
INTERNATIONAL PROGRAMME ON CHEMICAL SAFETY
ENVIRONMENTAL HEALTH CRITERIA 80
PYRROLIZIDINE ALKALOIDS
This report contains the collective views of an international group of
experts and does not necessarily represent the decisions or the stated
policy of either the World Health Organization or the United Nations
Environment Programme
Published under the joint sponsorship of
the United Nations Environment Programme
and the World Health Organization
World Health Organization
Geneva 1988
ISBN 92 4 154280 2
(c) World Health Organization 1988
Publications of the World Health Organization enjoy copyright
protection in accordance with the provisions of Protocol 2 of the
Universal Copyright Convention. For rights of reproduction or
translation of WHO publications, in part or in toto, application
should be made to the Office of Publications, World Health
Organization, Geneva, Switzerland. The World Health Organization
welcomes such applications.
The designations employed and the presentation of the material in
this publication do not imply the expression of any opinion whatsoever
on the part of the Secretariat of the World Health Organization
concerning the legal status of any country, territory, city or area or
of its authorities, or concerning the delimitation of its frontiers or
boundaries.
The mention of specific companies or of certain manufacturers'
products does not imply that they are endorsed or recommended by the
World Health Organization in preference to others of a similar nature
that are not mentioned. Errors and omissions excepted, the names of
proprietary products are distinguished by initial capital letters.
CONTENTS
ENVIRONMENTAL HEALTH CRITERIA FOR PYRROLIZIDINE ALKALOIDS
PREFACE
INTRODUCTION - PYRROLIZIDINE ALKALOIDS AND HUMAN HEALTH
1. SUMMARY AND RECOMMENDATIONS
1.1. Summary
1.2. Sources and chemical structure
1.3. Mechanisms and features of toxicity
1.4. Effects on man
1.4.1. Nature and extent of health risks
1.5. Methods for prevention
1.6. Recommendations
1.6.1. General recommendations
1.6.2. Recommendations for research
2. PROPERTIES AND ANALYTICAL METHODS
2.1. Chemical structure and properties
2.2. Analytical methods
2.2.1. Extraction
2.2.1.1 Plant tissue
2.2.1.2 Biological fluids and tissues
2.2.2. Analysis for pyrrolizidine alkaloids
2.2.2.1 Thin-layer chromatography (TLC)
2.2.2.2 High-performance liquid chromatography
(HPLC)
2.2.2.3 Gas chromatography (GC) and mass
spectrometry (MS)
2.2.2.4 Nuclear magnetic resonance (NMR)
spectrometry
2.2.2.5 The Ehrlich reaction
2.2.2.6 Indicator dyes
2.2.2.7 Direct weighing
2.3. Determination of metabolites in animal tissues
3. SOURCES AND PATHWAYS OF EXPOSURE
3.1. Hepatotoxic pyrrolizidine alkaloids and their sources
3.2. Pneumotoxic and other toxic pyrrolizidine alkaloids
3.3. Pathways of exposure
3.3.1. Contamination of staple food crops
3.3.2. Herbal infusions
3.3.3. Use of PA-containing plants as food
3.3.4. Contaminated honey
3.3.5. Milk
3.3.6. Meat
3.3.7. Use of PAs as chemotherapeutic agents for cancer
4. METABOLISM
4.1. Absorption, excretion, and tissue distribution
4.1.1. Absorption
4.1.2. Excretion and distribution
4.2. Metabolic routes
4.2.1. Hydrolysis
4.2.2. N-oxidation
4.2.3. Conversion to pyrrolic metabolites
4.3. Effects of treatments affecting metabolism
4.4. Other factors affecting metabolism
4.5. Other metabolic routes
4.6. Metabolism of pyrrolizidine N-oxides
4.7. Metabolism in man
5. MECHANISMS OF TOXICITY AND OTHER BIOLOGICAL ACTIONS
5.1. Metabolites responsible for toxicity
5.1.1. Metabolic basis of toxicity
5.1.2. Isolation of pyrrolic metabolites
5.1.3. Chemical aspects of pyrrolic metabolites
5.1.3.1 Preparation
5.1.3.2 Chemistry associated with toxic actions
5.1.4. Possible further metabolites
5.2. Toxic actions of pyrrolic metabolites
5.2.1. Animals
5.2.1.1 Pyrrolic esters (dehydro-alkaloids)
5.2.1.2 Pyrrolic alcohols (dehydro-necines)
5.2.2. Cell cultures
5.2.3. Possible participation of membrane lipid
peroxidation
5.3. Chemical and metabolic factors affecting toxicity
5.3.1. Structural features of a toxic alkaloid
5.3.2. Activation and detoxication
5.3.3. Factors affecting the toxicity of active
metabolites
5.3.3.1 Reactivity of the metabolite
5.3.3.2 The number of reactive groups
5.4. Metabolites associated with the biological actions of
pyrrolizidine alkaloids
5.4.1. Acute hepatotoxicity
5.4.2. Chronic hepatotoxicity
5.4.3. Pneumotoxicity
5.4.4. Toxicity in other tissues
5.4.5. Carcinogenicity
5.4.6. Antitumour activity
5.5. Prevention and treatment of pyrrolizidine poisoning
5.5.1. Modified diets
5.5.2. Pre-treatment to enhance the detoxication of active
metabolites
5.5.3. Other treatments
6. EFFECTS ON ANIMALS
6.1. Patterns of disease caused by different plant genera and
of organ involvement in different species
6.2. Field observations - outbreaks in farm animals
6.3. Studies on farm animals
6.4. Experimental animal studies
6.4.1. Effects on the liver
6.4.1.1 Relative hepatotoxicity of different PAs
and their N-oxides
6.4.1.2 Factors affecting hepatotoxicity
6.4.1.3 Acute effects
6.4.1.4 Mechanism of toxic action
6.4.1.5 Chronic effects
6.4.2. Effects on the lungs
6.4.2.1 Acute effects
6.4.2.2 Chronic effects
6.4.2.3 Mechanisms of toxic action
6.4.3. Effects on the central nervous system
6.4.4. Effects on other organs
6.4.5. Teratogenicity
6.4.6. Fetotoxicity
6.4.7. Mutagenicity
6.4.7.1 Chromosome damage
6.4.8. Carcinogenesis
6.4.8.1 Purified alkaloids
6.4.8.2 Plant materials
6.4.8.3 Pyrrolizidine alkaloid metabolites and
analogous synthetic compounds
6.4.8.4 Molecular structure and carcinogenic
activity
6.4.9. Antimitotic activity
6.4.10. Immunosuppression
6.4.11. Effects on mineral metabolism
6.4.12. Methods for the assessment of chronic
hepatotoxicity and pneumotoxicity
6.5. Effects on wild-life
6.5.1. Deer
6.5.2. Fish
6.5.3. Insects
7. EFFECTS ON MAN
7.1. Clinical features of veno-occlusive disease (VOD)
7.2. Salient pathological features of veno-occlusive disease
7.3. Human case reports of veno-occlusive disease
7.4. VOD and cirrhosis of the liver
7.5. Differences between VOD and Indian childhood cirrhosis
(ICC)
7.6. Chronic lung disease
7.7. Trichodesma poisoning
7.8. Relationship between dose level and toxic effects
7.9. Pyrrolizidine alkaloids as a chemotherapeutic agent for
cancer
7.10. Prevention of poisoning in man
8. BIOLOGICAL CONTROL
9. EVALUATION OF HUMAN HEALTH RISKS AND EFFECTS ON THE ENVIRONMENT
9.1. Human exposure conditions
9.1.1. Reported sources of human exposure
9.1.2. Plant species involved
9.1.3. Modes and pathways of exposure
9.1.3.1 Contamination of grain crops
9.1.3.2 Herbal medicines
9.1.3.3 PA-containing plants used as food and
beverages
9.1.3.4 Other food contaminated by PAs
9.1.4. Levels of intake
9.2. Acute effects of exposure
9.2.1. Acute liver disease
9.3. Chronic effects of exposure
9.3.1. Cirrhosis of the liver
9.3.2. Mutagenicity and teratogenicity
9.3.3. Cancer of the liver
9.3.4. Effects on other organs
9.4. Effects on the environment
9.4.1. Agriculture
9.4.2. Wild-life
9.4.3. Insects
9.4.4. Soil and water
REFERENCES
APPENDIX I. PYRROLIZIDINE ALKALOIDS AND THEIR PLANT SOURCES
APPENDIX II.
NOTE TO READERS OF THE CRITERIA DOCUMENTS
Every effort has been made to present information in the
criteria documents as accurately as possible without unduly
delaying their publication. In the interest of all users of the
environmental health criteria documents, readers are kindly
requested to communicate any errors that may have occurred to the
Manager of the International Programme on Chemical Safety, World
Health Organization, Geneva, Switzerland, in order that they may be
included in corrigenda, which will appear in subsequent volumes.
* * *
ENVIRONMENTAL HEALTH CRITERIA FOR PYRROLIZIDINE ALKALOIDS
A WHO Task Group on Environmental Health Criteria for
Pyrrolizidine Alkaloids met in Tashkent, USSR, on 1 - 5 December
1986. Dr M. Gounar opened the meeting on behalf of the three
co-sponsoring organizations of the IPCS (UNEP/ILO/WHO). The Task
Group reviewed and revised the draft criteria document and made an
evaluation of the health risks of exposure to pyrrolizidine
alkaloids.
Access to the original papers on the subject published in the
USSR was made possible by PROFESSOR M. ABDULLAHODJAEVA. DR A.R.
MATTOCKS wrote the first drafts of the sections on Properties and
Analytical Methods, Metabolism, and Mechanisms of Toxicity and
Other Biological Actions. DR C.C.J. CULVENOR, assisted PROFESSOR
H.D. TANDON in the finalization of the document after the Task
Group meeting. Dr J. Parizek, who was originally the IPCS staff
member responsible for the preparation of the document, and was to
be Secretary of the Task Group, could not attend the meeting
because of sudden illness, and the Task Group was assisted in his
place by Dr M. Gounar, former IPCS staff member. Dr A. Prost was
responsible for the final version of the document.
The Secretariat acknowledge the help of both Professor H.D.
Tandon and Dr C.C.J. Culvenor. The Task Group meeting in Tashkent
was organized by the Centre of International Projects, USSR State
Committee for Science and Technology.
The efforts of all who helped in the preparation and
finalization of the document are gratefully acknowledged.
* * *
Partial financial support for the publication of this criteria
document was kindly provided by the United States Department of
Health and Human Services, through a contract from the National
Institute of Environmental Health Sciences, Research Triangle Park,
North Carolina, USA - a WHO Collaborating Centre for Environmental
Health Effects.
* * *
A comprehensive data base on pyrrolizidine alkaloids has been
made available by CSIRO Division of Animal Health, Private Bag
No. 1, Parkville, Vic. 3052, Australia. The data base consists of
alkaloid occurrence tables and keyworded bibliography readable by
SCI-MATE software system (Bibliographic Manager, Institute for
Scientific Information), but adaptable to other systems. It is
available from CSIRO on IBM - PC diskettes; price on application to
L.W. Smith.
PREFACE
A disease caused by the consumption of plants containing
pyrrolizidine alkaloids (PAs) has been recognized independently as
an endemic disease in certain parts of the West Indies and in
Uzbekistan in the USSR. Outbreaks of the disease have affected
significant segments of populations or large numbers of people in
geographically confined areas in Afghanistan, India, and
Uzbekistan. The outbreaks have been caused through contamination
of the staple food crops with the seeds of plants containing PAs,
growing among the crops; such plants are likely to thrive following
periods of drought.
It is notable that the same family of plants that caused
endemic disease and large-scale outbreaks in Uzbekistan also caused
another outbreak of the disease in adjacent Afghanistan, long after
the chemical etiology of the disease (through consumption of toxic
seeds in the food) had been identified in the USSR. This happened
because there was a lack of general awareness of the causal
relationship between the chemical present in the plant and the
disease. Sporadic cases continue to occur in different parts of
the world through the consumption of seeds or plant parts
containing toxic PAs, as home remedies, beverages, or food.
The IPCS recognized that this was a health problem that might
be lethal, and that it was entirely preventable, provided that it
was recognized in time. It was also recognized that the
dissemination of knowledge, about both the disease and the sources
of the chemicals involved, would be a critical step in its
prevention.
Accordingly, the IPCS invited Professor H.D. Tandon, who was
responsible for establishing such a causal relationship in the
outbreaks in Afghanistan and India, to prepare a draft criteria
document and to assist in its further development and finalization
after the Task Group meeting, which was held in Tashkent, USSR, on
1 - 5 December, 1986.
In most episodes of toxic human disease caused by PAs, the
liver has been the principal target organ, except for an outbreak
in the USSR caused by Trichodesma alkaloids, in which the symptoms
were mostly extra-hepatic. The Environmental Health Criteria
document provides comprehensive coverage of the hepatotoxic PAS,
but lack of relevant documentation prevented the Task Group from
analysing the role of Trichodesma alkaloids in detail.
INTRODUCTION - PYRROLIZIDINE ALKALOIDS AND HUMAN HEALTH
Pyrrolizidine alkaloids (PAs) are found in plants growing in
most environments and all parts of the world. The main sources are
the families Boraginaceae (all genera), Compositae (tribes
Senecionae and Eupatoriae), and Leguminosae (genus Crotalaria), and
the potential number of alkaloid-containing species is as high as
6000, or 3% of the world's flowering plants (Culvenor, 1980). They
have long been known to be a health hazard for livestock, at least
since 1902 (Schoental, 1963), and loss of livestock in various
parts of the world has been traced to their grazing on certain
plants growing in pastures, especially following periods of drought
or in arid climates. They have been found to be toxic for all
species of animals tested (Schoental, 1963), though some species,
notably the guinea-pig, are resistant (Chesney & Allen, 1973a;
White et al., 1973). Human disease caused by PA toxicity has been
known to be endemic in the central Asian republics of the USSR, at
least since the early thirties (Ismailov, 1948a,b; Mnushkin, 1949)
when several outbreaks occurred, and the cause was discovered to be
the seeds of plants of Heliotropium species (Dubrovinskii, 1947,
1952; Khanin, 1948), which contaminated the staple food crops. A
spate of reports followed, mostly from the West Indies, of acute
and chronic liver disease (Bras et al., 1954, 1961; Bras & Hill,
1956; Stirling et al., 1962), associated with the ingestion by
people of herbal infusions for the treatment of certain ailments.
Schoental (1961) and Davidson (1963) suggested that, in view of the
evidence of the hepatotoxicity of PAs, consumption of plants
containing them could be of etiological significance in human liver
disease, especially in developing countries where they are consumed
as food or herbal medicines. In spite of this, and the fact that
such an ubiquitous source of toxic material is capable of producing
animal and human disease and that there have been more recent
reports, the PAs have not attracted much attention in the world as
a health hazard. In fact, a recent handbook on naturally occurring
toxic agents in food (Rechicigl, Jr, 1983) refers to them only in
passing and makes no mention of human disease caused by them.
Veno-occlusive disease (VOD) (Bras & Hill, 1956), which is
characterized by the dominant occlusive lesion of the centrilobular
veins of the liver lobule and is caused by these alkaloids, has
since been reported from all parts of the world, in both man and
animals (Hill, 1960; Bras, 1973). It has been attributed to the
accidental contamination of food by toxic plant products or the
ingestion of herbal infusions. There have been reports of stray
cases and of small outbreaks from both developing and developed
countries. However, in the most recent studies from Afghanistan
(Tandon & Tandon, 1975; Mohabbat et al., 1976; Tandon, B.N. et al.,
1978; Tandon, H.D. et al., 1978) and India (Tandon, B.N. et al.,
1976; Tandon, R.K. et al., 1976; Krishnamachari et al., 1977;
Tandon, H.D. et al., 1977; Tandon, B.N. et al., 1978), the disease
has been reported to affect large masses of the population,
resulting in high mortality, and has been attributed to the
accidental contamination of their staple food crops by PA-
containing seeds of plants, following periods of drought.
There is conclusive evidence from studies on experimental
animals that the effects of a single exposure to PAs may progress
relentlessly to advanced chronic liver disease and cirrhosis
(Schoental & Magee, 1957, 1959; Nolan et al., 1966), following a
long interval of apparent well-being, and without any other latent
or provocative factor (Schoental & Magee, 1959). The lowest levels
of such alkaloids administered thus far to experimental animals,
e.g., 1 - 4 mg/kg diet, have produced chronic liver disease and
tumours (Hooper & Scanlan, 1977; Culvenor & Jago, 1979).
Pyrrolizidine alkaloids have also been shown to act synergistically
with aflatoxin, another environmental toxin present in agricultural
products, in causing cirrhosis and hepatoma in primates (Lin et
al., 1974). Though there is no conclusive evidence yet of a
carcinogenic role of PAs in man, such a possibility has been
suspected on the basis of experimental data (Hill, 1960; Williams
et al., 1967; IARC, 1976, 1983; Huxtable, 1980; Culvenor, 1983),
and experimental studies have demonstrated carcinogenicity in rats
given dosages equivalent to those reported to have been ingested in
human cases (Cook et al., 1950; Culvenor, 1983).
Alkaloids/toxic metabolites have been shown to be secreted in
the milk of lactating dairy cattle (Dickinson et al., 1976) and
rats, and the young of both sexes have been shown to suffer toxic
damage, even when suckled by mothers treated with retrosine, who
apparently are not affected themselves (Schoental, 1959). Such
suckling animals may also be in apparent good health while the
livers show toxic effects. Protein-deficient and young suckling
animals are particularly vulnerable (Schoental, 1959).
Chromosomal aberrations have been demonstrated in rats and
humans with veno-occlusive disease (Martin et al., 1972).
Alkaloids have been found in the honey secreted by bees feeding
on the toxic plants (Deinzer et al., 1977). According to Culvenor
and his co-workers, populations in some countries are exposed to
low levels of alkaloids in commonly used foodstuffs, e.g., honey in
Australia (Culvenor et al., 1981; Culvenor, 1983, 1985) and comfrey
in many countries (Culvenor et al., 1980a; Culvenor, 1985).
Human cases of acute disease following the brief ingestion of
the alkaloids have been known to progress to cirrhosis (Stuart &
Bras, 1957; Braginskii & Bobokhadzaev, 1965; Stillman et al., 1977;
Tandon, B.N. et al., 1977; Tandon, H.D. et al., 1977) in as short a
period as 3 months from the acute phase (Stuart & Bras, 1957). The
initial disease may be cryptic (Braginskii & Bobokhadzaev, 1965)
and may not be ascribed to herbal consumption, and yet may progress
to cirrhosis (Huxtable, 1980). Veno-occlusive disease was stated
to be the most common cause of cirrhosis in infants in Jamaica
(Bras et al., 1961) and has been believed to be a significant
etiological factor for adult cirrhosis, especially in developing
countries (Gupta et al., 1963).
Plants known or suspected to contain toxic alkaloids are widely
used for medicinal purposes as home remedies all over the world,
without systematic testing for safety (Schoental, 1963; Smith &
Culvenor, 1981) and some are even used as food (Schoental & Coady,
1968; Culvenor, 1980). There are several reports of the continued
use of such herbs for medicinal purposes in technically advanced
countries (Culvenor, 1980). Senecio jacobaea continues to be sold
at herbalists shops in the United Kingdom (Schoental, 1963; Burns,
1972), and Symphytum spp. (comfrey) are still used as a vegetables,
beverages, or remedies (Mattocks, 1980). Both these herbs are
known to be carcinogenic (IARC, 1976; Hirono et al., 1978). Young
flower stalks of Petasites japonicus Maxim, the pre-bloom flower
of coltsfoot, Tussilago farfara, the leaf and root of comfrey,
Symphytum officinale, and the young leaves and stalks of Farfugium
japonicum and Senecio cannabifolius, which are all used in Japan
as human food or herbal remedies, are known to be carcinogenic for
rats (Hirono et al., 1983). Symphytum x uplandicum Nyman (Russian
comfrey), which contains several toxic PAs (Culvenor et al., 1980b)
echimidine and 7 acetylycopsamine being the main constituents, is
used as a salad plant, green drink, and medicinal herb. It has
been estimated that the rate of ingestion of alkaloids from this
herb may, over a period of time, exceed the levels reported to have
been taken during the Afghan outbreak. There is a report of at
least one patient who developed toxic effects as a result of
consuming a comfrey preparation (Culvenor et al., 1980a; Ridker et
al., 1985). Arseculeratne et al. (1981) found that 3 of the 50
medicinal herbs commonly used in Sri Lanka contained PAs that had
been proved to be hepatotoxic for animals. They suggested that
consumption of such herbs might contribute to the high incidence of
chronic liver disease, including primary liver cancer, in Asian and
African countries, especially as they may act synergistically with
aflatoxin and hepatitis B virus. The risk of toxic effects due to
these alkaloids may be particularly high in children (Schoental,
1959; Jago, 1970) and protein malnutrition, which exists in some
countries, may potentiate them (Schoental & Magee, 1957). Recent
studies from Hong Kong (Kumana et al., 1985; Culvenor et al.,
1986), the United Kingdom (McGee et. al, 1976; Ridker et al.,
1985), and the USA (Stillman et al., 1977; Fox et al., 1978; Ridker
et al., 1985) report instances of human disease that have been
caused by the use of such herbs, resulting in fatality or the
development of cirrhosis, even in countries with well-developed
health services and among the higher economic and educated strata
of society. Indeed, Stillman et al. (1977), from the USA, called PA
toxicosis the "iceberg disease", implying that cases of this
disease might be more frequent than reported in the USA, especially
among populations of Mexican-American origin. In general, the use
of herbal remedies is not elicited in the clinical history and
patients do not volunteer this information themselves.
Furthermore, the alkaloids are eliminated within 24 h (Huxtable,
1980) and, even though methods are available for their detection in
biological tissues and fluids, the suspicion cannot be confirmed,
as the symptoms may take several days or months to appear.
Contamination of food crops is particularly likely to occur in
parts of the world with arid climates, poor or uncertain rainfall,
poor irrigation facilities, and following periods of drought, all
of which promote the growth of the PA-containing plants that grow
as weeds among cultivated crops, as has been found in studies on
the outbreaks in Afghanistan, India, and the USSR (Terekhov, 1939;
Dubrovinskii, 1947; Ismailov, 1948a,b; Tandon & Tandon, 1975;
Mohabbat et al., 1976; Tandon, B.N. et al., 1976; Tandon, R.K. et
al., 1976; Tandon, H.D. et al., 1978) and in grazing pastures. The
use of traditional medicines is common in these countries and there
is insufficient awareness of this hazard, the disease condition,
and its diagnostic pathological picture. Furthermore, health
services are poorly developed. Thus, many of the cases or even
outbreaks may go unnoticed or unrecorded and may even be ascribed
to malnutrition (Lancet, 1984). Also, many of the reported cases
of so-called "Budd-Chiari syndrome", a condition associated with
obstruction of major hepatic veins and/or inferior vena cava, may
actually be cases of veno-occlusive disease (Sherlock, 1968), in
which only the central veins of the liver lobule or sublobular
veins are occluded.
Another type of PAs, Trichodesma alkaloids, has been known to
cause a human outbreak of disease in the USSR, through
contamination of the staple cereal with the seeds containing these
PAs; in this outbreak, the symptoms were principally extra-hepatic
(Ismailov et al., 1970).
This document is aimed at focusing on a health menace that is
insufficiently recognized, in order to evaluate the health risks on
the basis of published data, and to draft a set of recommendations
that would help in its recognition, prevention, and control.
1. SUMMARY AND RECOMMENDATIONS
1.1. Summary
The ingestion of pyrrolizidine alkaloids (PAs) in foods and
medicinal herbs results in acute and chronic effects in man,
affecting mainly the liver. Data from experimental animal studies
indicate that PAs represent a potential cause of cancer in man.
The alkaloids are produced by numerous plant species and occur
throughout the world. In the present document, the alkaloids and
their properties are described together with the sources of human
exposure and the diseases that they produce in man and animals.
The risks for human health are evaluated and recommendations are
made for reducing such risks.
1.2. Sources and Chemical Structure
The known pyrrolizidine alkaloids, most of which are
hepatotoxic, are produced by plant species within the following
families: Boraginaceae ( Heliotropium, Trichodesma, Symphytum, and
most other genera), Compositae ( Senecio, Eupatorium, and other
genera of the tribes Senecioneae and Eupatoriae), Leguminosae
(genus Crotalaria), and Scrophul-ariaceae (genus Castilleja).
These genera are mainly herbaceous and very widely distributed,
some species being found in most regions of the world. The
majority of the species within these genera have not yet been
investigated, but are expected to contain pyrrolizidine alkaloids.
The hepatotoxic alkaloids have a 1,2-double bond in the
pyrrolizidine ring and branched chain acids, esterifying a
9-hydroxyl and preferably also the 7-hydroxyl substituent. Modified
seco-pyrrolizidine alkaloids, in which the central bond between the
N and C8 atoms is broken, are also hepatotoxic. Some Senecio
species contain non-basic derivatives that are 5-oxopyrroles. The
toxicity of these derivatives may be similar to that of the
alkaloids, but this aspect has not been investigated. The
alkaloids occur as free bases and N-oxides. The latter are
reduced to the free bases in the gastrointestinal tract of animals
and have a similar toxicity when ingested orally.
Suitable analytical procedures are available for screening
plant species, including a simple field test for toxic alkaloids.
Thin-layer chromatography (TLC), high-performance liquid (HPLC),
gas chromatography (GC), and gas chromato-graphy-mass spectrometry
(GC-MS) have been applied for separating, characterizing, and
quantifying the alkaloids present. Effective use of these
procedures requires authentic alkaloids for standards, few of which
are available. Improved analytical methods are required for the
determination of very low levels of alkaloids in some foodstuffs.
1.3. Mechanisms and Features of Toxicity
The toxic effects of pyrrolizidine alkaloids are due to
activation in the liver. Metabolism of the alkaloids by mixed-
function oxidases leads to pyrrolic dehydro-alkaloids, which are
reactive alkylating agents. Reaction of initial metabolites with
constituents of the liver cell in which they are formed are
probably the main cause of liver cell necrosis. Metabolites are
released into the circulation and are believed to pass beyond the
liver to the lung causing vascular lesions characteristic of
primary pulmonary hypertension, especially when alkaloids, such as
monocrotaline, are administered to animals.
In experimental animals, PAs are quickly metabolized and are
almost completely excreted in 24 h, so that no residual products
are detectable in the biological fluids or body tissues after this
period.
The rate of formation of pyrrolic metabolites is influenced by
the induction or inhibition of the mixed-function oxidases in the
liver, but the relationship between the rate of metabolism and
expression of toxicity is uncertain.
Several pyrrolizidine alkaloid-derivatives and related
compounds are known to cause chromosome aberrations in plants,
leukocyte cell cultures of the marsupial (Potorus tridactylus),
and in hamster cell lines. Some pyrrolizidine alkaloids induce
micronuclei formation in erythrocytes in the bone marrow and fetal
liver in mice, sister chromatid exchanges in a Chinese hamster cell
line and human lymphocytes in vitro, and repair DNA synthesis in
rodent hepatocyte cell cultures. Chromosome aberrations have been
reported in the blood cells of children suffering from veno-
occlusive disease VOD, presumably caused by fulvine.
A number of pyrrolizidine alkaloids have been shown to be
mutagenic in the Salmonella typhimurium assay, after metabolic
activation. The carcinogenic activity of pyrrolizidine alkaloids
appears to parallel their mutagenic behaviour, but not their
hepatotoxicity.
Heliotrine at doses of 50 mg/kg body weight or more,
administered to rats during the second week of gestation, has been
shown to induce several abnormalities in the fetus. Doses of
200 mg/kg body weight resulted in intrauterine deaths or resorption
of fetuses. Dehydroheliotridine, the metabolic pyrrole derivative of
heliotrine, was 2.5 times more effective on a molar basis than its
parent PA in inducing teratogenic effects.
The ability of PAs to cross the placental barrier in the rat
and to induce premature delivery or death of litters has been
demonstrated. The embryo in utero appears to be more resistant to
the toxic effects of pyrrolizidine alkaloids than the neonate. PAs
are known to have passed through the mother's milk to the
sucklings.
Megalocytosis, the presence of enlarged hepatocytes containing
large, hyper-chromatic nuclei, is a characteristic feature of
pyrrolizidine alkaloid-induced chronic hepatotoxicity in
experimental animals. The enlarged hepatocytes arise through the
powerful antimitotic action of the pyrrole metabolites of
pyrrolizidine alkaloids. This change has not been observed in the
human liver, though human fetal liver cells in vitro culture
become enlarged when exposed to PAs, indicating susceptibility to
the antimitotic effect of the alkaloids.
In experimental animals, protein-rich and sucrose-only diets
have given some measure of protection against the effects of the
alkaloids, as has pre-treatment of animals with thiols, anti-
oxidants, or zinc chloride.
PAs are noted mainly for the poisoning of livestock due to the
animals grazing on PA-containing toxic weeds, and large-scale
outbreaks have been recorded. Such episodes have been reported
from most parts of the world, including those with temperate or
cold climates. Studies carried out on a wide variety of farm and
laboratory animals have revealed generally common features of
toxicity with some species variations. The liver is the principal
target organ. In small laboratory animals, doses approaching a
lethal dose produce a confluent, strictly zonal haemorrhagic
necrosis in the liver lobule, within 12 - 48 h of administration of
PAs. Simultaneously in non-human primates, or after a short time in
the rat, chicken, and swine, changes begin to occur, and later
become organized, in the subintima of the central or sublobular
veins in the liver resulting in their occlusion. The reticulin
framework in the central zone of the lobule collapses following
necrosis leading to scarring. Repeated administration of suitable
doses leads to chronic liver lesion characterized by megalocytosis,
and increasing fibrosis, which may result in cirrhosis. Chronic
liver disease including cirrhosis has been shown to develop in the
rat following administration of a single dose of a PA. In a number
of animal species, the lungs develop vascular lesions
characteristic of primary pulmonary hypertension with secondary
hypertrophy of the right ventricle of the heart. In rats,
appropriately low repeated doses of several alkaloids have been
shown to induce tumours, mainly in the liver. In some studies, a
single dose has been carcinogenic.
The central nervous system is the target organ of the toxic PAs
contained in Trichodesma, which produce spongy degeneration of the
brain.
1.4. Effects on Man
In man, PA poisoning is usually manifested as acute veno-
occlusive disease characterized by a dull dragging ache in the
right upper abdomen, rapidly filling ascites resulting in marked
distension of the abdomen, and sometimes associated with oliguria,
and massive pleural effusion. It can also manifest as subacute
disease with vague symptoms and persistent hepatomegaly. Children
are particularly vulnerable. Many cases progress to cirrhosis and,
in some cases, a single episode of acute disease has been
demonstrated to progress to cirrhosis, in spite of the fact that
the patient has been removed from the source of toxic exposure and
has been given symptomatic treatment. Mortality can be high with
death due to hepatic failure in the acute phase or due to
hematemesis resulting from ruptured oesophageal varices caused by
cirrhosis. Less severely affected cases may show clinical, or even
apparently complete, recovery. The Task Group was not aware of any
substantiated report of primary pulmonary hypertension resulting
from PA toxicity. However, in view of the evidence in experimental
animals and circumstantial evidence in one case report, the
possibility of the development of toxic pulmonary disease in man
cannot be ruled out. There is a report of an outbreak of
Trichodesma poisoning in the USSR in which the symptoms were mainly
neurological.
1.4.1. Nature and extent of health risks
The two main sources of pyrrolizidine alkaloid poisoning
reported in human beings are the consumption of cereal grain
contaminated by weeds containing the alkaloids and the use of
alkaloid-containing herbs for medicinal and dietary purposes. A
third form of exposure, with the potential to affect large
populations is the possible low-level contamination of some
foodstuffs, such as honey and milk, but the Task Group was not
aware of any cases of human toxicity having been caused through the
contamination of these foods.
Liver disease caused by the contamination of cereal grains has
been reported in rural populations in Afghanistan, India, South
Africa, and the USSR. A contributing factor appears to be
abnormally dry weather, resulting in the growth of an exceptionally
high proportion of the alkaloid-containing weeds in the crops, the
seeds of which contaminate the cereal grain on harvesting. The
weeds responsible for known outbreaks have been Heliotropium,
Trichodesma, Senecio, and Crotalaria species. Mortality in such
outbreaks has been reported to be high. In the largest reported
outbreak in northwestern Afghanistan, an estimated 8000 people were
affected in a total population of 35 000 with 1600 - 2000 deaths.
Human poisoning through the medicinal use of herbs containing
pyrrolizidine alkaloids has been reported from all parts of the
world. PAs were responsible for a common liver disease in children
in Jamaica, and individual cases in Ecuador, Hong Kong, India, the
United Kingdom, and the USA. The plants involved were species of
Crotalaria, Heliotropium, Senecio, Symphytum, and Gynura.
Symphytum-containing preparations present a particular hazard
because of their widespread use and the generally high levels of
individual exposures. The use of herbs is almost universal in
traditional folk medicine and is increasing in developed countries.
Some of the herbs used contain pyrrolizidine alkaloids and have a
long-term toxicity that is unsuspected by the people taking them.
Knowledge of the species used in herbal medicine and the frequency
of such use is very limited in the scientific literature. About 40
such species are listed in this report, about one-third of which
are in use in developed countries. They are often prescribed by
herbalists, naturopaths, and other non-orthodox practitioners. The
extent of the contribution to acute and chronic liver disease
cannot be accurately assessed. It may also constitute an
etiological factor in cirrhosis of the liver and, once this stage
is reached, it may not be possible to identify the cause as a PA.
PAs are known to be transmitted from the feed of dairy animals
into milk and to cause toxic damage in the suckling young. One
instance of large-scale contamination of honey is known to have
been caused by a common weed rich in PAs, which was the source of
nectar and pollen for the honey-secreting bees. No reports of
cases of acute toxicity caused by consumption of contaminated dairy
products or honey were available to the Task Group. Furthermore,
no information is available on the possible presence of PAs or
their metabolites in the meat of animals fed toxic weeds before
slaughter; however, the possibility of toxic disease being caused
through this medium is considered to be low.
There are no substantial, long-term follow-up data to assess
whether exposure to PAs results in increased incidence of chronic
liver disease or cancer in man. Available clinical and
experimental data suggest that a single episode of PA toxicity and
possibly also a long-term low level exposure may lead to cirrhosis
of the liver. PAs could also be possible carcinogens in man, since
a number of them have been demonstrated to induce cancer in
experimental animals, the main target organ being the liver. These
include some which have caused episodes of human toxicity, and some
others which are found in herbs traditionally used as items of
food. Also, in several instances of human toxicity, the reported
daily rates of intake of such PAs were in close range of those
known to induce tumours in rats. However, these risks cannot be
adequately assessed on a quantitative basis. There are indications
that PA intoxications leading to liver disease are more prevalent
than the reported frequency of cases would seem to indicate.
Because of their known involvement in human poisoning and their
possible carcinogenicity, exposure to pyrrolizidine alkaloids
should be kept as low as practically achievable. The setting of
regulatory tolerance levels for certain food products may be
required in some situations.
1.5. Methods for Prevention
The only known method of prevention is to avoid consumption of
the alkaloids. In the USSR, a set of agricultural (or
agrotechnical) legislative, phyto-sanitary and educational measures
has prevented new outbreaks of poisoning due to Heliotropium and
Trichodesma, since 1947.
1.6. Recommendations
1.6.1. General recommendations
1. Cereal crops should be assessed throughout the world for
possible contamination by weeds likely to contain pyrrolizidine
alkaloids. Appropriate grain inspection systems are desirable
in order to achieve near-zero levels of contamination by such
weeds.
2. There is a need to create awareness, among the general
population and those responsible for the delivery of health
services, with regard to the hazards of consuming such plants
as contaminants in food or as food, or for medicinal purposes.
Advice on hazards should include mention of possible increased
risks, if the alkaloid intake is associated with drug
treatment, (e.g. phenobarbitone) or foods which increase the
level of liver metabolizing enzymes.
3. Ethnobotanical and taxonomic studies are required in many
countries to provide specific information on the use of plant
species containing pyrrolizidine alkaloids for medicinal and
dietary purposes. There may be a need to control the sale of
some species, and their prescription by herbalists and other
practitioners of traditional systems of medicine.
4. Honey and dairy products, both local and bulk supplies, should
be assayed for pyrrolizidine alkaloids in all regions where a
risk of contamination of these foodstuffs has been identified.
1.6.2 Recommendations for research
1. Long-term follow-up studies of the survivors of both alkaloid
poisoning in human beings and animal outbreaks are required, in
order to determine the possible development of chronic liver
disease or cancer. Similar studies are also desirable on
individuals who regularly consume comfrey or other PA-
containing herbs over a substantial period of time.
2. Epidemiological studies should be carried out in countries with
a high incidence of primary liver cancer, in order to determine
whether there is an association with the intake of herbs
containing pyrrolizidine alkaloids.
3. A network of reference laboratories is needed to assist member
states in identifying plants and their seeds suspected of
producing toxic effects and for the assay and identification of
PAs. Provisions may be made for the easy availability of pure
alkaloids for use as reference standards for assays.
4. It is necessary to develop improved assay procedures, suitable
for the purposes of recommendation (4) in section 1.6.1,
particularly using fluorescence and immunochemical methods.
5. There is a need for further toxicological studies, such as
studies on the carcinogenicity of echimidine and the toxicity
of the 5-oxopyrrole constituents of Senecio species, and for
studies that would provide more quantitative information on the
various adverse biological effects of PAs. A study of the
carcinogenicity of the alkaloids in the pig is also indicated,
since the pig exhibits a high sensitivity to acute and subacute
toxicity similar to that seen in man.
6. Study is required of the possible alkaloid content of the meat,
organs, and fat of animals that have recently consumed plants
containing pyrrolizidine alkaloids.
7. Experimental studies are needed on the influence of nutritional
status on the metabolism, and acute and chronic effects of PAs.
8. Further metabolic studies are required to define more
specifically the enzymes involved in the microsomal activation
and detoxification of PAs, to determine whether organelles
other than microsomes are involved, and to explore further,
quantitative relationships between different routes of
metabolism.
9. The maximum no-observed-adverse-effect dose levels for repeated
long-term administration in the rat and the pig need to be
determined.
10. Experimental studies should be conducted to determine:
(a) whether pyrrolizidine alkaloid N-oxides may be
metabolized directly into the pyrrolic dehydroalkaloid
in mitochondria, especially in tumour cells; and
(b) which P450 enzymes are involved in the activation and
N-oxidation of PAs and thence in the selective
induction of N-oxidation enzymes.
11. A study might be conducted of human variability and its genetic
aspects in relation to factors that influence susceptibility to
PAs; for example, the study of mixed-function oxidase levels
in the liver by metabolism of appropriate test substances
recognized as harmless.
2. PROPERTIES AND ANALYTICAL METHODS
2.1 Chemical Structure and Properties
The chemical structure of PAs in relation to their toxic
effects has been reviewed recently by Mattocks (1986). The
pyrrolizidine alkaloids with which this document is concerned are
those that have previously been called "hepatotoxic" or
"nucleotoxic". Here it is proposed to refer to them as "toxic"
PAs, because of the weight of evidence now available that they
produce damage in other organs as well as the liver, and the need
to avoid a restrictive term. There are other types of
pyrrolizidine alkaloids, such as those that occur in the plant
family Orchidaceae, which are not toxic and are not discussed here.
The toxic PAs are esters of the amino-alcohols derived from the
heterocyclic nucleus. The pyrrolizidine molecule is made up of two
5-membered rings inclined to each other as shown in Fig. 1 so that
geometric isomerism is possible, and which share a common nitrogen
at position 4.
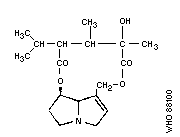
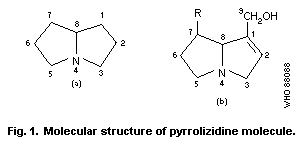 Most hepatotoxic alkaloids are esters of molecules similar to
that shown in Fig. 1(b) (1-hydroxymethyl-1:2-dehydro-
pyrrolizidine). However, a few hepatotoxic alkaloids are esters of
the amino-alcohol otonecine, e.g., petasitenine (Fig. 2, No.7).
The unsaturated pyrrolizidine nucleus itself is not toxic, but
esters of branched-chain acids are. Ester linkages may be at
positions 9, 7, or (rarely) 6. Some esters have an "open"
molecule, e.g., heliotrine, whereas others are macrocyclic
diesters, e.g., monocrotaline and retrosine. Examples of some
pyrrolizidine alkaloid structures are shown in Fig. 2.
The ring nucleus contains a double bond at the 1:2 position,
which is essential for the toxic effects of the alkaloid, but not
for unrelated effects.
1. Echimidine
Chemical structure:
Most hepatotoxic alkaloids are esters of molecules similar to
that shown in Fig. 1(b) (1-hydroxymethyl-1:2-dehydro-
pyrrolizidine). However, a few hepatotoxic alkaloids are esters of
the amino-alcohol otonecine, e.g., petasitenine (Fig. 2, No.7).
The unsaturated pyrrolizidine nucleus itself is not toxic, but
esters of branched-chain acids are. Ester linkages may be at
positions 9, 7, or (rarely) 6. Some esters have an "open"
molecule, e.g., heliotrine, whereas others are macrocyclic
diesters, e.g., monocrotaline and retrosine. Examples of some
pyrrolizidine alkaloid structures are shown in Fig. 2.
The ring nucleus contains a double bond at the 1:2 position,
which is essential for the toxic effects of the alkaloid, but not
for unrelated effects.
1. Echimidine
Chemical structure:
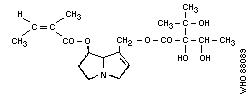 Chemical formula: C20H31NO7
Relative molecular mass: 397
CAS registry number: 520-68-3
2. Heliotrine
Chemical structure:
Chemical formula: C20H31NO7
Relative molecular mass: 397
CAS registry number: 520-68-3
2. Heliotrine
Chemical structure:
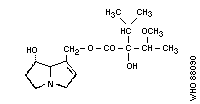 Chemical formula: C16H27NO5
Relative molecular mass: 313
CAS registry number: 303-33-3
3. Indicine- N -oxide
Chemical structure:
Chemical formula: C16H27NO5
Relative molecular mass: 313
CAS registry number: 303-33-3
3. Indicine- N -oxide
Chemical structure:
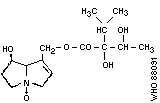 Chemical formula: C15H25NO6
Relative molecular mass: 315
CAS registry number: 41708-76-3
4. Jacobine
Chemical structure:
Chemical formula: C15H25NO6
Relative molecular mass: 315
CAS registry number: 41708-76-3
4. Jacobine
Chemical structure:
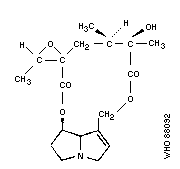 Chemical formula: C18H25NO6
Relative molecular mass: 351
CAS registry number: 6870-67-3
5. Lasiocarpine
Chemical formula: C18H25NO6
Relative molecular mass: 351
CAS registry number: 6870-67-3
5. Lasiocarpine
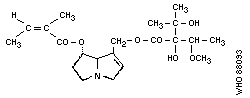 Chemical structure:
Chemical formula: C21H33NO7
Relative molecular mass: 411
CAS registry number: 303-34-4
6. Monocrotaline
Chemical structure:
Chemical formula: C21H33NO7
Relative molecular mass: 411
CAS registry number: 303-34-4
6. Monocrotaline
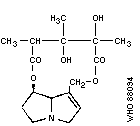 Chemical structure:
Chemical formula: C16H23NO6
Relative molecular mass: 325
CAS registry number: 315-22-0
7. Petasitenine
Chemical structure:
Chemical formula: C16H23NO6
Relative molecular mass: 325
CAS registry number: 315-22-0
7. Petasitenine
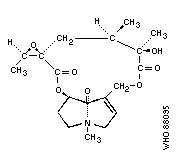 Chemical structure:
Chemical formula: C19H27NO7
Relative molecular mass: 381
CAS registry number: 60132-19-6
8. Retrorsine (retrosine N -oxide = isatidine)
Chemical structure:
Chemical formula: C19H27NO7
Relative molecular mass: 381
CAS registry number: 60132-19-6
8. Retrorsine (retrosine N -oxide = isatidine)
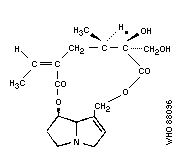 Chemical structure:
Chemical formula: C18H25NO6
Relative molecular mass: 351
CAS registry number: 480-54-6
9. Senecionine
Chemical structure:
Chemical formula: C18H25NO6
Relative molecular mass: 351
CAS registry number: 480-54-6
9. Senecionine
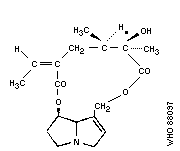 Chemical structure:
Chemical formula: C18H25NO5
Relative molecular mass: 335
CAS registry number: 130-01-8
10. Symphytine
Chemical structure:
Chemical formula: C18H25NO5
Relative molecular mass: 335
CAS registry number: 130-01-8
10. Symphytine
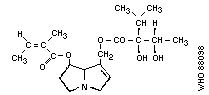 Chemical structure:
hemical formula: C20H31NO6
Relative molecular mass: 381
CAS registry number: 22571-95-5
11. Trichodesmine
Chemical structure:
hemical formula: C20H31NO6
Relative molecular mass: 381
CAS registry number: 22571-95-5
11. Trichodesmine
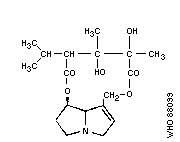 Chemical structure:
Chemical formula: C18H27NO6
Relative molecular mass: 353
CAS registry number: 548-90-3
12. Incanine
Chemical structure:
Chemical formula: C18H27NO6
Relative molecular mass: 353
CAS registry number: 548-90-3
12. Incanine
 Chemical structure:
Chemical formula: C18H27NO5
Relative molecular mass: 337
CAS registry number: 480-77-3
As the Task Group met in Tashkent, it is of historical interest
to recall that the structures of heliotrine and lasiocarpine, the
main alkaloids of Heliotropium lasiocarpum, were worked out by
Dr G.P. Men'shikov and associates in Moscow in the 1930s. This
work included determining the structure of heliotridine, the parent
compound of the amino-alcohol, heliotridane. Dr Men'shikov's
studies were carried out at essentially the same time, but
independently of studies by English and American authors on
retronecine-based alkaloids.
The alkaloids in plants are often found together with their
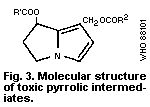
N-oxides, which are also toxic, when ingested orally. The
pyrrolizidine alkaloids acquire their toxic properties only through
the toxic pyrrolic intermediates (the general structure of which is
shown in Fig. 3) formed by the mixed-function oxidases of the
hepatocytes. To form these pyrrolic derivatives, the alkaloid
molecule should have:
(a) a double bond at the 1:2 position of the ring nucleus;
(b) esterified hydroxyl groups in the nucleus at the C 9
and/or C 7 positions; and
(c) a branched carbon chain in at least one of the ester side-
chains (McLean, 1974).
Chemical structure:
Chemical formula: C18H27NO5
Relative molecular mass: 337
CAS registry number: 480-77-3
As the Task Group met in Tashkent, it is of historical interest
to recall that the structures of heliotrine and lasiocarpine, the
main alkaloids of Heliotropium lasiocarpum, were worked out by
Dr G.P. Men'shikov and associates in Moscow in the 1930s. This
work included determining the structure of heliotridine, the parent
compound of the amino-alcohol, heliotridane. Dr Men'shikov's
studies were carried out at essentially the same time, but
independently of studies by English and American authors on
retronecine-based alkaloids.
The alkaloids in plants are often found together with their
N-oxides, which are also toxic, when ingested orally. The
pyrrolizidine alkaloids acquire their toxic properties only through
the toxic pyrrolic intermediates (the general structure of which is
shown in Fig. 3) formed by the mixed-function oxidases of the
hepatocytes. To form these pyrrolic derivatives, the alkaloid
molecule should have:
(a) a double bond at the 1:2 position of the ring nucleus;
(b) esterified hydroxyl groups in the nucleus at the C 9
and/or C 7 positions; and
(c) a branched carbon chain in at least one of the ester side-
chains (McLean, 1974).
 Substitution at the a position of the acid and esterification of
the C-7 hydroxy group both enhance the toxicity of the alkaloid
(Robins, 1982).
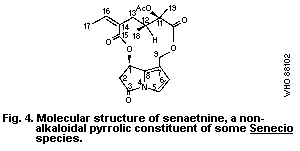
A group of related alkaloids, isolated from Senecio species by
Bohlmann et al. (1979), have non-basic pyrrolic structures similar
to those of toxic pyrrolizidine alkaloid metabolites, but they are
chemically deactivated by the presence of a carbonyl group at
position 3 of the pyrrolizidine nucleus, e.g., senaetnine (Fig. 4).
Senaetnine does not possess the acute hepatotoxic characteristics
of basic pyrrolizidine alkaloids. However, it had a direct
irritant action on tissues near the site of intraperitoneal
administration and caused damage to pulmonary vascular tissue when
given intraveinous to rats (Mattocks & Driver, 1987).
Substitution at the a position of the acid and esterification of
the C-7 hydroxy group both enhance the toxicity of the alkaloid
(Robins, 1982).
A group of related alkaloids, isolated from Senecio species by
Bohlmann et al. (1979), have non-basic pyrrolic structures similar
to those of toxic pyrrolizidine alkaloid metabolites, but they are
chemically deactivated by the presence of a carbonyl group at
position 3 of the pyrrolizidine nucleus, e.g., senaetnine (Fig. 4).
Senaetnine does not possess the acute hepatotoxic characteristics
of basic pyrrolizidine alkaloids. However, it had a direct
irritant action on tissues near the site of intraperitoneal
administration and caused damage to pulmonary vascular tissue when
given intraveinous to rats (Mattocks & Driver, 1987).
 The alkaloids are fairly stable chemically, but the ester
groups may undergo hydrolysis under alkaline conditions. Some
alkaloids in plant material may decompose during drying (Bull et
al., 1968), but others appear to be stable under similar conditions
(Pedersen, 1975; Birecka et al., 1980). The N-oxides of
unsaturated pyrrolizidines are more readily decomposed by heat than
the basic alkaloids, especially when dry. However, the stability
of the alkaloids and N-oxides in hot water as, for example, in
cooking, is not known.
Some pyrrolizidine alkaloids have a limited water solubility,
unless neutralized with acid; but others (e.g., indicine), and all
the N-oxides, are readily soluble.
2.2 Analytical Methods
When analysing for PAs, it is important to recognize that this
group consists of many different compounds (section 2.1) and that
these often occur as mixtures in plants or in materials of plant
origin. They may vary in structure, relative molecular mass,
response to analytical procedures, and toxicity. Both basic
alkaloids and corresponding N-oxides may be present at the same
time. Thus, where such mixtures are present, analyses will
inevitably be approximate, unless the individual components are
separated and identified.
Nevertheless, such estimates can be useful. In particular, all
hepatotoxic PAs are unsaturated in the sense that they possess a
1:2-double bond in the pyrrolizidine nucleus, and analytical
methods that are specific for this structure can be of value in
screening for potential toxicity. A simple qualitative field test
for screening plant materials for the presence of such alkaloids
and their N-oxides, without the need of high technology equipment,
is described in section 2.2.2.5.
2.2.1 Extraction
2.2.1.1 Plant tissue
Pyrrolizidine alkaloids are usually extracted from dried,
milled plant material with hot or cold alcohol. The alcohol is
evaporated, the bases taken up in dilute acid, and fats extracted
with ether or petroleum. It is usual, at this stage, to reduce any
N-oxides present to the corresponding basic alkaloids with zinc,
before making the solution alkaline and extracting the alkaloids
with chloroform (Koekemoer & Warren, 1951). Alternatively, alcohol
can be continuously circulated through the plant material and then
cation exchange resin, and the alkaloids subsequently eluted from
the resin (Mattocks, 1961; Deagen & Deinzer, 1977). PAs can also
be extracted by soaking plant material in dilute aqueous acid
(Briggs et al., 1965; Craig et al., 1984).
2.2.1.2 Biological fluids and tissues
Pyrrolizidine alkaloids have been extracted for analytical
purposes from honey (Deinzer et al., 1977), milk (Dickinson et al.,
1976), blood-plasma (Ames & Powis, 1978; McComish et al., 1980),
urine (Mattocks, 1967a; Jago et al., 1969; Evans et al., 1979), and
bile (Jago et al., 1969; Lafranconi et al., 1985).
When attempting to isolate PAs from animal tissues, it must be
appreciated that the toxic alkaloids are often metabolized very
rapidly in animals, so that the amounts that are recoverable
(except from urine), only a few hours after alkaloid ingestion, may
be extremely small. Various methods have been used to separate
PAs, but some mixtures are extremely difficult to separate. On the
analytical scale, the most useful methods are thin-layer
chromatography (TLC), high-performance liquid chromatography
(HPLC), and gas chromatography (GC) (section 2.2.2).
2.2.2 Analysis for pyrrolizidine alkaloids
2.2.2.1 Thin-layer chromatography (TLC)
For TLC, silica plates are usually used, eluted with chloroform:
methanol:aqueous ammonia mixtures (Sharma et al., 1965; Chalmers
et al., 1965); solvents suitable for the N-oxides, which
are more water-soluble, have been described by Mattocks (1967b)
and Wagner et al. (1981). The most sensitive methods for
detecting PAs on TLC are those using Ehrlich reagent
(4-dimethylaminobenzaldehyde) (Mattocks, 1967b). The unsaturated
alkaloids are best visualized by spraying the plates first with a
solution of orthochloranil, then with Ehrlich reagent, heating
after each spray (Molyneux & Roitman, 1980). The N-oxides of
unsaturated pyrrolizidines are detected by spraying a solution of
acetic anhydride, heating the plate, and then spraying Ehrlich
reagent (Mattocks, 1967b).
Pyrrolizidine alkaloids with a saturated base moiety must be
detected in other ways (which are not specific for pyrrolizidines),
e.g., by exposing the dried plates to iodine vapour, or by spraying
with an iodobismuth (Dragendorff) reagent (Munier, 1953).
2.2.2.2 High-performance liquid chromatography (HPLC)
Analytical or preparative scale HPLC separation of
pyrrolizidine alkaloids has been described by Segall (1979a,b) and
Dimenna et al. (1980), and an improved method has been reported by
Ramsdell & Buhler (1981). Alkaloids from Symphytum officinale
(comfrey) have been separated on an analytical scale by Tittel et
al. (1979), and partially separated on a preparative scale by
Huizing et al. (1981). UV detectors are usually used for the HPLC
of pyrrolizidine compounds (Mattocks, 1986).
2.2.2.3 Gas chromatography (GC) and mass spectrometry (MS)
The GC characterization of PAs using packed columns has been
described by Chalmers et al. (1965) and Wiedenfeld et al. (1981).
Mixtures of alkaloids from comfrey ( Symphytum sp.), normally hard
to separate, were resolved by Culvenor et al. (1980a) and Frahn et
al. (1980) by GC of the methylboronate derivatives.
Gas chromatography combined with mass spectrometry (GC-MS) has
become a valuable and highly sensitive means for both the
identification and the quantitative determination of pyrrolizidine
alkaloids. Thus, alkaloids extracted from honey were separated and
identified by Deinzer et al. (1977) and (as butylboronate
derivatives) by Culvenor et al. (1981). Deinzer et al. (1978)
described a method for the recognition (but not the individual
identification) of retronecine-based pyrrolizidine alkaloids, by
hydrolysing them to retronecine (the amino alcohol moiety) followed
by GC-MS of its bis-trifluoroacetate. The use of capillary GC has
greatly improved the sensitivity of pyrrolizidine alkaloid
analysis, especially when used with MS (Luthy et al., 1981). The
MS of pyrrolizidine compounds has been reviewed (Bull et al., 1968;
Mattocks, 1986).
Pyrrolizidine N-oxides generally undergo thermal decomposition,
when subjected to GC, but they can first be reduced to the
corresponding basic alkaloids (Koekemoer & Warren, 1951).
Alternatively they may be derivatised. Thus, trimethylsilylation
of indicine N-oxide or heliotrine N-oxide can lead either to the
trimethylsilyl (TMS) derivative of the parent alkaloid or to the
TMS derivative of the dehydro-alkaloid (pyrrolic derivative),
depending on the reagents used, and these products will run
successfully on GC-MS (Evans et al., 1979, 1980).
2.2.2.4 Nuclear magnetic resonance (NMR) spectrometry
A convenient, but relatively insensitive, method, specifically
for the determination of unsaturated PAs, has been described by
Molyneux et al. (1979). The basic alkaloids are extracted, then
subjected to NMR spectrometry along with an internal standard
( p-dinitrobenzene). This enables quantitative measurements to be
made of the signal(s) representing the H2 proton(s) in unsaturated
pyrrolizidines, and thus the alkaloid(s) can be determined.
Quantitative NMR analysis of pyrrolizidine alkaloid mixtures from
Senecio vulgaris has been described by Pieters & Vlietinck (1985)
and compared with an HPLC method by the same authors (1986).
Qualitative aspects of the NMR spectrometry of pyrrolizidine
alkaloids have been reviewed by Bull et al. (1968) and Mattocks
(1986).
2.2.2.5 The Ehrlich reaction
This method (Mattocks, 1967a, 1968b) is specific for
unsaturated pyrrolizidine alkaloids and is not suitable for other
alkaloids. Thus, it is the most useful colorimetric method for
potentially hepatotoxic pyrrolizidine compounds. The procedure
converts the alkaloid into its N-oxide, using hydrogen peroxide.
The product reacts with acetic anhydride to form a pyrrolic
derivative (dehydro-alkaloid) that gives a magenta colour with a
specially modified Ehrlich reagent. The latter contains boron
trifluoride to give maximum sensitivity. As little as 5 µg of most
unsaturated pyrrolizidines can be measured by this method. If the
oxidation stage is omitted, only the unsaturated pyrrolizidine
N-oxides can be determined. The determination of pyrrolizidine
N-oxides has also been discussed by Mattocks (1971b).
A simplification of the above colorimetric procedure was
described by Mattocks (1971d) to provide a qualitative test that
could be used to screen large numbers of plant samples for the
presence of unsaturated pyrrolizidine alkaloid N-oxides. An
improved version of this field test is now available (Mattocks &
Jukes, 1987). It is suitable for any plant parts, such as leaves,
stems, flowers, seeds, or roots, or materials of plant origin, such
as cereals or herbal teas, but has not yet been applied to cooked
food.
The plant material (0.2 - 1 g) is extracted by grinding it with
aqueous ascorbic acid (5%) and a small amount of sand. The
solution is filtered and divided into two equal portions ("test"
and "blank"). An aqueous solution (0.2 ml) of sodium nitroprusside
(5%) containing sodium hydroxide (10-3 mol) is added to the "test"
sample. Both portions are heated for approximately 1 min at 70 -
80 °C; then Ehrlich reagent is added and heating is continued for
1 min. The Ehrlich reagent contains 4-dimethylaminobenzaldehyde
(5 g) dissolved in a mixture of acetic acid (60 ml), water (30 ml),
and 60% perchloric acid (10 ml). A magenta colour in the "test"
compared with the "blank" indicates the presence of an unsaturated
PA N-oxide. The "blank" may show a colour if the plant contains
compounds, such as indoles or pyrroles, which can themselves give a
colour with Ehrlich reagent. The intensity of colour in the
"sample" compared with the "blank" can give a rough idea of the
amount of alkaloids present, and indicate whether further chemical
or toxicological testing of the plant material is adviseable.
In practice, the majority of PA-containing plants contain
enough alkaloid in the N-oxide form (often a large proportion) to
react positively in this test. The main exceptions are some seeds
(Crotalaria), which may contain much alkaloid base, but little or
no N-oxide. These (and any other sample not containing
chlorophyll) can be tested for basic PAs by grinding them with
chloroform, heating the filtered extract with a solution (0.1 ml)
of orthochloranil (0.5%) in acetonitrile, and then heating it with
Ehrlich reagent. A magenta colour indicates the presence of an
unsaturated PA. Non-toxic pyrrolizidine alkaloids having a
saturated pyrrolizidine nucleus, and pyrrolizidine alkaloids that
are otonecine esters, such as petasitenine, will not respond to
this test.
2.2.2.6 Indicator dyes
A method generally applicable to tertiary bases has been
adapted for pyrrolizidine alkaloids by Birecka et al. (1981). It
is sensitive, but is not specific for this group of alkaloids, and
it does not distinguish between the saturated and unsaturated
alkaloids. A chloroform solution of the alkaloid is shaken with
acidified aqueous methyl orange. The yellow alkaloid:dye complex
is subsequently released from the chloroform phase, using ethanolic
sulfuric acid, and measured spectrophotometrically.
2.2.2.7 Direct weighing
An insensitive way to determine the alkaloids in, for example,
a plant sample, providing enough is available, is to extract the
alkaloids (section 2.2.1) and weigh them. This will provide a
rough measure of the total bases present in the sample; however,
these may not necessarily be PAs. Nevertheless, the sample can
then be subjected to further tests, e.g., GC-MC, nuclear magnetic
resonance (NMR), or colorimetric analysis. Furthermore,
pyrrolizidine N-oxides are generally too water soluble to be
appreciably extractable from aqueous solution by chloroform. Thus,
if two portions of the sample are extracted, and one of them is
reduced to convert N-oxides to bases, the weight difference between
the two products will represent the alkaloid existing in the form
of N-oxide in the original sample.
2.3 Determination of Metabolites in Animal Tissues
Important metabolites of toxic pyrrolizidine alkaloids in
animals include "pyrrolic" derivatives (dehydro-alkaloids) and
N-oxides. A procedure for measuring pyrrolic metabolites in tissue
samples (such as liver or lung) has been described by Mattocks &
White (1970). The sample (usually 0.5 g) is homogenized in an
ethanolic solution of mercuric chloride; the solids are separated
by centrifugation and heated with Ehrlich reagent to give a soluble
colour that can be measured spectrophotometrically.
The measurement of pyrrolic and N-oxide metabolites, formed by
the action of hepatic microsomal preparations on PAs in vitro, is
an improvement described by Mattocks & Bird (1983).
3. SOURCES AND PATHWAYS OF EXPOSURE
3.1 Hepatotoxic Pyrrolizidine Alkaloids and Their Sources
Plants constitute the only natural source of pyrrolizidine
alkaloids (PAs) that cause toxic reactions in man and animals. PAs
occur in a number of species in the families Boraginaceae,
Compositae, Leguminosae (genus Crotalaria), Ranunculaceae (genus
Caltha), and Scrophulariaceae (genus Castilleja) (Table 1). The
most important genera of PA-containing toxic plants are Crotalaria
(Leguminosae), Senecio (Compositae), Heliotropium, Trichodesma,
Amsinckia, Echium, and Symphytum (Boraginaceae) (Hooper, 1978).
The recorded cases of human toxicity have mainly been caused by at
least 12 different pyrrolizidine alkaloids, mostly derived from
Heliotropium, Senecio, and Crotalaria genera. The Senecio spp.
grow throughout the world; the Crotalaria spp. are mainly found in
the tropics and subtropics (Culvenor, 1980).
Table 1. List of plant genera containing toxic pyrrolizidine alkaloids
(with number of species investigated)
-------------------------------------------------------------------------------------
Family Genera
-------------------------------------------------------------------------------------
Apocynaceae Fernaldia (1), Parsonsia (4),
Boraginaceae Alkanna (1), Amsinckia (4), Anchusa (2), Asperugo (1), Borago (1),
Caccinia (1), Cynoglossum (9), Echium (3), Hackelia (1),
Heliotropium (25), Lappula (2), Lindelofia (7), Lithosperum (1),
Macrotomia (1), Messerschmidtia (1), Myosotis (2), Paracaryum (1),
Paracynoglossum (1), Rindera (5), Solenanthus (4), Symphytum (7),
Tournefortia (2), Trachelanthus (2), Trichodesma (2), Ulugbekia (1)
Compositae Adenostyles (3), Brachyglottis (1), Cacalia (4), Conoclinium (1),
Crassocephalum (1), Doronicum (2), Echinacea (2), Emilia (2),
Erechtites (1), Eupatorium (8), Farfugium (1), Gynura (2),
Ligularia (5), Petasites (4), Senecio (142), Syneilesis (1),
Tussilago (1)
Leguminosae Crotalaria (60)
Ranunculaceae Caltha (2)
Scrophulariaceae Castilleja (1)
-------------------------------------------------------------------------------------
An alphabetical list of pyrrolizidine alkaloids with their
plant sources has been published by Smith & Culvenor (1981) and
Mattocks (1986). An updated version is attached as Appendix I.
The plant genera containing toxic PAs are listed in Table 1
indicating the number of species investigated for PAs. A
comprehensive list of species of plants belonging to each of these
genera, the alkaloids isolated from each, and the part of the plant
containing the alkaloid are presented in Appendix II. Table 1 in
Appendix II includes species known to contain alkaloids of proved
hepatotoxicity, or of a molecular structure that would make them
very probably hepatotoxic. Table 2 in Appendix II includes species
containing pyrrolizidine amino-alcohols or esters, which, while not
having all the features of hepatotoxicity, would need only minor
structural modifications to render them hepatotoxic. Plants of the
same taxonomic groups as the plants of proven hepatotoxicity are
listed in part (a) of the table. There is a possibility that, on
further examination, hepatotoxic alkaloids may be found, as minor
constituents, in strains or parts of these plants not yet
investigated or under specific conditions of growth. It should be
noted that the species that have been investigated and are listed
are only few compared with the total number of species in each
genera. It has been recommended by Smith & Culvenor (1981) that it
would be prudent to regard all species in the family Boraginaceae
and the genera Crotalaria, Senecio, and Eupatorium as potentially
hepatotoxic.
It is pertinent to note that the alkaloid content in different
parts of the plant (e.g., roots, leaves, stalks, flowers, and buds)
varies and is subject to fluctuations according to the climate,
soil conditions, and time of harvesting (Danninger et al., 1983;
Hartmann & Zimmer, 1986). Mattocks (1980) demonstrated that the
alkaloid content of the leaves of Symphytum spp. (Russian
comfrey), which are used as an item of food, varies with their
maturity. The toxic PA content is highest at the beginning of the
vegetative period and declines as the leaves mature. The PA
content of the roots is much higher than that of the leaves, and
dried leaves contain a higher concentration than fresh leaves
(Mattocks, 1986). According to Danninger et al. (1983), in some
species (Symphytum asperum), relatively long storage may lead to a
reduction in the alkaloid content, presumably because enzymes are
released during drying. Candrian et al. (1984b) studied the
stability of PAs in hay and silage containing various amounts of
Senecio alpinus. The PA content of hay remained constant for
several months, but the PAs in silage were mainly degraded.
However, the degradation of PAs was much less complete in the lower
concentration range. A quantitatively significant PA-degradation
product in silage was identified as retronecine. Silage with an
S. alpinus percentage of 3.5 - 23 still contained macrocyclic PAs at
a concentration of about 20 mg/kg wet weight. Such silage was not
considered safe for cattle bearing in mind that a 600-kg calf eats
about 30 kg silage/day, amounting approximately to a daily intake
of about 1 mg PAs/kg body weight. In feeding trials with Senecio
jacobaea, Johnson (1979) found that the minimum lethal dose for
cattle was between 1 and 2 mg PAs/kg body weight per day.
PAs known to have been associated with instances of human toxic
liver disease in different parts of the world are listed in Table
2. Two groups of alkaloids that, according to Culvenor (1983), are
consumed in significant amounts by people in different parts of the
world include:
(a) Echimidine, acetyllycopsamine, and related alkaloids
(many countries)
Leaves of plants of the Symphytum sp. ( Symphytum officinale
(comfrey) and Symphytum x uplandicum) are used traditionally as a
salad and as a medicinal herb in Australia, many countries of
Europe, and the USA. S. officinale has been shown to be
carcinogenic for rats (Hirono et al., 1978). Leaves of Russian
comfrey contain a concentration of alkaloids (mainly echimidine) of
0.1 - 1.5 g/kg. The highest level of daily consumption of the
alkaloids has been estimated to be 5 - 6 mg (Culvenor, 1983).
(b) Echimidine and related alkaloids (Australia)
PAs derived from Echium plantagineum, with echimidine as the
major component, have been found in honey secreted by bees feeding
on the plant (Culvenor et al., 1981). The plant is a major source
of honey (section 3.3.4).
3.2 Pneumotoxic and Other Toxic Pyrrolizidine Alkaloids
Not all hepatotoxic alkaloids are pneumotoxic. The commonest
ones used to produce experimental lung injury are fulvine (Barnes
et al., 1964; Kay et al., 1971a; Wagenvoort et al., 1974a,b) and
monocrotaline (Lalich & Ehrhart, 1962; Chesney & Allen, 1973b;
Huxtable et al., 1977). These are also the most active (Mattocks,
1986). The seeds of Crotalaria spectabilis, which contain
monocrotaline, have also been used to study pneumotoxic effects on
experimental animals (Turner & Lalich, 1965; Kay & Heath, 1966; Kay
et al., 1967a) and C. spectabilis has been called the pulmonary
hypertension plant (Kay & Heath, 1969), because of the pulmonary
hypertensionogenic properties of the PAs it contains. Culvenor et
al. (1976a) screened 62 PAs for hepatotoxicity and pneumotoxicity.
Chronic lung lesions were produced by most compounds that induced
chronic liver lesions, though high doses were required in some
instances. It is possible that chronic lung lesions may not occur
in experimental animals because of early death due to acute
toxicity. However, the authors identified a number of PAs that
were particularly prone to produce chronic lung damage in rats
including crispatine, senecionine, seneciphylline, and usaramine
(12-membered macrocyclic, retronecine diesters), anacrotine and
madurensine (crotonecine esters), and the heliotridine esters,
heliosupine, lasiocarpine, and rinderine.
The molecular structure-activity requirements for
pneumotoxicity are the same as those for hepatotoxicity. This is
consistent with their both being caused by the same toxic
metabolites and by the metabolic activation of the alkaloids in the
liver cells to form a reactive pyrrolic dehydro-alkaloid (Culvenor
et al., 1976a).
Trichodesmine and incanine, found in the seeds of Trichodesma
incanum (Yunusov & Plekhanova, 1959), are believed to have been
the causative factors of the "Ozhalangar encephalitis" that was
endemic in Uzbekistan, USSR (1942 - 51), in which the symptoms and
signs were related primarily to the central nervous system
(Shtenberg & Orlova, 1955) (section 7.7).
Table 2. Instances of human toxicity caused by pyrrolizidine alkaloidsa
Principal Plant Country/ Cause of intake Reference
alkaloid Region
Heliotrine and Heliotropium Afghanistan contamination Tandon & Tandon
other alkaloids popovii (1975); Tandon,
similar to B.N. et al.
lasiocarpine (1978); Tandon,
H.D. et al.
(1978);
Mohabbat et al.
(1976)
Senecionine Senecio South contamination Wilmot &
illiciformis; Africa Robertson
Senecio-burchelli (1920)
Senecio spp. South contamination Selzer &
Africa Parker (1951)
Alkaloids of Crotalaria Ecuador medicine Lyford et al.
trichodesmine juncea (1976)
and senecionine
type
Heliotrine and Heliotropium Hong Kong medicine Kumana et al.
lasiocarpine lasiocarpum (1985);
Culvenor et al.
(1986)
Table 2. (cont'd)
Principal Plant Country/ Cause of intake Reference
alkaloid Region
Crotananine and Crotalaria India contamination Tandon, R.K.
cronaburmine nana et al. (1976);
Krishnamachari
et al. (1977);
Siddiqui et al.
(1978a,b)
Heliotrine Heliotropium India medicine Datta et al.
N-oxide eichwaldii (1978a,b)
Monocrotaline Crotalaria West Indies medicine Bras et al.
fulvine retusa; (1954, 1957)
Crotalaria
fulva Stuart & Bras
(1957)
Ilex sp. United medicine McGee et al.
Kingdom (1976)
Riddelline Senecio USA medicine Stillman et al.
retrorsine longilobus (1977); Fox et
N-oxide al. (1978);
(with others) Huxtable (1980)
Indicine N-oxide purified USA medicine Letendre et al.
chemical (1984)
Symphytine, Symphytum sp. USA medicine Ridker et al.
symglandine, and (1985);
other symphytum Huxtable
alkaloids et al (1986)
Table 2. (cont'd)
Principal Plant Country/ Cause of intake Reference
alkaloid Region
Lasiocarpine and Heliotropium USSR contamination Dubrovinskii
heliotrine lasiocarpum (1952);
Mnushkin
(1952)
Trichodesmine and Trichodesma USSR contamination Shtenberg &
incanine incanum Orlova (1955);
Yunosov &
Plekhanova
(1959)
a Adapted from: Culvenor (1983) and Mattocks (1986). Refer also to Table 15 for
details and section 7.
3.3 Pathways of Exposure
Naturally-occurring animal disease is caused by the alkaloid-
containing plants growing in fields and pastures or being fed
accidentally as fodder. They are mostly herbaceous or small shrubs
and many thrive in dry and arid climates. One such plant
containing toxic PA alkaloids has been reported to grow in the
western desert of Egypt (Hammouda et al., 1984). The growth of
this group of plants is particularly prolific during, and
following, periods of drought, as has been reported in association
with the outbreaks of human disease in Afghanistan (Tandon &
Tandon, 1975; Mohabbat et al., 1976) and India (Tandon, B.N. et
al., 1976). Alkaloid-containing plants are widespread in the
tropics, especially Crotalaria, of which there are over 300
species in Africa. Ordinarily, the alkaloid-containing plants have
a bitter taste and grazing animals will reject them, unless their
normal fodder is scarce. However, PAs often occur largely as
N-oxides, which are said not to be bitter, and plants containing
PAs are readily eaten by some animal species.
Human intoxication may result from the ingestion of the toxic
substance in either contamined food or herbal infusion.
3.3.1 Contamination of staple food crops
The products of pyrrolizidine alkaloid-containing plants,
generally seeds, may contaminate the staple food and may be eaten
over long periods of time. The fact that these plants may cause
disease is generally not recognized by the people and such
contamination is known to have resulted in large-scale outbreaks of
poisoning (Dubrovinskii, 1952; Mnushkin, 1952; Shtenberg & Orlova,
1955; Tandon & Tandon, 1975; Mohabbat et al., 1976; Tandon, B.N. et
al., 1976, 1977; Tandon, R.K. et al., 1976; Krishnamachari et al.,
1977; Tandon, H.D. et al., 1977) (Table 2, section 3.1).
3.3.2 Herbal infusions
Plants have been used traditionally for medicinal purposes all
over the world. Herbs have been the mainstay of the indigenous
systems of medicine, especially in China, Greece, and India, since
ancient times. Table 3 includes a list of some plants that are
suspected, or known, to contain PAs and have been used as herbal
medicines in different countries (Mattocks, 1986).
Several PA-containing plants are included among the list of
plants used in indigenous systems of medicine in India (Chopra,
1933). As a part of a research study on the etiological factors of
chronic liver disease in Sri Lanka, Arseculeratne et al. (1981)
chemically screened the first 50 plants used in Sri Lanka's
traditional medicine pharmacopoaea, and found that 3 of them
contained PAs. All 3 were hepatotoxic in rats. Of the 3, the
presence of alkaloids in Cassia auriculata and that of PAs in
Hollarhena antidysenterica had not previously been recorded. It
should be noted that the amount of experimental plant material used
in this study was approximately 6.5 g/kg body weight per day, in
contrast to the approximate intake by a human being estimated to be
in the range of 0.3 - 0.6 g/kg body weight per day. Some, but not
all, of the plants reported to be etiological agents in human cases
of veno-occlusive disease can be found in an inventory of medicinal
plants used in different countries (WHO, 1980), which also
indicates the countries that they are used in. The above lists may
not be complete as many such plants may be used in folk medicine
but have not been mentioned in the scientific literature. However,
the lists do indicate the wide and varied use of such toxic herbs
in all parts of the world.
Lately, there has been a growing interest in the developed
countries in organically grown products for food, as well as home
remedies (Table 3), and some of the PA-containing herbs have been
freely available in herbal shops (Schoental, 1968; Burns, 1972).
Danninger et al. (1983) listed plants containing PAs that are
commonly used in the Federal Republic of Germany as medicaments
(Table 4). He also listed 9 plants in which alkaloids have only
been identified qualitatively, the toxicity of which has not been,
or has been insufficiently, investigated (Table 5). Similarly,
Roitman (1983) listed 10 plants, in which the presence of PAs is
suspected or has been proved and which are used as herbal teas in
the USA. The lists include 10 plants containing PAs, most of which
have been proved hepatotoxic experimentally, some having highly
carcinogenic promoter activity. Some of these alkaloids have been
associated with human case reports of PA toxicity. The more recent
reports (Table 2) of instances of PA poisoning through the use of
herbal medicines are from developed countries (Lyford et al., 1976;
Stillman et al., 1977; Fox et al., 1978; Kumana et al., 1985;
Ridker et al., 1985). Such use of the herbs is the reason that
veno-occlusive disease is endemic in Jamaica (Bras et al., 1954;
Jellife et al., 1954a,b; Bras & Watler, 1955; Stuart & Bras, 1955,
1957). There are obvious difficulties in exercising any kind of
control to restrict this use only to plants that have been tested
and certified as safe for human use. It is impossible to identify
many such herbs, as they are sold as plants or their amorphous
products in the herbal shops.
Table 3. Some plants containing (or suspected of containing) PAs, which have been used
by people either as herbal medicines (M) or foods (F)
Plant Mode Country Referencea
of use or region
BORAGINACEAE
Anchusa officinalis M Europe Broch-Due & Aasen (1980) B
Borago officinalis M USA Delorme et al. (1977) A
Cynoglossum M East Africa Schoental & Coady (1968) A
geometricum
Cynoglossum M Iran Coady (1973) B
officinale
Heliotropium M India Gandhi et al. (1966a); B
eichwaldii Datta et al. (1978a,b) A
H. europaeum M India, Greece IARC (1976) A
H. lasiocarpum M Hong Kong Kumana et al. (1985); A
Culvenor et al. (1986) A
H. indicum M India, Africa, Schoental (1968a); B
South America, Hoque et al. (1976) B
and elsewhere
H. ramossissimum M Arabia Macksad et al. (1970); B
(ramram) Coady (1973) B
H. supinum M Tanzania Schoental & Coady (1968) A
Table 3 (contd.)
Plant Mode Country Referencea
of use or region
Pulmonaria spp. M USA Delorme et al. (1977) A
Symphytum officinale F, M Japan and Hirono et al. (1978, 1979b) A
M USA Furuya & Hikichi (1971); A
Delorme et al. (1977) A
S. x uplandicum F, M General Hills (1976) B
USA Culvenor et al. (1980a,b) A
S. asperum M USA Pedersen (1975) A
COMPOSITAE
Cacalia decomposita M USA Sullivan (1981) B
(matarique)
C. yatabei F Japan Hikichi & Furuya (1978) B
Farfugium japonicum M Japan Furuya et al. (1971) B
Ligularia dentata F Japan Asada & Furuya (1984) B
Petasites japonicus F, M Japan Hirono et al. (1973, 1979b) A
Senecio abyssinicus M Nigeria Williams & Schoental (1970) B
S. aureus M USA Wade (1977) B
Table 3 (contd.)
Plant Mode Country Referencea
of use or region
S. bupleuroides M Africa Watt & Breyer-Brandwijk (1962) A
S. burchelli F, M South Africa Rose (1972) A
S. coronatus M South Africa Rose (1972) A
S. discolor M Jamaica Asprey & Thornton (1955) B
S. doronicum M Germany Roeder et al. (1980a) B
S. inaequidens F South Africa Rose (1972) B
S. jacobaea M Europe Schoental & Pullinger (1972); B
(ragwort) Wade (1977) B
S. longilobus M USA Stillman et al. (1977); A
(S. douglassi) Huxtable (1979a) B
S. monoensis M USA Huxtable (1980) A
S. nemorensis M Germany Habs et al. (1982) A
spp. fuchsii
S. pierotti F Japan Asada & Furuya (1982) B
S. retrorsus M South Africa Rose (1972) A
(S. latifolius)
Table 3 (contd.)
Plant Mode Country Referencea
of use or region
S. vulgaris M Europe Watt & Breyer-Brandwijk (1962) A
(common groundsel)
Netherlands Wade (1977) B
M Iran Coady (1973) B
Syneilesis palmata F Japan Hikichi & Furuya (1976) B
Trichodesma africana M Asia Omar et al. (1983) B
Tussilago farfara M Japan Culvenor et al. (1976a) A
(coltsfoot)
M China Hirono et al. (1976b) A
M Norway Borka & Onshuus (1979) B
M USA Borka & Onshuus (1979); B
Culvenor et al. (1976b); B
LEGUMINOSAE
Crotalaria brevidens F East Africa Coady (1973) B
C. fulva M Jamaica Barnes et al. (1964); A
McLean (1970, 1974) A
Table 3 (contd.)
Plant Mode Country Referencea
of use or region
C. incana M East Africa Schoental & Coady (1968) A
Watt & Breyer-Brandwijk A
(1962)
C. laburnifolia M Tanzania Schoental & Coady (1968) A
F Asia Coady (1973) B
C. mucronata M Tanzania Coady (1973) B
C. recta M, F Tanzania Schoental & Coady (1968); A
Coady (1973) B
C. retusa M, F Africa IARC (1976) A
India Watt & Breyer-Brandwijk (1962) A
C. verrucosa M Sri Lanka Arseculeratne et al. (1981) A
a A = Reference in the reference list of this document.
B = Reference in Mattocks (1986).
Manufactured preparations may also contain PA-containing herbs,
e.g., comfrey-pepsin capsules sold as a digestive aid (Huxtable et
al., 1986).
3.3.3 Use of PA-containing plants as food
Several PA-containing plants are used as food as can be seen in
Table 3 (Mattocks, 1986). Petasites japonicus Maxim, Tussilago
farfara L. (coltsfoot), and Symphytum officinale L. (comfrey or
Russian comfrey) are known as edible plants in Japan, and have been
proved to contain carcinogenic pyrrolizidine alkaloids (Hirono et
al., 1973, 1979a,b). The young flower-stalks of P. japonicus and
the buds of coltsfoot have been used in Japan as human food or
herbal remedies. The leaf and root of comfrey are also used as an
edible vegetable or tonic (Hirono et al., 1978) in Japan and other
countries (Culvenor, 1985). The carcinogenic PAs in these plants
are petasitenine (P. japonicus), senkirkine (coltsfoot), and the
group including symphytine (comfrey). They were also mutagenic in
the Ames system of Salmonella typhimurium and V79 hamster cell line
and induced transformation in cryo-preserved hamster embryonic
cells (Hirono et al., 1979b). Other such PA-containing plants,
used as food in Japan, include young leaves of Syneilesis palmata,
various Cacalia species, and young Senecio pierotti (Mattocks,
1986). According to Culvenor (1985), consumers of comfrey could be
ingesting up to 5 mg PAs per day. Rose (1972) listed a number of
plants of the genus Senecio that are used as spinach in South
Africa. These include S. burchelli, which is known to have caused
an episode of PA poisoning through the ingestion of contaminated
bread (Wilmot & Robertson, 1920).
3.3.4 Contaminated honey
In the USA, Deinzer et al. (1977) reported the presence of all
PAs contained in Senecio jacobaea (ragwort) and proved to be
hepatotoxic, in the honey secreted by bees feeding on the plant.
The total alkaloid content ranged from 0.3 to 3.9 mg/kg. It has
been estimated that an average annual human intake of honey (600 g)
at the highest alkaloid level quoted would contain less than 3 mg
of PAs (Mattocks, 1986). Culvenor et al. (1981) and Culvenor
(1983, 1985) drew attention to the same potential hazard in honey
from Echium plantagineum, a weed that grows widely in Southern
Australia and is a major source of honey, yielding an estimated
2000 - 3000 tonnes per annum for human consumption. Echimidine is
the major component of the alkaloids of Echium, which are present
in concentrations of up to 1 mg/kg. Culvenor (1983) estimated that
individuals may consume up to 80 g honey/day with a corresponding
alkaloid intake of 80 µg/day, if only the Echium honey were used.
No reports of acute human toxicity through this source are
available.
Table 4. Medicinal plants containing PAs of known hepatotoxicity, reported as commonly
used in the Federal Republic of Germany, and the PAs contained in thema
Family Genus Species Pyrrolizidine
alkaloids
Compositae Eupatorium E. cannabinum amabiline±
(hemp agrimony) supinineb
Petasites P. hybirdus senecionineb,c
integerrimineb
senkirkineb
Senecio S. nemorensis fuchsisenecionine
(groundsel) sp. fuchsii senecionineb,c
(Fuch's groundsel)
S. vulgaris senecionineb,c
(groundsel) seneciophyllineb
retrorsineb
riddellineb,c
S. Jacobaea jacobineb
(ragwort) senecionineb,c
seneciphyllineb
jacoline, jaconine
chlorinated PAsd
S. aureus senecionineb,c
(American golden
ragwort)
Tussilago T. farfara senkirkineb
(coltsfoot) (coltsfoot) senecionineb,c
tussilagine
Table 4 (contd.)
Family Genus Species Pyrrolizidine
alkaloids
Alkanna A. tinctoria 7-angelylretronecine
triangularine
dihydroxytriangularine
Anchusa A. officinalis lycopsamine
Boraginaceae Borago B. officinalis lycopsamine/intermedine±
(borage) acetyllycopsamine/
acetylintermedine
amabiline
supinine
Symphytum S. officinale symphytineb
(comfrey) (comfrey) echimidine(?)
lycopsamine
acetyllycopsamineb
lasiocarpineb,c
heliosupine N-oxide
S. peregrinum lycopsamineb
S. x uplandicum intermedineb
symphytineb
echimidineb
7-acetyllycopsamine
7-acetylintermedine
symlandine
uplandicine
S. asperum asperumine
(prickly comfrey) heliosupine N-oxide
echimidineb
echinatine
Table 4 (contd.)
Family Genus Species Pyrrolizidine
alkaloids
Cynoglossum C. officinale heliosupine N-oxide
(hound's (hound's tongue) echinatine
tongue) acetyl heliosupineb
O-7-angelylhelio-
tridineb
Heliotropium H. europaeum heliotrineb,c,e
(Heliotrope) (common heliotrope) lasiocarpineb,c,e
supinine
heleurine
europine
acetyllasiocarpineb
a Modified from: Danninger et al. (1983).
b Toxic alkaloids.
c Alkaloids known to have caused human toxicity.
d Alkaloids with highly carcinogenic promoter activity.
e Used only in homeopathy.
Table 5. Medicinal plants containing PAs, reported as
commonly used in the Federal Republic of Germany,
the toxicity of which has not been, or has been
insufficiently, investigateda
-----------------------------------------------------------
Family Genus Species
-----------------------------------------------------------
Compositae Eupatorium E. perforatum
Brachyglottis B. repens
Arnica A. montana (mountain arnica)
Boraginaceae Lappula L. intermedia (stickseed)
Pulmonaria P. officinalis (lungwort)
-----------------------------------------------------------
a Modified from: Danninger et al. (1983).
3.3.5 Milk
PAs have been shown to produce toxic effects via transference
into the milk of dams (Schoental, 1959). Retrorsine was
administered orally to 17, and intraperitoneally to 6, lactating
rats weighing 185 - 350 g in 5 - 10 mg doses daily, the first dose
being given during the first 24 h after parturition. The rats
received from 1 to 14 doses, the total intake amounting to
21 - 335 mg/kg body weight. The litters were separated from the
mothers for ´ h following the administration of PA to avoid direct
contamination of the former by licking. Apparently the milk
production was not affected as the stomachs of many of the young,
examined postmortem, were distended with milk. All animals whose
mothers had received a total dose of 138 mg PA or more died within
30 days. Many of the young whose mothers had received smaller
doses survived until they were killed at 6 months. Biopsy of the
liver of the young at various intervals or at autopsy showed marked
changes, even in cases where the mothers did not appear to be
affected. Animals dying at 18 - 30 days showed hydropic or fatty
vacuolation of liver cells. In the liver of animals dying or
killed later, various degrees of haemorrhagic necrosis and increase
in the centrilobular reticulin of the liver, and some thickening of
centrilobular veins were seen. In animals that survived 6 months,
the appearance was less abnormal, but some hyperplastic nodules and
bile-duct proliferation were seen. The lactating rats dosed with
the PAs generally survived longer than the suckling animals and
usually did not show any ill effects, suggesting that the
susceptibility of the suckling rats was greater than that of the
mothers.
Dickinson et al. (1976) demonstrated the presence of PAs in the
milk of dairy cattle fed or dosed with ragwort (Senecio jacobaea).
When 4 cows were administered the dried plant material at levels of
up to 10 g/kg body weight per day through rumen cannula, PA levels
of up to 0.84 mg/kg were observed in the milk. However, only one
(jacoline) of the several PAs contained in the plant was secreted.
Calves, bucket fed on the milk did not show any signs of PA
toxicity.
Dickinson (1980) repeated the study on goats. Four milk goats
were freshly prepared with rumen cannulae. The kids were separated
from their dams and were fed milk twice a day. Dried tansy ragwort
plant material with a PA content of 0.16% (dry weight) was
administered through the cannulae to each goat at a dosage rate of
10 g/kg body weight per day over 125 days. During this period,
each of the 4 kids received milk from their dams at approximately
125 ml/kg per day in addition to ad lib feeding on alfalfa grass
hay. Six PAs were isolated from the plant material: jacobine,
jaconine, jaconline, jacozine, senecionine, and seneciphylline.
Milk samples collected twice daily showed PA contents of
225 - 530 µg/litre with a mean of 381 µg/litre. No apparent health
effects were noted in the kids, and only mild hepatic damage was
suspected in the dams, on the basis of liver function tests. Fifty
percent of the kids were killed after 10 weeks. No lesions of PA
toxicity were seen. The dams were rebred and appeared normal
throughout the gestation period. However, three dams aborted at
almost full term, and the fetuses were born dead. One of the dams
died shortly after parturition and showed evidence of severe liver
damage characteristic of PA toxicity. Another, which delivered
normally, also showed a lesser degree of liver damage at biopsy.
Data relating to PA secretion were compared with similar
earlier data on cows. Mean secretion of PAs in cows appeared much
higher, e.g., 684 µg/litre. The authors concluded that the amount
of PAs secreted in the goat's milk did not cause any serious
deleterious effects in the kids.
Johnson (1976) fed long-term lethal doses of Senecio jacobaea,
by stomach tube, to 6 cows. Feeding started at term or within 30
days post-partum, and continued until what was considered to be a
lethal dose had been fed. The daily dose of the plant ranged from
1 to 4.4 g/kg body weight, the total amount fed representing 5 -
15% of body weight over a period of 54 - 126 days. Five cows died
within 98 days; one, in a moribund state, was killed on day 126.
The calves suckled for 30 - 126 days. Suckling started immediately
after birth in the case of 4 calves and 10 and 30 days later,
respectively, in the 2 remaining calves. Three calves were killed
with their dams or soon after, and 3 were retained for 1 year for
observation. Milk samples from 2 cows were collected and pooled in
14- to 16-day lots during 64 days of feeding of the Senecio plant.
Each pooled sample was administered intragastrically to a group of
rats in daily doses of 12 ml for 15 - 30 days. A control group of
rats were fed raw milk from cows not fed Senecio. Blood samples of
the dams and the calves were analysed for glutamic oxaloacetic
transaminase (GOT), lactic dehydrogenase (LDH), and gamma-glutamyl
transpeptidase (GGTP). Serum-enzyme levels in all cows indicated
statistically significant deviations suggesting liver dysfunction,
and the livers at autopsy had characteristic features of PA
toxicosis. The LDH and GOT levels in calves were generally
abnormal after 20 - 45 days of suckling. The abnormalities ranged
from mild to a 15- to 170-fold increase. One calf was autopsied at
the peak increase of serum-enzymes and was found to have mild focal
hepatitis. No significant pathological features were seen in the
livers of other animals, nor of the rats, some of which were
retained for up to 150 days.
Goeger et al. (1982) fed dried Senecio jacobaea (tansy ragwort)
to lactating goats in a proportion of 25% of the feed. The milk
contained 7.5 µg PA/kg dry weight. The milk produced by the goats
was pooled and then bottle fed to appetite to 2 Jersey bull calves
(1 day old) that also had access to tansy ragwort-free hay for 109
and 124 days, respectively. They were then weaned and given normal
feed and observed for 6 months, after which they were killed and
autopsied. In another study, rats were fed a diet containing the
freeze-dried milk at 80% level for 180 days with a calculated total
PA intake of 0.96 mg/rat. Other groups of rats were fed tansy
ragwort at dietary levels of 0.01 - 10 g/kg (corresponding to PA
intakes of 39.77, 5.04, 0.52, and 0.05 mg/rat). The calf livers
only showed very mild non-specific changes, but the livers of rats
fed tansy ragwort or the milk from tansy ragwort-fed goats
showed definite, but mild, changes including swollen
hepatocytes, megalocytosis, biliary hyperplasia, and fibrosis.
Histopathological changes in milk-fed rats were similar to those in
the group fed tansy ragwort in the diet at 0.01 g/kg. The authors
concluded that there was evidence of PA transfer into milk, which
proved hepatotoxic for rats. It was also noted that the goats had
been fed high levels of tansy ragwort at the upper limit of their
acceptance, and that the hepatic changes observed in rats fed high
levels of milk, for extensive periods, were slight.
Luthy et al. (1983) produced direct evidence of excretion of
macrocyclic esters of retronecine of the senecionine and
seneciphylline-type into rat milk. 3H-retronecine, an 3H-necic
acid-labelled senecionine, and seneciphylline were prepared
biosynthetically with seedlings of Senecio vulgaris L. Two
lactating rats (Ivanovas, Sprague Dawley), weighing 300 - 400 g,
were fed the first of the second compound by stomach tube, in doses
of 2.7 mg/kg and 5.5 mg/kg body weight, respectively. Samples of
blood were examined 1, 3, and 6 h after treatment, and those of
milk 1 and 3 h after. Animals were killed after 6 h. They were
found to have excreted approximately 0.08% of the applied
radioactivity in the milk within 3 h, mainly as unidentified
retronecine-derived metabolites, and approximately 0.02% as
unchanged PAs. The highest levels of PAs and metabolites in
tissues were found in the liver and lungs, 6 h after
administration.
Candrian et al. (1984a) also demonstrated that Drosophila
melanogaster flies fed on milk from lactating rats that had been
administered an oral dose of seneciphylline showed 1.2% sex-linked
recessive lethals, compared with 0.3% in controls, indicating the
transfer of the mutagenic properties of the PA via milk (section
6.4.7).
The implications of the above studies on the possibility of
carry-over of PAs into foodstuffs of animal origin are obvious.
However, no reports of human cases of acute PA toxicity, ascribed
to the consumption of contaminated milk, are available.
3.3.6 Meat
There have not been any reports of the detection of PAs in meat
products from livestock exposed to them.
3.3.7 Use of PAs as chemotherapeutic agents for cancer
An alkaloid of Heliotropium indicum L. (indicine N-oxide) has
been found to have antitumour activity and has been used in
experimental clinical chemotherapy for cancer (section 7.9).
4. METABOLISM
4.1 Absorption, Excretion, and Tissue Distribution
4.1.1 Absorption
There have been few studies on the absorption of PAs in man,
but absorption has been inferred from studies on tissue
distribution and the amounts of alkaloids and their metabolites
excreted in the urine, faeces, and bile of animals (section 4.1.2).
Swick et al. (1982c) measured the transfer of a mixture of
pyrrolizidine alkaloids extracted from Senecio jacobaea, across
isolated intestine and stomach segments from rabbits. The alkaloid
mixture contained seneciphylline, jacobine, jacozine, jacoline, and
senecionine. The alkaloids were transferred across the ileum and
jejunum, but not the stomach. Brauchli et al. (1982) compared the
oral and percutaneous absorption in rats of a crude alkaloid
mixture obtained from comfrey ( Symphytum officinale L.). The
mixture consisted of N-oxides of 7 alkaloids, principally 7-acetyl-
intermedine and 7-acetyl-lycopsamine. A dose of 194 mg/kg was
either given by gavage, or was applied to the shaved skin and left
for 44 h. After the dermal application, the excreted N-oxides in
urine (up to 48 h) amounted to 0.1 - 0.4% of the dose. After oral
dosage the excreted level of N-oxides and alkaloid bases was quoted
as being 20 - 50 times greater.
4.1.2 Excretion and distribution
The excretion and distribution of heliotrine in rats has been
reported in Bull et al., 1968. Young rats (150 g), given the LD50
of heliotrine by ip injection, were killed at intervals, bled
quickly, and the organs and tissues analysed. Heliotrine was
present in the liver after 2 min (3.7% of total dose), the level
peaking at 5 min (6.3%), and dropping to 2.2% at 1 h and 0.5% at
2.5 h. In adult rats, the level in the liver at 5 h was 0.07% of
the total dose. Five min after dosing, 30 - 40% of the initial
dose remained in the peritoneal cavity, and the blood level of
heliotrine was 60 mg/litre, dropping to 3 mg/litre at 1 h. The
urinary excretion of base and metabolites other than pyrrolic
metabolites, collected and measured 16 h after administration of
several alkaloids by ip injection, is shown in Table 6. The
proportion of base excreted unchanged increased with the
hydrophilicity of the alkaloid, being 62% for heliotrine N-oxide,
30% for heliotrine, and only 1 - 1.5% for lasiocarpine.
Heliotridine, the hydrolysis product from heliotrine and
lasiocarpine, was excreted in the form of the N-oxide in larger
quantities after the administration of each of these alkaloids.
The distribution and excretion of monocrotaline was studied in
rats by Hayashi (1966) who found that 50 - 70% was excreted in the
urine within the first day. However, the analysis was by a non-
specific chemical method that did not distinguish between the
unchanged alkaloid and its metabolites. Mattocks (1968a) gave
toxic pyrrolizidine alkaloids intraperitoneally to male rats and
measured the urinary excretion of the unchanged alkaloid, and of
N-oxide and pyrrolic metabolites. The excretion of N-oxide and
unchanged alkaloid was rapid and almost complete in the first 24 h.
Excretion of pyrroles was also rapid but continued for a little
longer. For example, in rats given retrosine (60 mg/kg body
weight), the urine in the first 24 h contained 10.6% unchanged
alkaloid, 13.3% N-oxide, and 13.4% pyrrolic metabolites. During
the second day, only 0.1% alkaloid, 0.2% N-oxide, and 1.8% pyrroles
were excreted. Biliary excretion also occurred. About one-quarter
of an iv dose of retrosine in rats was excreted in the bile as
pyrrolic metabolites, and 4% as unchanged alkaloid; most of this
excretion occurred during the first hour after the injection
(White, 1977).
Jago et al. (1969) gave heliotrine iv to sheep; urinary
excretion of the unchanged alkaloid together with metabolites
( N-oxide, and demethylation and hydrolysis products) occurred
rapidly and continued for up to 8 h. Excretion in the bile was
only 2% of that in the urine.
The tissue distribution of radioactivity from a tritiated toxic
pyrrolizidine alkaloid analogue was studied by Mattocks & White
(1976) using synthanecine A bis- N-ethylcarbamate (40 mg/kg body
weight). The highest concentrations of radioactivity were seen in
the liver (where metabolism occurs), lungs, kidneys, and spleen
(respectively, 3.9%, 0.19%, 0.18%, and 0.27% of the dose given),
and about 69% of the dose was eliminated in the urine during the
first day. Radioactivity in the expired air was negligible. The
binding of radioactivity in the liver, and especially the lungs,
was more persistent than in other organs. Similar results were
given by the semisynthetic pyrrolizidine alkaloid analogue,
retronecine bis- N-ethylcarbamate (Mattocks, 1977).
Table 6. Urinary metabolites of pyrrolizidine bases in the rat (16-h urine)a
Urine constituent (amount in percentage of dose injected)
Base Unchanged Base Heliotridine Heliotridine Heliotridine Heliotridine
administered base N-oxide trachelanthate trachelanthate N-oxide
(ip injection) N-oxide
Heliotrine 30 Trace 10 5 3 15
Heliotrine 62 (62) 2.7 ca. 6 ca. 1 ca. 10
N-oxide
Lasiocarpine 1-1.5 1.5-3 6
Heliotridine 35 ca. 1 (35) (ca. 1) 5 20
trachelanthate
Heliotridine 40 20 (40) (20)
a From: Bull et al. (1968).
The distribution of the uniformly 14C-labelled natural
pyrrolizidine alkaloid senecionine in lactating mice was studied by
Eastman et al. (1982). After 16 h, 75% of the radioactivity had
been recovered in the urine, 14% in the faeces, but only 0.04% was
in the milk; the liver contained 1.92%. The mice were milked using
teat cups. Candrian et al. (1985) studied the distribution of
radioactivity in rats given small doses of senecionine or
seneciphylline (0.3 - 3.3 mg/kg), tritiated in the pyrrolizidine
(retronecine) moiety. Most radioactivity was eliminated in the
urine and faeces within 4 days. Using mass spectrometry, Dickinson
et al. (1976) found a concentration of up to 0.84 mg PAs/litre in
the milk of cows fed Senecio jacobaea. Blood levels of senecionine
in rats given 0.1 LD50 ip were determined by Culvenor (1978). The
levels were 0.38, 0.32, and 0.14 mg/litre at 0.5, 1, and 2 h after
injection, respectively.
To summarize, the available evidence suggests that ingested
toxic pyrrolizidine alkaloids are rapidly metabolized and that the
excretion of unchanged alkaloid and of most metabolites is also
rapid. Thus, within a few hours, only a relatively small
proportion of the dose remains in the body, much of this in the
form of metabolites bound to tissue constituents. It appears
improbable that a significant amount of unchanged alkaloid will
remain in the body after the first day.
Pyrrolizidine N-oxides are much more water soluble than their
parent alkaloids. Indicine N-oxide (which is exceptionally water
soluble) is very rapidly excreted, either unchanged or conjugated.
Thus, indicine N-oxide given iv to mice, monkeys, or rabbits
disappeared from the serum with initial half-lives ranging from 3
to 20 min (Powis et al., 1979; El Dareer et al., 1982). Over 80%
of tritium-labelled indicine N-oxide given iv was excreted in the
urine of mice or monkeys within 24 h (El Dareer et al., 1982); at
2 h, the highest concentrations of radioactivity were in the
kidneys, liver, and intestines. Urinary excretion of indicine
N-oxide was also rapid in rabbits, but somewhat slower in human
beings (Powis et al., 1979).
4.2 Metabolic Routes
The major metabolic routes of unsaturated pyrrolizidine
alkaloids in animals are: (a) hydrolysis (of the ester groups);
(b) N-oxidation; and (c) dehydrogenation (of the pyrrolizidine
nucleus) to dehydro-alkaloids (pyrrolic derivatives). Other minor
routes of metabolism are known, but the three pathways account for
the major known toxic effects of these alkaloids (Fig. 5). Routes
(a) and (b) are believed to be detoxification mechanisms. Route
(c) leads to toxic metabolites and appears to be the major
activation mechanism. Route (a) may occur in various tissues,
including the liver and blood. Routes (b) and (c) are brought
about in the liver by the microsomal mixed-function oxidase system.
The alkaloids are fairly stable chemically, but the ester
groups may undergo hydrolysis under alkaline conditions. Some
alkaloids in plant material may decompose during drying (Bull et
al., 1968), but others appear to be stable under similar conditions
(Pedersen, 1975; Birecka et al., 1980). The N-oxides of
unsaturated pyrrolizidines are more readily decomposed by heat than
the basic alkaloids, especially when dry. However, the stability
of the alkaloids and N-oxides in hot water as, for example, in
cooking, is not known.
Some pyrrolizidine alkaloids have a limited water solubility,
unless neutralized with acid; but others (e.g., indicine), and all
the N-oxides, are readily soluble.
2.2 Analytical Methods
When analysing for PAs, it is important to recognize that this
group consists of many different compounds (section 2.1) and that
these often occur as mixtures in plants or in materials of plant
origin. They may vary in structure, relative molecular mass,
response to analytical procedures, and toxicity. Both basic
alkaloids and corresponding N-oxides may be present at the same
time. Thus, where such mixtures are present, analyses will
inevitably be approximate, unless the individual components are
separated and identified.
Nevertheless, such estimates can be useful. In particular, all
hepatotoxic PAs are unsaturated in the sense that they possess a
1:2-double bond in the pyrrolizidine nucleus, and analytical
methods that are specific for this structure can be of value in
screening for potential toxicity. A simple qualitative field test
for screening plant materials for the presence of such alkaloids
and their N-oxides, without the need of high technology equipment,
is described in section 2.2.2.5.
2.2.1 Extraction
2.2.1.1 Plant tissue
Pyrrolizidine alkaloids are usually extracted from dried,
milled plant material with hot or cold alcohol. The alcohol is
evaporated, the bases taken up in dilute acid, and fats extracted
with ether or petroleum. It is usual, at this stage, to reduce any
N-oxides present to the corresponding basic alkaloids with zinc,
before making the solution alkaline and extracting the alkaloids
with chloroform (Koekemoer & Warren, 1951). Alternatively, alcohol
can be continuously circulated through the plant material and then
cation exchange resin, and the alkaloids subsequently eluted from
the resin (Mattocks, 1961; Deagen & Deinzer, 1977). PAs can also
be extracted by soaking plant material in dilute aqueous acid
(Briggs et al., 1965; Craig et al., 1984).
2.2.1.2 Biological fluids and tissues
Pyrrolizidine alkaloids have been extracted for analytical
purposes from honey (Deinzer et al., 1977), milk (Dickinson et al.,
1976), blood-plasma (Ames & Powis, 1978; McComish et al., 1980),
urine (Mattocks, 1967a; Jago et al., 1969; Evans et al., 1979), and
bile (Jago et al., 1969; Lafranconi et al., 1985).
When attempting to isolate PAs from animal tissues, it must be
appreciated that the toxic alkaloids are often metabolized very
rapidly in animals, so that the amounts that are recoverable
(except from urine), only a few hours after alkaloid ingestion, may
be extremely small. Various methods have been used to separate
PAs, but some mixtures are extremely difficult to separate. On the
analytical scale, the most useful methods are thin-layer
chromatography (TLC), high-performance liquid chromatography
(HPLC), and gas chromatography (GC) (section 2.2.2).
2.2.2 Analysis for pyrrolizidine alkaloids
2.2.2.1 Thin-layer chromatography (TLC)
For TLC, silica plates are usually used, eluted with chloroform:
methanol:aqueous ammonia mixtures (Sharma et al., 1965; Chalmers
et al., 1965); solvents suitable for the N-oxides, which
are more water-soluble, have been described by Mattocks (1967b)
and Wagner et al. (1981). The most sensitive methods for
detecting PAs on TLC are those using Ehrlich reagent
(4-dimethylaminobenzaldehyde) (Mattocks, 1967b). The unsaturated
alkaloids are best visualized by spraying the plates first with a
solution of orthochloranil, then with Ehrlich reagent, heating
after each spray (Molyneux & Roitman, 1980). The N-oxides of
unsaturated pyrrolizidines are detected by spraying a solution of
acetic anhydride, heating the plate, and then spraying Ehrlich
reagent (Mattocks, 1967b).
Pyrrolizidine alkaloids with a saturated base moiety must be
detected in other ways (which are not specific for pyrrolizidines),
e.g., by exposing the dried plates to iodine vapour, or by spraying
with an iodobismuth (Dragendorff) reagent (Munier, 1953).
2.2.2.2 High-performance liquid chromatography (HPLC)
Analytical or preparative scale HPLC separation of
pyrrolizidine alkaloids has been described by Segall (1979a,b) and
Dimenna et al. (1980), and an improved method has been reported by
Ramsdell & Buhler (1981). Alkaloids from Symphytum officinale
(comfrey) have been separated on an analytical scale by Tittel et
al. (1979), and partially separated on a preparative scale by
Huizing et al. (1981). UV detectors are usually used for the HPLC
of pyrrolizidine compounds (Mattocks, 1986).
2.2.2.3 Gas chromatography (GC) and mass spectrometry (MS)
The GC characterization of PAs using packed columns has been
described by Chalmers et al. (1965) and Wiedenfeld et al. (1981).
Mixtures of alkaloids from comfrey ( Symphytum sp.), normally hard
to separate, were resolved by Culvenor et al. (1980a) and Frahn et
al. (1980) by GC of the methylboronate derivatives.
Gas chromatography combined with mass spectrometry (GC-MS) has
become a valuable and highly sensitive means for both the
identification and the quantitative determination of pyrrolizidine
alkaloids. Thus, alkaloids extracted from honey were separated and
identified by Deinzer et al. (1977) and (as butylboronate
derivatives) by Culvenor et al. (1981). Deinzer et al. (1978)
described a method for the recognition (but not the individual
identification) of retronecine-based pyrrolizidine alkaloids, by
hydrolysing them to retronecine (the amino alcohol moiety) followed
by GC-MS of its bis-trifluoroacetate. The use of capillary GC has
greatly improved the sensitivity of pyrrolizidine alkaloid
analysis, especially when used with MS (Luthy et al., 1981). The
MS of pyrrolizidine compounds has been reviewed (Bull et al., 1968;
Mattocks, 1986).
Pyrrolizidine N-oxides generally undergo thermal decomposition,
when subjected to GC, but they can first be reduced to the
corresponding basic alkaloids (Koekemoer & Warren, 1951).
Alternatively they may be derivatised. Thus, trimethylsilylation
of indicine N-oxide or heliotrine N-oxide can lead either to the
trimethylsilyl (TMS) derivative of the parent alkaloid or to the
TMS derivative of the dehydro-alkaloid (pyrrolic derivative),
depending on the reagents used, and these products will run
successfully on GC-MS (Evans et al., 1979, 1980).
2.2.2.4 Nuclear magnetic resonance (NMR) spectrometry
A convenient, but relatively insensitive, method, specifically
for the determination of unsaturated PAs, has been described by
Molyneux et al. (1979). The basic alkaloids are extracted, then
subjected to NMR spectrometry along with an internal standard
( p-dinitrobenzene). This enables quantitative measurements to be
made of the signal(s) representing the H2 proton(s) in unsaturated
pyrrolizidines, and thus the alkaloid(s) can be determined.
Quantitative NMR analysis of pyrrolizidine alkaloid mixtures from
Senecio vulgaris has been described by Pieters & Vlietinck (1985)
and compared with an HPLC method by the same authors (1986).
Qualitative aspects of the NMR spectrometry of pyrrolizidine
alkaloids have been reviewed by Bull et al. (1968) and Mattocks
(1986).
2.2.2.5 The Ehrlich reaction
This method (Mattocks, 1967a, 1968b) is specific for
unsaturated pyrrolizidine alkaloids and is not suitable for other
alkaloids. Thus, it is the most useful colorimetric method for
potentially hepatotoxic pyrrolizidine compounds. The procedure
converts the alkaloid into its N-oxide, using hydrogen peroxide.
The product reacts with acetic anhydride to form a pyrrolic
derivative (dehydro-alkaloid) that gives a magenta colour with a
specially modified Ehrlich reagent. The latter contains boron
trifluoride to give maximum sensitivity. As little as 5 µg of most
unsaturated pyrrolizidines can be measured by this method. If the
oxidation stage is omitted, only the unsaturated pyrrolizidine
N-oxides can be determined. The determination of pyrrolizidine
N-oxides has also been discussed by Mattocks (1971b).
A simplification of the above colorimetric procedure was
described by Mattocks (1971d) to provide a qualitative test that
could be used to screen large numbers of plant samples for the
presence of unsaturated pyrrolizidine alkaloid N-oxides. An
improved version of this field test is now available (Mattocks &
Jukes, 1987). It is suitable for any plant parts, such as leaves,
stems, flowers, seeds, or roots, or materials of plant origin, such
as cereals or herbal teas, but has not yet been applied to cooked
food.
The plant material (0.2 - 1 g) is extracted by grinding it with
aqueous ascorbic acid (5%) and a small amount of sand. The
solution is filtered and divided into two equal portions ("test"
and "blank"). An aqueous solution (0.2 ml) of sodium nitroprusside
(5%) containing sodium hydroxide (10-3 mol) is added to the "test"
sample. Both portions are heated for approximately 1 min at 70 -
80 °C; then Ehrlich reagent is added and heating is continued for
1 min. The Ehrlich reagent contains 4-dimethylaminobenzaldehyde
(5 g) dissolved in a mixture of acetic acid (60 ml), water (30 ml),
and 60% perchloric acid (10 ml). A magenta colour in the "test"
compared with the "blank" indicates the presence of an unsaturated
PA N-oxide. The "blank" may show a colour if the plant contains
compounds, such as indoles or pyrroles, which can themselves give a
colour with Ehrlich reagent. The intensity of colour in the
"sample" compared with the "blank" can give a rough idea of the
amount of alkaloids present, and indicate whether further chemical
or toxicological testing of the plant material is adviseable.
In practice, the majority of PA-containing plants contain
enough alkaloid in the N-oxide form (often a large proportion) to
react positively in this test. The main exceptions are some seeds
(Crotalaria), which may contain much alkaloid base, but little or
no N-oxide. These (and any other sample not containing
chlorophyll) can be tested for basic PAs by grinding them with
chloroform, heating the filtered extract with a solution (0.1 ml)
of orthochloranil (0.5%) in acetonitrile, and then heating it with
Ehrlich reagent. A magenta colour indicates the presence of an
unsaturated PA. Non-toxic pyrrolizidine alkaloids having a
saturated pyrrolizidine nucleus, and pyrrolizidine alkaloids that
are otonecine esters, such as petasitenine, will not respond to
this test.
2.2.2.6 Indicator dyes
A method generally applicable to tertiary bases has been
adapted for pyrrolizidine alkaloids by Birecka et al. (1981). It
is sensitive, but is not specific for this group of alkaloids, and
it does not distinguish between the saturated and unsaturated
alkaloids. A chloroform solution of the alkaloid is shaken with
acidified aqueous methyl orange. The yellow alkaloid:dye complex
is subsequently released from the chloroform phase, using ethanolic
sulfuric acid, and measured spectrophotometrically.
2.2.2.7 Direct weighing
An insensitive way to determine the alkaloids in, for example,
a plant sample, providing enough is available, is to extract the
alkaloids (section 2.2.1) and weigh them. This will provide a
rough measure of the total bases present in the sample; however,
these may not necessarily be PAs. Nevertheless, the sample can
then be subjected to further tests, e.g., GC-MC, nuclear magnetic
resonance (NMR), or colorimetric analysis. Furthermore,
pyrrolizidine N-oxides are generally too water soluble to be
appreciably extractable from aqueous solution by chloroform. Thus,
if two portions of the sample are extracted, and one of them is
reduced to convert N-oxides to bases, the weight difference between
the two products will represent the alkaloid existing in the form
of N-oxide in the original sample.
2.3 Determination of Metabolites in Animal Tissues
Important metabolites of toxic pyrrolizidine alkaloids in
animals include "pyrrolic" derivatives (dehydro-alkaloids) and
N-oxides. A procedure for measuring pyrrolic metabolites in tissue
samples (such as liver or lung) has been described by Mattocks &
White (1970). The sample (usually 0.5 g) is homogenized in an
ethanolic solution of mercuric chloride; the solids are separated
by centrifugation and heated with Ehrlich reagent to give a soluble
colour that can be measured spectrophotometrically.
The measurement of pyrrolic and N-oxide metabolites, formed by
the action of hepatic microsomal preparations on PAs in vitro, is
an improvement described by Mattocks & Bird (1983).
3. SOURCES AND PATHWAYS OF EXPOSURE
3.1 Hepatotoxic Pyrrolizidine Alkaloids and Their Sources
Plants constitute the only natural source of pyrrolizidine
alkaloids (PAs) that cause toxic reactions in man and animals. PAs
occur in a number of species in the families Boraginaceae,
Compositae, Leguminosae (genus Crotalaria), Ranunculaceae (genus
Caltha), and Scrophulariaceae (genus Castilleja) (Table 1). The
most important genera of PA-containing toxic plants are Crotalaria
(Leguminosae), Senecio (Compositae), Heliotropium, Trichodesma,
Amsinckia, Echium, and Symphytum (Boraginaceae) (Hooper, 1978).
The recorded cases of human toxicity have mainly been caused by at
least 12 different pyrrolizidine alkaloids, mostly derived from
Heliotropium, Senecio, and Crotalaria genera. The Senecio spp.
grow throughout the world; the Crotalaria spp. are mainly found in
the tropics and subtropics (Culvenor, 1980).
Table 1. List of plant genera containing toxic pyrrolizidine alkaloids
(with number of species investigated)
-------------------------------------------------------------------------------------
Family Genera
-------------------------------------------------------------------------------------
Apocynaceae Fernaldia (1), Parsonsia (4),
Boraginaceae Alkanna (1), Amsinckia (4), Anchusa (2), Asperugo (1), Borago (1),
Caccinia (1), Cynoglossum (9), Echium (3), Hackelia (1),
Heliotropium (25), Lappula (2), Lindelofia (7), Lithosperum (1),
Macrotomia (1), Messerschmidtia (1), Myosotis (2), Paracaryum (1),
Paracynoglossum (1), Rindera (5), Solenanthus (4), Symphytum (7),
Tournefortia (2), Trachelanthus (2), Trichodesma (2), Ulugbekia (1)
Compositae Adenostyles (3), Brachyglottis (1), Cacalia (4), Conoclinium (1),
Crassocephalum (1), Doronicum (2), Echinacea (2), Emilia (2),
Erechtites (1), Eupatorium (8), Farfugium (1), Gynura (2),
Ligularia (5), Petasites (4), Senecio (142), Syneilesis (1),
Tussilago (1)
Leguminosae Crotalaria (60)
Ranunculaceae Caltha (2)
Scrophulariaceae Castilleja (1)
-------------------------------------------------------------------------------------
An alphabetical list of pyrrolizidine alkaloids with their
plant sources has been published by Smith & Culvenor (1981) and
Mattocks (1986). An updated version is attached as Appendix I.
The plant genera containing toxic PAs are listed in Table 1
indicating the number of species investigated for PAs. A
comprehensive list of species of plants belonging to each of these
genera, the alkaloids isolated from each, and the part of the plant
containing the alkaloid are presented in Appendix II. Table 1 in
Appendix II includes species known to contain alkaloids of proved
hepatotoxicity, or of a molecular structure that would make them
very probably hepatotoxic. Table 2 in Appendix II includes species
containing pyrrolizidine amino-alcohols or esters, which, while not
having all the features of hepatotoxicity, would need only minor
structural modifications to render them hepatotoxic. Plants of the
same taxonomic groups as the plants of proven hepatotoxicity are
listed in part (a) of the table. There is a possibility that, on
further examination, hepatotoxic alkaloids may be found, as minor
constituents, in strains or parts of these plants not yet
investigated or under specific conditions of growth. It should be
noted that the species that have been investigated and are listed
are only few compared with the total number of species in each
genera. It has been recommended by Smith & Culvenor (1981) that it
would be prudent to regard all species in the family Boraginaceae
and the genera Crotalaria, Senecio, and Eupatorium as potentially
hepatotoxic.
It is pertinent to note that the alkaloid content in different
parts of the plant (e.g., roots, leaves, stalks, flowers, and buds)
varies and is subject to fluctuations according to the climate,
soil conditions, and time of harvesting (Danninger et al., 1983;
Hartmann & Zimmer, 1986). Mattocks (1980) demonstrated that the
alkaloid content of the leaves of Symphytum spp. (Russian
comfrey), which are used as an item of food, varies with their
maturity. The toxic PA content is highest at the beginning of the
vegetative period and declines as the leaves mature. The PA
content of the roots is much higher than that of the leaves, and
dried leaves contain a higher concentration than fresh leaves
(Mattocks, 1986). According to Danninger et al. (1983), in some
species (Symphytum asperum), relatively long storage may lead to a
reduction in the alkaloid content, presumably because enzymes are
released during drying. Candrian et al. (1984b) studied the
stability of PAs in hay and silage containing various amounts of
Senecio alpinus. The PA content of hay remained constant for
several months, but the PAs in silage were mainly degraded.
However, the degradation of PAs was much less complete in the lower
concentration range. A quantitatively significant PA-degradation
product in silage was identified as retronecine. Silage with an
S. alpinus percentage of 3.5 - 23 still contained macrocyclic PAs at
a concentration of about 20 mg/kg wet weight. Such silage was not
considered safe for cattle bearing in mind that a 600-kg calf eats
about 30 kg silage/day, amounting approximately to a daily intake
of about 1 mg PAs/kg body weight. In feeding trials with Senecio
jacobaea, Johnson (1979) found that the minimum lethal dose for
cattle was between 1 and 2 mg PAs/kg body weight per day.
PAs known to have been associated with instances of human toxic
liver disease in different parts of the world are listed in Table
2. Two groups of alkaloids that, according to Culvenor (1983), are
consumed in significant amounts by people in different parts of the
world include:
(a) Echimidine, acetyllycopsamine, and related alkaloids
(many countries)
Leaves of plants of the Symphytum sp. ( Symphytum officinale
(comfrey) and Symphytum x uplandicum) are used traditionally as a
salad and as a medicinal herb in Australia, many countries of
Europe, and the USA. S. officinale has been shown to be
carcinogenic for rats (Hirono et al., 1978). Leaves of Russian
comfrey contain a concentration of alkaloids (mainly echimidine) of
0.1 - 1.5 g/kg. The highest level of daily consumption of the
alkaloids has been estimated to be 5 - 6 mg (Culvenor, 1983).
(b) Echimidine and related alkaloids (Australia)
PAs derived from Echium plantagineum, with echimidine as the
major component, have been found in honey secreted by bees feeding
on the plant (Culvenor et al., 1981). The plant is a major source
of honey (section 3.3.4).
3.2 Pneumotoxic and Other Toxic Pyrrolizidine Alkaloids
Not all hepatotoxic alkaloids are pneumotoxic. The commonest
ones used to produce experimental lung injury are fulvine (Barnes
et al., 1964; Kay et al., 1971a; Wagenvoort et al., 1974a,b) and
monocrotaline (Lalich & Ehrhart, 1962; Chesney & Allen, 1973b;
Huxtable et al., 1977). These are also the most active (Mattocks,
1986). The seeds of Crotalaria spectabilis, which contain
monocrotaline, have also been used to study pneumotoxic effects on
experimental animals (Turner & Lalich, 1965; Kay & Heath, 1966; Kay
et al., 1967a) and C. spectabilis has been called the pulmonary
hypertension plant (Kay & Heath, 1969), because of the pulmonary
hypertensionogenic properties of the PAs it contains. Culvenor et
al. (1976a) screened 62 PAs for hepatotoxicity and pneumotoxicity.
Chronic lung lesions were produced by most compounds that induced
chronic liver lesions, though high doses were required in some
instances. It is possible that chronic lung lesions may not occur
in experimental animals because of early death due to acute
toxicity. However, the authors identified a number of PAs that
were particularly prone to produce chronic lung damage in rats
including crispatine, senecionine, seneciphylline, and usaramine
(12-membered macrocyclic, retronecine diesters), anacrotine and
madurensine (crotonecine esters), and the heliotridine esters,
heliosupine, lasiocarpine, and rinderine.
The molecular structure-activity requirements for
pneumotoxicity are the same as those for hepatotoxicity. This is
consistent with their both being caused by the same toxic
metabolites and by the metabolic activation of the alkaloids in the
liver cells to form a reactive pyrrolic dehydro-alkaloid (Culvenor
et al., 1976a).
Trichodesmine and incanine, found in the seeds of Trichodesma
incanum (Yunusov & Plekhanova, 1959), are believed to have been
the causative factors of the "Ozhalangar encephalitis" that was
endemic in Uzbekistan, USSR (1942 - 51), in which the symptoms and
signs were related primarily to the central nervous system
(Shtenberg & Orlova, 1955) (section 7.7).
Table 2. Instances of human toxicity caused by pyrrolizidine alkaloidsa
Principal Plant Country/ Cause of intake Reference
alkaloid Region
Heliotrine and Heliotropium Afghanistan contamination Tandon & Tandon
other alkaloids popovii (1975); Tandon,
similar to B.N. et al.
lasiocarpine (1978); Tandon,
H.D. et al.
(1978);
Mohabbat et al.
(1976)
Senecionine Senecio South contamination Wilmot &
illiciformis; Africa Robertson
Senecio-burchelli (1920)
Senecio spp. South contamination Selzer &
Africa Parker (1951)
Alkaloids of Crotalaria Ecuador medicine Lyford et al.
trichodesmine juncea (1976)
and senecionine
type
Heliotrine and Heliotropium Hong Kong medicine Kumana et al.
lasiocarpine lasiocarpum (1985);
Culvenor et al.
(1986)
Table 2. (cont'd)
Principal Plant Country/ Cause of intake Reference
alkaloid Region
Crotananine and Crotalaria India contamination Tandon, R.K.
cronaburmine nana et al. (1976);
Krishnamachari
et al. (1977);
Siddiqui et al.
(1978a,b)
Heliotrine Heliotropium India medicine Datta et al.
N-oxide eichwaldii (1978a,b)
Monocrotaline Crotalaria West Indies medicine Bras et al.
fulvine retusa; (1954, 1957)
Crotalaria
fulva Stuart & Bras
(1957)
Ilex sp. United medicine McGee et al.
Kingdom (1976)
Riddelline Senecio USA medicine Stillman et al.
retrorsine longilobus (1977); Fox et
N-oxide al. (1978);
(with others) Huxtable (1980)
Indicine N-oxide purified USA medicine Letendre et al.
chemical (1984)
Symphytine, Symphytum sp. USA medicine Ridker et al.
symglandine, and (1985);
other symphytum Huxtable
alkaloids et al (1986)
Table 2. (cont'd)
Principal Plant Country/ Cause of intake Reference
alkaloid Region
Lasiocarpine and Heliotropium USSR contamination Dubrovinskii
heliotrine lasiocarpum (1952);
Mnushkin
(1952)
Trichodesmine and Trichodesma USSR contamination Shtenberg &
incanine incanum Orlova (1955);
Yunosov &
Plekhanova
(1959)
a Adapted from: Culvenor (1983) and Mattocks (1986). Refer also to Table 15 for
details and section 7.
3.3 Pathways of Exposure
Naturally-occurring animal disease is caused by the alkaloid-
containing plants growing in fields and pastures or being fed
accidentally as fodder. They are mostly herbaceous or small shrubs
and many thrive in dry and arid climates. One such plant
containing toxic PA alkaloids has been reported to grow in the
western desert of Egypt (Hammouda et al., 1984). The growth of
this group of plants is particularly prolific during, and
following, periods of drought, as has been reported in association
with the outbreaks of human disease in Afghanistan (Tandon &
Tandon, 1975; Mohabbat et al., 1976) and India (Tandon, B.N. et
al., 1976). Alkaloid-containing plants are widespread in the
tropics, especially Crotalaria, of which there are over 300
species in Africa. Ordinarily, the alkaloid-containing plants have
a bitter taste and grazing animals will reject them, unless their
normal fodder is scarce. However, PAs often occur largely as
N-oxides, which are said not to be bitter, and plants containing
PAs are readily eaten by some animal species.
Human intoxication may result from the ingestion of the toxic
substance in either contamined food or herbal infusion.
3.3.1 Contamination of staple food crops
The products of pyrrolizidine alkaloid-containing plants,
generally seeds, may contaminate the staple food and may be eaten
over long periods of time. The fact that these plants may cause
disease is generally not recognized by the people and such
contamination is known to have resulted in large-scale outbreaks of
poisoning (Dubrovinskii, 1952; Mnushkin, 1952; Shtenberg & Orlova,
1955; Tandon & Tandon, 1975; Mohabbat et al., 1976; Tandon, B.N. et
al., 1976, 1977; Tandon, R.K. et al., 1976; Krishnamachari et al.,
1977; Tandon, H.D. et al., 1977) (Table 2, section 3.1).
3.3.2 Herbal infusions
Plants have been used traditionally for medicinal purposes all
over the world. Herbs have been the mainstay of the indigenous
systems of medicine, especially in China, Greece, and India, since
ancient times. Table 3 includes a list of some plants that are
suspected, or known, to contain PAs and have been used as herbal
medicines in different countries (Mattocks, 1986).
Several PA-containing plants are included among the list of
plants used in indigenous systems of medicine in India (Chopra,
1933). As a part of a research study on the etiological factors of
chronic liver disease in Sri Lanka, Arseculeratne et al. (1981)
chemically screened the first 50 plants used in Sri Lanka's
traditional medicine pharmacopoaea, and found that 3 of them
contained PAs. All 3 were hepatotoxic in rats. Of the 3, the
presence of alkaloids in Cassia auriculata and that of PAs in
Hollarhena antidysenterica had not previously been recorded. It
should be noted that the amount of experimental plant material used
in this study was approximately 6.5 g/kg body weight per day, in
contrast to the approximate intake by a human being estimated to be
in the range of 0.3 - 0.6 g/kg body weight per day. Some, but not
all, of the plants reported to be etiological agents in human cases
of veno-occlusive disease can be found in an inventory of medicinal
plants used in different countries (WHO, 1980), which also
indicates the countries that they are used in. The above lists may
not be complete as many such plants may be used in folk medicine
but have not been mentioned in the scientific literature. However,
the lists do indicate the wide and varied use of such toxic herbs
in all parts of the world.
Lately, there has been a growing interest in the developed
countries in organically grown products for food, as well as home
remedies (Table 3), and some of the PA-containing herbs have been
freely available in herbal shops (Schoental, 1968; Burns, 1972).
Danninger et al. (1983) listed plants containing PAs that are
commonly used in the Federal Republic of Germany as medicaments
(Table 4). He also listed 9 plants in which alkaloids have only
been identified qualitatively, the toxicity of which has not been,
or has been insufficiently, investigated (Table 5). Similarly,
Roitman (1983) listed 10 plants, in which the presence of PAs is
suspected or has been proved and which are used as herbal teas in
the USA. The lists include 10 plants containing PAs, most of which
have been proved hepatotoxic experimentally, some having highly
carcinogenic promoter activity. Some of these alkaloids have been
associated with human case reports of PA toxicity. The more recent
reports (Table 2) of instances of PA poisoning through the use of
herbal medicines are from developed countries (Lyford et al., 1976;
Stillman et al., 1977; Fox et al., 1978; Kumana et al., 1985;
Ridker et al., 1985). Such use of the herbs is the reason that
veno-occlusive disease is endemic in Jamaica (Bras et al., 1954;
Jellife et al., 1954a,b; Bras & Watler, 1955; Stuart & Bras, 1955,
1957). There are obvious difficulties in exercising any kind of
control to restrict this use only to plants that have been tested
and certified as safe for human use. It is impossible to identify
many such herbs, as they are sold as plants or their amorphous
products in the herbal shops.
Table 3. Some plants containing (or suspected of containing) PAs, which have been used
by people either as herbal medicines (M) or foods (F)
Plant Mode Country Referencea
of use or region
BORAGINACEAE
Anchusa officinalis M Europe Broch-Due & Aasen (1980) B
Borago officinalis M USA Delorme et al. (1977) A
Cynoglossum M East Africa Schoental & Coady (1968) A
geometricum
Cynoglossum M Iran Coady (1973) B
officinale
Heliotropium M India Gandhi et al. (1966a); B
eichwaldii Datta et al. (1978a,b) A
H. europaeum M India, Greece IARC (1976) A
H. lasiocarpum M Hong Kong Kumana et al. (1985); A
Culvenor et al. (1986) A
H. indicum M India, Africa, Schoental (1968a); B
South America, Hoque et al. (1976) B
and elsewhere
H. ramossissimum M Arabia Macksad et al. (1970); B
(ramram) Coady (1973) B
H. supinum M Tanzania Schoental & Coady (1968) A
Table 3 (contd.)
Plant Mode Country Referencea
of use or region
Pulmonaria spp. M USA Delorme et al. (1977) A
Symphytum officinale F, M Japan and Hirono et al. (1978, 1979b) A
M USA Furuya & Hikichi (1971); A
Delorme et al. (1977) A
S. x uplandicum F, M General Hills (1976) B
USA Culvenor et al. (1980a,b) A
S. asperum M USA Pedersen (1975) A
COMPOSITAE
Cacalia decomposita M USA Sullivan (1981) B
(matarique)
C. yatabei F Japan Hikichi & Furuya (1978) B
Farfugium japonicum M Japan Furuya et al. (1971) B
Ligularia dentata F Japan Asada & Furuya (1984) B
Petasites japonicus F, M Japan Hirono et al. (1973, 1979b) A
Senecio abyssinicus M Nigeria Williams & Schoental (1970) B
S. aureus M USA Wade (1977) B
Table 3 (contd.)
Plant Mode Country Referencea
of use or region
S. bupleuroides M Africa Watt & Breyer-Brandwijk (1962) A
S. burchelli F, M South Africa Rose (1972) A
S. coronatus M South Africa Rose (1972) A
S. discolor M Jamaica Asprey & Thornton (1955) B
S. doronicum M Germany Roeder et al. (1980a) B
S. inaequidens F South Africa Rose (1972) B
S. jacobaea M Europe Schoental & Pullinger (1972); B
(ragwort) Wade (1977) B
S. longilobus M USA Stillman et al. (1977); A
(S. douglassi) Huxtable (1979a) B
S. monoensis M USA Huxtable (1980) A
S. nemorensis M Germany Habs et al. (1982) A
spp. fuchsii
S. pierotti F Japan Asada & Furuya (1982) B
S. retrorsus M South Africa Rose (1972) A
(S. latifolius)
Table 3 (contd.)
Plant Mode Country Referencea
of use or region
S. vulgaris M Europe Watt & Breyer-Brandwijk (1962) A
(common groundsel)
Netherlands Wade (1977) B
M Iran Coady (1973) B
Syneilesis palmata F Japan Hikichi & Furuya (1976) B
Trichodesma africana M Asia Omar et al. (1983) B
Tussilago farfara M Japan Culvenor et al. (1976a) A
(coltsfoot)
M China Hirono et al. (1976b) A
M Norway Borka & Onshuus (1979) B
M USA Borka & Onshuus (1979); B
Culvenor et al. (1976b); B
LEGUMINOSAE
Crotalaria brevidens F East Africa Coady (1973) B
C. fulva M Jamaica Barnes et al. (1964); A
McLean (1970, 1974) A
Table 3 (contd.)
Plant Mode Country Referencea
of use or region
C. incana M East Africa Schoental & Coady (1968) A
Watt & Breyer-Brandwijk A
(1962)
C. laburnifolia M Tanzania Schoental & Coady (1968) A
F Asia Coady (1973) B
C. mucronata M Tanzania Coady (1973) B
C. recta M, F Tanzania Schoental & Coady (1968); A
Coady (1973) B
C. retusa M, F Africa IARC (1976) A
India Watt & Breyer-Brandwijk (1962) A
C. verrucosa M Sri Lanka Arseculeratne et al. (1981) A
a A = Reference in the reference list of this document.
B = Reference in Mattocks (1986).
Manufactured preparations may also contain PA-containing herbs,
e.g., comfrey-pepsin capsules sold as a digestive aid (Huxtable et
al., 1986).
3.3.3 Use of PA-containing plants as food
Several PA-containing plants are used as food as can be seen in
Table 3 (Mattocks, 1986). Petasites japonicus Maxim, Tussilago
farfara L. (coltsfoot), and Symphytum officinale L. (comfrey or
Russian comfrey) are known as edible plants in Japan, and have been
proved to contain carcinogenic pyrrolizidine alkaloids (Hirono et
al., 1973, 1979a,b). The young flower-stalks of P. japonicus and
the buds of coltsfoot have been used in Japan as human food or
herbal remedies. The leaf and root of comfrey are also used as an
edible vegetable or tonic (Hirono et al., 1978) in Japan and other
countries (Culvenor, 1985). The carcinogenic PAs in these plants
are petasitenine (P. japonicus), senkirkine (coltsfoot), and the
group including symphytine (comfrey). They were also mutagenic in
the Ames system of Salmonella typhimurium and V79 hamster cell line
and induced transformation in cryo-preserved hamster embryonic
cells (Hirono et al., 1979b). Other such PA-containing plants,
used as food in Japan, include young leaves of Syneilesis palmata,
various Cacalia species, and young Senecio pierotti (Mattocks,
1986). According to Culvenor (1985), consumers of comfrey could be
ingesting up to 5 mg PAs per day. Rose (1972) listed a number of
plants of the genus Senecio that are used as spinach in South
Africa. These include S. burchelli, which is known to have caused
an episode of PA poisoning through the ingestion of contaminated
bread (Wilmot & Robertson, 1920).
3.3.4 Contaminated honey
In the USA, Deinzer et al. (1977) reported the presence of all
PAs contained in Senecio jacobaea (ragwort) and proved to be
hepatotoxic, in the honey secreted by bees feeding on the plant.
The total alkaloid content ranged from 0.3 to 3.9 mg/kg. It has
been estimated that an average annual human intake of honey (600 g)
at the highest alkaloid level quoted would contain less than 3 mg
of PAs (Mattocks, 1986). Culvenor et al. (1981) and Culvenor
(1983, 1985) drew attention to the same potential hazard in honey
from Echium plantagineum, a weed that grows widely in Southern
Australia and is a major source of honey, yielding an estimated
2000 - 3000 tonnes per annum for human consumption. Echimidine is
the major component of the alkaloids of Echium, which are present
in concentrations of up to 1 mg/kg. Culvenor (1983) estimated that
individuals may consume up to 80 g honey/day with a corresponding
alkaloid intake of 80 µg/day, if only the Echium honey were used.
No reports of acute human toxicity through this source are
available.
Table 4. Medicinal plants containing PAs of known hepatotoxicity, reported as commonly
used in the Federal Republic of Germany, and the PAs contained in thema
Family Genus Species Pyrrolizidine
alkaloids
Compositae Eupatorium E. cannabinum amabiline±
(hemp agrimony) supinineb
Petasites P. hybirdus senecionineb,c
integerrimineb
senkirkineb
Senecio S. nemorensis fuchsisenecionine
(groundsel) sp. fuchsii senecionineb,c
(Fuch's groundsel)
S. vulgaris senecionineb,c
(groundsel) seneciophyllineb
retrorsineb
riddellineb,c
S. Jacobaea jacobineb
(ragwort) senecionineb,c
seneciphyllineb
jacoline, jaconine
chlorinated PAsd
S. aureus senecionineb,c
(American golden
ragwort)
Tussilago T. farfara senkirkineb
(coltsfoot) (coltsfoot) senecionineb,c
tussilagine
Table 4 (contd.)
Family Genus Species Pyrrolizidine
alkaloids
Alkanna A. tinctoria 7-angelylretronecine
triangularine
dihydroxytriangularine
Anchusa A. officinalis lycopsamine
Boraginaceae Borago B. officinalis lycopsamine/intermedine±
(borage) acetyllycopsamine/
acetylintermedine
amabiline
supinine
Symphytum S. officinale symphytineb
(comfrey) (comfrey) echimidine(?)
lycopsamine
acetyllycopsamineb
lasiocarpineb,c
heliosupine N-oxide
S. peregrinum lycopsamineb
S. x uplandicum intermedineb
symphytineb
echimidineb
7-acetyllycopsamine
7-acetylintermedine
symlandine
uplandicine
S. asperum asperumine
(prickly comfrey) heliosupine N-oxide
echimidineb
echinatine
Table 4 (contd.)
Family Genus Species Pyrrolizidine
alkaloids
Cynoglossum C. officinale heliosupine N-oxide
(hound's (hound's tongue) echinatine
tongue) acetyl heliosupineb
O-7-angelylhelio-
tridineb
Heliotropium H. europaeum heliotrineb,c,e
(Heliotrope) (common heliotrope) lasiocarpineb,c,e
supinine
heleurine
europine
acetyllasiocarpineb
a Modified from: Danninger et al. (1983).
b Toxic alkaloids.
c Alkaloids known to have caused human toxicity.
d Alkaloids with highly carcinogenic promoter activity.
e Used only in homeopathy.
Table 5. Medicinal plants containing PAs, reported as
commonly used in the Federal Republic of Germany,
the toxicity of which has not been, or has been
insufficiently, investigateda
-----------------------------------------------------------
Family Genus Species
-----------------------------------------------------------
Compositae Eupatorium E. perforatum
Brachyglottis B. repens
Arnica A. montana (mountain arnica)
Boraginaceae Lappula L. intermedia (stickseed)
Pulmonaria P. officinalis (lungwort)
-----------------------------------------------------------
a Modified from: Danninger et al. (1983).
3.3.5 Milk
PAs have been shown to produce toxic effects via transference
into the milk of dams (Schoental, 1959). Retrorsine was
administered orally to 17, and intraperitoneally to 6, lactating
rats weighing 185 - 350 g in 5 - 10 mg doses daily, the first dose
being given during the first 24 h after parturition. The rats
received from 1 to 14 doses, the total intake amounting to
21 - 335 mg/kg body weight. The litters were separated from the
mothers for ´ h following the administration of PA to avoid direct
contamination of the former by licking. Apparently the milk
production was not affected as the stomachs of many of the young,
examined postmortem, were distended with milk. All animals whose
mothers had received a total dose of 138 mg PA or more died within
30 days. Many of the young whose mothers had received smaller
doses survived until they were killed at 6 months. Biopsy of the
liver of the young at various intervals or at autopsy showed marked
changes, even in cases where the mothers did not appear to be
affected. Animals dying at 18 - 30 days showed hydropic or fatty
vacuolation of liver cells. In the liver of animals dying or
killed later, various degrees of haemorrhagic necrosis and increase
in the centrilobular reticulin of the liver, and some thickening of
centrilobular veins were seen. In animals that survived 6 months,
the appearance was less abnormal, but some hyperplastic nodules and
bile-duct proliferation were seen. The lactating rats dosed with
the PAs generally survived longer than the suckling animals and
usually did not show any ill effects, suggesting that the
susceptibility of the suckling rats was greater than that of the
mothers.
Dickinson et al. (1976) demonstrated the presence of PAs in the
milk of dairy cattle fed or dosed with ragwort (Senecio jacobaea).
When 4 cows were administered the dried plant material at levels of
up to 10 g/kg body weight per day through rumen cannula, PA levels
of up to 0.84 mg/kg were observed in the milk. However, only one
(jacoline) of the several PAs contained in the plant was secreted.
Calves, bucket fed on the milk did not show any signs of PA
toxicity.
Dickinson (1980) repeated the study on goats. Four milk goats
were freshly prepared with rumen cannulae. The kids were separated
from their dams and were fed milk twice a day. Dried tansy ragwort
plant material with a PA content of 0.16% (dry weight) was
administered through the cannulae to each goat at a dosage rate of
10 g/kg body weight per day over 125 days. During this period,
each of the 4 kids received milk from their dams at approximately
125 ml/kg per day in addition to ad lib feeding on alfalfa grass
hay. Six PAs were isolated from the plant material: jacobine,
jaconine, jaconline, jacozine, senecionine, and seneciphylline.
Milk samples collected twice daily showed PA contents of
225 - 530 µg/litre with a mean of 381 µg/litre. No apparent health
effects were noted in the kids, and only mild hepatic damage was
suspected in the dams, on the basis of liver function tests. Fifty
percent of the kids were killed after 10 weeks. No lesions of PA
toxicity were seen. The dams were rebred and appeared normal
throughout the gestation period. However, three dams aborted at
almost full term, and the fetuses were born dead. One of the dams
died shortly after parturition and showed evidence of severe liver
damage characteristic of PA toxicity. Another, which delivered
normally, also showed a lesser degree of liver damage at biopsy.
Data relating to PA secretion were compared with similar
earlier data on cows. Mean secretion of PAs in cows appeared much
higher, e.g., 684 µg/litre. The authors concluded that the amount
of PAs secreted in the goat's milk did not cause any serious
deleterious effects in the kids.
Johnson (1976) fed long-term lethal doses of Senecio jacobaea,
by stomach tube, to 6 cows. Feeding started at term or within 30
days post-partum, and continued until what was considered to be a
lethal dose had been fed. The daily dose of the plant ranged from
1 to 4.4 g/kg body weight, the total amount fed representing 5 -
15% of body weight over a period of 54 - 126 days. Five cows died
within 98 days; one, in a moribund state, was killed on day 126.
The calves suckled for 30 - 126 days. Suckling started immediately
after birth in the case of 4 calves and 10 and 30 days later,
respectively, in the 2 remaining calves. Three calves were killed
with their dams or soon after, and 3 were retained for 1 year for
observation. Milk samples from 2 cows were collected and pooled in
14- to 16-day lots during 64 days of feeding of the Senecio plant.
Each pooled sample was administered intragastrically to a group of
rats in daily doses of 12 ml for 15 - 30 days. A control group of
rats were fed raw milk from cows not fed Senecio. Blood samples of
the dams and the calves were analysed for glutamic oxaloacetic
transaminase (GOT), lactic dehydrogenase (LDH), and gamma-glutamyl
transpeptidase (GGTP). Serum-enzyme levels in all cows indicated
statistically significant deviations suggesting liver dysfunction,
and the livers at autopsy had characteristic features of PA
toxicosis. The LDH and GOT levels in calves were generally
abnormal after 20 - 45 days of suckling. The abnormalities ranged
from mild to a 15- to 170-fold increase. One calf was autopsied at
the peak increase of serum-enzymes and was found to have mild focal
hepatitis. No significant pathological features were seen in the
livers of other animals, nor of the rats, some of which were
retained for up to 150 days.
Goeger et al. (1982) fed dried Senecio jacobaea (tansy ragwort)
to lactating goats in a proportion of 25% of the feed. The milk
contained 7.5 µg PA/kg dry weight. The milk produced by the goats
was pooled and then bottle fed to appetite to 2 Jersey bull calves
(1 day old) that also had access to tansy ragwort-free hay for 109
and 124 days, respectively. They were then weaned and given normal
feed and observed for 6 months, after which they were killed and
autopsied. In another study, rats were fed a diet containing the
freeze-dried milk at 80% level for 180 days with a calculated total
PA intake of 0.96 mg/rat. Other groups of rats were fed tansy
ragwort at dietary levels of 0.01 - 10 g/kg (corresponding to PA
intakes of 39.77, 5.04, 0.52, and 0.05 mg/rat). The calf livers
only showed very mild non-specific changes, but the livers of rats
fed tansy ragwort or the milk from tansy ragwort-fed goats
showed definite, but mild, changes including swollen
hepatocytes, megalocytosis, biliary hyperplasia, and fibrosis.
Histopathological changes in milk-fed rats were similar to those in
the group fed tansy ragwort in the diet at 0.01 g/kg. The authors
concluded that there was evidence of PA transfer into milk, which
proved hepatotoxic for rats. It was also noted that the goats had
been fed high levels of tansy ragwort at the upper limit of their
acceptance, and that the hepatic changes observed in rats fed high
levels of milk, for extensive periods, were slight.
Luthy et al. (1983) produced direct evidence of excretion of
macrocyclic esters of retronecine of the senecionine and
seneciphylline-type into rat milk. 3H-retronecine, an 3H-necic
acid-labelled senecionine, and seneciphylline were prepared
biosynthetically with seedlings of Senecio vulgaris L. Two
lactating rats (Ivanovas, Sprague Dawley), weighing 300 - 400 g,
were fed the first of the second compound by stomach tube, in doses
of 2.7 mg/kg and 5.5 mg/kg body weight, respectively. Samples of
blood were examined 1, 3, and 6 h after treatment, and those of
milk 1 and 3 h after. Animals were killed after 6 h. They were
found to have excreted approximately 0.08% of the applied
radioactivity in the milk within 3 h, mainly as unidentified
retronecine-derived metabolites, and approximately 0.02% as
unchanged PAs. The highest levels of PAs and metabolites in
tissues were found in the liver and lungs, 6 h after
administration.
Candrian et al. (1984a) also demonstrated that Drosophila
melanogaster flies fed on milk from lactating rats that had been
administered an oral dose of seneciphylline showed 1.2% sex-linked
recessive lethals, compared with 0.3% in controls, indicating the
transfer of the mutagenic properties of the PA via milk (section
6.4.7).
The implications of the above studies on the possibility of
carry-over of PAs into foodstuffs of animal origin are obvious.
However, no reports of human cases of acute PA toxicity, ascribed
to the consumption of contaminated milk, are available.
3.3.6 Meat
There have not been any reports of the detection of PAs in meat
products from livestock exposed to them.
3.3.7 Use of PAs as chemotherapeutic agents for cancer
An alkaloid of Heliotropium indicum L. (indicine N-oxide) has
been found to have antitumour activity and has been used in
experimental clinical chemotherapy for cancer (section 7.9).
4. METABOLISM
4.1 Absorption, Excretion, and Tissue Distribution
4.1.1 Absorption
There have been few studies on the absorption of PAs in man,
but absorption has been inferred from studies on tissue
distribution and the amounts of alkaloids and their metabolites
excreted in the urine, faeces, and bile of animals (section 4.1.2).
Swick et al. (1982c) measured the transfer of a mixture of
pyrrolizidine alkaloids extracted from Senecio jacobaea, across
isolated intestine and stomach segments from rabbits. The alkaloid
mixture contained seneciphylline, jacobine, jacozine, jacoline, and
senecionine. The alkaloids were transferred across the ileum and
jejunum, but not the stomach. Brauchli et al. (1982) compared the
oral and percutaneous absorption in rats of a crude alkaloid
mixture obtained from comfrey ( Symphytum officinale L.). The
mixture consisted of N-oxides of 7 alkaloids, principally 7-acetyl-
intermedine and 7-acetyl-lycopsamine. A dose of 194 mg/kg was
either given by gavage, or was applied to the shaved skin and left
for 44 h. After the dermal application, the excreted N-oxides in
urine (up to 48 h) amounted to 0.1 - 0.4% of the dose. After oral
dosage the excreted level of N-oxides and alkaloid bases was quoted
as being 20 - 50 times greater.
4.1.2 Excretion and distribution
The excretion and distribution of heliotrine in rats has been
reported in Bull et al., 1968. Young rats (150 g), given the LD50
of heliotrine by ip injection, were killed at intervals, bled
quickly, and the organs and tissues analysed. Heliotrine was
present in the liver after 2 min (3.7% of total dose), the level
peaking at 5 min (6.3%), and dropping to 2.2% at 1 h and 0.5% at
2.5 h. In adult rats, the level in the liver at 5 h was 0.07% of
the total dose. Five min after dosing, 30 - 40% of the initial
dose remained in the peritoneal cavity, and the blood level of
heliotrine was 60 mg/litre, dropping to 3 mg/litre at 1 h. The
urinary excretion of base and metabolites other than pyrrolic
metabolites, collected and measured 16 h after administration of
several alkaloids by ip injection, is shown in Table 6. The
proportion of base excreted unchanged increased with the
hydrophilicity of the alkaloid, being 62% for heliotrine N-oxide,
30% for heliotrine, and only 1 - 1.5% for lasiocarpine.
Heliotridine, the hydrolysis product from heliotrine and
lasiocarpine, was excreted in the form of the N-oxide in larger
quantities after the administration of each of these alkaloids.
The distribution and excretion of monocrotaline was studied in
rats by Hayashi (1966) who found that 50 - 70% was excreted in the
urine within the first day. However, the analysis was by a non-
specific chemical method that did not distinguish between the
unchanged alkaloid and its metabolites. Mattocks (1968a) gave
toxic pyrrolizidine alkaloids intraperitoneally to male rats and
measured the urinary excretion of the unchanged alkaloid, and of
N-oxide and pyrrolic metabolites. The excretion of N-oxide and
unchanged alkaloid was rapid and almost complete in the first 24 h.
Excretion of pyrroles was also rapid but continued for a little
longer. For example, in rats given retrosine (60 mg/kg body
weight), the urine in the first 24 h contained 10.6% unchanged
alkaloid, 13.3% N-oxide, and 13.4% pyrrolic metabolites. During
the second day, only 0.1% alkaloid, 0.2% N-oxide, and 1.8% pyrroles
were excreted. Biliary excretion also occurred. About one-quarter
of an iv dose of retrosine in rats was excreted in the bile as
pyrrolic metabolites, and 4% as unchanged alkaloid; most of this
excretion occurred during the first hour after the injection
(White, 1977).
Jago et al. (1969) gave heliotrine iv to sheep; urinary
excretion of the unchanged alkaloid together with metabolites
( N-oxide, and demethylation and hydrolysis products) occurred
rapidly and continued for up to 8 h. Excretion in the bile was
only 2% of that in the urine.
The tissue distribution of radioactivity from a tritiated toxic
pyrrolizidine alkaloid analogue was studied by Mattocks & White
(1976) using synthanecine A bis- N-ethylcarbamate (40 mg/kg body
weight). The highest concentrations of radioactivity were seen in
the liver (where metabolism occurs), lungs, kidneys, and spleen
(respectively, 3.9%, 0.19%, 0.18%, and 0.27% of the dose given),
and about 69% of the dose was eliminated in the urine during the
first day. Radioactivity in the expired air was negligible. The
binding of radioactivity in the liver, and especially the lungs,
was more persistent than in other organs. Similar results were
given by the semisynthetic pyrrolizidine alkaloid analogue,
retronecine bis- N-ethylcarbamate (Mattocks, 1977).
Table 6. Urinary metabolites of pyrrolizidine bases in the rat (16-h urine)a
Urine constituent (amount in percentage of dose injected)
Base Unchanged Base Heliotridine Heliotridine Heliotridine Heliotridine
administered base N-oxide trachelanthate trachelanthate N-oxide
(ip injection) N-oxide
Heliotrine 30 Trace 10 5 3 15
Heliotrine 62 (62) 2.7 ca. 6 ca. 1 ca. 10
N-oxide
Lasiocarpine 1-1.5 1.5-3 6
Heliotridine 35 ca. 1 (35) (ca. 1) 5 20
trachelanthate
Heliotridine 40 20 (40) (20)
a From: Bull et al. (1968).
The distribution of the uniformly 14C-labelled natural
pyrrolizidine alkaloid senecionine in lactating mice was studied by
Eastman et al. (1982). After 16 h, 75% of the radioactivity had
been recovered in the urine, 14% in the faeces, but only 0.04% was
in the milk; the liver contained 1.92%. The mice were milked using
teat cups. Candrian et al. (1985) studied the distribution of
radioactivity in rats given small doses of senecionine or
seneciphylline (0.3 - 3.3 mg/kg), tritiated in the pyrrolizidine
(retronecine) moiety. Most radioactivity was eliminated in the
urine and faeces within 4 days. Using mass spectrometry, Dickinson
et al. (1976) found a concentration of up to 0.84 mg PAs/litre in
the milk of cows fed Senecio jacobaea. Blood levels of senecionine
in rats given 0.1 LD50 ip were determined by Culvenor (1978). The
levels were 0.38, 0.32, and 0.14 mg/litre at 0.5, 1, and 2 h after
injection, respectively.
To summarize, the available evidence suggests that ingested
toxic pyrrolizidine alkaloids are rapidly metabolized and that the
excretion of unchanged alkaloid and of most metabolites is also
rapid. Thus, within a few hours, only a relatively small
proportion of the dose remains in the body, much of this in the
form of metabolites bound to tissue constituents. It appears
improbable that a significant amount of unchanged alkaloid will
remain in the body after the first day.
Pyrrolizidine N-oxides are much more water soluble than their
parent alkaloids. Indicine N-oxide (which is exceptionally water
soluble) is very rapidly excreted, either unchanged or conjugated.
Thus, indicine N-oxide given iv to mice, monkeys, or rabbits
disappeared from the serum with initial half-lives ranging from 3
to 20 min (Powis et al., 1979; El Dareer et al., 1982). Over 80%
of tritium-labelled indicine N-oxide given iv was excreted in the
urine of mice or monkeys within 24 h (El Dareer et al., 1982); at
2 h, the highest concentrations of radioactivity were in the
kidneys, liver, and intestines. Urinary excretion of indicine
N-oxide was also rapid in rabbits, but somewhat slower in human
beings (Powis et al., 1979).
4.2 Metabolic Routes
The major metabolic routes of unsaturated pyrrolizidine
alkaloids in animals are: (a) hydrolysis (of the ester groups);
(b) N-oxidation; and (c) dehydrogenation (of the pyrrolizidine
nucleus) to dehydro-alkaloids (pyrrolic derivatives). Other minor
routes of metabolism are known, but the three pathways account for
the major known toxic effects of these alkaloids (Fig. 5). Routes
(a) and (b) are believed to be detoxification mechanisms. Route
(c) leads to toxic metabolites and appears to be the major
activation mechanism. Route (a) may occur in various tissues,
including the liver and blood. Routes (b) and (c) are brought
about in the liver by the microsomal mixed-function oxidase system.
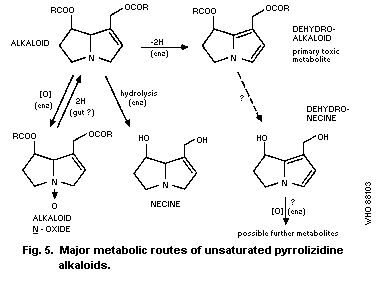 4.2.1 Hydrolysis
The hydrolysis of a PA leads to the formation of the amino-
alcohol moiety (necine base) and the acid moiety. Neither of these
is hepatotoxic (Schoental & Mattocks, 1960; Culvenor et al.,
1976a). The highly water-soluble necine base is readily excreted,
is not accessible to the microsomal system, and is not activated to
a toxic metabolite. Thus, pyrrolizidine alkaloids that are very
susceptible to (enzymic) hydrolysis have low toxicity (Mattocks,
1982). A major factor contributing to resistance to esterase is
the steric hindrance in the acid moiety. Thus, the chain branching
near the carbonyl groups slows hydrolysis allowing the formation of
relatively high levels of pyrrolic metabolites; a conformation of
the basic moiety, which brings the two ester groups close together,
thus leading to mutual steric hindrance, can also prevent
hydrolysis (Mattocks, 1981a).
The influence of hydrolysis in vivo on alternative metabolic
pathways is demonstrated by the fact that treatment of rats with an
esterase inhibitor, before giving pyrrolizidine alkaloids (or
synthetic analogues), can lead to greatly increased production of
pyrrolic metabolites from alkaloids that are normally susceptible
to hydrolysis, but little increase in those from alkaloids normally
resistant to hydrolysis (Mattocks, 1981a).
4.2.2 N-oxidation
The N-oxidation of pyrrolizidine alkaloids is induced by the
hepatic microsomal enzymes. The N-oxide metabolites are highly
water soluble and are rapidly excreted in the urine (Mattocks,
1968a). Pyrrolizidine N-oxides are not converted to any
significant extent to pyrrolic metabolites by microsomal enzymes
(Jago et al., 1970; Mattocks & White, 1971a), and there is no
evidence that they are toxic, unless first reduced to the
corresponding basic alkaloids, which can then be activated by the
microsomal system (Mattocks, 1971c). Thus, it appears that the
formation of N-oxides represents a detoxification pathway.
4.2.3 Conversion to pyrrolic metabolites
In laboratory animals, toxic pyrrolizidine alkaloids are
metabolized to pyrrolic derivatives, so-called because the
unsaturated ring of the pyrrolizidine system loses 2 hydrogen atoms
to form what is in effect a pyrrole ring (though the structure is
more correctly a dihydropyrrolizidine). Pyrrolic metabolites are
easily detectable in the tissues shortly after giving a toxic
pyrrolizidine alkaloid to an animal, by treating the tissue with an
Ehrlich reagent containing boron trifluoride, when a red colour is
produced; this reaction also occurs with the urine (Mattocks,
1968a; Mattocks & White, 1970). In rats given retrosine, pyrrolic
metabolites were found principally in the liver, with highest
levels associated with the microsomal and solid debris fractions
and less in the mitochondrial fraction; low levels were found in
the lungs, heart, spleen, and kidneys, within 4 h of giving
retrosine. Rats given 60 mg retrosine/kg body weight excreted 14%
of the dose in the urine, within 48 h.
Pyrrolic metabolites are formed by the hepatic mixed-function
oxidase system, with a requirement for cytochrome P450, oxygen, and
NADPH, as has been demonstrated in vitro (Jago et al., 1970;
Mattocks & White, 1971a). Conversion of pyrrolizidine alkaloids to
pyrrolic metabolites by the lung tissue of the human embryo
(Armstrong & Zuckerman, 1970), rat (Mattocks & White, 1971a;
Hilliker et al., 1983), or rabbit (Guengerich, 1977) was
negligible. The formation of pyrrolic metabolites does not proceed
via N-oxide intermediates, but appears to result from an initial
hydroxylation of the unsaturated pyrrolizidine ring adjacent to the
nitrogen atom (Mattocks & White, 1971a; Mattocks & Bird, 1983).
This would lead to a chemically unstable intermediate that would be
expected to decompose spontaneously to the pyrrolic product. The
primary pyrrolic metabolites (or dehydro-alkaloid) formed by
dehydrogenation of pyrrolizidine alkaloids are chemically
dehydropyrrolizidine esters (Fig. 5). These are highly reactive
compounds that can rapidly react with tissue constituents or
hydrolyse to the corresponding pyrrolic alcohols, or dehydro-
necines, which can thus be regarded as secondary metabolites. The
latter can also react with tissue constituents, but more slowly.
Because of their high chemical reactivity, the primary metabolites
would be expected to have a short life in the liver cell (minutes
or seconds) before they are hydrolysed or react with nucleophilic
tissue constituents. Some might escape into the blood stream and
reach other organs, especially the lungs. Dehydro-necines are more
stable and also more water soluble, and can become more widely
distributed throughout the body. However, they are also capable of
reacting with tissue constituents. Thus, measurements of pyrroles
formed from pyrrolizidine alkaloids in tissue samples, using a
colour reaction (Mattocks & White, 1970), will not represent a
single metabolite, but mixtures of the metabolites together with
various reaction products of these with tissue constituents. It
will be seen (section 5) that pyrrolic metabolites are believed to
be responsible for major toxic actions of pyrrolizidine alkaloids
(Mattocks, 1972a).
A pyrrolic metabolite with reactivity midway between that of
dehydromonocrotaline and dehydroretronecine has been reported to be
formed from monocrotaline in isolated, perfused rat liver
(Lafranconi et al., 1985). Studies on this metabolite (isolated
from bile) indicated that it is a monoester, and that it is toxic
in perfused rat lung. This suggests that monoester pyrrolic
metabolites may play a part in the toxic actions of PAs in extra-
hepatic tissues.
When rats or other laboratory animals are given a toxic
pyrrolizidine alkaloid, pyrrolic metabolites accumulate rapidly in
the liver (Mattocks, 1973; White et al., 1973), reaching a peak
within 1 - 2 h, then falling slowly during the next 24 h; the
metabolites may still be detectable after 2 days. Accumulation is
especially rapid after intraperitoneal injection, a very high level
of pyrrolic metabolites being attained within 20 min; this
indicates how rapid the metabolism of pyrrolizidine alkaloids can
be.
The level of pyrrolic metabolites in rat liver is generally
directly related to the amount of alkaloid given 2 h previously, at
least up to an acute LD50 dose. The pyrrole level depends on the
alkaloid used, and is related to the acute hepatotoxicity of the
alkaloid (Mattocks, 1972a).
To be converted to the type of chemically reactive, toxic
pyrrolic metabolites described above, an alkaloid must possess a
1-hydroxymethyl pyrrolizidine system, unsaturated in the
1,2-position (this makes the ring susceptible to dehydrogenation),
and at least one hydroxyl group must be esterified, usually by a
branched-chain acid. Otonecine esters are converted to similar
pyrrol metabolites by a different media involving N-demethylation.
Pyrrolizidine amino-alcohols (e.g., retronecine) are not
metabolized to more than small amounts of pyrroles (Jago et al.,
1970; Mattocks, 1981a), possibly because they are too water soluble
to reach the microsomal enzymes.
The metabolic formation of pyrroles is catalysed by cytochrome
P450 and specificity exists in the various isozymes (Guengerich,
1977; Juneja et al., 1984).
A few non-toxic pyrrolizidine alkaloids (e.g., rosmarinine and
hygrophylline) are converted to pyrrolic metabolites in vivo
(Mattocks, 1973). Such metabolites are chemically different from
the pyrroles of toxic alkaloids, and they are neither reactive nor
toxic (Mattocks & White, 1971b). The balance of structural
features necessary for a pyrrolizidine alkaloid to be converted to
give high concentrations of toxic pyrrolic metabolite has been
discussed by Mattocks (1981a); the optimum conditions appear to be
met in some alkaloids that are macrocyclic diesters, such as
retrosine.
4.3 Effects of Treatments Affecting Metabolism
The formation of pyrrolic metabolites (and of N-oxides) is
altered by treatments that affect the hepatic microsomal enzymes.
Such effects have been studied by measuring rates of metabolism of
pyrrolizidine alkaloids in vitro using microsomal preparations
from animals pre-treated in various ways (Table 7). For example,
microsomes from rats given the microsomal enzyme inducers
phenobarbitone or DDT (but not those from rats given
3-methylcholanthrene) induce greatly increased pyrrole formation
and smaller increases in N-oxide formation, from the alkaloid
retrorsine (Mattocks & White, 1971a). Enzyme preparations from
rats treated with inhibitors of microsomal enzymes, including SKF
525A and chloramphenicol, are much less active in converting
monocrotaline to pyrroles (Chesney et al., 1974). The ability to
metabolize retrorsine is diminished in microsomes from rats fed a
protein-free diet, or from rats acutely poisoned with retrorsine
(Mattocks & White, 1971a).
Table 7. Effect of pre-treatment of male rats on the conversion of PAs to
pyrrolic derivatives and to N-oxides by liver microsomes
in vitro
----------------------------------------------------------------------------
Alkaloid Pre-treatment, and Enzyme activity as Reference
time before enzyme % of control values
measurements for formation of:
Pyrroles N-oxides
----------------------------------------------------------------------------
retrorsine phenobarbitone, ip, 311 232 Mattocks &
3 x 100 mg/kg, White (1971a)
1 - 3 days
retrorsine DDT, ip, 75 mg/kg, 407 203 Mattocks &
3 days White (1971a)
retrorsine 3-methylcholanthrene, 95 (ns) 116 (ns) Mattocks &
ip, 3 x 20 mg/kg, White (1971a)
1 - 3 days
retrorsine retrorsine, ip, 63 - Mattocks &
35 mg/kg, 20 h White (1971a)
retrorsine protein-free diet, 39 - Mattocks &
3 days White (1971a)
monocrotaline phenobarbitone, sc, 448 165 Chesney et al.
4 x 75 mg/kg, (1974)
1 - 4 days
monocrotaline chloramphenicol, sc, 10 109 (ns) Chesney et al.
200 mg/kg, 1 h (1974)
monocrotaline SKF 525A, ip, 75 mg/kg, 10 87 (ns) Chesney et al.
1 h (1974)
----------------------------------------------------------------------------
ns = not significantly different from controls.
The effects of in vivo treatment with several types of enzyme
inducers on the toxicity of lasiocarpine and senecionine for
primary rat hepatocyte cultures was investigated by Hayes et al.
(1985). Pre-treatment with phenobarbitone potentiated the
cytotoxicity of senecionine towards the cultured cells, whereas
pre-treatment with 3-methylcholanthrene diminished the toxic action
of senecionine, but had little effect on lasiocarpine cytotoxicity.
The cytocidal effects of both alkaloids were substantially
inhibited in the presence of SKF 525A.
4.4 Other Factors Affecting Metabolism
Variations between animal species have been investigated by
White et al. (1973) and Shull et al. (1976). For instance,
metabolism to form pyrroles is high in rats and very low in guinea-
pigs, which, however, have higher rates of N-oxidation. For
example, 2 h after an ip dose of retrosine (100 mg/kg body weight),
the liver-pyrrole level in male rats was 13 times higher than that
in male guinea-pigs (White et al., 1973). Liver microsome
preparations from male rats were 28 times more active than
microsomes from male guinea-pigs in the dehydrogenation of
monocrotaline (Chesney & Allen, 1973a).
The development with age of the ability of Wistar rats to
metabolize retrosine was studied by Mattocks & White (1973). The
ability to form pyrroles is very low in new-born rats, but, by 5
days of age, it is nearly as high as in adult males. This activity
continues at a similar level in male rats, but, in females, it
falls after the age of about 20 days until, by 60 days, it is about
one-eighth that in males. Such a sex difference was not observed
in mice (White et al., 1973).
4.5 Other Metabolic Routes
The actions of hepatic microsomal enzymes on pyrrolizidine
alkaloids can produce other metabolites as well as pyrroles and
N-oxides, but there are few reports of these. Eastman & Segall
(1982) demonstrated hydroxylation of the acid moiety of senecionine
by liver microsomes from female mice. Such metabolism should not
prevent the subsequent conversion of the product to pyrrolic or
N-oxide metabolites. The formation of other microsomal metabolites
of senecionine has been reported by Segall et al. (1984).
The O-demethylation of the acid moiety of heliotrine has been
demonstrated by Jago et al. (1969) and represents a partial
detoxification mechanism, since the product is about half as toxic
as heliotrine. Other detoxification mechanisms exist in the rumen
of sheep (Dick et al., 1963; Lanigan & Smith, 1970a,b), which are,
thus, particularly resistant to the effects of pyrrolizidine
alkaloids.
4.6 Metabolism of Pyrrolizidine N-Oxides
As mentioned in section 4.2, the N-oxides of pyrrolizidine
alkaloids are not converted to pyrrolic metabolites by liver
microsomes. It appears that their main route of metabolism in
animals is reduction to the corresponding basic alkaloids, which
may then be further metabolized as already described. This
reduction has been shown to occur in the rat or rabbit gut
(Mattocks, 1971c; Powis et al., 1979), and may be brought about by
intestinal bacteria or possibly by gut enzymes. Such reduction can
also be brought about by hepatic microsomal fractions (Powis et
al., 1979) in the presence of NADH or of NADPH, and by sheep rumen
fluid (Lanigan et al., 1970a, b).
The reduction of pyrrolizidine N-oxides in vivo is of great
importance as a step in the bioactivation of these compounds
(Mattocks, 1971c), as shown in section 4.2.2.
4.7 Metabolism in Man
Powis et al. (1979) found that indicine N-oxide given iv to
3 human patients as an antitumour drug was partially reduced to
indicine base, detectable in the urine and plasma. Armstrong &
Zuckerman (1970) showed that human embryo liver slices, but not
lung slices, converted the pyrrolizidine alkaloids lasiocarpine,
retrorsine, and fulvine to pyrrolic metabolites in vitro.
5. MECHANISMS OF TOXICITY AND OTHER BIOLOGICAL ACTIONS
5.1 Metabolites Responsible for Toxicity
5.1.1 Metabolic basis of toxicity
The toxic effects of pyrrolizidine alkaloids are mediated
through their toxic metabolites and not by the alkaloids
themselves. The following observations are evidence for the above
statement (Mattocks, 1972a):
(a) The alkaloids are chemically rather unreactive and it is
hard to envisage reactions with cell constituents that they
could undergo readily under physiological conditions. On the
other hand, chemically prepared derivatives, similar or
identical to known metabolites of these alkaloids, are highly
reactive and are capable of causing toxic effects similar to
those of PAs, often at dose levels much lower than those
required by the alkaloids themselves.
(b) The liver is usually the main organ affected, whatever the
route of administration of the alkaloid. The alkaloids are
known to be metabolized in the liver.
(c) Direct application of these alkaloids to the skin does not
cause local toxic effects (Schoental et al., 1954), nor do
cytotoxic effects occur at sites of injection.
(d) The susceptibility of animals to the toxic actions of PAs
is related to the ability of the animal to metabolize the
alkaloids. For example, the hepatic microsomal enzymes of rats
less than 1 h old have very low activity towards retrorsine and
these rats are relatively resistant to it, whereas rats aged
several days have a high enzyme activity and are highly
susceptible to the alkaloid (Mattocks & White, 1973). Guinea-
pigs are very resistant to retrorsine, unless they have been
given phenobarbitone, which potentiates the enzymes that
metabolize it (White et al., 1973). Rats pre-treated with
microsomal enzyme inhibitors, such as SKF 525A or
chloramphenicol, have increased resistance to retrorsine or
monocrotaline (Allen et al., 1972; Mattocks, 1973). In
general, there is a good relationship between the rate of
hepatic metabolism of PAs to pyrrole in vitro (Shull et al.,
1976) and chronic toxicity. Highly resistant species, e.g.,
guinea-pigs, Japanese quail, and sheep, have a low rate of
pyrrole formation, while susceptible species, such as the
horse, cattle, and rat, have a high rate. Notable exceptions
are the rabbit and hamster, which have high rates of pyrrole
formation, but are resistant. It is possible that this may be
due to changes in the balance between activation and the
involvement of other factors, such as activity of
detoxification. For example, sheep have a high epoxide
hydrolase activity in the liver (Swick et al., 1983), which may
affect PA detoxification (Cheeke & Pierson-Goeger, 1983).
5.1.2 Isolation of pyrrolic metabolites
There is plenty of evidence that many unsaturated PAs are
converted into pyrrolic esters (dehydro-alkaloids) in the mammalian
liver (section 4.2.3). These primary pyrrolic metabolites cannot
be isolated, because of their high reactivity and rapid rate of
hydrolysis. However, their more stable hydrolysis products
(pyrrolic alcohols; dehydronecines) have been isolated and
identified. Thus dehydroheliotridine has been obtained from the
in vitro incubation of both the heliotridine-based alkaloids,
lasiocarpine and heliotrine, with rat liver microsomes (Jago et
al., 1970) and dehydro-retronecine was found to be the main
detectable pyrrolic metabolite in the liver, blood, and urine of
rats injected with the retronecine-based alkaloid, monocrotaline
(Hsu et al., 1973). There is evidence that these materials are
identical, i.e., the (±)-form resulting from racemization during
hydrolysis of the parent pyrrolic esters (Kedzierski & Buhler,
1985).
The results of these studies confirm that rat liver enzymes
convert PAs into metabolites with known cytotoxic activity (section
5.2), and imply that these metabolites are formed via the yet more
toxic and short-lived dehydro-alkaloids (Jago et al., 1970).
5.1.3 Chemical aspects of pyrrolic metabolites
5.1.3.1 Preparation
Chemical methods are available for converting unsaturated PAs
into pyrrolic esters (dehydro-alkaloids), the putative primary
toxic metabolites, enabling the physical, chemical, and
toxicological properties of the latter to be studied.
Small amounts of dehydro-pyrrolizidine alkaloids are usually
prepared by the reaction of the corresponding alkaloid N-oxides
with either acetic anhydride (Mattocks, 1969; Culvenor et al.,
1970a) or methanolic ferrous sulfate (Mattocks, 1969). The
products must be protected from moisture and from acids, which can
cause their immediate decomposition.
A variety of reagents can dehydrogenate the alkaloid bases to
pyrrolic derivatives, these include manganese dioxide (Culvenor et
al., 1970a,b; Mattocks, 1969), potassium permanganate (Culvenor et
al., 1970a), chloranil (Culvenor et al., 1970a), 2,3-dichloro-5,6-
dicyanobenzoquinone (Mattocks, 1969), iodine (Culvenor et al.,
1970b), and aryl thiols (Juneja et al., 1984). Some PAs are slowly
oxidized to pyrroles by molecular oxygen (Bick et al., 1975).
The more stable pyrrolic alcohol, dehydroretronecine (Fig. 6),
is prepared from retronecine using chloranil (Culvenor et al.,
1970a) or aqueous potassium nitro-sodisulfonate (Mattocks, 1981c)
or from retronecine N-oxide (isatinecine) using ferrous sulfate
(Mattocks, 1969). The enantiomeric dehydroheliotridine can be
prepared from heliotridine in similar ways. Racemic dehydro-
heliotridine has been synthesized (Viscontini & Gilhof-
Schaufelberger, 1971; Bohlmann et al., 1979).
4.2.1 Hydrolysis
The hydrolysis of a PA leads to the formation of the amino-
alcohol moiety (necine base) and the acid moiety. Neither of these
is hepatotoxic (Schoental & Mattocks, 1960; Culvenor et al.,
1976a). The highly water-soluble necine base is readily excreted,
is not accessible to the microsomal system, and is not activated to
a toxic metabolite. Thus, pyrrolizidine alkaloids that are very
susceptible to (enzymic) hydrolysis have low toxicity (Mattocks,
1982). A major factor contributing to resistance to esterase is
the steric hindrance in the acid moiety. Thus, the chain branching
near the carbonyl groups slows hydrolysis allowing the formation of
relatively high levels of pyrrolic metabolites; a conformation of
the basic moiety, which brings the two ester groups close together,
thus leading to mutual steric hindrance, can also prevent
hydrolysis (Mattocks, 1981a).
The influence of hydrolysis in vivo on alternative metabolic
pathways is demonstrated by the fact that treatment of rats with an
esterase inhibitor, before giving pyrrolizidine alkaloids (or
synthetic analogues), can lead to greatly increased production of
pyrrolic metabolites from alkaloids that are normally susceptible
to hydrolysis, but little increase in those from alkaloids normally
resistant to hydrolysis (Mattocks, 1981a).
4.2.2 N-oxidation
The N-oxidation of pyrrolizidine alkaloids is induced by the
hepatic microsomal enzymes. The N-oxide metabolites are highly
water soluble and are rapidly excreted in the urine (Mattocks,
1968a). Pyrrolizidine N-oxides are not converted to any
significant extent to pyrrolic metabolites by microsomal enzymes
(Jago et al., 1970; Mattocks & White, 1971a), and there is no
evidence that they are toxic, unless first reduced to the
corresponding basic alkaloids, which can then be activated by the
microsomal system (Mattocks, 1971c). Thus, it appears that the
formation of N-oxides represents a detoxification pathway.
4.2.3 Conversion to pyrrolic metabolites
In laboratory animals, toxic pyrrolizidine alkaloids are
metabolized to pyrrolic derivatives, so-called because the
unsaturated ring of the pyrrolizidine system loses 2 hydrogen atoms
to form what is in effect a pyrrole ring (though the structure is
more correctly a dihydropyrrolizidine). Pyrrolic metabolites are
easily detectable in the tissues shortly after giving a toxic
pyrrolizidine alkaloid to an animal, by treating the tissue with an
Ehrlich reagent containing boron trifluoride, when a red colour is
produced; this reaction also occurs with the urine (Mattocks,
1968a; Mattocks & White, 1970). In rats given retrosine, pyrrolic
metabolites were found principally in the liver, with highest
levels associated with the microsomal and solid debris fractions
and less in the mitochondrial fraction; low levels were found in
the lungs, heart, spleen, and kidneys, within 4 h of giving
retrosine. Rats given 60 mg retrosine/kg body weight excreted 14%
of the dose in the urine, within 48 h.
Pyrrolic metabolites are formed by the hepatic mixed-function
oxidase system, with a requirement for cytochrome P450, oxygen, and
NADPH, as has been demonstrated in vitro (Jago et al., 1970;
Mattocks & White, 1971a). Conversion of pyrrolizidine alkaloids to
pyrrolic metabolites by the lung tissue of the human embryo
(Armstrong & Zuckerman, 1970), rat (Mattocks & White, 1971a;
Hilliker et al., 1983), or rabbit (Guengerich, 1977) was
negligible. The formation of pyrrolic metabolites does not proceed
via N-oxide intermediates, but appears to result from an initial
hydroxylation of the unsaturated pyrrolizidine ring adjacent to the
nitrogen atom (Mattocks & White, 1971a; Mattocks & Bird, 1983).
This would lead to a chemically unstable intermediate that would be
expected to decompose spontaneously to the pyrrolic product. The
primary pyrrolic metabolites (or dehydro-alkaloid) formed by
dehydrogenation of pyrrolizidine alkaloids are chemically
dehydropyrrolizidine esters (Fig. 5). These are highly reactive
compounds that can rapidly react with tissue constituents or
hydrolyse to the corresponding pyrrolic alcohols, or dehydro-
necines, which can thus be regarded as secondary metabolites. The
latter can also react with tissue constituents, but more slowly.
Because of their high chemical reactivity, the primary metabolites
would be expected to have a short life in the liver cell (minutes
or seconds) before they are hydrolysed or react with nucleophilic
tissue constituents. Some might escape into the blood stream and
reach other organs, especially the lungs. Dehydro-necines are more
stable and also more water soluble, and can become more widely
distributed throughout the body. However, they are also capable of
reacting with tissue constituents. Thus, measurements of pyrroles
formed from pyrrolizidine alkaloids in tissue samples, using a
colour reaction (Mattocks & White, 1970), will not represent a
single metabolite, but mixtures of the metabolites together with
various reaction products of these with tissue constituents. It
will be seen (section 5) that pyrrolic metabolites are believed to
be responsible for major toxic actions of pyrrolizidine alkaloids
(Mattocks, 1972a).
A pyrrolic metabolite with reactivity midway between that of
dehydromonocrotaline and dehydroretronecine has been reported to be
formed from monocrotaline in isolated, perfused rat liver
(Lafranconi et al., 1985). Studies on this metabolite (isolated
from bile) indicated that it is a monoester, and that it is toxic
in perfused rat lung. This suggests that monoester pyrrolic
metabolites may play a part in the toxic actions of PAs in extra-
hepatic tissues.
When rats or other laboratory animals are given a toxic
pyrrolizidine alkaloid, pyrrolic metabolites accumulate rapidly in
the liver (Mattocks, 1973; White et al., 1973), reaching a peak
within 1 - 2 h, then falling slowly during the next 24 h; the
metabolites may still be detectable after 2 days. Accumulation is
especially rapid after intraperitoneal injection, a very high level
of pyrrolic metabolites being attained within 20 min; this
indicates how rapid the metabolism of pyrrolizidine alkaloids can
be.
The level of pyrrolic metabolites in rat liver is generally
directly related to the amount of alkaloid given 2 h previously, at
least up to an acute LD50 dose. The pyrrole level depends on the
alkaloid used, and is related to the acute hepatotoxicity of the
alkaloid (Mattocks, 1972a).
To be converted to the type of chemically reactive, toxic
pyrrolic metabolites described above, an alkaloid must possess a
1-hydroxymethyl pyrrolizidine system, unsaturated in the
1,2-position (this makes the ring susceptible to dehydrogenation),
and at least one hydroxyl group must be esterified, usually by a
branched-chain acid. Otonecine esters are converted to similar
pyrrol metabolites by a different media involving N-demethylation.
Pyrrolizidine amino-alcohols (e.g., retronecine) are not
metabolized to more than small amounts of pyrroles (Jago et al.,
1970; Mattocks, 1981a), possibly because they are too water soluble
to reach the microsomal enzymes.
The metabolic formation of pyrroles is catalysed by cytochrome
P450 and specificity exists in the various isozymes (Guengerich,
1977; Juneja et al., 1984).
A few non-toxic pyrrolizidine alkaloids (e.g., rosmarinine and
hygrophylline) are converted to pyrrolic metabolites in vivo
(Mattocks, 1973). Such metabolites are chemically different from
the pyrroles of toxic alkaloids, and they are neither reactive nor
toxic (Mattocks & White, 1971b). The balance of structural
features necessary for a pyrrolizidine alkaloid to be converted to
give high concentrations of toxic pyrrolic metabolite has been
discussed by Mattocks (1981a); the optimum conditions appear to be
met in some alkaloids that are macrocyclic diesters, such as
retrosine.
4.3 Effects of Treatments Affecting Metabolism
The formation of pyrrolic metabolites (and of N-oxides) is
altered by treatments that affect the hepatic microsomal enzymes.
Such effects have been studied by measuring rates of metabolism of
pyrrolizidine alkaloids in vitro using microsomal preparations
from animals pre-treated in various ways (Table 7). For example,
microsomes from rats given the microsomal enzyme inducers
phenobarbitone or DDT (but not those from rats given
3-methylcholanthrene) induce greatly increased pyrrole formation
and smaller increases in N-oxide formation, from the alkaloid
retrorsine (Mattocks & White, 1971a). Enzyme preparations from
rats treated with inhibitors of microsomal enzymes, including SKF
525A and chloramphenicol, are much less active in converting
monocrotaline to pyrroles (Chesney et al., 1974). The ability to
metabolize retrorsine is diminished in microsomes from rats fed a
protein-free diet, or from rats acutely poisoned with retrorsine
(Mattocks & White, 1971a).
Table 7. Effect of pre-treatment of male rats on the conversion of PAs to
pyrrolic derivatives and to N-oxides by liver microsomes
in vitro
----------------------------------------------------------------------------
Alkaloid Pre-treatment, and Enzyme activity as Reference
time before enzyme % of control values
measurements for formation of:
Pyrroles N-oxides
----------------------------------------------------------------------------
retrorsine phenobarbitone, ip, 311 232 Mattocks &
3 x 100 mg/kg, White (1971a)
1 - 3 days
retrorsine DDT, ip, 75 mg/kg, 407 203 Mattocks &
3 days White (1971a)
retrorsine 3-methylcholanthrene, 95 (ns) 116 (ns) Mattocks &
ip, 3 x 20 mg/kg, White (1971a)
1 - 3 days
retrorsine retrorsine, ip, 63 - Mattocks &
35 mg/kg, 20 h White (1971a)
retrorsine protein-free diet, 39 - Mattocks &
3 days White (1971a)
monocrotaline phenobarbitone, sc, 448 165 Chesney et al.
4 x 75 mg/kg, (1974)
1 - 4 days
monocrotaline chloramphenicol, sc, 10 109 (ns) Chesney et al.
200 mg/kg, 1 h (1974)
monocrotaline SKF 525A, ip, 75 mg/kg, 10 87 (ns) Chesney et al.
1 h (1974)
----------------------------------------------------------------------------
ns = not significantly different from controls.
The effects of in vivo treatment with several types of enzyme
inducers on the toxicity of lasiocarpine and senecionine for
primary rat hepatocyte cultures was investigated by Hayes et al.
(1985). Pre-treatment with phenobarbitone potentiated the
cytotoxicity of senecionine towards the cultured cells, whereas
pre-treatment with 3-methylcholanthrene diminished the toxic action
of senecionine, but had little effect on lasiocarpine cytotoxicity.
The cytocidal effects of both alkaloids were substantially
inhibited in the presence of SKF 525A.
4.4 Other Factors Affecting Metabolism
Variations between animal species have been investigated by
White et al. (1973) and Shull et al. (1976). For instance,
metabolism to form pyrroles is high in rats and very low in guinea-
pigs, which, however, have higher rates of N-oxidation. For
example, 2 h after an ip dose of retrosine (100 mg/kg body weight),
the liver-pyrrole level in male rats was 13 times higher than that
in male guinea-pigs (White et al., 1973). Liver microsome
preparations from male rats were 28 times more active than
microsomes from male guinea-pigs in the dehydrogenation of
monocrotaline (Chesney & Allen, 1973a).
The development with age of the ability of Wistar rats to
metabolize retrosine was studied by Mattocks & White (1973). The
ability to form pyrroles is very low in new-born rats, but, by 5
days of age, it is nearly as high as in adult males. This activity
continues at a similar level in male rats, but, in females, it
falls after the age of about 20 days until, by 60 days, it is about
one-eighth that in males. Such a sex difference was not observed
in mice (White et al., 1973).
4.5 Other Metabolic Routes
The actions of hepatic microsomal enzymes on pyrrolizidine
alkaloids can produce other metabolites as well as pyrroles and
N-oxides, but there are few reports of these. Eastman & Segall
(1982) demonstrated hydroxylation of the acid moiety of senecionine
by liver microsomes from female mice. Such metabolism should not
prevent the subsequent conversion of the product to pyrrolic or
N-oxide metabolites. The formation of other microsomal metabolites
of senecionine has been reported by Segall et al. (1984).
The O-demethylation of the acid moiety of heliotrine has been
demonstrated by Jago et al. (1969) and represents a partial
detoxification mechanism, since the product is about half as toxic
as heliotrine. Other detoxification mechanisms exist in the rumen
of sheep (Dick et al., 1963; Lanigan & Smith, 1970a,b), which are,
thus, particularly resistant to the effects of pyrrolizidine
alkaloids.
4.6 Metabolism of Pyrrolizidine N-Oxides
As mentioned in section 4.2, the N-oxides of pyrrolizidine
alkaloids are not converted to pyrrolic metabolites by liver
microsomes. It appears that their main route of metabolism in
animals is reduction to the corresponding basic alkaloids, which
may then be further metabolized as already described. This
reduction has been shown to occur in the rat or rabbit gut
(Mattocks, 1971c; Powis et al., 1979), and may be brought about by
intestinal bacteria or possibly by gut enzymes. Such reduction can
also be brought about by hepatic microsomal fractions (Powis et
al., 1979) in the presence of NADH or of NADPH, and by sheep rumen
fluid (Lanigan et al., 1970a, b).
The reduction of pyrrolizidine N-oxides in vivo is of great
importance as a step in the bioactivation of these compounds
(Mattocks, 1971c), as shown in section 4.2.2.
4.7 Metabolism in Man
Powis et al. (1979) found that indicine N-oxide given iv to
3 human patients as an antitumour drug was partially reduced to
indicine base, detectable in the urine and plasma. Armstrong &
Zuckerman (1970) showed that human embryo liver slices, but not
lung slices, converted the pyrrolizidine alkaloids lasiocarpine,
retrorsine, and fulvine to pyrrolic metabolites in vitro.
5. MECHANISMS OF TOXICITY AND OTHER BIOLOGICAL ACTIONS
5.1 Metabolites Responsible for Toxicity
5.1.1 Metabolic basis of toxicity
The toxic effects of pyrrolizidine alkaloids are mediated
through their toxic metabolites and not by the alkaloids
themselves. The following observations are evidence for the above
statement (Mattocks, 1972a):
(a) The alkaloids are chemically rather unreactive and it is
hard to envisage reactions with cell constituents that they
could undergo readily under physiological conditions. On the
other hand, chemically prepared derivatives, similar or
identical to known metabolites of these alkaloids, are highly
reactive and are capable of causing toxic effects similar to
those of PAs, often at dose levels much lower than those
required by the alkaloids themselves.
(b) The liver is usually the main organ affected, whatever the
route of administration of the alkaloid. The alkaloids are
known to be metabolized in the liver.
(c) Direct application of these alkaloids to the skin does not
cause local toxic effects (Schoental et al., 1954), nor do
cytotoxic effects occur at sites of injection.
(d) The susceptibility of animals to the toxic actions of PAs
is related to the ability of the animal to metabolize the
alkaloids. For example, the hepatic microsomal enzymes of rats
less than 1 h old have very low activity towards retrorsine and
these rats are relatively resistant to it, whereas rats aged
several days have a high enzyme activity and are highly
susceptible to the alkaloid (Mattocks & White, 1973). Guinea-
pigs are very resistant to retrorsine, unless they have been
given phenobarbitone, which potentiates the enzymes that
metabolize it (White et al., 1973). Rats pre-treated with
microsomal enzyme inhibitors, such as SKF 525A or
chloramphenicol, have increased resistance to retrorsine or
monocrotaline (Allen et al., 1972; Mattocks, 1973). In
general, there is a good relationship between the rate of
hepatic metabolism of PAs to pyrrole in vitro (Shull et al.,
1976) and chronic toxicity. Highly resistant species, e.g.,
guinea-pigs, Japanese quail, and sheep, have a low rate of
pyrrole formation, while susceptible species, such as the
horse, cattle, and rat, have a high rate. Notable exceptions
are the rabbit and hamster, which have high rates of pyrrole
formation, but are resistant. It is possible that this may be
due to changes in the balance between activation and the
involvement of other factors, such as activity of
detoxification. For example, sheep have a high epoxide
hydrolase activity in the liver (Swick et al., 1983), which may
affect PA detoxification (Cheeke & Pierson-Goeger, 1983).
5.1.2 Isolation of pyrrolic metabolites
There is plenty of evidence that many unsaturated PAs are
converted into pyrrolic esters (dehydro-alkaloids) in the mammalian
liver (section 4.2.3). These primary pyrrolic metabolites cannot
be isolated, because of their high reactivity and rapid rate of
hydrolysis. However, their more stable hydrolysis products
(pyrrolic alcohols; dehydronecines) have been isolated and
identified. Thus dehydroheliotridine has been obtained from the
in vitro incubation of both the heliotridine-based alkaloids,
lasiocarpine and heliotrine, with rat liver microsomes (Jago et
al., 1970) and dehydro-retronecine was found to be the main
detectable pyrrolic metabolite in the liver, blood, and urine of
rats injected with the retronecine-based alkaloid, monocrotaline
(Hsu et al., 1973). There is evidence that these materials are
identical, i.e., the (±)-form resulting from racemization during
hydrolysis of the parent pyrrolic esters (Kedzierski & Buhler,
1985).
The results of these studies confirm that rat liver enzymes
convert PAs into metabolites with known cytotoxic activity (section
5.2), and imply that these metabolites are formed via the yet more
toxic and short-lived dehydro-alkaloids (Jago et al., 1970).
5.1.3 Chemical aspects of pyrrolic metabolites
5.1.3.1 Preparation
Chemical methods are available for converting unsaturated PAs
into pyrrolic esters (dehydro-alkaloids), the putative primary
toxic metabolites, enabling the physical, chemical, and
toxicological properties of the latter to be studied.
Small amounts of dehydro-pyrrolizidine alkaloids are usually
prepared by the reaction of the corresponding alkaloid N-oxides
with either acetic anhydride (Mattocks, 1969; Culvenor et al.,
1970a) or methanolic ferrous sulfate (Mattocks, 1969). The
products must be protected from moisture and from acids, which can
cause their immediate decomposition.
A variety of reagents can dehydrogenate the alkaloid bases to
pyrrolic derivatives, these include manganese dioxide (Culvenor et
al., 1970a,b; Mattocks, 1969), potassium permanganate (Culvenor et
al., 1970a), chloranil (Culvenor et al., 1970a), 2,3-dichloro-5,6-
dicyanobenzoquinone (Mattocks, 1969), iodine (Culvenor et al.,
1970b), and aryl thiols (Juneja et al., 1984). Some PAs are slowly
oxidized to pyrroles by molecular oxygen (Bick et al., 1975).
The more stable pyrrolic alcohol, dehydroretronecine (Fig. 6),
is prepared from retronecine using chloranil (Culvenor et al.,
1970a) or aqueous potassium nitro-sodisulfonate (Mattocks, 1981c)
or from retronecine N-oxide (isatinecine) using ferrous sulfate
(Mattocks, 1969). The enantiomeric dehydroheliotridine can be
prepared from heliotridine in similar ways. Racemic dehydro-
heliotridine has been synthesized (Viscontini & Gilhof-
Schaufelberger, 1971; Bohlmann et al., 1979).
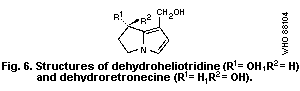 5.1.3.2 Chemistry associated with toxic actions
Dehydro-pyrrolizidine alkaloids and dehydronecines (pyrrolic
esters and alcohols) act chemically as alkylating (electrophilic)
agents, i.e., they can react with compounds possessing electron-
rich (nucleophilic) groups, such as amines, thiols, and some
hydroxyl compounds. The products of alkylation consist of the
"pyrrole" moiety covalently bonded to the substrate molecule. The
mechanism of alkylation is illustrated in Fig. 7 (Mattocks, 1972a).
An ester (R = COR) or hydroxyl group (R = H) attached to the
pyrrole ring via one carbon atom (i.e., at C7 or C9) is highly
reactive, being easily cleaved, leaving a positively charged
pyrrole moiety with a high affinity for electron-rich substrates.
Pyrrolic esters are the most reactive, RCOO being a better "leaving
group" than HO. When 2 oxygen functions are present (as
illustrated), either (in turn) can act as an alkylating centre.
Such bifunctional alkylation could lead to cross linking of
macromolecules (Mattocks, 1969; White & Mattocks, 1972; Petry et
al., 1984, 1986). When the groups (R) are the same or similar, C7
is the more reactive site. Examples of such alkylations using pure
chemicals (amines or alcohol) have been given by Mattocks (1969)
and Culvenor et al. (1970a). Mattocks & Bird (1983) showed that a
variety of nucleophiles of biological interest could be alkylated
by dehydroretronecine. Black & Jago (1970) demonstrated the
in vitro alkylation of DNA by dehydroheliotridine, and Robertson
(1982) and Wickramanayake et al. (1985) the alkylation of
deoxyguanosine by dehydroretronecine. The alkylation of mouse or
rat liver DNA by pyrrolizidine alkaloids has been shown in vivo by
Eastman et al. (1982) and Candrian et al. (1985).
5.1.3.2 Chemistry associated with toxic actions
Dehydro-pyrrolizidine alkaloids and dehydronecines (pyrrolic
esters and alcohols) act chemically as alkylating (electrophilic)
agents, i.e., they can react with compounds possessing electron-
rich (nucleophilic) groups, such as amines, thiols, and some
hydroxyl compounds. The products of alkylation consist of the
"pyrrole" moiety covalently bonded to the substrate molecule. The
mechanism of alkylation is illustrated in Fig. 7 (Mattocks, 1972a).
An ester (R = COR) or hydroxyl group (R = H) attached to the
pyrrole ring via one carbon atom (i.e., at C7 or C9) is highly
reactive, being easily cleaved, leaving a positively charged
pyrrole moiety with a high affinity for electron-rich substrates.
Pyrrolic esters are the most reactive, RCOO being a better "leaving
group" than HO. When 2 oxygen functions are present (as
illustrated), either (in turn) can act as an alkylating centre.
Such bifunctional alkylation could lead to cross linking of
macromolecules (Mattocks, 1969; White & Mattocks, 1972; Petry et
al., 1984, 1986). When the groups (R) are the same or similar, C7
is the more reactive site. Examples of such alkylations using pure
chemicals (amines or alcohol) have been given by Mattocks (1969)
and Culvenor et al. (1970a). Mattocks & Bird (1983) showed that a
variety of nucleophiles of biological interest could be alkylated
by dehydroretronecine. Black & Jago (1970) demonstrated the
in vitro alkylation of DNA by dehydroheliotridine, and Robertson
(1982) and Wickramanayake et al. (1985) the alkylation of
deoxyguanosine by dehydroretronecine. The alkylation of mouse or
rat liver DNA by pyrrolizidine alkaloids has been shown in vivo by
Eastman et al. (1982) and Candrian et al. (1985).
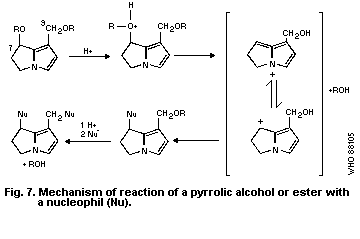 5.1.4 Possible further metabolites
The possibility that pyrrolic metabolites of PAs might
themselves be metabolized by microsomal enzymes to further
cytotoxic derivatives was suggested by Guengerich & Mitchell
(1980). These authors showed that the tritium-labelled model
compounds 1,2,3-trimethylpyrrole and 1-methyl-3-4 bishydroxy-
methylpyrrole could be metabolized in rats or by rat liver
microsomes to unidentified derivatives able to bind covalently to
proteins and nucleic acids. It is possible that liver damage, seen
in some rats given iv injections of pneumotoxic pyrrolic esters,
might have been due to metabolites of the latter formed in the
liver (Mattocks & Driver, 1983). Segall et al. (1985) have
identified trans-4-hydroxy-2-hexenal in an in vitro mouse liver
microsomal system metabolizing the PA senecionine and suggested
that it might have been formed from the alkaloid via a pyrrolic
intermediate. The compound is capable of causing liver damage and
might contribute to the acute hepatotoxicity of senecionine and
other alkaloids. However, this has not been proved, and the highly
reactive and toxic primary pyrrolic metabolites from PAs are
themselves capable of causing the known hepatotoxic effects of
these alkaloids.
5.2 Toxic Actions of Pyrrolic Metabolites
Pyrrolic derivatives prepared chemically from PAs, as well as
some analogous compounds, have been tested in experimental animals
and in vitro systems, and shown to have a variety of toxic actions.
5.2.1 Animals
5.2.1.1 Pyrrolic esters (dehydro-alkaloids)
Dehydro-pyrrolizidine alkaloids are very reactive and their
effects in vivo are largely confined to the first tissues they
encounter. When given orally to rats, they are destroyed almost
immediately in the aqueous acid of the stomach and show no toxic
action. When given ip, they cause severe local irritation and
peritonitis (Mattocks, 1968a; Butler et al., 1970); subcutaneous
injection leads to skin lesions (Hooson & Grasso, 1976). After iv
injection of pyrroles, such as dehydromonocrotaline (monocrotaline
pyrrole), into the tail veins of rats, the toxic injuries appear
principally in the lungs. Depending on the dose, these include
vascular lesions and pulmonary oedema (Plestina & Stoner, 1972); a
progressive alveolar proliferation similar to that produced by
very much larger doses of the parent alkaloid (Butler et al.,
1970) and pulmonary hypertension (Hilliker et al., 1983).
Dehydromonocrotaline does not require further metabolism to express
its pneumotoxicity, and it is rapidly rendered inactive after
exposure to aqueous media (Bruner et al., 1986). Similar
pneumotoxicity is produced by totally synthetic pyrrolic esters
having a simpler structure but the same type of chemical reactivity
as the alkaloid derivatives (Mattocks & Driver, 1983), thus
confirming the chemical mechanism of this action.
Injections of dehydro-pyrrolizidine alkaloids or synthetic
analogues into mesenteric veins of rats lead to liver damage after
smaller doses than the alkaloids themselves (Butler et al., 1970;
Shumaker et al., 1976). The liver damage differs somewhat from the
alkaloid damage, consistent with the toxin being introduced via the
hepatic vascular system rather than being produced within the
hepatocytes, as is the case with the alkaloids. Nevertheless, the
progressive liver lesions are very similar to those produced by PAs
(Butler et al., 1970). The lung damage after tail vein injections
bears a closer resemblance to pyrrolizidine damage, since the
latter is also believed to be caused by metabolites entering the
lungs via the bloodstream (Barnes et al., 1964).
5.2.1.2 Pyrrolic alcohols (dehydro-necines)
Dehydroheliotridine (Fig. 6), a secondary pyrrolic metabolite
from heliotridine-based PAs, such as heliotrine and lasiocarpine,
is less acutely toxic than its parent alkaloids; it has an LD50 (7
days) of about 250 mg/kg body weight in mice (Percy & Pierce,
1971). Its effects on 14-day-old rats were studied by Peterson et
al. (1972). All rats given ip doses of 0.4 mmol/kg body weight
survived, but a dose of 0.6 mmol/kg killed most animals within 10
days. Toxic effects were mainly found in rapidly developing
tissues. In young rats, it caused fur loss, tooth defects, and
atrophy of hair follicles, gut mucosa, spleen, thymus, testis, and
bone marrow. The lungs were not affected. Pathological effects in
the liver were confined to necrosis of isolated cells and
antimitotic action, which was manifested as a mild megalocytosis
(development of giant hepatocytes) in rats surviving 4 weeks or
more. The persistent antimitotic action of dehydroheliotridine and
of its parent alkaloid lasiocarpine in the liver of rats was
investigated by Samuel & Jago (1975), who located the mitotic block
as being either late in the DNA synthetic (S) phase or early in the
post synthetic (G2) phase of the cell cycle.
Dehydroheliotridine is also carcinogenic. Peterson et al.
(1983) showed that rats given 9 ip injections of this compound
(60 - 76.5 mg/kg body weight) over 23 weeks had a shorter life span
and suffered a significantly higher incidence of tumours than control
rats. The authors concluded that dehydroheliotridine is responsible
for some, or possibly all, of the carcinogenicity of its parent
alkaloids.
Dehydroheliotridine was found to be teratogenic when given ip
to female hooded rats on the 14th day of pregnancy. A dose of
40 mg/kg body weight produced effects similar to those produced by the
alkaloid heliotrine at a dose of 200 mg/kg (Peterson & Jago, 1980).
For the immunosuppressant activity of this compound, see section
6.4.10.
The toxic actions of dehydroretronecine (DHR) (Fig. 7) when
given sc to rats are similar to those of dehydroheliotridine (Hsu
et al., 1973; Shumaker et al., 1976). Repeated large doses also
caused ulceration of the glandular stomach. Daily sc doses
(4 mg/kg body weight), administered to rats for 1 week, caused lung
damage leading to right ventricular hypertrophy (Huxtable et al.,
1978). DHR was carcinogenic when applied repeatedly to mouse skin
(Johnson et al., 1978; Mattocks & Cabral, 1982).
5.2.2 Cell cultures
Dehydroheliotridine and dehydrosupinidine both have an
inhibitory action in cultures of KB cells (human epidermoid
carcinoma of the nasopharynx) with ED50 concentrations of 10-4 mol
and 10-5 mol, respectively (Culvernor et al., 1969).
Bick & Culvenor (1971) found dehydroheliotridine (DHR) to be
considerably more effective than the alkaloid heliotrine in
suppressing cell division and causing chromosome breaks, in
cultures of leukocytes from the marsupial Potorus tridactylus; at a
concentration of 6 x 10-5 mol, the mitotic index was zero, and more
than half the cells had disintegrated. In a study by Mattocks &
Legg (1980), dehydroretronecine and several synthetic analogues
completely inhibited cell division in a cultured rat liver cell
line at a concentration of 10-4 mol. Ord et al., (1985) found that
DHR induced sister chromatid exchange in human lymphocytes without
the need for metabolic activation. Analogous pyrroles with only
one functional (reactive) group were much less effective. DHR was
also weakly active in inducing mutations in the Salmonella
typhimurium base substitution strain, TA92, and gave positive
results in an in vitro cell transformation test using a culture
derived from hamster kidney cells (Styles et al., 1980).
The toxicity of the pyrrolic ester, dehydromonocrotaline, for
cultures of mouse fibroblasts was studied in vitro by Johnson
(1981). The level of exposure was approximately 1 ng per cell.
Cell death was preceded, first by the swelling and disruption of
organelles, including mitochondria, and then by the rupture of
plasma membranes with the release of cell components.
Bick et al., (1975) investigated whether the effects of PAs on
leukocyte cultures of Potorus tridactylus were due to pyrrolic
metabolites. Levels of dihydropyrrolizines, which could be
demonstrated in the culture media, were insufficient to account for
the observed effects of heliotrine, lasiocarpine, and monocrotaline
on the cells, but the actual amounts formed within the cells may
have been higher than those observed.
5.2.3 Possible participation of membrane lipid peroxidation
Distinct increases in NADPH- and ascorbate-dependent
peroxidation of microsomal membrane lipids were found in rats given
heliotrine subcutaneously (300 mg/kg body weight) (Savin 1983).
The primary biochemical interactions and cellular macromolecular
targets for the pathogenesis of PA-induced toxicity remain
unidentified.
5.3 Chemical and Metabolic Factors Affecting Toxicity
The toxicity of an alkaloid depends on the extent to which it
is converted into active metabolites and on the disposition and
reactivity of these metabolites, once formed.
5.3.1 Structural features of a toxic alkaloid
The essential structural features of a hepatotoxic PA (Fig. 8)
are:
(a) a 1-hydroxymethylpyrrolizidine ring system unsaturated in
the 1:2-position, with preferably a second hydroxyl group
in the 7-position;
(b) esterification of at least one of the hydroxyls, though
toxicity is much greater when both hydroxyls are
esterified; and
(c) ester groups that are resistant to enzymic hydrolysis,
which usually means that there is a high degree of chain
branching in the acid moiety.
The above requirements apply to natural PAs but, strictly
speaking, only the right hand (pyrroline) ring is essential, being
the ring that is metabolized to a pyrrole derivative. Thus, esters
of 2,3-bis-hydroxymethyl-1-methyl-3-pyrroline (synthanecine A)
(Fig. 9) have pyrrolizidine-like hepatotoxicity (Mattocks, 1971a;
Driver & Mattocks, 1984).
5.1.4 Possible further metabolites
The possibility that pyrrolic metabolites of PAs might
themselves be metabolized by microsomal enzymes to further
cytotoxic derivatives was suggested by Guengerich & Mitchell
(1980). These authors showed that the tritium-labelled model
compounds 1,2,3-trimethylpyrrole and 1-methyl-3-4 bishydroxy-
methylpyrrole could be metabolized in rats or by rat liver
microsomes to unidentified derivatives able to bind covalently to
proteins and nucleic acids. It is possible that liver damage, seen
in some rats given iv injections of pneumotoxic pyrrolic esters,
might have been due to metabolites of the latter formed in the
liver (Mattocks & Driver, 1983). Segall et al. (1985) have
identified trans-4-hydroxy-2-hexenal in an in vitro mouse liver
microsomal system metabolizing the PA senecionine and suggested
that it might have been formed from the alkaloid via a pyrrolic
intermediate. The compound is capable of causing liver damage and
might contribute to the acute hepatotoxicity of senecionine and
other alkaloids. However, this has not been proved, and the highly
reactive and toxic primary pyrrolic metabolites from PAs are
themselves capable of causing the known hepatotoxic effects of
these alkaloids.
5.2 Toxic Actions of Pyrrolic Metabolites
Pyrrolic derivatives prepared chemically from PAs, as well as
some analogous compounds, have been tested in experimental animals
and in vitro systems, and shown to have a variety of toxic actions.
5.2.1 Animals
5.2.1.1 Pyrrolic esters (dehydro-alkaloids)
Dehydro-pyrrolizidine alkaloids are very reactive and their
effects in vivo are largely confined to the first tissues they
encounter. When given orally to rats, they are destroyed almost
immediately in the aqueous acid of the stomach and show no toxic
action. When given ip, they cause severe local irritation and
peritonitis (Mattocks, 1968a; Butler et al., 1970); subcutaneous
injection leads to skin lesions (Hooson & Grasso, 1976). After iv
injection of pyrroles, such as dehydromonocrotaline (monocrotaline
pyrrole), into the tail veins of rats, the toxic injuries appear
principally in the lungs. Depending on the dose, these include
vascular lesions and pulmonary oedema (Plestina & Stoner, 1972); a
progressive alveolar proliferation similar to that produced by
very much larger doses of the parent alkaloid (Butler et al.,
1970) and pulmonary hypertension (Hilliker et al., 1983).
Dehydromonocrotaline does not require further metabolism to express
its pneumotoxicity, and it is rapidly rendered inactive after
exposure to aqueous media (Bruner et al., 1986). Similar
pneumotoxicity is produced by totally synthetic pyrrolic esters
having a simpler structure but the same type of chemical reactivity
as the alkaloid derivatives (Mattocks & Driver, 1983), thus
confirming the chemical mechanism of this action.
Injections of dehydro-pyrrolizidine alkaloids or synthetic
analogues into mesenteric veins of rats lead to liver damage after
smaller doses than the alkaloids themselves (Butler et al., 1970;
Shumaker et al., 1976). The liver damage differs somewhat from the
alkaloid damage, consistent with the toxin being introduced via the
hepatic vascular system rather than being produced within the
hepatocytes, as is the case with the alkaloids. Nevertheless, the
progressive liver lesions are very similar to those produced by PAs
(Butler et al., 1970). The lung damage after tail vein injections
bears a closer resemblance to pyrrolizidine damage, since the
latter is also believed to be caused by metabolites entering the
lungs via the bloodstream (Barnes et al., 1964).
5.2.1.2 Pyrrolic alcohols (dehydro-necines)
Dehydroheliotridine (Fig. 6), a secondary pyrrolic metabolite
from heliotridine-based PAs, such as heliotrine and lasiocarpine,
is less acutely toxic than its parent alkaloids; it has an LD50 (7
days) of about 250 mg/kg body weight in mice (Percy & Pierce,
1971). Its effects on 14-day-old rats were studied by Peterson et
al. (1972). All rats given ip doses of 0.4 mmol/kg body weight
survived, but a dose of 0.6 mmol/kg killed most animals within 10
days. Toxic effects were mainly found in rapidly developing
tissues. In young rats, it caused fur loss, tooth defects, and
atrophy of hair follicles, gut mucosa, spleen, thymus, testis, and
bone marrow. The lungs were not affected. Pathological effects in
the liver were confined to necrosis of isolated cells and
antimitotic action, which was manifested as a mild megalocytosis
(development of giant hepatocytes) in rats surviving 4 weeks or
more. The persistent antimitotic action of dehydroheliotridine and
of its parent alkaloid lasiocarpine in the liver of rats was
investigated by Samuel & Jago (1975), who located the mitotic block
as being either late in the DNA synthetic (S) phase or early in the
post synthetic (G2) phase of the cell cycle.
Dehydroheliotridine is also carcinogenic. Peterson et al.
(1983) showed that rats given 9 ip injections of this compound
(60 - 76.5 mg/kg body weight) over 23 weeks had a shorter life span
and suffered a significantly higher incidence of tumours than control
rats. The authors concluded that dehydroheliotridine is responsible
for some, or possibly all, of the carcinogenicity of its parent
alkaloids.
Dehydroheliotridine was found to be teratogenic when given ip
to female hooded rats on the 14th day of pregnancy. A dose of
40 mg/kg body weight produced effects similar to those produced by the
alkaloid heliotrine at a dose of 200 mg/kg (Peterson & Jago, 1980).
For the immunosuppressant activity of this compound, see section
6.4.10.
The toxic actions of dehydroretronecine (DHR) (Fig. 7) when
given sc to rats are similar to those of dehydroheliotridine (Hsu
et al., 1973; Shumaker et al., 1976). Repeated large doses also
caused ulceration of the glandular stomach. Daily sc doses
(4 mg/kg body weight), administered to rats for 1 week, caused lung
damage leading to right ventricular hypertrophy (Huxtable et al.,
1978). DHR was carcinogenic when applied repeatedly to mouse skin
(Johnson et al., 1978; Mattocks & Cabral, 1982).
5.2.2 Cell cultures
Dehydroheliotridine and dehydrosupinidine both have an
inhibitory action in cultures of KB cells (human epidermoid
carcinoma of the nasopharynx) with ED50 concentrations of 10-4 mol
and 10-5 mol, respectively (Culvernor et al., 1969).
Bick & Culvenor (1971) found dehydroheliotridine (DHR) to be
considerably more effective than the alkaloid heliotrine in
suppressing cell division and causing chromosome breaks, in
cultures of leukocytes from the marsupial Potorus tridactylus; at a
concentration of 6 x 10-5 mol, the mitotic index was zero, and more
than half the cells had disintegrated. In a study by Mattocks &
Legg (1980), dehydroretronecine and several synthetic analogues
completely inhibited cell division in a cultured rat liver cell
line at a concentration of 10-4 mol. Ord et al., (1985) found that
DHR induced sister chromatid exchange in human lymphocytes without
the need for metabolic activation. Analogous pyrroles with only
one functional (reactive) group were much less effective. DHR was
also weakly active in inducing mutations in the Salmonella
typhimurium base substitution strain, TA92, and gave positive
results in an in vitro cell transformation test using a culture
derived from hamster kidney cells (Styles et al., 1980).
The toxicity of the pyrrolic ester, dehydromonocrotaline, for
cultures of mouse fibroblasts was studied in vitro by Johnson
(1981). The level of exposure was approximately 1 ng per cell.
Cell death was preceded, first by the swelling and disruption of
organelles, including mitochondria, and then by the rupture of
plasma membranes with the release of cell components.
Bick et al., (1975) investigated whether the effects of PAs on
leukocyte cultures of Potorus tridactylus were due to pyrrolic
metabolites. Levels of dihydropyrrolizines, which could be
demonstrated in the culture media, were insufficient to account for
the observed effects of heliotrine, lasiocarpine, and monocrotaline
on the cells, but the actual amounts formed within the cells may
have been higher than those observed.
5.2.3 Possible participation of membrane lipid peroxidation
Distinct increases in NADPH- and ascorbate-dependent
peroxidation of microsomal membrane lipids were found in rats given
heliotrine subcutaneously (300 mg/kg body weight) (Savin 1983).
The primary biochemical interactions and cellular macromolecular
targets for the pathogenesis of PA-induced toxicity remain
unidentified.
5.3 Chemical and Metabolic Factors Affecting Toxicity
The toxicity of an alkaloid depends on the extent to which it
is converted into active metabolites and on the disposition and
reactivity of these metabolites, once formed.
5.3.1 Structural features of a toxic alkaloid
The essential structural features of a hepatotoxic PA (Fig. 8)
are:
(a) a 1-hydroxymethylpyrrolizidine ring system unsaturated in
the 1:2-position, with preferably a second hydroxyl group
in the 7-position;
(b) esterification of at least one of the hydroxyls, though
toxicity is much greater when both hydroxyls are
esterified; and
(c) ester groups that are resistant to enzymic hydrolysis,
which usually means that there is a high degree of chain
branching in the acid moiety.
The above requirements apply to natural PAs but, strictly
speaking, only the right hand (pyrroline) ring is essential, being
the ring that is metabolized to a pyrrole derivative. Thus, esters
of 2,3-bis-hydroxymethyl-1-methyl-3-pyrroline (synthanecine A)
(Fig. 9) have pyrrolizidine-like hepatotoxicity (Mattocks, 1971a;
Driver & Mattocks, 1984).
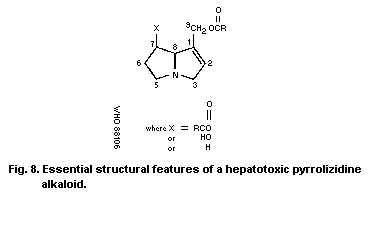 Structural requirements for N -oxides are the same as those for
the hepatotoxic alkaloids. However, it is important to note that a
PA N -oxide is not hepatotoxic itself; toxicity depends on it being
reduced to the corresponding basic alkaloid, chiefly in the gut
(Mattocks 1971c), but possibly in other organs, such as the liver
(Powis et al., 1979).
