
INTERNATIONAL PROGRAMME ON CHEMICAL SAFETY
ENVIRONMENTAL HEALTH CRITERIA 129
ISOBENZAN
This report contains the collective views of an international group of
experts and does not necessarily represent the decisions or the stated
policy of the United Nations Environment Programme, the International
Labour Organisation, or the World Health Organization.
Published under the joint sponsorship of
the United Nations Environment Programme,
the International Labour Organisation,
and the World Health Organization
First draft prepared by Dr E.A.H. van Heemstra-Lequin
and Dr G.J. van Esch, Netherlands
World Health Orgnization
Geneva, 1992
The International Programme on Chemical Safety (IPCS) is a
joint venture of the United Nations Environment Programme, the
International Labour Organisation, and the World Health
Organization. The main objective of the IPCS is to carry out and
disseminate evaluations of the effects of chemicals on human health
and the quality of the environment. Supporting activities include
the development of epidemiological, experimental laboratory, and
risk-assessment methods that could produce internationally
comparable results, and the development of manpower in the field of
toxicology. Other activities carried out by the IPCS include the
development of know-how for coping with chemical accidents,
coordination of laboratory testing and epidemiological studies, and
promotion of research on the mechanisms of the biological action of
chemicals.
WHO Library Cataloguing in Publication Data
Isobenzan.
(Environmental health criteria ; 129)
1.Insecticides, Organochlorine - toxicity 2.Environmental exposure
I.Series
ISBN 92 4 157129 2 (NLM Classification: WA 240)
ISSN 0250-863X
The World Health Organization welcomes requests for permission
to reproduce or translate its publications, in part or in full.
Applications and enquiries should be addressed to the Office of
Publications, World Health Organization, Geneva, Switzerland, which
will be glad to provide the latest information on any changes made
to the text, plans for new editions, and reprints and translations
already available.
(c) World Health Organization 1991
Publications of the World Health Organization enjoy copyright
protection in accordance with the provisions of Protocol 2 of the
Universal Copyright Convention. All rights reserved.
The designations employed and the presentation of the material
in this publication do not imply the expression of any opinion
whatsoever on the part of the Secretariat of the World Health
Organization concerning the legal status of any country, territory,
city or area or of its authorities, or concerning the delimitation
of its frontiers or boundaries.
The mention of specific companies or of certain manufacturers'
products does not imply that they are endorsed or recommended by the
World Health Organization in preference to others of a similar
nature that are not mentioned. Errors and omissions excepted, the
names of proprietary products are distinguished by initial capital
letters.
CONTENTS
ENVIRONMENTAL HEALTH CRITERIA FOR ISOBENZAN
1. SUMMARY AND EVALUATION; CONCLUSIONS AND RECOMMENDATIONS
1.1. Summary and evaluation
1.2. Conclusions and recommendations
2. IDENTITY, PHYSICAL AND CHEMICAL PROPERTIES, ANALYTICAL METHODS
2.1. Identity
2.2. Physical and chemical properties
2.3. Conversion factors
2.4. Analytical methods
3. SOURCES OF HUMAN AND ENVIRONMENTAL EXPOSURE
4. ENVIRONMENTAL TRANSPORT, DISTRIBUTION, AND TRANSFORMATION
4.1. Transport and distribution between media
4.2. Biotransformation
4.2.1. Soil
4.2.2. Water
5. ENVIRONMENTAL LEVELS AND HUMAN EXPOSURE
5.1. Environmental levels
5.1.1. Water
5.1.2. Soil
5.1.3. Food
5.1.3.1 Plant products
5.1.3.2 Products of domestic animals
5.1.3.3 Market surveys
5.1.4. Terrestrial and aquatic organisms
5.2. General population exposure
5.3. Occupational exposure
6. KINETICS AND METABOLISM
6.1. Absorption
6.2. Distribution
6.2.1. Rat
6.2.2. Dog
6.2.3. Domestic fowl
6.2.4. Cow
6.3. Metabolic transformation
6.3.1. Vertebrates
6.3.2. Invertebrates
6.3.3. Microorganisms
6.4. Elimination and excretion in expired air,
faeces, and urine
6.4.1. Oral administration
6.4.2. Parenteral administration
6.5. Retention and turnover
7. EFFECTS ON LABORATORY MAMMALS AND IN VITRO TEST SYSTEMS
7.1. Single exposure
7.1.1. Oral administration
7.1.2. Dermal administration
7.1.3. Parenteral administration
7.1.4. Formulated material
7.1.5. Metabolites
7.2. Short-term exposure
7.2.1. Oral administration
7.2.1.1 Mouse
7.2.1.2 Rat
7.2.1.3 Dog
7.2.2. Dermal administration
7.2.3. Intraperitoneal administration
7.3. Long-term exposure
7.3.1. Rat
7.4. Skin irritation
7.5. Reproductive toxicity, embryotoxicity, and
teratogenicity
7.5.1. Mouse
7.5.2. Rat
7.5.3. Dog
7.6. Mutagenicity and related end-points
7.7. Carcinogenicity
7.7.1. Mouse
7.7.2. Rat
7.8. Special studies
7.8.1. Biochemical studies
7.8.2. Neurotoxicity
7.8.3. Pharmacological studies
8. EFFECTS ON HUMANS
8.1. General population exposure
8.2. Occupational exposure
9. EFFECTS ON OTHER ORGANISMS IN THE LABORATORY AND FIELD
9.1. Microorganisms
9.2. Aquatic organisms
9.3. Terrestrial organisms
9.3.1. Soil invertebrates
9.3.2. Birds
9.3.2.1 Acute toxicity
9.3.2.2 Short-term toxicity
9.4. Population and ecosystem effects
9.4.1. Soil microorganisms
9.4.2. Soil invertebrates
REFERENCES
RESUME ET EVALUATION; CONCLUSIONS ET RECOMMANDATIONS
RESUMEN Y EVALUACION; CONCLUSIONES Y RECOMENDACIONES
WHO TASK GROUP ON ENVIRONMENTAL HEALTH CRITERIA FOR ISOBENZAN
Members
Dr L.A. Albert, Xalapa, Veracruz, Mexico
Dr V. Benes, Department of Toxicology and Reference Laboratory,
Institute of Hygiene and Epidemiology, Prague, Czechoslovakia
Dr S. Dobson, Institute of Terrestrial Ecology, Monks Wood
Experimental Station, Huntingdon, United Kingdom
Dr S.K. Kashyap, National Institute of Occupational Health,
Ahmedabad, India
Dr Y.I. Kundiev, Research Institute of Labour Hygiene and
Occupational Diseases, Kiev, USSR (Vice-Chairman)
Dr Y. Osman, Ministry of Health, Riyadh, Saudi Arabia
Dr H. Spencer, Office of Pesticides Programs, US Environmental
Protection Agency, Washington, D.C., USA (Chairman)
Dr G.J. van Esch, Bilthoven, Netherlands (Joint Rapporteur)
Dr E.A.H. van Heemstra-Lequin, Laren, Netherlands
(Joint Rapporteur)
Dr C. Winder, National Institute of Occupational Health and
Safety, Forest Lodge, New South Wales, Australia
Secretariat
Dr K.W. Jager, International Programme on Chemical Safety, World
Health Organization, Geneva, Switzerland (Secretary)
Ms B. Labarthe, International Register of Potentially Toxic
Chemicals, United Nations Environment Programme, Geneva,
Switzerland
Dr T.K. Ng, Office of Occupational Health, World Health
Organization, Geneva, Switzerland
NOTE TO READERS OF THE CRITERIA DOCUMENTS
Every effort has been made to present information in the
criteria documents as accurately as possible without unduly
delaying their publication. In the interest of all users of the
environmental health criteria documents, readers are kindly
requested to communicate any errors that may have occurred to the
Manager of the International Programme on Chemical Safety, World
Health Organization, Geneva, Switzerland, in order that they may be
included in corrigenda.
* * *
A detailed data profile and a legal file can be obtained from
the International Register of Potentially Toxic Chemicals, Palais
des Nations, 1211 Geneva 10, Switzerland (Telephone No. 7988400 or
7985850).
ENVIRONMENTAL HEALTH CRITERIA FOR ISOBENZAN
A WHO Task Group on Environmental Health Criteria for
Isobenzan met at the World Health Organization, Geneva, from 23 to
27 July 1990. Dr K.W. Jager welcomed the participants on behalf of
Dr M. Mercier, Manager of the IPCS, and the three IPCS cooperating
organizations (UNEP/ILO/WHO). The Task Group reviewed and revised
the draft document and made an evaluation of the risks for human
health and the environment from exposure to isobenzan.
The first draft of this document was prepared in cooperation
between Dr E.A.H. van Heemstra-Lequin and Dr G.J. van Esch of the
Netherlands. Dr van Esch prepared the second draft, incorporating
the comments received following circulation of the first draft to
the IPCS contact points for Environmental Health Criteria
documents. Dr K.W. Jager and Dr P.G. Jenkins, both members of the
IPCS Central Unit, were responsible for the scientific content and
technical editing, respectively.
The assistance of Shell in making available to the IPCS and the
Task Group its proprietary toxicological information on its products
is gratefully acknowledge. This allowed the Task Group to make their
evaluation on the basis of more complete data.
The efforts of all who helped in the preparation and
finalization of the document are gratefully acknowledged.
1. SUMMARY AND EVALUATION; CONCLUSIONS AND RECOMMENDATIONS
1.1 Summary and evaluation
As far as is known, isobenzan, an organochlorine insecticide,
was only manufactured during the period 1958-1965. It was used from
existing stocks for several years thereafter. At present, the only
major sources of exposure are believed to be the original
waste-disposal sites of industrial wastes or dredgings from
contaminated sediments.
After isobenzan is applied to soil, a rapid initial loss
occurs, after which the remaining compound decays at a much slower
rate. It persists in soil from 2 to 7 years depending on the type of
soil. Under laboratory conditions isobenzan decomposes in surface
water within a few weeks when exposed to natural or artificial
light.
Soil, ground water, and surface water from polders built up
using sediment contaminated with organochlorines, including
chlorinated cyclodiene compounds, still contained minor residues of
isobenzan some years later. In 1979-1980, no isobenzan was detected
(detection limit: 0.01 mg/kg dry weight) in the sediment of rivers
in the Netherlands. Following soil treatment, residues in crops are
usually low (below 0.05 mg/kg crop), but higher levels may be found
in some root crops (up to 0.2 mg/kg in carrots). In market surveys
conducted during the time of the agricultural use of isobenzan, no
residues were detected in the food items analysed (less than
0.01 mg/kg).
After cattle were allowed to graze pastures treated with
isobenzan, the resultant daily products contained residues of the
insecticide. Two samples of butter contained 0.07 and 0.15 mg
isobenzan/kg product, while the levels in whole milk were 0.005 to
0.07 mg/kg. Dried milk, however, contained only 0.005 mg/kg. During
the processing of dairy products, up to 50% of the residue was lost,
depending on the type of treatment.
No data are available on the levels of isobenzan in the blood
or adipose tissue of the general population. Operators exposed to
isobenzan in manufacturing and formulation plants had mean whole
blood levels of up to 0.041 mg/litre. In whole blood samples of
people living in the neighbourhood of one plant, the concentration
of isobenzan was below the limit of detection (0.001 mg/litre).
Isobenzan is readily absorbed through the gastrointestinal wall
and is transported in the blood as the unchanged compound.
Hydrophilic metabolites are formed, one of which has been identified
as isobenzan lactone. Isobenzan accumulates in the tissues and
organs of rats and dogs in the following order: fat > liver =
muscle > brain > blood. The tissue concentrations of female rats
are generally higher than those of males, especially in body fat.
The biological half-life in body fat was found to be 10.9 days in
male rats and 16.6 days in female rats. A female canine pup, whose
blood contained 0.09 mg isobenzan/litre, showed convulsions 15 days
after birth. The pup had only fed on the milk of its mother, a
Beagle hound that had been dosed with isobenzan and whose milk
contained 0.7 mg/litre. Similar effects on pups were seen in a rat
reproduction study. Isobenzan is excreted via the milk of cows.
Mosquito larvae and soil fungi metabolize isobenzan in the
same way as vertebrates, yielding isobenzan lactone as a
metabolite.
Isobenzan is very persistent in the environment and
bioaccumulates. It is highly toxic to fish, shrimps, and birds. In
the Netherlands, the country where isobenzan was manufactured,
residues in the eggs of terns living along the Dutch coast ranged up
to 0.45 mg/kg (mean, 0.09 mg/kg), while mean residues in mussels and
fish were 0.05 mg/kg in 1965. Earthworm numbers were found to be
reduced in field plots treated with isobenzan at 2 kg/ha.
Nitrification was reduced, with a consequent increase in inorganic
nitrogen, in soils treated with isobenzan in the field at 1 kg/ha,
although laboratory studies showed no effect on nitrification at
doses equivalent to 250 g/ha.
The acute toxicity of isobenzan to mammals is high, both by the
oral and percutaneous routes. The mode of action of its toxicity is
an overstimulation of the central nervous system, resulting in
convulsions. The acute toxicity of formulations of isobenzan
reflects the percentage of active ingredient present.
Isobenzan is not a skin irritant, but some formulated products
may cause irritation.
Limited short- and long-term oral studies in mice, rats, and
dogs have shown that isobenzan may cause histological changes of the
classical type of organochlorine intoxication in the liver. In a
long-term rat study, a no-observed-effect level of 5 mg/kg diet
(approximately 0.25 mg/kg body weight) was determined, and in a
2-year dog study the no-observed-adverse-effect level (NOAEL) was
0.025 mg/kg body weight.
A one-generation reproduction study in rats indicated a NOAEL
of 0.1 mg/kg diet (approximately 0.005 mg/kg body weight). At a
level of 1 mg/kg diet (approximately 0.05 mg/kg body weight) the
survival of pups decreased.
No teratogenicity or mutagenicity studies have been reported.
No carcinogenic potential was demonstrated in a 2-year oral
study on rats and in an oral study on mice, but these studies were
inadequate to evaluate carcinogenicity.
The toxicological data base for isobenzan is incomplete. In
general, the quality of the data is considered to be poor by today's
standards and inadequate for an evaluation of the hazards to human
health or the environment.
Data on exposed humans are limited to studies on workers in a
factory in the Netherlands during the manufacture and formulation of
isobenzan and related "chlorinated cyclodiene insecticides". No
cases of skin irritation were reported. In several cases of
intoxication, convulsions occurred but the changes in the EEG
pattern were reversible. The intoxication threshold level (for
convulsions) was estimated to be 0.015 mg isobenzan/litre blood, and
the biological half-life of isobenzan in human blood was estimated
to be of the order of 2.8 years.
1.2 Conclusions and recommendations
Isobenzan is highly toxic and very persistent. The available
information on the hazards of isobenzan is incomplete, but is,
nevertheless, sufficient to indicate that the hazard it poses to
those who handling it and to the environment is such that no human
or environmental exposure to this substance, used either as an
insecticide or for any other purpose, should be allowed.
2. IDENTITY, PHYSICAL AND CHEMICAL PROPERTIES, ANALYTICAL METHODS
2.1 Identity
Chemical formula C9H4OCl8
Chemical structure
and spatial
configuration
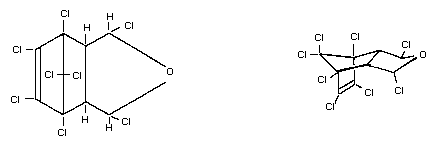 Chemical names 1,3,4,5,6,7,8,8-octachloro-4,7-methylene-
3a,4,7,7a-tetrahydro-isobenzofuran
Common synonyms BAS-4402; CP 14957; ENT-25 545; OMS-206;
OMS-618; SD-4402; WL 1650; preparation 948
Common trade name Telodrin (technical product), Omtan
Purity (technical) not less than 95% (w/w)
RTECS registry
number PC1225000
CAS registry number 297-78-9
2.2 Physical and chemical properties
Some physical and chemical properties of isobenzan are given in
Table 1.
Table 1. Some physical and chemical properties of isobenzan
Physical state crystalline powder
Colour whitish to light brown
Odour mild "chemical" odour
Relative molecular mass 411.73
Melting point (°C) 120-122
Flash point non-flammable
Explosion limits non-explosive
Relative density 1.87
Vapour pressure (20 °C) 6.7 x 10-4 Pa (5 x 10-6 mmHg)
Solubility in water practically insoluble
Solubility in organic slightly soluble in kerosene and ethanol; soluble
solvents in acetone, benzene, toluene, xylene, heavy aromatic
naphtha, and ethyl ether
Stability relatively stable to acids; dehydrochlorination may
occur under strong alkaline conditions
2.3 Conversion factors
1 ppm = 17 mg/m3 at 20 °C
1 mg/m3 = 0.06 ppm at 20 °C
2.4 Analytical methods
Analytical methods for the extraction, preparation, and
determination of residues of isobenzan in crops, animal products,
and soil using gas-liquid chromatography with electron-capture
detection have been described in detail by Elgar (1966) and Anon
(1974). The limit of determination is 0.01 mg/kg.
Kadoum (1968) described a rapid micromethod of sample clean-up
for gas-chromatographic analysis of isobenzan in ground water, soil,
and plant and animal extracts, using activated silica gel of high
purity. The percentage recovery was from 90% to 99% depending on the
quantity of eluate (305 v/v benzene in hexane) used. The limit of
determination in soil and plant or animal tissue was 0.01 mg/kg and
in water was 0.01 µg/litre.
Suzuki et al. (1974) analysed different types of pesticides
in extracts from crops or soil and separated them into a number of
groups by column chromatography (prior to thin-layer chromatography)
to obtain a systematic identification and determination of these
compounds. Silica gel was used for column chromatography and
thin-layer plates. For gas chromatographic separation, glass columns
packed with different absorbents were used. Electron capture
detection and a 63Ni source were used for the determination.
An advanced residue method, i.e. automated glass capillary gas
chromatography with electron-capture detection, was described by
Tuinstra & Traag (1979) for use with soil, vegetable material, milk
fat, and feed stuffs.
Wegman & Hofstee (1982) used capillary gas chromatography with
electron-capture determination for soil and river sediment samples.
The analysis of isobenzan in blood can be carried out according
to the method of Richardson et al. (1967) using gas-liquid
chromatography with electron-capture determination. The method is
sufficiently sensitive to detect isobenzan levels of less than
1 µg/litre blood.
Confirmation tests should be carried out using an appropriate
method (Anon, 1974).
3. SOURCES OF HUMAN AND ENVIRONMENTAL EXPOSURE
Telodrin was the registered trademark for the chlorinated
cyclodiene insecticide isobenzan. Isobenzan was only manufactured by
Shell during the period 1958-1965, but it was used for several years
thereafter from existing stocks. The major sources of exposure at
present appear to be the original waste disposal sites of industrial
wastes or dredged muds from contaminated areas.
4. ENVIRONMENTAL TRANSPORT, DISTRIBUTION, AND TRANSFORMATION
4.1 Transport and distribution between media
The environmental transport of isobenzan was investigated in a
slow sand filtration system used for the purification of ground
water. The first filter consisted of gravel and the second of sand.
Isobenzan was applied to the inlet water at a concentration of
1-10 µg/litre for 2 consecutive weeks. Fifty days elapsed after the
isobenzan treatment before it was no longer detectable in the
out-stream (Bauer, 1972).
4.2 Biotransformation
4.2.1 Soil
In a study by Bowman et al. (1965), the behaviour of
isobenzan in soil was investigated in laboratory tests. Eight types
of soil were used ranging from sand to sandy clay, the percentage of
sand varying from 93 to 56% and the percentage of clay from 4 to
35%. Following percolation with hexane, the percentage of isobenzan
in the eluate fraction was shown to decrease with increasing content
of clay in the soil (starting at about 30% clay). Except in the case
of sand with a high organic matter content (6-19%), no isobenzan was
recovered from the dry soils after 4-8 days of exposure at 45 °C.
When the soils were moistened with water and exposed at 45 °C for 4
days, degradation was markedly diminished, with the exception of the
sand with high organic matter.
After isobenzan is applied to soil (chalky loam, sandy loam,
and peat), a rapid initial loss occurs, probably due to sublimation.
The remaining compound then decays at a much lower rate, probably
having been adsorbed onto soil particles (Elgar, 1966).
The persistence of isobenzan in soil (95% disappearance) is 2-7
years (average 4 years) following an average dosage of 0.25-1 kg
isobenzan/ha (Edwards, 1965).
4.2.2 Water
When river water (pH 7.3) treated with 10 µg isobenzan/litre
was kept at room temperature in closed glass containers and exposed
to natural and artificial light, 25% of the isobenzan remained after
one week and 10% after 2 weeks. After the fourth week, no isobenzan
was detectable (detection limit: 50 ng/litre) (Eichelberger &
Lichtenberg, 1971).
5. ENVIRONMENTAL LEVELS AND HUMAN EXPOSURE
5.1 Environmental levels
5.1.1 Water
During the period 1969-1977, 1826 samples of surface water,
ground water, and rain water were taken at 99 sampling sites all
over the Netherlands, with special emphasis on the Rivers Rhine and
Meuse. Isobenzan was not present at measurable concentrations in any
of the samples (Wegman & Greve, 1980).
Soil samples were taken in 1977 to depths of 1-7 m from a
polder near Rotterdam that had been filled with material dredged
from the Rotterdam harbours between 1959 and 1976. In the ground
water, released from the samples by pressure, the concentration of
isobenzan in samples obtained from depths of 1, 3, and 5 m was less
than 0.05 µg/litre. The concentrations in those from depths of 2 and
7 m were 0.21 and 0.07 µg/litre, respectively. The surface water
taken from the drainage system of the polder contained
0.03 µg/litre. Most of the isobenzan in the polder was bound to
solids, the ratio of bound to dissolved isobenzan being over 11 000
to 1 (Kerdijk, 1981).
When 60 samples of water from 19 rivers and their estuaries
collected in Japan in 1974 were analysed for isobenzan, none was
detected (detection limit: < 0.1 µg/litre) (Japanese Environmental
Agency, 1975).
5.1.2 Soil
In a study by Elgar (1966), chalky loam, sandy loam, and peat
were treated with 0.5 kg isobenzan/ha (as emulsifiable concentrate)
once in 1961 or by three annual applications in 1961-1963. About 15%
of the initial residue remained after one year. More than half this
amount was still present 2 years later (Table 2).
In a Dutch monitoring programme, 145 sediment samples were
taken by dredge at 36 sampling sites in tributaries of the Rhine
River, Western Scheldt, and in some harbour basins of Rotterdam
during 1979-1980. None of the samples contained isobenzan (the
detection limit was 0.01 mg/kg soil on a dry weight basis) (Wegman &
Hofstee, 1982).
Sixty samples of bottom deposit from 19 rivers and their
estuaries in Japan collected in 1974 did not contain any isobenzan
(detection limit: 0.01 mg/kg) (Japanese Environmental Agency, 1975).
Table 2. Residues of isobenzan in 3 soil types after treatment with 0.5 kg isobenzan/ha
Time of sampling
Soil Years of Spring Autumn Spring Autumn Springa Autumn Spring
type treatment 1961 1961 1962 1962 1963 1963 1964
Chalky 1961 1.1 0.6 0.6 0.3 0.2 0.2 0.2
loam 1961, 1962, 1963 1.4 0.7 0.3/0.9 0.5 0.3
Sandy 1961 0.6 0.2 0.1 0.1 0.1 0.1 0.2
loam 1961, 1962, 1963 0.5 0.3 0.2/0.9 0.4 0.3
Peat 1961 4.2 0.3 0.2 0.2 0.3 0.3 0.3
soil 1961, 1962, 1963 1.2 0.7 0.2/1.6 1.1 0.5
a The two values represent residues before and after re-spraying.
5.1.3 Food
5.1.3.1 Plant products
Residue data, resulting from both foliar and soil treatment,
for a variety of crops have been reported (Shell, 1964).
a) Residues in crops: Foliar treatment
The magnitude of residues found on foliage or fruit is affected
by a number of factors. Crops having a large volume but a small
surface area tend to start off with a low concentration of
isobenzan. When crops have a rough (peach) or waxy (blackcurrant,
cabbage) surface, isobenzan appears to be less readily removed by
weathering than it is from smooth-skinned crops like tomatoes.
The rate of growth of the edible part of the crop is a
consistently significant factor, as is the application rate and
concentration. There is no evidence that isobenzan is translocated
or absorbed by plants and the rates of dissipation can be explained
by weathering of surface deposits, with some adsorption by cuticular
fats retarding this process. The period after the last application
required for the residue level to fall to below 0.05 mg/kg product
varies from 14 to 65 days, depending on the factors mentioned above.
Once a crop has been harvested, the residues present may be
further reduced or eliminated before consumption by various
subsequent processes. Washing can remove 60% of isobenzan residues
on broccoli. Isobenzan is removed when fruit and vegetables (such as
tomatoes) are processed to produce juice. Canning and freezing
processes (involving washing or blanching) also reduce residue
levels. Peeling of fruits (such as peaches) and discarding outer
leaves of vegetables (cabbage and lettuce) reduce the residue levels
significantly. In the case of tobacco, substantial quantities of
isobenzan are lost during both the curing and smoking. Cotton-seed
has very low levels of residues (of the order of 0.01 mg/kg product)
and the major part is found in the crude oil after processing
(Shell, 1964).
In 1964, cotton was treated up to 12 times with Telodrin dust
or emulsifiable concentrate at a dose of up to 350 g isobenzan/ha in
Guatemala, Mexico, and Nicaragua, and the seeds were harvested 17
days after the last treatment. The cotton seed oil contained
isobenzan residues of < 0.05 mg/kg (Elgar, 1965; Hughes, 1965).
Isobenzan residues of less than 0.05-1.5 mg/kg were found in
tobacco leaves grown in Australia during the period 1961-1964.
Cigarettes contained 0.2-0.3 mg/kg and pipe tobacco 0.06 mg/kg
(Buick, 1964).
b) Residues in crops resulting from soil treatment
When potatoes (in India and the United Kingdom) and sweet
potatoes (in South Africa) were grown in soil treated before
planting in 1964 with Telodrin dust or emulsifiable concentrate at
up to 3 kg isobenzan/ha or treated three times with Telodrin
emulsifiable concentrate at 200 g isobenzan/ha, the residues were
near the limit of detection (0.05 mg/kg crop) (Murphy & Standen,
1964; Buick, 1965; Buick & Cole, 1965).
In the United Kingdom, chalky loam, sandy loam, and peat were
treated with diluted 15% Telodrin emulsifiable concentrate at a
level of 0.5 kg isobenzan/ha. This was immediately incorporated by
harrow and, directly after, the plots were sown with cabbage,
carrot, onion, and sugar beet seed. Potatoes and celery were planted
at a later date. The plots were treated either in 1961 only or in
1961, 1962, and 1963. Residues were found in crops grown in both
loam soils, but not in those grown in peat, and only in root crops
(at a maximum of 0.05 mg/kg crop) after treatment in 1961. The
residues did not increase markedly with annual retreatment, the
maximum concentration found being 0.08 mg/kg in carrots (Elgar,
1966).
c) Residues in crops resulting from contaminated soils
Crops and the soil in which they had been grown were sampled at
harvest in 1976 from Dutch polders that had been built up during
1967-1969 with sediments dredged from the River Rhine and from a
harbour basin near a pesticide manufacturing plant. No residues of
isobenzan were detected in onions, brussel sprouts, or potatoes
(detection limit: 0.01 mg/kg), whereas carrots contained residues of
up to 0.09 mg/kg. The corresponding soil samples contained isobenzan
residues of between 0.01 and 3.5 mg/kg (dry weight basis). The ratio
of the concentration in carrots to that in soil, both calculated on
a dry weight basis, was 0.26 (Wegman et al., 1981).
5.1.3.2 Products of domestic animals
Pasture in Venezuela was treated with isobenzan at an average
dosage rate of 300 g/ha, and cattle were reintroduced 3-6 months
later. Analysis of the dairy products showed two samples of butter
containing residues of 0.07 and 0.15 mg/kg, respectively. In milk,
the residues ranged from 0.005 to 0.07 mg/kg, while dried milk
contained negligible residues (0.005 mg/kg) (Standen & Elgar, 1965).
Heat treatment of various dairy products manufactured from milk
containing 0.8 mg isobenzan/kg (18 mg/kg on fat basis) was found to
cause residue losses. Between 40% and 50% of the residues were
destroyed in evaporated milk and 10-20% of the residues during
processing of the milk for dry whole milk (Stemp & Liska, 1966).
No residues of isobenzan could be detected in chicken meat
after white meat containing 0.2 mg/kg or dark meat containing
0.5 mg/kg had been cooked (McCaskey et al., 1968).
In Victoria, Australia, in 1963, dairy pasture was sprayed with
140 g isobenzan/ha and left for 3 weeks before dairy cattle were
reintroduced. Within 2 days, toxic symptoms (circling, rolling of
eyes, salivation, convulsions) were observed in dairy cattle that
had consumed treated grass. Deaths occurred in cattle and calves and
in cats, rabbits, poultry, and dogs. A 10-month old baby, fed on
milk from the cows, developed an illness characterized by
irritability and persistent crying. Milk from one cow with signs of
intoxication had an isobenzan level of 1 mg/litre. Analysis of
isobenzan residues in farm milk (representing milk from many cows)
ranged up to 5 mg/litre, although most values were in the range of
0.05 to 0.2 mg/litre. Residues of isobenzan were still present in
some milk samples 14 months later. Isobenzan was also detected in
cow adipose tissue (10 mg/kg), cat liver (4.5 mg/kg), and calf liver
(7.5 mg/kg). Isobenzan levels in surface water used by the cattle
were very low (0.0002 mg/litre) (Shell, 1963).
5.1.3.3 Market surveys
Food items covering the important constituents of the local
diet (e.g., potatoes, rice, wheat, onions, different types of beans,
fruit, beef, lamb, milk, cheese, ground-nut products, maize, and
sugar cane) were collected in Venezuela (1966), Mexico (1967),
Nicaragua (1967), Spain (1967), and India (1968). Residues of
isobenzan in these products were below the limit of detection
(0.01 mg/kg) (Bull & Marlow, 1967; Bull & Ramsden, 1967; Elgar &
Holland, 1967; Marlow et al., 1968; Mathews, 1969).
Isobenzan was not detected in market-basket surveys conducted
by the Food and Drug Administration in the USA during the period
1980-1990 (Burse, 1990, personal communication to the IPCS).
Cured tobacco from Costa Rica contained 1.3 mg isobenzan/kg
(Mathews & Cole, 1966).
5.1.4 Terrestrial and aquatic organisms
Fish, mussels, and the eggs of various species of tern were
collected along the north coast of the Netherlands and analysed for
residues of chlorinated hydrocarbon insecticides. The mean isobenzan
residues in eggs were 0.09 mg/kg (range, 0.02-0.45 mg/kg) in 1965
and 0.06 mg/kg (range, 0.02-0.12 mg/kg) in 1966. The mean residues
in composite samples of fish (sprat, juvenile herring, and sand eel)
were 0.05 mg/kg (range, 0.04-0.07 mg/kg) in 1965 and 0.02 mg/kg
(range, 0.01-0.05 mg/kg) in 1966. Mussels (Mytilus edulis) sampled
in 1966 did not contain any residues of isobenzan (detection level:
0.003 mg/kg), but mussels sampled at one particular place in 1965
contained 0.11 mg/kg (Koeman et al., 1967, 1968).
Residues in the livers of sandwich terns found dead in the
Dutch Wadden Sea during the summers of 1965 and 1966 amounted to as
much as 3.8 mg/kg isobenzan (average, approximately 0.75 mg/kg)
(Koeman et al., 1967).
Sixty samples of fish and shellfish collected in 19 rivers and
their sea estuaries in Japan in 1974 contained no isobenzan
(detection limit: 0.005 mg/kg) (Japanese Environmental Agency,
1975).
5.2 General population exposure
A housing estate of about 800 houses and public buildings was
built directly on a 4-m thick layer of harbour sludge in the
Netherlands in 1983. The area was raised during the period 1962-1964
by sludge originating from about 20 harbour basins in Rotterdam and
the industrial area around the Nieuwe Waterweg. In the sludge,
organic solvents, polyaromatic hydrocarbons, heavy metals, and
chlorinated cyclodiene insecticides including isobenzan were
detected. One-third of the soil samples collected in the gardens
(71 locations), 0-40 cm below the surface, contained chlorinated
cyclodiene insecticides with a mean concentration of 1.2 mg/kg and a
maximum concentration of 19.5 mg/kg dry weight (Van Wijnen &
Stijkel, 1988).
From the residue data presented in section 5.1.3, it appears
that exposure of the general population via food was very low at the
time when isobenzan was used agriculturally. Exposure from
environmental sources must also have been minor. For example, the
concentration of isobenzan in the blood of 10 people living in the
vicinity of an isobenzan formulation plant in Venezuela was below
the limit of determination (0.001 mg/litre) (Davies, 1966a,b).
5.3 Occupational exposure
In a study carried out in 1965-1968 at a formulation plant in
Venezuela, 229 blood samples were taken from operators formulating
isobenzan and related chlorinated hydrocarbon insecticides. The
concentrations of isobenzan in blood fluctuated during this period
due to variations in the formulations being prepared at the plant.
The mean concentrations in whole blood ranged from 0.004 to
0.033 mg/litre, the maximum concentration being 0.045 mg/litre
(Davies, 1966a,b; Crabtree, 1968).
Isobenzan levels in the range of < 0.002 to 0.041 mg/litre
were found in the blood of operators at a manufacturing and
formulation plant in the Netherlands (Jager, 1970).
6. KINETICS AND METABOLISM
6.1 Absorption
In studies using everted sacs of rat ileum, colloidal solutions
and dispersions of isobenzan labelled with 14C were absorbed
through the intestinal wall. The maximum uptake occurred in the
middle segment of the ileum (Hathway, 1965).
Following an oral dose of 14C-isobenzan, only a small
proportion (< 10%) of the label was found in the thoracic lymph
duct, the remainder being in the hepatic portal blood (Hathway,
1965).
6.2 Distribution
Isobenzan is approximately 4000 times more soluble in rabbit
serum than in water, and it has been shown that, during transport in
the blood of rats and rabbits, isobenzan is associated with some
serum proteins (albumin and alpha-globulin in the rabbit and
albumins in the rat) and with constituents of the red blood cell,
mainly haemoglobin. The ratio of the distribution of the insecticide
between plasma and cells was shown to be approximately 2:1, this
ratio remaining constant at different intervals after administration
and at different concentrations. Gas chromatography showed that
unchanged isobenzan was transported in the blood (Moss & Hathway,
1964; Hathway, 1965).
6.2.1 Rat
In an extensive study, Carworth Farm rats were fed diets
containing isobenzan (96%) (5, 15.9, or 25 mg/kg) for periods of 44
to 224 days followed by a control diet for periods of up to 64 days.
Analyses of tissues at several intervals during feeding showed the
concentrations of isobenzan to be in the following order: fat >
liver = muscle > brain > blood. Concentrations in females were
higher than those in the males, especially in the fat. There was a
significant correlation between the concentration of isobenzan in
blood and in other organs, plausibly attributed to a dynamic
equilibrium. The biological half-life in body fat was 10.9 days in
male rats and 16.6 days in female rats (Robinson & Richardson,
1963).
When male and female rats were given a single intravenous
injection of 14C-isobenzan (15 µg/kg body weight), the
radio-activity in the blood of males 48 h later was 0.04% of the
applied dose and in females was 0.37%. The radioactivity in the
organs and tissues ranged between 0.01 and approximately 1.5% of the
applied dose, concentrations being lower in females than in males.
In abdominal fat, the concentrations were 19.2% in males and 26.6%
in females and, in muscle, 12.3% and 9.3%, respectively. The
concentration in subcutaneous fat was about half of that in
abdominal fat (Kaul et al., 1970).
Isobenzan rapidly crosses the placental barrier in pregnant
rats. Labelled isobenzan was found in fetal blood within 5 min of an
intravenous administration into the ear vein of the mother (Hathway,
1965).
Fetuses removed by caesarean section from Carworth Farm rats
fed a dietary isobenzan concentration of 5 mg/kg in a reproduction
study carried out by Chambers (1962a,b) (section 7.5.2) contained
0.1-0.13 mg isobenzan/kg tissue (Stevenson, 1964).
6.2.2 Dog
In a study by (Worden, 1969), groups of six Scottish terriers
(males and females) were given daily isobenzan doses of 0.025 or
0.1 mg/kg body weight by gavage for 2 years (section 7.2.1.3). At
the end of the study the distribution of isobenzan in the tissues
was examined, and the concentration was highest in body fat and
least in blood (Table 3). There was a significant correlation
between the concentrations in blood and those in other tissues. The
storage ratio (concentration in body fat/concentration in diet) did
not show any significant differences between the sexes or between
the two dose levels.
Table 3. Mean concentrations (mg/kg) of isobenzan in tissues of dogs
dosed orally with isobenzan for 2 years
Dose Fat Muscle Liver Brain Blood
0.025 mg/kg body 2.9 0.25 1.2 0.2 0.02
weight
0.1 mg/kg body 9.5 0.8 4.2 0.4 0.04
weight
The first litter from a female Beagle hound dosed with 0.08 mg
isobenzan/kg body weight per day (section 7.5.3) consisted of one
male, one female, and one still-born male. The concentrations of
isobenzan in the brain, liver, muscle, heart, and kidneys of the
still-born pup were below 0.2 mg/kg, with the liver containing the
highest level of 0.16 mg/kg. The female pup, which only fed on the
mother's milk, showed convulsions 15 days after birth and was killed
at 17 days of age. The blood contained 0.09 mg isobenzan per kg and
the urine 0.02 mg/kg. The concentrations in organs and tissues were
less than 1 mg/kg, the highest levels being in muscle (0.94 mg/kg),
liver (0.65 mg/kg), and fat (0.48 mg/kg). The milk from the mother
contained 0.7 mg/litre as whole milk and 3.4 mg/kg on a fat basis.
The remaining pup showed no ill effects. Four males and two females
in further litters also showed no ill effects (Brown & Richardson,
1964).
6.2.3 Domestic fowl
In a study by McCaskey et al. (1968), six Leghorn hens
received, in a gelatin capsule on each of 5 days, an amount of
isobenzan (94% purity) equivalent to 10-15 mg/kg of the average
weight of daily feed consumed. Eggs were collected on days 2-8, and
the hens were killed 3 days after the last dose. Residues in tissues
and eggs are given in Table 4.
Table 4. Concentrations of residues (mg/kg products) in tissues
and eggs of hens dosed orally with isobenzan
Tissue Residue concentration (mg/kg)
Abdominal fat 10.6
White meat 0.2 (on fat basis: 3.4)
Dark meat 0.5 (on fat basis: 4.3)
Egg yolk 0.1 on day 4; 0.4 on day 5; 0.5 on day 7
0.7 on day 8
6.2.4 Cow
Three Jersey cows were fed for 28 days at concentrations of
technical isobenzan of 0, 0.005, or 0.02 mg/kg in their daily ration
(average ration, 20 kg per cow). Residues found in the milk of the
cow fed 0.005 mg/kg increased from 0.4 µg/litre to 2 µg/litre at the
end of the feeding period and decreased rapidly thereafter. Higher
residues of up to 7.7 µg/litre were present in the milk from the cow
fed 0.02 mg/kg, which decreased to 1.5 µg/litre whole milk 10 weeks
after the last day of dosing (Hardee et al., 1964).
Following the consumption of feed contaminated with relatively
low concentrations of isobenzan, the concentration in milk rose
rapidly within a few hours to days, levelling off at a plateau
characteristic for each concentration in the diet. The average
milk/diet ratio for isobenzan was 0.4 to 0.5 for feeding levels of
0.005 to 0.02 mg/kg diet (Biehl & Buck, 1987).
In a study by Bishop & Huber (1964), four groups of three
lactating Holstein cows were fed 0, 0.02, 0.06, or 0.15 mg
isobenzan/kg (corresponding to 0.47, 1.38, or 3.38 mg/cow per day)
in their daily ration for 90 days. The concentrations in the milk
were directly proportional to the dietary level. Increases in
concentration were noted during the entire treatment period for the
0.06- and 0.15-mg/kg feeding levels, reaching 0.033 and
0.071 mg/litre of milk. Trace amounts (up to 0.008 mg/litre) were
found in the milk from cows fed 0.02 mg/kg. At the highest dose,
residues of 0.016 mg/litre were still present in the milk 60 days
after treatment, while in the groups fed 0.06 and 0.02 mg/kg, the
residues were then negligible. Residues in fat from biopsies taken
88 days after the treatment finished reflected the exposure levels,
being 0.11, 0.26, and 0.53 mg/kg tissue for the three levels.
6.3 Metabolic transformation
6.3.1 Vertebrates
The radioactive residue present in the urine and faeces of rats
treated with a single intravenous injection of 14C-isobenzan
consisted of a hydrophilic metabolite that, after hydrolysis, gave
isobenzan lactone (Kaul et al., 1970).
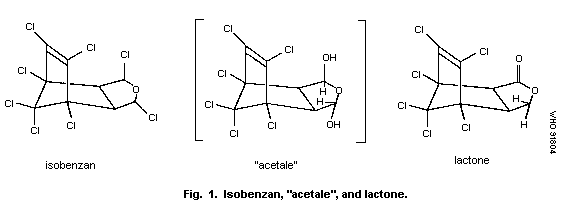
It is probable that both chlorine atoms on the tetrahydrofuran
ring are first replaced by hydroxyl groups. The resulting "acetale",
an unstable intermediate, is converted to the gamma-lactone (Korte,
1967) (Fig. 1).
6.3.2 Invertebrates
Mosquito larvae (Aedes aegypti) metabolize isobenzan,
labelled with 14C in the hexachloropentane ring or at the 1,3
position, to a metabolite more hydrophilic than that produced by
microorganisms. This metabolite consists of at least three
components. The hydrolytic product of one of these components was
identified as isobenzan lactone (Korte, 1963, 1967; Korte & Stiasni,
1964).
Chemical names 1,3,4,5,6,7,8,8-octachloro-4,7-methylene-
3a,4,7,7a-tetrahydro-isobenzofuran
Common synonyms BAS-4402; CP 14957; ENT-25 545; OMS-206;
OMS-618; SD-4402; WL 1650; preparation 948
Common trade name Telodrin (technical product), Omtan
Purity (technical) not less than 95% (w/w)
RTECS registry
number PC1225000
CAS registry number 297-78-9
2.2 Physical and chemical properties
Some physical and chemical properties of isobenzan are given in
Table 1.
