UMEMURA, T., HASEGAWA, R., & KUROKAWA, Y. (1990)
Significant increase of 8-hydroxydeoxyguanosine in liver DNA of rats
following short-term exposure to the peroxisome proliferators di(2-
ethylhexyl)phthalate and di(2-ethylhexyl)adipate Jpn. J. Cancer Res.,
81: 213-215.
TAKAGI, A., SAI, K., UMEMURA, T., HASEGAWA, R., & KUROKAWA, Y. (1991)
Short-term exposure to the peroxisome proliferators, perfluorooctanoic
acid and perfluorodecanoic acid, causes significant increase of 8-
hydroxydeoxyguanosine in liver DNA of rats. Cancer Lett., 57: 55-60.
TAMURA, H., IIDA, T., WATANABE, T., & SUGA, T., (1990) Long-term
effects of hypolipidemic peroxisome proliferator administration on
hepatic hydrogen peroxide metabolism in rats. Carcinogenesis, 11:
445-450.
TARR, B.D., BARRON, M.G., & HAYTON, W.L. (1990) Effect of body size on
the uptake and bioconcentration of di-2-ethylhexyl phthalate in
rainbow trout. Environ. Toxicol. Chem., 9: 989-995.
TAYLOR, B.F., CURRY, R.W., & CORCORAN, E.F. (1981) Potential for
biodegradation of phthalic acid esters in marine regions. Appl.
environ. Microbiol., 42: 590-595.
TEIRLYNCK, O.A. & BELPAIRE, F. (1985) Disposition of orally
administered di-(2-ethylhexyl) phthalate and mono-(2-ethylhexyl)
phthalate in the rat. Arch. Toxicol., 57: 226-230.
THIESS, A.M. & FLIEG, I. (1978) [Chromosomal studies in workers
exposed to di-2-ethylhexylphthalate (DOP).] Zentralbl. Arbeitsmed.,
28: 351-355 (in German).
THIESS, A.M., FRENTZEL-BEYME, R., & WIELAND, R. (1978a) [Mortality
study in workers exposed to di-2-ethylhexyl phthalate (DOP.] In:
[Possibilities and limits of biological monitoring - Problems in
occupational medicine of the industrial occupational health services.
Proceedings of a conference, Frankfurt, May 1978], Stuttgart, A.W.
Gertner, pp. 155-164 (in German).
THIESS, A.M., KORTE, A., & FLIEG, H. (1978b) [Morbidity studies in
workers exposed to di(2-ethylhexyl)phthalate (DEHP).] In:
[Possibilities and limits of biological monitoring - Problems in
occupational medicine of the industrial occupational health services.
Proceedings of a conference, Frankfurt, May 1978], Stuttgart, A.W.
Gertner, pp. 137-154 (in German).
THOMAS, G.H. (1973) Quantitative determination and confirmation of
identity of trace amounts of dialkylphthalates in environmental
samples. Environ. Health Perspect., 3: 23-28.
THOMAS, J.A., SCHEIN, L.G., GUPTA, P.K., MCCAFFERTY, R.E., FELICE,
P.R., & DONOVAN, M.P. (1979) Failure of monoethylhexyl phthalate to
cause teratogenic effects in offspring of rabbits. Toxicol. appl.
Pharmacol., 51: 523-528.
THUREN, A. (1986) Determination of phthalates in aquatic environments.
Bull. environ. Contam. Toxicol., 36: 33-40.
THUREN, A. & LARSSON, P. (1990) Phthalate esters in the Swedish
atmosphere. Environ. Sci. Technol., 24: 554-559.
THUREN, A. & WOIN, P. (1991) Effects of phthalate esters on the
locomotor activity of the freshwater amphipod Gammarus pulex. Bull
environ. Contam. Toxicol., 46: 159-166.
TIMOFIEVSKAJA, L.A., IRANOVA, N.I., & BALYNINA, E.S. (1980)
[Toxicology of esters of o-phthalic acid and hygienic standard for
them.] Gig. Tr. prof. Zabol., 3: 25-28 (in Russian).
TOMASZEWSKI, K.E., AGARWAL, D.K., & MELNICK, R.L. (1986) In vitro
steady-state levels of hydrogen peroxide after exposure of male F344
rats and female B6C3F1 mice to hepatic peroxisome proliferators.
Carcinogenicity, 7: 1871-1876.
TOMITA, I., NAKAMURA, Y., AOKI, N., & INUI, N. (1982a)
Mutagenic/carcinogenic potential of DEHP and MEHP. Environ. Health
Perspect., 45: 119-125.
TOMITA, I., NAKAMURA, Y., YAGI, Y., & TUTIKAWA, K. (1982b)
Teratogenicity/fetotoxicity of DEHP in mice. Environ. Health
Perspect., 45: 71-75.
TOMITA, I., NAKAMURA, Y., YAGI, Y., & TUTIKAWA, K. (1986) Fetotoxic
effects of mono-2-ethylhexyl phthalate (MEHP) in mice. Environ. Health
Perspect., 65: 249-254.
TRIMBLE, A.S., GOLDMAN, B.S., YAO, J.K., DOVATS, L.K., & BIGELOW, W.G.
(1966) Plastics - A source of chemical contamination in surgical
research. Surgery, 59: 857-859.
TYL, R.W., PRICE, C.J., MARR, M.C., & KIMMEL, C.A. (1988)
Developmental toxicity evaluation of dietary di(2-ethylhexyl)phthalate
in Fischer 344 rats and CD-1 mice. Fundam. appl. Toxicol., 10:
395-412.
VAINIOTALO, S. & PFÄFFLI, P. (1990) Air impurities in the PVC plastics
processing industry. Ann. occup. Hyg., 34: 585-590.
VILLENEUVE, D.C., FRANKLIN, C.A., CHU, I., YAGMINAS, A., MARINO, I.A.,
RITTER, L., & RUDDICK, J.A. (1978) Toxicity studies on mono-2-
ethylhexyl phthalate. Toxicol. appl. Pharmacol., 45: 250-251.
VON DÄNIKEN, A., LUTZ, W.K., JÄCKH, R., & SCHLATTER, C. (1984)
Investigation of the potential for binding of di(2-
ethylhexyl)phthalate (DEHP) and di-(2-ethylhexyl)adipate (DEHA) to
liver DNA in vivo. Toxicol. appl. Pharmacol., 73: 373-387.
WAALKES, M.P. & WARD, J.M. (1989) Induction of hepatic metallothionein
in male B6C3F1 mice exposed to hepatic tumor promotors: effects of
phenobarbital, acetaminophen, sodium barbital, and di(2-ethylhexyl)
phthalate. Toxicol. appl. Pharmacol., 100: 216-266.
WALDOCK, M.J. (1983) Determination of phthalate esters in samples from
the marine environment using gas chromatography mass spectrometry.
Chem. Ecol., 1: 261-277.
WALSETH, F., TOFTGÅRD, R., & NILSEN, O.G. (1982) Phthalate esters I:
Effects on cytochrome P-450 mediated metabolism in rat liver and lung,
serum enzymatic activities and serum protein levels. Arch. Toxicol.,
50: 1-10.
WAMS, T.J. (1987) Diethylhexylphthalate as an environmental
contaminant - A review. Sci. total Environ., 66: 1-16.
WARD, J.M., RICE, J.M., CREASIA, D., LYNCH, P., & RIGGS. C. (1983)
Dissimilar patterns of promotion by di(2-ethylhexyl)phthalate and
phenobarbital of hepatocellular neoplasia initiated by
diethylnitrosamine in B6C3F1 mice. Carcinogenesis, 4: 1021-1029.
WARD, J.M., DIWAN, B.A., OHSHIMA, M., HU, H., SCHULLER, H.M., & RICE,
J.M. (1986) Tumor-initiating and promoting activities of di(2-
ethylhexyl) phthalate in vivo and in vitro. Environ. Health Perspect.,
65: 279-291.
WEAST, R.C., ASTLE, M.J., & BEYER, W.H., ed. (1984) CRC handbook of
chemistry and physics, 65th ed., Boca Raton, Florida, CRC Press Inc.
WEBER, K. & ERNST, W. (1983) [Occurrence and fluctuation of organic
chemicals in major German estuaries.] Vom Wasser, 61: 111-123 (in
German).
WHITE, R.D., CARTER, D.E., EARNEST, D., & MUELLER, J. (1980)
Absorption and metabolism of 3 phthalate esters by the rat small
intestine. Food Cosmet. Toxicol., 18: 383-386.
WHO/FAO (1984a) Evaluation of certain food additives and contaminants
(Twenty-eighth report of the Joint FAO/WHO Expert Committee on Food
Additives), Geneva, World Health Organization, 44 pp (WHO Technical
Report Series, No. 710, and corrigendum).
WHO/FAO (1984b) Toxicological evaluation of certain food additives and
contaminants (Prepared by the Joint FAO/WHO Expert Committee on Food
Additives, Rome, 19-28 March, 1984), Geneva, World Health
Organization, 222 pp (WHO Food Additives Series No. 19).
WHO/FAO (1989a) Evaluation of certain food additives and contaminants
(Thirty-third report of the Joint FAO/WHO Expert Committee on Food
Additives), Geneva, World Health Organization, 64 pp (WHO Technical
Report Series No. 776).
WHO/FAO (1989b) Toxicological evaluation of certain food additives and
contaminants (Prepared by the 29th Meeting of the Joint FAO/WHO Expert
Committee on Food Additives), 362 pp. Cambridge, Cambridge University
Press (WHO Food Additives Series No. 24).
WILLIAMS, D.T. (1973) Dibutyl- and di-(2-ethylhexyl) phthalate in
fish. J. agric. food Chem., 21: 1128-1129.
WOFFORD, H.W., WILSEY, C.D., NEFF, G.S., GIAM, C.S., & NEFF, J.M.
(1981) Bioaccumulation and metabolism of phthalate esters by oysters,
brown shrimp, and sheepshead minnows. Ecotoxicol. environ. Saf., 5:
202-210.
WOIN, P. & LARSSON, P. (1987) Phthalate esters reduce predation
efficiency of dragonfly larvae, Odonata; Aeshna. Bull. environ.
Contam. Toxicol., 38: 220-225.
WOLFE, N.L., BURNS, L.A., & STEEN, W.C. (1980a) Use of linear free
energy relationships and an evaluative model to assess the fate and
transport of phthalate esters in the aquatic environment. Chemosphere,
9: 393-402.
WOLFE, N.L., STEEN, W.C., & BURNS, L.A. (1980b) Phthalate ester
hydrolysis: Linear free energy relationships. Chemosphere, 9: 403-408.
WOOD, D.L. & BITMAN, J. (1984) The effect of feeding di-(2-ethylhexyl)
phthalate and related compounds on lipids in the laying hen. Poult.
Sci., 63: 469-477.
WOODWARD, K.N., SMITH, A.M., MARISCOTTI, S.P., & TOMLINSON, N.J.
(1986) Review of the toxicity of the esters of o-phthalic acid
(phthalate esters), London, Health and Safety Executive, 183 pp
(Toxicity Review 14).
YAGI, Y., NAKAMURA, Y., TOMITA, I., TSUCHIKAWA, K., & SHIMOI, N.
(1980) Teratogenic potential of di- and mono-(2-ethylhexyl) phthalate
in mice. J. environ. Pathol. Toxicol., 4: 533-544.
YANAGITA, T., KOBAYASHI, K., & ENOMOTO, N. (1978) Accumulation of
hepatic phospholipids in rats fed di(2-ethylhexyl) phthalate. Biochem.
Pharmacol., 27: 2283-2288.
YANAGITA, T., KUZUHARA, S., ENOMOTO, N., SHIMAD, T., & SUGANO, M.
(1979) Effects of di(2-ethylhexyl) phthalate on the content of hepatic
mitochondrial and microsomal phospholipids in the rat. Biochem.
Pharmacol., 28: 3115-3121.
YOON, J.S., MASON, J.M., VALENCIA, R., WOODRUFF, R.C., & ZIMMERING, S.
(1985) Chemical mutagenesis testing in Drosophila. IV. Results of 45
coded compounds tested for the National Toxicology Program. Environ.
Mutagen., 7: 349-367.
YOSHIKAWA, K., TANAKA, A., YAMAHA, T., & KURATA, H. (1983)
Mutagenicity study of nine monoalkyl phthalates and a dialkyl
phthalate using Salmonella typhimurium and Escherichia coli. Food
chem. Toxicol., 21: 221-223.
ZEIGER, E., HAWORTH, S., MORTELMANS, K., & SPECK, W. (1985)
Mutagenicity testing of di(2-ethylhexyl) phthalate and related
chemicals in Salmonella. Environ. Mutagen., 7: 213-232.
ZIMMERING, S., MASON, J.M., & VALENCIA, R. (1989) Chemical mutagenesis
testing in drosophila. VII. Results of 22 coded compounds tested in
larval feeding experiments. Environ. mol. Mutagen., 14: 245-251.
ZIOGOU, K., KIRK, P.W.W., & LESTER, J.N. (1989) Behaviour of phthalic
acid esters during batch anaerobic digestion of sludge. Water Res.,
23: 743-748.
ZITKO, V. (1972) Determination, toxicity, and environmental levels of
phthalate plasticizers, St. Andrews, N.B., Biological Station,
Fisheries Research Board of Canada, 35 pp (Technical Report No. 344).
RESUME
1. Identité, propriétés physiques et chimiques et méthodes d'analyse
Le phtalate de di(2-éthylhexyle) est un ester de l'acide
benzènedicarboxylique qui se présente, à la température ambiante, sous
la forme d'un liquide huileux, incolore à jaune. Il est peu soluble
dans l'eau (0,3-0,4 mg/litre) et encore moins dans l'eau salée. Il est
miscible à la plupart des solvants organiques courants. Sa volatilité
est relativement faible (tension de vapeur: 8,6 x 10-4Pa).
On a mis au point, pour le dosage du DEHP dans différents
milieux, un grand nombre de méthodes de prélèvement et d'analyse. On
fait de plus en plus appel à des méthodes sensibles telles que la
chromatographie en phase gazeuse, la chromatographie en phase liquide
à haute performance et la spectrométrie de masse. La contamination due
au matériel en plastique utilisé lors des prélèvements et de l'analyse
complique le dosage du DEHP à faibles concentrations.
2. Sources d'exposition humaine et environnementale
Le DEHP présent dans l'environnement est, dans sa presque
totalité, d'origine artificielle.
La production mondiale de DEHP augmente depuis quelques décennies
et s'élève actuellement à environ un million de tonnes par an. Les
Etats-Unis d'Amérique et l'Europe entrent chacun pour un tiers dans
cette production.
Le DEHP est le plastifiant le plus largement utilisé pour
augmenter la fluidité des résines puisqu'il représente 50% de
l'ensemble des esters phtaliques utilisés comme plastifiants. Il peut
représenter jusqu'à 40% en poids ou davantage de la matière plastique.
Le DEHP est utilisé dans la fabrication du chlorure de polyvinyle
(PVC) utilisé dans l'industrie du bâtiment et de l'emballage ainsi que
pour la fabrication d'accessoires à usage médical. On l'utilise
également en plus petites quantités pour la préparation de peintures
industrielles ainsi que comme diélectrique dans les condensateurs.
Les produits contenant du plastique qui sont mis au rebut peuvent
être éliminés soit par incinération soit par enfouissement dans une
décharge. Lors de l'incinération à basse température, une proportion
importante du produit peut se dissiper dans l'atmosphère. On n'a pas
très bien étudié ce qu'il advient du DEHP déposé dans les décharges et
il n'est pas possible à ce stade de tirer des conclusions définitives.
3. Transport, distribution, et transformation dans l'environnement
C'est principalement en étant transportés dans l'atmosphère que
les phtalates pénètrent dans l'environnement. A partir de là, le DEHP
tombe sur le sol à moins d'être éliminé par les précipitations.
Le coefficient de partage du DEHP entre l'octanol et l'eau est
élevé, de sorte que l'équilibre entre l'eau et un sol ou un sédiment
riche en substances organiques s'établit aux dépens de la phase
aqueuse. Le DEHP s'adsorbe facilement sur les particules organiques et
du sol.
Bien que peu soluble dans l'eau, le DEHP peut se rencontrer en
quantités relativement élevées dans les eaux de surface en raison de
son adsorption sur les particules organiques et de son interaction
avec les substances organiques en solution. L'adsorption s'effectue
plus particulièrement sur les particules de petite taille et elle est
favorisée par la présence d'eau salée.
Dans l'atmosphère, le DEHP subit une photodégradation rapide mais
son hydrolyse dans l'environnement est pratiquement inexistante.
Plusieurs microorganismes terricoles effectuent la dégradation
aérobie du DEHP. Toutefois dans l'environnement, cette dégradation
microbienne s'effectue avec lenteur. La biodégradation commence par
l'hydrolyse du mono-ester qui est transformé en acide phtalique. Il y
a ensuite dégradation en pyruvate et succinate par ouverture du cycle
puis en CO2 et H2O, comme dans la dégradation métabolique de
l'acide benzoïque. La dégradation aérobie dépend de la température. Au
dessous de 10 °C la dégradation est peu importante. A température
supérieure, la biodégradation se produit dans la partie supérieure du
sol mais, à plus grande profondeur elle est pratiquement inexistante
du fait des conditions d'anaérobiose. La dégradation anaérobie, pour
autant qu'elle existe, est beaucoup plus lente que la dégradation
aérobie.
Le DEHP est très lipophile et modérément persistant. Le degré de
bioaccumulation dépend de la capacité de l'organisme qui l'absorbe à
métaboliser la substance. On a montré qu'il s'accumulait en forte
proportion chez divers invertébrés aquatiques, les poissons et les
amphibiens.
Après application de DEHP sur des feuilles de végétaux, on n'a
constaté qu'une faible perte de matière sur une période de 15 jours.
Le DEHP présent dans le sol ou dans les boues d'égout n'est que peu
fixé par les plantes.
4. Concentrations dans l'environnement et exposition humaine
Le DEHP est très répandu dans l'environnement et on le retrouve
dans la plupart des échantillons prélevés dans l'air, dans les
précipitations, l'eau, les sédiments, le sol et les biotes. C'est
généralement dans les zones industrielles que les teneurs sont les
plus fortes.
On a relevé des concentrations allant jusqu'à 300 ng/m3 dans
l'air des villes et en atmosphère polluée. Dans l'atmosphère
surmontant les océans, on a fait état de concentrations allant de 0,5
à 5 ng/m3 et les précipitations de ces régions en contenaient
jusqu'à 200 ng/litre. L'étude d'échantillons de précipitations
prélevés dans un secteur situé à proximité d'une usine produisant des
plastifiants, a montré que la vitesse de dépot à sec était de 0,7 à
4,7 µg/m2 et par jour.
Dans les rivières et les lacs, on a trouvé des concentrations de
DEHP allant jusqu'à 4 µg/litre, les teneurs les plus élevées étant
relevées au point de décharge d'effluents industriels. Dans la mer, la
concentration est inférieure à 1 µg/litre, les concentrations les plus
fortes étant observées dans les estuaires.
En raison de son caractère hydrophobe, le DEHP est facilement
absorbé par le sol, les sédiments et les particules. Dans les
sédiments de cours d'eau, des concentrations allant jusqu'à 70 mg/kg
(de poids à sec) ont été signalées, concentrations qui peuvent monter
jusqu'à 1480 mg/kg (de poids à sec) au voisinage des points de
décharge.
Dans les biotes, la concentration du DEHP varie de moins de 1 à
7000 µg/kg. On le rencontre dans divers types d'aliments: poisson,
coquillages, oeufs et fromages. L'exposition moyenne a été estimée à
environ 300 µg par personne et par jour aux Etats-Unis d'Amérique en
1974 et à 20 µg par personne et par jour au Royaume-Uni en 1986.
Les transfusions de sang et autres traitements utilisant du
matériel en plastique peuvent entraîner l'exposition involontaire des
malades au DEHP. C'est ainsi qu'on a relevé dans le poumon de malades
ainsi traités, des teneurs allant de 13,4 à 91,5 mg/kg de poids à sec.
Les quelques données disponibles indiquent que sur les lieux de
travail, la concentration de DEHP est généralement inférieure à 1
mg/m3.
5. Cinétique et métabolisme
Des données disponibles au sujet de l'administration par voie
orale indiquent que le DEHP est hydrolysé dans l'intestin par la
lipase pancréatique. Les métabolites qui se forment, c'est-à-dire le
phtalate de mono-2-éthylhexyle (MEHP) et le 2-éthylhexanol sont
rapidement absorbés. Après administration de DEHP marqué au 14C
(2,9 mg/kg) par voie orale à des rats, on a récupéré 50% de la dose
initiale dans l'urine et la bile de ces animaux. Il semble que la
biodisponibilité d'une dose orale de DEHP soit plus élevée chez les
jeunes animaux.
Après administration par voie orale, le DEHP est largement
hydrolysé dans l'intestin de certains animaux, par exemple les rats,
et se répartit dans l'organisme, principalement sous forme de phtalate
de monoéthylhexyle (MEHP). Toutefois chez les primates et l'homme,
l'hydrolyse est beaucoup moins importante. Il semble que ce soit
principalement au niveau du foie que s'effectue la métabolisation du
MEHP et du 2-éthylhexanol. On a identifié plusieurs autres
métabolites, l'omega et omega-1-oxydation étant les principales voies
métaboliques. Un ou plusieurs des produits résultant de l'omega-
oxydation peuvent encore être métabolisés par ß-oxydation. On a
constaté que l'omega-oxydation s'effectuait selon une cinétique non
linéaire. Le métabolisme du DEHP offre des différences considérables
selon les espèces; par exemple, l'omega-oxydation est moins importante
chez l'homme que chez le rat.
Une dose de DEHP de 2,9 mg/kg, administrée par voie orale à des
rats, a été récupérée une semaine plus tard presque intégralement dans
les matières fécales et l'urine des animaux. La bile et l'urine
constituent les principales voies d'excrétion. Lors d'une étude sur
l'homme, 15 à 25% d'une dose orale (0,45 mg/kg de DEHP) ont été
excrétés sous forme de MEHP et la majeure partie des produits
d'excrétion étaient constitués de métabolites oxydés.
6. Effets sur les mammifères de laboratoire et les systèmes
d'épreuve in vitro
La DL50 par voie orale du DEHP est d'environ 25 à 34 g/kg,
selon l'espèce, mais cette valeur est plus faible dans le cas du MEHP.
Lors d'études d'alimentation sur des rats et des souris, on a constaté
que des doses quotidiennes de DEHP supérieures à 3 g/kg entraînaient
la mort dans les 90 jours et qu'une dose de 0,4 g/kg réduisait le gain
de poids en l'espace de quelques jours. Dans d'autres études, c'est
une dose de 6,3-12,5 g/kg de nourriture qui a entraîné une réduction
du poids corporel.
Lors d'études à long terme, on a observé chez les animaux traités
une hépatomégalie accompagnée d'une augmentation du poids relatif des
reins. Dans l'une de ces études, on observait également une
hypertrophie cellulaire au niveau du lobe antérieur de l'hypophyse.
Un certain nombre d'études ont permis de mettre en évidence une
atrophie testiculaire, apparaîssant au bout de quelques jours, et liée
à l'administration de DEHP (doses dans l'alimentation allant de
10-20 g/kg). Les jeunes rats semblent plus vulnérables et les rats et
les souris plus sensibles que les hamsters et les ouistitis. On a
constaté que cette atrophie était réversible. Le MEHP exerce des
effets toxiques in vitro sur les cellules de Sertoli. Le DEHP de
même que le MEHP ont des propriétés tératogènes. Des malformations ont
été observées à des doses de 0,5-2 g/kg de nourriture chez la souris
et, à des doses supérieures à 10 g/kg, on a observé des effets
embryotoxiques.
Les tests de mutagénicité et autres manifestations du même genre
ont donné des résultats négatifs dans la plupart des études. Le DEHP
peut provoquer la transformation des cellules et on a montré qu'il
était cancérogène à des doses respectives de 6 et 12 g/kg de
nourriture chez le rat et de 3 et 6 g/kg de nourriture chez la souris.
On a constaté une augmentation, liée à la dose, des tumeurs
hépatocellulaires chez les deux sexes de l'une et l'autre espèce. La
prolifération des peroxysomes hépatiques et la réplication cellulaire
sont fortement liées aux effets cancérogènes sur le foie de certains
produits non génotoxiques comme le DEHP. Toutefois on a observé des
différences importantes entre les espèces animales en ce qui concerne
la prolifération des peroxysomes induite par le DEHP.
Contrairement à ce qui se passe dans le cas des hépatocytes de
rat, les métabolites du DEHP ne provoquent pas de prolifération des
peroxysomes dans les cultures d'hépatocytes humains.
7. Effets sur l'homme
On ne dispose que de données très limitées sur les effets que le
DEHP peut exercer sur l'homme. On a fait état chez deux sujets qui
avaient reçu 5 ou 10 g de DEHP respectivement, d'effets qui se
limitaient à de légers troubles gastriques.
8. Effets sur les autres êtres vivants en laboratoire et dans
leur milieu naturel
Dans la plupart des études, les valeurs nominales de la CL50
obtenues lors des tests de toxicité aiguë dépassent 10 mg/litre, ce
qui indique que le DEHP est peu toxique. Toutefois ces valeurs sont
supérieures à la solubilité du DEHP dans l'eau (0,3-0,4 mg/litre).
D'après une étude, une daphnie, Daphnia pulex, serait
particulièrement sensible, la CL50 à 48 heures étant dans son cas de
0,133 mg/litre. La seule épreuve de toxicité aiguë où l'on ait mesuré
des concentrations de DEHP a été effectuée sur un poisson, Pimephales
promelas, et elle a donné une CL50 à 96 heures supérieure à 0,33
mg/litre. Lors d'études de longue durée, on a obtenu une dose sans
effet nocif observable de 72 µg/litre pour Daphnia magna. Pour les
poissons adultes, cette dose était supérieure à 72 µg/litre. Une
exposition à une concentration de 14 µg/litre, pendant 12 jours avant
l'éclosion, a provoqué une importante augmentation de la mortalité
parmi des alevins de truites. Des concentrations comprises en 3,7 et
11 µg/litre ont entraîné une réduction de la teneur en collagène
vertébral chez les poissons.
La présence de DEHP dans la nourriture à raison de 50 mg/kg a eu
des effets nocifs sur la survie des alevins de Melambaphes zebra. A
la concentration de 25 mg/kg en poids dans les sédiments, on a
constaté une réduction de l'activité microbienne et du nombre d'
éclosions chez les tétards.
Le DEHP est peu toxique pour les algues, les végétaux, les
lombrics et les oiseaux.
9. Evaluation
Le DEHP exerce des effets cancérogènes sur le foie et affecte le
système reproducteur chez le rat et la souris.
Le principal effet au niveau des gonades consiste, chez le rat et
la souris, en une atrophie des testicules qui affecte davantage les
jeunes animaux. La prolifération des peroxysomes hépatiques et la
réplication cellulaire sont fortement liées à l'effet cancérogène
qu'exercent sur le foie certains produits non génotoxiques au nombre
desquels figure le DEHP. Toutefois, on a observé de fortes différences
selon les espèces animales pour ce qui est de la prolifération des
peroxysomes induite par le DEHP. On ne possède pas actuellement de
preuves suffisantes permettant de conclure que le DEHP est
potentiellement cancérogène pour l'homme.
Il n'existe pas de données attestées selon lesquelles le DEHP
serait dangereux pour la faune, si l'on s'en tient aux résultats
obtenus lors de l'exposition de poissons et de daphnies. Toutefois, on
a observé une réduction de l'activité microbienne dans les sédiments
aux doses auxquelles le DEHP est présent dans l'environnement. Si l'on
compare les doses de DEHP présentes dans l'environnement aux
concentrations qui sont susceptibles de produire des effets lors
d'études de longue durée, notamment sur les larves de poissons et
d'amphibiens, on ne peut exclure l'existence d'un risque pour
l'environnement, les effets s'exerçant notamment par l'intermédiaire
de l'eau et des sédiments. Des effets nocifs sur les êtres vivants
sont possibles dans les secteurs où l'eau et les sédiments sont
fortement pollués en raison de leur proximité des points de décharge.
Bien que peu d'études valables aient été effectuées, il semble
que le DEHP présente une faible toxicité aiguë pour les algues, les
végétaux, les lombrics et les oiseaux.
RESUMEN
1. Identidad, propiedades físicas y químicas y métodos analíticos
El di(2-etilhexil) ftalato (DEHF) es un éster del ácido
bencenodicarboxílico que a temperatura ambiente es un líquido oleoso
entre incoloro y amarillo. Su solubilidad en agua es baja (0,3-0,4
mg/litro) y aún más baja en agua salada. Es miscible con la mayor
parte de los disolventes orgánicos normales. La volatilidad del DEHF
es relativamente baja (8,6 x10-4 Pa).
Se han perfeccionado numerosos métodos de muestreo y análisis
para la determinación del DEHF en diferentes medios. Cada vez se
utilizan más métodos de gran sensibilidad, como la cromatografía de
gases, la cromatografía líquida de alto rendimiento y la
espectrometría de masas. El análisis de concentraciones bajas de DEHF
se complica por la contaminación debida al material de plástico
utilizado en el muestreo y el análisis.
2. Fuentes de exposición humana y ambiental
Casi todo el DEHF presente en el medio ambiente procede de
fuentes antropogénicas.
La producción mundial de DEHF ha ido aumentando durante los
últimos decenios y en la actualidad es de alrededor de 1 x 106
toneladas al año. Un tercio del total se produce en los Estados Unidos
y otro tercio en Europa.
El DEHF es el plastificante más utilizado (representa el 50% de
todos los plastificantes a base de ésteres de ftalato) para ablandar
las resinas. A él corresponde el 40% (p/p) o más del total de
plásticos. El DEHF se emplea en la fabricación de cloruro de
polivinilo (PVC) utilizado en los edificios, en la construcción, en
embalajes y en componentes de aparatos médicos. Se utiliza en
cantidades más pequeñas en las pinturas industriales y como fluido
dieléctrico en los condensadores.
Los productos plastificados de desecho se pueden eliminar por
incineración o terraplenado. Durante la incineración a baja
temperatura, se puede liberar un porcentaje elevado del DEHF a la
atmósfera. No se ha estudiado bien el destino medioambiental del DEHF
depositado en terraplenes, por lo que no se pueden sacar conclusiones
definitivas.
3. Transporte, distribución y transformación en el medio ambiente
El transporte en el aire es la vía más importante de
incorporación de los ftalatos al medio ambiente. De la atmósfera, el
DEHF precipita o es lavado por la lluvia.
El DEHF tiene un coeficiente de reparto octanol-agua elevado, de
manera que el equilibrio entre el agua y un suelo o sedimento rico en
materia orgánica está desplazado a favor de este último. Las
partículas orgánicas del suelo lo adsorben fácilmente.
Aunque la solubilidad del DEHF en agua es baja, la cantidad
presente en las aguas de superficie puede ser mayor debido a la
adsorción a las partículas orgánicas y a la interacción con la materia
orgánica disuelta. Las pequeñas partículas lo adsorben más fácilmente,
y el proceso se potencia en el agua salada.
La fotodegradación atmosférica del DEHF es rápida; en cambio, su
hidrólisis química en el medio ambiente es prácticamente inexistente.
Se ha observado que varios microorganismos del suelo llevan a
cabo la degradación aerobia. Sin embargo, se ha informado que la
degradación microbiana del DEHF en el medio ambiente es lenta. El
proceso de biodegradación comienza con la hidrólisis para formar el
monoéster, que se transforma luego en ácido ftálico. La degradación
con ruptura del anillo para dar primero piruvato y succinato y después
CO2 y H2O es análoga a la vía metabólica del ácido benzoico. La
degradación aerobia es dependiente de la temperatura. Por debajo de
los 10 °C es escasa. A temperaturas más altas se produce
biodegradación en la capa más superficial del suelo, pero es
prácticamente inexistente en las capas más profundas, donde las
condiciones son de anaerobiosis. La degradación anaerobia, si existe,
es mucho más lenta que la aerobia.
El DEHF es muy lipófilo y moderadamente persistente. El grado de
bioacumulación depende de la capacidad de los organismos para
metabolizarlo. Se ha observado un grado elevado de acumulación en
diversos invertebrados acuáticos, peces y anfibios.
Cuando se aplicó DEHF a hojas de plantas, la pérdida fue pequeña
durante un período de 15 días. Se ha observado que su absorción por
las plantas a partir del suelo o de los fangos cloacales es muy baja.
4. Niveles medioambientales y exposición humana
El DEHF está muy ampliamente distribuido en la naturaleza y se
encuentra en la mayor parte de las muestras, por ejemplo de aire,
precipitación, sedimento, suelo y biota. Las concentraciones más altas
aparecen generalmente en las zonas industrializadas.
En el aire urbano y contaminado se han encontrado concentraciones
de hasta 300 ng/m3. Se han comunicado de niveles comprendidos entre
0,5 y 5 ng/m3 en el aire de zonas oceánicas, y la lluvia de esas
zonas contenía hasta alrededor de 200 ng/litro. En muestras de
precipitación procedentes de una zona cercana a una fábrica de
plastificantes se observó que la tasa sedimentación seca era de 0,7 a
4,7 µg/m2 al día.
Se han encontrado concentraciones de DEHF en ríos y lagos de
hasta 4 µg/litro; los niveles más altos están asociados a los puntos
de descarga de efluentes industriales. La concentración en el mar es
inferior a 1 µg/litro, y los niveles más altos se detectan en los
estuarios.
Debido a su carácter hidrófobo, el DEHF se adsorbe muy fácilmente
al suelo, los sedimentos y la materia particulada. En los sedimentos
fluviales se han comunicado niveles de hasta 70 mg/kg (peso seco), y
cerca de los puntos de descarga ha llegado a ser de 1480 mg/kg (peso
seco).
La concentración de DEHF en la biota oscila entre menos de 1 y
7000 µg/kg. Se ha detectado en diversos tipos de alimentos, como
pescado, marisco, huevos y queso. En 1974 se estimó en los Estados
Unidos una exposición media de 300 µg/persona al día y en el Reino
Unido en 1986 de 20 µg/persona al día.
Las transfusiones de sangre y otros tipos de tratamiento médico
en los que se utilizan aparatos de plástico pueden dar lugar a una
exposición humana involuntaria al DEHF. En el tejido pulmonar de
algunos pacientes se han detectado concentraciones comprendidas entre
13,4 y 91,5 µg/kg (peso seco).
Los escasos datos disponibles indican que las concentraciones de
DEHF en el lugar de trabajo suelen estar por debajo de los 1 mg/m3.
5. Cinética y metabolismo
Los datos disponibles sobre la administración oral indican que la
lipasa pancreática hidroliza el DEHF en el intestino. Los metabolitos
formados, que son el mono (2-etilhexil) ftalato (MEHF) y el
2-etilhexanol, se absorben rápidamente. Cuando se administró por vía
oral a ratas DEHF marcado con 14C (2,9 mg/kg), se recuperó en la
orina o la bilis más del 50%. La biodisponibilidad de una dosis oral
de DEHF parece ser más elevada en las ratas jóvenes que en las más
viejas.
El DEHF administrado por vía oral se hidroliza en el intestino de
ciertos animales, por ejemplo las ratas, y se distribuye sobre todo
como monoetilhexil ftalato (MEHF). Sin embargo, la hidrólisis es mucho
menor en los primates y el ser humano. El MEHF se liga a las proteínas
del plasma. El hígado parece ser el principal órgano metabolizador del
MEHF y del 2-etilhexanol. Se han identificado algunos otros
metabolitos, siendo las principales vías metabólicas la omega-y la
omega-1-oxidación. Uno o varios productos de la omega-oxidación pueden
metabolizarse ulteriormente por ß-oxidación. En la omega-oxidación se
han observado cinéticas no lineales. En el metabolismo del DEHF se
registran considerables diferencias entre las especies; por ejemplo,
la omega-oxidación es menor en el hombre que en las ratas.
Una semana después de administrar una dosis oral de DEHF
(2,9 mg/kg) se recuperó casi el 100% en las heces y la orina de ratas.
La bilis y la orina son las principales vías de excreción. En un
estudio humano, del 15 al 25% de la dosis oral (0,45 mg/kg) de DEHF se
excretó en forma de MEHF; los metabolitos oxidados constituían la
parte más importante de los productos de excreción.
6. Efectos en los mamíferos de laboratorio y en sistemas de
ensayo in vitro
La DL50 del DEHF por vía oral es de alrededor de 25-34 g/kg,
según las especies, pero el valor para el MEHF es más bajo. En
estudios de alimentación en ratas y ratones, las dosis de DEHF
superiores a 3 g/kg al día causaron muertes en un plazo de 90 días, y
con una concentración de 0,4 g/kg al día se redujo el aumento de peso
a los pocos días. En otros estudios, con dosis de 6,3-12,5 g/kg de
alimentos se produjo una reducción del peso corporal.
En los animales tratados en estudios de larga duración se ha
observado hepatomegalia y un aumento del peso relativo de los riñones.
En un estudio se encontraron también células hipertróficas en el
lóbulo anterior de la hipófisis.
En varios estudios se ha detectado atrofia testicular, evidente
al cabo de pocos días, relacionada con la administración de DEHF
(concentraciones de 10-20 g de DEHF/kg de alimentos). Las ratas más
jóvenes parecen ser más susceptibles que las viejas, y las ratas y los
ratones parecen ser más sensibles que los titís y los hámsters. Se ha
observado que la atrofia es reversible. El MEHF tiene efecto tóxico
in vitro en las células de Sertoli. Tanto el DEHF como el MEHF
muestran propiedades teratogénicas. Con concentraciones de 0,5 a 2
g/kg de alimentos se observaron malformaciones en ratones, y con
niveles superiores a los 10 g/kg se apreciaron efectos embriotóxicos.
Las pruebas de mutagenicidad y puntos finales asociados han dado
resultados negativos en la mayor parte de los estudios. El DEHF puede
inducir la transformación celular y se ha demostrado que con dosis de
6 y 12 g/kg de alimentos en ratas y de 3 y 6 g/kg de alimentos en
ratones tiene efectos carcinógenos. En ambos sexos de las dos especies
se produjo un aumento de tumores hepatocelulares dependiente de la
dosis. La inducción de la proliferación de peroxisomas hepáticos y de
la replicación celular está fuertemente vinculada al efecto
carcinógeno en el hígado de determinados compuestos carcinógenos no
genotóxicos, entre los que figura el DEHF. Sin embargo, se han
observado notables diferencias entre distintas especies animales con
respecto a la proliferación de peroxisomas inducida por el DEHF.
A diferencia de lo que ocurre con los hepatocitos de rata, los
metabolitos del DEHF no inducen proliferación de peroxisomas en
cultivos de hepatocitos humanos.
7. Efectos en el ser humano
La información disponible sobre los efectos del DEHF en el ser
humano es muy escasa. Se han comunicado dos casos de trastornos
gástricos leves, con dosis de 5 y 10 g de DEHF, pero sin ningún otro
efecto nocivo.
8. Efectos en otros organismos en el laboratorio y en el
medio ambiente
En la mayoría de los estudios de toxicidad aguda se han obtenido
unos valores nominales para la CL50 que se sitúan por encima de los
10 mg/litro, por lo que el índice de toxicidad del DEHF es bajo. Sin
embargo, esos niveles son superiores a los correspondientes a su
solubilidad en agua (0,3-0,4 mg/litro). En un estudio se detectó en la
pulga de agua Daphnia pulex una sensibilidad mayor, con un valor
nominal de la CL50 a las 48 h de 0,133 mg/litro. La única prueba de
la toxicidad aguda realizada con concentraciones medidas de DEHF se
hizo en el pez Pimephales promelas y puso de manifiesto una CL50
> 0,33 mg/litro a las 96 h. En estudios prolongados, el nivel sin
observación de efectos (NOEL) en Daphnia magna fue de 72 µg/litro.
En peces adultos se determinó un NOEL > 62 µg/litro. Una exposición
a 14 µg/litro desde 12 días antes de la eclosión produjo un
significativo aumento de la mortalidad de los alevines de trucha. Las
concentraciones entre 3,7 y 11 µg/litro provocaron una reducción del
colágeno vertebral en peces.
Con concentraciones de 50 mg/kg de alimentos, el DEHF tiene
efectos adversos sobre la supervivencia de los alevines de
Brachydanio rerio. Concentraciones de 25 mg/kg (p/p) en el sedimento
redujeron de manera considerable la actividad microbiana y el número
de renacuajos que nacían.
La toxicidad aguda del DEHF en algas, plantas, lombrices de
tierra y aves es baja.
9. Evaluación
El DEHF tiene efectos reproductivos y hepatocarcinogénicos en
ratas y ratones.
El principal efecto sobre la reproducción en ratas y ratones es
la atrofia testicular; los animales jóvenes son más susceptibles que
los viejos. La inducción de la proliferación de peroxisomas hepáticos
y de la replicación celular está estrechamente relacionada con el
efecto carcinogénico en el hígado de determinados compuestos
carcinógenos no genotóxicos, incluido el DEHF. Sin embargo, se han
observado notables diferencias entre las distintas especies de
animales con respecto a la proliferación de peroxisomas inducida por
el DEHF. En la actualidad no hay pruebas suficientes que indiquen que
el DEHF sea un posible carcinógeno para el ser humano.
No hay información documentada de que el DEHF constituya un
riesgo, tomando como base la exposición aguda de peces y dáfnidos. Sin
embargo, se ha informado de una reducción de la actividad microbiana
en los sedimentos con los niveles de DEHF presentes en el medio
ambiente. La comparación entre los niveles medioambientales y las
concentraciones que producen efectos en estudios prolongados,
especialmente las pruebas en las fases vitales tempranas de peces y
anfibios, indica que no se puede descartar un cierto peligro para el
medio ambiente, sobre todo por conducto del agua y los sedimentos. Es
probable que se produzcan efectos adversos en los organismos en zonas
con agua y sedimentos muy contaminados que están próximos a las
fuentes de emisión.
Aunque son pocos los estudios que se conocen al respecto, la
toxicidad aguda del DEHF en algas, plantas, lombrices de tierra y aves
parece ser baja.
There are marked species differences in the metabolism of DEHP.
Thus omega-oxidation seems to play a dominante role in the rat and
guinea-pig (Albro et al., 1982; Lhuguenot & Elcombe, 1984; Lhuguenot
et al., 1985), but to be a minor pathway in the mouse, hamster, green
monkey, cynomolgus monkey, and marmoset (Albro et al., 1982; Lhguenot
& Elcombe, 1984). In guinea-pigs there are few omega-1 metabolites of
MEHP (Albro et al., 1982). This may have toxicological significance
because certain omega-1-oxidation metabolites have been identified as
active agents in peroxisomal proliferation in rat hepatocytes
(Mitchell et al., 1985a).
No conjugated metabolites were detected in the urine of
DEHP-treated rats, but a minor portion was conjugated in the urine of
hamsters (Albro et al., 1982; Lhuguenot & Elcombe, 1984). A major
portion of glucuronide conjugates was found in the urine of the
marmoset, mouse, guinea-pig, and green monkey, and in human urine
(Albro et al., 1982). Albro (1986) reported that glucuronidation of
DEHP metabolites was insignificant in rats. Studies in primates,
including the African green monkey (Albro et al., 1981), marmoset
(Rhodes et al., 1986), cynomolgus monkey (Short et al., 1987; Astill,
1989), and man (Schmid & Schlatter, 1985), demonstrated that
conjugation of DEHP can occur at the carboxylic acid moiety following
a single ester hydrolysis.
The level of unmetabolized MEHP excreted in urine also varies
considerably between species; it is low in the rat and hamster, but
high in the mouse, guinea-pig, green monkey, and man (Albro et al.,
1982).
Repeated oral administration of DEHP or MEHP at high doses (500
mg/kg) to rats leads to a change in the metabolic profile; there is an
increase in omega-oxidized metabolites and a decrease in
omega-1-oxidized metabolites (Lhuguenot et al., 1985). In rats given
2% DEHP in the diet for one week, a 4-fold increase in peroxisomal ß-
oxidation was found. ß-Oxidation of fatty acids induced by DEHP
appears to occur via mitochondrial and peroxisomal pathways that are
similar to normal pathways (Ganning et al., 1989). Drug-metabolizing
enzyme activities have been studied after DEHP administration, and in
some cases changes were observed (Walseth et al., 1982; Agarwal et
al., 1982; Gollamudi et al., 1985; Pollack et al., 1989).
The same metabolites as those found in rat urine can be detected
in human urine. One study on intravenously injected DEHP (Albro et
al., 1982) and one on orally administered DEHP (Schmid & Schlatter,
1985) indicated that humans metabolize DEHP by omega- and
omega-1-oxidation as well as by oxidation of the ethyl side chain.
However, the omega-oxidation-pathway seems to be a minor pathway in
man (Albro et al., 1982; Schmid & Schlatter, 1985). More than half of
the metabolites recovered in human urine are conjugated metabolites
(Albro et al., 1982; Schmid & Schlatter, 1985).
Time-averaged concentrations of DEHP, MEHP, and phthalic acid in
the blood of patients undergoing maintenance haemodialysis were 1.9,
1.3, and 5.2 mg/litre, respectively (Pollack et al., 1985a). Such
patients are considered to be at risk of potential DEHP toxicity
through prolonged contact with medical plastic products that contain
DEHP. The relatively high circulating level of phthalic acid may
indicate an altered metabolism of DEHP in uraemic patients (Pollack et
al., 1985a).
The levels of DEHP and MEHP in plasma have been studied in
newborn infants given blood exchange transfusions. In one case the
MEHP half-life was the same as for DEHP (about 12 h), indicating that
the hydrolysis of DEHP was the rate-limiting metabolic step. However,
in other children the half-time of MEHP was longer than that of DEHP
(Sjöberg et al., 1985b).
6.4 Elimination and excretion
Radioactivity from intravenously injected 14C-labelled DEHP is
mainly recovered in urine and faeces after 24 h (Schulz & Rubin,
1973), indicating that urine and bile are major excretory pathways.
When a low dose level (0.1 mg/kg) was given to rats, 50-60% of
injected radioactivity was recovered in urine and faeces after 24 h,
whereas at a high dose level (200 mg/kg) less than 50% was recovered
(Schulz & Rubin, 1973). Seven days after an oral dose (2.9 mg/kg) of
DEHP was given to rats, 42% of the radioactivity was recovered in the
urine and 57% in the faeces (Daniel & Bratt, 1974). Biliary excretion
was also measured in these experiments, and it was found that 14% of
the radioactivity was recovered in bile after 4 days (Daniel & Bratt,
1974). The almost 100% recovery reported by Daniel & Bratt (1974) has
been confirmed by Teirlynck & Belpaire (1985). Oral administration of
MEHP (50-500 mg/kg) gave a higher urinary recovery than orally
administered DEHP (50-500 mg/kg) as measured after 24 h (Lhuguenot et
al., 1985).
After the oral administration of non-radioactive DEHP (0.45
mg/kg) to human volunteers, it was found that 15-25% was excreted in
urine as MEHP or oxidized metabolites within 2-3 days (Schmid &
Schlatter, 1985).
In the rat no unmetabolized DEHP is excreted in the urine, but
small amounts are found in mouse or green monkey urine (Albro et al.,
1982). Major amounts of MEHP are excreted in mouse, guinea-pig, green
monkey, and human urine (Albro et al., 1982). However, oxidized
metabolites, either free or conjugated, constitute a major portion of
excretion products in rat, mouse, hamster, green monkey, and human
urine (Albro et al., 1982).
Changes in excretion pathways have been observed after prolonged
administration of DEHP. After oral dosing of rats without
pre-treatment with DEHP, the faecal excretion pathway dominated, while
in rats fed with DEHP for 7 days the urinary pathway dominated (Daniel
& Bratt, 1974).
6.5 Retention and turnover
6.5.1 Half-life and body burden
After the intravenous administration of radiolabelled DEHP, at
least two elimination phases of radioactivity, with short half-lives
(4.5-9 and 22 min, respectively), were observed in rat blood (Schulz
& Rubin, 1973). After 7 weeks of oral administration, the elimination
phase in the liver was considerably slower, the half-life being 3-5
days (Daniel & Bratt, 1974). No accumulation of DEHP or MEHP was
observed when the dosage was 2.8 g/kg per day for 7 days (Teirlynck &
Belpaire, 1985), nor was there any in a long-term (5-7 weeks) feeding
study at a dose level of 1 or 5 g/kg diet (corresponding to a daily
dose of about 50 and 250 mg/kg body weight) (Daniel & Bratt, 1974).
6.5.2 Indicator media
Analysis of the total amount of urinary metabolites, measured as
derivatized phthalic acid, indicate a weak positive correlation
between occupational exposure to phthalate and the presence of
metabolites in the urine (Nielsen et al., 1985; Liss et al., 1985). In
the study by Nielsen et al. (1985), workers were exposed mainly to
DEHP and diisodecyl phthalate, and the urinary level of phthalate
ester metabolites rose from the background level (17 µmol/litre) to
23-25 µmol/litre. In the study by Liss et al. (1985), workers were
exposed to DEHP and phthalic anhydride. Urinary phthalate
concentrations in exposed workers more than doubled after a workshift
and levels up to 44 µmol/litre were recorded. The authors concluded
that phthalic anhydride influenced the urinary level more than DEHP.
Phthalic acid is not a specific marker for DEHP exposure.
7. EFFECTS ON LABORATORY MAMMALS AND IN VITRO TEST SYSTEMS
7.1 Single exposure
Numerous LD50 values have been reported for DEHP. Oral LD50
values generally exceed 25 g/kg in rats and 30 g/kg in mice
(Stankevich et al., 1984; NIOSH, 1985b; Woodward et al., 1986); in the
rabbit it is 33.9 g/kg (Shaffer et al., 1945) and in the guinea-pig
26.3 g/kg (Krauskopf, 1973). Dermal LD50 values for guinea-pigs and
rabbits of 10 g/kg and 25 g/kg, respectively, have been reported
(NIOSH, 1985b).
The LD50 values after intraperitoneal administration were
30.7 g/kg in rats (Shaffer et al., 1945) and 14-75 g/kg in mice
(Lawrence et al., 1975; Woodward et al., 1986). LD50 values in the
range of 200-250 mg/kg were reported for the rat after intravenous
administration of DEHP solubilized with a nonionic detergent (Schmidt
et al., 1975; Rubin & Chang, 1978).
The main symptom of DEHP toxicity after single oral or
intraperitoneal dosing is diarrhoea (Hodge, 1943). An intraperitoneal
dose of 500 mg/kg in rats decreased spontaneous running activity,
thereby indicating behavioural changes (Rubin & Jaeger, 1973). After
intravenous dosing, lung lesions including oedema, haemorrhage, and
infiltrations of polymorphonuclear leucocytes were observed in rats at
doses as low as 50 mg/kg (Schulz et al., 1975). The etiology of the
lung lesions is unknown. It has, however, been suggested that some
changes could be due to the release of lysosomal enzymes from alveolar
macrophages, which was found to occur in vitro in rabbit alveolar
macrophages cultured with DEHP (Bally et al., 1980).
Rabbits treated intravenously with 350 mg DEHP/kg showed a
decrease in blood pressure and an increase in breathing rate. No
deaths occurred after doses up to 650 mg/kg were administered (Calley
et al., 1966).
The monoester, MEHP, may be more toxic than the diester but data
are very limited. In a short note by Villeneuve et al. (1978), the
oral LD50 of MEHP was reported to be 1.34 g/kg in female rats and
1.8 g/kg in males.
7.2 Short-term exposure
Doses of 3.4 g/kg body weight per day given by gavage (in olive
oil) for periods of up to 90 days caused the death of 15 out 20 rats
(Nikonorow et al., 1973). However, no deaths were reported among rats
fed 3% DEHP in the diet (1.9 g/kg body weight) for 90 days (Shaffer et
al., 1945) or in a rat study of the US National Toxicology Program
after dietary dosing (< 50 g/kg) for 14 days (NTP, 1982).
Oral administration of DEHP at a rate of > 0.4 g/kg body
weight per day resulted in a weight gain decrease in rats within a few
days (Nikonorow et al., 1973). In a 17-week feeding study where rats
were given 2, 10 or 20 g DEHP/kg diet, a decreased body weight was
observed (Gray et al., 1977). Reduction in body weight was also
observed in rats given dietary levels of 12.5 or 25 g/kg for 13 weeks.
Dosages of 1.6-6.3 g/kg resulted in either slight elevations of body
weight or no effect (NTP, 1982).
MEHP given at a level of 6.4 g/kg diet caused reduction in the
body weight gain of rats (Chu et al., 1981). No effects on body weight
occurred at dietary levels of 0.625 g/kg given for 3 months, but a
significant decrease in blood glucose was observed.
Reductions in haemoglobin, packed cell volume and erythrocyte
numbers were observed in rats given 10 or 20 g DEHP/kg diet for 17
weeks, but not when they were given 2 g/kg for the same period (Gray
et al., 1977).
Cystic kidneys and centrilobular necrosis were noted in one
strain of mice (ddY) fed 2.5 or 25 g DEHP/kg for 2 weeks, but not in
another strain (B6C3F1), even with a higher exposure level and a
longer exposure period (Woodward et al., 1986).
DEHP administered intravenously at a rate of 25-500 mg/kg per day
for 2-4 weeks to beagle dogs resulted in pulmonary haemorrhage and
inflammatory response similar in appearance to the "shock-lung" effect
(Woodward et al., 1986).
In an inhalation study, Wistar rats were exposed in a head-nose
inhalation system to DEHP aerosols of respirable particle size.
Exposure duration was 6 h/day, 5 days/week for 4 weeks at target
concentrations of 0, 0.01, 0.05, and 1.0 mg/litre. A statistically
significant increase in relative lung weights was found in the males
given the highest dosage, and this was accompanied by foam cell
proliferation and thickening of the alveolar septa (Klimisch et al.,
1991).
Another inhalation study has been reported, but this is
inadequate for assessment (Timofievskaja et al., 1980).
A discussion of the effects of DEHP on the liver is given in
section 7.9.
When rats were treated with DEHP by intraperitoneal injection
(Walseth et al., 1982) or by repeated oral dosing (Agarwal et al.,
1982), an increase in cytochrome P-450 levels was observed. The
increase in hepatic microsomal oxidation appears to be primarily due
to the deesterification products of DEHP, i.e. MEHP and
2-ethoxyhexanol, in long-term exposure, whereas DEHP and its two
metabolites may inhibit microsomal oxidation after acute exposure to
DEHP (Pollack et al., 1989). However, in vitro rat liver microsomal
cytochrome P-450 levels were not affected by DEHP (Gollamudi et al.,
1985).
Liver mitochondrial enzymes and mitochondrial morphology have
been reported to be influenced by DEHP administration (Ohyama, 1977;
Shindo et al., 1978). Recent results suggest that the in vitro
effects of DEHP (> 20 µmol/litre) on mitochondrial functions are
mainly related to the action on membrane lipids surrounding the
adenine nucleotide translocator, which reduces the rate of adenine
nucleotide exchange across the mitochondrial membrane (Kora et al.,
1989).
In male rats given DEHP in the diet, the urinary excretion of
zinc was enhanced and the testicular level of zinc decreased (Gray et
al., 1982; Oishi, 1985) (section 7.5). These changes in zinc
homeostasis could be due to altered levels of metallothionein. In mice
fed 6 or 12 g DEHP/kg diet for 24 weeks, hepatic levels of
metallothionein were increased up to 11-fold (Waalkes & Ward, 1989).
Several studies on rats have shown that DEHP given in the diet
(5-20 g/kg) decreases plasma triglyceride and cholesterol levels
(Yanagita et al., 1978; Sakurai et al., 1978; Bell et al., 1978a; Bell
et al., 1978b; Bell et al., 1979; Yanagita et al., 1979; Curstedt &
Sjövall, 1983). DEHP inhibits the biosynthesis of cholesterol, an
effect which is accompanied by phospholipidosis, and the same effects
have been observed with MEHP (Oishi & Hiraga, 1982).
7.3 Long-term exposure
In a 24-month study by Harris et al. (1956), three groups of
Wistar rats each comprising 43 males and 43 females were fed diets
containing 0, 1, and 5 g DEHP/kg, interim kills being made at 3, 6,
and 12 months. At the end of the study, only two control, four
low-dose, and seven high-dose animals were alive. During the first
year the body weights of the high-dose group were slightly reduced,
but by the second year the body weights of all groups were similar.
During the first 6 months an increase in relative liver and kidney
weights was seen in DEHP-treated animals but later they were similar
in control and treated animals. After 3 months of treatment, one out
of eight rats in the low-dose group was found to have mild renal
tubular atrophy. After 6, 12, and 24 months of treatment, no
compound-related pathological changes were evident. Because of high
mortality due to disease, this study is difficult to validate.
In another 24-month study (Carpenter et al., 1953), groups of
Sherman rats consisting of 32 males and 32 females were given diets
containing 0, 0.4, 1.3 or 4 g DEHP/kg. Owing to reduced life
expectancy due to disease and the small numbers of animals used, the
study was inadequate for assessing the chronic toxicity of DEHP.
In a 12-month study by Nikonorow et al. (1973), a group of 20
male and 20 female Wistar rats was given a diet containing 3.5 g
DEHP/kg, and a control group received the diet without DEHP. The only
gross or micropathological change noted in exposed animals at necropsy
was hepatomegaly. During the study, however, about 30% of the animals
died due to congestion of the small intestine and loss of the gastric
and/or intestinal mucosa, which was complicated by purulent pneumonia
and endometritis.
Crocker et al. (1988) described the renal effects of DEHP given
by gavage to young male rats at a dosage of 2.14 mg/kg body weight
three times per week for up to 12 months. A 50% reduction in
creatinine clearance and an increase in the severity of renal cyst
formation was observed. This lesion was consistent with spontaneous
nephropathy commonly observed in old rats; exposure may cause an onset
in younger rats. Furthermore, DEHP fed at 6 and 12 g/kg diet for two
years did not produce renal lesions in male and female F-344 rats
(NTP, 1982).
In a 2-year study (NTP, 1982; Kluwe et al., 1982), groups of
F-344 rats were given dietary levels of 0, 6, and 12 g DEHP/kg.
Decreased body weight in exposed groups was noted from week 30 until
the cessation of exposure. In addition to neoplastic effects (section
7.7) and testicular atrophy (section 7.5), a compound-related
hypertrophy of cells in the male anterior pituitary was noted in the
high-dose group. In both exposed groups an increased incidence of
clear changes in liver cells was observed. This study also
investigated B6C3F1 mice exposed to 3 or 6 g DEHP/kg diet. A
dose-related decrease of body weight was observed in female mice.
There was no increased incidence of non-neoplastic lesions except for
seminiferous tubular degeneration in the testes of male mice (section
7.5.1).
In a study on groups of male and female guinea-pigs fed diets
containing 0, 0.4 or 1.3 g DEHP/kg for 12 months (Carpenter et al.,
1953), the body weight of the low-dose animals was statistically
higher than that of controls, and liver weight relative to body weight
of dosed females slightly increased. No other exposure-related lesions
were found.
A study on groups of male ferrets fed diets containing 0 and 10
g DEHP/kg for 14 months revealed a reduction in body weight and an
increase in relative liver weight, but no evidence of peroxisome
proliferation (Lake et al., 1976).
7.4 Skin and eye irritation; sensitization
DEHP has been shown to be a weak irritant to mammalian skin when
administered topically or intradermally (0.2 ml of an emulsion of
100 g/litre) (Calley et al., 1966; Woodward et al., 1986).
In a study by Lawrence et al. (1975), no irritation occurred when
undiluted DEHP was instilled into the eye of rabbits.
No data are available on the potential for DEHP to induce skin
sensitization in animals.
7.5 Reproduction, embryotoxicity, and teratogenicity
7.5.1 Reproduction
The effects of DEHP on male reproductive organs have been studied
extensively. The majority of the studies have been carried out on rats
or mice given DEHP in the diet.
Seminiferous tubular atrophy, comprising a loss of spermatids and
spermatocytes, occurred when 4-week-old Wistar rats were given 2800 mg
DEHP/kg by oral intubation for 10 days (Gray & Butterworth, 1980). In
similarly treated 10-week-old rats, about 50% of the tubules were
atrophic and the remainder unaffected. However, no testicular damage
was detected in treated 15-week-old rats. When 20 g DEHP/kg was given
in the diet (approximately 1200 mg DEHP/kg per day) to 4-week-old
rats, the lesions produced were reversible whether treatment stopped
before or continued until after the control rats had reached sexual
maturity.
In rats given 10 or 20 g DEHP/kg diet, the testis atrophy was
dose dependent after approximately 2 weeks of feeding. This atrophy
was accompanied by pituitary changes, i.e. enlargement and
vacuolization of the basophils of the pars distalis, corresponding to
the formation of the so-called castration cells seen after gonadectomy
(Gray et al., 1977). In a subsequent study, there was a reduction in
testicular and prostatic zinc levels concomitant with increased
urinary excretion of zinc (Gray et al., 1982).
In a rat study by Oishi & Hiraga (1980a), the serum testoster-one
levels in rats fed 20 g DEHP/kg diet were reduced by approximately
50%. The total amount per testis decreased, but the concentration rose
to 150% of the original value because of the testicular atrophy.
Simultaneous administration of testosterone or zinc had no protective
effect on the atrophy but did prevent the weight reduction of the sex
organs such as the epididymis (Gray & Butterworth, 1980; Oishi &
Hiraga, 1983). In a further study, it was found that a low-zinc diet
aggravated the DEHP-induced testicular atrophy (Agarwal et al.,
1986a).
DEHP given to 7 young (5 weeks old) Wistar rats in the diet
(20 g/kg) for one week decreased the testicular weight significantly
(P < 0.05), compared to controls, and also the testicular zinc
concentration (Oishi, 1984). In a study by Oishi (1985), groups of 20
male rats were given DEHP (2.0 g/kg per day) by gavage for 14 days.
Ten rats were then killed and the remaining ten were kept for 45 days
on a DEHP-free diet. The histopathological changes of the testes seen
on day 15 were marked shrinkage of the seminiferous tubules, a
germinal epithelium consisting only of Sertoli cells, and very few
spermatogonia. After 45 days the percentage of spermatogenic tubules
had increased from 0 (at day 15) to 12.8%, indicating a limited
reversibility of the testicular atrophy.
Similar degeneration of the seminiferous tubules was observed
when 13-week-old Wistar rats were orally given 2 g DEHP/kg body weight
for 7 consecutive days (Saxena et al., 1985) .
In a dietary study, DEHP was administered to F-344 rats at 6 and
12 g/kg for 103 weeks (NTP, 1982). Degeneration of the seminiferous
tubules was observed only at the higher dose.
The testicular changes induced by oral DEHP administration appear
to be age dependent. A daily dosage of 2.8 g DEHP/kg body weight given
for 10 days caused seminiferous tubular atrophy in 4-week-old Wistar
rats, but this effect was less severe in 10-week-old rats and absent
in 15-week-old rats (Gray & Butterworth, 1980). Sjöberg et al. (1986)
showed that DEHP fed at a level of 1.7 g/kg diet caused reductions in
testis weight to 20%, 55%, and 92% of the control values in
Sprague-Dawley rats at 25, 40, and 60 days of age, respectively. This
was apparently not due to differences in pharmacokinetic parameters,
since the plasma levels of the metabolite MEHP were identical in the
25- and 40-day-old rats (Sjöberg et al., 1982).
Rats given DEHP intraperitoneally at a dosage of 100 mg/kg per
day for 5 days did not develop testicular atrophy (Curto et al.,
1982). However, this lack of response was probably a consequence of
the dose used, rather than the dose route, and there was a 30%
reduction in testicular zinc. Intraperitoneal administration of higher
doses (1-25 g/kg per day for 5 days) to Wistar rats decreased serum
testosterone levels (Oishi & Hiraga, 1979).
Some degenerated primary spermatocytes and altered Sertoli cells
were observed in Sprague-Dawley rats given 3-h intravenous infusions
of an emulsion at a rate of 1 ml/h, which corresponded to a daily dose
of 500 mg DEHP/kg (Sjöberg et al., 1985c). The infusions were given
every other day on six occasions. The emulsion contained DEHP,
fractionated egg yolk phosphatides, glycerol, and water. No effects
were observed when emulsions corresponding to 0, 5 or 50 mg DEHP/kg
were given.
In a study by Curto & Thomas (1982), groups of sexually mature
Swiss-Webster mice were given intraperitoneal injections of 50 or 100
mg DEHP/kg either daily for 5 days or alternate daily for 20 days (10
injections). The animals were killed 24 h after the last injection. No
significant alterations in testicular weight or zinc levels occurred.
Young rats were not more susceptible to testicular damage than
older ones following intravenous infusion of DEHP. It has been
suggested that the age-related difference observed in some studies may
be due to the fact that the rate of gastrointestinal absorption of the
DEHP-derived metabolite MEHP is higher in younger animals than in
older ones (Sjöberg et al., 1986).
In a NTP-sponsored study (Melnick et al., 1987), CD-1 mice given
3 g DEHP/kg diet showed significantly diminished testis and epididymis
weights compared to controls. In addition, the sperm concentration in
the cauda epididymis was reduced and the percentage of abnormal sperm
in the cauda was significantly higher in the treated mice than in the
controls.
A high oral dose (4.2 g/kg) of DEHP gave minimal tubular atrophy
in hamsters but did not produce any effects on urinary zinc excretion,
testicular zinc levels or testicular weights (Gray et al., 1982).
The effects of the monoester MEHP have not been as well studied
as those of DEHP. An oral dosage of 1 g MEHP/kg per day for 5 days
produced a significant decrease in rat testis weight and extensive
testicular atrophy (Gray et al., 1982). On the other hand, rats given
intraperitoneal doses of up to 100 mg MEHP/kg daily for 5 days showed
no abnormal histology (Curto et al., 1982) and an intraperitoneal dose
of 50 mg/kg given on alternate days for 20 days produced only a
reduction in prostatic zinc levels (Curto & Thomas, 1982).
Mice fed diets containing 20 g MEHP/kg for 1 week revealed
markedly reduced testicular zinc and testosterone levels, but there
were no reductions in testicular weight (Oishi & Hiraga, 1980b).
Hamsters given MEHP (1 g/kg per day) for 9 days showed more severe
testicular effects than those given DEHP at a level of 4.2 g/kg per
day for the same period (Gray et al., 1982). More recent studies
(Albro et al., 1989) indicate that testicular atrophy resulting from
DEHP exposure is most probably due to the formation of MEHP.
These studies indicate that the rat is the species most
susceptible to DEHP-induced testicular atrophy. The mechanism of
phthalate-induced testicular damage is not fully understood.
Testicular zinc depletion has been suggested to be a primary event
(Foster et al., 1982, 1983). However, Gray et al. (1982) showed that
an effect on zinc level is not always associated with testicular
atrophy in all species tested. Zinc is essential for normal testicular
function and its depletion is known to lead to testicular atrophy
(Barney et al., 1969). Inhibition of dehydrogenase enzymes, e.g.,
those controlling the biosynthesis of testosterone, leads to reduced
testosterone levels. DEHP administration has been shown to reduce the
level of serum testosterone in the rat (Oishi & Hiraga, 1979; Oishi &
Hiraga, 1980a) as well as in the mouse (Gray et al., 1982), although
in the mouse no testicular atrophy was observed. Administration of
testosterone or zinc did not prevent the testicular damage induced by
DEHP (Gray & Butterworth, 1980; Oishi & Hiraga, 1983).
Co-administration of DEHP and testosterone, on the other hand,
apparently enhanced the testicular damage caused by DEHP (Oishi,
1989a). In a similar study (Oishi, 1989b), luteinizing
hormone-releasing hormone (LRH) significantly decreased testis weight,
sulfhydryl content, and lactate dehydrogenase when given together with
DEHP, whereas LRH or DEHP alone had no effects.
Similar effects were observed with exogenously added follicle
stimulating hormone (FSH) in an in vitro study. Primary testicular
cell cultures pretreated with MEHP showed a dose-related reduction in
FSH-stimulated cyclic adenosine monophosphate (cAMP) production (Lloyd
& Foster, 1988). These results suggest that MEHP produces a
perturbation at the level of the FSH membrane receptor, causing an
inhibition of FSH action.
In vitro studies have indicated that the Sertoli cell is the
target cell. Mixed cultures of Sertoli and germ cells prepared from
rat testes were exposed to DEHP or MEHP (10-7-10-4 mol/litre)
(Gray & Beamand, 1984; Gray & Gangolli, 1986). DEHP had no effect, but
MEHP caused a dose-dependent increase in the rate of germ cell
detachment from Sertoli cells, accompanied by changes in Sertoli cell
morphology. More recent data indicate that Sertoli cell mitochondria
are a target for MEHP (Chapin et al., 1988). However, it has also
been shown that testicular mitochondrial respiratory functions are
decreased in rats given 2 g DEHP/kg body weight by gavage (Oishi,
1990).
In a study by Melnick et al. (1987), CD-1 mice were given 0, 0.1,
1 or 3 g DEHP/kg diet during a 7-day pre-mating period and a
subsequent 98-day cohabitation period. There was complete suppression
of fertility in the 3-g/kg group and a significant reduction in
fertility in the 1-g/kg group, compared to controls, but no effect on
fertility at 0.1 g/kg.
In a fertility study designed to investigate earlier significant
results (Singh et al., 1972), groups of male ICR mice were dosed
subcutaneously with undiluted DEHP at levels of 1, 2, 5, and 10 ml/kg
(equivalent to 0.99, 1.97, 4.93, and 9.86 g/kg) on days 1, 5, and 10
of the study (Agarwal et al., 1985a). They were subsequently mated
with virgin females on days 2, 6, 11, 16 and 21, and then at weekly
intervals until week 8. There were reductions in the incidence of
pregnancies in several groups, but these were dose related only at the
first mating (i.e. on day 2 of the experiment). Examination of
pregnant mice on day 13 of gestation showed that there were
pre-implantation losses corresponding to a DEHP dose level of 10 ml/kg
and a mating on day 6. There were consistent increases in early fetal
deaths relating to matings at day 21, week 4, and week 5. In a similar
study, male and female mice were treated subcutaneously with 1-100 ml
DEHP/kg, and this led to a reduction in testicular but not ovarian
weight. The ovaries exhibited histological injury at lower doses of
DEHP than the testes. Unlike the situation in the testes, there was no
significant dose-related increase in histopathological changes in the
ovaries (Agarwal et al., 1989).
In an investigation of the effects of phthalates on female
reproductive organs, three doses of 4.93 g DEHP/kg were given
intraperitoneally at 5-day intervals to female rats (Seth et al.,
1976). No histopathological changes in the ovaries were seen 22 days
after the first injection, but reductions in the activities of some
enzymes were noted.
The administration of DEHP (1000 mg/kg intraperitoneal or 2000
mg/kg oral) to marmosets daily for 14 days did not lead to testicular
atrophy (Rhodes et al., 1986).
Hence, it appears that rats and guinea-pigs are sensitive to
DEHP-induced testicular atrophy, while mice are fairly resistant and
hamsters and marmosets are highly resistant. The fact that at least
some of the effects associated with this atrophy can be produced
in vitro argues against a hormonally mediated indirect effect. The
earliest effects are seen in Sertoli cells and are described as
vacuolation (Albro, 1987).
7.5.2 Embryotoxicity and teratogenicity
In a study by Nikonorow et al. (1973) on female Wistar rats given
0.34 or 1.7 g DEHP/kg by gavage during the first 21 days of gestation,
the only untoward effect was a reduction in fetal body weight. When
DEHP (0, 5, 10, 15 or 20 g/kg) was administered orally to Fischer-344
rats on gestational days 0 to 20, maternal toxicity and reduced fetal
body weight per litter were observed at the three highest dose levels.
The number of fetuses per litter was unaffected by the treatment (Tyl
et al., 1988).
Intraperitoneal injections of 4.93 or 9.86 g DEHP/kg on days 5,
10, and 15 of gestation resulted in an increase in the number of
resorptions and reduced fetal weight in Sprague-Dawley rats (Singh et
al., 1972). In the highest-dose group, gross abnormalities, such as
twisted hind legs and anophthalmia, were noted but no skeletal defects
were observed.
Rat plasma soluble extracts of two PVC plastics containing DEHP
were administered intravenously to groups of pregnant Sprague-Dawley
rats daily from the 6th to the 15th day of gestation, and the animals
were killed at day 20 (Lewandowski et al., 1980). The daily doses of
DEHP were equivalent to 1.3 mg/kg and 5.2 mg/kg. No significant
teratogenic or embryotoxic effects were noted.
Groups of ICR mice were given DEHP in the diet at levels of 0.5
to 10 g/kg for the first 18 days of gestation and were then sacrificed
(Shiota et al., 1980; Shiota & Nishimura, 1982). The food intake was
an average of 7 g/day. At the 4 g/kg and 10 g/kg dose levels, no live
fetuses were found. At 2 g/kg, 40% of the fetuses had malformations
including exencephaly, spina bifida, and malformed tail. Delayed
ossification was seen in about 15% of the fetuses at 1 g/kg and
2 g/kg.
In a study by Tyl et al. (1988), DEHP was administered in the
diet to CD-1 mice on the first 17 days of gestation at levels of 0,
0.25, 0.5, 1, and 1.5 g/kg. At the two highest dose levels maternal
toxicity, increased resorptions and late fetal deaths, decreased
numbers of live fetuses, and reduced fetal body weight per litter were
observed. The number and percentage of malformed fetuses per litter
were elevated at the three highest dose levels.
A single oral administration of DEHP (0.1 ml/kg) on day 7 of
gestation to ddY-strain SPF (specific pathogen free) mice decreased
the number and the body weights of live fetuses (Tomita et al.,
1982b). In a study by Yagi et al. (1980), DEHP was given orally to SPF
mice on days 6, 7, 8, 9 or 10 of gestation. When 5.0 or 10.0 ml/kg was
given on day 7 there were no live fetuses, whereas 2.5 ml/kg
administered on the same day resulted in 14% live embryos and 1.0
ml/kg gave 40% live embryos. The percentages of live embryos when 10.0
ml/kg was given on day 8, 9 or 10 of gestation were 18, 92, and 95%,
respectively. Gross and skeletal abnormalities occurred in fetuses
given 2.5 and 7.5 ml/kg on day 7 or 8. The abnormalities included
exencephaly, open eyelid, and club-foot.
In a study by Shiota & Mima (1985), groups of ICR mice were given
DEHP by stomach intubation on days 7, 8, and 9 of gestation. The DEHP
doses (in olive oil) were 250, 500, 1000, and 2000 mg/kg, and the mice
were sacrificed on day 18. In the two highest dose groups, the numbers
of resorptions and malformed fetuses were significantly increased.
Fetal weights were significantly depressed. The most frequent
malformations were anencephaly and exencephaly. When doses of up to
8000 mg/kg were given by intraperitoneal injections on days 7,8, and
9 of gestation, no effects were noted. DEHP is highly embryotoxic and
terato-genic in mice when given orally but not when given
intraperitoneally.
The monoester MEHP, at oral doses of 225, 450, and 900 mg/kg per
day, produced significant signs of maternal toxicity when given to
pregnant Wistar rats on days 6-15 of gestation (Ruddick et al., 1981).
In the highest dose group a 73% mortality was observed in the dams.
There was a dose-related decrease in the number of litters and in the
litter weights of live pups.
Oral dosing with 0.1 or 1.0 g MEHP/kg on day 7 of gestation led
to increased incidence of early embryonic deaths in SPF mice (ddY
strain), but dosing on day 8 or 9 had less effect (Yagi et al., 1980;
Tomita et al., 1986). The fetuses had reduced body weight, and there
was a higher incidence of gross abnormalities, compared to controls,
when the higher dose level was given on day 8 or 9. The mice dosed on
day 8 produced fetuses with a high incidence of skeletal effects.
Intravenous injections of MEHP (11.38 mg/kg) to rabbits on days
6-17 of gestation gave a high incidence of resorptions (Thomas et al.,
1979). The incidence of fetal anomalies was similar to that in
controls.
When Nikonorow et al. (1973) administered DEHP (0.34 or 1.7 g/kg
per day) to female Wistar rats by gavage for 3 months prior to mating,
there was an increase in the number of resorptions but no effects on
fetal weights or the incidence of skeletal anomalies.
In utero administration of DEHP (1000 mg/kg body weight) to
rats daily from days 6 to 15 of gestation resulted in retardation of
fetal growth and an increase in fetal liver weight. There were
significant quantities of DEHP and decreased mitochondrial enzyme
activities in the fetal liver. These results indicate an effect on
fetal liver cell bioenergetics (Srivastava et al., 1989).
The mechanism of DEHP or MEHP teratogenicity is not known.
Teratogenic activity could result from zinc deficiency, which is known
to produce such effects (Swenerton & Hurley, 1971).
7.6 Mutagenicity and related end-points
The possible genotoxic effect of DEHP has been thoroughly
investigated in several different short-term tests. The effects of the
major metabolites of DEHP, i.e. MEHP and 2-ethylhexanol, as well as
phthalic acid and phthalic anhydride, have also been studied.
7.6.1 Mutation
Studies on the possible mutagenic effect of DEHP have been
performed in bacteria, fungi, and in cultured mammalian cells.
Drosophila melanogaster has also been used and results from a few
in vivo studies on mice have been reported.
7.6.1.1 Bacteria
Many studies have been performed using a variety of strains of
Salmonella typhimurium and DEHP doses of up to 10 mg/plate.
Incubations both with and without exogenous activation systems have
been performed. S9 mix from rats induced by Aroclor 1254 has
frequently been used, but other species and other inducers have also
been used to produce metabolic activation systems. With one exception
(Tomita et al., 1982a) these test results have all been negative
(Kirby et al., 1983; Yoshikawa et al., 1983; Zeiger et al., 1985;
Agarwal et al., 1985b), and in a IPCS collaborative study (Ashby et
al., 1985) all five laboratories reported negative results. Bacteria
other than S. typhimurium have also been used; negative results were
obtained with E. coli WP2 at doses of up to 2 mg per plate (Yoshikawa
et al., 1983).
The major metabolites of DEHP have also been tested for mutagenic
activity in bacteria. Concentrations of up to 3.333 mg per plate for
MEHP and phthalic anhydride (Zeiger et al., 1985) and 2 mg/plate for
2-ethylhexanol and phthalic acid (Agarwal et al., 1985b) have yielded
negative results in strains of Salmonella (see also Kirby et al.,
1983; Yoshikawa et al., 1983). However, Tomita et al. (1982a) reported
a significant increase in TA100 revertants following exposure to
either DEHP or MEHP (both with and without S9). It should be noted,
however, that MEHP mutagenicity is only demonstrable within a narrow
range of concentration, because it has a sterilizing effect at high
concentration and shows no mutagenic activity at low concentration.
Tomita et al. (1982a) also detected dose-dependent (0.4 and 0.5 mg
MEHP/plate) DNA damage in a B. subtilis Rec assay, while DEHP,
phthalic acid, and 2-ethylhexanol were all negative. In this study
MEHP also gave a positive result in E. coli WP2 b/r.
Negative results were obtained when pooled urine from rats,
treated with 2000 mg DEHP/kg per day for 15 days, was tested for
genotoxic activity. A direct plating procedure was used with
S. typhimurium strains TA98, TA100, TA1535, TA1537, and TA1538, both
with and without S9 and ß-glucuronidase/aryl sulfatase as the
activation system. When 2-ethylhexanol was tested according to the
same protocol, the result was also negative (Divincenzo et al., 1985).
7.6.1.2 Fungi
The induction of mutations by DEHP has been studied in various
fungal species. In the IPCS collaborative study on in vitro assay
systems (Ashby et al., 1985), DEHP was considered to be negative in
six out of seven assays. Positive results were obtained with
Saccharomyces cerevisiae, both with and without S9 activation, at
lowest effective concentrations of 1541 mg/litre and 3081 mg/litre,
respectively. However, other laboratories using different strains of
S. cerevisiae or Schizosaccharomyces pombe reported negative
results at a maximum tested concentration of 5000 mg/litre.
7.6.1.3 Mammalian cells
Mouse lymphoma cells (L5178Y), Chinese hamster V79 cells, and
human lymphoblasts have been used to study the mutagenic effect of
DEHP in cultured mammalian cells. Several investigators obtained
negative results, but a few positive results have also been reported.
In the IPCS collaborative study (Ashby et al., 1985), only one
out of ten investigators reported a positive response. Mouse lymphoma
cells were exposed to DEHP without S9, and two concentrations (7.5 and
20 mg/litre) gave positive results. In a separate study (Kirby et al.,
1983), where MEHP, 2-ethylhexanol, and DEHP were tested in the mouse
lymphoma cell assay, all three substances were found to be
non-mutagenic. The concentrations used were 0.016-1.0 ml/litre
(without S9) and 0.067-5.0 ml/litre (with S9) for DEHP, and 0.013-1.0
ml/litre for MEHP and 2-ethylhexanol.
7.6.1.4 Drosophila
DEHP has also been tested for mutagenicity in Drosophila
melanogaster using the sex-linked recessive lethal (SLRL) test and
various somatic recombination and mutation assays. SLRLs were not
induced by DEHP (20 µg/g) administered by injection (Yoon et al.,
1985). Negative results for DEHP were also reported in the SLRL
mutation assay on Drosophila melanogaster larvae (Zimmering et al.,
1989).
In the IPCS study (Ashby et al., 1985), DEHP gave a positive
response in the unstable eye mosaic test at a dose of 6.1 g/litre, in
two separate experiments, but neither lower nor higher concentrations
produced any response. No activity was seen in the wing spot test with
a single dose of 6.1 g/litre. It was concluded that DEHP exhibits
marginally positive mutagenicity (Ashby et al., 1985).
7.6.2 DNA damage
Various end-points, such as unscheduled DNA synthesis (UDS) and
single strand breaks, have been used to detect DNA damage induced by
DEHP in a variety of mammalian test systems. In the IPCS study (Ashby
et al., 1985), negative results were obtained when single strand
breaks were measured, either by alkaline elution in hepatocytes (up to
3.907 g/litre) or alkaline sucrose sedimentation in Chinese hamster
ovary (CHO) cells (up to 39 g/litre). UDS in either isolated
hepatocytes or cultured HeLa cells was investigated by four different
laboratories. One investigator detected a positive response using
isolated hepatocytes, but, since this result was only statistically
significant at one dose and not dose related, the consensus was that
DEHP does not cause UDS.
In a study by Butterworth et al. (1984), DEHP did not induce DNA
repair in primary rat hepatocytes. Similarly, neither DEHP nor MEHP
induced any DNA repair in primary human hepatocytes from three
different subjects. In this study, concentrations as high as 0.01 mol
DEHP/litre and 5 x 10-4 mol MEHP/litre were used and exposure
continued for 18 h. No induction of DNA repair or increased alkaline
elution of DNA was seen in hepatocytes from either female or male
F-344 rats treated with DEHP in vivo. UDS was not induced in male
rats treated with 500 mg DEHP/kg by gavage 2, 12, 24 or 48 h before
sacrifice. Furthermore, treatment of male rats with 150 mg/kg per day
by gavage for 14 days and treatment of female rats with dietary DEHP
(12 g/kg) for 30 days resulted in peroxisome proliferation, but no UDS
was induced.
Similar in vivo/in vitro results in studies of UDS in
hepatocytes were reported by Kornbrust et al. (1984). No UDS was
observed in primary rat hepatocytes exposed in vitro to 10-5 to
10-2 mol DEHP/litre or in vivo by a single gavage dose of 5 g/kg
2, 15 or 24 h prior to the isolation of hepatocytes. A dietary
concentration of 20 g DEHP/kg led to a marked proliferation of
peroxisomes after 4 weeks. Neither this treatment nor the additional
administration of a single gavage dose of 5 g/kg (15 h before
sacrifice) to animals fed the 20-g/kg diet for either 8 weeks or for
4 weeks with or without pretreatment with 3-amino-1,2,4-triazole (to
inhibit endogenous catalase activity) induced any detectable DNA
repair in hepatocytes. Indeed, the administration of DEHP and some
other proliferators has been shown to increase the levels of
8-hydroxy-deoxyguanosine in rat liver DNA (Takagi et al., 1990).