Structural requirements for N -oxides are the same as those for
the hepatotoxic alkaloids. However, it is important to note that a
PA N -oxide is not hepatotoxic itself; toxicity depends on it being
reduced to the corresponding basic alkaloid, chiefly in the gut
(Mattocks 1971c), but possibly in other organs, such as the liver
(Powis et al., 1979).
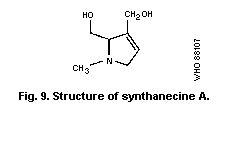 5.3.2 Activation and detoxication
Factors affecting the proportion of an ingested alkaloid that
is converted into toxic metabolites in an animal include the
following:
(a) Lipid solubility
Highly water-soluble alkaloids (such as indicine) are easily
excreted and have low toxicity. Alkaloids that are more lipophilic
are more open to activation by liver microsomes (Mattocks, 1981a).
(b) Subceptibility to hydrolysis
This is determined by the molecular structure and conformation
of the alkaloid (Mattocks, 1981a,b). If the alkaloid is open to
esterase attack, it may be largely detoxified by hydrolysis.
(c) Susceptibility to N-oxidation
The relative amounts of an alkaloid converted by hepatic
microsomal enzymes to N-oxide and to pyrrolic metabolites depends
on its molecular structure and conformation (Mattocks & Bird,
1983). N-oxidation represents a detoxication pathway (Mattocks,
1972b).
5.3.3 Factors affecting the toxicity of active metabolites
5.3.3.1 Reactivity of the metabolite
Toxic metabolites are formed in liver cells. Primary pyrrolic
metabolites (dehydro-alkaloids) are very reactive and, thus, are
quickly hydrolysed or deactivated by reaction with cell constituents.
To damage tissues other than the cells in which they are formed,
active metabolites must cross the cell membrane and survive while
being transported in the bloodstream. The more stable pyrrolic
metabolites, such as dehydromonocrotaline from the alkaloids
monocrotaline, are able to reach, and become bound to, lung tissue
(Mattocks, 1973). Thus, monocrotaline frequently damages the lungs,
whereas retrorsine, which yields a more reactive pyrrolic metabolite,
normally does not.
Secondary metabolites (pyrrolic alcohols, e.g., dehydroretronecine),
formed by the hydrolysis of primary pyrrolic metabolites, are water
soluble, relatively stable compounds that can become more widely
distributed throughout the body or excreted; these are not acutely
toxic.
5.3.3.2 The number of reactive groups
The toxicity of a pyrrolic alkylating agent is affected by the
number of reactive ester or hydroxyl groups (1 or 2) present as the
following examples show:
(a) Many pyrrolic esters can cause acute lung damage when
given iv to rats, but only bifunctional ones also cause
delayed effects on the lungs (Mattocks & Driver, 1983).
(b) Bifunctional pyrrolic alcohols are more effective
inhibitors of mitosis in cultured cells than monofunctional
pyrroles (Mattocks & Legg, 1980).
(c) Bifunctional pyrrolic alcohols are much better inducers
of sister chromatid exchange (SCE) in lymphocytes than
monoalcohols (Ord et al., 1985).
Reasons for these differences might be that the bifunctional
pyrroles are able to crosslink macromolecules or simply that they
can bind more strongly to target molecules.
5.4 Metabolites Associated with the Biological Actions of
Pyrrolizidine Alkaloids
5.4.1 Acute hepatotoxicity
The following is good evidence that acute liver necrosis is
caused by primary pyrrolic ester metabolites (dehydro-alkaloids):
(a) The liver, in which these metabolites are formed, is the
only organ exposed to them in relatively high concentrations.
(b) There are good correlations between amounts of pyrroles
bound to liver tissue and acute hepatotoxicity (Mattocks,
1973).
(c) Pyrrolic alcohols are not acutely hepatotoxic, even when
given to animals in very large amounts.
(d) Pyrrolic esters injected iv into the liver are much more
acutely hepatotoxic than the parent alkaloids (Butler et al.,
1970).
It is possible that other metabolites, such as 4-hydroxy
2,3-unsaturated aldehydes, might also contribute to the acute
hepatotoxicity of some PAs (Segall et al., 1985). However, this
has still to be confirmed.
5.4.2 Chronic hepatotoxicity
The persistent antimitotic action on the liver that leads to
the formation of giant hepatocytes can be produced both by pyrrolic
ester metabolites, such as dehydromonocrotaline (Hsu et al., 1973),
and by pyrrolic alcohols, such as dehydroheliotridine (Peterson et
al., 1972). Both kinds of metabolites can lead to similar
alkylation products and both are likely to be present in the liver
when the alkaloids are metabolized. Thus, either could be
responsible for chronic hepatotoxic effects. However, the
antimitotic action alone is not sufficient. It must be accompanied
or followed by a stimulus of cell division. This may be provided
by the acute necrotic effect of primary pyrrolic metabolites or by
any other cause of acute liver injury that leads to tissue
regeneration. In very young animals, the stimulus can be the
enhanced rate of replication that already exists in them.
5.4.3 Pneumotoxicity
Characteristic pyrrolizidine lung damage is produced by iv
injections of pyrrolic ester metabolites, which are effective at
much lower doses than the parent alkaloids. The latter are not
metabolized in lung tissue; thus, lung damage from PAs is believed
to be due to pyrrolic esters reaching the lungs from the liver
(Butler et al., 1970). Chronic lung damage appears to be caused by
bifunctional rather than by monofunctional pyrrolic alkylating
agents (Mattocks & Driver, 1983) (section 5.3.3.2).
There is some evidence that pyrrolic alcohol metabolites might
also be able to contribute to chronic (but not acute)
pneumotoxicity (Huxtable et al., 1978).
5.4.4 Toxicity in other tissues
Chronic heart damage including right ventricular hypertrophy is
a consequence of pyrrolizidine lung damage (pulmonary hypertension)
(Hayashi et al., 1967). Brain damage is attributed to ammonia
intoxication secondary to severe pyrrolizidine liver injury
(Hooper, 1972). This view has been contested and some PAs are
known to have direct effects on the central nervous system (section
6.4.3). There is no evidence that PAs are appreciably metabolized
in tissues other than the liver. Thus, damage to other organs is
probably due to metabolites transported from the liver. For
example, in the relatively uncommon cases of chronic kidney damage
after pyrrolizidine intoxication (Hooper, 1974; Hooper & Scanlan,
1977) megalocytosis in this organ suggests that pyrrolic
metabolites (either ester or alcohol) are involved. Overall,
patterns of disease, as observed in extrahepatic sites, are
consistent with a "spillover" effect of the pyrroles produced in
the liver (Hooper, 1978). Toxicity of an alkaloid reflects its
rate of metabolism to a pyrrole (Tuchweber et al., 1974) and so the
spillover effect is likely to be more evident at higher doses.
Studies of Culvenor et al. (1976a) suggest that the PAs that are
hepatotoxic for rats should also be pneumotoxic when administered
at higher doses. In acute poisoning, the hepatotoxic effects could
outweigh the pneumotoxic effects or those on other organs, to such
a degree that the latter are not manifested. Variation in
expression of disease (primarily hepatic or extrahepatic) also
depends on the reactions of host tissues in different species of
animals, in addition to the quantities of the pyrroles (Hooper,
1978). The sensitivity of the blood vessels might explain severe
interstitial pneumonias in some animals, or severe nephroses in
pigs (McGrath et al., 1975).
5.4.5 Carcinogenicity
The pyrrolic alcohols dehydroretronecine and dehydroheliotridine
are known carcinogens (Johnson et al., 1978; Peterson et al., 1983),
whereas the pyrrolic esters dehydromonocrotaline and dehydroretrorsine
are only carcinogenic in conjunction with a tumour promotor (Mattocks
& Cabral, 1979, 1982). This suggests that the more persistent
secondary metabolites (pyrrolic alcohols) might account for the rather
weak carcinogenicity of some PAs.
5.4.6 Antitumour activity
Some PAs and their N-oxides are active as tumour inhibitors in
test systems (Culvenor, 1968; Suffness & Cordell, 1985). Indicine
N-oxide, in particular, showed high activity against B16 melanoma,
mammary xenograft, M5076 sarcoma, P388 leukaemia, and Walker 256
carcinoma. In clinical studies, indicine N-oxide has shown
significant activity against some forms of leukaemia, with dosage
limited mainly by myelosuppression and sometimes by hepatotoxicity.
It is tempting to suppose that this action is related to the
powerful antimitotic action of their pyrrolic metabolites, even
though some of these alkaloids and derived pyrroles are themselves
carcinogenic. On the other hand, there is evidence suggesting that
indicine N-oxide owes it activity to a property of the compound
itself rather than to the pyrrolic metabolites, which could be
formed through reduction to indicine (Powis et al., 1979). The
evidence, that indicine is less effective than indicine N-oxide, is
not conclusive and other structure-activity data (Milkowsky, 1985)
point to a need for a structural capability to form a pyrrolic
metabolite. It is also possible that indicine N-oxide is converted
directly to dehydroindicine by mitochondrial enzymes in liver or
tumour cells, since the type of reaction required has been observed
in the mitochondrial metabolism of the N-oxides of tryptamine
alkaloids and certain methylated amino acids (Fish et al., 1956;
Smith et al., 1962).
5.5 Prevention and Treatment of Pyrrolizidine Poisoning
There is no known way to prevent pyrrolizidine liver damage,
once a hepatotoxic dose of the alkaloid has been ingested. A
number of dietary regimes have been found to partially protect
animals (chiefly rodents) from the acute effects of subsequent
alkaloids ingestion. None of these are of any practical use for
preventing pyrrolizidine intoxication in livestock. Furthermore,
chronic toxic effects in the liver or in other organs are sometimes
more severe in animals receiving higher doses of alkaloids after
being protected against acute hepatotoxicty.
5.5.1 Modified diets
The mechanism of action of modified diets is not clear, but
they may be associated with the decreased metabolic activation of
the alkaloids. Some examples follow:
(a) A protein-rich diet can give some protection to rats
against Senecio jacobaea alkaloids (Cheeke & Gorman, 1974).
Rats fed a high casein diet survived longer than rats given a
normal diet, when poisoned with retrorsine or riddelline, but
the survivors were more liable to develop liver tumours
(Schoental & Head, 1957). However, whether this was simply due
to a prolongation of life of the animals by the diet is open to
question.
(b) Male rats previously fed a sucrose-only diet for 4 days
were considerably protected against the acute hepatotoxicity of
retrorsine (LD50 120 mg/kg body weight compared with 34 mg/kg
in normal rats). However, lung damage, rare in control rats,
was frequently seen in "protected" rats given high doses of
retrorsine (Mattocks, 1973).
(c) Restriction of feed intake to 40% of normal attenuated the
increase in lung weight and lavage protein concentration in
cell-free bronchopulmonary lavage fluid and abolished the right
ventricular hypertrophy in monocrotaline-treated rats.
Furthermore, the percentage of diet-restricted animals that
survived was significantly higher than that in animals that had
eaten ad libitum up to day 28, but, from this time onwards,
there was no difference. Alterations of dietary sodium intake
alone did not affect the results of monocrotaline-induced
toxicity (Ganey et al., 1985).
5.5.2 Pretreatment to enhance the detoxication of active metabolites
Treatments that have afforded some protection against
pyrrolizidine hepatotoxicity (probably by increasing the liver
level of sulfydryl compounds, which are known to react with
pyrrolic metabolites) (White, 1976) include the following:
(a) Pre-treatment of rats with mercaptoethylamine (150 mg/kg
body weight ip) partially protected rats against the acute
hepatotoxicity of monocrotaline given 15 min later (Hayashi &
Lalich, 1968); it gave no protection when administered 2 h
after the alkaloid. Mercaptoethylamine, when given orally
(300 mg/kg body weight) at the same time as the lasiocarpine,
also increased the resistance of rats to the alkaloid (Rogers &
Newberne, 1971).
(b) Cysteine (1% in the diet) partially protected rats against
Senecio jacobaea alkaloids (Buckmaster et al., 1977) and mice
against monocrotaline (Miranda et al., 1981c).
(c) The antioxidant ethoxyquin fed at a level of 2.5 g/kg diet
to female mice for 38 days, increased the liver thiol
concentration and raised the acute LD50 of monocrotaline, given
ip on the 10th day, to 364 mg/kg compared with 243 mg/kg in
control mice (Miranda et al., 1981a).
(d) Rats or mice also had increased resistance to acute
pyrrolizidine hepatotoxicity when fed the antioxidant butylated
hydroxyanisole (BHA) (up to 7.5 g/kg diet) (Miranda et al.,
1981c, 1982a,b; Kim & Jones, 1982).
(e) Heliotrine-induced toxicity can be modified by the
co-administration of cupir (a copper-containing complex) at a
level of 1 mg/kg per day for 20 days. It prevented the exit of
hepatic cytosolic enzymes into the blood and improved all the
energy reactions studied in the mitochondria of heliotrine-
intoxicated rats (Yuldasheva & Sultanova, 1983). Inhibition of
lipid peroxidation by cytoplasmic copper was shown later
(Wittig & Stephen, 1964). Savin (1983) found that lethality to
rats of heliotrine (300 mg/kg sc) was completely prevented by
co-administration of alpha-tocopherol (6 ml/kg ip).
(f) Rats pre-treated with ip doses of zinc chloride (72 µmol/kg
body weight) had increased resistance to the hepatotoxicity of
Senecio jacobaea alkaloids, as assessed by histology and enzyme
measurements (Miranda et al., 1982c). The zinc treatment increased
the liver level of metallothionein, a sulfhydryl-rich protein that
might react with pyrrolic metabolites.
Metabolic inhibitors of the microsomal P450 mixed-function
oxidase system, SKF 525A, metyrapone, and allylisopropyl acetamide,
which inhibit the formation of toxic pyrroles in the liver, have
been tried successfully in the prevention of the toxic effects of
monocrotaline in rats (Eisenstein & Huxtable, 1979). The use of
P450 inhibitors was stated to show "potential therapeutic promise".
However, this would seem impracticable considering that, at least
in the rat, PAs undergo a high rate of metabolism commencing a few
minutes after ingestion (Mattocks, 1972b). In some instances, they
have been known to lead to an increase in toxicity, e.g., with
lasiocarpine as reported by Tuchweber et al. (1974).
5.5.3 Other treatments
Lanigan & Whittem (1970) attempted, unsuccessfully, to protect
sheep against Heliotropium europaeum poisoning by treating them
with cobalt, in the hope that this would enhance the vitamin
B12-mediated detoxication of the alkaloids in the rumen (Dick et
al., 1963).
Lanigan et al. (1978) found that the resistance of sheep to
dietary Heliotropium europaeum was increased by giving them large
daily doses of the antimethanogenic drug, iodoform. However, Swick
et al., (1983) found that Senecio jacobaea alkaloids were not
detoxified by incubation for 48 h with sheep rumen fluid in vitro.
6. EFFECTS ON ANIMALS
6.1 Patterns of Disease Caused by Different Plant Genera and of
Organ Involvement in Different Species
The most important genera of PA-containing plants listed in
section 3.1 are all hepatotoxic. Among these, Crotalaria spp.
cause damage in the broadest range of tissues in most domestic
species. In pigs, they are known to be severely nephrotoxic
(Peckham et al., 1974; McGrath et al., 1975; Hooper & Scanlan,
1977). Some species are known to be pneumotoxic for horses (Watt &
Breyer-Brandwijk, 1962; Gardiner et al., 1965), cattle (Sanders et
al., 1936; Berry & Bras, 1957), sheep (Laws, 1968), and pigs
(Peckham et al., 1974; Hooper & Scanlan, 1977), as well as
hepatotoxic.
Although several Crotalaria spp. are known to be pneumotoxic
for horses (Gardiner et al., 1965), C. retusa is an exception. It
is an important cause of disease in horses in northern Australia
(Hooper, 1978) and has been shown to be pneumotoxic for pigs in the
same area (Hooper & Scanlan, 1977); yet it produces only hepatic
disease in horses (Rose et al., 1957a,b).
Similarly, Senecio spp. are primarily hepatotoxic, but
S. jacobaea has been demonstrated to be pneumotoxic for pigs
(Harding et al., 1964), though it could probably be an inconsistent
change (Bull et al., 1968). This plant is also known to cause
pulmonary disease in rats and mice (Hooper, 1974). However, there
are no reports of its affecting the lungs in cattle, sheep, horses,
or chicken. Renal megalocytosis and mild nephrosis are reported in
most species poisoned with S. jacobaea (Harding et al., 1964; Bull
et al., 1968). Heliotropium spp., Amsinckia spp., and Echium spp.
are all mainly hepatotoxic.
Roitman (1983) summarized the pattern of organ involvement
observed in man and different species of farm and experimental
animals affected by pyrrolizidine alkaloids (Table 8). Even within
a single species, the nature of a toxic effect, as well as the
organ affected, can be altered by changing the dose rate and
duration.
6.2 Field Observations - Outbreaks in Farm Animals
The veterinary problem of PA toxicity has been reviewed by Bull
et al. (1968) and McLean (1970). Mattocks (1986) listed the cases
of livestock poisoning and feeding trials since 1968, and cited
relevant literature. Peterson & Culvenor (1983) produced a useful
and comprehensive table of the plant species known or suspected of
causing natural outbreaks of poisoning in each animal species. The
influence of factors such as species, age, sex, and diet, on
toxicity is also reviewed in the same paper.
Table 8. Animal species and organs affected by pyrrolizidine
alkaloidsa
---------------------------------------------------------------
Species Liver Lung Kidney Heart Pancreas Gastric Muscle
mucosa
---------------------------------------------------------------
Man +
Monkey + + + +
Horse + + +
Pig + + + + +
Sheep + + +
Goat + +
Cattle + +
Dog +
Mouse + +
Rat + + + +
Chicken + + + + +
Turkey + + +
---------------------------------------------------------------
a From: Roitman (1983).
The first cases of pyrrolizidine poisoning were described in
cattle as early as 1903 (Gilruth, 1903). Since then, there have
been numerous reports from most parts of the world, of poisoning
among farm animals caused by grazing or feeding on PA-containing
weeds (Bull et al., 1968; Mattocks, 1986). One of the first clues
to the etiology of the human disease in Jamaica, came from a study
in which calves fed with Crotalaria fulva (Bras et al., 1957)
developed characteristic veno-occlusive disease in the liver.
Laws (1968) described a field outbreak in sheep in a herd of
100 adult merino ewes, which developed within 2 weeks of moving
into a coastal farm in Australia, where they grazed Crotalaria
mucronata. The etiology was confirmed by feeding the plant to 6
sheep, 4 of which died within 24 h of feeding, with severe
pulmonary oedema. However, the rapidity of poisoning and the
atypical lung lesions suggest that possibly a toxin other than
pyrrolizidine alklaoids was also present.
An outbreak of Crotalaria retusa poisoning was observed in a
piggery near Darwin, Northern territory, Australia (Hooper &
Scanlan, 1977) containing approximately 350 sows. It was caused by
feeding sorghum contaminated by Crotalaria seeds at the rate of
about 0.1% by weight for about 3 weeks, and at a rate of about
0.05% for a further week. The disease was indicated by reduced
body weight gain and inappetence. The dominant pathological
features at autopsy were severe nephrosis with chronic uraemia, and
to a lesser degree, severe diffuse interstitial pneumonia. Both
were accompanied by microscopic disease in the liver, and both the
liver and kidney showed megalocytosis.
Walker & Kirkland (1981) reported outbreaks in the Hunter river
valley of New South Wales in Australia, in cattle that had been
grazing a pasture in which Senecio lautus was growing. There were
sporadic deaths among the cattle as well as two protracted
outbreaks affecting calves 3.5 months of age and older animals, in
which groups of 3 - 16 head of cattle died in addition to sporadic
deaths of animals over periods of 1 and 6 months. Clinical signs
were weakness, emaciation with recumbency, aimless wandering and
ataxia, which suggested neurological involvement. At autopsy, the
liver showed characteristic megalocytosis, periportal fibrosis, and
focal necroses. An aged animal had cirrhosis. The etiology of
S. lautus was proved in feeding studies on 3 calves.
Knight et al. (1984) reported the deaths of 10 horses during a
3-year period after being fed hay from the same pasture. The
animals became sick in the spring after being fed only the suspect
hay throughout the winter. The hay was found to be contaminated
with Cynoglossum officinale (hound's tongue), which contained two
PAs (heliosupine and echinatine) in much higher quantities than is
generally reported in Senecio species. The animals developed
weight loss, icterus, ataxia, and symptoms of hepatic failure. At
autopsy, there was diffuse severe megalocytosis, biliary
hyperplasia, and fibrosis. The C. officinale was proved as the
etiological factor in a feeding trial on a 14-year-old pony, that
developed clinical features and pathological changes in the liver
suggesting PA poisoning. The signs of PA toxicity in horses are
mostly neurological, though non-specific gastric and oesophageal
lesions have also been reported (McLean, 1970) (section 6.4.3).
Rose et al. (1957a,b) described a disease in which the dominant
symptom was "compulsive" walking in a straight line. It occurred
in areas where Crotalaria retusa was growing, and was ascribed to a
steep rise in blood-ammonia levels, which accompanied chronic liver
failure.
Farm animals differ widely in their sensitivity to PAs, sheep
and goats being fairly resistant, cattle and horses, less so, and
poultry and pigs, rather sensitive (section 6.4.1.2). Sheep are
not immediately affected and generally survive one season, after
feeding on heliotrope and Senecio (Bull & Dick, 1959). During the
second season of feeding, they die of neurological symptoms caused
presumably by rising blood-ammonia levels associated with chronic
liver disease, or with haemoglobinuria and very high copper levels
in the blood (Bull et al., 1956, 1958) (section 6.4.1.2). With
Crotalaria, the lung seems to be the target organ (Hooper, 1978).
Similar acute responses to a single feed of the plant Crotalaria
spectabilis were described in cattle by Emmel (1948) and in the
chicken by Piercy & Rusoff (1946).
Poisoning of cattle in northwestern USA has reached such
proportions that it has become a considerable economic problem
(Johnson, 1982). Culvenor (1985) has reviewed the problem of
livestock losses due to PA toxicosis in Australia, where it has
been estimated that about 10 million sheep are exposed to
heliotrope and Echium plantagineum (Paterson's curse) to a greater
or lesser extent and may suffer a shortening of their productive
life by as much as two years. Most of the PA-containing plants are
reported to grow in fallow fields and pastures and thrive
particularly in a dry climate or following periods of drought.
However, instances of cattle poisoning have been reported from most
parts of the world, including countries with temperate or cold
climates, which do not ordinarily suffer drought. Three herds of
cattle have been reported to have been affected in the
alpine/subalpine region of Switzerland, after they grazed pastures
that had Senecio alpinus growing on them. Nine different
hepatotoxic PAs were found in the weed, the main one being
seneciphylline. Analyses of urine samples from one cow confirmed
the presence of PA metabolites. Several cows had to be
slaughtered, because of cirrhosis of the liver (Luthy et al.,
1981).
6.3 Studies on Farm Animals
There are several reports of the production of disease
characteristic of PA toxicity in farm animals, by feeding them
PA-containing plants.
Senecio jacobaea (tansy ragwort) is a weed that commonly grows
in pastures and has been the cause of extensive livestock losses in
the United Kingdom (Forsyth, 1968) and the USA (Johnson, 1982).
Extensive studies on this plant have been carried out using a
variety of farm animals. Dickinson et al. (1976) fed tansy ragwort
to cows through a rumen canula at the rate of 10 mg/kg body weight
per day, for 2 weeks. Liver biopsies showed characteristic
megalocytosis and fibroplasia, and autopsy also showed
centrilobular necrosis. A PA, jacoline, was found in the milk, but
when the calves were bucket fed the milk, there were no detectable
effects on them. Thorpe & Ford (1968) made similar observations in
5 calves fed ragwort in their diet. Animals eventually dying of
toxicity showed characteristic necrosis, megalocytosis, and veno-
occlusive lesions. In a study by Goeger et al. (1982), goats were
fed dried ragwort mixed in the diet at 250 g/kg. Four of the 11
goat kids and lactating dairy goats died. Characteristic
megalocytosis was seen in the liver. Goats are more resistant to
tansy ragwort toxicosis than cattle and horses, the chronic lethal
dose for cattle or horses being 0.05 - 0.2 kg ragwort/kg body
weight and, for goats, 1.25 - 4.04 kg/kg body weight. The alkaloid
levels in the plant, and thus its toxicity, varies with season and
locality.
Hooper & Scanlan (1977) studied the long-term effects of
feeding very low levels of ground C. retusa seeds, mixed with the
feed, to pigs and chickens. Seven groups of 4 pigs each (sex not
mentioned) bred from Saddleback-large white cross sows and Large
White or Landrace boars, were maintained on diets containing 0
(control), 0.004%, 0.01%, 0.02%, 0.05%, 0.1%, or 0.5% body weight
ground seeds. Another 8 pigs were fed a diet containing 0.1%
C. retusa for 21 days followed by 0.05% for 7 days and then kept on
a C. retusa-free diet. Pigs either died or were killed when
moribund, or at the end of 136 days of feeding.
In a second study, groups each of 4 2-week-old chickens were
fed diets containing 0 (control), 0.005%, 0.01%, 0.05%, 0.1%, and
0.5% ground seeds of C. retusa. Chickens fed 0.5% started dying 12
days after the commencement of feeding and were all dead by day 45.
Five out of 8 birds fed 0.1% or more died between days 22 and 56.
No deaths occurred in animals fed 0.01% - 0.005%.
In the study on pigs, all animals died between days 63 and 107
except for 2 that survived 136 days. In these animals, pulmonary
disease was the main cause of death. Hepatic and renal
megalocytosis was seen in almost all animals in both the field
outbreak and study group. The lungs showed extensive consolidation
and oedema. Besides megalocytosis in the glomeruli and tubules,
the kidneys showed glomerular atrophy and tubular necrosis. In the
study on poultry, the major disease was hepatic necrosis of
irregular distribution. The kidneys showed mild megalocytosis.
In the above study, the low levels of contamination that
produced serious disease are worthy of note.
Johnson & Molyneux (1984) fed 55 cattle, by gastric lavage,
with hay mixed with threadleaf groundsel ( Senecio douglasii var.
longilobus), which grows commonly in the pastures of southwestern
USA. The PA dosage in different groups ranged from 5 to 40 mg/kg,
daily, and the total intake ranged from 80 to 284 mg/kg body
weight. The groups were fed for periods ranging from 2 to 20 days.
One hundred percent mortality occurred in 3 out of 9 groups, each
consisting of 2 - 8 calves, receiving doses of 13 mg/kg or more.
Mean survival time was generally inversely proportional to the
dosage received. All sick calves had typical clinical signs of
seneciosis. At autopsy, the principal lesion was seen in the liver
and consisted of swelling of the hepatocytes, necrosis, biliary
hyperplasia, and marked fibrosis. The estimated minimum lethal
dose of the PA was 13 mg/kg body weight for 15 days, or a total
intake of approximately 200 mg PA/kg, over a 15-day period. Cattle
that consumed up to 600 mg PA/kg, in hay, in a 20- to 100-day
period, were unaffected or only slightly affected. The authors
concluded that the time-dose relationship for PA toxicosis in
cattle is important and that there is a threshold level that must
be exceeded for the toxicosis to develop.
A similar study was conducted by Johnson et al. (1985) in which
the dry whole or ground leaves of Senecio riddelli, mixed with the
feed, were fed to calves in gelatin capsules or by gavage. Forty-two
female Hereford calves were divided into 3 groups. One group
of 12 was fed the leaves mixed with alfalfa hay feed estimated to
have 20 - 40 mg PA/kg body weight per day over a 20-day period in
different regimes, receiving a total of 400 - 800 mg PAs per
animal. The second group of 12 animals received the plant packed
into gelatin capsules, receiving an estimated PA content of
10 - 20 mg/kg body weight, daily, over 20 days, with a total of
200 - 400 mg per animal. The third group of 18 animals was
administered (by gavage) finely ground leaves in a water slurry at
various PA dosages ranging from 10 to 60 mg/kg body weight per day
and a total of 200 - 500 mg over 20 days.
Calves that received 10 mg PA/kg body weight per day for 20
days did not develop clinical signs of disease or show any changes
in serum-enzyme. However, feed containing the plant that provided
15 - 20 mg PA/kg per day or more, administered by gavage or fed in
capsules, resulted in high mortality. Malaise, depression, erratic
or unprecedented behaviour, aimless walking, and ataxia, were
observed in the affected calves; diarrhoea with tenesmus and rectal
collapse were frequently observed. The feed intake decreased
progressively and was negligible terminally. The animals that died
and those that were moribund or in a state of irreversible wasting,
were autopsied. Hepatobiliary lesions were present in all such
animals. The most consistent change was portal biliary hyperplasia
and periportal fibrosis. Centrilobular or zonal haemorrhage and
necrosis were observed in some lobules. Fibrosis of some central
veins was common, often encroaching on the lumen, resulting in
complete occlusion. Hepatocytes also showed nonspecific changes.
Central nervous system changes were present in all animals with
clinical signs of seneciosis, consisting mainly of spongy
degeneration of the brain.
The plant mixed in the hay ration was eaten slowly and
reluctantly and was tolerated at dosages > 20 mg/kg per day,
emphasizing that the toxicity depended on the rate at which the
dosage was consumed and that mortality was not necessarily
dependent on the cumulative dosage.
Burguera et al. (1983) produced the disease in turkey poults by
feeding them seeds of C. spectabilis. Simultaneous addition of
sodium selenite at doses of 0.1, 5, or 10 mg selenium/kg diet did
not provide any protection.
6.4 Experimental Animal Studies
6.4.1 Effects on liver
6.4.1.1 Relative hepatotoxicity of different PAs and their N-oxides
The LD50 values for rats, listed in Table 9, are for some of
the most commonly used hepatotoxic alkaloids, calculated from data
on animals dying from acute haemorrhagic necrosis of the liver, 3 - 7
days after intraperitoneal administration of a single dose. It
is evident that the toxicity varies widely between the alkaloids.
The most toxic are certain macrocyclic diesters of retrorsine and
the least toxic are the monoesters of heliotridine, retrorsine, and
supinine (Mattocks, 1986).
The relative toxicity of N-oxides compared with that of their
basic alkaloids depends on the route of administration. The
N-oxides of lasiocarpine, monocrotaline, and fulvine were reported
to be as toxic as their basic alkaloids (Schoental & Magee, 1959)
when administered orally; however, when given by the ip or
intravenous (iv) routes to rats, they were much less toxic
(Mattocks, 1971c). Similarly, the LD50 of retrorsine N-oxide when
administered ip to male rats was 250 mg/kg (Table 9) but when given
orally, it was 48 mg/kg. This has been explained by the
observations on the metabolic pathways of the basic alkaloids and
their N-oxides. The PAs or their N-oxides exert toxic effects only
after being metabolized to pyrroles by the hepatic microsomal
enzymes (section 5.1.1). Hepatic microsomes act directly on the
N-oxides (Mattocks & White, 1971b) only after they have been
converted to the basic alkaloids (Mattocks, 1986); this mainly
occurs in the gut (Mattocks, 1971c; Powis et al., 1979). This
matter is of practical importance as the alkaloids are often
present as their N-oxides in weeds grazed by farm animals.
Table 9. LD50s in male rats after a single intraperitoneal dose of
some hepatotoxic alkaloids
-------------------------------------------------------------------
Alkaloid LD50 Time range Reference
(mg/kg) (days)
-------------------------------------------------------------------
heliotrine 296 3 Bull et al. (1958)
lasiocarpine 77 3 Bull et al. (1958)
lasiocarpine N-oxide 547 3 Bull et al. (1958)
monocrotaline 175 3 Bull et al. (1968)
retrorsine 34 4 or 7 Mattocks (1972a)
retrorsine N-oxide 250 7 Mattocks (1972a)
senecionine 50 7 Mattocks (1972a)
seneciphylline 77 3 Bull et al. (1968)
senkirkine 220 -a Hirono et al. (1979a)
symphytine 130 -a Hirono et al. (1979a)
-------------------------------------------------------------------
a Not stated.
6.4.1.2 Factors affecting hepatotoxicity
These factors have been reviewed by Mattocks (1986).
(a) Route of administration
Most studies on LD50 values have been carried out using the ip
route, and very few experimental data are available on toxicity
using the oral route. There is a close similarity between the iv
data and the ip data. Furthermore, toxicity data on rats
administered PAs by the oral route (Schoental & Magee, 1959),
including retrorsine, lasiocarpine, heliotrine, and monocrotaline,
closely resemble those relating to the LD50 values for the same
strain administered PAs intraperitoneally (Mattocks, 1972b). Thus,
the hepatotoxicity of PAs in rats does not differ very much,
irrespective of the route of administration. However, rabbits
appear to be less susceptible to PAs in the plant Senecio jacobaea
when administered orally than when administered intravenously
(Pierson et al., 1977).
(b) Species
Wide differences have been observed in the hepatotoxic effects
of PAs and alkaloid-containing plants between different species of
both farm animals and laboratory animals, and in the same animal
exposed to PAs derived from different plants. Sheep are resistant
to PA-containing plants (section 6.2) and when fed Echium
plantagineum pellets containing 1.3 g alkaloid/kg as the sole diet
for 12 months, over a period of 2 years, showed almost no liver
damage (Culvenor et al., 1984). However, adult rats fed the same
pellets as only 50% of the diet for 14 days died 4 - 13 weeks later
(Peterson & Jago, 1984). Pigs were found to be 5 - 10 times as
susceptible to PAs in Crotalaria retusa as chickens (Hooper &
Scanlan, 1977). Overall, the approximate ratios of quantities of
plant material required to prove toxic in the various species
listed are about 200 for the sheep (approximately the same for the
goat), 150 for the mouse, 50 for the rat, 14 for cattle
(approximately the same for the horse), 5 for the chicken, and 1
for the pig (Hooper, 1978).
Cheeke & Pierson-Goeger (1983) studied the chronic toxicity of
Senecio jacobaea for several laboratory animals by feeding the
dried plant as a component of a mixed diet. The degree of
susceptibility to PA poisoning was compared in terms of the chronic
lethal dose of the dried plant as a percentage of the initial body
weight among the animals themselves, and with similar data on
livestock in other studies. Gerbils, hamsters, and guinea-pigs
were resistant to chronic toxicity, gerbils being the most
resistant, consuming over 35 times their body weight of the dried
plant. Comparison with similar data in other studies indicated
that the rabbit (Pierson et al., 1977), Japanese quail (Buckmaster
et al., 1977), and goat (Goeger et al., 1982) were resistant,
requiring a long-term lethal dose of the plant of 113% or more of
the initial body weight, whereas the rat was highly sensitive
requiring only 21% (Goeger et al., 1983). Chicks and turkey poults
were also susceptible with severe inhibition of growth occurring
when there was 5% and 10% contamination of the feed with the plant;
survival time was short (Cheeke & Pierson-Goeger, 1983).
In a study by Fushimi et al. (1978), on the carcinogenicity of
the flower stalks of Petasites japonicus Maxim in mice and Syrian
golden hamsters, species and strain differences were observed, not
only with regard to hepatotoxicity, but also with regard to the
carcinogenicity of PAs. Mice of ddN, Swiss, and C57BL/6 strains
and Syrian golden hamsters were fed on a diet containing young
flower stalks of the plant for 480 days. High incidences of lung
adenoma and adenocarcinoma were observed in ddN mice, but no
significant differences in tumour incidence were observed between
the experimental groups of Swiss mice and hamsters and the
corresponding control group. No tumours were induced in an
experimental group of C57BL/6 strain mice.
These differences have been explained by the differences in the
rate of metabolic conversion of PAs to toxic metabolites (pyrroles)
by the hepatocyte microsomes in the different animal species (White
et al., 1973; Shull et al., 1976; Peterson & Jago, 1984).
The resistance of sheep has been ascribed to destruction of the
alkaloids in the rumen by a reductive conversion into non-toxic
1-methylenepyrrolizidine derivatives (Bull et al., 1968; Lanigan,
1971, 1972). It has also been suggested that the resistance of
sheep is due to a low level of activation in liver cells (Shull et
al., 1976), but this factor was not prominent in some Australian
sheep, which were as sensitive as rats to PAs injected
intraperitoneally (Hooper, 1974).
Thus, it is possible for ruminants to graze plants containing
PAs for a period of months without evident harm, e.g., cattle
eating Crotalaria juncea in Africa (Srungboonmee & Maskasame,
1981), but long-term effects may arise in animals exposed over
several years.
Considerable differences in LD50 values have been reported for
the same alkaloids in different species. For example, the LD50 for
retrorsine varies from 34 mg/kg for male rats to 279 mg/kg for
quail and over 800 mg/kg body weight for guinea-pigs (White et al.,
1973). Guinea-pigs are also resistant to monocrotaline (Chesney &
Allen, 1973a), but not to jacobine or to mixed alkaloids of Senecio
jacobaea, which are highly toxic (Swick et al., 1982a).
(c) Sex
Significant differences in the hepatotoxicity of the same
alkaloid have been observed between sexes in some species. Male
rats are much more susceptible to the acute toxicity of retrorsine
or monocrotaline than females (Mattocks, 1972b). Mattocks & White
(1973) reported a higher level of metabolic transformation in young
male rats to form pyrroles from retrorsine, compared with females
(section 4.4). Jago (1971) reported a higher susceptibility in
male rats to the chronic hepatotoxic effects of heliotrine, while
female rats were more susceptible to lasiocarpine. It is possible
that this may be due to the potentiating effect of male sex
hormones. Campbell (1957a,b) reported that diethylstilboesterol
protects against the effects of seneciphylline and promotes repair
of damaged liver in poultry. Protein-deficient rats of both sexes,
or female animals pre-treated with testosterone, were more
susceptible to monocrotaline (Ratnoff & Mirick, 1949).
(d) Age
Available data on the effects of age are highly conflicting.
It has been stated that young rats, particularly suckling animals
(Schoental, 1959), are more susceptible than adults to the
hepatotoxic effects of some alkaloids (Jago, 1970), such as
monocrotaline (Schoental & Head, 1955), and retrorsine and
lasiocarpine (Schoental, 1959). Rats, 1 - 4 days old, were far
more susceptible to retrorsine and senkirkine than rats aged 25 - 30
days (Schoental, 1970); yet new-born rats (within 1 h of birth)
were relatively more resistant to the hepatotoxic effects of
retrorsine than 1- to 4-day-old rats (Mattocks & White, 1973).
McLean (1970) has critically reviewed the data. In comparing the
data on small animals from several studies, new-born and 4-week-old
animals appear to have about the same susceptibility as adults.
Data for the intervening period obtained by Harris et al. (1957),
Schoental (1959), and Hayashi & Lalich (1968) are conflicting,
suggesting decreased susceptibility in some studies (Harris et al.,
1957 and one series of Schoental's studies, 1959), and increased
susceptibility in others (Hayashi & Lalich, 1968 and the second
series of Schoental's studies, 1959). Furthermore, Jago (1971)
demonstrated that, while rats aged 1 - 2 weeks were more
susceptible to the acute effects of heliotrine and lasiocarpine
than older rats, sensitivity to the effects of long-term
administration of these alkaloids increased with age, after 2 - 3
months.
(e) Diet
Effects of both qualitative and quantitative changes in diet on
the hepatotoxicity of PAs have been investigated in several
studies. Restriction of protein levels in the diet enhanced the
acute hepatotoxic effects of retrorsine in rats (Selzer & Parker,
1951) and the chronic effects of a single dose of orally
administered lasiocarpine (Schoental & Magee, 1957) (section
6.4.5.1) as well as the toxicity of PAs in Senecio jacobaea,
whereas a high protein diet had a protective effect (Cheeke &
Gorman, 1974). Likewise, low lipotrope diet enhanced the toxic
effects of orally administered lasiocarpine in pregnant rats and
also in the fetal livers (Newberne, 1968). On the other hand, it
protected young male rats against the acute toxicity of
monocrotaline, because of the reduced metabolic conversion of the
alkaloid into pyrrolic metabolites (Newberne et al., 1971, 1974).
Caloric restriction reduced the cardiopulmonary toxicity of a
single dose of monocrotaline in rats (Hayashi et al., 1967). This
was ascribed to the reduced growth rate in animals on a restricted
diet rather than to a reduction in the rate of metabolic conversion
of the alkaloid, since dietary restriction started only after
administration of the alkaloid. When the animals were put back on
the ad libitum feeding regimen, they developed signs of increased
toxicity.
A high copper content in the diet has been shown to enhance the
toxic effects of PAs (Miranda et al., 1981b). Incorporation of
copper sulfate at 50 mg/kg in the diet containing the plant Senecio
jacobaea increased the hepatotoxicity in rats, as judged by enzyme
measurements. The implications of this observation are obvious if
some PA-containing plants being grazed by farm animals also have a
high copper content.
Mattocks (1972b) demonstrated the protective effects of sucrose
against the acute hepatotoxic effects of retrorsine in rats, if
administered for 3 days prior to alkaloid administration (section
5.5.1).
6.4.1.3 Acute effects
Experimental animal studies on the pathological effects of PAs
on the liver have been reviewed by Bull et al. (1968) and McLean
(1970). Most studies have been carried out on the rat (Schoental &
Magee, 1957, 1959; Bull & Dick, 1959; Schoental, 1963; Barnes et
al., 1964; McLean et al., 1964; Nolan et al., 1966; Jago, 1969;
Butler et al., 1970; Peterson & Jago, 1980), but several other
species of animals have been studied including the monkey (Wakim et
al., 1946; Rose et al., 1959; Allen & Carstens, 1968, 1971; Allen
et al., 1969), turkey (Allen et al., 1963), chicken (Allen et al.,
1960), hamster (Harris et al., 1957), mouse, guinea-pig (Chen et
al., 1940), quail, cat, rabbit, and pig (Emmel et al., 1935; Hooper
& Scanlan, 1977). All animals tested, except the guinea-pig (Chen,
1945), have been found to be susceptible in studies using purified
alkaloids and their N-oxides and crude extracts of PA-containing
plants.
Typically, the most common lesion produced in small laboratory
animals by doses close to the LD50 is a confluent haemorrhagic
necrosis in the liver, which appears within about 12 h of exposure
and peaks at 24 - 48 h. It is strictly zonal in distribution in
different species of animals but may vary within the same animal,
depending on the alkaloid used, species, nutritional status, and
pretreatment with other chemicals.
Retrorsine produces centrilobular necrosis in the rat, mouse,
and guinea-pig, periportal necrosis in the hamster, and focal
necrosis in the fowl and in the monkey (White et al., 1973). In
the monkey, monocrotaline produces centrilobular necrosis (Allen &
Carstens, 1968), but senecionine produces necrosis in the
periportal and midzonal areas of the liver lobule (Wakim et al.,
1946). Almost simultaneously, or shortly after the development of
acute haemorrhagic necrosis of the liver cells, various levels of
change appear in the central and sublobular veins of the liver
lobules, consisting of subintimal oedema or even necrosis, deposits
of fibrin, thrombosis, and occlusion of the lumen, which later
becomes organized. While haemorrhagic necrosis is a constant
feature, attempts to produce occlusive lesions in the veins of
experimental animals have produced variable results (Allen et al.,
1967). In man and non-human primates, hepatocellular necrosis and
venous occlusion occur simultaneously but, in the rat (McLean et
al., 1964), chicken (Allen et al., 1960), and swine (Emmel et al.,
1935), the vascular changes follow hepatic necrosis.
Selzer & Parker (1951) produced a lesion comparable to human
veno-occlusive disease in albino rats by administrating retrorsine
hydrochloride, the active alkaloid of Senecio ilicifolius, as well
as the crude plant extract. Four batches of rats were administered
alkaloids orally in a single dose of 1 - 1.5 mg/10 g body weight or
repeated doses of 5 - 50 mg/kg body weight for 31 days or as single
subcutaneous injection of 100 mg/kg body weight. One batch was fed
on a diet of Senecio ilicifolius constituting 10% of the ration as
crude plant or its extract; the animals lived for 21 - 84 days.
Some groups were kept on a normal diet, and others on a diet that
was protein-deficient. Animals, administered a single dose orally,
developed the earliest degenerative changes in the centrilobular
hepatocytes and sinusoidal dilatation, and the vascular lesion
appeared after 36 h. Protein deficiency enhanced the toxic effect.
Only 5 out of 9 animals administered repeated doses orally showed
early centrilobular fibrosis and none showed the vascular lesion,
possibly due to scarring.
Bull et al. (1958) studied the effects of a single ip LD70 dose
of heliotrine (320 mg/kg body weight), lasiocarpine (80 mg/kg), or
lasiocarpine N-oxide (629 mg/kg) in rats of a hooded strain of both
sexes. Eighty-one rats were used and 3 rats from each treatment
were killed at intervals of 4 - 36 h. Heliotrine produced marked
centrilobular necrosis at 24 h, but venous changes were not
evident, except for some aggregation of mononuclear macrophages on
the endothelium. With lasiocarpine, the hepatic changes were
similar, but the necrosis was not clearly centrilobular and, with
its N-oxide, it was midzonal at 34 - 49 h. The earliest toxic
effect of the PAs was manifested as a temporary loss of
mitochondria at 8 h. The authors concluded that PAs have an early
toxic effect on the hepatocytes and that this does not follow
vascular injury, as suggested by Davidson's earlier studies (1935).
McLean et al. (1964) administered an aqueous extract of
Crotalaria fulva to Wistar rats in a single intragastric dose of
0.8 - 1.5 g/kg body weight. Lesions identical to those of human
veno-occlusive disease were produced in the animals by adjusting
the dose to permit survival for 8 - 12 days. Loss of cytoplasmic
glycogen in the centrilobular cells occurred 3 h following
administration. Centrilobular necrosis, which occurred after 24 h,
increased with time. The central veins gradually filled up with
thickened endothelial cells at about 7 days, later progressing to
collagenization. Evidence was presented that the histological
occlusion of the central veins was preceded by several days by a
functional blocking of the blood flow.
Barnes et al. (1964) observed similar results in rats
administered a single oral dose of fulvine N-oxide at 50 mg/kg
body weight and studied at intervals of 1 - 4 days after the
administration. One hundred and thirty-five rats of both sexes
were used. Acute lesions resembling human disease were observed
during days 1 - 8. During days 19 - 21, 3 out of 25 animals showed
liver damage consisting of some centrilobular haemorrhage and
fibrous thickening of the central veins. Of the 78 animals studied
at 22 - 44 days, 50% still had centrilobular congestion and some
had fibrous thickening of the central veins.
The effects of pyrrolic derivatives of PAs on rats were studied
by Butler et al. (1970). Male albino rats of Porton strain were
administered solutions of pyrrole derivatives of monocrotaline
and retrorsine in dimethyl formamide as a single injection of
0.05 - 0.1 ml solution. When injected in the tail vein at a
concentration of 5 mg/kg body weight, it produced progressive
proliferation of alveolar epithelium of the lungs and the animals
developed signs of respiratory distress in 2 - 3 weeks. When injected
in the mesenteric vein at a concentration of 15 mg/kg body weight, as
a rule, the animals remained well in the postoperative period and
only 1/26 animals died of mesenteric vein thrombosis; the livers
developed multiple infarcts in the left lobes that developed into
multiple coarse nodules at 6 - 12 weeks. The above studies
substantiated the view that PAs act only when converted in
hepatocytes to pyrroles. When pyrroles were injected, they
affected the smaller vessels at the portal of entry; in animals
injected through the mesenteric vein, the main target was the
portal vein with only secondary damage to the parenchymal cells,
thus sparing the animals from the effects of hepatocellular injury.
On the other hand, pyrroles injected through the tail vein went
directly to the pulmonary arteries through the heart and damaged
the alveolar capillaries.
Acute veno-occlusive disease was produced in monkeys
administered monocrotaline (Allen et al., 1967, 1969) and ground
Crotalaria spectabilis seed (Allen & Carstens, 1968). In a study
published in 1967, these authors used 14 monkeys (Macaca speciosa)
of both sexes, each weighing approximately 4 kg. Seven of the
animals were administered 1 mg monocrotaline in distilled water by
gastric intubation on days 1 and 14. The remaining 7 were used as
controls and received distilled water only. Wedge biopsies of
liver were examined weekly. The survival time ranged from 14 to 38
days, the mean being 21 days. The livers of treated animals were
small and firm and showed changes characteristic of human veno-
occlusive disease including centrilobular necrosis, and vascular
changes in the central veins of liver lobules ranging from
subintimal oedema to progressive collagenization and extension of
collagen fibres into the sinusoids. Similar observations were made
in studies on Macaca mulatta monkeys (Allen & Carstens, 1968),
administered ground Crotalaria spectabilis seed. Sixty-four
animals, averaging 6.2 kg in weight, were divided into 3 groups.
Group I, comprising 10 experimental animals (4 control animals),
received seeds in the diet containing the equivalent of 0.074 mg
monocrotaline/kg body weight, daily, up to death. Group II,
consisting of 14 treated animals (4 control animals), received a
single dose of seeds containing 1.3 g monocrotaline/kg body weight,
and Group III, consisting of 26 experimental animals (6 controls),
received 3 weekly doses containing the equivalent of 0.116 g
monocrotaline/kg body weight, by gastric intubation. Liver
biopsies were carried out each month in Group I and each week in
Groups II and III. Animals of the last 2 groups were killed when
in extremis. The mean survival times for the groups were:
Group I, 269 days (176 - 425 days); Group II, 28 days (6 - 43
days); and Group III, 41 days (23 - 91 days). In Group I animals,
occlusive lesions of the central and sublobular veins of the liver
were seen in 7/10 animals at autopsy. These consisted of oedema,
haemorrhages, and fragmentation of the vessel walls, the lumina
being filled with fibrin, and degenerating liver cells. The
lobular pattern was distorted because of connective tissue bands
encircling small groups of liver cells, especially in the central
zones of the lobules. In Groups II and III, various levels of
focal or centrilobular necrosis were observed and the liver cells
were replaced by stromal tissue. Vascular lesions, as described
above, were seen in 25 monkeys, but no collagen was demonstrated.
In the studies of Wakim et al. (1946) on the rhesus monkey,
senecionine administered iv as a 2% solution at doses of 10 - 30 mg/kg
body weight to 4 animals, daily, until they appeared to be sick,
produced periportal, or midzonal necrosis in 3 animals accompanied
by haemorrhage. No mention was made of any vascular changes.
Electron microscopic studies
Svoboda & Soga (1966) studied the effects of lasiocarpine and
Crotalaria fulva on the livers of male Sprague Dawley rats weighing
110 - 150 g each. One group of 22 rats was given an ip injection
of lasiocarpine at 80 mg/kg body weight and pairs of animals were
killed at various intervals ranging from 15 min to 6 days. A
second group of 22 animals was administered a single dose of an
aqueous extract of Crotalaria fulva at 0.5 mg/g body weight, by
gastric tube, and killed at the same intervals. A third group of 8
rats was administered a total of 3.2 times the LD50 dose of
lasiocarpine in small doses, 3 times a week, and killed at 9 - 20
weeks. The changes primarily involved the nucleus and
interchromatin granules. The first change, seen after 30 min in
the nueleoli of the hepatocytes and Kupffer cells of animals
receiving lasiocarpine or crotalaria extract, consisted of a
separation of the fibrillar and granular components. The
hepatocyte nuclei had returned to normal after 72 h and remained so
throughout the rest of the study. In animals receiving a single
dose of lasiocarpine or crotalaria, round periodic acid schiff
(PAS) positive eosinophilic bodies appeared in the cytoplasm after
12 h, consisting of dense masses of cytoplasmic material. Five
days after treatment with crotalaria, large cells lined the luminal
surface of the central veins; the centrilobular cells had undergone
necrosis by this stage. Animals receiving 3.2 times the LD50 of
lasiocarpine developed megalocytosis after 9 weeks (section
6.4.1.5). The cytoplasm showed vesicles of smooth endoplasmic
reticulum with mitochondria of various shapes and sizes. The
appearance resembled an exaggerated regenerative response.
Allen et al. (1967, 1969) studied ultrastructural changes in
the liver tissue, in general, including the hepatic veins in Macaca
speciosa monkeys treated with PAs. In the study on hepatic veins
(Allen et al., 1969), 18 treated and 6 control adult animals were
used with an average weight of 5.8 kg. Animals were divided into 3
groups of 6 treated and 2 control animals each. The experimental
animals received 0.125 g monocrotaline/kg body weight by ip
injection. Liver wedge biopsies were examined at various intervals
in Group I during hours 1 - 48, in Group II at 4 - 12 days, and in
Group III at 16 - 32 days. The earliest changes, observed by light
microscopy, were seen at 24 h and consisted of progressive loss of
endothelial cells and other associated changes in the lumen and
wall leading to occlusion by collagenization by the third week.
Under the electron microscope, within 24 h of administration,
marked changes were observed in the endothelial cells resulting in
their rupture and release of organelles in the lumen. This was
followed by penetration of fluid though the vessel walls in the
first week and changes in the fibroblasts. By the third to fourth
week, hepatic veins showed various levels of occlusion and the
vessel was scattered with cell debris, free organelles, etc. The
authors concluded that, in this species, hepatocellular necrosis
was not the primary factor causing veno-occlusive disease, as the
association of cellular necrosis and venous occlusion occurred only
in the central area of liver lobules, and the hepatocytes
surrounding the sublobular and medium sized hepatic veins were
unaffected.
In their study of 1967, Allen et al. also investigated the
ultrastructural changes in the liver of M. speciosa monkeys after
administering 2 doses of 1 g monocrotaline each, on days 1 and 14.
At autopsy, after a mean survival time of 21 days, a wide spectrum
of changes was observed in the hepatocytic organelles, many of
which were lying, discharged into sinusoids, and also phagocytosed
by the Kupffer cells. By the third week, proliferation of
connective tissues had started in the sinusoids near the central
veins and also in the walls of central veins. The authors
concluded that the vascular and parenchymal cell changes were
simultaneous and appeared to be equally instrumental in the
development of the occlusive lesion.
6.4.1.4 Mechanism of toxic action
The mechanism of toxic action in acute pyrrolizidine
hepatotoxicity and the sequence of events, judged from the
collective experimental studies, appears to be as follows.
The PA, which is inactive as a cell poison by itself, becomes
cytotoxic through its metabolism in the hepatic parenchymal cells
to pyrroles, which act preferentially on the hepatocytes and the
endothelium of blood vessels in the liver or lungs. In the
hepatocytes, the immediate action is a rapid fall in cytoplasmic
protein synthesis reaching 30% of control levels at 15 min and 6%
at 1 h (Harris et al., 1969). This is manifested as disaggregation
of polyribosomes and is followed by failure of pyruvate oxidation,
loss of glycogen, structural damage to the mitochondria, lysosomal
activity, failure of mitochondrial nicotine-adenine-dinucleotide
(NAD) systems and nuclear NAD synthesis, and necrosis (McLean,
1970). The necrosis is zonal in the liver lobule, the particular
zone affected depending on the metabolic enzymic geography of the
lobule in the particular animal species, and also in man and monkey
on the vascular endothelium of the central and sublobular veins.
The sequence of events of the vascular lesion has been studied
by McLean et al (1964). After a single dose of Crotalaria fulva
extract in the rat, centrilobular necrosis is present after the
first day, but collagenous veno-occlusion of the central veins of
the liver lobule only appears between 7 and 10 days later.
Evidently, the necrosis of the liver cells is not secondary to
venous occlusion. Centrilobular haemorrhage is seen from day 2
onwards and signs of hepatic venous outflow tract obstruction
appear after 2 - 5 days (McLean & Hill, 1969).
Rappaport et al. (1967) and McLean (1969) demonstrated, through
transillumination studies on rats, that the outlet end of the
sinusoids is blocked by stationary columns of red cells, 16 - 24 h
following administration of PAs. The reaction is typically patchy
and results in stasis and extravasation of red cells spreading
backwards from the centre of the lobule. For at least 3 days, no
circulatory detail can be seen with transillumination. Portal
pressure is significantly raised 3 days after administration of
fulvine (Rappaport et al., 1967), notably before the first
appearance of collagenous venous occlusion at 7 days. McLean
(1969) observed that 6 - 10 days after PA administration, a new
irregular pattern of vascular flow, contrasting with the uniform
radial pattern of flow in the normal liver lobule, develops, which
corresponds to the bypass channels represented by dilated
paraseptal sinusoids, as observed in human liver biopsies (section
7.3). Segments of central vein into which the blocked sinusoids
open, are gradually abandoned in favour of such by-pass routes and
undergo occlusion first by oedematous connective tissue and then by
fibrosis. The mechanism of closure of the sinusoids is not clear.
A toxic action on the sinusoidal or venous endothelium, which
swells and occludes the lumen, seems possible, as suggested by
Allen & Carsten's (1968) electron microscopic studies on the
monkey, and studies on children (Brooks et al., 1970). The
endothelial lining of the vessels is denuded and replaced by a
fibrinous and proteinaceous precipitate, which, together with the
oedematous wall of the vessel, becomes organized and slowly
replaced by fibrous connective tissues. The occlusion of sinusoids
is further contributed by the discharge of cellular debris into the
space of Disse. The lumen of the sinusoids becomes occluded
simultaneously with the fibrosis occurring in the central vein.
Collagen fibres extend into the space of Disse and sinusoids
leading to a creeping fibrosis.
The proximate toxin that escapes from the liver is returned to
the heart, after which it damages the first portal of entry into
the alveolar capillaries of the lung and pulmonary arteries.
6.4.1.5 Chronic effects
The chronic hepatotoxic effects of PAs have been described in a
number of studies on a variety of animals and have been reviewed by
Bull et al. (1968) and McLean (1970). A notable feature is that an
appropriate single dose of PA has been demonstrated by Schoental
and Magee (1957, 1959) to lead to a relentlessly progressive course
and eventually kill the animal, more than 18 months after
administration. Schoental & Bensted (1963) demonstrated that rats
receiving a single dose of PA may develop chronic liver disease and
finally hepatocellular carcinoma more than 13 months after
administration. The morphological changes in the liver are similar
in a given species of animal, whether a single sublethal dose is
administered or multiple small doses.
Schoental & Magee (1957) studied the long-term effects of a
single dose of lasiocarpine on rats receiving normal and protein-
deficient diets. Albino rats of Porton strain were used. In the
first study, 66 rats fed a normal diet were administered a single
oral dose of lasiocarpine at one of 3 dose levels (50 - 74 mg/kg,
75 - 100 mg/kg, or 101 - 150 mg/kg body weight); 24 animals served
as controls. In another study, 46 young female rats were
administered a protein-deficient diet. Of these, 13 and 10 animals
received a single oral dose of lasiocarpine at 50 - 100 mg/kg and
50 - 75 mg/kg body weight, respectively. Each of these groups was
pair-fed with an identical number of animals that did not receive
any PA. Of the 66 rats fed a normal diet, very few died in the
first 10 days. Thirty-one animals survived longer than 3 months.
They continued to be in good health until shortly before death.
The numbers of animals that survived for 13 months after
administration of lasiocarpine were 8/10 males and 7/7 females
(lowest dose) and 5/25 males and 11/18 females (intermediate dose).
In the group that received the highest dose, neither of the 2 male
animals survived longer than 35 days, and only 1/4 female animals
survived longer than 3 months. In the animals that died or were
killed when moribund, parenchymal damage was invariably present
with prominent megalocytes, ductular proliferation, and invasion of
lobules by oval or elongated cells, thought to be derived from the
bile-duct epithelium or the reticuloendothelial cells. Animals
that survived showed various degrees of fibrosis and nodular
hyperplasia and, in some, a mild thickening of the central veins.
No obliterative lesions of the veins were seen. The 31 animals
that survived 13 months showed similar changes, but to a much
lesser extent. In the livers of animals that had repeat liver
biopsies, there was no tendency to regression of the lesions.
The above data indicate that very few animals died of acute
disease. In most animals, there was a latent period of 3 - 4
weeks, during which they remained well and showed little evidence
of liver cell injury, followed by a progressive course often
leading to fibrosis and nodular hyperplasia.
The low-protein diet adversely affected the growth of all the
rats in the control as well as the treated group. Only 3 out of 23
treated animals remained alive and in apparent good health, 8 - 11
months after the treatment. Liver biopsies taken at various
intervals between 4 and 10 months showed very severe fatty changes
in the liver cells. There was little fibrosis and no bile-duct
proliferation. In areas where the fatty changes were less severe,
characteristic megalocytes were seen. Control animals had either
normal livers or showed only slight fatty changes that were not
comparable in severity with those in the livers of PA-treated
animals. Thus protein deficiency in the diet was shown to enhance
the toxic effects of the PA.
Schoental & Magee (1959) extended these studies on young Wistar
rats using several other PAs including heliotrine, retrorsine,
riddelliine, seneciphylline, monocrotaline, and its N-oxide in
various dosages ranging from 25 to 300 mg/kg body weight; the
animals were studied at death from 1 - 10 days to 18 - 30 months.
Pathological changes were similar to those observed with
lasiocarpine in the previous study. Notable observations were that
necrosis did not necessarily precede subacute or chronic changes.
The livers of some animals became severely damaged and showed
nodular hyperplasia. Liver biopsy, 2 - 3 days after PA treatment,
did not show pathological changes in some animals, but a repeat
biopsy at 41 days showed characteristic changes. Fibrous
thickening of the central veins was observed in some animals, more
often with monocrotaline N-oxide, but no occlusion of the hepatic
veins was seen.
The studies of Nolan et al. (1966) confirmed the observations
of Schoental & Magee (1957, 1959). They gave a single dose of
lasiocarpine at 120 mg/kg body weight, by stomach tube, to 108
(equal numbers of both sexes) Sprague Dawley weanling rats (60 -
135 g body weight). Thirty animals of both sexes served as
controls. Groups of 10 animals, each consisting of 8 treated and 2
control animals, were killed at various intervals from 1 to 123
days. Of the 80 treated animals, 28 died within 26 days. No
delayed hepatic lesions were found in 59 rats between days 1 and
18. Between 19 and 123 days, delayed lesions were found in 34/49
rats. These 34 rats showed megalocytosis, but no ductular
proliferation or fibrosis.
In a second study, 127 twenty-one-day-old male Wistar rats were
given a single oral dose of lasiocarpine at 80 mg/kg body weight;
65 animals served as controls. Liver biopsy was carried out on day
3 in 108 and on day 9 in 15 of the treated animals. In 47 animals,
additional biopsies were carried out at intervals. Of the 127
PA-treated rats, 98 died during the first 9 days, and 29 after
10 - 50 days. In contrast to the first study, 32/58 survivors
exhibited delayed subacute and chronic lesions, as described by
Schoental & Magee (1957). Of these, 8 animals developed cirrhosis.
The observations indicated that the lesions of acute zonal
necrosis, which appeared on, or before, the third day, healed
without residual lesions. However, 55% of the 58 survivors
developed subacute/chronic lesions that tended to be progressive
after a latent period of 2 - 3 weeks. There appeared to be an
intimate relationship between chronic lesions and megalocytosis,
which was seen in 52/58 surviving animals. No obliterative
vascular changes were observed and so the lesions could not be
ascribed to impaired circulation.
Schoental (1959) demonstrated the toxic effects on the newborn
of PAs administered to lactating mother rats. Wistar rats
(200 - 300 g) were administered lasiocarpine, orally or by ip
injection, at 25 - 40 mg, in 5 - 10 doses of 5 - 10 mg, twice weekly
or more (24 rats), or retrorsine at 4 - 10 mg per dose, in 1 - 14
approximately daily doses (23 rats). The litters were left with
the mothers for 24 days or more (except for 1/2 hour separation
during the PA treatment). The litters were examined by biopsy at
frequent intervals or at autopsy when they died or were killed in a
moribund state. Litters of the lasiocarpine-treated rats showed
only insignificant fibrosis or some megalocytosis. In the
retrorsine-treated group, the majority of the young rats survived
for about 18 days, but all rats died before reaching the age of 30
days. The milk secretion of the mothers was apparently not affected
by the PA treatment. Of the 98 animals in 9 litters, 45 died by
the 20th day and 45 survived 30 days. Animals dying in the first
fortnight did not show gross liver lesions, but those that died at
weaning time or later, all showed signs of liver disease. Animals
dying at 18 - 30 days showed hydropic or fatty vacuolation of
hepatocytes and haemorrhage into distended sinusoids. The change
was severe in animals dying at 1 - 2 months, and some central veins
showed a narrowed lumen. The lactating rats that received the
alkaloids survived longer than their young, and most showed no ill
effects from the treatment. This evidently indicates either a high
susceptibility of suckling rats or a high concentration of PAs in
milk.
In studies by Allen et al. (1963), 2 groups of 4-week-old
turkeys, each consisting of 12 birds, were fed diets containing
ground Crotalaria spectabilis seed at 2.5 g/kg and 5 g/kg,
respectively, for 120 days. Twelve animals served as controls. At
the end of the study, 11/12 birds receiving 5 g/kg seed and 6/12
receiving 2.5 g/kg seed in the diet developed cirrhosis. The
minimum period of feeding required to produce cirrhosis was 75
days, provided the diet was reduced to a level that was not lethal.
Allen & Carstens (1971) induced the Budd-Chiari syndrome in
monkeys by monocrotaline. Six adult female and 9 adult male Macaca
speciosa monkeys, weighing 5.2 - 6.5 kg each, were divided into 2
groups, each comprising 5 control (3 males and 2 females) and 10
treated animals (6 males and 4 females). The treated group was
given a subcutaneous (sc) injection of monocrotaline at 60 mg/kg
body weight at monthly intervals for 3 months. Needle biopsy of
the liver was carried out every month for 5 months and laparotomy,
6 months after PA treatment.
The treated animals showed marked vascular changes and various
degrees of occlusion in the centrilobular and sublobular veins as
well as the larger vessels. There was also characteristic
haemorrhagic necrosis in the centrilobular zones and megalocytes
were seen. The portal venous pressures were raised. The animals
were autopsied at 6 months. The livers were markedly shrunken
weighing an average of 68 g in contrast to those of control
animals, which weighed 130 g. There were severe occlusive vascular
changes and irregular fibrosis in the lobules. The adjacent
sinusoids were dilated as a compensatory mechanism.
Swick et al. (1982a) studied the effects on guinea-pigs of
long-term dietary administration of Senecio jacobaea and compared
them with the toxic effects of single doses of injected Senecio
alkaloids and monocrotaline. The possible protective effect of
cysteine was also examined. Fifteen guinea-pigs of 250 - 300 g
initial body weight were divided into 2 treated groups, being fed
10% Senecio jacobaea, or 10% Senecio jacobaea plus 1% cysteine in
the diet, and a control group. The whole plant of Senecio jacobaea
was air dried and powdered for incorporation into the diets. The
animals were fed for 365 days. They were autopsied at death or at
the termination of the study. In a second study, 7 guinea-pigs of
500 g body weight were injected intraperitoneally with either
monocrotaline, jacobine, or mixed Senecio jacobaea PAs. The
chronic lethal dose LD100 of Senecio jacobaea was 1264 g/kg initial
body weight or 526% of the initial body weight with an average
survival time of 279 days. No mortality was observed in control
animals. This contrasts with the chronic LD100 of Senecio jacobaea
for rats of 58% of initial body weight (Swick et al., 1979) and
that of cattle equivalent to 5 - 20% body weight (Bull et al.,
1968). Addition of cysteine to the diet was only slightly, but not
significantly, protective. Pathological examination of the livers
of the guinea-pigs fed Senecio jacobaea revealed extensive
megalocytosis and severe cytoplasmic vacuolation with biliary
hyperplasia and fibrosis, primarily in periportal areas. The
centrilobular and midzonal areas were spared.
Monocrotaline was non-toxic at doses up to 1000 mg/kg body
weight, whereas jacobine and mixed alkaloids from Senecio jacobaea
were lethal at much lower levels. Similar results showing
resistance to monocrotaline in guinea-pigs were also reported by
Chesney & Allen (1973a). In in vitro studies, they related this
resistance to lack of conversion of PA to pyrroles by guinea-pig
microsomes.
A morphological peculiarity of chronic hepatotoxicity in a
large variety of laboratory and farm animals is megalocytosis
(Bull, 1955; McLean, 1970), i.e., the appearance of exceptionally
large hepatocytes, 10 - 30 times the volume of normal cells with
proportionately large nuclei. Relevant literature has been
reviewed by Jago (1969), McLean (1970), and Mattocks (1986).
Advanced megalocytosis was produced by Jago (1969) within 4 weeks
in 2-week-old rats by administering a single dose of lasiocarpine
at 76 µmol/kg body weight. Megalocytes tend to appear in the
periportal and midzones of the liver lobules with normal sized
cells around the central veins. The nuclear chromatin is
proportionately increased, but the cells appear incapable of
entering into mitosis, as only abnormal mitoses are seen. Jago
(1969) demonstrated a fall in the mitotic index (from 1.61 to 0.04)
in liver cells of 2-week-old rats, one day after injection of 50 µmol
lasiocarpine/kg. The electron microscopic appearance also
supports the above observations (Afzelius & Schoental, 1967). A
striking proliferation of rough endoplasmic reticulum and multiple
centrioles is seen in the cytoplasm, and the cytoplasmic organelles
are disorganized, suggesting increased metabolic activity but
inability of the cells to divide. Such cells may persist for the
life-time of the animal (up to 2 years in the rat) and the liver
never returns to normal (Mattocks, 1986). Megalocytes have also
been described in the kidney (Bull et al., 1968), the lung (Barnes
et al., 1964; Butler et al., 1970; Hooper, 1974), and the duodenum
(Hooper, 1975c).
Data on the total chronic lethal dose of heliotrine in rats
were discussed by Bull & Dick (1959) and Bull et al. (1968). For a
variety of dosing rates, and with withholding periods of 10 - 20
weeks interposed, the total doses ranged from 2.2 to 7.8 LD50. In
Table 10, these data are extended with results for other alkaloids.
The overall range of the total lethal dose is 1.2 - 10.3 LD50.
6.4.2 Effects on lungs
Current literature has been extensively reviewed by Kay & Heath
(1969), and Mattocks (1986). PAs have been shown to produce
pulmonary hypertension with associated vascular changes in the
pulmonary circulation in a number of experimental animal species
including the rat, mouse, frog, turkey, pig, sheep, rabbit, and
horse (McLean, 1970) as well as in non-human primates (Allen &
Chesney, 1972; Chesney & Allen, 1973b) and the dog (Miller et al.,
1978). The alkaloids have been administered by feeding the animals
with: PA-containing seeds of plants (notably Crotalaria
spectabilis) (Turner & Lalich, 1965; Kay & Heath, 1966; Kay et al.,
1967a) or the dried plant itself (e.g., Senecio jacobaea) (Burns,
1972), aqueous solutions of fulvine (Barnes et al., 1964;
Wagenvoort et al., 1974a,b) or monocrotaline (Lalich & Ehrhart,
1962; Huxtable et al., 1977), subcutaneous injections of
monocrotaline (Allen & Chesney, 1972; Chesney & Allen, 1973b) and
seneciphylline (Ohtsubo et al., 1977), or intravenous injections of
some pyrrolic esters and analogues of pyrrolizidine alkaloids and
their metabolites (Mattocks & Driver, 1983). Lafranconi & Huxtable
(1984) studied the hepatic metabolism and pulmonary toxicity of
monocrotaline in in vitro perfusion studies. Some of the
representative studies on the morphological effects of toxic lung
injury are listed in chronological order in Table 11.
Chronic lung lesions have been produced by most compounds that
produce chronic liver lesions, though higher doses were required in
some instances (Culvenor et al., 1976a). However, not all PAs that
are hepatotoxic are also pneumotoxic. Among the pneumotoxic
alkaloids, fulvine (Barnes et al., 1964) and monocrotaline are
particularly active (Mattocks, 1986). Molecular structure activity
requirements are the same as for hepatotoxicity, since both are
caused by the same toxic metabolites produced in the hepatocytes.
6.4.2.1 Acute effects
Pulmonary lesions produced by PAs have been extensively
investigated, mostly in rats, but also in non-human primates.
Monocrotaline has been the alkaloid most frequently used, but lung
lesions have also been seen in rats following fulvine and
seneciphylline administration. Besides pure alkaloids,
PA-containing seeds of some plants, most notably Crotalaria
spectabilis, have also been used.
Miller et al. (1978) gave a single iv injection of
monocrotaline at 60 mg/kg body weight to 10 mongrel dogs. Toxic
effects, recorded within 2 h, included ultrastructural changes in
the endothelial cells of the alveolar capillaries, prominent
accumulation of platelets, and the appearance of interstitial
oedema (Table 11). Valdivia et al. (1967a,b) used 25 Sprague
Dawley rats in their study and made similar observations on the rat
lung within 4 h of a single subcutaneous injection of monocrotaline
at a dose of 60 mg/kg body weight (Table 11). Interstitial oedema
and elastolysis of the alveolar wall, increase in number of mast
cells, and other associated changes were observed within 4 h of the
injection, followed by alterations in endothelial and interstitial
cells. All of the changes progressed steadily for up to 3 weeks.
It was concluded that the initial changes of destruction of
pulmonary capillaries and the other components of the alveolar wall
preceded the arteriolar hypertrophy and arteritis observed by other
investigators following monocrotaline administration, and alone was
sufficient to cause right ventricular hypertrophy. Sugita et al.
(1983a,b) administered a single dose of monocrotaline at 40 mg/kg
body weight to 5 Sprague Dawley rats and adduced further evidence,
by biochemical and radioisotopic studies, of microvascular leak in
the alveolar wall within the first 3 days of injury, which preceded
right ventricular hypertrophy observed 2 weeks following
administration (Table 11).
Table 10. Total chronic lethal doses in rats (ip administration, 2 or 3
times per week)
--------------------------------------------------------------------------
Dose (x LD50) Time to Total lethal Reference
death dose
(days) (x LD50)
--------------------------------------------------------------------------
Heliotrine (male rats, unless otherwise stated)
0.2 58 5 Bull & Dick (1959)
0.1 123 5.1
0.04 303 4.1
0.02 508 4.1
0.01 5.2 - 5.3 Bull & Dick (1960)
(with interval of 10 -
20 weeks after 21 days)
0.11 2.2 - 4.3 Bull & Dick (1959)
0.1 7.8 Jago (1971)
(35-day-old male rats)
0.1 4.7 Jago (1971)
(337-day-old male rats)
0.1 5.8 Jago (1971)
(35-day-old female rats)
0.1 4.5 Jago (1971)
(337-day-old female rats)
Lasiocarpine (male rats)
0.1 210 9 Culvenor & Jago (1979)
0.05 482 10.3
0.02 676 5.7
0.01 595 2.6
(0.005) (638) (1.4)
0.1 81 - 175 2.4 - 5 Bull & Dick (1959)
0.1 - 6.3 - 10.9 Jago (1971)
Lasiocarpine (female rats)
0.1 108 4.6 Culvenor & Jago (1979)
0.05 274 5.8
0.02 471 4
0.01 487 2.1
(0.005) (692) (1.5)
0.1 2.4 - 7 Jago (1971)
Monocrotaline
0.1 1.2 - 2.4 Bull et al. (1968)
0.05 2.5 - 4.4
Senecioninea
0.2 2 - 7.4 Bull et al. (1968)
0.1 1.7 - 5.7
0.04 (3 survived)
--------------------------------------------------------------------------
a Assuming LD50 mg/kg (c.f., Mattocks, 1986).
Table 11. Summary of experimental data on the morphological effects of toxic lung injury due to pyrrolizidine alkaloids (in
chronological order)
---------------------------------------------------------------------------------------------------------------------------------------
Animal/ Number Toxic agent Dose Administration Single/ Killed after Pathological References
strain/ of (mg/kg) dose multiple or survival effects
sex animals/ for
controls
---------------------------------------------------------------------------------------------------------------------------------------
Acute effects
Male 25/5 monocrotaline 60 subcutaneous single 2 - 48 h interstitial oedema, Valdivia et al.
Sprague (body 1 - 3 weeks endothelial cell (1967a,b)
Dawley rat weight) alterations,
elastolysis, etc.
Mongrel 10/0 monocrotaline 60 intravenous single 2 h interstitial oedema, Miller et al. (1978)
dog (body changes in endothelial
weight) cells
Male 5/3 monocrotaline 40 subcutaneous single 0 - 21 days microvascular leak, Sugita et al. (1983a)
Sprague (body right ventricular
Dawley rat weight) hypertrophy after 2
weeks
Chronic effects
Female 35/12 monocrotaline 10 - 30 ad libitum pulmonary arteritis Lalich & Ehrhart
Sprague (diet) feeding (with 20 - 30 mg dose (1962)
Dawley rat only)
6 alcohol- 2500 ad libitum 51 days none
extracted (diet) feeding
seeds of
Crotalaria
spectabilis
19/4 monocrotaline 10 - 75 ad libitum 26 - 232 Turner & Lalich (1965)
(diet) feeding days
---------------------------------------------------------------------------------------------------------------------------------------
Table 11. (contd.)
---------------------------------------------------------------------------------------------------------------------------------------
Animal/ Number Toxic agent Dose Administration Single/ Killed after Pathological References
strain/ of (mg/kg) dose multiple or survival effects
sex animals/ for
controls
---------------------------------------------------------------------------------------------------------------------------------------
Chronic effects (contd.)
Female 24/6 Crotalaria 200 - ad libitum 105 - 172 right ventricular Allen & Chesney (1972)
Wistar spectabilis 1600 feeding days hypertrophy and
Furth rat seeds (diet) dilatation, pulmonary
arterial hypertrophy,
pulmonary arteriolar
hypertrophy,
endocardial fibrosis
Female 10/34 Crotalaria 1000 ad libitum 30 - 60 days right ventricular Kay & Heath (1966)
weanling spectabilis (diet) feeding hypertrophy, pulmonary
Wistar rat seeds arterial hypertrophy,
pulmonary arteritis
Male 22/12 monocrotaline 120 subcutaneous single 20 - 47 days right ventricular Hayashi & Lalich
suckling (body hypertrophy, pulmonary (1967)
Sprague weight) arterial hypertrophy,
Dawley rat pulmonary arteritis,
fibrin thrombi
Female 30/10 fulvine 50 intraperitoneal single 3 - 37 days right ventricular Kay et al. (1971a)
Wistar rat (body hypertrophy, pulmonary
weight) arterial hypertrophy,
pulmonary arteriolar
hypertrophy, pulmonary
arteritis, arteriolar
thrombi
80 intragastric single 7 - 35 days
(body
weight)
---------------------------------------------------------------------------------------------------------------------------------------
Table 11. (contd.)
---------------------------------------------------------------------------------------------------------------------------------------
Animal/ Number Toxic agent Dose Administration Single/ Killed after Pathological References
strain/ of (mg/kg) dose multiple or survival effects
sex animals/ for
controls
---------------------------------------------------------------------------------------------------------------------------------------
Chronic effects (contd.)
Monkey 12 (30 monocrotaline 30 subcutaneous one 199 - 325 right ventricular Allen & Chesney (1972)
(Macaca days old) (body followed days hypertrophy and
arctoides) weight) by 3 (average, dilatation, left
(both (2, 4, 241) ventricular
sexes) and 6 hypertrophy, pulmonary
months arterial hypertrophy,
after pulmonary arteriolar
first hypertrophy, pulmonary
injection hypertension
12 (15 163 - 334 pulmonary arterial
months days hypertension (isolated,
old) (average, veno-occlusive disease
217) (liver)
Monkey 20/6 monocrotaline 30 subcutaneous as above 165 - 325 right ventricular Chesney & Allen
(Macaca (body days hypertrophy and (1973b)
arctoides) weight) (average, dilatation, pulmonary
(both 326) arterial hypertrophy,
sexes) pulmonary arteriolar
hypertrophy, pulmonary
arteritis, endocardial
fibrosis
Female 50/12 fulvine 80 intragastric single 1 - 6 weeks vasoconstriction, Wagenvoort et al.
Wistar rat (body pulmonary arterial (1974a,b)
weight) hypertrophy, pulmonary
or 50 arteriolar hypertrophy,
(body intraperitoneal right ventricular
weight) hypertrophy, thickening
of veins and venules
---------------------------------------------------------------------------------------------------------------------------------------
Table 11. (contd.)
---------------------------------------------------------------------------------------------------------------------------------------
Animal/ Number Toxic agent Dose Administration Single/ Killed after Pathological References
strain/ of (mg/kg) dose multiple or survival effects
sex animals/ for
controls
---------------------------------------------------------------------------------------------------------------------------------------
Chronic effects (contd.)
Male 16/0 seneciphylline 50 - 80 subcutaneous single 1 - 3 weeks pulmonary arteriolar Ohtsubo et al. (1977)
Wistar (body and right ventricular
rat (4 weight) hypertrophy (after 3
weeks old) weeks), right
ventricular dilatation
in 2 animals
Male 21/14 Crotalaria 1000 ad libitum 3 - 35 days pulmonary arterial Meyrick & Reid (1979,
Sprague spectabilis (diet) feeding hypertrophy and 1982)
Dawley rat pulmonary arteritis
(2/21); right
ventricular hypertrophy
---------------------------------------------------------------------------------------------------------------------------------------
A histological and electron microscopic study was made by
Hurley & Jago (1975) of the lungs of rats administered
dehydromonocrotaline. Female black and white hooded rats weighing
80 - 100 g were used. Dehydromonocrotaline dissolved in
dimethylformamide (DMF) was administered iv as a single dose at
30 mg/kg body weight to 12 rats and at 15 mg/kg body weight to 7 rats.
Four control rats were administered DMF alone. A colloidal
suspension of carbon black was injected iv, 6 - 18 h after
injection of dehydromonocrotaline, and the animals killed 19 - 44 h
after treatment.
After an interval of 6 - 8 h, there was a direct toxic effect
on the endothelial cells of pulmonary capillaries and small
venules. Many endothelial cells had prominent nuclei and thickened
cytoplasm, which contained more RNA granules than usual. There was
also an increase in the number of mitochondria. The endothelial
damage did not seem to have caused permanent disruption of the
small blood vessels, and, 2 days after injury, all vessels were
patent. Large numbers of mononuclear cells, which appeared in the
interstitial tissues of the lung 44 h after injury, seemed to be
altered emigrated blood monocytes.
6.4.2.2 Chronic effects
Lalich & Erhart (1962), fed 35 Sprague Dawley rats a diet
containing monocrotaline at 10 - 30 mg/kg (Table 11). Animals
receiving a daily dose of monocrotaline of 20 mg/kg diet or more,
showed progressive changes in the lungs after 24 days of feeding.
Of the 23 animals receiving 20 - 30 mg/kg, 12 showed pulmonary
arteritis, 4 of these even at the dose level of 20 mg/kg diet.
Pulmonary haemorrhages were observed in 16 animals. No changes
were observed in animals fed alcohol-extracted seeds or other
derivatives of monocrotaline.
Identical changes were observed in similar studies by Turner &
Lalich (1965) on two strains of rats, Sprague Dawley (19) and
Wistar Furth (24). The first group of 19 female Sprague Dawley
rats was fed a diet containing monocrotaline at an initial level of
10 mg/kg diet. Depending on the response of each individual
animal, the monocrotaline level was raised to a maximum of 75 mg/kg
diet (Table 11). Fourteen rats survived for more than 100 days and
8 reached the maximum dietary level of monocrotaline, the last
animal dying after 232 days. The second group of 24 female Wistar
Furth rats was fed a diet contaminated with Crotalaria spectabilis
seeds, initially at 0.2 mg/kg diet, gradually rising to 1.6 mg/kg
by increasing the levels by 0.2 mg/kg every week. All test animals
survived 100 days and 15 reached the maximum levels of Crotalaria
fed. The last animal died after 172 days of feeding. Animals
developed signs of toxicity and right ventricular strain, e.g.,
cyanosis, etc. Progressive thickening of media in the muscular
pulmonary arteries, progressive muscularization of arterioles, and
changes characteristic of pulmonary hypertension, were seen. Some
pulmonary arteries showed medial necrosis. No changes were
observed in the pulmonary veins. Significant hypertrophy of the
heart, as judged by the heart weight in relation to body weight,
was seen in almost all animals that survived 100 days or more
(32/38), and the right ventricles were dilated. The hypertrophy
affected the right side of the heart only, and generally
corresponded with the vascular changes. There was a marked
hyperplasia of the mast cells in the mediastinal lymph nodes and
around bronchi and pulmonary arteries. Similar observations were
made by Barnes et al. (1964) and Valdivia et al. (1967a,b).
Kay and his group studied cardiac and pulmonary vascular
changes in rats fed Crotalaria spectabilis seeds (Kay & Heath,
1966; Kay et al., 1967a,b) or administered fulvine (Kay et al.,
1971a).
A group of 10 female weanling Wistar albino rats were fed a
diet containing 1 g powdered seeds of Crotalaria spectabilis/kg
until they died of cardiorespiratory distress, after 36 - 60 days
of feeding. Thirty-four control rats were fed a normal diet. At
autopsy, the atria of the heart, the right ventricle, and the left
ventricle with the interventricular septum were weighed separately.
The medial thickness of the muscular pulmonary arteries was
measured, and expressed as percentage of external diameter. The
medial thickness of the muscular pulmonary arteries increased in
all test rats; acute or healing pulmonary arteritis was seen in 3
animals. Statistically significant cardiomegaly was present in all
rats fed the seeds, contributed chiefly by the right ventricle. The
readings from all the test rats were well outside the upper 95%
confidence limit. The increase in the medial thickness of the
pulmonary arteries correlated well with the weight of the right
ventricle (Fig. 10). Three rats showed pulmonary arteritis
(indicated by a solid triangle). It was presumed that the organic
basis for increased pulmonary resistance was the abnormal
muscularization of the radicles of the pulmonary arterial system.
Essentially similar results were obtained in an identical study
repeated on 8 test rats and 5 controls (Kay et al., 1967a). The
test rats developed pulmonary hypertension in 37 days, levels of
which were correlated with the medial thickness of the muscular
pulmonary arteries and that of the pulmonary trunk, as well as with
the weight of the right ventricle.
5.3.2 Activation and detoxication
Factors affecting the proportion of an ingested alkaloid that
is converted into toxic metabolites in an animal include the
following:
(a) Lipid solubility
Highly water-soluble alkaloids (such as indicine) are easily
excreted and have low toxicity. Alkaloids that are more lipophilic
are more open to activation by liver microsomes (Mattocks, 1981a).
(b) Subceptibility to hydrolysis
This is determined by the molecular structure and conformation
of the alkaloid (Mattocks, 1981a,b). If the alkaloid is open to
esterase attack, it may be largely detoxified by hydrolysis.
(c) Susceptibility to N-oxidation
The relative amounts of an alkaloid converted by hepatic
microsomal enzymes to N-oxide and to pyrrolic metabolites depends
on its molecular structure and conformation (Mattocks & Bird,
1983). N-oxidation represents a detoxication pathway (Mattocks,
1972b).
5.3.3 Factors affecting the toxicity of active metabolites
5.3.3.1 Reactivity of the metabolite
Toxic metabolites are formed in liver cells. Primary pyrrolic
metabolites (dehydro-alkaloids) are very reactive and, thus, are
quickly hydrolysed or deactivated by reaction with cell constituents.
To damage tissues other than the cells in which they are formed,
active metabolites must cross the cell membrane and survive while
being transported in the bloodstream. The more stable pyrrolic
metabolites, such as dehydromonocrotaline from the alkaloids
monocrotaline, are able to reach, and become bound to, lung tissue
(Mattocks, 1973). Thus, monocrotaline frequently damages the lungs,
whereas retrorsine, which yields a more reactive pyrrolic metabolite,
normally does not.
Secondary metabolites (pyrrolic alcohols, e.g., dehydroretronecine),
formed by the hydrolysis of primary pyrrolic metabolites, are water
soluble, relatively stable compounds that can become more widely
distributed throughout the body or excreted; these are not acutely
toxic.
5.3.3.2 The number of reactive groups
The toxicity of a pyrrolic alkylating agent is affected by the
number of reactive ester or hydroxyl groups (1 or 2) present as the
following examples show:
(a) Many pyrrolic esters can cause acute lung damage when
given iv to rats, but only bifunctional ones also cause
delayed effects on the lungs (Mattocks & Driver, 1983).
(b) Bifunctional pyrrolic alcohols are more effective
inhibitors of mitosis in cultured cells than monofunctional
pyrroles (Mattocks & Legg, 1980).
(c) Bifunctional pyrrolic alcohols are much better inducers
of sister chromatid exchange (SCE) in lymphocytes than
monoalcohols (Ord et al., 1985).
Reasons for these differences might be that the bifunctional
pyrroles are able to crosslink macromolecules or simply that they
can bind more strongly to target molecules.
5.4 Metabolites Associated with the Biological Actions of
Pyrrolizidine Alkaloids
5.4.1 Acute hepatotoxicity
The following is good evidence that acute liver necrosis is
caused by primary pyrrolic ester metabolites (dehydro-alkaloids):
(a) The liver, in which these metabolites are formed, is the
only organ exposed to them in relatively high concentrations.
(b) There are good correlations between amounts of pyrroles
bound to liver tissue and acute hepatotoxicity (Mattocks,
1973).
(c) Pyrrolic alcohols are not acutely hepatotoxic, even when
given to animals in very large amounts.
(d) Pyrrolic esters injected iv into the liver are much more
acutely hepatotoxic than the parent alkaloids (Butler et al.,
1970).
It is possible that other metabolites, such as 4-hydroxy
2,3-unsaturated aldehydes, might also contribute to the acute
hepatotoxicity of some PAs (Segall et al., 1985). However, this
has still to be confirmed.
5.4.2 Chronic hepatotoxicity
The persistent antimitotic action on the liver that leads to
the formation of giant hepatocytes can be produced both by pyrrolic
ester metabolites, such as dehydromonocrotaline (Hsu et al., 1973),
and by pyrrolic alcohols, such as dehydroheliotridine (Peterson et
al., 1972). Both kinds of metabolites can lead to similar
alkylation products and both are likely to be present in the liver
when the alkaloids are metabolized. Thus, either could be
responsible for chronic hepatotoxic effects. However, the
antimitotic action alone is not sufficient. It must be accompanied
or followed by a stimulus of cell division. This may be provided
by the acute necrotic effect of primary pyrrolic metabolites or by
any other cause of acute liver injury that leads to tissue
regeneration. In very young animals, the stimulus can be the
enhanced rate of replication that already exists in them.
5.4.3 Pneumotoxicity
Characteristic pyrrolizidine lung damage is produced by iv
injections of pyrrolic ester metabolites, which are effective at
much lower doses than the parent alkaloids. The latter are not
metabolized in lung tissue; thus, lung damage from PAs is believed
to be due to pyrrolic esters reaching the lungs from the liver
(Butler et al., 1970). Chronic lung damage appears to be caused by
bifunctional rather than by monofunctional pyrrolic alkylating
agents (Mattocks & Driver, 1983) (section 5.3.3.2).
There is some evidence that pyrrolic alcohol metabolites might
also be able to contribute to chronic (but not acute)
pneumotoxicity (Huxtable et al., 1978).
5.4.4 Toxicity in other tissues
Chronic heart damage including right ventricular hypertrophy is
a consequence of pyrrolizidine lung damage (pulmonary hypertension)
(Hayashi et al., 1967). Brain damage is attributed to ammonia
intoxication secondary to severe pyrrolizidine liver injury
(Hooper, 1972). This view has been contested and some PAs are
known to have direct effects on the central nervous system (section
6.4.3). There is no evidence that PAs are appreciably metabolized
in tissues other than the liver. Thus, damage to other organs is
probably due to metabolites transported from the liver. For
example, in the relatively uncommon cases of chronic kidney damage
after pyrrolizidine intoxication (Hooper, 1974; Hooper & Scanlan,
1977) megalocytosis in this organ suggests that pyrrolic
metabolites (either ester or alcohol) are involved. Overall,
patterns of disease, as observed in extrahepatic sites, are
consistent with a "spillover" effect of the pyrroles produced in
the liver (Hooper, 1978). Toxicity of an alkaloid reflects its
rate of metabolism to a pyrrole (Tuchweber et al., 1974) and so the
spillover effect is likely to be more evident at higher doses.
Studies of Culvenor et al. (1976a) suggest that the PAs that are
hepatotoxic for rats should also be pneumotoxic when administered
at higher doses. In acute poisoning, the hepatotoxic effects could
outweigh the pneumotoxic effects or those on other organs, to such
a degree that the latter are not manifested. Variation in
expression of disease (primarily hepatic or extrahepatic) also
depends on the reactions of host tissues in different species of
animals, in addition to the quantities of the pyrroles (Hooper,
1978). The sensitivity of the blood vessels might explain severe
interstitial pneumonias in some animals, or severe nephroses in
pigs (McGrath et al., 1975).
5.4.5 Carcinogenicity
The pyrrolic alcohols dehydroretronecine and dehydroheliotridine
are known carcinogens (Johnson et al., 1978; Peterson et al., 1983),
whereas the pyrrolic esters dehydromonocrotaline and dehydroretrorsine
are only carcinogenic in conjunction with a tumour promotor (Mattocks
& Cabral, 1979, 1982). This suggests that the more persistent
secondary metabolites (pyrrolic alcohols) might account for the rather
weak carcinogenicity of some PAs.
5.4.6 Antitumour activity
Some PAs and their N-oxides are active as tumour inhibitors in
test systems (Culvenor, 1968; Suffness & Cordell, 1985). Indicine
N-oxide, in particular, showed high activity against B16 melanoma,
mammary xenograft, M5076 sarcoma, P388 leukaemia, and Walker 256
carcinoma. In clinical studies, indicine N-oxide has shown
significant activity against some forms of leukaemia, with dosage
limited mainly by myelosuppression and sometimes by hepatotoxicity.
It is tempting to suppose that this action is related to the
powerful antimitotic action of their pyrrolic metabolites, even
though some of these alkaloids and derived pyrroles are themselves
carcinogenic. On the other hand, there is evidence suggesting that
indicine N-oxide owes it activity to a property of the compound
itself rather than to the pyrrolic metabolites, which could be
formed through reduction to indicine (Powis et al., 1979). The
evidence, that indicine is less effective than indicine N-oxide, is
not conclusive and other structure-activity data (Milkowsky, 1985)
point to a need for a structural capability to form a pyrrolic
metabolite. It is also possible that indicine N-oxide is converted
directly to dehydroindicine by mitochondrial enzymes in liver or
tumour cells, since the type of reaction required has been observed
in the mitochondrial metabolism of the N-oxides of tryptamine
alkaloids and certain methylated amino acids (Fish et al., 1956;
Smith et al., 1962).
5.5 Prevention and Treatment of Pyrrolizidine Poisoning
There is no known way to prevent pyrrolizidine liver damage,
once a hepatotoxic dose of the alkaloid has been ingested. A
number of dietary regimes have been found to partially protect
animals (chiefly rodents) from the acute effects of subsequent
alkaloids ingestion. None of these are of any practical use for
preventing pyrrolizidine intoxication in livestock. Furthermore,
chronic toxic effects in the liver or in other organs are sometimes
more severe in animals receiving higher doses of alkaloids after
being protected against acute hepatotoxicty.
5.5.1 Modified diets
The mechanism of action of modified diets is not clear, but
they may be associated with the decreased metabolic activation of
the alkaloids. Some examples follow:
(a) A protein-rich diet can give some protection to rats
against Senecio jacobaea alkaloids (Cheeke & Gorman, 1974).
Rats fed a high casein diet survived longer than rats given a
normal diet, when poisoned with retrorsine or riddelline, but
the survivors were more liable to develop liver tumours
(Schoental & Head, 1957). However, whether this was simply due
to a prolongation of life of the animals by the diet is open to
question.
(b) Male rats previously fed a sucrose-only diet for 4 days
were considerably protected against the acute hepatotoxicity of
retrorsine (LD50 120 mg/kg body weight compared with 34 mg/kg
in normal rats). However, lung damage, rare in control rats,
was frequently seen in "protected" rats given high doses of
retrorsine (Mattocks, 1973).
(c) Restriction of feed intake to 40% of normal attenuated the
increase in lung weight and lavage protein concentration in
cell-free bronchopulmonary lavage fluid and abolished the right
ventricular hypertrophy in monocrotaline-treated rats.
Furthermore, the percentage of diet-restricted animals that
survived was significantly higher than that in animals that had
eaten ad libitum up to day 28, but, from this time onwards,
there was no difference. Alterations of dietary sodium intake
alone did not affect the results of monocrotaline-induced
toxicity (Ganey et al., 1985).
5.5.2 Pretreatment to enhance the detoxication of active metabolites
Treatments that have afforded some protection against
pyrrolizidine hepatotoxicity (probably by increasing the liver
level of sulfydryl compounds, which are known to react with
pyrrolic metabolites) (White, 1976) include the following:
(a) Pre-treatment of rats with mercaptoethylamine (150 mg/kg
body weight ip) partially protected rats against the acute
hepatotoxicity of monocrotaline given 15 min later (Hayashi &
Lalich, 1968); it gave no protection when administered 2 h
after the alkaloid. Mercaptoethylamine, when given orally
(300 mg/kg body weight) at the same time as the lasiocarpine,
also increased the resistance of rats to the alkaloid (Rogers &
Newberne, 1971).
(b) Cysteine (1% in the diet) partially protected rats against
Senecio jacobaea alkaloids (Buckmaster et al., 1977) and mice
against monocrotaline (Miranda et al., 1981c).
(c) The antioxidant ethoxyquin fed at a level of 2.5 g/kg diet
to female mice for 38 days, increased the liver thiol
concentration and raised the acute LD50 of monocrotaline, given
ip on the 10th day, to 364 mg/kg compared with 243 mg/kg in
control mice (Miranda et al., 1981a).
(d) Rats or mice also had increased resistance to acute
pyrrolizidine hepatotoxicity when fed the antioxidant butylated
hydroxyanisole (BHA) (up to 7.5 g/kg diet) (Miranda et al.,
1981c, 1982a,b; Kim & Jones, 1982).
(e) Heliotrine-induced toxicity can be modified by the
co-administration of cupir (a copper-containing complex) at a
level of 1 mg/kg per day for 20 days. It prevented the exit of
hepatic cytosolic enzymes into the blood and improved all the
energy reactions studied in the mitochondria of heliotrine-
intoxicated rats (Yuldasheva & Sultanova, 1983). Inhibition of
lipid peroxidation by cytoplasmic copper was shown later
(Wittig & Stephen, 1964). Savin (1983) found that lethality to
rats of heliotrine (300 mg/kg sc) was completely prevented by
co-administration of alpha-tocopherol (6 ml/kg ip).
(f) Rats pre-treated with ip doses of zinc chloride (72 µmol/kg
body weight) had increased resistance to the hepatotoxicity of
Senecio jacobaea alkaloids, as assessed by histology and enzyme
measurements (Miranda et al., 1982c). The zinc treatment increased
the liver level of metallothionein, a sulfhydryl-rich protein that
might react with pyrrolic metabolites.
Metabolic inhibitors of the microsomal P450 mixed-function
oxidase system, SKF 525A, metyrapone, and allylisopropyl acetamide,
which inhibit the formation of toxic pyrroles in the liver, have
been tried successfully in the prevention of the toxic effects of
monocrotaline in rats (Eisenstein & Huxtable, 1979). The use of
P450 inhibitors was stated to show "potential therapeutic promise".
However, this would seem impracticable considering that, at least
in the rat, PAs undergo a high rate of metabolism commencing a few
minutes after ingestion (Mattocks, 1972b). In some instances, they
have been known to lead to an increase in toxicity, e.g., with
lasiocarpine as reported by Tuchweber et al. (1974).
5.5.3 Other treatments
Lanigan & Whittem (1970) attempted, unsuccessfully, to protect
sheep against Heliotropium europaeum poisoning by treating them
with cobalt, in the hope that this would enhance the vitamin
B12-mediated detoxication of the alkaloids in the rumen (Dick et
al., 1963).
Lanigan et al. (1978) found that the resistance of sheep to
dietary Heliotropium europaeum was increased by giving them large
daily doses of the antimethanogenic drug, iodoform. However, Swick
et al., (1983) found that Senecio jacobaea alkaloids were not
detoxified by incubation for 48 h with sheep rumen fluid in vitro.
6. EFFECTS ON ANIMALS
6.1 Patterns of Disease Caused by Different Plant Genera and of
Organ Involvement in Different Species
The most important genera of PA-containing plants listed in
section 3.1 are all hepatotoxic. Among these, Crotalaria spp.
cause damage in the broadest range of tissues in most domestic
species. In pigs, they are known to be severely nephrotoxic
(Peckham et al., 1974; McGrath et al., 1975; Hooper & Scanlan,
1977). Some species are known to be pneumotoxic for horses (Watt &
Breyer-Brandwijk, 1962; Gardiner et al., 1965), cattle (Sanders et
al., 1936; Berry & Bras, 1957), sheep (Laws, 1968), and pigs
(Peckham et al., 1974; Hooper & Scanlan, 1977), as well as
hepatotoxic.
Although several Crotalaria spp. are known to be pneumotoxic
for horses (Gardiner et al., 1965), C. retusa is an exception. It
is an important cause of disease in horses in northern Australia
(Hooper, 1978) and has been shown to be pneumotoxic for pigs in the
same area (Hooper & Scanlan, 1977); yet it produces only hepatic
disease in horses (Rose et al., 1957a,b).
Similarly, Senecio spp. are primarily hepatotoxic, but
S. jacobaea has been demonstrated to be pneumotoxic for pigs
(Harding et al., 1964), though it could probably be an inconsistent
change (Bull et al., 1968). This plant is also known to cause
pulmonary disease in rats and mice (Hooper, 1974). However, there
are no reports of its affecting the lungs in cattle, sheep, horses,
or chicken. Renal megalocytosis and mild nephrosis are reported in
most species poisoned with S. jacobaea (Harding et al., 1964; Bull
et al., 1968). Heliotropium spp., Amsinckia spp., and Echium spp.
are all mainly hepatotoxic.
Roitman (1983) summarized the pattern of organ involvement
observed in man and different species of farm and experimental
animals affected by pyrrolizidine alkaloids (Table 8). Even within
a single species, the nature of a toxic effect, as well as the
organ affected, can be altered by changing the dose rate and
duration.
6.2 Field Observations - Outbreaks in Farm Animals
The veterinary problem of PA toxicity has been reviewed by Bull
et al. (1968) and McLean (1970). Mattocks (1986) listed the cases
of livestock poisoning and feeding trials since 1968, and cited
relevant literature. Peterson & Culvenor (1983) produced a useful
and comprehensive table of the plant species known or suspected of
causing natural outbreaks of poisoning in each animal species. The
influence of factors such as species, age, sex, and diet, on
toxicity is also reviewed in the same paper.
Table 8. Animal species and organs affected by pyrrolizidine
alkaloidsa
---------------------------------------------------------------
Species Liver Lung Kidney Heart Pancreas Gastric Muscle
mucosa
---------------------------------------------------------------
Man +
Monkey + + + +
Horse + + +
Pig + + + + +
Sheep + + +
Goat + +
Cattle + +
Dog +
Mouse + +
Rat + + + +
Chicken + + + + +
Turkey + + +
---------------------------------------------------------------
a From: Roitman (1983).
The first cases of pyrrolizidine poisoning were described in
cattle as early as 1903 (Gilruth, 1903). Since then, there have
been numerous reports from most parts of the world, of poisoning
among farm animals caused by grazing or feeding on PA-containing
weeds (Bull et al., 1968; Mattocks, 1986). One of the first clues
to the etiology of the human disease in Jamaica, came from a study
in which calves fed with Crotalaria fulva (Bras et al., 1957)
developed characteristic veno-occlusive disease in the liver.
Laws (1968) described a field outbreak in sheep in a herd of
100 adult merino ewes, which developed within 2 weeks of moving
into a coastal farm in Australia, where they grazed Crotalaria
mucronata. The etiology was confirmed by feeding the plant to 6
sheep, 4 of which died within 24 h of feeding, with severe
pulmonary oedema. However, the rapidity of poisoning and the
atypical lung lesions suggest that possibly a toxin other than
pyrrolizidine alklaoids was also present.
An outbreak of Crotalaria retusa poisoning was observed in a
piggery near Darwin, Northern territory, Australia (Hooper &
Scanlan, 1977) containing approximately 350 sows. It was caused by
feeding sorghum contaminated by Crotalaria seeds at the rate of
about 0.1% by weight for about 3 weeks, and at a rate of about
0.05% for a further week. The disease was indicated by reduced
body weight gain and inappetence. The dominant pathological
features at autopsy were severe nephrosis with chronic uraemia, and
to a lesser degree, severe diffuse interstitial pneumonia. Both
were accompanied by microscopic disease in the liver, and both the
liver and kidney showed megalocytosis.
Walker & Kirkland (1981) reported outbreaks in the Hunter river
valley of New South Wales in Australia, in cattle that had been
grazing a pasture in which Senecio lautus was growing. There were
sporadic deaths among the cattle as well as two protracted
outbreaks affecting calves 3.5 months of age and older animals, in
which groups of 3 - 16 head of cattle died in addition to sporadic
deaths of animals over periods of 1 and 6 months. Clinical signs
were weakness, emaciation with recumbency, aimless wandering and
ataxia, which suggested neurological involvement. At autopsy, the
liver showed characteristic megalocytosis, periportal fibrosis, and
focal necroses. An aged animal had cirrhosis. The etiology of
S. lautus was proved in feeding studies on 3 calves.
Knight et al. (1984) reported the deaths of 10 horses during a
3-year period after being fed hay from the same pasture. The
animals became sick in the spring after being fed only the suspect
hay throughout the winter. The hay was found to be contaminated
with Cynoglossum officinale (hound's tongue), which contained two
PAs (heliosupine and echinatine) in much higher quantities than is
generally reported in Senecio species. The animals developed
weight loss, icterus, ataxia, and symptoms of hepatic failure. At
autopsy, there was diffuse severe megalocytosis, biliary
hyperplasia, and fibrosis. The C. officinale was proved as the
etiological factor in a feeding trial on a 14-year-old pony, that
developed clinical features and pathological changes in the liver
suggesting PA poisoning. The signs of PA toxicity in horses are
mostly neurological, though non-specific gastric and oesophageal
lesions have also been reported (McLean, 1970) (section 6.4.3).
Rose et al. (1957a,b) described a disease in which the dominant
symptom was "compulsive" walking in a straight line. It occurred
in areas where Crotalaria retusa was growing, and was ascribed to a
steep rise in blood-ammonia levels, which accompanied chronic liver
failure.
Farm animals differ widely in their sensitivity to PAs, sheep
and goats being fairly resistant, cattle and horses, less so, and
poultry and pigs, rather sensitive (section 6.4.1.2). Sheep are
not immediately affected and generally survive one season, after
feeding on heliotrope and Senecio (Bull & Dick, 1959). During the
second season of feeding, they die of neurological symptoms caused
presumably by rising blood-ammonia levels associated with chronic
liver disease, or with haemoglobinuria and very high copper levels
in the blood (Bull et al., 1956, 1958) (section 6.4.1.2). With
Crotalaria, the lung seems to be the target organ (Hooper, 1978).
Similar acute responses to a single feed of the plant Crotalaria
spectabilis were described in cattle by Emmel (1948) and in the
chicken by Piercy & Rusoff (1946).
Poisoning of cattle in northwestern USA has reached such
proportions that it has become a considerable economic problem
(Johnson, 1982). Culvenor (1985) has reviewed the problem of
livestock losses due to PA toxicosis in Australia, where it has
been estimated that about 10 million sheep are exposed to
heliotrope and Echium plantagineum (Paterson's curse) to a greater
or lesser extent and may suffer a shortening of their productive
life by as much as two years. Most of the PA-containing plants are
reported to grow in fallow fields and pastures and thrive
particularly in a dry climate or following periods of drought.
However, instances of cattle poisoning have been reported from most
parts of the world, including countries with temperate or cold
climates, which do not ordinarily suffer drought. Three herds of
cattle have been reported to have been affected in the
alpine/subalpine region of Switzerland, after they grazed pastures
that had Senecio alpinus growing on them. Nine different
hepatotoxic PAs were found in the weed, the main one being
seneciphylline. Analyses of urine samples from one cow confirmed
the presence of PA metabolites. Several cows had to be
slaughtered, because of cirrhosis of the liver (Luthy et al.,
1981).
6.3 Studies on Farm Animals
There are several reports of the production of disease
characteristic of PA toxicity in farm animals, by feeding them
PA-containing plants.
Senecio jacobaea (tansy ragwort) is a weed that commonly grows
in pastures and has been the cause of extensive livestock losses in
the United Kingdom (Forsyth, 1968) and the USA (Johnson, 1982).
Extensive studies on this plant have been carried out using a
variety of farm animals. Dickinson et al. (1976) fed tansy ragwort
to cows through a rumen canula at the rate of 10 mg/kg body weight
per day, for 2 weeks. Liver biopsies showed characteristic
megalocytosis and fibroplasia, and autopsy also showed
centrilobular necrosis. A PA, jacoline, was found in the milk, but
when the calves were bucket fed the milk, there were no detectable
effects on them. Thorpe & Ford (1968) made similar observations in
5 calves fed ragwort in their diet. Animals eventually dying of
toxicity showed characteristic necrosis, megalocytosis, and veno-
occlusive lesions. In a study by Goeger et al. (1982), goats were
fed dried ragwort mixed in the diet at 250 g/kg. Four of the 11
goat kids and lactating dairy goats died. Characteristic
megalocytosis was seen in the liver. Goats are more resistant to
tansy ragwort toxicosis than cattle and horses, the chronic lethal
dose for cattle or horses being 0.05 - 0.2 kg ragwort/kg body
weight and, for goats, 1.25 - 4.04 kg/kg body weight. The alkaloid
levels in the plant, and thus its toxicity, varies with season and
locality.
Hooper & Scanlan (1977) studied the long-term effects of
feeding very low levels of ground C. retusa seeds, mixed with the
feed, to pigs and chickens. Seven groups of 4 pigs each (sex not
mentioned) bred from Saddleback-large white cross sows and Large
White or Landrace boars, were maintained on diets containing 0
(control), 0.004%, 0.01%, 0.02%, 0.05%, 0.1%, or 0.5% body weight
ground seeds. Another 8 pigs were fed a diet containing 0.1%
C. retusa for 21 days followed by 0.05% for 7 days and then kept on
a C. retusa-free diet. Pigs either died or were killed when
moribund, or at the end of 136 days of feeding.
In a second study, groups each of 4 2-week-old chickens were
fed diets containing 0 (control), 0.005%, 0.01%, 0.05%, 0.1%, and
0.5% ground seeds of C. retusa. Chickens fed 0.5% started dying 12
days after the commencement of feeding and were all dead by day 45.
Five out of 8 birds fed 0.1% or more died between days 22 and 56.
No deaths occurred in animals fed 0.01% - 0.005%.
In the study on pigs, all animals died between days 63 and 107
except for 2 that survived 136 days. In these animals, pulmonary
disease was the main cause of death. Hepatic and renal
megalocytosis was seen in almost all animals in both the field
outbreak and study group. The lungs showed extensive consolidation
and oedema. Besides megalocytosis in the glomeruli and tubules,
the kidneys showed glomerular atrophy and tubular necrosis. In the
study on poultry, the major disease was hepatic necrosis of
irregular distribution. The kidneys showed mild megalocytosis.
In the above study, the low levels of contamination that
produced serious disease are worthy of note.
Johnson & Molyneux (1984) fed 55 cattle, by gastric lavage,
with hay mixed with threadleaf groundsel ( Senecio douglasii var.
longilobus), which grows commonly in the pastures of southwestern
USA. The PA dosage in different groups ranged from 5 to 40 mg/kg,
daily, and the total intake ranged from 80 to 284 mg/kg body
weight. The groups were fed for periods ranging from 2 to 20 days.
One hundred percent mortality occurred in 3 out of 9 groups, each
consisting of 2 - 8 calves, receiving doses of 13 mg/kg or more.
Mean survival time was generally inversely proportional to the
dosage received. All sick calves had typical clinical signs of
seneciosis. At autopsy, the principal lesion was seen in the liver
and consisted of swelling of the hepatocytes, necrosis, biliary
hyperplasia, and marked fibrosis. The estimated minimum lethal
dose of the PA was 13 mg/kg body weight for 15 days, or a total
intake of approximately 200 mg PA/kg, over a 15-day period. Cattle
that consumed up to 600 mg PA/kg, in hay, in a 20- to 100-day
period, were unaffected or only slightly affected. The authors
concluded that the time-dose relationship for PA toxicosis in
cattle is important and that there is a threshold level that must
be exceeded for the toxicosis to develop.
A similar study was conducted by Johnson et al. (1985) in which
the dry whole or ground leaves of Senecio riddelli, mixed with the
feed, were fed to calves in gelatin capsules or by gavage. Forty-two
female Hereford calves were divided into 3 groups. One group
of 12 was fed the leaves mixed with alfalfa hay feed estimated to
have 20 - 40 mg PA/kg body weight per day over a 20-day period in
different regimes, receiving a total of 400 - 800 mg PAs per
animal. The second group of 12 animals received the plant packed
into gelatin capsules, receiving an estimated PA content of
10 - 20 mg/kg body weight, daily, over 20 days, with a total of
200 - 400 mg per animal. The third group of 18 animals was
administered (by gavage) finely ground leaves in a water slurry at
various PA dosages ranging from 10 to 60 mg/kg body weight per day
and a total of 200 - 500 mg over 20 days.
Calves that received 10 mg PA/kg body weight per day for 20
days did not develop clinical signs of disease or show any changes
in serum-enzyme. However, feed containing the plant that provided
15 - 20 mg PA/kg per day or more, administered by gavage or fed in
capsules, resulted in high mortality. Malaise, depression, erratic
or unprecedented behaviour, aimless walking, and ataxia, were
observed in the affected calves; diarrhoea with tenesmus and rectal
collapse were frequently observed. The feed intake decreased
progressively and was negligible terminally. The animals that died
and those that were moribund or in a state of irreversible wasting,
were autopsied. Hepatobiliary lesions were present in all such
animals. The most consistent change was portal biliary hyperplasia
and periportal fibrosis. Centrilobular or zonal haemorrhage and
necrosis were observed in some lobules. Fibrosis of some central
veins was common, often encroaching on the lumen, resulting in
complete occlusion. Hepatocytes also showed nonspecific changes.
Central nervous system changes were present in all animals with
clinical signs of seneciosis, consisting mainly of spongy
degeneration of the brain.
The plant mixed in the hay ration was eaten slowly and
reluctantly and was tolerated at dosages > 20 mg/kg per day,
emphasizing that the toxicity depended on the rate at which the
dosage was consumed and that mortality was not necessarily
dependent on the cumulative dosage.
Burguera et al. (1983) produced the disease in turkey poults by
feeding them seeds of C. spectabilis. Simultaneous addition of
sodium selenite at doses of 0.1, 5, or 10 mg selenium/kg diet did
not provide any protection.
6.4 Experimental Animal Studies
6.4.1 Effects on liver
6.4.1.1 Relative hepatotoxicity of different PAs and their N-oxides
The LD50 values for rats, listed in Table 9, are for some of
the most commonly used hepatotoxic alkaloids, calculated from data
on animals dying from acute haemorrhagic necrosis of the liver, 3 - 7
days after intraperitoneal administration of a single dose. It
is evident that the toxicity varies widely between the alkaloids.
The most toxic are certain macrocyclic diesters of retrorsine and
the least toxic are the monoesters of heliotridine, retrorsine, and
supinine (Mattocks, 1986).
The relative toxicity of N-oxides compared with that of their
basic alkaloids depends on the route of administration. The
N-oxides of lasiocarpine, monocrotaline, and fulvine were reported
to be as toxic as their basic alkaloids (Schoental & Magee, 1959)
when administered orally; however, when given by the ip or
intravenous (iv) routes to rats, they were much less toxic
(Mattocks, 1971c). Similarly, the LD50 of retrorsine N-oxide when
administered ip to male rats was 250 mg/kg (Table 9) but when given
orally, it was 48 mg/kg. This has been explained by the
observations on the metabolic pathways of the basic alkaloids and
their N-oxides. The PAs or their N-oxides exert toxic effects only
after being metabolized to pyrroles by the hepatic microsomal
enzymes (section 5.1.1). Hepatic microsomes act directly on the
N-oxides (Mattocks & White, 1971b) only after they have been
converted to the basic alkaloids (Mattocks, 1986); this mainly
occurs in the gut (Mattocks, 1971c; Powis et al., 1979). This
matter is of practical importance as the alkaloids are often
present as their N-oxides in weeds grazed by farm animals.
Table 9. LD50s in male rats after a single intraperitoneal dose of
some hepatotoxic alkaloids
-------------------------------------------------------------------
Alkaloid LD50 Time range Reference
(mg/kg) (days)
-------------------------------------------------------------------
heliotrine 296 3 Bull et al. (1958)
lasiocarpine 77 3 Bull et al. (1958)
lasiocarpine N-oxide 547 3 Bull et al. (1958)
monocrotaline 175 3 Bull et al. (1968)
retrorsine 34 4 or 7 Mattocks (1972a)
retrorsine N-oxide 250 7 Mattocks (1972a)
senecionine 50 7 Mattocks (1972a)
seneciphylline 77 3 Bull et al. (1968)
senkirkine 220 -a Hirono et al. (1979a)
symphytine 130 -a Hirono et al. (1979a)
-------------------------------------------------------------------
a Not stated.
6.4.1.2 Factors affecting hepatotoxicity
These factors have been reviewed by Mattocks (1986).
(a) Route of administration
Most studies on LD50 values have been carried out using the ip
route, and very few experimental data are available on toxicity
using the oral route. There is a close similarity between the iv
data and the ip data. Furthermore, toxicity data on rats
administered PAs by the oral route (Schoental & Magee, 1959),
including retrorsine, lasiocarpine, heliotrine, and monocrotaline,
closely resemble those relating to the LD50 values for the same
strain administered PAs intraperitoneally (Mattocks, 1972b). Thus,
the hepatotoxicity of PAs in rats does not differ very much,
irrespective of the route of administration. However, rabbits
appear to be less susceptible to PAs in the plant Senecio jacobaea
when administered orally than when administered intravenously
(Pierson et al., 1977).
(b) Species
Wide differences have been observed in the hepatotoxic effects
of PAs and alkaloid-containing plants between different species of
both farm animals and laboratory animals, and in the same animal
exposed to PAs derived from different plants. Sheep are resistant
to PA-containing plants (section 6.2) and when fed Echium
plantagineum pellets containing 1.3 g alkaloid/kg as the sole diet
for 12 months, over a period of 2 years, showed almost no liver
damage (Culvenor et al., 1984). However, adult rats fed the same
pellets as only 50% of the diet for 14 days died 4 - 13 weeks later
(Peterson & Jago, 1984). Pigs were found to be 5 - 10 times as
susceptible to PAs in Crotalaria retusa as chickens (Hooper &
Scanlan, 1977). Overall, the approximate ratios of quantities of
plant material required to prove toxic in the various species
listed are about 200 for the sheep (approximately the same for the
goat), 150 for the mouse, 50 for the rat, 14 for cattle
(approximately the same for the horse), 5 for the chicken, and 1
for the pig (Hooper, 1978).
Cheeke & Pierson-Goeger (1983) studied the chronic toxicity of
Senecio jacobaea for several laboratory animals by feeding the
dried plant as a component of a mixed diet. The degree of
susceptibility to PA poisoning was compared in terms of the chronic
lethal dose of the dried plant as a percentage of the initial body
weight among the animals themselves, and with similar data on
livestock in other studies. Gerbils, hamsters, and guinea-pigs
were resistant to chronic toxicity, gerbils being the most
resistant, consuming over 35 times their body weight of the dried
plant. Comparison with similar data in other studies indicated
that the rabbit (Pierson et al., 1977), Japanese quail (Buckmaster
et al., 1977), and goat (Goeger et al., 1982) were resistant,
requiring a long-term lethal dose of the plant of 113% or more of
the initial body weight, whereas the rat was highly sensitive
requiring only 21% (Goeger et al., 1983). Chicks and turkey poults
were also susceptible with severe inhibition of growth occurring
when there was 5% and 10% contamination of the feed with the plant;
survival time was short (Cheeke & Pierson-Goeger, 1983).
In a study by Fushimi et al. (1978), on the carcinogenicity of
the flower stalks of Petasites japonicus Maxim in mice and Syrian
golden hamsters, species and strain differences were observed, not
only with regard to hepatotoxicity, but also with regard to the
carcinogenicity of PAs. Mice of ddN, Swiss, and C57BL/6 strains
and Syrian golden hamsters were fed on a diet containing young
flower stalks of the plant for 480 days. High incidences of lung
adenoma and adenocarcinoma were observed in ddN mice, but no
significant differences in tumour incidence were observed between
the experimental groups of Swiss mice and hamsters and the
corresponding control group. No tumours were induced in an
experimental group of C57BL/6 strain mice.
These differences have been explained by the differences in the
rate of metabolic conversion of PAs to toxic metabolites (pyrroles)
by the hepatocyte microsomes in the different animal species (White
et al., 1973; Shull et al., 1976; Peterson & Jago, 1984).
The resistance of sheep has been ascribed to destruction of the
alkaloids in the rumen by a reductive conversion into non-toxic
1-methylenepyrrolizidine derivatives (Bull et al., 1968; Lanigan,
1971, 1972). It has also been suggested that the resistance of
sheep is due to a low level of activation in liver cells (Shull et
al., 1976), but this factor was not prominent in some Australian
sheep, which were as sensitive as rats to PAs injected
intraperitoneally (Hooper, 1974).
Thus, it is possible for ruminants to graze plants containing
PAs for a period of months without evident harm, e.g., cattle
eating Crotalaria juncea in Africa (Srungboonmee & Maskasame,
1981), but long-term effects may arise in animals exposed over
several years.
Considerable differences in LD50 values have been reported for
the same alkaloids in different species. For example, the LD50 for
retrorsine varies from 34 mg/kg for male rats to 279 mg/kg for
quail and over 800 mg/kg body weight for guinea-pigs (White et al.,
1973). Guinea-pigs are also resistant to monocrotaline (Chesney &
Allen, 1973a), but not to jacobine or to mixed alkaloids of Senecio
jacobaea, which are highly toxic (Swick et al., 1982a).
(c) Sex
Significant differences in the hepatotoxicity of the same
alkaloid have been observed between sexes in some species. Male
rats are much more susceptible to the acute toxicity of retrorsine
or monocrotaline than females (Mattocks, 1972b). Mattocks & White
(1973) reported a higher level of metabolic transformation in young
male rats to form pyrroles from retrorsine, compared with females
(section 4.4). Jago (1971) reported a higher susceptibility in
male rats to the chronic hepatotoxic effects of heliotrine, while
female rats were more susceptible to lasiocarpine. It is possible
that this may be due to the potentiating effect of male sex
hormones. Campbell (1957a,b) reported that diethylstilboesterol
protects against the effects of seneciphylline and promotes repair
of damaged liver in poultry. Protein-deficient rats of both sexes,
or female animals pre-treated with testosterone, were more
susceptible to monocrotaline (Ratnoff & Mirick, 1949).
(d) Age
Available data on the effects of age are highly conflicting.
It has been stated that young rats, particularly suckling animals
(Schoental, 1959), are more susceptible than adults to the
hepatotoxic effects of some alkaloids (Jago, 1970), such as
monocrotaline (Schoental & Head, 1955), and retrorsine and
lasiocarpine (Schoental, 1959). Rats, 1 - 4 days old, were far
more susceptible to retrorsine and senkirkine than rats aged 25 - 30
days (Schoental, 1970); yet new-born rats (within 1 h of birth)
were relatively more resistant to the hepatotoxic effects of
retrorsine than 1- to 4-day-old rats (Mattocks & White, 1973).
McLean (1970) has critically reviewed the data. In comparing the
data on small animals from several studies, new-born and 4-week-old
animals appear to have about the same susceptibility as adults.
Data for the intervening period obtained by Harris et al. (1957),
Schoental (1959), and Hayashi & Lalich (1968) are conflicting,
suggesting decreased susceptibility in some studies (Harris et al.,
1957 and one series of Schoental's studies, 1959), and increased
susceptibility in others (Hayashi & Lalich, 1968 and the second
series of Schoental's studies, 1959). Furthermore, Jago (1971)
demonstrated that, while rats aged 1 - 2 weeks were more
susceptible to the acute effects of heliotrine and lasiocarpine
than older rats, sensitivity to the effects of long-term
administration of these alkaloids increased with age, after 2 - 3
months.
(e) Diet
Effects of both qualitative and quantitative changes in diet on
the hepatotoxicity of PAs have been investigated in several
studies. Restriction of protein levels in the diet enhanced the
acute hepatotoxic effects of retrorsine in rats (Selzer & Parker,
1951) and the chronic effects of a single dose of orally
administered lasiocarpine (Schoental & Magee, 1957) (section
6.4.5.1) as well as the toxicity of PAs in Senecio jacobaea,
whereas a high protein diet had a protective effect (Cheeke &
Gorman, 1974). Likewise, low lipotrope diet enhanced the toxic
effects of orally administered lasiocarpine in pregnant rats and
also in the fetal livers (Newberne, 1968). On the other hand, it
protected young male rats against the acute toxicity of
monocrotaline, because of the reduced metabolic conversion of the
alkaloid into pyrrolic metabolites (Newberne et al., 1971, 1974).
Caloric restriction reduced the cardiopulmonary toxicity of a
single dose of monocrotaline in rats (Hayashi et al., 1967). This
was ascribed to the reduced growth rate in animals on a restricted
diet rather than to a reduction in the rate of metabolic conversion
of the alkaloid, since dietary restriction started only after
administration of the alkaloid. When the animals were put back on
the ad libitum feeding regimen, they developed signs of increased
toxicity.
A high copper content in the diet has been shown to enhance the
toxic effects of PAs (Miranda et al., 1981b). Incorporation of
copper sulfate at 50 mg/kg in the diet containing the plant Senecio
jacobaea increased the hepatotoxicity in rats, as judged by enzyme
measurements. The implications of this observation are obvious if
some PA-containing plants being grazed by farm animals also have a
high copper content.
Mattocks (1972b) demonstrated the protective effects of sucrose
against the acute hepatotoxic effects of retrorsine in rats, if
administered for 3 days prior to alkaloid administration (section
5.5.1).
6.4.1.3 Acute effects
Experimental animal studies on the pathological effects of PAs
on the liver have been reviewed by Bull et al. (1968) and McLean
(1970). Most studies have been carried out on the rat (Schoental &
Magee, 1957, 1959; Bull & Dick, 1959; Schoental, 1963; Barnes et
al., 1964; McLean et al., 1964; Nolan et al., 1966; Jago, 1969;
Butler et al., 1970; Peterson & Jago, 1980), but several other
species of animals have been studied including the monkey (Wakim et
al., 1946; Rose et al., 1959; Allen & Carstens, 1968, 1971; Allen
et al., 1969), turkey (Allen et al., 1963), chicken (Allen et al.,
1960), hamster (Harris et al., 1957), mouse, guinea-pig (Chen et
al., 1940), quail, cat, rabbit, and pig (Emmel et al., 1935; Hooper
& Scanlan, 1977). All animals tested, except the guinea-pig (Chen,
1945), have been found to be susceptible in studies using purified
alkaloids and their N-oxides and crude extracts of PA-containing
plants.
Typically, the most common lesion produced in small laboratory
animals by doses close to the LD50 is a confluent haemorrhagic
necrosis in the liver, which appears within about 12 h of exposure
and peaks at 24 - 48 h. It is strictly zonal in distribution in
different species of animals but may vary within the same animal,
depending on the alkaloid used, species, nutritional status, and
pretreatment with other chemicals.
Retrorsine produces centrilobular necrosis in the rat, mouse,
and guinea-pig, periportal necrosis in the hamster, and focal
necrosis in the fowl and in the monkey (White et al., 1973). In
the monkey, monocrotaline produces centrilobular necrosis (Allen &
Carstens, 1968), but senecionine produces necrosis in the
periportal and midzonal areas of the liver lobule (Wakim et al.,
1946). Almost simultaneously, or shortly after the development of
acute haemorrhagic necrosis of the liver cells, various levels of
change appear in the central and sublobular veins of the liver
lobules, consisting of subintimal oedema or even necrosis, deposits
of fibrin, thrombosis, and occlusion of the lumen, which later
becomes organized. While haemorrhagic necrosis is a constant
feature, attempts to produce occlusive lesions in the veins of
experimental animals have produced variable results (Allen et al.,
1967). In man and non-human primates, hepatocellular necrosis and
venous occlusion occur simultaneously but, in the rat (McLean et
al., 1964), chicken (Allen et al., 1960), and swine (Emmel et al.,
1935), the vascular changes follow hepatic necrosis.
Selzer & Parker (1951) produced a lesion comparable to human
veno-occlusive disease in albino rats by administrating retrorsine
hydrochloride, the active alkaloid of Senecio ilicifolius, as well
as the crude plant extract. Four batches of rats were administered
alkaloids orally in a single dose of 1 - 1.5 mg/10 g body weight or
repeated doses of 5 - 50 mg/kg body weight for 31 days or as single
subcutaneous injection of 100 mg/kg body weight. One batch was fed
on a diet of Senecio ilicifolius constituting 10% of the ration as
crude plant or its extract; the animals lived for 21 - 84 days.
Some groups were kept on a normal diet, and others on a diet that
was protein-deficient. Animals, administered a single dose orally,
developed the earliest degenerative changes in the centrilobular
hepatocytes and sinusoidal dilatation, and the vascular lesion
appeared after 36 h. Protein deficiency enhanced the toxic effect.
Only 5 out of 9 animals administered repeated doses orally showed
early centrilobular fibrosis and none showed the vascular lesion,
possibly due to scarring.
Bull et al. (1958) studied the effects of a single ip LD70 dose
of heliotrine (320 mg/kg body weight), lasiocarpine (80 mg/kg), or
lasiocarpine N-oxide (629 mg/kg) in rats of a hooded strain of both
sexes. Eighty-one rats were used and 3 rats from each treatment
were killed at intervals of 4 - 36 h. Heliotrine produced marked
centrilobular necrosis at 24 h, but venous changes were not
evident, except for some aggregation of mononuclear macrophages on
the endothelium. With lasiocarpine, the hepatic changes were
similar, but the necrosis was not clearly centrilobular and, with
its N-oxide, it was midzonal at 34 - 49 h. The earliest toxic
effect of the PAs was manifested as a temporary loss of
mitochondria at 8 h. The authors concluded that PAs have an early
toxic effect on the hepatocytes and that this does not follow
vascular injury, as suggested by Davidson's earlier studies (1935).
McLean et al. (1964) administered an aqueous extract of
Crotalaria fulva to Wistar rats in a single intragastric dose of
0.8 - 1.5 g/kg body weight. Lesions identical to those of human
veno-occlusive disease were produced in the animals by adjusting
the dose to permit survival for 8 - 12 days. Loss of cytoplasmic
glycogen in the centrilobular cells occurred 3 h following
administration. Centrilobular necrosis, which occurred after 24 h,
increased with time. The central veins gradually filled up with
thickened endothelial cells at about 7 days, later progressing to
collagenization. Evidence was presented that the histological
occlusion of the central veins was preceded by several days by a
functional blocking of the blood flow.
Barnes et al. (1964) observed similar results in rats
administered a single oral dose of fulvine N-oxide at 50 mg/kg
body weight and studied at intervals of 1 - 4 days after the
administration. One hundred and thirty-five rats of both sexes
were used. Acute lesions resembling human disease were observed
during days 1 - 8. During days 19 - 21, 3 out of 25 animals showed
liver damage consisting of some centrilobular haemorrhage and
fibrous thickening of the central veins. Of the 78 animals studied
at 22 - 44 days, 50% still had centrilobular congestion and some
had fibrous thickening of the central veins.
The effects of pyrrolic derivatives of PAs on rats were studied
by Butler et al. (1970). Male albino rats of Porton strain were
administered solutions of pyrrole derivatives of monocrotaline
and retrorsine in dimethyl formamide as a single injection of
0.05 - 0.1 ml solution. When injected in the tail vein at a
concentration of 5 mg/kg body weight, it produced progressive
proliferation of alveolar epithelium of the lungs and the animals
developed signs of respiratory distress in 2 - 3 weeks. When injected
in the mesenteric vein at a concentration of 15 mg/kg body weight, as
a rule, the animals remained well in the postoperative period and
only 1/26 animals died of mesenteric vein thrombosis; the livers
developed multiple infarcts in the left lobes that developed into
multiple coarse nodules at 6 - 12 weeks. The above studies
substantiated the view that PAs act only when converted in
hepatocytes to pyrroles. When pyrroles were injected, they
affected the smaller vessels at the portal of entry; in animals
injected through the mesenteric vein, the main target was the
portal vein with only secondary damage to the parenchymal cells,
thus sparing the animals from the effects of hepatocellular injury.
On the other hand, pyrroles injected through the tail vein went
directly to the pulmonary arteries through the heart and damaged
the alveolar capillaries.
Acute veno-occlusive disease was produced in monkeys
administered monocrotaline (Allen et al., 1967, 1969) and ground
Crotalaria spectabilis seed (Allen & Carstens, 1968). In a study
published in 1967, these authors used 14 monkeys (Macaca speciosa)
of both sexes, each weighing approximately 4 kg. Seven of the
animals were administered 1 mg monocrotaline in distilled water by
gastric intubation on days 1 and 14. The remaining 7 were used as
controls and received distilled water only. Wedge biopsies of
liver were examined weekly. The survival time ranged from 14 to 38
days, the mean being 21 days. The livers of treated animals were
small and firm and showed changes characteristic of human veno-
occlusive disease including centrilobular necrosis, and vascular
changes in the central veins of liver lobules ranging from
subintimal oedema to progressive collagenization and extension of
collagen fibres into the sinusoids. Similar observations were made
in studies on Macaca mulatta monkeys (Allen & Carstens, 1968),
administered ground Crotalaria spectabilis seed. Sixty-four
animals, averaging 6.2 kg in weight, were divided into 3 groups.
Group I, comprising 10 experimental animals (4 control animals),
received seeds in the diet containing the equivalent of 0.074 mg
monocrotaline/kg body weight, daily, up to death. Group II,
consisting of 14 treated animals (4 control animals), received a
single dose of seeds containing 1.3 g monocrotaline/kg body weight,
and Group III, consisting of 26 experimental animals (6 controls),
received 3 weekly doses containing the equivalent of 0.116 g
monocrotaline/kg body weight, by gastric intubation. Liver
biopsies were carried out each month in Group I and each week in
Groups II and III. Animals of the last 2 groups were killed when
in extremis. The mean survival times for the groups were:
Group I, 269 days (176 - 425 days); Group II, 28 days (6 - 43
days); and Group III, 41 days (23 - 91 days). In Group I animals,
occlusive lesions of the central and sublobular veins of the liver
were seen in 7/10 animals at autopsy. These consisted of oedema,
haemorrhages, and fragmentation of the vessel walls, the lumina
being filled with fibrin, and degenerating liver cells. The
lobular pattern was distorted because of connective tissue bands
encircling small groups of liver cells, especially in the central
zones of the lobules. In Groups II and III, various levels of
focal or centrilobular necrosis were observed and the liver cells
were replaced by stromal tissue. Vascular lesions, as described
above, were seen in 25 monkeys, but no collagen was demonstrated.
In the studies of Wakim et al. (1946) on the rhesus monkey,
senecionine administered iv as a 2% solution at doses of 10 - 30 mg/kg
body weight to 4 animals, daily, until they appeared to be sick,
produced periportal, or midzonal necrosis in 3 animals accompanied
by haemorrhage. No mention was made of any vascular changes.
Electron microscopic studies
Svoboda & Soga (1966) studied the effects of lasiocarpine and
Crotalaria fulva on the livers of male Sprague Dawley rats weighing
110 - 150 g each. One group of 22 rats was given an ip injection
of lasiocarpine at 80 mg/kg body weight and pairs of animals were
killed at various intervals ranging from 15 min to 6 days. A
second group of 22 animals was administered a single dose of an
aqueous extract of Crotalaria fulva at 0.5 mg/g body weight, by
gastric tube, and killed at the same intervals. A third group of 8
rats was administered a total of 3.2 times the LD50 dose of
lasiocarpine in small doses, 3 times a week, and killed at 9 - 20
weeks. The changes primarily involved the nucleus and
interchromatin granules. The first change, seen after 30 min in
the nueleoli of the hepatocytes and Kupffer cells of animals
receiving lasiocarpine or crotalaria extract, consisted of a
separation of the fibrillar and granular components. The
hepatocyte nuclei had returned to normal after 72 h and remained so
throughout the rest of the study. In animals receiving a single
dose of lasiocarpine or crotalaria, round periodic acid schiff
(PAS) positive eosinophilic bodies appeared in the cytoplasm after
12 h, consisting of dense masses of cytoplasmic material. Five
days after treatment with crotalaria, large cells lined the luminal
surface of the central veins; the centrilobular cells had undergone
necrosis by this stage. Animals receiving 3.2 times the LD50 of
lasiocarpine developed megalocytosis after 9 weeks (section
6.4.1.5). The cytoplasm showed vesicles of smooth endoplasmic
reticulum with mitochondria of various shapes and sizes. The
appearance resembled an exaggerated regenerative response.
Allen et al. (1967, 1969) studied ultrastructural changes in
the liver tissue, in general, including the hepatic veins in Macaca
speciosa monkeys treated with PAs. In the study on hepatic veins
(Allen et al., 1969), 18 treated and 6 control adult animals were
used with an average weight of 5.8 kg. Animals were divided into 3
groups of 6 treated and 2 control animals each. The experimental
animals received 0.125 g monocrotaline/kg body weight by ip
injection. Liver wedge biopsies were examined at various intervals
in Group I during hours 1 - 48, in Group II at 4 - 12 days, and in
Group III at 16 - 32 days. The earliest changes, observed by light
microscopy, were seen at 24 h and consisted of progressive loss of
endothelial cells and other associated changes in the lumen and
wall leading to occlusion by collagenization by the third week.
Under the electron microscope, within 24 h of administration,
marked changes were observed in the endothelial cells resulting in
their rupture and release of organelles in the lumen. This was
followed by penetration of fluid though the vessel walls in the
first week and changes in the fibroblasts. By the third to fourth
week, hepatic veins showed various levels of occlusion and the
vessel was scattered with cell debris, free organelles, etc. The
authors concluded that, in this species, hepatocellular necrosis
was not the primary factor causing veno-occlusive disease, as the
association of cellular necrosis and venous occlusion occurred only
in the central area of liver lobules, and the hepatocytes
surrounding the sublobular and medium sized hepatic veins were
unaffected.
In their study of 1967, Allen et al. also investigated the
ultrastructural changes in the liver of M. speciosa monkeys after
administering 2 doses of 1 g monocrotaline each, on days 1 and 14.
At autopsy, after a mean survival time of 21 days, a wide spectrum
of changes was observed in the hepatocytic organelles, many of
which were lying, discharged into sinusoids, and also phagocytosed
by the Kupffer cells. By the third week, proliferation of
connective tissues had started in the sinusoids near the central
veins and also in the walls of central veins. The authors
concluded that the vascular and parenchymal cell changes were
simultaneous and appeared to be equally instrumental in the
development of the occlusive lesion.
6.4.1.4 Mechanism of toxic action
The mechanism of toxic action in acute pyrrolizidine
hepatotoxicity and the sequence of events, judged from the
collective experimental studies, appears to be as follows.
The PA, which is inactive as a cell poison by itself, becomes
cytotoxic through its metabolism in the hepatic parenchymal cells
to pyrroles, which act preferentially on the hepatocytes and the
endothelium of blood vessels in the liver or lungs. In the
hepatocytes, the immediate action is a rapid fall in cytoplasmic
protein synthesis reaching 30% of control levels at 15 min and 6%
at 1 h (Harris et al., 1969). This is manifested as disaggregation
of polyribosomes and is followed by failure of pyruvate oxidation,
loss of glycogen, structural damage to the mitochondria, lysosomal
activity, failure of mitochondrial nicotine-adenine-dinucleotide
(NAD) systems and nuclear NAD synthesis, and necrosis (McLean,
1970). The necrosis is zonal in the liver lobule, the particular
zone affected depending on the metabolic enzymic geography of the
lobule in the particular animal species, and also in man and monkey
on the vascular endothelium of the central and sublobular veins.
The sequence of events of the vascular lesion has been studied
by McLean et al (1964). After a single dose of Crotalaria fulva
extract in the rat, centrilobular necrosis is present after the
first day, but collagenous veno-occlusion of the central veins of
the liver lobule only appears between 7 and 10 days later.
Evidently, the necrosis of the liver cells is not secondary to
venous occlusion. Centrilobular haemorrhage is seen from day 2
onwards and signs of hepatic venous outflow tract obstruction
appear after 2 - 5 days (McLean & Hill, 1969).
Rappaport et al. (1967) and McLean (1969) demonstrated, through
transillumination studies on rats, that the outlet end of the
sinusoids is blocked by stationary columns of red cells, 16 - 24 h
following administration of PAs. The reaction is typically patchy
and results in stasis and extravasation of red cells spreading
backwards from the centre of the lobule. For at least 3 days, no
circulatory detail can be seen with transillumination. Portal
pressure is significantly raised 3 days after administration of
fulvine (Rappaport et al., 1967), notably before the first
appearance of collagenous venous occlusion at 7 days. McLean
(1969) observed that 6 - 10 days after PA administration, a new
irregular pattern of vascular flow, contrasting with the uniform
radial pattern of flow in the normal liver lobule, develops, which
corresponds to the bypass channels represented by dilated
paraseptal sinusoids, as observed in human liver biopsies (section
7.3). Segments of central vein into which the blocked sinusoids
open, are gradually abandoned in favour of such by-pass routes and
undergo occlusion first by oedematous connective tissue and then by
fibrosis. The mechanism of closure of the sinusoids is not clear.
A toxic action on the sinusoidal or venous endothelium, which
swells and occludes the lumen, seems possible, as suggested by
Allen & Carsten's (1968) electron microscopic studies on the
monkey, and studies on children (Brooks et al., 1970). The
endothelial lining of the vessels is denuded and replaced by a
fibrinous and proteinaceous precipitate, which, together with the
oedematous wall of the vessel, becomes organized and slowly
replaced by fibrous connective tissues. The occlusion of sinusoids
is further contributed by the discharge of cellular debris into the
space of Disse. The lumen of the sinusoids becomes occluded
simultaneously with the fibrosis occurring in the central vein.
Collagen fibres extend into the space of Disse and sinusoids
leading to a creeping fibrosis.
The proximate toxin that escapes from the liver is returned to
the heart, after which it damages the first portal of entry into
the alveolar capillaries of the lung and pulmonary arteries.
6.4.1.5 Chronic effects
The chronic hepatotoxic effects of PAs have been described in a
number of studies on a variety of animals and have been reviewed by
Bull et al. (1968) and McLean (1970). A notable feature is that an
appropriate single dose of PA has been demonstrated by Schoental
and Magee (1957, 1959) to lead to a relentlessly progressive course
and eventually kill the animal, more than 18 months after
administration. Schoental & Bensted (1963) demonstrated that rats
receiving a single dose of PA may develop chronic liver disease and
finally hepatocellular carcinoma more than 13 months after
administration. The morphological changes in the liver are similar
in a given species of animal, whether a single sublethal dose is
administered or multiple small doses.
Schoental & Magee (1957) studied the long-term effects of a
single dose of lasiocarpine on rats receiving normal and protein-
deficient diets. Albino rats of Porton strain were used. In the
first study, 66 rats fed a normal diet were administered a single
oral dose of lasiocarpine at one of 3 dose levels (50 - 74 mg/kg,
75 - 100 mg/kg, or 101 - 150 mg/kg body weight); 24 animals served
as controls. In another study, 46 young female rats were
administered a protein-deficient diet. Of these, 13 and 10 animals
received a single oral dose of lasiocarpine at 50 - 100 mg/kg and
50 - 75 mg/kg body weight, respectively. Each of these groups was
pair-fed with an identical number of animals that did not receive
any PA. Of the 66 rats fed a normal diet, very few died in the
first 10 days. Thirty-one animals survived longer than 3 months.
They continued to be in good health until shortly before death.
The numbers of animals that survived for 13 months after
administration of lasiocarpine were 8/10 males and 7/7 females
(lowest dose) and 5/25 males and 11/18 females (intermediate dose).
In the group that received the highest dose, neither of the 2 male
animals survived longer than 35 days, and only 1/4 female animals
survived longer than 3 months. In the animals that died or were
killed when moribund, parenchymal damage was invariably present
with prominent megalocytes, ductular proliferation, and invasion of
lobules by oval or elongated cells, thought to be derived from the
bile-duct epithelium or the reticuloendothelial cells. Animals
that survived showed various degrees of fibrosis and nodular
hyperplasia and, in some, a mild thickening of the central veins.
No obliterative lesions of the veins were seen. The 31 animals
that survived 13 months showed similar changes, but to a much
lesser extent. In the livers of animals that had repeat liver
biopsies, there was no tendency to regression of the lesions.
The above data indicate that very few animals died of acute
disease. In most animals, there was a latent period of 3 - 4
weeks, during which they remained well and showed little evidence
of liver cell injury, followed by a progressive course often
leading to fibrosis and nodular hyperplasia.
The low-protein diet adversely affected the growth of all the
rats in the control as well as the treated group. Only 3 out of 23
treated animals remained alive and in apparent good health, 8 - 11
months after the treatment. Liver biopsies taken at various
intervals between 4 and 10 months showed very severe fatty changes
in the liver cells. There was little fibrosis and no bile-duct
proliferation. In areas where the fatty changes were less severe,
characteristic megalocytes were seen. Control animals had either
normal livers or showed only slight fatty changes that were not
comparable in severity with those in the livers of PA-treated
animals. Thus protein deficiency in the diet was shown to enhance
the toxic effects of the PA.
Schoental & Magee (1959) extended these studies on young Wistar
rats using several other PAs including heliotrine, retrorsine,
riddelliine, seneciphylline, monocrotaline, and its N-oxide in
various dosages ranging from 25 to 300 mg/kg body weight; the
animals were studied at death from 1 - 10 days to 18 - 30 months.
Pathological changes were similar to those observed with
lasiocarpine in the previous study. Notable observations were that
necrosis did not necessarily precede subacute or chronic changes.
The livers of some animals became severely damaged and showed
nodular hyperplasia. Liver biopsy, 2 - 3 days after PA treatment,
did not show pathological changes in some animals, but a repeat
biopsy at 41 days showed characteristic changes. Fibrous
thickening of the central veins was observed in some animals, more
often with monocrotaline N-oxide, but no occlusion of the hepatic
veins was seen.
The studies of Nolan et al. (1966) confirmed the observations
of Schoental & Magee (1957, 1959). They gave a single dose of
lasiocarpine at 120 mg/kg body weight, by stomach tube, to 108
(equal numbers of both sexes) Sprague Dawley weanling rats (60 -
135 g body weight). Thirty animals of both sexes served as
controls. Groups of 10 animals, each consisting of 8 treated and 2
control animals, were killed at various intervals from 1 to 123
days. Of the 80 treated animals, 28 died within 26 days. No
delayed hepatic lesions were found in 59 rats between days 1 and
18. Between 19 and 123 days, delayed lesions were found in 34/49
rats. These 34 rats showed megalocytosis, but no ductular
proliferation or fibrosis.
In a second study, 127 twenty-one-day-old male Wistar rats were
given a single oral dose of lasiocarpine at 80 mg/kg body weight;
65 animals served as controls. Liver biopsy was carried out on day
3 in 108 and on day 9 in 15 of the treated animals. In 47 animals,
additional biopsies were carried out at intervals. Of the 127
PA-treated rats, 98 died during the first 9 days, and 29 after
10 - 50 days. In contrast to the first study, 32/58 survivors
exhibited delayed subacute and chronic lesions, as described by
Schoental & Magee (1957). Of these, 8 animals developed cirrhosis.
The observations indicated that the lesions of acute zonal
necrosis, which appeared on, or before, the third day, healed
without residual lesions. However, 55% of the 58 survivors
developed subacute/chronic lesions that tended to be progressive
after a latent period of 2 - 3 weeks. There appeared to be an
intimate relationship between chronic lesions and megalocytosis,
which was seen in 52/58 surviving animals. No obliterative
vascular changes were observed and so the lesions could not be
ascribed to impaired circulation.
Schoental (1959) demonstrated the toxic effects on the newborn
of PAs administered to lactating mother rats. Wistar rats
(200 - 300 g) were administered lasiocarpine, orally or by ip
injection, at 25 - 40 mg, in 5 - 10 doses of 5 - 10 mg, twice weekly
or more (24 rats), or retrorsine at 4 - 10 mg per dose, in 1 - 14
approximately daily doses (23 rats). The litters were left with
the mothers for 24 days or more (except for 1/2 hour separation
during the PA treatment). The litters were examined by biopsy at
frequent intervals or at autopsy when they died or were killed in a
moribund state. Litters of the lasiocarpine-treated rats showed
only insignificant fibrosis or some megalocytosis. In the
retrorsine-treated group, the majority of the young rats survived
for about 18 days, but all rats died before reaching the age of 30
days. The milk secretion of the mothers was apparently not affected
by the PA treatment. Of the 98 animals in 9 litters, 45 died by
the 20th day and 45 survived 30 days. Animals dying in the first
fortnight did not show gross liver lesions, but those that died at
weaning time or later, all showed signs of liver disease. Animals
dying at 18 - 30 days showed hydropic or fatty vacuolation of
hepatocytes and haemorrhage into distended sinusoids. The change
was severe in animals dying at 1 - 2 months, and some central veins
showed a narrowed lumen. The lactating rats that received the
alkaloids survived longer than their young, and most showed no ill
effects from the treatment. This evidently indicates either a high
susceptibility of suckling rats or a high concentration of PAs in
milk.
In studies by Allen et al. (1963), 2 groups of 4-week-old
turkeys, each consisting of 12 birds, were fed diets containing
ground Crotalaria spectabilis seed at 2.5 g/kg and 5 g/kg,
respectively, for 120 days. Twelve animals served as controls. At
the end of the study, 11/12 birds receiving 5 g/kg seed and 6/12
receiving 2.5 g/kg seed in the diet developed cirrhosis. The
minimum period of feeding required to produce cirrhosis was 75
days, provided the diet was reduced to a level that was not lethal.
Allen & Carstens (1971) induced the Budd-Chiari syndrome in
monkeys by monocrotaline. Six adult female and 9 adult male Macaca
speciosa monkeys, weighing 5.2 - 6.5 kg each, were divided into 2
groups, each comprising 5 control (3 males and 2 females) and 10
treated animals (6 males and 4 females). The treated group was
given a subcutaneous (sc) injection of monocrotaline at 60 mg/kg
body weight at monthly intervals for 3 months. Needle biopsy of
the liver was carried out every month for 5 months and laparotomy,
6 months after PA treatment.
The treated animals showed marked vascular changes and various
degrees of occlusion in the centrilobular and sublobular veins as
well as the larger vessels. There was also characteristic
haemorrhagic necrosis in the centrilobular zones and megalocytes
were seen. The portal venous pressures were raised. The animals
were autopsied at 6 months. The livers were markedly shrunken
weighing an average of 68 g in contrast to those of control
animals, which weighed 130 g. There were severe occlusive vascular
changes and irregular fibrosis in the lobules. The adjacent
sinusoids were dilated as a compensatory mechanism.
Swick et al. (1982a) studied the effects on guinea-pigs of
long-term dietary administration of Senecio jacobaea and compared
them with the toxic effects of single doses of injected Senecio
alkaloids and monocrotaline. The possible protective effect of
cysteine was also examined. Fifteen guinea-pigs of 250 - 300 g
initial body weight were divided into 2 treated groups, being fed
10% Senecio jacobaea, or 10% Senecio jacobaea plus 1% cysteine in
the diet, and a control group. The whole plant of Senecio jacobaea
was air dried and powdered for incorporation into the diets. The
animals were fed for 365 days. They were autopsied at death or at
the termination of the study. In a second study, 7 guinea-pigs of
500 g body weight were injected intraperitoneally with either
monocrotaline, jacobine, or mixed Senecio jacobaea PAs. The
chronic lethal dose LD100 of Senecio jacobaea was 1264 g/kg initial
body weight or 526% of the initial body weight with an average
survival time of 279 days. No mortality was observed in control
animals. This contrasts with the chronic LD100 of Senecio jacobaea
for rats of 58% of initial body weight (Swick et al., 1979) and
that of cattle equivalent to 5 - 20% body weight (Bull et al.,
1968). Addition of cysteine to the diet was only slightly, but not
significantly, protective. Pathological examination of the livers
of the guinea-pigs fed Senecio jacobaea revealed extensive
megalocytosis and severe cytoplasmic vacuolation with biliary
hyperplasia and fibrosis, primarily in periportal areas. The
centrilobular and midzonal areas were spared.
Monocrotaline was non-toxic at doses up to 1000 mg/kg body
weight, whereas jacobine and mixed alkaloids from Senecio jacobaea
were lethal at much lower levels. Similar results showing
resistance to monocrotaline in guinea-pigs were also reported by
Chesney & Allen (1973a). In in vitro studies, they related this
resistance to lack of conversion of PA to pyrroles by guinea-pig
microsomes.
A morphological peculiarity of chronic hepatotoxicity in a
large variety of laboratory and farm animals is megalocytosis
(Bull, 1955; McLean, 1970), i.e., the appearance of exceptionally
large hepatocytes, 10 - 30 times the volume of normal cells with
proportionately large nuclei. Relevant literature has been
reviewed by Jago (1969), McLean (1970), and Mattocks (1986).
Advanced megalocytosis was produced by Jago (1969) within 4 weeks
in 2-week-old rats by administering a single dose of lasiocarpine
at 76 µmol/kg body weight. Megalocytes tend to appear in the
periportal and midzones of the liver lobules with normal sized
cells around the central veins. The nuclear chromatin is
proportionately increased, but the cells appear incapable of
entering into mitosis, as only abnormal mitoses are seen. Jago
(1969) demonstrated a fall in the mitotic index (from 1.61 to 0.04)
in liver cells of 2-week-old rats, one day after injection of 50 µmol
lasiocarpine/kg. The electron microscopic appearance also
supports the above observations (Afzelius & Schoental, 1967). A
striking proliferation of rough endoplasmic reticulum and multiple
centrioles is seen in the cytoplasm, and the cytoplasmic organelles
are disorganized, suggesting increased metabolic activity but
inability of the cells to divide. Such cells may persist for the
life-time of the animal (up to 2 years in the rat) and the liver
never returns to normal (Mattocks, 1986). Megalocytes have also
been described in the kidney (Bull et al., 1968), the lung (Barnes
et al., 1964; Butler et al., 1970; Hooper, 1974), and the duodenum
(Hooper, 1975c).
Data on the total chronic lethal dose of heliotrine in rats
were discussed by Bull & Dick (1959) and Bull et al. (1968). For a
variety of dosing rates, and with withholding periods of 10 - 20
weeks interposed, the total doses ranged from 2.2 to 7.8 LD50. In
Table 10, these data are extended with results for other alkaloids.
The overall range of the total lethal dose is 1.2 - 10.3 LD50.
6.4.2 Effects on lungs
Current literature has been extensively reviewed by Kay & Heath
(1969), and Mattocks (1986). PAs have been shown to produce
pulmonary hypertension with associated vascular changes in the
pulmonary circulation in a number of experimental animal species
including the rat, mouse, frog, turkey, pig, sheep, rabbit, and
horse (McLean, 1970) as well as in non-human primates (Allen &
Chesney, 1972; Chesney & Allen, 1973b) and the dog (Miller et al.,
1978). The alkaloids have been administered by feeding the animals
with: PA-containing seeds of plants (notably Crotalaria
spectabilis) (Turner & Lalich, 1965; Kay & Heath, 1966; Kay et al.,
1967a) or the dried plant itself (e.g., Senecio jacobaea) (Burns,
1972), aqueous solutions of fulvine (Barnes et al., 1964;
Wagenvoort et al., 1974a,b) or monocrotaline (Lalich & Ehrhart,
1962; Huxtable et al., 1977), subcutaneous injections of
monocrotaline (Allen & Chesney, 1972; Chesney & Allen, 1973b) and
seneciphylline (Ohtsubo et al., 1977), or intravenous injections of
some pyrrolic esters and analogues of pyrrolizidine alkaloids and
their metabolites (Mattocks & Driver, 1983). Lafranconi & Huxtable
(1984) studied the hepatic metabolism and pulmonary toxicity of
monocrotaline in in vitro perfusion studies. Some of the
representative studies on the morphological effects of toxic lung
injury are listed in chronological order in Table 11.
Chronic lung lesions have been produced by most compounds that
produce chronic liver lesions, though higher doses were required in
some instances (Culvenor et al., 1976a). However, not all PAs that
are hepatotoxic are also pneumotoxic. Among the pneumotoxic
alkaloids, fulvine (Barnes et al., 1964) and monocrotaline are
particularly active (Mattocks, 1986). Molecular structure activity
requirements are the same as for hepatotoxicity, since both are
caused by the same toxic metabolites produced in the hepatocytes.
6.4.2.1 Acute effects
Pulmonary lesions produced by PAs have been extensively
investigated, mostly in rats, but also in non-human primates.
Monocrotaline has been the alkaloid most frequently used, but lung
lesions have also been seen in rats following fulvine and
seneciphylline administration. Besides pure alkaloids,
PA-containing seeds of some plants, most notably Crotalaria
spectabilis, have also been used.
Miller et al. (1978) gave a single iv injection of
monocrotaline at 60 mg/kg body weight to 10 mongrel dogs. Toxic
effects, recorded within 2 h, included ultrastructural changes in
the endothelial cells of the alveolar capillaries, prominent
accumulation of platelets, and the appearance of interstitial
oedema (Table 11). Valdivia et al. (1967a,b) used 25 Sprague
Dawley rats in their study and made similar observations on the rat
lung within 4 h of a single subcutaneous injection of monocrotaline
at a dose of 60 mg/kg body weight (Table 11). Interstitial oedema
and elastolysis of the alveolar wall, increase in number of mast
cells, and other associated changes were observed within 4 h of the
injection, followed by alterations in endothelial and interstitial
cells. All of the changes progressed steadily for up to 3 weeks.
It was concluded that the initial changes of destruction of
pulmonary capillaries and the other components of the alveolar wall
preceded the arteriolar hypertrophy and arteritis observed by other
investigators following monocrotaline administration, and alone was
sufficient to cause right ventricular hypertrophy. Sugita et al.
(1983a,b) administered a single dose of monocrotaline at 40 mg/kg
body weight to 5 Sprague Dawley rats and adduced further evidence,
by biochemical and radioisotopic studies, of microvascular leak in
the alveolar wall within the first 3 days of injury, which preceded
right ventricular hypertrophy observed 2 weeks following
administration (Table 11).
Table 10. Total chronic lethal doses in rats (ip administration, 2 or 3
times per week)
--------------------------------------------------------------------------
Dose (x LD50) Time to Total lethal Reference
death dose
(days) (x LD50)
--------------------------------------------------------------------------
Heliotrine (male rats, unless otherwise stated)
0.2 58 5 Bull & Dick (1959)
0.1 123 5.1
0.04 303 4.1
0.02 508 4.1
0.01 5.2 - 5.3 Bull & Dick (1960)
(with interval of 10 -
20 weeks after 21 days)
0.11 2.2 - 4.3 Bull & Dick (1959)
0.1 7.8 Jago (1971)
(35-day-old male rats)
0.1 4.7 Jago (1971)
(337-day-old male rats)
0.1 5.8 Jago (1971)
(35-day-old female rats)
0.1 4.5 Jago (1971)
(337-day-old female rats)
Lasiocarpine (male rats)
0.1 210 9 Culvenor & Jago (1979)
0.05 482 10.3
0.02 676 5.7
0.01 595 2.6
(0.005) (638) (1.4)
0.1 81 - 175 2.4 - 5 Bull & Dick (1959)
0.1 - 6.3 - 10.9 Jago (1971)
Lasiocarpine (female rats)
0.1 108 4.6 Culvenor & Jago (1979)
0.05 274 5.8
0.02 471 4
0.01 487 2.1
(0.005) (692) (1.5)
0.1 2.4 - 7 Jago (1971)
Monocrotaline
0.1 1.2 - 2.4 Bull et al. (1968)
0.05 2.5 - 4.4
Senecioninea
0.2 2 - 7.4 Bull et al. (1968)
0.1 1.7 - 5.7
0.04 (3 survived)
--------------------------------------------------------------------------
a Assuming LD50 mg/kg (c.f., Mattocks, 1986).
Table 11. Summary of experimental data on the morphological effects of toxic lung injury due to pyrrolizidine alkaloids (in
chronological order)
---------------------------------------------------------------------------------------------------------------------------------------
Animal/ Number Toxic agent Dose Administration Single/ Killed after Pathological References
strain/ of (mg/kg) dose multiple or survival effects
sex animals/ for
controls
---------------------------------------------------------------------------------------------------------------------------------------
Acute effects
Male 25/5 monocrotaline 60 subcutaneous single 2 - 48 h interstitial oedema, Valdivia et al.
Sprague (body 1 - 3 weeks endothelial cell (1967a,b)
Dawley rat weight) alterations,
elastolysis, etc.
Mongrel 10/0 monocrotaline 60 intravenous single 2 h interstitial oedema, Miller et al. (1978)
dog (body changes in endothelial
weight) cells
Male 5/3 monocrotaline 40 subcutaneous single 0 - 21 days microvascular leak, Sugita et al. (1983a)
Sprague (body right ventricular
Dawley rat weight) hypertrophy after 2
weeks
Chronic effects
Female 35/12 monocrotaline 10 - 30 ad libitum pulmonary arteritis Lalich & Ehrhart
Sprague (diet) feeding (with 20 - 30 mg dose (1962)
Dawley rat only)
6 alcohol- 2500 ad libitum 51 days none
extracted (diet) feeding
seeds of
Crotalaria
spectabilis
19/4 monocrotaline 10 - 75 ad libitum 26 - 232 Turner & Lalich (1965)
(diet) feeding days
---------------------------------------------------------------------------------------------------------------------------------------
Table 11. (contd.)
---------------------------------------------------------------------------------------------------------------------------------------
Animal/ Number Toxic agent Dose Administration Single/ Killed after Pathological References
strain/ of (mg/kg) dose multiple or survival effects
sex animals/ for
controls
---------------------------------------------------------------------------------------------------------------------------------------
Chronic effects (contd.)
Female 24/6 Crotalaria 200 - ad libitum 105 - 172 right ventricular Allen & Chesney (1972)
Wistar spectabilis 1600 feeding days hypertrophy and
Furth rat seeds (diet) dilatation, pulmonary
arterial hypertrophy,
pulmonary arteriolar
hypertrophy,
endocardial fibrosis
Female 10/34 Crotalaria 1000 ad libitum 30 - 60 days right ventricular Kay & Heath (1966)
weanling spectabilis (diet) feeding hypertrophy, pulmonary
Wistar rat seeds arterial hypertrophy,
pulmonary arteritis
Male 22/12 monocrotaline 120 subcutaneous single 20 - 47 days right ventricular Hayashi & Lalich
suckling (body hypertrophy, pulmonary (1967)
Sprague weight) arterial hypertrophy,
Dawley rat pulmonary arteritis,
fibrin thrombi
Female 30/10 fulvine 50 intraperitoneal single 3 - 37 days right ventricular Kay et al. (1971a)
Wistar rat (body hypertrophy, pulmonary
weight) arterial hypertrophy,
pulmonary arteriolar
hypertrophy, pulmonary
arteritis, arteriolar
thrombi
80 intragastric single 7 - 35 days
(body
weight)
---------------------------------------------------------------------------------------------------------------------------------------
Table 11. (contd.)
---------------------------------------------------------------------------------------------------------------------------------------
Animal/ Number Toxic agent Dose Administration Single/ Killed after Pathological References
strain/ of (mg/kg) dose multiple or survival effects
sex animals/ for
controls
---------------------------------------------------------------------------------------------------------------------------------------
Chronic effects (contd.)
Monkey 12 (30 monocrotaline 30 subcutaneous one 199 - 325 right ventricular Allen & Chesney (1972)
(Macaca days old) (body followed days hypertrophy and
arctoides) weight) by 3 (average, dilatation, left
(both (2, 4, 241) ventricular
sexes) and 6 hypertrophy, pulmonary
months arterial hypertrophy,
after pulmonary arteriolar
first hypertrophy, pulmonary
injection hypertension
12 (15 163 - 334 pulmonary arterial
months days hypertension (isolated,
old) (average, veno-occlusive disease
217) (liver)
Monkey 20/6 monocrotaline 30 subcutaneous as above 165 - 325 right ventricular Chesney & Allen
(Macaca (body days hypertrophy and (1973b)
arctoides) weight) (average, dilatation, pulmonary
(both 326) arterial hypertrophy,
sexes) pulmonary arteriolar
hypertrophy, pulmonary
arteritis, endocardial
fibrosis
Female 50/12 fulvine 80 intragastric single 1 - 6 weeks vasoconstriction, Wagenvoort et al.
Wistar rat (body pulmonary arterial (1974a,b)
weight) hypertrophy, pulmonary
or 50 arteriolar hypertrophy,
(body intraperitoneal right ventricular
weight) hypertrophy, thickening
of veins and venules
---------------------------------------------------------------------------------------------------------------------------------------
Table 11. (contd.)
---------------------------------------------------------------------------------------------------------------------------------------
Animal/ Number Toxic agent Dose Administration Single/ Killed after Pathological References
strain/ of (mg/kg) dose multiple or survival effects
sex animals/ for
controls
---------------------------------------------------------------------------------------------------------------------------------------
Chronic effects (contd.)
Male 16/0 seneciphylline 50 - 80 subcutaneous single 1 - 3 weeks pulmonary arteriolar Ohtsubo et al. (1977)
Wistar (body and right ventricular
rat (4 weight) hypertrophy (after 3
weeks old) weeks), right
ventricular dilatation
in 2 animals
Male 21/14 Crotalaria 1000 ad libitum 3 - 35 days pulmonary arterial Meyrick & Reid (1979,
Sprague spectabilis (diet) feeding hypertrophy and 1982)
Dawley rat pulmonary arteritis
(2/21); right
ventricular hypertrophy
---------------------------------------------------------------------------------------------------------------------------------------
A histological and electron microscopic study was made by
Hurley & Jago (1975) of the lungs of rats administered
dehydromonocrotaline. Female black and white hooded rats weighing
80 - 100 g were used. Dehydromonocrotaline dissolved in
dimethylformamide (DMF) was administered iv as a single dose at
30 mg/kg body weight to 12 rats and at 15 mg/kg body weight to 7 rats.
Four control rats were administered DMF alone. A colloidal
suspension of carbon black was injected iv, 6 - 18 h after
injection of dehydromonocrotaline, and the animals killed 19 - 44 h
after treatment.
After an interval of 6 - 8 h, there was a direct toxic effect
on the endothelial cells of pulmonary capillaries and small
venules. Many endothelial cells had prominent nuclei and thickened
cytoplasm, which contained more RNA granules than usual. There was
also an increase in the number of mitochondria. The endothelial
damage did not seem to have caused permanent disruption of the
small blood vessels, and, 2 days after injury, all vessels were
patent. Large numbers of mononuclear cells, which appeared in the
interstitial tissues of the lung 44 h after injury, seemed to be
altered emigrated blood monocytes.
6.4.2.2 Chronic effects
Lalich & Erhart (1962), fed 35 Sprague Dawley rats a diet
containing monocrotaline at 10 - 30 mg/kg (Table 11). Animals
receiving a daily dose of monocrotaline of 20 mg/kg diet or more,
showed progressive changes in the lungs after 24 days of feeding.
Of the 23 animals receiving 20 - 30 mg/kg, 12 showed pulmonary
arteritis, 4 of these even at the dose level of 20 mg/kg diet.
Pulmonary haemorrhages were observed in 16 animals. No changes
were observed in animals fed alcohol-extracted seeds or other
derivatives of monocrotaline.
Identical changes were observed in similar studies by Turner &
Lalich (1965) on two strains of rats, Sprague Dawley (19) and
Wistar Furth (24). The first group of 19 female Sprague Dawley
rats was fed a diet containing monocrotaline at an initial level of
10 mg/kg diet. Depending on the response of each individual
animal, the monocrotaline level was raised to a maximum of 75 mg/kg
diet (Table 11). Fourteen rats survived for more than 100 days and
8 reached the maximum dietary level of monocrotaline, the last
animal dying after 232 days. The second group of 24 female Wistar
Furth rats was fed a diet contaminated with Crotalaria spectabilis
seeds, initially at 0.2 mg/kg diet, gradually rising to 1.6 mg/kg
by increasing the levels by 0.2 mg/kg every week. All test animals
survived 100 days and 15 reached the maximum levels of Crotalaria
fed. The last animal died after 172 days of feeding. Animals
developed signs of toxicity and right ventricular strain, e.g.,
cyanosis, etc. Progressive thickening of media in the muscular
pulmonary arteries, progressive muscularization of arterioles, and
changes characteristic of pulmonary hypertension, were seen. Some
pulmonary arteries showed medial necrosis. No changes were
observed in the pulmonary veins. Significant hypertrophy of the
heart, as judged by the heart weight in relation to body weight,
was seen in almost all animals that survived 100 days or more
(32/38), and the right ventricles were dilated. The hypertrophy
affected the right side of the heart only, and generally
corresponded with the vascular changes. There was a marked
hyperplasia of the mast cells in the mediastinal lymph nodes and
around bronchi and pulmonary arteries. Similar observations were
made by Barnes et al. (1964) and Valdivia et al. (1967a,b).
Kay and his group studied cardiac and pulmonary vascular
changes in rats fed Crotalaria spectabilis seeds (Kay & Heath,
1966; Kay et al., 1967a,b) or administered fulvine (Kay et al.,
1971a).
A group of 10 female weanling Wistar albino rats were fed a
diet containing 1 g powdered seeds of Crotalaria spectabilis/kg
until they died of cardiorespiratory distress, after 36 - 60 days
of feeding. Thirty-four control rats were fed a normal diet. At
autopsy, the atria of the heart, the right ventricle, and the left
ventricle with the interventricular septum were weighed separately.
The medial thickness of the muscular pulmonary arteries was
measured, and expressed as percentage of external diameter. The
medial thickness of the muscular pulmonary arteries increased in
all test rats; acute or healing pulmonary arteritis was seen in 3
animals. Statistically significant cardiomegaly was present in all
rats fed the seeds, contributed chiefly by the right ventricle. The
readings from all the test rats were well outside the upper 95%
confidence limit. The increase in the medial thickness of the
pulmonary arteries correlated well with the weight of the right
ventricle (Fig. 10). Three rats showed pulmonary arteritis
(indicated by a solid triangle). It was presumed that the organic
basis for increased pulmonary resistance was the abnormal
muscularization of the radicles of the pulmonary arterial system.
Essentially similar results were obtained in an identical study
repeated on 8 test rats and 5 controls (Kay et al., 1967a). The
test rats developed pulmonary hypertension in 37 days, levels of
which were correlated with the medial thickness of the muscular
pulmonary arteries and that of the pulmonary trunk, as well as with
the weight of the right ventricle.
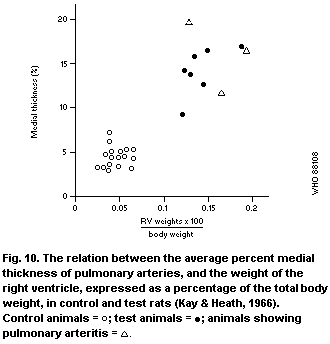 Ghodsi & Will (1981) made similar observations in Sprague
Dawley rats given a single subcutaneous injection of monocrotaline
at 60 mg/kg body weight. Forty rats weighing 180 - 200 g were
used; of these, 20 constituted the control group. The control
animals received the same volume of saline. Each week, 3 rats from
each group were catheterized and pulmonary artery pressures were
measured. In the treated group, 2 out of 5 animals showed a mild
increase in pulmonary artery pressure at the end of 8 days. A
further 4 out of 5 animals showed a mild to moderate rise in
pulmonary artery pressure after 2 weeks. The highest value
recorded in test rats was 56 mmHg compared with a normal upper
limit of 22 mmHg. The medial thickness of pulmonary arteries was
correlated with pulmonary artery pressures ( P < 0.02) as was the
thickness of the right ventricle. The correlation between the
pulmonary artery pressures and right ventricular hypertrophy was
statistically significant ( P < 0.05).
Kay et al. (1971a) administered a single dose of fulvine to
rats, intraperitoneally at 50 mg/kg body weight, or through a
stomach tube at 80 mg/kg. Of the 30 treated rats, 17 survived 23
days. All of the animals showed changes characteristic of
hypertensive pulmonary vascular disease with right ventricular
hypertrophy and muscular hypertrophy of the pulmonary trunk and the
muscular pulmonary arteries. Pulmonary arterioles were also
muscularized and contained fibrin thrombi. Four animals showed
pulmonary arteritis.
Essentially similar changes in the pulmonary arterial system
were produced within 20 - 28 days by Hayashi & Lalich (1967) in 22
male suckling rats administered a single injection of monocrotaline
at 120 mg/kg body weight, and within 28 days by Ohtsubo et al.
(1977) in 16 male, 4-week-old rats given a single injection of
seneciphylline at 80 mg/kg body weight. Hooper (1974) did not find
any such effect on feeding powdered Senecio jacobaea at
100 - 200 mg/kg diet to 9 male white mice for up to 193 days.
In studies by Allen & Chesney (1972), non-human primates
(Macaca arctoides) were administered 4 doses of monocrotaline at
30 - 60 mg/kg body weight by subcutaneous injection (Table 11).
Twelve infant monkeys (30 days old) and 12 adults (15 months old)
were studied with different results. Vascular changes,
characteristic of pulmonary hypertension and resultant cor
pulmonale, were observed in the infant monkeys, as described by
earlier workers in the rat. Only isolated small hepatic veins were
occluded. On the other hand, the adult animals showed a more
severe involvement of the liver with changes characteristic of
veno-occlusive disease and only an occasional pulmonary blood
vessel was involved. The authors postulated that the different
responses in infant and adult animals were due to the different
stages of maturation of the enzyme systems of the hepatocyte in the
two age groups. It is possible that the different reactions in the
liver and lung in the 2 groups may be due to the fact that the
enzymatic pathways responsible for producing metabolites that cause
hepatic damage are poorly developed in the infant, but those
responsible for causing pulmonary lesions are better developed.
Chesney & Allen (1973b) made observations similar to those of
Allen & Chesney (1972) in twenty, 30-day-old monkeys in a similar
study using monocrotaline injections and recorded, in addition,
endocardial fibrosis of the right heart. The treated animals
developed classical clinical features of cardiopulmonary distress,
which was also evidenced by changes in the blood-gas parameters.
The raised right heart pressures were confirmed by actual
measurements of the blood pressure in the right ventricle,
pulmonary artery, and descending aorta. The authors considered
this study to be a good experimental model to investigate
hypertensive pulmonary vascular disease, or pulmonary and
endocardial fibrosis. The type of vascular changes seen in the
animals were comparable with those associated with pulmonary
hypertension in man in cardiopulmonary disease (Barnes et al.,
1964; Kay & Heath, 1966; Kay et al., 1967a; Chesney & Allen,
1973b).
Wagenvoort et al. (1974a,b) made light microscopic and ultra-
structural studies on 50 female Wistar rats, 1 - 6 weeks following
a single oral dose of fulvine at 80 mg/kg body weight or an ip dose
at 60 mg/kg body weight. Twelve animals served as controls.
Vasoconstriction of muscular pulmonary arteries and arterioles was
seen initially, one week following administration. This was
evident by the coiled appearance of the muscular nuclei and
excessive crenation of the internal elastic lamina. The nuclei of
smooth muscle cells as well as those of endothelial cells were
partly squeezed between the folds of the lamina. After 3 - 4
weeks, these blood vessels began to thicken, with muscular
hypertrophy and fibrinoid necrosis of the arterial muscle. Animals
surviving administration of fulvine developed right ventricular
hypertrophy, proliferation of endothelial cells in the arteries and
even thickening of the veins.
In a study by Meyrick and Reid (1979), 21 Sprague Dawley rats
were fed a diet containing 1 g powdered seeds of Crotalaria
spectabilis/kg for various periods ranging from 3 to 35 days. The
earliest demonstrable change in the pulmonary arterial system of
the animals was seen on day 3 and consisted of the appearance of
muscle in normally non-muscular arteries of the lung. The muscular
pulmonary arteries began to show hypertrophy of the media from day
7, which reached statistically significant levels on day 10 in
smaller arteries and on day 14 in the larger arteries. Significant
right ventricular hypertrophy was seen on day 21. These changes
were confirmed by 3H-thymidine uptake studies (Meyrick & Reid,
1982).
6.4.2.3 Mechanisms of toxic action
Considerable progress has been made recently in the
understanding of biochemical and pharmacological changes that occur
in PA-induced lung disease.
Turner & Lalich (1965) and Takeoka et al. (1962) postulated
that pulmonary hypertension was mediated by the release of
5-hydroxytryptamine from the mast cells, which became hyperplastic
in the mediastinal lymph nodes and around bronchi and pulmonary
arteries (Turner & Lalich, 1965) (section 6.4.2.2) following
administration of monocrotaline, causing vasoconstriction. On the
other hand, Kay et al. (1967b) found that the number of mast cells
corresponded with the severity of exudative changes in the lung and
were not related to the genesis of pulmonary hyperplasia.
Besides the medial muscular hypertrophy of pulmonary arteries
reported in several studies cited above, swelling and lysis of the
endothelial cells, contributing to luminal narrowing and thickening
of the wall with fibrosis, have been described (Allen & Carstens,
1970).
Weanling rats are more susceptible to these changes than older
animals, and the changes follow a strict temporal sequence. Oral
administration of monocrotaline to rats at 20 mg/litre in drinking-
water produced a sequence of changes over 3 weeks, that included an
increase in lung mass, which was significant by day 9, stimulation
of pulmonary RNA and protein synthesis (maximal on day 10),
increased pulmonary arterial blood pressure (significant by day
10), and right ventricular hypertrophy by day 14 (Huxtable et al.,
1978; Lafranconi et al., 1984). The increase in the lung mass was
not accompanied by change in the total collagen content and was
contributed possibly by hypertrophy of endothelial cells, but the
increased mass of the right ventricle was associated with a 4-fold
increase in collagen content (Lafranconi et al., 1985).
An early event is inhibition of serotonin removal by pulmonary
endothelium (Huxtable et al., 1978). This phenomemon, combined
with the increased release of serotonin by mast cells that has been
observed, may be involved in the development of pulmonary
hypertension (Carillo & Aviado, 1969). Right ventricular
hypertrophy is blocked by propanolol, whereas the development of
pulmonary hypertension is unaffected (Huxtable et al., 1977).
Novel metabolites have been found to be released by livers perfused
with monocrotaline in vitro, and these metabolites block serotonin
transport in vitro, when perfused through isolated lungs
(Lafranconi & Huxtable, 1984). These data suggest that the slow
release of metabolites from the liver into the circulation
following low-level exposure to monocrotaline results in specific
inhibition of endothelial cell function (Huxtable et al., 1978).
The effect of monocrotaline treatment on pulmonary angiotensin
converting enzyme (ACE) activity in the rat is disputed. Hayashi
et al. (1984) observed a reduction in the ACE activity of pulmonary
tissue in pyrrolizidine-exposed rats in parallel with the
development of pulmonary alterations, while the ACE activity of the
plasma remained unchanged. However, other authors have reported
that, though the specific activity of ACE falls in the isolated
perfused lungs of monocrotaline-treated rats, or in lung
homogenates from such animals, when activity is expressed as total
activity per lung, there is no significant alteration in the lungs
of treated animals compared with those of untreated animals
(Huxtable et al., 1978; Lafranconi & Huxtable, 1983). Therefore,
the significance of changes in ACE activity is open to question.
Molteni et al. (1984) also found evidence of endothelial cell
damage by monocrotaline in their ultrastructural and biochemical
studies on rats. Eighty male Sprague Dawley rats were used; half
were administered monocrotaline at 20 mg/litre in the drinking-
water and half were given plain water. The average daily water
consumption was 35 ml/rat. Thus, the treated rats were estimated
to have received 2 mg/kg per day. Five animals each from the
treated and control groups were killed at intervals of 1 - 12 weeks
after the start of the study. The endothelial damage was measured
by ACE activity, plasminogen-activator (PLA) activity, and
prostacyclin (PGI2) production. These were correlated with
pulmonary arterial perfusion and ultrastructural changes in the
lung. In the treated groups, after an initial rise at 1 week, the
ACE activity showed a steady decline from 1 to 6 weeks, after which
it plateaued at 55% of normal. PLA activity did not change for 2
weeks, but decreased by 59 and 79% of the control value after 6 and
12 weeks, respectively. On the other hand, the PGI2 production
increased progressively reaching 140 and 270% of the control level
after 6 and 12 weeks, respectively. These endothelial functional
changes were not accompanied by significant changes in pulmonary
arterial perfusion as visualized by 99mTc-labelled macroaggregated
albumin perfusion studies. The activities of ACE and PLA and the
production of PGI2 are considered sensitive indices of endothelial
function in rats. The above results indicated endothelial cell
dysfunction. The ultrastructural studies also revealed oedema of
capillary subendothelial, perivenous and periarterial tissues at 1
week, and interstitial inflammatory infiltrates at 2 weeks. At
6 - 12 weeks, there was thickening of the pulmonary arteries and
enlargement of right side of the heart.
Stenmark et al. (1985) studied the role of alveolar
inflammation and arachidonate metabolism in monocrotaline-induced
pulmonary hypertension in rats. Five groups of male Sprague Dawley
rats were treated as follows: (a) 20 rats received 40 mg
monocrotaline/kg body weight, sc; (b) 20 rats received
monocrotaline, 40 mg/kg sc plus diethylcarbamazine (DEC) 100 mg/kg
sc, every 12 h; in addition, 250 mg DEC was added to 100 ml of
drinking-water. This treatment started 2 days prior to the start
of the study and was continued daily for 3 weeks; (c) 12 control
rats received normal saline plus monocrotaline at 40 mg/kg sc; (d)
12 rats received indomethacin at 2 mg/kg sc for 2 days, prior to
receiving monocrotaline at 40 mg/kg and then daily for 3 weeks; (e)
6 animals each received a single sc injection of normal saline and
served as additional controls.
One, 2, and 3 weeks after monocrotaline or saline injection,
lung lavage was carried out for cell counts and assay for enzyme
activity and cyclooxygenase metabolites, the degradation products
of prostacyclin (PGI2) and thromboxane A2(TXA2), as 6-keto-
prostaglandin (PGF1alpha) and TXB2, respectively. At 3 weeks, the
animals were anaesthetized, right ventricular pressures measured by
catheterization and the heart removed. The 2 ventricles were
separated and weighed for the determination of heart-weight ratio
(right ventricle/left ventricle + septums RV/LV + S) an indicator
of right ventricular hypertrophy.
The right ventricle showed hypertrophy at 2 weeks and the right
ventricular pressure was increased at 3 weeks following
monocrotaline administration (Fig. 11). The leukocyte count in the
lavage fluid increased at 3 weeks, with a rise in the percentage of
polymorphonuclear leukocytes and large, abnormal alveolar
macrophages in the test animals. B- N-acetyl-D-glucoseaminidose
activity was also elevated at 3 weeks, indicating activation of
leukocytes. There was also a rise in the concentration of 6-keto-
PGF1alpha at 1 and 3 weeks, as well as in TXB2 at 3 weeks, compared
with those in control animals.
The administration of DEC inhibited both the increase in heart-
weight ratio (RV/LV + S) and the increase in pulmonary artery
pressure (Fig. 11) that occurred 3 weeks after monocrotaline
administration, and reduced the percentage of polymorphonuclear
cells, abnormal alveolar macrophages, and hexoseaminidase activity
in the lavage fluid, compared with that from animals that had
received monocrotaline only. The rise in the levels of 6-keto-
PGF1alpha was inhibited ( P < 0.05) by 73% and that of TXB2 by 74%
in the lung lavage.
Ghodsi & Will (1981) made similar observations in Sprague
Dawley rats given a single subcutaneous injection of monocrotaline
at 60 mg/kg body weight. Forty rats weighing 180 - 200 g were
used; of these, 20 constituted the control group. The control
animals received the same volume of saline. Each week, 3 rats from
each group were catheterized and pulmonary artery pressures were
measured. In the treated group, 2 out of 5 animals showed a mild
increase in pulmonary artery pressure at the end of 8 days. A
further 4 out of 5 animals showed a mild to moderate rise in
pulmonary artery pressure after 2 weeks. The highest value
recorded in test rats was 56 mmHg compared with a normal upper
limit of 22 mmHg. The medial thickness of pulmonary arteries was
correlated with pulmonary artery pressures ( P < 0.02) as was the
thickness of the right ventricle. The correlation between the
pulmonary artery pressures and right ventricular hypertrophy was
statistically significant ( P < 0.05).
Kay et al. (1971a) administered a single dose of fulvine to
rats, intraperitoneally at 50 mg/kg body weight, or through a
stomach tube at 80 mg/kg. Of the 30 treated rats, 17 survived 23
days. All of the animals showed changes characteristic of
hypertensive pulmonary vascular disease with right ventricular
hypertrophy and muscular hypertrophy of the pulmonary trunk and the
muscular pulmonary arteries. Pulmonary arterioles were also
muscularized and contained fibrin thrombi. Four animals showed
pulmonary arteritis.
Essentially similar changes in the pulmonary arterial system
were produced within 20 - 28 days by Hayashi & Lalich (1967) in 22
male suckling rats administered a single injection of monocrotaline
at 120 mg/kg body weight, and within 28 days by Ohtsubo et al.
(1977) in 16 male, 4-week-old rats given a single injection of
seneciphylline at 80 mg/kg body weight. Hooper (1974) did not find
any such effect on feeding powdered Senecio jacobaea at
100 - 200 mg/kg diet to 9 male white mice for up to 193 days.
In studies by Allen & Chesney (1972), non-human primates
(Macaca arctoides) were administered 4 doses of monocrotaline at
30 - 60 mg/kg body weight by subcutaneous injection (Table 11).
Twelve infant monkeys (30 days old) and 12 adults (15 months old)
were studied with different results. Vascular changes,
characteristic of pulmonary hypertension and resultant cor
pulmonale, were observed in the infant monkeys, as described by
earlier workers in the rat. Only isolated small hepatic veins were
occluded. On the other hand, the adult animals showed a more
severe involvement of the liver with changes characteristic of
veno-occlusive disease and only an occasional pulmonary blood
vessel was involved. The authors postulated that the different
responses in infant and adult animals were due to the different
stages of maturation of the enzyme systems of the hepatocyte in the
two age groups. It is possible that the different reactions in the
liver and lung in the 2 groups may be due to the fact that the
enzymatic pathways responsible for producing metabolites that cause
hepatic damage are poorly developed in the infant, but those
responsible for causing pulmonary lesions are better developed.
Chesney & Allen (1973b) made observations similar to those of
Allen & Chesney (1972) in twenty, 30-day-old monkeys in a similar
study using monocrotaline injections and recorded, in addition,
endocardial fibrosis of the right heart. The treated animals
developed classical clinical features of cardiopulmonary distress,
which was also evidenced by changes in the blood-gas parameters.
The raised right heart pressures were confirmed by actual
measurements of the blood pressure in the right ventricle,
pulmonary artery, and descending aorta. The authors considered
this study to be a good experimental model to investigate
hypertensive pulmonary vascular disease, or pulmonary and
endocardial fibrosis. The type of vascular changes seen in the
animals were comparable with those associated with pulmonary
hypertension in man in cardiopulmonary disease (Barnes et al.,
1964; Kay & Heath, 1966; Kay et al., 1967a; Chesney & Allen,
1973b).
Wagenvoort et al. (1974a,b) made light microscopic and ultra-
structural studies on 50 female Wistar rats, 1 - 6 weeks following
a single oral dose of fulvine at 80 mg/kg body weight or an ip dose
at 60 mg/kg body weight. Twelve animals served as controls.
Vasoconstriction of muscular pulmonary arteries and arterioles was
seen initially, one week following administration. This was
evident by the coiled appearance of the muscular nuclei and
excessive crenation of the internal elastic lamina. The nuclei of
smooth muscle cells as well as those of endothelial cells were
partly squeezed between the folds of the lamina. After 3 - 4
weeks, these blood vessels began to thicken, with muscular
hypertrophy and fibrinoid necrosis of the arterial muscle. Animals
surviving administration of fulvine developed right ventricular
hypertrophy, proliferation of endothelial cells in the arteries and
even thickening of the veins.
In a study by Meyrick and Reid (1979), 21 Sprague Dawley rats
were fed a diet containing 1 g powdered seeds of Crotalaria
spectabilis/kg for various periods ranging from 3 to 35 days. The
earliest demonstrable change in the pulmonary arterial system of
the animals was seen on day 3 and consisted of the appearance of
muscle in normally non-muscular arteries of the lung. The muscular
pulmonary arteries began to show hypertrophy of the media from day
7, which reached statistically significant levels on day 10 in
smaller arteries and on day 14 in the larger arteries. Significant
right ventricular hypertrophy was seen on day 21. These changes
were confirmed by 3H-thymidine uptake studies (Meyrick & Reid,
1982).
6.4.2.3 Mechanisms of toxic action
Considerable progress has been made recently in the
understanding of biochemical and pharmacological changes that occur
in PA-induced lung disease.
Turner & Lalich (1965) and Takeoka et al. (1962) postulated
that pulmonary hypertension was mediated by the release of
5-hydroxytryptamine from the mast cells, which became hyperplastic
in the mediastinal lymph nodes and around bronchi and pulmonary
arteries (Turner & Lalich, 1965) (section 6.4.2.2) following
administration of monocrotaline, causing vasoconstriction. On the
other hand, Kay et al. (1967b) found that the number of mast cells
corresponded with the severity of exudative changes in the lung and
were not related to the genesis of pulmonary hyperplasia.
Besides the medial muscular hypertrophy of pulmonary arteries
reported in several studies cited above, swelling and lysis of the
endothelial cells, contributing to luminal narrowing and thickening
of the wall with fibrosis, have been described (Allen & Carstens,
1970).
Weanling rats are more susceptible to these changes than older
animals, and the changes follow a strict temporal sequence. Oral
administration of monocrotaline to rats at 20 mg/litre in drinking-
water produced a sequence of changes over 3 weeks, that included an
increase in lung mass, which was significant by day 9, stimulation
of pulmonary RNA and protein synthesis (maximal on day 10),
increased pulmonary arterial blood pressure (significant by day
10), and right ventricular hypertrophy by day 14 (Huxtable et al.,
1978; Lafranconi et al., 1984). The increase in the lung mass was
not accompanied by change in the total collagen content and was
contributed possibly by hypertrophy of endothelial cells, but the
increased mass of the right ventricle was associated with a 4-fold
increase in collagen content (Lafranconi et al., 1985).
An early event is inhibition of serotonin removal by pulmonary
endothelium (Huxtable et al., 1978). This phenomemon, combined
with the increased release of serotonin by mast cells that has been
observed, may be involved in the development of pulmonary
hypertension (Carillo & Aviado, 1969). Right ventricular
hypertrophy is blocked by propanolol, whereas the development of
pulmonary hypertension is unaffected (Huxtable et al., 1977).
Novel metabolites have been found to be released by livers perfused
with monocrotaline in vitro, and these metabolites block serotonin
transport in vitro, when perfused through isolated lungs
(Lafranconi & Huxtable, 1984). These data suggest that the slow
release of metabolites from the liver into the circulation
following low-level exposure to monocrotaline results in specific
inhibition of endothelial cell function (Huxtable et al., 1978).
The effect of monocrotaline treatment on pulmonary angiotensin
converting enzyme (ACE) activity in the rat is disputed. Hayashi
et al. (1984) observed a reduction in the ACE activity of pulmonary
tissue in pyrrolizidine-exposed rats in parallel with the
development of pulmonary alterations, while the ACE activity of the
plasma remained unchanged. However, other authors have reported
that, though the specific activity of ACE falls in the isolated
perfused lungs of monocrotaline-treated rats, or in lung
homogenates from such animals, when activity is expressed as total
activity per lung, there is no significant alteration in the lungs
of treated animals compared with those of untreated animals
(Huxtable et al., 1978; Lafranconi & Huxtable, 1983). Therefore,
the significance of changes in ACE activity is open to question.
Molteni et al. (1984) also found evidence of endothelial cell
damage by monocrotaline in their ultrastructural and biochemical
studies on rats. Eighty male Sprague Dawley rats were used; half
were administered monocrotaline at 20 mg/litre in the drinking-
water and half were given plain water. The average daily water
consumption was 35 ml/rat. Thus, the treated rats were estimated
to have received 2 mg/kg per day. Five animals each from the
treated and control groups were killed at intervals of 1 - 12 weeks
after the start of the study. The endothelial damage was measured
by ACE activity, plasminogen-activator (PLA) activity, and
prostacyclin (PGI2) production. These were correlated with
pulmonary arterial perfusion and ultrastructural changes in the
lung. In the treated groups, after an initial rise at 1 week, the
ACE activity showed a steady decline from 1 to 6 weeks, after which
it plateaued at 55% of normal. PLA activity did not change for 2
weeks, but decreased by 59 and 79% of the control value after 6 and
12 weeks, respectively. On the other hand, the PGI2 production
increased progressively reaching 140 and 270% of the control level
after 6 and 12 weeks, respectively. These endothelial functional
changes were not accompanied by significant changes in pulmonary
arterial perfusion as visualized by 99mTc-labelled macroaggregated
albumin perfusion studies. The activities of ACE and PLA and the
production of PGI2 are considered sensitive indices of endothelial
function in rats. The above results indicated endothelial cell
dysfunction. The ultrastructural studies also revealed oedema of
capillary subendothelial, perivenous and periarterial tissues at 1
week, and interstitial inflammatory infiltrates at 2 weeks. At
6 - 12 weeks, there was thickening of the pulmonary arteries and
enlargement of right side of the heart.
Stenmark et al. (1985) studied the role of alveolar
inflammation and arachidonate metabolism in monocrotaline-induced
pulmonary hypertension in rats. Five groups of male Sprague Dawley
rats were treated as follows: (a) 20 rats received 40 mg
monocrotaline/kg body weight, sc; (b) 20 rats received
monocrotaline, 40 mg/kg sc plus diethylcarbamazine (DEC) 100 mg/kg
sc, every 12 h; in addition, 250 mg DEC was added to 100 ml of
drinking-water. This treatment started 2 days prior to the start
of the study and was continued daily for 3 weeks; (c) 12 control
rats received normal saline plus monocrotaline at 40 mg/kg sc; (d)
12 rats received indomethacin at 2 mg/kg sc for 2 days, prior to
receiving monocrotaline at 40 mg/kg and then daily for 3 weeks; (e)
6 animals each received a single sc injection of normal saline and
served as additional controls.
One, 2, and 3 weeks after monocrotaline or saline injection,
lung lavage was carried out for cell counts and assay for enzyme
activity and cyclooxygenase metabolites, the degradation products
of prostacyclin (PGI2) and thromboxane A2(TXA2), as 6-keto-
prostaglandin (PGF1alpha) and TXB2, respectively. At 3 weeks, the
animals were anaesthetized, right ventricular pressures measured by
catheterization and the heart removed. The 2 ventricles were
separated and weighed for the determination of heart-weight ratio
(right ventricle/left ventricle + septums RV/LV + S) an indicator
of right ventricular hypertrophy.
The right ventricle showed hypertrophy at 2 weeks and the right
ventricular pressure was increased at 3 weeks following
monocrotaline administration (Fig. 11). The leukocyte count in the
lavage fluid increased at 3 weeks, with a rise in the percentage of
polymorphonuclear leukocytes and large, abnormal alveolar
macrophages in the test animals. B- N-acetyl-D-glucoseaminidose
activity was also elevated at 3 weeks, indicating activation of
leukocytes. There was also a rise in the concentration of 6-keto-
PGF1alpha at 1 and 3 weeks, as well as in TXB2 at 3 weeks, compared
with those in control animals.
The administration of DEC inhibited both the increase in heart-
weight ratio (RV/LV + S) and the increase in pulmonary artery
pressure (Fig. 11) that occurred 3 weeks after monocrotaline
administration, and reduced the percentage of polymorphonuclear
cells, abnormal alveolar macrophages, and hexoseaminidase activity
in the lavage fluid, compared with that from animals that had
received monocrotaline only. The rise in the levels of 6-keto-
PGF1alpha was inhibited ( P < 0.05) by 73% and that of TXB2 by 74%
in the lung lavage.
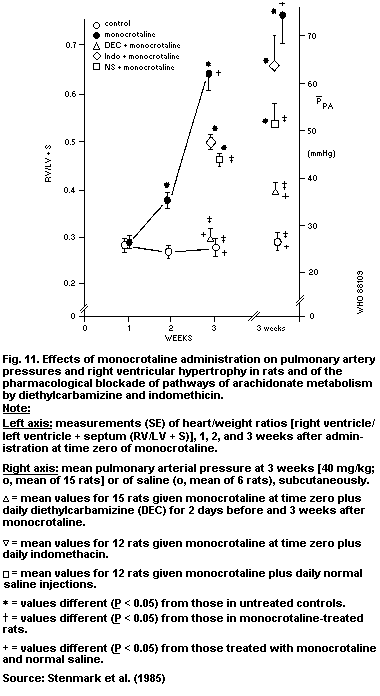 The administration of indomethacin did not have any effects on
either the heart-weight ratio or the pulmonary arterial pressure 3
weeks after monocrotaline administration (Fig. 11), but it
inhibited ( P < 0.05) the rises in 6-keto-PGF1alpha (by 90%) and
TXB2 (by 91%) that occurred in the lung lavage of monocrotaline-
treated animals at 3 weeks.
The above studies indicate that both the cyclo-oxygenase and
the lipo-oxygenase pathways of arachidonate metabolism are
activated by monocrotaline as early events in its toxic effect.
Activation of the cyclo-oxyenase pathway, demonstrated by increased
concentrations of the prostaglandin metabolites 6-keto-PGF1alpha
and TXB2 in lavage fluid, was inhibited by indomethacin, but this
inhibition did not prevent the monocrotaline-induced injury. DEC
attenuated both the inflammatory response and pulmonary
hypertension and inhibited the formation of slow reacting
substances including leukotriene D4. Since DEC produces a
pharmacological blockade of the lipo-oxygenase pathway, it seems
that the latter, rather than the cyclo-oxygenase pathway, is
responsible for perpetuating the pathophysiological mechanism
leading to monocrotaline-induced pulmonary hypertension.
Hilliker et al. (1984) demonstrated that antibody-induced
thrombocytopaenia attenuates right ventricular hypertrophy induced
by monocrotaline in rats. In another study, Hilliker & Roth (1984)
also produced evidence that hydrallazine, a vasodilator and
inhibitor of platelet prostaglandin synthesis, dexamethason, an
antiinflammatory agent and inhibitor of phospholipase, and
sulfinopyrazone, an inhibitor of platelet prostaglandin synthesis
inhibited monocrotaline-induced right ventricular hypertrophy in
rats, supporting the hypothesis that platelets and vasoconstrictor
agents play a role in monocrotaline-induced pulmonary hypertension.
Likewise, prior chemical sympathectomy with 6 hydroxydopamine
(100 mg/kg) or inhibition of serotonin synthesis with
p-chlorophenylalanine (500 mg/kg) reduced the degree of
monocrotaline-induced right ventricular hypertrophy in rats, but
did not prevent or reduce pulmonary vascular muscularization
(Tucker et al., 1983). Thus, the sympathetic nervous system and
serotogenic mechanisms seemed to be involved in the development of
right ventricular hypertrophy, but not in the development of the
pulmonary vascular lesion induced by monocrotaline. Kay et
al. (1985) also demonstrated that pretreatment with
p-chlorophenylalanine, which inhibits 5-hydroxytryptamine (5HP)
synthesis, also significantly ( P < 0.05) reduced right ventricular
systolic pressure, right ventricular hypertrophy, and medial
thickness of muscular pulmonary arteries in monocrotaline-treated
rats. Similar observations were made in rats exposed to hypoxia.
It was therefore suggested that 5HP might play a role in
monocrotaline-induced or chronic hypoxic pulmonary hypertension.
The biosynthesis of rat lung polyamines, putrescine,
spermidine, and spermine, generally considered to be important
regulators of cell growth and differentiation, is increased prior
to the evolution of monocrotaline-induced pulmonary hypertension in
rats. Continuous administration of alpha-difluoromethylornithine
(DFMO), which is a highly specific irreversible inhibitor of
ornithine decarboxylase (DDC), a rate-limiting enzyme in polyamine
biosynthesis, attenuated the development of monocrotaline-induced
pulmonary hypertension in rats (Olson et al., 1984). This effect
was mediated by the DFMO, by inhibiting the synthesis of putrescine
and spermidine, and not by blocking the hepatic metabolism of
monocrotaline to pyrroles (Olson et al., 1985). Thus, it was
suggested that lung polyamine biosynthesis might be essential for
the expression of monocrotaline-induced perivascular oedema as well
as medial thickening in the development of monocrotaline-induced
pulmonary hypertension vascular disease.
On the basis of the preceding studies, mostly on the rat, the
mechanism of chronic long-term injury to the lung by monocrotaline
seems to be as follows. Within hours of PA administration, there
is damage to the pulmonary endothelial cells accompanied by
vascular leak leading to pulmonary oedema. Platelet aggregation
also occurs. The endothelial damage indicated by ultrastructural
and biochemical studies activates the production of prostacyclin
and lipogenic products, which mediate increases in vascular
permeability and inflammatory reaction. There is simultaneous
production of 5 hydroxytryptamine and several polyamines. The
injected monocrotaline is completely metabolized within hours, and
no significant quantity is found in the body at 24 h (Hayashi,
1966) and, though some active metabolites may still be detectable
by isotope studies, even at 14 days (Hsu et al., 1974), the rats do
not have any lung lesions. The slow evolution of vascular changes
suggests that it is not caused by monocrotaline but through
biological pathways activated by the initial injury.
Methylprednisolone (MP), which reduces acute lung oedema caused
by monocrotaline (MCT), has been shown to reduce MCT-induced
pulmonary hypertensive vascular changes in rats and the resultant
right ventricular hypertrophy (Langleben & Reid, 1985). Daily ip
administration of MP at 5 mg/kg body weight, was found to be more
effective than 2 large doses of MP at 30 mg/kg, 2 h before and 2 h
after a single sc injection of MCT at 60 mg/kg. It was suggested
that secondary changes, though triggered by the acute MCT injury,
become self sustaining and are more significant for vascular
structural remodelling.
Structural arterial remodelling with vasoconstriction, medial
hypertrophy of the muscular pulmonary arteries, and muscularization
of the pulmonary arterioles follow as late effects, resulting in
pulmonary hypertension and right ventricular hypertrophy of the
heart.
The results of the above studies suggest a direct toxic effect
of the alkaloid on the endothelial cells of the alveolar
capillaries and on the pulmonary arteries, as well as a pulmonary
hypertensive effect on the heart.
6.4.3 Effects on the central nervous system
The dominant signs of pyrrolizidine poisoning in horses are
neurological (Rose et al., 1957a,b; McLean, 1970). Similar signs
can also occur in cattle and sheep. It has been claimed that such
signs are probably non-specific secondary effects following primary
liver disease resulting in hyperammonaemia (Rose et al., 1957a).
However, neurological abnormalities in which animals walk in a
straight line until they come to an object, and then stand with
their heads pressed against the object, indicate specific lesions
in the central nervous system. Spongy degeneration of the central
nervous system occurs in cattle, sheep, and pigs (Hooper et al.,
1974; Hooper, 1975a,b).
Trichodesma alkaloids, in particular, appear to be neurotoxic.
There is a considerable body of literature in the USSR on
Trichodesma intoxication of mice, rabbits, and dogs, which has
been reviewed (Ismailov et al., 1970). Mice given Trichodesma
alkaloids subcutaneously at 0.5 mg/kg develop paresis of the hind
limbs within 12 - 17 days. Opisthotonus and clonic convulsions are
also seen. Doses of 10 - 15 mg/kg of alkaloids produces death in
all animals within 2 - 6 h, as the result of respiratory
depression. Higher doses produce immediate death.
6.4.4 Effects on other organs
Right ventricular hypertrophy, secondary to the primary effects
on the pulmonary arteries, and the resultant pulmonary hypertension
in animals treated with PAs or PA-containing plants have been dealt
with in section 6.4.2. Lalich & Merkow (1961) reported myocarditis
consisting of focal oedema and infiltration with a minimal number
of lymphocytes and mononuclear cells in some rats fed Crotalaria
spectabilis seeds mixed with the diet at a concentration of
0.13 - 2 g/kg. Treated groups consisted of 11 - 24 animals each;
there were 12 controls. The changes were seen in all groups of
animals, but the maximum number of rats (10 out of an unspecified
number of the group) showing these changes was in the group that
received 0.5 g/kg diet for 20 - 31 days. Generally, there was a
close correlation with the presence of pulmonary arteritis.
Renal changes have been described by a number of investigators.
Hayashi & Lalich (1967) observed mild to moderate changes in renal
glomeruli consisting of necrosis, capillary thrombosis, and
degenerative changes in the epithelial and mesangial cells,
thickening of interlobular arteries, and arterial thrombosis in
suckling, male Sprague Dawley rats given a single sc dose of
monocrotaline at 120 mg/kg body weight. Renal changes were seen,
to some extent, in all animals surviving for 41 - 47 days.
Carstens & Allen (1970) studied the effects of feeding
Crotalaria spectabilis seed on the rat kidney. Fifty male Sprague
Dawley rats were fed a diet containing ground Crotalaria
spectabilis seed at 0.2 - 0.8 g/kg for 8 months. The seeds were
estimated to contain approximately 3.5 g monocrotaline/kg; 10
animals served as controls. Renal changes were seen in 33/50
PA-treated rats. In 22 rats, over 75% of the glomeruli were
hyalinized and capsules thickened. In the less severely affected
kidneys, the glomerular basement membrane was thickened and
homogeneous deposits were seen in mesangial areas. Afferent
arterioles and interlobular arteries were markedly thickened. In
the most severely affected vessels, the internal elastic lamina was
necrosed and the larger arteries showed fibrinoid necrosis.
Renal tubular megalocytosis was the dominant lesion described
by Hooper (1974) in mice. Nine male white mice, 10 weeks of age,
were fed Senecio jacobaea, which contained a concentration of
alkaloids (jaconine, jacobine, and seneciphylline) of 2.7 g/kg and
a concentration of N-oxide of 0.9 g/kg, mixed with the diet. The
S. jacobaea was given at 100 g/kg diet for 9 weeks, before being
raised to 200 g/kg diet. Five animals served as controls. The
animals were killed from 63 to 193 days after the start of the
study. All treated animals, except 2 killed on day 63, showed
changes. The large cells occurred in both the proximal tubules and
the loop of Henle. Similar cells were seen in the alveolar and
bronchiolar epithelium. No glomerular lesions were described. The
author mentioned having seen the above changes in rats given
repeated sublethal injections of fulvine and spectabiline. On the
other hand, Kurozumi et al. (1983) observed glomerular lesions in
rats given a single injection of monocrotaline.
A variety of renal lesions has been observed in pigs, a common
pathological feature being renal megalocytosis, which was observed
in pigs poisoned by at least 4 different plant genera containing a
variety of toxic alkaloids (Harding et al., 1964; Peckham et al.,
1974) and has also been observed in wild pigs grazing in areas rich
in PA-containing plants in northern Australia (Hooper, 1978).
McGrath et al. (1975) described glomerular lesions in pigs given
Crotalaria spectabilis seed daily for 43 days. Severe renal
lesions comprising tubular dilatation, megalocytosis, and necrosis
of tubular epithelial cells with casts in the lumen, interstitial
and periglomerular fibrosis, and glomerular hyalinization were
reported by Hooper & Scanlan (1977) in pigs fed Crotalaria retusa
seeds containing monocrotaline. Renal megalocytosis has also been
reported in C. retusa poisoning in horses, sheep, and mice poisoned
by S. jacobaea but not by H. europaeum, and in vervet monkeys with
chronic retrorsine poisoning (Van der Watt et al., 1972).
Lesions have been reported in the stomach and intestines in
field and experimental animals after poisoning with pyrrolizidine
alkaloids, but are difficult to identify as specific PA injury.
Hooper (1975c) conducted studies on sheep, rats, and mice. In the
study on sheep, 12 male cross-bred lambs, 7 - 8 weeks of age, were
newly weaned on to a standard commercial calf grower diet.
Lasiocarpine was administered at the rate of 15 - 20 mg/kg body
weight every 2 - 4 days. Each animal was killed when in terminal
coma. Survival time ranged from 4 to 17 days. In the rat study,
young Wistar-Furth rats (sex not stated) weighing 150 - 200 g were
used. In one group of 11 rats, each animal received an ip
injection of lasiocarpine at the rate of 40 mg/kg body weight.
Three animals received isotonic saline. Animals were killed or
died 2 - 6 days after the injection. A second group of 13 rats
received a dose of 35 mg lasiocarpine/kg body weight; 4 control
animals received saline. All rats received a second injection
48 h later. They were killed 3 - 60 days after the second
administration of lasiocarpine. In the mouse study, 3 mature male
white mice received 6 injections each of lasiocarpine at the rate
of 45 mg/kg body weight followed by 4 injections of 90 mg/kg body
weight at 48-h intervals. There was one control animal.
All animals showed characteristic hepatic lesions. Sheep also
showed severe oedema, haemorrhage, and epithelial necrosis in the
gall bladder; lesions were also found in the central nervous system
and occasionally in the kidney. All animals showed severe
intestinal atrophy. There was inhibition of crypt cell mitosis
leading to mitotic irregularities, abnormal large cells and
syncitial cells, especially in the duodenum of sheep, and severe
villous atrophy with ulceration. Lesions in the intestines were
similar to those caused by radiation and radiomimetic agents. It
was suggested that the local intestinal radiomimetic effect was due
to local exposure to the pyrrole metabolite of lasiocarpine after
excretion through the bile duct. It was proposed that a more
conspicuous and rapid development of duodenal megalocytosis was due
to very rapid turnover of cells in the duodenum.
Other probably secondary effects included haemolysis in sheep
in association with advanced liver disease and high liver-copper
levels (Bull et al., 1956), anaemias and disturbance in iron
metabolism and haematopoiesis (Schoental & Magee, 1959; Schoental,
1963; Peckham et al., 1974; Hooper & Scanlan, 1977) (section
6.4.11), pancreatic oedema and fibrosis (Bras & Hill, 1956;
Schoental & Magee, 1959), cerebral oedema, haemorrhage, and
congestion in the rat brain (Davidson, 1935; Rosenfield & Beath,
1945).
Tumours in the different organs have been dealt with separately
under carcinogenesis (section 6.4.8).
6.4.5 Teratogenicity
The teratogenic potential of PAs was demonstrated by Green &
Christie (1961) who produced a variety of dose-related fetal
abnormalities in the rat, with a single intraperitoneal injection
of heliotrine administered during the second week of gestation.
The dosages ranged from 15 to 300 mg/kg maternal body weight.
Litters exposed to a dose of less than 50 mg did not show any
abnormalities, but abnormalities were observed in litters exposed
to higher doses, and increased in frequency and severity with
increasing dose. The abnormalities included retardation of
development, musculoskeletal defects, especially hypoplasia of the
lower jaw, cleft palate, and other abnormalities. Doses above 200 mg
resulted in the intrauterine death or resorption of many fetuses.
Similar studies were performed by Peterson & Jago (1980) who
compared the effects of heliotrine with its metabolic pyrrole
derivative dehydroheliotridine (DHH), when administered in a single
ip injection to rats on the 14th day of gestation. Heliotrine was
administered at 200 mg/kg body weight and DHH at 30 - 90 mg/kg, 14
days after conception. Effects on embryos, evaluated on the 20th
day, showed that both heliotrine and DHH retarded growth and were
teratogenic, but that the effects of a 40 mg/kg dose of DHH were
equivalent to those of 200 mg/kg heliotrine, i.e., the metabolite
was 2.5 times as effective on a molar basis. DHH produced a number
of skeletal abnormalities including retarded ossification,
distorted ribs, long bones, cleft palate, and feet defects. At
higher doses, growth almost ceased in many tissues and the fetuses
were very immature. However, the embryonic liver parenchyma did
not show the antimitotic effects of DHH.
The teratogenic properties of heliotrine were also demonstrated
in Drosophila larvae fed low levels of the alkaloid (Brink, 1982).
6.4.6 Fetotoxicity
The subject of fetotoxicity has been reviewed by Mattocks
(1986). Sundareson (1942) demonstrated the ability of
pyrrolizidine alkaloids to cross the rat placenta. Twice weekly
injections of the PA, starting at, or after, the 12th day of
gestation, resulted in premature delivery of some litters and many
were born dead. The same author showed that the alkaloids
themselves and not just the pyrroles formed in the dams' livers,
could pass the placental barrier by injecting senecionine into
19-day-old rat fetuses in utero, which produced the characteristic
toxic lesions in the dams. The fetuses were also found to be more
resistant to the lethal effects of the PA than the mother rats.
When 4 fetuses were each administered 1.25 mg of PA, representing
about 200 - 400 mg/kg body weight, which is much higher than the
LD50 for an adult rat, 3 of them were still alive after 2 days.
Green & Christie (1961) did not find any liver damage in fetuses
from pregnant rats given teratogenic doses of heliotrine. Only
mild liver damage was found in the embryo rats whose mothers had
been injected with PAs (heliotrine, lasiocarpine, retrorsine, or
monocrotaline) (Bhattacharya, 1965). In contrast, Schoental (1959)
demonstrated that lasiocarpine and retrorsine, when administered to
lactating rats produced little effect on the mothers, but produced
acute liver lesions in the suckling infants. The lesions were most
severe in 3- to 7-week-old animals. It was suggested that the
infants were affected by the milk from the lactating mothers, which
possibly contained the metabolic products of the PAs.
It would seem from the above studies that the embryo is
relatively more resistant to the toxic effects of PAs in utero than
it is after birth. Mattocks & White (1973) postulated that this
could be due to the low capacity for the metabolic activation of
PAs of the embryo liver, as they had shown that the ability of
liver enzymes to convert retrorsine to toxic metabolites was low in
rats, immediately after birth, but picked up rapidly afterwards.
The susceptibilities of rats of various ages to the hepatotoxic
effects of the PAs was proportional to their capacity to form and
retain the pyrrolic metabolites. Twenty-day-old rats were found to
be more sensitive than older animals.
The effects of fulvine administration on pregnant rats between
9 and 12 days of gestation were studied by Persaud & Hoyte (1974).
Dose-related fetal resorptions were observed, but no hepatic
lesions were seen in the fetuses. On the other hand, Newberne
(1968) observed damage, in both the maternal and fetal livers, when
lasiocarpine was administered to pregnant rats. Acute liver
necrosis was observed in the livers of mothers as well as fetuses
in animals that had received 100 mg lasiocarpine/kg body weight on
day 13 of gestation. However, in animals that received 2 doses of
35 mg/kg body weight on days 13 and 17 of pregnancy, liver necrosis
was seen in the fetal liver but not in that of the mother. It is
not known why lasiocarpine acts differently from other alkaloids
and has a greater effect on the fetal liver. Mattocks (1986) has
postulated the possibility that fetotoxicity was caused chiefly by
toxic metabolites formed in the maternal liver, and that a greater
proportion of such metabolites reached the fetus from lasiocarpine
than from other PAs.
6.4.7 Mutagenicity
A number of PAs that have been shown to be powerful dose
dependent mutagens in Drosophila melanogaster have been listed by
Mattocks (1986). All the compounds are hepatotoxic though the
degree of mutagenicity is not necessarily proportional. Table 12
provides a summary of the mutagenicity tests on different PAs,
related compounds and plant extracts. Clark (1959) demonstrated
the mutagenic effect of heliotrine in Drosophila, in which a
considerable increase in sex-linked recessive lethals was produced,
apparently by interfering with the maturation of germ cells, so
that as soon as the available spermatozoa were used, the males were
no longer capable of breeding. The cell damage was irreversible.
The mutagenic effect of feeding Drosophila males for 24 h with a
medium containing 10-3 mol monocrotaline was comparable to about
1000 R of X rays (Clark, 1976). The Basc test with Drosophila
melanogaster is considered a highly sensitive mutagenicity test
for PAs (Candrian et al., 1984a).
Seneciphylline and senkirkine, known to occur in animal feeds
and medicinal herbs, respectively, were tested for their ability to
produce sex-linked recessive lethals in males of Drosophila
melanogaster using the Basc (3-day feeding method) by Candrian et
al. (1984a). Seneciphylline was found to be mutagenic at
concentrations of 10-5, 10-4, and 10-3 mol, which produced 3.8%
(983 chromosomes tested), 9% (708 chromosomes tested), and 15.3%
(327 chromosomes tested) sex-linked recessive lethals,
respectively. Senkirkine (10-5 mol) was found to produce 4.4% sex-
linked recessive lethals (2541 chromosomes tested) against 0.17%
maximum sensitivity in the late spermatid stage of spermatogenesis
indicating that PAs act as indirect mutagens. Flies fed with milk
from lactating rats given an oral dose of 25 mg seneciphylline/kg
showed 1.2% sex-linked recessive lethals (1477 chromosomes tested)
compared with 0.3% (1533 chromosomes tested) in controls.