Table 1. Some physical and chemical properties of isobenzan
Physical state crystalline powder
Colour whitish to light brown
Odour mild "chemical" odour
Relative molecular mass 411.73
Melting point (°C) 120-122
Flash point non-flammable
Explosion limits non-explosive
Relative density 1.87
Vapour pressure (20 °C) 6.7 x 10-4 Pa (5 x 10-6 mmHg)
Solubility in water practically insoluble
Solubility in organic slightly soluble in kerosene and ethanol; soluble
solvents in acetone, benzene, toluene, xylene, heavy aromatic
naphtha, and ethyl ether
Stability relatively stable to acids; dehydrochlorination may
occur under strong alkaline conditions
2.3 Conversion factors
1 ppm = 17 mg/m3 at 20 °C
1 mg/m3 = 0.06 ppm at 20 °C
2.4 Analytical methods
Analytical methods for the extraction, preparation, and
determination of residues of isobenzan in crops, animal products,
and soil using gas-liquid chromatography with electron-capture
detection have been described in detail by Elgar (1966) and Anon
(1974). The limit of determination is 0.01 mg/kg.
Kadoum (1968) described a rapid micromethod of sample clean-up
for gas-chromatographic analysis of isobenzan in ground water, soil,
and plant and animal extracts, using activated silica gel of high
purity. The percentage recovery was from 90% to 99% depending on the
quantity of eluate (305 v/v benzene in hexane) used. The limit of
determination in soil and plant or animal tissue was 0.01 mg/kg and
in water was 0.01 µg/litre.
Suzuki et al. (1974) analysed different types of pesticides
in extracts from crops or soil and separated them into a number of
groups by column chromatography (prior to thin-layer chromatography)
to obtain a systematic identification and determination of these
compounds. Silica gel was used for column chromatography and
thin-layer plates. For gas chromatographic separation, glass columns
packed with different absorbents were used. Electron capture
detection and a 63Ni source were used for the determination.
An advanced residue method, i.e. automated glass capillary gas
chromatography with electron-capture detection, was described by
Tuinstra & Traag (1979) for use with soil, vegetable material, milk
fat, and feed stuffs.
Wegman & Hofstee (1982) used capillary gas chromatography with
electron-capture determination for soil and river sediment samples.
The analysis of isobenzan in blood can be carried out according
to the method of Richardson et al. (1967) using gas-liquid
chromatography with electron-capture determination. The method is
sufficiently sensitive to detect isobenzan levels of less than
1 µg/litre blood.
Confirmation tests should be carried out using an appropriate
method (Anon, 1974).
3. SOURCES OF HUMAN AND ENVIRONMENTAL EXPOSURE
Telodrin was the registered trademark for the chlorinated
cyclodiene insecticide isobenzan. Isobenzan was only manufactured by
Shell during the period 1958-1965, but it was used for several years
thereafter from existing stocks. The major sources of exposure at
present appear to be the original waste disposal sites of industrial
wastes or dredged muds from contaminated areas.
4. ENVIRONMENTAL TRANSPORT, DISTRIBUTION, AND TRANSFORMATION
4.1 Transport and distribution between media
The environmental transport of isobenzan was investigated in a
slow sand filtration system used for the purification of ground
water. The first filter consisted of gravel and the second of sand.
Isobenzan was applied to the inlet water at a concentration of
1-10 µg/litre for 2 consecutive weeks. Fifty days elapsed after the
isobenzan treatment before it was no longer detectable in the
out-stream (Bauer, 1972).
4.2 Biotransformation
4.2.1 Soil
In a study by Bowman et al. (1965), the behaviour of
isobenzan in soil was investigated in laboratory tests. Eight types
of soil were used ranging from sand to sandy clay, the percentage of
sand varying from 93 to 56% and the percentage of clay from 4 to
35%. Following percolation with hexane, the percentage of isobenzan
in the eluate fraction was shown to decrease with increasing content
of clay in the soil (starting at about 30% clay). Except in the case
of sand with a high organic matter content (6-19%), no isobenzan was
recovered from the dry soils after 4-8 days of exposure at 45 °C.
When the soils were moistened with water and exposed at 45 °C for 4
days, degradation was markedly diminished, with the exception of the
sand with high organic matter.
After isobenzan is applied to soil (chalky loam, sandy loam,
and peat), a rapid initial loss occurs, probably due to sublimation.
The remaining compound then decays at a much lower rate, probably
having been adsorbed onto soil particles (Elgar, 1966).
The persistence of isobenzan in soil (95% disappearance) is 2-7
years (average 4 years) following an average dosage of 0.25-1 kg
isobenzan/ha (Edwards, 1965).
4.2.2 Water
When river water (pH 7.3) treated with 10 µg isobenzan/litre
was kept at room temperature in closed glass containers and exposed
to natural and artificial light, 25% of the isobenzan remained after
one week and 10% after 2 weeks. After the fourth week, no isobenzan
was detectable (detection limit: 50 ng/litre) (Eichelberger &
Lichtenberg, 1971).
5. ENVIRONMENTAL LEVELS AND HUMAN EXPOSURE
5.1 Environmental levels
5.1.1 Water
During the period 1969-1977, 1826 samples of surface water,
ground water, and rain water were taken at 99 sampling sites all
over the Netherlands, with special emphasis on the Rivers Rhine and
Meuse. Isobenzan was not present at measurable concentrations in any
of the samples (Wegman & Greve, 1980).
Soil samples were taken in 1977 to depths of 1-7 m from a
polder near Rotterdam that had been filled with material dredged
from the Rotterdam harbours between 1959 and 1976. In the ground
water, released from the samples by pressure, the concentration of
isobenzan in samples obtained from depths of 1, 3, and 5 m was less
than 0.05 µg/litre. The concentrations in those from depths of 2 and
7 m were 0.21 and 0.07 µg/litre, respectively. The surface water
taken from the drainage system of the polder contained
0.03 µg/litre. Most of the isobenzan in the polder was bound to
solids, the ratio of bound to dissolved isobenzan being over 11 000
to 1 (Kerdijk, 1981).
When 60 samples of water from 19 rivers and their estuaries
collected in Japan in 1974 were analysed for isobenzan, none was
detected (detection limit: < 0.1 µg/litre) (Japanese Environmental
Agency, 1975).
5.1.2 Soil
In a study by Elgar (1966), chalky loam, sandy loam, and peat
were treated with 0.5 kg isobenzan/ha (as emulsifiable concentrate)
once in 1961 or by three annual applications in 1961-1963. About 15%
of the initial residue remained after one year. More than half this
amount was still present 2 years later (Table 2).
In a Dutch monitoring programme, 145 sediment samples were
taken by dredge at 36 sampling sites in tributaries of the Rhine
River, Western Scheldt, and in some harbour basins of Rotterdam
during 1979-1980. None of the samples contained isobenzan (the
detection limit was 0.01 mg/kg soil on a dry weight basis) (Wegman &
Hofstee, 1982).
Sixty samples of bottom deposit from 19 rivers and their
estuaries in Japan collected in 1974 did not contain any isobenzan
(detection limit: 0.01 mg/kg) (Japanese Environmental Agency, 1975).
Table 2. Residues of isobenzan in 3 soil types after treatment with 0.5 kg isobenzan/ha
Time of sampling
Soil Years of Spring Autumn Spring Autumn Springa Autumn Spring
type treatment 1961 1961 1962 1962 1963 1963 1964
Chalky 1961 1.1 0.6 0.6 0.3 0.2 0.2 0.2
loam 1961, 1962, 1963 1.4 0.7 0.3/0.9 0.5 0.3
Sandy 1961 0.6 0.2 0.1 0.1 0.1 0.1 0.2
loam 1961, 1962, 1963 0.5 0.3 0.2/0.9 0.4 0.3
Peat 1961 4.2 0.3 0.2 0.2 0.3 0.3 0.3
soil 1961, 1962, 1963 1.2 0.7 0.2/1.6 1.1 0.5
a The two values represent residues before and after re-spraying.
5.1.3 Food
5.1.3.1 Plant products
Residue data, resulting from both foliar and soil treatment,
for a variety of crops have been reported (Shell, 1964).
a) Residues in crops: Foliar treatment
The magnitude of residues found on foliage or fruit is affected
by a number of factors. Crops having a large volume but a small
surface area tend to start off with a low concentration of
isobenzan. When crops have a rough (peach) or waxy (blackcurrant,
cabbage) surface, isobenzan appears to be less readily removed by
weathering than it is from smooth-skinned crops like tomatoes.
The rate of growth of the edible part of the crop is a
consistently significant factor, as is the application rate and
concentration. There is no evidence that isobenzan is translocated
or absorbed by plants and the rates of dissipation can be explained
by weathering of surface deposits, with some adsorption by cuticular
fats retarding this process. The period after the last application
required for the residue level to fall to below 0.05 mg/kg product
varies from 14 to 65 days, depending on the factors mentioned above.
Once a crop has been harvested, the residues present may be
further reduced or eliminated before consumption by various
subsequent processes. Washing can remove 60% of isobenzan residues
on broccoli. Isobenzan is removed when fruit and vegetables (such as
tomatoes) are processed to produce juice. Canning and freezing
processes (involving washing or blanching) also reduce residue
levels. Peeling of fruits (such as peaches) and discarding outer
leaves of vegetables (cabbage and lettuce) reduce the residue levels
significantly. In the case of tobacco, substantial quantities of
isobenzan are lost during both the curing and smoking. Cotton-seed
has very low levels of residues (of the order of 0.01 mg/kg product)
and the major part is found in the crude oil after processing
(Shell, 1964).
In 1964, cotton was treated up to 12 times with Telodrin dust
or emulsifiable concentrate at a dose of up to 350 g isobenzan/ha in
Guatemala, Mexico, and Nicaragua, and the seeds were harvested 17
days after the last treatment. The cotton seed oil contained
isobenzan residues of < 0.05 mg/kg (Elgar, 1965; Hughes, 1965).
Isobenzan residues of less than 0.05-1.5 mg/kg were found in
tobacco leaves grown in Australia during the period 1961-1964.
Cigarettes contained 0.2-0.3 mg/kg and pipe tobacco 0.06 mg/kg
(Buick, 1964).
b) Residues in crops resulting from soil treatment
When potatoes (in India and the United Kingdom) and sweet
potatoes (in South Africa) were grown in soil treated before
planting in 1964 with Telodrin dust or emulsifiable concentrate at
up to 3 kg isobenzan/ha or treated three times with Telodrin
emulsifiable concentrate at 200 g isobenzan/ha, the residues were
near the limit of detection (0.05 mg/kg crop) (Murphy & Standen,
1964; Buick, 1965; Buick & Cole, 1965).
In the United Kingdom, chalky loam, sandy loam, and peat were
treated with diluted 15% Telodrin emulsifiable concentrate at a
level of 0.5 kg isobenzan/ha. This was immediately incorporated by
harrow and, directly after, the plots were sown with cabbage,
carrot, onion, and sugar beet seed. Potatoes and celery were planted
at a later date. The plots were treated either in 1961 only or in
1961, 1962, and 1963. Residues were found in crops grown in both
loam soils, but not in those grown in peat, and only in root crops
(at a maximum of 0.05 mg/kg crop) after treatment in 1961. The
residues did not increase markedly with annual retreatment, the
maximum concentration found being 0.08 mg/kg in carrots (Elgar,
1966).
c) Residues in crops resulting from contaminated soils
Crops and the soil in which they had been grown were sampled at
harvest in 1976 from Dutch polders that had been built up during
1967-1969 with sediments dredged from the River Rhine and from a
harbour basin near a pesticide manufacturing plant. No residues of
isobenzan were detected in onions, brussel sprouts, or potatoes
(detection limit: 0.01 mg/kg), whereas carrots contained residues of
up to 0.09 mg/kg. The corresponding soil samples contained isobenzan
residues of between 0.01 and 3.5 mg/kg (dry weight basis). The ratio
of the concentration in carrots to that in soil, both calculated on
a dry weight basis, was 0.26 (Wegman et al., 1981).
5.1.3.2 Products of domestic animals
Pasture in Venezuela was treated with isobenzan at an average
dosage rate of 300 g/ha, and cattle were reintroduced 3-6 months
later. Analysis of the dairy products showed two samples of butter
containing residues of 0.07 and 0.15 mg/kg, respectively. In milk,
the residues ranged from 0.005 to 0.07 mg/kg, while dried milk
contained negligible residues (0.005 mg/kg) (Standen & Elgar, 1965).
Heat treatment of various dairy products manufactured from milk
containing 0.8 mg isobenzan/kg (18 mg/kg on fat basis) was found to
cause residue losses. Between 40% and 50% of the residues were
destroyed in evaporated milk and 10-20% of the residues during
processing of the milk for dry whole milk (Stemp & Liska, 1966).
No residues of isobenzan could be detected in chicken meat
after white meat containing 0.2 mg/kg or dark meat containing
0.5 mg/kg had been cooked (McCaskey et al., 1968).
In Victoria, Australia, in 1963, dairy pasture was sprayed with
140 g isobenzan/ha and left for 3 weeks before dairy cattle were
reintroduced. Within 2 days, toxic symptoms (circling, rolling of
eyes, salivation, convulsions) were observed in dairy cattle that
had consumed treated grass. Deaths occurred in cattle and calves and
in cats, rabbits, poultry, and dogs. A 10-month old baby, fed on
milk from the cows, developed an illness characterized by
irritability and persistent crying. Milk from one cow with signs of
intoxication had an isobenzan level of 1 mg/litre. Analysis of
isobenzan residues in farm milk (representing milk from many cows)
ranged up to 5 mg/litre, although most values were in the range of
0.05 to 0.2 mg/litre. Residues of isobenzan were still present in
some milk samples 14 months later. Isobenzan was also detected in
cow adipose tissue (10 mg/kg), cat liver (4.5 mg/kg), and calf liver
(7.5 mg/kg). Isobenzan levels in surface water used by the cattle
were very low (0.0002 mg/litre) (Shell, 1963).
5.1.3.3 Market surveys
Food items covering the important constituents of the local
diet (e.g., potatoes, rice, wheat, onions, different types of beans,
fruit, beef, lamb, milk, cheese, ground-nut products, maize, and
sugar cane) were collected in Venezuela (1966), Mexico (1967),
Nicaragua (1967), Spain (1967), and India (1968). Residues of
isobenzan in these products were below the limit of detection
(0.01 mg/kg) (Bull & Marlow, 1967; Bull & Ramsden, 1967; Elgar &
Holland, 1967; Marlow et al., 1968; Mathews, 1969).
Isobenzan was not detected in market-basket surveys conducted
by the Food and Drug Administration in the USA during the period
1980-1990 (Burse, 1990, personal communication to the IPCS).
Cured tobacco from Costa Rica contained 1.3 mg isobenzan/kg
(Mathews & Cole, 1966).
5.1.4 Terrestrial and aquatic organisms
Fish, mussels, and the eggs of various species of tern were
collected along the north coast of the Netherlands and analysed for
residues of chlorinated hydrocarbon insecticides. The mean isobenzan
residues in eggs were 0.09 mg/kg (range, 0.02-0.45 mg/kg) in 1965
and 0.06 mg/kg (range, 0.02-0.12 mg/kg) in 1966. The mean residues
in composite samples of fish (sprat, juvenile herring, and sand eel)
were 0.05 mg/kg (range, 0.04-0.07 mg/kg) in 1965 and 0.02 mg/kg
(range, 0.01-0.05 mg/kg) in 1966. Mussels (Mytilus edulis) sampled
in 1966 did not contain any residues of isobenzan (detection level:
0.003 mg/kg), but mussels sampled at one particular place in 1965
contained 0.11 mg/kg (Koeman et al., 1967, 1968).
Residues in the livers of sandwich terns found dead in the
Dutch Wadden Sea during the summers of 1965 and 1966 amounted to as
much as 3.8 mg/kg isobenzan (average, approximately 0.75 mg/kg)
(Koeman et al., 1967).
Sixty samples of fish and shellfish collected in 19 rivers and
their sea estuaries in Japan in 1974 contained no isobenzan
(detection limit: 0.005 mg/kg) (Japanese Environmental Agency,
1975).
5.2 General population exposure
A housing estate of about 800 houses and public buildings was
built directly on a 4-m thick layer of harbour sludge in the
Netherlands in 1983. The area was raised during the period 1962-1964
by sludge originating from about 20 harbour basins in Rotterdam and
the industrial area around the Nieuwe Waterweg. In the sludge,
organic solvents, polyaromatic hydrocarbons, heavy metals, and
chlorinated cyclodiene insecticides including isobenzan were
detected. One-third of the soil samples collected in the gardens
(71 locations), 0-40 cm below the surface, contained chlorinated
cyclodiene insecticides with a mean concentration of 1.2 mg/kg and a
maximum concentration of 19.5 mg/kg dry weight (Van Wijnen &
Stijkel, 1988).
From the residue data presented in section 5.1.3, it appears
that exposure of the general population via food was very low at the
time when isobenzan was used agriculturally. Exposure from
environmental sources must also have been minor. For example, the
concentration of isobenzan in the blood of 10 people living in the
vicinity of an isobenzan formulation plant in Venezuela was below
the limit of determination (0.001 mg/litre) (Davies, 1966a,b).
5.3 Occupational exposure
In a study carried out in 1965-1968 at a formulation plant in
Venezuela, 229 blood samples were taken from operators formulating
isobenzan and related chlorinated hydrocarbon insecticides. The
concentrations of isobenzan in blood fluctuated during this period
due to variations in the formulations being prepared at the plant.
The mean concentrations in whole blood ranged from 0.004 to
0.033 mg/litre, the maximum concentration being 0.045 mg/litre
(Davies, 1966a,b; Crabtree, 1968).
Isobenzan levels in the range of < 0.002 to 0.041 mg/litre
were found in the blood of operators at a manufacturing and
formulation plant in the Netherlands (Jager, 1970).
6. KINETICS AND METABOLISM
6.1 Absorption
In studies using everted sacs of rat ileum, colloidal solutions
and dispersions of isobenzan labelled with 14C were absorbed
through the intestinal wall. The maximum uptake occurred in the
middle segment of the ileum (Hathway, 1965).
Following an oral dose of 14C-isobenzan, only a small
proportion (< 10%) of the label was found in the thoracic lymph
duct, the remainder being in the hepatic portal blood (Hathway,
1965).
6.2 Distribution
Isobenzan is approximately 4000 times more soluble in rabbit
serum than in water, and it has been shown that, during transport in
the blood of rats and rabbits, isobenzan is associated with some
serum proteins (albumin and alpha-globulin in the rabbit and
albumins in the rat) and with constituents of the red blood cell,
mainly haemoglobin. The ratio of the distribution of the insecticide
between plasma and cells was shown to be approximately 2:1, this
ratio remaining constant at different intervals after administration
and at different concentrations. Gas chromatography showed that
unchanged isobenzan was transported in the blood (Moss & Hathway,
1964; Hathway, 1965).
6.2.1 Rat
In an extensive study, Carworth Farm rats were fed diets
containing isobenzan (96%) (5, 15.9, or 25 mg/kg) for periods of 44
to 224 days followed by a control diet for periods of up to 64 days.
Analyses of tissues at several intervals during feeding showed the
concentrations of isobenzan to be in the following order: fat >
liver = muscle > brain > blood. Concentrations in females were
higher than those in the males, especially in the fat. There was a
significant correlation between the concentration of isobenzan in
blood and in other organs, plausibly attributed to a dynamic
equilibrium. The biological half-life in body fat was 10.9 days in
male rats and 16.6 days in female rats (Robinson & Richardson,
1963).
When male and female rats were given a single intravenous
injection of 14C-isobenzan (15 µg/kg body weight), the
radio-activity in the blood of males 48 h later was 0.04% of the
applied dose and in females was 0.37%. The radioactivity in the
organs and tissues ranged between 0.01 and approximately 1.5% of the
applied dose, concentrations being lower in females than in males.
In abdominal fat, the concentrations were 19.2% in males and 26.6%
in females and, in muscle, 12.3% and 9.3%, respectively. The
concentration in subcutaneous fat was about half of that in
abdominal fat (Kaul et al., 1970).
Isobenzan rapidly crosses the placental barrier in pregnant
rats. Labelled isobenzan was found in fetal blood within 5 min of an
intravenous administration into the ear vein of the mother (Hathway,
1965).
Fetuses removed by caesarean section from Carworth Farm rats
fed a dietary isobenzan concentration of 5 mg/kg in a reproduction
study carried out by Chambers (1962a,b) (section 7.5.2) contained
0.1-0.13 mg isobenzan/kg tissue (Stevenson, 1964).
6.2.2 Dog
In a study by (Worden, 1969), groups of six Scottish terriers
(males and females) were given daily isobenzan doses of 0.025 or
0.1 mg/kg body weight by gavage for 2 years (section 7.2.1.3). At
the end of the study the distribution of isobenzan in the tissues
was examined, and the concentration was highest in body fat and
least in blood (Table 3). There was a significant correlation
between the concentrations in blood and those in other tissues. The
storage ratio (concentration in body fat/concentration in diet) did
not show any significant differences between the sexes or between
the two dose levels.
Table 3. Mean concentrations (mg/kg) of isobenzan in tissues of dogs
dosed orally with isobenzan for 2 years
Dose Fat Muscle Liver Brain Blood
0.025 mg/kg body 2.9 0.25 1.2 0.2 0.02
weight
0.1 mg/kg body 9.5 0.8 4.2 0.4 0.04
weight
The first litter from a female Beagle hound dosed with 0.08 mg
isobenzan/kg body weight per day (section 7.5.3) consisted of one
male, one female, and one still-born male. The concentrations of
isobenzan in the brain, liver, muscle, heart, and kidneys of the
still-born pup were below 0.2 mg/kg, with the liver containing the
highest level of 0.16 mg/kg. The female pup, which only fed on the
mother's milk, showed convulsions 15 days after birth and was killed
at 17 days of age. The blood contained 0.09 mg isobenzan per kg and
the urine 0.02 mg/kg. The concentrations in organs and tissues were
less than 1 mg/kg, the highest levels being in muscle (0.94 mg/kg),
liver (0.65 mg/kg), and fat (0.48 mg/kg). The milk from the mother
contained 0.7 mg/litre as whole milk and 3.4 mg/kg on a fat basis.
The remaining pup showed no ill effects. Four males and two females
in further litters also showed no ill effects (Brown & Richardson,
1964).
6.2.3 Domestic fowl
In a study by McCaskey et al. (1968), six Leghorn hens
received, in a gelatin capsule on each of 5 days, an amount of
isobenzan (94% purity) equivalent to 10-15 mg/kg of the average
weight of daily feed consumed. Eggs were collected on days 2-8, and
the hens were killed 3 days after the last dose. Residues in tissues
and eggs are given in Table 4.
Table 4. Concentrations of residues (mg/kg products) in tissues
and eggs of hens dosed orally with isobenzan
Tissue Residue concentration (mg/kg)
Abdominal fat 10.6
White meat 0.2 (on fat basis: 3.4)
Dark meat 0.5 (on fat basis: 4.3)
Egg yolk 0.1 on day 4; 0.4 on day 5; 0.5 on day 7
0.7 on day 8
6.2.4 Cow
Three Jersey cows were fed for 28 days at concentrations of
technical isobenzan of 0, 0.005, or 0.02 mg/kg in their daily ration
(average ration, 20 kg per cow). Residues found in the milk of the
cow fed 0.005 mg/kg increased from 0.4 µg/litre to 2 µg/litre at the
end of the feeding period and decreased rapidly thereafter. Higher
residues of up to 7.7 µg/litre were present in the milk from the cow
fed 0.02 mg/kg, which decreased to 1.5 µg/litre whole milk 10 weeks
after the last day of dosing (Hardee et al., 1964).
Following the consumption of feed contaminated with relatively
low concentrations of isobenzan, the concentration in milk rose
rapidly within a few hours to days, levelling off at a plateau
characteristic for each concentration in the diet. The average
milk/diet ratio for isobenzan was 0.4 to 0.5 for feeding levels of
0.005 to 0.02 mg/kg diet (Biehl & Buck, 1987).
In a study by Bishop & Huber (1964), four groups of three
lactating Holstein cows were fed 0, 0.02, 0.06, or 0.15 mg
isobenzan/kg (corresponding to 0.47, 1.38, or 3.38 mg/cow per day)
in their daily ration for 90 days. The concentrations in the milk
were directly proportional to the dietary level. Increases in
concentration were noted during the entire treatment period for the
0.06- and 0.15-mg/kg feeding levels, reaching 0.033 and
0.071 mg/litre of milk. Trace amounts (up to 0.008 mg/litre) were
found in the milk from cows fed 0.02 mg/kg. At the highest dose,
residues of 0.016 mg/litre were still present in the milk 60 days
after treatment, while in the groups fed 0.06 and 0.02 mg/kg, the
residues were then negligible. Residues in fat from biopsies taken
88 days after the treatment finished reflected the exposure levels,
being 0.11, 0.26, and 0.53 mg/kg tissue for the three levels.
6.3 Metabolic transformation
6.3.1 Vertebrates
The radioactive residue present in the urine and faeces of rats
treated with a single intravenous injection of 14C-isobenzan
consisted of a hydrophilic metabolite that, after hydrolysis, gave
isobenzan lactone (Kaul et al., 1970).
It is probable that both chlorine atoms on the tetrahydrofuran
ring are first replaced by hydroxyl groups. The resulting "acetale",
an unstable intermediate, is converted to the gamma-lactone (Korte,
1967) (Fig. 1).
6.3.2 Invertebrates
Mosquito larvae (Aedes aegypti) metabolize isobenzan,
labelled with 14C in the hexachloropentane ring or at the 1,3
position, to a metabolite more hydrophilic than that produced by
microorganisms. This metabolite consists of at least three
components. The hydrolytic product of one of these components was
identified as isobenzan lactone (Korte, 1963, 1967; Korte & Stiasni,
1964).
 6.3.3 Microorganisms
Isobenzan labelled with 14C in the hexachloropentane ring or
at the 1,3 position is metabolized by the fungi Aspergillus niger,
Aspergillus flavus, Penicillium chrysogenum, and Penicillium
notatum to isobenzan lactone, the same metabolite as found in
animal metabolism studies (Korte, 1963; Korte & Stiasni, 1964).
6.4 Elimination and excretion in expired air, faeces, and urine
6.4.1 Oral administration
In a study by Korte (1963), ten rabbits received in their diet
2 mg 14C-isobenzan, diluted with non-radioactive isobenzan, every
2 days to a total amount of 25-30 mg per rabbit. After 3 months,
about 50% of the total radioactivity administered had been excreted,
mainly in the urine. On the other hand, rats excreted most of the
radioactivity via bile into the gastrointestinal tract and excreted
these products with the faeces. No unchanged isobenzan was excreted,
only hydrophilic metabolites.
6.4.2 Parenteral administration
When Carworth Farm rats received daily intraperitoneal
injections of isobenzan (98% purity) of 0.25, 1, or 2 mg/kg body
weight 5 days per week for 2 weeks (section 7.2.3), they excreted
less than 1% of the daily dose as unchanged isobenzan in the faeces
(Brown et al., 1962).
In a study by Kaul et al. (1970), male and female rats
received a single intravenous injection of 15 µg 14C-isobenzan/kg
body weight. The male rats excreted 1% of the applied radioactivity
in the urine and 12% in the faeces within 48 h, while the females
excreted 5% and 11%, respectively.
After having been administered intravenously to rats with
cannulated bile ducts, 14C-isobenzan was excreted as hydrophilic
metabolites in the bile (Korte, 1963, 1967).
Male rabbits given an intravenous injection of 14C-isobenzan
(241 µg/kg body weight) excreted 12% in the urine and 1% in the
faeces within 96 h (Kaul et al., 1970).
6.5 Retention and turnover
The absorption of isobenzan into the body is determined by
measuring the insecticide in the blood. No human studies exist
relating the concentration of isobenzan in the blood at the state of
equilibrium to the total daily intake or relating the concentration
of isobenzan in the blood to that in the tissues. However, in
experimental animals, such relationships have been determined. Thus,
in human beings, measurement of the blood concentration of the
insecticide at the state of equilibrium is assumed to reflect total
absorption by all routes (skin, pulmonary, and oral exposure) as
well as the storage level in adipose tissue, thereby providing a
measure of the total body burden of isobenzan.
From human data, it is estimated that the biological half-life
of isobenzan in blood is of the order of 2.8 years (Jager, 1970)
(section 8.2).
7. EFFECTS ON LABORATORY MAMMALS AND IN VITRO TEST SYSTEMS
7.1 Single exposure
7.1.1 Oral administration
In rats, the first signs of toxicity were evident approximately
1 h after the administration of a lethal dose and consisted of
lethargy followed by muscular twitching, laboured breathing, and,
finally, general convulsions. The majority of deaths occurred within
the first 20 h after administration. No specific changes were
observed in the organs of fatally intoxicated animals. Signs of
intoxication were similar in mice, rats, guinea-pigs, cats, dogs,
and chickens. Surviving animals recovered completely (Brown et al.,
1962; Worden, 1969).
Oral LD50 values for the various animal species range from
1.6 to 10 mg/kg and are summarized in Table 5.
Table 5. Oral LD50 values for technical isobenzan
Species Vehicle LD50 Reference
(mg/kg body
weight)
Mouse arachis oil 10a,b Worden (1969)
Mouse corn oil 12.5 Spynu (1964)
Rat corn oil 4.8 Howard et al. (1957)
Rat corn oil 14.4 Spynu (1964)
Rat carboxymethyl cellulose 5.4 Stevenson (1964)
Rat dimethylsulfoxide 7.2 Stevenson (1964)
Rat arachis oil 10b Worden (1969)
Rat unknown 8.0 Layton et al. (1987)
Guinea-pig arachis oil 2.5a,b Worden (1969)
Golden hamster arachis oil 20b Worden (1969)
Dog unknown 1.6 Stevenson (1964)
a Average of males and females.
b Isobenzan 99.5%.
7.1.2 Dermal administration
Signs of intoxication are the same as those described for acute
oral intoxication but they develop more slowly (Brown et al.,
1962). The LD50 values are summarized in Table 6.
Table 6. Dermal LD50 values for technical isobenzan
Species Vehicle LD50 Reference
(mg/kg body
weight)
Rat arachis oil occluded, 4 Zavon (1961)
non-occluded, 10
Rat corn oil non-occluded, 8.5 Spynu (1964)
Rat crystalline form occluded, 60 Stevenson (1964)
(approximately)
7.1.3 Parenteral administration
The acute toxicity of isobenzan administered parenterally is
similar to that following oral administration (Table 7). The signs
of intoxication are similar to those observed after acute oral
toxicity but develop more rapidly, starting less than 1 h after the
injection (Brown et al., 1962; Worden, 1969).
Table 7. Parenteral LD50 values for technical isobenzan
Route Species Vehicle LD50 Reference
(mg/kg body
weight)
Intraperitoneal mouse xylene emulsion 8.2 Stevenson (1964)
Intraperitoneal mouse methoxytriglycol 6.0 Cole & Casida (1986)
Intraperitoneal rat dimethylsulfoxide 3.6 Stevenson (1964)
Subcutaneous rat, arachis oil 6-10 Worden (1969)
mouse
Intravenous rat unknown 1.7 Zavon (1961)
7.1.4 Formulated material
A 50% wettable powder formulation and a 15% emulsifiable
concentrate (in mixed petroleum xylenes) were tested for their acute
oral toxicity to rats, mice, rabbits, hamsters, cats, and dogs. The
LD50 values, when expressed as active material, were comparable
with those of isobenzan itself (Stevenson, 1964; Worden, 1969). The
dermal LD50 value for the 15% emulsifiable concentrate was
25-35 mg isobenzan/kg body weight for Hooded-Lister rats and 6 mg
isobenzan/kg body weight for New Zealand white rabbits, whereas in
the case of the 50% wettable powder the LD50 was 41 mg
isobenzan/kg body weight for rabbits (Stevenson, 1964). Rabbits
exposed dermally to the 15% emulsifiable concentrate formulation
behaved differently to rats similarly exposed. The rabbits generally
lost weight due to anorexia and a failure to drink, and they would
then convulse, even as much as 3 weeks after the exposure. However,
if feeding and drinking were resumed quickly, the rabbits did not
convulse (Brown, 1963, 1964).
7.1.5 Metabolites
The acute oral and intravenous toxicities of the metabolite
isobenzan lactone for mice were 30 times lower than those of
isobenzan. The oral and intravenous LD50 values were 306 mg/kg
body weight and > 100 mg/kg body weight, respectively (Korte,
1967).
7.2 Short-term exposure
The short-term, long-term, and reproduction studies that were
used for establishing a no-observed-effect level are summarized in
Table 8.
7.2.1 Oral administration
7.2.1.1 Mouse
In a study by Brown et al. (1962), groups of five male and
five female Carworth Farm No. 1 mice were fed diets containing
isobenzan (98%) at levels of 1, 2, 3, 4, or 5 mg/kg for up to 3
weeks. No mortality was observed at 1 mg/kg, but at the higher
concentrations the mortality was dose related, reaching 100% at
5 mg/kg diet.
7.2.1.2 Rat
Groups of eight male and eight female Carworth Farm rats were
intubated with isobenzan (98%) in dimethylsulfoxide (0.25, 1, or
2.5 mg/kg body weight per day) 5 days/week for 2 weeks. All rats
dosed with 2.5 mg/kg body weight died within 5 days. All the females
and two of the males dosed with 1 mg/kg body weight died after 5-7
doses, whereas only one female dosed with 0.25 mg/kg body weight
died after five doses. Weight loss preceded the death of the animals
at the highest dose, this being the result of reduced food intake.
No gross or microscopic changes were found in the rats dying from
intoxication or in the survivors sacrificed 14 days after the last
treatment (no details were reported concerning the parameters
studied) (Brown et al., 1962).
In a study by Howard et al. (1957), groups of 10 male and 10
female Sprague-Dawley rats were fed diets containing 0, 2.5, 12.5,
or 25 mg isobenzan/kg diet for 30 days. One female in the
highest-dose group died. Some of the rats fed with levels of 12.5 or
25 mg/kg were nervous and irritable, and the reduced body weight
gain in both these groups correlated with diminished food
consumption. At autopsy, there were some areas of necrosis of heart
cells and haemorrhages in the heart muscle of animals fed with
levels of 12.5 or 25 mg/kg diet.
Table 8. Short- and long-term oral exposure to isobenzan
Animal (strain) Exposure period NOAELa LOAELa References
(mg/kg diet) (mg/kgdiet)
Rat (Sprague-Dawley) 30 days 2.5 (0.12) 12.5 (0.62): irritability, Howard et al. (1957)
decreased body weight gain,
histopathology changes in heart
Dog (Beagle) 2 years (0.08) (0.125): convulsion, mortality Brown & Richardson (1964)
Dog (Scottish terrier) 2 years (0.025) (0.1): decreased body weight gain, Worden (1969)
increased liver weight
Rat (Hooded-Lister) 2 years 5 (0.25) 17.5 (0.87): convulsions Worden (1969)
Reproduction study
Rat (Carworth Farm) one generation 0.1 (0.005) 1 (0.05): decreased survival of pups Chambers (1962a,b)
(2 litters) (pups) 5 (0.25): convulsions
1.0 (0.05)
(parents)
a Figures in parentheses are values for exposure concentration in mg/kg body weight.
In a study by Worden (1969), groups of five male and five
female Hooded-Lister rats were fed diets containing isobenzan
(99.5%) (25, 35, 50, or 100 mg/kg diet) as a 15% emulsifiable
concentrate (in mixed petroleum xylenes). After 37 days on the test
diets, half of the surviving animals of each group were transferred
to a control diet, while the remainder continued on the test diets
for a further 42 days. During the first period, all rats fed with
100 mg/kg diet died except for one male. One male and two females
fed with 50 mg/kg diet died as did three females fed with 35 mg/kg
diet. There were no further deaths among the rats after dosing was
discontinued. During the second period of feeding with the test
diet, one female fed at the lowest level died. In one rat only, a
female fed with 50 mg/kg, the classical signs of organochlorine
intoxication were seen in the liver. These have been described by
Hodge et al. (1967) as "enlarged centrolobular hepatic cells with
cytoplasmic oxyphilia and somewhat increased and peripheral
migration of the basophilic granules".
7.2.1.3 Dog
Pairs of Beagle hounds (one male and one female) were given
daily oral doses of 0, 0.08, 0.125, or 0.2 mg isobenzan (98%)/kg
body weight in olive oil in gelatin capsules for 2 years. The dogs
given the two highest doses had several convulsive episodes during
the course of the study. When a dog exhibited a convulsion, dosing
was discontinued for 8 weeks. The male dog dosed with 0.125 mg/kg
died one week after a third convulsive episode, which followed a
sudden fall in body weight. No signs of intoxication were observed
in the dogs dosed with 0.08 mg/kg body weight. During this study,
blood isobenzan concentrations were measured regularly, in
particular at the time of convulsions. Concentrations in muscle
tissue were determined 3 days after the convulsion, and the results
are given in Table 9. The blood concentrations in the dogs receiving
0.08 mg/kg body weight for 2 years without convulsions indicated
that a plateau was reached after a relatively short exposure. The
mean concentration was approximately 0.02 mg isobenzan/litre and the
maximum concentration 0.04-0.05 mg/litre. The two highest doses
caused convulsions (Brown & Richardson, 1964).
Groups of three male and three female Scottish terriers (2.5-9
kg body weight) received daily gavage doses of isobenzan (0, 0.025,
or 0.1 mg/kg body weight) as 15% emulsifiable concentrate (in mixed
petroleum xylenes) for 2 years. No deaths and no signs of
intoxication resulted from the isobenzan administration. Body weight
gain, urinalysis, and haematological and clinical chemistry values
for the dogs fed with 0.025 mg/kg body weight were comparable with
those of the control animals. At a dose level of 0.1 mg/kg body
weight, a decrease in body weight gain, a slight increase in serum
Table 9. Concentration of isobenzan in the blood and muscle of dogs
Dose (mg/kg Sex Number of Blood concentration Muscle tissue
body weight) convulsions at time of convulsion (mg/kg wet
(mg/litre) weight)
0.08 male none 0.04 (maximum
concentration)
female none 0.05 (maximum
concentration)
0.125 male first two 0.06
third (death) 0.11 16
female 4 0.03-0.06 0.18
0.2 male 9 0.02-0.11 0.36
female 6 0.07-0.08 0.68
alkaline phosphatase values during the second year, and an increase
in liver weight were noted. No evidence of histopathological lesions
attributable to isobenzan was found at either treatment level. The
no-observed-effect level resulting from this study was 0.025 mg/kg
body weight (Worden, 1969). The distribution of isobenzan in the
tissues examined is summarized in section 6.2.2.
7.2.2 Dermal administration
When groups of five female albino rabbits were given daily
applications of isobenzan in corn oil (0, 5, 10, 20, 30, or 40 mg
per rabbit) to the shaven skin for 3 weeks, mortality was high at
all dose levels, reaching 100% after 2 weeks for rabbits given doses
of 30 or 40 mg. Histopathological examination revealed necrosis of
the heart muscle, non-dose-related lesions of the liver, and
degenerative changes in cells of the central nervous system in a few
animals (Howard et al., 1957).
7.2.3 Intraperitoneal administration
Groups of five male and five female Carworth Farm rats were
injected intraperitoneally with isobenzan (98%) in
dimethyl-sulfoxide (0.25, 1, or 2 mg/kg body weight per day) 5
days/week for 2 weeks. All rats given the lowest dose survived, but
two rats of each sex given 1 mg/kg and five males and four females
given 2 mg/kg died after 5-10 doses. No gross or microscopic lesions
were found in fatally intoxicated rats or in the survivors killed
7-14 days after the last treatment (no details concerning
parameters studied were reported) (Brown et al., 1962).
7.3 Long-term exposure
7.3.1 Rat
Groups of 25 male and 25 female Hooded-Lister rats were fed, at
dietary concentrations of 5, 17.5, or 30 mg/kg (equivalent to 0.25,
0.875, or 1.5 mg/kg body weight), isobenzan as a 15% emulsifiable
concentrate (in mixed petroleum xylenes) for 2 years. Three control
groups, each consisting of 25 male and 25 female rats, were used.
Additional groups of rats, fed at the same dose levels and
accompanied by separate control groups, were used for liver and
kidney function tests after 20, 66, and 104 weeks. Five females fed
at the highest dose level died during the first 3 weeks of the
study. Signs of intoxication (such as ruffled coat, lethargy,
muscular twitches, and mild to violent convulsions) were observed in
animals given 17.5 or 30 mg/kg diet, mainly during the first few
weeks. There were no adverse effects on body weight gain, food
conversion ratios, haematological parameters, serum alkaline
phosphatase, serum glutamic-pyruvic transaminase, total serum
protein, or albumin/globulin ratios. Absolute liver weight was
increased in the animals given the highest dose level. Gross and
microscopic examinations did not reveal any compound-related
changes, with the possible exception of thyroid hyperplasia recorded
in four males and six females at a level of 30 mg/kg diet. The liver
function test (bromsulfophthalein) and kidney function test (phenol
red) showed no deviations from control values. No significant
increase in the number and type of tumours was found. The
no-observed-effect level for toxicological effects was 5 mg
isobenzan/kg diet (equivalent to 0.25 mg/kg body weight) for 2 years
(Worden, 1969).
7.4 Skin irritation
In a study by Worden (1969), four male guinea-pigs received a
single application (2 mg/kg body weight) of isobenzan (99.5%) as a
0.2% w/v solution in arachis oil to the shaved skin. The material
was not removed from the skin. The animals were kept under
observation for 21 days, but no irritation was observed.
Two male and two female rabbits were given 12 successive daily
applications (0.5 mg/kg body weight) of isobenzan (99.5%) and six
female rats received 30 successive daily applications (0.3 mg/kg
body weight) of isobenzan as a 0.2% w/v solution in arachis oil to
the shaved skin. The material was left on the skin and the
observation period was 21 days after the last application. No
irritation was observed (Worden, 1969).
7.5 Reproductive toxicity, embryotoxicity, and teratogenicity
No studies on the potential teratogenicity of isobenzan have
been reported.
7.5.1 Mouse
In a range-finding test, groups of 25 male and 25 female BALB/C
mice were fed diets containing 0, 1, 2.5, 5, or 10 mg isobenzan
(94%)/kg. The mice in the 5- and 10-mg/kg dose groups all died
within 64 and 24 days, respectively. Only 20% of those in the
2.5-mg/kg group survived for 120 days. The 1 mg/kg group survived
and reproduced normally (no further data were reported). In the main
study, groups of 108 mice of each sex of Swiss strain BALB/C were
fed control diet and 106 mice of each sex were fed 1 mg isobenzan/kg
diet for 30 days, after which they were randomly paired and
continued on the same diet for 90 days. The number of litters
produced, litter size, sex ratio, and mortality were recorded in
this one-generation reproduction study. There were no statistically
significant differences between the control and isobenzan-treated
animals for any of the parameters measured (Ware & Good, 1967).