7.6.3 DNA binding
In an in vivo study by Albro et al. (1982), radioactivity from
carbonyl-labelled DEHP did not associate with purified protein, RNA or
DNA from rat liver. Label from 2-ethyl-(1-14C)-hexyl-labelled DEHP
or MEHP appeared to associate strongly with purified DNA, but this was
not the case with label from free 14C-labelled 2-ethylhexanol.
According to Albro (1987), although there is incorporation into normal
nucleosides, there is no evidence of alkylation.
In a similar study (von Däniken et al., 1984; Lutz, 1986), DEHP
radiolabelled in different positions was administered orally to female
F-344 rats with or without pretreatment with unlabelled DEHP (10 g/kg
diet) for 4 weeks. The administration of 14C-carboxylate-labelled
DEHP resulted in no measurable DNA radioactivity, whereas
radioactivity was clearly measurable after the administration of DEHP,
14C- or 3H-labelled in the alcohol moiety, or of
2-ethyl(1-14C)hexanol. HPLC analysis showed that the normal
nucleosides had incorporated radiolabel, but fractions expected to
contain carcinogen-modified nucleoside adducts did not contain any
radioactivity.
DNA isolated from the livers of male F-344 rats administered 2000
mg DEHP/kg daily by gavage for 3 days was analysed for possible
carcinogen-DNA adducts by the 32P-postlabelling technique (Gupta et
al., 1985). No adducts were detected in the DNA, which also was the
case when DNA from hepatocytes exposed to 10-3 mol DEHP/litre
in vitro for 4 h was analysed.
7.6.4 Chromosomal effects
Chromosomal effects of DEHP have mainly been studied in vitro,
although some studies on the induction of micronuclei in the
peripheral blood erythrocytes of mice have been published.
DEHP did not induce any increase in the level of sister-chromatid
exchange (SCE) in Chinese hamster ovary (CHO) cells, treated for 1 h,
either with or without S9, at levels of up to 0.01 mol/litre (Douglas
et al., 1986). On the other hand, MEHP has been reported to induce SCE
in V79 cells treated with 25 or 50 mg/litre for 24 h or 1500 mg/litre
for 3 h (Tomita et al., 1982a). This metabolite also induced
chromosomal aberrations in CHO cells and RL4 cells (from rat liver),
but only at cytotoxic concentrations in the CHO cells (1.0 and 1.3
mmol/litre, with or without S9). MEHP was less toxic to RL4 cells
and concentrations between 2.0 and 5.0 mmol/litre gave a dose-related
increase in chromosomal aberrations (Phillips et al., 1986). According
to the authors, observations on changes in CHO cell structure and
permeability and on the haemolytic effects of phthalate monoesters
suggest that MEHP cytotoxicity may be due primarily to an action on
cell membranes.
The induction of aneuploidy by DEHP was investigated both in
mammalian cells and in fungi in the IPCS study (Ashby et al., 1985).
The mammalian assays gave positive responses at 50 mg/litre in a
fibroblast cell line from Chinese hamster liver and at levels of
between 25 and 50 mg/litre in Chinese hamster primary liver cells. Two
out of four studies using fungi were also positive and the consensus
was that DEHP is capable of inducing aneuploidy in vitro in both
fungi and mammalian cells.
In a study by Ahmed et al. (1990), male Wistar rats were fed for
alternate 7-day periods with a diet containing 20 g DEHP/kg and a
control diet. The rats were examined after 3 days on the DEHP diet or
after 7 days on the control diet. An analysis of nuclear size gave
results consistent with an increase in tetraploid hepatocytes after
treatment with DEHP, which was reversed when the rats returned to the
control diet.
7.6.5 Cell transformation
DEHP-induced cellular transformation has been studied in several
different experimental systems. In a test programme (Astill et al.,
1986), the BALB/3T3 cell transformation assay was used both with and
without rat primary hepatocytes. Both DEHP (0.875-1 µl/litre) and the
two metabolites MEHP and 2-ethylhexanol gave negative results. On the
other hand, the majority of transformation tests in the IPCS study
(Ashby et al., 1985) were positive for DEHP. Negative results were
obtained with BALB/c-3T3 cells, while a study measuring the
enhancement of viral transformation of Syrian hamster embryo (SHE)
cells was considered to be inconclusive. Positive responses were
obtained by four other investigators using SHE cells at 1-300 mg/litre
(two different laboratories), embryonic mouse fibroblasts at 1000
mg/litre with S9 and 10 mg/litre without S9, or retrovirus-infected
Fischer rat embryo cells at 2000 mg/litre (the highest dose tested).
In a separate study (Tomita et al., 1982a), both DEHP (7.5 and 15
g/kg) and MEHP (375 and 750 mg/kg) induced morphological
transformation, as well as chromosomal aberrations, in SHE cells after
transplacental administration.
In a study by Diwan et al. (1985), anchorage-independent growth
of JB6 mouse epidermal cells was enhanced by DEHP at concentrations of
500 to 20 000 ppm/ml (the Task Group noted that the unit used in
Diwan's paper is ppm/ml, but it should read ppm or mg/litre). Ward et
al. (1986) used the same model system and reported that both DEHP
(1.3-51 x 10-6 mol/litre) and MEHP (2-5 x 10-8 mol/litre) were
effective, while 2-ethylhexanol (4-77 x 10-7 mol/litre) was without
effect. The morphological transformation in the SHE cell system
induced by DEHP, MEHP, and other hepatic peroxisome proliferators
seemed not to be correlated with increased peroxisomal ß-oxidation,
increased production of oxidative radicals or peroxisome proliferation
(Mikalsen et al., 1990a; Mikalsen et al., 1990b; Mikalsen et al.,
1990c).
A few studies on DEHP-induced inhibition of metabolic
cooperation, which may be indicative of the promoting potential of a
substance, have been reported. Metabolic cooperation in Chinese
hamster V79 cells was not inhibited by DEHP at non-cytotoxic
concentrations, i.e. 3 x 10-4 mol/litre (0.12 mg/litre) or less
(Kornbrust et al., 1984). In the IPCS study (Ashby et al., 1985), one
investigator reported inhibition in V79 cells at non-cytotoxic
concentrations of DEHP (25-200 mg/litre in two separate experiments
and 5-25 mg/litre in another), while another investigator, using V79
cells in a microassay method, detected a slight (but non-significant)
increased inhibition at doses of between 10-5 and 2 x 10-4
mol/litre (3.9-78 mg/litre).
In a study by Malcolm & Mills (1989) on V79 cells, DEHP inhibited
intercellular communication (gap junctions) at non-cytotoxic
concentrations (10-30 mg/litre).
7.6.6 In vivo effects
Putman et al. (1982) dosed male Fischer-344 rats orally for 5
days with DEHP (5.0, 1.7, and 0.5 g/kg per day), MEHP (0.14, 0.05, and
0.01 g/kg per day), and 2-ethylhexyl (0.21, 0.07, and 0.02 g/kg per
day), and bone marrow metaphase cells were examined. No significant
increases were observed in gap breaks or structural rearrangements. In
addition, the mitotic index was unaffected by treatment.
A micronucleus assay on mouse (B6C3F1) peripheral blood
erythrocytes (intraperitoneal doses of 0.6, 3.0, and 6.0 g/kg per day
for 5 days), sampled at 0, 2, and 4 weeks after the last treatment,
yielded negative results (Douglas et al., 1986). A sperm morphology
assay in B6C3F1 mice and Sprague-Dawley rats at the same dose levels
also gave negative results (Douglas et al., 1986). However, Agarwal
et al. (1986b) reported increases in sperm morphology changes in adult
F-344 rats given 20 g DEHP/kg for 60 consecutive days.
Two studies have yielded negative results in dominant lethal
tests (Hamano et al., 1979; Rushbrook et al., 1982). Hamano et al.
(1979) administered MEHP and DEHP orally, and the observation period
was six weeks. Rushbrook et al. (1982) dosed ICR/SRM mice orally for
5 days with DEHP (2465, 4930, and 9860 mg/kg per day), MEHP (50, 100,
and 200 mg/kg per day) or 2-ethylhexanol (250, 500, and 1000 mg/kg per
day). Each male was mated weekly with virgin females for 8 consecutive
weeks, and females were evaluated for pregnancy, live fetuses, and
early and late fetal deaths. All data were within the normal ranges.
DEHP and its major metabolites have also been tested for their
potential to induce micronuclei. Exposure to 0.6, 3.0 or 6.0 g DEHP/kg
per day for 5 days over a 4-week period did not induce micronuclei in
the peripheral blood erythrocytes of B6C3F1 male mice (Douglas et
al., 1986). Negative results were also obtained in another mouse
micronucleus test after either a single or daily doses of 5 g DEHP/kg.
In this study MEHP and 2-ethylhexanol also gave negative results
(Astill et al., 1986).
7.7 Carcinogenicity
In a carcinogenicity study (NTP, 1982; Kluwe et al., 1982),
groups of 50 male and 50 female Fischer-344 rats were fed diets
containing 6 or 12 g DEHP/kg diet, and 50 male and 50 female B6C3F1
mice were fed diets containing 3 or 6 g DEHP/kg for 103 consecutive
weeks. Concurrent controls (50 of each sex and species) were fed a
diet without the addition of DEHP. Food and water were supplied
ad libitum. All animals were given the control diet for 1-2 weeks
after 103 weeks of treatment and were then killed and examined both
grossly and microscopically. The administered concentrations of DEHP
were estimated to be equal to, or one half of, the maximum tolerated
doses. Under the conditions studied, DEHP caused an increased
incidence of hepatocellular carcinomas in female rats and male and
female mice, and an increased incidence of hepatocellular carcinomas
and neoplastic nodules in female rats (Table 8). Twenty of the 57
hepatocellular carcinomas in the DEHP-treated mice (sexes and doses
combined) had metastasized to the lung. The figure of nine
hepatocellular carcinomas in control male mice is considered to be
within the normal range (NTP, 1982).
The reported decreased incidence of tumours of the thyroid,
pituitary, and testis could be related to an increased endocrine
activity of the pituitary gland (NTP, 1982).
The carcinogenicity of DEHP in rats has been confirmed in two
further studies. Rao et al. (1990) found a 78.5% incidence in a group
of 14 male Fischer-344 rats fed a diet containing 20 g DEHP/kg for up
to 108 weeks, whereas the incidence in the 10 controls was 10%. Popp
et al. (1987) found either hepatocellular carcinomas or neoplastic
nodules in 6 out of 20 animals after female Fischer-344 rats were
exposed for 2 years to a diet containing 12 g/kg.
Table 8. Carcinogenic effects of DEHP on the livera
Control Low dose High dose P valueb
Hepatocellular carcinoma
Male rats 1/50 1/49 5/49 < 0.05
Female rats 0/50 2/49 8/50 < 0.005
Male mice 9/50 14/48 19/50 < 0.05
Female mice 0/50 7/50 17/50 < 0.0001
Neoplastic nodules
Male rats 2/50 5/49 7/49 n.s.
Female rats 0/50 4/49 5/50 < 0.05
Hepatocellular adenoma
Male mice 6/50 11/48 10/50 n.s
Female mice 1/50 5/50 1/50 n.s
a From: NTP (1982)
b Probability level in Codiran-Armitage test for linear trend when
P < 0.05; otherwise not significant (n.s.)
Two other long-term studies on DEHP have been performed by
Carpenter et al. (1953) and Harris et al. (1956), but, due to the
small numbers of animals used, the studies are inadequate to assess
the carcinogenic potential.
DEHP has been investigated in two life-time studies on Syrian
hamsters (Schmezer et al., 1988). In one study, groups of 25 male and
25 female 6-week-old hamsters were assigned to each of six groups:
untreated control; 3 g DEHP/kg body weight given intraperitoneally
once every week for 18 weeks; the same dose once every two weeks for
18 weeks; the same dose once every four weeks for 32 weeks; the same
dose once every four weeks for 32 weeks plus N-dimethylnitrosamine
(NDMA) at 1.67 mg/kg body weight given orally once per week; and the
same dose of NDMA without DEHP treatment. NDMA increased the tumour
rate for malignant liver tumours, mainly haemangioendotheliomas.
Co-administration of DEHP with NDMA neither increased nor decreased
the tumour rates. No significant differences of tumour rates were
observed in groups treated with DEHP alone compared with controls.
Schmezer et al. (1988) also investigated the life-time, whole-
body exposure of Syrian hamsters to DEHP vapour alone in air or in
combination with orally administered NDMA. The low doses of DEHP
(15 µg/m3, resulting in a total exposure of about 7.5 µg/kg body
weight) in this study render it inadequate for the assessment of the
long-term effects of DEHP alone. In combination with NDMA, this low
level of DEHP was associated with highly significant (P < 0.001)
decreases in hepatic haemangioendothe-lioma and fibrosarcomas combined
in males and females combined. This unexpected result should be
further investigated to evaluate its significance.
7.8 Special studies
Since DEHP lacks genotoxic activity in most test systems, it has
been suggested that the carcinogenic effect is exerted during the
promotion phase of hepatocarcinogenicity. DEHP has therefore been
tested in several initiation/promotion experiments in rats and mice
where the end-point has been the number and/or volume of altered liver
cell foci. As expected, DEHP lacked initiating activity in these
studies (Ward et al., 1986; Popp et al., 1987).
DEHP appears to be a tumour promotor in mouse liver. In male
B6C3F1 mice given an intraperitoneal initiating dose of
diethylnitrosamine (80 mg/kg), DEHP at levels of 6 and 12 g/kg diet
caused accelerated growth of foci and increased incidence of
hepatocellular adenomas (Ward et al., 1983; Ward et al., 1986).
However, in a 6-month study, DEHP at a level of 12 g/kg diet did not
appear to promote altered foci in male F-344 rats given an
intraperitoneal initiating dose of diethylnitrosamine of 150 mg/kg
(Popp et al., 1987). In other promotion studies DEHP appeared to
accelerate the regression or inhibit the appearance of some kinds of
foci in rats (DeAngelo & Garrett, 1983; DeAngelo et al., 1984).
However, other studies have shown that peroxisome proliferators can
promote the development of altered foci and tumours in rat liver.
These results are somewhat different from those of other liver tumour
promotors with respect to the histological phenotype of the foci and
the relatively low frequency of the foci (Cattley & Popp, 1989). Thus,
further studies are required to demonstrate that DEHP can promote
altered foci in rat liver.
7.9 Mechanisms of hepatotoxicity
Together with a number of structurally diverse chemicals,
including certain hypolipidaemic drugs, herbicides, and chlorinated
solvents, DEHP has been shown to produce hepatic peroxisome
proliferation in rats and mice (Reddy & Lalwani, 1983; Lock et al.,
1989). This proliferation is accompanied by liver enlargement,
stimulation of replicative DNA synthesis and cell division, and the
induction of peroxisomal and microsomal fatty acid oxidizing enzyme
activities (Reddy & Lalwani, 1983; Marsman et al., 1988; Lock et al.,
1989). The increase in microsomal fatty acid oxidation is due to the
induction of a cytochrome P-450 IVA1 (Lock et al., 1989). Peroxisome
proliferation and induction of peroxisomal and microsomal fatty acid
oxidizing enzyme activities can be observed in primary hepatocyte
in vitro cultures, as well as in the intact animal (Elcombe &
Mitchell, 1986; Lake et al., 1986). Both in vivo and in vitro
studies with rat hepatocytes have demonstrated wide compound potency
differences (Reddy et al., 1986; Barber et al., 1987; Lake et al.,
1988). Compared to some other compounds, DEHP is not considered to be
a particularly potent peroxisome proliferator in rodent liver (Reddy
et al., 1986; Barber et al., 1987). Possible mechanisms of peroxisome
proliferation include a perturbation of hepatic lipid metabolism
and/or the presence of a receptor protein (Lalwani et al., 1987; Lock
et al., 1989; Issemann & Green, 1990).
Certain peroxisome proliferators, including DEHP, have been shown
to increase the incidence of liver tumours in rats and mice (Reddy &
Lalwani, 1983; Reddy et al., 1986; Butterworth et al., 1987).
Generally, there is a good correlation between the potency of a
compound to produce peroxisome proliferation in rat and mouse liver
and its hepatocarcinogenic properties (Reddy et al., 1986). However,
the magnitude of peroxisome induction does not necessarily always
correlate with the carcinogenic response when different compounds are
compared (Marsman et al., 1988). Since the peroxisome proliferators
known to date are non-genotoxic carcinogens, Reddy and coworkers
(Reddy & Lalwani, 1983; Rao & Reddy, 1987; Reddy & Rao, 1989) have
suggested that liver tumour formation arises from a sustained
oxidative stress to the hepatocytes due to an imbalance in the
production and degradation of hydrogen peroxide (H2O2). DEHP
administration results in increased peroxisomal production of H2O2
in hepatocytes (Tomaszewski et al., 1986). There is an imbalance in
H2O2 production and degradation due to the fact that catalase is
induced to a much lesser extent than peroxisomal ß-oxidation enzymes.
In addition, the level of cytosolic glutathione peroxidase is reduced
on prolonged exposure to DEHP (Lake et al., 1987; Marsman et al.,
1988; Conway et al., 1989b; Tamura et al., 1990). Treatment with
peroxisome proliferators also results in a reduction of superoxide
dismutase and glutathione S-transferase activities (Ciriolo et al.,
1982; Goel et al., 1986; Lake et al., 1987). It has been suggested
that the increased level of H2O2 in hepatocytes, either directly or
via other reactive oxygen species (e.g., hydroxyl radical), damages
intracellular membranes and/or DNA (Reddy & Rao, 1989). Indeed chronic
administration of DEHP and other peroxisome proliferators results in
increased lipid peroxidation and lipofuscin deposition in rat
hepatocytes (Mitchell et al., 1985b; Goel et al., 1986; Cattley et
al., 1987; Lake et al., 1987; Reddy & Rao, 1989; Conway et al.,
1989b). Oxygen radicals may damage DNA (Bridges, 1985), leading to
increased levels of 8-hydroxydeoxy-guanosine and other modified bases
in DNA (Reddy & Rao, 1989). Indeed, the administration of DEHP and
some other peroxisome proliferators has been shown to increase levels
of 8-hydroxyde-oxyguanosine in rat liver DNA (Kasai et al., 1989;
Takagi et al., 1989, 1990, 1991). Apart from a role in peroxisome
proliferation, leading to oxidative stress, recent studies on DEHP
have demonstrated a role in the stimulation of replicative DNA
synthesis in the hepatocarcinogenicity of peroxisome proliferators
(Butterworth et al., 1987; Smith-Oliver & Butterworth, 1987; Marsman
et al., 1988). Increased cellular division may result in spontaneous
mutational events or promotional effects (Bridges, 1985; Butterworth
et al., 1987), including the promotion of spontaneously initiated
cells found in the livers of aging rodents (Schulte-Hermann et al.,
1989). A comparative study of the hepatic effects of Wy-14643
([4-chloro-6 (2,3-xylidino) 2-pyrimidinylthio]acetic acid), a potent
peroxisome proliferator, and DEHP indicated that, although both
compounds produced a similar induction of peroxisome proliferation at
the dose levels administered, only Wy-14643 produced liver tumours
within one year (Marsman et al., 1988; Conway et al., 1989b). However,
analysis of these data demonstrates that Wy-14643 produces both a more
marked increase in lipofuscin deposition (presumably reflecting
oxidative stress) and a sustained stimulation of replicative DNA
synthesis. These data suggest a role for both oxidative stress and
increased cell replication in the hepatocarcinogenicity of DEHP and
other peroxisome proliferators.
The mechanism of hepatocarcinogenicity induced by peroxisome
proliferators could include both enhanced cell replication and DNA
damage induced by oxygen radicals. Thus, at dose levels that do not
produce either significant peroxisome proliferation or cell
replication, it is unlikely that DEHP or other peroxisome
proliferators would elicit hepatic tumour formation. In this context
data on threshold values for liver effects in sensitive species (e.g.,
the rat and mouse), together with an assessment of species differences
in response (see below), should be considered. The value for the
subchronic liver effects of DEHP (e.g., peroxisome proliferation and
DNA synthesis) in the rat appears to be around 50 mg/kg per day (Lake
et al., 1984, 1991; Mitchell et al., 1985b; Tomaszewski et al., 1986).
Furthermore, a two-year study of DEHP in female rats demonstrated no
effects at 0.3 g/kg diet, a value which corresponds well to that noted
above (Cattley et al., 1987; Popp et al., 1987).
Many studies have shown dramatic species differences in response
to peroxisome proliferators including DEHP (ECETOC, 1992). For
example, DEHP threshold values for peroxisome proliferation of 25 and
250 mg/kg, respectively, were found in rats and hamsters after 14 days
of daily gastric intubation (Lake et al., 1984). Several other studies
showing species differences in the response to DEHP have been
reported. One experiment demonstrated a large increase in hepatic
peroxisomes after 14 days of oral DEHP administration (2000 mg/kg per
day) to rats, but the same treatment of marmosets resulted in no
peroxisome proliferation (Rhodes et al., 1986). A limited study in
cynomolgus monkeys also revealed no evidence of peroxisome
proliferation due to DEHP (Short et al., 1987). In addition, DEHP did
not elicit peroxisome proliferation in guinea-pigs (Osumi & Hashimoto,
1978; Mitchell et al., 1985a). Some studies have indicated that
intrinsic species differences in hepatocellular sensitivity exist. For
example, MEHP and MEHP metabolite VI (mono(2-ethyl-5-oxohexyl)
phthalate), which is a proximate peroxisome proliferator in the rat,
were good peroxisome proliferators in cultured rat hepatocytes, but
had little or no effect in guinea-pig, marmoset or human hepatocytes
(Mitchell et al., 1985a; Elcombe & Mitchell, 1986). More recently,
Bichet et al. (1990) and Butterworth et al. (1989) have also
demonstrated the inability of MEHP to elicit peroxisome proliferation
in human hepatocyte cultures. Studies using a variety of other
peroxisome proliferators (e.g., clofibric acid, beclobric acid,
methylclofenapate, trichloroacetic acid, ciprofibrate, and
benzbromarone) have also failed to demonstrate peroxisome
proliferation in cultured human hepatocytes, in spite of a good
response in rat hepatocytes (Elcombe, 1985; Allen et al., 1987;
Elcombe & Styles, 1989; Butterworth et al., 1989; Bichet et al., 1990;
Blaauboer et al., 1990).
Species differences in cytochrome P-450 IVA1 induction and
stimulation of replicative DNA synthesis have been less well studied.
However, MEHP and metabolite VI did not induce P-450 IVA1-mediated
lauric acid hydroxylation in vivo in marmosets (Rhodes et al., 1986)
or in cultured marmoset or human hepato-cytes (Elcombe & Mitchell,
1986).
In conclusion, it appears that the livers of rats and mice are
exquisitely sensitive to peroxisome proliferators, including DEHP,
while those of guinea-pigs, monkeys, and humans show minimal or no
response (ECETOC, 1992).
8. EFFECTS ON HUMANS
8.1 General population exposure
Two adults who swallowed 5 or 10 g DEHP experienced no untoward
effects apart from mild gastric disturbances and moderate diarrhoea
with 10 g DEHP (Shaffer et al., 1945). Three cases of nonspecific
hepatitis were described among 27 haemodialysis patients with terminal
renal failure. The PVC blood tubing used released DEHP at a
concentration of 10-20 mg/litre of perfusate. The symptoms and signs
of hepatitis disappeared rapidly when the use of tubing that did not
contain DEHP was resumed (Neergaard et al., 1971).
In three pre-term infants artificially ventilated with PVC
respiratory tubes, unusual lung disorders (opacification of the lung)
were observed during the fourth week of life. It was assumed by the
authors that these lung disorders were causually related to the
exposure to DEHP released from the respiratory tubes (Roth et al.,
1988).
8.2 Occupational exposure
There are very few data on the effects of occupational exposure
specifically to DEHP.
Two studies reported symptoms and signs of polyneuropathy among
47 out of 147 workers at a PVC-processing plant in the USSR and 12 out
of 23 workers at a plant for phthalate production in Italy. The
workers were exposed to a mixture of phthalates and DEHP was a minor
constituent, at least in the USSR plant. Furthermore, tricresyl
phosphate (a neurotoxin) was a component of the incombustible
materials produced in 10-20% of machines assigned to various workers
(Milkov et al., 1973). In the Italian study there was no corresponding
unexposed control group and the authors concluded that no definite
conclusion could be drawn from the study because of the limited number
of workers examined (Gilioli et al., 1978). The total phthalate air
concentrations recorded varied between 1.7 and 66 mg/m3 in the USSR
and 1 and 60 mg/m3 in Italy (Milkov et al., 1973; Gilioli et al.,
1978).
In a study involving a Swedish PVC-processing factory, peripheral
nervous system symptoms and signs were investigated among 54 male
workers exposed mainly to DEHP, diisodecylphthalate, and some
butylbenzylphthalate. The workers were divided into three groups of
approximately equal size and with mean phthalate exposures of 0.1,
0.2, and 0.7 mg/m3. Some workers displayed various peripheral
nervous system symptoms and signs, but these were not related to the
level of exposure. None of the workers reported symptoms indicating
work-related obstructive lung disease. Conventional lung function
tests also showed no association with exposure (Nielsen et al., 1985).
However, several biochemical parameters showed significant
associations with exposure. There was a slight decrease in the
haemoglobin level with longevity of employment as well as with
exposure in the last year. The serum ý-1-antitrypsin level increased
slightly with length of employment and the serum immunoglobulin A
level rose with rising exposure during the last year (Nielsen et al.,
1985).
One case of occupational asthma due to DEHP was reported in
worker at a PVC-processing plant (Brunetti & Moscato, 1984).
Diagnosis was made by exposing the patient to DEHP vapour in an
inhalation chamber; this evoked a dual asthmatic reaction. The action
was inhibited by prior administration of sodium chromoglycate.
A study of blood lipids, serum activities of liver enzymes, and
routine haematological tests was carried out among workers at a German
plant for DEHP production. The results were negative and uninformative
due to very low exposure levels (below 0.16 mg/m3) and the lack of
a control group (Thiess et al., 1978b). Thiess & Flieg (1978)
investigated the frequency of chromosome aberrations in 10 workers who
were employed from 10 to 30 years in this DEHP production plant. The
exposure levels were very low (0.09-0.16 mg/m3), and there was no
increase in chromosome aberrations compared to the control group. A
mortality study of 221 workers exposed to DEHP in the same plant was
also conducted. Eight deaths occurred in the cohort compared with
expected values of 15.9 and 17.0 from the city and county data,
respectively (Thiess et al., 1978a).
9. EFFECTS ON OTHER ORGANISMS IN THE LABORATORY AND FIELD
9.1 Toxicity to microorganisms
Bringmann & Kühn (1980) calculated toxic thresholds from cell
multiplication inhibition tests and found thresholds of > 400
mg/litre for the bacterium Pseudomonas putida, 10 mg/litre for the
alga Scenedesmus quadricaudaia, and 19 mg/litre for the protozoan
Entosiphon sulcatum.
Mathur (1974b) reported that DEHP added to soil (3 g/kg)
inhibited respiration, whereas addition of the same amount of DEHP to
soil preincubated for 14 weeks with DEHP or dioctyl phthalate had no
effect on respiration rate. The experiments were conducted at 22 °C
since no degradation of DEHP occurred at 4 °C or 10 °C. In an
intermittent flow-through hydrosoil microcosm, DEHP (at 1 and 100
mg/litre) produced no significant effect on the numbers or
physiological activity of the microflora monitored, nor on any of the
overall activities that were studied (Mutz & Jones 1977).
Larsson et al. (1986) studied the effect of DEHP on the microbial
activity in sediments. Sediment cores were taken, together with the
overlying water, from a eutrophic lake and the sediment was spiked
with between 25 and 400 mg DEHP/kg at a level of 5 mm below the
surface. Uncontaminated sediment samples had a significantly higher
oxygen uptake than sediment containing DEHP. Microbial activity, as
estimated from decreased oxygen saturation in the soil column,
correlated inversely with increasing levels of DEHP in the sediment.
9.2 Toxicity to aquatic organisms
Many of the toxicity values given in this section are above the
water solubility of DEHP, which ranges from 0.3 to 0.4 mg/litre (see
section 2.2). DEHP adsorbs strongly to sediment, food, dissolved
organic carbon, and even the testing vessels. Many of the toxicity
tests are also based on nominal concentrations. Therefore, the actual
exposure of the organisms is very difficult to determine and care must
be taken when interpreting the results.
9.2.1 Invertebrates
Acute toxicity to aquatic invertebrates is summarized in Table 9.
Table 9. Toxicity of DEHP to aquatic invertebratesa
Organism Age Temperature Hardnessb pH Duration LC50 Reference
(° C) (h) (mg/litre)d
Water flea < 24 h 17 48 0.133 Passino & Smith (1987)
(Daphnia pulex)
Water flea < 24 h 21-23 173 7.4-9.4 24 > 68 Leblanc (1980)
(Daphnia magna) < 24 h 21-23 173 7.4-9.4 48 11 Leblanc (1980)
Crayfish 96 > 10 Mayer & Sanders (1973)
(Orconectes nais)
Harpacticoid adult 20-22 7c 7.8 96 > 300 Linden et al. (1979)
(Nitocra spinipes)
Scud 96 > 32 Sanders et al. (1973)
(Gammarus pseudolimnaeus)
Midge larvae 21-23 270 7.4 48 > 18 Streufert et al. (1980)
(Chironomus plumosus)
a All experiments were performed using static conditions (water unchanged for duration of test)
b Hardness is expressed as mg calcium carbonate per litre. The tests were performed in fresh water
except where stated otherwise
c Salinity (%)
d Nominal values
Brown & Thompson (1982a) found no mortality of Daphnia magna
over an exposure period of 48 h and at DEHP dose levels up to 320
µg/litre. However, at levels of 180 µg/litre or more, the daphnids
were seen to float in the surface layer. The authors suggested that
this observation was connected with the solubility of DEHP (which
tends to precipitate out at > 180 µg/litre, possibly onto the
daphnids, thereby causing them to float). Stephenson (1983) found no
mortality in Gammarus pulex exposed for up to 120 h to a DEHP
concentration (400 µg/litre) in excess of its solubility.
Laughlin et al. (1978) exposed grass shrimps (Palaemonetes
pugio) to DEHP concentrations of up to 1 mg/litre (well above the
solubility limit of the ester) and found no significant difference in
mortality between the control and treated shrimps during the 28-day
exposure period. DEHP had no significant effect on the development
rate of larvae from hatching to moult. No apparent effects were noted
when mussels (Mytilus edulis) were exposed to 50 µg/litre for 28
days (Brown & Thompson, 1982b).
Hobson et al. (1984) found no significant mortality of penaeid
shrimps (Penaeus vannamei) fed a diet containing up to 50 g DEHP/kg
for 14 days, this representing 4% of the body weight per day.
Histological examination revealed no changes and no significant dose-
related effects were observed on moulting.
Sanders et al. (1973) and Mayer & Sanders (1973) exposed Daphnia
magna for a complete life cycle (21 days) to 3, 10 or 30 µg
DEHP/litre in an intermittent-flow system. Reproduction was
significantly inhibited at all concentrations (60, 70, and 80%
inhibition at the three dose levels, respectively). In contrast, Brown
& Thompson (1982a) found no effect on the reproduction of Daphnia
magna at concentrations of up to 100 µg/litre and suggested that the
difference in the numbers of young born to the controls in the two
studies, i.e. 11 offspring per parent (Mayer & Sanders, 1973) compared
with 170 per parent (Brown & Thompson, 1982a), could account for these
conflicting results.
When Knowles et al. (1987) exposed Daphnia magna to DEHP
concentrations of between 12 and 811 µg/litre for up to 21 days under
flow-through conditions (the reproduction rate was approximately 200
offspring per adult), survival was not affected at levels of < 158
µg/litre. At 811 µg/litre, however, survival was significantly reduced
after both 7 and 21 days. The mean number of young per surviving adult
was also reduced at this dose level. The levels of major biochemical
components such as protein, RNA, DNA, and glycogen were all
significantly reduced after 7 days at 811 µg/litre, but all except
glycogen were unaffected after 21 days. The authors concluded that the
maximum acceptable toxicant concentration (MATC), based on survival
and reproduction, was between 158 and 811 µg/litre. A no-observed-
effect level (NOEL) of 72 µg/litre was identified both by DNA content
and RNA/DNA ratio at day 7 and by surfacing of Daphnia at day 0.
Thuren & Woin (1991) exposed the freshwater amphipod Gammarus
pulex to DEHP at concentrations of 100 or 500 µg/litre for 10 days
under flow-through conditions. There was a 5 day pre- and post-
exposure period. The overall locomotor activity of G. pulex was
significantly decreased at the higher exposure level, and the effect
persisted throughout the post-exposure period. No significant effects
were observed at the lower dose level.
Flow-through chronic toxicity tests in both sand and hydrosoil
showed that DEHP concentrations as high as 360 µg/litre (sand) and 240
µg/litre (hydrosoil) had no significant effects on the growth or
development of midge larvae (Chironomus plumosus) over a 35-day
period. The continuous exposure of first-generation midge eggs in sand
substrate to mean DEHP concentrations of between 140 and 360 µg/litre
had no significant effect on hatchability (Streufert et al., 1980).
Woin & Larsson (1987) found that dragonfly larvae ( Aeshna sp.)
caught significantly fewer prey (Chaborus larvae) per attempt when
exposed to sediment DEHP concentrations of approximately 600 mg/kg for
between 3 and 9 weeks.
9.2.2 Fish
The acute toxicity to fish is summarized in Table 10 and the
toxicity to the embryo-larval stages of fish is shown in Table 11.
DEHP caused no deaths among one-year-old rainbow trout (Salmo
gairdneri) exposed to between 1 and 1000 mg/litre for 48 h (Silvo,
1974) or juvenile Atlantic salmon (Salmo salar) exposed to 100
mg/litre for 96 h (Zitko, 1972).
Mehrle & Mayer (1976) reported no effects on growth or survival
of adult fathead minnows (Pimephales promelas) exposed to between 1
and 62 µg/litre for 56 days. They also exposed rainbow trout eggs to
DEHP levels of 5, 14, and 54 µg/litre for 12 days prior to hatching,
but there was no significant effect on hatchability. The resulting fry
were continuously exposed to DEHP for a further 90 days. The two
highest concentrations caused a significant, but not dose related,
increase in the mortality of sac fry within 5 days of hatching. After
yolk absorption (at 24 days), DEHP caused no significant mortality or
effects on growth and development for the remainder of the exposure
period.
Table 10. Toxicity (96-h LC50 values) of DEHP to fish
Organism Size Stat/ Temperature Hardnessb pH LC50 Reference
flowa (° C) (mg/litre)c
Bluegill sunfish > 10 Mayer & Sanders (1973)
(Lepomis macrochirus) 0.32-1.2 g stat 21-23 32-48 6.7-7.8 > 770 Buccafusco et al. (1981)
Fathead minnow > 10 Mayer & Sanders (1973)
(Pimephales promelas) 0.055-0.25 g 24-26 44-46 7-8 > 0.33d Defoe et al. (1990)
Channel catfish > 10 Mayer & Sanders (1973)
(Ictalurus punctatus)
Rainbow trout fingerling stat 14-16 540 Hrudey et al. (1976)
(Oncorhynchus mykiss)
> 10 Mayer & Sanders (1973)
a Stat = static conditions (water unchanged for duration of test)
b Hardness is expressed as mg calcium carbonate per litre. The tests were performed in fresh water
except where stated otherwise
c Nominal values unless stated otherwise
d Measured value
Table 11. Toxicity of DEHP to the embryo-larval stages of fisha
Organism Stat/flowb Hardnessc pH LC50 (mg/litre) 95% confidence limits
Channel catfish stat 90-115 7.6-8.1 0.69 0.55-0.86
(Ictalurus punctatus)
Redear sunfish stat 90-115 7.6-8.1 6.18 4.65-8.04
(Lepomis microlophus)
Largemouth bass flow 45-55 7.5-8.0 42.1 28.8-77.8
(Micropterus salmoides)
flow 190-225 7.5-8.0 32.9 19.8-58.9
Rainbow trout flow 45-55 7.5-8.0 139.5 123.2-165.2
(Oncorhynchus mykiss)
flow 190-225 7.5-8.0 149.2 125.8-203.8
a From: Birge et al. (1978). Exposure was initiated 2 to 6 h after spawning (except in the case of rainbow trout where exposure
was initiated 15 min after fertilisation) and continued until 4 days after hatching. The hatching times were 22 days for rainbow
trout (13.5-14.3 °C), 3 days for catfish (29-31 °C), and 3-4 days for bass and sunfish (20-24 °C).
b Stat = static conditions, but water renewed every 12 h; flow = flow-through conditions (DEHP concentration in water continuously
maintained)
c Hardness is expressed as mg calcium carbonate per litre. The tests were performed in fresh water except where stated otherwise.
DeFoe et al. (1990) exposed rainbow trout (Oncorhynchus mykiss)
and Japanese medaka (Oryzias latipes) to DEHP using concentrations
of up to 0.502 mg/litre for a 90-day trout embryo-larval test and
0.554 mg/litre for a 168-day larval test on medaka. No significant
adverse effects were observed on trout hatchability, survival or
growth, but there was a significant reduction in the growth of
Japanese medaka.
Mayer & Sanders (1973) studied the effects of DEHP on the
reproduction of zebra fish (Brachydanio rerio) and guppies
(Lebistes reticulatus). During the 90-day dietary exposure, zebra
fish were fed on 50 or 100 mg DEHP/kg food, and guppies were fed 100
mg/kg. Although the treated zebra fish spawned more frequently than
the control fish, the latter produced more eggs per spawn. Fry
survival was significantly reduced by DEHP. Treated guppies produced
fewer fry per adult and had an 8% incidence of abortions, whereas
there were no abortions in the control group (no statistics were
given).
In a study of fish exposed to DEHP, Mayer et al. (1977) found
reduced vertebral collagen levels after 150 days at a DEHP
concentration of 3.7 µg/litre in adult brook trout (Salvelinus
fontinalis), after 127 days at 11 µg/litre in fathead minnow, and
after 90 days at 5 µg/litre in rainbow trout. However, there was no
effect on fish growth. Pfuderer & Francis (1975) found that DEHP had
no effect on the heart rate of goldfish when the fish were exposed to
200 mg/litre for 10 min.
9.2.3 Amphibians
The acute toxicity of DEHP to tadpoles of Fowler's toad and the
leopard frog is given in Table 12.
When Larsson & Thuren (1987) exposed eggs of the moorfrog (Rana
arvalis) to sediment concentrations of between 10 and 800 mg DEHP/kg
(fresh weight), the number of tadpoles hatching decreased with
increasing exposure concentration. The percentage hatch was 90% for
the controls eggs, 50% at 150 mg DEHP/kg, and < 30% at > 400 mg/kg.
The lowest DEHP concentration that caused a significant decrease in
the number of tadpoles hatching was 25 mg/kg. After hatching, the
survival of tadpoles was unaffected. There were no delays in hatching
and no abnormalities in the developing tadpoles exposed to the various
DEHP concentrations.
Table 12. Toxicity of DEHP to frog and toad tadpolesa
Organism LC50 (mg/litre) 95% confidence limits
Fowler's toad 3.88 3.08-4.84
(Bufo fowleri)
Leopard frog 4.44 3.65-5.37
(Rana pipiens)
a From: Birge et al. (1978). Exposure occurred under static
conditions, but water was renewed every 12 h. It was initiated 2
to 6 h after spawning and continued until 4 days after hatching.
The hatching times varied between 3 and 4 days, and so the
exposure period varied between 7 and 8 days. The temperature was
20-24 °C, hardness 90-115 mg CaCO3/litre, and pH 7.6-8.1.
Wams (1987) quoted an unpublished Dutch report which stated that
an exposure of 2 mg DEHP/litre caused a reduction in the growth rate
of clawed toad (Xenopus laevis) larvae.
9.3 Toxicity to terrestrial organisms
9.3.1 Plants
When Herring & Bering (1988) grew spinach and pea plants from
seed for 14 to 16 days in soil containing 100 mg DEHP/kg, there was no
effect on plant growth, as measured by height. The effect of DEHP on
seed germination was observed by placing seeds in petri dishes
containing a solution of 100 mg/litre. A reduction of 40% to 50% in
the number of seeds germinating was found.
Lokke & Rasmussen (1983) reported that DEHP had no visible effect
on Sinapis alba, Brassica napusor or Achillea millefolium when
sprayed in the field at concentrations of up to 87.5 kg/ha. According
to Stanley & Tapp (1982), DEHP at a level of 1000 mg/kg soil had no
effect on the growth of rape (Brassica rapa) and only a slight
effect on that of oats (Avena sativa).
9.3.2 Earthworms
In a series of contact toxicity tests, red earthworms (Eisenia
foetida) were exposed to DEHP via filter paper in glass vials. An
LD50 could not be calculated because DEHP was not toxic even at the
highest dose of 25 mg/cm2 (Neuhauser et al., 1986).
9.3.3 Insects
Al-Badry & Knowles (1980) found DEHP to be non-toxic to female
houseflies (Musca domestica) when applied topically or by injection
at a concentration of 20 µg/fly (equivalent to 1000 mg/kg). When it
was topically applied simultaneously with various organophosphates, an
antagonistic interaction was apparent; mortality of 85% to 95% was
reduced to less than 10% by the DEHP. However, when DEHP was topically
applied 30 min before exposure to an organophosphate concentration
that caused 10% to 30% mortality, the resulting interaction was
synergistic with mortality rising to over 60% and in most cases to
over 80%.
9.3.4 Birds
Hill et al. (1975) found no mortality among 10-day-old ring-
necked pheasants or mallard fed up to 5000 mg DEHP/kg for 5 days
followed by 3 days on a normal diet.
When Wood & Bitman (1984) fed broiler hens a diet containing 2000
mg DEHP/kg (226 mg DEHP/hen per day) for 4 weeks, egg production and
body weights were significantly decreased. Ishida et al. (1982)
reported the cessation of egg production and an abnormality of the
ovaries in laying hens fed 5 or 10 g DEHP/kg diet for up to 230 days.
In a study by O'Shea & Stafford (1980), starlings (Sturnus
vulgaris) were fed on a diet containing 25 or 250 mg DEHP/kg for a
30-day period. At both dose levels, the treated birds gained
significantly more body weight than controls during the exposure
period, but at the lower dose level food consumption was significantly
reduced compared to controls.
When Peakall (1974) fed ring doves (Streptopelia risoria) on a
diet containing 10 mg/kg, there were no effects on the eggshell
thickness, ashed egg weight, rate of water loss, surface area or
permeability of the eggs laid.
10. EVALUATION OF HUMAN HEALTH RISKS AND EFFECTS ON THE ENVIRONMENT
10.1 Evaluation of human health risks
10.1.1 Exposure levels
DEHP concentrations of up to 300 ng/m3 have been measured in
urban air, but usually the levels in ambient air are well below 100
ng/m3. Data on occupational exposure levels are limited.
Concentrations of up to 4.1 mg/m3 have been reported but they are
usually below 1 mg/m3. Total phthalate exposure levels have been
reported to be between 0.1 and 60 mg/m3. Clinical treatment,
including transfusion, haemodialysis, extracorporal circulation, and
artificial respiration, may lead to DEHP exposure.
The exposure to DEHP from drinking-water and food is low.
10.1.2 Toxic effects
Dose-dependent kinetics of DEHP or its metabolites and species
differences in the metabolism have been confirmed in several studies.
In animals some inhalation studies have been performed. However,
no consistent findings are reported.
The oral and intraperitoneal LD50 values exceed 25 g/kg,
indicating that DEHP has low acute toxicity.
In rats and mice, DEHP administration produces hepatic
hyperplasia and hypertrophy, which is characterized by peroxisome
proliferation. The threshold dose level for hepatic peroxisome
proliferation in the rat is approximately 50 mg/kg per day. High DEHP
dose levels (12 g DEHP/kg diet in rats; 6 g DEHP/kg diet in mice) in
a feeding study resulted in an increased incidence of hepatic tumours
in rats and mice.
Marked species differences in DEHP-induced hepatomegaly and
peroxisome proliferation exist. Rats and mice are very responsive,
Syrian hamsters less responsive, and guinea-pigs, marmosets, and
cynomolgus monkeys non-responsive. Studies with primary hepatocyte
cultures have shown an excellent in vitro/in vivo correlation
concerning responsiveness to DEHP/DEHP metabolites. Several studies
have demonstrated the non-responsive nature of cultured human
hepatocytes to DEHP metabolites and other peroxisome proliferators.
The induction of peroxisome proliferation and cell replication is
strongly associated with the development of hepatic tumours in rats
and mice. Thus, the available data suggest that species which do not
respond to the hepatic effects of DEHP are unlikely to be susceptible
to the development of hepatic tumours.
Testicular atrophy is one of the most consistent effects of DEHP
in in vivo studies on a variety of experimental animal species. Such
effects have been observed in rats fed 6 g DEHP/kg diet and mice fed
3 g/kg diet, and they are more pronounced in young animals. Hamsters
and marmosets appear to be more resistant to the testicular effects of
DEHP.
When both male and female CD-1 mice were treated with 1 g DEHP/kg
diet, a significant reduction in fertility was observed.
A dietary level of 20 g DEHP/kg throughout gestation produced an
increased incidence of resorptions in rats but no malformations. In
the mouse, however, 1 g/kg throughout pregnancy increased the
incidence of embryolethality and abnormalities. Sensitivity was
greatest on days 7-9 of gestation. The dose level (0.5 g/kg) that
induced fetotoxicity in mice did not induce maternal toxicity. Results
from several different genotoxicity tests indicate that DEHP and its
major metabolites do not exhibit any direct genotoxic effect in either
bacteria or in vitro mammalian cells. This has been confirmed in
in vivo binding studies, which indicated that DEHP and its
metabolites do not interact covalently with DNA. However, it has been
established that DEHP has the potential of inducing aneuploidy in
fungi as well as in in vitro mammalian cells. There are no
consistent results from dominant lethal studies.
Few data on the human health effects of DEHP exposure have been
reported. There have been a few studies on workers exposed to
phthalate mixtures, but no consistent health effects that could be
directly related to DEHP have been reported.
10.1.3 Conclusion
DEHP causes reproductive and hepatocarcinogenic effects in rats
and mice.
Testicular atrophy is the main reproductive effect in rats and
mice, and young animals are more susceptible than older ones to this
effect. The induction of hepatic peroxisome proliferation and cell
replication are strongly associated with the liver carcinogenic effect
of certain non-genotoxic carcinogens including DEHP. However, marked
differences have been observed among animal species with respect to
DEHP-induced peroxisome proliferation. Currently there is not
sufficient evidence to suggest that DEHP is a potential human
carcinogen.
10.2 Evaluation of effects on the environment
10.2.1 Exposure levels
DEHP exists widely in the environment, being released during
production, processing, usage, and disposal. Transport in the air is
the major route by which it enters the environment. It can be
deposited by dry or wet deposition and has been found to leach into
ground water.
DEHP is readily photodegraded in the atmosphere. In the
laboratory, aerobic biodegradation has been shown to occur under
certain circumstances. However, in the environment aerobic
biodegradation seems to be slow and anaerobic degradation is even
slower.
Based on a measured log Pow of about 5 and measured
bioaccumulation factors for biota and sediment, it can be seen that
DEHP is highly bioaccumulative.
In rivers and lakes, DEHP concentrations of up to 4 µg/litre have
been measured.
Due to its hydrophobic nature, DEHP adsorbs readily to soil,
sediment, and particulate matter. Levels of up to 70 mg/kg dry weight
have been found in river sediments, but near to a discharge point
levels of up to 1480 mg/kg dry weight have been reported.
10.2.2 Toxic effects
Most acute toxicity studies on aquatic organisms show DEHP to be
of low toxicity. However, one study suggested a greater sensitivity
for the water flea (Daphnia pulex), the nominal 48-h LC50 being
133 µg/litre.
In a 21-day study on Daphnia magna, the NOEL for biochemical
and behavioural effects was 72 µg/litre. A significant increase in
mortality was recorded in trout fry exposed to 14 µg DEHP/litre from
12 days prior to hatching. At concentrations of 3.7 to 11 µg/litre,
reductions in vertebral collagen were reported in three species of
fish exposed for 150 days.
Zebra fish fry survival and guppy reproduction were adversely
affected at DEHP concentrations in food of 50 and 100 mg/kg,
respectively, during a 90-day exposure period.
In two separate studies, DEHP concentrations of 25 mg/kg
(wet weight) of sediment significantly reduced microbial activity and
the number of frog tadpoles hatching.
Available studies indicate that the acute toxicity of DEHP to
algae, plants, earthworms, and birds is low. There are no data
relating to effects upon wild mammals.
10.2.3 Conclusion
There is no documented information that DEHP presents any hazard,
based on acute exposure to fish and daphnids. However, a reduction of
microbial activity in sediment at environmental levels of DEHP was
reported. A comparison between environmental levels and the
concentrations that produce effects in prolonged studies, especially
early life-stage tests on fish and amphibians, indicates that a hazard
for the environment, particularly via water and sediment, cannot be
excluded. Adverse effects on organisms are likely in areas with highly
contaminated water and sediments which are near to point emission
sources.
Although few relevant studies have been reported, the acute
toxicity of DEHP to algae, plants, earthworms, and birds appears to be
low.
11. RECOMMENDATIONS FOR PROTECTION OF HUMAN HEALTH AND THE
ENVIRONMENT
a) Current disposal practises should be improved.
b) Measures must be undertaken to reduce the release of DEHP to the
environment.
c) Medical devices and products that contribute to the body burden
of DEHP must be scrutinized to reduce exposure to DEHP via the
intravenous route.
12. FURTHER RESEARCH
More research is needed on the effects of DEHP on the ecosystem
of sediments and on the subject of DEHP leaching, particularly from
landfill to ground water.
Since DEHP occurs in the air, including workplace air, further
inhalation studies are needed.
Epidemiological surveys on exposed populations should be
encouraged. Further studies are needed on the mechanism of peroxisome-
proliferator-induced hepatocarcinogenicity, with emphasis on human
health effects.
13. PREVIOUS EVALUATIONS BY INTERNATIONAL BODIES
DEHP was one of the substances discussed by a working group of
the International Agency for Research on Cancer in 1981 (IARC, 1982).
The evaluation by the group was that there was sufficient evidence for
the carcinogenicity of DEHP in mice and rats, but that no adequate
epidemiological study was available. The working group concluded that
DEHP is possibly carcinogenic to humans and placed it in group 2B
(IARC, 1987).
DEHP was evaluated at the 28th meeting of the Joint FAO/WHO
Expert Committee on Food Additives. The recommendation was to reduce
human exposure to DEHP from food-contact materials to the lowest level
technologically attainable (WHO/FAO, 1984a,b). During the 33rd meeting
of the Joint FAO/WHO Expert Committee on Food Additives, DEHP was
again evaluated and the recommendation was the same: DEHP in food
should be reduced to the lowest level attainable. The Committee
considered that this might be achieved by using alternative
plasticizers or alternatives to plastic material containing DEHP
(WHO/FAO, 1989a,b).
REFERENCES
AGARWAL, D.K. (1986) Letters to the editor. Toxicol. appl. Pharmacol.,
82: 383-387.
AGARWAL, D.K., AGARWAL, S., & SETH, P.K. (1982) Effect of di-(2-
ethylhexyl) phthalate on drug metabolism, lipid peroxidation, and
sulfhydryl content of rat liver. Drug Metab. Disp., 10: 77-80.
AGARWAL, D.K., LAWRENCE, W.H., & AUTIAN, J. (1985a) Antifertility and
mutagenic effects in mice from parenteral administration of di-2-
ethylhexyl phthalate (DEHP). J. Toxicol. environ. Health, 16: 71-84.
AGARWAL, D.K., LAWRENCE, W.H., NUNEZ, L.J., & AUTIAN, J. (1985b)
Mutagenicity evaluation of phthalic acid esters and metabolites in
Salmonella typhimurium cultures. J. Toxicol. environ. Health, 16:
61-69.
AGARWAL, D.K., EUSTIS, S., LAMB J.C., IV, JAMESON, C.W., & KLUWE, W.M.
(1986a) Influence of dietary zinc on di(2-ethylhexyl)phthalate-induced
testicular atrophy and zinc depletion in adult rats. Toxicol. appl.
Pharmacol., 84: 12-24.
AGARWAL, D.K., EUSTIS, S., LAMB J.C., IV, REEL, J.R., & KLUWE, W.M.
(1986b) Effects of di(2-ethylhexyl)phthalate on the gonadal patho-
physiology, sperm morphology, and reproductive performance of male
rats. Environ. Health Perspect., 65: 343-350.
AGARWAL, D.K., LAWRENCE, W.H., TURNER, J.E., & AUTIAN, J. (1989)
Effects of parenteral di(2-ethylhexyl)phthalate (DEHP) on gonadal
biochemistry, pathology and reproductive performance of mice. J.
Toxicol. environ. Health, 26: 39-59.
AHMED, R.S., PRICE, S.C., GRASSO, P., & HINTON, R.H. (1990) Hepatic
nuclear and cytoplasmic effects following intermittent feeding of rats
with di(2-ethylhexyl)phthalate. Food chem. Toxicol., 28: 427-434.
AL-BADRY, M. & KNOWLES, C.O. (1980) Phthalate-organophosphate
interactions: Toxicity, penetration, and metabolism studies with house
flies. Arch. environ. Contam. Toxicol., 9: 147-161.
ALBRO, P.W. (1986) Absorption, metabolism, and excretion of di(2-
ethylhexyl) phthalate by rats and mice. Environ. Health Perspect., 65:
293-298.
ALBRO, P.W. (1987) The biochemical toxicology of di-(2-ethylhexyl) and
related phthalates: testicular atrophy and hepatocarcinogenesis. In:
Hodgson, E., Bend, J.R., & Philpot, R.M., ed. Reviews in biochemical
toxicology, Amsterdam, Oxford, New York, Elsevier Science Publishers,
Vol. 8, pp. 73-119.
ALBRO, P.W. & LAVENHAR, S.R. (1989) Metabolism of di(2-ethylhexyl)
phthalate. Drug Metabol. Rev., 21: 13-34.
ALBRO, P.W. & THOMAS, R.O. (1973) Enzymatic hydrolysis of di(2-
ethylhexyl) phthalate by lipases. Biochim. Biophys Actaw, 360:
380-390.
ALBRO, P.W., THOMAS, R., & FISHBEIN, L. (1973) Metabolism of
diethylhexyl phthalate by rats. Isolation and characterization of the
urinary metabolites. J. Chromatogr., 76: 321-330.
ALBRO, P.W., HAAS, J.R., PECK, C.C., ODOM, D.G., CORBETT, J.T.,
BAILEY, F.J., BLATT, H.E., & BARRETT, B.B. (1981) Identification of
the metabolites of di-(2-ethyl-hexyl) phthalate in urine from the
African green monkey, Drug Met. Disp., 9: 223-225.
ALBRO, P.W., CORBETT, J.T., SCHROEDER, J.L., JORDAN, S., & MATTHEWS,
H.B. (1982) Pharmacokinetics, interactions with macromolecules and
species differences in metabolism of DEHP. Environ. Health Perspect.,
45: 19-25.
ALBRO, P.W., CHAPIN, R.E., CORBETT, J.T., SCHROEDER, J., & PHELPS,
J.L. (1989) Mono-2-ethylhexylphthalate, a metabolite of di-(2-
ethylhexyl)phthalate, causally linked to testicular atrophy in rats.
Toxicol. appl. Pharmacol., 100: 193-200.
ALLEN, K.L., GREEN, C.E., & TYSON, C.A. (1987) Comparative studies of
peroxisomal enzyme induction in hepatocytes from rat, cynomolgus
monkey and human by hypolipidemic drugs. Toxicologist, 7: 63.
AL-OMRAN, L.A. & PRESTON, M.R. (1987) The interactions of phthalate
esters with suspended particulate material in fresh and marine waters.
Environ. Pollut., 46: 177-186.
ANTONYUK, O.K. (1975) Toxicology of complex ethers of phthalic acid (A
review of literature), Hyg. Labour occup. Dis., 1: 32-35 (in Russian).
ARANDA, J.M., O'CONNOR, G.A., & EICEMAN, G.A. (1989) Effects of sewage
sludge on di-(2-ethylhexyl) phthalate uptake by plants J. environ.
Qual., 18: 45-50.
ARBIN, A. & ÖSTELIUS, J. (1980) Determination by electron-capture gas
chromatography of mono- and di(2-ethylhexyl) phthalate in intravenous
solutions stored in polyvinyl chloride bags. J. Chromatogr., 193:
405-412.
ASHBY, J., DE SERRES, F.J., DRAPER, M., ISHIDATE, M., Jr, MARGOLIN,
B.H., MATTER, B.E., & SHELBY, M.D., ed. (1985) Evaluation of short-
term tests for carcinogens. Report of the International Programme on
Chemical Safety's collaborative study on in vitro assays, Amsterdam,
Oxford, New York, Elsevier Science Publishers, 752 pp (Progress in
Mutation Research, Vol. 5).
ASTILL, B. (1989) Metabolism of DEHP: Effects of prefeeding and dose
variation, and comparative studies in rodents and the cynomolgus
monkey (CMA studies). Drug Metab. Rev., 21: 35-53.
ASTILL, B., BARBER, E., LINGTON, A., MORAN, E., MULHOLLAND, A.,
ROBINSON, E., & SCHEIDER, B. (1986) Chemical industry voluntary test
program for phthalate esters: Health effect studies. Environ. Health
Perspect., 65: 329-336.
ATLAS, E. & GIAM, C.S. (1981) Global transport of organic pollutants:
Ambient concentrations in the remote marine atmosphere. Science, 211:
163-165.
ATSDR (1988) Toxicological profile for di(2-ethylhexyl) phthalate,
Atlanta, Georgia, Agency for Toxic Substances and Disease Registry, US
Public Health Service (Draft for public comment).
BALLY, M.B., OPHEIM, D.J., & SHERTZER, H.G. (1980) Di-(2-ethylhexyl)
phthalate enhances the release of lysosomal enzymes from alveolar
macrophages during phagocytosis. Toxicology, 18: 49-60.
BARBER, E.D., ASTILL, B.D., MORAN, E.J., SCHNEIDER, B.F., GRAY,
T.J.B., LAKE, B.G., & EVANS, J.G. (1987) Peroxisome induction studies
on seven phthalate esters. Toxicol. ind. Health, 3: 7-24.
BARNEY, G.H., ORGEBIN-CRIST, M.C., & MACAPINLAC, M.P. (1969) Genesis
of esophageal parakeratosis and histologic changes in the testes of
the zinc-deficient rat and their reversal by zinc repletion. J. Nutr.,
95: 526-534.
BAUGHMAN, G.L., PARIS, D.F., & STEEN, W.C. (1980) Quantitative
expression of biotransformation. In: Maki, A.W., Dickson, K.L., &
Cairns, J., ed. Biotransformation and fate of chemicals in the
environment, Washington, DC, American Society for Microbiology, pp.
105-111.
BELISLE, A.A., REICHEL, W.L., & SPANN, J.W. (1975) Analysis of tissues
of mallard ducks fed two phthalate esters. Bull. environ. Contam.
Toxicol., 13: 129-132.
BELL, F.P., PATT, C.S., BRUNDAGE, B., GILLIES, P.J., & PHILIPS, W.A.
(1978a) Studies on lipid biosynthesis and cholesterol content of liver
and serum lipoproteins in rats fed various phthalate esters. Lipids,
13: 66-74.
BELL, F.P., PATT, C.S., & GILLIES, P.J. (1978b) Effect of phthalate
esters on serum cholesterol and lipid biosynthesis in liver, testes,
and epididymal fat in the rat and rabbit. Lipids, 13: 673-678.
BELL, F.P., WANG, S., MENDOZA, A.R., & NISHIZAWA, E.E. (1979) Platelet
function and platelet lipid biosynthesis in rats and rabbits fed the
plasticiser DEHP. Bull. environ. Contam. Toxicol., 23: 306-310.
BICHET, N., CAHARD, D., FABRE, G., REMANDET, B., GOUY, D., & CANO,
J.-P. (1990) Toxicological studies on a benzofuran derivative. III.
Comparison of peroxisome proliferation in rat and human hepatocytes in
primary culture. Toxicol. appl. Pharmacol., 106: 509-517.
BIRGE, W.J., BLACK, J.A., & WESTERMAN, A.G. (1978) Effects of
polychlorinated biphenyl compounds and proposed PCB-replacement
products on embryo-larval stages of fish and amphibians, Washington,
DC, US Department of the Interior, 33 pp (Research Report No. 118).
BLAAUBOER, B.J., VAN HOLSTEIJN, C.W.M., BLEUMINK, R., MENNES, W.C.,
VAN PELT, F.N.A.M., YAP, S.H., VAN PELT, J.F., VAN IERSEL, A.A.J.,
TIMMERMANN, A., & SCHMID, B.P. (1990) The effect of beclobric acid and
clofibric acid on peroxisomal beta-oxidation and peroxisome
proliferation in primary cultures of rat, monkey and human
hepatocytes. Biochem. Pharmacol., 40: 521-528.
BOVE, J.L., DALVEN, P., & KUKREJA, V.P. (1978) Airborne di-butyl and
di(2-ethylhexyl)-phthalate at three New York city air sampling
stations. Int. J. environ. anal. Chem., 5: 189-194.
BRIDGES, J.W. (1985) Frontiers in biochemical toxicology. Trends
pharmacol. Sci., 6 (suppl.): 11-15.
BRINGMANN, G. & KÜHN, R. (1980) Comparison of the toxicity thresholds
of water pollutants to bacteria, algae, and protozoa in the cell
multiplication inhibition test. Water Res., 14: 231-241.
BROWN, D. & THOMPSON, R.S. (1982a) Phthalates and the aquatic
environment: Part I. The effect of di-2-ethylhexyl phthalate (DEHP)
and di-isodecyl phthalate (DIDP) on the reproduction of Daphnia magna
and observations on their bioaccumulation. Chemosphere, 11: 417-426.
BROWN, D. & THOMPSON, R.S. (1982b) Phthalates and the aquatic
environment: Part II. The bioconcentration and depuration of di-2-
ethylhexyl phthalate (DEHP) and di-isodecyl phthalate (DIDP) in
mussels (Mytilus edulis). Chemosphere, 11: 427-435.
BRUNETTI, G. & MOSCATO, G. (1984) [Bronchial asthma due to
occupational exposure to dioctyl phthalate.] Med. Lav., 75: 120-124
(in Italian).
BUCCAFUSCO, R.J., ELLS, S.J., & LEBLANC, G.A. (1981) Acute toxicity of
priority pollutants to bluegill (Lepomis macrochirus). Bull. environ.
Contam. Toxicol., 26: 446-452.
BUTTERWORTH, B.E., BERMUDEZ, E., SMITH-OLIVER, T., EARLE, L., CATTLEY,
R., MARTIN, J., POPP, J.A., STROM, S., JIRTLE, R., & MICHALOPOULOS, G.
(1984) Lack of genotoxic activity of di(2-ethylhexyl) phthalate (DEHP)
in rat and human hepatocytes. Carcinogenesis, 5: 1329-1335.
BUTTERWORTH, B.E., LOURY, D.J., SMITH-OLIVER, T., & CATTLEY, R.C.
(1987) The potential role of chemically induced hyperplasia in the
carcinogenic activity of the hypolipidemic carcinogens. Toxicol. ind.
Health, 3: 129-148.
BUTTERWORTH, B.E., SMITH-OLIVER, T., EARLE, L., LOURY, D.J., WHITE,
R.D., DOOLITTLE, D.J., WORKING, P.K., CATTLEY, R.C., JIRTLE, R.,
MICHALOPOULOS, G., & STROM, S. (1989) Use of primary cultures of human
hepatocytes in toxicology studies. Cancer Res., 49: 1075-1084.
CALLEY, D., AUTIAN, J., & GUESS, W.L. (1966) Toxicology of a series of
phthalate esters. J. pharm. Sci., 55: 158-162.
CARPENTER, C.P., WEIL, C.S., & SMYTH, H.F. (1953) Chronic oral
toxicity of di(2-ethylhexyl) phthalate for rats, guinea-pigs, and
dogs. Arch. ind. Hyg., 8: 219-226.
CATTLEY, R.C. & POPP, J.A. (1989) Differences between the promoting
activities of the peroxisome proliferator WY14,643 and phenobarbital
in rat liver. Cancer Res., 49: 3246-3251.
CATTLEY, R.C., CONWAY, J.G., & POPP, J.A. (1987) Association of
persistent peroxisome proliferation and oxidative injury with
hepatocarcinogenicity in female F-344 rats fed di(2-
ethylhexyl)phthalate for 2 years. Cancer Lett., 38: 15-27.
CAUTREELS, W. & VAN CAUWENBERGHE, K. (1978) Experiments on the
distribution of organic pollutants between airborne particulate matter
and the corresponding gas phase. Atmos. Environ., 12: 1133-1141.
CAUTREELS, W., VAN CAUWENBERGHE, K., & GUZMAN, L.A. (1977) Comparison
between the organic fraction of suspended matter at a background and
an urban station. Sci. total Environ., 8: 79-88.
CERBULIS, J. & ARD, J.S. (1967) Method for isolation and detection of
dioctyl phthalate from milk lipids. J. Assoc. Off. Anal. Chem., 50:
646-650.
CHAPIN, R.E., GRAY, T.J.B., PHELPS, J.L., & DUTTON, S.L. (1988) The
effects of mono-(2-ethylhexyl)-phthalate on rat sertoli cell-enriched
primary cultures. Toxicol. appl. Pharmacol., 92: 467-479.
CHEN, W.S., KERKAY, J., PEARSON, K.H., PAGANINI, E.P., & NAKAMOTO, S.
(1979a) Determination of urinary bis(2-ethylhexyl) phthalate levels in
non-uremic subjects by gas chromatography. Anal. Lett., 12: 1501-1515.
CHEN, W.S., KERKAY, J., PEARSON, K.H., PAGANINI, E.P., & NAKAMOTO, S.
(1979b) Tissue bis(2-ethylhexyl) phthalate levels in uremic subjects.
Anal. Lett., 12: 1517-1535.
CHU, I., SECOURS, V.E., MARINO, I.A., VILLENEUVE, D.C., & VALLI, V.E.
(1981) Sub-acute and sub-chronic toxicity of mono-2-ethylhexyl
phthalate in the rat. Arch. environ. Contam. Toxicol., 10: 271-280.
CIRIOLO, M.R., MAVELLI, I., ROTILIO, G., BORZATTA, V., CRISTOFARI, M.,
& STANZANI, L. (1982) Decrease of superoxide dismutase and glutathione
peroxidase in liver of rats treated with hypolipidemic drugs. FEBS
Lett., 144: 264-268.
CLAYTON, G.D. & CLAYTON, F.E. (1981) Patty's industrial hygiene and
toxicology, 3rd ed., New York, Wiley-Interscience, pp. 2342-2353.
CONTRERAS, T.J., SHELBLEY, R.H., & VALERI, C.R. (1974) Accumulation of
di-2-ethylhexyl phthalate (DEHP) in whole blood, platelet
concentrates, and platelet-poor plasma. Transfusion, 14: 34-46.
CONWAY, J.G., CATTLEY, R.C., POPP, J.A., & BUTTERWORTH, B.E. (1989a)
Possible mechanisms in hepatocarcinogenesis by the peroxisome
proliferator di(2-ethylhexyl)phthalate. Drug Metab. Rev., 21: 65-102.
CONWAY, J.G., TOMASZEWSKI, K.E., OLSON, M.J., CATTLEY, R.C., MARSMAN,
D.S., & POPP, J.A. (1989b) Relationship of oxidative damage to the
hepatocarcinogenicity of the peroxisome proliferators di(2-ethylhexyl)
phthalate and Wy-14, 643. Carcinogenesis, 10: 513-519.
CROCKER, J.F.S., SAFE, S.H., & ACOTT, P. (1988) Effects of chronic
phthalate exposure on the kidney. J. Toxicol. environ. Health, 23:
433-444.
CURSTEDT, T. & SJÖVALL, J. (1983) Individual molecular species of
phosphatidylcholines and phosphatidylinositols in livers of rats fed
bis(2-ethylhexyl) phthalate. Med. Biol., 61: 219-222.
CURTO, K.A. & THOMAS, J.A. (1982) Comparative effects of diethylhexyl
phthalate or monoethylhexyl phthalate on male mouse and rat
reproductive organs. Toxicol. appl. Pharmacol., 62: 121-125.
CURTO, K.A., MCCAFFERTY, R.E., DONOVAN, M.P., & THOMAS, J.A. (1982)
Further studies on the effects of the phthalate acid esters (PAE) on
rat male reproductive organs. Toxicologist, 2: 71.
DANIEL, J.W. & BRATT, H. (1974) The absorption, metabolism and tissue
distribution of di(2-ethylhexyl) phthalate in rats. Toxicology, 2:
51-65.
DEANGELO, A.B. & GARRETT, C.T. (1983) Inhibition of development of
preneoplastic lesions in the livers of rats fed a weakly carcinogenic
environmental contaminant. Cancer Lett., 20: 199-205.
DEANGELO, A.B., GARRETT, C.T., & QUERAL, A.E. (1984) Inhibition of
phenobarbital and dietary choline deficiency promoted preneoplastic
lesions in rat liver by environmental contaminant di(2-ethylhexyl)
phthalate. Cancer Lett., 23: 323-330.
DEFOE, D.L., HOLCOMBE, G.W., HAMMERMEISTER, D.E., & BIESINGER, K.E.
(1990) Solubility and toxicity of eight phthalate esters to four
aquatic organisms.Environ. Toxicol. Chem. 9: 623-636.
DIVINCENZO, G.D., HAMILTON, M.L., MUELLER, K.R., DONISH, W.H., &
BARBER, E.D. (1985) Bacterial mutagenicity testing of urine from rats
dosed with 2-ethylhexanol derived plasticizers. Toxicology, 34:
247-259.
DIWAN, B.A., WARD, J.M., RICE, J.M., COLBURN, N.H., & SPANGLER, E.F.
(1985) Tumor-promoting effects of di(2-ethylhexyl) phthalate in JB6
mouse epidermal cells and mouse skin. Carcinogenesis, 6: 343-347.
DOUGLAS, R.G., HUGENHOLTZ, A.P., & BLAKEY, D.H. (1986) Genetic
toxicology of phthalate esters: mutagenic and other genotoxic effects.
Environ. Health Perspect., 65: 255-262.
ECETOC (1985) An assessment of the occurrence and effects of dialkyl
ortho-phthalates in the environment, Brussels, European Chemical
Industry Ecology and Toxicology Centre, 63 pp (Technical Report No.
19).
ECETOC (1992) Hepatic peroxisome proliferation, Brussels, European
Chemical Industry Ecology and Toxicology Centre, (Monograph No. 17).
EGLINTON, G., SIMONEIT, B.R.T., & ZORO, J.A. (1975) The recognition of
organic pollutants in aquatic sediments. Proc. R. Soc. Lond., B189:
415-442.
EISENREICH, S.J., LOONEY, B.B., & THORNTON, J.D. (1981) Airborne
organic contaminants in the Great Lakes ecosystem. Environ. Sci.
Technol., 15: 30-38.
ELCOMBE, C.R. (1985) Species differences in carcinogenicity and
peroxisome proliferation due to trichloroethylene: A biochemical human
hazard assessment.Arch. Toxicol., Suppl. 8: 6-17.
ELCOMBE, C.R. & MITCHELL, A.M. (1986) Peroxisome proliferation due to
di(2-ethylhexyl)phthalate (DEHP): Species differences and possible
mechanisms. Environ. Health Perspect., 70: 211-219.
ELCOMBE, C.R. & STYLES J.A. (1989) Species differences in peroxisome
proliferation. Toxicologist, 9: 63.
ELSISI, A.E., CARTER, D.E., & SIPES, I.G. (1989) Dermal absorption of
phthalate diesters in rats. Fundam. appl. Toxicol., 12: 70-77.
ENGELHARDT, G. & WALLNOFER, P.R. (1978) Metabolism of di- and mono-N-
butyl phthalate by soil bacteria. Appl. environ. Microbiol., 35:
243-246.
ENGELHARDT, G., WALLNOFER, P.R., & HUTZINGER, O. (1975) The microbial
metabolism of di-n-butyl phthalate and related dialkyl phthalates.
Bull. environ. Contam. Toxicol., 13: 342-347.
ENGELHARDT, G., TILLMANNS, G., WALLNOFER, P.R., & HUTZINGER, O. (1977)
Biodegradation of di-iso-butyl phthalate and related dialkyl
phthalates by Penicillium lilacinum. Chemosphere, 6: 347-354.
ENVIRONMENT AGENCY OF JAPAN (1989) [Chemicals in the environment],
Tokyo, Environment Agency of Japan, p. 418 (in Japanese).
FATOKI, O.S. & VERNON, F. (1990) Phthalate esters in rivers of the
greater Manchester area, U.K. Sci. total Environ., 95: 221-232.
FISHBEIN, L. & ALBRO, P.W. (1972) Chromatographic and biological
aspects of the phthalate esters. J. Chromatogr., 70: 365-412.
FOSTER, P.M.D., LAKE, B.G., COOK, M.W., THOMAS, L.V., & GANGOLLI, S.D.
(1982) Structure-activity requirements for the induction of testicular
atrophy by butyl phthalates in immature rats: effects on testicular
zinc content. Adv. exp. Med. Biol., 136A: 445-452.
FOSTER, P.M.D., THOMAS, L.V., COOK, M.W., & WALTERS, D.G. (1983)
Effect of di-n-pentyl phthalate treatment on testicular steroidogenic
enzymes and cytochrome P-450 in the rat. Toxicol. Lett., 15: 265-271.
GANNING, A.E., OLLSON, M.J., PETERSON, E., & DALLNER., G. (1989) Fatty
acid oxidation in hepatic peroxisomes and mitochondria after treatment
of rats with di(2-ethylhexyl) phthalate. Pharmacol. Toxicol., 65:
265-268.
GIAM, C.S., CHAN, H.S., NEFF, G.S., & ATLAS, E.L. (1978) Phthalate
ester plasticizers: A new class of marine pollutant. Science, 199:
419-421.
GIAM, C.S., ATLAS, E., CHAN, H.S., & NEFF, G.S. (1980) Phthalate
esters, PCB and DDT residues in the Gulf of Mexico atmosphere. Atmos.
Environ., 14: 65-69.
GILIOLI, R., BULGHERONI, C., TERRANA, T., FILIPPINI, G., MASSETTO, N.,
& BOERI, R. (1978) [A neurological, electromyographic and
electroneurographic study in subjects working at the production of
phthalate plasticizers.] Med. Lav., 69: 620-631 (in Italian).
GOEL, S.K., LALWANI, N.D., & REDDY, J.K. (1986) Peroxisome
proliferation and lipid peroxidation in rat liver. Cancer Res., 46:
1324-1330.
GOLLAMUDI, R., LAWRENCE, W.H., RAO, R.H., & AUTIAN, J. (1985) Effects
of phthalic acid esters on drug metabolizing enzymes of rat liver. J.
appl. Toxicol., 5: 368-371.
GOTO, M. (1979) Monitoring environmental materials and specimen
banking - State-of-the-art of Japanese experience and knowledge. In:
Luepke, N.-P., ed. Monitoring environmental materials and specimen
banking. Proceedings of an International Workshop, Berlin (West),
23-28 October, 1978, pp. 271-288.
GRAHAM, P.R. (1973) Phthalate ester plasticizers - Why and how they
are used. Environ. Health Perspect., 3: 3-12.
GRAY, T.J.B. & BEAMAND, J.A. (1984) Effect of some phthalate esters
and other testicular toxins on primary cultures of testicular cells.
Food chem. Toxicol., 22: 123-131. GRAY, T.J.B. & BUTTERWORTH, K.R.
(1980) Testicular atrophy produced by phthalate esters. Arch.
Toxicol., Suppl. 4: 452-455.
GRAY, T.J.B. & GANGOLLI, S.D. (1986) Aspects of the testicular
toxicity of phthalate esters. Environ. Health Perspect., 65: 229-235.
GRAY, T.J.B., BUTTERWORTH, K.R., GAUNT, I.F., GRASSO, P., & GANGOLLI,
S.D. (1977) Short-term toxicity study of di-(2-ethylhexyl) phthalate
in rats. Food Cosmet. Toxicol., 15: 389-399.
GRAY, T.J.B., ROWLAND, I.R., FOSTER, P.M.D., & GANGOLLI, S.D. (1982)
Species differences in the testicular toxicity of phthalate esters.
Toxicol. Lett., 11: 141-147.
GUESS, W.L. & HABERMAN, S. (1968) Toxicity profiles of vinyl and
polyolefinic plastics and their additives. J. biomed. Mater. Res., 2:
313-335.
GUPTA, R.C., GOEL, S.K., EARLEY, K., SINGH, B., & REDDY, J.K. (1985)
32P-Postlabeling analysis of peroxisome proliferator-DNA adduct
formation in rat liver in vivo and hepatocytes in vitro.
Carcinogenesis, 6: 933-936.
HAMANO, Y., INOUE, K., ODA, Y., YAMAMOTO, H., & KUNITA, N. (1979)
[Studies on the toxicity of phthalic acid esters. Part 2. Dominant
lethal tests for DEHP and MEHP in mice, Osaka, Osaka Public Health and
Sanitation Research Centre, pp. 1-4 (Food Hygiene Series No. 10) (in
Japanese).
HARRIS, R.S., HODGE, H.C., MAYNARD, E.A., & BLANCHET, H.J., Jr (1956)
Chronic oral toxicity of 2-ethylhexyl phthalate in rats and dogs. Am.
Med. Assoc. Arch. ind. Health, 13: 259-264.
HERRING, R. & BERING, C.L. (1988) Effects of phthalate esters on plant
seedlings and reversal by a soil microorganism. Bull. environ. Contam.
Toxicol., 40: 626-632.
HILL, E.F., HEATH, R.G., SPANN, J.W., & WILLIAMS, J.D. (1975) Lethal
dietary toxicities of environmental pollutants to birds, Washington,
DC, US Fish and Wildlife Service, 61 pp (Special Scientific Report
Wildlife No. 191).
HOBSON, J.F., CARTER, D.E., & LIGHTNER, D.V. (1984) Toxicity of a
phthalate ester in the diet of a penaied shrimp. J. Toxicol. environ.
Health, 13: 959-968.
HODGE, H.C. (1943) Acute toxicity for rats and mice of di-2-ethylhexyl
phthalate with a note upon the mechanism. Proc. Soc. Exp. Biol. Med.,
53: 20-23.
HOLLIFIELD, H.C. (1979) Rapid nephelometric estimate of water
solubility of highly insoluble organic chemicals of environmental
interest. Bull. environ. Contam. Toxicol., 23: 579-586.
HOWARD, P.H., BANERJEE, S., & ROBILLARD, K.H. (1985) Measurement of
water solubilities, octanol/water partition coefficients and vapour
pressures of commercial phthalate esters. Environ. Toxicol. Chem., 4:
653-661.
HRUDEY, S.E., SERGY, G.A., & THACKERAY, T. (1976) Toxicity of oil
sands plant wastewaters and associated organic contaminants. In:
Proceedings of the 11th Canadian Symposium on Water Pollution
Research, Ottawa, Water Pollution Research Canada, pp. 34-45.
IARC (1982) Di(2-ethylhexyl) phthalate. In: Some industrial chemicals
and dyestuffs, Lyon, International Agency for Research on Cancer, pp.
269-294 (IARC Monographs on the Evaluation of the Carcinogenic Risks
of Chemicals to Humans, Vol. 29).
IARC (1987) Overall evaluations of carcinogenicity: An updating of
IARC monographs volumes 1 to 42, Lyon, International Agency for
Research on Cancer, 440 pp (IARC Monographs on the Evaluation of
Carcinogenic Risks to Humans, Supplement 7).
ISHIDA, M., SUYAMA, K., & ADACHI, S. (1980) Background contamination
by phthalates commonly encountered in the chromatographic analysis of
lipid samples. J. Chromatogr., 189: 421-424.
ISHIDA, M., SUYAMA, K., & ADACHI, S. (1981) Occurrence of dibutyl and
di-2-ethylhexyl phthalate in chicken eggs. J. agric. food Chem., 29:
72-74.
ISHIDA, M., SUYAMA, K., ADACHI, S., & HOSHINO, T. (1982) Distribution
of orally administered diethylhexyl phthalate in laying hens. Poult.
Sci., 61: 262-267.
ISSEMANN, I. & GREEN, S. (1990) Activation of a member of the steroid
hormone receptor superfamily by peroxisome proliferators.
Nature(Lond.): 347: 645-650.
JAEGER, R.J. & RUBIN, R.J. (1972) Migration of a phthalate ester
plasticizer from polyvinyl chloride blood bags into stored human blood
and its localization in human tissues. New Engl. J. Med., 287:
1114-1118.
JOHNSON, B.T. & LULVES, W. (1975) Biodegradation of di-butyl phthalate
and di-2-ethylhexyl phthalate in freshwater hydrosoil. J. Fish Res.
Board Can., 32: 333-339.
JOHNSON, B.T., HEITKAMP, M.A., & JONES, J.R. (1984) Environmental and
chemical factors influencing the biodegradation of phthalic acid
esters in freshwater sediments. Environ. Pollut., B8: 101-118.
KAMATA, I., TSUTSUI, G., TAKANA, J., & SHIRAI, T. (1978) [Phthalic
acid esters in fish in the Uji river.] Kyoto-fu Eisei Kogai Kenkyusho
Nempo, 22: 114 (in Japanese).
KARARA, A.H. & HAYTON, W.L. (1989) A pharmacokinetic analysis of the
effect of temperature on the accumulation of di-2-ethylhexyl phthalate
(DEHP) in sheepshead minnow. Aquat. Toxicol., 15: 27-36.
KARASEK, F.W., DENNEY, D.W., CHAN, K.W., & CLEMENT, R.E. (1978)
Analysis of complex organic mixtures on airborne particulate matter.
Anal. Chem., 50: 82-87.
KASAI, H., OKADA, Y., NISHIMURA, S., RAO, M.S., & REDDY, J.K. (1989)
Formation of 8-hydroxydeoxyguanosine in liver DNA of rats following
long-term exposure to a peroxisome proliferator.Cancer Res., 49:
2603-2605.
KATAEVA, S.W. (1988) [The method for determination of dioctyl
phthalates in water and model media imitating foodstuffs.] Gig. i
Sanit., 1: 52-53 (in Russian).
KEYSER, P., PUJAR, G.P., EATON, R.W., & RIBBONS, D.W. (1976)
Biodegradation of the phthalates and their esters by bacteria.
Environ. Health Perspect., 18: 159-166.
KIRBY, P.E., PIZZARELLO, R.F., LAWLOR, T.E., HAWORTH, S.E., & HODGSON,
J.R. (1983) Evaluation of di(2-ethylhexyl) phthalate and its major
metabolites in the Ames test and L5178Y mouse lymphoma mutagenicity
assay. Environ. Mutagen., 5: 657-663.
KLIMISCH, H.-J., HELLWIG, J., KAUFMANN, W., & JÄCKH, R. (1991) Di-(2-
ethylhexyl)phthalate (DEHP): Investigation of inhalation toxicity in
rats after repeated exposure (28 d). Hum. exp. Toxicol., 3: 68.
KLOPFFER, W., KAUFMANN, G., RIPPEN, G., & POREMSKI, H.J. (1982) A
laboratory method for testing the volatility from aqueous solution:
First results and comparison with theory. Ecotoxicol. environ. Saf.,
6: 545.