Table 12. Mutagenicity tests on pyrrolizidine alkaloids,
related compounds, and source plants
---------------------------------------------------------
Compound or material Type of testa Responseb
---------------------------------------------------------
Clivorine A +
HPC +
Echimidine D +
Echinatine D +
Fulvine D +
Heliotrine D +
P +
F +
CC +
A +
B +
TM +
CM/CC +
Integerrimine D +
Jacobine D ±
P +
Lasiocarpine D +
P +
F +
A +
HPC +
CM/CC +
TM +
Ligularidine A +
Lindelofine A 0
Lycopsamine A 0
Monocrotaline D +
P +
CC +
B +
A 0
HPC +
CT +
Petasitenine (fukinotoxin) A +
HPC +
CM +
---------------------------------------------------------
Table 12. (contd.)
---------------------------------------------------------
Compound or material Type of testa Responseb
---------------------------------------------------------
Platyphylline D 0
Retrorsine A +
D +
CT +
Rosmarinine CT 0
Senecionine D +
A 0
Seneciphylline A 0
P +
D +
Senkirkine A +
HPC +
CM/CC +
D +
Supinine D ±
P +
Mixed alkaloids from
Senecio jacobaea A 0
Senecio numorensis
spp. fuchsii (extract) CM +
A 0
A +
Senecio jacobaea (extract) A +
Senecio longilobus (extract) A 0
Symphytum officinale
(comfrey extract) A 0
Retronecine bis-p-chloro- P +
benzoate
Synthanecine A bis-N-ethyl- CT +
carbamate
Retronecine A 0
Heliotridine D 0
Viridofloric acid A 0
---------------------------------------------------------
Table 12. (contd.)
---------------------------------------------------------
Compound or material Type of testa Responseb
---------------------------------------------------------
Heliotric (heliotrinic) acid D ±
HPC +
Dehydroretronecine CT +
A ±
SCE +
Dehydroheliotridine CM +
Pyrrole HPC 0
2,3-Bishydroxymethyl-1- CT +
methylpyrrole A ±
SCE +
2-Hydroxylmethyl-1-methyl- A 0
pyrrole SCE ±
3-Hydroxylmethyl-1-methyl- A +
pyrrole SCE ±
---------------------------------------------------------
a A = Salmonella ("Ames") test.
B = Other bacterial tests.
CC = Clastogenic activity in cultured cells.
CM = Mutagenicity in cultered mammalian cells.
CT = Cell transformation test.
D = Mutagenicity in Drosophila.
F = Tests in fungus (Aspergillus nidulans).
HPC = Hepatocyte primary culture/DNA repair test.
P = Chromosomal aberrations in plant cells.
SCE = Sister chromatid exchange.
TM = Transplacental micronucleus test.
b + = active.
± = marginally active.
0 = inactive.
Mutagenic properties of 7 PAs extracted from plants to
Salmonella typhimurium TA100 have been demonstrated by a modified
Ames method by Yamanaka et al. (1979). The PAs were clivorine,
fukinotoxin, heliotrine, lasiocarpine, ligularidine, LXC201, and
senkirkine. Pre-incubation of these alkaloids with liver S9 mix
and bacteria in liquid medium was essential for demonstration of
the property. PAs in the heliotridine and otonecine family were
mutagens, while retronecine bases were inactive. Monocrotaline and
heliotrine were not active mutagens to Escherichia coli WP2, even
though they were quite cytotoxic (Green & Muriel, 1975). They were
active in repair deficient strains. Retrorsine was active in
inducing mutations on the Ames Salmonella/microsome assay (Wehner
et al., 1979). Extracts from medicinal plants and noxious weeds
were mutagenic towards Salmonella in the Ames assay (Pool, 1982;
White et al., 1983; Koletsky et al., 1978).
From the limited data available, it seems that the carcinogenic
activity of individual alkaloids parallels their mutagenic
behaviour, but not their relative hepatotoxicities (Culvenor &
Jago, 1979).
6.4.7.1 Chromosome damage
Pyrrolizidine alkaloids have been shown to be capable of
damaging chromosomes in plants, fungi, bacteria, tissue cell
cultures, and the fruit fly (Drosophila melanogaster). Literature
on this topic has been reviewed by Bull et al. (1968), McLean
(1970), and Mattocks (1986).
Several PAs are known for their ability to damage the
chromosomes of growing plant cells (Mattocks, 1986). Similar
properties have been demonstrated in leukocyte cultures from the
marsupial (Potorus tridactylus) (Bick & Jackson, 1968; Bick, 1970).
Bick & Culvenor (1971) found dehydroheliotridine, a metabolite of
heliotrine, to be 10 times more active than the alkaloid.
Infusions of Symphytum officinale L., described in Polish
pharmacopoeia as Radix symphyti, are recommended as expectorants,
especially for children. Furmanowa et al. (1983) demonstrated the
mutagenic effects of an alkaloidal fraction and infusion in this
plant in the meristematic cells of the lateral roots of Vicia faba
L. var minor. Lasiocarpine, a proven carcinogen, served as a
positive control.
Chromosome damage by PAs in the hamster lung cell line was
demonstrated by Takanashi et al. (1980). Stoyel & Clark (1980)
used the transplacental micronucleus test in pregnant female mice
and showed the chromosome damaging properties of heliotrine (225 mg/kg
body weight) and lasiocarpine (86 mg/kg) within 20 h of the injection.
The genotoxicity of heliotrine, monocrotaline, seneciphylline,
and senkirkine was studied by Bruggeman & Van der Hoeven (1985)
using the sister-chromatid exchange (SCE) assay in V79 Chinese
hamster cells co-cultured with primary chick embryo hepatocytes.
Exposure to these PAs resulted in the high induction of SCEs, a
more than 5-fold increase in the SCE rate with 2.5 mg
heliotrine/litre, 4-fold with monocrotaline at 5 mg/litre, 8-fold
with seneciphyline at 1.2 mg/litre, and more than 5-fold response
with senkirkine at 2.5 mg/litre. For all compounds, a dose-
response relationship was observed at concentrations that did not
seriously affect survival. PAs are also known to induce DNA repair
in rodent hepatocytes (Green et al., 1981; Mori et al., 1985). DNA
repair synthesis was elicited by 15 alkaloids, including 11 of
unknown carcinogenic potential (Mori et al., 1985).
There are also a few reports of chromosome damage by PAs in
man. Martin et al. (1972) found chromosome damage in the blood
cells of children with veno-occlusive disease, probably caused by
fulvine. It has also been shown by Ord et al. (1985) that
dehydroretronecine, is able to induce SCE in human lymphocytes.
Kraus et al. (1985) studied the PAs senkirkine and tussilagine,
which occur in a medicinal plant Tussilago farfara, for their
ability to induce chromosome damage in human lymphocytes in vitro.
They were not found to enhance the number of chromosome aberrations
up to concentrations of 1000 µmol. However, heliotrine, used for
comparison, induced chromosomal aberrations at concentrations of
100 µmol. In addition, heliotrine was also found to be capable of
damaging unstimulated eg Go-phase lymphocytes.
6.4.8 Carcinogenesis
Carcinogenesis has been reviewed by McLean (1970), IARC (1976,
1983), and Mattocks (1986). A number of purified PAs, purified or
crude extracts of plants containing them in a mixture or the actual
plant, dried and milled, and several PA metabolites or synthetic
analogue compounds have been tested for carcinogenecity. However,
these include only relatively few of the known cytotoxic PAs. Data
relating to some of the representative studies on rats are
summarized in Table 13. Studies on liver tumours found in rats
given PAs and plant materials are summarized in Table 14. All
experimental animal studies, with the exception of one on chickens
(Campbell, 1956) and one on Syrian golden hamsters (Fushimi et al.,
1978), have been carried out on rats.
The liver is the most common organ involved in experimental
studies. Tumours produced are mostly of epithelial origin, but a
significant number are also vascular. Lack of precision and
diversity of terms used to describe similar or identical tumours
makes it difficult to compare the types of carcinogenic effect in
different studies. Some terms have been used interchangeably,
e.g., hepatomas, hepatocellular carcinomas, haemangiogenic and
cholangiogenic tumours; nodular hyperplasia, pre-neoplasma,
neoplastic nodules, and hepatocellular tumours. In most studies,
there are no supporting photomicrographs to draw any inference as
to whether the tumours were malignant. Difficulties in the
interpretation of data have been commented on by Schoental et al.
(1954) and McLean (1970).
Lasiocarpine has produced the largest yield of tumours. In the
studies of Svoboda & Reddy (1972), 16/18 animals surviving for more
than 56 weeks after receiving ip multidoses of lasiocarpine
developed malignant tumours of the liver. Of these, 10 animals had
more than one tumour. Continuous feeding of rats on a regimen
containing lasiocarpine resulted in all animals (24/24) developing
tumours (NCI, 1978). In one study, a single oral administration of
retrorsine (Schoental & Bensted, 1963) to weanling rats resulted in
7 of the 29 animals that survived for more than one year developing
11 tumours of a wide variety, at least of 5 which were malignant.
It is of note that this PA is known to have caused two cases of
human toxicity together with riddelline in two cases, though the
total intake was proportionately lower (Stillman et al., 1977; Fox
et al., 1978; Huxtable, 1980) (Table 15).
Tumours produced covered a very wide range in unrelated tissues
and organs, for example, the pancreas, urinary bladder, pituitary,
bone, retro-peritoneal tissues, and skin, among others.
Hepatocellular carcinoma and haemangiosarcoma were the most common.
Crude extracts of plants or whole plants have also been
demonstrated to produce a variety of tumours. For example, Senecio
longilobus has been shown to produce tumours in a significantly
large proportion of experimental animals (Harris & Chen, 1970).
Hirono et al. (1973, 1976, 1978, 1979b) demonstrated the
carcinogenic properties of a number of plants, used as food in
Japan, in the rat.
A summary of the relevant experimental data follows.
6.4.8.1 Purified alkaloids
Kuhara et al. (1980) administered clivorine in the drinking-
water at a concentration of 0.05 g/litre to 12 rats of both sexes,
continuously for 340 days followed by plain water. There were 20
control rats. All the treated rats survived 440 days. Eight of
the 12 animals developed liver tumours including 2
haemangioendothelial sarcomas and 6 that were described as
"neoplastic nodules". No liver tumours were seen in the control
animals (Table 13).
Schoental (1975) tested the carcinogenic properties of
heliotrine, with and without prior administration of nicotinamide.
Nicotinamide protects against liver necrosis and so may enhance
tumour yeild, as shown by Rakietin et al. (1971), who studied
pancreatic tumours in rats given streptozootocin.
Heliotrine was administered intragastrically to 4 groups of 26
male weanling rats in 1 or 2 doses of 230 mg/kg and 300 mg/kg body
weight; 2 groups also received nicotinamide at 350 - 500 mg/kg body
weight, administered ip 10 - 15 min before, and 2.5 h after
administration of heliotrine, as per the dosing regimen shown in
Table 13. There were 8 controls. All animals administered the
higher dose of heliotrine (300 mg/kg body weight) died within 5
months of the PA administration. The livers showed lesions
characteristic of PA toxicity. No tumours were seen. Only 1 out
of 4 animals receiving heliotrine alone, survived 27 months and it
showed an islet cell adenoma as well as adenoma of pituitary, but
so did 3 of the 8 controls. In the group receiving 230 mg
heliotrine/kg and also treated with nicotinamide, 4 animals died
within 5.5 months (one with a fibrosarcoma and the other in a
moribund condition), chiefly from toxic liver disease, and two more
had to be killed. Of the 6 animals surviving more than 22 months
after heliotrine treatment, islet cell adenoma was seen in 3
together with other tumours as shown in the table. This tumour is
stated to be extremely rare in the animal strain used. Hepatoma
was seen in only one animal. The role of nicotinamide in this
study is not clear.
Table 13. Summary of data on the carcinogenic action of PAs and PA-containing plants in rats (in chronological order)
Material Strain, Route of Other Duration Period of Tumours Tumour Reference
tested number, administration treatment of observation produced incidence
and sex of and dosing treatment in
animals regimen surviving
animals
Alkaloids Wistar rat drinking-water 1 week until death only nodular Schoental
of Senecio (13 males, (0.05 g/litre) (males) hyperplasia et al. (1954)
jacobaea 12 females) 2 weeks of liver
(females);
followed by gap of
7 weeks
9 males, 0.03 g/litre, until
1 female 3 days/week death
(surviving)
Retrorsine 10 males, 0.03 g/litre, until hepatomas 4/14
4 females 3 days/week death
Isatidine 8 males, 0.05 g/litre
14 females followed by
0.03 g/litre, 20 months until death hepatomas 10/22
3 days/week
3 males, as above choline until hepatomas 3/7
4 females (0.5% in death
drinking-
water), 4
days/week
Table 13. (contd.)
Material Strain, Route of Other Duration Period of Tumours Tumour Reference
tested number, administration treatment of observation produced incidence
and sex of and dosing treatment in
animals regimen surviving
animals
Isatidine 2 males, single ip Schoental
(contd.) 3 females injection of et al. (1954)
2 mg in 0.2 ml
tricaprilyn
followed by
skin application 15 months 15 months hepatoma (?) 1/5
tion of 0.5%
solution, 3
days/week
controls not mentioned
(7 males,
7 females)
Retrorsine Porton single 400 r until death hepatomas 5/25 Schoental &
Wistar intragastric radiation Bensted (1963)
weanling dose of 30 mg/kg to 31/50 hepatocellular 1/25
rat body weight surviving
(50 males) for 100 carcinoma
days; 4/13 with
had head metastases
shielded
mammary 2/25
tumours
Table 13. (contd.)
Material Strain, Route of Other Duration Period of Tumours Tumour Reference
tested number, administration treatment of observation produced incidence
and sex of and dosing treatment in
animals regimen surviving
animals
Retrorsine lung carcinoma 1/25
(contd.)
renal 2/25
carcinomas
colonic 1/25
carcinomas
Porton as above as above splenic 1/25 Schoental &
Wistar rat haemangio- Bensted (1963)
(50 males) endothelioma
osteosarcoma 1/25
bone
leukaemia 1/25
"spindle cell" 1/25
tumour (neck)
Table 13. (contd.)
Material Strain, Route of Other Duration Period of Tumours Tumour Reference
tested number, administration treatment of observation produced incidence
and sex of and dosing treatment in
animals regimen surviving
animals
Retrorsine 95 males, single oral dose until death hepatomas 5/29
(contd.) 95 females of 30 mg/kg body
(weanling) weight mammary tumour 1/29
(controls)
lung carcinoma 1/29
splenic 1/29
haemangio-
endothelioma
uterine 1/29
carcinoma
Porton retroperitoneal 1/29 Schoental &
Wistar rat sarcoma Bensted (1963)
(controls)
squamous cell 1/29
carcinoma
(jaw)
Table 13. (contd.)
Material Strain, Route of Other Duration Period of Tumours Tumour Reference
tested number, administration treatment of observation produced incidence
and sex of and dosing treatment in
animals regimen surviving
animals
Retrorsine 6 males no PAs 400 r until death leukaemia 2/6
(contd.) (weanling) radiation
osteosarcoma 1/6
renal adenoma 1/6
10 males single partial until death hepatomas 2/9
(weanling) intragastric hepatectomy
dose (30 mg/kg squamous cell 1/9
body weight) carcinoma
(9 days after (jaw)
partial
hepatectomy)
Table 13. (contd.)
Material Strain, Route of Other Duration Period of Tumours Tumour Reference
tested number, administration treatment of observation produced incidence
and sex of and dosing treatment in
animals regimen surviving
animals
Mixed PAs Randomly single more than 1 pancreatic 1/15 Schoental et al.
from seeds bred from intragastric dose year? islet cell (1970)
of Porton at 500-1500 mg/kg adenomaa
Amsinckia Wistar body weight
intermedia weanling "papillary 1/15
(intermedine rat tumour"a of
medine and (15 males) urinary
lycopsamine) bladder
pituitary 1/15
adenomaa
as above as above pancreatic 1/15 Schoental et al.
islet cell (1970)
adenocarcinoma
exocrine 1/15
pancreatic
adenoma
Leaves and 2 males 10% mixed with 1 month until death pancreatic 1/2
stems of (weanling) diet (period not islet cell
Heliotropium stated) adenoma
supinum L.
Table 13. (contd.)
Material Strain, Route of Other Duration Period of Tumours Tumour Reference
tested number, administration treatment of observation produced incidence
and sex of and dosing treatment in
animals regimen surviving
animals
Heliotropium 6 males single until death pancreatic 1/6
supinum L. intragastric dose (longer islet cell
(contd.) at 200-300 mg/kg than 1 adenoma
body weight of year)
crude alkaloidal
fraction
controls not stated
Senecio Harlan rat 0.75% of diet until death none Harris & Chen
longilobus 50 males, within 131 (1970)
50 females days
50 males, 0.5% of diet until death none Harris & Chen
50 females within 200 (1970)
days
Table 13. (contd.)
Material Strain, Route of Other Duration Period of Tumours Tumour Reference
tested number, administration treatment of observation produced incidence
and sex of and dosing treatment in
animals regimen surviving
animals
Senecio 40 males, 0.5% of diet for 1 year 428-657 liver cell 4/23
longilobus 40 females 1 month days carcinomas
(contd.) alternating with
normal diet for peritoneal 1/23
2 weeks mesothelioma
50 males, 0.5% of diet 54 weeks 217-470 liver cell 16/47
50 females for 1 week days carcinomas
alternating with
normal diet for angiosarcoma 1/47
1 week
controls not stated
(10 males,
10 females)
Lasiocarpine Fischer rat intraperitoneal till 60-76 hepatocellular 10/18 Svoboda & Reddy
(25 males) injection at moribund weeks carcinomas (1972)
7.8 mg/kg body or had
weight, twice palpable
weekly for 4 tumours cholangio- 1/18
weeks, then carcinoma
once a week
for an additional lung adenomas 5/18
52 weeks
Table 13. (contd.)
Material Strain, Route of Other Duration Period of Tumours Tumour Reference
tested number, administration treatment of observation produced incidence
and sex of and dosing treatment in
animals regimen surviving
animals
Lasiocarpine skin squamous 6/18
(contd.) cell
carcinomas
Fischer rat as above ileal 2/18 Svoboda & Reddy
(25 males) adenocarcinoma (1972)
ileal 1/18
adenomyoma
testicular 1/18
interstitial
cell tumour
controls lung adenomas 2/25
(25 males)
Table 13. (contd.)
Material Strain, Route of Other Duration Period of Tumours Tumour Reference
tested number, administration treatment of observation produced incidence
and sex of and dosing treatment in
animals regimen surviving
animals
Retronecine, Porton single sc spinal cord 1/10 Schoental &
hydrochlorine Wistar injection of ependymo- Cavanagh (1972)
newborn rat 300-1000 mg/kg blastoma
(6 males, body weight
6 females)
"pituitary 5/10
tumour"
"mammary 1/10
tumour"
Hydroxy- 5 males single ip brain 1/5 Schoental &
senkirkine (weanling) injection of astrocytoma Cavanagh (1972)
100-300 mg/kg
body weight
Table 13. (contd.)
Material Strain, Route of Other Duration Period of Tumours Tumour Reference
tested number, administration treatment of observation produced incidence
and sex of and dosing treatment in
animals regimen surviving
animals
Heliotropium 5 females 5% of diet fed Schwann cell 1/5
ramosissimum to 1 pregnant tumour of
rat during 1st spinal cord
15 days of
pregnancy and
from 10th day
of parturition
until weaning;
female off-
spring (5) fed
on experimental
diet for 10 days
at 6 months of
age
controls not stated
Table 13. (contd.)
Material Strain, Route of Other Duration Period of Tumours Tumour Reference
tested number, administration treatment of observation produced incidence
and sex of and dosing treatment in
animals regimen surviving
animals
Groups
I II
Petasites ACI rat 4% of diet for until 480 days liver cell Hirono et al.
japonicus Group I: 6 months; death or until adenomas 6/27 4/19 (1973)
(young 12 males, subsequently, moribund
flower 15 females 8% of diet liver 3/27 8/19
stalks) alternating haemangio-
weekly with endotheliomas
normal diet
liver cell 2/27 1/19
carcinomas
Group II: 4% of diet
11 males,
8 females
controls normal diet none
7 females/
8 females
Table 13. (contd.)
Material Strain, Route of Other Duration Period of Tumours Tumour Reference
tested number, administration treatment of observation produced incidence
and sex of and dosing treatment in
animals regimen surviving
animals
Mono- Sprague gastric intubation 72 weeks liver cell 10/42 Newberne &
crotaline Dawley rat weekly of 25 mg/kg carcinomas Rogers (1973)
(50 males) body weight for
4 weeks, then
8 mg/kg body
weight for
38 weeks
50 males as above, with liver cell 14/33
a diet deficient carcinomas
in lipotropes
Heliotrine Porton intragastric Schoental (1975)
Wistar
weanling
rat
4 males 300 mg/kg body until death none
weight; repeated (5 months)
after 3 weeks to
2 surviving rats
Table 13. (contd.)
Material Strain, Route of Other Duration Period of Tumours Tumour Reference
tested number, administration treatment of observation produced incidence
and sex of and dosing treatment in
animals regimen surviving
animals
Heliotrine 6 males 300 mg/kg body nicotinamide until death none Schoental (1975)
(contd.) weight; repeated (500 mg/kg (5 months)
after 3 weeks body weight),
ip, before
and after
each heliotrine
administration
4 males 230 mg/kg body 27 months pancreatic 1/1
weight; repeated islet cell
to 2/4 surviving adenoma
after 5 days
pituitary 1/1
adenoma
Table 13. (contd.)
Material Strain, Route of Other Duration Period of Tumours Tumour Reference
tested number, administration treatment of observation produced incidence
and sex of and dosing treatment in
animals regimen surviving
animals
Heliotrine 12 males 230 mg/kg body nicotinamide until death fibrosarcoma 1/8
(contd.) weight; repeated (350 mg/kg or killed of jaw
6.5 days later body weight), when moribund
ip, before, up to 27.5 pancreatic 3/6
plus 2 doses months islet cell
after each adenomasb
heliotrine
administration hepatomab 1/6
urinary 1/6
bladder
papillomab
12 males testicular 1/6
interstitial
cell tumourb
2 males ip injection nicotinamide 19-27.5 pituitary 1/2 Schoental (1975)
at 350 mg/kg months adenoma
body weight
Table 13. (contd.)
Material Strain, Route of Other Duration Period of Tumours Tumour Reference
tested number, administration treatment of observation produced incidence
and sex of and dosing treatment in
animals regimen surviving
animals
Heliotrine controls no treatment 19-27.5 pituitary 3/6
(contd.) (6 males) months adenomas
Tussilago ACI rat 32% in diet for 600 days 600 days haemangio- 8/12 Hirono et al.
farfara (6 males, 4 days, then 16% endothelioma (1976)
(coltsfoot) 6 females) until end of
(preblooming (1.5 months study liver cell 1/12
flowers) old) adenomac
hepatocellular 1/12
carcinomac
bladder 1/12
papillomac
Table 13. (contd.)
Material Strain, Route of Other Duration Period of Tumours Tumour Reference
tested number, administration treatment of observation produced incidence
and sex of and dosing treatment in
animals regimen surviving
animals
Tussilago 5 males, 8% in diet 600 days 600 days liver 1/9
farfara 5 females haemangio-
(contd.) endothelioma
6 males, 4% in diet 600 days 600 days none Hirono et al.
5 females (1976)
controls none 600 days 600 days none
(8 males,
8 females)
Dehydro- Sprague sc injection partial 12 months 10 months rhabdomyo- 36/60 Allen et al.
retronecine Dawley rat bi-weekly for 4 hepatectomy sarcomas (5 (1975)
Group I: months at on 15 with
75 males 20 mg/kg body animals metastases)
weight, followed after 4
by 10 mg/kg body months
weight for 8
months
Table 13. (contd.)
Material Strain, Route of Other Duration Period of Tumours Tumour Reference
tested number, administration treatment of observation produced incidence
and sex of and dosing treatment in
animals regimen surviving
animals
Monocrotaline Group II: sc injection partial 12 months 10 months rhabdomyo- 2/60
75 males bi-weekly at hepatectomy sarcomas
5 mg/kg body on 15
weight for 12 animals hepatocellular 2/60
months after 4 carcinomas
months
acute myeloid 2/60
leukaemia
pulmonary 2/60
adenomas
controls sc injection partial 12 months 10 months none mentioned Allen et al.
(50 males) bi-weekly, hepatectomy (1975)
0.1 mol on 5 animals
phosphate after 4
buffer, pH7 months
Table 13. (contd.)
Material Strain, Route of Other Duration Period of Tumours Tumour Reference
tested number, administration treatment of observation produced incidence
and sex of and dosing treatment in
animals regimen surviving
animals
Monocrotaline Sprague sc injection on 12 months any tumours 17/60 Shumaker et al.
(contd.) Dawley rat alternate weeks (several (1976)
(60 males) (5 mg/kg body animals had
weight) more than 1
tumour)
pulmonary 11/60
carcinomas
hepatocellular 5/60
carcinomas
acute myeloid 3/60
leukaemia
rhabdomyo- 4/60
sarcomas
adrenal 8/60
adenomas
kidney adenoma 1/60
Table 13. (contd.)
Material Strain, Route of Other Duration Period of Tumours Tumour Reference
tested number, administration treatment of observation produced incidence
and sex of and dosing treatment in
animals regimen surviving
animals
Dehydro- 60 males sc injection on 12 months 12 months rhabdomyo- 39/60
retronecine alternate weeks until sarcomas at
at 20 mg/kg body moribund site of
weight followed injection
by 10 mg/kg
body weight,
alternate weeks
for 8 months
controls adrenal 2/45
(45 males) adenomas
(same group
as above)
Mono- Sprague single sc pancreatic 16/23 Hayashi et al.
crotaline Dawley rat injection of insulinomas (1977)
(80 males) 40 mg/kg body
weight
Petasitenine ACI rat drinking-water 45 days 72 days none Hirono et al.
(3 males) (0.05% solution) (1977)
(1 month
old)
Table 13. (contd.)
Material Strain, Route of Other Duration Period of Tumours Tumour Reference
tested number, administration treatment of observation produced incidence
and sex of and dosing treatment in
animals regimen surviving
animals
Petasitenine 5 males, 0.01% solution until up to 16 haemangio- 5/10d
(contd.) 6 females death months endothelial
or sarcomas
moribund
liver adenomas 5/10d
controls none fibrosarcoma 1/10 Hirono et al.
(10 males, (subcutaneous) (1977)
9 females)
Lasiocarpine Fischer rat mixed with 55 weeks 59 weeks liver 9/20 Rao & Reddy
(20 males) feed at a angiosarcomas (1978)
concentration of
50 mg/kg hepatocellular 7/20
carcinomas
malignant 1/20e
adnexal tumour
of skin
malignant 1/20e
lymphoma
Table 13. (contd.)
Material Strain, Route of Other Duration Period of Tumours Tumour Reference
tested number, administration treatment of observation produced incidence
and sex of and dosing treatment in
animals regimen surviving
animals
Lasiocarpine controls none
(contd.) (10 males)
Petasites ddN mice 4% of diet as 480 days 480 days lung adenomas 24/39 Fushimi et al.
japonicus 24 males dried flower (1978)
(flower 21 females stalks lung 6/39
stalks) adenocarcinoma
liver 4/39
reticullum
cell sarcoma
liver 1/39
haemangio-
endothelial
sarcoma
thymoma 1/39
leukemia 2/39
Table 13. (contd.)
Material Strain, Route of Other Duration Period of Tumours Tumour Reference
tested number, administration treatment of observation produced incidence
and sex of and dosing treatment in
animals regimen surviving
animals
Petasites control nil 480 days 480 days lung 1g
japonicus 23 males kidney
(contd.) 27 females haemangio-
endothelial
sarcoma 1g
spleen
haemangioma 1g
Swiss 4% diet as 480 days 480 days lung 5/26 Fushimi et al.
strain mice dried flower adenoma (1978)
20 males stalks
20 females leukemia 1/26
controls nil 480 days 480 days liver 1g
23 males haemangio-
20 females endothelioma
breast 1g
carcinoma
Table 13. (contd.)
Material Strain, Route of Other Duration Period of Tumours Tumour Reference
tested number, administration treatment of observation produced incidence
and sex of and dosing treatment in
animals regimen surviving
animals
Petasites lung adenoma 3g
japonicus
(contd.)
leukemia 2g
C57BL/6 4% diet as 480 days 480 days no tumours
mice dried flower
20 males stalks
20 females
controls none 480 days 480 days lung adenoma 1g
20 males
20 females
Syrian 4% diet as 480 days 480 days adrenal 1/25 Fushimi et al.
golden flower stalks cortical (1978)
hamsters adenoma &
13 males breast
17 females carcinoma
Table 13. (contd.)
Material Strain, Route of Other Duration Period of Tumours Tumour Reference
tested number, administration treatment of observation produced incidence
and sex of and dosing treatment in
animals regimen surviving
animals
Petasites controls none 480 days 480 days no tumours
japonicus 12 males
(contd.) 9 females
Symphytum ACI rat 33% of diet as 480 days until death liver adenomas 5/19 Hirono et al.
officinale (1 - 1.5 leaves or moribund (1979b)
(leaves or months old)
root) urinary 1/19
Group I.1: bladder
11 males, papilloma
8 females
urinary 2/19
bladder
carcinomas
(rats with 5/19
tumours)
Table 13. (contd.)
Material Strain, Route of Other Duration Period of Tumours Tumour Reference
tested number, administration treatment of observation produced incidence
and sex of and dosing treatment in
animals regimen surviving
animals
Symphytum Group I.2: 33% of diet as 600 days until death liver adenomas 11/19
officinale 10 males, leaves or moribund
(contd.) 10 females
urinary 2/19
bladder
papillomas
(rats with 11/19
tumours)
Group II: 16% of diet as 600 days until death liver adenomas 7/21 Hirono et al.
11 males, leaves or moribund (1979b)
10 females
haemangio- 1/21
endothelial
sarcoma
Table 13. (contd.)
Material Strain, Route of Other Duration Period of Tumours Tumour Reference
tested number, administration treatment of observation produced incidence
and sex of and dosing treatment in
animals regimen surviving
animals
Symphytum urinary 2/21
officinale bladder
(contd.) papillomas
urinary 1/21
bladder
carcinoma
lymphatic 1/21
leukaemia
colonic 1/21
adenoma
pituitary 1/21
adenoma
(rats with 7/21
tumours)
Table 13. (contd.)
Material Strain, Route of Other Duration Period of Tumours Tumour Reference
tested number, administration treatment of observation produced incidence
and sex of and dosing treatment in
animals regimen surviving
animals
Symphytum Group III: 8% of diet as 600 days until death liver adenoma 1/25 Hirono et al.
officinale 14 males, leaves or moribund (1979b)
(contd.) 14 females
Group IV: 8% of diet as until liver adenomas 19/22
12 males, root death
12 females
urinary 2/19
bladder
papilloma
(rats with 19/22
tumours)
Group V: 4% of diet as until until death liver adenomas 16/42
24 males, root reduced by death or moribund
24 females stages to basal
diet after 180 urinary 1/42
days bladder
carcinoma
Table 13. (contd.)
Material Strain, Route of Other Duration Period of Tumours Tumour Reference
tested number, administration treatment of observation produced incidence
and sex of and dosing treatment in
animals regimen surviving
animals
Symphytum adrenal 2/42
officinale cortical
(contd.) adenomas
(rats with 16/42
tumours)
Group VI: 2% of diet as 280 days liver adenomas 10/23
12 males, root for 190
12 females days reduced by
stages to basal
diet
Group VII: 1% of diet as until liver adenomas 16/24 Hirono et al.
15 males, root for 275 death (1979b)
15 females days and
subsequently a liver 4/24
basal diet haemangio-
alternating sarcomas
with 0.5% at
3-week intervals cholangio- 1/24
carcinoma
Table 13. (contd.)
Material Strain, Route of Other Duration Period of Tumours Tumour Reference
tested number, administration treatment of observation produced incidence
and sex of and dosing treatment in
animals regimen surviving
animals
Symphytum adrenal 1/24
officinale cortical
(contd.) adenomas
(rats with 17/24
tumours)
Group VIII: 0.5% of diet as entire liver adenomas 8/30
15 males, root study
15 females
liver 9/30
haemangio-
sarcomas
pituitary 1/30
adenoma
uterine 1/30
adrenocarcinoma
Table 13. (contd.)
Material Strain, Route of Other Duration Period of Tumours Tumour Reference
tested number, administration treatment of observation produced incidence
and sex of and dosing treatment in
animals regimen surviving
animals
Symphytum gonadal 1/30 Hirono et al.
officinale stromal tumour (1979b)
(contd.)
(rats with 10/30
tumours)
controls none till the urinary 1
(65 males, end bladder
65 females) papilloma
subcutaneous 1
fibrosarcoma
caecal adenoma 1
mammary 1
fibroadenoma
retroperitoneal 1
teratoma
Table 13. (contd.)
Material Strain, Route of Other Duration Period of Tumours Tumour Reference
tested number, administration treatment of observation produced incidence
and sex of and dosing treatment in
animals regimen surviving
animals
Senkirkine ACI rat ip injection of 4 weeks, 650 days liver adenomas 9/20 Hirono et al.
(20 males) 22 mg/kg body 52 weeks or till (1979a,b)
weight, twice moribund myeloid 1/20
weekly, then leukaemia
once weekly
testis
interstitial 1/20
tumour
Symphytine 20 males ip injection of 4 weeks, 650 days liver adenoma 1/20
22 mg/kg body 52 weeks or till
weight, twice death or liver 3/20
weekly, then moribund haemangio-
once weekly sarcomas
controls myeloid 1/20
(20 males) leukemia
Table 13. (contd.)
Material Strain, Route of Other Duration Period of Tumours Tumour Reference
tested number, administration treatment of observation produced incidence
and sex of and dosing treatment in
animals regimen surviving
animals
Symphytine fibroma-soft 1/20
(contd.) tissues
testis- 1/20
interstitial
tumour
Clivorine ACI rat drinking-water 340 days 480 days liver 2/12 Kuhara et al.
(6 males, (0.005%) haemangio- (1980)
6 females) sarcomas
ACI rat drinking-water hepatic 6/12
(6 males, (0.005%) neoplastic
6 females) nodules
testicular 3/12
interstitial
cell tumour
Table 13. (contd.)
Material Strain, Route of Other Duration Period of Tumours Tumour Reference
tested number, administration treatment of observation produced incidence
and sex of and dosing treatment in
animals regimen surviving
animals
Clivorine 10 males, pituitary 1/20
(contd.) 10 females adenoma
testicular 3/20f Kuhara et al.
interstitial
cell tumour
adreno- 1/20f
cortical
adenoma
pancreatic 1/20
aunar cell
adenoma
Table 13. (contd.)
Material Strain, Route of Other Duration Period of Tumours Tumour Reference
tested number, administration treatment of observation produced incidence
and sex of and dosing treatment in
animals regimen surviving
animals
Crude Sprague intragastric 104 weeks 114 weeks liver tumours 13/40 (2 Habs et al.
alkaloidal Dawley rat dose of 8 mg/kg (all types) males, 11 (1982)
extract (20 males, body weight, 5 females)
from 20 females) times per week
Senecio other tumours 11/40
numorensis (all types) (5 males,
fuchsii 6 females)
20 males, intragastric 104 weeks 114 weeks liver tumours 34/40
20 females dose of 40 mg/kg (all types) (5 males,
body weight, 5 29 females)
times per week
other tumours 10/40
(all types) (7 males,
3 females)
controls liver tumour 1/40
(20 males, (male)
20 females)
other tumours 10/40
(all types) (4 males,
6 females)
Table 13. (contd.)
Material Strain, Route of Other Duration Period of Tumours Tumour Reference
tested number, administration treatment of observation produced incidence
and sex of and dosing treatment in
animals regimen surviving
animals
Farfugium AC1 rat diet (20%) 480 days till death liver 6/29 Hirono et al.
japonicum (15 males, or end of haemangio- (1983)
(leaves 14 females) study sarcomas
and
stalks) liver adenomas 7/29
adrenalcortical 7/29
adenomas
adrenal 1/29
phaeochromo-
cytoma
urinary 2/29
bladder
papillomas
ACI rat diet (20%) testicular 2/29
(15 males, interstitial
14 females) tumours
ileal 1/29
adenocarcinoma
Table 13. (contd.)
Material Strain, Route of Other Duration Period of Tumours Tumour Reference
tested number, administration treatment of observation produced incidence
and sex of and dosing treatment in
animals regimen surviving
Senecio 15 males, diet (8%) none Hirono et al
cannabifolius 15 females survived (1983)
(leaves and > 240 days
stalks)
14 males, diet (4%) none
14 females survived
> 240 days
12 males, diet (1%) 480 days till dead liver 1/23
12 females haemangio-
sarcoma
liver adenomas 13/23
adrenal 5/23
cortical
adenomas
urinary 1/23
bladder
papilloma
Table 13. (contd.)
Material Strain, Route of Other Duration Period of Tumours Tumour Reference
tested number, administration treatment of observation produced incidence
and sex of and dosing treatment in
animals regimen surviving
animals
Senecio testicular 1/23
cannabifolius interstitial
(contd.) tumour
12 males, diet (0.2%) 480 days till death liver 8/24
12 females haemangio-
sarcomas
liver cell 3/24
adenomas
adreno- 3/24
cortical
adenomas
adrenal 1/24 Hirono et al.
phaeochromo- (1983)
cytoma
testicular 2/24
interstitial
tumours
pituitary 1/24
adenoma
caecal 1/24
fibrosarcoma
Table 13. (contd.)
Material Strain, Route of Other Duration Period of Tumours Tumour Reference
tested number, administration treatment of observation produced incidence
and sex of and dosing treatment in
animals regimen surviving
animals
Senecio controls basal diet 560 days cortical 3/49
cannabifolius (25 males, adenomas of
(contd.) 24 females adrenal
controls basal diet testicular 3/49 Hirono et al.
(25 males, interstitial (1983)
24 females) tumours
pituitary 2/49
adenomas
a These tumours were present in the same animal.
b Each coexisted in one animal (seen in animals surviving 22 - 27.5 months).
c Together with haemangioendothelioma of the liver.
d Two animals had both tumours.
e Found in the same animal with angiosarcoma.
f In the same animal.
g No data available on number of surviving animals in the control groups.
Note: Figures in column 2 indicate the number of animals used at the start of the study. In column 8, animals developing a tumour out of
the number surviving up to the end of study/or dying with tumour are indicated. Animals developing more than one tumour are
indicated separately in a footnote. Different terms have been used in different studies to describe the same tumour in the liver,
e.g., haemangiosarcoma, angiosarcoma, haemangioendothelioma, haemangioendothelial sarcoma, and haemangiogenic sarcoma.
Table 14. Liver tumours found in rats given PAs and plant materials (condensed results from various authors)a
Alkaloid or plant Alkaloid type Route of Number of Number and types of liver Reference
given Necine Type of administration rats at tumours found
ester autopsy
Clivorine otonecine macrocyclic oral 12 2 haemangiosarcomas Kuhara et al. (1980)
diester 6 neoplastic nodules
Lasiocarpine heliotridine "open" ip 18 11 hepatocellular carcinomas Svoboda & Reddy
diester oral 20 9 angiosarcomas (1972);
7 hepatocellular carcinomas Rao & Reddy (1978)
Monocrotaline retronecine macrocyclic oral 75 24 hepatocellular carcinomas Newberne & Rogers
diester (1973)
Petasitenine otonecine macrocyclic oral 11 5 haemangiosarcomas Hirono et al. (1977)
diester 5 adenomas
Retrorsine retronecine macrocyclic various 14 4 "hepatomas" Schoental et al.
diester (1954);
Schoental & Head
(1957)
Isatidine retronecine macrocyclic various 7 4 "hepatomas" and Schoental et al.
diester "nodular hyperplasia" (1954);
Schoental & Head
(1957)
Senecionine retronecine macrocyclic oral 80 19 hepatocellular tumours Habs et al. (1982)
(+ fuchsisenecionine) (platynecine) diester, 16 cholangiogenic tumours
monoester 12 "haemangiogenic" tumours
Table 14 (cont'd)
Alkaloid or plant Alkaloid type Route of Number of Number and types of liver Reference
given Necine Type of administration rats at tumours found
ester autopsy
Senkirkine otonecine macrocyclic ip 20 9 adenomas Hirono et al.
diester (1979a)
Symphytine retronecine "open" ip 20 1 adenoma Hirono et al.
diester 3 haemangiosarcomas (1979a)
Senecio longilobus macrocyclic oral 47 16 hepatocellular carcinomas Harris & Chen
(seneciphylline, (retronecine) diester 1 angiosarcoma (1970)
retrorsine, etc.)
Petasites japonicus macrocyclic oral 46 11 haemangiosarcomas Hirono et al.
(petasitenine) (otonecine) diester 10 adenomas (1973)
3 hepatocellular carcinomas
Tussilago farfara macrocyclic oral 12 8 haemangiosarcomas Hirono et al.
(senkirkine) (otonecine) diester (1976)
Symphytum officinale monoester + oral 175 81 adenomas Hirono et al.
(various) (retronecine) "open" 3 haemangiosarcomas (1978)
diester
Farfugium japonicum senkirkine oral 29 7 adenomas Hirono et al.
6 haemangioangiosarcomas (1983)
Senecio cannabifolius petasitenine oral 21 16 adenomas Hirono et al.
9 haemangioangiosarcomas (1983)
a From: Mattocks (1986).
Svoboda & Reddy and their group have carried out 2 studies
using lasiocarpine (Svoboda & Reddy, 1972, 1974; Rao & Reddy,
1978). Svoboda & Reddy (1972, 1974) gave repeated ip injections to
25 rats at a dose of 7.8 mg/kg body weight (0.1 LD50) for 56 weeks
as per regimen shown in Table 13. Three rats died of acute liver
necrosis in the initial 4 weeks. Eighteen rats survived 56 weeks,
by which time each animal had received an average cumulative dose
of 125 mg lasiocarpine. Of these, 16 animals developed a variety
of tumours 60 - 76 weeks after the beginning of the study (Table
13). Ten of the 16 animals had more than 1 tumour. Hepatocellular
carcinoma was the most common. The squamous cell carcinoma of the
skin was found to be transplantable. When the same PA, mixed with
the diet at the rate of 50 mg/kg (Rao & Reddy, 1978) (Table 13),
was administered to 20 rats for 55 weeks, 17 animals developed
tumours. Angiosarcoma of the liver emerged as the most common
tumour (9/20 animals), even though hepatocellular carcinomas were
also frequently seen (7/20 animals); squamous cell carcinoma of the
skin was not found, but there was a malignant adnexal tumour of the
skin. The average cumulative dose of the alkaloid was estimated to
be 190 - 200 mg per rat.
Monocrotaline has been studied for its carcinogenic activity in
rats by Schoental & Head (1955), Newberne & Rogers (1973), Allen et
al. (1975), Shumaker et al. (1976), and Hayashi et al. (1977),
using different routes of administration.
Allen et al. (1975) studied the long-term effects on rats of
repeated sc injections of monocrotaline or its major detectable
metabolite, dehydroretronecine. Male Sprague Dawley rats were
given biweekly injections of monocrotaline, at 5 mg/kg body weight
(75 animals) for 12 months, or dehydroretronecine at 20 mg/kg body
weight (75 animals) for 4 months followed by 10 mg/kg body weight
for 8 months. Fifty control animals received phosphate buffer
(Table 13). Partial hepatectomy was performed on 15 animals in
each of the treated groups and 5 in the control group. They were
observed for 10 months following cessation of the injections. Of
the 60 animals surviving in each of the treated groups, those
receiving monocrotaline showed rhabdomyosarcoma at the injection
site (2 animals), hepatocellular carcinoma (2 animals), acute
myelocytic leukaemia (2 animals), and pulmonary adenoma (2
animals). In the group receiving dehydroretronecine, 36 animals
developed rhabdomyosarcomas, and 5 of these animals developed
metastases. None of the control group developed tumours. Tissues
obtained from partial hepatectomies showed that both compounds
caused inhibition of mitotic division in regenerating liver. The
results of the study illustrate the dual alkylating and antimitotic
properties of these agents, commented on by Culvenor et al. (1969).
In a similar study by Shumaker et al. (1976), rats were
administered monocrotaline at 5 mg/kg body weight on alternate
weeks or its metabolite dehydroretronecine at 20 mg/kg body weight,
on alternate weeks for 4 months, followed by a dose of 10 mg/kg on
alternate weeks in the succeeding 8 months (Table 13). Of the 60
rats receiving monocrotaline, 17 developed one or more tumours, but
not until after the treatment was discontinued. The time interval
was not stated. The most common tumour was carcinoma of the lung
(11 animals) followed by hepatocellular carcinoma (5 animals). A
wide variety of other tumours was also seen. A notable feature was
that the metabolite dehydroretronecine did not produce any tumours
by systemic action, but only at the site of injection, where
significant numbers of rhabdomyosarcomas (39/60 animals) were seen.
The marked difference in tumour sites is explained by the fact that
the parent alkaloid monocrotaline has to be metabolized before it
becomes a carcinogen. For this reason, the tumours are distributed
in several organs of the body, whereas dehydroretronecine is itself
carcinogenic and so acts at the site of injection. When
monocrotaline was delivered in a higher dose, but as a single
subcutaneous injection (40 mg/kg body weight) (Hayashi et al.,
1977) to 40 rats there were no malignant tumours but only adenomas
of the islets in the pancreas in 16/23 surviving animals. The
results of the above studies indicate that monocrotaline is
tumorigenic, but the type of tumour and the malignancy both depend
on the route of administration and the dosage used.
Hirono et al. (1977) studied petasitenine, the pure alkaloid
isolated from the flower stalks of the plant, Petasites japonicus
Maxim, which has been found to be carcinogenic for rats (Hirono et
al., 1973). Two groups of ACI rats of both sexes, Group I of 3
animals and Group II of 11 animals, were given the alkaloid in in
the drinking-water, at concentrations of 0.5 g/kg and 0.1 g/kg,
respectively (Table 13). There were 19 controls. All 3 animals in
group I died within 72 days showing marked hepatocellular damage;
no tumours were seen. In Group II, 10 out of 11 rats survived for
more than 160 days. Eight of the 10 animals developed tumours -
liver cell adenomas (5) and haemangiosarcomas (5) (Table 10). Two
animals had both types of tumours. The authors concluded that the
carcinogenicity of the plant was due to petasitenine.
Retrorsine and its N-oxide (isatidine) have been used in
several studies. Schoental et al. (1954) administered retrorsine
at a concentration of 3 mg/litre in the drinking-water to 14 rats
and isatidine at concentrations of 5 mg/litre followed by 3 mg/litre
to 22 rats until death. Twenty-five rats were administered the
alkaloid mixture from Senecio jacobaea Lin at a concentration of
500 mg/litre followed by 300 mg/litre in the drinking-water. Dosing
regimens are shown in Table 13. One group of 7 animals receiving
isatidine received supplementary 0.5% choline in the drinking-water
and another group of 5 animals received isatidine 2 mg as a single ip
administration in 0.2 ml of tricaprilyn, followed by skin application
of alkalids as 0.5% solution for 3 days/week.
The animals receiving mixed alkaloids of the plant showed
extensive liver damage followed by marked nodular hyperplasia; no
tumours developed. The nodules were earlier interpreted as
hepatomas, but later only as an early stage in the progression from
hyperplasia to neoplasia.
The retrorsine group showed extensive liver damage associated
with cirrhosis and nodular hyperplasia. In 4 rats, they were
interpreted as hepatomas.
In the isatidine group, 10 out of the 22 rats developed
hepatomas. Tumours were present in 3 out of 7 animals receiving
isatidine plus choline indicating that the latter had no protective
role. One out of the 5 animals receiving the alkaloid ip and then
through dermal application developed a tumour, which was also
interpreted as a hepatoma.
In another study (Schoental & Bensted, 1963), 95 weanling rats
were administered a single dose of retrorsine at 30 mg/kg body
weight, by stomach tube (Group II). One comparable group of 50
weanling rats (Group I) received 400 r radiation in animals
surviving 100 days after retrorsine administration (31/50). A
third group (Group III) of 6 animals received 400 r radiation
alone. Another group of 10 weanling rats received the PA, 9 days
after partial hepatectomy (Group IV) (Table 13). The additional
treatments were given to study whether they would act as
co-carcinogens and induce neoplasia in hepatocytes, which are known to
show injurious effects for long periods following PA treatment. In
Group I, 19 of the 50 animals receiving a single dose of the PA
died before radiation could be given. Of the 31 remaining animals
that received radiation after the PA, 25 survived 12 months. Among
these, 19 tumours of a wide variety were seen (Table 13). Of the 6
tumours in the liver, only 1 was malignant, having metastasized.
Most of the other tumours were malignant, including those of the
breast, one of which had also metastasized.
In Group II, 29 out of 95 animals that had received one dose of
PA and no radiation survived for more than a year, with a mean
survival time of 23 months. Among these, 7 animals developed
tumours of a wide variety (Table 13). Five tumours in the liver
were benign. Most of the others were malignant. Two tumours, a
cystic tumour of the breast and a carcinoma of the uterus, were
present in one animal. Tumours seen in Groups III and IV, which
were found in animals surviving 12 months or more after the start
of the study, are shown in the Table 13. The 2 tumours of the
liver in Group IV were also benign. The number of control animals,
if any, were not indicated.
The authors concluded that the above studies did not provide
definite evidence of synergistic action in the carcinogenicity of
retrorsine by whole body radiation or partial hepatectomy.
Hirono et al. (1979a) studied the carcinogenic properties of
senkirkine extracted from the dried milled buds of Tussilago
farfara (coltsfoot) and symphytine extracted from dried milled
roots of Symphytum officinale (comfrey). Both Tussilago farfara
(Hirono et al., 1976) and Symphytum officinale (Hirono et al.,
1978) had earlier been demonstrated to have carcinogenic
properties.
Sixty inbred ACI strain male rats were divided into 3 groups of
20 animals each and received repeated ip injections of senkirkine
at 22 mg/kg body weight or symphytine at 13 mg/kg body weight as
per schedule given in Table 13. All animals treated with
senkirkine survived 290 days. Nine out of 20 rats developed liver
adenomas mostly after 350 - 450 days from start of the study.
Cirrhosis of liver was frequently observed. Of the symphytine
group, all animals survived 330 days after the start of the study.
Three animals developed haemangioendothelial sarcoma and one,
liver adenoma. The sarcomas were noted at least 518 days after the
start of the study.
Schoental & Cavanagh (1972) used two alkaloids, retronecine and
hydroxysenkirkine isolated from Crotalaria laburnifolia, which was
injected ip in single doses ranging from 100 to 300 mg/kg body
weight in 5 weanling Porton Wistar rats (Table 13). One animal
that had received the 300 mg/kg dose developed astrocytoma of the
brain after 14 1/2 months. Retronecine hydrochloride was
administered in doses ranging from 300 to 1000 mg/kg body weight by
single subcutaneous injection to 10 new-born rats. One male rat,
which was found to be paraplegic 6.6 months after receiving a dose
of 600 mg PA/kg, was also found to have ependymoblastoma of the
spinal cord. Among the litter mates of this rat, 1 male that had
received a dose of 1000 mg/kg died. The remaining 6 females and 2
males were killed within 22 months of being dosed. Of the females,
5 had pituitary tumours and 1 had a mammary tumour (type not
stated). Two males did not show any significant abnormalities.
One group of 5, 6-month-old female rats, born to dams that had
been fed on a diet containing 50 g dried and powdered Heliotropium
ramosissimum/kg diet, a tribal remedy, used during pregnancy and
parturition, were then themselves fed on the same diet at 6 months
of age, as per the regimen indicated in Table 13. One female rat
was found to be paraplegic at 7 months of age. A tumour, possibly
of Schwann or satellite cell origin, was found.
6.4.8.2 Plant materials
A number of plant materials have been tested for their
carcinogenicity by administering either a mixture of extracted
alkaloids (Cook et al., 1950; Schoental et al., 1954) or, more
often, the dried and milled plant mixed with the diet. The only
study in which the plant alone was fed was that of Campbell (1956),
who produced tumours in chickens by feeding dried and milled
Senecio jacobaea plant. It is notable that the plants tested are
almost all those that have been reported to be used as herbal
medicines and/or food, some of which have been reported to cause
human toxicity.
Schoental et al. (1970) used mixed PAs (intermedine and
lycopsamine) extracted from seeds of Amsinckia intermedia, known to
cause livestock losses in the USA, and leaves and stems from
Heliotropium supinum L., known to be used by women in East Africa
as a herbal medicine, after childbirth. The dosing regimen and
mode of administration are indicated in Table 13. Of the 15 male
weanling rats that had received a single treatment with the
Amsinckia PAs and survived for more than 1 year, 3 rats showed one
adenoma, one adenocarcinoma of the islet cells, and one adenoma of
the exocrine pancreas, respectively (Table 13). In addition, one
of the animals also developed a pituitary adenoma and papillary
tumour of the urinary bladder. Eight animals received the
treatment with Heliotropium supinum L. (Table 13). One out of the
2 weanling rats fed on the plant with the diet and one out of the 6
animals that received a single intragastric dose of the crude
alkaloid fraction developed islet cell adenoma of the pancreas.
The number of control animals, if any, used in the study is not
stated.
Harris & Chen (1970) tested the carcinogenicity of Senecio
longilobus, which has been associated with cases of human toxicity
(Stillman et al., 1977; Huxtable, 1980; Fox et al., 1978). Harlan
rats were divided into 4 groups (equal numbers of both sexes) and
fed diets containing dried and powdered stems and leaves of the
plant in the proportion of 5 -7.5 g/kg. The number of animals in
each group and the feeding regimen are shown in Table 13.
Continuous feeding did not produce any tumours, presumably because
of the comparatively low survival rate of animals. Significant
results were obtained when the animals were fed contaminated diet
alternating with normal diet (Group IV). Of the 100 animals fed on
this regimen for 54 weeks, 47 survived for more than 200 days.
Seventeen of these animals developed malignant tumours in the
livers - hepatocellular carcinomas in 16 and an angiosarcoma in 1
animal after a minimum feeding period of 217 days. In Group III,
fed a contaminated diet for 1 year, 23 rats lived for more than 200
days. Four rats (3 males and 1 female) developed hepatocellular
carcinomas and one, a peritoneal mesothelioma, after a minimum
feeding period of more than 428 days. The tumour-bearing animals
were predominantly male. The authors emphasized the relative
rarity of liver tumours in the strain of animals used. Results
demonstrated that S. longilobus is carcinogenic for rats.
For a study of Schoental & Cavanagh (1972), using dried and
powdered Heliotropium ramosissimum, see section 6.4.8.1.
Hirono et al. tested the carcinogenic effects of 3 widely-used
herbs containing PAs. Hirono et al. (1973) studied the possible
carcinogenic effects of the young flower stalks of Petasites
japonicus Maxim, which has long been used in Japan as food or
herbal remedy as well as the PA, petasitenine, isolated from it.
The flower stalks of the plant were dried, milled, and fed to young
ACI rats of both sexes mixed with the basal diet in the proportion
of 40 - 80 g/kg, as indicated in the feeding regimen in Table 13,
until the animals were moribund or dead. One group of 27 rats was
fed the plant mixed in the proportion of 40 g/kg diet for 6 months
followed by 80 g/kg diet on alternate weeks. The second group of
19 rats was fed a contaminated diet (40 g/kg) continually. Three
animals in Group I died of pneumonia. Eleven out of the remaining
24 rats in this group developed liver tumours after 15 - 16 months
of feeding. There were 2 hepatocellular carcinomas, 6 liver cell
adenomas, and 3 haemangiosarcomas, of which 2 had metastasized. In
Group II, 2 animals died from non-tumorous causes. Of the
remaining 17, 8 developed haemangiosarcomas, 4 liver cell adenomas
and 1 had a hepatocellular carcinoma. The incidence of
haemangiosarcoma was statistically higher in Group II.
In a similar study on mice and hamsters (Fushimi et al. (1978),
groups comprising 20 - 24 male and 20 - 21 female 6-week-old ddN,
Swiss, and C57BL/6 mice, and 13 male and 17 female hamsters, were
fed a diet combining 4% young, dried, and milled flower stalks of
Petasites japonicus Maxim for 480 days. All surviving animals were
killed at the end of the study. Lung adenomas and adenocarcinomas
were found in 30/39 surviving male and female ddN mice combined
(compared with 1/50 in the respective controls) in addition to
other tumours (Table 13). No significant differences in tumour
incidence were observed between treated Swiss and C57BL/6 mice and
hamsters, and the corresponding controls. No data were given on
the tumour incidence according to sex, in treated and control
animals or on survival in the controls.
In studies on rats (Hirono et al., 1976), the dried and
powdered flower buds of Tussilago farfara (coltsfoot) were mixed
with the diet. Forty-nine inbred ACI strain rats were divided into
4 groups and fed diets containing 40 - 320 g T. farfara/kg for up
to 600 days. The number of animals in each group and the dosage
and feeding regimen are given in Table 13. Two groups receiving a
diet containing 80 - 320 g/kg on a continual basis developed
tumours in the liver of the same types as animals in the studies
using Petasites japonicus, e.g., haemangioendotheliomas, liver
cell adenomas, and hepatocellular carcinomas (Table 13). All 12
animals in Group I survived for more than 380 days after the start
of the study. Of these, 8 (5 males, 3 females) developed
haemangioendothelial sarcoma of the liver. In addition, 3 of the 8
rats developed simultaneously hepatocellular adenoma, hepatocellular
carcinoma, or urinary bladder papilloma. In Group II receiving
coltsfoot at 80 g/kg in the diet, 9 out of 10 animals that survived
for more than 420 days developed a haemangioendothelial sarcoma.
Hirono et al. (1978) fed Symphytum officinale, similarly dried
and milled, to the ACI strain of inbred rats of both sexes at
different levels ranging from 80 to 330 g/kg as leaves or 5 - 40 g/kg
as root, for 280 days or more. Eight groups of animals of
19 - 48 animals each were used on different regimens of feeding.
The feeding regimen, the number of animals in each group, and the
duration of treatment are given in Table 13. Sixty-five males and
64 females served as controls. Tumours were induced in all groups
receiving leaves or roots. The most common tumour was liver
adenoma. Haemangiosarcomas were observed but infrequently (3
animals in the whole study). No carcinomas of the liver were seen,
but there was a wide variety of other tumours. It is noteworthy
that, in Group VII, 14 of 15 animals fed the lowest dose of 10 g/kg
for 275 days followed by 5 g/kg or basal diet alternating every 3
weeks, developed tumours, 2 of which were malignant (haemangiosarcomas).
In the control group, single animals each had papilloma of urinary
bladder, caecal adenoma, subcutaneous fibrosarcoma, mammary
fibroadenoma, or retroperitoneal teratoma. The livers of animals that
did not have the tumours showed other features commonly encountered in
animals administered PAs, e.g., megalocytosis, liver cirrhosis,
hyperplastic nodules, etc., suggesting that they were induced by the
PAs contained in the plant. The authors concluded that the
carcinogenic activity of this plant was weaker than that observed in
animals fed Petasites (coltsfoot). Hirono et al. (1979b) have
summarized the above studies on PAs found in edible plants in Japan.
Habs (1982) and Habs et al. (1982) tested a crude alkaloid
extract from the plant Senecio numorensis sp. fuchsii containing
fuchsisenecionine (500 g/kg) and senecionine (10 g/kg). The
extract was administered intragastrically in 2 doses of 8 mg and
40 mg/kg body weight, respectively, to 2 groups of rats of both sexes,
comprising 40 animals each, 5 times a week for 104 weeks (Table
13). A large, dose-related number of liver tumours was produced,
originating in the liver cell and the sinusoidal system, and
predominantly affecting the female animals. The tumour incidence
was higher in Group II (dose, 40 mg/kg) with 34 tumours compared
with 13 in Group I. In Group I (dose, 8 mg/kg), 2 tumours were
found in 20 males compared with 11 tumours among 20 female rats.
Similarly, in Group II, only 6 tumours were found in 20 males
compared with 29 among 20 female rats. The tumours included 19 of
"hepatocellular origin", 16 of "cholangigenic origin", and 12 of
"haemangiogenic origin" (Table 14). Besides the liver tumours, 12
males and 9 females in the treated groups and 4 males and 6 females
in the control group developed a variety of extra-hepatic tumours.
Senecionine is known to be hepatotoxic and capable of being
converted in the rat liver into cytotoxic metabolites.
Fuchsisenecionine is a saturated PA, not previously known to be
cytotoxic. It is therefore likely that senecionine was the
hepatotoxic component and that perhaps the two PAs acted
synergistically with each other, though there is no actual evidence
for synergism. However, this needs confirmation. Moreover, there
appeared to have been other unknown components in the mixture
tested (Mattocks, 1986).
Hirono et al. (1983) studied the carcinogenicity of 2 more
plants (Farfugium japonicum and Senecio cannabifolius) of the tribe
Senecioneae in the family Compositae, the leaves and stalks of
which are used in Japan as human food. Fresh leaves and stalks of
the plants were dried, milled, and mixed with the basal diet.
Inbred strain ACI rats of both sexes, with preponderance of
females, 1.5 months old, were divided into 6 groups. They were fed
diets containing various proportions of the dried plant materials,
as indicated in Table 13. The study was terminated at 480 days,
except for one group, which was studied for 560 days. Besides the
groups shown in the tables, 2 more groups of 30 and 28 animals of
equal numbers of both sexes were fed 8% and 4% of Senecio
cannabifolius, respectively. None of these animals survived more
than 177 days and all died of hepatotoxicity. A wide range of
tumours was observed, mostly in the liver, as shown in Table 13,
the most common being haemangiosarcomas, which were not encountered
in the control group.
The carcinogenicity of Farfugium japonicum is considered to be
due to senkirkine and petasitenine. Senecio cannabifolius contains
senecicannabine, a new macrocyclic PA, seneciphylline, and
jacozine. It is probable that the carcinogenicity of Senecio
cannabifolius is due to these PAs (Hirono, et al., 1983).
Hayes et al. (1985) studied the biological mechanisms by which
PAs initiate carcinogenesis in male Fischer 344 rats. Lasiocarpine
(single or double injection of up to 80 µmol/kg body weight) and
senecionine (single or double injection of up to 160 µmol/kg) were
inactive as initiators of Y-GT-positive nodules in rats exposed to
2-acetylaminofluorene and partial hepatectomy. Administration of
lasiocarpine or senecionine 12 h after partial hepatectomy resulted
in the development of very few nodules. Lasiocarpine given in a
single or double dose (up to 80 µmol/kg) delayed hepatic
regeneration by at least 8 weeks after partial hepatectomy, and
pretreatment with this PA reduced the initiating capacity of
diethylnitrosamine and N-nitrosomethylurea in rats subsequently
selected with 2-acetylaminofluorene and partial hepatectomy.
Resistant nodules selected with lasiocarpine also had the typical
resistant nodule phenotype (positive for Y-GT and epoxide
hydrolase) and also lacked PA-induced megalocytosis. Lasiocarpine
treatment also resulted in small regenerative nodules that were
distinct from resistant nodules, because they were negative for
Y-GT and epoxide hydrolase.
6.4.8.3 Pyrrolizidine alkaloid metabolites and analogous synthetic
compounds
The subject has been reviewed by Mattocks (1986). The
pyrrolizidine alkaloids are converted into pyrrolic esters in the
hepatocytes. These may then be hydrolysed into pyrrolic alcohols,
which are more water soluble and less active than the esters. The
esters may be widely distributed throughout the body. Some of
these compounds and their analogues have been tested for
carcinogenicity.
(a) Pyrrolic esters
(i) Dehydromonocrotaline (monocrotaline pyrrole)
Mattocks & Cabral (1979) tested dehydromonocrotaline on the
skin of mice. In their first study on male BALB/c mice, they
applied the compound on the back at 1- to 2-week intervals.
Thirty-three applications of 1 µmol did not produce any skin
tumours in 16 mice, but 2 developed lung adenomas; no tumours
occurred in 14 control mice treated with the solvent (acetone). In
the second study (Mattocks & Cabral, 1982), 11 female LACA mice
each received 47 applications of 2.5 µmol dehydromonocrotaline; one
animal developed a malignant skin tumour. A second batch of 10
mice was given similar treatment and subsequently received 61
twice-weekly applications of croton oil at the same site. Half of
these animals developed tumours. In the control group of 10 mice,
only 1 tumour was seen. The results of these studies indicate that
dehydromonocrotaline requires the action of a promoter to manifest
carcinogenic potential.
(ii) Dehydroretrorsine
This supposed pyrrolic metabolite of retrorsine was applied to
the skin of male BALB/c mice at 1- to 2-week intervals; 33
treatments of 0.5 or 1 µmol failed to produce tumours in 15 mice
that survived for up to 60 weeks (Mattocks & Cabral, 1979).
(iii) 1-Methyl-2,3-bistrimethylacetoxymethylpyrrole
Mattocks & Cabral (1979, 1982) made 2 studies on this compound.
In the second study, which was more significant, 22 female LACA
mice received 47 dermal applications of 0.5 µmol each. This caused
marked skin damage with ulceration and scarring. Malignant skin
tumours developed in 19 out of 21 surviving animals. Two
hydrolysis products of this ester, pivalic acid and 1-methyl-2,3-
bishydroxymethylpyrrole, were similarly tested (Mattocks, 1986).
Tumours were produced, though fewer.
The results of the above studies suggest that the intact
pyrrolic ester is a carcinogen.
(b) Pyrrolic alcohols
Studies have been conducted using dehydroretronecine
(retronecine pyrrole) and dehydroheliotridine, which are secondary
metabolites of monocrotaline and heliotridine-based PAs,
respectively. Originally they were regarded as (+)- and (-)-
forms, respectively, of dihydro-7-hydroxy-1-hydroxy-methyl-5H-
pyrrolizine. Kadzierski & Buhler (1985, 1986) showed that the
metabolite from monocrotaline is racemic and concluded that the
product from all heliotridine and retro-necine esters is the same
(±)- form.
Johnson et al. (1978) painted the skin on the back of 16 female
Swiss mice with dehydroretronecine, each dose equalling 20 mg/kg
body weight or about 5 µmol per mouse, once a week for 4 weeks and
then twice more after 6 months. Six mice developed skin tumours.
Subcutaneous injections of the same dose yielded tumours in 13 out
of 21 mice. Twenty-eight out of 55 mice given both topical
applications and sc injections developed skin tumours.
When the same study was repeated on 34 BALB/c mice, only one
skin tumour developed (Mattocks & Cabral, 1982). When 17 animals
were given the same dose at more frequent intervals, e.g., 65
weekly doses, no tumours developed.
Similar results were obtained in a study by Shumaker et al.
(1976), already desecribed in section 6.4.8.1, in which 39/60 rats
given repeated subcutaneous injections of dehydro-retronecine
developed rhabdomyosarcomas at the site of injection. The compound
appears to be a direct-acting carcinogen for rats and mice, though
the susceptibility of various strains of mice varies.
Peterson et al. (1983) gave 9 ip injections (60 - 76.5 mg/kg
body weight) of dehydroheliotridine to rats over a 32-week period.
A large variety of tumours was produced. It was concluded that
this metabolite may be responsible for the carcinogenicity of its
parent PA.
6.4.8.4 Molecular structure and carcinogenic activity
Mattocks (1986) has reviewed the present position concerning
the relationship between molecular structure and carcinogenic
activity. Data available at present are not adequate for any
strict correlation to be established between the molecular
structure of PAs and the types of tumours produced by them in the
rat. However, the common determinants in the molecular structure
of all carcinogenic PAs are that they are macrocyclic or "open"
diesters, in which the amino-alcohol moiety is retronecine,
heliotridine, or otonecine. These are all esters of unsaturated
necines and are capable of being metabolized to pyrrolic esters in
the mammalian liver. Studies on pyrrolic esters, the toxic
metabolic product of PAs, have yielded equivocal results.
Monocrotaline has been shown to bind covalently to DNA, which is
associated with the carcinogenic activity of the pyrrolizidine
alkaloids (Robertson, 1982). Dehydromonocrotaline, the primary
metabolite of monocrotaline has been found to be an incomplete
dermal carcinogen (Hooson & Grasso, 1976; Mattocks & Cabral,
1982), whereas the synthetic compound 1-methyl-2,3-
bistrimethylacetoxymethylpyrrole, which is chemically similar to
dehydromonocrotaline, is clearly carcinogenic (Mattocks & Cabral,
1982). Dehydroretronecine (DHR), a second metabolite of
monocrotaline and possibly other retronecine-based PAs, is also
carcinogenic for the skin and is considered a proximate
carcinogenic metabolite, since, unlike monocrotaline, it acts at
the site of application directly and not at remote sites.
6.4.9 Antimitotic activity
Literature on this phenomenon has been reviewed by Jago (1969),
McLean (1970), and Mattocks (1986). The most characteristic
feature of the chronic hepatotoxicity of the PAs is the presence in
the liver of megalocytes (Bull & Dick, 1959; McLean, 1970), which
are generally enlarged hepatocytes containing large, hyperchromatic
nuclei (section 6.4.1.5). These appear to be the result of a
combined action of PAs on the hepatocytes, a stimulus to regenerate
following parenchymal cell injury, and the powerful antimitotic
action of the pyrrole metabolites of the PAs (Mattocks, 1986; Jago,
1969). This property has served as the basis for using a PA
(indicine- N-oxide) as a chemotherapeutic agent for cancer (Letendre
et al., 1981, 1984). Peterson (1965) showed that the number of
mitoses following partial hepatectomy was reduced to 50% or less of
normal values by prior administration of hepatotoxic alkaloids, and
that the effect was dose dependent. The hepatocytes seemed to
continue to grow without dividing. The effect can be produced by a
single sublethal dose of the alkaloids (Schoental & Magee, 1957) or
can be a cumulative effect of small doses (Bull & Dick, 1959). The
lesion appears within a few weeks and may persist for the lifetime of
the animal (Mattocks, 1986). It was characteristically described in
the liver of the rat, but has also been reported in a number of other
animals, e.g., mouse, sheep, horse, and pig, and in some other organs,
e.g., kidney and lung (McLean, 1970). It has not been observed in
human livers (Tandon, H.D. et al., 1978; Mattocks, 1986), but it has
been observed that cultured human fetal liver cells become enlarged
when exposed to PAs (Armstrong et al., 1972) indicating a
susceptibility to the antimitotic effect of PAs. The lesion is not
specific for PAs but has been reported to be produced by a number of
toxins (McLean, 1970), including semisynthetic derivatives of PAs but
not non-hepatotoxic PAs, such as platyphyline (Jago, 1970). The
ultrastructural features of megalocytes are controversial.
However, consistent with other functional and metabolic features,
the cells show morphological characters suggesting increased
metabolic activity with increased exchange of material between the
nucleus and cytoplasm.
This unique reaction of the hepatocytes to PA has been used by
Jago (1970) to develop a method for assessing hepato-toxicity and
by Culvenor et al. (1976a) for screening 62 PAs for acute and
chronic hepatotoxicity and pneumotoxicity.
Mattocks (1986) suggested a scheme for the antimitotic action
of PAs in vivo, consistent with observations of the phenomenon in
animals. The PA is irreversibly metabolized by the hepatic
microsomes into a pyrrolic ester, which can be hydrolysed to a
pyrrolic alcohol. The latter is the agent that inhibits mitosis.
The more reactive primary metabolite may also do this, by reacting
with tissue constituents to give products identical to pyrrolic
alcohols, inhibiting mitosis. On the other hand, it can also
produce acute injury to the cells, which stimulates regeneration.
Thus administration of the PA or the pyrrolic ester can induce
megalocytosis to a much greater extent than a secondary metabolite
alone.
Antimitotic activity does not seem to be directly related to
inhibition of DNA synthesis, since the latter recovers within a
week while mitotic inhibition continues for up to a period of 4
weeks (Peterson, 1965; Armstrong & Zuckerman, 1970). Mattocks &
Legg (1980) have shown that the level of DNA synthesis is reduced
in cells that do not divide, but is not totally inhibited. Samuel
& Jago (1975) investigated the position in the cell cycle of the
antimitotic action of lasiocarpine and of its pyrrolic metabolite,
dehydroheliotridine. Their studies indicated that the alkaloid
acts during the late S or early G2 phase of the cell cycle.
6.4.10 Immunosuppression
Dehydroheliotridine (DHH), a pyrrolic metabolite, has a
significant immunosuppressant activity on the primary response in
young mice; when injected ip shortly before the antigenic stimulus
(Percy & Pierce, 1971). The secondary response to antigenic
stimuli; as measured by the reduction in the number of 7S and 19S
specific antibody-synthesizing cells of the spleen, was suppressed
when DHH was administered at the time of secondary stimulus, but
not when it was given 24 or 36 h after the antigenic stimulus. It
was suggested that dehydroheliotridine selectively destroys or
inactivates cells involved in the initial stages of antigen
recognition and processing.
6.4.11 Effects on mineral metabolism
Aberrations of mineral metabolism have been observed in several
species of animals. The most notable among them relate to
haemolysis and copper metabolism. Anaemia has been reported to
occur in rats following PA poisoning (Schoental & Magee, 1959;
Schoental, 1963) and the kidney and liver show haemosiderosis
(Hayashi & Lalich, 1967). Besides the haemolysis, PAs have been
found to exert a direct inhibitory effect on haematopoiesis in the
livers of new-born rats (Sundaresan, 1942). Disturbances of iron
metabolism and haematopoiesis have also been demonstrated in rats
fed Senecio jacobaea and supplementary copper (Swick et al., 1982d)
and they have been found to develop raised copper levels in the
liver and spleen, when fed on this plant (Swick et al., 1982b).
Miranda et al. (1979) reported elevated levels of iron in the liver
and spleen and of copper in the liver in rats fed on tansy ragwort.
Studies with radioactive iron also indicated a specific inhibitory
effect of PAs on haematopoiesis. High copper levels in the liver
associated with haemoglobinuria have been reported in sheep grazing
on heliotrope (Bull et al., 1956), signs of disease closely
resembling those of chronic copper poisoning. St. George-Grambauer
& Rac (1962) reported a similar outbreak of fatal jaundice due to
haemolytic crisis of chronic copper poisoning in sheep that had
grazed Echium plantagineum over 2 or more seasons. The
pathological changes in the liver were indistinguishable from those
of Heliotropium, and the livers had a high copper content. Studies
of Miranda et al. (1981b) indicate that dietary copper can enhance
PA hepatotoxicity in rats. It has been suggested that the
hepatotoxic effects of some PAs may interfere with the excretion of
copper (Bull & Dick, 1959; Farrington & Gallagher, 1960). Similar
effects have been observed in pigs fed Senecio (Harding et al.,
1964). White et al. (1984) did not observe any rise in hepatic
copper levels in sheep fed Senecio jacobaea.
6.4.12 Methods for the assessment of chronic hepatotoxicity and
pneumotoxicity
Pyrrolizidine alkaloids produce acute as well as chronic liver
damage. The acute effect is seen as extensive necrosis (Schoental
& Magee, 1957), while the chronic effect in rats is manifested
characteristically by the presence of greatly enlarged parenchymal
cells (Schoental & Magee, 1957; Bull & Dick, 1959), which persist
long after a single exposure (Schoental & Magee, 1959). The latter
effect of megalocytosis may manifest without the liver cells going
through the process of necrosis (Schoental & Magee, 1959). This
property has been used by Jago (1970) to develop a method for the
assessment of the relative chronic hepatotoxicity of different
alkaloids. It has since been used by other investigators (Culvenor
et al., 1976a). It consists of the intraperitoneal administration
of a single dose of between 0.025 and 3.2 µmol of the alkaloid per
kg body weight in 0.2 ml aqueous solution to 14-day-old suckling
hooded Wistar rats of both sexes. The litters are randomized among
the mothers, one day before the administration of the toxin, and
then weaned at 28 days. Animals are killed 4 weeks after the
injection. The relative acute toxicity is indicated by the dose
levels that cause death within approximately a week and chronic
hepatotoxicity by those that produce hepatic megalocytosis within 4
weeks of the injection. With this method, it is not only possible
to evaluate the hepatotoxicity of a given alkaloid but also to
compare the hepatotoxic effects of different compounds in relation
to each other on a molar basis. Chronic effects on the lungs can
also be assessed by the same method. Culvenor et al. (1976a) found
this method satisfactory for compounds of medium to high
hepatotoxicity but failed to detect toxicity in certain compounds
of known, low hepatotoxicity.
6.5 Effects on Wild-Life
6.5.1 Deer
There is very little information on the consumption of
PA-containing plants by non-domesticated animals, birds, and other
wild-life. In one instance, the deaths of white-tailed deer in
coastal marshes in Louisiana in 1967, was ascribed to the
consumption of Crotalaria and/or Heliotropium species in a period
of feed scarcity (Seger et al., 1969). The animals had thin and
watery blood, abnormal bone marrow, and serious atrophy of cardiac
and mesenteric adipose tissue. In a 9-year-old doe, the liver was
dull and somewhat granular and evinced megalocytosis of the
hepatocytes. In another 4-year-old animal, the liver appeared
normal but with microscopic evidence of early megalocytosis, with
considerable vacuolization in the centrilobular hepatocytes.
Plants of the genera Crotalaria and Heliotropium were abundant and
there were some Senecio species. There were signs of ingestion of
the plants by the deer.
Senecio jacobaea (tansy ragwort) has been fed experimentally to
black-tailed deer (Odocoileus hemionus columbianus) in Oregon to
determine their susceptibility to poisoning (Dean & Winward, 1974).
The ragwort was given ad libitum together with different levels of
basal ration (85% alfalfa, 10% barley, 5% molasses) to captive
deer. The ragwort was eaten, only when the basal ration was
inadequate. One group, not given any basal ration for 6 days,
began eating ragwort and consumed 5.4 kg dry weight per animal in
42 days. This represented 24% of the animal body weight. The
animals did not show any toxic signs, and blood levels of SGOT and
bilirubin were normal.
6.5.2 Fish
The effects of S. jacobaea alkaloids on rainbow trout (Salmo
gairdneri) fingerlings has been investigated (Hendricks et al.,
1981). Duplicate groups of 80 fingerlings were fed for up to 12
months on diets containing 20 or 100 mg alkaloid/kg. The alkaloid
comprised 91% jacobine, 3% jacazine, 2.5% senecionine, and 2.5%
seneciphylline. The 100 mg/kg diet resulted in severe growth
depression and mortality, which began at 3 - 4 months. Both levels
of PAs produced severe hepatic lesions. The livers from these fish
were shrivelled, mottled yellow or whitish in colour, nodular,
fibrous, and sometimes haemorrhagic. Microscopically, there was
megalocytosis, severe fibrosis, and bile-duct proliferation.
Characteristic veno-occlusive changes were seen in the centrilobular
and hepatic veins, which, in the case of fish receiving the 100 mg/kg
diet, appeared after only 2 months on the diet.
6.5.3 Insects
There are no reports of the toxicity of PAs for insects, but
there is substantial literature on the use of PAs by certain insect
families that have evolved with the ability to store the alkaloids
as defensive chemicals and to convert them into pheromones and
other signalling chemicals (see recent reviews by Brown (1984) and
Boppré (1986), and an earlier complementary paper by Edgar (1982)).
In some species, such as moths of the family Arctiidae, the larvae
feed on PA-containing plants. In other families, such as Nymphalid
butterflies of the sub-families Danainae and Ithomiinae, the larvae
of most species live on other plants, but the adult males seek out
PA-containing species and contrive to ingest alkaloids from
wilting, dead, or damaged plant material or from nectar. The
alkaloids so acquired have a functional role as defensive chemicals
against predators and, in some species, are also converted into
pheromones and other signalling chemicals involved in mating. The
alkaloid derivatives may be pyrrolic compounds related to
dehydroretronecine or derivatives of the esterifying acids. In one
Arctiid genus, Creatonotus, the alkaloids have a morphogenetic or
hormonal effect, determining the size of the pheromone-disseminating
organ. Thus, for some insect species, PA-containing plants may be
necessary for survival.
7. EFFECTS ON MAN
The toxic effects of pyrrolizidine alkaloids are principally on
the liver. The toxic disease, produced by consuming PAs derived
from certain plants, is called veno-occlusive disease (VOD), the
pivotal and pathognomonic lesion being the occlusion of the central
and sublobular hepatic veins in the liver. The larger hepatic vein
tributaries are characteristically unaffected in contrast with the
findings in Budd-Chiari syndrome (Bras, 1973).
7.1 Clinical Features of Veno-Occlusive Disease (VOD)
There are several good clinical accounts of the disease, mostly
in the earlier reports from Jamaica, in children (Jelliffe et al.,
1954a,b), and adults (Stuart & Bras, 1955), which have been
summarized by Stuart & Bras (1957). Maksudov (1952) has described
the clinical features among children in outbreaks in the USSR,
where it was called toxic hepatitis with ascites, and Ismailov
(1948a,b), Mnushkin (1949, 1952), and Zheltova (1952) have
described them among adults. Srivastava et al. (1978) also
described the clinical findings among children in a large outbreak.
Children seem to be particularly vulnerable as is evident from the
report of Stuart & Bras (1957) and that of Mohabbat et al. (1976)
concerning a large outbreak, though the disease is rare before the
age of one or two years. Frequently, more than one member of the
family becomes affected (Stuart & Bras, 1957; Mohabbat et al.,
1976; Tandon et al., 1976; Arora et al., 1981) within days or weeks
of each other.
The disease generally has an acute onset, characterized by
rapidly developing and progressing symptoms of upper abdominal
discomfort, dragging pain in right hypochondrium, ascites, and
sometimes oliguria and oedema of the feet. Nausea and vomiting may
be present. Jaundice and fever are rare. There is generally
gross, tender, smooth hepatomegaly often accompanied by massive
pleural effusion, and sometimes slight splenomegaly and minimal
ankle oedema. Liver function tests may show only mild disturbance.
The acute disease is associated with high mortality and a subacute
or chronic onset may lead to cirrhosis. Death often occurs after
oesophageal haemorrhage.
Stuart & Bras (1957) summarized the clinical data of 84
patients ranging in age from 6 months to 53 years. The highest
incidence occurred between the ages of 1 and 3 years (39%), and 26%
of patients belonged to the 3- to 6-year age group. Thus, children
up to 6 years accounted for 65% of total cases. Although the VOD
was relatively uncommon at the 2 extremes of age, early infancy and
adult life, mortality was highest in these groups, being 60% and
54%, respectively. Hepatomegaly and some degree of ascites were
invariably present in acute cases; in 48 out of 64 patients,
ascites was acute enough to require paracentesis. In 38 patients,
hepatomegaly was grossly severe, reaching more than half way down
to the umbilicus. Jaundice was relatively uncommon. Among the
liver function tests, the most significant and consistent changes
were found in the serum-cholinesterase (t = 2.67, 0.02 > P > 0.01)
and serum-albumin levels (t = 2.82, 0.01 > P). Mortality rates
associated with signs of parenchymal liver damage were 74% with
clinical jaundice or high levels of serum-bilirubin, 62% with
diminished serum-cholinesterase, and 58% with considerable anorexia
and apathy. The mortality rates among cases with acute, subacute,
or chronic disease were reported to be 27%, 17%, and 57%,
respectively. Death was mostly due to hepatocellular failure (71%)
in the acute phase and haemetemesis in the chronic phase (75%).
More recent publications (Lyford et al., 1976; McGee et al., 1976;
Tandon, B.N. et al., 1977; Datta et al., 1978a,b) also describe the
haemodynamic data of the hepatic blood flow and the results of
portovenographic studies that suggest outflow tract obstruction in
the liver at the post-sinusoidal level, and irregularity and
obstruction/distortion of the hepatic venous radicles,
respectively.
The clinical course of the disease has been shown schematically
by Stuart & Bras (1957) (Fig. 12). However, the temporal
relationships in the different phases of the disease are not
precise, and their account, at best, represents a trend.
The administration of indomethacin did not have any effects on
either the heart-weight ratio or the pulmonary arterial pressure 3
weeks after monocrotaline administration (Fig. 11), but it
inhibited ( P < 0.05) the rises in 6-keto-PGF1alpha (by 90%) and
TXB2 (by 91%) that occurred in the lung lavage of monocrotaline-
treated animals at 3 weeks.
The above studies indicate that both the cyclo-oxygenase and
the lipo-oxygenase pathways of arachidonate metabolism are
activated by monocrotaline as early events in its toxic effect.
Activation of the cyclo-oxyenase pathway, demonstrated by increased
concentrations of the prostaglandin metabolites 6-keto-PGF1alpha
and TXB2 in lavage fluid, was inhibited by indomethacin, but this
inhibition did not prevent the monocrotaline-induced injury. DEC
attenuated both the inflammatory response and pulmonary
hypertension and inhibited the formation of slow reacting
substances including leukotriene D4. Since DEC produces a
pharmacological blockade of the lipo-oxygenase pathway, it seems
that the latter, rather than the cyclo-oxygenase pathway, is
responsible for perpetuating the pathophysiological mechanism
leading to monocrotaline-induced pulmonary hypertension.
Hilliker et al. (1984) demonstrated that antibody-induced
thrombocytopaenia attenuates right ventricular hypertrophy induced
by monocrotaline in rats. In another study, Hilliker & Roth (1984)
also produced evidence that hydrallazine, a vasodilator and
inhibitor of platelet prostaglandin synthesis, dexamethason, an
antiinflammatory agent and inhibitor of phospholipase, and
sulfinopyrazone, an inhibitor of platelet prostaglandin synthesis
inhibited monocrotaline-induced right ventricular hypertrophy in
rats, supporting the hypothesis that platelets and vasoconstrictor
agents play a role in monocrotaline-induced pulmonary hypertension.
Likewise, prior chemical sympathectomy with 6 hydroxydopamine
(100 mg/kg) or inhibition of serotonin synthesis with
p-chlorophenylalanine (500 mg/kg) reduced the degree of
monocrotaline-induced right ventricular hypertrophy in rats, but
did not prevent or reduce pulmonary vascular muscularization
(Tucker et al., 1983). Thus, the sympathetic nervous system and
serotogenic mechanisms seemed to be involved in the development of
right ventricular hypertrophy, but not in the development of the
pulmonary vascular lesion induced by monocrotaline. Kay et
al. (1985) also demonstrated that pretreatment with
p-chlorophenylalanine, which inhibits 5-hydroxytryptamine (5HP)
synthesis, also significantly ( P < 0.05) reduced right ventricular
systolic pressure, right ventricular hypertrophy, and medial
thickness of muscular pulmonary arteries in monocrotaline-treated
rats. Similar observations were made in rats exposed to hypoxia.
It was therefore suggested that 5HP might play a role in
monocrotaline-induced or chronic hypoxic pulmonary hypertension.
The biosynthesis of rat lung polyamines, putrescine,
spermidine, and spermine, generally considered to be important
regulators of cell growth and differentiation, is increased prior
to the evolution of monocrotaline-induced pulmonary hypertension in
rats. Continuous administration of alpha-difluoromethylornithine
(DFMO), which is a highly specific irreversible inhibitor of
ornithine decarboxylase (DDC), a rate-limiting enzyme in polyamine
biosynthesis, attenuated the development of monocrotaline-induced
pulmonary hypertension in rats (Olson et al., 1984). This effect
was mediated by the DFMO, by inhibiting the synthesis of putrescine
and spermidine, and not by blocking the hepatic metabolism of
monocrotaline to pyrroles (Olson et al., 1985). Thus, it was
suggested that lung polyamine biosynthesis might be essential for
the expression of monocrotaline-induced perivascular oedema as well
as medial thickening in the development of monocrotaline-induced
pulmonary hypertension vascular disease.
On the basis of the preceding studies, mostly on the rat, the
mechanism of chronic long-term injury to the lung by monocrotaline
seems to be as follows. Within hours of PA administration, there
is damage to the pulmonary endothelial cells accompanied by
vascular leak leading to pulmonary oedema. Platelet aggregation
also occurs. The endothelial damage indicated by ultrastructural
and biochemical studies activates the production of prostacyclin
and lipogenic products, which mediate increases in vascular
permeability and inflammatory reaction. There is simultaneous
production of 5 hydroxytryptamine and several polyamines. The
injected monocrotaline is completely metabolized within hours, and
no significant quantity is found in the body at 24 h (Hayashi,
1966) and, though some active metabolites may still be detectable
by isotope studies, even at 14 days (Hsu et al., 1974), the rats do
not have any lung lesions. The slow evolution of vascular changes
suggests that it is not caused by monocrotaline but through
biological pathways activated by the initial injury.
Methylprednisolone (MP), which reduces acute lung oedema caused
by monocrotaline (MCT), has been shown to reduce MCT-induced
pulmonary hypertensive vascular changes in rats and the resultant
right ventricular hypertrophy (Langleben & Reid, 1985). Daily ip
administration of MP at 5 mg/kg body weight, was found to be more
effective than 2 large doses of MP at 30 mg/kg, 2 h before and 2 h
after a single sc injection of MCT at 60 mg/kg. It was suggested
that secondary changes, though triggered by the acute MCT injury,
become self sustaining and are more significant for vascular
structural remodelling.
Structural arterial remodelling with vasoconstriction, medial
hypertrophy of the muscular pulmonary arteries, and muscularization
of the pulmonary arterioles follow as late effects, resulting in
pulmonary hypertension and right ventricular hypertrophy of the
heart.
The results of the above studies suggest a direct toxic effect
of the alkaloid on the endothelial cells of the alveolar
capillaries and on the pulmonary arteries, as well as a pulmonary
hypertensive effect on the heart.
6.4.3 Effects on the central nervous system
The dominant signs of pyrrolizidine poisoning in horses are
neurological (Rose et al., 1957a,b; McLean, 1970). Similar signs
can also occur in cattle and sheep. It has been claimed that such
signs are probably non-specific secondary effects following primary
liver disease resulting in hyperammonaemia (Rose et al., 1957a).
However, neurological abnormalities in which animals walk in a
straight line until they come to an object, and then stand with
their heads pressed against the object, indicate specific lesions
in the central nervous system. Spongy degeneration of the central
nervous system occurs in cattle, sheep, and pigs (Hooper et al.,
1974; Hooper, 1975a,b).
Trichodesma alkaloids, in particular, appear to be neurotoxic.
There is a considerable body of literature in the USSR on
Trichodesma intoxication of mice, rabbits, and dogs, which has
been reviewed (Ismailov et al., 1970). Mice given Trichodesma
alkaloids subcutaneously at 0.5 mg/kg develop paresis of the hind
limbs within 12 - 17 days. Opisthotonus and clonic convulsions are
also seen. Doses of 10 - 15 mg/kg of alkaloids produces death in
all animals within 2 - 6 h, as the result of respiratory
depression. Higher doses produce immediate death.
6.4.4 Effects on other organs
Right ventricular hypertrophy, secondary to the primary effects
on the pulmonary arteries, and the resultant pulmonary hypertension
in animals treated with PAs or PA-containing plants have been dealt
with in section 6.4.2. Lalich & Merkow (1961) reported myocarditis
consisting of focal oedema and infiltration with a minimal number
of lymphocytes and mononuclear cells in some rats fed Crotalaria
spectabilis seeds mixed with the diet at a concentration of
0.13 - 2 g/kg. Treated groups consisted of 11 - 24 animals each;
there were 12 controls. The changes were seen in all groups of
animals, but the maximum number of rats (10 out of an unspecified
number of the group) showing these changes was in the group that
received 0.5 g/kg diet for 20 - 31 days. Generally, there was a
close correlation with the presence of pulmonary arteritis.
Renal changes have been described by a number of investigators.
Hayashi & Lalich (1967) observed mild to moderate changes in renal
glomeruli consisting of necrosis, capillary thrombosis, and
degenerative changes in the epithelial and mesangial cells,
thickening of interlobular arteries, and arterial thrombosis in
suckling, male Sprague Dawley rats given a single sc dose of
monocrotaline at 120 mg/kg body weight. Renal changes were seen,
to some extent, in all animals surviving for 41 - 47 days.
Carstens & Allen (1970) studied the effects of feeding
Crotalaria spectabilis seed on the rat kidney. Fifty male Sprague
Dawley rats were fed a diet containing ground Crotalaria
spectabilis seed at 0.2 - 0.8 g/kg for 8 months. The seeds were
estimated to contain approximately 3.5 g monocrotaline/kg; 10
animals served as controls. Renal changes were seen in 33/50
PA-treated rats. In 22 rats, over 75% of the glomeruli were
hyalinized and capsules thickened. In the less severely affected
kidneys, the glomerular basement membrane was thickened and
homogeneous deposits were seen in mesangial areas. Afferent
arterioles and interlobular arteries were markedly thickened. In
the most severely affected vessels, the internal elastic lamina was
necrosed and the larger arteries showed fibrinoid necrosis.
Renal tubular megalocytosis was the dominant lesion described
by Hooper (1974) in mice. Nine male white mice, 10 weeks of age,
were fed Senecio jacobaea, which contained a concentration of
alkaloids (jaconine, jacobine, and seneciphylline) of 2.7 g/kg and
a concentration of N-oxide of 0.9 g/kg, mixed with the diet. The
S. jacobaea was given at 100 g/kg diet for 9 weeks, before being
raised to 200 g/kg diet. Five animals served as controls. The
animals were killed from 63 to 193 days after the start of the
study. All treated animals, except 2 killed on day 63, showed
changes. The large cells occurred in both the proximal tubules and
the loop of Henle. Similar cells were seen in the alveolar and
bronchiolar epithelium. No glomerular lesions were described. The
author mentioned having seen the above changes in rats given
repeated sublethal injections of fulvine and spectabiline. On the
other hand, Kurozumi et al. (1983) observed glomerular lesions in
rats given a single injection of monocrotaline.
A variety of renal lesions has been observed in pigs, a common
pathological feature being renal megalocytosis, which was observed
in pigs poisoned by at least 4 different plant genera containing a
variety of toxic alkaloids (Harding et al., 1964; Peckham et al.,
1974) and has also been observed in wild pigs grazing in areas rich
in PA-containing plants in northern Australia (Hooper, 1978).
McGrath et al. (1975) described glomerular lesions in pigs given
Crotalaria spectabilis seed daily for 43 days. Severe renal
lesions comprising tubular dilatation, megalocytosis, and necrosis
of tubular epithelial cells with casts in the lumen, interstitial
and periglomerular fibrosis, and glomerular hyalinization were
reported by Hooper & Scanlan (1977) in pigs fed Crotalaria retusa
seeds containing monocrotaline. Renal megalocytosis has also been
reported in C. retusa poisoning in horses, sheep, and mice poisoned
by S. jacobaea but not by H. europaeum, and in vervet monkeys with
chronic retrorsine poisoning (Van der Watt et al., 1972).
Lesions have been reported in the stomach and intestines in
field and experimental animals after poisoning with pyrrolizidine
alkaloids, but are difficult to identify as specific PA injury.
Hooper (1975c) conducted studies on sheep, rats, and mice. In the
study on sheep, 12 male cross-bred lambs, 7 - 8 weeks of age, were
newly weaned on to a standard commercial calf grower diet.
Lasiocarpine was administered at the rate of 15 - 20 mg/kg body
weight every 2 - 4 days. Each animal was killed when in terminal
coma. Survival time ranged from 4 to 17 days. In the rat study,
young Wistar-Furth rats (sex not stated) weighing 150 - 200 g were
used. In one group of 11 rats, each animal received an ip
injection of lasiocarpine at the rate of 40 mg/kg body weight.
Three animals received isotonic saline. Animals were killed or
died 2 - 6 days after the injection. A second group of 13 rats
received a dose of 35 mg lasiocarpine/kg body weight; 4 control
animals received saline. All rats received a second injection
48 h later. They were killed 3 - 60 days after the second
administration of lasiocarpine. In the mouse study, 3 mature male
white mice received 6 injections each of lasiocarpine at the rate
of 45 mg/kg body weight followed by 4 injections of 90 mg/kg body
weight at 48-h intervals. There was one control animal.
All animals showed characteristic hepatic lesions. Sheep also
showed severe oedema, haemorrhage, and epithelial necrosis in the
gall bladder; lesions were also found in the central nervous system
and occasionally in the kidney. All animals showed severe
intestinal atrophy. There was inhibition of crypt cell mitosis
leading to mitotic irregularities, abnormal large cells and
syncitial cells, especially in the duodenum of sheep, and severe
villous atrophy with ulceration. Lesions in the intestines were
similar to those caused by radiation and radiomimetic agents. It
was suggested that the local intestinal radiomimetic effect was due
to local exposure to the pyrrole metabolite of lasiocarpine after
excretion through the bile duct. It was proposed that a more
conspicuous and rapid development of duodenal megalocytosis was due
to very rapid turnover of cells in the duodenum.
Other probably secondary effects included haemolysis in sheep
in association with advanced liver disease and high liver-copper
levels (Bull et al., 1956), anaemias and disturbance in iron
metabolism and haematopoiesis (Schoental & Magee, 1959; Schoental,
1963; Peckham et al., 1974; Hooper & Scanlan, 1977) (section
6.4.11), pancreatic oedema and fibrosis (Bras & Hill, 1956;
Schoental & Magee, 1959), cerebral oedema, haemorrhage, and
congestion in the rat brain (Davidson, 1935; Rosenfield & Beath,
1945).
Tumours in the different organs have been dealt with separately
under carcinogenesis (section 6.4.8).
6.4.5 Teratogenicity
The teratogenic potential of PAs was demonstrated by Green &
Christie (1961) who produced a variety of dose-related fetal
abnormalities in the rat, with a single intraperitoneal injection
of heliotrine administered during the second week of gestation.
The dosages ranged from 15 to 300 mg/kg maternal body weight.
Litters exposed to a dose of less than 50 mg did not show any
abnormalities, but abnormalities were observed in litters exposed
to higher doses, and increased in frequency and severity with
increasing dose. The abnormalities included retardation of
development, musculoskeletal defects, especially hypoplasia of the
lower jaw, cleft palate, and other abnormalities. Doses above 200 mg
resulted in the intrauterine death or resorption of many fetuses.
Similar studies were performed by Peterson & Jago (1980) who
compared the effects of heliotrine with its metabolic pyrrole
derivative dehydroheliotridine (DHH), when administered in a single
ip injection to rats on the 14th day of gestation. Heliotrine was
administered at 200 mg/kg body weight and DHH at 30 - 90 mg/kg, 14
days after conception. Effects on embryos, evaluated on the 20th
day, showed that both heliotrine and DHH retarded growth and were
teratogenic, but that the effects of a 40 mg/kg dose of DHH were
equivalent to those of 200 mg/kg heliotrine, i.e., the metabolite
was 2.5 times as effective on a molar basis. DHH produced a number
of skeletal abnormalities including retarded ossification,
distorted ribs, long bones, cleft palate, and feet defects. At
higher doses, growth almost ceased in many tissues and the fetuses
were very immature. However, the embryonic liver parenchyma did
not show the antimitotic effects of DHH.
The teratogenic properties of heliotrine were also demonstrated
in Drosophila larvae fed low levels of the alkaloid (Brink, 1982).
6.4.6 Fetotoxicity
The subject of fetotoxicity has been reviewed by Mattocks
(1986). Sundareson (1942) demonstrated the ability of
pyrrolizidine alkaloids to cross the rat placenta. Twice weekly
injections of the PA, starting at, or after, the 12th day of
gestation, resulted in premature delivery of some litters and many
were born dead. The same author showed that the alkaloids
themselves and not just the pyrroles formed in the dams' livers,
could pass the placental barrier by injecting senecionine into
19-day-old rat fetuses in utero, which produced the characteristic
toxic lesions in the dams. The fetuses were also found to be more
resistant to the lethal effects of the PA than the mother rats.
When 4 fetuses were each administered 1.25 mg of PA, representing
about 200 - 400 mg/kg body weight, which is much higher than the
LD50 for an adult rat, 3 of them were still alive after 2 days.
Green & Christie (1961) did not find any liver damage in fetuses
from pregnant rats given teratogenic doses of heliotrine. Only
mild liver damage was found in the embryo rats whose mothers had
been injected with PAs (heliotrine, lasiocarpine, retrorsine, or
monocrotaline) (Bhattacharya, 1965). In contrast, Schoental (1959)
demonstrated that lasiocarpine and retrorsine, when administered to
lactating rats produced little effect on the mothers, but produced
acute liver lesions in the suckling infants. The lesions were most
severe in 3- to 7-week-old animals. It was suggested that the
infants were affected by the milk from the lactating mothers, which
possibly contained the metabolic products of the PAs.
It would seem from the above studies that the embryo is
relatively more resistant to the toxic effects of PAs in utero than
it is after birth. Mattocks & White (1973) postulated that this
could be due to the low capacity for the metabolic activation of
PAs of the embryo liver, as they had shown that the ability of
liver enzymes to convert retrorsine to toxic metabolites was low in
rats, immediately after birth, but picked up rapidly afterwards.
The susceptibilities of rats of various ages to the hepatotoxic
effects of the PAs was proportional to their capacity to form and
retain the pyrrolic metabolites. Twenty-day-old rats were found to
be more sensitive than older animals.
The effects of fulvine administration on pregnant rats between
9 and 12 days of gestation were studied by Persaud & Hoyte (1974).
Dose-related fetal resorptions were observed, but no hepatic
lesions were seen in the fetuses. On the other hand, Newberne
(1968) observed damage, in both the maternal and fetal livers, when
lasiocarpine was administered to pregnant rats. Acute liver
necrosis was observed in the livers of mothers as well as fetuses
in animals that had received 100 mg lasiocarpine/kg body weight on
day 13 of gestation. However, in animals that received 2 doses of
35 mg/kg body weight on days 13 and 17 of pregnancy, liver necrosis
was seen in the fetal liver but not in that of the mother. It is
not known why lasiocarpine acts differently from other alkaloids
and has a greater effect on the fetal liver. Mattocks (1986) has
postulated the possibility that fetotoxicity was caused chiefly by
toxic metabolites formed in the maternal liver, and that a greater
proportion of such metabolites reached the fetus from lasiocarpine
than from other PAs.
6.4.7 Mutagenicity
A number of PAs that have been shown to be powerful dose
dependent mutagens in Drosophila melanogaster have been listed by
Mattocks (1986). All the compounds are hepatotoxic though the
degree of mutagenicity is not necessarily proportional. Table 12
provides a summary of the mutagenicity tests on different PAs,
related compounds and plant extracts. Clark (1959) demonstrated
the mutagenic effect of heliotrine in Drosophila, in which a
considerable increase in sex-linked recessive lethals was produced,
apparently by interfering with the maturation of germ cells, so
that as soon as the available spermatozoa were used, the males were
no longer capable of breeding. The cell damage was irreversible.
The mutagenic effect of feeding Drosophila males for 24 h with a
medium containing 10-3 mol monocrotaline was comparable to about
1000 R of X rays (Clark, 1976). The Basc test with Drosophila
melanogaster is considered a highly sensitive mutagenicity test
for PAs (Candrian et al., 1984a).
Seneciphylline and senkirkine, known to occur in animal feeds
and medicinal herbs, respectively, were tested for their ability to
produce sex-linked recessive lethals in males of Drosophila
melanogaster using the Basc (3-day feeding method) by Candrian et
al. (1984a). Seneciphylline was found to be mutagenic at
concentrations of 10-5, 10-4, and 10-3 mol, which produced 3.8%
(983 chromosomes tested), 9% (708 chromosomes tested), and 15.3%
(327 chromosomes tested) sex-linked recessive lethals,
respectively. Senkirkine (10-5 mol) was found to produce 4.4% sex-
linked recessive lethals (2541 chromosomes tested) against 0.17%
maximum sensitivity in the late spermatid stage of spermatogenesis
indicating that PAs act as indirect mutagens. Flies fed with milk
from lactating rats given an oral dose of 25 mg seneciphylline/kg
showed 1.2% sex-linked recessive lethals (1477 chromosomes tested)
compared with 0.3% (1533 chromosomes tested) in controls.
Table 12. Mutagenicity tests on pyrrolizidine alkaloids,
related compounds, and source plants
---------------------------------------------------------
Compound or material Type of testa Responseb
---------------------------------------------------------
Clivorine A +
HPC +
Echimidine D +
Echinatine D +
Fulvine D +
Heliotrine D +
P +
F +
CC +
A +
B +
TM +
CM/CC +
Integerrimine D +
Jacobine D ±
P +
Lasiocarpine D +
P +
F +
A +
HPC +
CM/CC +
TM +
Ligularidine A +
Lindelofine A 0
Lycopsamine A 0
Monocrotaline D +
P +
CC +
B +
A 0
HPC +
CT +
Petasitenine (fukinotoxin) A +
HPC +
CM +
---------------------------------------------------------
Table 12. (contd.)
---------------------------------------------------------
Compound or material Type of testa Responseb
---------------------------------------------------------
Platyphylline D 0
Retrorsine A +
D +
CT +
Rosmarinine CT 0
Senecionine D +
A 0
Seneciphylline A 0
P +
D +
Senkirkine A +
HPC +
CM/CC +
D +
Supinine D ±
P +
Mixed alkaloids from
Senecio jacobaea A 0
Senecio numorensis
spp. fuchsii (extract) CM +
A 0
A +
Senecio jacobaea (extract) A +
Senecio longilobus (extract) A 0
Symphytum officinale
(comfrey extract) A 0
Retronecine bis-p-chloro- P +
benzoate
Synthanecine A bis-N-ethyl- CT +
carbamate
Retronecine A 0
Heliotridine D 0
Viridofloric acid A 0
---------------------------------------------------------
Table 12. (contd.)
---------------------------------------------------------
Compound or material Type of testa Responseb
---------------------------------------------------------
Heliotric (heliotrinic) acid D ±
HPC +
Dehydroretronecine CT +
A ±
SCE +
Dehydroheliotridine CM +
Pyrrole HPC 0
2,3-Bishydroxymethyl-1- CT +
methylpyrrole A ±
SCE +
2-Hydroxylmethyl-1-methyl- A 0
pyrrole SCE ±
3-Hydroxylmethyl-1-methyl- A +
pyrrole SCE ±
---------------------------------------------------------
a A = Salmonella ("Ames") test.
B = Other bacterial tests.
CC = Clastogenic activity in cultured cells.
CM = Mutagenicity in cultered mammalian cells.
CT = Cell transformation test.
D = Mutagenicity in Drosophila.
F = Tests in fungus (Aspergillus nidulans).
HPC = Hepatocyte primary culture/DNA repair test.
P = Chromosomal aberrations in plant cells.
SCE = Sister chromatid exchange.
TM = Transplacental micronucleus test.
b + = active.
± = marginally active.
0 = inactive.
Mutagenic properties of 7 PAs extracted from plants to
Salmonella typhimurium TA100 have been demonstrated by a modified
Ames method by Yamanaka et al. (1979). The PAs were clivorine,
fukinotoxin, heliotrine, lasiocarpine, ligularidine, LXC201, and
senkirkine. Pre-incubation of these alkaloids with liver S9 mix
and bacteria in liquid medium was essential for demonstration of
the property. PAs in the heliotridine and otonecine family were
mutagens, while retronecine bases were inactive. Monocrotaline and
heliotrine were not active mutagens to Escherichia coli WP2, even
though they were quite cytotoxic (Green & Muriel, 1975). They were
active in repair deficient strains. Retrorsine was active in
inducing mutations on the Ames Salmonella/microsome assay (Wehner
et al., 1979). Extracts from medicinal plants and noxious weeds
were mutagenic towards Salmonella in the Ames assay (Pool, 1982;
White et al., 1983; Koletsky et al., 1978).
From the limited data available, it seems that the carcinogenic
activity of individual alkaloids parallels their mutagenic
behaviour, but not their relative hepatotoxicities (Culvenor &
Jago, 1979).
6.4.7.1 Chromosome damage
Pyrrolizidine alkaloids have been shown to be capable of
damaging chromosomes in plants, fungi, bacteria, tissue cell
cultures, and the fruit fly (Drosophila melanogaster). Literature
on this topic has been reviewed by Bull et al. (1968), McLean
(1970), and Mattocks (1986).
Several PAs are known for their ability to damage the
chromosomes of growing plant cells (Mattocks, 1986). Similar
properties have been demonstrated in leukocyte cultures from the
marsupial (Potorus tridactylus) (Bick & Jackson, 1968; Bick, 1970).
Bick & Culvenor (1971) found dehydroheliotridine, a metabolite of
heliotrine, to be 10 times more active than the alkaloid.
Infusions of Symphytum officinale L., described in Polish
pharmacopoeia as Radix symphyti, are recommended as expectorants,
especially for children. Furmanowa et al. (1983) demonstrated the
mutagenic effects of an alkaloidal fraction and infusion in this
plant in the meristematic cells of the lateral roots of Vicia faba
L. var minor. Lasiocarpine, a proven carcinogen, served as a
positive control.
Chromosome damage by PAs in the hamster lung cell line was
demonstrated by Takanashi et al. (1980). Stoyel & Clark (1980)
used the transplacental micronucleus test in pregnant female mice
and showed the chromosome damaging properties of heliotrine (225 mg/kg
body weight) and lasiocarpine (86 mg/kg) within 20 h of the injection.
The genotoxicity of heliotrine, monocrotaline, seneciphylline,
and senkirkine was studied by Bruggeman & Van der Hoeven (1985)
using the sister-chromatid exchange (SCE) assay in V79 Chinese
hamster cells co-cultured with primary chick embryo hepatocytes.
Exposure to these PAs resulted in the high induction of SCEs, a
more than 5-fold increase in the SCE rate with 2.5 mg
heliotrine/litre, 4-fold with monocrotaline at 5 mg/litre, 8-fold
with seneciphyline at 1.2 mg/litre, and more than 5-fold response
with senkirkine at 2.5 mg/litre. For all compounds, a dose-
response relationship was observed at concentrations that did not
seriously affect survival. PAs are also known to induce DNA repair
in rodent hepatocytes (Green et al., 1981; Mori et al., 1985). DNA
repair synthesis was elicited by 15 alkaloids, including 11 of
unknown carcinogenic potential (Mori et al., 1985).
There are also a few reports of chromosome damage by PAs in
man. Martin et al. (1972) found chromosome damage in the blood
cells of children with veno-occlusive disease, probably caused by
fulvine. It has also been shown by Ord et al. (1985) that
dehydroretronecine, is able to induce SCE in human lymphocytes.
Kraus et al. (1985) studied the PAs senkirkine and tussilagine,
which occur in a medicinal plant Tussilago farfara, for their
ability to induce chromosome damage in human lymphocytes in vitro.
They were not found to enhance the number of chromosome aberrations
up to concentrations of 1000 µmol. However, heliotrine, used for
comparison, induced chromosomal aberrations at concentrations of
100 µmol. In addition, heliotrine was also found to be capable of
damaging unstimulated eg Go-phase lymphocytes.
6.4.8 Carcinogenesis
Carcinogenesis has been reviewed by McLean (1970), IARC (1976,
1983), and Mattocks (1986). A number of purified PAs, purified or
crude extracts of plants containing them in a mixture or the actual
plant, dried and milled, and several PA metabolites or synthetic
analogue compounds have been tested for carcinogenecity. However,
these include only relatively few of the known cytotoxic PAs. Data
relating to some of the representative studies on rats are
summarized in Table 13. Studies on liver tumours found in rats
given PAs and plant materials are summarized in Table 14. All
experimental animal studies, with the exception of one on chickens
(Campbell, 1956) and one on Syrian golden hamsters (Fushimi et al.,
1978), have been carried out on rats.
The liver is the most common organ involved in experimental
studies. Tumours produced are mostly of epithelial origin, but a
significant number are also vascular. Lack of precision and
diversity of terms used to describe similar or identical tumours
makes it difficult to compare the types of carcinogenic effect in
different studies. Some terms have been used interchangeably,
e.g., hepatomas, hepatocellular carcinomas, haemangiogenic and
cholangiogenic tumours; nodular hyperplasia, pre-neoplasma,
neoplastic nodules, and hepatocellular tumours. In most studies,
there are no supporting photomicrographs to draw any inference as
to whether the tumours were malignant. Difficulties in the
interpretation of data have been commented on by Schoental et al.
(1954) and McLean (1970).
Lasiocarpine has produced the largest yield of tumours. In the
studies of Svoboda & Reddy (1972), 16/18 animals surviving for more
than 56 weeks after receiving ip multidoses of lasiocarpine
developed malignant tumours of the liver. Of these, 10 animals had
more than one tumour. Continuous feeding of rats on a regimen
containing lasiocarpine resulted in all animals (24/24) developing
tumours (NCI, 1978). In one study, a single oral administration of
retrorsine (Schoental & Bensted, 1963) to weanling rats resulted in
7 of the 29 animals that survived for more than one year developing
11 tumours of a wide variety, at least of 5 which were malignant.
It is of note that this PA is known to have caused two cases of
human toxicity together with riddelline in two cases, though the
total intake was proportionately lower (Stillman et al., 1977; Fox
et al., 1978; Huxtable, 1980) (Table 15).
Tumours produced covered a very wide range in unrelated tissues
and organs, for example, the pancreas, urinary bladder, pituitary,
bone, retro-peritoneal tissues, and skin, among others.
Hepatocellular carcinoma and haemangiosarcoma were the most common.
Crude extracts of plants or whole plants have also been
demonstrated to produce a variety of tumours. For example, Senecio
longilobus has been shown to produce tumours in a significantly
large proportion of experimental animals (Harris & Chen, 1970).
Hirono et al. (1973, 1976, 1978, 1979b) demonstrated the
carcinogenic properties of a number of plants, used as food in
Japan, in the rat.
A summary of the relevant experimental data follows.
6.4.8.1 Purified alkaloids
Kuhara et al. (1980) administered clivorine in the drinking-
water at a concentration of 0.05 g/litre to 12 rats of both sexes,
continuously for 340 days followed by plain water. There were 20
control rats. All the treated rats survived 440 days. Eight of
the 12 animals developed liver tumours including 2
haemangioendothelial sarcomas and 6 that were described as
"neoplastic nodules". No liver tumours were seen in the control
animals (Table 13).
Schoental (1975) tested the carcinogenic properties of
heliotrine, with and without prior administration of nicotinamide.
Nicotinamide protects against liver necrosis and so may enhance
tumour yeild, as shown by Rakietin et al. (1971), who studied
pancreatic tumours in rats given streptozootocin.
Heliotrine was administered intragastrically to 4 groups of 26
male weanling rats in 1 or 2 doses of 230 mg/kg and 300 mg/kg body
weight; 2 groups also received nicotinamide at 350 - 500 mg/kg body
weight, administered ip 10 - 15 min before, and 2.5 h after
administration of heliotrine, as per the dosing regimen shown in
Table 13. There were 8 controls. All animals administered the
higher dose of heliotrine (300 mg/kg body weight) died within 5
months of the PA administration. The livers showed lesions
characteristic of PA toxicity. No tumours were seen. Only 1 out
of 4 animals receiving heliotrine alone, survived 27 months and it
showed an islet cell adenoma as well as adenoma of pituitary, but
so did 3 of the 8 controls. In the group receiving 230 mg
heliotrine/kg and also treated with nicotinamide, 4 animals died
within 5.5 months (one with a fibrosarcoma and the other in a
moribund condition), chiefly from toxic liver disease, and two more
had to be killed. Of the 6 animals surviving more than 22 months
after heliotrine treatment, islet cell adenoma was seen in 3
together with other tumours as shown in the table. This tumour is
stated to be extremely rare in the animal strain used. Hepatoma
was seen in only one animal. The role of nicotinamide in this
study is not clear.
Table 13. Summary of data on the carcinogenic action of PAs and PA-containing plants in rats (in chronological order)
Material Strain, Route of Other Duration Period of Tumours Tumour Reference
tested number, administration treatment of observation produced incidence
and sex of and dosing treatment in
animals regimen surviving
animals
Alkaloids Wistar rat drinking-water 1 week until death only nodular Schoental
of Senecio (13 males, (0.05 g/litre) (males) hyperplasia et al. (1954)
jacobaea 12 females) 2 weeks of liver
(females);
followed by gap of
7 weeks
9 males, 0.03 g/litre, until
1 female 3 days/week death
(surviving)
Retrorsine 10 males, 0.03 g/litre, until hepatomas 4/14
4 females 3 days/week death
Isatidine 8 males, 0.05 g/litre
14 females followed by
0.03 g/litre, 20 months until death hepatomas 10/22
3 days/week
3 males, as above choline until hepatomas 3/7
4 females (0.5% in death
drinking-
water), 4
days/week
Table 13. (contd.)
Material Strain, Route of Other Duration Period of Tumours Tumour Reference
tested number, administration treatment of observation produced incidence
and sex of and dosing treatment in
animals regimen surviving
animals
Isatidine 2 males, single ip Schoental
(contd.) 3 females injection of et al. (1954)
2 mg in 0.2 ml
tricaprilyn
followed by
skin application 15 months 15 months hepatoma (?) 1/5
tion of 0.5%
solution, 3
days/week
controls not mentioned
(7 males,
7 females)
Retrorsine Porton single 400 r until death hepatomas 5/25 Schoental &
Wistar intragastric radiation Bensted (1963)
weanling dose of 30 mg/kg to 31/50 hepatocellular 1/25
rat body weight surviving
(50 males) for 100 carcinoma
days; 4/13 with
had head metastases
shielded
mammary 2/25
tumours
Table 13. (contd.)
Material Strain, Route of Other Duration Period of Tumours Tumour Reference
tested number, administration treatment of observation produced incidence
and sex of and dosing treatment in
animals regimen surviving
animals
Retrorsine lung carcinoma 1/25
(contd.)
renal 2/25
carcinomas
colonic 1/25
carcinomas
Porton as above as above splenic 1/25 Schoental &
Wistar rat haemangio- Bensted (1963)
(50 males) endothelioma
osteosarcoma 1/25
bone
leukaemia 1/25
"spindle cell" 1/25
tumour (neck)
Table 13. (contd.)
Material Strain, Route of Other Duration Period of Tumours Tumour Reference
tested number, administration treatment of observation produced incidence
and sex of and dosing treatment in
animals regimen surviving
animals
Retrorsine 95 males, single oral dose until death hepatomas 5/29
(contd.) 95 females of 30 mg/kg body
(weanling) weight mammary tumour 1/29
(controls)
lung carcinoma 1/29
splenic 1/29
haemangio-
endothelioma
uterine 1/29
carcinoma
Porton retroperitoneal 1/29 Schoental &
Wistar rat sarcoma Bensted (1963)
(controls)
squamous cell 1/29
carcinoma
(jaw)
Table 13. (contd.)
Material Strain, Route of Other Duration Period of Tumours Tumour Reference
tested number, administration treatment of observation produced incidence
and sex of and dosing treatment in
animals regimen surviving
animals
Retrorsine 6 males no PAs 400 r until death leukaemia 2/6
(contd.) (weanling) radiation
osteosarcoma 1/6
renal adenoma 1/6
10 males single partial until death hepatomas 2/9
(weanling) intragastric hepatectomy
dose (30 mg/kg squamous cell 1/9
body weight) carcinoma
(9 days after (jaw)
partial
hepatectomy)
Table 13. (contd.)
Material Strain, Route of Other Duration Period of Tumours Tumour Reference
tested number, administration treatment of observation produced incidence
and sex of and dosing treatment in
animals regimen surviving
animals
Mixed PAs Randomly single more than 1 pancreatic 1/15 Schoental et al.
from seeds bred from intragastric dose year? islet cell (1970)
of Porton at 500-1500 mg/kg adenomaa
Amsinckia Wistar body weight
intermedia weanling "papillary 1/15
(intermedine rat tumour"a of
medine and (15 males) urinary
lycopsamine) bladder
pituitary 1/15
adenomaa
as above as above pancreatic 1/15 Schoental et al.
islet cell (1970)
adenocarcinoma
exocrine 1/15
pancreatic
adenoma
Leaves and 2 males 10% mixed with 1 month until death pancreatic 1/2
stems of (weanling) diet (period not islet cell
Heliotropium stated) adenoma
supinum L.
Table 13. (contd.)
Material Strain, Route of Other Duration Period of Tumours Tumour Reference
tested number, administration treatment of observation produced incidence
and sex of and dosing treatment in
animals regimen surviving
animals
Heliotropium 6 males single until death pancreatic 1/6
supinum L. intragastric dose (longer islet cell
(contd.) at 200-300 mg/kg than 1 adenoma
body weight of year)
crude alkaloidal
fraction
controls not stated
Senecio Harlan rat 0.75% of diet until death none Harris & Chen
longilobus 50 males, within 131 (1970)
50 females days
50 males, 0.5% of diet until death none Harris & Chen
50 females within 200 (1970)
days
Table 13. (contd.)
Material Strain, Route of Other Duration Period of Tumours Tumour Reference
tested number, administration treatment of observation produced incidence
and sex of and dosing treatment in
animals regimen surviving
animals
Senecio 40 males, 0.5% of diet for 1 year 428-657 liver cell 4/23
longilobus 40 females 1 month days carcinomas
(contd.) alternating with
normal diet for peritoneal 1/23
2 weeks mesothelioma
50 males, 0.5% of diet 54 weeks 217-470 liver cell 16/47
50 females for 1 week days carcinomas
alternating with
normal diet for angiosarcoma 1/47
1 week
controls not stated
(10 males,
10 females)
Lasiocarpine Fischer rat intraperitoneal till 60-76 hepatocellular 10/18 Svoboda & Reddy
(25 males) injection at moribund weeks carcinomas (1972)
7.8 mg/kg body or had
weight, twice palpable
weekly for 4 tumours cholangio- 1/18
weeks, then carcinoma
once a week
for an additional lung adenomas 5/18
52 weeks
Table 13. (contd.)
Material Strain, Route of Other Duration Period of Tumours Tumour Reference
tested number, administration treatment of observation produced incidence
and sex of and dosing treatment in
animals regimen surviving
animals
Lasiocarpine skin squamous 6/18
(contd.) cell
carcinomas
Fischer rat as above ileal 2/18 Svoboda & Reddy
(25 males) adenocarcinoma (1972)
ileal 1/18
adenomyoma
testicular 1/18
interstitial
cell tumour
controls lung adenomas 2/25
(25 males)
Table 13. (contd.)
Material Strain, Route of Other Duration Period of Tumours Tumour Reference
tested number, administration treatment of observation produced incidence
and sex of and dosing treatment in
animals regimen surviving
animals
Retronecine, Porton single sc spinal cord 1/10 Schoental &
hydrochlorine Wistar injection of ependymo- Cavanagh (1972)
newborn rat 300-1000 mg/kg blastoma
(6 males, body weight
6 females)
"pituitary 5/10
tumour"
"mammary 1/10
tumour"
Hydroxy- 5 males single ip brain 1/5 Schoental &
senkirkine (weanling) injection of astrocytoma Cavanagh (1972)
100-300 mg/kg
body weight
Table 13. (contd.)
Material Strain, Route of Other Duration Period of Tumours Tumour Reference
tested number, administration treatment of observation produced incidence
and sex of and dosing treatment in
animals regimen surviving
animals
Heliotropium 5 females 5% of diet fed Schwann cell 1/5
ramosissimum to 1 pregnant tumour of
rat during 1st spinal cord
15 days of
pregnancy and
from 10th day
of parturition
until weaning;
female off-
spring (5) fed
on experimental
diet for 10 days
at 6 months of
age
controls not stated
Table 13. (contd.)
Material Strain, Route of Other Duration Period of Tumours Tumour Reference
tested number, administration treatment of observation produced incidence
and sex of and dosing treatment in
animals regimen surviving
animals
Groups
I II
Petasites ACI rat 4% of diet for until 480 days liver cell Hirono et al.
japonicus Group I: 6 months; death or until adenomas 6/27 4/19 (1973)
(young 12 males, subsequently, moribund
flower 15 females 8% of diet liver 3/27 8/19
stalks) alternating haemangio-
weekly with endotheliomas
normal diet
liver cell 2/27 1/19
carcinomas
Group II: 4% of diet
11 males,
8 females
controls normal diet none
7 females/
8 females
Table 13. (contd.)
Material Strain, Route of Other Duration Period of Tumours Tumour Reference
tested number, administration treatment of observation produced incidence
and sex of and dosing treatment in
animals regimen surviving
animals
Mono- Sprague gastric intubation 72 weeks liver cell 10/42 Newberne &
crotaline Dawley rat weekly of 25 mg/kg carcinomas Rogers (1973)
(50 males) body weight for
4 weeks, then
8 mg/kg body
weight for
38 weeks
50 males as above, with liver cell 14/33
a diet deficient carcinomas
in lipotropes
Heliotrine Porton intragastric Schoental (1975)
Wistar
weanling
rat
4 males 300 mg/kg body until death none
weight; repeated (5 months)
after 3 weeks to
2 surviving rats
Table 13. (contd.)
Material Strain, Route of Other Duration Period of Tumours Tumour Reference
tested number, administration treatment of observation produced incidence
and sex of and dosing treatment in
animals regimen surviving
animals
Heliotrine 6 males 300 mg/kg body nicotinamide until death none Schoental (1975)
(contd.) weight; repeated (500 mg/kg (5 months)
after 3 weeks body weight),
ip, before
and after
each heliotrine
administration
4 males 230 mg/kg body 27 months pancreatic 1/1
weight; repeated islet cell
to 2/4 surviving adenoma
after 5 days
pituitary 1/1
adenoma
Table 13. (contd.)
Material Strain, Route of Other Duration Period of Tumours Tumour Reference
tested number, administration treatment of observation produced incidence
and sex of and dosing treatment in
animals regimen surviving
animals
Heliotrine 12 males 230 mg/kg body nicotinamide until death fibrosarcoma 1/8
(contd.) weight; repeated (350 mg/kg or killed of jaw
6.5 days later body weight), when moribund
ip, before, up to 27.5 pancreatic 3/6
plus 2 doses months islet cell
after each adenomasb
heliotrine
administration hepatomab 1/6
urinary 1/6
bladder
papillomab
12 males testicular 1/6
interstitial
cell tumourb
2 males ip injection nicotinamide 19-27.5 pituitary 1/2 Schoental (1975)
at 350 mg/kg months adenoma
body weight
Table 13. (contd.)
Material Strain, Route of Other Duration Period of Tumours Tumour Reference
tested number, administration treatment of observation produced incidence
and sex of and dosing treatment in
animals regimen surviving
animals
Heliotrine controls no treatment 19-27.5 pituitary 3/6
(contd.) (6 males) months adenomas
Tussilago ACI rat 32% in diet for 600 days 600 days haemangio- 8/12 Hirono et al.
farfara (6 males, 4 days, then 16% endothelioma (1976)
(coltsfoot) 6 females) until end of
(preblooming (1.5 months study liver cell 1/12
flowers) old) adenomac
hepatocellular 1/12
carcinomac
bladder 1/12
papillomac
Table 13. (contd.)
Material Strain, Route of Other Duration Period of Tumours Tumour Reference
tested number, administration treatment of observation produced incidence
and sex of and dosing treatment in
animals regimen surviving
animals
Tussilago 5 males, 8% in diet 600 days 600 days liver 1/9
farfara 5 females haemangio-
(contd.) endothelioma
6 males, 4% in diet 600 days 600 days none Hirono et al.
5 females (1976)
controls none 600 days 600 days none
(8 males,
8 females)
Dehydro- Sprague sc injection partial 12 months 10 months rhabdomyo- 36/60 Allen et al.
retronecine Dawley rat bi-weekly for 4 hepatectomy sarcomas (5 (1975)
Group I: months at on 15 with
75 males 20 mg/kg body animals metastases)
weight, followed after 4
by 10 mg/kg body months
weight for 8
months
Table 13. (contd.)
Material Strain, Route of Other Duration Period of Tumours Tumour Reference
tested number, administration treatment of observation produced incidence
and sex of and dosing treatment in
animals regimen surviving
animals
Monocrotaline Group II: sc injection partial 12 months 10 months rhabdomyo- 2/60
75 males bi-weekly at hepatectomy sarcomas
5 mg/kg body on 15
weight for 12 animals hepatocellular 2/60
months after 4 carcinomas
months
acute myeloid 2/60
leukaemia
pulmonary 2/60
adenomas
controls sc injection partial 12 months 10 months none mentioned Allen et al.
(50 males) bi-weekly, hepatectomy (1975)
0.1 mol on 5 animals
phosphate after 4
buffer, pH7 months
Table 13. (contd.)
Material Strain, Route of Other Duration Period of Tumours Tumour Reference
tested number, administration treatment of observation produced incidence
and sex of and dosing treatment in
animals regimen surviving
animals
Monocrotaline Sprague sc injection on 12 months any tumours 17/60 Shumaker et al.
(contd.) Dawley rat alternate weeks (several (1976)
(60 males) (5 mg/kg body animals had
weight) more than 1
tumour)
pulmonary 11/60
carcinomas
hepatocellular 5/60
carcinomas
acute myeloid 3/60
leukaemia
rhabdomyo- 4/60
sarcomas
adrenal 8/60
adenomas
kidney adenoma 1/60
Table 13. (contd.)
Material Strain, Route of Other Duration Period of Tumours Tumour Reference
tested number, administration treatment of observation produced incidence
and sex of and dosing treatment in
animals regimen surviving
animals
Dehydro- 60 males sc injection on 12 months 12 months rhabdomyo- 39/60
retronecine alternate weeks until sarcomas at
at 20 mg/kg body moribund site of
weight followed injection
by 10 mg/kg
body weight,
alternate weeks
for 8 months
controls adrenal 2/45
(45 males) adenomas
(same group
as above)
Mono- Sprague single sc pancreatic 16/23 Hayashi et al.
crotaline Dawley rat injection of insulinomas (1977)
(80 males) 40 mg/kg body
weight
Petasitenine ACI rat drinking-water 45 days 72 days none Hirono et al.
(3 males) (0.05% solution) (1977)
(1 month
old)
Table 13. (contd.)
Material Strain, Route of Other Duration Period of Tumours Tumour Reference
tested number, administration treatment of observation produced incidence
and sex of and dosing treatment in
animals regimen surviving
animals
Petasitenine 5 males, 0.01% solution until up to 16 haemangio- 5/10d
(contd.) 6 females death months endothelial
or sarcomas
moribund
liver adenomas 5/10d
controls none fibrosarcoma 1/10 Hirono et al.
(10 males, (subcutaneous) (1977)
9 females)
Lasiocarpine Fischer rat mixed with 55 weeks 59 weeks liver 9/20 Rao & Reddy
(20 males) feed at a angiosarcomas (1978)
concentration of
50 mg/kg hepatocellular 7/20
carcinomas
malignant 1/20e
adnexal tumour
of skin
malignant 1/20e
lymphoma
Table 13. (contd.)
Material Strain, Route of Other Duration Period of Tumours Tumour Reference
tested number, administration treatment of observation produced incidence
and sex of and dosing treatment in
animals regimen surviving
animals
Lasiocarpine controls none
(contd.) (10 males)
Petasites ddN mice 4% of diet as 480 days 480 days lung adenomas 24/39 Fushimi et al.
japonicus 24 males dried flower (1978)
(flower 21 females stalks lung 6/39
stalks) adenocarcinoma
liver 4/39
reticullum
cell sarcoma
liver 1/39
haemangio-
endothelial
sarcoma
thymoma 1/39
leukemia 2/39
Table 13. (contd.)
Material Strain, Route of Other Duration Period of Tumours Tumour Reference
tested number, administration treatment of observation produced incidence
and sex of and dosing treatment in
animals regimen surviving
animals
Petasites control nil 480 days 480 days lung 1g
japonicus 23 males kidney
(contd.) 27 females haemangio-
endothelial
sarcoma 1g
spleen
haemangioma 1g
Swiss 4% diet as 480 days 480 days lung 5/26 Fushimi et al.
strain mice dried flower adenoma (1978)
20 males stalks
20 females leukemia 1/26
controls nil 480 days 480 days liver 1g
23 males haemangio-
20 females endothelioma
breast 1g
carcinoma
Table 13. (contd.)
Material Strain, Route of Other Duration Period of Tumours Tumour Reference
tested number, administration treatment of observation produced incidence
and sex of and dosing treatment in
animals regimen surviving
animals
Petasites lung adenoma 3g
japonicus
(contd.)
leukemia 2g
C57BL/6 4% diet as 480 days 480 days no tumours
mice dried flower
20 males stalks
20 females
controls none 480 days 480 days lung adenoma 1g
20 males
20 females
Syrian 4% diet as 480 days 480 days adrenal 1/25 Fushimi et al.
golden flower stalks cortical (1978)
hamsters adenoma &
13 males breast
17 females carcinoma
Table 13. (contd.)
Material Strain, Route of Other Duration Period of Tumours Tumour Reference
tested number, administration treatment of observation produced incidence
and sex of and dosing treatment in
animals regimen surviving
animals
Petasites controls none 480 days 480 days no tumours
japonicus 12 males
(contd.) 9 females
Symphytum ACI rat 33% of diet as 480 days until death liver adenomas 5/19 Hirono et al.
officinale (1 - 1.5 leaves or moribund (1979b)
(leaves or months old)
root) urinary 1/19
Group I.1: bladder
11 males, papilloma
8 females
urinary 2/19
bladder
carcinomas
(rats with 5/19
tumours)
Table 13. (contd.)
Material Strain, Route of Other Duration Period of Tumours Tumour Reference
tested number, administration treatment of observation produced incidence
and sex of and dosing treatment in
animals regimen surviving
animals
Symphytum Group I.2: 33% of diet as 600 days until death liver adenomas 11/19
officinale 10 males, leaves or moribund
(contd.) 10 females
urinary 2/19
bladder
papillomas
(rats with 11/19
tumours)
Group II: 16% of diet as 600 days until death liver adenomas 7/21 Hirono et al.
11 males, leaves or moribund (1979b)
10 females
haemangio- 1/21
endothelial
sarcoma
Table 13. (contd.)
Material Strain, Route of Other Duration Period of Tumours Tumour Reference
tested number, administration treatment of observation produced incidence
and sex of and dosing treatment in
animals regimen surviving
animals
Symphytum urinary 2/21
officinale bladder
(contd.) papillomas
urinary 1/21
bladder
carcinoma
lymphatic 1/21
leukaemia
colonic 1/21
adenoma
pituitary 1/21
adenoma
(rats with 7/21
tumours)
Table 13. (contd.)
Material Strain, Route of Other Duration Period of Tumours Tumour Reference
tested number, administration treatment of observation produced incidence
and sex of and dosing treatment in
animals regimen surviving
animals
Symphytum Group III: 8% of diet as 600 days until death liver adenoma 1/25 Hirono et al.
officinale 14 males, leaves or moribund (1979b)
(contd.) 14 females
Group IV: 8% of diet as until liver adenomas 19/22
12 males, root death
12 females
urinary 2/19
bladder
papilloma
(rats with 19/22
tumours)
Group V: 4% of diet as until until death liver adenomas 16/42
24 males, root reduced by death or moribund
24 females stages to basal
diet after 180 urinary 1/42
days bladder
carcinoma
Table 13. (contd.)
Material Strain, Route of Other Duration Period of Tumours Tumour Reference
tested number, administration treatment of observation produced incidence
and sex of and dosing treatment in
animals regimen surviving
animals
Symphytum adrenal 2/42
officinale cortical
(contd.) adenomas
(rats with 16/42
tumours)
Group VI: 2% of diet as 280 days liver adenomas 10/23
12 males, root for 190
12 females days reduced by
stages to basal
diet
Group VII: 1% of diet as until liver adenomas 16/24 Hirono et al.
15 males, root for 275 death (1979b)
15 females days and
subsequently a liver 4/24
basal diet haemangio-
alternating sarcomas
with 0.5% at
3-week intervals cholangio- 1/24
carcinoma
Table 13. (contd.)
Material Strain, Route of Other Duration Period of Tumours Tumour Reference
tested number, administration treatment of observation produced incidence
and sex of and dosing treatment in
animals regimen surviving
animals
Symphytum adrenal 1/24
officinale cortical
(contd.) adenomas
(rats with 17/24
tumours)
Group VIII: 0.5% of diet as entire liver adenomas 8/30
15 males, root study
15 females
liver 9/30
haemangio-
sarcomas
pituitary 1/30
adenoma
uterine 1/30
adrenocarcinoma
Table 13. (contd.)
Material Strain, Route of Other Duration Period of Tumours Tumour Reference
tested number, administration treatment of observation produced incidence
and sex of and dosing treatment in
animals regimen surviving
animals
Symphytum gonadal 1/30 Hirono et al.
officinale stromal tumour (1979b)
(contd.)
(rats with 10/30
tumours)
controls none till the urinary 1
(65 males, end bladder
65 females) papilloma
subcutaneous 1
fibrosarcoma
caecal adenoma 1
mammary 1
fibroadenoma
retroperitoneal 1
teratoma
Table 13. (contd.)
Material Strain, Route of Other Duration Period of Tumours Tumour Reference
tested number, administration treatment of observation produced incidence
and sex of and dosing treatment in
animals regimen surviving
animals
Senkirkine ACI rat ip injection of 4 weeks, 650 days liver adenomas 9/20 Hirono et al.
(20 males) 22 mg/kg body 52 weeks or till (1979a,b)
weight, twice moribund myeloid 1/20
weekly, then leukaemia
once weekly
testis
interstitial 1/20
tumour
Symphytine 20 males ip injection of 4 weeks, 650 days liver adenoma 1/20
22 mg/kg body 52 weeks or till
weight, twice death or liver 3/20
weekly, then moribund haemangio-
once weekly sarcomas
controls myeloid 1/20
(20 males) leukemia
Table 13. (contd.)
Material Strain, Route of Other Duration Period of Tumours Tumour Reference
tested number, administration treatment of observation produced incidence
and sex of and dosing treatment in
animals regimen surviving
animals
Symphytine fibroma-soft 1/20
(contd.) tissues
testis- 1/20
interstitial
tumour
Clivorine ACI rat drinking-water 340 days 480 days liver 2/12 Kuhara et al.
(6 males, (0.005%) haemangio- (1980)
6 females) sarcomas
ACI rat drinking-water hepatic 6/12
(6 males, (0.005%) neoplastic
6 females) nodules
testicular 3/12
interstitial
cell tumour
Table 13. (contd.)
Material Strain, Route of Other Duration Period of Tumours Tumour Reference
tested number, administration treatment of observation produced incidence
and sex of and dosing treatment in
animals regimen surviving
animals
Clivorine 10 males, pituitary 1/20
(contd.) 10 females adenoma
testicular 3/20f Kuhara et al.
interstitial
cell tumour
adreno- 1/20f
cortical
adenoma
pancreatic 1/20
aunar cell
adenoma
Table 13. (contd.)
Material Strain, Route of Other Duration Period of Tumours Tumour Reference
tested number, administration treatment of observation produced incidence
and sex of and dosing treatment in
animals regimen surviving
animals
Crude Sprague intragastric 104 weeks 114 weeks liver tumours 13/40 (2 Habs et al.
alkaloidal Dawley rat dose of 8 mg/kg (all types) males, 11 (1982)
extract (20 males, body weight, 5 females)
from 20 females) times per week
Senecio other tumours 11/40
numorensis (all types) (5 males,
fuchsii 6 females)
20 males, intragastric 104 weeks 114 weeks liver tumours 34/40
20 females dose of 40 mg/kg (all types) (5 males,
body weight, 5 29 females)
times per week
other tumours 10/40
(all types) (7 males,
3 females)
controls liver tumour 1/40
(20 males, (male)
20 females)
other tumours 10/40
(all types) (4 males,
6 females)
Table 13. (contd.)
Material Strain, Route of Other Duration Period of Tumours Tumour Reference
tested number, administration treatment of observation produced incidence
and sex of and dosing treatment in
animals regimen surviving
animals
Farfugium AC1 rat diet (20%) 480 days till death liver 6/29 Hirono et al.
japonicum (15 males, or end of haemangio- (1983)
(leaves 14 females) study sarcomas
and
stalks) liver adenomas 7/29
adrenalcortical 7/29
adenomas
adrenal 1/29
phaeochromo-
cytoma
urinary 2/29
bladder
papillomas
ACI rat diet (20%) testicular 2/29
(15 males, interstitial
14 females) tumours
ileal 1/29
adenocarcinoma
Table 13. (contd.)
Material Strain, Route of Other Duration Period of Tumours Tumour Reference
tested number, administration treatment of observation produced incidence
and sex of and dosing treatment in
animals regimen surviving
Senecio 15 males, diet (8%) none Hirono et al
cannabifolius 15 females survived (1983)
(leaves and > 240 days
stalks)
14 males, diet (4%) none
14 females survived
> 240 days
12 males, diet (1%) 480 days till dead liver 1/23
12 females haemangio-
sarcoma
liver adenomas 13/23
adrenal 5/23
cortical
adenomas
urinary 1/23
bladder
papilloma
Table 13. (contd.)
Material Strain, Route of Other Duration Period of Tumours Tumour Reference
tested number, administration treatment of observation produced incidence
and sex of and dosing treatment in
animals regimen surviving
animals
Senecio testicular 1/23
cannabifolius interstitial
(contd.) tumour
12 males, diet (0.2%) 480 days till death liver 8/24
12 females haemangio-
sarcomas
liver cell 3/24
adenomas
adreno- 3/24
cortical
adenomas
adrenal 1/24 Hirono et al.
phaeochromo- (1983)
cytoma
testicular 2/24
interstitial
tumours
pituitary 1/24
adenoma
caecal 1/24
fibrosarcoma
Table 13. (contd.)
Material Strain, Route of Other Duration Period of Tumours Tumour Reference
tested number, administration treatment of observation produced incidence
and sex of and dosing treatment in
animals regimen surviving
animals
Senecio controls basal diet 560 days cortical 3/49
cannabifolius (25 males, adenomas of
(contd.) 24 females adrenal
controls basal diet testicular 3/49 Hirono et al.
(25 males, interstitial (1983)
24 females) tumours
pituitary 2/49
adenomas
a These tumours were present in the same animal.
b Each coexisted in one animal (seen in animals surviving 22 - 27.5 months).
c Together with haemangioendothelioma of the liver.
d Two animals had both tumours.
e Found in the same animal with angiosarcoma.
f In the same animal.
g No data available on number of surviving animals in the control groups.
Note: Figures in column 2 indicate the number of animals used at the start of the study. In column 8, animals developing a tumour out of
the number surviving up to the end of study/or dying with tumour are indicated. Animals developing more than one tumour are
indicated separately in a footnote. Different terms have been used in different studies to describe the same tumour in the liver,
e.g., haemangiosarcoma, angiosarcoma, haemangioendothelioma, haemangioendothelial sarcoma, and haemangiogenic sarcoma.
Table 14. Liver tumours found in rats given PAs and plant materials (condensed results from various authors)a
Alkaloid or plant Alkaloid type Route of Number of Number and types of liver Reference
given Necine Type of administration rats at tumours found
ester autopsy
Clivorine otonecine macrocyclic oral 12 2 haemangiosarcomas Kuhara et al. (1980)
diester 6 neoplastic nodules
Lasiocarpine heliotridine "open" ip 18 11 hepatocellular carcinomas Svoboda & Reddy
diester oral 20 9 angiosarcomas (1972);
7 hepatocellular carcinomas Rao & Reddy (1978)
Monocrotaline retronecine macrocyclic oral 75 24 hepatocellular carcinomas Newberne & Rogers
diester (1973)
Petasitenine otonecine macrocyclic oral 11 5 haemangiosarcomas Hirono et al. (1977)
diester 5 adenomas
Retrorsine retronecine macrocyclic various 14 4 "hepatomas" Schoental et al.
diester (1954);
Schoental & Head
(1957)
Isatidine retronecine macrocyclic various 7 4 "hepatomas" and Schoental et al.
diester "nodular hyperplasia" (1954);
Schoental & Head
(1957)
Senecionine retronecine macrocyclic oral 80 19 hepatocellular tumours Habs et al. (1982)
(+ fuchsisenecionine) (platynecine) diester, 16 cholangiogenic tumours
monoester 12 "haemangiogenic" tumours
Table 14 (cont'd)
Alkaloid or plant Alkaloid type Route of Number of Number and types of liver Reference
given Necine Type of administration rats at tumours found
ester autopsy
Senkirkine otonecine macrocyclic ip 20 9 adenomas Hirono et al.
diester (1979a)
Symphytine retronecine "open" ip 20 1 adenoma Hirono et al.
diester 3 haemangiosarcomas (1979a)
Senecio longilobus macrocyclic oral 47 16 hepatocellular carcinomas Harris & Chen
(seneciphylline, (retronecine) diester 1 angiosarcoma (1970)
retrorsine, etc.)
Petasites japonicus macrocyclic oral 46 11 haemangiosarcomas Hirono et al.
(petasitenine) (otonecine) diester 10 adenomas (1973)
3 hepatocellular carcinomas
Tussilago farfara macrocyclic oral 12 8 haemangiosarcomas Hirono et al.
(senkirkine) (otonecine) diester (1976)
Symphytum officinale monoester + oral 175 81 adenomas Hirono et al.
(various) (retronecine) "open" 3 haemangiosarcomas (1978)
diester
Farfugium japonicum senkirkine oral 29 7 adenomas Hirono et al.
6 haemangioangiosarcomas (1983)
Senecio cannabifolius petasitenine oral 21 16 adenomas Hirono et al.
9 haemangioangiosarcomas (1983)
a From: Mattocks (1986).
Svoboda & Reddy and their group have carried out 2 studies
using lasiocarpine (Svoboda & Reddy, 1972, 1974; Rao & Reddy,
1978). Svoboda & Reddy (1972, 1974) gave repeated ip injections to
25 rats at a dose of 7.8 mg/kg body weight (0.1 LD50) for 56 weeks
as per regimen shown in Table 13. Three rats died of acute liver
necrosis in the initial 4 weeks. Eighteen rats survived 56 weeks,
by which time each animal had received an average cumulative dose
of 125 mg lasiocarpine. Of these, 16 animals developed a variety
of tumours 60 - 76 weeks after the beginning of the study (Table
13). Ten of the 16 animals had more than 1 tumour. Hepatocellular
carcinoma was the most common. The squamous cell carcinoma of the
skin was found to be transplantable. When the same PA, mixed with
the diet at the rate of 50 mg/kg (Rao & Reddy, 1978) (Table 13),
was administered to 20 rats for 55 weeks, 17 animals developed
tumours. Angiosarcoma of the liver emerged as the most common
tumour (9/20 animals), even though hepatocellular carcinomas were
also frequently seen (7/20 animals); squamous cell carcinoma of the
skin was not found, but there was a malignant adnexal tumour of the
skin. The average cumulative dose of the alkaloid was estimated to
be 190 - 200 mg per rat.
Monocrotaline has been studied for its carcinogenic activity in
rats by Schoental & Head (1955), Newberne & Rogers (1973), Allen et
al. (1975), Shumaker et al. (1976), and Hayashi et al. (1977),
using different routes of administration.
Allen et al. (1975) studied the long-term effects on rats of
repeated sc injections of monocrotaline or its major detectable
metabolite, dehydroretronecine. Male Sprague Dawley rats were
given biweekly injections of monocrotaline, at 5 mg/kg body weight
(75 animals) for 12 months, or dehydroretronecine at 20 mg/kg body
weight (75 animals) for 4 months followed by 10 mg/kg body weight
for 8 months. Fifty control animals received phosphate buffer
(Table 13). Partial hepatectomy was performed on 15 animals in
each of the treated groups and 5 in the control group. They were
observed for 10 months following cessation of the injections. Of
the 60 animals surviving in each of the treated groups, those
receiving monocrotaline showed rhabdomyosarcoma at the injection
site (2 animals), hepatocellular carcinoma (2 animals), acute
myelocytic leukaemia (2 animals), and pulmonary adenoma (2
animals). In the group receiving dehydroretronecine, 36 animals
developed rhabdomyosarcomas, and 5 of these animals developed
metastases. None of the control group developed tumours. Tissues
obtained from partial hepatectomies showed that both compounds
caused inhibition of mitotic division in regenerating liver. The
results of the study illustrate the dual alkylating and antimitotic
properties of these agents, commented on by Culvenor et al. (1969).
In a similar study by Shumaker et al. (1976), rats were
administered monocrotaline at 5 mg/kg body weight on alternate
weeks or its metabolite dehydroretronecine at 20 mg/kg body weight,
on alternate weeks for 4 months, followed by a dose of 10 mg/kg on
alternate weeks in the succeeding 8 months (Table 13). Of the 60
rats receiving monocrotaline, 17 developed one or more tumours, but
not until after the treatment was discontinued. The time interval
was not stated. The most common tumour was carcinoma of the lung
(11 animals) followed by hepatocellular carcinoma (5 animals). A
wide variety of other tumours was also seen. A notable feature was
that the metabolite dehydroretronecine did not produce any tumours
by systemic action, but only at the site of injection, where
significant numbers of rhabdomyosarcomas (39/60 animals) were seen.
The marked difference in tumour sites is explained by the fact that
the parent alkaloid monocrotaline has to be metabolized before it
becomes a carcinogen. For this reason, the tumours are distributed
in several organs of the body, whereas dehydroretronecine is itself
carcinogenic and so acts at the site of injection. When
monocrotaline was delivered in a higher dose, but as a single
subcutaneous injection (40 mg/kg body weight) (Hayashi et al.,
1977) to 40 rats there were no malignant tumours but only adenomas
of the islets in the pancreas in 16/23 surviving animals. The
results of the above studies indicate that monocrotaline is
tumorigenic, but the type of tumour and the malignancy both depend
on the route of administration and the dosage used.
Hirono et al. (1977) studied petasitenine, the pure alkaloid
isolated from the flower stalks of the plant, Petasites japonicus
Maxim, which has been found to be carcinogenic for rats (Hirono et
al., 1973). Two groups of ACI rats of both sexes, Group I of 3
animals and Group II of 11 animals, were given the alkaloid in in
the drinking-water, at concentrations of 0.5 g/kg and 0.1 g/kg,
respectively (Table 13). There were 19 controls. All 3 animals in
group I died within 72 days showing marked hepatocellular damage;
no tumours were seen. In Group II, 10 out of 11 rats survived for
more than 160 days. Eight of the 10 animals developed tumours -
liver cell adenomas (5) and haemangiosarcomas (5) (Table 10). Two
animals had both types of tumours. The authors concluded that the
carcinogenicity of the plant was due to petasitenine.
Retrorsine and its N-oxide (isatidine) have been used in
several studies. Schoental et al. (1954) administered retrorsine
at a concentration of 3 mg/litre in the drinking-water to 14 rats
and isatidine at concentrations of 5 mg/litre followed by 3 mg/litre
to 22 rats until death. Twenty-five rats were administered the
alkaloid mixture from Senecio jacobaea Lin at a concentration of
500 mg/litre followed by 300 mg/litre in the drinking-water. Dosing
regimens are shown in Table 13. One group of 7 animals receiving
isatidine received supplementary 0.5% choline in the drinking-water
and another group of 5 animals received isatidine 2 mg as a single ip
administration in 0.2 ml of tricaprilyn, followed by skin application
of alkalids as 0.5% solution for 3 days/week.
The animals receiving mixed alkaloids of the plant showed
extensive liver damage followed by marked nodular hyperplasia; no
tumours developed. The nodules were earlier interpreted as
hepatomas, but later only as an early stage in the progression from
hyperplasia to neoplasia.
The retrorsine group showed extensive liver damage associated
with cirrhosis and nodular hyperplasia. In 4 rats, they were
interpreted as hepatomas.
In the isatidine group, 10 out of the 22 rats developed
hepatomas. Tumours were present in 3 out of 7 animals receiving
isatidine plus choline indicating that the latter had no protective
role. One out of the 5 animals receiving the alkaloid ip and then
through dermal application developed a tumour, which was also
interpreted as a hepatoma.
In another study (Schoental & Bensted, 1963), 95 weanling rats
were administered a single dose of retrorsine at 30 mg/kg body
weight, by stomach tube (Group II). One comparable group of 50
weanling rats (Group I) received 400 r radiation in animals
surviving 100 days after retrorsine administration (31/50). A
third group (Group III) of 6 animals received 400 r radiation
alone. Another group of 10 weanling rats received the PA, 9 days
after partial hepatectomy (Group IV) (Table 13). The additional
treatments were given to study whether they would act as
co-carcinogens and induce neoplasia in hepatocytes, which are known to
show injurious effects for long periods following PA treatment. In
Group I, 19 of the 50 animals receiving a single dose of the PA
died before radiation could be given. Of the 31 remaining animals
that received radiation after the PA, 25 survived 12 months. Among
these, 19 tumours of a wide variety were seen (Table 13). Of the 6
tumours in the liver, only 1 was malignant, having metastasized.
Most of the other tumours were malignant, including those of the
breast, one of which had also metastasized.
In Group II, 29 out of 95 animals that had received one dose of
PA and no radiation survived for more than a year, with a mean
survival time of 23 months. Among these, 7 animals developed
tumours of a wide variety (Table 13). Five tumours in the liver
were benign. Most of the others were malignant. Two tumours, a
cystic tumour of the breast and a carcinoma of the uterus, were
present in one animal. Tumours seen in Groups III and IV, which
were found in animals surviving 12 months or more after the start
of the study, are shown in the Table 13. The 2 tumours of the
liver in Group IV were also benign. The number of control animals,
if any, were not indicated.
The authors concluded that the above studies did not provide
definite evidence of synergistic action in the carcinogenicity of
retrorsine by whole body radiation or partial hepatectomy.
Hirono et al. (1979a) studied the carcinogenic properties of
senkirkine extracted from the dried milled buds of Tussilago
farfara (coltsfoot) and symphytine extracted from dried milled
roots of Symphytum officinale (comfrey). Both Tussilago farfara
(Hirono et al., 1976) and Symphytum officinale (Hirono et al.,
1978) had earlier been demonstrated to have carcinogenic
properties.
Sixty inbred ACI strain male rats were divided into 3 groups of
20 animals each and received repeated ip injections of senkirkine
at 22 mg/kg body weight or symphytine at 13 mg/kg body weight as
per schedule given in Table 13. All animals treated with
senkirkine survived 290 days. Nine out of 20 rats developed liver
adenomas mostly after 350 - 450 days from start of the study.
Cirrhosis of liver was frequently observed. Of the symphytine
group, all animals survived 330 days after the start of the study.
Three animals developed haemangioendothelial sarcoma and one,
liver adenoma. The sarcomas were noted at least 518 days after the
start of the study.
Schoental & Cavanagh (1972) used two alkaloids, retronecine and
hydroxysenkirkine isolated from Crotalaria laburnifolia, which was
injected ip in single doses ranging from 100 to 300 mg/kg body
weight in 5 weanling Porton Wistar rats (Table 13). One animal
that had received the 300 mg/kg dose developed astrocytoma of the
brain after 14 1/2 months. Retronecine hydrochloride was
administered in doses ranging from 300 to 1000 mg/kg body weight by
single subcutaneous injection to 10 new-born rats. One male rat,
which was found to be paraplegic 6.6 months after receiving a dose
of 600 mg PA/kg, was also found to have ependymoblastoma of the
spinal cord. Among the litter mates of this rat, 1 male that had
received a dose of 1000 mg/kg died. The remaining 6 females and 2
males were killed within 22 months of being dosed. Of the females,
5 had pituitary tumours and 1 had a mammary tumour (type not
stated). Two males did not show any significant abnormalities.
One group of 5, 6-month-old female rats, born to dams that had
been fed on a diet containing 50 g dried and powdered Heliotropium
ramosissimum/kg diet, a tribal remedy, used during pregnancy and
parturition, were then themselves fed on the same diet at 6 months
of age, as per the regimen indicated in Table 13. One female rat
was found to be paraplegic at 7 months of age. A tumour, possibly
of Schwann or satellite cell origin, was found.
6.4.8.2 Plant materials
A number of plant materials have been tested for their
carcinogenicity by administering either a mixture of extracted
alkaloids (Cook et al., 1950; Schoental et al., 1954) or, more
often, the dried and milled plant mixed with the diet. The only
study in which the plant alone was fed was that of Campbell (1956),
who produced tumours in chickens by feeding dried and milled
Senecio jacobaea plant. It is notable that the plants tested are
almost all those that have been reported to be used as herbal
medicines and/or food, some of which have been reported to cause
human toxicity.
Schoental et al. (1970) used mixed PAs (intermedine and
lycopsamine) extracted from seeds of Amsinckia intermedia, known to
cause livestock losses in the USA, and leaves and stems from
Heliotropium supinum L., known to be used by women in East Africa
as a herbal medicine, after childbirth. The dosing regimen and
mode of administration are indicated in Table 13. Of the 15 male
weanling rats that had received a single treatment with the
Amsinckia PAs and survived for more than 1 year, 3 rats showed one
adenoma, one adenocarcinoma of the islet cells, and one adenoma of
the exocrine pancreas, respectively (Table 13). In addition, one
of the animals also developed a pituitary adenoma and papillary
tumour of the urinary bladder. Eight animals received the
treatment with Heliotropium supinum L. (Table 13). One out of the
2 weanling rats fed on the plant with the diet and one out of the 6
animals that received a single intragastric dose of the crude
alkaloid fraction developed islet cell adenoma of the pancreas.
The number of control animals, if any, used in the study is not
stated.
Harris & Chen (1970) tested the carcinogenicity of Senecio
longilobus, which has been associated with cases of human toxicity
(Stillman et al., 1977; Huxtable, 1980; Fox et al., 1978). Harlan
rats were divided into 4 groups (equal numbers of both sexes) and
fed diets containing dried and powdered stems and leaves of the
plant in the proportion of 5 -7.5 g/kg. The number of animals in
each group and the feeding regimen are shown in Table 13.
Continuous feeding did not produce any tumours, presumably because
of the comparatively low survival rate of animals. Significant
results were obtained when the animals were fed contaminated diet
alternating with normal diet (Group IV). Of the 100 animals fed on
this regimen for 54 weeks, 47 survived for more than 200 days.
Seventeen of these animals developed malignant tumours in the
livers - hepatocellular carcinomas in 16 and an angiosarcoma in 1
animal after a minimum feeding period of 217 days. In Group III,
fed a contaminated diet for 1 year, 23 rats lived for more than 200
days. Four rats (3 males and 1 female) developed hepatocellular
carcinomas and one, a peritoneal mesothelioma, after a minimum
feeding period of more than 428 days. The tumour-bearing animals
were predominantly male. The authors emphasized the relative
rarity of liver tumours in the strain of animals used. Results
demonstrated that S. longilobus is carcinogenic for rats.
For a study of Schoental & Cavanagh (1972), using dried and
powdered Heliotropium ramosissimum, see section 6.4.8.1.
Hirono et al. tested the carcinogenic effects of 3 widely-used
herbs containing PAs. Hirono et al. (1973) studied the possible
carcinogenic effects of the young flower stalks of Petasites
japonicus Maxim, which has long been used in Japan as food or
herbal remedy as well as the PA, petasitenine, isolated from it.
The flower stalks of the plant were dried, milled, and fed to young
ACI rats of both sexes mixed with the basal diet in the proportion
of 40 - 80 g/kg, as indicated in the feeding regimen in Table 13,
until the animals were moribund or dead. One group of 27 rats was
fed the plant mixed in the proportion of 40 g/kg diet for 6 months
followed by 80 g/kg diet on alternate weeks. The second group of
19 rats was fed a contaminated diet (40 g/kg) continually. Three
animals in Group I died of pneumonia. Eleven out of the remaining
24 rats in this group developed liver tumours after 15 - 16 months
of feeding. There were 2 hepatocellular carcinomas, 6 liver cell
adenomas, and 3 haemangiosarcomas, of which 2 had metastasized. In
Group II, 2 animals died from non-tumorous causes. Of the
remaining 17, 8 developed haemangiosarcomas, 4 liver cell adenomas
and 1 had a hepatocellular carcinoma. The incidence of
haemangiosarcoma was statistically higher in Group II.
In a similar study on mice and hamsters (Fushimi et al. (1978),
groups comprising 20 - 24 male and 20 - 21 female 6-week-old ddN,
Swiss, and C57BL/6 mice, and 13 male and 17 female hamsters, were
fed a diet combining 4% young, dried, and milled flower stalks of
Petasites japonicus Maxim for 480 days. All surviving animals were
killed at the end of the study. Lung adenomas and adenocarcinomas
were found in 30/39 surviving male and female ddN mice combined
(compared with 1/50 in the respective controls) in addition to
other tumours (Table 13). No significant differences in tumour
incidence were observed between treated Swiss and C57BL/6 mice and
hamsters, and the corresponding controls. No data were given on
the tumour incidence according to sex, in treated and control
animals or on survival in the controls.
In studies on rats (Hirono et al., 1976), the dried and
powdered flower buds of Tussilago farfara (coltsfoot) were mixed
with the diet. Forty-nine inbred ACI strain rats were divided into
4 groups and fed diets containing 40 - 320 g T. farfara/kg for up
to 600 days. The number of animals in each group and the dosage
and feeding regimen are given in Table 13. Two groups receiving a
diet containing 80 - 320 g/kg on a continual basis developed
tumours in the liver of the same types as animals in the studies
using Petasites japonicus, e.g., haemangioendotheliomas, liver
cell adenomas, and hepatocellular carcinomas (Table 13). All 12
animals in Group I survived for more than 380 days after the start
of the study. Of these, 8 (5 males, 3 females) developed
haemangioendothelial sarcoma of the liver. In addition, 3 of the 8
rats developed simultaneously hepatocellular adenoma, hepatocellular
carcinoma, or urinary bladder papilloma. In Group II receiving
coltsfoot at 80 g/kg in the diet, 9 out of 10 animals that survived
for more than 420 days developed a haemangioendothelial sarcoma.
Hirono et al. (1978) fed Symphytum officinale, similarly dried
and milled, to the ACI strain of inbred rats of both sexes at
different levels ranging from 80 to 330 g/kg as leaves or 5 - 40 g/kg
as root, for 280 days or more. Eight groups of animals of
19 - 48 animals each were used on different regimens of feeding.
The feeding regimen, the number of animals in each group, and the
duration of treatment are given in Table 13. Sixty-five males and
64 females served as controls. Tumours were induced in all groups
receiving leaves or roots. The most common tumour was liver
adenoma. Haemangiosarcomas were observed but infrequently (3
animals in the whole study). No carcinomas of the liver were seen,
but there was a wide variety of other tumours. It is noteworthy
that, in Group VII, 14 of 15 animals fed the lowest dose of 10 g/kg
for 275 days followed by 5 g/kg or basal diet alternating every 3
weeks, developed tumours, 2 of which were malignant (haemangiosarcomas).
In the control group, single animals each had papilloma of urinary
bladder, caecal adenoma, subcutaneous fibrosarcoma, mammary
fibroadenoma, or retroperitoneal teratoma. The livers of animals that
did not have the tumours showed other features commonly encountered in
animals administered PAs, e.g., megalocytosis, liver cirrhosis,
hyperplastic nodules, etc., suggesting that they were induced by the
PAs contained in the plant. The authors concluded that the
carcinogenic activity of this plant was weaker than that observed in
animals fed Petasites (coltsfoot). Hirono et al. (1979b) have
summarized the above studies on PAs found in edible plants in Japan.
Habs (1982) and Habs et al. (1982) tested a crude alkaloid
extract from the plant Senecio numorensis sp. fuchsii containing
fuchsisenecionine (500 g/kg) and senecionine (10 g/kg). The
extract was administered intragastrically in 2 doses of 8 mg and
40 mg/kg body weight, respectively, to 2 groups of rats of both sexes,
comprising 40 animals each, 5 times a week for 104 weeks (Table
13). A large, dose-related number of liver tumours was produced,
originating in the liver cell and the sinusoidal system, and
predominantly affecting the female animals. The tumour incidence
was higher in Group II (dose, 40 mg/kg) with 34 tumours compared
with 13 in Group I. In Group I (dose, 8 mg/kg), 2 tumours were
found in 20 males compared with 11 tumours among 20 female rats.
Similarly, in Group II, only 6 tumours were found in 20 males
compared with 29 among 20 female rats. The tumours included 19 of
"hepatocellular origin", 16 of "cholangigenic origin", and 12 of
"haemangiogenic origin" (Table 14). Besides the liver tumours, 12
males and 9 females in the treated groups and 4 males and 6 females
in the control group developed a variety of extra-hepatic tumours.
Senecionine is known to be hepatotoxic and capable of being
converted in the rat liver into cytotoxic metabolites.
Fuchsisenecionine is a saturated PA, not previously known to be
cytotoxic. It is therefore likely that senecionine was the
hepatotoxic component and that perhaps the two PAs acted
synergistically with each other, though there is no actual evidence
for synergism. However, this needs confirmation. Moreover, there
appeared to have been other unknown components in the mixture
tested (Mattocks, 1986).
Hirono et al. (1983) studied the carcinogenicity of 2 more
plants (Farfugium japonicum and Senecio cannabifolius) of the tribe
Senecioneae in the family Compositae, the leaves and stalks of
which are used in Japan as human food. Fresh leaves and stalks of
the plants were dried, milled, and mixed with the basal diet.
Inbred strain ACI rats of both sexes, with preponderance of
females, 1.5 months old, were divided into 6 groups. They were fed
diets containing various proportions of the dried plant materials,
as indicated in Table 13. The study was terminated at 480 days,
except for one group, which was studied for 560 days. Besides the
groups shown in the tables, 2 more groups of 30 and 28 animals of
equal numbers of both sexes were fed 8% and 4% of Senecio
cannabifolius, respectively. None of these animals survived more
than 177 days and all died of hepatotoxicity. A wide range of
tumours was observed, mostly in the liver, as shown in Table 13,
the most common being haemangiosarcomas, which were not encountered
in the control group.
The carcinogenicity of Farfugium japonicum is considered to be
due to senkirkine and petasitenine. Senecio cannabifolius contains
senecicannabine, a new macrocyclic PA, seneciphylline, and
jacozine. It is probable that the carcinogenicity of Senecio
cannabifolius is due to these PAs (Hirono, et al., 1983).
Hayes et al. (1985) studied the biological mechanisms by which
PAs initiate carcinogenesis in male Fischer 344 rats. Lasiocarpine
(single or double injection of up to 80 µmol/kg body weight) and
senecionine (single or double injection of up to 160 µmol/kg) were
inactive as initiators of Y-GT-positive nodules in rats exposed to
2-acetylaminofluorene and partial hepatectomy. Administration of
lasiocarpine or senecionine 12 h after partial hepatectomy resulted
in the development of very few nodules. Lasiocarpine given in a
single or double dose (up to 80 µmol/kg) delayed hepatic
regeneration by at least 8 weeks after partial hepatectomy, and
pretreatment with this PA reduced the initiating capacity of
diethylnitrosamine and N-nitrosomethylurea in rats subsequently
selected with 2-acetylaminofluorene and partial hepatectomy.
Resistant nodules selected with lasiocarpine also had the typical
resistant nodule phenotype (positive for Y-GT and epoxide
hydrolase) and also lacked PA-induced megalocytosis. Lasiocarpine
treatment also resulted in small regenerative nodules that were
distinct from resistant nodules, because they were negative for
Y-GT and epoxide hydrolase.
6.4.8.3 Pyrrolizidine alkaloid metabolites and analogous synthetic
compounds
The subject has been reviewed by Mattocks (1986). The
pyrrolizidine alkaloids are converted into pyrrolic esters in the
hepatocytes. These may then be hydrolysed into pyrrolic alcohols,
which are more water soluble and less active than the esters. The
esters may be widely distributed throughout the body. Some of
these compounds and their analogues have been tested for
carcinogenicity.
(a) Pyrrolic esters
(i) Dehydromonocrotaline (monocrotaline pyrrole)
Mattocks & Cabral (1979) tested dehydromonocrotaline on the
skin of mice. In their first study on male BALB/c mice, they
applied the compound on the back at 1- to 2-week intervals.
Thirty-three applications of 1 µmol did not produce any skin
tumours in 16 mice, but 2 developed lung adenomas; no tumours
occurred in 14 control mice treated with the solvent (acetone). In
the second study (Mattocks & Cabral, 1982), 11 female LACA mice
each received 47 applications of 2.5 µmol dehydromonocrotaline; one
animal developed a malignant skin tumour. A second batch of 10
mice was given similar treatment and subsequently received 61
twice-weekly applications of croton oil at the same site. Half of
these animals developed tumours. In the control group of 10 mice,
only 1 tumour was seen. The results of these studies indicate that
dehydromonocrotaline requires the action of a promoter to manifest
carcinogenic potential.
(ii) Dehydroretrorsine
This supposed pyrrolic metabolite of retrorsine was applied to
the skin of male BALB/c mice at 1- to 2-week intervals; 33
treatments of 0.5 or 1 µmol failed to produce tumours in 15 mice
that survived for up to 60 weeks (Mattocks & Cabral, 1979).
(iii) 1-Methyl-2,3-bistrimethylacetoxymethylpyrrole
Mattocks & Cabral (1979, 1982) made 2 studies on this compound.
In the second study, which was more significant, 22 female LACA
mice received 47 dermal applications of 0.5 µmol each. This caused
marked skin damage with ulceration and scarring. Malignant skin
tumours developed in 19 out of 21 surviving animals. Two
hydrolysis products of this ester, pivalic acid and 1-methyl-2,3-
bishydroxymethylpyrrole, were similarly tested (Mattocks, 1986).
Tumours were produced, though fewer.
The results of the above studies suggest that the intact
pyrrolic ester is a carcinogen.
(b) Pyrrolic alcohols
Studies have been conducted using dehydroretronecine
(retronecine pyrrole) and dehydroheliotridine, which are secondary
metabolites of monocrotaline and heliotridine-based PAs,
respectively. Originally they were regarded as (+)- and (-)-
forms, respectively, of dihydro-7-hydroxy-1-hydroxy-methyl-5H-
pyrrolizine. Kadzierski & Buhler (1985, 1986) showed that the
metabolite from monocrotaline is racemic and concluded that the
product from all heliotridine and retro-necine esters is the same
(±)- form.
Johnson et al. (1978) painted the skin on the back of 16 female
Swiss mice with dehydroretronecine, each dose equalling 20 mg/kg
body weight or about 5 µmol per mouse, once a week for 4 weeks and
then twice more after 6 months. Six mice developed skin tumours.
Subcutaneous injections of the same dose yielded tumours in 13 out
of 21 mice. Twenty-eight out of 55 mice given both topical
applications and sc injections developed skin tumours.
When the same study was repeated on 34 BALB/c mice, only one
skin tumour developed (Mattocks & Cabral, 1982). When 17 animals
were given the same dose at more frequent intervals, e.g., 65
weekly doses, no tumours developed.
Similar results were obtained in a study by Shumaker et al.
(1976), already desecribed in section 6.4.8.1, in which 39/60 rats
given repeated subcutaneous injections of dehydro-retronecine
developed rhabdomyosarcomas at the site of injection. The compound
appears to be a direct-acting carcinogen for rats and mice, though
the susceptibility of various strains of mice varies.
Peterson et al. (1983) gave 9 ip injections (60 - 76.5 mg/kg
body weight) of dehydroheliotridine to rats over a 32-week period.
A large variety of tumours was produced. It was concluded that
this metabolite may be responsible for the carcinogenicity of its
parent PA.
6.4.8.4 Molecular structure and carcinogenic activity
Mattocks (1986) has reviewed the present position concerning
the relationship between molecular structure and carcinogenic
activity. Data available at present are not adequate for any
strict correlation to be established between the molecular
structure of PAs and the types of tumours produced by them in the
rat. However, the common determinants in the molecular structure
of all carcinogenic PAs are that they are macrocyclic or "open"
diesters, in which the amino-alcohol moiety is retronecine,
heliotridine, or otonecine. These are all esters of unsaturated
necines and are capable of being metabolized to pyrrolic esters in
the mammalian liver. Studies on pyrrolic esters, the toxic
metabolic product of PAs, have yielded equivocal results.
Monocrotaline has been shown to bind covalently to DNA, which is
associated with the carcinogenic activity of the pyrrolizidine
alkaloids (Robertson, 1982). Dehydromonocrotaline, the primary
metabolite of monocrotaline has been found to be an incomplete
dermal carcinogen (Hooson & Grasso, 1976; Mattocks & Cabral,
1982), whereas the synthetic compound 1-methyl-2,3-
bistrimethylacetoxymethylpyrrole, which is chemically similar to
dehydromonocrotaline, is clearly carcinogenic (Mattocks & Cabral,
1982). Dehydroretronecine (DHR), a second metabolite of
monocrotaline and possibly other retronecine-based PAs, is also
carcinogenic for the skin and is considered a proximate
carcinogenic metabolite, since, unlike monocrotaline, it acts at
the site of application directly and not at remote sites.
6.4.9 Antimitotic activity
Literature on this phenomenon has been reviewed by Jago (1969),
McLean (1970), and Mattocks (1986). The most characteristic
feature of the chronic hepatotoxicity of the PAs is the presence in
the liver of megalocytes (Bull & Dick, 1959; McLean, 1970), which
are generally enlarged hepatocytes containing large, hyperchromatic
nuclei (section 6.4.1.5). These appear to be the result of a
combined action of PAs on the hepatocytes, a stimulus to regenerate
following parenchymal cell injury, and the powerful antimitotic
action of the pyrrole metabolites of the PAs (Mattocks, 1986; Jago,
1969). This property has served as the basis for using a PA
(indicine- N-oxide) as a chemotherapeutic agent for cancer (Letendre
et al., 1981, 1984). Peterson (1965) showed that the number of
mitoses following partial hepatectomy was reduced to 50% or less of
normal values by prior administration of hepatotoxic alkaloids, and
that the effect was dose dependent. The hepatocytes seemed to
continue to grow without dividing. The effect can be produced by a
single sublethal dose of the alkaloids (Schoental & Magee, 1957) or
can be a cumulative effect of small doses (Bull & Dick, 1959). The
lesion appears within a few weeks and may persist for the lifetime of
the animal (Mattocks, 1986). It was characteristically described in
the liver of the rat, but has also been reported in a number of other
animals, e.g., mouse, sheep, horse, and pig, and in some other organs,
e.g., kidney and lung (McLean, 1970). It has not been observed in
human livers (Tandon, H.D. et al., 1978; Mattocks, 1986), but it has
been observed that cultured human fetal liver cells become enlarged
when exposed to PAs (Armstrong et al., 1972) indicating a
susceptibility to the antimitotic effect of PAs. The lesion is not
specific for PAs but has been reported to be produced by a number of
toxins (McLean, 1970), including semisynthetic derivatives of PAs but
not non-hepatotoxic PAs, such as platyphyline (Jago, 1970). The
ultrastructural features of megalocytes are controversial.
However, consistent with other functional and metabolic features,
the cells show morphological characters suggesting increased
metabolic activity with increased exchange of material between the
nucleus and cytoplasm.
This unique reaction of the hepatocytes to PA has been used by
Jago (1970) to develop a method for assessing hepato-toxicity and
by Culvenor et al. (1976a) for screening 62 PAs for acute and
chronic hepatotoxicity and pneumotoxicity.
Mattocks (1986) suggested a scheme for the antimitotic action
of PAs in vivo, consistent with observations of the phenomenon in
animals. The PA is irreversibly metabolized by the hepatic
microsomes into a pyrrolic ester, which can be hydrolysed to a
pyrrolic alcohol. The latter is the agent that inhibits mitosis.
The more reactive primary metabolite may also do this, by reacting
with tissue constituents to give products identical to pyrrolic
alcohols, inhibiting mitosis. On the other hand, it can also
produce acute injury to the cells, which stimulates regeneration.
Thus administration of the PA or the pyrrolic ester can induce
megalocytosis to a much greater extent than a secondary metabolite
alone.
Antimitotic activity does not seem to be directly related to
inhibition of DNA synthesis, since the latter recovers within a
week while mitotic inhibition continues for up to a period of 4
weeks (Peterson, 1965; Armstrong & Zuckerman, 1970). Mattocks &
Legg (1980) have shown that the level of DNA synthesis is reduced
in cells that do not divide, but is not totally inhibited. Samuel
& Jago (1975) investigated the position in the cell cycle of the
antimitotic action of lasiocarpine and of its pyrrolic metabolite,
dehydroheliotridine. Their studies indicated that the alkaloid
acts during the late S or early G2 phase of the cell cycle.
6.4.10 Immunosuppression
Dehydroheliotridine (DHH), a pyrrolic metabolite, has a
significant immunosuppressant activity on the primary response in
young mice; when injected ip shortly before the antigenic stimulus
(Percy & Pierce, 1971). The secondary response to antigenic
stimuli; as measured by the reduction in the number of 7S and 19S
specific antibody-synthesizing cells of the spleen, was suppressed
when DHH was administered at the time of secondary stimulus, but
not when it was given 24 or 36 h after the antigenic stimulus. It
was suggested that dehydroheliotridine selectively destroys or
inactivates cells involved in the initial stages of antigen
recognition and processing.
6.4.11 Effects on mineral metabolism
Aberrations of mineral metabolism have been observed in several
species of animals. The most notable among them relate to
haemolysis and copper metabolism. Anaemia has been reported to
occur in rats following PA poisoning (Schoental & Magee, 1959;
Schoental, 1963) and the kidney and liver show haemosiderosis
(Hayashi & Lalich, 1967). Besides the haemolysis, PAs have been
found to exert a direct inhibitory effect on haematopoiesis in the
livers of new-born rats (Sundaresan, 1942). Disturbances of iron
metabolism and haematopoiesis have also been demonstrated in rats
fed Senecio jacobaea and supplementary copper (Swick et al., 1982d)
and they have been found to develop raised copper levels in the
liver and spleen, when fed on this plant (Swick et al., 1982b).
Miranda et al. (1979) reported elevated levels of iron in the liver
and spleen and of copper in the liver in rats fed on tansy ragwort.
Studies with radioactive iron also indicated a specific inhibitory
effect of PAs on haematopoiesis. High copper levels in the liver
associated with haemoglobinuria have been reported in sheep grazing
on heliotrope (Bull et al., 1956), signs of disease closely
resembling those of chronic copper poisoning. St. George-Grambauer
& Rac (1962) reported a similar outbreak of fatal jaundice due to
haemolytic crisis of chronic copper poisoning in sheep that had
grazed Echium plantagineum over 2 or more seasons. The
pathological changes in the liver were indistinguishable from those
of Heliotropium, and the livers had a high copper content. Studies
of Miranda et al. (1981b) indicate that dietary copper can enhance
PA hepatotoxicity in rats. It has been suggested that the
hepatotoxic effects of some PAs may interfere with the excretion of
copper (Bull & Dick, 1959; Farrington & Gallagher, 1960). Similar
effects have been observed in pigs fed Senecio (Harding et al.,
1964). White et al. (1984) did not observe any rise in hepatic
copper levels in sheep fed Senecio jacobaea.
6.4.12 Methods for the assessment of chronic hepatotoxicity and
pneumotoxicity
Pyrrolizidine alkaloids produce acute as well as chronic liver
damage. The acute effect is seen as extensive necrosis (Schoental
& Magee, 1957), while the chronic effect in rats is manifested
characteristically by the presence of greatly enlarged parenchymal
cells (Schoental & Magee, 1957; Bull & Dick, 1959), which persist
long after a single exposure (Schoental & Magee, 1959). The latter
effect of megalocytosis may manifest without the liver cells going
through the process of necrosis (Schoental & Magee, 1959). This
property has been used by Jago (1970) to develop a method for the
assessment of the relative chronic hepatotoxicity of different
alkaloids. It has since been used by other investigators (Culvenor
et al., 1976a). It consists of the intraperitoneal administration
of a single dose of between 0.025 and 3.2 µmol of the alkaloid per
kg body weight in 0.2 ml aqueous solution to 14-day-old suckling
hooded Wistar rats of both sexes. The litters are randomized among
the mothers, one day before the administration of the toxin, and
then weaned at 28 days. Animals are killed 4 weeks after the
injection. The relative acute toxicity is indicated by the dose
levels that cause death within approximately a week and chronic
hepatotoxicity by those that produce hepatic megalocytosis within 4
weeks of the injection. With this method, it is not only possible
to evaluate the hepatotoxicity of a given alkaloid but also to
compare the hepatotoxic effects of different compounds in relation
to each other on a molar basis. Chronic effects on the lungs can
also be assessed by the same method. Culvenor et al. (1976a) found
this method satisfactory for compounds of medium to high
hepatotoxicity but failed to detect toxicity in certain compounds
of known, low hepatotoxicity.
6.5 Effects on Wild-Life
6.5.1 Deer
There is very little information on the consumption of
PA-containing plants by non-domesticated animals, birds, and other
wild-life. In one instance, the deaths of white-tailed deer in
coastal marshes in Louisiana in 1967, was ascribed to the
consumption of Crotalaria and/or Heliotropium species in a period
of feed scarcity (Seger et al., 1969). The animals had thin and
watery blood, abnormal bone marrow, and serious atrophy of cardiac
and mesenteric adipose tissue. In a 9-year-old doe, the liver was
dull and somewhat granular and evinced megalocytosis of the
hepatocytes. In another 4-year-old animal, the liver appeared
normal but with microscopic evidence of early megalocytosis, with
considerable vacuolization in the centrilobular hepatocytes.
Plants of the genera Crotalaria and Heliotropium were abundant and
there were some Senecio species. There were signs of ingestion of
the plants by the deer.
Senecio jacobaea (tansy ragwort) has been fed experimentally to
black-tailed deer (Odocoileus hemionus columbianus) in Oregon to
determine their susceptibility to poisoning (Dean & Winward, 1974).
The ragwort was given ad libitum together with different levels of
basal ration (85% alfalfa, 10% barley, 5% molasses) to captive
deer. The ragwort was eaten, only when the basal ration was
inadequate. One group, not given any basal ration for 6 days,
began eating ragwort and consumed 5.4 kg dry weight per animal in
42 days. This represented 24% of the animal body weight. The
animals did not show any toxic signs, and blood levels of SGOT and
bilirubin were normal.
6.5.2 Fish
The effects of S. jacobaea alkaloids on rainbow trout (Salmo
gairdneri) fingerlings has been investigated (Hendricks et al.,
1981). Duplicate groups of 80 fingerlings were fed for up to 12
months on diets containing 20 or 100 mg alkaloid/kg. The alkaloid
comprised 91% jacobine, 3% jacazine, 2.5% senecionine, and 2.5%
seneciphylline. The 100 mg/kg diet resulted in severe growth
depression and mortality, which began at 3 - 4 months. Both levels
of PAs produced severe hepatic lesions. The livers from these fish
were shrivelled, mottled yellow or whitish in colour, nodular,
fibrous, and sometimes haemorrhagic. Microscopically, there was
megalocytosis, severe fibrosis, and bile-duct proliferation.
Characteristic veno-occlusive changes were seen in the centrilobular
and hepatic veins, which, in the case of fish receiving the 100 mg/kg
diet, appeared after only 2 months on the diet.
6.5.3 Insects
There are no reports of the toxicity of PAs for insects, but
there is substantial literature on the use of PAs by certain insect
families that have evolved with the ability to store the alkaloids
as defensive chemicals and to convert them into pheromones and
other signalling chemicals (see recent reviews by Brown (1984) and
Boppré (1986), and an earlier complementary paper by Edgar (1982)).
In some species, such as moths of the family Arctiidae, the larvae
feed on PA-containing plants. In other families, such as Nymphalid
butterflies of the sub-families Danainae and Ithomiinae, the larvae
of most species live on other plants, but the adult males seek out
PA-containing species and contrive to ingest alkaloids from
wilting, dead, or damaged plant material or from nectar. The
alkaloids so acquired have a functional role as defensive chemicals
against predators and, in some species, are also converted into
pheromones and other signalling chemicals involved in mating. The
alkaloid derivatives may be pyrrolic compounds related to
dehydroretronecine or derivatives of the esterifying acids. In one
Arctiid genus, Creatonotus, the alkaloids have a morphogenetic or
hormonal effect, determining the size of the pheromone-disseminating
organ. Thus, for some insect species, PA-containing plants may be
necessary for survival.
7. EFFECTS ON MAN
The toxic effects of pyrrolizidine alkaloids are principally on
the liver. The toxic disease, produced by consuming PAs derived
from certain plants, is called veno-occlusive disease (VOD), the
pivotal and pathognomonic lesion being the occlusion of the central
and sublobular hepatic veins in the liver. The larger hepatic vein
tributaries are characteristically unaffected in contrast with the
findings in Budd-Chiari syndrome (Bras, 1973).
7.1 Clinical Features of Veno-Occlusive Disease (VOD)
There are several good clinical accounts of the disease, mostly
in the earlier reports from Jamaica, in children (Jelliffe et al.,
1954a,b), and adults (Stuart & Bras, 1955), which have been
summarized by Stuart & Bras (1957). Maksudov (1952) has described
the clinical features among children in outbreaks in the USSR,
where it was called toxic hepatitis with ascites, and Ismailov
(1948a,b), Mnushkin (1949, 1952), and Zheltova (1952) have
described them among adults. Srivastava et al. (1978) also
described the clinical findings among children in a large outbreak.
Children seem to be particularly vulnerable as is evident from the
report of Stuart & Bras (1957) and that of Mohabbat et al. (1976)
concerning a large outbreak, though the disease is rare before the
age of one or two years. Frequently, more than one member of the
family becomes affected (Stuart & Bras, 1957; Mohabbat et al.,
1976; Tandon et al., 1976; Arora et al., 1981) within days or weeks
of each other.
The disease generally has an acute onset, characterized by
rapidly developing and progressing symptoms of upper abdominal
discomfort, dragging pain in right hypochondrium, ascites, and
sometimes oliguria and oedema of the feet. Nausea and vomiting may
be present. Jaundice and fever are rare. There is generally
gross, tender, smooth hepatomegaly often accompanied by massive
pleural effusion, and sometimes slight splenomegaly and minimal
ankle oedema. Liver function tests may show only mild disturbance.
The acute disease is associated with high mortality and a subacute
or chronic onset may lead to cirrhosis. Death often occurs after
oesophageal haemorrhage.
Stuart & Bras (1957) summarized the clinical data of 84
patients ranging in age from 6 months to 53 years. The highest
incidence occurred between the ages of 1 and 3 years (39%), and 26%
of patients belonged to the 3- to 6-year age group. Thus, children
up to 6 years accounted for 65% of total cases. Although the VOD
was relatively uncommon at the 2 extremes of age, early infancy and
adult life, mortality was highest in these groups, being 60% and
54%, respectively. Hepatomegaly and some degree of ascites were
invariably present in acute cases; in 48 out of 64 patients,
ascites was acute enough to require paracentesis. In 38 patients,
hepatomegaly was grossly severe, reaching more than half way down
to the umbilicus. Jaundice was relatively uncommon. Among the
liver function tests, the most significant and consistent changes
were found in the serum-cholinesterase (t = 2.67, 0.02 > P > 0.01)
and serum-albumin levels (t = 2.82, 0.01 > P). Mortality rates
associated with signs of parenchymal liver damage were 74% with
clinical jaundice or high levels of serum-bilirubin, 62% with
diminished serum-cholinesterase, and 58% with considerable anorexia
and apathy. The mortality rates among cases with acute, subacute,
or chronic disease were reported to be 27%, 17%, and 57%,
respectively. Death was mostly due to hepatocellular failure (71%)
in the acute phase and haemetemesis in the chronic phase (75%).
More recent publications (Lyford et al., 1976; McGee et al., 1976;
Tandon, B.N. et al., 1977; Datta et al., 1978a,b) also describe the
haemodynamic data of the hepatic blood flow and the results of
portovenographic studies that suggest outflow tract obstruction in
the liver at the post-sinusoidal level, and irregularity and
obstruction/distortion of the hepatic venous radicles,
respectively.
The clinical course of the disease has been shown schematically
by Stuart & Bras (1957) (Fig. 12). However, the temporal
relationships in the different phases of the disease are not
precise, and their account, at best, represents a trend.
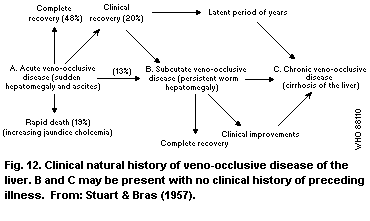 The onset of the disease may be sudden (acute) or insidious
(subacute or chronic). The acute disease may recover completely or
result in death. A few patients may go on to the subacute phase,
with almost none or very few symptoms, but a persistent
hepatomegaly. The patient may subsequently recover completely, or
may, after or without apparent clinical improvement, go on to the
chronic phase of disease, mostly ending up in cirrhosis. Some
patients with the acute disease may go on to the subacute phase,
even after clinical recovery, or as postulated by the authors,
after a latent period of several years, progress to cirrhosis.
Braginskii & Bobokhadzaev (1965) related an experience
concerning the evolution of this disease that was similar to that
observed in the USSR. About 50 - 60% of cases made a full recovery
and 35% made a partial recovery with continuing hepatomegaly.
About 2% of cases developed persistent ascites with "loss of
working capacity". It has been suggested that these cases may
develop cirrhosis.
7.2 Salient Pathological Features of Veno-Occlusive Disease
The pathological features of the disease at different stages
have been described in detail (Terekhov, 1939, 1952; Dolinskaya,
1952; Bras et al., 1954; Bras & Watler, 1955; Bras & Hill, 1956;
Stuart & Bras, 1957; Stirling et al., 1962; Aikat et al., 1978;
Tandon, B.N. et al., 1978; Tandon, H.D. et al., 1978). The
following description of Bras & Watler (1955) characterizes the
morphological changes at different stages of the disease described
by most investigators.
Morphological features of the liver at autopsy in 19 patients
from Jamaica who died at various stages of VOD have been described.
The ages ranged from 10 months to 45 years. Fourteen patients were
below the age of 14 years. Of the 14 patients, 9 had developed
cirrhosis, 3 died of acute disease, and 7 had various levels of
fibrosis superimposed over acute VOD. In the acute stages, there
was acute centrilobular congestion. The centrilobular and
sublobular veins showed different degrees of thickening of the wall
and occlusion of the lumen, mainly due to subintimal swelling
composed of loose reticular tissues and a few cells including
endothelial cells, occasional histocytes, lymphocytes, and
polymorphs, suggesting an acute exudative process. In addition,
there was a small amount of fibrin. Organization of this exudate
gradually led to collagenization and thickening of the wall. The
centrilobular congestion resulted in compression and even
disappearance of liver cell cords. The reticular framework of the
liver lobule was frequently preserved but was ruptured at places.
Clearly recognizable thrombi occurred sporadically but were not
commonly seen. The portal veins and hepatic arteries were normal.
Stirling et al. (1962) in their description of the early lesion
of VOD of the liver also emphasized that thrombosis of the hepatic
veins was not an important histogenetic factor in the evolution of
the lesion. In the later stages of the disease, hepatic venous
occlusion was chronically established. Perivenous reticular
collapse and the resultant condensation and reduplication of the
reticular framework resulted in non-portal fibrosis and later
cirrhosis. Macroscopically, the cirrhosis looked like Laennec's
cirrhosis. The cirrhotic process, which was non-portal to begin
with, involved portal tracts which also became incorporated in the
connective tissue septa. The presence of oesophageal varices was a
common finding. Extra-hepatic collateral vessels and congestive
splenomegaly were frequently seen. Within the liver, intrahepatic
collateral circulation was established with the coalescence of
sinusoids. The larger hepatic veins and inferior vena cava did not
show any thrombi or other pathological change. The subintimal
swelling observed in the hepatic veins was not seen in any other
vessels in the body. The authors concluded that acute VOD
gradually leads to Laennec's cirrhosis, but, in the initial phases,
it is non-portal.
No clear dose or temporal relationships between the liver and
parenchymal and vascular changes in the liver lobules are evident
from the available human case reports.
It is notable that megalocytosis, which is the hallmark of
chronic PA toxicity in experimental animals and a morphological
manifestation of the antimitotic effect of PAs, has not been
observed in human subjects (Tandon, H.D. et al., 1978) (section
6.4.9).
Tandon H.D. (personal communication) has analysed all published
and unpublished observations including liver biopsies and autopsies
derived from 3 outbreaks of VOD of which two occurred in India
(Tandon, B.N. et al., 1976, 1977; Tandon, R.K. et al., 1976;
Tandon, H.D. et al., 1977) and one occurred in Afghanistan (Tandon,
B.N. et al., 1978; Tandon, H.D. et al., 1978). He commented on the
frequency with which the characteristic veno-occlusion may not be
seen in the needle biopsy in the acute phase of the disease, though
it is invariably observed in the autopsy material. Haemorrhages
may persist for up to 1 year after subsidence of acute symptoms.
There is no significant inflammation accompanying the veno-
occlusive changes. Cirrhosis was reported to have developed within
3 months in one patient, after an initial biopsy had shown no
fibrosis (Stuart & Bras, 1957).
Brooks et al (1970) made an ultrastructural study of the liver
in VOD in Jamaican children. They found extensive damage in the
sinusoidal epithelium resulting in entra-vasation of red cells in
Disse's space and between hepatocytes. The closed structure of the
vessels, the absence of fenestrations, the existence of a basement
membrane, and the presence of collagen in the wall contribute to
the resistance to cellular debris and erythrocytes which track up
Disse's space and tend to narrow the lumen of the sinusoid where it
enters the vein. Fibrin may be present in this location and
occasionally in the sinusoids, but not within hepatic veins
themselves. The venous block, therefore, does not appear to be the
result of thrombosis.
Pancreatic changes similar to those in Kwashiorkor were stated
to be commonly observed (Bras & Hill, 1956; Stuart & Bras, 1957).
7.3 Human Case Reports of Veno-Occlusive Disease
Available data on human cases are summarized in Table 15, with
the countries they have been reported from in alphabetical order.
The first report of this disease in man and its relationship with
consumption of wheat flour contaminated by seeds of a plant of the
genus Senecio was made by Albertjin in 1918 in a report made to the
government of South Africa, as quoted by Wilmot & Robertson (1920),
who gave the first account of this disease in scientific literature
from that country. It is possible that it may have existed even
earlier, as its occurrence in farm animals had been described since
the beginning of the century in veterinary literature (Bull et al.,
1968). Willmot & Robertson (1920) recorded the occurrence of
'bread poisoning' in South Africa for a period of about 10 years,
during which 80 cases had been observed, mostly resulting in death.
The detailed account of clinical data and pathological findings in
11 cases was consistent with what is known as VOD today. The flour
from which the bread was prepared was found to be contaminated with
the flower heads and seeds of Senecio spp.
Isolated case reports are available in the literature (Hashem,
1939; Wurm, 1939) describing changes in childrens' liver
characteristic of veno-occlusive disease; however, in these
studies, no mention was made of possible etiological agents.
A pattern of disease described in the Soviet literature as
dystrophy of the liver, and later as toxic hepatitis with ascites
has been known in the Central Asian republics of the USSR
especially Uzbekistan and Tadjikistan for a long time (Terekhov,
1952). The local inhabitants called the disease, characterized by
rapidly filling ascites, the "camel-belly" syndrome and are
reported to have long distrusted a weed with fine seeds, known
locally as "Kharmyk", as a source of the disease (Dubrovinskii,
1952) (it is notable that, in the Afghan outbreak, which occurred
close to the area in Uzbekistan where the disease had been known,
the toxic seeds were known as "Charmak" among the local
population). The "camel-belly" disease was associated with the use
of bread with a bitter taste (presumably caused by the mixing and
grinding of toxic seeds with the wheat grains). Two waves of
outbreaks occurred, the first between 1931-35 (Terekhov, 1939) and
the second between 1945 - 46 (Dubrovinskii, 1947; Ismailov,
1948a,b). In the first wave of outbreaks, approximately 1000 -
1500 subjects were estimated to have been affected with an overall
mortality rate of 13 - 15%. The age of the affected subjects
ranged from 3 to 50 years. In the second wave of outbreaks, 60 -
70% of the population in agricultural areas were estimated to have
been affected (Ismailov, 1948a,b). Domestic animals also suffered
from the disease (Dubrovinskii, 1947). During the second wave of
outbreaks, the disease was found to have been caused by
contamination of food crops with seeds of Heliotropium lasiocarpum
(Dubrovinskii, 1947; Khanin, 1948). The etiology was confirmed by
studies on a variety of experimental animals using the suspect seed
(Khanin, 1948; Kampanzev, 1952). Pathological features of the
disease in children have been described by Dolinskaya (1952) and in
all age groups by Terekhov (1939, 1952). First an acute form of
the disease was recognized, followed by hepatic cirrhosis, which
was found to result as a consequence of the initial disease
(Ismailov, 1948a,b; Savvina, 1952). The disease has since been
largely eradicated by the agricultural and public health measures
taken.
Table 15. Pyrrolizidine alkaloid poisoning and human reports of veno-occlusive disease (VOD)
(in alphabetical order of the countries reported from)
Country/ Age Suspected Name of Alkaloid Alkaloid Nature of Outcome Reference
number group vehicle of plant concentration lesion (cirrhosis/
cases intoxication and intake died)
Afghanistan
8000 <14-80 contaminated Heliotropium mainly 1.32-1.49 g/kg all stages; many died Tandon &
(approximately) years wheat flour popovii heliotrine, (in the mostly acute Tandon (1975);
gillianum in addition seeds) daily to subacute Mohabbat
to 1 or 2 intake, 2 mg; et al. (1976)
alkaloids total
similar to consumption,
lasiocarpine up to 1.46 g
(75 - 100%
as N-oxides)
Barbados
3 2-4 herbal Crotalaria not analysed acute lesions no details Stuart & Bras
years infusion retusa (1956)
China, People's Republic of
2 49-57 herbal Gynura not analysed acute lesions died Hou (1980)
years infusion segetum
(Compositae)
Ecuador
1 35 years herbal Crotalaria not analysed acute lesions recovered Lyford et al.
infusion juncea (1976)
Table 15 (cont'd)
Country/ Age Suspected Name of Alkaloid Alkaloid Nature of Outcome Reference
number group vehicle of plant concentration lesion (cirrhosis/
cases intoxication and intake died)
Egypt
3 not known Hashem (1939)
59 1-12 plant seed not acute lesions cirrhosis Safouh &
years decoction identified in one Shehata (1965)
in 17
Federal Republic of Germany
8 75-116 not known acute lesions all died Wurm (1939)
days (alimentary
toxaemia)
Hong Kong
4 23-28 herbal Heliotropium pyrrolizidine herbs-alkaloid acute VOD 1 died Kumana et al.
years infusion lasiocarpum alkaloids base 0.42 g/kg; (1983, 1985);
N-oxides Culvenor et al.
1.4 g/kg; daily (1986)
intake, 12 mg
base, 18 mg
N-oxide; total
intake, 570 -
1350 mg up to
development of
symptoms in 3
patients over
19-45 days,
1300 mg up to
Table 15 (cont'd)
Country/ Age Suspected Name of Alkaloid Alkaloid Nature of Outcome Reference
number group vehicle of plant concentration lesion (cirrhosis/
cases intoxication and intake died)
Hong Kong (cont'd)
death in 1
patient. 630 mg
in one case who
remained
asymptomatic.
India
2 25 and herbal not unknown acute recovered Gupta et al.
35 years decoction identified (1963)
and pills
25 12-38 possibly not not analysed acute 12 died; Tandon, R.K.
years 6 contaminated identified lesions; 1 cirrhosis et al. (1976);
patients cereal cirrhosis Tandon, H.D.
studied) in one et al. (1977)
108 3-60+ contaminated Crotalaria crotananine 0.81 g/kg various up to 63% Tandon, B.N.
cereal nana and stages of died et al.
crotaburmine disease (1976, 1977)a
Siddiqui
et al.
(1978a,b)
Table 15 (cont'd)
Country/ Age Suspected Name of Alkaloid Alkaloid Nature of Outcome Reference
number group vehicle of plant concentration lesion (cirrhosis/
cases intoxication and intake died)
India (cont'd)
35 1- > 25 contaminated Crotalaria as above 5.3 g/kg seed acute as above Krishnamachari
years cereal (0 - 1.9% of et al.
seeds in (1977)a;
cereal); daily Siddiqui et al.
intake, 40 mg (1978a,b)
6 20-70 herbal Heliotropium heliotrine 12 - 20 g/kg; acute 3 died Aikat et al.
medicine in eichwaldii as N-oxide total intake, lesions; (2 with (1978);
4 cases by 3 4 g and 10 g cirrhosis fulminant Datta et al.
patients in patients who in one disease) (1978a,b)
died of organizing
fulminant thrombi in
disease hepatic veins
in 4 cases
Iraq
9 3-10 possibly possibly not acute 1 died Al-Hasany &
years wheat flour Senecio identified Mohamed (1970)
South Africa
11 11-19 wheat flour Senecio not centrilobular "majority Wilmot &
(only 5 years as bread ilicifolius; identified haemorrhages; died" Robertson
described) S. burchelli senecionine? central vein (1920)
"dilated"
Table 15 (cont'd)
Country/ Age Suspected Name of Alkaloid Alkaloid Nature of Outcome Reference
number group vehicle of plant concentration lesion (cirrhosis/
cases intoxication and intake died)
South Africa (cont'd)
12 wheat not not centrilobular Selzer & Parker
flour identified; identified haemorrhages; (1951)
"possibly organizing
Senecio" thrombi in
hepatic veins;
3 cases of VOD
12 5-30 herbal not none found "acute" in 3 died of Stein &
months medicines identified in herbal 5 cases; disease; Isaacson (1962)
admitted in medicines "subacute" no follow-up Stein (1957)
4 cases; in 7 cases; on 5
food not no detail
analysed
United Kingdom
1 26 years herbal Maté-Paraguay identified "trace acute with died McGee et al.
infusion tea only as amounts" veno-occlusion (1976)
pyrrolizidine going on to
alkaloids chronic with
fibrosis
1 13 years herbal Symphytum not analysed not known "thrombotic" Weston et al.
infusion officinale variant of (1987)
veno-occlusive
disease
Table 15 (cont'd)
Country/ Age Suspected Name of Alkaloid Alkaloid Nature of Outcome Reference
number group vehicle of plant concentration lesion (cirrhosis/
cases intoxication and intake died)
USA
1 2 months herbal Senecio riddelline 15 g/kg as acute lesions died Fox et al.
infusion longilobus and N-oxides alkaloids; (1978)
of retrorsine, total intake,
seneciphylline, 66 mg
and senecionine
1 6 months herbal Senecio as above 3 g free acute lesions cirrhosis Stillman et al.
infusion longilobus alkaloid plus (1977);
10.5 g N-oxide Huxtable (1980)
per kg; total
intake,
70-147 mg of
combined
alkaloid
and N-oxide
1 49 years herbal Symphytum sp. symphytum 14.1 µg/kg acute lesions recovered Ridker et al.
infusion pyrrolizidine body weight after (1985);
alkaloids per day; total short Huxtable et al.
intake, 85 mg surgery (1986)
USSR
1000 - 1500 3-50 contamination Heliotropium heliotrine, acute lesions 13-15% Mirochnik
years of wheat lasiocarpum lasiocarpine died; (1938);
flour cirrhosis Terekhov
in many (1939)
Table 15 (cont'd)
Country/ Age Suspected Name of Alkaloid Alkaloid Nature of Outcome Reference
number group vehicle of plant concentration lesion (cirrhosis/
cases intoxication and intake died)
USSR (cont'd)
"large contamination Heliotropium heliotrine Dubrovinskii
number" of wheat lasiocarpium lasiocarpine (1947);
flour Ismailov
(1948a,b)
Venezuela
1 5 years "earth and not acute lesions died Grases & Beker
plant eating identified (1972)
habits"
West Indies
11 14 months not known, acute to data Jelliffe et al.
to possibly cirrhosis unclear; (1954b)
9 years herbal cirrhosis
infusions in some
5 23 months not known, not 1 Bras et al.
to 18 possibility identified cirrhosis (1954)
years of "bush
teas"
4 16-45 history of not acute to 3 died Stuart & Bras
years herbal identified acute-on- (1955)
infusion in chronic
one case
Table 15 (cont'd)
Country/ Age Suspected Name of Alkaloid Alkaloid Nature of Outcome Reference
number group vehicle of plant concentration lesion (cirrhosis/
cases intoxication and intake died)
West Indies (cont'd)
84 0.5-53 probably not 11% had Stuart & Bras
years "bush teas" identified; cirrhosis; (1957)
(Senecio and 27% died
Crotalaria)
23 possibly not stated not analysed acute to Bras & Hill
bush teas "cirrhosis" (1956)
(no breakup)
23 1-50 no precise no analysis cirrhosis Bras et al.
years identification; (1961)
"Crotalaria
fulva and
possibly
other toxic
factors"
3 "children" "toxic not acute died Stirling et al.
substances identified (1962)
in herbal
remedies"
a Pertains to the same outbreak.
Selzer & Parker (1951), from South Africa, described 12 cases,
10 of whom came from 3 families that had eaten bread made of flour
of "imperfectly winnowed wheat". Five cases who were autopsied
showed characteristic occlusion of the central vein of the liver
lobules.
Attention on veno-occlusive disease as an entity followed a
spate of reports, mostly from Jamaica, on the occurrence of this
disease, mainly among children (Bras et al., 1954, 1961; Jelliffe
et al., 1954a,b; Bras & Watler, 1955; Bras & Hill, 1956; Stirling
et al., 1962) but also among adults (Stuart & Bras, 1955, 1957).
The youngest patient reported was a 3-month-old infant (Stein &
Isaacson, 1962), but the disease was stated even to have been
observed in the new-born (Stein, 1957). Very often, several
members of the family were affected by disease, which presented
primarily as rapidly filling ascites, within a matter of days,
sometimes accompanied by fever, and leading to hepatic failure. It
was considered an important cause of cirrhosis among children in
Jamaica (Jelliffe et al., 1954a,b). On the basis of evidence of an
almost identical disease occurring among grazing animals (Hill,
1960), and a prevalent practice among the Jamaicans of using herbal
infusions for treating a variety of ailments as a home remedy,
herbs, notably of the Senecio and Crotalaria groups, were suspected
of being a contributory factor (Bras & Hill, 1956; Bras et al.,
1957, 1961; Stuart & Bras, 1957).
Hill (1960) gave a comprehensive account of current knowledge
up to that time of the world-wide distribution of seneciosis in man
and animals in which mention is made of this disease only in Egypt,
the Federal Republic of Germany, South Africa, and the West Indies.
Pyrrolizidine alkaloids were identified as the active element in
the toxic factor in the plants.
However, there are earlier reports of this disease from
elsewhere. Bras (1973) cited a number of reports from Europe from
1905 to 1949, including reports by Wurm (1939) and Teilum (1949),
of an entity in infants and children that was called Endophlebitis
hepatica obliterans. He reported having studied some tissue
sections of liver in Austrian children aged 7 months - 1 year, who
had died with clinical and histological patterns of VOD. Some of
these conformed to the Budd-Chiari syndrome whereas others were
consistent with VOD. The case of a 36-year-old woman reported by
Teilum (1949) does not, however, fall in the usual category of
cases accepted as veno-occlusive, since thrombotic changes were
also seen in the larger hepatic veins and several, in the
peripheral parts of the body, as well as the portal vein.
A comprehensive account of all available published reports of
this disease to date from different parts of the world is given in
Table 15. A clear distinction between the acute and chronic
effects of exposure is not always possible from the published data,
since, in most patients, the history of onset of disease with
intake of the toxic substance is not generally volunteered or
available, and so a precise temporal relationship is difficult to
establish. However, this information is available in a reasonably
accurate form in more recent reports (Stillman et al., 1977; Datta
et al., 1978a,b; Fox et al., 1978; Kumana et al., 1985).
Gupta et al. (1963) from India described 2 cases who had drunk
some herbal infusions for the treatment of skin disorders. The
liver biopsies confirmed changes typical of acute VOD. However,
the herb was not identified and no analysis was made of the
infusion for alkaloids.
The cases of 59 children in Egypt who had symptoms of rapidly
developing abdominal distension and hepatomegaly were reported by
Safouh & Shehata (1965). Their ages ranged between 1 and 12 years,
47 being below 4 years of age. A dietary survey carried out on 17
patients indicated ingestion of drinks made by boiling some common
plant seeds, but these were not identified. Wedge biopsies of
liver were performed in 6 patients and 16 postmortem examinations
were made. In all the autopsy cases, there was thrombotic
obliteration of the main hepatic veins and their ostia. The
central and sublobular veins were not uniformly thickened as they
were frequently dilated or disrupted and some contained fresh
thrombi. There was centrilobular necrosis of the liver lobules.
One patient developed decompensated cirrhosis in 3 years. The
authors observed that the clinical and pathological features
closely resembled those of VOD in Jamaica, but thrombosis was an
unusual feature of the latter disease.
Al-Hasany & Mohamed (1970) described a short outbreak in Iraq,
occurring during a 3-month period, affecting 9 children, all except
one being below 12 years of age, and belonging to 3 Bedouin
families living outside Baghdad. One of the children died.
Autopsy of this case and biopsies on the others showed changes
characteristic of VOD. Poisoning through the wheat flour
contaminated by seeds of some PA-containing plant was suspected,
but no analysis of the food was carried out.
Three of the largest outbreaks of the disease have been
reported from South Asia, two from the same site in central India
(Tandon, B.N. et al., 1976, 1977; Tandon, R.K. et al., 1976;
Krishnamachari et al., 1977; Tandon, H.D. et al., 1977) and one
from North-West Afghanistan (Tandon & Tandon, 1975; Mohabbat et
al., 1976; Tandon, B.N. et al., 1978; Tandon, H.D. et al., 1978).
The first Indian outbreak was reported by Tandon, B.N. et al.
(1976) and Tandon, R.K. et al. (1976) and Tandon, B.N. et al.
(1977) and Tandon, H.D. et al. (1977). It occurred in a group of 5
tribal villages in central India in 1972 - 73. The epidemiology of
the outbreak was later described by Arora et al. (1981). Out of a
total population of 2060 in these villages, 71 households with 366
members were investigated. Among these, 39 cases had developed and
19 had died before commencement of the investigations. The
incidence rate was 1.1% and the case fatality rate was 50%. All
cases occurred among 20 households. In many households, several
members were affected. In one household, 4 out of 5 cases died.
Six sick patients were investigated in detail with repeated liver
biopsies. One patient died 17 months after onset and was
autopsied. The clinical onset was characteristic of disease.
Haemodynamic and radiographic studies suggested a combined post and
perisinusoidal block. Liver biopsy studies showed features
characteristic of acute centrilobular haemorrhagic necrosis with
progressive fibrosis, hepatic veno-occlusion, and non-portal
cirrhosis in one case who survived 17 months after the acute onset.
The etiological factor of this outbreak was not established, though
dietary contamination with pyrrolizidine alkaloids was considered.
A second outbreak occurred at the same site in 1975 and was
studied and reported independently by Krishnamachari et al. (1977)
and Tandon, B.N. et al. (1976, 1977). A total population of 486
was affected, 67 cases were reported, of whom 28 (46%) had died.
There was a strong family history (Tandon, B.N. et al., 1976). In
a later survey, 108 patients were studied and the mortality rate
was estimated to be 63% (Tandon, B.N. et al., 1977). This time the
etiological factor was identified as the plant Crotalaria nana
Burm, which had been growing in the fields of millet (Panicum
miliare), their staple food crop. The seeds of this plant became
mixed with the cereal grain during harvesting. The toxic seeds
contained pyrrolizidine alkaloids that were identified as a
macrocyclic ester closely similar to monocrotaline. The total
alkaloid content was estimated to be 5.3 g/kg of seed, expressed as
monocrotaline. The levels of contamination of the millet with
seeds were reported to be 0 - 3.4 g/kg in the unaffected and
0 - 19 g/kg in the affected households (Krishnamachari et al., 1977).
A precise identification could not be made, but the same material,
independently studied by Siddiqui et al. (1978a,b), were reported
to contain 2 new alkaloids, cronaburmine and crotananine. The seed
contained an alkaloid level of 26 g/kg. The levels of
contamination of the millet were up to 20 g/kg and the amount of
alkaloid ingested was estimated to be up to 40 mg per day.
The largest outbreak reported to date occurred in the Gulran
district of Herat Province in northwest Afghanistan, close to the
border of the USSR. Tandon & Tandon (1975) identified the plant as
being causative factor of the disease, which was surveyed and
reported in detail by Mohabbat et al. (1976). The outbreak, was
estimated to have affected a population of approximately 35 000 in
98 villages. Examination of 7200 inhabitants of the affected
villages showed evidence of disease in 22.6%, which was more
serious in 15%. Thus, it was estimated that approximately 8000
subjects suffered from the disease including 5000 who were
seriously affected. All age groups were affected, but 46% of
subjects were below 14 years of age. However, no sign of disease
was found in children below 2 years of age. A detailed report
concerning the pathological material obtained from 14 liver
biopsies and 8 autopsies was made by Tandon, B.N. et al. (1978) and
Tandon, H.D. et al. (1978). The time interval between the onset of
symptoms and the biopsy/autopsy was not indicated. Pathological
findings were characteristic, ranging from acute disease with
characteristic veno-occlusion to non-portal cirrhosis, which was
observed in 5 of the above 22 cases. The outbreak was ascribed to
massive contamination of wheat, the staple food crop, following 2
years of drought, with the seeds of Heliotropium popovii H. Riedl
subsp. gillianum H. Riedl, which had been growing profusely among
the wheat crop. The seeds contained pyrrolizidine alkaloids at
concentrations reported by 2 laboratories to be 7.2 and 13.2 -
14.9 g/kg, identified as mainly as the N-oxide of heliotrine (74%)
(Mattocks, personal communication), and one or two other compounds
similar in character to lasiocarpine. Samples of wheat from
several villages contained an average of 40 seeds/kg, i.e., 0.03%
by weight. It was estimated that an adult consumed at least 700 g
flour/day, containing approximately 2 mg alkaloid (based on a mean
of the seed analyses). There is some uncertainty about the
estimate, since Mohabbat et al. (1976) also stated that the samples
of the wheat flour were assayed and contained 0.186 - 0.50%
alkaloid. This analysis and the 0.72% result for alkaloid in the
H. popovii seed were from the same laboratory in Kabul (R.N.
Srivastava, personal communication), and together, imply 13 - 36%
seed in the wheat. If correctly reported, the result for the flour
conflicts with the estimated proportion of the seed in the wheat
and can scarcely have been representative.
Tandon & Tandon (1975) stated in their report of the survey
during which the causative factor of the Afghan outbreak was
discovered, that such cases had always been observed by Government
physicians posted in this area in the past, sometimes in
significant numbers, but neither the population nor the physicians
remembered that the disease had occurred in the form of an
outbreak.
There was no mention of VOD in the hepatic lesions observed by
Sobin et al. (1969) among the 121 specimens of liver obtained at
medico-legal autopsies or by needle biopsies at Kabul, though 6.6%
of the 89 autopsy specimens showed "acute passive congestion with
necrosis". The ages of these subjects was not stated and the
authors did not discuss the cause of the lesion.
Following these outbreaks, a number of isolated cases have been
described following the use of herbal medicines. McGee et al.
(1976) reported a case from the United Kingdom of a 26-year-old
woman who had consumed herbal tea containing pyrrolizidine
alkaloids. The one sample examined contained only trace amounts of
alkaloids. Neither the plant nor the alkaloids were further
characterized. The patient had been taking very large quantities
of the tea for about 2 years and it is possible that some of the
earlier batches may have been more heavily contaminated. Maté or
Paraguay tea ( Ilex species), which she was drinking, is stated to
be a popular drink in Brazil and is not believed to contain
pyrrolizidine alkaloids. It has been stated (Huxtable, 1980) that
possibly she ingested the pyrrolizidine alkaloids from some other
unidentified source. The clinical course of the disease progressed
rapidly. Three biopsies carried out within one month and the
autopsy showed characteristic changes including centrizonal
fibrosis, but no cirrhosis. It is notable that some involvement of
muscular pulmonary arteries was also seen.
Lyford et al. (1976) reported the case of a 35-year-old woman
from Ecuador, who had ingested herbal tea prepared from Crotalaria
juncea. She had consumed 1 - 2 litres of this infusion daily for 6
weeks, but no qualitative or quantitative analysis for
pyrrolizidine alkaloids was made. She had had arthralgias for 3
years, for which she had received treatment with indomethacin and
phenylbutazone. The liver biopsy showed characteristic changes of
acute VOD from which she recovered completely as proved by a repeat
biopsy carried out one year later.
The occurrence of acute disease was reported in 2 infants in
Arizona in the USA, aged 6 months and 2 months, respectively,
following ingestion of infusions of a herb, locally called the
Gordolobo Yerba by the Mexican-American population and identified
as Senecio longilobus (Stillman et al., 1977; Fox et al., 1978;
Huxtable, 1980). The plant from which this infusion had been
prepared was found to contain pyrrolizidine alkaloids identified as
riddelline and N-oxides of retrorsine, seneciphylline, and
senecionine (Huxtable, 1980), in a concentration of 3 g free
alkaloid and 10.5 g N-oxides/kg (Stillman et al., 1977). It was
estimated that, during a period of 2 weeks, the 6-month-old infant
received a total dose of between 70 and 147 mg of combined alkaloid
and N-oxide derivative. The liver biopsy showed characteristic
features of acute disease, which had progressed to extensive
central, portal, and sinusoidal fibrosis (Stillman et al., 1977).