7.5.2 Rat
A one-generation, 2-litter reproduction study was carried out
with groups of 20 male and 20 female weanling Carworth Farm rats
that were fed diets containing isobenzan at levels of 0, 0.1, 1, 5,
or 10 mg/kg for 100 days and then mated. Convulsions were seen
during the mating period and pregnancy in females fed 10 mg/kg, and
during the lactation period in one female fed 5 mg/kg. Pups of the
second mating from both these treatment groups were also seen to
convulse. Mean litter size and survival of pups were markedly
reduced in the 10-mg/kg group and, to a lesser extent, in the
5-mg/kg group. No clinical signs were observed in the 1-mg/kg group.
At this dose level, the mean litter size was comparable with
controls, but survival of the pups at 21 days was decreased. No
effects attributable to isobenzan were found in the 0.1-mg/kg group
(Chambers, 1962a,b).
7.5.3 Dog
During a 2-year study (section 7.2.1.3), three litters were
born to a female Beagle hound dosed daily with 0.08 mg isobenzan/kg
body weight five days per week. A male Beagle dog, dosed at the same
level, was sire for several of the litters. The first litter
consisted of one male and one female, both normal, and one
still-born male. Fifteen days after birth, the female pup began to
convulse, having only fed on the mother's milk (which contained
0.7 mg isobenzan/litre) and at 17 days the pup was sacrificed.
Concentrations of isobenzan in tissues are given in section 6.2.2.
Two further litters of pups were born to the original dam (two male
pups in the first litter and two pups of each sex in the second).
These animals all appeared normal and healthy and showed no ill
effects from the ingestion of maternal milk. One litter of pups, one
male and one female, was born to a female Beagle dog fed 0.125 mg/kg
body weight per day for two years. These pups did not exhibit any
signs of intoxication and were killed when 26 days old. Autopsy did
not reveal any structural changes. It did not appear that isobenzan
interfered with either the male or female reproduction function
(Brown & Richardson, 1964).
7.6 Mutagenicity and related end-points
No information on mutagenicity is available.
7.7 Carcinogenicity
No adequate carcinogenicity studies have been reported.
7.7.1 Mouse
Groups of 18 male and 18 female mice of two hybrid strains (the
F1 hybrids C57Bl/6 x C3H/Anf and C57Bl/6 x AKR) were given the
maximum tolerated dose of isobenzan in 0.5% gelatin (0.215 mg/kg
body weight) daily from 7 to 28 days of age by stomach tube.
Thereafter the isobenzan was mixed in the diet to a concentration of
0.646 mg/kg, and the mice were killed at 18 months of age. No
significant increase in tumour incidence was found (Innes et al.,
1969).
7.7.2 Rat
In a long-term feeding study on rats (section 7.4.1), no
evidence of carcinogenicity was found as a result of feeding diets
containing up to 30 mg isobenzan/kg for 2 years (Worden, 1969).
7.8 Special studies
7.8.1 Biochemical studies
Mehrotra et al. (1982) studied the comparative effects of
cyclodiene compounds on different ATPase activities in beef and rat
brain synaptosomal fractions in vitro. Isobenzan significantly
inhibited Na+-K+-ATPase in rat brain synaptosomes. A
dose-related response was observed at up to 80 µmol/litre, but no
increase in inhibition was observed with a further increase in the
concentration of the compound. Oligomycin-sensitive Mg2+-ATPase in
rat brain synaptosomes was significantly inhibited by isobenzan, a
maximum of 64% inhibition occurring at 120 µmol/litre. In addition,
the oligomycin-insensitive Mg2+-ATPase in rat brain synaptosomes
was inhibited, and the inhibition was concentration dependent.
Isobenzan did not have any effect on K+-stimulated
p-nitrophenylphosphatase, an enzyme which is known to represent
the dephosphorylation step in the overall reaction of the
Na+-K+-ATPase. Oligomycin-sensitive Mg2+-ATPase in beef heart
mitochondria was significantly inhibited.
Isobenzan did not affect the activity of adenylate cyclase or
phosphodiesterase in Sprague-Dawley rat brain synaptosomes in vitro
at concentrations of up to 200 µmol/litre (Kodavanti et al.,
1988).
Several studies into the effect of isobenzan on the transfer of
ammonia in brain have indicated that isobenzan acts by increasing
brain ammonia levels before and during convulsions. Glutamic acid,
glutamine, and alpha-ketoglutaric acid, which are utilized in an
ammonia-binding mechanism, become overwhelmed, resulting in free
ammonia accumulating in the cerebral tissues (Hathway & Mallinson,
1964; Hathway, 1965; Hathway et al., 1965).
7.8.2 Neurotoxicity
In vitro studies using fresh rat brain synaptic membranes
showed isobenzan to be a potent inhibitor of the binding of the
convulsant tert-butylbicyclophosphorothionate (TBPS) to
brain-specific sites, thereby indicating an action at the
gamma-aminobutyric acid (GABA)-regulated chloride channel. Metabolic
activation by rat liver microsomes did not enhance the potency for
inhibition. This inhibition indicates that isobenzan binds to the
same site as TBPS, suggesting that isobenzan acts in a manner
similar to non-competitive GABA-A antagonists and providing a basis
for its convulsant action in mammals (Lawrence & Casida, 1984).
Bloomquist et al. (1986) produced a concentration-dependent
inhibition of 36Cl uptake into mouse brain vesicles by adding
isobenzan to mouse brain homogenate. The inhibitory activity was
confined to that portion of 36Cl uptake that was GABA dependent.
The insecticide concentration producing 50% inhibition (I50) of
36Cl uptake was 2.0 (0.83 to 5.1) µmol/litre, and the inhibitory
potency (I50) value for 35S-TBPS binding in rat brain
synaptosomes was 0.30 µmol/litre (Lawrence & Casida, 1984).
Cole & Casida (1986) confirmed in a study with male
Swiss-Webster mice administered isobenzan intraperitoneally that a
correlation also exist in vivo between binding to mouse brain GABA
receptors and convulsive activity. The inhibitory potency (IC50)
for in vitro TBPS binding to mouse brain synaptosomes is
0.03 µmol/litre. It was shown that the inhibitory potencies of
cyclodienes, including isobenzan, parallel their acute oral
toxicities. Isobenzan was slightly more potent than endrin and
produced virtually complete inhibition of GABA-dependent chloride
uptake at 30 µmol/litre. There was a significant linear correlation
between the 36Cl flux and 35S-TBPS-binding assays.
7.8.3 Pharmacological studies
Pharmacological studies on the function of organ systems in
various animal species (such as rats, guinea-pigs, rabbits, cats,
and frogs) after the administration of isobenzan by different routes
showed that the only significant effect was a disturbance of the
central nervous system associated with convulsions. This effect was
due to stimulation of the higher brain centres at the level of the
medulla and above. Changes in respiratory rate, heart rate, and
salivary secretion were probably mediated by the central nervous
system as a secondary effect of central nervous stimulation.
Barbiturates were found to control these convulsions. The
stimulation of brain activity was reflected in the occurrence of
electroencephalographic changes in the pre-convulsive stage and
during the convulsive episodes. This corresponds to the situation in
cases of human intoxication by occupational exposure to cyclodiene
insecticides as described by Hoogendam et al. (1962, 1965) and
Chambers (1962c).
Ibrahim (1964) showed that isobenzan injected intraperitoneally
at a toxic dose (7 mg/kg body weight) into male Wistar rats produced
a higher tension of contraction in the gastrocnemius muscle at lower
frequencies of stimulation than in controls. The maximum tetanic
tension was also attained at a lower frequency. An increase in the
duration of the "active state" of the muscle was considered to be
the most like explanation.
8. EFFECTS ON HUMANS
8.1 General population exposure
No poisoning incidents or untoward effects of long-term
exposure of the general population have been reported.
8.2 Occupational exposure
Isobenzan was initially manufactured and handled in the
Netherlands between 1958 and 1965. Aldrin, dieldrin, and endrin were
also produced in the same manufacturing plant and, consequently, in
many cases the exposure was mixed. Routine medical examination of
233 workers, who were exposed for more than 4 years and who followed
normal procedures during that period, did not reveal any
abnormalities in EEG, clinical chemistry or haematological
parameters, or liver microsomal enzyme induction (Jager, 1970;
Versteeg & Jager, 1973). The mean isobenzan concentration in the
blood of 20 operators after cessation of exposure decreased from
22 µg/litre in 1965 to 7 µg/litre in 1969. The biological half-life
of isobenzan in the blood (at the state of equilibrium of isobenzan
in body tissues) was estimated to be of the order of 2.8 years
(Jager, 1970).
In the 7 years of isobenzan production, 15 cases of clinical
intoxication, including eight cases with convulsions, were reported.
The mean concentration of isobenzan in the blood of nine workers at
the time of intoxication was 23 µg/litre, the range being
17-30 µg/litre. Although these workers recovered fully, it took
longer than with the related cyclodiene insecticides. In three
cases, certain typical complaints, such as headache, dizziness,
drowsiness, and irritability, persisted for 6 months, and the return
to normal of the modified EEG pattern sometimes took more than a
year. In one case of acute over-exposure, without signs of
intoxication, the blood isobenzan concentration decreased from
8 µg/litre to less than 2 µg/litre within 3 days (Jager, 1970).
The data from plant workers indicated a threshold level of
isobenzan in blood below which no signs or symptoms of intoxication
occur. This level was found to be 15 µg/litre (Jager, 1970).
Ribbens (1985) carried out a mortality study on the
above-mentioned industrial workers exposed to aldrin, dieldrin,
endrin, and isobenzan. Vital status and cause of death were assessed
for 232 of the total population of more than 1000 workers. This
group was selected for follow-up on account of the high degree of
exposure in the initial years of manufacturing and formulation and
the long exposure (mean 11 years) and observation (mean 24 years)
periods. Total observed mortality was 25 as opposed to 38 expected
on the basis of death statistics for the male Dutch population. Of
the nine cancer deaths, three were caused by lung cancer, while the
remaining six were each of a different nature. The author concluded
that although exposures in this group were high and exposure as well
as observation periods long, this study did not reveal any
indication of a specific carcinogenic activity of these pesticides.
9. EFFECTS ON OTHER ORGANISMS IN THE LABORATORY AND FIELD
9.1 Microorganisms
In laboratory studies, sandy loam soil was treated with 250 or
2500 mg isobenzan/kg (concentrations that exceeded the recommended
rates). At both concentrations, the carbon dioxide production was
inhibited to almost the same extent (21 and 24%, respectively)
during a 30-day test period. The addition of glucose to the soil
reduced the inhibitory effect to 5 and 4%, respectively. Isobenzan
at 250 mg/kg did not influence nitrification in the soil up to 18
days after treatment (Bartha et al., 1967).
Screening studies on microorganisms in pure culture were
carried out on nutrient agar plates with isobenzan either
incorporated in a uniformly emulsified form (1000 mg/kg) or as a
thin surface film (1 mg/cm2). The growth of the gram-positive
Bacillus megaterium was inhibited, but not that of various
gram-negative organisms such as several Pseudomonas strains,
Escherichia coli, Klebsiella aerogenes W5, or Achromobacter
butyri (Trudgill & Widdus, 1970).
9.2 Aquatic organisms
Groups of five adult Harlequin fish (Rasbora heteromorpha)
were exposed for 2 h at a temperature of 20 °C to water (pH 7.2)
containing isobenzan (99%), dissolved in DMSO, at a concentration of
0.01, 0.1, or 1 mg/litre. The fish were then transferred to clean
water and observed for an additional 48 h. The treatment with
1 mg/litre caused disorientation and the fish became excited by
external stimuli, lost the ability to swim, and, finally, all died
within 1 h. In some cases, they appeared to convulse. Isobenzan at
0.1 mg/litre caused similar symptoms; within 2 h of exposure, all
five fish died. No fish died at 0.01 mg/litre, but slight changes in
swimming behaviour were observed (Brown et al., 1962). When
guppies (Poecilia reticulata) were tested in the same way, the
symptoms of intoxication and susceptibility were similar to those in
Harlequin fish, but the guppies were slower to react (Brown et al.,
1962).
Data on the acute toxicity of isobenzan for aquatic organisms
in flow-through tests are given in Table 10.
Table 10. Acute toxicity of isobenzan (technical grade, 94%) for aquatic organisms
Organism Developmental Temperature Parameter Concentration Reference
stage (°C) (µg/litre)
Brown shrimp juvenile 17 48-h EC50 0.034a US EPA
(Penaeus (1987)
aztecus)
Eastern oyster juvenile 18 96-h EC50 32b US EPA
(Crassostrea (1987)
virginica)
Sheepshead minnow juvenile 17 48-h LC50 2.0a US EPA
(Cyprinodon (1987)
variegatus)
Spot juvenile 13 48-h LC50 0.32c US EPA
(Leiostomus (1987)
xanthurus)
a Salinity 30 ng/litre.
b Salinity 33 ng/litre.
c Salinity 22 ng/litre.
9.3 Terrestrial organisms
9.3.1 Soil invertebrates
In laboratory studies, plain-field sand was treated once with
0.05 mg isobenzan/kg and stored at 13 or 24 °C. The springtail
(Folsomia candida) was used for bioassays lasting up to 16 weeks.
The biological activity of isobenzan persisted slightly longer at
the higher temperature, killing 100% of the insects after 16 weeks
(Thompson, 1973).
9.3.2 Birds
9.3.2.1 Acute toxicity
Isobenzan (> 99%) is highly toxic to birds when administered
as a single oral dose (Table 11). LD50 values range from
1-10 mg/kg.
9.3.2.2 Short-term toxicity
When groups of five male and five female Japanese quail
(Coturnix coturnix japonica) were fed diets containing 2 or 10 mg
isobenzan/kg, the mean survival time in the high-dose group was 6.9
days (range, 2-20 days). The residues in the liver and brain
averaged 3.4 mg/kg and 1.4 mg/kg, respectively. In the low-dose
group, the mean survival time was 45.9 days (range, 19-65 days) and
the average residues in liver and brain were 6 mg/kg and 1.6 mg/kg,
respectively. The concentration of isobenzan in the liver of birds
fed 2 mg/kg was significantly higher than that in the high-dose
group, but the concentration in the brain showed no difference
(Koeman, 1971).
Table 11. Oral LD50 values of isobenzan for birds
Species LD50 (mg/kg Reference
body weight)
Mallard duck (female) 4.15 Hudson et al. (1984)
(Anas platyrhynchos) (2.47-6.97)
Coturnix quail (female) 4.2 Schafer & Brunton (1979)
(Coturnix coturnix)
Grackle 1.3 Schafer & Brunton (1979)
(Quiscalus quiscula)
Pigeon 10 Schafer & Brunton (1979)
(Columba livia)
Red-winged blackbird (male) 3.2 Schafer & Brunton (1979)
(Agelaius phoeniceus)
Sparrow 1 Schafer & Brunton (1979)
(Passer domesticus)
Starling 2.4 Schafer & Brunton (1979)
(Sturnus vulgaris)
When groups of 20 Rhode Island Red laying hens were fed
isobenzan (15% emulsifiable concentrate) at levels of 0.1, 0.25,
0.75, or 1 mg/kg diet for 14 weeks, no effects were seen on food
consumption, egg production, egg weight, or egg fertility (Verma
et al., 1967).
9.4 Population and ecosystem effects
9.4.1 Soil microorganisms
In a sugar cane field in India, Srivastava (1966) studied the
effect of isobenzan (1 kg/ha) on nitrification of ammonium sulfate
in the soil. The insecticide-treated soil contained higher amounts
of total inorganic nitrogen and ammonium nitrogen (80% increase) up
to 60 days after treatment than did untreated soil samples,
indicating impaired nitrification.
9.4.2 Soil invertebrates
In a study by Kelsey & Arlidge (1968), five sandy loam plots of
pasture in New Zealand were treated with Telodrin (15% emulsifiable
concentrate) at a rate of 2 kg isobenzan/ha in June 1962. The
populations of all recorded groups (grass grub, porina, Collembola,
Diptera, Hemiptera, Coleoptera, mites, and earthworms), except
nematodes, were drastically reduced. There was no recovery in the
populations of any of the affected groups during the period up to
October 1965. In silt loam plots treated with isobenzan granules
(1 kg/ha) in April 1964, the populations of Coleoptera, Collembola,
Diptera, mites, and earthworms were all reduced, but to a lesser
extent than in the test using 2 kg/ha. However, grass grub and
Hemiptera were not significantly affected, and nematode numbers were
elevated 2 years after the treatment. Studies on root development
showed that 90% of roots with root hairs were located within a mat
of plant debris above the soil, whereas in controls 99% were located
in the soil. Plant growth was retarded. The moisture content of the
treated plots was significantly less than that of control plots,
indicating that the capacity of soil to absorb and retain water had
been reduced.
When loose sandy soil in New Zealand was treated with 2.25 kg
isobenzan/ha as 5% granules, the population reduction, 1-18 weeks
after treatment, was 90-100% for larval Coleoptera and Lepidoptera
and 75% for Diptera and earthworms. The number of surface
arthropods, 5-15 days after treatment, was reduced by 45%. Six
months after treatment, little effect was found on nematodes
(13% reduction), bacteria (18% reduction), and fungi (7% increase)
(Moeed, 1975).
REFERENCES
ANON (1974) Aldrin, dieldrin, and endrin. Determination of residues
of organochlorine insecticides in crops, soils, and animal products,
Sittingbourne, Shell Research (Residue Analytical Method WAMS 60-1).
BARTHA, R., LANZILOTTA, R.P., & PRAMER, D. (1967) Stability and
effects of some pesticides in soil. Appl. Microbiol., 15(1): 67-75.
BAUER, U. (1972) [Behaviour of a series of plant-protecting agents
during the treatment of groundwater by slow sand filtration.]
Schriftenr. Ver. Wasser-Boden-Lufthyg. (Berlin-Dahlem), 37: 91-102
(in German).
BIEHL, M.L. & BUCK, H.B. (1987) Chemical contaminants: their
metabolism and their residues. J. Food Prot., 50(12): 1058-1073.
BISHOP, J.L. & HUBER, J.T. (1964) Secretion of Telodrin in the milk
of cows fed varying levels of Telodrin. J. dairy Sci., 47: 552-554.
BLOOMQUIST, J.R., ADAMS, P.M., & SÖDERLAND, D.M. (1986) Inhibition
of gamma-aminobutyric acid stimulated chloride flux in mouse brain
vesicles by polychlorocycloalkane and pyrethroid insecticides.
Neurotoxicology, 7(3): 11-20.
BOWMAN, M.C., SCHECHTER, M.S., & CARTER, R.L. (1965) Behavior of
chlorinated insecticides in a broad spectrum of soil types. J.
agric. food Chem., 13: 360-365.
BROWN, V.K.H. (1963) Agricultural chemicals: the relative
percutaneous toxicities of Nitrophoska fertiliser granules
impregnated with aldrin and Telodrin, Sittingbourne, Shell Research
(Technical Memorandum TOX 10/63).
BROWN, V.K.H. (1964) Some effects of percutaneously absorbed
Telodrin in rabbits, Sittingbourne, Shell Research (Report No. PPR
TL/2/64).
BROWN, V.K.H. & RICHARDSON, A. (1964) Chronic oral exposure of
beagle hounds to Telodrin and dieldrin: report on the first two
years, Sittingbourne, Shell Research (Report No. PPR TL/1/64).
BROWN, V.K.H., CHAMBERS, P.L., HUNTER, C.G., & STEVENSON, D.E.
(1962) The toxicity of Telodrin for vertebrates, Sittingbourne,
Shell Research (Report No. R(T)-2-62).
BUICK, A.R. (1964) Telodrin residues on tobacco from Australia,
Sittingbourne, Shell International Chemical Company, Ltd (Technical
Memorandum 132/64)
BUICK, A.R. (1965) Telodrin residues in potatoes from India,
Sittingbourne, Shell International Chemical Company, Ltd (Technical
Memorandum 25/65).
BUICK, A.R. & COLE, E.R. (1965) Telodrin residues in sweet potatoes
from South Africa, Sittingbourne, Shell International Chemical
Company, Ltd (Technical Memorandum 25/65).
BULL, M.S. & MARLOW, R.G. (1967) Insecticide residues in diet
samples from Nicaragua, Sittingbourne, Shell International Chemical
Company, Ltd (Technical Service Note 85/67).
BULL, M.S. & RAMSDEN, D.P. (1967) Residues of organochlorine
insecticides in samples from Venezuela, Sittingbourne, Shell
International Chemical Company, Ltd (Technical Service Note 86/67).
CHAMBERS, P.L. (1962a) Agricultural chemicals: the effect of
Telodrin on reproduction in the rat when fed at various levels in
the diet. Report No. 3, Sittingbourne, Shell International Chemical
Company, Ltd (Technical Memorandum TOX 24/62).
CHAMBERS, P.L. (1962b) Agricultural chemicals: the effect of
Telodrin on reproduction in the rat when fed at various levels in
the diet. Report No. 4, Sittingbourne, Shell International Chemical
Company, Ltd (Technical Memorandum TOX 33/62).
CHAMBERS, P.L. (1962c) The physiological and pharmacological effects
of Telodrin, Sittingbourne, Shell Research (Report No. M(T)-4-62).
COLE, L.M. & CASIDA, J.E. (1986) Polychlorocycloalkane
insecticide-induced convulsions in mice in relation to disruption of
the GABA-regulated chloride ionophore. Life Sci., 39: 1855-1862.
CRABTREE, A.N. (1968) Concentration of chlorinated insecticides in
the whole blood of the CAPSA plant in Venezuela, Sittingbourne,
Shell Research (Report No. TLGR.0032.68).
DAVIES, J.M. (1966a) Chlorinated insecticide content of the blood of
formulators in the CAPSA plant in Venezuela, Sittingbourne, Shell
Research (Report No. IRR TL/14/66).
DAVIES, J.M. (1966b) Concentration of chlorinated insecticides in
the whole blood of formulators of the CAPSA plant in Venezuela,
Sittingbourne, Shell Research (Report No. IRR TL/37/66).
EDWARDS, C.A. (1965) Effects of pesticide residues on soil
invertebrates and plants. In: Proceedings of the 5th Symposium of
the British Ecological Society, Ecology and Industrial Society.
EICHELBERGER, J.W. & LICHTENBERG, J.J. (1971) Persistence of
pesticides in river water. Environ. Sci. Technol., 5(6): 541-544.
ELGAR, K.E. (1965) Insecticide residues in samples of cottonseed
from Mexico, Sittingbourne, Shell International Chemical Company,
Ltd (Technical Memorandum 99/65).
ELGAR, K.E. (1966) Analysis of crops and soils for residues of the
soil insecticides aldrin and Telodrin. J. Sci. Food Agric., 17:
541-545.
ELGAR, K.E. & HOLLAND, A.A. (1967) Residues of organochlorine
insecticides in potatoes from Mexico, Sittingbourne, Shell
International Chemical Company, Ltd (Technical Service Note 82/67).
HARDEE, D.D., GUTENMANN, W.H., KEENAN, G.I., GYRISCO, G.G., LISK,
D.J., FOX, F.H., TRIMBERGER, G.W., & HOLLAND, R.F. (1964) Residues
of heptachlor epoxide and Telodrin in milk from cows fed at parts
per billion insecticide levels. J. econ. Entomol., 56(3): 404-407.
HATHWAY, D.E. (1965) The biochemistry of dieldrin and Telodrin.
Arch. environ. Health, 11: 380-388.
HATHWAY, D.E. & MALLINSON, A. (1964) Chemical studies in relation to
convulsive conditions: effect of Telodrin on the liberation and
utilization of ammonia in rat brain. Biochem. J., 90: 51-60.
HATHWAY, D.E., MALLINSON, A., & AKINTONWA, D.A.A. (1965) Effects of
dieldrin, picrotoxin, and Telodrin on the metabolism of ammonia in
brain. Biochem. J., 94: 676-686.
HODGE, H.C., BOYCE, A.M., DEICHMANN, W.B., & KRAYBILL, H.F. (1967)
Toxicology and no-effect levels of aldrin and dieldrin. Toxicol.
appl. Pharmacol., 10(3): 613-637.
HOOGENDAM, I., VERSTEEG, J.P.J., & VLIEGER, M., DE (1962)
Electroencephalograms in insecticide toxicity. Arch. environ.
Health, 4: 86-94.
HOOGENDAM, I., VERSTEEG, J.P.J., & VLIEGER, M., DE (1965) Nine years
toxicity control in insecticide plants. Arch. environ. Health, 10:
441-448.
HOWARD, J.A., BUXTON, J.A., ATER, F.B., OVERBECK, R.C., FOOTE, W.L.,
ROBINSON, R.F., & DAVIDSON, R.S. (1957) Final report on toxicology
of BAS-4402 to the Shell Development Corporation, Columbus, Ohio,
Battelle Memorial Institute.
HUDSON, R.H., TUCKER, R.K., & HAEGELE, M.A. (1984) Handbook of
toxicity of pesticides to wildlife. 2nd ed., Washington, DC, US
Department of the Interior, Fish and Wildlife Service (Resource
Publication No. 153).
HUGHES, D.G. (1965) Insecticide residues in cottonseed oil,
Sittingbourne, Shell International Chemical Company, Ltd (Technical
Memorandum 23/65).
IBRAHIM, T.M. (1964) A toxicological study of the action of the
insecticide dieldrin and related substances on the contraction of
striated muscle in the rat, University of Utrecht (Thesis).
INNES, J.R.M., ULLAND, B.M., VALERIO, M.G., PETRUCELLI, L.,
FISHBEIN, L., HART, E.R., PALLOTTA, A.J., BATES, R.R., FALK, H.L.,
GART, J.J., KLEIN, M., MITCHELL, I., & PETERS, J. (1969) Bioassay of
pesticides and industrial chemicals for tumorigenicity in mice: a
preliminary note. J. Natl Cancer Inst., 42(6): 1101-1114.
JAGER, K.W. (1970) Aldrin, dieldrin, endrin, and Telodrin: an
epidemiological and toxicological study of long-term occupational
exposure, Amsterdam, London, New York, Elsevier Science Publishers.
JAPANESE ENVIRONMENTAL AGENCY (1975) Environmental survey report on
chemical substances in FY 1974, Tokyo, Environmental Health
Department, Planning and Coordination Bureau (Unpublished report).
KADOUM, A.M. (1968) Application of the rapid micro method of sample
clean-up for gas chromatographic analysis of common organic
pesticides in ground water, soil, plant and animal extracts. Bull.
environ. Contam. Toxicol., 3(2): 65-70.
KAUL, R., KLEIN, W., & KORTE, F. (1970) [Contributions to ecological
chemistry. XX. Distribution, elimination, and metabolism of Telodrin
and heptachlor in rats and male rabbits, end-product of the
metabolism of heptachlor in mammals.] Tetrahedron, 26: 331-337 (in
German).
KELSEY, J.M. & ARLIDGE, E.Z. (1968) Effects of isobenzan on soil
fauna and soil structure. N. Z. J. agric. Res., 11: 245-260.
KERDIJK, H.N. (1981) Groundwater pollution by heavy metals and
pesticides from a dredge spoil dump. Stud. environ. Sci., 17:
279-286.
KODAVANTI, P.R.S., MEHROTRA, R.D., CHETTY, S.C., & DESAIAH, D.
(1988) Effect of selected insecticides on rat brain synaptosomal
adenylate cyclase and phosphodiesterase. J. Toxicol. environ.
Health, 25: 207-215.
KOEMAN, J.H. (1971) [The occurrence and toxicological implications
of some chlorinated hydrocarbons in the Dutch coastal area in the
period from 1965 to 1970], University of Utrecht, pp. 30-31 (Thesis)
(in Dutch).
KOEMAN, J.H., OSKAMP, A.A.G., VEEN, J., BROUWER, E., ROOTH, J.,
ZWART, P., VAN DE BROEK, E., & VAN GENDEREN, H. (1967) Insecticides
as a factor in the mortality of the sandwich tern (Sterna
sandvicensis): a preliminary communication. Meded. Landbouwwet.
Hogesch. Gent., 32: 841-854.
KOEMAN, J.H., VEEN, J, BROUWER, E., HUISMAN-DE BROUWER, L., &
KOOLEN, J.L. (1968) Residues of chlorinated hydrocarbon insecticides
in the North Sea environment. Helgoländer wiss. Meerunters., 17:
375-380.
KORTE, F. (1963) Review of metabolism studies with 14C-labelled
insecticides from 1958 to March 1963. Unpublished paper presented at
a Toxicological Meeting, New York, 25-27 March.
KORTE, F. (1967) Metabolism of 14C-labelled insecticides in
microorganisms, insects, and mammals. Botyu-Kagaku, 32(2): 46-59.
KORTE, F. & STIASNI, M. (1964) [Metabolism of 14C-Telodrin in
microorganisms and mosquito larvae.] Ann. Chem., 673: 146-152 (in
German).
LAWRENCE, L.J. & CASIDA, J.E. (1984) Interactions of lindane,
toxaphene, and cyclodienes with brain-specific
t-butylbicyclophosphorothionate receptor. Life Sci., 35: 171-178.
LAYTON, D.W., MALLON, B.J., ROSENBLATT, D.H., & SMALL, M.J. (1987)
Deriving allowable daily intakes for systemic toxicants lacking
chronic toxicity data. Regul. Toxicol. Pharmacol., 7: 96-112.
MCCASKEY, T.A., STEMP, A.R., LISKA, B.J., & STADELMAN, W.J. (1968)
Residues in egg yolks and raw and cooked tissues from laying hens
administered selected chlorinated hydrocarbon insecticides. Poultry
Sci., 47: 564-569.
MARLOW, R.G., BULL, M.S., & WILLIAMS, S. (1968) Residues of
organochlorine insecticides in foodstuffs from Spain, Sittingbourne,
Shell International Chemical Company, Ltd (Technical Service Report
WKTR.0058.68).
MATHEWS, B.L. (1969) Residues of organochlorine insecticides in
foodstuffs from India, Sittingbourne, Shell Research (Report No.
WKGR.0102.69).
MATHEWS, B.L. & COLE, E.R. (1966) Residues of Telodrin,
methylparathion, and DDT on tobacco from Costa Rica, Sittingbourne,
Shell International Chemical Company, Ltd (Technical Service Note
123/66).
MEHROTRA, B.D., BANSAL, S.K., & DESAIAH, D. (1982) Comparative
effects of structurally-related cyclodiene pesticides on ATPases. J.
appl. Toxicol., 2(6): 278-283.
MOEED, A. (1975) Effects of isobenzan, fensulfothion, and diazinon
on invertebrates and microorganisms. N. Z. J. exp. Agric., 3:
181-185.
MOSS, J.A. & HATHWAY, D.E. (1964) Transport of organic compounds in
the mammal. Partition of dieldrin and Telodrin between the cellular
components and soluble proteins of blood. Biochem. J., 91: 384-393.
MURPHY, M.W. & STANDEN, M.E. (1964) The translocation of Telodrin
into potatoes, Sittingbourne, Shell International Chemical Company,
Ltd (Technical Memorandum 184/64).
RIBBENS, P.H. (1985) Mortality study of industrial workers exposed
to aldrin, dieldrin and endrin. Arch. occup. environ. Health, 56(2):
75-79.
RICHARDSON, A., ROBINSON, J., BUSH, B., & DAVIES, J.M. (1967)
Determination of dieldrin (HEOD) in blood. Arch. environ. Health,
14(5): 703-708.
ROBINSON, J. & RICHARDSON, A.R. (1963) The distribution, storage,
and elimination of Telodrin in rats fed this insecticide in their
diet, Sittingbourne, Shell Research (Report No. R(T)-1-63).
SCHAFER, E.W. & BRUNTON, R.B. (1979) Indicator bird species for
toxicity determinations: is the technique usable in test method
development? In: Beck, J.R., ed. Vertebrate pest control and
management materials, Philadelphia, American Society for Testing and
Materials, pp. 157-168 (ASTM STP 680).
SHELL (1963) An episode of pesticide contamination of pasture and
dairy products - Isobenzan in Victoria, Australia, in 1963.
Melbourne, Shell Chemical Australia (Unpublished report No.
90111501WP5).
SHELL (1964) Telodrin residues. A summary, London, Shell
International Chemical Company (Unpublished report).
SPYNU, E.I. (1964) On the toxicology of new organic chloride
insecticides obtained by diene synthesis on the basis of
hexachlorocyclopentadiene. Gig. Tr. prof. Zabol., 4: 30-35.
SRIVASTAVA, S.C. (1966) The effect of Telodrin on nitrification of
ammonia in soil and its implication on nitrogen nutrition of
sugarcane. Plant Soil, 25(3): 471-473.
STANDEN, M.E. & ELGAR, K.E. (1965) Telodrin residues in dairy
products from Venezuela, Sittingbourne, Shell International Chemical
Company, Ltd (Technical Memorandum 36/65).
STEMP, A.R. & LISKA, B.J. (1966) Effects of processing and storage
of dairy products on Telodrin and methoxychlor residues. J. diary
Sci., 49: 1006-1008.
STEVENSON, D.E. (1964) The toxicology of Telodrin. Meded. Fac.
Landbouwwet. Rijksuniv. Gent, 29: 1198-1207.
SUZUKI, K., NAGAYOSHI, H., & KASHIWA, T. (1974) The systematic
separation and identification of pesticides in the first division.
Agric. biol. Chem., 38(2): 279-285.
THOMPSON, A.R. (1973) Persistence of biological activity of seven
insecticides in soil assayed with Folsomia candida. J. econ.
Entomol., 66(4): 855-857.
TRUDGILL, P.W. & WIDDUS, R. (1970) Effects of chlorinated
insecticides on metabolic processes in bacteria. Biochem. J., 118:
48-49.
TUINSTRA, L.G.M.TH. & TRAAG, W.A. (1979) Automated glass capillary
gas chromatographic analysis of PCB and organochlorine pesticide
residues in agricultural products. J. high Resolut. Chromatogr.
Chromatogr. Commun., 2(2): 723-728.
US EPA (1987) Acute toxicity handbook of chemicals to estuarine
organisms. Springfield, Virginia, US Department of Commerce,
National Technical Information Service (EPA/600/8-87/017).
VAN WIJNEN, J.H. & STIJKEL, A. (1988) Health risk assessment of
residents living on harbour sludge. Int. Arch. occup. environ.
Health, 61: 77-87.
VERMA, M.P., BAHGA, H.S., & SONI, B.K. (1967) Effect of prolonged
administration of the insecticide Telodrin in poultry. Indian vet.
J., 44: 962-966.
VERSTEEG, J.P.J. & JAGER, K.W. (1973) Long-term occupational
exposure to the insecticides aldrin, dieldrin, endrin, and Telodrin.
Br. J. ind. Med., 30: 201-202.
WARE, G.W. & GOOD, E.E. (1967) Effects of insecticides on
reproduction in the laboratory mouse. II. Mirex, Telodrin, and DDT.
Toxicol. appl. Pharmacol., 10(1): 54-61.
WEGMAN, R.C.C. & GREVE, P.A. (1980) Halogenated hydrocarbons in
Dutch water samples over the years 1969-77. Environ. Sci. Res., 16:
405-415.
WEGMAN, R.C.C. & HOFSTEE, A.W.M. (1982) Determination of
organochlorines in river sediment by capillary gas chromatography.
Water Res., 16: 1265-1272.
WEGMAN, R.C.C., HOFSTEE, A.W.M., & GREVE, P.A. (1981) Uptake of
organochlorines by plants growing on river and basin sediment.
Meded. Fac. Landbouwwet. Rijksuniv. Gent, 46(1): 359-365.
WORDEN, A.N. (1969) Toxicity of Telodrin. Toxicol. appl. Pharmacol.,
14: 556-573.
ZAVON, M.R. (1961) Toxicology and pharmacology of Telodrin
insecticide (compound SD 4402), Cincinnati, Ohio, The Kettering
Laboratory (Unpublished report).
RESUME ET EVALUATION; CONCLUSIONS ET RECOMMANDATIONS
1. Résumé et évaluation
Autant qu'on sache, l'isobenzan, un insecticide organochloré
n'a été fabriqué que pendant la période 1958-1965. Plusieurs années
aprés, on puisait toujours sur les stocks existants. A l'heure
actuelle, les seules sources importantes d'exposition sont
vraisemblablement les sites de décharge initiaux de déchets
industriels ou les boues de dragage provenant de sédiments
contaminés.
Une fois épandu sur le sol, la majeure partie de l'isobenzan
disparaît rapidement. Après quoi, la fraction restante se décompose
beaucoup plus lentement. Elle persiste dans le sol de deux à sept
ans selon la nature de celui-ci. Au laboratoire, l'isobenzan se
décompose dans les eaux de surface en l'espace de quelques semaines
lorsqu'il est exposé à la lumière naturelle ou artificielle.
Le sol, les eaux souterraines et superficielles provenant des
polders constitués de sédiments contaminés par des organochlorés,
notamment des dérivés cyclodiéniques, contenaient encore plusieurs
années après, de faibles résidus d'isobenzan. En 1979-1980, on n'a
pas détecté d'isobenzan (limite de détection 0,01 mg/kg de poids
sec) dans les sédiments des cours d'eau des Pays-Bas. Après
traitement du sol, les résidus qui subsistent sur les récoltes sont
généralement faibles (inférieurs à 0,05 mg/kg de végétaux), mais on
peut en trouver des quantités plus fortes sur certaines racines
(jusqu'à 0,2 mg/kg dans les carottes). Des enquêtes de type "panier
de la ménagère" effectuées lorsqu'on utilisait de l'isobenzan en
agriculture, n'ont pas permis de déceler de résidus dans les denrées
contrôlées (moins de 0,01 mg/kg).
Chaque fois qu'on a laissé paître des bovins dans des pâturages
traités par de l'isobenzan, on a constaté que les produits laitiers
obtenus contenaient des résidus de cet insecticide. C'est ainsi que
dans deux échantillons de beurre, on a trouvé 0,07 et 0,15 mg
d'isobenzan par kg de produit, les concentrations dans le lait
entier allant de 0,005 à 0,07 mg/kg. Le lait en poudre n'en
contenait toutefois que 0,005 mg/kg. Lors du traitement industriel
des produits laitiers, plus de 50% du résidu disparaît selon le type
de traitement.
On ne dispose d'aucune donnée sur les quantités d'isobenzan
présentes dans le sang ou les tissus adipeux de la population
générale. Des travailleurs exposés à l'isobenzan lors de la
fabrication ou de la formulation de cet insecticide, présentaient
des taux sanguins (sang total) allant jusqu'à 0,041 mg/litre. Dans
les échantillons de sang total prélevés sur des personnes vivant à
proximité d'une unité de production, la concentration d'isobenzan
était inférieure à la limite de détection (0,001 mg/litre).
L'isobenzan est facilement résorbé par la paroi du tube
digestif et il passe dans le sang sans modification. Il se forme des
métabolites hydrophiles et notamment une lactone. L'isobenzan
s'accumule dans les tissus et les organes des rats et des chiens
selon l'ordre: graisses > foie = muscle > cerveau > sang. Les
concentrations tissulaires chez les rattes sont généralement plus
faibles que chez les rats, spécialement dans les graisses. La
demi-vie biologique dans le tissu adipeux était ainsi de 10,9 jours
chez les rats et de 16,6 jours chez les rattes. Chez un chiot
femelle dont le sang contenait 0,09 mg d'isobenzan par litre, on a
observé des convulsions 15 jours après la naissance. L'animal
n'avait été nourri qu'avec le lait de sa mère, une chienne Beagle à
qui l'on avait fait absorber de l'isobenzan et dont le lait en
contenait 0,7 mg/litre. Des effets analogues sur les petits ont été
observés lors d'une étude de reproduction sur le rat. Chez la vache,
l'isobenzan est excrété dans le lait.
Les larves de moustiques et les champignons terricoles
métabolisent l'isobenzan de la même manière que les vertébrés,
notamment sous forme de lactone. L'isobenzan est très persistant
dans l'environnement et s'accumule dans les organismes vivants. Il
est extrêmement toxique pour les poissons, les crevettes et les
oiseaux. Aux Pays-Bas, pays où l'on fabriquait l'isobenzan, on a
trouvé des résidus dans des oeufs de sternes vivant sur la côte
hollandaise qui atteignaient 0,45 mg/kg (moyenne, 0,09 mg/kg). Dans
les moules et le poisson, les résidus moyens étaient de 0,05 mg/kg
en 1965. Dans les parcelles traitées par de l'isobenzan à raison de
2 kg/ha, on a constaté une réduction du nombre de lombrics. Il y
avait réduction de la nitrification avec accroissement corrélatif de
l'azote minéral dans les sols traités par l'isobenzan à raison
d'1 kg/ha; en revanche, les études en laboratoire n'ont mis en
évidence aucun effet sur la nitrification à des doses correspondant
à 250 g/ha.
L'isobenzan présente une forte toxicité aiguë pour les
mammifères, que ce soit par la voie orale ou par la voie percutanée.
Le mode d'action de l'isobenzan consiste en une stimulation
excessive du système nerveaux central conduisant à des convulsions.
La toxicité aiguë des diverses formulations d'isobenzan correspond à
la proportion de matière active.
L'isobenzan n'est pas irritant pour la peau mais certaines de
ses formulations peuvent l'être.
Des études limitées à court et à long terme, au cours
desquelles on a administré par voie orale de l'isobenzan à des
souris, à des rats et à des chiens, ont montré que ce composé
pouvait induire des lésions histologiques au niveau du foie, du type
de celles qu'on observe classiquement avec les organochlorés. Une
étude de longue durée sur des rats a permis de déterminer que la
dose sans effet observable était de 5 mg/kg de nourriture (soit
l'équivalent de 0,25 mg/kg de poids corporel). Chez le chien, la
dose sans effet nocif observable, déterminée à la suite d'une étude
de deux ans, était de 0,025 mg/kg de poids corporel.
Une étude de reproduction portant sur une génération de rats a
montré que la dose sans effet nocif observable était de 0,1 mg/kg de
nourriture (soit l'équivalent de 0,05 mg/kg de poids corporel. A la
dose de 1 mg/kg de nourriture (soit l'équivalent de 0,05 mg/kg de
poids corporel), il y a eu réduction de la survie des ratons.
Aucune étude de tératogénicité ni de mutagénicité n'a été
rapportée.
Une étude de deux ans sur des rats (administration par voie
orale) et une étude sur des souris n'ont pas permis de mettre en
évidence un pouvoir cancérogène quelconque, mais ces études
n'étaient pas adaptées à une telle évaluation.
La base de données toxicologiques sur l'isobenzan est
incomplète. En général on estime que, d'après les critères actuels,
les données sont d'une qualité médiocre et sont en tous cas
insuffisantes pour permettre une évaluation du risque que ce composé
présente pour la santé humaine ou l'environnement.
Les données concernant l'exposition humaine se limitent à des
études effectuées sur des travailleurs d'une usine hollandaise qui
étaient employés à la fabrication et à la formulation d'isobenzan et
d'insecticides cyclodiéniques apparentés. Aucun cas d'irritation
cutanée n'a été signalé. Dans plusieurs cas d'intoxication, on a
observé des convulsions mais les anomalies du tracé
électro-encéphalographique étaient réversibles. Le seuil limite
d'intoxication (pour les convulsions), a été estimé à 0,015 mg
d'isobenzan par litre de sang et la demi-vie biologique de ce
composé dans le sang humain est, semble-t-il, de l'ordre de 2,8
années.