KLUWE, W., HASEMAN, J.K., DOUGLAS, J.F., & HUFF, J.E. (1982) The
carcinogenicity of dietary di(2-ethylhexyl) phthalate (DEHP) in Fisher
344 rats and B6C3F1 mice. J. Toxicol. environ. Health, 10: 797-815.
KNOWLES, C.O., MCKEE, M.J., & PALAWSKI, D.U. (1987) Chronic effects of
di-2-ethylhexyl phthalate on biochemical composition, survival and
reproduction of Daphnia magna. Environ. Toxicol. Chem., 6: 201-208.
KODAMA, T. & TAKAI, Y. (1974) [Studies on the determination of
phthalic acid esters, IV. Analysis of phthalic acid ester in fish
meat.] Kogai To Taisaku, 10: 850-852 (in Japanese).
KODAMA, T., KOBAYASKI, K., EBA, H., OGAWA, T., TAKAI, Y., NAITO, N.,
OKADA, N., & SAWANO, Y. (1975) [Studies on the determination of
phthalic acid esters: VI. Investigations in Aichi Prefecture.] Kogai
To Taisaku, 11: 341-350 (in Japanese).
KORA, S., SADO, M., KOLKE, H., & TERADA, H. (1989) Protective effect
of the plasticizer di(2-ethylhexyl) phthalate against damage of the
mitochondrial membrane induced by calcium: possible participation of
the adenine nucleotide translocator. Biochim. Biophys. Acta, 985:
286-292.
KORNBRUST, D.J., BARFKNECHT, T.R., INGRAM, P., & SHELBURNE, J.D.
(1984) Effect of di(2-ethylhexyl)phthalate on DNA repair and lipid
peroxidation in rat hepatocytes and on metabolic cooperation in
Chinese hamster V-79 cells. J. Toxicol. environ. Health, 13: 99-116.
KRAUSKOPF, L.G. (1973) Studies on the toxicity of phthalates via
ingestion. Environ. Health Perspect., 3: 61-72.
KURANE, R., SUZUKI, T., & TAKAHARA, Y. (1977) Isolation of
microorganisms growing on phthalate esters and degradation of
phthalate esters by Pseudomonas acidovorans 256-1. Agric. biol.
Chem., 41: 2119-2123.
KURANE, R., SUZUKI, T., & TAKAHARA, Y. (1978) Removal of phthalate
esters in soil columns inoculated with micro-organisms. Agric. biol.
Chem., 42: 1469-1478.
KURANE, R., SUZUKI, T., & TAKAHARA, Y. (1979) Microbial population and
identification of phthalate ester-utilising micro-organisms in
activated sludge with micro-organisms. Agric. biol. Chem., 43:
907-917.
KURANE, R., SUZUKI, T., & TAKAHARA, Y. (1980) Induction of enzymes
involved in phthalate esters metabolism in Nocardia erythropolis and
enzymatic hydrolysis of phthalate esters by commercial lipases. Agric.
biol. Chem., 44: 529-536.
KURANE, R., SUZUKI, T., TAKAHARA, Y., & FUKUOKA, S. (1984)
Protocatechuate 3,4-dioxygenase from Nocardia erythropolis. Agric.
biol. Chem., 48: 2105-2111.
LAKE, B.G., BRANTOM, P.G., GANGOLLI, S.H., BUTTERWORTH, K.R., &
GRASSO, P. (1976) Studies on the effects of orally administered di(2-
ethylhexyl)phthalate in the ferret. Toxicology, 6: 341-356.
LAKE, B.G., GRAY, T.J.B., FOSTER, J.R., STUBBERFIELD, C.R., &
GANGOLLI, S.D. (1984) Comparative studies on di-(2-ethylhexyl)
phthalate-induced hepatic peroxisome proliferation in the rat and
hamster. Toxicol. appl. Pharmacol., 72: 46-60.
LAKE, B.G., GRAY, T.J.B., & GANGOLLI, S.D. (1986) Hepatic effects of
phthalate esters and related compounds - in vivo and in vitro
correlations. Environ. Health Perspect., 67: 283-290.
LAKE, B.G., KOZLEN, S.L., EVANS, J.G., GRAY, T.J.B., YOUNG, P.J., &
GANGOLLI, S.D. (1987) Effect of prolonged administration of clofibric
acid and di-(2-ethylhexyl) phthalate on hepatic enzyme activities and
lipid peroxidation in the rat. Toxicology, 44: 213-228.
LAKE, B.G., LEWIS, D.F.V. & GRAY, T.J.B. (1988) Structure-activity
relationships for hepatic peroxisome proliferation. Arch. Toxicol.,
Suppl. 12: 217-224.
LAKE, B.G., COOK, W.M., WORRELL, N.R., CUNNINGHAME, M.E., EVANS, J.G.,
PRICE, R.J., YOUNG, P.J., & CARPANINI, F.M.B. (1991) Dose-response
relationships for induction of hepatic peroxisome proliferation and
testicular atrophy by phthalate esters in the rat. Hum. exp. Toxicol.,
10(1): 67.
LALWANI, N.D., ALVARES, K., REDDY, M.K., REDDY, M.N., PARIKH, I., &
REDDY, J.K. (1987) Peroxisome proliferator-binding protein:
Identification and partial characterization of nafenopin-, clofibric
acid-, and clofibrate-binding proteins from rat liver. Proc. Natl
Acad. Sci. (USA), 84: 5242-5246.
LARSSON, P. & THUREN, A. (1987) Di-2-ethylhexylphthalate inhibits the
hatching of frog eggs and is bioaccumulated by tadpoles. Environ.
Toxicol. Chem., 6: 417-422.
LARSSON, P., THUREN, A., & GAHNSTROM, G. (1986) Phthalate esters
inhibit microbial activity in aquatic sediments. Environ. Pollut., 42:
223-231.
LAUGHLIN, R.B., NEFF, J.M., HRUNG, Y.C., GOODWIN, T.C., & GIAM, C.S.
(1978) The effects of three phthalate esters on the larval development
of the grass shrimp Palaemonetes pugio (Holthuis). Water Air Soil
Pollut., 9: 323-336.
LAWRENCE, W.H., MALIK, M., TURNER, J.E., SINGH, A.R., & AUTIAN, J.
(1975) A toxicological investigation of some acute, short-term, and
chronic effects of administering di-2-ethylhexyl phthalate (DEHP) and
other phthalate esters. Environ. Res., 9: 1-11.
LEBLANC, G.A. (1980) Acute toxicity of priority pollutants to water
flea (Daphnia magna). Bull. environ. Contam. Toxicol., 24: 684-691.
LEWANDOWSKI, M., FERNANDES, J., & CHEN, T.S. (1980) Assessment of the
teratogenic potential of plasma-soluble extracts of diethylhexyl
phthalate plasticized polyvinyl chloride plastics in rats. Toxicol.
appl. Pharmacol., 54: 141-147.
LEWIS, L.M., FLECHTNER, T.W., KERKAY, J., PEARSON, K.H., CHEN, W.T.,
POPOWNIAK, K.L., & NAKAMOTO, S. (1977) Determination of plasticizer
levels in serum of hemodialysis patients. Trans. Am. Soc. Artif.
Intern. Organs, 23: 566-572.
LEYDER, F. & BOULANGER, P. (1983) Ultraviolet absorption, aqueous
solubility, and octanol-water partition for several phthalates. Bull.
environ. Contam. Toxicol., 30: 152-157.
LHUGUENOT, J.C. & ELCOMBE, C.R. (1984) Variation selon l'espèce des
phénomènes de conjugasion lors du métabolisme mono-(éthyl-2-hexyl)
phthalate (MEHP). Sci. Aliments, 4: 227-232.
LHUGUENOT, J.C., MITCHELL, A.M., MILNER, G., LOCK, E.A., & ELCOMBE,
C.R. (1985) The metabolism of di(2-ethylhexyl)phthalate (DEHP) and
mono-(2-ethylhexyl) phthalate (MEHP) in rats: In vivo and in vitro
dose and time dependency of metabolism. Toxicol. appl. Pharmacol., 80:
11-22.
LINDEN, E., BENGTSSON, B.-E., SVANBERG, O., & SUNDSTROM, G. (1979) The
acute toxicity of 78 chemicals and pesticide formulations against two
brackish water organisms, the bleak (Alburnus alburnus) and the
harpacticoid (Nitocra spinipes). Chemosphere, 8: 843-851.
LISS, G.M., ALBRO, P.W., HARTLE, R.W., & STRINGER, W.T. (1985) Urine
phthalate determinations as an index of occupational exposure to
phthalic anhydride and di(2-ethylhexyl) phthalate. Scand. J. Work
Environ. Health, 11: 381-387.
LLOYD, S.C. & FOSTER, P.M.D. (1988) Effect of mono-(2-ethylhexyl)
phthalate on follicle-stimulating hormone responsiveness of cultured
rat Sertoli cells. Toxicol. appl. Pharmacol., 95: 484-489.
LOCK, E.A., MITCHELL, A.M.R., & ELCOMBE, C.R. (1989) Biochemical
mechanisms of induction of hepatic peroxisome proliferation. Annu.
Rev. Pharmacol. Toxicol., 29: 145-168.
LOKKE, H. & BRO-RASMUSSEN, F. (1981) Studies of mobility of di-iso-
butyl phthalate (DiBP), di-n-butyl phthalate (DBP), and di-(2-
ethylhexyl) phthalate (DEHP) by plant foliage treatment in a closed
terrestrial simulation chamber. Chemosphere, 10: 1223-1235.
LOKKE, H. & RASMUSSEN, L. (1983) Phytotoxicological effects of di-(2-
ethylhexyl)-phthalate and n-butyl-phthalate on higher plants in
laboratory and field experiments. Environ. Pollut., 32: 179-199.
LUTZ, W.K. (1986) Investigation of the potential for binding of di(2-
ethylhexyl) phthalate (DEHP) to rat liver DNA in vivo. Environ.
Health Perspect., 65: 267-269.
MACEK, K.J., PETROCELLI, S.R., & SLEIGHT, B.H., III (1979)
Considerations in assessing the potential for, and significance of,
biomagnification of chemical residues in aquatic food chains. In:
Aquatic toxicology, Philadelphia, American Society for Testing and
Materials, pp. 251-268 (ASTM STP 667).
MAFF (1987) Survey of plasticiser levels in food contact materials and
in foods, London, Ministry of Agriculture, Fisheries and Food (Food
Surveillance Paper No. 21).
MALCOLM, A.R. & MILLS, L.J. (1989) Inhibition of gap-junctional
intercellular communication between Chinese hamster lung fibroblasts
by di(2-ethylhexyl) phthalate (DEHP) and trisodium nitrilotriacetate
monohydrate (NTA). Cell Biol. Toxicol., 5: 145-153.
MANANDHAR, M.D., SHOEB, A., KAPIL, R.S., & POPLI, S.P. (1979) Studies
in medicinal plants. Part V, Di-2-n-propyl pentyl phthalate and
reticulene from Cryptocaryo amyggdalina Nees. Indian J. Chem., 178:
411-412.
MARCEL, Y.L. & NOEL, S.P. (1970) Contamination of blood stored in
plastic packs. Lancet, 1: 35-36.
MARSMAN, D.S., CATTLEY, R.C., CONWAY, J.G., & POPP, J.A. (1988)
Relationships of hepatic peroxisome proliferation and replicative DNA
synthesis to the hepatocarcinogenicity of the peroxisome proliferators
di(2-ethylhexyl) phthalate and [4-chloro-6-(2,3-xylidino)-2-
pyrimidinylthio]acetic acid (Wy-14,643) in rats. Cancer Res., 48:
6739-6744.
MATHUR, S.P. (1974a) Phthalate esters in the environment: Pollutants
or natural products. J. environ. Qual., 3: 189.
MATHUR, S.P. (1974b) Respirometric evidence of the utilization in soil
of di-octyl and di-2-ethylhexyl phthalate plasticizers. J. environ.
Qual., 3: 207-209.
MATSUDA, K. & SCHNITZER, M. (1971) Reactions between fulvic acid, a
soil humic material, and dialkyl phthalates. Bull. environ. Contam.
Toxicol., 6: 200-204.
MAYER, F.L. (1976) Residue dynamics of di-2-ethylhexyl phthalate in
fathead minnows (Pimephales promelas). J. Fish Res. Board Can., 33:
2610-2613.
MAYER, F.L. & SANDERS, H.O. (1973) Toxicology of phthalic acid esters
in aquatic organisms. Environ. Health Perspect., 3: 153-157.
MAYER, F.L., STALLING, D.L., & JOHNSON, J.L. (1972) Phthalate esters
as environmental contaminants. Nature (Lond.), 238: 411-413.
MAYER, F.L., MEHRLE, P.M., & SCHOETTGER, R.A. (1977) Collagen
metabolism in fish exposed to organic chemicals. In: Symposium on
Recent Advances in Fish Toxicology, Washington, DC, US Environmental
Protection Agency, p. 31 (EPA 600/3-77-085).
MEHRLE, P.M. & MAYER, F.L. (1976) Di-2-ethylhexyl phthalate: Residue
dynamics and biological effects in rainbow trout and fathead minnow.
Trace Subst. environ. Health, 10: 519-524.
MELANCON, M.J. (1979) Metabolism of phthalate esters in aquatic
species. In: Pesticides and xenobiotic metabolism in aquatic
organisms, Washington, DC, American Chemical Society, pp. 77-94 (ACS
Symposium Series No. 99).
MELNICK, R.L., MORRISSEY, R.E., & TOMASZEWSKI, K.E. (1987) Studies by
the National Toxicology Program on di(2-ethylhexyl) phthalate.
Toxicol. ind. Health, 3: 99-118.
MES, J., COFFIN, D.E., & CAMPBELL, D.S. (1974) Di-n-butyl and di-2-
ethylhexyl phthalate in human adipose tissue. Bull. environ. Contam.
Toxicol., 12: 721-725.
METCALF, R.L., BOOTH, G.M., SCHUTH, C.K., HANSEN, D.J., & LU, P.-Y.
(1973) Uptake and fate of di-2-ethylhexyl phthalate in aquatic
organisms and in a model ecosystem. Environ. Health Perspect., 4:
27-34.
MIKALSEN, S.O., HOLEN, I., & SANNER, T. (1990a) Morphological
transformation and catalase activity of Syrian hamster embryo cells
treated with hepatic peroxisome proliferators, TPA and nickel
sulphate. Cell Biol. Toxicol., 6: 1-13.
MIKALSEN, S.O., KAALHUS, O., REITH, A., & SANNER, T. (1990b) Role of
catalase and oxidative stress in hepatic peroxisome proliferator-
induced morphological transformation of Syrian hamster embryo cell.
Int. J. Cancer, 46: 950-957.
MIKALSEN, S.O., REUTER, B., & SANNER, T. (1990c) Effects of hepatic
peroxisome proliferators and 12-O-tetradecanoyl phorbol-13-acetate on
catalase and other enzyme activities of embryonic cells in vitro.
Biochem. Pharmacol., 39: 527-535.
MILKOV, L.E., ALDYREVA, M.V., POPOVA, T.B., LOPUKHOVA, K.A.,
MAKARENKO, Y.L., MALYAR, L.M., & SHAKHOVA, T.K. (1973) Health status
of workers exposed to phthalate plasticizers in the manufacture of
artificial leather and films based on PVC resins. Environ. Health
Perspect., 3: 175-178.
MITCHELL, A.M., LHUGUENOT, J.-C., BRIDGES, J.W., & ELCOMBE, C.R.
(1985a) Identification of the proximate peroxisome proliferator(s)
derived from di(2-ethylhexyl) phthalate. Toxicol. appl. Pharmacol.,
80: 23-32.
MITCHELL, F.E., PRICE, S.C., HINTON, R.H., GRASSO, P., & BRIDGES, J.W.
(1985b) Time and dose-response study of the effects on rats of the
plasticizer di(2-ethylhexyl) phthalate. Toxicol. appl. Pharmacol., 81:
371-392.
MORI, S. (1976) Identification and determination of phthalate esters
in river water by high-performance liquid chromatography. J.
Chromatogr., 129: 53-60.
MUSIAL, C.J. & UTHE, J.F. (1980) Studies on phthalate esters in
Western Atlantic finfish. Report to International Council for the
Exploration of the Sea (ICES), Copenhagen, International Council for
the Exploration of the Sea, pp. 1-6 (Report E:11).
MUTZ, R.C. & JONES, J.R. (1977) The effects of phthalate esters on
geochemical cycles in freshwater hydrosoil. Trans. Missouri Acad.
Sci., 10-11: 296.
AZIR, D.J., ALCARAZ, A.P., BIERL, B.A., BEROZA, M., & NAIR, P.P.
(1971) Isolation, identification, and specific localization of di-2-
ethylhexyl phthalate in bovine heart muscle mitochondria.
Biochemistry, 10: 4228-4232.
NEERGAARD, J., NIELSEN, B., FAURBY, V., CHRISTENSEN, D.H., & NIELSEN,
O.F. (1971) Plasticizers in P.V.C. and the occurrence of hepatitis in
a haemodialysis unit. Scand. J. Urol. Nephrol., 5: 141-145.
NEUHAUSER, E.F., LOER, R.C., & MALECKI, M.R. (1986) Contact and
artificial soil tests using earthworms to evaluate the impact of
wastes in soil. In: Petros, J.K., Jr, Lacy, W.J., & Conway, R.A., ed.
Hazardous and industrial solid waste testing: Fourth symposium,
Philadelphia, American Society for Testing and Materials, pp. 192-203
(ASTM STP 866).
NIELSEN, J., ÅKESSON, B., & SKERFVING, S. (1985) Phthalate ester
exposure - air levels and health of workers processing
polyvinylchloride. Am. Ind. Hyg. Assoc. J., 46: 643-647.
NIKONOROW, M., MAZUR, H., & PIEKACZ, H. (1973) Effect of orally
administered plasticizers and polyvinyl chloride stabilizers in the
rat. Toxicol. appl. Pharmacol., 26: 253-259.
NIOSH (1977) Manual of analytical methods, 2nd ed., Cincinnati, Ohio,
National Institute for Occupational Safety and Health, Vol. 2, pp.
S40/1-S40/9.
NIOSH (1985a) Manual of analytical methods, 3rd ed., Cincinnati, Ohio,
National Institute for Occupational Safety and Health, Vol. 1, pp.
5020/1-5020/4.
NIOSH (1985b) Registry of toxic effects of chemical substances:
1983-84 Supplement, Cincinnati, Ohio, National Institute for
Occupational Safety and Health, Vol. II, p. 1442.
NTP (1982) Technical report on the carcinogenicity bioassay of di(2-
ethylhexyl) phthalate, Research Triangle Park, NC, National Toxicology
Programme (DHHS Publ. No. (NIH) 82-1773).
O'CONNOR, O.A., RIVERA, M.D., & YOUNG, L.Y. (1989) Toxicity and
biodegradation of phthalic acid esters under methanogenic conditions.
Environ. Toxicol. Chem., 8: 569-576.
OHYAMA, T. (1977) Effects of phthalate esters on glucose-6-phosphate
dehydrogenase and other enzymes in vitro. Toxicol. appl. Pharmacol.,
40: 355-364.
OISHI, S. (1984) Testicular atrophy of rats induced by di-2-ethylhexyl
phthalate: effects of vitamin A and zinc concentrations in the testis,
liver and serum. Toxicol. Lett., 20: 75-78.
OISHI, S. (1985) Reversibility of testicular atrophy induced by di(2-
ethylhexyl) phthalate in rats. Environ. Res., 36: 160-169.
OISHI, S. (1989a) Effects of co-administration of di(2-ethylhexyl)
phthalate and testosterone on several parameters in the testis and
pharmacokinetics of its mono-deesterified metabolite. Arch. Toxicol.,
63: 289-295.
OISHI, S. (1989b) Enhancing effects of luteinizing hormone-releasing
hormone on testicular damage induced by di(2-ethylhexyl) phthalate in
rats. Toxicol. Lett., 47: 271-277.
OISHI, S. (1990) Effects of phthalic acid esters on testicular
mitochondrial functions in the rat. Appl. Toxicol., 64: 143-147.
OISHI, S. & HIRAGA, K. (1979) Effect of phthalic acid esters on
gonadal function in male rats. Bull. environ. Contam. Toxicol., 21:
65-67.
OISHI, S. & HIRAGA, K. (1980a) Testicular atrophy induced by phthalic
esters: effect on testosterone and zinc concentrations. Toxicol. appl.
Pharmacol., 53: 35-41.
OISHI, S. & HIRAGA, K. (1980b) Effect of phthalic acid monoesters on
mouse testes. Toxicol. Lett., 6: 239-242.
OISHI, S. & HIRAGA, K. (1982) Effects of monoesters of o-phthalic acid
on serum lipid composition in rats. Toxicol. Lett., 14: 79-84.
OISHI, S. & HIRAGA, K. (1983) Testicular atrophy induced by di-2-
ethylhexyl phthalate: effect of zinc supplement. Toxicol. appl.
Pharmacol., 70: 43-48.
O'SHEA, T.J. & STAFFORD, C.J. (1980) Phthalate plasticizers:
Accumulation and effects on weight and food consumption in captive
starlings. Bull. environ. Contam. Toxicol., 25: 345-352.
OSUMI, T. & HASHIMOTO, T. (1978) Enhancement of fatty acyl-CoA
oxidizing activity in rat liver peroxisomes by di-(2-
ethylhexyl)phthalate. J. Biochem., 83: 1361-1365.
PARE, J.R.J., BELANGER, J., & JANKOWSKI, K. (1981) Asymmetric
phthalate from Sansevieria trifasciata. J. nat. Prod., 44: 490-492.
PASSINO, D.R.M. & SMITH, S.B. (1987) Acute bioassays and hazard
evaluation of representative contaminants detected in Great Lakes
fish. Environ. Toxicol. Chem., 6: 901-907.
PEAKALL, D.B. (1974) Effects of di-n-butyl and di-2-ethylhexyl
phthalate on the eggs of ring doves. Bull. environ. Contam. Toxicol.,
12: 698-702.
PEAKALL, D.B. (1975) Phthalate esters: Occurrence and biological
effects. Res. Rev., 54: 1-40.
PERERA, M.I.R., KATYAL, S.L., & SHINOZUKA, H. (1986) Suppression of
choline-deficient diet-induced hepatocyte membrane lipid peroxidation
in rats by the peroxisome proliferators 4-chloro-6-(2,3-xylidino)-2-
pyrimidinylthio(N-ß-hydroxyethyl)acetamide and di(2-
ethylhexyl)phthalate. Cancer Res., 46: 3304-3308.
PERKINS, E.G. (1967) Characterization of the nonvolatile compounds
formed during the thermal oxidation of corn oil. II. Phthalate esters.
J. Am. Oil Chem. Soc., 44: 197-199.
PERSSON, P.-E., PENTTINEN, H., & NUORTEVA, P. (1978) DEHP in the
vicinity of an industrial area in Finland. Environ. Pollut., 16:
163-166.
PETERSON, J.C. & FREEMAN, D.H. (1984) Variations of phthalate ester
concentrations in sediments from the Chester river, Maryland. Int. J.
environ. anal. Chem., 18: 237-252.
PFUDERER, P. & FRANCIS, A.A. (1975) Phthalate esters: Heartrate
depressors in the goldfish. Bull. environ. Contam. Toxicol., 13:
275-279.
PHILLIPS, B.J., ANDERSON, D., & GANGOLLI, S.D. (1986) Studies on the
genetic effects of phthalic acid esters on cells in culture. Environ.
Health Perspect., 65: 263-266.
PIECHOCKI, J.T. & PURDY, W.C. (1973) Determination of di-(2-
ethylhexyl)-phthalate (DEHP) in human plasma. Clin. Chim. Acta, 48:
385-391.
POLLACK, G.M., BUCHANAN, J.F., SLAUGHTER, R.L., KOHLI, R.K., & SHEN,
D.D. (1985a) Circulating concentrations of di(2-ethylhexyl) phthalate
and its de-esterified phthalic acid products following plasticizer
exposure in patients receiving hemodialysis. Toxicol. appl.
Pharmacol., 79: 257-267.
POLLACK, G.M., LI, R.C.K., ERMER, J.C., & SHEN, D.D. (1985b) Effects
of route of administration and repetitive dosing on the disposition
kinetics of di(2-ethylhexyl) phthalate and its mono-de-esterified
metabolite in rats. Toxicol. appl. Pharmacol., 79: 246-256.
POLLACK, G.M., SHEN, D.D., & DORR, M.B. (1989) Contribution of
metabolites to the route- and time-dependent hepatic effects of di-(2-
ethylhexyl)phthalate in the rat. J. Pharmacol. exp. Ther., 248:
176-181.
POPP, J.A., GARVEY, L.K., & CATTLEY, R.C. (1987) In vivo studies on
the mechanism of di(2-ethylhexyl) phthalate carcinogenesis. Toxicol.
ind. Health, 3: 151-163.
PRESTON, M.R. & AL-OMRAN, L.A. (1986) Dissolved and particulate
phthalate esters in the river Mersey estuary. Mar. Pollut. Bull., 17:
548-553.
PRESTON, M.R. & AL-OMRAN, L.A. (1989) Phthalate ester speciation in
estuarine water, suspended particulates and sediments. Environ.
Pollut., 62: 183-193.
PUTMAN, D.L., MOORE, W.A., SCHECHTMAN, L.M., & HODGSON, J.R. (1982)
Cytogenetic evaluation of di-(2-ethylhexyl)phthalate and its major
metabolites in Fischer 344 rats. Environ. Mutagen., 4: 387.
RAO, M.S. & REDDY, J.K. (1987) Peroxisome proliferation and
hepatocellular hepatocarcinogenesis. Carcinogenesis, 8: 631-636.
RAO, M.S., YELDANDI, A.V.P., & SUBBARAO, V. (1990) Quantitative
analysis of hepatocellular lesions induced by di(2-
ethylhexyl)phthalate in F344 rats. J. Toxicol. environ. Health, 30:
85-89.
RAO, P.S.C., HORNSBY, A.G., & JESSUP, R.E. (1985) Indices for ranking
the potential for pesticide contamination of groundwater. Soil Crop
Sci. Soc. Proc., 44: 1-8.
RAY, L.E., MURRAY, H.E., & GIAM, C.S. (1983a) Organic pollutants in
marine samples from Portland, Maine. Chemosphere, 12: 1031-1038.
RAY, L.E., MURRAY, H.E., & GIAM, C.S. (1983b) Analysis of water and
sediment from the Nueces estuary/Corpus Christi bay (Texas) for
selected organic pollutants. Chemosphere, 12: 1039-1045.
REDDY, J.K. & LALWANI, N.D. (1983) Carcinogenesis by hepatic
peroxisome proliferators: evaluation of the risk of hypolipidemic
drugs and industrial plasticizers to humans. CRC crit. Rev. Toxicol.,
12: 1-58.
REDDY, J.K. & RAO, M.S. (1989) Oxidative DNA damage caused by
persistent peroxisome proliferation: its role in hepatocarcinogenesis.
Mutat. Res., 214: 63-68.
REDDY, J.K., REDDY, M.K., USMAN, M.I., LALWANI, N.D., & RAO, M.S.
(1986) Comparison of hepatic peroxisome proliferative effect and its
implication for hepatocarcinogenicity of phthalate esters, di(2-
ethylhexyl)phthalate, and di(2-ethylhexyl) adipate with a
hypolipidemic drug. Environ. Health Perspect., 65: 317-327.
RHODES, C., ORTON, T.C., PRATT, I.S., BATTEN, P.L., BRATT, H.,
JACKSON, S.J., & ELCOMBE, C.R. (1986) Comparative pharmacokinetics and
subacute toxicity of di(2-ethylhexyl)phthalate (DEHP) in rats and
marmosets: extrapolation of effects in rodents to man. Environ. Health
Perspect., 65: 299-308.
RITSEMA, R., COFINO, W.P., FRINTROP, P.C.M., & BRINKMAN, V.A.T. (1989)
Trace-level analysis of phthalate esters in surface water and
suspended particulate matter by means of capillary gas chromatography
with electron-capture and mass-selective detection. Chemosphere, 18:
2161-2175.
ROTH, B., HERKENRATH, P., LEHMANN, H.J., OHLES, H.D., HÖMIG, H.J.,
BENZ-BOHM, G., KREUDER, J., & YOUNOSSI-HARTENSTEIN, A. (1988) Di-(2-
ethylhexyl)phthalate as plasticizer in PVC respiratory tubing systems:
indications of hazardous effects on pulmonary function in mechanically
ventilated, preterm infants. Eur. J. Pediatr., 147: 41-46.
RUBIN, R.J. & CHANG, J.F.C. (1978) Effect of the intravenous
administration of the solubilized plasticizer di(2-ethylhexyl)
phthalate on the lung and on survival of transfused rats. Toxicol.
appl. Pharmacol., 45: 230.
RUBIN, R.J. & JAEGER, R.J. (1973) Some pharmacologic and toxicologic
effects of di-2-ethylhexyl phthalate (DEHP) and other plasticizers.
Environ. Health Perspect., 3: 53-59.
RUBIN, R.J. & NAIR, P.P. (1972) Plasticizers in human tissues. New
Engl. J. Med., 288: 915-916.
RUDDICK, J.A., VILLENEUVE, D.C., CHU, I., NESTMANN, E., & MILES, D.
(1981) An assessment of the teratogenicity in the rat and mutagenicity
in Salmonella of mono-2-ethylhexyl phthalate. Bull. environ. Contam.
Toxicol., 27: 181-186.
RUSHBROOK, C.L., JORGENSON, T.A., & HODGSON, J.R. (1982) Dominant
lethal study of di-(2-ethylhexyl)phthalate and its major metabolites
in ICR/SIM mice. Environ. Mutagen., 4: 387.
SAEGER, V.W. & TUCKER, E.S. (1973) Biodegradation of phthalate esters.
In: Flexible vinyls and human safety: An objective analysis. Presented
at the Regional Technical Conference of the Society of Plastics
Engineers, Palisades Section, Kiamesha Lake, New York, 20-22 March,
1973, pp. 105-133.
SAEGER, V.W. & TUCKER, E.S. (1976) Biodegradation of phthalic acid
esters in river water and activated sludge. Appl. environ. Microbiol.,
31: 29-34.
SAKURAI, T., MIYAZAWA, S., & HASHIMOTO, T. (1978) Effect of di(2-
ethylhexyl) phthalate administration on carbohydrate and fatty acid
metabolism in rat liver. J. Biochem., 83: 313-320.
SANDERS, H.O., MAYER, F.L., & WALSH, D.F. (1973) Toxicity, residue
dynamics, and reproductive effects of phthalate esters in aquatic
invertebrates. Environ. Res., 6: 84-90.
SAX, N.I. (1984) Dangerous properties of industrial materials, 6th
ed., New York, Van Nostrand Reinhold Company.
SAXENA, D.K., SRIVASTAVA, S.P., CHANDRA, S.V., & SETH, P.K. (1985)
Testicular effects of di(2-ethylhexyl) phthalate (DEHP): histochemical
and histopathological alterations. Ind. Health, 23: 191-198.
SCHMEZER, P., POOL, B.L., KLEIN, R.G., KOMITOWSKI, D., & SCHMÄHL, D.,
(1988) Various short-term assays and two long-term studies with the
plasticizer di(2-ethylhexyl) phthalate in the Syrian hamster.
Carcinogenesis, 9: 37-43.
SCHMID, P. & SCHLATTER, C. (1985) Excretion and metabolism of di(2-
ethyl-hexyl) phthalate in man. Xenobiotica, 15: 251-256.
SCHMIDT, J.G., GARVIN, P.J., & LEESTMA, J.E. (1975) Effect of vehicle
on the response to intravenous di-(2-ethylhexyl) phthalate (DEHP) in
rats. Toxicol. appl. Pharmacol., 33: 169.
SCHMITZER, J.L., SCHEUNERT, I., & KORTE, F. (1988) Fate of bis(2-
ethylhexyl) [14C] phthalate in laboratory and outdoor soil-plant
systems. J. agric. food Chem., 36: 210-215.
SCHNEIDER, B., SCHENA, J., TROUG, R., JACOBSON, M., & KEVY, S. (1989)
Exposure to di(2-ethylhexyl)phthalate in infants receiving
extracorporeal membrane oxygenation. New Engl. J. Med., 320: 1563.
SCHULTE-HERMANN, R., KRAUPP-GRASL, B., BURSCH, W., GERBRACHT, U., &
TIMMERMANN-TROSIENER, I. (1989) Effects of non-genotoxic hepato-
carcinogens phenobarbital and nafenopin on phenotype and growth of
different populations of altered foci in rat liver. Toxicol. Pathol.,
17: 642-650.
SCHULZ, C.O. (1989) Assessing human health risks from exposure to
di(2-ethylhexyl)phthalate (DEHP) and related phthalates: scientific
issues. Drug Metab. Rev., 21: 111-120.
SCHULZ, C.O. & RUBIN, R.J. (1973) Distribution, metabolism, and
excretion of di-2-ethylhexyl phthalate in the rat. Environ. Health
Perspect., 3: 123-129.
SCHULZ, C.O., RUBIN, R.J., & HUTCHINS, G.M. (1975) Acute lung toxicity
and sudden death in rats following the intravenous administration of
the plasticizer, di(2-ethylhexyl)-phthalate, solubilized with Tween
surfactants. Toxicol. appl. Pharmacol., 33: 514-525.
SCHWARTZ, H.E., ANZION, C.J.M., VAN VLIET, H.P.M., COPIUS PEEREBOOMS,
J.W., & BRINMAN, U.A.T. (1979) Analysis of phthalate esters in
sediments from Dutch rivers by means of high performance liquid
chromatography. Intern. J. environ. anal. Chem., 6: 133-144.
SETH, P.K., SRIVASTAVA, S.P., AGARWAL, D.K., & CHANDRA, S.V. (1976)
Effect of di-2-ethylhexyl phthalate (DEHP) on rat gonads. Environ.
Res., 12: 131-138.
SHAFFER, C.B., CARPENTER, C.P., & SMYTH, H.F., Jr (1945) Acute and
subacute toxicity of di(2-ethylhexyl) phthalate with note upon its
metabolism. J. ind. Hyg. Toxicol., 27: 130-135.
SHANKER, R., RAMAKRISHNA, C., & SETH, P.K. (1985) Degradation of some
phthalic acid esters in soil. Environ. Pollut., 39: 1-7.
SHELDON, L.S. & HITES, R.A. (1979) Sources and movement of organic
chemicals in the Delaware river. Environ. Sci. Technol., 13: 574-579.
SHINDO, Y., OSUMI, T., & HASHIMOTO, T. (1978) Effects of
administration of di(2-ethylhexyl) phthalate on rat liver
mitochondria. Biochem. Pharmacol., 27: 2683-2688.
SHIOTA, K. & MIMA, S. (1985) Assessment of the teratogenicity of di(2-
ethylhexyl) phthalate and mono(2-ethylhexyl) phthalate in mice. Arch.
Toxicol., 56: 263-266.
SHIOTA, K. & NISHIMURA, H. (1982) Teratogenicity of di(2-ethylhexyl)
phthalate (DEHP) and di-n-butyl phthalate (DBP) in mice. Environ.
Health Perspect., 45: 65-70. SHIOTA, K., CHOU, M.J., & NISHIMURA, H.
(1980) Embryotoxic effects of di-2-ethylhexyl phthalate (DEHP) and di-
n-butyl phthalate (DBP) in mice. Environ. Res., 22: 245-253.
SHORT, R.D., ROBINSON, E.C., LINGTON, A.W., & CHIN, A.E. (1987)
Metabolic and peroxisome proliferation studies with di(2-ethylhexyl)
phthalate in rats and monkeys. Toxicol. ind. Health, 3: 185-195.
SHUKLA, R.R., ALBRO, P.W., CORBETT, J.T., & SCHROEDER, J.L. (1989) In
vivo studies of the inhibition of protein kinase C from rat brain by
di-(2-ethylhexyl) phthalate. Chem.-biol. Interact., 69: 73-85.
SILVO, O.E.J. (1974) [Preliminary studies on the acute toxicity of
dioctylphthalate (DOP) to rainbow trout ( Salmo gairdneri Richardson)
and its effect upon phytoplankton and oxygen content of the water.]
Suomen Kalatalous, 47: 19-25 (in Finnish).
SINGH, A.R., LAWRENCE, W.H., & AUTIAN, J. (1972) Teratogenicity of
phthalate esters in rats. J. pharm. Sci., 61: 51-55.
SITTIG, M. (1985) Handbook of toxic and hazardous chemicals and
carcinogens, 2nd ed., Park Ridge, New Jersey, Noyes Publications.
SJÖBERG, P., PLÖEN, L., BONDESSON, U., & LINDQUIST, N.G. (1982)
Testicular toxicity of di-(2-ethylhexyl) phthalate in rats of
different ages. Teratology, 26: 20A.
SJÖBERG, P., BONDESSON, U., KJELLEN, L., LINDQUIST, N.-G., MONTIN, G.,
& PLÖEN, L. (1985a) Kinetics of di-(2-ethylhexyl) phthalate in
immature and mature rats and effect on testis. Acta pharmacol.
toxicol., 56: 30-37.
SJÖBERG, P., BONDESSON, U., SEDIN, G., & GUSTAFSSON, J. (1985b)
Dispositions of di- and mono-(2-ethylhexyl) phthalate in newborn
infants subjected to exchange transfusions. Eur. J. clin. Invest., 15:
430-436.
SJÖBERG, P., LINDQUIST, N.G., MONTIN, G., & PLÖEN, L. (1985c) Effect
of repeated intravenous infusions of the plasticizer di-(2-ethylhexyl)
phthalate in young male rats. Arch. Toxicol., 58: 78-83.
SJÖBERG, P., LINDQUIST, N.G., & PLÖEN, L. (1986) Age-dependent
response of the rat testes to di(2-ethylhexyl) phthalate. Environ.
Health Perspect., 65: 237-242.
SMISTAD, G., WAALER, T., & ROKSVAAG, P.O. (1989) Migration of plastic
additives from soft polyvinyl chloride bags into normal saline and
glucose infusions. Acta pharm. Nord., 1: 287-290.
SMITH-OLIVER, T. & BUTTERWORTH, B.E. (1987) Correlation of the
carcinogenic potential of di(2-ethylhexyl)phthalate (DEHP) with
induced hyperplasia rather than with genotoxic activity. Mutat. Res.,
188: 21-28.
SODERGREN, A. (1982) Significance of interfaces in the distribution
and metabolism of di-2-ethylhexyl phthalate in an aquatic laboratory
model ecosystem. Environ. Pollut., 27: 263-274.
SRI (1985) Chemical economics handbook, Menlo Park, California, SRI
International.
SRIVASTAVA, S., AWASTHI, V.K., SRIVASTAVA, S.P., & SETH, P.K. (1989)
Biochemical alterations in rat fetal liver following in utero exposure
to di(2-ethylhexyl)phthalate (DEHP). Indian J. exp. Biol., 27:
885-888.
STANKEVICH, V.V. & ZAREMBO, O.K. (1978) [Toxicological and hygienic
characteristics of PVC materials plasticizers.] In: [Modern problems
of hygiene of PVC application], Moscow, VNIIMI, pp. 10-16 (Series
Gigiena No. 4) (in Russian).
STANKEVICH, V.V., ZAREMBO, O.K., GUMENNOY, V.S., & LEUITSKAYA, V.N.
(1984) [Present state of the regulation program relating to vinyl
chloride monomers and other additives used as polyvinyl chloride
packing material (A review)], Moscow, VNIIMI, pp. 26-36 (Series
Gigiena) (in Russian).
STANLEY, R.D. & TAPP, J.F. (1982) An assessment of ecological test
methods. VIII. The effect of nine chemicals on the growth of Avena
saliva and Brassica rapa, United Kingdom, ICI Brixham Laboratory
(Internal Report No. BL/A/2164).
STEPHENSON, R.R. (1983) Effects of water hardness, water temperature,
and size of the test organism on the susceptibility of the freshwater
shrimp, Gammarus pulex (L.), to toxicants. Bull. environ. Contam.
Toxicol., 31: 459-466.
STREUFERT, J.M., JONES, J.R., & SANDERS, H.O. (1980) Toxicity and
biological effects of phthalate esters on midges (Chironomus
plumosus). Trans. Missouri Acad. Sci., 14: 33-40.
SUGATT, R.H., O'GRADY, D.P., BANERJEE, S., HOWARD, P.H., & GLEDMILL,
W.E. (1984) Shake flask biodegradation of 14 commercial phthalate
esters. Appl. environ. Microbiol., 47: 601-606.
SWENERTON, H. & HURLEY, L.S. (1971) Teratogenic effects of a chelating
agent and their prevention by zinc. Science, 173: 62-63.
TABORSKY, R.G. (1967) Isolation studies on a lipoidal portion of the
bovine pineal gland. J. agric. food Chem., 15: 1073-1076.
TAKAGI, A., SAI, K., UMEMURA, T., KUROKAWA, Y., & KASAI, H. (1989)
Production of 8-hydroxydeoxyguanosine in rat liver DNA by oral
administration of peroxisome proliferators. Mutat. Res., 216: 379.
TAKAGI, A., SAI, K., UMEMURA, T., HASEGAWA, R., & KUROKAWA, Y. (1990)
Significant increase of 8-hydroxydeoxyguanosine in liver DNA of rats
following short-term exposure to the peroxisome proliferators di(2-
ethylhexyl)phthalate and di(2-ethylhexyl)adipate Jpn. J. Cancer Res.,
81: 213-215.
TAKAGI, A., SAI, K., UMEMURA, T., HASEGAWA, R., & KUROKAWA, Y. (1991)
Short-term exposure to the peroxisome proliferators, perfluorooctanoic
acid and perfluorodecanoic acid, causes significant increase of 8-
hydroxydeoxyguanosine in liver DNA of rats. Cancer Lett., 57: 55-60.
TAMURA, H., IIDA, T., WATANABE, T., & SUGA, T., (1990) Long-term
effects of hypolipidemic peroxisome proliferator administration on
hepatic hydrogen peroxide metabolism in rats. Carcinogenesis, 11:
445-450.
TARR, B.D., BARRON, M.G., & HAYTON, W.L. (1990) Effect of body size on
the uptake and bioconcentration of di-2-ethylhexyl phthalate in
rainbow trout. Environ. Toxicol. Chem., 9: 989-995.
TAYLOR, B.F., CURRY, R.W., & CORCORAN, E.F. (1981) Potential for
biodegradation of phthalic acid esters in marine regions. Appl.
environ. Microbiol., 42: 590-595.
TEIRLYNCK, O.A. & BELPAIRE, F. (1985) Disposition of orally
administered di-(2-ethylhexyl) phthalate and mono-(2-ethylhexyl)
phthalate in the rat. Arch. Toxicol., 57: 226-230.
THIESS, A.M. & FLIEG, I. (1978) [Chromosomal studies in workers
exposed to di-2-ethylhexylphthalate (DOP).] Zentralbl. Arbeitsmed.,
28: 351-355 (in German).
THIESS, A.M., FRENTZEL-BEYME, R., & WIELAND, R. (1978a) [Mortality
study in workers exposed to di-2-ethylhexyl phthalate (DOP.] In:
[Possibilities and limits of biological monitoring - Problems in
occupational medicine of the industrial occupational health services.
Proceedings of a conference, Frankfurt, May 1978], Stuttgart, A.W.
Gertner, pp. 155-164 (in German).
THIESS, A.M., KORTE, A., & FLIEG, H. (1978b) [Morbidity studies in
workers exposed to di(2-ethylhexyl)phthalate (DEHP).] In:
[Possibilities and limits of biological monitoring - Problems in
occupational medicine of the industrial occupational health services.
Proceedings of a conference, Frankfurt, May 1978], Stuttgart, A.W.
Gertner, pp. 137-154 (in German).
THOMAS, G.H. (1973) Quantitative determination and confirmation of
identity of trace amounts of dialkylphthalates in environmental
samples. Environ. Health Perspect., 3: 23-28.
THOMAS, J.A., SCHEIN, L.G., GUPTA, P.K., MCCAFFERTY, R.E., FELICE,
P.R., & DONOVAN, M.P. (1979) Failure of monoethylhexyl phthalate to
cause teratogenic effects in offspring of rabbits. Toxicol. appl.
Pharmacol., 51: 523-528.
THUREN, A. (1986) Determination of phthalates in aquatic environments.
Bull. environ. Contam. Toxicol., 36: 33-40.
THUREN, A. & LARSSON, P. (1990) Phthalate esters in the Swedish
atmosphere. Environ. Sci. Technol., 24: 554-559.
THUREN, A. & WOIN, P. (1991) Effects of phthalate esters on the
locomotor activity of the freshwater amphipod Gammarus pulex. Bull
environ. Contam. Toxicol., 46: 159-166.
TIMOFIEVSKAJA, L.A., IRANOVA, N.I., & BALYNINA, E.S. (1980)
[Toxicology of esters of o-phthalic acid and hygienic standard for
them.] Gig. Tr. prof. Zabol., 3: 25-28 (in Russian).
TOMASZEWSKI, K.E., AGARWAL, D.K., & MELNICK, R.L. (1986) In vitro
steady-state levels of hydrogen peroxide after exposure of male F344
rats and female B6C3F1 mice to hepatic peroxisome proliferators.
Carcinogenicity, 7: 1871-1876.
TOMITA, I., NAKAMURA, Y., AOKI, N., & INUI, N. (1982a)
Mutagenic/carcinogenic potential of DEHP and MEHP. Environ. Health
Perspect., 45: 119-125.
TOMITA, I., NAKAMURA, Y., YAGI, Y., & TUTIKAWA, K. (1982b)
Teratogenicity/fetotoxicity of DEHP in mice. Environ. Health
Perspect., 45: 71-75.
TOMITA, I., NAKAMURA, Y., YAGI, Y., & TUTIKAWA, K. (1986) Fetotoxic
effects of mono-2-ethylhexyl phthalate (MEHP) in mice. Environ. Health
Perspect., 65: 249-254.
TRIMBLE, A.S., GOLDMAN, B.S., YAO, J.K., DOVATS, L.K., & BIGELOW, W.G.
(1966) Plastics - A source of chemical contamination in surgical
research. Surgery, 59: 857-859.
TYL, R.W., PRICE, C.J., MARR, M.C., & KIMMEL, C.A. (1988)
Developmental toxicity evaluation of dietary di(2-ethylhexyl)phthalate
in Fischer 344 rats and CD-1 mice. Fundam. appl. Toxicol., 10:
395-412.
VAINIOTALO, S. & PFÄFFLI, P. (1990) Air impurities in the PVC plastics
processing industry. Ann. occup. Hyg., 34: 585-590.
VILLENEUVE, D.C., FRANKLIN, C.A., CHU, I., YAGMINAS, A., MARINO, I.A.,
RITTER, L., & RUDDICK, J.A. (1978) Toxicity studies on mono-2-
ethylhexyl phthalate. Toxicol. appl. Pharmacol., 45: 250-251.
VON DÄNIKEN, A., LUTZ, W.K., JÄCKH, R., & SCHLATTER, C. (1984)
Investigation of the potential for binding of di(2-
ethylhexyl)phthalate (DEHP) and di-(2-ethylhexyl)adipate (DEHA) to
liver DNA in vivo. Toxicol. appl. Pharmacol., 73: 373-387.
WAALKES, M.P. & WARD, J.M. (1989) Induction of hepatic metallothionein
in male B6C3F1 mice exposed to hepatic tumor promotors: effects of
phenobarbital, acetaminophen, sodium barbital, and di(2-ethylhexyl)
phthalate. Toxicol. appl. Pharmacol., 100: 216-266.
WALDOCK, M.J. (1983) Determination of phthalate esters in samples from
the marine environment using gas chromatography mass spectrometry.
Chem. Ecol., 1: 261-277.
WALSETH, F., TOFTGÅRD, R., & NILSEN, O.G. (1982) Phthalate esters I:
Effects on cytochrome P-450 mediated metabolism in rat liver and lung,
serum enzymatic activities and serum protein levels. Arch. Toxicol.,
50: 1-10.
WAMS, T.J. (1987) Diethylhexylphthalate as an environmental
contaminant - A review. Sci. total Environ., 66: 1-16.
WARD, J.M., RICE, J.M., CREASIA, D., LYNCH, P., & RIGGS. C. (1983)
Dissimilar patterns of promotion by di(2-ethylhexyl)phthalate and
phenobarbital of hepatocellular neoplasia initiated by
diethylnitrosamine in B6C3F1 mice. Carcinogenesis, 4: 1021-1029.
WARD, J.M., DIWAN, B.A., OHSHIMA, M., HU, H., SCHULLER, H.M., & RICE,
J.M. (1986) Tumor-initiating and promoting activities of di(2-
ethylhexyl) phthalate in vivo and in vitro. Environ. Health Perspect.,
65: 279-291.
WEAST, R.C., ASTLE, M.J., & BEYER, W.H., ed. (1984) CRC handbook of
chemistry and physics, 65th ed., Boca Raton, Florida, CRC Press Inc.
WEBER, K. & ERNST, W. (1983) [Occurrence and fluctuation of organic
chemicals in major German estuaries.] Vom Wasser, 61: 111-123 (in
German).
WHITE, R.D., CARTER, D.E., EARNEST, D., & MUELLER, J. (1980)
Absorption and metabolism of 3 phthalate esters by the rat small
intestine. Food Cosmet. Toxicol., 18: 383-386.
WHO/FAO (1984a) Evaluation of certain food additives and contaminants
(Twenty-eighth report of the Joint FAO/WHO Expert Committee on Food
Additives), Geneva, World Health Organization, 44 pp (WHO Technical
Report Series, No. 710, and corrigendum).
WHO/FAO (1984b) Toxicological evaluation of certain food additives and
contaminants (Prepared by the Joint FAO/WHO Expert Committee on Food
Additives, Rome, 19-28 March, 1984), Geneva, World Health
Organization, 222 pp (WHO Food Additives Series No. 19).
WHO/FAO (1989a) Evaluation of certain food additives and contaminants
(Thirty-third report of the Joint FAO/WHO Expert Committee on Food
Additives), Geneva, World Health Organization, 64 pp (WHO Technical
Report Series No. 776).
WHO/FAO (1989b) Toxicological evaluation of certain food additives and
contaminants (Prepared by the 29th Meeting of the Joint FAO/WHO Expert
Committee on Food Additives), 362 pp. Cambridge, Cambridge University
Press (WHO Food Additives Series No. 24).
WILLIAMS, D.T. (1973) Dibutyl- and di-(2-ethylhexyl) phthalate in
fish. J. agric. food Chem., 21: 1128-1129.
WOFFORD, H.W., WILSEY, C.D., NEFF, G.S., GIAM, C.S., & NEFF, J.M.
(1981) Bioaccumulation and metabolism of phthalate esters by oysters,
brown shrimp, and sheepshead minnows. Ecotoxicol. environ. Saf., 5:
202-210.
WOIN, P. & LARSSON, P. (1987) Phthalate esters reduce predation
efficiency of dragonfly larvae, Odonata; Aeshna. Bull. environ.
Contam. Toxicol., 38: 220-225.
WOLFE, N.L., BURNS, L.A., & STEEN, W.C. (1980a) Use of linear free
energy relationships and an evaluative model to assess the fate and
transport of phthalate esters in the aquatic environment. Chemosphere,
9: 393-402.
WOLFE, N.L., STEEN, W.C., & BURNS, L.A. (1980b) Phthalate ester
hydrolysis: Linear free energy relationships. Chemosphere, 9: 403-408.
WOOD, D.L. & BITMAN, J. (1984) The effect of feeding di-(2-ethylhexyl)
phthalate and related compounds on lipids in the laying hen. Poult.
Sci., 63: 469-477.
WOODWARD, K.N., SMITH, A.M., MARISCOTTI, S.P., & TOMLINSON, N.J.
(1986) Review of the toxicity of the esters of o-phthalic acid
(phthalate esters), London, Health and Safety Executive, 183 pp
(Toxicity Review 14).
YAGI, Y., NAKAMURA, Y., TOMITA, I., TSUCHIKAWA, K., & SHIMOI, N.
(1980) Teratogenic potential of di- and mono-(2-ethylhexyl) phthalate
in mice. J. environ. Pathol. Toxicol., 4: 533-544.
YANAGITA, T., KOBAYASHI, K., & ENOMOTO, N. (1978) Accumulation of
hepatic phospholipids in rats fed di(2-ethylhexyl) phthalate. Biochem.
Pharmacol., 27: 2283-2288.
YANAGITA, T., KUZUHARA, S., ENOMOTO, N., SHIMAD, T., & SUGANO, M.
(1979) Effects of di(2-ethylhexyl) phthalate on the content of hepatic
mitochondrial and microsomal phospholipids in the rat. Biochem.
Pharmacol., 28: 3115-3121.
YOON, J.S., MASON, J.M., VALENCIA, R., WOODRUFF, R.C., & ZIMMERING, S.
(1985) Chemical mutagenesis testing in Drosophila. IV. Results of 45
coded compounds tested for the National Toxicology Program. Environ.
Mutagen., 7: 349-367.
YOSHIKAWA, K., TANAKA, A., YAMAHA, T., & KURATA, H. (1983)
Mutagenicity study of nine monoalkyl phthalates and a dialkyl
phthalate using Salmonella typhimurium and Escherichia coli. Food
chem. Toxicol., 21: 221-223.
ZEIGER, E., HAWORTH, S., MORTELMANS, K., & SPECK, W. (1985)
Mutagenicity testing of di(2-ethylhexyl) phthalate and related
chemicals in Salmonella. Environ. Mutagen., 7: 213-232.
ZIMMERING, S., MASON, J.M., & VALENCIA, R. (1989) Chemical mutagenesis
testing in drosophila. VII. Results of 22 coded compounds tested in
larval feeding experiments. Environ. mol. Mutagen., 14: 245-251.
ZIOGOU, K., KIRK, P.W.W., & LESTER, J.N. (1989) Behaviour of phthalic
acid esters during batch anaerobic digestion of sludge. Water Res.,
23: 743-748.
ZITKO, V. (1972) Determination, toxicity, and environmental levels of
phthalate plasticizers, St. Andrews, N.B., Biological Station,
Fisheries Research Board of Canada, 35 pp (Technical Report No. 344).
RESUME
1. Identité, propriétés physiques et chimiques et méthodes d'analyse
Le phtalate de di(2-éthylhexyle) est un ester de l'acide
benzènedicarboxylique qui se présente, à la température ambiante, sous
la forme d'un liquide huileux, incolore à jaune. Il est peu soluble
dans l'eau (0,3-0,4 mg/litre) et encore moins dans l'eau salée. Il est
miscible à la plupart des solvants organiques courants. Sa volatilité
est relativement faible (tension de vapeur: 8,6 x 10-4Pa).
On a mis au point, pour le dosage du DEHP dans différents
milieux, un grand nombre de méthodes de prélèvement et d'analyse. On
fait de plus en plus appel à des méthodes sensibles telles que la
chromatographie en phase gazeuse, la chromatographie en phase liquide
à haute performance et la spectrométrie de masse. La contamination due
au matériel en plastique utilisé lors des prélèvements et de l'analyse
complique le dosage du DEHP à faibles concentrations.
2. Sources d'exposition humaine et environnementale
Le DEHP présent dans l'environnement est, dans sa presque
totalité, d'origine artificielle.
La production mondiale de DEHP augmente depuis quelques décennies
et s'élève actuellement à environ un million de tonnes par an. Les
Etats-Unis d'Amérique et l'Europe entrent chacun pour un tiers dans
cette production.
Le DEHP est le plastifiant le plus largement utilisé pour
augmenter la fluidité des résines puisqu'il représente 50% de
l'ensemble des esters phtaliques utilisés comme plastifiants. Il peut
représenter jusqu'à 40% en poids ou davantage de la matière plastique.
Le DEHP est utilisé dans la fabrication du chlorure de polyvinyle
(PVC) utilisé dans l'industrie du bâtiment et de l'emballage ainsi que
pour la fabrication d'accessoires à usage médical. On l'utilise
également en plus petites quantités pour la préparation de peintures
industrielles ainsi que comme diélectrique dans les condensateurs.
Les produits contenant du plastique qui sont mis au rebut peuvent
être éliminés soit par incinération soit par enfouissement dans une
décharge. Lors de l'incinération à basse température, une proportion
importante du produit peut se dissiper dans l'atmosphère. On n'a pas
très bien étudié ce qu'il advient du DEHP déposé dans les décharges et
il n'est pas possible à ce stade de tirer des conclusions définitives.
3. Transport, distribution, et transformation dans l'environnement
C'est principalement en étant transportés dans l'atmosphère que
les phtalates pénètrent dans l'environnement. A partir de là, le DEHP
tombe sur le sol à moins d'être éliminé par les précipitations.
Le coefficient de partage du DEHP entre l'octanol et l'eau est
élevé, de sorte que l'équilibre entre l'eau et un sol ou un sédiment
riche en substances organiques s'établit aux dépens de la phase
aqueuse. Le DEHP s'adsorbe facilement sur les particules organiques et
du sol.
Bien que peu soluble dans l'eau, le DEHP peut se rencontrer en
quantités relativement élevées dans les eaux de surface en raison de
son adsorption sur les particules organiques et de son interaction
avec les substances organiques en solution. L'adsorption s'effectue
plus particulièrement sur les particules de petite taille et elle est
favorisée par la présence d'eau salée.
Dans l'atmosphère, le DEHP subit une photodégradation rapide mais
son hydrolyse dans l'environnement est pratiquement inexistante.
Plusieurs microorganismes terricoles effectuent la dégradation
aérobie du DEHP. Toutefois dans l'environnement, cette dégradation
microbienne s'effectue avec lenteur. La biodégradation commence par
l'hydrolyse du mono-ester qui est transformé en acide phtalique. Il y
a ensuite dégradation en pyruvate et succinate par ouverture du cycle
puis en CO2 et H2O, comme dans la dégradation métabolique de
l'acide benzoïque. La dégradation aérobie dépend de la température. Au
dessous de 10 °C la dégradation est peu importante. A température
supérieure, la biodégradation se produit dans la partie supérieure du
sol mais, à plus grande profondeur elle est pratiquement inexistante
du fait des conditions d'anaérobiose. La dégradation anaérobie, pour
autant qu'elle existe, est beaucoup plus lente que la dégradation
aérobie.
Le DEHP est très lipophile et modérément persistant. Le degré de
bioaccumulation dépend de la capacité de l'organisme qui l'absorbe à
métaboliser la substance. On a montré qu'il s'accumulait en forte
proportion chez divers invertébrés aquatiques, les poissons et les
amphibiens.
Après application de DEHP sur des feuilles de végétaux, on n'a
constaté qu'une faible perte de matière sur une période de 15 jours.
Le DEHP présent dans le sol ou dans les boues d'égout n'est que peu
fixé par les plantes.
4. Concentrations dans l'environnement et exposition humaine
Le DEHP est très répandu dans l'environnement et on le retrouve
dans la plupart des échantillons prélevés dans l'air, dans les
précipitations, l'eau, les sédiments, le sol et les biotes. C'est
généralement dans les zones industrielles que les teneurs sont les
plus fortes.
On a relevé des concentrations allant jusqu'à 300 ng/m3 dans
l'air des villes et en atmosphère polluée. Dans l'atmosphère
surmontant les océans, on a fait état de concentrations allant de 0,5
à 5 ng/m3 et les précipitations de ces régions en contenaient
jusqu'à 200 ng/litre. L'étude d'échantillons de précipitations
prélevés dans un secteur situé à proximité d'une usine produisant des
plastifiants, a montré que la vitesse de dépot à sec était de 0,7 à
4,7 µg/m2 et par jour.
Dans les rivières et les lacs, on a trouvé des concentrations de
DEHP allant jusqu'à 4 µg/litre, les teneurs les plus élevées étant
relevées au point de décharge d'effluents industriels. Dans la mer, la
concentration est inférieure à 1 µg/litre, les concentrations les plus
fortes étant observées dans les estuaires.
En raison de son caractère hydrophobe, le DEHP est facilement
absorbé par le sol, les sédiments et les particules. Dans les
sédiments de cours d'eau, des concentrations allant jusqu'à 70 mg/kg
(de poids à sec) ont été signalées, concentrations qui peuvent monter
jusqu'à 1480 mg/kg (de poids à sec) au voisinage des points de
décharge.
Dans les biotes, la concentration du DEHP varie de moins de 1 à
7000 µg/kg. On le rencontre dans divers types d'aliments: poisson,
coquillages, oeufs et fromages. L'exposition moyenne a été estimée à
environ 300 µg par personne et par jour aux Etats-Unis d'Amérique en
1974 et à 20 µg par personne et par jour au Royaume-Uni en 1986.
Les transfusions de sang et autres traitements utilisant du
matériel en plastique peuvent entraîner l'exposition involontaire des
malades au DEHP. C'est ainsi qu'on a relevé dans le poumon de malades
ainsi traités, des teneurs allant de 13,4 à 91,5 mg/kg de poids à sec.
Les quelques données disponibles indiquent que sur les lieux de
travail, la concentration de DEHP est généralement inférieure à 1
mg/m3.
5. Cinétique et métabolisme
Des données disponibles au sujet de l'administration par voie
orale indiquent que le DEHP est hydrolysé dans l'intestin par la
lipase pancréatique. Les métabolites qui se forment, c'est-à-dire le
phtalate de mono-2-éthylhexyle (MEHP) et le 2-éthylhexanol sont
rapidement absorbés. Après administration de DEHP marqué au 14C
(2,9 mg/kg) par voie orale à des rats, on a récupéré 50% de la dose
initiale dans l'urine et la bile de ces animaux. Il semble que la
biodisponibilité d'une dose orale de DEHP soit plus élevée chez les
jeunes animaux.
Après administration par voie orale, le DEHP est largement
hydrolysé dans l'intestin de certains animaux, par exemple les rats,
et se répartit dans l'organisme, principalement sous forme de phtalate
de monoéthylhexyle (MEHP). Toutefois chez les primates et l'homme,
l'hydrolyse est beaucoup moins importante. Il semble que ce soit
principalement au niveau du foie que s'effectue la métabolisation du
MEHP et du 2-éthylhexanol. On a identifié plusieurs autres
métabolites, l'omega et omega-1-oxydation étant les principales voies
métaboliques. Un ou plusieurs des produits résultant de l'omega-
oxydation peuvent encore être métabolisés par ß-oxydation. On a
constaté que l'omega-oxydation s'effectuait selon une cinétique non
linéaire. Le métabolisme du DEHP offre des différences considérables
selon les espèces; par exemple, l'omega-oxydation est moins importante
chez l'homme que chez le rat.
Une dose de DEHP de 2,9 mg/kg, administrée par voie orale à des
rats, a été récupérée une semaine plus tard presque intégralement dans
les matières fécales et l'urine des animaux. La bile et l'urine
constituent les principales voies d'excrétion. Lors d'une étude sur
l'homme, 15 à 25% d'une dose orale (0,45 mg/kg de DEHP) ont été
excrétés sous forme de MEHP et la majeure partie des produits
d'excrétion étaient constitués de métabolites oxydés.
6. Effets sur les mammifères de laboratoire et les systèmes
d'épreuve in vitro
La DL50 par voie orale du DEHP est d'environ 25 à 34 g/kg,
selon l'espèce, mais cette valeur est plus faible dans le cas du MEHP.
Lors d'études d'alimentation sur des rats et des souris, on a constaté
que des doses quotidiennes de DEHP supérieures à 3 g/kg entraînaient
la mort dans les 90 jours et qu'une dose de 0,4 g/kg réduisait le gain
de poids en l'espace de quelques jours. Dans d'autres études, c'est
une dose de 6,3-12,5 g/kg de nourriture qui a entraîné une réduction
du poids corporel.
Lors d'études à long terme, on a observé chez les animaux traités
une hépatomégalie accompagnée d'une augmentation du poids relatif des
reins. Dans l'une de ces études, on observait également une
hypertrophie cellulaire au niveau du lobe antérieur de l'hypophyse.
Un certain nombre d'études ont permis de mettre en évidence une
atrophie testiculaire, apparaîssant au bout de quelques jours, et liée
à l'administration de DEHP (doses dans l'alimentation allant de
10-20 g/kg). Les jeunes rats semblent plus vulnérables et les rats et
les souris plus sensibles que les hamsters et les ouistitis. On a
constaté que cette atrophie était réversible. Le MEHP exerce des
effets toxiques in vitro sur les cellules de Sertoli. Le DEHP de
même que le MEHP ont des propriétés tératogènes. Des malformations ont
été observées à des doses de 0,5-2 g/kg de nourriture chez la souris
et, à des doses supérieures à 10 g/kg, on a observé des effets
embryotoxiques.
Les tests de mutagénicité et autres manifestations du même genre
ont donné des résultats négatifs dans la plupart des études. Le DEHP
peut provoquer la transformation des cellules et on a montré qu'il
était cancérogène à des doses respectives de 6 et 12 g/kg de
nourriture chez le rat et de 3 et 6 g/kg de nourriture chez la souris.
On a constaté une augmentation, liée à la dose, des tumeurs
hépatocellulaires chez les deux sexes de l'une et l'autre espèce. La
prolifération des peroxysomes hépatiques et la réplication cellulaire
sont fortement liées aux effets cancérogènes sur le foie de certains
produits non génotoxiques comme le DEHP. Toutefois on a observé des
différences importantes entre les espèces animales en ce qui concerne
la prolifération des peroxysomes induite par le DEHP.
Contrairement à ce qui se passe dans le cas des hépatocytes de
rat, les métabolites du DEHP ne provoquent pas de prolifération des
peroxysomes dans les cultures d'hépatocytes humains.
7. Effets sur l'homme
On ne dispose que de données très limitées sur les effets que le
DEHP peut exercer sur l'homme. On a fait état chez deux sujets qui
avaient reçu 5 ou 10 g de DEHP respectivement, d'effets qui se
limitaient à de légers troubles gastriques.
8. Effets sur les autres êtres vivants en laboratoire et dans
leur milieu naturel
Dans la plupart des études, les valeurs nominales de la CL50
obtenues lors des tests de toxicité aiguë dépassent 10 mg/litre, ce
qui indique que le DEHP est peu toxique. Toutefois ces valeurs sont
supérieures à la solubilité du DEHP dans l'eau (0,3-0,4 mg/litre).
D'après une étude, une daphnie, Daphnia pulex, serait
particulièrement sensible, la CL50 à 48 heures étant dans son cas de
0,133 mg/litre. La seule épreuve de toxicité aiguë où l'on ait mesuré
des concentrations de DEHP a été effectuée sur un poisson, Pimephales
promelas, et elle a donné une CL50 à 96 heures supérieure à 0,33
mg/litre. Lors d'études de longue durée, on a obtenu une dose sans
effet nocif observable de 72 µg/litre pour Daphnia magna. Pour les
poissons adultes, cette dose était supérieure à 72 µg/litre. Une
exposition à une concentration de 14 µg/litre, pendant 12 jours avant
l'éclosion, a provoqué une importante augmentation de la mortalité
parmi des alevins de truites. Des concentrations comprises en 3,7 et
11 µg/litre ont entraîné une réduction de la teneur en collagène
vertébral chez les poissons.
La présence de DEHP dans la nourriture à raison de 50 mg/kg a eu
des effets nocifs sur la survie des alevins de Melambaphes zebra. A
la concentration de 25 mg/kg en poids dans les sédiments, on a
constaté une réduction de l'activité microbienne et du nombre d'
éclosions chez les tétards.
Le DEHP est peu toxique pour les algues, les végétaux, les
lombrics et les oiseaux.
9. Evaluation
Le DEHP exerce des effets cancérogènes sur le foie et affecte le
système reproducteur chez le rat et la souris.
Le principal effet au niveau des gonades consiste, chez le rat et
la souris, en une atrophie des testicules qui affecte davantage les
jeunes animaux. La prolifération des peroxysomes hépatiques et la
réplication cellulaire sont fortement liées à l'effet cancérogène
qu'exercent sur le foie certains produits non génotoxiques au nombre
desquels figure le DEHP. Toutefois, on a observé de fortes différences
selon les espèces animales pour ce qui est de la prolifération des
peroxysomes induite par le DEHP. On ne possède pas actuellement de
preuves suffisantes permettant de conclure que le DEHP est
potentiellement cancérogène pour l'homme.
Il n'existe pas de données attestées selon lesquelles le DEHP
serait dangereux pour la faune, si l'on s'en tient aux résultats
obtenus lors de l'exposition de poissons et de daphnies. Toutefois, on
a observé une réduction de l'activité microbienne dans les sédiments
aux doses auxquelles le DEHP est présent dans l'environnement. Si l'on
compare les doses de DEHP présentes dans l'environnement aux
concentrations qui sont susceptibles de produire des effets lors
d'études de longue durée, notamment sur les larves de poissons et
d'amphibiens, on ne peut exclure l'existence d'un risque pour
l'environnement, les effets s'exerçant notamment par l'intermédiaire
de l'eau et des sédiments. Des effets nocifs sur les êtres vivants
sont possibles dans les secteurs où l'eau et les sédiments sont
fortement pollués en raison de leur proximité des points de décharge.
Bien que peu d'études valables aient été effectuées, il semble
que le DEHP présente une faible toxicité aiguë pour les algues, les
végétaux, les lombrics et les oiseaux.
RESUMEN
1. Identidad, propiedades físicas y químicas y métodos analíticos
El di(2-etilhexil) ftalato (DEHF) es un éster del ácido
bencenodicarboxílico que a temperatura ambiente es un líquido oleoso
entre incoloro y amarillo. Su solubilidad en agua es baja (0,3-0,4
mg/litro) y aún más baja en agua salada. Es miscible con la mayor
parte de los disolventes orgánicos normales. La volatilidad del DEHF
es relativamente baja (8,6 x10-4 Pa).
Se han perfeccionado numerosos métodos de muestreo y análisis
para la determinación del DEHF en diferentes medios. Cada vez se
utilizan más métodos de gran sensibilidad, como la cromatografía de
gases, la cromatografía líquida de alto rendimiento y la
espectrometría de masas. El análisis de concentraciones bajas de DEHF
se complica por la contaminación debida al material de plástico
utilizado en el muestreo y el análisis.
2. Fuentes de exposición humana y ambiental
Casi todo el DEHF presente en el medio ambiente procede de
fuentes antropogénicas.
La producción mundial de DEHF ha ido aumentando durante los
últimos decenios y en la actualidad es de alrededor de 1 x 106
toneladas al año. Un tercio del total se produce en los Estados Unidos
y otro tercio en Europa.
El DEHF es el plastificante más utilizado (representa el 50% de
todos los plastificantes a base de ésteres de ftalato) para ablandar
las resinas. A él corresponde el 40% (p/p) o más del total de
plásticos. El DEHF se emplea en la fabricación de cloruro de
polivinilo (PVC) utilizado en los edificios, en la construcción, en
embalajes y en componentes de aparatos médicos. Se utiliza en
cantidades más pequeñas en las pinturas industriales y como fluido
dieléctrico en los condensadores.
Los productos plastificados de desecho se pueden eliminar por
incineración o terraplenado. Durante la incineración a baja
temperatura, se puede liberar un porcentaje elevado del DEHF a la
atmósfera. No se ha estudiado bien el destino medioambiental del DEHF
depositado en terraplenes, por lo que no se pueden sacar conclusiones
definitivas.
3. Transporte, distribución y transformación en el medio ambiente
El transporte en el aire es la vía más importante de
incorporación de los ftalatos al medio ambiente. De la atmósfera, el
DEHF precipita o es lavado por la lluvia.
El DEHF tiene un coeficiente de reparto octanol-agua elevado, de
manera que el equilibrio entre el agua y un suelo o sedimento rico en
materia orgánica está desplazado a favor de este último. Las
partículas orgánicas del suelo lo adsorben fácilmente.
Aunque la solubilidad del DEHF en agua es baja, la cantidad
presente en las aguas de superficie puede ser mayor debido a la
adsorción a las partículas orgánicas y a la interacción con la materia
orgánica disuelta. Las pequeñas partículas lo adsorben más fácilmente,
y el proceso se potencia en el agua salada.
La fotodegradación atmosférica del DEHF es rápida; en cambio, su
hidrólisis química en el medio ambiente es prácticamente inexistente.
Se ha observado que varios microorganismos del suelo llevan a
cabo la degradación aerobia. Sin embargo, se ha informado que la
degradación microbiana del DEHF en el medio ambiente es lenta. El
proceso de biodegradación comienza con la hidrólisis para formar el
monoéster, que se transforma luego en ácido ftálico. La degradación
con ruptura del anillo para dar primero piruvato y succinato y después
CO2 y H2O es análoga a la vía metabólica del ácido benzoico. La
degradación aerobia es dependiente de la temperatura. Por debajo de
los 10 °C es escasa. A temperaturas más altas se produce
biodegradación en la capa más superficial del suelo, pero es
prácticamente inexistente en las capas más profundas, donde las
condiciones son de anaerobiosis. La degradación anaerobia, si existe,
es mucho más lenta que la aerobia.
El DEHF es muy lipófilo y moderadamente persistente. El grado de
bioacumulación depende de la capacidad de los organismos para
metabolizarlo. Se ha observado un grado elevado de acumulación en
diversos invertebrados acuáticos, peces y anfibios.
Cuando se aplicó DEHF a hojas de plantas, la pérdida fue pequeña
durante un período de 15 días. Se ha observado que su absorción por
las plantas a partir del suelo o de los fangos cloacales es muy baja.
4. Niveles medioambientales y exposición humana
El DEHF está muy ampliamente distribuido en la naturaleza y se
encuentra en la mayor parte de las muestras, por ejemplo de aire,
precipitación, sedimento, suelo y biota. Las concentraciones más altas
aparecen generalmente en las zonas industrializadas.
En el aire urbano y contaminado se han encontrado concentraciones
de hasta 300 ng/m3. Se han comunicado de niveles comprendidos entre
0,5 y 5 ng/m3 en el aire de zonas oceánicas, y la lluvia de esas
zonas contenía hasta alrededor de 200 ng/litro. En muestras de
precipitación procedentes de una zona cercana a una fábrica de
plastificantes se observó que la tasa sedimentación seca era de 0,7 a
4,7 µg/m2 al día.
Se han encontrado concentraciones de DEHF en ríos y lagos de
hasta 4 µg/litro; los niveles más altos están asociados a los puntos
de descarga de efluentes industriales. La concentración en el mar es
inferior a 1 µg/litro, y los niveles más altos se detectan en los
estuarios.
Debido a su carácter hidrófobo, el DEHF se adsorbe muy fácilmente
al suelo, los sedimentos y la materia particulada. En los sedimentos
fluviales se han comunicado niveles de hasta 70 mg/kg (peso seco), y
cerca de los puntos de descarga ha llegado a ser de 1480 mg/kg (peso
seco).
La concentración de DEHF en la biota oscila entre menos de 1 y
7000 µg/kg. Se ha detectado en diversos tipos de alimentos, como
pescado, marisco, huevos y queso. En 1974 se estimó en los Estados
Unidos una exposición media de 300 µg/persona al día y en el Reino
Unido en 1986 de 20 µg/persona al día.
Las transfusiones de sangre y otros tipos de tratamiento médico
en los que se utilizan aparatos de plástico pueden dar lugar a una
exposición humana involuntaria al DEHF. En el tejido pulmonar de
algunos pacientes se han detectado concentraciones comprendidas entre
13,4 y 91,5 µg/kg (peso seco).
Los escasos datos disponibles indican que las concentraciones de
DEHF en el lugar de trabajo suelen estar por debajo de los 1 mg/m3.
5. Cinética y metabolismo
Los datos disponibles sobre la administración oral indican que la
lipasa pancreática hidroliza el DEHF en el intestino. Los metabolitos
formados, que son el mono (2-etilhexil) ftalato (MEHF) y el
2-etilhexanol, se absorben rápidamente. Cuando se administró por vía
oral a ratas DEHF marcado con 14C (2,9 mg/kg), se recuperó en la
orina o la bilis más del 50%. La biodisponibilidad de una dosis oral
de DEHF parece ser más elevada en las ratas jóvenes que en las más
viejas.
El DEHF administrado por vía oral se hidroliza en el intestino de
ciertos animales, por ejemplo las ratas, y se distribuye sobre todo
como monoetilhexil ftalato (MEHF). Sin embargo, la hidrólisis es mucho
menor en los primates y el ser humano. El MEHF se liga a las proteínas
del plasma. El hígado parece ser el principal órgano metabolizador del
MEHF y del 2-etilhexanol. Se han identificado algunos otros
metabolitos, siendo las principales vías metabólicas la omega-y la
omega-1-oxidación. Uno o varios productos de la omega-oxidación pueden
metabolizarse ulteriormente por ß-oxidación. En la omega-oxidación se
han observado cinéticas no lineales. En el metabolismo del DEHF se
registran considerables diferencias entre las especies; por ejemplo,
la omega-oxidación es menor en el hombre que en las ratas.
Una semana después de administrar una dosis oral de DEHF
(2,9 mg/kg) se recuperó casi el 100% en las heces y la orina de ratas.
La bilis y la orina son las principales vías de excreción. En un
estudio humano, del 15 al 25% de la dosis oral (0,45 mg/kg) de DEHF se
excretó en forma de MEHF; los metabolitos oxidados constituían la
parte más importante de los productos de excreción.
6. Efectos en los mamíferos de laboratorio y en sistemas de
ensayo in vitro
La DL50 del DEHF por vía oral es de alrededor de 25-34 g/kg,
según las especies, pero el valor para el MEHF es más bajo. En
estudios de alimentación en ratas y ratones, las dosis de DEHF
superiores a 3 g/kg al día causaron muertes en un plazo de 90 días, y
con una concentración de 0,4 g/kg al día se redujo el aumento de peso
a los pocos días. En otros estudios, con dosis de 6,3-12,5 g/kg de
alimentos se produjo una reducción del peso corporal.
En los animales tratados en estudios de larga duración se ha
observado hepatomegalia y un aumento del peso relativo de los riñones.
En un estudio se encontraron también células hipertróficas en el
lóbulo anterior de la hipófisis.
En varios estudios se ha detectado atrofia testicular, evidente
al cabo de pocos días, relacionada con la administración de DEHF
(concentraciones de 10-20 g de DEHF/kg de alimentos). Las ratas más
jóvenes parecen ser más susceptibles que las viejas, y las ratas y los
ratones parecen ser más sensibles que los titís y los hámsters. Se ha
observado que la atrofia es reversible. El MEHF tiene efecto tóxico
in vitro en las células de Sertoli. Tanto el DEHF como el MEHF
muestran propiedades teratogénicas. Con concentraciones de 0,5 a 2
g/kg de alimentos se observaron malformaciones en ratones, y con
niveles superiores a los 10 g/kg se apreciaron efectos embriotóxicos.
Las pruebas de mutagenicidad y puntos finales asociados han dado
resultados negativos en la mayor parte de los estudios. El DEHF puede
inducir la transformación celular y se ha demostrado que con dosis de
6 y 12 g/kg de alimentos en ratas y de 3 y 6 g/kg de alimentos en
ratones tiene efectos carcinógenos. En ambos sexos de las dos especies
se produjo un aumento de tumores hepatocelulares dependiente de la
dosis. La inducción de la proliferación de peroxisomas hepáticos y de
la replicación celular está fuertemente vinculada al efecto
carcinógeno en el hígado de determinados compuestos carcinógenos no
genotóxicos, entre los que figura el DEHF. Sin embargo, se han
observado notables diferencias entre distintas especies animales con
respecto a la proliferación de peroxisomas inducida por el DEHF.
A diferencia de lo que ocurre con los hepatocitos de rata, los
metabolitos del DEHF no inducen proliferación de peroxisomas en
cultivos de hepatocitos humanos.
7. Efectos en el ser humano
La información disponible sobre los efectos del DEHF en el ser
humano es muy escasa. Se han comunicado dos casos de trastornos
gástricos leves, con dosis de 5 y 10 g de DEHF, pero sin ningún otro
efecto nocivo.
8. Efectos en otros organismos en el laboratorio y en el
medio ambiente
En la mayoría de los estudios de toxicidad aguda se han obtenido
unos valores nominales para la CL50 que se sitúan por encima de los
10 mg/litro, por lo que el índice de toxicidad del DEHF es bajo. Sin
embargo, esos niveles son superiores a los correspondientes a su
solubilidad en agua (0,3-0,4 mg/litro). En un estudio se detectó en la
pulga de agua Daphnia pulex una sensibilidad mayor, con un valor
nominal de la CL50 a las 48 h de 0,133 mg/litro. La única prueba de
la toxicidad aguda realizada con concentraciones medidas de DEHF se
hizo en el pez Pimephales promelas y puso de manifiesto una CL50
> 0,33 mg/litro a las 96 h. En estudios prolongados, el nivel sin
observación de efectos (NOEL) en Daphnia magna fue de 72 µg/litro.
En peces adultos se determinó un NOEL > 62 µg/litro. Una exposición
a 14 µg/litro desde 12 días antes de la eclosión produjo un
significativo aumento de la mortalidad de los alevines de trucha. Las
concentraciones entre 3,7 y 11 µg/litro provocaron una reducción del
colágeno vertebral en peces.
Con concentraciones de 50 mg/kg de alimentos, el DEHF tiene
efectos adversos sobre la supervivencia de los alevines de
Brachydanio rerio. Concentraciones de 25 mg/kg (p/p) en el sedimento
redujeron de manera considerable la actividad microbiana y el número
de renacuajos que nacían.
La toxicidad aguda del DEHF en algas, plantas, lombrices de
tierra y aves es baja.
9. Evaluación
El DEHF tiene efectos reproductivos y hepatocarcinogénicos en
ratas y ratones.
El principal efecto sobre la reproducción en ratas y ratones es
la atrofia testicular; los animales jóvenes son más susceptibles que
los viejos. La inducción de la proliferación de peroxisomas hepáticos
y de la replicación celular está estrechamente relacionada con el
efecto carcinogénico en el hígado de determinados compuestos
carcinógenos no genotóxicos, incluido el DEHF. Sin embargo, se han
observado notables diferencias entre las distintas especies de
animales con respecto a la proliferación de peroxisomas inducida por
el DEHF. En la actualidad no hay pruebas suficientes que indiquen que
el DEHF sea un posible carcinógeno para el ser humano.
No hay información documentada de que el DEHF constituya un
riesgo, tomando como base la exposición aguda de peces y dáfnidos. Sin
embargo, se ha informado de una reducción de la actividad microbiana
en los sedimentos con los niveles de DEHF presentes en el medio
ambiente. La comparación entre los niveles medioambientales y las
concentraciones que producen efectos en estudios prolongados,
especialmente las pruebas en las fases vitales tempranas de peces y
anfibios, indica que no se puede descartar un cierto peligro para el
medio ambiente, sobre todo por conducto del agua y los sedimentos. Es
probable que se produzcan efectos adversos en los organismos en zonas
con agua y sedimentos muy contaminados que están próximos a las
fuentes de emisión.
Aunque son pocos los estudios que se conocen al respecto, la
toxicidad aguda del DEHF en algas, plantas, lombrices de tierra y aves
parece ser baja.