The child subsequently developed cirrhosis over the next 8-month
period. The 2-month-old infant was administered an infusion of the
same herb for 4 days, after which he became progressively more ill
and stuporous (Fox et al., 1978). On admission he was diagnosed as
a case of Reye's syndrome, but subsequently developed jaundice and
possibly ascites and died. The sample of herb contained a
concentration of alkaloids of 15 g/kg. It was estimated that the
infant had received a total of 66 mg of mixed alkaloids over the
4-day period. At autopsy, extensive centrilobular haemorrhagic
necrosis of the liver was seen, which is characteristic of the
acute disease. However, no occlusive lesions of the central vein
of the lobules were described, and no obstructive lesions were seen
in the larger hepatic veins or inferior vena cava. No mention was
made of ascites. The basal ganglia showed kernicterus.
Datta et al. (1978 a,b) reported 6 cases that occurred between
1974 and 1977. All of the patients had taken herbal medicines,
identified in 1 case as Heliotropium eichwaldii, which contained
N-oxides of heliotrine. Two patients took the herb as an extract
of the whole plant, which contained an alkaloid concentration of
20 g/kg, for 20 and 50 days, respectively, and developed symptoms
after a time lag of 45 and 90 days, respectively. They were both
estimated to have consumed 200 mg of heliotrine per day, the total
alkaloid intake being 4 g and 10 g, respectively. They had taken
the herb for treatment of epilepsy, and were admitted with acute
onset of symptoms of abdominal pain, ascites, jaundice, hepatic
encephalopathy, and gastrointestinal bleeding, which suggested
fulminant viral hepatitis. They died within 2 - 12 weeks of the
onset of symptoms. Only a brief description of the main autopsy
findings in the liver was given, which indicated that there were
changes characteristic of acute veno-occlusive disease of liver,
including marked centrilobular haemorrhagic necrosis of the liver
lobules, and occlusive lesions of the central and sublobular veins.
It is interesting to note that both patients had also been on long-
term anticonvulsant phenobarbitone therapy. The remaining 4
patients had a chronic insidious onset of disease suggesting
cirrhosis of the liver in 3, and alcoholic liver disease in one.
One of the former had been taking some indigenous powder,
presumably prepared from a herb, the indication and nature of which
are not known. The patient died from hepatic encephalopathy. No
detailed description of the autopsy findings was given, but it was
stated that the central and sublobular veins of the liver showed
chronic occlusive changes. The inferior vena cava and large
hepatic veins were patent. There was non-portal cirrhosis. A
notable feature was that the arsenic levels in the liver tissue
were high (500 µg/kg; normal, 1 µg/kg). There is no mention of
arsenic in the report on the analysis of the indigenous powder
taken by the patient. Two of the remaining 3 patients with chronic
disease, had taken herbal medicine for vitiligo, and one for
diabetes mellitus. The herb was identified as Heliotropium
eichwaldii in the case of one of the vitiligo patients, who had
taken it for 10 days and in whom the onset of symptoms occurred
within 10 days. The herb was taken in the form of seed with an
alkaloid content of 12 g/kg. The daily intake was estimated to be
500 mg of alkaloid and the total intake, 5 g. This patient was
admitted with a clinical diagnosis of cirrhosis of the liver. The
liver biopsy showed changes characteristic of acute VOD. Follow-up
data are not known. The herb taken by the other 2 patients was not
identified. The diabetic patient was being treated with oral
hypoglycaemic drugs and was also known to be an alcoholic. The
indigenous powder being taken by him contained a high concentration
of arsenic (5 mg/kg). The results of haemo-dynamic studies
suggested hepatic venous outflow tract obstruction of the
intrahepatic post-sinusoidal type in the smallest hepatic veins.
Liver biopsy showed characteristic centrilobular haemorrhages.
Central veins could not be recognized. There were mild changes in
the liver cells, but no alcoholic hyaline was seen. The liver
biopsy of the third patient showed characteristic features of acute
disease with veno-occlusion.
Two cases have been reported from China (Hou et al., 1980).
Both were adults who were taken ill after taking medicinal
infusions prepared from Gynura segetum of the family Compositae
(tribe, Senecioneae). The presenting symptoms and the cause of
disease, as well as the pathological findings, were characteristic,
except that one patient had jaundice and also portal vein
thrombosis, which is not a usual feature of the disease. No
chemical analysis of the plant was made and the alkaloid was not
precisely identified. Furthermore, the total intake of alkaloid
was not calculated. It has been stated that this was the first
report of such a case, but that it was possible that the disease
might occur among adults more frequently without being reported.
Ghanem & Hershko (1981) reported 3 cases of Arabs, one 3-year-
old child and 2 adults, who were diagnosed as having VOD of the
liver, on the basis of the clinical findings and morphological
features of liver biopsies. One more patient had occlusive lesions
of both the small and large hepatic vein radicles. They were among
29 patients with clinical features of hepatic vein thrombosis (HVT)
found on a retrospective analysis of data from 9 major hospitals in
Israel. Of these patients, 15 were Jews and 14 were Arabs.
Notable features were that all Jewish patients were adults, whereas
the majority of Arab patients were children below 10 years of age
and primary HVT was 2.4 times more common among the latter. No
analysis of the diet was made for PAs and their etiological role
was suspected only on a presumptive basis. A survey of stored
wheat grain in 9 villages showed that 2 samples were contaminated
with seeds of Lolium, belonging to the Graminae family, which were
found to contain 2 PAs (loline and norloline). However, these PAs
are not known to be hepatotoxic. The authors argued that, even
though in a classical case of VOD there should not be thrombosis of
the larger hepatic vein radicles, the difference in the anatomical
appearance of VOD and that of primary HVT of the near-east type is
not due to a different etiological agent but rather to a difference
in the dose and rate of absorption of the ingested toxic compounds.
A further report of the disease from Hong Kong, by Kumana et
al. (1985), described it in 4 young Chinese women with psoriasis
who took infusions of a herbal remedy, the toxic component of which
has since been identified as Heliotropium lasiocarpum (Culvenor et
al., 1986). They developed symptoms 19 - 45 days after starting
the herbal treatment, and were examined 61 - 68 days after its
initiation. The condition of patient No. 2, who continued taking
the herb for 16 days after the onset of symptoms, deteriorated and
she died of hepatic failure and was autopsied. The liver biopsies
and autopsy confirmed the presence of acute disease in all
patients. Patient No. 4 stopped taking the herb after 21 days, on
account of a new rash. When assessed 77 days later, she had mild
hepatomegaly only. A detailed analysis of the alkaloid content was
carried out for each case. The pyrrolizidine alkaloids were
quantified as if senecionine based. The herb contained 0.42 g
alkaloid/kg and 1.4 g N-oxide/kg. The daily intakes of alkaloid
base and N-oxide were estimated to be 12 ± 1 mg and 18 ± 4 mg,
respectively. The respective cumulative doses of alkaloid (base
and N-oxide) consumed by patients Nos 1, 2, and 3, up to onset of
symptoms, were calculated to be 1350 mg over 45 days, 900 mg over
30 days, and 570 over 19 days, respectively. Patient No. 4 who had
irrefutable histological evidence of disease but did not develop
symptomatic disease, must have consumed 630 mg over 21 days.
Patient No. 2, who died, was estimated to have taken a total amount
of 1380 mg alkaloid over 46 days. The estimated cumulative intake
per kg body weight before the development of symptoms for patients
Nos. 1, 2, 3, and 4 was 26, 15, 12, and 15 mg/kg, respectively. It
should be noted that, in patient No. 2, who died, the cumulative
dose until the onset of symptoms was the same as in patient No. 4,
who was asymptomatic. Moreover, the total intake by patient No. 2,
was 23 mg/kg, which was lower than the intake by patient No. 1, who
survived. The authors compared the intake data of their patients
with those of the Arizona children reported by Stillman et al.
(1977) and Fox et al. (1978). The 6-month-old baby, who survived
but developed cirrhosis, and the 2-month-old baby, who died, are
estimated by comparison to have taken cumulative doses of 12 - 25
and 11 mg/kg, respectively. The above data suggest marked
variation in susceptibility among individual subjects. It is also
known from experimental animal studies that the young and new-born
animals are particularly vulnerable (Jago, 1970).
A case reported from the USA is ascribed to the consumption of
comfrey ( Symphytum sp.) powder, sold as a digestive aid (Ridker et
al., 1985). A 49-year-old woman presented with classical symptoms
and signs of VOD. The haemodynamic data showed a hepatic vein
wedge pressure of 3.07 kPa (23 mmHg) with a sinusoidal pressure of
2.27 kPa (17 mmHg). Hepatic venograms showed near obliteration of
the smaller radicles of the hepatic veins during balloon distension
of one of the intrahepatic venous tributaries, and there was extra-
vasation of the dye into the hepatic parenchyma. A porta-caval
shunt was carried out and the operative findings confirmed the
presence of a post-sinusoidal block. The liver biopsy showed
marked centrilobular necrosis and congestion with dilatation of the
central veins and sinusoids, consistent with hepatic venous outflow
tract obstruction. According to the clinical history, the patient
had been a heavy consumer of food supplements. Apart from several
vitamins and minerals, she had been drinking 3 cups of camomile tea
per week and for 6 months before admission had consumed 1 g/day of
a commercially available herbal tea. For 4 months before
admission, she had taken 2 capsules of "comfrey-pepsin" pills with
each meal. The herbal tea and the pills were analysed for PAs.
Pyrrolizidine alkaloids and their N-oxides were found, but the
compounds were not precisely identified. On the basis of the
analysis of the PA content, the patient was estimated to have
consumed a total of at least 85 mg of PA (Huxtable et al., 1986)
(14.1 µg/kg body weight per day, Ridker et al., 1985). The authors
emphasized that the total PA consumption was relatively low. It
was possible that the patient had other sources of exposure and
probably she had been consuming PA-containing supplements for
longer than the periods stated by her in the clinical history.
The latest is the report of a 13 year old boy from the U.K. who
is stated to have developed symptoms of toxicity following
administration of herbal tea prepared from comfrey leaf (Symphytum
officinale) for treatment of inflammatory bowel disease for two or
three years (Weston et al., 1987). The exact quantity of leaves
consumed and frequency of administration were not known. The liver
biopsy is stated to have shown a "thrombotic variant" of veno-
occlusive disease, though the inferior vena cava and the major
hepatic veins were patent on Doppler ultrasound and percutaneous
phlebography. He had earlier been treated with predinisolone
and sulphasalazine. The case is unusual in so far as the
thrombosis of the central veins of the liver lobules, which is not
a usual feature of veno-occlusive disease of the liver.
7.4 VOD and Cirrhosis of the Liver
Jelliffe et al. (1954b) were the first to draw attention to VOD
being an important cause of cirrhosis among Jamaican children.
Prior to this, Hashem (1939), while reviewing the records of all
cases of cirrhosis admitted to a children's hospital in Egypt since
1933, described 3 cases of a special type of cirrhosis that was
rare in adults. The clinical features and pathological findings
were similar to those in cases of VOD. They speculated that the
cause was some metabolic toxins of gastrointestinal origin. Royes
(1948) suggested that the cirrhosis in the Jamaican children was
very like the disease described from India and Egypt.
The clinical and pathological features of 100 cases of VOD
among Jamaican children were described by Bras et al. (1954).
Sixty-five of the cases were below 12 years of age. None of the
100 patients had cirrhosis initially, but 5 showed occlusive
lesions in hepatic veins and features of non-portal fibrosis. Four
of these 5 cases later developed cirrhosis. The authors concluded
that VOD contributed to a substantial number of all cases of
cirrhosis in this age group. Stuart & Bras (1957) studied 84
patients with VOD including 64 acute, 6 subacute, and 14 chronic
cases. Twenty-three patients were followed up, some for up to 5
years. Autopsy performed on 21/26 cases showed cirrhosis in 11
cases. Notable features were that 1 of the 6 cases of acute
disease described in detail, developed cirrhosis. Of the 2 cases
with chronic disease, 1 developed cirrhosis within 3 months of a
liver biopsy for acute disease, at which time the liver had shown
hepatic venous occlusive lesions but no fibrosis. Autopsy findings
were described by Bras & Watler (1955) in 19 patients aged 10
months - 45 years in different stages of the disease. Nine
patients had cirrhosis that was non-portal to begin with but
finally became indistinguishable from Laennec's portal cirrhosis.
Rhodes (1957) studied the pattern of liver disease among Jamaican
children. A total of 193 liver biopsies was studied derived from
39 children who had one biopsy and 59 who had more than one at
intervals of 1 week - 3 years. Of the 14 autopsies on cases of
VOD, 12 had cirrhosis. A notable feature was that the disease
could occur asymptomatically with hepatomegaly. The autopsy
material from the University College Hospital, Jamaica was analysed
by Bras et al. (1961). Of the 1560 autopsies, 28.5% cases
concerned infants of less than one year old, mostly from poor,
predominantly black families. Cirrhosis was seen in 77 autopsies.
Approximately 30% of these 77 cases were diagnosed as post-VOD.
The authors postulated that they might have resulted from the
ingestion of Crotalaria fulva or some other toxic substances.
In a follow-up study of 61 patients who developed the disease
in an outbreak in the USSR in 1958, 28 developed "Hepatoleinal
syndrome" (Braginski & Bobokhadzaev, 1965). Two of these cases, in
whom the disease in the initial stages was not particularly severe,
developed cirrhosis within 4 years.
Tandon, H.D. (personal communication) has analysed the
pathological data derived from the Afghan and Indian outbreaks on
the basis of 61 liver biopsies and 17 autopsies, including repeat
biopsy studies on 15 patients who were followed-up for 1 month - 3
years after onset in the Indian outbreaks. Three of the 11
patients, followed up for 16 months or longer for persistent
clinical evidence of disease, ended up with cirrhosis and 2 more
had marked fibrosis with equivocal changes of cirrhosis in the
biopsy. Notable features of the study were that the disease
progressed to cirrhosis in patients who were put on a normal diet,
free from alkaloids, after appearance of symptoms of acute disease
and did not receive any subsequent exposure. Impact of alcohol
intake was excluded. There was a poor correlation between the
clinical and pathological severity of disease. Centrilobular
haemorrhages, which are a sign of acute disease, were seen to
persist for over one year in patients, some of whom were apparently
well. At needle biopsy, characteristic hepatic venous occlusions
were not seen in many patients in the acute phase of the disease,
though they were seen in all livers at autopsy and most of them
showed persistent centrilobular haemorrhages. Biopsy findings in
cirrhotic livers were often not histopathologically characteristic
for any specific form of cirrhosis. Features that might suggest
the veno-occlusive etiology of cirrhosis at biopsy included
paraseptal dilatation of sinusoids and persistent haemorrhages or
haemosiderin in the septa.
In studies by Aikat et al. (1978) and Datta et al. (1978a,b), 6
cases of VOD were reported following ingestion of herbal medicines
containing PAs. Four of the patients had symptoms of chronic
disease and one of them developed non-portal cirrhosis.
One of the 2 infants from Arizona, USA, who suffered from VOD
following the administration of PA-containing herbal medicine,
developed cirrhosis during the 8-month period following the
appearance of acute symptoms (Stillman et al., 1977; Fox et al.,
1978; Huxtable, 1980). Huxtable (1980) mentioned the death from
cirrhosis of liver of a 62-year-old woman, who had consumed the
same herb as the 2 infants for 6 months prior to her death.
However, there was no confirmation of the diagnosis of cirrhosis.
7.5 Differences Between VOD and Indian Childhood Cirrhosis (ICC)
A type of cirrhosis of liver, peculiar to the people of Indian
origin, Indian Childhood Cirrhosis (ICC), has been ascribed to PA
toxic etiology (Bras et al., 1954; Rhodes, 1957), owing to the
observation of occlusive changes in the central and sublobular
veins of the liver, by some investigators (Radhakrishna Rao, 1935;
Prabhu, 1940; Jelliffe et al., 1957; Ramalingaswami & Nayak, 1969),
though this is not a characteristic feature of the disease. The
presence of copper-positive granules in the hepatocytes (Salaspuro
& Sipponen, 1976) has added to such a conjecture (Tanner &
Portmann, 1981), because of the reported aberration of copper
metabolism in experimental animals exposed to PAs (section 6.4.11).
However, ICC and VOD are clinically and pathologically distinct.
ICC, confined to infants and children, often affects siblings.
Jaundice is a common sign and hepatosplenomegaly is a common
feature. The disease is almost invariably fatal due to rapidly
developing hepatocellular failure. Liver parenchymal changes are
characterized by marked ballooning degeneration of hepatocytes,
prominent deposits of alcoholic hyaline, severe cholestasis, and
aggressive, pericellular fibrosis (Nayak et al., 1969), all
features not characteristic of VOD. Moreover, occlusive changes in
the hepatic veins are very rare and were not observed by any member
of the liver diseases subcommittee of the Indian Council of Medical
Research (1955), who made a critical study of the disease.
7.6 Chronic Lung Disease
Heath et al. (1975) reported the case of a 19-year-old African
man who had died of congestive cardiac failure, and who was
suspected of having ingested a herbal remedy containing the seeds
of Crotalaria laburnoides. Histopathological examination of the
lungs showed characteristic vascular changes of severe primary
pulmonary hypertension. Powdered seeds of the plant were fed in
the diet to Wistar albino rats for 60 days (Table 11).
Characteristic features of pulmonary hypertension including
hypertensive vascular changes in the lung and right ventricular
hypertrophy of the heart were produced in the animals showing that
the seeds contained an agent capable of inducing pulmonary
hypertension in rats. Apart from this indirect evidence, there was
no proof of such a causal relationship with the pulmonary
hypertensive disease in the patient.
A brief mention is made by McGee et al. (1976) of finding
changes "somewhat similar but rather more mature" (than those seen
in the hepatic veins) involving some branches of the pulmonary
artery in the lower lobe of the left lung, in a case of veno-
occlusive disease of the liver caused by ingestion of PA-containing
herbal teas. The alkaloids were not further characterized. The
changes were also stated to be similar to those produced in
experimental animals by PAs. Apart from these 2 cases, there is no
mention of involvement of the pulmonary arterial system in any of
the case reports available.
The possibility that diet-mediated agents might induce
pulmonary hypertension in man has been discussed at length by
Fishman (1974). A parallel was drawn with the epidemic of
pulmonary hypertension that occurred in Austria, the Federal
Republic of Germany, and Switzerland between 1966 and 1968 (Kay et
al., 1971b), which was suspected of being caused by Aminorex, a
compound that resembles epinephrine and amphetamine in chemical
structure. Although the etiological role of Aminorex could not be
conclusively proved on epidemiological or experimental grounds,
there continues to be a suspicion that agents taken by mouth can
evoke pulmonary hypertension in man. Levine et al. (1973) reported
the cases of 3 children aged 5 1/2 - 13 years, and 11 months,
respectively, with portal hypertension, who developed progressive
pulmonary hypertension resulting in cor pulmonale and death. In
all 3 cases, there was evidence of extra-hepatic portal vein
obstruction confirmed at autopsy, and the symptoms and signs of
portal obstruction had appeared in early childhood. They developed
symptoms of cardio-respiratory failure. Studies of pulmonary and
cardiovascular function including haemodynamic studies of pulmonary
circulation in 2 of the children suggested pulmonary vascular
obstructive disease. At autopsy, no primary parenchymal lung
disease was found. There were vascular changes of advanced
pulmonary hypertension (plexiform lesions), but no evidence of
thromboembolism was found. No factor responsible for pulmonary
vasoconstriction was identified. In one case, the liver was stated
to show coarse nodularity at autopsy, but the microscopic
examination showed only patchy areas of portal fibrosis and
regeneration. In the other 2 cases, there were only non-specific
changes, with fibrosis in one. However, centrilobular congestion
was present in 2 cases. It is possible that some metabolites of
toxic agents, such as PAs, which are metabolized in the liver,
might have blocked a metabolic pathway that ordinarily exerts a
pulmonary antihypertensive effect, or that, by damaging the liver,
vasoactive substances, such as histamine, serotonin, and
catecholamines, might escape metabolic pathways to reach the lungs
and injure the pulmonary vessels. However, no such agents were
identified.
Kay et al. (1971b) made a plea that, on the basis of the
experimental data available, including the ability of several
agents to produce pulmonary hypertension in experimental animals,
and, in spite of the fact that pulmonary vascular disease has never
been demonstrated in human cases of veno-occlusive disease, careful
enquiries should be made on the possibility of patients presenting
with unexplained pulmonary hypertension having ingested a plant
product. A similar plea was made by Heath et al. (1975).
7.7 Trichodesma Poisoning
The disease "Ozhalanger encephalitis", which occurred in the
Samarkand region of Uzbekistan, USSR in the period 1942 - 51, is
believed to have been caused by contamination of food grain with
the seeds of Trichodesma incanum (Shtenberg & Orlova, 1955;
Ismailov et al., 1970), which contain 1.5 - 3.1% alkaloids, mainly
trichodesmine and incanine (Yunusov & Plekhanova, 1959). Clinical
features of the disease have been described by Ismailov et al.
(1970). This outbreak differed from the others described above in
that the primary symptomatology was extra-hepatic. Over 200
patients were affected, not including children below the age of 10,
or the breast-fed infants of the affected mothers. Following
exposure, there was a latent period of about 10 days, then vertigo
and recurring headaches developed leading to nausea and vomiting.
This was followed by generalized malaise, which progressed to
delirium and loss of consciousness. Physical signs included
pathological reflexes in 59% of the patients and paresis of the
extremities and the facial nerve. Death was stated to have been
caused mostly by respiratory depression. Of the 200 patients
affected, 44 died. Autopsy findings were relatively non-specific
degenerative and necrotic lesions scattered in several organs
including the central nervous system. No report of such a disease
is available from outside the USSR.
7.8 Relationship Between Dose Level and Toxic Effects
In some recent human case reports, the PAs consumed have ben
identified and estimates made of the daily and total intakes (Table
16). The relationship between these intake levels and the known
toxicity of the alkaloids in rats has been discussed by Culvenor
(1983) and Mattocks (1986).
Discussion of the relationship between the dose level and toxic
effects in human cases is complicated, because the poisoning is
generally due to a mixture of alkaloids found in naturally-
occurring plant products, consumed as herbal remedies or food, and
different plant species. There are wide differences in the acute
toxicities of the alkaloids, which are the best available measure
of comparative effects due to long-term intake as well.
Furthermore, estimates of intake can, at best, be approximate.
When a large population is affected through the contamination of a
food crop, as happened in the Afghan and Indian outbreaks (section
7.3), no precise estimates are possible regarding the extent of
contamination in different households, the amount of the
contaminated grain consumed, the length of exposure resulting in
the appearance of symptoms or signs of toxicity, or death, in the
cases studied. There may also be various other contributing
factors that are not apparent, e.g., food or cooking habits, on
account of which no conclusive generalizations regarding the
causative role of the toxic agent can be made. Reports on chemical
analysis for the toxic agent and, hence, the amounts ingested, may
not always be reliable (refer to the controversy in the Afghan
outbreak in section 7.3). In cases, such as the one reported by
Ridker et al. (1985), when the total dose of PAs estimated to have
been received by the patient before the disease developed, was only
a fraction of that of other alkaloids causing episodes of human
toxicity (Table 16), it is not certain whether that was the only
toxic alkaloid agent that the patient was exposed to, or whether
there were other contributing factors, particularly as the patient
was stated to be a heavy consumer of herbs, vitamins, and natural
food supplements.
In the known instances of human toxicity the principal
alkaloids involved are heliotrine from Heliotropium, echimidine and
related alkaloids from Symphytum, riddelline and retrosine from
Senecio longilobus, and crotananine and cronaburmine from
Crotalaria nana (Table 16). Approximate acute toxicity values
(LD50 in rats) for these alkaloid mixtures are 300, 500, and
50 mg/kg, respectively. For the mixture from C. nana, for which
there are no experimental data, the acute toxicity was assumed to
be similar to that of monocrotaline, 100 mg/kg. These relativities
need to be taken into account in discussing dose-effect
relationships for the PAs as a group. This has been done by
discussing first the dose estimates for heliotrine, since it was
the main alkaloid in 1 epidemic and in 6 case reports (Table 16).
Then in discussing the other alkaloids, reference is made to a
heliotrine-equivalent dose as well as the actual dose. The
heliotrine equivalent is:
LD50 of heliotrine
actual dose x ----------------------------------
LD50 of alkaloid mixture concerned
The estimated daily intake in poisoning by heliotrine ranges from
0.033 mg/kg body weight in the Afghan epidemic (Mohabbat et al.,
1976), which after a period of about 180 days and a total intake of
about 6 mg/kg caused fatalities, to 3.3 mg/kg, which led, in 2
cases in India (Datta et al., 1978a,b), to death after 20 and 50
days and total intakes of 67 and 167 mg/kg, respectively. In
between are 4 cases in Hong Kong with estimated daily intakes
ranging from 0.49 to 0.71 mg/kg (Kumana et al., 1983, 1985;
Culvenor et al., 1986). Three cases were non-fatal at total doses
of 11 - 27 mg/kg body weight and one was fatal at a total dose of
23 mg/kg. These heliotrine cases imply that daily intakes are
cumulative down to 0.033 mg/kg and may be fairly rapidly fatal
above 0.5 mg/kg. Above a total dose of 6 - 15 mg/kg, VOD may
become evident and sometimes fatal.
In the 2 cases due to riddelline and retrorsine in Senecio
longilobus (Stillman et al., 1977; Fox et al., 1978; Huxtable,
1980), the estimated daily intakes were 0.8 - 0.17 and 3 mg/kg body
weight (equivalent to 3 and 1 mg heliotrine/kg, respectively, and
the total doses were 12 - 25 and 12 mg/kg (equivalent to 72 - 150
and 72 mg heliotrine/kg, respectively). These levels are
comparable with the highest reported intakes of heliotrine and, in
infants, led to the rapid development of VOD and, in one case,
death.
In the epidemic due to mixed crotananine and cronaburmine in
Crotalaria nana (Tandon, B.N. et al., 1976; Tandon, R.K. et al.,
1976; Krishnamachari et al., 1977), the estimated daily intake of
0.66 mg/kg and the total intake of 40 mg/kg (equivalent to 2 and
120 mg heliotrine/kg, respectively) also corresponded to the
highest intake of heliotrine.
In the case of Symphytum poisoning (Ridker et al., 1985), the
estimated daily intake of echimidine and related alkaloids was
0.015 mg/kg and the total dose 1.7 mg/kg (equivalent to 0.009 and
1.0 mg/kg heliotrine, respectively). The dose levels are lower in
equivalent terms than the lowest estimates in cases due to
heliotrine by a factor of about 4 for daily intake and 6 for total
intake. The estimates were based on questioning of the patient and
assay of the material concerned. It seems prudent to conclude that
a daily intake of pyrrolizidine alkaloid as low as the equivalent
of 0.01 mg/kg heliotrine may lead to disease in humans.
Table 16. Estimated intakes of PAs in human beings
-----------------------------------------------------------------------------------------
Principal Age of Daily Period Total dose Toxic Reference
alkaloids(s)a subject intake (days) mg mg/kg effectc
(years) (mg/kg)b
-----------------------------------------------------------------------------------------
1. Heliotrine variousd 0.033 180e 360 6e VOD, Mohabbat et al.
death (1976)
2. Heliotrine (a) 20 3.3 20 4000 67 death Datta et al.
(b) 23 3.3 50 10000 167 death (1978a,b)
3. Riddelline, 0.5 0.8-1.7b 14 70-147 12-25 VOD Stillman et al.
retrorsine (1977); Huxtable
(1980)
4. Riddelline, 0.17 3.0b 4 66 12 death Fox et al. (1978)
retrorsine
5. Crotananine, variousd 0.66 c. 60e 2400 40 VOD, Tandon, B.N. et al.
crotaburmine death (1976); Tandon,
R.K. et al. (1976);
Krishnamachari et
al. (1977)
6. Heliotrine (a) 28 0.59 45 1350 27 VOD Kumana et al.
(b) 26 0.49 46 1380 23 death (1983, 1985);
(c) 23 0.60 19 570 11 VOD Culvenor et al.
(d) 27 0.71 21 630 15 VOD (1986)
7. Echimidine 49 0.015 120 94 1.7 VOD Ridker et al.
(1985)
-----------------------------------------------------------------------------------------
a The principal alkaloid is recorded as the free base, even if there was evidence of its
presence in the plant mainly as N-oxide.
b Calculated for a 60-kg adult, unless definite information available. The 0.5-year
infant was said to be 6 kg, and
c When mentioned, death was a consequence of severe liver damage.
d Epidemic.
e Estimate based on unpublished information available to the Task Group.
There is substantial overlap between intake rates and total
intakes for fatal and non-fatal poisoning. This presumably
reflects the influences of a number of factors, such as individual
sensitivity, age, nutritional status, and general health, but it is
also due to the progressive nature of pyrrolizidine toxicity and
the effects of time. In the epidemics, in which some people died,
only an estimated average intake is available and some who were
alive at the time of investigation may have died later. Comparing
the total intakes for human toxicity with the total doses up to
death observed in the long-term administration of PAs to rats,
1.2 - 10.9 times the LD50 dose, equivalent to 360 - 3270 mg
heliotrine/kg (Table 10, section 6.4.1.5), it is evident that human
beings are more susceptible to the acute and chronic effects of the
alkaloids than rats, sometimes markedly so.
These considerations of the toxic effects in human beings of
various intake levels could provide a basis for some assessment of
the likely hazard from other types of exposure to PAs. For
example, the consumption of comfrey root tea, estimated by Roitman
(1981) to contain 8.5 mg alkaloid per cup, at the rate of 3 cups
per day, or the ingestion of comfrey leaf at the rate of one leaf
per day, could lead to alkaloid ingestion rates of 0.40 and
0.016 mg/kg. These rates are respectively, much greater than, and
equal to, the lowest daily rate causing veno-occlusive disease. Lower
levels of exposure arising from such sources as the consumption of
milk from cows eating PA-containing plants or of honey derived from
such plants, seems unlikely, in practice, to cause acute or
subacute liver disease. However, care should be exercised. In an
experimental situation in which cows were fed Senecio jacobaea,
the milk was reported to contain up to 0.84 mg alkaloid/litre. A
30-kg child drinking 0.5 litre/day of this milk could ingest
0.014 mg/kg alkaloid, equivalent to 0.028 mg heliotrine/kg (assuming
an LD50 of 150 mg/kg for S. jacobaea alkaloid). This is above the
lowest daily rate leading to veno-occlusive disease and the lowest
estimated total toxic dose would be achieved in 36 days. This
level of contamination of milk is undoubtedly extreme and there is
no knowledge of any contamination of commercial milk supplies.
Honey derived from Echium plantagineum has been reported to contain
up to 1 mg alkaloid/kg (Culvenor et al., 1981). A 30-kg child
consuming 30 g/day of honey (a high consumption rate) would ingest
0.001 mg alkaloid/kg body weight. The lowest estimated total toxic
dose (1.7 mg comfrey alkaloid/kg, very similar to Echium alkaloid)
would be achieved in 1700 days. Although it seems likely that
consumption of contaminated milk and honey would lead to acute or
subacute liver disease, the possibility remains that they may
contribute to chronic liver disease or liver tumours.
The possibility of carcinogenic effects due to long-term
exposure to PA-containing plants has been discussed by Culvenor
(1983). Some of the PAs involved in instances of human poisoning
have been found to be carcinogenic in experimental animals (Table
13). Data from some of the significant experimental studies were
summarized by Culvenor (1983) with approximate estimates of PA
dosages administered to rats in terms of mg/kg body weight per day
(Table 17). The dose rates that were carcinogenic for rats (Table
17) ranged from 2 to 6 mg/kg per day for an initial period and
0.2 - 3 mg/kg per day for a remaining period of about 12 months,
except in one study in which a dose of 10 mg/kg per day was used.
It can be seen that, in all except two instances of human
poisonings summarized in Table 16, the estimated daily rates of
intake ranging from 0.015 to 3.3 mg/kg body weight per day are
within close range of those known to induce tumours in rats. In
other reports, the consumption rates are above and below this
range.
Epidemiological studies to assess the carcinogenic role of PAs
for man are not available. In countries with a high incidence of
primary liver cancer, it is possible that PAs may have an additive
effect with those attributed to aflatoxin (Newberne & Rogers, 1973)
and hepatitis B virus. The total evidence now available warrants
long-term studies of the survivors of poisoning outbreaks,
especially where a substantial number of people were affected, as
in the Afghanistan outbreak.
7.9 Pyrrolizidine Alkaloids as a Chemotherapeutic Agent for Cancer
The PA, indicine N-oxide derived from Heliotropium indicum, a
widely used indigenous drug in Ayurvedic medicine, has been found
to have an antitumour activity and has been used in clinical trials
as a chemotherapeutic agent for leukaemia (Letendre et al., 1981,
1984; Cook et al., 1983) and solid tumours (Kovach et al., 1979a,b;
Nichols et al., 1981; Ohnuma et al., 1982; Taylor et al., 1983).
Dosing schedules typically were 5 consecutive intravenous doses of
0.15 - 3 g/m2 body surface area (approximately 2.5 - 5 mg/kg body
weight) repeated at 4- or 6-week intervals (Kovach et al., 1979a;
Letendre et al., 1981; Ohnuma et al., 1982). Hepatic toxicity, as
judged by increased SGOP levels, was infrequent and mild. However,
subsequent trials with this agent have indicated more serious
hepatotoxicity. In a more recent report by the same workers
(Letendre et al., 1984), 5 out of 22 cases of refractory acute
leukaemia, treated with indicine N-oxide, had severe
hepatotoxicity, presumably induced by the drug. One of these
patients had been treated for 18 months with methyl-testosterone, 4
months prior to receiving indicine N-oxide. Symptoms of severe
hepatocellular failure appeared in 3 patients after the initial
course of treatment. This occurred after 4 daily doses of 3 g/m2
surface area in one patient and after 5 daily doses of 3.75 g/m2
surface area in 2 patients. Two other patients who had received
3 g/m2 surface area daily for 5 days developed hepatocellular failure
after the second course of treatment, one at 3.3 g/m2 and the other
at 3.75 g/m2 surface area, daily, for 5 days. In each patient, the
onset of hepatic disease was rapid and the course was downhill.
Livers of 4 of these patients examined at post-mortem showed severe
centrilobular vascular congestion with necrosis of parenchymal
cells, and, in one patient, a few sublobular veins were found to be
occluded.
Miser et al. (1982) reported severe hepatotoxicity in 4 of 45
children treated with indicine N-oxide for refractory leukaemia or
advanced solid tumours. Similarly, Cook et al. (1983) reported the
case of a 5-year-old child with acute myeloid leukaemia who
developed severe hepatic failure within 3 days of starting the
treatment. Autopsy showed massive hepatic necrosis.
However, it should be noted that no hepatic failure was
reported in more than 100 adults with solid tumours, treated with
the same agent (Kovach et al., 1979a,b; Nichols et al., 1981;
Taylor et al., 1983). No hepatotoxic effects were reported by
Ohnuma et al. (1982) among 37 patients who received this drug for
solid tumours. The major toxic effect was myelosuppression (Kovach
et al., 1979b).
Table 17. Rates of administration of PAs leading to tumours in ratsa
------------------------------------------------------------------------------------------
Alkaloid Dosing schedule Approximate Number of Reference
equivalent rateb rats
(mg/kg per day) developing
tumours
------------------------------------------------------------------------------------------
Lasiocarpine (a) 7 mg/kg diet, 24 months 0.70 23/24 Nat. Cancer
Institute
(1978)
(b) 15 mg/kg diet, 24 months 1.50 24/24 Nat. Cancer
Institute
(1978)
(c) 7.8 mg/kg, ip, 2/week for 2.2 for 4 weeks, 16/18 Svoboda &
4 weeks and 1/week for then 1.1 for Reddy (1972)
52 weeks 52 weeks
(d) 50 mg/kg diet, 55 weeks 5.0 18/20 Rao & Reddy
(1978)
(e) 0.39 mg/kg, ip, 3/week, 0.2 2/7 Culvenor &
to death Jago (1979)
Monocrotaline (a) 25 mg/kg, ip, 1/week for 3.5 for 4 weeks, 10/50 Newberne &
4 weeks, and 8 mg/kg, then 1.1 for Rogers (1973)
for 38 weeks 38 weeks
(b) 5 mg/kg sc, once per 0.36 43/60 Shumaker et
2 weeks for 52 weeks al. (1976)
Retrorsine (a) 30 mg/kg, ip, single dose - 7/29 Schoental &
Bensted (1963)
(b) 30 mg/litre in water, 1.3 4/14 Schoental et
3 days/week, to death al. (1954)
(c) 30 - 50 mg N-oxide/litre 1.3 - 2.0 10/22 Schoental et
in water, 3 days/week al. (1954)
for 20 months
------------------------------------------------------------------------------------------
Table 17. (contd)
------------------------------------------------------------------------------------------
Alkaloid Dosing schedule Approximate Number of Reference
equivalent rateb rats
(mg/kg per day) developing
tumours
------------------------------------------------------------------------------------------
Petasitenine 0.1 g/litre in water, 10 8/10 Hirono et
up to 16 months al. (1977)
Senkirkine 22 mg/kg, ip, 2/week for 6 for 4 weeks, 11/24 Hirono et
4 weeks and 1/week for then 3 for al. (1979a)
52 weeks 25 weeks
Symphytine 13 mg/kg, ip, 2/week for 3.7 for 4 weeks, 5/24 Hirono et
4 weeks and 1/week for then 1.9 for al. (1979a)
52 weeks 52 weeks
------------------------------------------------------------------------------------------
a From: Culvenor (1983).
b Where necessary, estimates assume a daily rat food intake of 100 g/kg body weight, and
water intake 100 ml/kg body weight. Injected doses are given pro rata, for daily
administration.
7.10 Prevention of Poisoning in Man
At present, prevention of poisoning can be achieved only by
reducing or eliminating ingestion of the alkaloids. The two
effective procedures are control of PA-containing plants in
agricultural areas and educational programmes directed to the
populations at risk.
The control of plant populations for this purpose has been
carried out only in Uzbekistan, USSR, following the epidemics of
human disease due to contamination of grain by seeds of
Heliotropium lasiocarpum and Trichodesma incanum. The following
measures were introduced and have been effective in preventing
further outbreaks:
1. A state standard was set for the quality of seed grain, which
must be certified by a State Seed Inspectorate. Current
standards prohibit the sowing of wheat, rye, barley, or oats
contaminated by seed of Heliotropium lasiocarpum or Trichodesma
incanum.
2. A state standard was set for the quality of grain stored for
food. The limits for Heliotropium lasiocarpum and
Trichodesma incanum seeds are 0.2% and zero, respectively.
3. Agricultural (agritechnical) measures to ensure minimum
contamination of crops and harvested grain, including
specification of the most suitable methods and timing of
cultivation, use of clean seed for sowing, weeding of crops
prior to maturing of the grain (towards the end of May), and
mechanical cleaning of grain.
4. Methods for monitoring levels of contamination of flour, bread,
and similar products.
5. Publication of educational booklets describing the biological,
environmental, and morphological characteristics of the toxic
weeds, their pathways of distribution and the causes of the
toxicoses experienced.
6. Promotion of weed control by governmental authorities and
provision of legislation to enforce the control measures.
In other countries, the control of some PA-containing weeds in
crops is practised by cultivation and herbicide treatment, in order
to maximize yield and the general quality of the grain. In
pastures, animal management and herbicide treatments are used to
increase pastures and reduce poisoning of animals. Specific
treatment methods differ according to the plant species and the
circumstances. General references were not available to the Task
Group.
In Australia, where Heliotropium europaeum and Echium
plantagineum are widespread weeds in wheat-growing areas but where
normal agricultural practices prevent all but occasional minor
contamination, relevant tolerance standards for wheat delivered at
storage silos are not specific. Heliotropium europaeum seed is
rarely seen and would form part of the "unmillable material" the
seed component of which can be up to 1% of the volume of the wheat.
Seed of Echium plantagineum is occasionally seen in delivered grain
at levels of up to 10 seeds per half litre, the tolerance level for
this seed fraction being 50 seeds per half litre.
8. BIOLOGICAL CONTROL
Biological control methods have been investigated for several
PA-containing plant species, notably Senecio jacobaea, Heliotropium
europaeum, Echium plantagineum, and Trichodesma incanum. The
effectiveness of such methods is variable and good results may be
confined to certain regions where favourable conditions exist. For
example, in control programmes against S. jacobaea in Australia,
Canada, New Zealand, and the USA, using 3 insect species, results
varied from virtually nil to nearly 100% control (Julien 1982).
The effects of the introduction of the cinnabar moth ( Tyria
jacobaea L.) on S. jacobaea in these countries have been summarized
as in Table 18.
Table 18. Results of the attempted control of Senecio
jacobaea (ragwort) with the cinnabar moth
------------------------------------------------------------
Country or region Result
------------------------------------------------------------
Australia Establishment precluded by predation,
parasitism and disease
Western Canada Moth populations stabilized below that
required for control
Eastern Canada Establishment and subsequent notable
reductions in ragwort levels
New Zealand Marginal establishment, moth population
limited by predation and parasitism,
little impact on ragwort
USA Widespread establishment, ragwort levels
sometimes reduced at localities near the
limits of its distribution
------------------------------------------------------------
Several agents are being tested in Australia for the control of
H. europaeum and one species, a flee beetle Longitarsus albineus,
has been released (Julien, 1982; Delfosse, 1985). There are good
prospects in this country for the biological control of Echium
plantagineum and 2 other Echium spp., with 8 insect species
approved for release, when legal restrictions are lifted (Delfosse
& Cullen, 1985a,b). Preliminary studies have been made on the
biological control of Amsinckia and other Senecio species (e.g.,
Pantone et al., 1985).
Given adequate funding, PA-containing plants are a suitable
target for biological control.
9. EVALUATION OF HUMAN HEALTH RISKS AND EFFECTS ON THE ENVIRONMENT
9.1 Human Exposure Conditions
9.1.1 Reported sources of human exposure
The two main sources of exposure of human beings to toxic PAs
that have led to major outbreaks of poisoning with high mortalities
as well as to individual cases in several countries are:
(a) the contamination of cereal grains, such as wheat and
millet, with the seed or other parts of plants containing
alkaloids; and
(b) the consumption for medicinal or dietary purposes of herbs
containing the alkaloids, either as the plant itself or as
infusions.
Consumption of contaminated grain is more likely to occur in
regions where food is in short supply, and particularly when
drought favours infestation of the grain crop by PA-containing
weeds. A qualitative field test for detecting the presence of
toxic pyrrolizidine alkaloids in plant materials, using simple
laboratory methods, is now available (section 2.2.2.5).
9.1.2 Plant species involved
Plant species containing toxic PAs occur throughout the world
and are known in 47 genera in 6 plant families. As many as 6000
species are potentially PA-containing. The most important genera
responsible for human and animal disease are Senecio and other
genera of the tribe Senecioneae (family Compositae), Crotalaria
(family Leguminosae) and Heliotropium, Trichodesma, and other
genera of the family Boraginaceae (sections 3.1 and 3.2).
Approximately 150 different toxic PAs have been isolated from
about 360 plant species that have been investigated and contain
this type of alkaloid. Of these, about 12 have been involved in
instances of human toxicity (section 3.1). The molecular
structures of almost all of these alkaloids are known and the main
outlines of structure-toxicity relationships have been established
(section 2.1).
The alkaloids may occur in all plant parts and are often
present as the N-oxide derivatives, which are also toxic when
ingested orally. Alkaloid contents vary from low (0.1 g/kg dry
weight) to very high (40 g/kg up to the maximum recorded of
180 g/kg in Senecio riddelli). Levels vary with stage of growth,
locality, and other circumstances. In some, but not all, species,
the alkaloid is partly decomposed during the drying or storage of
the plant. The decomposition is largely enzymic and once the plant
material is dry, the alkaloid is fairly stable.
9.1.3 Modes and pathways of exposure
9.1.3.1 Contamination of grain crops
Large outbreaks of poisoning have occurred through
contamination of wheat crops in Afghanistan, India, and the USSR.
In particular, 3 species of Boraginaceae (Heliotropium lasiocarpum,
H. popovii, and H. europaeum) are well adapted to vigorous growth
under the climatic conditions in which wheat is usually grown.
Contamination can be effectively controlled in wheat produced using
modern harvesting techniques and grain seed that is inspected and
controlled for weed seed contamination, but control of
contamination is more difficult where these conditions cannot be
met. Contamination of staple food grain is of particular concern,
since entire populations are exposed, and control may not be
possible, if the people are not aware of the hazard that
PA-containing weeds present.
9.1.3.2 Herbal medicines
Herbal preparations containing PAs are used as tonics,
treatments, preventatives, and food supplements. Such usages are
so widespread that they are nearly universal. Many are
traditional, while others reflect a rejection of, or lack of access
to, standard health care services (section 3.3.2).
Veno-occlusive disease was first recognized as a clinical
entity in Jamaica as a result of the medicinal use of PA-containing
herbs prepared from Crotalaria. Crotalaria-containing herbs have
also been responsible for human poisonings in Barbados, Equador,
and other locations in the West Indies. Heliotropium herbs have
been reported to cause poisoning in Hong Kong and India.
Symphytum- and Senecio-containing herbs have given rise to case
reports in the USA. Other reports of PA poisoning are known in
which the herbs used were not botanically identified. PA-poisoning
has been associated with both home-prepared and commercially
available herbs, the latter including prescriptions by herbalists
(Weston et al., 1987).
Various other genera of PA-containing plants in the families
Boraginaceae, Compositae, and Leguminosae are also widely used as
herbs. No case reports are available for these genera.
Reported cases of PA poisoning due to the use of the herbs are
geographically widespread, but few in number. However, the scale
on which PA-containing plants are used as herbs, the typically
delayed effects of long-term exposure, and the difficulties of
diagnosis led the Task Group to conclude that there is every
indication of under-reporting of intoxications from the use of such
herbs. Symphytum root preparations, in particular, represent a
hazard, and certain user groups are routinely exposed to levels of
Symphytum alkaloids that are higher than those at which
intoxications have been reported.
The risks associated with the use of PA-containing herbs are
accentuated by the difficulties of controlling this use.
9.1.3.3 PA-containing plants used as food and beverages
Some PA-containing plants are used for food or the making of
beverages in many countries, including developed countries. The
following species are known to be used (though many other plants
are also probably used in this way): Cacalia yatabei, Symphytum
species, Ligularia dentata, Petasites japonicus, Senecio burchellii,
S. inadequidens, S. pierotti, Syneilesis palmata, Crotalaria
anagyrodies, C. brevidens, C. juncea, C. laburnifolia, C. pumila,
C. recta, and C. retusa. No information is available on
the extent to which the different types are consumed (section 3.3.3).
9.1.3.4 Other foods contaminated by PAs
Several species of Boraginaceae are nectar and pollen sources
for bees. Echium plantagineum, in particular, is a widespread weed
in some countries and a substantial source of honey containing a
low level of alkaloid. Senecio species are also visited by bees
and yield alkaloid-containing honey, though Senecio-derived honey
is not known to be produced in quantity for sale. Thus, some
regional and local populations are exposed to a low-level intake
through the presence of PAs in honey, and surveillance may be
desirable in countries producing honey (section 3.3.4).
Under experimental conditions, PAs are transmitted from the
feed of dairy cows and goats into the milk. Some PA-containing
species, such as Senecio jacobaea, S. lautus, and Echium plantagineum,
are weeds in dairy pastures in some countries and are eaten by cattle
under certain conditions. There are no published reports of alkaloid
in milk supplies for human consumption (section 3.3.5).
The Task Group was not aware of any reported cases of
pyrrolizidine toxicity that had been ascribed to either honey or
dairy products.
No information was available to the Task Group on the possible
presence of alkaloids or their metabolites in meat from animals
that had consumed PA-containing plants shortly before slaughter.
The results of metabolic studies in rats have indicated that the
alkaloid is rapidly cleared from the body and, therefore, the
levels of PAs in meat are expected to be very low. However, there
is no information on the possibility of alkaloid accumulating in
storage sites.
9.1.4 Levels of intake
Reliable estimates of levels of intake of PAs, especially in
outbreaks of disease caused by the contamination of cereal crops
with the seeds of toxic plants, are extremely difficult to make.
Sampling of the contaminated grain may not be strictly
representative, since the extent of the contamination may vary in
different sites and households, as is evident from the estimates of
PA intake in the Indian and Afghan outbreaks reported in section
7.3. Furthermore, no accurate record is possible of the amount of
contaminated food consumed over an uncertain length of time. No
records of the levels of toxic PA intake are available in the
earlier reports of human toxicity. Where available, estimates of
intake in outbreaks caused by the contamination of staple food
crops have been made on the basis of random sampling of the
contaminated grain in food stores, and rough estimates of daily
consumption by average adults. Food-on-the-plate analyses have not
been made. The estimated lengths of exposure, and hence the amount
of total intake, are also approximate.
The contamination of cereal grains with the seed of
PA-containing plants has caused major epidemics of human poisoning,
though, in the two instances where estimates of alkaloid intake are
available, the intakes were lower than in some exposures due to the
use of herbal medicines. The estimated intakes are summarized in
Table 16. In an outbreak in India, millet contaminated with
Crotalaria nana seed had an average alkaloid content of 0.5 g/kg,
and the estimated daily intake by the population was 0.66 mg/kg
body weight. In a larger outbreak in Afghanistan, due to the seeds
of Heliotropium popovii in wheat, the level of contamination was
probably variable; representative samples of wheat contained
alkaloid at 0.04 g/kg. The estimated daily intake was 0.033 mg/kg
body weight. These intakes, sustained for periods of approximately
2 and 6 months, respectively, resulted in typical acute and
subacute veno-occlusive disease.
The highest intake rates have been associated with the use or
misuse of medicinal herbs and have resulted in acute liver damage
and death. In two occasions, the consumption of Heliotropium
eichwaldii as a treatment for epilepsy led to an estimated intake
of 3.3 mg/kg body weight daily for 20 or 50 days, and the use of
extracts of Senecio longilobus as medicine for young children led
to estimated intakes of 3 and 0.8 - 1.7 mg/kg body weight. The
highest intake led to extensive liver necrosis. However, it is
possible that, in the above case of poisoning by Heliotropium
eichwaldii, the toxicity was enhanced due to simultaneous
administration of phenobarbitone, which has a potentiating effect
on the microsomal enzymes in the liver cells that convert the PAs
to toxic metabolites.
The use of Heliotropium lasiocarpum as a component of a herbal
treatment for psoriasis involved somewhat lower daily intake rates
of 0.49 - 0.71 mg/kg body weight in 4 patients, who, after periods
of 19 - 46 days, developed veno-occlusive disease. The patient
with the longest intake period and a total intake of 1.4 g alkaloid
or 23 mg/kg body weight died.
The lowest ingestion rate leading to a case of veno-occlusive
disease was also due to medicinal herbal treatment or, more
specifically, to the use of a digestive aid containing a
preparation of comfrey root. Commercial herb and food supplement
preparations containing comfrey leaf or root are on sale in many
countries. Limited assays of one comfrey-pepsin preparation
prepared from comfrey root indicated a PA content of 2.9 g/kg.
Another preparation made from comfrey leaf contained up to 0.27 g
alkaloid/kg. The consumption of these preparations led to an
estimated daily intake of 0.015 mg/kg body weight. Veno-occlusive
disease was diagnosed after a 4- to 6-month period.
The consumption of Symphytum officinale (comfrey) and S. x
uplandicum (Russian comfrey) in the form of food, infusions, or
other preparations is widespread, though the full extent cannot be
estimated. A high level of consumption as salad appears to be
about 5 - 6 leaves per day and consumption as comfrey tea probably
reaches a similar level. Limited assays indicate that the average
alkaloid content of the leaf is about 1 mg/leaf, the concentration
being higher in the younger, smaller leaves. The alkaloid intake
from comfrey leaves could therefore vary from a low value, up to
about 6 mg/day, or 0.1 mg/kg body weight for an adult, an intake
within the range producing veno-occlusive disease. However, the
Task Group noted that some people claim to have consumed comfrey at
such a rate without suffering any disease.
Overall, the estimates of intake of PAs by human beings (Table
16) indicate that ingestion rates above 0.015 mg/kg body weight for
the mixture of echimidine and related alkaloids in comfrey may lead
to acute or subacute liver disease. If expressed in terms of
equivalent doses of heliotrine (section 7.8), the estimated total
doses in the known outbreaks or cases of veno-occlusive disease
range from 1 to 167 mg/kg body weight. There is little real
difference in the ranges of estimated total doses in non-fatal
cases (1 - 120 mg/kg body weight) and those leading to death
(6 - 167 mg/kg). These figures, when compared with the total
lethal dose of several PAs in rats, i.e., 1.2 - 10.9 times the LD50
dose (equivalent to 360 - 3270 mg heliotrine/kg) (Table 10), would
seem to indicate that man is markedly more sensitive than the rat
to the toxic effects of PAs with regard to the development of acute
and chronic effects on the liver. It should be noted that these
estimates are based on limited raw data and a number of
assumptions, and so are of uncertain reliability.
The dose estimates indicate strongly that the effects of PAs in
human beings are cumulative at very low intake rates. Lower rates
of intake of PAs may lead to chronic forms of intoxication, though,
at present, there is no evidence on which the degree of risk in
these circumstances can be evaluated. The information available on
dose-response relationships is very limited, but the data support
the conclusion that even low rates of intake of PAs over a period
of time may present a health risk and that exposure should be
minimized wherever possible.
There has not been any systematic monitoring of PAs in cereal
grains, food products, and herbal medicines. Analytical surveys of
these materials are feasible, but it would be difficult to design
surveys that would give direct estimates of the dietary intake of
PAs.
9.2 Acute Effects of Exposure
9.2.1 Acute liver disease
All cases of human intoxication in reported accounts have been
in the acute phase of the disease, the dominant symptom being
rapidly filling ascites. The disease can affect large
subpopulations and, in one study, up to 22.6% of the population was
affected.
Children appear to be the most vulnerable group and mortality
can be high at the extremes of age. The liver is the principal
target organ. In the acute stage of the disease, the liver shows a
characteristic centrilobular haemorrhagic necrosis, which in man is
accompanied by occlusion of the hepatic veins. However,
characteristic veno-occlusive lesions, seen in the central veins of
hepatic lobules, may not always be evident in the needle biopsy
examination of the liver, but are always apparent on examination of
the autopsy material.
9.3 Chronic Effects of Exposure
9.3.1 Cirrhosis of the liver
There is evidence that the administration of a single dose of
PA to experimental animals or a single acute episode of illness in
man, following brief consumption of PA-containing herbs or
PA-contaminated food, may lead to progressive chronic liver disease
resulting in cirrhosis. Cirrhosis may also be a consequence of
long-term low-dose administration of PAs to experimental animals
and possibly also of long-term low intake of PAs by human beings,
though there is no proof of the latter. Cirrhosis resulting from
the toxic effects of PAs in the advanced stage, may not be
distinguishable from that resulting from other causes (sections
6.4.1.5 and 7.4). The Task Group did not find any evidence
suggesting that PAs are a causative factor of the specific disease,
Indian Childhood Cirrhosis (section 7.5).
9.3.2 Mutagenicity and teratogenicity
Several PAs, PA-derivatives, and related compounds have been
shown to produce chromosome aberrations in plants and several cell
culture systems, mutagenic effects ( Salmonella ("Ame's"), sister
chromatid exchanges, and other tests), and teratogenic and
fetotoxic effects in experimental animals (sections 6.4.5, 6.4.6,
6.4.7). Chromosomal aberrations have been reported in the blood
cells of children suffering from veno-occlusive disease, believed
to have been caused by fulvine. The Task Group was not aware of
data on the teratogenic/fetotoxic effects of PAs on human beings
and was unable to evaluate the potential for these effects in PA
exposure.
9.3.3 Cancer of the liver
A relatively large number of people have been exposed, in the
past, to PAs and have suffered acute and chronic toxic effects.
However, no information is available on the long-term follow-up of
these populations, to ascertain whether this type of exposure could
have resulted in an increased incidence of liver cancer or other
types of cancer. Because of this lack of knowledge, it is not
possible, at present, to make an evaluation of the cancer risk due
to PAs. However, various PAs have been shown to be carcinogenic
for experimental animals, which implies that a potential cancer
risk for human beings should be seriously considered.
Of several PAs evaluated for carcinogenicity by IARC (1976,
1983), there is "sufficient or limited evidence" for the
carcinogenicity in experimental animals (IARC, 1976) of
monocrotaline, retrorsine, isatidine, lasiocarpine, petasitenine,
senkirkine, and of extracts of the PA-containing plants Petasites
japonicum, Tussilago farara, Symphytum officinale, Senecio
longilobus, Senecio numorensis, Farfugium japonicum, and Senecio
cannabifolius. These studies were carried out mainly on rats, with
few studies on mice or hamsters (section 6.4.8). The
carcinogenicity data obtained with other PAs are difficult to
evaluate, because of the limited number of treated animals and the
lack of adequate numbers as controls. The main target organ is the
liver, where liver cell tumours and haemangioendothelial sarcomas
were observed. In some instances, tumours in extra-hepatic tissues
(lung, pancreas, intestine) were also observed, namely with
monocrotaline, retrorsine, and lasiocarpine. Some PAs, for
example, retrorsine, have been shown to be carcinogenic after a
single dose. The pyrrolic metabolites have also been shown to be
carcinogenic for rats.
It may be recalled that several of the PAs involved in human
poisoning include the above compounds. It is notable that the dose
rates that have been effective in inducing tumours in rats, mostly
equivalent to 0.2 - 3 mg/kg body weight per day (Table 17), are
roughly similar in magnitude to estimated intake rates (0.49 -
3.3 mg/kg body weight per day) (Table 16) in several episodes of human
toxicity. Comparison of the total intakes resulting in human
toxicity with the total doses to death observed in the chronic
toxicity studies on rats indicates that human beings are more
susceptible (section 7.8) and suggests that human beings may
survive for sufficient time to develop cancer after only a brief
exposure at this level or a longer exposure at a markedly lower
level. A more quantitative assessment is not possible on the basis
of the available information, and the Task Group stressed the need
for appropriate epidemiological studies.
9.3.4 Effects on other organs
Substantiated reports of PA-induced extra-hepatic injury in man
are limited to Trichodesma intoxication, in which symptoms and
signs were predominantly neurological. The range of organs
affected by other PAs in experimental and farm animals suggests
that exposure of human beings to other PAs may also carry the
potential for extra-hepatic injury.
There are extensive reports of experimental studies in which
PAs have been demonstrated to produce the characteristic vascular
changes of primary pulmonary hypertension and consequent right
ventricular hypertrophy of the heart in rats and non-human primates
(section 6.4.2). Susceptibility is age dependent, weanling rats
being more vulnerable than older animals. There is only
circumstantial evidence of PA-induced pulmonary vascular disease in
one patient (section 7.6), but judging by the experimental evidence
available, it is possible that human beings may be susceptible to
PA-induced cardiopulmonary changes.
In the opinion of the Task Group, the neurological involvement
which is a dominant feature in PA-intoxicated horses and is also
seen in cows and sheep, cannot be explained solely as a consequence
of liver damage. Central nervous system lesions have been
demonstrated in sheep, pigs, and rats. Distribution studies of the
radiolabelled metabolite, 3H-dehydroretronecine, show increasing
accumulation of radioactivity in the brain with time.
Trichodesma alkaloids, structurally related to monocrotaline,
are neurotoxic agents. Trichodesma toxicosis in man has been
reported only from the USSR, together with several studies on
experimental animals. Detailed reports on the pathological
findings were not available to the Task Group, but the information
available indicated that the central nervous system was the primary
target organ (sections 6.4.3 and 7.7).
Stomach and intestinal lesions have been shown in PA-exposed
sheep, mice, cows, and rats. Distribution studies with
radiolabelled pyrroles showed a high retention of radioactivity in
the stomach, consistent with the acid-sensitive nature of the
pyrroles. In rats, pyrrolic metabolites are secreted in high
concentrations in the bile.
Kidney changes following to PA administration have been shown
in mice, pigs, horses, sheep, and monkeys. Pyrrolizidine
metabolites have been found covalently bound to kidney DNA in rats.
Urinary excretion is a major route of excretion of metabolic
products of PAs in rats.
There is no evidence of involvement of organs other than the
liver and central nervous system ascribed primarily to PA toxicity
in any of the published human case reports. It is possible that
under some circumstances, other major organ systems may also be at
risk. As bioactivation of PAs has been demonstrated only in the
liver, the risk of damage should be expected to be lower in the
organs.
9.4 Effects on the Environment
9.4.1 Agriculture
In some countries, PA-containing weeds densely cover areas of
up to thousands of square kilometres. Their adverse effects
include the covering of pastures, additional costs in agricultural
production, and the poisoning of farm animals. The toxicity of PAs
for farm animals, including sheep, cattle, horses, pigs, goats, and
poultry, which has been the inspiration for much of the investigation
of PA toxicity. In Australia, for example, Heliotropium europaeum
and Echium plantagineum cause the death of thousands of animals
annually (section 6.2).
9.4.2 Wild-life
By contrast, little is known about the consumption of PA-containing
plants by wild-life, or of their individual sensitivities. The death
of deer in Louisiana has been ascribed to eating Heliotropium or
Crotalaria species, and an experimental study has shown that the
rainbow trout (Salmo gairdneri) is sensitive to Senecio jacobaea
alkaloids (sections 6.5.1 and 6.5.2).
There is no information on the effects of the alkaloids on
field rodents or other seed-eating mammals and birds that might be
expected to consume seeds of PA-containing plants and to suffer
toxic effects.
9.4.3 Insects
Many species of insects, such as some moths of the family
Arctidae and butterflies of the sub-families Danainae and
Ithominae, have become dependent on PA-containing plants, using the
alkaloids as defensive chemicals and derivatives of them as
pheromones and other signalling chemicals. Thus, complete
elimination of PA-containing plants in a region might lead to a
marked reduction in the local population of insects of this type
(section 6.5.3).
9.4.4 Soil and water
There have not been any studies on the fate of PAs when the
plants in which they occur wilt and age. If alkaloid is leached
into soil or water, it is probably readily degraded by microorganisms
since, as a base and ester, it is subject to oxidative and hydrolytic
reactions.
REFERENCES
AFZELIUS, B.A. & SCHOENTAL, R. (1967) The ultrastructure of
enlarged hepatocytes induced in rats with a single oral dose of
retrorsine, pyrrolizidine (Senecio) alkaloids. J. ultra-struct.
Res., 20: 328-345.
AIKAT, B.K., BHUSNURMATH, S.R., DATTA, D.V., & CHHUTTANI, P.N.
(1978) Veno-occlusive disease in north-west India. Indian J.
Pathol. Microbiol., 21: 203-211.
AL-HASANY, M. & MOHAMED, A.S. (1970) Veno-occlusive disease of the
liver in Iraq. Arch. dis. Child., 45: 722-724.
ALLEN, J.R. & CARSTENS, L.A. (1968) Veno-occlusive disease in
rhesus monkeys. Am. J. vet. Res., 29: 1681-1694.
ALLEN, J.R. & CARSTENS, L.A. (1970) Pulmonary vascular occlusions
initiated by endothelial lysis in monocrotaline-intoxicated rats.
Exp. mol. Pathol.., 13: 159-171.
ALLEN, J.R. & CARSTENS, L.A. (1971) Monocrotaline induced Budd-
Chiari syndrome in monkeys. Am. J. dig. Dis., 16: 111-121.
ALLEN, J.R. & CHESNEY, C.F. (1972) Effect of age on development of
cor pulmonale in non-human primates following pyrrolizidine
alkaloid intoxication. Exp. mol. Pathol., 17: 220-232.
ALLEN, J.R., CHILDS, G.R., & CRAVENS, W.W. (1960) Crotalaria
spectabilis toxicity in chickens. Proc. Soc. Exp. Biol. Med., 104:
434-436.
ALLEN, J.R., LALICH, J.J., & SCHMUTTLE, S.M. (1963) Crotalaria
spectabilis induced cirrhosis in turkeys. Lab. Invest., 12: 512-517.
ALLEN, J.R., CARSTENS, L.A., & OLSON, B.E. (1967) Veno-occlusive
disease in Macaca speciosa monkeys. Am. J. Pathol., 50: 653-667.
ALLEN, J.R., CARSTENS, L.A., & KATAGIRI, G.J. (1969) Hepatic veins
of monkeys with veno-occlusive disease-sequential ultrastructural
changes. Arch. Pathol., 87: 279-289.
ALLEN, J.R., CHESNEY, C.F., & FRAZEE, W.J. (1972) Modifications of
pyrrolizidine alkaloids intoxication resulting from altered
microsomal enzymes. Toxicol. appl. Pharmacol., 23: 470-479.
ALLEN, J.R., HSU, I.-C., & CARSTENS, L.A. (1975) Dehydroretronecine
induced rhabdomyosarcomas in rats. Cancer Res., 35: 997-1002.
AMES, M.M. & POWIS, G. (1978) Determination of indicine N-oxide
and indicine in plasma and urine by electron-capture gas-liquid
chromatography. J. Chromatogr., 166: 519-526.
ARMSTRONG, S.J. & ZUCKERMAN, A.J. (1970) Production of pyrroles
from pyrrolizidine alkaloids by human embryo tissue. Nature
(Lond.), 228: 569-570.
ARMSTRONG, S.J., ZUCKERMAN, A.J., & BIRD, R.G. (1972) Induction of
morphological changes in human embryo liver cells by the
pyrrolizidine alkaloid lasiocarpine. Br. J. exp. Pathol., 53:
145-149.
ARORA, R.R., PYARELAL, GHOSH, T.K., MATHUR, K.K., & TANDON, B.N.
(1981) Epidemiology of veno-occlusive disease in tribal population
of Madhya Pradesh and Bihar. J. commun. Dis. (India), 13: 147-151.
ARSECULERATNE, S.N., GUNATILAKA, A.A.L., & PANABOKKE, R.G. (1981)
Studies on medicinal plants of Sri Lanka: occurrence of
pyrrolizidine alkaloids and hepatotoxic properties in some
traditional medicinal herbs. J. Ethnopharmacol., 4: 159-177.
ASADA, Y., FURUYA, T., & MURAKAMI, N. (1981) Pyrrolizidine
alkaloids from Ligularia japonica. Planta Med., 42: 202-203.
ASADA, Y., SHIRAISHI, M., TAKEUCHI, T., OSAWA, Y., & FURUYA, T.
(1985) Pyrrolizidine alkaloids from Crassocephalum crepidioides.
Planta Med., 51: 539-540.
BARNES, J.M., MAGEE, P.N., & SCHOENTAL, R. (1964) Lesions in the
lungs and livers of rats poisoned with pyrrolizidine alkaloids
fulvine and its N-oxide. J. Pathol. Bacteriol., 88: 521-531.
BERRY, D.M. & BRAS, G. (1957) Venous occlusion of the liver in
Crotalaria and Senecio poisoning. North Am. Vet., 38: 323-327.
BHATTACHARYA, K.J. (1965) Foetal and neonatal responses to
hepatotoxic agents. J. Pathol. Bacteriol., 90: 151-161.
BICCHI, C., D'AMATO, A., & CAPPELLETTI, E. (1985) Determination
of pyrrolizidine alkaloids in Senecio inaequidens D.C. by
capillary gas chromatography. J. Chromatogr., 349: 23-29.
BICK, Y.A.E. (1970) Comparison of the effects of LSD, heliotrine,
and X-irradiation on chromosome breakage and the effects of LSD on
the rats of cell division. Nature (Lond.), 226: 1165-1167.
BICK, Y.A.E. & CULVENOR, C.C.J. (1971) Effects of dehydro-
heliotridine, a metabolite of pyrrolizidine alkaloids on chromosome
structure and cell division in cultures of animal cells. Cytobios,
3: 245-255.
BICK, Y.A.E. & JACKSON, W.D. (1968) Effects of the pyrrolizidine
alkaloid heliotrine on cell division and chromosome breakage in
cultures of leucocytes from the marsupial Potorus tridactylus.
Aust. J. biol. Sci., 21: 469-481.
BICK, Y.A.E., CULVENOR, C.C.J., & JAGO, M.V. (1975) Comparative
effects of pyrrolizidine alkaloids and related compounds on
leukocyte cultures from Potorus tridactylus. Cytobios, 14: 151-160.
BIRECKA, H., FROHLICH, M.W., HULL, L., & CHASKES, M.J. (1980)
Pyrrolizidine alkaloids of Heliotropium from Mexico and adjacent
USA. Phytochemistry, 19: 421-426.
BIRECKA, H., CATALFAMO, J.L., & EISEN, R.N. (1981) A sensitive
method for detection and quantitative determination of
pyrrolizidine alkaloids. Phytochemistry, 20: 343-344.
BLACK, D.N. & JAGO, M.V. (1970) Interaction of
dehydroheliotridine, a metabolite of heliotridine based
pyrrolizidine alkaloids, with natural and heat denatured RNA.
Biochem. J., 118: 347-353.
BOHLMANN, F., KLOSE, W., & NIKISCH, K. (1979) [Synthesis of
dehydroheliotridine and 3-oxy-dehydroheliotridine.] Tetrahedr.
Lett., 1979: 3699-3702 (in German).
BOHLMANN, F., ZDERO, C., JAKUPOVIC, J., GRENZ, M., CASTRO, V.,
KING, R.M., ROBINSON, H., & VINCENT, L.P.D. (1986) Further
pyrrolizidine alkaloids and furoeremophilanes from Senecio species.
Phytochemistry, 15: 1151-1159.
BOPPRE, M. (1986) Insects pharmacophagously utilizing defensive
plant chemicals (pyrrolizidine alkaloids). Naturwiss enschaften, 73:
17-26.
BRAGINSKII, B.M. & BOBOKHADZAEV, I. (1965) [Hepatosplenomegaly
against the background of heliotropic toxicosis.] Sov. Med., 28:
57-60 (in Russian).
BRAS, G. (1973) Aspects of hepatic vascular diseases. In: Gall,
E.A. & Mostofi, F.K., ed. The liver: International Academy of
Pathology monograph, Baltimore, Maryland, Williams and Wilkins,
pp. 406-530.
BRAS, G. & HILL, K.R. (1956) Veno-occlusive disease of the liver -
essential pathology. Lancet, 2: 161-163.
BRAS, G. & WATLER, D.C. (1955) Further observations on the
morphology of veno-occlusive disease of the liver in Jamaica. West
Indian med. J., 4: 201-211.
BRAS, G., JELLIFFE, D.B., & STUART, K.L. (1954) Veno-occlusive
disease of the liver with nonportal type of cirrhosis occurring in
Jamaica. Arch. Pathol., 57: 285-300.
BRAS, G., BERRY, D.M., & GYORGI, P. (1957) Plants as etiological
factor in veno-occlusive disease of liver. Lancet, 1: 960-962.
BRAS, G., BROOKS, S.E.H., & WATLER, D.C. (1961) Cirrhosis of liver
in Jamaica. J. Pathol. Bacteriol., 82: 503-512.
BRAUCHLI, J., LUTHY, J., ZWEIFEL, U., & SCHLATTER, C. (1982)
Pyrrolizidine alkaloids from Symphytum officinale L. and their
percutaneous absorption in rats. Experientia (Basel), 38:
1085-1087.
BRIGGS, L.H., CAMBIE, R.C., CANDY, B.J., O'DONOVAN, G.M., RUSSELL,
R.A., & SEELYE, R.N. (1965) Alkaloids of New Zealand Senecio
species. Part II: senkirkine. J. Chem. Soc., C1965: 2492-2498.
BRINK, N.G. (1982) Somatic and teratogenic effects induced by
heliotrine in Drosophilia. Mutat. Res., 104: 105-111.
BROOKS, W.E.H., MILLER, C.G., MCKENZIE, K., AUDRETSCH, J.J., &
BRAS, G. (1970) Acute veno-occlusive disease of the liver. Fine
structure in Jamaican children. Arch. Pathol., 89: 507-529.
BROWN, K.S. (1984) Chemical ecology of dehydropyrrolizidine
alkaloids in adult Ithomiinae (Lepidoptera: Nymphalidae). Rev.
Bras. Biol., 44: 435-440.
BRUGGEMAN, I.M. & VAN DER HOEVEN, J.C.M. (1985) Induction of SCEs
by some pyrrolizidine alkaloids in V79 Chinese hamster cells co-
cultured with chick embryo hepatocytes. Mutat. Res., 142: 209-212.
BRUNER, L.H., CARPENTER, L.J., HAMLOW, P., & ROTH, R.A. (1986)
Effect of a mixed-function oxidase inducer and inhibitor on
monocrotaline pyrrole pneumotoxicity. Toxicol. appl. Pharmacol., 85:
416-427.
BUCKMASTER, G.W., CHEEKE, P.R., ARSCOTT, G.H., DICKINSON, E.D.,
PIERSON, M.L., & SHULL, L.R. (1977) Response of Japanese quail to
dietary and injected pyrrolizidine (Senecio) alkaloid. J. anim.
Sci., 45(6): 1322-1325.
BULL, L.B. (1955) The histological evidence of liver damage from
pyrrolizidine alkaloids. Aust. vet. J., 31: 33-40.
BULL, L.B. & DICK, A.T. (1959) The chronic pathological effects on
the liver of the rat of the pyrrolizidine alkaloids heliotrine,
lasiocarpine, and their N-oxides. J. Pathol. Bacteriol., 78: 483-502.
BULL, L.B. & DICK, A.T. (1960) The function of total dose in the
production of chronic lethal disease in rats by periodic injections
of the pyrrolizidine alkaloid heliotrine. Aust. J. exp. Biol. med.
Sci., 38: 515.
BULL, L.B., DICK, A.T., KEAST, J.C., & EDGAR, G. (1956) An
experimental investigation of the hepatotoxic and other effects on
sheep of consumption of Heliotropium europaeum L. Heliotropium
poisoning of sheep. Aust. J. agric. Res., 7: 281-332.
BULL, L.B., DICK, A.T., & MCKENZIE, J.S. (1958) The acute effects
of heliotrine and lasiocarpine and their N-oxides on the rat. J.
Pathol. Bacteriol., 75: 17-25.
BULL, L.B., CULVENOR, C.C.J., & DICK, A.T. (1968) The
pyrrolizidine alkaloids, Amsterdam, North Holland Publishing Co.
BURGUERA, J.A., EDDS, G.T., & OSUNA, O. (1983) Influence of
selenium on aflatoxin B1 or Crotalaria toxicity in turkey poults.
Am. J. vet. Res., 44: 1714-1717.
BURNS, J. (1972) The heart and pulmonary arteries in rat fed on
Senecio jacobaea. J. Pathol., 106: 187-194.
BUTLER, W.H., MATTOCKS, A.R., & BARNES, J.M. (1970) Lesions in the
liver and lungs of rats given pyrrole derivatives of pyrrolizidine
alkaloids. J. Pathol., 100: 169-175.
CAMPBELL, J.G. (1956) An investigation of the hepatotoxic effects
in the fowl of ragwort ( Senecio jacobaea Linn) with special
reference to the induction of liver tumours with seneciphylline.
Proc. R. Soc. Edinburgh, B66: 111-130.
CAMPBELL, J.G. (1957a) Studies on the influence of sex hormones on
the avian liver. II. Acute liver damage in the male fowl and the
protective effect of oestrogen, as determined by a liver function
test. J. Endocrinol., 15: 346-350.
CAMPBELL, J.G. (1957b) Studies on the influence of sex hormones on
avian liver. III. Oestrogen-induced regeneration of the chronically
damaged liver. J. Endocrinol., 15: 351-354.
CANDRIAN, U., LUTHY, J., GRAF, U., & SCHLATTER, CH. (1984a)
Mutagenic activity of the pyrrolizidine alkaloids seneciphylline
and senkirkine in Drosophila and their transfer into rat milk.
Food chem. Toxicol., 22: 223-225.
CANDRIAN, U., LUTHY, J., SCHMID, P., SCHLATTER, CH., & GALLASZ, E.
(1984b) Stability of pyrrolizidine alkaloids in hay and silage. J.
agric. food Chem., 32: 935-937.
CANDRIAN, U., LUTHY, J., & SCHLATTER, CH. (1985) In vivo binding
of retronecine-labelled (3H), seneciphylline, and 3H-senecionine to
DNA of rat liver, lung and kidney. Chem.-biol. Interact., 54: 57-69.
CARILLO, L. & AVIADO, D.M. (1969) Monocrotaline induced pulmonary
hypertension and p-chlorophenylalanine. Lab. Invest., 20: 213-218.
CARSTENS, L.A. & ALLEN, J.R. (1970) Arterial degeneration and
glomerular hyalinization in the kidney of monocrotaline intoxicated
rats. Am. J. Pathol., 60: 75-92.
CHALMERS, A.H., CULVENOR, C.C.J., & SMITH, L.W. (1965)
Characterization of pyrrolizidine alkaloids by gas, thin layer, and
paper chromatography. J. Chromatogr., 20: 270-277.
CHEEKE, P.R. & GORMAN, G.R. (1974) Influence of dietary protein
and sulphur amino-acid levels on the toxicity of Senecio jacobaea
(tansy ragwort) to rats. Nutr. Rep. Int., 9: 197-207.
CHEEKE, P.R. & PIERSON-GOEGER, M.L. (1983) Toxicity of Senecio
jacobaea and pyrrolizidine alkaloids in various laboratory animals
and avian species. Toxicol. Lett., 18: 343-349.
CHEN, K.K. (1945) Pharmacology. Hepatotoxic alkaloids. Ann. Rev.
Physiol., 7: 695-697.
CHEN, K.K., HARRIS, P.N., & ROSE, C.L. (1940) The action and
toxicity of platyphylline and seneciphylline. J. Pharmacol. exp.
Ther., 68: 130-140.
CHESNEY, C.F. & ALLEN, J.R. (1973a) Resistance of the guinea pig
to pyrrolizidine alkaloid intoxication. Toxicol. appl. Pharmacol.,
26: 385-392.
CHESNEY, C.F. & ALLEN, J.R. (1973b) Endocardial fibrosis
associated with monocrotaline induced pulmonary hypertension in
non-human primates (Macaca arctoides). Am. J. vet. Res., 34:
1577-1581.
CHESNEY, C.F., HSU, I.C., & ALLEN, J.R. (1974) Modifications of
the in vitro metabolism of the hepatotoxic pyrrolizidine alkaloid,
monocrotaline. Res. Commun. chem. Pathol. Pharmacol., 8: 567-570.
CHOPRA, R.N., ed. (1933) Indigenous drugs of India, Calcutta, The
Art Press.
CLARK, A.M. (1959) Mutagenic activity of the alkaloid heliotrine
in Drosophila. Nature (Lond.), 183: 731-732.
CLARK, A.M. (1976) Naturally occurring mutagens. Mutat. Res., 32:
361-174.
COOK, B.A., SINNHUBER, J.R., THOMAS, P.J., OLSON, T.A., SILVERMAN,
T.A., JONES, R., WHITEHEAD, V.M., & ROYMANN, F.B. (1983) Hepatic
failure secondary to indicine N-oxide toxicity. A pediatric
oncology group study. Cancer, 52: 61-63.
COOK, J.W., DUFFY, E., & SCHOENTAL, R. (1950) Primary liver
tumours in rats following feeding with alkaloids of Senecio
jacobaea. Br. J. Cancer, 4: 405-410.
CRAIG, A.M., SHEGGEBY, G., & WICKS, C.E. (1984) Large scale
extraction of pyrrolizidine alkaloids from tansy ragwort (Senecio
jacobaea). Vet. hum. Toxicol., 26: 108-111.
CULVENOR, C.C.J. (1968) Tumour inhibitory activity of
pyrrolizidine alkaloids. J. pharm. Sci., 57: 1112-1117.
CULVENOR, C.C.J. (1978) Pyrrolizidine alkaloids: occurrence and
systematic importance in angiosperms. Bot. Notiser, 131: 473-486.
CULVENOR, C.C.J. (1980) Alkaloids and human disease. In: Smith,
R.L. & Bababunmi, E.A., ed. Toxicology in the tropics, London,
Taylor & Francis Ltd., pp. 124-141.
CULVENOR, C.C.J. (1983) Estimated intakes of pyrrolizidine
alkaloids by humans. A comparison with dose rates causing tumours
in rats. J. Toxicol. environ. Health, 11: 625-635.
CULVENOR, C.C.J. (1985) Pyrrolizidine alkaloids: some aspects of the
Australian involvement. Trends pharmacol. Sci., 6: 18-22.
CULVENOR, C.C.J. & JAGO, M.V. (1979) Carcinogenic plant products
and DNA. In: Grover, P.L., ed. Chemical carcinogens and DNA, Boca
Raton, Florida, CRC Press, Vol. 1, 161 pp.
CULVENOR, C.C.J., DOWNING, D.T., EDGAR, J.A., & JAGO, M.V. (1969)
Pyrrolizidine alkaloids as alkylating and antimitotic agents. N.Y.
Acad. Sci., 163: 837-847.
CULVENOR, C.C.J., EDGAR, J.A., SMITH, L.W., & TWEEDDALE, H.J.
(1970a) Dihydropyrrolizines. III. Preparation and reactions of
derivatives related to pyrrolizidine alkaloids. Aust. J. Chem., 23:
1853-1867.
CULVENOR, C.C.J., EDGAR, J.A., SMITH, L.W., & TWEEDDALE, H.J.
(1970b) Dehydrolizines. IV. Manganese dioxide oxidation of
1,2-dihydropyrrolizidines. Aust. J. Chem., 23: 1869-1879.
CULVENOR, C.C.J., EDGAR, J.A., JAGO, M.V., OUTTERIDGE, A., PETERSON
J.E., & SMITH, L.W. (1976a) Hepato- and pneumotoxicity of
pyrrolizidine alkaloids and derivatives in relation to molecular
structure. Chem.-biol. Interact., 12: 299-324.
CULVENOR, C.C.J., EDGAR, J.A., SMITH, L.W., & HIRONO, I. (1976b)
The occurrence of senkirkine in Tussilago farfara. Aust. J. Chem.,
29: 229-233.
CULVENOR, C.C.J., CLARKE, M., EDGAR, J.A., FRAHN, J.L., JAGO, M.V.,
PETERSON, J.E., & SMITH, L.W. (1980a) Structure and toxicity of
the alkaloids of Russian comfrey ( Symphytum x uplandicum Nyman), a
medicinal herb and item of human diet. Experientia (Basel), 36:
377-389.
CULVENOR, C.C.J., EDGAR, J.A., FRAHN, J.L., & SMITH, L.W. (1980b)
The alkaloids of Symphytum x uplandicum (Russian comfrey). Aust.
J. Chem., 33: 1105-1113.
CULVENOR, C.C.J., EDGAR, J.A., & SMITH, L.W. (1981) Pyrrolizidine
alkaloids in honey from Echium plantagineum L. J. agric. food
Chem., 29: 958-960.
CULVENOR, C.C.J., JAGO, M.V., PETERSON, J.E., SMITH, L.W., PAYNE,
A.L, CAMPBELL, D.G., EDGAR, J.A., & FRAHN, J.L. (1984) Toxicity of
Echium plantagineum (Paterson's curse). I. Marginal toxic effects
of Merino wethers from long-term feeding. Aust. J. agric. Res., 35:
293-304.
CULVENOR, C.C.J., EDGAR, J.A., SMITH, L.W., KUMANA, C.R., & LIN,
H.J. (1986) Heliotropium lasiocarpum Fisch and Mey identified as
cause of veno-occlusive disease due to a herbal tea. Lancet, 1: 978.
DANN, A.T. (1960) Detection of N-oxides of the pyrrolizidine
alkaloids. Nature, 4730: 1051.
DANNINGER, T., HAGEMANN, U., SCHMIDT, V., & SCHOENHOEFER, P.S.
(1983) Toxicity of pyrrolizidine alkaloid-containing medicinal
plants. Pharm. Ztg, 128: 289-303.
DATTA, D.V., KHUROO, M.S., MATTOCKS, A.R., AIKAT, B.K., &
CHHUTTANI, P.N. (1978a) Veno-occlusive disease of liver due to
Heliotropium plant used as medicinal herb (report of six cases with
review of literature). J. Assoc. Phys. India, 26(5): 383-393.
DATTA, D.V., KHUROO, M.S., MATTOCKS, A.R., AIKAT, B.K., &
CHHUTTANNI, P.N. (1978b) Herbal medicines and veno-occlusive
disease in India. Postgrad. med. J., 54: 511-515.
DAVIDSON, C.S. (1963) Plants and fungi as etiologic agents of
cirrhosis. New Engl. J. Med., 268: 1072-1073.
DAVIDSON, J. (1935) The action of retrorsine on rat's liver. J.
Pathol. Bacteriol., 40: 285-295.
DEAGEN, J.T. & DEINZER, M.L. (1977) Improvement in the extraction
of pyrrolizidine alkaloids. Lloydia, 40: 395-397.
DEAN, R.E. & WINWARD, A.H. (1974) An investigation into the
possibility of tansy ragwort poisoning of black-tailed deer. J.
wildl. Dis., 10: 166-169
DEINZER, M.L., THOMSON, P.A., BURGETT, D.M., & ISAACSON, D.L.
(1977) Pyrrolizidine alkaloids: their occurrence in honey from
tansy ragwort (S. jacobaea). Science, 195: 497-499.
DEINZER, M.L., THOMSON, P.A., GRIFFIN, D., & DICKINSON, E. (1978)
Sensitive analytical method for pyrrolizidine alkaloids. The mass
spectra of retronecine derivatives. Biomed. mass Spectrom., 5:
175-179.
DELFOSSE, E.S. (1985) Re-evaluation of the biological control
program for Heliotropium europaeum in Australia. In: Delfosse,
E.S., ed. Proceedings of the VI International Symposium on
Biological Control of Weeds, 19-25 August 1984, Vancouver, Canada,
Ottawa Agriculture Canada, pp. 735-42.
DELFOSSE, E.S. & CULLEN, J.M. (1985a) CSIRO division of Entomology
Submission to the enquiries into biological control of Echium
plantagineum L., Paterson's curse/Salvation Jane. Plant Prot. Q.,
1: 24-40.
DELFOSSE, E.S. & CULLEN, J.M. (1985b) Echium plantagineum:
catalyst for conflict and change in Australia. In: Delfosse, E.S.,
ed. Proceedings of the VI International Symposium on Biological
Control of Weeds, 19-25 August, 1984, Vancouver, Canada, Ottawa
Agriculture Canada pp. 249-292.
DELORME, P., JAY, M., & FERRY, S. (1977) Phytochemical inventory
in indigenous Boraginaceae: study of the alkaloids and polyphenolic
compounds (anthocyanic and flavonoid compounds). Plant. Méd.
Phytothér., 11: 5-11. (in french)
DE WAAL, H.L. & VAN TWISK, P. (1964) The chemical composition of
four Senecio species from Kruger National Park. Koedoe (South
Africa), 7: 40-42.
DICK, A.T., DANN, A.T., BULL, L.B., & CULVENOR, C.C.J. (1963)
Vitamin B12 and the detoxication of hepatotoxic pyrrolizidine
alkaloids in rumen liquor. Nature (Lond.), 197: 207-208.
DICKINSON, J.O. (1980) Release of pyrrolizidine alkaloids into
milk. Proc. West. Pharmacol. Soc., 23: 377-379.
DICKINSON, J.O., COOKE, M.P., KING, R.R., & MOHAMED, P.A. (1976)
Milk transfer of pyrrolizidine alkaloids in cattle. J. Am. Vet.
Med. Assoc., 169: 1192-1196.
DIMENNA, G.P., KRICK, T.P., & SEGALL, H.J. (1980) Rapid high
performance liquid chromatography isolation of monoesters,
diesters, and macrocyclic diesters and pyrrolizidine alkaloids from
Senecio jacobaea and Amsinckia intermedia. J. Chromatogr., 192:
474-478.
DOLINSKAYA, K.N. (1952) [Pathomorphology of heliotropic hepatic
dystrophy in children.] In: Milenkov, S.M. & Kizhaikin, Y., ed.
[Collection of scientific papers on Toxic Hepatitis with Ascites,]
Tashkent, Publishing House of the University of Central Asia,
pp. 165-172 (in Russian).
DREIFUSS, P.A. (1984) A study of the mass spectra of
pyrrolizidine alkaloids by negative ion chemical ionization and
ms/ms: the identification of a monoester pyrrolizidine alkaloid in
Eupatorium rugosum. Diss. Abstr. Int. B., 45:'1183-1184.
DRIVER, H.E. & MATTOCKS, A.R. (1984) The toxic effects in rats of
some synthanecine carbamate and phosphate esters analogous to
hepatotoxic pyrrolizidine alkaloids. Chem.-biol. Interact., 51:
201-218.
DUBROVINSKII, S.B. (1946) [About the alimentary toxicosis caused
by heliotrope.] J. Sov. Prot. Health, 6: 17-21 (in Russian).
DUBROVINSKII, S.B. (1952) [The etiology of toxic hepatitis with
ascites.] In: Millenkov, S.M. & Kizhaikin, Y., ed. [Collection of
scientific papers on Toxic Hepatitis with Ascites, Tashkent, USSR,]
Tashkent, Publishing House of the University of Central Asia,
pp. 9-25 (in Russian).
EASTMAN, D.F. & SEGALL, H.J. (1982) A new pyrrolizidine alkaloid
metabolite, 19-hydroxysenecionine, isolated from in vitro mouse
hepatic microsomes using high performance liquid chromatography.
Drug Metab. Disp., 10: 696-699.
EASTMAN, D.F., DIMENNA, G.P., & SEGALL, H.J. (1982) Covalent
binding of two pyrrolizidine alkaloids, senecionine, and
seneciphylline to hepatic macromolecules and their distribution,
excretion and transfer into milk of lactating mice. Drug Metab.
Disp., 10: 236-240.
EDGAR, J.A. (1982) Pyrrolizidine alkaloids sequestered by Solomon
Island Danaine butterflies. The feeding preferences of the Danainae
and Ithomiinae. J. Zool. (Lond.), 196: 385-399.
EDGAR, J.A. (1985) Gas chromatography of pyrrolizidine alkaloids.
In: Seawright, A.A., Hegarty, M.P., James, L.F., & Keeler, R.F.,
ed. Plant toxicology. Proceedings of the Australia - USA Poisonous
Plants Symposium, Brisbane, Australia, 14-18 May 1984, Brisbane,
Queensland Poisonous Plants Committee, pp. 227-234.
EISENSTEIN, D. & HUXTABLE, R.J. (1979) Approaches to the treatment
of pyrrolizidine toxicosis. In: Cheeke, P.R., ed. Proceedings of
the Symposium on Pyrrolizidine (Senecio) Alkaloids: Toxicity,
Metabolism, and Poisonous Plant Control Measures, Corvallis,
Oregon, USA, 23-24 February 1979, Oregon, Nutrition Research
Institute, pp. 109-113.
EL DAREER, S.M., TILLERY, K.F., LLOYD, H.H., & HILL, D.L. (1982)
Disposition of indicine N-oxide in mice and monkeys. Cancer Treat.
Rep., 66: 183-186.
EMMEL, M.W. (1948) Crotalaria poisoning in cattle. J. Am. Vet.
Med. Assoc., 113: 164.
EMMEL, M.W., SANDERS, D.A., & HENLEY, W.W. (1935) Crotalaria
spectabilis seed poisoning in swine. J. Am. Vet. Med. Assoc., 86:
43-49.
EVANS, J.V., PENG, A., & NIELSEN, C.J. (1979) The gas
chromatographic mass spectrometric analysis of the new anti-tumour
drug indicine-N-oxide utilizing a novel reaction accompanying
trimethysilylation. Biomed. mass Spectrom., 6: 38-43.
EVANS, J.V., DALEY, S.K., MCCLUSKY, G.A., & NIELSEN, C.J. (1980)
Direct quantitative analysis of indicine N-oxide in cancer patient
samples by gas chromatography using the internal standard
heliotrine N-oxide including a mass spectral comparison of their
trimethylsilyl derivatives. Biomed. mass Spectrom., 7: 65-73.
FARRINGTON, K.J. & GALLAGHER, C.H. (1960) Complexes of copper with
some pyrrolizidine alkaloids and with some of their esterifying
acids. Aust. J. biol. Sci., 13: 600-603.
FISH, M.S., SWEELEY, C.C., JOHNSON, N.M., LAWRENCE, E.P., &
HORNING, E.C. (1956) Chemical and enzymic rearrangements of
N,N-dimethylaminoacid oxides. Biochem. Biophys. Acta, 21: 196-197.
FISHMAN, A.P. (1974) Dietary pulmonary hypertension. Circ. Res.,
35: 657-660.
FORSYTH, A.A. (1968) British poisonous plants, London, Her
Majesty's Stationery Office.
FOX, D.W., HART, M.C., BERGESON, P.S., JARRETT, P.B., STILLMAN,
A.E., & HUXTABLE, R.J. (1978) Pyrrolizidine (Senecio)
intoxication mimicking Reye's syndrome. J. Paediatr., 93: 980-982.
FRAHN, J.L., CULVENOR, C.C.J., & MILLS, J.A. (1980) Preparative
separation of the pyrrolizidine alkaloids, intermedine and
lycopsamine, as their borate complexes. J. Chromatogr., 195:
379-383.
FURMANOWA, M., GUZEWSKA, J., & BELDOWSKA, B. (1983) Mutagenic
effects of aqueous extracts of Symphytum officinale L. and of its
alkaloidal fractions. J. appl. Toxicol., 3: 127-130.
FURUYA, T. & HIKICHI, M. (1971) Alkaloids and triterpenoids of
Symphytum officinale. Phytochemistry, 10: 2217-2220.
FUSHIMI, K., KATO, K., KATO, T., MATSUBARA, N., & HIRONO, I. (1978)
Carcinogenicity of flower stalks of Petasites japonicus Maxim in
mice and Syrian golden hamsters. Toxicol. Lett., 1: 291-294.
GADELLA, T.W.J., KLIPHUIS, E., & HUIZING, H.J. (1983) Cyto- and
chemotaxonomical studies on the sections officinalia and coerulea
of the genus Symphytum. Bot. Helv., 93: 169-192.
GANEY, P.E., FINK, G.D., & ROTH, R.A. (1985) The effect of dietary
restriction and altered sodium intake on the cardiopulmonary
toxicity of monocrotaline pyrrole. Toxicol. appl. Pharmacol., 78:
55-62.
GARDINER, M.R., ROYCE, R., & BOKOR, A. (1965) Studies on
Crotalaria crispata, a newly recognized cause of Kimberley horse
disease. J. Pathol. Bacteriol., 89: 43-55.
GHANEM, J. & HERSHKO, C. (1981) Veno-occlusive disease and primary
hepatic vein thrombosis in Israeli Arabs. Isr. J. med. Sci., 17:
339-347.
GHODSI, F. & WILL, J.A. (1981) Changes in pulmonary structure and
function induced by monocrotaline intoxication. Am. J. Physiol.,
240: H149-H155.
GILRUTH, J.A. (1903) Hepatic cirrhosis affecting horses and cattle
(so-called "Winton disease"), Wellington, New Zealand Department
of Agriculture, pp. 228-279 (11th annual report).
GOEGER, D.E., CHEEKE, P.R., SCHMITZ, J.A., & BUHLER, D.R. (1982)
Toxicity of tansy ragwort (Senecio jacobaea) to goats. Am. J. vet.
Res., 43: 252-254.
GOEGER, D.E., CHEEKE, P.R., RAMSDELL, H.S., NICHOLSON, S.S., &
BUHLER, D.R. (1983) Comparison of the toxicities of Senecio
jacobaea, Senecio vulgaris and Senecio glabellus in rats.
Toxicol. Lett., 15: 19-23.
GONZALEZ, A.G., DE LA FUENTE, G., REINA, M., & LOYOLA, L.A. (1986a)
Pyrrolizidine alkaloids from Senecio phillipicus and Senecio
illinitus. Planta Med., 52: 160.
GONZALEZ, E., GARCIA, R., LEMUS, I., & ERAZO, S. (1986b)
Pharmacological studies on senecionine, a pyrrolizidine alkaloid
from Senecio fistulosus Poepp. Ex less. (hualtata). An. R. Acad.
Farm., 52(1): 123-132.
GRASES, P.J. & BEKER, S.G. (1972) Veno-occlusive disease of the
liver. A case from Venezuela. Am. J. Med., 53: 511-516.
GRAY, A.I., NIC, A.N., TSAOIR, E., & WATERMAN, P.G. (1983)
Hepatotoxic alkaloids and allantoin in Symphytum tuberosum. J.
Pharm. Pharmacol., 35(Suppl): 13.
GREEN, C.R. & CHRISTIE, G.S. (1961) Malformations in foetal rats
induced by the pyrrolizidine alkaloid, heliotrine. Br. J. exp.
Pathol., 42: 369-378.
GREEN, M.H.L. & MURIEL, W.J. (1975) Use of repair-deficient
strains of Escherichia coli and liver microsomes to detect and
characterise DNA damage caused by the pyrrolizidine alkaloids
heliotrine and monocrotaline. Mutat. Res., 28: 331-336.
GREEN, C.E., SEGALL, H.J., & BYARD, J.L. (1981) Metabolism,
cytotoxicity, and genotoxicity of the pyrrolizidine alkaloid
senecionine in primary cultures of rat hepatocytes. Toxicol. appl.
Pharmacol., 60: 176-185.
GUENGERICH, F.P. (1977) Separation and purification of multiple
forms of microsomal cytochrome P-450. J. biol. Chem., 252:
3970-3979.
GUENGERICH, F.P. & MITCHELL, M.B. (1980) Metabolic activation of
model pyrroles by cytochrome P-450. Drug Metab. Disp., 8: 34-38.
GUIDUGLI, F.H., PESTCHANKER, M.J., DESALMERON, M.S.A., & GIORDANO,
O.S. (1986) 1-hydroxyplatyphyllide, a norsequiterpene lactone
from Senecio gilliesiano. Phytochemistry, 25: 1923-1926.
GUNER, N. (1986) Alkaloids of Heliotropium suaveolens. J. nat.
Prod., 49: 369.
GUPTA, P.S., GUPTA, G.D., & SHARMA, M.L. (1963) Veno-occlusive
disease of the liver. Br. med. J., 1: 1184-1186.
HABS, H. (1982) Senecio numorensis fuchsii. Carcinogenic and
mutagenic activity of an alkaloidal extract from a phytotherapeutic
drug. Dtsch. Apoth. Ztg, 122: 799-804.
HABS, H., HABS, M., MARQUARDT, H., RODER, E., SCHMAHL, D., &
WIEDENFELD, H. (1982) Carcinogenic and mutagenic activity of an
alkaloidal extract of Senecio numorensis spp. fuchsii.
Arzneimittelforsch, 32: 144-148.
HAGGLUND, K.M., L'EMPEREUR, K.M., ROBY, M.R., & STERMITZ, F.R.
(1985) Latifoline and latifoline N-oxide from Hackelia
floribunda, the western false forget-me-not. J. nat. Prod., 48:
638-639.
HAMMOUDA, F.M., RIZK, A.M., ISMAIL, S.I., ATTEYA, S.Z., GHALEB,
H.A., MADKOUR, M.K., POWAND, A.E., & WOOD, G. (1984) Poisonous
plants contaminating edible ones and toxic substances in plant
foods. Part 3: pyrrolizidine alkaloids from Heliotropium digynum
Forssk (= H. luteum, Poir). Pharmazie, 39: 703-705.
HARDING, J.D.J., LEWIS, G., DONE, J.T., & ALLCROFT, R. (1964)
Experimental poisoning by Senecio jacobaea in pigs. Pathol. Vet.,
1: 204-220.
HARRIS, C., REDDY, J., CHIGA, M., & SVOBODA, D. (1969)
Polyribosomal disaggregation by lasiocarpine. Biophys. Acta 180:
587-589.
HARRIS, P.N. & CHEN, K.K. (1970) Development of hepatic tumours in
rats following ingestion of Senecio longilobus. Cancer Res., 30:
2881-2886.
HARRIS, P.N., ROSE, C.L., & CHEN, K.K. (1957) Hepatotoxic and
pharmacological properties of heliotrine. Arch. Pathol., 64:
152-157.
HARTMANN, T. & ZIMMER, M. (1986) Organ-specific distribution and
accumulation of pyrrolizidine alkaloids during the life history of
2 annual Senecio species. J. plant Physiol., 122: 67-80.
HASHEM, M. (1939) Etiology and pathology of types of liver
cirrhosis in Egyptian children. J. Egypt. Med. Assoc., 22: 319-354.
HAYASHI, Y. (1966) Excretion and alteration of monocrotaline in
rats after a subcutaneous injection (abstract). Fed. Proc., 25: 688.
HAYASHI, Y. & LALICH, J.J. (1967) Renal and pulmonary alterations
induced by a single injection of monocrotaline. Proc. Soc. Exp.
Biol. Med., 124: 392-396.
HAYASHI, Y. & LALICH, J.J. (1968) Protective effect of
mercaptoethylamine and cysteine against monocrotaline intoxication
in rats. Toxicol. appl. Pharmacol., 12: 36-43.
HAYASHI, Y., HUSSA, J.F., & LALICH, J.J. (1967) Cor pulmonale in
rats. Lab. Invest., 16: 875-881.
HAYASHI, Y., KATO, M., OTSUKA, H. (1979) Inhibitory effects of
diet reduction on Monocrotaline intoxication in rats. Toxicol.
Lett., 3: 151-155.
HAYASHI, Y., SHIMADA, M., & KATAYAMA, H. (1977) Experimental
insulinoma in rats after a single administration of monocrotaline.
Toxicol. Lett., 1: 41-44.
HAYASHI, Y., KOKUBO, T., TAKAHASHI, M., FURUKAWA, F., OTSUKA, H., &
HASHIMOTO, K. (1984) Correlative morphological and biochemical
studies on monocrotaline-induced pulmonary alterations in rats.
Toxicol. Lett., 21: 65-77.
HAYES, M.A., ROBERTS, E., & FARBER, E. (1985) Initiation and
selection of resistant hepatocyte nodules in rats given the
pyrrolizidine alkaloids lasiocarpine and senecionine. Cancer Res.,
45: 3726-3734.
HEATH, D., SHABA, J., WILLIAMS, A., SMITH, P., & KOMBE, A. (1975)
A pulmonary hypertension-producing plant from Tanzania. Thorax, 30:
399-404.
HENDRICKS, J.D., SINNHUBER, R.O., HENDERSON, M.D., & BUHLER, D.R.
(1981) Liver and kidney pathology in rainbow trout (Salmo
gairdneri) exposed to dietary pyrrolizidine (Senecio) alkaloids.
Exp. mol. Pathol., 35: 170.183.
HILL, K.R. (1960) Worldwide distribution of seneciosis in man and
animals. Proc. R. Soc. Med., 53: 281-282.
HILLIKER, K.S. & ROTH, R.A. (1984) Alteration of monocrotaline
pyrrole-induced cardiopulmonary effects in rats by hydrallazine,
dexamethasone or sulphinpyrazone. Br. J. Pharmacol., 82: 375-380.
HILLIKER, K.S., BELL, T.G., & ROTH, R.A. (1983) Monocrotaline
pyrrole-induced pulmonary hypertension in fawn-hooded rats with
platelet storage pool deficiency: 5 hydroxytryptamine uptake by
isolated, perfused lungs. Thromb. Haemostasis (Stuttgart), 50:
844-847.
HILLIKER, K.S., BELL, T.G., LORIMER, D., & ROTH, R.A. (1984)
Effects of thrombocytopaenia in monocrotaline pyrrole-induced
pulmonary hypertension. Am. J. Physiol., 246: H747-H753.
HIRONO, I., SHIMIZU, M., FUSHIMI, K., MORI, H., & KATO, K. (1973)
Carcinogenic activity of Petasites japonicus Maxim, a kind of
coltsfoot. Gann Monogr. Cancer Res., 64: 527-528.
HIRONO, I., MORI, H., & CULVENOR, C.C.J. (1976) Carcinogenic
activity of coltsfoot, Tussilago farfara L. Gann Monogr. Cancer
Res., 67: 125-129.
HIRONO, I., MORI, H., YAMADA, K., HIRATA, Y., HAGA, M., TATEMATSU,
H., & KANIE, S. (1977) Carcinogenic activity of petasitenine, a
new pyrrolizidine alkaloid isolated from Petasites japonicus
Maxim. J. Natl Cancer Inst., 58: 1155-1157.
HIRONO, I., MORI, H., & HAGA, M. (1978) Carcinogenic activity of
Symphytum officinale. J. Natl Cancer Inst., 61: 865-869.
HIRONO, I., HAGA, M., FUJII, M., MATSUURA, S., MATSUBARA, N.,
NAKAYAMA, M., FURUYA, T., HIKICHI, M., TAKANASHI, H., UCHIDA, E.,
HOSAKA, S., & UENO, I. (1979a) Induction of hepatic tumours in
rats by senkirkine and symphytine. J. Natl Cancer Inst., 63: 469-472.
HIRONO, I., MORI, H., HAGA, M., FUJII, M., YAMADA, K., TAKANASHI,
H., UCHIDA, E., HOSAKA, S., UENO, I., MATSUSHIMA, I., UMEZAVA, K.,
& SHIRAI, A. (1979b) Edible plants containing carcinogenic
pyrrolizidine alkaloids in Japan. In: Miller, B.C. ET AL., ed.
Naturally occurring carcinogens, mutagens, and modulators of
carcinogenesis, Baltimore, Maryland, University Park Press, pp.
79-87.
HIRONO, I., UENO, I., AISO, S., YAMAJI, T., & HAGA, M. (1983)
Carcinogenic activity of Farfagium japonicum and Senecio
cannabifolius. Cancer Lett., 20: 191-198.
HOET, P., ASTIGUETA, M., & FRISQUE, A.M. (1981) Phytochemical
study of Crotalaria nitens. Rev. Latinoam. Quim., 12: 34-35.
HOOPER, P.T. (1972) Spongy degeneration in the brain in relation
to hepatic disease and ammonia toxicity in domestic animals. Vet.
Res., 90: 37-38.
HOOPER, P.T. (1974) The pathology of Senecio jacobaea poisoning of
mice. J. Pathol., 113: 227-230.
HOOPER, P.T. (1975a) Spongy degeneration in the central nervous
system of domestic animals. Part I: morphology. Acta neuropathol.
(Berlin), 31: 325-334.
HOOPER, P.T. (1975b) Spongy degeneration in the central nervous
system of domestic animals. Part III: occurrence and pathogenesis -
hepatocerebral disease caused by hyper-ammonaemia. Acta
neuropathol. (Berlin), 31: 343-351.
HOOPER, P.T. (1975c) Experimental acute gastro-intestinal disease
caused by the pyrrolizidine alkaloid, lasiocarpine. J. comp.
Pathol., 85: 341-349.
HOOPER, P.T. (1978) Pyrrolizidine alkaloid poisoning-pathology
with particular reference to differences in animal and plant
species. In: Keeler, R.F., Van Kampen, K.R., & James, L.F., ed.
Effects of poisonous plants on livestock, New York, Academic
Press, pp. 161-176.
HOOPER, P.T. & SCANLAN, W.A. (1977) Crotalaria retusa poisoning in
pigs and poultry. Aust. vet. J., 53: 109-114.
HOOPER, P.T., BEST, S.M., & MURRAY, D.R. (1974) Hyper-ammonaemia
and spongy degeneration of the brain in sheep affected with hepatic
necrosis. Res. vet. Sci., 16: 216-222.
HOOSON, J. & GRASSO, P. (1976) Cytotoxic and carcinogenic response
to monocrotaline pyrrole. J. Pathol., 118: 121-127.
HOU JINQUI, XIA YUDIN, YU ZHANSHI, AN YAN, & TANG YIXAI (1980)
[Veno-occlusive disease of the liver with report of 2 cases.] Chung
Hua Nei ko Tsa Chih, 19: 187-191 (in Chinese).
HSU, I.C., ALLEN, J.R., & CHESNEY, C.F. (1973) Identification and
toxicological effects of dehydroretronecine, a metabolite of
monocrotaline. Proc. Soc. Exp. Biol. Med., 144: 834-838.
HSU, I.C., SHUMAKER, R.C., & ALLEN, J.R. (1974) Tissue
distribution of tritium labelled dehydroretronecine. Chem.-biol.
Interact., 8: 163.
HUIZING, H.J., DE BOER, R., & MALINGRE, T.M. (1981) Preparative
ion-pair high performance liquid chromatography and gas
chromatography of pyrrolizidine alkaloids from comfrey. J.
Chromatogr., 214: 257-262.
HURLEY, J.V. & JAGO, M.V. (1975) Pulmonary oedema in rats given
dehydromonocrotaline: a topographic and electron-microscopic
study. J. Pathol., 117: 23-32.
HUXTABLE, R.J. (1980) Herbal teas and toxins: novel aspects of
pyrrolizidine poisoning in the United States. Perspect. Biol. Med.,
24: 1-14.
HUXTABLE, R., PAPLANUS, S., & LAUGHARN, J. (1977) The prevention
of monocrotaline induced right ventricular hypertrophy. Chest, 71:
308-310.
HUXTABLE, R.J., CIARAMITARO, D., & EISENSTEIN, D. (1978) The
effect of a pyrrolizidine alkaloid, monocrotaline and a pyrrole,
dehydroretronecine, on the biochemical functions of the pulmonary
endothelium. Mol. Pharmacol., 14: 1189-1203.
HUXTABLE, R.J., LUTHY, J., & ZWEIFEL, V. (1986) Toxicity of
comfrey-pepsin preparations: letters to the editor. New Engl. J.
Med., 315: 1095.
IARC (1976) Pyrrolizidine alkaloids. In: Some naturally occurring
substances, Lyons, International Agency for Research on Cancer,
pp. 265-342 (IARC Monograph on the Evaluation of Carcinogenic Risk
of Chemicals to Man, Vol. 10).
IARC (1983) Some food additives, feed additives and naturally
occurring substances, Lyons, International Agency for Research on
Cancer, pp. 207-245 (IARC Monograph on the Evaluation of
Carcinogenic Risk of Chemicals to Humans, Vol. 31).
INDIAN COUNCIL OF MEDICAL RESEARCH (1955) Infantile cirrhosis of
the liver in India. Indian J. med. Res., 43: 723-747.
ISMAILOV, N.I. (1948a) [On clinical features, etiology and
pathogenesis of heliotropic dystrophy (toxic hepatitis with
ascites).] Clin. Med., 8: 28 (in Russian).
ISMAILOV, N.I. (1948b) [Heliotropic toxicosis (toxic hepatitis
with ascites),] Tashkent, Academy of Sciences of Uzbekistan,
pp. 1-120 (in Russian).
ISMAILOV, N.I., MADZHIDOV, N.M., MAGRUPOV, A.I., MAKHKAMOV, G.M., &
MUKMINOVA, S.G. (1970) [Clinical signs, diagnosis and treatment of
Trichodesma toxicosis (alimentary toxic encephalopathy),]
Tashkent, Meditsina, p. 85 (in Russian).
JAGO, M.V. (1969) The development of the hepatic megalocytosis
of chronic pyrrolizidine alkaloid poisoning. Am. J. Pathol., 56:
405-422.
JAGO, M.V. (1970) A method for the assessment of the chronic
hepatotoxicity of pyrrolizidine alkaloids. Aust. J. exp. Biol. med.
Sci., 48: 93-103.
JAGO, M.V. (1971) Factors affecting the chronic hepatotoxicity
of pyrrolizidine alkaloids. J. Pathol., 105: 1-11.
JAGO, M.V., LANIGAN, G.W., BINGLEY, J.B., PIEREY, D.W.T., WHITTEN,
J.H., & TITCHEN, D.A. (1969) Excretion of the pyrrolizidine
alkaloid heliotrine in the urine and bile of sheep. J. Pathol., 98:
115-128.
JAGO, M.V., EDGAR, J.A., SMITH, L.W., & CULVENOR, C.C.J. (1970)
Metabolic conversion of heliotrine based pyrrolizidine alkaloids to
dehydroheliotridine. Mol. Pharmacol., 6: 402-406.
JELLIFFE, D.B., BRAS, G., & STUART, K.L. (1954a) Veno-occlusive
disease of the liver. Paediatrics, 14: 334-339.
JELLIFFE, D.B., BRAS, G., & STUART, K.L. (1954b) The clinical
picture of veno-occlusive disease of the liver in Jamaican
children. Ann. trop. Med. Parasitol., 48: 386-396.
JELLIFFE, D.B., BRAS, G., & MUKHERJEE, K.L. (1957) Veno-occlusive
disease of the liver and Indian childhood cirrhosis. Arch. dis.
Child., 32: 369-385.
JOHNSON, A.E. (1976) Changes in calves and rats consuming milk
from cows fed chronic lethal doses of Senecio jacobaea (tansy
ragwort). Am. J. vet. Res., 37: 107-110.
JOHNSON, A.E. (1979) Toxicity of tansy ragwort to cattle. In:
Cheeke, P.R., ed. Proceedings of the Symposium on Pyrrolizidine
(Senecio) Alkaloids: Toxicity, Metabolism, and Poisonous Plant
Control Measures, Corvallis, Oregon, USA, 23-24 February 1979,
Oregon, Nutrition Research Institute, pp. 129-134.
JOHNSON, A.E. (1982) Failure of mineral-vitamin supplements to
prevent tansy ragwort (Senecio jacobaea) toxicosis in cattle. Am.
J. vet. Res., 43: 718-723.
JOHNSON, A.E. & MOLYNEUX, R.J. (1984) Toxicity of thread leaf
groundsel (Senecio douglasii var. longilobus) to cattle. Am. J.
vet. Res., 45: 26-31.
JOHNSON, A.E., MOLYNEUX, R.J., & STUART, L.D. (1985) Toxicity of
Riddel's groundsel (Senecio riddelli) to cattle. Am. J. vet. Res.,
46: 577-582.
JOHNSON, W.D. (1981) Mechanism of in vitro acute toxicity of
dehydromonocrotaline a metabolite of pyrrolizidine alkaloid
monocrotaline. Toxicologist, 1: 107-108.
JOHNSON, W.D., ROBERTSON, K.A., POUNDS, J.G., & ALLEN, J.R. (1978)
Dehydroretronecine-induced skin tumours in mice. J. Natl Cancer
Inst., 61: 85-89.
JULIEN, M.H., ed. (1982) Biological control of weeds. A world
catalog of agents and their target weeds, Curepe, Trinidad and
Toabago, Commonwealth Institute of Biological Control.
JUNEJA, T.R., GUPTA, R.L., & SAMANTA, S. (1984) Activation of
monocrotaline, fulvine and their derivatives to toxic pyrroles by
some thiols. Toxicol. Lett., 21: 185-189.
KAMPANZEV, N.N. (1952) [Experimental heliotropic hepatitis.] In:
Milenkov, S.M. & Kizhaikin, Y., ed. [Collection of scientific
papers on Toxic Hepatitis with Ascites,] Tashkent, Publishing
House of the University of Central Asia, pp. 165-172 (in Russian).
KAY, J.M. & HEATH, D. (1966) Observation on the pulmonary arteries
and heart weight of rats fed on Crotalaria spectabilis seeds. J.
Pathol., 92: 385-394.
KAY, J.M. & HEATH, D. (1969) Crotalaria spectabilis: the pulmonary
hypertension plant, Springfield, Illinois, Charles C. Thomas, p.
38.
KAY, J.M., HARRIS, P., & HEATH, D. (1967a) Pulmonary hypertension
produced in rats by ingestion of Crotalaria spectabilis seeds.
Thorax, 22: 176-179.
KAY, J.M., GILLUND, T.D., & HEATH, D. (1967b) Mast cells in the
lungs of rats fed on Crotalaria spectabilis seeds. Am. J. Pathol.,
51: 1031-1044.
KAY, J.M., HEATH, D., SMITH, P., BRAS, G., & SUMMERELL, J. (1971a)
Fulvine and pulmonary circulation. Thorax, 26: 249-261.
KAY, J.M., SMITH, P., & HEATH, D. (1971b) Aminorex and the
pulmonary circulation. Thorax, 26: 262-270.
KAY, J.M., KEANE, P.M., & SUYAMA, K.L. (1985) Pulmonary
hypertension induced in rats by monocrotaline and chronic hypoxia
is reduced by p-chlorophenylalanine. Respiration, 47: 48-56.
KEDZIERSKI, B. & BUHLER, D.R. (1985) Configuration of necine
pyrroles - toxic metabolites of pyrrolizidine alkaloids. Toxicol.
Lett., 25: 115-119.
KEDZIERSKI, B. & BUHLER, D.R. (1986) The formation of 6,7-di-
hydro-7-hydroxy-1-hydroxymethyl-5H-pyrrolizine, a metabolite of
pyrrolizidine alkaloids. Chem.-biol. Interact., 57: 217-222.
KHANIN, M.N. (1948) [The etiology of toxic hepatitis with
ascites.] Arch. Pathol. USSR, 1: 42-47 (in Russian).
KIM, H.L. & JONES, L.P. (1982) Protective effects of butylated
anisole, ethoxyquin and disulfiran on acute pyrrolizidine alkaloid
poisoning in mice. Res. Comm. chem. Pathol. Pharmacol., 36: 341-344.
KIRFEL, A., WILL, G., WIEDENFELD, H. & RODER, E. (1980)
(1aR,6bR,10R,11R)-9,15-dioxo-10-hydroxy-10,11,13-trimethyl-1a,2,
3,6b-tetrabydro-5H-pyrrolizino-[1a,6b,6a,c],8-dioxa-15-cis-tride
cene,C18H25NO5. Cryst. Styruct. Comm., 9: 353-361.
KNIGHT, A.P., KIMBERLING, C.V., STERMITZ, F.R., & ROBY, M.R. (1984)
Cynoglossum officinale (hound's tongue) - a cause of pyrrolizidine
alkaloid poisoning in horses. J. Am. Vet. Med. Assoc., 185: 647-650.
KOEKEMOER, M.J. & WARREN, F.L. (1951) The occurrence and
preparation of the N -oxides. An improved method of extraction of
the Senecio alkaloids. J. Chem. Soc., C1951: pp. 66-68.
KOLETSKY, A., OYASU, R., & REDDY, J.K. (1978) Mutagenicity of the
pyrrolizidine (Senecio) alkaloid, lasiocarpine in the
salmonella/microsome test. Lab. Invest., 38: 352 (Abstract).
KOVACH, J.S., MOERTEL, C.G., & HAHN, R.G. (1979a) A phase 1 study
of indicine- N-oxide. Proc. Am. Assoc. Cancer Res., 20: 357.
KOVACH, J.S., AMES, M.M., POWIS, G., MOERTEL, C.G., HAHN, R.G., &
CREAGAN E.T. (1979b) Toxicity and pharmacokinetics of a
pyrrolizidine alkaloid, indicine- N-oxide, in humans. Cancer Res.,
39: 4540-4544.
KRAUS, C., ABEL, G., & SCHIMMER, O. (1985) [Studies on the
chromosome damaging effect of some pyrrolizidine alkaloids in human
lymphocytes in vitro.] Planta Med., 51: 89-91 (in German).
KRISHNAMACHARI, K.A.V.R., BHAT, R.V., KRISHNAMURTHY, D.,
KRISHNASWAMY, K., & NAGARAJAN (1977) Aetiopathogenesis of endemic
ascites in Sarguja district of Madhya Pradesh. Indian J. med. Res.,
65: 672-678.
KUHARA, K., TAKANASHI, H., HIRONO, I., FURUYA, T., & ASADA, Y.
(1980) Carcinogenic activity of clivorine, a pyrrolizidine
alkaloid isolated from Ligularia dentata. Cancer Lett., 10: 117-122.
KUMANA, C.R., NG, M., LIN, H.J., KO, W., WU, P.C., & TODD, D.
(1983) Hepatic veno-occlusive disease due to toxic alkaloid herbal
tea - letter to the editor. Lancet, 10 December: 1360-1361.
KUMANA, C.R., NG, M., LIN, H.J., KO, W., WU, P.C., & TODD, D.
(1985) Herbal tea induced hepatic veno-occlusive disease:
quantification of toxic alkaloid exposure in adults. Gut, 26:
101-104.
KUROZUMI, T., TANAKA, K., KIDO, M., & SHOYAMA, Y. (1983)
Monocrotaline-induced renal lesions. Exp. mol. Pathol., 39: 377-386.
LAFRANCONI, W.M. & HUXTABLE, R.J. (1983) Changes in angiotensin-
converting enzyme activity in lungs damaged by the pyrrolizidine
alkaloid, monocrotaline. Thorax, 38: 307-309.
LAFRANCONI, W.M. & HUXTABLE, R.J. (1984) Hepatic metabolism and
pulmonary toxicity of monocrotaline using isolated perfused liver
and lung. Biochem. Pharmacol., 33: 2479-2484.
LAFRANCONI, W.M., DUHAMEL, R.C., BRENDEL, K., & HUXTABLE,R.J.
(1984) Differentiation of the cardiac and pulmonary toxicity of
monocrotaline, a pyrrolizidine alkaloid. Biochem. Pharmacol., 33:
191-197.
LAFRANCONI, W.M., OHNUMA, S., & HUXTABLE, R.S. (1985) Biliary
excretion of novel pneumotoxic metabolites of the pyrrolizidine
alkaloid monocrotaline. Toxicon, 23: 903-992.
LALICH, J.J. & EHRHART, L.A. (1962) Monocrotaline induced
pulmonary arteritis in rats. J. atheroscler. Res., 2: 482-492.
LALICH, J.J. & MERKOW, L. (1961) Pulmonary arteritis produced in
rats by feeding Crotalaria spectabilis. Lab. Invest., 10: 744-750.
LANCET (1984) Pyrrolizidine alkaloids (editorial). Lancet, 1:
201-202.
LANGLEBEN, D. & REID, L.M. (1985) Effect of methylprednisolone on
monocrotaline-induced pulmonary vascular disease and right
ventricular hypertrophy. Lab. Invest., 52: 298-303.
LANIGAN, G.W. (1971) Metabolism of pyrrolizidine alkaloids in the
ovine rumen. III. The competitive relationship between heliotrine
metabolism and methanogenesis in rumen fluid in vitro. Aust. J.
agric. Res., 22: 123-130.
LANIGAN, G.W. (1972) Metabolism of pyrrolizidine alkaloids in
ovine rumen. IV. Effects of chloral hydrate and halogenated
methanes on rumen methanogenesis and alkaloid metabolism in
fistulated sheep. Aust. J. agric. Res., 23: 1085-1091.
LANIGAN, G.W. & SMITH, L.W. (1970a) Metabolism of pyrrolizidine
alkaloids in the ovine rumen. I. Formation of 7 alpha-hydroxy-
1alpha-methyl-8alpha-pyrrolizidine from heliotrine and
lasiocarpine. Aust. J. agric. Res., 21: 493-500.
LANIGAN, G.W. & SMITH, L.W. (1970b) Metabolism of pyrrolizidine
alkaloids in the ovine rumen. II. Formation of 7alpha-hydroxy-
1alpha-methyl-8alpha-pyrrolizidine from heliotrine and
lasiocarpine. Aust. J. agric. Res., 22: 123-130.
LANIGAN, G.W. & WHITTEM, J.H. (1970) Cobalt pellets and
Heliotropium europaeum poisoning in penned sheep. Aust. vet. J.,
46: 17-21.
LANIGAN, G.W., PAYNE, A.L., & PETERSON, J.E. (1978) Anti-
methanogenic drugs and Heliotropium europaeum poisoning in penned
sheep. Aust. J. Agric. Res., 29: 1281-1292.
LAWS, L. (1968) Toxicity of Crotalaria mucronata to sheep. Aust.
vet. J., 44: 453-455.
LETENDRE, L., SMITHSON, W.A., GILCHRIST, G.S., BURGERT, E.O., Jr,
HOGLAND, C.H., AMES, M.M., POWIS, G., & KOVACH, J.S. (1981)
Activity of indicine- N-oxide in refractory acute leukemia. Cancer,
47: 437-441.
LETENDRE, L., LUDWIG, J., PERRAULT, J., SMITHSON, W.A., & KOVACH,
J.S. (1984) Hepatocellular toxicity during the treatment of
refractory acute leukemia with indicine- N-oxide. Cancer, 54:
1256-1259.
LEVINE, O.R., HARRIS, R.C., BLANCE, W.A., & MELLINS, R.B. (1973)
Progressive pulmonary hypertension in children with portal
hypertension. J. Pediatr., 83: 964-972.
LIN, J.J., LIU, C., & SVOBODA, D.J. (1974) Long-term effects of
aflatoxin B1 and vocal hepatitis on marmoset liver: a preliminary
report. Lab. Invest., 30: 267-278.
LUTHY, J., ZWEIFEL, U., SCHLATTER, C., & BENN, M.H. (1980)
[Pyrrolizidine alkaloids in coltsfoot ( Tussilago farfara L.) of
various sources.] Mitt. Geb. Lebensm. Hyg., 71: 73-80 (in German
with English summary).
LUTHY, J., ZWEIFEL, U., KARLHUBER, B., & SCHLATTER, C. (1981)
Pyrrolizidine alkaloids of Senecio alpinus L. and their detection
in feedstuffs. J. agric. food Chem., 29: 302-305.
LUTHY, J., HEIM, TH., & SCHLATTER, CH. (1983) Transfer of [3H]
pyrrolizidine alkaloids from Senecio vulgaris L. and metabolites
into rat milk and tissues. Toxicol. Lett., 17: 283-288.
LUTHY, J., BRAUCHLI, J., ZWEIFEL, U., SCHMID, P., & SCHLATTER, CH.
(1984) [Pyrrolizidine alkaloid in arzneipflanzen der Boraginaceen:
Borago officinalis L. and Pulmonaria officinalis L.] Pharm. Acta
Helv., 59: 242-246 (in German).
LYFORD, C.L., VERGAVA, G.G., & MOELLER, D.D. (1976) Hepatic veno-
occlusive disease originating in Ecuador. Gastro-enterology, 70:
105-108.
MCCOMISH, M., BODEK, I., & BRAUFMAN, A.R. (1980) Quantitation of
the antineoplastic agent indicine- N-oxide in human plasma by
differential pulse polarography. J. pharmacol. Sci., 69: 727-729.
MCCOY, J.W., ROBY, M.R., & STERMITZ, F.R. (1983) Analysis of plant
alkaloid mixtures by ammonia chemical ionization mass spectroscopy.
J. nat. Prod., 46: 894-900.
MCGEE, J.O'D., PATRICK, R.S., WOOD, C.B., & BLUMGART, L.H. (1976)
A case of veno-occlusive disease of the liver in Britain associated
with herbal tea consumption. J. clin. Pathol., 29: 788-794.
MCGRATH, J.P.M., DUNCAN, J.R., & MUNNELL, J.F. (1975) Crotalaria
spectabilis toxicity in swine: characterization of the renal
glomerular lesion. J. comp. Pathol., 85(2): 185-194.
MCLEAN, E.K. (1969) The early sinusoidal lesion in experimental
veno-occlusive disease. Br. J. exp. Pathol., 50: 223-229.
MCLEAN, E.K. (1970) The toxic actions of pyrrolizidine (Senecio)
alkaloids. Pharmacol. Rev., 22: 429-483.
MCLEAN, E.K. (1974) Senecio and other plants as liver poisons.
Isr. J. med. Sci., 10: 436-440.
MCLEAN, E.K. & HILL, K.R. (1969) Portal hypertension in acute
experimental veno-occulsive disease of the liver in rats. Br. J.
exp. Pathol., 50: 37-41.
MCLEAN, E.K., BRAS, G., & GYORGY, P. (1964) Veno-occlusive lesions
in livers of rats fed Crotalaria fulva. Br. J. exp. Pathol., 45:
242-247.
MAKSUDOV, A.M. (1952) [Heliotropic toxic hepatitis with ascites.]
In: Milenkov, S.M. & Kizhaikin, Y., ed. [Collection of scientific
papers on Toxic Hepatitis with Ascites,] Tashkent, Publishing House
of the University of Central Asia, pp. 103-116 (in Russian).
MARTIN, P.A., THORBURNE, M.K., HUTCHINSON, S., BRAS, G., & MILLER,
C.G. (1972) Preliminary findings of chromosome studies in rats and
humans with veno-occlusive disease. Br. J. exp. Pathol., 53, 374-380.
MATTOCKS, A.R. (1961) Extraction of heat-labile alkaloids from
plants. Nature (Lond.), 191: 1281-1282.
MATTOCKS, A.R. (1967a) Spectrophotometric determination of
unsaturated pyrrolizidine alkaloids. Anal. Chem., 39: 443-447.
MATTOCKS, A.R. (1967b) Detection of pyrrolizidine alkaloids on
thin-layer chromatograms. J. Chromatogr., 27: 505-508.
MATTOCKS, A.R. (1968a) Toxicity of pyrrolizidine alkaloids. Nature
(Lond.), 217: 723-728.
MATTOCKS, A.R. (1968b) Spectrophotometric determination of
pyrrolizidine alkaloids: some improvements. Anal. Chem., 40: 1749.
MATTOCKS, A.R. (1969) Dehydropyrrolizine derivatives from
unsaturated pyrrolizidine alkaloids. J. Chem. Soc., C1969:
1155-1162.
MATTOCKS, A.R. (1971a) Synthetic compounds with toxic properties
similar to those of pyrrolizidine alkaloids and their pyrrolic
metabolites. Nature (Lond.), 232: 476.
MATTOCKS, A.R. (1971b) The occurrence and analysis of
pyrrolizidine alkaloid N-oxides. Xenobiotica, 1: 451-453.
MATTOCKS, A.R. (1971c) Hepatotoxic effects due to pyrrolizidine
alkaloid N-oxides. Xenobiotica, 1: 563-565.
MATTOCKS, A.R. (1971d) A field test for N-oxides of unsaturated
pyrrolizidine alkaloids. Trop. Sci., 13: 65-70.
MATTOCKS, A.R. (1972a) Toxicity and metabolism of Senecio
alkaloids. In: Harborne, J.B., ed. Phytochemical ecology, London,
New York, Academic Press, pp. 179-200.
MATTOCKS, A.R. (1972b) Acute hepatotoxicity and pyrrolic
metabolites in rats dosed with pyrrolizidine alkaloids. Chem.-biol.
Interact., 5: 227-242.
MATTOCKS, A.R. (1973) Mechanisms of pyrrolizidine alkaloid
toxicity. Pharmacology and the future of man. In: Proceedings of
the 5th International Congress on Pharmacology, San Francisco,
1972, Basel, Karger, Vol. 2, pp. 114-123.
MATTOCKS, A.R. (1977) Tissue distribution of radioactivity in rats
given tritiated analogues of hepatotoxic pyrrolizidine alkaloids.
Xenobiotica, 7: 665-670.
MATTOCKS, A.R. (1980) Toxic pyrrolizidine alkaloids in comfrey.
Lancet, 22 November: 1136-1137.
MATTOCKS, A.R. (1981a) Liver cell enlargement in rats given
hydroxymethyl pyrroles analogous to pyrrolizidine alkaloid
metabolites, followed later by the hepatotoxin dimethylnitrosamine.
Toxicol. Lett., 8: 201-205.
MATTOCKS, A.R. (1981b) Relation of structural features to pyrrolic
metabolites in livers of rats given pyrrolizidine alkaloids and
derivatives. Chem.-biol. Interact., 35: 301-310.
MATTOCKS, A.R. (1981c) A simple preparation of dehydroretronecine
using potassium nitrosodisulphonate. Chem. Ind., 7: 251.
MATTOCKS, A.R. (1982) Hydrolysis and hepatotoxicity of retronecine
diesters. Toxicol. Lett., 14: 111-116.
MATTOCKS, A.R. (1986) Chemistry and toxicology of pyrrolizidine
alkaloids, London, New York, Academic Press.
MATTOCKS, A.R. & BIRD, I. (1983) Pyrrolic and N-oxide metabolites
formed from pyrrolizidine alkaloids by hepatic microsomes in vitro:
relevance to in vivo hepatotoxicity. Chem.-biol. Interact., 43:
209-222.
MATTOCKS, A.R. & CABRAL, J.R.P. (1979) Effects of some pyrrolic
and dehydropyrrolizine esters on mouse skin: a preliminary study.
Tumori, 65: 289-293.
MATTOCKS, A.R. & CABRAL, J.R.P. (1982) Carcinogenicity of some
pyrrolizidine alkaloid metabolites and analogues. Cancer Lett., 17:
61-66.
MATTOCKS, A.R. & DRIVER, H.E. (1983) A comparison of the
pneumotoxicity of some pyrrolic esters and similar compounds
analogous to pyrrolizidine alkaloid metabolites given intravenously
to rats. Toxicology, 27: 159-177.
MATTOCKS, A.R. & DRIVER, H.E. (1987) Toxic actions of senaetnine,
a new pyrrolizidine alkaloid, in rats. Toxicol. Lett., 38: 315-319.
MATTOCKS, A.R. & JUKES, R. (1987) New improved field test for
toxic pyrrolizidine alkaloids. J. nat. Prod., 50: 161-166.
MATTOCKS, A.R. & LEGG, R.F. (1980) Antimitotic activity of
dehydroretronecine, a pyrrolizidine alkaloid metabolite, and some
analogous compounds in a rat liver parenchymal cell line. Chem.-
biol. Interact., 30: 325-336.
MATTOCKS, A.R. & WHITE, I.N.H. (1970) Estimation of metabolites
of pyrrolizidine alkaloids in animal tissues. Anal. Biochem., 38:
529-535.
MATTOCKS, A.R. & WHITE, I.N.H. (1971a) The conversion of
pyrrolizidine alkaloids to dihydropyrrolizine derivatives by rat-
liver microsomes in vitro. Chem.-biol. Interact., 3: 383-396.
MATTOCKS, A.R. & WHITE, I.N.H. (1971b) Pyrrolic metabolites from
non-toxic pyrrolizidine alkaloids. Nat. new Biol., 231: 114-115.
MATTOCKS, A.R. & WHITE, I.N.H. (1973) Toxic effects and pyrrolic
metabolites in the liver of young rats given the pyrrolizidine
alkaloid retrorsine. Chem.-biol. Interact., 6: 297-306.
MATTOCKS, A.R. & WHITE, I.N.H. (1976) The distribution of
[3H]-synthanecine A bis- n-ethylcarbamate and its metabolites
in the rat. Chem.-biol. Interact., 15: 173-184.
MEYRICK, B.O. & REID, L.M. (1979) Development of pulmonary
arterial changes in rats fed Crotalaria spectabilis. Am. J.
Pathol., 94: 37-50.
MEYRICK, B.O. & REID, L.M. (1982) Crotalaria-induced pulmonary
hypertension: uptake of 3H-thymidine by the cells of the pulmonary
circulation and alveolar walls. Am. J. Pathol., 106: 84-94.
MILKOWSKY, A.S. (1985) The synthesis and anti-tumour activity of
monocyclic analogs of indicine- N-oxide. Diss. Abstr. Int., 45(3):
844.
MILLER, W.C., RICE, D.L., KREUSEL, R.G., & BEDROSSIAN, C.W.M.
(1978) Monocrotaline model of non-cardiogenic pulmonary edema in
dogs. J. appl. Physiol., 45: 962-965.
MIRANDA, C.L., BUHLER, D.R., & CHEEKE, P.R. (1979) The effect of
tansy ragwort consumption on mineral metabolism in the rat. In:
Cheeke, P.R., ed. Proceedings of the Symposium on Pyrrolizidine
(Senecio) Alkaloids: Toxicity, Metabolism, and Poisonous Plant
Control Measures, Corvallis, Oregon, USA, 23-24 February 1979,
Oregon, Nutrition Research Institute, pp. 61-64.
MIRANDA, C.L., CARPENTER, H.M., CHEEKE, P.R., & BUHLER, D.R.
(1981a) Effect of ethoxyquin on the toxicity of pyrrolizidine
alkaloid monocrotaline and on hepatic long metabolism in mice.
Chem.-biol. Interact., 37: 95-107.
MIRANDA, C.L., HENDERSON, M.C., & BUHLER, D.R. (1981b) Dietary
copper enhances the hepatotoxicity of Senecio jacobaea in rats.
Toxicol. appl. Pharmacol., 60(3): 418-423.
MIRANDA, C.L., REED, R.L., CHEEKE, P.R., & BUHLER, D.R. (1981c)
Protective effects of butylated hydroxyanisole against the acute
toxicity of monocrotaline in mice. Toxicol. appl. Pharmacol., 59:
424-430.
MIRANDA, C.L., BUHLER, D.R., RAMSDELL, H.S., CHEEKE, P.R., &
SCHMITZ, J.A. (1982a) Modifications of chronic hepatotoxicity of
pyrrolizidine (Senecio) alkaloids by butylated hydroxyanisole
and cysteine. Toxicol. Lett., 10: 177-182.
MIRANDA, C.L., HENDERSON, M.C., BUHLER, D.R., & SCHMITZ, J.A.
(1982b) Comparative effects of antioxidants on the toxicity of
mixed pyrrolizidine alkaloids from Senecio jacobaea in mice. J.
Toxicol. environ. Health, 9(5-6): 933-939.
MIRANDA, C.L., HENDERSON, M.C., REED, R.L., SCHMITZ, J.A., &
BUHLER, D.R. (1982c) Protective action of zinc against
pyrrolizidine alkaloid-induced hepatotoxicity in rats. J. Toxicol.
environ. Health, 9: 359-366.
MIROCHNIK, M.F. (1938) [Clinical course and etiopathogenesis of
toxic hepatites with ascites.] Medical Institute, scientific
papers of the second therapeutic clinic, Vol I, Tashkent, Medical
Literature Publishers.
MISER, J.S., MISER, A.W., SMITHSON, W.A., COCCIA, P.F., AMES, M.M.,
DAVIS, D.M., HUGHES, C.S., & GILCHRIST, G.S. (1982) A phase I
trial of indicine- N-oxide in childhood malignancy. Proc. Am. Soc.
Clin. Oncol., 1: 137.
MNUSHKIN, A.S. (1949) [Some materials to clinical features and
pathogenesis of toxic hepatitis with ascites.] Sov. Med., 1: 8-9
(in Russian).
MNUSHKIN, A.S. (1952) [The clinical features, pathogenesis and
treatment of toxic hepatitis with ascites.] In: Milenkov, S.M. &
Kizhaikin, Y., ed. [Collection of scientific papers on Toxic
Hepatitis with Ascites,] Tashkent, Publishing House of the
University of Central Asia, pp. 91-98 (in Russian).
MOHABBAT, O., SRIVASTAVA, R.N., YOUNOS, M.S., SEDIQ, G.G., MENZAD,
A.A., & ARAM, G.N. (1976) An outbreak of hepatic veno-occlusive
disease in north-western Afghanistan. Lancet, 7 August: 269-271.
MOLTENI, A., WARD, W.F., TS'AO, C.-S., PORT, C.D., & SOLLIDAY, N.H.
(1984) Monocrotaline-induced pulmonary endothelial dysfunction in
rats. Proc. Soc. Exp. Biol. Med., 176: 88-94.
MOLYNEUX, R.J. & ROITMAN, J.N. (1980) Specific detection of
pyrrolizidine alkaloids on thin-layer chromatograms. J.
Chromatogr., 195: 412-415.
MOLYNEUX, R.J., JOHNSON, A.E., ROITMAN, J.N., & BENSON, M.E. (1979)
Chemistry of toxic range plants. Determination of pyrrolizidine
alkaloid content and composition in Senecio species by nuclear
magnetic resonance spectroscopy. J. agric. food Chem., 27: 494-499.
MORI, H., SUGIE, S., YOSHIMI, N., ASADA, Y., FURUYA, T., &
WILLIAMS, G.M. (1985) Genotoxicity of a variety of pyrrolizidine
alkaloids in the hepatocyte primary culture-DNA repair test using
rat, mouse, and hamster hepatocytes. Cancer Res., 45: 3125-3129.
MUNIER, R. (1953) Separation of alkaloids from their N-oxides by
paper chromatography. Bull. Soc. Chim. Biol., 35: 1225.
NARDI, N.B., GIMMLER, M.C., & LEITE, B.G. (1980) Antimitotic
action of integerrimine in rats. Rev. Bras. Genet., 3: 387-392.
NATIONAL CANCER INSTITUTE (1978) Bioassay of lasiocarpine for
possible carcinogenecity, Bethesda, Maryland, National Cancer
Institute, US Department of Health, Education and Welfare (Report
of Carcinogenesis Testing Programme, Division of Cancer Cause and
Prevention).
NAYAK, N.C., SAGREIYA, K., & RAMALINGASWAMI, V. (1969) Indian
childhood cirrhosis - the nature and significance of cytoplasmic
hyaline of hepatocytes. Arch. Pathol., 88: 631-637.
NEWBERNE, P.M. (1968) The influence of a low isotrope diet on
response of maternal and fetal rats to lasiocarpine. Cancer Res.,
28: 2327-2337.
NEWBERNE, P.M. & ROGERS, A.E. (1973) Nutrition, monocrotaline and
aflatoxin B1 in liver carcinogenesis. Plant Food Man, 1: 23-31.
NEWBERNE, P.M., WILSON, R., & ROGERS, A.E. (1971) Effects of a
low-lipotrope diet on the response of young male rats to the
pyrrolizidine alkaloid monocrotaline. Toxicol. appl. Pharmacol., 18:
387-397.
NEWBERNE, P.M., CHAN, W.C., & ROGERS, A.E. (1974) Influence of
light, riboflavin and carotene on the response of rats to the acute
toxicity of aflatoxin and monocrotaline. Toxicol. appl. Pharmacol.,
28: 200-208.
NICHOLS, W.C., MOERTL, C.G., RUBIN, J., SCHULT, A.J., & BRITTEL,
J.C. (1981) Phase II trial of indicine- N-oxide (NSC132319) in
patients with advanced colorectal carcinoma. Cancer Treat. Rep., 65:
337-339.
NIWA, H., ISHIWATA, H., & YAMADA, K. (1985) Isolation of
petasitenine, a carcinogenic pyrrolizidine alkaloid from Farfugium
japonicum. J. nat. Prod., 48: 10083-1004.
NOLAN, J.P., SCHEIG, R.L., & KLATSKIN, G. (1966) Delayed hepatitis
and cirrhosis in weanling rats following a single small dose of the
Senecio alkaloid, lasiocarpine. Am. J. Pathol., 49: 129-151.
OHNUMA, T., SRIDHAR, K.S., RATNER, L.H., & HOLLAND, J.F. (1982)
Phase I study of indicine- N-oxide in patients with advanced cancer.
Cancer Treat. Rep., 66(7): 1509-1515.
OHTSUBO, K., ITO, Y., SAITO, M., FURUYA, T., & HIKICHI, M. (1977)
Hypertrophy of pulmonary arteries and arterioles with cor pulmonale
in rats induced by seneciphylline, a pyrrolizidine alkaloid.
Experientia (Basel), 33: 498-499.
OLSON, J.W., HACKER, A.D., ALTIERE, R.J., & GILLESPIE, M.N. (1984)
Polyamines and the development of monocrotaline-induced pulmonary
hypertension. Am. J. Physiol., 247: H682-H685.
OLSON, J.W., ATKINSON, J.E., HACKER, A.D., ALTIERE, R.J., &
GILLESPIE, M.N. (1985) Suppression of polyamine biosynthesis
prevents monocrotaline induced pulmonary oedema and arterial medial
thickening. Toxicol. appl. Pharmacol., 81: 91-99.
ORD, M.J., HERBERT, A., & MATTOCKS, A.R. (1985) The ability of
bifunctional and monofunctional pyrrole compounds to induce sister
chromatid exchange (SCE) in human lymphocytes and mutations in
Salmonella typhimurium. Mutat. Res., 149: 485-493.
PANTONE, D.J., BROWN, S.M., & WOMERSLEY, C. (1985) Biological
control of fiddleneck. California Agric., 39: 4-9.
PECKHAM, J.C., SANGSTER, L.P., & JONES, O.H. (1974) Crotalaria
spectabilis poisoning in swine. J. Am. Vet. Med. Assoc., 165:
633-638.
PEDERSEN, E. (1975) Pyrrolizidine alkaloids in Danish species of
the family Boraginaceae. Arch. Pharm. Chem. Sci. Ed., 3: 55-64.
PERCY, J.J. & PIERCE, A.E. (1971) Immunosuppressive activity of
the pyrrolizidine alkaloid metabolite dehydroheliotridine.
Immunology, 21: 273-280.
PERSAUD, J.V. & HOYTE, D.A. (1974) Pregnancy and progeny in rats
treated with the pyrrolizidine alkaloid fulvine. Exp. Pathol., 9:
59-63.
PESTCHANKER, M.J. & GIORDANO, O.S. (1986) Pyrrolizidine alkaloids
from five Senecio species. J. nat. Prod., 49: 722-723.
PESTCHANKER, M.J., ASCHERI, M.S., & GIORDANO, O.S. (1985a)
Uspallatine, a pyrrolizidine alkaloid from Senecio uspallatensis.
Phytochemistry, 24: 1622-1624.
PESTCHANKER, M.J., ASCHERI, M.S., & GIORDANO, O.S. (1985b)
Pyrrolizidine alkaloids from Senecio subulatus and S. glandulosus.
Planta Med., 51: 165-166.
PETERSON, J.E. (1965) Effects of pyrrolizidine alkaloid
lasiocarpine N -oxide on nuclear and cell division in the liver of
rats. J. Pathol. Bacteriol., 89: 153-171.
PETERSON, J.E. & CULVENOR, C.C.J. (1983) Plant and fungal toxins.
In: Keeler, R.F. & Tu, A.T., ed. Handbook of natural toxins, New
York, Marcel Dekker, Vol. 1, pp. 637-681.
PETERSON, J.E. & JAGO, M.V. (1980) Comparison of the toxic effects
of dehydroheliotridine and heliotrine in pregnant rats and their
embryos. J. Pathol., 131: 339-355.
PETERSON, J.E. & JAGO, M.V. (1984) Toxicity of Echium plantagineum
(Paterson's curse): pyrrolizidine alkaloid poisoning in rats. Aust.
J. agric. Res., 35: 305-316.
PETERSON, J.E., SAMUEL, A., & JAGO, M.V. (1972) Pathological
effects of dehydroheliotridine, a metabolite of heliotridine-based
pyrrolizidine alkaloids in the young rat. J. Pathol., 107: 107-189.
PETERSON, J.E., JAGO, M.V., REDDY, J.K., & JARRETT, R.G. (1983)
Neoplasia and chronic disease associated with the prolonged
administration of dehydroheliotridine to rats. J. Natl Cancer
Inst., 70: 381-386.
PETRY, T.W., BOWDEN, G.P., HUXTABLE, R.J., & SIPES, I.G. (1984)
Characterization of hepatic DNA damage induced in rats by the
pyrrolizidine alkaloid monocrotaline. Cancer Res., 44: 1505-1509.
PETRY, T.W., BOWDEN, G.P., BUHLER, D.R., & SIPES, I.G. (1986)
Genotoxicity of the pyrrolizidine alkaloid jacobine in rats.
Toxicol. Lett., 32: 275-281.
PIERCY, P.L. & RUSOFF, L.L. (1946) Crotalaria spectabilis
poisoning in Louisiana livestock. J. Am. Vet. Med. Assoc., 108:
69-73.
PIERSON, M.L., CHEEKE, P.R., & DICKINSON, E.O. (1977) Resistance
of the rabbit to dietary pyrrolizidine (Senecio) alkaloid. Res.
Commun. chem. Pathol. Pharmacol., 16: 561-564.
PIETERS, L.A.C. & VLIETINCK, A.J. (1985) Quantitative 'H Fourier
transform nuclear magnetic resonance spectroscopic analysis of
mixtures of pyrrolizidine alkaloids from Senecio vulgaris.
Fresenius' Z. Anal. Chem., 321: 355-358.
PIETERS, L.A.C. & VLIETINCK, A.J. (1986) Comparison of high-
performance liquid chromatography with 1H nuclear magnetic
resonance spectrometry for the quantitative analysis of
pyrrolizidine alkaloids from Senecio vulgaris. J. liq. Chromatogr.,
9: 745-755.
PLESTINA, R. & STONER, H.B. (1972) Pulmonary oedema in rats given
monocrotaline pyrrole. J. Pathol., 106: 235-249.
POOL, B.L. (1982) Genotoxic activity of an alkaloidal extract of
Senecio nemorensis spp. fuchsii in Salmonella typhimurium and
Escherichia coli systems (letter to the editor). Toxicology, 24:
351-355.
POWIS, G., AMES, M.M., & KOVACH, J.S. (1979) Metabolic conversion
of indicine- N-oxide to indicine in rabbits and humans. Cancer
Res., 39: 3564-3570.
PRABHU, M.B. (1940) Infantile cirrhosis of liver. Indian J.
Pediatr., 7: 121-134.
RADHAKRISHNA RAO, M.V. (1935) Histopathology of the liver in
infantile biliary cirrhosis. Indian J. med. Res., 23: 69-90.
RAJAGOPALAN, T.R. & NEGI, R.K.S. (1985) Alkaloids from Doronicum
pardalianches Linn. Indian J. Chem., 24B, 882.
RAKIETEN, N., GORDON, B.S., BEATTY, A., COONEY, D.A., DAVIS, R.D.,
& SCHIEN, P.S. (1971) Pancreatic islet cell tumours produced by
the combined action of streptozotocin and nicotinamide. Proc. Soc.
Exp. Biol. Med., 137: 280-283.
RAMALINGASWAMI, V. & NAYAK, N.C. (1969) Liver disease in India.
In: Popper, H. & Schaffner, F., ed. Progress in liver diseases,
New York, London, Grune and Stratton, pp. 222-235.
RAMSDELL, A.S. & BUHLER, D.R. (1981) High performance liquid
chromatographic analysis of pyrrolizidine (Senecio) alkaloids
using a reversed phase styrene-divinylbenzene resin column. J.
Chromatogr., 210: 154-158.
RAO, M.S. & REDDY, J.K. (1978) Malignant neoplasms in rats fed
lasiocarpine. Br. J. Cancer, 37: 289-293.
RAPPAPORT, A.M., KNOBLAUCH, M., SUMMERSKILL, J., & BRAS, G. (1967)
Experimental veno-occlusive disease. Gastroenterology, 54: 164.
RATNOFF, O.D. & MIRICK, G.S. (1949) Influence of sex upon the
lethal effects of an hepatotoxic alkaloid, monocrotaline. Bull.
Johns Hopkins Hosp., 84: 507-25.
RECHICIGL, M., Jr, ed. (1983) CRC Handbook on naturally occurring
food toxicants, Boca Raton, Florida, CRC Press, 266 pp.
RESCH, J.F. & MEINWALD, J. (1982) A revised structure for
acetylheliosupine. Phytochemistry, 21: 2340-2431.
RHODES, K. (1957) Two types of liver disease in Jamaican children.
West Indian med. J., 6: 1-29.
RIDKER, P.M., OHKUMA, S., MCDERMOTT, W.V., TREY, C., & HUXTABLE,
R.J. (1985) Hepatic veno-occlusive disease associated with
consumption of pyrrolizidine alkaloid containing dietary
supplements. Gastroenterology, 88: 1050-1054.
RIZK, A.M., HAMMOUDA, F.M., ISMAIL, S.I., GHALEB, H.A., MADKOUR,
M.K., POHLAND, A.E., & WOOD, G. (1983) Poisonous plants
contaminating edible ones and toxic substances in plant foods. I.
Alkaloids from Senecio desfontainei. Fitoterapia, 54: 115-121.
ROBERTSON, K.A. (1982) Alkylation of N2 in deoxyguanosine by
dehydroretronecine, a carcinogenic metabolite of the pyrrolizidine
alkaloid monocrotaline. Cancer Res., 42: 8-14.
ROBINS, D.J. (1982) The pyrrolizidine alkaloids. In: Herz, W.,
Grisebach, H., & Kirby, G.W., ed. Progress in the chemistry of
organic natural products, Vienna, New York, Springer-Verlag, pp.
115-203.
RODER, E., WEIDENFELD, H., & STENGL, P. (1981) Pyrrolizidine
alkaloids senecionine and retrorsine from Senecio inaequidens.
Planta Med., 41: 412-413.
RODER, E., WIEDENFELD, H., & KNOZINGER-FISCHER (1984a)
Pyrrolizidine alkaloid from Senecio abrotanifolius spp.
abroatanifolius, spp. abrotanifolius, and var. tiroliensis.
Planta Med., 41: 412-413.
RODER, E., WIEDENFELD, H., & BRITZ-KIRSTGEN, R. (1984b)
Pyrrolizidine alkaloids from Senecio cacaliaster. Phytochemistry,
23: 1761-1763.
ROGERS, A.E. & NEWBERNE, P.M. (1971) Lasiocarpine factors
influencing its toxicity and effects in liver cell division.
Toxicol. appl. Pharmacol., 18: 356-366.
ROITMAN, J.N. (1981) Comfrey and liver damage. Lancet, 25 April:
944.
ROITMAN, J.N. (1983) Ingestion of pyrrolizidine alkaloids: a
health hazard of global proportions. In: Finlay, J.W. & Schwass,
D.E., ed. Xenobiotics in foods and feeds, Washington DC, American
Chemical Society, pp. 345-378 (ACS Series).
ROSE, A.L., GARDNER, C.A., MCDONNELL, J.D., & BULL, L.B. (1957a)
Field and experimental investigation of "walk-about" disease of
horses (Kimberley horse disease) in northern Australia: Crotalaria
poisoning in horses. Part II. Aust. vet. J., 33: 49-62.
ROSE, A.L., GARDNER, C.A., MCDONNELL, J.D., & BULL, L.B. (1957b)
Field and experimental investigation of "walk about" disease of
horses (Kimberley horse disease) in northern Australia. Part I.
Aust. vet. J., 33: 25-33.
ROSE, C.L., HARRIS, P.N., & CHEN, K.K. (1959) Some pharmacological
action of supinine and lasiocarpine. J. Pharmacol. exp. Ther., 126:
179-184.
ROSE, E.F. (1972) Senecio species: toxic plants used as food and
medicine in the Transkei. S. Afr. Med. J., 46: 1039-1043.
ROSENFIELD, I. & BEATH, O.A. (1945) Tissue changes induced by
Senecio riddellii. Am. J. clin. Pathol., 15: 407-412.
ROTH, R.A., DOTZLAF, L.A., BARANYI, B., & HOOK, J.B. (1981) Effect
of monocrotaline ingestion on liver, kidney and lung of rat.
Toxicol. appl. Pharmacol., 60: 193-203.
ROYES, K. (1948) Infantile hepatic cirrhosis in Jamaica. Caribb.
med. J., 10: 16-48.
SAFOUH, M. & SHEHATA, A.H. (1965) Hepatic vein occlusion disease
of Egyptian children. J. Pediatr., 67: 415-432.
ST. GEORGE-GRAMBAUER, T.D. & RAC, R. (1962) Hepatogenous chronic
copper poisoning in sheep in south Australia due to consumption of
Echium plantagineum L. (Salvation Jane). Aust. vet. J., 38: 288-293.
SALASPURO, M. & SIPPONEN, P. (1976) Demonstration of an
intracellular copper-binding protein in orcein staining in long
standing cholestatic liver diseases. Gut, 17: 787-790.
SAMUEL, A. & JAGO, M.V. (1975) Localization in the cell cycle of
the antimitotic action of the pyrrolizidine alkaloid, lasiocarpine,
and its metabolite, dehydroheliotridine. Chem.-biol. Interact., 10:
185-197.
SANDERS, D.A., SHEALY, A.L., & EMMEL, M.W. (1936) The pathology of
Crotalaria spectabilis Roth poisoning in cattle. J. Am. Vet. Med.
Assoc., 89: 150-156.
SAVIN, I.G., (1983) [Research of the influence of heliotrin on
the microsomal mono oxygenase systems of the rat liver.] N.E.
Pyrogov 2nd Moscow State Medical Institute. (Thesis in biological
chemistry) (in Russian).
SAVVINA, K.I. (1952) Pathological anatomy of atrophic hepatic
cirrhosis. Arch. Pathol., 14: 65-70.
SCHOENTAL, R. (1959) Liver lesions in young rats suckled by
mothers treated with the pyrrolizidine (Senecio) alkaloids,
lasiocarpine, and retrorsine. J. Pathol. Bacteriol., 77: 485-504.
SCHOENTAL, R. (1961) Herbal medicines and liver disease. J. exp.
med. Sci., 4: 126-135.
SCHOENTAL, R. (1963) Liver disease and 'natural' hepatotoxins.
Bull. World Health Organ., 29: 823-833.
SCHOENTAL, R. (1968) Toxicology and carcinogenic action of
pyrrolizidine alkaloids. Cancer Res., 28: 2237-2246.
SCHOENTAL, R. (1970) Hepatotoxic activity of retrorsine,
senkirkine and hydroxysenkirkine in newborn rats, and the role of
epoxides in carcinogenesis by pyrrolizidine alkaloids and
aflatoxins. Nature (Lond.), 227: 401-402.
SCHOENTAL, R. (1975) Pancreatic islet-cell and other tumours in
rats given heliotrine, a monoester pyrrolizidine alkaloid, and
nicotinamide. Cancer Res., 35: 2020-2024.
SCHOENTAL, R. & BENSTED, J.P.M. (1963) Effects of whole body
irradiation and of partial hepatectomy on the liver lesions induced
in rats by a single dose of retrorsine, a pyrrolizidine (Senecio)
alkaloid. Br. J. Cancer, 17: 242-251.
SCHOENTAL, R. & CAVANAGH, J.B. (1972) Brain and spinal cord
tumours in rats treated with pyrrolizidine alkaloids. J. Natl
Cancer Inst., 49: 665-671.
SCHOENTAL, R. & COADY, A. (1968) The hepatotoxicity of some
Ethiopian and east African plants including some used as
traditional medicines. East Afr. med. J., 45: 577-579.
SCHOENTAL, R. & HEAD, M.A. (1955) Pathological changes in rats as
result of treatment with monocrotaline. Br. J. Cancer, 9: 229-237.
SCHOENTAL, R. & HEAD, M.A. (1957) Progression of liver lesions
produced in rats by temporary treatment with pyrrolizidine
(Senecio) alkaloids, and the effects of betaine and high casein
diet. Br. J. Cancer, 11: 535-544.
SCHOENTAL, R. & MAGEE, P.N. (1957) Chronic liver changes in rats
after a single dose of lasiocarpine, a pyrrolizidine (Senecio)
alkaloid. J. Pathol. Bacteriol., 74: 305-319.
SCHOENTAL, R. & MAGEE, P.N. (1959) Further observation on the
subacute and chronic liver changes rats after a single dose of
various pyrrolizidine (Senecio) alkaloids. J. Pathol. Bacteriol.,
78: 471-482.
SCHOENTAL, R. & MATTOCKS, A.R. (1960) Hepatotoxic activity of
semisynthetic analogues of pyrrolizidine alkaloids. Nature (Lond.),
185: 842-843.
SCHOENTAL, R., HEAD, M.A., & PEACOCK, P.R. (1954) Senecio
alkaloids: primary liver tumours in rats as a result of treatment
with (1) mixture of alkaloids from S. jacobaea Lin, (2)
retrorsine, (3) isatidine. Br. J. Cancer, 8: 458-465.
SCHOENTAL, R., FOWLER, M.E., & COADY, A. (1970) Islet cell tumours
of the pancreas found in rats given pyrrolizidine alkaloids from
Amsinckia intermedia Fisch and Mey and from Heliotropium supinum.
Cancer Res., 30: 2127-2131.
SEGALL, H.J. (1979a) Reversed phase isolation of pyrrolizidine
alkaloids. Liq. Chromatogr., 2: 429-436.
SEGALL, H.J. (1979b) Preparative isolation of pyrrolizidine
alkaloids derived from Senecio vulgaris. Liq. Chromatogr., 2:
1319-1323.
SEGALL, H.J., DALLAS, J.L., & HADDON, W.F. (1984) Two
dihydropyrrolizine alkaloid metabolites isolated from mouse hepatic
microsomes in vitro. Drug Metab. Disp., 12: 68-71.
SEGALL, H.J., WILSON, D.W., DALLAS, J.L., & HADDON, W.F. (1985)
Trans-4-hydroxy-2-hexenol: a reactive metabolite from the
macrocyclic pyrrolizidine alkaloid senecionine. Science, 229: 472-475.
SEGER, C.L., NEWSON, J.D., ROTH, E.E., & HUTCHINSON, W.R. (1969)
Chronic toxic hepatitis in deer from a Louisiana coastal marsh.
Bull. Wildlife Disease Assoc. Proc. Ann. Conf., pp. 295-6.
SELZER, G. & PARKER, R.G.F. (1951) Senecio poisoning exhibiting
as Chiari's syndrome: a report of 12 cases. Am. J. Pathol., 27:
885-907.
SENER, B., TEMIZER, H., TEMIZER, A., & KARAKAYA, A.E. (1986) High
performance liquid chromatographic determination of alkaloids in
Senecio vernalis. J. Pharm. Belg., 41: 115-117.
SHARMA, R.K., KHAJURIA, G.S., & ATAL, C.K. (1965) Thin layer
chromatography of pyrrolizidine alkaloids. J. Chromatogr., 19:
433-434.
SHERLOCK, S. (1968) Diseases of liver and biliary system, 4th
ed., Oxford, Edinburgh, Blackwell Scientific Publishers, 250 pp.
SHTENBERG, A.I. & ORLOVA, N.V. (1955) [The question of the
etiology of the so-called "Ozhalangar Encephalitis".] Vopr. Pitan.,
14: 27-31 (in Russian).
SHULL, L.R., BUCKMASTER, G.W., & CHEEKE, P.R. (1976) Factors
influencing pyrrolizidine (Senecio) alkaloid metabolism: species,
liver sulphydryls and rumen fermentation. J. anim. Sci., 43: 1247-1253.
SHUMAKER, R.C., ROBERTSON, K.A., HSU, I.C., & ALLEN, J.R. (1976)
Neoplastic transformation in tissues of rats exposed to
monocrotaline or dehydroretronecine. J. Natl Cancer Inst., 56: 787-789.
SIDDIQI, M.A., SURI, K.A., SURI, M.P., & ATAL, C.K. (1978a) Novel
pyrrolizidine alkaloid from Crotalaria nana. Phytochemistry, 17:
2143-2144.
SIDDIQI, M.A., SURI, K.A., SURI, O.P., & ATAL, C.K. (1978b) Genus
Crotalaria. Part 34. Cronaburmine, a new pyrrolizidine alkaloid
from Crotalaria nana Burm. Indian J. Chem., 16B: 1132-1133.
SMITH, L.W. & CULVENOR, C.C.J. (1981) Plant sources of
hepatotoxic pyrrolizidine alkaloids. J. nat. Prod., 44: 129-152.
SMITH, T.E., WEISBACH, H., & UDENFRIEND S. (1962) Studies on the
mechanism of monoaminooxidase: metabolism of N,N-dimethyltryptamine
and N,N-dimethyltryptamine- N-oxide. Biochemistry, 1: 137.
SOBIN, L.H., FETRAT, M.E., & ANWER, M.A. (1969) Hepatic lesions in
Afghanistan. Trop. geogr. Med., 21: 27-29.
SRIVASTAVA, R.N., MOHABBAT, O., GHANI, A.R., & ARAM, G.N. (1978)
Veno-occlusive disease of the liver. Indian. Paediatr., 15: 143-146.
SRUNGBOONMEE, S. & MASKASAME, C. (1981) A preliminary study on
the toxicity of Crotalaria juncea to cattle. Sattawaphaet San, 32:
91-107.
STEIN, H. (1957) Veno-occlusive disease of liver in African
children. Br. med. J., 29 June: 1496-1499.
STEIN, H. & ISAACSON, C. (1962) Veno-occlusive disease of the
liver. Br. med. J., 10 February: 372-374.
STENMARK, K.R., MORGANROTH, M.L., REMIGIO, L.K., VOELKEL, N.F.,
MURPHY, R.C., HENSON, P.M., MATHIAS, M.M., & REEVES, J. (1985)
Alveolar inflammation and arachidonate metabolism in monocrotaline-
induced pulmonary hypertension. Am. J. Physiol., 248: H859-H866.
STILLMAN, A.E., HUXTABLE, R.J., CONSROE, P., KOHNEN, P., & SMITH,
S. (1977) Hepatic veno-occlusive disease due to pyrrolizidine
poisoning in Arizona. Gastroenterology, 73: 349-352.
STIRLING, G.A., BRAS, G., & URQUHART, A.E. (1962) The early
lesions in veno-occlusive disease of the liver. Arch. dis. Child.,
37: 535-538.
STOYEL, C. & CLARK, A.M. (1980) The transplacental micronucleus
test. Mutat. Res., 74: 393-398.
STUART, K.L. & BRAS, G. (1955) Clinical observations on veno-
occlusive disease of the liver in Jamaican adults. Br. med. J., 2:
348-352.
STUART, K.L. & BRAS, G. (1956) Veno-occlusive disease of the liver
in Barbados. West Indian med. J., 5: 33-36.
STUART, K.L. & BRAS, G. (1957) Veno-occlusive disease of the
liver. Q. J. Med., 26: 291-315.
STYLES, J., ASHBY, J., & MATTOCKS, A.R. (1980) Evaluation in vitro
of several pyrrolizidine alkaloid carcinogens: observation on the
essential pyrrolic nucleus. Carcinogenesis, 1: 161-164.
SUFFNESS, M. & CORDELL, G.A. (1985) Antitumour alkaloids. In:
Brossi, A., ed. The alkaloids, New York, Academic Press,
pp. 1-347.
SUGITA, T., HYERS, T.M., DAUBER, I.M., WAGNER, W.W., MCMURTRY,
I.F., & REEVES, J.T. (1983a) Lung leak precedes right ventricular
hypertrophy in monocrotaline treated rats. J. appl. Physiol., 54:
371-374.
SUGITA, T., STENMARK, K.R., WAGNER, W.W., HENSON, P.M., HENSON,
J.E., HYERS, T.M., & REEVES, J.T. (1983b) Abnormal alveolar cells
in monocrotaline induced pulmonary hypertension. Exp. lung Res.,
5: 201-215.
SUNDARESON, A.E. (1942) An experimental study of placental
permeability to cirrhogenic poisons. J. Pathol. Bacteriol., 54:
289-298.
SVOBODA, D. & REDDY, J.K. (1972) Malignant tumours in rats given
lasiocarpine. Cancer Res., 32: 908-912.
SVOBODA, D. & REDDY, J.K. (1974) Lasiocarpine induced,
transplantable squamous cell carcinoma of rat skin. J. Natl Cancer
Inst., 53: 1415-1418.
SVOBODA, D. & SOGA, J. (1966) Early effects of pyrrolizidine
alkaloids on the fine structure of rat liver cells. Am. J. Pathol.,
48: 347-373.
SWICK, R.A., CHEEKE, P.R., & BUHLER, D.R. (1979) Factors affecting
the toxicity of dietary tansy ragwort to rats. In: Cheeke, P.R.,
ed. Proceedings of the Symposium on Pyrrolizidine (Senecio)
Alkaloids: Toxicity, Metabolism, and Poisonous Plant Control
Measures, Corvallis, Oregon, USA, 23-24 February 1979, Oregon,
Nutrition Research Institute.
SWICK, R.A., CHEEKE, P.R., GOEGER, D.E., & BUHLER, D.R. (1982a)
Effect of dietary Senecio jacobaea and injected Senecio alkaloids
and monocrotaline on guinea pigs. J. anim. Sci., 55: 1411-1416.
SWICK, R.A., CHEEKE, P.R., & BUHLER, D.R. (1982b) Subcellular
distribution of hepatic copper, zinc and iron and serum
ceruloplasmin in rats intoxicated by oral pyrrolizidine (Senecio)
alkaloids. J. anim. Sci., 55(6): 1425-1430.
SWICK, R.A., CHEEKE, P.R., PATTON, N.M., & BUHLER, D.R. (1982c)
Absorption and excretion of pyrrolizidine (Senecio) alkaloids and
their effects on mineral metabolism in rabbits. J. anim. Sci., 55:
1417-1424.
SWICK, R.A., CHEEKE, P.R., MIRANDA, C.L., & BUHLER, D.R. (1982d)
The effect of consumption of the pyrrolizidine alkaloid containing
plant Senecio jacobaea on iron and copper metabolism in the rat.
J. Toxicol. environ. Health, 10: 757-768.
SWICK, R.A., CHEEKE, P.R., RAMSDELL, H.S., & BUHLER, D.R. (1983)
Effect of sheep rumen fermentation and methane inhibition on the
toxicity of Senecio jacobaea. J. anim. Sci., 56: 645-651.
TAKANASHI, H., UMEDA, M., & HIRONO, I. (1980) Chromosomal
aberrations and mutation in cultured mammalian cells induced by
pyrrolizidine alkaloids. Mutat. Res., 78: 67-77.
TAKEOKA, O., ANGEVINE, M., & LALICH, J. (1962) Stimulation of mast
cells in rat fed various chemicals. Am. J. Pathol., 40: 545-554.
TANDON, B.N., TANDON, H.D., TANDON, R.K., NARENDRANATHAN, M., &
JOSHI, Y.K. (1976) Epidemic of veno-occlusive disease in central
India. Lancet, 7 August: 271-272.
TANDON, B.N., TANDON, H.D., KOSHY, A., NARENDRANATHAN, M., JOSHI,
Y.K., TANDON, R.K., BHARGAVA, S., RAJANI, M., BHATIA, M.L.,
MANCHANDA, S.C., & KASTURI, T.E. (1977) Epidemiological,
clinical, biochemical and haemodynamic study of veno-occlusive
disease of the liver due to Crotalaria alkaloids in India. J. All
Ind. Inst. Med. Sci., 3: 165-175.
TANDON, B.N., TANDON, H.D., & MATTOCKS, A.R. (1978) Study of an
epidemic of veno-occlusive disease in Afghanistan. Indian J. med.
Res., 68: 84-90.
TANDON, H.D. & TANDON, B.N. (1975) Epidemic of liver disease -
Gulran District, Herat Province, Afghanistan, Alexandria, World
Health Organization, Regional Office for the Eastern Mediterranean
(Assignment report No. EM/AFG/OCD/001/RB).
TANDON, H.D., TANDON, B.N., TANDON, R., & NAYAK, N.C. (1977) A
pathological study of the liver in an epidemic outbreak of veno-
occlusive disease. Indian J. med. Res., 65: 679-684.
TANDON, H.D., TANDON, B.N., & MATTOCKS, A.R. (1978) An epidemic of
veno-occlusive disease of the liver in Afghanistan. Am. J.
Gastroenterol., 72: 607-613.
TANDON, R.K., TANDON, B.N., TANDON, H.D., BHATIA, M.L., BHARGAVA,
S., LAL, P., & ARORA, R.R. (1976) Study of an epidemic of veno-
occlusive disease in India. Gut, 17: 849-855.
TANNER, M.S. & PORTMANN, B. (1981) Indian childhood cirrhosis.
Arch. dis. Child., 56: 4-6.
TAYLOR, S., BELT, R.J., HAAS, C.D., & HOOGSTRATEN, B. (1983) Phase
I trial of indicine- N-oxide on two dose schedules. Cancer, 51:
1988-1991.
TEILUM, G. (1949) Endophlebitis hepatica obliterans. Acta pathol.
microbiol., 26: 147-166.
TEMIZER, A., ONAR, A.N., SENER, B., TEMIZER, H., & KARAKAYA, A.E.
(1985) Determination of alkaloids by differential pulse
polarography. I. Senecio alkaloids. J. Pharm. Belg., 40(2): 75-78.
TEREKHOV, G.N. (1939) [Pathomorphology of toxic hepatitis with
ascites.] In: Mirochnik, M.F., ed. [Functional, diagnostic and
pathological changes of toxic hepatitis with ascites,] Tashkent,
State Publishing House of Science Technology and Socio-economic
Literature of Uzbekistan, pp. 145-200 (in Russian).
TEREKHOV, G.N. (1952) [The pathomorphology of alimentary toxic
dystrophy with ascites.] In: Milenkov, S.M. & Kizhaikin, Y., ed.
[Collection of scientific papers on Toxic Hepatitis with Ascites,]
Tashkent, Publishing House of the University of Central Asia, pp.
148-164 (in Russian).
THORPE, E. & FORD, E.J.H. (1968) Development of hepatic lesions in
calves fed with ragwort (Senecio jacobaea). J. comp. Pathol., 78:
195-205.
TITTEL, G., HINZ, H., & WAGNER, H. (1979) Quantitative
determination of pyrrolizidine alkaloids in Symphyti radix by
HPLC. Planta Med., 37: 1-8.
TUCHWEBER, B., KOVAKS, K., JAGO, M.V., & BEAULIEU, T. (1974)
Effect of steroidal and non-steroidal microsomal enzyme inducers on
the hepatotoxicity of pyrrolizidine alkaloids in rats. Res. Commun
chem. Pathol. Pharmacol., 7: 459-480.
TUCKER, A., BRYANT, S.E., FROST, H.H., & MIGALLY, N. (1983)
Chemical sympathectomy and serotonin inhibition reduce
monocrotaline-induced right ventricular hypertrophy in rats. Can.
J. Physiol. Pharmacol., 61: 356-362.
TURNER, J.H. & LALICH, J.J. (1965) Experimental cor pulmonale in
the rat. Arch. Pathol., 79: 409-418.
VALDIVIA, E., SONNAD, J., HAYASHI, Y., & LALICH, J.J. (1967a)
Experimental interstitial pulmonary oedema. Angiology, 18: 378-383.
VALDIVIA, E., LALICH, J., HAYASHI, Y., & SONNAD, J. (1967b)
Alterations in pulmonary alveoli after a single injection of
monocrotaline. Arch. Pathol., 84: 64-76.
VAN DER WATT, J.J., PURCHASE, I.F.H., & TUSTIN, R.C. (1972) The
chronic toxicity of retrorsine, a pyrrolizidine alkaloid, in vervet
monkeys. J. Pathol., 107: 279-287.
VILLARROEL, L.H., TORRES, R.G., NAVARRO, J.M., & FAJARDO, V.M.
(1985) Senecionine and seneciphylline from Senecio patagonicus.
Fitoterapia, 56: 250-251.
VISCONTINI, M. & GILHOF-SCHAUFELBERGER, H. (1971) Synthesis of (±)
dehydroheliotridine. Helv. chim. Acta, 54: 449-456.
WAGENVOORT, C.A., DINGEMANS, K.P., & LOTGERING, G.G. (1974a)
Electron microscopy of pulmonary vasculature after application of
fulvine. Thorax, 29: 511-521.
WAGENVOORT, C.A., WAGENVOORT, N., & DIJK, H.J. (1974b) Effect of
fulvine on pulmonary arteries and veins of the rat. Thorax, 29:
522-529.
WAGNER, H., NEIDHARDT, U., & TITTEL, G. (1981) TLC and HPLC
analysis of pyrrolizidine N-oxide alkaloids of Symphyti radix.
Planta Med., 41: 232-239.
WAKIM, K.G., HARRIS, P.N., & CHEN, K.K. (1946) The effects of
senecionine on the monkey. J. Pharmacol. exp. Ther., 87: 38-45.
WALKER, K.H. & KIRKLAND, P.D. (1981) Senecio lautus toxicity in
cattle. Aust. vet. J., 57: 1-7.
WATT, J.M. & BREYER-BRANDWIJK, M.G. (1962) Medicinal and poisonous
plants of southern and eastern Africa, Edinburgh, London, E. and
S. Livingstone, 584 p.
WEHNER, F.C., THIEL, P.G., & VAN RENSBURG, S.J. (1979)
Mutagenicity of alkaloids in the Salmonella/microsome system.
Mutat. Res., 66: 187-190.
WESTON, C.F.M., COOPER, B.T., DAVIES, J.D., & LEVINE, D.F. (1987)
Veno-occlusive disease of the liver secondary to ingestion of
comfrey. Br. med. J., 295: 183.
WHITE, I.N.H. (1976) The role of liver glutathion in the acute
toxicity of retrorsine to rats. Chem.-biol. Interact., 13: 333-342.
WHITE, I.N.H. (1977) Excretion of pyrrolic metabolites in bile of
rats given retrorsine or the bis- n-ethylcarbamate of synthanecine
A. Chem.-biol. Interact., 16: 169-180.
WHITE, I.N.H. & MATTOCKS, A.R. (1971) Some factors affecting the
conversion of pyrrolizidine alkaloids to N-oxides and to pyrrolic
derivatives in vitro. Xenobiotica, 1: 503-505.
WHITE, I.N.H. & MATTOCKS, A.R. (1972) Reactions of dihydro-
pyrrolizidine with deoxyribonucleic acid in vitro. Biochem.
J., 128: 291-297.
WHITE, I.N.H., MATTOCKS, A.R., & BUTLER, W.H. (1973) The
conversion of the pyrrolizidine alkaloid retrorsine to pyrrolic
derivatives in vivo and in vitro and its acute toxicity to
various animal species. Chem.-biol. Interact., 6: 207-218.
WHITE, R.D., KRUMPERMAN, P.H., CHEEKE, P.R., & BUHLER, D.R. (1983)
An evaluation of acetone extracts from six plants in the Ames
mutagenicity test. Toxicol. Lett., 15: 25-31.
WHITE, R.D., SWICK, R.A., CHEEKE, P.R. (1984) Effect of dietary
copper and molybdenum on tansy ragwort (Senecio Jacobaea) toxicity
in sheep. Am. J. vet. Res., 45: 159-161.
WHO (1980) Inventory of medicinal plants used in the different
countries, Geneva, World Health Organization (Document No.
DPM/80.3).
WICKRAMANAYAKE, P.P., ARBOGAST, B.L., BUHLER, D.R., DEINZER, M.L.,
& BURLINGAME, A.L. (1985) Alkylation of nucleosides and
nucleotides by dehydroretronecine: characterization of covalent
adducts by liquid secondary ion mass spectrometry. J. Am. Chem.
Soc., 107: 2485-2488.
WIEDENFELD, H. (1982) Two pyrrolizidine alkaloids from Gynura
scandens. Phytochemistry, 21: 2767-2768.
WIEDENFELD, H., PASTEWKA, U., STENGL, P., & ROEDER, E. (1981) On
the gas chromatographical determination of the pyrrolizidine
alkaloids of some Senecio species. Planta Med., 41: 124-128.
WIEDENFELD, H., RODER, E., & ANDERS, E. (1985) Pyrrolizidine
alkaloids from seeds of Crotalaria scassellatii. Phytochemistry,
24: 376-378.
WILLIAMS, A.O., EDINGTON, G.M., & OBAKPONOVWE, P.C. (1967)
Hepatocellular carcinoma in infancy and childhood in Ibadan,
western Nigeria. Br. J. Cancer, 21(3): 474-482.
WILMOT, F.C. & ROBERTSON, G.W. (1920) Senecio disease or
cirrhosis of the liver due to Senecio poisoning. Lancet,
23 October: 848-849.
WITTIG, M. & STEPHEN, C. (1964) Modification of microsomal lipid
peroxidation and drug metabolism by cytoplasmic copper. Res.
Commun. Chem. Pathol. Pharmacol., 44: 477-493.
WONG, R.Y. & ROITMAN, J.N. (1984) Structure and absolute
configuration of (+)-doronine-benzene (1:1). Acta Crystallogr.,
Sect. C: Cryst. Struc. Commun., C40: 163-166.
WURM, H. (1939) [A cluster of endophlebilis hepatica obliterans in
the age group of suckling infants.] Klin. Wochenschr., 18: 1527-1531
(in German).
YAMANAKA, H., NAGAO, M., SUGIMURA, T., FURUYA, T., SHIRAI, A., &
MATSUSHIMA, T. (1979) Mutagenicity of pyrrolizidine alkaloids in
the Salmonella/mammalian microsome test. Mutat. Res., 68: 211-216.
YULDASHEVA, L.N. & SULTANOVA, R.G. (1983) [Oxidative reactions in
rat liver tissue under conditions of chronic heliotrine hepatitis.]
Vopr. Med. Khim., 29: 81-85 (in Russian).
YUNUSOV, S.YU. & PLEKHANOVA, N.V. (1959) [The alkaloids of
Trichodesma incanum. The structure of incanine and trichesmine.]
Zh. Obshch. Khim., 29: 677-684 (in Russian).
ZHELTOVA, L.I. (1952) [The clinical course of toxic hepatitis.]
In: Milenkov, S.M. & Kizhaikin, Y., ed. [Collection of scientific
papers on Toxic Hepatitis with Ascites,] Tashkent, Publishing
House of the University of Central Asia, pp. 76-90 (in Russian).
APPENDIX I
Pyrrolizidine Alkaloids and Their Plant Sources
ISOLATIONS OF TOXIC PYRROLIZIDINE ALKALOIDS - (8701081148)
Alkaloid Plant Sources Reference Reference
Location
19-Acetoxysenkirkine Senecio laricifolius H.B.K. Bohlmann et al. (1986) A
6-Acetylanacrotine Crotalaria agatiflora Schweinf. Culvenor & Smith (1972) B281
6-Acetyl-trans-anacrotine Crotalaria agatiflora Schweinf. Culvenor & Smith (1972) B281
Acetylcrotaverrine Crotalaria verrucosa L. O.P. Suri et al. (1976) B339
C. walkeri Arnott K.A. Suri et al. (1976) B340
7-Acetylechinatine Cynoglossum amabile Stapf. and Drummond Culvenor & Smith, unpubl.
Lindelofia spectabilis Lehm. Rao et al. (1974) B62
Symphytum asperum Lepech. Pedersen (1975b) A
S. officinale Linn. Pedersen (1975b) A
Acetylgynuramine Gynura scandens O. Hoffm. Wiedenfeld (1982) C
Acetylheliosupine Cynoglossum officinale L. Pedersen (1970); Resch & Meinwald (1982) B43, A
Myosotis sylvatica Hoffm. Resch & Meinwald (1982) A
Symphytum asperum Lepech. Pedersen (1975) A
S. officinale Linn. Pedersen (1975) A
Acetylindicine Heliotropium indicum L. Mattocks (1967a) B71
7-Acetylintermedine Borago officinale L. Luthy et al. (1984) A
Symphytum aspera Roitman (1981) A
S. officinale Linn. Roder et al. (1982) C
S. x uplandicum Nyman Culvenor et al. (1980a), (1980b) A, A
Alkaloid Plant Sources Reference Reference
Location
Acetyllasiocarpine Heliotropium europaeum L. Culvenor et al. (1975) B69
7-Acetyllycopsamine Amsinckia menziesii (Lehm.) Nels Roitman, (1983a) C
& Macbr.
Anchusa officinalis L. Pedersen (1975); Broch-Due & A, B32
Aasen (1980)
Borago officinale L. Luthy et al. (1984) A
Symphytum aspera Roitman (1981) A
S. officinale Linn. Huizing & Malingre (1981) C
S. x uplandicum Nyman Culvenor et al. (1980a, 1980b) A, A
3a'-Acetyllycopsamine Amsinckia menziesii (Lehm.) Nels. Roitman (1983a C
& Macbr.
7-Acetylmadurensine Crotalaria agatiflora Schweinf. Culvenor & Smith (1972) B281
7-Acetyl-cis-madurensine Crotalaria agatiflora Schweinf. Culvenor & Smith (1972) B281
7-Acetylscorpioidine Myosotis scorpioides L. Resch et al. (1982) C
Acetylseneciphylline Senecio pterophorus DC. Edgar et al. (1976) B246
18-Acetylsenkirkine Senecio illinitus Phill. Gonzalez et al. (1986a) A
S. kirkii Hook. f. ex Kirk Briggs et al. (1965) A
S. tenuifolius Burm. Bhakuni & Gupta (1982) C
Acetylsyneilesine Syneilesis palmata Maxim. Hikichi & Furuya (1976) B279
Amabiline Borago officinale L. Luthy et al. (1984) A
Cynoglossum amabile Stapf. et Drummond Culvenor & Smith (1967) B33
C. glochidiatum Wall. ex Lindl. K.A. Suri et al. (1975a) B36
Eupatorium cannabinum L. Luthy et al. (1984) A
Lindelofia angustifolia (Schrenk) Brand. K.A. Suri et al. (1975a) B36
Alkaloid Plant Sources Reference Reference
Location
Anacrotine Crotalaria agatifolia Schweinf. Culvenor & Smith (1972) B281
C. incana L. Mattocks (1968) B302
C. laburnifolia L. Snehelata et al. (1966); Sawhney B309,
et al. (1967) 291
C. laburnifolia L. subsp. eldomae Crout (1972) B312
C. micans Link. Atal et al. (1966a) B280
C. verrucosa L. Subramanian & Nagarajan (1967) B338
Anadoline Symphytum orientale Ulubelen & Doganca (1971); Culvenor B103,
et al. (1975) 104
S. tuberosum L. Ulubelen & Ocal (1977) B105
Angelylechimidine (or Symphytum asperum Lepech. Gadella et al. (1983) A
isomer) S. x uplandicum Nyman Gadella et al. (1983) A
7-Angelylheliotridine Heliotropium supinum L. Crowley & Culvenor (1959) B83
trachelanthate
7-Angelylheliotridine Heliotropium supinum L. Crowley & Culvenor (1959) B83
viridiflorate
7-Angelylheliotrine Heliotropium digynum Forssk. Hammouda et al. (1984) A
H. eichwaldii Steud ex DC. O.P. Suri et al. (1975) B63
7-Angelyl-9-sarracinyl- Senecio triangularis Hook. Rueger & Benn (1983) C
retronecine
6-Angelyl-trans-anacrotine Crotalaria agatiflora Schweinf. Culvenor & Smith (1972) B281
Asperumine Echium vulgare L. Karimov et al. (1975) B50
Symphytum asperum Lepech. Man'ko et al. (1969); Man'ko & B95, 96
Kotowskii (1970); Man'ko et al. 1970 B97
S. caucasicum Bieb. Man'ko et al. (1972); Mel'kumova B98, 99
et al. 1974
Alkaloid Plant Sources Reference Reference
Location
Axillaridine Crotalaria axillaris Ait. Crout (1968b, 1969) B287, 288
C. scassellatii Chiov Wiedenfeld et al. (1985) A
Axillarine Crotalaria axillaris Ait. Crout (1968b, 1969) B287, 288
C. scassellatii Chiov Wiedenfeld et al. (1985) A
Bisline Senecio othonniformis Fourcade Coucourakis & Gordon-Gray (1970) B224
S. petasitis DC. Gonzalez et al. (1973) B225
Brachyglottine Brachyglottis repanda Forst. et Forst. White, pers. commun.
Carategine Lindelofia tschimganica Akramov et al. (1965) B87
Rindera oblongifolia M. Pop. Akramov et al. (1965) B87
Solenanthus karategenius Lipsky Akramov et al. (1964) B93
Chlorodeoxysceleratine Senecio latifolius DC. (S. sceleratus) Gordon-Gray (1967) B261
Clivorine Ligularia brachyphylla Hand. Mazz. Klasek et al. (1971) B134
L. clivorum Klasek et al. (1967, 1969, 1970); B135,
Birnbaum et al. (1971) 136,
137,
138
L. dentata (A. Gray) Hara Klasek et al. (1971) B134
L. elegans (Cass.) Klasek et al. (1971) B134
Crispatine Crotalaria candicans W. & A. Suri et al. (1982) C
C. crispata F. Muell. ex Benth. Culvenor & Smith (1963) B294
C. lunata Beddome ex Polhill Rothschild et al. (1979) C
C. madurensis R. Wight Habib et al. (1971) B315
Crobarbatine Crotalaria barbata R. Graham ex R. Wight Puri et al. (1973) B289
Walk.-Arn.
Alkaloid Plant Sources Reference Reference
Location
Cromadurine Crotalaria madurensis Wight Rao et al. (1974, 1975b) B62, 316
Cronaburmine Crotalaria nana Burm. Siddiqi et al. (1978b) A
Crosemperine Crotalaria aegyptiaca Benth. Zalkow et al. (1979) B419
C. semperflorens Vent. Atal et al. (1967) B331
Crotaflorine Crotalaria agatiflora Schweinf. Culvenor & Smith (1972) B281
Crotafoline Crotalaria laburnifolia L. subsp. eldomae Crout (1972) B312
Crotalarine Crotalaria burhia Buch-Ham. Ali & Adil (1973); Rao et al. (1975a) B292,
293
Crotaleschenine Crotalaria leschenaultii Suri & Atal (1967); Smith et al. (1988) B313
Crotananine Crotalaria nana Burm. Siddiqi et al. (1978a) A
Crotastriatine Crotalaria pallida Ait. (syn. C. Gandhi et al. (1968); Batra et al. (1975) B323,
mucronata Desv., C. striata DC.) 324
Crotaverrine Crotalaria verrucosa L. O.P. Suri et al. (1976) B339
C. walkeri Arnott K.A. Suri et al. (1976) B340
Cruentine A Senecio cruentus DC. Chu & Chu (1964) B172
Cruentine B Senecio cruentus DC. Chu & Chu (1964) B172
Curassavinine Heliotropium curassavicum Linn. Mohanraj et al. (1982a) C
Cynaustine Borago officinale L. (or amabiline ?) Larson et al. (1984) C
Cynoglossum australe R. Br. Culvenor & Smith, 1967 B33
C. lanceolatum Forsk. Suri et al. 1975a B36
Alkaloid Plant Sources Reference Reference
Location
Deoxyaxillarine Crotalaria scassellatii Chiov Wiedenfeld et al. 1985 A
Diacetyllycopsamine Amsinckia menziesii (Lehm.) Nels. & Macbr. Roitman, 1983a C
Dibenzoylretronecine Caccinea glauca Savi. Siddiqi et al. 1978a B346
Dicrotaline Crotalaria dura J.M. Wood et Evans Marais, 1944; Adams & Van Duuren, 1953a B295, 296
C. globifera E. Mey. Marais, 1944; Adams & Van B295,
Duuren, 1953a 296
N-(Dihydropyrrolizino- Heliotropium europaeum L. Culvenor & Smith, 1969 B68
methyl) heliotrine chloride
15,20-Dihydroxyerucifoline Senecio dolichodoryius Cuatr. Bohlmann et al. 1986 A
Dihydroxytriangularine Alkanna tinctoria Tausch Roder et al. 1984b C
Doronenine Senecio abrotanifolius ssp. Roder et al. 1984a A
abrotanifolius
S. abrotanifolius ssp. abrotanifolius Roder et al. 1984a A
var tiroliensis
S. doronicum L. Roder et al. 1979a, 1980; Kirfel B176, C
et al. 1980 A
Doronine Doronicum macrophyllum Alieva et al. 1976 B122
Senecio abrotanifolius ssp. Roder et al. 1984a A
abrotanifolius
S. abrotanifolius ssp. abrotanifolius Roder et al. 1984a A
var tiroliensis
S. clevelandii E.L. Greene Wong & Roitman, 1984 A
S. othonnae Bieb. Khalilov et al. 1977 B223
Alkaloid Plant Sources Reference Reference
Location
H. suaveolens Bieb. Guner, 1986 A
H. supinum L. Crowley & Culvenor, 1959 B83
Lappula glochidiata Suri et al. 1978 B84
Lindelofia angustifolia (Schrenk) Brand. Rao et al. 1974; Suri et al. 1975a B62, 36
L. spectabilis Lehm. Rao et al. 1974; Suri et al. 1975a B62, 36
L. stylosa (Kar. et Kir.) Brand. Kiyamitdinova et al. 1967 B75
L. tschimganica Akramov et al. 1965 B87
Paracynoglossum imeritinum (Kusn.) M. Pop. Man'ko & Marchenko, 1971b B88
Prestonia sp. Edgar, 1985 A
Rindera austroechinata M. Pop. Akramov et al. 1965 B87
R. baldshuanica Kusnezov Akramov et al. 1965 B87
R. cyclodonta Akramov et al. 1967a B59
R. echinata Regel Men'shikov & Denisova, 1953 B92
R. oblongifolia M. Pop. Akramov et al. 1965 B87
Solenanthus circinnatus Ledeb. Akramov et al. 1964 B93
S. coronatus Kiyamitdinova et al. 1967 B75
S. karateginus Lipsky Akramov et al. 1964 B93
Symphytum asperum Lepech. Man'ko et al. 1970b B97
S. caucasicum Bieb. Man'ko et al. 1972 B98
S. officinale Linn. Man'ko et al. 1970a; Huizing & B101, C
Malingre, 1979
Echiumine Amsinckia hispida (Ruiz et Pav.) Culvenor & Smith, 1966a B30
I.M. Johnston
A. intermedia Fisch et C. Mey. Culvenor & Smith, 1966a B30
A. lycopsoides Lehm. Culvenor & Smith, 1966a B30
Echium plantagineum L. Culvenor, 1956 B48
Emiline Emilia flammea Cass. Kohlmunzer & Tomczyk, 1969; Tomczyk & B123, 124
Kohlmunzer, 1971; Kohlmunzer B125
et al. 1971
Alkaloid Plant Sources Reference Reference
Location
13,19-Epoxyseneciphylline Senecio megaphyllus Green. Bohlmann et al. 1986 A
S. usgorensis Cuatr. Bohlmann et al. 1986 A
13,19-Epoxyspartiodine Senecio megaphyllus Green. Bohlmann et al. 1986 A
Erucifoline Senecio aegypticus L. Klasek et al. 1968a B145
S. erraticus Berthol. subsp. Schroter & Santavy, 1960; Sedmera B179,
barbaraeifolius Krock. et al. 1972 180
S. erucifolius L. Kompis & Santavy, 1962; Sedmera B183,
et al. 1972 180
Europine Heliotropium arbainense Zalkow et al. 1979 B419
H. digynum Forssk. Hammouda et al. 1984 A
H. europaeum L. Culvenor, 1954 B66
H. lasiocarpum Fisch and Mey. Culvenor et al. 1986 A
H. maris-mortui Zalkow et al. 1978 B74
H. rotundifolium Zalkow et al. 1978 B74
H. suaveolens Bieb. Guner, 1986 A
Trichodesma africana Zalkow et al. 1979 B419
Floricaline Cacalia floridana Cava et al. 1968 B119
Floridanine Cacalia floridana Cava et al. 1968 B119
Doronicum macrophyllum Alieva et al. 1976 B122
Senecio aureus L. Roder et al. 1983 C
S. erraticus Berthol. Gaiduk et al. 1974 B177
S. othonnae Bieb. Khalilov & Telezhenetskaya, 1973b B222
Florosenine Cacalia floridana Cava et al. 1968 B119
Senecio aureus L. Roder et al. 1983 C
S. fluviatilis Wallr. Klasek et al. 1973b B185
S. quebradensis Greem. Bohlmann et al. 1986 A
Alkaloid Plant Sources Reference Reference
Location
Fulvine Crotalaria berteroana DC. (C. fulva Roxb.) Schoental, 1963 B297
C. crispata F. Muell. ex Benth. Culvenor & Smith, 1963 B294
C. madurensis R. Wight Atal et al. 1966a; Habib et al. 1971 B280, 315
C. paniculata Willd. Subramanium et al. 1968 B325
Globiferine Crotalaria globifera E. Mey. Brown et al. 1984 C
Grahamine Crotalaria grahamiana R. Wight et Atal et al. 1969 B299
Walk.-Arn.
Grantaline Crotalaria globifera E. Mey Brawn et al., 1984 C
C. virgulata subsp. grantiana (Harv.) Smith & Culvenor, 1984 C
Polhill (C. grantiana Harvey)
Grantianine Crotalaria globifera E. Mey. Brown et al. 1984 C
C. virgulata subsp. grantiana (Harv.) Adams et al. 1942b; Adams & B301
Polhill (C. grantiana Harvey) Gianturco, 1956b; Smith & B259, C
Culvenor, 1984
Gynuramine Gynura scandens O. Hoffm. Wiedenfeld, 1982 A
Heleurine Heliotropium europaeum L. Culvenor, 1954 B66
H. indicum L. Hoque et al. 1976 B72
H. lasiocarpum Fisch and Mey. Culvenor et al. 1986 A
Heliosupine Cynoglossum creticum Zalkow et al. 1979 B419
C. officinale Man'ko & Borisyuk, 1957; Man'ko, 1959; B38, 39
Sykulska, 1961 B40
C. pictum Man'ko & Marchenko, 1971b, 1972b B44, 45
C. viridiflorum Pallas ex Lehm. Man'ko, 1972 B34
Echium vulgare L. Man'ko, 1964 B49
Heliotropium supinum L. Denisova et al. 1953; Crowley & B82, 83
Culvenor, 1959
Alkaloid Plant Sources Reference Reference
Location
Myosotis sylvatica Hoffm. Culvenor & Smith, unpubl.
Paracynoglossum imeritinum (Kusn.) Man'ko & Marchenko, 1971 B88
Symphytum asperum Lepech. Man'ko et al. 1970b B97
S. officinale Linn. Man'ko et al. 1970a B101
Heliotrine Heliotropium acutiflorum Akramov et al. 1968 B51
H. arbainense Zalkow et al. 1979 B419
H. arguzioides Kar. et Kir. Zolotavina, 1963 B53
H. curassavicum Linn. Rajagopalan & Batra, 1977b B56
H. dasycarpum Ledeb. Akramov et al. 1961a B54
H. digynum Forssk. Hammouda et al. 1984 A
H. eichwaldi Steud. ex DC. Gandhi et al. 1966a B60
H. europaeum L. Trautner & Neufeld, 1949; Culvenor B64, 65
et al. 1954
H. indicum L. Hoque et al. 1976 B72
H. lasiocarpum Fisch et C. Mey. Men'shikov, 1932 B73
H. olgae Kiyamitdinova et al. 1967; Sheveleva B75, 76
et al. 1969
H. popovii H. Riedl. subsp. gillianum Mohabbet et al. 1976 B77
H. ramosissimum Habib, 1975; Schoental & B79, 78
Cavanagh, 1972
H. suaveolens Bieb. Guner, 1986 A
H. supinum L. Pandey et al. 1983 C
H. transoxanum Akramov et al. 1968 B51
Heliovinine Heliotropium curassavicum Linn. Mohanraj et al. 1982c C
Heterophylline Parsonsia heterophylla A. Cunn. Edgar et al. 1980 B418
P. spiralis Edgar et al. 1980 B418
18-Hydroxysenkirkine Crotalaria laburnifolia L. subsp. Crout, 1972 B312
19-Hydroxysenkirkine Senecio laricifolius H.B.K. Bohlmann et al. 1986 A
Alkaloid Plant Sources Reference Reference
Location
Incanine Heliotropium olgae Sheveleva et al. 1969 B76
Trichodesma incanum Alph. DC. Yunusov & Plekhanova, 1953, 1957, B110, 111
1959; Tashkhodzhaev et al. 1979a B112, C
Indicine Heliotropium amplexicaule Vahl. Ketterer et al. 1987, (in press)
H. indicum L. Mattocks et al. 1961 B70
Prestonia sp. Edgar, 1985 A
Integerrimine Cacalia hastata L. subsp. orientalis Hayashi et al. 1972 B120
Kitamura
Crotalaria brevidens Benth. var. Suri et al. 1975b B304
intermedia (Kotschy) Polhill
C. brevifolia Sawhney & Atal, 1966 B290
C. incana L. Adams & Van Duuren, 1953b B187
C. pallida Ait. Sawhney et al. 1967 B291
C. tetragona Roxb. Puri et al. 1974 B314
C. zanzibarica Benth. (C. usaramoensis) Culvenor & Smith, 1966b B337
Petasites hybridus L. Luthy et al. 1983 C
Senecio alpinus L. Luthy et al. 1981 A
S. antieuphorbium (L.) Sch. Bip. Rodriguez & Gonzalez, 1969 B152
S. brasiliensis DC. Motidome & Ferreira, 1966a B163
S. brasiliensis Less. var tripartitus Nardi et al. 1980 A
S. durieui Gay Panizo & Rodriguez, 1974 B157
S. erraticus Berthol. subsp. Santavy et al. 1962 B182
barbaraeifolius Krock.
S. faberi Hemsl. Wei et al. 1982 C
S. formosus Munoz Quevedos, 1976 B186
S. glandulosus Don ex Hook. et Arn. Pestchanker et al. 1985b A
S. inaequidens DC. Bicchi et al. 1985 A
Alkaloid Plant Sources Reference Reference
Location
S. incanus L. subsp. carniolicus Klasek et al. 1968b B147
(Willd.) Br.-Bl.
S. integerrimus Manske, 1939a; Roitman et al. 1979 B156, 420
S. kleinia Sch. Bip. Gonzalez & Calero, 1958b B205
S. leucostachys Baker Pestchanker & Giordano, 1986 A
S. magnificus F. Muell. Gellert & Mate, 1964 B216
S. morrisonensis Hayata Lu, Sheng-Teh, 1972 B217
S. nebrodensis L. var sicula Plescia et al. 1976 B218
S. ragonesei Cabr. Pestcharnker & Giordano, 1986 A
S. spathulatus A. Rich. White, 1969 B158
S. squalidus L. Kropman & Warren, 1950; Gonzalez & B265,
Calero, 1958a 205
S. tenuifolius Burm. Bhakuni & Gupta, 1982 C
S. triangularis Hook. Roitman, 1983b C
S. vernalis Walst. et Kit. Sener et al. 1986; Hartmann & A, A
Zimmer, 1986
S. viscosus L. Barger & Blackie, 1936; Santavy et al. B264,
1962 182
S. vulgaris L. Pieters & Vlietinck, 1986 A
Intermedine Amsinckia hispida (Ruiz et Pav.) Culvenor & Smith, 1966a B30
I.M. Johnston
A. intermedia Fisch et C. Mey. Culvenor & Smith, 1966a B30
A. lycopsoides Lehm. Culvenor & Smith, 1966a B30
A. menziesii (Lehm.) Nels. & Macbr. Roitman, 1983 C
Borago officinale L. Luthy et al. 1984 A
Conoclinium coelestinium (L.) DC Herz et al. 1981 C
Eupatorium compositifolium Walt. Herz et al. 1981 C
Symphytum aspera Roitman, 1981 A
S. officinale Linn. Roder et al. 1982 C
S. x uplandicum Nyman Culvenor et al. 1980a, 1980b A, A
Trichodesma africana Zalkow et al. 1979 B419
Alkaloid Plant Sources Reference Reference
Location
Isocromadurine Crotalaria candicans W. and A. Suri et al. 1982 C
C. madurensis R. Wight ]Rao et al. 1975c B317
Isoline Senecio othonniformis Fourcade Coucourakis & Gordon-Gray, 1970; B224
Coucourakis et al. 1972 B225
Jacobine Crassocephalum crepidioides Asada et al. 1985 A
Senecio alpinus L. Luthy et al. 1981 A
S. brasiliensis DC. Adams & Gianturco, 1956e B148
S. cineraria DC. Barger & Blackie, 1937 B167
S. jacobaea L. Manske, 1931; Blackie, 1937; B194, 153
Bradbury & Culvenor, 1954 B198
S. paludosus L. Blackie, 1937; Dorosh & Alekseev, 1960 B153, 227
Alekseev, 1961b B228
Jacoline Crassocephalum crepidioides Asada et al. 1985 A
Senecio alpinus L. Luthy et al. 1981 A
S. jacobaea L. Bradbury & Culvenor, 1954 B198
Jaconine Senecio alpinus L. Luthy et al. 1981 A
S. jacobaea L. Bradbury & Culvenor, 1954 B198
Jacozine Senecio alpinus L. Luthy et al. 1981 A
S. cannabifolius Less Asada et al. 1982 C
S.jacobaea L. Bradbury & Culvenor, 1954; Culvenor, B198,
1964 202
Junceine Crotalaria juncea L. Adams & Gianturco, 1956a, 1956b, 1956c B305,
B306
B307
C. wightiana Grah. ex Wight & Arn. Atal et al. 1966b B329
Alkaloid Plant Sources Reference Reference
Location
Lasiocarpine Heliotropium arbainense Zalkow et al. 1979 B419
H. arborescens L. Carcamo-Marquez, 1961 B52
H. curassavicum Linn. Rajagopalan & Batra, 1977b B56
H. digynum Forssk. Hammouda et al. 1984 A
H. eichwaldii Steud. ex DC. Rao et al. 1974 B62
H. europaeum L. Culvenor et al. 1954 B65
H. indicum Linn. Hoque et al. 1976 B72
H. lasiocarpum Fisch. et C. Mey. Men'shikov, 1932 B73
H. maris mortui Zalkow et al. 1979 B419
H. suaveolens Bieb. Guner, 1986 A
H. supinum L. Pandey et al. 1983 C
Lappula intermedia Man'ko & Vasil'kov, 1968 B85
Symphytum caucasicum Man'ko et al. 1969 B95
S. officinale Linn. Man'ko et al. 1969, 1970a B95, 101
Latifoline Cynoglossum latifolium R. Br. Crowley & Culvenor, 1962 B37
Hackelia floribunda Hagglund et al. 1985 A
Ligudentine Ligularia brachyphylla Hand.-Mazz. Klasek et al. 1971 B134
L. dentata (A. Gray) Hara. Klasek et al. 1971 B134
Ligularidine Ligularia dentata (A. Gray) Hara Hikichi et al. 1979, Asada & B436, C
Furuya, 1984a
Ligularine Ligularia brachyphylla Hand.-Mazz. Klasek et al. 1971 B134
L. dentata (A. Gray) Hara. Klasek et al. 1971 B134
L. elegans Cass. Klasek et al. 1971 B134
Ligularizine Ligularia dentata (A. Gray) Hara. Asada & Furuya, 1984a C
Alkaloid Plant Sources Reference Reference
Location
Lycopsamine Amsinckia hispida (Ruiz et Pav.) Culvenor & Smith, 1966a B30
I.M. Johnston
A. intermedia Fisch. et C. Mey. Culvenor & Smith, 1966a B30
A. lycopsoides Lehm. Culvenor & Smith, 1966a B30
A. menziesii (Lehm.) Nels. & Macbr. Roitman, 1983a C
Anchusa officinalis L. Broch-Due & Aasen, 1980 B32
Borago officinale L. Larson et al. 1984; Luthy et al. 1984 C, A
Eupatorium compositifolium Walt. Herz et al. 1981 C
Heliotropium steudneri Vatke Schneider et al. 1975 B8O
Messerschmidia sibirica Hikichi et al. 1980 C
Parsonsia eucalyptophylla F. Muell. Edgar & Culvenor, 1975 B28
P. straminea (R. Br.) F. Muell. Edgar & Culvenor, 1975 B28
Prestonia sp. Edgar, 1985 A
Symphytum asperum Lepech. Roitman, 1981 A
Symphytum officinale Linn. Huizing & Malingre, 1981 C
Symphytum x uplandicum Nyman Culvenor et al. 1980a, 1980b A, A
Madurensine Crotalaria agatiflora Schweinf. Atal et al. 1966a B280
C. laburnifolia L. subsp. eldomae Crout, 1972 B312
C. madurensis R. Wight Atal et al. 1966a; Mahran et al. 1979 B280, 426
Merenskine Senecio latifolius DC. Bredenkamp et al. 1985 C
Monocrotaline Crotalaria aegyptiaca Benth. Mahran et al. 1979; Zalkow et al. 1979 B426, 419
C. assamica Benth. Crotalaria Research Group, 1974 B286
C. burhia Rao et al. 1975 B293
C. cephalotes Steud. ex A. Rich Pilbeam et al. 1983 C
C. crispata F. Muell. ex Benth. Culvenor & Smith, 1963 B294
C. cunninghamii R. Br. Pilbeam et al. 1983 C
C. grahamiana R. Wight ex Walk.-Arn. Gandhi et al. 1966b; Atal et al. 1969 B298, 299
C. leschenaultii DC. Suri & Atal, 1967 B313
Alkaloid Plant Sources Reference Reference
Location
C. leiloba Bartl. Puri et al. 1974 B314
C. mitchellii Benth. Culvenor et al. 1967b B318
C. mysorensis Roth. Sawhney & Atal, 1968 B319
C. nitens Kunth. Hoet et al. 1981 A
C. novae-hollandiae DC. subsp. Culvenor et al. 1967b B318
lasiophylla (Benth.) A. Lee
C. paulina schrank. Pilbeam et al. 1983 C
C. quinquefolia L. Pilbeam et al. 1983 C
C. recta Steud. ex A. Rich Crout, 1968a B326
C. retusa L. Adams & Rogers, 1939; Culvenor & B327,
Smith, 1957a 328
C. sagittalis L. Willette & Cammarata, 1972 B330
C. spectabilis Roth. Neal et al. 1935; Adams & Rogers, B325,
1939 327
C. stipularia Desv. Puri et al. 1974 B314
Lindelofia spectabilis Lehm. Rao et al. 1974 B62
Monocrotalinine Crotalaria grahamiana Wight et Arn. Rajagopalan & Batra, 1977a B300
Myoscorpine Myosotis scorpioides L. Resch et al. 1982 C
Symphytum officinale Linn. Resch et al. 1982C C
Neoligularidine Ligularia dentata (A. Gray) Hara. Asada & Furuya, 1984a C
Neopetasitenine Ligularia japonica Asada et al. 1981 A
Petasites japonicus Maxim. Yamada et al. 1976a B19
Neosenkirkine Senecio auricola Bourg. Panizo & Rodriguez, 1974 B157
S. grandifolius Less. Bohlmann et al. 1986 A
S. pierotii Asada & Furuya, 1982 C
Neotriangularine Senecio triangularis Hook. Roitman, 1983b C
Alkaloid Plant Sources Reference Reference
Location
Nilgirine Crotalaria pallida Ait. Atal et al. 1968 B322
Onetine Senecio othonnae Bieb. Danilova et al. 1962 B221
Otosenine Cacalia floridana Cava et al. 1968 B119
Doronicum macrophyllum Alieva et al. 1976 B122
D. pardalianches Linn. Rajagopalan & Negi, 1985 A
Emilia flammea Cass. Kohlmunzer & Tomczyk, 1969 B123
Senecio aegypticus L. Klasek et al. 1968a B145
S. aureus L. Resch et al. 1983, Roder et al. 1983 C, C
S. cineraria DC. Habib, 1974 B170
S. desfontainei Druce Haddad et al. 1963; Klasek B173,
et al. 1968a 145
S. erraticus Berthol. Gaiduk et al. 1974 B177
S. erraticus subsp. barbaraeifolius Krock. Santavy, 1958; Schroter & Santavy, 1960 B178, 179
S. fluviatilis Wallr. Klasek et al. 1973b B185
S. jacobaea L. Akramov et al. 1968 B51
S. othonnae Bieb. Zhdanovich & Men'shikov, 1941; B220
Danilova et al. 1962 B221
S. renardii Winkl. Danilova & Konovalova, 1950 B249
S. tomentosus Adams et al. 1956; Schroter & B271,
Santavy, 1960 179
Parsonsine Parsonsia heterophylla A. Cunn. Eggers & Gainsford, 1979; Edgar et al. B417,
1980 418
P. spiralis Edgar et al. 1980 B418
Petasinine Petasites japonicus Maxim. Yamada et al. 1978b B141
Petasitenine Farfugium japonicum Kitam Niwa et al. 1985 A
Petasites japonicus Maxim. Yamada et al. 1976a, 1976b; B19, 139
Furuya et al. 1976 B20
Alkaloid Plant Sources Reference Reference
Location
Retroisosenine Senecio nemorensis L. var. bulgaricus Nghia et al. 1976 B219
(Vel.) Stoj. et Stef.
S. nemorensis L. var subdecurrens Klasek et al. 1980a B422
Retrorsine Crotalaria spartioides DC. Bruemmerhoff & de Waal, 1961 B334
C. zanzibarica Benth. (C. usaramoensis) Culvenor & Smith, 1966b B337
Senecio ambrosioides Adams & Gianturco, 1956e B148
S. ampullaceus Hook. Adams & Govindachari, 1949b; Adams & B149, 151
Looker, 1951; Warren et al. 1950 B150
S. bipinnatisectus Belcher White, 1969 B158
S. brasiliensis DC. Motidome & Ferreira, 1966a B163
S. bupleuroides DC. Sapiro, 1949 B164
S. cineraria DC. Klasek et al. 1975 B171
S. cruentus DC. Asada et al. 1982 C
S. cymbaroides Roitman et al. 1979 B420
S. desfontainei Druce Rizk et al. 1983 A
S. discolor DC. Schoental, 1960; Hennig, 1961 B174, 175
S. douglasii DC. Adams & Govindachari, 1949b; Adams B149,
& Looker, 1951 151
S. eremophilus Richards Adams & Govindachari, 1949b; Adams B149,
& Looker, 1951 151
S. erucifolius L. Ferry & Brazier, 1976 B184
S. filaginoides (H. et A.) DC. Pestchanker & Giordano, 1986 A
S. formosus Munoz Quevedo, 1976 B186
S. gilliesiano Guidugli et al. 1986 A
S. glaberrimus DC. Blackie, 1937 B153
S. glandulosus Don ex Hook. et Arn. Pestchanker et al. 1985b A
S. graminifolius N.J. Jacq. de Waal, 1941 B188
S. griesbachii Motidome & Ferreira, 1966b B190
S. ilicifolius Thunb. de Waal, 1940a, 1940b, 1941; Culvenor B191, 192
& Smith, 1954 B188, 127
Alkaloid Plant Sources Reference Reference
Location
S. inaequidens DC. Roder et al. 1981 A
S. isatideus DC. Blackie, 1937; de Waal, 1939 B153, 193
S. jacobaea L. Ferry & Brazier, 1976 B184
S. latifolius DC. Watt, 1909; Barger et al. 1935 B211, 212
S. longilobus Benth. Adams & Govindachari, 1949b; Adams & B149, 151
Looker, 1951; Warren et al. 1950 B150
S. paucicalyculatus Klatt Pretorius, 1949 B230
S. phillipicus Roegel et Koern Gonzalez et al. 1986a A
S. pterophorus DC. de Waal, 1940b, 1941; Culvenor & B192, 188
Smith, 1954 127
S. quadridentatus Labill. Culvenor & Smith, 1955 B247
S. ragonesei Cabr. Pestchanker & Giordano, 1986 A
S. retrorsus DC. Manske, 1931; de Waal, 1939 B194, 193
S. riddellii Torr. et A. Gray Roitman et al. 1979 B420
S. riddellii Torr. et A. Gray var. Adams & Govindachari, 1949b B149
parksii Cory.
S. ruderalis Harvey Leisegang, 1950 B254
S. seratophiloides Griseb. Pestchanker & Giordano, 1986 A
S. spartioides Roitman et al. 1979 B420
S. subulatus Don ex Hook. et Arn Pestchanker et al. 1985b A
var erectus
S. swaziensis Compton Gordon-Gray et al. 1972; Gordon-Gray B268,
& Wells, 1974 270
S. triangularis Hook. Roitman, 1983b C
S. uspallatensis Pestchanker et al. 1985a A
S. venosus Harvey Blackie, 1937 B153
S. vernalis Waldst. et Kit. Roder et al. 1979b C
S. viminalis Bremek. de Waal & van Twisk, 1964 A
S. vulgaris L. Tschu Shun et al. 1960 B277
S. werneriaefolius Roitman et al. 1979 B420
Alkaloid Plant Sources Reference Reference
Location
Retusamine Crotalaria mitchellii Benth. Culvenor et al. 1967b B318
C. mitchellii Benth. subsp. laevis Culvenor et al. 1967b B318
A. Lee
C. novae-hollandiae DC. subsp. Culvenor et al. 1967b B318
lasiophylla Benth. A. Lee
C. novae-hollandiae DC. subsp. Culvenor et al. 1967b B318
novae-hollandiae
C. retusa L. Culvenor & Smith, 1957a; Wunderlich, B328, C
1962
Riddelliine Crotalaria juncea L. Adams & Gianturco, 1956a B305
Senecio aegypticus L. Klasek et al. 1968a B145
S. ambrosioides Roitman et al. 1979 B420
S. cruentus DC. Asada et al. 1982 C
S. cymbaroides Roitman et al. 1979 B420
S. desfontainei Druce Haddad et al. 1963; Klasek et al. B173,
1968a 145
S. douglassi DC. Adams & Govindachari, 1949b; Adams & B149,
Looker, 1951 151
S. eremophilus Richards Adams & Govindachari, 1949b; Adams & B149,
Looker, 1951 151
S. longilobus Benth. Adams & Govindachari, 1949b; Adams & B149,
Looker, 1951; 151
Warren et al. 1950 B150
S. riddellii Torr. et A. Gray Manske, 1939a; Adams et al. 1942c B156, 252
S. riddellii Torr. et A. Gray var. Adams & Govindachari, 1949b B149
parksii (Cory)
S. spartioides Roitman et al. 1979 B420
S. vernalis Walst. et Kit. Sener et al. 1986 A
S. vulgaris Roitman et al. 1979 B420
Alkaloid Plant Sources Reference Reference
Location
Rinderine Eupatorium altissimum L. Herz et al. 1981 C
E. serotinum Michx. Locock et al. 1966 B131
Prestonia sp. Edgar, 1985 A
Rindera baldshuanica Kusnezov Akramov et al. 1961c B91
Solenanthus turkestanicus Regel Akramov et al. 1962 B94
et Smirnov
(Kusnezov)
Sceleratine Senecio latifolius DC. (S. sceleratus) de Waal & Pretorius, 1941; de Waal B257,
et al. 1963 260
Scorpioidine Myosotis scorpioides L. Resch et al. 1982 C
Sencalenine Senecio cacaliaster (Lam.) Roder et al. 1984b A
Senecicannabine Senecio cannabifolius Less. Asada et al. 1982 C
Senecionine Brachyglottis repanda Forst. et Forst. Mortimer & White, 1967 B117
Caltha biflora Stermitz & Adamovics, 1977 B341
C. leptosepala Stermitz & Adamovics, 1977 B341
Castilleja rhexifolia Rydb. Stermitz & Suess, 1978 B342
Castilleja "rhexifolia aff. miniata" Roby & Stermitz, 1984 C
Crotalaria juncea L. Adams & Gianturco, 1956a B305
C. micans Link. Sethi & Atal, 1964 B284
C. zanzibarica Benth. (C. usaramoensis) Culvenor & Smith, 1966b B337
Emilia sonchifolia DC. Culvenor, unpubl.