2. Conclusions et recommandations
L'isobenzan est extrêmement toxique et très persistant. Les
données dont on dispose sur le danger qu'il représente sont
incomplètes mais néanmoins suffisantes pour montrer que ce danger
est réel pour les personnes qui le manipulent ainsi que pour
l'environnement, de sorte qu'il faut éviter toute contamination
humaine ou environnementale qui résulterait de l'utilisation de ce
produit comme insecticide ou autre.
RESUMEN Y EVALUACION; CONCLUSIONES Y RECOMENDACIONES
1. Resumen y evaluación
Según los datos de que se dispone, el isobenzano, un
insecticida organoclorado, sólo se fabricó durante el periodo
1958-1965. Durante los años siguientes se utilizaron las existencias
almacenadas. En la actualidad, se cree que las únicas fuentes
importantes de exposición son los lugares donde originalmente se
evacuaron los desechos industriales o los materiales dragados de
sedimentos contaminados.
Cuando se aplica el isobenzano al suelo, se produce una rápida
pérdida inicial; después, el resto del compuesto se degrada mucho
más despacio. Persiste en el suelo durante 2 a 7 años, según el tipo
de suelo. En condiciones de laboratorio, el isobenzano se descompone
en las aguas de superficie en pocas semanas cuando se expone a la
luz natural o artificial.
El suelo, las aguas subterráneas y las aguas superficiales de
pólders construidos con sedimentos contaminados por sustancias
organocloradas, inclusive compuestos de ciclodieno clorado, aún
contenían pequeños residuos de isobenzano algunos años después. En
1979-1980, no se detectó isobenzano (límite de detección: 0,01 mg/kg
de peso seco) en el sedimento de ríos de los Países Bajos. Tras el
tratamiento del suelo, los residuos en las cosechas suelen ser bajos
(menos de 0,05 mg/kg de cosecha), pero pueden encontrarse
concentraciones superiores en algunos tubérculos (hasta 0,2 mg/kg en
zanahorias). En las encuestas de mercado realizadas durante la época
de uso agrícola del isobenzano no se detectaron residuos en los
alimentos analizados (menos de 0,01 mg/kg).
En los productos lácteos procedentes de ganado que se alimentó
en pastos tratados con isobenzano se encontraron residuos del
insecticida. Dos muestras de mantequilla contenían 0,07 y 0,15 mg de
isobenzano/kg de producto, mientras que los niveles en la leche
entera fueron de 0,005 mg/kg a 0,07 mg/kg. En la leche deshidratada,
no obstante, se encontraron sólo 0,005 mg/kg. Durante la elaboración
de los productos lácteos se perdía hasta el 50% del residuo, según
el tipo de tratamiento.
No se dispone de datos sobre los niveles de isobenzano en la
sangre o el tejido adiposo de la población general. Los operarios
expuestos al isobenzano en las plantas de fabricación y elaboración
presentaron niveles medios en sangre entera de hasta 0,041 mg/litro.
En muestras de sangre entera procedente de personas que vivían en
las proximidades de una de las plantas, la concentración de
isobenzano estaba por debajo del límite de detección
(0,001 mg/litro).
El isobenzano es absorbido rápidamente a través de la pared
gastrointestinal y es transportado por la sangre sin alteraciones.
Se forman metabolitos hidrófìlos de los que se ha identificado uno,
la lactona de isobenzano. El isobenzano se acumula en los tejidos y
los órganos de ratas y perros en el orden siguiente: grasa > hígado
= músculo > cerebro > sangre. Las concentraciones tisulares en las
hembras de rata son en general superiores a las que aparecen en los
machos, sobre todo en la grasa. En la rata se determinó que la
semivida biológica en la grasa del organismo es de 10,9 días en los
machos y 16,6 días en las hembras. En un cachorro hembra de perro,
cuya sangre contenía 0,09 mg de isobenzano/litro, se observaron
convulsiones a los 15 días del nacimiento. El cachorro sólo se había
alimentado de leche de su madre, una Beagle a la que se había
administrado isobenzano y cuya leche contenía 0,7 mg/litro. En un
estudio de reproducción en ratas se observaron efectos similares en
las crías. Las vacas excretan isobenzano con la leche.
Las larvas de mosquito y los hongos del suelo metabolizan el
isobenzano del mismo modo que los vertebrados, dando lactona de
isobenzano como metabolito.
El isobenzano es muy persistente en el medio ambiente y se
bioacumula. Es sumamente tóxico para los peces, los camarones y las
aves. En los Países Bajos, país en el que se fabricaba el
isobenzano, los residuos encontrados en los huevos de golondrinas de
mar de las costas holandesas ascendieron a 0,45 mg/kg (promedio:
0,09 mg/kg), mientras que el promedio de residuos encontrados en
mejillones y peces fue de 0,05 mg/kg en 1965. Se observó una
disminución del número de lombrices en terrenos tratados con
isobenzano a razón de 2 kg/ha. Se redujo la nitrificación, con el
consiguiente aumento del nitrógeno inorgánico, en los suelos
tratados con isobenzano sobre el terreno a razón de 1 kg/ha, si bien
en estudios de laboratorio no se demostró efecto alguno en la
nitrificación con dosis equivalentes a 250 g/ha.
La toxicidad aguda del isobenzano para los mamíferos es elevada
por las vías oral y percutánea. La forma de acción de su toxicidad
es la sobreestimulación del sistema nervioso central, que da lugar a
convulsiones. La toxicidad aguda de las preparaciones de isobenzano
refleja el porcentaje de ingrediente activo presente.
Aunque el isobenzano no irrita la piel, algunos productos
preparados a partir de él pueden causar irritación.
En estudios limitados a corto y largo plazo de administración
oral realizados en ratones, ratas y perros se ha demostrado que el
isobenzano puede provocar en el hígado cambios histológicos que
reponden al tipo clásico de intoxicación por compuestos
organo-clorados. En un estudio realizado a largo plazo en ratas, se
determinó un nivel efectos sin observados de 5 mg/kg de dieta
(equivalente a 0,25 mg/kg de peso corporal), y en un estudio en
perros de dos años de duración se determinó un nivel sin observación
de efectos adversos de 0,025 mg/kg de peso corporal.
En un estudio de reproducción en una generación de ratas se
obtuvo un nivel sin efectos adversos observados de 0,1 mg/kg de
dieta (equivalente a 0,05 mg/kg de peso corporal). Con un nivel de
1 mg/kg de dieta (equivalente a 0,05 mg/kg de peso corporal)
disminuyó la supervivencia de las crías.
No se han comunicado estudios sobre la teratogenicidad ni la
mutagenicidad del compuesto.
No se observó potencial carcinogénico en un estudio de
administración oral a ratas durante dos años ni en un estudio de
administración oral a ratones, pero esos estudios no eran apropiados
para evaluar la carcinogenicidad.
La base de datos toxicológicos correspondiente al isobenzano es
incompleta. En general, actualmente se considera que la calidad de
los datos es mediocre e insuficiente para evaluar los riesgos que
representa para la salud humana o el medio ambiente.
Los datos sobre personas expuestas se limitan a estudios
realizados en trabajadores de una fábrica de los Países Bajos
durante la fabricación y la elaboración de preparaciones de
isobenzano y otros insecticidas de ciclodieno clorado afines. No se
comunicaron casos de irritación cutánea. En varios casos de
intoxicación, se produjeron convulsiones pero los cambios del
trazado electroencefalográfico resultaron ser reversibles. El nivel
umbral de intoxicación (en el caso de las convulsiones) se estimó en
0,015 mg de isobenzano/litro de sangre, y se calculó que la semivida
biológica del isobenzano en la sangre humana es del orden de 2,8
años.
2. Conclusiones y recomendaciones
El isobenzano es sumamente tóxico y muy persistente. La
información de que se dispone sobre los riegos del isobenzano es
incompleta, pero, aún así, basta para indicar que el riesgo que
supone para los que lo manipulan y para el medio ambiente es tan
elevado que no debería permitirse la exposición humana o del medio
ambiente a esta sustancia, ya sea utilizada como insecticida o para
cualquier otro fin.
6.3.3 Microorganisms
Isobenzan labelled with 14C in the hexachloropentane ring or
at the 1,3 position is metabolized by the fungi Aspergillus niger,
Aspergillus flavus, Penicillium chrysogenum, and Penicillium
notatum to isobenzan lactone, the same metabolite as found in
animal metabolism studies (Korte, 1963; Korte & Stiasni, 1964).
6.4 Elimination and excretion in expired air, faeces, and urine
6.4.1 Oral administration
In a study by Korte (1963), ten rabbits received in their diet
2 mg 14C-isobenzan, diluted with non-radioactive isobenzan, every
2 days to a total amount of 25-30 mg per rabbit. After 3 months,
about 50% of the total radioactivity administered had been excreted,
mainly in the urine. On the other hand, rats excreted most of the
radioactivity via bile into the gastrointestinal tract and excreted
these products with the faeces. No unchanged isobenzan was excreted,
only hydrophilic metabolites.
6.4.2 Parenteral administration
When Carworth Farm rats received daily intraperitoneal
injections of isobenzan (98% purity) of 0.25, 1, or 2 mg/kg body
weight 5 days per week for 2 weeks (section 7.2.3), they excreted
less than 1% of the daily dose as unchanged isobenzan in the faeces
(Brown et al., 1962).
In a study by Kaul et al. (1970), male and female rats
received a single intravenous injection of 15 µg 14C-isobenzan/kg
body weight. The male rats excreted 1% of the applied radioactivity
in the urine and 12% in the faeces within 48 h, while the females
excreted 5% and 11%, respectively.
After having been administered intravenously to rats with
cannulated bile ducts, 14C-isobenzan was excreted as hydrophilic
metabolites in the bile (Korte, 1963, 1967).
Male rabbits given an intravenous injection of 14C-isobenzan
(241 µg/kg body weight) excreted 12% in the urine and 1% in the
faeces within 96 h (Kaul et al., 1970).
6.5 Retention and turnover
The absorption of isobenzan into the body is determined by
measuring the insecticide in the blood. No human studies exist
relating the concentration of isobenzan in the blood at the state of
equilibrium to the total daily intake or relating the concentration
of isobenzan in the blood to that in the tissues. However, in
experimental animals, such relationships have been determined. Thus,
in human beings, measurement of the blood concentration of the
insecticide at the state of equilibrium is assumed to reflect total
absorption by all routes (skin, pulmonary, and oral exposure) as
well as the storage level in adipose tissue, thereby providing a
measure of the total body burden of isobenzan.
From human data, it is estimated that the biological half-life
of isobenzan in blood is of the order of 2.8 years (Jager, 1970)
(section 8.2).
7. EFFECTS ON LABORATORY MAMMALS AND IN VITRO TEST SYSTEMS
7.1 Single exposure
7.1.1 Oral administration
In rats, the first signs of toxicity were evident approximately
1 h after the administration of a lethal dose and consisted of
lethargy followed by muscular twitching, laboured breathing, and,
finally, general convulsions. The majority of deaths occurred within
the first 20 h after administration. No specific changes were
observed in the organs of fatally intoxicated animals. Signs of
intoxication were similar in mice, rats, guinea-pigs, cats, dogs,
and chickens. Surviving animals recovered completely (Brown et al.,
1962; Worden, 1969).
Oral LD50 values for the various animal species range from
1.6 to 10 mg/kg and are summarized in Table 5.
Table 5. Oral LD50 values for technical isobenzan
Species Vehicle LD50 Reference
(mg/kg body
weight)
Mouse arachis oil 10a,b Worden (1969)
Mouse corn oil 12.5 Spynu (1964)
Rat corn oil 4.8 Howard et al. (1957)
Rat corn oil 14.4 Spynu (1964)
Rat carboxymethyl cellulose 5.4 Stevenson (1964)
Rat dimethylsulfoxide 7.2 Stevenson (1964)
Rat arachis oil 10b Worden (1969)
Rat unknown 8.0 Layton et al. (1987)
Guinea-pig arachis oil 2.5a,b Worden (1969)
Golden hamster arachis oil 20b Worden (1969)
Dog unknown 1.6 Stevenson (1964)
a Average of males and females.
b Isobenzan 99.5%.
7.1.2 Dermal administration
Signs of intoxication are the same as those described for acute
oral intoxication but they develop more slowly (Brown et al.,
1962). The LD50 values are summarized in Table 6.
Table 6. Dermal LD50 values for technical isobenzan
Species Vehicle LD50 Reference
(mg/kg body
weight)
Rat arachis oil occluded, 4 Zavon (1961)
non-occluded, 10
Rat corn oil non-occluded, 8.5 Spynu (1964)
Rat crystalline form occluded, 60 Stevenson (1964)
(approximately)
7.1.3 Parenteral administration
The acute toxicity of isobenzan administered parenterally is
similar to that following oral administration (Table 7). The signs
of intoxication are similar to those observed after acute oral
toxicity but develop more rapidly, starting less than 1 h after the
injection (Brown et al., 1962; Worden, 1969).
Table 7. Parenteral LD50 values for technical isobenzan
Route Species Vehicle LD50 Reference
(mg/kg body
weight)
Intraperitoneal mouse xylene emulsion 8.2 Stevenson (1964)
Intraperitoneal mouse methoxytriglycol 6.0 Cole & Casida (1986)
Intraperitoneal rat dimethylsulfoxide 3.6 Stevenson (1964)
Subcutaneous rat, arachis oil 6-10 Worden (1969)
mouse
Intravenous rat unknown 1.7 Zavon (1961)
7.1.4 Formulated material
A 50% wettable powder formulation and a 15% emulsifiable
concentrate (in mixed petroleum xylenes) were tested for their acute
oral toxicity to rats, mice, rabbits, hamsters, cats, and dogs. The
LD50 values, when expressed as active material, were comparable
with those of isobenzan itself (Stevenson, 1964; Worden, 1969). The
dermal LD50 value for the 15% emulsifiable concentrate was
25-35 mg isobenzan/kg body weight for Hooded-Lister rats and 6 mg
isobenzan/kg body weight for New Zealand white rabbits, whereas in
the case of the 50% wettable powder the LD50 was 41 mg
isobenzan/kg body weight for rabbits (Stevenson, 1964). Rabbits
exposed dermally to the 15% emulsifiable concentrate formulation
behaved differently to rats similarly exposed. The rabbits generally
lost weight due to anorexia and a failure to drink, and they would
then convulse, even as much as 3 weeks after the exposure. However,
if feeding and drinking were resumed quickly, the rabbits did not
convulse (Brown, 1963, 1964).
7.1.5 Metabolites
The acute oral and intravenous toxicities of the metabolite
isobenzan lactone for mice were 30 times lower than those of
isobenzan. The oral and intravenous LD50 values were 306 mg/kg
body weight and > 100 mg/kg body weight, respectively (Korte,
1967).
7.2 Short-term exposure
The short-term, long-term, and reproduction studies that were
used for establishing a no-observed-effect level are summarized in
Table 8.
7.2.1 Oral administration
7.2.1.1 Mouse
In a study by Brown et al. (1962), groups of five male and
five female Carworth Farm No. 1 mice were fed diets containing
isobenzan (98%) at levels of 1, 2, 3, 4, or 5 mg/kg for up to 3
weeks. No mortality was observed at 1 mg/kg, but at the higher
concentrations the mortality was dose related, reaching 100% at
5 mg/kg diet.
7.2.1.2 Rat
Groups of eight male and eight female Carworth Farm rats were
intubated with isobenzan (98%) in dimethylsulfoxide (0.25, 1, or
2.5 mg/kg body weight per day) 5 days/week for 2 weeks. All rats
dosed with 2.5 mg/kg body weight died within 5 days. All the females
and two of the males dosed with 1 mg/kg body weight died after 5-7
doses, whereas only one female dosed with 0.25 mg/kg body weight
died after five doses. Weight loss preceded the death of the animals
at the highest dose, this being the result of reduced food intake.
No gross or microscopic changes were found in the rats dying from
intoxication or in the survivors sacrificed 14 days after the last
treatment (no details were reported concerning the parameters
studied) (Brown et al., 1962).
In a study by Howard et al. (1957), groups of 10 male and 10
female Sprague-Dawley rats were fed diets containing 0, 2.5, 12.5,
or 25 mg isobenzan/kg diet for 30 days. One female in the
highest-dose group died. Some of the rats fed with levels of 12.5 or
25 mg/kg were nervous and irritable, and the reduced body weight
gain in both these groups correlated with diminished food
consumption. At autopsy, there were some areas of necrosis of heart
cells and haemorrhages in the heart muscle of animals fed with
levels of 12.5 or 25 mg/kg diet.
Table 8. Short- and long-term oral exposure to isobenzan
Animal (strain) Exposure period NOAELa LOAELa References
(mg/kg diet) (mg/kgdiet)
Rat (Sprague-Dawley) 30 days 2.5 (0.12) 12.5 (0.62): irritability, Howard et al. (1957)
decreased body weight gain,
histopathology changes in heart
Dog (Beagle) 2 years (0.08) (0.125): convulsion, mortality Brown & Richardson (1964)
Dog (Scottish terrier) 2 years (0.025) (0.1): decreased body weight gain, Worden (1969)
increased liver weight
Rat (Hooded-Lister) 2 years 5 (0.25) 17.5 (0.87): convulsions Worden (1969)
Reproduction study
Rat (Carworth Farm) one generation 0.1 (0.005) 1 (0.05): decreased survival of pups Chambers (1962a,b)
(2 litters) (pups) 5 (0.25): convulsions
1.0 (0.05)
(parents)
a Figures in parentheses are values for exposure concentration in mg/kg body weight.
In a study by Worden (1969), groups of five male and five
female Hooded-Lister rats were fed diets containing isobenzan
(99.5%) (25, 35, 50, or 100 mg/kg diet) as a 15% emulsifiable
concentrate (in mixed petroleum xylenes). After 37 days on the test
diets, half of the surviving animals of each group were transferred
to a control diet, while the remainder continued on the test diets
for a further 42 days. During the first period, all rats fed with
100 mg/kg diet died except for one male. One male and two females
fed with 50 mg/kg diet died as did three females fed with 35 mg/kg
diet. There were no further deaths among the rats after dosing was
discontinued. During the second period of feeding with the test
diet, one female fed at the lowest level died. In one rat only, a
female fed with 50 mg/kg, the classical signs of organochlorine
intoxication were seen in the liver. These have been described by
Hodge et al. (1967) as "enlarged centrolobular hepatic cells with
cytoplasmic oxyphilia and somewhat increased and peripheral
migration of the basophilic granules".
7.2.1.3 Dog
Pairs of Beagle hounds (one male and one female) were given
daily oral doses of 0, 0.08, 0.125, or 0.2 mg isobenzan (98%)/kg
body weight in olive oil in gelatin capsules for 2 years. The dogs
given the two highest doses had several convulsive episodes during
the course of the study. When a dog exhibited a convulsion, dosing
was discontinued for 8 weeks. The male dog dosed with 0.125 mg/kg
died one week after a third convulsive episode, which followed a
sudden fall in body weight. No signs of intoxication were observed
in the dogs dosed with 0.08 mg/kg body weight. During this study,
blood isobenzan concentrations were measured regularly, in
particular at the time of convulsions. Concentrations in muscle
tissue were determined 3 days after the convulsion, and the results
are given in Table 9. The blood concentrations in the dogs receiving
0.08 mg/kg body weight for 2 years without convulsions indicated
that a plateau was reached after a relatively short exposure. The
mean concentration was approximately 0.02 mg isobenzan/litre and the
maximum concentration 0.04-0.05 mg/litre. The two highest doses
caused convulsions (Brown & Richardson, 1964).
Groups of three male and three female Scottish terriers (2.5-9
kg body weight) received daily gavage doses of isobenzan (0, 0.025,
or 0.1 mg/kg body weight) as 15% emulsifiable concentrate (in mixed
petroleum xylenes) for 2 years. No deaths and no signs of
intoxication resulted from the isobenzan administration. Body weight
gain, urinalysis, and haematological and clinical chemistry values
for the dogs fed with 0.025 mg/kg body weight were comparable with
those of the control animals. At a dose level of 0.1 mg/kg body
weight, a decrease in body weight gain, a slight increase in serum
Table 9. Concentration of isobenzan in the blood and muscle of dogs
Dose (mg/kg Sex Number of Blood concentration Muscle tissue
body weight) convulsions at time of convulsion (mg/kg wet
(mg/litre) weight)
0.08 male none 0.04 (maximum
concentration)
female none 0.05 (maximum
concentration)
0.125 male first two 0.06
third (death) 0.11 16
female 4 0.03-0.06 0.18
0.2 male 9 0.02-0.11 0.36
female 6 0.07-0.08 0.68
alkaline phosphatase values during the second year, and an increase
in liver weight were noted. No evidence of histopathological lesions
attributable to isobenzan was found at either treatment level. The
no-observed-effect level resulting from this study was 0.025 mg/kg
body weight (Worden, 1969). The distribution of isobenzan in the
tissues examined is summarized in section 6.2.2.
7.2.2 Dermal administration
When groups of five female albino rabbits were given daily
applications of isobenzan in corn oil (0, 5, 10, 20, 30, or 40 mg
per rabbit) to the shaven skin for 3 weeks, mortality was high at
all dose levels, reaching 100% after 2 weeks for rabbits given doses
of 30 or 40 mg. Histopathological examination revealed necrosis of
the heart muscle, non-dose-related lesions of the liver, and
degenerative changes in cells of the central nervous system in a few
animals (Howard et al., 1957).
7.2.3 Intraperitoneal administration
Groups of five male and five female Carworth Farm rats were
injected intraperitoneally with isobenzan (98%) in
dimethyl-sulfoxide (0.25, 1, or 2 mg/kg body weight per day) 5
days/week for 2 weeks. All rats given the lowest dose survived, but
two rats of each sex given 1 mg/kg and five males and four females
given 2 mg/kg died after 5-10 doses. No gross or microscopic lesions
were found in fatally intoxicated rats or in the survivors killed
7-14 days after the last treatment (no details concerning
parameters studied were reported) (Brown et al., 1962).
7.3 Long-term exposure
7.3.1 Rat
Groups of 25 male and 25 female Hooded-Lister rats were fed, at
dietary concentrations of 5, 17.5, or 30 mg/kg (equivalent to 0.25,
0.875, or 1.5 mg/kg body weight), isobenzan as a 15% emulsifiable
concentrate (in mixed petroleum xylenes) for 2 years. Three control
groups, each consisting of 25 male and 25 female rats, were used.
Additional groups of rats, fed at the same dose levels and
accompanied by separate control groups, were used for liver and
kidney function tests after 20, 66, and 104 weeks. Five females fed
at the highest dose level died during the first 3 weeks of the
study. Signs of intoxication (such as ruffled coat, lethargy,
muscular twitches, and mild to violent convulsions) were observed in
animals given 17.5 or 30 mg/kg diet, mainly during the first few
weeks. There were no adverse effects on body weight gain, food
conversion ratios, haematological parameters, serum alkaline
phosphatase, serum glutamic-pyruvic transaminase, total serum
protein, or albumin/globulin ratios. Absolute liver weight was
increased in the animals given the highest dose level. Gross and
microscopic examinations did not reveal any compound-related
changes, with the possible exception of thyroid hyperplasia recorded
in four males and six females at a level of 30 mg/kg diet. The liver
function test (bromsulfophthalein) and kidney function test (phenol
red) showed no deviations from control values. No significant
increase in the number and type of tumours was found. The
no-observed-effect level for toxicological effects was 5 mg
isobenzan/kg diet (equivalent to 0.25 mg/kg body weight) for 2 years
(Worden, 1969).
7.4 Skin irritation
In a study by Worden (1969), four male guinea-pigs received a
single application (2 mg/kg body weight) of isobenzan (99.5%) as a
0.2% w/v solution in arachis oil to the shaved skin. The material
was not removed from the skin. The animals were kept under
observation for 21 days, but no irritation was observed.
Two male and two female rabbits were given 12 successive daily
applications (0.5 mg/kg body weight) of isobenzan (99.5%) and six
female rats received 30 successive daily applications (0.3 mg/kg
body weight) of isobenzan as a 0.2% w/v solution in arachis oil to
the shaved skin. The material was left on the skin and the
observation period was 21 days after the last application. No
irritation was observed (Worden, 1969).
7.5 Reproductive toxicity, embryotoxicity, and teratogenicity
No studies on the potential teratogenicity of isobenzan have
been reported.
7.5.1 Mouse
In a range-finding test, groups of 25 male and 25 female BALB/C
mice were fed diets containing 0, 1, 2.5, 5, or 10 mg isobenzan
(94%)/kg. The mice in the 5- and 10-mg/kg dose groups all died
within 64 and 24 days, respectively. Only 20% of those in the
2.5-mg/kg group survived for 120 days. The 1 mg/kg group survived
and reproduced normally (no further data were reported). In the main
study, groups of 108 mice of each sex of Swiss strain BALB/C were
fed control diet and 106 mice of each sex were fed 1 mg isobenzan/kg
diet for 30 days, after which they were randomly paired and
continued on the same diet for 90 days. The number of litters
produced, litter size, sex ratio, and mortality were recorded in
this one-generation reproduction study. There were no statistically
significant differences between the control and isobenzan-treated
animals for any of the parameters measured (Ware & Good, 1967).
7.5.2 Rat
A one-generation, 2-litter reproduction study was carried out
with groups of 20 male and 20 female weanling Carworth Farm rats
that were fed diets containing isobenzan at levels of 0, 0.1, 1, 5,
or 10 mg/kg for 100 days and then mated. Convulsions were seen
during the mating period and pregnancy in females fed 10 mg/kg, and
during the lactation period in one female fed 5 mg/kg. Pups of the
second mating from both these treatment groups were also seen to
convulse. Mean litter size and survival of pups were markedly
reduced in the 10-mg/kg group and, to a lesser extent, in the
5-mg/kg group. No clinical signs were observed in the 1-mg/kg group.
At this dose level, the mean litter size was comparable with
controls, but survival of the pups at 21 days was decreased. No
effects attributable to isobenzan were found in the 0.1-mg/kg group
(Chambers, 1962a,b).
7.5.3 Dog
During a 2-year study (section 7.2.1.3), three litters were
born to a female Beagle hound dosed daily with 0.08 mg isobenzan/kg
body weight five days per week. A male Beagle dog, dosed at the same
level, was sire for several of the litters. The first litter
consisted of one male and one female, both normal, and one
still-born male. Fifteen days after birth, the female pup began to
convulse, having only fed on the mother's milk (which contained
0.7 mg isobenzan/litre) and at 17 days the pup was sacrificed.
Concentrations of isobenzan in tissues are given in section 6.2.2.
Two further litters of pups were born to the original dam (two male
pups in the first litter and two pups of each sex in the second).
These animals all appeared normal and healthy and showed no ill
effects from the ingestion of maternal milk. One litter of pups, one
male and one female, was born to a female Beagle dog fed 0.125 mg/kg
body weight per day for two years. These pups did not exhibit any
signs of intoxication and were killed when 26 days old. Autopsy did
not reveal any structural changes. It did not appear that isobenzan
interfered with either the male or female reproduction function
(Brown & Richardson, 1964).
7.6 Mutagenicity and related end-points
No information on mutagenicity is available.
7.7 Carcinogenicity
No adequate carcinogenicity studies have been reported.
7.7.1 Mouse
Groups of 18 male and 18 female mice of two hybrid strains (the
F1 hybrids C57Bl/6 x C3H/Anf and C57Bl/6 x AKR) were given the
maximum tolerated dose of isobenzan in 0.5% gelatin (0.215 mg/kg
body weight) daily from 7 to 28 days of age by stomach tube.
Thereafter the isobenzan was mixed in the diet to a concentration of
0.646 mg/kg, and the mice were killed at 18 months of age. No
significant increase in tumour incidence was found (Innes et al.,
1969).
7.7.2 Rat
In a long-term feeding study on rats (section 7.4.1), no
evidence of carcinogenicity was found as a result of feeding diets
containing up to 30 mg isobenzan/kg for 2 years (Worden, 1969).
7.8 Special studies
7.8.1 Biochemical studies
Mehrotra et al. (1982) studied the comparative effects of
cyclodiene compounds on different ATPase activities in beef and rat
brain synaptosomal fractions in vitro. Isobenzan significantly
inhibited Na+-K+-ATPase in rat brain synaptosomes. A
dose-related response was observed at up to 80 µmol/litre, but no
increase in inhibition was observed with a further increase in the
concentration of the compound. Oligomycin-sensitive Mg2+-ATPase in
rat brain synaptosomes was significantly inhibited by isobenzan, a
maximum of 64% inhibition occurring at 120 µmol/litre. In addition,
the oligomycin-insensitive Mg2+-ATPase in rat brain synaptosomes
was inhibited, and the inhibition was concentration dependent.
Isobenzan did not have any effect on K+-stimulated
p-nitrophenylphosphatase, an enzyme which is known to represent
the dephosphorylation step in the overall reaction of the
Na+-K+-ATPase. Oligomycin-sensitive Mg2+-ATPase in beef heart
mitochondria was significantly inhibited.
Isobenzan did not affect the activity of adenylate cyclase or
phosphodiesterase in Sprague-Dawley rat brain synaptosomes in vitro
at concentrations of up to 200 µmol/litre (Kodavanti et al.,
1988).
Several studies into the effect of isobenzan on the transfer of
ammonia in brain have indicated that isobenzan acts by increasing
brain ammonia levels before and during convulsions. Glutamic acid,
glutamine, and alpha-ketoglutaric acid, which are utilized in an
ammonia-binding mechanism, become overwhelmed, resulting in free
ammonia accumulating in the cerebral tissues (Hathway & Mallinson,
1964; Hathway, 1965; Hathway et al., 1965).
7.8.2 Neurotoxicity
In vitro studies using fresh rat brain synaptic membranes
showed isobenzan to be a potent inhibitor of the binding of the
convulsant tert-butylbicyclophosphorothionate (TBPS) to
brain-specific sites, thereby indicating an action at the
gamma-aminobutyric acid (GABA)-regulated chloride channel. Metabolic
activation by rat liver microsomes did not enhance the potency for
inhibition. This inhibition indicates that isobenzan binds to the
same site as TBPS, suggesting that isobenzan acts in a manner
similar to non-competitive GABA-A antagonists and providing a basis
for its convulsant action in mammals (Lawrence & Casida, 1984).
Bloomquist et al. (1986) produced a concentration-dependent
inhibition of 36Cl uptake into mouse brain vesicles by adding
isobenzan to mouse brain homogenate. The inhibitory activity was
confined to that portion of 36Cl uptake that was GABA dependent.
The insecticide concentration producing 50% inhibition (I50) of
36Cl uptake was 2.0 (0.83 to 5.1) µmol/litre, and the inhibitory
potency (I50) value for 35S-TBPS binding in rat brain
synaptosomes was 0.30 µmol/litre (Lawrence & Casida, 1984).
Cole & Casida (1986) confirmed in a study with male
Swiss-Webster mice administered isobenzan intraperitoneally that a
correlation also exist in vivo between binding to mouse brain GABA
receptors and convulsive activity. The inhibitory potency (IC50)
for in vitro TBPS binding to mouse brain synaptosomes is
0.03 µmol/litre. It was shown that the inhibitory potencies of
cyclodienes, including isobenzan, parallel their acute oral
toxicities. Isobenzan was slightly more potent than endrin and
produced virtually complete inhibition of GABA-dependent chloride
uptake at 30 µmol/litre. There was a significant linear correlation
between the 36Cl flux and 35S-TBPS-binding assays.
7.8.3 Pharmacological studies
Pharmacological studies on the function of organ systems in
various animal species (such as rats, guinea-pigs, rabbits, cats,
and frogs) after the administration of isobenzan by different routes
showed that the only significant effect was a disturbance of the
central nervous system associated with convulsions. This effect was
due to stimulation of the higher brain centres at the level of the
medulla and above. Changes in respiratory rate, heart rate, and
salivary secretion were probably mediated by the central nervous
system as a secondary effect of central nervous stimulation.
Barbiturates were found to control these convulsions. The
stimulation of brain activity was reflected in the occurrence of
electroencephalographic changes in the pre-convulsive stage and
during the convulsive episodes. This corresponds to the situation in
cases of human intoxication by occupational exposure to cyclodiene
insecticides as described by Hoogendam et al. (1962, 1965) and
Chambers (1962c).
Ibrahim (1964) showed that isobenzan injected intraperitoneally
at a toxic dose (7 mg/kg body weight) into male Wistar rats produced
a higher tension of contraction in the gastrocnemius muscle at lower
frequencies of stimulation than in controls. The maximum tetanic
tension was also attained at a lower frequency. An increase in the
duration of the "active state" of the muscle was considered to be
the most like explanation.
8. EFFECTS ON HUMANS
8.1 General population exposure
No poisoning incidents or untoward effects of long-term
exposure of the general population have been reported.
8.2 Occupational exposure
Isobenzan was initially manufactured and handled in the
Netherlands between 1958 and 1965. Aldrin, dieldrin, and endrin were
also produced in the same manufacturing plant and, consequently, in
many cases the exposure was mixed. Routine medical examination of
233 workers, who were exposed for more than 4 years and who followed
normal procedures during that period, did not reveal any
abnormalities in EEG, clinical chemistry or haematological
parameters, or liver microsomal enzyme induction (Jager, 1970;
Versteeg & Jager, 1973). The mean isobenzan concentration in the
blood of 20 operators after cessation of exposure decreased from
22 µg/litre in 1965 to 7 µg/litre in 1969. The biological half-life
of isobenzan in the blood (at the state of equilibrium of isobenzan
in body tissues) was estimated to be of the order of 2.8 years
(Jager, 1970).
In the 7 years of isobenzan production, 15 cases of clinical
intoxication, including eight cases with convulsions, were reported.
The mean concentration of isobenzan in the blood of nine workers at
the time of intoxication was 23 µg/litre, the range being
17-30 µg/litre. Although these workers recovered fully, it took
longer than with the related cyclodiene insecticides. In three
cases, certain typical complaints, such as headache, dizziness,
drowsiness, and irritability, persisted for 6 months, and the return
to normal of the modified EEG pattern sometimes took more than a
year. In one case of acute over-exposure, without signs of
intoxication, the blood isobenzan concentration decreased from
8 µg/litre to less than 2 µg/litre within 3 days (Jager, 1970).
The data from plant workers indicated a threshold level of
isobenzan in blood below which no signs or symptoms of intoxication
occur. This level was found to be 15 µg/litre (Jager, 1970).
Ribbens (1985) carried out a mortality study on the
above-mentioned industrial workers exposed to aldrin, dieldrin,
endrin, and isobenzan. Vital status and cause of death were assessed
for 232 of the total population of more than 1000 workers. This
group was selected for follow-up on account of the high degree of
exposure in the initial years of manufacturing and formulation and
the long exposure (mean 11 years) and observation (mean 24 years)
periods. Total observed mortality was 25 as opposed to 38 expected
on the basis of death statistics for the male Dutch population. Of
the nine cancer deaths, three were caused by lung cancer, while the
remaining six were each of a different nature. The author concluded
that although exposures in this group were high and exposure as well
as observation periods long, this study did not reveal any
indication of a specific carcinogenic activity of these pesticides.
9. EFFECTS ON OTHER ORGANISMS IN THE LABORATORY AND FIELD
9.1 Microorganisms
In laboratory studies, sandy loam soil was treated with 250 or
2500 mg isobenzan/kg (concentrations that exceeded the recommended
rates). At both concentrations, the carbon dioxide production was
inhibited to almost the same extent (21 and 24%, respectively)
during a 30-day test period. The addition of glucose to the soil
reduced the inhibitory effect to 5 and 4%, respectively. Isobenzan
at 250 mg/kg did not influence nitrification in the soil up to 18
days after treatment (Bartha et al., 1967).
Screening studies on microorganisms in pure culture were
carried out on nutrient agar plates with isobenzan either
incorporated in a uniformly emulsified form (1000 mg/kg) or as a
thin surface film (1 mg/cm2). The growth of the gram-positive
Bacillus megaterium was inhibited, but not that of various
gram-negative organisms such as several Pseudomonas strains,
Escherichia coli, Klebsiella aerogenes W5, or Achromobacter
butyri (Trudgill & Widdus, 1970).
9.2 Aquatic organisms
Groups of five adult Harlequin fish (Rasbora heteromorpha)
were exposed for 2 h at a temperature of 20 °C to water (pH 7.2)
containing isobenzan (99%), dissolved in DMSO, at a concentration of
0.01, 0.1, or 1 mg/litre. The fish were then transferred to clean
water and observed for an additional 48 h. The treatment with
1 mg/litre caused disorientation and the fish became excited by
external stimuli, lost the ability to swim, and, finally, all died
within 1 h. In some cases, they appeared to convulse. Isobenzan at
0.1 mg/litre caused similar symptoms; within 2 h of exposure, all
five fish died. No fish died at 0.01 mg/litre, but slight changes in
swimming behaviour were observed (Brown et al., 1962). When
guppies (Poecilia reticulata) were tested in the same way, the
symptoms of intoxication and susceptibility were similar to those in
Harlequin fish, but the guppies were slower to react (Brown et al.,
1962).
Data on the acute toxicity of isobenzan for aquatic organisms
in flow-through tests are given in Table 10.
Table 10. Acute toxicity of isobenzan (technical grade, 94%) for aquatic organisms
Organism Developmental Temperature Parameter Concentration Reference
stage (°C) (µg/litre)
Brown shrimp juvenile 17 48-h EC50 0.034a US EPA
(Penaeus (1987)
aztecus)
Eastern oyster juvenile 18 96-h EC50 32b US EPA
(Crassostrea (1987)
virginica)
Sheepshead minnow juvenile 17 48-h LC50 2.0a US EPA
(Cyprinodon (1987)
variegatus)
Spot juvenile 13 48-h LC50 0.32c US EPA
(Leiostomus (1987)
xanthurus)
a Salinity 30 ng/litre.
b Salinity 33 ng/litre.
c Salinity 22 ng/litre.
9.3 Terrestrial organisms
9.3.1 Soil invertebrates
In laboratory studies, plain-field sand was treated once with
0.05 mg isobenzan/kg and stored at 13 or 24 °C. The springtail
(Folsomia candida) was used for bioassays lasting up to 16 weeks.
The biological activity of isobenzan persisted slightly longer at
the higher temperature, killing 100% of the insects after 16 weeks
(Thompson, 1973).
9.3.2 Birds
9.3.2.1 Acute toxicity
Isobenzan (> 99%) is highly toxic to birds when administered
as a single oral dose (Table 11). LD50 values range from
1-10 mg/kg.
9.3.2.2 Short-term toxicity
When groups of five male and five female Japanese quail
(Coturnix coturnix japonica) were fed diets containing 2 or 10 mg
isobenzan/kg, the mean survival time in the high-dose group was 6.9
days (range, 2-20 days). The residues in the liver and brain
averaged 3.4 mg/kg and 1.4 mg/kg, respectively. In the low-dose
group, the mean survival time was 45.9 days (range, 19-65 days) and
the average residues in liver and brain were 6 mg/kg and 1.6 mg/kg,
respectively. The concentration of isobenzan in the liver of birds
fed 2 mg/kg was significantly higher than that in the high-dose
group, but the concentration in the brain showed no difference
(Koeman, 1971).
Table 11. Oral LD50 values of isobenzan for birds
Species LD50 (mg/kg Reference
body weight)
Mallard duck (female) 4.15 Hudson et al. (1984)
(Anas platyrhynchos) (2.47-6.97)
Coturnix quail (female) 4.2 Schafer & Brunton (1979)
(Coturnix coturnix)
Grackle 1.3 Schafer & Brunton (1979)
(Quiscalus quiscula)
Pigeon 10 Schafer & Brunton (1979)
(Columba livia)
Red-winged blackbird (male) 3.2 Schafer & Brunton (1979)
(Agelaius phoeniceus)
Sparrow 1 Schafer & Brunton (1979)
(Passer domesticus)
Starling 2.4 Schafer & Brunton (1979)
(Sturnus vulgaris)
When groups of 20 Rhode Island Red laying hens were fed
isobenzan (15% emulsifiable concentrate) at levels of 0.1, 0.25,
0.75, or 1 mg/kg diet for 14 weeks, no effects were seen on food
consumption, egg production, egg weight, or egg fertility (Verma
et al., 1967).
9.4 Population and ecosystem effects
9.4.1 Soil microorganisms
In a sugar cane field in India, Srivastava (1966) studied the
effect of isobenzan (1 kg/ha) on nitrification of ammonium sulfate
in the soil. The insecticide-treated soil contained higher amounts
of total inorganic nitrogen and ammonium nitrogen (80% increase) up
to 60 days after treatment than did untreated soil samples,
indicating impaired nitrification.
9.4.2 Soil invertebrates
In a study by Kelsey & Arlidge (1968), five sandy loam plots of
pasture in New Zealand were treated with Telodrin (15% emulsifiable
concentrate) at a rate of 2 kg isobenzan/ha in June 1962. The
populations of all recorded groups (grass grub, porina, Collembola,
Diptera, Hemiptera, Coleoptera, mites, and earthworms), except
nematodes, were drastically reduced. There was no recovery in the
populations of any of the affected groups during the period up to
October 1965. In silt loam plots treated with isobenzan granules
(1 kg/ha) in April 1964, the populations of Coleoptera, Collembola,
Diptera, mites, and earthworms were all reduced, but to a lesser
extent than in the test using 2 kg/ha. However, grass grub and
Hemiptera were not significantly affected, and nematode numbers were
elevated 2 years after the treatment. Studies on root development
showed that 90% of roots with root hairs were located within a mat
of plant debris above the soil, whereas in controls 99% were located
in the soil. Plant growth was retarded. The moisture content of the
treated plots was significantly less than that of control plots,
indicating that the capacity of soil to absorb and retain water had
been reduced.
When loose sandy soil in New Zealand was treated with 2.25 kg
isobenzan/ha as 5% granules, the population reduction, 1-18 weeks
after treatment, was 90-100% for larval Coleoptera and Lepidoptera
and 75% for Diptera and earthworms. The number of surface
arthropods, 5-15 days after treatment, was reduced by 45%. Six
months after treatment, little effect was found on nematodes
(13% reduction), bacteria (18% reduction), and fungi (7% increase)
(Moeed, 1975).
REFERENCES
ANON (1974) Aldrin, dieldrin, and endrin. Determination of residues
of organochlorine insecticides in crops, soils, and animal products,
Sittingbourne, Shell Research (Residue Analytical Method WAMS 60-1).
BARTHA, R., LANZILOTTA, R.P., & PRAMER, D. (1967) Stability and
effects of some pesticides in soil. Appl. Microbiol., 15(1): 67-75.
BAUER, U. (1972) [Behaviour of a series of plant-protecting agents
during the treatment of groundwater by slow sand filtration.]