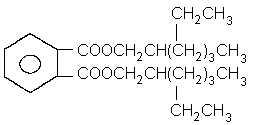 Empirical formula: C24H38O4
Abbreviation: DEHP
Relative molecular
mass: 390.57
Common synonyms:
1,2-benzenedicarboxylic acid bis(2-ethylhexyl) ester (CAS name);
phthalic acid bis(2-ethylhexyl) ester (IUPAC name); BEHP;
1,2-benzenedicarboxylic acid bis(ethylhexyl) ester;
bis(2-ethylhexyl) 1,2-benzenedicarboxylate; bis(2-ethyl-hexyl)
ester of phthalic acid; bis(2-ethylhexyl) phthalate;
di(2-ethylhexyl) ortho-phthalate; di(ethylhexyl) phthalate;
dioctyl phthalate; DOP; ethylhexyl phthalate; 2-ethylhexyl
phthalate; octyl phthalate; di- sec-octyl phthalate; phthalic
acid dioctyl ester
Common trade names:
Bisoflex 81; Bisoflex DOP; Compound 889; DAF 68; Ergoplast FDO;
Eviplast 80; Eviplast 81; Fleximel; Flexol DOP; Goodrite GP 264;
Hatcol DOP; Kodaflex DOP; Mollan O; Nuoplaz DOP; Octoil;
Palatinol AH; Platinol DOP; Pittsburgh; PX-138; Reomol DOP;
Reomol D 79P; Sicol 150; Staflex DOP; Truflex DOP; Vestinol AH;
Vinicizer 80; Witcizer 312 (IARC, 1982; NIOSH, 1985b)
CAS registry
number:
Empirical formula: C24H38O4
Abbreviation: DEHP
Relative molecular
mass: 390.57
Common synonyms:
1,2-benzenedicarboxylic acid bis(2-ethylhexyl) ester (CAS name);
phthalic acid bis(2-ethylhexyl) ester (IUPAC name); BEHP;
1,2-benzenedicarboxylic acid bis(ethylhexyl) ester;
bis(2-ethylhexyl) 1,2-benzenedicarboxylate; bis(2-ethyl-hexyl)
ester of phthalic acid; bis(2-ethylhexyl) phthalate;
di(2-ethylhexyl) ortho-phthalate; di(ethylhexyl) phthalate;
dioctyl phthalate; DOP; ethylhexyl phthalate; 2-ethylhexyl
phthalate; octyl phthalate; di- sec-octyl phthalate; phthalic
acid dioctyl ester
Common trade names:
Bisoflex 81; Bisoflex DOP; Compound 889; DAF 68; Ergoplast FDO;
Eviplast 80; Eviplast 81; Fleximel; Flexol DOP; Goodrite GP 264;
Hatcol DOP; Kodaflex DOP; Mollan O; Nuoplaz DOP; Octoil;
Palatinol AH; Platinol DOP; Pittsburgh; PX-138; Reomol DOP;
Reomol D 79P; Sicol 150; Staflex DOP; Truflex DOP; Vestinol AH;
Vinicizer 80; Witcizer 312 (IARC, 1982; NIOSH, 1985b)
CAS registry
number:  There are marked species differences in the metabolism of DEHP.
Thus omega-oxidation seems to play a dominante role in the rat and
guinea-pig (Albro et al., 1982; Lhuguenot & Elcombe, 1984; Lhuguenot
et al., 1985), but to be a minor pathway in the mouse, hamster, green
monkey, cynomolgus monkey, and marmoset (Albro et al., 1982; Lhguenot
& Elcombe, 1984). In guinea-pigs there are few omega-1 metabolites of
MEHP (Albro et al., 1982). This may have toxicological significance
because certain omega-1-oxidation metabolites have been identified as
active agents in peroxisomal proliferation in rat hepatocytes
(Mitchell et al., 1985a).
No conjugated metabolites were detected in the urine of
DEHP-treated rats, but a minor portion was conjugated in the urine of
hamsters (Albro et al., 1982; Lhuguenot & Elcombe, 1984). A major
portion of glucuronide conjugates was found in the urine of the
marmoset, mouse, guinea-pig, and green monkey, and in human urine
(Albro et al., 1982). Albro (1986) reported that glucuronidation of
DEHP metabolites was insignificant in rats. Studies in primates,
including the African green monkey (Albro et al., 1981), marmoset
(Rhodes et al., 1986), cynomolgus monkey (Short et al., 1987; Astill,
1989), and man (Schmid & Schlatter, 1985), demonstrated that
conjugation of DEHP can occur at the carboxylic acid moiety following
a single ester hydrolysis.
The level of unmetabolized MEHP excreted in urine also varies
considerably between species; it is low in the rat and hamster, but
high in the mouse, guinea-pig, green monkey, and man (Albro et al.,
1982).
Repeated oral administration of DEHP or MEHP at high doses (500
mg/kg) to rats leads to a change in the metabolic profile; there is an
increase in omega-oxidized metabolites and a decrease in
omega-1-oxidized metabolites (Lhuguenot et al., 1985). In rats given
2% DEHP in the diet for one week, a 4-fold increase in peroxisomal ß-
oxidation was found. ß-Oxidation of fatty acids induced by DEHP
appears to occur via mitochondrial and peroxisomal pathways that are
similar to normal pathways (Ganning et al., 1989). Drug-metabolizing
enzyme activities have been studied after DEHP administration, and in
some cases changes were observed (Walseth et al., 1982; Agarwal et
al., 1982; Gollamudi et al., 1985; Pollack et al., 1989).
The same metabolites as those found in rat urine can be detected
in human urine. One study on intravenously injected DEHP (Albro et
al., 1982) and one on orally administered DEHP (Schmid & Schlatter,
1985) indicated that humans metabolize DEHP by omega- and
omega-1-oxidation as well as by oxidation of the ethyl side chain.
However, the omega-oxidation-pathway seems to be a minor pathway in
man (Albro et al., 1982; Schmid & Schlatter, 1985). More than half of
the metabolites recovered in human urine are conjugated metabolites
(Albro et al., 1982; Schmid & Schlatter, 1985).
Time-averaged concentrations of DEHP, MEHP, and phthalic acid in
the blood of patients undergoing maintenance haemodialysis were 1.9,
1.3, and 5.2 mg/litre, respectively (Pollack et al., 1985a). Such
patients are considered to be at risk of potential DEHP toxicity
through prolonged contact with medical plastic products that contain
DEHP. The relatively high circulating level of phthalic acid may
indicate an altered metabolism of DEHP in uraemic patients (Pollack et
al., 1985a).
The levels of DEHP and MEHP in plasma have been studied in
newborn infants given blood exchange transfusions. In one case the
MEHP half-life was the same as for DEHP (about 12 h), indicating that
the hydrolysis of DEHP was the rate-limiting metabolic step. However,
in other children the half-time of MEHP was longer than that of DEHP
(Sjöberg et al., 1985b).
6.4 Elimination and excretion
Radioactivity from intravenously injected 14C-labelled DEHP is
mainly recovered in urine and faeces after 24 h (Schulz & Rubin,
1973), indicating that urine and bile are major excretory pathways.
When a low dose level (0.1 mg/kg) was given to rats, 50-60% of
injected radioactivity was recovered in urine and faeces after 24 h,
whereas at a high dose level (200 mg/kg) less than 50% was recovered
(Schulz & Rubin, 1973). Seven days after an oral dose (2.9 mg/kg) of
DEHP was given to rats, 42% of the radioactivity was recovered in the
urine and 57% in the faeces (Daniel & Bratt, 1974). Biliary excretion
was also measured in these experiments, and it was found that 14% of
the radioactivity was recovered in bile after 4 days (Daniel & Bratt,
1974). The almost 100% recovery reported by Daniel & Bratt (1974) has
been confirmed by Teirlynck & Belpaire (1985). Oral administration of
MEHP (50-500 mg/kg) gave a higher urinary recovery than orally
administered DEHP (50-500 mg/kg) as measured after 24 h (Lhuguenot et
al., 1985).
After the oral administration of non-radioactive DEHP (0.45
mg/kg) to human volunteers, it was found that 15-25% was excreted in
urine as MEHP or oxidized metabolites within 2-3 days (Schmid &
Schlatter, 1985).
In the rat no unmetabolized DEHP is excreted in the urine, but
small amounts are found in mouse or green monkey urine (Albro et al.,
1982). Major amounts of MEHP are excreted in mouse, guinea-pig, green
monkey, and human urine (Albro et al., 1982). However, oxidized
metabolites, either free or conjugated, constitute a major portion of
excretion products in rat, mouse, hamster, green monkey, and human
urine (Albro et al., 1982).
Changes in excretion pathways have been observed after prolonged
administration of DEHP. After oral dosing of rats without
pre-treatment with DEHP, the faecal excretion pathway dominated, while
in rats fed with DEHP for 7 days the urinary pathway dominated (Daniel
& Bratt, 1974).
6.5 Retention and turnover
6.5.1 Half-life and body burden
After the intravenous administration of radiolabelled DEHP, at
least two elimination phases of radioactivity, with short half-lives
(4.5-9 and 22 min, respectively), were observed in rat blood (Schulz
& Rubin, 1973). After 7 weeks of oral administration, the elimination
phase in the liver was considerably slower, the half-life being 3-5
days (Daniel & Bratt, 1974). No accumulation of DEHP or MEHP was
observed when the dosage was 2.8 g/kg per day for 7 days (Teirlynck &
Belpaire, 1985), nor was there any in a long-term (5-7 weeks) feeding
study at a dose level of 1 or 5 g/kg diet (corresponding to a daily
dose of about 50 and 250 mg/kg body weight) (Daniel & Bratt, 1974).
6.5.2 Indicator media
Analysis of the total amount of urinary metabolites, measured as
derivatized phthalic acid, indicate a weak positive correlation
between occupational exposure to phthalate and the presence of
metabolites in the urine (Nielsen et al., 1985; Liss et al., 1985). In
the study by Nielsen et al. (1985), workers were exposed mainly to
DEHP and diisodecyl phthalate, and the urinary level of phthalate
ester metabolites rose from the background level (17 µmol/litre) to
23-25 µmol/litre. In the study by Liss et al. (1985), workers were
exposed to DEHP and phthalic anhydride. Urinary phthalate
concentrations in exposed workers more than doubled after a workshift
and levels up to 44 µmol/litre were recorded. The authors concluded
that phthalic anhydride influenced the urinary level more than DEHP.
Phthalic acid is not a specific marker for DEHP exposure.
7. EFFECTS ON LABORATORY MAMMALS AND IN VITRO TEST SYSTEMS
7.1 Single exposure
Numerous LD50 values have been reported for DEHP. Oral LD50
values generally exceed 25 g/kg in rats and 30 g/kg in mice
(Stankevich et al., 1984; NIOSH, 1985b; Woodward et al., 1986); in the
rabbit it is 33.9 g/kg (Shaffer et al., 1945) and in the guinea-pig
26.3 g/kg (Krauskopf, 1973). Dermal LD50 values for guinea-pigs and
rabbits of 10 g/kg and 25 g/kg, respectively, have been reported
(NIOSH, 1985b).
The LD50 values after intraperitoneal administration were
30.7 g/kg in rats (Shaffer et al., 1945) and 14-75 g/kg in mice
(Lawrence et al., 1975; Woodward et al., 1986). LD50 values in the
range of 200-250 mg/kg were reported for the rat after intravenous
administration of DEHP solubilized with a nonionic detergent (Schmidt
et al., 1975; Rubin & Chang, 1978).
The main symptom of DEHP toxicity after single oral or
intraperitoneal dosing is diarrhoea (Hodge, 1943). An intraperitoneal
dose of 500 mg/kg in rats decreased spontaneous running activity,
thereby indicating behavioural changes (Rubin & Jaeger, 1973). After
intravenous dosing, lung lesions including oedema, haemorrhage, and
infiltrations of polymorphonuclear leucocytes were observed in rats at
doses as low as 50 mg/kg (Schulz et al., 1975). The etiology of the
lung lesions is unknown. It has, however, been suggested that some
changes could be due to the release of lysosomal enzymes from alveolar
macrophages, which was found to occur in vitro in rabbit alveolar
macrophages cultured with DEHP (Bally et al., 1980).
Rabbits treated intravenously with 350 mg DEHP/kg showed a
decrease in blood pressure and an increase in breathing rate. No
deaths occurred after doses up to 650 mg/kg were administered (Calley
et al., 1966).
The monoester, MEHP, may be more toxic than the diester but data
are very limited. In a short note by Villeneuve et al. (1978), the
oral LD50 of MEHP was reported to be 1.34 g/kg in female rats and
1.8 g/kg in males.
7.2 Short-term exposure
Doses of 3.4 g/kg body weight per day given by gavage (in olive
oil) for periods of up to 90 days caused the death of 15 out 20 rats
(Nikonorow et al., 1973). However, no deaths were reported among rats
fed 3% DEHP in the diet (1.9 g/kg body weight) for 90 days (Shaffer et
al., 1945) or in a rat study of the US National Toxicology Program
after dietary dosing (< 50 g/kg) for 14 days (NTP, 1982).
Oral administration of DEHP at a rate of > 0.4 g/kg body
weight per day resulted in a weight gain decrease in rats within a few
days (Nikonorow et al., 1973). In a 17-week feeding study where rats
were given 2, 10 or 20 g DEHP/kg diet, a decreased body weight was
observed (Gray et al., 1977). Reduction in body weight was also
observed in rats given dietary levels of 12.5 or 25 g/kg for 13 weeks.
Dosages of 1.6-6.3 g/kg resulted in either slight elevations of body
weight or no effect (NTP, 1982).
MEHP given at a level of 6.4 g/kg diet caused reduction in the
body weight gain of rats (Chu et al., 1981). No effects on body weight
occurred at dietary levels of 0.625 g/kg given for 3 months, but a
significant decrease in blood glucose was observed.
Reductions in haemoglobin, packed cell volume and erythrocyte
numbers were observed in rats given 10 or 20 g DEHP/kg diet for 17
weeks, but not when they were given 2 g/kg for the same period (Gray
et al., 1977).
Cystic kidneys and centrilobular necrosis were noted in one
strain of mice (ddY) fed 2.5 or 25 g DEHP/kg for 2 weeks, but not in
another strain (B6C3F1), even with a higher exposure level and a
longer exposure period (Woodward et al., 1986).
DEHP administered intravenously at a rate of 25-500 mg/kg per day
for 2-4 weeks to beagle dogs resulted in pulmonary haemorrhage and
inflammatory response similar in appearance to the "shock-lung" effect
(Woodward et al., 1986).
In an inhalation study, Wistar rats were exposed in a head-nose
inhalation system to DEHP aerosols of respirable particle size.
Exposure duration was 6 h/day, 5 days/week for 4 weeks at target
concentrations of 0, 0.01, 0.05, and 1.0 mg/litre. A statistically
significant increase in relative lung weights was found in the males
given the highest dosage, and this was accompanied by foam cell
proliferation and thickening of the alveolar septa (Klimisch et al.,
1991).
Another inhalation study has been reported, but this is
inadequate for assessment (Timofievskaja et al., 1980).
A discussion of the effects of DEHP on the liver is given in
section 7.9.
When rats were treated with DEHP by intraperitoneal injection
(Walseth et al., 1982) or by repeated oral dosing (Agarwal et al.,
1982), an increase in cytochrome P-450 levels was observed. The
increase in hepatic microsomal oxidation appears to be primarily due
to the deesterification products of DEHP, i.e. MEHP and
2-ethoxyhexanol, in long-term exposure, whereas DEHP and its two
metabolites may inhibit microsomal oxidation after acute exposure to
DEHP (Pollack et al., 1989). However, in vitro rat liver microsomal
cytochrome P-450 levels were not affected by DEHP (Gollamudi et al.,
1985).
Liver mitochondrial enzymes and mitochondrial morphology have
been reported to be influenced by DEHP administration (Ohyama, 1977;
Shindo et al., 1978). Recent results suggest that the in vitro
effects of DEHP (> 20 µmol/litre) on mitochondrial functions are
mainly related to the action on membrane lipids surrounding the
adenine nucleotide translocator, which reduces the rate of adenine
nucleotide exchange across the mitochondrial membrane (Kora et al.,
1989).
In male rats given DEHP in the diet, the urinary excretion of
zinc was enhanced and the testicular level of zinc decreased (Gray et
al., 1982; Oishi, 1985) (section 7.5). These changes in zinc
homeostasis could be due to altered levels of metallothionein. In mice
fed 6 or 12 g DEHP/kg diet for 24 weeks, hepatic levels of
metallothionein were increased up to 11-fold (Waalkes & Ward, 1989).
Several studies on rats have shown that DEHP given in the diet
(5-20 g/kg) decreases plasma triglyceride and cholesterol levels
(Yanagita et al., 1978; Sakurai et al., 1978; Bell et al., 1978a; Bell
et al., 1978b; Bell et al., 1979; Yanagita et al., 1979; Curstedt &
Sjövall, 1983). DEHP inhibits the biosynthesis of cholesterol, an
effect which is accompanied by phospholipidosis, and the same effects
have been observed with MEHP (Oishi & Hiraga, 1982).
7.3 Long-term exposure
In a 24-month study by Harris et al. (1956), three groups of
Wistar rats each comprising 43 males and 43 females were fed diets
containing 0, 1, and 5 g DEHP/kg, interim kills being made at 3, 6,
and 12 months. At the end of the study, only two control, four
low-dose, and seven high-dose animals were alive. During the first
year the body weights of the high-dose group were slightly reduced,
but by the second year the body weights of all groups were similar.
During the first 6 months an increase in relative liver and kidney
weights was seen in DEHP-treated animals but later they were similar
in control and treated animals. After 3 months of treatment, one out
of eight rats in the low-dose group was found to have mild renal
tubular atrophy. After 6, 12, and 24 months of treatment, no
compound-related pathological changes were evident. Because of high
mortality due to disease, this study is difficult to validate.
In another 24-month study (Carpenter et al., 1953), groups of
Sherman rats consisting of 32 males and 32 females were given diets
containing 0, 0.4, 1.3 or 4 g DEHP/kg. Owing to reduced life
expectancy due to disease and the small numbers of animals used, the
study was inadequate for assessing the chronic toxicity of DEHP.
In a 12-month study by Nikonorow et al. (1973), a group of 20
male and 20 female Wistar rats was given a diet containing 3.5 g
DEHP/kg, and a control group received the diet without DEHP. The only
gross or micropathological change noted in exposed animals at necropsy
was hepatomegaly. During the study, however, about 30% of the animals
died due to congestion of the small intestine and loss of the gastric
and/or intestinal mucosa, which was complicated by purulent pneumonia
and endometritis.
Crocker et al. (1988) described the renal effects of DEHP given
by gavage to young male rats at a dosage of 2.14 mg/kg body weight
three times per week for up to 12 months. A 50% reduction in
creatinine clearance and an increase in the severity of renal cyst
formation was observed. This lesion was consistent with spontaneous
nephropathy commonly observed in old rats; exposure may cause an onset
in younger rats. Furthermore, DEHP fed at 6 and 12 g/kg diet for two
years did not produce renal lesions in male and female F-344 rats
(NTP, 1982).
In a 2-year study (NTP, 1982; Kluwe et al., 1982), groups of
F-344 rats were given dietary levels of 0, 6, and 12 g DEHP/kg.
Decreased body weight in exposed groups was noted from week 30 until
the cessation of exposure. In addition to neoplastic effects (section
7.7) and testicular atrophy (section 7.5), a compound-related
hypertrophy of cells in the male anterior pituitary was noted in the
high-dose group. In both exposed groups an increased incidence of
clear changes in liver cells was observed. This study also
investigated B6C3F1 mice exposed to 3 or 6 g DEHP/kg diet. A
dose-related decrease of body weight was observed in female mice.
There was no increased incidence of non-neoplastic lesions except for
seminiferous tubular degeneration in the testes of male mice (section
7.5.1).
In a study on groups of male and female guinea-pigs fed diets
containing 0, 0.4 or 1.3 g DEHP/kg for 12 months (Carpenter et al.,
1953), the body weight of the low-dose animals was statistically
higher than that of controls, and liver weight relative to body weight
of dosed females slightly increased. No other exposure-related lesions
were found.
A study on groups of male ferrets fed diets containing 0 and 10
g DEHP/kg for 14 months revealed a reduction in body weight and an
increase in relative liver weight, but no evidence of peroxisome
proliferation (Lake et al., 1976).
7.4 Skin and eye irritation; sensitization
DEHP has been shown to be a weak irritant to mammalian skin when
administered topically or intradermally (0.2 ml of an emulsion of
100 g/litre) (Calley et al., 1966; Woodward et al., 1986).
In a study by Lawrence et al. (1975), no irritation occurred when
undiluted DEHP was instilled into the eye of rabbits.
No data are available on the potential for DEHP to induce skin
sensitization in animals.
7.5 Reproduction, embryotoxicity, and teratogenicity
7.5.1 Reproduction
The effects of DEHP on male reproductive organs have been studied
extensively. The majority of the studies have been carried out on rats
or mice given DEHP in the diet.
Seminiferous tubular atrophy, comprising a loss of spermatids and
spermatocytes, occurred when 4-week-old Wistar rats were given 2800 mg
DEHP/kg by oral intubation for 10 days (Gray & Butterworth, 1980). In
similarly treated 10-week-old rats, about 50% of the tubules were
atrophic and the remainder unaffected. However, no testicular damage
was detected in treated 15-week-old rats. When 20 g DEHP/kg was given
in the diet (approximately 1200 mg DEHP/kg per day) to 4-week-old
rats, the lesions produced were reversible whether treatment stopped
before or continued until after the control rats had reached sexual
maturity.
In rats given 10 or 20 g DEHP/kg diet, the testis atrophy was
dose dependent after approximately 2 weeks of feeding. This atrophy
was accompanied by pituitary changes, i.e. enlargement and
vacuolization of the basophils of the pars distalis, corresponding to
the formation of the so-called castration cells seen after gonadectomy
(Gray et al., 1977). In a subsequent study, there was a reduction in
testicular and prostatic zinc levels concomitant with increased
urinary excretion of zinc (Gray et al., 1982).
In a rat study by Oishi & Hiraga (1980a), the serum testoster-one
levels in rats fed 20 g DEHP/kg diet were reduced by approximately
50%. The total amount per testis decreased, but the concentration rose
to 150% of the original value because of the testicular atrophy.
Simultaneous administration of testosterone or zinc had no protective
effect on the atrophy but did prevent the weight reduction of the sex
organs such as the epididymis (Gray & Butterworth, 1980; Oishi &
Hiraga, 1983). In a further study, it was found that a low-zinc diet
aggravated the DEHP-induced testicular atrophy (Agarwal et al.,
1986a).
DEHP given to 7 young (5 weeks old) Wistar rats in the diet
(20 g/kg) for one week decreased the testicular weight significantly
(P < 0.05), compared to controls, and also the testicular zinc
concentration (Oishi, 1984). In a study by Oishi (1985), groups of 20
male rats were given DEHP (2.0 g/kg per day) by gavage for 14 days.
Ten rats were then killed and the remaining ten were kept for 45 days
on a DEHP-free diet. The histopathological changes of the testes seen
on day 15 were marked shrinkage of the seminiferous tubules, a
germinal epithelium consisting only of Sertoli cells, and very few
spermatogonia. After 45 days the percentage of spermatogenic tubules
had increased from 0 (at day 15) to 12.8%, indicating a limited
reversibility of the testicular atrophy.
Similar degeneration of the seminiferous tubules was observed
when 13-week-old Wistar rats were orally given 2 g DEHP/kg body weight
for 7 consecutive days (Saxena et al., 1985) .
In a dietary study, DEHP was administered to F-344 rats at 6 and
12 g/kg for 103 weeks (NTP, 1982). Degeneration of the seminiferous
tubules was observed only at the higher dose.
The testicular changes induced by oral DEHP administration appear
to be age dependent. A daily dosage of 2.8 g DEHP/kg body weight given
for 10 days caused seminiferous tubular atrophy in 4-week-old Wistar
rats, but this effect was less severe in 10-week-old rats and absent
in 15-week-old rats (Gray & Butterworth, 1980). Sjöberg et al. (1986)
showed that DEHP fed at a level of 1.7 g/kg diet caused reductions in
testis weight to 20%, 55%, and 92% of the control values in
Sprague-Dawley rats at 25, 40, and 60 days of age, respectively. This
was apparently not due to differences in pharmacokinetic parameters,
since the plasma levels of the metabolite MEHP were identical in the
25- and 40-day-old rats (Sjöberg et al., 1982).
Rats given DEHP intraperitoneally at a dosage of 100 mg/kg per
day for 5 days did not develop testicular atrophy (Curto et al.,
1982). However, this lack of response was probably a consequence of
the dose used, rather than the dose route, and there was a 30%
reduction in testicular zinc. Intraperitoneal administration of higher
doses (1-25 g/kg per day for 5 days) to Wistar rats decreased serum
testosterone levels (Oishi & Hiraga, 1979).
Some degenerated primary spermatocytes and altered Sertoli cells
were observed in Sprague-Dawley rats given 3-h intravenous infusions
of an emulsion at a rate of 1 ml/h, which corresponded to a daily dose
of 500 mg DEHP/kg (Sjöberg et al., 1985c). The infusions were given
every other day on six occasions. The emulsion contained DEHP,
fractionated egg yolk phosphatides, glycerol, and water. No effects
were observed when emulsions corresponding to 0, 5 or 50 mg DEHP/kg
were given.
In a study by Curto & Thomas (1982), groups of sexually mature
Swiss-Webster mice were given intraperitoneal injections of 50 or 100
mg DEHP/kg either daily for 5 days or alternate daily for 20 days (10
injections). The animals were killed 24 h after the last injection. No
significant alterations in testicular weight or zinc levels occurred.
Young rats were not more susceptible to testicular damage than
older ones following intravenous infusion of DEHP. It has been
suggested that the age-related difference observed in some studies may
be due to the fact that the rate of gastrointestinal absorption of the
DEHP-derived metabolite MEHP is higher in younger animals than in
older ones (Sjöberg et al., 1986).
In a NTP-sponsored study (Melnick et al., 1987), CD-1 mice given
3 g DEHP/kg diet showed significantly diminished testis and epididymis
weights compared to controls. In addition, the sperm concentration in
the cauda epididymis was reduced and the percentage of abnormal sperm
in the cauda was significantly higher in the treated mice than in the
controls.
A high oral dose (4.2 g/kg) of DEHP gave minimal tubular atrophy
in hamsters but did not produce any effects on urinary zinc excretion,
testicular zinc levels or testicular weights (Gray et al., 1982).
The effects of the monoester MEHP have not been as well studied
as those of DEHP. An oral dosage of 1 g MEHP/kg per day for 5 days
produced a significant decrease in rat testis weight and extensive
testicular atrophy (Gray et al., 1982). On the other hand, rats given
intraperitoneal doses of up to 100 mg MEHP/kg daily for 5 days showed
no abnormal histology (Curto et al., 1982) and an intraperitoneal dose
of 50 mg/kg given on alternate days for 20 days produced only a
reduction in prostatic zinc levels (Curto & Thomas, 1982).
Mice fed diets containing 20 g MEHP/kg for 1 week revealed
markedly reduced testicular zinc and testosterone levels, but there
were no reductions in testicular weight (Oishi & Hiraga, 1980b).
Hamsters given MEHP (1 g/kg per day) for 9 days showed more severe
testicular effects than those given DEHP at a level of 4.2 g/kg per
day for the same period (Gray et al., 1982). More recent studies
(Albro et al., 1989) indicate that testicular atrophy resulting from
DEHP exposure is most probably due to the formation of MEHP.
These studies indicate that the rat is the species most
susceptible to DEHP-induced testicular atrophy. The mechanism of
phthalate-induced testicular damage is not fully understood.
Testicular zinc depletion has been suggested to be a primary event
(Foster et al., 1982, 1983). However, Gray et al. (1982) showed that
an effect on zinc level is not always associated with testicular
atrophy in all species tested. Zinc is essential for normal testicular
function and its depletion is known to lead to testicular atrophy
(Barney et al., 1969). Inhibition of dehydrogenase enzymes, e.g.,
those controlling the biosynthesis of testosterone, leads to reduced
testosterone levels. DEHP administration has been shown to reduce the
level of serum testosterone in the rat (Oishi & Hiraga, 1979; Oishi &
Hiraga, 1980a) as well as in the mouse (Gray et al., 1982), although
in the mouse no testicular atrophy was observed. Administration of
testosterone or zinc did not prevent the testicular damage induced by
DEHP (Gray & Butterworth, 1980; Oishi & Hiraga, 1983).
Co-administration of DEHP and testosterone, on the other hand,
apparently enhanced the testicular damage caused by DEHP (Oishi,
1989a). In a similar study (Oishi, 1989b), luteinizing
hormone-releasing hormone (LRH) significantly decreased testis weight,
sulfhydryl content, and lactate dehydrogenase when given together with
DEHP, whereas LRH or DEHP alone had no effects.
Similar effects were observed with exogenously added follicle
stimulating hormone (FSH) in an in vitro study. Primary testicular
cell cultures pretreated with MEHP showed a dose-related reduction in
FSH-stimulated cyclic adenosine monophosphate (cAMP) production (Lloyd
& Foster, 1988). These results suggest that MEHP produces a
perturbation at the level of the FSH membrane receptor, causing an
inhibition of FSH action.
In vitro studies have indicated that the Sertoli cell is the
target cell. Mixed cultures of Sertoli and germ cells prepared from
rat testes were exposed to DEHP or MEHP (10-7-10-4 mol/litre)
(Gray & Beamand, 1984; Gray & Gangolli, 1986). DEHP had no effect, but
MEHP caused a dose-dependent increase in the rate of germ cell
detachment from Sertoli cells, accompanied by changes in Sertoli cell
morphology. More recent data indicate that Sertoli cell mitochondria
are a target for MEHP (Chapin et al., 1988). However, it has also
been shown that testicular mitochondrial respiratory functions are
decreased in rats given 2 g DEHP/kg body weight by gavage (Oishi,
1990).
In a study by Melnick et al. (1987), CD-1 mice were given 0, 0.1,
1 or 3 g DEHP/kg diet during a 7-day pre-mating period and a
subsequent 98-day cohabitation period. There was complete suppression
of fertility in the 3-g/kg group and a significant reduction in
fertility in the 1-g/kg group, compared to controls, but no effect on
fertility at 0.1 g/kg.
In a fertility study designed to investigate earlier significant
results (Singh et al., 1972), groups of male ICR mice were dosed
subcutaneously with undiluted DEHP at levels of 1, 2, 5, and 10 ml/kg
(equivalent to 0.99, 1.97, 4.93, and 9.86 g/kg) on days 1, 5, and 10
of the study (Agarwal et al., 1985a). They were subsequently mated
with virgin females on days 2, 6, 11, 16 and 21, and then at weekly
intervals until week 8. There were reductions in the incidence of
pregnancies in several groups, but these were dose related only at the
first mating (i.e. on day 2 of the experiment). Examination of
pregnant mice on day 13 of gestation showed that there were
pre-implantation losses corresponding to a DEHP dose level of 10 ml/kg
and a mating on day 6. There were consistent increases in early fetal
deaths relating to matings at day 21, week 4, and week 5. In a similar
study, male and female mice were treated subcutaneously with 1-100 ml
DEHP/kg, and this led to a reduction in testicular but not ovarian
weight. The ovaries exhibited histological injury at lower doses of
DEHP than the testes. Unlike the situation in the testes, there was no
significant dose-related increase in histopathological changes in the
ovaries (Agarwal et al., 1989).
In an investigation of the effects of phthalates on female
reproductive organs, three doses of 4.93 g DEHP/kg were given
intraperitoneally at 5-day intervals to female rats (Seth et al.,
1976). No histopathological changes in the ovaries were seen 22 days
after the first injection, but reductions in the activities of some
enzymes were noted.
The administration of DEHP (1000 mg/kg intraperitoneal or 2000
mg/kg oral) to marmosets daily for 14 days did not lead to testicular
atrophy (Rhodes et al., 1986).
Hence, it appears that rats and guinea-pigs are sensitive to
DEHP-induced testicular atrophy, while mice are fairly resistant and
hamsters and marmosets are highly resistant. The fact that at least
some of the effects associated with this atrophy can be produced
in vitro argues against a hormonally mediated indirect effect. The
earliest effects are seen in Sertoli cells and are described as
vacuolation (Albro, 1987).
7.5.2 Embryotoxicity and teratogenicity
In a study by Nikonorow et al. (1973) on female Wistar rats given
0.34 or 1.7 g DEHP/kg by gavage during the first 21 days of gestation,
the only untoward effect was a reduction in fetal body weight. When
DEHP (0, 5, 10, 15 or 20 g/kg) was administered orally to Fischer-344
rats on gestational days 0 to 20, maternal toxicity and reduced fetal
body weight per litter were observed at the three highest dose levels.
The number of fetuses per litter was unaffected by the treatment (Tyl
et al., 1988).
Intraperitoneal injections of 4.93 or 9.86 g DEHP/kg on days 5,
10, and 15 of gestation resulted in an increase in the number of
resorptions and reduced fetal weight in Sprague-Dawley rats (Singh et
al., 1972). In the highest-dose group, gross abnormalities, such as
twisted hind legs and anophthalmia, were noted but no skeletal defects
were observed.
Rat plasma soluble extracts of two PVC plastics containing DEHP
were administered intravenously to groups of pregnant Sprague-Dawley
rats daily from the 6th to the 15th day of gestation, and the animals
were killed at day 20 (Lewandowski et al., 1980). The daily doses of
DEHP were equivalent to 1.3 mg/kg and 5.2 mg/kg. No significant
teratogenic or embryotoxic effects were noted.
Groups of ICR mice were given DEHP in the diet at levels of 0.5
to 10 g/kg for the first 18 days of gestation and were then sacrificed
(Shiota et al., 1980; Shiota & Nishimura, 1982). The food intake was
an average of 7 g/day. At the 4 g/kg and 10 g/kg dose levels, no live
fetuses were found. At 2 g/kg, 40% of the fetuses had malformations
including exencephaly, spina bifida, and malformed tail. Delayed
ossification was seen in about 15% of the fetuses at 1 g/kg and
2 g/kg.
In a study by Tyl et al. (1988), DEHP was administered in the
diet to CD-1 mice on the first 17 days of gestation at levels of 0,
0.25, 0.5, 1, and 1.5 g/kg. At the two highest dose levels maternal
toxicity, increased resorptions and late fetal deaths, decreased
numbers of live fetuses, and reduced fetal body weight per litter were
observed. The number and percentage of malformed fetuses per litter
were elevated at the three highest dose levels.
A single oral administration of DEHP (0.1 ml/kg) on day 7 of
gestation to ddY-strain SPF (specific pathogen free) mice decreased
the number and the body weights of live fetuses (Tomita et al.,
1982b). In a study by Yagi et al. (1980), DEHP was given orally to SPF
mice on days 6, 7, 8, 9 or 10 of gestation. When 5.0 or 10.0 ml/kg was
given on day 7 there were no live fetuses, whereas 2.5 ml/kg
administered on the same day resulted in 14% live embryos and 1.0
ml/kg gave 40% live embryos. The percentages of live embryos when 10.0
ml/kg was given on day 8, 9 or 10 of gestation were 18, 92, and 95%,
respectively. Gross and skeletal abnormalities occurred in fetuses
given 2.5 and 7.5 ml/kg on day 7 or 8. The abnormalities included
exencephaly, open eyelid, and club-foot.
In a study by Shiota & Mima (1985), groups of ICR mice were given
DEHP by stomach intubation on days 7, 8, and 9 of gestation. The DEHP
doses (in olive oil) were 250, 500, 1000, and 2000 mg/kg, and the mice
were sacrificed on day 18. In the two highest dose groups, the numbers
of resorptions and malformed fetuses were significantly increased.
Fetal weights were significantly depressed. The most frequent
malformations were anencephaly and exencephaly. When doses of up to
8000 mg/kg were given by intraperitoneal injections on days 7,8, and
9 of gestation, no effects were noted. DEHP is highly embryotoxic and
terato-genic in mice when given orally but not when given
intraperitoneally.
The monoester MEHP, at oral doses of 225, 450, and 900 mg/kg per
day, produced significant signs of maternal toxicity when given to
pregnant Wistar rats on days 6-15 of gestation (Ruddick et al., 1981).
In the highest dose group a 73% mortality was observed in the dams.
There was a dose-related decrease in the number of litters and in the
litter weights of live pups.
Oral dosing with 0.1 or 1.0 g MEHP/kg on day 7 of gestation led
to increased incidence of early embryonic deaths in SPF mice (ddY
strain), but dosing on day 8 or 9 had less effect (Yagi et al., 1980;
Tomita et al., 1986). The fetuses had reduced body weight, and there
was a higher incidence of gross abnormalities, compared to controls,
when the higher dose level was given on day 8 or 9. The mice dosed on
day 8 produced fetuses with a high incidence of skeletal effects.
Intravenous injections of MEHP (11.38 mg/kg) to rabbits on days
6-17 of gestation gave a high incidence of resorptions (Thomas et al.,
1979). The incidence of fetal anomalies was similar to that in
controls.
When Nikonorow et al. (1973) administered DEHP (0.34 or 1.7 g/kg
per day) to female Wistar rats by gavage for 3 months prior to mating,
there was an increase in the number of resorptions but no effects on
fetal weights or the incidence of skeletal anomalies.
In utero administration of DEHP (1000 mg/kg body weight) to
rats daily from days 6 to 15 of gestation resulted in retardation of
fetal growth and an increase in fetal liver weight. There were
significant quantities of DEHP and decreased mitochondrial enzyme
activities in the fetal liver. These results indicate an effect on
fetal liver cell bioenergetics (Srivastava et al., 1989).
The mechanism of DEHP or MEHP teratogenicity is not known.
Teratogenic activity could result from zinc deficiency, which is known
to produce such effects (Swenerton & Hurley, 1971).
7.6 Mutagenicity and related end-points
The possible genotoxic effect of DEHP has been thoroughly
investigated in several different short-term tests. The effects of the
major metabolites of DEHP, i.e. MEHP and 2-ethylhexanol, as well as
phthalic acid and phthalic anhydride, have also been studied.
7.6.1 Mutation
Studies on the possible mutagenic effect of DEHP have been
performed in bacteria, fungi, and in cultured mammalian cells.
Drosophila melanogaster has also been used and results from a few
in vivo studies on mice have been reported.
7.6.1.1 Bacteria
Many studies have been performed using a variety of strains of
Salmonella typhimurium and DEHP doses of up to 10 mg/plate.
Incubations both with and without exogenous activation systems have
been performed. S9 mix from rats induced by Aroclor 1254 has
frequently been used, but other species and other inducers have also
been used to produce metabolic activation systems. With one exception
(Tomita et al., 1982a) these test results have all been negative
(Kirby et al., 1983; Yoshikawa et al., 1983; Zeiger et al., 1985;
Agarwal et al., 1985b), and in a IPCS collaborative study (Ashby et
al., 1985) all five laboratories reported negative results. Bacteria
other than S. typhimurium have also been used; negative results were
obtained with E. coli WP2 at doses of up to 2 mg per plate (Yoshikawa
et al., 1983).
The major metabolites of DEHP have also been tested for mutagenic
activity in bacteria. Concentrations of up to 3.333 mg per plate for
MEHP and phthalic anhydride (Zeiger et al., 1985) and 2 mg/plate for
2-ethylhexanol and phthalic acid (Agarwal et al., 1985b) have yielded
negative results in strains of Salmonella (see also Kirby et al.,
1983; Yoshikawa et al., 1983). However, Tomita et al. (1982a) reported
a significant increase in TA100 revertants following exposure to
either DEHP or MEHP (both with and without S9). It should be noted,
however, that MEHP mutagenicity is only demonstrable within a narrow
range of concentration, because it has a sterilizing effect at high
concentration and shows no mutagenic activity at low concentration.
Tomita et al. (1982a) also detected dose-dependent (0.4 and 0.5 mg
MEHP/plate) DNA damage in a B. subtilis Rec assay, while DEHP,
phthalic acid, and 2-ethylhexanol were all negative. In this study
MEHP also gave a positive result in E. coli WP2 b/r.
Negative results were obtained when pooled urine from rats,
treated with 2000 mg DEHP/kg per day for 15 days, was tested for
genotoxic activity. A direct plating procedure was used with
S. typhimurium strains TA98, TA100, TA1535, TA1537, and TA1538, both
with and without S9 and ß-glucuronidase/aryl sulfatase as the
activation system. When 2-ethylhexanol was tested according to the
same protocol, the result was also negative (Divincenzo et al., 1985).
7.6.1.2 Fungi
The induction of mutations by DEHP has been studied in various
fungal species. In the IPCS collaborative study on in vitro assay
systems (Ashby et al., 1985), DEHP was considered to be negative in
six out of seven assays. Positive results were obtained with
Saccharomyces cerevisiae, both with and without S9 activation, at
lowest effective concentrations of 1541 mg/litre and 3081 mg/litre,
respectively. However, other laboratories using different strains of
S. cerevisiae or Schizosaccharomyces pombe reported negative
results at a maximum tested concentration of 5000 mg/litre.
7.6.1.3 Mammalian cells
Mouse lymphoma cells (L5178Y), Chinese hamster V79 cells, and
human lymphoblasts have been used to study the mutagenic effect of
DEHP in cultured mammalian cells. Several investigators obtained
negative results, but a few positive results have also been reported.
In the IPCS collaborative study (Ashby et al., 1985), only one
out of ten investigators reported a positive response. Mouse lymphoma
cells were exposed to DEHP without S9, and two concentrations (7.5 and
20 mg/litre) gave positive results. In a separate study (Kirby et al.,
1983), where MEHP, 2-ethylhexanol, and DEHP were tested in the mouse
lymphoma cell assay, all three substances were found to be
non-mutagenic. The concentrations used were 0.016-1.0 ml/litre
(without S9) and 0.067-5.0 ml/litre (with S9) for DEHP, and 0.013-1.0
ml/litre for MEHP and 2-ethylhexanol.
7.6.1.4 Drosophila
DEHP has also been tested for mutagenicity in Drosophila
melanogaster using the sex-linked recessive lethal (SLRL) test and
various somatic recombination and mutation assays. SLRLs were not
induced by DEHP (20 µg/g) administered by injection (Yoon et al.,
1985). Negative results for DEHP were also reported in the SLRL
mutation assay on Drosophila melanogaster larvae (Zimmering et al.,
1989).
In the IPCS study (Ashby et al., 1985), DEHP gave a positive
response in the unstable eye mosaic test at a dose of 6.1 g/litre, in
two separate experiments, but neither lower nor higher concentrations
produced any response. No activity was seen in the wing spot test with
a single dose of 6.1 g/litre. It was concluded that DEHP exhibits
marginally positive mutagenicity (Ashby et al., 1985).
7.6.2 DNA damage
Various end-points, such as unscheduled DNA synthesis (UDS) and
single strand breaks, have been used to detect DNA damage induced by
DEHP in a variety of mammalian test systems. In the IPCS study (Ashby
et al., 1985), negative results were obtained when single strand
breaks were measured, either by alkaline elution in hepatocytes (up to
3.907 g/litre) or alkaline sucrose sedimentation in Chinese hamster
ovary (CHO) cells (up to 39 g/litre). UDS in either isolated
hepatocytes or cultured HeLa cells was investigated by four different
laboratories. One investigator detected a positive response using
isolated hepatocytes, but, since this result was only statistically
significant at one dose and not dose related, the consensus was that
DEHP does not cause UDS.
In a study by Butterworth et al. (1984), DEHP did not induce DNA
repair in primary rat hepatocytes. Similarly, neither DEHP nor MEHP
induced any DNA repair in primary human hepatocytes from three
different subjects. In this study, concentrations as high as 0.01 mol
DEHP/litre and 5 x 10-4 mol MEHP/litre were used and exposure
continued for 18 h. No induction of DNA repair or increased alkaline
elution of DNA was seen in hepatocytes from either female or male
F-344 rats treated with DEHP in vivo. UDS was not induced in male
rats treated with 500 mg DEHP/kg by gavage 2, 12, 24 or 48 h before
sacrifice. Furthermore, treatment of male rats with 150 mg/kg per day
by gavage for 14 days and treatment of female rats with dietary DEHP
(12 g/kg) for 30 days resulted in peroxisome proliferation, but no UDS
was induced.
Similar in vivo/in vitro results in studies of UDS in
hepatocytes were reported by Kornbrust et al. (1984). No UDS was
observed in primary rat hepatocytes exposed in vitro to 10-5 to
10-2 mol DEHP/litre or in vivo by a single gavage dose of 5 g/kg
2, 15 or 24 h prior to the isolation of hepatocytes. A dietary
concentration of 20 g DEHP/kg led to a marked proliferation of
peroxisomes after 4 weeks. Neither this treatment nor the additional
administration of a single gavage dose of 5 g/kg (15 h before
sacrifice) to animals fed the 20-g/kg diet for either 8 weeks or for
4 weeks with or without pretreatment with 3-amino-1,2,4-triazole (to
inhibit endogenous catalase activity) induced any detectable DNA
repair in hepatocytes. Indeed, the administration of DEHP and some
other proliferators has been shown to increase the levels of
8-hydroxy-deoxyguanosine in rat liver DNA (Takagi et al., 1990).
7.6.3 DNA binding
In an in vivo study by Albro et al. (1982), radioactivity from
carbonyl-labelled DEHP did not associate with purified protein, RNA or
DNA from rat liver. Label from 2-ethyl-(1-14C)-hexyl-labelled DEHP
or MEHP appeared to associate strongly with purified DNA, but this was
not the case with label from free 14C-labelled 2-ethylhexanol.
According to Albro (1987), although there is incorporation into normal
nucleosides, there is no evidence of alkylation.
In a similar study (von Däniken et al., 1984; Lutz, 1986), DEHP
radiolabelled in different positions was administered orally to female
F-344 rats with or without pretreatment with unlabelled DEHP (10 g/kg
diet) for 4 weeks. The administration of 14C-carboxylate-labelled
DEHP resulted in no measurable DNA radioactivity, whereas
radioactivity was clearly measurable after the administration of DEHP,
14C- or 3H-labelled in the alcohol moiety, or of
2-ethyl(1-14C)hexanol. HPLC analysis showed that the normal
nucleosides had incorporated radiolabel, but fractions expected to
contain carcinogen-modified nucleoside adducts did not contain any
radioactivity.
DNA isolated from the livers of male F-344 rats administered 2000
mg DEHP/kg daily by gavage for 3 days was analysed for possible
carcinogen-DNA adducts by the 32P-postlabelling technique (Gupta et
al., 1985). No adducts were detected in the DNA, which also was the
case when DNA from hepatocytes exposed to 10-3 mol DEHP/litre
in vitro for 4 h was analysed.
7.6.4 Chromosomal effects
Chromosomal effects of DEHP have mainly been studied in vitro,
although some studies on the induction of micronuclei in the
peripheral blood erythrocytes of mice have been published.
DEHP did not induce any increase in the level of sister-chromatid
exchange (SCE) in Chinese hamster ovary (CHO) cells, treated for 1 h,
either with or without S9, at levels of up to 0.01 mol/litre (Douglas
et al., 1986). On the other hand, MEHP has been reported to induce SCE
in V79 cells treated with 25 or 50 mg/litre for 24 h or 1500 mg/litre
for 3 h (Tomita et al., 1982a). This metabolite also induced
chromosomal aberrations in CHO cells and RL4 cells (from rat liver),
but only at cytotoxic concentrations in the CHO cells (1.0 and 1.3
mmol/litre, with or without S9). MEHP was less toxic to RL4 cells
and concentrations between 2.0 and 5.0 mmol/litre gave a dose-related
increase in chromosomal aberrations (Phillips et al., 1986). According
to the authors, observations on changes in CHO cell structure and
permeability and on the haemolytic effects of phthalate monoesters
suggest that MEHP cytotoxicity may be due primarily to an action on
cell membranes.
The induction of aneuploidy by DEHP was investigated both in
mammalian cells and in fungi in the IPCS study (Ashby et al., 1985).
The mammalian assays gave positive responses at 50 mg/litre in a
fibroblast cell line from Chinese hamster liver and at levels of
between 25 and 50 mg/litre in Chinese hamster primary liver cells. Two
out of four studies using fungi were also positive and the consensus
was that DEHP is capable of inducing aneuploidy in vitro in both
fungi and mammalian cells.
In a study by Ahmed et al. (1990), male Wistar rats were fed for
alternate 7-day periods with a diet containing 20 g DEHP/kg and a
control diet. The rats were examined after 3 days on the DEHP diet or
after 7 days on the control diet. An analysis of nuclear size gave
results consistent with an increase in tetraploid hepatocytes after
treatment with DEHP, which was reversed when the rats returned to the
control diet.
7.6.5 Cell transformation
DEHP-induced cellular transformation has been studied in several
different experimental systems. In a test programme (Astill et al.,
1986), the BALB/3T3 cell transformation assay was used both with and
without rat primary hepatocytes. Both DEHP (0.875-1 µl/litre) and the
two metabolites MEHP and 2-ethylhexanol gave negative results. On the
other hand, the majority of transformation tests in the IPCS study
(Ashby et al., 1985) were positive for DEHP. Negative results were
obtained with BALB/c-3T3 cells, while a study measuring the
enhancement of viral transformation of Syrian hamster embryo (SHE)
cells was considered to be inconclusive. Positive responses were
obtained by four other investigators using SHE cells at 1-300 mg/litre
(two different laboratories), embryonic mouse fibroblasts at 1000
mg/litre with S9 and 10 mg/litre without S9, or retrovirus-infected
Fischer rat embryo cells at 2000 mg/litre (the highest dose tested).
In a separate study (Tomita et al., 1982a), both DEHP (7.5 and 15
g/kg) and MEHP (375 and 750 mg/kg) induced morphological
transformation, as well as chromosomal aberrations, in SHE cells after
transplacental administration.
In a study by Diwan et al. (1985), anchorage-independent growth
of JB6 mouse epidermal cells was enhanced by DEHP at concentrations of
500 to 20 000 ppm/ml (the Task Group noted that the unit used in
Diwan's paper is ppm/ml, but it should read ppm or mg/litre). Ward et
al. (1986) used the same model system and reported that both DEHP
(1.3-51 x 10-6 mol/litre) and MEHP (2-5 x 10-8 mol/litre) were
effective, while 2-ethylhexanol (4-77 x 10-7 mol/litre) was without
effect. The morphological transformation in the SHE cell system
induced by DEHP, MEHP, and other hepatic peroxisome proliferators
seemed not to be correlated with increased peroxisomal ß-oxidation,
increased production of oxidative radicals or peroxisome proliferation
(Mikalsen et al., 1990a; Mikalsen et al., 1990b; Mikalsen et al.,
1990c).
A few studies on DEHP-induced inhibition of metabolic
cooperation, which may be indicative of the promoting potential of a
substance, have been reported. Metabolic cooperation in Chinese
hamster V79 cells was not inhibited by DEHP at non-cytotoxic
concentrations, i.e. 3 x 10-4 mol/litre (0.12 mg/litre) or less
(Kornbrust et al., 1984). In the IPCS study (Ashby et al., 1985), one
investigator reported inhibition in V79 cells at non-cytotoxic
concentrations of DEHP (25-200 mg/litre in two separate experiments
and 5-25 mg/litre in another), while another investigator, using V79
cells in a microassay method, detected a slight (but non-significant)
increased inhibition at doses of between 10-5 and 2 x 10-4
mol/litre (3.9-78 mg/litre).
In a study by Malcolm & Mills (1989) on V79 cells, DEHP inhibited
intercellular communication (gap junctions) at non-cytotoxic
concentrations (10-30 mg/litre).
7.6.6 In vivo effects
Putman et al. (1982) dosed male Fischer-344 rats orally for 5
days with DEHP (5.0, 1.7, and 0.5 g/kg per day), MEHP (0.14, 0.05, and
0.01 g/kg per day), and 2-ethylhexyl (0.21, 0.07, and 0.02 g/kg per
day), and bone marrow metaphase cells were examined. No significant
increases were observed in gap breaks or structural rearrangements. In
addition, the mitotic index was unaffected by treatment.
A micronucleus assay on mouse (B6C3F1) peripheral blood
erythrocytes (intraperitoneal doses of 0.6, 3.0, and 6.0 g/kg per day
for 5 days), sampled at 0, 2, and 4 weeks after the last treatment,
yielded negative results (Douglas et al., 1986). A sperm morphology
assay in B6C3F1 mice and Sprague-Dawley rats at the same dose levels
also gave negative results (Douglas et al., 1986). However, Agarwal
et al. (1986b) reported increases in sperm morphology changes in adult
F-344 rats given 20 g DEHP/kg for 60 consecutive days.
Two studies have yielded negative results in dominant lethal
tests (Hamano et al., 1979; Rushbrook et al., 1982). Hamano et al.
(1979) administered MEHP and DEHP orally, and the observation period
was six weeks. Rushbrook et al. (1982) dosed ICR/SRM mice orally for
5 days with DEHP (2465, 4930, and 9860 mg/kg per day), MEHP (50, 100,
and 200 mg/kg per day) or 2-ethylhexanol (250, 500, and 1000 mg/kg per
day). Each male was mated weekly with virgin females for 8 consecutive
weeks, and females were evaluated for pregnancy, live fetuses, and
early and late fetal deaths. All data were within the normal ranges.
DEHP and its major metabolites have also been tested for their
potential to induce micronuclei. Exposure to 0.6, 3.0 or 6.0 g DEHP/kg
per day for 5 days over a 4-week period did not induce micronuclei in
the peripheral blood erythrocytes of B6C3F1 male mice (Douglas et
al., 1986). Negative results were also obtained in another mouse
micronucleus test after either a single or daily doses of 5 g DEHP/kg.
In this study MEHP and 2-ethylhexanol also gave negative results
(Astill et al., 1986).
7.7 Carcinogenicity
In a carcinogenicity study (NTP, 1982; Kluwe et al., 1982),
groups of 50 male and 50 female Fischer-344 rats were fed diets
containing 6 or 12 g DEHP/kg diet, and 50 male and 50 female B6C3F1
mice were fed diets containing 3 or 6 g DEHP/kg for 103 consecutive
weeks. Concurrent controls (50 of each sex and species) were fed a
diet without the addition of DEHP. Food and water were supplied
ad libitum. All animals were given the control diet for 1-2 weeks
after 103 weeks of treatment and were then killed and examined both
grossly and microscopically. The administered concentrations of DEHP
were estimated to be equal to, or one half of, the maximum tolerated
doses. Under the conditions studied, DEHP caused an increased
incidence of hepatocellular carcinomas in female rats and male and
female mice, and an increased incidence of hepatocellular carcinomas
and neoplastic nodules in female rats (Table 8). Twenty of the 57
hepatocellular carcinomas in the DEHP-treated mice (sexes and doses
combined) had metastasized to the lung. The figure of nine
hepatocellular carcinomas in control male mice is considered to be
within the normal range (NTP, 1982).
The reported decreased incidence of tumours of the thyroid,
pituitary, and testis could be related to an increased endocrine
activity of the pituitary gland (NTP, 1982).
The carcinogenicity of DEHP in rats has been confirmed in two
further studies. Rao et al. (1990) found a 78.5% incidence in a group
of 14 male Fischer-344 rats fed a diet containing 20 g DEHP/kg for up
to 108 weeks, whereas the incidence in the 10 controls was 10%. Popp
et al. (1987) found either hepatocellular carcinomas or neoplastic
nodules in 6 out of 20 animals after female Fischer-344 rats were
exposed for 2 years to a diet containing 12 g/kg.
Table 8. Carcinogenic effects of DEHP on the livera
Control Low dose High dose P valueb
Hepatocellular carcinoma
Male rats 1/50 1/49 5/49 < 0.05
Female rats 0/50 2/49 8/50 < 0.005
Male mice 9/50 14/48 19/50 < 0.05
Female mice 0/50 7/50 17/50 < 0.0001
Neoplastic nodules
Male rats 2/50 5/49 7/49 n.s.
Female rats 0/50 4/49 5/50 < 0.05
Hepatocellular adenoma
Male mice 6/50 11/48 10/50 n.s
Female mice 1/50 5/50 1/50 n.s
a From: NTP (1982)
b Probability level in Codiran-Armitage test for linear trend when
P < 0.05; otherwise not significant (n.s.)
Two other long-term studies on DEHP have been performed by
Carpenter et al. (1953) and Harris et al. (1956), but, due to the
small numbers of animals used, the studies are inadequate to assess
the carcinogenic potential.
DEHP has been investigated in two life-time studies on Syrian
hamsters (Schmezer et al., 1988). In one study, groups of 25 male and
25 female 6-week-old hamsters were assigned to each of six groups:
untreated control; 3 g DEHP/kg body weight given intraperitoneally
once every week for 18 weeks; the same dose once every two weeks for
18 weeks; the same dose once every four weeks for 32 weeks; the same
dose once every four weeks for 32 weeks plus N-dimethylnitrosamine
(NDMA) at 1.67 mg/kg body weight given orally once per week; and the
same dose of NDMA without DEHP treatment. NDMA increased the tumour
rate for malignant liver tumours, mainly haemangioendotheliomas.
Co-administration of DEHP with NDMA neither increased nor decreased
the tumour rates. No significant differences of tumour rates were
observed in groups treated with DEHP alone compared with controls.
Schmezer et al. (1988) also investigated the life-time, whole-
body exposure of Syrian hamsters to DEHP vapour alone in air or in
combination with orally administered NDMA. The low doses of DEHP
(15 µg/m3, resulting in a total exposure of about 7.5 µg/kg body
weight) in this study render it inadequate for the assessment of the
long-term effects of DEHP alone. In combination with NDMA, this low
level of DEHP was associated with highly significant (P < 0.001)
decreases in hepatic haemangioendothe-lioma and fibrosarcomas combined
in males and females combined. This unexpected result should be
further investigated to evaluate its significance.
7.8 Special studies
Since DEHP lacks genotoxic activity in most test systems, it has
been suggested that the carcinogenic effect is exerted during the
promotion phase of hepatocarcinogenicity. DEHP has therefore been
tested in several initiation/promotion experiments in rats and mice
where the end-point has been the number and/or volume of altered liver
cell foci. As expected, DEHP lacked initiating activity in these
studies (Ward et al., 1986; Popp et al., 1987).
DEHP appears to be a tumour promotor in mouse liver. In male
B6C3F1 mice given an intraperitoneal initiating dose of
diethylnitrosamine (80 mg/kg), DEHP at levels of 6 and 12 g/kg diet
caused accelerated growth of foci and increased incidence of
hepatocellular adenomas (Ward et al., 1983; Ward et al., 1986).
However, in a 6-month study, DEHP at a level of 12 g/kg diet did not
appear to promote altered foci in male F-344 rats given an
intraperitoneal initiating dose of diethylnitrosamine of 150 mg/kg
(Popp et al., 1987). In other promotion studies DEHP appeared to
accelerate the regression or inhibit the appearance of some kinds of
foci in rats (DeAngelo & Garrett, 1983; DeAngelo et al., 1984).
However, other studies have shown that peroxisome proliferators can
promote the development of altered foci and tumours in rat liver.
These results are somewhat different from those of other liver tumour
promotors with respect to the histological phenotype of the foci and
the relatively low frequency of the foci (Cattley & Popp, 1989). Thus,
further studies are required to demonstrate that DEHP can promote
altered foci in rat liver.
7.9 Mechanisms of hepatotoxicity
Together with a number of structurally diverse chemicals,
including certain hypolipidaemic drugs, herbicides, and chlorinated
solvents, DEHP has been shown to produce hepatic peroxisome
proliferation in rats and mice (Reddy & Lalwani, 1983; Lock et al.,
1989). This proliferation is accompanied by liver enlargement,
stimulation of replicative DNA synthesis and cell division, and the
induction of peroxisomal and microsomal fatty acid oxidizing enzyme
activities (Reddy & Lalwani, 1983; Marsman et al., 1988; Lock et al.,
1989). The increase in microsomal fatty acid oxidation is due to the
induction of a cytochrome P-450 IVA1 (Lock et al., 1989). Peroxisome
proliferation and induction of peroxisomal and microsomal fatty acid
oxidizing enzyme activities can be observed in primary hepatocyte
in vitro cultures, as well as in the intact animal (Elcombe &
Mitchell, 1986; Lake et al., 1986). Both in vivo and in vitro
studies with rat hepatocytes have demonstrated wide compound potency
differences (Reddy et al., 1986; Barber et al., 1987; Lake et al.,
1988). Compared to some other compounds, DEHP is not considered to be
a particularly potent peroxisome proliferator in rodent liver (Reddy
et al., 1986; Barber et al., 1987). Possible mechanisms of peroxisome
proliferation include a perturbation of hepatic lipid metabolism
and/or the presence of a receptor protein (Lalwani et al., 1987; Lock
et al., 1989; Issemann & Green, 1990).
Certain peroxisome proliferators, including DEHP, have been shown
to increase the incidence of liver tumours in rats and mice (Reddy &
Lalwani, 1983; Reddy et al., 1986; Butterworth et al., 1987).
Generally, there is a good correlation between the potency of a
compound to produce peroxisome proliferation in rat and mouse liver
and its hepatocarcinogenic properties (Reddy et al., 1986). However,
the magnitude of peroxisome induction does not necessarily always
correlate with the carcinogenic response when different compounds are
compared (Marsman et al., 1988). Since the peroxisome proliferators
known to date are non-genotoxic carcinogens, Reddy and coworkers
(Reddy & Lalwani, 1983; Rao & Reddy, 1987; Reddy & Rao, 1989) have
suggested that liver tumour formation arises from a sustained
oxidative stress to the hepatocytes due to an imbalance in the
production and degradation of hydrogen peroxide (H2O2). DEHP
administration results in increased peroxisomal production of H2O2
in hepatocytes (Tomaszewski et al., 1986). There is an imbalance in
H2O2 production and degradation due to the fact that catalase is
induced to a much lesser extent than peroxisomal ß-oxidation enzymes.
In addition, the level of cytosolic glutathione peroxidase is reduced
on prolonged exposure to DEHP (Lake et al., 1987; Marsman et al.,
1988; Conway et al., 1989b; Tamura et al., 1990). Treatment with
peroxisome proliferators also results in a reduction of superoxide
dismutase and glutathione S-transferase activities (Ciriolo et al.,
1982; Goel et al., 1986; Lake et al., 1987). It has been suggested
that the increased level of H2O2 in hepatocytes, either directly or
via other reactive oxygen species (e.g., hydroxyl radical), damages
intracellular membranes and/or DNA (Reddy & Rao, 1989). Indeed chronic
administration of DEHP and other peroxisome proliferators results in
increased lipid peroxidation and lipofuscin deposition in rat
hepatocytes (Mitchell et al., 1985b; Goel et al., 1986; Cattley et
al., 1987; Lake et al., 1987; Reddy & Rao, 1989; Conway et al.,
1989b). Oxygen radicals may damage DNA (Bridges, 1985), leading to
increased levels of 8-hydroxydeoxy-guanosine and other modified bases
in DNA (Reddy & Rao, 1989). Indeed, the administration of DEHP and
some other peroxisome proliferators has been shown to increase levels
of 8-hydroxyde-oxyguanosine in rat liver DNA (Kasai et al., 1989;
Takagi et al., 1989, 1990, 1991). Apart from a role in peroxisome
proliferation, leading to oxidative stress, recent studies on DEHP
have demonstrated a role in the stimulation of replicative DNA
synthesis in the hepatocarcinogenicity of peroxisome proliferators
(Butterworth et al., 1987; Smith-Oliver & Butterworth, 1987; Marsman
et al., 1988). Increased cellular division may result in spontaneous
mutational events or promotional effects (Bridges, 1985; Butterworth
et al., 1987), including the promotion of spontaneously initiated
cells found in the livers of aging rodents (Schulte-Hermann et al.,
1989). A comparative study of the hepatic effects of Wy-14643
([4-chloro-6 (2,3-xylidino) 2-pyrimidinylthio]acetic acid), a potent
peroxisome proliferator, and DEHP indicated that, although both
compounds produced a similar induction of peroxisome proliferation at
the dose levels administered, only Wy-14643 produced liver tumours
within one year (Marsman et al., 1988; Conway et al., 1989b). However,
analysis of these data demonstrates that Wy-14643 produces both a more
marked increase in lipofuscin deposition (presumably reflecting
oxidative stress) and a sustained stimulation of replicative DNA
synthesis. These data suggest a role for both oxidative stress and
increased cell replication in the hepatocarcinogenicity of DEHP and
other peroxisome proliferators.
The mechanism of hepatocarcinogenicity induced by peroxisome
proliferators could include both enhanced cell replication and DNA
damage induced by oxygen radicals. Thus, at dose levels that do not
produce either significant peroxisome proliferation or cell
replication, it is unlikely that DEHP or other peroxisome
proliferators would elicit hepatic tumour formation. In this context
data on threshold values for liver effects in sensitive species (e.g.,
the rat and mouse), together with an assessment of species differences
in response (see below), should be considered. The value for the
subchronic liver effects of DEHP (e.g., peroxisome proliferation and
DNA synthesis) in the rat appears to be around 50 mg/kg per day (Lake
et al., 1984, 1991; Mitchell et al., 1985b; Tomaszewski et al., 1986).
Furthermore, a two-year study of DEHP in female rats demonstrated no
effects at 0.3 g/kg diet, a value which corresponds well to that noted
above (Cattley et al., 1987; Popp et al., 1987).
Many studies have shown dramatic species differences in response
to peroxisome proliferators including DEHP (ECETOC, 1992). For
example, DEHP threshold values for peroxisome proliferation of 25 and
250 mg/kg, respectively, were found in rats and hamsters after 14 days
of daily gastric intubation (Lake et al., 1984). Several other studies
showing species differences in the response to DEHP have been
reported. One experiment demonstrated a large increase in hepatic
peroxisomes after 14 days of oral DEHP administration (2000 mg/kg per
day) to rats, but the same treatment of marmosets resulted in no
peroxisome proliferation (Rhodes et al., 1986). A limited study in
cynomolgus monkeys also revealed no evidence of peroxisome
proliferation due to DEHP (Short et al., 1987). In addition, DEHP did
not elicit peroxisome proliferation in guinea-pigs (Osumi & Hashimoto,
1978; Mitchell et al., 1985a). Some studies have indicated that
intrinsic species differences in hepatocellular sensitivity exist. For
example, MEHP and MEHP metabolite VI (mono(2-ethyl-5-oxohexyl)
phthalate), which is a proximate peroxisome proliferator in the rat,
were good peroxisome proliferators in cultured rat hepatocytes, but
had little or no effect in guinea-pig, marmoset or human hepatocytes
(Mitchell et al., 1985a; Elcombe & Mitchell, 1986). More recently,
Bichet et al. (1990) and Butterworth et al. (1989) have also
demonstrated the inability of MEHP to elicit peroxisome proliferation
in human hepatocyte cultures. Studies using a variety of other
peroxisome proliferators (e.g., clofibric acid, beclobric acid,
methylclofenapate, trichloroacetic acid, ciprofibrate, and
benzbromarone) have also failed to demonstrate peroxisome
proliferation in cultured human hepatocytes, in spite of a good
response in rat hepatocytes (Elcombe, 1985; Allen et al., 1987;
Elcombe & Styles, 1989; Butterworth et al., 1989; Bichet et al., 1990;
Blaauboer et al., 1990).
Species differences in cytochrome P-450 IVA1 induction and
stimulation of replicative DNA synthesis have been less well studied.
However, MEHP and metabolite VI did not induce P-450 IVA1-mediated
lauric acid hydroxylation in vivo in marmosets (Rhodes et al., 1986)
or in cultured marmoset or human hepato-cytes (Elcombe & Mitchell,
1986).
In conclusion, it appears that the livers of rats and mice are
exquisitely sensitive to peroxisome proliferators, including DEHP,
while those of guinea-pigs, monkeys, and humans show minimal or no
response (ECETOC, 1992).
8. EFFECTS ON HUMANS
8.1 General population exposure
Two adults who swallowed 5 or 10 g DEHP experienced no untoward
effects apart from mild gastric disturbances and moderate diarrhoea
with 10 g DEHP (Shaffer et al., 1945). Three cases of nonspecific
hepatitis were described among 27 haemodialysis patients with terminal
renal failure. The PVC blood tubing used released DEHP at a
concentration of 10-20 mg/litre of perfusate. The symptoms and signs
of hepatitis disappeared rapidly when the use of tubing that did not
contain DEHP was resumed (Neergaard et al., 1971).
In three pre-term infants artificially ventilated with PVC
respiratory tubes, unusual lung disorders (opacification of the lung)
were observed during the fourth week of life. It was assumed by the
authors that these lung disorders were causually related to the
exposure to DEHP released from the respiratory tubes (Roth et al.,
1988).
8.2 Occupational exposure
There are very few data on the effects of occupational exposure
specifically to DEHP.
Two studies reported symptoms and signs of polyneuropathy among
47 out of 147 workers at a PVC-processing plant in the USSR and 12 out
of 23 workers at a plant for phthalate production in Italy. The
workers were exposed to a mixture of phthalates and DEHP was a minor
constituent, at least in the USSR plant. Furthermore, tricresyl
phosphate (a neurotoxin) was a component of the incombustible
materials produced in 10-20% of machines assigned to various workers
(Milkov et al., 1973). In the Italian study there was no corresponding
unexposed control group and the authors concluded that no definite
conclusion could be drawn from the study because of the limited number
of workers examined (Gilioli et al., 1978). The total phthalate air
concentrations recorded varied between 1.7 and 66 mg/m3 in the USSR
and 1 and 60 mg/m3 in Italy (Milkov et al., 1973; Gilioli et al.,
1978).
In a study involving a Swedish PVC-processing factory, peripheral
nervous system symptoms and signs were investigated among 54 male
workers exposed mainly to DEHP, diisodecylphthalate, and some
butylbenzylphthalate. The workers were divided into three groups of
approximately equal size and with mean phthalate exposures of 0.1,
0.2, and 0.7 mg/m3. Some workers displayed various peripheral
nervous system symptoms and signs, but these were not related to the
level of exposure. None of the workers reported symptoms indicating
work-related obstructive lung disease. Conventional lung function
tests also showed no association with exposure (Nielsen et al., 1985).
However, several biochemical parameters showed significant
associations with exposure. There was a slight decrease in the
haemoglobin level with longevity of employment as well as with
exposure in the last year. The serum ý-1-antitrypsin level increased
slightly with length of employment and the serum immunoglobulin A
level rose with rising exposure during the last year (Nielsen et al.,
1985).
One case of occupational asthma due to DEHP was reported in
worker at a PVC-processing plant (Brunetti & Moscato, 1984).
Diagnosis was made by exposing the patient to DEHP vapour in an
inhalation chamber; this evoked a dual asthmatic reaction. The action
was inhibited by prior administration of sodium chromoglycate.
A study of blood lipids, serum activities of liver enzymes, and
routine haematological tests was carried out among workers at a German
plant for DEHP production. The results were negative and uninformative
due to very low exposure levels (below 0.16 mg/m3) and the lack of
a control group (Thiess et al., 1978b). Thiess & Flieg (1978)
investigated the frequency of chromosome aberrations in 10 workers who
were employed from 10 to 30 years in this DEHP production plant. The
exposure levels were very low (0.09-0.16 mg/m3), and there was no
increase in chromosome aberrations compared to the control group. A
mortality study of 221 workers exposed to DEHP in the same plant was
also conducted. Eight deaths occurred in the cohort compared with
expected values of 15.9 and 17.0 from the city and county data,
respectively (Thiess et al., 1978a).
9. EFFECTS ON OTHER ORGANISMS IN THE LABORATORY AND FIELD
9.1 Toxicity to microorganisms
Bringmann & Kühn (1980) calculated toxic thresholds from cell
multiplication inhibition tests and found thresholds of > 400
mg/litre for the bacterium Pseudomonas putida, 10 mg/litre for the
alga Scenedesmus quadricaudaia, and 19 mg/litre for the protozoan
Entosiphon sulcatum.
Mathur (1974b) reported that DEHP added to soil (3 g/kg)
inhibited respiration, whereas addition of the same amount of DEHP to
soil preincubated for 14 weeks with DEHP or dioctyl phthalate had no
effect on respiration rate. The experiments were conducted at 22 °C
since no degradation of DEHP occurred at 4 °C or 10 °C. In an
intermittent flow-through hydrosoil microcosm, DEHP (at 1 and 100
mg/litre) produced no significant effect on the numbers or
physiological activity of the microflora monitored, nor on any of the
overall activities that were studied (Mutz & Jones 1977).
Larsson et al. (1986) studied the effect of DEHP on the microbial
activity in sediments. Sediment cores were taken, together with the
overlying water, from a eutrophic lake and the sediment was spiked
with between 25 and 400 mg DEHP/kg at a level of 5 mm below the
surface. Uncontaminated sediment samples had a significantly higher
oxygen uptake than sediment containing DEHP. Microbial activity, as
estimated from decreased oxygen saturation in the soil column,
correlated inversely with increasing levels of DEHP in the sediment.
9.2 Toxicity to aquatic organisms
Many of the toxicity values given in this section are above the
water solubility of DEHP, which ranges from 0.3 to 0.4 mg/litre (see
section 2.2). DEHP adsorbs strongly to sediment, food, dissolved
organic carbon, and even the testing vessels. Many of the toxicity
tests are also based on nominal concentrations. Therefore, the actual
exposure of the organisms is very difficult to determine and care must
be taken when interpreting the results.
9.2.1 Invertebrates
Acute toxicity to aquatic invertebrates is summarized in Table 9.
Table 9. Toxicity of DEHP to aquatic invertebratesa
Organism Age Temperature Hardnessb pH Duration LC50 Reference
(° C) (h) (mg/litre)d
Water flea < 24 h 17 48 0.133 Passino & Smith (1987)
(Daphnia pulex)
Water flea < 24 h 21-23 173 7.4-9.4 24 > 68 Leblanc (1980)
(Daphnia magna) < 24 h 21-23 173 7.4-9.4 48 11 Leblanc (1980)
Crayfish 96 > 10 Mayer & Sanders (1973)
(Orconectes nais)
Harpacticoid adult 20-22 7c 7.8 96 > 300 Linden et al. (1979)
(Nitocra spinipes)
Scud 96 > 32 Sanders et al. (1973)
(Gammarus pseudolimnaeus)
Midge larvae 21-23 270 7.4 48 > 18 Streufert et al. (1980)
(Chironomus plumosus)
a All experiments were performed using static conditions (water unchanged for duration of test)
b Hardness is expressed as mg calcium carbonate per litre. The tests were performed in fresh water
except where stated otherwise
c Salinity (%)
d Nominal values
Brown & Thompson (1982a) found no mortality of Daphnia magna
over an exposure period of 48 h and at DEHP dose levels up to 320
µg/litre. However, at levels of 180 µg/litre or more, the daphnids
were seen to float in the surface layer. The authors suggested that
this observation was connected with the solubility of DEHP (which
tends to precipitate out at > 180 µg/litre, possibly onto the
daphnids, thereby causing them to float). Stephenson (1983) found no
mortality in Gammarus pulex exposed for up to 120 h to a DEHP
concentration (400 µg/litre) in excess of its solubility.
Laughlin et al. (1978) exposed grass shrimps (Palaemonetes
pugio) to DEHP concentrations of up to 1 mg/litre (well above the
solubility limit of the ester) and found no significant difference in
mortality between the control and treated shrimps during the 28-day
exposure period. DEHP had no significant effect on the development
rate of larvae from hatching to moult. No apparent effects were noted
when mussels (Mytilus edulis) were exposed to 50 µg/litre for 28
days (Brown & Thompson, 1982b).
Hobson et al. (1984) found no significant mortality of penaeid
shrimps (Penaeus vannamei) fed a diet containing up to 50 g DEHP/kg
for 14 days, this representing 4% of the body weight per day.
Histological examination revealed no changes and no significant dose-
related effects were observed on moulting.
Sanders et al. (1973) and Mayer & Sanders (1973) exposed Daphnia
magna for a complete life cycle (21 days) to 3, 10 or 30 µg
DEHP/litre in an intermittent-flow system. Reproduction was
significantly inhibited at all concentrations (60, 70, and 80%
inhibition at the three dose levels, respectively). In contrast, Brown
& Thompson (1982a) found no effect on the reproduction of Daphnia
magna at concentrations of up to 100 µg/litre and suggested that the
difference in the numbers of young born to the controls in the two
studies, i.e. 11 offspring per parent (Mayer & Sanders, 1973) compared
with 170 per parent (Brown & Thompson, 1982a), could account for these
conflicting results.
When Knowles et al. (1987) exposed Daphnia magna to DEHP
concentrations of between 12 and 811 µg/litre for up to 21 days under
flow-through conditions (the reproduction rate was approximately 200
offspring per adult), survival was not affected at levels of < 158
µg/litre. At 811 µg/litre, however, survival was significantly reduced
after both 7 and 21 days. The mean number of young per surviving adult
was also reduced at this dose level. The levels of major biochemical
components such as protein, RNA, DNA, and glycogen were all
significantly reduced after 7 days at 811 µg/litre, but all except
glycogen were unaffected after 21 days. The authors concluded that the
maximum acceptable toxicant concentration (MATC), based on survival
and reproduction, was between 158 and 811 µg/litre. A no-observed-
effect level (NOEL) of 72 µg/litre was identified both by DNA content
and RNA/DNA ratio at day 7 and by surfacing of Daphnia at day 0.
Thuren & Woin (1991) exposed the freshwater amphipod Gammarus
pulex to DEHP at concentrations of 100 or 500 µg/litre for 10 days
under flow-through conditions. There was a 5 day pre- and post-
exposure period. The overall locomotor activity of G. pulex was
significantly decreased at the higher exposure level, and the effect
persisted throughout the post-exposure period. No significant effects
were observed at the lower dose level.
Flow-through chronic toxicity tests in both sand and hydrosoil
showed that DEHP concentrations as high as 360 µg/litre (sand) and 240
µg/litre (hydrosoil) had no significant effects on the growth or
development of midge larvae (Chironomus plumosus) over a 35-day
period. The continuous exposure of first-generation midge eggs in sand
substrate to mean DEHP concentrations of between 140 and 360 µg/litre
had no significant effect on hatchability (Streufert et al., 1980).
Woin & Larsson (1987) found that dragonfly larvae ( Aeshna sp.)
caught significantly fewer prey (Chaborus larvae) per attempt when
exposed to sediment DEHP concentrations of approximately 600 mg/kg for
between 3 and 9 weeks.
9.2.2 Fish
The acute toxicity to fish is summarized in Table 10 and the
toxicity to the embryo-larval stages of fish is shown in Table 11.
DEHP caused no deaths among one-year-old rainbow trout (Salmo
gairdneri) exposed to between 1 and 1000 mg/litre for 48 h (Silvo,
1974) or juvenile Atlantic salmon (Salmo salar) exposed to 100
mg/litre for 96 h (Zitko, 1972).
Mehrle & Mayer (1976) reported no effects on growth or survival
of adult fathead minnows (Pimephales promelas) exposed to between 1
and 62 µg/litre for 56 days. They also exposed rainbow trout eggs to
DEHP levels of 5, 14, and 54 µg/litre for 12 days prior to hatching,
but there was no significant effect on hatchability. The resulting fry
were continuously exposed to DEHP for a further 90 days. The two
highest concentrations caused a significant, but not dose related,
increase in the mortality of sac fry within 5 days of hatching. After
yolk absorption (at 24 days), DEHP caused no significant mortality or
effects on growth and development for the remainder of the exposure
period.
Table 10. Toxicity (96-h LC50 values) of DEHP to fish
Organism Size Stat/ Temperature Hardnessb pH LC50 Reference
flowa (° C) (mg/litre)c
Bluegill sunfish > 10 Mayer & Sanders (1973)
(Lepomis macrochirus) 0.32-1.2 g stat 21-23 32-48 6.7-7.8 > 770 Buccafusco et al. (1981)
Fathead minnow > 10 Mayer & Sanders (1973)
(Pimephales promelas) 0.055-0.25 g 24-26 44-46 7-8 > 0.33d Defoe et al. (1990)
Channel catfish > 10 Mayer & Sanders (1973)
(Ictalurus punctatus)
Rainbow trout fingerling stat 14-16 540 Hrudey et al. (1976)
(Oncorhynchus mykiss)
> 10 Mayer & Sanders (1973)
a Stat = static conditions (water unchanged for duration of test)
b Hardness is expressed as mg calcium carbonate per litre. The tests were performed in fresh water
except where stated otherwise
c Nominal values unless stated otherwise
d Measured value
Table 11. Toxicity of DEHP to the embryo-larval stages of fisha
Organism Stat/flowb Hardnessc pH LC50 (mg/litre) 95% confidence limits
Channel catfish stat 90-115 7.6-8.1 0.69 0.55-0.86
(Ictalurus punctatus)
Redear sunfish stat 90-115 7.6-8.1 6.18 4.65-8.04
(Lepomis microlophus)
Largemouth bass flow 45-55 7.5-8.0 42.1 28.8-77.8
(Micropterus salmoides)
flow 190-225 7.5-8.0 32.9 19.8-58.9
Rainbow trout flow 45-55 7.5-8.0 139.5 123.2-165.2
(Oncorhynchus mykiss)
flow 190-225 7.5-8.0 149.2 125.8-203.8
a From: Birge et al. (1978). Exposure was initiated 2 to 6 h after spawning (except in the case of rainbow trout where exposure
was initiated 15 min after fertilisation) and continued until 4 days after hatching. The hatching times were 22 days for rainbow
trout (13.5-14.3 °C), 3 days for catfish (29-31 °C), and 3-4 days for bass and sunfish (20-24 °C).
b Stat = static conditions, but water renewed every 12 h; flow = flow-through conditions (DEHP concentration in water continuously
maintained)
c Hardness is expressed as mg calcium carbonate per litre. The tests were performed in fresh water except where stated otherwise.
DeFoe et al. (1990) exposed rainbow trout (Oncorhynchus mykiss)
and Japanese medaka (Oryzias latipes) to DEHP using concentrations
of up to 0.502 mg/litre for a 90-day trout embryo-larval test and
0.554 mg/litre for a 168-day larval test on medaka. No significant
adverse effects were observed on trout hatchability, survival or
growth, but there was a significant reduction in the growth of
Japanese medaka.
Mayer & Sanders (1973) studied the effects of DEHP on the
reproduction of zebra fish (Brachydanio rerio) and guppies
(Lebistes reticulatus). During the 90-day dietary exposure, zebra
fish were fed on 50 or 100 mg DEHP/kg food, and guppies were fed 100
mg/kg. Although the treated zebra fish spawned more frequently than
the control fish, the latter produced more eggs per spawn. Fry
survival was significantly reduced by DEHP. Treated guppies produced
fewer fry per adult and had an 8% incidence of abortions, whereas
there were no abortions in the control group (no statistics were
given).
In a study of fish exposed to DEHP, Mayer et al. (1977) found
reduced vertebral collagen levels after 150 days at a DEHP
concentration of 3.7 µg/litre in adult brook trout (Salvelinus
fontinalis), after 127 days at 11 µg/litre in fathead minnow, and
after 90 days at 5 µg/litre in rainbow trout. However, there was no
effect on fish growth. Pfuderer & Francis (1975) found that DEHP had
no effect on the heart rate of goldfish when the fish were exposed to
200 mg/litre for 10 min.
9.2.3 Amphibians
The acute toxicity of DEHP to tadpoles of Fowler's toad and the
leopard frog is given in Table 12.
When Larsson & Thuren (1987) exposed eggs of the moorfrog (Rana
arvalis) to sediment concentrations of between 10 and 800 mg DEHP/kg
(fresh weight), the number of tadpoles hatching decreased with
increasing exposure concentration. The percentage hatch was 90% for
the controls eggs, 50% at 150 mg DEHP/kg, and < 30% at > 400 mg/kg.
The lowest DEHP concentration that caused a significant decrease in
the number of tadpoles hatching was 25 mg/kg. After hatching, the
survival of tadpoles was unaffected. There were no delays in hatching
and no abnormalities in the developing tadpoles exposed to the various
DEHP concentrations.
Table 12. Toxicity of DEHP to frog and toad tadpolesa
Organism LC50 (mg/litre) 95% confidence limits
Fowler's toad 3.88 3.08-4.84
(Bufo fowleri)
Leopard frog 4.44 3.65-5.37
(Rana pipiens)
a From: Birge et al. (1978). Exposure occurred under static
conditions, but water was renewed every 12 h. It was initiated 2
to 6 h after spawning and continued until 4 days after hatching.
The hatching times varied between 3 and 4 days, and so the
exposure period varied between 7 and 8 days. The temperature was
20-24 °C, hardness 90-115 mg CaCO3/litre, and pH 7.6-8.1.
Wams (1987) quoted an unpublished Dutch report which stated that
an exposure of 2 mg DEHP/litre caused a reduction in the growth rate
of clawed toad (Xenopus laevis) larvae.