Erechtites hieracifolia (L.) Raf. ex DC. Manske, 1939b; Culvenor & Smith, 1954 B126, 127
Gynura segetum (Lour.) Merr. Hua et al. 1983; Liang & Roder, 1984 C, C
Ligularia japonica Asada et al. 1981 A
Petasites hybridus L. Luthy et al. 1983 C
P. laevigatus (Willd.) Reichenb. Massagetov & Kuzovkov, 1953 B142
Senecio aegypticus L. Klasek et al. 1968a; Gharbo & B145,
Habib, 1969 146
Alkaloid Plant Sources Reference Reference
Location
S. alpinus (L.) Scop. Luthy et al. 1981 A
S. ambrosioides Adams & Gianturco, 1956e B148
S. ampullaceus Hook. Adams & Govindachari, 1949b; Adams & B149,
Looker, 1951; 151
Warren et al. 1950 B150
S. argentino Baker (vira-vira Hieron) Pestchanker & Giordano, 1986 A
S. aureus L. Manske, 1936, 1939a B155, 156
S. brasiliensis DC. Fonseca, 1951; Novelli & De Varella, B162,
1945; Adams & Gianturco, 1956e 161
B148
S. carthamoides Greene Adams & Govindachari, 1949b; Adams B149,
& Looker, 1951 151
S. cineraria DC. Barger & Blackie, 1937; Adams & B167,
Govindachari, 1949a; 168
Alekseev et al. 1962a B169
S. congestus (R.Br.) DC. Roder et al. 1982 C
S. cruentus DC. Asada et al. 1982 C
S. cymbaroides Roitman et al. 1979 B420
S. desfontainei Druce Klasek et al. 1968a B145
S. discolor DC. Schoental, 1960; Hennig, 1961 B174, 175
S. douglasii DC. Adams & Govindachari, 1949b; Adams B149,
& Looker, 1951 151
S. eremophilus Richards Adams & Govindachari, 1949b; Adams B149,
& Looker, 1951 151
S. erraticus Berthol. Gaiduk et al. 1974 B177
S. erraticus Berthol. subsp. Santavy, 1958; Schroter & Santavy, B178,
barbaraeifolius Krock. 1960; Kompis et al. 1960 179
B181
S. erucifolius L. Kompis & Santavy, 1962 B183
S. filaginoides (H. et A.) DC. Pestchanker & Giordano, 1986 A
Alkaloid Plant Sources Reference Reference
Location
S. fistulosus Poepp. ex Less. Gonzalez et al. 1986b A
S. fremontii Torr. et A. Gray Adams & Gianturco, 1956e B148
S. gilliesiano Guidugli et al. 1986 A
S. glabellus Turcz. DC. Adams & Van Duuren, 1953b B187
S. ilicifolius Thunb. de Waal, 1940a, 1940b, 1941; Culvenor B191,
& Smith, 1954 192
B188,
127
S. illinitus Phill. Gonzalez et al. 1986a A
S. inaequidens DC. Roder et al. 1981 A
S. integerrimus Nutt. Manske, 1939a B156
S. jacobaea L. Bradbury & Mosbauer, 1956 B199
S. laricifolius H.B.K. Bohlmann et al. 1986 A
S. lautus Forst. f. ex Willd. Culvenor unpubl.
S. leucostachys Baker Pestchanker & Giordano, 1986 A
S. longiflorus Sch. Bip. de Waal & van Twisk, 1964 A
S. magnificus F. Muell. Culvenor, 1962 B215
S. multilobatus MaCoy et al. 1983 A
S. multivenius Benth. in Oerst Bohlmann et al. 1986 A
S. nebrodensis L. var. sicula Plescia et al. 1976 B218
S. nemorensis L. subsp. fuchsii Gmel. Wiedenfeld & Roder, 1979 B423
S. pampeanus Cabrera Novelli, 1958 B229
S. pancicii Degen var arnautorum Jizba et al. 1982 C
(Velen.) Stoj. Stef. et Kit.
S. pancicii Degen var pancicii Jizba et al. 1982 C
S. patagonicus Hook. and Arn. Villarroel et al. 1985 A
S. petasitis DC. Gharbo & Habib, 1969 B146
S. pimpinellifolius H.B.K. Bohlmann et al. 1986 A
S. pseudo-arnica Less. Manske, 1939a B156
S. pterophorus DC. de Waal, 1940b, 1941; Culvenor & B192,
Smith, 1954 188
B127
Alkaloid Plant Sources Reference Reference
Location
S. quadridentatus Labill. Culvenor & Smith, 1955 B247
S. sandrasicus Temizer et al. 1985 A
S. scandens Batra & Rajagopalan, 1977 B256
S. seratophiloides Griseb. Pestchanker & Giordano, 1986 A
S. spartioides Manske, 1939a; Adams & Gianturco, B156,
1957b 262
S. spathulatus A. Rich White, 1969 B158
S. squalidus L. Barger & Blackie, 1936; Kropman B264,
& Warren, 1950 265
S. subalpinus C. Koch Trivedi & Santavy, 1963 B267
S. subulatus Don ex Hook. et Arn Pestchanker et al. 1985b A
var erectus
S. tenuifolius Burm. Bhakuni & Gupta, 1982 C
S. tomentosus Adams et al. 1956 B271
S. triangularis Hook. Kupchan & Suffness, 1967 B272
S. uintahensis Roitman et al. 1979 B420
S. vernalis Waldst. et Kit. Roder et al. 1979b C
S. viminalis Bremek. de Waal & van Twisk, 1964 A
S. viscosus L. Barger & Blackie, 1936; Santavy et al. B264,
1962 182
S. vulgaris L. Grandval & Lajoux, 1895; Barger & B275,
Blackie, 1936; Konovalova & 264
Orekhov, 1937a; Tschu Shun B276
et al. 1960 B277
S. wernariaefolius Roitman et al. 1979 B420
Syneilesis palmata Maxim. Hikichi & Furuya, 1976 B279
Tussilago farfara Rosberger et al. 1981 C
7-Senecioyl-9-(2-hydroxy- Senecio caudatus DC. Bohlmann et al. 1986 A
3-acetylbutyrl)retronecine
Alkaloid Plant Sources Reference Reference
Location
7-Senecioyl-9-(2-hydroxy- Senecio caudatus DC. Bohlmann et al. 1986 A
methyl-2,3-dihydroxy-
butyrylretronecine
7-Senecioyl-9-(2-methyl- Senecio caudatus DC. Bohlmann et al. 1986 A
2,3-dihydroxybutyryl-
retronecine
7-Senecioylretronecine Senecio cacaliaster (Lam.) Roder et al. 1984b C
S. caudatus DC. Bohlmann et al. 1986 A
S. triangularis Hook. Rueger & Benn, 1983a C
S. variabilis Sch. Bip. Bohlmann et al. 1986 A
9-Senecioylretronecine Senecio caudatus DC. Bohlmann et al. 1986 A
S. variabilis Sch. Bip. Bohlmann et al. 1986 A
7-Senecioyl-9-sarracinyl- Senecio cacaliaster Roder et al. 1984b C
retronecine S. caudatus DC. Bohlmann et al. 1986 A
S. triangularis Hook. Rueger & Benn, 1983 C
S. ungeniensis Thell. Bohlmann et al. 1986 A
S. variabilis Sch. Bip. Bohlmann et al. 1986 A
Seneciphylline Adenostyles alliarae Yakhontova et al. 1976 B115
A. glabra Wiedenfeld et al. 1984 C
A. rhombifolius (Willd.) M. Pimen.subsp. Pimenov et al. 1975 B116
platyphylloides
Crotalaria juncea L. Adams & Gianturco, 1956a B305
Erechtites hieracifolia (L.) Raf. ex DC. Manske, 1939a; Culvenor & Smith, 1954 B126, 127
Senecio alpinus (L.) Scop. Klasek et al. 1968b; Luthy et al. 1981 B147, A
S. ambrosioides Adams & Gianturco, 1956e B148
Alkaloid Plant Sources Reference Reference
Location
S. ampullaceus Hook. Adams & Govindachari, 1949b; Adams & B149,
Looker, 1951; Warren et al. 1950 151
B150
S. aquaticus Hill Blackie, 1937; Evans & Evans, 1949 B153, 154
S. borysthenicus Red'ko, 1956; Alekseev, 1961a B159, 160
S. brasiliensis DC. Fonseca, 1951; Novelli & de Varella, B162,
161
1945; Adams & Gianturco, 1956e B148
S. cannabifolius Less. Alekseev, 1964; Asada et al. 1982 B165, C
S. carthamoides Greene Adams & Govindachari, 1949b; Adams & B149,
Looker, 1951 151
S. chrysanthemoides Wali & Handa, 1964 B166
S. cineraria DC. Barger & Blackie, 1937; Adams & B167,
Govindachari, 1949a 168
S. cruentus DC. Asada et al. 1982 C
S. cymbaroides Roitman et al. 1979 B420
S. desfontainei Druce Gharbo & Habib, 1969 B146
S. douglasii DC. Adams & Govindachari, 1949b; Adams & B149,
Looker, 1951 151
S. eremophilus Richards Adams & Govindachari, 1949b; Adams & B149,
Looker, 1951 151
S. erraticus Berthol. subsp. Kompis et al. 1960; Santavy B181,
barbaraeifolius Krock. et al. 1962 182
S. erucifolius L. Kompis & Santavy, 1962 B183
S. fluviatilis Wallr. Klasek et al. 1973b B185
S. fremontii Torr. et A. Gray Adams & Gianturco, 1956e B148
S. grandifolia Glonti, 1958 B189
S. ilicifolius Thunb. de Waal, 1940a, 1940b, 1941; B191, 192
Culvenor & Smith, 1954 B188,
127
Alkaloid Plant Sources Reference Reference
Location
S. incanus L. subsp. carniolus Klasek et al. 1968b B147
(Willd.) Br.-Bl.
S. jacobaea L. Blackie, 1937; Bradbury & B153,
Culvenor, 1954 198
S. krylovii Sapunova & Ban'kovskii, 1968 B207
S. kubensis Grossh. Khalilov & Telezhenetskaya, 1973a B208
S. lampsanoides Khalilov & Damirov, 1974 B210
S. laricifolius H.B.K. Bohlmann et al. 1986 A
S. latifolius DC. Danilova et al. 1960 B213
S. longiflorus Sch. Bip. de Waal & van Twisk, 1964 A
S. longilobus Benth. Adams & Govindachari, 1949b; Adams & B149,
Looker, 1951 151
S. minimus Poir. White, 1969 B158
S. multivenius Benth. in Oerst Bohlmann et al. 1986 A
S. othonnae Bieb. Zhdanovich & Men'shikov, 1941; B220
Danilova et al. 1962 B221
S. palmatus Pall. Alekseev, 1960 B226
S. paludosus L. Blackie, 1937; Dorosh & Alekseev, B153,
1960; 227
Alekseev, 1961b B228
S. pancicii Degen var arnautorum Jizba et al. 1982 C
(Velen.) Stoj. Stef. et Kit.
S. pancicii Degen var panicii Jizba et al. 1982 C
S. patagonicus Hook. and Arn. Villarroel et al. 1985 A
S. paucifolius S.G. Gmel. Alekseev & Ban'kovskii, 1965 B231
S. phillipicusRoegel et Koern Gonzalez et al. 1986a A
S. platyphylloides Somm. et Lev. Murav'eva, 1964b, 1965; Dauksha, 1970 B233, 234
B235
S. platyphyllus (Bieb.) DC. Orekhov, 1935; Konovalova & B236,
Orekhov, 1938; 237
Konovalova, 1951 B238
Alkaloid Plant Sources Reference Reference
Location
S. pojarkovae Chernova & Murav'eva, 1974 B243
S. propinquus Ait. Khalilov et al. 1972 B245
S. pterophorus DC. de Waal, 1940b, 1941; Culvenor & B192,
Smith, 1954 188
B127
S. quadridentatus Labill. Culvenor & Smith, 1955 B247
S. racemosus Khmel, 1961 B248
S. renardii Winkl. Danilova & Konovalova, 1950 B249
S. rhombifolius (Willd.) Sch. Bip. Khalilov & Telezhenetskaya, 1973a B208
S. scandens Batra & Rajagopalan, 1977 B256
S. spartioides Torr. et A. Gray Manske, 1939a; Adams & Gianturco, B156,
1957b 262
S. spathulatus Benn et al. 1979 B263
S. stenocephalus Maxim. Konovalova & Orekhov, 1937b B266
S. subalpinus C. Koch. Trivedi & Santavy, 1963; Klasek B267,
et al. 1968b 147
S. vernalis Walst. et Kit. Sener et al. 1986; Hartmann & A, A
Zimmer, 1986
S. vulgaris L. Barger & Blackie, 1936; Konovalova & B264,
Orenkhov, 1937a; 276
Tschu Shun et al. 1960 B277
Senecivernine Senecio inaequidens Bicchi et al. 1985 A
S. seratophylloides Griseb. Pestchanker & Giordano, 1986 A
S. vernalis Waldst. et Kit. Roder et al. 1979b C
Senkirkine Brachyglottis repanda Forst. et Forst. Mortimer & White, 1967 B117
Crotalaria laburnifolia subsp. eldomae Crout, 1972 B312
Farfugium japonicum Kitam. Furuya et al. 1971 B133
Petasites albus L. Luthy et al. 1983 C
P. hybridus L. Luthy et al. 1983 C
P. japonicus Maxim. Yamada et al. 1978a B140
Alkaloid Plant Sources Reference Reference
Location
P. laevigatus (Willd.) Reichenb. Massagetov & Kuzovkov, 1953 B142
Senecio antieuphorbium (L.) Sch. Bip. Rodriguez & Gonzalez, 1969 B152
S. desfontainei Druce Rizk et al. 1983 A
S. grandifolius Less. Bohlmann et al. 1986 A
S. illinitus Phill. Gonzalez et al. 1986a A
S. jacobaea L. Akramov et al. 1968 B51
S. kirkii Hook. f. ex Kirk. Briggs et al. 1948; Briggs et al. 1965 B203, 204
S. kleinia Sch. Bip. Rodriguez et al. 1967 B206
S. laricifolius H.B.K. Bohlmann et al. 1986 A
S. pierotii Asada & Furuya, 1982 C
S. procerus L. var. procerus Stoj. Jovceva et al. 1978 B244
Stef. et Kit.
S. quebradensis Greenm. Bohlmann et al. 1986 A
S. renardii Winkl. Danilova & Konovalova, 1950; Briggs B249,
et al. 1965 204
S. tenuifolius Burm. Bhakuni & Gupta, 1982 C
S. vernalis Waldst. et Kit. Roder et al. 1979b C
S. uintahensis Roitman et al. 1979 B420
Tussilago farfara Culvenor et al. 1976b; Borka & A, B425,
Onshuus, 1979; Luthy et al, 1980 A
Sincamidine Amsinckia intermedia Fisch. et C. Mey. Culvenor & Smith, 1966a B30
Spartioidine Senecio spartioides Torr. et A. Gray Manske, 1939a; Adams & Gianturco, 1957 B156,
262
S. vulgaris L. Pieters & Vlietinck, 1986 A
Spectabiline Crotalaria spectabilis Roth Culvenor & Smith, 1957b B336
Spiracine Parsonsia spiralis Wall. Edgar et al. 1980 B418
Spiraline Parsonsia spiralis Wall. Edgar et al. 1980 B418
Alkaloid Plant Sources Reference Reference
Location
Spiranine Parsonsia spiralis Wall. Edgar et al. 1980 B418
Supinine Borago officinale L. Luthy et al. 1984 A
Eupatorium cannabinum L. Pederson, 1975a B128
E. serotinum Michx. Locock et al. 1966 B131
E. stoechadosmum Hanse Furuya & Hikichi, 1973 B132
Heliotropium europeum L. Culvenor, 1954 B66
H. indicum L. Hoque et al. 1976 B72
H. supinum L. Men'shikov & Gurevich, 1949; Crowley & B81, 83
Culvenor, 1959
Tournefortia sarmentosa Lam. Crowley & Culvenor, 1955 B107
Trichodesma zeylanicum (Burm. f.) R. Br. O'Kelly & Sargeant, 1961 B113
Swazine Senecio barbellatus DC. Gordon-Gray & Wells, 1974 B270
S. swaziensis Compton Gordon-Gray et al. 1972; Laing & B268,
Sommerville, 1972 269
Symlandine Symphytum asperum Lepech. Roitman, 1981 A
S. officinale Linn. Roder et al. 1982 C
S. tuberosum L. Gray et al. 1983 A
S. x uplandicum Nyman Culvenor et al. 1980a, 1980b A, A
Symphytine Myosotis scorpioides L. Resch et al. 1982 C
Symphytum aspera Roitman, 1981 A
S. officinale Linn. Furuya & Araki, 1968; Furuya & B15, 16
Hikichi, 1971
S. peregrinum Ledeb. Gadella et al. 1983 A
S. x uplandicum Nyman Culvenor et al. 1980a, 1980b A, A
Syneilesine Syneilesis palmata Maxim. Hikichi & Furuya, 1974, 1976 B278, 279
Alkaloid Plant Sources Reference Reference
Location
Triangularine Alkanna tinctoria Tausch Roder et al. 1984b C
Senecio triangularis Hook. Roitman, 1983b C
Trichodesmine Crotalaria globifera E. Mey. Brown et al. 1984 C
C. juncea L. Adams & Gianturco, 1956a B305
C. lunata Beddome ex Polhill Rothschild et al. 1979 C
C. recta Steud ex A. Rich. Crout, 1968a B326
C. wightiana Grah. ex Wight & Arn. Atal et al. 1966b B329
C. tetragona Roxb. Puri et al. 1974 B314
Heliotropium arguzioides Kar. et Kir. Akramov et al. 1961aB54
Trichodesma incanum Alph. DC. Men'shikov & Rubinstein, 1935; B108
Men'shikov, 1936; Yunusov & B109,
Plekhanova, 1957, 1959; B111, 112
Tashkhodzhaev et al. 1979 C
Uluganine Ulugbeckia tschimganica (B.Fedtsch.) Zak. Khasanova et al. 1974 B114
Uplandicine Symphytum x uplandicum Nyman Culvenor et al. 1980a, 1980b A, A
Usaramine Crotalaria brevidens Benth. var. Suri et al. 1975b B304
intermedia (Kotschy) Polhill
C. brevifolia Sawhney et al. 1967 B291
C. incana L. Sawhney & Atal, 1970a B303
C. pallida Ait. Sawhney et al. 1967 B291
C. zanzibarica Benth. (C. usaramoensis) Culvenor & Smith, 1966b B337
Senecio glandulosus Don ex Hook. et Arn. Pestchanker et al. 1985b A
S. seratophylloides Griseb Pestchanker & Giordano, 1986 A
S. vulgaris L. Pieters & Vlietinck, 1986 A
Alkaloid Plant Sources Reference Reference
Location
Uspallatine Senecio argentino Baker (vira-vira Hieron) Pestchanker et al. 1985a A
S. leucostachys Baker Pestchanker & Giordano, 1986 A
S. seratophiloides Griseb Pestchanker & Giordano, 1986 A
S. uspallatensis Pestchanker et al. 1985a A
Yamataimine Cacalia yatabei Maxim. Hikichi et al. 1978 B121
A - References in this publication.
B - References in Smith & Culvenor, J. Nat. Prod., 44, 129-152 (1981), with reference number.
C - References in Mattocks, Chemistry and Toxicology of Pyrrolizidine Alkaloids, 1986.
APPENDIX II
Table 1. Plants containing hepatotoxic pyrrolizidine alkaloids
------------------------------------------------------------------------------------------
Plant Constituent alkaloids Plant part Referencea
------------------------------------------------------------------------------------------
Apocynaceae
Fernaldia pandurata loroquin root B 29
(syn. Urechites karwinsky
Mueller)
Parsonsia eucalyptophylla lycopsamine aeriel B 28
(F. Muell.)
Parsonsia heterophylla parsonsine whole B 417
A. Cunn. heterophylline B 418
Parsonsia spiralis Wall. heterophylline leaf B 418
parsonsine
spiracine
spiranine
spiraline
Parsonsia straminea (R. Br.) lycopsamine aerial B 28
F. Muell.
Parsonsia estonia sp. echinatine A Edgar (1985)
Boraginaceae
Alkanna tinctoria Tausch 7-angelylretronecine C Roder et al.
dihydroxytriangularine (1984b)
triangularine
Amsinckia hispida (Ruiz intermedine whole B 30
et Pav.) M. Johnston lycopsamine
echiumine
Amsinckia intermedia Fisch intermedine whole B 30
et C. Mey lycopsamine
echiumine
sincamidine
echimidine
Amsinckia lycopsoides Lehm. intermedine whole B 30
lycopsamine
echiumine
Amsinckia menziesii (Lehm.) 7-acetyllycopsamine aerial C Roitman (1983a)
Nels and Macbr. 3'-acetyllycopsamine
diacetyllycopsamine
lycopsamine
intermedine
------------------------------------------------------------------------------------------
APPENDIX II Table 1. (contd.)
------------------------------------------------------------------------------------------
Plant Constituent alkaloids Plant part Referencea
------------------------------------------------------------------------------------------
Boraginaceae (contd.)
Anchusa arvensis ( L.) Bieb. echinatine whole B 31
(or diastereoisomer)
Anchusa officinalis L. 7-acetyllycopsamine whole B 31
(or diastereoisomer)
lycopsamine B 32
Asperugo procumbens L. supinine (or whole B 31
diastereoisomer)
lycopsamine (or
diastereoisomer)
Borago officinalis L. lycopsamine aerial, root C Larson et al.
amabiline (1984)
supinine A Luthy et al.
intermedine (1984)
acetylintermedine
acetyllycopsamine
thesinine seed, flower
Cynoglossum amabile Stapf amabiline whole B 33, 34
& Drummond echinatine
Cynoglossum australe R. Br. cynaustine whole B 33
cynaustraline
heliosupine
Cynoglossum creticum heliosupine aerial B 419
echinatine
Cynoglossum glochidiatum amabiline whole B 36
Wall. ex Lindl.
Cynoglossum lanceolatum cynaustraline whole B 36
Forsk cynaustine
Cynoglossum latifolium latifoline aerial B 37
R. Br. 7-angelylretronecine
Cynoglossum officinale L. heliosupine aerial B 38, 39, 40
echinatine root, aerial B 41, 42
acetylheliosupine aerial B 43
7-angelylheliotridine
Cynoglossum pictum Ait. heliosupine root, aerial B 44, 45
echinatine
pictumine aerial B 46
------------------------------------------------------------------------------------------
APPENDIX II Table 1. (contd.)
------------------------------------------------------------------------------------------
Plant Constituent alkaloids Plant part Referencea
------------------------------------------------------------------------------------------
Boraginaceae (contd.)
Cynoglossum viridiflorum viridiflorine root B 47
Pallas ex Lehm. heliosupine B 34
Echium plantagineum L. echiumine aerial B 48
(Echium lycopsis L.) echimidine
Echium vulgare L. heliosupine aerial B 49
asperumine aerial B 50
echinatine (or whole B 31
diastereoisomer)
Hackelia floribunda latifoline A Hagglund et al.
(1985)
Heliotropium acutiflorum heliotrine aerial B 51
Heliotropium arbainense heliotrine aerial B 419
europine
lasiocarpine
Heliotropium arborescens L. lasiocarpine aerial B 52
(Heliotropium peruvianum L.)
Heliotropium arguzioides heliotrine aerial B 53
Kar. et Kir. trichodesmine aerial, root B 54, 55
Heliotropium curassavicum heliotrine whole B 56
Linn. lasiocarpine
angelylheliotridine
curassavine aerial B 57
heliovicine
trachelanthamidine B 58
acetylcurassavine aerial C Mohanraj et al.
heliocurassavinine (1982)
heliocurassavine
heliocoromandaline
heliocurassavicine
curassanecine
curassavinine
coromandalinine
heliovinine
Heliotropium dasycarpum heliotrine aerial, root B 54
Ledeb. seed B 59
------------------------------------------------------------------------------------------
APPENDIX II Table 1. (contd.)
------------------------------------------------------------------------------------------
Plant Constituent alkaloids Plant part Referencea
------------------------------------------------------------------------------------------
Boraginaceae (contd.)
Heliotropium digynum heliotrine A Hammouda et al.
(Heliotropium luteum) lasiocarpine (1984)
europine
angelylheliotrine
Heliotropium eichwaldi heliotrine whole B 60, 61
Steud. ex DC. lasiocarpine aerial B 62
7-angelylheliotrine aerial B 63
Heliotropium europaeum heliotrine whole B 64, 65
lasiocarpine
europine whole B 66, 67
supinine
heleurine
N-dihydropyrrolizino- whole B 68
methyl-
heliotrine chloride
acetyllasiocarpine whole B 69
Heliotropium indicum L. indicine aerial B 70
acetylindicine aerial B 71
indicinine
echinatine aerial B 72
supinine
heleurine
lasiocarpine
Heliotropium lasiocarpum heliotrine aerial B 73
Fisch. et Mey. lasiocarpine
europine A Culvenor et al.
heleurine (1986)
Heliotropium maris-mortui europine aerial B 74
lasiocarpine aerial B 419
Heliotropium olgae heliotrine aerial, root B 75
Heliotropium popovii H. heliotrine seed B 77
Riedl. subsp. gillianum
H. Riedl.
Heliotropium ramosissimum heliotrine aerial B 78, 79
(Lehm.) Dec. (syn. heleurine
Heliotropium persicum L., supinine
Heliotropium undulatum) lasiocarpine
Heliotropium rotundifolium europine aerial B 74
------------------------------------------------------------------------------------------
APPENDIX II Table 1. (contd.)
------------------------------------------------------------------------------------------
Plant Constituent alkaloids Plant part Referencea
------------------------------------------------------------------------------------------
Boraginaceae (contd.)
Heliotropium steudneri Vatke lycopsamine leaf B 80
Heliotropium suaveolens Bieb. heliotrine aerial A Guner (1986)
lasiocarpine
europine
echinatine
Heliotropium supinum L. supinine root B 81
heliosupine root B 82
echinatine whole B 83
7-angelylheliotridine
7-angelylheliotridine
viridiflorate
7-angelylheliotridine
trachelanthate
heliotrine seed, leaf C Pandey et al.
lasiocarpine (1983)
Heliotropium transoxanum heliotrine aerial B 51
Lappula glochidiata echinatine aerial B 84
Lappula intermedia lasiocarpine aerial B 85
Lindelofia angustifolia echinatine aerial B 62, 36
(Schrenk) Brand. amabiline
Lindelofia spectabilis echinatine aerial B 62, 63
Lehm. 7-acetylechinatine
monocrotaline
Lindelofia stylosa viridiflorine aerial B 86
(Kar. et Kir.) Brand echinatine seed B 75
lindelofine
Lindelofia tschimganica carategine aerial B 87
echinatine
viridiflorine
Lithospermum officinale L. acetylechimidinyl- whole B 31
retronecine
(or diastereoisomer)
Messerschmidia sibirica lycopsamine whole C Hikichi et al.
angelylretronecine (1980)
------------------------------------------------------------------------------------------
APPENDIX II Table 1. (contd.)
------------------------------------------------------------------------------------------
Plant Constituent alkaloids Plant part Referencea
------------------------------------------------------------------------------------------
Boraginaceae (contd.)
Myosotis scorpioides L. 7-acetylscorpioidine aerial C Resch et al.
(syn. Myosotis scorpioidine (1982)
palustris L.) symphytine
myoscorpine
Paracynoglossum imeritinum heliosupine aerial, root B 88
(Kusn.) M. Pop. echinatine B 89, 90
Rindera austroechinata echinatine whole, seed B 87
M. Pop.
Rindera baldshuanica rinderine aerial B 91
Kusnezov echinatine aerial B 87
trachelanthamine
turkestanine
Rindera cyclodonta Bge. echinatine aerial B 59
Rindera echinata Regel echinatine aerial B 92
trachelanthamine aerial B 59
Rindera oblongifolia carategine aerial B 87
M. Pop. echinatine
turkestanine
Solenanthus circinnatus echinatine seed, aerial, B 93
Ledeb. root
Solenanthus coronatus echinatine aerial B 75
Solenanthus karateginius carategine aerial B 93
Lipsky echinatine
Solenanthus turkestanicus rinderine aerial B 94
turkestanine
Symphytum asperum Lepech. asperumine aerial, root B 95, 96
echinatine aerial, root B 97
acetylheliosupine whole B 31
(or diastereoisomer)
7-acetyllycopsamine leaf, root C Roitman (1981)
intermedine
symlandine
7-acetylintermedine
symphytine
lycopsamine
echimidine
angelylechimidine root A Gadella et al.
(or diastereoisomer) (1983)
------------------------------------------------------------------------------------------
APPENDIX II Table 1. (contd.)
------------------------------------------------------------------------------------------
Plant Constituent alkaloids Plant part Referencea
------------------------------------------------------------------------------------------
Boraginaceae (contd.)
Symphytum caucasicum Bieb. lasiocarpine aerial, root B 95
asperumine aerial, root B 98, 99
echinatine
echimidine
Symphytum officinale Linn. symphytine root B 15, 16
echimidine B 100
lasiocarpine aerial, root B 95
heliosupine aerial, root B 101
viridiflorine root
echinatine root
acetylechimidine B 31
(or diastereoisomer)
7-acetyllycopsamine aerial, root A Huizing et al.
lycopsamine (1981)
intermedine
7-acetylintermedine
symlandine C Roder et al.
myoscorpine root (1982a)
C Resch et al.
(1982)
Symphytum orientale anadoline whole B 102, 103, 104
symphytine
echimidine
Symphytum peregrinum Ledeb. echimidine root A Gadella et al.
symphytine (1983)
Symphytum tuberosum L. echimidine whole B 105
anadoline
symlandine root A Gray et al.
(1983)
Symphytum x uplandicum Nyman lycopsamine aerial B 31, 17, 106
intermedine
uplandicine
7-acetyllycopsamine
7-acetylintermedine
echimidine
symphytine
symlandine
angelylechimidine root A Gadella et al.
(or diastereoisomer) (1983)
Tournefortia sarmentosa Lam. supinine leaf, stem B 107
------------------------------------------------------------------------------------------
APPENDIX II Table 1. (contd.)
------------------------------------------------------------------------------------------
Plant Constituent alkaloids Plant part Referencea
------------------------------------------------------------------------------------------
Boraginaceae (contd.)
Trichodesma africana intermedine aerial B 419
europine
Trichodesma incanum Alph. trichodesmine aerial B 108, 109
DC. incanine seed, aerial, B 110, 111, 112
root
Trichodesma zeylanicum supinine seed B 113
(Burm. f) R. Br.
Ulugbekia tschimganica uluganine B 114
Compositae
Adenostyles alliariae platyphylline root B 115
seneciphylline
Adenostyles glabra seneciphylline C Wiedenfeld et al.
(1984)
Adenostyles rhombifolius platyphylline aerial B 116
(Willd.) M. Pimen. ssp. seneciphylline
platyphylloides
Brachyglottis repanda senecionine aerial B 117
Forst. et Forst. senkirkine
brachyglottine B 118
Cacalia floridana (= Senecio otosenine aerial B 119
floridanus Sch. Bip.) florosenine
floridanine
floricaline
Cacalia hastata L. subsp. integerrimine root B 120
orientalis Kitamura
Cacalia yatabei Maxim yamataimine root B 121
Conoclinium coelestinium intermedine C Herz et al. (1981)
(L.) DC
Crassocephalum crepidioides jacobine aerial A Asada et al.
jacoline (1985)
Doronicum macrophyllum otosenine root B 122
floridanine
doronine
------------------------------------------------------------------------------------------
APPENDIX II Table 1. (contd.)
------------------------------------------------------------------------------------------
Plant Constituent alkaloids Plant part Referencea
------------------------------------------------------------------------------------------
Compositae (contd.)
Doronicum pardalianches otosenine root A Rajagopalan & Negi
Linn. (1985)
Echinacea angustifolia DC. tussilagine whole C
isotussilagine
Echinacea purpurea M. tussilagine whole C
isotussilagine
Emilia flammea Cass. otosenine aerial, root B 123, 124, 125
emiline
Erechtites hieracifolia (L.) senecionine aerial B 126, 127
Raf. ex DC. seneciphylline
Eupatorium altissimum L. rinderine C Herz et al. (1981)
angelylheliotridine
Eupatorium cannabinum L. echinatine aerial B 128
supinine
amabiline A Luthy et al.
(1984)
Eupatorium compositifolium intermedine C Herz et al. (1981)
Walt. lycopsamine
Eupatorium maculatum L. echinatine root B 129
trachelanthamidine
Eupatorium purpureum probably echinatine aerial B 130
Eupatorium serotinum Michx. supinine aerial B 131
rinderine
Eupatorium stoechadosmum lindelofine root B 132
Hance supinine
Farfugium japonicum Kitam. senkirkine root, leaf B 133
farfugine whole C Niwa et al. (1983)
petasitenine whole A Niwa et al. (1985)
Gynura scandens O. Hoffm. gynuramine C Wiedenfeld (1982)
acetylgynuramine
Gynura segetum (Lour.) Merr. senecionine C Liang & Roder
(1984)
------------------------------------------------------------------------------------------
APPENDIX II Table 1. (contd.)
------------------------------------------------------------------------------------------
Plant Constituent alkaloids Plant part Referencea
------------------------------------------------------------------------------------------
Compositae (contd.)
Ligularia brachyphylla clivorine aerial B 134
Hand.-Mazz. ligularine
ligudentine
Ligularia clivorum clivorine aerial B 135, 136,
137, 138
Ligularia dentata clivorine aerial B 134
(A. Gray) Hara ligularine
ligudentine
ligularidine whole B 436
ligularinine aerial, root C Asada & Furuya
ligularizine (1984a)
neoligularidine
Ligularia elegans (Cass.) clivorine aerial B 134
[syn. Ligularia macrophylla ligularine
(Ledeb. DC.)]
Ligularia japonica senecionine root A Asada et al.
neopetasitenine (1981)
platyphylline
Petasites albus L. senkirkine aerial C Luthy et al.
(1983)
Petasites hybridus L. senecionine aerial C Luthy et al.
integerrimine (1983)
senkirkine
Petasites japonicus Maxim. petasitenine aerial B 20, 19, 139
(fukinotoxin)
neopetasitenine
senkirkine stem B 140
petasinine aerial B 141
petasinoside
Petasites laevigatus (Willd.) platyphylline aerial B 142
Reichenb. [syn. Nardosmia senkirkine (renardine) aerial B 143, 144
laevigata (Willd.) DC.] senecionine
Senecio abrotanifolius doronine aerial A Roder et al.
ssp. abrotanifolius doronenine (1984a)
bulgarsenine
Senecio abrotanifolius doronine aerial A Roder et al.
ssp. abrotanifolius var. doronenine (1984a)
tiroliensis bulgarsenine
------------------------------------------------------------------------------------------
APPENDIX II Table 1. (contd.)
------------------------------------------------------------------------------------------
Plant Constituent alkaloids Plant part Referencea
------------------------------------------------------------------------------------------
Senecio aegypticus L. senecionine whole B 145, 146
otosenine aerial
riddelliine
erucifoline
Senecio alpinus (L.) Scop. seneciphylline aerial B 147
jacozine
jacobine whole A Luthy et al.
integerrimine (1981)
jacoline
senecionine
jaconine
Senecio ambrosioides retrorsine whole B 148
(= Senecio brasiliensis seneciphylline
Less.) senecionine
riddelliine whole B 420
Senecio ampullaceus Hook. senecionine whole B 149, 150, 151
seneciphylline
retrorsine
Senecio antieuphorbium integerrimine aerial B 152
(L.) Sch. Bip. senkirkine
Senecio aquaticus Hill seneciphylline aerial B 153, 154
Senecio aureus L. senecionine aerial B 155, 156
otosenine aerial C Resch et al.
floridanine (1983)
florosenine C Roder et al.
(1983)
Senecio auricola Bourg. neosenkirkine aerial B 157
Senecio barbellatus DC. swazine B 270
retrorsine
Senecio bipinnatisectus retrorsine aerial, root B 158
Belcher (syn.
Erechtites atkinsoniae)
Senecio borysthenicus seneciphylline aerial, root B 159, 160
(= Senecio prealtus
Berthol.)
Senecio brasiliensis senecionine leaf B 161, 162
DC. (syn. seneciphylline B 148, 163
Senecio ambrosioides) jacobine
integerrimine
retrorsine
------------------------------------------------------------------------------------------
APPENDIX II Table 1. (contd.)
------------------------------------------------------------------------------------------
Plant Constituent alkaloids Plant part Referencea
------------------------------------------------------------------------------------------
Compositae (contd.)
Senecio brasiliensis Less integerrimine A Nardi et al.
var tripartitus (1980)
Senecio bupleuroides DC. retrorsine aerial B 164
Senecio cacaliaster (Lam.) sencalenine A Roder et al.
bulgarsenine (1984b)
7-senecioylretrone-
cine
7-senecioyl-9-sarra-
cinoylretronecine
Senecio cannabifolius Less. seneciphylline aerial B 165
senecicannabine aerial, root C Asada et al.
jacozine (1982a)
Senecio carthamoides Greene senecionine whole B 149, 151
seneciphylline
Senecio caudatus DC. 7-senecioylretrone- aerial A Bohlmann et al.
cine (1986)
9-senecioylretrone-
cine
7-senecioyl-9-sarra-
cinylretronecine
7-senecioyl-9-(2-
methyl-2,3-dihyroxy-
butyryl)retronecine
7-senecioyl-9-(2-
methy-2-hydroxy-3-
acetoxybutyryl)
retronecine
retronecine
2-senecioyl-(-)-
macronecine
9-senecioyl-(-)-
macronecine
senecicaudatine-9-
senecioate
senecidaudatine-9-
isovalerate
norsenecicaudatine-
9-sencioate
senecicaudatinal
semiacetal
Senecio chrysanthemoides seneciphylline B 166
------------------------------------------------------------------------------------------
APPENDIX II Table 1. (contd.)
------------------------------------------------------------------------------------------
Plant Constituent alkaloids Plant part Referencea
------------------------------------------------------------------------------------------
Compositae (contd.)
Senecio cineraria DC. jacobine aerial B 153, 167
senecionine seed B 168
seneciphylline aerial B 169
otosenine aerial B 170
retrorsine aerial B 171
Senecio congestus (R. Br.) senecionine C Roder et al.
DC. [syn. Senecio palustris neoplatyphylline (1982b)
(L.) Hooker, Senecio platyphylline
tubicaulis Mansfeld)
Senecio cruentus DC. senecionine C Asada et al.
seneciphylline (1982b)
retrorsine
riddelliine
Senecio cymbaroides senecionine whole B 420
seneciphylline
riddelliine
retrorsine
Senecio desfonainei Druce senecionine aerial B 173, 145
otosenine
riddelliine
seneciphylline aerial B 146
retrorsine A Rizk et al. (1983)
senkirkine
angelylretronecine
Senecio discolor DC. retrorsine leaf B 174
senecionine aerial B 175
Senecio dolichodoryius 15,20-dihyroxyeruci- aerial A Bohlmann et al.
Cuatr. foline (1986)
Senecio doronicum L. bulgarsenine leaf B 176
doronenine
Senecio douglasii DC. retrorsine whole B 149, 150, 151
riddelliine
seneciphylline
senecionine
Senecio durieui Gay integerrimine whole B 157
Senecio eremophilus senecionine aerial B 149, 150, 151
Richards seneciphylline
retrorsine
riddelliine
------------------------------------------------------------------------------------------
APPENDIX II Table 1. (contd.)
------------------------------------------------------------------------------------------
Plant Constituent alkaloids Plant part Referencea
------------------------------------------------------------------------------------------
Compositae (contd.)
Senecio erraticus Berthol. senecionine aerial B 177
otosenine
floridanine
Senecio erraticus Berthol. senecionine B 178, 179, 180
subsp. barbaraeifolius Krock otosenine
erucifoline
seneciphylline leaf B 181
integerrimine aerial B 182
Senecio erucifolius L. senecionine aerial B 153
seneciphylline aerial B 183, 180
erucifoline
retrorsine aerial B 184
Senecio faberi Hemsl. integerrimine C Wei et al. (1982)
Senecio filaginoides senecionine root A Pestchanker &
(H. et A.) DC. retrorsine Giordano (1986)
Senecio fistulosus senecionine A Gonzalez et al.
Poepp. ex Less. (1986b)
Senecio fluviatilis Wallr. seneciphylline aerial B 185
otosenine
florosenine
Senecio formosus integerrimine aerial B 186
retrorsine
Senecio fremontii Torr. seneciphylline whole B 148
et A. Gray senecionine
Senecio gilliesiano senecionine root A Guidugli et al.
retrorsine (1986)
Senecio glabellus senecionine whole B 187
(Turcz.) DC.
Senecio glaberrimus DC. retrorsine aerial B 153
Senecio glandulosus integerrimine root A Pestchanker et al.
Don ex Hook. et Arn. retrorsine (1985b)
usaramine
Senecio graminifolius retrorsine aerial B 188
N.J. Jacq. graminifoline
------------------------------------------------------------------------------------------
APPENDIX II Table 1. (contd.)
------------------------------------------------------------------------------------------
Plant Constituent alkaloids Plant part Referencea
------------------------------------------------------------------------------------------
Compositae (contd.)
Senecio grandifolia Jaqu. platyphylline root, leaf, B 189
seneciphylline stem
Senecio grandifolius Less. senkirkine aerial A Bohlmann et al.
neosenkirkine (1986)
Senecio griesbachii Baker retrorsine aerial B 190
Senecio ilicifolius Thunb. senecionine aerial B 191, 192, 188, 127
seneciphylline
retrorsine
Senecio illinitus Phill. senkirkine aerial A Gonzalez et al.
O-acetylsenkirkine (1986a)
senecionine
Senecio inaequidens DC. retrorsine A Roder et al.
senecionine (1981)
senecivernine aerial A Bicchi et al.
integerrimine (1985)
Senecio incanus L. seneciphylline aerial B 147
subsp. carniolicus integerrimine
(Willd.) Br.-Bl.
Senecio integerrimus Nutt. integerrimine aerial B 156
senecionine
neoplatyphylline whole B 420
platyphylline
Senecio isatideus DC. retrorsine aerial B 153, 193
Senecio jacobaea L. seneciphylline aerial B 194, 195
senecionine B 196, 197
jacobine B 153, 197, 198
jaconine B 199, 200, 201
jacozine
otosenine aerial B 51
senkirkine
retrorsine aerial B 184
Senecio kirkii Hook. f. senkirkine bark, leaf B 203
ex Kirk O-acetylsenkirkine leaf B 204
Senecio kleinia Sch. Bip. integerrimine stem B 205
senkirkine stem B 206
Senecio krylovii seneciphylline aerial B 207
------------------------------------------------------------------------------------------
APPENDIX II Table 1. (contd.)
------------------------------------------------------------------------------------------
Plant Constituent alkaloids Plant part Referencea
------------------------------------------------------------------------------------------
Compositae (contd.)
Senecio kubensis Grossh. seneciphylline aerial B 208
Senecio lampsanoides seneciphylline aerial, root B 209, 210
Senecio laricifolius senecionine aerial A Bohlmann et al.
H.B.K. seneciphylline (1986)
senkirkine
19-hydroxysenkirkine
19-acetoxysenkirkine
Senecio latifolius DC. retrorsine aerial B 211, 212
(syn. Senecio sceleratus seneciphylline aerial B 213
Schweikerdt) platyphylline
sceleratine aerial B 260
chlorodeoxysceleratine aerial B 261;
(merenskine) C Bredenkamp et al.
(1985)
Senecio leucostachys Baker senecionine root A Pestchanker &
Giordano (1986)
Senecio longilobus Benth. seneciphylline whole B 156, 214, 150
retrorsine B 151
riddelliine whole B 149
Senecio magnificus F. Muell. senecionine aerial B 215
integerrimine B 216
Senecio megaphyllus Green. 13,19-epoxyseneci- aerial A Bohlmann et al.
phylline (1986)
13,19-epoxysparti-
oidine
Senecio minimus Poir seneciphylline aerial B 158
------------------------------------------------------------------------------------------
APPENDIX II Table 1. (contd.)
------------------------------------------------------------------------------------------
Plant Constituent alkaloids Plant part Referencea
------------------------------------------------------------------------------------------
Compositae (contd.)
Senecio morrisonensis Hayata integerrimine whole B 217
Senecio multilobatus senecionine A McCoy et al.
(1983)
Senecio multivenius seneciphylline aerial A Bohlmann et al.
Benth. in Oerst. senecionine (1986)
Senecio nebrodensis L. integerrimine whole B 218
var sicula senecionine
Senecio nemorensis L. bulgarsenine leaf B 219
var bulgaricus retrosisosenine
(Vel) Stoj. nemorensine
Senecio nemorensis L. ssp. fuchsisenecionine B 362, 363, 364, 365
fuchsii Gmel.
senecionine B 423
Senecio nemorensis L. nemorensine aerial, root B 371
subdecurrens Griseb. retroisosenine aerial B 422
bulgarsenine
Senecio othonnae Bieb. otosenine aerial, root B 220
onetine root B 221
seneciphylline
floridanine aerial, root B 222
doronine aerial B 223
Senecio othonniformis bisline aerial B 22, 225
Fourcade isoline
Senecio palmatus Pall. seneciphylline root B 226
Senecio paludosus L. seneciphylline root, aerial B 153, 227, 228
Senecio pampeanus Cabrera senecionine aerial B 229
Senecio pancicii Degen senecionine whole C Jizba et al.
var arnautorum (Velen.) seneciphylline (1982)
Stoj., Stef. et Kit.
Senecio pancicii Degen senecionine whole C Jizba et al.
var pancicii (1982)
Senecio patagonicus senecionine aerial A Villaroel et al.
Hook. and Arn. seneciphylline (1985)
------------------------------------------------------------------------------------------
APPENDIX II Table 1. (contd.)
------------------------------------------------------------------------------------------
Plant Constituent alkaloids Plant part Referencea
------------------------------------------------------------------------------------------
Compositae (contd.)
Senecio paucicalyculatus paucicaline whole B 230
(Klatt.) retrorsine
Senecio paucifolius seneciphylline B 231
S.G. Gmel.
Senecio petasitis DC. senecionine leaf B 146
bisline (?) aerial B 232
Senecio phillipicus retrorsine aerial A Gonzalez et al.
Rogel et Koern. (1986a)
Senecio pierotii neosenkirkine aerial, root C Asada & Furuya
senkirkine (1982)
Senecio pimpinellifolius senecionine aerial A Bohlmann et al.
H.B.K. (1986)
Senecio platyphylloides platyphylline root B 233, 234, 235
Somm. et Lev. seneciphylline
Senecio platyphyllus platyphylline root, aerial B 236, 237, 238
(Bieb.) DC. seneciphylline leaf B 239
neoplatyphylline root B 240, 241
sarracine root B 242
Senecio pojarkovae sarracine root B 243
seneciphylline
Senecio procerus L. senkirkine aerial, root B 244
var procerus procerine
Stoj. Stef. et Kit.
Senecio propinquus Ait. seneciphylline aerial, root B 209, 245
Senecio pseudo-arnica Less. senecionine aerial B 156
Senecio pterophorus senecionine aerial B 192, 188, 127
seneciphylline
retrorsine
rosmarinine
acetylseneciphylline
Senecio quadridentatus senecionine aerial B 247
Labill. (syn. Erechtites seneciphylline
quadridentata DC.) retrorsine
------------------------------------------------------------------------------------------
APPENDIX II Table 1. (contd.)
------------------------------------------------------------------------------------------
Plant Constituent alkaloids Plant part Referencea
------------------------------------------------------------------------------------------
Compositae (contd.)
Senecio quebradensis senkirkine aerial A Bohlmann et al.
Greenm. florosenine (1986)
Senecio racemosus DC. seneciphylline root B 248
Senecio renardii Winkl. seneciphylline aerial B 249, 250
senkirkine (renardine)
otosenine
Senecio retrorsus DC. retrorsine aerial B 194, 193
Senecio rhombifolius sarracine root B 251, 233
(Willd.) Sch. Bip. platyphylline aerial, root B 208
seneciphylline
neoplatyphylline
Senecio riddellii riddelliine aerial B 156
Torr. et A. Gray whole B 252, 253
retrorsine B 420
Senecio riddellii retrorsine whole B 149
Torr. et A. Gray riddelliine
var. parksii (Cory)
Senecio ruderalis Harvey retrorsine aerial B 254
Senecio ruwenzoriensis ruwenine whole B 255
S. Moore ruzorine
Senecio sandrasicus senecionine A Temizer et al.
(1985)
Senecio scandens senecionine whole B 256
seneciphylline
Senecio seratophiloides senecionine root A Pestchanker &
Griseb. senecivernine Giordano (1986)
usaramine
retrorsine
uspallatine
Senecio spartioides seneciphylline aerial B 156, 262
Torr. et A. Gray senecionine
spartiodine
riddelliine whole B 420
retrorsine
------------------------------------------------------------------------------------------
APPENDIX II Table 1. (contd.)
------------------------------------------------------------------------------------------
Plant Constituent alkaloids Plant part Referencea
------------------------------------------------------------------------------------------
Compositae (contd.)
Senecio spathulatus senecionine aerial, root B 158
A. Rich. integerrimine
seneciphylline B 263
Senecio squalidus L. senecionine aerial B 153, 264, 265
integerrimine B 205
Senecio stenocephalus seneciphylline aerial B 266
Maxim.
Senecio subalpinus senecionine leaf B 267
C. Koch. seneciphylline aerial B 147
integerrimine
Senecio subulatus dihydroretrosine root A Pestchanker et
Don ex Hook. retrorsine al. (1985b)
et Arn. var. erectus senecionine
Senecio swaziensis retrorsine aerial B 268, 269, 270
Compton swazine
Senecio tenuifolius senecionine aerial C Bhakuni & Gupta
Burm. integerrimine (1982)
senkirkine
o-acetylsenkirkine
Senecio tomentosus senecionine aerial B 271, 179
otosenine
(tomentosine)
Senecio triangularis senecionine aerial B 272
Hook. integerrimine C Roitman (1983b)
platyphylline
rosmarinine
retrorsine
triangularine
neotriangularine
7-angelylretronecine whole C Rueger & Benn
7-senecioylretrone- (1973)
cine
7-angelyl-9-sarra-
cinylretronecine
7-senecioyl-9-sarra-
cinylretronecine
Senecio uintahensis senkirkine whole B 420
senecionine
------------------------------------------------------------------------------------------
APPENDIX II Table 1. (contd.)
------------------------------------------------------------------------------------------
Plant Constituent alkaloids Plant part Referencea
------------------------------------------------------------------------------------------
Compositae (contd.)
Senecio umgeniensis 7-senecioyl-9-sarra- aerial A Bohlmann et al.
Thell. cinylretronecine (1986)
Senecio usgorensis 13,19-epoxyseneci- aerial A Bohlmann et al.
Cuatr. phylline (1986)
Senecio uspallatensis retrorsine root A Pestchanker et
uspallatine al. (1985a)
Senecio variabilis Sch. 7-senecioylretrone- aerial, root A Bohlmann et al.
Bip. cine (1986)
9-senecioylretrone-
cine
7-senecioyl-9-sarra-
cinylretronecine
Senecio venosus Harvey retrorsine aerial B 153
Senecio vernalis Walst. retrorsine aerial B 273, 274
et Kit. senecionine
senkirkine
senecivernine
integerrimine A Sener et al.
seneciphylline (1986)
riddelliine
retronecine
Senecio viscosus L. senecionine aerial B 264, 153
integerrimine aerial B 182
Senecio vulgaris L. senecionine aerial B 275, 264, 153
seneciphylline aerial B 155, 276, 277
retrorsine
riddelliine whole B 420
integerrimine A Pieters &
spartioidine Vlietinck (1986)
usaramine
Senecio senecionine whole B 420
werneriaefolius retrorsine
Syneilesis palmata syneilesine aerial, root B 278, 279
Maxim. acetylsyneilesine
senecionine
------------------------------------------------------------------------------------------
APPENDIX II Table 1. (contd.)
------------------------------------------------------------------------------------------
Plant Constituent alkaloids Plant part Referencea
------------------------------------------------------------------------------------------
Compositae (contd.)
Tussilago farfara L. senkirkine flower B 18, 425
leaf, stem C Rosberger et al.
(1981)
senecionine leaf, stem A Luthy et al.
(1980)
tussilagine C Roder et al.
(1981b)
Leguminosae
Crotalaria aegyptica crosemperine B 419
Benth. monocrotaline
7beta-hydroxy-1- B 426
methylene-8alpha-
pyrrolizidine
Crotalaria agatiflora maduraensine aerial B 280
Schweinf. anacrotine aerial B 281
7-acetylmadurensine
6-acetylanacrotine
7-acetyl-cis-
madurensine
6-acetyl-trans-
anacrotine
crotaflorine
6-angelyl-trans-
anacrotine
Crotalaria assamica Benth. monocrotaline B 285, 286
Crotalaria axillaris Ait. axillarine seed B 287, 288
axillaridine
Crotalaria barbata R. Graham crobarbatine seed B 289
ex R. Wight et Walk.-Arn.
Crotalaria berteroana DC. fulvine aerial B 297
(syn. Crotalaria fulva Roxb.)
Crotalaria brevidens integerrimine seed B 304
Benth. var. intermedia usaramine
(Kotschy) Polhill(syn.
Crotalaria intermedia
Kotschy)
Crotalaria breviflora DC. integerrimine seed B 290, 291
usaramine
------------------------------------------------------------------------------------------
APPENDIX II Table 1. (contd.)
------------------------------------------------------------------------------------------
Plant Constituent alkaloids Plant part Referencea
------------------------------------------------------------------------------------------
Leguminosae (contd.)
Crotalaria burhia Ham. crotalarine aerial B 292, 293
ex Benth. monocrotaline
Crotalaria candicans crocandine seed B 380
W. and A. isocorcandine
isocromadurine seed C Suri et al.
crispatine (1982)
turneforcidine
cropodine seed C Haksar et al.
(1982)
Crotalaria cephalotes Steud. monocrotaline seed C Pilbeam et al.
ex A. Rich (1983)
Crotalaria crispata monocrotaline whole B 294
F. Muell. ex Benth. fulvine
crispatine
Crotalaria cunninghamii monocrotaline seed C Pilbeam et al.
R. Br. (1983)
Crotalaria dura dicrotaline aerial B 295, 296
J.M. Wood et Evans
Crotalaria fulva Roxb.
(see Crotalaria
berteroana DC.)
Crotalaria globifera E. Mey dicrotaline aerial B 295, 296
globiferine seed C Brown et al.
grantianine (1984)
grantaline
trichodesmine
Crotalaria grahamiana monocrotaline seed B 298
Wight & Arn. grahamine seed B 299
monocrotalinine whole B 300
Crotalaria incana L. integerrimine seed B 187
anacrotine aerial B 302
usaramine seed B 303
Crotalaria juncea L. senecionine seed B 305, 306, 307
seneciphylline
riddelliine
trichodesmine
junceine
------------------------------------------------------------------------------------------
APPENDIX II Table 1. (contd.)
------------------------------------------------------------------------------------------
Plant Constituent alkaloids Plant part Referencea
------------------------------------------------------------------------------------------
Leguminosae (contd.)
Crotalaria laburnifolia L. anacrotine seed B 308, 309, 310,
(crotalaburnine) 291, 311
Crotalaria laburnifolia L. madurensine aerial B 312
subsp. eldomae anacrotine
senkirkine
hydroxysenkirkine
crotafoline
Crotalaria leschenaultii monocrotaline seed B 313
Crotalaria leiloba Bartl. monocrotaline seed B 314
(syn. Crotalaria
ferruginea Wall.)
Crotalaria madurensis madurensine seed, flower, B 280
R. Wight leaf
crispatine aerial B 315
fulvine
cromadurine seed B 62, 316
isocromadurine seed B 317
Crotalaria micans Link. 1-methylenepyrroliz- seed B 282, 283
(syn. Crotalaria anagyroides idine seed B 284
Humb. et al.) senecionine B 280
anacrotine
Crotalaria mitchellii Benth. monocrotaline whole B 318
retusamine whole
Crotalaria mitchellii Benth. retusamine whole B 318
subsp. laevis A. Lee,
published as
"sp. aff. mitchellii")
Crotalaria mysorensis Roth. monocrotaline seed B 319
Crotalaria nana Burm. crotananine seed B 320
cronaburmine seed B 427
Crotalaria nitens Kunth. monocrotaline A Hoet et al.
(1981)
Crotalaria novae-hollandiae monocrotaline whole B 318
DC. subsp. lasiophylla retusamine
(Benth) A. Lee
------------------------------------------------------------------------------------------
APPENDIX II Table 1. (contd.)
------------------------------------------------------------------------------------------
Plant Constituent alkaloids Plant part Referencea
------------------------------------------------------------------------------------------
Leguminosae (contd.)
Crotalaria novae-hollandiae retusamine seed B 318
DC. subsp. novae-hollandiae
(syn. Crotalaria
crassipes Hook.)
Crotalaria pallida Ait. usaramine seed B 291
(syn. Crotalaria mucronata, nilgirine seed B 322
Crotalaria striata) crotastriatine seed B 323, 324
Crotalaria paniculata Willd. fulvine seed B 325
Crotalaria paulina Schrank monocrotaline seed C Pilbeam et al.
(1983)
Crotalaria quinquefolia L. monocrotaline seed C Pilbeam et al.
(1983)
Crotalaria recta Steud. monocrotaline aerial B 326
ex A. Rich
Crotalaria retusa L. monocrotaline seed B 327
retusine seed, aerial B 328
retusamine
retronecine
Crotalaria sagittalis L. monocrotaline seed B 330
Crotalaria scassellatii axillaridine seed A Wiedenfeld et
Chiov. axillarine al. (1985)
deoxyaxillarine
Crotalaria semperflorens crosemperine seed B 331
Vent.
Crotalaria spartioides DC. retrorsine aerial B 334
Crotalaria spectabilis Roth. monocrotaline seed B 335, 227
(syn. Crotalaria sericea spectabiline seed, whole B 336
Retz)
Crotalaria stipularia Desv. monocrotaline seed B 314
Crotalaria tetragona Roxb. integerrimine seed B 314
trichodesmine
Crotalaria verrucosa L. anacrotine seed B 338
crotaverrine seed B 339
acetylcrotaverrine
------------------------------------------------------------------------------------------
APPENDIX II Table 1. (contd.)
------------------------------------------------------------------------------------------
Plant Constituent alkaloids Plant part Referencea
------------------------------------------------------------------------------------------
Leguminosae (contd.)
Crotalaria virgulata grantianine seed B 301, 259
subsp. grantiana (Harv.) grantaline C Smith & Culvenor
Polhill (syn. Crotalaria 1-hydroxymethyl- (1984)
grantiana Harv.) 1beta,2beta-epoxy-
pyrrolizidine
Crotalaria walkeri Arn. crotaverrine seed B 340
acetylcrotaverrine
Crotalaria wightiana Grah. junceine seed B 329
ex Wight & Arn. (syn. trichodesmine
Crotalaria rubiginosa Willd.
var. wightiana J.G. Baker)
Crotalaria zanzibarica integerrimine seed B 187
Benth. (syn. Crotalaria usaramine seed B 337
usaramoensis E.G. Baker) senecionine
retrorsine
Ranunculaceae
Caltha biflora DC. senecionine aerial B 341
Caltha leptosepala DC. senecionine aerial, root B 341
Scrophulariaceae
Castilleja rhexifolia senecionine B 342
Rydb. sarracine C Roby & Stermitz
indicine or isomer (1984)
------------------------------------------------------------------------------------------
a A = References in the reference list of this document.
B = References in Smith & Culvenor (1981), J. nat. Prod., 44: 129-152 (with reference
number).
C = References in Mattocks (1986), Chemistry and toxicology of pyrrolizidine alkaloids.
APPENDIX II
Table 2. Plants containing known alkaloids that are non-hepatotoxic (aminoalcohols and esters)
------------------------------------------------------------------------------------------
Plant Constituent alkaloids Plant part Referencea
------------------------------------------------------------------------------------------
A. Families in which hepatotoxic alkaloids also occur
Apocynaceae
Alafia multiflora alafine seed B 343
Anodendron affine Druce alloanodendrine aerial B 344, 345
anodendrin
Boraginaceae
Caccinea glauca Savi 7,9-dibenzoylretrone- fl B 346
cine
Ehretia aspera Willd. ehretinine leaf A Suri et al.
(1980)
Heliotropium angiospermum 1-hydroxymethyl- whole C Birecka et al.
Murray 1beta,2beta-epoxy- (1983)
pyrrolizidine
Heliotropium ovalifolium heliofoline whole C Mohanraj et al.
Forsk retronecine (1981)
Heliotropium spathulatum acetylcurassavine aerial A Birecka et al.
Rydb. curassavine (1980)
Heliotropium strigosum Willd. strigosine aerial B 347
Lindelofia macrostyla (Bunge) lindelofine aerial B 348
M. Pop. (syn. Lindelofia lindelofamine
anchusoides,Paracaryum
heliocarpum Kern.)
Lindelofia olgae (Regel et viridiflorine aerial B 94
Smirnov) Brand
Lindelofia pterocarpa (Rupr.) viridiflorine aerial B 93
M. Pop.
Macrotomia echioides Boiss. macrotomine aerial B 350
Paracaryum himalayense viridiflorine aerial B 93
(Klotsch) C.B. Clark
Tournefortia sibirica L. turneforcine aerial B 351
Trachelanthus hissoricus viridiflorine leaf B 352
Lipsky trachelanthamine
------------------------------------------------------------------------------------------
APPENDIX II Table 2. (contd.)
------------------------------------------------------------------------------------------
Plant Constituent alkaloids Plant part Referencea
------------------------------------------------------------------------------------------
Boraginaceae (contd.)
Trachelanthus korolkovii trachelanthamine aerial B 353, 354, 355, 94
(Lipsky) B. Fedtsch.
Celastraceae
Bhesa archboldiana 9-angelylretronecine bk B 356
(Merr. & Perry) Ding Hou
[syn. Kurrimia archboldiana
(Merr. & Perry)]
Compositae
Adenostyles rhombifolius sarracine aerial B 116
(Willd.) M. Pimen. ssp.
rhombifolia chemovar.
sarracinifera
Adenostyles rhombifolius platyphylline aerial B 116
(Willd.) M. Pimen. ssp.
rhombifolia chemovar.
platyphyllinifera
Cacalia hastata L. hastacine root B 357, 241
Cacalia robusta hastacine B 358
Senecio amphibolus macrophylline aerial B 359
Senecio angulatus L. angularine whole B 360
rosmarinine
Senecio aronicoides hygrophylline whole B 420
Senecio brachypodus DC. rosmarinine aerial, root B 361
Senecio francheti Winkl. sarracine aerial B 352
franchetine
Senecio glastifolius sarracine A Mortimer & White
(1975)
Senecio hygrophyllus R.A. platyphylline aerial B 366, 361
Dyer et C.A. Smith (syn. rosmarinine
Senecio adnatus DC.) hygrophylline
Senecio macrophyllus Bieb. macrophylline aerial B 368
Senecio mikanioides Otto. ex sarracine aerial B 155, 369, 370
Walp.
------------------------------------------------------------------------------------------
APPENDIX II Table 2. (contd.)
------------------------------------------------------------------------------------------
Plant Constituent alkaloids Plant part Referencea
------------------------------------------------------------------------------------------
Compositae (contd.)
Senecio nemorensis L. ssp. nemorensine aerial B 371
fuchsii var. nova (Zlatnik)
Senecio nemorensis L. ssp. nemorensine aerial B 371
jaquinianus (Rchb.) Durand
Senicio ovirensis ssp. angelylheliotridine A Roder et al.
gaudinil (1980)
Senecio pauciligulatus rosmarinine aerial B 361
Dyer et Sm.
Senecio rivularis DC. 7-angelylheliotridine aerial B 182, 135
Senecio rosmarinifolius Linn. rosmarinine aerial B 191, 192, 188
Senecio salignus DC. 7-angelylheliotridine aerial A Bohlmann et al.
(1986)
Senecio sarracenius L. sarracine aerial B 153, 372, 373
Senecio schvetsovii Korsh macrophylline aerial B 231
Senecio sylvaticus L. silvasenecine aerial B 362, 153
sarracine aerial B 374
Senecio taiwanensis Hayata rosmarinine aerial B 217
Senecio tournefortii Lap. platyphylline aerial B 375
Leguminosae
Crotalaria albida Heyne ex croalbidine aerial B 376, 377
Roth (syn. Crotalaria montana
Roxb.)
Crotalaria aridicola Domin. 1-methoxymethyl-1,2- aerial B 378
dehydropyrrolizidine
7beta-hydroxy-1-
methoxymethyl-1,2-
dehydropyrrolizidine
7beta-acetoxy-1- whole B 379
methoxymethyl-1,2-
dehydropyrrolizidine
Crotalaria damarensis Engl. 1-methylenepyrrolizi- whole, seed B 381, 283
dine
------------------------------------------------------------------------------------------
APPENDIX II Table 2. (contd.)
------------------------------------------------------------------------------------------
Plant Constituent alkaloids Plant part Referencea
------------------------------------------------------------------------------------------
Leguminosae (contd.)
Crotalaria goreensis Guill. 7beta-hydroxy-1- aerial, seed B 382
et Perr. methylene-8beta-
pyrrolizidine
7beta-hydroxy-1-
methylene-8alpha-
pyrrolizidine
Crotalaria grandistipulata 1-methylenepyrrolizi- seed B 434
Harms dine
Crotalaria lachnophora 1-methylenepyrrolizi- seed B 434
A. Rich dine
Crotalaria maypurensis Humb 7beta-hydroxy-1- aerial B 383
et al. methylene-8beta-
pyrrolizidine
7beta-hydroxy-1-
methylene-8alpha-
pyrrolizidine
Crotalaria medicaginea Lam. 1-methoxymethyl-1,2- whole, seed B 378
dehydropyrrolizidine
7beta-hydroxy-1-
methoxymethyl-1,2-
dehydropyrrolizidine
1alpha-methoxymethyl- whole, seed
1beta,2beta-
epoxypyrrolizidine
7alpha-hydroxy-1- seed
methoxymethyl-1,2-
dehydropyrrolizidine
1alpha-hydroxymethyl- aerial
1beta,2beta-
epoxypyrrolizidine
Crotalaria natalitia Meissner 1-methylenepyrrolizi- seed B 434
dine
Crotalaria podocarpa DC. 7-hydroxy-1-methylene- seed B 435
pyrrolizidine
Crotalaria stolzii (Bak. f.) 1-methylenepyrrolizi- seed B 434
Milne-Redh. ex Polhill dine
Ranunculacae laburnum L. laburnine seed B 387, 388, 389
1-hydroxymethyl-7-e B 390
hydroxypyrrolizidin
------------------------------------------------------------------------------------------
APPENDIX II Table 2. (contd.)
------------------------------------------------------------------------------------------
Plant Constituent alkaloids Plant part Referencea
------------------------------------------------------------------------------------------
Scrophulariacea
Castilleja "rhexifolia aff. sarracine C Roby & Stermitz
miniata" 7-angelylplatynecine (1984)
8-angelylplatynecine
B. Families in which hepatotoxic alkaloids are not known to occur
Orchidaceae
Chysis bractescens Lindl. 1alpha-methoxycar- whole B 391, 392
bonyl-8beta-
pyrrolizidine
1alpha-ethoxycar-
bonyl-8beta-
pyrrolizidine
Doritis pulcherrima phalaenopsine La or T whole B 393
(syn. Phalaenopsis esmerelda)
Hammarbya paludosa (L.) O.K. paludosine whole B 394
hammarbine whole B 395
Kingiella taenialis (Lindl.) phalaenopsine La whole B 396
Rolfe
Liparis auriculata (Blume) auriculine whole B 397
Liparis bicallosa Schltr. laburnine whole B 397
malaxine whole B 398, 399
Liparis hachijoensis Nakai laburnine whole B 397
malaxine whole B 398
Liparis keitaoensis Hay. keitaoine whole B 395
keitine
Liparis kumokiri F. Maekawa kumokirine whole B 400, 398
Liparis loeselii (L.) L.C. auriculine whole B 394
Rich
Liparis nervosa Lindl. nervosine whole B 398, 401
Malaxis congesta comb. nov. malaxin whole B 402
(Rchb. f.)
Malaxis grandifolia Schltr. grandifoline whole B 403
Phalaenopsis amabilis Bl. phalaenopsine T whole B 404, 405, 393
Phalaenopsis amboinensis phalaenopsine La whole B 393
------------------------------------------------------------------------------------------
APPENDIX II Table 2. (contd.)
------------------------------------------------------------------------------------------
Plant Constituent alkaloids Plant part Referencea
------------------------------------------------------------------------------------------
Orchidaceae (contd.)
Phalaenopsis aphrodite phalaenopsine T whole B 393
Phalaenopsis cornu-cervi cornucervine whole B 404, 393
Rchb. f.
Phalaenopsis equestris phalaenopsin ls whole B 393
Rchb. f. phalaenopsin T
Phalaenopsis fimbriata phalaenopsine T whole B 393
Phalaenopsis hieroglyfica phalaenopsine T or La whole B 393
Phalaenopsis lueddemanniana phalaenopsine T or La whole B 393
Phalaenopsis mannii Rchb. f. phalaenopsine La whole B 393
Phalaenopsis sanderiana phalaenopsine La whole B 393
Rchb. f. phalaenopsine T
Phalaenopsis schilleriana phalaenopsine La whole B 393
Phalaenopsis stuartiana phalaenopsine La whole B 393
Rchb. f. phalaenopsine T
Phalaenopsis sumatrana phalaenopsis La whole B 393
Phalaenopsis violacea phalaenopsis La or T whole B 393
Vanda cristata Lindl. acetyllaburnine whole B 407
Vanda helvola Bl. laburnine whole B 408
acetyllaburnine
Vanda hindsii Lindl. acetyllaburnine whole B 408
Vanda luzonica Loher acetyllaburnine whole B 408
Vandopsis gigantea Pfitz. laburnine whole B 408
lindelofidine
acetyllaburnine
acetyllindelofidine
Vandopsis lissochiloides laburnine whole B 408
Pfitz. lindelofidine
acetyllaburnine
acetyllindelofidine
------------------------------------------------------------------------------------------
APPENDIX II Table 2. (contd.)
------------------------------------------------------------------------------------------
Plant Constituent alkaloids Plant part Referencea
------------------------------------------------------------------------------------------
Rhizophoraceae
Cassipourea gummiflua Tulasne cassipourine stem, leaf B 409
var. verticellata Lewis bk B 410
Santalaceae
Thesium minkwitzianum thesine aerial B 411
B. Fedtsch. thesinine B 412, 413
thesinicine
isoretronecanol root
Sapotaceae
Mimusops elengi L. 1-hydroxymethyl- B 414
pyrrolizidine
tiglate
Planchonella anteridifera planchonelline leaf B 415
(C.T. White et W.D. Francis) tiglyllaburnine
H.J. Lamb benzoyllaburnine
Planchonella thyrsoidea C.T. planchonelline leaf B 415
White ex F.S. Walker tiglyllaburnine
benzoyllaburnine
Planchonella sp. (NGF 24722) trans-beta-thio- leaf B 416
acrylyl-(-)-iso-
retronecanol
tiglylisoretronecanol
------------------------------------------------------------------------------------------
a A = References in the reference list of this document.
B = References in Smith & Culvenor (1981), J. nat. Prod., 44: 129-152 (with reference
number).
C = References in Mattocks (1986), Chemistry and toxicology of pyrrolizidine alkaloids.
The onset of the disease may be sudden (acute) or insidious
(subacute or chronic). The acute disease may recover completely or
result in death. A few patients may go on to the subacute phase,
with almost none or very few symptoms, but a persistent
hepatomegaly. The patient may subsequently recover completely, or
may, after or without apparent clinical improvement, go on to the
chronic phase of disease, mostly ending up in cirrhosis. Some
patients with the acute disease may go on to the subacute phase,
even after clinical recovery, or as postulated by the authors,
after a latent period of several years, progress to cirrhosis.
Braginskii & Bobokhadzaev (1965) related an experience
concerning the evolution of this disease that was similar to that
observed in the USSR. About 50 - 60% of cases made a full recovery
and 35% made a partial recovery with continuing hepatomegaly.
About 2% of cases developed persistent ascites with "loss of
working capacity". It has been suggested that these cases may
develop cirrhosis.
7.2 Salient Pathological Features of Veno-Occlusive Disease
The pathological features of the disease at different stages
have been described in detail (Terekhov, 1939, 1952; Dolinskaya,
1952; Bras et al., 1954; Bras & Watler, 1955; Bras & Hill, 1956;
Stuart & Bras, 1957; Stirling et al., 1962; Aikat et al., 1978;
Tandon, B.N. et al., 1978; Tandon, H.D. et al., 1978). The
following description of Bras & Watler (1955) characterizes the
morphological changes at different stages of the disease described
by most investigators.
Morphological features of the liver at autopsy in 19 patients
from Jamaica who died at various stages of VOD have been described.
The ages ranged from 10 months to 45 years. Fourteen patients were
below the age of 14 years. Of the 14 patients, 9 had developed
cirrhosis, 3 died of acute disease, and 7 had various levels of
fibrosis superimposed over acute VOD. In the acute stages, there
was acute centrilobular congestion. The centrilobular and
sublobular veins showed different degrees of thickening of the wall
and occlusion of the lumen, mainly due to subintimal swelling
composed of loose reticular tissues and a few cells including
endothelial cells, occasional histocytes, lymphocytes, and
polymorphs, suggesting an acute exudative process. In addition,
there was a small amount of fibrin. Organization of this exudate
gradually led to collagenization and thickening of the wall. The
centrilobular congestion resulted in compression and even
disappearance of liver cell cords. The reticular framework of the
liver lobule was frequently preserved but was ruptured at places.
Clearly recognizable thrombi occurred sporadically but were not
commonly seen. The portal veins and hepatic arteries were normal.
Stirling et al. (1962) in their description of the early lesion
of VOD of the liver also emphasized that thrombosis of the hepatic
veins was not an important histogenetic factor in the evolution of
the lesion. In the later stages of the disease, hepatic venous
occlusion was chronically established. Perivenous reticular
collapse and the resultant condensation and reduplication of the
reticular framework resulted in non-portal fibrosis and later
cirrhosis. Macroscopically, the cirrhosis looked like Laennec's
cirrhosis. The cirrhotic process, which was non-portal to begin
with, involved portal tracts which also became incorporated in the
connective tissue septa. The presence of oesophageal varices was a
common finding. Extra-hepatic collateral vessels and congestive
splenomegaly were frequently seen. Within the liver, intrahepatic
collateral circulation was established with the coalescence of
sinusoids. The larger hepatic veins and inferior vena cava did not
show any thrombi or other pathological change. The subintimal
swelling observed in the hepatic veins was not seen in any other
vessels in the body. The authors concluded that acute VOD
gradually leads to Laennec's cirrhosis, but, in the initial phases,
it is non-portal.
No clear dose or temporal relationships between the liver and
parenchymal and vascular changes in the liver lobules are evident
from the available human case reports.
It is notable that megalocytosis, which is the hallmark of
chronic PA toxicity in experimental animals and a morphological
manifestation of the antimitotic effect of PAs, has not been
observed in human subjects (Tandon, H.D. et al., 1978) (section
6.4.9).
Tandon H.D. (personal communication) has analysed all published
and unpublished observations including liver biopsies and autopsies
derived from 3 outbreaks of VOD of which two occurred in India
(Tandon, B.N. et al., 1976, 1977; Tandon, R.K. et al., 1976;
Tandon, H.D. et al., 1977) and one occurred in Afghanistan (Tandon,
B.N. et al., 1978; Tandon, H.D. et al., 1978). He commented on the
frequency with which the characteristic veno-occlusion may not be
seen in the needle biopsy in the acute phase of the disease, though
it is invariably observed in the autopsy material. Haemorrhages
may persist for up to 1 year after subsidence of acute symptoms.
There is no significant inflammation accompanying the veno-
occlusive changes. Cirrhosis was reported to have developed within
3 months in one patient, after an initial biopsy had shown no
fibrosis (Stuart & Bras, 1957).
Brooks et al (1970) made an ultrastructural study of the liver
in VOD in Jamaican children. They found extensive damage in the
sinusoidal epithelium resulting in entra-vasation of red cells in
Disse's space and between hepatocytes. The closed structure of the
vessels, the absence of fenestrations, the existence of a basement
membrane, and the presence of collagen in the wall contribute to
the resistance to cellular debris and erythrocytes which track up
Disse's space and tend to narrow the lumen of the sinusoid where it
enters the vein. Fibrin may be present in this location and
occasionally in the sinusoids, but not within hepatic veins
themselves. The venous block, therefore, does not appear to be the
result of thrombosis.
Pancreatic changes similar to those in Kwashiorkor were stated
to be commonly observed (Bras & Hill, 1956; Stuart & Bras, 1957).
7.3 Human Case Reports of Veno-Occlusive Disease
Available data on human cases are summarized in Table 15, with
the countries they have been reported from in alphabetical order.
The first report of this disease in man and its relationship with
consumption of wheat flour contaminated by seeds of a plant of the
genus Senecio was made by Albertjin in 1918 in a report made to the
government of South Africa, as quoted by Wilmot & Robertson (1920),
who gave the first account of this disease in scientific literature
from that country. It is possible that it may have existed even
earlier, as its occurrence in farm animals had been described since
the beginning of the century in veterinary literature (Bull et al.,
1968). Willmot & Robertson (1920) recorded the occurrence of
'bread poisoning' in South Africa for a period of about 10 years,
during which 80 cases had been observed, mostly resulting in death.
The detailed account of clinical data and pathological findings in
11 cases was consistent with what is known as VOD today. The flour
from which the bread was prepared was found to be contaminated with
the flower heads and seeds of Senecio spp.
Isolated case reports are available in the literature (Hashem,
1939; Wurm, 1939) describing changes in childrens' liver
characteristic of veno-occlusive disease; however, in these
studies, no mention was made of possible etiological agents.
A pattern of disease described in the Soviet literature as
dystrophy of the liver, and later as toxic hepatitis with ascites
has been known in the Central Asian republics of the USSR
especially Uzbekistan and Tadjikistan for a long time (Terekhov,
1952). The local inhabitants called the disease, characterized by
rapidly filling ascites, the "camel-belly" syndrome and are
reported to have long distrusted a weed with fine seeds, known
locally as "Kharmyk", as a source of the disease (Dubrovinskii,
1952) (it is notable that, in the Afghan outbreak, which occurred
close to the area in Uzbekistan where the disease had been known,
the toxic seeds were known as "Charmak" among the local
population). The "camel-belly" disease was associated with the use
of bread with a bitter taste (presumably caused by the mixing and
grinding of toxic seeds with the wheat grains). Two waves of
outbreaks occurred, the first between 1931-35 (Terekhov, 1939) and
the second between 1945 - 46 (Dubrovinskii, 1947; Ismailov,
1948a,b). In the first wave of outbreaks, approximately 1000 -
1500 subjects were estimated to have been affected with an overall
mortality rate of 13 - 15%. The age of the affected subjects
ranged from 3 to 50 years. In the second wave of outbreaks, 60 -
70% of the population in agricultural areas were estimated to have
been affected (Ismailov, 1948a,b). Domestic animals also suffered
from the disease (Dubrovinskii, 1947). During the second wave of
outbreaks, the disease was found to have been caused by
contamination of food crops with seeds of Heliotropium lasiocarpum
(Dubrovinskii, 1947; Khanin, 1948). The etiology was confirmed by
studies on a variety of experimental animals using the suspect seed
(Khanin, 1948; Kampanzev, 1952). Pathological features of the
disease in children have been described by Dolinskaya (1952) and in
all age groups by Terekhov (1939, 1952). First an acute form of
the disease was recognized, followed by hepatic cirrhosis, which
was found to result as a consequence of the initial disease
(Ismailov, 1948a,b; Savvina, 1952). The disease has since been
largely eradicated by the agricultural and public health measures
taken.
Table 15. Pyrrolizidine alkaloid poisoning and human reports of veno-occlusive disease (VOD)
(in alphabetical order of the countries reported from)
Country/ Age Suspected Name of Alkaloid Alkaloid Nature of Outcome Reference
number group vehicle of plant concentration lesion (cirrhosis/
cases intoxication and intake died)
Afghanistan
8000 <14-80 contaminated Heliotropium mainly 1.32-1.49 g/kg all stages; many died Tandon &
(approximately) years wheat flour popovii heliotrine, (in the mostly acute Tandon (1975);
gillianum in addition seeds) daily to subacute Mohabbat
to 1 or 2 intake, 2 mg; et al. (1976)
alkaloids total
similar to consumption,
lasiocarpine up to 1.46 g
(75 - 100%
as N-oxides)
Barbados
3 2-4 herbal Crotalaria not analysed acute lesions no details Stuart & Bras
years infusion retusa (1956)
China, People's Republic of
2 49-57 herbal Gynura not analysed acute lesions died Hou (1980)
years infusion segetum
(Compositae)
Ecuador
1 35 years herbal Crotalaria not analysed acute lesions recovered Lyford et al.
infusion juncea (1976)
Table 15 (cont'd)
Country/ Age Suspected Name of Alkaloid Alkaloid Nature of Outcome Reference
number group vehicle of plant concentration lesion (cirrhosis/
cases intoxication and intake died)
Egypt
3 not known Hashem (1939)
59 1-12 plant seed not acute lesions cirrhosis Safouh &
years decoction identified in one Shehata (1965)
in 17
Federal Republic of Germany
8 75-116 not known acute lesions all died Wurm (1939)
days (alimentary
toxaemia)
Hong Kong
4 23-28 herbal Heliotropium pyrrolizidine herbs-alkaloid acute VOD 1 died Kumana et al.
years infusion lasiocarpum alkaloids base 0.42 g/kg; (1983, 1985);
N-oxides Culvenor et al.
1.4 g/kg; daily (1986)
intake, 12 mg
base, 18 mg
N-oxide; total
intake, 570 -
1350 mg up to
development of
symptoms in 3
patients over
19-45 days,
1300 mg up to
Table 15 (cont'd)
Country/ Age Suspected Name of Alkaloid Alkaloid Nature of Outcome Reference
number group vehicle of plant concentration lesion (cirrhosis/
cases intoxication and intake died)
Hong Kong (cont'd)
death in 1
patient. 630 mg
in one case who
remained
asymptomatic.
India
2 25 and herbal not unknown acute recovered Gupta et al.
35 years decoction identified (1963)
and pills
25 12-38 possibly not not analysed acute 12 died; Tandon, R.K.
years 6 contaminated identified lesions; 1 cirrhosis et al. (1976);
patients cereal cirrhosis Tandon, H.D.
studied) in one et al. (1977)
108 3-60+ contaminated Crotalaria crotananine 0.81 g/kg various up to 63% Tandon, B.N.
cereal nana and stages of died et al.
crotaburmine disease (1976, 1977)a
Siddiqui
et al.
(1978a,b)
Table 15 (cont'd)
Country/ Age Suspected Name of Alkaloid Alkaloid Nature of Outcome Reference
number group vehicle of plant concentration lesion (cirrhosis/
cases intoxication and intake died)
India (cont'd)
35 1- > 25 contaminated Crotalaria as above 5.3 g/kg seed acute as above Krishnamachari
years cereal (0 - 1.9% of et al.
seeds in (1977)a;
cereal); daily Siddiqui et al.
intake, 40 mg (1978a,b)
6 20-70 herbal Heliotropium heliotrine 12 - 20 g/kg; acute 3 died Aikat et al.
medicine in eichwaldii as N-oxide total intake, lesions; (2 with (1978);
4 cases by 3 4 g and 10 g cirrhosis fulminant Datta et al.
patients in patients who in one disease) (1978a,b)
died of organizing
fulminant thrombi in
disease hepatic veins
in 4 cases
Iraq
9 3-10 possibly possibly not acute 1 died Al-Hasany &
years wheat flour Senecio identified Mohamed (1970)
South Africa
11 11-19 wheat flour Senecio not centrilobular "majority Wilmot &
(only 5 years as bread ilicifolius; identified haemorrhages; died" Robertson
described) S. burchelli senecionine? central vein (1920)
"dilated"
Table 15 (cont'd)
Country/ Age Suspected Name of Alkaloid Alkaloid Nature of Outcome Reference
number group vehicle of plant concentration lesion (cirrhosis/
cases intoxication and intake died)
South Africa (cont'd)
12 wheat not not centrilobular Selzer & Parker
flour identified; identified haemorrhages; (1951)
"possibly organizing
Senecio" thrombi in
hepatic veins;
3 cases of VOD
12 5-30 herbal not none found "acute" in 3 died of Stein &
months medicines identified in herbal 5 cases; disease; Isaacson (1962)
admitted in medicines "subacute" no follow-up Stein (1957)
4 cases; in 7 cases; on 5
food not no detail
analysed
United Kingdom
1 26 years herbal Maté-Paraguay identified "trace acute with died McGee et al.
infusion tea only as amounts" veno-occlusion (1976)
pyrrolizidine going on to
alkaloids chronic with
fibrosis
1 13 years herbal Symphytum not analysed not known "thrombotic" Weston et al.
infusion officinale variant of (1987)
veno-occlusive
disease
Table 15 (cont'd)
Country/ Age Suspected Name of Alkaloid Alkaloid Nature of Outcome Reference
number group vehicle of plant concentration lesion (cirrhosis/
cases intoxication and intake died)
USA
1 2 months herbal Senecio riddelline 15 g/kg as acute lesions died Fox et al.
infusion longilobus and N-oxides alkaloids; (1978)
of retrorsine, total intake,
seneciphylline, 66 mg
and senecionine
1 6 months herbal Senecio as above 3 g free acute lesions cirrhosis Stillman et al.
infusion longilobus alkaloid plus (1977);
10.5 g N-oxide Huxtable (1980)
per kg; total
intake,
70-147 mg of
combined
alkaloid
and N-oxide
1 49 years herbal Symphytum sp. symphytum 14.1 µg/kg acute lesions recovered Ridker et al.
infusion pyrrolizidine body weight after (1985);
alkaloids per day; total short Huxtable et al.
intake, 85 mg surgery (1986)
USSR
1000 - 1500 3-50 contamination Heliotropium heliotrine, acute lesions 13-15% Mirochnik
years of wheat lasiocarpum lasiocarpine died; (1938);
flour cirrhosis Terekhov
in many (1939)
Table 15 (cont'd)
Country/ Age Suspected Name of Alkaloid Alkaloid Nature of Outcome Reference
number group vehicle of plant concentration lesion (cirrhosis/
cases intoxication and intake died)
USSR (cont'd)
"large contamination Heliotropium heliotrine Dubrovinskii
number" of wheat lasiocarpium lasiocarpine (1947);
flour Ismailov
(1948a,b)
Venezuela
1 5 years "earth and not acute lesions died Grases & Beker
plant eating identified (1972)
habits"
West Indies
11 14 months not known, acute to data Jelliffe et al.
to possibly cirrhosis unclear; (1954b)
9 years herbal cirrhosis
infusions in some
5 23 months not known, not 1 Bras et al.
to 18 possibility identified cirrhosis (1954)
years of "bush
teas"
4 16-45 history of not acute to 3 died Stuart & Bras
years herbal identified acute-on- (1955)
infusion in chronic
one case
Table 15 (cont'd)
Country/ Age Suspected Name of Alkaloid Alkaloid Nature of Outcome Reference
number group vehicle of plant concentration lesion (cirrhosis/
cases intoxication and intake died)
West Indies (cont'd)
84 0.5-53 probably not 11% had Stuart & Bras
years "bush teas" identified; cirrhosis; (1957)
(Senecio and 27% died
Crotalaria)
23 possibly not stated not analysed acute to Bras & Hill
bush teas "cirrhosis" (1956)
(no breakup)
23 1-50 no precise no analysis cirrhosis Bras et al.
years identification; (1961)
"Crotalaria
fulva and
possibly
other toxic
factors"
3 "children" "toxic not acute died Stirling et al.
substances identified (1962)
in herbal
remedies"
a Pertains to the same outbreak.
Selzer & Parker (1951), from South Africa, described 12 cases,
10 of whom came from 3 families that had eaten bread made of flour
of "imperfectly winnowed wheat". Five cases who were autopsied
showed characteristic occlusion of the central vein of the liver
lobules.
Attention on veno-occlusive disease as an entity followed a
spate of reports, mostly from Jamaica, on the occurrence of this
disease, mainly among children (Bras et al., 1954, 1961; Jelliffe
et al., 1954a,b; Bras & Watler, 1955; Bras & Hill, 1956; Stirling
et al., 1962) but also among adults (Stuart & Bras, 1955, 1957).
The youngest patient reported was a 3-month-old infant (Stein &
Isaacson, 1962), but the disease was stated even to have been
observed in the new-born (Stein, 1957). Very often, several
members of the family were affected by disease, which presented
primarily as rapidly filling ascites, within a matter of days,
sometimes accompanied by fever, and leading to hepatic failure. It
was considered an important cause of cirrhosis among children in
Jamaica (Jelliffe et al., 1954a,b). On the basis of evidence of an
almost identical disease occurring among grazing animals (Hill,
1960), and a prevalent practice among the Jamaicans of using herbal
infusions for treating a variety of ailments as a home remedy,
herbs, notably of the Senecio and Crotalaria groups, were suspected
of being a contributory factor (Bras & Hill, 1956; Bras et al.,
1957, 1961; Stuart & Bras, 1957).
Hill (1960) gave a comprehensive account of current knowledge
up to that time of the world-wide distribution of seneciosis in man
and animals in which mention is made of this disease only in Egypt,
the Federal Republic of Germany, South Africa, and the West Indies.
Pyrrolizidine alkaloids were identified as the active element in
the toxic factor in the plants.
However, there are earlier reports of this disease from
elsewhere. Bras (1973) cited a number of reports from Europe from
1905 to 1949, including reports by Wurm (1939) and Teilum (1949),
of an entity in infants and children that was called Endophlebitis
hepatica obliterans. He reported having studied some tissue
sections of liver in Austrian children aged 7 months - 1 year, who
had died with clinical and histological patterns of VOD. Some of
these conformed to the Budd-Chiari syndrome whereas others were
consistent with VOD. The case of a 36-year-old woman reported by
Teilum (1949) does not, however, fall in the usual category of
cases accepted as veno-occlusive, since thrombotic changes were
also seen in the larger hepatic veins and several, in the
peripheral parts of the body, as well as the portal vein.
A comprehensive account of all available published reports of
this disease to date from different parts of the world is given in
Table 15. A clear distinction between the acute and chronic
effects of exposure is not always possible from the published data,
since, in most patients, the history of onset of disease with
intake of the toxic substance is not generally volunteered or
available, and so a precise temporal relationship is difficult to
establish. However, this information is available in a reasonably
accurate form in more recent reports (Stillman et al., 1977; Datta
et al., 1978a,b; Fox et al., 1978; Kumana et al., 1985).
Gupta et al. (1963) from India described 2 cases who had drunk
some herbal infusions for the treatment of skin disorders. The
liver biopsies confirmed changes typical of acute VOD. However,
the herb was not identified and no analysis was made of the
infusion for alkaloids.
The cases of 59 children in Egypt who had symptoms of rapidly
developing abdominal distension and hepatomegaly were reported by
Safouh & Shehata (1965). Their ages ranged between 1 and 12 years,
47 being below 4 years of age. A dietary survey carried out on 17
patients indicated ingestion of drinks made by boiling some common
plant seeds, but these were not identified. Wedge biopsies of
liver were performed in 6 patients and 16 postmortem examinations
were made. In all the autopsy cases, there was thrombotic
obliteration of the main hepatic veins and their ostia. The
central and sublobular veins were not uniformly thickened as they
were frequently dilated or disrupted and some contained fresh
thrombi. There was centrilobular necrosis of the liver lobules.
One patient developed decompensated cirrhosis in 3 years. The
authors observed that the clinical and pathological features
closely resembled those of VOD in Jamaica, but thrombosis was an
unusual feature of the latter disease.
Al-Hasany & Mohamed (1970) described a short outbreak in Iraq,
occurring during a 3-month period, affecting 9 children, all except
one being below 12 years of age, and belonging to 3 Bedouin
families living outside Baghdad. One of the children died.
Autopsy of this case and biopsies on the others showed changes
characteristic of VOD. Poisoning through the wheat flour
contaminated by seeds of some PA-containing plant was suspected,
but no analysis of the food was carried out.
Three of the largest outbreaks of the disease have been
reported from South Asia, two from the same site in central India
(Tandon, B.N. et al., 1976, 1977; Tandon, R.K. et al., 1976;
Krishnamachari et al., 1977; Tandon, H.D. et al., 1977) and one
from North-West Afghanistan (Tandon & Tandon, 1975; Mohabbat et
al., 1976; Tandon, B.N. et al., 1978; Tandon, H.D. et al., 1978).
The first Indian outbreak was reported by Tandon, B.N. et al.
(1976) and Tandon, R.K. et al. (1976) and Tandon, B.N. et al.
(1977) and Tandon, H.D. et al. (1977). It occurred in a group of 5
tribal villages in central India in 1972 - 73. The epidemiology of
the outbreak was later described by Arora et al. (1981). Out of a
total population of 2060 in these villages, 71 households with 366
members were investigated. Among these, 39 cases had developed and
19 had died before commencement of the investigations. The
incidence rate was 1.1% and the case fatality rate was 50%. All
cases occurred among 20 households. In many households, several
members were affected. In one household, 4 out of 5 cases died.
Six sick patients were investigated in detail with repeated liver
biopsies. One patient died 17 months after onset and was
autopsied. The clinical onset was characteristic of disease.
Haemodynamic and radiographic studies suggested a combined post and
perisinusoidal block. Liver biopsy studies showed features
characteristic of acute centrilobular haemorrhagic necrosis with
progressive fibrosis, hepatic veno-occlusion, and non-portal
cirrhosis in one case who survived 17 months after the acute onset.
The etiological factor of this outbreak was not established, though
dietary contamination with pyrrolizidine alkaloids was considered.
A second outbreak occurred at the same site in 1975 and was
studied and reported independently by Krishnamachari et al. (1977)
and Tandon, B.N. et al. (1976, 1977). A total population of 486
was affected, 67 cases were reported, of whom 28 (46%) had died.
There was a strong family history (Tandon, B.N. et al., 1976). In
a later survey, 108 patients were studied and the mortality rate
was estimated to be 63% (Tandon, B.N. et al., 1977). This time the
etiological factor was identified as the plant Crotalaria nana
Burm, which had been growing in the fields of millet (Panicum
miliare), their staple food crop. The seeds of this plant became
mixed with the cereal grain during harvesting. The toxic seeds
contained pyrrolizidine alkaloids that were identified as a
macrocyclic ester closely similar to monocrotaline. The total
alkaloid content was estimated to be 5.3 g/kg of seed, expressed as
monocrotaline. The levels of contamination of the millet with
seeds were reported to be 0 - 3.4 g/kg in the unaffected and
0 - 19 g/kg in the affected households (Krishnamachari et al., 1977).
A precise identification could not be made, but the same material,
independently studied by Siddiqui et al. (1978a,b), were reported
to contain 2 new alkaloids, cronaburmine and crotananine. The seed
contained an alkaloid level of 26 g/kg. The levels of
contamination of the millet were up to 20 g/kg and the amount of
alkaloid ingested was estimated to be up to 40 mg per day.
The largest outbreak reported to date occurred in the Gulran
district of Herat Province in northwest Afghanistan, close to the
border of the USSR. Tandon & Tandon (1975) identified the plant as
being causative factor of the disease, which was surveyed and
reported in detail by Mohabbat et al. (1976). The outbreak, was
estimated to have affected a population of approximately 35 000 in
98 villages. Examination of 7200 inhabitants of the affected
villages showed evidence of disease in 22.6%, which was more
serious in 15%. Thus, it was estimated that approximately 8000
subjects suffered from the disease including 5000 who were
seriously affected. All age groups were affected, but 46% of
subjects were below 14 years of age. However, no sign of disease
was found in children below 2 years of age. A detailed report
concerning the pathological material obtained from 14 liver
biopsies and 8 autopsies was made by Tandon, B.N. et al. (1978) and
Tandon, H.D. et al. (1978). The time interval between the onset of
symptoms and the biopsy/autopsy was not indicated. Pathological
findings were characteristic, ranging from acute disease with
characteristic veno-occlusion to non-portal cirrhosis, which was
observed in 5 of the above 22 cases. The outbreak was ascribed to
massive contamination of wheat, the staple food crop, following 2
years of drought, with the seeds of Heliotropium popovii H. Riedl
subsp. gillianum H. Riedl, which had been growing profusely among
the wheat crop. The seeds contained pyrrolizidine alkaloids at
concentrations reported by 2 laboratories to be 7.2 and 13.2 -
14.9 g/kg, identified as mainly as the N-oxide of heliotrine (74%)
(Mattocks, personal communication), and one or two other compounds
similar in character to lasiocarpine. Samples of wheat from
several villages contained an average of 40 seeds/kg, i.e., 0.03%
by weight. It was estimated that an adult consumed at least 700 g
flour/day, containing approximately 2 mg alkaloid (based on a mean
of the seed analyses). There is some uncertainty about the
estimate, since Mohabbat et al. (1976) also stated that the samples
of the wheat flour were assayed and contained 0.186 - 0.50%
alkaloid. This analysis and the 0.72% result for alkaloid in the
H. popovii seed were from the same laboratory in Kabul (R.N.
Srivastava, personal communication), and together, imply 13 - 36%
seed in the wheat. If correctly reported, the result for the flour
conflicts with the estimated proportion of the seed in the wheat
and can scarcely have been representative.
Tandon & Tandon (1975) stated in their report of the survey
during which the causative factor of the Afghan outbreak was
discovered, that such cases had always been observed by Government
physicians posted in this area in the past, sometimes in
significant numbers, but neither the population nor the physicians
remembered that the disease had occurred in the form of an
outbreak.
There was no mention of VOD in the hepatic lesions observed by
Sobin et al. (1969) among the 121 specimens of liver obtained at
medico-legal autopsies or by needle biopsies at Kabul, though 6.6%
of the 89 autopsy specimens showed "acute passive congestion with
necrosis". The ages of these subjects was not stated and the
authors did not discuss the cause of the lesion.
Following these outbreaks, a number of isolated cases have been
described following the use of herbal medicines. McGee et al.
(1976) reported a case from the United Kingdom of a 26-year-old
woman who had consumed herbal tea containing pyrrolizidine
alkaloids. The one sample examined contained only trace amounts of
alkaloids. Neither the plant nor the alkaloids were further
characterized. The patient had been taking very large quantities
of the tea for about 2 years and it is possible that some of the
earlier batches may have been more heavily contaminated. Maté or
Paraguay tea ( Ilex species), which she was drinking, is stated to
be a popular drink in Brazil and is not believed to contain
pyrrolizidine alkaloids. It has been stated (Huxtable, 1980) that
possibly she ingested the pyrrolizidine alkaloids from some other
unidentified source. The clinical course of the disease progressed
rapidly. Three biopsies carried out within one month and the
autopsy showed characteristic changes including centrizonal
fibrosis, but no cirrhosis. It is notable that some involvement of
muscular pulmonary arteries was also seen.
Lyford et al. (1976) reported the case of a 35-year-old woman
from Ecuador, who had ingested herbal tea prepared from Crotalaria
juncea. She had consumed 1 - 2 litres of this infusion daily for 6
weeks, but no qualitative or quantitative analysis for
pyrrolizidine alkaloids was made. She had had arthralgias for 3
years, for which she had received treatment with indomethacin and
phenylbutazone. The liver biopsy showed characteristic changes of
acute VOD from which she recovered completely as proved by a repeat
biopsy carried out one year later.
The occurrence of acute disease was reported in 2 infants in
Arizona in the USA, aged 6 months and 2 months, respectively,
following ingestion of infusions of a herb, locally called the
Gordolobo Yerba by the Mexican-American population and identified
as Senecio longilobus (Stillman et al., 1977; Fox et al., 1978;
Huxtable, 1980). The plant from which this infusion had been
prepared was found to contain pyrrolizidine alkaloids identified as
riddelline and N-oxides of retrorsine, seneciphylline, and
senecionine (Huxtable, 1980), in a concentration of 3 g free
alkaloid and 10.5 g N-oxides/kg (Stillman et al., 1977). It was
estimated that, during a period of 2 weeks, the 6-month-old infant
received a total dose of between 70 and 147 mg of combined alkaloid
and N-oxide derivative. The liver biopsy showed characteristic
features of acute disease, which had progressed to extensive
central, portal, and sinusoidal fibrosis (Stillman et al., 1977).
The child subsequently developed cirrhosis over the next 8-month
period. The 2-month-old infant was administered an infusion of the
same herb for 4 days, after which he became progressively more ill
and stuporous (Fox et al., 1978). On admission he was diagnosed as
a case of Reye's syndrome, but subsequently developed jaundice and
possibly ascites and died. The sample of herb contained a
concentration of alkaloids of 15 g/kg. It was estimated that the
infant had received a total of 66 mg of mixed alkaloids over the
4-day period. At autopsy, extensive centrilobular haemorrhagic
necrosis of the liver was seen, which is characteristic of the
acute disease. However, no occlusive lesions of the central vein
of the lobules were described, and no obstructive lesions were seen
in the larger hepatic veins or inferior vena cava. No mention was
made of ascites. The basal ganglia showed kernicterus.
Datta et al. (1978 a,b) reported 6 cases that occurred between
1974 and 1977. All of the patients had taken herbal medicines,
identified in 1 case as Heliotropium eichwaldii, which contained
N-oxides of heliotrine. Two patients took the herb as an extract
of the whole plant, which contained an alkaloid concentration of
20 g/kg, for 20 and 50 days, respectively, and developed symptoms
after a time lag of 45 and 90 days, respectively. They were both
estimated to have consumed 200 mg of heliotrine per day, the total
alkaloid intake being 4 g and 10 g, respectively. They had taken
the herb for treatment of epilepsy, and were admitted with acute
onset of symptoms of abdominal pain, ascites, jaundice, hepatic
encephalopathy, and gastrointestinal bleeding, which suggested
fulminant viral hepatitis. They died within 2 - 12 weeks of the
onset of symptoms. Only a brief description of the main autopsy
findings in the liver was given, which indicated that there were
changes characteristic of acute veno-occlusive disease of liver,
including marked centrilobular haemorrhagic necrosis of the liver
lobules, and occlusive lesions of the central and sublobular veins.
It is interesting to note that both patients had also been on long-
term anticonvulsant phenobarbitone therapy. The remaining 4
patients had a chronic insidious onset of disease suggesting
cirrhosis of the liver in 3, and alcoholic liver disease in one.
One of the former had been taking some indigenous powder,
presumably prepared from a herb, the indication and nature of which
are not known. The patient died from hepatic encephalopathy. No
detailed description of the autopsy findings was given, but it was
stated that the central and sublobular veins of the liver showed
chronic occlusive changes. The inferior vena cava and large
hepatic veins were patent. There was non-portal cirrhosis. A
notable feature was that the arsenic levels in the liver tissue
were high (500 µg/kg; normal, 1 µg/kg). There is no mention of
arsenic in the report on the analysis of the indigenous powder
taken by the patient. Two of the remaining 3 patients with chronic
disease, had taken herbal medicine for vitiligo, and one for
diabetes mellitus. The herb was identified as Heliotropium
eichwaldii in the case of one of the vitiligo patients, who had
taken it for 10 days and in whom the onset of symptoms occurred
within 10 days. The herb was taken in the form of seed with an
alkaloid content of 12 g/kg. The daily intake was estimated to be
500 mg of alkaloid and the total intake, 5 g. This patient was
admitted with a clinical diagnosis of cirrhosis of the liver. The
liver biopsy showed changes characteristic of acute VOD. Follow-up
data are not known. The herb taken by the other 2 patients was not
identified. The diabetic patient was being treated with oral
hypoglycaemic drugs and was also known to be an alcoholic. The
indigenous powder being taken by him contained a high concentration
of arsenic (5 mg/kg). The results of haemo-dynamic studies
suggested hepatic venous outflow tract obstruction of the
intrahepatic post-sinusoidal type in the smallest hepatic veins.
Liver biopsy showed characteristic centrilobular haemorrhages.
Central veins could not be recognized. There were mild changes in
the liver cells, but no alcoholic hyaline was seen. The liver
biopsy of the third patient showed characteristic features of acute
disease with veno-occlusion.
Two cases have been reported from China (Hou et al., 1980).
Both were adults who were taken ill after taking medicinal
infusions prepared from Gynura segetum of the family Compositae
(tribe, Senecioneae). The presenting symptoms and the cause of
disease, as well as the pathological findings, were characteristic,
except that one patient had jaundice and also portal vein
thrombosis, which is not a usual feature of the disease. No
chemical analysis of the plant was made and the alkaloid was not
precisely identified. Furthermore, the total intake of alkaloid
was not calculated. It has been stated that this was the first
report of such a case, but that it was possible that the disease
might occur among adults more frequently without being reported.
Ghanem & Hershko (1981) reported 3 cases of Arabs, one 3-year-
old child and 2 adults, who were diagnosed as having VOD of the
liver, on the basis of the clinical findings and morphological
features of liver biopsies. One more patient had occlusive lesions
of both the small and large hepatic vein radicles. They were among
29 patients with clinical features of hepatic vein thrombosis (HVT)
found on a retrospective analysis of data from 9 major hospitals in
Israel. Of these patients, 15 were Jews and 14 were Arabs.
Notable features were that all Jewish patients were adults, whereas
the majority of Arab patients were children below 10 years of age
and primary HVT was 2.4 times more common among the latter. No
analysis of the diet was made for PAs and their etiological role
was suspected only on a presumptive basis. A survey of stored
wheat grain in 9 villages showed that 2 samples were contaminated
with seeds of Lolium, belonging to the Graminae family, which were
found to contain 2 PAs (loline and norloline). However, these PAs
are not known to be hepatotoxic. The authors argued that, even
though in a classical case of VOD there should not be thrombosis of
the larger hepatic vein radicles, the difference in the anatomical
appearance of VOD and that of primary HVT of the near-east type is
not due to a different etiological agent but rather to a difference
in the dose and rate of absorption of the ingested toxic compounds.
A further report of the disease from Hong Kong, by Kumana et
al. (1985), described it in 4 young Chinese women with psoriasis
who took infusions of a herbal remedy, the toxic component of which
has since been identified as Heliotropium lasiocarpum (Culvenor et
al., 1986). They developed symptoms 19 - 45 days after starting
the herbal treatment, and were examined 61 - 68 days after its
initiation. The condition of patient No. 2, who continued taking
the herb for 16 days after the onset of symptoms, deteriorated and
she died of hepatic failure and was autopsied. The liver biopsies
and autopsy confirmed the presence of acute disease in all
patients. Patient No. 4 stopped taking the herb after 21 days, on
account of a new rash. When assessed 77 days later, she had mild
hepatomegaly only. A detailed analysis of the alkaloid content was
carried out for each case. The pyrrolizidine alkaloids were
quantified as if senecionine based. The herb contained 0.42 g
alkaloid/kg and 1.4 g N-oxide/kg. The daily intakes of alkaloid
base and N-oxide were estimated to be 12 ± 1 mg and 18 ± 4 mg,
respectively. The respective cumulative doses of alkaloid (base
and N-oxide) consumed by patients Nos 1, 2, and 3, up to onset of
symptoms, were calculated to be 1350 mg over 45 days, 900 mg over
30 days, and 570 over 19 days, respectively. Patient No. 4 who had
irrefutable histological evidence of disease but did not develop
symptomatic disease, must have consumed 630 mg over 21 days.
Patient No. 2, who died, was estimated to have taken a total amount
of 1380 mg alkaloid over 46 days. The estimated cumulative intake
per kg body weight before the development of symptoms for patients
Nos. 1, 2, 3, and 4 was 26, 15, 12, and 15 mg/kg, respectively. It
should be noted that, in patient No. 2, who died, the cumulative
dose until the onset of symptoms was the same as in patient No. 4,
who was asymptomatic. Moreover, the total intake by patient No. 2,
was 23 mg/kg, which was lower than the intake by patient No. 1, who
survived. The authors compared the intake data of their patients
with those of the Arizona children reported by Stillman et al.
(1977) and Fox et al. (1978). The 6-month-old baby, who survived
but developed cirrhosis, and the 2-month-old baby, who died, are
estimated by comparison to have taken cumulative doses of 12 - 25
and 11 mg/kg, respectively. The above data suggest marked
variation in susceptibility among individual subjects. It is also
known from experimental animal studies that the young and new-born
animals are particularly vulnerable (Jago, 1970).
A case reported from the USA is ascribed to the consumption of
comfrey ( Symphytum sp.) powder, sold as a digestive aid (Ridker et
al., 1985). A 49-year-old woman presented with classical symptoms
and signs of VOD. The haemodynamic data showed a hepatic vein
wedge pressure of 3.07 kPa (23 mmHg) with a sinusoidal pressure of
2.27 kPa (17 mmHg). Hepatic venograms showed near obliteration of
the smaller radicles of the hepatic veins during balloon distension
of one of the intrahepatic venous tributaries, and there was extra-
vasation of the dye into the hepatic parenchyma. A porta-caval
shunt was carried out and the operative findings confirmed the
presence of a post-sinusoidal block. The liver biopsy showed
marked centrilobular necrosis and congestion with dilatation of the
central veins and sinusoids, consistent with hepatic venous outflow
tract obstruction. According to the clinical history, the patient
had been a heavy consumer of food supplements. Apart from several
vitamins and minerals, she had been drinking 3 cups of camomile tea
per week and for 6 months before admission had consumed 1 g/day of
a commercially available herbal tea. For 4 months before
admission, she had taken 2 capsules of "comfrey-pepsin" pills with
each meal. The herbal tea and the pills were analysed for PAs.
Pyrrolizidine alkaloids and their N-oxides were found, but the
compounds were not precisely identified. On the basis of the
analysis of the PA content, the patient was estimated to have
consumed a total of at least 85 mg of PA (Huxtable et al., 1986)
(14.1 µg/kg body weight per day, Ridker et al., 1985). The authors
emphasized that the total PA consumption was relatively low. It
was possible that the patient had other sources of exposure and
probably she had been consuming PA-containing supplements for
longer than the periods stated by her in the clinical history.
The latest is the report of a 13 year old boy from the U.K. who
is stated to have developed symptoms of toxicity following
administration of herbal tea prepared from comfrey leaf (Symphytum
officinale) for treatment of inflammatory bowel disease for two or
three years (Weston et al., 1987). The exact quantity of leaves
consumed and frequency of administration were not known. The liver
biopsy is stated to have shown a "thrombotic variant" of veno-
occlusive disease, though the inferior vena cava and the major
hepatic veins were patent on Doppler ultrasound and percutaneous
phlebography. He had earlier been treated with predinisolone
and sulphasalazine. The case is unusual in so far as the
thrombosis of the central veins of the liver lobules, which is not
a usual feature of veno-occlusive disease of the liver.
7.4 VOD and Cirrhosis of the Liver
Jelliffe et al. (1954b) were the first to draw attention to VOD
being an important cause of cirrhosis among Jamaican children.
Prior to this, Hashem (1939), while reviewing the records of all
cases of cirrhosis admitted to a children's hospital in Egypt since
1933, described 3 cases of a special type of cirrhosis that was
rare in adults. The clinical features and pathological findings
were similar to those in cases of VOD. They speculated that the
cause was some metabolic toxins of gastrointestinal origin. Royes
(1948) suggested that the cirrhosis in the Jamaican children was
very like the disease described from India and Egypt.
The clinical and pathological features of 100 cases of VOD
among Jamaican children were described by Bras et al. (1954).
Sixty-five of the cases were below 12 years of age. None of the
100 patients had cirrhosis initially, but 5 showed occlusive
lesions in hepatic veins and features of non-portal fibrosis. Four
of these 5 cases later developed cirrhosis. The authors concluded
that VOD contributed to a substantial number of all cases of
cirrhosis in this age group. Stuart & Bras (1957) studied 84
patients with VOD including 64 acute, 6 subacute, and 14 chronic
cases. Twenty-three patients were followed up, some for up to 5
years. Autopsy performed on 21/26 cases showed cirrhosis in 11
cases. Notable features were that 1 of the 6 cases of acute
disease described in detail, developed cirrhosis. Of the 2 cases
with chronic disease, 1 developed cirrhosis within 3 months of a
liver biopsy for acute disease, at which time the liver had shown
hepatic venous occlusive lesions but no fibrosis. Autopsy findings
were described by Bras & Watler (1955) in 19 patients aged 10
months - 45 years in different stages of the disease. Nine
patients had cirrhosis that was non-portal to begin with but
finally became indistinguishable from Laennec's portal cirrhosis.
Rhodes (1957) studied the pattern of liver disease among Jamaican
children. A total of 193 liver biopsies was studied derived from
39 children who had one biopsy and 59 who had more than one at
intervals of 1 week - 3 years. Of the 14 autopsies on cases of
VOD, 12 had cirrhosis. A notable feature was that the disease
could occur asymptomatically with hepatomegaly. The autopsy
material from the University College Hospital, Jamaica was analysed
by Bras et al. (1961). Of the 1560 autopsies, 28.5% cases
concerned infants of less than one year old, mostly from poor,
predominantly black families. Cirrhosis was seen in 77 autopsies.
Approximately 30% of these 77 cases were diagnosed as post-VOD.
The authors postulated that they might have resulted from the
ingestion of Crotalaria fulva or some other toxic substances.
In a follow-up study of 61 patients who developed the disease
in an outbreak in the USSR in 1958, 28 developed "Hepatoleinal
syndrome" (Braginski & Bobokhadzaev, 1965). Two of these cases, in
whom the disease in the initial stages was not particularly severe,
developed cirrhosis within 4 years.
Tandon, H.D. (personal communication) has analysed the
pathological data derived from the Afghan and Indian outbreaks on
the basis of 61 liver biopsies and 17 autopsies, including repeat
biopsy studies on 15 patients who were followed-up for 1 month - 3
years after onset in the Indian outbreaks. Three of the 11
patients, followed up for 16 months or longer for persistent
clinical evidence of disease, ended up with cirrhosis and 2 more
had marked fibrosis with equivocal changes of cirrhosis in the
biopsy. Notable features of the study were that the disease
progressed to cirrhosis in patients who were put on a normal diet,
free from alkaloids, after appearance of symptoms of acute disease
and did not receive any subsequent exposure. Impact of alcohol
intake was excluded. There was a poor correlation between the
clinical and pathological severity of disease. Centrilobular
haemorrhages, which are a sign of acute disease, were seen to
persist for over one year in patients, some of whom were apparently
well. At needle biopsy, characteristic hepatic venous occlusions
were not seen in many patients in the acute phase of the disease,
though they were seen in all livers at autopsy and most of them
showed persistent centrilobular haemorrhages. Biopsy findings in
cirrhotic livers were often not histopathologically characteristic
for any specific form of cirrhosis. Features that might suggest
the veno-occlusive etiology of cirrhosis at biopsy included
paraseptal dilatation of sinusoids and persistent haemorrhages or
haemosiderin in the septa.
In studies by Aikat et al. (1978) and Datta et al. (1978a,b), 6
cases of VOD were reported following ingestion of herbal medicines
containing PAs. Four of the patients had symptoms of chronic
disease and one of them developed non-portal cirrhosis.
One of the 2 infants from Arizona, USA, who suffered from VOD
following the administration of PA-containing herbal medicine,
developed cirrhosis during the 8-month period following the
appearance of acute symptoms (Stillman et al., 1977; Fox et al.,
1978; Huxtable, 1980). Huxtable (1980) mentioned the death from
cirrhosis of liver of a 62-year-old woman, who had consumed the
same herb as the 2 infants for 6 months prior to her death.
However, there was no confirmation of the diagnosis of cirrhosis.
7.5 Differences Between VOD and Indian Childhood Cirrhosis (ICC)
A type of cirrhosis of liver, peculiar to the people of Indian
origin, Indian Childhood Cirrhosis (ICC), has been ascribed to PA
toxic etiology (Bras et al., 1954; Rhodes, 1957), owing to the
observation of occlusive changes in the central and sublobular
veins of the liver, by some investigators (Radhakrishna Rao, 1935;
Prabhu, 1940; Jelliffe et al., 1957; Ramalingaswami & Nayak, 1969),
though this is not a characteristic feature of the disease. The
presence of copper-positive granules in the hepatocytes (Salaspuro
& Sipponen, 1976) has added to such a conjecture (Tanner &
Portmann, 1981), because of the reported aberration of copper
metabolism in experimental animals exposed to PAs (section 6.4.11).
However, ICC and VOD are clinically and pathologically distinct.
ICC, confined to infants and children, often affects siblings.
Jaundice is a common sign and hepatosplenomegaly is a common
feature. The disease is almost invariably fatal due to rapidly
developing hepatocellular failure. Liver parenchymal changes are
characterized by marked ballooning degeneration of hepatocytes,
prominent deposits of alcoholic hyaline, severe cholestasis, and
aggressive, pericellular fibrosis (Nayak et al., 1969), all
features not characteristic of VOD. Moreover, occlusive changes in
the hepatic veins are very rare and were not observed by any member
of the liver diseases subcommittee of the Indian Council of Medical
Research (1955), who made a critical study of the disease.
7.6 Chronic Lung Disease
Heath et al. (1975) reported the case of a 19-year-old African
man who had died of congestive cardiac failure, and who was
suspected of having ingested a herbal remedy containing the seeds
of Crotalaria laburnoides. Histopathological examination of the
lungs showed characteristic vascular changes of severe primary
pulmonary hypertension. Powdered seeds of the plant were fed in
the diet to Wistar albino rats for 60 days (Table 11).
Characteristic features of pulmonary hypertension including
hypertensive vascular changes in the lung and right ventricular
hypertrophy of the heart were produced in the animals showing that
the seeds contained an agent capable of inducing pulmonary
hypertension in rats. Apart from this indirect evidence, there was
no proof of such a causal relationship with the pulmonary
hypertensive disease in the patient.
A brief mention is made by McGee et al. (1976) of finding
changes "somewhat similar but rather more mature" (than those seen
in the hepatic veins) involving some branches of the pulmonary
artery in the lower lobe of the left lung, in a case of veno-
occlusive disease of the liver caused by ingestion of PA-containing
herbal teas. The alkaloids were not further characterized. The
changes were also stated to be similar to those produced in
experimental animals by PAs. Apart from these 2 cases, there is no
mention of involvement of the pulmonary arterial system in any of
the case reports available.
The possibility that diet-mediated agents might induce
pulmonary hypertension in man has been discussed at length by
Fishman (1974). A parallel was drawn with the epidemic of
pulmonary hypertension that occurred in Austria, the Federal
Republic of Germany, and Switzerland between 1966 and 1968 (Kay et
al., 1971b), which was suspected of being caused by Aminorex, a
compound that resembles epinephrine and amphetamine in chemical
structure. Although the etiological role of Aminorex could not be
conclusively proved on epidemiological or experimental grounds,
there continues to be a suspicion that agents taken by mouth can
evoke pulmonary hypertension in man. Levine et al. (1973) reported
the cases of 3 children aged 5 1/2 - 13 years, and 11 months,
respectively, with portal hypertension, who developed progressive
pulmonary hypertension resulting in cor pulmonale and death. In
all 3 cases, there was evidence of extra-hepatic portal vein
obstruction confirmed at autopsy, and the symptoms and signs of
portal obstruction had appeared in early childhood. They developed
symptoms of cardio-respiratory failure. Studies of pulmonary and
cardiovascular function including haemodynamic studies of pulmonary
circulation in 2 of the children suggested pulmonary vascular
obstructive disease. At autopsy, no primary parenchymal lung
disease was found. There were vascular changes of advanced
pulmonary hypertension (plexiform lesions), but no evidence of
thromboembolism was found. No factor responsible for pulmonary
vasoconstriction was identified. In one case, the liver was stated
to show coarse nodularity at autopsy, but the microscopic
examination showed only patchy areas of portal fibrosis and
regeneration. In the other 2 cases, there were only non-specific
changes, with fibrosis in one. However, centrilobular congestion
was present in 2 cases. It is possible that some metabolites of
toxic agents, such as PAs, which are metabolized in the liver,
might have blocked a metabolic pathway that ordinarily exerts a
pulmonary antihypertensive effect, or that, by damaging the liver,
vasoactive substances, such as histamine, serotonin, and
catecholamines, might escape metabolic pathways to reach the lungs
and injure the pulmonary vessels. However, no such agents were
identified.
Kay et al. (1971b) made a plea that, on the basis of the
experimental data available, including the ability of several
agents to produce pulmonary hypertension in experimental animals,
and, in spite of the fact that pulmonary vascular disease has never
been demonstrated in human cases of veno-occlusive disease, careful
enquiries should be made on the possibility of patients presenting
with unexplained pulmonary hypertension having ingested a plant
product. A similar plea was made by Heath et al. (1975).
7.7 Trichodesma Poisoning
The disease "Ozhalanger encephalitis", which occurred in the
Samarkand region of Uzbekistan, USSR in the period 1942 - 51, is
believed to have been caused by contamination of food grain with
the seeds of Trichodesma incanum (Shtenberg & Orlova, 1955;
Ismailov et al., 1970), which contain 1.5 - 3.1% alkaloids, mainly
trichodesmine and incanine (Yunusov & Plekhanova, 1959). Clinical
features of the disease have been described by Ismailov et al.
(1970). This outbreak differed from the others described above in
that the primary symptomatology was extra-hepatic. Over 200
patients were affected, not including children below the age of 10,
or the breast-fed infants of the affected mothers. Following
exposure, there was a latent period of about 10 days, then vertigo
and recurring headaches developed leading to nausea and vomiting.
This was followed by generalized malaise, which progressed to
delirium and loss of consciousness. Physical signs included
pathological reflexes in 59% of the patients and paresis of the
extremities and the facial nerve. Death was stated to have been
caused mostly by respiratory depression. Of the 200 patients
affected, 44 died. Autopsy findings were relatively non-specific
degenerative and necrotic lesions scattered in several organs
including the central nervous system. No report of such a disease
is available from outside the USSR.
7.8 Relationship Between Dose Level and Toxic Effects
In some recent human case reports, the PAs consumed have ben
identified and estimates made of the daily and total intakes (Table
16). The relationship between these intake levels and the known
toxicity of the alkaloids in rats has been discussed by Culvenor
(1983) and Mattocks (1986).
Discussion of the relationship between the dose level and toxic
effects in human cases is complicated, because the poisoning is
generally due to a mixture of alkaloids found in naturally-
occurring plant products, consumed as herbal remedies or food, and
different plant species. There are wide differences in the acute
toxicities of the alkaloids, which are the best available measure
of comparative effects due to long-term intake as well.
Furthermore, estimates of intake can, at best, be approximate.
When a large population is affected through the contamination of a
food crop, as happened in the Afghan and Indian outbreaks (section
7.3), no precise estimates are possible regarding the extent of
contamination in different households, the amount of the
contaminated grain consumed, the length of exposure resulting in
the appearance of symptoms or signs of toxicity, or death, in the
cases studied. There may also be various other contributing
factors that are not apparent, e.g., food or cooking habits, on
account of which no conclusive generalizations regarding the
causative role of the toxic agent can be made. Reports on chemical
analysis for the toxic agent and, hence, the amounts ingested, may
not always be reliable (refer to the controversy in the Afghan
outbreak in section 7.3). In cases, such as the one reported by
Ridker et al. (1985), when the total dose of PAs estimated to have
been received by the patient before the disease developed, was only
a fraction of that of other alkaloids causing episodes of human
toxicity (Table 16), it is not certain whether that was the only
toxic alkaloid agent that the patient was exposed to, or whether
there were other contributing factors, particularly as the patient
was stated to be a heavy consumer of herbs, vitamins, and natural
food supplements.
In the known instances of human toxicity the principal
alkaloids involved are heliotrine from Heliotropium, echimidine and
related alkaloids from Symphytum, riddelline and retrosine from
Senecio longilobus, and crotananine and cronaburmine from
Crotalaria nana (Table 16). Approximate acute toxicity values
(LD50 in rats) for these alkaloid mixtures are 300, 500, and
50 mg/kg, respectively. For the mixture from C. nana, for which
there are no experimental data, the acute toxicity was assumed to
be similar to that of monocrotaline, 100 mg/kg. These relativities
need to be taken into account in discussing dose-effect
relationships for the PAs as a group. This has been done by
discussing first the dose estimates for heliotrine, since it was
the main alkaloid in 1 epidemic and in 6 case reports (Table 16).
Then in discussing the other alkaloids, reference is made to a
heliotrine-equivalent dose as well as the actual dose. The
heliotrine equivalent is:
LD50 of heliotrine
actual dose x ----------------------------------
LD50 of alkaloid mixture concerned
The estimated daily intake in poisoning by heliotrine ranges from
0.033 mg/kg body weight in the Afghan epidemic (Mohabbat et al.,
1976), which after a period of about 180 days and a total intake of
about 6 mg/kg caused fatalities, to 3.3 mg/kg, which led, in 2
cases in India (Datta et al., 1978a,b), to death after 20 and 50
days and total intakes of 67 and 167 mg/kg, respectively. In
between are 4 cases in Hong Kong with estimated daily intakes
ranging from 0.49 to 0.71 mg/kg (Kumana et al., 1983, 1985;
Culvenor et al., 1986). Three cases were non-fatal at total doses
of 11 - 27 mg/kg body weight and one was fatal at a total dose of
23 mg/kg. These heliotrine cases imply that daily intakes are
cumulative down to 0.033 mg/kg and may be fairly rapidly fatal
above 0.5 mg/kg. Above a total dose of 6 - 15 mg/kg, VOD may
become evident and sometimes fatal.
In the 2 cases due to riddelline and retrorsine in Senecio
longilobus (Stillman et al., 1977; Fox et al., 1978; Huxtable,
1980), the estimated daily intakes were 0.8 - 0.17 and 3 mg/kg body
weight (equivalent to 3 and 1 mg heliotrine/kg, respectively, and
the total doses were 12 - 25 and 12 mg/kg (equivalent to 72 - 150
and 72 mg heliotrine/kg, respectively). These levels are
comparable with the highest reported intakes of heliotrine and, in
infants, led to the rapid development of VOD and, in one case,
death.
In the epidemic due to mixed crotananine and cronaburmine in
Crotalaria nana (Tandon, B.N. et al., 1976; Tandon, R.K. et al.,
1976; Krishnamachari et al., 1977), the estimated daily intake of
0.66 mg/kg and the total intake of 40 mg/kg (equivalent to 2 and
120 mg heliotrine/kg, respectively) also corresponded to the
highest intake of heliotrine.
In the case of Symphytum poisoning (Ridker et al., 1985), the
estimated daily intake of echimidine and related alkaloids was
0.015 mg/kg and the total dose 1.7 mg/kg (equivalent to 0.009 and
1.0 mg/kg heliotrine, respectively). The dose levels are lower in
equivalent terms than the lowest estimates in cases due to
heliotrine by a factor of about 4 for daily intake and 6 for total
intake. The estimates were based on questioning of the patient and
assay of the material concerned. It seems prudent to conclude that
a daily intake of pyrrolizidine alkaloid as low as the equivalent
of 0.01 mg/kg heliotrine may lead to disease in humans.
Table 16. Estimated intakes of PAs in human beings
-----------------------------------------------------------------------------------------
Principal Age of Daily Period Total dose Toxic Reference
alkaloids(s)a subject intake (days) mg mg/kg effectc
(years) (mg/kg)b
-----------------------------------------------------------------------------------------
1. Heliotrine variousd 0.033 180e 360 6e VOD, Mohabbat et al.
death (1976)
2. Heliotrine (a) 20 3.3 20 4000 67 death Datta et al.
(b) 23 3.3 50 10000 167 death (1978a,b)
3. Riddelline, 0.5 0.8-1.7b 14 70-147 12-25 VOD Stillman et al.
retrorsine (1977); Huxtable
(1980)
4. Riddelline, 0.17 3.0b 4 66 12 death Fox et al. (1978)
retrorsine
5. Crotananine, variousd 0.66 c. 60e 2400 40 VOD, Tandon, B.N. et al.
crotaburmine death (1976); Tandon,
R.K. et al. (1976);
Krishnamachari et
al. (1977)
6. Heliotrine (a) 28 0.59 45 1350 27 VOD Kumana et al.
(b) 26 0.49 46 1380 23 death (1983, 1985);
(c) 23 0.60 19 570 11 VOD Culvenor et al.
(d) 27 0.71 21 630 15 VOD (1986)
7. Echimidine 49 0.015 120 94 1.7 VOD Ridker et al.
(1985)
-----------------------------------------------------------------------------------------
a The principal alkaloid is recorded as the free base, even if there was evidence of its
presence in the plant mainly as N-oxide.
b Calculated for a 60-kg adult, unless definite information available. The 0.5-year
infant was said to be 6 kg, and
c When mentioned, death was a consequence of severe liver damage.
d Epidemic.
e Estimate based on unpublished information available to the Task Group.
There is substantial overlap between intake rates and total
intakes for fatal and non-fatal poisoning. This presumably
reflects the influences of a number of factors, such as individual
sensitivity, age, nutritional status, and general health, but it is
also due to the progressive nature of pyrrolizidine toxicity and
the effects of time. In the epidemics, in which some people died,
only an estimated average intake is available and some who were
alive at the time of investigation may have died later. Comparing
the total intakes for human toxicity with the total doses up to
death observed in the long-term administration of PAs to rats,
1.2 - 10.9 times the LD50 dose, equivalent to 360 - 3270 mg
heliotrine/kg (Table 10, section 6.4.1.5), it is evident that human
beings are more susceptible to the acute and chronic effects of the
alkaloids than rats, sometimes markedly so.
These considerations of the toxic effects in human beings of
various intake levels could provide a basis for some assessment of
the likely hazard from other types of exposure to PAs. For
example, the consumption of comfrey root tea, estimated by Roitman
(1981) to contain 8.5 mg alkaloid per cup, at the rate of 3 cups
per day, or the ingestion of comfrey leaf at the rate of one leaf
per day, could lead to alkaloid ingestion rates of 0.40 and
0.016 mg/kg. These rates are respectively, much greater than, and
equal to, the lowest daily rate causing veno-occlusive disease. Lower
levels of exposure arising from such sources as the consumption of
milk from cows eating PA-containing plants or of honey derived from
such plants, seems unlikely, in practice, to cause acute or
subacute liver disease. However, care should be exercised. In an
experimental situation in which cows were fed Senecio jacobaea,
the milk was reported to contain up to 0.84 mg alkaloid/litre. A
30-kg child drinking 0.5 litre/day of this milk could ingest
0.014 mg/kg alkaloid, equivalent to 0.028 mg heliotrine/kg (assuming
an LD50 of 150 mg/kg for S. jacobaea alkaloid). This is above the
lowest daily rate leading to veno-occlusive disease and the lowest
estimated total toxic dose would be achieved in 36 days. This
level of contamination of milk is undoubtedly extreme and there is
no knowledge of any contamination of commercial milk supplies.
Honey derived from Echium plantagineum has been reported to contain
up to 1 mg alkaloid/kg (Culvenor et al., 1981). A 30-kg child
consuming 30 g/day of honey (a high consumption rate) would ingest
0.001 mg alkaloid/kg body weight. The lowest estimated total toxic
dose (1.7 mg comfrey alkaloid/kg, very similar to Echium alkaloid)
would be achieved in 1700 days. Although it seems likely that
consumption of contaminated milk and honey would lead to acute or
subacute liver disease, the possibility remains that they may
contribute to chronic liver disease or liver tumours.
The possibility of carcinogenic effects due to long-term
exposure to PA-containing plants has been discussed by Culvenor
(1983). Some of the PAs involved in instances of human poisoning
have been found to be carcinogenic in experimental animals (Table
13). Data from some of the significant experimental studies were
summarized by Culvenor (1983) with approximate estimates of PA
dosages administered to rats in terms of mg/kg body weight per day
(Table 17). The dose rates that were carcinogenic for rats (Table
17) ranged from 2 to 6 mg/kg per day for an initial period and
0.2 - 3 mg/kg per day for a remaining period of about 12 months,
except in one study in which a dose of 10 mg/kg per day was used.
It can be seen that, in all except two instances of human
poisonings summarized in Table 16, the estimated daily rates of
intake ranging from 0.015 to 3.3 mg/kg body weight per day are
within close range of those known to induce tumours in rats. In
other reports, the consumption rates are above and below this
range.
Epidemiological studies to assess the carcinogenic role of PAs
for man are not available. In countries with a high incidence of
primary liver cancer, it is possible that PAs may have an additive
effect with those attributed to aflatoxin (Newberne & Rogers, 1973)
and hepatitis B virus. The total evidence now available warrants
long-term studies of the survivors of poisoning outbreaks,
especially where a substantial number of people were affected, as
in the Afghanistan outbreak.
7.9 Pyrrolizidine Alkaloids as a Chemotherapeutic Agent for Cancer
The PA, indicine N-oxide derived from Heliotropium indicum, a
widely used indigenous drug in Ayurvedic medicine, has been found
to have an antitumour activity and has been used in clinical trials
as a chemotherapeutic agent for leukaemia (Letendre et al., 1981,
1984; Cook et al., 1983) and solid tumours (Kovach et al., 1979a,b;
Nichols et al., 1981; Ohnuma et al., 1982; Taylor et al., 1983).
Dosing schedules typically were 5 consecutive intravenous doses of
0.15 - 3 g/m2 body surface area (approximately 2.5 - 5 mg/kg body
weight) repeated at 4- or 6-week intervals (Kovach et al., 1979a;
Letendre et al., 1981; Ohnuma et al., 1982). Hepatic toxicity, as
judged by increased SGOP levels, was infrequent and mild. However,
subsequent trials with this agent have indicated more serious
hepatotoxicity. In a more recent report by the same workers
(Letendre et al., 1984), 5 out of 22 cases of refractory acute
leukaemia, treated with indicine N-oxide, had severe
hepatotoxicity, presumably induced by the drug. One of these
patients had been treated for 18 months with methyl-testosterone, 4
months prior to receiving indicine N-oxide. Symptoms of severe
hepatocellular failure appeared in 3 patients after the initial
course of treatment. This occurred after 4 daily doses of 3 g/m2
surface area in one patient and after 5 daily doses of 3.75 g/m2
surface area in 2 patients. Two other patients who had received
3 g/m2 surface area daily for 5 days developed hepatocellular failure
after the second course of treatment, one at 3.3 g/m2 and the other
at 3.75 g/m2 surface area, daily, for 5 days. In each patient, the
onset of hepatic disease was rapid and the course was downhill.
Livers of 4 of these patients examined at post-mortem showed severe
centrilobular vascular congestion with necrosis of parenchymal
cells, and, in one patient, a few sublobular veins were found to be
occluded.
Miser et al. (1982) reported severe hepatotoxicity in 4 of 45
children treated with indicine N-oxide for refractory leukaemia or
advanced solid tumours. Similarly, Cook et al. (1983) reported the
case of a 5-year-old child with acute myeloid leukaemia who
developed severe hepatic failure within 3 days of starting the
treatment. Autopsy showed massive hepatic necrosis.
However, it should be noted that no hepatic failure was
reported in more than 100 adults with solid tumours, treated with
the same agent (Kovach et al., 1979a,b; Nichols et al., 1981;
Taylor et al., 1983). No hepatotoxic effects were reported by
Ohnuma et al. (1982) among 37 patients who received this drug for
solid tumours. The major toxic effect was myelosuppression (Kovach
et al., 1979b).
Table 17. Rates of administration of PAs leading to tumours in ratsa
------------------------------------------------------------------------------------------
Alkaloid Dosing schedule Approximate Number of Reference
equivalent rateb rats
(mg/kg per day) developing
tumours
------------------------------------------------------------------------------------------
Lasiocarpine (a) 7 mg/kg diet, 24 months 0.70 23/24 Nat. Cancer
Institute
(1978)
(b) 15 mg/kg diet, 24 months 1.50 24/24 Nat. Cancer
Institute
(1978)
(c) 7.8 mg/kg, ip, 2/week for 2.2 for 4 weeks, 16/18 Svoboda &
4 weeks and 1/week for then 1.1 for Reddy (1972)
52 weeks 52 weeks
(d) 50 mg/kg diet, 55 weeks 5.0 18/20 Rao & Reddy
(1978)
(e) 0.39 mg/kg, ip, 3/week, 0.2 2/7 Culvenor &
to death Jago (1979)
Monocrotaline (a) 25 mg/kg, ip, 1/week for 3.5 for 4 weeks, 10/50 Newberne &
4 weeks, and 8 mg/kg, then 1.1 for Rogers (1973)
for 38 weeks 38 weeks
(b) 5 mg/kg sc, once per 0.36 43/60 Shumaker et
2 weeks for 52 weeks al. (1976)
Retrorsine (a) 30 mg/kg, ip, single dose - 7/29 Schoental &
Bensted (1963)
(b) 30 mg/litre in water, 1.3 4/14 Schoental et
3 days/week, to death al. (1954)
(c) 30 - 50 mg N-oxide/litre 1.3 - 2.0 10/22 Schoental et
in water, 3 days/week al. (1954)
for 20 months
------------------------------------------------------------------------------------------
Table 17. (contd)
------------------------------------------------------------------------------------------
Alkaloid Dosing schedule Approximate Number of Reference
equivalent rateb rats
(mg/kg per day) developing
tumours
------------------------------------------------------------------------------------------
Petasitenine 0.1 g/litre in water, 10 8/10 Hirono et
up to 16 months al. (1977)
Senkirkine 22 mg/kg, ip, 2/week for 6 for 4 weeks, 11/24 Hirono et
4 weeks and 1/week for then 3 for al. (1979a)
52 weeks 25 weeks
Symphytine 13 mg/kg, ip, 2/week for 3.7 for 4 weeks, 5/24 Hirono et
4 weeks and 1/week for then 1.9 for al. (1979a)
52 weeks 52 weeks
------------------------------------------------------------------------------------------
a From: Culvenor (1983).
b Where necessary, estimates assume a daily rat food intake of 100 g/kg body weight, and
water intake 100 ml/kg body weight. Injected doses are given pro rata, for daily
administration.
7.10 Prevention of Poisoning in Man
At present, prevention of poisoning can be achieved only by
reducing or eliminating ingestion of the alkaloids. The two
effective procedures are control of PA-containing plants in
agricultural areas and educational programmes directed to the
populations at risk.
The control of plant populations for this purpose has been
carried out only in Uzbekistan, USSR, following the epidemics of
human disease due to contamination of grain by seeds of
Heliotropium lasiocarpum and Trichodesma incanum. The following
measures were introduced and have been effective in preventing
further outbreaks:
1. A state standard was set for the quality of seed grain, which
must be certified by a State Seed Inspectorate. Current
standards prohibit the sowing of wheat, rye, barley, or oats
contaminated by seed of Heliotropium lasiocarpum or Trichodesma
incanum.
2. A state standard was set for the quality of grain stored for
food. The limits for Heliotropium lasiocarpum and
Trichodesma incanum seeds are 0.2% and zero, respectively.
3. Agricultural (agritechnical) measures to ensure minimum
contamination of crops and harvested grain, including
specification of the most suitable methods and timing of
cultivation, use of clean seed for sowing, weeding of crops
prior to maturing of the grain (towards the end of May), and
mechanical cleaning of grain.
4. Methods for monitoring levels of contamination of flour, bread,
and similar products.
5. Publication of educational booklets describing the biological,
environmental, and morphological characteristics of the toxic
weeds, their pathways of distribution and the causes of the
toxicoses experienced.
6. Promotion of weed control by governmental authorities and
provision of legislation to enforce the control measures.
In other countries, the control of some PA-containing weeds in
crops is practised by cultivation and herbicide treatment, in order
to maximize yield and the general quality of the grain. In
pastures, animal management and herbicide treatments are used to
increase pastures and reduce poisoning of animals. Specific
treatment methods differ according to the plant species and the
circumstances. General references were not available to the Task
Group.
In Australia, where Heliotropium europaeum and Echium
plantagineum are widespread weeds in wheat-growing areas but where
normal agricultural practices prevent all but occasional minor
contamination, relevant tolerance standards for wheat delivered at
storage silos are not specific. Heliotropium europaeum seed is
rarely seen and would form part of the "unmillable material" the
seed component of which can be up to 1% of the volume of the wheat.
Seed of Echium plantagineum is occasionally seen in delivered grain
at levels of up to 10 seeds per half litre, the tolerance level for
this seed fraction being 50 seeds per half litre.
8. BIOLOGICAL CONTROL
Biological control methods have been investigated for several
PA-containing plant species, notably Senecio jacobaea, Heliotropium
europaeum, Echium plantagineum, and Trichodesma incanum. The
effectiveness of such methods is variable and good results may be
confined to certain regions where favourable conditions exist. For
example, in control programmes against S. jacobaea in Australia,
Canada, New Zealand, and the USA, using 3 insect species, results
varied from virtually nil to nearly 100% control (Julien 1982).
The effects of the introduction of the cinnabar moth ( Tyria
jacobaea L.) on S. jacobaea in these countries have been summarized
as in Table 18.
Table 18. Results of the attempted control of Senecio
jacobaea (ragwort) with the cinnabar moth
------------------------------------------------------------
Country or region Result
------------------------------------------------------------
Australia Establishment precluded by predation,
parasitism and disease
Western Canada Moth populations stabilized below that
required for control
Eastern Canada Establishment and subsequent notable
reductions in ragwort levels
New Zealand Marginal establishment, moth population
limited by predation and parasitism,
little impact on ragwort
USA Widespread establishment, ragwort levels
sometimes reduced at localities near the
limits of its distribution
------------------------------------------------------------
Several agents are being tested in Australia for the control of
H. europaeum and one species, a flee beetle Longitarsus albineus,
has been released (Julien, 1982; Delfosse, 1985). There are good
prospects in this country for the biological control of Echium
plantagineum and 2 other Echium spp., with 8 insect species
approved for release, when legal restrictions are lifted (Delfosse
& Cullen, 1985a,b). Preliminary studies have been made on the
biological control of Amsinckia and other Senecio species (e.g.,
Pantone et al., 1985).
Given adequate funding, PA-containing plants are a suitable
target for biological control.
9. EVALUATION OF HUMAN HEALTH RISKS AND EFFECTS ON THE ENVIRONMENT
9.1 Human Exposure Conditions
9.1.1 Reported sources of human exposure
The two main sources of exposure of human beings to toxic PAs
that have led to major outbreaks of poisoning with high mortalities
as well as to individual cases in several countries are:
(a) the contamination of cereal grains, such as wheat and
millet, with the seed or other parts of plants containing
alkaloids; and
(b) the consumption for medicinal or dietary purposes of herbs
containing the alkaloids, either as the plant itself or as
infusions.
Consumption of contaminated grain is more likely to occur in
regions where food is in short supply, and particularly when
drought favours infestation of the grain crop by PA-containing
weeds. A qualitative field test for detecting the presence of
toxic pyrrolizidine alkaloids in plant materials, using simple
laboratory methods, is now available (section 2.2.2.5).
9.1.2 Plant species involved
Plant species containing toxic PAs occur throughout the world
and are known in 47 genera in 6 plant families. As many as 6000
species are potentially PA-containing. The most important genera
responsible for human and animal disease are Senecio and other
genera of the tribe Senecioneae (family Compositae), Crotalaria
(family Leguminosae) and Heliotropium, Trichodesma, and other
genera of the family Boraginaceae (sections 3.1 and 3.2).
Approximately 150 different toxic PAs have been isolated from
about 360 plant species that have been investigated and contain
this type of alkaloid. Of these, about 12 have been involved in
instances of human toxicity (section 3.1). The molecular
structures of almost all of these alkaloids are known and the main
outlines of structure-toxicity relationships have been established
(section 2.1).
The alkaloids may occur in all plant parts and are often
present as the N-oxide derivatives, which are also toxic when
ingested orally. Alkaloid contents vary from low (0.1 g/kg dry
weight) to very high (40 g/kg up to the maximum recorded of
180 g/kg in Senecio riddelli). Levels vary with stage of growth,
locality, and other circumstances. In some, but not all, species,
the alkaloid is partly decomposed during the drying or storage of
the plant. The decomposition is largely enzymic and once the plant
material is dry, the alkaloid is fairly stable.
9.1.3 Modes and pathways of exposure
9.1.3.1 Contamination of grain crops
Large outbreaks of poisoning have occurred through
contamination of wheat crops in Afghanistan, India, and the USSR.
In particular, 3 species of Boraginaceae (Heliotropium lasiocarpum,
H. popovii, and H. europaeum) are well adapted to vigorous growth
under the climatic conditions in which wheat is usually grown.
Contamination can be effectively controlled in wheat produced using
modern harvesting techniques and grain seed that is inspected and
controlled for weed seed contamination, but control of
contamination is more difficult where these conditions cannot be
met. Contamination of staple food grain is of particular concern,
since entire populations are exposed, and control may not be
possible, if the people are not aware of the hazard that
PA-containing weeds present.
9.1.3.2 Herbal medicines
Herbal preparations containing PAs are used as tonics,
treatments, preventatives, and food supplements. Such usages are
so widespread that they are nearly universal. Many are
traditional, while others reflect a rejection of, or lack of access
to, standard health care services (section 3.3.2).
Veno-occlusive disease was first recognized as a clinical
entity in Jamaica as a result of the medicinal use of PA-containing
herbs prepared from Crotalaria. Crotalaria-containing herbs have
also been responsible for human poisonings in Barbados, Equador,
and other locations in the West Indies. Heliotropium herbs have
been reported to cause poisoning in Hong Kong and India.
Symphytum- and Senecio-containing herbs have given rise to case
reports in the USA. Other reports of PA poisoning are known in
which the herbs used were not botanically identified. PA-poisoning
has been associated with both home-prepared and commercially
available herbs, the latter including prescriptions by herbalists
(Weston et al., 1987).
Various other genera of PA-containing plants in the families
Boraginaceae, Compositae, and Leguminosae are also widely used as
herbs. No case reports are available for these genera.
Reported cases of PA poisoning due to the use of the herbs are
geographically widespread, but few in number. However, the scale
on which PA-containing plants are used as herbs, the typically
delayed effects of long-term exposure, and the difficulties of
diagnosis led the Task Group to conclude that there is every
indication of under-reporting of intoxications from the use of such
herbs. Symphytum root preparations, in particular, represent a
hazard, and certain user groups are routinely exposed to levels of
Symphytum alkaloids that are higher than those at which
intoxications have been reported.
The risks associated with the use of PA-containing herbs are
accentuated by the difficulties of controlling this use.
9.1.3.3 PA-containing plants used as food and beverages
Some PA-containing plants are used for food or the making of
beverages in many countries, including developed countries. The
following species are known to be used (though many other plants
are also probably used in this way): Cacalia yatabei, Symphytum
species, Ligularia dentata, Petasites japonicus, Senecio burchellii,
S. inadequidens, S. pierotti, Syneilesis palmata, Crotalaria
anagyrodies, C. brevidens, C. juncea, C. laburnifolia, C. pumila,
C. recta, and C. retusa. No information is available on
the extent to which the different types are consumed (section 3.3.3).
9.1.3.4 Other foods contaminated by PAs
Several species of Boraginaceae are nectar and pollen sources
for bees. Echium plantagineum, in particular, is a widespread weed
in some countries and a substantial source of honey containing a
low level of alkaloid. Senecio species are also visited by bees
and yield alkaloid-containing honey, though Senecio-derived honey
is not known to be produced in quantity for sale. Thus, some
regional and local populations are exposed to a low-level intake
through the presence of PAs in honey, and surveillance may be
desirable in countries producing honey (section 3.3.4).
Under experimental conditions, PAs are transmitted from the
feed of dairy cows and goats into the milk. Some PA-containing
species, such as Senecio jacobaea, S. lautus, and Echium plantagineum,
are weeds in dairy pastures in some countries and are eaten by cattle
under certain conditions. There are no published reports of alkaloid
in milk supplies for human consumption (section 3.3.5).
The Task Group was not aware of any reported cases of
pyrrolizidine toxicity that had been ascribed to either honey or
dairy products.
No information was available to the Task Group on the possible
presence of alkaloids or their metabolites in meat from animals
that had consumed PA-containing plants shortly before slaughter.
The results of metabolic studies in rats have indicated that the
alkaloid is rapidly cleared from the body and, therefore, the
levels of PAs in meat are expected to be very low. However, there
is no information on the possibility of alkaloid accumulating in
storage sites.
9.1.4 Levels of intake
Reliable estimates of levels of intake of PAs, especially in
outbreaks of disease caused by the contamination of cereal crops
with the seeds of toxic plants, are extremely difficult to make.
Sampling of the contaminated grain may not be strictly
representative, since the extent of the contamination may vary in
different sites and households, as is evident from the estimates of
PA intake in the Indian and Afghan outbreaks reported in section
7.3. Furthermore, no accurate record is possible of the amount of
contaminated food consumed over an uncertain length of time. No
records of the levels of toxic PA intake are available in the
earlier reports of human toxicity. Where available, estimates of
intake in outbreaks caused by the contamination of staple food
crops have been made on the basis of random sampling of the
contaminated grain in food stores, and rough estimates of daily
consumption by average adults. Food-on-the-plate analyses have not
been made. The estimated lengths of exposure, and hence the amount
of total intake, are also approximate.
The contamination of cereal grains with the seed of
PA-containing plants has caused major epidemics of human poisoning,
though, in the two instances where estimates of alkaloid intake are
available, the intakes were lower than in some exposures due to the
use of herbal medicines. The estimated intakes are summarized in
Table 16. In an outbreak in India, millet contaminated with
Crotalaria nana seed had an average alkaloid content of 0.5 g/kg,
and the estimated daily intake by the population was 0.66 mg/kg
body weight. In a larger outbreak in Afghanistan, due to the seeds
of Heliotropium popovii in wheat, the level of contamination was
probably variable; representative samples of wheat contained
alkaloid at 0.04 g/kg. The estimated daily intake was 0.033 mg/kg
body weight. These intakes, sustained for periods of approximately
2 and 6 months, respectively, resulted in typical acute and
subacute veno-occlusive disease.
The highest intake rates have been associated with the use or
misuse of medicinal herbs and have resulted in acute liver damage
and death. In two occasions, the consumption of Heliotropium
eichwaldii as a treatment for epilepsy led to an estimated intake
of 3.3 mg/kg body weight daily for 20 or 50 days, and the use of
extracts of Senecio longilobus as medicine for young children led
to estimated intakes of 3 and 0.8 - 1.7 mg/kg body weight. The
highest intake led to extensive liver necrosis. However, it is
possible that, in the above case of poisoning by Heliotropium
eichwaldii, the toxicity was enhanced due to simultaneous
administration of phenobarbitone, which has a potentiating effect
on the microsomal enzymes in the liver cells that convert the PAs
to toxic metabolites.
The use of Heliotropium lasiocarpum as a component of a herbal
treatment for psoriasis involved somewhat lower daily intake rates
of 0.49 - 0.71 mg/kg body weight in 4 patients, who, after periods
of 19 - 46 days, developed veno-occlusive disease. The patient
with the longest intake period and a total intake of 1.4 g alkaloid
or 23 mg/kg body weight died.
The lowest ingestion rate leading to a case of veno-occlusive
disease was also due to medicinal herbal treatment or, more
specifically, to the use of a digestive aid containing a
preparation of comfrey root. Commercial herb and food supplement
preparations containing comfrey leaf or root are on sale in many
countries. Limited assays of one comfrey-pepsin preparation
prepared from comfrey root indicated a PA content of 2.9 g/kg.
Another preparation made from comfrey leaf contained up to 0.27 g
alkaloid/kg. The consumption of these preparations led to an
estimated daily intake of 0.015 mg/kg body weight. Veno-occlusive
disease was diagnosed after a 4- to 6-month period.
The consumption of Symphytum officinale (comfrey) and S. x
uplandicum (Russian comfrey) in the form of food, infusions, or
other preparations is widespread, though the full extent cannot be
estimated. A high level of consumption as salad appears to be
about 5 - 6 leaves per day and consumption as comfrey tea probably
reaches a similar level. Limited assays indicate that the average
alkaloid content of the leaf is about 1 mg/leaf, the concentration
being higher in the younger, smaller leaves. The alkaloid intake
from comfrey leaves could therefore vary from a low value, up to
about 6 mg/day, or 0.1 mg/kg body weight for an adult, an intake
within the range producing veno-occlusive disease. However, the
Task Group noted that some people claim to have consumed comfrey at
such a rate without suffering any disease.
Overall, the estimates of intake of PAs by human beings (Table
16) indicate that ingestion rates above 0.015 mg/kg body weight for
the mixture of echimidine and related alkaloids in comfrey may lead
to acute or subacute liver disease. If expressed in terms of
equivalent doses of heliotrine (section 7.8), the estimated total
doses in the known outbreaks or cases of veno-occlusive disease
range from 1 to 167 mg/kg body weight. There is little real
difference in the ranges of estimated total doses in non-fatal
cases (1 - 120 mg/kg body weight) and those leading to death
(6 - 167 mg/kg). These figures, when compared with the total
lethal dose of several PAs in rats, i.e., 1.2 - 10.9 times the LD50
dose (equivalent to 360 - 3270 mg heliotrine/kg) (Table 10), would
seem to indicate that man is markedly more sensitive than the rat
to the toxic effects of PAs with regard to the development of acute
and chronic effects on the liver. It should be noted that these
estimates are based on limited raw data and a number of
assumptions, and so are of uncertain reliability.
The dose estimates indicate strongly that the effects of PAs in
human beings are cumulative at very low intake rates. Lower rates
of intake of PAs may lead to chronic forms of intoxication, though,
at present, there is no evidence on which the degree of risk in
these circumstances can be evaluated. The information available on
dose-response relationships is very limited, but the data support
the conclusion that even low rates of intake of PAs over a period
of time may present a health risk and that exposure should be
minimized wherever possible.
There has not been any systematic monitoring of PAs in cereal
grains, food products, and herbal medicines. Analytical surveys of
these materials are feasible, but it would be difficult to design
surveys that would give direct estimates of the dietary intake of
PAs.
9.2 Acute Effects of Exposure
9.2.1 Acute liver disease
All cases of human intoxication in reported accounts have been
in the acute phase of the disease, the dominant symptom being
rapidly filling ascites. The disease can affect large
subpopulations and, in one study, up to 22.6% of the population was
affected.
Children appear to be the most vulnerable group and mortality
can be high at the extremes of age. The liver is the principal
target organ. In the acute stage of the disease, the liver shows a
characteristic centrilobular haemorrhagic necrosis, which in man is
accompanied by occlusion of the hepatic veins. However,
characteristic veno-occlusive lesions, seen in the central veins of
hepatic lobules, may not always be evident in the needle biopsy
examination of the liver, but are always apparent on examination of
the autopsy material.
9.3 Chronic Effects of Exposure
9.3.1 Cirrhosis of the liver
There is evidence that the administration of a single dose of
PA to experimental animals or a single acute episode of illness in
man, following brief consumption of PA-containing herbs or
PA-contaminated food, may lead to progressive chronic liver disease
resulting in cirrhosis. Cirrhosis may also be a consequence of
long-term low-dose administration of PAs to experimental animals
and possibly also of long-term low intake of PAs by human beings,
though there is no proof of the latter. Cirrhosis resulting from
the toxic effects of PAs in the advanced stage, may not be
distinguishable from that resulting from other causes (sections
6.4.1.5 and 7.4). The Task Group did not find any evidence
suggesting that PAs are a causative factor of the specific disease,
Indian Childhood Cirrhosis (section 7.5).
9.3.2 Mutagenicity and teratogenicity
Several PAs, PA-derivatives, and related compounds have been
shown to produce chromosome aberrations in plants and several cell
culture systems, mutagenic effects ( Salmonella ("Ame's"), sister
chromatid exchanges, and other tests), and teratogenic and
fetotoxic effects in experimental animals (sections 6.4.5, 6.4.6,
6.4.7). Chromosomal aberrations have been reported in the blood
cells of children suffering from veno-occlusive disease, believed
to have been caused by fulvine. The Task Group was not aware of
data on the teratogenic/fetotoxic effects of PAs on human beings
and was unable to evaluate the potential for these effects in PA
exposure.
9.3.3 Cancer of the liver
A relatively large number of people have been exposed, in the
past, to PAs and have suffered acute and chronic toxic effects.
However, no information is available on the long-term follow-up of
these populations, to ascertain whether this type of exposure could
have resulted in an increased incidence of liver cancer or other
types of cancer. Because of this lack of knowledge, it is not
possible, at present, to make an evaluation of the cancer risk due
to PAs. However, various PAs have been shown to be carcinogenic
for experimental animals, which implies that a potential cancer
risk for human beings should be seriously considered.
Of several PAs evaluated for carcinogenicity by IARC (1976,
1983), there is "sufficient or limited evidence" for the
carcinogenicity in experimental animals (IARC, 1976) of
monocrotaline, retrorsine, isatidine, lasiocarpine, petasitenine,
senkirkine, and of extracts of the PA-containing plants Petasites
japonicum, Tussilago farara, Symphytum officinale, Senecio
longilobus, Senecio numorensis, Farfugium japonicum, and Senecio
cannabifolius. These studies were carried out mainly on rats, with
few studies on mice or hamsters (section 6.4.8). The
carcinogenicity data obtained with other PAs are difficult to
evaluate, because of the limited number of treated animals and the
lack of adequate numbers as controls. The main target organ is the
liver, where liver cell tumours and haemangioendothelial sarcomas
were observed. In some instances, tumours in extra-hepatic tissues
(lung, pancreas, intestine) were also observed, namely with
monocrotaline, retrorsine, and lasiocarpine. Some PAs, for
example, retrorsine, have been shown to be carcinogenic after a
single dose. The pyrrolic metabolites have also been shown to be
carcinogenic for rats.
It may be recalled that several of the PAs involved in human
poisoning include the above compounds. It is notable that the dose
rates that have been effective in inducing tumours in rats, mostly
equivalent to 0.2 - 3 mg/kg body weight per day (Table 17), are
roughly similar in magnitude to estimated intake rates (0.49 -
3.3 mg/kg body weight per day) (Table 16) in several episodes of human
toxicity. Comparison of the total intakes resulting in human
toxicity with the total doses to death observed in the chronic
toxicity studies on rats indicates that human beings are more
susceptible (section 7.8) and suggests that human beings may
survive for sufficient time to develop cancer after only a brief
exposure at this level or a longer exposure at a markedly lower
level. A more quantitative assessment is not possible on the basis
of the available information, and the Task Group stressed the need
for appropriate epidemiological studies.
9.3.4 Effects on other organs
Substantiated reports of PA-induced extra-hepatic injury in man
are limited to Trichodesma intoxication, in which symptoms and
signs were predominantly neurological. The range of organs
affected by other PAs in experimental and farm animals suggests
that exposure of human beings to other PAs may also carry the
potential for extra-hepatic injury.
There are extensive reports of experimental studies in which
PAs have been demonstrated to produce the characteristic vascular
changes of primary pulmonary hypertension and consequent right
ventricular hypertrophy of the heart in rats and non-human primates
(section 6.4.2). Susceptibility is age dependent, weanling rats
being more vulnerable than older animals. There is only
circumstantial evidence of PA-induced pulmonary vascular disease in
one patient (section 7.6), but judging by the experimental evidence
available, it is possible that human beings may be susceptible to
PA-induced cardiopulmonary changes.
In the opinion of the Task Group, the neurological involvement
which is a dominant feature in PA-intoxicated horses and is also
seen in cows and sheep, cannot be explained solely as a consequence
of liver damage. Central nervous system lesions have been
demonstrated in sheep, pigs, and rats. Distribution studies of the
radiolabelled metabolite, 3H-dehydroretronecine, show increasing
accumulation of radioactivity in the brain with time.
Trichodesma alkaloids, structurally related to monocrotaline,
are neurotoxic agents. Trichodesma toxicosis in man has been
reported only from the USSR, together with several studies on
experimental animals. Detailed reports on the pathological
findings were not available to the Task Group, but the information
available indicated that the central nervous system was the primary
target organ (sections 6.4.3 and 7.7).
Stomach and intestinal lesions have been shown in PA-exposed
sheep, mice, cows, and rats. Distribution studies with
radiolabelled pyrroles showed a high retention of radioactivity in
the stomach, consistent with the acid-sensitive nature of the
pyrroles. In rats, pyrrolic metabolites are secreted in high
concentrations in the bile.
Kidney changes following to PA administration have been shown
in mice, pigs, horses, sheep, and monkeys. Pyrrolizidine
metabolites have been found covalently bound to kidney DNA in rats.
Urinary excretion is a major route of excretion of metabolic
products of PAs in rats.
There is no evidence of involvement of organs other than the
liver and central nervous system ascribed primarily to PA toxicity
in any of the published human case reports. It is possible that
under some circumstances, other major organ systems may also be at
risk. As bioactivation of PAs has been demonstrated only in the
liver, the risk of damage should be expected to be lower in the
organs.
9.4 Effects on the Environment
9.4.1 Agriculture
In some countries, PA-containing weeds densely cover areas of
up to thousands of square kilometres. Their adverse effects
include the covering of pastures, additional costs in agricultural
production, and the poisoning of farm animals. The toxicity of PAs
for farm animals, including sheep, cattle, horses, pigs, goats, and
poultry, which has been the inspiration for much of the investigation
of PA toxicity. In Australia, for example, Heliotropium europaeum
and Echium plantagineum cause the death of thousands of animals
annually (section 6.2).
9.4.2 Wild-life
By contrast, little is known about the consumption of PA-containing
plants by wild-life, or of their individual sensitivities. The death
of deer in Louisiana has been ascribed to eating Heliotropium or
Crotalaria species, and an experimental study has shown that the
rainbow trout (Salmo gairdneri) is sensitive to Senecio jacobaea
alkaloids (sections 6.5.1 and 6.5.2).
There is no information on the effects of the alkaloids on
field rodents or other seed-eating mammals and birds that might be
expected to consume seeds of PA-containing plants and to suffer
toxic effects.
9.4.3 Insects
Many species of insects, such as some moths of the family
Arctidae and butterflies of the sub-families Danainae and
Ithominae, have become dependent on PA-containing plants, using the
alkaloids as defensive chemicals and derivatives of them as
pheromones and other signalling chemicals. Thus, complete
elimination of PA-containing plants in a region might lead to a
marked reduction in the local population of insects of this type
(section 6.5.3).
9.4.4 Soil and water
There have not been any studies on the fate of PAs when the
plants in which they occur wilt and age. If alkaloid is leached
into soil or water, it is probably readily degraded by microorganisms
since, as a base and ester, it is subject to oxidative and hydrolytic
reactions.
REFERENCES
AFZELIUS, B.A. & SCHOENTAL, R. (1967) The ultrastructure of
enlarged hepatocytes induced in rats with a single oral dose of
retrorsine, pyrrolizidine (Senecio) alkaloids. J. ultra-struct.
Res., 20: 328-345.
AIKAT, B.K., BHUSNURMATH, S.R., DATTA, D.V., & CHHUTTANI, P.N.
(1978) Veno-occlusive disease in north-west India. Indian J.
Pathol. Microbiol., 21: 203-211.
AL-HASANY, M. & MOHAMED, A.S. (1970) Veno-occlusive disease of the
liver in Iraq. Arch. dis. Child., 45: 722-724.
ALLEN, J.R. & CARSTENS, L.A. (1968) Veno-occlusive disease in
rhesus monkeys. Am. J. vet. Res., 29: 1681-1694.
ALLEN, J.R. & CARSTENS, L.A. (1970) Pulmonary vascular occlusions
initiated by endothelial lysis in monocrotaline-intoxicated rats.
Exp. mol. Pathol.., 13: 159-171.
ALLEN, J.R. & CARSTENS, L.A. (1971) Monocrotaline induced Budd-
Chiari syndrome in monkeys. Am. J. dig. Dis., 16: 111-121.
ALLEN, J.R. & CHESNEY, C.F. (1972) Effect of age on development of
cor pulmonale in non-human primates following pyrrolizidine
alkaloid intoxication. Exp. mol. Pathol., 17: 220-232.
ALLEN, J.R., CHILDS, G.R., & CRAVENS, W.W. (1960) Crotalaria
spectabilis toxicity in chickens. Proc. Soc. Exp. Biol. Med., 104:
434-436.
ALLEN, J.R., LALICH, J.J., & SCHMUTTLE, S.M. (1963) Crotalaria
spectabilis induced cirrhosis in turkeys. Lab. Invest., 12: 512-517.
ALLEN, J.R., CARSTENS, L.A., & OLSON, B.E. (1967) Veno-occlusive
disease in Macaca speciosa monkeys. Am. J. Pathol., 50: 653-667.
ALLEN, J.R., CARSTENS, L.A., & KATAGIRI, G.J. (1969) Hepatic veins
of monkeys with veno-occlusive disease-sequential ultrastructural
changes. Arch. Pathol., 87: 279-289.
ALLEN, J.R., CHESNEY, C.F., & FRAZEE, W.J. (1972) Modifications of
pyrrolizidine alkaloids intoxication resulting from altered
microsomal enzymes. Toxicol. appl. Pharmacol., 23: 470-479.
ALLEN, J.R., HSU, I.-C., & CARSTENS, L.A. (1975) Dehydroretronecine
induced rhabdomyosarcomas in rats. Cancer Res., 35: 997-1002.
AMES, M.M. & POWIS, G. (1978) Determination of indicine N-oxide
and indicine in plasma and urine by electron-capture gas-liquid
chromatography. J. Chromatogr., 166: 519-526.
ARMSTRONG, S.J. & ZUCKERMAN, A.J. (1970) Production of pyrroles
from pyrrolizidine alkaloids by human embryo tissue. Nature
(Lond.), 228: 569-570.
ARMSTRONG, S.J., ZUCKERMAN, A.J., & BIRD, R.G. (1972) Induction of
morphological changes in human embryo liver cells by the
pyrrolizidine alkaloid lasiocarpine. Br. J. exp. Pathol., 53:
145-149.
ARORA, R.R., PYARELAL, GHOSH, T.K., MATHUR, K.K., & TANDON, B.N.
(1981) Epidemiology of veno-occlusive disease in tribal population
of Madhya Pradesh and Bihar. J. commun. Dis. (India), 13: 147-151.
ARSECULERATNE, S.N., GUNATILAKA, A.A.L., & PANABOKKE, R.G. (1981)
Studies on medicinal plants of Sri Lanka: occurrence of
pyrrolizidine alkaloids and hepatotoxic properties in some
traditional medicinal herbs. J. Ethnopharmacol., 4: 159-177.
ASADA, Y., FURUYA, T., & MURAKAMI, N. (1981) Pyrrolizidine
alkaloids from Ligularia japonica. Planta Med., 42: 202-203.
ASADA, Y., SHIRAISHI, M., TAKEUCHI, T., OSAWA, Y., & FURUYA, T.
(1985) Pyrrolizidine alkaloids from Crassocephalum crepidioides.
Planta Med., 51: 539-540.
BARNES, J.M., MAGEE, P.N., & SCHOENTAL, R. (1964) Lesions in the
lungs and livers of rats poisoned with pyrrolizidine alkaloids
fulvine and its N-oxide. J. Pathol. Bacteriol., 88: 521-531.
BERRY, D.M. & BRAS, G. (1957) Venous occlusion of the liver in
Crotalaria and Senecio poisoning. North Am. Vet., 38: 323-327.
BHATTACHARYA, K.J. (1965) Foetal and neonatal responses to
hepatotoxic agents. J. Pathol. Bacteriol., 90: 151-161.
BICCHI, C., D'AMATO, A., & CAPPELLETTI, E. (1985) Determination
of pyrrolizidine alkaloids in Senecio inaequidens D.C. by
capillary gas chromatography. J. Chromatogr., 349: 23-29.
BICK, Y.A.E. (1970) Comparison of the effects of LSD, heliotrine,
and X-irradiation on chromosome breakage and the effects of LSD on
the rats of cell division. Nature (Lond.), 226: 1165-1167.
BICK, Y.A.E. & CULVENOR, C.C.J. (1971) Effects of dehydro-
heliotridine, a metabolite of pyrrolizidine alkaloids on chromosome
structure and cell division in cultures of animal cells. Cytobios,
3: 245-255.
BICK, Y.A.E. & JACKSON, W.D. (1968) Effects of the pyrrolizidine
alkaloid heliotrine on cell division and chromosome breakage in
cultures of leucocytes from the marsupial Potorus tridactylus.
Aust. J. biol. Sci., 21: 469-481.
BICK, Y.A.E., CULVENOR, C.C.J., & JAGO, M.V. (1975) Comparative
effects of pyrrolizidine alkaloids and related compounds on
leukocyte cultures from Potorus tridactylus. Cytobios, 14: 151-160.
BIRECKA, H., FROHLICH, M.W., HULL, L., & CHASKES, M.J. (1980)
Pyrrolizidine alkaloids of Heliotropium from Mexico and adjacent
USA. Phytochemistry, 19: 421-426.
BIRECKA, H., CATALFAMO, J.L., & EISEN, R.N. (1981) A sensitive
method for detection and quantitative determination of
pyrrolizidine alkaloids. Phytochemistry, 20: 343-344.
BLACK, D.N. & JAGO, M.V. (1970) Interaction of
dehydroheliotridine, a metabolite of heliotridine based
pyrrolizidine alkaloids, with natural and heat denatured RNA.
Biochem. J., 118: 347-353.
BOHLMANN, F., KLOSE, W., & NIKISCH, K. (1979) [Synthesis of
dehydroheliotridine and 3-oxy-dehydroheliotridine.] Tetrahedr.
Lett., 1979: 3699-3702 (in German).
BOHLMANN, F., ZDERO, C., JAKUPOVIC, J., GRENZ, M., CASTRO, V.,
KING, R.M., ROBINSON, H., & VINCENT, L.P.D. (1986) Further
pyrrolizidine alkaloids and furoeremophilanes from Senecio species.
Phytochemistry, 15: 1151-1159.
BOPPRE, M. (1986) Insects pharmacophagously utilizing defensive
plant chemicals (pyrrolizidine alkaloids). Naturwiss enschaften, 73:
17-26.
BRAGINSKII, B.M. & BOBOKHADZAEV, I. (1965) [Hepatosplenomegaly
against the background of heliotropic toxicosis.] Sov. Med., 28:
57-60 (in Russian).
BRAS, G. (1973) Aspects of hepatic vascular diseases. In: Gall,
E.A. & Mostofi, F.K., ed. The liver: International Academy of
Pathology monograph, Baltimore, Maryland, Williams and Wilkins,
pp. 406-530.
BRAS, G. & HILL, K.R. (1956) Veno-occlusive disease of the liver -
essential pathology. Lancet, 2: 161-163.
BRAS, G. & WATLER, D.C. (1955) Further observations on the
morphology of veno-occlusive disease of the liver in Jamaica. West
Indian med. J., 4: 201-211.
BRAS, G., JELLIFFE, D.B., & STUART, K.L. (1954) Veno-occlusive
disease of the liver with nonportal type of cirrhosis occurring in
Jamaica. Arch. Pathol., 57: 285-300.
BRAS, G., BERRY, D.M., & GYORGI, P. (1957) Plants as etiological
factor in veno-occlusive disease of liver. Lancet, 1: 960-962.
BRAS, G., BROOKS, S.E.H., & WATLER, D.C. (1961) Cirrhosis of liver
in Jamaica. J. Pathol. Bacteriol., 82: 503-512.
BRAUCHLI, J., LUTHY, J., ZWEIFEL, U., & SCHLATTER, C. (1982)
Pyrrolizidine alkaloids from Symphytum officinale L. and their
percutaneous absorption in rats. Experientia (Basel), 38:
1085-1087.
BRIGGS, L.H., CAMBIE, R.C., CANDY, B.J., O'DONOVAN, G.M., RUSSELL,
R.A., & SEELYE, R.N. (1965) Alkaloids of New Zealand Senecio
species. Part II: senkirkine. J. Chem. Soc., C1965: 2492-2498.
BRINK, N.G. (1982) Somatic and teratogenic effects induced by
heliotrine in Drosophilia. Mutat. Res., 104: 105-111.
BROOKS, W.E.H., MILLER, C.G., MCKENZIE, K., AUDRETSCH, J.J., &
BRAS, G. (1970) Acute veno-occlusive disease of the liver. Fine
structure in Jamaican children. Arch. Pathol., 89: 507-529.
BROWN, K.S. (1984) Chemical ecology of dehydropyrrolizidine
alkaloids in adult Ithomiinae (Lepidoptera: Nymphalidae). Rev.
Bras. Biol., 44: 435-440.
BRUGGEMAN, I.M. & VAN DER HOEVEN, J.C.M. (1985) Induction of SCEs
by some pyrrolizidine alkaloids in V79 Chinese hamster cells co-
cultured with chick embryo hepatocytes. Mutat. Res., 142: 209-212.
BRUNER, L.H., CARPENTER, L.J., HAMLOW, P., & ROTH, R.A. (1986)
Effect of a mixed-function oxidase inducer and inhibitor on
monocrotaline pyrrole pneumotoxicity. Toxicol. appl. Pharmacol., 85:
416-427.
BUCKMASTER, G.W., CHEEKE, P.R., ARSCOTT, G.H., DICKINSON, E.D.,
PIERSON, M.L., & SHULL, L.R. (1977) Response of Japanese quail to
dietary and injected pyrrolizidine (Senecio) alkaloid. J. anim.
Sci., 45(6): 1322-1325.
BULL, L.B. (1955) The histological evidence of liver damage from
pyrrolizidine alkaloids. Aust. vet. J., 31: 33-40.
BULL, L.B. & DICK, A.T. (1959) The chronic pathological effects on
the liver of the rat of the pyrrolizidine alkaloids heliotrine,
lasiocarpine, and their N-oxides. J. Pathol. Bacteriol., 78: 483-502.
BULL, L.B. & DICK, A.T. (1960) The function of total dose in the
production of chronic lethal disease in rats by periodic injections
of the pyrrolizidine alkaloid heliotrine. Aust. J. exp. Biol. med.
Sci., 38: 515.
BULL, L.B., DICK, A.T., KEAST, J.C., & EDGAR, G. (1956) An
experimental investigation of the hepatotoxic and other effects on
sheep of consumption of Heliotropium europaeum L. Heliotropium
poisoning of sheep. Aust. J. agric. Res., 7: 281-332.
BULL, L.B., DICK, A.T., & MCKENZIE, J.S. (1958) The acute effects
of heliotrine and lasiocarpine and their N-oxides on the rat. J.
Pathol. Bacteriol., 75: 17-25.
BULL, L.B., CULVENOR, C.C.J., & DICK, A.T. (1968) The
pyrrolizidine alkaloids, Amsterdam, North Holland Publishing Co.
BURGUERA, J.A., EDDS, G.T., & OSUNA, O. (1983) Influence of
selenium on aflatoxin B1 or Crotalaria toxicity in turkey poults.
Am. J. vet. Res., 44: 1714-1717.
BURNS, J. (1972) The heart and pulmonary arteries in rat fed on
Senecio jacobaea. J. Pathol., 106: 187-194.
BUTLER, W.H., MATTOCKS, A.R., & BARNES, J.M. (1970) Lesions in the
liver and lungs of rats given pyrrole derivatives of pyrrolizidine
alkaloids. J. Pathol., 100: 169-175.
CAMPBELL, J.G. (1956) An investigation of the hepatotoxic effects
in the fowl of ragwort ( Senecio jacobaea Linn) with special
reference to the induction of liver tumours with seneciphylline.
Proc. R. Soc. Edinburgh, B66: 111-130.
CAMPBELL, J.G. (1957a) Studies on the influence of sex hormones on
the avian liver. II. Acute liver damage in the male fowl and the
protective effect of oestrogen, as determined by a liver function
test. J. Endocrinol., 15: 346-350.
CAMPBELL, J.G. (1957b) Studies on the influence of sex hormones on
avian liver. III. Oestrogen-induced regeneration of the chronically
damaged liver. J. Endocrinol., 15: 351-354.
CANDRIAN, U., LUTHY, J., GRAF, U., & SCHLATTER, CH. (1984a)
Mutagenic activity of the pyrrolizidine alkaloids seneciphylline
and senkirkine in Drosophila and their transfer into rat milk.
Food chem. Toxicol., 22: 223-225.
CANDRIAN, U., LUTHY, J., SCHMID, P., SCHLATTER, CH., & GALLASZ, E.
(1984b) Stability of pyrrolizidine alkaloids in hay and silage. J.
agric. food Chem., 32: 935-937.
CANDRIAN, U., LUTHY, J., & SCHLATTER, CH. (1985) In vivo binding
of retronecine-labelled (3H), seneciphylline, and 3H-senecionine to
DNA of rat liver, lung and kidney. Chem.-biol. Interact., 54: 57-69.
CARILLO, L. & AVIADO, D.M. (1969) Monocrotaline induced pulmonary
hypertension and p-chlorophenylalanine. Lab. Invest., 20: 213-218.
CARSTENS, L.A. & ALLEN, J.R. (1970) Arterial degeneration and
glomerular hyalinization in the kidney of monocrotaline intoxicated
rats. Am. J. Pathol., 60: 75-92.
CHALMERS, A.H., CULVENOR, C.C.J., & SMITH, L.W. (1965)
Characterization of pyrrolizidine alkaloids by gas, thin layer, and
paper chromatography. J. Chromatogr., 20: 270-277.
CHEEKE, P.R. & GORMAN, G.R. (1974) Influence of dietary protein
and sulphur amino-acid levels on the toxicity of Senecio jacobaea
(tansy ragwort) to rats. Nutr. Rep. Int., 9: 197-207.
CHEEKE, P.R. & PIERSON-GOEGER, M.L. (1983) Toxicity of Senecio
jacobaea and pyrrolizidine alkaloids in various laboratory animals
and avian species. Toxicol. Lett., 18: 343-349.
CHEN, K.K. (1945) Pharmacology. Hepatotoxic alkaloids. Ann. Rev.
Physiol., 7: 695-697.
CHEN, K.K., HARRIS, P.N., & ROSE, C.L. (1940) The action and
toxicity of platyphylline and seneciphylline. J. Pharmacol. exp.
Ther., 68: 130-140.
CHESNEY, C.F. & ALLEN, J.R. (1973a) Resistance of the guinea pig
to pyrrolizidine alkaloid intoxication. Toxicol. appl. Pharmacol.,
26: 385-392.
CHESNEY, C.F. & ALLEN, J.R. (1973b) Endocardial fibrosis
associated with monocrotaline induced pulmonary hypertension in
non-human primates (Macaca arctoides). Am. J. vet. Res., 34:
1577-1581.
CHESNEY, C.F., HSU, I.C., & ALLEN, J.R. (1974) Modifications of
the in vitro metabolism of the hepatotoxic pyrrolizidine alkaloid,
monocrotaline. Res. Commun. chem. Pathol. Pharmacol., 8: 567-570.
CHOPRA, R.N., ed. (1933) Indigenous drugs of India, Calcutta, The
Art Press.
CLARK, A.M. (1959) Mutagenic activity of the alkaloid heliotrine
in Drosophila. Nature (Lond.), 183: 731-732.
CLARK, A.M. (1976) Naturally occurring mutagens. Mutat. Res., 32:
361-174.
COOK, B.A., SINNHUBER, J.R., THOMAS, P.J., OLSON, T.A., SILVERMAN,
T.A., JONES, R., WHITEHEAD, V.M., & ROYMANN, F.B. (1983) Hepatic
failure secondary to indicine N-oxide toxicity. A pediatric
oncology group study. Cancer, 52: 61-63.
COOK, J.W., DUFFY, E., & SCHOENTAL, R. (1950) Primary liver
tumours in rats following feeding with alkaloids of Senecio
jacobaea. Br. J. Cancer, 4: 405-410.
CRAIG, A.M., SHEGGEBY, G., & WICKS, C.E. (1984) Large scale
extraction of pyrrolizidine alkaloids from tansy ragwort (Senecio
jacobaea). Vet. hum. Toxicol., 26: 108-111.
CULVENOR, C.C.J. (1968) Tumour inhibitory activity of
pyrrolizidine alkaloids. J. pharm. Sci., 57: 1112-1117.
CULVENOR, C.C.J. (1978) Pyrrolizidine alkaloids: occurrence and
systematic importance in angiosperms. Bot. Notiser, 131: 473-486.
CULVENOR, C.C.J. (1980) Alkaloids and human disease. In: Smith,
R.L. & Bababunmi, E.A., ed. Toxicology in the tropics, London,
Taylor & Francis Ltd., pp. 124-141.
CULVENOR, C.C.J. (1983) Estimated intakes of pyrrolizidine
alkaloids by humans. A comparison with dose rates causing tumours
in rats. J. Toxicol. environ. Health, 11: 625-635.
CULVENOR, C.C.J. (1985) Pyrrolizidine alkaloids: some aspects of the
Australian involvement. Trends pharmacol. Sci., 6: 18-22.
CULVENOR, C.C.J. & JAGO, M.V. (1979) Carcinogenic plant products
and DNA. In: Grover, P.L., ed. Chemical carcinogens and DNA, Boca
Raton, Florida, CRC Press, Vol. 1, 161 pp.
CULVENOR, C.C.J., DOWNING, D.T., EDGAR, J.A., & JAGO, M.V. (1969)
Pyrrolizidine alkaloids as alkylating and antimitotic agents. N.Y.
Acad. Sci., 163: 837-847.
CULVENOR, C.C.J., EDGAR, J.A., SMITH, L.W., & TWEEDDALE, H.J.
(1970a) Dihydropyrrolizines. III. Preparation and reactions of
derivatives related to pyrrolizidine alkaloids. Aust. J. Chem., 23:
1853-1867.
CULVENOR, C.C.J., EDGAR, J.A., SMITH, L.W., & TWEEDDALE, H.J.
(1970b) Dehydrolizines. IV. Manganese dioxide oxidation of
1,2-dihydropyrrolizidines. Aust. J. Chem., 23: 1869-1879.
CULVENOR, C.C.J., EDGAR, J.A., JAGO, M.V., OUTTERIDGE, A., PETERSON
J.E., & SMITH, L.W. (1976a) Hepato- and pneumotoxicity of
pyrrolizidine alkaloids and derivatives in relation to molecular
structure. Chem.-biol. Interact., 12: 299-324.
CULVENOR, C.C.J., EDGAR, J.A., SMITH, L.W., & HIRONO, I. (1976b)
The occurrence of senkirkine in Tussilago farfara. Aust. J. Chem.,
29: 229-233.
CULVENOR, C.C.J., CLARKE, M., EDGAR, J.A., FRAHN, J.L., JAGO, M.V.,
PETERSON, J.E., & SMITH, L.W. (1980a) Structure and toxicity of
the alkaloids of Russian comfrey ( Symphytum x uplandicum Nyman), a
medicinal herb and item of human diet. Experientia (Basel), 36:
377-389.
CULVENOR, C.C.J., EDGAR, J.A., FRAHN, J.L., & SMITH, L.W. (1980b)
The alkaloids of Symphytum x uplandicum (Russian comfrey). Aust.
J. Chem., 33: 1105-1113.
CULVENOR, C.C.J., EDGAR, J.A., & SMITH, L.W. (1981) Pyrrolizidine
alkaloids in honey from Echium plantagineum L. J. agric. food
Chem., 29: 958-960.
CULVENOR, C.C.J., JAGO, M.V., PETERSON, J.E., SMITH, L.W., PAYNE,
A.L, CAMPBELL, D.G., EDGAR, J.A., & FRAHN, J.L. (1984) Toxicity of
Echium plantagineum (Paterson's curse). I. Marginal toxic effects
of Merino wethers from long-term feeding. Aust. J. agric. Res., 35:
293-304.
CULVENOR, C.C.J., EDGAR, J.A., SMITH, L.W., KUMANA, C.R., & LIN,
H.J. (1986) Heliotropium lasiocarpum Fisch and Mey identified as
cause of veno-occlusive disease due to a herbal tea. Lancet, 1: 978.
DANN, A.T. (1960) Detection of N-oxides of the pyrrolizidine
alkaloids. Nature, 4730: 1051.
DANNINGER, T., HAGEMANN, U., SCHMIDT, V., & SCHOENHOEFER, P.S.
(1983) Toxicity of pyrrolizidine alkaloid-containing medicinal
plants. Pharm. Ztg, 128: 289-303.
DATTA, D.V., KHUROO, M.S., MATTOCKS, A.R., AIKAT, B.K., &
CHHUTTANI, P.N. (1978a) Veno-occlusive disease of liver due to
Heliotropium plant used as medicinal herb (report of six cases with
review of literature). J. Assoc. Phys. India, 26(5): 383-393.
DATTA, D.V., KHUROO, M.S., MATTOCKS, A.R., AIKAT, B.K., &
CHHUTTANNI, P.N. (1978b) Herbal medicines and veno-occlusive
disease in India. Postgrad. med. J., 54: 511-515.
DAVIDSON, C.S. (1963) Plants and fungi as etiologic agents of
cirrhosis. New Engl. J. Med., 268: 1072-1073.
DAVIDSON, J. (1935) The action of retrorsine on rat's liver. J.
Pathol. Bacteriol., 40: 285-295.
DEAGEN, J.T. & DEINZER, M.L. (1977) Improvement in the extraction
of pyrrolizidine alkaloids. Lloydia, 40: 395-397.
DEAN, R.E. & WINWARD, A.H. (1974) An investigation into the
possibility of tansy ragwort poisoning of black-tailed deer. J.
wildl. Dis., 10: 166-169
DEINZER, M.L., THOMSON, P.A., BURGETT, D.M., & ISAACSON, D.L.
(1977) Pyrrolizidine alkaloids: their occurrence in honey from
tansy ragwort (S. jacobaea). Science, 195: 497-499.
DEINZER, M.L., THOMSON, P.A., GRIFFIN, D., & DICKINSON, E. (1978)
Sensitive analytical method for pyrrolizidine alkaloids. The mass
spectra of retronecine derivatives. Biomed. mass Spectrom., 5:
175-179.
DELFOSSE, E.S. (1985) Re-evaluation of the biological control
program for Heliotropium europaeum in Australia. In: Delfosse,
E.S., ed. Proceedings of the VI International Symposium on
Biological Control of Weeds, 19-25 August 1984, Vancouver, Canada,
Ottawa Agriculture Canada, pp. 735-42.
DELFOSSE, E.S. & CULLEN, J.M. (1985a) CSIRO division of Entomology
Submission to the enquiries into biological control of Echium
plantagineum L., Paterson's curse/Salvation Jane. Plant Prot. Q.,
1: 24-40.
DELFOSSE, E.S. & CULLEN, J.M. (1985b) Echium plantagineum:
catalyst for conflict and change in Australia. In: Delfosse, E.S.,
ed. Proceedings of the VI International Symposium on Biological
Control of Weeds, 19-25 August, 1984, Vancouver, Canada, Ottawa
Agriculture Canada pp. 249-292.
DELORME, P., JAY, M., & FERRY, S. (1977) Phytochemical inventory
in indigenous Boraginaceae: study of the alkaloids and polyphenolic
compounds (anthocyanic and flavonoid compounds). Plant. Méd.
Phytothér., 11: 5-11. (in french)
DE WAAL, H.L. & VAN TWISK, P. (1964) The chemical composition of
four Senecio species from Kruger National Park. Koedoe (South
Africa), 7: 40-42.
DICK, A.T., DANN, A.T., BULL, L.B., & CULVENOR, C.C.J. (1963)
Vitamin B12 and the detoxication of hepatotoxic pyrrolizidine
alkaloids in rumen liquor. Nature (Lond.), 197: 207-208.
DICKINSON, J.O. (1980) Release of pyrrolizidine alkaloids into
milk. Proc. West. Pharmacol. Soc., 23: 377-379.
DICKINSON, J.O., COOKE, M.P., KING, R.R., & MOHAMED, P.A. (1976)
Milk transfer of pyrrolizidine alkaloids in cattle. J. Am. Vet.
Med. Assoc., 169: 1192-1196.
DIMENNA, G.P., KRICK, T.P., & SEGALL, H.J. (1980) Rapid high
performance liquid chromatography isolation of monoesters,
diesters, and macrocyclic diesters and pyrrolizidine alkaloids from
Senecio jacobaea and Amsinckia intermedia. J. Chromatogr., 192:
474-478.
DOLINSKAYA, K.N. (1952) [Pathomorphology of heliotropic hepatic
dystrophy in children.] In: Milenkov, S.M. & Kizhaikin, Y., ed.
[Collection of scientific papers on Toxic Hepatitis with Ascites,]
Tashkent, Publishing House of the University of Central Asia,
pp. 165-172 (in Russian).
DREIFUSS, P.A. (1984) A study of the mass spectra of
pyrrolizidine alkaloids by negative ion chemical ionization and
ms/ms: the identification of a monoester pyrrolizidine alkaloid in
Eupatorium rugosum. Diss. Abstr. Int. B., 45:'1183-1184.
DRIVER, H.E. & MATTOCKS, A.R. (1984) The toxic effects in rats of
some synthanecine carbamate and phosphate esters analogous to
hepatotoxic pyrrolizidine alkaloids. Chem.-biol. Interact., 51:
201-218.
DUBROVINSKII, S.B. (1946) [About the alimentary toxicosis caused
by heliotrope.] J. Sov. Prot. Health, 6: 17-21 (in Russian).
DUBROVINSKII, S.B. (1952) [The etiology of toxic hepatitis with
ascites.] In: Millenkov, S.M. & Kizhaikin, Y., ed. [Collection of
scientific papers on Toxic Hepatitis with Ascites, Tashkent, USSR,]
Tashkent, Publishing House of the University of Central Asia,
pp. 9-25 (in Russian).
EASTMAN, D.F. & SEGALL, H.J. (1982) A new pyrrolizidine alkaloid
metabolite, 19-hydroxysenecionine, isolated from in vitro mouse
hepatic microsomes using high performance liquid chromatography.
Drug Metab. Disp., 10: 696-699.
EASTMAN, D.F., DIMENNA, G.P., & SEGALL, H.J. (1982) Covalent
binding of two pyrrolizidine alkaloids, senecionine, and
seneciphylline to hepatic macromolecules and their distribution,
excretion and transfer into milk of lactating mice. Drug Metab.
Disp., 10: 236-240.
EDGAR, J.A. (1982) Pyrrolizidine alkaloids sequestered by Solomon
Island Danaine butterflies. The feeding preferences of the Danainae
and Ithomiinae. J. Zool. (Lond.), 196: 385-399.
EDGAR, J.A. (1985) Gas chromatography of pyrrolizidine alkaloids.
In: Seawright, A.A., Hegarty, M.P., James, L.F., & Keeler, R.F.,
ed. Plant toxicology. Proceedings of the Australia - USA Poisonous
Plants Symposium, Brisbane, Australia, 14-18 May 1984, Brisbane,
Queensland Poisonous Plants Committee, pp. 227-234.
EISENSTEIN, D. & HUXTABLE, R.J. (1979) Approaches to the treatment
of pyrrolizidine toxicosis. In: Cheeke, P.R., ed. Proceedings of
the Symposium on Pyrrolizidine (Senecio) Alkaloids: Toxicity,
Metabolism, and Poisonous Plant Control Measures, Corvallis,
Oregon, USA, 23-24 February 1979, Oregon, Nutrition Research
Institute, pp. 109-113.
EL DAREER, S.M., TILLERY, K.F., LLOYD, H.H., & HILL, D.L. (1982)
Disposition of indicine N-oxide in mice and monkeys. Cancer Treat.
Rep., 66: 183-186.
EMMEL, M.W. (1948) Crotalaria poisoning in cattle. J. Am. Vet.
Med. Assoc., 113: 164.
EMMEL, M.W., SANDERS, D.A., & HENLEY, W.W. (1935) Crotalaria
spectabilis seed poisoning in swine. J. Am. Vet. Med. Assoc., 86:
43-49.
EVANS, J.V., PENG, A., & NIELSEN, C.J. (1979) The gas
chromatographic mass spectrometric analysis of the new anti-tumour
drug indicine-N-oxide utilizing a novel reaction accompanying
trimethysilylation. Biomed. mass Spectrom., 6: 38-43.
EVANS, J.V., DALEY, S.K., MCCLUSKY, G.A., & NIELSEN, C.J. (1980)
Direct quantitative analysis of indicine N-oxide in cancer patient
samples by gas chromatography using the internal standard
heliotrine N-oxide including a mass spectral comparison of their
trimethylsilyl derivatives. Biomed. mass Spectrom., 7: 65-73.
FARRINGTON, K.J. & GALLAGHER, C.H. (1960) Complexes of copper with
some pyrrolizidine alkaloids and with some of their esterifying
acids. Aust. J. biol. Sci., 13: 600-603.
FISH, M.S., SWEELEY, C.C., JOHNSON, N.M., LAWRENCE, E.P., &
HORNING, E.C. (1956) Chemical and enzymic rearrangements of
N,N-dimethylaminoacid oxides. Biochem. Biophys. Acta, 21: 196-197.
FISHMAN, A.P. (1974) Dietary pulmonary hypertension. Circ. Res.,
35: 657-660.
FORSYTH, A.A. (1968) British poisonous plants, London, Her
Majesty's Stationery Office.
FOX, D.W., HART, M.C., BERGESON, P.S., JARRETT, P.B., STILLMAN,
A.E., & HUXTABLE, R.J. (1978) Pyrrolizidine (Senecio)
intoxication mimicking Reye's syndrome. J. Paediatr., 93: 980-982.
FRAHN, J.L., CULVENOR, C.C.J., & MILLS, J.A. (1980) Preparative
separation of the pyrrolizidine alkaloids, intermedine and
lycopsamine, as their borate complexes. J. Chromatogr., 195:
379-383.
FURMANOWA, M., GUZEWSKA, J., & BELDOWSKA, B. (1983) Mutagenic
effects of aqueous extracts of Symphytum officinale L. and of its
alkaloidal fractions. J. appl. Toxicol., 3: 127-130.
FURUYA, T. & HIKICHI, M. (1971) Alkaloids and triterpenoids of
Symphytum officinale. Phytochemistry, 10: 2217-2220.
FUSHIMI, K., KATO, K., KATO, T., MATSUBARA, N., & HIRONO, I. (1978)
Carcinogenicity of flower stalks of Petasites japonicus Maxim in
mice and Syrian golden hamsters. Toxicol. Lett., 1: 291-294.
GADELLA, T.W.J., KLIPHUIS, E., & HUIZING, H.J. (1983) Cyto- and
chemotaxonomical studies on the sections officinalia and coerulea
of the genus Symphytum. Bot. Helv., 93: 169-192.
GANEY, P.E., FINK, G.D., & ROTH, R.A. (1985) The effect of dietary
restriction and altered sodium intake on the cardiopulmonary
toxicity of monocrotaline pyrrole. Toxicol. appl. Pharmacol., 78:
55-62.
GARDINER, M.R., ROYCE, R., & BOKOR, A. (1965) Studies on
Crotalaria crispata, a newly recognized cause of Kimberley horse
disease. J. Pathol. Bacteriol., 89: 43-55.
GHANEM, J. & HERSHKO, C. (1981) Veno-occlusive disease and primary
hepatic vein thrombosis in Israeli Arabs. Isr. J. med. Sci., 17:
339-347.
GHODSI, F. & WILL, J.A. (1981) Changes in pulmonary structure and
function induced by monocrotaline intoxication. Am. J. Physiol.,
240: H149-H155.
GILRUTH, J.A. (1903) Hepatic cirrhosis affecting horses and cattle
(so-called "Winton disease"), Wellington, New Zealand Department
of Agriculture, pp. 228-279 (11th annual report).
GOEGER, D.E., CHEEKE, P.R., SCHMITZ, J.A., & BUHLER, D.R. (1982)
Toxicity of tansy ragwort (Senecio jacobaea) to goats. Am. J. vet.
Res., 43: 252-254.
GOEGER, D.E., CHEEKE, P.R., RAMSDELL, H.S., NICHOLSON, S.S., &
BUHLER, D.R. (1983) Comparison of the toxicities of Senecio
jacobaea, Senecio vulgaris and Senecio glabellus in rats.
Toxicol. Lett., 15: 19-23.
GONZALEZ, A.G., DE LA FUENTE, G., REINA, M., & LOYOLA, L.A. (1986a)
Pyrrolizidine alkaloids from Senecio phillipicus and Senecio
illinitus. Planta Med., 52: 160.
GONZALEZ, E., GARCIA, R., LEMUS, I., & ERAZO, S. (1986b)
Pharmacological studies on senecionine, a pyrrolizidine alkaloid
from Senecio fistulosus Poepp. Ex less. (hualtata). An. R. Acad.
Farm., 52(1): 123-132.
GRASES, P.J. & BEKER, S.G. (1972) Veno-occlusive disease of the
liver. A case from Venezuela. Am. J. Med., 53: 511-516.
GRAY, A.I., NIC, A.N., TSAOIR, E., & WATERMAN, P.G. (1983)
Hepatotoxic alkaloids and allantoin in Symphytum tuberosum. J.
Pharm. Pharmacol., 35(Suppl): 13.
GREEN, C.R. & CHRISTIE, G.S. (1961) Malformations in foetal rats
induced by the pyrrolizidine alkaloid, heliotrine. Br. J. exp.
Pathol., 42: 369-378.
GREEN, M.H.L. & MURIEL, W.J. (1975) Use of repair-deficient
strains of Escherichia coli and liver microsomes to detect and
characterise DNA damage caused by the pyrrolizidine alkaloids
heliotrine and monocrotaline. Mutat. Res., 28: 331-336.
GREEN, C.E., SEGALL, H.J., & BYARD, J.L. (1981) Metabolism,
cytotoxicity, and genotoxicity of the pyrrolizidine alkaloid
senecionine in primary cultures of rat hepatocytes. Toxicol. appl.
Pharmacol., 60: 176-185.
GUENGERICH, F.P. (1977) Separation and purification of multiple
forms of microsomal cytochrome P-450. J. biol. Chem., 252:
3970-3979.
GUENGERICH, F.P. & MITCHELL, M.B. (1980) Metabolic activation of
model pyrroles by cytochrome P-450. Drug Metab. Disp., 8: 34-38.
GUIDUGLI, F.H., PESTCHANKER, M.J., DESALMERON, M.S.A., & GIORDANO,
O.S. (1986) 1-hydroxyplatyphyllide, a norsequiterpene lactone
from Senecio gilliesiano. Phytochemistry, 25: 1923-1926.
GUNER, N. (1986) Alkaloids of Heliotropium suaveolens. J. nat.
Prod., 49: 369.
GUPTA, P.S., GUPTA, G.D., & SHARMA, M.L. (1963) Veno-occlusive
disease of the liver. Br. med. J., 1: 1184-1186.
HABS, H. (1982) Senecio numorensis fuchsii. Carcinogenic and
mutagenic activity of an alkaloidal extract from a phytotherapeutic
drug. Dtsch. Apoth. Ztg, 122: 799-804.
HABS, H., HABS, M., MARQUARDT, H., RODER, E., SCHMAHL, D., &
WIEDENFELD, H. (1982) Carcinogenic and mutagenic activity of an
alkaloidal extract of Senecio numorensis spp. fuchsii.
Arzneimittelforsch, 32: 144-148.
HAGGLUND, K.M., L'EMPEREUR, K.M., ROBY, M.R., & STERMITZ, F.R.
(1985) Latifoline and latifoline N-oxide from Hackelia
floribunda, the western false forget-me-not. J. nat. Prod., 48:
638-639.
HAMMOUDA, F.M., RIZK, A.M., ISMAIL, S.I., ATTEYA, S.Z., GHALEB,
H.A., MADKOUR, M.K., POWAND, A.E., & WOOD, G. (1984) Poisonous
plants contaminating edible ones and toxic substances in plant
foods. Part 3: pyrrolizidine alkaloids from Heliotropium digynum
Forssk (= H. luteum, Poir). Pharmazie, 39: 703-705.
HARDING, J.D.J., LEWIS, G., DONE, J.T., & ALLCROFT, R. (1964)
Experimental poisoning by Senecio jacobaea in pigs. Pathol. Vet.,
1: 204-220.
HARRIS, C., REDDY, J., CHIGA, M., & SVOBODA, D. (1969)
Polyribosomal disaggregation by lasiocarpine. Biophys. Acta 180:
587-589.
HARRIS, P.N. & CHEN, K.K. (1970) Development of hepatic tumours in
rats following ingestion of Senecio longilobus. Cancer Res., 30:
2881-2886.
HARRIS, P.N., ROSE, C.L., & CHEN, K.K. (1957) Hepatotoxic and
pharmacological properties of heliotrine. Arch. Pathol., 64:
152-157.
HARTMANN, T. & ZIMMER, M. (1986) Organ-specific distribution and
accumulation of pyrrolizidine alkaloids during the life history of
2 annual Senecio species. J. plant Physiol., 122: 67-80.
HASHEM, M. (1939) Etiology and pathology of types of liver
cirrhosis in Egyptian children. J. Egypt. Med. Assoc., 22: 319-354.
HAYASHI, Y. (1966) Excretion and alteration of monocrotaline in
rats after a subcutaneous injection (abstract). Fed. Proc., 25: 688.
HAYASHI, Y. & LALICH, J.J. (1967) Renal and pulmonary alterations
induced by a single injection of monocrotaline. Proc. Soc. Exp.
Biol. Med., 124: 392-396.
HAYASHI, Y. & LALICH, J.J. (1968) Protective effect of
mercaptoethylamine and cysteine against monocrotaline intoxication
in rats. Toxicol. appl. Pharmacol., 12: 36-43.
HAYASHI, Y., HUSSA, J.F., & LALICH, J.J. (1967) Cor pulmonale in
rats. Lab. Invest., 16: 875-881.
HAYASHI, Y., KATO, M., OTSUKA, H. (1979) Inhibitory effects of
diet reduction on Monocrotaline intoxication in rats. Toxicol.
Lett., 3: 151-155.
HAYASHI, Y., SHIMADA, M., & KATAYAMA, H. (1977) Experimental
insulinoma in rats after a single administration of monocrotaline.
Toxicol. Lett., 1: 41-44.
HAYASHI, Y., KOKUBO, T., TAKAHASHI, M., FURUKAWA, F., OTSUKA, H., &
HASHIMOTO, K. (1984) Correlative morphological and biochemical
studies on monocrotaline-induced pulmonary alterations in rats.
Toxicol. Lett., 21: 65-77.
HAYES, M.A., ROBERTS, E., & FARBER, E. (1985) Initiation and
selection of resistant hepatocyte nodules in rats given the
pyrrolizidine alkaloids lasiocarpine and senecionine. Cancer Res.,
45: 3726-3734.
HEATH, D., SHABA, J., WILLIAMS, A., SMITH, P., & KOMBE, A. (1975)
A pulmonary hypertension-producing plant from Tanzania. Thorax, 30:
399-404.
HENDRICKS, J.D., SINNHUBER, R.O., HENDERSON, M.D., & BUHLER, D.R.
(1981) Liver and kidney pathology in rainbow trout (Salmo
gairdneri) exposed to dietary pyrrolizidine (Senecio) alkaloids.
Exp. mol. Pathol., 35: 170.183.
HILL, K.R. (1960) Worldwide distribution of seneciosis in man and
animals. Proc. R. Soc. Med., 53: 281-282.
HILLIKER, K.S. & ROTH, R.A. (1984) Alteration of monocrotaline
pyrrole-induced cardiopulmonary effects in rats by hydrallazine,
dexamethasone or sulphinpyrazone. Br. J. Pharmacol., 82: 375-380.
HILLIKER, K.S., BELL, T.G., & ROTH, R.A. (1983) Monocrotaline
pyrrole-induced pulmonary hypertension in fawn-hooded rats with
platelet storage pool deficiency: 5 hydroxytryptamine uptake by
isolated, perfused lungs. Thromb. Haemostasis (Stuttgart), 50:
844-847.
HILLIKER, K.S., BELL, T.G., LORIMER, D., & ROTH, R.A. (1984)
Effects of thrombocytopaenia in monocrotaline pyrrole-induced
pulmonary hypertension. Am. J. Physiol., 246: H747-H753.
HIRONO, I., SHIMIZU, M., FUSHIMI, K., MORI, H., & KATO, K. (1973)
Carcinogenic activity of Petasites japonicus Maxim, a kind of
coltsfoot. Gann Monogr. Cancer Res., 64: 527-528.
HIRONO, I., MORI, H., & CULVENOR, C.C.J. (1976) Carcinogenic
activity of coltsfoot, Tussilago farfara L. Gann Monogr. Cancer
Res., 67: 125-129.
HIRONO, I., MORI, H., YAMADA, K., HIRATA, Y., HAGA, M., TATEMATSU,
H., & KANIE, S. (1977) Carcinogenic activity of petasitenine, a
new pyrrolizidine alkaloid isolated from Petasites japonicus
Maxim. J. Natl Cancer Inst., 58: 1155-1157.
HIRONO, I., MORI, H., & HAGA, M. (1978) Carcinogenic activity of
Symphytum officinale. J. Natl Cancer Inst., 61: 865-869.
HIRONO, I., HAGA, M., FUJII, M., MATSUURA, S., MATSUBARA, N.,
NAKAYAMA, M., FURUYA, T., HIKICHI, M., TAKANASHI, H., UCHIDA, E.,
HOSAKA, S., & UENO, I. (1979a) Induction of hepatic tumours in
rats by senkirkine and symphytine. J. Natl Cancer Inst., 63: 469-472.
HIRONO, I., MORI, H., HAGA, M., FUJII, M., YAMADA, K., TAKANASHI,
H., UCHIDA, E., HOSAKA, S., UENO, I., MATSUSHIMA, I., UMEZAVA, K.,
& SHIRAI, A. (1979b) Edible plants containing carcinogenic
pyrrolizidine alkaloids in Japan. In: Miller, B.C. ET AL., ed.
Naturally occurring carcinogens, mutagens, and modulators of
carcinogenesis, Baltimore, Maryland, University Park Press, pp.
79-87.
HIRONO, I., UENO, I., AISO, S., YAMAJI, T., & HAGA, M. (1983)
Carcinogenic activity of Farfagium japonicum and Senecio
cannabifolius. Cancer Lett., 20: 191-198.
HOET, P., ASTIGUETA, M., & FRISQUE, A.M. (1981) Phytochemical
study of Crotalaria nitens. Rev. Latinoam. Quim., 12: 34-35.
HOOPER, P.T. (1972) Spongy degeneration in the brain in relation
to hepatic disease and ammonia toxicity in domestic animals. Vet.
Res., 90: 37-38.
HOOPER, P.T. (1974) The pathology of Senecio jacobaea poisoning of
mice. J. Pathol., 113: 227-230.
HOOPER, P.T. (1975a) Spongy degeneration in the central nervous
system of domestic animals. Part I: morphology. Acta neuropathol.
(Berlin), 31: 325-334.
HOOPER, P.T. (1975b) Spongy degeneration in the central nervous
system of domestic animals. Part III: occurrence and pathogenesis -
hepatocerebral disease caused by hyper-ammonaemia. Acta
neuropathol. (Berlin), 31: 343-351.
HOOPER, P.T. (1975c) Experimental acute gastro-intestinal disease
caused by the pyrrolizidine alkaloid, lasiocarpine. J. comp.
Pathol., 85: 341-349.
HOOPER, P.T. (1978) Pyrrolizidine alkaloid poisoning-pathology
with particular reference to differences in animal and plant
species. In: Keeler, R.F., Van Kampen, K.R., & James, L.F., ed.
Effects of poisonous plants on livestock, New York, Academic
Press, pp. 161-176.
HOOPER, P.T. & SCANLAN, W.A. (1977) Crotalaria retusa poisoning in
pigs and poultry. Aust. vet. J., 53: 109-114.
HOOPER, P.T., BEST, S.M., & MURRAY, D.R. (1974) Hyper-ammonaemia
and spongy degeneration of the brain in sheep affected with hepatic
necrosis. Res. vet. Sci., 16: 216-222.
HOOSON, J. & GRASSO, P. (1976) Cytotoxic and carcinogenic response
to monocrotaline pyrrole. J. Pathol., 118: 121-127.
HOU JINQUI, XIA YUDIN, YU ZHANSHI, AN YAN, & TANG YIXAI (1980)
[Veno-occlusive disease of the liver with report of 2 cases.] Chung
Hua Nei ko Tsa Chih, 19: 187-191 (in Chinese).
HSU, I.C., ALLEN, J.R., & CHESNEY, C.F. (1973) Identification and
toxicological effects of dehydroretronecine, a metabolite of
monocrotaline. Proc. Soc. Exp. Biol. Med., 144: 834-838.
HSU, I.C., SHUMAKER, R.C., & ALLEN, J.R. (1974) Tissue
distribution of tritium labelled dehydroretronecine. Chem.-biol.
Interact., 8: 163.
HUIZING, H.J., DE BOER, R., & MALINGRE, T.M. (1981) Preparative
ion-pair high performance liquid chromatography and gas
chromatography of pyrrolizidine alkaloids from comfrey. J.
Chromatogr., 214: 257-262.
HURLEY, J.V. & JAGO, M.V. (1975) Pulmonary oedema in rats given
dehydromonocrotaline: a topographic and electron-microscopic
study. J. Pathol., 117: 23-32.
HUXTABLE, R.J. (1980) Herbal teas and toxins: novel aspects of
pyrrolizidine poisoning in the United States. Perspect. Biol. Med.,
24: 1-14.
HUXTABLE, R., PAPLANUS, S., & LAUGHARN, J. (1977) The prevention
of monocrotaline induced right ventricular hypertrophy. Chest, 71:
308-310.
HUXTABLE, R.J., CIARAMITARO, D., & EISENSTEIN, D. (1978) The
effect of a pyrrolizidine alkaloid, monocrotaline and a pyrrole,
dehydroretronecine, on the biochemical functions of the pulmonary
endothelium. Mol. Pharmacol., 14: 1189-1203.
HUXTABLE, R.J., LUTHY, J., & ZWEIFEL, V. (1986) Toxicity of
comfrey-pepsin preparations: letters to the editor. New Engl. J.
Med., 315: 1095.
IARC (1976) Pyrrolizidine alkaloids. In: Some naturally occurring
substances, Lyons, International Agency for Research on Cancer,
pp. 265-342 (IARC Monograph on the Evaluation of Carcinogenic Risk
of Chemicals to Man, Vol. 10).
IARC (1983) Some food additives, feed additives and naturally
occurring substances, Lyons, International Agency for Research on
Cancer, pp. 207-245 (IARC Monograph on the Evaluation of
Carcinogenic Risk of Chemicals to Humans, Vol. 31).
INDIAN COUNCIL OF MEDICAL RESEARCH (1955) Infantile cirrhosis of
the liver in India. Indian J. med. Res., 43: 723-747.
ISMAILOV, N.I. (1948a) [On clinical features, etiology and
pathogenesis of heliotropic dystrophy (toxic hepatitis with
ascites).] Clin. Med., 8: 28 (in Russian).
ISMAILOV, N.I. (1948b) [Heliotropic toxicosis (toxic hepatitis
with ascites),] Tashkent, Academy of Sciences of Uzbekistan,
pp. 1-120 (in Russian).
ISMAILOV, N.I., MADZHIDOV, N.M., MAGRUPOV, A.I., MAKHKAMOV, G.M., &
MUKMINOVA, S.G. (1970) [Clinical signs, diagnosis and treatment of
Trichodesma toxicosis (alimentary toxic encephalopathy),]
Tashkent, Meditsina, p. 85 (in Russian).
JAGO, M.V. (1969) The development of the hepatic megalocytosis
of chronic pyrrolizidine alkaloid poisoning. Am. J. Pathol., 56:
405-422.
JAGO, M.V. (1970) A method for the assessment of the chronic
hepatotoxicity of pyrrolizidine alkaloids. Aust. J. exp. Biol. med.
Sci., 48: 93-103.
JAGO, M.V. (1971) Factors affecting the chronic hepatotoxicity
of pyrrolizidine alkaloids. J. Pathol., 105: 1-11.
JAGO, M.V., LANIGAN, G.W., BINGLEY, J.B., PIEREY, D.W.T., WHITTEN,
J.H., & TITCHEN, D.A. (1969) Excretion of the pyrrolizidine
alkaloid heliotrine in the urine and bile of sheep. J. Pathol., 98:
115-128.
JAGO, M.V., EDGAR, J.A., SMITH, L.W., & CULVENOR, C.C.J. (1970)
Metabolic conversion of heliotrine based pyrrolizidine alkaloids to
dehydroheliotridine. Mol. Pharmacol., 6: 402-406.
JELLIFFE, D.B., BRAS, G., & STUART, K.L. (1954a) Veno-occlusive
disease of the liver. Paediatrics, 14: 334-339.
JELLIFFE, D.B., BRAS, G., & STUART, K.L. (1954b) The clinical
picture of veno-occlusive disease of the liver in Jamaican
children. Ann. trop. Med. Parasitol., 48: 386-396.
JELLIFFE, D.B., BRAS, G., & MUKHERJEE, K.L. (1957) Veno-occlusive
disease of the liver and Indian childhood cirrhosis. Arch. dis.
Child., 32: 369-385.
JOHNSON, A.E. (1976) Changes in calves and rats consuming milk
from cows fed chronic lethal doses of Senecio jacobaea (tansy
ragwort). Am. J. vet. Res., 37: 107-110.
JOHNSON, A.E. (1979) Toxicity of tansy ragwort to cattle. In:
Cheeke, P.R., ed. Proceedings of the Symposium on Pyrrolizidine
(Senecio) Alkaloids: Toxicity, Metabolism, and Poisonous Plant
Control Measures, Corvallis, Oregon, USA, 23-24 February 1979,
Oregon, Nutrition Research Institute, pp. 129-134.
JOHNSON, A.E. (1982) Failure of mineral-vitamin supplements to
prevent tansy ragwort (Senecio jacobaea) toxicosis in cattle. Am.
J. vet. Res., 43: 718-723.
JOHNSON, A.E. & MOLYNEUX, R.J. (1984) Toxicity of thread leaf
groundsel (Senecio douglasii var. longilobus) to cattle. Am. J.
vet. Res., 45: 26-31.
JOHNSON, A.E., MOLYNEUX, R.J., & STUART, L.D. (1985) Toxicity of
Riddel's groundsel (Senecio riddelli) to cattle. Am. J. vet. Res.,
46: 577-582.
JOHNSON, W.D. (1981) Mechanism of in vitro acute toxicity of
dehydromonocrotaline a metabolite of pyrrolizidine alkaloid
monocrotaline. Toxicologist, 1: 107-108.
JOHNSON, W.D., ROBERTSON, K.A., POUNDS, J.G., & ALLEN, J.R. (1978)
Dehydroretronecine-induced skin tumours in mice. J. Natl Cancer
Inst., 61: 85-89.
JULIEN, M.H., ed. (1982) Biological control of weeds. A world
catalog of agents and their target weeds, Curepe, Trinidad and
Toabago, Commonwealth Institute of Biological Control.
JUNEJA, T.R., GUPTA, R.L., & SAMANTA, S. (1984) Activation of
monocrotaline, fulvine and their derivatives to toxic pyrroles by
some thiols. Toxicol. Lett., 21: 185-189.
KAMPANZEV, N.N. (1952) [Experimental heliotropic hepatitis.] In:
Milenkov, S.M. & Kizhaikin, Y., ed. [Collection of scientific
papers on Toxic Hepatitis with Ascites,] Tashkent, Publishing
House of the University of Central Asia, pp. 165-172 (in Russian).
KAY, J.M. & HEATH, D. (1966) Observation on the pulmonary arteries
and heart weight of rats fed on Crotalaria spectabilis seeds. J.
Pathol., 92: 385-394.
KAY, J.M. & HEATH, D. (1969) Crotalaria spectabilis: the pulmonary
hypertension plant, Springfield, Illinois, Charles C. Thomas, p.
38.
KAY, J.M., HARRIS, P., & HEATH, D. (1967a) Pulmonary hypertension
produced in rats by ingestion of Crotalaria spectabilis seeds.
Thorax, 22: 176-179.
KAY, J.M., GILLUND, T.D., & HEATH, D. (1967b) Mast cells in the
lungs of rats fed on Crotalaria spectabilis seeds. Am. J. Pathol.,
51: 1031-1044.
KAY, J.M., HEATH, D., SMITH, P., BRAS, G., & SUMMERELL, J. (1971a)
Fulvine and pulmonary circulation. Thorax, 26: 249-261.
KAY, J.M., SMITH, P., & HEATH, D. (1971b) Aminorex and the
pulmonary circulation. Thorax, 26: 262-270.
KAY, J.M., KEANE, P.M., & SUYAMA, K.L. (1985) Pulmonary
hypertension induced in rats by monocrotaline and chronic hypoxia
is reduced by p-chlorophenylalanine. Respiration, 47: 48-56.