Schriftenr. Ver. Wasser-Boden-Lufthyg. (Berlin-Dahlem), 37: 91-102
(in German).
BIEHL, M.L. & BUCK, H.B. (1987) Chemical contaminants: their
metabolism and their residues. J. Food Prot., 50(12): 1058-1073.
BISHOP, J.L. & HUBER, J.T. (1964) Secretion of Telodrin in the milk
of cows fed varying levels of Telodrin. J. dairy Sci., 47: 552-554.
BLOOMQUIST, J.R., ADAMS, P.M., & SÖDERLAND, D.M. (1986) Inhibition
of gamma-aminobutyric acid stimulated chloride flux in mouse brain
vesicles by polychlorocycloalkane and pyrethroid insecticides.
Neurotoxicology, 7(3): 11-20.
BOWMAN, M.C., SCHECHTER, M.S., & CARTER, R.L. (1965) Behavior of
chlorinated insecticides in a broad spectrum of soil types. J.
agric. food Chem., 13: 360-365.
BROWN, V.K.H. (1963) Agricultural chemicals: the relative
percutaneous toxicities of Nitrophoska fertiliser granules
impregnated with aldrin and Telodrin, Sittingbourne, Shell Research
(Technical Memorandum TOX 10/63).
BROWN, V.K.H. (1964) Some effects of percutaneously absorbed
Telodrin in rabbits, Sittingbourne, Shell Research (Report No. PPR
TL/2/64).
BROWN, V.K.H. & RICHARDSON, A. (1964) Chronic oral exposure of
beagle hounds to Telodrin and dieldrin: report on the first two
years, Sittingbourne, Shell Research (Report No. PPR TL/1/64).
BROWN, V.K.H., CHAMBERS, P.L., HUNTER, C.G., & STEVENSON, D.E.
(1962) The toxicity of Telodrin for vertebrates, Sittingbourne,
Shell Research (Report No. R(T)-2-62).
BUICK, A.R. (1964) Telodrin residues on tobacco from Australia,
Sittingbourne, Shell International Chemical Company, Ltd (Technical
Memorandum 132/64)
BUICK, A.R. (1965) Telodrin residues in potatoes from India,
Sittingbourne, Shell International Chemical Company, Ltd (Technical
Memorandum 25/65).
BUICK, A.R. & COLE, E.R. (1965) Telodrin residues in sweet potatoes
from South Africa, Sittingbourne, Shell International Chemical
Company, Ltd (Technical Memorandum 25/65).
BULL, M.S. & MARLOW, R.G. (1967) Insecticide residues in diet
samples from Nicaragua, Sittingbourne, Shell International Chemical
Company, Ltd (Technical Service Note 85/67).
BULL, M.S. & RAMSDEN, D.P. (1967) Residues of organochlorine
insecticides in samples from Venezuela, Sittingbourne, Shell
International Chemical Company, Ltd (Technical Service Note 86/67).
CHAMBERS, P.L. (1962a) Agricultural chemicals: the effect of
Telodrin on reproduction in the rat when fed at various levels in
the diet. Report No. 3, Sittingbourne, Shell International Chemical
Company, Ltd (Technical Memorandum TOX 24/62).
CHAMBERS, P.L. (1962b) Agricultural chemicals: the effect of
Telodrin on reproduction in the rat when fed at various levels in
the diet. Report No. 4, Sittingbourne, Shell International Chemical
Company, Ltd (Technical Memorandum TOX 33/62).
CHAMBERS, P.L. (1962c) The physiological and pharmacological effects
of Telodrin, Sittingbourne, Shell Research (Report No. M(T)-4-62).
COLE, L.M. & CASIDA, J.E. (1986) Polychlorocycloalkane
insecticide-induced convulsions in mice in relation to disruption of
the GABA-regulated chloride ionophore. Life Sci., 39: 1855-1862.
CRABTREE, A.N. (1968) Concentration of chlorinated insecticides in
the whole blood of the CAPSA plant in Venezuela, Sittingbourne,
Shell Research (Report No. TLGR.0032.68).
DAVIES, J.M. (1966a) Chlorinated insecticide content of the blood of
formulators in the CAPSA plant in Venezuela, Sittingbourne, Shell
Research (Report No. IRR TL/14/66).
DAVIES, J.M. (1966b) Concentration of chlorinated insecticides in
the whole blood of formulators of the CAPSA plant in Venezuela,
Sittingbourne, Shell Research (Report No. IRR TL/37/66).
EDWARDS, C.A. (1965) Effects of pesticide residues on soil
invertebrates and plants. In: Proceedings of the 5th Symposium of
the British Ecological Society, Ecology and Industrial Society.
EICHELBERGER, J.W. & LICHTENBERG, J.J. (1971) Persistence of
pesticides in river water. Environ. Sci. Technol., 5(6): 541-544.
ELGAR, K.E. (1965) Insecticide residues in samples of cottonseed
from Mexico, Sittingbourne, Shell International Chemical Company,
Ltd (Technical Memorandum 99/65).
ELGAR, K.E. (1966) Analysis of crops and soils for residues of the
soil insecticides aldrin and Telodrin. J. Sci. Food Agric., 17:
541-545.
ELGAR, K.E. & HOLLAND, A.A. (1967) Residues of organochlorine
insecticides in potatoes from Mexico, Sittingbourne, Shell
International Chemical Company, Ltd (Technical Service Note 82/67).
HARDEE, D.D., GUTENMANN, W.H., KEENAN, G.I., GYRISCO, G.G., LISK,
D.J., FOX, F.H., TRIMBERGER, G.W., & HOLLAND, R.F. (1964) Residues
of heptachlor epoxide and Telodrin in milk from cows fed at parts
per billion insecticide levels. J. econ. Entomol., 56(3): 404-407.
HATHWAY, D.E. (1965) The biochemistry of dieldrin and Telodrin.
Arch. environ. Health, 11: 380-388.
HATHWAY, D.E. & MALLINSON, A. (1964) Chemical studies in relation to
convulsive conditions: effect of Telodrin on the liberation and
utilization of ammonia in rat brain. Biochem. J., 90: 51-60.
HATHWAY, D.E., MALLINSON, A., & AKINTONWA, D.A.A. (1965) Effects of
dieldrin, picrotoxin, and Telodrin on the metabolism of ammonia in
brain. Biochem. J., 94: 676-686.
HODGE, H.C., BOYCE, A.M., DEICHMANN, W.B., & KRAYBILL, H.F. (1967)
Toxicology and no-effect levels of aldrin and dieldrin. Toxicol.
appl. Pharmacol., 10(3): 613-637.
HOOGENDAM, I., VERSTEEG, J.P.J., & VLIEGER, M., DE (1962)
Electroencephalograms in insecticide toxicity. Arch. environ.
Health, 4: 86-94.
HOOGENDAM, I., VERSTEEG, J.P.J., & VLIEGER, M., DE (1965) Nine years
toxicity control in insecticide plants. Arch. environ. Health, 10:
441-448.
HOWARD, J.A., BUXTON, J.A., ATER, F.B., OVERBECK, R.C., FOOTE, W.L.,
ROBINSON, R.F., & DAVIDSON, R.S. (1957) Final report on toxicology
of BAS-4402 to the Shell Development Corporation, Columbus, Ohio,
Battelle Memorial Institute.
HUDSON, R.H., TUCKER, R.K., & HAEGELE, M.A. (1984) Handbook of
toxicity of pesticides to wildlife. 2nd ed., Washington, DC, US
Department of the Interior, Fish and Wildlife Service (Resource
Publication No. 153).
HUGHES, D.G. (1965) Insecticide residues in cottonseed oil,
Sittingbourne, Shell International Chemical Company, Ltd (Technical
Memorandum 23/65).
IBRAHIM, T.M. (1964) A toxicological study of the action of the
insecticide dieldrin and related substances on the contraction of
striated muscle in the rat, University of Utrecht (Thesis).
INNES, J.R.M., ULLAND, B.M., VALERIO, M.G., PETRUCELLI, L.,
FISHBEIN, L., HART, E.R., PALLOTTA, A.J., BATES, R.R., FALK, H.L.,
GART, J.J., KLEIN, M., MITCHELL, I., & PETERS, J. (1969) Bioassay of
pesticides and industrial chemicals for tumorigenicity in mice: a
preliminary note. J. Natl Cancer Inst., 42(6): 1101-1114.
JAGER, K.W. (1970) Aldrin, dieldrin, endrin, and Telodrin: an
epidemiological and toxicological study of long-term occupational
exposure, Amsterdam, London, New York, Elsevier Science Publishers.
JAPANESE ENVIRONMENTAL AGENCY (1975) Environmental survey report on
chemical substances in FY 1974, Tokyo, Environmental Health
Department, Planning and Coordination Bureau (Unpublished report).
KADOUM, A.M. (1968) Application of the rapid micro method of sample
clean-up for gas chromatographic analysis of common organic
pesticides in ground water, soil, plant and animal extracts. Bull.
environ. Contam. Toxicol., 3(2): 65-70.
KAUL, R., KLEIN, W., & KORTE, F. (1970) [Contributions to ecological
chemistry. XX. Distribution, elimination, and metabolism of Telodrin
and heptachlor in rats and male rabbits, end-product of the
metabolism of heptachlor in mammals.] Tetrahedron, 26: 331-337 (in
German).
KELSEY, J.M. & ARLIDGE, E.Z. (1968) Effects of isobenzan on soil
fauna and soil structure. N. Z. J. agric. Res., 11: 245-260.
KERDIJK, H.N. (1981) Groundwater pollution by heavy metals and
pesticides from a dredge spoil dump. Stud. environ. Sci., 17:
279-286.
KODAVANTI, P.R.S., MEHROTRA, R.D., CHETTY, S.C., & DESAIAH, D.
(1988) Effect of selected insecticides on rat brain synaptosomal
adenylate cyclase and phosphodiesterase. J. Toxicol. environ.
Health, 25: 207-215.
KOEMAN, J.H. (1971) [The occurrence and toxicological implications
of some chlorinated hydrocarbons in the Dutch coastal area in the
period from 1965 to 1970], University of Utrecht, pp. 30-31 (Thesis)
(in Dutch).
KOEMAN, J.H., OSKAMP, A.A.G., VEEN, J., BROUWER, E., ROOTH, J.,
ZWART, P., VAN DE BROEK, E., & VAN GENDEREN, H. (1967) Insecticides
as a factor in the mortality of the sandwich tern (Sterna
sandvicensis): a preliminary communication. Meded. Landbouwwet.
Hogesch. Gent., 32: 841-854.
KOEMAN, J.H., VEEN, J, BROUWER, E., HUISMAN-DE BROUWER, L., &
KOOLEN, J.L. (1968) Residues of chlorinated hydrocarbon insecticides
in the North Sea environment. Helgoländer wiss. Meerunters., 17:
375-380.
KORTE, F. (1963) Review of metabolism studies with 14C-labelled
insecticides from 1958 to March 1963. Unpublished paper presented at
a Toxicological Meeting, New York, 25-27 March.
KORTE, F. (1967) Metabolism of 14C-labelled insecticides in
microorganisms, insects, and mammals. Botyu-Kagaku, 32(2): 46-59.
KORTE, F. & STIASNI, M. (1964) [Metabolism of 14C-Telodrin in
microorganisms and mosquito larvae.] Ann. Chem., 673: 146-152 (in
German).
LAWRENCE, L.J. & CASIDA, J.E. (1984) Interactions of lindane,
toxaphene, and cyclodienes with brain-specific
t-butylbicyclophosphorothionate receptor. Life Sci., 35: 171-178.
LAYTON, D.W., MALLON, B.J., ROSENBLATT, D.H., & SMALL, M.J. (1987)
Deriving allowable daily intakes for systemic toxicants lacking
chronic toxicity data. Regul. Toxicol. Pharmacol., 7: 96-112.
MCCASKEY, T.A., STEMP, A.R., LISKA, B.J., & STADELMAN, W.J. (1968)
Residues in egg yolks and raw and cooked tissues from laying hens
administered selected chlorinated hydrocarbon insecticides. Poultry
Sci., 47: 564-569.
MARLOW, R.G., BULL, M.S., & WILLIAMS, S. (1968) Residues of
organochlorine insecticides in foodstuffs from Spain, Sittingbourne,
Shell International Chemical Company, Ltd (Technical Service Report
WKTR.0058.68).
MATHEWS, B.L. (1969) Residues of organochlorine insecticides in
foodstuffs from India, Sittingbourne, Shell Research (Report No.
WKGR.0102.69).
MATHEWS, B.L. & COLE, E.R. (1966) Residues of Telodrin,
methylparathion, and DDT on tobacco from Costa Rica, Sittingbourne,
Shell International Chemical Company, Ltd (Technical Service Note
123/66).
MEHROTRA, B.D., BANSAL, S.K., & DESAIAH, D. (1982) Comparative
effects of structurally-related cyclodiene pesticides on ATPases. J.
appl. Toxicol., 2(6): 278-283.
MOEED, A. (1975) Effects of isobenzan, fensulfothion, and diazinon
on invertebrates and microorganisms. N. Z. J. exp. Agric., 3:
181-185.
MOSS, J.A. & HATHWAY, D.E. (1964) Transport of organic compounds in
the mammal. Partition of dieldrin and Telodrin between the cellular
components and soluble proteins of blood. Biochem. J., 91: 384-393.
MURPHY, M.W. & STANDEN, M.E. (1964) The translocation of Telodrin
into potatoes, Sittingbourne, Shell International Chemical Company,
Ltd (Technical Memorandum 184/64).
RIBBENS, P.H. (1985) Mortality study of industrial workers exposed
to aldrin, dieldrin and endrin. Arch. occup. environ. Health, 56(2):
75-79.
RICHARDSON, A., ROBINSON, J., BUSH, B., & DAVIES, J.M. (1967)
Determination of dieldrin (HEOD) in blood. Arch. environ. Health,
14(5): 703-708.
ROBINSON, J. & RICHARDSON, A.R. (1963) The distribution, storage,
and elimination of Telodrin in rats fed this insecticide in their
diet, Sittingbourne, Shell Research (Report No. R(T)-1-63).
SCHAFER, E.W. & BRUNTON, R.B. (1979) Indicator bird species for
toxicity determinations: is the technique usable in test method
development? In: Beck, J.R., ed. Vertebrate pest control and
management materials, Philadelphia, American Society for Testing and
Materials, pp. 157-168 (ASTM STP 680).
SHELL (1963) An episode of pesticide contamination of pasture and
dairy products - Isobenzan in Victoria, Australia, in 1963.
Melbourne, Shell Chemical Australia (Unpublished report No.
90111501WP5).
SHELL (1964) Telodrin residues. A summary, London, Shell
International Chemical Company (Unpublished report).
SPYNU, E.I. (1964) On the toxicology of new organic chloride
insecticides obtained by diene synthesis on the basis of
hexachlorocyclopentadiene. Gig. Tr. prof. Zabol., 4: 30-35.
SRIVASTAVA, S.C. (1966) The effect of Telodrin on nitrification of
ammonia in soil and its implication on nitrogen nutrition of
sugarcane. Plant Soil, 25(3): 471-473.
STANDEN, M.E. & ELGAR, K.E. (1965) Telodrin residues in dairy
products from Venezuela, Sittingbourne, Shell International Chemical
Company, Ltd (Technical Memorandum 36/65).
STEMP, A.R. & LISKA, B.J. (1966) Effects of processing and storage
of dairy products on Telodrin and methoxychlor residues. J. diary
Sci., 49: 1006-1008.
STEVENSON, D.E. (1964) The toxicology of Telodrin. Meded. Fac.
Landbouwwet. Rijksuniv. Gent, 29: 1198-1207.
SUZUKI, K., NAGAYOSHI, H., & KASHIWA, T. (1974) The systematic
separation and identification of pesticides in the first division.
Agric. biol. Chem., 38(2): 279-285.
THOMPSON, A.R. (1973) Persistence of biological activity of seven
insecticides in soil assayed with Folsomia candida. J. econ.
Entomol., 66(4): 855-857.
TRUDGILL, P.W. & WIDDUS, R. (1970) Effects of chlorinated
insecticides on metabolic processes in bacteria. Biochem. J., 118:
48-49.
TUINSTRA, L.G.M.TH. & TRAAG, W.A. (1979) Automated glass capillary
gas chromatographic analysis of PCB and organochlorine pesticide
residues in agricultural products. J. high Resolut. Chromatogr.
Chromatogr. Commun., 2(2): 723-728.
US EPA (1987) Acute toxicity handbook of chemicals to estuarine
organisms. Springfield, Virginia, US Department of Commerce,
National Technical Information Service (EPA/600/8-87/017).
VAN WIJNEN, J.H. & STIJKEL, A. (1988) Health risk assessment of
residents living on harbour sludge. Int. Arch. occup. environ.
Health, 61: 77-87.
VERMA, M.P., BAHGA, H.S., & SONI, B.K. (1967) Effect of prolonged
administration of the insecticide Telodrin in poultry. Indian vet.
J., 44: 962-966.
VERSTEEG, J.P.J. & JAGER, K.W. (1973) Long-term occupational
exposure to the insecticides aldrin, dieldrin, endrin, and Telodrin.
Br. J. ind. Med., 30: 201-202.
WARE, G.W. & GOOD, E.E. (1967) Effects of insecticides on
reproduction in the laboratory mouse. II. Mirex, Telodrin, and DDT.
Toxicol. appl. Pharmacol., 10(1): 54-61.
WEGMAN, R.C.C. & GREVE, P.A. (1980) Halogenated hydrocarbons in
Dutch water samples over the years 1969-77. Environ. Sci. Res., 16:
405-415.
WEGMAN, R.C.C. & HOFSTEE, A.W.M. (1982) Determination of
organochlorines in river sediment by capillary gas chromatography.
Water Res., 16: 1265-1272.
WEGMAN, R.C.C., HOFSTEE, A.W.M., & GREVE, P.A. (1981) Uptake of
organochlorines by plants growing on river and basin sediment.
Meded. Fac. Landbouwwet. Rijksuniv. Gent, 46(1): 359-365.
WORDEN, A.N. (1969) Toxicity of Telodrin. Toxicol. appl. Pharmacol.,
14: 556-573.
ZAVON, M.R. (1961) Toxicology and pharmacology of Telodrin
insecticide (compound SD 4402), Cincinnati, Ohio, The Kettering
Laboratory (Unpublished report).
RESUME ET EVALUATION; CONCLUSIONS ET RECOMMANDATIONS
1. Résumé et évaluation
Autant qu'on sache, l'isobenzan, un insecticide organochloré
n'a été fabriqué que pendant la période 1958-1965. Plusieurs années
aprés, on puisait toujours sur les stocks existants. A l'heure
actuelle, les seules sources importantes d'exposition sont
vraisemblablement les sites de décharge initiaux de déchets
industriels ou les boues de dragage provenant de sédiments
contaminés.
Une fois épandu sur le sol, la majeure partie de l'isobenzan
disparaît rapidement. Après quoi, la fraction restante se décompose
beaucoup plus lentement. Elle persiste dans le sol de deux à sept
ans selon la nature de celui-ci. Au laboratoire, l'isobenzan se
décompose dans les eaux de surface en l'espace de quelques semaines
lorsqu'il est exposé à la lumière naturelle ou artificielle.
Le sol, les eaux souterraines et superficielles provenant des
polders constitués de sédiments contaminés par des organochlorés,
notamment des dérivés cyclodiéniques, contenaient encore plusieurs
années après, de faibles résidus d'isobenzan. En 1979-1980, on n'a
pas détecté d'isobenzan (limite de détection 0,01 mg/kg de poids
sec) dans les sédiments des cours d'eau des Pays-Bas. Après
traitement du sol, les résidus qui subsistent sur les récoltes sont
généralement faibles (inférieurs à 0,05 mg/kg de végétaux), mais on
peut en trouver des quantités plus fortes sur certaines racines
(jusqu'à 0,2 mg/kg dans les carottes). Des enquêtes de type "panier
de la ménagère" effectuées lorsqu'on utilisait de l'isobenzan en
agriculture, n'ont pas permis de déceler de résidus dans les denrées
contrôlées (moins de 0,01 mg/kg).
Chaque fois qu'on a laissé paître des bovins dans des pâturages
traités par de l'isobenzan, on a constaté que les produits laitiers
obtenus contenaient des résidus de cet insecticide. C'est ainsi que
dans deux échantillons de beurre, on a trouvé 0,07 et 0,15 mg
d'isobenzan par kg de produit, les concentrations dans le lait
entier allant de 0,005 à 0,07 mg/kg. Le lait en poudre n'en
contenait toutefois que 0,005 mg/kg. Lors du traitement industriel
des produits laitiers, plus de 50% du résidu disparaît selon le type
de traitement.
On ne dispose d'aucune donnée sur les quantités d'isobenzan
présentes dans le sang ou les tissus adipeux de la population
générale. Des travailleurs exposés à l'isobenzan lors de la
fabrication ou de la formulation de cet insecticide, présentaient
des taux sanguins (sang total) allant jusqu'à 0,041 mg/litre. Dans
les échantillons de sang total prélevés sur des personnes vivant à
proximité d'une unité de production, la concentration d'isobenzan
était inférieure à la limite de détection (0,001 mg/litre).
L'isobenzan est facilement résorbé par la paroi du tube
digestif et il passe dans le sang sans modification. Il se forme des
métabolites hydrophiles et notamment une lactone. L'isobenzan
s'accumule dans les tissus et les organes des rats et des chiens
selon l'ordre: graisses > foie = muscle > cerveau > sang. Les
concentrations tissulaires chez les rattes sont généralement plus
faibles que chez les rats, spécialement dans les graisses. La
demi-vie biologique dans le tissu adipeux était ainsi de 10,9 jours
chez les rats et de 16,6 jours chez les rattes. Chez un chiot
femelle dont le sang contenait 0,09 mg d'isobenzan par litre, on a
observé des convulsions 15 jours après la naissance. L'animal
n'avait été nourri qu'avec le lait de sa mère, une chienne Beagle à
qui l'on avait fait absorber de l'isobenzan et dont le lait en
contenait 0,7 mg/litre. Des effets analogues sur les petits ont été
observés lors d'une étude de reproduction sur le rat. Chez la vache,
l'isobenzan est excrété dans le lait.
Les larves de moustiques et les champignons terricoles
métabolisent l'isobenzan de la même manière que les vertébrés,
notamment sous forme de lactone. L'isobenzan est très persistant
dans l'environnement et s'accumule dans les organismes vivants. Il
est extrêmement toxique pour les poissons, les crevettes et les
oiseaux. Aux Pays-Bas, pays où l'on fabriquait l'isobenzan, on a
trouvé des résidus dans des oeufs de sternes vivant sur la côte
hollandaise qui atteignaient 0,45 mg/kg (moyenne, 0,09 mg/kg). Dans
les moules et le poisson, les résidus moyens étaient de 0,05 mg/kg
en 1965. Dans les parcelles traitées par de l'isobenzan à raison de
2 kg/ha, on a constaté une réduction du nombre de lombrics. Il y
avait réduction de la nitrification avec accroissement corrélatif de
l'azote minéral dans les sols traités par l'isobenzan à raison
d'1 kg/ha; en revanche, les études en laboratoire n'ont mis en
évidence aucun effet sur la nitrification à des doses correspondant
à 250 g/ha.
L'isobenzan présente une forte toxicité aiguë pour les
mammifères, que ce soit par la voie orale ou par la voie percutanée.
Le mode d'action de l'isobenzan consiste en une stimulation
excessive du système nerveaux central conduisant à des convulsions.
La toxicité aiguë des diverses formulations d'isobenzan correspond à
la proportion de matière active.
L'isobenzan n'est pas irritant pour la peau mais certaines de
ses formulations peuvent l'être.
Des études limitées à court et à long terme, au cours
desquelles on a administré par voie orale de l'isobenzan à des
souris, à des rats et à des chiens, ont montré que ce composé
pouvait induire des lésions histologiques au niveau du foie, du type
de celles qu'on observe classiquement avec les organochlorés. Une
étude de longue durée sur des rats a permis de déterminer que la
dose sans effet observable était de 5 mg/kg de nourriture (soit
l'équivalent de 0,25 mg/kg de poids corporel). Chez le chien, la
dose sans effet nocif observable, déterminée à la suite d'une étude
de deux ans, était de 0,025 mg/kg de poids corporel.
Une étude de reproduction portant sur une génération de rats a
montré que la dose sans effet nocif observable était de 0,1 mg/kg de
nourriture (soit l'équivalent de 0,05 mg/kg de poids corporel. A la
dose de 1 mg/kg de nourriture (soit l'équivalent de 0,05 mg/kg de
poids corporel), il y a eu réduction de la survie des ratons.
Aucune étude de tératogénicité ni de mutagénicité n'a été
rapportée.
Une étude de deux ans sur des rats (administration par voie
orale) et une étude sur des souris n'ont pas permis de mettre en
évidence un pouvoir cancérogène quelconque, mais ces études
n'étaient pas adaptées à une telle évaluation.
La base de données toxicologiques sur l'isobenzan est
incomplète. En général on estime que, d'après les critères actuels,
les données sont d'une qualité médiocre et sont en tous cas
insuffisantes pour permettre une évaluation du risque que ce composé
présente pour la santé humaine ou l'environnement.
Les données concernant l'exposition humaine se limitent à des
études effectuées sur des travailleurs d'une usine hollandaise qui
étaient employés à la fabrication et à la formulation d'isobenzan et
d'insecticides cyclodiéniques apparentés. Aucun cas d'irritation
cutanée n'a été signalé. Dans plusieurs cas d'intoxication, on a
observé des convulsions mais les anomalies du tracé
électro-encéphalographique étaient réversibles. Le seuil limite
d'intoxication (pour les convulsions), a été estimé à 0,015 mg
d'isobenzan par litre de sang et la demi-vie biologique de ce
composé dans le sang humain est, semble-t-il, de l'ordre de 2,8
années.
2. Conclusions et recommandations
L'isobenzan est extrêmement toxique et très persistant. Les
données dont on dispose sur le danger qu'il représente sont
incomplètes mais néanmoins suffisantes pour montrer que ce danger
est réel pour les personnes qui le manipulent ainsi que pour
l'environnement, de sorte qu'il faut éviter toute contamination
humaine ou environnementale qui résulterait de l'utilisation de ce
produit comme insecticide ou autre.
RESUMEN Y EVALUACION; CONCLUSIONES Y RECOMENDACIONES
1. Resumen y evaluación
Según los datos de que se dispone, el isobenzano, un
insecticida organoclorado, sólo se fabricó durante el periodo
1958-1965. Durante los años siguientes se utilizaron las existencias
almacenadas. En la actualidad, se cree que las únicas fuentes
importantes de exposición son los lugares donde originalmente se
evacuaron los desechos industriales o los materiales dragados de
sedimentos contaminados.
Cuando se aplica el isobenzano al suelo, se produce una rápida
pérdida inicial; después, el resto del compuesto se degrada mucho
más despacio. Persiste en el suelo durante 2 a 7 años, según el tipo
de suelo. En condiciones de laboratorio, el isobenzano se descompone
en las aguas de superficie en pocas semanas cuando se expone a la
luz natural o artificial.
El suelo, las aguas subterráneas y las aguas superficiales de
pólders construidos con sedimentos contaminados por sustancias
organocloradas, inclusive compuestos de ciclodieno clorado, aún
contenían pequeños residuos de isobenzano algunos años después. En
1979-1980, no se detectó isobenzano (límite de detección: 0,01 mg/kg
de peso seco) en el sedimento de ríos de los Países Bajos. Tras el
tratamiento del suelo, los residuos en las cosechas suelen ser bajos
(menos de 0,05 mg/kg de cosecha), pero pueden encontrarse
concentraciones superiores en algunos tubérculos (hasta 0,2 mg/kg en
zanahorias). En las encuestas de mercado realizadas durante la época
de uso agrícola del isobenzano no se detectaron residuos en los
alimentos analizados (menos de 0,01 mg/kg).
En los productos lácteos procedentes de ganado que se alimentó
en pastos tratados con isobenzano se encontraron residuos del
insecticida. Dos muestras de mantequilla contenían 0,07 y 0,15 mg de
isobenzano/kg de producto, mientras que los niveles en la leche
entera fueron de 0,005 mg/kg a 0,07 mg/kg. En la leche deshidratada,
no obstante, se encontraron sólo 0,005 mg/kg. Durante la elaboración
de los productos lácteos se perdía hasta el 50% del residuo, según
el tipo de tratamiento.
No se dispone de datos sobre los niveles de isobenzano en la
sangre o el tejido adiposo de la población general. Los operarios
expuestos al isobenzano en las plantas de fabricación y elaboración
presentaron niveles medios en sangre entera de hasta 0,041 mg/litro.
En muestras de sangre entera procedente de personas que vivían en
las proximidades de una de las plantas, la concentración de
isobenzano estaba por debajo del límite de detección
(0,001 mg/litro).
El isobenzano es absorbido rápidamente a través de la pared
gastrointestinal y es transportado por la sangre sin alteraciones.
Se forman metabolitos hidrófìlos de los que se ha identificado uno,
la lactona de isobenzano. El isobenzano se acumula en los tejidos y
los órganos de ratas y perros en el orden siguiente: grasa > hígado
= músculo > cerebro > sangre. Las concentraciones tisulares en las
hembras de rata son en general superiores a las que aparecen en los
machos, sobre todo en la grasa. En la rata se determinó que la
semivida biológica en la grasa del organismo es de 10,9 días en los
machos y 16,6 días en las hembras. En un cachorro hembra de perro,
cuya sangre contenía 0,09 mg de isobenzano/litro, se observaron
convulsiones a los 15 días del nacimiento. El cachorro sólo se había
alimentado de leche de su madre, una Beagle a la que se había
administrado isobenzano y cuya leche contenía 0,7 mg/litro. En un
estudio de reproducción en ratas se observaron efectos similares en
las crías. Las vacas excretan isobenzano con la leche.
Las larvas de mosquito y los hongos del suelo metabolizan el
isobenzano del mismo modo que los vertebrados, dando lactona de
isobenzano como metabolito.
El isobenzano es muy persistente en el medio ambiente y se
bioacumula. Es sumamente tóxico para los peces, los camarones y las
aves. En los Países Bajos, país en el que se fabricaba el
isobenzano, los residuos encontrados en los huevos de golondrinas de
mar de las costas holandesas ascendieron a 0,45 mg/kg (promedio:
0,09 mg/kg), mientras que el promedio de residuos encontrados en
mejillones y peces fue de 0,05 mg/kg en 1965. Se observó una
disminución del número de lombrices en terrenos tratados con
isobenzano a razón de 2 kg/ha. Se redujo la nitrificación, con el
consiguiente aumento del nitrógeno inorgánico, en los suelos
tratados con isobenzano sobre el terreno a razón de 1 kg/ha, si bien
en estudios de laboratorio no se demostró efecto alguno en la
nitrificación con dosis equivalentes a 250 g/ha.
La toxicidad aguda del isobenzano para los mamíferos es elevada
por las vías oral y percutánea. La forma de acción de su toxicidad
es la sobreestimulación del sistema nervioso central, que da lugar a
convulsiones. La toxicidad aguda de las preparaciones de isobenzano
refleja el porcentaje de ingrediente activo presente.
Aunque el isobenzano no irrita la piel, algunos productos
preparados a partir de él pueden causar irritación.
En estudios limitados a corto y largo plazo de administración
oral realizados en ratones, ratas y perros se ha demostrado que el
isobenzano puede provocar en el hígado cambios histológicos que
reponden al tipo clásico de intoxicación por compuestos
organo-clorados. En un estudio realizado a largo plazo en ratas, se
determinó un nivel efectos sin observados de 5 mg/kg de dieta
(equivalente a 0,25 mg/kg de peso corporal), y en un estudio en
perros de dos años de duración se determinó un nivel sin observación
de efectos adversos de 0,025 mg/kg de peso corporal.
En un estudio de reproducción en una generación de ratas se
obtuvo un nivel sin efectos adversos observados de 0,1 mg/kg de
dieta (equivalente a 0,05 mg/kg de peso corporal). Con un nivel de
1 mg/kg de dieta (equivalente a 0,05 mg/kg de peso corporal)
disminuyó la supervivencia de las crías.
No se han comunicado estudios sobre la teratogenicidad ni la
mutagenicidad del compuesto.
No se observó potencial carcinogénico en un estudio de
administración oral a ratas durante dos años ni en un estudio de
administración oral a ratones, pero esos estudios no eran apropiados
para evaluar la carcinogenicidad.
La base de datos toxicológicos correspondiente al isobenzano es
incompleta. En general, actualmente se considera que la calidad de
los datos es mediocre e insuficiente para evaluar los riesgos que
representa para la salud humana o el medio ambiente.
Los datos sobre personas expuestas se limitan a estudios
realizados en trabajadores de una fábrica de los Países Bajos
durante la fabricación y la elaboración de preparaciones de
isobenzano y otros insecticidas de ciclodieno clorado afines. No se
comunicaron casos de irritación cutánea. En varios casos de
intoxicación, se produjeron convulsiones pero los cambios del
trazado electroencefalográfico resultaron ser reversibles. El nivel
umbral de intoxicación (en el caso de las convulsiones) se estimó en
0,015 mg de isobenzano/litro de sangre, y se calculó que la semivida
biológica del isobenzano en la sangre humana es del orden de 2,8
años.
2. Conclusiones y recomendaciones
El isobenzano es sumamente tóxico y muy persistente. La
información de que se dispone sobre los riegos del isobenzano es
incompleta, pero, aún así, basta para indicar que el riesgo que
supone para los que lo manipulan y para el medio ambiente es tan
elevado que no debería permitirse la exposición humana o del medio
ambiente a esta sustancia, ya sea utilizada como insecticida o para
cualquier otro fin.
 Chemical names 1,3,4,5,6,7,8,8-octachloro-4,7-methylene-
3a,4,7,7a-tetrahydro-isobenzofuran
Common synonyms BAS-4402; CP 14957; ENT-25 545; OMS-206;
OMS-618; SD-4402; WL 1650; preparation 948
Common trade name Telodrin (technical product), Omtan
Purity (technical) not less than 95% (w/w)
RTECS registry
number PC1225000
CAS registry number
Chemical names 1,3,4,5,6,7,8,8-octachloro-4,7-methylene-
3a,4,7,7a-tetrahydro-isobenzofuran
Common synonyms BAS-4402; CP 14957; ENT-25 545; OMS-206;
OMS-618; SD-4402; WL 1650; preparation 948
Common trade name Telodrin (technical product), Omtan
Purity (technical) not less than 95% (w/w)
RTECS registry
number PC1225000
CAS registry number  6.3.3 Microorganisms
Isobenzan labelled with 14C in the hexachloropentane ring or
at the 1,3 position is metabolized by the fungi Aspergillus niger,
Aspergillus flavus, Penicillium chrysogenum, and Penicillium
notatum to isobenzan lactone, the same metabolite as found in
animal metabolism studies (Korte, 1963; Korte & Stiasni, 1964).
6.4 Elimination and excretion in expired air, faeces, and urine
6.4.1 Oral administration
In a study by Korte (1963), ten rabbits received in their diet
2 mg 14C-isobenzan, diluted with non-radioactive isobenzan, every
2 days to a total amount of 25-30 mg per rabbit. After 3 months,
about 50% of the total radioactivity administered had been excreted,
mainly in the urine. On the other hand, rats excreted most of the
radioactivity via bile into the gastrointestinal tract and excreted
these products with the faeces. No unchanged isobenzan was excreted,
only hydrophilic metabolites.
6.4.2 Parenteral administration
When Carworth Farm rats received daily intraperitoneal
injections of isobenzan (98% purity) of 0.25, 1, or 2 mg/kg body
weight 5 days per week for 2 weeks (section 7.2.3), they excreted
less than 1% of the daily dose as unchanged isobenzan in the faeces
(Brown et al., 1962).
In a study by Kaul et al. (1970), male and female rats
received a single intravenous injection of 15 µg 14C-isobenzan/kg
body weight. The male rats excreted 1% of the applied radioactivity
in the urine and 12% in the faeces within 48 h, while the females
excreted 5% and 11%, respectively.
After having been administered intravenously to rats with
cannulated bile ducts, 14C-isobenzan was excreted as hydrophilic
metabolites in the bile (Korte, 1963, 1967).
Male rabbits given an intravenous injection of 14C-isobenzan
(241 µg/kg body weight) excreted 12% in the urine and 1% in the
faeces within 96 h (Kaul et al., 1970).
6.5 Retention and turnover
The absorption of isobenzan into the body is determined by
measuring the insecticide in the blood. No human studies exist
relating the concentration of isobenzan in the blood at the state of
equilibrium to the total daily intake or relating the concentration
of isobenzan in the blood to that in the tissues. However, in
experimental animals, such relationships have been determined. Thus,
in human beings, measurement of the blood concentration of the
insecticide at the state of equilibrium is assumed to reflect total
absorption by all routes (skin, pulmonary, and oral exposure) as
well as the storage level in adipose tissue, thereby providing a
measure of the total body burden of isobenzan.
From human data, it is estimated that the biological half-life
of isobenzan in blood is of the order of 2.8 years (Jager, 1970)
(section 8.2).
7. EFFECTS ON LABORATORY MAMMALS AND IN VITRO TEST SYSTEMS
7.1 Single exposure
7.1.1 Oral administration
In rats, the first signs of toxicity were evident approximately
1 h after the administration of a lethal dose and consisted of
lethargy followed by muscular twitching, laboured breathing, and,
finally, general convulsions. The majority of deaths occurred within
the first 20 h after administration. No specific changes were
observed in the organs of fatally intoxicated animals. Signs of
intoxication were similar in mice, rats, guinea-pigs, cats, dogs,
and chickens. Surviving animals recovered completely (Brown et al.,
1962; Worden, 1969).
Oral LD50 values for the various animal species range from
1.6 to 10 mg/kg and are summarized in Table 5.
Table 5. Oral LD50 values for technical isobenzan
Species Vehicle LD50 Reference
(mg/kg body
weight)
Mouse arachis oil 10a,b Worden (1969)
Mouse corn oil 12.5 Spynu (1964)
Rat corn oil 4.8 Howard et al. (1957)
Rat corn oil 14.4 Spynu (1964)
Rat carboxymethyl cellulose 5.4 Stevenson (1964)
Rat dimethylsulfoxide 7.2 Stevenson (1964)
Rat arachis oil 10b Worden (1969)
Rat unknown 8.0 Layton et al. (1987)
Guinea-pig arachis oil 2.5a,b Worden (1969)
Golden hamster arachis oil 20b Worden (1969)
Dog unknown 1.6 Stevenson (1964)
a Average of males and females.
b Isobenzan 99.5%.
7.1.2 Dermal administration
Signs of intoxication are the same as those described for acute
oral intoxication but they develop more slowly (Brown et al.,
1962). The LD50 values are summarized in Table 6.
Table 6. Dermal LD50 values for technical isobenzan
Species Vehicle LD50 Reference
(mg/kg body
weight)
Rat arachis oil occluded, 4 Zavon (1961)
non-occluded, 10
Rat corn oil non-occluded, 8.5 Spynu (1964)
Rat crystalline form occluded, 60 Stevenson (1964)
(approximately)
7.1.3 Parenteral administration
The acute toxicity of isobenzan administered parenterally is
similar to that following oral administration (Table 7). The signs
of intoxication are similar to those observed after acute oral
toxicity but develop more rapidly, starting less than 1 h after the
injection (Brown et al., 1962; Worden, 1969).
Table 7. Parenteral LD50 values for technical isobenzan
Route Species Vehicle LD50 Reference
(mg/kg body
weight)
Intraperitoneal mouse xylene emulsion 8.2 Stevenson (1964)
Intraperitoneal mouse methoxytriglycol 6.0 Cole & Casida (1986)
Intraperitoneal rat dimethylsulfoxide 3.6 Stevenson (1964)
Subcutaneous rat, arachis oil 6-10 Worden (1969)
mouse
Intravenous rat unknown 1.7 Zavon (1961)
7.1.4 Formulated material
A 50% wettable powder formulation and a 15% emulsifiable
concentrate (in mixed petroleum xylenes) were tested for their acute
oral toxicity to rats, mice, rabbits, hamsters, cats, and dogs. The
LD50 values, when expressed as active material, were comparable
with those of isobenzan itself (Stevenson, 1964; Worden, 1969). The
dermal LD50 value for the 15% emulsifiable concentrate was
25-35 mg isobenzan/kg body weight for Hooded-Lister rats and 6 mg
isobenzan/kg body weight for New Zealand white rabbits, whereas in
the case of the 50% wettable powder the LD50 was 41 mg
isobenzan/kg body weight for rabbits (Stevenson, 1964). Rabbits
exposed dermally to the 15% emulsifiable concentrate formulation
behaved differently to rats similarly exposed. The rabbits generally
lost weight due to anorexia and a failure to drink, and they would
then convulse, even as much as 3 weeks after the exposure. However,
if feeding and drinking were resumed quickly, the rabbits did not
convulse (Brown, 1963, 1964).
7.1.5 Metabolites
The acute oral and intravenous toxicities of the metabolite
isobenzan lactone for mice were 30 times lower than those of
isobenzan. The oral and intravenous LD50 values were 306 mg/kg
body weight and > 100 mg/kg body weight, respectively (Korte,
1967).
7.2 Short-term exposure
The short-term, long-term, and reproduction studies that were
used for establishing a no-observed-effect level are summarized in
Table 8.
7.2.1 Oral administration
7.2.1.1 Mouse
In a study by Brown et al. (1962), groups of five male and
five female Carworth Farm No. 1 mice were fed diets containing
isobenzan (98%) at levels of 1, 2, 3, 4, or 5 mg/kg for up to 3
weeks. No mortality was observed at 1 mg/kg, but at the higher
concentrations the mortality was dose related, reaching 100% at
5 mg/kg diet.
7.2.1.2 Rat
Groups of eight male and eight female Carworth Farm rats were
intubated with isobenzan (98%) in dimethylsulfoxide (0.25, 1, or
2.5 mg/kg body weight per day) 5 days/week for 2 weeks. All rats
dosed with 2.5 mg/kg body weight died within 5 days. All the females
and two of the males dosed with 1 mg/kg body weight died after 5-7
doses, whereas only one female dosed with 0.25 mg/kg body weight
died after five doses. Weight loss preceded the death of the animals
at the highest dose, this being the result of reduced food intake.
No gross or microscopic changes were found in the rats dying from
intoxication or in the survivors sacrificed 14 days after the last
treatment (no details were reported concerning the parameters
studied) (Brown et al., 1962).
In a study by Howard et al. (1957), groups of 10 male and 10
female Sprague-Dawley rats were fed diets containing 0, 2.5, 12.5,
or 25 mg isobenzan/kg diet for 30 days. One female in the
highest-dose group died. Some of the rats fed with levels of 12.5 or
25 mg/kg were nervous and irritable, and the reduced body weight
gain in both these groups correlated with diminished food
consumption. At autopsy, there were some areas of necrosis of heart
cells and haemorrhages in the heart muscle of animals fed with
levels of 12.5 or 25 mg/kg diet.