9.3 Toxicity to terrestrial organisms
9.3.1 Plants
When Herring & Bering (1988) grew spinach and pea plants from
seed for 14 to 16 days in soil containing 100 mg DEHP/kg, there was no
effect on plant growth, as measured by height. The effect of DEHP on
seed germination was observed by placing seeds in petri dishes
containing a solution of 100 mg/litre. A reduction of 40% to 50% in
the number of seeds germinating was found.
Lokke & Rasmussen (1983) reported that DEHP had no visible effect
on Sinapis alba, Brassica napusor or Achillea millefolium when
sprayed in the field at concentrations of up to 87.5 kg/ha. According
to Stanley & Tapp (1982), DEHP at a level of 1000 mg/kg soil had no
effect on the growth of rape (Brassica rapa) and only a slight
effect on that of oats (Avena sativa).
9.3.2 Earthworms
In a series of contact toxicity tests, red earthworms (Eisenia
foetida) were exposed to DEHP via filter paper in glass vials. An
LD50 could not be calculated because DEHP was not toxic even at the
highest dose of 25 mg/cm2 (Neuhauser et al., 1986).
9.3.3 Insects
Al-Badry & Knowles (1980) found DEHP to be non-toxic to female
houseflies (Musca domestica) when applied topically or by injection
at a concentration of 20 µg/fly (equivalent to 1000 mg/kg). When it
was topically applied simultaneously with various organophosphates, an
antagonistic interaction was apparent; mortality of 85% to 95% was
reduced to less than 10% by the DEHP. However, when DEHP was topically
applied 30 min before exposure to an organophosphate concentration
that caused 10% to 30% mortality, the resulting interaction was
synergistic with mortality rising to over 60% and in most cases to
over 80%.
9.3.4 Birds
Hill et al. (1975) found no mortality among 10-day-old ring-
necked pheasants or mallard fed up to 5000 mg DEHP/kg for 5 days
followed by 3 days on a normal diet.
When Wood & Bitman (1984) fed broiler hens a diet containing 2000
mg DEHP/kg (226 mg DEHP/hen per day) for 4 weeks, egg production and
body weights were significantly decreased. Ishida et al. (1982)
reported the cessation of egg production and an abnormality of the
ovaries in laying hens fed 5 or 10 g DEHP/kg diet for up to 230 days.
In a study by O'Shea & Stafford (1980), starlings (Sturnus
vulgaris) were fed on a diet containing 25 or 250 mg DEHP/kg for a
30-day period. At both dose levels, the treated birds gained
significantly more body weight than controls during the exposure
period, but at the lower dose level food consumption was significantly
reduced compared to controls.
When Peakall (1974) fed ring doves (Streptopelia risoria) on a
diet containing 10 mg/kg, there were no effects on the eggshell
thickness, ashed egg weight, rate of water loss, surface area or
permeability of the eggs laid.
10. EVALUATION OF HUMAN HEALTH RISKS AND EFFECTS ON THE ENVIRONMENT
10.1 Evaluation of human health risks
10.1.1 Exposure levels
DEHP concentrations of up to 300 ng/m3 have been measured in
urban air, but usually the levels in ambient air are well below 100
ng/m3. Data on occupational exposure levels are limited.
Concentrations of up to 4.1 mg/m3 have been reported but they are
usually below 1 mg/m3. Total phthalate exposure levels have been
reported to be between 0.1 and 60 mg/m3. Clinical treatment,
including transfusion, haemodialysis, extracorporal circulation, and
artificial respiration, may lead to DEHP exposure.
The exposure to DEHP from drinking-water and food is low.
10.1.2 Toxic effects
Dose-dependent kinetics of DEHP or its metabolites and species
differences in the metabolism have been confirmed in several studies.
In animals some inhalation studies have been performed. However,
no consistent findings are reported.
The oral and intraperitoneal LD50 values exceed 25 g/kg,
indicating that DEHP has low acute toxicity.
In rats and mice, DEHP administration produces hepatic
hyperplasia and hypertrophy, which is characterized by peroxisome
proliferation. The threshold dose level for hepatic peroxisome
proliferation in the rat is approximately 50 mg/kg per day. High DEHP
dose levels (12 g DEHP/kg diet in rats; 6 g DEHP/kg diet in mice) in
a feeding study resulted in an increased incidence of hepatic tumours
in rats and mice.
Marked species differences in DEHP-induced hepatomegaly and
peroxisome proliferation exist. Rats and mice are very responsive,
Syrian hamsters less responsive, and guinea-pigs, marmosets, and
cynomolgus monkeys non-responsive. Studies with primary hepatocyte
cultures have shown an excellent in vitro/in vivo correlation
concerning responsiveness to DEHP/DEHP metabolites. Several studies
have demonstrated the non-responsive nature of cultured human
hepatocytes to DEHP metabolites and other peroxisome proliferators.
The induction of peroxisome proliferation and cell replication is
strongly associated with the development of hepatic tumours in rats
and mice. Thus, the available data suggest that species which do not
respond to the hepatic effects of DEHP are unlikely to be susceptible
to the development of hepatic tumours.
Testicular atrophy is one of the most consistent effects of DEHP
in in vivo studies on a variety of experimental animal species. Such
effects have been observed in rats fed 6 g DEHP/kg diet and mice fed
3 g/kg diet, and they are more pronounced in young animals. Hamsters
and marmosets appear to be more resistant to the testicular effects of
DEHP.
When both male and female CD-1 mice were treated with 1 g DEHP/kg
diet, a significant reduction in fertility was observed.
A dietary level of 20 g DEHP/kg throughout gestation produced an
increased incidence of resorptions in rats but no malformations. In
the mouse, however, 1 g/kg throughout pregnancy increased the
incidence of embryolethality and abnormalities. Sensitivity was
greatest on days 7-9 of gestation. The dose level (0.5 g/kg) that
induced fetotoxicity in mice did not induce maternal toxicity. Results
from several different genotoxicity tests indicate that DEHP and its
major metabolites do not exhibit any direct genotoxic effect in either
bacteria or in vitro mammalian cells. This has been confirmed in
in vivo binding studies, which indicated that DEHP and its
metabolites do not interact covalently with DNA. However, it has been
established that DEHP has the potential of inducing aneuploidy in
fungi as well as in in vitro mammalian cells. There are no
consistent results from dominant lethal studies.
Few data on the human health effects of DEHP exposure have been
reported. There have been a few studies on workers exposed to
phthalate mixtures, but no consistent health effects that could be
directly related to DEHP have been reported.
10.1.3 Conclusion
DEHP causes reproductive and hepatocarcinogenic effects in rats
and mice.
Testicular atrophy is the main reproductive effect in rats and
mice, and young animals are more susceptible than older ones to this
effect. The induction of hepatic peroxisome proliferation and cell
replication are strongly associated with the liver carcinogenic effect
of certain non-genotoxic carcinogens including DEHP. However, marked
differences have been observed among animal species with respect to
DEHP-induced peroxisome proliferation. Currently there is not
sufficient evidence to suggest that DEHP is a potential human
carcinogen.
10.2 Evaluation of effects on the environment
10.2.1 Exposure levels
DEHP exists widely in the environment, being released during
production, processing, usage, and disposal. Transport in the air is
the major route by which it enters the environment. It can be
deposited by dry or wet deposition and has been found to leach into
ground water.
DEHP is readily photodegraded in the atmosphere. In the
laboratory, aerobic biodegradation has been shown to occur under
certain circumstances. However, in the environment aerobic
biodegradation seems to be slow and anaerobic degradation is even
slower.
Based on a measured log Pow of about 5 and measured
bioaccumulation factors for biota and sediment, it can be seen that
DEHP is highly bioaccumulative.
In rivers and lakes, DEHP concentrations of up to 4 µg/litre have
been measured.
Due to its hydrophobic nature, DEHP adsorbs readily to soil,
sediment, and particulate matter. Levels of up to 70 mg/kg dry weight
have been found in river sediments, but near to a discharge point
levels of up to 1480 mg/kg dry weight have been reported.
10.2.2 Toxic effects
Most acute toxicity studies on aquatic organisms show DEHP to be
of low toxicity. However, one study suggested a greater sensitivity
for the water flea (Daphnia pulex), the nominal 48-h LC50 being
133 µg/litre.
In a 21-day study on Daphnia magna, the NOEL for biochemical
and behavioural effects was 72 µg/litre. A significant increase in
mortality was recorded in trout fry exposed to 14 µg DEHP/litre from
12 days prior to hatching. At concentrations of 3.7 to 11 µg/litre,
reductions in vertebral collagen were reported in three species of
fish exposed for 150 days.
Zebra fish fry survival and guppy reproduction were adversely
affected at DEHP concentrations in food of 50 and 100 mg/kg,
respectively, during a 90-day exposure period.
In two separate studies, DEHP concentrations of 25 mg/kg
(wet weight) of sediment significantly reduced microbial activity and
the number of frog tadpoles hatching.
Available studies indicate that the acute toxicity of DEHP to
algae, plants, earthworms, and birds is low. There are no data
relating to effects upon wild mammals.
10.2.3 Conclusion
There is no documented information that DEHP presents any hazard,
based on acute exposure to fish and daphnids. However, a reduction of
microbial activity in sediment at environmental levels of DEHP was
reported. A comparison between environmental levels and the
concentrations that produce effects in prolonged studies, especially
early life-stage tests on fish and amphibians, indicates that a hazard
for the environment, particularly via water and sediment, cannot be
excluded. Adverse effects on organisms are likely in areas with highly
contaminated water and sediments which are near to point emission
sources.
Although few relevant studies have been reported, the acute
toxicity of DEHP to algae, plants, earthworms, and birds appears to be
low.
11. RECOMMENDATIONS FOR PROTECTION OF HUMAN HEALTH AND THE
ENVIRONMENT
a) Current disposal practises should be improved.
b) Measures must be undertaken to reduce the release of DEHP to the
environment.
c) Medical devices and products that contribute to the body burden
of DEHP must be scrutinized to reduce exposure to DEHP via the
intravenous route.
12. FURTHER RESEARCH
More research is needed on the effects of DEHP on the ecosystem
of sediments and on the subject of DEHP leaching, particularly from
landfill to ground water.
Since DEHP occurs in the air, including workplace air, further
inhalation studies are needed.
Epidemiological surveys on exposed populations should be
encouraged. Further studies are needed on the mechanism of peroxisome-
proliferator-induced hepatocarcinogenicity, with emphasis on human
health effects.
13. PREVIOUS EVALUATIONS BY INTERNATIONAL BODIES
DEHP was one of the substances discussed by a working group of
the International Agency for Research on Cancer in 1981 (IARC, 1982).
The evaluation by the group was that there was sufficient evidence for
the carcinogenicity of DEHP in mice and rats, but that no adequate
epidemiological study was available. The working group concluded that
DEHP is possibly carcinogenic to humans and placed it in group 2B
(IARC, 1987).
DEHP was evaluated at the 28th meeting of the Joint FAO/WHO
Expert Committee on Food Additives. The recommendation was to reduce
human exposure to DEHP from food-contact materials to the lowest level
technologically attainable (WHO/FAO, 1984a,b). During the 33rd meeting
of the Joint FAO/WHO Expert Committee on Food Additives, DEHP was
again evaluated and the recommendation was the same: DEHP in food
should be reduced to the lowest level attainable. The Committee
considered that this might be achieved by using alternative
plasticizers or alternatives to plastic material containing DEHP
(WHO/FAO, 1989a,b).
REFERENCES
AGARWAL, D.K. (1986) Letters to the editor. Toxicol. appl. Pharmacol.,
82: 383-387.
AGARWAL, D.K., AGARWAL, S., & SETH, P.K. (1982) Effect of di-(2-
ethylhexyl) phthalate on drug metabolism, lipid peroxidation, and
sulfhydryl content of rat liver. Drug Metab. Disp., 10: 77-80.
AGARWAL, D.K., LAWRENCE, W.H., & AUTIAN, J. (1985a) Antifertility and
mutagenic effects in mice from parenteral administration of di-2-
ethylhexyl phthalate (DEHP). J. Toxicol. environ. Health, 16: 71-84.
AGARWAL, D.K., LAWRENCE, W.H., NUNEZ, L.J., & AUTIAN, J. (1985b)
Mutagenicity evaluation of phthalic acid esters and metabolites in
Salmonella typhimurium cultures. J. Toxicol. environ. Health, 16:
61-69.
AGARWAL, D.K., EUSTIS, S., LAMB J.C., IV, JAMESON, C.W., & KLUWE, W.M.
(1986a) Influence of dietary zinc on di(2-ethylhexyl)phthalate-induced
testicular atrophy and zinc depletion in adult rats. Toxicol. appl.
Pharmacol., 84: 12-24.
AGARWAL, D.K., EUSTIS, S., LAMB J.C., IV, REEL, J.R., & KLUWE, W.M.
(1986b) Effects of di(2-ethylhexyl)phthalate on the gonadal patho-
physiology, sperm morphology, and reproductive performance of male
rats. Environ. Health Perspect., 65: 343-350.
AGARWAL, D.K., LAWRENCE, W.H., TURNER, J.E., & AUTIAN, J. (1989)
Effects of parenteral di(2-ethylhexyl)phthalate (DEHP) on gonadal
biochemistry, pathology and reproductive performance of mice. J.
Toxicol. environ. Health, 26: 39-59.
AHMED, R.S., PRICE, S.C., GRASSO, P., & HINTON, R.H. (1990) Hepatic
nuclear and cytoplasmic effects following intermittent feeding of rats
with di(2-ethylhexyl)phthalate. Food chem. Toxicol., 28: 427-434.
AL-BADRY, M. & KNOWLES, C.O. (1980) Phthalate-organophosphate
interactions: Toxicity, penetration, and metabolism studies with house
flies. Arch. environ. Contam. Toxicol., 9: 147-161.
ALBRO, P.W. (1986) Absorption, metabolism, and excretion of di(2-
ethylhexyl) phthalate by rats and mice. Environ. Health Perspect., 65:
293-298.
ALBRO, P.W. (1987) The biochemical toxicology of di-(2-ethylhexyl) and
related phthalates: testicular atrophy and hepatocarcinogenesis. In:
Hodgson, E., Bend, J.R., & Philpot, R.M., ed. Reviews in biochemical
toxicology, Amsterdam, Oxford, New York, Elsevier Science Publishers,
Vol. 8, pp. 73-119.
ALBRO, P.W. & LAVENHAR, S.R. (1989) Metabolism of di(2-ethylhexyl)
phthalate. Drug Metabol. Rev., 21: 13-34.
ALBRO, P.W. & THOMAS, R.O. (1973) Enzymatic hydrolysis of di(2-
ethylhexyl) phthalate by lipases. Biochim. Biophys Actaw, 360:
380-390.
ALBRO, P.W., THOMAS, R., & FISHBEIN, L. (1973) Metabolism of
diethylhexyl phthalate by rats. Isolation and characterization of the
urinary metabolites. J. Chromatogr., 76: 321-330.
ALBRO, P.W., HAAS, J.R., PECK, C.C., ODOM, D.G., CORBETT, J.T.,
BAILEY, F.J., BLATT, H.E., & BARRETT, B.B. (1981) Identification of
the metabolites of di-(2-ethyl-hexyl) phthalate in urine from the
African green monkey, Drug Met. Disp., 9: 223-225.
ALBRO, P.W., CORBETT, J.T., SCHROEDER, J.L., JORDAN, S., & MATTHEWS,
H.B. (1982) Pharmacokinetics, interactions with macromolecules and
species differences in metabolism of DEHP. Environ. Health Perspect.,
45: 19-25.
ALBRO, P.W., CHAPIN, R.E., CORBETT, J.T., SCHROEDER, J., & PHELPS,
J.L. (1989) Mono-2-ethylhexylphthalate, a metabolite of di-(2-
ethylhexyl)phthalate, causally linked to testicular atrophy in rats.
Toxicol. appl. Pharmacol., 100: 193-200.
ALLEN, K.L., GREEN, C.E., & TYSON, C.A. (1987) Comparative studies of
peroxisomal enzyme induction in hepatocytes from rat, cynomolgus
monkey and human by hypolipidemic drugs. Toxicologist, 7: 63.
AL-OMRAN, L.A. & PRESTON, M.R. (1987) The interactions of phthalate
esters with suspended particulate material in fresh and marine waters.
Environ. Pollut., 46: 177-186.
ANTONYUK, O.K. (1975) Toxicology of complex ethers of phthalic acid (A
review of literature), Hyg. Labour occup. Dis., 1: 32-35 (in Russian).
ARANDA, J.M., O'CONNOR, G.A., & EICEMAN, G.A. (1989) Effects of sewage
sludge on di-(2-ethylhexyl) phthalate uptake by plants J. environ.
Qual., 18: 45-50.
ARBIN, A. & ÖSTELIUS, J. (1980) Determination by electron-capture gas
chromatography of mono- and di(2-ethylhexyl) phthalate in intravenous
solutions stored in polyvinyl chloride bags. J. Chromatogr., 193:
405-412.
ASHBY, J., DE SERRES, F.J., DRAPER, M., ISHIDATE, M., Jr, MARGOLIN,
B.H., MATTER, B.E., & SHELBY, M.D., ed. (1985) Evaluation of short-
term tests for carcinogens. Report of the International Programme on
Chemical Safety's collaborative study on in vitro assays, Amsterdam,
Oxford, New York, Elsevier Science Publishers, 752 pp (Progress in
Mutation Research, Vol. 5).
ASTILL, B. (1989) Metabolism of DEHP: Effects of prefeeding and dose
variation, and comparative studies in rodents and the cynomolgus
monkey (CMA studies). Drug Metab. Rev., 21: 35-53.
ASTILL, B., BARBER, E., LINGTON, A., MORAN, E., MULHOLLAND, A.,
ROBINSON, E., & SCHEIDER, B. (1986) Chemical industry voluntary test
program for phthalate esters: Health effect studies. Environ. Health
Perspect., 65: 329-336.
ATLAS, E. & GIAM, C.S. (1981) Global transport of organic pollutants:
Ambient concentrations in the remote marine atmosphere. Science, 211:
163-165.
ATSDR (1988) Toxicological profile for di(2-ethylhexyl) phthalate,
Atlanta, Georgia, Agency for Toxic Substances and Disease Registry, US
Public Health Service (Draft for public comment).
BALLY, M.B., OPHEIM, D.J., & SHERTZER, H.G. (1980) Di-(2-ethylhexyl)
phthalate enhances the release of lysosomal enzymes from alveolar
macrophages during phagocytosis. Toxicology, 18: 49-60.
BARBER, E.D., ASTILL, B.D., MORAN, E.J., SCHNEIDER, B.F., GRAY,
T.J.B., LAKE, B.G., & EVANS, J.G. (1987) Peroxisome induction studies
on seven phthalate esters. Toxicol. ind. Health, 3: 7-24.
BARNEY, G.H., ORGEBIN-CRIST, M.C., & MACAPINLAC, M.P. (1969) Genesis
of esophageal parakeratosis and histologic changes in the testes of
the zinc-deficient rat and their reversal by zinc repletion. J. Nutr.,
95: 526-534.
BAUGHMAN, G.L., PARIS, D.F., & STEEN, W.C. (1980) Quantitative
expression of biotransformation. In: Maki, A.W., Dickson, K.L., &
Cairns, J., ed. Biotransformation and fate of chemicals in the
environment, Washington, DC, American Society for Microbiology, pp.
105-111.
BELISLE, A.A., REICHEL, W.L., & SPANN, J.W. (1975) Analysis of tissues
of mallard ducks fed two phthalate esters. Bull. environ. Contam.
Toxicol., 13: 129-132.
BELL, F.P., PATT, C.S., BRUNDAGE, B., GILLIES, P.J., & PHILIPS, W.A.
(1978a) Studies on lipid biosynthesis and cholesterol content of liver
and serum lipoproteins in rats fed various phthalate esters. Lipids,
13: 66-74.
BELL, F.P., PATT, C.S., & GILLIES, P.J. (1978b) Effect of phthalate
esters on serum cholesterol and lipid biosynthesis in liver, testes,
and epididymal fat in the rat and rabbit. Lipids, 13: 673-678.
BELL, F.P., WANG, S., MENDOZA, A.R., & NISHIZAWA, E.E. (1979) Platelet
function and platelet lipid biosynthesis in rats and rabbits fed the
plasticiser DEHP. Bull. environ. Contam. Toxicol., 23: 306-310.
BICHET, N., CAHARD, D., FABRE, G., REMANDET, B., GOUY, D., & CANO,
J.-P. (1990) Toxicological studies on a benzofuran derivative. III.
Comparison of peroxisome proliferation in rat and human hepatocytes in
primary culture. Toxicol. appl. Pharmacol., 106: 509-517.
BIRGE, W.J., BLACK, J.A., & WESTERMAN, A.G. (1978) Effects of
polychlorinated biphenyl compounds and proposed PCB-replacement
products on embryo-larval stages of fish and amphibians, Washington,
DC, US Department of the Interior, 33 pp (Research Report No. 118).
BLAAUBOER, B.J., VAN HOLSTEIJN, C.W.M., BLEUMINK, R., MENNES, W.C.,
VAN PELT, F.N.A.M., YAP, S.H., VAN PELT, J.F., VAN IERSEL, A.A.J.,
TIMMERMANN, A., & SCHMID, B.P. (1990) The effect of beclobric acid and
clofibric acid on peroxisomal beta-oxidation and peroxisome
proliferation in primary cultures of rat, monkey and human
hepatocytes. Biochem. Pharmacol., 40: 521-528.
BOVE, J.L., DALVEN, P., & KUKREJA, V.P. (1978) Airborne di-butyl and
di(2-ethylhexyl)-phthalate at three New York city air sampling
stations. Int. J. environ. anal. Chem., 5: 189-194.
BRIDGES, J.W. (1985) Frontiers in biochemical toxicology. Trends
pharmacol. Sci., 6 (suppl.): 11-15.
BRINGMANN, G. & KÜHN, R. (1980) Comparison of the toxicity thresholds
of water pollutants to bacteria, algae, and protozoa in the cell
multiplication inhibition test. Water Res., 14: 231-241.
BROWN, D. & THOMPSON, R.S. (1982a) Phthalates and the aquatic
environment: Part I. The effect of di-2-ethylhexyl phthalate (DEHP)
and di-isodecyl phthalate (DIDP) on the reproduction of Daphnia magna
and observations on their bioaccumulation. Chemosphere, 11: 417-426.
BROWN, D. & THOMPSON, R.S. (1982b) Phthalates and the aquatic
environment: Part II. The bioconcentration and depuration of di-2-
ethylhexyl phthalate (DEHP) and di-isodecyl phthalate (DIDP) in
mussels (Mytilus edulis). Chemosphere, 11: 427-435.
BRUNETTI, G. & MOSCATO, G. (1984) [Bronchial asthma due to
occupational exposure to dioctyl phthalate.] Med. Lav., 75: 120-124
(in Italian).
BUCCAFUSCO, R.J., ELLS, S.J., & LEBLANC, G.A. (1981) Acute toxicity of
priority pollutants to bluegill (Lepomis macrochirus). Bull. environ.
Contam. Toxicol., 26: 446-452.
BUTTERWORTH, B.E., BERMUDEZ, E., SMITH-OLIVER, T., EARLE, L., CATTLEY,
R., MARTIN, J., POPP, J.A., STROM, S., JIRTLE, R., & MICHALOPOULOS, G.
(1984) Lack of genotoxic activity of di(2-ethylhexyl) phthalate (DEHP)
in rat and human hepatocytes. Carcinogenesis, 5: 1329-1335.
BUTTERWORTH, B.E., LOURY, D.J., SMITH-OLIVER, T., & CATTLEY, R.C.
(1987) The potential role of chemically induced hyperplasia in the
carcinogenic activity of the hypolipidemic carcinogens. Toxicol. ind.
Health, 3: 129-148.
BUTTERWORTH, B.E., SMITH-OLIVER, T., EARLE, L., LOURY, D.J., WHITE,
R.D., DOOLITTLE, D.J., WORKING, P.K., CATTLEY, R.C., JIRTLE, R.,
MICHALOPOULOS, G., & STROM, S. (1989) Use of primary cultures of human
hepatocytes in toxicology studies. Cancer Res., 49: 1075-1084.
CALLEY, D., AUTIAN, J., & GUESS, W.L. (1966) Toxicology of a series of
phthalate esters. J. pharm. Sci., 55: 158-162.
CARPENTER, C.P., WEIL, C.S., & SMYTH, H.F. (1953) Chronic oral
toxicity of di(2-ethylhexyl) phthalate for rats, guinea-pigs, and
dogs. Arch. ind. Hyg., 8: 219-226.
CATTLEY, R.C. & POPP, J.A. (1989) Differences between the promoting
activities of the peroxisome proliferator WY14,643 and phenobarbital
in rat liver. Cancer Res., 49: 3246-3251.
CATTLEY, R.C., CONWAY, J.G., & POPP, J.A. (1987) Association of
persistent peroxisome proliferation and oxidative injury with
hepatocarcinogenicity in female F-344 rats fed di(2-
ethylhexyl)phthalate for 2 years. Cancer Lett., 38: 15-27.
CAUTREELS, W. & VAN CAUWENBERGHE, K. (1978) Experiments on the
distribution of organic pollutants between airborne particulate matter
and the corresponding gas phase. Atmos. Environ., 12: 1133-1141.
CAUTREELS, W., VAN CAUWENBERGHE, K., & GUZMAN, L.A. (1977) Comparison
between the organic fraction of suspended matter at a background and
an urban station. Sci. total Environ., 8: 79-88.
CERBULIS, J. & ARD, J.S. (1967) Method for isolation and detection of
dioctyl phthalate from milk lipids. J. Assoc. Off. Anal. Chem., 50:
646-650.
CHAPIN, R.E., GRAY, T.J.B., PHELPS, J.L., & DUTTON, S.L. (1988) The
effects of mono-(2-ethylhexyl)-phthalate on rat sertoli cell-enriched
primary cultures. Toxicol. appl. Pharmacol., 92: 467-479.
CHEN, W.S., KERKAY, J., PEARSON, K.H., PAGANINI, E.P., & NAKAMOTO, S.
(1979a) Determination of urinary bis(2-ethylhexyl) phthalate levels in
non-uremic subjects by gas chromatography. Anal. Lett., 12: 1501-1515.
CHEN, W.S., KERKAY, J., PEARSON, K.H., PAGANINI, E.P., & NAKAMOTO, S.
(1979b) Tissue bis(2-ethylhexyl) phthalate levels in uremic subjects.
Anal. Lett., 12: 1517-1535.
CHU, I., SECOURS, V.E., MARINO, I.A., VILLENEUVE, D.C., & VALLI, V.E.
(1981) Sub-acute and sub-chronic toxicity of mono-2-ethylhexyl
phthalate in the rat. Arch. environ. Contam. Toxicol., 10: 271-280.
CIRIOLO, M.R., MAVELLI, I., ROTILIO, G., BORZATTA, V., CRISTOFARI, M.,
& STANZANI, L. (1982) Decrease of superoxide dismutase and glutathione
peroxidase in liver of rats treated with hypolipidemic drugs. FEBS
Lett., 144: 264-268.
CLAYTON, G.D. & CLAYTON, F.E. (1981) Patty's industrial hygiene and
toxicology, 3rd ed., New York, Wiley-Interscience, pp. 2342-2353.
CONTRERAS, T.J., SHELBLEY, R.H., & VALERI, C.R. (1974) Accumulation of
di-2-ethylhexyl phthalate (DEHP) in whole blood, platelet
concentrates, and platelet-poor plasma. Transfusion, 14: 34-46.
CONWAY, J.G., CATTLEY, R.C., POPP, J.A., & BUTTERWORTH, B.E. (1989a)
Possible mechanisms in hepatocarcinogenesis by the peroxisome
proliferator di(2-ethylhexyl)phthalate. Drug Metab. Rev., 21: 65-102.
CONWAY, J.G., TOMASZEWSKI, K.E., OLSON, M.J., CATTLEY, R.C., MARSMAN,
D.S., & POPP, J.A. (1989b) Relationship of oxidative damage to the
hepatocarcinogenicity of the peroxisome proliferators di(2-ethylhexyl)
phthalate and Wy-14, 643. Carcinogenesis, 10: 513-519.
CROCKER, J.F.S., SAFE, S.H., & ACOTT, P. (1988) Effects of chronic
phthalate exposure on the kidney. J. Toxicol. environ. Health, 23:
433-444.
CURSTEDT, T. & SJÖVALL, J. (1983) Individual molecular species of
phosphatidylcholines and phosphatidylinositols in livers of rats fed
bis(2-ethylhexyl) phthalate. Med. Biol., 61: 219-222.
CURTO, K.A. & THOMAS, J.A. (1982) Comparative effects of diethylhexyl
phthalate or monoethylhexyl phthalate on male mouse and rat
reproductive organs. Toxicol. appl. Pharmacol., 62: 121-125.
CURTO, K.A., MCCAFFERTY, R.E., DONOVAN, M.P., & THOMAS, J.A. (1982)
Further studies on the effects of the phthalate acid esters (PAE) on
rat male reproductive organs. Toxicologist, 2: 71.
DANIEL, J.W. & BRATT, H. (1974) The absorption, metabolism and tissue
distribution of di(2-ethylhexyl) phthalate in rats. Toxicology, 2:
51-65.
DEANGELO, A.B. & GARRETT, C.T. (1983) Inhibition of development of
preneoplastic lesions in the livers of rats fed a weakly carcinogenic
environmental contaminant. Cancer Lett., 20: 199-205.
DEANGELO, A.B., GARRETT, C.T., & QUERAL, A.E. (1984) Inhibition of
phenobarbital and dietary choline deficiency promoted preneoplastic
lesions in rat liver by environmental contaminant di(2-ethylhexyl)
phthalate. Cancer Lett., 23: 323-330.
DEFOE, D.L., HOLCOMBE, G.W., HAMMERMEISTER, D.E., & BIESINGER, K.E.
(1990) Solubility and toxicity of eight phthalate esters to four
aquatic organisms.Environ. Toxicol. Chem. 9: 623-636.
DIVINCENZO, G.D., HAMILTON, M.L., MUELLER, K.R., DONISH, W.H., &
BARBER, E.D. (1985) Bacterial mutagenicity testing of urine from rats
dosed with 2-ethylhexanol derived plasticizers. Toxicology, 34:
247-259.
DIWAN, B.A., WARD, J.M., RICE, J.M., COLBURN, N.H., & SPANGLER, E.F.
(1985) Tumor-promoting effects of di(2-ethylhexyl) phthalate in JB6
mouse epidermal cells and mouse skin. Carcinogenesis, 6: 343-347.
DOUGLAS, R.G., HUGENHOLTZ, A.P., & BLAKEY, D.H. (1986) Genetic
toxicology of phthalate esters: mutagenic and other genotoxic effects.
Environ. Health Perspect., 65: 255-262.
ECETOC (1985) An assessment of the occurrence and effects of dialkyl
ortho-phthalates in the environment, Brussels, European Chemical
Industry Ecology and Toxicology Centre, 63 pp (Technical Report No.
19).
ECETOC (1992) Hepatic peroxisome proliferation, Brussels, European
Chemical Industry Ecology and Toxicology Centre, (Monograph No. 17).
EGLINTON, G., SIMONEIT, B.R.T., & ZORO, J.A. (1975) The recognition of
organic pollutants in aquatic sediments. Proc. R. Soc. Lond., B189:
415-442.
EISENREICH, S.J., LOONEY, B.B., & THORNTON, J.D. (1981) Airborne
organic contaminants in the Great Lakes ecosystem. Environ. Sci.
Technol., 15: 30-38.
ELCOMBE, C.R. (1985) Species differences in carcinogenicity and
peroxisome proliferation due to trichloroethylene: A biochemical human
hazard assessment.Arch. Toxicol., Suppl. 8: 6-17.
ELCOMBE, C.R. & MITCHELL, A.M. (1986) Peroxisome proliferation due to
di(2-ethylhexyl)phthalate (DEHP): Species differences and possible
mechanisms. Environ. Health Perspect., 70: 211-219.
ELCOMBE, C.R. & STYLES J.A. (1989) Species differences in peroxisome
proliferation. Toxicologist, 9: 63.
ELSISI, A.E., CARTER, D.E., & SIPES, I.G. (1989) Dermal absorption of
phthalate diesters in rats. Fundam. appl. Toxicol., 12: 70-77.
ENGELHARDT, G. & WALLNOFER, P.R. (1978) Metabolism of di- and mono-N-
butyl phthalate by soil bacteria. Appl. environ. Microbiol., 35:
243-246.
ENGELHARDT, G., WALLNOFER, P.R., & HUTZINGER, O. (1975) The microbial
metabolism of di-n-butyl phthalate and related dialkyl phthalates.
Bull. environ. Contam. Toxicol., 13: 342-347.
ENGELHARDT, G., TILLMANNS, G., WALLNOFER, P.R., & HUTZINGER, O. (1977)
Biodegradation of di-iso-butyl phthalate and related dialkyl
phthalates by Penicillium lilacinum. Chemosphere, 6: 347-354.
ENVIRONMENT AGENCY OF JAPAN (1989) [Chemicals in the environment],
Tokyo, Environment Agency of Japan, p. 418 (in Japanese).
FATOKI, O.S. & VERNON, F. (1990) Phthalate esters in rivers of the
greater Manchester area, U.K. Sci. total Environ., 95: 221-232.
FISHBEIN, L. & ALBRO, P.W. (1972) Chromatographic and biological
aspects of the phthalate esters. J. Chromatogr., 70: 365-412.
FOSTER, P.M.D., LAKE, B.G., COOK, M.W., THOMAS, L.V., & GANGOLLI, S.D.
(1982) Structure-activity requirements for the induction of testicular
atrophy by butyl phthalates in immature rats: effects on testicular
zinc content. Adv. exp. Med. Biol., 136A: 445-452.
FOSTER, P.M.D., THOMAS, L.V., COOK, M.W., & WALTERS, D.G. (1983)
Effect of di-n-pentyl phthalate treatment on testicular steroidogenic
enzymes and cytochrome P-450 in the rat. Toxicol. Lett., 15: 265-271.
GANNING, A.E., OLLSON, M.J., PETERSON, E., & DALLNER., G. (1989) Fatty
acid oxidation in hepatic peroxisomes and mitochondria after treatment
of rats with di(2-ethylhexyl) phthalate. Pharmacol. Toxicol., 65:
265-268.
GIAM, C.S., CHAN, H.S., NEFF, G.S., & ATLAS, E.L. (1978) Phthalate
ester plasticizers: A new class of marine pollutant. Science, 199:
419-421.
GIAM, C.S., ATLAS, E., CHAN, H.S., & NEFF, G.S. (1980) Phthalate
esters, PCB and DDT residues in the Gulf of Mexico atmosphere. Atmos.
Environ., 14: 65-69.
GILIOLI, R., BULGHERONI, C., TERRANA, T., FILIPPINI, G., MASSETTO, N.,
& BOERI, R. (1978) [A neurological, electromyographic and
electroneurographic study in subjects working at the production of
phthalate plasticizers.] Med. Lav., 69: 620-631 (in Italian).
GOEL, S.K., LALWANI, N.D., & REDDY, J.K. (1986) Peroxisome
proliferation and lipid peroxidation in rat liver. Cancer Res., 46:
1324-1330.
GOLLAMUDI, R., LAWRENCE, W.H., RAO, R.H., & AUTIAN, J. (1985) Effects
of phthalic acid esters on drug metabolizing enzymes of rat liver. J.
appl. Toxicol., 5: 368-371.
GOTO, M. (1979) Monitoring environmental materials and specimen
banking - State-of-the-art of Japanese experience and knowledge. In:
Luepke, N.-P., ed. Monitoring environmental materials and specimen
banking. Proceedings of an International Workshop, Berlin (West),
23-28 October, 1978, pp. 271-288.
GRAHAM, P.R. (1973) Phthalate ester plasticizers - Why and how they
are used. Environ. Health Perspect., 3: 3-12.
GRAY, T.J.B. & BEAMAND, J.A. (1984) Effect of some phthalate esters
and other testicular toxins on primary cultures of testicular cells.
Food chem. Toxicol., 22: 123-131. GRAY, T.J.B. & BUTTERWORTH, K.R.
(1980) Testicular atrophy produced by phthalate esters. Arch.
Toxicol., Suppl. 4: 452-455.
GRAY, T.J.B. & GANGOLLI, S.D. (1986) Aspects of the testicular
toxicity of phthalate esters. Environ. Health Perspect., 65: 229-235.
GRAY, T.J.B., BUTTERWORTH, K.R., GAUNT, I.F., GRASSO, P., & GANGOLLI,
S.D. (1977) Short-term toxicity study of di-(2-ethylhexyl) phthalate
in rats. Food Cosmet. Toxicol., 15: 389-399.
GRAY, T.J.B., ROWLAND, I.R., FOSTER, P.M.D., & GANGOLLI, S.D. (1982)
Species differences in the testicular toxicity of phthalate esters.
Toxicol. Lett., 11: 141-147.
GUESS, W.L. & HABERMAN, S. (1968) Toxicity profiles of vinyl and
polyolefinic plastics and their additives. J. biomed. Mater. Res., 2:
313-335.
GUPTA, R.C., GOEL, S.K., EARLEY, K., SINGH, B., & REDDY, J.K. (1985)
32P-Postlabeling analysis of peroxisome proliferator-DNA adduct
formation in rat liver in vivo and hepatocytes in vitro.
Carcinogenesis, 6: 933-936.
HAMANO, Y., INOUE, K., ODA, Y., YAMAMOTO, H., & KUNITA, N. (1979)
[Studies on the toxicity of phthalic acid esters. Part 2. Dominant
lethal tests for DEHP and MEHP in mice, Osaka, Osaka Public Health and
Sanitation Research Centre, pp. 1-4 (Food Hygiene Series No. 10) (in
Japanese).
HARRIS, R.S., HODGE, H.C., MAYNARD, E.A., & BLANCHET, H.J., Jr (1956)
Chronic oral toxicity of 2-ethylhexyl phthalate in rats and dogs. Am.
Med. Assoc. Arch. ind. Health, 13: 259-264.
HERRING, R. & BERING, C.L. (1988) Effects of phthalate esters on plant
seedlings and reversal by a soil microorganism. Bull. environ. Contam.
Toxicol., 40: 626-632.
HILL, E.F., HEATH, R.G., SPANN, J.W., & WILLIAMS, J.D. (1975) Lethal
dietary toxicities of environmental pollutants to birds, Washington,
DC, US Fish and Wildlife Service, 61 pp (Special Scientific Report
Wildlife No. 191).
HOBSON, J.F., CARTER, D.E., & LIGHTNER, D.V. (1984) Toxicity of a
phthalate ester in the diet of a penaied shrimp. J. Toxicol. environ.
Health, 13: 959-968.
HODGE, H.C. (1943) Acute toxicity for rats and mice of di-2-ethylhexyl
phthalate with a note upon the mechanism. Proc. Soc. Exp. Biol. Med.,
53: 20-23.
HOLLIFIELD, H.C. (1979) Rapid nephelometric estimate of water
solubility of highly insoluble organic chemicals of environmental
interest. Bull. environ. Contam. Toxicol., 23: 579-586.
HOWARD, P.H., BANERJEE, S., & ROBILLARD, K.H. (1985) Measurement of
water solubilities, octanol/water partition coefficients and vapour
pressures of commercial phthalate esters. Environ. Toxicol. Chem., 4:
653-661.
HRUDEY, S.E., SERGY, G.A., & THACKERAY, T. (1976) Toxicity of oil
sands plant wastewaters and associated organic contaminants. In:
Proceedings of the 11th Canadian Symposium on Water Pollution
Research, Ottawa, Water Pollution Research Canada, pp. 34-45.
IARC (1982) Di(2-ethylhexyl) phthalate. In: Some industrial chemicals
and dyestuffs, Lyon, International Agency for Research on Cancer, pp.
269-294 (IARC Monographs on the Evaluation of the Carcinogenic Risks
of Chemicals to Humans, Vol. 29).
IARC (1987) Overall evaluations of carcinogenicity: An updating of
IARC monographs volumes 1 to 42, Lyon, International Agency for
Research on Cancer, 440 pp (IARC Monographs on the Evaluation of
Carcinogenic Risks to Humans, Supplement 7).
ISHIDA, M., SUYAMA, K., & ADACHI, S. (1980) Background contamination
by phthalates commonly encountered in the chromatographic analysis of
lipid samples. J. Chromatogr., 189: 421-424.
ISHIDA, M., SUYAMA, K., & ADACHI, S. (1981) Occurrence of dibutyl and
di-2-ethylhexyl phthalate in chicken eggs. J. agric. food Chem., 29:
72-74.
ISHIDA, M., SUYAMA, K., ADACHI, S., & HOSHINO, T. (1982) Distribution
of orally administered diethylhexyl phthalate in laying hens. Poult.
Sci., 61: 262-267.
ISSEMANN, I. & GREEN, S. (1990) Activation of a member of the steroid
hormone receptor superfamily by peroxisome proliferators.
Nature(Lond.): 347: 645-650.
JAEGER, R.J. & RUBIN, R.J. (1972) Migration of a phthalate ester
plasticizer from polyvinyl chloride blood bags into stored human blood
and its localization in human tissues. New Engl. J. Med., 287:
1114-1118.
JOHNSON, B.T. & LULVES, W. (1975) Biodegradation of di-butyl phthalate
and di-2-ethylhexyl phthalate in freshwater hydrosoil. J. Fish Res.
Board Can., 32: 333-339.
JOHNSON, B.T., HEITKAMP, M.A., & JONES, J.R. (1984) Environmental and
chemical factors influencing the biodegradation of phthalic acid
esters in freshwater sediments. Environ. Pollut., B8: 101-118.
KAMATA, I., TSUTSUI, G., TAKANA, J., & SHIRAI, T. (1978) [Phthalic
acid esters in fish in the Uji river.] Kyoto-fu Eisei Kogai Kenkyusho
Nempo, 22: 114 (in Japanese).
KARARA, A.H. & HAYTON, W.L. (1989) A pharmacokinetic analysis of the
effect of temperature on the accumulation of di-2-ethylhexyl phthalate
(DEHP) in sheepshead minnow. Aquat. Toxicol., 15: 27-36.
KARASEK, F.W., DENNEY, D.W., CHAN, K.W., & CLEMENT, R.E. (1978)
Analysis of complex organic mixtures on airborne particulate matter.
Anal. Chem., 50: 82-87.
KASAI, H., OKADA, Y., NISHIMURA, S., RAO, M.S., & REDDY, J.K. (1989)
Formation of 8-hydroxydeoxyguanosine in liver DNA of rats following
long-term exposure to a peroxisome proliferator.Cancer Res., 49:
2603-2605.
KATAEVA, S.W. (1988) [The method for determination of dioctyl
phthalates in water and model media imitating foodstuffs.] Gig. i
Sanit., 1: 52-53 (in Russian).
KEYSER, P., PUJAR, G.P., EATON, R.W., & RIBBONS, D.W. (1976)
Biodegradation of the phthalates and their esters by bacteria.
Environ. Health Perspect., 18: 159-166.
KIRBY, P.E., PIZZARELLO, R.F., LAWLOR, T.E., HAWORTH, S.E., & HODGSON,
J.R. (1983) Evaluation of di(2-ethylhexyl) phthalate and its major
metabolites in the Ames test and L5178Y mouse lymphoma mutagenicity
assay. Environ. Mutagen., 5: 657-663.
KLIMISCH, H.-J., HELLWIG, J., KAUFMANN, W., & JÄCKH, R. (1991) Di-(2-
ethylhexyl)phthalate (DEHP): Investigation of inhalation toxicity in
rats after repeated exposure (28 d). Hum. exp. Toxicol., 3: 68.
KLOPFFER, W., KAUFMANN, G., RIPPEN, G., & POREMSKI, H.J. (1982) A
laboratory method for testing the volatility from aqueous solution:
First results and comparison with theory. Ecotoxicol. environ. Saf.,
6: 545.
KLUWE, W., HASEMAN, J.K., DOUGLAS, J.F., & HUFF, J.E. (1982) The
carcinogenicity of dietary di(2-ethylhexyl) phthalate (DEHP) in Fisher
344 rats and B6C3F1 mice. J. Toxicol. environ. Health, 10: 797-815.
KNOWLES, C.O., MCKEE, M.J., & PALAWSKI, D.U. (1987) Chronic effects of
di-2-ethylhexyl phthalate on biochemical composition, survival and
reproduction of Daphnia magna. Environ. Toxicol. Chem., 6: 201-208.
KODAMA, T. & TAKAI, Y. (1974) [Studies on the determination of
phthalic acid esters, IV. Analysis of phthalic acid ester in fish
meat.] Kogai To Taisaku, 10: 850-852 (in Japanese).
KODAMA, T., KOBAYASKI, K., EBA, H., OGAWA, T., TAKAI, Y., NAITO, N.,
OKADA, N., & SAWANO, Y. (1975) [Studies on the determination of
phthalic acid esters: VI. Investigations in Aichi Prefecture.] Kogai
To Taisaku, 11: 341-350 (in Japanese).
KORA, S., SADO, M., KOLKE, H., & TERADA, H. (1989) Protective effect
of the plasticizer di(2-ethylhexyl) phthalate against damage of the
mitochondrial membrane induced by calcium: possible participation of
the adenine nucleotide translocator. Biochim. Biophys. Acta, 985:
286-292.
KORNBRUST, D.J., BARFKNECHT, T.R., INGRAM, P., & SHELBURNE, J.D.
(1984) Effect of di(2-ethylhexyl)phthalate on DNA repair and lipid
peroxidation in rat hepatocytes and on metabolic cooperation in
Chinese hamster V-79 cells. J. Toxicol. environ. Health, 13: 99-116.
KRAUSKOPF, L.G. (1973) Studies on the toxicity of phthalates via
ingestion. Environ. Health Perspect., 3: 61-72.
KURANE, R., SUZUKI, T., & TAKAHARA, Y. (1977) Isolation of
microorganisms growing on phthalate esters and degradation of
phthalate esters by Pseudomonas acidovorans 256-1. Agric. biol.
Chem., 41: 2119-2123.
KURANE, R., SUZUKI, T., & TAKAHARA, Y. (1978) Removal of phthalate
esters in soil columns inoculated with micro-organisms. Agric. biol.
Chem., 42: 1469-1478.
KURANE, R., SUZUKI, T., & TAKAHARA, Y. (1979) Microbial population and
identification of phthalate ester-utilising micro-organisms in
activated sludge with micro-organisms. Agric. biol. Chem., 43:
907-917.
KURANE, R., SUZUKI, T., & TAKAHARA, Y. (1980) Induction of enzymes
involved in phthalate esters metabolism in Nocardia erythropolis and
enzymatic hydrolysis of phthalate esters by commercial lipases. Agric.
biol. Chem., 44: 529-536.
KURANE, R., SUZUKI, T., TAKAHARA, Y., & FUKUOKA, S. (1984)
Protocatechuate 3,4-dioxygenase from Nocardia erythropolis. Agric.
biol. Chem., 48: 2105-2111.
LAKE, B.G., BRANTOM, P.G., GANGOLLI, S.H., BUTTERWORTH, K.R., &
GRASSO, P. (1976) Studies on the effects of orally administered di(2-
ethylhexyl)phthalate in the ferret. Toxicology, 6: 341-356.
LAKE, B.G., GRAY, T.J.B., FOSTER, J.R., STUBBERFIELD, C.R., &
GANGOLLI, S.D. (1984) Comparative studies on di-(2-ethylhexyl)
phthalate-induced hepatic peroxisome proliferation in the rat and
hamster. Toxicol. appl. Pharmacol., 72: 46-60.
LAKE, B.G., GRAY, T.J.B., & GANGOLLI, S.D. (1986) Hepatic effects of
phthalate esters and related compounds - in vivo and in vitro
correlations. Environ. Health Perspect., 67: 283-290.
LAKE, B.G., KOZLEN, S.L., EVANS, J.G., GRAY, T.J.B., YOUNG, P.J., &
GANGOLLI, S.D. (1987) Effect of prolonged administration of clofibric
acid and di-(2-ethylhexyl) phthalate on hepatic enzyme activities and
lipid peroxidation in the rat. Toxicology, 44: 213-228.
LAKE, B.G., LEWIS, D.F.V. & GRAY, T.J.B. (1988) Structure-activity
relationships for hepatic peroxisome proliferation. Arch. Toxicol.,
Suppl. 12: 217-224.
LAKE, B.G., COOK, W.M., WORRELL, N.R., CUNNINGHAME, M.E., EVANS, J.G.,
PRICE, R.J., YOUNG, P.J., & CARPANINI, F.M.B. (1991) Dose-response
relationships for induction of hepatic peroxisome proliferation and
testicular atrophy by phthalate esters in the rat. Hum. exp. Toxicol.,
10(1): 67.
LALWANI, N.D., ALVARES, K., REDDY, M.K., REDDY, M.N., PARIKH, I., &
REDDY, J.K. (1987) Peroxisome proliferator-binding protein:
Identification and partial characterization of nafenopin-, clofibric
acid-, and clofibrate-binding proteins from rat liver. Proc. Natl
Acad. Sci. (USA), 84: 5242-5246.
LARSSON, P. & THUREN, A. (1987) Di-2-ethylhexylphthalate inhibits the
hatching of frog eggs and is bioaccumulated by tadpoles. Environ.
Toxicol. Chem., 6: 417-422.
LARSSON, P., THUREN, A., & GAHNSTROM, G. (1986) Phthalate esters
inhibit microbial activity in aquatic sediments. Environ. Pollut., 42:
223-231.
LAUGHLIN, R.B., NEFF, J.M., HRUNG, Y.C., GOODWIN, T.C., & GIAM, C.S.
(1978) The effects of three phthalate esters on the larval development
of the grass shrimp Palaemonetes pugio (Holthuis). Water Air Soil
Pollut., 9: 323-336.
LAWRENCE, W.H., MALIK, M., TURNER, J.E., SINGH, A.R., & AUTIAN, J.
(1975) A toxicological investigation of some acute, short-term, and
chronic effects of administering di-2-ethylhexyl phthalate (DEHP) and
other phthalate esters. Environ. Res., 9: 1-11.
LEBLANC, G.A. (1980) Acute toxicity of priority pollutants to water
flea (Daphnia magna). Bull. environ. Contam. Toxicol., 24: 684-691.
LEWANDOWSKI, M., FERNANDES, J., & CHEN, T.S. (1980) Assessment of the
teratogenic potential of plasma-soluble extracts of diethylhexyl
phthalate plasticized polyvinyl chloride plastics in rats. Toxicol.
appl. Pharmacol., 54: 141-147.
LEWIS, L.M., FLECHTNER, T.W., KERKAY, J., PEARSON, K.H., CHEN, W.T.,
POPOWNIAK, K.L., & NAKAMOTO, S. (1977) Determination of plasticizer
levels in serum of hemodialysis patients. Trans. Am. Soc. Artif.
Intern. Organs, 23: 566-572.
LEYDER, F. & BOULANGER, P. (1983) Ultraviolet absorption, aqueous
solubility, and octanol-water partition for several phthalates. Bull.
environ. Contam. Toxicol., 30: 152-157.
LHUGUENOT, J.C. & ELCOMBE, C.R. (1984) Variation selon l'espèce des
phénomènes de conjugasion lors du métabolisme mono-(éthyl-2-hexyl)
phthalate (MEHP). Sci. Aliments, 4: 227-232.
LHUGUENOT, J.C., MITCHELL, A.M., MILNER, G., LOCK, E.A., & ELCOMBE,
C.R. (1985) The metabolism of di(2-ethylhexyl)phthalate (DEHP) and
mono-(2-ethylhexyl) phthalate (MEHP) in rats: In vivo and in vitro
dose and time dependency of metabolism. Toxicol. appl. Pharmacol., 80:
11-22.
LINDEN, E., BENGTSSON, B.-E., SVANBERG, O., & SUNDSTROM, G. (1979) The
acute toxicity of 78 chemicals and pesticide formulations against two
brackish water organisms, the bleak (Alburnus alburnus) and the
harpacticoid (Nitocra spinipes). Chemosphere, 8: 843-851.
LISS, G.M., ALBRO, P.W., HARTLE, R.W., & STRINGER, W.T. (1985) Urine
phthalate determinations as an index of occupational exposure to
phthalic anhydride and di(2-ethylhexyl) phthalate. Scand. J. Work
Environ. Health, 11: 381-387.
LLOYD, S.C. & FOSTER, P.M.D. (1988) Effect of mono-(2-ethylhexyl)
phthalate on follicle-stimulating hormone responsiveness of cultured
rat Sertoli cells. Toxicol. appl. Pharmacol., 95: 484-489.
LOCK, E.A., MITCHELL, A.M.R., & ELCOMBE, C.R. (1989) Biochemical
mechanisms of induction of hepatic peroxisome proliferation. Annu.
Rev. Pharmacol. Toxicol., 29: 145-168.
LOKKE, H. & BRO-RASMUSSEN, F. (1981) Studies of mobility of di-iso-
butyl phthalate (DiBP), di-n-butyl phthalate (DBP), and di-(2-
ethylhexyl) phthalate (DEHP) by plant foliage treatment in a closed
terrestrial simulation chamber. Chemosphere, 10: 1223-1235.
LOKKE, H. & RASMUSSEN, L. (1983) Phytotoxicological effects of di-(2-
ethylhexyl)-phthalate and n-butyl-phthalate on higher plants in
laboratory and field experiments. Environ. Pollut., 32: 179-199.
LUTZ, W.K. (1986) Investigation of the potential for binding of di(2-
ethylhexyl) phthalate (DEHP) to rat liver DNA in vivo. Environ.
Health Perspect., 65: 267-269.
MACEK, K.J., PETROCELLI, S.R., & SLEIGHT, B.H., III (1979)
Considerations in assessing the potential for, and significance of,
biomagnification of chemical residues in aquatic food chains. In:
Aquatic toxicology, Philadelphia, American Society for Testing and
Materials, pp. 251-268 (ASTM STP 667).
MAFF (1987) Survey of plasticiser levels in food contact materials and
in foods, London, Ministry of Agriculture, Fisheries and Food (Food
Surveillance Paper No. 21).
MALCOLM, A.R. & MILLS, L.J. (1989) Inhibition of gap-junctional
intercellular communication between Chinese hamster lung fibroblasts
by di(2-ethylhexyl) phthalate (DEHP) and trisodium nitrilotriacetate
monohydrate (NTA). Cell Biol. Toxicol., 5: 145-153.
MANANDHAR, M.D., SHOEB, A., KAPIL, R.S., & POPLI, S.P. (1979) Studies
in medicinal plants. Part V, Di-2-n-propyl pentyl phthalate and
reticulene from Cryptocaryo amyggdalina Nees. Indian J. Chem., 178:
411-412.
MARCEL, Y.L. & NOEL, S.P. (1970) Contamination of blood stored in
plastic packs. Lancet, 1: 35-36.
MARSMAN, D.S., CATTLEY, R.C., CONWAY, J.G., & POPP, J.A. (1988)
Relationships of hepatic peroxisome proliferation and replicative DNA
synthesis to the hepatocarcinogenicity of the peroxisome proliferators
di(2-ethylhexyl) phthalate and [4-chloro-6-(2,3-xylidino)-2-
pyrimidinylthio]acetic acid (Wy-14,643) in rats. Cancer Res., 48:
6739-6744.
MATHUR, S.P. (1974a) Phthalate esters in the environment: Pollutants
or natural products. J. environ. Qual., 3: 189.
MATHUR, S.P. (1974b) Respirometric evidence of the utilization in soil
of di-octyl and di-2-ethylhexyl phthalate plasticizers. J. environ.
Qual., 3: 207-209.
MATSUDA, K. & SCHNITZER, M. (1971) Reactions between fulvic acid, a
soil humic material, and dialkyl phthalates. Bull. environ. Contam.
Toxicol., 6: 200-204.
MAYER, F.L. (1976) Residue dynamics of di-2-ethylhexyl phthalate in
fathead minnows (Pimephales promelas). J. Fish Res. Board Can., 33:
2610-2613.
MAYER, F.L. & SANDERS, H.O. (1973) Toxicology of phthalic acid esters
in aquatic organisms. Environ. Health Perspect., 3: 153-157.
MAYER, F.L., STALLING, D.L., & JOHNSON, J.L. (1972) Phthalate esters
as environmental contaminants. Nature (Lond.), 238: 411-413.
MAYER, F.L., MEHRLE, P.M., & SCHOETTGER, R.A. (1977) Collagen
metabolism in fish exposed to organic chemicals. In: Symposium on
Recent Advances in Fish Toxicology, Washington, DC, US Environmental
Protection Agency, p. 31 (EPA 600/3-77-085).
MEHRLE, P.M. & MAYER, F.L. (1976) Di-2-ethylhexyl phthalate: Residue
dynamics and biological effects in rainbow trout and fathead minnow.
Trace Subst. environ. Health, 10: 519-524.
MELANCON, M.J. (1979) Metabolism of phthalate esters in aquatic
species. In: Pesticides and xenobiotic metabolism in aquatic
organisms, Washington, DC, American Chemical Society, pp. 77-94 (ACS
Symposium Series No. 99).
MELNICK, R.L., MORRISSEY, R.E., & TOMASZEWSKI, K.E. (1987) Studies by
the National Toxicology Program on di(2-ethylhexyl) phthalate.
Toxicol. ind. Health, 3: 99-118.
MES, J., COFFIN, D.E., & CAMPBELL, D.S. (1974) Di-n-butyl and di-2-
ethylhexyl phthalate in human adipose tissue. Bull. environ. Contam.
Toxicol., 12: 721-725.
METCALF, R.L., BOOTH, G.M., SCHUTH, C.K., HANSEN, D.J., & LU, P.-Y.
(1973) Uptake and fate of di-2-ethylhexyl phthalate in aquatic
organisms and in a model ecosystem. Environ. Health Perspect., 4:
27-34.
MIKALSEN, S.O., HOLEN, I., & SANNER, T. (1990a) Morphological
transformation and catalase activity of Syrian hamster embryo cells
treated with hepatic peroxisome proliferators, TPA and nickel
sulphate. Cell Biol. Toxicol., 6: 1-13.
MIKALSEN, S.O., KAALHUS, O., REITH, A., & SANNER, T. (1990b) Role of
catalase and oxidative stress in hepatic peroxisome proliferator-
induced morphological transformation of Syrian hamster embryo cell.
Int. J. Cancer, 46: 950-957.
MIKALSEN, S.O., REUTER, B., & SANNER, T. (1990c) Effects of hepatic
peroxisome proliferators and 12-O-tetradecanoyl phorbol-13-acetate on
catalase and other enzyme activities of embryonic cells in vitro.
Biochem. Pharmacol., 39: 527-535.
MILKOV, L.E., ALDYREVA, M.V., POPOVA, T.B., LOPUKHOVA, K.A.,
MAKARENKO, Y.L., MALYAR, L.M., & SHAKHOVA, T.K. (1973) Health status
of workers exposed to phthalate plasticizers in the manufacture of
artificial leather and films based on PVC resins. Environ. Health
Perspect., 3: 175-178.
MITCHELL, A.M., LHUGUENOT, J.-C., BRIDGES, J.W., & ELCOMBE, C.R.
(1985a) Identification of the proximate peroxisome proliferator(s)
derived from di(2-ethylhexyl) phthalate. Toxicol. appl. Pharmacol.,
80: 23-32.
MITCHELL, F.E., PRICE, S.C., HINTON, R.H., GRASSO, P., & BRIDGES, J.W.
(1985b) Time and dose-response study of the effects on rats of the
plasticizer di(2-ethylhexyl) phthalate. Toxicol. appl. Pharmacol., 81:
371-392.
MORI, S. (1976) Identification and determination of phthalate esters
in river water by high-performance liquid chromatography. J.
Chromatogr., 129: 53-60.
MUSIAL, C.J. & UTHE, J.F. (1980) Studies on phthalate esters in
Western Atlantic finfish. Report to International Council for the
Exploration of the Sea (ICES), Copenhagen, International Council for
the Exploration of the Sea, pp. 1-6 (Report E:11).
MUTZ, R.C. & JONES, J.R. (1977) The effects of phthalate esters on
geochemical cycles in freshwater hydrosoil. Trans. Missouri Acad.
Sci., 10-11: 296.
AZIR, D.J., ALCARAZ, A.P., BIERL, B.A., BEROZA, M., & NAIR, P.P.
(1971) Isolation, identification, and specific localization of di-2-
ethylhexyl phthalate in bovine heart muscle mitochondria.
Biochemistry, 10: 4228-4232.
NEERGAARD, J., NIELSEN, B., FAURBY, V., CHRISTENSEN, D.H., & NIELSEN,
O.F. (1971) Plasticizers in P.V.C. and the occurrence of hepatitis in
a haemodialysis unit. Scand. J. Urol. Nephrol., 5: 141-145.
NEUHAUSER, E.F., LOER, R.C., & MALECKI, M.R. (1986) Contact and
artificial soil tests using earthworms to evaluate the impact of
wastes in soil. In: Petros, J.K., Jr, Lacy, W.J., & Conway, R.A., ed.
Hazardous and industrial solid waste testing: Fourth symposium,
Philadelphia, American Society for Testing and Materials, pp. 192-203
(ASTM STP 866).
NIELSEN, J., ÅKESSON, B., & SKERFVING, S. (1985) Phthalate ester
exposure - air levels and health of workers processing
polyvinylchloride. Am. Ind. Hyg. Assoc. J., 46: 643-647.
NIKONOROW, M., MAZUR, H., & PIEKACZ, H. (1973) Effect of orally
administered plasticizers and polyvinyl chloride stabilizers in the
rat. Toxicol. appl. Pharmacol., 26: 253-259.
NIOSH (1977) Manual of analytical methods, 2nd ed., Cincinnati, Ohio,
National Institute for Occupational Safety and Health, Vol. 2, pp.
S40/1-S40/9.
NIOSH (1985a) Manual of analytical methods, 3rd ed., Cincinnati, Ohio,
National Institute for Occupational Safety and Health, Vol. 1, pp.
5020/1-5020/4.
NIOSH (1985b) Registry of toxic effects of chemical substances:
1983-84 Supplement, Cincinnati, Ohio, National Institute for
Occupational Safety and Health, Vol. II, p. 1442.
NTP (1982) Technical report on the carcinogenicity bioassay of di(2-
ethylhexyl) phthalate, Research Triangle Park, NC, National Toxicology
Programme (DHHS Publ. No. (NIH) 82-1773).
O'CONNOR, O.A., RIVERA, M.D., & YOUNG, L.Y. (1989) Toxicity and
biodegradation of phthalic acid esters under methanogenic conditions.
Environ. Toxicol. Chem., 8: 569-576.
OHYAMA, T. (1977) Effects of phthalate esters on glucose-6-phosphate
dehydrogenase and other enzymes in vitro. Toxicol. appl. Pharmacol.,
40: 355-364.
OISHI, S. (1984) Testicular atrophy of rats induced by di-2-ethylhexyl
phthalate: effects of vitamin A and zinc concentrations in the testis,
liver and serum. Toxicol. Lett., 20: 75-78.
OISHI, S. (1985) Reversibility of testicular atrophy induced by di(2-
ethylhexyl) phthalate in rats. Environ. Res., 36: 160-169.
OISHI, S. (1989a) Effects of co-administration of di(2-ethylhexyl)
phthalate and testosterone on several parameters in the testis and
pharmacokinetics of its mono-deesterified metabolite. Arch. Toxicol.,
63: 289-295.
OISHI, S. (1989b) Enhancing effects of luteinizing hormone-releasing
hormone on testicular damage induced by di(2-ethylhexyl) phthalate in
rats. Toxicol. Lett., 47: 271-277.
OISHI, S. (1990) Effects of phthalic acid esters on testicular
mitochondrial functions in the rat. Appl. Toxicol., 64: 143-147.
OISHI, S. & HIRAGA, K. (1979) Effect of phthalic acid esters on
gonadal function in male rats. Bull. environ. Contam. Toxicol., 21:
65-67.
OISHI, S. & HIRAGA, K. (1980a) Testicular atrophy induced by phthalic
esters: effect on testosterone and zinc concentrations. Toxicol. appl.
Pharmacol., 53: 35-41.
OISHI, S. & HIRAGA, K. (1980b) Effect of phthalic acid monoesters on
mouse testes. Toxicol. Lett., 6: 239-242.
OISHI, S. & HIRAGA, K. (1982) Effects of monoesters of o-phthalic acid
on serum lipid composition in rats. Toxicol. Lett., 14: 79-84.
OISHI, S. & HIRAGA, K. (1983) Testicular atrophy induced by di-2-
ethylhexyl phthalate: effect of zinc supplement. Toxicol. appl.
Pharmacol., 70: 43-48.
O'SHEA, T.J. & STAFFORD, C.J. (1980) Phthalate plasticizers:
Accumulation and effects on weight and food consumption in captive
starlings. Bull. environ. Contam. Toxicol., 25: 345-352.
OSUMI, T. & HASHIMOTO, T. (1978) Enhancement of fatty acyl-CoA
oxidizing activity in rat liver peroxisomes by di-(2-
ethylhexyl)phthalate. J. Biochem., 83: 1361-1365.
PARE, J.R.J., BELANGER, J., & JANKOWSKI, K. (1981) Asymmetric
phthalate from Sansevieria trifasciata. J. nat. Prod., 44: 490-492.
PASSINO, D.R.M. & SMITH, S.B. (1987) Acute bioassays and hazard
evaluation of representative contaminants detected in Great Lakes
fish. Environ. Toxicol. Chem., 6: 901-907.
PEAKALL, D.B. (1974) Effects of di-n-butyl and di-2-ethylhexyl
phthalate on the eggs of ring doves. Bull. environ. Contam. Toxicol.,
12: 698-702.
PEAKALL, D.B. (1975) Phthalate esters: Occurrence and biological
effects. Res. Rev., 54: 1-40.
PERERA, M.I.R., KATYAL, S.L., & SHINOZUKA, H. (1986) Suppression of
choline-deficient diet-induced hepatocyte membrane lipid peroxidation
in rats by the peroxisome proliferators 4-chloro-6-(2,3-xylidino)-2-
pyrimidinylthio(N-ß-hydroxyethyl)acetamide and di(2-
ethylhexyl)phthalate. Cancer Res., 46: 3304-3308.
PERKINS, E.G. (1967) Characterization of the nonvolatile compounds
formed during the thermal oxidation of corn oil. II. Phthalate esters.
J. Am. Oil Chem. Soc., 44: 197-199.
PERSSON, P.-E., PENTTINEN, H., & NUORTEVA, P. (1978) DEHP in the
vicinity of an industrial area in Finland. Environ. Pollut., 16:
163-166.
PETERSON, J.C. & FREEMAN, D.H. (1984) Variations of phthalate ester
concentrations in sediments from the Chester river, Maryland. Int. J.
environ. anal. Chem., 18: 237-252.
PFUDERER, P. & FRANCIS, A.A. (1975) Phthalate esters: Heartrate
depressors in the goldfish. Bull. environ. Contam. Toxicol., 13:
275-279.
PHILLIPS, B.J., ANDERSON, D., & GANGOLLI, S.D. (1986) Studies on the
genetic effects of phthalic acid esters on cells in culture. Environ.
Health Perspect., 65: 263-266.
PIECHOCKI, J.T. & PURDY, W.C. (1973) Determination of di-(2-
ethylhexyl)-phthalate (DEHP) in human plasma. Clin. Chim. Acta, 48:
385-391.
POLLACK, G.M., BUCHANAN, J.F., SLAUGHTER, R.L., KOHLI, R.K., & SHEN,
D.D. (1985a) Circulating concentrations of di(2-ethylhexyl) phthalate
and its de-esterified phthalic acid products following plasticizer
exposure in patients receiving hemodialysis. Toxicol. appl.
Pharmacol., 79: 257-267.
POLLACK, G.M., LI, R.C.K., ERMER, J.C., & SHEN, D.D. (1985b) Effects
of route of administration and repetitive dosing on the disposition
kinetics of di(2-ethylhexyl) phthalate and its mono-de-esterified
metabolite in rats. Toxicol. appl. Pharmacol., 79: 246-256.
POLLACK, G.M., SHEN, D.D., & DORR, M.B. (1989) Contribution of
metabolites to the route- and time-dependent hepatic effects of di-(2-
ethylhexyl)phthalate in the rat. J. Pharmacol. exp. Ther., 248:
176-181.
POPP, J.A., GARVEY, L.K., & CATTLEY, R.C. (1987) In vivo studies on
the mechanism of di(2-ethylhexyl) phthalate carcinogenesis. Toxicol.
ind. Health, 3: 151-163.
PRESTON, M.R. & AL-OMRAN, L.A. (1986) Dissolved and particulate
phthalate esters in the river Mersey estuary. Mar. Pollut. Bull., 17:
548-553.
PRESTON, M.R. & AL-OMRAN, L.A. (1989) Phthalate ester speciation in
estuarine water, suspended particulates and sediments. Environ.
Pollut., 62: 183-193.
PUTMAN, D.L., MOORE, W.A., SCHECHTMAN, L.M., & HODGSON, J.R. (1982)
Cytogenetic evaluation of di-(2-ethylhexyl)phthalate and its major
metabolites in Fischer 344 rats. Environ. Mutagen., 4: 387.
RAO, M.S. & REDDY, J.K. (1987) Peroxisome proliferation and
hepatocellular hepatocarcinogenesis. Carcinogenesis, 8: 631-636.
RAO, M.S., YELDANDI, A.V.P., & SUBBARAO, V. (1990) Quantitative
analysis of hepatocellular lesions induced by di(2-
ethylhexyl)phthalate in F344 rats. J. Toxicol. environ. Health, 30:
85-89.
RAO, P.S.C., HORNSBY, A.G., & JESSUP, R.E. (1985) Indices for ranking
the potential for pesticide contamination of groundwater. Soil Crop
Sci. Soc. Proc., 44: 1-8.
RAY, L.E., MURRAY, H.E., & GIAM, C.S. (1983a) Organic pollutants in
marine samples from Portland, Maine. Chemosphere, 12: 1031-1038.
RAY, L.E., MURRAY, H.E., & GIAM, C.S. (1983b) Analysis of water and
sediment from the Nueces estuary/Corpus Christi bay (Texas) for
selected organic pollutants. Chemosphere, 12: 1039-1045.
REDDY, J.K. & LALWANI, N.D. (1983) Carcinogenesis by hepatic
peroxisome proliferators: evaluation of the risk of hypolipidemic
drugs and industrial plasticizers to humans. CRC crit. Rev. Toxicol.,
12: 1-58.
REDDY, J.K. & RAO, M.S. (1989) Oxidative DNA damage caused by
persistent peroxisome proliferation: its role in hepatocarcinogenesis.
Mutat. Res., 214: 63-68.
REDDY, J.K., REDDY, M.K., USMAN, M.I., LALWANI, N.D., & RAO, M.S.
(1986) Comparison of hepatic peroxisome proliferative effect and its
implication for hepatocarcinogenicity of phthalate esters, di(2-
ethylhexyl)phthalate, and di(2-ethylhexyl) adipate with a
hypolipidemic drug. Environ. Health Perspect., 65: 317-327.
RHODES, C., ORTON, T.C., PRATT, I.S., BATTEN, P.L., BRATT, H.,
JACKSON, S.J., & ELCOMBE, C.R. (1986) Comparative pharmacokinetics and
subacute toxicity of di(2-ethylhexyl)phthalate (DEHP) in rats and
marmosets: extrapolation of effects in rodents to man. Environ. Health
Perspect., 65: 299-308.
RITSEMA, R., COFINO, W.P., FRINTROP, P.C.M., & BRINKMAN, V.A.T. (1989)
Trace-level analysis of phthalate esters in surface water and
suspended particulate matter by means of capillary gas chromatography
with electron-capture and mass-selective detection. Chemosphere, 18:
2161-2175.
ROTH, B., HERKENRATH, P., LEHMANN, H.J., OHLES, H.D., HÖMIG, H.J.,
BENZ-BOHM, G., KREUDER, J., & YOUNOSSI-HARTENSTEIN, A. (1988) Di-(2-
ethylhexyl)phthalate as plasticizer in PVC respiratory tubing systems:
indications of hazardous effects on pulmonary function in mechanically
ventilated, preterm infants. Eur. J. Pediatr., 147: 41-46.
RUBIN, R.J. & CHANG, J.F.C. (1978) Effect of the intravenous
administration of the solubilized plasticizer di(2-ethylhexyl)
phthalate on the lung and on survival of transfused rats. Toxicol.
appl. Pharmacol., 45: 230.
RUBIN, R.J. & JAEGER, R.J. (1973) Some pharmacologic and toxicologic
effects of di-2-ethylhexyl phthalate (DEHP) and other plasticizers.
Environ. Health Perspect., 3: 53-59.
RUBIN, R.J. & NAIR, P.P. (1972) Plasticizers in human tissues. New
Engl. J. Med., 288: 915-916.
RUDDICK, J.A., VILLENEUVE, D.C., CHU, I., NESTMANN, E., & MILES, D.
(1981) An assessment of the teratogenicity in the rat and mutagenicity
in Salmonella of mono-2-ethylhexyl phthalate. Bull. environ. Contam.
Toxicol., 27: 181-186.
RUSHBROOK, C.L., JORGENSON, T.A., & HODGSON, J.R. (1982) Dominant
lethal study of di-(2-ethylhexyl)phthalate and its major metabolites
in ICR/SIM mice. Environ. Mutagen., 4: 387.
SAEGER, V.W. & TUCKER, E.S. (1973) Biodegradation of phthalate esters.
In: Flexible vinyls and human safety: An objective analysis. Presented
at the Regional Technical Conference of the Society of Plastics
Engineers, Palisades Section, Kiamesha Lake, New York, 20-22 March,
1973, pp. 105-133.
SAEGER, V.W. & TUCKER, E.S. (1976) Biodegradation of phthalic acid
esters in river water and activated sludge. Appl. environ. Microbiol.,
31: 29-34.
SAKURAI, T., MIYAZAWA, S., & HASHIMOTO, T. (1978) Effect of di(2-
ethylhexyl) phthalate administration on carbohydrate and fatty acid
metabolism in rat liver. J. Biochem., 83: 313-320.
SANDERS, H.O., MAYER, F.L., & WALSH, D.F. (1973) Toxicity, residue
dynamics, and reproductive effects of phthalate esters in aquatic
invertebrates. Environ. Res., 6: 84-90.
SAX, N.I. (1984) Dangerous properties of industrial materials, 6th
ed., New York, Van Nostrand Reinhold Company.
SAXENA, D.K., SRIVASTAVA, S.P., CHANDRA, S.V., & SETH, P.K. (1985)
Testicular effects of di(2-ethylhexyl) phthalate (DEHP): histochemical
and histopathological alterations. Ind. Health, 23: 191-198.
SCHMEZER, P., POOL, B.L., KLEIN, R.G., KOMITOWSKI, D., & SCHMÄHL, D.,
(1988) Various short-term assays and two long-term studies with the
plasticizer di(2-ethylhexyl) phthalate in the Syrian hamster.
Carcinogenesis, 9: 37-43.
SCHMID, P. & SCHLATTER, C. (1985) Excretion and metabolism of di(2-
ethyl-hexyl) phthalate in man. Xenobiotica, 15: 251-256.
SCHMIDT, J.G., GARVIN, P.J., & LEESTMA, J.E. (1975) Effect of vehicle
on the response to intravenous di-(2-ethylhexyl) phthalate (DEHP) in
rats. Toxicol. appl. Pharmacol., 33: 169.
SCHMITZER, J.L., SCHEUNERT, I., & KORTE, F. (1988) Fate of bis(2-
ethylhexyl) [14C] phthalate in laboratory and outdoor soil-plant
systems. J. agric. food Chem., 36: 210-215.
SCHNEIDER, B., SCHENA, J., TROUG, R., JACOBSON, M., & KEVY, S. (1989)
Exposure to di(2-ethylhexyl)phthalate in infants receiving
extracorporeal membrane oxygenation. New Engl. J. Med., 320: 1563.
SCHULTE-HERMANN, R., KRAUPP-GRASL, B., BURSCH, W., GERBRACHT, U., &
TIMMERMANN-TROSIENER, I. (1989) Effects of non-genotoxic hepato-
carcinogens phenobarbital and nafenopin on phenotype and growth of
different populations of altered foci in rat liver. Toxicol. Pathol.,
17: 642-650.
SCHULZ, C.O. (1989) Assessing human health risks from exposure to
di(2-ethylhexyl)phthalate (DEHP) and related phthalates: scientific
issues. Drug Metab. Rev., 21: 111-120.
SCHULZ, C.O. & RUBIN, R.J. (1973) Distribution, metabolism, and
excretion of di-2-ethylhexyl phthalate in the rat. Environ. Health
Perspect., 3: 123-129.
SCHULZ, C.O., RUBIN, R.J., & HUTCHINS, G.M. (1975) Acute lung toxicity
and sudden death in rats following the intravenous administration of
the plasticizer, di(2-ethylhexyl)-phthalate, solubilized with Tween
surfactants. Toxicol. appl. Pharmacol., 33: 514-525.
SCHWARTZ, H.E., ANZION, C.J.M., VAN VLIET, H.P.M., COPIUS PEEREBOOMS,
J.W., & BRINMAN, U.A.T. (1979) Analysis of phthalate esters in
sediments from Dutch rivers by means of high performance liquid
chromatography. Intern. J. environ. anal. Chem., 6: 133-144.
SETH, P.K., SRIVASTAVA, S.P., AGARWAL, D.K., & CHANDRA, S.V. (1976)
Effect of di-2-ethylhexyl phthalate (DEHP) on rat gonads. Environ.
Res., 12: 131-138.
SHAFFER, C.B., CARPENTER, C.P., & SMYTH, H.F., Jr (1945) Acute and
subacute toxicity of di(2-ethylhexyl) phthalate with note upon its
metabolism. J. ind. Hyg. Toxicol., 27: 130-135.
SHANKER, R., RAMAKRISHNA, C., & SETH, P.K. (1985) Degradation of some
phthalic acid esters in soil. Environ. Pollut., 39: 1-7.
SHELDON, L.S. & HITES, R.A. (1979) Sources and movement of organic
chemicals in the Delaware river. Environ. Sci. Technol., 13: 574-579.
SHINDO, Y., OSUMI, T., & HASHIMOTO, T. (1978) Effects of
administration of di(2-ethylhexyl) phthalate on rat liver
mitochondria. Biochem. Pharmacol., 27: 2683-2688.
SHIOTA, K. & MIMA, S. (1985) Assessment of the teratogenicity of di(2-
ethylhexyl) phthalate and mono(2-ethylhexyl) phthalate in mice. Arch.
Toxicol., 56: 263-266.
SHIOTA, K. & NISHIMURA, H. (1982) Teratogenicity of di(2-ethylhexyl)
phthalate (DEHP) and di-n-butyl phthalate (DBP) in mice. Environ.
Health Perspect., 45: 65-70. SHIOTA, K., CHOU, M.J., & NISHIMURA, H.
(1980) Embryotoxic effects of di-2-ethylhexyl phthalate (DEHP) and di-
n-butyl phthalate (DBP) in mice. Environ. Res., 22: 245-253.
SHORT, R.D., ROBINSON, E.C., LINGTON, A.W., & CHIN, A.E. (1987)
Metabolic and peroxisome proliferation studies with di(2-ethylhexyl)
phthalate in rats and monkeys. Toxicol. ind. Health, 3: 185-195.
SHUKLA, R.R., ALBRO, P.W., CORBETT, J.T., & SCHROEDER, J.L. (1989) In
vivo studies of the inhibition of protein kinase C from rat brain by
di-(2-ethylhexyl) phthalate. Chem.-biol. Interact., 69: 73-85.
SILVO, O.E.J. (1974) [Preliminary studies on the acute toxicity of
dioctylphthalate (DOP) to rainbow trout ( Salmo gairdneri Richardson)
and its effect upon phytoplankton and oxygen content of the water.]
Suomen Kalatalous, 47: 19-25 (in Finnish).
SINGH, A.R., LAWRENCE, W.H., & AUTIAN, J. (1972) Teratogenicity of
phthalate esters in rats. J. pharm. Sci., 61: 51-55.
SITTIG, M. (1985) Handbook of toxic and hazardous chemicals and
carcinogens, 2nd ed., Park Ridge, New Jersey, Noyes Publications.
SJÖBERG, P., PLÖEN, L., BONDESSON, U., & LINDQUIST, N.G. (1982)
Testicular toxicity of di-(2-ethylhexyl) phthalate in rats of
different ages. Teratology, 26: 20A.
SJÖBERG, P., BONDESSON, U., KJELLEN, L., LINDQUIST, N.-G., MONTIN, G.,
& PLÖEN, L. (1985a) Kinetics of di-(2-ethylhexyl) phthalate in
immature and mature rats and effect on testis. Acta pharmacol.
toxicol., 56: 30-37.
SJÖBERG, P., BONDESSON, U., SEDIN, G., & GUSTAFSSON, J. (1985b)
Dispositions of di- and mono-(2-ethylhexyl) phthalate in newborn
infants subjected to exchange transfusions. Eur. J. clin. Invest., 15:
430-436.
SJÖBERG, P., LINDQUIST, N.G., MONTIN, G., & PLÖEN, L. (1985c) Effect
of repeated intravenous infusions of the plasticizer di-(2-ethylhexyl)
phthalate in young male rats. Arch. Toxicol., 58: 78-83.
SJÖBERG, P., LINDQUIST, N.G., & PLÖEN, L. (1986) Age-dependent
response of the rat testes to di(2-ethylhexyl) phthalate. Environ.
Health Perspect., 65: 237-242.
SMISTAD, G., WAALER, T., & ROKSVAAG, P.O. (1989) Migration of plastic
additives from soft polyvinyl chloride bags into normal saline and
glucose infusions. Acta pharm. Nord., 1: 287-290.
SMITH-OLIVER, T. & BUTTERWORTH, B.E. (1987) Correlation of the
carcinogenic potential of di(2-ethylhexyl)phthalate (DEHP) with
induced hyperplasia rather than with genotoxic activity. Mutat. Res.,
188: 21-28.
SODERGREN, A. (1982) Significance of interfaces in the distribution
and metabolism of di-2-ethylhexyl phthalate in an aquatic laboratory
model ecosystem. Environ. Pollut., 27: 263-274.
SRI (1985) Chemical economics handbook, Menlo Park, California, SRI
International.
SRIVASTAVA, S., AWASTHI, V.K., SRIVASTAVA, S.P., & SETH, P.K. (1989)
Biochemical alterations in rat fetal liver following in utero exposure
to di(2-ethylhexyl)phthalate (DEHP). Indian J. exp. Biol., 27:
885-888.
STANKEVICH, V.V. & ZAREMBO, O.K. (1978) [Toxicological and hygienic
characteristics of PVC materials plasticizers.] In: [Modern problems
of hygiene of PVC application], Moscow, VNIIMI, pp. 10-16 (Series
Gigiena No. 4) (in Russian).
STANKEVICH, V.V., ZAREMBO, O.K., GUMENNOY, V.S., & LEUITSKAYA, V.N.
(1984) [Present state of the regulation program relating to vinyl
chloride monomers and other additives used as polyvinyl chloride
packing material (A review)], Moscow, VNIIMI, pp. 26-36 (Series
Gigiena) (in Russian).
STANLEY, R.D. & TAPP, J.F. (1982) An assessment of ecological test
methods. VIII. The effect of nine chemicals on the growth of Avena
saliva and Brassica rapa, United Kingdom, ICI Brixham Laboratory
(Internal Report No. BL/A/2164).
STEPHENSON, R.R. (1983) Effects of water hardness, water temperature,
and size of the test organism on the susceptibility of the freshwater
shrimp, Gammarus pulex (L.), to toxicants. Bull. environ. Contam.
Toxicol., 31: 459-466.