KEDZIERSKI, B. & BUHLER, D.R. (1985) Configuration of necine
pyrroles - toxic metabolites of pyrrolizidine alkaloids. Toxicol.
Lett., 25: 115-119.
KEDZIERSKI, B. & BUHLER, D.R. (1986) The formation of 6,7-di-
hydro-7-hydroxy-1-hydroxymethyl-5H-pyrrolizine, a metabolite of
pyrrolizidine alkaloids. Chem.-biol. Interact., 57: 217-222.
KHANIN, M.N. (1948) [The etiology of toxic hepatitis with
ascites.] Arch. Pathol. USSR, 1: 42-47 (in Russian).
KIM, H.L. & JONES, L.P. (1982) Protective effects of butylated
anisole, ethoxyquin and disulfiran on acute pyrrolizidine alkaloid
poisoning in mice. Res. Comm. chem. Pathol. Pharmacol., 36: 341-344.
KIRFEL, A., WILL, G., WIEDENFELD, H. & RODER, E. (1980)
(1aR,6bR,10R,11R)-9,15-dioxo-10-hydroxy-10,11,13-trimethyl-1a,2,
3,6b-tetrabydro-5H-pyrrolizino-[1a,6b,6a,c],8-dioxa-15-cis-tride
cene,C18H25NO5. Cryst. Styruct. Comm., 9: 353-361.
KNIGHT, A.P., KIMBERLING, C.V., STERMITZ, F.R., & ROBY, M.R. (1984)
Cynoglossum officinale (hound's tongue) - a cause of pyrrolizidine
alkaloid poisoning in horses. J. Am. Vet. Med. Assoc., 185: 647-650.
KOEKEMOER, M.J. & WARREN, F.L. (1951) The occurrence and
preparation of the N -oxides. An improved method of extraction of
the Senecio alkaloids. J. Chem. Soc., C1951: pp. 66-68.
KOLETSKY, A., OYASU, R., & REDDY, J.K. (1978) Mutagenicity of the
pyrrolizidine (Senecio) alkaloid, lasiocarpine in the
salmonella/microsome test. Lab. Invest., 38: 352 (Abstract).
KOVACH, J.S., MOERTEL, C.G., & HAHN, R.G. (1979a) A phase 1 study
of indicine- N-oxide. Proc. Am. Assoc. Cancer Res., 20: 357.
KOVACH, J.S., AMES, M.M., POWIS, G., MOERTEL, C.G., HAHN, R.G., &
CREAGAN E.T. (1979b) Toxicity and pharmacokinetics of a
pyrrolizidine alkaloid, indicine- N-oxide, in humans. Cancer Res.,
39: 4540-4544.
KRAUS, C., ABEL, G., & SCHIMMER, O. (1985) [Studies on the
chromosome damaging effect of some pyrrolizidine alkaloids in human
lymphocytes in vitro.] Planta Med., 51: 89-91 (in German).
KRISHNAMACHARI, K.A.V.R., BHAT, R.V., KRISHNAMURTHY, D.,
KRISHNASWAMY, K., & NAGARAJAN (1977) Aetiopathogenesis of endemic
ascites in Sarguja district of Madhya Pradesh. Indian J. med. Res.,
65: 672-678.
KUHARA, K., TAKANASHI, H., HIRONO, I., FURUYA, T., & ASADA, Y.
(1980) Carcinogenic activity of clivorine, a pyrrolizidine
alkaloid isolated from Ligularia dentata. Cancer Lett., 10: 117-122.
KUMANA, C.R., NG, M., LIN, H.J., KO, W., WU, P.C., & TODD, D.
(1983) Hepatic veno-occlusive disease due to toxic alkaloid herbal
tea - letter to the editor. Lancet, 10 December: 1360-1361.
KUMANA, C.R., NG, M., LIN, H.J., KO, W., WU, P.C., & TODD, D.
(1985) Herbal tea induced hepatic veno-occlusive disease:
quantification of toxic alkaloid exposure in adults. Gut, 26:
101-104.
KUROZUMI, T., TANAKA, K., KIDO, M., & SHOYAMA, Y. (1983)
Monocrotaline-induced renal lesions. Exp. mol. Pathol., 39: 377-386.
LAFRANCONI, W.M. & HUXTABLE, R.J. (1983) Changes in angiotensin-
converting enzyme activity in lungs damaged by the pyrrolizidine
alkaloid, monocrotaline. Thorax, 38: 307-309.
LAFRANCONI, W.M. & HUXTABLE, R.J. (1984) Hepatic metabolism and
pulmonary toxicity of monocrotaline using isolated perfused liver
and lung. Biochem. Pharmacol., 33: 2479-2484.
LAFRANCONI, W.M., DUHAMEL, R.C., BRENDEL, K., & HUXTABLE,R.J.
(1984) Differentiation of the cardiac and pulmonary toxicity of
monocrotaline, a pyrrolizidine alkaloid. Biochem. Pharmacol., 33:
191-197.
LAFRANCONI, W.M., OHNUMA, S., & HUXTABLE, R.S. (1985) Biliary
excretion of novel pneumotoxic metabolites of the pyrrolizidine
alkaloid monocrotaline. Toxicon, 23: 903-992.
LALICH, J.J. & EHRHART, L.A. (1962) Monocrotaline induced
pulmonary arteritis in rats. J. atheroscler. Res., 2: 482-492.
LALICH, J.J. & MERKOW, L. (1961) Pulmonary arteritis produced in
rats by feeding Crotalaria spectabilis. Lab. Invest., 10: 744-750.
LANCET (1984) Pyrrolizidine alkaloids (editorial). Lancet, 1:
201-202.
LANGLEBEN, D. & REID, L.M. (1985) Effect of methylprednisolone on
monocrotaline-induced pulmonary vascular disease and right
ventricular hypertrophy. Lab. Invest., 52: 298-303.
LANIGAN, G.W. (1971) Metabolism of pyrrolizidine alkaloids in the
ovine rumen. III. The competitive relationship between heliotrine
metabolism and methanogenesis in rumen fluid in vitro. Aust. J.
agric. Res., 22: 123-130.
LANIGAN, G.W. (1972) Metabolism of pyrrolizidine alkaloids in
ovine rumen. IV. Effects of chloral hydrate and halogenated
methanes on rumen methanogenesis and alkaloid metabolism in
fistulated sheep. Aust. J. agric. Res., 23: 1085-1091.
LANIGAN, G.W. & SMITH, L.W. (1970a) Metabolism of pyrrolizidine
alkaloids in the ovine rumen. I. Formation of 7 alpha-hydroxy-
1alpha-methyl-8alpha-pyrrolizidine from heliotrine and
lasiocarpine. Aust. J. agric. Res., 21: 493-500.
LANIGAN, G.W. & SMITH, L.W. (1970b) Metabolism of pyrrolizidine
alkaloids in the ovine rumen. II. Formation of 7alpha-hydroxy-
1alpha-methyl-8alpha-pyrrolizidine from heliotrine and
lasiocarpine. Aust. J. agric. Res., 22: 123-130.
LANIGAN, G.W. & WHITTEM, J.H. (1970) Cobalt pellets and
Heliotropium europaeum poisoning in penned sheep. Aust. vet. J.,
46: 17-21.
LANIGAN, G.W., PAYNE, A.L., & PETERSON, J.E. (1978) Anti-
methanogenic drugs and Heliotropium europaeum poisoning in penned
sheep. Aust. J. Agric. Res., 29: 1281-1292.
LAWS, L. (1968) Toxicity of Crotalaria mucronata to sheep. Aust.
vet. J., 44: 453-455.
LETENDRE, L., SMITHSON, W.A., GILCHRIST, G.S., BURGERT, E.O., Jr,
HOGLAND, C.H., AMES, M.M., POWIS, G., & KOVACH, J.S. (1981)
Activity of indicine- N-oxide in refractory acute leukemia. Cancer,
47: 437-441.
LETENDRE, L., LUDWIG, J., PERRAULT, J., SMITHSON, W.A., & KOVACH,
J.S. (1984) Hepatocellular toxicity during the treatment of
refractory acute leukemia with indicine- N-oxide. Cancer, 54:
1256-1259.
LEVINE, O.R., HARRIS, R.C., BLANCE, W.A., & MELLINS, R.B. (1973)
Progressive pulmonary hypertension in children with portal
hypertension. J. Pediatr., 83: 964-972.
LIN, J.J., LIU, C., & SVOBODA, D.J. (1974) Long-term effects of
aflatoxin B1 and vocal hepatitis on marmoset liver: a preliminary
report. Lab. Invest., 30: 267-278.
LUTHY, J., ZWEIFEL, U., SCHLATTER, C., & BENN, M.H. (1980)
[Pyrrolizidine alkaloids in coltsfoot ( Tussilago farfara L.) of
various sources.] Mitt. Geb. Lebensm. Hyg., 71: 73-80 (in German
with English summary).
LUTHY, J., ZWEIFEL, U., KARLHUBER, B., & SCHLATTER, C. (1981)
Pyrrolizidine alkaloids of Senecio alpinus L. and their detection
in feedstuffs. J. agric. food Chem., 29: 302-305.
LUTHY, J., HEIM, TH., & SCHLATTER, CH. (1983) Transfer of [3H]
pyrrolizidine alkaloids from Senecio vulgaris L. and metabolites
into rat milk and tissues. Toxicol. Lett., 17: 283-288.
LUTHY, J., BRAUCHLI, J., ZWEIFEL, U., SCHMID, P., & SCHLATTER, CH.
(1984) [Pyrrolizidine alkaloid in arzneipflanzen der Boraginaceen:
Borago officinalis L. and Pulmonaria officinalis L.] Pharm. Acta
Helv., 59: 242-246 (in German).
LYFORD, C.L., VERGAVA, G.G., & MOELLER, D.D. (1976) Hepatic veno-
occlusive disease originating in Ecuador. Gastro-enterology, 70:
105-108.
MCCOMISH, M., BODEK, I., & BRAUFMAN, A.R. (1980) Quantitation of
the antineoplastic agent indicine- N-oxide in human plasma by
differential pulse polarography. J. pharmacol. Sci., 69: 727-729.
MCCOY, J.W., ROBY, M.R., & STERMITZ, F.R. (1983) Analysis of plant
alkaloid mixtures by ammonia chemical ionization mass spectroscopy.
J. nat. Prod., 46: 894-900.
MCGEE, J.O'D., PATRICK, R.S., WOOD, C.B., & BLUMGART, L.H. (1976)
A case of veno-occlusive disease of the liver in Britain associated
with herbal tea consumption. J. clin. Pathol., 29: 788-794.
MCGRATH, J.P.M., DUNCAN, J.R., & MUNNELL, J.F. (1975) Crotalaria
spectabilis toxicity in swine: characterization of the renal
glomerular lesion. J. comp. Pathol., 85(2): 185-194.
MCLEAN, E.K. (1969) The early sinusoidal lesion in experimental
veno-occlusive disease. Br. J. exp. Pathol., 50: 223-229.
MCLEAN, E.K. (1970) The toxic actions of pyrrolizidine (Senecio)
alkaloids. Pharmacol. Rev., 22: 429-483.
MCLEAN, E.K. (1974) Senecio and other plants as liver poisons.
Isr. J. med. Sci., 10: 436-440.
MCLEAN, E.K. & HILL, K.R. (1969) Portal hypertension in acute
experimental veno-occulsive disease of the liver in rats. Br. J.
exp. Pathol., 50: 37-41.
MCLEAN, E.K., BRAS, G., & GYORGY, P. (1964) Veno-occlusive lesions
in livers of rats fed Crotalaria fulva. Br. J. exp. Pathol., 45:
242-247.
MAKSUDOV, A.M. (1952) [Heliotropic toxic hepatitis with ascites.]
In: Milenkov, S.M. & Kizhaikin, Y., ed. [Collection of scientific
papers on Toxic Hepatitis with Ascites,] Tashkent, Publishing House
of the University of Central Asia, pp. 103-116 (in Russian).
MARTIN, P.A., THORBURNE, M.K., HUTCHINSON, S., BRAS, G., & MILLER,
C.G. (1972) Preliminary findings of chromosome studies in rats and
humans with veno-occlusive disease. Br. J. exp. Pathol., 53, 374-380.
MATTOCKS, A.R. (1961) Extraction of heat-labile alkaloids from
plants. Nature (Lond.), 191: 1281-1282.
MATTOCKS, A.R. (1967a) Spectrophotometric determination of
unsaturated pyrrolizidine alkaloids. Anal. Chem., 39: 443-447.
MATTOCKS, A.R. (1967b) Detection of pyrrolizidine alkaloids on
thin-layer chromatograms. J. Chromatogr., 27: 505-508.
MATTOCKS, A.R. (1968a) Toxicity of pyrrolizidine alkaloids. Nature
(Lond.), 217: 723-728.
MATTOCKS, A.R. (1968b) Spectrophotometric determination of
pyrrolizidine alkaloids: some improvements. Anal. Chem., 40: 1749.
MATTOCKS, A.R. (1969) Dehydropyrrolizine derivatives from
unsaturated pyrrolizidine alkaloids. J. Chem. Soc., C1969:
1155-1162.
MATTOCKS, A.R. (1971a) Synthetic compounds with toxic properties
similar to those of pyrrolizidine alkaloids and their pyrrolic
metabolites. Nature (Lond.), 232: 476.
MATTOCKS, A.R. (1971b) The occurrence and analysis of
pyrrolizidine alkaloid N-oxides. Xenobiotica, 1: 451-453.
MATTOCKS, A.R. (1971c) Hepatotoxic effects due to pyrrolizidine
alkaloid N-oxides. Xenobiotica, 1: 563-565.
MATTOCKS, A.R. (1971d) A field test for N-oxides of unsaturated
pyrrolizidine alkaloids. Trop. Sci., 13: 65-70.
MATTOCKS, A.R. (1972a) Toxicity and metabolism of Senecio
alkaloids. In: Harborne, J.B., ed. Phytochemical ecology, London,
New York, Academic Press, pp. 179-200.
MATTOCKS, A.R. (1972b) Acute hepatotoxicity and pyrrolic
metabolites in rats dosed with pyrrolizidine alkaloids. Chem.-biol.
Interact., 5: 227-242.
MATTOCKS, A.R. (1973) Mechanisms of pyrrolizidine alkaloid
toxicity. Pharmacology and the future of man. In: Proceedings of
the 5th International Congress on Pharmacology, San Francisco,
1972, Basel, Karger, Vol. 2, pp. 114-123.
MATTOCKS, A.R. (1977) Tissue distribution of radioactivity in rats
given tritiated analogues of hepatotoxic pyrrolizidine alkaloids.
Xenobiotica, 7: 665-670.
MATTOCKS, A.R. (1980) Toxic pyrrolizidine alkaloids in comfrey.
Lancet, 22 November: 1136-1137.
MATTOCKS, A.R. (1981a) Liver cell enlargement in rats given
hydroxymethyl pyrroles analogous to pyrrolizidine alkaloid
metabolites, followed later by the hepatotoxin dimethylnitrosamine.
Toxicol. Lett., 8: 201-205.
MATTOCKS, A.R. (1981b) Relation of structural features to pyrrolic
metabolites in livers of rats given pyrrolizidine alkaloids and
derivatives. Chem.-biol. Interact., 35: 301-310.
MATTOCKS, A.R. (1981c) A simple preparation of dehydroretronecine
using potassium nitrosodisulphonate. Chem. Ind., 7: 251.
MATTOCKS, A.R. (1982) Hydrolysis and hepatotoxicity of retronecine
diesters. Toxicol. Lett., 14: 111-116.
MATTOCKS, A.R. (1986) Chemistry and toxicology of pyrrolizidine
alkaloids, London, New York, Academic Press.
MATTOCKS, A.R. & BIRD, I. (1983) Pyrrolic and N-oxide metabolites
formed from pyrrolizidine alkaloids by hepatic microsomes in vitro:
relevance to in vivo hepatotoxicity. Chem.-biol. Interact., 43:
209-222.
MATTOCKS, A.R. & CABRAL, J.R.P. (1979) Effects of some pyrrolic
and dehydropyrrolizine esters on mouse skin: a preliminary study.
Tumori, 65: 289-293.
MATTOCKS, A.R. & CABRAL, J.R.P. (1982) Carcinogenicity of some
pyrrolizidine alkaloid metabolites and analogues. Cancer Lett., 17:
61-66.
MATTOCKS, A.R. & DRIVER, H.E. (1983) A comparison of the
pneumotoxicity of some pyrrolic esters and similar compounds
analogous to pyrrolizidine alkaloid metabolites given intravenously
to rats. Toxicology, 27: 159-177.
MATTOCKS, A.R. & DRIVER, H.E. (1987) Toxic actions of senaetnine,
a new pyrrolizidine alkaloid, in rats. Toxicol. Lett., 38: 315-319.
MATTOCKS, A.R. & JUKES, R. (1987) New improved field test for
toxic pyrrolizidine alkaloids. J. nat. Prod., 50: 161-166.
MATTOCKS, A.R. & LEGG, R.F. (1980) Antimitotic activity of
dehydroretronecine, a pyrrolizidine alkaloid metabolite, and some
analogous compounds in a rat liver parenchymal cell line. Chem.-
biol. Interact., 30: 325-336.
MATTOCKS, A.R. & WHITE, I.N.H. (1970) Estimation of metabolites
of pyrrolizidine alkaloids in animal tissues. Anal. Biochem., 38:
529-535.
MATTOCKS, A.R. & WHITE, I.N.H. (1971a) The conversion of
pyrrolizidine alkaloids to dihydropyrrolizine derivatives by rat-
liver microsomes in vitro. Chem.-biol. Interact., 3: 383-396.
MATTOCKS, A.R. & WHITE, I.N.H. (1971b) Pyrrolic metabolites from
non-toxic pyrrolizidine alkaloids. Nat. new Biol., 231: 114-115.
MATTOCKS, A.R. & WHITE, I.N.H. (1973) Toxic effects and pyrrolic
metabolites in the liver of young rats given the pyrrolizidine
alkaloid retrorsine. Chem.-biol. Interact., 6: 297-306.
MATTOCKS, A.R. & WHITE, I.N.H. (1976) The distribution of
[3H]-synthanecine A bis- n-ethylcarbamate and its metabolites
in the rat. Chem.-biol. Interact., 15: 173-184.
MEYRICK, B.O. & REID, L.M. (1979) Development of pulmonary
arterial changes in rats fed Crotalaria spectabilis. Am. J.
Pathol., 94: 37-50.
MEYRICK, B.O. & REID, L.M. (1982) Crotalaria-induced pulmonary
hypertension: uptake of 3H-thymidine by the cells of the pulmonary
circulation and alveolar walls. Am. J. Pathol., 106: 84-94.
MILKOWSKY, A.S. (1985) The synthesis and anti-tumour activity of
monocyclic analogs of indicine- N-oxide. Diss. Abstr. Int., 45(3):
844.
MILLER, W.C., RICE, D.L., KREUSEL, R.G., & BEDROSSIAN, C.W.M.
(1978) Monocrotaline model of non-cardiogenic pulmonary edema in
dogs. J. appl. Physiol., 45: 962-965.
MIRANDA, C.L., BUHLER, D.R., & CHEEKE, P.R. (1979) The effect of
tansy ragwort consumption on mineral metabolism in the rat. In:
Cheeke, P.R., ed. Proceedings of the Symposium on Pyrrolizidine
(Senecio) Alkaloids: Toxicity, Metabolism, and Poisonous Plant
Control Measures, Corvallis, Oregon, USA, 23-24 February 1979,
Oregon, Nutrition Research Institute, pp. 61-64.
MIRANDA, C.L., CARPENTER, H.M., CHEEKE, P.R., & BUHLER, D.R.
(1981a) Effect of ethoxyquin on the toxicity of pyrrolizidine
alkaloid monocrotaline and on hepatic long metabolism in mice.
Chem.-biol. Interact., 37: 95-107.
MIRANDA, C.L., HENDERSON, M.C., & BUHLER, D.R. (1981b) Dietary
copper enhances the hepatotoxicity of Senecio jacobaea in rats.
Toxicol. appl. Pharmacol., 60(3): 418-423.
MIRANDA, C.L., REED, R.L., CHEEKE, P.R., & BUHLER, D.R. (1981c)
Protective effects of butylated hydroxyanisole against the acute
toxicity of monocrotaline in mice. Toxicol. appl. Pharmacol., 59:
424-430.
MIRANDA, C.L., BUHLER, D.R., RAMSDELL, H.S., CHEEKE, P.R., &
SCHMITZ, J.A. (1982a) Modifications of chronic hepatotoxicity of
pyrrolizidine (Senecio) alkaloids by butylated hydroxyanisole
and cysteine. Toxicol. Lett., 10: 177-182.
MIRANDA, C.L., HENDERSON, M.C., BUHLER, D.R., & SCHMITZ, J.A.
(1982b) Comparative effects of antioxidants on the toxicity of
mixed pyrrolizidine alkaloids from Senecio jacobaea in mice. J.
Toxicol. environ. Health, 9(5-6): 933-939.
MIRANDA, C.L., HENDERSON, M.C., REED, R.L., SCHMITZ, J.A., &
BUHLER, D.R. (1982c) Protective action of zinc against
pyrrolizidine alkaloid-induced hepatotoxicity in rats. J. Toxicol.
environ. Health, 9: 359-366.
MIROCHNIK, M.F. (1938) [Clinical course and etiopathogenesis of
toxic hepatites with ascites.] Medical Institute, scientific
papers of the second therapeutic clinic, Vol I, Tashkent, Medical
Literature Publishers.
MISER, J.S., MISER, A.W., SMITHSON, W.A., COCCIA, P.F., AMES, M.M.,
DAVIS, D.M., HUGHES, C.S., & GILCHRIST, G.S. (1982) A phase I
trial of indicine- N-oxide in childhood malignancy. Proc. Am. Soc.
Clin. Oncol., 1: 137.
MNUSHKIN, A.S. (1949) [Some materials to clinical features and
pathogenesis of toxic hepatitis with ascites.] Sov. Med., 1: 8-9
(in Russian).
MNUSHKIN, A.S. (1952) [The clinical features, pathogenesis and
treatment of toxic hepatitis with ascites.] In: Milenkov, S.M. &
Kizhaikin, Y., ed. [Collection of scientific papers on Toxic
Hepatitis with Ascites,] Tashkent, Publishing House of the
University of Central Asia, pp. 91-98 (in Russian).
MOHABBAT, O., SRIVASTAVA, R.N., YOUNOS, M.S., SEDIQ, G.G., MENZAD,
A.A., & ARAM, G.N. (1976) An outbreak of hepatic veno-occlusive
disease in north-western Afghanistan. Lancet, 7 August: 269-271.
MOLTENI, A., WARD, W.F., TS'AO, C.-S., PORT, C.D., & SOLLIDAY, N.H.
(1984) Monocrotaline-induced pulmonary endothelial dysfunction in
rats. Proc. Soc. Exp. Biol. Med., 176: 88-94.
MOLYNEUX, R.J. & ROITMAN, J.N. (1980) Specific detection of
pyrrolizidine alkaloids on thin-layer chromatograms. J.
Chromatogr., 195: 412-415.
MOLYNEUX, R.J., JOHNSON, A.E., ROITMAN, J.N., & BENSON, M.E. (1979)
Chemistry of toxic range plants. Determination of pyrrolizidine
alkaloid content and composition in Senecio species by nuclear
magnetic resonance spectroscopy. J. agric. food Chem., 27: 494-499.
MORI, H., SUGIE, S., YOSHIMI, N., ASADA, Y., FURUYA, T., &
WILLIAMS, G.M. (1985) Genotoxicity of a variety of pyrrolizidine
alkaloids in the hepatocyte primary culture-DNA repair test using
rat, mouse, and hamster hepatocytes. Cancer Res., 45: 3125-3129.
MUNIER, R. (1953) Separation of alkaloids from their N-oxides by
paper chromatography. Bull. Soc. Chim. Biol., 35: 1225.
NARDI, N.B., GIMMLER, M.C., & LEITE, B.G. (1980) Antimitotic
action of integerrimine in rats. Rev. Bras. Genet., 3: 387-392.
NATIONAL CANCER INSTITUTE (1978) Bioassay of lasiocarpine for
possible carcinogenecity, Bethesda, Maryland, National Cancer
Institute, US Department of Health, Education and Welfare (Report
of Carcinogenesis Testing Programme, Division of Cancer Cause and
Prevention).
NAYAK, N.C., SAGREIYA, K., & RAMALINGASWAMI, V. (1969) Indian
childhood cirrhosis - the nature and significance of cytoplasmic
hyaline of hepatocytes. Arch. Pathol., 88: 631-637.
NEWBERNE, P.M. (1968) The influence of a low isotrope diet on
response of maternal and fetal rats to lasiocarpine. Cancer Res.,
28: 2327-2337.
NEWBERNE, P.M. & ROGERS, A.E. (1973) Nutrition, monocrotaline and
aflatoxin B1 in liver carcinogenesis. Plant Food Man, 1: 23-31.
NEWBERNE, P.M., WILSON, R., & ROGERS, A.E. (1971) Effects of a
low-lipotrope diet on the response of young male rats to the
pyrrolizidine alkaloid monocrotaline. Toxicol. appl. Pharmacol., 18:
387-397.
NEWBERNE, P.M., CHAN, W.C., & ROGERS, A.E. (1974) Influence of
light, riboflavin and carotene on the response of rats to the acute
toxicity of aflatoxin and monocrotaline. Toxicol. appl. Pharmacol.,
28: 200-208.
NICHOLS, W.C., MOERTL, C.G., RUBIN, J., SCHULT, A.J., & BRITTEL,
J.C. (1981) Phase II trial of indicine- N-oxide (NSC132319) in
patients with advanced colorectal carcinoma. Cancer Treat. Rep., 65:
337-339.
NIWA, H., ISHIWATA, H., & YAMADA, K. (1985) Isolation of
petasitenine, a carcinogenic pyrrolizidine alkaloid from Farfugium
japonicum. J. nat. Prod., 48: 10083-1004.
NOLAN, J.P., SCHEIG, R.L., & KLATSKIN, G. (1966) Delayed hepatitis
and cirrhosis in weanling rats following a single small dose of the
Senecio alkaloid, lasiocarpine. Am. J. Pathol., 49: 129-151.
OHNUMA, T., SRIDHAR, K.S., RATNER, L.H., & HOLLAND, J.F. (1982)
Phase I study of indicine- N-oxide in patients with advanced cancer.
Cancer Treat. Rep., 66(7): 1509-1515.
OHTSUBO, K., ITO, Y., SAITO, M., FURUYA, T., & HIKICHI, M. (1977)
Hypertrophy of pulmonary arteries and arterioles with cor pulmonale
in rats induced by seneciphylline, a pyrrolizidine alkaloid.
Experientia (Basel), 33: 498-499.
OLSON, J.W., HACKER, A.D., ALTIERE, R.J., & GILLESPIE, M.N. (1984)
Polyamines and the development of monocrotaline-induced pulmonary
hypertension. Am. J. Physiol., 247: H682-H685.
OLSON, J.W., ATKINSON, J.E., HACKER, A.D., ALTIERE, R.J., &
GILLESPIE, M.N. (1985) Suppression of polyamine biosynthesis
prevents monocrotaline induced pulmonary oedema and arterial medial
thickening. Toxicol. appl. Pharmacol., 81: 91-99.
ORD, M.J., HERBERT, A., & MATTOCKS, A.R. (1985) The ability of
bifunctional and monofunctional pyrrole compounds to induce sister
chromatid exchange (SCE) in human lymphocytes and mutations in
Salmonella typhimurium. Mutat. Res., 149: 485-493.
PANTONE, D.J., BROWN, S.M., & WOMERSLEY, C. (1985) Biological
control of fiddleneck. California Agric., 39: 4-9.
PECKHAM, J.C., SANGSTER, L.P., & JONES, O.H. (1974) Crotalaria
spectabilis poisoning in swine. J. Am. Vet. Med. Assoc., 165:
633-638.
PEDERSEN, E. (1975) Pyrrolizidine alkaloids in Danish species of
the family Boraginaceae. Arch. Pharm. Chem. Sci. Ed., 3: 55-64.
PERCY, J.J. & PIERCE, A.E. (1971) Immunosuppressive activity of
the pyrrolizidine alkaloid metabolite dehydroheliotridine.
Immunology, 21: 273-280.
PERSAUD, J.V. & HOYTE, D.A. (1974) Pregnancy and progeny in rats
treated with the pyrrolizidine alkaloid fulvine. Exp. Pathol., 9:
59-63.
PESTCHANKER, M.J. & GIORDANO, O.S. (1986) Pyrrolizidine alkaloids
from five Senecio species. J. nat. Prod., 49: 722-723.
PESTCHANKER, M.J., ASCHERI, M.S., & GIORDANO, O.S. (1985a)
Uspallatine, a pyrrolizidine alkaloid from Senecio uspallatensis.
Phytochemistry, 24: 1622-1624.
PESTCHANKER, M.J., ASCHERI, M.S., & GIORDANO, O.S. (1985b)
Pyrrolizidine alkaloids from Senecio subulatus and S. glandulosus.
Planta Med., 51: 165-166.
PETERSON, J.E. (1965) Effects of pyrrolizidine alkaloid
lasiocarpine N -oxide on nuclear and cell division in the liver of
rats. J. Pathol. Bacteriol., 89: 153-171.
PETERSON, J.E. & CULVENOR, C.C.J. (1983) Plant and fungal toxins.
In: Keeler, R.F. & Tu, A.T., ed. Handbook of natural toxins, New
York, Marcel Dekker, Vol. 1, pp. 637-681.
PETERSON, J.E. & JAGO, M.V. (1980) Comparison of the toxic effects
of dehydroheliotridine and heliotrine in pregnant rats and their
embryos. J. Pathol., 131: 339-355.
PETERSON, J.E. & JAGO, M.V. (1984) Toxicity of Echium plantagineum
(Paterson's curse): pyrrolizidine alkaloid poisoning in rats. Aust.
J. agric. Res., 35: 305-316.
PETERSON, J.E., SAMUEL, A., & JAGO, M.V. (1972) Pathological
effects of dehydroheliotridine, a metabolite of heliotridine-based
pyrrolizidine alkaloids in the young rat. J. Pathol., 107: 107-189.
PETERSON, J.E., JAGO, M.V., REDDY, J.K., & JARRETT, R.G. (1983)
Neoplasia and chronic disease associated with the prolonged
administration of dehydroheliotridine to rats. J. Natl Cancer
Inst., 70: 381-386.
PETRY, T.W., BOWDEN, G.P., HUXTABLE, R.J., & SIPES, I.G. (1984)
Characterization of hepatic DNA damage induced in rats by the
pyrrolizidine alkaloid monocrotaline. Cancer Res., 44: 1505-1509.
PETRY, T.W., BOWDEN, G.P., BUHLER, D.R., & SIPES, I.G. (1986)
Genotoxicity of the pyrrolizidine alkaloid jacobine in rats.
Toxicol. Lett., 32: 275-281.
PIERCY, P.L. & RUSOFF, L.L. (1946) Crotalaria spectabilis
poisoning in Louisiana livestock. J. Am. Vet. Med. Assoc., 108:
69-73.
PIERSON, M.L., CHEEKE, P.R., & DICKINSON, E.O. (1977) Resistance
of the rabbit to dietary pyrrolizidine (Senecio) alkaloid. Res.
Commun. chem. Pathol. Pharmacol., 16: 561-564.
PIETERS, L.A.C. & VLIETINCK, A.J. (1985) Quantitative 'H Fourier
transform nuclear magnetic resonance spectroscopic analysis of
mixtures of pyrrolizidine alkaloids from Senecio vulgaris.
Fresenius' Z. Anal. Chem., 321: 355-358.
PIETERS, L.A.C. & VLIETINCK, A.J. (1986) Comparison of high-
performance liquid chromatography with 1H nuclear magnetic
resonance spectrometry for the quantitative analysis of
pyrrolizidine alkaloids from Senecio vulgaris. J. liq. Chromatogr.,
9: 745-755.
PLESTINA, R. & STONER, H.B. (1972) Pulmonary oedema in rats given
monocrotaline pyrrole. J. Pathol., 106: 235-249.
POOL, B.L. (1982) Genotoxic activity of an alkaloidal extract of
Senecio nemorensis spp. fuchsii in Salmonella typhimurium and
Escherichia coli systems (letter to the editor). Toxicology, 24:
351-355.
POWIS, G., AMES, M.M., & KOVACH, J.S. (1979) Metabolic conversion
of indicine- N-oxide to indicine in rabbits and humans. Cancer
Res., 39: 3564-3570.
PRABHU, M.B. (1940) Infantile cirrhosis of liver. Indian J.
Pediatr., 7: 121-134.
RADHAKRISHNA RAO, M.V. (1935) Histopathology of the liver in
infantile biliary cirrhosis. Indian J. med. Res., 23: 69-90.
RAJAGOPALAN, T.R. & NEGI, R.K.S. (1985) Alkaloids from Doronicum
pardalianches Linn. Indian J. Chem., 24B, 882.
RAKIETEN, N., GORDON, B.S., BEATTY, A., COONEY, D.A., DAVIS, R.D.,
& SCHIEN, P.S. (1971) Pancreatic islet cell tumours produced by
the combined action of streptozotocin and nicotinamide. Proc. Soc.
Exp. Biol. Med., 137: 280-283.
RAMALINGASWAMI, V. & NAYAK, N.C. (1969) Liver disease in India.
In: Popper, H. & Schaffner, F., ed. Progress in liver diseases,
New York, London, Grune and Stratton, pp. 222-235.
RAMSDELL, A.S. & BUHLER, D.R. (1981) High performance liquid
chromatographic analysis of pyrrolizidine (Senecio) alkaloids
using a reversed phase styrene-divinylbenzene resin column. J.
Chromatogr., 210: 154-158.
RAO, M.S. & REDDY, J.K. (1978) Malignant neoplasms in rats fed
lasiocarpine. Br. J. Cancer, 37: 289-293.
RAPPAPORT, A.M., KNOBLAUCH, M., SUMMERSKILL, J., & BRAS, G. (1967)
Experimental veno-occlusive disease. Gastroenterology, 54: 164.
RATNOFF, O.D. & MIRICK, G.S. (1949) Influence of sex upon the
lethal effects of an hepatotoxic alkaloid, monocrotaline. Bull.
Johns Hopkins Hosp., 84: 507-25.
RECHICIGL, M., Jr, ed. (1983) CRC Handbook on naturally occurring
food toxicants, Boca Raton, Florida, CRC Press, 266 pp.
RESCH, J.F. & MEINWALD, J. (1982) A revised structure for
acetylheliosupine. Phytochemistry, 21: 2340-2431.
RHODES, K. (1957) Two types of liver disease in Jamaican children.
West Indian med. J., 6: 1-29.
RIDKER, P.M., OHKUMA, S., MCDERMOTT, W.V., TREY, C., & HUXTABLE,
R.J. (1985) Hepatic veno-occlusive disease associated with
consumption of pyrrolizidine alkaloid containing dietary
supplements. Gastroenterology, 88: 1050-1054.
RIZK, A.M., HAMMOUDA, F.M., ISMAIL, S.I., GHALEB, H.A., MADKOUR,
M.K., POHLAND, A.E., & WOOD, G. (1983) Poisonous plants
contaminating edible ones and toxic substances in plant foods. I.
Alkaloids from Senecio desfontainei. Fitoterapia, 54: 115-121.
ROBERTSON, K.A. (1982) Alkylation of N2 in deoxyguanosine by
dehydroretronecine, a carcinogenic metabolite of the pyrrolizidine
alkaloid monocrotaline. Cancer Res., 42: 8-14.
ROBINS, D.J. (1982) The pyrrolizidine alkaloids. In: Herz, W.,
Grisebach, H., & Kirby, G.W., ed. Progress in the chemistry of
organic natural products, Vienna, New York, Springer-Verlag, pp.
115-203.
RODER, E., WEIDENFELD, H., & STENGL, P. (1981) Pyrrolizidine
alkaloids senecionine and retrorsine from Senecio inaequidens.
Planta Med., 41: 412-413.
RODER, E., WIEDENFELD, H., & KNOZINGER-FISCHER (1984a)
Pyrrolizidine alkaloid from Senecio abrotanifolius spp.
abroatanifolius, spp. abrotanifolius, and var. tiroliensis.
Planta Med., 41: 412-413.
RODER, E., WIEDENFELD, H., & BRITZ-KIRSTGEN, R. (1984b)
Pyrrolizidine alkaloids from Senecio cacaliaster. Phytochemistry,
23: 1761-1763.
ROGERS, A.E. & NEWBERNE, P.M. (1971) Lasiocarpine factors
influencing its toxicity and effects in liver cell division.
Toxicol. appl. Pharmacol., 18: 356-366.
ROITMAN, J.N. (1981) Comfrey and liver damage. Lancet, 25 April:
944.
ROITMAN, J.N. (1983) Ingestion of pyrrolizidine alkaloids: a
health hazard of global proportions. In: Finlay, J.W. & Schwass,
D.E., ed. Xenobiotics in foods and feeds, Washington DC, American
Chemical Society, pp. 345-378 (ACS Series).
ROSE, A.L., GARDNER, C.A., MCDONNELL, J.D., & BULL, L.B. (1957a)
Field and experimental investigation of "walk-about" disease of
horses (Kimberley horse disease) in northern Australia: Crotalaria
poisoning in horses. Part II. Aust. vet. J., 33: 49-62.
ROSE, A.L., GARDNER, C.A., MCDONNELL, J.D., & BULL, L.B. (1957b)
Field and experimental investigation of "walk about" disease of
horses (Kimberley horse disease) in northern Australia. Part I.
Aust. vet. J., 33: 25-33.
ROSE, C.L., HARRIS, P.N., & CHEN, K.K. (1959) Some pharmacological
action of supinine and lasiocarpine. J. Pharmacol. exp. Ther., 126:
179-184.
ROSE, E.F. (1972) Senecio species: toxic plants used as food and
medicine in the Transkei. S. Afr. Med. J., 46: 1039-1043.
ROSENFIELD, I. & BEATH, O.A. (1945) Tissue changes induced by
Senecio riddellii. Am. J. clin. Pathol., 15: 407-412.
ROTH, R.A., DOTZLAF, L.A., BARANYI, B., & HOOK, J.B. (1981) Effect
of monocrotaline ingestion on liver, kidney and lung of rat.
Toxicol. appl. Pharmacol., 60: 193-203.
ROYES, K. (1948) Infantile hepatic cirrhosis in Jamaica. Caribb.
med. J., 10: 16-48.
SAFOUH, M. & SHEHATA, A.H. (1965) Hepatic vein occlusion disease
of Egyptian children. J. Pediatr., 67: 415-432.
ST. GEORGE-GRAMBAUER, T.D. & RAC, R. (1962) Hepatogenous chronic
copper poisoning in sheep in south Australia due to consumption of
Echium plantagineum L. (Salvation Jane). Aust. vet. J., 38: 288-293.
SALASPURO, M. & SIPPONEN, P. (1976) Demonstration of an
intracellular copper-binding protein in orcein staining in long
standing cholestatic liver diseases. Gut, 17: 787-790.
SAMUEL, A. & JAGO, M.V. (1975) Localization in the cell cycle of
the antimitotic action of the pyrrolizidine alkaloid, lasiocarpine,
and its metabolite, dehydroheliotridine. Chem.-biol. Interact., 10:
185-197.
SANDERS, D.A., SHEALY, A.L., & EMMEL, M.W. (1936) The pathology of
Crotalaria spectabilis Roth poisoning in cattle. J. Am. Vet. Med.
Assoc., 89: 150-156.
SAVIN, I.G., (1983) [Research of the influence of heliotrin on
the microsomal mono oxygenase systems of the rat liver.] N.E.
Pyrogov 2nd Moscow State Medical Institute. (Thesis in biological
chemistry) (in Russian).
SAVVINA, K.I. (1952) Pathological anatomy of atrophic hepatic
cirrhosis. Arch. Pathol., 14: 65-70.
SCHOENTAL, R. (1959) Liver lesions in young rats suckled by
mothers treated with the pyrrolizidine (Senecio) alkaloids,
lasiocarpine, and retrorsine. J. Pathol. Bacteriol., 77: 485-504.
SCHOENTAL, R. (1961) Herbal medicines and liver disease. J. exp.
med. Sci., 4: 126-135.
SCHOENTAL, R. (1963) Liver disease and 'natural' hepatotoxins.
Bull. World Health Organ., 29: 823-833.
SCHOENTAL, R. (1968) Toxicology and carcinogenic action of
pyrrolizidine alkaloids. Cancer Res., 28: 2237-2246.
SCHOENTAL, R. (1970) Hepatotoxic activity of retrorsine,
senkirkine and hydroxysenkirkine in newborn rats, and the role of
epoxides in carcinogenesis by pyrrolizidine alkaloids and
aflatoxins. Nature (Lond.), 227: 401-402.
SCHOENTAL, R. (1975) Pancreatic islet-cell and other tumours in
rats given heliotrine, a monoester pyrrolizidine alkaloid, and
nicotinamide. Cancer Res., 35: 2020-2024.
SCHOENTAL, R. & BENSTED, J.P.M. (1963) Effects of whole body
irradiation and of partial hepatectomy on the liver lesions induced
in rats by a single dose of retrorsine, a pyrrolizidine (Senecio)
alkaloid. Br. J. Cancer, 17: 242-251.
SCHOENTAL, R. & CAVANAGH, J.B. (1972) Brain and spinal cord
tumours in rats treated with pyrrolizidine alkaloids. J. Natl
Cancer Inst., 49: 665-671.
SCHOENTAL, R. & COADY, A. (1968) The hepatotoxicity of some
Ethiopian and east African plants including some used as
traditional medicines. East Afr. med. J., 45: 577-579.
SCHOENTAL, R. & HEAD, M.A. (1955) Pathological changes in rats as
result of treatment with monocrotaline. Br. J. Cancer, 9: 229-237.
SCHOENTAL, R. & HEAD, M.A. (1957) Progression of liver lesions
produced in rats by temporary treatment with pyrrolizidine
(Senecio) alkaloids, and the effects of betaine and high casein
diet. Br. J. Cancer, 11: 535-544.
SCHOENTAL, R. & MAGEE, P.N. (1957) Chronic liver changes in rats
after a single dose of lasiocarpine, a pyrrolizidine (Senecio)
alkaloid. J. Pathol. Bacteriol., 74: 305-319.
SCHOENTAL, R. & MAGEE, P.N. (1959) Further observation on the
subacute and chronic liver changes rats after a single dose of
various pyrrolizidine (Senecio) alkaloids. J. Pathol. Bacteriol.,
78: 471-482.
SCHOENTAL, R. & MATTOCKS, A.R. (1960) Hepatotoxic activity of
semisynthetic analogues of pyrrolizidine alkaloids. Nature (Lond.),
185: 842-843.
SCHOENTAL, R., HEAD, M.A., & PEACOCK, P.R. (1954) Senecio
alkaloids: primary liver tumours in rats as a result of treatment
with (1) mixture of alkaloids from S. jacobaea Lin, (2)
retrorsine, (3) isatidine. Br. J. Cancer, 8: 458-465.
SCHOENTAL, R., FOWLER, M.E., & COADY, A. (1970) Islet cell tumours
of the pancreas found in rats given pyrrolizidine alkaloids from
Amsinckia intermedia Fisch and Mey and from Heliotropium supinum.
Cancer Res., 30: 2127-2131.
SEGALL, H.J. (1979a) Reversed phase isolation of pyrrolizidine
alkaloids. Liq. Chromatogr., 2: 429-436.
SEGALL, H.J. (1979b) Preparative isolation of pyrrolizidine
alkaloids derived from Senecio vulgaris. Liq. Chromatogr., 2:
1319-1323.
SEGALL, H.J., DALLAS, J.L., & HADDON, W.F. (1984) Two
dihydropyrrolizine alkaloid metabolites isolated from mouse hepatic
microsomes in vitro. Drug Metab. Disp., 12: 68-71.
SEGALL, H.J., WILSON, D.W., DALLAS, J.L., & HADDON, W.F. (1985)
Trans-4-hydroxy-2-hexenol: a reactive metabolite from the
macrocyclic pyrrolizidine alkaloid senecionine. Science, 229: 472-475.
SEGER, C.L., NEWSON, J.D., ROTH, E.E., & HUTCHINSON, W.R. (1969)
Chronic toxic hepatitis in deer from a Louisiana coastal marsh.
Bull. Wildlife Disease Assoc. Proc. Ann. Conf., pp. 295-6.
SELZER, G. & PARKER, R.G.F. (1951) Senecio poisoning exhibiting
as Chiari's syndrome: a report of 12 cases. Am. J. Pathol., 27:
885-907.
SENER, B., TEMIZER, H., TEMIZER, A., & KARAKAYA, A.E. (1986) High
performance liquid chromatographic determination of alkaloids in
Senecio vernalis. J. Pharm. Belg., 41: 115-117.
SHARMA, R.K., KHAJURIA, G.S., & ATAL, C.K. (1965) Thin layer
chromatography of pyrrolizidine alkaloids. J. Chromatogr., 19:
433-434.
SHERLOCK, S. (1968) Diseases of liver and biliary system, 4th
ed., Oxford, Edinburgh, Blackwell Scientific Publishers, 250 pp.
SHTENBERG, A.I. & ORLOVA, N.V. (1955) [The question of the
etiology of the so-called "Ozhalangar Encephalitis".] Vopr. Pitan.,
14: 27-31 (in Russian).
SHULL, L.R., BUCKMASTER, G.W., & CHEEKE, P.R. (1976) Factors
influencing pyrrolizidine (Senecio) alkaloid metabolism: species,
liver sulphydryls and rumen fermentation. J. anim. Sci., 43: 1247-1253.
SHUMAKER, R.C., ROBERTSON, K.A., HSU, I.C., & ALLEN, J.R. (1976)
Neoplastic transformation in tissues of rats exposed to
monocrotaline or dehydroretronecine. J. Natl Cancer Inst., 56: 787-789.
SIDDIQI, M.A., SURI, K.A., SURI, M.P., & ATAL, C.K. (1978a) Novel
pyrrolizidine alkaloid from Crotalaria nana. Phytochemistry, 17:
2143-2144.
SIDDIQI, M.A., SURI, K.A., SURI, O.P., & ATAL, C.K. (1978b) Genus
Crotalaria. Part 34. Cronaburmine, a new pyrrolizidine alkaloid
from Crotalaria nana Burm. Indian J. Chem., 16B: 1132-1133.
SMITH, L.W. & CULVENOR, C.C.J. (1981) Plant sources of
hepatotoxic pyrrolizidine alkaloids. J. nat. Prod., 44: 129-152.
SMITH, T.E., WEISBACH, H., & UDENFRIEND S. (1962) Studies on the
mechanism of monoaminooxidase: metabolism of N,N-dimethyltryptamine
and N,N-dimethyltryptamine- N-oxide. Biochemistry, 1: 137.
SOBIN, L.H., FETRAT, M.E., & ANWER, M.A. (1969) Hepatic lesions in
Afghanistan. Trop. geogr. Med., 21: 27-29.
SRIVASTAVA, R.N., MOHABBAT, O., GHANI, A.R., & ARAM, G.N. (1978)
Veno-occlusive disease of the liver. Indian. Paediatr., 15: 143-146.
SRUNGBOONMEE, S. & MASKASAME, C. (1981) A preliminary study on
the toxicity of Crotalaria juncea to cattle. Sattawaphaet San, 32:
91-107.
STEIN, H. (1957) Veno-occlusive disease of liver in African
children. Br. med. J., 29 June: 1496-1499.
STEIN, H. & ISAACSON, C. (1962) Veno-occlusive disease of the
liver. Br. med. J., 10 February: 372-374.
STENMARK, K.R., MORGANROTH, M.L., REMIGIO, L.K., VOELKEL, N.F.,
MURPHY, R.C., HENSON, P.M., MATHIAS, M.M., & REEVES, J. (1985)
Alveolar inflammation and arachidonate metabolism in monocrotaline-
induced pulmonary hypertension. Am. J. Physiol., 248: H859-H866.
STILLMAN, A.E., HUXTABLE, R.J., CONSROE, P., KOHNEN, P., & SMITH,
S. (1977) Hepatic veno-occlusive disease due to pyrrolizidine
poisoning in Arizona. Gastroenterology, 73: 349-352.
STIRLING, G.A., BRAS, G., & URQUHART, A.E. (1962) The early
lesions in veno-occlusive disease of the liver. Arch. dis. Child.,
37: 535-538.
STOYEL, C. & CLARK, A.M. (1980) The transplacental micronucleus
test. Mutat. Res., 74: 393-398.
STUART, K.L. & BRAS, G. (1955) Clinical observations on veno-
occlusive disease of the liver in Jamaican adults. Br. med. J., 2:
348-352.
STUART, K.L. & BRAS, G. (1956) Veno-occlusive disease of the liver
in Barbados. West Indian med. J., 5: 33-36.
STUART, K.L. & BRAS, G. (1957) Veno-occlusive disease of the
liver. Q. J. Med., 26: 291-315.
STYLES, J., ASHBY, J., & MATTOCKS, A.R. (1980) Evaluation in vitro
of several pyrrolizidine alkaloid carcinogens: observation on the
essential pyrrolic nucleus. Carcinogenesis, 1: 161-164.
SUFFNESS, M. & CORDELL, G.A. (1985) Antitumour alkaloids. In:
Brossi, A., ed. The alkaloids, New York, Academic Press,
pp. 1-347.
SUGITA, T., HYERS, T.M., DAUBER, I.M., WAGNER, W.W., MCMURTRY,
I.F., & REEVES, J.T. (1983a) Lung leak precedes right ventricular
hypertrophy in monocrotaline treated rats. J. appl. Physiol., 54:
371-374.
SUGITA, T., STENMARK, K.R., WAGNER, W.W., HENSON, P.M., HENSON,
J.E., HYERS, T.M., & REEVES, J.T. (1983b) Abnormal alveolar cells
in monocrotaline induced pulmonary hypertension. Exp. lung Res.,
5: 201-215.
SUNDARESON, A.E. (1942) An experimental study of placental
permeability to cirrhogenic poisons. J. Pathol. Bacteriol., 54:
289-298.
SVOBODA, D. & REDDY, J.K. (1972) Malignant tumours in rats given
lasiocarpine. Cancer Res., 32: 908-912.
SVOBODA, D. & REDDY, J.K. (1974) Lasiocarpine induced,
transplantable squamous cell carcinoma of rat skin. J. Natl Cancer
Inst., 53: 1415-1418.
SVOBODA, D. & SOGA, J. (1966) Early effects of pyrrolizidine
alkaloids on the fine structure of rat liver cells. Am. J. Pathol.,
48: 347-373.
SWICK, R.A., CHEEKE, P.R., & BUHLER, D.R. (1979) Factors affecting
the toxicity of dietary tansy ragwort to rats. In: Cheeke, P.R.,
ed. Proceedings of the Symposium on Pyrrolizidine (Senecio)
Alkaloids: Toxicity, Metabolism, and Poisonous Plant Control
Measures, Corvallis, Oregon, USA, 23-24 February 1979, Oregon,
Nutrition Research Institute.
SWICK, R.A., CHEEKE, P.R., GOEGER, D.E., & BUHLER, D.R. (1982a)
Effect of dietary Senecio jacobaea and injected Senecio alkaloids
and monocrotaline on guinea pigs. J. anim. Sci., 55: 1411-1416.
SWICK, R.A., CHEEKE, P.R., & BUHLER, D.R. (1982b) Subcellular
distribution of hepatic copper, zinc and iron and serum
ceruloplasmin in rats intoxicated by oral pyrrolizidine (Senecio)
alkaloids. J. anim. Sci., 55(6): 1425-1430.
SWICK, R.A., CHEEKE, P.R., PATTON, N.M., & BUHLER, D.R. (1982c)
Absorption and excretion of pyrrolizidine (Senecio) alkaloids and
their effects on mineral metabolism in rabbits. J. anim. Sci., 55:
1417-1424.
SWICK, R.A., CHEEKE, P.R., MIRANDA, C.L., & BUHLER, D.R. (1982d)
The effect of consumption of the pyrrolizidine alkaloid containing
plant Senecio jacobaea on iron and copper metabolism in the rat.
J. Toxicol. environ. Health, 10: 757-768.
SWICK, R.A., CHEEKE, P.R., RAMSDELL, H.S., & BUHLER, D.R. (1983)
Effect of sheep rumen fermentation and methane inhibition on the
toxicity of Senecio jacobaea. J. anim. Sci., 56: 645-651.
TAKANASHI, H., UMEDA, M., & HIRONO, I. (1980) Chromosomal
aberrations and mutation in cultured mammalian cells induced by
pyrrolizidine alkaloids. Mutat. Res., 78: 67-77.
TAKEOKA, O., ANGEVINE, M., & LALICH, J. (1962) Stimulation of mast
cells in rat fed various chemicals. Am. J. Pathol., 40: 545-554.
TANDON, B.N., TANDON, H.D., TANDON, R.K., NARENDRANATHAN, M., &
JOSHI, Y.K. (1976) Epidemic of veno-occlusive disease in central
India. Lancet, 7 August: 271-272.
TANDON, B.N., TANDON, H.D., KOSHY, A., NARENDRANATHAN, M., JOSHI,
Y.K., TANDON, R.K., BHARGAVA, S., RAJANI, M., BHATIA, M.L.,
MANCHANDA, S.C., & KASTURI, T.E. (1977) Epidemiological,
clinical, biochemical and haemodynamic study of veno-occlusive
disease of the liver due to Crotalaria alkaloids in India. J. All
Ind. Inst. Med. Sci., 3: 165-175.
TANDON, B.N., TANDON, H.D., & MATTOCKS, A.R. (1978) Study of an
epidemic of veno-occlusive disease in Afghanistan. Indian J. med.
Res., 68: 84-90.
TANDON, H.D. & TANDON, B.N. (1975) Epidemic of liver disease -
Gulran District, Herat Province, Afghanistan, Alexandria, World
Health Organization, Regional Office for the Eastern Mediterranean
(Assignment report No. EM/AFG/OCD/001/RB).
TANDON, H.D., TANDON, B.N., TANDON, R., & NAYAK, N.C. (1977) A
pathological study of the liver in an epidemic outbreak of veno-
occlusive disease. Indian J. med. Res., 65: 679-684.
TANDON, H.D., TANDON, B.N., & MATTOCKS, A.R. (1978) An epidemic of
veno-occlusive disease of the liver in Afghanistan. Am. J.
Gastroenterol., 72: 607-613.
TANDON, R.K., TANDON, B.N., TANDON, H.D., BHATIA, M.L., BHARGAVA,
S., LAL, P., & ARORA, R.R. (1976) Study of an epidemic of veno-
occlusive disease in India. Gut, 17: 849-855.
TANNER, M.S. & PORTMANN, B. (1981) Indian childhood cirrhosis.
Arch. dis. Child., 56: 4-6.
TAYLOR, S., BELT, R.J., HAAS, C.D., & HOOGSTRATEN, B. (1983) Phase
I trial of indicine- N-oxide on two dose schedules. Cancer, 51:
1988-1991.
TEILUM, G. (1949) Endophlebitis hepatica obliterans. Acta pathol.
microbiol., 26: 147-166.
TEMIZER, A., ONAR, A.N., SENER, B., TEMIZER, H., & KARAKAYA, A.E.
(1985) Determination of alkaloids by differential pulse
polarography. I. Senecio alkaloids. J. Pharm. Belg., 40(2): 75-78.
TEREKHOV, G.N. (1939) [Pathomorphology of toxic hepatitis with
ascites.] In: Mirochnik, M.F., ed. [Functional, diagnostic and
pathological changes of toxic hepatitis with ascites,] Tashkent,
State Publishing House of Science Technology and Socio-economic
Literature of Uzbekistan, pp. 145-200 (in Russian).
TEREKHOV, G.N. (1952) [The pathomorphology of alimentary toxic
dystrophy with ascites.] In: Milenkov, S.M. & Kizhaikin, Y., ed.
[Collection of scientific papers on Toxic Hepatitis with Ascites,]
Tashkent, Publishing House of the University of Central Asia, pp.
148-164 (in Russian).
THORPE, E. & FORD, E.J.H. (1968) Development of hepatic lesions in
calves fed with ragwort (Senecio jacobaea). J. comp. Pathol., 78:
195-205.
TITTEL, G., HINZ, H., & WAGNER, H. (1979) Quantitative
determination of pyrrolizidine alkaloids in Symphyti radix by
HPLC. Planta Med., 37: 1-8.
TUCHWEBER, B., KOVAKS, K., JAGO, M.V., & BEAULIEU, T. (1974)
Effect of steroidal and non-steroidal microsomal enzyme inducers on
the hepatotoxicity of pyrrolizidine alkaloids in rats. Res. Commun
chem. Pathol. Pharmacol., 7: 459-480.
TUCKER, A., BRYANT, S.E., FROST, H.H., & MIGALLY, N. (1983)
Chemical sympathectomy and serotonin inhibition reduce
monocrotaline-induced right ventricular hypertrophy in rats. Can.
J. Physiol. Pharmacol., 61: 356-362.
TURNER, J.H. & LALICH, J.J. (1965) Experimental cor pulmonale in
the rat. Arch. Pathol., 79: 409-418.
VALDIVIA, E., SONNAD, J., HAYASHI, Y., & LALICH, J.J. (1967a)
Experimental interstitial pulmonary oedema. Angiology, 18: 378-383.
VALDIVIA, E., LALICH, J., HAYASHI, Y., & SONNAD, J. (1967b)
Alterations in pulmonary alveoli after a single injection of
monocrotaline. Arch. Pathol., 84: 64-76.
VAN DER WATT, J.J., PURCHASE, I.F.H., & TUSTIN, R.C. (1972) The
chronic toxicity of retrorsine, a pyrrolizidine alkaloid, in vervet
monkeys. J. Pathol., 107: 279-287.
VILLARROEL, L.H., TORRES, R.G., NAVARRO, J.M., & FAJARDO, V.M.
(1985) Senecionine and seneciphylline from Senecio patagonicus.
Fitoterapia, 56: 250-251.
VISCONTINI, M. & GILHOF-SCHAUFELBERGER, H. (1971) Synthesis of (±)
dehydroheliotridine. Helv. chim. Acta, 54: 449-456.
WAGENVOORT, C.A., DINGEMANS, K.P., & LOTGERING, G.G. (1974a)
Electron microscopy of pulmonary vasculature after application of
fulvine. Thorax, 29: 511-521.
WAGENVOORT, C.A., WAGENVOORT, N., & DIJK, H.J. (1974b) Effect of
fulvine on pulmonary arteries and veins of the rat. Thorax, 29:
522-529.
WAGNER, H., NEIDHARDT, U., & TITTEL, G. (1981) TLC and HPLC
analysis of pyrrolizidine N-oxide alkaloids of Symphyti radix.
Planta Med., 41: 232-239.
WAKIM, K.G., HARRIS, P.N., & CHEN, K.K. (1946) The effects of
senecionine on the monkey. J. Pharmacol. exp. Ther., 87: 38-45.
WALKER, K.H. & KIRKLAND, P.D. (1981) Senecio lautus toxicity in
cattle. Aust. vet. J., 57: 1-7.
WATT, J.M. & BREYER-BRANDWIJK, M.G. (1962) Medicinal and poisonous
plants of southern and eastern Africa, Edinburgh, London, E. and
S. Livingstone, 584 p.
WEHNER, F.C., THIEL, P.G., & VAN RENSBURG, S.J. (1979)
Mutagenicity of alkaloids in the Salmonella/microsome system.
Mutat. Res., 66: 187-190.
WESTON, C.F.M., COOPER, B.T., DAVIES, J.D., & LEVINE, D.F. (1987)
Veno-occlusive disease of the liver secondary to ingestion of
comfrey. Br. med. J., 295: 183.
WHITE, I.N.H. (1976) The role of liver glutathion in the acute
toxicity of retrorsine to rats. Chem.-biol. Interact., 13: 333-342.
WHITE, I.N.H. (1977) Excretion of pyrrolic metabolites in bile of
rats given retrorsine or the bis- n-ethylcarbamate of synthanecine
A. Chem.-biol. Interact., 16: 169-180.
WHITE, I.N.H. & MATTOCKS, A.R. (1971) Some factors affecting the
conversion of pyrrolizidine alkaloids to N-oxides and to pyrrolic
derivatives in vitro. Xenobiotica, 1: 503-505.
WHITE, I.N.H. & MATTOCKS, A.R. (1972) Reactions of dihydro-
pyrrolizidine with deoxyribonucleic acid in vitro. Biochem.
J., 128: 291-297.
WHITE, I.N.H., MATTOCKS, A.R., & BUTLER, W.H. (1973) The
conversion of the pyrrolizidine alkaloid retrorsine to pyrrolic
derivatives in vivo and in vitro and its acute toxicity to
various animal species. Chem.-biol. Interact., 6: 207-218.
WHITE, R.D., KRUMPERMAN, P.H., CHEEKE, P.R., & BUHLER, D.R. (1983)
An evaluation of acetone extracts from six plants in the Ames
mutagenicity test. Toxicol. Lett., 15: 25-31.
WHITE, R.D., SWICK, R.A., CHEEKE, P.R. (1984) Effect of dietary
copper and molybdenum on tansy ragwort (Senecio Jacobaea) toxicity
in sheep. Am. J. vet. Res., 45: 159-161.
WHO (1980) Inventory of medicinal plants used in the different
countries, Geneva, World Health Organization (Document No.
DPM/80.3).
WICKRAMANAYAKE, P.P., ARBOGAST, B.L., BUHLER, D.R., DEINZER, M.L.,
& BURLINGAME, A.L. (1985) Alkylation of nucleosides and
nucleotides by dehydroretronecine: characterization of covalent
adducts by liquid secondary ion mass spectrometry. J. Am. Chem.
Soc., 107: 2485-2488.
WIEDENFELD, H. (1982) Two pyrrolizidine alkaloids from Gynura
scandens. Phytochemistry, 21: 2767-2768.
WIEDENFELD, H., PASTEWKA, U., STENGL, P., & ROEDER, E. (1981) On
the gas chromatographical determination of the pyrrolizidine
alkaloids of some Senecio species. Planta Med., 41: 124-128.
WIEDENFELD, H., RODER, E., & ANDERS, E. (1985) Pyrrolizidine
alkaloids from seeds of Crotalaria scassellatii. Phytochemistry,
24: 376-378.
WILLIAMS, A.O., EDINGTON, G.M., & OBAKPONOVWE, P.C. (1967)
Hepatocellular carcinoma in infancy and childhood in Ibadan,
western Nigeria. Br. J. Cancer, 21(3): 474-482.
WILMOT, F.C. & ROBERTSON, G.W. (1920) Senecio disease or
cirrhosis of the liver due to Senecio poisoning. Lancet,
23 October: 848-849.
WITTIG, M. & STEPHEN, C. (1964) Modification of microsomal lipid
peroxidation and drug metabolism by cytoplasmic copper. Res.
Commun. Chem. Pathol. Pharmacol., 44: 477-493.
WONG, R.Y. & ROITMAN, J.N. (1984) Structure and absolute
configuration of (+)-doronine-benzene (1:1). Acta Crystallogr.,
Sect. C: Cryst. Struc. Commun., C40: 163-166.
WURM, H. (1939) [A cluster of endophlebilis hepatica obliterans in
the age group of suckling infants.] Klin. Wochenschr., 18: 1527-1531
(in German).
YAMANAKA, H., NAGAO, M., SUGIMURA, T., FURUYA, T., SHIRAI, A., &
MATSUSHIMA, T. (1979) Mutagenicity of pyrrolizidine alkaloids in
the Salmonella/mammalian microsome test. Mutat. Res., 68: 211-216.
YULDASHEVA, L.N. & SULTANOVA, R.G. (1983) [Oxidative reactions in
rat liver tissue under conditions of chronic heliotrine hepatitis.]
Vopr. Med. Khim., 29: 81-85 (in Russian).
YUNUSOV, S.YU. & PLEKHANOVA, N.V. (1959) [The alkaloids of
Trichodesma incanum. The structure of incanine and trichesmine.]
Zh. Obshch. Khim., 29: 677-684 (in Russian).
ZHELTOVA, L.I. (1952) [The clinical course of toxic hepatitis.]
In: Milenkov, S.M. & Kizhaikin, Y., ed. [Collection of scientific
papers on Toxic Hepatitis with Ascites,] Tashkent, Publishing
House of the University of Central Asia, pp. 76-90 (in Russian).
APPENDIX I
Pyrrolizidine Alkaloids and Their Plant Sources
ISOLATIONS OF TOXIC PYRROLIZIDINE ALKALOIDS - (8701081148)
Alkaloid Plant Sources Reference Reference
Location
19-Acetoxysenkirkine Senecio laricifolius H.B.K. Bohlmann et al. (1986) A
6-Acetylanacrotine Crotalaria agatiflora Schweinf. Culvenor & Smith (1972) B281
6-Acetyl-trans-anacrotine Crotalaria agatiflora Schweinf. Culvenor & Smith (1972) B281
Acetylcrotaverrine Crotalaria verrucosa L. O.P. Suri et al. (1976) B339
C. walkeri Arnott K.A. Suri et al. (1976) B340
7-Acetylechinatine Cynoglossum amabile Stapf. and Drummond Culvenor & Smith, unpubl.
Lindelofia spectabilis Lehm. Rao et al. (1974) B62
Symphytum asperum Lepech. Pedersen (1975b) A
S. officinale Linn. Pedersen (1975b) A
Acetylgynuramine Gynura scandens O. Hoffm. Wiedenfeld (1982) C
Acetylheliosupine Cynoglossum officinale L. Pedersen (1970); Resch & Meinwald (1982) B43, A
Myosotis sylvatica Hoffm. Resch & Meinwald (1982) A
Symphytum asperum Lepech. Pedersen (1975) A
S. officinale Linn. Pedersen (1975) A
Acetylindicine Heliotropium indicum L. Mattocks (1967a) B71
7-Acetylintermedine Borago officinale L. Luthy et al. (1984) A
Symphytum aspera Roitman (1981) A
S. officinale Linn. Roder et al. (1982) C
S. x uplandicum Nyman Culvenor et al. (1980a), (1980b) A, A
Alkaloid Plant Sources Reference Reference
Location
Acetyllasiocarpine Heliotropium europaeum L. Culvenor et al. (1975) B69
7-Acetyllycopsamine Amsinckia menziesii (Lehm.) Nels Roitman, (1983a) C
& Macbr.
Anchusa officinalis L. Pedersen (1975); Broch-Due & A, B32
Aasen (1980)
Borago officinale L. Luthy et al. (1984) A
Symphytum aspera Roitman (1981) A
S. officinale Linn. Huizing & Malingre (1981) C
S. x uplandicum Nyman Culvenor et al. (1980a, 1980b) A, A
3a'-Acetyllycopsamine Amsinckia menziesii (Lehm.) Nels. Roitman (1983a C
& Macbr.
7-Acetylmadurensine Crotalaria agatiflora Schweinf. Culvenor & Smith (1972) B281
7-Acetyl-cis-madurensine Crotalaria agatiflora Schweinf. Culvenor & Smith (1972) B281
7-Acetylscorpioidine Myosotis scorpioides L. Resch et al. (1982) C
Acetylseneciphylline Senecio pterophorus DC. Edgar et al. (1976) B246
18-Acetylsenkirkine Senecio illinitus Phill. Gonzalez et al. (1986a) A
S. kirkii Hook. f. ex Kirk Briggs et al. (1965) A
S. tenuifolius Burm. Bhakuni & Gupta (1982) C
Acetylsyneilesine Syneilesis palmata Maxim. Hikichi & Furuya (1976) B279
Amabiline Borago officinale L. Luthy et al. (1984) A
Cynoglossum amabile Stapf. et Drummond Culvenor & Smith (1967) B33
C. glochidiatum Wall. ex Lindl. K.A. Suri et al. (1975a) B36
Eupatorium cannabinum L. Luthy et al. (1984) A
Lindelofia angustifolia (Schrenk) Brand. K.A. Suri et al. (1975a) B36
Alkaloid Plant Sources Reference Reference
Location
Anacrotine Crotalaria agatifolia Schweinf. Culvenor & Smith (1972) B281
C. incana L. Mattocks (1968) B302
C. laburnifolia L. Snehelata et al. (1966); Sawhney B309,
et al. (1967) 291
C. laburnifolia L. subsp. eldomae Crout (1972) B312
C. micans Link. Atal et al. (1966a) B280
C. verrucosa L. Subramanian & Nagarajan (1967) B338
Anadoline Symphytum orientale Ulubelen & Doganca (1971); Culvenor B103,
et al. (1975) 104
S. tuberosum L. Ulubelen & Ocal (1977) B105
Angelylechimidine (or Symphytum asperum Lepech. Gadella et al. (1983) A
isomer) S. x uplandicum Nyman Gadella et al. (1983) A
7-Angelylheliotridine Heliotropium supinum L. Crowley & Culvenor (1959) B83
trachelanthate
7-Angelylheliotridine Heliotropium supinum L. Crowley & Culvenor (1959) B83
viridiflorate
7-Angelylheliotrine Heliotropium digynum Forssk. Hammouda et al. (1984) A
H. eichwaldii Steud ex DC. O.P. Suri et al. (1975) B63
7-Angelyl-9-sarracinyl- Senecio triangularis Hook. Rueger & Benn (1983) C
retronecine
6-Angelyl-trans-anacrotine Crotalaria agatiflora Schweinf. Culvenor & Smith (1972) B281
Asperumine Echium vulgare L. Karimov et al. (1975) B50
Symphytum asperum Lepech. Man'ko et al. (1969); Man'ko & B95, 96
Kotowskii (1970); Man'ko et al. 1970 B97
S. caucasicum Bieb. Man'ko et al. (1972); Mel'kumova B98, 99
et al. 1974
Alkaloid Plant Sources Reference Reference
Location
Axillaridine Crotalaria axillaris Ait. Crout (1968b, 1969) B287, 288
C. scassellatii Chiov Wiedenfeld et al. (1985) A
Axillarine Crotalaria axillaris Ait. Crout (1968b, 1969) B287, 288
C. scassellatii Chiov Wiedenfeld et al. (1985) A
Bisline Senecio othonniformis Fourcade Coucourakis & Gordon-Gray (1970) B224
S. petasitis DC. Gonzalez et al. (1973) B225
Brachyglottine Brachyglottis repanda Forst. et Forst. White, pers. commun.
Carategine Lindelofia tschimganica Akramov et al. (1965) B87
Rindera oblongifolia M. Pop. Akramov et al. (1965) B87
Solenanthus karategenius Lipsky Akramov et al. (1964) B93
Chlorodeoxysceleratine Senecio latifolius DC. (S. sceleratus) Gordon-Gray (1967) B261
Clivorine Ligularia brachyphylla Hand. Mazz. Klasek et al. (1971) B134
L. clivorum Klasek et al. (1967, 1969, 1970); B135,
Birnbaum et al. (1971) 136,
137,
138
L. dentata (A. Gray) Hara Klasek et al. (1971) B134
L. elegans (Cass.) Klasek et al. (1971) B134
Crispatine Crotalaria candicans W. & A. Suri et al. (1982) C
C. crispata F. Muell. ex Benth. Culvenor & Smith (1963) B294
C. lunata Beddome ex Polhill Rothschild et al. (1979) C
C. madurensis R. Wight Habib et al. (1971) B315
Crobarbatine Crotalaria barbata R. Graham ex R. Wight Puri et al. (1973) B289
Walk.-Arn.
Alkaloid Plant Sources Reference Reference
Location
Cromadurine Crotalaria madurensis Wight Rao et al. (1974, 1975b) B62, 316
Cronaburmine Crotalaria nana Burm. Siddiqi et al. (1978b) A
Crosemperine Crotalaria aegyptiaca Benth. Zalkow et al. (1979) B419
C. semperflorens Vent. Atal et al. (1967) B331
Crotaflorine Crotalaria agatiflora Schweinf. Culvenor & Smith (1972) B281
Crotafoline Crotalaria laburnifolia L. subsp. eldomae Crout (1972) B312
Crotalarine Crotalaria burhia Buch-Ham. Ali & Adil (1973); Rao et al. (1975a) B292,
293
Crotaleschenine Crotalaria leschenaultii Suri & Atal (1967); Smith et al. (1988) B313
Crotananine Crotalaria nana Burm. Siddiqi et al. (1978a) A
Crotastriatine Crotalaria pallida Ait. (syn. C. Gandhi et al. (1968); Batra et al. (1975) B323,
mucronata Desv., C. striata DC.) 324
Crotaverrine Crotalaria verrucosa L. O.P. Suri et al. (1976) B339
C. walkeri Arnott K.A. Suri et al. (1976) B340
Cruentine A Senecio cruentus DC. Chu & Chu (1964) B172
Cruentine B Senecio cruentus DC. Chu & Chu (1964) B172
Curassavinine Heliotropium curassavicum Linn. Mohanraj et al. (1982a) C
Cynaustine Borago officinale L. (or amabiline ?) Larson et al. (1984) C
Cynoglossum australe R. Br. Culvenor & Smith, 1967 B33
C. lanceolatum Forsk. Suri et al. 1975a B36
Alkaloid Plant Sources Reference Reference
Location
Deoxyaxillarine Crotalaria scassellatii Chiov Wiedenfeld et al. 1985 A
Diacetyllycopsamine Amsinckia menziesii (Lehm.) Nels. & Macbr. Roitman, 1983a C
Dibenzoylretronecine Caccinea glauca Savi. Siddiqi et al. 1978a B346
Dicrotaline Crotalaria dura J.M. Wood et Evans Marais, 1944; Adams & Van Duuren, 1953a B295, 296
C. globifera E. Mey. Marais, 1944; Adams & Van B295,
Duuren, 1953a 296
N-(Dihydropyrrolizino- Heliotropium europaeum L. Culvenor & Smith, 1969 B68
methyl) heliotrine chloride
15,20-Dihydroxyerucifoline Senecio dolichodoryius Cuatr. Bohlmann et al. 1986 A
Dihydroxytriangularine Alkanna tinctoria Tausch Roder et al. 1984b C
Doronenine Senecio abrotanifolius ssp. Roder et al. 1984a A
abrotanifolius
S. abrotanifolius ssp. abrotanifolius Roder et al. 1984a A
var tiroliensis
S. doronicum L. Roder et al. 1979a, 1980; Kirfel B176, C
et al. 1980 A
Doronine Doronicum macrophyllum Alieva et al. 1976 B122
Senecio abrotanifolius ssp. Roder et al. 1984a A
abrotanifolius
S. abrotanifolius ssp. abrotanifolius Roder et al. 1984a A
var tiroliensis
S. clevelandii E.L. Greene Wong & Roitman, 1984 A
S. othonnae Bieb. Khalilov et al. 1977 B223
Alkaloid Plant Sources Reference Reference
Location
H. suaveolens Bieb. Guner, 1986 A
H. supinum L. Crowley & Culvenor, 1959 B83
Lappula glochidiata Suri et al. 1978 B84
Lindelofia angustifolia (Schrenk) Brand. Rao et al. 1974; Suri et al. 1975a B62, 36
L. spectabilis Lehm. Rao et al. 1974; Suri et al. 1975a B62, 36
L. stylosa (Kar. et Kir.) Brand. Kiyamitdinova et al. 1967 B75
L. tschimganica Akramov et al. 1965 B87
Paracynoglossum imeritinum (Kusn.) M. Pop. Man'ko & Marchenko, 1971b B88
Prestonia sp. Edgar, 1985 A
Rindera austroechinata M. Pop. Akramov et al. 1965 B87
R. baldshuanica Kusnezov Akramov et al. 1965 B87
R. cyclodonta Akramov et al. 1967a B59
R. echinata Regel Men'shikov & Denisova, 1953 B92
R. oblongifolia M. Pop. Akramov et al. 1965 B87
Solenanthus circinnatus Ledeb. Akramov et al. 1964 B93
S. coronatus Kiyamitdinova et al. 1967 B75
S. karateginus Lipsky Akramov et al. 1964 B93
Symphytum asperum Lepech. Man'ko et al. 1970b B97
S. caucasicum Bieb. Man'ko et al. 1972 B98
S. officinale Linn. Man'ko et al. 1970a; Huizing & B101, C
Malingre, 1979
Echiumine Amsinckia hispida (Ruiz et Pav.) Culvenor & Smith, 1966a B30
I.M. Johnston
A. intermedia Fisch et C. Mey. Culvenor & Smith, 1966a B30
A. lycopsoides Lehm. Culvenor & Smith, 1966a B30
Echium plantagineum L. Culvenor, 1956 B48
Emiline Emilia flammea Cass. Kohlmunzer & Tomczyk, 1969; Tomczyk & B123, 124
Kohlmunzer, 1971; Kohlmunzer B125
et al. 1971
Alkaloid Plant Sources Reference Reference
Location
13,19-Epoxyseneciphylline Senecio megaphyllus Green. Bohlmann et al. 1986 A
S. usgorensis Cuatr. Bohlmann et al. 1986 A
13,19-Epoxyspartiodine Senecio megaphyllus Green. Bohlmann et al. 1986 A
Erucifoline Senecio aegypticus L. Klasek et al. 1968a B145
S. erraticus Berthol. subsp. Schroter & Santavy, 1960; Sedmera B179,
barbaraeifolius Krock. et al. 1972 180
S. erucifolius L. Kompis & Santavy, 1962; Sedmera B183,
et al. 1972 180
Europine Heliotropium arbainense Zalkow et al. 1979 B419
H. digynum Forssk. Hammouda et al. 1984 A
H. europaeum L. Culvenor, 1954 B66
H. lasiocarpum Fisch and Mey. Culvenor et al. 1986 A
H. maris-mortui Zalkow et al. 1978 B74
H. rotundifolium Zalkow et al. 1978 B74
H. suaveolens Bieb. Guner, 1986 A
Trichodesma africana Zalkow et al. 1979 B419
Floricaline Cacalia floridana Cava et al. 1968 B119
Floridanine Cacalia floridana Cava et al. 1968 B119
Doronicum macrophyllum Alieva et al. 1976 B122
Senecio aureus L. Roder et al. 1983 C
S. erraticus Berthol. Gaiduk et al. 1974 B177
S. othonnae Bieb. Khalilov & Telezhenetskaya, 1973b B222
Florosenine Cacalia floridana Cava et al. 1968 B119
Senecio aureus L. Roder et al. 1983 C
S. fluviatilis Wallr. Klasek et al. 1973b B185
S. quebradensis Greem. Bohlmann et al. 1986 A
Alkaloid Plant Sources Reference Reference
Location
Fulvine Crotalaria berteroana DC. (C. fulva Roxb.) Schoental, 1963 B297
C. crispata F. Muell. ex Benth. Culvenor & Smith, 1963 B294
C. madurensis R. Wight Atal et al. 1966a; Habib et al. 1971 B280, 315
C. paniculata Willd. Subramanium et al. 1968 B325
Globiferine Crotalaria globifera E. Mey. Brown et al. 1984 C
Grahamine Crotalaria grahamiana R. Wight et Atal et al. 1969 B299
Walk.-Arn.
Grantaline Crotalaria globifera E. Mey Brawn et al., 1984 C
C. virgulata subsp. grantiana (Harv.) Smith & Culvenor, 1984 C
Polhill (C. grantiana Harvey)
Grantianine Crotalaria globifera E. Mey. Brown et al. 1984 C
C. virgulata subsp. grantiana (Harv.) Adams et al. 1942b; Adams & B301
Polhill (C. grantiana Harvey) Gianturco, 1956b; Smith & B259, C
Culvenor, 1984
Gynuramine Gynura scandens O. Hoffm. Wiedenfeld, 1982 A
Heleurine Heliotropium europaeum L. Culvenor, 1954 B66
H. indicum L. Hoque et al. 1976 B72
H. lasiocarpum Fisch and Mey. Culvenor et al. 1986 A
Heliosupine Cynoglossum creticum Zalkow et al. 1979 B419
C. officinale Man'ko & Borisyuk, 1957; Man'ko, 1959; B38, 39
Sykulska, 1961 B40
C. pictum Man'ko & Marchenko, 1971b, 1972b B44, 45
C. viridiflorum Pallas ex Lehm. Man'ko, 1972 B34
Echium vulgare L. Man'ko, 1964 B49
Heliotropium supinum L. Denisova et al. 1953; Crowley & B82, 83
Culvenor, 1959
Alkaloid Plant Sources Reference Reference
Location
Myosotis sylvatica Hoffm. Culvenor & Smith, unpubl.
Paracynoglossum imeritinum (Kusn.) Man'ko & Marchenko, 1971 B88
Symphytum asperum Lepech. Man'ko et al. 1970b B97
S. officinale Linn. Man'ko et al. 1970a B101
Heliotrine Heliotropium acutiflorum Akramov et al. 1968 B51
H. arbainense Zalkow et al. 1979 B419
H. arguzioides Kar. et Kir. Zolotavina, 1963 B53
H. curassavicum Linn. Rajagopalan & Batra, 1977b B56
H. dasycarpum Ledeb. Akramov et al. 1961a B54
H. digynum Forssk. Hammouda et al. 1984 A
H. eichwaldi Steud. ex DC. Gandhi et al. 1966a B60
H. europaeum L. Trautner & Neufeld, 1949; Culvenor B64, 65
et al. 1954
H. indicum L. Hoque et al. 1976 B72
H. lasiocarpum Fisch et C. Mey. Men'shikov, 1932 B73
H. olgae Kiyamitdinova et al. 1967; Sheveleva B75, 76
et al. 1969
H. popovii H. Riedl. subsp. gillianum Mohabbet et al. 1976 B77
H. ramosissimum Habib, 1975; Schoental & B79, 78
Cavanagh, 1972
H. suaveolens Bieb. Guner, 1986 A
H. supinum L. Pandey et al. 1983 C
H. transoxanum Akramov et al. 1968 B51
Heliovinine Heliotropium curassavicum Linn. Mohanraj et al. 1982c C
Heterophylline Parsonsia heterophylla A. Cunn. Edgar et al. 1980 B418
P. spiralis Edgar et al. 1980 B418
18-Hydroxysenkirkine Crotalaria laburnifolia L. subsp. Crout, 1972 B312
19-Hydroxysenkirkine Senecio laricifolius H.B.K. Bohlmann et al. 1986 A
Alkaloid Plant Sources Reference Reference
Location
Incanine Heliotropium olgae Sheveleva et al. 1969 B76
Trichodesma incanum Alph. DC. Yunusov & Plekhanova, 1953, 1957, B110, 111
1959; Tashkhodzhaev et al. 1979a B112, C
Indicine Heliotropium amplexicaule Vahl. Ketterer et al. 1987, (in press)
H. indicum L. Mattocks et al. 1961 B70
Prestonia sp. Edgar, 1985 A
Integerrimine Cacalia hastata L. subsp. orientalis Hayashi et al. 1972 B120
Kitamura
Crotalaria brevidens Benth. var. Suri et al. 1975b B304
intermedia (Kotschy) Polhill
C. brevifolia Sawhney & Atal, 1966 B290
C. incana L. Adams & Van Duuren, 1953b B187
C. pallida Ait. Sawhney et al. 1967 B291
C. tetragona Roxb. Puri et al. 1974 B314
C. zanzibarica Benth. (C. usaramoensis) Culvenor & Smith, 1966b B337
Petasites hybridus L. Luthy et al. 1983 C
Senecio alpinus L. Luthy et al. 1981 A
S. antieuphorbium (L.) Sch. Bip. Rodriguez & Gonzalez, 1969 B152
S. brasiliensis DC. Motidome & Ferreira, 1966a B163
S. brasiliensis Less. var tripartitus Nardi et al. 1980 A
S. durieui Gay Panizo & Rodriguez, 1974 B157
S. erraticus Berthol. subsp. Santavy et al. 1962 B182
barbaraeifolius Krock.
S. faberi Hemsl. Wei et al. 1982 C
S. formosus Munoz Quevedos, 1976 B186
S. glandulosus Don ex Hook. et Arn. Pestchanker et al. 1985b A
S. inaequidens DC. Bicchi et al. 1985 A
Alkaloid Plant Sources Reference Reference
Location
S. incanus L. subsp. carniolicus Klasek et al. 1968b B147
(Willd.) Br.-Bl.
S. integerrimus Manske, 1939a; Roitman et al. 1979 B156, 420
S. kleinia Sch. Bip. Gonzalez & Calero, 1958b B205
S. leucostachys Baker Pestchanker & Giordano, 1986 A
S. magnificus F. Muell. Gellert & Mate, 1964 B216
S. morrisonensis Hayata Lu, Sheng-Teh, 1972 B217
S. nebrodensis L. var sicula Plescia et al. 1976 B218
S. ragonesei Cabr. Pestcharnker & Giordano, 1986 A
S. spathulatus A. Rich. White, 1969 B158
S. squalidus L. Kropman & Warren, 1950; Gonzalez & B265,
Calero, 1958a 205
S. tenuifolius Burm. Bhakuni & Gupta, 1982 C
S. triangularis Hook. Roitman, 1983b C
S. vernalis Walst. et Kit. Sener et al. 1986; Hartmann & A, A
Zimmer, 1986
S. viscosus L. Barger & Blackie, 1936; Santavy et al. B264,
1962 182
S. vulgaris L. Pieters & Vlietinck, 1986 A
Intermedine Amsinckia hispida (Ruiz et Pav.) Culvenor & Smith, 1966a B30
I.M. Johnston
A. intermedia Fisch et C. Mey. Culvenor & Smith, 1966a B30
A. lycopsoides Lehm. Culvenor & Smith, 1966a B30
A. menziesii (Lehm.) Nels. & Macbr. Roitman, 1983 C
Borago officinale L. Luthy et al. 1984 A
Conoclinium coelestinium (L.) DC Herz et al. 1981 C
Eupatorium compositifolium Walt. Herz et al. 1981 C
Symphytum aspera Roitman, 1981 A
S. officinale Linn. Roder et al. 1982 C
S. x uplandicum Nyman Culvenor et al. 1980a, 1980b A, A
Trichodesma africana Zalkow et al. 1979 B419
Alkaloid Plant Sources Reference Reference
Location
Isocromadurine Crotalaria candicans W. and A. Suri et al. 1982 C
C. madurensis R. Wight ]Rao et al. 1975c B317
Isoline Senecio othonniformis Fourcade Coucourakis & Gordon-Gray, 1970; B224
Coucourakis et al. 1972 B225
Jacobine Crassocephalum crepidioides Asada et al. 1985 A
Senecio alpinus L. Luthy et al. 1981 A
S. brasiliensis DC. Adams & Gianturco, 1956e B148
S. cineraria DC. Barger & Blackie, 1937 B167
S. jacobaea L. Manske, 1931; Blackie, 1937; B194, 153
Bradbury & Culvenor, 1954 B198
S. paludosus L. Blackie, 1937; Dorosh & Alekseev, 1960 B153, 227
Alekseev, 1961b B228
Jacoline Crassocephalum crepidioides Asada et al. 1985 A
Senecio alpinus L. Luthy et al. 1981 A
S. jacobaea L. Bradbury & Culvenor, 1954 B198
Jaconine Senecio alpinus L. Luthy et al. 1981 A
S. jacobaea L. Bradbury & Culvenor, 1954 B198
Jacozine Senecio alpinus L. Luthy et al. 1981 A
S. cannabifolius Less Asada et al. 1982 C
S.jacobaea L. Bradbury & Culvenor, 1954; Culvenor, B198,
1964 202
Junceine Crotalaria juncea L. Adams & Gianturco, 1956a, 1956b, 1956c B305,
B306
B307
C. wightiana Grah. ex Wight & Arn. Atal et al. 1966b B329
Alkaloid Plant Sources Reference Reference
Location
Lasiocarpine Heliotropium arbainense Zalkow et al. 1979 B419
H. arborescens L. Carcamo-Marquez, 1961 B52
H. curassavicum Linn. Rajagopalan & Batra, 1977b B56
H. digynum Forssk. Hammouda et al. 1984 A
H. eichwaldii Steud. ex DC. Rao et al. 1974 B62
H. europaeum L. Culvenor et al. 1954 B65
H. indicum Linn. Hoque et al. 1976 B72
H. lasiocarpum Fisch. et C. Mey. Men'shikov, 1932 B73
H. maris mortui Zalkow et al. 1979 B419
H. suaveolens Bieb. Guner, 1986 A
H. supinum L. Pandey et al. 1983 C
Lappula intermedia Man'ko & Vasil'kov, 1968 B85
Symphytum caucasicum Man'ko et al. 1969 B95
S. officinale Linn. Man'ko et al. 1969, 1970a B95, 101
Latifoline Cynoglossum latifolium R. Br. Crowley & Culvenor, 1962 B37
Hackelia floribunda Hagglund et al. 1985 A
Ligudentine Ligularia brachyphylla Hand.-Mazz. Klasek et al. 1971 B134
L. dentata (A. Gray) Hara. Klasek et al. 1971 B134
Ligularidine Ligularia dentata (A. Gray) Hara Hikichi et al. 1979, Asada & B436, C
Furuya, 1984a
Ligularine Ligularia brachyphylla Hand.-Mazz. Klasek et al. 1971 B134
L. dentata (A. Gray) Hara. Klasek et al. 1971 B134
L. elegans Cass. Klasek et al. 1971 B134
Ligularizine Ligularia dentata (A. Gray) Hara. Asada & Furuya, 1984a C
Alkaloid Plant Sources Reference Reference
Location
Lycopsamine Amsinckia hispida (Ruiz et Pav.) Culvenor & Smith, 1966a B30
I.M. Johnston
A. intermedia Fisch. et C. Mey. Culvenor & Smith, 1966a B30
A. lycopsoides Lehm. Culvenor & Smith, 1966a B30
A. menziesii (Lehm.) Nels. & Macbr. Roitman, 1983a C
Anchusa officinalis L. Broch-Due & Aasen, 1980 B32
Borago officinale L. Larson et al. 1984; Luthy et al. 1984 C, A
Eupatorium compositifolium Walt. Herz et al. 1981 C
Heliotropium steudneri Vatke Schneider et al. 1975 B8O
Messerschmidia sibirica Hikichi et al. 1980 C
Parsonsia eucalyptophylla F. Muell. Edgar & Culvenor, 1975 B28
P. straminea (R. Br.) F. Muell. Edgar & Culvenor, 1975 B28
Prestonia sp. Edgar, 1985 A
Symphytum asperum Lepech. Roitman, 1981 A
Symphytum officinale Linn. Huizing & Malingre, 1981 C
Symphytum x uplandicum Nyman Culvenor et al. 1980a, 1980b A, A
Madurensine Crotalaria agatiflora Schweinf. Atal et al. 1966a B280
C. laburnifolia L. subsp. eldomae Crout, 1972 B312
C. madurensis R. Wight Atal et al. 1966a; Mahran et al. 1979 B280, 426
Merenskine Senecio latifolius DC. Bredenkamp et al. 1985 C
Monocrotaline Crotalaria aegyptiaca Benth. Mahran et al. 1979; Zalkow et al. 1979 B426, 419
C. assamica Benth. Crotalaria Research Group, 1974 B286
C. burhia Rao et al. 1975 B293
C. cephalotes Steud. ex A. Rich Pilbeam et al. 1983 C
C. crispata F. Muell. ex Benth. Culvenor & Smith, 1963 B294
C. cunninghamii R. Br. Pilbeam et al. 1983 C
C. grahamiana R. Wight ex Walk.-Arn. Gandhi et al. 1966b; Atal et al. 1969 B298, 299
C. leschenaultii DC. Suri & Atal, 1967 B313
Alkaloid Plant Sources Reference Reference
Location
C. leiloba Bartl. Puri et al. 1974 B314
C. mitchellii Benth. Culvenor et al. 1967b B318
C. mysorensis Roth. Sawhney & Atal, 1968 B319
C. nitens Kunth. Hoet et al. 1981 A
C. novae-hollandiae DC. subsp. Culvenor et al. 1967b B318
lasiophylla (Benth.) A. Lee
C. paulina schrank. Pilbeam et al. 1983 C
C. quinquefolia L. Pilbeam et al. 1983 C
C. recta Steud. ex A. Rich Crout, 1968a B326
C. retusa L. Adams & Rogers, 1939; Culvenor & B327,
Smith, 1957a 328
C. sagittalis L. Willette & Cammarata, 1972 B330
C. spectabilis Roth. Neal et al. 1935; Adams & Rogers, B325,
1939 327
C. stipularia Desv. Puri et al. 1974 B314
Lindelofia spectabilis Lehm. Rao et al. 1974 B62
Monocrotalinine Crotalaria grahamiana Wight et Arn. Rajagopalan & Batra, 1977a B300
Myoscorpine Myosotis scorpioides L. Resch et al. 1982 C
Symphytum officinale Linn. Resch et al. 1982C C
Neoligularidine Ligularia dentata (A. Gray) Hara. Asada & Furuya, 1984a C
Neopetasitenine Ligularia japonica Asada et al. 1981 A
Petasites japonicus Maxim. Yamada et al. 1976a B19
Neosenkirkine Senecio auricola Bourg. Panizo & Rodriguez, 1974 B157
S. grandifolius Less. Bohlmann et al. 1986 A
S. pierotii Asada & Furuya, 1982 C
Neotriangularine Senecio triangularis Hook. Roitman, 1983b C
Alkaloid Plant Sources Reference Reference
Location
Nilgirine Crotalaria pallida Ait. Atal et al. 1968 B322
Onetine Senecio othonnae Bieb. Danilova et al. 1962 B221
Otosenine Cacalia floridana Cava et al. 1968 B119
Doronicum macrophyllum Alieva et al. 1976 B122
D. pardalianches Linn. Rajagopalan & Negi, 1985 A
Emilia flammea Cass. Kohlmunzer & Tomczyk, 1969 B123
Senecio aegypticus L. Klasek et al. 1968a B145
S. aureus L. Resch et al. 1983, Roder et al. 1983 C, C
S. cineraria DC. Habib, 1974 B170
S. desfontainei Druce Haddad et al. 1963; Klasek B173,
et al. 1968a 145
S. erraticus Berthol. Gaiduk et al. 1974 B177
S. erraticus subsp. barbaraeifolius Krock. Santavy, 1958; Schroter & Santavy, 1960 B178, 179
S. fluviatilis Wallr. Klasek et al. 1973b B185
S. jacobaea L. Akramov et al. 1968 B51
S. othonnae Bieb. Zhdanovich & Men'shikov, 1941; B220
Danilova et al. 1962 B221
S. renardii Winkl. Danilova & Konovalova, 1950 B249
S. tomentosus Adams et al. 1956; Schroter & B271,
Santavy, 1960 179
Parsonsine Parsonsia heterophylla A. Cunn. Eggers & Gainsford, 1979; Edgar et al. B417,
1980 418
P. spiralis Edgar et al. 1980 B418
Petasinine Petasites japonicus Maxim. Yamada et al. 1978b B141
Petasitenine Farfugium japonicum Kitam Niwa et al. 1985 A
Petasites japonicus Maxim. Yamada et al. 1976a, 1976b; B19, 139
Furuya et al. 1976 B20
Alkaloid Plant Sources Reference Reference
Location
Retroisosenine Senecio nemorensis L. var. bulgaricus Nghia et al. 1976 B219
(Vel.) Stoj. et Stef.
S. nemorensis L. var subdecurrens Klasek et al. 1980a B422
Retrorsine Crotalaria spartioides DC. Bruemmerhoff & de Waal, 1961 B334
C. zanzibarica Benth. (C. usaramoensis) Culvenor & Smith, 1966b B337
Senecio ambrosioides Adams & Gianturco, 1956e B148
S. ampullaceus Hook. Adams & Govindachari, 1949b; Adams & B149, 151
Looker, 1951; Warren et al. 1950 B150
S. bipinnatisectus Belcher White, 1969 B158
S. brasiliensis DC. Motidome & Ferreira, 1966a B163
S. bupleuroides DC. Sapiro, 1949 B164
S. cineraria DC. Klasek et al. 1975 B171
S. cruentus DC. Asada et al. 1982 C
S. cymbaroides Roitman et al. 1979 B420
S. desfontainei Druce Rizk et al. 1983 A
S. discolor DC. Schoental, 1960; Hennig, 1961 B174, 175
S. douglasii DC. Adams & Govindachari, 1949b; Adams B149,
& Looker, 1951 151
S. eremophilus Richards Adams & Govindachari, 1949b; Adams B149,
& Looker, 1951 151
S. erucifolius L. Ferry & Brazier, 1976 B184
S. filaginoides (H. et A.) DC. Pestchanker & Giordano, 1986 A
S. formosus Munoz Quevedo, 1976 B186
S. gilliesiano Guidugli et al. 1986 A
S. glaberrimus DC. Blackie, 1937 B153
S. glandulosus Don ex Hook. et Arn. Pestchanker et al. 1985b A
S. graminifolius N.J. Jacq. de Waal, 1941 B188
S. griesbachii Motidome & Ferreira, 1966b B190
S. ilicifolius Thunb. de Waal, 1940a, 1940b, 1941; Culvenor B191, 192
& Smith, 1954 B188, 127
Alkaloid Plant Sources Reference Reference
Location
S. inaequidens DC. Roder et al. 1981 A
S. isatideus DC. Blackie, 1937; de Waal, 1939 B153, 193
S. jacobaea L. Ferry & Brazier, 1976 B184
S. latifolius DC. Watt, 1909; Barger et al. 1935 B211, 212
S. longilobus Benth. Adams & Govindachari, 1949b; Adams & B149, 151
Looker, 1951; Warren et al. 1950 B150
S. paucicalyculatus Klatt Pretorius, 1949 B230
S. phillipicus Roegel et Koern Gonzalez et al. 1986a A
S. pterophorus DC. de Waal, 1940b, 1941; Culvenor & B192, 188
Smith, 1954 127
S. quadridentatus Labill. Culvenor & Smith, 1955 B247
S. ragonesei Cabr. Pestchanker & Giordano, 1986 A
S. retrorsus DC. Manske, 1931; de Waal, 1939 B194, 193
S. riddellii Torr. et A. Gray Roitman et al. 1979 B420
S. riddellii Torr. et A. Gray var. Adams & Govindachari, 1949b B149
parksii Cory.
S. ruderalis Harvey Leisegang, 1950 B254
S. seratophiloides Griseb. Pestchanker & Giordano, 1986 A
S. spartioides Roitman et al. 1979 B420
S. subulatus Don ex Hook. et Arn Pestchanker et al. 1985b A
var erectus
S. swaziensis Compton Gordon-Gray et al. 1972; Gordon-Gray B268,
& Wells, 1974 270
S. triangularis Hook. Roitman, 1983b C
S. uspallatensis Pestchanker et al. 1985a A
S. venosus Harvey Blackie, 1937 B153
S. vernalis Waldst. et Kit. Roder et al. 1979b C
S. viminalis Bremek. de Waal & van Twisk, 1964 A
S. vulgaris L. Tschu Shun et al. 1960 B277
S. werneriaefolius Roitman et al. 1979 B420
Alkaloid Plant Sources Reference Reference
Location
Retusamine Crotalaria mitchellii Benth. Culvenor et al. 1967b B318
C. mitchellii Benth. subsp. laevis Culvenor et al. 1967b B318
A. Lee
C. novae-hollandiae DC. subsp. Culvenor et al. 1967b B318
lasiophylla Benth. A. Lee
C. novae-hollandiae DC. subsp. Culvenor et al. 1967b B318
novae-hollandiae
C. retusa L. Culvenor & Smith, 1957a; Wunderlich, B328, C
1962
Riddelliine Crotalaria juncea L. Adams & Gianturco, 1956a B305
Senecio aegypticus L. Klasek et al. 1968a B145
S. ambrosioides Roitman et al. 1979 B420
S. cruentus DC. Asada et al. 1982 C
S. cymbaroides Roitman et al. 1979 B420
S. desfontainei Druce Haddad et al. 1963; Klasek et al. B173,
1968a 145
S. douglassi DC. Adams & Govindachari, 1949b; Adams & B149,
Looker, 1951 151
S. eremophilus Richards Adams & Govindachari, 1949b; Adams & B149,
Looker, 1951 151
S. longilobus Benth. Adams & Govindachari, 1949b; Adams & B149,
Looker, 1951; 151
Warren et al. 1950 B150
S. riddellii Torr. et A. Gray Manske, 1939a; Adams et al. 1942c B156, 252
S. riddellii Torr. et A. Gray var. Adams & Govindachari, 1949b B149
parksii (Cory)
S. spartioides Roitman et al. 1979 B420
S. vernalis Walst. et Kit. Sener et al. 1986 A
S. vulgaris Roitman et al. 1979 B420
Alkaloid Plant Sources Reference Reference
Location
Rinderine Eupatorium altissimum L. Herz et al. 1981 C
E. serotinum Michx. Locock et al. 1966 B131
Prestonia sp. Edgar, 1985 A
Rindera baldshuanica Kusnezov Akramov et al. 1961c B91
Solenanthus turkestanicus Regel Akramov et al. 1962 B94
et Smirnov
(Kusnezov)
Sceleratine Senecio latifolius DC. (S. sceleratus) de Waal & Pretorius, 1941; de Waal B257,
et al. 1963 260
Scorpioidine Myosotis scorpioides L. Resch et al. 1982 C
Sencalenine Senecio cacaliaster (Lam.) Roder et al. 1984b A
Senecicannabine Senecio cannabifolius Less. Asada et al. 1982 C
Senecionine Brachyglottis repanda Forst. et Forst. Mortimer & White, 1967 B117
Caltha biflora Stermitz & Adamovics, 1977 B341
C. leptosepala Stermitz & Adamovics, 1977 B341
Castilleja rhexifolia Rydb. Stermitz & Suess, 1978 B342
Castilleja "rhexifolia aff. miniata" Roby & Stermitz, 1984 C
Crotalaria juncea L. Adams & Gianturco, 1956a B305
C. micans Link. Sethi & Atal, 1964 B284
C. zanzibarica Benth. (C. usaramoensis) Culvenor & Smith, 1966b B337
Emilia sonchifolia DC. Culvenor, unpubl.
Erechtites hieracifolia (L.) Raf. ex DC. Manske, 1939b; Culvenor & Smith, 1954 B126, 127
Gynura segetum (Lour.) Merr. Hua et al. 1983; Liang & Roder, 1984 C, C
Ligularia japonica Asada et al. 1981 A
Petasites hybridus L. Luthy et al. 1983 C
P. laevigatus (Willd.) Reichenb. Massagetov & Kuzovkov, 1953 B142
Senecio aegypticus L. Klasek et al. 1968a; Gharbo & B145,
Habib, 1969 146
Alkaloid Plant Sources Reference Reference
Location
S. alpinus (L.) Scop. Luthy et al. 1981 A
S. ambrosioides Adams & Gianturco, 1956e B148
S. ampullaceus Hook. Adams & Govindachari, 1949b; Adams & B149,
Looker, 1951; 151
Warren et al. 1950 B150
S. argentino Baker (vira-vira Hieron) Pestchanker & Giordano, 1986 A
S. aureus L. Manske, 1936, 1939a B155, 156
S. brasiliensis DC. Fonseca, 1951; Novelli & De Varella, B162,
1945; Adams & Gianturco, 1956e 161
B148
S. carthamoides Greene Adams & Govindachari, 1949b; Adams B149,
& Looker, 1951 151
S. cineraria DC. Barger & Blackie, 1937; Adams & B167,
Govindachari, 1949a; 168
Alekseev et al. 1962a B169
S. congestus (R.Br.) DC. Roder et al. 1982 C
S. cruentus DC. Asada et al. 1982 C
S. cymbaroides Roitman et al. 1979 B420
S. desfontainei Druce Klasek et al. 1968a B145
S. discolor DC. Schoental, 1960; Hennig, 1961 B174, 175
S. douglasii DC. Adams & Govindachari, 1949b; Adams B149,
& Looker, 1951 151
S. eremophilus Richards Adams & Govindachari, 1949b; Adams B149,
& Looker, 1951 151
S. erraticus Berthol. Gaiduk et al. 1974 B177
S. erraticus Berthol. subsp. Santavy, 1958; Schroter & Santavy, B178,
barbaraeifolius Krock. 1960; Kompis et al. 1960 179
B181
S. erucifolius L. Kompis & Santavy, 1962 B183
S. filaginoides (H. et A.) DC. Pestchanker & Giordano, 1986 A
Alkaloid Plant Sources Reference Reference
Location
S. fistulosus Poepp. ex Less. Gonzalez et al. 1986b A
S. fremontii Torr. et A. Gray Adams & Gianturco, 1956e B148
S. gilliesiano Guidugli et al. 1986 A
S. glabellus Turcz. DC. Adams & Van Duuren, 1953b B187
S. ilicifolius Thunb. de Waal, 1940a, 1940b, 1941; Culvenor B191,
& Smith, 1954 192
B188,
127
S. illinitus Phill. Gonzalez et al. 1986a A
S. inaequidens DC. Roder et al. 1981 A
S. integerrimus Nutt. Manske, 1939a B156
S. jacobaea L. Bradbury & Mosbauer, 1956 B199
S. laricifolius H.B.K. Bohlmann et al. 1986 A
S. lautus Forst. f. ex Willd. Culvenor unpubl.
S. leucostachys Baker Pestchanker & Giordano, 1986 A
S. longiflorus Sch. Bip. de Waal & van Twisk, 1964 A
S. magnificus F. Muell. Culvenor, 1962 B215
S. multilobatus MaCoy et al. 1983 A
S. multivenius Benth. in Oerst Bohlmann et al. 1986 A
S. nebrodensis L. var. sicula Plescia et al. 1976 B218
S. nemorensis L. subsp. fuchsii Gmel. Wiedenfeld & Roder, 1979 B423
S. pampeanus Cabrera Novelli, 1958 B229
S. pancicii Degen var arnautorum Jizba et al. 1982 C
(Velen.) Stoj. Stef. et Kit.
S. pancicii Degen var pancicii Jizba et al. 1982 C
S. patagonicus Hook. and Arn. Villarroel et al. 1985 A
S. petasitis DC. Gharbo & Habib, 1969 B146
S. pimpinellifolius H.B.K. Bohlmann et al. 1986 A
S. pseudo-arnica Less. Manske, 1939a B156
S. pterophorus DC. de Waal, 1940b, 1941; Culvenor & B192,
Smith, 1954 188
B127
Alkaloid Plant Sources Reference Reference
Location
S. quadridentatus Labill. Culvenor & Smith, 1955 B247
S. sandrasicus Temizer et al. 1985 A
S. scandens Batra & Rajagopalan, 1977 B256
S. seratophiloides Griseb. Pestchanker & Giordano, 1986 A
S. spartioides Manske, 1939a; Adams & Gianturco, B156,
1957b 262
S. spathulatus A. Rich White, 1969 B158
S. squalidus L. Barger & Blackie, 1936; Kropman B264,
& Warren, 1950 265
S. subalpinus C. Koch Trivedi & Santavy, 1963 B267
S. subulatus Don ex Hook. et Arn Pestchanker et al. 1985b A
var erectus
S. tenuifolius Burm. Bhakuni & Gupta, 1982 C
S. tomentosus Adams et al. 1956 B271
S. triangularis Hook. Kupchan & Suffness, 1967 B272
S. uintahensis Roitman et al. 1979 B420
S. vernalis Waldst. et Kit. Roder et al. 1979b C
S. viminalis Bremek. de Waal & van Twisk, 1964 A
S. viscosus L. Barger & Blackie, 1936; Santavy et al. B264,
1962 182
S. vulgaris L. Grandval & Lajoux, 1895; Barger & B275,
Blackie, 1936; Konovalova & 264
Orekhov, 1937a; Tschu Shun B276
et al. 1960 B277
S. wernariaefolius Roitman et al. 1979 B420
Syneilesis palmata Maxim. Hikichi & Furuya, 1976 B279
Tussilago farfara Rosberger et al. 1981 C
7-Senecioyl-9-(2-hydroxy- Senecio caudatus DC. Bohlmann et al. 1986 A
3-acetylbutyrl)retronecine
Alkaloid Plant Sources Reference Reference
Location
7-Senecioyl-9-(2-hydroxy- Senecio caudatus DC. Bohlmann et al. 1986 A
methyl-2,3-dihydroxy-
butyrylretronecine
7-Senecioyl-9-(2-methyl- Senecio caudatus DC. Bohlmann et al. 1986 A
2,3-dihydroxybutyryl-
retronecine
7-Senecioylretronecine Senecio cacaliaster (Lam.) Roder et al. 1984b C
S. caudatus DC. Bohlmann et al. 1986 A
S. triangularis Hook. Rueger & Benn, 1983a C
S. variabilis Sch. Bip. Bohlmann et al. 1986 A
9-Senecioylretronecine Senecio caudatus DC. Bohlmann et al. 1986 A
S. variabilis Sch. Bip. Bohlmann et al. 1986 A
7-Senecioyl-9-sarracinyl- Senecio cacaliaster Roder et al. 1984b C
retronecine S. caudatus DC. Bohlmann et al. 1986 A
S. triangularis Hook. Rueger & Benn, 1983 C
S. ungeniensis Thell. Bohlmann et al. 1986 A
S. variabilis Sch. Bip. Bohlmann et al. 1986 A
Seneciphylline Adenostyles alliarae Yakhontova et al. 1976 B115
A. glabra Wiedenfeld et al. 1984 C
A. rhombifolius (Willd.) M. Pimen.subsp. Pimenov et al. 1975 B116
platyphylloides
Crotalaria juncea L. Adams & Gianturco, 1956a B305
Erechtites hieracifolia (L.) Raf. ex DC. Manske, 1939a; Culvenor & Smith, 1954 B126, 127
Senecio alpinus (L.) Scop. Klasek et al. 1968b; Luthy et al. 1981 B147, A
S. ambrosioides Adams & Gianturco, 1956e B148
Alkaloid Plant Sources Reference Reference
Location
S. ampullaceus Hook. Adams & Govindachari, 1949b; Adams & B149,
Looker, 1951; Warren et al. 1950 151
B150
S. aquaticus Hill Blackie, 1937; Evans & Evans, 1949 B153, 154
S. borysthenicus Red'ko, 1956; Alekseev, 1961a B159, 160
S. brasiliensis DC. Fonseca, 1951; Novelli & de Varella, B162,
161
1945; Adams & Gianturco, 1956e B148
S. cannabifolius Less. Alekseev, 1964; Asada et al. 1982 B165, C
S. carthamoides Greene Adams & Govindachari, 1949b; Adams & B149,
Looker, 1951 151
S. chrysanthemoides Wali & Handa, 1964 B166
S. cineraria DC. Barger & Blackie, 1937; Adams & B167,
Govindachari, 1949a 168
S. cruentus DC. Asada et al. 1982 C
S. cymbaroides Roitman et al. 1979 B420
S. desfontainei Druce Gharbo & Habib, 1969 B146
S. douglasii DC. Adams & Govindachari, 1949b; Adams & B149,
Looker, 1951 151
S. eremophilus Richards Adams & Govindachari, 1949b; Adams & B149,
Looker, 1951 151
S. erraticus Berthol. subsp. Kompis et al. 1960; Santavy B181,
barbaraeifolius Krock. et al. 1962 182
S. erucifolius L. Kompis & Santavy, 1962 B183
S. fluviatilis Wallr. Klasek et al. 1973b B185
S. fremontii Torr. et A. Gray Adams & Gianturco, 1956e B148
S. grandifolia Glonti, 1958 B189
S. ilicifolius Thunb. de Waal, 1940a, 1940b, 1941; B191, 192
Culvenor & Smith, 1954 B188,
127
Alkaloid Plant Sources Reference Reference
Location
S. incanus L. subsp. carniolus Klasek et al. 1968b B147
(Willd.) Br.-Bl.
S. jacobaea L. Blackie, 1937; Bradbury & B153,
Culvenor, 1954 198
S. krylovii Sapunova & Ban'kovskii, 1968 B207
S. kubensis Grossh. Khalilov & Telezhenetskaya, 1973a B208
S. lampsanoides Khalilov & Damirov, 1974 B210
S. laricifolius H.B.K. Bohlmann et al. 1986 A
S. latifolius DC. Danilova et al. 1960 B213
S. longiflorus Sch. Bip. de Waal & van Twisk, 1964 A
S. longilobus Benth. Adams & Govindachari, 1949b; Adams & B149,
Looker, 1951 151
S. minimus Poir. White, 1969 B158
S. multivenius Benth. in Oerst Bohlmann et al. 1986 A
S. othonnae Bieb. Zhdanovich & Men'shikov, 1941; B220
Danilova et al. 1962 B221
S. palmatus Pall. Alekseev, 1960 B226
S. paludosus L. Blackie, 1937; Dorosh & Alekseev, B153,
1960; 227
Alekseev, 1961b B228
S. pancicii Degen var arnautorum Jizba et al. 1982 C
(Velen.) Stoj. Stef. et Kit.
S. pancicii Degen var panicii Jizba et al. 1982 C
S. patagonicus Hook. and Arn. Villarroel et al. 1985 A
S. paucifolius S.G. Gmel. Alekseev & Ban'kovskii, 1965 B231
S. phillipicusRoegel et Koern Gonzalez et al. 1986a A
S. platyphylloides Somm. et Lev. Murav'eva, 1964b, 1965; Dauksha, 1970 B233, 234
B235
S. platyphyllus (Bieb.) DC. Orekhov, 1935; Konovalova & B236,
Orekhov, 1938; 237
Konovalova, 1951 B238
Alkaloid Plant Sources Reference Reference
Location
S. pojarkovae Chernova & Murav'eva, 1974 B243
S. propinquus Ait. Khalilov et al. 1972 B245
S. pterophorus DC. de Waal, 1940b, 1941; Culvenor & B192,
Smith, 1954 188
B127
S. quadridentatus Labill. Culvenor & Smith, 1955 B247
S. racemosus Khmel, 1961 B248
S. renardii Winkl. Danilova & Konovalova, 1950 B249
S. rhombifolius (Willd.) Sch. Bip. Khalilov & Telezhenetskaya, 1973a B208
S. scandens Batra & Rajagopalan, 1977 B256
S. spartioides Torr. et A. Gray Manske, 1939a; Adams & Gianturco, B156,
1957b 262
S. spathulatus Benn et al. 1979 B263
S. stenocephalus Maxim. Konovalova & Orekhov, 1937b B266
S. subalpinus C. Koch. Trivedi & Santavy, 1963; Klasek B267,
et al. 1968b 147
S. vernalis Walst. et Kit. Sener et al. 1986; Hartmann & A, A
Zimmer, 1986
S. vulgaris L. Barger & Blackie, 1936; Konovalova & B264,
Orenkhov, 1937a; 276
Tschu Shun et al. 1960 B277
Senecivernine Senecio inaequidens Bicchi et al. 1985 A
S. seratophylloides Griseb. Pestchanker & Giordano, 1986 A
S. vernalis Waldst. et Kit. Roder et al. 1979b C
Senkirkine Brachyglottis repanda Forst. et Forst. Mortimer & White, 1967 B117
Crotalaria laburnifolia subsp. eldomae Crout, 1972 B312
Farfugium japonicum Kitam. Furuya et al. 1971 B133
Petasites albus L. Luthy et al. 1983 C
P. hybridus L. Luthy et al. 1983 C
P. japonicus Maxim. Yamada et al. 1978a B140
Alkaloid Plant Sources Reference Reference
Location
P. laevigatus (Willd.) Reichenb. Massagetov & Kuzovkov, 1953 B142
Senecio antieuphorbium (L.) Sch. Bip. Rodriguez & Gonzalez, 1969 B152
S. desfontainei Druce Rizk et al. 1983 A
S. grandifolius Less. Bohlmann et al. 1986 A
S. illinitus Phill. Gonzalez et al. 1986a A
S. jacobaea L. Akramov et al. 1968 B51
S. kirkii Hook. f. ex Kirk. Briggs et al. 1948; Briggs et al. 1965 B203, 204
S. kleinia Sch. Bip. Rodriguez et al. 1967 B206
S. laricifolius H.B.K. Bohlmann et al. 1986 A
S. pierotii Asada & Furuya, 1982 C
S. procerus L. var. procerus Stoj. Jovceva et al. 1978 B244
Stef. et Kit.
S. quebradensis Greenm. Bohlmann et al. 1986 A
S. renardii Winkl. Danilova & Konovalova, 1950; Briggs B249,
et al. 1965 204
S. tenuifolius Burm. Bhakuni & Gupta, 1982 C
S. vernalis Waldst. et Kit. Roder et al. 1979b C
S. uintahensis Roitman et al. 1979 B420
Tussilago farfara Culvenor et al. 1976b; Borka & A, B425,
Onshuus, 1979; Luthy et al, 1980 A
Sincamidine Amsinckia intermedia Fisch. et C. Mey. Culvenor & Smith, 1966a B30
Spartioidine Senecio spartioides Torr. et A. Gray Manske, 1939a; Adams & Gianturco, 1957 B156,
262
S. vulgaris L. Pieters & Vlietinck, 1986 A
Spectabiline Crotalaria spectabilis Roth Culvenor & Smith, 1957b B336
Spiracine Parsonsia spiralis Wall. Edgar et al. 1980 B418
Spiraline Parsonsia spiralis Wall. Edgar et al. 1980 B418
Alkaloid Plant Sources Reference Reference
Location
Spiranine Parsonsia spiralis Wall. Edgar et al. 1980 B418
Supinine Borago officinale L. Luthy et al. 1984 A
Eupatorium cannabinum L. Pederson, 1975a B128
E. serotinum Michx. Locock et al. 1966 B131
E. stoechadosmum Hanse Furuya & Hikichi, 1973 B132
Heliotropium europeum L. Culvenor, 1954 B66
H. indicum L. Hoque et al. 1976 B72
H. supinum L. Men'shikov & Gurevich, 1949; Crowley & B81, 83
Culvenor, 1959
Tournefortia sarmentosa Lam. Crowley & Culvenor, 1955 B107
Trichodesma zeylanicum (Burm. f.) R. Br. O'Kelly & Sargeant, 1961 B113
Swazine Senecio barbellatus DC. Gordon-Gray & Wells, 1974 B270
S. swaziensis Compton Gordon-Gray et al. 1972; Laing & B268,
Sommerville, 1972 269
Symlandine Symphytum asperum Lepech. Roitman, 1981 A
S. officinale Linn. Roder et al. 1982 C
S. tuberosum L. Gray et al. 1983 A
S. x uplandicum Nyman Culvenor et al. 1980a, 1980b A, A
Symphytine Myosotis scorpioides L. Resch et al. 1982 C
Symphytum aspera Roitman, 1981 A
S. officinale Linn. Furuya & Araki, 1968; Furuya & B15, 16
Hikichi, 1971
S. peregrinum Ledeb. Gadella et al. 1983 A
S. x uplandicum Nyman Culvenor et al. 1980a, 1980b A, A
Syneilesine Syneilesis palmata Maxim. Hikichi & Furuya, 1974, 1976 B278, 279
Alkaloid Plant Sources Reference Reference
Location
Triangularine Alkanna tinctoria Tausch Roder et al. 1984b C
Senecio triangularis Hook. Roitman, 1983b C
Trichodesmine Crotalaria globifera E. Mey. Brown et al. 1984 C
C. juncea L. Adams & Gianturco, 1956a B305
C. lunata Beddome ex Polhill Rothschild et al. 1979 C
C. recta Steud ex A. Rich. Crout, 1968a B326
C. wightiana Grah. ex Wight & Arn. Atal et al. 1966b B329
C. tetragona Roxb. Puri et al. 1974 B314
Heliotropium arguzioides Kar. et Kir. Akramov et al. 1961aB54
Trichodesma incanum Alph. DC. Men'shikov & Rubinstein, 1935; B108
Men'shikov, 1936; Yunusov & B109,
Plekhanova, 1957, 1959; B111, 112
Tashkhodzhaev et al. 1979 C
Uluganine Ulugbeckia tschimganica (B.Fedtsch.) Zak. Khasanova et al. 1974 B114
Uplandicine Symphytum x uplandicum Nyman Culvenor et al. 1980a, 1980b A, A
Usaramine Crotalaria brevidens Benth. var. Suri et al. 1975b B304
intermedia (Kotschy) Polhill
C. brevifolia Sawhney et al. 1967 B291
C. incana L. Sawhney & Atal, 1970a B303
C. pallida Ait. Sawhney et al. 1967 B291
C. zanzibarica Benth. (C. usaramoensis) Culvenor & Smith, 1966b B337
Senecio glandulosus Don ex Hook. et Arn. Pestchanker et al. 1985b A
S. seratophylloides Griseb Pestchanker & Giordano, 1986 A
S. vulgaris L. Pieters & Vlietinck, 1986 A
Alkaloid Plant Sources Reference Reference
Location
Uspallatine Senecio argentino Baker (vira-vira Hieron) Pestchanker et al. 1985a A
S. leucostachys Baker Pestchanker & Giordano, 1986 A
S. seratophiloides Griseb Pestchanker & Giordano, 1986 A
S. uspallatensis Pestchanker et al. 1985a A
Yamataimine Cacalia yatabei Maxim. Hikichi et al. 1978 B121
A - References in this publication.
B - References in Smith & Culvenor, J. Nat. Prod., 44, 129-152 (1981), with reference number.
C - References in Mattocks, Chemistry and Toxicology of Pyrrolizidine Alkaloids, 1986.
APPENDIX II
Table 1. Plants containing hepatotoxic pyrrolizidine alkaloids
------------------------------------------------------------------------------------------
Plant Constituent alkaloids Plant part Referencea
------------------------------------------------------------------------------------------
Apocynaceae
Fernaldia pandurata loroquin root B 29
(syn. Urechites karwinsky
Mueller)
Parsonsia eucalyptophylla lycopsamine aeriel B 28
(F. Muell.)
Parsonsia heterophylla parsonsine whole B 417
A. Cunn. heterophylline B 418
Parsonsia spiralis Wall. heterophylline leaf B 418
parsonsine
spiracine
spiranine
spiraline
Parsonsia straminea (R. Br.) lycopsamine aerial B 28
F. Muell.
Parsonsia estonia sp. echinatine A Edgar (1985)
Boraginaceae
Alkanna tinctoria Tausch 7-angelylretronecine C Roder et al.
dihydroxytriangularine (1984b)
triangularine
Amsinckia hispida (Ruiz intermedine whole B 30
et Pav.) M. Johnston lycopsamine
echiumine
Amsinckia intermedia Fisch intermedine whole B 30
et C. Mey lycopsamine
echiumine
sincamidine
echimidine
Amsinckia lycopsoides Lehm. intermedine whole B 30
lycopsamine
echiumine
Amsinckia menziesii (Lehm.) 7-acetyllycopsamine aerial C Roitman (1983a)
Nels and Macbr. 3'-acetyllycopsamine
diacetyllycopsamine
lycopsamine
intermedine
------------------------------------------------------------------------------------------
APPENDIX II Table 1. (contd.)
------------------------------------------------------------------------------------------
Plant Constituent alkaloids Plant part Referencea
------------------------------------------------------------------------------------------
Boraginaceae (contd.)
Anchusa arvensis ( L.) Bieb. echinatine whole B 31
(or diastereoisomer)
Anchusa officinalis L. 7-acetyllycopsamine whole B 31
(or diastereoisomer)
lycopsamine B 32
Asperugo procumbens L. supinine (or whole B 31
diastereoisomer)
lycopsamine (or
diastereoisomer)
Borago officinalis L. lycopsamine aerial, root C Larson et al.
amabiline (1984)
supinine A Luthy et al.
intermedine (1984)
acetylintermedine
acetyllycopsamine
thesinine seed, flower
Cynoglossum amabile Stapf amabiline whole B 33, 34
& Drummond echinatine
Cynoglossum australe R. Br. cynaustine whole B 33
cynaustraline
heliosupine
Cynoglossum creticum heliosupine aerial B 419
echinatine
Cynoglossum glochidiatum amabiline whole B 36
Wall. ex Lindl.
Cynoglossum lanceolatum cynaustraline whole B 36
Forsk cynaustine
Cynoglossum latifolium latifoline aerial B 37
R. Br. 7-angelylretronecine
Cynoglossum officinale L. heliosupine aerial B 38, 39, 40
echinatine root, aerial B 41, 42
acetylheliosupine aerial B 43
7-angelylheliotridine
Cynoglossum pictum Ait. heliosupine root, aerial B 44, 45
echinatine
pictumine aerial B 46
------------------------------------------------------------------------------------------
APPENDIX II Table 1. (contd.)
------------------------------------------------------------------------------------------
Plant Constituent alkaloids Plant part Referencea
------------------------------------------------------------------------------------------
Boraginaceae (contd.)
Cynoglossum viridiflorum viridiflorine root B 47
Pallas ex Lehm. heliosupine B 34
Echium plantagineum L. echiumine aerial B 48
(Echium lycopsis L.) echimidine
Echium vulgare L. heliosupine aerial B 49
asperumine aerial B 50
echinatine (or whole B 31
diastereoisomer)
Hackelia floribunda latifoline A Hagglund et al.
(1985)
Heliotropium acutiflorum heliotrine aerial B 51
Heliotropium arbainense heliotrine aerial B 419
europine
lasiocarpine
Heliotropium arborescens L. lasiocarpine aerial B 52
(Heliotropium peruvianum L.)
Heliotropium arguzioides heliotrine aerial B 53
Kar. et Kir. trichodesmine aerial, root B 54, 55
Heliotropium curassavicum heliotrine whole B 56
Linn. lasiocarpine
angelylheliotridine
curassavine aerial B 57
heliovicine
trachelanthamidine B 58
acetylcurassavine aerial C Mohanraj et al.
heliocurassavinine (1982)
heliocurassavine
heliocoromandaline
heliocurassavicine
curassanecine
curassavinine
coromandalinine
heliovinine
Heliotropium dasycarpum heliotrine aerial, root B 54
Ledeb. seed B 59
------------------------------------------------------------------------------------------
APPENDIX II Table 1. (contd.)
------------------------------------------------------------------------------------------
Plant Constituent alkaloids Plant part Referencea
------------------------------------------------------------------------------------------
Boraginaceae (contd.)
Heliotropium digynum heliotrine A Hammouda et al.
(Heliotropium luteum) lasiocarpine (1984)
europine
angelylheliotrine
Heliotropium eichwaldi heliotrine whole B 60, 61
Steud. ex DC. lasiocarpine aerial B 62
7-angelylheliotrine aerial B 63
Heliotropium europaeum heliotrine whole B 64, 65
lasiocarpine
europine whole B 66, 67
supinine
heleurine
N-dihydropyrrolizino- whole B 68
methyl-
heliotrine chloride
acetyllasiocarpine whole B 69
Heliotropium indicum L. indicine aerial B 70
acetylindicine aerial B 71
indicinine
echinatine aerial B 72
supinine
heleurine
lasiocarpine
Heliotropium lasiocarpum heliotrine aerial B 73
Fisch. et Mey. lasiocarpine
europine A Culvenor et al.
heleurine (1986)
Heliotropium maris-mortui europine aerial B 74
lasiocarpine aerial B 419
Heliotropium olgae heliotrine aerial, root B 75
Heliotropium popovii H. heliotrine seed B 77
Riedl. subsp. gillianum
H. Riedl.
Heliotropium ramosissimum heliotrine aerial B 78, 79
(Lehm.) Dec. (syn. heleurine
Heliotropium persicum L., supinine
Heliotropium undulatum) lasiocarpine
Heliotropium rotundifolium europine aerial B 74
------------------------------------------------------------------------------------------
APPENDIX II Table 1. (contd.)
------------------------------------------------------------------------------------------
Plant Constituent alkaloids Plant part Referencea
------------------------------------------------------------------------------------------
Boraginaceae (contd.)
Heliotropium steudneri Vatke lycopsamine leaf B 80
Heliotropium suaveolens Bieb. heliotrine aerial A Guner (1986)
lasiocarpine
europine
echinatine
Heliotropium supinum L. supinine root B 81
heliosupine root B 82
echinatine whole B 83
7-angelylheliotridine
7-angelylheliotridine
viridiflorate
7-angelylheliotridine
trachelanthate
heliotrine seed, leaf C Pandey et al.
lasiocarpine (1983)
Heliotropium transoxanum heliotrine aerial B 51
Lappula glochidiata echinatine aerial B 84
Lappula intermedia lasiocarpine aerial B 85
Lindelofia angustifolia echinatine aerial B 62, 36
(Schrenk) Brand. amabiline
Lindelofia spectabilis echinatine aerial B 62, 63
Lehm. 7-acetylechinatine
monocrotaline
Lindelofia stylosa viridiflorine aerial B 86
(Kar. et Kir.) Brand echinatine seed B 75
lindelofine
Lindelofia tschimganica carategine aerial B 87
echinatine
viridiflorine
Lithospermum officinale L. acetylechimidinyl- whole B 31
retronecine
(or diastereoisomer)
Messerschmidia sibirica lycopsamine whole C Hikichi et al.
angelylretronecine (1980)
------------------------------------------------------------------------------------------
APPENDIX II Table 1. (contd.)
------------------------------------------------------------------------------------------
Plant Constituent alkaloids Plant part Referencea
------------------------------------------------------------------------------------------
Boraginaceae (contd.)
Myosotis scorpioides L. 7-acetylscorpioidine aerial C Resch et al.
(syn. Myosotis scorpioidine (1982)
palustris L.) symphytine
myoscorpine
Paracynoglossum imeritinum heliosupine aerial, root B 88
(Kusn.) M. Pop. echinatine B 89, 90
Rindera austroechinata echinatine whole, seed B 87
M. Pop.
Rindera baldshuanica rinderine aerial B 91
Kusnezov echinatine aerial B 87
trachelanthamine
turkestanine
Rindera cyclodonta Bge. echinatine aerial B 59
Rindera echinata Regel echinatine aerial B 92
trachelanthamine aerial B 59
Rindera oblongifolia carategine aerial B 87
M. Pop. echinatine
turkestanine
Solenanthus circinnatus echinatine seed, aerial, B 93
Ledeb. root
Solenanthus coronatus echinatine aerial B 75
Solenanthus karateginius carategine aerial B 93
Lipsky echinatine
Solenanthus turkestanicus rinderine aerial B 94
turkestanine
Symphytum asperum Lepech. asperumine aerial, root B 95, 96
echinatine aerial, root B 97
acetylheliosupine whole B 31
(or diastereoisomer)
7-acetyllycopsamine leaf, root C Roitman (1981)
intermedine
symlandine
7-acetylintermedine
symphytine
lycopsamine
echimidine
angelylechimidine root A Gadella et al.
(or diastereoisomer) (1983)
------------------------------------------------------------------------------------------
APPENDIX II Table 1. (contd.)
------------------------------------------------------------------------------------------
Plant Constituent alkaloids Plant part Referencea
------------------------------------------------------------------------------------------
Boraginaceae (contd.)
Symphytum caucasicum Bieb. lasiocarpine aerial, root B 95
asperumine aerial, root B 98, 99
echinatine
echimidine
Symphytum officinale Linn. symphytine root B 15, 16
echimidine B 100
lasiocarpine aerial, root B 95
heliosupine aerial, root B 101
viridiflorine root
echinatine root
acetylechimidine B 31
(or diastereoisomer)
7-acetyllycopsamine aerial, root A Huizing et al.
lycopsamine (1981)
intermedine
7-acetylintermedine
symlandine C Roder et al.
myoscorpine root (1982a)
C Resch et al.
(1982)
Symphytum orientale anadoline whole B 102, 103, 104
symphytine
echimidine
Symphytum peregrinum Ledeb. echimidine root A Gadella et al.
symphytine (1983)
Symphytum tuberosum L. echimidine whole B 105
anadoline
symlandine root A Gray et al.
(1983)
Symphytum x uplandicum Nyman lycopsamine aerial B 31, 17, 106
intermedine
uplandicine
7-acetyllycopsamine
7-acetylintermedine
echimidine
symphytine
symlandine
angelylechimidine root A Gadella et al.
(or diastereoisomer) (1983)
Tournefortia sarmentosa Lam. supinine leaf, stem B 107
------------------------------------------------------------------------------------------
APPENDIX II Table 1. (contd.)
------------------------------------------------------------------------------------------
Plant Constituent alkaloids Plant part Referencea
------------------------------------------------------------------------------------------
Boraginaceae (contd.)
Trichodesma africana intermedine aerial B 419
europine
Trichodesma incanum Alph. trichodesmine aerial B 108, 109
DC. incanine seed, aerial, B 110, 111, 112
root
Trichodesma zeylanicum supinine seed B 113
(Burm. f) R. Br.
Ulugbekia tschimganica uluganine B 114
Compositae
Adenostyles alliariae platyphylline root B 115
seneciphylline
Adenostyles glabra seneciphylline C Wiedenfeld et al.
(1984)
Adenostyles rhombifolius platyphylline aerial B 116
(Willd.) M. Pimen. ssp. seneciphylline
platyphylloides
Brachyglottis repanda senecionine aerial B 117
Forst. et Forst. senkirkine
brachyglottine B 118
Cacalia floridana (= Senecio otosenine aerial B 119
floridanus Sch. Bip.) florosenine
floridanine
floricaline
Cacalia hastata L. subsp. integerrimine root B 120
orientalis Kitamura
Cacalia yatabei Maxim yamataimine root B 121
Conoclinium coelestinium intermedine C Herz et al. (1981)
(L.) DC
Crassocephalum crepidioides jacobine aerial A Asada et al.
jacoline (1985)
Doronicum macrophyllum otosenine root B 122
floridanine
doronine
------------------------------------------------------------------------------------------
APPENDIX II Table 1. (contd.)
------------------------------------------------------------------------------------------
Plant Constituent alkaloids Plant part Referencea
------------------------------------------------------------------------------------------
Compositae (contd.)
Doronicum pardalianches otosenine root A Rajagopalan & Negi
Linn. (1985)
Echinacea angustifolia DC. tussilagine whole C
isotussilagine
Echinacea purpurea M. tussilagine whole C
isotussilagine
Emilia flammea Cass. otosenine aerial, root B 123, 124, 125
emiline
Erechtites hieracifolia (L.) senecionine aerial B 126, 127
Raf. ex DC. seneciphylline
Eupatorium altissimum L. rinderine C Herz et al. (1981)
angelylheliotridine
Eupatorium cannabinum L. echinatine aerial B 128
supinine
amabiline A Luthy et al.
(1984)
Eupatorium compositifolium intermedine C Herz et al. (1981)
Walt. lycopsamine
Eupatorium maculatum L. echinatine root B 129
trachelanthamidine
Eupatorium purpureum probably echinatine aerial B 130
Eupatorium serotinum Michx. supinine aerial B 131
rinderine
Eupatorium stoechadosmum lindelofine root B 132
Hance supinine
Farfugium japonicum Kitam. senkirkine root, leaf B 133
farfugine whole C Niwa et al. (1983)
petasitenine whole A Niwa et al. (1985)
Gynura scandens O. Hoffm. gynuramine C Wiedenfeld (1982)
acetylgynuramine
Gynura segetum (Lour.) Merr. senecionine C Liang & Roder
(1984)
------------------------------------------------------------------------------------------
APPENDIX II Table 1. (contd.)
------------------------------------------------------------------------------------------
Plant Constituent alkaloids Plant part Referencea
------------------------------------------------------------------------------------------
Compositae (contd.)
Ligularia brachyphylla clivorine aerial B 134
Hand.-Mazz. ligularine
ligudentine
Ligularia clivorum clivorine aerial B 135, 136,
137, 138
Ligularia dentata clivorine aerial B 134
(A. Gray) Hara ligularine
ligudentine
ligularidine whole B 436
ligularinine aerial, root C Asada & Furuya
ligularizine (1984a)
neoligularidine
Ligularia elegans (Cass.) clivorine aerial B 134
[syn. Ligularia macrophylla ligularine
(Ledeb. DC.)]
Ligularia japonica senecionine root A Asada et al.
neopetasitenine (1981)
platyphylline
Petasites albus L. senkirkine aerial C Luthy et al.
(1983)
Petasites hybridus L. senecionine aerial C Luthy et al.
integerrimine (1983)
senkirkine
Petasites japonicus Maxim. petasitenine aerial B 20, 19, 139
(fukinotoxin)
neopetasitenine
senkirkine stem B 140
petasinine aerial B 141
petasinoside
Petasites laevigatus (Willd.) platyphylline aerial B 142
Reichenb. [syn. Nardosmia senkirkine (renardine) aerial B 143, 144
laevigata (Willd.) DC.] senecionine
Senecio abrotanifolius doronine aerial A Roder et al.
ssp. abrotanifolius doronenine (1984a)
bulgarsenine
Senecio abrotanifolius doronine aerial A Roder et al.
ssp. abrotanifolius var. doronenine (1984a)
tiroliensis bulgarsenine
------------------------------------------------------------------------------------------
APPENDIX II Table 1. (contd.)
------------------------------------------------------------------------------------------
Plant Constituent alkaloids Plant part Referencea
------------------------------------------------------------------------------------------
Senecio aegypticus L. senecionine whole B 145, 146
otosenine aerial
riddelliine
erucifoline
Senecio alpinus (L.) Scop. seneciphylline aerial B 147
jacozine
jacobine whole A Luthy et al.
integerrimine (1981)
jacoline
senecionine
jaconine
Senecio ambrosioides retrorsine whole B 148
(= Senecio brasiliensis seneciphylline
Less.) senecionine
riddelliine whole B 420
Senecio ampullaceus Hook. senecionine whole B 149, 150, 151
seneciphylline
retrorsine
Senecio antieuphorbium integerrimine aerial B 152
(L.) Sch. Bip. senkirkine
Senecio aquaticus Hill seneciphylline aerial B 153, 154
Senecio aureus L. senecionine aerial B 155, 156
otosenine aerial C Resch et al.
floridanine (1983)
florosenine C Roder et al.
(1983)
Senecio auricola Bourg. neosenkirkine aerial B 157
Senecio barbellatus DC. swazine B 270
retrorsine
Senecio bipinnatisectus retrorsine aerial, root B 158
Belcher (syn.
Erechtites atkinsoniae)
Senecio borysthenicus seneciphylline aerial, root B 159, 160
(= Senecio prealtus
Berthol.)
Senecio brasiliensis senecionine leaf B 161, 162
DC. (syn. seneciphylline B 148, 163
Senecio ambrosioides) jacobine
integerrimine
retrorsine
------------------------------------------------------------------------------------------
APPENDIX II Table 1. (contd.)
------------------------------------------------------------------------------------------
Plant Constituent alkaloids Plant part Referencea
------------------------------------------------------------------------------------------
Compositae (contd.)
Senecio brasiliensis Less integerrimine A Nardi et al.
var tripartitus (1980)
Senecio bupleuroides DC. retrorsine aerial B 164
Senecio cacaliaster (Lam.) sencalenine A Roder et al.
bulgarsenine (1984b)
7-senecioylretrone-
cine
7-senecioyl-9-sarra-
cinoylretronecine
Senecio cannabifolius Less. seneciphylline aerial B 165
senecicannabine aerial, root C Asada et al.
jacozine (1982a)
Senecio carthamoides Greene senecionine whole B 149, 151
seneciphylline
Senecio caudatus DC. 7-senecioylretrone- aerial A Bohlmann et al.
cine (1986)
9-senecioylretrone-
cine
7-senecioyl-9-sarra-
cinylretronecine
7-senecioyl-9-(2-
methyl-2,3-dihyroxy-
butyryl)retronecine
7-senecioyl-9-(2-
methy-2-hydroxy-3-
acetoxybutyryl)
retronecine
retronecine
2-senecioyl-(-)-
macronecine
9-senecioyl-(-)-
macronecine
senecicaudatine-9-
senecioate
senecidaudatine-9-
isovalerate
norsenecicaudatine-
9-sencioate
senecicaudatinal
semiacetal
Senecio chrysanthemoides seneciphylline B 166
------------------------------------------------------------------------------------------
APPENDIX II Table 1. (contd.)
------------------------------------------------------------------------------------------
Plant Constituent alkaloids Plant part Referencea
------------------------------------------------------------------------------------------
Compositae (contd.)
Senecio cineraria DC. jacobine aerial B 153, 167
senecionine seed B 168
seneciphylline aerial B 169
otosenine aerial B 170
retrorsine aerial B 171
Senecio congestus (R. Br.) senecionine C Roder et al.
DC. [syn. Senecio palustris neoplatyphylline (1982b)
(L.) Hooker, Senecio platyphylline
tubicaulis Mansfeld)
Senecio cruentus DC. senecionine C Asada et al.
seneciphylline (1982b)
retrorsine
riddelliine
Senecio cymbaroides senecionine whole B 420
seneciphylline
riddelliine
retrorsine
Senecio desfonainei Druce senecionine aerial B 173, 145
otosenine
riddelliine
seneciphylline aerial B 146
retrorsine A Rizk et al. (1983)
senkirkine
angelylretronecine
Senecio discolor DC. retrorsine leaf B 174
senecionine aerial B 175
Senecio dolichodoryius 15,20-dihyroxyeruci- aerial A Bohlmann et al.
Cuatr. foline (1986)
Senecio doronicum L. bulgarsenine leaf B 176
doronenine
Senecio douglasii DC. retrorsine whole B 149, 150, 151
riddelliine
seneciphylline
senecionine
Senecio durieui Gay integerrimine whole B 157
Senecio eremophilus senecionine aerial B 149, 150, 151
Richards seneciphylline
retrorsine
riddelliine
------------------------------------------------------------------------------------------
APPENDIX II Table 1. (contd.)
------------------------------------------------------------------------------------------
Plant Constituent alkaloids Plant part Referencea
------------------------------------------------------------------------------------------
Compositae (contd.)
Senecio erraticus Berthol. senecionine aerial B 177
otosenine
floridanine
Senecio erraticus Berthol. senecionine B 178, 179, 180
subsp. barbaraeifolius Krock otosenine
erucifoline
seneciphylline leaf B 181
integerrimine aerial B 182
Senecio erucifolius L. senecionine aerial B 153
seneciphylline aerial B 183, 180
erucifoline
retrorsine aerial B 184
Senecio faberi Hemsl. integerrimine C Wei et al. (1982)
Senecio filaginoides senecionine root A Pestchanker &
(H. et A.) DC. retrorsine Giordano (1986)
Senecio fistulosus senecionine A Gonzalez et al.
Poepp. ex Less. (1986b)
Senecio fluviatilis Wallr. seneciphylline aerial B 185
otosenine
florosenine
Senecio formosus integerrimine aerial B 186
retrorsine
Senecio fremontii Torr. seneciphylline whole B 148
et A. Gray senecionine
Senecio gilliesiano senecionine root A Guidugli et al.
retrorsine (1986)
Senecio glabellus senecionine whole B 187
(Turcz.) DC.
Senecio glaberrimus DC. retrorsine aerial B 153
Senecio glandulosus integerrimine root A Pestchanker et al.
Don ex Hook. et Arn. retrorsine (1985b)
usaramine
Senecio graminifolius retrorsine aerial B 188
N.J. Jacq. graminifoline
------------------------------------------------------------------------------------------
APPENDIX II Table 1. (contd.)
------------------------------------------------------------------------------------------
Plant Constituent alkaloids Plant part Referencea
------------------------------------------------------------------------------------------
Compositae (contd.)
Senecio grandifolia Jaqu. platyphylline root, leaf, B 189
seneciphylline stem
Senecio grandifolius Less. senkirkine aerial A Bohlmann et al.
neosenkirkine (1986)
Senecio griesbachii Baker retrorsine aerial B 190
Senecio ilicifolius Thunb. senecionine aerial B 191, 192, 188, 127
seneciphylline
retrorsine
Senecio illinitus Phill. senkirkine aerial A Gonzalez et al.
O-acetylsenkirkine (1986a)
senecionine
Senecio inaequidens DC. retrorsine A Roder et al.
senecionine (1981)
senecivernine aerial A Bicchi et al.
integerrimine (1985)
Senecio incanus L. seneciphylline aerial B 147
subsp. carniolicus integerrimine
(Willd.) Br.-Bl.
Senecio integerrimus Nutt. integerrimine aerial B 156
senecionine
neoplatyphylline whole B 420
platyphylline
Senecio isatideus DC. retrorsine aerial B 153, 193
Senecio jacobaea L. seneciphylline aerial B 194, 195
senecionine B 196, 197
jacobine B 153, 197, 198
jaconine B 199, 200, 201
jacozine
otosenine aerial B 51
senkirkine
retrorsine aerial B 184
Senecio kirkii Hook. f. senkirkine bark, leaf B 203
ex Kirk O-acetylsenkirkine leaf B 204
Senecio kleinia Sch. Bip. integerrimine stem B 205
senkirkine stem B 206
Senecio krylovii seneciphylline aerial B 207
------------------------------------------------------------------------------------------
APPENDIX II Table 1. (contd.)
------------------------------------------------------------------------------------------
Plant Constituent alkaloids Plant part Referencea
------------------------------------------------------------------------------------------
Compositae (contd.)
Senecio kubensis Grossh. seneciphylline aerial B 208
Senecio lampsanoides seneciphylline aerial, root B 209, 210
Senecio laricifolius senecionine aerial A Bohlmann et al.
H.B.K. seneciphylline (1986)
senkirkine
19-hydroxysenkirkine
19-acetoxysenkirkine
Senecio latifolius DC. retrorsine aerial B 211, 212
(syn. Senecio sceleratus seneciphylline aerial B 213
Schweikerdt) platyphylline
sceleratine aerial B 260
chlorodeoxysceleratine aerial B 261;
(merenskine) C Bredenkamp et al.
(1985)
Senecio leucostachys Baker senecionine root A Pestchanker &
Giordano (1986)
Senecio longilobus Benth. seneciphylline whole B 156, 214, 150
retrorsine B 151
riddelliine whole B 149
Senecio magnificus F. Muell. senecionine aerial B 215
integerrimine B 216
Senecio megaphyllus Green. 13,19-epoxyseneci- aerial A Bohlmann et al.
phylline (1986)
13,19-epoxysparti-
oidine
Senecio minimus Poir seneciphylline aerial B 158
------------------------------------------------------------------------------------------
APPENDIX II Table 1. (contd.)
------------------------------------------------------------------------------------------
Plant Constituent alkaloids Plant part Referencea
------------------------------------------------------------------------------------------
Compositae (contd.)
Senecio morrisonensis Hayata integerrimine whole B 217
Senecio multilobatus senecionine A McCoy et al.
(1983)
Senecio multivenius seneciphylline aerial A Bohlmann et al.
Benth. in Oerst. senecionine (1986)
Senecio nebrodensis L. integerrimine whole B 218
var sicula senecionine
Senecio nemorensis L. bulgarsenine leaf B 219
var bulgaricus retrosisosenine
(Vel) Stoj. nemorensine
Senecio nemorensis L. ssp. fuchsisenecionine B 362, 363, 364, 365
fuchsii Gmel.
senecionine B 423
Senecio nemorensis L. nemorensine aerial, root B 371
subdecurrens Griseb. retroisosenine aerial B 422
bulgarsenine
Senecio othonnae Bieb. otosenine aerial, root B 220
onetine root B 221
seneciphylline
floridanine aerial, root B 222
doronine aerial B 223
Senecio othonniformis bisline aerial B 22, 225
Fourcade isoline
Senecio palmatus Pall. seneciphylline root B 226
Senecio paludosus L. seneciphylline root, aerial B 153, 227, 228
Senecio pampeanus Cabrera senecionine aerial B 229
Senecio pancicii Degen senecionine whole C Jizba et al.
var arnautorum (Velen.) seneciphylline (1982)
Stoj., Stef. et Kit.
Senecio pancicii Degen senecionine whole C Jizba et al.
var pancicii (1982)
Senecio patagonicus senecionine aerial A Villaroel et al.
Hook. and Arn. seneciphylline (1985)
------------------------------------------------------------------------------------------
APPENDIX II Table 1. (contd.)
------------------------------------------------------------------------------------------
Plant Constituent alkaloids Plant part Referencea
------------------------------------------------------------------------------------------
Compositae (contd.)
Senecio paucicalyculatus paucicaline whole B 230
(Klatt.) retrorsine
Senecio paucifolius seneciphylline B 231
S.G. Gmel.
Senecio petasitis DC. senecionine leaf B 146
bisline (?) aerial B 232
Senecio phillipicus retrorsine aerial A Gonzalez et al.
Rogel et Koern. (1986a)
Senecio pierotii neosenkirkine aerial, root C Asada & Furuya
senkirkine (1982)
Senecio pimpinellifolius senecionine aerial A Bohlmann et al.
H.B.K. (1986)
Senecio platyphylloides platyphylline root B 233, 234, 235
Somm. et Lev. seneciphylline
Senecio platyphyllus platyphylline root, aerial B 236, 237, 238
(Bieb.) DC. seneciphylline leaf B 239
neoplatyphylline root B 240, 241
sarracine root B 242
Senecio pojarkovae sarracine root B 243
seneciphylline
Senecio procerus L. senkirkine aerial, root B 244
var procerus procerine
Stoj. Stef. et Kit.
Senecio propinquus Ait. seneciphylline aerial, root B 209, 245
Senecio pseudo-arnica Less. senecionine aerial B 156
Senecio pterophorus senecionine aerial B 192, 188, 127
seneciphylline
retrorsine
rosmarinine
acetylseneciphylline
Senecio quadridentatus senecionine aerial B 247
Labill. (syn. Erechtites seneciphylline
quadridentata DC.) retrorsine
------------------------------------------------------------------------------------------
APPENDIX II Table 1. (contd.)
------------------------------------------------------------------------------------------
Plant Constituent alkaloids Plant part Referencea
------------------------------------------------------------------------------------------
Compositae (contd.)
Senecio quebradensis senkirkine aerial A Bohlmann et al.
Greenm. florosenine (1986)
Senecio racemosus DC. seneciphylline root B 248
Senecio renardii Winkl. seneciphylline aerial B 249, 250
senkirkine (renardine)
otosenine
Senecio retrorsus DC. retrorsine aerial B 194, 193
Senecio rhombifolius sarracine root B 251, 233
(Willd.) Sch. Bip. platyphylline aerial, root B 208
seneciphylline
neoplatyphylline
Senecio riddellii riddelliine aerial B 156
Torr. et A. Gray whole B 252, 253
retrorsine B 420
Senecio riddellii retrorsine whole B 149
Torr. et A. Gray riddelliine
var. parksii (Cory)
Senecio ruderalis Harvey retrorsine aerial B 254
Senecio ruwenzoriensis ruwenine whole B 255
S. Moore ruzorine
Senecio sandrasicus senecionine A Temizer et al.
(1985)
Senecio scandens senecionine whole B 256
seneciphylline
Senecio seratophiloides senecionine root A Pestchanker &
Griseb. senecivernine Giordano (1986)
usaramine
retrorsine
uspallatine
Senecio spartioides seneciphylline aerial B 156, 262
Torr. et A. Gray senecionine
spartiodine
riddelliine whole B 420
retrorsine
------------------------------------------------------------------------------------------
APPENDIX II Table 1. (contd.)
------------------------------------------------------------------------------------------
Plant Constituent alkaloids Plant part Referencea
------------------------------------------------------------------------------------------
Compositae (contd.)
Senecio spathulatus senecionine aerial, root B 158
A. Rich. integerrimine
seneciphylline B 263
Senecio squalidus L. senecionine aerial B 153, 264, 265
integerrimine B 205
Senecio stenocephalus seneciphylline aerial B 266
Maxim.
Senecio subalpinus senecionine leaf B 267
C. Koch. seneciphylline aerial B 147
integerrimine
Senecio subulatus dihydroretrosine root A Pestchanker et
Don ex Hook. retrorsine al. (1985b)
et Arn. var. erectus senecionine
Senecio swaziensis retrorsine aerial B 268, 269, 270
Compton swazine
Senecio tenuifolius senecionine aerial C Bhakuni & Gupta
Burm. integerrimine (1982)
senkirkine
o-acetylsenkirkine
Senecio tomentosus senecionine aerial B 271, 179
otosenine
(tomentosine)
Senecio triangularis senecionine aerial B 272
Hook. integerrimine C Roitman (1983b)
platyphylline
rosmarinine
retrorsine
triangularine
neotriangularine
7-angelylretronecine whole C Rueger & Benn
7-senecioylretrone- (1973)
cine
7-angelyl-9-sarra-
cinylretronecine
7-senecioyl-9-sarra-
cinylretronecine
Senecio uintahensis senkirkine whole B 420
senecionine
------------------------------------------------------------------------------------------
APPENDIX II Table 1. (contd.)
------------------------------------------------------------------------------------------
Plant Constituent alkaloids Plant part Referencea
------------------------------------------------------------------------------------------
Compositae (contd.)
Senecio umgeniensis 7-senecioyl-9-sarra- aerial A Bohlmann et al.
Thell. cinylretronecine (1986)
Senecio usgorensis 13,19-epoxyseneci- aerial A Bohlmann et al.
Cuatr. phylline (1986)
Senecio uspallatensis retrorsine root A Pestchanker et
uspallatine al. (1985a)
Senecio variabilis Sch. 7-senecioylretrone- aerial, root A Bohlmann et al.
Bip. cine (1986)
9-senecioylretrone-
cine
7-senecioyl-9-sarra-
cinylretronecine
Senecio venosus Harvey retrorsine aerial B 153
Senecio vernalis Walst. retrorsine aerial B 273, 274
et Kit. senecionine
senkirkine
senecivernine
integerrimine A Sener et al.
seneciphylline (1986)
riddelliine
retronecine
Senecio viscosus L. senecionine aerial B 264, 153
integerrimine aerial B 182
Senecio vulgaris L. senecionine aerial B 275, 264, 153
seneciphylline aerial B 155, 276, 277
retrorsine
riddelliine whole B 420
integerrimine A Pieters &
spartioidine Vlietinck (1986)
usaramine
Senecio senecionine whole B 420
werneriaefolius retrorsine
Syneilesis palmata syneilesine aerial, root B 278, 279
Maxim. acetylsyneilesine
senecionine
------------------------------------------------------------------------------------------
APPENDIX II Table 1. (contd.)
------------------------------------------------------------------------------------------
Plant Constituent alkaloids Plant part Referencea
------------------------------------------------------------------------------------------
Compositae (contd.)
Tussilago farfara L. senkirkine flower B 18, 425
leaf, stem C Rosberger et al.
(1981)
senecionine leaf, stem A Luthy et al.
(1980)
tussilagine C Roder et al.
(1981b)
Leguminosae
Crotalaria aegyptica crosemperine B 419
Benth. monocrotaline
7beta-hydroxy-1- B 426
methylene-8alpha-
pyrrolizidine
Crotalaria agatiflora maduraensine aerial B 280
Schweinf. anacrotine aerial B 281
7-acetylmadurensine
6-acetylanacrotine
7-acetyl-cis-
madurensine
6-acetyl-trans-
anacrotine
crotaflorine
6-angelyl-trans-
anacrotine
Crotalaria assamica Benth. monocrotaline B 285, 286
Crotalaria axillaris Ait. axillarine seed B 287, 288
axillaridine
Crotalaria barbata R. Graham crobarbatine seed B 289
ex R. Wight et Walk.-Arn.
Crotalaria berteroana DC. fulvine aerial B 297
(syn. Crotalaria fulva Roxb.)
Crotalaria brevidens integerrimine seed B 304
Benth. var. intermedia usaramine
(Kotschy) Polhill(syn.
Crotalaria intermedia
Kotschy)
Crotalaria breviflora DC. integerrimine seed B 290, 291
usaramine
------------------------------------------------------------------------------------------
APPENDIX II Table 1. (contd.)
------------------------------------------------------------------------------------------
Plant Constituent alkaloids Plant part Referencea
------------------------------------------------------------------------------------------
Leguminosae (contd.)
Crotalaria burhia Ham. crotalarine aerial B 292, 293
ex Benth. monocrotaline
Crotalaria candicans crocandine seed B 380
W. and A. isocorcandine
isocromadurine seed C Suri et al.
crispatine (1982)
turneforcidine
cropodine seed C Haksar et al.
(1982)
Crotalaria cephalotes Steud. monocrotaline seed C Pilbeam et al.
ex A. Rich (1983)
Crotalaria crispata monocrotaline whole B 294
F. Muell. ex Benth. fulvine
crispatine
Crotalaria cunninghamii monocrotaline seed C Pilbeam et al.
R. Br. (1983)
Crotalaria dura dicrotaline aerial B 295, 296
J.M. Wood et Evans
Crotalaria fulva Roxb.
(see Crotalaria
berteroana DC.)
Crotalaria globifera E. Mey dicrotaline aerial B 295, 296
globiferine seed C Brown et al.
grantianine (1984)
grantaline
trichodesmine
Crotalaria grahamiana monocrotaline seed B 298
Wight & Arn. grahamine seed B 299
monocrotalinine whole B 300
Crotalaria incana L. integerrimine seed B 187
anacrotine aerial B 302
usaramine seed B 303
Crotalaria juncea L. senecionine seed B 305, 306, 307
seneciphylline
riddelliine
trichodesmine
junceine
------------------------------------------------------------------------------------------
APPENDIX II Table 1. (contd.)
------------------------------------------------------------------------------------------
Plant Constituent alkaloids Plant part Referencea
------------------------------------------------------------------------------------------
Leguminosae (contd.)
Crotalaria laburnifolia L. anacrotine seed B 308, 309, 310,
(crotalaburnine) 291, 311
Crotalaria laburnifolia L. madurensine aerial B 312
subsp. eldomae anacrotine
senkirkine
hydroxysenkirkine
crotafoline
Crotalaria leschenaultii monocrotaline seed B 313
Crotalaria leiloba Bartl. monocrotaline seed B 314
(syn. Crotalaria
ferruginea Wall.)
Crotalaria madurensis madurensine seed, flower, B 280
R. Wight leaf
crispatine aerial B 315
fulvine
cromadurine seed B 62, 316
isocromadurine seed B 317
Crotalaria micans Link. 1-methylenepyrroliz- seed B 282, 283
(syn. Crotalaria anagyroides idine seed B 284
Humb. et al.) senecionine B 280
anacrotine
Crotalaria mitchellii Benth. monocrotaline whole B 318
retusamine whole
Crotalaria mitchellii Benth. retusamine whole B 318
subsp. laevis A. Lee,
published as
"sp. aff. mitchellii")
Crotalaria mysorensis Roth. monocrotaline seed B 319
Crotalaria nana Burm. crotananine seed B 320
cronaburmine seed B 427
Crotalaria nitens Kunth. monocrotaline A Hoet et al.
(1981)
Crotalaria novae-hollandiae monocrotaline whole B 318
DC. subsp. lasiophylla retusamine
(Benth) A. Lee
------------------------------------------------------------------------------------------
APPENDIX II Table 1. (contd.)
------------------------------------------------------------------------------------------
Plant Constituent alkaloids Plant part Referencea
------------------------------------------------------------------------------------------
Leguminosae (contd.)
Crotalaria novae-hollandiae retusamine seed B 318
DC. subsp. novae-hollandiae
(syn. Crotalaria
crassipes Hook.)
Crotalaria pallida Ait. usaramine seed B 291
(syn. Crotalaria mucronata, nilgirine seed B 322
Crotalaria striata) crotastriatine seed B 323, 324
Crotalaria paniculata Willd. fulvine seed B 325
Crotalaria paulina Schrank monocrotaline seed C Pilbeam et al.
(1983)
Crotalaria quinquefolia L. monocrotaline seed C Pilbeam et al.
(1983)
Crotalaria recta Steud. monocrotaline aerial B 326
ex A. Rich
Crotalaria retusa L. monocrotaline seed B 327
retusine seed, aerial B 328
retusamine
retronecine
Crotalaria sagittalis L. monocrotaline seed B 330
Crotalaria scassellatii axillaridine seed A Wiedenfeld et
Chiov. axillarine al. (1985)
deoxyaxillarine
Crotalaria semperflorens crosemperine seed B 331
Vent.
Crotalaria spartioides DC. retrorsine aerial B 334
Crotalaria spectabilis Roth. monocrotaline seed B 335, 227
(syn. Crotalaria sericea spectabiline seed, whole B 336
Retz)
Crotalaria stipularia Desv. monocrotaline seed B 314
Crotalaria tetragona Roxb. integerrimine seed B 314
trichodesmine
Crotalaria verrucosa L. anacrotine seed B 338
crotaverrine seed B 339
acetylcrotaverrine
------------------------------------------------------------------------------------------
APPENDIX II Table 1. (contd.)
------------------------------------------------------------------------------------------
Plant Constituent alkaloids Plant part Referencea
------------------------------------------------------------------------------------------
Leguminosae (contd.)
Crotalaria virgulata grantianine seed B 301, 259
subsp. grantiana (Harv.) grantaline C Smith & Culvenor
Polhill (syn. Crotalaria 1-hydroxymethyl- (1984)
grantiana Harv.) 1beta,2beta-epoxy-
pyrrolizidine
Crotalaria walkeri Arn. crotaverrine seed B 340
acetylcrotaverrine
Crotalaria wightiana Grah. junceine seed B 329
ex Wight & Arn. (syn. trichodesmine
Crotalaria rubiginosa Willd.
var. wightiana J.G. Baker)
Crotalaria zanzibarica integerrimine seed B 187
Benth. (syn. Crotalaria usaramine seed B 337
usaramoensis E.G. Baker) senecionine
retrorsine
Ranunculaceae
Caltha biflora DC. senecionine aerial B 341
Caltha leptosepala DC. senecionine aerial, root B 341
Scrophulariaceae
Castilleja rhexifolia senecionine B 342
Rydb. sarracine C Roby & Stermitz
indicine or isomer (1984)
------------------------------------------------------------------------------------------
a A = References in the reference list of this document.
B = References in Smith & Culvenor (1981), J. nat. Prod., 44: 129-152 (with reference
number).
C = References in Mattocks (1986), Chemistry and toxicology of pyrrolizidine alkaloids.
APPENDIX II
Table 2. Plants containing known alkaloids that are non-hepatotoxic (aminoalcohols and esters)
------------------------------------------------------------------------------------------
Plant Constituent alkaloids Plant part Referencea
------------------------------------------------------------------------------------------
A. Families in which hepatotoxic alkaloids also occur
Apocynaceae
Alafia multiflora alafine seed B 343
Anodendron affine Druce alloanodendrine aerial B 344, 345
anodendrin
Boraginaceae
Caccinea glauca Savi 7,9-dibenzoylretrone- fl B 346
cine
Ehretia aspera Willd. ehretinine leaf A Suri et al.
(1980)
Heliotropium angiospermum 1-hydroxymethyl- whole C Birecka et al.
Murray 1beta,2beta-epoxy- (1983)
pyrrolizidine
Heliotropium ovalifolium heliofoline whole C Mohanraj et al.
Forsk retronecine (1981)
Heliotropium spathulatum acetylcurassavine aerial A Birecka et al.
Rydb. curassavine (1980)
Heliotropium strigosum Willd. strigosine aerial B 347
Lindelofia macrostyla (Bunge) lindelofine aerial B 348
M. Pop. (syn. Lindelofia lindelofamine
anchusoides,Paracaryum
heliocarpum Kern.)
Lindelofia olgae (Regel et viridiflorine aerial B 94
Smirnov) Brand
Lindelofia pterocarpa (Rupr.) viridiflorine aerial B 93
M. Pop.
Macrotomia echioides Boiss. macrotomine aerial B 350
Paracaryum himalayense viridiflorine aerial B 93
(Klotsch) C.B. Clark
Tournefortia sibirica L. turneforcine aerial B 351
Trachelanthus hissoricus viridiflorine leaf B 352
Lipsky trachelanthamine
------------------------------------------------------------------------------------------
APPENDIX II Table 2. (contd.)
------------------------------------------------------------------------------------------
Plant Constituent alkaloids Plant part Referencea
------------------------------------------------------------------------------------------
Boraginaceae (contd.)
Trachelanthus korolkovii trachelanthamine aerial B 353, 354, 355, 94
(Lipsky) B. Fedtsch.
Celastraceae
Bhesa archboldiana 9-angelylretronecine bk B 356
(Merr. & Perry) Ding Hou
[syn. Kurrimia archboldiana
(Merr. & Perry)]
Compositae
Adenostyles rhombifolius sarracine aerial B 116
(Willd.) M. Pimen. ssp.
rhombifolia chemovar.
sarracinifera
Adenostyles rhombifolius platyphylline aerial B 116
(Willd.) M. Pimen. ssp.
rhombifolia chemovar.
platyphyllinifera
Cacalia hastata L. hastacine root B 357, 241
Cacalia robusta hastacine B 358
Senecio amphibolus macrophylline aerial B 359
Senecio angulatus L. angularine whole B 360
rosmarinine
Senecio aronicoides hygrophylline whole B 420
Senecio brachypodus DC. rosmarinine aerial, root B 361
Senecio francheti Winkl. sarracine aerial B 352
franchetine
Senecio glastifolius sarracine A Mortimer & White
(1975)
Senecio hygrophyllus R.A. platyphylline aerial B 366, 361
Dyer et C.A. Smith (syn. rosmarinine
Senecio adnatus DC.) hygrophylline
Senecio macrophyllus Bieb. macrophylline aerial B 368
Senecio mikanioides Otto. ex sarracine aerial B 155, 369, 370
Walp.
------------------------------------------------------------------------------------------
APPENDIX II Table 2. (contd.)
------------------------------------------------------------------------------------------
Plant Constituent alkaloids Plant part Referencea
------------------------------------------------------------------------------------------
Compositae (contd.)
Senecio nemorensis L. ssp. nemorensine aerial B 371
fuchsii var. nova (Zlatnik)
Senecio nemorensis L. ssp. nemorensine aerial B 371
jaquinianus (Rchb.) Durand
Senicio ovirensis ssp. angelylheliotridine A Roder et al.
gaudinil (1980)
Senecio pauciligulatus rosmarinine aerial B 361
Dyer et Sm.
Senecio rivularis DC. 7-angelylheliotridine aerial B 182, 135
Senecio rosmarinifolius Linn. rosmarinine aerial B 191, 192, 188
Senecio salignus DC. 7-angelylheliotridine aerial A Bohlmann et al.
(1986)
Senecio sarracenius L. sarracine aerial B 153, 372, 373
Senecio schvetsovii Korsh macrophylline aerial B 231
Senecio sylvaticus L. silvasenecine aerial B 362, 153
sarracine aerial B 374
Senecio taiwanensis Hayata rosmarinine aerial B 217
Senecio tournefortii Lap. platyphylline aerial B 375
Leguminosae
Crotalaria albida Heyne ex croalbidine aerial B 376, 377
Roth (syn. Crotalaria montana
Roxb.)
Crotalaria aridicola Domin. 1-methoxymethyl-1,2- aerial B 378
dehydropyrrolizidine
7beta-hydroxy-1-
methoxymethyl-1,2-
dehydropyrrolizidine
7beta-acetoxy-1- whole B 379
methoxymethyl-1,2-
dehydropyrrolizidine
Crotalaria damarensis Engl. 1-methylenepyrrolizi- whole, seed B 381, 283
dine
------------------------------------------------------------------------------------------
APPENDIX II Table 2. (contd.)
------------------------------------------------------------------------------------------
Plant Constituent alkaloids Plant part Referencea
------------------------------------------------------------------------------------------
Leguminosae (contd.)
Crotalaria goreensis Guill. 7beta-hydroxy-1- aerial, seed B 382
et Perr. methylene-8beta-
pyrrolizidine
7beta-hydroxy-1-
methylene-8alpha-
pyrrolizidine
Crotalaria grandistipulata 1-methylenepyrrolizi- seed B 434
Harms dine
Crotalaria lachnophora 1-methylenepyrrolizi- seed B 434
A. Rich dine
Crotalaria maypurensis Humb 7beta-hydroxy-1- aerial B 383
et al. methylene-8beta-
pyrrolizidine
7beta-hydroxy-1-
methylene-8alpha-
pyrrolizidine
Crotalaria medicaginea Lam. 1-methoxymethyl-1,2- whole, seed B 378
dehydropyrrolizidine
7beta-hydroxy-1-
methoxymethyl-1,2-
dehydropyrrolizidine
1alpha-methoxymethyl- whole, seed
1beta,2beta-
epoxypyrrolizidine
7alpha-hydroxy-1- seed
methoxymethyl-1,2-
dehydropyrrolizidine
1alpha-hydroxymethyl- aerial
1beta,2beta-
epoxypyrrolizidine
Crotalaria natalitia Meissner 1-methylenepyrrolizi- seed B 434
dine
Crotalaria podocarpa DC. 7-hydroxy-1-methylene- seed B 435
pyrrolizidine
Crotalaria stolzii (Bak. f.) 1-methylenepyrrolizi- seed B 434
Milne-Redh. ex Polhill dine
Ranunculacae laburnum L. laburnine seed B 387, 388, 389
1-hydroxymethyl-7-e B 390
hydroxypyrrolizidin
------------------------------------------------------------------------------------------
APPENDIX II Table 2. (contd.)
------------------------------------------------------------------------------------------
Plant Constituent alkaloids Plant part Referencea
------------------------------------------------------------------------------------------
Scrophulariacea
Castilleja "rhexifolia aff. sarracine C Roby & Stermitz
miniata" 7-angelylplatynecine (1984)
8-angelylplatynecine
B. Families in which hepatotoxic alkaloids are not known to occur
Orchidaceae
Chysis bractescens Lindl. 1alpha-methoxycar- whole B 391, 392
bonyl-8beta-
pyrrolizidine
1alpha-ethoxycar-
bonyl-8beta-
pyrrolizidine
Doritis pulcherrima phalaenopsine La or T whole B 393
(syn. Phalaenopsis esmerelda)
Hammarbya paludosa (L.) O.K. paludosine whole B 394
hammarbine whole B 395
Kingiella taenialis (Lindl.) phalaenopsine La whole B 396
Rolfe
Liparis auriculata (Blume) auriculine whole B 397
Liparis bicallosa Schltr. laburnine whole B 397
malaxine whole B 398, 399
Liparis hachijoensis Nakai laburnine whole B 397
malaxine whole B 398
Liparis keitaoensis Hay. keitaoine whole B 395
keitine
Liparis kumokiri F. Maekawa kumokirine whole B 400, 398
Liparis loeselii (L.) L.C. auriculine whole B 394
Rich
Liparis nervosa Lindl. nervosine whole B 398, 401
Malaxis congesta comb. nov. malaxin whole B 402
(Rchb. f.)
Malaxis grandifolia Schltr. grandifoline whole B 403
Phalaenopsis amabilis Bl. phalaenopsine T whole B 404, 405, 393
Phalaenopsis amboinensis phalaenopsine La whole B 393
------------------------------------------------------------------------------------------
APPENDIX II Table 2. (contd.)
------------------------------------------------------------------------------------------
Plant Constituent alkaloids Plant part Referencea
------------------------------------------------------------------------------------------
Orchidaceae (contd.)
Phalaenopsis aphrodite phalaenopsine T whole B 393
Phalaenopsis cornu-cervi cornucervine whole B 404, 393
Rchb. f.
Phalaenopsis equestris phalaenopsin ls whole B 393
Rchb. f. phalaenopsin T
Phalaenopsis fimbriata phalaenopsine T whole B 393
Phalaenopsis hieroglyfica phalaenopsine T or La whole B 393
Phalaenopsis lueddemanniana phalaenopsine T or La whole B 393
Phalaenopsis mannii Rchb. f. phalaenopsine La whole B 393
Phalaenopsis sanderiana phalaenopsine La whole B 393
Rchb. f. phalaenopsine T
Phalaenopsis schilleriana phalaenopsine La whole B 393
Phalaenopsis stuartiana phalaenopsine La whole B 393
Rchb. f. phalaenopsine T
Phalaenopsis sumatrana phalaenopsis La whole B 393
Phalaenopsis violacea phalaenopsis La or T whole B 393
Vanda cristata Lindl. acetyllaburnine whole B 407
Vanda helvola Bl. laburnine whole B 408
acetyllaburnine
Vanda hindsii Lindl. acetyllaburnine whole B 408
Vanda luzonica Loher acetyllaburnine whole B 408
Vandopsis gigantea Pfitz. laburnine whole B 408
lindelofidine
acetyllaburnine
acetyllindelofidine
Vandopsis lissochiloides laburnine whole B 408
Pfitz. lindelofidine
acetyllaburnine
acetyllindelofidine
------------------------------------------------------------------------------------------
APPENDIX II Table 2. (contd.)
------------------------------------------------------------------------------------------
Plant Constituent alkaloids Plant part Referencea
------------------------------------------------------------------------------------------
Rhizophoraceae
Cassipourea gummiflua Tulasne cassipourine stem, leaf B 409
var. verticellata Lewis bk B 410
Santalaceae
Thesium minkwitzianum thesine aerial B 411
B. Fedtsch. thesinine B 412, 413
thesinicine
isoretronecanol root
Sapotaceae
Mimusops elengi L. 1-hydroxymethyl- B 414
pyrrolizidine
tiglate
Planchonella anteridifera planchonelline leaf B 415
(C.T. White et W.D. Francis) tiglyllaburnine
H.J. Lamb benzoyllaburnine
Planchonella thyrsoidea C.T. planchonelline leaf B 415
White ex F.S. Walker tiglyllaburnine
benzoyllaburnine
Planchonella sp. (NGF 24722) trans-beta-thio- leaf B 416
acrylyl-(-)-iso-
retronecanol
tiglylisoretronecanol
------------------------------------------------------------------------------------------
a A = References in the reference list of this document.
B = References in Smith & Culvenor (1981), J. nat. Prod., 44: 129-152 (with reference
number).
C = References in Mattocks (1986), Chemistry and toxicology of pyrrolizidine alkaloids.
 Most hepatotoxic alkaloids are esters of molecules similar to
that shown in Fig. 1(b) (1-hydroxymethyl-1:2-dehydro-
pyrrolizidine). However, a few hepatotoxic alkaloids are esters of
the amino-alcohol otonecine, e.g., petasitenine (Fig. 2, No.7).
The unsaturated pyrrolizidine nucleus itself is not toxic, but
esters of branched-chain acids are. Ester linkages may be at
positions 9, 7, or (rarely) 6. Some esters have an "open"
molecule, e.g., heliotrine, whereas others are macrocyclic
diesters, e.g., monocrotaline and retrosine. Examples of some
pyrrolizidine alkaloid structures are shown in Fig. 2.
The ring nucleus contains a double bond at the 1:2 position,
which is essential for the toxic effects of the alkaloid, but not
for unrelated effects.
1. Echimidine
Chemical structure:
Most hepatotoxic alkaloids are esters of molecules similar to
that shown in Fig. 1(b) (1-hydroxymethyl-1:2-dehydro-
pyrrolizidine). However, a few hepatotoxic alkaloids are esters of
the amino-alcohol otonecine, e.g., petasitenine (Fig. 2, No.7).
The unsaturated pyrrolizidine nucleus itself is not toxic, but
esters of branched-chain acids are. Ester linkages may be at
positions 9, 7, or (rarely) 6. Some esters have an "open"
molecule, e.g., heliotrine, whereas others are macrocyclic
diesters, e.g., monocrotaline and retrosine. Examples of some
pyrrolizidine alkaloid structures are shown in Fig. 2.
The ring nucleus contains a double bond at the 1:2 position,
which is essential for the toxic effects of the alkaloid, but not
for unrelated effects.
1. Echimidine
Chemical structure:
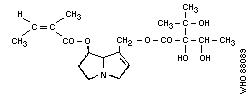 Chemical formula: C20H31NO7
Relative molecular mass: 397
CAS registry number:
Chemical formula: C20H31NO7
Relative molecular mass: 397
CAS registry number:  Chemical formula: C16H27NO5
Relative molecular mass: 313
CAS registry number:
Chemical formula: C16H27NO5
Relative molecular mass: 313
CAS registry number:  Chemical formula: C15H25NO6
Relative molecular mass: 315
CAS registry number:
Chemical formula: C15H25NO6
Relative molecular mass: 315
CAS registry number:  Chemical formula: C18H25NO6
Relative molecular mass: 351
CAS registry number:
Chemical formula: C18H25NO6
Relative molecular mass: 351
CAS registry number:  Chemical structure:
Chemical formula: C21H33NO7
Relative molecular mass: 411
CAS registry number:
Chemical structure:
Chemical formula: C21H33NO7
Relative molecular mass: 411
CAS registry number:  Chemical structure:
Chemical formula: C16H23NO6
Relative molecular mass: 325
CAS registry number:
Chemical structure:
Chemical formula: C16H23NO6
Relative molecular mass: 325
CAS registry number:  Chemical structure:
Chemical formula: C19H27NO7
Relative molecular mass: 381
CAS registry number:
Chemical structure:
Chemical formula: C19H27NO7
Relative molecular mass: 381
CAS registry number:  Chemical structure:
Chemical formula: C18H25NO6
Relative molecular mass: 351
CAS registry number:
Chemical structure:
Chemical formula: C18H25NO6
Relative molecular mass: 351
CAS registry number:  Chemical structure:
Chemical formula: C18H25NO5
Relative molecular mass: 335
CAS registry number:
Chemical structure:
Chemical formula: C18H25NO5
Relative molecular mass: 335
CAS registry number:  Chemical structure:
hemical formula: C20H31NO6
Relative molecular mass: 381
CAS registry number:
Chemical structure:
hemical formula: C20H31NO6
Relative molecular mass: 381
CAS registry number:  Chemical structure:
Chemical formula: C18H27NO6
Relative molecular mass: 353
CAS registry number:
Chemical structure:
Chemical formula: C18H27NO6
Relative molecular mass: 353
CAS registry number:  Chemical structure:
Chemical formula: C18H27NO5
Relative molecular mass: 337
CAS registry number:
Chemical structure:
Chemical formula: C18H27NO5
Relative molecular mass: 337
CAS registry number:  Substitution at the a position of the acid and esterification of
the C-7 hydroxy group both enhance the toxicity of the alkaloid
(Robins, 1982).
A group of related alkaloids, isolated from Senecio species by
Bohlmann et al. (1979), have non-basic pyrrolic structures similar
to those of toxic pyrrolizidine alkaloid metabolites, but they are
chemically deactivated by the presence of a carbonyl group at
position 3 of the pyrrolizidine nucleus, e.g., senaetnine (Fig. 4).
Senaetnine does not possess the acute hepatotoxic characteristics
of basic pyrrolizidine alkaloids. However, it had a direct
irritant action on tissues near the site of intraperitoneal
administration and caused damage to pulmonary vascular tissue when
given intraveinous to rats (Mattocks & Driver, 1987).
Substitution at the a position of the acid and esterification of
the C-7 hydroxy group both enhance the toxicity of the alkaloid
(Robins, 1982).
A group of related alkaloids, isolated from Senecio species by
Bohlmann et al. (1979), have non-basic pyrrolic structures similar
to those of toxic pyrrolizidine alkaloid metabolites, but they are
chemically deactivated by the presence of a carbonyl group at
position 3 of the pyrrolizidine nucleus, e.g., senaetnine (Fig. 4).
Senaetnine does not possess the acute hepatotoxic characteristics
of basic pyrrolizidine alkaloids. However, it had a direct
irritant action on tissues near the site of intraperitoneal
administration and caused damage to pulmonary vascular tissue when
given intraveinous to rats (Mattocks & Driver, 1987).
 The alkaloids are fairly stable chemically, but the ester
groups may undergo hydrolysis under alkaline conditions. Some
alkaloids in plant material may decompose during drying (Bull et
al., 1968), but others appear to be stable under similar conditions
(Pedersen, 1975; Birecka et al., 1980). The N-oxides of
unsaturated pyrrolizidines are more readily decomposed by heat than
the basic alkaloids, especially when dry. However, the stability
of the alkaloids and N-oxides in hot water as, for example, in
cooking, is not known.
Some pyrrolizidine alkaloids have a limited water solubility,
unless neutralized with acid; but others (e.g., indicine), and all
the N-oxides, are readily soluble.
2.2 Analytical Methods
When analysing for PAs, it is important to recognize that this
group consists of many different compounds (section 2.1) and that
these often occur as mixtures in plants or in materials of plant
origin. They may vary in structure, relative molecular mass,
response to analytical procedures, and toxicity. Both basic
alkaloids and corresponding N-oxides may be present at the same
time. Thus, where such mixtures are present, analyses will
inevitably be approximate, unless the individual components are
separated and identified.
Nevertheless, such estimates can be useful. In particular, all
hepatotoxic PAs are unsaturated in the sense that they possess a
1:2-double bond in the pyrrolizidine nucleus, and analytical
methods that are specific for this structure can be of value in
screening for potential toxicity. A simple qualitative field test
for screening plant materials for the presence of such alkaloids
and their N-oxides, without the need of high technology equipment,
is described in section 2.2.2.5.
2.2.1 Extraction
2.2.1.1 Plant tissue
Pyrrolizidine alkaloids are usually extracted from dried,
milled plant material with hot or cold alcohol. The alcohol is
evaporated, the bases taken up in dilute acid, and fats extracted
with ether or petroleum. It is usual, at this stage, to reduce any
N-oxides present to the corresponding basic alkaloids with zinc,
before making the solution alkaline and extracting the alkaloids
with chloroform (Koekemoer & Warren, 1951). Alternatively, alcohol
can be continuously circulated through the plant material and then
cation exchange resin, and the alkaloids subsequently eluted from
the resin (Mattocks, 1961; Deagen & Deinzer, 1977). PAs can also
be extracted by soaking plant material in dilute aqueous acid
(Briggs et al., 1965; Craig et al., 1984).
2.2.1.2 Biological fluids and tissues
Pyrrolizidine alkaloids have been extracted for analytical
purposes from honey (Deinzer et al., 1977), milk (Dickinson et al.,
1976), blood-plasma (Ames & Powis, 1978; McComish et al., 1980),
urine (Mattocks, 1967a; Jago et al., 1969; Evans et al., 1979), and
bile (Jago et al., 1969; Lafranconi et al., 1985).
When attempting to isolate PAs from animal tissues, it must be
appreciated that the toxic alkaloids are often metabolized very
rapidly in animals, so that the amounts that are recoverable
(except from urine), only a few hours after alkaloid ingestion, may
be extremely small. Various methods have been used to separate
PAs, but some mixtures are extremely difficult to separate. On the
analytical scale, the most useful methods are thin-layer
chromatography (TLC), high-performance liquid chromatography
(HPLC), and gas chromatography (GC) (section 2.2.2).
2.2.2 Analysis for pyrrolizidine alkaloids
2.2.2.1 Thin-layer chromatography (TLC)
For TLC, silica plates are usually used, eluted with chloroform:
methanol:aqueous ammonia mixtures (Sharma et al., 1965; Chalmers
et al., 1965); solvents suitable for the N-oxides, which
are more water-soluble, have been described by Mattocks (1967b)
and Wagner et al. (1981). The most sensitive methods for
detecting PAs on TLC are those using Ehrlich reagent
(4-dimethylaminobenzaldehyde) (Mattocks, 1967b). The unsaturated
alkaloids are best visualized by spraying the plates first with a
solution of orthochloranil, then with Ehrlich reagent, heating
after each spray (Molyneux & Roitman, 1980). The N-oxides of
unsaturated pyrrolizidines are detected by spraying a solution of
acetic anhydride, heating the plate, and then spraying Ehrlich
reagent (Mattocks, 1967b).
Pyrrolizidine alkaloids with a saturated base moiety must be
detected in other ways (which are not specific for pyrrolizidines),
e.g., by exposing the dried plates to iodine vapour, or by spraying
with an iodobismuth (Dragendorff) reagent (Munier, 1953).
2.2.2.2 High-performance liquid chromatography (HPLC)
Analytical or preparative scale HPLC separation of
pyrrolizidine alkaloids has been described by Segall (1979a,b) and
Dimenna et al. (1980), and an improved method has been reported by
Ramsdell & Buhler (1981). Alkaloids from Symphytum officinale
(comfrey) have been separated on an analytical scale by Tittel et
al. (1979), and partially separated on a preparative scale by
Huizing et al. (1981). UV detectors are usually used for the HPLC
of pyrrolizidine compounds (Mattocks, 1986).
2.2.2.3 Gas chromatography (GC) and mass spectrometry (MS)
The GC characterization of PAs using packed columns has been
described by Chalmers et al. (1965) and Wiedenfeld et al. (1981).
Mixtures of alkaloids from comfrey ( Symphytum sp.), normally hard
to separate, were resolved by Culvenor et al. (1980a) and Frahn et
al. (1980) by GC of the methylboronate derivatives.
Gas chromatography combined with mass spectrometry (GC-MS) has
become a valuable and highly sensitive means for both the
identification and the quantitative determination of pyrrolizidine
alkaloids. Thus, alkaloids extracted from honey were separated and
identified by Deinzer et al. (1977) and (as butylboronate
derivatives) by Culvenor et al. (1981). Deinzer et al. (1978)
described a method for the recognition (but not the individual
identification) of retronecine-based pyrrolizidine alkaloids, by
hydrolysing them to retronecine (the amino alcohol moiety) followed
by GC-MS of its bis-trifluoroacetate. The use of capillary GC has
greatly improved the sensitivity of pyrrolizidine alkaloid
analysis, especially when used with MS (Luthy et al., 1981). The
MS of pyrrolizidine compounds has been reviewed (Bull et al., 1968;
Mattocks, 1986).
Pyrrolizidine N-oxides generally undergo thermal decomposition,
when subjected to GC, but they can first be reduced to the
corresponding basic alkaloids (Koekemoer & Warren, 1951).
Alternatively they may be derivatised. Thus, trimethylsilylation
of indicine N-oxide or heliotrine N-oxide can lead either to the
trimethylsilyl (TMS) derivative of the parent alkaloid or to the
TMS derivative of the dehydro-alkaloid (pyrrolic derivative),
depending on the reagents used, and these products will run
successfully on GC-MS (Evans et al., 1979, 1980).
2.2.2.4 Nuclear magnetic resonance (NMR) spectrometry
A convenient, but relatively insensitive, method, specifically
for the determination of unsaturated PAs, has been described by
Molyneux et al. (1979). The basic alkaloids are extracted, then
subjected to NMR spectrometry along with an internal standard
( p-dinitrobenzene). This enables quantitative measurements to be
made of the signal(s) representing the H2 proton(s) in unsaturated
pyrrolizidines, and thus the alkaloid(s) can be determined.
Quantitative NMR analysis of pyrrolizidine alkaloid mixtures from
Senecio vulgaris has been described by Pieters & Vlietinck (1985)
and compared with an HPLC method by the same authors (1986).
Qualitative aspects of the NMR spectrometry of pyrrolizidine
alkaloids have been reviewed by Bull et al. (1968) and Mattocks
(1986).
2.2.2.5 The Ehrlich reaction
This method (Mattocks, 1967a, 1968b) is specific for
unsaturated pyrrolizidine alkaloids and is not suitable for other
alkaloids. Thus, it is the most useful colorimetric method for
potentially hepatotoxic pyrrolizidine compounds. The procedure
converts the alkaloid into its N-oxide, using hydrogen peroxide.
The product reacts with acetic anhydride to form a pyrrolic
derivative (dehydro-alkaloid) that gives a magenta colour with a
specially modified Ehrlich reagent. The latter contains boron
trifluoride to give maximum sensitivity. As little as 5 µg of most
unsaturated pyrrolizidines can be measured by this method. If the
oxidation stage is omitted, only the unsaturated pyrrolizidine
N-oxides can be determined. The determination of pyrrolizidine
N-oxides has also been discussed by Mattocks (1971b).
A simplification of the above colorimetric procedure was
described by Mattocks (1971d) to provide a qualitative test that
could be used to screen large numbers of plant samples for the
presence of unsaturated pyrrolizidine alkaloid N-oxides. An
improved version of this field test is now available (Mattocks &
Jukes, 1987). It is suitable for any plant parts, such as leaves,
stems, flowers, seeds, or roots, or materials of plant origin, such
as cereals or herbal teas, but has not yet been applied to cooked
food.
The plant material (0.2 - 1 g) is extracted by grinding it with
aqueous ascorbic acid (5%) and a small amount of sand. The
solution is filtered and divided into two equal portions ("test"
and "blank"). An aqueous solution (0.2 ml) of sodium nitroprusside
(5%) containing sodium hydroxide (10-3 mol) is added to the "test"
sample. Both portions are heated for approximately 1 min at 70 -
80 °C; then Ehrlich reagent is added and heating is continued for
1 min. The Ehrlich reagent contains 4-dimethylaminobenzaldehyde
(5 g) dissolved in a mixture of acetic acid (60 ml), water (30 ml),
and 60% perchloric acid (10 ml). A magenta colour in the "test"
compared with the "blank" indicates the presence of an unsaturated
PA N-oxide. The "blank" may show a colour if the plant contains
compounds, such as indoles or pyrroles, which can themselves give a
colour with Ehrlich reagent. The intensity of colour in the
"sample" compared with the "blank" can give a rough idea of the
amount of alkaloids present, and indicate whether further chemical
or toxicological testing of the plant material is adviseable.
In practice, the majority of PA-containing plants contain
enough alkaloid in the N-oxide form (often a large proportion) to
react positively in this test. The main exceptions are some seeds
(Crotalaria), which may contain much alkaloid base, but little or
no N-oxide. These (and any other sample not containing
chlorophyll) can be tested for basic PAs by grinding them with
chloroform, heating the filtered extract with a solution (0.1 ml)
of orthochloranil (0.5%) in acetonitrile, and then heating it with
Ehrlich reagent. A magenta colour indicates the presence of an
unsaturated PA. Non-toxic pyrrolizidine alkaloids having a
saturated pyrrolizidine nucleus, and pyrrolizidine alkaloids that
are otonecine esters, such as petasitenine, will not respond to
this test.
2.2.2.6 Indicator dyes
A method generally applicable to tertiary bases has been
adapted for pyrrolizidine alkaloids by Birecka et al. (1981). It
is sensitive, but is not specific for this group of alkaloids, and
it does not distinguish between the saturated and unsaturated
alkaloids. A chloroform solution of the alkaloid is shaken with
acidified aqueous methyl orange. The yellow alkaloid:dye complex
is subsequently released from the chloroform phase, using ethanolic
sulfuric acid, and measured spectrophotometrically.
2.2.2.7 Direct weighing
An insensitive way to determine the alkaloids in, for example,
a plant sample, providing enough is available, is to extract the
alkaloids (section 2.2.1) and weigh them. This will provide a
rough measure of the total bases present in the sample; however,
these may not necessarily be PAs. Nevertheless, the sample can
then be subjected to further tests, e.g., GC-MC, nuclear magnetic
resonance (NMR), or colorimetric analysis. Furthermore,
pyrrolizidine N-oxides are generally too water soluble to be
appreciably extractable from aqueous solution by chloroform. Thus,
if two portions of the sample are extracted, and one of them is
reduced to convert N-oxides to bases, the weight difference between
the two products will represent the alkaloid existing in the form
of N-oxide in the original sample.
2.3 Determination of Metabolites in Animal Tissues
Important metabolites of toxic pyrrolizidine alkaloids in
animals include "pyrrolic" derivatives (dehydro-alkaloids) and
N-oxides. A procedure for measuring pyrrolic metabolites in tissue
samples (such as liver or lung) has been described by Mattocks &
White (1970). The sample (usually 0.5 g) is homogenized in an
ethanolic solution of mercuric chloride; the solids are separated
by centrifugation and heated with Ehrlich reagent to give a soluble
colour that can be measured spectrophotometrically.
The measurement of pyrrolic and N-oxide metabolites, formed by
the action of hepatic microsomal preparations on PAs in vitro, is
an improvement described by Mattocks & Bird (1983).
3. SOURCES AND PATHWAYS OF EXPOSURE
3.1 Hepatotoxic Pyrrolizidine Alkaloids and Their Sources
Plants constitute the only natural source of pyrrolizidine
alkaloids (PAs) that cause toxic reactions in man and animals. PAs
occur in a number of species in the families Boraginaceae,
Compositae, Leguminosae (genus Crotalaria), Ranunculaceae (genus
Caltha), and Scrophulariaceae (genus Castilleja) (Table 1). The
most important genera of PA-containing toxic plants are Crotalaria
(Leguminosae), Senecio (Compositae), Heliotropium, Trichodesma,
Amsinckia, Echium, and Symphytum (Boraginaceae) (Hooper, 1978).
The recorded cases of human toxicity have mainly been caused by at
least 12 different pyrrolizidine alkaloids, mostly derived from
Heliotropium, Senecio, and Crotalaria genera. The Senecio spp.
grow throughout the world; the Crotalaria spp. are mainly found in
the tropics and subtropics (Culvenor, 1980).
Table 1. List of plant genera containing toxic pyrrolizidine alkaloids
(with number of species investigated)
-------------------------------------------------------------------------------------
Family Genera
-------------------------------------------------------------------------------------
Apocynaceae Fernaldia (1), Parsonsia (4),
Boraginaceae Alkanna (1), Amsinckia (4), Anchusa (2), Asperugo (1), Borago (1),
Caccinia (1), Cynoglossum (9), Echium (3), Hackelia (1),
Heliotropium (25), Lappula (2), Lindelofia (7), Lithosperum (1),
Macrotomia (1), Messerschmidtia (1), Myosotis (2), Paracaryum (1),
Paracynoglossum (1), Rindera (5), Solenanthus (4), Symphytum (7),
Tournefortia (2), Trachelanthus (2), Trichodesma (2), Ulugbekia (1)
Compositae Adenostyles (3), Brachyglottis (1), Cacalia (4), Conoclinium (1),
Crassocephalum (1), Doronicum (2), Echinacea (2), Emilia (2),
Erechtites (1), Eupatorium (8), Farfugium (1), Gynura (2),
Ligularia (5), Petasites (4), Senecio (142), Syneilesis (1),
Tussilago (1)
Leguminosae Crotalaria (60)
Ranunculaceae Caltha (2)
Scrophulariaceae Castilleja (1)
-------------------------------------------------------------------------------------
An alphabetical list of pyrrolizidine alkaloids with their
plant sources has been published by Smith & Culvenor (1981) and
Mattocks (1986). An updated version is attached as Appendix I.
The plant genera containing toxic PAs are listed in Table 1
indicating the number of species investigated for PAs. A
comprehensive list of species of plants belonging to each of these
genera, the alkaloids isolated from each, and the part of the plant
containing the alkaloid are presented in Appendix II. Table 1 in
Appendix II includes species known to contain alkaloids of proved
hepatotoxicity, or of a molecular structure that would make them
very probably hepatotoxic. Table 2 in Appendix II includes species
containing pyrrolizidine amino-alcohols or esters, which, while not
having all the features of hepatotoxicity, would need only minor
structural modifications to render them hepatotoxic. Plants of the
same taxonomic groups as the plants of proven hepatotoxicity are
listed in part (a) of the table. There is a possibility that, on
further examination, hepatotoxic alkaloids may be found, as minor
constituents, in strains or parts of these plants not yet
investigated or under specific conditions of growth. It should be
noted that the species that have been investigated and are listed
are only few compared with the total number of species in each
genera. It has been recommended by Smith & Culvenor (1981) that it
would be prudent to regard all species in the family Boraginaceae
and the genera Crotalaria, Senecio, and Eupatorium as potentially
hepatotoxic.
It is pertinent to note that the alkaloid content in different
parts of the plant (e.g., roots, leaves, stalks, flowers, and buds)
varies and is subject to fluctuations according to the climate,
soil conditions, and time of harvesting (Danninger et al., 1983;
Hartmann & Zimmer, 1986). Mattocks (1980) demonstrated that the
alkaloid content of the leaves of Symphytum spp. (Russian
comfrey), which are used as an item of food, varies with their
maturity. The toxic PA content is highest at the beginning of the
vegetative period and declines as the leaves mature. The PA
content of the roots is much higher than that of the leaves, and
dried leaves contain a higher concentration than fresh leaves
(Mattocks, 1986). According to Danninger et al. (1983), in some
species (Symphytum asperum), relatively long storage may lead to a
reduction in the alkaloid content, presumably because enzymes are
released during drying. Candrian et al. (1984b) studied the
stability of PAs in hay and silage containing various amounts of
Senecio alpinus. The PA content of hay remained constant for
several months, but the PAs in silage were mainly degraded.
However, the degradation of PAs was much less complete in the lower
concentration range. A quantitatively significant PA-degradation
product in silage was identified as retronecine. Silage with an
S. alpinus percentage of 3.5 - 23 still contained macrocyclic PAs at
a concentration of about 20 mg/kg wet weight. Such silage was not
considered safe for cattle bearing in mind that a 600-kg calf eats
about 30 kg silage/day, amounting approximately to a daily intake
of about 1 mg PAs/kg body weight. In feeding trials with Senecio
jacobaea, Johnson (1979) found that the minimum lethal dose for
cattle was between 1 and 2 mg PAs/kg body weight per day.
PAs known to have been associated with instances of human toxic
liver disease in different parts of the world are listed in Table
2. Two groups of alkaloids that, according to Culvenor (1983), are
consumed in significant amounts by people in different parts of the
world include:
(a) Echimidine, acetyllycopsamine, and related alkaloids
(many countries)
Leaves of plants of the Symphytum sp. ( Symphytum officinale
(comfrey) and Symphytum x uplandicum) are used traditionally as a
salad and as a medicinal herb in Australia, many countries of
Europe, and the USA. S. officinale has been shown to be
carcinogenic for rats (Hirono et al., 1978). Leaves of Russian
comfrey contain a concentration of alkaloids (mainly echimidine) of
0.1 - 1.5 g/kg. The highest level of daily consumption of the
alkaloids has been estimated to be 5 - 6 mg (Culvenor, 1983).
(b) Echimidine and related alkaloids (Australia)
PAs derived from Echium plantagineum, with echimidine as the
major component, have been found in honey secreted by bees feeding
on the plant (Culvenor et al., 1981). The plant is a major source
of honey (section 3.3.4).
3.2 Pneumotoxic and Other Toxic Pyrrolizidine Alkaloids
Not all hepatotoxic alkaloids are pneumotoxic. The commonest
ones used to produce experimental lung injury are fulvine (Barnes
et al., 1964; Kay et al., 1971a; Wagenvoort et al., 1974a,b) and
monocrotaline (Lalich & Ehrhart, 1962; Chesney & Allen, 1973b;
Huxtable et al., 1977). These are also the most active (Mattocks,
1986). The seeds of Crotalaria spectabilis, which contain
monocrotaline, have also been used to study pneumotoxic effects on
experimental animals (Turner & Lalich, 1965; Kay & Heath, 1966; Kay
et al., 1967a) and C. spectabilis has been called the pulmonary
hypertension plant (Kay & Heath, 1969), because of the pulmonary
hypertensionogenic properties of the PAs it contains. Culvenor et
al. (1976a) screened 62 PAs for hepatotoxicity and pneumotoxicity.
Chronic lung lesions were produced by most compounds that induced
chronic liver lesions, though high doses were required in some
instances. It is possible that chronic lung lesions may not occur
in experimental animals because of early death due to acute
toxicity. However, the authors identified a number of PAs that
were particularly prone to produce chronic lung damage in rats
including crispatine, senecionine, seneciphylline, and usaramine
(12-membered macrocyclic, retronecine diesters), anacrotine and
madurensine (crotonecine esters), and the heliotridine esters,
heliosupine, lasiocarpine, and rinderine.
The molecular structure-activity requirements for
pneumotoxicity are the same as those for hepatotoxicity. This is
consistent with their both being caused by the same toxic
metabolites and by the metabolic activation of the alkaloids in the
liver cells to form a reactive pyrrolic dehydro-alkaloid (Culvenor
et al., 1976a).
Trichodesmine and incanine, found in the seeds of Trichodesma
incanum (Yunusov & Plekhanova, 1959), are believed to have been
the causative factors of the "Ozhalangar encephalitis" that was
endemic in Uzbekistan, USSR (1942 - 51), in which the symptoms and
signs were related primarily to the central nervous system
(Shtenberg & Orlova, 1955) (section 7.7).
Table 2. Instances of human toxicity caused by pyrrolizidine alkaloidsa
Principal Plant Country/ Cause of intake Reference
alkaloid Region
Heliotrine and Heliotropium Afghanistan contamination Tandon & Tandon
other alkaloids popovii (1975); Tandon,
similar to B.N. et al.
lasiocarpine (1978); Tandon,
H.D. et al.
(1978);
Mohabbat et al.
(1976)
Senecionine Senecio South contamination Wilmot &
illiciformis; Africa Robertson
Senecio-burchelli (1920)
Senecio spp. South contamination Selzer &
Africa Parker (1951)
Alkaloids of Crotalaria Ecuador medicine Lyford et al.
trichodesmine juncea (1976)
and senecionine
type
Heliotrine and Heliotropium Hong Kong medicine Kumana et al.
lasiocarpine lasiocarpum (1985);
Culvenor et al.
(1986)
Table 2. (cont'd)
Principal Plant Country/ Cause of intake Reference
alkaloid Region
Crotananine and Crotalaria India contamination Tandon, R.K.
cronaburmine nana et al. (1976);
Krishnamachari
et al. (1977);
Siddiqui et al.
(1978a,b)
Heliotrine Heliotropium India medicine Datta et al.
N-oxide eichwaldii (1978a,b)
Monocrotaline Crotalaria West Indies medicine Bras et al.
fulvine retusa; (1954, 1957)
Crotalaria
fulva Stuart & Bras
(1957)
Ilex sp. United medicine McGee et al.
Kingdom (1976)
Riddelline Senecio USA medicine Stillman et al.
retrorsine longilobus (1977); Fox et
N-oxide al. (1978);
(with others) Huxtable (1980)
Indicine N-oxide purified USA medicine Letendre et al.
chemical (1984)
Symphytine, Symphytum sp. USA medicine Ridker et al.
symglandine, and (1985);
other symphytum Huxtable
alkaloids et al (1986)
Table 2. (cont'd)
Principal Plant Country/ Cause of intake Reference
alkaloid Region
Lasiocarpine and Heliotropium USSR contamination Dubrovinskii
heliotrine lasiocarpum (1952);
Mnushkin
(1952)
Trichodesmine and Trichodesma USSR contamination Shtenberg &
incanine incanum Orlova (1955);
Yunosov &
Plekhanova
(1959)
a Adapted from: Culvenor (1983) and Mattocks (1986). Refer also to Table 15 for
details and section 7.
3.3 Pathways of Exposure
Naturally-occurring animal disease is caused by the alkaloid-
containing plants growing in fields and pastures or being fed
accidentally as fodder. They are mostly herbaceous or small shrubs
and many thrive in dry and arid climates. One such plant
containing toxic PA alkaloids has been reported to grow in the
western desert of Egypt (Hammouda et al., 1984). The growth of
this group of plants is particularly prolific during, and
following, periods of drought, as has been reported in association
with the outbreaks of human disease in Afghanistan (Tandon &
Tandon, 1975; Mohabbat et al., 1976) and India (Tandon, B.N. et
al., 1976). Alkaloid-containing plants are widespread in the
tropics, especially Crotalaria, of which there are over 300
species in Africa. Ordinarily, the alkaloid-containing plants have
a bitter taste and grazing animals will reject them, unless their
normal fodder is scarce. However, PAs often occur largely as
N-oxides, which are said not to be bitter, and plants containing
PAs are readily eaten by some animal species.
Human intoxication may result from the ingestion of the toxic
substance in either contamined food or herbal infusion.
3.3.1 Contamination of staple food crops
The products of pyrrolizidine alkaloid-containing plants,
generally seeds, may contaminate the staple food and may be eaten
over long periods of time. The fact that these plants may cause
disease is generally not recognized by the people and such
contamination is known to have resulted in large-scale outbreaks of
poisoning (Dubrovinskii, 1952; Mnushkin, 1952; Shtenberg & Orlova,
1955; Tandon & Tandon, 1975; Mohabbat et al., 1976; Tandon, B.N. et
al., 1976, 1977; Tandon, R.K. et al., 1976; Krishnamachari et al.,
1977; Tandon, H.D. et al., 1977) (Table 2, section 3.1).
3.3.2 Herbal infusions
Plants have been used traditionally for medicinal purposes all
over the world. Herbs have been the mainstay of the indigenous
systems of medicine, especially in China, Greece, and India, since
ancient times. Table 3 includes a list of some plants that are
suspected, or known, to contain PAs and have been used as herbal
medicines in different countries (Mattocks, 1986).
Several PA-containing plants are included among the list of
plants used in indigenous systems of medicine in India (Chopra,
1933). As a part of a research study on the etiological factors of
chronic liver disease in Sri Lanka, Arseculeratne et al. (1981)
chemically screened the first 50 plants used in Sri Lanka's
traditional medicine pharmacopoaea, and found that 3 of them
contained PAs. All 3 were hepatotoxic in rats. Of the 3, the
presence of alkaloids in Cassia auriculata and that of PAs in
Hollarhena antidysenterica had not previously been recorded. It
should be noted that the amount of experimental plant material used
in this study was approximately 6.5 g/kg body weight per day, in
contrast to the approximate intake by a human being estimated to be
in the range of 0.3 - 0.6 g/kg body weight per day. Some, but not
all, of the plants reported to be etiological agents in human cases
of veno-occlusive disease can be found in an inventory of medicinal
plants used in different countries (WHO, 1980), which also
indicates the countries that they are used in. The above lists may
not be complete as many such plants may be used in folk medicine
but have not been mentioned in the scientific literature. However,
the lists do indicate the wide and varied use of such toxic herbs
in all parts of the world.
Lately, there has been a growing interest in the developed
countries in organically grown products for food, as well as home
remedies (Table 3), and some of the PA-containing herbs have been
freely available in herbal shops (Schoental, 1968; Burns, 1972).
Danninger et al. (1983) listed plants containing PAs that are
commonly used in the Federal Republic of Germany as medicaments
(Table 4). He also listed 9 plants in which alkaloids have only
been identified qualitatively, the toxicity of which has not been,
or has been insufficiently, investigated (Table 5). Similarly,
Roitman (1983) listed 10 plants, in which the presence of PAs is
suspected or has been proved and which are used as herbal teas in
the USA. The lists include 10 plants containing PAs, most of which
have been proved hepatotoxic experimentally, some having highly
carcinogenic promoter activity. Some of these alkaloids have been
associated with human case reports of PA toxicity. The more recent
reports (Table 2) of instances of PA poisoning through the use of
herbal medicines are from developed countries (Lyford et al., 1976;
Stillman et al., 1977; Fox et al., 1978; Kumana et al., 1985;
Ridker et al., 1985). Such use of the herbs is the reason that
veno-occlusive disease is endemic in Jamaica (Bras et al., 1954;
Jellife et al., 1954a,b; Bras & Watler, 1955; Stuart & Bras, 1955,
1957). There are obvious difficulties in exercising any kind of
control to restrict this use only to plants that have been tested
and certified as safe for human use. It is impossible to identify
many such herbs, as they are sold as plants or their amorphous
products in the herbal shops.
Table 3. Some plants containing (or suspected of containing) PAs, which have been used
by people either as herbal medicines (M) or foods (F)
Plant Mode Country Referencea
of use or region
BORAGINACEAE
Anchusa officinalis M Europe Broch-Due & Aasen (1980) B
Borago officinalis M USA Delorme et al. (1977) A
Cynoglossum M East Africa Schoental & Coady (1968) A
geometricum
Cynoglossum M Iran Coady (1973) B
officinale
Heliotropium M India Gandhi et al. (1966a); B
eichwaldii Datta et al. (1978a,b) A
H. europaeum M India, Greece IARC (1976) A
H. lasiocarpum M Hong Kong Kumana et al. (1985); A
Culvenor et al. (1986) A
H. indicum M India, Africa, Schoental (1968a); B
South America, Hoque et al. (1976) B
and elsewhere
H. ramossissimum M Arabia Macksad et al. (1970); B
(ramram) Coady (1973) B
H. supinum M Tanzania Schoental & Coady (1968) A
Table 3 (contd.)
Plant Mode Country Referencea
of use or region
Pulmonaria spp. M USA Delorme et al. (1977) A
Symphytum officinale F, M Japan and Hirono et al. (1978, 1979b) A
M USA Furuya & Hikichi (1971); A
Delorme et al. (1977) A
S. x uplandicum F, M General Hills (1976) B
USA Culvenor et al. (1980a,b) A
S. asperum M USA Pedersen (1975) A
COMPOSITAE
Cacalia decomposita M USA Sullivan (1981) B
(matarique)
C. yatabei F Japan Hikichi & Furuya (1978) B
Farfugium japonicum M Japan Furuya et al. (1971) B
Ligularia dentata F Japan Asada & Furuya (1984) B
Petasites japonicus F, M Japan Hirono et al. (1973, 1979b) A
Senecio abyssinicus M Nigeria Williams & Schoental (1970) B
S. aureus M USA Wade (1977) B
Table 3 (contd.)
Plant Mode Country Referencea
of use or region
S. bupleuroides M Africa Watt & Breyer-Brandwijk (1962) A
S. burchelli F, M South Africa Rose (1972) A
S. coronatus M South Africa Rose (1972) A
S. discolor M Jamaica Asprey & Thornton (1955) B
S. doronicum M Germany Roeder et al. (1980a) B
S. inaequidens F South Africa Rose (1972) B
S. jacobaea M Europe Schoental & Pullinger (1972); B
(ragwort) Wade (1977) B
S. longilobus M USA Stillman et al. (1977); A
(S. douglassi) Huxtable (1979a) B
S. monoensis M USA Huxtable (1980) A
S. nemorensis M Germany Habs et al. (1982) A
spp. fuchsii
S. pierotti F Japan Asada & Furuya (1982) B
S. retrorsus M South Africa Rose (1972) A
(S. latifolius)
Table 3 (contd.)
Plant Mode Country Referencea
of use or region
S. vulgaris M Europe Watt & Breyer-Brandwijk (1962) A
(common groundsel)
Netherlands Wade (1977) B
M Iran Coady (1973) B
Syneilesis palmata F Japan Hikichi & Furuya (1976) B
Trichodesma africana M Asia Omar et al. (1983) B
Tussilago farfara M Japan Culvenor et al. (1976a) A
(coltsfoot)
M China Hirono et al. (1976b) A
M Norway Borka & Onshuus (1979) B
M USA Borka & Onshuus (1979); B
Culvenor et al. (1976b); B
LEGUMINOSAE
Crotalaria brevidens F East Africa Coady (1973) B
C. fulva M Jamaica Barnes et al. (1964); A
McLean (1970, 1974) A
Table 3 (contd.)
Plant Mode Country Referencea
of use or region
C. incana M East Africa Schoental & Coady (1968) A
Watt & Breyer-Brandwijk A
(1962)
C. laburnifolia M Tanzania Schoental & Coady (1968) A
F Asia Coady (1973) B
C. mucronata M Tanzania Coady (1973) B
C. recta M, F Tanzania Schoental & Coady (1968); A
Coady (1973) B
C. retusa M, F Africa IARC (1976) A
India Watt & Breyer-Brandwijk (1962) A
C. verrucosa M Sri Lanka Arseculeratne et al. (1981) A
a A = Reference in the reference list of this document.
B = Reference in Mattocks (1986).
Manufactured preparations may also contain PA-containing herbs,
e.g., comfrey-pepsin capsules sold as a digestive aid (Huxtable et
al., 1986).
3.3.3 Use of PA-containing plants as food
Several PA-containing plants are used as food as can be seen in
Table 3 (Mattocks, 1986). Petasites japonicus Maxim, Tussilago
farfara L. (coltsfoot), and Symphytum officinale L. (comfrey or
Russian comfrey) are known as edible plants in Japan, and have been
proved to contain carcinogenic pyrrolizidine alkaloids (Hirono et
al., 1973, 1979a,b). The young flower-stalks of P. japonicus and
the buds of coltsfoot have been used in Japan as human food or
herbal remedies. The leaf and root of comfrey are also used as an
edible vegetable or tonic (Hirono et al., 1978) in Japan and other
countries (Culvenor, 1985). The carcinogenic PAs in these plants
are petasitenine (P. japonicus), senkirkine (coltsfoot), and the
group including symphytine (comfrey). They were also mutagenic in
the Ames system of Salmonella typhimurium and V79 hamster cell line
and induced transformation in cryo-preserved hamster embryonic
cells (Hirono et al., 1979b). Other such PA-containing plants,
used as food in Japan, include young leaves of Syneilesis palmata,
various Cacalia species, and young Senecio pierotti (Mattocks,
1986). According to Culvenor (1985), consumers of comfrey could be
ingesting up to 5 mg PAs per day. Rose (1972) listed a number of
plants of the genus Senecio that are used as spinach in South
Africa. These include S. burchelli, which is known to have caused
an episode of PA poisoning through the ingestion of contaminated
bread (Wilmot & Robertson, 1920).
3.3.4 Contaminated honey
In the USA, Deinzer et al. (1977) reported the presence of all
PAs contained in Senecio jacobaea (ragwort) and proved to be
hepatotoxic, in the honey secreted by bees feeding on the plant.
The total alkaloid content ranged from 0.3 to 3.9 mg/kg. It has
been estimated that an average annual human intake of honey (600 g)
at the highest alkaloid level quoted would contain less than 3 mg
of PAs (Mattocks, 1986). Culvenor et al. (1981) and Culvenor
(1983, 1985) drew attention to the same potential hazard in honey
from Echium plantagineum, a weed that grows widely in Southern
Australia and is a major source of honey, yielding an estimated
2000 - 3000 tonnes per annum for human consumption. Echimidine is
the major component of the alkaloids of Echium, which are present
in concentrations of up to 1 mg/kg. Culvenor (1983) estimated that
individuals may consume up to 80 g honey/day with a corresponding
alkaloid intake of 80 µg/day, if only the Echium honey were used.
No reports of acute human toxicity through this source are
available.
Table 4. Medicinal plants containing PAs of known hepatotoxicity, reported as commonly
used in the Federal Republic of Germany, and the PAs contained in thema
Family Genus Species Pyrrolizidine
alkaloids
Compositae Eupatorium E. cannabinum amabiline±
(hemp agrimony) supinineb
Petasites P. hybirdus senecionineb,c
integerrimineb
senkirkineb
Senecio S. nemorensis fuchsisenecionine
(groundsel) sp. fuchsii senecionineb,c
(Fuch's groundsel)
S. vulgaris senecionineb,c
(groundsel) seneciophyllineb
retrorsineb
riddellineb,c
S. Jacobaea jacobineb
(ragwort) senecionineb,c
seneciphyllineb
jacoline, jaconine
chlorinated PAsd
S. aureus senecionineb,c
(American golden
ragwort)
Tussilago T. farfara senkirkineb
(coltsfoot) (coltsfoot) senecionineb,c
tussilagine
Table 4 (contd.)
Family Genus Species Pyrrolizidine
alkaloids
Alkanna A. tinctoria 7-angelylretronecine
triangularine
dihydroxytriangularine
Anchusa A. officinalis lycopsamine
Boraginaceae Borago B. officinalis lycopsamine/intermedine±
(borage) acetyllycopsamine/
acetylintermedine
amabiline
supinine
Symphytum S. officinale symphytineb
(comfrey) (comfrey) echimidine(?)
lycopsamine
acetyllycopsamineb
lasiocarpineb,c
heliosupine N-oxide
S. peregrinum lycopsamineb
S. x uplandicum intermedineb
symphytineb
echimidineb
7-acetyllycopsamine
7-acetylintermedine
symlandine
uplandicine
S. asperum asperumine
(prickly comfrey) heliosupine N-oxide
echimidineb
echinatine
Table 4 (contd.)
Family Genus Species Pyrrolizidine
alkaloids
Cynoglossum C. officinale heliosupine N-oxide
(hound's (hound's tongue) echinatine
tongue) acetyl heliosupineb
O-7-angelylhelio-
tridineb
Heliotropium H. europaeum heliotrineb,c,e
(Heliotrope) (common heliotrope) lasiocarpineb,c,e
supinine
heleurine
europine
acetyllasiocarpineb
a Modified from: Danninger et al. (1983).
b Toxic alkaloids.
c Alkaloids known to have caused human toxicity.
d Alkaloids with highly carcinogenic promoter activity.
e Used only in homeopathy.
Table 5. Medicinal plants containing PAs, reported as
commonly used in the Federal Republic of Germany,
the toxicity of which has not been, or has been
insufficiently, investigateda
-----------------------------------------------------------
Family Genus Species
-----------------------------------------------------------
Compositae Eupatorium E. perforatum
Brachyglottis B. repens
Arnica A. montana (mountain arnica)
Boraginaceae Lappula L. intermedia (stickseed)
Pulmonaria P. officinalis (lungwort)
-----------------------------------------------------------
a Modified from: Danninger et al. (1983).
3.3.5 Milk
PAs have been shown to produce toxic effects via transference
into the milk of dams (Schoental, 1959). Retrorsine was
administered orally to 17, and intraperitoneally to 6, lactating
rats weighing 185 - 350 g in 5 - 10 mg doses daily, the first dose
being given during the first 24 h after parturition. The rats
received from 1 to 14 doses, the total intake amounting to
21 - 335 mg/kg body weight. The litters were separated from the
mothers for ´ h following the administration of PA to avoid direct
contamination of the former by licking. Apparently the milk
production was not affected as the stomachs of many of the young,
examined postmortem, were distended with milk. All animals whose
mothers had received a total dose of 138 mg PA or more died within
30 days. Many of the young whose mothers had received smaller
doses survived until they were killed at 6 months. Biopsy of the
liver of the young at various intervals or at autopsy showed marked
changes, even in cases where the mothers did not appear to be
affected. Animals dying at 18 - 30 days showed hydropic or fatty
vacuolation of liver cells. In the liver of animals dying or
killed later, various degrees of haemorrhagic necrosis and increase
in the centrilobular reticulin of the liver, and some thickening of
centrilobular veins were seen. In animals that survived 6 months,
the appearance was less abnormal, but some hyperplastic nodules and
bile-duct proliferation were seen. The lactating rats dosed with
the PAs generally survived longer than the suckling animals and
usually did not show any ill effects, suggesting that the
susceptibility of the suckling rats was greater than that of the
mothers.