Table 8. Short- and long-term oral exposure to isobenzan
Animal (strain) Exposure period NOAELa LOAELa References
(mg/kg diet) (mg/kgdiet)
Rat (Sprague-Dawley) 30 days 2.5 (0.12) 12.5 (0.62): irritability, Howard et al. (1957)
decreased body weight gain,
histopathology changes in heart
Dog (Beagle) 2 years (0.08) (0.125): convulsion, mortality Brown & Richardson (1964)
Dog (Scottish terrier) 2 years (0.025) (0.1): decreased body weight gain, Worden (1969)
increased liver weight
Rat (Hooded-Lister) 2 years 5 (0.25) 17.5 (0.87): convulsions Worden (1969)
Reproduction study
Rat (Carworth Farm) one generation 0.1 (0.005) 1 (0.05): decreased survival of pups Chambers (1962a,b)
(2 litters) (pups) 5 (0.25): convulsions
1.0 (0.05)
(parents)
a Figures in parentheses are values for exposure concentration in mg/kg body weight.
In a study by Worden (1969), groups of five male and five
female Hooded-Lister rats were fed diets containing isobenzan
(99.5%) (25, 35, 50, or 100 mg/kg diet) as a 15% emulsifiable
concentrate (in mixed petroleum xylenes). After 37 days on the test
diets, half of the surviving animals of each group were transferred
to a control diet, while the remainder continued on the test diets
for a further 42 days. During the first period, all rats fed with
100 mg/kg diet died except for one male. One male and two females
fed with 50 mg/kg diet died as did three females fed with 35 mg/kg
diet. There were no further deaths among the rats after dosing was
discontinued. During the second period of feeding with the test
diet, one female fed at the lowest level died. In one rat only, a
female fed with 50 mg/kg, the classical signs of organochlorine
intoxication were seen in the liver. These have been described by
Hodge et al. (1967) as "enlarged centrolobular hepatic cells with
cytoplasmic oxyphilia and somewhat increased and peripheral
migration of the basophilic granules".
7.2.1.3 Dog
Pairs of Beagle hounds (one male and one female) were given
daily oral doses of 0, 0.08, 0.125, or 0.2 mg isobenzan (98%)/kg
body weight in olive oil in gelatin capsules for 2 years. The dogs
given the two highest doses had several convulsive episodes during
the course of the study. When a dog exhibited a convulsion, dosing
was discontinued for 8 weeks. The male dog dosed with 0.125 mg/kg
died one week after a third convulsive episode, which followed a
sudden fall in body weight. No signs of intoxication were observed
in the dogs dosed with 0.08 mg/kg body weight. During this study,
blood isobenzan concentrations were measured regularly, in
particular at the time of convulsions. Concentrations in muscle
tissue were determined 3 days after the convulsion, and the results
are given in Table 9. The blood concentrations in the dogs receiving
0.08 mg/kg body weight for 2 years without convulsions indicated
that a plateau was reached after a relatively short exposure. The
mean concentration was approximately 0.02 mg isobenzan/litre and the
maximum concentration 0.04-0.05 mg/litre. The two highest doses
caused convulsions (Brown & Richardson, 1964).
Groups of three male and three female Scottish terriers (2.5-9
kg body weight) received daily gavage doses of isobenzan (0, 0.025,
or 0.1 mg/kg body weight) as 15% emulsifiable concentrate (in mixed
petroleum xylenes) for 2 years. No deaths and no signs of
intoxication resulted from the isobenzan administration. Body weight
gain, urinalysis, and haematological and clinical chemistry values
for the dogs fed with 0.025 mg/kg body weight were comparable with
those of the control animals. At a dose level of 0.1 mg/kg body
weight, a decrease in body weight gain, a slight increase in serum
Table 9. Concentration of isobenzan in the blood and muscle of dogs
Dose (mg/kg Sex Number of Blood concentration Muscle tissue
body weight) convulsions at time of convulsion (mg/kg wet
(mg/litre) weight)
0.08 male none 0.04 (maximum
concentration)
female none 0.05 (maximum
concentration)
0.125 male first two 0.06
third (death) 0.11 16
female 4 0.03-0.06 0.18
0.2 male 9 0.02-0.11 0.36
female 6 0.07-0.08 0.68
alkaline phosphatase values during the second year, and an increase
in liver weight were noted. No evidence of histopathological lesions
attributable to isobenzan was found at either treatment level. The
no-observed-effect level resulting from this study was 0.025 mg/kg
body weight (Worden, 1969). The distribution of isobenzan in the
tissues examined is summarized in section 6.2.2.
7.2.2 Dermal administration
When groups of five female albino rabbits were given daily
applications of isobenzan in corn oil (0, 5, 10, 20, 30, or 40 mg
per rabbit) to the shaven skin for 3 weeks, mortality was high at
all dose levels, reaching 100% after 2 weeks for rabbits given doses
of 30 or 40 mg. Histopathological examination revealed necrosis of
the heart muscle, non-dose-related lesions of the liver, and
degenerative changes in cells of the central nervous system in a few
animals (Howard et al., 1957).
7.2.3 Intraperitoneal administration
Groups of five male and five female Carworth Farm rats were
injected intraperitoneally with isobenzan (98%) in
dimethyl-sulfoxide (0.25, 1, or 2 mg/kg body weight per day) 5
days/week for 2 weeks. All rats given the lowest dose survived, but
two rats of each sex given 1 mg/kg and five males and four females
given 2 mg/kg died after 5-10 doses. No gross or microscopic lesions
were found in fatally intoxicated rats or in the survivors killed
7-14 days after the last treatment (no details concerning
parameters studied were reported) (Brown et al., 1962).
7.3 Long-term exposure
7.3.1 Rat
Groups of 25 male and 25 female Hooded-Lister rats were fed, at
dietary concentrations of 5, 17.5, or 30 mg/kg (equivalent to 0.25,
0.875, or 1.5 mg/kg body weight), isobenzan as a 15% emulsifiable
concentrate (in mixed petroleum xylenes) for 2 years. Three control
groups, each consisting of 25 male and 25 female rats, were used.
Additional groups of rats, fed at the same dose levels and
accompanied by separate control groups, were used for liver and
kidney function tests after 20, 66, and 104 weeks. Five females fed
at the highest dose level died during the first 3 weeks of the
study. Signs of intoxication (such as ruffled coat, lethargy,
muscular twitches, and mild to violent convulsions) were observed in
animals given 17.5 or 30 mg/kg diet, mainly during the first few
weeks. There were no adverse effects on body weight gain, food
conversion ratios, haematological parameters, serum alkaline
phosphatase, serum glutamic-pyruvic transaminase, total serum
protein, or albumin/globulin ratios. Absolute liver weight was
increased in the animals given the highest dose level. Gross and
microscopic examinations did not reveal any compound-related
changes, with the possible exception of thyroid hyperplasia recorded
in four males and six females at a level of 30 mg/kg diet. The liver
function test (bromsulfophthalein) and kidney function test (phenol
red) showed no deviations from control values. No significant
increase in the number and type of tumours was found. The
no-observed-effect level for toxicological effects was 5 mg
isobenzan/kg diet (equivalent to 0.25 mg/kg body weight) for 2 years
(Worden, 1969).
7.4 Skin irritation
In a study by Worden (1969), four male guinea-pigs received a
single application (2 mg/kg body weight) of isobenzan (99.5%) as a
0.2% w/v solution in arachis oil to the shaved skin. The material
was not removed from the skin. The animals were kept under
observation for 21 days, but no irritation was observed.
Two male and two female rabbits were given 12 successive daily
applications (0.5 mg/kg body weight) of isobenzan (99.5%) and six
female rats received 30 successive daily applications (0.3 mg/kg
body weight) of isobenzan as a 0.2% w/v solution in arachis oil to
the shaved skin. The material was left on the skin and the
observation period was 21 days after the last application. No
irritation was observed (Worden, 1969).
7.5 Reproductive toxicity, embryotoxicity, and teratogenicity
No studies on the potential teratogenicity of isobenzan have
been reported.
7.5.1 Mouse
In a range-finding test, groups of 25 male and 25 female BALB/C
mice were fed diets containing 0, 1, 2.5, 5, or 10 mg isobenzan
(94%)/kg. The mice in the 5- and 10-mg/kg dose groups all died
within 64 and 24 days, respectively. Only 20% of those in the
2.5-mg/kg group survived for 120 days. The 1 mg/kg group survived
and reproduced normally (no further data were reported). In the main
study, groups of 108 mice of each sex of Swiss strain BALB/C were
fed control diet and 106 mice of each sex were fed 1 mg isobenzan/kg
diet for 30 days, after which they were randomly paired and
continued on the same diet for 90 days. The number of litters
produced, litter size, sex ratio, and mortality were recorded in
this one-generation reproduction study. There were no statistically
significant differences between the control and isobenzan-treated
animals for any of the parameters measured (Ware & Good, 1967).
7.5.2 Rat
A one-generation, 2-litter reproduction study was carried out
with groups of 20 male and 20 female weanling Carworth Farm rats
that were fed diets containing isobenzan at levels of 0, 0.1, 1, 5,
or 10 mg/kg for 100 days and then mated. Convulsions were seen
during the mating period and pregnancy in females fed 10 mg/kg, and
during the lactation period in one female fed 5 mg/kg. Pups of the
second mating from both these treatment groups were also seen to
convulse. Mean litter size and survival of pups were markedly
reduced in the 10-mg/kg group and, to a lesser extent, in the
5-mg/kg group. No clinical signs were observed in the 1-mg/kg group.
At this dose level, the mean litter size was comparable with
controls, but survival of the pups at 21 days was decreased. No
effects attributable to isobenzan were found in the 0.1-mg/kg group
(Chambers, 1962a,b).
7.5.3 Dog
During a 2-year study (section 7.2.1.3), three litters were
born to a female Beagle hound dosed daily with 0.08 mg isobenzan/kg
body weight five days per week. A male Beagle dog, dosed at the same
level, was sire for several of the litters. The first litter
consisted of one male and one female, both normal, and one
still-born male. Fifteen days after birth, the female pup began to
convulse, having only fed on the mother's milk (which contained
0.7 mg isobenzan/litre) and at 17 days the pup was sacrificed.
Concentrations of isobenzan in tissues are given in section 6.2.2.
Two further litters of pups were born to the original dam (two male
pups in the first litter and two pups of each sex in the second).
These animals all appeared normal and healthy and showed no ill
effects from the ingestion of maternal milk. One litter of pups, one
male and one female, was born to a female Beagle dog fed 0.125 mg/kg
body weight per day for two years. These pups did not exhibit any
signs of intoxication and were killed when 26 days old. Autopsy did
not reveal any structural changes. It did not appear that isobenzan
interfered with either the male or female reproduction function
(Brown & Richardson, 1964).
7.6 Mutagenicity and related end-points
No information on mutagenicity is available.
7.7 Carcinogenicity
No adequate carcinogenicity studies have been reported.
7.7.1 Mouse
Groups of 18 male and 18 female mice of two hybrid strains (the
F1 hybrids C57Bl/6 x C3H/Anf and C57Bl/6 x AKR) were given the
maximum tolerated dose of isobenzan in 0.5% gelatin (0.215 mg/kg
body weight) daily from 7 to 28 days of age by stomach tube.
Thereafter the isobenzan was mixed in the diet to a concentration of
0.646 mg/kg, and the mice were killed at 18 months of age. No
significant increase in tumour incidence was found (Innes et al.,
1969).
7.7.2 Rat
In a long-term feeding study on rats (section 7.4.1), no
evidence of carcinogenicity was found as a result of feeding diets
containing up to 30 mg isobenzan/kg for 2 years (Worden, 1969).
7.8 Special studies
7.8.1 Biochemical studies
Mehrotra et al. (1982) studied the comparative effects of
cyclodiene compounds on different ATPase activities in beef and rat
brain synaptosomal fractions in vitro. Isobenzan significantly
inhibited Na+-K+-ATPase in rat brain synaptosomes. A
dose-related response was observed at up to 80 µmol/litre, but no
increase in inhibition was observed with a further increase in the
concentration of the compound. Oligomycin-sensitive Mg2+-ATPase in
rat brain synaptosomes was significantly inhibited by isobenzan, a
maximum of 64% inhibition occurring at 120 µmol/litre. In addition,
the oligomycin-insensitive Mg2+-ATPase in rat brain synaptosomes
was inhibited, and the inhibition was concentration dependent.
Isobenzan did not have any effect on K+-stimulated
p-nitrophenylphosphatase, an enzyme which is known to represent
the dephosphorylation step in the overall reaction of the
Na+-K+-ATPase. Oligomycin-sensitive Mg2+-ATPase in beef heart
mitochondria was significantly inhibited.
Isobenzan did not affect the activity of adenylate cyclase or
phosphodiesterase in Sprague-Dawley rat brain synaptosomes in vitro
at concentrations of up to 200 µmol/litre (Kodavanti et al.,
1988).
Several studies into the effect of isobenzan on the transfer of
ammonia in brain have indicated that isobenzan acts by increasing
brain ammonia levels before and during convulsions. Glutamic acid,
glutamine, and alpha-ketoglutaric acid, which are utilized in an
ammonia-binding mechanism, become overwhelmed, resulting in free
ammonia accumulating in the cerebral tissues (Hathway & Mallinson,
1964; Hathway, 1965; Hathway et al., 1965).
7.8.2 Neurotoxicity
In vitro studies using fresh rat brain synaptic membranes
showed isobenzan to be a potent inhibitor of the binding of the
convulsant tert-butylbicyclophosphorothionate (TBPS) to
brain-specific sites, thereby indicating an action at the
gamma-aminobutyric acid (GABA)-regulated chloride channel. Metabolic
activation by rat liver microsomes did not enhance the potency for
inhibition. This inhibition indicates that isobenzan binds to the
same site as TBPS, suggesting that isobenzan acts in a manner
similar to non-competitive GABA-A antagonists and providing a basis
for its convulsant action in mammals (Lawrence & Casida, 1984).
Bloomquist et al. (1986) produced a concentration-dependent
inhibition of 36Cl uptake into mouse brain vesicles by adding
isobenzan to mouse brain homogenate. The inhibitory activity was
confined to that portion of 36Cl uptake that was GABA dependent.
The insecticide concentration producing 50% inhibition (I50) of
36Cl uptake was 2.0 (0.83 to 5.1) µmol/litre, and the inhibitory
potency (I50) value for 35S-TBPS binding in rat brain
synaptosomes was 0.30 µmol/litre (Lawrence & Casida, 1984).
Cole & Casida (1986) confirmed in a study with male
Swiss-Webster mice administered isobenzan intraperitoneally that a
correlation also exist in vivo between binding to mouse brain GABA
receptors and convulsive activity. The inhibitory potency (IC50)
for in vitro TBPS binding to mouse brain synaptosomes is
0.03 µmol/litre. It was shown that the inhibitory potencies of
cyclodienes, including isobenzan, parallel their acute oral
toxicities. Isobenzan was slightly more potent than endrin and
produced virtually complete inhibition of GABA-dependent chloride
uptake at 30 µmol/litre. There was a significant linear correlation
between the 36Cl flux and 35S-TBPS-binding assays.
7.8.3 Pharmacological studies
Pharmacological studies on the function of organ systems in
various animal species (such as rats, guinea-pigs, rabbits, cats,
and frogs) after the administration of isobenzan by different routes
showed that the only significant effect was a disturbance of the
central nervous system associated with convulsions. This effect was
due to stimulation of the higher brain centres at the level of the
medulla and above. Changes in respiratory rate, heart rate, and
salivary secretion were probably mediated by the central nervous
system as a secondary effect of central nervous stimulation.
Barbiturates were found to control these convulsions. The
stimulation of brain activity was reflected in the occurrence of
electroencephalographic changes in the pre-convulsive stage and
during the convulsive episodes. This corresponds to the situation in
cases of human intoxication by occupational exposure to cyclodiene
insecticides as described by Hoogendam et al. (1962, 1965) and
Chambers (1962c).
Ibrahim (1964) showed that isobenzan injected intraperitoneally
at a toxic dose (7 mg/kg body weight) into male Wistar rats produced
a higher tension of contraction in the gastrocnemius muscle at lower
frequencies of stimulation than in controls. The maximum tetanic
tension was also attained at a lower frequency. An increase in the
duration of the "active state" of the muscle was considered to be
the most like explanation.
8. EFFECTS ON HUMANS
8.1 General population exposure
No poisoning incidents or untoward effects of long-term
exposure of the general population have been reported.
8.2 Occupational exposure
Isobenzan was initially manufactured and handled in the
Netherlands between 1958 and 1965. Aldrin, dieldrin, and endrin were
also produced in the same manufacturing plant and, consequently, in
many cases the exposure was mixed. Routine medical examination of
233 workers, who were exposed for more than 4 years and who followed
normal procedures during that period, did not reveal any
abnormalities in EEG, clinical chemistry or haematological
parameters, or liver microsomal enzyme induction (Jager, 1970;
Versteeg & Jager, 1973). The mean isobenzan concentration in the
blood of 20 operators after cessation of exposure decreased from
22 µg/litre in 1965 to 7 µg/litre in 1969. The biological half-life
of isobenzan in the blood (at the state of equilibrium of isobenzan
in body tissues) was estimated to be of the order of 2.8 years
(Jager, 1970).
In the 7 years of isobenzan production, 15 cases of clinical
intoxication, including eight cases with convulsions, were reported.
The mean concentration of isobenzan in the blood of nine workers at
the time of intoxication was 23 µg/litre, the range being
17-30 µg/litre. Although these workers recovered fully, it took
longer than with the related cyclodiene insecticides. In three
cases, certain typical complaints, such as headache, dizziness,
drowsiness, and irritability, persisted for 6 months, and the return
to normal of the modified EEG pattern sometimes took more than a
year. In one case of acute over-exposure, without signs of
intoxication, the blood isobenzan concentration decreased from
8 µg/litre to less than 2 µg/litre within 3 days (Jager, 1970).
The data from plant workers indicated a threshold level of
isobenzan in blood below which no signs or symptoms of intoxication
occur. This level was found to be 15 µg/litre (Jager, 1970).
Ribbens (1985) carried out a mortality study on the
above-mentioned industrial workers exposed to aldrin, dieldrin,
endrin, and isobenzan. Vital status and cause of death were assessed
for 232 of the total population of more than 1000 workers. This
group was selected for follow-up on account of the high degree of
exposure in the initial years of manufacturing and formulation and
the long exposure (mean 11 years) and observation (mean 24 years)
periods. Total observed mortality was 25 as opposed to 38 expected
on the basis of death statistics for the male Dutch population. Of
the nine cancer deaths, three were caused by lung cancer, while the
remaining six were each of a different nature. The author concluded
that although exposures in this group were high and exposure as well
as observation periods long, this study did not reveal any
indication of a specific carcinogenic activity of these pesticides.
9. EFFECTS ON OTHER ORGANISMS IN THE LABORATORY AND FIELD
9.1 Microorganisms
In laboratory studies, sandy loam soil was treated with 250 or
2500 mg isobenzan/kg (concentrations that exceeded the recommended
rates). At both concentrations, the carbon dioxide production was
inhibited to almost the same extent (21 and 24%, respectively)
during a 30-day test period. The addition of glucose to the soil
reduced the inhibitory effect to 5 and 4%, respectively. Isobenzan
at 250 mg/kg did not influence nitrification in the soil up to 18
days after treatment (Bartha et al., 1967).
Screening studies on microorganisms in pure culture were
carried out on nutrient agar plates with isobenzan either
incorporated in a uniformly emulsified form (1000 mg/kg) or as a
thin surface film (1 mg/cm2). The growth of the gram-positive
Bacillus megaterium was inhibited, but not that of various
gram-negative organisms such as several Pseudomonas strains,
Escherichia coli, Klebsiella aerogenes W5, or Achromobacter
butyri (Trudgill & Widdus, 1970).
9.2 Aquatic organisms
Groups of five adult Harlequin fish (Rasbora heteromorpha)
were exposed for 2 h at a temperature of 20 °C to water (pH 7.2)
containing isobenzan (99%), dissolved in DMSO, at a concentration of
0.01, 0.1, or 1 mg/litre. The fish were then transferred to clean
water and observed for an additional 48 h. The treatment with
1 mg/litre caused disorientation and the fish became excited by
external stimuli, lost the ability to swim, and, finally, all died
within 1 h. In some cases, they appeared to convulse. Isobenzan at
0.1 mg/litre caused similar symptoms; within 2 h of exposure, all
five fish died. No fish died at 0.01 mg/litre, but slight changes in
swimming behaviour were observed (Brown et al., 1962). When
guppies (Poecilia reticulata) were tested in the same way, the
symptoms of intoxication and susceptibility were similar to those in
Harlequin fish, but the guppies were slower to react (Brown et al.,
1962).
Data on the acute toxicity of isobenzan for aquatic organisms
in flow-through tests are given in Table 10.
Table 10. Acute toxicity of isobenzan (technical grade, 94%) for aquatic organisms
Organism Developmental Temperature Parameter Concentration Reference
stage (°C) (µg/litre)
Brown shrimp juvenile 17 48-h EC50 0.034a US EPA
(Penaeus (1987)
aztecus)
Eastern oyster juvenile 18 96-h EC50 32b US EPA
(Crassostrea (1987)
virginica)
Sheepshead minnow juvenile 17 48-h LC50 2.0a US EPA
(Cyprinodon (1987)
variegatus)
Spot juvenile 13 48-h LC50 0.32c US EPA
(Leiostomus (1987)
xanthurus)
a Salinity 30 ng/litre.
b Salinity 33 ng/litre.
c Salinity 22 ng/litre.
9.3 Terrestrial organisms
9.3.1 Soil invertebrates
In laboratory studies, plain-field sand was treated once with
0.05 mg isobenzan/kg and stored at 13 or 24 °C. The springtail
(Folsomia candida) was used for bioassays lasting up to 16 weeks.
The biological activity of isobenzan persisted slightly longer at
the higher temperature, killing 100% of the insects after 16 weeks
(Thompson, 1973).
9.3.2 Birds
9.3.2.1 Acute toxicity
Isobenzan (> 99%) is highly toxic to birds when administered
as a single oral dose (Table 11). LD50 values range from
1-10 mg/kg.
9.3.2.2 Short-term toxicity
When groups of five male and five female Japanese quail
(Coturnix coturnix japonica) were fed diets containing 2 or 10 mg
isobenzan/kg, the mean survival time in the high-dose group was 6.9
days (range, 2-20 days). The residues in the liver and brain
averaged 3.4 mg/kg and 1.4 mg/kg, respectively. In the low-dose
group, the mean survival time was 45.9 days (range, 19-65 days) and
the average residues in liver and brain were 6 mg/kg and 1.6 mg/kg,
respectively. The concentration of isobenzan in the liver of birds
fed 2 mg/kg was significantly higher than that in the high-dose
group, but the concentration in the brain showed no difference
(Koeman, 1971).
Table 11. Oral LD50 values of isobenzan for birds
Species LD50 (mg/kg Reference
body weight)
Mallard duck (female) 4.15 Hudson et al. (1984)
(Anas platyrhynchos) (2.47-6.97)
Coturnix quail (female) 4.2 Schafer & Brunton (1979)
(Coturnix coturnix)
Grackle 1.3 Schafer & Brunton (1979)
(Quiscalus quiscula)
Pigeon 10 Schafer & Brunton (1979)
(Columba livia)
Red-winged blackbird (male) 3.2 Schafer & Brunton (1979)
(Agelaius phoeniceus)
Sparrow 1 Schafer & Brunton (1979)
(Passer domesticus)
Starling 2.4 Schafer & Brunton (1979)
(Sturnus vulgaris)
When groups of 20 Rhode Island Red laying hens were fed
isobenzan (15% emulsifiable concentrate) at levels of 0.1, 0.25,
0.75, or 1 mg/kg diet for 14 weeks, no effects were seen on food
consumption, egg production, egg weight, or egg fertility (Verma
et al., 1967).
9.4 Population and ecosystem effects
9.4.1 Soil microorganisms
In a sugar cane field in India, Srivastava (1966) studied the
effect of isobenzan (1 kg/ha) on nitrification of ammonium sulfate
in the soil. The insecticide-treated soil contained higher amounts
of total inorganic nitrogen and ammonium nitrogen (80% increase) up
to 60 days after treatment than did untreated soil samples,
indicating impaired nitrification.
9.4.2 Soil invertebrates
In a study by Kelsey & Arlidge (1968), five sandy loam plots of
pasture in New Zealand were treated with Telodrin (15% emulsifiable
concentrate) at a rate of 2 kg isobenzan/ha in June 1962. The
populations of all recorded groups (grass grub, porina, Collembola,
Diptera, Hemiptera, Coleoptera, mites, and earthworms), except
nematodes, were drastically reduced. There was no recovery in the
populations of any of the affected groups during the period up to
October 1965. In silt loam plots treated with isobenzan granules
(1 kg/ha) in April 1964, the populations of Coleoptera, Collembola,
Diptera, mites, and earthworms were all reduced, but to a lesser
extent than in the test using 2 kg/ha. However, grass grub and
Hemiptera were not significantly affected, and nematode numbers were
elevated 2 years after the treatment. Studies on root development
showed that 90% of roots with root hairs were located within a mat
of plant debris above the soil, whereas in controls 99% were located
in the soil. Plant growth was retarded. The moisture content of the
treated plots was significantly less than that of control plots,
indicating that the capacity of soil to absorb and retain water had
been reduced.
When loose sandy soil in New Zealand was treated with 2.25 kg
isobenzan/ha as 5% granules, the population reduction, 1-18 weeks
after treatment, was 90-100% for larval Coleoptera and Lepidoptera
and 75% for Diptera and earthworms. The number of surface
arthropods, 5-15 days after treatment, was reduced by 45%. Six
months after treatment, little effect was found on nematodes
(13% reduction), bacteria (18% reduction), and fungi (7% increase)
(Moeed, 1975).
REFERENCES
ANON (1974) Aldrin, dieldrin, and endrin. Determination of residues
of organochlorine insecticides in crops, soils, and animal products,
Sittingbourne, Shell Research (Residue Analytical Method WAMS 60-1).
BARTHA, R., LANZILOTTA, R.P., & PRAMER, D. (1967) Stability and
effects of some pesticides in soil. Appl. Microbiol., 15(1): 67-75.
BAUER, U. (1972) [Behaviour of a series of plant-protecting agents
during the treatment of groundwater by slow sand filtration.]
Schriftenr. Ver. Wasser-Boden-Lufthyg. (Berlin-Dahlem), 37: 91-102
(in German).
BIEHL, M.L. & BUCK, H.B. (1987) Chemical contaminants: their
metabolism and their residues. J. Food Prot., 50(12): 1058-1073.
BISHOP, J.L. & HUBER, J.T. (1964) Secretion of Telodrin in the milk
of cows fed varying levels of Telodrin. J. dairy Sci., 47: 552-554.
BLOOMQUIST, J.R., ADAMS, P.M., & SÖDERLAND, D.M. (1986) Inhibition
of gamma-aminobutyric acid stimulated chloride flux in mouse brain
vesicles by polychlorocycloalkane and pyrethroid insecticides.
Neurotoxicology, 7(3): 11-20.
BOWMAN, M.C., SCHECHTER, M.S., & CARTER, R.L. (1965) Behavior of
chlorinated insecticides in a broad spectrum of soil types. J.
agric. food Chem., 13: 360-365.
BROWN, V.K.H. (1963) Agricultural chemicals: the relative
percutaneous toxicities of Nitrophoska fertiliser granules
impregnated with aldrin and Telodrin, Sittingbourne, Shell Research
(Technical Memorandum TOX 10/63).
BROWN, V.K.H. (1964) Some effects of percutaneously absorbed
Telodrin in rabbits, Sittingbourne, Shell Research (Report No. PPR
TL/2/64).
BROWN, V.K.H. & RICHARDSON, A. (1964) Chronic oral exposure of
beagle hounds to Telodrin and dieldrin: report on the first two
years, Sittingbourne, Shell Research (Report No. PPR TL/1/64).
BROWN, V.K.H., CHAMBERS, P.L., HUNTER, C.G., & STEVENSON, D.E.
(1962) The toxicity of Telodrin for vertebrates, Sittingbourne,
Shell Research (Report No. R(T)-2-62).
BUICK, A.R. (1964) Telodrin residues on tobacco from Australia,
Sittingbourne, Shell International Chemical Company, Ltd (Technical
Memorandum 132/64)
BUICK, A.R. (1965) Telodrin residues in potatoes from India,
Sittingbourne, Shell International Chemical Company, Ltd (Technical
Memorandum 25/65).
BUICK, A.R. & COLE, E.R. (1965) Telodrin residues in sweet potatoes
from South Africa, Sittingbourne, Shell International Chemical
Company, Ltd (Technical Memorandum 25/65).
BULL, M.S. & MARLOW, R.G. (1967) Insecticide residues in diet
samples from Nicaragua, Sittingbourne, Shell International Chemical
Company, Ltd (Technical Service Note 85/67).
BULL, M.S. & RAMSDEN, D.P. (1967) Residues of organochlorine
insecticides in samples from Venezuela, Sittingbourne, Shell
International Chemical Company, Ltd (Technical Service Note 86/67).
CHAMBERS, P.L. (1962a) Agricultural chemicals: the effect of
Telodrin on reproduction in the rat when fed at various levels in
the diet. Report No. 3, Sittingbourne, Shell International Chemical
Company, Ltd (Technical Memorandum TOX 24/62).
CHAMBERS, P.L. (1962b) Agricultural chemicals: the effect of
Telodrin on reproduction in the rat when fed at various levels in
the diet. Report No. 4, Sittingbourne, Shell International Chemical
Company, Ltd (Technical Memorandum TOX 33/62).
CHAMBERS, P.L. (1962c) The physiological and pharmacological effects
of Telodrin, Sittingbourne, Shell Research (Report No. M(T)-4-62).
COLE, L.M. & CASIDA, J.E. (1986) Polychlorocycloalkane
insecticide-induced convulsions in mice in relation to disruption of
the GABA-regulated chloride ionophore. Life Sci., 39: 1855-1862.
CRABTREE, A.N. (1968) Concentration of chlorinated insecticides in
the whole blood of the CAPSA plant in Venezuela, Sittingbourne,
Shell Research (Report No. TLGR.0032.68).
DAVIES, J.M. (1966a) Chlorinated insecticide content of the blood of
formulators in the CAPSA plant in Venezuela, Sittingbourne, Shell
Research (Report No. IRR TL/14/66).
DAVIES, J.M. (1966b) Concentration of chlorinated insecticides in
the whole blood of formulators of the CAPSA plant in Venezuela,
Sittingbourne, Shell Research (Report No. IRR TL/37/66).
EDWARDS, C.A. (1965) Effects of pesticide residues on soil
invertebrates and plants. In: Proceedings of the 5th Symposium of
the British Ecological Society, Ecology and Industrial Society.
EICHELBERGER, J.W. & LICHTENBERG, J.J. (1971) Persistence of
pesticides in river water. Environ. Sci. Technol., 5(6): 541-544.
ELGAR, K.E. (1965) Insecticide residues in samples of cottonseed
from Mexico, Sittingbourne, Shell International Chemical Company,
Ltd (Technical Memorandum 99/65).
ELGAR, K.E. (1966) Analysis of crops and soils for residues of the
soil insecticides aldrin and Telodrin. J. Sci. Food Agric., 17:
541-545.
ELGAR, K.E. & HOLLAND, A.A. (1967) Residues of organochlorine
insecticides in potatoes from Mexico, Sittingbourne, Shell
International Chemical Company, Ltd (Technical Service Note 82/67).
HARDEE, D.D., GUTENMANN, W.H., KEENAN, G.I., GYRISCO, G.G., LISK,
D.J., FOX, F.H., TRIMBERGER, G.W., & HOLLAND, R.F. (1964) Residues
of heptachlor epoxide and Telodrin in milk from cows fed at parts
per billion insecticide levels. J. econ. Entomol., 56(3): 404-407.
HATHWAY, D.E. (1965) The biochemistry of dieldrin and Telodrin.
Arch. environ. Health, 11: 380-388.
HATHWAY, D.E. & MALLINSON, A. (1964) Chemical studies in relation to
convulsive conditions: effect of Telodrin on the liberation and
utilization of ammonia in rat brain. Biochem. J., 90: 51-60.
HATHWAY, D.E., MALLINSON, A., & AKINTONWA, D.A.A. (1965) Effects of
dieldrin, picrotoxin, and Telodrin on the metabolism of ammonia in
brain. Biochem. J., 94: 676-686.
HODGE, H.C., BOYCE, A.M., DEICHMANN, W.B., & KRAYBILL, H.F. (1967)
Toxicology and no-effect levels of aldrin and dieldrin. Toxicol.
appl. Pharmacol., 10(3): 613-637.
HOOGENDAM, I., VERSTEEG, J.P.J., & VLIEGER, M., DE (1962)
Electroencephalograms in insecticide toxicity. Arch. environ.
Health, 4: 86-94.
HOOGENDAM, I., VERSTEEG, J.P.J., & VLIEGER, M., DE (1965) Nine years
toxicity control in insecticide plants. Arch. environ. Health, 10:
441-448.
HOWARD, J.A., BUXTON, J.A., ATER, F.B., OVERBECK, R.C., FOOTE, W.L.,
ROBINSON, R.F., & DAVIDSON, R.S. (1957) Final report on toxicology
of BAS-4402 to the Shell Development Corporation, Columbus, Ohio,
Battelle Memorial Institute.
HUDSON, R.H., TUCKER, R.K., & HAEGELE, M.A. (1984) Handbook of
toxicity of pesticides to wildlife. 2nd ed., Washington, DC, US
Department of the Interior, Fish and Wildlife Service (Resource
Publication No. 153).
HUGHES, D.G. (1965) Insecticide residues in cottonseed oil,
Sittingbourne, Shell International Chemical Company, Ltd (Technical
Memorandum 23/65).
IBRAHIM, T.M. (1964) A toxicological study of the action of the
insecticide dieldrin and related substances on the contraction of
striated muscle in the rat, University of Utrecht (Thesis).
INNES, J.R.M., ULLAND, B.M., VALERIO, M.G., PETRUCELLI, L.,
FISHBEIN, L., HART, E.R., PALLOTTA, A.J., BATES, R.R., FALK, H.L.,
GART, J.J., KLEIN, M., MITCHELL, I., & PETERS, J. (1969) Bioassay of
pesticides and industrial chemicals for tumorigenicity in mice: a
preliminary note. J. Natl Cancer Inst., 42(6): 1101-1114.
JAGER, K.W. (1970) Aldrin, dieldrin, endrin, and Telodrin: an
epidemiological and toxicological study of long-term occupational
exposure, Amsterdam, London, New York, Elsevier Science Publishers.
JAPANESE ENVIRONMENTAL AGENCY (1975) Environmental survey report on
chemical substances in FY 1974, Tokyo, Environmental Health
Department, Planning and Coordination Bureau (Unpublished report).
KADOUM, A.M. (1968) Application of the rapid micro method of sample
clean-up for gas chromatographic analysis of common organic
pesticides in ground water, soil, plant and animal extracts. Bull.
environ. Contam. Toxicol., 3(2): 65-70.
KAUL, R., KLEIN, W., & KORTE, F. (1970) [Contributions to ecological
chemistry. XX. Distribution, elimination, and metabolism of Telodrin
and heptachlor in rats and male rabbits, end-product of the
metabolism of heptachlor in mammals.] Tetrahedron, 26: 331-337 (in
German).
KELSEY, J.M. & ARLIDGE, E.Z. (1968) Effects of isobenzan on soil
fauna and soil structure. N. Z. J. agric. Res., 11: 245-260.
KERDIJK, H.N. (1981) Groundwater pollution by heavy metals and
pesticides from a dredge spoil dump. Stud. environ. Sci., 17:
279-286.
KODAVANTI, P.R.S., MEHROTRA, R.D., CHETTY, S.C., & DESAIAH, D.
(1988) Effect of selected insecticides on rat brain synaptosomal
adenylate cyclase and phosphodiesterase. J. Toxicol. environ.
Health, 25: 207-215.
KOEMAN, J.H. (1971) [The occurrence and toxicological implications
of some chlorinated hydrocarbons in the Dutch coastal area in the
period from 1965 to 1970], University of Utrecht, pp. 30-31 (Thesis)
(in Dutch).
KOEMAN, J.H., OSKAMP, A.A.G., VEEN, J., BROUWER, E., ROOTH, J.,
ZWART, P., VAN DE BROEK, E., & VAN GENDEREN, H. (1967) Insecticides
as a factor in the mortality of the sandwich tern (Sterna
sandvicensis): a preliminary communication. Meded. Landbouwwet.
Hogesch. Gent., 32: 841-854.
KOEMAN, J.H., VEEN, J, BROUWER, E., HUISMAN-DE BROUWER, L., &
KOOLEN, J.L. (1968) Residues of chlorinated hydrocarbon insecticides
in the North Sea environment. Helgoländer wiss. Meerunters., 17:
375-380.
KORTE, F. (1963) Review of metabolism studies with 14C-labelled
insecticides from 1958 to March 1963. Unpublished paper presented at
a Toxicological Meeting, New York, 25-27 March.
KORTE, F. (1967) Metabolism of 14C-labelled insecticides in
microorganisms, insects, and mammals. Botyu-Kagaku, 32(2): 46-59.
KORTE, F. & STIASNI, M. (1964) [Metabolism of 14C-Telodrin in
microorganisms and mosquito larvae.] Ann. Chem., 673: 146-152 (in
German).
LAWRENCE, L.J. & CASIDA, J.E. (1984) Interactions of lindane,
toxaphene, and cyclodienes with brain-specific
t-butylbicyclophosphorothionate receptor. Life Sci., 35: 171-178.
LAYTON, D.W., MALLON, B.J., ROSENBLATT, D.H., & SMALL, M.J. (1987)
Deriving allowable daily intakes for systemic toxicants lacking
chronic toxicity data. Regul. Toxicol. Pharmacol., 7: 96-112.
MCCASKEY, T.A., STEMP, A.R., LISKA, B.J., & STADELMAN, W.J. (1968)
Residues in egg yolks and raw and cooked tissues from laying hens
administered selected chlorinated hydrocarbon insecticides. Poultry
Sci., 47: 564-569.
MARLOW, R.G., BULL, M.S., & WILLIAMS, S. (1968) Residues of
organochlorine insecticides in foodstuffs from Spain, Sittingbourne,
Shell International Chemical Company, Ltd (Technical Service Report
WKTR.0058.68).
MATHEWS, B.L. (1969) Residues of organochlorine insecticides in
foodstuffs from India, Sittingbourne, Shell Research (Report No.
WKGR.0102.69).
MATHEWS, B.L. & COLE, E.R. (1966) Residues of Telodrin,
methylparathion, and DDT on tobacco from Costa Rica, Sittingbourne,
Shell International Chemical Company, Ltd (Technical Service Note
123/66).
MEHROTRA, B.D., BANSAL, S.K., & DESAIAH, D. (1982) Comparative
effects of structurally-related cyclodiene pesticides on ATPases. J.
appl. Toxicol., 2(6): 278-283.
MOEED, A. (1975) Effects of isobenzan, fensulfothion, and diazinon
on invertebrates and microorganisms. N. Z. J. exp. Agric., 3:
181-185.
MOSS, J.A. & HATHWAY, D.E. (1964) Transport of organic compounds in
the mammal. Partition of dieldrin and Telodrin between the cellular
components and soluble proteins of blood. Biochem. J., 91: 384-393.
MURPHY, M.W. & STANDEN, M.E. (1964) The translocation of Telodrin
into potatoes, Sittingbourne, Shell International Chemical Company,
Ltd (Technical Memorandum 184/64).
RIBBENS, P.H. (1985) Mortality study of industrial workers exposed
to aldrin, dieldrin and endrin. Arch. occup. environ. Health, 56(2):
75-79.
RICHARDSON, A., ROBINSON, J., BUSH, B., & DAVIES, J.M. (1967)
Determination of dieldrin (HEOD) in blood. Arch. environ. Health,
14(5): 703-708.
ROBINSON, J. & RICHARDSON, A.R. (1963) The distribution, storage,
and elimination of Telodrin in rats fed this insecticide in their
diet, Sittingbourne, Shell Research (Report No. R(T)-1-63).
SCHAFER, E.W. & BRUNTON, R.B. (1979) Indicator bird species for
toxicity determinations: is the technique usable in test method
development? In: Beck, J.R., ed. Vertebrate pest control and
management materials, Philadelphia, American Society for Testing and
Materials, pp. 157-168 (ASTM STP 680).
SHELL (1963) An episode of pesticide contamination of pasture and
dairy products - Isobenzan in Victoria, Australia, in 1963.
Melbourne, Shell Chemical Australia (Unpublished report No.
90111501WP5).
SHELL (1964) Telodrin residues. A summary, London, Shell
International Chemical Company (Unpublished report).
SPYNU, E.I. (1964) On the toxicology of new organic chloride
insecticides obtained by diene synthesis on the basis of
hexachlorocyclopentadiene. Gig. Tr. prof. Zabol., 4: 30-35.
SRIVASTAVA, S.C. (1966) The effect of Telodrin on nitrification of
ammonia in soil and its implication on nitrogen nutrition of
sugarcane. Plant Soil, 25(3): 471-473.
STANDEN, M.E. & ELGAR, K.E. (1965) Telodrin residues in dairy
products from Venezuela, Sittingbourne, Shell International Chemical
Company, Ltd (Technical Memorandum 36/65).
STEMP, A.R. & LISKA, B.J. (1966) Effects of processing and storage
of dairy products on Telodrin and methoxychlor residues. J. diary
Sci., 49: 1006-1008.
STEVENSON, D.E. (1964) The toxicology of Telodrin. Meded. Fac.
Landbouwwet. Rijksuniv. Gent, 29: 1198-1207.
SUZUKI, K., NAGAYOSHI, H., & KASHIWA, T. (1974) The systematic
separation and identification of pesticides in the first division.
Agric. biol. Chem., 38(2): 279-285.
THOMPSON, A.R. (1973) Persistence of biological activity of seven
insecticides in soil assayed with Folsomia candida. J. econ.
Entomol., 66(4): 855-857.
TRUDGILL, P.W. & WIDDUS, R. (1970) Effects of chlorinated
insecticides on metabolic processes in bacteria. Biochem. J., 118:
48-49.
TUINSTRA, L.G.M.TH. & TRAAG, W.A. (1979) Automated glass capillary
gas chromatographic analysis of PCB and organochlorine pesticide
residues in agricultural products. J. high Resolut. Chromatogr.
Chromatogr. Commun., 2(2): 723-728.
US EPA (1987) Acute toxicity handbook of chemicals to estuarine
organisms. Springfield, Virginia, US Department of Commerce,
National Technical Information Service (EPA/600/8-87/017).