STREUFERT, J.M., JONES, J.R., & SANDERS, H.O. (1980) Toxicity and
biological effects of phthalate esters on midges (Chironomus
plumosus). Trans. Missouri Acad. Sci., 14: 33-40.
SUGATT, R.H., O'GRADY, D.P., BANERJEE, S., HOWARD, P.H., & GLEDMILL,
W.E. (1984) Shake flask biodegradation of 14 commercial phthalate
esters. Appl. environ. Microbiol., 47: 601-606.
SWENERTON, H. & HURLEY, L.S. (1971) Teratogenic effects of a chelating
agent and their prevention by zinc. Science, 173: 62-63.
TABORSKY, R.G. (1967) Isolation studies on a lipoidal portion of the
bovine pineal gland. J. agric. food Chem., 15: 1073-1076.
TAKAGI, A., SAI, K., UMEMURA, T., KUROKAWA, Y., & KASAI, H. (1989)
Production of 8-hydroxydeoxyguanosine in rat liver DNA by oral
administration of peroxisome proliferators. Mutat. Res., 216: 379.
TAKAGI, A., SAI, K., UMEMURA, T., HASEGAWA, R., & KUROKAWA, Y. (1990)
Significant increase of 8-hydroxydeoxyguanosine in liver DNA of rats
following short-term exposure to the peroxisome proliferators di(2-
ethylhexyl)phthalate and di(2-ethylhexyl)adipate Jpn. J. Cancer Res.,
81: 213-215.
TAKAGI, A., SAI, K., UMEMURA, T., HASEGAWA, R., & KUROKAWA, Y. (1991)
Short-term exposure to the peroxisome proliferators, perfluorooctanoic
acid and perfluorodecanoic acid, causes significant increase of 8-
hydroxydeoxyguanosine in liver DNA of rats. Cancer Lett., 57: 55-60.
TAMURA, H., IIDA, T., WATANABE, T., & SUGA, T., (1990) Long-term
effects of hypolipidemic peroxisome proliferator administration on
hepatic hydrogen peroxide metabolism in rats. Carcinogenesis, 11:
445-450.
TARR, B.D., BARRON, M.G., & HAYTON, W.L. (1990) Effect of body size on
the uptake and bioconcentration of di-2-ethylhexyl phthalate in
rainbow trout. Environ. Toxicol. Chem., 9: 989-995.
TAYLOR, B.F., CURRY, R.W., & CORCORAN, E.F. (1981) Potential for
biodegradation of phthalic acid esters in marine regions. Appl.
environ. Microbiol., 42: 590-595.
TEIRLYNCK, O.A. & BELPAIRE, F. (1985) Disposition of orally
administered di-(2-ethylhexyl) phthalate and mono-(2-ethylhexyl)
phthalate in the rat. Arch. Toxicol., 57: 226-230.
THIESS, A.M. & FLIEG, I. (1978) [Chromosomal studies in workers
exposed to di-2-ethylhexylphthalate (DOP).] Zentralbl. Arbeitsmed.,
28: 351-355 (in German).
THIESS, A.M., FRENTZEL-BEYME, R., & WIELAND, R. (1978a) [Mortality
study in workers exposed to di-2-ethylhexyl phthalate (DOP.] In:
[Possibilities and limits of biological monitoring - Problems in
occupational medicine of the industrial occupational health services.
Proceedings of a conference, Frankfurt, May 1978], Stuttgart, A.W.
Gertner, pp. 155-164 (in German).
THIESS, A.M., KORTE, A., & FLIEG, H. (1978b) [Morbidity studies in
workers exposed to di(2-ethylhexyl)phthalate (DEHP).] In:
[Possibilities and limits of biological monitoring - Problems in
occupational medicine of the industrial occupational health services.
Proceedings of a conference, Frankfurt, May 1978], Stuttgart, A.W.
Gertner, pp. 137-154 (in German).
THOMAS, G.H. (1973) Quantitative determination and confirmation of
identity of trace amounts of dialkylphthalates in environmental
samples. Environ. Health Perspect., 3: 23-28.
THOMAS, J.A., SCHEIN, L.G., GUPTA, P.K., MCCAFFERTY, R.E., FELICE,
P.R., & DONOVAN, M.P. (1979) Failure of monoethylhexyl phthalate to
cause teratogenic effects in offspring of rabbits. Toxicol. appl.
Pharmacol., 51: 523-528.
THUREN, A. (1986) Determination of phthalates in aquatic environments.
Bull. environ. Contam. Toxicol., 36: 33-40.
THUREN, A. & LARSSON, P. (1990) Phthalate esters in the Swedish
atmosphere. Environ. Sci. Technol., 24: 554-559.
THUREN, A. & WOIN, P. (1991) Effects of phthalate esters on the
locomotor activity of the freshwater amphipod Gammarus pulex. Bull
environ. Contam. Toxicol., 46: 159-166.
TIMOFIEVSKAJA, L.A., IRANOVA, N.I., & BALYNINA, E.S. (1980)
[Toxicology of esters of o-phthalic acid and hygienic standard for
them.] Gig. Tr. prof. Zabol., 3: 25-28 (in Russian).
TOMASZEWSKI, K.E., AGARWAL, D.K., & MELNICK, R.L. (1986) In vitro
steady-state levels of hydrogen peroxide after exposure of male F344
rats and female B6C3F1 mice to hepatic peroxisome proliferators.
Carcinogenicity, 7: 1871-1876.
TOMITA, I., NAKAMURA, Y., AOKI, N., & INUI, N. (1982a)
Mutagenic/carcinogenic potential of DEHP and MEHP. Environ. Health
Perspect., 45: 119-125.
TOMITA, I., NAKAMURA, Y., YAGI, Y., & TUTIKAWA, K. (1982b)
Teratogenicity/fetotoxicity of DEHP in mice. Environ. Health
Perspect., 45: 71-75.
TOMITA, I., NAKAMURA, Y., YAGI, Y., & TUTIKAWA, K. (1986) Fetotoxic
effects of mono-2-ethylhexyl phthalate (MEHP) in mice. Environ. Health
Perspect., 65: 249-254.
TRIMBLE, A.S., GOLDMAN, B.S., YAO, J.K., DOVATS, L.K., & BIGELOW, W.G.
(1966) Plastics - A source of chemical contamination in surgical
research. Surgery, 59: 857-859.
TYL, R.W., PRICE, C.J., MARR, M.C., & KIMMEL, C.A. (1988)
Developmental toxicity evaluation of dietary di(2-ethylhexyl)phthalate
in Fischer 344 rats and CD-1 mice. Fundam. appl. Toxicol., 10:
395-412.
VAINIOTALO, S. & PFÄFFLI, P. (1990) Air impurities in the PVC plastics
processing industry. Ann. occup. Hyg., 34: 585-590.
VILLENEUVE, D.C., FRANKLIN, C.A., CHU, I., YAGMINAS, A., MARINO, I.A.,
RITTER, L., & RUDDICK, J.A. (1978) Toxicity studies on mono-2-
ethylhexyl phthalate. Toxicol. appl. Pharmacol., 45: 250-251.
VON DÄNIKEN, A., LUTZ, W.K., JÄCKH, R., & SCHLATTER, C. (1984)
Investigation of the potential for binding of di(2-
ethylhexyl)phthalate (DEHP) and di-(2-ethylhexyl)adipate (DEHA) to
liver DNA in vivo. Toxicol. appl. Pharmacol., 73: 373-387.
WAALKES, M.P. & WARD, J.M. (1989) Induction of hepatic metallothionein
in male B6C3F1 mice exposed to hepatic tumor promotors: effects of
phenobarbital, acetaminophen, sodium barbital, and di(2-ethylhexyl)
phthalate. Toxicol. appl. Pharmacol., 100: 216-266.
WALDOCK, M.J. (1983) Determination of phthalate esters in samples from
the marine environment using gas chromatography mass spectrometry.
Chem. Ecol., 1: 261-277.
WALSETH, F., TOFTGÅRD, R., & NILSEN, O.G. (1982) Phthalate esters I:
Effects on cytochrome P-450 mediated metabolism in rat liver and lung,
serum enzymatic activities and serum protein levels. Arch. Toxicol.,
50: 1-10.
WAMS, T.J. (1987) Diethylhexylphthalate as an environmental
contaminant - A review. Sci. total Environ., 66: 1-16.
WARD, J.M., RICE, J.M., CREASIA, D., LYNCH, P., & RIGGS. C. (1983)
Dissimilar patterns of promotion by di(2-ethylhexyl)phthalate and
phenobarbital of hepatocellular neoplasia initiated by
diethylnitrosamine in B6C3F1 mice. Carcinogenesis, 4: 1021-1029.
WARD, J.M., DIWAN, B.A., OHSHIMA, M., HU, H., SCHULLER, H.M., & RICE,
J.M. (1986) Tumor-initiating and promoting activities of di(2-
ethylhexyl) phthalate in vivo and in vitro. Environ. Health Perspect.,
65: 279-291.
WEAST, R.C., ASTLE, M.J., & BEYER, W.H., ed. (1984) CRC handbook of
chemistry and physics, 65th ed., Boca Raton, Florida, CRC Press Inc.
WEBER, K. & ERNST, W. (1983) [Occurrence and fluctuation of organic
chemicals in major German estuaries.] Vom Wasser, 61: 111-123 (in
German).
WHITE, R.D., CARTER, D.E., EARNEST, D., & MUELLER, J. (1980)
Absorption and metabolism of 3 phthalate esters by the rat small
intestine. Food Cosmet. Toxicol., 18: 383-386.
WHO/FAO (1984a) Evaluation of certain food additives and contaminants
(Twenty-eighth report of the Joint FAO/WHO Expert Committee on Food
Additives), Geneva, World Health Organization, 44 pp (WHO Technical
Report Series, No. 710, and corrigendum).
WHO/FAO (1984b) Toxicological evaluation of certain food additives and
contaminants (Prepared by the Joint FAO/WHO Expert Committee on Food
Additives, Rome, 19-28 March, 1984), Geneva, World Health
Organization, 222 pp (WHO Food Additives Series No. 19).
WHO/FAO (1989a) Evaluation of certain food additives and contaminants
(Thirty-third report of the Joint FAO/WHO Expert Committee on Food
Additives), Geneva, World Health Organization, 64 pp (WHO Technical
Report Series No. 776).
WHO/FAO (1989b) Toxicological evaluation of certain food additives and
contaminants (Prepared by the 29th Meeting of the Joint FAO/WHO Expert
Committee on Food Additives), 362 pp. Cambridge, Cambridge University
Press (WHO Food Additives Series No. 24).
WILLIAMS, D.T. (1973) Dibutyl- and di-(2-ethylhexyl) phthalate in
fish. J. agric. food Chem., 21: 1128-1129.
WOFFORD, H.W., WILSEY, C.D., NEFF, G.S., GIAM, C.S., & NEFF, J.M.
(1981) Bioaccumulation and metabolism of phthalate esters by oysters,
brown shrimp, and sheepshead minnows. Ecotoxicol. environ. Saf., 5:
202-210.
WOIN, P. & LARSSON, P. (1987) Phthalate esters reduce predation
efficiency of dragonfly larvae, Odonata; Aeshna. Bull. environ.
Contam. Toxicol., 38: 220-225.
WOLFE, N.L., BURNS, L.A., & STEEN, W.C. (1980a) Use of linear free
energy relationships and an evaluative model to assess the fate and
transport of phthalate esters in the aquatic environment. Chemosphere,
9: 393-402.
WOLFE, N.L., STEEN, W.C., & BURNS, L.A. (1980b) Phthalate ester
hydrolysis: Linear free energy relationships. Chemosphere, 9: 403-408.
WOOD, D.L. & BITMAN, J. (1984) The effect of feeding di-(2-ethylhexyl)
phthalate and related compounds on lipids in the laying hen. Poult.
Sci., 63: 469-477.
WOODWARD, K.N., SMITH, A.M., MARISCOTTI, S.P., & TOMLINSON, N.J.
(1986) Review of the toxicity of the esters of o-phthalic acid
(phthalate esters), London, Health and Safety Executive, 183 pp
(Toxicity Review 14).
YAGI, Y., NAKAMURA, Y., TOMITA, I., TSUCHIKAWA, K., & SHIMOI, N.
(1980) Teratogenic potential of di- and mono-(2-ethylhexyl) phthalate
in mice. J. environ. Pathol. Toxicol., 4: 533-544.
YANAGITA, T., KOBAYASHI, K., & ENOMOTO, N. (1978) Accumulation of
hepatic phospholipids in rats fed di(2-ethylhexyl) phthalate. Biochem.
Pharmacol., 27: 2283-2288.
YANAGITA, T., KUZUHARA, S., ENOMOTO, N., SHIMAD, T., & SUGANO, M.
(1979) Effects of di(2-ethylhexyl) phthalate on the content of hepatic
mitochondrial and microsomal phospholipids in the rat. Biochem.
Pharmacol., 28: 3115-3121.
YOON, J.S., MASON, J.M., VALENCIA, R., WOODRUFF, R.C., & ZIMMERING, S.
(1985) Chemical mutagenesis testing in Drosophila. IV. Results of 45
coded compounds tested for the National Toxicology Program. Environ.
Mutagen., 7: 349-367.
YOSHIKAWA, K., TANAKA, A., YAMAHA, T., & KURATA, H. (1983)
Mutagenicity study of nine monoalkyl phthalates and a dialkyl
phthalate using Salmonella typhimurium and Escherichia coli. Food
chem. Toxicol., 21: 221-223.
ZEIGER, E., HAWORTH, S., MORTELMANS, K., & SPECK, W. (1985)
Mutagenicity testing of di(2-ethylhexyl) phthalate and related
chemicals in Salmonella. Environ. Mutagen., 7: 213-232.
ZIMMERING, S., MASON, J.M., & VALENCIA, R. (1989) Chemical mutagenesis
testing in drosophila. VII. Results of 22 coded compounds tested in
larval feeding experiments. Environ. mol. Mutagen., 14: 245-251.
ZIOGOU, K., KIRK, P.W.W., & LESTER, J.N. (1989) Behaviour of phthalic
acid esters during batch anaerobic digestion of sludge. Water Res.,
23: 743-748.
ZITKO, V. (1972) Determination, toxicity, and environmental levels of
phthalate plasticizers, St. Andrews, N.B., Biological Station,
Fisheries Research Board of Canada, 35 pp (Technical Report No. 344).
RESUME
1. Identité, propriétés physiques et chimiques et méthodes d'analyse
Le phtalate de di(2-éthylhexyle) est un ester de l'acide
benzènedicarboxylique qui se présente, à la température ambiante, sous
la forme d'un liquide huileux, incolore à jaune. Il est peu soluble
dans l'eau (0,3-0,4 mg/litre) et encore moins dans l'eau salée. Il est
miscible à la plupart des solvants organiques courants. Sa volatilité
est relativement faible (tension de vapeur: 8,6 x 10-4Pa).
On a mis au point, pour le dosage du DEHP dans différents
milieux, un grand nombre de méthodes de prélèvement et d'analyse. On
fait de plus en plus appel à des méthodes sensibles telles que la
chromatographie en phase gazeuse, la chromatographie en phase liquide
à haute performance et la spectrométrie de masse. La contamination due
au matériel en plastique utilisé lors des prélèvements et de l'analyse
complique le dosage du DEHP à faibles concentrations.
2. Sources d'exposition humaine et environnementale
Le DEHP présent dans l'environnement est, dans sa presque
totalité, d'origine artificielle.
La production mondiale de DEHP augmente depuis quelques décennies
et s'élève actuellement à environ un million de tonnes par an. Les
Etats-Unis d'Amérique et l'Europe entrent chacun pour un tiers dans
cette production.
Le DEHP est le plastifiant le plus largement utilisé pour
augmenter la fluidité des résines puisqu'il représente 50% de
l'ensemble des esters phtaliques utilisés comme plastifiants. Il peut
représenter jusqu'à 40% en poids ou davantage de la matière plastique.
Le DEHP est utilisé dans la fabrication du chlorure de polyvinyle
(PVC) utilisé dans l'industrie du bâtiment et de l'emballage ainsi que
pour la fabrication d'accessoires à usage médical. On l'utilise
également en plus petites quantités pour la préparation de peintures
industrielles ainsi que comme diélectrique dans les condensateurs.
Les produits contenant du plastique qui sont mis au rebut peuvent
être éliminés soit par incinération soit par enfouissement dans une
décharge. Lors de l'incinération à basse température, une proportion
importante du produit peut se dissiper dans l'atmosphère. On n'a pas
très bien étudié ce qu'il advient du DEHP déposé dans les décharges et
il n'est pas possible à ce stade de tirer des conclusions définitives.
3. Transport, distribution, et transformation dans l'environnement
C'est principalement en étant transportés dans l'atmosphère que
les phtalates pénètrent dans l'environnement. A partir de là, le DEHP
tombe sur le sol à moins d'être éliminé par les précipitations.
Le coefficient de partage du DEHP entre l'octanol et l'eau est
élevé, de sorte que l'équilibre entre l'eau et un sol ou un sédiment
riche en substances organiques s'établit aux dépens de la phase
aqueuse. Le DEHP s'adsorbe facilement sur les particules organiques et
du sol.
Bien que peu soluble dans l'eau, le DEHP peut se rencontrer en
quantités relativement élevées dans les eaux de surface en raison de
son adsorption sur les particules organiques et de son interaction
avec les substances organiques en solution. L'adsorption s'effectue
plus particulièrement sur les particules de petite taille et elle est
favorisée par la présence d'eau salée.
Dans l'atmosphère, le DEHP subit une photodégradation rapide mais
son hydrolyse dans l'environnement est pratiquement inexistante.
Plusieurs microorganismes terricoles effectuent la dégradation
aérobie du DEHP. Toutefois dans l'environnement, cette dégradation
microbienne s'effectue avec lenteur. La biodégradation commence par
l'hydrolyse du mono-ester qui est transformé en acide phtalique. Il y
a ensuite dégradation en pyruvate et succinate par ouverture du cycle
puis en CO2 et H2O, comme dans la dégradation métabolique de
l'acide benzoïque. La dégradation aérobie dépend de la température. Au
dessous de 10 °C la dégradation est peu importante. A température
supérieure, la biodégradation se produit dans la partie supérieure du
sol mais, à plus grande profondeur elle est pratiquement inexistante
du fait des conditions d'anaérobiose. La dégradation anaérobie, pour
autant qu'elle existe, est beaucoup plus lente que la dégradation
aérobie.
Le DEHP est très lipophile et modérément persistant. Le degré de
bioaccumulation dépend de la capacité de l'organisme qui l'absorbe à
métaboliser la substance. On a montré qu'il s'accumulait en forte
proportion chez divers invertébrés aquatiques, les poissons et les
amphibiens.
Après application de DEHP sur des feuilles de végétaux, on n'a
constaté qu'une faible perte de matière sur une période de 15 jours.
Le DEHP présent dans le sol ou dans les boues d'égout n'est que peu
fixé par les plantes.
4. Concentrations dans l'environnement et exposition humaine
Le DEHP est très répandu dans l'environnement et on le retrouve
dans la plupart des échantillons prélevés dans l'air, dans les
précipitations, l'eau, les sédiments, le sol et les biotes. C'est
généralement dans les zones industrielles que les teneurs sont les
plus fortes.
On a relevé des concentrations allant jusqu'à 300 ng/m3 dans
l'air des villes et en atmosphère polluée. Dans l'atmosphère
surmontant les océans, on a fait état de concentrations allant de 0,5
à 5 ng/m3 et les précipitations de ces régions en contenaient
jusqu'à 200 ng/litre. L'étude d'échantillons de précipitations
prélevés dans un secteur situé à proximité d'une usine produisant des
plastifiants, a montré que la vitesse de dépot à sec était de 0,7 à
4,7 µg/m2 et par jour.
Dans les rivières et les lacs, on a trouvé des concentrations de
DEHP allant jusqu'à 4 µg/litre, les teneurs les plus élevées étant
relevées au point de décharge d'effluents industriels. Dans la mer, la
concentration est inférieure à 1 µg/litre, les concentrations les plus
fortes étant observées dans les estuaires.
En raison de son caractère hydrophobe, le DEHP est facilement
absorbé par le sol, les sédiments et les particules. Dans les
sédiments de cours d'eau, des concentrations allant jusqu'à 70 mg/kg
(de poids à sec) ont été signalées, concentrations qui peuvent monter
jusqu'à 1480 mg/kg (de poids à sec) au voisinage des points de
décharge.
Dans les biotes, la concentration du DEHP varie de moins de 1 à
7000 µg/kg. On le rencontre dans divers types d'aliments: poisson,
coquillages, oeufs et fromages. L'exposition moyenne a été estimée à
environ 300 µg par personne et par jour aux Etats-Unis d'Amérique en
1974 et à 20 µg par personne et par jour au Royaume-Uni en 1986.
Les transfusions de sang et autres traitements utilisant du
matériel en plastique peuvent entraîner l'exposition involontaire des
malades au DEHP. C'est ainsi qu'on a relevé dans le poumon de malades
ainsi traités, des teneurs allant de 13,4 à 91,5 mg/kg de poids à sec.
Les quelques données disponibles indiquent que sur les lieux de
travail, la concentration de DEHP est généralement inférieure à 1
mg/m3.
5. Cinétique et métabolisme
Des données disponibles au sujet de l'administration par voie
orale indiquent que le DEHP est hydrolysé dans l'intestin par la
lipase pancréatique. Les métabolites qui se forment, c'est-à-dire le
phtalate de mono-2-éthylhexyle (MEHP) et le 2-éthylhexanol sont
rapidement absorbés. Après administration de DEHP marqué au 14C
(2,9 mg/kg) par voie orale à des rats, on a récupéré 50% de la dose
initiale dans l'urine et la bile de ces animaux. Il semble que la
biodisponibilité d'une dose orale de DEHP soit plus élevée chez les
jeunes animaux.
Après administration par voie orale, le DEHP est largement
hydrolysé dans l'intestin de certains animaux, par exemple les rats,
et se répartit dans l'organisme, principalement sous forme de phtalate
de monoéthylhexyle (MEHP). Toutefois chez les primates et l'homme,
l'hydrolyse est beaucoup moins importante. Il semble que ce soit
principalement au niveau du foie que s'effectue la métabolisation du
MEHP et du 2-éthylhexanol. On a identifié plusieurs autres
métabolites, l'omega et omega-1-oxydation étant les principales voies
métaboliques. Un ou plusieurs des produits résultant de l'omega-
oxydation peuvent encore être métabolisés par ß-oxydation. On a
constaté que l'omega-oxydation s'effectuait selon une cinétique non
linéaire. Le métabolisme du DEHP offre des différences considérables
selon les espèces; par exemple, l'omega-oxydation est moins importante
chez l'homme que chez le rat.
Une dose de DEHP de 2,9 mg/kg, administrée par voie orale à des
rats, a été récupérée une semaine plus tard presque intégralement dans
les matières fécales et l'urine des animaux. La bile et l'urine
constituent les principales voies d'excrétion. Lors d'une étude sur
l'homme, 15 à 25% d'une dose orale (0,45 mg/kg de DEHP) ont été
excrétés sous forme de MEHP et la majeure partie des produits
d'excrétion étaient constitués de métabolites oxydés.
6. Effets sur les mammifères de laboratoire et les systèmes
d'épreuve in vitro
La DL50 par voie orale du DEHP est d'environ 25 à 34 g/kg,
selon l'espèce, mais cette valeur est plus faible dans le cas du MEHP.
Lors d'études d'alimentation sur des rats et des souris, on a constaté
que des doses quotidiennes de DEHP supérieures à 3 g/kg entraînaient
la mort dans les 90 jours et qu'une dose de 0,4 g/kg réduisait le gain
de poids en l'espace de quelques jours. Dans d'autres études, c'est
une dose de 6,3-12,5 g/kg de nourriture qui a entraîné une réduction
du poids corporel.
Lors d'études à long terme, on a observé chez les animaux traités
une hépatomégalie accompagnée d'une augmentation du poids relatif des
reins. Dans l'une de ces études, on observait également une
hypertrophie cellulaire au niveau du lobe antérieur de l'hypophyse.
Un certain nombre d'études ont permis de mettre en évidence une
atrophie testiculaire, apparaîssant au bout de quelques jours, et liée
à l'administration de DEHP (doses dans l'alimentation allant de
10-20 g/kg). Les jeunes rats semblent plus vulnérables et les rats et
les souris plus sensibles que les hamsters et les ouistitis. On a
constaté que cette atrophie était réversible. Le MEHP exerce des
effets toxiques in vitro sur les cellules de Sertoli. Le DEHP de
même que le MEHP ont des propriétés tératogènes. Des malformations ont
été observées à des doses de 0,5-2 g/kg de nourriture chez la souris
et, à des doses supérieures à 10 g/kg, on a observé des effets
embryotoxiques.
Les tests de mutagénicité et autres manifestations du même genre
ont donné des résultats négatifs dans la plupart des études. Le DEHP
peut provoquer la transformation des cellules et on a montré qu'il
était cancérogène à des doses respectives de 6 et 12 g/kg de
nourriture chez le rat et de 3 et 6 g/kg de nourriture chez la souris.
On a constaté une augmentation, liée à la dose, des tumeurs
hépatocellulaires chez les deux sexes de l'une et l'autre espèce. La
prolifération des peroxysomes hépatiques et la réplication cellulaire
sont fortement liées aux effets cancérogènes sur le foie de certains
produits non génotoxiques comme le DEHP. Toutefois on a observé des
différences importantes entre les espèces animales en ce qui concerne
la prolifération des peroxysomes induite par le DEHP.
Contrairement à ce qui se passe dans le cas des hépatocytes de
rat, les métabolites du DEHP ne provoquent pas de prolifération des
peroxysomes dans les cultures d'hépatocytes humains.
7. Effets sur l'homme
On ne dispose que de données très limitées sur les effets que le
DEHP peut exercer sur l'homme. On a fait état chez deux sujets qui
avaient reçu 5 ou 10 g de DEHP respectivement, d'effets qui se
limitaient à de légers troubles gastriques.
8. Effets sur les autres êtres vivants en laboratoire et dans
leur milieu naturel
Dans la plupart des études, les valeurs nominales de la CL50
obtenues lors des tests de toxicité aiguë dépassent 10 mg/litre, ce
qui indique que le DEHP est peu toxique. Toutefois ces valeurs sont
supérieures à la solubilité du DEHP dans l'eau (0,3-0,4 mg/litre).
D'après une étude, une daphnie, Daphnia pulex, serait
particulièrement sensible, la CL50 à 48 heures étant dans son cas de
0,133 mg/litre. La seule épreuve de toxicité aiguë où l'on ait mesuré
des concentrations de DEHP a été effectuée sur un poisson, Pimephales
promelas, et elle a donné une CL50 à 96 heures supérieure à 0,33
mg/litre. Lors d'études de longue durée, on a obtenu une dose sans
effet nocif observable de 72 µg/litre pour Daphnia magna. Pour les
poissons adultes, cette dose était supérieure à 72 µg/litre. Une
exposition à une concentration de 14 µg/litre, pendant 12 jours avant
l'éclosion, a provoqué une importante augmentation de la mortalité
parmi des alevins de truites. Des concentrations comprises en 3,7 et
11 µg/litre ont entraîné une réduction de la teneur en collagène
vertébral chez les poissons.
La présence de DEHP dans la nourriture à raison de 50 mg/kg a eu
des effets nocifs sur la survie des alevins de Melambaphes zebra. A
la concentration de 25 mg/kg en poids dans les sédiments, on a
constaté une réduction de l'activité microbienne et du nombre d'
éclosions chez les tétards.
Le DEHP est peu toxique pour les algues, les végétaux, les
lombrics et les oiseaux.
9. Evaluation
Le DEHP exerce des effets cancérogènes sur le foie et affecte le
système reproducteur chez le rat et la souris.
Le principal effet au niveau des gonades consiste, chez le rat et
la souris, en une atrophie des testicules qui affecte davantage les
jeunes animaux. La prolifération des peroxysomes hépatiques et la
réplication cellulaire sont fortement liées à l'effet cancérogène
qu'exercent sur le foie certains produits non génotoxiques au nombre
desquels figure le DEHP. Toutefois, on a observé de fortes différences
selon les espèces animales pour ce qui est de la prolifération des
peroxysomes induite par le DEHP. On ne possède pas actuellement de
preuves suffisantes permettant de conclure que le DEHP est
potentiellement cancérogène pour l'homme.
Il n'existe pas de données attestées selon lesquelles le DEHP
serait dangereux pour la faune, si l'on s'en tient aux résultats
obtenus lors de l'exposition de poissons et de daphnies. Toutefois, on
a observé une réduction de l'activité microbienne dans les sédiments
aux doses auxquelles le DEHP est présent dans l'environnement. Si l'on
compare les doses de DEHP présentes dans l'environnement aux
concentrations qui sont susceptibles de produire des effets lors
d'études de longue durée, notamment sur les larves de poissons et
d'amphibiens, on ne peut exclure l'existence d'un risque pour
l'environnement, les effets s'exerçant notamment par l'intermédiaire
de l'eau et des sédiments. Des effets nocifs sur les êtres vivants
sont possibles dans les secteurs où l'eau et les sédiments sont
fortement pollués en raison de leur proximité des points de décharge.
Bien que peu d'études valables aient été effectuées, il semble
que le DEHP présente une faible toxicité aiguë pour les algues, les
végétaux, les lombrics et les oiseaux.
RESUMEN
1. Identidad, propiedades físicas y químicas y métodos analíticos
El di(2-etilhexil) ftalato (DEHF) es un éster del ácido
bencenodicarboxílico que a temperatura ambiente es un líquido oleoso
entre incoloro y amarillo. Su solubilidad en agua es baja (0,3-0,4
mg/litro) y aún más baja en agua salada. Es miscible con la mayor
parte de los disolventes orgánicos normales. La volatilidad del DEHF
es relativamente baja (8,6 x10-4 Pa).
Se han perfeccionado numerosos métodos de muestreo y análisis
para la determinación del DEHF en diferentes medios. Cada vez se
utilizan más métodos de gran sensibilidad, como la cromatografía de
gases, la cromatografía líquida de alto rendimiento y la
espectrometría de masas. El análisis de concentraciones bajas de DEHF
se complica por la contaminación debida al material de plástico
utilizado en el muestreo y el análisis.
2. Fuentes de exposición humana y ambiental
Casi todo el DEHF presente en el medio ambiente procede de
fuentes antropogénicas.
La producción mundial de DEHF ha ido aumentando durante los
últimos decenios y en la actualidad es de alrededor de 1 x 106
toneladas al año. Un tercio del total se produce en los Estados Unidos
y otro tercio en Europa.
El DEHF es el plastificante más utilizado (representa el 50% de
todos los plastificantes a base de ésteres de ftalato) para ablandar
las resinas. A él corresponde el 40% (p/p) o más del total de
plásticos. El DEHF se emplea en la fabricación de cloruro de
polivinilo (PVC) utilizado en los edificios, en la construcción, en
embalajes y en componentes de aparatos médicos. Se utiliza en
cantidades más pequeñas en las pinturas industriales y como fluido
dieléctrico en los condensadores.
Los productos plastificados de desecho se pueden eliminar por
incineración o terraplenado. Durante la incineración a baja
temperatura, se puede liberar un porcentaje elevado del DEHF a la
atmósfera. No se ha estudiado bien el destino medioambiental del DEHF
depositado en terraplenes, por lo que no se pueden sacar conclusiones
definitivas.
3. Transporte, distribución y transformación en el medio ambiente
El transporte en el aire es la vía más importante de
incorporación de los ftalatos al medio ambiente. De la atmósfera, el
DEHF precipita o es lavado por la lluvia.
El DEHF tiene un coeficiente de reparto octanol-agua elevado, de
manera que el equilibrio entre el agua y un suelo o sedimento rico en
materia orgánica está desplazado a favor de este último. Las
partículas orgánicas del suelo lo adsorben fácilmente.
Aunque la solubilidad del DEHF en agua es baja, la cantidad
presente en las aguas de superficie puede ser mayor debido a la
adsorción a las partículas orgánicas y a la interacción con la materia
orgánica disuelta. Las pequeñas partículas lo adsorben más fácilmente,
y el proceso se potencia en el agua salada.
La fotodegradación atmosférica del DEHF es rápida; en cambio, su
hidrólisis química en el medio ambiente es prácticamente inexistente.
Se ha observado que varios microorganismos del suelo llevan a
cabo la degradación aerobia. Sin embargo, se ha informado que la
degradación microbiana del DEHF en el medio ambiente es lenta. El
proceso de biodegradación comienza con la hidrólisis para formar el
monoéster, que se transforma luego en ácido ftálico. La degradación
con ruptura del anillo para dar primero piruvato y succinato y después
CO2 y H2O es análoga a la vía metabólica del ácido benzoico. La
degradación aerobia es dependiente de la temperatura. Por debajo de
los 10 °C es escasa. A temperaturas más altas se produce
biodegradación en la capa más superficial del suelo, pero es
prácticamente inexistente en las capas más profundas, donde las
condiciones son de anaerobiosis. La degradación anaerobia, si existe,
es mucho más lenta que la aerobia.
El DEHF es muy lipófilo y moderadamente persistente. El grado de
bioacumulación depende de la capacidad de los organismos para
metabolizarlo. Se ha observado un grado elevado de acumulación en
diversos invertebrados acuáticos, peces y anfibios.
Cuando se aplicó DEHF a hojas de plantas, la pérdida fue pequeña
durante un período de 15 días. Se ha observado que su absorción por
las plantas a partir del suelo o de los fangos cloacales es muy baja.
4. Niveles medioambientales y exposición humana
El DEHF está muy ampliamente distribuido en la naturaleza y se
encuentra en la mayor parte de las muestras, por ejemplo de aire,
precipitación, sedimento, suelo y biota. Las concentraciones más altas
aparecen generalmente en las zonas industrializadas.
En el aire urbano y contaminado se han encontrado concentraciones
de hasta 300 ng/m3. Se han comunicado de niveles comprendidos entre
0,5 y 5 ng/m3 en el aire de zonas oceánicas, y la lluvia de esas
zonas contenía hasta alrededor de 200 ng/litro. En muestras de
precipitación procedentes de una zona cercana a una fábrica de
plastificantes se observó que la tasa sedimentación seca era de 0,7 a
4,7 µg/m2 al día.
Se han encontrado concentraciones de DEHF en ríos y lagos de
hasta 4 µg/litro; los niveles más altos están asociados a los puntos
de descarga de efluentes industriales. La concentración en el mar es
inferior a 1 µg/litro, y los niveles más altos se detectan en los
estuarios.
Debido a su carácter hidrófobo, el DEHF se adsorbe muy fácilmente
al suelo, los sedimentos y la materia particulada. En los sedimentos
fluviales se han comunicado niveles de hasta 70 mg/kg (peso seco), y
cerca de los puntos de descarga ha llegado a ser de 1480 mg/kg (peso
seco).
La concentración de DEHF en la biota oscila entre menos de 1 y
7000 µg/kg. Se ha detectado en diversos tipos de alimentos, como
pescado, marisco, huevos y queso. En 1974 se estimó en los Estados
Unidos una exposición media de 300 µg/persona al día y en el Reino
Unido en 1986 de 20 µg/persona al día.
Las transfusiones de sangre y otros tipos de tratamiento médico
en los que se utilizan aparatos de plástico pueden dar lugar a una
exposición humana involuntaria al DEHF. En el tejido pulmonar de
algunos pacientes se han detectado concentraciones comprendidas entre
13,4 y 91,5 µg/kg (peso seco).
Los escasos datos disponibles indican que las concentraciones de
DEHF en el lugar de trabajo suelen estar por debajo de los 1 mg/m3.
5. Cinética y metabolismo
Los datos disponibles sobre la administración oral indican que la
lipasa pancreática hidroliza el DEHF en el intestino. Los metabolitos
formados, que son el mono (2-etilhexil) ftalato (MEHF) y el
2-etilhexanol, se absorben rápidamente. Cuando se administró por vía
oral a ratas DEHF marcado con 14C (2,9 mg/kg), se recuperó en la
orina o la bilis más del 50%. La biodisponibilidad de una dosis oral
de DEHF parece ser más elevada en las ratas jóvenes que en las más
viejas.
El DEHF administrado por vía oral se hidroliza en el intestino de
ciertos animales, por ejemplo las ratas, y se distribuye sobre todo
como monoetilhexil ftalato (MEHF). Sin embargo, la hidrólisis es mucho
menor en los primates y el ser humano. El MEHF se liga a las proteínas
del plasma. El hígado parece ser el principal órgano metabolizador del
MEHF y del 2-etilhexanol. Se han identificado algunos otros
metabolitos, siendo las principales vías metabólicas la omega-y la
omega-1-oxidación. Uno o varios productos de la omega-oxidación pueden
metabolizarse ulteriormente por ß-oxidación. En la omega-oxidación se
han observado cinéticas no lineales. En el metabolismo del DEHF se
registran considerables diferencias entre las especies; por ejemplo,
la omega-oxidación es menor en el hombre que en las ratas.
Una semana después de administrar una dosis oral de DEHF
(2,9 mg/kg) se recuperó casi el 100% en las heces y la orina de ratas.
La bilis y la orina son las principales vías de excreción. En un
estudio humano, del 15 al 25% de la dosis oral (0,45 mg/kg) de DEHF se
excretó en forma de MEHF; los metabolitos oxidados constituían la
parte más importante de los productos de excreción.
6. Efectos en los mamíferos de laboratorio y en sistemas de
ensayo in vitro
La DL50 del DEHF por vía oral es de alrededor de 25-34 g/kg,
según las especies, pero el valor para el MEHF es más bajo. En
estudios de alimentación en ratas y ratones, las dosis de DEHF
superiores a 3 g/kg al día causaron muertes en un plazo de 90 días, y
con una concentración de 0,4 g/kg al día se redujo el aumento de peso
a los pocos días. En otros estudios, con dosis de 6,3-12,5 g/kg de
alimentos se produjo una reducción del peso corporal.
En los animales tratados en estudios de larga duración se ha
observado hepatomegalia y un aumento del peso relativo de los riñones.
En un estudio se encontraron también células hipertróficas en el
lóbulo anterior de la hipófisis.
En varios estudios se ha detectado atrofia testicular, evidente
al cabo de pocos días, relacionada con la administración de DEHF
(concentraciones de 10-20 g de DEHF/kg de alimentos). Las ratas más
jóvenes parecen ser más susceptibles que las viejas, y las ratas y los
ratones parecen ser más sensibles que los titís y los hámsters. Se ha
observado que la atrofia es reversible. El MEHF tiene efecto tóxico
in vitro en las células de Sertoli. Tanto el DEHF como el MEHF
muestran propiedades teratogénicas. Con concentraciones de 0,5 a 2
g/kg de alimentos se observaron malformaciones en ratones, y con
niveles superiores a los 10 g/kg se apreciaron efectos embriotóxicos.
Las pruebas de mutagenicidad y puntos finales asociados han dado
resultados negativos en la mayor parte de los estudios. El DEHF puede
inducir la transformación celular y se ha demostrado que con dosis de
6 y 12 g/kg de alimentos en ratas y de 3 y 6 g/kg de alimentos en
ratones tiene efectos carcinógenos. En ambos sexos de las dos especies
se produjo un aumento de tumores hepatocelulares dependiente de la
dosis. La inducción de la proliferación de peroxisomas hepáticos y de
la replicación celular está fuertemente vinculada al efecto
carcinógeno en el hígado de determinados compuestos carcinógenos no
genotóxicos, entre los que figura el DEHF. Sin embargo, se han
observado notables diferencias entre distintas especies animales con
respecto a la proliferación de peroxisomas inducida por el DEHF.
A diferencia de lo que ocurre con los hepatocitos de rata, los
metabolitos del DEHF no inducen proliferación de peroxisomas en
cultivos de hepatocitos humanos.
7. Efectos en el ser humano
La información disponible sobre los efectos del DEHF en el ser
humano es muy escasa. Se han comunicado dos casos de trastornos
gástricos leves, con dosis de 5 y 10 g de DEHF, pero sin ningún otro
efecto nocivo.
8. Efectos en otros organismos en el laboratorio y en el
medio ambiente
En la mayoría de los estudios de toxicidad aguda se han obtenido
unos valores nominales para la CL50 que se sitúan por encima de los
10 mg/litro, por lo que el índice de toxicidad del DEHF es bajo. Sin
embargo, esos niveles son superiores a los correspondientes a su
solubilidad en agua (0,3-0,4 mg/litro). En un estudio se detectó en la
pulga de agua Daphnia pulex una sensibilidad mayor, con un valor
nominal de la CL50 a las 48 h de 0,133 mg/litro. La única prueba de
la toxicidad aguda realizada con concentraciones medidas de DEHF se
hizo en el pez Pimephales promelas y puso de manifiesto una CL50
> 0,33 mg/litro a las 96 h. En estudios prolongados, el nivel sin
observación de efectos (NOEL) en Daphnia magna fue de 72 µg/litro.
En peces adultos se determinó un NOEL > 62 µg/litro. Una exposición
a 14 µg/litro desde 12 días antes de la eclosión produjo un
significativo aumento de la mortalidad de los alevines de trucha. Las
concentraciones entre 3,7 y 11 µg/litro provocaron una reducción del
colágeno vertebral en peces.
Con concentraciones de 50 mg/kg de alimentos, el DEHF tiene
efectos adversos sobre la supervivencia de los alevines de
Brachydanio rerio. Concentraciones de 25 mg/kg (p/p) en el sedimento
redujeron de manera considerable la actividad microbiana y el número
de renacuajos que nacían.
La toxicidad aguda del DEHF en algas, plantas, lombrices de
tierra y aves es baja.
9. Evaluación
El DEHF tiene efectos reproductivos y hepatocarcinogénicos en
ratas y ratones.
El principal efecto sobre la reproducción en ratas y ratones es
la atrofia testicular; los animales jóvenes son más susceptibles que
los viejos. La inducción de la proliferación de peroxisomas hepáticos
y de la replicación celular está estrechamente relacionada con el
efecto carcinogénico en el hígado de determinados compuestos
carcinógenos no genotóxicos, incluido el DEHF. Sin embargo, se han
observado notables diferencias entre las distintas especies de
animales con respecto a la proliferación de peroxisomas inducida por
el DEHF. En la actualidad no hay pruebas suficientes que indiquen que
el DEHF sea un posible carcinógeno para el ser humano.
No hay información documentada de que el DEHF constituya un
riesgo, tomando como base la exposición aguda de peces y dáfnidos. Sin
embargo, se ha informado de una reducción de la actividad microbiana
en los sedimentos con los niveles de DEHF presentes en el medio
ambiente. La comparación entre los niveles medioambientales y las
concentraciones que producen efectos en estudios prolongados,
especialmente las pruebas en las fases vitales tempranas de peces y
anfibios, indica que no se puede descartar un cierto peligro para el
medio ambiente, sobre todo por conducto del agua y los sedimentos. Es
probable que se produzcan efectos adversos en los organismos en zonas
con agua y sedimentos muy contaminados que están próximos a las
fuentes de emisión.
Aunque son pocos los estudios que se conocen al respecto, la
toxicidad aguda del DEHF en algas, plantas, lombrices de tierra y aves
parece ser baja.
There are marked species differences in the metabolism of DEHP.
Thus omega-oxidation seems to play a dominante role in the rat and
guinea-pig (Albro et al., 1982; Lhuguenot & Elcombe, 1984; Lhuguenot
et al., 1985), but to be a minor pathway in the mouse, hamster, green
monkey, cynomolgus monkey, and marmoset (Albro et al., 1982; Lhguenot
& Elcombe, 1984). In guinea-pigs there are few omega-1 metabolites of
MEHP (Albro et al., 1982). This may have toxicological significance
because certain omega-1-oxidation metabolites have been identified as
active agents in peroxisomal proliferation in rat hepatocytes
(Mitchell et al., 1985a).
No conjugated metabolites were detected in the urine of
DEHP-treated rats, but a minor portion was conjugated in the urine of
hamsters (Albro et al., 1982; Lhuguenot & Elcombe, 1984). A major
portion of glucuronide conjugates was found in the urine of the
marmoset, mouse, guinea-pig, and green monkey, and in human urine
(Albro et al., 1982). Albro (1986) reported that glucuronidation of
DEHP metabolites was insignificant in rats. Studies in primates,
including the African green monkey (Albro et al., 1981), marmoset
(Rhodes et al., 1986), cynomolgus monkey (Short et al., 1987; Astill,
1989), and man (Schmid & Schlatter, 1985), demonstrated that
conjugation of DEHP can occur at the carboxylic acid moiety following
a single ester hydrolysis.
The level of unmetabolized MEHP excreted in urine also varies
considerably between species; it is low in the rat and hamster, but
high in the mouse, guinea-pig, green monkey, and man (Albro et al.,
1982).
Repeated oral administration of DEHP or MEHP at high doses (500
mg/kg) to rats leads to a change in the metabolic profile; there is an
increase in omega-oxidized metabolites and a decrease in
omega-1-oxidized metabolites (Lhuguenot et al., 1985). In rats given
2% DEHP in the diet for one week, a 4-fold increase in peroxisomal ß-
oxidation was found. ß-Oxidation of fatty acids induced by DEHP
appears to occur via mitochondrial and peroxisomal pathways that are
similar to normal pathways (Ganning et al., 1989). Drug-metabolizing
enzyme activities have been studied after DEHP administration, and in
some cases changes were observed (Walseth et al., 1982; Agarwal et
al., 1982; Gollamudi et al., 1985; Pollack et al., 1989).
The same metabolites as those found in rat urine can be detected
in human urine. One study on intravenously injected DEHP (Albro et
al., 1982) and one on orally administered DEHP (Schmid & Schlatter,
1985) indicated that humans metabolize DEHP by omega- and
omega-1-oxidation as well as by oxidation of the ethyl side chain.
However, the omega-oxidation-pathway seems to be a minor pathway in
man (Albro et al., 1982; Schmid & Schlatter, 1985). More than half of
the metabolites recovered in human urine are conjugated metabolites
(Albro et al., 1982; Schmid & Schlatter, 1985).
Time-averaged concentrations of DEHP, MEHP, and phthalic acid in
the blood of patients undergoing maintenance haemodialysis were 1.9,
1.3, and 5.2 mg/litre, respectively (Pollack et al., 1985a). Such
patients are considered to be at risk of potential DEHP toxicity
through prolonged contact with medical plastic products that contain
DEHP. The relatively high circulating level of phthalic acid may
indicate an altered metabolism of DEHP in uraemic patients (Pollack et
al., 1985a).
The levels of DEHP and MEHP in plasma have been studied in
newborn infants given blood exchange transfusions. In one case the
MEHP half-life was the same as for DEHP (about 12 h), indicating that
the hydrolysis of DEHP was the rate-limiting metabolic step. However,
in other children the half-time of MEHP was longer than that of DEHP
(Sjöberg et al., 1985b).
6.4 Elimination and excretion
Radioactivity from intravenously injected 14C-labelled DEHP is
mainly recovered in urine and faeces after 24 h (Schulz & Rubin,
1973), indicating that urine and bile are major excretory pathways.
When a low dose level (0.1 mg/kg) was given to rats, 50-60% of
injected radioactivity was recovered in urine and faeces after 24 h,
whereas at a high dose level (200 mg/kg) less than 50% was recovered
(Schulz & Rubin, 1973). Seven days after an oral dose (2.9 mg/kg) of
DEHP was given to rats, 42% of the radioactivity was recovered in the
urine and 57% in the faeces (Daniel & Bratt, 1974). Biliary excretion
was also measured in these experiments, and it was found that 14% of
the radioactivity was recovered in bile after 4 days (Daniel & Bratt,
1974). The almost 100% recovery reported by Daniel & Bratt (1974) has
been confirmed by Teirlynck & Belpaire (1985). Oral administration of
MEHP (50-500 mg/kg) gave a higher urinary recovery than orally
administered DEHP (50-500 mg/kg) as measured after 24 h (Lhuguenot et
al., 1985).
After the oral administration of non-radioactive DEHP (0.45
mg/kg) to human volunteers, it was found that 15-25% was excreted in
urine as MEHP or oxidized metabolites within 2-3 days (Schmid &
Schlatter, 1985).
In the rat no unmetabolized DEHP is excreted in the urine, but
small amounts are found in mouse or green monkey urine (Albro et al.,
1982). Major amounts of MEHP are excreted in mouse, guinea-pig, green
monkey, and human urine (Albro et al., 1982). However, oxidized
metabolites, either free or conjugated, constitute a major portion of
excretion products in rat, mouse, hamster, green monkey, and human
urine (Albro et al., 1982).
Changes in excretion pathways have been observed after prolonged
administration of DEHP. After oral dosing of rats without
pre-treatment with DEHP, the faecal excretion pathway dominated, while
in rats fed with DEHP for 7 days the urinary pathway dominated (Daniel
& Bratt, 1974).
6.5 Retention and turnover
6.5.1 Half-life and body burden
After the intravenous administration of radiolabelled DEHP, at
least two elimination phases of radioactivity, with short half-lives
(4.5-9 and 22 min, respectively), were observed in rat blood (Schulz
& Rubin, 1973). After 7 weeks of oral administration, the elimination
phase in the liver was considerably slower, the half-life being 3-5
days (Daniel & Bratt, 1974). No accumulation of DEHP or MEHP was
observed when the dosage was 2.8 g/kg per day for 7 days (Teirlynck &
Belpaire, 1985), nor was there any in a long-term (5-7 weeks) feeding
study at a dose level of 1 or 5 g/kg diet (corresponding to a daily
dose of about 50 and 250 mg/kg body weight) (Daniel & Bratt, 1974).
6.5.2 Indicator media
Analysis of the total amount of urinary metabolites, measured as
derivatized phthalic acid, indicate a weak positive correlation
between occupational exposure to phthalate and the presence of
metabolites in the urine (Nielsen et al., 1985; Liss et al., 1985). In
the study by Nielsen et al. (1985), workers were exposed mainly to
DEHP and diisodecyl phthalate, and the urinary level of phthalate
ester metabolites rose from the background level (17 µmol/litre) to
23-25 µmol/litre. In the study by Liss et al. (1985), workers were
exposed to DEHP and phthalic anhydride. Urinary phthalate
concentrations in exposed workers more than doubled after a workshift
and levels up to 44 µmol/litre were recorded. The authors concluded
that phthalic anhydride influenced the urinary level more than DEHP.
Phthalic acid is not a specific marker for DEHP exposure.
7. EFFECTS ON LABORATORY MAMMALS AND IN VITRO TEST SYSTEMS
7.1 Single exposure
Numerous LD50 values have been reported for DEHP. Oral LD50
values generally exceed 25 g/kg in rats and 30 g/kg in mice
(Stankevich et al., 1984; NIOSH, 1985b; Woodward et al., 1986); in the
rabbit it is 33.9 g/kg (Shaffer et al., 1945) and in the guinea-pig
26.3 g/kg (Krauskopf, 1973). Dermal LD50 values for guinea-pigs and
rabbits of 10 g/kg and 25 g/kg, respectively, have been reported
(NIOSH, 1985b).
The LD50 values after intraperitoneal administration were
30.7 g/kg in rats (Shaffer et al., 1945) and 14-75 g/kg in mice
(Lawrence et al., 1975; Woodward et al., 1986). LD50 values in the
range of 200-250 mg/kg were reported for the rat after intravenous
administration of DEHP solubilized with a nonionic detergent (Schmidt
et al., 1975; Rubin & Chang, 1978).
The main symptom of DEHP toxicity after single oral or
intraperitoneal dosing is diarrhoea (Hodge, 1943). An intraperitoneal
dose of 500 mg/kg in rats decreased spontaneous running activity,
thereby indicating behavioural changes (Rubin & Jaeger, 1973). After
intravenous dosing, lung lesions including oedema, haemorrhage, and
infiltrations of polymorphonuclear leucocytes were observed in rats at
doses as low as 50 mg/kg (Schulz et al., 1975). The etiology of the
lung lesions is unknown. It has, however, been suggested that some
changes could be due to the release of lysosomal enzymes from alveolar
macrophages, which was found to occur in vitro in rabbit alveolar
macrophages cultured with DEHP (Bally et al., 1980).
Rabbits treated intravenously with 350 mg DEHP/kg showed a
decrease in blood pressure and an increase in breathing rate. No
deaths occurred after doses up to 650 mg/kg were administered (Calley
et al., 1966).
The monoester, MEHP, may be more toxic than the diester but data
are very limited. In a short note by Villeneuve et al. (1978), the
oral LD50 of MEHP was reported to be 1.34 g/kg in female rats and
1.8 g/kg in males.
7.2 Short-term exposure
Doses of 3.4 g/kg body weight per day given by gavage (in olive
oil) for periods of up to 90 days caused the death of 15 out 20 rats
(Nikonorow et al., 1973). However, no deaths were reported among rats
fed 3% DEHP in the diet (1.9 g/kg body weight) for 90 days (Shaffer et
al., 1945) or in a rat study of the US National Toxicology Program
after dietary dosing (< 50 g/kg) for 14 days (NTP, 1982).
Oral administration of DEHP at a rate of > 0.4 g/kg body
weight per day resulted in a weight gain decrease in rats within a few
days (Nikonorow et al., 1973). In a 17-week feeding study where rats
were given 2, 10 or 20 g DEHP/kg diet, a decreased body weight was
observed (Gray et al., 1977). Reduction in body weight was also
observed in rats given dietary levels of 12.5 or 25 g/kg for 13 weeks.
Dosages of 1.6-6.3 g/kg resulted in either slight elevations of body
weight or no effect (NTP, 1982).
MEHP given at a level of 6.4 g/kg diet caused reduction in the
body weight gain of rats (Chu et al., 1981). No effects on body weight
occurred at dietary levels of 0.625 g/kg given for 3 months, but a
significant decrease in blood glucose was observed.
Reductions in haemoglobin, packed cell volume and erythrocyte
numbers were observed in rats given 10 or 20 g DEHP/kg diet for 17
weeks, but not when they were given 2 g/kg for the same period (Gray
et al., 1977).
Cystic kidneys and centrilobular necrosis were noted in one
strain of mice (ddY) fed 2.5 or 25 g DEHP/kg for 2 weeks, but not in
another strain (B6C3F1), even with a higher exposure level and a
longer exposure period (Woodward et al., 1986).
DEHP administered intravenously at a rate of 25-500 mg/kg per day
for 2-4 weeks to beagle dogs resulted in pulmonary haemorrhage and
inflammatory response similar in appearance to the "shock-lung" effect
(Woodward et al., 1986).
In an inhalation study, Wistar rats were exposed in a head-nose
inhalation system to DEHP aerosols of respirable particle size.
Exposure duration was 6 h/day, 5 days/week for 4 weeks at target
concentrations of 0, 0.01, 0.05, and 1.0 mg/litre. A statistically
significant increase in relative lung weights was found in the males
given the highest dosage, and this was accompanied by foam cell
proliferation and thickening of the alveolar septa (Klimisch et al.,
1991).
Another inhalation study has been reported, but this is
inadequate for assessment (Timofievskaja et al., 1980).
A discussion of the effects of DEHP on the liver is given in
section 7.9.
When rats were treated with DEHP by intraperitoneal injection
(Walseth et al., 1982) or by repeated oral dosing (Agarwal et al.,
1982), an increase in cytochrome P-450 levels was observed. The
increase in hepatic microsomal oxidation appears to be primarily due
to the deesterification products of DEHP, i.e. MEHP and
2-ethoxyhexanol, in long-term exposure, whereas DEHP and its two
metabolites may inhibit microsomal oxidation after acute exposure to
DEHP (Pollack et al., 1989). However, in vitro rat liver microsomal
cytochrome P-450 levels were not affected by DEHP (Gollamudi et al.,
1985).
Liver mitochondrial enzymes and mitochondrial morphology have
been reported to be influenced by DEHP administration (Ohyama, 1977;
Shindo et al., 1978). Recent results suggest that the in vitro
effects of DEHP (> 20 µmol/litre) on mitochondrial functions are
mainly related to the action on membrane lipids surrounding the
adenine nucleotide translocator, which reduces the rate of adenine
nucleotide exchange across the mitochondrial membrane (Kora et al.,
1989).
In male rats given DEHP in the diet, the urinary excretion of
zinc was enhanced and the testicular level of zinc decreased (Gray et
al., 1982; Oishi, 1985) (section 7.5). These changes in zinc
homeostasis could be due to altered levels of metallothionein. In mice
fed 6 or 12 g DEHP/kg diet for 24 weeks, hepatic levels of
metallothionein were increased up to 11-fold (Waalkes & Ward, 1989).
Several studies on rats have shown that DEHP given in the diet
(5-20 g/kg) decreases plasma triglyceride and cholesterol levels
(Yanagita et al., 1978; Sakurai et al., 1978; Bell et al., 1978a; Bell
et al., 1978b; Bell et al., 1979; Yanagita et al., 1979; Curstedt &
Sjövall, 1983). DEHP inhibits the biosynthesis of cholesterol, an
effect which is accompanied by phospholipidosis, and the same effects
have been observed with MEHP (Oishi & Hiraga, 1982).
7.3 Long-term exposure
In a 24-month study by Harris et al. (1956), three groups of
Wistar rats each comprising 43 males and 43 females were fed diets
containing 0, 1, and 5 g DEHP/kg, interim kills being made at 3, 6,
and 12 months. At the end of the study, only two control, four
low-dose, and seven high-dose animals were alive. During the first
year the body weights of the high-dose group were slightly reduced,
but by the second year the body weights of all groups were similar.
During the first 6 months an increase in relative liver and kidney
weights was seen in DEHP-treated animals but later they were similar
in control and treated animals. After 3 months of treatment, one out
of eight rats in the low-dose group was found to have mild renal
tubular atrophy. After 6, 12, and 24 months of treatment, no
compound-related pathological changes were evident. Because of high
mortality due to disease, this study is difficult to validate.
In another 24-month study (Carpenter et al., 1953), groups of
Sherman rats consisting of 32 males and 32 females were given diets
containing 0, 0.4, 1.3 or 4 g DEHP/kg. Owing to reduced life
expectancy due to disease and the small numbers of animals used, the
study was inadequate for assessing the chronic toxicity of DEHP.
In a 12-month study by Nikonorow et al. (1973), a group of 20
male and 20 female Wistar rats was given a diet containing 3.5 g
DEHP/kg, and a control group received the diet without DEHP. The only
gross or micropathological change noted in exposed animals at necropsy
was hepatomegaly. During the study, however, about 30% of the animals
died due to congestion of the small intestine and loss of the gastric
and/or intestinal mucosa, which was complicated by purulent pneumonia
and endometritis.
Crocker et al. (1988) described the renal effects of DEHP given
by gavage to young male rats at a dosage of 2.14 mg/kg body weight
three times per week for up to 12 months. A 50% reduction in
creatinine clearance and an increase in the severity of renal cyst
formation was observed. This lesion was consistent with spontaneous
nephropathy commonly observed in old rats; exposure may cause an onset
in younger rats. Furthermore, DEHP fed at 6 and 12 g/kg diet for two
years did not produce renal lesions in male and female F-344 rats
(NTP, 1982).
In a 2-year study (NTP, 1982; Kluwe et al., 1982), groups of
F-344 rats were given dietary levels of 0, 6, and 12 g DEHP/kg.
Decreased body weight in exposed groups was noted from week 30 until
the cessation of exposure. In addition to neoplastic effects (section
7.7) and testicular atrophy (section 7.5), a compound-related
hypertrophy of cells in the male anterior pituitary was noted in the
high-dose group. In both exposed groups an increased incidence of
clear changes in liver cells was observed. This study also
investigated B6C3F1 mice exposed to 3 or 6 g DEHP/kg diet. A
dose-related decrease of body weight was observed in female mice.
There was no increased incidence of non-neoplastic lesions except for
seminiferous tubular degeneration in the testes of male mice (section
7.5.1).
In a study on groups of male and female guinea-pigs fed diets
containing 0, 0.4 or 1.3 g DEHP/kg for 12 months (Carpenter et al.,
1953), the body weight of the low-dose animals was statistically
higher than that of controls, and liver weight relative to body weight
of dosed females slightly increased. No other exposure-related lesions
were found.
A study on groups of male ferrets fed diets containing 0 and 10
g DEHP/kg for 14 months revealed a reduction in body weight and an
increase in relative liver weight, but no evidence of peroxisome
proliferation (Lake et al., 1976).
7.4 Skin and eye irritation; sensitization
DEHP has been shown to be a weak irritant to mammalian skin when
administered topically or intradermally (0.2 ml of an emulsion of
100 g/litre) (Calley et al., 1966; Woodward et al., 1986).
In a study by Lawrence et al. (1975), no irritation occurred when
undiluted DEHP was instilled into the eye of rabbits.
No data are available on the potential for DEHP to induce skin
sensitization in animals.
7.5 Reproduction, embryotoxicity, and teratogenicity
7.5.1 Reproduction
The effects of DEHP on male reproductive organs have been studied
extensively. The majority of the studies have been carried out on rats
or mice given DEHP in the diet.
Seminiferous tubular atrophy, comprising a loss of spermatids and
spermatocytes, occurred when 4-week-old Wistar rats were given 2800 mg
DEHP/kg by oral intubation for 10 days (Gray & Butterworth, 1980). In
similarly treated 10-week-old rats, about 50% of the tubules were
atrophic and the remainder unaffected. However, no testicular damage
was detected in treated 15-week-old rats. When 20 g DEHP/kg was given
in the diet (approximately 1200 mg DEHP/kg per day) to 4-week-old
rats, the lesions produced were reversible whether treatment stopped
before or continued until after the control rats had reached sexual
maturity.
In rats given 10 or 20 g DEHP/kg diet, the testis atrophy was
dose dependent after approximately 2 weeks of feeding. This atrophy
was accompanied by pituitary changes, i.e. enlargement and
vacuolization of the basophils of the pars distalis, corresponding to
the formation of the so-called castration cells seen after gonadectomy
(Gray et al., 1977). In a subsequent study, there was a reduction in
testicular and prostatic zinc levels concomitant with increased
urinary excretion of zinc (Gray et al., 1982).
In a rat study by Oishi & Hiraga (1980a), the serum testoster-one
levels in rats fed 20 g DEHP/kg diet were reduced by approximately
50%. The total amount per testis decreased, but the concentration rose
to 150% of the original value because of the testicular atrophy.
Simultaneous administration of testosterone or zinc had no protective
effect on the atrophy but did prevent the weight reduction of the sex
organs such as the epididymis (Gray & Butterworth, 1980; Oishi &
Hiraga, 1983). In a further study, it was found that a low-zinc diet
aggravated the DEHP-induced testicular atrophy (Agarwal et al.,
1986a).
DEHP given to 7 young (5 weeks old) Wistar rats in the diet
(20 g/kg) for one week decreased the testicular weight significantly
(P < 0.05), compared to controls, and also the testicular zinc
concentration (Oishi, 1984). In a study by Oishi (1985), groups of 20
male rats were given DEHP (2.0 g/kg per day) by gavage for 14 days.
Ten rats were then killed and the remaining ten were kept for 45 days
on a DEHP-free diet. The histopathological changes of the testes seen
on day 15 were marked shrinkage of the seminiferous tubules, a
germinal epithelium consisting only of Sertoli cells, and very few
spermatogonia. After 45 days the percentage of spermatogenic tubules
had increased from 0 (at day 15) to 12.8%, indicating a limited
reversibility of the testicular atrophy.
Similar degeneration of the seminiferous tubules was observed
when 13-week-old Wistar rats were orally given 2 g DEHP/kg body weight
for 7 consecutive days (Saxena et al., 1985) .
In a dietary study, DEHP was administered to F-344 rats at 6 and
12 g/kg for 103 weeks (NTP, 1982). Degeneration of the seminiferous
tubules was observed only at the higher dose.
The testicular changes induced by oral DEHP administration appear
to be age dependent. A daily dosage of 2.8 g DEHP/kg body weight given
for 10 days caused seminiferous tubular atrophy in 4-week-old Wistar
rats, but this effect was less severe in 10-week-old rats and absent
in 15-week-old rats (Gray & Butterworth, 1980). Sjöberg et al. (1986)
showed that DEHP fed at a level of 1.7 g/kg diet caused reductions in
testis weight to 20%, 55%, and 92% of the control values in
Sprague-Dawley rats at 25, 40, and 60 days of age, respectively. This
was apparently not due to differences in pharmacokinetic parameters,
since the plasma levels of the metabolite MEHP were identical in the
25- and 40-day-old rats (Sjöberg et al., 1982).
Rats given DEHP intraperitoneally at a dosage of 100 mg/kg per
day for 5 days did not develop testicular atrophy (Curto et al.,
1982). However, this lack of response was probably a consequence of
the dose used, rather than the dose route, and there was a 30%
reduction in testicular zinc. Intraperitoneal administration of higher
doses (1-25 g/kg per day for 5 days) to Wistar rats decreased serum
testosterone levels (Oishi & Hiraga, 1979).
Some degenerated primary spermatocytes and altered Sertoli cells
were observed in Sprague-Dawley rats given 3-h intravenous infusions
of an emulsion at a rate of 1 ml/h, which corresponded to a daily dose
of 500 mg DEHP/kg (Sjöberg et al., 1985c). The infusions were given
every other day on six occasions. The emulsion contained DEHP,
fractionated egg yolk phosphatides, glycerol, and water. No effects
were observed when emulsions corresponding to 0, 5 or 50 mg DEHP/kg
were given.
In a study by Curto & Thomas (1982), groups of sexually mature
Swiss-Webster mice were given intraperitoneal injections of 50 or 100
mg DEHP/kg either daily for 5 days or alternate daily for 20 days (10
injections). The animals were killed 24 h after the last injection. No
significant alterations in testicular weight or zinc levels occurred.
Young rats were not more susceptible to testicular damage than
older ones following intravenous infusion of DEHP. It has been
suggested that the age-related difference observed in some studies may
be due to the fact that the rate of gastrointestinal absorption of the
DEHP-derived metabolite MEHP is higher in younger animals than in
older ones (Sjöberg et al., 1986).
In a NTP-sponsored study (Melnick et al., 1987), CD-1 mice given
3 g DEHP/kg diet showed significantly diminished testis and epididymis
weights compared to controls. In addition, the sperm concentration in
the cauda epididymis was reduced and the percentage of abnormal sperm
in the cauda was significantly higher in the treated mice than in the
controls.
A high oral dose (4.2 g/kg) of DEHP gave minimal tubular atrophy
in hamsters but did not produce any effects on urinary zinc excretion,
testicular zinc levels or testicular weights (Gray et al., 1982).
The effects of the monoester MEHP have not been as well studied
as those of DEHP. An oral dosage of 1 g MEHP/kg per day for 5 days
produced a significant decrease in rat testis weight and extensive
testicular atrophy (Gray et al., 1982). On the other hand, rats given
intraperitoneal doses of up to 100 mg MEHP/kg daily for 5 days showed
no abnormal histology (Curto et al., 1982) and an intraperitoneal dose
of 50 mg/kg given on alternate days for 20 days produced only a
reduction in prostatic zinc levels (Curto & Thomas, 1982).
Mice fed diets containing 20 g MEHP/kg for 1 week revealed
markedly reduced testicular zinc and testosterone levels, but there
were no reductions in testicular weight (Oishi & Hiraga, 1980b).
Hamsters given MEHP (1 g/kg per day) for 9 days showed more severe
testicular effects than those given DEHP at a level of 4.2 g/kg per
day for the same period (Gray et al., 1982). More recent studies
(Albro et al., 1989) indicate that testicular atrophy resulting from
DEHP exposure is most probably due to the formation of MEHP.
These studies indicate that the rat is the species most
susceptible to DEHP-induced testicular atrophy. The mechanism of
phthalate-induced testicular damage is not fully understood.
Testicular zinc depletion has been suggested to be a primary event
(Foster et al., 1982, 1983). However, Gray et al. (1982) showed that
an effect on zinc level is not always associated with testicular
atrophy in all species tested. Zinc is essential for normal testicular
function and its depletion is known to lead to testicular atrophy
(Barney et al., 1969). Inhibition of dehydrogenase enzymes, e.g.,
those controlling the biosynthesis of testosterone, leads to reduced
testosterone levels. DEHP administration has been shown to reduce the
level of serum testosterone in the rat (Oishi & Hiraga, 1979; Oishi &
Hiraga, 1980a) as well as in the mouse (Gray et al., 1982), although
in the mouse no testicular atrophy was observed. Administration of
testosterone or zinc did not prevent the testicular damage induced by
DEHP (Gray & Butterworth, 1980; Oishi & Hiraga, 1983).
Co-administration of DEHP and testosterone, on the other hand,
apparently enhanced the testicular damage caused by DEHP (Oishi,
1989a). In a similar study (Oishi, 1989b), luteinizing
hormone-releasing hormone (LRH) significantly decreased testis weight,
sulfhydryl content, and lactate dehydrogenase when given together with
DEHP, whereas LRH or DEHP alone had no effects.
Similar effects were observed with exogenously added follicle
stimulating hormone (FSH) in an in vitro study. Primary testicular
cell cultures pretreated with MEHP showed a dose-related reduction in
FSH-stimulated cyclic adenosine monophosphate (cAMP) production (Lloyd
& Foster, 1988). These results suggest that MEHP produces a
perturbation at the level of the FSH membrane receptor, causing an
inhibition of FSH action.
In vitro studies have indicated that the Sertoli cell is the
target cell. Mixed cultures of Sertoli and germ cells prepared from
rat testes were exposed to DEHP or MEHP (10-7-10-4 mol/litre)
(Gray & Beamand, 1984; Gray & Gangolli, 1986). DEHP had no effect, but
MEHP caused a dose-dependent increase in the rate of germ cell
detachment from Sertoli cells, accompanied by changes in Sertoli cell
morphology. More recent data indicate that Sertoli cell mitochondria
are a target for MEHP (Chapin et al., 1988). However, it has also
been shown that testicular mitochondrial respiratory functions are
decreased in rats given 2 g DEHP/kg body weight by gavage (Oishi,
1990).
In a study by Melnick et al. (1987), CD-1 mice were given 0, 0.1,
1 or 3 g DEHP/kg diet during a 7-day pre-mating period and a
subsequent 98-day cohabitation period. There was complete suppression
of fertility in the 3-g/kg group and a significant reduction in
fertility in the 1-g/kg group, compared to controls, but no effect on
fertility at 0.1 g/kg.
In a fertility study designed to investigate earlier significant
results (Singh et al., 1972), groups of male ICR mice were dosed
subcutaneously with undiluted DEHP at levels of 1, 2, 5, and 10 ml/kg
(equivalent to 0.99, 1.97, 4.93, and 9.86 g/kg) on days 1, 5, and 10
of the study (Agarwal et al., 1985a). They were subsequently mated
with virgin females on days 2, 6, 11, 16 and 21, and then at weekly
intervals until week 8. There were reductions in the incidence of
pregnancies in several groups, but these were dose related only at the
first mating (i.e. on day 2 of the experiment). Examination of
pregnant mice on day 13 of gestation showed that there were
pre-implantation losses corresponding to a DEHP dose level of 10 ml/kg
and a mating on day 6. There were consistent increases in early fetal
deaths relating to matings at day 21, week 4, and week 5. In a similar
study, male and female mice were treated subcutaneously with 1-100 ml
DEHP/kg, and this led to a reduction in testicular but not ovarian
weight. The ovaries exhibited histological injury at lower doses of
DEHP than the testes. Unlike the situation in the testes, there was no
significant dose-related increase in histopathological changes in the
ovaries (Agarwal et al., 1989).
In an investigation of the effects of phthalates on female
reproductive organs, three doses of 4.93 g DEHP/kg were given
intraperitoneally at 5-day intervals to female rats (Seth et al.,
1976). No histopathological changes in the ovaries were seen 22 days
after the first injection, but reductions in the activities of some
enzymes were noted.
The administration of DEHP (1000 mg/kg intraperitoneal or 2000
mg/kg oral) to marmosets daily for 14 days did not lead to testicular
atrophy (Rhodes et al., 1986).
Hence, it appears that rats and guinea-pigs are sensitive to
DEHP-induced testicular atrophy, while mice are fairly resistant and
hamsters and marmosets are highly resistant. The fact that at least
some of the effects associated with this atrophy can be produced
in vitro argues against a hormonally mediated indirect effect. The
earliest effects are seen in Sertoli cells and are described as
vacuolation (Albro, 1987).
7.5.2 Embryotoxicity and teratogenicity
In a study by Nikonorow et al. (1973) on female Wistar rats given
0.34 or 1.7 g DEHP/kg by gavage during the first 21 days of gestation,
the only untoward effect was a reduction in fetal body weight. When
DEHP (0, 5, 10, 15 or 20 g/kg) was administered orally to Fischer-344
rats on gestational days 0 to 20, maternal toxicity and reduced fetal
body weight per litter were observed at the three highest dose levels.
The number of fetuses per litter was unaffected by the treatment (Tyl
et al., 1988).
Intraperitoneal injections of 4.93 or 9.86 g DEHP/kg on days 5,
10, and 15 of gestation resulted in an increase in the number of
resorptions and reduced fetal weight in Sprague-Dawley rats (Singh et
al., 1972). In the highest-dose group, gross abnormalities, such as
twisted hind legs and anophthalmia, were noted but no skeletal defects
were observed.
Rat plasma soluble extracts of two PVC plastics containing DEHP
were administered intravenously to groups of pregnant Sprague-Dawley
rats daily from the 6th to the 15th day of gestation, and the animals
were killed at day 20 (Lewandowski et al., 1980). The daily doses of
DEHP were equivalent to 1.3 mg/kg and 5.2 mg/kg. No significant
teratogenic or embryotoxic effects were noted.
Groups of ICR mice were given DEHP in the diet at levels of 0.5
to 10 g/kg for the first 18 days of gestation and were then sacrificed
(Shiota et al., 1980; Shiota & Nishimura, 1982). The food intake was
an average of 7 g/day. At the 4 g/kg and 10 g/kg dose levels, no live
fetuses were found. At 2 g/kg, 40% of the fetuses had malformations
including exencephaly, spina bifida, and malformed tail. Delayed
ossification was seen in about 15% of the fetuses at 1 g/kg and
2 g/kg.
In a study by Tyl et al. (1988), DEHP was administered in the
diet to CD-1 mice on the first 17 days of gestation at levels of 0,
0.25, 0.5, 1, and 1.5 g/kg. At the two highest dose levels maternal
toxicity, increased resorptions and late fetal deaths, decreased
numbers of live fetuses, and reduced fetal body weight per litter were
observed. The number and percentage of malformed fetuses per litter
were elevated at the three highest dose levels.
A single oral administration of DEHP (0.1 ml/kg) on day 7 of
gestation to ddY-strain SPF (specific pathogen free) mice decreased
the number and the body weights of live fetuses (Tomita et al.,
1982b). In a study by Yagi et al. (1980), DEHP was given orally to SPF
mice on days 6, 7, 8, 9 or 10 of gestation. When 5.0 or 10.0 ml/kg was
given on day 7 there were no live fetuses, whereas 2.5 ml/kg
administered on the same day resulted in 14% live embryos and 1.0
ml/kg gave 40% live embryos. The percentages of live embryos when 10.0
ml/kg was given on day 8, 9 or 10 of gestation were 18, 92, and 95%,
respectively. Gross and skeletal abnormalities occurred in fetuses
given 2.5 and 7.5 ml/kg on day 7 or 8. The abnormalities included
exencephaly, open eyelid, and club-foot.
In a study by Shiota & Mima (1985), groups of ICR mice were given
DEHP by stomach intubation on days 7, 8, and 9 of gestation. The DEHP
doses (in olive oil) were 250, 500, 1000, and 2000 mg/kg, and the mice
were sacrificed on day 18. In the two highest dose groups, the numbers
of resorptions and malformed fetuses were significantly increased.
Fetal weights were significantly depressed. The most frequent
malformations were anencephaly and exencephaly. When doses of up to
8000 mg/kg were given by intraperitoneal injections on days 7,8, and
9 of gestation, no effects were noted. DEHP is highly embryotoxic and
terato-genic in mice when given orally but not when given
intraperitoneally.
The monoester MEHP, at oral doses of 225, 450, and 900 mg/kg per
day, produced significant signs of maternal toxicity when given to
pregnant Wistar rats on days 6-15 of gestation (Ruddick et al., 1981).
In the highest dose group a 73% mortality was observed in the dams.
There was a dose-related decrease in the number of litters and in the
litter weights of live pups.
Oral dosing with 0.1 or 1.0 g MEHP/kg on day 7 of gestation led
to increased incidence of early embryonic deaths in SPF mice (ddY
strain), but dosing on day 8 or 9 had less effect (Yagi et al., 1980;
Tomita et al., 1986). The fetuses had reduced body weight, and there
was a higher incidence of gross abnormalities, compared to controls,
when the higher dose level was given on day 8 or 9. The mice dosed on
day 8 produced fetuses with a high incidence of skeletal effects.
Intravenous injections of MEHP (11.38 mg/kg) to rabbits on days
6-17 of gestation gave a high incidence of resorptions (Thomas et al.,
1979). The incidence of fetal anomalies was similar to that in
controls.
When Nikonorow et al. (1973) administered DEHP (0.34 or 1.7 g/kg
per day) to female Wistar rats by gavage for 3 months prior to mating,
there was an increase in the number of resorptions but no effects on
fetal weights or the incidence of skeletal anomalies.
In utero administration of DEHP (1000 mg/kg body weight) to
rats daily from days 6 to 15 of gestation resulted in retardation of
fetal growth and an increase in fetal liver weight. There were
significant quantities of DEHP and decreased mitochondrial enzyme
activities in the fetal liver. These results indicate an effect on
fetal liver cell bioenergetics (Srivastava et al., 1989).
The mechanism of DEHP or MEHP teratogenicity is not known.
Teratogenic activity could result from zinc deficiency, which is known
to produce such effects (Swenerton & Hurley, 1971).
7.6 Mutagenicity and related end-points
The possible genotoxic effect of DEHP has been thoroughly
investigated in several different short-term tests. The effects of the
major metabolites of DEHP, i.e. MEHP and 2-ethylhexanol, as well as
phthalic acid and phthalic anhydride, have also been studied.
7.6.1 Mutation
Studies on the possible mutagenic effect of DEHP have been
performed in bacteria, fungi, and in cultured mammalian cells.
Drosophila melanogaster has also been used and results from a few
in vivo studies on mice have been reported.
7.6.1.1 Bacteria
Many studies have been performed using a variety of strains of
Salmonella typhimurium and DEHP doses of up to 10 mg/plate.
Incubations both with and without exogenous activation systems have
been performed. S9 mix from rats induced by Aroclor 1254 has
frequently been used, but other species and other inducers have also
been used to produce metabolic activation systems. With one exception
(Tomita et al., 1982a) these test results have all been negative
(Kirby et al., 1983; Yoshikawa et al., 1983; Zeiger et al., 1985;
Agarwal et al., 1985b), and in a IPCS collaborative study (Ashby et
al., 1985) all five laboratories reported negative results. Bacteria
other than S. typhimurium have also been used; negative results were
obtained with E. coli WP2 at doses of up to 2 mg per plate (Yoshikawa
et al., 1983).
The major metabolites of DEHP have also been tested for mutagenic
activity in bacteria. Concentrations of up to 3.333 mg per plate for
MEHP and phthalic anhydride (Zeiger et al., 1985) and 2 mg/plate for
2-ethylhexanol and phthalic acid (Agarwal et al., 1985b) have yielded
negative results in strains of Salmonella (see also Kirby et al.,
1983; Yoshikawa et al., 1983). However, Tomita et al. (1982a) reported
a significant increase in TA100 revertants following exposure to
either DEHP or MEHP (both with and without S9). It should be noted,
however, that MEHP mutagenicity is only demonstrable within a narrow
range of concentration, because it has a sterilizing effect at high
concentration and shows no mutagenic activity at low concentration.
Tomita et al. (1982a) also detected dose-dependent (0.4 and 0.5 mg
MEHP/plate) DNA damage in a B. subtilis Rec assay, while DEHP,
phthalic acid, and 2-ethylhexanol were all negative. In this study
MEHP also gave a positive result in E. coli WP2 b/r.
Negative results were obtained when pooled urine from rats,
treated with 2000 mg DEHP/kg per day for 15 days, was tested for
genotoxic activity. A direct plating procedure was used with
S. typhimurium strains TA98, TA100, TA1535, TA1537, and TA1538, both
with and without S9 and ß-glucuronidase/aryl sulfatase as the
activation system. When 2-ethylhexanol was tested according to the
same protocol, the result was also negative (Divincenzo et al., 1985).
7.6.1.2 Fungi
The induction of mutations by DEHP has been studied in various
fungal species. In the IPCS collaborative study on in vitro assay
systems (Ashby et al., 1985), DEHP was considered to be negative in
six out of seven assays. Positive results were obtained with
Saccharomyces cerevisiae, both with and without S9 activation, at
lowest effective concentrations of 1541 mg/litre and 3081 mg/litre,
respectively. However, other laboratories using different strains of
S. cerevisiae or Schizosaccharomyces pombe reported negative
results at a maximum tested concentration of 5000 mg/litre.
7.6.1.3 Mammalian cells
Mouse lymphoma cells (L5178Y), Chinese hamster V79 cells, and
human lymphoblasts have been used to study the mutagenic effect of
DEHP in cultured mammalian cells. Several investigators obtained
negative results, but a few positive results have also been reported.
In the IPCS collaborative study (Ashby et al., 1985), only one
out of ten investigators reported a positive response. Mouse lymphoma
cells were exposed to DEHP without S9, and two concentrations (7.5 and
20 mg/litre) gave positive results. In a separate study (Kirby et al.,
1983), where MEHP, 2-ethylhexanol, and DEHP were tested in the mouse
lymphoma cell assay, all three substances were found to be
non-mutagenic. The concentrations used were 0.016-1.0 ml/litre
(without S9) and 0.067-5.0 ml/litre (with S9) for DEHP, and 0.013-1.0
ml/litre for MEHP and 2-ethylhexanol.
7.6.1.4 Drosophila
DEHP has also been tested for mutagenicity in Drosophila
melanogaster using the sex-linked recessive lethal (SLRL) test and
various somatic recombination and mutation assays. SLRLs were not
induced by DEHP (20 µg/g) administered by injection (Yoon et al.,
1985). Negative results for DEHP were also reported in the SLRL
mutation assay on Drosophila melanogaster larvae (Zimmering et al.,
1989).
In the IPCS study (Ashby et al., 1985), DEHP gave a positive
response in the unstable eye mosaic test at a dose of 6.1 g/litre, in
two separate experiments, but neither lower nor higher concentrations
produced any response. No activity was seen in the wing spot test with
a single dose of 6.1 g/litre. It was concluded that DEHP exhibits
marginally positive mutagenicity (Ashby et al., 1985).
7.6.2 DNA damage
Various end-points, such as unscheduled DNA synthesis (UDS) and
single strand breaks, have been used to detect DNA damage induced by
DEHP in a variety of mammalian test systems. In the IPCS study (Ashby
et al., 1985), negative results were obtained when single strand
breaks were measured, either by alkaline elution in hepatocytes (up to
3.907 g/litre) or alkaline sucrose sedimentation in Chinese hamster
ovary (CHO) cells (up to 39 g/litre). UDS in either isolated
hepatocytes or cultured HeLa cells was investigated by four different
laboratories. One investigator detected a positive response using
isolated hepatocytes, but, since this result was only statistically
significant at one dose and not dose related, the consensus was that
DEHP does not cause UDS.
In a study by Butterworth et al. (1984), DEHP did not induce DNA
repair in primary rat hepatocytes. Similarly, neither DEHP nor MEHP
induced any DNA repair in primary human hepatocytes from three
different subjects. In this study, concentrations as high as 0.01 mol
DEHP/litre and 5 x 10-4 mol MEHP/litre were used and exposure
continued for 18 h. No induction of DNA repair or increased alkaline
elution of DNA was seen in hepatocytes from either female or male
F-344 rats treated with DEHP in vivo. UDS was not induced in male
rats treated with 500 mg DEHP/kg by gavage 2, 12, 24 or 48 h before
sacrifice. Furthermore, treatment of male rats with 150 mg/kg per day
by gavage for 14 days and treatment of female rats with dietary DEHP
(12 g/kg) for 30 days resulted in peroxisome proliferation, but no UDS
was induced.