Dickinson et al. (1976) demonstrated the presence of PAs in the
milk of dairy cattle fed or dosed with ragwort (Senecio jacobaea).
When 4 cows were administered the dried plant material at levels of
up to 10 g/kg body weight per day through rumen cannula, PA levels
of up to 0.84 mg/kg were observed in the milk. However, only one
(jacoline) of the several PAs contained in the plant was secreted.
Calves, bucket fed on the milk did not show any signs of PA
toxicity.
Dickinson (1980) repeated the study on goats. Four milk goats
were freshly prepared with rumen cannulae. The kids were separated
from their dams and were fed milk twice a day. Dried tansy ragwort
plant material with a PA content of 0.16% (dry weight) was
administered through the cannulae to each goat at a dosage rate of
10 g/kg body weight per day over 125 days. During this period,
each of the 4 kids received milk from their dams at approximately
125 ml/kg per day in addition to ad lib feeding on alfalfa grass
hay. Six PAs were isolated from the plant material: jacobine,
jaconine, jaconline, jacozine, senecionine, and seneciphylline.
Milk samples collected twice daily showed PA contents of
225 - 530 µg/litre with a mean of 381 µg/litre. No apparent health
effects were noted in the kids, and only mild hepatic damage was
suspected in the dams, on the basis of liver function tests. Fifty
percent of the kids were killed after 10 weeks. No lesions of PA
toxicity were seen. The dams were rebred and appeared normal
throughout the gestation period. However, three dams aborted at
almost full term, and the fetuses were born dead. One of the dams
died shortly after parturition and showed evidence of severe liver
damage characteristic of PA toxicity. Another, which delivered
normally, also showed a lesser degree of liver damage at biopsy.
Data relating to PA secretion were compared with similar
earlier data on cows. Mean secretion of PAs in cows appeared much
higher, e.g., 684 µg/litre. The authors concluded that the amount
of PAs secreted in the goat's milk did not cause any serious
deleterious effects in the kids.
Johnson (1976) fed long-term lethal doses of Senecio jacobaea,
by stomach tube, to 6 cows. Feeding started at term or within 30
days post-partum, and continued until what was considered to be a
lethal dose had been fed. The daily dose of the plant ranged from
1 to 4.4 g/kg body weight, the total amount fed representing 5 -
15% of body weight over a period of 54 - 126 days. Five cows died
within 98 days; one, in a moribund state, was killed on day 126.
The calves suckled for 30 - 126 days. Suckling started immediately
after birth in the case of 4 calves and 10 and 30 days later,
respectively, in the 2 remaining calves. Three calves were killed
with their dams or soon after, and 3 were retained for 1 year for
observation. Milk samples from 2 cows were collected and pooled in
14- to 16-day lots during 64 days of feeding of the Senecio plant.
Each pooled sample was administered intragastrically to a group of
rats in daily doses of 12 ml for 15 - 30 days. A control group of
rats were fed raw milk from cows not fed Senecio. Blood samples of
the dams and the calves were analysed for glutamic oxaloacetic
transaminase (GOT), lactic dehydrogenase (LDH), and gamma-glutamyl
transpeptidase (GGTP). Serum-enzyme levels in all cows indicated
statistically significant deviations suggesting liver dysfunction,
and the livers at autopsy had characteristic features of PA
toxicosis. The LDH and GOT levels in calves were generally
abnormal after 20 - 45 days of suckling. The abnormalities ranged
from mild to a 15- to 170-fold increase. One calf was autopsied at
the peak increase of serum-enzymes and was found to have mild focal
hepatitis. No significant pathological features were seen in the
livers of other animals, nor of the rats, some of which were
retained for up to 150 days.
Goeger et al. (1982) fed dried Senecio jacobaea (tansy ragwort)
to lactating goats in a proportion of 25% of the feed. The milk
contained 7.5 µg PA/kg dry weight. The milk produced by the goats
was pooled and then bottle fed to appetite to 2 Jersey bull calves
(1 day old) that also had access to tansy ragwort-free hay for 109
and 124 days, respectively. They were then weaned and given normal
feed and observed for 6 months, after which they were killed and
autopsied. In another study, rats were fed a diet containing the
freeze-dried milk at 80% level for 180 days with a calculated total
PA intake of 0.96 mg/rat. Other groups of rats were fed tansy
ragwort at dietary levels of 0.01 - 10 g/kg (corresponding to PA
intakes of 39.77, 5.04, 0.52, and 0.05 mg/rat). The calf livers
only showed very mild non-specific changes, but the livers of rats
fed tansy ragwort or the milk from tansy ragwort-fed goats
showed definite, but mild, changes including swollen
hepatocytes, megalocytosis, biliary hyperplasia, and fibrosis.
Histopathological changes in milk-fed rats were similar to those in
the group fed tansy ragwort in the diet at 0.01 g/kg. The authors
concluded that there was evidence of PA transfer into milk, which
proved hepatotoxic for rats. It was also noted that the goats had
been fed high levels of tansy ragwort at the upper limit of their
acceptance, and that the hepatic changes observed in rats fed high
levels of milk, for extensive periods, were slight.
Luthy et al. (1983) produced direct evidence of excretion of
macrocyclic esters of retronecine of the senecionine and
seneciphylline-type into rat milk. 3H-retronecine, an 3H-necic
acid-labelled senecionine, and seneciphylline were prepared
biosynthetically with seedlings of Senecio vulgaris L. Two
lactating rats (Ivanovas, Sprague Dawley), weighing 300 - 400 g,
were fed the first of the second compound by stomach tube, in doses
of 2.7 mg/kg and 5.5 mg/kg body weight, respectively. Samples of
blood were examined 1, 3, and 6 h after treatment, and those of
milk 1 and 3 h after. Animals were killed after 6 h. They were
found to have excreted approximately 0.08% of the applied
radioactivity in the milk within 3 h, mainly as unidentified
retronecine-derived metabolites, and approximately 0.02% as
unchanged PAs. The highest levels of PAs and metabolites in
tissues were found in the liver and lungs, 6 h after
administration.
Candrian et al. (1984a) also demonstrated that Drosophila
melanogaster flies fed on milk from lactating rats that had been
administered an oral dose of seneciphylline showed 1.2% sex-linked
recessive lethals, compared with 0.3% in controls, indicating the
transfer of the mutagenic properties of the PA via milk (section
6.4.7).
The implications of the above studies on the possibility of
carry-over of PAs into foodstuffs of animal origin are obvious.
However, no reports of human cases of acute PA toxicity, ascribed
to the consumption of contaminated milk, are available.
3.3.6 Meat
There have not been any reports of the detection of PAs in meat
products from livestock exposed to them.
3.3.7 Use of PAs as chemotherapeutic agents for cancer
An alkaloid of Heliotropium indicum L. (indicine N-oxide) has
been found to have antitumour activity and has been used in
experimental clinical chemotherapy for cancer (section 7.9).
4. METABOLISM
4.1 Absorption, Excretion, and Tissue Distribution
4.1.1 Absorption
There have been few studies on the absorption of PAs in man,
but absorption has been inferred from studies on tissue
distribution and the amounts of alkaloids and their metabolites
excreted in the urine, faeces, and bile of animals (section 4.1.2).
Swick et al. (1982c) measured the transfer of a mixture of
pyrrolizidine alkaloids extracted from Senecio jacobaea, across
isolated intestine and stomach segments from rabbits. The alkaloid
mixture contained seneciphylline, jacobine, jacozine, jacoline, and
senecionine. The alkaloids were transferred across the ileum and
jejunum, but not the stomach. Brauchli et al. (1982) compared the
oral and percutaneous absorption in rats of a crude alkaloid
mixture obtained from comfrey ( Symphytum officinale L.). The
mixture consisted of N-oxides of 7 alkaloids, principally 7-acetyl-
intermedine and 7-acetyl-lycopsamine. A dose of 194 mg/kg was
either given by gavage, or was applied to the shaved skin and left
for 44 h. After the dermal application, the excreted N-oxides in
urine (up to 48 h) amounted to 0.1 - 0.4% of the dose. After oral
dosage the excreted level of N-oxides and alkaloid bases was quoted
as being 20 - 50 times greater.
4.1.2 Excretion and distribution
The excretion and distribution of heliotrine in rats has been
reported in Bull et al., 1968. Young rats (150 g), given the LD50
of heliotrine by ip injection, were killed at intervals, bled
quickly, and the organs and tissues analysed. Heliotrine was
present in the liver after 2 min (3.7% of total dose), the level
peaking at 5 min (6.3%), and dropping to 2.2% at 1 h and 0.5% at
2.5 h. In adult rats, the level in the liver at 5 h was 0.07% of
the total dose. Five min after dosing, 30 - 40% of the initial
dose remained in the peritoneal cavity, and the blood level of
heliotrine was 60 mg/litre, dropping to 3 mg/litre at 1 h. The
urinary excretion of base and metabolites other than pyrrolic
metabolites, collected and measured 16 h after administration of
several alkaloids by ip injection, is shown in Table 6. The
proportion of base excreted unchanged increased with the
hydrophilicity of the alkaloid, being 62% for heliotrine N-oxide,
30% for heliotrine, and only 1 - 1.5% for lasiocarpine.
Heliotridine, the hydrolysis product from heliotrine and
lasiocarpine, was excreted in the form of the N-oxide in larger
quantities after the administration of each of these alkaloids.
The distribution and excretion of monocrotaline was studied in
rats by Hayashi (1966) who found that 50 - 70% was excreted in the
urine within the first day. However, the analysis was by a non-
specific chemical method that did not distinguish between the
unchanged alkaloid and its metabolites. Mattocks (1968a) gave
toxic pyrrolizidine alkaloids intraperitoneally to male rats and
measured the urinary excretion of the unchanged alkaloid, and of
N-oxide and pyrrolic metabolites. The excretion of N-oxide and
unchanged alkaloid was rapid and almost complete in the first 24 h.
Excretion of pyrroles was also rapid but continued for a little
longer. For example, in rats given retrosine (60 mg/kg body
weight), the urine in the first 24 h contained 10.6% unchanged
alkaloid, 13.3% N-oxide, and 13.4% pyrrolic metabolites. During
the second day, only 0.1% alkaloid, 0.2% N-oxide, and 1.8% pyrroles
were excreted. Biliary excretion also occurred. About one-quarter
of an iv dose of retrosine in rats was excreted in the bile as
pyrrolic metabolites, and 4% as unchanged alkaloid; most of this
excretion occurred during the first hour after the injection
(White, 1977).
Jago et al. (1969) gave heliotrine iv to sheep; urinary
excretion of the unchanged alkaloid together with metabolites
( N-oxide, and demethylation and hydrolysis products) occurred
rapidly and continued for up to 8 h. Excretion in the bile was
only 2% of that in the urine.
The tissue distribution of radioactivity from a tritiated toxic
pyrrolizidine alkaloid analogue was studied by Mattocks & White
(1976) using synthanecine A bis- N-ethylcarbamate (40 mg/kg body
weight). The highest concentrations of radioactivity were seen in
the liver (where metabolism occurs), lungs, kidneys, and spleen
(respectively, 3.9%, 0.19%, 0.18%, and 0.27% of the dose given),
and about 69% of the dose was eliminated in the urine during the
first day. Radioactivity in the expired air was negligible. The
binding of radioactivity in the liver, and especially the lungs,
was more persistent than in other organs. Similar results were
given by the semisynthetic pyrrolizidine alkaloid analogue,
retronecine bis- N-ethylcarbamate (Mattocks, 1977).
Table 6. Urinary metabolites of pyrrolizidine bases in the rat (16-h urine)a
Urine constituent (amount in percentage of dose injected)
Base Unchanged Base Heliotridine Heliotridine Heliotridine Heliotridine
administered base N-oxide trachelanthate trachelanthate N-oxide
(ip injection) N-oxide
Heliotrine 30 Trace 10 5 3 15
Heliotrine 62 (62) 2.7 ca. 6 ca. 1 ca. 10
N-oxide
Lasiocarpine 1-1.5 1.5-3 6
Heliotridine 35 ca. 1 (35) (ca. 1) 5 20
trachelanthate
Heliotridine 40 20 (40) (20)
a From: Bull et al. (1968).
The distribution of the uniformly 14C-labelled natural
pyrrolizidine alkaloid senecionine in lactating mice was studied by
Eastman et al. (1982). After 16 h, 75% of the radioactivity had
been recovered in the urine, 14% in the faeces, but only 0.04% was
in the milk; the liver contained 1.92%. The mice were milked using
teat cups. Candrian et al. (1985) studied the distribution of
radioactivity in rats given small doses of senecionine or
seneciphylline (0.3 - 3.3 mg/kg), tritiated in the pyrrolizidine
(retronecine) moiety. Most radioactivity was eliminated in the
urine and faeces within 4 days. Using mass spectrometry, Dickinson
et al. (1976) found a concentration of up to 0.84 mg PAs/litre in
the milk of cows fed Senecio jacobaea. Blood levels of senecionine
in rats given 0.1 LD50 ip were determined by Culvenor (1978). The
levels were 0.38, 0.32, and 0.14 mg/litre at 0.5, 1, and 2 h after
injection, respectively.
To summarize, the available evidence suggests that ingested
toxic pyrrolizidine alkaloids are rapidly metabolized and that the
excretion of unchanged alkaloid and of most metabolites is also
rapid. Thus, within a few hours, only a relatively small
proportion of the dose remains in the body, much of this in the
form of metabolites bound to tissue constituents. It appears
improbable that a significant amount of unchanged alkaloid will
remain in the body after the first day.
Pyrrolizidine N-oxides are much more water soluble than their
parent alkaloids. Indicine N-oxide (which is exceptionally water
soluble) is very rapidly excreted, either unchanged or conjugated.
Thus, indicine N-oxide given iv to mice, monkeys, or rabbits
disappeared from the serum with initial half-lives ranging from 3
to 20 min (Powis et al., 1979; El Dareer et al., 1982). Over 80%
of tritium-labelled indicine N-oxide given iv was excreted in the
urine of mice or monkeys within 24 h (El Dareer et al., 1982); at
2 h, the highest concentrations of radioactivity were in the
kidneys, liver, and intestines. Urinary excretion of indicine
N-oxide was also rapid in rabbits, but somewhat slower in human
beings (Powis et al., 1979).
4.2 Metabolic Routes
The major metabolic routes of unsaturated pyrrolizidine
alkaloids in animals are: (a) hydrolysis (of the ester groups);
(b) N-oxidation; and (c) dehydrogenation (of the pyrrolizidine
nucleus) to dehydro-alkaloids (pyrrolic derivatives). Other minor
routes of metabolism are known, but the three pathways account for
the major known toxic effects of these alkaloids (Fig. 5). Routes
(a) and (b) are believed to be detoxification mechanisms. Route
(c) leads to toxic metabolites and appears to be the major
activation mechanism. Route (a) may occur in various tissues,
including the liver and blood. Routes (b) and (c) are brought
about in the liver by the microsomal mixed-function oxidase system.
The alkaloids are fairly stable chemically, but the ester
groups may undergo hydrolysis under alkaline conditions. Some
alkaloids in plant material may decompose during drying (Bull et
al., 1968), but others appear to be stable under similar conditions
(Pedersen, 1975; Birecka et al., 1980). The N-oxides of
unsaturated pyrrolizidines are more readily decomposed by heat than
the basic alkaloids, especially when dry. However, the stability
of the alkaloids and N-oxides in hot water as, for example, in
cooking, is not known.
Some pyrrolizidine alkaloids have a limited water solubility,
unless neutralized with acid; but others (e.g., indicine), and all
the N-oxides, are readily soluble.
2.2 Analytical Methods
When analysing for PAs, it is important to recognize that this
group consists of many different compounds (section 2.1) and that
these often occur as mixtures in plants or in materials of plant
origin. They may vary in structure, relative molecular mass,
response to analytical procedures, and toxicity. Both basic
alkaloids and corresponding N-oxides may be present at the same
time. Thus, where such mixtures are present, analyses will
inevitably be approximate, unless the individual components are
separated and identified.
Nevertheless, such estimates can be useful. In particular, all
hepatotoxic PAs are unsaturated in the sense that they possess a
1:2-double bond in the pyrrolizidine nucleus, and analytical
methods that are specific for this structure can be of value in
screening for potential toxicity. A simple qualitative field test
for screening plant materials for the presence of such alkaloids
and their N-oxides, without the need of high technology equipment,
is described in section 2.2.2.5.
2.2.1 Extraction
2.2.1.1 Plant tissue
Pyrrolizidine alkaloids are usually extracted from dried,
milled plant material with hot or cold alcohol. The alcohol is
evaporated, the bases taken up in dilute acid, and fats extracted
with ether or petroleum. It is usual, at this stage, to reduce any
N-oxides present to the corresponding basic alkaloids with zinc,
before making the solution alkaline and extracting the alkaloids
with chloroform (Koekemoer & Warren, 1951). Alternatively, alcohol
can be continuously circulated through the plant material and then
cation exchange resin, and the alkaloids subsequently eluted from
the resin (Mattocks, 1961; Deagen & Deinzer, 1977). PAs can also
be extracted by soaking plant material in dilute aqueous acid
(Briggs et al., 1965; Craig et al., 1984).
2.2.1.2 Biological fluids and tissues
Pyrrolizidine alkaloids have been extracted for analytical
purposes from honey (Deinzer et al., 1977), milk (Dickinson et al.,
1976), blood-plasma (Ames & Powis, 1978; McComish et al., 1980),
urine (Mattocks, 1967a; Jago et al., 1969; Evans et al., 1979), and
bile (Jago et al., 1969; Lafranconi et al., 1985).
When attempting to isolate PAs from animal tissues, it must be
appreciated that the toxic alkaloids are often metabolized very
rapidly in animals, so that the amounts that are recoverable
(except from urine), only a few hours after alkaloid ingestion, may
be extremely small. Various methods have been used to separate
PAs, but some mixtures are extremely difficult to separate. On the
analytical scale, the most useful methods are thin-layer
chromatography (TLC), high-performance liquid chromatography
(HPLC), and gas chromatography (GC) (section 2.2.2).
2.2.2 Analysis for pyrrolizidine alkaloids
2.2.2.1 Thin-layer chromatography (TLC)
For TLC, silica plates are usually used, eluted with chloroform:
methanol:aqueous ammonia mixtures (Sharma et al., 1965; Chalmers
et al., 1965); solvents suitable for the N-oxides, which
are more water-soluble, have been described by Mattocks (1967b)
and Wagner et al. (1981). The most sensitive methods for
detecting PAs on TLC are those using Ehrlich reagent
(4-dimethylaminobenzaldehyde) (Mattocks, 1967b). The unsaturated
alkaloids are best visualized by spraying the plates first with a
solution of orthochloranil, then with Ehrlich reagent, heating
after each spray (Molyneux & Roitman, 1980). The N-oxides of
unsaturated pyrrolizidines are detected by spraying a solution of
acetic anhydride, heating the plate, and then spraying Ehrlich
reagent (Mattocks, 1967b).
Pyrrolizidine alkaloids with a saturated base moiety must be
detected in other ways (which are not specific for pyrrolizidines),
e.g., by exposing the dried plates to iodine vapour, or by spraying
with an iodobismuth (Dragendorff) reagent (Munier, 1953).
2.2.2.2 High-performance liquid chromatography (HPLC)
Analytical or preparative scale HPLC separation of
pyrrolizidine alkaloids has been described by Segall (1979a,b) and
Dimenna et al. (1980), and an improved method has been reported by
Ramsdell & Buhler (1981). Alkaloids from Symphytum officinale
(comfrey) have been separated on an analytical scale by Tittel et
al. (1979), and partially separated on a preparative scale by
Huizing et al. (1981). UV detectors are usually used for the HPLC
of pyrrolizidine compounds (Mattocks, 1986).
2.2.2.3 Gas chromatography (GC) and mass spectrometry (MS)
The GC characterization of PAs using packed columns has been
described by Chalmers et al. (1965) and Wiedenfeld et al. (1981).
Mixtures of alkaloids from comfrey ( Symphytum sp.), normally hard
to separate, were resolved by Culvenor et al. (1980a) and Frahn et
al. (1980) by GC of the methylboronate derivatives.
Gas chromatography combined with mass spectrometry (GC-MS) has
become a valuable and highly sensitive means for both the
identification and the quantitative determination of pyrrolizidine
alkaloids. Thus, alkaloids extracted from honey were separated and
identified by Deinzer et al. (1977) and (as butylboronate
derivatives) by Culvenor et al. (1981). Deinzer et al. (1978)
described a method for the recognition (but not the individual
identification) of retronecine-based pyrrolizidine alkaloids, by
hydrolysing them to retronecine (the amino alcohol moiety) followed
by GC-MS of its bis-trifluoroacetate. The use of capillary GC has
greatly improved the sensitivity of pyrrolizidine alkaloid
analysis, especially when used with MS (Luthy et al., 1981). The
MS of pyrrolizidine compounds has been reviewed (Bull et al., 1968;
Mattocks, 1986).
Pyrrolizidine N-oxides generally undergo thermal decomposition,
when subjected to GC, but they can first be reduced to the
corresponding basic alkaloids (Koekemoer & Warren, 1951).
Alternatively they may be derivatised. Thus, trimethylsilylation
of indicine N-oxide or heliotrine N-oxide can lead either to the
trimethylsilyl (TMS) derivative of the parent alkaloid or to the
TMS derivative of the dehydro-alkaloid (pyrrolic derivative),
depending on the reagents used, and these products will run
successfully on GC-MS (Evans et al., 1979, 1980).
2.2.2.4 Nuclear magnetic resonance (NMR) spectrometry
A convenient, but relatively insensitive, method, specifically
for the determination of unsaturated PAs, has been described by
Molyneux et al. (1979). The basic alkaloids are extracted, then
subjected to NMR spectrometry along with an internal standard
( p-dinitrobenzene). This enables quantitative measurements to be
made of the signal(s) representing the H2 proton(s) in unsaturated
pyrrolizidines, and thus the alkaloid(s) can be determined.
Quantitative NMR analysis of pyrrolizidine alkaloid mixtures from
Senecio vulgaris has been described by Pieters & Vlietinck (1985)
and compared with an HPLC method by the same authors (1986).
Qualitative aspects of the NMR spectrometry of pyrrolizidine
alkaloids have been reviewed by Bull et al. (1968) and Mattocks
(1986).
2.2.2.5 The Ehrlich reaction
This method (Mattocks, 1967a, 1968b) is specific for
unsaturated pyrrolizidine alkaloids and is not suitable for other
alkaloids. Thus, it is the most useful colorimetric method for
potentially hepatotoxic pyrrolizidine compounds. The procedure
converts the alkaloid into its N-oxide, using hydrogen peroxide.
The product reacts with acetic anhydride to form a pyrrolic
derivative (dehydro-alkaloid) that gives a magenta colour with a
specially modified Ehrlich reagent. The latter contains boron
trifluoride to give maximum sensitivity. As little as 5 µg of most
unsaturated pyrrolizidines can be measured by this method. If the
oxidation stage is omitted, only the unsaturated pyrrolizidine
N-oxides can be determined. The determination of pyrrolizidine
N-oxides has also been discussed by Mattocks (1971b).
A simplification of the above colorimetric procedure was
described by Mattocks (1971d) to provide a qualitative test that
could be used to screen large numbers of plant samples for the
presence of unsaturated pyrrolizidine alkaloid N-oxides. An
improved version of this field test is now available (Mattocks &
Jukes, 1987). It is suitable for any plant parts, such as leaves,
stems, flowers, seeds, or roots, or materials of plant origin, such
as cereals or herbal teas, but has not yet been applied to cooked
food.
The plant material (0.2 - 1 g) is extracted by grinding it with
aqueous ascorbic acid (5%) and a small amount of sand. The
solution is filtered and divided into two equal portions ("test"
and "blank"). An aqueous solution (0.2 ml) of sodium nitroprusside
(5%) containing sodium hydroxide (10-3 mol) is added to the "test"
sample. Both portions are heated for approximately 1 min at 70 -
80 °C; then Ehrlich reagent is added and heating is continued for
1 min. The Ehrlich reagent contains 4-dimethylaminobenzaldehyde
(5 g) dissolved in a mixture of acetic acid (60 ml), water (30 ml),
and 60% perchloric acid (10 ml). A magenta colour in the "test"
compared with the "blank" indicates the presence of an unsaturated
PA N-oxide. The "blank" may show a colour if the plant contains
compounds, such as indoles or pyrroles, which can themselves give a
colour with Ehrlich reagent. The intensity of colour in the
"sample" compared with the "blank" can give a rough idea of the
amount of alkaloids present, and indicate whether further chemical
or toxicological testing of the plant material is adviseable.
In practice, the majority of PA-containing plants contain
enough alkaloid in the N-oxide form (often a large proportion) to
react positively in this test. The main exceptions are some seeds
(Crotalaria), which may contain much alkaloid base, but little or
no N-oxide. These (and any other sample not containing
chlorophyll) can be tested for basic PAs by grinding them with
chloroform, heating the filtered extract with a solution (0.1 ml)
of orthochloranil (0.5%) in acetonitrile, and then heating it with
Ehrlich reagent. A magenta colour indicates the presence of an
unsaturated PA. Non-toxic pyrrolizidine alkaloids having a
saturated pyrrolizidine nucleus, and pyrrolizidine alkaloids that
are otonecine esters, such as petasitenine, will not respond to
this test.
2.2.2.6 Indicator dyes
A method generally applicable to tertiary bases has been
adapted for pyrrolizidine alkaloids by Birecka et al. (1981). It
is sensitive, but is not specific for this group of alkaloids, and
it does not distinguish between the saturated and unsaturated
alkaloids. A chloroform solution of the alkaloid is shaken with
acidified aqueous methyl orange. The yellow alkaloid:dye complex
is subsequently released from the chloroform phase, using ethanolic
sulfuric acid, and measured spectrophotometrically.
2.2.2.7 Direct weighing
An insensitive way to determine the alkaloids in, for example,
a plant sample, providing enough is available, is to extract the
alkaloids (section 2.2.1) and weigh them. This will provide a
rough measure of the total bases present in the sample; however,
these may not necessarily be PAs. Nevertheless, the sample can
then be subjected to further tests, e.g., GC-MC, nuclear magnetic
resonance (NMR), or colorimetric analysis. Furthermore,
pyrrolizidine N-oxides are generally too water soluble to be
appreciably extractable from aqueous solution by chloroform. Thus,
if two portions of the sample are extracted, and one of them is
reduced to convert N-oxides to bases, the weight difference between
the two products will represent the alkaloid existing in the form
of N-oxide in the original sample.
2.3 Determination of Metabolites in Animal Tissues
Important metabolites of toxic pyrrolizidine alkaloids in
animals include "pyrrolic" derivatives (dehydro-alkaloids) and
N-oxides. A procedure for measuring pyrrolic metabolites in tissue
samples (such as liver or lung) has been described by Mattocks &
White (1970). The sample (usually 0.5 g) is homogenized in an
ethanolic solution of mercuric chloride; the solids are separated
by centrifugation and heated with Ehrlich reagent to give a soluble
colour that can be measured spectrophotometrically.
The measurement of pyrrolic and N-oxide metabolites, formed by
the action of hepatic microsomal preparations on PAs in vitro, is
an improvement described by Mattocks & Bird (1983).
3. SOURCES AND PATHWAYS OF EXPOSURE
3.1 Hepatotoxic Pyrrolizidine Alkaloids and Their Sources
Plants constitute the only natural source of pyrrolizidine
alkaloids (PAs) that cause toxic reactions in man and animals. PAs
occur in a number of species in the families Boraginaceae,
Compositae, Leguminosae (genus Crotalaria), Ranunculaceae (genus
Caltha), and Scrophulariaceae (genus Castilleja) (Table 1). The
most important genera of PA-containing toxic plants are Crotalaria
(Leguminosae), Senecio (Compositae), Heliotropium, Trichodesma,
Amsinckia, Echium, and Symphytum (Boraginaceae) (Hooper, 1978).
The recorded cases of human toxicity have mainly been caused by at
least 12 different pyrrolizidine alkaloids, mostly derived from
Heliotropium, Senecio, and Crotalaria genera. The Senecio spp.
grow throughout the world; the Crotalaria spp. are mainly found in
the tropics and subtropics (Culvenor, 1980).
Table 1. List of plant genera containing toxic pyrrolizidine alkaloids
(with number of species investigated)
-------------------------------------------------------------------------------------
Family Genera
-------------------------------------------------------------------------------------
Apocynaceae Fernaldia (1), Parsonsia (4),
Boraginaceae Alkanna (1), Amsinckia (4), Anchusa (2), Asperugo (1), Borago (1),
Caccinia (1), Cynoglossum (9), Echium (3), Hackelia (1),
Heliotropium (25), Lappula (2), Lindelofia (7), Lithosperum (1),
Macrotomia (1), Messerschmidtia (1), Myosotis (2), Paracaryum (1),
Paracynoglossum (1), Rindera (5), Solenanthus (4), Symphytum (7),
Tournefortia (2), Trachelanthus (2), Trichodesma (2), Ulugbekia (1)
Compositae Adenostyles (3), Brachyglottis (1), Cacalia (4), Conoclinium (1),
Crassocephalum (1), Doronicum (2), Echinacea (2), Emilia (2),
Erechtites (1), Eupatorium (8), Farfugium (1), Gynura (2),
Ligularia (5), Petasites (4), Senecio (142), Syneilesis (1),
Tussilago (1)
Leguminosae Crotalaria (60)
Ranunculaceae Caltha (2)
Scrophulariaceae Castilleja (1)
-------------------------------------------------------------------------------------
An alphabetical list of pyrrolizidine alkaloids with their
plant sources has been published by Smith & Culvenor (1981) and
Mattocks (1986). An updated version is attached as Appendix I.
The plant genera containing toxic PAs are listed in Table 1
indicating the number of species investigated for PAs. A
comprehensive list of species of plants belonging to each of these
genera, the alkaloids isolated from each, and the part of the plant
containing the alkaloid are presented in Appendix II. Table 1 in
Appendix II includes species known to contain alkaloids of proved
hepatotoxicity, or of a molecular structure that would make them
very probably hepatotoxic. Table 2 in Appendix II includes species
containing pyrrolizidine amino-alcohols or esters, which, while not
having all the features of hepatotoxicity, would need only minor
structural modifications to render them hepatotoxic. Plants of the
same taxonomic groups as the plants of proven hepatotoxicity are
listed in part (a) of the table. There is a possibility that, on
further examination, hepatotoxic alkaloids may be found, as minor
constituents, in strains or parts of these plants not yet
investigated or under specific conditions of growth. It should be
noted that the species that have been investigated and are listed
are only few compared with the total number of species in each
genera. It has been recommended by Smith & Culvenor (1981) that it
would be prudent to regard all species in the family Boraginaceae
and the genera Crotalaria, Senecio, and Eupatorium as potentially
hepatotoxic.
It is pertinent to note that the alkaloid content in different
parts of the plant (e.g., roots, leaves, stalks, flowers, and buds)
varies and is subject to fluctuations according to the climate,
soil conditions, and time of harvesting (Danninger et al., 1983;
Hartmann & Zimmer, 1986). Mattocks (1980) demonstrated that the
alkaloid content of the leaves of Symphytum spp. (Russian
comfrey), which are used as an item of food, varies with their
maturity. The toxic PA content is highest at the beginning of the
vegetative period and declines as the leaves mature. The PA
content of the roots is much higher than that of the leaves, and
dried leaves contain a higher concentration than fresh leaves
(Mattocks, 1986). According to Danninger et al. (1983), in some
species (Symphytum asperum), relatively long storage may lead to a
reduction in the alkaloid content, presumably because enzymes are
released during drying. Candrian et al. (1984b) studied the
stability of PAs in hay and silage containing various amounts of
Senecio alpinus. The PA content of hay remained constant for
several months, but the PAs in silage were mainly degraded.
However, the degradation of PAs was much less complete in the lower
concentration range. A quantitatively significant PA-degradation
product in silage was identified as retronecine. Silage with an
S. alpinus percentage of 3.5 - 23 still contained macrocyclic PAs at
a concentration of about 20 mg/kg wet weight. Such silage was not
considered safe for cattle bearing in mind that a 600-kg calf eats
about 30 kg silage/day, amounting approximately to a daily intake
of about 1 mg PAs/kg body weight. In feeding trials with Senecio
jacobaea, Johnson (1979) found that the minimum lethal dose for
cattle was between 1 and 2 mg PAs/kg body weight per day.
PAs known to have been associated with instances of human toxic
liver disease in different parts of the world are listed in Table
2. Two groups of alkaloids that, according to Culvenor (1983), are
consumed in significant amounts by people in different parts of the
world include:
(a) Echimidine, acetyllycopsamine, and related alkaloids
(many countries)
Leaves of plants of the Symphytum sp. ( Symphytum officinale
(comfrey) and Symphytum x uplandicum) are used traditionally as a
salad and as a medicinal herb in Australia, many countries of
Europe, and the USA. S. officinale has been shown to be
carcinogenic for rats (Hirono et al., 1978). Leaves of Russian
comfrey contain a concentration of alkaloids (mainly echimidine) of
0.1 - 1.5 g/kg. The highest level of daily consumption of the
alkaloids has been estimated to be 5 - 6 mg (Culvenor, 1983).
(b) Echimidine and related alkaloids (Australia)
PAs derived from Echium plantagineum, with echimidine as the
major component, have been found in honey secreted by bees feeding
on the plant (Culvenor et al., 1981). The plant is a major source
of honey (section 3.3.4).
3.2 Pneumotoxic and Other Toxic Pyrrolizidine Alkaloids
Not all hepatotoxic alkaloids are pneumotoxic. The commonest
ones used to produce experimental lung injury are fulvine (Barnes
et al., 1964; Kay et al., 1971a; Wagenvoort et al., 1974a,b) and
monocrotaline (Lalich & Ehrhart, 1962; Chesney & Allen, 1973b;
Huxtable et al., 1977). These are also the most active (Mattocks,
1986). The seeds of Crotalaria spectabilis, which contain
monocrotaline, have also been used to study pneumotoxic effects on
experimental animals (Turner & Lalich, 1965; Kay & Heath, 1966; Kay
et al., 1967a) and C. spectabilis has been called the pulmonary
hypertension plant (Kay & Heath, 1969), because of the pulmonary
hypertensionogenic properties of the PAs it contains. Culvenor et
al. (1976a) screened 62 PAs for hepatotoxicity and pneumotoxicity.
Chronic lung lesions were produced by most compounds that induced
chronic liver lesions, though high doses were required in some
instances. It is possible that chronic lung lesions may not occur
in experimental animals because of early death due to acute
toxicity. However, the authors identified a number of PAs that
were particularly prone to produce chronic lung damage in rats
including crispatine, senecionine, seneciphylline, and usaramine
(12-membered macrocyclic, retronecine diesters), anacrotine and
madurensine (crotonecine esters), and the heliotridine esters,
heliosupine, lasiocarpine, and rinderine.
The molecular structure-activity requirements for
pneumotoxicity are the same as those for hepatotoxicity. This is
consistent with their both being caused by the same toxic
metabolites and by the metabolic activation of the alkaloids in the
liver cells to form a reactive pyrrolic dehydro-alkaloid (Culvenor
et al., 1976a).
Trichodesmine and incanine, found in the seeds of Trichodesma
incanum (Yunusov & Plekhanova, 1959), are believed to have been
the causative factors of the "Ozhalangar encephalitis" that was
endemic in Uzbekistan, USSR (1942 - 51), in which the symptoms and
signs were related primarily to the central nervous system
(Shtenberg & Orlova, 1955) (section 7.7).
Table 2. Instances of human toxicity caused by pyrrolizidine alkaloidsa
Principal Plant Country/ Cause of intake Reference
alkaloid Region
Heliotrine and Heliotropium Afghanistan contamination Tandon & Tandon
other alkaloids popovii (1975); Tandon,
similar to B.N. et al.
lasiocarpine (1978); Tandon,
H.D. et al.
(1978);
Mohabbat et al.
(1976)
Senecionine Senecio South contamination Wilmot &
illiciformis; Africa Robertson
Senecio-burchelli (1920)
Senecio spp. South contamination Selzer &
Africa Parker (1951)
Alkaloids of Crotalaria Ecuador medicine Lyford et al.
trichodesmine juncea (1976)
and senecionine
type
Heliotrine and Heliotropium Hong Kong medicine Kumana et al.
lasiocarpine lasiocarpum (1985);
Culvenor et al.
(1986)
Table 2. (cont'd)
Principal Plant Country/ Cause of intake Reference
alkaloid Region
Crotananine and Crotalaria India contamination Tandon, R.K.
cronaburmine nana et al. (1976);
Krishnamachari
et al. (1977);
Siddiqui et al.
(1978a,b)
Heliotrine Heliotropium India medicine Datta et al.
N-oxide eichwaldii (1978a,b)
Monocrotaline Crotalaria West Indies medicine Bras et al.
fulvine retusa; (1954, 1957)
Crotalaria
fulva Stuart & Bras
(1957)
Ilex sp. United medicine McGee et al.
Kingdom (1976)
Riddelline Senecio USA medicine Stillman et al.
retrorsine longilobus (1977); Fox et
N-oxide al. (1978);
(with others) Huxtable (1980)
Indicine N-oxide purified USA medicine Letendre et al.
chemical (1984)
Symphytine, Symphytum sp. USA medicine Ridker et al.
symglandine, and (1985);
other symphytum Huxtable
alkaloids et al (1986)
Table 2. (cont'd)
Principal Plant Country/ Cause of intake Reference
alkaloid Region
Lasiocarpine and Heliotropium USSR contamination Dubrovinskii
heliotrine lasiocarpum (1952);
Mnushkin
(1952)
Trichodesmine and Trichodesma USSR contamination Shtenberg &
incanine incanum Orlova (1955);
Yunosov &
Plekhanova
(1959)
a Adapted from: Culvenor (1983) and Mattocks (1986). Refer also to Table 15 for
details and section 7.
3.3 Pathways of Exposure
Naturally-occurring animal disease is caused by the alkaloid-
containing plants growing in fields and pastures or being fed
accidentally as fodder. They are mostly herbaceous or small shrubs
and many thrive in dry and arid climates. One such plant
containing toxic PA alkaloids has been reported to grow in the
western desert of Egypt (Hammouda et al., 1984). The growth of
this group of plants is particularly prolific during, and
following, periods of drought, as has been reported in association
with the outbreaks of human disease in Afghanistan (Tandon &
Tandon, 1975; Mohabbat et al., 1976) and India (Tandon, B.N. et
al., 1976). Alkaloid-containing plants are widespread in the
tropics, especially Crotalaria, of which there are over 300
species in Africa. Ordinarily, the alkaloid-containing plants have
a bitter taste and grazing animals will reject them, unless their
normal fodder is scarce. However, PAs often occur largely as
N-oxides, which are said not to be bitter, and plants containing
PAs are readily eaten by some animal species.
Human intoxication may result from the ingestion of the toxic
substance in either contamined food or herbal infusion.
3.3.1 Contamination of staple food crops
The products of pyrrolizidine alkaloid-containing plants,
generally seeds, may contaminate the staple food and may be eaten
over long periods of time. The fact that these plants may cause
disease is generally not recognized by the people and such
contamination is known to have resulted in large-scale outbreaks of
poisoning (Dubrovinskii, 1952; Mnushkin, 1952; Shtenberg & Orlova,
1955; Tandon & Tandon, 1975; Mohabbat et al., 1976; Tandon, B.N. et
al., 1976, 1977; Tandon, R.K. et al., 1976; Krishnamachari et al.,
1977; Tandon, H.D. et al., 1977) (Table 2, section 3.1).
3.3.2 Herbal infusions
Plants have been used traditionally for medicinal purposes all
over the world. Herbs have been the mainstay of the indigenous
systems of medicine, especially in China, Greece, and India, since
ancient times. Table 3 includes a list of some plants that are
suspected, or known, to contain PAs and have been used as herbal
medicines in different countries (Mattocks, 1986).
Several PA-containing plants are included among the list of
plants used in indigenous systems of medicine in India (Chopra,
1933). As a part of a research study on the etiological factors of
chronic liver disease in Sri Lanka, Arseculeratne et al. (1981)
chemically screened the first 50 plants used in Sri Lanka's
traditional medicine pharmacopoaea, and found that 3 of them
contained PAs. All 3 were hepatotoxic in rats. Of the 3, the
presence of alkaloids in Cassia auriculata and that of PAs in
Hollarhena antidysenterica had not previously been recorded. It
should be noted that the amount of experimental plant material used
in this study was approximately 6.5 g/kg body weight per day, in
contrast to the approximate intake by a human being estimated to be
in the range of 0.3 - 0.6 g/kg body weight per day. Some, but not
all, of the plants reported to be etiological agents in human cases
of veno-occlusive disease can be found in an inventory of medicinal
plants used in different countries (WHO, 1980), which also
indicates the countries that they are used in. The above lists may
not be complete as many such plants may be used in folk medicine
but have not been mentioned in the scientific literature. However,
the lists do indicate the wide and varied use of such toxic herbs
in all parts of the world.
Lately, there has been a growing interest in the developed
countries in organically grown products for food, as well as home
remedies (Table 3), and some of the PA-containing herbs have been
freely available in herbal shops (Schoental, 1968; Burns, 1972).
Danninger et al. (1983) listed plants containing PAs that are
commonly used in the Federal Republic of Germany as medicaments
(Table 4). He also listed 9 plants in which alkaloids have only
been identified qualitatively, the toxicity of which has not been,
or has been insufficiently, investigated (Table 5). Similarly,
Roitman (1983) listed 10 plants, in which the presence of PAs is
suspected or has been proved and which are used as herbal teas in
the USA. The lists include 10 plants containing PAs, most of which
have been proved hepatotoxic experimentally, some having highly
carcinogenic promoter activity. Some of these alkaloids have been
associated with human case reports of PA toxicity. The more recent
reports (Table 2) of instances of PA poisoning through the use of
herbal medicines are from developed countries (Lyford et al., 1976;
Stillman et al., 1977; Fox et al., 1978; Kumana et al., 1985;
Ridker et al., 1985). Such use of the herbs is the reason that
veno-occlusive disease is endemic in Jamaica (Bras et al., 1954;
Jellife et al., 1954a,b; Bras & Watler, 1955; Stuart & Bras, 1955,
1957). There are obvious difficulties in exercising any kind of
control to restrict this use only to plants that have been tested
and certified as safe for human use. It is impossible to identify
many such herbs, as they are sold as plants or their amorphous
products in the herbal shops.
Table 3. Some plants containing (or suspected of containing) PAs, which have been used
by people either as herbal medicines (M) or foods (F)
Plant Mode Country Referencea
of use or region
BORAGINACEAE
Anchusa officinalis M Europe Broch-Due & Aasen (1980) B
Borago officinalis M USA Delorme et al. (1977) A
Cynoglossum M East Africa Schoental & Coady (1968) A
geometricum
Cynoglossum M Iran Coady (1973) B
officinale
Heliotropium M India Gandhi et al. (1966a); B
eichwaldii Datta et al. (1978a,b) A
H. europaeum M India, Greece IARC (1976) A
H. lasiocarpum M Hong Kong Kumana et al. (1985); A
Culvenor et al. (1986) A
H. indicum M India, Africa, Schoental (1968a); B
South America, Hoque et al. (1976) B
and elsewhere
H. ramossissimum M Arabia Macksad et al. (1970); B
(ramram) Coady (1973) B
H. supinum M Tanzania Schoental & Coady (1968) A
Table 3 (contd.)
Plant Mode Country Referencea
of use or region
Pulmonaria spp. M USA Delorme et al. (1977) A
Symphytum officinale F, M Japan and Hirono et al. (1978, 1979b) A
M USA Furuya & Hikichi (1971); A
Delorme et al. (1977) A
S. x uplandicum F, M General Hills (1976) B
USA Culvenor et al. (1980a,b) A
S. asperum M USA Pedersen (1975) A
COMPOSITAE
Cacalia decomposita M USA Sullivan (1981) B
(matarique)
C. yatabei F Japan Hikichi & Furuya (1978) B
Farfugium japonicum M Japan Furuya et al. (1971) B
Ligularia dentata F Japan Asada & Furuya (1984) B
Petasites japonicus F, M Japan Hirono et al. (1973, 1979b) A
Senecio abyssinicus M Nigeria Williams & Schoental (1970) B
S. aureus M USA Wade (1977) B
Table 3 (contd.)
Plant Mode Country Referencea
of use or region
S. bupleuroides M Africa Watt & Breyer-Brandwijk (1962) A
S. burchelli F, M South Africa Rose (1972) A
S. coronatus M South Africa Rose (1972) A
S. discolor M Jamaica Asprey & Thornton (1955) B
S. doronicum M Germany Roeder et al. (1980a) B
S. inaequidens F South Africa Rose (1972) B
S. jacobaea M Europe Schoental & Pullinger (1972); B
(ragwort) Wade (1977) B
S. longilobus M USA Stillman et al. (1977); A
(S. douglassi) Huxtable (1979a) B
S. monoensis M USA Huxtable (1980) A
S. nemorensis M Germany Habs et al. (1982) A
spp. fuchsii
S. pierotti F Japan Asada & Furuya (1982) B
S. retrorsus M South Africa Rose (1972) A
(S. latifolius)
Table 3 (contd.)
Plant Mode Country Referencea
of use or region
S. vulgaris M Europe Watt & Breyer-Brandwijk (1962) A
(common groundsel)
Netherlands Wade (1977) B
M Iran Coady (1973) B
Syneilesis palmata F Japan Hikichi & Furuya (1976) B
Trichodesma africana M Asia Omar et al. (1983) B
Tussilago farfara M Japan Culvenor et al. (1976a) A
(coltsfoot)
M China Hirono et al. (1976b) A
M Norway Borka & Onshuus (1979) B
M USA Borka & Onshuus (1979); B
Culvenor et al. (1976b); B
LEGUMINOSAE
Crotalaria brevidens F East Africa Coady (1973) B
C. fulva M Jamaica Barnes et al. (1964); A
McLean (1970, 1974) A
Table 3 (contd.)
Plant Mode Country Referencea
of use or region
C. incana M East Africa Schoental & Coady (1968) A
Watt & Breyer-Brandwijk A
(1962)
C. laburnifolia M Tanzania Schoental & Coady (1968) A
F Asia Coady (1973) B
C. mucronata M Tanzania Coady (1973) B
C. recta M, F Tanzania Schoental & Coady (1968); A
Coady (1973) B
C. retusa M, F Africa IARC (1976) A
India Watt & Breyer-Brandwijk (1962) A
C. verrucosa M Sri Lanka Arseculeratne et al. (1981) A
a A = Reference in the reference list of this document.
B = Reference in Mattocks (1986).
Manufactured preparations may also contain PA-containing herbs,
e.g., comfrey-pepsin capsules sold as a digestive aid (Huxtable et
al., 1986).
3.3.3 Use of PA-containing plants as food
Several PA-containing plants are used as food as can be seen in
Table 3 (Mattocks, 1986). Petasites japonicus Maxim, Tussilago
farfara L. (coltsfoot), and Symphytum officinale L. (comfrey or
Russian comfrey) are known as edible plants in Japan, and have been
proved to contain carcinogenic pyrrolizidine alkaloids (Hirono et
al., 1973, 1979a,b). The young flower-stalks of P. japonicus and
the buds of coltsfoot have been used in Japan as human food or
herbal remedies. The leaf and root of comfrey are also used as an
edible vegetable or tonic (Hirono et al., 1978) in Japan and other
countries (Culvenor, 1985). The carcinogenic PAs in these plants
are petasitenine (P. japonicus), senkirkine (coltsfoot), and the
group including symphytine (comfrey). They were also mutagenic in
the Ames system of Salmonella typhimurium and V79 hamster cell line
and induced transformation in cryo-preserved hamster embryonic
cells (Hirono et al., 1979b). Other such PA-containing plants,
used as food in Japan, include young leaves of Syneilesis palmata,
various Cacalia species, and young Senecio pierotti (Mattocks,
1986). According to Culvenor (1985), consumers of comfrey could be
ingesting up to 5 mg PAs per day. Rose (1972) listed a number of
plants of the genus Senecio that are used as spinach in South
Africa. These include S. burchelli, which is known to have caused
an episode of PA poisoning through the ingestion of contaminated
bread (Wilmot & Robertson, 1920).
3.3.4 Contaminated honey
In the USA, Deinzer et al. (1977) reported the presence of all
PAs contained in Senecio jacobaea (ragwort) and proved to be
hepatotoxic, in the honey secreted by bees feeding on the plant.
The total alkaloid content ranged from 0.3 to 3.9 mg/kg. It has
been estimated that an average annual human intake of honey (600 g)
at the highest alkaloid level quoted would contain less than 3 mg
of PAs (Mattocks, 1986). Culvenor et al. (1981) and Culvenor
(1983, 1985) drew attention to the same potential hazard in honey
from Echium plantagineum, a weed that grows widely in Southern
Australia and is a major source of honey, yielding an estimated
2000 - 3000 tonnes per annum for human consumption. Echimidine is
the major component of the alkaloids of Echium, which are present
in concentrations of up to 1 mg/kg. Culvenor (1983) estimated that
individuals may consume up to 80 g honey/day with a corresponding
alkaloid intake of 80 µg/day, if only the Echium honey were used.
No reports of acute human toxicity through this source are
available.
Table 4. Medicinal plants containing PAs of known hepatotoxicity, reported as commonly
used in the Federal Republic of Germany, and the PAs contained in thema
Family Genus Species Pyrrolizidine
alkaloids
Compositae Eupatorium E. cannabinum amabiline±
(hemp agrimony) supinineb
Petasites P. hybirdus senecionineb,c
integerrimineb
senkirkineb
Senecio S. nemorensis fuchsisenecionine
(groundsel) sp. fuchsii senecionineb,c
(Fuch's groundsel)
S. vulgaris senecionineb,c
(groundsel) seneciophyllineb
retrorsineb
riddellineb,c
S. Jacobaea jacobineb
(ragwort) senecionineb,c
seneciphyllineb
jacoline, jaconine
chlorinated PAsd
S. aureus senecionineb,c
(American golden
ragwort)
Tussilago T. farfara senkirkineb
(coltsfoot) (coltsfoot) senecionineb,c
tussilagine
Table 4 (contd.)
Family Genus Species Pyrrolizidine
alkaloids
Alkanna A. tinctoria 7-angelylretronecine
triangularine
dihydroxytriangularine
Anchusa A. officinalis lycopsamine
Boraginaceae Borago B. officinalis lycopsamine/intermedine±
(borage) acetyllycopsamine/
acetylintermedine
amabiline
supinine
Symphytum S. officinale symphytineb
(comfrey) (comfrey) echimidine(?)
lycopsamine
acetyllycopsamineb
lasiocarpineb,c
heliosupine N-oxide
S. peregrinum lycopsamineb
S. x uplandicum intermedineb
symphytineb
echimidineb
7-acetyllycopsamine
7-acetylintermedine
symlandine
uplandicine
S. asperum asperumine
(prickly comfrey) heliosupine N-oxide
echimidineb
echinatine
Table 4 (contd.)
Family Genus Species Pyrrolizidine
alkaloids
Cynoglossum C. officinale heliosupine N-oxide
(hound's (hound's tongue) echinatine
tongue) acetyl heliosupineb
O-7-angelylhelio-
tridineb
Heliotropium H. europaeum heliotrineb,c,e
(Heliotrope) (common heliotrope) lasiocarpineb,c,e
supinine
heleurine
europine
acetyllasiocarpineb
a Modified from: Danninger et al. (1983).
b Toxic alkaloids.
c Alkaloids known to have caused human toxicity.
d Alkaloids with highly carcinogenic promoter activity.
e Used only in homeopathy.
Table 5. Medicinal plants containing PAs, reported as
commonly used in the Federal Republic of Germany,
the toxicity of which has not been, or has been
insufficiently, investigateda
-----------------------------------------------------------
Family Genus Species
-----------------------------------------------------------
Compositae Eupatorium E. perforatum
Brachyglottis B. repens
Arnica A. montana (mountain arnica)
Boraginaceae Lappula L. intermedia (stickseed)
Pulmonaria P. officinalis (lungwort)
-----------------------------------------------------------
a Modified from: Danninger et al. (1983).
3.3.5 Milk
PAs have been shown to produce toxic effects via transference
into the milk of dams (Schoental, 1959). Retrorsine was
administered orally to 17, and intraperitoneally to 6, lactating
rats weighing 185 - 350 g in 5 - 10 mg doses daily, the first dose
being given during the first 24 h after parturition. The rats
received from 1 to 14 doses, the total intake amounting to
21 - 335 mg/kg body weight. The litters were separated from the
mothers for ´ h following the administration of PA to avoid direct
contamination of the former by licking. Apparently the milk
production was not affected as the stomachs of many of the young,
examined postmortem, were distended with milk. All animals whose
mothers had received a total dose of 138 mg PA or more died within
30 days. Many of the young whose mothers had received smaller
doses survived until they were killed at 6 months. Biopsy of the
liver of the young at various intervals or at autopsy showed marked
changes, even in cases where the mothers did not appear to be
affected. Animals dying at 18 - 30 days showed hydropic or fatty
vacuolation of liver cells. In the liver of animals dying or
killed later, various degrees of haemorrhagic necrosis and increase
in the centrilobular reticulin of the liver, and some thickening of
centrilobular veins were seen. In animals that survived 6 months,
the appearance was less abnormal, but some hyperplastic nodules and
bile-duct proliferation were seen. The lactating rats dosed with
the PAs generally survived longer than the suckling animals and
usually did not show any ill effects, suggesting that the
susceptibility of the suckling rats was greater than that of the
mothers.
Dickinson et al. (1976) demonstrated the presence of PAs in the
milk of dairy cattle fed or dosed with ragwort (Senecio jacobaea).
When 4 cows were administered the dried plant material at levels of
up to 10 g/kg body weight per day through rumen cannula, PA levels
of up to 0.84 mg/kg were observed in the milk. However, only one
(jacoline) of the several PAs contained in the plant was secreted.
Calves, bucket fed on the milk did not show any signs of PA
toxicity.
Dickinson (1980) repeated the study on goats. Four milk goats
were freshly prepared with rumen cannulae. The kids were separated
from their dams and were fed milk twice a day. Dried tansy ragwort
plant material with a PA content of 0.16% (dry weight) was
administered through the cannulae to each goat at a dosage rate of
10 g/kg body weight per day over 125 days. During this period,
each of the 4 kids received milk from their dams at approximately
125 ml/kg per day in addition to ad lib feeding on alfalfa grass
hay. Six PAs were isolated from the plant material: jacobine,
jaconine, jaconline, jacozine, senecionine, and seneciphylline.
Milk samples collected twice daily showed PA contents of
225 - 530 µg/litre with a mean of 381 µg/litre. No apparent health
effects were noted in the kids, and only mild hepatic damage was
suspected in the dams, on the basis of liver function tests. Fifty
percent of the kids were killed after 10 weeks. No lesions of PA
toxicity were seen. The dams were rebred and appeared normal
throughout the gestation period. However, three dams aborted at
almost full term, and the fetuses were born dead. One of the dams
died shortly after parturition and showed evidence of severe liver
damage characteristic of PA toxicity. Another, which delivered
normally, also showed a lesser degree of liver damage at biopsy.
Data relating to PA secretion were compared with similar
earlier data on cows. Mean secretion of PAs in cows appeared much
higher, e.g., 684 µg/litre. The authors concluded that the amount
of PAs secreted in the goat's milk did not cause any serious
deleterious effects in the kids.
Johnson (1976) fed long-term lethal doses of Senecio jacobaea,
by stomach tube, to 6 cows. Feeding started at term or within 30
days post-partum, and continued until what was considered to be a
lethal dose had been fed. The daily dose of the plant ranged from
1 to 4.4 g/kg body weight, the total amount fed representing 5 -
15% of body weight over a period of 54 - 126 days. Five cows died
within 98 days; one, in a moribund state, was killed on day 126.
The calves suckled for 30 - 126 days. Suckling started immediately
after birth in the case of 4 calves and 10 and 30 days later,
respectively, in the 2 remaining calves. Three calves were killed
with their dams or soon after, and 3 were retained for 1 year for
observation. Milk samples from 2 cows were collected and pooled in
14- to 16-day lots during 64 days of feeding of the Senecio plant.
Each pooled sample was administered intragastrically to a group of
rats in daily doses of 12 ml for 15 - 30 days. A control group of
rats were fed raw milk from cows not fed Senecio. Blood samples of
the dams and the calves were analysed for glutamic oxaloacetic
transaminase (GOT), lactic dehydrogenase (LDH), and gamma-glutamyl
transpeptidase (GGTP). Serum-enzyme levels in all cows indicated
statistically significant deviations suggesting liver dysfunction,
and the livers at autopsy had characteristic features of PA
toxicosis. The LDH and GOT levels in calves were generally
abnormal after 20 - 45 days of suckling. The abnormalities ranged
from mild to a 15- to 170-fold increase. One calf was autopsied at
the peak increase of serum-enzymes and was found to have mild focal
hepatitis. No significant pathological features were seen in the
livers of other animals, nor of the rats, some of which were
retained for up to 150 days.
Goeger et al. (1982) fed dried Senecio jacobaea (tansy ragwort)
to lactating goats in a proportion of 25% of the feed. The milk
contained 7.5 µg PA/kg dry weight. The milk produced by the goats
was pooled and then bottle fed to appetite to 2 Jersey bull calves
(1 day old) that also had access to tansy ragwort-free hay for 109
and 124 days, respectively. They were then weaned and given normal
feed and observed for 6 months, after which they were killed and
autopsied. In another study, rats were fed a diet containing the
freeze-dried milk at 80% level for 180 days with a calculated total
PA intake of 0.96 mg/rat. Other groups of rats were fed tansy
ragwort at dietary levels of 0.01 - 10 g/kg (corresponding to PA
intakes of 39.77, 5.04, 0.52, and 0.05 mg/rat). The calf livers
only showed very mild non-specific changes, but the livers of rats
fed tansy ragwort or the milk from tansy ragwort-fed goats
showed definite, but mild, changes including swollen
hepatocytes, megalocytosis, biliary hyperplasia, and fibrosis.
Histopathological changes in milk-fed rats were similar to those in
the group fed tansy ragwort in the diet at 0.01 g/kg. The authors
concluded that there was evidence of PA transfer into milk, which
proved hepatotoxic for rats. It was also noted that the goats had
been fed high levels of tansy ragwort at the upper limit of their
acceptance, and that the hepatic changes observed in rats fed high
levels of milk, for extensive periods, were slight.
Luthy et al. (1983) produced direct evidence of excretion of
macrocyclic esters of retronecine of the senecionine and
seneciphylline-type into rat milk. 3H-retronecine, an 3H-necic
acid-labelled senecionine, and seneciphylline were prepared
biosynthetically with seedlings of Senecio vulgaris L. Two
lactating rats (Ivanovas, Sprague Dawley), weighing 300 - 400 g,
were fed the first of the second compound by stomach tube, in doses
of 2.7 mg/kg and 5.5 mg/kg body weight, respectively. Samples of
blood were examined 1, 3, and 6 h after treatment, and those of
milk 1 and 3 h after. Animals were killed after 6 h. They were
found to have excreted approximately 0.08% of the applied
radioactivity in the milk within 3 h, mainly as unidentified
retronecine-derived metabolites, and approximately 0.02% as
unchanged PAs. The highest levels of PAs and metabolites in
tissues were found in the liver and lungs, 6 h after
administration.
Candrian et al. (1984a) also demonstrated that Drosophila
melanogaster flies fed on milk from lactating rats that had been
administered an oral dose of seneciphylline showed 1.2% sex-linked
recessive lethals, compared with 0.3% in controls, indicating the
transfer of the mutagenic properties of the PA via milk (section
6.4.7).
The implications of the above studies on the possibility of
carry-over of PAs into foodstuffs of animal origin are obvious.
However, no reports of human cases of acute PA toxicity, ascribed
to the consumption of contaminated milk, are available.
3.3.6 Meat
There have not been any reports of the detection of PAs in meat
products from livestock exposed to them.
3.3.7 Use of PAs as chemotherapeutic agents for cancer
An alkaloid of Heliotropium indicum L. (indicine N-oxide) has
been found to have antitumour activity and has been used in
experimental clinical chemotherapy for cancer (section 7.9).
4. METABOLISM
4.1 Absorption, Excretion, and Tissue Distribution
4.1.1 Absorption
There have been few studies on the absorption of PAs in man,
but absorption has been inferred from studies on tissue
distribution and the amounts of alkaloids and their metabolites
excreted in the urine, faeces, and bile of animals (section 4.1.2).
Swick et al. (1982c) measured the transfer of a mixture of
pyrrolizidine alkaloids extracted from Senecio jacobaea, across
isolated intestine and stomach segments from rabbits. The alkaloid
mixture contained seneciphylline, jacobine, jacozine, jacoline, and
senecionine. The alkaloids were transferred across the ileum and
jejunum, but not the stomach. Brauchli et al. (1982) compared the
oral and percutaneous absorption in rats of a crude alkaloid
mixture obtained from comfrey ( Symphytum officinale L.). The
mixture consisted of N-oxides of 7 alkaloids, principally 7-acetyl-
intermedine and 7-acetyl-lycopsamine. A dose of 194 mg/kg was
either given by gavage, or was applied to the shaved skin and left
for 44 h. After the dermal application, the excreted N-oxides in
urine (up to 48 h) amounted to 0.1 - 0.4% of the dose. After oral
dosage the excreted level of N-oxides and alkaloid bases was quoted
as being 20 - 50 times greater.
4.1.2 Excretion and distribution
The excretion and distribution of heliotrine in rats has been
reported in Bull et al., 1968. Young rats (150 g), given the LD50
of heliotrine by ip injection, were killed at intervals, bled
quickly, and the organs and tissues analysed. Heliotrine was
present in the liver after 2 min (3.7% of total dose), the level
peaking at 5 min (6.3%), and dropping to 2.2% at 1 h and 0.5% at
2.5 h. In adult rats, the level in the liver at 5 h was 0.07% of
the total dose. Five min after dosing, 30 - 40% of the initial
dose remained in the peritoneal cavity, and the blood level of
heliotrine was 60 mg/litre, dropping to 3 mg/litre at 1 h. The
urinary excretion of base and metabolites other than pyrrolic
metabolites, collected and measured 16 h after administration of
several alkaloids by ip injection, is shown in Table 6. The
proportion of base excreted unchanged increased with the
hydrophilicity of the alkaloid, being 62% for heliotrine N-oxide,
30% for heliotrine, and only 1 - 1.5% for lasiocarpine.
Heliotridine, the hydrolysis product from heliotrine and
lasiocarpine, was excreted in the form of the N-oxide in larger
quantities after the administration of each of these alkaloids.
The distribution and excretion of monocrotaline was studied in
rats by Hayashi (1966) who found that 50 - 70% was excreted in the
urine within the first day. However, the analysis was by a non-
specific chemical method that did not distinguish between the
unchanged alkaloid and its metabolites. Mattocks (1968a) gave
toxic pyrrolizidine alkaloids intraperitoneally to male rats and
measured the urinary excretion of the unchanged alkaloid, and of
N-oxide and pyrrolic metabolites. The excretion of N-oxide and
unchanged alkaloid was rapid and almost complete in the first 24 h.
Excretion of pyrroles was also rapid but continued for a little
longer. For example, in rats given retrosine (60 mg/kg body
weight), the urine in the first 24 h contained 10.6% unchanged
alkaloid, 13.3% N-oxide, and 13.4% pyrrolic metabolites. During
the second day, only 0.1% alkaloid, 0.2% N-oxide, and 1.8% pyrroles
were excreted. Biliary excretion also occurred. About one-quarter
of an iv dose of retrosine in rats was excreted in the bile as
pyrrolic metabolites, and 4% as unchanged alkaloid; most of this
excretion occurred during the first hour after the injection
(White, 1977).
Jago et al. (1969) gave heliotrine iv to sheep; urinary
excretion of the unchanged alkaloid together with metabolites
( N-oxide, and demethylation and hydrolysis products) occurred
rapidly and continued for up to 8 h. Excretion in the bile was
only 2% of that in the urine.
The tissue distribution of radioactivity from a tritiated toxic
pyrrolizidine alkaloid analogue was studied by Mattocks & White
(1976) using synthanecine A bis- N-ethylcarbamate (40 mg/kg body
weight). The highest concentrations of radioactivity were seen in
the liver (where metabolism occurs), lungs, kidneys, and spleen
(respectively, 3.9%, 0.19%, 0.18%, and 0.27% of the dose given),
and about 69% of the dose was eliminated in the urine during the
first day. Radioactivity in the expired air was negligible. The
binding of radioactivity in the liver, and especially the lungs,
was more persistent than in other organs. Similar results were
given by the semisynthetic pyrrolizidine alkaloid analogue,
retronecine bis- N-ethylcarbamate (Mattocks, 1977).
Table 6. Urinary metabolites of pyrrolizidine bases in the rat (16-h urine)a
Urine constituent (amount in percentage of dose injected)
Base Unchanged Base Heliotridine Heliotridine Heliotridine Heliotridine
administered base N-oxide trachelanthate trachelanthate N-oxide
(ip injection) N-oxide
Heliotrine 30 Trace 10 5 3 15
Heliotrine 62 (62) 2.7 ca. 6 ca. 1 ca. 10
N-oxide
Lasiocarpine 1-1.5 1.5-3 6
Heliotridine 35 ca. 1 (35) (ca. 1) 5 20
trachelanthate
Heliotridine 40 20 (40) (20)
a From: Bull et al. (1968).
The distribution of the uniformly 14C-labelled natural
pyrrolizidine alkaloid senecionine in lactating mice was studied by
Eastman et al. (1982). After 16 h, 75% of the radioactivity had
been recovered in the urine, 14% in the faeces, but only 0.04% was
in the milk; the liver contained 1.92%. The mice were milked using
teat cups. Candrian et al. (1985) studied the distribution of
radioactivity in rats given small doses of senecionine or
seneciphylline (0.3 - 3.3 mg/kg), tritiated in the pyrrolizidine
(retronecine) moiety. Most radioactivity was eliminated in the
urine and faeces within 4 days. Using mass spectrometry, Dickinson
et al. (1976) found a concentration of up to 0.84 mg PAs/litre in
the milk of cows fed Senecio jacobaea. Blood levels of senecionine
in rats given 0.1 LD50 ip were determined by Culvenor (1978). The
levels were 0.38, 0.32, and 0.14 mg/litre at 0.5, 1, and 2 h after
injection, respectively.
To summarize, the available evidence suggests that ingested
toxic pyrrolizidine alkaloids are rapidly metabolized and that the
excretion of unchanged alkaloid and of most metabolites is also
rapid. Thus, within a few hours, only a relatively small
proportion of the dose remains in the body, much of this in the
form of metabolites bound to tissue constituents. It appears
improbable that a significant amount of unchanged alkaloid will
remain in the body after the first day.
Pyrrolizidine N-oxides are much more water soluble than their
parent alkaloids. Indicine N-oxide (which is exceptionally water
soluble) is very rapidly excreted, either unchanged or conjugated.
Thus, indicine N-oxide given iv to mice, monkeys, or rabbits
disappeared from the serum with initial half-lives ranging from 3
to 20 min (Powis et al., 1979; El Dareer et al., 1982). Over 80%
of tritium-labelled indicine N-oxide given iv was excreted in the
urine of mice or monkeys within 24 h (El Dareer et al., 1982); at
2 h, the highest concentrations of radioactivity were in the
kidneys, liver, and intestines. Urinary excretion of indicine
N-oxide was also rapid in rabbits, but somewhat slower in human
beings (Powis et al., 1979).
4.2 Metabolic Routes
The major metabolic routes of unsaturated pyrrolizidine
alkaloids in animals are: (a) hydrolysis (of the ester groups);
(b) N-oxidation; and (c) dehydrogenation (of the pyrrolizidine
nucleus) to dehydro-alkaloids (pyrrolic derivatives). Other minor
routes of metabolism are known, but the three pathways account for
the major known toxic effects of these alkaloids (Fig. 5). Routes
(a) and (b) are believed to be detoxification mechanisms. Route
(c) leads to toxic metabolites and appears to be the major
activation mechanism. Route (a) may occur in various tissues,
including the liver and blood. Routes (b) and (c) are brought
about in the liver by the microsomal mixed-function oxidase system.
 4.2.1 Hydrolysis
The hydrolysis of a PA leads to the formation of the amino-
alcohol moiety (necine base) and the acid moiety. Neither of these
is hepatotoxic (Schoental & Mattocks, 1960; Culvenor et al.,
1976a). The highly water-soluble necine base is readily excreted,
is not accessible to the microsomal system, and is not activated to
a toxic metabolite. Thus, pyrrolizidine alkaloids that are very
susceptible to (enzymic) hydrolysis have low toxicity (Mattocks,
1982). A major factor contributing to resistance to esterase is
the steric hindrance in the acid moiety. Thus, the chain branching
near the carbonyl groups slows hydrolysis allowing the formation of
relatively high levels of pyrrolic metabolites; a conformation of
the basic moiety, which brings the two ester groups close together,
thus leading to mutual steric hindrance, can also prevent
hydrolysis (Mattocks, 1981a).
The influence of hydrolysis in vivo on alternative metabolic
pathways is demonstrated by the fact that treatment of rats with an
esterase inhibitor, before giving pyrrolizidine alkaloids (or
synthetic analogues), can lead to greatly increased production of
pyrrolic metabolites from alkaloids that are normally susceptible
to hydrolysis, but little increase in those from alkaloids normally
resistant to hydrolysis (Mattocks, 1981a).
4.2.2 N-oxidation
The N-oxidation of pyrrolizidine alkaloids is induced by the
hepatic microsomal enzymes. The N-oxide metabolites are highly
water soluble and are rapidly excreted in the urine (Mattocks,
1968a). Pyrrolizidine N-oxides are not converted to any
significant extent to pyrrolic metabolites by microsomal enzymes
(Jago et al., 1970; Mattocks & White, 1971a), and there is no
evidence that they are toxic, unless first reduced to the
corresponding basic alkaloids, which can then be activated by the
microsomal system (Mattocks, 1971c). Thus, it appears that the
formation of N-oxides represents a detoxification pathway.
4.2.3 Conversion to pyrrolic metabolites
In laboratory animals, toxic pyrrolizidine alkaloids are
metabolized to pyrrolic derivatives, so-called because the
unsaturated ring of the pyrrolizidine system loses 2 hydrogen atoms
to form what is in effect a pyrrole ring (though the structure is
more correctly a dihydropyrrolizidine). Pyrrolic metabolites are
easily detectable in the tissues shortly after giving a toxic
pyrrolizidine alkaloid to an animal, by treating the tissue with an
Ehrlich reagent containing boron trifluoride, when a red colour is
produced; this reaction also occurs with the urine (Mattocks,
1968a; Mattocks & White, 1970). In rats given retrosine, pyrrolic
metabolites were found principally in the liver, with highest
levels associated with the microsomal and solid debris fractions
and less in the mitochondrial fraction; low levels were found in
the lungs, heart, spleen, and kidneys, within 4 h of giving
retrosine. Rats given 60 mg retrosine/kg body weight excreted 14%
of the dose in the urine, within 48 h.
Pyrrolic metabolites are formed by the hepatic mixed-function
oxidase system, with a requirement for cytochrome P450, oxygen, and
NADPH, as has been demonstrated in vitro (Jago et al., 1970;
Mattocks & White, 1971a). Conversion of pyrrolizidine alkaloids to
pyrrolic metabolites by the lung tissue of the human embryo
(Armstrong & Zuckerman, 1970), rat (Mattocks & White, 1971a;
Hilliker et al., 1983), or rabbit (Guengerich, 1977) was
negligible. The formation of pyrrolic metabolites does not proceed
via N-oxide intermediates, but appears to result from an initial
hydroxylation of the unsaturated pyrrolizidine ring adjacent to the
nitrogen atom (Mattocks & White, 1971a; Mattocks & Bird, 1983).
This would lead to a chemically unstable intermediate that would be
expected to decompose spontaneously to the pyrrolic product. The
primary pyrrolic metabolites (or dehydro-alkaloid) formed by
dehydrogenation of pyrrolizidine alkaloids are chemically
dehydropyrrolizidine esters (Fig. 5). These are highly reactive
compounds that can rapidly react with tissue constituents or
hydrolyse to the corresponding pyrrolic alcohols, or dehydro-
necines, which can thus be regarded as secondary metabolites. The
latter can also react with tissue constituents, but more slowly.
Because of their high chemical reactivity, the primary metabolites
would be expected to have a short life in the liver cell (minutes
or seconds) before they are hydrolysed or react with nucleophilic
tissue constituents. Some might escape into the blood stream and
reach other organs, especially the lungs. Dehydro-necines are more
stable and also more water soluble, and can become more widely
distributed throughout the body. However, they are also capable of
reacting with tissue constituents. Thus, measurements of pyrroles
formed from pyrrolizidine alkaloids in tissue samples, using a
colour reaction (Mattocks & White, 1970), will not represent a
single metabolite, but mixtures of the metabolites together with
various reaction products of these with tissue constituents. It
will be seen (section 5) that pyrrolic metabolites are believed to
be responsible for major toxic actions of pyrrolizidine alkaloids
(Mattocks, 1972a).
A pyrrolic metabolite with reactivity midway between that of
dehydromonocrotaline and dehydroretronecine has been reported to be
formed from monocrotaline in isolated, perfused rat liver
(Lafranconi et al., 1985). Studies on this metabolite (isolated
from bile) indicated that it is a monoester, and that it is toxic
in perfused rat lung. This suggests that monoester pyrrolic
metabolites may play a part in the toxic actions of PAs in extra-
hepatic tissues.
When rats or other laboratory animals are given a toxic
pyrrolizidine alkaloid, pyrrolic metabolites accumulate rapidly in
the liver (Mattocks, 1973; White et al., 1973), reaching a peak
within 1 - 2 h, then falling slowly during the next 24 h; the
metabolites may still be detectable after 2 days. Accumulation is
especially rapid after intraperitoneal injection, a very high level
of pyrrolic metabolites being attained within 20 min; this
indicates how rapid the metabolism of pyrrolizidine alkaloids can
be.
The level of pyrrolic metabolites in rat liver is generally
directly related to the amount of alkaloid given 2 h previously, at
least up to an acute LD50 dose. The pyrrole level depends on the
alkaloid used, and is related to the acute hepatotoxicity of the
alkaloid (Mattocks, 1972a).
To be converted to the type of chemically reactive, toxic
pyrrolic metabolites described above, an alkaloid must possess a
1-hydroxymethyl pyrrolizidine system, unsaturated in the
1,2-position (this makes the ring susceptible to dehydrogenation),
and at least one hydroxyl group must be esterified, usually by a
branched-chain acid. Otonecine esters are converted to similar
pyrrol metabolites by a different media involving N-demethylation.
Pyrrolizidine amino-alcohols (e.g., retronecine) are not
metabolized to more than small amounts of pyrroles (Jago et al.,
1970; Mattocks, 1981a), possibly because they are too water soluble
to reach the microsomal enzymes.
The metabolic formation of pyrroles is catalysed by cytochrome
P450 and specificity exists in the various isozymes (Guengerich,
1977; Juneja et al., 1984).
A few non-toxic pyrrolizidine alkaloids (e.g., rosmarinine and
hygrophylline) are converted to pyrrolic metabolites in vivo
(Mattocks, 1973). Such metabolites are chemically different from
the pyrroles of toxic alkaloids, and they are neither reactive nor
toxic (Mattocks & White, 1971b). The balance of structural
features necessary for a pyrrolizidine alkaloid to be converted to
give high concentrations of toxic pyrrolic metabolite has been
discussed by Mattocks (1981a); the optimum conditions appear to be
met in some alkaloids that are macrocyclic diesters, such as
retrosine.
4.3 Effects of Treatments Affecting Metabolism
The formation of pyrrolic metabolites (and of N-oxides) is
altered by treatments that affect the hepatic microsomal enzymes.
Such effects have been studied by measuring rates of metabolism of
pyrrolizidine alkaloids in vitro using microsomal preparations
from animals pre-treated in various ways (Table 7). For example,
microsomes from rats given the microsomal enzyme inducers
phenobarbitone or DDT (but not those from rats given
3-methylcholanthrene) induce greatly increased pyrrole formation
and smaller increases in N-oxide formation, from the alkaloid
retrorsine (Mattocks & White, 1971a). Enzyme preparations from
rats treated with inhibitors of microsomal enzymes, including SKF
525A and chloramphenicol, are much less active in converting
monocrotaline to pyrroles (Chesney et al., 1974). The ability to
metabolize retrorsine is diminished in microsomes from rats fed a
protein-free diet, or from rats acutely poisoned with retrorsine
(Mattocks & White, 1971a).
Table 7. Effect of pre-treatment of male rats on the conversion of PAs to
pyrrolic derivatives and to N-oxides by liver microsomes
in vitro
----------------------------------------------------------------------------
Alkaloid Pre-treatment, and Enzyme activity as Reference
time before enzyme % of control values
measurements for formation of:
Pyrroles N-oxides
----------------------------------------------------------------------------
retrorsine phenobarbitone, ip, 311 232 Mattocks &
3 x 100 mg/kg, White (1971a)
1 - 3 days
retrorsine DDT, ip, 75 mg/kg, 407 203 Mattocks &
3 days White (1971a)
retrorsine 3-methylcholanthrene, 95 (ns) 116 (ns) Mattocks &
ip, 3 x 20 mg/kg, White (1971a)
1 - 3 days
retrorsine retrorsine, ip, 63 - Mattocks &
35 mg/kg, 20 h White (1971a)
retrorsine protein-free diet, 39 - Mattocks &
3 days White (1971a)
monocrotaline phenobarbitone, sc, 448 165 Chesney et al.
4 x 75 mg/kg, (1974)
1 - 4 days
monocrotaline chloramphenicol, sc, 10 109 (ns) Chesney et al.
200 mg/kg, 1 h (1974)
monocrotaline SKF 525A, ip, 75 mg/kg, 10 87 (ns) Chesney et al.
1 h (1974)
----------------------------------------------------------------------------
ns = not significantly different from controls.
The effects of in vivo treatment with several types of enzyme
inducers on the toxicity of lasiocarpine and senecionine for
primary rat hepatocyte cultures was investigated by Hayes et al.
(1985). Pre-treatment with phenobarbitone potentiated the
cytotoxicity of senecionine towards the cultured cells, whereas
pre-treatment with 3-methylcholanthrene diminished the toxic action
of senecionine, but had little effect on lasiocarpine cytotoxicity.
The cytocidal effects of both alkaloids were substantially
inhibited in the presence of SKF 525A.
4.4 Other Factors Affecting Metabolism
Variations between animal species have been investigated by
White et al. (1973) and Shull et al. (1976). For instance,
metabolism to form pyrroles is high in rats and very low in guinea-
pigs, which, however, have higher rates of N-oxidation. For
example, 2 h after an ip dose of retrosine (100 mg/kg body weight),
the liver-pyrrole level in male rats was 13 times higher than that
in male guinea-pigs (White et al., 1973). Liver microsome
preparations from male rats were 28 times more active than
microsomes from male guinea-pigs in the dehydrogenation of
monocrotaline (Chesney & Allen, 1973a).
The development with age of the ability of Wistar rats to
metabolize retrosine was studied by Mattocks & White (1973). The
ability to form pyrroles is very low in new-born rats, but, by 5
days of age, it is nearly as high as in adult males. This activity
continues at a similar level in male rats, but, in females, it
falls after the age of about 20 days until, by 60 days, it is about
one-eighth that in males. Such a sex difference was not observed
in mice (White et al., 1973).
4.5 Other Metabolic Routes
The actions of hepatic microsomal enzymes on pyrrolizidine
alkaloids can produce other metabolites as well as pyrroles and
N-oxides, but there are few reports of these. Eastman & Segall
(1982) demonstrated hydroxylation of the acid moiety of senecionine
by liver microsomes from female mice. Such metabolism should not
prevent the subsequent conversion of the product to pyrrolic or
N-oxide metabolites. The formation of other microsomal metabolites
of senecionine has been reported by Segall et al. (1984).
The O-demethylation of the acid moiety of heliotrine has been
demonstrated by Jago et al. (1969) and represents a partial
detoxification mechanism, since the product is about half as toxic
as heliotrine. Other detoxification mechanisms exist in the rumen
of sheep (Dick et al., 1963; Lanigan & Smith, 1970a,b), which are,
thus, particularly resistant to the effects of pyrrolizidine
alkaloids.
4.6 Metabolism of Pyrrolizidine N-Oxides
As mentioned in section 4.2, the N-oxides of pyrrolizidine
alkaloids are not converted to pyrrolic metabolites by liver
microsomes. It appears that their main route of metabolism in
animals is reduction to the corresponding basic alkaloids, which
may then be further metabolized as already described. This
reduction has been shown to occur in the rat or rabbit gut
(Mattocks, 1971c; Powis et al., 1979), and may be brought about by
intestinal bacteria or possibly by gut enzymes. Such reduction can
also be brought about by hepatic microsomal fractions (Powis et
al., 1979) in the presence of NADH or of NADPH, and by sheep rumen
fluid (Lanigan et al., 1970a, b).
The reduction of pyrrolizidine N-oxides in vivo is of great
importance as a step in the bioactivation of these compounds
(Mattocks, 1971c), as shown in section 4.2.2.
4.7 Metabolism in Man
Powis et al. (1979) found that indicine N-oxide given iv to
3 human patients as an antitumour drug was partially reduced to
indicine base, detectable in the urine and plasma. Armstrong &
Zuckerman (1970) showed that human embryo liver slices, but not
lung slices, converted the pyrrolizidine alkaloids lasiocarpine,
retrorsine, and fulvine to pyrrolic metabolites in vitro.
5. MECHANISMS OF TOXICITY AND OTHER BIOLOGICAL ACTIONS
5.1 Metabolites Responsible for Toxicity
5.1.1 Metabolic basis of toxicity
The toxic effects of pyrrolizidine alkaloids are mediated
through their toxic metabolites and not by the alkaloids
themselves. The following observations are evidence for the above
statement (Mattocks, 1972a):
(a) The alkaloids are chemically rather unreactive and it is
hard to envisage reactions with cell constituents that they
could undergo readily under physiological conditions. On the
other hand, chemically prepared derivatives, similar or
identical to known metabolites of these alkaloids, are highly
reactive and are capable of causing toxic effects similar to
those of PAs, often at dose levels much lower than those
required by the alkaloids themselves.
(b) The liver is usually the main organ affected, whatever the
route of administration of the alkaloid. The alkaloids are
known to be metabolized in the liver.
(c) Direct application of these alkaloids to the skin does not
cause local toxic effects (Schoental et al., 1954), nor do
cytotoxic effects occur at sites of injection.
(d) The susceptibility of animals to the toxic actions of PAs
is related to the ability of the animal to metabolize the
alkaloids. For example, the hepatic microsomal enzymes of rats
less than 1 h old have very low activity towards retrorsine and
these rats are relatively resistant to it, whereas rats aged
several days have a high enzyme activity and are highly
susceptible to the alkaloid (Mattocks & White, 1973). Guinea-
pigs are very resistant to retrorsine, unless they have been
given phenobarbitone, which potentiates the enzymes that
metabolize it (White et al., 1973). Rats pre-treated with
microsomal enzyme inhibitors, such as SKF 525A or
chloramphenicol, have increased resistance to retrorsine or
monocrotaline (Allen et al., 1972; Mattocks, 1973). In
general, there is a good relationship between the rate of
hepatic metabolism of PAs to pyrrole in vitro (Shull et al.,
1976) and chronic toxicity. Highly resistant species, e.g.,
guinea-pigs, Japanese quail, and sheep, have a low rate of
pyrrole formation, while susceptible species, such as the
horse, cattle, and rat, have a high rate. Notable exceptions
are the rabbit and hamster, which have high rates of pyrrole
formation, but are resistant. It is possible that this may be
due to changes in the balance between activation and the
involvement of other factors, such as activity of
detoxification. For example, sheep have a high epoxide
hydrolase activity in the liver (Swick et al., 1983), which may
affect PA detoxification (Cheeke & Pierson-Goeger, 1983).
5.1.2 Isolation of pyrrolic metabolites
There is plenty of evidence that many unsaturated PAs are
converted into pyrrolic esters (dehydro-alkaloids) in the mammalian
liver (section 4.2.3). These primary pyrrolic metabolites cannot
be isolated, because of their high reactivity and rapid rate of
hydrolysis. However, their more stable hydrolysis products
(pyrrolic alcohols; dehydronecines) have been isolated and
identified. Thus dehydroheliotridine has been obtained from the
in vitro incubation of both the heliotridine-based alkaloids,
lasiocarpine and heliotrine, with rat liver microsomes (Jago et
al., 1970) and dehydro-retronecine was found to be the main
detectable pyrrolic metabolite in the liver, blood, and urine of
rats injected with the retronecine-based alkaloid, monocrotaline
(Hsu et al., 1973). There is evidence that these materials are
identical, i.e., the (±)-form resulting from racemization during
hydrolysis of the parent pyrrolic esters (Kedzierski & Buhler,
1985).
The results of these studies confirm that rat liver enzymes
convert PAs into metabolites with known cytotoxic activity (section
5.2), and imply that these metabolites are formed via the yet more
toxic and short-lived dehydro-alkaloids (Jago et al., 1970).
5.1.3 Chemical aspects of pyrrolic metabolites
5.1.3.1 Preparation
Chemical methods are available for converting unsaturated PAs
into pyrrolic esters (dehydro-alkaloids), the putative primary
toxic metabolites, enabling the physical, chemical, and
toxicological properties of the latter to be studied.
Small amounts of dehydro-pyrrolizidine alkaloids are usually
prepared by the reaction of the corresponding alkaloid N-oxides
with either acetic anhydride (Mattocks, 1969; Culvenor et al.,
1970a) or methanolic ferrous sulfate (Mattocks, 1969). The
products must be protected from moisture and from acids, which can
cause their immediate decomposition.
A variety of reagents can dehydrogenate the alkaloid bases to
pyrrolic derivatives, these include manganese dioxide (Culvenor et
al., 1970a,b; Mattocks, 1969), potassium permanganate (Culvenor et
al., 1970a), chloranil (Culvenor et al., 1970a), 2,3-dichloro-5,6-
dicyanobenzoquinone (Mattocks, 1969), iodine (Culvenor et al.,
1970b), and aryl thiols (Juneja et al., 1984). Some PAs are slowly
oxidized to pyrroles by molecular oxygen (Bick et al., 1975).
The more stable pyrrolic alcohol, dehydroretronecine (Fig. 6),
is prepared from retronecine using chloranil (Culvenor et al.,
1970a) or aqueous potassium nitro-sodisulfonate (Mattocks, 1981c)
or from retronecine N-oxide (isatinecine) using ferrous sulfate
(Mattocks, 1969). The enantiomeric dehydroheliotridine can be
prepared from heliotridine in similar ways. Racemic dehydro-
heliotridine has been synthesized (Viscontini & Gilhof-
Schaufelberger, 1971; Bohlmann et al., 1979).
4.2.1 Hydrolysis
The hydrolysis of a PA leads to the formation of the amino-
alcohol moiety (necine base) and the acid moiety. Neither of these
is hepatotoxic (Schoental & Mattocks, 1960; Culvenor et al.,
1976a). The highly water-soluble necine base is readily excreted,
is not accessible to the microsomal system, and is not activated to
a toxic metabolite. Thus, pyrrolizidine alkaloids that are very
susceptible to (enzymic) hydrolysis have low toxicity (Mattocks,
1982). A major factor contributing to resistance to esterase is
the steric hindrance in the acid moiety. Thus, the chain branching
near the carbonyl groups slows hydrolysis allowing the formation of
relatively high levels of pyrrolic metabolites; a conformation of
the basic moiety, which brings the two ester groups close together,
thus leading to mutual steric hindrance, can also prevent
hydrolysis (Mattocks, 1981a).
The influence of hydrolysis in vivo on alternative metabolic
pathways is demonstrated by the fact that treatment of rats with an
esterase inhibitor, before giving pyrrolizidine alkaloids (or
synthetic analogues), can lead to greatly increased production of
pyrrolic metabolites from alkaloids that are normally susceptible
to hydrolysis, but little increase in those from alkaloids normally
resistant to hydrolysis (Mattocks, 1981a).
4.2.2 N-oxidation
The N-oxidation of pyrrolizidine alkaloids is induced by the
hepatic microsomal enzymes. The N-oxide metabolites are highly
water soluble and are rapidly excreted in the urine (Mattocks,
1968a). Pyrrolizidine N-oxides are not converted to any
significant extent to pyrrolic metabolites by microsomal enzymes
(Jago et al., 1970; Mattocks & White, 1971a), and there is no
evidence that they are toxic, unless first reduced to the
corresponding basic alkaloids, which can then be activated by the
microsomal system (Mattocks, 1971c). Thus, it appears that the
formation of N-oxides represents a detoxification pathway.
4.2.3 Conversion to pyrrolic metabolites
In laboratory animals, toxic pyrrolizidine alkaloids are
metabolized to pyrrolic derivatives, so-called because the
unsaturated ring of the pyrrolizidine system loses 2 hydrogen atoms
to form what is in effect a pyrrole ring (though the structure is
more correctly a dihydropyrrolizidine). Pyrrolic metabolites are
easily detectable in the tissues shortly after giving a toxic
pyrrolizidine alkaloid to an animal, by treating the tissue with an
Ehrlich reagent containing boron trifluoride, when a red colour is
produced; this reaction also occurs with the urine (Mattocks,
1968a; Mattocks & White, 1970). In rats given retrosine, pyrrolic
metabolites were found principally in the liver, with highest
levels associated with the microsomal and solid debris fractions
and less in the mitochondrial fraction; low levels were found in
the lungs, heart, spleen, and kidneys, within 4 h of giving
retrosine. Rats given 60 mg retrosine/kg body weight excreted 14%
of the dose in the urine, within 48 h.
Pyrrolic metabolites are formed by the hepatic mixed-function
oxidase system, with a requirement for cytochrome P450, oxygen, and
NADPH, as has been demonstrated in vitro (Jago et al., 1970;
Mattocks & White, 1971a). Conversion of pyrrolizidine alkaloids to
pyrrolic metabolites by the lung tissue of the human embryo
(Armstrong & Zuckerman, 1970), rat (Mattocks & White, 1971a;
Hilliker et al., 1983), or rabbit (Guengerich, 1977) was
negligible. The formation of pyrrolic metabolites does not proceed
via N-oxide intermediates, but appears to result from an initial
hydroxylation of the unsaturated pyrrolizidine ring adjacent to the
nitrogen atom (Mattocks & White, 1971a; Mattocks & Bird, 1983).
This would lead to a chemically unstable intermediate that would be
expected to decompose spontaneously to the pyrrolic product. The
primary pyrrolic metabolites (or dehydro-alkaloid) formed by
dehydrogenation of pyrrolizidine alkaloids are chemically
dehydropyrrolizidine esters (Fig. 5). These are highly reactive
compounds that can rapidly react with tissue constituents or
hydrolyse to the corresponding pyrrolic alcohols, or dehydro-
necines, which can thus be regarded as secondary metabolites. The
latter can also react with tissue constituents, but more slowly.
Because of their high chemical reactivity, the primary metabolites
would be expected to have a short life in the liver cell (minutes
or seconds) before they are hydrolysed or react with nucleophilic
tissue constituents. Some might escape into the blood stream and
reach other organs, especially the lungs. Dehydro-necines are more
stable and also more water soluble, and can become more widely
distributed throughout the body. However, they are also capable of
reacting with tissue constituents. Thus, measurements of pyrroles
formed from pyrrolizidine alkaloids in tissue samples, using a
colour reaction (Mattocks & White, 1970), will not represent a
single metabolite, but mixtures of the metabolites together with
various reaction products of these with tissue constituents. It
will be seen (section 5) that pyrrolic metabolites are believed to
be responsible for major toxic actions of pyrrolizidine alkaloids
(Mattocks, 1972a).
A pyrrolic metabolite with reactivity midway between that of
dehydromonocrotaline and dehydroretronecine has been reported to be
formed from monocrotaline in isolated, perfused rat liver
(Lafranconi et al., 1985). Studies on this metabolite (isolated
from bile) indicated that it is a monoester, and that it is toxic
in perfused rat lung. This suggests that monoester pyrrolic
metabolites may play a part in the toxic actions of PAs in extra-
hepatic tissues.
When rats or other laboratory animals are given a toxic
pyrrolizidine alkaloid, pyrrolic metabolites accumulate rapidly in
the liver (Mattocks, 1973; White et al., 1973), reaching a peak
within 1 - 2 h, then falling slowly during the next 24 h; the
metabolites may still be detectable after 2 days. Accumulation is
especially rapid after intraperitoneal injection, a very high level
of pyrrolic metabolites being attained within 20 min; this
indicates how rapid the metabolism of pyrrolizidine alkaloids can
be.
The level of pyrrolic metabolites in rat liver is generally
directly related to the amount of alkaloid given 2 h previously, at
least up to an acute LD50 dose. The pyrrole level depends on the
alkaloid used, and is related to the acute hepatotoxicity of the
alkaloid (Mattocks, 1972a).
To be converted to the type of chemically reactive, toxic
pyrrolic metabolites described above, an alkaloid must possess a
1-hydroxymethyl pyrrolizidine system, unsaturated in the
1,2-position (this makes the ring susceptible to dehydrogenation),
and at least one hydroxyl group must be esterified, usually by a
branched-chain acid. Otonecine esters are converted to similar
pyrrol metabolites by a different media involving N-demethylation.
Pyrrolizidine amino-alcohols (e.g., retronecine) are not
metabolized to more than small amounts of pyrroles (Jago et al.,
1970; Mattocks, 1981a), possibly because they are too water soluble
to reach the microsomal enzymes.
The metabolic formation of pyrroles is catalysed by cytochrome
P450 and specificity exists in the various isozymes (Guengerich,
1977; Juneja et al., 1984).
A few non-toxic pyrrolizidine alkaloids (e.g., rosmarinine and
hygrophylline) are converted to pyrrolic metabolites in vivo
(Mattocks, 1973). Such metabolites are chemically different from
the pyrroles of toxic alkaloids, and they are neither reactive nor
toxic (Mattocks & White, 1971b). The balance of structural
features necessary for a pyrrolizidine alkaloid to be converted to
give high concentrations of toxic pyrrolic metabolite has been
discussed by Mattocks (1981a); the optimum conditions appear to be
met in some alkaloids that are macrocyclic diesters, such as
retrosine.
4.3 Effects of Treatments Affecting Metabolism
The formation of pyrrolic metabolites (and of N-oxides) is
altered by treatments that affect the hepatic microsomal enzymes.
Such effects have been studied by measuring rates of metabolism of
pyrrolizidine alkaloids in vitro using microsomal preparations
from animals pre-treated in various ways (Table 7). For example,
microsomes from rats given the microsomal enzyme inducers
phenobarbitone or DDT (but not those from rats given
3-methylcholanthrene) induce greatly increased pyrrole formation
and smaller increases in N-oxide formation, from the alkaloid
retrorsine (Mattocks & White, 1971a). Enzyme preparations from
rats treated with inhibitors of microsomal enzymes, including SKF
525A and chloramphenicol, are much less active in converting
monocrotaline to pyrroles (Chesney et al., 1974). The ability to
metabolize retrorsine is diminished in microsomes from rats fed a
protein-free diet, or from rats acutely poisoned with retrorsine
(Mattocks & White, 1971a).
Table 7. Effect of pre-treatment of male rats on the conversion of PAs to
pyrrolic derivatives and to N-oxides by liver microsomes
in vitro
----------------------------------------------------------------------------
Alkaloid Pre-treatment, and Enzyme activity as Reference
time before enzyme % of control values
measurements for formation of:
Pyrroles N-oxides
----------------------------------------------------------------------------
retrorsine phenobarbitone, ip, 311 232 Mattocks &
3 x 100 mg/kg, White (1971a)
1 - 3 days
retrorsine DDT, ip, 75 mg/kg, 407 203 Mattocks &
3 days White (1971a)
retrorsine 3-methylcholanthrene, 95 (ns) 116 (ns) Mattocks &
ip, 3 x 20 mg/kg, White (1971a)
1 - 3 days
retrorsine retrorsine, ip, 63 - Mattocks &
35 mg/kg, 20 h White (1971a)
retrorsine protein-free diet, 39 - Mattocks &
3 days White (1971a)
monocrotaline phenobarbitone, sc, 448 165 Chesney et al.
4 x 75 mg/kg, (1974)
1 - 4 days
monocrotaline chloramphenicol, sc, 10 109 (ns) Chesney et al.
200 mg/kg, 1 h (1974)
monocrotaline SKF 525A, ip, 75 mg/kg, 10 87 (ns) Chesney et al.
1 h (1974)
----------------------------------------------------------------------------
ns = not significantly different from controls.
The effects of in vivo treatment with several types of enzyme
inducers on the toxicity of lasiocarpine and senecionine for
primary rat hepatocyte cultures was investigated by Hayes et al.
(1985). Pre-treatment with phenobarbitone potentiated the
cytotoxicity of senecionine towards the cultured cells, whereas
pre-treatment with 3-methylcholanthrene diminished the toxic action
of senecionine, but had little effect on lasiocarpine cytotoxicity.
The cytocidal effects of both alkaloids were substantially
inhibited in the presence of SKF 525A.
4.4 Other Factors Affecting Metabolism
Variations between animal species have been investigated by
White et al. (1973) and Shull et al. (1976). For instance,
metabolism to form pyrroles is high in rats and very low in guinea-
pigs, which, however, have higher rates of N-oxidation. For
example, 2 h after an ip dose of retrosine (100 mg/kg body weight),
the liver-pyrrole level in male rats was 13 times higher than that
in male guinea-pigs (White et al., 1973). Liver microsome
preparations from male rats were 28 times more active than
microsomes from male guinea-pigs in the dehydrogenation of
monocrotaline (Chesney & Allen, 1973a).
The development with age of the ability of Wistar rats to
metabolize retrosine was studied by Mattocks & White (1973). The
ability to form pyrroles is very low in new-born rats, but, by 5
days of age, it is nearly as high as in adult males. This activity
continues at a similar level in male rats, but, in females, it
falls after the age of about 20 days until, by 60 days, it is about
one-eighth that in males. Such a sex difference was not observed
in mice (White et al., 1973).
4.5 Other Metabolic Routes
The actions of hepatic microsomal enzymes on pyrrolizidine
alkaloids can produce other metabolites as well as pyrroles and
N-oxides, but there are few reports of these. Eastman & Segall
(1982) demonstrated hydroxylation of the acid moiety of senecionine
by liver microsomes from female mice. Such metabolism should not
prevent the subsequent conversion of the product to pyrrolic or
N-oxide metabolites. The formation of other microsomal metabolites
of senecionine has been reported by Segall et al. (1984).
The O-demethylation of the acid moiety of heliotrine has been
demonstrated by Jago et al. (1969) and represents a partial
detoxification mechanism, since the product is about half as toxic
as heliotrine. Other detoxification mechanisms exist in the rumen
of sheep (Dick et al., 1963; Lanigan & Smith, 1970a,b), which are,
thus, particularly resistant to the effects of pyrrolizidine
alkaloids.
4.6 Metabolism of Pyrrolizidine N-Oxides
As mentioned in section 4.2, the N-oxides of pyrrolizidine
alkaloids are not converted to pyrrolic metabolites by liver
microsomes. It appears that their main route of metabolism in
animals is reduction to the corresponding basic alkaloids, which
may then be further metabolized as already described. This
reduction has been shown to occur in the rat or rabbit gut
(Mattocks, 1971c; Powis et al., 1979), and may be brought about by
intestinal bacteria or possibly by gut enzymes. Such reduction can
also be brought about by hepatic microsomal fractions (Powis et
al., 1979) in the presence of NADH or of NADPH, and by sheep rumen
fluid (Lanigan et al., 1970a, b).
The reduction of pyrrolizidine N-oxides in vivo is of great
importance as a step in the bioactivation of these compounds
(Mattocks, 1971c), as shown in section 4.2.2.
4.7 Metabolism in Man
Powis et al. (1979) found that indicine N-oxide given iv to
3 human patients as an antitumour drug was partially reduced to
indicine base, detectable in the urine and plasma. Armstrong &
Zuckerman (1970) showed that human embryo liver slices, but not
lung slices, converted the pyrrolizidine alkaloids lasiocarpine,
retrorsine, and fulvine to pyrrolic metabolites in vitro.
5. MECHANISMS OF TOXICITY AND OTHER BIOLOGICAL ACTIONS
5.1 Metabolites Responsible for Toxicity
5.1.1 Metabolic basis of toxicity
The toxic effects of pyrrolizidine alkaloids are mediated
through their toxic metabolites and not by the alkaloids
themselves. The following observations are evidence for the above
statement (Mattocks, 1972a):
(a) The alkaloids are chemically rather unreactive and it is
hard to envisage reactions with cell constituents that they
could undergo readily under physiological conditions. On the
other hand, chemically prepared derivatives, similar or
identical to known metabolites of these alkaloids, are highly
reactive and are capable of causing toxic effects similar to
those of PAs, often at dose levels much lower than those
required by the alkaloids themselves.
(b) The liver is usually the main organ affected, whatever the
route of administration of the alkaloid. The alkaloids are
known to be metabolized in the liver.
(c) Direct application of these alkaloids to the skin does not
cause local toxic effects (Schoental et al., 1954), nor do
cytotoxic effects occur at sites of injection.
(d) The susceptibility of animals to the toxic actions of PAs
is related to the ability of the animal to metabolize the
alkaloids. For example, the hepatic microsomal enzymes of rats
less than 1 h old have very low activity towards retrorsine and
these rats are relatively resistant to it, whereas rats aged
several days have a high enzyme activity and are highly
susceptible to the alkaloid (Mattocks & White, 1973). Guinea-
pigs are very resistant to retrorsine, unless they have been
given phenobarbitone, which potentiates the enzymes that
metabolize it (White et al., 1973). Rats pre-treated with
microsomal enzyme inhibitors, such as SKF 525A or
chloramphenicol, have increased resistance to retrorsine or
monocrotaline (Allen et al., 1972; Mattocks, 1973). In
general, there is a good relationship between the rate of
hepatic metabolism of PAs to pyrrole in vitro (Shull et al.,
1976) and chronic toxicity. Highly resistant species, e.g.,
guinea-pigs, Japanese quail, and sheep, have a low rate of
pyrrole formation, while susceptible species, such as the
horse, cattle, and rat, have a high rate. Notable exceptions
are the rabbit and hamster, which have high rates of pyrrole
formation, but are resistant. It is possible that this may be
due to changes in the balance between activation and the
involvement of other factors, such as activity of
detoxification. For example, sheep have a high epoxide
hydrolase activity in the liver (Swick et al., 1983), which may
affect PA detoxification (Cheeke & Pierson-Goeger, 1983).
5.1.2 Isolation of pyrrolic metabolites
There is plenty of evidence that many unsaturated PAs are
converted into pyrrolic esters (dehydro-alkaloids) in the mammalian
liver (section 4.2.3). These primary pyrrolic metabolites cannot
be isolated, because of their high reactivity and rapid rate of
hydrolysis. However, their more stable hydrolysis products
(pyrrolic alcohols; dehydronecines) have been isolated and
identified. Thus dehydroheliotridine has been obtained from the
in vitro incubation of both the heliotridine-based alkaloids,
lasiocarpine and heliotrine, with rat liver microsomes (Jago et
al., 1970) and dehydro-retronecine was found to be the main
detectable pyrrolic metabolite in the liver, blood, and urine of
rats injected with the retronecine-based alkaloid, monocrotaline
(Hsu et al., 1973). There is evidence that these materials are
identical, i.e., the (±)-form resulting from racemization during
hydrolysis of the parent pyrrolic esters (Kedzierski & Buhler,
1985).
The results of these studies confirm that rat liver enzymes
convert PAs into metabolites with known cytotoxic activity (section
5.2), and imply that these metabolites are formed via the yet more
toxic and short-lived dehydro-alkaloids (Jago et al., 1970).
5.1.3 Chemical aspects of pyrrolic metabolites
5.1.3.1 Preparation
Chemical methods are available for converting unsaturated PAs
into pyrrolic esters (dehydro-alkaloids), the putative primary
toxic metabolites, enabling the physical, chemical, and
toxicological properties of the latter to be studied.
Small amounts of dehydro-pyrrolizidine alkaloids are usually
prepared by the reaction of the corresponding alkaloid N-oxides
with either acetic anhydride (Mattocks, 1969; Culvenor et al.,
1970a) or methanolic ferrous sulfate (Mattocks, 1969). The
products must be protected from moisture and from acids, which can
cause their immediate decomposition.
A variety of reagents can dehydrogenate the alkaloid bases to
pyrrolic derivatives, these include manganese dioxide (Culvenor et
al., 1970a,b; Mattocks, 1969), potassium permanganate (Culvenor et
al., 1970a), chloranil (Culvenor et al., 1970a), 2,3-dichloro-5,6-
dicyanobenzoquinone (Mattocks, 1969), iodine (Culvenor et al.,
1970b), and aryl thiols (Juneja et al., 1984). Some PAs are slowly
oxidized to pyrroles by molecular oxygen (Bick et al., 1975).
The more stable pyrrolic alcohol, dehydroretronecine (Fig. 6),
is prepared from retronecine using chloranil (Culvenor et al.,
1970a) or aqueous potassium nitro-sodisulfonate (Mattocks, 1981c)
or from retronecine N-oxide (isatinecine) using ferrous sulfate
(Mattocks, 1969). The enantiomeric dehydroheliotridine can be
prepared from heliotridine in similar ways. Racemic dehydro-
heliotridine has been synthesized (Viscontini & Gilhof-
Schaufelberger, 1971; Bohlmann et al., 1979).
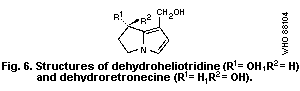 5.1.3.2 Chemistry associated with toxic actions
Dehydro-pyrrolizidine alkaloids and dehydronecines (pyrrolic
esters and alcohols) act chemically as alkylating (electrophilic)
agents, i.e., they can react with compounds possessing electron-
rich (nucleophilic) groups, such as amines, thiols, and some
hydroxyl compounds. The products of alkylation consist of the
"pyrrole" moiety covalently bonded to the substrate molecule. The
mechanism of alkylation is illustrated in Fig. 7 (Mattocks, 1972a).
An ester (R = COR) or hydroxyl group (R = H) attached to the
pyrrole ring via one carbon atom (i.e., at C7 or C9) is highly
reactive, being easily cleaved, leaving a positively charged
pyrrole moiety with a high affinity for electron-rich substrates.
Pyrrolic esters are the most reactive, RCOO being a better "leaving
group" than HO. When 2 oxygen functions are present (as
illustrated), either (in turn) can act as an alkylating centre.
Such bifunctional alkylation could lead to cross linking of
macromolecules (Mattocks, 1969; White & Mattocks, 1972; Petry et
al., 1984, 1986). When the groups (R) are the same or similar, C7
is the more reactive site. Examples of such alkylations using pure
chemicals (amines or alcohol) have been given by Mattocks (1969)
and Culvenor et al. (1970a). Mattocks & Bird (1983) showed that a
variety of nucleophiles of biological interest could be alkylated
by dehydroretronecine. Black & Jago (1970) demonstrated the
in vitro alkylation of DNA by dehydroheliotridine, and Robertson
(1982) and Wickramanayake et al. (1985) the alkylation of
deoxyguanosine by dehydroretronecine. The alkylation of mouse or
rat liver DNA by pyrrolizidine alkaloids has been shown in vivo by
Eastman et al. (1982) and Candrian et al. (1985).
5.1.3.2 Chemistry associated with toxic actions
Dehydro-pyrrolizidine alkaloids and dehydronecines (pyrrolic
esters and alcohols) act chemically as alkylating (electrophilic)
agents, i.e., they can react with compounds possessing electron-
rich (nucleophilic) groups, such as amines, thiols, and some
hydroxyl compounds. The products of alkylation consist of the
"pyrrole" moiety covalently bonded to the substrate molecule. The
mechanism of alkylation is illustrated in Fig. 7 (Mattocks, 1972a).
An ester (R = COR) or hydroxyl group (R = H) attached to the
pyrrole ring via one carbon atom (i.e., at C7 or C9) is highly
reactive, being easily cleaved, leaving a positively charged
pyrrole moiety with a high affinity for electron-rich substrates.
Pyrrolic esters are the most reactive, RCOO being a better "leaving
group" than HO. When 2 oxygen functions are present (as
illustrated), either (in turn) can act as an alkylating centre.
Such bifunctional alkylation could lead to cross linking of
macromolecules (Mattocks, 1969; White & Mattocks, 1972; Petry et
al., 1984, 1986). When the groups (R) are the same or similar, C7
is the more reactive site. Examples of such alkylations using pure
chemicals (amines or alcohol) have been given by Mattocks (1969)
and Culvenor et al. (1970a). Mattocks & Bird (1983) showed that a
variety of nucleophiles of biological interest could be alkylated
by dehydroretronecine. Black & Jago (1970) demonstrated the
in vitro alkylation of DNA by dehydroheliotridine, and Robertson
(1982) and Wickramanayake et al. (1985) the alkylation of
deoxyguanosine by dehydroretronecine. The alkylation of mouse or
rat liver DNA by pyrrolizidine alkaloids has been shown in vivo by
Eastman et al. (1982) and Candrian et al. (1985).
 5.1.4 Possible further metabolites
The possibility that pyrrolic metabolites of PAs might
themselves be metabolized by microsomal enzymes to further
cytotoxic derivatives was suggested by Guengerich & Mitchell
(1980). These authors showed that the tritium-labelled model
compounds 1,2,3-trimethylpyrrole and 1-methyl-3-4 bishydroxy-
methylpyrrole could be metabolized in rats or by rat liver
microsomes to unidentified derivatives able to bind covalently to
proteins and nucleic acids. It is possible that liver damage, seen
in some rats given iv injections of pneumotoxic pyrrolic esters,
might have been due to metabolites of the latter formed in the
liver (Mattocks & Driver, 1983). Segall et al. (1985) have
identified trans-4-hydroxy-2-hexenal in an in vitro mouse liver
microsomal system metabolizing the PA senecionine and suggested
that it might have been formed from the alkaloid via a pyrrolic
intermediate. The compound is capable of causing liver damage and
might contribute to the acute hepatotoxicity of senecionine and
other alkaloids. However, this has not been proved, and the highly
reactive and toxic primary pyrrolic metabolites from PAs are
themselves capable of causing the known hepatotoxic effects of
these alkaloids.
5.2 Toxic Actions of Pyrrolic Metabolites
Pyrrolic derivatives prepared chemically from PAs, as well as
some analogous compounds, have been tested in experimental animals
and in vitro systems, and shown to have a variety of toxic actions.
5.2.1 Animals
5.2.1.1 Pyrrolic esters (dehydro-alkaloids)
Dehydro-pyrrolizidine alkaloids are very reactive and their
effects in vivo are largely confined to the first tissues they
encounter. When given orally to rats, they are destroyed almost
immediately in the aqueous acid of the stomach and show no toxic
action. When given ip, they cause severe local irritation and
peritonitis (Mattocks, 1968a; Butler et al., 1970); subcutaneous
injection leads to skin lesions (Hooson & Grasso, 1976). After iv
injection of pyrroles, such as dehydromonocrotaline (monocrotaline
pyrrole), into the tail veins of rats, the toxic injuries appear
principally in the lungs. Depending on the dose, these include
vascular lesions and pulmonary oedema (Plestina & Stoner, 1972); a
progressive alveolar proliferation similar to that produced by
very much larger doses of the parent alkaloid (Butler et al.,
1970) and pulmonary hypertension (Hilliker et al., 1983).
Dehydromonocrotaline does not require further metabolism to express
its pneumotoxicity, and it is rapidly rendered inactive after
exposure to aqueous media (Bruner et al., 1986). Similar
pneumotoxicity is produced by totally synthetic pyrrolic esters
having a simpler structure but the same type of chemical reactivity
as the alkaloid derivatives (Mattocks & Driver, 1983), thus
confirming the chemical mechanism of this action.
Injections of dehydro-pyrrolizidine alkaloids or synthetic
analogues into mesenteric veins of rats lead to liver damage after
smaller doses than the alkaloids themselves (Butler et al., 1970;
Shumaker et al., 1976). The liver damage differs somewhat from the
alkaloid damage, consistent with the toxin being introduced via the
hepatic vascular system rather than being produced within the
hepatocytes, as is the case with the alkaloids. Nevertheless, the
progressive liver lesions are very similar to those produced by PAs
(Butler et al., 1970). The lung damage after tail vein injections
bears a closer resemblance to pyrrolizidine damage, since the
latter is also believed to be caused by metabolites entering the
lungs via the bloodstream (Barnes et al., 1964).
5.2.1.2 Pyrrolic alcohols (dehydro-necines)
Dehydroheliotridine (Fig. 6), a secondary pyrrolic metabolite
from heliotridine-based PAs, such as heliotrine and lasiocarpine,
is less acutely toxic than its parent alkaloids; it has an LD50 (7
days) of about 250 mg/kg body weight in mice (Percy & Pierce,
1971). Its effects on 14-day-old rats were studied by Peterson et
al. (1972). All rats given ip doses of 0.4 mmol/kg body weight
survived, but a dose of 0.6 mmol/kg killed most animals within 10
days. Toxic effects were mainly found in rapidly developing
tissues. In young rats, it caused fur loss, tooth defects, and
atrophy of hair follicles, gut mucosa, spleen, thymus, testis, and
bone marrow. The lungs were not affected. Pathological effects in
the liver were confined to necrosis of isolated cells and
antimitotic action, which was manifested as a mild megalocytosis
(development of giant hepatocytes) in rats surviving 4 weeks or
more. The persistent antimitotic action of dehydroheliotridine and
of its parent alkaloid lasiocarpine in the liver of rats was
investigated by Samuel & Jago (1975), who located the mitotic block
as being either late in the DNA synthetic (S) phase or early in the
post synthetic (G2) phase of the cell cycle.
Dehydroheliotridine is also carcinogenic. Peterson et al.
(1983) showed that rats given 9 ip injections of this compound
(60 - 76.5 mg/kg body weight) over 23 weeks had a shorter life span
and suffered a significantly higher incidence of tumours than control
rats. The authors concluded that dehydroheliotridine is responsible
for some, or possibly all, of the carcinogenicity of its parent
alkaloids.
Dehydroheliotridine was found to be teratogenic when given ip
to female hooded rats on the 14th day of pregnancy. A dose of
40 mg/kg body weight produced effects similar to those produced by the
alkaloid heliotrine at a dose of 200 mg/kg (Peterson & Jago, 1980).
For the immunosuppressant activity of this compound, see section
6.4.10.
The toxic actions of dehydroretronecine (DHR) (Fig. 7) when
given sc to rats are similar to those of dehydroheliotridine (Hsu
et al., 1973; Shumaker et al., 1976). Repeated large doses also
caused ulceration of the glandular stomach. Daily sc doses
(4 mg/kg body weight), administered to rats for 1 week, caused lung
damage leading to right ventricular hypertrophy (Huxtable et al.,
1978). DHR was carcinogenic when applied repeatedly to mouse skin
(Johnson et al., 1978; Mattocks & Cabral, 1982).
5.2.2 Cell cultures
Dehydroheliotridine and dehydrosupinidine both have an
inhibitory action in cultures of KB cells (human epidermoid
carcinoma of the nasopharynx) with ED50 concentrations of 10-4 mol
and 10-5 mol, respectively (Culvernor et al., 1969).
Bick & Culvenor (1971) found dehydroheliotridine (DHR) to be
considerably more effective than the alkaloid heliotrine in
suppressing cell division and causing chromosome breaks, in
cultures of leukocytes from the marsupial Potorus tridactylus; at a
concentration of 6 x 10-5 mol, the mitotic index was zero, and more
than half the cells had disintegrated. In a study by Mattocks &
Legg (1980), dehydroretronecine and several synthetic analogues
completely inhibited cell division in a cultured rat liver cell
line at a concentration of 10-4 mol. Ord et al., (1985) found that
DHR induced sister chromatid exchange in human lymphocytes without
the need for metabolic activation. Analogous pyrroles with only
one functional (reactive) group were much less effective. DHR was
also weakly active in inducing mutations in the Salmonella
typhimurium base substitution strain, TA92, and gave positive
results in an in vitro cell transformation test using a culture
derived from hamster kidney cells (Styles et al., 1980).
The toxicity of the pyrrolic ester, dehydromonocrotaline, for
cultures of mouse fibroblasts was studied in vitro by Johnson
(1981). The level of exposure was approximately 1 ng per cell.
Cell death was preceded, first by the swelling and disruption of
organelles, including mitochondria, and then by the rupture of
plasma membranes with the release of cell components.
Bick et al., (1975) investigated whether the effects of PAs on
leukocyte cultures of Potorus tridactylus were due to pyrrolic
metabolites. Levels of dihydropyrrolizines, which could be
demonstrated in the culture media, were insufficient to account for
the observed effects of heliotrine, lasiocarpine, and monocrotaline
on the cells, but the actual amounts formed within the cells may
have been higher than those observed.
5.2.3 Possible participation of membrane lipid peroxidation
Distinct increases in NADPH- and ascorbate-dependent
peroxidation of microsomal membrane lipids were found in rats given
heliotrine subcutaneously (300 mg/kg body weight) (Savin 1983).
The primary biochemical interactions and cellular macromolecular
targets for the pathogenesis of PA-induced toxicity remain
unidentified.
5.3 Chemical and Metabolic Factors Affecting Toxicity
The toxicity of an alkaloid depends on the extent to which it
is converted into active metabolites and on the disposition and
reactivity of these metabolites, once formed.
5.3.1 Structural features of a toxic alkaloid
The essential structural features of a hepatotoxic PA (Fig. 8)
are:
(a) a 1-hydroxymethylpyrrolizidine ring system unsaturated in
the 1:2-position, with preferably a second hydroxyl group
in the 7-position;
(b) esterification of at least one of the hydroxyls, though
toxicity is much greater when both hydroxyls are
esterified; and
(c) ester groups that are resistant to enzymic hydrolysis,
which usually means that there is a high degree of chain
branching in the acid moiety.
The above requirements apply to natural PAs but, strictly
speaking, only the right hand (pyrroline) ring is essential, being
the ring that is metabolized to a pyrrole derivative. Thus, esters
of 2,3-bis-hydroxymethyl-1-methyl-3-pyrroline (synthanecine A)
(Fig. 9) have pyrrolizidine-like hepatotoxicity (Mattocks, 1971a;
Driver & Mattocks, 1984).
5.1.4 Possible further metabolites
The possibility that pyrrolic metabolites of PAs might
themselves be metabolized by microsomal enzymes to further
cytotoxic derivatives was suggested by Guengerich & Mitchell
(1980). These authors showed that the tritium-labelled model
compounds 1,2,3-trimethylpyrrole and 1-methyl-3-4 bishydroxy-
methylpyrrole could be metabolized in rats or by rat liver
microsomes to unidentified derivatives able to bind covalently to
proteins and nucleic acids. It is possible that liver damage, seen
in some rats given iv injections of pneumotoxic pyrrolic esters,
might have been due to metabolites of the latter formed in the
liver (Mattocks & Driver, 1983). Segall et al. (1985) have
identified trans-4-hydroxy-2-hexenal in an in vitro mouse liver
microsomal system metabolizing the PA senecionine and suggested
that it might have been formed from the alkaloid via a pyrrolic
intermediate. The compound is capable of causing liver damage and
might contribute to the acute hepatotoxicity of senecionine and
other alkaloids. However, this has not been proved, and the highly
reactive and toxic primary pyrrolic metabolites from PAs are
themselves capable of causing the known hepatotoxic effects of
these alkaloids.
5.2 Toxic Actions of Pyrrolic Metabolites
Pyrrolic derivatives prepared chemically from PAs, as well as
some analogous compounds, have been tested in experimental animals
and in vitro systems, and shown to have a variety of toxic actions.
5.2.1 Animals
5.2.1.1 Pyrrolic esters (dehydro-alkaloids)
Dehydro-pyrrolizidine alkaloids are very reactive and their
effects in vivo are largely confined to the first tissues they
encounter. When given orally to rats, they are destroyed almost
immediately in the aqueous acid of the stomach and show no toxic
action. When given ip, they cause severe local irritation and
peritonitis (Mattocks, 1968a; Butler et al., 1970); subcutaneous
injection leads to skin lesions (Hooson & Grasso, 1976). After iv
injection of pyrroles, such as dehydromonocrotaline (monocrotaline
pyrrole), into the tail veins of rats, the toxic injuries appear
principally in the lungs. Depending on the dose, these include
vascular lesions and pulmonary oedema (Plestina & Stoner, 1972); a
progressive alveolar proliferation similar to that produced by
very much larger doses of the parent alkaloid (Butler et al.,
1970) and pulmonary hypertension (Hilliker et al., 1983).
Dehydromonocrotaline does not require further metabolism to express
its pneumotoxicity, and it is rapidly rendered inactive after
exposure to aqueous media (Bruner et al., 1986). Similar
pneumotoxicity is produced by totally synthetic pyrrolic esters
having a simpler structure but the same type of chemical reactivity
as the alkaloid derivatives (Mattocks & Driver, 1983), thus
confirming the chemical mechanism of this action.
Injections of dehydro-pyrrolizidine alkaloids or synthetic
analogues into mesenteric veins of rats lead to liver damage after
smaller doses than the alkaloids themselves (Butler et al., 1970;
Shumaker et al., 1976). The liver damage differs somewhat from the
alkaloid damage, consistent with the toxin being introduced via the
hepatic vascular system rather than being produced within the
hepatocytes, as is the case with the alkaloids. Nevertheless, the
progressive liver lesions are very similar to those produced by PAs
(Butler et al., 1970). The lung damage after tail vein injections
bears a closer resemblance to pyrrolizidine damage, since the
latter is also believed to be caused by metabolites entering the
lungs via the bloodstream (Barnes et al., 1964).
5.2.1.2 Pyrrolic alcohols (dehydro-necines)
Dehydroheliotridine (Fig. 6), a secondary pyrrolic metabolite
from heliotridine-based PAs, such as heliotrine and lasiocarpine,
is less acutely toxic than its parent alkaloids; it has an LD50 (7
days) of about 250 mg/kg body weight in mice (Percy & Pierce,
1971). Its effects on 14-day-old rats were studied by Peterson et
al. (1972). All rats given ip doses of 0.4 mmol/kg body weight
survived, but a dose of 0.6 mmol/kg killed most animals within 10
days. Toxic effects were mainly found in rapidly developing
tissues. In young rats, it caused fur loss, tooth defects, and
atrophy of hair follicles, gut mucosa, spleen, thymus, testis, and
bone marrow. The lungs were not affected. Pathological effects in
the liver were confined to necrosis of isolated cells and
antimitotic action, which was manifested as a mild megalocytosis
(development of giant hepatocytes) in rats surviving 4 weeks or
more. The persistent antimitotic action of dehydroheliotridine and
of its parent alkaloid lasiocarpine in the liver of rats was
investigated by Samuel & Jago (1975), who located the mitotic block
as being either late in the DNA synthetic (S) phase or early in the
post synthetic (G2) phase of the cell cycle.
Dehydroheliotridine is also carcinogenic. Peterson et al.
(1983) showed that rats given 9 ip injections of this compound
(60 - 76.5 mg/kg body weight) over 23 weeks had a shorter life span
and suffered a significantly higher incidence of tumours than control
rats. The authors concluded that dehydroheliotridine is responsible
for some, or possibly all, of the carcinogenicity of its parent
alkaloids.
Dehydroheliotridine was found to be teratogenic when given ip
to female hooded rats on the 14th day of pregnancy. A dose of
40 mg/kg body weight produced effects similar to those produced by the
alkaloid heliotrine at a dose of 200 mg/kg (Peterson & Jago, 1980).
For the immunosuppressant activity of this compound, see section
6.4.10.
The toxic actions of dehydroretronecine (DHR) (Fig. 7) when
given sc to rats are similar to those of dehydroheliotridine (Hsu
et al., 1973; Shumaker et al., 1976). Repeated large doses also
caused ulceration of the glandular stomach. Daily sc doses
(4 mg/kg body weight), administered to rats for 1 week, caused lung
damage leading to right ventricular hypertrophy (Huxtable et al.,
1978). DHR was carcinogenic when applied repeatedly to mouse skin
(Johnson et al., 1978; Mattocks & Cabral, 1982).
5.2.2 Cell cultures
Dehydroheliotridine and dehydrosupinidine both have an
inhibitory action in cultures of KB cells (human epidermoid
carcinoma of the nasopharynx) with ED50 concentrations of 10-4 mol
and 10-5 mol, respectively (Culvernor et al., 1969).
Bick & Culvenor (1971) found dehydroheliotridine (DHR) to be
considerably more effective than the alkaloid heliotrine in
suppressing cell division and causing chromosome breaks, in
cultures of leukocytes from the marsupial Potorus tridactylus; at a
concentration of 6 x 10-5 mol, the mitotic index was zero, and more
than half the cells had disintegrated. In a study by Mattocks &
Legg (1980), dehydroretronecine and several synthetic analogues
completely inhibited cell division in a cultured rat liver cell
line at a concentration of 10-4 mol. Ord et al., (1985) found that
DHR induced sister chromatid exchange in human lymphocytes without
the need for metabolic activation. Analogous pyrroles with only
one functional (reactive) group were much less effective. DHR was
also weakly active in inducing mutations in the Salmonella
typhimurium base substitution strain, TA92, and gave positive
results in an in vitro cell transformation test using a culture
derived from hamster kidney cells (Styles et al., 1980).
The toxicity of the pyrrolic ester, dehydromonocrotaline, for
cultures of mouse fibroblasts was studied in vitro by Johnson
(1981). The level of exposure was approximately 1 ng per cell.
Cell death was preceded, first by the swelling and disruption of
organelles, including mitochondria, and then by the rupture of
plasma membranes with the release of cell components.
Bick et al., (1975) investigated whether the effects of PAs on
leukocyte cultures of Potorus tridactylus were due to pyrrolic
metabolites. Levels of dihydropyrrolizines, which could be
demonstrated in the culture media, were insufficient to account for
the observed effects of heliotrine, lasiocarpine, and monocrotaline
on the cells, but the actual amounts formed within the cells may
have been higher than those observed.
5.2.3 Possible participation of membrane lipid peroxidation
Distinct increases in NADPH- and ascorbate-dependent
peroxidation of microsomal membrane lipids were found in rats given
heliotrine subcutaneously (300 mg/kg body weight) (Savin 1983).
The primary biochemical interactions and cellular macromolecular
targets for the pathogenesis of PA-induced toxicity remain
unidentified.
5.3 Chemical and Metabolic Factors Affecting Toxicity
The toxicity of an alkaloid depends on the extent to which it
is converted into active metabolites and on the disposition and
reactivity of these metabolites, once formed.
5.3.1 Structural features of a toxic alkaloid
The essential structural features of a hepatotoxic PA (Fig. 8)
are:
(a) a 1-hydroxymethylpyrrolizidine ring system unsaturated in
the 1:2-position, with preferably a second hydroxyl group
in the 7-position;
(b) esterification of at least one of the hydroxyls, though
toxicity is much greater when both hydroxyls are
esterified; and
(c) ester groups that are resistant to enzymic hydrolysis,
which usually means that there is a high degree of chain
branching in the acid moiety.
The above requirements apply to natural PAs but, strictly
speaking, only the right hand (pyrroline) ring is essential, being
the ring that is metabolized to a pyrrole derivative. Thus, esters
of 2,3-bis-hydroxymethyl-1-methyl-3-pyrroline (synthanecine A)
(Fig. 9) have pyrrolizidine-like hepatotoxicity (Mattocks, 1971a;
Driver & Mattocks, 1984).
 Structural requirements for N -oxides are the same as those for
the hepatotoxic alkaloids. However, it is important to note that a
PA N -oxide is not hepatotoxic itself; toxicity depends on it being
reduced to the corresponding basic alkaloid, chiefly in the gut
(Mattocks 1971c), but possibly in other organs, such as the liver
(Powis et al., 1979).
Structural requirements for N -oxides are the same as those for
the hepatotoxic alkaloids. However, it is important to note that a
PA N -oxide is not hepatotoxic itself; toxicity depends on it being
reduced to the corresponding basic alkaloid, chiefly in the gut
(Mattocks 1971c), but possibly in other organs, such as the liver
(Powis et al., 1979).
 5.3.2 Activation and detoxication
Factors affecting the proportion of an ingested alkaloid that
is converted into toxic metabolites in an animal include the
following:
(a) Lipid solubility
Highly water-soluble alkaloids (such as indicine) are easily
excreted and have low toxicity. Alkaloids that are more lipophilic
are more open to activation by liver microsomes (Mattocks, 1981a).
(b) Subceptibility to hydrolysis
This is determined by the molecular structure and conformation
of the alkaloid (Mattocks, 1981a,b). If the alkaloid is open to
esterase attack, it may be largely detoxified by hydrolysis.
(c) Susceptibility to N-oxidation
The relative amounts of an alkaloid converted by hepatic
microsomal enzymes to N-oxide and to pyrrolic metabolites depends
on its molecular structure and conformation (Mattocks & Bird,
1983). N-oxidation represents a detoxication pathway (Mattocks,
1972b).
5.3.3 Factors affecting the toxicity of active metabolites
5.3.3.1 Reactivity of the metabolite
Toxic metabolites are formed in liver cells. Primary pyrrolic
metabolites (dehydro-alkaloids) are very reactive and, thus, are
quickly hydrolysed or deactivated by reaction with cell constituents.
To damage tissues other than the cells in which they are formed,
active metabolites must cross the cell membrane and survive while
being transported in the bloodstream. The more stable pyrrolic
metabolites, such as dehydromonocrotaline from the alkaloids
monocrotaline, are able to reach, and become bound to, lung tissue
(Mattocks, 1973). Thus, monocrotaline frequently damages the lungs,
whereas retrorsine, which yields a more reactive pyrrolic metabolite,
normally does not.
Secondary metabolites (pyrrolic alcohols, e.g., dehydroretronecine),
formed by the hydrolysis of primary pyrrolic metabolites, are water
soluble, relatively stable compounds that can become more widely
distributed throughout the body or excreted; these are not acutely
toxic.
5.3.3.2 The number of reactive groups
The toxicity of a pyrrolic alkylating agent is affected by the
number of reactive ester or hydroxyl groups (1 or 2) present as the
following examples show:
(a) Many pyrrolic esters can cause acute lung damage when
given iv to rats, but only bifunctional ones also cause
delayed effects on the lungs (Mattocks & Driver, 1983).
(b) Bifunctional pyrrolic alcohols are more effective
inhibitors of mitosis in cultured cells than monofunctional
pyrroles (Mattocks & Legg, 1980).
(c) Bifunctional pyrrolic alcohols are much better inducers
of sister chromatid exchange (SCE) in lymphocytes than
monoalcohols (Ord et al., 1985).
Reasons for these differences might be that the bifunctional
pyrroles are able to crosslink macromolecules or simply that they
can bind more strongly to target molecules.
5.4 Metabolites Associated with the Biological Actions of
Pyrrolizidine Alkaloids
5.4.1 Acute hepatotoxicity
The following is good evidence that acute liver necrosis is
caused by primary pyrrolic ester metabolites (dehydro-alkaloids):
(a) The liver, in which these metabolites are formed, is the
only organ exposed to them in relatively high concentrations.
(b) There are good correlations between amounts of pyrroles
bound to liver tissue and acute hepatotoxicity (Mattocks,
1973).
(c) Pyrrolic alcohols are not acutely hepatotoxic, even when
given to animals in very large amounts.
(d) Pyrrolic esters injected iv into the liver are much more
acutely hepatotoxic than the parent alkaloids (Butler et al.,
1970).
It is possible that other metabolites, such as 4-hydroxy
2,3-unsaturated aldehydes, might also contribute to the acute
hepatotoxicity of some PAs (Segall et al., 1985). However, this
has still to be confirmed.
5.4.2 Chronic hepatotoxicity
The persistent antimitotic action on the liver that leads to
the formation of giant hepatocytes can be produced both by pyrrolic
ester metabolites, such as dehydromonocrotaline (Hsu et al., 1973),
and by pyrrolic alcohols, such as dehydroheliotridine (Peterson et
al., 1972). Both kinds of metabolites can lead to similar
alkylation products and both are likely to be present in the liver
when the alkaloids are metabolized. Thus, either could be
responsible for chronic hepatotoxic effects. However, the
antimitotic action alone is not sufficient. It must be accompanied
or followed by a stimulus of cell division. This may be provided
by the acute necrotic effect of primary pyrrolic metabolites or by
any other cause of acute liver injury that leads to tissue
regeneration. In very young animals, the stimulus can be the
enhanced rate of replication that already exists in them.
5.4.3 Pneumotoxicity
Characteristic pyrrolizidine lung damage is produced by iv
injections of pyrrolic ester metabolites, which are effective at
much lower doses than the parent alkaloids. The latter are not
metabolized in lung tissue; thus, lung damage from PAs is believed
to be due to pyrrolic esters reaching the lungs from the liver
(Butler et al., 1970). Chronic lung damage appears to be caused by
bifunctional rather than by monofunctional pyrrolic alkylating
agents (Mattocks & Driver, 1983) (section 5.3.3.2).
There is some evidence that pyrrolic alcohol metabolites might
also be able to contribute to chronic (but not acute)
pneumotoxicity (Huxtable et al., 1978).
5.4.4 Toxicity in other tissues
Chronic heart damage including right ventricular hypertrophy is
a consequence of pyrrolizidine lung damage (pulmonary hypertension)
(Hayashi et al., 1967). Brain damage is attributed to ammonia
intoxication secondary to severe pyrrolizidine liver injury
(Hooper, 1972). This view has been contested and some PAs are
known to have direct effects on the central nervous system (section
6.4.3). There is no evidence that PAs are appreciably metabolized
in tissues other than the liver. Thus, damage to other organs is
probably due to metabolites transported from the liver. For
example, in the relatively uncommon cases of chronic kidney damage
after pyrrolizidine intoxication (Hooper, 1974; Hooper & Scanlan,
1977) megalocytosis in this organ suggests that pyrrolic
metabolites (either ester or alcohol) are involved. Overall,
patterns of disease, as observed in extrahepatic sites, are
consistent with a "spillover" effect of the pyrroles produced in
the liver (Hooper, 1978). Toxicity of an alkaloid reflects its
rate of metabolism to a pyrrole (Tuchweber et al., 1974) and so the
spillover effect is likely to be more evident at higher doses.
Studies of Culvenor et al. (1976a) suggest that the PAs that are
hepatotoxic for rats should also be pneumotoxic when administered
at higher doses. In acute poisoning, the hepatotoxic effects could
outweigh the pneumotoxic effects or those on other organs, to such
a degree that the latter are not manifested. Variation in
expression of disease (primarily hepatic or extrahepatic) also
depends on the reactions of host tissues in different species of
animals, in addition to the quantities of the pyrroles (Hooper,
1978). The sensitivity of the blood vessels might explain severe
interstitial pneumonias in some animals, or severe nephroses in
pigs (McGrath et al., 1975).
5.4.5 Carcinogenicity
The pyrrolic alcohols dehydroretronecine and dehydroheliotridine
are known carcinogens (Johnson et al., 1978; Peterson et al., 1983),
whereas the pyrrolic esters dehydromonocrotaline and dehydroretrorsine
are only carcinogenic in conjunction with a tumour promotor (Mattocks
& Cabral, 1979, 1982). This suggests that the more persistent
secondary metabolites (pyrrolic alcohols) might account for the rather
weak carcinogenicity of some PAs.
5.4.6 Antitumour activity
Some PAs and their N-oxides are active as tumour inhibitors in
test systems (Culvenor, 1968; Suffness & Cordell, 1985). Indicine
N-oxide, in particular, showed high activity against B16 melanoma,
mammary xenograft, M5076 sarcoma, P388 leukaemia, and Walker 256
carcinoma. In clinical studies, indicine N-oxide has shown
significant activity against some forms of leukaemia, with dosage
limited mainly by myelosuppression and sometimes by hepatotoxicity.
It is tempting to suppose that this action is related to the
powerful antimitotic action of their pyrrolic metabolites, even
though some of these alkaloids and derived pyrroles are themselves
carcinogenic. On the other hand, there is evidence suggesting that
indicine N-oxide owes it activity to a property of the compound
itself rather than to the pyrrolic metabolites, which could be
formed through reduction to indicine (Powis et al., 1979). The
evidence, that indicine is less effective than indicine N-oxide, is
not conclusive and other structure-activity data (Milkowsky, 1985)
point to a need for a structural capability to form a pyrrolic
metabolite. It is also possible that indicine N-oxide is converted
directly to dehydroindicine by mitochondrial enzymes in liver or
tumour cells, since the type of reaction required has been observed
in the mitochondrial metabolism of the N-oxides of tryptamine
alkaloids and certain methylated amino acids (Fish et al., 1956;
Smith et al., 1962).
5.5 Prevention and Treatment of Pyrrolizidine Poisoning
There is no known way to prevent pyrrolizidine liver damage,
once a hepatotoxic dose of the alkaloid has been ingested. A
number of dietary regimes have been found to partially protect
animals (chiefly rodents) from the acute effects of subsequent
alkaloids ingestion. None of these are of any practical use for
preventing pyrrolizidine intoxication in livestock. Furthermore,
chronic toxic effects in the liver or in other organs are sometimes
more severe in animals receiving higher doses of alkaloids after
being protected against acute hepatotoxicty.
5.5.1 Modified diets
The mechanism of action of modified diets is not clear, but
they may be associated with the decreased metabolic activation of
the alkaloids. Some examples follow:
(a) A protein-rich diet can give some protection to rats
against Senecio jacobaea alkaloids (Cheeke & Gorman, 1974).
Rats fed a high casein diet survived longer than rats given a
normal diet, when poisoned with retrorsine or riddelline, but
the survivors were more liable to develop liver tumours
(Schoental & Head, 1957). However, whether this was simply due
to a prolongation of life of the animals by the diet is open to
question.
(b) Male rats previously fed a sucrose-only diet for 4 days
were considerably protected against the acute hepatotoxicity of
retrorsine (LD50 120 mg/kg body weight compared with 34 mg/kg
in normal rats). However, lung damage, rare in control rats,
was frequently seen in "protected" rats given high doses of
retrorsine (Mattocks, 1973).
(c) Restriction of feed intake to 40% of normal attenuated the
increase in lung weight and lavage protein concentration in
cell-free bronchopulmonary lavage fluid and abolished the right
ventricular hypertrophy in monocrotaline-treated rats.
Furthermore, the percentage of diet-restricted animals that
survived was significantly higher than that in animals that had
eaten ad libitum up to day 28, but, from this time onwards,
there was no difference. Alterations of dietary sodium intake
alone did not affect the results of monocrotaline-induced
toxicity (Ganey et al., 1985).
5.5.2 Pretreatment to enhance the detoxication of active metabolites
Treatments that have afforded some protection against
pyrrolizidine hepatotoxicity (probably by increasing the liver
level of sulfydryl compounds, which are known to react with
pyrrolic metabolites) (White, 1976) include the following:
(a) Pre-treatment of rats with mercaptoethylamine (150 mg/kg
body weight ip) partially protected rats against the acute
hepatotoxicity of monocrotaline given 15 min later (Hayashi &
Lalich, 1968); it gave no protection when administered 2 h
after the alkaloid. Mercaptoethylamine, when given orally
(300 mg/kg body weight) at the same time as the lasiocarpine,
also increased the resistance of rats to the alkaloid (Rogers &
Newberne, 1971).
(b) Cysteine (1% in the diet) partially protected rats against
Senecio jacobaea alkaloids (Buckmaster et al., 1977) and mice
against monocrotaline (Miranda et al., 1981c).
(c) The antioxidant ethoxyquin fed at a level of 2.5 g/kg diet
to female mice for 38 days, increased the liver thiol
concentration and raised the acute LD50 of monocrotaline, given
ip on the 10th day, to 364 mg/kg compared with 243 mg/kg in
control mice (Miranda et al., 1981a).
(d) Rats or mice also had increased resistance to acute
pyrrolizidine hepatotoxicity when fed the antioxidant butylated
hydroxyanisole (BHA) (up to 7.5 g/kg diet) (Miranda et al.,
1981c, 1982a,b; Kim & Jones, 1982).
(e) Heliotrine-induced toxicity can be modified by the
co-administration of cupir (a copper-containing complex) at a
level of 1 mg/kg per day for 20 days. It prevented the exit of
hepatic cytosolic enzymes into the blood and improved all the
energy reactions studied in the mitochondria of heliotrine-
intoxicated rats (Yuldasheva & Sultanova, 1983). Inhibition of
lipid peroxidation by cytoplasmic copper was shown later
(Wittig & Stephen, 1964). Savin (1983) found that lethality to
rats of heliotrine (300 mg/kg sc) was completely prevented by
co-administration of alpha-tocopherol (6 ml/kg ip).
(f) Rats pre-treated with ip doses of zinc chloride (72 µmol/kg
body weight) had increased resistance to the hepatotoxicity of
Senecio jacobaea alkaloids, as assessed by histology and enzyme
measurements (Miranda et al., 1982c). The zinc treatment increased
the liver level of metallothionein, a sulfhydryl-rich protein that
might react with pyrrolic metabolites.
Metabolic inhibitors of the microsomal P450 mixed-function
oxidase system, SKF 525A, metyrapone, and allylisopropyl acetamide,
which inhibit the formation of toxic pyrroles in the liver, have
been tried successfully in the prevention of the toxic effects of
monocrotaline in rats (Eisenstein & Huxtable, 1979). The use of
P450 inhibitors was stated to show "potential therapeutic promise".
However, this would seem impracticable considering that, at least
in the rat, PAs undergo a high rate of metabolism commencing a few
minutes after ingestion (Mattocks, 1972b). In some instances, they
have been known to lead to an increase in toxicity, e.g., with
lasiocarpine as reported by Tuchweber et al. (1974).
5.5.3 Other treatments
Lanigan & Whittem (1970) attempted, unsuccessfully, to protect
sheep against Heliotropium europaeum poisoning by treating them
with cobalt, in the hope that this would enhance the vitamin
B12-mediated detoxication of the alkaloids in the rumen (Dick et
al., 1963).
Lanigan et al. (1978) found that the resistance of sheep to
dietary Heliotropium europaeum was increased by giving them large
daily doses of the antimethanogenic drug, iodoform. However, Swick
et al., (1983) found that Senecio jacobaea alkaloids were not
detoxified by incubation for 48 h with sheep rumen fluid in vitro.
6. EFFECTS ON ANIMALS
6.1 Patterns of Disease Caused by Different Plant Genera and of
Organ Involvement in Different Species
The most important genera of PA-containing plants listed in
section 3.1 are all hepatotoxic. Among these, Crotalaria spp.
cause damage in the broadest range of tissues in most domestic
species. In pigs, they are known to be severely nephrotoxic
(Peckham et al., 1974; McGrath et al., 1975; Hooper & Scanlan,
1977). Some species are known to be pneumotoxic for horses (Watt &
Breyer-Brandwijk, 1962; Gardiner et al., 1965), cattle (Sanders et
al., 1936; Berry & Bras, 1957), sheep (Laws, 1968), and pigs
(Peckham et al., 1974; Hooper & Scanlan, 1977), as well as
hepatotoxic.
Although several Crotalaria spp. are known to be pneumotoxic
for horses (Gardiner et al., 1965), C. retusa is an exception. It
is an important cause of disease in horses in northern Australia
(Hooper, 1978) and has been shown to be pneumotoxic for pigs in the
same area (Hooper & Scanlan, 1977); yet it produces only hepatic
disease in horses (Rose et al., 1957a,b).
Similarly, Senecio spp. are primarily hepatotoxic, but
S. jacobaea has been demonstrated to be pneumotoxic for pigs
(Harding et al., 1964), though it could probably be an inconsistent
change (Bull et al., 1968). This plant is also known to cause
pulmonary disease in rats and mice (Hooper, 1974). However, there
are no reports of its affecting the lungs in cattle, sheep, horses,
or chicken. Renal megalocytosis and mild nephrosis are reported in
most species poisoned with S. jacobaea (Harding et al., 1964; Bull
et al., 1968). Heliotropium spp., Amsinckia spp., and Echium spp.
are all mainly hepatotoxic.
Roitman (1983) summarized the pattern of organ involvement
observed in man and different species of farm and experimental
animals affected by pyrrolizidine alkaloids (Table 8). Even within
a single species, the nature of a toxic effect, as well as the
organ affected, can be altered by changing the dose rate and
duration.
6.2 Field Observations - Outbreaks in Farm Animals
The veterinary problem of PA toxicity has been reviewed by Bull
et al. (1968) and McLean (1970). Mattocks (1986) listed the cases
of livestock poisoning and feeding trials since 1968, and cited
relevant literature. Peterson & Culvenor (1983) produced a useful
and comprehensive table of the plant species known or suspected of
causing natural outbreaks of poisoning in each animal species. The
influence of factors such as species, age, sex, and diet, on
toxicity is also reviewed in the same paper.
Table 8. Animal species and organs affected by pyrrolizidine
alkaloidsa
---------------------------------------------------------------
Species Liver Lung Kidney Heart Pancreas Gastric Muscle
mucosa
---------------------------------------------------------------
Man +
Monkey + + + +
Horse + + +
Pig + + + + +
Sheep + + +
Goat + +
Cattle + +
Dog +
Mouse + +
Rat + + + +
Chicken + + + + +
Turkey + + +
---------------------------------------------------------------
a From: Roitman (1983).
The first cases of pyrrolizidine poisoning were described in
cattle as early as 1903 (Gilruth, 1903). Since then, there have
been numerous reports from most parts of the world, of poisoning
among farm animals caused by grazing or feeding on PA-containing
weeds (Bull et al., 1968; Mattocks, 1986). One of the first clues
to the etiology of the human disease in Jamaica, came from a study
in which calves fed with Crotalaria fulva (Bras et al., 1957)
developed characteristic veno-occlusive disease in the liver.
Laws (1968) described a field outbreak in sheep in a herd of
100 adult merino ewes, which developed within 2 weeks of moving
into a coastal farm in Australia, where they grazed Crotalaria
mucronata. The etiology was confirmed by feeding the plant to 6
sheep, 4 of which died within 24 h of feeding, with severe
pulmonary oedema. However, the rapidity of poisoning and the
atypical lung lesions suggest that possibly a toxin other than
pyrrolizidine alklaoids was also present.
An outbreak of Crotalaria retusa poisoning was observed in a
piggery near Darwin, Northern territory, Australia (Hooper &
Scanlan, 1977) containing approximately 350 sows. It was caused by
feeding sorghum contaminated by Crotalaria seeds at the rate of
about 0.1% by weight for about 3 weeks, and at a rate of about
0.05% for a further week. The disease was indicated by reduced
body weight gain and inappetence. The dominant pathological
features at autopsy were severe nephrosis with chronic uraemia, and
to a lesser degree, severe diffuse interstitial pneumonia. Both
were accompanied by microscopic disease in the liver, and both the
liver and kidney showed megalocytosis.
Walker & Kirkland (1981) reported outbreaks in the Hunter river
valley of New South Wales in Australia, in cattle that had been
grazing a pasture in which Senecio lautus was growing. There were
sporadic deaths among the cattle as well as two protracted
outbreaks affecting calves 3.5 months of age and older animals, in
which groups of 3 - 16 head of cattle died in addition to sporadic
deaths of animals over periods of 1 and 6 months. Clinical signs
were weakness, emaciation with recumbency, aimless wandering and
ataxia, which suggested neurological involvement. At autopsy, the
liver showed characteristic megalocytosis, periportal fibrosis, and
focal necroses. An aged animal had cirrhosis. The etiology of
S. lautus was proved in feeding studies on 3 calves.
Knight et al. (1984) reported the deaths of 10 horses during a
3-year period after being fed hay from the same pasture. The
animals became sick in the spring after being fed only the suspect
hay throughout the winter. The hay was found to be contaminated
with Cynoglossum officinale (hound's tongue), which contained two
PAs (heliosupine and echinatine) in much higher quantities than is
generally reported in Senecio species. The animals developed
weight loss, icterus, ataxia, and symptoms of hepatic failure. At
autopsy, there was diffuse severe megalocytosis, biliary
hyperplasia, and fibrosis. The C. officinale was proved as the
etiological factor in a feeding trial on a 14-year-old pony, that
developed clinical features and pathological changes in the liver
suggesting PA poisoning. The signs of PA toxicity in horses are
mostly neurological, though non-specific gastric and oesophageal
lesions have also been reported (McLean, 1970) (section 6.4.3).
Rose et al. (1957a,b) described a disease in which the dominant
symptom was "compulsive" walking in a straight line. It occurred
in areas where Crotalaria retusa was growing, and was ascribed to a
steep rise in blood-ammonia levels, which accompanied chronic liver
failure.
Farm animals differ widely in their sensitivity to PAs, sheep
and goats being fairly resistant, cattle and horses, less so, and
poultry and pigs, rather sensitive (section 6.4.1.2). Sheep are
not immediately affected and generally survive one season, after
feeding on heliotrope and Senecio (Bull & Dick, 1959). During the
second season of feeding, they die of neurological symptoms caused
presumably by rising blood-ammonia levels associated with chronic
liver disease, or with haemoglobinuria and very high copper levels
in the blood (Bull et al., 1956, 1958) (section 6.4.1.2). With
Crotalaria, the lung seems to be the target organ (Hooper, 1978).
Similar acute responses to a single feed of the plant Crotalaria
spectabilis were described in cattle by Emmel (1948) and in the
chicken by Piercy & Rusoff (1946).
Poisoning of cattle in northwestern USA has reached such
proportions that it has become a considerable economic problem
(Johnson, 1982). Culvenor (1985) has reviewed the problem of
livestock losses due to PA toxicosis in Australia, where it has
been estimated that about 10 million sheep are exposed to
heliotrope and Echium plantagineum (Paterson's curse) to a greater
or lesser extent and may suffer a shortening of their productive
life by as much as two years. Most of the PA-containing plants are
reported to grow in fallow fields and pastures and thrive
particularly in a dry climate or following periods of drought.
However, instances of cattle poisoning have been reported from most
parts of the world, including countries with temperate or cold
climates, which do not ordinarily suffer drought. Three herds of
cattle have been reported to have been affected in the
alpine/subalpine region of Switzerland, after they grazed pastures
that had Senecio alpinus growing on them. Nine different
hepatotoxic PAs were found in the weed, the main one being
seneciphylline. Analyses of urine samples from one cow confirmed
the presence of PA metabolites. Several cows had to be
slaughtered, because of cirrhosis of the liver (Luthy et al.,
1981).
6.3 Studies on Farm Animals
There are several reports of the production of disease
characteristic of PA toxicity in farm animals, by feeding them
PA-containing plants.
Senecio jacobaea (tansy ragwort) is a weed that commonly grows
in pastures and has been the cause of extensive livestock losses in
the United Kingdom (Forsyth, 1968) and the USA (Johnson, 1982).
Extensive studies on this plant have been carried out using a
variety of farm animals. Dickinson et al. (1976) fed tansy ragwort
to cows through a rumen canula at the rate of 10 mg/kg body weight
per day, for 2 weeks. Liver biopsies showed characteristic
megalocytosis and fibroplasia, and autopsy also showed
centrilobular necrosis. A PA, jacoline, was found in the milk, but
when the calves were bucket fed the milk, there were no detectable
effects on them. Thorpe & Ford (1968) made similar observations in
5 calves fed ragwort in their diet. Animals eventually dying of
toxicity showed characteristic necrosis, megalocytosis, and veno-
occlusive lesions. In a study by Goeger et al. (1982), goats were
fed dried ragwort mixed in the diet at 250 g/kg. Four of the 11
goat kids and lactating dairy goats died. Characteristic
megalocytosis was seen in the liver. Goats are more resistant to
tansy ragwort toxicosis than cattle and horses, the chronic lethal
dose for cattle or horses being 0.05 - 0.2 kg ragwort/kg body
weight and, for goats, 1.25 - 4.04 kg/kg body weight. The alkaloid
levels in the plant, and thus its toxicity, varies with season and
locality.
Hooper & Scanlan (1977) studied the long-term effects of
feeding very low levels of ground C. retusa seeds, mixed with the
feed, to pigs and chickens. Seven groups of 4 pigs each (sex not
mentioned) bred from Saddleback-large white cross sows and Large
White or Landrace boars, were maintained on diets containing 0
(control), 0.004%, 0.01%, 0.02%, 0.05%, 0.1%, or 0.5% body weight
ground seeds. Another 8 pigs were fed a diet containing 0.1%
C. retusa for 21 days followed by 0.05% for 7 days and then kept on
a C. retusa-free diet. Pigs either died or were killed when
moribund, or at the end of 136 days of feeding.
In a second study, groups each of 4 2-week-old chickens were
fed diets containing 0 (control), 0.005%, 0.01%, 0.05%, 0.1%, and
0.5% ground seeds of C. retusa. Chickens fed 0.5% started dying 12
days after the commencement of feeding and were all dead by day 45.
Five out of 8 birds fed 0.1% or more died between days 22 and 56.
No deaths occurred in animals fed 0.01% - 0.005%.
In the study on pigs, all animals died between days 63 and 107
except for 2 that survived 136 days. In these animals, pulmonary
disease was the main cause of death. Hepatic and renal
megalocytosis was seen in almost all animals in both the field
outbreak and study group. The lungs showed extensive consolidation
and oedema. Besides megalocytosis in the glomeruli and tubules,
the kidneys showed glomerular atrophy and tubular necrosis. In the
study on poultry, the major disease was hepatic necrosis of
irregular distribution. The kidneys showed mild megalocytosis.
In the above study, the low levels of contamination that
produced serious disease are worthy of note.
Johnson & Molyneux (1984) fed 55 cattle, by gastric lavage,
with hay mixed with threadleaf groundsel ( Senecio douglasii var.
longilobus), which grows commonly in the pastures of southwestern
USA. The PA dosage in different groups ranged from 5 to 40 mg/kg,
daily, and the total intake ranged from 80 to 284 mg/kg body
weight. The groups were fed for periods ranging from 2 to 20 days.
One hundred percent mortality occurred in 3 out of 9 groups, each
consisting of 2 - 8 calves, receiving doses of 13 mg/kg or more.
Mean survival time was generally inversely proportional to the
dosage received. All sick calves had typical clinical signs of
seneciosis. At autopsy, the principal lesion was seen in the liver
and consisted of swelling of the hepatocytes, necrosis, biliary
hyperplasia, and marked fibrosis. The estimated minimum lethal
dose of the PA was 13 mg/kg body weight for 15 days, or a total
intake of approximately 200 mg PA/kg, over a 15-day period. Cattle
that consumed up to 600 mg PA/kg, in hay, in a 20- to 100-day
period, were unaffected or only slightly affected. The authors
concluded that the time-dose relationship for PA toxicosis in
cattle is important and that there is a threshold level that must
be exceeded for the toxicosis to develop.
A similar study was conducted by Johnson et al. (1985) in which
the dry whole or ground leaves of Senecio riddelli, mixed with the
feed, were fed to calves in gelatin capsules or by gavage. Forty-two
female Hereford calves were divided into 3 groups. One group
of 12 was fed the leaves mixed with alfalfa hay feed estimated to
have 20 - 40 mg PA/kg body weight per day over a 20-day period in
different regimes, receiving a total of 400 - 800 mg PAs per
animal. The second group of 12 animals received the plant packed
into gelatin capsules, receiving an estimated PA content of
10 - 20 mg/kg body weight, daily, over 20 days, with a total of
200 - 400 mg per animal. The third group of 18 animals was
administered (by gavage) finely ground leaves in a water slurry at
various PA dosages ranging from 10 to 60 mg/kg body weight per day
and a total of 200 - 500 mg over 20 days.
Calves that received 10 mg PA/kg body weight per day for 20
days did not develop clinical signs of disease or show any changes
in serum-enzyme. However, feed containing the plant that provided
15 - 20 mg PA/kg per day or more, administered by gavage or fed in
capsules, resulted in high mortality. Malaise, depression, erratic
or unprecedented behaviour, aimless walking, and ataxia, were
observed in the affected calves; diarrhoea with tenesmus and rectal
collapse were frequently observed. The feed intake decreased
progressively and was negligible terminally. The animals that died
and those that were moribund or in a state of irreversible wasting,
were autopsied. Hepatobiliary lesions were present in all such
animals. The most consistent change was portal biliary hyperplasia
and periportal fibrosis. Centrilobular or zonal haemorrhage and
necrosis were observed in some lobules. Fibrosis of some central
veins was common, often encroaching on the lumen, resulting in
complete occlusion. Hepatocytes also showed nonspecific changes.
Central nervous system changes were present in all animals with
clinical signs of seneciosis, consisting mainly of spongy
degeneration of the brain.
The plant mixed in the hay ration was eaten slowly and
reluctantly and was tolerated at dosages > 20 mg/kg per day,
emphasizing that the toxicity depended on the rate at which the
dosage was consumed and that mortality was not necessarily
dependent on the cumulative dosage.
Burguera et al. (1983) produced the disease in turkey poults by
feeding them seeds of C. spectabilis. Simultaneous addition of
sodium selenite at doses of 0.1, 5, or 10 mg selenium/kg diet did
not provide any protection.
6.4 Experimental Animal Studies
6.4.1 Effects on liver
6.4.1.1 Relative hepatotoxicity of different PAs and their N-oxides
The LD50 values for rats, listed in Table 9, are for some of
the most commonly used hepatotoxic alkaloids, calculated from data
on animals dying from acute haemorrhagic necrosis of the liver, 3 - 7
days after intraperitoneal administration of a single dose. It
is evident that the toxicity varies widely between the alkaloids.
The most toxic are certain macrocyclic diesters of retrorsine and
the least toxic are the monoesters of heliotridine, retrorsine, and
supinine (Mattocks, 1986).
The relative toxicity of N-oxides compared with that of their
basic alkaloids depends on the route of administration. The
N-oxides of lasiocarpine, monocrotaline, and fulvine were reported
to be as toxic as their basic alkaloids (Schoental & Magee, 1959)
when administered orally; however, when given by the ip or
intravenous (iv) routes to rats, they were much less toxic
(Mattocks, 1971c). Similarly, the LD50 of retrorsine N-oxide when
administered ip to male rats was 250 mg/kg (Table 9) but when given
orally, it was 48 mg/kg. This has been explained by the
observations on the metabolic pathways of the basic alkaloids and
their N-oxides. The PAs or their N-oxides exert toxic effects only
after being metabolized to pyrroles by the hepatic microsomal
enzymes (section 5.1.1). Hepatic microsomes act directly on the
N-oxides (Mattocks & White, 1971b) only after they have been
converted to the basic alkaloids (Mattocks, 1986); this mainly
occurs in the gut (Mattocks, 1971c; Powis et al., 1979). This
matter is of practical importance as the alkaloids are often
present as their N-oxides in weeds grazed by farm animals.
Table 9. LD50s in male rats after a single intraperitoneal dose of
some hepatotoxic alkaloids
-------------------------------------------------------------------
Alkaloid LD50 Time range Reference
(mg/kg) (days)
-------------------------------------------------------------------
heliotrine 296 3 Bull et al. (1958)
lasiocarpine 77 3 Bull et al. (1958)
lasiocarpine N-oxide 547 3 Bull et al. (1958)
monocrotaline 175 3 Bull et al. (1968)
retrorsine 34 4 or 7 Mattocks (1972a)
retrorsine N-oxide 250 7 Mattocks (1972a)
senecionine 50 7 Mattocks (1972a)
seneciphylline 77 3 Bull et al. (1968)
senkirkine 220 -a Hirono et al. (1979a)
symphytine 130 -a Hirono et al. (1979a)
-------------------------------------------------------------------
a Not stated.
6.4.1.2 Factors affecting hepatotoxicity
These factors have been reviewed by Mattocks (1986).
(a) Route of administration
Most studies on LD50 values have been carried out using the ip
route, and very few experimental data are available on toxicity
using the oral route. There is a close similarity between the iv
data and the ip data. Furthermore, toxicity data on rats
administered PAs by the oral route (Schoental & Magee, 1959),
including retrorsine, lasiocarpine, heliotrine, and monocrotaline,
closely resemble those relating to the LD50 values for the same
strain administered PAs intraperitoneally (Mattocks, 1972b). Thus,
the hepatotoxicity of PAs in rats does not differ very much,
irrespective of the route of administration. However, rabbits
appear to be less susceptible to PAs in the plant Senecio jacobaea
when administered orally than when administered intravenously
(Pierson et al., 1977).
(b) Species
Wide differences have been observed in the hepatotoxic effects
of PAs and alkaloid-containing plants between different species of
both farm animals and laboratory animals, and in the same animal
exposed to PAs derived from different plants. Sheep are resistant
to PA-containing plants (section 6.2) and when fed Echium
plantagineum pellets containing 1.3 g alkaloid/kg as the sole diet
for 12 months, over a period of 2 years, showed almost no liver
damage (Culvenor et al., 1984). However, adult rats fed the same
pellets as only 50% of the diet for 14 days died 4 - 13 weeks later
(Peterson & Jago, 1984). Pigs were found to be 5 - 10 times as
susceptible to PAs in Crotalaria retusa as chickens (Hooper &
Scanlan, 1977). Overall, the approximate ratios of quantities of
plant material required to prove toxic in the various species
listed are about 200 for the sheep (approximately the same for the
goat), 150 for the mouse, 50 for the rat, 14 for cattle
(approximately the same for the horse), 5 for the chicken, and 1
for the pig (Hooper, 1978).
Cheeke & Pierson-Goeger (1983) studied the chronic toxicity of
Senecio jacobaea for several laboratory animals by feeding the
dried plant as a component of a mixed diet. The degree of
susceptibility to PA poisoning was compared in terms of the chronic
lethal dose of the dried plant as a percentage of the initial body
weight among the animals themselves, and with similar data on
livestock in other studies. Gerbils, hamsters, and guinea-pigs
were resistant to chronic toxicity, gerbils being the most
resistant, consuming over 35 times their body weight of the dried
plant. Comparison with similar data in other studies indicated
that the rabbit (Pierson et al., 1977), Japanese quail (Buckmaster
et al., 1977), and goat (Goeger et al., 1982) were resistant,
requiring a long-term lethal dose of the plant of 113% or more of
the initial body weight, whereas the rat was highly sensitive
requiring only 21% (Goeger et al., 1983). Chicks and turkey poults
were also susceptible with severe inhibition of growth occurring
when there was 5% and 10% contamination of the feed with the plant;
survival time was short (Cheeke & Pierson-Goeger, 1983).
In a study by Fushimi et al. (1978), on the carcinogenicity of
the flower stalks of Petasites japonicus Maxim in mice and Syrian
golden hamsters, species and strain differences were observed, not
only with regard to hepatotoxicity, but also with regard to the
carcinogenicity of PAs. Mice of ddN, Swiss, and C57BL/6 strains
and Syrian golden hamsters were fed on a diet containing young
flower stalks of the plant for 480 days. High incidences of lung
adenoma and adenocarcinoma were observed in ddN mice, but no
significant differences in tumour incidence were observed between
the experimental groups of Swiss mice and hamsters and the
corresponding control group. No tumours were induced in an
experimental group of C57BL/6 strain mice.
These differences have been explained by the differences in the
rate of metabolic conversion of PAs to toxic metabolites (pyrroles)
by the hepatocyte microsomes in the different animal species (White
et al., 1973; Shull et al., 1976; Peterson & Jago, 1984).
The resistance of sheep has been ascribed to destruction of the
alkaloids in the rumen by a reductive conversion into non-toxic
1-methylenepyrrolizidine derivatives (Bull et al., 1968; Lanigan,
1971, 1972). It has also been suggested that the resistance of
sheep is due to a low level of activation in liver cells (Shull et
al., 1976), but this factor was not prominent in some Australian
sheep, which were as sensitive as rats to PAs injected
intraperitoneally (Hooper, 1974).
Thus, it is possible for ruminants to graze plants containing
PAs for a period of months without evident harm, e.g., cattle
eating Crotalaria juncea in Africa (Srungboonmee & Maskasame,
1981), but long-term effects may arise in animals exposed over
several years.
Considerable differences in LD50 values have been reported for
the same alkaloids in different species. For example, the LD50 for
retrorsine varies from 34 mg/kg for male rats to 279 mg/kg for
quail and over 800 mg/kg body weight for guinea-pigs (White et al.,
1973). Guinea-pigs are also resistant to monocrotaline (Chesney &
Allen, 1973a), but not to jacobine or to mixed alkaloids of Senecio
jacobaea, which are highly toxic (Swick et al., 1982a).
(c) Sex
Significant differences in the hepatotoxicity of the same
alkaloid have been observed between sexes in some species. Male
rats are much more susceptible to the acute toxicity of retrorsine
or monocrotaline than females (Mattocks, 1972b). Mattocks & White
(1973) reported a higher level of metabolic transformation in young
male rats to form pyrroles from retrorsine, compared with females
(section 4.4). Jago (1971) reported a higher susceptibility in
male rats to the chronic hepatotoxic effects of heliotrine, while
female rats were more susceptible to lasiocarpine. It is possible
that this may be due to the potentiating effect of male sex
hormones. Campbell (1957a,b) reported that diethylstilboesterol
protects against the effects of seneciphylline and promotes repair
of damaged liver in poultry. Protein-deficient rats of both sexes,
or female animals pre-treated with testosterone, were more
susceptible to monocrotaline (Ratnoff & Mirick, 1949).
(d) Age
Available data on the effects of age are highly conflicting.
It has been stated that young rats, particularly suckling animals
(Schoental, 1959), are more susceptible than adults to the
hepatotoxic effects of some alkaloids (Jago, 1970), such as
monocrotaline (Schoental & Head, 1955), and retrorsine and
lasiocarpine (Schoental, 1959). Rats, 1 - 4 days old, were far
more susceptible to retrorsine and senkirkine than rats aged 25 - 30
days (Schoental, 1970); yet new-born rats (within 1 h of birth)
were relatively more resistant to the hepatotoxic effects of
retrorsine than 1- to 4-day-old rats (Mattocks & White, 1973).
McLean (1970) has critically reviewed the data. In comparing the
data on small animals from several studies, new-born and 4-week-old
animals appear to have about the same susceptibility as adults.
Data for the intervening period obtained by Harris et al. (1957),
Schoental (1959), and Hayashi & Lalich (1968) are conflicting,
suggesting decreased susceptibility in some studies (Harris et al.,
1957 and one series of Schoental's studies, 1959), and increased
susceptibility in others (Hayashi & Lalich, 1968 and the second
series of Schoental's studies, 1959). Furthermore, Jago (1971)
demonstrated that, while rats aged 1 - 2 weeks were more
susceptible to the acute effects of heliotrine and lasiocarpine
than older rats, sensitivity to the effects of long-term
administration of these alkaloids increased with age, after 2 - 3
months.
(e) Diet
Effects of both qualitative and quantitative changes in diet on
the hepatotoxicity of PAs have been investigated in several
studies. Restriction of protein levels in the diet enhanced the
acute hepatotoxic effects of retrorsine in rats (Selzer & Parker,
1951) and the chronic effects of a single dose of orally
administered lasiocarpine (Schoental & Magee, 1957) (section
6.4.5.1) as well as the toxicity of PAs in Senecio jacobaea,
whereas a high protein diet had a protective effect (Cheeke &
Gorman, 1974). Likewise, low lipotrope diet enhanced the toxic
effects of orally administered lasiocarpine in pregnant rats and
also in the fetal livers (Newberne, 1968). On the other hand, it
protected young male rats against the acute toxicity of
monocrotaline, because of the reduced metabolic conversion of the
alkaloid into pyrrolic metabolites (Newberne et al., 1971, 1974).
Caloric restriction reduced the cardiopulmonary toxicity of a
single dose of monocrotaline in rats (Hayashi et al., 1967). This
was ascribed to the reduced growth rate in animals on a restricted
diet rather than to a reduction in the rate of metabolic conversion
of the alkaloid, since dietary restriction started only after
administration of the alkaloid. When the animals were put back on
the ad libitum feeding regimen, they developed signs of increased
toxicity.
A high copper content in the diet has been shown to enhance the
toxic effects of PAs (Miranda et al., 1981b). Incorporation of
copper sulfate at 50 mg/kg in the diet containing the plant Senecio
jacobaea increased the hepatotoxicity in rats, as judged by enzyme
measurements. The implications of this observation are obvious if
some PA-containing plants being grazed by farm animals also have a
high copper content.
Mattocks (1972b) demonstrated the protective effects of sucrose
against the acute hepatotoxic effects of retrorsine in rats, if
administered for 3 days prior to alkaloid administration (section
5.5.1).
6.4.1.3 Acute effects
Experimental animal studies on the pathological effects of PAs
on the liver have been reviewed by Bull et al. (1968) and McLean
(1970). Most studies have been carried out on the rat (Schoental &
Magee, 1957, 1959; Bull & Dick, 1959; Schoental, 1963; Barnes et
al., 1964; McLean et al., 1964; Nolan et al., 1966; Jago, 1969;
Butler et al., 1970; Peterson & Jago, 1980), but several other
species of animals have been studied including the monkey (Wakim et
al., 1946; Rose et al., 1959; Allen & Carstens, 1968, 1971; Allen
et al., 1969), turkey (Allen et al., 1963), chicken (Allen et al.,
1960), hamster (Harris et al., 1957), mouse, guinea-pig (Chen et
al., 1940), quail, cat, rabbit, and pig (Emmel et al., 1935; Hooper
& Scanlan, 1977). All animals tested, except the guinea-pig (Chen,
1945), have been found to be susceptible in studies using purified
alkaloids and their N-oxides and crude extracts of PA-containing
plants.
Typically, the most common lesion produced in small laboratory
animals by doses close to the LD50 is a confluent haemorrhagic
necrosis in the liver, which appears within about 12 h of exposure
and peaks at 24 - 48 h. It is strictly zonal in distribution in
different species of animals but may vary within the same animal,
depending on the alkaloid used, species, nutritional status, and
pretreatment with other chemicals.
Retrorsine produces centrilobular necrosis in the rat, mouse,
and guinea-pig, periportal necrosis in the hamster, and focal
necrosis in the fowl and in the monkey (White et al., 1973). In
the monkey, monocrotaline produces centrilobular necrosis (Allen &
Carstens, 1968), but senecionine produces necrosis in the
periportal and midzonal areas of the liver lobule (Wakim et al.,
1946). Almost simultaneously, or shortly after the development of
acute haemorrhagic necrosis of the liver cells, various levels of
change appear in the central and sublobular veins of the liver
lobules, consisting of subintimal oedema or even necrosis, deposits
of fibrin, thrombosis, and occlusion of the lumen, which later
becomes organized. While haemorrhagic necrosis is a constant
feature, attempts to produce occlusive lesions in the veins of
experimental animals have produced variable results (Allen et al.,
1967). In man and non-human primates, hepatocellular necrosis and
venous occlusion occur simultaneously but, in the rat (McLean et
al., 1964), chicken (Allen et al., 1960), and swine (Emmel et al.,
1935), the vascular changes follow hepatic necrosis.
Selzer & Parker (1951) produced a lesion comparable to human
veno-occlusive disease in albino rats by administrating retrorsine
hydrochloride, the active alkaloid of Senecio ilicifolius, as well
as the crude plant extract. Four batches of rats were administered
alkaloids orally in a single dose of 1 - 1.5 mg/10 g body weight or
repeated doses of 5 - 50 mg/kg body weight for 31 days or as single
subcutaneous injection of 100 mg/kg body weight. One batch was fed
on a diet of Senecio ilicifolius constituting 10% of the ration as
crude plant or its extract; the animals lived for 21 - 84 days.
Some groups were kept on a normal diet, and others on a diet that
was protein-deficient. Animals, administered a single dose orally,
developed the earliest degenerative changes in the centrilobular
hepatocytes and sinusoidal dilatation, and the vascular lesion
appeared after 36 h. Protein deficiency enhanced the toxic effect.
Only 5 out of 9 animals administered repeated doses orally showed
early centrilobular fibrosis and none showed the vascular lesion,
possibly due to scarring.
Bull et al. (1958) studied the effects of a single ip LD70 dose
of heliotrine (320 mg/kg body weight), lasiocarpine (80 mg/kg), or
lasiocarpine N-oxide (629 mg/kg) in rats of a hooded strain of both
sexes. Eighty-one rats were used and 3 rats from each treatment
were killed at intervals of 4 - 36 h. Heliotrine produced marked
centrilobular necrosis at 24 h, but venous changes were not
evident, except for some aggregation of mononuclear macrophages on
the endothelium. With lasiocarpine, the hepatic changes were
similar, but the necrosis was not clearly centrilobular and, with
its N-oxide, it was midzonal at 34 - 49 h. The earliest toxic
effect of the PAs was manifested as a temporary loss of
mitochondria at 8 h. The authors concluded that PAs have an early
toxic effect on the hepatocytes and that this does not follow
vascular injury, as suggested by Davidson's earlier studies (1935).
McLean et al. (1964) administered an aqueous extract of
Crotalaria fulva to Wistar rats in a single intragastric dose of
0.8 - 1.5 g/kg body weight. Lesions identical to those of human
veno-occlusive disease were produced in the animals by adjusting
the dose to permit survival for 8 - 12 days. Loss of cytoplasmic
glycogen in the centrilobular cells occurred 3 h following
administration. Centrilobular necrosis, which occurred after 24 h,
increased with time. The central veins gradually filled up with
thickened endothelial cells at about 7 days, later progressing to
collagenization. Evidence was presented that the histological
occlusion of the central veins was preceded by several days by a
functional blocking of the blood flow.
Barnes et al. (1964) observed similar results in rats
administered a single oral dose of fulvine N-oxide at 50 mg/kg
body weight and studied at intervals of 1 - 4 days after the
administration. One hundred and thirty-five rats of both sexes
were used. Acute lesions resembling human disease were observed
during days 1 - 8. During days 19 - 21, 3 out of 25 animals showed
liver damage consisting of some centrilobular haemorrhage and
fibrous thickening of the central veins. Of the 78 animals studied
at 22 - 44 days, 50% still had centrilobular congestion and some
had fibrous thickening of the central veins.
The effects of pyrrolic derivatives of PAs on rats were studied
by Butler et al. (1970). Male albino rats of Porton strain were
administered solutions of pyrrole derivatives of monocrotaline
and retrorsine in dimethyl formamide as a single injection of
0.05 - 0.1 ml solution. When injected in the tail vein at a
concentration of 5 mg/kg body weight, it produced progressive
proliferation of alveolar epithelium of the lungs and the animals
developed signs of respiratory distress in 2 - 3 weeks. When injected
in the mesenteric vein at a concentration of 15 mg/kg body weight, as
a rule, the animals remained well in the postoperative period and
only 1/26 animals died of mesenteric vein thrombosis; the livers
developed multiple infarcts in the left lobes that developed into
multiple coarse nodules at 6 - 12 weeks. The above studies
substantiated the view that PAs act only when converted in
hepatocytes to pyrroles. When pyrroles were injected, they
affected the smaller vessels at the portal of entry; in animals
injected through the mesenteric vein, the main target was the
portal vein with only secondary damage to the parenchymal cells,
thus sparing the animals from the effects of hepatocellular injury.
On the other hand, pyrroles injected through the tail vein went
directly to the pulmonary arteries through the heart and damaged
the alveolar capillaries.
Acute veno-occlusive disease was produced in monkeys
administered monocrotaline (Allen et al., 1967, 1969) and ground
Crotalaria spectabilis seed (Allen & Carstens, 1968). In a study
published in 1967, these authors used 14 monkeys (Macaca speciosa)
of both sexes, each weighing approximately 4 kg. Seven of the
animals were administered 1 mg monocrotaline in distilled water by
gastric intubation on days 1 and 14. The remaining 7 were used as
controls and received distilled water only. Wedge biopsies of
liver were examined weekly. The survival time ranged from 14 to 38
days, the mean being 21 days. The livers of treated animals were
small and firm and showed changes characteristic of human veno-
occlusive disease including centrilobular necrosis, and vascular
changes in the central veins of liver lobules ranging from
subintimal oedema to progressive collagenization and extension of
collagen fibres into the sinusoids. Similar observations were made
in studies on Macaca mulatta monkeys (Allen & Carstens, 1968),
administered ground Crotalaria spectabilis seed. Sixty-four
animals, averaging 6.2 kg in weight, were divided into 3 groups.
Group I, comprising 10 experimental animals (4 control animals),
received seeds in the diet containing the equivalent of 0.074 mg
monocrotaline/kg body weight, daily, up to death. Group II,
consisting of 14 treated animals (4 control animals), received a
single dose of seeds containing 1.3 g monocrotaline/kg body weight,
and Group III, consisting of 26 experimental animals (6 controls),
received 3 weekly doses containing the equivalent of 0.116 g
monocrotaline/kg body weight, by gastric intubation. Liver
biopsies were carried out each month in Group I and each week in
Groups II and III. Animals of the last 2 groups were killed when
in extremis. The mean survival times for the groups were:
Group I, 269 days (176 - 425 days); Group II, 28 days (6 - 43
days); and Group III, 41 days (23 - 91 days). In Group I animals,
occlusive lesions of the central and sublobular veins of the liver
were seen in 7/10 animals at autopsy. These consisted of oedema,
haemorrhages, and fragmentation of the vessel walls, the lumina
being filled with fibrin, and degenerating liver cells. The
lobular pattern was distorted because of connective tissue bands
encircling small groups of liver cells, especially in the central
zones of the lobules. In Groups II and III, various levels of
focal or centrilobular necrosis were observed and the liver cells
were replaced by stromal tissue. Vascular lesions, as described
above, were seen in 25 monkeys, but no collagen was demonstrated.
In the studies of Wakim et al. (1946) on the rhesus monkey,
senecionine administered iv as a 2% solution at doses of 10 - 30 mg/kg
body weight to 4 animals, daily, until they appeared to be sick,
produced periportal, or midzonal necrosis in 3 animals accompanied
by haemorrhage. No mention was made of any vascular changes.
Electron microscopic studies
Svoboda & Soga (1966) studied the effects of lasiocarpine and
Crotalaria fulva on the livers of male Sprague Dawley rats weighing
110 - 150 g each. One group of 22 rats was given an ip injection
of lasiocarpine at 80 mg/kg body weight and pairs of animals were
killed at various intervals ranging from 15 min to 6 days. A
second group of 22 animals was administered a single dose of an
aqueous extract of Crotalaria fulva at 0.5 mg/g body weight, by
gastric tube, and killed at the same intervals. A third group of 8
rats was administered a total of 3.2 times the LD50 dose of
lasiocarpine in small doses, 3 times a week, and killed at 9 - 20
weeks. The changes primarily involved the nucleus and
interchromatin granules. The first change, seen after 30 min in
the nueleoli of the hepatocytes and Kupffer cells of animals
receiving lasiocarpine or crotalaria extract, consisted of a
separation of the fibrillar and granular components. The
hepatocyte nuclei had returned to normal after 72 h and remained so
throughout the rest of the study. In animals receiving a single
dose of lasiocarpine or crotalaria, round periodic acid schiff
(PAS) positive eosinophilic bodies appeared in the cytoplasm after
12 h, consisting of dense masses of cytoplasmic material. Five
days after treatment with crotalaria, large cells lined the luminal
surface of the central veins; the centrilobular cells had undergone
necrosis by this stage. Animals receiving 3.2 times the LD50 of
lasiocarpine developed megalocytosis after 9 weeks (section
6.4.1.5). The cytoplasm showed vesicles of smooth endoplasmic
reticulum with mitochondria of various shapes and sizes. The
appearance resembled an exaggerated regenerative response.
Allen et al. (1967, 1969) studied ultrastructural changes in
the liver tissue, in general, including the hepatic veins in Macaca
speciosa monkeys treated with PAs. In the study on hepatic veins
(Allen et al., 1969), 18 treated and 6 control adult animals were
used with an average weight of 5.8 kg. Animals were divided into 3
groups of 6 treated and 2 control animals each. The experimental
animals received 0.125 g monocrotaline/kg body weight by ip
injection. Liver wedge biopsies were examined at various intervals
in Group I during hours 1 - 48, in Group II at 4 - 12 days, and in
Group III at 16 - 32 days. The earliest changes, observed by light
microscopy, were seen at 24 h and consisted of progressive loss of
endothelial cells and other associated changes in the lumen and
wall leading to occlusion by collagenization by the third week.
Under the electron microscope, within 24 h of administration,
marked changes were observed in the endothelial cells resulting in
their rupture and release of organelles in the lumen. This was
followed by penetration of fluid though the vessel walls in the
first week and changes in the fibroblasts. By the third to fourth
week, hepatic veins showed various levels of occlusion and the
vessel was scattered with cell debris, free organelles, etc. The
authors concluded that, in this species, hepatocellular necrosis
was not the primary factor causing veno-occlusive disease, as the
association of cellular necrosis and venous occlusion occurred only
in the central area of liver lobules, and the hepatocytes
surrounding the sublobular and medium sized hepatic veins were
unaffected.
In their study of 1967, Allen et al. also investigated the
ultrastructural changes in the liver of M. speciosa monkeys after
administering 2 doses of 1 g monocrotaline each, on days 1 and 14.
At autopsy, after a mean survival time of 21 days, a wide spectrum
of changes was observed in the hepatocytic organelles, many of
which were lying, discharged into sinusoids, and also phagocytosed
by the Kupffer cells. By the third week, proliferation of
connective tissues had started in the sinusoids near the central
veins and also in the walls of central veins. The authors
concluded that the vascular and parenchymal cell changes were
simultaneous and appeared to be equally instrumental in the
development of the occlusive lesion.
6.4.1.4 Mechanism of toxic action
The mechanism of toxic action in acute pyrrolizidine
hepatotoxicity and the sequence of events, judged from the
collective experimental studies, appears to be as follows.
The PA, which is inactive as a cell poison by itself, becomes
cytotoxic through its metabolism in the hepatic parenchymal cells
to pyrroles, which act preferentially on the hepatocytes and the
endothelium of blood vessels in the liver or lungs. In the
hepatocytes, the immediate action is a rapid fall in cytoplasmic
protein synthesis reaching 30% of control levels at 15 min and 6%
at 1 h (Harris et al., 1969). This is manifested as disaggregation
of polyribosomes and is followed by failure of pyruvate oxidation,
loss of glycogen, structural damage to the mitochondria, lysosomal
activity, failure of mitochondrial nicotine-adenine-dinucleotide
(NAD) systems and nuclear NAD synthesis, and necrosis (McLean,
1970). The necrosis is zonal in the liver lobule, the particular
zone affected depending on the metabolic enzymic geography of the
lobule in the particular animal species, and also in man and monkey
on the vascular endothelium of the central and sublobular veins.
The sequence of events of the vascular lesion has been studied
by McLean et al (1964). After a single dose of Crotalaria fulva
extract in the rat, centrilobular necrosis is present after the
first day, but collagenous veno-occlusion of the central veins of
the liver lobule only appears between 7 and 10 days later.
Evidently, the necrosis of the liver cells is not secondary to
venous occlusion. Centrilobular haemorrhage is seen from day 2
onwards and signs of hepatic venous outflow tract obstruction
appear after 2 - 5 days (McLean & Hill, 1969).
Rappaport et al. (1967) and McLean (1969) demonstrated, through
transillumination studies on rats, that the outlet end of the
sinusoids is blocked by stationary columns of red cells, 16 - 24 h
following administration of PAs. The reaction is typically patchy
and results in stasis and extravasation of red cells spreading
backwards from the centre of the lobule. For at least 3 days, no
circulatory detail can be seen with transillumination. Portal
pressure is significantly raised 3 days after administration of
fulvine (Rappaport et al., 1967), notably before the first
appearance of collagenous venous occlusion at 7 days. McLean
(1969) observed that 6 - 10 days after PA administration, a new
irregular pattern of vascular flow, contrasting with the uniform
radial pattern of flow in the normal liver lobule, develops, which
corresponds to the bypass channels represented by dilated
paraseptal sinusoids, as observed in human liver biopsies (section
7.3). Segments of central vein into which the blocked sinusoids
open, are gradually abandoned in favour of such by-pass routes and
undergo occlusion first by oedematous connective tissue and then by
fibrosis. The mechanism of closure of the sinusoids is not clear.
A toxic action on the sinusoidal or venous endothelium, which
swells and occludes the lumen, seems possible, as suggested by
Allen & Carsten's (1968) electron microscopic studies on the
monkey, and studies on children (Brooks et al., 1970). The
endothelial lining of the vessels is denuded and replaced by a
fibrinous and proteinaceous precipitate, which, together with the
oedematous wall of the vessel, becomes organized and slowly
replaced by fibrous connective tissues. The occlusion of sinusoids
is further contributed by the discharge of cellular debris into the
space of Disse. The lumen of the sinusoids becomes occluded
simultaneously with the fibrosis occurring in the central vein.
Collagen fibres extend into the space of Disse and sinusoids
leading to a creeping fibrosis.
The proximate toxin that escapes from the liver is returned to
the heart, after which it damages the first portal of entry into
the alveolar capillaries of the lung and pulmonary arteries.
6.4.1.5 Chronic effects
The chronic hepatotoxic effects of PAs have been described in a
number of studies on a variety of animals and have been reviewed by
Bull et al. (1968) and McLean (1970). A notable feature is that an
appropriate single dose of PA has been demonstrated by Schoental
and Magee (1957, 1959) to lead to a relentlessly progressive course
and eventually kill the animal, more than 18 months after
administration. Schoental & Bensted (1963) demonstrated that rats
receiving a single dose of PA may develop chronic liver disease and
finally hepatocellular carcinoma more than 13 months after
administration. The morphological changes in the liver are similar
in a given species of animal, whether a single sublethal dose is
administered or multiple small doses.
Schoental & Magee (1957) studied the long-term effects of a
single dose of lasiocarpine on rats receiving normal and protein-
deficient diets. Albino rats of Porton strain were used. In the
first study, 66 rats fed a normal diet were administered a single
oral dose of lasiocarpine at one of 3 dose levels (50 - 74 mg/kg,
75 - 100 mg/kg, or 101 - 150 mg/kg body weight); 24 animals served
as controls. In another study, 46 young female rats were
administered a protein-deficient diet. Of these, 13 and 10 animals
received a single oral dose of lasiocarpine at 50 - 100 mg/kg and
50 - 75 mg/kg body weight, respectively. Each of these groups was
pair-fed with an identical number of animals that did not receive
any PA. Of the 66 rats fed a normal diet, very few died in the
first 10 days. Thirty-one animals survived longer than 3 months.
They continued to be in good health until shortly before death.
The numbers of animals that survived for 13 months after
administration of lasiocarpine were 8/10 males and 7/7 females
(lowest dose) and 5/25 males and 11/18 females (intermediate dose).
In the group that received the highest dose, neither of the 2 male
animals survived longer than 35 days, and only 1/4 female animals
survived longer than 3 months. In the animals that died or were
killed when moribund, parenchymal damage was invariably present
with prominent megalocytes, ductular proliferation, and invasion of
lobules by oval or elongated cells, thought to be derived from the
bile-duct epithelium or the reticuloendothelial cells. Animals
that survived showed various degrees of fibrosis and nodular
hyperplasia and, in some, a mild thickening of the central veins.
No obliterative lesions of the veins were seen. The 31 animals
that survived 13 months showed similar changes, but to a much
lesser extent. In the livers of animals that had repeat liver
biopsies, there was no tendency to regression of the lesions.
The above data indicate that very few animals died of acute
disease. In most animals, there was a latent period of 3 - 4
weeks, during which they remained well and showed little evidence
of liver cell injury, followed by a progressive course often
leading to fibrosis and nodular hyperplasia.
The low-protein diet adversely affected the growth of all the
rats in the control as well as the treated group. Only 3 out of 23
treated animals remained alive and in apparent good health, 8 - 11
months after the treatment. Liver biopsies taken at various
intervals between 4 and 10 months showed very severe fatty changes
in the liver cells. There was little fibrosis and no bile-duct
proliferation. In areas where the fatty changes were less severe,
characteristic megalocytes were seen. Control animals had either
normal livers or showed only slight fatty changes that were not
comparable in severity with those in the livers of PA-treated
animals. Thus protein deficiency in the diet was shown to enhance
the toxic effects of the PA.
Schoental & Magee (1959) extended these studies on young Wistar
rats using several other PAs including heliotrine, retrorsine,
riddelliine, seneciphylline, monocrotaline, and its N-oxide in
various dosages ranging from 25 to 300 mg/kg body weight; the
animals were studied at death from 1 - 10 days to 18 - 30 months.
Pathological changes were similar to those observed with
lasiocarpine in the previous study. Notable observations were that
necrosis did not necessarily precede subacute or chronic changes.
The livers of some animals became severely damaged and showed
nodular hyperplasia. Liver biopsy, 2 - 3 days after PA treatment,
did not show pathological changes in some animals, but a repeat
biopsy at 41 days showed characteristic changes. Fibrous
thickening of the central veins was observed in some animals, more
often with monocrotaline N-oxide, but no occlusion of the hepatic
veins was seen.
The studies of Nolan et al. (1966) confirmed the observations
of Schoental & Magee (1957, 1959). They gave a single dose of
lasiocarpine at 120 mg/kg body weight, by stomach tube, to 108
(equal numbers of both sexes) Sprague Dawley weanling rats (60 -
135 g body weight). Thirty animals of both sexes served as
controls. Groups of 10 animals, each consisting of 8 treated and 2
control animals, were killed at various intervals from 1 to 123
days. Of the 80 treated animals, 28 died within 26 days. No
delayed hepatic lesions were found in 59 rats between days 1 and
18. Between 19 and 123 days, delayed lesions were found in 34/49
rats. These 34 rats showed megalocytosis, but no ductular
proliferation or fibrosis.
In a second study, 127 twenty-one-day-old male Wistar rats were
given a single oral dose of lasiocarpine at 80 mg/kg body weight;
65 animals served as controls. Liver biopsy was carried out on day
3 in 108 and on day 9 in 15 of the treated animals. In 47 animals,
additional biopsies were carried out at intervals. Of the 127
PA-treated rats, 98 died during the first 9 days, and 29 after
10 - 50 days. In contrast to the first study, 32/58 survivors
exhibited delayed subacute and chronic lesions, as described by
Schoental & Magee (1957). Of these, 8 animals developed cirrhosis.
The observations indicated that the lesions of acute zonal
necrosis, which appeared on, or before, the third day, healed
without residual lesions. However, 55% of the 58 survivors
developed subacute/chronic lesions that tended to be progressive
after a latent period of 2 - 3 weeks. There appeared to be an
intimate relationship between chronic lesions and megalocytosis,
which was seen in 52/58 surviving animals. No obliterative
vascular changes were observed and so the lesions could not be
ascribed to impaired circulation.
Schoental (1959) demonstrated the toxic effects on the newborn
of PAs administered to lactating mother rats. Wistar rats
(200 - 300 g) were administered lasiocarpine, orally or by ip
injection, at 25 - 40 mg, in 5 - 10 doses of 5 - 10 mg, twice weekly
or more (24 rats), or retrorsine at 4 - 10 mg per dose, in 1 - 14
approximately daily doses (23 rats). The litters were left with
the mothers for 24 days or more (except for 1/2 hour separation
during the PA treatment). The litters were examined by biopsy at
frequent intervals or at autopsy when they died or were killed in a
moribund state. Litters of the lasiocarpine-treated rats showed
only insignificant fibrosis or some megalocytosis. In the
retrorsine-treated group, the majority of the young rats survived
for about 18 days, but all rats died before reaching the age of 30
days. The milk secretion of the mothers was apparently not affected
by the PA treatment. Of the 98 animals in 9 litters, 45 died by
the 20th day and 45 survived 30 days. Animals dying in the first
fortnight did not show gross liver lesions, but those that died at
weaning time or later, all showed signs of liver disease. Animals
dying at 18 - 30 days showed hydropic or fatty vacuolation of
hepatocytes and haemorrhage into distended sinusoids. The change
was severe in animals dying at 1 - 2 months, and some central veins
showed a narrowed lumen. The lactating rats that received the
alkaloids survived longer than their young, and most showed no ill
effects from the treatment. This evidently indicates either a high
susceptibility of suckling rats or a high concentration of PAs in
milk.
In studies by Allen et al. (1963), 2 groups of 4-week-old
turkeys, each consisting of 12 birds, were fed diets containing
ground Crotalaria spectabilis seed at 2.5 g/kg and 5 g/kg,
respectively, for 120 days. Twelve animals served as controls. At
the end of the study, 11/12 birds receiving 5 g/kg seed and 6/12
receiving 2.5 g/kg seed in the diet developed cirrhosis. The
minimum period of feeding required to produce cirrhosis was 75
days, provided the diet was reduced to a level that was not lethal.
Allen & Carstens (1971) induced the Budd-Chiari syndrome in
monkeys by monocrotaline. Six adult female and 9 adult male Macaca
speciosa monkeys, weighing 5.2 - 6.5 kg each, were divided into 2
groups, each comprising 5 control (3 males and 2 females) and 10
treated animals (6 males and 4 females). The treated group was
given a subcutaneous (sc) injection of monocrotaline at 60 mg/kg
body weight at monthly intervals for 3 months. Needle biopsy of
the liver was carried out every month for 5 months and laparotomy,
6 months after PA treatment.
The treated animals showed marked vascular changes and various
degrees of occlusion in the centrilobular and sublobular veins as
well as the larger vessels. There was also characteristic
haemorrhagic necrosis in the centrilobular zones and megalocytes
were seen. The portal venous pressures were raised. The animals
were autopsied at 6 months. The livers were markedly shrunken
weighing an average of 68 g in contrast to those of control
animals, which weighed 130 g. There were severe occlusive vascular
changes and irregular fibrosis in the lobules. The adjacent
sinusoids were dilated as a compensatory mechanism.
Swick et al. (1982a) studied the effects on guinea-pigs of
long-term dietary administration of Senecio jacobaea and compared
them with the toxic effects of single doses of injected Senecio
alkaloids and monocrotaline. The possible protective effect of
cysteine was also examined. Fifteen guinea-pigs of 250 - 300 g
initial body weight were divided into 2 treated groups, being fed
10% Senecio jacobaea, or 10% Senecio jacobaea plus 1% cysteine in
the diet, and a control group. The whole plant of Senecio jacobaea
was air dried and powdered for incorporation into the diets. The
animals were fed for 365 days. They were autopsied at death or at
the termination of the study. In a second study, 7 guinea-pigs of
500 g body weight were injected intraperitoneally with either
monocrotaline, jacobine, or mixed Senecio jacobaea PAs. The
chronic lethal dose LD100 of Senecio jacobaea was 1264 g/kg initial
body weight or 526% of the initial body weight with an average
survival time of 279 days. No mortality was observed in control
animals. This contrasts with the chronic LD100 of Senecio jacobaea
for rats of 58% of initial body weight (Swick et al., 1979) and
that of cattle equivalent to 5 - 20% body weight (Bull et al.,
1968). Addition of cysteine to the diet was only slightly, but not
significantly, protective. Pathological examination of the livers
of the guinea-pigs fed Senecio jacobaea revealed extensive
megalocytosis and severe cytoplasmic vacuolation with biliary
hyperplasia and fibrosis, primarily in periportal areas. The
centrilobular and midzonal areas were spared.
Monocrotaline was non-toxic at doses up to 1000 mg/kg body
weight, whereas jacobine and mixed alkaloids from Senecio jacobaea
were lethal at much lower levels. Similar results showing
resistance to monocrotaline in guinea-pigs were also reported by
Chesney & Allen (1973a). In in vitro studies, they related this
resistance to lack of conversion of PA to pyrroles by guinea-pig
microsomes.
A morphological peculiarity of chronic hepatotoxicity in a
large variety of laboratory and farm animals is megalocytosis
(Bull, 1955; McLean, 1970), i.e., the appearance of exceptionally
large hepatocytes, 10 - 30 times the volume of normal cells with
proportionately large nuclei. Relevant literature has been
reviewed by Jago (1969), McLean (1970), and Mattocks (1986).
Advanced megalocytosis was produced by Jago (1969) within 4 weeks
in 2-week-old rats by administering a single dose of lasiocarpine
at 76 µmol/kg body weight. Megalocytes tend to appear in the
periportal and midzones of the liver lobules with normal sized
cells around the central veins. The nuclear chromatin is
proportionately increased, but the cells appear incapable of
entering into mitosis, as only abnormal mitoses are seen. Jago
(1969) demonstrated a fall in the mitotic index (from 1.61 to 0.04)
in liver cells of 2-week-old rats, one day after injection of 50 µmol
lasiocarpine/kg. The electron microscopic appearance also
supports the above observations (Afzelius & Schoental, 1967). A
striking proliferation of rough endoplasmic reticulum and multiple
centrioles is seen in the cytoplasm, and the cytoplasmic organelles
are disorganized, suggesting increased metabolic activity but
inability of the cells to divide. Such cells may persist for the
life-time of the animal (up to 2 years in the rat) and the liver
never returns to normal (Mattocks, 1986). Megalocytes have also
been described in the kidney (Bull et al., 1968), the lung (Barnes
et al., 1964; Butler et al., 1970; Hooper, 1974), and the duodenum
(Hooper, 1975c).
Data on the total chronic lethal dose of heliotrine in rats
were discussed by Bull & Dick (1959) and Bull et al. (1968). For a
variety of dosing rates, and with withholding periods of 10 - 20
weeks interposed, the total doses ranged from 2.2 to 7.8 LD50. In
Table 10, these data are extended with results for other alkaloids.
The overall range of the total lethal dose is 1.2 - 10.3 LD50.
6.4.2 Effects on lungs
Current literature has been extensively reviewed by Kay & Heath
(1969), and Mattocks (1986). PAs have been shown to produce
pulmonary hypertension with associated vascular changes in the
pulmonary circulation in a number of experimental animal species
including the rat, mouse, frog, turkey, pig, sheep, rabbit, and
horse (McLean, 1970) as well as in non-human primates (Allen &
Chesney, 1972; Chesney & Allen, 1973b) and the dog (Miller et al.,
1978). The alkaloids have been administered by feeding the animals
with: PA-containing seeds of plants (notably Crotalaria
spectabilis) (Turner & Lalich, 1965; Kay & Heath, 1966; Kay et al.,
1967a) or the dried plant itself (e.g., Senecio jacobaea) (Burns,
1972), aqueous solutions of fulvine (Barnes et al., 1964;
Wagenvoort et al., 1974a,b) or monocrotaline (Lalich & Ehrhart,
1962; Huxtable et al., 1977), subcutaneous injections of
monocrotaline (Allen & Chesney, 1972; Chesney & Allen, 1973b) and
seneciphylline (Ohtsubo et al., 1977), or intravenous injections of
some pyrrolic esters and analogues of pyrrolizidine alkaloids and
their metabolites (Mattocks & Driver, 1983). Lafranconi & Huxtable
(1984) studied the hepatic metabolism and pulmonary toxicity of
monocrotaline in in vitro perfusion studies. Some of the
representative studies on the morphological effects of toxic lung
injury are listed in chronological order in Table 11.
Chronic lung lesions have been produced by most compounds that
produce chronic liver lesions, though higher doses were required in
some instances (Culvenor et al., 1976a). However, not all PAs that
are hepatotoxic are also pneumotoxic. Among the pneumotoxic
alkaloids, fulvine (Barnes et al., 1964) and monocrotaline are
particularly active (Mattocks, 1986). Molecular structure activity
requirements are the same as for hepatotoxicity, since both are
caused by the same toxic metabolites produced in the hepatocytes.
6.4.2.1 Acute effects
Pulmonary lesions produced by PAs have been extensively
investigated, mostly in rats, but also in non-human primates.
Monocrotaline has been the alkaloid most frequently used, but lung
lesions have also been seen in rats following fulvine and
seneciphylline administration. Besides pure alkaloids,
PA-containing seeds of some plants, most notably Crotalaria
spectabilis, have also been used.
Miller et al. (1978) gave a single iv injection of
monocrotaline at 60 mg/kg body weight to 10 mongrel dogs. Toxic
effects, recorded within 2 h, included ultrastructural changes in
the endothelial cells of the alveolar capillaries, prominent
accumulation of platelets, and the appearance of interstitial
oedema (Table 11). Valdivia et al. (1967a,b) used 25 Sprague
Dawley rats in their study and made similar observations on the rat
lung within 4 h of a single subcutaneous injection of monocrotaline
at a dose of 60 mg/kg body weight (Table 11). Interstitial oedema
and elastolysis of the alveolar wall, increase in number of mast
cells, and other associated changes were observed within 4 h of the
injection, followed by alterations in endothelial and interstitial
cells. All of the changes progressed steadily for up to 3 weeks.
It was concluded that the initial changes of destruction of
pulmonary capillaries and the other components of the alveolar wall
preceded the arteriolar hypertrophy and arteritis observed by other
investigators following monocrotaline administration, and alone was
sufficient to cause right ventricular hypertrophy. Sugita et al.
(1983a,b) administered a single dose of monocrotaline at 40 mg/kg
body weight to 5 Sprague Dawley rats and adduced further evidence,
by biochemical and radioisotopic studies, of microvascular leak in
the alveolar wall within the first 3 days of injury, which preceded
right ventricular hypertrophy observed 2 weeks following
administration (Table 11).
Table 10. Total chronic lethal doses in rats (ip administration, 2 or 3
times per week)
--------------------------------------------------------------------------
Dose (x LD50) Time to Total lethal Reference
death dose
(days) (x LD50)
--------------------------------------------------------------------------
Heliotrine (male rats, unless otherwise stated)
0.2 58 5 Bull & Dick (1959)
0.1 123 5.1
0.04 303 4.1
0.02 508 4.1
0.01 5.2 - 5.3 Bull & Dick (1960)
(with interval of 10 -
20 weeks after 21 days)
0.11 2.2 - 4.3 Bull & Dick (1959)
0.1 7.8 Jago (1971)
(35-day-old male rats)
0.1 4.7 Jago (1971)
(337-day-old male rats)
0.1 5.8 Jago (1971)
(35-day-old female rats)
0.1 4.5 Jago (1971)
(337-day-old female rats)
Lasiocarpine (male rats)
0.1 210 9 Culvenor & Jago (1979)
0.05 482 10.3
0.02 676 5.7
0.01 595 2.6
(0.005) (638) (1.4)
0.1 81 - 175 2.4 - 5 Bull & Dick (1959)
0.1 - 6.3 - 10.9 Jago (1971)
Lasiocarpine (female rats)
0.1 108 4.6 Culvenor & Jago (1979)
0.05 274 5.8
0.02 471 4
0.01 487 2.1
(0.005) (692) (1.5)
0.1 2.4 - 7 Jago (1971)
Monocrotaline
0.1 1.2 - 2.4 Bull et al. (1968)
0.05 2.5 - 4.4
Senecioninea
0.2 2 - 7.4 Bull et al. (1968)
0.1 1.7 - 5.7
0.04 (3 survived)
--------------------------------------------------------------------------
a Assuming LD50 mg/kg (c.f., Mattocks, 1986).
Table 11. Summary of experimental data on the morphological effects of toxic lung injury due to pyrrolizidine alkaloids (in
chronological order)
---------------------------------------------------------------------------------------------------------------------------------------
Animal/ Number Toxic agent Dose Administration Single/ Killed after Pathological References
strain/ of (mg/kg) dose multiple or survival effects
sex animals/ for
controls
---------------------------------------------------------------------------------------------------------------------------------------
Acute effects
Male 25/5 monocrotaline 60 subcutaneous single 2 - 48 h interstitial oedema, Valdivia et al.
Sprague (body 1 - 3 weeks endothelial cell (1967a,b)
Dawley rat weight) alterations,
elastolysis, etc.
Mongrel 10/0 monocrotaline 60 intravenous single 2 h interstitial oedema, Miller et al. (1978)
dog (body changes in endothelial
weight) cells
Male 5/3 monocrotaline 40 subcutaneous single 0 - 21 days microvascular leak, Sugita et al. (1983a)
Sprague (body right ventricular
Dawley rat weight) hypertrophy after 2
weeks
Chronic effects
Female 35/12 monocrotaline 10 - 30 ad libitum pulmonary arteritis Lalich & Ehrhart
Sprague (diet) feeding (with 20 - 30 mg dose (1962)
Dawley rat only)
6 alcohol- 2500 ad libitum 51 days none
extracted (diet) feeding
seeds of
Crotalaria
spectabilis
19/4 monocrotaline 10 - 75 ad libitum 26 - 232 Turner & Lalich (1965)
(diet) feeding days
---------------------------------------------------------------------------------------------------------------------------------------
Table 11. (contd.)
---------------------------------------------------------------------------------------------------------------------------------------
Animal/ Number Toxic agent Dose Administration Single/ Killed after Pathological References
strain/ of (mg/kg) dose multiple or survival effects
sex animals/ for
controls
---------------------------------------------------------------------------------------------------------------------------------------
Chronic effects (contd.)
Female 24/6 Crotalaria 200 - ad libitum 105 - 172 right ventricular Allen & Chesney (1972)
Wistar spectabilis 1600 feeding days hypertrophy and
Furth rat seeds (diet) dilatation, pulmonary
arterial hypertrophy,
pulmonary arteriolar
hypertrophy,
endocardial fibrosis
Female 10/34 Crotalaria 1000 ad libitum 30 - 60 days right ventricular Kay & Heath (1966)
weanling spectabilis (diet) feeding hypertrophy, pulmonary
Wistar rat seeds arterial hypertrophy,
pulmonary arteritis
Male 22/12 monocrotaline 120 subcutaneous single 20 - 47 days right ventricular Hayashi & Lalich
suckling (body hypertrophy, pulmonary (1967)
Sprague weight) arterial hypertrophy,
Dawley rat pulmonary arteritis,
fibrin thrombi
Female 30/10 fulvine 50 intraperitoneal single 3 - 37 days right ventricular Kay et al. (1971a)
Wistar rat (body hypertrophy, pulmonary
weight) arterial hypertrophy,
pulmonary arteriolar
hypertrophy, pulmonary
arteritis, arteriolar
thrombi
80 intragastric single 7 - 35 days
(body
weight)
---------------------------------------------------------------------------------------------------------------------------------------
Table 11. (contd.)
---------------------------------------------------------------------------------------------------------------------------------------
Animal/ Number Toxic agent Dose Administration Single/ Killed after Pathological References
strain/ of (mg/kg) dose multiple or survival effects
sex animals/ for
controls
---------------------------------------------------------------------------------------------------------------------------------------
Chronic effects (contd.)
Monkey 12 (30 monocrotaline 30 subcutaneous one 199 - 325 right ventricular Allen & Chesney (1972)
(Macaca days old) (body followed days hypertrophy and
arctoides) weight) by 3 (average, dilatation, left
(both (2, 4, 241) ventricular
sexes) and 6 hypertrophy, pulmonary
months arterial hypertrophy,
after pulmonary arteriolar
first hypertrophy, pulmonary
injection hypertension
12 (15 163 - 334 pulmonary arterial
months days hypertension (isolated,
old) (average, veno-occlusive disease
217) (liver)
Monkey 20/6 monocrotaline 30 subcutaneous as above 165 - 325 right ventricular Chesney & Allen
(Macaca (body days hypertrophy and (1973b)
arctoides) weight) (average, dilatation, pulmonary
(both 326) arterial hypertrophy,
sexes) pulmonary arteriolar
hypertrophy, pulmonary
arteritis, endocardial
fibrosis
Female 50/12 fulvine 80 intragastric single 1 - 6 weeks vasoconstriction, Wagenvoort et al.
Wistar rat (body pulmonary arterial (1974a,b)
weight) hypertrophy, pulmonary
or 50 arteriolar hypertrophy,
(body intraperitoneal right ventricular
weight) hypertrophy, thickening
of veins and venules
---------------------------------------------------------------------------------------------------------------------------------------
Table 11. (contd.)
---------------------------------------------------------------------------------------------------------------------------------------
Animal/ Number Toxic agent Dose Administration Single/ Killed after Pathological References
strain/ of (mg/kg) dose multiple or survival effects
sex animals/ for
controls
---------------------------------------------------------------------------------------------------------------------------------------
Chronic effects (contd.)
Male 16/0 seneciphylline 50 - 80 subcutaneous single 1 - 3 weeks pulmonary arteriolar Ohtsubo et al. (1977)
Wistar (body and right ventricular
rat (4 weight) hypertrophy (after 3
weeks old) weeks), right
ventricular dilatation
in 2 animals
Male 21/14 Crotalaria 1000 ad libitum 3 - 35 days pulmonary arterial Meyrick & Reid (1979,
Sprague spectabilis (diet) feeding hypertrophy and 1982)
Dawley rat pulmonary arteritis
(2/21); right
ventricular hypertrophy
---------------------------------------------------------------------------------------------------------------------------------------
A histological and electron microscopic study was made by
Hurley & Jago (1975) of the lungs of rats administered
dehydromonocrotaline. Female black and white hooded rats weighing
80 - 100 g were used. Dehydromonocrotaline dissolved in
dimethylformamide (DMF) was administered iv as a single dose at
30 mg/kg body weight to 12 rats and at 15 mg/kg body weight to 7 rats.
Four control rats were administered DMF alone. A colloidal
suspension of carbon black was injected iv, 6 - 18 h after
injection of dehydromonocrotaline, and the animals killed 19 - 44 h
after treatment.
After an interval of 6 - 8 h, there was a direct toxic effect
on the endothelial cells of pulmonary capillaries and small
venules. Many endothelial cells had prominent nuclei and thickened
cytoplasm, which contained more RNA granules than usual. There was
also an increase in the number of mitochondria. The endothelial
damage did not seem to have caused permanent disruption of the
small blood vessels, and, 2 days after injury, all vessels were
patent. Large numbers of mononuclear cells, which appeared in the
interstitial tissues of the lung 44 h after injury, seemed to be
altered emigrated blood monocytes.
6.4.2.2 Chronic effects
Lalich & Erhart (1962), fed 35 Sprague Dawley rats a diet
containing monocrotaline at 10 - 30 mg/kg (Table 11). Animals
receiving a daily dose of monocrotaline of 20 mg/kg diet or more,
showed progressive changes in the lungs after 24 days of feeding.
Of the 23 animals receiving 20 - 30 mg/kg, 12 showed pulmonary
arteritis, 4 of these even at the dose level of 20 mg/kg diet.
Pulmonary haemorrhages were observed in 16 animals. No changes
were observed in animals fed alcohol-extracted seeds or other
derivatives of monocrotaline.
Identical changes were observed in similar studies by Turner &
Lalich (1965) on two strains of rats, Sprague Dawley (19) and
Wistar Furth (24). The first group of 19 female Sprague Dawley
rats was fed a diet containing monocrotaline at an initial level of
10 mg/kg diet. Depending on the response of each individual
animal, the monocrotaline level was raised to a maximum of 75 mg/kg
diet (Table 11). Fourteen rats survived for more than 100 days and
8 reached the maximum dietary level of monocrotaline, the last
animal dying after 232 days. The second group of 24 female Wistar
Furth rats was fed a diet contaminated with Crotalaria spectabilis
seeds, initially at 0.2 mg/kg diet, gradually rising to 1.6 mg/kg
by increasing the levels by 0.2 mg/kg every week. All test animals
survived 100 days and 15 reached the maximum levels of Crotalaria
fed. The last animal died after 172 days of feeding. Animals
developed signs of toxicity and right ventricular strain, e.g.,
cyanosis, etc. Progressive thickening of media in the muscular
pulmonary arteries, progressive muscularization of arterioles, and
changes characteristic of pulmonary hypertension, were seen. Some
pulmonary arteries showed medial necrosis. No changes were
observed in the pulmonary veins. Significant hypertrophy of the
heart, as judged by the heart weight in relation to body weight,
was seen in almost all animals that survived 100 days or more
(32/38), and the right ventricles were dilated. The hypertrophy
affected the right side of the heart only, and generally
corresponded with the vascular changes. There was a marked
hyperplasia of the mast cells in the mediastinal lymph nodes and
around bronchi and pulmonary arteries. Similar observations were
made by Barnes et al. (1964) and Valdivia et al. (1967a,b).
Kay and his group studied cardiac and pulmonary vascular
changes in rats fed Crotalaria spectabilis seeds (Kay & Heath,
1966; Kay et al., 1967a,b) or administered fulvine (Kay et al.,
1971a).
A group of 10 female weanling Wistar albino rats were fed a
diet containing 1 g powdered seeds of Crotalaria spectabilis/kg
until they died of cardiorespiratory distress, after 36 - 60 days
of feeding. Thirty-four control rats were fed a normal diet. At
autopsy, the atria of the heart, the right ventricle, and the left
ventricle with the interventricular septum were weighed separately.
The medial thickness of the muscular pulmonary arteries was
measured, and expressed as percentage of external diameter. The
medial thickness of the muscular pulmonary arteries increased in
all test rats; acute or healing pulmonary arteritis was seen in 3
animals. Statistically significant cardiomegaly was present in all
rats fed the seeds, contributed chiefly by the right ventricle. The
readings from all the test rats were well outside the upper 95%
confidence limit. The increase in the medial thickness of the
pulmonary arteries correlated well with the weight of the right
ventricle (Fig. 10). Three rats showed pulmonary arteritis
(indicated by a solid triangle). It was presumed that the organic
basis for increased pulmonary resistance was the abnormal
muscularization of the radicles of the pulmonary arterial system.
Essentially similar results were obtained in an identical study
repeated on 8 test rats and 5 controls (Kay et al., 1967a). The
test rats developed pulmonary hypertension in 37 days, levels of
which were correlated with the medial thickness of the muscular
pulmonary arteries and that of the pulmonary trunk, as well as with
the weight of the right ventricle.
5.3.2 Activation and detoxication
Factors affecting the proportion of an ingested alkaloid that
is converted into toxic metabolites in an animal include the
following:
(a) Lipid solubility
Highly water-soluble alkaloids (such as indicine) are easily
excreted and have low toxicity. Alkaloids that are more lipophilic
are more open to activation by liver microsomes (Mattocks, 1981a).
(b) Subceptibility to hydrolysis
This is determined by the molecular structure and conformation
of the alkaloid (Mattocks, 1981a,b). If the alkaloid is open to
esterase attack, it may be largely detoxified by hydrolysis.
(c) Susceptibility to N-oxidation
The relative amounts of an alkaloid converted by hepatic
microsomal enzymes to N-oxide and to pyrrolic metabolites depends
on its molecular structure and conformation (Mattocks & Bird,
1983). N-oxidation represents a detoxication pathway (Mattocks,
1972b).
5.3.3 Factors affecting the toxicity of active metabolites
5.3.3.1 Reactivity of the metabolite
Toxic metabolites are formed in liver cells. Primary pyrrolic
metabolites (dehydro-alkaloids) are very reactive and, thus, are
quickly hydrolysed or deactivated by reaction with cell constituents.
To damage tissues other than the cells in which they are formed,
active metabolites must cross the cell membrane and survive while
being transported in the bloodstream. The more stable pyrrolic
metabolites, such as dehydromonocrotaline from the alkaloids
monocrotaline, are able to reach, and become bound to, lung tissue
(Mattocks, 1973). Thus, monocrotaline frequently damages the lungs,
whereas retrorsine, which yields a more reactive pyrrolic metabolite,
normally does not.
Secondary metabolites (pyrrolic alcohols, e.g., dehydroretronecine),
formed by the hydrolysis of primary pyrrolic metabolites, are water
soluble, relatively stable compounds that can become more widely
distributed throughout the body or excreted; these are not acutely
toxic.
5.3.3.2 The number of reactive groups
The toxicity of a pyrrolic alkylating agent is affected by the
number of reactive ester or hydroxyl groups (1 or 2) present as the
following examples show:
(a) Many pyrrolic esters can cause acute lung damage when
given iv to rats, but only bifunctional ones also cause
delayed effects on the lungs (Mattocks & Driver, 1983).
(b) Bifunctional pyrrolic alcohols are more effective
inhibitors of mitosis in cultured cells than monofunctional
pyrroles (Mattocks & Legg, 1980).
(c) Bifunctional pyrrolic alcohols are much better inducers
of sister chromatid exchange (SCE) in lymphocytes than
monoalcohols (Ord et al., 1985).
Reasons for these differences might be that the bifunctional
pyrroles are able to crosslink macromolecules or simply that they
can bind more strongly to target molecules.
5.4 Metabolites Associated with the Biological Actions of
Pyrrolizidine Alkaloids
5.4.1 Acute hepatotoxicity
The following is good evidence that acute liver necrosis is
caused by primary pyrrolic ester metabolites (dehydro-alkaloids):
(a) The liver, in which these metabolites are formed, is the
only organ exposed to them in relatively high concentrations.
(b) There are good correlations between amounts of pyrroles
bound to liver tissue and acute hepatotoxicity (Mattocks,
1973).
(c) Pyrrolic alcohols are not acutely hepatotoxic, even when
given to animals in very large amounts.
(d) Pyrrolic esters injected iv into the liver are much more
acutely hepatotoxic than the parent alkaloids (Butler et al.,
1970).