VAN WIJNEN, J.H. & STIJKEL, A. (1988) Health risk assessment of
residents living on harbour sludge. Int. Arch. occup. environ.
Health, 61: 77-87.
VERMA, M.P., BAHGA, H.S., & SONI, B.K. (1967) Effect of prolonged
administration of the insecticide Telodrin in poultry. Indian vet.
J., 44: 962-966.
VERSTEEG, J.P.J. & JAGER, K.W. (1973) Long-term occupational
exposure to the insecticides aldrin, dieldrin, endrin, and Telodrin.
Br. J. ind. Med., 30: 201-202.
WARE, G.W. & GOOD, E.E. (1967) Effects of insecticides on
reproduction in the laboratory mouse. II. Mirex, Telodrin, and DDT.
Toxicol. appl. Pharmacol., 10(1): 54-61.
WEGMAN, R.C.C. & GREVE, P.A. (1980) Halogenated hydrocarbons in
Dutch water samples over the years 1969-77. Environ. Sci. Res., 16:
405-415.
WEGMAN, R.C.C. & HOFSTEE, A.W.M. (1982) Determination of
organochlorines in river sediment by capillary gas chromatography.
Water Res., 16: 1265-1272.
WEGMAN, R.C.C., HOFSTEE, A.W.M., & GREVE, P.A. (1981) Uptake of
organochlorines by plants growing on river and basin sediment.
Meded. Fac. Landbouwwet. Rijksuniv. Gent, 46(1): 359-365.
WORDEN, A.N. (1969) Toxicity of Telodrin. Toxicol. appl. Pharmacol.,
14: 556-573.
ZAVON, M.R. (1961) Toxicology and pharmacology of Telodrin
insecticide (compound SD 4402), Cincinnati, Ohio, The Kettering
Laboratory (Unpublished report).
RESUME ET EVALUATION; CONCLUSIONS ET RECOMMANDATIONS
1. Résumé et évaluation
Autant qu'on sache, l'isobenzan, un insecticide organochloré
n'a été fabriqué que pendant la période 1958-1965. Plusieurs années
aprés, on puisait toujours sur les stocks existants. A l'heure
actuelle, les seules sources importantes d'exposition sont
vraisemblablement les sites de décharge initiaux de déchets
industriels ou les boues de dragage provenant de sédiments
contaminés.
Une fois épandu sur le sol, la majeure partie de l'isobenzan
disparaît rapidement. Après quoi, la fraction restante se décompose
beaucoup plus lentement. Elle persiste dans le sol de deux à sept
ans selon la nature de celui-ci. Au laboratoire, l'isobenzan se
décompose dans les eaux de surface en l'espace de quelques semaines
lorsqu'il est exposé à la lumière naturelle ou artificielle.
Le sol, les eaux souterraines et superficielles provenant des
polders constitués de sédiments contaminés par des organochlorés,
notamment des dérivés cyclodiéniques, contenaient encore plusieurs
années après, de faibles résidus d'isobenzan. En 1979-1980, on n'a
pas détecté d'isobenzan (limite de détection 0,01 mg/kg de poids
sec) dans les sédiments des cours d'eau des Pays-Bas. Après
traitement du sol, les résidus qui subsistent sur les récoltes sont
généralement faibles (inférieurs à 0,05 mg/kg de végétaux), mais on
peut en trouver des quantités plus fortes sur certaines racines
(jusqu'à 0,2 mg/kg dans les carottes). Des enquêtes de type "panier
de la ménagère" effectuées lorsqu'on utilisait de l'isobenzan en
agriculture, n'ont pas permis de déceler de résidus dans les denrées
contrôlées (moins de 0,01 mg/kg).
Chaque fois qu'on a laissé paître des bovins dans des pâturages
traités par de l'isobenzan, on a constaté que les produits laitiers
obtenus contenaient des résidus de cet insecticide. C'est ainsi que
dans deux échantillons de beurre, on a trouvé 0,07 et 0,15 mg
d'isobenzan par kg de produit, les concentrations dans le lait
entier allant de 0,005 à 0,07 mg/kg. Le lait en poudre n'en
contenait toutefois que 0,005 mg/kg. Lors du traitement industriel
des produits laitiers, plus de 50% du résidu disparaît selon le type
de traitement.
On ne dispose d'aucune donnée sur les quantités d'isobenzan
présentes dans le sang ou les tissus adipeux de la population
générale. Des travailleurs exposés à l'isobenzan lors de la
fabrication ou de la formulation de cet insecticide, présentaient
des taux sanguins (sang total) allant jusqu'à 0,041 mg/litre. Dans
les échantillons de sang total prélevés sur des personnes vivant à
proximité d'une unité de production, la concentration d'isobenzan
était inférieure à la limite de détection (0,001 mg/litre).
L'isobenzan est facilement résorbé par la paroi du tube
digestif et il passe dans le sang sans modification. Il se forme des
métabolites hydrophiles et notamment une lactone. L'isobenzan
s'accumule dans les tissus et les organes des rats et des chiens
selon l'ordre: graisses > foie = muscle > cerveau > sang. Les
concentrations tissulaires chez les rattes sont généralement plus
faibles que chez les rats, spécialement dans les graisses. La
demi-vie biologique dans le tissu adipeux était ainsi de 10,9 jours
chez les rats et de 16,6 jours chez les rattes. Chez un chiot
femelle dont le sang contenait 0,09 mg d'isobenzan par litre, on a
observé des convulsions 15 jours après la naissance. L'animal
n'avait été nourri qu'avec le lait de sa mère, une chienne Beagle à
qui l'on avait fait absorber de l'isobenzan et dont le lait en
contenait 0,7 mg/litre. Des effets analogues sur les petits ont été
observés lors d'une étude de reproduction sur le rat. Chez la vache,
l'isobenzan est excrété dans le lait.
Les larves de moustiques et les champignons terricoles
métabolisent l'isobenzan de la même manière que les vertébrés,
notamment sous forme de lactone. L'isobenzan est très persistant
dans l'environnement et s'accumule dans les organismes vivants. Il
est extrêmement toxique pour les poissons, les crevettes et les
oiseaux. Aux Pays-Bas, pays où l'on fabriquait l'isobenzan, on a
trouvé des résidus dans des oeufs de sternes vivant sur la côte
hollandaise qui atteignaient 0,45 mg/kg (moyenne, 0,09 mg/kg). Dans
les moules et le poisson, les résidus moyens étaient de 0,05 mg/kg
en 1965. Dans les parcelles traitées par de l'isobenzan à raison de
2 kg/ha, on a constaté une réduction du nombre de lombrics. Il y
avait réduction de la nitrification avec accroissement corrélatif de
l'azote minéral dans les sols traités par l'isobenzan à raison
d'1 kg/ha; en revanche, les études en laboratoire n'ont mis en
évidence aucun effet sur la nitrification à des doses correspondant
à 250 g/ha.
L'isobenzan présente une forte toxicité aiguë pour les
mammifères, que ce soit par la voie orale ou par la voie percutanée.
Le mode d'action de l'isobenzan consiste en une stimulation
excessive du système nerveaux central conduisant à des convulsions.
La toxicité aiguë des diverses formulations d'isobenzan correspond à
la proportion de matière active.
L'isobenzan n'est pas irritant pour la peau mais certaines de
ses formulations peuvent l'être.
Des études limitées à court et à long terme, au cours
desquelles on a administré par voie orale de l'isobenzan à des
souris, à des rats et à des chiens, ont montré que ce composé
pouvait induire des lésions histologiques au niveau du foie, du type
de celles qu'on observe classiquement avec les organochlorés. Une
étude de longue durée sur des rats a permis de déterminer que la
dose sans effet observable était de 5 mg/kg de nourriture (soit
l'équivalent de 0,25 mg/kg de poids corporel). Chez le chien, la
dose sans effet nocif observable, déterminée à la suite d'une étude
de deux ans, était de 0,025 mg/kg de poids corporel.
Une étude de reproduction portant sur une génération de rats a
montré que la dose sans effet nocif observable était de 0,1 mg/kg de
nourriture (soit l'équivalent de 0,05 mg/kg de poids corporel. A la
dose de 1 mg/kg de nourriture (soit l'équivalent de 0,05 mg/kg de
poids corporel), il y a eu réduction de la survie des ratons.
Aucune étude de tératogénicité ni de mutagénicité n'a été
rapportée.
Une étude de deux ans sur des rats (administration par voie
orale) et une étude sur des souris n'ont pas permis de mettre en
évidence un pouvoir cancérogène quelconque, mais ces études
n'étaient pas adaptées à une telle évaluation.
La base de données toxicologiques sur l'isobenzan est
incomplète. En général on estime que, d'après les critères actuels,
les données sont d'une qualité médiocre et sont en tous cas
insuffisantes pour permettre une évaluation du risque que ce composé
présente pour la santé humaine ou l'environnement.
Les données concernant l'exposition humaine se limitent à des
études effectuées sur des travailleurs d'une usine hollandaise qui
étaient employés à la fabrication et à la formulation d'isobenzan et
d'insecticides cyclodiéniques apparentés. Aucun cas d'irritation
cutanée n'a été signalé. Dans plusieurs cas d'intoxication, on a
observé des convulsions mais les anomalies du tracé
électro-encéphalographique étaient réversibles. Le seuil limite
d'intoxication (pour les convulsions), a été estimé à 0,015 mg
d'isobenzan par litre de sang et la demi-vie biologique de ce
composé dans le sang humain est, semble-t-il, de l'ordre de 2,8
années.
2. Conclusions et recommandations
L'isobenzan est extrêmement toxique et très persistant. Les
données dont on dispose sur le danger qu'il représente sont
incomplètes mais néanmoins suffisantes pour montrer que ce danger
est réel pour les personnes qui le manipulent ainsi que pour
l'environnement, de sorte qu'il faut éviter toute contamination
humaine ou environnementale qui résulterait de l'utilisation de ce
produit comme insecticide ou autre.
RESUMEN Y EVALUACION; CONCLUSIONES Y RECOMENDACIONES
1. Resumen y evaluación
Según los datos de que se dispone, el isobenzano, un
insecticida organoclorado, sólo se fabricó durante el periodo
1958-1965. Durante los años siguientes se utilizaron las existencias
almacenadas. En la actualidad, se cree que las únicas fuentes
importantes de exposición son los lugares donde originalmente se
evacuaron los desechos industriales o los materiales dragados de
sedimentos contaminados.
Cuando se aplica el isobenzano al suelo, se produce una rápida
pérdida inicial; después, el resto del compuesto se degrada mucho
más despacio. Persiste en el suelo durante 2 a 7 años, según el tipo
de suelo. En condiciones de laboratorio, el isobenzano se descompone
en las aguas de superficie en pocas semanas cuando se expone a la
luz natural o artificial.
El suelo, las aguas subterráneas y las aguas superficiales de
pólders construidos con sedimentos contaminados por sustancias
organocloradas, inclusive compuestos de ciclodieno clorado, aún
contenían pequeños residuos de isobenzano algunos años después. En
1979-1980, no se detectó isobenzano (límite de detección: 0,01 mg/kg
de peso seco) en el sedimento de ríos de los Países Bajos. Tras el
tratamiento del suelo, los residuos en las cosechas suelen ser bajos
(menos de 0,05 mg/kg de cosecha), pero pueden encontrarse
concentraciones superiores en algunos tubérculos (hasta 0,2 mg/kg en
zanahorias). En las encuestas de mercado realizadas durante la época
de uso agrícola del isobenzano no se detectaron residuos en los
alimentos analizados (menos de 0,01 mg/kg).
En los productos lácteos procedentes de ganado que se alimentó
en pastos tratados con isobenzano se encontraron residuos del
insecticida. Dos muestras de mantequilla contenían 0,07 y 0,15 mg de
isobenzano/kg de producto, mientras que los niveles en la leche
entera fueron de 0,005 mg/kg a 0,07 mg/kg. En la leche deshidratada,
no obstante, se encontraron sólo 0,005 mg/kg. Durante la elaboración
de los productos lácteos se perdía hasta el 50% del residuo, según
el tipo de tratamiento.
No se dispone de datos sobre los niveles de isobenzano en la
sangre o el tejido adiposo de la población general. Los operarios
expuestos al isobenzano en las plantas de fabricación y elaboración
presentaron niveles medios en sangre entera de hasta 0,041 mg/litro.
En muestras de sangre entera procedente de personas que vivían en
las proximidades de una de las plantas, la concentración de
isobenzano estaba por debajo del límite de detección
(0,001 mg/litro).
El isobenzano es absorbido rápidamente a través de la pared
gastrointestinal y es transportado por la sangre sin alteraciones.
Se forman metabolitos hidrófìlos de los que se ha identificado uno,
la lactona de isobenzano. El isobenzano se acumula en los tejidos y
los órganos de ratas y perros en el orden siguiente: grasa > hígado
= músculo > cerebro > sangre. Las concentraciones tisulares en las
hembras de rata son en general superiores a las que aparecen en los
machos, sobre todo en la grasa. En la rata se determinó que la
semivida biológica en la grasa del organismo es de 10,9 días en los
machos y 16,6 días en las hembras. En un cachorro hembra de perro,
cuya sangre contenía 0,09 mg de isobenzano/litro, se observaron
convulsiones a los 15 días del nacimiento. El cachorro sólo se había
alimentado de leche de su madre, una Beagle a la que se había
administrado isobenzano y cuya leche contenía 0,7 mg/litro. En un
estudio de reproducción en ratas se observaron efectos similares en
las crías. Las vacas excretan isobenzano con la leche.
Las larvas de mosquito y los hongos del suelo metabolizan el
isobenzano del mismo modo que los vertebrados, dando lactona de
isobenzano como metabolito.
El isobenzano es muy persistente en el medio ambiente y se
bioacumula. Es sumamente tóxico para los peces, los camarones y las
aves. En los Países Bajos, país en el que se fabricaba el
isobenzano, los residuos encontrados en los huevos de golondrinas de
mar de las costas holandesas ascendieron a 0,45 mg/kg (promedio:
0,09 mg/kg), mientras que el promedio de residuos encontrados en
mejillones y peces fue de 0,05 mg/kg en 1965. Se observó una
disminución del número de lombrices en terrenos tratados con
isobenzano a razón de 2 kg/ha. Se redujo la nitrificación, con el
consiguiente aumento del nitrógeno inorgánico, en los suelos
tratados con isobenzano sobre el terreno a razón de 1 kg/ha, si bien
en estudios de laboratorio no se demostró efecto alguno en la
nitrificación con dosis equivalentes a 250 g/ha.
La toxicidad aguda del isobenzano para los mamíferos es elevada
por las vías oral y percutánea. La forma de acción de su toxicidad
es la sobreestimulación del sistema nervioso central, que da lugar a
convulsiones. La toxicidad aguda de las preparaciones de isobenzano
refleja el porcentaje de ingrediente activo presente.
Aunque el isobenzano no irrita la piel, algunos productos
preparados a partir de él pueden causar irritación.
En estudios limitados a corto y largo plazo de administración
oral realizados en ratones, ratas y perros se ha demostrado que el
isobenzano puede provocar en el hígado cambios histológicos que
reponden al tipo clásico de intoxicación por compuestos
organo-clorados. En un estudio realizado a largo plazo en ratas, se
determinó un nivel efectos sin observados de 5 mg/kg de dieta
(equivalente a 0,25 mg/kg de peso corporal), y en un estudio en
perros de dos años de duración se determinó un nivel sin observación
de efectos adversos de 0,025 mg/kg de peso corporal.
En un estudio de reproducción en una generación de ratas se
obtuvo un nivel sin efectos adversos observados de 0,1 mg/kg de
dieta (equivalente a 0,05 mg/kg de peso corporal). Con un nivel de
1 mg/kg de dieta (equivalente a 0,05 mg/kg de peso corporal)
disminuyó la supervivencia de las crías.
No se han comunicado estudios sobre la teratogenicidad ni la
mutagenicidad del compuesto.
No se observó potencial carcinogénico en un estudio de
administración oral a ratas durante dos años ni en un estudio de
administración oral a ratones, pero esos estudios no eran apropiados
para evaluar la carcinogenicidad.
La base de datos toxicológicos correspondiente al isobenzano es
incompleta. En general, actualmente se considera que la calidad de
los datos es mediocre e insuficiente para evaluar los riesgos que
representa para la salud humana o el medio ambiente.
Los datos sobre personas expuestas se limitan a estudios
realizados en trabajadores de una fábrica de los Países Bajos
durante la fabricación y la elaboración de preparaciones de
isobenzano y otros insecticidas de ciclodieno clorado afines. No se
comunicaron casos de irritación cutánea. En varios casos de
intoxicación, se produjeron convulsiones pero los cambios del
trazado electroencefalográfico resultaron ser reversibles. El nivel
umbral de intoxicación (en el caso de las convulsiones) se estimó en
0,015 mg de isobenzano/litro de sangre, y se calculó que la semivida
biológica del isobenzano en la sangre humana es del orden de 2,8
años.
2. Conclusiones y recomendaciones
El isobenzano es sumamente tóxico y muy persistente. La
información de que se dispone sobre los riegos del isobenzano es
incompleta, pero, aún así, basta para indicar que el riesgo que
supone para los que lo manipulan y para el medio ambiente es tan
elevado que no debería permitirse la exposición humana o del medio
ambiente a esta sustancia, ya sea utilizada como insecticida o para
cualquier otro fin.
6.3.3 Microorganisms
Isobenzan labelled with 14C in the hexachloropentane ring or
at the 1,3 position is metabolized by the fungi Aspergillus niger,
Aspergillus flavus, Penicillium chrysogenum, and Penicillium
notatum to isobenzan lactone, the same metabolite as found in
animal metabolism studies (Korte, 1963; Korte & Stiasni, 1964).
6.4 Elimination and excretion in expired air, faeces, and urine
6.4.1 Oral administration
In a study by Korte (1963), ten rabbits received in their diet
2 mg 14C-isobenzan, diluted with non-radioactive isobenzan, every
2 days to a total amount of 25-30 mg per rabbit. After 3 months,
about 50% of the total radioactivity administered had been excreted,
mainly in the urine. On the other hand, rats excreted most of the
radioactivity via bile into the gastrointestinal tract and excreted
these products with the faeces. No unchanged isobenzan was excreted,
only hydrophilic metabolites.
6.4.2 Parenteral administration
When Carworth Farm rats received daily intraperitoneal
injections of isobenzan (98% purity) of 0.25, 1, or 2 mg/kg body
weight 5 days per week for 2 weeks (section 7.2.3), they excreted
less than 1% of the daily dose as unchanged isobenzan in the faeces
(Brown et al., 1962).
In a study by Kaul et al. (1970), male and female rats
received a single intravenous injection of 15 µg 14C-isobenzan/kg
body weight. The male rats excreted 1% of the applied radioactivity
in the urine and 12% in the faeces within 48 h, while the females
excreted 5% and 11%, respectively.
After having been administered intravenously to rats with
cannulated bile ducts, 14C-isobenzan was excreted as hydrophilic
metabolites in the bile (Korte, 1963, 1967).
Male rabbits given an intravenous injection of 14C-isobenzan
(241 µg/kg body weight) excreted 12% in the urine and 1% in the
faeces within 96 h (Kaul et al., 1970).
6.5 Retention and turnover
The absorption of isobenzan into the body is determined by
measuring the insecticide in the blood. No human studies exist
relating the concentration of isobenzan in the blood at the state of
equilibrium to the total daily intake or relating the concentration
of isobenzan in the blood to that in the tissues. However, in
experimental animals, such relationships have been determined. Thus,
in human beings, measurement of the blood concentration of the
insecticide at the state of equilibrium is assumed to reflect total
absorption by all routes (skin, pulmonary, and oral exposure) as
well as the storage level in adipose tissue, thereby providing a
measure of the total body burden of isobenzan.
From human data, it is estimated that the biological half-life
of isobenzan in blood is of the order of 2.8 years (Jager, 1970)
(section 8.2).
7. EFFECTS ON LABORATORY MAMMALS AND IN VITRO TEST SYSTEMS
7.1 Single exposure
7.1.1 Oral administration
In rats, the first signs of toxicity were evident approximately
1 h after the administration of a lethal dose and consisted of
lethargy followed by muscular twitching, laboured breathing, and,
finally, general convulsions. The majority of deaths occurred within
the first 20 h after administration. No specific changes were
observed in the organs of fatally intoxicated animals. Signs of
intoxication were similar in mice, rats, guinea-pigs, cats, dogs,
and chickens. Surviving animals recovered completely (Brown et al.,
1962; Worden, 1969).
Oral LD50 values for the various animal species range from
1.6 to 10 mg/kg and are summarized in Table 5.
Table 5. Oral LD50 values for technical isobenzan
Species Vehicle LD50 Reference
(mg/kg body
weight)
Mouse arachis oil 10a,b Worden (1969)
Mouse corn oil 12.5 Spynu (1964)
Rat corn oil 4.8 Howard et al. (1957)
Rat corn oil 14.4 Spynu (1964)
Rat carboxymethyl cellulose 5.4 Stevenson (1964)
Rat dimethylsulfoxide 7.2 Stevenson (1964)
Rat arachis oil 10b Worden (1969)
Rat unknown 8.0 Layton et al. (1987)
Guinea-pig arachis oil 2.5a,b Worden (1969)
Golden hamster arachis oil 20b Worden (1969)
Dog unknown 1.6 Stevenson (1964)
a Average of males and females.
b Isobenzan 99.5%.
7.1.2 Dermal administration
Signs of intoxication are the same as those described for acute
oral intoxication but they develop more slowly (Brown et al.,
1962). The LD50 values are summarized in Table 6.
Table 6. Dermal LD50 values for technical isobenzan
Species Vehicle LD50 Reference
(mg/kg body
weight)
Rat arachis oil occluded, 4 Zavon (1961)
non-occluded, 10
Rat corn oil non-occluded, 8.5 Spynu (1964)
Rat crystalline form occluded, 60 Stevenson (1964)
(approximately)
7.1.3 Parenteral administration
The acute toxicity of isobenzan administered parenterally is
similar to that following oral administration (Table 7). The signs
of intoxication are similar to those observed after acute oral
toxicity but develop more rapidly, starting less than 1 h after the
injection (Brown et al., 1962; Worden, 1969).
Table 7. Parenteral LD50 values for technical isobenzan
Route Species Vehicle LD50 Reference
(mg/kg body
weight)
Intraperitoneal mouse xylene emulsion 8.2 Stevenson (1964)
Intraperitoneal mouse methoxytriglycol 6.0 Cole & Casida (1986)
Intraperitoneal rat dimethylsulfoxide 3.6 Stevenson (1964)
Subcutaneous rat, arachis oil 6-10 Worden (1969)
mouse
Intravenous rat unknown 1.7 Zavon (1961)
7.1.4 Formulated material
A 50% wettable powder formulation and a 15% emulsifiable
concentrate (in mixed petroleum xylenes) were tested for their acute
oral toxicity to rats, mice, rabbits, hamsters, cats, and dogs. The
LD50 values, when expressed as active material, were comparable
with those of isobenzan itself (Stevenson, 1964; Worden, 1969). The
dermal LD50 value for the 15% emulsifiable concentrate was
25-35 mg isobenzan/kg body weight for Hooded-Lister rats and 6 mg
isobenzan/kg body weight for New Zealand white rabbits, whereas in
the case of the 50% wettable powder the LD50 was 41 mg
isobenzan/kg body weight for rabbits (Stevenson, 1964). Rabbits
exposed dermally to the 15% emulsifiable concentrate formulation
behaved differently to rats similarly exposed. The rabbits generally
lost weight due to anorexia and a failure to drink, and they would
then convulse, even as much as 3 weeks after the exposure. However,
if feeding and drinking were resumed quickly, the rabbits did not
convulse (Brown, 1963, 1964).
7.1.5 Metabolites
The acute oral and intravenous toxicities of the metabolite
isobenzan lactone for mice were 30 times lower than those of
isobenzan. The oral and intravenous LD50 values were 306 mg/kg
body weight and > 100 mg/kg body weight, respectively (Korte,
1967).
7.2 Short-term exposure
The short-term, long-term, and reproduction studies that were
used for establishing a no-observed-effect level are summarized in
Table 8.
7.2.1 Oral administration
7.2.1.1 Mouse
In a study by Brown et al. (1962), groups of five male and
five female Carworth Farm No. 1 mice were fed diets containing
isobenzan (98%) at levels of 1, 2, 3, 4, or 5 mg/kg for up to 3
weeks. No mortality was observed at 1 mg/kg, but at the higher
concentrations the mortality was dose related, reaching 100% at
5 mg/kg diet.
7.2.1.2 Rat
Groups of eight male and eight female Carworth Farm rats were
intubated with isobenzan (98%) in dimethylsulfoxide (0.25, 1, or
2.5 mg/kg body weight per day) 5 days/week for 2 weeks. All rats
dosed with 2.5 mg/kg body weight died within 5 days. All the females
and two of the males dosed with 1 mg/kg body weight died after 5-7
doses, whereas only one female dosed with 0.25 mg/kg body weight
died after five doses. Weight loss preceded the death of the animals
at the highest dose, this being the result of reduced food intake.
No gross or microscopic changes were found in the rats dying from
intoxication or in the survivors sacrificed 14 days after the last
treatment (no details were reported concerning the parameters
studied) (Brown et al., 1962).
In a study by Howard et al. (1957), groups of 10 male and 10
female Sprague-Dawley rats were fed diets containing 0, 2.5, 12.5,
or 25 mg isobenzan/kg diet for 30 days. One female in the
highest-dose group died. Some of the rats fed with levels of 12.5 or
25 mg/kg were nervous and irritable, and the reduced body weight
gain in both these groups correlated with diminished food
consumption. At autopsy, there were some areas of necrosis of heart
cells and haemorrhages in the heart muscle of animals fed with
levels of 12.5 or 25 mg/kg diet.
Table 8. Short- and long-term oral exposure to isobenzan
Animal (strain) Exposure period NOAELa LOAELa References
(mg/kg diet) (mg/kgdiet)
Rat (Sprague-Dawley) 30 days 2.5 (0.12) 12.5 (0.62): irritability, Howard et al. (1957)
decreased body weight gain,
histopathology changes in heart
Dog (Beagle) 2 years (0.08) (0.125): convulsion, mortality Brown & Richardson (1964)
Dog (Scottish terrier) 2 years (0.025) (0.1): decreased body weight gain, Worden (1969)
increased liver weight
Rat (Hooded-Lister) 2 years 5 (0.25) 17.5 (0.87): convulsions Worden (1969)
Reproduction study
Rat (Carworth Farm) one generation 0.1 (0.005) 1 (0.05): decreased survival of pups Chambers (1962a,b)
(2 litters) (pups) 5 (0.25): convulsions
1.0 (0.05)
(parents)
a Figures in parentheses are values for exposure concentration in mg/kg body weight.
In a study by Worden (1969), groups of five male and five
female Hooded-Lister rats were fed diets containing isobenzan
(99.5%) (25, 35, 50, or 100 mg/kg diet) as a 15% emulsifiable
concentrate (in mixed petroleum xylenes). After 37 days on the test
diets, half of the surviving animals of each group were transferred
to a control diet, while the remainder continued on the test diets
for a further 42 days. During the first period, all rats fed with
100 mg/kg diet died except for one male. One male and two females
fed with 50 mg/kg diet died as did three females fed with 35 mg/kg
diet. There were no further deaths among the rats after dosing was
discontinued. During the second period of feeding with the test
diet, one female fed at the lowest level died. In one rat only, a
female fed with 50 mg/kg, the classical signs of organochlorine
intoxication were seen in the liver. These have been described by
Hodge et al. (1967) as "enlarged centrolobular hepatic cells with
cytoplasmic oxyphilia and somewhat increased and peripheral
migration of the basophilic granules".
7.2.1.3 Dog
Pairs of Beagle hounds (one male and one female) were given
daily oral doses of 0, 0.08, 0.125, or 0.2 mg isobenzan (98%)/kg
body weight in olive oil in gelatin capsules for 2 years. The dogs
given the two highest doses had several convulsive episodes during
the course of the study. When a dog exhibited a convulsion, dosing
was discontinued for 8 weeks. The male dog dosed with 0.125 mg/kg
died one week after a third convulsive episode, which followed a
sudden fall in body weight. No signs of intoxication were observed
in the dogs dosed with 0.08 mg/kg body weight. During this study,
blood isobenzan concentrations were measured regularly, in
particular at the time of convulsions. Concentrations in muscle
tissue were determined 3 days after the convulsion, and the results
are given in Table 9. The blood concentrations in the dogs receiving
0.08 mg/kg body weight for 2 years without convulsions indicated
that a plateau was reached after a relatively short exposure. The
mean concentration was approximately 0.02 mg isobenzan/litre and the
maximum concentration 0.04-0.05 mg/litre. The two highest doses
caused convulsions (Brown & Richardson, 1964).
Groups of three male and three female Scottish terriers (2.5-9
kg body weight) received daily gavage doses of isobenzan (0, 0.025,
or 0.1 mg/kg body weight) as 15% emulsifiable concentrate (in mixed
petroleum xylenes) for 2 years. No deaths and no signs of
intoxication resulted from the isobenzan administration. Body weight
gain, urinalysis, and haematological and clinical chemistry values
for the dogs fed with 0.025 mg/kg body weight were comparable with
those of the control animals. At a dose level of 0.1 mg/kg body
weight, a decrease in body weight gain, a slight increase in serum
Table 9. Concentration of isobenzan in the blood and muscle of dogs
Dose (mg/kg Sex Number of Blood concentration Muscle tissue
body weight) convulsions at time of convulsion (mg/kg wet
(mg/litre) weight)
0.08 male none 0.04 (maximum
concentration)
female none 0.05 (maximum
concentration)
0.125 male first two 0.06
third (death) 0.11 16
female 4 0.03-0.06 0.18
0.2 male 9 0.02-0.11 0.36
female 6 0.07-0.08 0.68
alkaline phosphatase values during the second year, and an increase
in liver weight were noted. No evidence of histopathological lesions
attributable to isobenzan was found at either treatment level. The
no-observed-effect level resulting from this study was 0.025 mg/kg
body weight (Worden, 1969). The distribution of isobenzan in the
tissues examined is summarized in section 6.2.2.
7.2.2 Dermal administration
When groups of five female albino rabbits were given daily
applications of isobenzan in corn oil (0, 5, 10, 20, 30, or 40 mg
per rabbit) to the shaven skin for 3 weeks, mortality was high at
all dose levels, reaching 100% after 2 weeks for rabbits given doses
of 30 or 40 mg. Histopathological examination revealed necrosis of
the heart muscle, non-dose-related lesions of the liver, and
degenerative changes in cells of the central nervous system in a few
animals (Howard et al., 1957).
7.2.3 Intraperitoneal administration
Groups of five male and five female Carworth Farm rats were
injected intraperitoneally with isobenzan (98%) in
dimethyl-sulfoxide (0.25, 1, or 2 mg/kg body weight per day) 5
days/week for 2 weeks. All rats given the lowest dose survived, but
two rats of each sex given 1 mg/kg and five males and four females
given 2 mg/kg died after 5-10 doses. No gross or microscopic lesions
were found in fatally intoxicated rats or in the survivors killed
7-14 days after the last treatment (no details concerning
parameters studied were reported) (Brown et al., 1962).
7.3 Long-term exposure
7.3.1 Rat
Groups of 25 male and 25 female Hooded-Lister rats were fed, at
dietary concentrations of 5, 17.5, or 30 mg/kg (equivalent to 0.25,
0.875, or 1.5 mg/kg body weight), isobenzan as a 15% emulsifiable
concentrate (in mixed petroleum xylenes) for 2 years. Three control
groups, each consisting of 25 male and 25 female rats, were used.
Additional groups of rats, fed at the same dose levels and
accompanied by separate control groups, were used for liver and
kidney function tests after 20, 66, and 104 weeks. Five females fed
at the highest dose level died during the first 3 weeks of the
study. Signs of intoxication (such as ruffled coat, lethargy,
muscular twitches, and mild to violent convulsions) were observed in
animals given 17.5 or 30 mg/kg diet, mainly during the first few
weeks. There were no adverse effects on body weight gain, food
conversion ratios, haematological parameters, serum alkaline
phosphatase, serum glutamic-pyruvic transaminase, total serum
protein, or albumin/globulin ratios. Absolute liver weight was
increased in the animals given the highest dose level. Gross and
microscopic examinations did not reveal any compound-related
changes, with the possible exception of thyroid hyperplasia recorded
in four males and six females at a level of 30 mg/kg diet. The liver
function test (bromsulfophthalein) and kidney function test (phenol
red) showed no deviations from control values. No significant
increase in the number and type of tumours was found. The
no-observed-effect level for toxicological effects was 5 mg
isobenzan/kg diet (equivalent to 0.25 mg/kg body weight) for 2 years
(Worden, 1969).
7.4 Skin irritation
In a study by Worden (1969), four male guinea-pigs received a
single application (2 mg/kg body weight) of isobenzan (99.5%) as a
0.2% w/v solution in arachis oil to the shaved skin. The material
was not removed from the skin. The animals were kept under
observation for 21 days, but no irritation was observed.
Two male and two female rabbits were given 12 successive daily
applications (0.5 mg/kg body weight) of isobenzan (99.5%) and six
female rats received 30 successive daily applications (0.3 mg/kg
body weight) of isobenzan as a 0.2% w/v solution in arachis oil to
the shaved skin. The material was left on the skin and the
observation period was 21 days after the last application. No
irritation was observed (Worden, 1969).
7.5 Reproductive toxicity, embryotoxicity, and teratogenicity
No studies on the potential teratogenicity of isobenzan have
been reported.
7.5.1 Mouse
In a range-finding test, groups of 25 male and 25 female BALB/C
mice were fed diets containing 0, 1, 2.5, 5, or 10 mg isobenzan
(94%)/kg. The mice in the 5- and 10-mg/kg dose groups all died
within 64 and 24 days, respectively. Only 20% of those in the
2.5-mg/kg group survived for 120 days. The 1 mg/kg group survived
and reproduced normally (no further data were reported). In the main
study, groups of 108 mice of each sex of Swiss strain BALB/C were
fed control diet and 106 mice of each sex were fed 1 mg isobenzan/kg
diet for 30 days, after which they were randomly paired and
continued on the same diet for 90 days. The number of litters
produced, litter size, sex ratio, and mortality were recorded in
this one-generation reproduction study. There were no statistically
significant differences between the control and isobenzan-treated
animals for any of the parameters measured (Ware & Good, 1967).
7.5.2 Rat
A one-generation, 2-litter reproduction study was carried out
with groups of 20 male and 20 female weanling Carworth Farm rats
that were fed diets containing isobenzan at levels of 0, 0.1, 1, 5,
or 10 mg/kg for 100 days and then mated. Convulsions were seen
during the mating period and pregnancy in females fed 10 mg/kg, and
during the lactation period in one female fed 5 mg/kg. Pups of the
second mating from both these treatment groups were also seen to
convulse. Mean litter size and survival of pups were markedly
reduced in the 10-mg/kg group and, to a lesser extent, in the
5-mg/kg group. No clinical signs were observed in the 1-mg/kg group.
At this dose level, the mean litter size was comparable with
controls, but survival of the pups at 21 days was decreased. No
effects attributable to isobenzan were found in the 0.1-mg/kg group
(Chambers, 1962a,b).
7.5.3 Dog
During a 2-year study (section 7.2.1.3), three litters were
born to a female Beagle hound dosed daily with 0.08 mg isobenzan/kg
body weight five days per week. A male Beagle dog, dosed at the same
level, was sire for several of the litters. The first litter
consisted of one male and one female, both normal, and one
still-born male. Fifteen days after birth, the female pup began to
convulse, having only fed on the mother's milk (which contained
0.7 mg isobenzan/litre) and at 17 days the pup was sacrificed.
Concentrations of isobenzan in tissues are given in section 6.2.2.
Two further litters of pups were born to the original dam (two male
pups in the first litter and two pups of each sex in the second).
These animals all appeared normal and healthy and showed no ill
effects from the ingestion of maternal milk. One litter of pups, one
male and one female, was born to a female Beagle dog fed 0.125 mg/kg
body weight per day for two years. These pups did not exhibit any
signs of intoxication and were killed when 26 days old. Autopsy did
not reveal any structural changes. It did not appear that isobenzan
interfered with either the male or female reproduction function
(Brown & Richardson, 1964).
7.6 Mutagenicity and related end-points
No information on mutagenicity is available.
7.7 Carcinogenicity
No adequate carcinogenicity studies have been reported.
7.7.1 Mouse
Groups of 18 male and 18 female mice of two hybrid strains (the
F1 hybrids C57Bl/6 x C3H/Anf and C57Bl/6 x AKR) were given the
maximum tolerated dose of isobenzan in 0.5% gelatin (0.215 mg/kg
body weight) daily from 7 to 28 days of age by stomach tube.
Thereafter the isobenzan was mixed in the diet to a concentration of
0.646 mg/kg, and the mice were killed at 18 months of age. No
significant increase in tumour incidence was found (Innes et al.,
1969).
7.7.2 Rat
In a long-term feeding study on rats (section 7.4.1), no
evidence of carcinogenicity was found as a result of feeding diets
containing up to 30 mg isobenzan/kg for 2 years (Worden, 1969).
7.8 Special studies
7.8.1 Biochemical studies
Mehrotra et al. (1982) studied the comparative effects of
cyclodiene compounds on different ATPase activities in beef and rat
brain synaptosomal fractions in vitro. Isobenzan significantly
inhibited Na+-K+-ATPase in rat brain synaptosomes. A
dose-related response was observed at up to 80 µmol/litre, but no
increase in inhibition was observed with a further increase in the
concentration of the compound. Oligomycin-sensitive Mg2+-ATPase in
rat brain synaptosomes was significantly inhibited by isobenzan, a
maximum of 64% inhibition occurring at 120 µmol/litre. In addition,
the oligomycin-insensitive Mg2+-ATPase in rat brain synaptosomes
was inhibited, and the inhibition was concentration dependent.
Isobenzan did not have any effect on K+-stimulated
p-nitrophenylphosphatase, an enzyme which is known to represent
the dephosphorylation step in the overall reaction of the
Na+-K+-ATPase. Oligomycin-sensitive Mg2+-ATPase in beef heart
mitochondria was significantly inhibited.
Isobenzan did not affect the activity of adenylate cyclase or
phosphodiesterase in Sprague-Dawley rat brain synaptosomes in vitro
at concentrations of up to 200 µmol/litre (Kodavanti et al.,
1988).
Several studies into the effect of isobenzan on the transfer of
ammonia in brain have indicated that isobenzan acts by increasing
brain ammonia levels before and during convulsions. Glutamic acid,
glutamine, and alpha-ketoglutaric acid, which are utilized in an
ammonia-binding mechanism, become overwhelmed, resulting in free
ammonia accumulating in the cerebral tissues (Hathway & Mallinson,
1964; Hathway, 1965; Hathway et al., 1965).
7.8.2 Neurotoxicity
In vitro studies using fresh rat brain synaptic membranes
showed isobenzan to be a potent inhibitor of the binding of the
convulsant tert-butylbicyclophosphorothionate (TBPS) to
brain-specific sites, thereby indicating an action at the
gamma-aminobutyric acid (GABA)-regulated chloride channel. Metabolic
activation by rat liver microsomes did not enhance the potency for
inhibition. This inhibition indicates that isobenzan binds to the
same site as TBPS, suggesting that isobenzan acts in a manner
similar to non-competitive GABA-A antagonists and providing a basis
for its convulsant action in mammals (Lawrence & Casida, 1984).
Bloomquist et al. (1986) produced a concentration-dependent
inhibition of 36Cl uptake into mouse brain vesicles by adding
isobenzan to mouse brain homogenate. The inhibitory activity was
confined to that portion of 36Cl uptake that was GABA dependent.
The insecticide concentration producing 50% inhibition (I50) of
36Cl uptake was 2.0 (0.83 to 5.1) µmol/litre, and the inhibitory
potency (I50) value for 35S-TBPS binding in rat brain
synaptosomes was 0.30 µmol/litre (Lawrence & Casida, 1984).
Cole & Casida (1986) confirmed in a study with male
Swiss-Webster mice administered isobenzan intraperitoneally that a
correlation also exist in vivo between binding to mouse brain GABA
receptors and convulsive activity. The inhibitory potency (IC50)
for in vitro TBPS binding to mouse brain synaptosomes is
0.03 µmol/litre. It was shown that the inhibitory potencies of
cyclodienes, including isobenzan, parallel their acute oral
toxicities. Isobenzan was slightly more potent than endrin and
produced virtually complete inhibition of GABA-dependent chloride
uptake at 30 µmol/litre. There was a significant linear correlation
between the 36Cl flux and 35S-TBPS-binding assays.
7.8.3 Pharmacological studies
Pharmacological studies on the function of organ systems in
various animal species (such as rats, guinea-pigs, rabbits, cats,
and frogs) after the administration of isobenzan by different routes
showed that the only significant effect was a disturbance of the
central nervous system associated with convulsions. This effect was
due to stimulation of the higher brain centres at the level of the
medulla and above. Changes in respiratory rate, heart rate, and
salivary secretion were probably mediated by the central nervous
system as a secondary effect of central nervous stimulation.
Barbiturates were found to control these convulsions. The
stimulation of brain activity was reflected in the occurrence of
electroencephalographic changes in the pre-convulsive stage and
during the convulsive episodes. This corresponds to the situation in
cases of human intoxication by occupational exposure to cyclodiene
insecticides as described by Hoogendam et al. (1962, 1965) and
Chambers (1962c).
Ibrahim (1964) showed that isobenzan injected intraperitoneally
at a toxic dose (7 mg/kg body weight) into male Wistar rats produced
a higher tension of contraction in the gastrocnemius muscle at lower
frequencies of stimulation than in controls. The maximum tetanic
tension was also attained at a lower frequency. An increase in the
duration of the "active state" of the muscle was considered to be
the most like explanation.
8. EFFECTS ON HUMANS
8.1 General population exposure
No poisoning incidents or untoward effects of long-term
exposure of the general population have been reported.
8.2 Occupational exposure
Isobenzan was initially manufactured and handled in the
Netherlands between 1958 and 1965. Aldrin, dieldrin, and endrin were
also produced in the same manufacturing plant and, consequently, in
many cases the exposure was mixed. Routine medical examination of
233 workers, who were exposed for more than 4 years and who followed
normal procedures during that period, did not reveal any
abnormalities in EEG, clinical chemistry or haematological
parameters, or liver microsomal enzyme induction (Jager, 1970;
Versteeg & Jager, 1973). The mean isobenzan concentration in the
blood of 20 operators after cessation of exposure decreased from
22 µg/litre in 1965 to 7 µg/litre in 1969. The biological half-life
of isobenzan in the blood (at the state of equilibrium of isobenzan
in body tissues) was estimated to be of the order of 2.8 years
(Jager, 1970).