Similar in vivo/in vitro results in studies of UDS in
hepatocytes were reported by Kornbrust et al. (1984). No UDS was
observed in primary rat hepatocytes exposed in vitro to 10-5 to
10-2 mol DEHP/litre or in vivo by a single gavage dose of 5 g/kg
2, 15 or 24 h prior to the isolation of hepatocytes. A dietary
concentration of 20 g DEHP/kg led to a marked proliferation of
peroxisomes after 4 weeks. Neither this treatment nor the additional
administration of a single gavage dose of 5 g/kg (15 h before
sacrifice) to animals fed the 20-g/kg diet for either 8 weeks or for
4 weeks with or without pretreatment with 3-amino-1,2,4-triazole (to
inhibit endogenous catalase activity) induced any detectable DNA
repair in hepatocytes. Indeed, the administration of DEHP and some
other proliferators has been shown to increase the levels of
8-hydroxy-deoxyguanosine in rat liver DNA (Takagi et al., 1990).
7.6.3 DNA binding
In an in vivo study by Albro et al. (1982), radioactivity from
carbonyl-labelled DEHP did not associate with purified protein, RNA or
DNA from rat liver. Label from 2-ethyl-(1-14C)-hexyl-labelled DEHP
or MEHP appeared to associate strongly with purified DNA, but this was
not the case with label from free 14C-labelled 2-ethylhexanol.
According to Albro (1987), although there is incorporation into normal
nucleosides, there is no evidence of alkylation.
In a similar study (von Däniken et al., 1984; Lutz, 1986), DEHP
radiolabelled in different positions was administered orally to female
F-344 rats with or without pretreatment with unlabelled DEHP (10 g/kg
diet) for 4 weeks. The administration of 14C-carboxylate-labelled
DEHP resulted in no measurable DNA radioactivity, whereas
radioactivity was clearly measurable after the administration of DEHP,
14C- or 3H-labelled in the alcohol moiety, or of
2-ethyl(1-14C)hexanol. HPLC analysis showed that the normal
nucleosides had incorporated radiolabel, but fractions expected to
contain carcinogen-modified nucleoside adducts did not contain any
radioactivity.
DNA isolated from the livers of male F-344 rats administered 2000
mg DEHP/kg daily by gavage for 3 days was analysed for possible
carcinogen-DNA adducts by the 32P-postlabelling technique (Gupta et
al., 1985). No adducts were detected in the DNA, which also was the
case when DNA from hepatocytes exposed to 10-3 mol DEHP/litre
in vitro for 4 h was analysed.
7.6.4 Chromosomal effects
Chromosomal effects of DEHP have mainly been studied in vitro,
although some studies on the induction of micronuclei in the
peripheral blood erythrocytes of mice have been published.
DEHP did not induce any increase in the level of sister-chromatid
exchange (SCE) in Chinese hamster ovary (CHO) cells, treated for 1 h,
either with or without S9, at levels of up to 0.01 mol/litre (Douglas
et al., 1986). On the other hand, MEHP has been reported to induce SCE
in V79 cells treated with 25 or 50 mg/litre for 24 h or 1500 mg/litre
for 3 h (Tomita et al., 1982a). This metabolite also induced
chromosomal aberrations in CHO cells and RL4 cells (from rat liver),
but only at cytotoxic concentrations in the CHO cells (1.0 and 1.3
mmol/litre, with or without S9). MEHP was less toxic to RL4 cells
and concentrations between 2.0 and 5.0 mmol/litre gave a dose-related
increase in chromosomal aberrations (Phillips et al., 1986). According
to the authors, observations on changes in CHO cell structure and
permeability and on the haemolytic effects of phthalate monoesters
suggest that MEHP cytotoxicity may be due primarily to an action on
cell membranes.
The induction of aneuploidy by DEHP was investigated both in
mammalian cells and in fungi in the IPCS study (Ashby et al., 1985).
The mammalian assays gave positive responses at 50 mg/litre in a
fibroblast cell line from Chinese hamster liver and at levels of
between 25 and 50 mg/litre in Chinese hamster primary liver cells. Two
out of four studies using fungi were also positive and the consensus
was that DEHP is capable of inducing aneuploidy in vitro in both
fungi and mammalian cells.
In a study by Ahmed et al. (1990), male Wistar rats were fed for
alternate 7-day periods with a diet containing 20 g DEHP/kg and a
control diet. The rats were examined after 3 days on the DEHP diet or
after 7 days on the control diet. An analysis of nuclear size gave
results consistent with an increase in tetraploid hepatocytes after
treatment with DEHP, which was reversed when the rats returned to the
control diet.
7.6.5 Cell transformation
DEHP-induced cellular transformation has been studied in several
different experimental systems. In a test programme (Astill et al.,
1986), the BALB/3T3 cell transformation assay was used both with and
without rat primary hepatocytes. Both DEHP (0.875-1 µl/litre) and the
two metabolites MEHP and 2-ethylhexanol gave negative results. On the
other hand, the majority of transformation tests in the IPCS study
(Ashby et al., 1985) were positive for DEHP. Negative results were
obtained with BALB/c-3T3 cells, while a study measuring the
enhancement of viral transformation of Syrian hamster embryo (SHE)
cells was considered to be inconclusive. Positive responses were
obtained by four other investigators using SHE cells at 1-300 mg/litre
(two different laboratories), embryonic mouse fibroblasts at 1000
mg/litre with S9 and 10 mg/litre without S9, or retrovirus-infected
Fischer rat embryo cells at 2000 mg/litre (the highest dose tested).
In a separate study (Tomita et al., 1982a), both DEHP (7.5 and 15
g/kg) and MEHP (375 and 750 mg/kg) induced morphological
transformation, as well as chromosomal aberrations, in SHE cells after
transplacental administration.
In a study by Diwan et al. (1985), anchorage-independent growth
of JB6 mouse epidermal cells was enhanced by DEHP at concentrations of
500 to 20 000 ppm/ml (the Task Group noted that the unit used in
Diwan's paper is ppm/ml, but it should read ppm or mg/litre). Ward et
al. (1986) used the same model system and reported that both DEHP
(1.3-51 x 10-6 mol/litre) and MEHP (2-5 x 10-8 mol/litre) were
effective, while 2-ethylhexanol (4-77 x 10-7 mol/litre) was without
effect. The morphological transformation in the SHE cell system
induced by DEHP, MEHP, and other hepatic peroxisome proliferators
seemed not to be correlated with increased peroxisomal ß-oxidation,
increased production of oxidative radicals or peroxisome proliferation
(Mikalsen et al., 1990a; Mikalsen et al., 1990b; Mikalsen et al.,
1990c).
A few studies on DEHP-induced inhibition of metabolic
cooperation, which may be indicative of the promoting potential of a
substance, have been reported. Metabolic cooperation in Chinese
hamster V79 cells was not inhibited by DEHP at non-cytotoxic
concentrations, i.e. 3 x 10-4 mol/litre (0.12 mg/litre) or less
(Kornbrust et al., 1984). In the IPCS study (Ashby et al., 1985), one
investigator reported inhibition in V79 cells at non-cytotoxic
concentrations of DEHP (25-200 mg/litre in two separate experiments
and 5-25 mg/litre in another), while another investigator, using V79
cells in a microassay method, detected a slight (but non-significant)
increased inhibition at doses of between 10-5 and 2 x 10-4
mol/litre (3.9-78 mg/litre).
In a study by Malcolm & Mills (1989) on V79 cells, DEHP inhibited
intercellular communication (gap junctions) at non-cytotoxic
concentrations (10-30 mg/litre).
7.6.6 In vivo effects
Putman et al. (1982) dosed male Fischer-344 rats orally for 5
days with DEHP (5.0, 1.7, and 0.5 g/kg per day), MEHP (0.14, 0.05, and
0.01 g/kg per day), and 2-ethylhexyl (0.21, 0.07, and 0.02 g/kg per
day), and bone marrow metaphase cells were examined. No significant
increases were observed in gap breaks or structural rearrangements. In
addition, the mitotic index was unaffected by treatment.
A micronucleus assay on mouse (B6C3F1) peripheral blood
erythrocytes (intraperitoneal doses of 0.6, 3.0, and 6.0 g/kg per day
for 5 days), sampled at 0, 2, and 4 weeks after the last treatment,
yielded negative results (Douglas et al., 1986). A sperm morphology
assay in B6C3F1 mice and Sprague-Dawley rats at the same dose levels
also gave negative results (Douglas et al., 1986). However, Agarwal
et al. (1986b) reported increases in sperm morphology changes in adult
F-344 rats given 20 g DEHP/kg for 60 consecutive days.
Two studies have yielded negative results in dominant lethal
tests (Hamano et al., 1979; Rushbrook et al., 1982). Hamano et al.
(1979) administered MEHP and DEHP orally, and the observation period
was six weeks. Rushbrook et al. (1982) dosed ICR/SRM mice orally for
5 days with DEHP (2465, 4930, and 9860 mg/kg per day), MEHP (50, 100,
and 200 mg/kg per day) or 2-ethylhexanol (250, 500, and 1000 mg/kg per
day). Each male was mated weekly with virgin females for 8 consecutive
weeks, and females were evaluated for pregnancy, live fetuses, and
early and late fetal deaths. All data were within the normal ranges.
DEHP and its major metabolites have also been tested for their
potential to induce micronuclei. Exposure to 0.6, 3.0 or 6.0 g DEHP/kg
per day for 5 days over a 4-week period did not induce micronuclei in
the peripheral blood erythrocytes of B6C3F1 male mice (Douglas et
al., 1986). Negative results were also obtained in another mouse
micronucleus test after either a single or daily doses of 5 g DEHP/kg.
In this study MEHP and 2-ethylhexanol also gave negative results
(Astill et al., 1986).
7.7 Carcinogenicity
In a carcinogenicity study (NTP, 1982; Kluwe et al., 1982),
groups of 50 male and 50 female Fischer-344 rats were fed diets
containing 6 or 12 g DEHP/kg diet, and 50 male and 50 female B6C3F1
mice were fed diets containing 3 or 6 g DEHP/kg for 103 consecutive
weeks. Concurrent controls (50 of each sex and species) were fed a
diet without the addition of DEHP. Food and water were supplied
ad libitum. All animals were given the control diet for 1-2 weeks
after 103 weeks of treatment and were then killed and examined both
grossly and microscopically. The administered concentrations of DEHP
were estimated to be equal to, or one half of, the maximum tolerated
doses. Under the conditions studied, DEHP caused an increased
incidence of hepatocellular carcinomas in female rats and male and
female mice, and an increased incidence of hepatocellular carcinomas
and neoplastic nodules in female rats (Table 8). Twenty of the 57
hepatocellular carcinomas in the DEHP-treated mice (sexes and doses
combined) had metastasized to the lung. The figure of nine
hepatocellular carcinomas in control male mice is considered to be
within the normal range (NTP, 1982).
The reported decreased incidence of tumours of the thyroid,
pituitary, and testis could be related to an increased endocrine
activity of the pituitary gland (NTP, 1982).
The carcinogenicity of DEHP in rats has been confirmed in two
further studies. Rao et al. (1990) found a 78.5% incidence in a group
of 14 male Fischer-344 rats fed a diet containing 20 g DEHP/kg for up
to 108 weeks, whereas the incidence in the 10 controls was 10%. Popp
et al. (1987) found either hepatocellular carcinomas or neoplastic
nodules in 6 out of 20 animals after female Fischer-344 rats were
exposed for 2 years to a diet containing 12 g/kg.
Table 8. Carcinogenic effects of DEHP on the livera
Control Low dose High dose P valueb
Hepatocellular carcinoma
Male rats 1/50 1/49 5/49 < 0.05
Female rats 0/50 2/49 8/50 < 0.005
Male mice 9/50 14/48 19/50 < 0.05
Female mice 0/50 7/50 17/50 < 0.0001
Neoplastic nodules
Male rats 2/50 5/49 7/49 n.s.
Female rats 0/50 4/49 5/50 < 0.05
Hepatocellular adenoma
Male mice 6/50 11/48 10/50 n.s
Female mice 1/50 5/50 1/50 n.s
a From: NTP (1982)
b Probability level in Codiran-Armitage test for linear trend when
P < 0.05; otherwise not significant (n.s.)
Two other long-term studies on DEHP have been performed by
Carpenter et al. (1953) and Harris et al. (1956), but, due to the
small numbers of animals used, the studies are inadequate to assess
the carcinogenic potential.
DEHP has been investigated in two life-time studies on Syrian
hamsters (Schmezer et al., 1988). In one study, groups of 25 male and
25 female 6-week-old hamsters were assigned to each of six groups:
untreated control; 3 g DEHP/kg body weight given intraperitoneally
once every week for 18 weeks; the same dose once every two weeks for
18 weeks; the same dose once every four weeks for 32 weeks; the same
dose once every four weeks for 32 weeks plus N-dimethylnitrosamine
(NDMA) at 1.67 mg/kg body weight given orally once per week; and the
same dose of NDMA without DEHP treatment. NDMA increased the tumour
rate for malignant liver tumours, mainly haemangioendotheliomas.
Co-administration of DEHP with NDMA neither increased nor decreased
the tumour rates. No significant differences of tumour rates were
observed in groups treated with DEHP alone compared with controls.
Schmezer et al. (1988) also investigated the life-time, whole-
body exposure of Syrian hamsters to DEHP vapour alone in air or in
combination with orally administered NDMA. The low doses of DEHP
(15 µg/m3, resulting in a total exposure of about 7.5 µg/kg body
weight) in this study render it inadequate for the assessment of the
long-term effects of DEHP alone. In combination with NDMA, this low
level of DEHP was associated with highly significant (P < 0.001)
decreases in hepatic haemangioendothe-lioma and fibrosarcomas combined
in males and females combined. This unexpected result should be
further investigated to evaluate its significance.
7.8 Special studies
Since DEHP lacks genotoxic activity in most test systems, it has
been suggested that the carcinogenic effect is exerted during the
promotion phase of hepatocarcinogenicity. DEHP has therefore been
tested in several initiation/promotion experiments in rats and mice
where the end-point has been the number and/or volume of altered liver
cell foci. As expected, DEHP lacked initiating activity in these
studies (Ward et al., 1986; Popp et al., 1987).
DEHP appears to be a tumour promotor in mouse liver. In male
B6C3F1 mice given an intraperitoneal initiating dose of
diethylnitrosamine (80 mg/kg), DEHP at levels of 6 and 12 g/kg diet
caused accelerated growth of foci and increased incidence of
hepatocellular adenomas (Ward et al., 1983; Ward et al., 1986).
However, in a 6-month study, DEHP at a level of 12 g/kg diet did not
appear to promote altered foci in male F-344 rats given an
intraperitoneal initiating dose of diethylnitrosamine of 150 mg/kg
(Popp et al., 1987). In other promotion studies DEHP appeared to
accelerate the regression or inhibit the appearance of some kinds of
foci in rats (DeAngelo & Garrett, 1983; DeAngelo et al., 1984).
However, other studies have shown that peroxisome proliferators can
promote the development of altered foci and tumours in rat liver.
These results are somewhat different from those of other liver tumour
promotors with respect to the histological phenotype of the foci and
the relatively low frequency of the foci (Cattley & Popp, 1989). Thus,
further studies are required to demonstrate that DEHP can promote
altered foci in rat liver.
7.9 Mechanisms of hepatotoxicity
Together with a number of structurally diverse chemicals,
including certain hypolipidaemic drugs, herbicides, and chlorinated
solvents, DEHP has been shown to produce hepatic peroxisome
proliferation in rats and mice (Reddy & Lalwani, 1983; Lock et al.,
1989). This proliferation is accompanied by liver enlargement,
stimulation of replicative DNA synthesis and cell division, and the
induction of peroxisomal and microsomal fatty acid oxidizing enzyme
activities (Reddy & Lalwani, 1983; Marsman et al., 1988; Lock et al.,
1989). The increase in microsomal fatty acid oxidation is due to the
induction of a cytochrome P-450 IVA1 (Lock et al., 1989). Peroxisome
proliferation and induction of peroxisomal and microsomal fatty acid
oxidizing enzyme activities can be observed in primary hepatocyte
in vitro cultures, as well as in the intact animal (Elcombe &
Mitchell, 1986; Lake et al., 1986). Both in vivo and in vitro
studies with rat hepatocytes have demonstrated wide compound potency
differences (Reddy et al., 1986; Barber et al., 1987; Lake et al.,
1988). Compared to some other compounds, DEHP is not considered to be
a particularly potent peroxisome proliferator in rodent liver (Reddy
et al., 1986; Barber et al., 1987). Possible mechanisms of peroxisome
proliferation include a perturbation of hepatic lipid metabolism
and/or the presence of a receptor protein (Lalwani et al., 1987; Lock
et al., 1989; Issemann & Green, 1990).
Certain peroxisome proliferators, including DEHP, have been shown
to increase the incidence of liver tumours in rats and mice (Reddy &
Lalwani, 1983; Reddy et al., 1986; Butterworth et al., 1987).
Generally, there is a good correlation between the potency of a
compound to produce peroxisome proliferation in rat and mouse liver
and its hepatocarcinogenic properties (Reddy et al., 1986). However,
the magnitude of peroxisome induction does not necessarily always
correlate with the carcinogenic response when different compounds are
compared (Marsman et al., 1988). Since the peroxisome proliferators
known to date are non-genotoxic carcinogens, Reddy and coworkers
(Reddy & Lalwani, 1983; Rao & Reddy, 1987; Reddy & Rao, 1989) have
suggested that liver tumour formation arises from a sustained
oxidative stress to the hepatocytes due to an imbalance in the
production and degradation of hydrogen peroxide (H2O2). DEHP
administration results in increased peroxisomal production of H2O2
in hepatocytes (Tomaszewski et al., 1986). There is an imbalance in
H2O2 production and degradation due to the fact that catalase is
induced to a much lesser extent than peroxisomal ß-oxidation enzymes.
In addition, the level of cytosolic glutathione peroxidase is reduced
on prolonged exposure to DEHP (Lake et al., 1987; Marsman et al.,
1988; Conway et al., 1989b; Tamura et al., 1990). Treatment with
peroxisome proliferators also results in a reduction of superoxide
dismutase and glutathione S-transferase activities (Ciriolo et al.,
1982; Goel et al., 1986; Lake et al., 1987). It has been suggested
that the increased level of H2O2 in hepatocytes, either directly or
via other reactive oxygen species (e.g., hydroxyl radical), damages
intracellular membranes and/or DNA (Reddy & Rao, 1989). Indeed chronic
administration of DEHP and other peroxisome proliferators results in
increased lipid peroxidation and lipofuscin deposition in rat
hepatocytes (Mitchell et al., 1985b; Goel et al., 1986; Cattley et
al., 1987; Lake et al., 1987; Reddy & Rao, 1989; Conway et al.,
1989b). Oxygen radicals may damage DNA (Bridges, 1985), leading to
increased levels of 8-hydroxydeoxy-guanosine and other modified bases
in DNA (Reddy & Rao, 1989). Indeed, the administration of DEHP and
some other peroxisome proliferators has been shown to increase levels
of 8-hydroxyde-oxyguanosine in rat liver DNA (Kasai et al., 1989;
Takagi et al., 1989, 1990, 1991). Apart from a role in peroxisome
proliferation, leading to oxidative stress, recent studies on DEHP
have demonstrated a role in the stimulation of replicative DNA
synthesis in the hepatocarcinogenicity of peroxisome proliferators
(Butterworth et al., 1987; Smith-Oliver & Butterworth, 1987; Marsman
et al., 1988). Increased cellular division may result in spontaneous
mutational events or promotional effects (Bridges, 1985; Butterworth
et al., 1987), including the promotion of spontaneously initiated
cells found in the livers of aging rodents (Schulte-Hermann et al.,
1989). A comparative study of the hepatic effects of Wy-14643
([4-chloro-6 (2,3-xylidino) 2-pyrimidinylthio]acetic acid), a potent
peroxisome proliferator, and DEHP indicated that, although both
compounds produced a similar induction of peroxisome proliferation at
the dose levels administered, only Wy-14643 produced liver tumours
within one year (Marsman et al., 1988; Conway et al., 1989b). However,
analysis of these data demonstrates that Wy-14643 produces both a more
marked increase in lipofuscin deposition (presumably reflecting
oxidative stress) and a sustained stimulation of replicative DNA
synthesis. These data suggest a role for both oxidative stress and
increased cell replication in the hepatocarcinogenicity of DEHP and
other peroxisome proliferators.
The mechanism of hepatocarcinogenicity induced by peroxisome
proliferators could include both enhanced cell replication and DNA
damage induced by oxygen radicals. Thus, at dose levels that do not
produce either significant peroxisome proliferation or cell
replication, it is unlikely that DEHP or other peroxisome
proliferators would elicit hepatic tumour formation. In this context
data on threshold values for liver effects in sensitive species (e.g.,
the rat and mouse), together with an assessment of species differences
in response (see below), should be considered. The value for the
subchronic liver effects of DEHP (e.g., peroxisome proliferation and
DNA synthesis) in the rat appears to be around 50 mg/kg per day (Lake
et al., 1984, 1991; Mitchell et al., 1985b; Tomaszewski et al., 1986).
Furthermore, a two-year study of DEHP in female rats demonstrated no
effects at 0.3 g/kg diet, a value which corresponds well to that noted
above (Cattley et al., 1987; Popp et al., 1987).
Many studies have shown dramatic species differences in response
to peroxisome proliferators including DEHP (ECETOC, 1992). For
example, DEHP threshold values for peroxisome proliferation of 25 and
250 mg/kg, respectively, were found in rats and hamsters after 14 days
of daily gastric intubation (Lake et al., 1984). Several other studies
showing species differences in the response to DEHP have been
reported. One experiment demonstrated a large increase in hepatic
peroxisomes after 14 days of oral DEHP administration (2000 mg/kg per
day) to rats, but the same treatment of marmosets resulted in no
peroxisome proliferation (Rhodes et al., 1986). A limited study in
cynomolgus monkeys also revealed no evidence of peroxisome
proliferation due to DEHP (Short et al., 1987). In addition, DEHP did
not elicit peroxisome proliferation in guinea-pigs (Osumi & Hashimoto,
1978; Mitchell et al., 1985a). Some studies have indicated that
intrinsic species differences in hepatocellular sensitivity exist. For
example, MEHP and MEHP metabolite VI (mono(2-ethyl-5-oxohexyl)
phthalate), which is a proximate peroxisome proliferator in the rat,
were good peroxisome proliferators in cultured rat hepatocytes, but
had little or no effect in guinea-pig, marmoset or human hepatocytes
(Mitchell et al., 1985a; Elcombe & Mitchell, 1986). More recently,
Bichet et al. (1990) and Butterworth et al. (1989) have also
demonstrated the inability of MEHP to elicit peroxisome proliferation
in human hepatocyte cultures. Studies using a variety of other
peroxisome proliferators (e.g., clofibric acid, beclobric acid,
methylclofenapate, trichloroacetic acid, ciprofibrate, and
benzbromarone) have also failed to demonstrate peroxisome
proliferation in cultured human hepatocytes, in spite of a good
response in rat hepatocytes (Elcombe, 1985; Allen et al., 1987;
Elcombe & Styles, 1989; Butterworth et al., 1989; Bichet et al., 1990;
Blaauboer et al., 1990).
Species differences in cytochrome P-450 IVA1 induction and
stimulation of replicative DNA synthesis have been less well studied.
However, MEHP and metabolite VI did not induce P-450 IVA1-mediated
lauric acid hydroxylation in vivo in marmosets (Rhodes et al., 1986)
or in cultured marmoset or human hepato-cytes (Elcombe & Mitchell,
1986).
In conclusion, it appears that the livers of rats and mice are
exquisitely sensitive to peroxisome proliferators, including DEHP,
while those of guinea-pigs, monkeys, and humans show minimal or no
response (ECETOC, 1992).
8. EFFECTS ON HUMANS
8.1 General population exposure
Two adults who swallowed 5 or 10 g DEHP experienced no untoward
effects apart from mild gastric disturbances and moderate diarrhoea
with 10 g DEHP (Shaffer et al., 1945). Three cases of nonspecific
hepatitis were described among 27 haemodialysis patients with terminal
renal failure. The PVC blood tubing used released DEHP at a
concentration of 10-20 mg/litre of perfusate. The symptoms and signs
of hepatitis disappeared rapidly when the use of tubing that did not
contain DEHP was resumed (Neergaard et al., 1971).
In three pre-term infants artificially ventilated with PVC
respiratory tubes, unusual lung disorders (opacification of the lung)
were observed during the fourth week of life. It was assumed by the
authors that these lung disorders were causually related to the
exposure to DEHP released from the respiratory tubes (Roth et al.,
1988).
8.2 Occupational exposure
There are very few data on the effects of occupational exposure
specifically to DEHP.
Two studies reported symptoms and signs of polyneuropathy among
47 out of 147 workers at a PVC-processing plant in the USSR and 12 out
of 23 workers at a plant for phthalate production in Italy. The
workers were exposed to a mixture of phthalates and DEHP was a minor
constituent, at least in the USSR plant. Furthermore, tricresyl
phosphate (a neurotoxin) was a component of the incombustible
materials produced in 10-20% of machines assigned to various workers
(Milkov et al., 1973). In the Italian study there was no corresponding
unexposed control group and the authors concluded that no definite
conclusion could be drawn from the study because of the limited number
of workers examined (Gilioli et al., 1978). The total phthalate air
concentrations recorded varied between 1.7 and 66 mg/m3 in the USSR
and 1 and 60 mg/m3 in Italy (Milkov et al., 1973; Gilioli et al.,
1978).
In a study involving a Swedish PVC-processing factory, peripheral
nervous system symptoms and signs were investigated among 54 male
workers exposed mainly to DEHP, diisodecylphthalate, and some
butylbenzylphthalate. The workers were divided into three groups of
approximately equal size and with mean phthalate exposures of 0.1,
0.2, and 0.7 mg/m3. Some workers displayed various peripheral
nervous system symptoms and signs, but these were not related to the
level of exposure. None of the workers reported symptoms indicating
work-related obstructive lung disease. Conventional lung function
tests also showed no association with exposure (Nielsen et al., 1985).
However, several biochemical parameters showed significant
associations with exposure. There was a slight decrease in the
haemoglobin level with longevity of employment as well as with
exposure in the last year. The serum ý-1-antitrypsin level increased
slightly with length of employment and the serum immunoglobulin A
level rose with rising exposure during the last year (Nielsen et al.,
1985).
One case of occupational asthma due to DEHP was reported in
worker at a PVC-processing plant (Brunetti & Moscato, 1984).
Diagnosis was made by exposing the patient to DEHP vapour in an
inhalation chamber; this evoked a dual asthmatic reaction. The action
was inhibited by prior administration of sodium chromoglycate.
A study of blood lipids, serum activities of liver enzymes, and
routine haematological tests was carried out among workers at a German
plant for DEHP production. The results were negative and uninformative
due to very low exposure levels (below 0.16 mg/m3) and the lack of
a control group (Thiess et al., 1978b). Thiess & Flieg (1978)
investigated the frequency of chromosome aberrations in 10 workers who
were employed from 10 to 30 years in this DEHP production plant. The
exposure levels were very low (0.09-0.16 mg/m3), and there was no
increase in chromosome aberrations compared to the control group. A
mortality study of 221 workers exposed to DEHP in the same plant was
also conducted. Eight deaths occurred in the cohort compared with
expected values of 15.9 and 17.0 from the city and county data,
respectively (Thiess et al., 1978a).
9. EFFECTS ON OTHER ORGANISMS IN THE LABORATORY AND FIELD
9.1 Toxicity to microorganisms
Bringmann & Kühn (1980) calculated toxic thresholds from cell
multiplication inhibition tests and found thresholds of > 400
mg/litre for the bacterium Pseudomonas putida, 10 mg/litre for the
alga Scenedesmus quadricaudaia, and 19 mg/litre for the protozoan
Entosiphon sulcatum.
Mathur (1974b) reported that DEHP added to soil (3 g/kg)
inhibited respiration, whereas addition of the same amount of DEHP to
soil preincubated for 14 weeks with DEHP or dioctyl phthalate had no
effect on respiration rate. The experiments were conducted at 22 °C
since no degradation of DEHP occurred at 4 °C or 10 °C. In an
intermittent flow-through hydrosoil microcosm, DEHP (at 1 and 100
mg/litre) produced no significant effect on the numbers or
physiological activity of the microflora monitored, nor on any of the
overall activities that were studied (Mutz & Jones 1977).
Larsson et al. (1986) studied the effect of DEHP on the microbial
activity in sediments. Sediment cores were taken, together with the
overlying water, from a eutrophic lake and the sediment was spiked
with between 25 and 400 mg DEHP/kg at a level of 5 mm below the
surface. Uncontaminated sediment samples had a significantly higher
oxygen uptake than sediment containing DEHP. Microbial activity, as
estimated from decreased oxygen saturation in the soil column,
correlated inversely with increasing levels of DEHP in the sediment.
9.2 Toxicity to aquatic organisms
Many of the toxicity values given in this section are above the
water solubility of DEHP, which ranges from 0.3 to 0.4 mg/litre (see
section 2.2). DEHP adsorbs strongly to sediment, food, dissolved
organic carbon, and even the testing vessels. Many of the toxicity
tests are also based on nominal concentrations. Therefore, the actual
exposure of the organisms is very difficult to determine and care must
be taken when interpreting the results.
9.2.1 Invertebrates
Acute toxicity to aquatic invertebrates is summarized in Table 9.
Table 9. Toxicity of DEHP to aquatic invertebratesa
Organism Age Temperature Hardnessb pH Duration LC50 Reference
(° C) (h) (mg/litre)d
Water flea < 24 h 17 48 0.133 Passino & Smith (1987)
(Daphnia pulex)
Water flea < 24 h 21-23 173 7.4-9.4 24 > 68 Leblanc (1980)
(Daphnia magna) < 24 h 21-23 173 7.4-9.4 48 11 Leblanc (1980)
Crayfish 96 > 10 Mayer & Sanders (1973)
(Orconectes nais)
Harpacticoid adult 20-22 7c 7.8 96 > 300 Linden et al. (1979)
(Nitocra spinipes)
Scud 96 > 32 Sanders et al. (1973)
(Gammarus pseudolimnaeus)
Midge larvae 21-23 270 7.4 48 > 18 Streufert et al. (1980)
(Chironomus plumosus)
a All experiments were performed using static conditions (water unchanged for duration of test)
b Hardness is expressed as mg calcium carbonate per litre. The tests were performed in fresh water
except where stated otherwise
c Salinity (%)
d Nominal values
Brown & Thompson (1982a) found no mortality of Daphnia magna
over an exposure period of 48 h and at DEHP dose levels up to 320
µg/litre. However, at levels of 180 µg/litre or more, the daphnids
were seen to float in the surface layer. The authors suggested that
this observation was connected with the solubility of DEHP (which
tends to precipitate out at > 180 µg/litre, possibly onto the
daphnids, thereby causing them to float). Stephenson (1983) found no
mortality in Gammarus pulex exposed for up to 120 h to a DEHP
concentration (400 µg/litre) in excess of its solubility.
Laughlin et al. (1978) exposed grass shrimps (Palaemonetes
pugio) to DEHP concentrations of up to 1 mg/litre (well above the
solubility limit of the ester) and found no significant difference in
mortality between the control and treated shrimps during the 28-day
exposure period. DEHP had no significant effect on the development
rate of larvae from hatching to moult. No apparent effects were noted
when mussels (Mytilus edulis) were exposed to 50 µg/litre for 28
days (Brown & Thompson, 1982b).
Hobson et al. (1984) found no significant mortality of penaeid
shrimps (Penaeus vannamei) fed a diet containing up to 50 g DEHP/kg
for 14 days, this representing 4% of the body weight per day.
Histological examination revealed no changes and no significant dose-
related effects were observed on moulting.
Sanders et al. (1973) and Mayer & Sanders (1973) exposed Daphnia
magna for a complete life cycle (21 days) to 3, 10 or 30 µg
DEHP/litre in an intermittent-flow system. Reproduction was
significantly inhibited at all concentrations (60, 70, and 80%
inhibition at the three dose levels, respectively). In contrast, Brown
& Thompson (1982a) found no effect on the reproduction of Daphnia
magna at concentrations of up to 100 µg/litre and suggested that the
difference in the numbers of young born to the controls in the two
studies, i.e. 11 offspring per parent (Mayer & Sanders, 1973) compared
with 170 per parent (Brown & Thompson, 1982a), could account for these
conflicting results.
When Knowles et al. (1987) exposed Daphnia magna to DEHP
concentrations of between 12 and 811 µg/litre for up to 21 days under
flow-through conditions (the reproduction rate was approximately 200
offspring per adult), survival was not affected at levels of < 158
µg/litre. At 811 µg/litre, however, survival was significantly reduced
after both 7 and 21 days. The mean number of young per surviving adult
was also reduced at this dose level. The levels of major biochemical
components such as protein, RNA, DNA, and glycogen were all
significantly reduced after 7 days at 811 µg/litre, but all except
glycogen were unaffected after 21 days. The authors concluded that the
maximum acceptable toxicant concentration (MATC), based on survival
and reproduction, was between 158 and 811 µg/litre. A no-observed-
effect level (NOEL) of 72 µg/litre was identified both by DNA content
and RNA/DNA ratio at day 7 and by surfacing of Daphnia at day 0.
Thuren & Woin (1991) exposed the freshwater amphipod Gammarus
pulex to DEHP at concentrations of 100 or 500 µg/litre for 10 days
under flow-through conditions. There was a 5 day pre- and post-
exposure period. The overall locomotor activity of G. pulex was
significantly decreased at the higher exposure level, and the effect
persisted throughout the post-exposure period. No significant effects
were observed at the lower dose level.
Flow-through chronic toxicity tests in both sand and hydrosoil
showed that DEHP concentrations as high as 360 µg/litre (sand) and 240
µg/litre (hydrosoil) had no significant effects on the growth or
development of midge larvae (Chironomus plumosus) over a 35-day
period. The continuous exposure of first-generation midge eggs in sand
substrate to mean DEHP concentrations of between 140 and 360 µg/litre
had no significant effect on hatchability (Streufert et al., 1980).
Woin & Larsson (1987) found that dragonfly larvae ( Aeshna sp.)
caught significantly fewer prey (Chaborus larvae) per attempt when
exposed to sediment DEHP concentrations of approximately 600 mg/kg for
between 3 and 9 weeks.
9.2.2 Fish
The acute toxicity to fish is summarized in Table 10 and the
toxicity to the embryo-larval stages of fish is shown in Table 11.
DEHP caused no deaths among one-year-old rainbow trout (Salmo
gairdneri) exposed to between 1 and 1000 mg/litre for 48 h (Silvo,
1974) or juvenile Atlantic salmon (Salmo salar) exposed to 100
mg/litre for 96 h (Zitko, 1972).
Mehrle & Mayer (1976) reported no effects on growth or survival
of adult fathead minnows (Pimephales promelas) exposed to between 1
and 62 µg/litre for 56 days. They also exposed rainbow trout eggs to
DEHP levels of 5, 14, and 54 µg/litre for 12 days prior to hatching,
but there was no significant effect on hatchability. The resulting fry
were continuously exposed to DEHP for a further 90 days. The two
highest concentrations caused a significant, but not dose related,
increase in the mortality of sac fry within 5 days of hatching. After
yolk absorption (at 24 days), DEHP caused no significant mortality or
effects on growth and development for the remainder of the exposure
period.
Table 10. Toxicity (96-h LC50 values) of DEHP to fish
Organism Size Stat/ Temperature Hardnessb pH LC50 Reference
flowa (° C) (mg/litre)c
Bluegill sunfish > 10 Mayer & Sanders (1973)
(Lepomis macrochirus) 0.32-1.2 g stat 21-23 32-48 6.7-7.8 > 770 Buccafusco et al. (1981)
Fathead minnow > 10 Mayer & Sanders (1973)
(Pimephales promelas) 0.055-0.25 g 24-26 44-46 7-8 > 0.33d Defoe et al. (1990)
Channel catfish > 10 Mayer & Sanders (1973)
(Ictalurus punctatus)
Rainbow trout fingerling stat 14-16 540 Hrudey et al. (1976)
(Oncorhynchus mykiss)
> 10 Mayer & Sanders (1973)
a Stat = static conditions (water unchanged for duration of test)
b Hardness is expressed as mg calcium carbonate per litre. The tests were performed in fresh water
except where stated otherwise
c Nominal values unless stated otherwise
d Measured value
Table 11. Toxicity of DEHP to the embryo-larval stages of fisha
Organism Stat/flowb Hardnessc pH LC50 (mg/litre) 95% confidence limits
Channel catfish stat 90-115 7.6-8.1 0.69 0.55-0.86
(Ictalurus punctatus)
Redear sunfish stat 90-115 7.6-8.1 6.18 4.65-8.04
(Lepomis microlophus)
Largemouth bass flow 45-55 7.5-8.0 42.1 28.8-77.8
(Micropterus salmoides)
flow 190-225 7.5-8.0 32.9 19.8-58.9
Rainbow trout flow 45-55 7.5-8.0 139.5 123.2-165.2
(Oncorhynchus mykiss)
flow 190-225 7.5-8.0 149.2 125.8-203.8
a From: Birge et al. (1978). Exposure was initiated 2 to 6 h after spawning (except in the case of rainbow trout where exposure
was initiated 15 min after fertilisation) and continued until 4 days after hatching. The hatching times were 22 days for rainbow
trout (13.5-14.3 °C), 3 days for catfish (29-31 °C), and 3-4 days for bass and sunfish (20-24 °C).
b Stat = static conditions, but water renewed every 12 h; flow = flow-through conditions (DEHP concentration in water continuously
maintained)
c Hardness is expressed as mg calcium carbonate per litre. The tests were performed in fresh water except where stated otherwise.
DeFoe et al. (1990) exposed rainbow trout (Oncorhynchus mykiss)
and Japanese medaka (Oryzias latipes) to DEHP using concentrations
of up to 0.502 mg/litre for a 90-day trout embryo-larval test and
0.554 mg/litre for a 168-day larval test on medaka. No significant
adverse effects were observed on trout hatchability, survival or
growth, but there was a significant reduction in the growth of
Japanese medaka.
Mayer & Sanders (1973) studied the effects of DEHP on the
reproduction of zebra fish (Brachydanio rerio) and guppies
(Lebistes reticulatus). During the 90-day dietary exposure, zebra
fish were fed on 50 or 100 mg DEHP/kg food, and guppies were fed 100
mg/kg. Although the treated zebra fish spawned more frequently than
the control fish, the latter produced more eggs per spawn. Fry
survival was significantly reduced by DEHP. Treated guppies produced
fewer fry per adult and had an 8% incidence of abortions, whereas
there were no abortions in the control group (no statistics were
given).
In a study of fish exposed to DEHP, Mayer et al. (1977) found
reduced vertebral collagen levels after 150 days at a DEHP
concentration of 3.7 µg/litre in adult brook trout (Salvelinus
fontinalis), after 127 days at 11 µg/litre in fathead minnow, and
after 90 days at 5 µg/litre in rainbow trout. However, there was no
effect on fish growth. Pfuderer & Francis (1975) found that DEHP had
no effect on the heart rate of goldfish when the fish were exposed to
200 mg/litre for 10 min.
9.2.3 Amphibians
The acute toxicity of DEHP to tadpoles of Fowler's toad and the
leopard frog is given in Table 12.
When Larsson & Thuren (1987) exposed eggs of the moorfrog (Rana
arvalis) to sediment concentrations of between 10 and 800 mg DEHP/kg
(fresh weight), the number of tadpoles hatching decreased with
increasing exposure concentration. The percentage hatch was 90% for
the controls eggs, 50% at 150 mg DEHP/kg, and < 30% at > 400 mg/kg.
The lowest DEHP concentration that caused a significant decrease in
the number of tadpoles hatching was 25 mg/kg. After hatching, the
survival of tadpoles was unaffected. There were no delays in hatching
and no abnormalities in the developing tadpoles exposed to the various
DEHP concentrations.
Table 12. Toxicity of DEHP to frog and toad tadpolesa
Organism LC50 (mg/litre) 95% confidence limits
Fowler's toad 3.88 3.08-4.84
(Bufo fowleri)
Leopard frog 4.44 3.65-5.37
(Rana pipiens)
a From: Birge et al. (1978). Exposure occurred under static
conditions, but water was renewed every 12 h. It was initiated 2
to 6 h after spawning and continued until 4 days after hatching.
The hatching times varied between 3 and 4 days, and so the
exposure period varied between 7 and 8 days. The temperature was
20-24 °C, hardness 90-115 mg CaCO3/litre, and pH 7.6-8.1.
Wams (1987) quoted an unpublished Dutch report which stated that
an exposure of 2 mg DEHP/litre caused a reduction in the growth rate
of clawed toad (Xenopus laevis) larvae.
9.3 Toxicity to terrestrial organisms
9.3.1 Plants
When Herring & Bering (1988) grew spinach and pea plants from
seed for 14 to 16 days in soil containing 100 mg DEHP/kg, there was no
effect on plant growth, as measured by height. The effect of DEHP on
seed germination was observed by placing seeds in petri dishes
containing a solution of 100 mg/litre. A reduction of 40% to 50% in
the number of seeds germinating was found.
Lokke & Rasmussen (1983) reported that DEHP had no visible effect
on Sinapis alba, Brassica napusor or Achillea millefolium when
sprayed in the field at concentrations of up to 87.5 kg/ha. According
to Stanley & Tapp (1982), DEHP at a level of 1000 mg/kg soil had no
effect on the growth of rape (Brassica rapa) and only a slight
effect on that of oats (Avena sativa).
9.3.2 Earthworms
In a series of contact toxicity tests, red earthworms (Eisenia
foetida) were exposed to DEHP via filter paper in glass vials. An
LD50 could not be calculated because DEHP was not toxic even at the
highest dose of 25 mg/cm2 (Neuhauser et al., 1986).
9.3.3 Insects
Al-Badry & Knowles (1980) found DEHP to be non-toxic to female
houseflies (Musca domestica) when applied topically or by injection
at a concentration of 20 µg/fly (equivalent to 1000 mg/kg). When it
was topically applied simultaneously with various organophosphates, an
antagonistic interaction was apparent; mortality of 85% to 95% was
reduced to less than 10% by the DEHP. However, when DEHP was topically
applied 30 min before exposure to an organophosphate concentration
that caused 10% to 30% mortality, the resulting interaction was
synergistic with mortality rising to over 60% and in most cases to
over 80%.
9.3.4 Birds
Hill et al. (1975) found no mortality among 10-day-old ring-
necked pheasants or mallard fed up to 5000 mg DEHP/kg for 5 days
followed by 3 days on a normal diet.
When Wood & Bitman (1984) fed broiler hens a diet containing 2000
mg DEHP/kg (226 mg DEHP/hen per day) for 4 weeks, egg production and
body weights were significantly decreased. Ishida et al. (1982)
reported the cessation of egg production and an abnormality of the
ovaries in laying hens fed 5 or 10 g DEHP/kg diet for up to 230 days.
In a study by O'Shea & Stafford (1980), starlings (Sturnus
vulgaris) were fed on a diet containing 25 or 250 mg DEHP/kg for a
30-day period. At both dose levels, the treated birds gained
significantly more body weight than controls during the exposure
period, but at the lower dose level food consumption was significantly
reduced compared to controls.
When Peakall (1974) fed ring doves (Streptopelia risoria) on a
diet containing 10 mg/kg, there were no effects on the eggshell
thickness, ashed egg weight, rate of water loss, surface area or
permeability of the eggs laid.
10. EVALUATION OF HUMAN HEALTH RISKS AND EFFECTS ON THE ENVIRONMENT
10.1 Evaluation of human health risks
10.1.1 Exposure levels
DEHP concentrations of up to 300 ng/m3 have been measured in
urban air, but usually the levels in ambient air are well below 100
ng/m3. Data on occupational exposure levels are limited.
Concentrations of up to 4.1 mg/m3 have been reported but they are
usually below 1 mg/m3. Total phthalate exposure levels have been
reported to be between 0.1 and 60 mg/m3. Clinical treatment,
including transfusion, haemodialysis, extracorporal circulation, and
artificial respiration, may lead to DEHP exposure.
The exposure to DEHP from drinking-water and food is low.
10.1.2 Toxic effects
Dose-dependent kinetics of DEHP or its metabolites and species
differences in the metabolism have been confirmed in several studies.
In animals some inhalation studies have been performed. However,
no consistent findings are reported.
The oral and intraperitoneal LD50 values exceed 25 g/kg,
indicating that DEHP has low acute toxicity.
In rats and mice, DEHP administration produces hepatic
hyperplasia and hypertrophy, which is characterized by peroxisome
proliferation. The threshold dose level for hepatic peroxisome
proliferation in the rat is approximately 50 mg/kg per day. High DEHP
dose levels (12 g DEHP/kg diet in rats; 6 g DEHP/kg diet in mice) in
a feeding study resulted in an increased incidence of hepatic tumours
in rats and mice.
Marked species differences in DEHP-induced hepatomegaly and
peroxisome proliferation exist. Rats and mice are very responsive,
Syrian hamsters less responsive, and guinea-pigs, marmosets, and
cynomolgus monkeys non-responsive. Studies with primary hepatocyte
cultures have shown an excellent in vitro/in vivo correlation
concerning responsiveness to DEHP/DEHP metabolites. Several studies
have demonstrated the non-responsive nature of cultured human
hepatocytes to DEHP metabolites and other peroxisome proliferators.
The induction of peroxisome proliferation and cell replication is
strongly associated with the development of hepatic tumours in rats
and mice. Thus, the available data suggest that species which do not
respond to the hepatic effects of DEHP are unlikely to be susceptible
to the development of hepatic tumours.
Testicular atrophy is one of the most consistent effects of DEHP
in in vivo studies on a variety of experimental animal species. Such
effects have been observed in rats fed 6 g DEHP/kg diet and mice fed
3 g/kg diet, and they are more pronounced in young animals. Hamsters
and marmosets appear to be more resistant to the testicular effects of
DEHP.
When both male and female CD-1 mice were treated with 1 g DEHP/kg
diet, a significant reduction in fertility was observed.
A dietary level of 20 g DEHP/kg throughout gestation produced an
increased incidence of resorptions in rats but no malformations. In
the mouse, however, 1 g/kg throughout pregnancy increased the
incidence of embryolethality and abnormalities. Sensitivity was
greatest on days 7-9 of gestation. The dose level (0.5 g/kg) that
induced fetotoxicity in mice did not induce maternal toxicity. Results
from several different genotoxicity tests indicate that DEHP and its
major metabolites do not exhibit any direct genotoxic effect in either
bacteria or in vitro mammalian cells. This has been confirmed in
in vivo binding studies, which indicated that DEHP and its
metabolites do not interact covalently with DNA. However, it has been
established that DEHP has the potential of inducing aneuploidy in
fungi as well as in in vitro mammalian cells. There are no
consistent results from dominant lethal studies.
Few data on the human health effects of DEHP exposure have been
reported. There have been a few studies on workers exposed to
phthalate mixtures, but no consistent health effects that could be
directly related to DEHP have been reported.
10.1.3 Conclusion
DEHP causes reproductive and hepatocarcinogenic effects in rats
and mice.
Testicular atrophy is the main reproductive effect in rats and
mice, and young animals are more susceptible than older ones to this
effect. The induction of hepatic peroxisome proliferation and cell
replication are strongly associated with the liver carcinogenic effect
of certain non-genotoxic carcinogens including DEHP. However, marked
differences have been observed among animal species with respect to
DEHP-induced peroxisome proliferation. Currently there is not
sufficient evidence to suggest that DEHP is a potential human
carcinogen.
10.2 Evaluation of effects on the environment
10.2.1 Exposure levels
DEHP exists widely in the environment, being released during
production, processing, usage, and disposal. Transport in the air is
the major route by which it enters the environment. It can be
deposited by dry or wet deposition and has been found to leach into
ground water.
DEHP is readily photodegraded in the atmosphere. In the
laboratory, aerobic biodegradation has been shown to occur under
certain circumstances. However, in the environment aerobic
biodegradation seems to be slow and anaerobic degradation is even
slower.
Based on a measured log Pow of about 5 and measured
bioaccumulation factors for biota and sediment, it can be seen that
DEHP is highly bioaccumulative.
In rivers and lakes, DEHP concentrations of up to 4 µg/litre have
been measured.
Due to its hydrophobic nature, DEHP adsorbs readily to soil,
sediment, and particulate matter. Levels of up to 70 mg/kg dry weight
have been found in river sediments, but near to a discharge point
levels of up to 1480 mg/kg dry weight have been reported.
10.2.2 Toxic effects
Most acute toxicity studies on aquatic organisms show DEHP to be
of low toxicity. However, one study suggested a greater sensitivity
for the water flea (Daphnia pulex), the nominal 48-h LC50 being
133 µg/litre.
In a 21-day study on Daphnia magna, the NOEL for biochemical
and behavioural effects was 72 µg/litre. A significant increase in
mortality was recorded in trout fry exposed to 14 µg DEHP/litre from
12 days prior to hatching. At concentrations of 3.7 to 11 µg/litre,
reductions in vertebral collagen were reported in three species of
fish exposed for 150 days.
Zebra fish fry survival and guppy reproduction were adversely
affected at DEHP concentrations in food of 50 and 100 mg/kg,
respectively, during a 90-day exposure period.
In two separate studies, DEHP concentrations of 25 mg/kg
(wet weight) of sediment significantly reduced microbial activity and
the number of frog tadpoles hatching.
Available studies indicate that the acute toxicity of DEHP to
algae, plants, earthworms, and birds is low. There are no data
relating to effects upon wild mammals.
10.2.3 Conclusion
There is no documented information that DEHP presents any hazard,
based on acute exposure to fish and daphnids. However, a reduction of
microbial activity in sediment at environmental levels of DEHP was
reported. A comparison between environmental levels and the
concentrations that produce effects in prolonged studies, especially
early life-stage tests on fish and amphibians, indicates that a hazard
for the environment, particularly via water and sediment, cannot be
excluded. Adverse effects on organisms are likely in areas with highly
contaminated water and sediments which are near to point emission
sources.
Although few relevant studies have been reported, the acute
toxicity of DEHP to algae, plants, earthworms, and birds appears to be
low.
11. RECOMMENDATIONS FOR PROTECTION OF HUMAN HEALTH AND THE
ENVIRONMENT
a) Current disposal practises should be improved.
b) Measures must be undertaken to reduce the release of DEHP to the
environment.
c) Medical devices and products that contribute to the body burden
of DEHP must be scrutinized to reduce exposure to DEHP via the
intravenous route.
12. FURTHER RESEARCH
More research is needed on the effects of DEHP on the ecosystem
of sediments and on the subject of DEHP leaching, particularly from
landfill to ground water.
Since DEHP occurs in the air, including workplace air, further
inhalation studies are needed.
Epidemiological surveys on exposed populations should be
encouraged. Further studies are needed on the mechanism of peroxisome-
proliferator-induced hepatocarcinogenicity, with emphasis on human
health effects.
13. PREVIOUS EVALUATIONS BY INTERNATIONAL BODIES
DEHP was one of the substances discussed by a working group of
the International Agency for Research on Cancer in 1981 (IARC, 1982).
The evaluation by the group was that there was sufficient evidence for
the carcinogenicity of DEHP in mice and rats, but that no adequate
epidemiological study was available. The working group concluded that
DEHP is possibly carcinogenic to humans and placed it in group 2B
(IARC, 1987).
DEHP was evaluated at the 28th meeting of the Joint FAO/WHO
Expert Committee on Food Additives. The recommendation was to reduce
human exposure to DEHP from food-contact materials to the lowest level
technologically attainable (WHO/FAO, 1984a,b). During the 33rd meeting
of the Joint FAO/WHO Expert Committee on Food Additives, DEHP was
again evaluated and the recommendation was the same: DEHP in food
should be reduced to the lowest level attainable. The Committee
considered that this might be achieved by using alternative
plasticizers or alternatives to plastic material containing DEHP
(WHO/FAO, 1989a,b).
REFERENCES
AGARWAL, D.K. (1986) Letters to the editor. Toxicol. appl. Pharmacol.,
82: 383-387.
AGARWAL, D.K., AGARWAL, S., & SETH, P.K. (1982) Effect of di-(2-
ethylhexyl) phthalate on drug metabolism, lipid peroxidation, and
sulfhydryl content of rat liver. Drug Metab. Disp., 10: 77-80.
AGARWAL, D.K., LAWRENCE, W.H., & AUTIAN, J. (1985a) Antifertility and
mutagenic effects in mice from parenteral administration of di-2-
ethylhexyl phthalate (DEHP). J. Toxicol. environ. Health, 16: 71-84.
AGARWAL, D.K., LAWRENCE, W.H., NUNEZ, L.J., & AUTIAN, J. (1985b)
Mutagenicity evaluation of phthalic acid esters and metabolites in
Salmonella typhimurium cultures. J. Toxicol. environ. Health, 16:
61-69.
AGARWAL, D.K., EUSTIS, S., LAMB J.C., IV, JAMESON, C.W., & KLUWE, W.M.
(1986a) Influence of dietary zinc on di(2-ethylhexyl)phthalate-induced
testicular atrophy and zinc depletion in adult rats. Toxicol. appl.
Pharmacol., 84: 12-24.
AGARWAL, D.K., EUSTIS, S., LAMB J.C., IV, REEL, J.R., & KLUWE, W.M.
(1986b) Effects of di(2-ethylhexyl)phthalate on the gonadal patho-
physiology, sperm morphology, and reproductive performance of male
rats. Environ. Health Perspect., 65: 343-350.
AGARWAL, D.K., LAWRENCE, W.H., TURNER, J.E., & AUTIAN, J. (1989)
Effects of parenteral di(2-ethylhexyl)phthalate (DEHP) on gonadal
biochemistry, pathology and reproductive performance of mice. J.
Toxicol. environ. Health, 26: 39-59.
AHMED, R.S., PRICE, S.C., GRASSO, P., & HINTON, R.H. (1990) Hepatic
nuclear and cytoplasmic effects following intermittent feeding of rats
with di(2-ethylhexyl)phthalate. Food chem. Toxicol., 28: 427-434.
AL-BADRY, M. & KNOWLES, C.O. (1980) Phthalate-organophosphate
interactions: Toxicity, penetration, and metabolism studies with house
flies. Arch. environ. Contam. Toxicol., 9: 147-161.
ALBRO, P.W. (1986) Absorption, metabolism, and excretion of di(2-
ethylhexyl) phthalate by rats and mice. Environ. Health Perspect., 65:
293-298.
ALBRO, P.W. (1987) The biochemical toxicology of di-(2-ethylhexyl) and
related phthalates: testicular atrophy and hepatocarcinogenesis. In:
Hodgson, E., Bend, J.R., & Philpot, R.M., ed. Reviews in biochemical
toxicology, Amsterdam, Oxford, New York, Elsevier Science Publishers,
Vol. 8, pp. 73-119.
ALBRO, P.W. & LAVENHAR, S.R. (1989) Metabolism of di(2-ethylhexyl)
phthalate. Drug Metabol. Rev., 21: 13-34.
ALBRO, P.W. & THOMAS, R.O. (1973) Enzymatic hydrolysis of di(2-
ethylhexyl) phthalate by lipases. Biochim. Biophys Actaw, 360:
380-390.
ALBRO, P.W., THOMAS, R., & FISHBEIN, L. (1973) Metabolism of
diethylhexyl phthalate by rats. Isolation and characterization of the
urinary metabolites. J. Chromatogr., 76: 321-330.
ALBRO, P.W., HAAS, J.R., PECK, C.C., ODOM, D.G., CORBETT, J.T.,
BAILEY, F.J., BLATT, H.E., & BARRETT, B.B. (1981) Identification of
the metabolites of di-(2-ethyl-hexyl) phthalate in urine from the
African green monkey, Drug Met. Disp., 9: 223-225.
ALBRO, P.W., CORBETT, J.T., SCHROEDER, J.L., JORDAN, S., & MATTHEWS,
H.B. (1982) Pharmacokinetics, interactions with macromolecules and
species differences in metabolism of DEHP. Environ. Health Perspect.,
45: 19-25.
ALBRO, P.W., CHAPIN, R.E., CORBETT, J.T., SCHROEDER, J., & PHELPS,
J.L. (1989) Mono-2-ethylhexylphthalate, a metabolite of di-(2-
ethylhexyl)phthalate, causally linked to testicular atrophy in rats.
Toxicol. appl. Pharmacol., 100: 193-200.
ALLEN, K.L., GREEN, C.E., & TYSON, C.A. (1987) Comparative studies of
peroxisomal enzyme induction in hepatocytes from rat, cynomolgus
monkey and human by hypolipidemic drugs. Toxicologist, 7: 63.
AL-OMRAN, L.A. & PRESTON, M.R. (1987) The interactions of phthalate
esters with suspended particulate material in fresh and marine waters.
Environ. Pollut., 46: 177-186.
ANTONYUK, O.K. (1975) Toxicology of complex ethers of phthalic acid (A
review of literature), Hyg. Labour occup. Dis., 1: 32-35 (in Russian).
ARANDA, J.M., O'CONNOR, G.A., & EICEMAN, G.A. (1989) Effects of sewage
sludge on di-(2-ethylhexyl) phthalate uptake by plants J. environ.
Qual., 18: 45-50.
ARBIN, A. & ÖSTELIUS, J. (1980) Determination by electron-capture gas
chromatography of mono- and di(2-ethylhexyl) phthalate in intravenous
solutions stored in polyvinyl chloride bags. J. Chromatogr., 193:
405-412.
ASHBY, J., DE SERRES, F.J., DRAPER, M., ISHIDATE, M., Jr, MARGOLIN,
B.H., MATTER, B.E., & SHELBY, M.D., ed. (1985) Evaluation of short-
term tests for carcinogens. Report of the International Programme on
Chemical Safety's collaborative study on in vitro assays, Amsterdam,
Oxford, New York, Elsevier Science Publishers, 752 pp (Progress in
Mutation Research, Vol. 5).
ASTILL, B. (1989) Metabolism of DEHP: Effects of prefeeding and dose
variation, and comparative studies in rodents and the cynomolgus
monkey (CMA studies). Drug Metab. Rev., 21: 35-53.
ASTILL, B., BARBER, E., LINGTON, A., MORAN, E., MULHOLLAND, A.,
ROBINSON, E., & SCHEIDER, B. (1986) Chemical industry voluntary test
program for phthalate esters: Health effect studies. Environ. Health
Perspect., 65: 329-336.
ATLAS, E. & GIAM, C.S. (1981) Global transport of organic pollutants:
Ambient concentrations in the remote marine atmosphere. Science, 211:
163-165.
ATSDR (1988) Toxicological profile for di(2-ethylhexyl) phthalate,
Atlanta, Georgia, Agency for Toxic Substances and Disease Registry, US
Public Health Service (Draft for public comment).
BALLY, M.B., OPHEIM, D.J., & SHERTZER, H.G. (1980) Di-(2-ethylhexyl)
phthalate enhances the release of lysosomal enzymes from alveolar
macrophages during phagocytosis. Toxicology, 18: 49-60.
BARBER, E.D., ASTILL, B.D., MORAN, E.J., SCHNEIDER, B.F., GRAY,
T.J.B., LAKE, B.G., & EVANS, J.G. (1987) Peroxisome induction studies
on seven phthalate esters. Toxicol. ind. Health, 3: 7-24.
BARNEY, G.H., ORGEBIN-CRIST, M.C., & MACAPINLAC, M.P. (1969) Genesis
of esophageal parakeratosis and histologic changes in the testes of
the zinc-deficient rat and their reversal by zinc repletion. J. Nutr.,
95: 526-534.
BAUGHMAN, G.L., PARIS, D.F., & STEEN, W.C. (1980) Quantitative
expression of biotransformation. In: Maki, A.W., Dickson, K.L., &
Cairns, J., ed. Biotransformation and fate of chemicals in the
environment, Washington, DC, American Society for Microbiology, pp.
105-111.
BELISLE, A.A., REICHEL, W.L., & SPANN, J.W. (1975) Analysis of tissues
of mallard ducks fed two phthalate esters. Bull. environ. Contam.
Toxicol., 13: 129-132.
BELL, F.P., PATT, C.S., BRUNDAGE, B., GILLIES, P.J., & PHILIPS, W.A.
(1978a) Studies on lipid biosynthesis and cholesterol content of liver
and serum lipoproteins in rats fed various phthalate esters. Lipids,
13: 66-74.
BELL, F.P., PATT, C.S., & GILLIES, P.J. (1978b) Effect of phthalate
esters on serum cholesterol and lipid biosynthesis in liver, testes,
and epididymal fat in the rat and rabbit. Lipids, 13: 673-678.
BELL, F.P., WANG, S., MENDOZA, A.R., & NISHIZAWA, E.E. (1979) Platelet
function and platelet lipid biosynthesis in rats and rabbits fed the
plasticiser DEHP. Bull. environ. Contam. Toxicol., 23: 306-310.
BICHET, N., CAHARD, D., FABRE, G., REMANDET, B., GOUY, D., & CANO,
J.-P. (1990) Toxicological studies on a benzofuran derivative. III.
Comparison of peroxisome proliferation in rat and human hepatocytes in
primary culture. Toxicol. appl. Pharmacol., 106: 509-517.
BIRGE, W.J., BLACK, J.A., & WESTERMAN, A.G. (1978) Effects of
polychlorinated biphenyl compounds and proposed PCB-replacement
products on embryo-larval stages of fish and amphibians, Washington,
DC, US Department of the Interior, 33 pp (Research Report No. 118).
BLAAUBOER, B.J., VAN HOLSTEIJN, C.W.M., BLEUMINK, R., MENNES, W.C.,
VAN PELT, F.N.A.M., YAP, S.H., VAN PELT, J.F., VAN IERSEL, A.A.J.,
TIMMERMANN, A., & SCHMID, B.P. (1990) The effect of beclobric acid and
clofibric acid on peroxisomal beta-oxidation and peroxisome
proliferation in primary cultures of rat, monkey and human
hepatocytes. Biochem. Pharmacol., 40: 521-528.
BOVE, J.L., DALVEN, P., & KUKREJA, V.P. (1978) Airborne di-butyl and
di(2-ethylhexyl)-phthalate at three New York city air sampling
stations. Int. J. environ. anal. Chem., 5: 189-194.
BRIDGES, J.W. (1985) Frontiers in biochemical toxicology. Trends
pharmacol. Sci., 6 (suppl.): 11-15.
BRINGMANN, G. & KÜHN, R. (1980) Comparison of the toxicity thresholds
of water pollutants to bacteria, algae, and protozoa in the cell
multiplication inhibition test. Water Res., 14: 231-241.
BROWN, D. & THOMPSON, R.S. (1982a) Phthalates and the aquatic
environment: Part I. The effect of di-2-ethylhexyl phthalate (DEHP)
and di-isodecyl phthalate (DIDP) on the reproduction of Daphnia magna
and observations on their bioaccumulation. Chemosphere, 11: 417-426.
BROWN, D. & THOMPSON, R.S. (1982b) Phthalates and the aquatic
environment: Part II. The bioconcentration and depuration of di-2-
ethylhexyl phthalate (DEHP) and di-isodecyl phthalate (DIDP) in
mussels (Mytilus edulis). Chemosphere, 11: 427-435.
BRUNETTI, G. & MOSCATO, G. (1984) [Bronchial asthma due to
occupational exposure to dioctyl phthalate.] Med. Lav., 75: 120-124
(in Italian).
BUCCAFUSCO, R.J., ELLS, S.J., & LEBLANC, G.A. (1981) Acute toxicity of
priority pollutants to bluegill (Lepomis macrochirus). Bull. environ.
Contam. Toxicol., 26: 446-452.
BUTTERWORTH, B.E., BERMUDEZ, E., SMITH-OLIVER, T., EARLE, L., CATTLEY,
R., MARTIN, J., POPP, J.A., STROM, S., JIRTLE, R., & MICHALOPOULOS, G.
(1984) Lack of genotoxic activity of di(2-ethylhexyl) phthalate (DEHP)
in rat and human hepatocytes. Carcinogenesis, 5: 1329-1335.
BUTTERWORTH, B.E., LOURY, D.J., SMITH-OLIVER, T., & CATTLEY, R.C.
(1987) The potential role of chemically induced hyperplasia in the
carcinogenic activity of the hypolipidemic carcinogens. Toxicol. ind.
Health, 3: 129-148.
BUTTERWORTH, B.E., SMITH-OLIVER, T., EARLE, L., LOURY, D.J., WHITE,
R.D., DOOLITTLE, D.J., WORKING, P.K., CATTLEY, R.C., JIRTLE, R.,
MICHALOPOULOS, G., & STROM, S. (1989) Use of primary cultures of human
hepatocytes in toxicology studies. Cancer Res., 49: 1075-1084.
CALLEY, D., AUTIAN, J., & GUESS, W.L. (1966) Toxicology of a series of
phthalate esters. J. pharm. Sci., 55: 158-162.
CARPENTER, C.P., WEIL, C.S., & SMYTH, H.F. (1953) Chronic oral
toxicity of di(2-ethylhexyl) phthalate for rats, guinea-pigs, and
dogs. Arch. ind. Hyg., 8: 219-226.
CATTLEY, R.C. & POPP, J.A. (1989) Differences between the promoting
activities of the peroxisome proliferator WY14,643 and phenobarbital
in rat liver. Cancer Res., 49: 3246-3251.
CATTLEY, R.C., CONWAY, J.G., & POPP, J.A. (1987) Association of
persistent peroxisome proliferation and oxidative injury with
hepatocarcinogenicity in female F-344 rats fed di(2-
ethylhexyl)phthalate for 2 years. Cancer Lett., 38: 15-27.
CAUTREELS, W. & VAN CAUWENBERGHE, K. (1978) Experiments on the
distribution of organic pollutants between airborne particulate matter
and the corresponding gas phase. Atmos. Environ., 12: 1133-1141.
CAUTREELS, W., VAN CAUWENBERGHE, K., & GUZMAN, L.A. (1977) Comparison
between the organic fraction of suspended matter at a background and
an urban station. Sci. total Environ., 8: 79-88.
CERBULIS, J. & ARD, J.S. (1967) Method for isolation and detection of
dioctyl phthalate from milk lipids. J. Assoc. Off. Anal. Chem., 50:
646-650.
CHAPIN, R.E., GRAY, T.J.B., PHELPS, J.L., & DUTTON, S.L. (1988) The
effects of mono-(2-ethylhexyl)-phthalate on rat sertoli cell-enriched
primary cultures. Toxicol. appl. Pharmacol., 92: 467-479.
CHEN, W.S., KERKAY, J., PEARSON, K.H., PAGANINI, E.P., & NAKAMOTO, S.
(1979a) Determination of urinary bis(2-ethylhexyl) phthalate levels in
non-uremic subjects by gas chromatography. Anal. Lett., 12: 1501-1515.
CHEN, W.S., KERKAY, J., PEARSON, K.H., PAGANINI, E.P., & NAKAMOTO, S.
(1979b) Tissue bis(2-ethylhexyl) phthalate levels in uremic subjects.
Anal. Lett., 12: 1517-1535.
CHU, I., SECOURS, V.E., MARINO, I.A., VILLENEUVE, D.C., & VALLI, V.E.
(1981) Sub-acute and sub-chronic toxicity of mono-2-ethylhexyl
phthalate in the rat. Arch. environ. Contam. Toxicol., 10: 271-280.
CIRIOLO, M.R., MAVELLI, I., ROTILIO, G., BORZATTA, V., CRISTOFARI, M.,
& STANZANI, L. (1982) Decrease of superoxide dismutase and glutathione
peroxidase in liver of rats treated with hypolipidemic drugs. FEBS
Lett., 144: 264-268.
CLAYTON, G.D. & CLAYTON, F.E. (1981) Patty's industrial hygiene and
toxicology, 3rd ed., New York, Wiley-Interscience, pp. 2342-2353.
CONTRERAS, T.J., SHELBLEY, R.H., & VALERI, C.R. (1974) Accumulation of
di-2-ethylhexyl phthalate (DEHP) in whole blood, platelet
concentrates, and platelet-poor plasma. Transfusion, 14: 34-46.
CONWAY, J.G., CATTLEY, R.C., POPP, J.A., & BUTTERWORTH, B.E. (1989a)
Possible mechanisms in hepatocarcinogenesis by the peroxisome
proliferator di(2-ethylhexyl)phthalate. Drug Metab. Rev., 21: 65-102.
CONWAY, J.G., TOMASZEWSKI, K.E., OLSON, M.J., CATTLEY, R.C., MARSMAN,
D.S., & POPP, J.A. (1989b) Relationship of oxidative damage to the
hepatocarcinogenicity of the peroxisome proliferators di(2-ethylhexyl)
phthalate and Wy-14, 643. Carcinogenesis, 10: 513-519.
CROCKER, J.F.S., SAFE, S.H., & ACOTT, P. (1988) Effects of chronic
phthalate exposure on the kidney. J. Toxicol. environ. Health, 23:
433-444.
CURSTEDT, T. & SJÖVALL, J. (1983) Individual molecular species of
phosphatidylcholines and phosphatidylinositols in livers of rats fed
bis(2-ethylhexyl) phthalate. Med. Biol., 61: 219-222.
CURTO, K.A. & THOMAS, J.A. (1982) Comparative effects of diethylhexyl
phthalate or monoethylhexyl phthalate on male mouse and rat
reproductive organs. Toxicol. appl. Pharmacol., 62: 121-125.
CURTO, K.A., MCCAFFERTY, R.E., DONOVAN, M.P., & THOMAS, J.A. (1982)
Further studies on the effects of the phthalate acid esters (PAE) on
rat male reproductive organs. Toxicologist, 2: 71.
DANIEL, J.W. & BRATT, H. (1974) The absorption, metabolism and tissue
distribution of di(2-ethylhexyl) phthalate in rats. Toxicology, 2:
51-65.
DEANGELO, A.B. & GARRETT, C.T. (1983) Inhibition of development of
preneoplastic lesions in the livers of rats fed a weakly carcinogenic
environmental contaminant. Cancer Lett., 20: 199-205.
DEANGELO, A.B., GARRETT, C.T., & QUERAL, A.E. (1984) Inhibition of
phenobarbital and dietary choline deficiency promoted preneoplastic
lesions in rat liver by environmental contaminant di(2-ethylhexyl)
phthalate. Cancer Lett., 23: 323-330.
DEFOE, D.L., HOLCOMBE, G.W., HAMMERMEISTER, D.E., & BIESINGER, K.E.
(1990) Solubility and toxicity of eight phthalate esters to four
aquatic organisms.Environ. Toxicol. Chem. 9: 623-636.
DIVINCENZO, G.D., HAMILTON, M.L., MUELLER, K.R., DONISH, W.H., &
BARBER, E.D. (1985) Bacterial mutagenicity testing of urine from rats
dosed with 2-ethylhexanol derived plasticizers. Toxicology, 34:
247-259.
DIWAN, B.A., WARD, J.M., RICE, J.M., COLBURN, N.H., & SPANGLER, E.F.
(1985) Tumor-promoting effects of di(2-ethylhexyl) phthalate in JB6
mouse epidermal cells and mouse skin. Carcinogenesis, 6: 343-347.
DOUGLAS, R.G., HUGENHOLTZ, A.P., & BLAKEY, D.H. (1986) Genetic
toxicology of phthalate esters: mutagenic and other genotoxic effects.
Environ. Health Perspect., 65: 255-262.
ECETOC (1985) An assessment of the occurrence and effects of dialkyl
ortho-phthalates in the environment, Brussels, European Chemical
Industry Ecology and Toxicology Centre, 63 pp (Technical Report No.
19).
ECETOC (1992) Hepatic peroxisome proliferation, Brussels, European
Chemical Industry Ecology and Toxicology Centre, (Monograph No. 17).
EGLINTON, G., SIMONEIT, B.R.T., & ZORO, J.A. (1975) The recognition of
organic pollutants in aquatic sediments. Proc. R. Soc. Lond., B189:
415-442.
EISENREICH, S.J., LOONEY, B.B., & THORNTON, J.D. (1981) Airborne
organic contaminants in the Great Lakes ecosystem. Environ. Sci.
Technol., 15: 30-38.
ELCOMBE, C.R. (1985) Species differences in carcinogenicity and
peroxisome proliferation due to trichloroethylene: A biochemical human
hazard assessment.Arch. Toxicol., Suppl. 8: 6-17.
ELCOMBE, C.R. & MITCHELL, A.M. (1986) Peroxisome proliferation due to
di(2-ethylhexyl)phthalate (DEHP): Species differences and possible
mechanisms. Environ. Health Perspect., 70: 211-219.
ELCOMBE, C.R. & STYLES J.A. (1989) Species differences in peroxisome
proliferation. Toxicologist, 9: 63.
ELSISI, A.E., CARTER, D.E., & SIPES, I.G. (1989) Dermal absorption of
phthalate diesters in rats. Fundam. appl. Toxicol., 12: 70-77.
ENGELHARDT, G. & WALLNOFER, P.R. (1978) Metabolism of di- and mono-N-
butyl phthalate by soil bacteria. Appl. environ. Microbiol., 35:
243-246.
ENGELHARDT, G., WALLNOFER, P.R., & HUTZINGER, O. (1975) The microbial
metabolism of di-n-butyl phthalate and related dialkyl phthalates.
Bull. environ. Contam. Toxicol., 13: 342-347.
ENGELHARDT, G., TILLMANNS, G., WALLNOFER, P.R., & HUTZINGER, O. (1977)
Biodegradation of di-iso-butyl phthalate and related dialkyl
phthalates by Penicillium lilacinum. Chemosphere, 6: 347-354.
ENVIRONMENT AGENCY OF JAPAN (1989) [Chemicals in the environment],
Tokyo, Environment Agency of Japan, p. 418 (in Japanese).
FATOKI, O.S. & VERNON, F. (1990) Phthalate esters in rivers of the
greater Manchester area, U.K. Sci. total Environ., 95: 221-232.
FISHBEIN, L. & ALBRO, P.W. (1972) Chromatographic and biological
aspects of the phthalate esters. J. Chromatogr., 70: 365-412.
FOSTER, P.M.D., LAKE, B.G., COOK, M.W., THOMAS, L.V., & GANGOLLI, S.D.
(1982) Structure-activity requirements for the induction of testicular
atrophy by butyl phthalates in immature rats: effects on testicular
zinc content. Adv. exp. Med. Biol., 136A: 445-452.
FOSTER, P.M.D., THOMAS, L.V., COOK, M.W., & WALTERS, D.G. (1983)
Effect of di-n-pentyl phthalate treatment on testicular steroidogenic
enzymes and cytochrome P-450 in the rat. Toxicol. Lett., 15: 265-271.
GANNING, A.E., OLLSON, M.J., PETERSON, E., & DALLNER., G. (1989) Fatty
acid oxidation in hepatic peroxisomes and mitochondria after treatment
of rats with di(2-ethylhexyl) phthalate. Pharmacol. Toxicol., 65:
265-268.
GIAM, C.S., CHAN, H.S., NEFF, G.S., & ATLAS, E.L. (1978) Phthalate
ester plasticizers: A new class of marine pollutant. Science, 199:
419-421.
GIAM, C.S., ATLAS, E., CHAN, H.S., & NEFF, G.S. (1980) Phthalate
esters, PCB and DDT residues in the Gulf of Mexico atmosphere. Atmos.
Environ., 14: 65-69.
GILIOLI, R., BULGHERONI, C., TERRANA, T., FILIPPINI, G., MASSETTO, N.,
& BOERI, R. (1978) [A neurological, electromyographic and
electroneurographic study in subjects working at the production of
phthalate plasticizers.] Med. Lav., 69: 620-631 (in Italian).
GOEL, S.K., LALWANI, N.D., & REDDY, J.K. (1986) Peroxisome
proliferation and lipid peroxidation in rat liver. Cancer Res., 46:
1324-1330.
GOLLAMUDI, R., LAWRENCE, W.H., RAO, R.H., & AUTIAN, J. (1985) Effects
of phthalic acid esters on drug metabolizing enzymes of rat liver. J.
appl. Toxicol., 5: 368-371.
GOTO, M. (1979) Monitoring environmental materials and specimen
banking - State-of-the-art of Japanese experience and knowledge. In:
Luepke, N.-P., ed. Monitoring environmental materials and specimen
banking. Proceedings of an International Workshop, Berlin (West),
23-28 October, 1978, pp. 271-288.
GRAHAM, P.R. (1973) Phthalate ester plasticizers - Why and how they
are used. Environ. Health Perspect., 3: 3-12.
GRAY, T.J.B. & BEAMAND, J.A. (1984) Effect of some phthalate esters
and other testicular toxins on primary cultures of testicular cells.
Food chem. Toxicol., 22: 123-131. GRAY, T.J.B. & BUTTERWORTH, K.R.
(1980) Testicular atrophy produced by phthalate esters. Arch.
Toxicol., Suppl. 4: 452-455.
GRAY, T.J.B. & GANGOLLI, S.D. (1986) Aspects of the testicular
toxicity of phthalate esters. Environ. Health Perspect., 65: 229-235.
GRAY, T.J.B., BUTTERWORTH, K.R., GAUNT, I.F., GRASSO, P., & GANGOLLI,
S.D. (1977) Short-term toxicity study of di-(2-ethylhexyl) phthalate
in rats. Food Cosmet. Toxicol., 15: 389-399.
GRAY, T.J.B., ROWLAND, I.R., FOSTER, P.M.D., & GANGOLLI, S.D. (1982)
Species differences in the testicular toxicity of phthalate esters.
Toxicol. Lett., 11: 141-147.
GUESS, W.L. & HABERMAN, S. (1968) Toxicity profiles of vinyl and
polyolefinic plastics and their additives. J. biomed. Mater. Res., 2:
313-335.
GUPTA, R.C., GOEL, S.K., EARLEY, K., SINGH, B., & REDDY, J.K. (1985)
32P-Postlabeling analysis of peroxisome proliferator-DNA adduct
formation in rat liver in vivo and hepatocytes in vitro.
Carcinogenesis, 6: 933-936.
HAMANO, Y., INOUE, K., ODA, Y., YAMAMOTO, H., & KUNITA, N. (1979)
[Studies on the toxicity of phthalic acid esters. Part 2. Dominant
lethal tests for DEHP and MEHP in mice, Osaka, Osaka Public Health and
Sanitation Research Centre, pp. 1-4 (Food Hygiene Series No. 10) (in
Japanese).
HARRIS, R.S., HODGE, H.C., MAYNARD, E.A., & BLANCHET, H.J., Jr (1956)
Chronic oral toxicity of 2-ethylhexyl phthalate in rats and dogs. Am.
Med. Assoc. Arch. ind. Health, 13: 259-264.
HERRING, R. & BERING, C.L. (1988) Effects of phthalate esters on plant
seedlings and reversal by a soil microorganism. Bull. environ. Contam.
Toxicol., 40: 626-632.
HILL, E.F., HEATH, R.G., SPANN, J.W., & WILLIAMS, J.D. (1975) Lethal
dietary toxicities of environmental pollutants to birds, Washington,
DC, US Fish and Wildlife Service, 61 pp (Special Scientific Report
Wildlife No. 191).
HOBSON, J.F., CARTER, D.E., & LIGHTNER, D.V. (1984) Toxicity of a
phthalate ester in the diet of a penaied shrimp. J. Toxicol. environ.
Health, 13: 959-968.
HODGE, H.C. (1943) Acute toxicity for rats and mice of di-2-ethylhexyl
phthalate with a note upon the mechanism. Proc. Soc. Exp. Biol. Med.,
53: 20-23.
HOLLIFIELD, H.C. (1979) Rapid nephelometric estimate of water
solubility of highly insoluble organic chemicals of environmental
interest. Bull. environ. Contam. Toxicol., 23: 579-586.
HOWARD, P.H., BANERJEE, S., & ROBILLARD, K.H. (1985) Measurement of
water solubilities, octanol/water partition coefficients and vapour
pressures of commercial phthalate esters. Environ. Toxicol. Chem., 4:
653-661.
HRUDEY, S.E., SERGY, G.A., & THACKERAY, T. (1976) Toxicity of oil
sands plant wastewaters and associated organic contaminants. In:
Proceedings of the 11th Canadian Symposium on Water Pollution
Research, Ottawa, Water Pollution Research Canada, pp. 34-45.
IARC (1982) Di(2-ethylhexyl) phthalate. In: Some industrial chemicals
and dyestuffs, Lyon, International Agency for Research on Cancer, pp.
269-294 (IARC Monographs on the Evaluation of the Carcinogenic Risks
of Chemicals to Humans, Vol. 29).
IARC (1987) Overall evaluations of carcinogenicity: An updating of
IARC monographs volumes 1 to 42, Lyon, International Agency for
Research on Cancer, 440 pp (IARC Monographs on the Evaluation of
Carcinogenic Risks to Humans, Supplement 7).
ISHIDA, M., SUYAMA, K., & ADACHI, S. (1980) Background contamination
by phthalates commonly encountered in the chromatographic analysis of
lipid samples. J. Chromatogr., 189: 421-424.
ISHIDA, M., SUYAMA, K., & ADACHI, S. (1981) Occurrence of dibutyl and
di-2-ethylhexyl phthalate in chicken eggs. J. agric. food Chem., 29:
72-74.
ISHIDA, M., SUYAMA, K., ADACHI, S., & HOSHINO, T. (1982) Distribution
of orally administered diethylhexyl phthalate in laying hens. Poult.
Sci., 61: 262-267.
ISSEMANN, I. & GREEN, S. (1990) Activation of a member of the steroid
hormone receptor superfamily by peroxisome proliferators.
Nature(Lond.): 347: 645-650.
JAEGER, R.J. & RUBIN, R.J. (1972) Migration of a phthalate ester
plasticizer from polyvinyl chloride blood bags into stored human blood
and its localization in human tissues. New Engl. J. Med., 287:
1114-1118.
JOHNSON, B.T. & LULVES, W. (1975) Biodegradation of di-butyl phthalate
and di-2-ethylhexyl phthalate in freshwater hydrosoil. J. Fish Res.
Board Can., 32: 333-339.
JOHNSON, B.T., HEITKAMP, M.A., & JONES, J.R. (1984) Environmental and
chemical factors influencing the biodegradation of phthalic acid
esters in freshwater sediments. Environ. Pollut., B8: 101-118.
KAMATA, I., TSUTSUI, G., TAKANA, J., & SHIRAI, T. (1978) [Phthalic
acid esters in fish in the Uji river.] Kyoto-fu Eisei Kogai Kenkyusho
Nempo, 22: 114 (in Japanese).
KARARA, A.H. & HAYTON, W.L. (1989) A pharmacokinetic analysis of the
effect of temperature on the accumulation of di-2-ethylhexyl phthalate
(DEHP) in sheepshead minnow. Aquat. Toxicol., 15: 27-36.
KARASEK, F.W., DENNEY, D.W., CHAN, K.W., & CLEMENT, R.E. (1978)
Analysis of complex organic mixtures on airborne particulate matter.
Anal. Chem., 50: 82-87.
KASAI, H., OKADA, Y., NISHIMURA, S., RAO, M.S., & REDDY, J.K. (1989)
Formation of 8-hydroxydeoxyguanosine in liver DNA of rats following
long-term exposure to a peroxisome proliferator.Cancer Res., 49:
2603-2605.
KATAEVA, S.W. (1988) [The method for determination of dioctyl
phthalates in water and model media imitating foodstuffs.] Gig. i
Sanit., 1: 52-53 (in Russian).
KEYSER, P., PUJAR, G.P., EATON, R.W., & RIBBONS, D.W. (1976)
Biodegradation of the phthalates and their esters by bacteria.
Environ. Health Perspect., 18: 159-166.
KIRBY, P.E., PIZZARELLO, R.F., LAWLOR, T.E., HAWORTH, S.E., & HODGSON,
J.R. (1983) Evaluation of di(2-ethylhexyl) phthalate and its major
metabolites in the Ames test and L5178Y mouse lymphoma mutagenicity
assay. Environ. Mutagen., 5: 657-663.
KLIMISCH, H.-J., HELLWIG, J., KAUFMANN, W., & JÄCKH, R. (1991) Di-(2-
ethylhexyl)phthalate (DEHP): Investigation of inhalation toxicity in
rats after repeated exposure (28 d). Hum. exp. Toxicol., 3: 68.
KLOPFFER, W., KAUFMANN, G., RIPPEN, G., & POREMSKI, H.J. (1982) A
laboratory method for testing the volatility from aqueous solution:
First results and comparison with theory. Ecotoxicol. environ. Saf.,
6: 545.
KLUWE, W., HASEMAN, J.K., DOUGLAS, J.F., & HUFF, J.E. (1982) The
carcinogenicity of dietary di(2-ethylhexyl) phthalate (DEHP) in Fisher
344 rats and B6C3F1 mice. J. Toxicol. environ. Health, 10: 797-815.
KNOWLES, C.O., MCKEE, M.J., & PALAWSKI, D.U. (1987) Chronic effects of
di-2-ethylhexyl phthalate on biochemical composition, survival and
reproduction of Daphnia magna. Environ. Toxicol. Chem., 6: 201-208.
KODAMA, T. & TAKAI, Y. (1974) [Studies on the determination of
phthalic acid esters, IV. Analysis of phthalic acid ester in fish
meat.] Kogai To Taisaku, 10: 850-852 (in Japanese).
KODAMA, T., KOBAYASKI, K., EBA, H., OGAWA, T., TAKAI, Y., NAITO, N.,
OKADA, N., & SAWANO, Y. (1975) [Studies on the determination of
phthalic acid esters: VI. Investigations in Aichi Prefecture.] Kogai
To Taisaku, 11: 341-350 (in Japanese).
KORA, S., SADO, M., KOLKE, H., & TERADA, H. (1989) Protective effect
of the plasticizer di(2-ethylhexyl) phthalate against damage of the
mitochondrial membrane induced by calcium: possible participation of
the adenine nucleotide translocator. Biochim. Biophys. Acta, 985:
286-292.
KORNBRUST, D.J., BARFKNECHT, T.R., INGRAM, P., & SHELBURNE, J.D.
(1984) Effect of di(2-ethylhexyl)phthalate on DNA repair and lipid
peroxidation in rat hepatocytes and on metabolic cooperation in
Chinese hamster V-79 cells. J. Toxicol. environ. Health, 13: 99-116.
KRAUSKOPF, L.G. (1973) Studies on the toxicity of phthalates via
ingestion. Environ. Health Perspect., 3: 61-72.
KURANE, R., SUZUKI, T., & TAKAHARA, Y. (1977) Isolation of
microorganisms growing on phthalate esters and degradation of
phthalate esters by Pseudomonas acidovorans 256-1. Agric. biol.
Chem., 41: 2119-2123.
KURANE, R., SUZUKI, T., & TAKAHARA, Y. (1978) Removal of phthalate
esters in soil columns inoculated with micro-organisms. Agric. biol.
Chem., 42: 1469-1478.
KURANE, R., SUZUKI, T., & TAKAHARA, Y. (1979) Microbial population and
identification of phthalate ester-utilising micro-organisms in
activated sludge with micro-organisms. Agric. biol. Chem., 43:
907-917.
KURANE, R., SUZUKI, T., & TAKAHARA, Y. (1980) Induction of enzymes
involved in phthalate esters metabolism in Nocardia erythropolis and
enzymatic hydrolysis of phthalate esters by commercial lipases. Agric.
biol. Chem., 44: 529-536.
KURANE, R., SUZUKI, T., TAKAHARA, Y., & FUKUOKA, S. (1984)
Protocatechuate 3,4-dioxygenase from Nocardia erythropolis. Agric.
biol. Chem., 48: 2105-2111.
LAKE, B.G., BRANTOM, P.G., GANGOLLI, S.H., BUTTERWORTH, K.R., &
GRASSO, P. (1976) Studies on the effects of orally administered di(2-
ethylhexyl)phthalate in the ferret. Toxicology, 6: 341-356.
LAKE, B.G., GRAY, T.J.B., FOSTER, J.R., STUBBERFIELD, C.R., &
GANGOLLI, S.D. (1984) Comparative studies on di-(2-ethylhexyl)
phthalate-induced hepatic peroxisome proliferation in the rat and
hamster. Toxicol. appl. Pharmacol., 72: 46-60.
LAKE, B.G., GRAY, T.J.B., & GANGOLLI, S.D. (1986) Hepatic effects of
phthalate esters and related compounds - in vivo and in vitro
correlations. Environ. Health Perspect., 67: 283-290.
LAKE, B.G., KOZLEN, S.L., EVANS, J.G., GRAY, T.J.B., YOUNG, P.J., &
GANGOLLI, S.D. (1987) Effect of prolonged administration of clofibric
acid and di-(2-ethylhexyl) phthalate on hepatic enzyme activities and
lipid peroxidation in the rat. Toxicology, 44: 213-228.
LAKE, B.G., LEWIS, D.F.V. & GRAY, T.J.B. (1988) Structure-activity
relationships for hepatic peroxisome proliferation. Arch. Toxicol.,
Suppl. 12: 217-224.
LAKE, B.G., COOK, W.M., WORRELL, N.R., CUNNINGHAME, M.E., EVANS, J.G.,
PRICE, R.J., YOUNG, P.J., & CARPANINI, F.M.B. (1991) Dose-response
relationships for induction of hepatic peroxisome proliferation and
testicular atrophy by phthalate esters in the rat. Hum. exp. Toxicol.,
10(1): 67.
LALWANI, N.D., ALVARES, K., REDDY, M.K., REDDY, M.N., PARIKH, I., &
REDDY, J.K. (1987) Peroxisome proliferator-binding protein:
Identification and partial characterization of nafenopin-, clofibric
acid-, and clofibrate-binding proteins from rat liver. Proc. Natl
Acad. Sci. (USA), 84: 5242-5246.
LARSSON, P. & THUREN, A. (1987) Di-2-ethylhexylphthalate inhibits the
hatching of frog eggs and is bioaccumulated by tadpoles. Environ.
Toxicol. Chem., 6: 417-422.
LARSSON, P., THUREN, A., & GAHNSTROM, G. (1986) Phthalate esters
inhibit microbial activity in aquatic sediments. Environ. Pollut., 42:
223-231.
LAUGHLIN, R.B., NEFF, J.M., HRUNG, Y.C., GOODWIN, T.C., & GIAM, C.S.
(1978) The effects of three phthalate esters on the larval development
of the grass shrimp Palaemonetes pugio (Holthuis). Water Air Soil
Pollut., 9: 323-336.
LAWRENCE, W.H., MALIK, M., TURNER, J.E., SINGH, A.R., & AUTIAN, J.
(1975) A toxicological investigation of some acute, short-term, and
chronic effects of administering di-2-ethylhexyl phthalate (DEHP) and
other phthalate esters. Environ. Res., 9: 1-11.
LEBLANC, G.A. (1980) Acute toxicity of priority pollutants to water
flea (Daphnia magna). Bull. environ. Contam. Toxicol., 24: 684-691.
LEWANDOWSKI, M., FERNANDES, J., & CHEN, T.S. (1980) Assessment of the
teratogenic potential of plasma-soluble extracts of diethylhexyl
phthalate plasticized polyvinyl chloride plastics in rats. Toxicol.
appl. Pharmacol., 54: 141-147.
LEWIS, L.M., FLECHTNER, T.W., KERKAY, J., PEARSON, K.H., CHEN, W.T.,
POPOWNIAK, K.L., & NAKAMOTO, S. (1977) Determination of plasticizer
levels in serum of hemodialysis patients. Trans. Am. Soc. Artif.
Intern. Organs, 23: 566-572.
LEYDER, F. & BOULANGER, P. (1983) Ultraviolet absorption, aqueous
solubility, and octanol-water partition for several phthalates. Bull.
environ. Contam. Toxicol., 30: 152-157.
LHUGUENOT, J.C. & ELCOMBE, C.R. (1984) Variation selon l'espèce des
phénomènes de conjugasion lors du métabolisme mono-(éthyl-2-hexyl)
phthalate (MEHP). Sci. Aliments, 4: 227-232.
LHUGUENOT, J.C., MITCHELL, A.M., MILNER, G., LOCK, E.A., & ELCOMBE,
C.R. (1985) The metabolism of di(2-ethylhexyl)phthalate (DEHP) and
mono-(2-ethylhexyl) phthalate (MEHP) in rats: In vivo and in vitro
dose and time dependency of metabolism. Toxicol. appl. Pharmacol., 80:
11-22.
LINDEN, E., BENGTSSON, B.-E., SVANBERG, O., & SUNDSTROM, G. (1979) The
acute toxicity of 78 chemicals and pesticide formulations against two
brackish water organisms, the bleak (Alburnus alburnus) and the
harpacticoid (Nitocra spinipes). Chemosphere, 8: 843-851.
LISS, G.M., ALBRO, P.W., HARTLE, R.W., & STRINGER, W.T. (1985) Urine
phthalate determinations as an index of occupational exposure to
phthalic anhydride and di(2-ethylhexyl) phthalate. Scand. J. Work
Environ. Health, 11: 381-387.
LLOYD, S.C. & FOSTER, P.M.D. (1988) Effect of mono-(2-ethylhexyl)
phthalate on follicle-stimulating hormone responsiveness of cultured
rat Sertoli cells. Toxicol. appl. Pharmacol., 95: 484-489.
LOCK, E.A., MITCHELL, A.M.R., & ELCOMBE, C.R. (1989) Biochemical
mechanisms of induction of hepatic peroxisome proliferation. Annu.
Rev. Pharmacol. Toxicol., 29: 145-168.
LOKKE, H. & BRO-RASMUSSEN, F. (1981) Studies of mobility of di-iso-
butyl phthalate (DiBP), di-n-butyl phthalate (DBP), and di-(2-
ethylhexyl) phthalate (DEHP) by plant foliage treatment in a closed
terrestrial simulation chamber. Chemosphere, 10: 1223-1235.
LOKKE, H. & RASMUSSEN, L. (1983) Phytotoxicological effects of di-(2-
ethylhexyl)-phthalate and n-butyl-phthalate on higher plants in
laboratory and field experiments. Environ. Pollut., 32: 179-199.
LUTZ, W.K. (1986) Investigation of the potential for binding of di(2-
ethylhexyl) phthalate (DEHP) to rat liver DNA in vivo. Environ.
Health Perspect., 65: 267-269.
MACEK, K.J., PETROCELLI, S.R., & SLEIGHT, B.H., III (1979)
Considerations in assessing the potential for, and significance of,
biomagnification of chemical residues in aquatic food chains. In:
Aquatic toxicology, Philadelphia, American Society for Testing and
Materials, pp. 251-268 (ASTM STP 667).
MAFF (1987) Survey of plasticiser levels in food contact materials and
in foods, London, Ministry of Agriculture, Fisheries and Food (Food
Surveillance Paper No. 21).
MALCOLM, A.R. & MILLS, L.J. (1989) Inhibition of gap-junctional
intercellular communication between Chinese hamster lung fibroblasts
by di(2-ethylhexyl) phthalate (DEHP) and trisodium nitrilotriacetate
monohydrate (NTA). Cell Biol. Toxicol., 5: 145-153.
MANANDHAR, M.D., SHOEB, A., KAPIL, R.S., & POPLI, S.P. (1979) Studies
in medicinal plants. Part V, Di-2-n-propyl pentyl phthalate and
reticulene from Cryptocaryo amyggdalina Nees. Indian J. Chem., 178:
411-412.
MARCEL, Y.L. & NOEL, S.P. (1970) Contamination of blood stored in
plastic packs. Lancet, 1: 35-36.
MARSMAN, D.S., CATTLEY, R.C., CONWAY, J.G., & POPP, J.A. (1988)
Relationships of hepatic peroxisome proliferation and replicative DNA
synthesis to the hepatocarcinogenicity of the peroxisome proliferators
di(2-ethylhexyl) phthalate and [4-chloro-6-(2,3-xylidino)-2-
pyrimidinylthio]acetic acid (Wy-14,643) in rats. Cancer Res., 48:
6739-6744.
MATHUR, S.P. (1974a) Phthalate esters in the environment: Pollutants
or natural products. J. environ. Qual., 3: 189.
MATHUR, S.P. (1974b) Respirometric evidence of the utilization in soil
of di-octyl and di-2-ethylhexyl phthalate plasticizers. J. environ.
Qual., 3: 207-209.
MATSUDA, K. & SCHNITZER, M. (1971) Reactions between fulvic acid, a
soil humic material, and dialkyl phthalates. Bull. environ. Contam.
Toxicol., 6: 200-204.
MAYER, F.L. (1976) Residue dynamics of di-2-ethylhexyl phthalate in
fathead minnows (Pimephales promelas). J. Fish Res. Board Can., 33:
2610-2613.
MAYER, F.L. & SANDERS, H.O. (1973) Toxicology of phthalic acid esters
in aquatic organisms. Environ. Health Perspect., 3: 153-157.
MAYER, F.L., STALLING, D.L., & JOHNSON, J.L. (1972) Phthalate esters
as environmental contaminants. Nature (Lond.), 238: 411-413.
MAYER, F.L., MEHRLE, P.M., & SCHOETTGER, R.A. (1977) Collagen
metabolism in fish exposed to organic chemicals. In: Symposium on
Recent Advances in Fish Toxicology, Washington, DC, US Environmental
Protection Agency, p. 31 (EPA 600/3-77-085).
MEHRLE, P.M. & MAYER, F.L. (1976) Di-2-ethylhexyl phthalate: Residue
dynamics and biological effects in rainbow trout and fathead minnow.
Trace Subst. environ. Health, 10: 519-524.
MELANCON, M.J. (1979) Metabolism of phthalate esters in aquatic
species. In: Pesticides and xenobiotic metabolism in aquatic
organisms, Washington, DC, American Chemical Society, pp. 77-94 (ACS
Symposium Series No. 99).
MELNICK, R.L., MORRISSEY, R.E., & TOMASZEWSKI, K.E. (1987) Studies by
the National Toxicology Program on di(2-ethylhexyl) phthalate.
Toxicol. ind. Health, 3: 99-118.
MES, J., COFFIN, D.E., & CAMPBELL, D.S. (1974) Di-n-butyl and di-2-
ethylhexyl phthalate in human adipose tissue. Bull. environ. Contam.
Toxicol., 12: 721-725.
METCALF, R.L., BOOTH, G.M., SCHUTH, C.K., HANSEN, D.J., & LU, P.-Y.
(1973) Uptake and fate of di-2-ethylhexyl phthalate in aquatic
organisms and in a model ecosystem. Environ. Health Perspect., 4:
27-34.
MIKALSEN, S.O., HOLEN, I., & SANNER, T. (1990a) Morphological
transformation and catalase activity of Syrian hamster embryo cells
treated with hepatic peroxisome proliferators, TPA and nickel
sulphate. Cell Biol. Toxicol., 6: 1-13.
MIKALSEN, S.O., KAALHUS, O., REITH, A., & SANNER, T. (1990b) Role of
catalase and oxidative stress in hepatic peroxisome proliferator-
induced morphological transformation of Syrian hamster embryo cell.
Int. J. Cancer, 46: 950-957.
MIKALSEN, S.O., REUTER, B., & SANNER, T. (1990c) Effects of hepatic
peroxisome proliferators and 12-O-tetradecanoyl phorbol-13-acetate on
catalase and other enzyme activities of embryonic cells in vitro.
Biochem. Pharmacol., 39: 527-535.
MILKOV, L.E., ALDYREVA, M.V., POPOVA, T.B., LOPUKHOVA, K.A.,
MAKARENKO, Y.L., MALYAR, L.M., & SHAKHOVA, T.K. (1973) Health status
of workers exposed to phthalate plasticizers in the manufacture of
artificial leather and films based on PVC resins. Environ. Health
Perspect., 3: 175-178.
MITCHELL, A.M., LHUGUENOT, J.-C., BRIDGES, J.W., & ELCOMBE, C.R.
(1985a) Identification of the proximate peroxisome proliferator(s)
derived from di(2-ethylhexyl) phthalate. Toxicol. appl. Pharmacol.,
80: 23-32.
MITCHELL, F.E., PRICE, S.C., HINTON, R.H., GRASSO, P., & BRIDGES, J.W.
(1985b) Time and dose-response study of the effects on rats of the
plasticizer di(2-ethylhexyl) phthalate. Toxicol. appl. Pharmacol., 81:
371-392.
MORI, S. (1976) Identification and determination of phthalate esters
in river water by high-performance liquid chromatography. J.
Chromatogr., 129: 53-60.
MUSIAL, C.J. & UTHE, J.F. (1980) Studies on phthalate esters in
Western Atlantic finfish. Report to International Council for the
Exploration of the Sea (ICES), Copenhagen, International Council for
the Exploration of the Sea, pp. 1-6 (Report E:11).
MUTZ, R.C. & JONES, J.R. (1977) The effects of phthalate esters on
geochemical cycles in freshwater hydrosoil. Trans. Missouri Acad.
Sci., 10-11: 296.
AZIR, D.J., ALCARAZ, A.P., BIERL, B.A., BEROZA, M., & NAIR, P.P.
(1971) Isolation, identification, and specific localization of di-2-
ethylhexyl phthalate in bovine heart muscle mitochondria.
Biochemistry, 10: 4228-4232.
NEERGAARD, J., NIELSEN, B., FAURBY, V., CHRISTENSEN, D.H., & NIELSEN,
O.F. (1971) Plasticizers in P.V.C. and the occurrence of hepatitis in
a haemodialysis unit. Scand. J. Urol. Nephrol., 5: 141-145.
NEUHAUSER, E.F., LOER, R.C., & MALECKI, M.R. (1986) Contact and
artificial soil tests using earthworms to evaluate the impact of
wastes in soil. In: Petros, J.K., Jr, Lacy, W.J., & Conway, R.A., ed.
Hazardous and industrial solid waste testing: Fourth symposium,
Philadelphia, American Society for Testing and Materials, pp. 192-203
(ASTM STP 866).
NIELSEN, J., ÅKESSON, B., & SKERFVING, S. (1985) Phthalate ester
exposure - air levels and health of workers processing
polyvinylchloride. Am. Ind. Hyg. Assoc. J., 46: 643-647.
NIKONOROW, M., MAZUR, H., & PIEKACZ, H. (1973) Effect of orally
administered plasticizers and polyvinyl chloride stabilizers in the
rat. Toxicol. appl. Pharmacol., 26: 253-259.
NIOSH (1977) Manual of analytical methods, 2nd ed., Cincinnati, Ohio,
National Institute for Occupational Safety and Health, Vol. 2, pp.
S40/1-S40/9.
NIOSH (1985a) Manual of analytical methods, 3rd ed., Cincinnati, Ohio,
National Institute for Occupational Safety and Health, Vol. 1, pp.
5020/1-5020/4.
NIOSH (1985b) Registry of toxic effects of chemical substances:
1983-84 Supplement, Cincinnati, Ohio, National Institute for
Occupational Safety and Health, Vol. II, p. 1442.
NTP (1982) Technical report on the carcinogenicity bioassay of di(2-
ethylhexyl) phthalate, Research Triangle Park, NC, National Toxicology
Programme (DHHS Publ. No. (NIH) 82-1773).
O'CONNOR, O.A., RIVERA, M.D., & YOUNG, L.Y. (1989) Toxicity and
biodegradation of phthalic acid esters under methanogenic conditions.
Environ. Toxicol. Chem., 8: 569-576.
OHYAMA, T. (1977) Effects of phthalate esters on glucose-6-phosphate
dehydrogenase and other enzymes in vitro. Toxicol. appl. Pharmacol.,
40: 355-364.
OISHI, S. (1984) Testicular atrophy of rats induced by di-2-ethylhexyl
phthalate: effects of vitamin A and zinc concentrations in the testis,
liver and serum. Toxicol. Lett., 20: 75-78.
OISHI, S. (1985) Reversibility of testicular atrophy induced by di(2-
ethylhexyl) phthalate in rats. Environ. Res., 36: 160-169.
OISHI, S. (1989a) Effects of co-administration of di(2-ethylhexyl)
phthalate and testosterone on several parameters in the testis and
pharmacokinetics of its mono-deesterified metabolite. Arch. Toxicol.,
63: 289-295.
OISHI, S. (1989b) Enhancing effects of luteinizing hormone-releasing
hormone on testicular damage induced by di(2-ethylhexyl) phthalate in
rats. Toxicol. Lett., 47: 271-277.
OISHI, S. (1990) Effects of phthalic acid esters on testicular
mitochondrial functions in the rat. Appl. Toxicol., 64: 143-147.
OISHI, S. & HIRAGA, K. (1979) Effect of phthalic acid esters on
gonadal function in male rats. Bull. environ. Contam. Toxicol., 21:
65-67.
OISHI, S. & HIRAGA, K. (1980a) Testicular atrophy induced by phthalic
esters: effect on testosterone and zinc concentrations. Toxicol. appl.
Pharmacol., 53: 35-41.
OISHI, S. & HIRAGA, K. (1980b) Effect of phthalic acid monoesters on
mouse testes. Toxicol. Lett., 6: 239-242.
OISHI, S. & HIRAGA, K. (1982) Effects of monoesters of o-phthalic acid
on serum lipid composition in rats. Toxicol. Lett., 14: 79-84.
OISHI, S. & HIRAGA, K. (1983) Testicular atrophy induced by di-2-
ethylhexyl phthalate: effect of zinc supplement. Toxicol. appl.
Pharmacol., 70: 43-48.
O'SHEA, T.J. & STAFFORD, C.J. (1980) Phthalate plasticizers:
Accumulation and effects on weight and food consumption in captive
starlings. Bull. environ. Contam. Toxicol., 25: 345-352.
OSUMI, T. & HASHIMOTO, T. (1978) Enhancement of fatty acyl-CoA
oxidizing activity in rat liver peroxisomes by di-(2-
ethylhexyl)phthalate. J. Biochem., 83: 1361-1365.
PARE, J.R.J., BELANGER, J., & JANKOWSKI, K. (1981) Asymmetric
phthalate from Sansevieria trifasciata. J. nat. Prod., 44: 490-492.
PASSINO, D.R.M. & SMITH, S.B. (1987) Acute bioassays and hazard
evaluation of representative contaminants detected in Great Lakes
fish. Environ. Toxicol. Chem., 6: 901-907.
PEAKALL, D.B. (1974) Effects of di-n-butyl and di-2-ethylhexyl
phthalate on the eggs of ring doves. Bull. environ. Contam. Toxicol.,
12: 698-702.
PEAKALL, D.B. (1975) Phthalate esters: Occurrence and biological
effects. Res. Rev., 54: 1-40.
PERERA, M.I.R., KATYAL, S.L., & SHINOZUKA, H. (1986) Suppression of
choline-deficient diet-induced hepatocyte membrane lipid peroxidation
in rats by the peroxisome proliferators 4-chloro-6-(2,3-xylidino)-2-
pyrimidinylthio(N-ß-hydroxyethyl)acetamide and di(2-
ethylhexyl)phthalate. Cancer Res., 46: 3304-3308.
PERKINS, E.G. (1967) Characterization of the nonvolatile compounds
formed during the thermal oxidation of corn oil. II. Phthalate esters.
J. Am. Oil Chem. Soc., 44: 197-199.
PERSSON, P.-E., PENTTINEN, H., & NUORTEVA, P. (1978) DEHP in the
vicinity of an industrial area in Finland. Environ. Pollut., 16:
163-166.
PETERSON, J.C. & FREEMAN, D.H. (1984) Variations of phthalate ester
concentrations in sediments from the Chester river, Maryland. Int. J.
environ. anal. Chem., 18: 237-252.
PFUDERER, P. & FRANCIS, A.A. (1975) Phthalate esters: Heartrate
depressors in the goldfish. Bull. environ. Contam. Toxicol., 13:
275-279.
PHILLIPS, B.J., ANDERSON, D., & GANGOLLI, S.D. (1986) Studies on the
genetic effects of phthalic acid esters on cells in culture. Environ.
Health Perspect., 65: 263-266.
PIECHOCKI, J.T. & PURDY, W.C. (1973) Determination of di-(2-
ethylhexyl)-phthalate (DEHP) in human plasma. Clin. Chim. Acta, 48:
385-391.
POLLACK, G.M., BUCHANAN, J.F., SLAUGHTER, R.L., KOHLI, R.K., & SHEN,
D.D. (1985a) Circulating concentrations of di(2-ethylhexyl) phthalate
and its de-esterified phthalic acid products following plasticizer
exposure in patients receiving hemodialysis. Toxicol. appl.
Pharmacol., 79: 257-267.
POLLACK, G.M., LI, R.C.K., ERMER, J.C., & SHEN, D.D. (1985b) Effects
of route of administration and repetitive dosing on the disposition
kinetics of di(2-ethylhexyl) phthalate and its mono-de-esterified
metabolite in rats. Toxicol. appl. Pharmacol., 79: 246-256.
POLLACK, G.M., SHEN, D.D., & DORR, M.B. (1989) Contribution of
metabolites to the route- and time-dependent hepatic effects of di-(2-
ethylhexyl)phthalate in the rat. J. Pharmacol. exp. Ther., 248:
176-181.
POPP, J.A., GARVEY, L.K., & CATTLEY, R.C. (1987) In vivo studies on
the mechanism of di(2-ethylhexyl) phthalate carcinogenesis. Toxicol.
ind. Health, 3: 151-163.
PRESTON, M.R. & AL-OMRAN, L.A. (1986) Dissolved and particulate
phthalate esters in the river Mersey estuary. Mar. Pollut. Bull., 17:
548-553.
PRESTON, M.R. & AL-OMRAN, L.A. (1989) Phthalate ester speciation in
estuarine water, suspended particulates and sediments. Environ.
Pollut., 62: 183-193.
PUTMAN, D.L., MOORE, W.A., SCHECHTMAN, L.M., & HODGSON, J.R. (1982)
Cytogenetic evaluation of di-(2-ethylhexyl)phthalate and its major
metabolites in Fischer 344 rats. Environ. Mutagen., 4: 387.
RAO, M.S. & REDDY, J.K. (1987) Peroxisome proliferation and
hepatocellular hepatocarcinogenesis. Carcinogenesis, 8: 631-636.
RAO, M.S., YELDANDI, A.V.P., & SUBBARAO, V. (1990) Quantitative
analysis of hepatocellular lesions induced by di(2-
ethylhexyl)phthalate in F344 rats. J. Toxicol. environ. Health, 30:
85-89.
RAO, P.S.C., HORNSBY, A.G., & JESSUP, R.E. (1985) Indices for ranking
the potential for pesticide contamination of groundwater. Soil Crop
Sci. Soc. Proc., 44: 1-8.
RAY, L.E., MURRAY, H.E., & GIAM, C.S. (1983a) Organic pollutants in
marine samples from Portland, Maine. Chemosphere, 12: 1031-1038.
RAY, L.E., MURRAY, H.E., & GIAM, C.S. (1983b) Analysis of water and
sediment from the Nueces estuary/Corpus Christi bay (Texas) for
selected organic pollutants. Chemosphere, 12: 1039-1045.
REDDY, J.K. & LALWANI, N.D. (1983) Carcinogenesis by hepatic
peroxisome proliferators: evaluation of the risk of hypolipidemic
drugs and industrial plasticizers to humans. CRC crit. Rev. Toxicol.,
12: 1-58.
REDDY, J.K. & RAO, M.S. (1989) Oxidative DNA damage caused by
persistent peroxisome proliferation: its role in hepatocarcinogenesis.
Mutat. Res., 214: 63-68.
REDDY, J.K., REDDY, M.K., USMAN, M.I., LALWANI, N.D., & RAO, M.S.
(1986) Comparison of hepatic peroxisome proliferative effect and its
implication for hepatocarcinogenicity of phthalate esters, di(2-
ethylhexyl)phthalate, and di(2-ethylhexyl) adipate with a
hypolipidemic drug. Environ. Health Perspect., 65: 317-327.
RHODES, C., ORTON, T.C., PRATT, I.S., BATTEN, P.L., BRATT, H.,
JACKSON, S.J., & ELCOMBE, C.R. (1986) Comparative pharmacokinetics and
subacute toxicity of di(2-ethylhexyl)phthalate (DEHP) in rats and
marmosets: extrapolation of effects in rodents to man. Environ. Health
Perspect., 65: 299-308.
RITSEMA, R., COFINO, W.P., FRINTROP, P.C.M., & BRINKMAN, V.A.T. (1989)
Trace-level analysis of phthalate esters in surface water and
suspended particulate matter by means of capillary gas chromatography
with electron-capture and mass-selective detection. Chemosphere, 18:
2161-2175.
ROTH, B., HERKENRATH, P., LEHMANN, H.J., OHLES, H.D., HÖMIG, H.J.,
BENZ-BOHM, G., KREUDER, J., & YOUNOSSI-HARTENSTEIN, A. (1988) Di-(2-
ethylhexyl)phthalate as plasticizer in PVC respiratory tubing systems:
indications of hazardous effects on pulmonary function in mechanically
ventilated, preterm infants. Eur. J. Pediatr., 147: 41-46.
RUBIN, R.J. & CHANG, J.F.C. (1978) Effect of the intravenous
administration of the solubilized plasticizer di(2-ethylhexyl)
phthalate on the lung and on survival of transfused rats. Toxicol.
appl. Pharmacol., 45: 230.
RUBIN, R.J. & JAEGER, R.J. (1973) Some pharmacologic and toxicologic
effects of di-2-ethylhexyl phthalate (DEHP) and other plasticizers.
Environ. Health Perspect., 3: 53-59.
RUBIN, R.J. & NAIR, P.P. (1972) Plasticizers in human tissues. New
Engl. J. Med., 288: 915-916.
RUDDICK, J.A., VILLENEUVE, D.C., CHU, I., NESTMANN, E., & MILES, D.
(1981) An assessment of the teratogenicity in the rat and mutagenicity
in Salmonella of mono-2-ethylhexyl phthalate. Bull. environ. Contam.
Toxicol., 27: 181-186.
RUSHBROOK, C.L., JORGENSON, T.A., & HODGSON, J.R. (1982) Dominant
lethal study of di-(2-ethylhexyl)phthalate and its major metabolites
in ICR/SIM mice. Environ. Mutagen., 4: 387.
SAEGER, V.W. & TUCKER, E.S. (1973) Biodegradation of phthalate esters.
In: Flexible vinyls and human safety: An objective analysis. Presented
at the Regional Technical Conference of the Society of Plastics
Engineers, Palisades Section, Kiamesha Lake, New York, 20-22 March,
1973, pp. 105-133.
SAEGER, V.W. & TUCKER, E.S. (1976) Biodegradation of phthalic acid
esters in river water and activated sludge. Appl. environ. Microbiol.,
31: 29-34.
SAKURAI, T., MIYAZAWA, S., & HASHIMOTO, T. (1978) Effect of di(2-
ethylhexyl) phthalate administration on carbohydrate and fatty acid
metabolism in rat liver. J. Biochem., 83: 313-320.
SANDERS, H.O., MAYER, F.L., & WALSH, D.F. (1973) Toxicity, residue
dynamics, and reproductive effects of phthalate esters in aquatic
invertebrates. Environ. Res., 6: 84-90.
SAX, N.I. (1984) Dangerous properties of industrial materials, 6th
ed., New York, Van Nostrand Reinhold Company.
SAXENA, D.K., SRIVASTAVA, S.P., CHANDRA, S.V., & SETH, P.K. (1985)
Testicular effects of di(2-ethylhexyl) phthalate (DEHP): histochemical
and histopathological alterations. Ind. Health, 23: 191-198.
SCHMEZER, P., POOL, B.L., KLEIN, R.G., KOMITOWSKI, D., & SCHMÄHL, D.,
(1988) Various short-term assays and two long-term studies with the
plasticizer di(2-ethylhexyl) phthalate in the Syrian hamster.
Carcinogenesis, 9: 37-43.
SCHMID, P. & SCHLATTER, C. (1985) Excretion and metabolism of di(2-
ethyl-hexyl) phthalate in man. Xenobiotica, 15: 251-256.
SCHMIDT, J.G., GARVIN, P.J., & LEESTMA, J.E. (1975) Effect of vehicle
on the response to intravenous di-(2-ethylhexyl) phthalate (DEHP) in
rats. Toxicol. appl. Pharmacol., 33: 169.
SCHMITZER, J.L., SCHEUNERT, I., & KORTE, F. (1988) Fate of bis(2-
ethylhexyl) [14C] phthalate in laboratory and outdoor soil-plant
systems. J. agric. food Chem., 36: 210-215.
SCHNEIDER, B., SCHENA, J., TROUG, R., JACOBSON, M., & KEVY, S. (1989)
Exposure to di(2-ethylhexyl)phthalate in infants receiving
extracorporeal membrane oxygenation. New Engl. J. Med., 320: 1563.
SCHULTE-HERMANN, R., KRAUPP-GRASL, B., BURSCH, W., GERBRACHT, U., &
TIMMERMANN-TROSIENER, I. (1989) Effects of non-genotoxic hepato-
carcinogens phenobarbital and nafenopin on phenotype and growth of
different populations of altered foci in rat liver. Toxicol. Pathol.,
17: 642-650.
SCHULZ, C.O. (1989) Assessing human health risks from exposure to
di(2-ethylhexyl)phthalate (DEHP) and related phthalates: scientific
issues. Drug Metab. Rev., 21: 111-120.
SCHULZ, C.O. & RUBIN, R.J. (1973) Distribution, metabolism, and
excretion of di-2-ethylhexyl phthalate in the rat. Environ. Health
Perspect., 3: 123-129.
SCHULZ, C.O., RUBIN, R.J., & HUTCHINS, G.M. (1975) Acute lung toxicity
and sudden death in rats following the intravenous administration of
the plasticizer, di(2-ethylhexyl)-phthalate, solubilized with Tween
surfactants. Toxicol. appl. Pharmacol., 33: 514-525.
SCHWARTZ, H.E., ANZION, C.J.M., VAN VLIET, H.P.M., COPIUS PEEREBOOMS,
J.W., & BRINMAN, U.A.T. (1979) Analysis of phthalate esters in
sediments from Dutch rivers by means of high performance liquid
chromatography. Intern. J. environ. anal. Chem., 6: 133-144.
SETH, P.K., SRIVASTAVA, S.P., AGARWAL, D.K., & CHANDRA, S.V. (1976)
Effect of di-2-ethylhexyl phthalate (DEHP) on rat gonads. Environ.
Res., 12: 131-138.
SHAFFER, C.B., CARPENTER, C.P., & SMYTH, H.F., Jr (1945) Acute and
subacute toxicity of di(2-ethylhexyl) phthalate with note upon its
metabolism. J. ind. Hyg. Toxicol., 27: 130-135.
SHANKER, R., RAMAKRISHNA, C., & SETH, P.K. (1985) Degradation of some
phthalic acid esters in soil. Environ. Pollut., 39: 1-7.
SHELDON, L.S. & HITES, R.A. (1979) Sources and movement of organic
chemicals in the Delaware river. Environ. Sci. Technol., 13: 574-579.
SHINDO, Y., OSUMI, T., & HASHIMOTO, T. (1978) Effects of
administration of di(2-ethylhexyl) phthalate on rat liver
mitochondria. Biochem. Pharmacol., 27: 2683-2688.
SHIOTA, K. & MIMA, S. (1985) Assessment of the teratogenicity of di(2-
ethylhexyl) phthalate and mono(2-ethylhexyl) phthalate in mice. Arch.
Toxicol., 56: 263-266.
SHIOTA, K. & NISHIMURA, H. (1982) Teratogenicity of di(2-ethylhexyl)
phthalate (DEHP) and di-n-butyl phthalate (DBP) in mice. Environ.
Health Perspect., 45: 65-70. SHIOTA, K., CHOU, M.J., & NISHIMURA, H.
(1980) Embryotoxic effects of di-2-ethylhexyl phthalate (DEHP) and di-
n-butyl phthalate (DBP) in mice. Environ. Res., 22: 245-253.
SHORT, R.D., ROBINSON, E.C., LINGTON, A.W., & CHIN, A.E. (1987)
Metabolic and peroxisome proliferation studies with di(2-ethylhexyl)
phthalate in rats and monkeys. Toxicol. ind. Health, 3: 185-195.
SHUKLA, R.R., ALBRO, P.W., CORBETT, J.T., & SCHROEDER, J.L. (1989) In
vivo studies of the inhibition of protein kinase C from rat brain by
di-(2-ethylhexyl) phthalate. Chem.-biol. Interact., 69: 73-85.
SILVO, O.E.J. (1974) [Preliminary studies on the acute toxicity of
dioctylphthalate (DOP) to rainbow trout ( Salmo gairdneri Richardson)
and its effect upon phytoplankton and oxygen content of the water.]
Suomen Kalatalous, 47: 19-25 (in Finnish).
SINGH, A.R., LAWRENCE, W.H., & AUTIAN, J. (1972) Teratogenicity of
phthalate esters in rats. J. pharm. Sci., 61: 51-55.
SITTIG, M. (1985) Handbook of toxic and hazardous chemicals and
carcinogens, 2nd ed., Park Ridge, New Jersey, Noyes Publications.
SJÖBERG, P., PLÖEN, L., BONDESSON, U., & LINDQUIST, N.G. (1982)
Testicular toxicity of di-(2-ethylhexyl) phthalate in rats of
different ages. Teratology, 26: 20A.
SJÖBERG, P., BONDESSON, U., KJELLEN, L., LINDQUIST, N.-G., MONTIN, G.,
& PLÖEN, L. (1985a) Kinetics of di-(2-ethylhexyl) phthalate in
immature and mature rats and effect on testis. Acta pharmacol.
toxicol., 56: 30-37.
SJÖBERG, P., BONDESSON, U., SEDIN, G., & GUSTAFSSON, J. (1985b)
Dispositions of di- and mono-(2-ethylhexyl) phthalate in newborn
infants subjected to exchange transfusions. Eur. J. clin. Invest., 15:
430-436.
SJÖBERG, P., LINDQUIST, N.G., MONTIN, G., & PLÖEN, L. (1985c) Effect
of repeated intravenous infusions of the plasticizer di-(2-ethylhexyl)
phthalate in young male rats. Arch. Toxicol., 58: 78-83.
SJÖBERG, P., LINDQUIST, N.G., & PLÖEN, L. (1986) Age-dependent
response of the rat testes to di(2-ethylhexyl) phthalate. Environ.
Health Perspect., 65: 237-242.
SMISTAD, G., WAALER, T., & ROKSVAAG, P.O. (1989) Migration of plastic
additives from soft polyvinyl chloride bags into normal saline and
glucose infusions. Acta pharm. Nord., 1: 287-290.
SMITH-OLIVER, T. & BUTTERWORTH, B.E. (1987) Correlation of the
carcinogenic potential of di(2-ethylhexyl)phthalate (DEHP) with
induced hyperplasia rather than with genotoxic activity. Mutat. Res.,
188: 21-28.
SODERGREN, A. (1982) Significance of interfaces in the distribution
and metabolism of di-2-ethylhexyl phthalate in an aquatic laboratory
model ecosystem. Environ. Pollut., 27: 263-274.
SRI (1985) Chemical economics handbook, Menlo Park, California, SRI
International.
SRIVASTAVA, S., AWASTHI, V.K., SRIVASTAVA, S.P., & SETH, P.K. (1989)
Biochemical alterations in rat fetal liver following in utero exposure
to di(2-ethylhexyl)phthalate (DEHP). Indian J. exp. Biol., 27:
885-888.
STANKEVICH, V.V. & ZAREMBO, O.K. (1978) [Toxicological and hygienic
characteristics of PVC materials plasticizers.] In: [Modern problems
of hygiene of PVC application], Moscow, VNIIMI, pp. 10-16 (Series
Gigiena No. 4) (in Russian).
STANKEVICH, V.V., ZAREMBO, O.K., GUMENNOY, V.S., & LEUITSKAYA, V.N.
(1984) [Present state of the regulation program relating to vinyl
chloride monomers and other additives used as polyvinyl chloride
packing material (A review)], Moscow, VNIIMI, pp. 26-36 (Series
Gigiena) (in Russian).
STANLEY, R.D. & TAPP, J.F. (1982) An assessment of ecological test
methods. VIII. The effect of nine chemicals on the growth of Avena
saliva and Brassica rapa, United Kingdom, ICI Brixham Laboratory
(Internal Report No. BL/A/2164).
STEPHENSON, R.R. (1983) Effects of water hardness, water temperature,
and size of the test organism on the susceptibility of the freshwater
shrimp, Gammarus pulex (L.), to toxicants. Bull. environ. Contam.
Toxicol., 31: 459-466.
STREUFERT, J.M., JONES, J.R., & SANDERS, H.O. (1980) Toxicity and
biological effects of phthalate esters on midges (Chironomus
plumosus). Trans. Missouri Acad. Sci., 14: 33-40.
SUGATT, R.H., O'GRADY, D.P., BANERJEE, S., HOWARD, P.H., & GLEDMILL,
W.E. (1984) Shake flask biodegradation of 14 commercial phthalate
esters. Appl. environ. Microbiol., 47: 601-606.
SWENERTON, H. & HURLEY, L.S. (1971) Teratogenic effects of a chelating
agent and their prevention by zinc. Science, 173: 62-63.
TABORSKY, R.G. (1967) Isolation studies on a lipoidal portion of the
bovine pineal gland. J. agric. food Chem., 15: 1073-1076.
TAKAGI, A., SAI, K., UMEMURA, T., KUROKAWA, Y., & KASAI, H. (1989)
Production of 8-hydroxydeoxyguanosine in rat liver DNA by oral
administration of peroxisome proliferators. Mutat. Res., 216: 379.
TAKAGI, A., SAI, K., UMEMURA, T., HASEGAWA, R., & KUROKAWA, Y. (1990)
Significant increase of 8-hydroxydeoxyguanosine in liver DNA of rats
following short-term exposure to the peroxisome proliferators di(2-
ethylhexyl)phthalate and di(2-ethylhexyl)adipate Jpn. J. Cancer Res.,
81: 213-215.
TAKAGI, A., SAI, K., UMEMURA, T., HASEGAWA, R., & KUROKAWA, Y. (1991)
Short-term exposure to the peroxisome proliferators, perfluorooctanoic
acid and perfluorodecanoic acid, causes significant increase of 8-
hydroxydeoxyguanosine in liver DNA of rats. Cancer Lett., 57: 55-60.
TAMURA, H., IIDA, T., WATANABE, T., & SUGA, T., (1990) Long-term
effects of hypolipidemic peroxisome proliferator administration on
hepatic hydrogen peroxide metabolism in rats. Carcinogenesis, 11:
445-450.
TARR, B.D., BARRON, M.G., & HAYTON, W.L. (1990) Effect of body size on
the uptake and bioconcentration of di-2-ethylhexyl phthalate in
rainbow trout. Environ. Toxicol. Chem., 9: 989-995.
TAYLOR, B.F., CURRY, R.W., & CORCORAN, E.F. (1981) Potential for
biodegradation of phthalic acid esters in marine regions. Appl.
environ. Microbiol., 42: 590-595.
TEIRLYNCK, O.A. & BELPAIRE, F. (1985) Disposition of orally
administered di-(2-ethylhexyl) phthalate and mono-(2-ethylhexyl)
phthalate in the rat. Arch. Toxicol., 57: 226-230.
THIESS, A.M. & FLIEG, I. (1978) [Chromosomal studies in workers
exposed to di-2-ethylhexylphthalate (DOP).] Zentralbl. Arbeitsmed.,
28: 351-355 (in German).
THIESS, A.M., FRENTZEL-BEYME, R., & WIELAND, R. (1978a) [Mortality
study in workers exposed to di-2-ethylhexyl phthalate (DOP.] In:
[Possibilities and limits of biological monitoring - Problems in
occupational medicine of the industrial occupational health services.
Proceedings of a conference, Frankfurt, May 1978], Stuttgart, A.W.
Gertner, pp. 155-164 (in German).
THIESS, A.M., KORTE, A., & FLIEG, H. (1978b) [Morbidity studies in
workers exposed to di(2-ethylhexyl)phthalate (DEHP).] In:
[Possibilities and limits of biological monitoring - Problems in
occupational medicine of the industrial occupational health services.
Proceedings of a conference, Frankfurt, May 1978], Stuttgart, A.W.
Gertner, pp. 137-154 (in German).
THOMAS, G.H. (1973) Quantitative determination and confirmation of
identity of trace amounts of dialkylphthalates in environmental
samples. Environ. Health Perspect., 3: 23-28.
THOMAS, J.A., SCHEIN, L.G., GUPTA, P.K., MCCAFFERTY, R.E., FELICE,
P.R., & DONOVAN, M.P. (1979) Failure of monoethylhexyl phthalate to
cause teratogenic effects in offspring of rabbits. Toxicol. appl.
Pharmacol., 51: 523-528.
THUREN, A. (1986) Determination of phthalates in aquatic environments.
Bull. environ. Contam. Toxicol., 36: 33-40.
THUREN, A. & LARSSON, P. (1990) Phthalate esters in the Swedish
atmosphere. Environ. Sci. Technol., 24: 554-559.
THUREN, A. & WOIN, P. (1991) Effects of phthalate esters on the
locomotor activity of the freshwater amphipod Gammarus pulex. Bull
environ. Contam. Toxicol., 46: 159-166.
TIMOFIEVSKAJA, L.A., IRANOVA, N.I., & BALYNINA, E.S. (1980)
[Toxicology of esters of o-phthalic acid and hygienic standard for
them.] Gig. Tr. prof. Zabol., 3: 25-28 (in Russian).
TOMASZEWSKI, K.E., AGARWAL, D.K., & MELNICK, R.L. (1986) In vitro
steady-state levels of hydrogen peroxide after exposure of male F344
rats and female B6C3F1 mice to hepatic peroxisome proliferators.
Carcinogenicity, 7: 1871-1876.
TOMITA, I., NAKAMURA, Y., AOKI, N., & INUI, N. (1982a)
Mutagenic/carcinogenic potential of DEHP and MEHP. Environ. Health
Perspect., 45: 119-125.
TOMITA, I., NAKAMURA, Y., YAGI, Y., & TUTIKAWA, K. (1982b)
Teratogenicity/fetotoxicity of DEHP in mice. Environ. Health
Perspect., 45: 71-75.
TOMITA, I., NAKAMURA, Y., YAGI, Y., & TUTIKAWA, K. (1986) Fetotoxic
effects of mono-2-ethylhexyl phthalate (MEHP) in mice. Environ. Health
Perspect., 65: 249-254.
TRIMBLE, A.S., GOLDMAN, B.S., YAO, J.K., DOVATS, L.K., & BIGELOW, W.G.
(1966) Plastics - A source of chemical contamination in surgical
research. Surgery, 59: 857-859.
TYL, R.W., PRICE, C.J., MARR, M.C., & KIMMEL, C.A. (1988)
Developmental toxicity evaluation of dietary di(2-ethylhexyl)phthalate
in Fischer 344 rats and CD-1 mice. Fundam. appl. Toxicol., 10:
395-412.
VAINIOTALO, S. & PFÄFFLI, P. (1990) Air impurities in the PVC plastics
processing industry. Ann. occup. Hyg., 34: 585-590.
VILLENEUVE, D.C., FRANKLIN, C.A., CHU, I., YAGMINAS, A., MARINO, I.A.,
RITTER, L., & RUDDICK, J.A. (1978) Toxicity studies on mono-2-
ethylhexyl phthalate. Toxicol. appl. Pharmacol., 45: 250-251.
VON DÄNIKEN, A., LUTZ, W.K., JÄCKH, R., & SCHLATTER, C. (1984)
Investigation of the potential for binding of di(2-
ethylhexyl)phthalate (DEHP) and di-(2-ethylhexyl)adipate (DEHA) to
liver DNA in vivo. Toxicol. appl. Pharmacol., 73: 373-387.
WAALKES, M.P. & WARD, J.M. (1989) Induction of hepatic metallothionein
in male B6C3F1 mice exposed to hepatic tumor promotors: effects of
phenobarbital, acetaminophen, sodium barbital, and di(2-ethylhexyl)
phthalate. Toxicol. appl. Pharmacol., 100: 216-266.
WALDOCK, M.J. (1983) Determination of phthalate esters in samples from
the marine environment using gas chromatography mass spectrometry.
Chem. Ecol., 1: 261-277.
WALSETH, F., TOFTGÅRD, R., & NILSEN, O.G. (1982) Phthalate esters I:
Effects on cytochrome P-450 mediated metabolism in rat liver and lung,
serum enzymatic activities and serum protein levels. Arch. Toxicol.,
50: 1-10.
WAMS, T.J. (1987) Diethylhexylphthalate as an environmental
contaminant - A review. Sci. total Environ., 66: 1-16.
WARD, J.M., RICE, J.M., CREASIA, D., LYNCH, P., & RIGGS. C. (1983)
Dissimilar patterns of promotion by di(2-ethylhexyl)phthalate and
phenobarbital of hepatocellular neoplasia initiated by
diethylnitrosamine in B6C3F1 mice. Carcinogenesis, 4: 1021-1029.
WARD, J.M., DIWAN, B.A., OHSHIMA, M., HU, H., SCHULLER, H.M., & RICE,
J.M. (1986) Tumor-initiating and promoting activities of di(2-
ethylhexyl) phthalate in vivo and in vitro. Environ. Health Perspect.,
65: 279-291.
WEAST, R.C., ASTLE, M.J., & BEYER, W.H., ed. (1984) CRC handbook of
chemistry and physics, 65th ed., Boca Raton, Florida, CRC Press Inc.
WEBER, K. & ERNST, W. (1983) [Occurrence and fluctuation of organic
chemicals in major German estuaries.] Vom Wasser, 61: 111-123 (in
German).
WHITE, R.D., CARTER, D.E., EARNEST, D., & MUELLER, J. (1980)
Absorption and metabolism of 3 phthalate esters by the rat small
intestine. Food Cosmet. Toxicol., 18: 383-386.
WHO/FAO (1984a) Evaluation of certain food additives and contaminants
(Twenty-eighth report of the Joint FAO/WHO Expert Committee on Food
Additives), Geneva, World Health Organization, 44 pp (WHO Technical
Report Series, No. 710, and corrigendum).
WHO/FAO (1984b) Toxicological evaluation of certain food additives and
contaminants (Prepared by the Joint FAO/WHO Expert Committee on Food
Additives, Rome, 19-28 March, 1984), Geneva, World Health
Organization, 222 pp (WHO Food Additives Series No. 19).
WHO/FAO (1989a) Evaluation of certain food additives and contaminants
(Thirty-third report of the Joint FAO/WHO Expert Committee on Food
Additives), Geneva, World Health Organization, 64 pp (WHO Technical
Report Series No. 776).
WHO/FAO (1989b) Toxicological evaluation of certain food additives and
contaminants (Prepared by the 29th Meeting of the Joint FAO/WHO Expert
Committee on Food Additives), 362 pp. Cambridge, Cambridge University
Press (WHO Food Additives Series No. 24).
WILLIAMS, D.T. (1973) Dibutyl- and di-(2-ethylhexyl) phthalate in
fish. J. agric. food Chem., 21: 1128-1129.
WOFFORD, H.W., WILSEY, C.D., NEFF, G.S., GIAM, C.S., & NEFF, J.M.
(1981) Bioaccumulation and metabolism of phthalate esters by oysters,
brown shrimp, and sheepshead minnows. Ecotoxicol. environ. Saf., 5:
202-210.
WOIN, P. & LARSSON, P. (1987) Phthalate esters reduce predation
efficiency of dragonfly larvae, Odonata; Aeshna. Bull. environ.
Contam. Toxicol., 38: 220-225.
WOLFE, N.L., BURNS, L.A., & STEEN, W.C. (1980a) Use of linear free
energy relationships and an evaluative model to assess the fate and
transport of phthalate esters in the aquatic environment. Chemosphere,
9: 393-402.
WOLFE, N.L., STEEN, W.C., & BURNS, L.A. (1980b) Phthalate ester
hydrolysis: Linear free energy relationships. Chemosphere, 9: 403-408.
WOOD, D.L. & BITMAN, J. (1984) The effect of feeding di-(2-ethylhexyl)
phthalate and related compounds on lipids in the laying hen. Poult.
Sci., 63: 469-477.
WOODWARD, K.N., SMITH, A.M., MARISCOTTI, S.P., & TOMLINSON, N.J.
(1986) Review of the toxicity of the esters of o-phthalic acid
(phthalate esters), London, Health and Safety Executive, 183 pp
(Toxicity Review 14).
YAGI, Y., NAKAMURA, Y., TOMITA, I., TSUCHIKAWA, K., & SHIMOI, N.
(1980) Teratogenic potential of di- and mono-(2-ethylhexyl) phthalate
in mice. J. environ. Pathol. Toxicol., 4: 533-544.
YANAGITA, T., KOBAYASHI, K., & ENOMOTO, N. (1978) Accumulation of
hepatic phospholipids in rats fed di(2-ethylhexyl) phthalate. Biochem.
Pharmacol., 27: 2283-2288.
YANAGITA, T., KUZUHARA, S., ENOMOTO, N., SHIMAD, T., & SUGANO, M.
(1979) Effects of di(2-ethylhexyl) phthalate on the content of hepatic
mitochondrial and microsomal phospholipids in the rat. Biochem.
Pharmacol., 28: 3115-3121.
YOON, J.S., MASON, J.M., VALENCIA, R., WOODRUFF, R.C., & ZIMMERING, S.
(1985) Chemical mutagenesis testing in Drosophila. IV. Results of 45
coded compounds tested for the National Toxicology Program. Environ.
Mutagen., 7: 349-367.
YOSHIKAWA, K., TANAKA, A., YAMAHA, T., & KURATA, H. (1983)
Mutagenicity study of nine monoalkyl phthalates and a dialkyl
phthalate using Salmonella typhimurium and Escherichia coli. Food
chem. Toxicol., 21: 221-223.
ZEIGER, E., HAWORTH, S., MORTELMANS, K., & SPECK, W. (1985)
Mutagenicity testing of di(2-ethylhexyl) phthalate and related
chemicals in Salmonella. Environ. Mutagen., 7: 213-232.
ZIMMERING, S., MASON, J.M., & VALENCIA, R. (1989) Chemical mutagenesis
testing in drosophila. VII. Results of 22 coded compounds tested in
larval feeding experiments. Environ. mol. Mutagen., 14: 245-251.
ZIOGOU, K., KIRK, P.W.W., & LESTER, J.N. (1989) Behaviour of phthalic
acid esters during batch anaerobic digestion of sludge. Water Res.,
23: 743-748.
ZITKO, V. (1972) Determination, toxicity, and environmental levels of
phthalate plasticizers, St. Andrews, N.B., Biological Station,
Fisheries Research Board of Canada, 35 pp (Technical Report No. 344).
RESUME
1. Identité, propriétés physiques et chimiques et méthodes d'analyse
Le phtalate de di(2-éthylhexyle) est un ester de l'acide
benzènedicarboxylique qui se présente, à la température ambiante, sous
la forme d'un liquide huileux, incolore à jaune. Il est peu soluble
dans l'eau (0,3-0,4 mg/litre) et encore moins dans l'eau salée. Il est
miscible à la plupart des solvants organiques courants. Sa volatilité
est relativement faible (tension de vapeur: 8,6 x 10-4Pa).
On a mis au point, pour le dosage du DEHP dans différents
milieux, un grand nombre de méthodes de prélèvement et d'analyse. On
fait de plus en plus appel à des méthodes sensibles telles que la
chromatographie en phase gazeuse, la chromatographie en phase liquide
à haute performance et la spectrométrie de masse. La contamination due
au matériel en plastique utilisé lors des prélèvements et de l'analyse
complique le dosage du DEHP à faibles concentrations.
2. Sources d'exposition humaine et environnementale
Le DEHP présent dans l'environnement est, dans sa presque
totalité, d'origine artificielle.
La production mondiale de DEHP augmente depuis quelques décennies
et s'élève actuellement à environ un million de tonnes par an. Les
Etats-Unis d'Amérique et l'Europe entrent chacun pour un tiers dans
cette production.
Le DEHP est le plastifiant le plus largement utilisé pour
augmenter la fluidité des résines puisqu'il représente 50% de
l'ensemble des esters phtaliques utilisés comme plastifiants. Il peut
représenter jusqu'à 40% en poids ou davantage de la matière plastique.
Le DEHP est utilisé dans la fabrication du chlorure de polyvinyle
(PVC) utilisé dans l'industrie du bâtiment et de l'emballage ainsi que
pour la fabrication d'accessoires à usage médical. On l'utilise
également en plus petites quantités pour la préparation de peintures
industrielles ainsi que comme diélectrique dans les condensateurs.
Les produits contenant du plastique qui sont mis au rebut peuvent
être éliminés soit par incinération soit par enfouissement dans une
décharge. Lors de l'incinération à basse température, une proportion
importante du produit peut se dissiper dans l'atmosphère. On n'a pas
très bien étudié ce qu'il advient du DEHP déposé dans les décharges et
il n'est pas possible à ce stade de tirer des conclusions définitives.
3. Transport, distribution, et transformation dans l'environnement
C'est principalement en étant transportés dans l'atmosphère que
les phtalates pénètrent dans l'environnement. A partir de là, le DEHP
tombe sur le sol à moins d'être éliminé par les précipitations.
Le coefficient de partage du DEHP entre l'octanol et l'eau est
élevé, de sorte que l'équilibre entre l'eau et un sol ou un sédiment
riche en substances organiques s'établit aux dépens de la phase
aqueuse. Le DEHP s'adsorbe facilement sur les particules organiques et
du sol.
Bien que peu soluble dans l'eau, le DEHP peut se rencontrer en
quantités relativement élevées dans les eaux de surface en raison de
son adsorption sur les particules organiques et de son interaction
avec les substances organiques en solution. L'adsorption s'effectue
plus particulièrement sur les particules de petite taille et elle est
favorisée par la présence d'eau salée.
Dans l'atmosphère, le DEHP subit une photodégradation rapide mais
son hydrolyse dans l'environnement est pratiquement inexistante.
Plusieurs microorganismes terricoles effectuent la dégradation
aérobie du DEHP. Toutefois dans l'environnement, cette dégradation
microbienne s'effectue avec lenteur. La biodégradation commence par
l'hydrolyse du mono-ester qui est transformé en acide phtalique. Il y
a ensuite dégradation en pyruvate et succinate par ouverture du cycle
puis en CO2 et H2O, comme dans la dégradation métabolique de
l'acide benzoïque. La dégradation aérobie dépend de la température. Au
dessous de 10 °C la dégradation est peu importante. A température
supérieure, la biodégradation se produit dans la partie supérieure du
sol mais, à plus grande profondeur elle est pratiquement inexistante
du fait des conditions d'anaérobiose. La dégradation anaérobie, pour
autant qu'elle existe, est beaucoup plus lente que la dégradation
aérobie.
Le DEHP est très lipophile et modérément persistant. Le degré de
bioaccumulation dépend de la capacité de l'organisme qui l'absorbe à
métaboliser la substance. On a montré qu'il s'accumulait en forte
proportion chez divers invertébrés aquatiques, les poissons et les
amphibiens.
Après application de DEHP sur des feuilles de végétaux, on n'a
constaté qu'une faible perte de matière sur une période de 15 jours.
Le DEHP présent dans le sol ou dans les boues d'égout n'est que peu
fixé par les plantes.
4. Concentrations dans l'environnement et exposition humaine
Le DEHP est très répandu dans l'environnement et on le retrouve
dans la plupart des échantillons prélevés dans l'air, dans les
précipitations, l'eau, les sédiments, le sol et les biotes. C'est
généralement dans les zones industrielles que les teneurs sont les
plus fortes.
On a relevé des concentrations allant jusqu'à 300 ng/m3 dans
l'air des villes et en atmosphère polluée. Dans l'atmosphère
surmontant les océans, on a fait état de concentrations allant de 0,5
à 5 ng/m3 et les précipitations de ces régions en contenaient
jusqu'à 200 ng/litre. L'étude d'échantillons de précipitations
prélevés dans un secteur situé à proximité d'une usine produisant des
plastifiants, a montré que la vitesse de dépot à sec était de 0,7 à
4,7 µg/m2 et par jour.
Dans les rivières et les lacs, on a trouvé des concentrations de
DEHP allant jusqu'à 4 µg/litre, les teneurs les plus élevées étant
relevées au point de décharge d'effluents industriels. Dans la mer, la
concentration est inférieure à 1 µg/litre, les concentrations les plus
fortes étant observées dans les estuaires.
En raison de son caractère hydrophobe, le DEHP est facilement
absorbé par le sol, les sédiments et les particules. Dans les
sédiments de cours d'eau, des concentrations allant jusqu'à 70 mg/kg
(de poids à sec) ont été signalées, concentrations qui peuvent monter
jusqu'à 1480 mg/kg (de poids à sec) au voisinage des points de
décharge.
Dans les biotes, la concentration du DEHP varie de moins de 1 à
7000 µg/kg. On le rencontre dans divers types d'aliments: poisson,
coquillages, oeufs et fromages. L'exposition moyenne a été estimée à
environ 300 µg par personne et par jour aux Etats-Unis d'Amérique en
1974 et à 20 µg par personne et par jour au Royaume-Uni en 1986.
Les transfusions de sang et autres traitements utilisant du
matériel en plastique peuvent entraîner l'exposition involontaire des
malades au DEHP. C'est ainsi qu'on a relevé dans le poumon de malades
ainsi traités, des teneurs allant de 13,4 à 91,5 mg/kg de poids à sec.
Les quelques données disponibles indiquent que sur les lieux de
travail, la concentration de DEHP est généralement inférieure à 1
mg/m3.
5. Cinétique et métabolisme
Des données disponibles au sujet de l'administration par voie
orale indiquent que le DEHP est hydrolysé dans l'intestin par la
lipase pancréatique. Les métabolites qui se forment, c'est-à-dire le
phtalate de mono-2-éthylhexyle (MEHP) et le 2-éthylhexanol sont
rapidement absorbés. Après administration de DEHP marqué au 14C
(2,9 mg/kg) par voie orale à des rats, on a récupéré 50% de la dose
initiale dans l'urine et la bile de ces animaux. Il semble que la
biodisponibilité d'une dose orale de DEHP soit plus élevée chez les
jeunes animaux.
Après administration par voie orale, le DEHP est largement
hydrolysé dans l'intestin de certains animaux, par exemple les rats,
et se répartit dans l'organisme, principalement sous forme de phtalate
de monoéthylhexyle (MEHP). Toutefois chez les primates et l'homme,
l'hydrolyse est beaucoup moins importante. Il semble que ce soit
principalement au niveau du foie que s'effectue la métabolisation du
MEHP et du 2-éthylhexanol. On a identifié plusieurs autres
métabolites, l'omega et omega-1-oxydation étant les principales voies
métaboliques. Un ou plusieurs des produits résultant de l'omega-
oxydation peuvent encore être métabolisés par ß-oxydation. On a
constaté que l'omega-oxydation s'effectuait selon une cinétique non
linéaire. Le métabolisme du DEHP offre des différences considérables
selon les espèces; par exemple, l'omega-oxydation est moins importante
chez l'homme que chez le rat.
Une dose de DEHP de 2,9 mg/kg, administrée par voie orale à des
rats, a été récupérée une semaine plus tard presque intégralement dans
les matières fécales et l'urine des animaux. La bile et l'urine
constituent les principales voies d'excrétion. Lors d'une étude sur
l'homme, 15 à 25% d'une dose orale (0,45 mg/kg de DEHP) ont été
excrétés sous forme de MEHP et la majeure partie des produits
d'excrétion étaient constitués de métabolites oxydés.
6. Effets sur les mammifères de laboratoire et les systèmes
d'épreuve in vitro
La DL50 par voie orale du DEHP est d'environ 25 à 34 g/kg,
selon l'espèce, mais cette valeur est plus faible dans le cas du MEHP.
Lors d'études d'alimentation sur des rats et des souris, on a constaté
que des doses quotidiennes de DEHP supérieures à 3 g/kg entraînaient
la mort dans les 90 jours et qu'une dose de 0,4 g/kg réduisait le gain
de poids en l'espace de quelques jours. Dans d'autres études, c'est
une dose de 6,3-12,5 g/kg de nourriture qui a entraîné une réduction
du poids corporel.
Lors d'études à long terme, on a observé chez les animaux traités
une hépatomégalie accompagnée d'une augmentation du poids relatif des
reins. Dans l'une de ces études, on observait également une
hypertrophie cellulaire au niveau du lobe antérieur de l'hypophyse.
Un certain nombre d'études ont permis de mettre en évidence une
atrophie testiculaire, apparaîssant au bout de quelques jours, et liée
à l'administration de DEHP (doses dans l'alimentation allant de
10-20 g/kg). Les jeunes rats semblent plus vulnérables et les rats et
les souris plus sensibles que les hamsters et les ouistitis. On a
constaté que cette atrophie était réversible. Le MEHP exerce des
effets toxiques in vitro sur les cellules de Sertoli. Le DEHP de
même que le MEHP ont des propriétés tératogènes. Des malformations ont
été observées à des doses de 0,5-2 g/kg de nourriture chez la souris
et, à des doses supérieures à 10 g/kg, on a observé des effets
embryotoxiques.
Les tests de mutagénicité et autres manifestations du même genre
ont donné des résultats négatifs dans la plupart des études. Le DEHP
peut provoquer la transformation des cellules et on a montré qu'il
était cancérogène à des doses respectives de 6 et 12 g/kg de
nourriture chez le rat et de 3 et 6 g/kg de nourriture chez la souris.
On a constaté une augmentation, liée à la dose, des tumeurs
hépatocellulaires chez les deux sexes de l'une et l'autre espèce. La
prolifération des peroxysomes hépatiques et la réplication cellulaire
sont fortement liées aux effets cancérogènes sur le foie de certains
produits non génotoxiques comme le DEHP. Toutefois on a observé des
différences importantes entre les espèces animales en ce qui concerne
la prolifération des peroxysomes induite par le DEHP.
Contrairement à ce qui se passe dans le cas des hépatocytes de
rat, les métabolites du DEHP ne provoquent pas de prolifération des
peroxysomes dans les cultures d'hépatocytes humains.
7. Effets sur l'homme
On ne dispose que de données très limitées sur les effets que le
DEHP peut exercer sur l'homme. On a fait état chez deux sujets qui
avaient reçu 5 ou 10 g de DEHP respectivement, d'effets qui se
limitaient à de légers troubles gastriques.
8. Effets sur les autres êtres vivants en laboratoire et dans
leur milieu naturel
Dans la plupart des études, les valeurs nominales de la CL50
obtenues lors des tests de toxicité aiguë dépassent 10 mg/litre, ce
qui indique que le DEHP est peu toxique. Toutefois ces valeurs sont
supérieures à la solubilité du DEHP dans l'eau (0,3-0,4 mg/litre).
D'après une étude, une daphnie, Daphnia pulex, serait
particulièrement sensible, la CL50 à 48 heures étant dans son cas de
0,133 mg/litre. La seule épreuve de toxicité aiguë où l'on ait mesuré
des concentrations de DEHP a été effectuée sur un poisson, Pimephales
promelas, et elle a donné une CL50 à 96 heures supérieure à 0,33
mg/litre. Lors d'études de longue durée, on a obtenu une dose sans
effet nocif observable de 72 µg/litre pour Daphnia magna. Pour les
poissons adultes, cette dose était supérieure à 72 µg/litre. Une
exposition à une concentration de 14 µg/litre, pendant 12 jours avant
l'éclosion, a provoqué une importante augmentation de la mortalité
parmi des alevins de truites. Des concentrations comprises en 3,7 et
11 µg/litre ont entraîné une réduction de la teneur en collagène
vertébral chez les poissons.
La présence de DEHP dans la nourriture à raison de 50 mg/kg a eu
des effets nocifs sur la survie des alevins de Melambaphes zebra. A
la concentration de 25 mg/kg en poids dans les sédiments, on a
constaté une réduction de l'activité microbienne et du nombre d'
éclosions chez les tétards.
Le DEHP est peu toxique pour les algues, les végétaux, les
lombrics et les oiseaux.
9. Evaluation
Le DEHP exerce des effets cancérogènes sur le foie et affecte le
système reproducteur chez le rat et la souris.
Le principal effet au niveau des gonades consiste, chez le rat et
la souris, en une atrophie des testicules qui affecte davantage les
jeunes animaux. La prolifération des peroxysomes hépatiques et la
réplication cellulaire sont fortement liées à l'effet cancérogène
qu'exercent sur le foie certains produits non génotoxiques au nombre
desquels figure le DEHP. Toutefois, on a observé de fortes différences
selon les espèces animales pour ce qui est de la prolifération des
peroxysomes induite par le DEHP. On ne possède pas actuellement de
preuves suffisantes permettant de conclure que le DEHP est
potentiellement cancérogène pour l'homme.
Il n'existe pas de données attestées selon lesquelles le DEHP
serait dangereux pour la faune, si l'on s'en tient aux résultats
obtenus lors de l'exposition de poissons et de daphnies. Toutefois, on
a observé une réduction de l'activité microbienne dans les sédiments
aux doses auxquelles le DEHP est présent dans l'environnement. Si l'on
compare les doses de DEHP présentes dans l'environnement aux
concentrations qui sont susceptibles de produire des effets lors
d'études de longue durée, notamment sur les larves de poissons et
d'amphibiens, on ne peut exclure l'existence d'un risque pour
l'environnement, les effets s'exerçant notamment par l'intermédiaire
de l'eau et des sédiments. Des effets nocifs sur les êtres vivants
sont possibles dans les secteurs où l'eau et les sédiments sont
fortement pollués en raison de leur proximité des points de décharge.
Bien que peu d'études valables aient été effectuées, il semble
que le DEHP présente une faible toxicité aiguë pour les algues, les
végétaux, les lombrics et les oiseaux.
RESUMEN
1. Identidad, propiedades físicas y químicas y métodos analíticos
El di(2-etilhexil) ftalato (DEHF) es un éster del ácido
bencenodicarboxílico que a temperatura ambiente es un líquido oleoso
entre incoloro y amarillo. Su solubilidad en agua es baja (0,3-0,4
mg/litro) y aún más baja en agua salada. Es miscible con la mayor
parte de los disolventes orgánicos normales. La volatilidad del DEHF
es relativamente baja (8,6 x10-4 Pa).
Se han perfeccionado numerosos métodos de muestreo y análisis
para la determinación del DEHF en diferentes medios. Cada vez se
utilizan más métodos de gran sensibilidad, como la cromatografía de
gases, la cromatografía líquida de alto rendimiento y la
espectrometría de masas. El análisis de concentraciones bajas de DEHF
se complica por la contaminación debida al material de plástico
utilizado en el muestreo y el análisis.
2. Fuentes de exposición humana y ambiental
Casi todo el DEHF presente en el medio ambiente procede de
fuentes antropogénicas.
La producción mundial de DEHF ha ido aumentando durante los
últimos decenios y en la actualidad es de alrededor de 1 x 106
toneladas al año. Un tercio del total se produce en los Estados Unidos
y otro tercio en Europa.
El DEHF es el plastificante más utilizado (representa el 50% de
todos los plastificantes a base de ésteres de ftalato) para ablandar
las resinas. A él corresponde el 40% (p/p) o más del total de
plásticos. El DEHF se emplea en la fabricación de cloruro de
polivinilo (PVC) utilizado en los edificios, en la construcción, en
embalajes y en componentes de aparatos médicos. Se utiliza en
cantidades más pequeñas en las pinturas industriales y como fluido
dieléctrico en los condensadores.
Los productos plastificados de desecho se pueden eliminar por
incineración o terraplenado. Durante la incineración a baja
temperatura, se puede liberar un porcentaje elevado del DEHF a la
atmósfera. No se ha estudiado bien el destino medioambiental del DEHF
depositado en terraplenes, por lo que no se pueden sacar conclusiones
definitivas.
3. Transporte, distribución y transformación en el medio ambiente
El transporte en el aire es la vía más importante de
incorporación de los ftalatos al medio ambiente. De la atmósfera, el
DEHF precipita o es lavado por la lluvia.
El DEHF tiene un coeficiente de reparto octanol-agua elevado, de
manera que el equilibrio entre el agua y un suelo o sedimento rico en
materia orgánica está desplazado a favor de este último. Las
partículas orgánicas del suelo lo adsorben fácilmente.
Aunque la solubilidad del DEHF en agua es baja, la cantidad
presente en las aguas de superficie puede ser mayor debido a la
adsorción a las partículas orgánicas y a la interacción con la materia
orgánica disuelta. Las pequeñas partículas lo adsorben más fácilmente,
y el proceso se potencia en el agua salada.
La fotodegradación atmosférica del DEHF es rápida; en cambio, su
hidrólisis química en el medio ambiente es prácticamente inexistente.
Se ha observado que varios microorganismos del suelo llevan a
cabo la degradación aerobia. Sin embargo, se ha informado que la
degradación microbiana del DEHF en el medio ambiente es lenta. El
proceso de biodegradación comienza con la hidrólisis para formar el
monoéster, que se transforma luego en ácido ftálico. La degradación
con ruptura del anillo para dar primero piruvato y succinato y después
CO2 y H2O es análoga a la vía metabólica del ácido benzoico. La
degradación aerobia es dependiente de la temperatura. Por debajo de
los 10 °C es escasa. A temperaturas más altas se produce
biodegradación en la capa más superficial del suelo, pero es
prácticamente inexistente en las capas más profundas, donde las
condiciones son de anaerobiosis. La degradación anaerobia, si existe,
es mucho más lenta que la aerobia.
El DEHF es muy lipófilo y moderadamente persistente. El grado de
bioacumulación depende de la capacidad de los organismos para
metabolizarlo. Se ha observado un grado elevado de acumulación en
diversos invertebrados acuáticos, peces y anfibios.
Cuando se aplicó DEHF a hojas de plantas, la pérdida fue pequeña
durante un período de 15 días. Se ha observado que su absorción por
las plantas a partir del suelo o de los fangos cloacales es muy baja.
4. Niveles medioambientales y exposición humana
El DEHF está muy ampliamente distribuido en la naturaleza y se
encuentra en la mayor parte de las muestras, por ejemplo de aire,
precipitación, sedimento, suelo y biota. Las concentraciones más altas
aparecen generalmente en las zonas industrializadas.
En el aire urbano y contaminado se han encontrado concentraciones
de hasta 300 ng/m3. Se han comunicado de niveles comprendidos entre
0,5 y 5 ng/m3 en el aire de zonas oceánicas, y la lluvia de esas
zonas contenía hasta alrededor de 200 ng/litro. En muestras de
precipitación procedentes de una zona cercana a una fábrica de
plastificantes se observó que la tasa sedimentación seca era de 0,7 a
4,7 µg/m2 al día.
Se han encontrado concentraciones de DEHF en ríos y lagos de
hasta 4 µg/litro; los niveles más altos están asociados a los puntos
de descarga de efluentes industriales. La concentración en el mar es
inferior a 1 µg/litro, y los niveles más altos se detectan en los
estuarios.
Debido a su carácter hidrófobo, el DEHF se adsorbe muy fácilmente
al suelo, los sedimentos y la materia particulada. En los sedimentos
fluviales se han comunicado niveles de hasta 70 mg/kg (peso seco), y
cerca de los puntos de descarga ha llegado a ser de 1480 mg/kg (peso
seco).
La concentración de DEHF en la biota oscila entre menos de 1 y
7000 µg/kg. Se ha detectado en diversos tipos de alimentos, como
pescado, marisco, huevos y queso. En 1974 se estimó en los Estados
Unidos una exposición media de 300 µg/persona al día y en el Reino
Unido en 1986 de 20 µg/persona al día.
Las transfusiones de sangre y otros tipos de tratamiento médico
en los que se utilizan aparatos de plástico pueden dar lugar a una
exposición humana involuntaria al DEHF. En el tejido pulmonar de
algunos pacientes se han detectado concentraciones comprendidas entre
13,4 y 91,5 µg/kg (peso seco).
Los escasos datos disponibles indican que las concentraciones de
DEHF en el lugar de trabajo suelen estar por debajo de los 1 mg/m3.
5. Cinética y metabolismo
Los datos disponibles sobre la administración oral indican que la
lipasa pancreática hidroliza el DEHF en el intestino. Los metabolitos
formados, que son el mono (2-etilhexil) ftalato (MEHF) y el
2-etilhexanol, se absorben rápidamente. Cuando se administró por vía
oral a ratas DEHF marcado con 14C (2,9 mg/kg), se recuperó en la
orina o la bilis más del 50%. La biodisponibilidad de una dosis oral
de DEHF parece ser más elevada en las ratas jóvenes que en las más
viejas.
El DEHF administrado por vía oral se hidroliza en el intestino de
ciertos animales, por ejemplo las ratas, y se distribuye sobre todo
como monoetilhexil ftalato (MEHF). Sin embargo, la hidrólisis es mucho
menor en los primates y el ser humano. El MEHF se liga a las proteínas
del plasma. El hígado parece ser el principal órgano metabolizador del
MEHF y del 2-etilhexanol. Se han identificado algunos otros
metabolitos, siendo las principales vías metabólicas la omega-y la
omega-1-oxidación. Uno o varios productos de la omega-oxidación pueden
metabolizarse ulteriormente por ß-oxidación. En la omega-oxidación se
han observado cinéticas no lineales. En el metabolismo del DEHF se
registran considerables diferencias entre las especies; por ejemplo,
la omega-oxidación es menor en el hombre que en las ratas.
Una semana después de administrar una dosis oral de DEHF
(2,9 mg/kg) se recuperó casi el 100% en las heces y la orina de ratas.
La bilis y la orina son las principales vías de excreción. En un
estudio humano, del 15 al 25% de la dosis oral (0,45 mg/kg) de DEHF se
excretó en forma de MEHF; los metabolitos oxidados constituían la
parte más importante de los productos de excreción.
6. Efectos en los mamíferos de laboratorio y en sistemas de
ensayo in vitro
La DL50 del DEHF por vía oral es de alrededor de 25-34 g/kg,
según las especies, pero el valor para el MEHF es más bajo. En
estudios de alimentación en ratas y ratones, las dosis de DEHF
superiores a 3 g/kg al día causaron muertes en un plazo de 90 días, y
con una concentración de 0,4 g/kg al día se redujo el aumento de peso
a los pocos días. En otros estudios, con dosis de 6,3-12,5 g/kg de
alimentos se produjo una reducción del peso corporal.
En los animales tratados en estudios de larga duración se ha
observado hepatomegalia y un aumento del peso relativo de los riñones.
En un estudio se encontraron también células hipertróficas en el
lóbulo anterior de la hipófisis.
En varios estudios se ha detectado atrofia testicular, evidente
al cabo de pocos días, relacionada con la administración de DEHF
(concentraciones de 10-20 g de DEHF/kg de alimentos). Las ratas más
jóvenes parecen ser más susceptibles que las viejas, y las ratas y los
ratones parecen ser más sensibles que los titís y los hámsters. Se ha
observado que la atrofia es reversible. El MEHF tiene efecto tóxico
in vitro en las células de Sertoli. Tanto el DEHF como el MEHF
muestran propiedades teratogénicas. Con concentraciones de 0,5 a 2
g/kg de alimentos se observaron malformaciones en ratones, y con
niveles superiores a los 10 g/kg se apreciaron efectos embriotóxicos.
Las pruebas de mutagenicidad y puntos finales asociados han dado
resultados negativos en la mayor parte de los estudios. El DEHF puede
inducir la transformación celular y se ha demostrado que con dosis de
6 y 12 g/kg de alimentos en ratas y de 3 y 6 g/kg de alimentos en
ratones tiene efectos carcinógenos. En ambos sexos de las dos especies
se produjo un aumento de tumores hepatocelulares dependiente de la
dosis. La inducción de la proliferación de peroxisomas hepáticos y de
la replicación celular está fuertemente vinculada al efecto
carcinógeno en el hígado de determinados compuestos carcinógenos no
genotóxicos, entre los que figura el DEHF. Sin embargo, se han
observado notables diferencias entre distintas especies animales con
respecto a la proliferación de peroxisomas inducida por el DEHF.
A diferencia de lo que ocurre con los hepatocitos de rata, los
metabolitos del DEHF no inducen proliferación de peroxisomas en
cultivos de hepatocitos humanos.
7. Efectos en el ser humano
La información disponible sobre los efectos del DEHF en el ser
humano es muy escasa. Se han comunicado dos casos de trastornos
gástricos leves, con dosis de 5 y 10 g de DEHF, pero sin ningún otro
efecto nocivo.
8. Efectos en otros organismos en el laboratorio y en el
medio ambiente
En la mayoría de los estudios de toxicidad aguda se han obtenido
unos valores nominales para la CL50 que se sitúan por encima de los
10 mg/litro, por lo que el índice de toxicidad del DEHF es bajo. Sin
embargo, esos niveles son superiores a los correspondientes a su
solubilidad en agua (0,3-0,4 mg/litro). En un estudio se detectó en la
pulga de agua Daphnia pulex una sensibilidad mayor, con un valor
nominal de la CL50 a las 48 h de 0,133 mg/litro. La única prueba de
la toxicidad aguda realizada con concentraciones medidas de DEHF se
hizo en el pez Pimephales promelas y puso de manifiesto una CL50
> 0,33 mg/litro a las 96 h. En estudios prolongados, el nivel sin
observación de efectos (NOEL) en Daphnia magna fue de 72 µg/litro.
En peces adultos se determinó un NOEL > 62 µg/litro. Una exposición
a 14 µg/litro desde 12 días antes de la eclosión produjo un
significativo aumento de la mortalidad de los alevines de trucha. Las
concentraciones entre 3,7 y 11 µg/litro provocaron una reducción del
colágeno vertebral en peces.
Con concentraciones de 50 mg/kg de alimentos, el DEHF tiene
efectos adversos sobre la supervivencia de los alevines de
Brachydanio rerio. Concentraciones de 25 mg/kg (p/p) en el sedimento
redujeron de manera considerable la actividad microbiana y el número
de renacuajos que nacían.
La toxicidad aguda del DEHF en algas, plantas, lombrices de
tierra y aves es baja.
9. Evaluación
El DEHF tiene efectos reproductivos y hepatocarcinogénicos en
ratas y ratones.
El principal efecto sobre la reproducción en ratas y ratones es
la atrofia testicular; los animales jóvenes son más susceptibles que
los viejos. La inducción de la proliferación de peroxisomas hepáticos
y de la replicación celular está estrechamente relacionada con el
efecto carcinogénico en el hígado de determinados compuestos
carcinógenos no genotóxicos, incluido el DEHF. Sin embargo, se han
observado notables diferencias entre las distintas especies de
animales con respecto a la proliferación de peroxisomas inducida por
el DEHF. En la actualidad no hay pruebas suficientes que indiquen que
el DEHF sea un posible carcinógeno para el ser humano.
No hay información documentada de que el DEHF constituya un
riesgo, tomando como base la exposición aguda de peces y dáfnidos. Sin
embargo, se ha informado de una reducción de la actividad microbiana
en los sedimentos con los niveles de DEHF presentes en el medio
ambiente. La comparación entre los niveles medioambientales y las
concentraciones que producen efectos en estudios prolongados,
especialmente las pruebas en las fases vitales tempranas de peces y
anfibios, indica que no se puede descartar un cierto peligro para el
medio ambiente, sobre todo por conducto del agua y los sedimentos. Es
probable que se produzcan efectos adversos en los organismos en zonas
con agua y sedimentos muy contaminados que están próximos a las
fuentes de emisión.
Aunque son pocos los estudios que se conocen al respecto, la
toxicidad aguda del DEHF en algas, plantas, lombrices de tierra y aves
parece ser baja.