In the 7 years of isobenzan production, 15 cases of clinical
intoxication, including eight cases with convulsions, were reported.
The mean concentration of isobenzan in the blood of nine workers at
the time of intoxication was 23 µg/litre, the range being
17-30 µg/litre. Although these workers recovered fully, it took
longer than with the related cyclodiene insecticides. In three
cases, certain typical complaints, such as headache, dizziness,
drowsiness, and irritability, persisted for 6 months, and the return
to normal of the modified EEG pattern sometimes took more than a
year. In one case of acute over-exposure, without signs of
intoxication, the blood isobenzan concentration decreased from
8 µg/litre to less than 2 µg/litre within 3 days (Jager, 1970).
The data from plant workers indicated a threshold level of
isobenzan in blood below which no signs or symptoms of intoxication
occur. This level was found to be 15 µg/litre (Jager, 1970).
Ribbens (1985) carried out a mortality study on the
above-mentioned industrial workers exposed to aldrin, dieldrin,
endrin, and isobenzan. Vital status and cause of death were assessed
for 232 of the total population of more than 1000 workers. This
group was selected for follow-up on account of the high degree of
exposure in the initial years of manufacturing and formulation and
the long exposure (mean 11 years) and observation (mean 24 years)
periods. Total observed mortality was 25 as opposed to 38 expected
on the basis of death statistics for the male Dutch population. Of
the nine cancer deaths, three were caused by lung cancer, while the
remaining six were each of a different nature. The author concluded
that although exposures in this group were high and exposure as well
as observation periods long, this study did not reveal any
indication of a specific carcinogenic activity of these pesticides.
9. EFFECTS ON OTHER ORGANISMS IN THE LABORATORY AND FIELD
9.1 Microorganisms
In laboratory studies, sandy loam soil was treated with 250 or
2500 mg isobenzan/kg (concentrations that exceeded the recommended
rates). At both concentrations, the carbon dioxide production was
inhibited to almost the same extent (21 and 24%, respectively)
during a 30-day test period. The addition of glucose to the soil
reduced the inhibitory effect to 5 and 4%, respectively. Isobenzan
at 250 mg/kg did not influence nitrification in the soil up to 18
days after treatment (Bartha et al., 1967).
Screening studies on microorganisms in pure culture were
carried out on nutrient agar plates with isobenzan either
incorporated in a uniformly emulsified form (1000 mg/kg) or as a
thin surface film (1 mg/cm2). The growth of the gram-positive
Bacillus megaterium was inhibited, but not that of various
gram-negative organisms such as several Pseudomonas strains,
Escherichia coli, Klebsiella aerogenes W5, or Achromobacter
butyri (Trudgill & Widdus, 1970).
9.2 Aquatic organisms
Groups of five adult Harlequin fish (Rasbora heteromorpha)
were exposed for 2 h at a temperature of 20 °C to water (pH 7.2)
containing isobenzan (99%), dissolved in DMSO, at a concentration of
0.01, 0.1, or 1 mg/litre. The fish were then transferred to clean
water and observed for an additional 48 h. The treatment with
1 mg/litre caused disorientation and the fish became excited by
external stimuli, lost the ability to swim, and, finally, all died
within 1 h. In some cases, they appeared to convulse. Isobenzan at
0.1 mg/litre caused similar symptoms; within 2 h of exposure, all
five fish died. No fish died at 0.01 mg/litre, but slight changes in
swimming behaviour were observed (Brown et al., 1962). When
guppies (Poecilia reticulata) were tested in the same way, the
symptoms of intoxication and susceptibility were similar to those in
Harlequin fish, but the guppies were slower to react (Brown et al.,
1962).
Data on the acute toxicity of isobenzan for aquatic organisms
in flow-through tests are given in Table 10.
Table 10. Acute toxicity of isobenzan (technical grade, 94%) for aquatic organisms
Organism Developmental Temperature Parameter Concentration Reference
stage (°C) (µg/litre)
Brown shrimp juvenile 17 48-h EC50 0.034a US EPA
(Penaeus (1987)
aztecus)
Eastern oyster juvenile 18 96-h EC50 32b US EPA
(Crassostrea (1987)
virginica)
Sheepshead minnow juvenile 17 48-h LC50 2.0a US EPA
(Cyprinodon (1987)
variegatus)
Spot juvenile 13 48-h LC50 0.32c US EPA
(Leiostomus (1987)
xanthurus)
a Salinity 30 ng/litre.
b Salinity 33 ng/litre.
c Salinity 22 ng/litre.
9.3 Terrestrial organisms
9.3.1 Soil invertebrates
In laboratory studies, plain-field sand was treated once with
0.05 mg isobenzan/kg and stored at 13 or 24 °C. The springtail
(Folsomia candida) was used for bioassays lasting up to 16 weeks.
The biological activity of isobenzan persisted slightly longer at
the higher temperature, killing 100% of the insects after 16 weeks
(Thompson, 1973).
9.3.2 Birds
9.3.2.1 Acute toxicity
Isobenzan (> 99%) is highly toxic to birds when administered
as a single oral dose (Table 11). LD50 values range from
1-10 mg/kg.
9.3.2.2 Short-term toxicity
When groups of five male and five female Japanese quail
(Coturnix coturnix japonica) were fed diets containing 2 or 10 mg
isobenzan/kg, the mean survival time in the high-dose group was 6.9
days (range, 2-20 days). The residues in the liver and brain
averaged 3.4 mg/kg and 1.4 mg/kg, respectively. In the low-dose
group, the mean survival time was 45.9 days (range, 19-65 days) and
the average residues in liver and brain were 6 mg/kg and 1.6 mg/kg,
respectively. The concentration of isobenzan in the liver of birds
fed 2 mg/kg was significantly higher than that in the high-dose
group, but the concentration in the brain showed no difference
(Koeman, 1971).
Table 11. Oral LD50 values of isobenzan for birds
Species LD50 (mg/kg Reference
body weight)
Mallard duck (female) 4.15 Hudson et al. (1984)
(Anas platyrhynchos) (2.47-6.97)
Coturnix quail (female) 4.2 Schafer & Brunton (1979)
(Coturnix coturnix)
Grackle 1.3 Schafer & Brunton (1979)
(Quiscalus quiscula)
Pigeon 10 Schafer & Brunton (1979)
(Columba livia)
Red-winged blackbird (male) 3.2 Schafer & Brunton (1979)
(Agelaius phoeniceus)
Sparrow 1 Schafer & Brunton (1979)
(Passer domesticus)
Starling 2.4 Schafer & Brunton (1979)
(Sturnus vulgaris)
When groups of 20 Rhode Island Red laying hens were fed
isobenzan (15% emulsifiable concentrate) at levels of 0.1, 0.25,
0.75, or 1 mg/kg diet for 14 weeks, no effects were seen on food
consumption, egg production, egg weight, or egg fertility (Verma
et al., 1967).
9.4 Population and ecosystem effects
9.4.1 Soil microorganisms
In a sugar cane field in India, Srivastava (1966) studied the
effect of isobenzan (1 kg/ha) on nitrification of ammonium sulfate
in the soil. The insecticide-treated soil contained higher amounts
of total inorganic nitrogen and ammonium nitrogen (80% increase) up
to 60 days after treatment than did untreated soil samples,
indicating impaired nitrification.
9.4.2 Soil invertebrates
In a study by Kelsey & Arlidge (1968), five sandy loam plots of
pasture in New Zealand were treated with Telodrin (15% emulsifiable
concentrate) at a rate of 2 kg isobenzan/ha in June 1962. The
populations of all recorded groups (grass grub, porina, Collembola,
Diptera, Hemiptera, Coleoptera, mites, and earthworms), except
nematodes, were drastically reduced. There was no recovery in the
populations of any of the affected groups during the period up to
October 1965. In silt loam plots treated with isobenzan granules
(1 kg/ha) in April 1964, the populations of Coleoptera, Collembola,
Diptera, mites, and earthworms were all reduced, but to a lesser
extent than in the test using 2 kg/ha. However, grass grub and
Hemiptera were not significantly affected, and nematode numbers were
elevated 2 years after the treatment. Studies on root development
showed that 90% of roots with root hairs were located within a mat
of plant debris above the soil, whereas in controls 99% were located
in the soil. Plant growth was retarded. The moisture content of the
treated plots was significantly less than that of control plots,
indicating that the capacity of soil to absorb and retain water had
been reduced.
When loose sandy soil in New Zealand was treated with 2.25 kg
isobenzan/ha as 5% granules, the population reduction, 1-18 weeks
after treatment, was 90-100% for larval Coleoptera and Lepidoptera
and 75% for Diptera and earthworms. The number of surface
arthropods, 5-15 days after treatment, was reduced by 45%. Six
months after treatment, little effect was found on nematodes
(13% reduction), bacteria (18% reduction), and fungi (7% increase)
(Moeed, 1975).
REFERENCES
ANON (1974) Aldrin, dieldrin, and endrin. Determination of residues
of organochlorine insecticides in crops, soils, and animal products,
Sittingbourne, Shell Research (Residue Analytical Method WAMS 60-1).
BARTHA, R., LANZILOTTA, R.P., & PRAMER, D. (1967) Stability and
effects of some pesticides in soil. Appl. Microbiol., 15(1): 67-75.
BAUER, U. (1972) [Behaviour of a series of plant-protecting agents
during the treatment of groundwater by slow sand filtration.]
Schriftenr. Ver. Wasser-Boden-Lufthyg. (Berlin-Dahlem), 37: 91-102
(in German).
BIEHL, M.L. & BUCK, H.B. (1987) Chemical contaminants: their
metabolism and their residues. J. Food Prot., 50(12): 1058-1073.
BISHOP, J.L. & HUBER, J.T. (1964) Secretion of Telodrin in the milk
of cows fed varying levels of Telodrin. J. dairy Sci., 47: 552-554.
BLOOMQUIST, J.R., ADAMS, P.M., & SÖDERLAND, D.M. (1986) Inhibition
of gamma-aminobutyric acid stimulated chloride flux in mouse brain
vesicles by polychlorocycloalkane and pyrethroid insecticides.
Neurotoxicology, 7(3): 11-20.
BOWMAN, M.C., SCHECHTER, M.S., & CARTER, R.L. (1965) Behavior of
chlorinated insecticides in a broad spectrum of soil types. J.
agric. food Chem., 13: 360-365.
BROWN, V.K.H. (1963) Agricultural chemicals: the relative
percutaneous toxicities of Nitrophoska fertiliser granules
impregnated with aldrin and Telodrin, Sittingbourne, Shell Research
(Technical Memorandum TOX 10/63).
BROWN, V.K.H. (1964) Some effects of percutaneously absorbed
Telodrin in rabbits, Sittingbourne, Shell Research (Report No. PPR
TL/2/64).
BROWN, V.K.H. & RICHARDSON, A. (1964) Chronic oral exposure of
beagle hounds to Telodrin and dieldrin: report on the first two
years, Sittingbourne, Shell Research (Report No. PPR TL/1/64).
BROWN, V.K.H., CHAMBERS, P.L., HUNTER, C.G., & STEVENSON, D.E.
(1962) The toxicity of Telodrin for vertebrates, Sittingbourne,
Shell Research (Report No. R(T)-2-62).
BUICK, A.R. (1964) Telodrin residues on tobacco from Australia,
Sittingbourne, Shell International Chemical Company, Ltd (Technical
Memorandum 132/64)
BUICK, A.R. (1965) Telodrin residues in potatoes from India,
Sittingbourne, Shell International Chemical Company, Ltd (Technical
Memorandum 25/65).
BUICK, A.R. & COLE, E.R. (1965) Telodrin residues in sweet potatoes
from South Africa, Sittingbourne, Shell International Chemical
Company, Ltd (Technical Memorandum 25/65).
BULL, M.S. & MARLOW, R.G. (1967) Insecticide residues in diet
samples from Nicaragua, Sittingbourne, Shell International Chemical
Company, Ltd (Technical Service Note 85/67).
BULL, M.S. & RAMSDEN, D.P. (1967) Residues of organochlorine
insecticides in samples from Venezuela, Sittingbourne, Shell
International Chemical Company, Ltd (Technical Service Note 86/67).
CHAMBERS, P.L. (1962a) Agricultural chemicals: the effect of
Telodrin on reproduction in the rat when fed at various levels in
the diet. Report No. 3, Sittingbourne, Shell International Chemical
Company, Ltd (Technical Memorandum TOX 24/62).
CHAMBERS, P.L. (1962b) Agricultural chemicals: the effect of
Telodrin on reproduction in the rat when fed at various levels in
the diet. Report No. 4, Sittingbourne, Shell International Chemical
Company, Ltd (Technical Memorandum TOX 33/62).
CHAMBERS, P.L. (1962c) The physiological and pharmacological effects
of Telodrin, Sittingbourne, Shell Research (Report No. M(T)-4-62).
COLE, L.M. & CASIDA, J.E. (1986) Polychlorocycloalkane
insecticide-induced convulsions in mice in relation to disruption of
the GABA-regulated chloride ionophore. Life Sci., 39: 1855-1862.
CRABTREE, A.N. (1968) Concentration of chlorinated insecticides in
the whole blood of the CAPSA plant in Venezuela, Sittingbourne,
Shell Research (Report No. TLGR.0032.68).
DAVIES, J.M. (1966a) Chlorinated insecticide content of the blood of
formulators in the CAPSA plant in Venezuela, Sittingbourne, Shell
Research (Report No. IRR TL/14/66).
DAVIES, J.M. (1966b) Concentration of chlorinated insecticides in
the whole blood of formulators of the CAPSA plant in Venezuela,
Sittingbourne, Shell Research (Report No. IRR TL/37/66).
EDWARDS, C.A. (1965) Effects of pesticide residues on soil
invertebrates and plants. In: Proceedings of the 5th Symposium of
the British Ecological Society, Ecology and Industrial Society.
EICHELBERGER, J.W. & LICHTENBERG, J.J. (1971) Persistence of
pesticides in river water. Environ. Sci. Technol., 5(6): 541-544.
ELGAR, K.E. (1965) Insecticide residues in samples of cottonseed
from Mexico, Sittingbourne, Shell International Chemical Company,
Ltd (Technical Memorandum 99/65).
ELGAR, K.E. (1966) Analysis of crops and soils for residues of the
soil insecticides aldrin and Telodrin. J. Sci. Food Agric., 17:
541-545.
ELGAR, K.E. & HOLLAND, A.A. (1967) Residues of organochlorine
insecticides in potatoes from Mexico, Sittingbourne, Shell
International Chemical Company, Ltd (Technical Service Note 82/67).
HARDEE, D.D., GUTENMANN, W.H., KEENAN, G.I., GYRISCO, G.G., LISK,
D.J., FOX, F.H., TRIMBERGER, G.W., & HOLLAND, R.F. (1964) Residues
of heptachlor epoxide and Telodrin in milk from cows fed at parts
per billion insecticide levels. J. econ. Entomol., 56(3): 404-407.
HATHWAY, D.E. (1965) The biochemistry of dieldrin and Telodrin.
Arch. environ. Health, 11: 380-388.
HATHWAY, D.E. & MALLINSON, A. (1964) Chemical studies in relation to
convulsive conditions: effect of Telodrin on the liberation and
utilization of ammonia in rat brain. Biochem. J., 90: 51-60.
HATHWAY, D.E., MALLINSON, A., & AKINTONWA, D.A.A. (1965) Effects of
dieldrin, picrotoxin, and Telodrin on the metabolism of ammonia in
brain. Biochem. J., 94: 676-686.
HODGE, H.C., BOYCE, A.M., DEICHMANN, W.B., & KRAYBILL, H.F. (1967)
Toxicology and no-effect levels of aldrin and dieldrin. Toxicol.
appl. Pharmacol., 10(3): 613-637.
HOOGENDAM, I., VERSTEEG, J.P.J., & VLIEGER, M., DE (1962)
Electroencephalograms in insecticide toxicity. Arch. environ.
Health, 4: 86-94.
HOOGENDAM, I., VERSTEEG, J.P.J., & VLIEGER, M., DE (1965) Nine years
toxicity control in insecticide plants. Arch. environ. Health, 10:
441-448.
HOWARD, J.A., BUXTON, J.A., ATER, F.B., OVERBECK, R.C., FOOTE, W.L.,
ROBINSON, R.F., & DAVIDSON, R.S. (1957) Final report on toxicology
of BAS-4402 to the Shell Development Corporation, Columbus, Ohio,
Battelle Memorial Institute.
HUDSON, R.H., TUCKER, R.K., & HAEGELE, M.A. (1984) Handbook of
toxicity of pesticides to wildlife. 2nd ed., Washington, DC, US
Department of the Interior, Fish and Wildlife Service (Resource
Publication No. 153).
HUGHES, D.G. (1965) Insecticide residues in cottonseed oil,
Sittingbourne, Shell International Chemical Company, Ltd (Technical
Memorandum 23/65).
IBRAHIM, T.M. (1964) A toxicological study of the action of the
insecticide dieldrin and related substances on the contraction of
striated muscle in the rat, University of Utrecht (Thesis).
INNES, J.R.M., ULLAND, B.M., VALERIO, M.G., PETRUCELLI, L.,
FISHBEIN, L., HART, E.R., PALLOTTA, A.J., BATES, R.R., FALK, H.L.,
GART, J.J., KLEIN, M., MITCHELL, I., & PETERS, J. (1969) Bioassay of
pesticides and industrial chemicals for tumorigenicity in mice: a
preliminary note. J. Natl Cancer Inst., 42(6): 1101-1114.
JAGER, K.W. (1970) Aldrin, dieldrin, endrin, and Telodrin: an
epidemiological and toxicological study of long-term occupational
exposure, Amsterdam, London, New York, Elsevier Science Publishers.
JAPANESE ENVIRONMENTAL AGENCY (1975) Environmental survey report on
chemical substances in FY 1974, Tokyo, Environmental Health
Department, Planning and Coordination Bureau (Unpublished report).
KADOUM, A.M. (1968) Application of the rapid micro method of sample
clean-up for gas chromatographic analysis of common organic
pesticides in ground water, soil, plant and animal extracts. Bull.
environ. Contam. Toxicol., 3(2): 65-70.
KAUL, R., KLEIN, W., & KORTE, F. (1970) [Contributions to ecological
chemistry. XX. Distribution, elimination, and metabolism of Telodrin
and heptachlor in rats and male rabbits, end-product of the
metabolism of heptachlor in mammals.] Tetrahedron, 26: 331-337 (in
German).
KELSEY, J.M. & ARLIDGE, E.Z. (1968) Effects of isobenzan on soil
fauna and soil structure. N. Z. J. agric. Res., 11: 245-260.
KERDIJK, H.N. (1981) Groundwater pollution by heavy metals and
pesticides from a dredge spoil dump. Stud. environ. Sci., 17:
279-286.
KODAVANTI, P.R.S., MEHROTRA, R.D., CHETTY, S.C., & DESAIAH, D.
(1988) Effect of selected insecticides on rat brain synaptosomal
adenylate cyclase and phosphodiesterase. J. Toxicol. environ.
Health, 25: 207-215.
KOEMAN, J.H. (1971) [The occurrence and toxicological implications
of some chlorinated hydrocarbons in the Dutch coastal area in the
period from 1965 to 1970], University of Utrecht, pp. 30-31 (Thesis)
(in Dutch).
KOEMAN, J.H., OSKAMP, A.A.G., VEEN, J., BROUWER, E., ROOTH, J.,
ZWART, P., VAN DE BROEK, E., & VAN GENDEREN, H. (1967) Insecticides
as a factor in the mortality of the sandwich tern (Sterna
sandvicensis): a preliminary communication. Meded. Landbouwwet.
Hogesch. Gent., 32: 841-854.
KOEMAN, J.H., VEEN, J, BROUWER, E., HUISMAN-DE BROUWER, L., &
KOOLEN, J.L. (1968) Residues of chlorinated hydrocarbon insecticides
in the North Sea environment. Helgoländer wiss. Meerunters., 17:
375-380.
KORTE, F. (1963) Review of metabolism studies with 14C-labelled
insecticides from 1958 to March 1963. Unpublished paper presented at
a Toxicological Meeting, New York, 25-27 March.
KORTE, F. (1967) Metabolism of 14C-labelled insecticides in
microorganisms, insects, and mammals. Botyu-Kagaku, 32(2): 46-59.
KORTE, F. & STIASNI, M. (1964) [Metabolism of 14C-Telodrin in
microorganisms and mosquito larvae.] Ann. Chem., 673: 146-152 (in
German).
LAWRENCE, L.J. & CASIDA, J.E. (1984) Interactions of lindane,
toxaphene, and cyclodienes with brain-specific
t-butylbicyclophosphorothionate receptor. Life Sci., 35: 171-178.
LAYTON, D.W., MALLON, B.J., ROSENBLATT, D.H., & SMALL, M.J. (1987)
Deriving allowable daily intakes for systemic toxicants lacking
chronic toxicity data. Regul. Toxicol. Pharmacol., 7: 96-112.
MCCASKEY, T.A., STEMP, A.R., LISKA, B.J., & STADELMAN, W.J. (1968)
Residues in egg yolks and raw and cooked tissues from laying hens
administered selected chlorinated hydrocarbon insecticides. Poultry
Sci., 47: 564-569.
MARLOW, R.G., BULL, M.S., & WILLIAMS, S. (1968) Residues of
organochlorine insecticides in foodstuffs from Spain, Sittingbourne,
Shell International Chemical Company, Ltd (Technical Service Report
WKTR.0058.68).
MATHEWS, B.L. (1969) Residues of organochlorine insecticides in
foodstuffs from India, Sittingbourne, Shell Research (Report No.
WKGR.0102.69).
MATHEWS, B.L. & COLE, E.R. (1966) Residues of Telodrin,
methylparathion, and DDT on tobacco from Costa Rica, Sittingbourne,
Shell International Chemical Company, Ltd (Technical Service Note
123/66).
MEHROTRA, B.D., BANSAL, S.K., & DESAIAH, D. (1982) Comparative
effects of structurally-related cyclodiene pesticides on ATPases. J.
appl. Toxicol., 2(6): 278-283.
MOEED, A. (1975) Effects of isobenzan, fensulfothion, and diazinon
on invertebrates and microorganisms. N. Z. J. exp. Agric., 3:
181-185.
MOSS, J.A. & HATHWAY, D.E. (1964) Transport of organic compounds in
the mammal. Partition of dieldrin and Telodrin between the cellular
components and soluble proteins of blood. Biochem. J., 91: 384-393.
MURPHY, M.W. & STANDEN, M.E. (1964) The translocation of Telodrin
into potatoes, Sittingbourne, Shell International Chemical Company,
Ltd (Technical Memorandum 184/64).
RIBBENS, P.H. (1985) Mortality study of industrial workers exposed
to aldrin, dieldrin and endrin. Arch. occup. environ. Health, 56(2):
75-79.
RICHARDSON, A., ROBINSON, J., BUSH, B., & DAVIES, J.M. (1967)
Determination of dieldrin (HEOD) in blood. Arch. environ. Health,
14(5): 703-708.
ROBINSON, J. & RICHARDSON, A.R. (1963) The distribution, storage,
and elimination of Telodrin in rats fed this insecticide in their
diet, Sittingbourne, Shell Research (Report No. R(T)-1-63).
SCHAFER, E.W. & BRUNTON, R.B. (1979) Indicator bird species for
toxicity determinations: is the technique usable in test method
development? In: Beck, J.R., ed. Vertebrate pest control and
management materials, Philadelphia, American Society for Testing and
Materials, pp. 157-168 (ASTM STP 680).
SHELL (1963) An episode of pesticide contamination of pasture and
dairy products - Isobenzan in Victoria, Australia, in 1963.
Melbourne, Shell Chemical Australia (Unpublished report No.
90111501WP5).
SHELL (1964) Telodrin residues. A summary, London, Shell
International Chemical Company (Unpublished report).
SPYNU, E.I. (1964) On the toxicology of new organic chloride
insecticides obtained by diene synthesis on the basis of
hexachlorocyclopentadiene. Gig. Tr. prof. Zabol., 4: 30-35.
SRIVASTAVA, S.C. (1966) The effect of Telodrin on nitrification of
ammonia in soil and its implication on nitrogen nutrition of
sugarcane. Plant Soil, 25(3): 471-473.
STANDEN, M.E. & ELGAR, K.E. (1965) Telodrin residues in dairy
products from Venezuela, Sittingbourne, Shell International Chemical
Company, Ltd (Technical Memorandum 36/65).
STEMP, A.R. & LISKA, B.J. (1966) Effects of processing and storage
of dairy products on Telodrin and methoxychlor residues. J. diary
Sci., 49: 1006-1008.
STEVENSON, D.E. (1964) The toxicology of Telodrin. Meded. Fac.
Landbouwwet. Rijksuniv. Gent, 29: 1198-1207.
SUZUKI, K., NAGAYOSHI, H., & KASHIWA, T. (1974) The systematic
separation and identification of pesticides in the first division.
Agric. biol. Chem., 38(2): 279-285.
THOMPSON, A.R. (1973) Persistence of biological activity of seven
insecticides in soil assayed with Folsomia candida. J. econ.
Entomol., 66(4): 855-857.
TRUDGILL, P.W. & WIDDUS, R. (1970) Effects of chlorinated
insecticides on metabolic processes in bacteria. Biochem. J., 118:
48-49.
TUINSTRA, L.G.M.TH. & TRAAG, W.A. (1979) Automated glass capillary
gas chromatographic analysis of PCB and organochlorine pesticide
residues in agricultural products. J. high Resolut. Chromatogr.
Chromatogr. Commun., 2(2): 723-728.
US EPA (1987) Acute toxicity handbook of chemicals to estuarine
organisms. Springfield, Virginia, US Department of Commerce,
National Technical Information Service (EPA/600/8-87/017).
VAN WIJNEN, J.H. & STIJKEL, A. (1988) Health risk assessment of
residents living on harbour sludge. Int. Arch. occup. environ.
Health, 61: 77-87.
VERMA, M.P., BAHGA, H.S., & SONI, B.K. (1967) Effect of prolonged
administration of the insecticide Telodrin in poultry. Indian vet.
J., 44: 962-966.
VERSTEEG, J.P.J. & JAGER, K.W. (1973) Long-term occupational
exposure to the insecticides aldrin, dieldrin, endrin, and Telodrin.
Br. J. ind. Med., 30: 201-202.
WARE, G.W. & GOOD, E.E. (1967) Effects of insecticides on
reproduction in the laboratory mouse. II. Mirex, Telodrin, and DDT.
Toxicol. appl. Pharmacol., 10(1): 54-61.
WEGMAN, R.C.C. & GREVE, P.A. (1980) Halogenated hydrocarbons in
Dutch water samples over the years 1969-77. Environ. Sci. Res., 16:
405-415.
WEGMAN, R.C.C. & HOFSTEE, A.W.M. (1982) Determination of
organochlorines in river sediment by capillary gas chromatography.
Water Res., 16: 1265-1272.
WEGMAN, R.C.C., HOFSTEE, A.W.M., & GREVE, P.A. (1981) Uptake of
organochlorines by plants growing on river and basin sediment.
Meded. Fac. Landbouwwet. Rijksuniv. Gent, 46(1): 359-365.
WORDEN, A.N. (1969) Toxicity of Telodrin. Toxicol. appl. Pharmacol.,
14: 556-573.
ZAVON, M.R. (1961) Toxicology and pharmacology of Telodrin
insecticide (compound SD 4402), Cincinnati, Ohio, The Kettering
Laboratory (Unpublished report).
RESUME ET EVALUATION; CONCLUSIONS ET RECOMMANDATIONS
1. Résumé et évaluation
Autant qu'on sache, l'isobenzan, un insecticide organochloré
n'a été fabriqué que pendant la période 1958-1965. Plusieurs années
aprés, on puisait toujours sur les stocks existants. A l'heure
actuelle, les seules sources importantes d'exposition sont
vraisemblablement les sites de décharge initiaux de déchets
industriels ou les boues de dragage provenant de sédiments
contaminés.
Une fois épandu sur le sol, la majeure partie de l'isobenzan
disparaît rapidement. Après quoi, la fraction restante se décompose
beaucoup plus lentement. Elle persiste dans le sol de deux à sept
ans selon la nature de celui-ci. Au laboratoire, l'isobenzan se
décompose dans les eaux de surface en l'espace de quelques semaines
lorsqu'il est exposé à la lumière naturelle ou artificielle.
Le sol, les eaux souterraines et superficielles provenant des
polders constitués de sédiments contaminés par des organochlorés,
notamment des dérivés cyclodiéniques, contenaient encore plusieurs
années après, de faibles résidus d'isobenzan. En 1979-1980, on n'a
pas détecté d'isobenzan (limite de détection 0,01 mg/kg de poids
sec) dans les sédiments des cours d'eau des Pays-Bas. Après
traitement du sol, les résidus qui subsistent sur les récoltes sont
généralement faibles (inférieurs à 0,05 mg/kg de végétaux), mais on
peut en trouver des quantités plus fortes sur certaines racines
(jusqu'à 0,2 mg/kg dans les carottes). Des enquêtes de type "panier
de la ménagère" effectuées lorsqu'on utilisait de l'isobenzan en
agriculture, n'ont pas permis de déceler de résidus dans les denrées
contrôlées (moins de 0,01 mg/kg).
Chaque fois qu'on a laissé paître des bovins dans des pâturages
traités par de l'isobenzan, on a constaté que les produits laitiers
obtenus contenaient des résidus de cet insecticide. C'est ainsi que
dans deux échantillons de beurre, on a trouvé 0,07 et 0,15 mg
d'isobenzan par kg de produit, les concentrations dans le lait
entier allant de 0,005 à 0,07 mg/kg. Le lait en poudre n'en
contenait toutefois que 0,005 mg/kg. Lors du traitement industriel
des produits laitiers, plus de 50% du résidu disparaît selon le type
de traitement.
On ne dispose d'aucune donnée sur les quantités d'isobenzan
présentes dans le sang ou les tissus adipeux de la population
générale. Des travailleurs exposés à l'isobenzan lors de la
fabrication ou de la formulation de cet insecticide, présentaient
des taux sanguins (sang total) allant jusqu'à 0,041 mg/litre. Dans
les échantillons de sang total prélevés sur des personnes vivant à
proximité d'une unité de production, la concentration d'isobenzan
était inférieure à la limite de détection (0,001 mg/litre).
L'isobenzan est facilement résorbé par la paroi du tube
digestif et il passe dans le sang sans modification. Il se forme des
métabolites hydrophiles et notamment une lactone. L'isobenzan
s'accumule dans les tissus et les organes des rats et des chiens
selon l'ordre: graisses > foie = muscle > cerveau > sang. Les
concentrations tissulaires chez les rattes sont généralement plus
faibles que chez les rats, spécialement dans les graisses. La
demi-vie biologique dans le tissu adipeux était ainsi de 10,9 jours
chez les rats et de 16,6 jours chez les rattes. Chez un chiot
femelle dont le sang contenait 0,09 mg d'isobenzan par litre, on a
observé des convulsions 15 jours après la naissance. L'animal
n'avait été nourri qu'avec le lait de sa mère, une chienne Beagle à
qui l'on avait fait absorber de l'isobenzan et dont le lait en
contenait 0,7 mg/litre. Des effets analogues sur les petits ont été
observés lors d'une étude de reproduction sur le rat. Chez la vache,
l'isobenzan est excrété dans le lait.
Les larves de moustiques et les champignons terricoles
métabolisent l'isobenzan de la même manière que les vertébrés,
notamment sous forme de lactone. L'isobenzan est très persistant
dans l'environnement et s'accumule dans les organismes vivants. Il
est extrêmement toxique pour les poissons, les crevettes et les
oiseaux. Aux Pays-Bas, pays où l'on fabriquait l'isobenzan, on a
trouvé des résidus dans des oeufs de sternes vivant sur la côte
hollandaise qui atteignaient 0,45 mg/kg (moyenne, 0,09 mg/kg). Dans
les moules et le poisson, les résidus moyens étaient de 0,05 mg/kg
en 1965. Dans les parcelles traitées par de l'isobenzan à raison de
2 kg/ha, on a constaté une réduction du nombre de lombrics. Il y
avait réduction de la nitrification avec accroissement corrélatif de
l'azote minéral dans les sols traités par l'isobenzan à raison
d'1 kg/ha; en revanche, les études en laboratoire n'ont mis en
évidence aucun effet sur la nitrification à des doses correspondant
à 250 g/ha.
L'isobenzan présente une forte toxicité aiguë pour les
mammifères, que ce soit par la voie orale ou par la voie percutanée.
Le mode d'action de l'isobenzan consiste en une stimulation
excessive du système nerveaux central conduisant à des convulsions.
La toxicité aiguë des diverses formulations d'isobenzan correspond à
la proportion de matière active.
L'isobenzan n'est pas irritant pour la peau mais certaines de
ses formulations peuvent l'être.
Des études limitées à court et à long terme, au cours
desquelles on a administré par voie orale de l'isobenzan à des
souris, à des rats et à des chiens, ont montré que ce composé
pouvait induire des lésions histologiques au niveau du foie, du type
de celles qu'on observe classiquement avec les organochlorés. Une
étude de longue durée sur des rats a permis de déterminer que la
dose sans effet observable était de 5 mg/kg de nourriture (soit
l'équivalent de 0,25 mg/kg de poids corporel). Chez le chien, la
dose sans effet nocif observable, déterminée à la suite d'une étude
de deux ans, était de 0,025 mg/kg de poids corporel.
Une étude de reproduction portant sur une génération de rats a
montré que la dose sans effet nocif observable était de 0,1 mg/kg de
nourriture (soit l'équivalent de 0,05 mg/kg de poids corporel. A la
dose de 1 mg/kg de nourriture (soit l'équivalent de 0,05 mg/kg de
poids corporel), il y a eu réduction de la survie des ratons.
Aucune étude de tératogénicité ni de mutagénicité n'a été
rapportée.
Une étude de deux ans sur des rats (administration par voie
orale) et une étude sur des souris n'ont pas permis de mettre en
évidence un pouvoir cancérogène quelconque, mais ces études
n'étaient pas adaptées à une telle évaluation.
La base de données toxicologiques sur l'isobenzan est
incomplète. En général on estime que, d'après les critères actuels,
les données sont d'une qualité médiocre et sont en tous cas
insuffisantes pour permettre une évaluation du risque que ce composé
présente pour la santé humaine ou l'environnement.
Les données concernant l'exposition humaine se limitent à des
études effectuées sur des travailleurs d'une usine hollandaise qui
étaient employés à la fabrication et à la formulation d'isobenzan et
d'insecticides cyclodiéniques apparentés. Aucun cas d'irritation
cutanée n'a été signalé. Dans plusieurs cas d'intoxication, on a
observé des convulsions mais les anomalies du tracé
électro-encéphalographique étaient réversibles. Le seuil limite
d'intoxication (pour les convulsions), a été estimé à 0,015 mg
d'isobenzan par litre de sang et la demi-vie biologique de ce
composé dans le sang humain est, semble-t-il, de l'ordre de 2,8
années.
2. Conclusions et recommandations
L'isobenzan est extrêmement toxique et très persistant. Les
données dont on dispose sur le danger qu'il représente sont
incomplètes mais néanmoins suffisantes pour montrer que ce danger
est réel pour les personnes qui le manipulent ainsi que pour
l'environnement, de sorte qu'il faut éviter toute contamination
humaine ou environnementale qui résulterait de l'utilisation de ce
produit comme insecticide ou autre.
RESUMEN Y EVALUACION; CONCLUSIONES Y RECOMENDACIONES
1. Resumen y evaluación
Según los datos de que se dispone, el isobenzano, un
insecticida organoclorado, sólo se fabricó durante el periodo
1958-1965. Durante los años siguientes se utilizaron las existencias
almacenadas. En la actualidad, se cree que las únicas fuentes
importantes de exposición son los lugares donde originalmente se
evacuaron los desechos industriales o los materiales dragados de
sedimentos contaminados.
Cuando se aplica el isobenzano al suelo, se produce una rápida
pérdida inicial; después, el resto del compuesto se degrada mucho
más despacio. Persiste en el suelo durante 2 a 7 años, según el tipo
de suelo. En condiciones de laboratorio, el isobenzano se descompone
en las aguas de superficie en pocas semanas cuando se expone a la
luz natural o artificial.
El suelo, las aguas subterráneas y las aguas superficiales de
pólders construidos con sedimentos contaminados por sustancias
organocloradas, inclusive compuestos de ciclodieno clorado, aún
contenían pequeños residuos de isobenzano algunos años después. En
1979-1980, no se detectó isobenzano (límite de detección: 0,01 mg/kg
de peso seco) en el sedimento de ríos de los Países Bajos. Tras el
tratamiento del suelo, los residuos en las cosechas suelen ser bajos
(menos de 0,05 mg/kg de cosecha), pero pueden encontrarse
concentraciones superiores en algunos tubérculos (hasta 0,2 mg/kg en
zanahorias). En las encuestas de mercado realizadas durante la época
de uso agrícola del isobenzano no se detectaron residuos en los
alimentos analizados (menos de 0,01 mg/kg).
En los productos lácteos procedentes de ganado que se alimentó
en pastos tratados con isobenzano se encontraron residuos del
insecticida. Dos muestras de mantequilla contenían 0,07 y 0,15 mg de
isobenzano/kg de producto, mientras que los niveles en la leche
entera fueron de 0,005 mg/kg a 0,07 mg/kg. En la leche deshidratada,
no obstante, se encontraron sólo 0,005 mg/kg. Durante la elaboración
de los productos lácteos se perdía hasta el 50% del residuo, según
el tipo de tratamiento.
No se dispone de datos sobre los niveles de isobenzano en la
sangre o el tejido adiposo de la población general. Los operarios
expuestos al isobenzano en las plantas de fabricación y elaboración
presentaron niveles medios en sangre entera de hasta 0,041 mg/litro.
En muestras de sangre entera procedente de personas que vivían en
las proximidades de una de las plantas, la concentración de
isobenzano estaba por debajo del límite de detección
(0,001 mg/litro).
El isobenzano es absorbido rápidamente a través de la pared
gastrointestinal y es transportado por la sangre sin alteraciones.
Se forman metabolitos hidrófìlos de los que se ha identificado uno,
la lactona de isobenzano. El isobenzano se acumula en los tejidos y
los órganos de ratas y perros en el orden siguiente: grasa > hígado
= músculo > cerebro > sangre. Las concentraciones tisulares en las
hembras de rata son en general superiores a las que aparecen en los
machos, sobre todo en la grasa. En la rata se determinó que la
semivida biológica en la grasa del organismo es de 10,9 días en los
machos y 16,6 días en las hembras. En un cachorro hembra de perro,
cuya sangre contenía 0,09 mg de isobenzano/litro, se observaron
convulsiones a los 15 días del nacimiento. El cachorro sólo se había
alimentado de leche de su madre, una Beagle a la que se había
administrado isobenzano y cuya leche contenía 0,7 mg/litro. En un
estudio de reproducción en ratas se observaron efectos similares en
las crías. Las vacas excretan isobenzano con la leche.
Las larvas de mosquito y los hongos del suelo metabolizan el
isobenzano del mismo modo que los vertebrados, dando lactona de
isobenzano como metabolito.
El isobenzano es muy persistente en el medio ambiente y se
bioacumula. Es sumamente tóxico para los peces, los camarones y las
aves. En los Países Bajos, país en el que se fabricaba el
isobenzano, los residuos encontrados en los huevos de golondrinas de
mar de las costas holandesas ascendieron a 0,45 mg/kg (promedio:
0,09 mg/kg), mientras que el promedio de residuos encontrados en
mejillones y peces fue de 0,05 mg/kg en 1965. Se observó una
disminución del número de lombrices en terrenos tratados con
isobenzano a razón de 2 kg/ha. Se redujo la nitrificación, con el
consiguiente aumento del nitrógeno inorgánico, en los suelos
tratados con isobenzano sobre el terreno a razón de 1 kg/ha, si bien
en estudios de laboratorio no se demostró efecto alguno en la
nitrificación con dosis equivalentes a 250 g/ha.
La toxicidad aguda del isobenzano para los mamíferos es elevada
por las vías oral y percutánea. La forma de acción de su toxicidad
es la sobreestimulación del sistema nervioso central, que da lugar a
convulsiones. La toxicidad aguda de las preparaciones de isobenzano
refleja el porcentaje de ingrediente activo presente.
Aunque el isobenzano no irrita la piel, algunos productos
preparados a partir de él pueden causar irritación.
En estudios limitados a corto y largo plazo de administración
oral realizados en ratones, ratas y perros se ha demostrado que el
isobenzano puede provocar en el hígado cambios histológicos que
reponden al tipo clásico de intoxicación por compuestos
organo-clorados. En un estudio realizado a largo plazo en ratas, se
determinó un nivel efectos sin observados de 5 mg/kg de dieta
(equivalente a 0,25 mg/kg de peso corporal), y en un estudio en
perros de dos años de duración se determinó un nivel sin observación
de efectos adversos de 0,025 mg/kg de peso corporal.
En un estudio de reproducción en una generación de ratas se
obtuvo un nivel sin efectos adversos observados de 0,1 mg/kg de
dieta (equivalente a 0,05 mg/kg de peso corporal). Con un nivel de
1 mg/kg de dieta (equivalente a 0,05 mg/kg de peso corporal)
disminuyó la supervivencia de las crías.
No se han comunicado estudios sobre la teratogenicidad ni la
mutagenicidad del compuesto.
No se observó potencial carcinogénico en un estudio de
administración oral a ratas durante dos años ni en un estudio de
administración oral a ratones, pero esos estudios no eran apropiados
para evaluar la carcinogenicidad.
La base de datos toxicológicos correspondiente al isobenzano es
incompleta. En general, actualmente se considera que la calidad de
los datos es mediocre e insuficiente para evaluar los riesgos que
representa para la salud humana o el medio ambiente.
Los datos sobre personas expuestas se limitan a estudios
realizados en trabajadores de una fábrica de los Países Bajos
durante la fabricación y la elaboración de preparaciones de
isobenzano y otros insecticidas de ciclodieno clorado afines. No se
comunicaron casos de irritación cutánea. En varios casos de
intoxicación, se produjeron convulsiones pero los cambios del
trazado electroencefalográfico resultaron ser reversibles. El nivel
umbral de intoxicación (en el caso de las convulsiones) se estimó en
0,015 mg de isobenzano/litro de sangre, y se calculó que la semivida
biológica del isobenzano en la sangre humana es del orden de 2,8
años.
2. Conclusiones y recomendaciones
El isobenzano es sumamente tóxico y muy persistente. La
información de que se dispone sobre los riegos del isobenzano es
incompleta, pero, aún así, basta para indicar que el riesgo que
supone para los que lo manipulan y para el medio ambiente es tan
elevado que no debería permitirse la exposición humana o del medio
ambiente a esta sustancia, ya sea utilizada como insecticida o para
cualquier otro fin.