It is possible that other metabolites, such as 4-hydroxy
2,3-unsaturated aldehydes, might also contribute to the acute
hepatotoxicity of some PAs (Segall et al., 1985). However, this
has still to be confirmed.
5.4.2 Chronic hepatotoxicity
The persistent antimitotic action on the liver that leads to
the formation of giant hepatocytes can be produced both by pyrrolic
ester metabolites, such as dehydromonocrotaline (Hsu et al., 1973),
and by pyrrolic alcohols, such as dehydroheliotridine (Peterson et
al., 1972). Both kinds of metabolites can lead to similar
alkylation products and both are likely to be present in the liver
when the alkaloids are metabolized. Thus, either could be
responsible for chronic hepatotoxic effects. However, the
antimitotic action alone is not sufficient. It must be accompanied
or followed by a stimulus of cell division. This may be provided
by the acute necrotic effect of primary pyrrolic metabolites or by
any other cause of acute liver injury that leads to tissue
regeneration. In very young animals, the stimulus can be the
enhanced rate of replication that already exists in them.
5.4.3 Pneumotoxicity
Characteristic pyrrolizidine lung damage is produced by iv
injections of pyrrolic ester metabolites, which are effective at
much lower doses than the parent alkaloids. The latter are not
metabolized in lung tissue; thus, lung damage from PAs is believed
to be due to pyrrolic esters reaching the lungs from the liver
(Butler et al., 1970). Chronic lung damage appears to be caused by
bifunctional rather than by monofunctional pyrrolic alkylating
agents (Mattocks & Driver, 1983) (section 5.3.3.2).
There is some evidence that pyrrolic alcohol metabolites might
also be able to contribute to chronic (but not acute)
pneumotoxicity (Huxtable et al., 1978).
5.4.4 Toxicity in other tissues
Chronic heart damage including right ventricular hypertrophy is
a consequence of pyrrolizidine lung damage (pulmonary hypertension)
(Hayashi et al., 1967). Brain damage is attributed to ammonia
intoxication secondary to severe pyrrolizidine liver injury
(Hooper, 1972). This view has been contested and some PAs are
known to have direct effects on the central nervous system (section
6.4.3). There is no evidence that PAs are appreciably metabolized
in tissues other than the liver. Thus, damage to other organs is
probably due to metabolites transported from the liver. For
example, in the relatively uncommon cases of chronic kidney damage
after pyrrolizidine intoxication (Hooper, 1974; Hooper & Scanlan,
1977) megalocytosis in this organ suggests that pyrrolic
metabolites (either ester or alcohol) are involved. Overall,
patterns of disease, as observed in extrahepatic sites, are
consistent with a "spillover" effect of the pyrroles produced in
the liver (Hooper, 1978). Toxicity of an alkaloid reflects its
rate of metabolism to a pyrrole (Tuchweber et al., 1974) and so the
spillover effect is likely to be more evident at higher doses.
Studies of Culvenor et al. (1976a) suggest that the PAs that are
hepatotoxic for rats should also be pneumotoxic when administered
at higher doses. In acute poisoning, the hepatotoxic effects could
outweigh the pneumotoxic effects or those on other organs, to such
a degree that the latter are not manifested. Variation in
expression of disease (primarily hepatic or extrahepatic) also
depends on the reactions of host tissues in different species of
animals, in addition to the quantities of the pyrroles (Hooper,
1978). The sensitivity of the blood vessels might explain severe
interstitial pneumonias in some animals, or severe nephroses in
pigs (McGrath et al., 1975).
5.4.5 Carcinogenicity
The pyrrolic alcohols dehydroretronecine and dehydroheliotridine
are known carcinogens (Johnson et al., 1978; Peterson et al., 1983),
whereas the pyrrolic esters dehydromonocrotaline and dehydroretrorsine
are only carcinogenic in conjunction with a tumour promotor (Mattocks
& Cabral, 1979, 1982). This suggests that the more persistent
secondary metabolites (pyrrolic alcohols) might account for the rather
weak carcinogenicity of some PAs.
5.4.6 Antitumour activity
Some PAs and their N-oxides are active as tumour inhibitors in
test systems (Culvenor, 1968; Suffness & Cordell, 1985). Indicine
N-oxide, in particular, showed high activity against B16 melanoma,
mammary xenograft, M5076 sarcoma, P388 leukaemia, and Walker 256
carcinoma. In clinical studies, indicine N-oxide has shown
significant activity against some forms of leukaemia, with dosage
limited mainly by myelosuppression and sometimes by hepatotoxicity.
It is tempting to suppose that this action is related to the
powerful antimitotic action of their pyrrolic metabolites, even
though some of these alkaloids and derived pyrroles are themselves
carcinogenic. On the other hand, there is evidence suggesting that
indicine N-oxide owes it activity to a property of the compound
itself rather than to the pyrrolic metabolites, which could be
formed through reduction to indicine (Powis et al., 1979). The
evidence, that indicine is less effective than indicine N-oxide, is
not conclusive and other structure-activity data (Milkowsky, 1985)
point to a need for a structural capability to form a pyrrolic
metabolite. It is also possible that indicine N-oxide is converted
directly to dehydroindicine by mitochondrial enzymes in liver or
tumour cells, since the type of reaction required has been observed
in the mitochondrial metabolism of the N-oxides of tryptamine
alkaloids and certain methylated amino acids (Fish et al., 1956;
Smith et al., 1962).
5.5 Prevention and Treatment of Pyrrolizidine Poisoning
There is no known way to prevent pyrrolizidine liver damage,
once a hepatotoxic dose of the alkaloid has been ingested. A
number of dietary regimes have been found to partially protect
animals (chiefly rodents) from the acute effects of subsequent
alkaloids ingestion. None of these are of any practical use for
preventing pyrrolizidine intoxication in livestock. Furthermore,
chronic toxic effects in the liver or in other organs are sometimes
more severe in animals receiving higher doses of alkaloids after
being protected against acute hepatotoxicty.
5.5.1 Modified diets
The mechanism of action of modified diets is not clear, but
they may be associated with the decreased metabolic activation of
the alkaloids. Some examples follow:
(a) A protein-rich diet can give some protection to rats
against Senecio jacobaea alkaloids (Cheeke & Gorman, 1974).
Rats fed a high casein diet survived longer than rats given a
normal diet, when poisoned with retrorsine or riddelline, but
the survivors were more liable to develop liver tumours
(Schoental & Head, 1957). However, whether this was simply due
to a prolongation of life of the animals by the diet is open to
question.
(b) Male rats previously fed a sucrose-only diet for 4 days
were considerably protected against the acute hepatotoxicity of
retrorsine (LD50 120 mg/kg body weight compared with 34 mg/kg
in normal rats). However, lung damage, rare in control rats,
was frequently seen in "protected" rats given high doses of
retrorsine (Mattocks, 1973).
(c) Restriction of feed intake to 40% of normal attenuated the
increase in lung weight and lavage protein concentration in
cell-free bronchopulmonary lavage fluid and abolished the right
ventricular hypertrophy in monocrotaline-treated rats.
Furthermore, the percentage of diet-restricted animals that
survived was significantly higher than that in animals that had
eaten ad libitum up to day 28, but, from this time onwards,
there was no difference. Alterations of dietary sodium intake
alone did not affect the results of monocrotaline-induced
toxicity (Ganey et al., 1985).
5.5.2 Pretreatment to enhance the detoxication of active metabolites
Treatments that have afforded some protection against
pyrrolizidine hepatotoxicity (probably by increasing the liver
level of sulfydryl compounds, which are known to react with
pyrrolic metabolites) (White, 1976) include the following:
(a) Pre-treatment of rats with mercaptoethylamine (150 mg/kg
body weight ip) partially protected rats against the acute
hepatotoxicity of monocrotaline given 15 min later (Hayashi &
Lalich, 1968); it gave no protection when administered 2 h
after the alkaloid. Mercaptoethylamine, when given orally
(300 mg/kg body weight) at the same time as the lasiocarpine,
also increased the resistance of rats to the alkaloid (Rogers &
Newberne, 1971).
(b) Cysteine (1% in the diet) partially protected rats against
Senecio jacobaea alkaloids (Buckmaster et al., 1977) and mice
against monocrotaline (Miranda et al., 1981c).
(c) The antioxidant ethoxyquin fed at a level of 2.5 g/kg diet
to female mice for 38 days, increased the liver thiol
concentration and raised the acute LD50 of monocrotaline, given
ip on the 10th day, to 364 mg/kg compared with 243 mg/kg in
control mice (Miranda et al., 1981a).
(d) Rats or mice also had increased resistance to acute
pyrrolizidine hepatotoxicity when fed the antioxidant butylated
hydroxyanisole (BHA) (up to 7.5 g/kg diet) (Miranda et al.,
1981c, 1982a,b; Kim & Jones, 1982).
(e) Heliotrine-induced toxicity can be modified by the
co-administration of cupir (a copper-containing complex) at a
level of 1 mg/kg per day for 20 days. It prevented the exit of
hepatic cytosolic enzymes into the blood and improved all the
energy reactions studied in the mitochondria of heliotrine-
intoxicated rats (Yuldasheva & Sultanova, 1983). Inhibition of
lipid peroxidation by cytoplasmic copper was shown later
(Wittig & Stephen, 1964). Savin (1983) found that lethality to
rats of heliotrine (300 mg/kg sc) was completely prevented by
co-administration of alpha-tocopherol (6 ml/kg ip).
(f) Rats pre-treated with ip doses of zinc chloride (72 µmol/kg
body weight) had increased resistance to the hepatotoxicity of
Senecio jacobaea alkaloids, as assessed by histology and enzyme
measurements (Miranda et al., 1982c). The zinc treatment increased
the liver level of metallothionein, a sulfhydryl-rich protein that
might react with pyrrolic metabolites.
Metabolic inhibitors of the microsomal P450 mixed-function
oxidase system, SKF 525A, metyrapone, and allylisopropyl acetamide,
which inhibit the formation of toxic pyrroles in the liver, have
been tried successfully in the prevention of the toxic effects of
monocrotaline in rats (Eisenstein & Huxtable, 1979). The use of
P450 inhibitors was stated to show "potential therapeutic promise".
However, this would seem impracticable considering that, at least
in the rat, PAs undergo a high rate of metabolism commencing a few
minutes after ingestion (Mattocks, 1972b). In some instances, they
have been known to lead to an increase in toxicity, e.g., with
lasiocarpine as reported by Tuchweber et al. (1974).
5.5.3 Other treatments
Lanigan & Whittem (1970) attempted, unsuccessfully, to protect
sheep against Heliotropium europaeum poisoning by treating them
with cobalt, in the hope that this would enhance the vitamin
B12-mediated detoxication of the alkaloids in the rumen (Dick et
al., 1963).
Lanigan et al. (1978) found that the resistance of sheep to
dietary Heliotropium europaeum was increased by giving them large
daily doses of the antimethanogenic drug, iodoform. However, Swick
et al., (1983) found that Senecio jacobaea alkaloids were not
detoxified by incubation for 48 h with sheep rumen fluid in vitro.
6. EFFECTS ON ANIMALS
6.1 Patterns of Disease Caused by Different Plant Genera and of
Organ Involvement in Different Species
The most important genera of PA-containing plants listed in
section 3.1 are all hepatotoxic. Among these, Crotalaria spp.
cause damage in the broadest range of tissues in most domestic
species. In pigs, they are known to be severely nephrotoxic
(Peckham et al., 1974; McGrath et al., 1975; Hooper & Scanlan,
1977). Some species are known to be pneumotoxic for horses (Watt &
Breyer-Brandwijk, 1962; Gardiner et al., 1965), cattle (Sanders et
al., 1936; Berry & Bras, 1957), sheep (Laws, 1968), and pigs
(Peckham et al., 1974; Hooper & Scanlan, 1977), as well as
hepatotoxic.
Although several Crotalaria spp. are known to be pneumotoxic
for horses (Gardiner et al., 1965), C. retusa is an exception. It
is an important cause of disease in horses in northern Australia
(Hooper, 1978) and has been shown to be pneumotoxic for pigs in the
same area (Hooper & Scanlan, 1977); yet it produces only hepatic
disease in horses (Rose et al., 1957a,b).
Similarly, Senecio spp. are primarily hepatotoxic, but
S. jacobaea has been demonstrated to be pneumotoxic for pigs
(Harding et al., 1964), though it could probably be an inconsistent
change (Bull et al., 1968). This plant is also known to cause
pulmonary disease in rats and mice (Hooper, 1974). However, there
are no reports of its affecting the lungs in cattle, sheep, horses,
or chicken. Renal megalocytosis and mild nephrosis are reported in
most species poisoned with S. jacobaea (Harding et al., 1964; Bull
et al., 1968). Heliotropium spp., Amsinckia spp., and Echium spp.
are all mainly hepatotoxic.
Roitman (1983) summarized the pattern of organ involvement
observed in man and different species of farm and experimental
animals affected by pyrrolizidine alkaloids (Table 8). Even within
a single species, the nature of a toxic effect, as well as the
organ affected, can be altered by changing the dose rate and
duration.
6.2 Field Observations - Outbreaks in Farm Animals
The veterinary problem of PA toxicity has been reviewed by Bull
et al. (1968) and McLean (1970). Mattocks (1986) listed the cases
of livestock poisoning and feeding trials since 1968, and cited
relevant literature. Peterson & Culvenor (1983) produced a useful
and comprehensive table of the plant species known or suspected of
causing natural outbreaks of poisoning in each animal species. The
influence of factors such as species, age, sex, and diet, on
toxicity is also reviewed in the same paper.
Table 8. Animal species and organs affected by pyrrolizidine
alkaloidsa
---------------------------------------------------------------
Species Liver Lung Kidney Heart Pancreas Gastric Muscle
mucosa
---------------------------------------------------------------
Man +
Monkey + + + +
Horse + + +
Pig + + + + +
Sheep + + +
Goat + +
Cattle + +
Dog +
Mouse + +
Rat + + + +
Chicken + + + + +
Turkey + + +
---------------------------------------------------------------
a From: Roitman (1983).
The first cases of pyrrolizidine poisoning were described in
cattle as early as 1903 (Gilruth, 1903). Since then, there have
been numerous reports from most parts of the world, of poisoning
among farm animals caused by grazing or feeding on PA-containing
weeds (Bull et al., 1968; Mattocks, 1986). One of the first clues
to the etiology of the human disease in Jamaica, came from a study
in which calves fed with Crotalaria fulva (Bras et al., 1957)
developed characteristic veno-occlusive disease in the liver.
Laws (1968) described a field outbreak in sheep in a herd of
100 adult merino ewes, which developed within 2 weeks of moving
into a coastal farm in Australia, where they grazed Crotalaria
mucronata. The etiology was confirmed by feeding the plant to 6
sheep, 4 of which died within 24 h of feeding, with severe
pulmonary oedema. However, the rapidity of poisoning and the
atypical lung lesions suggest that possibly a toxin other than
pyrrolizidine alklaoids was also present.
An outbreak of Crotalaria retusa poisoning was observed in a
piggery near Darwin, Northern territory, Australia (Hooper &
Scanlan, 1977) containing approximately 350 sows. It was caused by
feeding sorghum contaminated by Crotalaria seeds at the rate of
about 0.1% by weight for about 3 weeks, and at a rate of about
0.05% for a further week. The disease was indicated by reduced
body weight gain and inappetence. The dominant pathological
features at autopsy were severe nephrosis with chronic uraemia, and
to a lesser degree, severe diffuse interstitial pneumonia. Both
were accompanied by microscopic disease in the liver, and both the
liver and kidney showed megalocytosis.
Walker & Kirkland (1981) reported outbreaks in the Hunter river
valley of New South Wales in Australia, in cattle that had been
grazing a pasture in which Senecio lautus was growing. There were
sporadic deaths among the cattle as well as two protracted
outbreaks affecting calves 3.5 months of age and older animals, in
which groups of 3 - 16 head of cattle died in addition to sporadic
deaths of animals over periods of 1 and 6 months. Clinical signs
were weakness, emaciation with recumbency, aimless wandering and
ataxia, which suggested neurological involvement. At autopsy, the
liver showed characteristic megalocytosis, periportal fibrosis, and
focal necroses. An aged animal had cirrhosis. The etiology of
S. lautus was proved in feeding studies on 3 calves.
Knight et al. (1984) reported the deaths of 10 horses during a
3-year period after being fed hay from the same pasture. The
animals became sick in the spring after being fed only the suspect
hay throughout the winter. The hay was found to be contaminated
with Cynoglossum officinale (hound's tongue), which contained two
PAs (heliosupine and echinatine) in much higher quantities than is
generally reported in Senecio species. The animals developed
weight loss, icterus, ataxia, and symptoms of hepatic failure. At
autopsy, there was diffuse severe megalocytosis, biliary
hyperplasia, and fibrosis. The C. officinale was proved as the
etiological factor in a feeding trial on a 14-year-old pony, that
developed clinical features and pathological changes in the liver
suggesting PA poisoning. The signs of PA toxicity in horses are
mostly neurological, though non-specific gastric and oesophageal
lesions have also been reported (McLean, 1970) (section 6.4.3).
Rose et al. (1957a,b) described a disease in which the dominant
symptom was "compulsive" walking in a straight line. It occurred
in areas where Crotalaria retusa was growing, and was ascribed to a
steep rise in blood-ammonia levels, which accompanied chronic liver
failure.
Farm animals differ widely in their sensitivity to PAs, sheep
and goats being fairly resistant, cattle and horses, less so, and
poultry and pigs, rather sensitive (section 6.4.1.2). Sheep are
not immediately affected and generally survive one season, after
feeding on heliotrope and Senecio (Bull & Dick, 1959). During the
second season of feeding, they die of neurological symptoms caused
presumably by rising blood-ammonia levels associated with chronic
liver disease, or with haemoglobinuria and very high copper levels
in the blood (Bull et al., 1956, 1958) (section 6.4.1.2). With
Crotalaria, the lung seems to be the target organ (Hooper, 1978).
Similar acute responses to a single feed of the plant Crotalaria
spectabilis were described in cattle by Emmel (1948) and in the
chicken by Piercy & Rusoff (1946).
Poisoning of cattle in northwestern USA has reached such
proportions that it has become a considerable economic problem
(Johnson, 1982). Culvenor (1985) has reviewed the problem of
livestock losses due to PA toxicosis in Australia, where it has
been estimated that about 10 million sheep are exposed to
heliotrope and Echium plantagineum (Paterson's curse) to a greater
or lesser extent and may suffer a shortening of their productive
life by as much as two years. Most of the PA-containing plants are
reported to grow in fallow fields and pastures and thrive
particularly in a dry climate or following periods of drought.
However, instances of cattle poisoning have been reported from most
parts of the world, including countries with temperate or cold
climates, which do not ordinarily suffer drought. Three herds of
cattle have been reported to have been affected in the
alpine/subalpine region of Switzerland, after they grazed pastures
that had Senecio alpinus growing on them. Nine different
hepatotoxic PAs were found in the weed, the main one being
seneciphylline. Analyses of urine samples from one cow confirmed
the presence of PA metabolites. Several cows had to be
slaughtered, because of cirrhosis of the liver (Luthy et al.,
1981).
6.3 Studies on Farm Animals
There are several reports of the production of disease
characteristic of PA toxicity in farm animals, by feeding them
PA-containing plants.
Senecio jacobaea (tansy ragwort) is a weed that commonly grows
in pastures and has been the cause of extensive livestock losses in
the United Kingdom (Forsyth, 1968) and the USA (Johnson, 1982).
Extensive studies on this plant have been carried out using a
variety of farm animals. Dickinson et al. (1976) fed tansy ragwort
to cows through a rumen canula at the rate of 10 mg/kg body weight
per day, for 2 weeks. Liver biopsies showed characteristic
megalocytosis and fibroplasia, and autopsy also showed
centrilobular necrosis. A PA, jacoline, was found in the milk, but
when the calves were bucket fed the milk, there were no detectable
effects on them. Thorpe & Ford (1968) made similar observations in
5 calves fed ragwort in their diet. Animals eventually dying of
toxicity showed characteristic necrosis, megalocytosis, and veno-
occlusive lesions. In a study by Goeger et al. (1982), goats were
fed dried ragwort mixed in the diet at 250 g/kg. Four of the 11
goat kids and lactating dairy goats died. Characteristic
megalocytosis was seen in the liver. Goats are more resistant to
tansy ragwort toxicosis than cattle and horses, the chronic lethal
dose for cattle or horses being 0.05 - 0.2 kg ragwort/kg body
weight and, for goats, 1.25 - 4.04 kg/kg body weight. The alkaloid
levels in the plant, and thus its toxicity, varies with season and
locality.
Hooper & Scanlan (1977) studied the long-term effects of
feeding very low levels of ground C. retusa seeds, mixed with the
feed, to pigs and chickens. Seven groups of 4 pigs each (sex not
mentioned) bred from Saddleback-large white cross sows and Large
White or Landrace boars, were maintained on diets containing 0
(control), 0.004%, 0.01%, 0.02%, 0.05%, 0.1%, or 0.5% body weight
ground seeds. Another 8 pigs were fed a diet containing 0.1%
C. retusa for 21 days followed by 0.05% for 7 days and then kept on
a C. retusa-free diet. Pigs either died or were killed when
moribund, or at the end of 136 days of feeding.
In a second study, groups each of 4 2-week-old chickens were
fed diets containing 0 (control), 0.005%, 0.01%, 0.05%, 0.1%, and
0.5% ground seeds of C. retusa. Chickens fed 0.5% started dying 12
days after the commencement of feeding and were all dead by day 45.
Five out of 8 birds fed 0.1% or more died between days 22 and 56.
No deaths occurred in animals fed 0.01% - 0.005%.
In the study on pigs, all animals died between days 63 and 107
except for 2 that survived 136 days. In these animals, pulmonary
disease was the main cause of death. Hepatic and renal
megalocytosis was seen in almost all animals in both the field
outbreak and study group. The lungs showed extensive consolidation
and oedema. Besides megalocytosis in the glomeruli and tubules,
the kidneys showed glomerular atrophy and tubular necrosis. In the
study on poultry, the major disease was hepatic necrosis of
irregular distribution. The kidneys showed mild megalocytosis.
In the above study, the low levels of contamination that
produced serious disease are worthy of note.
Johnson & Molyneux (1984) fed 55 cattle, by gastric lavage,
with hay mixed with threadleaf groundsel ( Senecio douglasii var.
longilobus), which grows commonly in the pastures of southwestern
USA. The PA dosage in different groups ranged from 5 to 40 mg/kg,
daily, and the total intake ranged from 80 to 284 mg/kg body
weight. The groups were fed for periods ranging from 2 to 20 days.
One hundred percent mortality occurred in 3 out of 9 groups, each
consisting of 2 - 8 calves, receiving doses of 13 mg/kg or more.
Mean survival time was generally inversely proportional to the
dosage received. All sick calves had typical clinical signs of
seneciosis. At autopsy, the principal lesion was seen in the liver
and consisted of swelling of the hepatocytes, necrosis, biliary
hyperplasia, and marked fibrosis. The estimated minimum lethal
dose of the PA was 13 mg/kg body weight for 15 days, or a total
intake of approximately 200 mg PA/kg, over a 15-day period. Cattle
that consumed up to 600 mg PA/kg, in hay, in a 20- to 100-day
period, were unaffected or only slightly affected. The authors
concluded that the time-dose relationship for PA toxicosis in
cattle is important and that there is a threshold level that must
be exceeded for the toxicosis to develop.
A similar study was conducted by Johnson et al. (1985) in which
the dry whole or ground leaves of Senecio riddelli, mixed with the
feed, were fed to calves in gelatin capsules or by gavage. Forty-two
female Hereford calves were divided into 3 groups. One group
of 12 was fed the leaves mixed with alfalfa hay feed estimated to
have 20 - 40 mg PA/kg body weight per day over a 20-day period in
different regimes, receiving a total of 400 - 800 mg PAs per
animal. The second group of 12 animals received the plant packed
into gelatin capsules, receiving an estimated PA content of
10 - 20 mg/kg body weight, daily, over 20 days, with a total of
200 - 400 mg per animal. The third group of 18 animals was
administered (by gavage) finely ground leaves in a water slurry at
various PA dosages ranging from 10 to 60 mg/kg body weight per day
and a total of 200 - 500 mg over 20 days.
Calves that received 10 mg PA/kg body weight per day for 20
days did not develop clinical signs of disease or show any changes
in serum-enzyme. However, feed containing the plant that provided
15 - 20 mg PA/kg per day or more, administered by gavage or fed in
capsules, resulted in high mortality. Malaise, depression, erratic
or unprecedented behaviour, aimless walking, and ataxia, were
observed in the affected calves; diarrhoea with tenesmus and rectal
collapse were frequently observed. The feed intake decreased
progressively and was negligible terminally. The animals that died
and those that were moribund or in a state of irreversible wasting,
were autopsied. Hepatobiliary lesions were present in all such
animals. The most consistent change was portal biliary hyperplasia
and periportal fibrosis. Centrilobular or zonal haemorrhage and
necrosis were observed in some lobules. Fibrosis of some central
veins was common, often encroaching on the lumen, resulting in
complete occlusion. Hepatocytes also showed nonspecific changes.
Central nervous system changes were present in all animals with
clinical signs of seneciosis, consisting mainly of spongy
degeneration of the brain.
The plant mixed in the hay ration was eaten slowly and
reluctantly and was tolerated at dosages > 20 mg/kg per day,
emphasizing that the toxicity depended on the rate at which the
dosage was consumed and that mortality was not necessarily
dependent on the cumulative dosage.
Burguera et al. (1983) produced the disease in turkey poults by
feeding them seeds of C. spectabilis. Simultaneous addition of
sodium selenite at doses of 0.1, 5, or 10 mg selenium/kg diet did
not provide any protection.
6.4 Experimental Animal Studies
6.4.1 Effects on liver
6.4.1.1 Relative hepatotoxicity of different PAs and their N-oxides
The LD50 values for rats, listed in Table 9, are for some of
the most commonly used hepatotoxic alkaloids, calculated from data
on animals dying from acute haemorrhagic necrosis of the liver, 3 - 7
days after intraperitoneal administration of a single dose. It
is evident that the toxicity varies widely between the alkaloids.
The most toxic are certain macrocyclic diesters of retrorsine and
the least toxic are the monoesters of heliotridine, retrorsine, and
supinine (Mattocks, 1986).
The relative toxicity of N-oxides compared with that of their
basic alkaloids depends on the route of administration. The
N-oxides of lasiocarpine, monocrotaline, and fulvine were reported
to be as toxic as their basic alkaloids (Schoental & Magee, 1959)
when administered orally; however, when given by the ip or
intravenous (iv) routes to rats, they were much less toxic
(Mattocks, 1971c). Similarly, the LD50 of retrorsine N-oxide when
administered ip to male rats was 250 mg/kg (Table 9) but when given
orally, it was 48 mg/kg. This has been explained by the
observations on the metabolic pathways of the basic alkaloids and
their N-oxides. The PAs or their N-oxides exert toxic effects only
after being metabolized to pyrroles by the hepatic microsomal
enzymes (section 5.1.1). Hepatic microsomes act directly on the
N-oxides (Mattocks & White, 1971b) only after they have been
converted to the basic alkaloids (Mattocks, 1986); this mainly
occurs in the gut (Mattocks, 1971c; Powis et al., 1979). This
matter is of practical importance as the alkaloids are often
present as their N-oxides in weeds grazed by farm animals.
Table 9. LD50s in male rats after a single intraperitoneal dose of
some hepatotoxic alkaloids
-------------------------------------------------------------------
Alkaloid LD50 Time range Reference
(mg/kg) (days)
-------------------------------------------------------------------
heliotrine 296 3 Bull et al. (1958)
lasiocarpine 77 3 Bull et al. (1958)
lasiocarpine N-oxide 547 3 Bull et al. (1958)
monocrotaline 175 3 Bull et al. (1968)
retrorsine 34 4 or 7 Mattocks (1972a)
retrorsine N-oxide 250 7 Mattocks (1972a)
senecionine 50 7 Mattocks (1972a)
seneciphylline 77 3 Bull et al. (1968)
senkirkine 220 -a Hirono et al. (1979a)
symphytine 130 -a Hirono et al. (1979a)
-------------------------------------------------------------------
a Not stated.
6.4.1.2 Factors affecting hepatotoxicity
These factors have been reviewed by Mattocks (1986).
(a) Route of administration
Most studies on LD50 values have been carried out using the ip
route, and very few experimental data are available on toxicity
using the oral route. There is a close similarity between the iv
data and the ip data. Furthermore, toxicity data on rats
administered PAs by the oral route (Schoental & Magee, 1959),
including retrorsine, lasiocarpine, heliotrine, and monocrotaline,
closely resemble those relating to the LD50 values for the same
strain administered PAs intraperitoneally (Mattocks, 1972b). Thus,
the hepatotoxicity of PAs in rats does not differ very much,
irrespective of the route of administration. However, rabbits
appear to be less susceptible to PAs in the plant Senecio jacobaea
when administered orally than when administered intravenously
(Pierson et al., 1977).
(b) Species
Wide differences have been observed in the hepatotoxic effects
of PAs and alkaloid-containing plants between different species of
both farm animals and laboratory animals, and in the same animal
exposed to PAs derived from different plants. Sheep are resistant
to PA-containing plants (section 6.2) and when fed Echium
plantagineum pellets containing 1.3 g alkaloid/kg as the sole diet
for 12 months, over a period of 2 years, showed almost no liver
damage (Culvenor et al., 1984). However, adult rats fed the same
pellets as only 50% of the diet for 14 days died 4 - 13 weeks later
(Peterson & Jago, 1984). Pigs were found to be 5 - 10 times as
susceptible to PAs in Crotalaria retusa as chickens (Hooper &
Scanlan, 1977). Overall, the approximate ratios of quantities of
plant material required to prove toxic in the various species
listed are about 200 for the sheep (approximately the same for the
goat), 150 for the mouse, 50 for the rat, 14 for cattle
(approximately the same for the horse), 5 for the chicken, and 1
for the pig (Hooper, 1978).
Cheeke & Pierson-Goeger (1983) studied the chronic toxicity of
Senecio jacobaea for several laboratory animals by feeding the
dried plant as a component of a mixed diet. The degree of
susceptibility to PA poisoning was compared in terms of the chronic
lethal dose of the dried plant as a percentage of the initial body
weight among the animals themselves, and with similar data on
livestock in other studies. Gerbils, hamsters, and guinea-pigs
were resistant to chronic toxicity, gerbils being the most
resistant, consuming over 35 times their body weight of the dried
plant. Comparison with similar data in other studies indicated
that the rabbit (Pierson et al., 1977), Japanese quail (Buckmaster
et al., 1977), and goat (Goeger et al., 1982) were resistant,
requiring a long-term lethal dose of the plant of 113% or more of
the initial body weight, whereas the rat was highly sensitive
requiring only 21% (Goeger et al., 1983). Chicks and turkey poults
were also susceptible with severe inhibition of growth occurring
when there was 5% and 10% contamination of the feed with the plant;
survival time was short (Cheeke & Pierson-Goeger, 1983).
In a study by Fushimi et al. (1978), on the carcinogenicity of
the flower stalks of Petasites japonicus Maxim in mice and Syrian
golden hamsters, species and strain differences were observed, not
only with regard to hepatotoxicity, but also with regard to the
carcinogenicity of PAs. Mice of ddN, Swiss, and C57BL/6 strains
and Syrian golden hamsters were fed on a diet containing young
flower stalks of the plant for 480 days. High incidences of lung
adenoma and adenocarcinoma were observed in ddN mice, but no
significant differences in tumour incidence were observed between
the experimental groups of Swiss mice and hamsters and the
corresponding control group. No tumours were induced in an
experimental group of C57BL/6 strain mice.
These differences have been explained by the differences in the
rate of metabolic conversion of PAs to toxic metabolites (pyrroles)
by the hepatocyte microsomes in the different animal species (White
et al., 1973; Shull et al., 1976; Peterson & Jago, 1984).
The resistance of sheep has been ascribed to destruction of the
alkaloids in the rumen by a reductive conversion into non-toxic
1-methylenepyrrolizidine derivatives (Bull et al., 1968; Lanigan,
1971, 1972). It has also been suggested that the resistance of
sheep is due to a low level of activation in liver cells (Shull et
al., 1976), but this factor was not prominent in some Australian
sheep, which were as sensitive as rats to PAs injected
intraperitoneally (Hooper, 1974).
Thus, it is possible for ruminants to graze plants containing
PAs for a period of months without evident harm, e.g., cattle
eating Crotalaria juncea in Africa (Srungboonmee & Maskasame,
1981), but long-term effects may arise in animals exposed over
several years.
Considerable differences in LD50 values have been reported for
the same alkaloids in different species. For example, the LD50 for
retrorsine varies from 34 mg/kg for male rats to 279 mg/kg for
quail and over 800 mg/kg body weight for guinea-pigs (White et al.,
1973). Guinea-pigs are also resistant to monocrotaline (Chesney &
Allen, 1973a), but not to jacobine or to mixed alkaloids of Senecio
jacobaea, which are highly toxic (Swick et al., 1982a).
(c) Sex
Significant differences in the hepatotoxicity of the same
alkaloid have been observed between sexes in some species. Male
rats are much more susceptible to the acute toxicity of retrorsine
or monocrotaline than females (Mattocks, 1972b). Mattocks & White
(1973) reported a higher level of metabolic transformation in young
male rats to form pyrroles from retrorsine, compared with females
(section 4.4). Jago (1971) reported a higher susceptibility in
male rats to the chronic hepatotoxic effects of heliotrine, while
female rats were more susceptible to lasiocarpine. It is possible
that this may be due to the potentiating effect of male sex
hormones. Campbell (1957a,b) reported that diethylstilboesterol
protects against the effects of seneciphylline and promotes repair
of damaged liver in poultry. Protein-deficient rats of both sexes,
or female animals pre-treated with testosterone, were more
susceptible to monocrotaline (Ratnoff & Mirick, 1949).
(d) Age
Available data on the effects of age are highly conflicting.
It has been stated that young rats, particularly suckling animals
(Schoental, 1959), are more susceptible than adults to the
hepatotoxic effects of some alkaloids (Jago, 1970), such as
monocrotaline (Schoental & Head, 1955), and retrorsine and
lasiocarpine (Schoental, 1959). Rats, 1 - 4 days old, were far
more susceptible to retrorsine and senkirkine than rats aged 25 - 30
days (Schoental, 1970); yet new-born rats (within 1 h of birth)
were relatively more resistant to the hepatotoxic effects of
retrorsine than 1- to 4-day-old rats (Mattocks & White, 1973).
McLean (1970) has critically reviewed the data. In comparing the
data on small animals from several studies, new-born and 4-week-old
animals appear to have about the same susceptibility as adults.
Data for the intervening period obtained by Harris et al. (1957),
Schoental (1959), and Hayashi & Lalich (1968) are conflicting,
suggesting decreased susceptibility in some studies (Harris et al.,
1957 and one series of Schoental's studies, 1959), and increased
susceptibility in others (Hayashi & Lalich, 1968 and the second
series of Schoental's studies, 1959). Furthermore, Jago (1971)
demonstrated that, while rats aged 1 - 2 weeks were more
susceptible to the acute effects of heliotrine and lasiocarpine
than older rats, sensitivity to the effects of long-term
administration of these alkaloids increased with age, after 2 - 3
months.
(e) Diet
Effects of both qualitative and quantitative changes in diet on
the hepatotoxicity of PAs have been investigated in several
studies. Restriction of protein levels in the diet enhanced the
acute hepatotoxic effects of retrorsine in rats (Selzer & Parker,
1951) and the chronic effects of a single dose of orally
administered lasiocarpine (Schoental & Magee, 1957) (section
6.4.5.1) as well as the toxicity of PAs in Senecio jacobaea,
whereas a high protein diet had a protective effect (Cheeke &
Gorman, 1974). Likewise, low lipotrope diet enhanced the toxic
effects of orally administered lasiocarpine in pregnant rats and
also in the fetal livers (Newberne, 1968). On the other hand, it
protected young male rats against the acute toxicity of
monocrotaline, because of the reduced metabolic conversion of the
alkaloid into pyrrolic metabolites (Newberne et al., 1971, 1974).
Caloric restriction reduced the cardiopulmonary toxicity of a
single dose of monocrotaline in rats (Hayashi et al., 1967). This
was ascribed to the reduced growth rate in animals on a restricted
diet rather than to a reduction in the rate of metabolic conversion
of the alkaloid, since dietary restriction started only after
administration of the alkaloid. When the animals were put back on
the ad libitum feeding regimen, they developed signs of increased
toxicity.
A high copper content in the diet has been shown to enhance the
toxic effects of PAs (Miranda et al., 1981b). Incorporation of
copper sulfate at 50 mg/kg in the diet containing the plant Senecio
jacobaea increased the hepatotoxicity in rats, as judged by enzyme
measurements. The implications of this observation are obvious if
some PA-containing plants being grazed by farm animals also have a
high copper content.
Mattocks (1972b) demonstrated the protective effects of sucrose
against the acute hepatotoxic effects of retrorsine in rats, if
administered for 3 days prior to alkaloid administration (section
5.5.1).
6.4.1.3 Acute effects
Experimental animal studies on the pathological effects of PAs
on the liver have been reviewed by Bull et al. (1968) and McLean
(1970). Most studies have been carried out on the rat (Schoental &
Magee, 1957, 1959; Bull & Dick, 1959; Schoental, 1963; Barnes et
al., 1964; McLean et al., 1964; Nolan et al., 1966; Jago, 1969;
Butler et al., 1970; Peterson & Jago, 1980), but several other
species of animals have been studied including the monkey (Wakim et
al., 1946; Rose et al., 1959; Allen & Carstens, 1968, 1971; Allen
et al., 1969), turkey (Allen et al., 1963), chicken (Allen et al.,
1960), hamster (Harris et al., 1957), mouse, guinea-pig (Chen et
al., 1940), quail, cat, rabbit, and pig (Emmel et al., 1935; Hooper
& Scanlan, 1977). All animals tested, except the guinea-pig (Chen,
1945), have been found to be susceptible in studies using purified
alkaloids and their N-oxides and crude extracts of PA-containing
plants.
Typically, the most common lesion produced in small laboratory
animals by doses close to the LD50 is a confluent haemorrhagic
necrosis in the liver, which appears within about 12 h of exposure
and peaks at 24 - 48 h. It is strictly zonal in distribution in
different species of animals but may vary within the same animal,
depending on the alkaloid used, species, nutritional status, and
pretreatment with other chemicals.
Retrorsine produces centrilobular necrosis in the rat, mouse,
and guinea-pig, periportal necrosis in the hamster, and focal
necrosis in the fowl and in the monkey (White et al., 1973). In
the monkey, monocrotaline produces centrilobular necrosis (Allen &
Carstens, 1968), but senecionine produces necrosis in the
periportal and midzonal areas of the liver lobule (Wakim et al.,
1946). Almost simultaneously, or shortly after the development of
acute haemorrhagic necrosis of the liver cells, various levels of
change appear in the central and sublobular veins of the liver
lobules, consisting of subintimal oedema or even necrosis, deposits
of fibrin, thrombosis, and occlusion of the lumen, which later
becomes organized. While haemorrhagic necrosis is a constant
feature, attempts to produce occlusive lesions in the veins of
experimental animals have produced variable results (Allen et al.,
1967). In man and non-human primates, hepatocellular necrosis and
venous occlusion occur simultaneously but, in the rat (McLean et
al., 1964), chicken (Allen et al., 1960), and swine (Emmel et al.,
1935), the vascular changes follow hepatic necrosis.
Selzer & Parker (1951) produced a lesion comparable to human
veno-occlusive disease in albino rats by administrating retrorsine
hydrochloride, the active alkaloid of Senecio ilicifolius, as well
as the crude plant extract. Four batches of rats were administered
alkaloids orally in a single dose of 1 - 1.5 mg/10 g body weight or
repeated doses of 5 - 50 mg/kg body weight for 31 days or as single
subcutaneous injection of 100 mg/kg body weight. One batch was fed
on a diet of Senecio ilicifolius constituting 10% of the ration as
crude plant or its extract; the animals lived for 21 - 84 days.
Some groups were kept on a normal diet, and others on a diet that
was protein-deficient. Animals, administered a single dose orally,
developed the earliest degenerative changes in the centrilobular
hepatocytes and sinusoidal dilatation, and the vascular lesion
appeared after 36 h. Protein deficiency enhanced the toxic effect.
Only 5 out of 9 animals administered repeated doses orally showed
early centrilobular fibrosis and none showed the vascular lesion,
possibly due to scarring.
Bull et al. (1958) studied the effects of a single ip LD70 dose
of heliotrine (320 mg/kg body weight), lasiocarpine (80 mg/kg), or
lasiocarpine N-oxide (629 mg/kg) in rats of a hooded strain of both
sexes. Eighty-one rats were used and 3 rats from each treatment
were killed at intervals of 4 - 36 h. Heliotrine produced marked
centrilobular necrosis at 24 h, but venous changes were not
evident, except for some aggregation of mononuclear macrophages on
the endothelium. With lasiocarpine, the hepatic changes were
similar, but the necrosis was not clearly centrilobular and, with
its N-oxide, it was midzonal at 34 - 49 h. The earliest toxic
effect of the PAs was manifested as a temporary loss of
mitochondria at 8 h. The authors concluded that PAs have an early
toxic effect on the hepatocytes and that this does not follow
vascular injury, as suggested by Davidson's earlier studies (1935).
McLean et al. (1964) administered an aqueous extract of
Crotalaria fulva to Wistar rats in a single intragastric dose of
0.8 - 1.5 g/kg body weight. Lesions identical to those of human
veno-occlusive disease were produced in the animals by adjusting
the dose to permit survival for 8 - 12 days. Loss of cytoplasmic
glycogen in the centrilobular cells occurred 3 h following
administration. Centrilobular necrosis, which occurred after 24 h,
increased with time. The central veins gradually filled up with
thickened endothelial cells at about 7 days, later progressing to
collagenization. Evidence was presented that the histological
occlusion of the central veins was preceded by several days by a
functional blocking of the blood flow.
Barnes et al. (1964) observed similar results in rats
administered a single oral dose of fulvine N-oxide at 50 mg/kg
body weight and studied at intervals of 1 - 4 days after the
administration. One hundred and thirty-five rats of both sexes
were used. Acute lesions resembling human disease were observed
during days 1 - 8. During days 19 - 21, 3 out of 25 animals showed
liver damage consisting of some centrilobular haemorrhage and
fibrous thickening of the central veins. Of the 78 animals studied
at 22 - 44 days, 50% still had centrilobular congestion and some
had fibrous thickening of the central veins.
The effects of pyrrolic derivatives of PAs on rats were studied
by Butler et al. (1970). Male albino rats of Porton strain were
administered solutions of pyrrole derivatives of monocrotaline
and retrorsine in dimethyl formamide as a single injection of
0.05 - 0.1 ml solution. When injected in the tail vein at a
concentration of 5 mg/kg body weight, it produced progressive
proliferation of alveolar epithelium of the lungs and the animals
developed signs of respiratory distress in 2 - 3 weeks. When injected
in the mesenteric vein at a concentration of 15 mg/kg body weight, as
a rule, the animals remained well in the postoperative period and
only 1/26 animals died of mesenteric vein thrombosis; the livers
developed multiple infarcts in the left lobes that developed into
multiple coarse nodules at 6 - 12 weeks. The above studies
substantiated the view that PAs act only when converted in
hepatocytes to pyrroles. When pyrroles were injected, they
affected the smaller vessels at the portal of entry; in animals
injected through the mesenteric vein, the main target was the
portal vein with only secondary damage to the parenchymal cells,
thus sparing the animals from the effects of hepatocellular injury.
On the other hand, pyrroles injected through the tail vein went
directly to the pulmonary arteries through the heart and damaged
the alveolar capillaries.
Acute veno-occlusive disease was produced in monkeys
administered monocrotaline (Allen et al., 1967, 1969) and ground
Crotalaria spectabilis seed (Allen & Carstens, 1968). In a study
published in 1967, these authors used 14 monkeys (Macaca speciosa)
of both sexes, each weighing approximately 4 kg. Seven of the
animals were administered 1 mg monocrotaline in distilled water by
gastric intubation on days 1 and 14. The remaining 7 were used as
controls and received distilled water only. Wedge biopsies of
liver were examined weekly. The survival time ranged from 14 to 38
days, the mean being 21 days. The livers of treated animals were
small and firm and showed changes characteristic of human veno-
occlusive disease including centrilobular necrosis, and vascular
changes in the central veins of liver lobules ranging from
subintimal oedema to progressive collagenization and extension of
collagen fibres into the sinusoids. Similar observations were made
in studies on Macaca mulatta monkeys (Allen & Carstens, 1968),
administered ground Crotalaria spectabilis seed. Sixty-four
animals, averaging 6.2 kg in weight, were divided into 3 groups.
Group I, comprising 10 experimental animals (4 control animals),
received seeds in the diet containing the equivalent of 0.074 mg
monocrotaline/kg body weight, daily, up to death. Group II,
consisting of 14 treated animals (4 control animals), received a
single dose of seeds containing 1.3 g monocrotaline/kg body weight,
and Group III, consisting of 26 experimental animals (6 controls),
received 3 weekly doses containing the equivalent of 0.116 g
monocrotaline/kg body weight, by gastric intubation. Liver
biopsies were carried out each month in Group I and each week in
Groups II and III. Animals of the last 2 groups were killed when
in extremis. The mean survival times for the groups were:
Group I, 269 days (176 - 425 days); Group II, 28 days (6 - 43
days); and Group III, 41 days (23 - 91 days). In Group I animals,
occlusive lesions of the central and sublobular veins of the liver
were seen in 7/10 animals at autopsy. These consisted of oedema,
haemorrhages, and fragmentation of the vessel walls, the lumina
being filled with fibrin, and degenerating liver cells. The
lobular pattern was distorted because of connective tissue bands
encircling small groups of liver cells, especially in the central
zones of the lobules. In Groups II and III, various levels of
focal or centrilobular necrosis were observed and the liver cells
were replaced by stromal tissue. Vascular lesions, as described
above, were seen in 25 monkeys, but no collagen was demonstrated.
In the studies of Wakim et al. (1946) on the rhesus monkey,
senecionine administered iv as a 2% solution at doses of 10 - 30 mg/kg
body weight to 4 animals, daily, until they appeared to be sick,
produced periportal, or midzonal necrosis in 3 animals accompanied
by haemorrhage. No mention was made of any vascular changes.
Electron microscopic studies
Svoboda & Soga (1966) studied the effects of lasiocarpine and
Crotalaria fulva on the livers of male Sprague Dawley rats weighing
110 - 150 g each. One group of 22 rats was given an ip injection
of lasiocarpine at 80 mg/kg body weight and pairs of animals were
killed at various intervals ranging from 15 min to 6 days. A
second group of 22 animals was administered a single dose of an
aqueous extract of Crotalaria fulva at 0.5 mg/g body weight, by
gastric tube, and killed at the same intervals. A third group of 8
rats was administered a total of 3.2 times the LD50 dose of
lasiocarpine in small doses, 3 times a week, and killed at 9 - 20
weeks. The changes primarily involved the nucleus and
interchromatin granules. The first change, seen after 30 min in
the nueleoli of the hepatocytes and Kupffer cells of animals
receiving lasiocarpine or crotalaria extract, consisted of a
separation of the fibrillar and granular components. The
hepatocyte nuclei had returned to normal after 72 h and remained so
throughout the rest of the study. In animals receiving a single
dose of lasiocarpine or crotalaria, round periodic acid schiff
(PAS) positive eosinophilic bodies appeared in the cytoplasm after
12 h, consisting of dense masses of cytoplasmic material. Five
days after treatment with crotalaria, large cells lined the luminal
surface of the central veins; the centrilobular cells had undergone
necrosis by this stage. Animals receiving 3.2 times the LD50 of
lasiocarpine developed megalocytosis after 9 weeks (section
6.4.1.5). The cytoplasm showed vesicles of smooth endoplasmic
reticulum with mitochondria of various shapes and sizes. The
appearance resembled an exaggerated regenerative response.
Allen et al. (1967, 1969) studied ultrastructural changes in
the liver tissue, in general, including the hepatic veins in Macaca
speciosa monkeys treated with PAs. In the study on hepatic veins
(Allen et al., 1969), 18 treated and 6 control adult animals were
used with an average weight of 5.8 kg. Animals were divided into 3
groups of 6 treated and 2 control animals each. The experimental
animals received 0.125 g monocrotaline/kg body weight by ip
injection. Liver wedge biopsies were examined at various intervals
in Group I during hours 1 - 48, in Group II at 4 - 12 days, and in
Group III at 16 - 32 days. The earliest changes, observed by light
microscopy, were seen at 24 h and consisted of progressive loss of
endothelial cells and other associated changes in the lumen and
wall leading to occlusion by collagenization by the third week.
Under the electron microscope, within 24 h of administration,
marked changes were observed in the endothelial cells resulting in
their rupture and release of organelles in the lumen. This was
followed by penetration of fluid though the vessel walls in the
first week and changes in the fibroblasts. By the third to fourth
week, hepatic veins showed various levels of occlusion and the
vessel was scattered with cell debris, free organelles, etc. The
authors concluded that, in this species, hepatocellular necrosis
was not the primary factor causing veno-occlusive disease, as the
association of cellular necrosis and venous occlusion occurred only
in the central area of liver lobules, and the hepatocytes
surrounding the sublobular and medium sized hepatic veins were
unaffected.
In their study of 1967, Allen et al. also investigated the
ultrastructural changes in the liver of M. speciosa monkeys after
administering 2 doses of 1 g monocrotaline each, on days 1 and 14.
At autopsy, after a mean survival time of 21 days, a wide spectrum
of changes was observed in the hepatocytic organelles, many of
which were lying, discharged into sinusoids, and also phagocytosed
by the Kupffer cells. By the third week, proliferation of
connective tissues had started in the sinusoids near the central
veins and also in the walls of central veins. The authors
concluded that the vascular and parenchymal cell changes were
simultaneous and appeared to be equally instrumental in the
development of the occlusive lesion.
6.4.1.4 Mechanism of toxic action
The mechanism of toxic action in acute pyrrolizidine
hepatotoxicity and the sequence of events, judged from the
collective experimental studies, appears to be as follows.
The PA, which is inactive as a cell poison by itself, becomes
cytotoxic through its metabolism in the hepatic parenchymal cells
to pyrroles, which act preferentially on the hepatocytes and the
endothelium of blood vessels in the liver or lungs. In the
hepatocytes, the immediate action is a rapid fall in cytoplasmic
protein synthesis reaching 30% of control levels at 15 min and 6%
at 1 h (Harris et al., 1969). This is manifested as disaggregation
of polyribosomes and is followed by failure of pyruvate oxidation,
loss of glycogen, structural damage to the mitochondria, lysosomal
activity, failure of mitochondrial nicotine-adenine-dinucleotide
(NAD) systems and nuclear NAD synthesis, and necrosis (McLean,
1970). The necrosis is zonal in the liver lobule, the particular
zone affected depending on the metabolic enzymic geography of the
lobule in the particular animal species, and also in man and monkey
on the vascular endothelium of the central and sublobular veins.
The sequence of events of the vascular lesion has been studied
by McLean et al (1964). After a single dose of Crotalaria fulva
extract in the rat, centrilobular necrosis is present after the
first day, but collagenous veno-occlusion of the central veins of
the liver lobule only appears between 7 and 10 days later.
Evidently, the necrosis of the liver cells is not secondary to
venous occlusion. Centrilobular haemorrhage is seen from day 2
onwards and signs of hepatic venous outflow tract obstruction
appear after 2 - 5 days (McLean & Hill, 1969).
Rappaport et al. (1967) and McLean (1969) demonstrated, through
transillumination studies on rats, that the outlet end of the
sinusoids is blocked by stationary columns of red cells, 16 - 24 h
following administration of PAs. The reaction is typically patchy
and results in stasis and extravasation of red cells spreading
backwards from the centre of the lobule. For at least 3 days, no
circulatory detail can be seen with transillumination. Portal
pressure is significantly raised 3 days after administration of
fulvine (Rappaport et al., 1967), notably before the first
appearance of collagenous venous occlusion at 7 days. McLean
(1969) observed that 6 - 10 days after PA administration, a new
irregular pattern of vascular flow, contrasting with the uniform
radial pattern of flow in the normal liver lobule, develops, which
corresponds to the bypass channels represented by dilated
paraseptal sinusoids, as observed in human liver biopsies (section
7.3). Segments of central vein into which the blocked sinusoids
open, are gradually abandoned in favour of such by-pass routes and
undergo occlusion first by oedematous connective tissue and then by
fibrosis. The mechanism of closure of the sinusoids is not clear.
A toxic action on the sinusoidal or venous endothelium, which
swells and occludes the lumen, seems possible, as suggested by
Allen & Carsten's (1968) electron microscopic studies on the
monkey, and studies on children (Brooks et al., 1970). The
endothelial lining of the vessels is denuded and replaced by a
fibrinous and proteinaceous precipitate, which, together with the
oedematous wall of the vessel, becomes organized and slowly
replaced by fibrous connective tissues. The occlusion of sinusoids
is further contributed by the discharge of cellular debris into the
space of Disse. The lumen of the sinusoids becomes occluded
simultaneously with the fibrosis occurring in the central vein.
Collagen fibres extend into the space of Disse and sinusoids
leading to a creeping fibrosis.
The proximate toxin that escapes from the liver is returned to
the heart, after which it damages the first portal of entry into
the alveolar capillaries of the lung and pulmonary arteries.
6.4.1.5 Chronic effects
The chronic hepatotoxic effects of PAs have been described in a
number of studies on a variety of animals and have been reviewed by
Bull et al. (1968) and McLean (1970). A notable feature is that an
appropriate single dose of PA has been demonstrated by Schoental
and Magee (1957, 1959) to lead to a relentlessly progressive course
and eventually kill the animal, more than 18 months after
administration. Schoental & Bensted (1963) demonstrated that rats
receiving a single dose of PA may develop chronic liver disease and
finally hepatocellular carcinoma more than 13 months after
administration. The morphological changes in the liver are similar
in a given species of animal, whether a single sublethal dose is
administered or multiple small doses.
Schoental & Magee (1957) studied the long-term effects of a
single dose of lasiocarpine on rats receiving normal and protein-
deficient diets. Albino rats of Porton strain were used. In the
first study, 66 rats fed a normal diet were administered a single
oral dose of lasiocarpine at one of 3 dose levels (50 - 74 mg/kg,
75 - 100 mg/kg, or 101 - 150 mg/kg body weight); 24 animals served
as controls. In another study, 46 young female rats were
administered a protein-deficient diet. Of these, 13 and 10 animals
received a single oral dose of lasiocarpine at 50 - 100 mg/kg and
50 - 75 mg/kg body weight, respectively. Each of these groups was
pair-fed with an identical number of animals that did not receive
any PA. Of the 66 rats fed a normal diet, very few died in the
first 10 days. Thirty-one animals survived longer than 3 months.
They continued to be in good health until shortly before death.
The numbers of animals that survived for 13 months after
administration of lasiocarpine were 8/10 males and 7/7 females
(lowest dose) and 5/25 males and 11/18 females (intermediate dose).
In the group that received the highest dose, neither of the 2 male
animals survived longer than 35 days, and only 1/4 female animals
survived longer than 3 months. In the animals that died or were
killed when moribund, parenchymal damage was invariably present
with prominent megalocytes, ductular proliferation, and invasion of
lobules by oval or elongated cells, thought to be derived from the
bile-duct epithelium or the reticuloendothelial cells. Animals
that survived showed various degrees of fibrosis and nodular
hyperplasia and, in some, a mild thickening of the central veins.
No obliterative lesions of the veins were seen. The 31 animals
that survived 13 months showed similar changes, but to a much
lesser extent. In the livers of animals that had repeat liver
biopsies, there was no tendency to regression of the lesions.
The above data indicate that very few animals died of acute
disease. In most animals, there was a latent period of 3 - 4
weeks, during which they remained well and showed little evidence
of liver cell injury, followed by a progressive course often
leading to fibrosis and nodular hyperplasia.
The low-protein diet adversely affected the growth of all the
rats in the control as well as the treated group. Only 3 out of 23
treated animals remained alive and in apparent good health, 8 - 11
months after the treatment. Liver biopsies taken at various
intervals between 4 and 10 months showed very severe fatty changes
in the liver cells. There was little fibrosis and no bile-duct
proliferation. In areas where the fatty changes were less severe,
characteristic megalocytes were seen. Control animals had either
normal livers or showed only slight fatty changes that were not
comparable in severity with those in the livers of PA-treated
animals. Thus protein deficiency in the diet was shown to enhance
the toxic effects of the PA.
Schoental & Magee (1959) extended these studies on young Wistar
rats using several other PAs including heliotrine, retrorsine,
riddelliine, seneciphylline, monocrotaline, and its N-oxide in
various dosages ranging from 25 to 300 mg/kg body weight; the
animals were studied at death from 1 - 10 days to 18 - 30 months.
Pathological changes were similar to those observed with
lasiocarpine in the previous study. Notable observations were that
necrosis did not necessarily precede subacute or chronic changes.
The livers of some animals became severely damaged and showed
nodular hyperplasia. Liver biopsy, 2 - 3 days after PA treatment,
did not show pathological changes in some animals, but a repeat
biopsy at 41 days showed characteristic changes. Fibrous
thickening of the central veins was observed in some animals, more
often with monocrotaline N-oxide, but no occlusion of the hepatic
veins was seen.
The studies of Nolan et al. (1966) confirmed the observations
of Schoental & Magee (1957, 1959). They gave a single dose of
lasiocarpine at 120 mg/kg body weight, by stomach tube, to 108
(equal numbers of both sexes) Sprague Dawley weanling rats (60 -
135 g body weight). Thirty animals of both sexes served as
controls. Groups of 10 animals, each consisting of 8 treated and 2
control animals, were killed at various intervals from 1 to 123
days. Of the 80 treated animals, 28 died within 26 days. No
delayed hepatic lesions were found in 59 rats between days 1 and
18. Between 19 and 123 days, delayed lesions were found in 34/49
rats. These 34 rats showed megalocytosis, but no ductular
proliferation or fibrosis.
In a second study, 127 twenty-one-day-old male Wistar rats were
given a single oral dose of lasiocarpine at 80 mg/kg body weight;
65 animals served as controls. Liver biopsy was carried out on day
3 in 108 and on day 9 in 15 of the treated animals. In 47 animals,
additional biopsies were carried out at intervals. Of the 127
PA-treated rats, 98 died during the first 9 days, and 29 after
10 - 50 days. In contrast to the first study, 32/58 survivors
exhibited delayed subacute and chronic lesions, as described by
Schoental & Magee (1957). Of these, 8 animals developed cirrhosis.
The observations indicated that the lesions of acute zonal
necrosis, which appeared on, or before, the third day, healed
without residual lesions. However, 55% of the 58 survivors
developed subacute/chronic lesions that tended to be progressive
after a latent period of 2 - 3 weeks. There appeared to be an
intimate relationship between chronic lesions and megalocytosis,
which was seen in 52/58 surviving animals. No obliterative
vascular changes were observed and so the lesions could not be
ascribed to impaired circulation.
Schoental (1959) demonstrated the toxic effects on the newborn
of PAs administered to lactating mother rats. Wistar rats
(200 - 300 g) were administered lasiocarpine, orally or by ip
injection, at 25 - 40 mg, in 5 - 10 doses of 5 - 10 mg, twice weekly
or more (24 rats), or retrorsine at 4 - 10 mg per dose, in 1 - 14
approximately daily doses (23 rats). The litters were left with
the mothers for 24 days or more (except for 1/2 hour separation
during the PA treatment). The litters were examined by biopsy at
frequent intervals or at autopsy when they died or were killed in a
moribund state. Litters of the lasiocarpine-treated rats showed
only insignificant fibrosis or some megalocytosis. In the
retrorsine-treated group, the majority of the young rats survived
for about 18 days, but all rats died before reaching the age of 30
days. The milk secretion of the mothers was apparently not affected
by the PA treatment. Of the 98 animals in 9 litters, 45 died by
the 20th day and 45 survived 30 days. Animals dying in the first
fortnight did not show gross liver lesions, but those that died at
weaning time or later, all showed signs of liver disease. Animals
dying at 18 - 30 days showed hydropic or fatty vacuolation of
hepatocytes and haemorrhage into distended sinusoids. The change
was severe in animals dying at 1 - 2 months, and some central veins
showed a narrowed lumen. The lactating rats that received the
alkaloids survived longer than their young, and most showed no ill
effects from the treatment. This evidently indicates either a high
susceptibility of suckling rats or a high concentration of PAs in
milk.
In studies by Allen et al. (1963), 2 groups of 4-week-old
turkeys, each consisting of 12 birds, were fed diets containing
ground Crotalaria spectabilis seed at 2.5 g/kg and 5 g/kg,
respectively, for 120 days. Twelve animals served as controls. At
the end of the study, 11/12 birds receiving 5 g/kg seed and 6/12
receiving 2.5 g/kg seed in the diet developed cirrhosis. The
minimum period of feeding required to produce cirrhosis was 75
days, provided the diet was reduced to a level that was not lethal.
Allen & Carstens (1971) induced the Budd-Chiari syndrome in
monkeys by monocrotaline. Six adult female and 9 adult male Macaca
speciosa monkeys, weighing 5.2 - 6.5 kg each, were divided into 2
groups, each comprising 5 control (3 males and 2 females) and 10
treated animals (6 males and 4 females). The treated group was
given a subcutaneous (sc) injection of monocrotaline at 60 mg/kg
body weight at monthly intervals for 3 months. Needle biopsy of
the liver was carried out every month for 5 months and laparotomy,
6 months after PA treatment.
The treated animals showed marked vascular changes and various
degrees of occlusion in the centrilobular and sublobular veins as
well as the larger vessels. There was also characteristic
haemorrhagic necrosis in the centrilobular zones and megalocytes
were seen. The portal venous pressures were raised. The animals
were autopsied at 6 months. The livers were markedly shrunken
weighing an average of 68 g in contrast to those of control
animals, which weighed 130 g. There were severe occlusive vascular
changes and irregular fibrosis in the lobules. The adjacent
sinusoids were dilated as a compensatory mechanism.
Swick et al. (1982a) studied the effects on guinea-pigs of
long-term dietary administration of Senecio jacobaea and compared
them with the toxic effects of single doses of injected Senecio
alkaloids and monocrotaline. The possible protective effect of
cysteine was also examined. Fifteen guinea-pigs of 250 - 300 g
initial body weight were divided into 2 treated groups, being fed
10% Senecio jacobaea, or 10% Senecio jacobaea plus 1% cysteine in
the diet, and a control group. The whole plant of Senecio jacobaea
was air dried and powdered for incorporation into the diets. The
animals were fed for 365 days. They were autopsied at death or at
the termination of the study. In a second study, 7 guinea-pigs of
500 g body weight were injected intraperitoneally with either
monocrotaline, jacobine, or mixed Senecio jacobaea PAs. The
chronic lethal dose LD100 of Senecio jacobaea was 1264 g/kg initial
body weight or 526% of the initial body weight with an average
survival time of 279 days. No mortality was observed in control
animals. This contrasts with the chronic LD100 of Senecio jacobaea
for rats of 58% of initial body weight (Swick et al., 1979) and
that of cattle equivalent to 5 - 20% body weight (Bull et al.,
1968). Addition of cysteine to the diet was only slightly, but not
significantly, protective. Pathological examination of the livers
of the guinea-pigs fed Senecio jacobaea revealed extensive
megalocytosis and severe cytoplasmic vacuolation with biliary
hyperplasia and fibrosis, primarily in periportal areas. The
centrilobular and midzonal areas were spared.
Monocrotaline was non-toxic at doses up to 1000 mg/kg body
weight, whereas jacobine and mixed alkaloids from Senecio jacobaea
were lethal at much lower levels. Similar results showing
resistance to monocrotaline in guinea-pigs were also reported by
Chesney & Allen (1973a). In in vitro studies, they related this
resistance to lack of conversion of PA to pyrroles by guinea-pig
microsomes.
A morphological peculiarity of chronic hepatotoxicity in a
large variety of laboratory and farm animals is megalocytosis
(Bull, 1955; McLean, 1970), i.e., the appearance of exceptionally
large hepatocytes, 10 - 30 times the volume of normal cells with
proportionately large nuclei. Relevant literature has been
reviewed by Jago (1969), McLean (1970), and Mattocks (1986).
Advanced megalocytosis was produced by Jago (1969) within 4 weeks
in 2-week-old rats by administering a single dose of lasiocarpine
at 76 µmol/kg body weight. Megalocytes tend to appear in the
periportal and midzones of the liver lobules with normal sized
cells around the central veins. The nuclear chromatin is
proportionately increased, but the cells appear incapable of
entering into mitosis, as only abnormal mitoses are seen. Jago
(1969) demonstrated a fall in the mitotic index (from 1.61 to 0.04)
in liver cells of 2-week-old rats, one day after injection of 50 µmol
lasiocarpine/kg. The electron microscopic appearance also
supports the above observations (Afzelius & Schoental, 1967). A
striking proliferation of rough endoplasmic reticulum and multiple
centrioles is seen in the cytoplasm, and the cytoplasmic organelles
are disorganized, suggesting increased metabolic activity but
inability of the cells to divide. Such cells may persist for the
life-time of the animal (up to 2 years in the rat) and the liver
never returns to normal (Mattocks, 1986). Megalocytes have also
been described in the kidney (Bull et al., 1968), the lung (Barnes
et al., 1964; Butler et al., 1970; Hooper, 1974), and the duodenum
(Hooper, 1975c).
Data on the total chronic lethal dose of heliotrine in rats
were discussed by Bull & Dick (1959) and Bull et al. (1968). For a
variety of dosing rates, and with withholding periods of 10 - 20
weeks interposed, the total doses ranged from 2.2 to 7.8 LD50. In
Table 10, these data are extended with results for other alkaloids.
The overall range of the total lethal dose is 1.2 - 10.3 LD50.
6.4.2 Effects on lungs
Current literature has been extensively reviewed by Kay & Heath
(1969), and Mattocks (1986). PAs have been shown to produce
pulmonary hypertension with associated vascular changes in the
pulmonary circulation in a number of experimental animal species
including the rat, mouse, frog, turkey, pig, sheep, rabbit, and
horse (McLean, 1970) as well as in non-human primates (Allen &
Chesney, 1972; Chesney & Allen, 1973b) and the dog (Miller et al.,
1978). The alkaloids have been administered by feeding the animals
with: PA-containing seeds of plants (notably Crotalaria
spectabilis) (Turner & Lalich, 1965; Kay & Heath, 1966; Kay et al.,
1967a) or the dried plant itself (e.g., Senecio jacobaea) (Burns,
1972), aqueous solutions of fulvine (Barnes et al., 1964;
Wagenvoort et al., 1974a,b) or monocrotaline (Lalich & Ehrhart,
1962; Huxtable et al., 1977), subcutaneous injections of
monocrotaline (Allen & Chesney, 1972; Chesney & Allen, 1973b) and
seneciphylline (Ohtsubo et al., 1977), or intravenous injections of
some pyrrolic esters and analogues of pyrrolizidine alkaloids and
their metabolites (Mattocks & Driver, 1983). Lafranconi & Huxtable
(1984) studied the hepatic metabolism and pulmonary toxicity of
monocrotaline in in vitro perfusion studies. Some of the
representative studies on the morphological effects of toxic lung
injury are listed in chronological order in Table 11.
Chronic lung lesions have been produced by most compounds that
produce chronic liver lesions, though higher doses were required in
some instances (Culvenor et al., 1976a). However, not all PAs that
are hepatotoxic are also pneumotoxic. Among the pneumotoxic
alkaloids, fulvine (Barnes et al., 1964) and monocrotaline are
particularly active (Mattocks, 1986). Molecular structure activity
requirements are the same as for hepatotoxicity, since both are
caused by the same toxic metabolites produced in the hepatocytes.
6.4.2.1 Acute effects
Pulmonary lesions produced by PAs have been extensively
investigated, mostly in rats, but also in non-human primates.
Monocrotaline has been the alkaloid most frequently used, but lung
lesions have also been seen in rats following fulvine and
seneciphylline administration. Besides pure alkaloids,
PA-containing seeds of some plants, most notably Crotalaria
spectabilis, have also been used.
Miller et al. (1978) gave a single iv injection of
monocrotaline at 60 mg/kg body weight to 10 mongrel dogs. Toxic
effects, recorded within 2 h, included ultrastructural changes in
the endothelial cells of the alveolar capillaries, prominent
accumulation of platelets, and the appearance of interstitial
oedema (Table 11). Valdivia et al. (1967a,b) used 25 Sprague
Dawley rats in their study and made similar observations on the rat
lung within 4 h of a single subcutaneous injection of monocrotaline
at a dose of 60 mg/kg body weight (Table 11). Interstitial oedema
and elastolysis of the alveolar wall, increase in number of mast
cells, and other associated changes were observed within 4 h of the
injection, followed by alterations in endothelial and interstitial
cells. All of the changes progressed steadily for up to 3 weeks.
It was concluded that the initial changes of destruction of
pulmonary capillaries and the other components of the alveolar wall
preceded the arteriolar hypertrophy and arteritis observed by other
investigators following monocrotaline administration, and alone was
sufficient to cause right ventricular hypertrophy. Sugita et al.
(1983a,b) administered a single dose of monocrotaline at 40 mg/kg
body weight to 5 Sprague Dawley rats and adduced further evidence,
by biochemical and radioisotopic studies, of microvascular leak in
the alveolar wall within the first 3 days of injury, which preceded
right ventricular hypertrophy observed 2 weeks following
administration (Table 11).
Table 10. Total chronic lethal doses in rats (ip administration, 2 or 3
times per week)
--------------------------------------------------------------------------
Dose (x LD50) Time to Total lethal Reference
death dose
(days) (x LD50)
--------------------------------------------------------------------------
Heliotrine (male rats, unless otherwise stated)
0.2 58 5 Bull & Dick (1959)
0.1 123 5.1
0.04 303 4.1
0.02 508 4.1
0.01 5.2 - 5.3 Bull & Dick (1960)
(with interval of 10 -
20 weeks after 21 days)
0.11 2.2 - 4.3 Bull & Dick (1959)
0.1 7.8 Jago (1971)
(35-day-old male rats)
0.1 4.7 Jago (1971)
(337-day-old male rats)
0.1 5.8 Jago (1971)
(35-day-old female rats)
0.1 4.5 Jago (1971)
(337-day-old female rats)
Lasiocarpine (male rats)
0.1 210 9 Culvenor & Jago (1979)
0.05 482 10.3
0.02 676 5.7
0.01 595 2.6
(0.005) (638) (1.4)
0.1 81 - 175 2.4 - 5 Bull & Dick (1959)
0.1 - 6.3 - 10.9 Jago (1971)
Lasiocarpine (female rats)
0.1 108 4.6 Culvenor & Jago (1979)
0.05 274 5.8
0.02 471 4
0.01 487 2.1
(0.005) (692) (1.5)
0.1 2.4 - 7 Jago (1971)
Monocrotaline
0.1 1.2 - 2.4 Bull et al. (1968)
0.05 2.5 - 4.4
Senecioninea
0.2 2 - 7.4 Bull et al. (1968)
0.1 1.7 - 5.7
0.04 (3 survived)
--------------------------------------------------------------------------
a Assuming LD50 mg/kg (c.f., Mattocks, 1986).
Table 11. Summary of experimental data on the morphological effects of toxic lung injury due to pyrrolizidine alkaloids (in
chronological order)
---------------------------------------------------------------------------------------------------------------------------------------
Animal/ Number Toxic agent Dose Administration Single/ Killed after Pathological References
strain/ of (mg/kg) dose multiple or survival effects
sex animals/ for
controls
---------------------------------------------------------------------------------------------------------------------------------------
Acute effects
Male 25/5 monocrotaline 60 subcutaneous single 2 - 48 h interstitial oedema, Valdivia et al.
Sprague (body 1 - 3 weeks endothelial cell (1967a,b)
Dawley rat weight) alterations,
elastolysis, etc.
Mongrel 10/0 monocrotaline 60 intravenous single 2 h interstitial oedema, Miller et al. (1978)
dog (body changes in endothelial
weight) cells
Male 5/3 monocrotaline 40 subcutaneous single 0 - 21 days microvascular leak, Sugita et al. (1983a)
Sprague (body right ventricular
Dawley rat weight) hypertrophy after 2
weeks
Chronic effects
Female 35/12 monocrotaline 10 - 30 ad libitum pulmonary arteritis Lalich & Ehrhart
Sprague (diet) feeding (with 20 - 30 mg dose (1962)
Dawley rat only)
6 alcohol- 2500 ad libitum 51 days none
extracted (diet) feeding
seeds of
Crotalaria
spectabilis
19/4 monocrotaline 10 - 75 ad libitum 26 - 232 Turner & Lalich (1965)
(diet) feeding days
---------------------------------------------------------------------------------------------------------------------------------------
Table 11. (contd.)
---------------------------------------------------------------------------------------------------------------------------------------
Animal/ Number Toxic agent Dose Administration Single/ Killed after Pathological References
strain/ of (mg/kg) dose multiple or survival effects
sex animals/ for
controls
---------------------------------------------------------------------------------------------------------------------------------------
Chronic effects (contd.)
Female 24/6 Crotalaria 200 - ad libitum 105 - 172 right ventricular Allen & Chesney (1972)
Wistar spectabilis 1600 feeding days hypertrophy and
Furth rat seeds (diet) dilatation, pulmonary
arterial hypertrophy,
pulmonary arteriolar
hypertrophy,
endocardial fibrosis
Female 10/34 Crotalaria 1000 ad libitum 30 - 60 days right ventricular Kay & Heath (1966)
weanling spectabilis (diet) feeding hypertrophy, pulmonary
Wistar rat seeds arterial hypertrophy,
pulmonary arteritis
Male 22/12 monocrotaline 120 subcutaneous single 20 - 47 days right ventricular Hayashi & Lalich
suckling (body hypertrophy, pulmonary (1967)
Sprague weight) arterial hypertrophy,
Dawley rat pulmonary arteritis,
fibrin thrombi
Female 30/10 fulvine 50 intraperitoneal single 3 - 37 days right ventricular Kay et al. (1971a)
Wistar rat (body hypertrophy, pulmonary
weight) arterial hypertrophy,
pulmonary arteriolar
hypertrophy, pulmonary
arteritis, arteriolar
thrombi
80 intragastric single 7 - 35 days
(body
weight)
---------------------------------------------------------------------------------------------------------------------------------------
Table 11. (contd.)
---------------------------------------------------------------------------------------------------------------------------------------
Animal/ Number Toxic agent Dose Administration Single/ Killed after Pathological References
strain/ of (mg/kg) dose multiple or survival effects
sex animals/ for
controls
---------------------------------------------------------------------------------------------------------------------------------------
Chronic effects (contd.)
Monkey 12 (30 monocrotaline 30 subcutaneous one 199 - 325 right ventricular Allen & Chesney (1972)
(Macaca days old) (body followed days hypertrophy and
arctoides) weight) by 3 (average, dilatation, left
(both (2, 4, 241) ventricular
sexes) and 6 hypertrophy, pulmonary
months arterial hypertrophy,
after pulmonary arteriolar
first hypertrophy, pulmonary
injection hypertension
12 (15 163 - 334 pulmonary arterial
months days hypertension (isolated,
old) (average, veno-occlusive disease
217) (liver)
Monkey 20/6 monocrotaline 30 subcutaneous as above 165 - 325 right ventricular Chesney & Allen
(Macaca (body days hypertrophy and (1973b)
arctoides) weight) (average, dilatation, pulmonary
(both 326) arterial hypertrophy,
sexes) pulmonary arteriolar
hypertrophy, pulmonary
arteritis, endocardial
fibrosis
Female 50/12 fulvine 80 intragastric single 1 - 6 weeks vasoconstriction, Wagenvoort et al.
Wistar rat (body pulmonary arterial (1974a,b)
weight) hypertrophy, pulmonary
or 50 arteriolar hypertrophy,
(body intraperitoneal right ventricular
weight) hypertrophy, thickening
of veins and venules
---------------------------------------------------------------------------------------------------------------------------------------
Table 11. (contd.)
---------------------------------------------------------------------------------------------------------------------------------------
Animal/ Number Toxic agent Dose Administration Single/ Killed after Pathological References
strain/ of (mg/kg) dose multiple or survival effects
sex animals/ for
controls
---------------------------------------------------------------------------------------------------------------------------------------
Chronic effects (contd.)
Male 16/0 seneciphylline 50 - 80 subcutaneous single 1 - 3 weeks pulmonary arteriolar Ohtsubo et al. (1977)
Wistar (body and right ventricular
rat (4 weight) hypertrophy (after 3
weeks old) weeks), right
ventricular dilatation
in 2 animals
Male 21/14 Crotalaria 1000 ad libitum 3 - 35 days pulmonary arterial Meyrick & Reid (1979,
Sprague spectabilis (diet) feeding hypertrophy and 1982)
Dawley rat pulmonary arteritis
(2/21); right
ventricular hypertrophy
---------------------------------------------------------------------------------------------------------------------------------------
A histological and electron microscopic study was made by
Hurley & Jago (1975) of the lungs of rats administered
dehydromonocrotaline. Female black and white hooded rats weighing
80 - 100 g were used. Dehydromonocrotaline dissolved in
dimethylformamide (DMF) was administered iv as a single dose at
30 mg/kg body weight to 12 rats and at 15 mg/kg body weight to 7 rats.
Four control rats were administered DMF alone. A colloidal
suspension of carbon black was injected iv, 6 - 18 h after
injection of dehydromonocrotaline, and the animals killed 19 - 44 h
after treatment.
After an interval of 6 - 8 h, there was a direct toxic effect
on the endothelial cells of pulmonary capillaries and small
venules. Many endothelial cells had prominent nuclei and thickened
cytoplasm, which contained more RNA granules than usual. There was
also an increase in the number of mitochondria. The endothelial
damage did not seem to have caused permanent disruption of the
small blood vessels, and, 2 days after injury, all vessels were
patent. Large numbers of mononuclear cells, which appeared in the
interstitial tissues of the lung 44 h after injury, seemed to be
altered emigrated blood monocytes.
6.4.2.2 Chronic effects
Lalich & Erhart (1962), fed 35 Sprague Dawley rats a diet
containing monocrotaline at 10 - 30 mg/kg (Table 11). Animals
receiving a daily dose of monocrotaline of 20 mg/kg diet or more,
showed progressive changes in the lungs after 24 days of feeding.
Of the 23 animals receiving 20 - 30 mg/kg, 12 showed pulmonary
arteritis, 4 of these even at the dose level of 20 mg/kg diet.
Pulmonary haemorrhages were observed in 16 animals. No changes
were observed in animals fed alcohol-extracted seeds or other
derivatives of monocrotaline.
Identical changes were observed in similar studies by Turner &
Lalich (1965) on two strains of rats, Sprague Dawley (19) and
Wistar Furth (24). The first group of 19 female Sprague Dawley
rats was fed a diet containing monocrotaline at an initial level of
10 mg/kg diet. Depending on the response of each individual
animal, the monocrotaline level was raised to a maximum of 75 mg/kg
diet (Table 11). Fourteen rats survived for more than 100 days and
8 reached the maximum dietary level of monocrotaline, the last
animal dying after 232 days. The second group of 24 female Wistar
Furth rats was fed a diet contaminated with Crotalaria spectabilis
seeds, initially at 0.2 mg/kg diet, gradually rising to 1.6 mg/kg
by increasing the levels by 0.2 mg/kg every week. All test animals
survived 100 days and 15 reached the maximum levels of Crotalaria
fed. The last animal died after 172 days of feeding. Animals
developed signs of toxicity and right ventricular strain, e.g.,
cyanosis, etc. Progressive thickening of media in the muscular
pulmonary arteries, progressive muscularization of arterioles, and
changes characteristic of pulmonary hypertension, were seen. Some
pulmonary arteries showed medial necrosis. No changes were
observed in the pulmonary veins. Significant hypertrophy of the
heart, as judged by the heart weight in relation to body weight,
was seen in almost all animals that survived 100 days or more
(32/38), and the right ventricles were dilated. The hypertrophy
affected the right side of the heart only, and generally
corresponded with the vascular changes. There was a marked
hyperplasia of the mast cells in the mediastinal lymph nodes and
around bronchi and pulmonary arteries. Similar observations were
made by Barnes et al. (1964) and Valdivia et al. (1967a,b).
Kay and his group studied cardiac and pulmonary vascular
changes in rats fed Crotalaria spectabilis seeds (Kay & Heath,
1966; Kay et al., 1967a,b) or administered fulvine (Kay et al.,
1971a).
A group of 10 female weanling Wistar albino rats were fed a
diet containing 1 g powdered seeds of Crotalaria spectabilis/kg
until they died of cardiorespiratory distress, after 36 - 60 days
of feeding. Thirty-four control rats were fed a normal diet. At
autopsy, the atria of the heart, the right ventricle, and the left
ventricle with the interventricular septum were weighed separately.
The medial thickness of the muscular pulmonary arteries was
measured, and expressed as percentage of external diameter. The
medial thickness of the muscular pulmonary arteries increased in
all test rats; acute or healing pulmonary arteritis was seen in 3
animals. Statistically significant cardiomegaly was present in all
rats fed the seeds, contributed chiefly by the right ventricle. The
readings from all the test rats were well outside the upper 95%
confidence limit. The increase in the medial thickness of the
pulmonary arteries correlated well with the weight of the right
ventricle (Fig. 10). Three rats showed pulmonary arteritis
(indicated by a solid triangle). It was presumed that the organic
basis for increased pulmonary resistance was the abnormal
muscularization of the radicles of the pulmonary arterial system.
Essentially similar results were obtained in an identical study
repeated on 8 test rats and 5 controls (Kay et al., 1967a). The
test rats developed pulmonary hypertension in 37 days, levels of
which were correlated with the medial thickness of the muscular
pulmonary arteries and that of the pulmonary trunk, as well as with
the weight of the right ventricle.
 Ghodsi & Will (1981) made similar observations in Sprague
Dawley rats given a single subcutaneous injection of monocrotaline
at 60 mg/kg body weight. Forty rats weighing 180 - 200 g were
used; of these, 20 constituted the control group. The control
animals received the same volume of saline. Each week, 3 rats from
each group were catheterized and pulmonary artery pressures were
measured. In the treated group, 2 out of 5 animals showed a mild
increase in pulmonary artery pressure at the end of 8 days. A
further 4 out of 5 animals showed a mild to moderate rise in
pulmonary artery pressure after 2 weeks. The highest value
recorded in test rats was 56 mmHg compared with a normal upper
limit of 22 mmHg. The medial thickness of pulmonary arteries was
correlated with pulmonary artery pressures ( P < 0.02) as was the
thickness of the right ventricle. The correlation between the
pulmonary artery pressures and right ventricular hypertrophy was
statistically significant ( P < 0.05).
Kay et al. (1971a) administered a single dose of fulvine to
rats, intraperitoneally at 50 mg/kg body weight, or through a
stomach tube at 80 mg/kg. Of the 30 treated rats, 17 survived 23
days. All of the animals showed changes characteristic of
hypertensive pulmonary vascular disease with right ventricular
hypertrophy and muscular hypertrophy of the pulmonary trunk and the
muscular pulmonary arteries. Pulmonary arterioles were also
muscularized and contained fibrin thrombi. Four animals showed
pulmonary arteritis.
Essentially similar changes in the pulmonary arterial system
were produced within 20 - 28 days by Hayashi & Lalich (1967) in 22
male suckling rats administered a single injection of monocrotaline
at 120 mg/kg body weight, and within 28 days by Ohtsubo et al.
(1977) in 16 male, 4-week-old rats given a single injection of
seneciphylline at 80 mg/kg body weight. Hooper (1974) did not find
any such effect on feeding powdered Senecio jacobaea at
100 - 200 mg/kg diet to 9 male white mice for up to 193 days.
In studies by Allen & Chesney (1972), non-human primates
(Macaca arctoides) were administered 4 doses of monocrotaline at
30 - 60 mg/kg body weight by subcutaneous injection (Table 11).
Twelve infant monkeys (30 days old) and 12 adults (15 months old)
were studied with different results. Vascular changes,
characteristic of pulmonary hypertension and resultant cor
pulmonale, were observed in the infant monkeys, as described by
earlier workers in the rat. Only isolated small hepatic veins were
occluded. On the other hand, the adult animals showed a more
severe involvement of the liver with changes characteristic of
veno-occlusive disease and only an occasional pulmonary blood
vessel was involved. The authors postulated that the different
responses in infant and adult animals were due to the different
stages of maturation of the enzyme systems of the hepatocyte in the
two age groups. It is possible that the different reactions in the
liver and lung in the 2 groups may be due to the fact that the
enzymatic pathways responsible for producing metabolites that cause
hepatic damage are poorly developed in the infant, but those
responsible for causing pulmonary lesions are better developed.
Chesney & Allen (1973b) made observations similar to those of
Allen & Chesney (1972) in twenty, 30-day-old monkeys in a similar
study using monocrotaline injections and recorded, in addition,
endocardial fibrosis of the right heart. The treated animals
developed classical clinical features of cardiopulmonary distress,
which was also evidenced by changes in the blood-gas parameters.
The raised right heart pressures were confirmed by actual
measurements of the blood pressure in the right ventricle,
pulmonary artery, and descending aorta. The authors considered
this study to be a good experimental model to investigate
hypertensive pulmonary vascular disease, or pulmonary and
endocardial fibrosis. The type of vascular changes seen in the
animals were comparable with those associated with pulmonary
hypertension in man in cardiopulmonary disease (Barnes et al.,
1964; Kay & Heath, 1966; Kay et al., 1967a; Chesney & Allen,
1973b).
Wagenvoort et al. (1974a,b) made light microscopic and ultra-
structural studies on 50 female Wistar rats, 1 - 6 weeks following
a single oral dose of fulvine at 80 mg/kg body weight or an ip dose
at 60 mg/kg body weight. Twelve animals served as controls.
Vasoconstriction of muscular pulmonary arteries and arterioles was
seen initially, one week following administration. This was
evident by the coiled appearance of the muscular nuclei and
excessive crenation of the internal elastic lamina. The nuclei of
smooth muscle cells as well as those of endothelial cells were
partly squeezed between the folds of the lamina. After 3 - 4
weeks, these blood vessels began to thicken, with muscular
hypertrophy and fibrinoid necrosis of the arterial muscle. Animals
surviving administration of fulvine developed right ventricular
hypertrophy, proliferation of endothelial cells in the arteries and
even thickening of the veins.
In a study by Meyrick and Reid (1979), 21 Sprague Dawley rats
were fed a diet containing 1 g powdered seeds of Crotalaria
spectabilis/kg for various periods ranging from 3 to 35 days. The
earliest demonstrable change in the pulmonary arterial system of
the animals was seen on day 3 and consisted of the appearance of
muscle in normally non-muscular arteries of the lung. The muscular
pulmonary arteries began to show hypertrophy of the media from day
7, which reached statistically significant levels on day 10 in
smaller arteries and on day 14 in the larger arteries. Significant
right ventricular hypertrophy was seen on day 21. These changes
were confirmed by 3H-thymidine uptake studies (Meyrick & Reid,
1982).
6.4.2.3 Mechanisms of toxic action
Considerable progress has been made recently in the
understanding of biochemical and pharmacological changes that occur
in PA-induced lung disease.
Turner & Lalich (1965) and Takeoka et al. (1962) postulated
that pulmonary hypertension was mediated by the release of
5-hydroxytryptamine from the mast cells, which became hyperplastic
in the mediastinal lymph nodes and around bronchi and pulmonary
arteries (Turner & Lalich, 1965) (section 6.4.2.2) following
administration of monocrotaline, causing vasoconstriction. On the
other hand, Kay et al. (1967b) found that the number of mast cells
corresponded with the severity of exudative changes in the lung and
were not related to the genesis of pulmonary hyperplasia.
Besides the medial muscular hypertrophy of pulmonary arteries
reported in several studies cited above, swelling and lysis of the
endothelial cells, contributing to luminal narrowing and thickening
of the wall with fibrosis, have been described (Allen & Carstens,
1970).
Weanling rats are more susceptible to these changes than older
animals, and the changes follow a strict temporal sequence. Oral
administration of monocrotaline to rats at 20 mg/litre in drinking-
water produced a sequence of changes over 3 weeks, that included an
increase in lung mass, which was significant by day 9, stimulation
of pulmonary RNA and protein synthesis (maximal on day 10),
increased pulmonary arterial blood pressure (significant by day
10), and right ventricular hypertrophy by day 14 (Huxtable et al.,
1978; Lafranconi et al., 1984). The increase in the lung mass was
not accompanied by change in the total collagen content and was
contributed possibly by hypertrophy of endothelial cells, but the
increased mass of the right ventricle was associated with a 4-fold
increase in collagen content (Lafranconi et al., 1985).
An early event is inhibition of serotonin removal by pulmonary
endothelium (Huxtable et al., 1978). This phenomemon, combined
with the increased release of serotonin by mast cells that has been
observed, may be involved in the development of pulmonary
hypertension (Carillo & Aviado, 1969). Right ventricular
hypertrophy is blocked by propanolol, whereas the development of
pulmonary hypertension is unaffected (Huxtable et al., 1977).
Novel metabolites have been found to be released by livers perfused
with monocrotaline in vitro, and these metabolites block serotonin
transport in vitro, when perfused through isolated lungs
(Lafranconi & Huxtable, 1984). These data suggest that the slow
release of metabolites from the liver into the circulation
following low-level exposure to monocrotaline results in specific
inhibition of endothelial cell function (Huxtable et al., 1978).
The effect of monocrotaline treatment on pulmonary angiotensin
converting enzyme (ACE) activity in the rat is disputed. Hayashi
et al. (1984) observed a reduction in the ACE activity of pulmonary
tissue in pyrrolizidine-exposed rats in parallel with the
development of pulmonary alterations, while the ACE activity of the
plasma remained unchanged. However, other authors have reported
that, though the specific activity of ACE falls in the isolated
perfused lungs of monocrotaline-treated rats, or in lung
homogenates from such animals, when activity is expressed as total
activity per lung, there is no significant alteration in the lungs
of treated animals compared with those of untreated animals
(Huxtable et al., 1978; Lafranconi & Huxtable, 1983). Therefore,
the significance of changes in ACE activity is open to question.
Molteni et al. (1984) also found evidence of endothelial cell
damage by monocrotaline in their ultrastructural and biochemical
studies on rats. Eighty male Sprague Dawley rats were used; half
were administered monocrotaline at 20 mg/litre in the drinking-
water and half were given plain water. The average daily water
consumption was 35 ml/rat. Thus, the treated rats were estimated
to have received 2 mg/kg per day. Five animals each from the
treated and control groups were killed at intervals of 1 - 12 weeks
after the start of the study. The endothelial damage was measured
by ACE activity, plasminogen-activator (PLA) activity, and
prostacyclin (PGI2) production. These were correlated with
pulmonary arterial perfusion and ultrastructural changes in the
lung. In the treated groups, after an initial rise at 1 week, the
ACE activity showed a steady decline from 1 to 6 weeks, after which
it plateaued at 55% of normal. PLA activity did not change for 2
weeks, but decreased by 59 and 79% of the control value after 6 and
12 weeks, respectively. On the other hand, the PGI2 production
increased progressively reaching 140 and 270% of the control level
after 6 and 12 weeks, respectively. These endothelial functional
changes were not accompanied by significant changes in pulmonary
arterial perfusion as visualized by 99mTc-labelled macroaggregated
albumin perfusion studies. The activities of ACE and PLA and the
production of PGI2 are considered sensitive indices of endothelial
function in rats. The above results indicated endothelial cell
dysfunction. The ultrastructural studies also revealed oedema of
capillary subendothelial, perivenous and periarterial tissues at 1
week, and interstitial inflammatory infiltrates at 2 weeks. At
6 - 12 weeks, there was thickening of the pulmonary arteries and
enlargement of right side of the heart.
Stenmark et al. (1985) studied the role of alveolar
inflammation and arachidonate metabolism in monocrotaline-induced
pulmonary hypertension in rats. Five groups of male Sprague Dawley
rats were treated as follows: (a) 20 rats received 40 mg
monocrotaline/kg body weight, sc; (b) 20 rats received
monocrotaline, 40 mg/kg sc plus diethylcarbamazine (DEC) 100 mg/kg
sc, every 12 h; in addition, 250 mg DEC was added to 100 ml of
drinking-water. This treatment started 2 days prior to the start
of the study and was continued daily for 3 weeks; (c) 12 control
rats received normal saline plus monocrotaline at 40 mg/kg sc; (d)
12 rats received indomethacin at 2 mg/kg sc for 2 days, prior to
receiving monocrotaline at 40 mg/kg and then daily for 3 weeks; (e)
6 animals each received a single sc injection of normal saline and
served as additional controls.
One, 2, and 3 weeks after monocrotaline or saline injection,
lung lavage was carried out for cell counts and assay for enzyme
activity and cyclooxygenase metabolites, the degradation products
of prostacyclin (PGI2) and thromboxane A2(TXA2), as 6-keto-
prostaglandin (PGF1alpha) and TXB2, respectively. At 3 weeks, the
animals were anaesthetized, right ventricular pressures measured by
catheterization and the heart removed. The 2 ventricles were
separated and weighed for the determination of heart-weight ratio
(right ventricle/left ventricle + septums RV/LV + S) an indicator
of right ventricular hypertrophy.
The right ventricle showed hypertrophy at 2 weeks and the right
ventricular pressure was increased at 3 weeks following
monocrotaline administration (Fig. 11). The leukocyte count in the
lavage fluid increased at 3 weeks, with a rise in the percentage of
polymorphonuclear leukocytes and large, abnormal alveolar
macrophages in the test animals. B- N-acetyl-D-glucoseaminidose
activity was also elevated at 3 weeks, indicating activation of
leukocytes. There was also a rise in the concentration of 6-keto-
PGF1alpha at 1 and 3 weeks, as well as in TXB2 at 3 weeks, compared
with those in control animals.
The administration of DEC inhibited both the increase in heart-
weight ratio (RV/LV + S) and the increase in pulmonary artery
pressure (Fig. 11) that occurred 3 weeks after monocrotaline
administration, and reduced the percentage of polymorphonuclear
cells, abnormal alveolar macrophages, and hexoseaminidase activity
in the lavage fluid, compared with that from animals that had
received monocrotaline only. The rise in the levels of 6-keto-
PGF1alpha was inhibited ( P < 0.05) by 73% and that of TXB2 by 74%
in the lung lavage.
Ghodsi & Will (1981) made similar observations in Sprague
Dawley rats given a single subcutaneous injection of monocrotaline
at 60 mg/kg body weight. Forty rats weighing 180 - 200 g were
used; of these, 20 constituted the control group. The control
animals received the same volume of saline. Each week, 3 rats from
each group were catheterized and pulmonary artery pressures were
measured. In the treated group, 2 out of 5 animals showed a mild
increase in pulmonary artery pressure at the end of 8 days. A
further 4 out of 5 animals showed a mild to moderate rise in
pulmonary artery pressure after 2 weeks. The highest value
recorded in test rats was 56 mmHg compared with a normal upper
limit of 22 mmHg. The medial thickness of pulmonary arteries was
correlated with pulmonary artery pressures ( P < 0.02) as was the
thickness of the right ventricle. The correlation between the
pulmonary artery pressures and right ventricular hypertrophy was
statistically significant ( P < 0.05).
Kay et al. (1971a) administered a single dose of fulvine to
rats, intraperitoneally at 50 mg/kg body weight, or through a
stomach tube at 80 mg/kg. Of the 30 treated rats, 17 survived 23
days. All of the animals showed changes characteristic of
hypertensive pulmonary vascular disease with right ventricular
hypertrophy and muscular hypertrophy of the pulmonary trunk and the
muscular pulmonary arteries. Pulmonary arterioles were also
muscularized and contained fibrin thrombi. Four animals showed
pulmonary arteritis.
Essentially similar changes in the pulmonary arterial system
were produced within 20 - 28 days by Hayashi & Lalich (1967) in 22
male suckling rats administered a single injection of monocrotaline
at 120 mg/kg body weight, and within 28 days by Ohtsubo et al.
(1977) in 16 male, 4-week-old rats given a single injection of
seneciphylline at 80 mg/kg body weight. Hooper (1974) did not find
any such effect on feeding powdered Senecio jacobaea at
100 - 200 mg/kg diet to 9 male white mice for up to 193 days.
In studies by Allen & Chesney (1972), non-human primates
(Macaca arctoides) were administered 4 doses of monocrotaline at
30 - 60 mg/kg body weight by subcutaneous injection (Table 11).
Twelve infant monkeys (30 days old) and 12 adults (15 months old)
were studied with different results. Vascular changes,
characteristic of pulmonary hypertension and resultant cor
pulmonale, were observed in the infant monkeys, as described by
earlier workers in the rat. Only isolated small hepatic veins were
occluded. On the other hand, the adult animals showed a more
severe involvement of the liver with changes characteristic of
veno-occlusive disease and only an occasional pulmonary blood
vessel was involved. The authors postulated that the different
responses in infant and adult animals were due to the different
stages of maturation of the enzyme systems of the hepatocyte in the
two age groups. It is possible that the different reactions in the
liver and lung in the 2 groups may be due to the fact that the
enzymatic pathways responsible for producing metabolites that cause
hepatic damage are poorly developed in the infant, but those
responsible for causing pulmonary lesions are better developed.
Chesney & Allen (1973b) made observations similar to those of
Allen & Chesney (1972) in twenty, 30-day-old monkeys in a similar
study using monocrotaline injections and recorded, in addition,
endocardial fibrosis of the right heart. The treated animals
developed classical clinical features of cardiopulmonary distress,
which was also evidenced by changes in the blood-gas parameters.
The raised right heart pressures were confirmed by actual
measurements of the blood pressure in the right ventricle,
pulmonary artery, and descending aorta. The authors considered
this study to be a good experimental model to investigate
hypertensive pulmonary vascular disease, or pulmonary and
endocardial fibrosis. The type of vascular changes seen in the
animals were comparable with those associated with pulmonary
hypertension in man in cardiopulmonary disease (Barnes et al.,
1964; Kay & Heath, 1966; Kay et al., 1967a; Chesney & Allen,
1973b).
Wagenvoort et al. (1974a,b) made light microscopic and ultra-
structural studies on 50 female Wistar rats, 1 - 6 weeks following
a single oral dose of fulvine at 80 mg/kg body weight or an ip dose
at 60 mg/kg body weight. Twelve animals served as controls.
Vasoconstriction of muscular pulmonary arteries and arterioles was
seen initially, one week following administration. This was
evident by the coiled appearance of the muscular nuclei and
excessive crenation of the internal elastic lamina. The nuclei of
smooth muscle cells as well as those of endothelial cells were
partly squeezed between the folds of the lamina. After 3 - 4
weeks, these blood vessels began to thicken, with muscular
hypertrophy and fibrinoid necrosis of the arterial muscle. Animals
surviving administration of fulvine developed right ventricular
hypertrophy, proliferation of endothelial cells in the arteries and
even thickening of the veins.
In a study by Meyrick and Reid (1979), 21 Sprague Dawley rats
were fed a diet containing 1 g powdered seeds of Crotalaria
spectabilis/kg for various periods ranging from 3 to 35 days. The
earliest demonstrable change in the pulmonary arterial system of
the animals was seen on day 3 and consisted of the appearance of
muscle in normally non-muscular arteries of the lung. The muscular
pulmonary arteries began to show hypertrophy of the media from day
7, which reached statistically significant levels on day 10 in
smaller arteries and on day 14 in the larger arteries. Significant
right ventricular hypertrophy was seen on day 21. These changes
were confirmed by 3H-thymidine uptake studies (Meyrick & Reid,
1982).
6.4.2.3 Mechanisms of toxic action
Considerable progress has been made recently in the
understanding of biochemical and pharmacological changes that occur
in PA-induced lung disease.
Turner & Lalich (1965) and Takeoka et al. (1962) postulated
that pulmonary hypertension was mediated by the release of
5-hydroxytryptamine from the mast cells, which became hyperplastic
in the mediastinal lymph nodes and around bronchi and pulmonary
arteries (Turner & Lalich, 1965) (section 6.4.2.2) following
administration of monocrotaline, causing vasoconstriction. On the
other hand, Kay et al. (1967b) found that the number of mast cells
corresponded with the severity of exudative changes in the lung and
were not related to the genesis of pulmonary hyperplasia.
Besides the medial muscular hypertrophy of pulmonary arteries
reported in several studies cited above, swelling and lysis of the
endothelial cells, contributing to luminal narrowing and thickening
of the wall with fibrosis, have been described (Allen & Carstens,
1970).
Weanling rats are more susceptible to these changes than older
animals, and the changes follow a strict temporal sequence. Oral
administration of monocrotaline to rats at 20 mg/litre in drinking-
water produced a sequence of changes over 3 weeks, that included an
increase in lung mass, which was significant by day 9, stimulation
of pulmonary RNA and protein synthesis (maximal on day 10),
increased pulmonary arterial blood pressure (significant by day
10), and right ventricular hypertrophy by day 14 (Huxtable et al.,
1978; Lafranconi et al., 1984). The increase in the lung mass was
not accompanied by change in the total collagen content and was
contributed possibly by hypertrophy of endothelial cells, but the
increased mass of the right ventricle was associated with a 4-fold
increase in collagen content (Lafranconi et al., 1985).
An early event is inhibition of serotonin removal by pulmonary
endothelium (Huxtable et al., 1978). This phenomemon, combined
with the increased release of serotonin by mast cells that has been
observed, may be involved in the development of pulmonary
hypertension (Carillo & Aviado, 1969). Right ventricular
hypertrophy is blocked by propanolol, whereas the development of
pulmonary hypertension is unaffected (Huxtable et al., 1977).
Novel metabolites have been found to be released by livers perfused
with monocrotaline in vitro, and these metabolites block serotonin
transport in vitro, when perfused through isolated lungs
(Lafranconi & Huxtable, 1984). These data suggest that the slow
release of metabolites from the liver into the circulation
following low-level exposure to monocrotaline results in specific
inhibition of endothelial cell function (Huxtable et al., 1978).
The effect of monocrotaline treatment on pulmonary angiotensin
converting enzyme (ACE) activity in the rat is disputed. Hayashi
et al. (1984) observed a reduction in the ACE activity of pulmonary
tissue in pyrrolizidine-exposed rats in parallel with the
development of pulmonary alterations, while the ACE activity of the
plasma remained unchanged. However, other authors have reported
that, though the specific activity of ACE falls in the isolated
perfused lungs of monocrotaline-treated rats, or in lung
homogenates from such animals, when activity is expressed as total
activity per lung, there is no significant alteration in the lungs
of treated animals compared with those of untreated animals
(Huxtable et al., 1978; Lafranconi & Huxtable, 1983). Therefore,
the significance of changes in ACE activity is open to question.
Molteni et al. (1984) also found evidence of endothelial cell
damage by monocrotaline in their ultrastructural and biochemical
studies on rats. Eighty male Sprague Dawley rats were used; half
were administered monocrotaline at 20 mg/litre in the drinking-
water and half were given plain water. The average daily water
consumption was 35 ml/rat. Thus, the treated rats were estimated
to have received 2 mg/kg per day. Five animals each from the
treated and control groups were killed at intervals of 1 - 12 weeks
after the start of the study. The endothelial damage was measured
by ACE activity, plasminogen-activator (PLA) activity, and
prostacyclin (PGI2) production. These were correlated with
pulmonary arterial perfusion and ultrastructural changes in the
lung. In the treated groups, after an initial rise at 1 week, the
ACE activity showed a steady decline from 1 to 6 weeks, after which
it plateaued at 55% of normal. PLA activity did not change for 2
weeks, but decreased by 59 and 79% of the control value after 6 and
12 weeks, respectively. On the other hand, the PGI2 production
increased progressively reaching 140 and 270% of the control level
after 6 and 12 weeks, respectively. These endothelial functional
changes were not accompanied by significant changes in pulmonary
arterial perfusion as visualized by 99mTc-labelled macroaggregated
albumin perfusion studies. The activities of ACE and PLA and the
production of PGI2 are considered sensitive indices of endothelial
function in rats. The above results indicated endothelial cell
dysfunction. The ultrastructural studies also revealed oedema of
capillary subendothelial, perivenous and periarterial tissues at 1
week, and interstitial inflammatory infiltrates at 2 weeks. At
6 - 12 weeks, there was thickening of the pulmonary arteries and
enlargement of right side of the heart.
Stenmark et al. (1985) studied the role of alveolar
inflammation and arachidonate metabolism in monocrotaline-induced
pulmonary hypertension in rats. Five groups of male Sprague Dawley
rats were treated as follows: (a) 20 rats received 40 mg
monocrotaline/kg body weight, sc; (b) 20 rats received
monocrotaline, 40 mg/kg sc plus diethylcarbamazine (DEC) 100 mg/kg
sc, every 12 h; in addition, 250 mg DEC was added to 100 ml of
drinking-water. This treatment started 2 days prior to the start
of the study and was continued daily for 3 weeks; (c) 12 control
rats received normal saline plus monocrotaline at 40 mg/kg sc; (d)
12 rats received indomethacin at 2 mg/kg sc for 2 days, prior to
receiving monocrotaline at 40 mg/kg and then daily for 3 weeks; (e)
6 animals each received a single sc injection of normal saline and
served as additional controls.
One, 2, and 3 weeks after monocrotaline or saline injection,
lung lavage was carried out for cell counts and assay for enzyme
activity and cyclooxygenase metabolites, the degradation products
of prostacyclin (PGI2) and thromboxane A2(TXA2), as 6-keto-
prostaglandin (PGF1alpha) and TXB2, respectively. At 3 weeks, the
animals were anaesthetized, right ventricular pressures measured by
catheterization and the heart removed. The 2 ventricles were
separated and weighed for the determination of heart-weight ratio
(right ventricle/left ventricle + septums RV/LV + S) an indicator
of right ventricular hypertrophy.
The right ventricle showed hypertrophy at 2 weeks and the right
ventricular pressure was increased at 3 weeks following
monocrotaline administration (Fig. 11). The leukocyte count in the
lavage fluid increased at 3 weeks, with a rise in the percentage of
polymorphonuclear leukocytes and large, abnormal alveolar
macrophages in the test animals. B- N-acetyl-D-glucoseaminidose
activity was also elevated at 3 weeks, indicating activation of
leukocytes. There was also a rise in the concentration of 6-keto-
PGF1alpha at 1 and 3 weeks, as well as in TXB2 at 3 weeks, compared
with those in control animals.
The administration of DEC inhibited both the increase in heart-
weight ratio (RV/LV + S) and the increase in pulmonary artery
pressure (Fig. 11) that occurred 3 weeks after monocrotaline
administration, and reduced the percentage of polymorphonuclear
cells, abnormal alveolar macrophages, and hexoseaminidase activity
in the lavage fluid, compared with that from animals that had
received monocrotaline only. The rise in the levels of 6-keto-
PGF1alpha was inhibited ( P < 0.05) by 73% and that of TXB2 by 74%
in the lung lavage.
 The administration of indomethacin did not have any effects on
either the heart-weight ratio or the pulmonary arterial pressure 3
weeks after monocrotaline administration (Fig. 11), but it
inhibited ( P < 0.05) the rises in 6-keto-PGF1alpha (by 90%) and
TXB2 (by 91%) that occurred in the lung lavage of monocrotaline-
treated animals at 3 weeks.
The above studies indicate that both the cyclo-oxygenase and
the lipo-oxygenase pathways of arachidonate metabolism are
activated by monocrotaline as early events in its toxic effect.
Activation of the cyclo-oxyenase pathway, demonstrated by increased
concentrations of the prostaglandin metabolites 6-keto-PGF1alpha
and TXB2 in lavage fluid, was inhibited by indomethacin, but this
inhibition did not prevent the monocrotaline-induced injury. DEC
attenuated both the inflammatory response and pulmonary
hypertension and inhibited the formation of slow reacting
substances including leukotriene D4. Since DEC produces a
pharmacological blockade of the lipo-oxygenase pathway, it seems
that the latter, rather than the cyclo-oxygenase pathway, is
responsible for perpetuating the pathophysiological mechanism
leading to monocrotaline-induced pulmonary hypertension.
Hilliker et al. (1984) demonstrated that antibody-induced
thrombocytopaenia attenuates right ventricular hypertrophy induced
by monocrotaline in rats. In another study, Hilliker & Roth (1984)
also produced evidence that hydrallazine, a vasodilator and
inhibitor of platelet prostaglandin synthesis, dexamethason, an
antiinflammatory agent and inhibitor of phospholipase, and
sulfinopyrazone, an inhibitor of platelet prostaglandin synthesis
inhibited monocrotaline-induced right ventricular hypertrophy in
rats, supporting the hypothesis that platelets and vasoconstrictor
agents play a role in monocrotaline-induced pulmonary hypertension.
Likewise, prior chemical sympathectomy with 6 hydroxydopamine
(100 mg/kg) or inhibition of serotonin synthesis with
p-chlorophenylalanine (500 mg/kg) reduced the degree of
monocrotaline-induced right ventricular hypertrophy in rats, but
did not prevent or reduce pulmonary vascular muscularization
(Tucker et al., 1983). Thus, the sympathetic nervous system and
serotogenic mechanisms seemed to be involved in the development of
right ventricular hypertrophy, but not in the development of the
pulmonary vascular lesion induced by monocrotaline. Kay et
al. (1985) also demonstrated that pretreatment with
p-chlorophenylalanine, which inhibits 5-hydroxytryptamine (5HP)
synthesis, also significantly ( P < 0.05) reduced right ventricular
systolic pressure, right ventricular hypertrophy, and medial
thickness of muscular pulmonary arteries in monocrotaline-treated
rats. Similar observations were made in rats exposed to hypoxia.
It was therefore suggested that 5HP might play a role in
monocrotaline-induced or chronic hypoxic pulmonary hypertension.
The biosynthesis of rat lung polyamines, putrescine,
spermidine, and spermine, generally considered to be important
regulators of cell growth and differentiation, is increased prior
to the evolution of monocrotaline-induced pulmonary hypertension in
rats. Continuous administration of alpha-difluoromethylornithine
(DFMO), which is a highly specific irreversible inhibitor of
ornithine decarboxylase (DDC), a rate-limiting enzyme in polyamine
biosynthesis, attenuated the development of monocrotaline-induced
pulmonary hypertension in rats (Olson et al., 1984). This effect
was mediated by the DFMO, by inhibiting the synthesis of putrescine
and spermidine, and not by blocking the hepatic metabolism of
monocrotaline to pyrroles (Olson et al., 1985). Thus, it was
suggested that lung polyamine biosynthesis might be essential for
the expression of monocrotaline-induced perivascular oedema as well
as medial thickening in the development of monocrotaline-induced
pulmonary hypertension vascular disease.
On the basis of the preceding studies, mostly on the rat, the
mechanism of chronic long-term injury to the lung by monocrotaline
seems to be as follows. Within hours of PA administration, there
is damage to the pulmonary endothelial cells accompanied by
vascular leak leading to pulmonary oedema. Platelet aggregation
also occurs. The endothelial damage indicated by ultrastructural
and biochemical studies activates the production of prostacyclin
and lipogenic products, which mediate increases in vascular
permeability and inflammatory reaction. There is simultaneous
production of 5 hydroxytryptamine and several polyamines. The
injected monocrotaline is completely metabolized within hours, and
no significant quantity is found in the body at 24 h (Hayashi,
1966) and, though some active metabolites may still be detectable
by isotope studies, even at 14 days (Hsu et al., 1974), the rats do
not have any lung lesions. The slow evolution of vascular changes
suggests that it is not caused by monocrotaline but through
biological pathways activated by the initial injury.
Methylprednisolone (MP), which reduces acute lung oedema caused
by monocrotaline (MCT), has been shown to reduce MCT-induced
pulmonary hypertensive vascular changes in rats and the resultant
right ventricular hypertrophy (Langleben & Reid, 1985). Daily ip
administration of MP at 5 mg/kg body weight, was found to be more
effective than 2 large doses of MP at 30 mg/kg, 2 h before and 2 h
after a single sc injection of MCT at 60 mg/kg. It was suggested
that secondary changes, though triggered by the acute MCT injury,
become self sustaining and are more significant for vascular
structural remodelling.
Structural arterial remodelling with vasoconstriction, medial
hypertrophy of the muscular pulmonary arteries, and muscularization
of the pulmonary arterioles follow as late effects, resulting in
pulmonary hypertension and right ventricular hypertrophy of the
heart.
The results of the above studies suggest a direct toxic effect
of the alkaloid on the endothelial cells of the alveolar
capillaries and on the pulmonary arteries, as well as a pulmonary
hypertensive effect on the heart.
6.4.3 Effects on the central nervous system
The dominant signs of pyrrolizidine poisoning in horses are
neurological (Rose et al., 1957a,b; McLean, 1970). Similar signs
can also occur in cattle and sheep. It has been claimed that such
signs are probably non-specific secondary effects following primary
liver disease resulting in hyperammonaemia (Rose et al., 1957a).
However, neurological abnormalities in which animals walk in a
straight line until they come to an object, and then stand with
their heads pressed against the object, indicate specific lesions
in the central nervous system. Spongy degeneration of the central
nervous system occurs in cattle, sheep, and pigs (Hooper et al.,
1974; Hooper, 1975a,b).
Trichodesma alkaloids, in particular, appear to be neurotoxic.
There is a considerable body of literature in the USSR on
Trichodesma intoxication of mice, rabbits, and dogs, which has
been reviewed (Ismailov et al., 1970). Mice given Trichodesma
alkaloids subcutaneously at 0.5 mg/kg develop paresis of the hind
limbs within 12 - 17 days. Opisthotonus and clonic convulsions are
also seen. Doses of 10 - 15 mg/kg of alkaloids produces death in
all animals within 2 - 6 h, as the result of respiratory
depression. Higher doses produce immediate death.
6.4.4 Effects on other organs
Right ventricular hypertrophy, secondary to the primary effects
on the pulmonary arteries, and the resultant pulmonary hypertension
in animals treated with PAs or PA-containing plants have been dealt
with in section 6.4.2. Lalich & Merkow (1961) reported myocarditis
consisting of focal oedema and infiltration with a minimal number
of lymphocytes and mononuclear cells in some rats fed Crotalaria
spectabilis seeds mixed with the diet at a concentration of
0.13 - 2 g/kg. Treated groups consisted of 11 - 24 animals each;
there were 12 controls. The changes were seen in all groups of
animals, but the maximum number of rats (10 out of an unspecified
number of the group) showing these changes was in the group that
received 0.5 g/kg diet for 20 - 31 days. Generally, there was a
close correlation with the presence of pulmonary arteritis.
Renal changes have been described by a number of investigators.
Hayashi & Lalich (1967) observed mild to moderate changes in renal
glomeruli consisting of necrosis, capillary thrombosis, and
degenerative changes in the epithelial and mesangial cells,
thickening of interlobular arteries, and arterial thrombosis in
suckling, male Sprague Dawley rats given a single sc dose of
monocrotaline at 120 mg/kg body weight. Renal changes were seen,
to some extent, in all animals surviving for 41 - 47 days.
Carstens & Allen (1970) studied the effects of feeding
Crotalaria spectabilis seed on the rat kidney. Fifty male Sprague
Dawley rats were fed a diet containing ground Crotalaria
spectabilis seed at 0.2 - 0.8 g/kg for 8 months. The seeds were
estimated to contain approximately 3.5 g monocrotaline/kg; 10
animals served as controls. Renal changes were seen in 33/50
PA-treated rats. In 22 rats, over 75% of the glomeruli were
hyalinized and capsules thickened. In the less severely affected
kidneys, the glomerular basement membrane was thickened and
homogeneous deposits were seen in mesangial areas. Afferent
arterioles and interlobular arteries were markedly thickened. In
the most severely affected vessels, the internal elastic lamina was
necrosed and the larger arteries showed fibrinoid necrosis.
Renal tubular megalocytosis was the dominant lesion described
by Hooper (1974) in mice. Nine male white mice, 10 weeks of age,
were fed Senecio jacobaea, which contained a concentration of
alkaloids (jaconine, jacobine, and seneciphylline) of 2.7 g/kg and
a concentration of N-oxide of 0.9 g/kg, mixed with the diet. The
S. jacobaea was given at 100 g/kg diet for 9 weeks, before being
raised to 200 g/kg diet. Five animals served as controls. The
animals were killed from 63 to 193 days after the start of the
study. All treated animals, except 2 killed on day 63, showed
changes. The large cells occurred in both the proximal tubules and
the loop of Henle. Similar cells were seen in the alveolar and
bronchiolar epithelium. No glomerular lesions were described. The
author mentioned having seen the above changes in rats given
repeated sublethal injections of fulvine and spectabiline. On the
other hand, Kurozumi et al. (1983) observed glomerular lesions in
rats given a single injection of monocrotaline.
A variety of renal lesions has been observed in pigs, a common
pathological feature being renal megalocytosis, which was observed
in pigs poisoned by at least 4 different plant genera containing a
variety of toxic alkaloids (Harding et al., 1964; Peckham et al.,
1974) and has also been observed in wild pigs grazing in areas rich
in PA-containing plants in northern Australia (Hooper, 1978).
McGrath et al. (1975) described glomerular lesions in pigs given
Crotalaria spectabilis seed daily for 43 days. Severe renal
lesions comprising tubular dilatation, megalocytosis, and necrosis
of tubular epithelial cells with casts in the lumen, interstitial
and periglomerular fibrosis, and glomerular hyalinization were
reported by Hooper & Scanlan (1977) in pigs fed Crotalaria retusa
seeds containing monocrotaline. Renal megalocytosis has also been
reported in C. retusa poisoning in horses, sheep, and mice poisoned
by S. jacobaea but not by H. europaeum, and in vervet monkeys with
chronic retrorsine poisoning (Van der Watt et al., 1972).
Lesions have been reported in the stomach and intestines in
field and experimental animals after poisoning with pyrrolizidine
alkaloids, but are difficult to identify as specific PA injury.
Hooper (1975c) conducted studies on sheep, rats, and mice. In the
study on sheep, 12 male cross-bred lambs, 7 - 8 weeks of age, were
newly weaned on to a standard commercial calf grower diet.
Lasiocarpine was administered at the rate of 15 - 20 mg/kg body
weight every 2 - 4 days. Each animal was killed when in terminal
coma. Survival time ranged from 4 to 17 days. In the rat study,
young Wistar-Furth rats (sex not stated) weighing 150 - 200 g were
used. In one group of 11 rats, each animal received an ip
injection of lasiocarpine at the rate of 40 mg/kg body weight.
Three animals received isotonic saline. Animals were killed or
died 2 - 6 days after the injection. A second group of 13 rats
received a dose of 35 mg lasiocarpine/kg body weight; 4 control
animals received saline. All rats received a second injection
48 h later. They were killed 3 - 60 days after the second
administration of lasiocarpine. In the mouse study, 3 mature male
white mice received 6 injections each of lasiocarpine at the rate
of 45 mg/kg body weight followed by 4 injections of 90 mg/kg body
weight at 48-h intervals. There was one control animal.
All animals showed characteristic hepatic lesions. Sheep also
showed severe oedema, haemorrhage, and epithelial necrosis in the
gall bladder; lesions were also found in the central nervous system
and occasionally in the kidney. All animals showed severe
intestinal atrophy. There was inhibition of crypt cell mitosis
leading to mitotic irregularities, abnormal large cells and
syncitial cells, especially in the duodenum of sheep, and severe
villous atrophy with ulceration. Lesions in the intestines were
similar to those caused by radiation and radiomimetic agents. It
was suggested that the local intestinal radiomimetic effect was due
to local exposure to the pyrrole metabolite of lasiocarpine after
excretion through the bile duct. It was proposed that a more
conspicuous and rapid development of duodenal megalocytosis was due
to very rapid turnover of cells in the duodenum.
Other probably secondary effects included haemolysis in sheep
in association with advanced liver disease and high liver-copper
levels (Bull et al., 1956), anaemias and disturbance in iron
metabolism and haematopoiesis (Schoental & Magee, 1959; Schoental,
1963; Peckham et al., 1974; Hooper & Scanlan, 1977) (section
6.4.11), pancreatic oedema and fibrosis (Bras & Hill, 1956;
Schoental & Magee, 1959), cerebral oedema, haemorrhage, and
congestion in the rat brain (Davidson, 1935; Rosenfield & Beath,
1945).
Tumours in the different organs have been dealt with separately
under carcinogenesis (section 6.4.8).
6.4.5 Teratogenicity
The teratogenic potential of PAs was demonstrated by Green &
Christie (1961) who produced a variety of dose-related fetal
abnormalities in the rat, with a single intraperitoneal injection
of heliotrine administered during the second week of gestation.
The dosages ranged from 15 to 300 mg/kg maternal body weight.
Litters exposed to a dose of less than 50 mg did not show any
abnormalities, but abnormalities were observed in litters exposed
to higher doses, and increased in frequency and severity with
increasing dose. The abnormalities included retardation of
development, musculoskeletal defects, especially hypoplasia of the
lower jaw, cleft palate, and other abnormalities. Doses above 200 mg
resulted in the intrauterine death or resorption of many fetuses.
Similar studies were performed by Peterson & Jago (1980) who
compared the effects of heliotrine with its metabolic pyrrole
derivative dehydroheliotridine (DHH), when administered in a single
ip injection to rats on the 14th day of gestation. Heliotrine was
administered at 200 mg/kg body weight and DHH at 30 - 90 mg/kg, 14
days after conception. Effects on embryos, evaluated on the 20th
day, showed that both heliotrine and DHH retarded growth and were
teratogenic, but that the effects of a 40 mg/kg dose of DHH were
equivalent to those of 200 mg/kg heliotrine, i.e., the metabolite
was 2.5 times as effective on a molar basis. DHH produced a number
of skeletal abnormalities including retarded ossification,
distorted ribs, long bones, cleft palate, and feet defects. At
higher doses, growth almost ceased in many tissues and the fetuses
were very immature. However, the embryonic liver parenchyma did
not show the antimitotic effects of DHH.
The teratogenic properties of heliotrine were also demonstrated
in Drosophila larvae fed low levels of the alkaloid (Brink, 1982).
6.4.6 Fetotoxicity
The subject of fetotoxicity has been reviewed by Mattocks
(1986). Sundareson (1942) demonstrated the ability of
pyrrolizidine alkaloids to cross the rat placenta. Twice weekly
injections of the PA, starting at, or after, the 12th day of
gestation, resulted in premature delivery of some litters and many
were born dead. The same author showed that the alkaloids
themselves and not just the pyrroles formed in the dams' livers,
could pass the placental barrier by injecting senecionine into
19-day-old rat fetuses in utero, which produced the characteristic
toxic lesions in the dams. The fetuses were also found to be more
resistant to the lethal effects of the PA than the mother rats.
When 4 fetuses were each administered 1.25 mg of PA, representing
about 200 - 400 mg/kg body weight, which is much higher than the
LD50 for an adult rat, 3 of them were still alive after 2 days.
Green & Christie (1961) did not find any liver damage in fetuses
from pregnant rats given teratogenic doses of heliotrine. Only
mild liver damage was found in the embryo rats whose mothers had
been injected with PAs (heliotrine, lasiocarpine, retrorsine, or
monocrotaline) (Bhattacharya, 1965). In contrast, Schoental (1959)
demonstrated that lasiocarpine and retrorsine, when administered to
lactating rats produced little effect on the mothers, but produced
acute liver lesions in the suckling infants. The lesions were most
severe in 3- to 7-week-old animals. It was suggested that the
infants were affected by the milk from the lactating mothers, which
possibly contained the metabolic products of the PAs.
It would seem from the above studies that the embryo is
relatively more resistant to the toxic effects of PAs in utero than
it is after birth. Mattocks & White (1973) postulated that this
could be due to the low capacity for the metabolic activation of
PAs of the embryo liver, as they had shown that the ability of
liver enzymes to convert retrorsine to toxic metabolites was low in
rats, immediately after birth, but picked up rapidly afterwards.
The susceptibilities of rats of various ages to the hepatotoxic
effects of the PAs was proportional to their capacity to form and
retain the pyrrolic metabolites. Twenty-day-old rats were found to
be more sensitive than older animals.
The effects of fulvine administration on pregnant rats between
9 and 12 days of gestation were studied by Persaud & Hoyte (1974).
Dose-related fetal resorptions were observed, but no hepatic
lesions were seen in the fetuses. On the other hand, Newberne
(1968) observed damage, in both the maternal and fetal livers, when
lasiocarpine was administered to pregnant rats. Acute liver
necrosis was observed in the livers of mothers as well as fetuses
in animals that had received 100 mg lasiocarpine/kg body weight on
day 13 of gestation. However, in animals that received 2 doses of
35 mg/kg body weight on days 13 and 17 of pregnancy, liver necrosis
was seen in the fetal liver but not in that of the mother. It is
not known why lasiocarpine acts differently from other alkaloids
and has a greater effect on the fetal liver. Mattocks (1986) has
postulated the possibility that fetotoxicity was caused chiefly by
toxic metabolites formed in the maternal liver, and that a greater
proportion of such metabolites reached the fetus from lasiocarpine
than from other PAs.
6.4.7 Mutagenicity
A number of PAs that have been shown to be powerful dose
dependent mutagens in Drosophila melanogaster have been listed by
Mattocks (1986). All the compounds are hepatotoxic though the
degree of mutagenicity is not necessarily proportional. Table 12
provides a summary of the mutagenicity tests on different PAs,
related compounds and plant extracts. Clark (1959) demonstrated
the mutagenic effect of heliotrine in Drosophila, in which a
considerable increase in sex-linked recessive lethals was produced,
apparently by interfering with the maturation of germ cells, so
that as soon as the available spermatozoa were used, the males were
no longer capable of breeding. The cell damage was irreversible.
The mutagenic effect of feeding Drosophila males for 24 h with a
medium containing 10-3 mol monocrotaline was comparable to about
1000 R of X rays (Clark, 1976). The Basc test with Drosophila
melanogaster is considered a highly sensitive mutagenicity test
for PAs (Candrian et al., 1984a).
Seneciphylline and senkirkine, known to occur in animal feeds
and medicinal herbs, respectively, were tested for their ability to
produce sex-linked recessive lethals in males of Drosophila
melanogaster using the Basc (3-day feeding method) by Candrian et
al. (1984a). Seneciphylline was found to be mutagenic at
concentrations of 10-5, 10-4, and 10-3 mol, which produced 3.8%
(983 chromosomes tested), 9% (708 chromosomes tested), and 15.3%
(327 chromosomes tested) sex-linked recessive lethals,
respectively. Senkirkine (10-5 mol) was found to produce 4.4% sex-
linked recessive lethals (2541 chromosomes tested) against 0.17%
maximum sensitivity in the late spermatid stage of spermatogenesis
indicating that PAs act as indirect mutagens. Flies fed with milk
from lactating rats given an oral dose of 25 mg seneciphylline/kg
showed 1.2% sex-linked recessive lethals (1477 chromosomes tested)
compared with 0.3% (1533 chromosomes tested) in controls.
Table 12. Mutagenicity tests on pyrrolizidine alkaloids,
related compounds, and source plants
---------------------------------------------------------
Compound or material Type of testa Responseb
---------------------------------------------------------
Clivorine A +
HPC +
Echimidine D +
Echinatine D +
Fulvine D +
Heliotrine D +
P +
F +
CC +
A +
B +
TM +
CM/CC +
Integerrimine D +
Jacobine D ±
P +
Lasiocarpine D +
P +
F +
A +
HPC +
CM/CC +
TM +
Ligularidine A +
Lindelofine A 0
Lycopsamine A 0
Monocrotaline D +
P +
CC +
B +
A 0
HPC +
CT +
Petasitenine (fukinotoxin) A +
HPC +
CM +
---------------------------------------------------------
Table 12. (contd.)
---------------------------------------------------------
Compound or material Type of testa Responseb
---------------------------------------------------------
Platyphylline D 0
Retrorsine A +
D +
CT +
Rosmarinine CT 0
Senecionine D +
A 0
Seneciphylline A 0
P +
D +
Senkirkine A +
HPC +
CM/CC +
D +
Supinine D ±
P +
Mixed alkaloids from
Senecio jacobaea A 0
Senecio numorensis
spp. fuchsii (extract) CM +
A 0
A +
Senecio jacobaea (extract) A +
Senecio longilobus (extract) A 0
Symphytum officinale
(comfrey extract) A 0
Retronecine bis-p-chloro- P +
benzoate
Synthanecine A bis-N-ethyl- CT +
carbamate
Retronecine A 0
Heliotridine D 0
Viridofloric acid A 0
---------------------------------------------------------
Table 12. (contd.)
---------------------------------------------------------
Compound or material Type of testa Responseb
---------------------------------------------------------
Heliotric (heliotrinic) acid D ±
HPC +
Dehydroretronecine CT +
A ±
SCE +
Dehydroheliotridine CM +
Pyrrole HPC 0
2,3-Bishydroxymethyl-1- CT +
methylpyrrole A ±
SCE +
2-Hydroxylmethyl-1-methyl- A 0
pyrrole SCE ±
3-Hydroxylmethyl-1-methyl- A +
pyrrole SCE ±
---------------------------------------------------------
a A = Salmonella ("Ames") test.
B = Other bacterial tests.
CC = Clastogenic activity in cultured cells.
CM = Mutagenicity in cultered mammalian cells.
CT = Cell transformation test.
D = Mutagenicity in Drosophila.
F = Tests in fungus (Aspergillus nidulans).
HPC = Hepatocyte primary culture/DNA repair test.
P = Chromosomal aberrations in plant cells.
SCE = Sister chromatid exchange.
TM = Transplacental micronucleus test.
b + = active.
± = marginally active.
0 = inactive.
Mutagenic properties of 7 PAs extracted from plants to
Salmonella typhimurium TA100 have been demonstrated by a modified
Ames method by Yamanaka et al. (1979). The PAs were clivorine,
fukinotoxin, heliotrine, lasiocarpine, ligularidine, LXC201, and
senkirkine. Pre-incubation of these alkaloids with liver S9 mix
and bacteria in liquid medium was essential for demonstration of
the property. PAs in the heliotridine and otonecine family were
mutagens, while retronecine bases were inactive. Monocrotaline and
heliotrine were not active mutagens to Escherichia coli WP2, even
though they were quite cytotoxic (Green & Muriel, 1975). They were
active in repair deficient strains. Retrorsine was active in
inducing mutations on the Ames Salmonella/microsome assay (Wehner
et al., 1979). Extracts from medicinal plants and noxious weeds
were mutagenic towards Salmonella in the Ames assay (Pool, 1982;
White et al., 1983; Koletsky et al., 1978).
From the limited data available, it seems that the carcinogenic
activity of individual alkaloids parallels their mutagenic
behaviour, but not their relative hepatotoxicities (Culvenor &
Jago, 1979).
6.4.7.1 Chromosome damage
Pyrrolizidine alkaloids have been shown to be capable of
damaging chromosomes in plants, fungi, bacteria, tissue cell
cultures, and the fruit fly (Drosophila melanogaster). Literature
on this topic has been reviewed by Bull et al. (1968), McLean
(1970), and Mattocks (1986).
Several PAs are known for their ability to damage the
chromosomes of growing plant cells (Mattocks, 1986). Similar
properties have been demonstrated in leukocyte cultures from the
marsupial (Potorus tridactylus) (Bick & Jackson, 1968; Bick, 1970).
Bick & Culvenor (1971) found dehydroheliotridine, a metabolite of
heliotrine, to be 10 times more active than the alkaloid.
Infusions of Symphytum officinale L., described in Polish
pharmacopoeia as Radix symphyti, are recommended as expectorants,
especially for children. Furmanowa et al. (1983) demonstrated the
mutagenic effects of an alkaloidal fraction and infusion in this
plant in the meristematic cells of the lateral roots of Vicia faba
L. var minor. Lasiocarpine, a proven carcinogen, served as a
positive control.
Chromosome damage by PAs in the hamster lung cell line was
demonstrated by Takanashi et al. (1980). Stoyel & Clark (1980)
used the transplacental micronucleus test in pregnant female mice
and showed the chromosome damaging properties of heliotrine (225 mg/kg
body weight) and lasiocarpine (86 mg/kg) within 20 h of the injection.
The genotoxicity of heliotrine, monocrotaline, seneciphylline,
and senkirkine was studied by Bruggeman & Van der Hoeven (1985)
using the sister-chromatid exchange (SCE) assay in V79 Chinese
hamster cells co-cultured with primary chick embryo hepatocytes.
Exposure to these PAs resulted in the high induction of SCEs, a
more than 5-fold increase in the SCE rate with 2.5 mg
heliotrine/litre, 4-fold with monocrotaline at 5 mg/litre, 8-fold
with seneciphyline at 1.2 mg/litre, and more than 5-fold response
with senkirkine at 2.5 mg/litre. For all compounds, a dose-
response relationship was observed at concentrations that did not
seriously affect survival. PAs are also known to induce DNA repair
in rodent hepatocytes (Green et al., 1981; Mori et al., 1985). DNA
repair synthesis was elicited by 15 alkaloids, including 11 of
unknown carcinogenic potential (Mori et al., 1985).
There are also a few reports of chromosome damage by PAs in
man. Martin et al. (1972) found chromosome damage in the blood
cells of children with veno-occlusive disease, probably caused by
fulvine. It has also been shown by Ord et al. (1985) that
dehydroretronecine, is able to induce SCE in human lymphocytes.
Kraus et al. (1985) studied the PAs senkirkine and tussilagine,
which occur in a medicinal plant Tussilago farfara, for their
ability to induce chromosome damage in human lymphocytes in vitro.
They were not found to enhance the number of chromosome aberrations
up to concentrations of 1000 µmol. However, heliotrine, used for
comparison, induced chromosomal aberrations at concentrations of
100 µmol. In addition, heliotrine was also found to be capable of
damaging unstimulated eg Go-phase lymphocytes.
6.4.8 Carcinogenesis
Carcinogenesis has been reviewed by McLean (1970), IARC (1976,
1983), and Mattocks (1986). A number of purified PAs, purified or
crude extracts of plants containing them in a mixture or the actual
plant, dried and milled, and several PA metabolites or synthetic
analogue compounds have been tested for carcinogenecity. However,
these include only relatively few of the known cytotoxic PAs. Data
relating to some of the representative studies on rats are
summarized in Table 13. Studies on liver tumours found in rats
given PAs and plant materials are summarized in Table 14. All
experimental animal studies, with the exception of one on chickens
(Campbell, 1956) and one on Syrian golden hamsters (Fushimi et al.,
1978), have been carried out on rats.
The liver is the most common organ involved in experimental
studies. Tumours produced are mostly of epithelial origin, but a
significant number are also vascular. Lack of precision and
diversity of terms used to describe similar or identical tumours
makes it difficult to compare the types of carcinogenic effect in
different studies. Some terms have been used interchangeably,
e.g., hepatomas, hepatocellular carcinomas, haemangiogenic and
cholangiogenic tumours; nodular hyperplasia, pre-neoplasma,
neoplastic nodules, and hepatocellular tumours. In most studies,
there are no supporting photomicrographs to draw any inference as
to whether the tumours were malignant. Difficulties in the
interpretation of data have been commented on by Schoental et al.
(1954) and McLean (1970).
Lasiocarpine has produced the largest yield of tumours. In the
studies of Svoboda & Reddy (1972), 16/18 animals surviving for more
than 56 weeks after receiving ip multidoses of lasiocarpine
developed malignant tumours of the liver. Of these, 10 animals had
more than one tumour. Continuous feeding of rats on a regimen
containing lasiocarpine resulted in all animals (24/24) developing
tumours (NCI, 1978). In one study, a single oral administration of
retrorsine (Schoental & Bensted, 1963) to weanling rats resulted in
7 of the 29 animals that survived for more than one year developing
11 tumours of a wide variety, at least of 5 which were malignant.
It is of note that this PA is known to have caused two cases of
human toxicity together with riddelline in two cases, though the
total intake was proportionately lower (Stillman et al., 1977; Fox
et al., 1978; Huxtable, 1980) (Table 15).
Tumours produced covered a very wide range in unrelated tissues
and organs, for example, the pancreas, urinary bladder, pituitary,
bone, retro-peritoneal tissues, and skin, among others.
Hepatocellular carcinoma and haemangiosarcoma were the most common.
Crude extracts of plants or whole plants have also been
demonstrated to produce a variety of tumours. For example, Senecio
longilobus has been shown to produce tumours in a significantly
large proportion of experimental animals (Harris & Chen, 1970).
Hirono et al. (1973, 1976, 1978, 1979b) demonstrated the
carcinogenic properties of a number of plants, used as food in
Japan, in the rat.
A summary of the relevant experimental data follows.
6.4.8.1 Purified alkaloids
Kuhara et al. (1980) administered clivorine in the drinking-
water at a concentration of 0.05 g/litre to 12 rats of both sexes,
continuously for 340 days followed by plain water. There were 20
control rats. All the treated rats survived 440 days. Eight of
the 12 animals developed liver tumours including 2
haemangioendothelial sarcomas and 6 that were described as
"neoplastic nodules". No liver tumours were seen in the control
animals (Table 13).
Schoental (1975) tested the carcinogenic properties of
heliotrine, with and without prior administration of nicotinamide.
Nicotinamide protects against liver necrosis and so may enhance
tumour yeild, as shown by Rakietin et al. (1971), who studied
pancreatic tumours in rats given streptozootocin.
Heliotrine was administered intragastrically to 4 groups of 26
male weanling rats in 1 or 2 doses of 230 mg/kg and 300 mg/kg body
weight; 2 groups also received nicotinamide at 350 - 500 mg/kg body
weight, administered ip 10 - 15 min before, and 2.5 h after
administration of heliotrine, as per the dosing regimen shown in
Table 13. There were 8 controls. All animals administered the
higher dose of heliotrine (300 mg/kg body weight) died within 5
months of the PA administration. The livers showed lesions
characteristic of PA toxicity. No tumours were seen. Only 1 out
of 4 animals receiving heliotrine alone, survived 27 months and it
showed an islet cell adenoma as well as adenoma of pituitary, but
so did 3 of the 8 controls. In the group receiving 230 mg
heliotrine/kg and also treated with nicotinamide, 4 animals died
within 5.5 months (one with a fibrosarcoma and the other in a
moribund condition), chiefly from toxic liver disease, and two more
had to be killed. Of the 6 animals surviving more than 22 months
after heliotrine treatment, islet cell adenoma was seen in 3
together with other tumours as shown in the table. This tumour is
stated to be extremely rare in the animal strain used. Hepatoma
was seen in only one animal. The role of nicotinamide in this
study is not clear.
Table 13. Summary of data on the carcinogenic action of PAs and PA-containing plants in rats (in chronological order)
Material Strain, Route of Other Duration Period of Tumours Tumour Reference
tested number, administration treatment of observation produced incidence
and sex of and dosing treatment in
animals regimen surviving
animals
Alkaloids Wistar rat drinking-water 1 week until death only nodular Schoental
of Senecio (13 males, (0.05 g/litre) (males) hyperplasia et al. (1954)
jacobaea 12 females) 2 weeks of liver
(females);
followed by gap of
7 weeks
9 males, 0.03 g/litre, until
1 female 3 days/week death
(surviving)
Retrorsine 10 males, 0.03 g/litre, until hepatomas 4/14
4 females 3 days/week death
Isatidine 8 males, 0.05 g/litre
14 females followed by
0.03 g/litre, 20 months until death hepatomas 10/22
3 days/week
3 males, as above choline until hepatomas 3/7
4 females (0.5% in death
drinking-
water), 4
days/week
Table 13. (contd.)
Material Strain, Route of Other Duration Period of Tumours Tumour Reference
tested number, administration treatment of observation produced incidence
and sex of and dosing treatment in
animals regimen surviving
animals
Isatidine 2 males, single ip Schoental
(contd.) 3 females injection of et al. (1954)
2 mg in 0.2 ml
tricaprilyn
followed by
skin application 15 months 15 months hepatoma (?) 1/5
tion of 0.5%
solution, 3
days/week
controls not mentioned
(7 males,
7 females)
Retrorsine Porton single 400 r until death hepatomas 5/25 Schoental &
Wistar intragastric radiation Bensted (1963)
weanling dose of 30 mg/kg to 31/50 hepatocellular 1/25
rat body weight surviving
(50 males) for 100 carcinoma
days; 4/13 with
had head metastases
shielded
mammary 2/25
tumours
Table 13. (contd.)
Material Strain, Route of Other Duration Period of Tumours Tumour Reference
tested number, administration treatment of observation produced incidence
and sex of and dosing treatment in
animals regimen surviving
animals
Retrorsine lung carcinoma 1/25
(contd.)
renal 2/25
carcinomas
colonic 1/25
carcinomas
Porton as above as above splenic 1/25 Schoental &
Wistar rat haemangio- Bensted (1963)
(50 males) endothelioma
osteosarcoma 1/25
bone
leukaemia 1/25
"spindle cell" 1/25
tumour (neck)
Table 13. (contd.)
Material Strain, Route of Other Duration Period of Tumours Tumour Reference
tested number, administration treatment of observation produced incidence
and sex of and dosing treatment in
animals regimen surviving
animals
Retrorsine 95 males, single oral dose until death hepatomas 5/29
(contd.) 95 females of 30 mg/kg body
(weanling) weight mammary tumour 1/29
(controls)
lung carcinoma 1/29
splenic 1/29
haemangio-
endothelioma
uterine 1/29
carcinoma
Porton retroperitoneal 1/29 Schoental &
Wistar rat sarcoma Bensted (1963)
(controls)
squamous cell 1/29
carcinoma
(jaw)
Table 13. (contd.)
Material Strain, Route of Other Duration Period of Tumours Tumour Reference
tested number, administration treatment of observation produced incidence
and sex of and dosing treatment in
animals regimen surviving
animals
Retrorsine 6 males no PAs 400 r until death leukaemia 2/6
(contd.) (weanling) radiation
osteosarcoma 1/6
renal adenoma 1/6
10 males single partial until death hepatomas 2/9
(weanling) intragastric hepatectomy
dose (30 mg/kg squamous cell 1/9
body weight) carcinoma
(9 days after (jaw)
partial
hepatectomy)
Table 13. (contd.)
Material Strain, Route of Other Duration Period of Tumours Tumour Reference
tested number, administration treatment of observation produced incidence
and sex of and dosing treatment in
animals regimen surviving
animals
Mixed PAs Randomly single more than 1 pancreatic 1/15 Schoental et al.
from seeds bred from intragastric dose year? islet cell (1970)
of Porton at 500-1500 mg/kg adenomaa
Amsinckia Wistar body weight
intermedia weanling "papillary 1/15
(intermedine rat tumour"a of
medine and (15 males) urinary
lycopsamine) bladder
pituitary 1/15
adenomaa
as above as above pancreatic 1/15 Schoental et al.
islet cell (1970)
adenocarcinoma
exocrine 1/15
pancreatic
adenoma
Leaves and 2 males 10% mixed with 1 month until death pancreatic 1/2
stems of (weanling) diet (period not islet cell
Heliotropium stated) adenoma
supinum L.
Table 13. (contd.)
Material Strain, Route of Other Duration Period of Tumours Tumour Reference
tested number, administration treatment of observation produced incidence
and sex of and dosing treatment in
animals regimen surviving
animals
Heliotropium 6 males single until death pancreatic 1/6
supinum L. intragastric dose (longer islet cell
(contd.) at 200-300 mg/kg than 1 adenoma
body weight of year)
crude alkaloidal
fraction
controls not stated
Senecio Harlan rat 0.75% of diet until death none Harris & Chen
longilobus 50 males, within 131 (1970)
50 females days
50 males, 0.5% of diet until death none Harris & Chen
50 females within 200 (1970)
days
Table 13. (contd.)
Material Strain, Route of Other Duration Period of Tumours Tumour Reference
tested number, administration treatment of observation produced incidence
and sex of and dosing treatment in
animals regimen surviving
animals
Senecio 40 males, 0.5% of diet for 1 year 428-657 liver cell 4/23
longilobus 40 females 1 month days carcinomas
(contd.) alternating with
normal diet for peritoneal 1/23
2 weeks mesothelioma
50 males, 0.5% of diet 54 weeks 217-470 liver cell 16/47
50 females for 1 week days carcinomas
alternating with
normal diet for angiosarcoma 1/47
1 week
controls not stated
(10 males,
10 females)
Lasiocarpine Fischer rat intraperitoneal till 60-76 hepatocellular 10/18 Svoboda & Reddy
(25 males) injection at moribund weeks carcinomas (1972)
7.8 mg/kg body or had
weight, twice palpable
weekly for 4 tumours cholangio- 1/18
weeks, then carcinoma
once a week
for an additional lung adenomas 5/18
52 weeks
Table 13. (contd.)
Material Strain, Route of Other Duration Period of Tumours Tumour Reference
tested number, administration treatment of observation produced incidence
and sex of and dosing treatment in
animals regimen surviving
animals
Lasiocarpine skin squamous 6/18
(contd.) cell
carcinomas
Fischer rat as above ileal 2/18 Svoboda & Reddy
(25 males) adenocarcinoma (1972)
ileal 1/18
adenomyoma
testicular 1/18
interstitial
cell tumour
controls lung adenomas 2/25
(25 males)
Table 13. (contd.)
Material Strain, Route of Other Duration Period of Tumours Tumour Reference
tested number, administration treatment of observation produced incidence
and sex of and dosing treatment in
animals regimen surviving
animals
Retronecine, Porton single sc spinal cord 1/10 Schoental &
hydrochlorine Wistar injection of ependymo- Cavanagh (1972)
newborn rat 300-1000 mg/kg blastoma
(6 males, body weight
6 females)
"pituitary 5/10
tumour"
"mammary 1/10
tumour"
Hydroxy- 5 males single ip brain 1/5 Schoental &
senkirkine (weanling) injection of astrocytoma Cavanagh (1972)
100-300 mg/kg
body weight
Table 13. (contd.)
Material Strain, Route of Other Duration Period of Tumours Tumour Reference
tested number, administration treatment of observation produced incidence
and sex of and dosing treatment in
animals regimen surviving
animals
Heliotropium 5 females 5% of diet fed Schwann cell 1/5
ramosissimum to 1 pregnant tumour of
rat during 1st spinal cord
15 days of
pregnancy and
from 10th day
of parturition
until weaning;
female off-
spring (5) fed
on experimental
diet for 10 days
at 6 months of
age
controls not stated
Table 13. (contd.)
Material Strain, Route of Other Duration Period of Tumours Tumour Reference
tested number, administration treatment of observation produced incidence
and sex of and dosing treatment in
animals regimen surviving
animals
Groups
I II
Petasites ACI rat 4% of diet for until 480 days liver cell Hirono et al.
japonicus Group I: 6 months; death or until adenomas 6/27 4/19 (1973)
(young 12 males, subsequently, moribund
flower 15 females 8% of diet liver 3/27 8/19
stalks) alternating haemangio-
weekly with endotheliomas
normal diet
liver cell 2/27 1/19
carcinomas
Group II: 4% of diet
11 males,
8 females
controls normal diet none
7 females/
8 females
Table 13. (contd.)
Material Strain, Route of Other Duration Period of Tumours Tumour Reference
tested number, administration treatment of observation produced incidence
and sex of and dosing treatment in
animals regimen surviving
animals
Mono- Sprague gastric intubation 72 weeks liver cell 10/42 Newberne &
crotaline Dawley rat weekly of 25 mg/kg carcinomas Rogers (1973)
(50 males) body weight for
4 weeks, then
8 mg/kg body
weight for
38 weeks
50 males as above, with liver cell 14/33
a diet deficient carcinomas
in lipotropes
Heliotrine Porton intragastric Schoental (1975)
Wistar
weanling
rat
4 males 300 mg/kg body until death none
weight; repeated (5 months)
after 3 weeks to
2 surviving rats
Table 13. (contd.)
Material Strain, Route of Other Duration Period of Tumours Tumour Reference
tested number, administration treatment of observation produced incidence
and sex of and dosing treatment in
animals regimen surviving
animals
Heliotrine 6 males 300 mg/kg body nicotinamide until death none Schoental (1975)
(contd.) weight; repeated (500 mg/kg (5 months)
after 3 weeks body weight),
ip, before
and after
each heliotrine
administration
4 males 230 mg/kg body 27 months pancreatic 1/1
weight; repeated islet cell
to 2/4 surviving adenoma
after 5 days
pituitary 1/1
adenoma
Table 13. (contd.)
Material Strain, Route of Other Duration Period of Tumours Tumour Reference
tested number, administration treatment of observation produced incidence
and sex of and dosing treatment in
animals regimen surviving
animals
Heliotrine 12 males 230 mg/kg body nicotinamide until death fibrosarcoma 1/8
(contd.) weight; repeated (350 mg/kg or killed of jaw
6.5 days later body weight), when moribund
ip, before, up to 27.5 pancreatic 3/6
plus 2 doses months islet cell
after each adenomasb
heliotrine
administration hepatomab 1/6
urinary 1/6
bladder
papillomab
12 males testicular 1/6
interstitial
cell tumourb
2 males ip injection nicotinamide 19-27.5 pituitary 1/2 Schoental (1975)
at 350 mg/kg months adenoma
body weight
Table 13. (contd.)
Material Strain, Route of Other Duration Period of Tumours Tumour Reference
tested number, administration treatment of observation produced incidence
and sex of and dosing treatment in
animals regimen surviving
animals
Heliotrine controls no treatment 19-27.5 pituitary 3/6
(contd.) (6 males) months adenomas
Tussilago ACI rat 32% in diet for 600 days 600 days haemangio- 8/12 Hirono et al.
farfara (6 males, 4 days, then 16% endothelioma (1976)
(coltsfoot) 6 females) until end of
(preblooming (1.5 months study liver cell 1/12
flowers) old) adenomac
hepatocellular 1/12
carcinomac
bladder 1/12
papillomac
Table 13. (contd.)
Material Strain, Route of Other Duration Period of Tumours Tumour Reference
tested number, administration treatment of observation produced incidence
and sex of and dosing treatment in
animals regimen surviving
animals
Tussilago 5 males, 8% in diet 600 days 600 days liver 1/9
farfara 5 females haemangio-
(contd.) endothelioma
6 males, 4% in diet 600 days 600 days none Hirono et al.
5 females (1976)
controls none 600 days 600 days none
(8 males,
8 females)
Dehydro- Sprague sc injection partial 12 months 10 months rhabdomyo- 36/60 Allen et al.
retronecine Dawley rat bi-weekly for 4 hepatectomy sarcomas (5 (1975)
Group I: months at on 15 with
75 males 20 mg/kg body animals metastases)
weight, followed after 4
by 10 mg/kg body months
weight for 8
months
Table 13. (contd.)
Material Strain, Route of Other Duration Period of Tumours Tumour Reference
tested number, administration treatment of observation produced incidence
and sex of and dosing treatment in
animals regimen surviving
animals
Monocrotaline Group II: sc injection partial 12 months 10 months rhabdomyo- 2/60
75 males bi-weekly at hepatectomy sarcomas
5 mg/kg body on 15
weight for 12 animals hepatocellular 2/60
months after 4 carcinomas
months
acute myeloid 2/60
leukaemia
pulmonary 2/60
adenomas
controls sc injection partial 12 months 10 months none mentioned Allen et al.
(50 males) bi-weekly, hepatectomy (1975)
0.1 mol on 5 animals
phosphate after 4
buffer, pH7 months
Table 13. (contd.)
Material Strain, Route of Other Duration Period of Tumours Tumour Reference
tested number, administration treatment of observation produced incidence
and sex of and dosing treatment in
animals regimen surviving
animals
Monocrotaline Sprague sc injection on 12 months any tumours 17/60 Shumaker et al.
(contd.) Dawley rat alternate weeks (several (1976)
(60 males) (5 mg/kg body animals had
weight) more than 1
tumour)
pulmonary 11/60
carcinomas
hepatocellular 5/60
carcinomas
acute myeloid 3/60
leukaemia
rhabdomyo- 4/60
sarcomas
adrenal 8/60
adenomas
kidney adenoma 1/60
Table 13. (contd.)
Material Strain, Route of Other Duration Period of Tumours Tumour Reference
tested number, administration treatment of observation produced incidence
and sex of and dosing treatment in
animals regimen surviving
animals
Dehydro- 60 males sc injection on 12 months 12 months rhabdomyo- 39/60
retronecine alternate weeks until sarcomas at
at 20 mg/kg body moribund site of
weight followed injection
by 10 mg/kg
body weight,
alternate weeks
for 8 months
controls adrenal 2/45
(45 males) adenomas
(same group
as above)
Mono- Sprague single sc pancreatic 16/23 Hayashi et al.
crotaline Dawley rat injection of insulinomas (1977)
(80 males) 40 mg/kg body
weight
Petasitenine ACI rat drinking-water 45 days 72 days none Hirono et al.
(3 males) (0.05% solution) (1977)
(1 month
old)
Table 13. (contd.)
Material Strain, Route of Other Duration Period of Tumours Tumour Reference
tested number, administration treatment of observation produced incidence
and sex of and dosing treatment in
animals regimen surviving
animals
Petasitenine 5 males, 0.01% solution until up to 16 haemangio- 5/10d
(contd.) 6 females death months endothelial
or sarcomas
moribund
liver adenomas 5/10d
controls none fibrosarcoma 1/10 Hirono et al.
(10 males, (subcutaneous) (1977)
9 females)
Lasiocarpine Fischer rat mixed with 55 weeks 59 weeks liver 9/20 Rao & Reddy
(20 males) feed at a angiosarcomas (1978)
concentration of
50 mg/kg hepatocellular 7/20
carcinomas
malignant 1/20e
adnexal tumour
of skin
malignant 1/20e
lymphoma
Table 13. (contd.)
Material Strain, Route of Other Duration Period of Tumours Tumour Reference
tested number, administration treatment of observation produced incidence
and sex of and dosing treatment in
animals regimen surviving
animals
Lasiocarpine controls none
(contd.) (10 males)
Petasites ddN mice 4% of diet as 480 days 480 days lung adenomas 24/39 Fushimi et al.
japonicus 24 males dried flower (1978)
(flower 21 females stalks lung 6/39
stalks) adenocarcinoma
liver 4/39
reticullum
cell sarcoma
liver 1/39
haemangio-
endothelial
sarcoma
thymoma 1/39
leukemia 2/39
Table 13. (contd.)
Material Strain, Route of Other Duration Period of Tumours Tumour Reference
tested number, administration treatment of observation produced incidence
and sex of and dosing treatment in
animals regimen surviving
animals
Petasites control nil 480 days 480 days lung 1g
japonicus 23 males kidney
(contd.) 27 females haemangio-
endothelial
sarcoma 1g
spleen
haemangioma 1g
Swiss 4% diet as 480 days 480 days lung 5/26 Fushimi et al.
strain mice dried flower adenoma (1978)
20 males stalks
20 females leukemia 1/26
controls nil 480 days 480 days liver 1g
23 males haemangio-
20 females endothelioma
breast 1g
carcinoma
Table 13. (contd.)
Material Strain, Route of Other Duration Period of Tumours Tumour Reference
tested number, administration treatment of observation produced incidence
and sex of and dosing treatment in
animals regimen surviving
animals
Petasites lung adenoma 3g
japonicus
(contd.)
leukemia 2g
C57BL/6 4% diet as 480 days 480 days no tumours
mice dried flower
20 males stalks
20 females
controls none 480 days 480 days lung adenoma 1g
20 males
20 females
Syrian 4% diet as 480 days 480 days adrenal 1/25 Fushimi et al.
golden flower stalks cortical (1978)
hamsters adenoma &
13 males breast
17 females carcinoma
Table 13. (contd.)
Material Strain, Route of Other Duration Period of Tumours Tumour Reference
tested number, administration treatment of observation produced incidence
and sex of and dosing treatment in
animals regimen surviving
animals
Petasites controls none 480 days 480 days no tumours
japonicus 12 males
(contd.) 9 females
Symphytum ACI rat 33% of diet as 480 days until death liver adenomas 5/19 Hirono et al.
officinale (1 - 1.5 leaves or moribund (1979b)
(leaves or months old)
root) urinary 1/19
Group I.1: bladder
11 males, papilloma
8 females
urinary 2/19
bladder
carcinomas
(rats with 5/19
tumours)
Table 13. (contd.)
Material Strain, Route of Other Duration Period of Tumours Tumour Reference
tested number, administration treatment of observation produced incidence
and sex of and dosing treatment in
animals regimen surviving
animals
Symphytum Group I.2: 33% of diet as 600 days until death liver adenomas 11/19
officinale 10 males, leaves or moribund
(contd.) 10 females
urinary 2/19
bladder
papillomas
(rats with 11/19
tumours)
Group II: 16% of diet as 600 days until death liver adenomas 7/21 Hirono et al.
11 males, leaves or moribund (1979b)
10 females
haemangio- 1/21
endothelial
sarcoma
Table 13. (contd.)
Material Strain, Route of Other Duration Period of Tumours Tumour Reference
tested number, administration treatment of observation produced incidence
and sex of and dosing treatment in
animals regimen surviving
animals
Symphytum urinary 2/21
officinale bladder
(contd.) papillomas
urinary 1/21
bladder
carcinoma
lymphatic 1/21
leukaemia
colonic 1/21
adenoma
pituitary 1/21
adenoma
(rats with 7/21
tumours)
Table 13. (contd.)
Material Strain, Route of Other Duration Period of Tumours Tumour Reference
tested number, administration treatment of observation produced incidence
and sex of and dosing treatment in
animals regimen surviving
animals
Symphytum Group III: 8% of diet as 600 days until death liver adenoma 1/25 Hirono et al.
officinale 14 males, leaves or moribund (1979b)
(contd.) 14 females
Group IV: 8% of diet as until liver adenomas 19/22
12 males, root death
12 females
urinary 2/19
bladder
papilloma
(rats with 19/22
tumours)
Group V: 4% of diet as until until death liver adenomas 16/42
24 males, root reduced by death or moribund
24 females stages to basal
diet after 180 urinary 1/42
days bladder
carcinoma
Table 13. (contd.)
Material Strain, Route of Other Duration Period of Tumours Tumour Reference
tested number, administration treatment of observation produced incidence
and sex of and dosing treatment in
animals regimen surviving
animals
Symphytum adrenal 2/42
officinale cortical
(contd.) adenomas
(rats with 16/42
tumours)
Group VI: 2% of diet as 280 days liver adenomas 10/23
12 males, root for 190
12 females days reduced by
stages to basal
diet
Group VII: 1% of diet as until liver adenomas 16/24 Hirono et al.
15 males, root for 275 death (1979b)
15 females days and
subsequently a liver 4/24
basal diet haemangio-
alternating sarcomas
with 0.5% at
3-week intervals cholangio- 1/24
carcinoma
Table 13. (contd.)
Material Strain, Route of Other Duration Period of Tumours Tumour Reference
tested number, administration treatment of observation produced incidence
and sex of and dosing treatment in
animals regimen surviving
animals
Symphytum adrenal 1/24
officinale cortical
(contd.) adenomas
(rats with 17/24
tumours)
Group VIII: 0.5% of diet as entire liver adenomas 8/30
15 males, root study
15 females
liver 9/30
haemangio-
sarcomas
pituitary 1/30
adenoma
uterine 1/30
adrenocarcinoma
Table 13. (contd.)
Material Strain, Route of Other Duration Period of Tumours Tumour Reference
tested number, administration treatment of observation produced incidence
and sex of and dosing treatment in
animals regimen surviving
animals
Symphytum gonadal 1/30 Hirono et al.
officinale stromal tumour (1979b)
(contd.)
(rats with 10/30
tumours)
controls none till the urinary 1
(65 males, end bladder
65 females) papilloma
subcutaneous 1
fibrosarcoma
caecal adenoma 1
mammary 1
fibroadenoma
retroperitoneal 1
teratoma
Table 13. (contd.)
Material Strain, Route of Other Duration Period of Tumours Tumour Reference
tested number, administration treatment of observation produced incidence
and sex of and dosing treatment in
animals regimen surviving
animals
Senkirkine ACI rat ip injection of 4 weeks, 650 days liver adenomas 9/20 Hirono et al.
(20 males) 22 mg/kg body 52 weeks or till (1979a,b)
weight, twice moribund myeloid 1/20
weekly, then leukaemia
once weekly
testis
interstitial 1/20
tumour
Symphytine 20 males ip injection of 4 weeks, 650 days liver adenoma 1/20
22 mg/kg body 52 weeks or till
weight, twice death or liver 3/20
weekly, then moribund haemangio-
once weekly sarcomas
controls myeloid 1/20
(20 males) leukemia
Table 13. (contd.)
Material Strain, Route of Other Duration Period of Tumours Tumour Reference
tested number, administration treatment of observation produced incidence
and sex of and dosing treatment in
animals regimen surviving
animals
Symphytine fibroma-soft 1/20
(contd.) tissues
testis- 1/20
interstitial
tumour
Clivorine ACI rat drinking-water 340 days 480 days liver 2/12 Kuhara et al.
(6 males, (0.005%) haemangio- (1980)
6 females) sarcomas
ACI rat drinking-water hepatic 6/12
(6 males, (0.005%) neoplastic
6 females) nodules
testicular 3/12
interstitial
cell tumour
Table 13. (contd.)
Material Strain, Route of Other Duration Period of Tumours Tumour Reference
tested number, administration treatment of observation produced incidence
and sex of and dosing treatment in
animals regimen surviving
animals
Clivorine 10 males, pituitary 1/20
(contd.) 10 females adenoma
testicular 3/20f Kuhara et al.
interstitial
cell tumour
adreno- 1/20f
cortical
adenoma
pancreatic 1/20
aunar cell
adenoma
Table 13. (contd.)
Material Strain, Route of Other Duration Period of Tumours Tumour Reference
tested number, administration treatment of observation produced incidence
and sex of and dosing treatment in
animals regimen surviving
animals
Crude Sprague intragastric 104 weeks 114 weeks liver tumours 13/40 (2 Habs et al.
alkaloidal Dawley rat dose of 8 mg/kg (all types) males, 11 (1982)
extract (20 males, body weight, 5 females)
from 20 females) times per week
Senecio other tumours 11/40
numorensis (all types) (5 males,
fuchsii 6 females)
20 males, intragastric 104 weeks 114 weeks liver tumours 34/40
20 females dose of 40 mg/kg (all types) (5 males,
body weight, 5 29 females)
times per week
other tumours 10/40
(all types) (7 males,
3 females)
controls liver tumour 1/40
(20 males, (male)
20 females)
other tumours 10/40
(all types) (4 males,
6 females)
Table 13. (contd.)
Material Strain, Route of Other Duration Period of Tumours Tumour Reference
tested number, administration treatment of observation produced incidence
and sex of and dosing treatment in
animals regimen surviving
animals
Farfugium AC1 rat diet (20%) 480 days till death liver 6/29 Hirono et al.
japonicum (15 males, or end of haemangio- (1983)
(leaves 14 females) study sarcomas
and
stalks) liver adenomas 7/29
adrenalcortical 7/29
adenomas
adrenal 1/29
phaeochromo-
cytoma
urinary 2/29
bladder
papillomas
ACI rat diet (20%) testicular 2/29
(15 males, interstitial
14 females) tumours
ileal 1/29
adenocarcinoma
Table 13. (contd.)
Material Strain, Route of Other Duration Period of Tumours Tumour Reference
tested number, administration treatment of observation produced incidence
and sex of and dosing treatment in
animals regimen surviving
Senecio 15 males, diet (8%) none Hirono et al
cannabifolius 15 females survived (1983)
(leaves and > 240 days
stalks)
14 males, diet (4%) none
14 females survived
> 240 days
12 males, diet (1%) 480 days till dead liver 1/23
12 females haemangio-
sarcoma
liver adenomas 13/23
adrenal 5/23
cortical
adenomas
urinary 1/23
bladder
papilloma
Table 13. (contd.)
Material Strain, Route of Other Duration Period of Tumours Tumour Reference
tested number, administration treatment of observation produced incidence
and sex of and dosing treatment in
animals regimen surviving
animals
Senecio testicular 1/23
cannabifolius interstitial
(contd.) tumour
12 males, diet (0.2%) 480 days till death liver 8/24
12 females haemangio-
sarcomas
liver cell 3/24
adenomas
adreno- 3/24
cortical
adenomas
adrenal 1/24 Hirono et al.
phaeochromo- (1983)
cytoma
testicular 2/24
interstitial
tumours
pituitary 1/24
adenoma
caecal 1/24
fibrosarcoma
Table 13. (contd.)
Material Strain, Route of Other Duration Period of Tumours Tumour Reference
tested number, administration treatment of observation produced incidence
and sex of and dosing treatment in
animals regimen surviving
animals
Senecio controls basal diet 560 days cortical 3/49
cannabifolius (25 males, adenomas of
(contd.) 24 females adrenal
controls basal diet testicular 3/49 Hirono et al.
(25 males, interstitial (1983)
24 females) tumours
pituitary 2/49
adenomas
a These tumours were present in the same animal.
b Each coexisted in one animal (seen in animals surviving 22 - 27.5 months).
c Together with haemangioendothelioma of the liver.
d Two animals had both tumours.
e Found in the same animal with angiosarcoma.
f In the same animal.
g No data available on number of surviving animals in the control groups.
Note: Figures in column 2 indicate the number of animals used at the start of the study. In column 8, animals developing a tumour out of
the number surviving up to the end of study/or dying with tumour are indicated. Animals developing more than one tumour are
indicated separately in a footnote. Different terms have been used in different studies to describe the same tumour in the liver,
e.g., haemangiosarcoma, angiosarcoma, haemangioendothelioma, haemangioendothelial sarcoma, and haemangiogenic sarcoma.
Table 14. Liver tumours found in rats given PAs and plant materials (condensed results from various authors)a
Alkaloid or plant Alkaloid type Route of Number of Number and types of liver Reference
given Necine Type of administration rats at tumours found
ester autopsy
Clivorine otonecine macrocyclic oral 12 2 haemangiosarcomas Kuhara et al. (1980)
diester 6 neoplastic nodules
Lasiocarpine heliotridine "open" ip 18 11 hepatocellular carcinomas Svoboda & Reddy
diester oral 20 9 angiosarcomas (1972);
7 hepatocellular carcinomas Rao & Reddy (1978)
Monocrotaline retronecine macrocyclic oral 75 24 hepatocellular carcinomas Newberne & Rogers
diester (1973)
Petasitenine otonecine macrocyclic oral 11 5 haemangiosarcomas Hirono et al. (1977)
diester 5 adenomas
Retrorsine retronecine macrocyclic various 14 4 "hepatomas" Schoental et al.
diester (1954);
Schoental & Head
(1957)
Isatidine retronecine macrocyclic various 7 4 "hepatomas" and Schoental et al.
diester "nodular hyperplasia" (1954);
Schoental & Head
(1957)
Senecionine retronecine macrocyclic oral 80 19 hepatocellular tumours Habs et al. (1982)
(+ fuchsisenecionine) (platynecine) diester, 16 cholangiogenic tumours
monoester 12 "haemangiogenic" tumours
Table 14 (cont'd)
Alkaloid or plant Alkaloid type Route of Number of Number and types of liver Reference
given Necine Type of administration rats at tumours found
ester autopsy
Senkirkine otonecine macrocyclic ip 20 9 adenomas Hirono et al.
diester (1979a)
Symphytine retronecine "open" ip 20 1 adenoma Hirono et al.
diester 3 haemangiosarcomas (1979a)
Senecio longilobus macrocyclic oral 47 16 hepatocellular carcinomas Harris & Chen
(seneciphylline, (retronecine) diester 1 angiosarcoma (1970)
retrorsine, etc.)
Petasites japonicus macrocyclic oral 46 11 haemangiosarcomas Hirono et al.
(petasitenine) (otonecine) diester 10 adenomas (1973)
3 hepatocellular carcinomas
Tussilago farfara macrocyclic oral 12 8 haemangiosarcomas Hirono et al.
(senkirkine) (otonecine) diester (1976)
Symphytum officinale monoester + oral 175 81 adenomas Hirono et al.
(various) (retronecine) "open" 3 haemangiosarcomas (1978)
diester
Farfugium japonicum senkirkine oral 29 7 adenomas Hirono et al.
6 haemangioangiosarcomas (1983)
Senecio cannabifolius petasitenine oral 21 16 adenomas Hirono et al.
9 haemangioangiosarcomas (1983)
a From: Mattocks (1986).
Svoboda & Reddy and their group have carried out 2 studies
using lasiocarpine (Svoboda & Reddy, 1972, 1974; Rao & Reddy,
1978). Svoboda & Reddy (1972, 1974) gave repeated ip injections to
25 rats at a dose of 7.8 mg/kg body weight (0.1 LD50) for 56 weeks
as per regimen shown in Table 13. Three rats died of acute liver
necrosis in the initial 4 weeks. Eighteen rats survived 56 weeks,
by which time each animal had received an average cumulative dose
of 125 mg lasiocarpine. Of these, 16 animals developed a variety
of tumours 60 - 76 weeks after the beginning of the study (Table
13). Ten of the 16 animals had more than 1 tumour. Hepatocellular
carcinoma was the most common. The squamous cell carcinoma of the
skin was found to be transplantable. When the same PA, mixed with
the diet at the rate of 50 mg/kg (Rao & Reddy, 1978) (Table 13),
was administered to 20 rats for 55 weeks, 17 animals developed
tumours. Angiosarcoma of the liver emerged as the most common
tumour (9/20 animals), even though hepatocellular carcinomas were
also frequently seen (7/20 animals); squamous cell carcinoma of the
skin was not found, but there was a malignant adnexal tumour of the
skin. The average cumulative dose of the alkaloid was estimated to
be 190 - 200 mg per rat.
Monocrotaline has been studied for its carcinogenic activity in
rats by Schoental & Head (1955), Newberne & Rogers (1973), Allen et
al. (1975), Shumaker et al. (1976), and Hayashi et al. (1977),
using different routes of administration.
Allen et al. (1975) studied the long-term effects on rats of
repeated sc injections of monocrotaline or its major detectable
metabolite, dehydroretronecine. Male Sprague Dawley rats were
given biweekly injections of monocrotaline, at 5 mg/kg body weight
(75 animals) for 12 months, or dehydroretronecine at 20 mg/kg body
weight (75 animals) for 4 months followed by 10 mg/kg body weight
for 8 months. Fifty control animals received phosphate buffer
(Table 13). Partial hepatectomy was performed on 15 animals in
each of the treated groups and 5 in the control group. They were
observed for 10 months following cessation of the injections. Of
the 60 animals surviving in each of the treated groups, those
receiving monocrotaline showed rhabdomyosarcoma at the injection
site (2 animals), hepatocellular carcinoma (2 animals), acute
myelocytic leukaemia (2 animals), and pulmonary adenoma (2
animals). In the group receiving dehydroretronecine, 36 animals
developed rhabdomyosarcomas, and 5 of these animals developed
metastases. None of the control group developed tumours. Tissues
obtained from partial hepatectomies showed that both compounds
caused inhibition of mitotic division in regenerating liver. The
results of the study illustrate the dual alkylating and antimitotic
properties of these agents, commented on by Culvenor et al. (1969).
In a similar study by Shumaker et al. (1976), rats were
administered monocrotaline at 5 mg/kg body weight on alternate
weeks or its metabolite dehydroretronecine at 20 mg/kg body weight,
on alternate weeks for 4 months, followed by a dose of 10 mg/kg on
alternate weeks in the succeeding 8 months (Table 13). Of the 60
rats receiving monocrotaline, 17 developed one or more tumours, but
not until after the treatment was discontinued. The time interval
was not stated. The most common tumour was carcinoma of the lung
(11 animals) followed by hepatocellular carcinoma (5 animals). A
wide variety of other tumours was also seen. A notable feature was
that the metabolite dehydroretronecine did not produce any tumours
by systemic action, but only at the site of injection, where
significant numbers of rhabdomyosarcomas (39/60 animals) were seen.
The marked difference in tumour sites is explained by the fact that
the parent alkaloid monocrotaline has to be metabolized before it
becomes a carcinogen. For this reason, the tumours are distributed
in several organs of the body, whereas dehydroretronecine is itself
carcinogenic and so acts at the site of injection. When
monocrotaline was delivered in a higher dose, but as a single
subcutaneous injection (40 mg/kg body weight) (Hayashi et al.,
1977) to 40 rats there were no malignant tumours but only adenomas
of the islets in the pancreas in 16/23 surviving animals. The
results of the above studies indicate that monocrotaline is
tumorigenic, but the type of tumour and the malignancy both depend
on the route of administration and the dosage used.
Hirono et al. (1977) studied petasitenine, the pure alkaloid
isolated from the flower stalks of the plant, Petasites japonicus
Maxim, which has been found to be carcinogenic for rats (Hirono et
al., 1973). Two groups of ACI rats of both sexes, Group I of 3
animals and Group II of 11 animals, were given the alkaloid in in
the drinking-water, at concentrations of 0.5 g/kg and 0.1 g/kg,
respectively (Table 13). There were 19 controls. All 3 animals in
group I died within 72 days showing marked hepatocellular damage;
no tumours were seen. In Group II, 10 out of 11 rats survived for
more than 160 days. Eight of the 10 animals developed tumours -
liver cell adenomas (5) and haemangiosarcomas (5) (Table 10). Two
animals had both types of tumours. The authors concluded that the
carcinogenicity of the plant was due to petasitenine.
Retrorsine and its N-oxide (isatidine) have been used in
several studies. Schoental et al. (1954) administered retrorsine
at a concentration of 3 mg/litre in the drinking-water to 14 rats
and isatidine at concentrations of 5 mg/litre followed by 3 mg/litre
to 22 rats until death. Twenty-five rats were administered the
alkaloid mixture from Senecio jacobaea Lin at a concentration of
500 mg/litre followed by 300 mg/litre in the drinking-water. Dosing
regimens are shown in Table 13. One group of 7 animals receiving
isatidine received supplementary 0.5% choline in the drinking-water
and another group of 5 animals received isatidine 2 mg as a single ip
administration in 0.2 ml of tricaprilyn, followed by skin application
of alkalids as 0.5% solution for 3 days/week.
The animals receiving mixed alkaloids of the plant showed
extensive liver damage followed by marked nodular hyperplasia; no
tumours developed. The nodules were earlier interpreted as
hepatomas, but later only as an early stage in the progression from
hyperplasia to neoplasia.
The retrorsine group showed extensive liver damage associated
with cirrhosis and nodular hyperplasia. In 4 rats, they were
interpreted as hepatomas.
In the isatidine group, 10 out of the 22 rats developed
hepatomas. Tumours were present in 3 out of 7 animals receiving
isatidine plus choline indicating that the latter had no protective
role. One out of the 5 animals receiving the alkaloid ip and then
through dermal application developed a tumour, which was also
interpreted as a hepatoma.
In another study (Schoental & Bensted, 1963), 95 weanling rats
were administered a single dose of retrorsine at 30 mg/kg body
weight, by stomach tube (Group II). One comparable group of 50
weanling rats (Group I) received 400 r radiation in animals
surviving 100 days after retrorsine administration (31/50). A
third group (Group III) of 6 animals received 400 r radiation
alone. Another group of 10 weanling rats received the PA, 9 days
after partial hepatectomy (Group IV) (Table 13). The additional
treatments were given to study whether they would act as
co-carcinogens and induce neoplasia in hepatocytes, which are known to
show injurious effects for long periods following PA treatment. In
Group I, 19 of the 50 animals receiving a single dose of the PA
died before radiation could be given. Of the 31 remaining animals
that received radiation after the PA, 25 survived 12 months. Among
these, 19 tumours of a wide variety were seen (Table 13). Of the 6
tumours in the liver, only 1 was malignant, having metastasized.
Most of the other tumours were malignant, including those of the
breast, one of which had also metastasized.
In Group II, 29 out of 95 animals that had received one dose of
PA and no radiation survived for more than a year, with a mean
survival time of 23 months. Among these, 7 animals developed
tumours of a wide variety (Table 13). Five tumours in the liver
were benign. Most of the others were malignant. Two tumours, a
cystic tumour of the breast and a carcinoma of the uterus, were
present in one animal. Tumours seen in Groups III and IV, which
were found in animals surviving 12 months or more after the start
of the study, are shown in the Table 13. The 2 tumours of the
liver in Group IV were also benign. The number of control animals,
if any, were not indicated.
The authors concluded that the above studies did not provide
definite evidence of synergistic action in the carcinogenicity of
retrorsine by whole body radiation or partial hepatectomy.
Hirono et al. (1979a) studied the carcinogenic properties of
senkirkine extracted from the dried milled buds of Tussilago
farfara (coltsfoot) and symphytine extracted from dried milled
roots of Symphytum officinale (comfrey). Both Tussilago farfara
(Hirono et al., 1976) and Symphytum officinale (Hirono et al.,
1978) had earlier been demonstrated to have carcinogenic
properties.
Sixty inbred ACI strain male rats were divided into 3 groups of
20 animals each and received repeated ip injections of senkirkine
at 22 mg/kg body weight or symphytine at 13 mg/kg body weight as
per schedule given in Table 13. All animals treated with
senkirkine survived 290 days. Nine out of 20 rats developed liver
adenomas mostly after 350 - 450 days from start of the study.
Cirrhosis of liver was frequently observed. Of the symphytine
group, all animals survived 330 days after the start of the study.
Three animals developed haemangioendothelial sarcoma and one,
liver adenoma. The sarcomas were noted at least 518 days after the
start of the study.
Schoental & Cavanagh (1972) used two alkaloids, retronecine and
hydroxysenkirkine isolated from Crotalaria laburnifolia, which was
injected ip in single doses ranging from 100 to 300 mg/kg body
weight in 5 weanling Porton Wistar rats (Table 13). One animal
that had received the 300 mg/kg dose developed astrocytoma of the
brain after 14 1/2 months. Retronecine hydrochloride was
administered in doses ranging from 300 to 1000 mg/kg body weight by
single subcutaneous injection to 10 new-born rats. One male rat,
which was found to be paraplegic 6.6 months after receiving a dose
of 600 mg PA/kg, was also found to have ependymoblastoma of the
spinal cord. Among the litter mates of this rat, 1 male that had
received a dose of 1000 mg/kg died. The remaining 6 females and 2
males were killed within 22 months of being dosed. Of the females,
5 had pituitary tumours and 1 had a mammary tumour (type not
stated). Two males did not show any significant abnormalities.
One group of 5, 6-month-old female rats, born to dams that had
been fed on a diet containing 50 g dried and powdered Heliotropium
ramosissimum/kg diet, a tribal remedy, used during pregnancy and
parturition, were then themselves fed on the same diet at 6 months
of age, as per the regimen indicated in Table 13. One female rat
was found to be paraplegic at 7 months of age. A tumour, possibly
of Schwann or satellite cell origin, was found.
6.4.8.2 Plant materials
A number of plant materials have been tested for their
carcinogenicity by administering either a mixture of extracted
alkaloids (Cook et al., 1950; Schoental et al., 1954) or, more
often, the dried and milled plant mixed with the diet. The only
study in which the plant alone was fed was that of Campbell (1956),
who produced tumours in chickens by feeding dried and milled
Senecio jacobaea plant. It is notable that the plants tested are
almost all those that have been reported to be used as herbal
medicines and/or food, some of which have been reported to cause
human toxicity.
Schoental et al. (1970) used mixed PAs (intermedine and
lycopsamine) extracted from seeds of Amsinckia intermedia, known to
cause livestock losses in the USA, and leaves and stems from
Heliotropium supinum L., known to be used by women in East Africa
as a herbal medicine, after childbirth. The dosing regimen and
mode of administration are indicated in Table 13. Of the 15 male
weanling rats that had received a single treatment with the
Amsinckia PAs and survived for more than 1 year, 3 rats showed one
adenoma, one adenocarcinoma of the islet cells, and one adenoma of
the exocrine pancreas, respectively (Table 13). In addition, one
of the animals also developed a pituitary adenoma and papillary
tumour of the urinary bladder. Eight animals received the
treatment with Heliotropium supinum L. (Table 13). One out of the
2 weanling rats fed on the plant with the diet and one out of the 6
animals that received a single intragastric dose of the crude
alkaloid fraction developed islet cell adenoma of the pancreas.
The number of control animals, if any, used in the study is not
stated.
Harris & Chen (1970) tested the carcinogenicity of Senecio
longilobus, which has been associated with cases of human toxicity
(Stillman et al., 1977; Huxtable, 1980; Fox et al., 1978). Harlan
rats were divided into 4 groups (equal numbers of both sexes) and
fed diets containing dried and powdered stems and leaves of the
plant in the proportion of 5 -7.5 g/kg. The number of animals in
each group and the feeding regimen are shown in Table 13.
Continuous feeding did not produce any tumours, presumably because
of the comparatively low survival rate of animals. Significant
results were obtained when the animals were fed contaminated diet
alternating with normal diet (Group IV). Of the 100 animals fed on
this regimen for 54 weeks, 47 survived for more than 200 days.
Seventeen of these animals developed malignant tumours in the
livers - hepatocellular carcinomas in 16 and an angiosarcoma in 1
animal after a minimum feeding period of 217 days. In Group III,
fed a contaminated diet for 1 year, 23 rats lived for more than 200
days. Four rats (3 males and 1 female) developed hepatocellular
carcinomas and one, a peritoneal mesothelioma, after a minimum
feeding period of more than 428 days. The tumour-bearing animals
were predominantly male. The authors emphasized the relative
rarity of liver tumours in the strain of animals used. Results
demonstrated that S. longilobus is carcinogenic for rats.
For a study of Schoental & Cavanagh (1972), using dried and
powdered Heliotropium ramosissimum, see section 6.4.8.1.
Hirono et al. tested the carcinogenic effects of 3 widely-used
herbs containing PAs. Hirono et al. (1973) studied the possible
carcinogenic effects of the young flower stalks of Petasites
japonicus Maxim, which has long been used in Japan as food or
herbal remedy as well as the PA, petasitenine, isolated from it.
The flower stalks of the plant were dried, milled, and fed to young
ACI rats of both sexes mixed with the basal diet in the proportion
of 40 - 80 g/kg, as indicated in the feeding regimen in Table 13,
until the animals were moribund or dead. One group of 27 rats was
fed the plant mixed in the proportion of 40 g/kg diet for 6 months
followed by 80 g/kg diet on alternate weeks. The second group of
19 rats was fed a contaminated diet (40 g/kg) continually. Three
animals in Group I died of pneumonia. Eleven out of the remaining
24 rats in this group developed liver tumours after 15 - 16 months
of feeding. There were 2 hepatocellular carcinomas, 6 liver cell
adenomas, and 3 haemangiosarcomas, of which 2 had metastasized. In
Group II, 2 animals died from non-tumorous causes. Of the
remaining 17, 8 developed haemangiosarcomas, 4 liver cell adenomas
and 1 had a hepatocellular carcinoma. The incidence of
haemangiosarcoma was statistically higher in Group II.
In a similar study on mice and hamsters (Fushimi et al. (1978),
groups comprising 20 - 24 male and 20 - 21 female 6-week-old ddN,
Swiss, and C57BL/6 mice, and 13 male and 17 female hamsters, were
fed a diet combining 4% young, dried, and milled flower stalks of
Petasites japonicus Maxim for 480 days. All surviving animals were
killed at the end of the study. Lung adenomas and adenocarcinomas
were found in 30/39 surviving male and female ddN mice combined
(compared with 1/50 in the respective controls) in addition to
other tumours (Table 13). No significant differences in tumour
incidence were observed between treated Swiss and C57BL/6 mice and
hamsters, and the corresponding controls. No data were given on
the tumour incidence according to sex, in treated and control
animals or on survival in the controls.
In studies on rats (Hirono et al., 1976), the dried and
powdered flower buds of Tussilago farfara (coltsfoot) were mixed
with the diet. Forty-nine inbred ACI strain rats were divided into
4 groups and fed diets containing 40 - 320 g T. farfara/kg for up
to 600 days. The number of animals in each group and the dosage
and feeding regimen are given in Table 13. Two groups receiving a
diet containing 80 - 320 g/kg on a continual basis developed
tumours in the liver of the same types as animals in the studies
using Petasites japonicus, e.g., haemangioendotheliomas, liver
cell adenomas, and hepatocellular carcinomas (Table 13). All 12
animals in Group I survived for more than 380 days after the start
of the study. Of these, 8 (5 males, 3 females) developed
haemangioendothelial sarcoma of the liver. In addition, 3 of the 8
rats developed simultaneously hepatocellular adenoma, hepatocellular
carcinoma, or urinary bladder papilloma. In Group II receiving
coltsfoot at 80 g/kg in the diet, 9 out of 10 animals that survived
for more than 420 days developed a haemangioendothelial sarcoma.
Hirono et al. (1978) fed Symphytum officinale, similarly dried
and milled, to the ACI strain of inbred rats of both sexes at
different levels ranging from 80 to 330 g/kg as leaves or 5 - 40 g/kg
as root, for 280 days or more. Eight groups of animals of
19 - 48 animals each were used on different regimens of feeding.
The feeding regimen, the number of animals in each group, and the
duration of treatment are given in Table 13. Sixty-five males and
64 females served as controls. Tumours were induced in all groups
receiving leaves or roots. The most common tumour was liver
adenoma. Haemangiosarcomas were observed but infrequently (3
animals in the whole study). No carcinomas of the liver were seen,
but there was a wide variety of other tumours. It is noteworthy
that, in Group VII, 14 of 15 animals fed the lowest dose of 10 g/kg
for 275 days followed by 5 g/kg or basal diet alternating every 3
weeks, developed tumours, 2 of which were malignant (haemangiosarcomas).
In the control group, single animals each had papilloma of urinary
bladder, caecal adenoma, subcutaneous fibrosarcoma, mammary
fibroadenoma, or retroperitoneal teratoma. The livers of animals that
did not have the tumours showed other features commonly encountered in
animals administered PAs, e.g., megalocytosis, liver cirrhosis,
hyperplastic nodules, etc., suggesting that they were induced by the
PAs contained in the plant. The authors concluded that the
carcinogenic activity of this plant was weaker than that observed in
animals fed Petasites (coltsfoot). Hirono et al. (1979b) have
summarized the above studies on PAs found in edible plants in Japan.
Habs (1982) and Habs et al. (1982) tested a crude alkaloid
extract from the plant Senecio numorensis sp. fuchsii containing
fuchsisenecionine (500 g/kg) and senecionine (10 g/kg). The
extract was administered intragastrically in 2 doses of 8 mg and
40 mg/kg body weight, respectively, to 2 groups of rats of both sexes,
comprising 40 animals each, 5 times a week for 104 weeks (Table
13). A large, dose-related number of liver tumours was produced,
originating in the liver cell and the sinusoidal system, and
predominantly affecting the female animals. The tumour incidence
was higher in Group II (dose, 40 mg/kg) with 34 tumours compared
with 13 in Group I. In Group I (dose, 8 mg/kg), 2 tumours were
found in 20 males compared with 11 tumours among 20 female rats.
Similarly, in Group II, only 6 tumours were found in 20 males
compared with 29 among 20 female rats. The tumours included 19 of
"hepatocellular origin", 16 of "cholangigenic origin", and 12 of
"haemangiogenic origin" (Table 14). Besides the liver tumours, 12
males and 9 females in the treated groups and 4 males and 6 females
in the control group developed a variety of extra-hepatic tumours.
Senecionine is known to be hepatotoxic and capable of being
converted in the rat liver into cytotoxic metabolites.
Fuchsisenecionine is a saturated PA, not previously known to be
cytotoxic. It is therefore likely that senecionine was the
hepatotoxic component and that perhaps the two PAs acted
synergistically with each other, though there is no actual evidence
for synergism. However, this needs confirmation. Moreover, there
appeared to have been other unknown components in the mixture
tested (Mattocks, 1986).
Hirono et al. (1983) studied the carcinogenicity of 2 more
plants (Farfugium japonicum and Senecio cannabifolius) of the tribe
Senecioneae in the family Compositae, the leaves and stalks of
which are used in Japan as human food. Fresh leaves and stalks of
the plants were dried, milled, and mixed with the basal diet.
Inbred strain ACI rats of both sexes, with preponderance of
females, 1.5 months old, were divided into 6 groups. They were fed
diets containing various proportions of the dried plant materials,
as indicated in Table 13. The study was terminated at 480 days,
except for one group, which was studied for 560 days. Besides the
groups shown in the tables, 2 more groups of 30 and 28 animals of
equal numbers of both sexes were fed 8% and 4% of Senecio
cannabifolius, respectively. None of these animals survived more
than 177 days and all died of hepatotoxicity. A wide range of
tumours was observed, mostly in the liver, as shown in Table 13,
the most common being haemangiosarcomas, which were not encountered
in the control group.
The carcinogenicity of Farfugium japonicum is considered to be
due to senkirkine and petasitenine. Senecio cannabifolius contains
senecicannabine, a new macrocyclic PA, seneciphylline, and
jacozine. It is probable that the carcinogenicity of Senecio
cannabifolius is due to these PAs (Hirono, et al., 1983).
Hayes et al. (1985) studied the biological mechanisms by which
PAs initiate carcinogenesis in male Fischer 344 rats. Lasiocarpine
(single or double injection of up to 80 µmol/kg body weight) and
senecionine (single or double injection of up to 160 µmol/kg) were
inactive as initiators of Y-GT-positive nodules in rats exposed to
2-acetylaminofluorene and partial hepatectomy. Administration of
lasiocarpine or senecionine 12 h after partial hepatectomy resulted
in the development of very few nodules. Lasiocarpine given in a
single or double dose (up to 80 µmol/kg) delayed hepatic
regeneration by at least 8 weeks after partial hepatectomy, and
pretreatment with this PA reduced the initiating capacity of
diethylnitrosamine and N-nitrosomethylurea in rats subsequently
selected with 2-acetylaminofluorene and partial hepatectomy.
Resistant nodules selected with lasiocarpine also had the typical
resistant nodule phenotype (positive for Y-GT and epoxide
hydrolase) and also lacked PA-induced megalocytosis. Lasiocarpine
treatment also resulted in small regenerative nodules that were
distinct from resistant nodules, because they were negative for
Y-GT and epoxide hydrolase.
6.4.8.3 Pyrrolizidine alkaloid metabolites and analogous synthetic
compounds
The subject has been reviewed by Mattocks (1986). The
pyrrolizidine alkaloids are converted into pyrrolic esters in the
hepatocytes. These may then be hydrolysed into pyrrolic alcohols,
which are more water soluble and less active than the esters. The
esters may be widely distributed throughout the body. Some of
these compounds and their analogues have been tested for
carcinogenicity.
(a) Pyrrolic esters
(i) Dehydromonocrotaline (monocrotaline pyrrole)
Mattocks & Cabral (1979) tested dehydromonocrotaline on the
skin of mice. In their first study on male BALB/c mice, they
applied the compound on the back at 1- to 2-week intervals.
Thirty-three applications of 1 µmol did not produce any skin
tumours in 16 mice, but 2 developed lung adenomas; no tumours
occurred in 14 control mice treated with the solvent (acetone). In
the second study (Mattocks & Cabral, 1982), 11 female LACA mice
each received 47 applications of 2.5 µmol dehydromonocrotaline; one
animal developed a malignant skin tumour. A second batch of 10
mice was given similar treatment and subsequently received 61
twice-weekly applications of croton oil at the same site. Half of
these animals developed tumours. In the control group of 10 mice,
only 1 tumour was seen. The results of these studies indicate that
dehydromonocrotaline requires the action of a promoter to manifest
carcinogenic potential.
(ii) Dehydroretrorsine
This supposed pyrrolic metabolite of retrorsine was applied to
the skin of male BALB/c mice at 1- to 2-week intervals; 33
treatments of 0.5 or 1 µmol failed to produce tumours in 15 mice
that survived for up to 60 weeks (Mattocks & Cabral, 1979).
(iii) 1-Methyl-2,3-bistrimethylacetoxymethylpyrrole
Mattocks & Cabral (1979, 1982) made 2 studies on this compound.
In the second study, which was more significant, 22 female LACA
mice received 47 dermal applications of 0.5 µmol each. This caused
marked skin damage with ulceration and scarring. Malignant skin
tumours developed in 19 out of 21 surviving animals. Two
hydrolysis products of this ester, pivalic acid and 1-methyl-2,3-
bishydroxymethylpyrrole, were similarly tested (Mattocks, 1986).
Tumours were produced, though fewer.
The results of the above studies suggest that the intact
pyrrolic ester is a carcinogen.
(b) Pyrrolic alcohols
Studies have been conducted using dehydroretronecine
(retronecine pyrrole) and dehydroheliotridine, which are secondary
metabolites of monocrotaline and heliotridine-based PAs,
respectively. Originally they were regarded as (+)- and (-)-
forms, respectively, of dihydro-7-hydroxy-1-hydroxy-methyl-5H-
pyrrolizine. Kadzierski & Buhler (1985, 1986) showed that the
metabolite from monocrotaline is racemic and concluded that the
product from all heliotridine and retro-necine esters is the same
(±)- form.
Johnson et al. (1978) painted the skin on the back of 16 female
Swiss mice with dehydroretronecine, each dose equalling 20 mg/kg
body weight or about 5 µmol per mouse, once a week for 4 weeks and
then twice more after 6 months. Six mice developed skin tumours.
Subcutaneous injections of the same dose yielded tumours in 13 out
of 21 mice. Twenty-eight out of 55 mice given both topical
applications and sc injections developed skin tumours.
When the same study was repeated on 34 BALB/c mice, only one
skin tumour developed (Mattocks & Cabral, 1982). When 17 animals
were given the same dose at more frequent intervals, e.g., 65
weekly doses, no tumours developed.
Similar results were obtained in a study by Shumaker et al.
(1976), already desecribed in section 6.4.8.1, in which 39/60 rats
given repeated subcutaneous injections of dehydro-retronecine
developed rhabdomyosarcomas at the site of injection. The compound
appears to be a direct-acting carcinogen for rats and mice, though
the susceptibility of various strains of mice varies.
Peterson et al. (1983) gave 9 ip injections (60 - 76.5 mg/kg
body weight) of dehydroheliotridine to rats over a 32-week period.
A large variety of tumours was produced. It was concluded that
this metabolite may be responsible for the carcinogenicity of its
parent PA.
6.4.8.4 Molecular structure and carcinogenic activity
Mattocks (1986) has reviewed the present position concerning
the relationship between molecular structure and carcinogenic
activity. Data available at present are not adequate for any
strict correlation to be established between the molecular
structure of PAs and the types of tumours produced by them in the
rat. However, the common determinants in the molecular structure
of all carcinogenic PAs are that they are macrocyclic or "open"
diesters, in which the amino-alcohol moiety is retronecine,
heliotridine, or otonecine. These are all esters of unsaturated
necines and are capable of being metabolized to pyrrolic esters in
the mammalian liver. Studies on pyrrolic esters, the toxic
metabolic product of PAs, have yielded equivocal results.
Monocrotaline has been shown to bind covalently to DNA, which is
associated with the carcinogenic activity of the pyrrolizidine
alkaloids (Robertson, 1982). Dehydromonocrotaline, the primary
metabolite of monocrotaline has been found to be an incomplete
dermal carcinogen (Hooson & Grasso, 1976; Mattocks & Cabral,
1982), whereas the synthetic compound 1-methyl-2,3-
bistrimethylacetoxymethylpyrrole, which is chemically similar to
dehydromonocrotaline, is clearly carcinogenic (Mattocks & Cabral,
1982). Dehydroretronecine (DHR), a second metabolite of
monocrotaline and possibly other retronecine-based PAs, is also
carcinogenic for the skin and is considered a proximate
carcinogenic metabolite, since, unlike monocrotaline, it acts at
the site of application directly and not at remote sites.
6.4.9 Antimitotic activity
Literature on this phenomenon has been reviewed by Jago (1969),
McLean (1970), and Mattocks (1986). The most characteristic
feature of the chronic hepatotoxicity of the PAs is the presence in
the liver of megalocytes (Bull & Dick, 1959; McLean, 1970), which
are generally enlarged hepatocytes containing large, hyperchromatic
nuclei (section 6.4.1.5). These appear to be the result of a
combined action of PAs on the hepatocytes, a stimulus to regenerate
following parenchymal cell injury, and the powerful antimitotic
action of the pyrrole metabolites of the PAs (Mattocks, 1986; Jago,
1969). This property has served as the basis for using a PA
(indicine- N-oxide) as a chemotherapeutic agent for cancer (Letendre
et al., 1981, 1984). Peterson (1965) showed that the number of
mitoses following partial hepatectomy was reduced to 50% or less of
normal values by prior administration of hepatotoxic alkaloids, and
that the effect was dose dependent. The hepatocytes seemed to
continue to grow without dividing. The effect can be produced by a
single sublethal dose of the alkaloids (Schoental & Magee, 1957) or
can be a cumulative effect of small doses (Bull & Dick, 1959). The
lesion appears within a few weeks and may persist for the lifetime of
the animal (Mattocks, 1986). It was characteristically described in
the liver of the rat, but has also been reported in a number of other
animals, e.g., mouse, sheep, horse, and pig, and in some other organs,
e.g., kidney and lung (McLean, 1970). It has not been observed in
human livers (Tandon, H.D. et al., 1978; Mattocks, 1986), but it has
been observed that cultured human fetal liver cells become enlarged
when exposed to PAs (Armstrong et al., 1972) indicating a
susceptibility to the antimitotic effect of PAs. The lesion is not
specific for PAs but has been reported to be produced by a number of
toxins (McLean, 1970), including semisynthetic derivatives of PAs but
not non-hepatotoxic PAs, such as platyphyline (Jago, 1970). The
ultrastructural features of megalocytes are controversial.
However, consistent with other functional and metabolic features,
the cells show morphological characters suggesting increased
metabolic activity with increased exchange of material between the
nucleus and cytoplasm.
This unique reaction of the hepatocytes to PA has been used by
Jago (1970) to develop a method for assessing hepato-toxicity and
by Culvenor et al. (1976a) for screening 62 PAs for acute and
chronic hepatotoxicity and pneumotoxicity.
Mattocks (1986) suggested a scheme for the antimitotic action
of PAs in vivo, consistent with observations of the phenomenon in
animals. The PA is irreversibly metabolized by the hepatic
microsomes into a pyrrolic ester, which can be hydrolysed to a
pyrrolic alcohol. The latter is the agent that inhibits mitosis.
The more reactive primary metabolite may also do this, by reacting
with tissue constituents to give products identical to pyrrolic
alcohols, inhibiting mitosis. On the other hand, it can also
produce acute injury to the cells, which stimulates regeneration.
Thus administration of the PA or the pyrrolic ester can induce
megalocytosis to a much greater extent than a secondary metabolite
alone.
Antimitotic activity does not seem to be directly related to
inhibition of DNA synthesis, since the latter recovers within a
week while mitotic inhibition continues for up to a period of 4
weeks (Peterson, 1965; Armstrong & Zuckerman, 1970). Mattocks &
Legg (1980) have shown that the level of DNA synthesis is reduced
in cells that do not divide, but is not totally inhibited. Samuel
& Jago (1975) investigated the position in the cell cycle of the
antimitotic action of lasiocarpine and of its pyrrolic metabolite,
dehydroheliotridine. Their studies indicated that the alkaloid
acts during the late S or early G2 phase of the cell cycle.
6.4.10 Immunosuppression
Dehydroheliotridine (DHH), a pyrrolic metabolite, has a
significant immunosuppressant activity on the primary response in
young mice; when injected ip shortly before the antigenic stimulus
(Percy & Pierce, 1971). The secondary response to antigenic
stimuli; as measured by the reduction in the number of 7S and 19S
specific antibody-synthesizing cells of the spleen, was suppressed
when DHH was administered at the time of secondary stimulus, but
not when it was given 24 or 36 h after the antigenic stimulus. It
was suggested that dehydroheliotridine selectively destroys or
inactivates cells involved in the initial stages of antigen
recognition and processing.
6.4.11 Effects on mineral metabolism
Aberrations of mineral metabolism have been observed in several
species of animals. The most notable among them relate to
haemolysis and copper metabolism. Anaemia has been reported to
occur in rats following PA poisoning (Schoental & Magee, 1959;
Schoental, 1963) and the kidney and liver show haemosiderosis
(Hayashi & Lalich, 1967). Besides the haemolysis, PAs have been
found to exert a direct inhibitory effect on haematopoiesis in the
livers of new-born rats (Sundaresan, 1942). Disturbances of iron
metabolism and haematopoiesis have also been demonstrated in rats
fed Senecio jacobaea and supplementary copper (Swick et al., 1982d)
and they have been found to develop raised copper levels in the
liver and spleen, when fed on this plant (Swick et al., 1982b).
Miranda et al. (1979) reported elevated levels of iron in the liver
and spleen and of copper in the liver in rats fed on tansy ragwort.
Studies with radioactive iron also indicated a specific inhibitory
effect of PAs on haematopoiesis. High copper levels in the liver
associated with haemoglobinuria have been reported in sheep grazing
on heliotrope (Bull et al., 1956), signs of disease closely
resembling those of chronic copper poisoning. St. George-Grambauer
& Rac (1962) reported a similar outbreak of fatal jaundice due to
haemolytic crisis of chronic copper poisoning in sheep that had
grazed Echium plantagineum over 2 or more seasons. The
pathological changes in the liver were indistinguishable from those
of Heliotropium, and the livers had a high copper content. Studies
of Miranda et al. (1981b) indicate that dietary copper can enhance
PA hepatotoxicity in rats. It has been suggested that the
hepatotoxic effects of some PAs may interfere with the excretion of
copper (Bull & Dick, 1959; Farrington & Gallagher, 1960). Similar
effects have been observed in pigs fed Senecio (Harding et al.,
1964). White et al. (1984) did not observe any rise in hepatic
copper levels in sheep fed Senecio jacobaea.
6.4.12 Methods for the assessment of chronic hepatotoxicity and
pneumotoxicity
Pyrrolizidine alkaloids produce acute as well as chronic liver
damage. The acute effect is seen as extensive necrosis (Schoental
& Magee, 1957), while the chronic effect in rats is manifested
characteristically by the presence of greatly enlarged parenchymal
cells (Schoental & Magee, 1957; Bull & Dick, 1959), which persist
long after a single exposure (Schoental & Magee, 1959). The latter
effect of megalocytosis may manifest without the liver cells going
through the process of necrosis (Schoental & Magee, 1959). This
property has been used by Jago (1970) to develop a method for the
assessment of the relative chronic hepatotoxicity of different
alkaloids. It has since been used by other investigators (Culvenor
et al., 1976a). It consists of the intraperitoneal administration
of a single dose of between 0.025 and 3.2 µmol of the alkaloid per
kg body weight in 0.2 ml aqueous solution to 14-day-old suckling
hooded Wistar rats of both sexes. The litters are randomized among
the mothers, one day before the administration of the toxin, and
then weaned at 28 days. Animals are killed 4 weeks after the
injection. The relative acute toxicity is indicated by the dose
levels that cause death within approximately a week and chronic
hepatotoxicity by those that produce hepatic megalocytosis within 4
weeks of the injection. With this method, it is not only possible
to evaluate the hepatotoxicity of a given alkaloid but also to
compare the hepatotoxic effects of different compounds in relation
to each other on a molar basis. Chronic effects on the lungs can
also be assessed by the same method. Culvenor et al. (1976a) found
this method satisfactory for compounds of medium to high
hepatotoxicity but failed to detect toxicity in certain compounds
of known, low hepatotoxicity.
6.5 Effects on Wild-Life
6.5.1 Deer
There is very little information on the consumption of
PA-containing plants by non-domesticated animals, birds, and other
wild-life. In one instance, the deaths of white-tailed deer in
coastal marshes in Louisiana in 1967, was ascribed to the
consumption of Crotalaria and/or Heliotropium species in a period
of feed scarcity (Seger et al., 1969). The animals had thin and
watery blood, abnormal bone marrow, and serious atrophy of cardiac
and mesenteric adipose tissue. In a 9-year-old doe, the liver was
dull and somewhat granular and evinced megalocytosis of the
hepatocytes. In another 4-year-old animal, the liver appeared
normal but with microscopic evidence of early megalocytosis, with
considerable vacuolization in the centrilobular hepatocytes.
Plants of the genera Crotalaria and Heliotropium were abundant and
there were some Senecio species. There were signs of ingestion of
the plants by the deer.
Senecio jacobaea (tansy ragwort) has been fed experimentally to
black-tailed deer (Odocoileus hemionus columbianus) in Oregon to
determine their susceptibility to poisoning (Dean & Winward, 1974).
The ragwort was given ad libitum together with different levels of
basal ration (85% alfalfa, 10% barley, 5% molasses) to captive
deer. The ragwort was eaten, only when the basal ration was
inadequate. One group, not given any basal ration for 6 days,
began eating ragwort and consumed 5.4 kg dry weight per animal in
42 days. This represented 24% of the animal body weight. The
animals did not show any toxic signs, and blood levels of SGOT and
bilirubin were normal.
6.5.2 Fish
The effects of S. jacobaea alkaloids on rainbow trout (Salmo
gairdneri) fingerlings has been investigated (Hendricks et al.,
1981). Duplicate groups of 80 fingerlings were fed for up to 12
months on diets containing 20 or 100 mg alkaloid/kg. The alkaloid
comprised 91% jacobine, 3% jacazine, 2.5% senecionine, and 2.5%
seneciphylline. The 100 mg/kg diet resulted in severe growth
depression and mortality, which began at 3 - 4 months. Both levels
of PAs produced severe hepatic lesions. The livers from these fish
were shrivelled, mottled yellow or whitish in colour, nodular,
fibrous, and sometimes haemorrhagic. Microscopically, there was
megalocytosis, severe fibrosis, and bile-duct proliferation.
Characteristic veno-occlusive changes were seen in the centrilobular
and hepatic veins, which, in the case of fish receiving the 100 mg/kg
diet, appeared after only 2 months on the diet.
6.5.3 Insects
There are no reports of the toxicity of PAs for insects, but
there is substantial literature on the use of PAs by certain insect
families that have evolved with the ability to store the alkaloids
as defensive chemicals and to convert them into pheromones and
other signalling chemicals (see recent reviews by Brown (1984) and
Boppré (1986), and an earlier complementary paper by Edgar (1982)).
In some species, such as moths of the family Arctiidae, the larvae
feed on PA-containing plants. In other families, such as Nymphalid
butterflies of the sub-families Danainae and Ithomiinae, the larvae
of most species live on other plants, but the adult males seek out
PA-containing species and contrive to ingest alkaloids from
wilting, dead, or damaged plant material or from nectar. The
alkaloids so acquired have a functional role as defensive chemicals
against predators and, in some species, are also converted into
pheromones and other signalling chemicals involved in mating. The
alkaloid derivatives may be pyrrolic compounds related to
dehydroretronecine or derivatives of the esterifying acids. In one
Arctiid genus, Creatonotus, the alkaloids have a morphogenetic or
hormonal effect, determining the size of the pheromone-disseminating
organ. Thus, for some insect species, PA-containing plants may be
necessary for survival.
7. EFFECTS ON MAN
The toxic effects of pyrrolizidine alkaloids are principally on
the liver. The toxic disease, produced by consuming PAs derived
from certain plants, is called veno-occlusive disease (VOD), the
pivotal and pathognomonic lesion being the occlusion of the central
and sublobular hepatic veins in the liver. The larger hepatic vein
tributaries are characteristically unaffected in contrast with the
findings in Budd-Chiari syndrome (Bras, 1973).
7.1 Clinical Features of Veno-Occlusive Disease (VOD)
There are several good clinical accounts of the disease, mostly
in the earlier reports from Jamaica, in children (Jelliffe et al.,
1954a,b), and adults (Stuart & Bras, 1955), which have been
summarized by Stuart & Bras (1957). Maksudov (1952) has described
the clinical features among children in outbreaks in the USSR,
where it was called toxic hepatitis with ascites, and Ismailov
(1948a,b), Mnushkin (1949, 1952), and Zheltova (1952) have
described them among adults. Srivastava et al. (1978) also
described the clinical findings among children in a large outbreak.
Children seem to be particularly vulnerable as is evident from the
report of Stuart & Bras (1957) and that of Mohabbat et al. (1976)
concerning a large outbreak, though the disease is rare before the
age of one or two years. Frequently, more than one member of the
family becomes affected (Stuart & Bras, 1957; Mohabbat et al.,
1976; Tandon et al., 1976; Arora et al., 1981) within days or weeks
of each other.
The disease generally has an acute onset, characterized by
rapidly developing and progressing symptoms of upper abdominal
discomfort, dragging pain in right hypochondrium, ascites, and
sometimes oliguria and oedema of the feet. Nausea and vomiting may
be present. Jaundice and fever are rare. There is generally
gross, tender, smooth hepatomegaly often accompanied by massive
pleural effusion, and sometimes slight splenomegaly and minimal
ankle oedema. Liver function tests may show only mild disturbance.
The acute disease is associated with high mortality and a subacute
or chronic onset may lead to cirrhosis. Death often occurs after
oesophageal haemorrhage.
Stuart & Bras (1957) summarized the clinical data of 84
patients ranging in age from 6 months to 53 years. The highest
incidence occurred between the ages of 1 and 3 years (39%), and 26%
of patients belonged to the 3- to 6-year age group. Thus, children
up to 6 years accounted for 65% of total cases. Although the VOD
was relatively uncommon at the 2 extremes of age, early infancy and
adult life, mortality was highest in these groups, being 60% and
54%, respectively. Hepatomegaly and some degree of ascites were
invariably present in acute cases; in 48 out of 64 patients,
ascites was acute enough to require paracentesis. In 38 patients,
hepatomegaly was grossly severe, reaching more than half way down
to the umbilicus. Jaundice was relatively uncommon. Among the
liver function tests, the most significant and consistent changes
were found in the serum-cholinesterase (t = 2.67, 0.02 > P > 0.01)
and serum-albumin levels (t = 2.82, 0.01 > P). Mortality rates
associated with signs of parenchymal liver damage were 74% with
clinical jaundice or high levels of serum-bilirubin, 62% with
diminished serum-cholinesterase, and 58% with considerable anorexia
and apathy. The mortality rates among cases with acute, subacute,
or chronic disease were reported to be 27%, 17%, and 57%,
respectively. Death was mostly due to hepatocellular failure (71%)
in the acute phase and haemetemesis in the chronic phase (75%).
More recent publications (Lyford et al., 1976; McGee et al., 1976;
Tandon, B.N. et al., 1977; Datta et al., 1978a,b) also describe the
haemodynamic data of the hepatic blood flow and the results of
portovenographic studies that suggest outflow tract obstruction in
the liver at the post-sinusoidal level, and irregularity and
obstruction/distortion of the hepatic venous radicles,
respectively.
The clinical course of the disease has been shown schematically
by Stuart & Bras (1957) (Fig. 12). However, the temporal
relationships in the different phases of the disease are not
precise, and their account, at best, represents a trend.
The administration of indomethacin did not have any effects on
either the heart-weight ratio or the pulmonary arterial pressure 3
weeks after monocrotaline administration (Fig. 11), but it
inhibited ( P < 0.05) the rises in 6-keto-PGF1alpha (by 90%) and
TXB2 (by 91%) that occurred in the lung lavage of monocrotaline-
treated animals at 3 weeks.
The above studies indicate that both the cyclo-oxygenase and
the lipo-oxygenase pathways of arachidonate metabolism are
activated by monocrotaline as early events in its toxic effect.
Activation of the cyclo-oxyenase pathway, demonstrated by increased
concentrations of the prostaglandin metabolites 6-keto-PGF1alpha
and TXB2 in lavage fluid, was inhibited by indomethacin, but this
inhibition did not prevent the monocrotaline-induced injury. DEC
attenuated both the inflammatory response and pulmonary
hypertension and inhibited the formation of slow reacting
substances including leukotriene D4. Since DEC produces a
pharmacological blockade of the lipo-oxygenase pathway, it seems
that the latter, rather than the cyclo-oxygenase pathway, is
responsible for perpetuating the pathophysiological mechanism
leading to monocrotaline-induced pulmonary hypertension.
Hilliker et al. (1984) demonstrated that antibody-induced
thrombocytopaenia attenuates right ventricular hypertrophy induced
by monocrotaline in rats. In another study, Hilliker & Roth (1984)
also produced evidence that hydrallazine, a vasodilator and
inhibitor of platelet prostaglandin synthesis, dexamethason, an
antiinflammatory agent and inhibitor of phospholipase, and
sulfinopyrazone, an inhibitor of platelet prostaglandin synthesis
inhibited monocrotaline-induced right ventricular hypertrophy in
rats, supporting the hypothesis that platelets and vasoconstrictor
agents play a role in monocrotaline-induced pulmonary hypertension.
Likewise, prior chemical sympathectomy with 6 hydroxydopamine
(100 mg/kg) or inhibition of serotonin synthesis with
p-chlorophenylalanine (500 mg/kg) reduced the degree of
monocrotaline-induced right ventricular hypertrophy in rats, but
did not prevent or reduce pulmonary vascular muscularization
(Tucker et al., 1983). Thus, the sympathetic nervous system and
serotogenic mechanisms seemed to be involved in the development of
right ventricular hypertrophy, but not in the development of the
pulmonary vascular lesion induced by monocrotaline. Kay et
al. (1985) also demonstrated that pretreatment with
p-chlorophenylalanine, which inhibits 5-hydroxytryptamine (5HP)
synthesis, also significantly ( P < 0.05) reduced right ventricular
systolic pressure, right ventricular hypertrophy, and medial
thickness of muscular pulmonary arteries in monocrotaline-treated
rats. Similar observations were made in rats exposed to hypoxia.
It was therefore suggested that 5HP might play a role in
monocrotaline-induced or chronic hypoxic pulmonary hypertension.
The biosynthesis of rat lung polyamines, putrescine,
spermidine, and spermine, generally considered to be important
regulators of cell growth and differentiation, is increased prior
to the evolution of monocrotaline-induced pulmonary hypertension in
rats. Continuous administration of alpha-difluoromethylornithine
(DFMO), which is a highly specific irreversible inhibitor of
ornithine decarboxylase (DDC), a rate-limiting enzyme in polyamine
biosynthesis, attenuated the development of monocrotaline-induced
pulmonary hypertension in rats (Olson et al., 1984). This effect
was mediated by the DFMO, by inhibiting the synthesis of putrescine
and spermidine, and not by blocking the hepatic metabolism of
monocrotaline to pyrroles (Olson et al., 1985). Thus, it was
suggested that lung polyamine biosynthesis might be essential for
the expression of monocrotaline-induced perivascular oedema as well
as medial thickening in the development of monocrotaline-induced
pulmonary hypertension vascular disease.
On the basis of the preceding studies, mostly on the rat, the
mechanism of chronic long-term injury to the lung by monocrotaline
seems to be as follows. Within hours of PA administration, there
is damage to the pulmonary endothelial cells accompanied by
vascular leak leading to pulmonary oedema. Platelet aggregation
also occurs. The endothelial damage indicated by ultrastructural
and biochemical studies activates the production of prostacyclin
and lipogenic products, which mediate increases in vascular
permeability and inflammatory reaction. There is simultaneous
production of 5 hydroxytryptamine and several polyamines. The
injected monocrotaline is completely metabolized within hours, and
no significant quantity is found in the body at 24 h (Hayashi,
1966) and, though some active metabolites may still be detectable
by isotope studies, even at 14 days (Hsu et al., 1974), the rats do
not have any lung lesions. The slow evolution of vascular changes
suggests that it is not caused by monocrotaline but through
biological pathways activated by the initial injury.
Methylprednisolone (MP), which reduces acute lung oedema caused
by monocrotaline (MCT), has been shown to reduce MCT-induced
pulmonary hypertensive vascular changes in rats and the resultant
right ventricular hypertrophy (Langleben & Reid, 1985). Daily ip
administration of MP at 5 mg/kg body weight, was found to be more
effective than 2 large doses of MP at 30 mg/kg, 2 h before and 2 h
after a single sc injection of MCT at 60 mg/kg. It was suggested
that secondary changes, though triggered by the acute MCT injury,
become self sustaining and are more significant for vascular
structural remodelling.
Structural arterial remodelling with vasoconstriction, medial
hypertrophy of the muscular pulmonary arteries, and muscularization
of the pulmonary arterioles follow as late effects, resulting in
pulmonary hypertension and right ventricular hypertrophy of the
heart.
The results of the above studies suggest a direct toxic effect
of the alkaloid on the endothelial cells of the alveolar
capillaries and on the pulmonary arteries, as well as a pulmonary
hypertensive effect on the heart.
6.4.3 Effects on the central nervous system
The dominant signs of pyrrolizidine poisoning in horses are
neurological (Rose et al., 1957a,b; McLean, 1970). Similar signs
can also occur in cattle and sheep. It has been claimed that such
signs are probably non-specific secondary effects following primary
liver disease resulting in hyperammonaemia (Rose et al., 1957a).
However, neurological abnormalities in which animals walk in a
straight line until they come to an object, and then stand with
their heads pressed against the object, indicate specific lesions
in the central nervous system. Spongy degeneration of the central
nervous system occurs in cattle, sheep, and pigs (Hooper et al.,
1974; Hooper, 1975a,b).
Trichodesma alkaloids, in particular, appear to be neurotoxic.
There is a considerable body of literature in the USSR on
Trichodesma intoxication of mice, rabbits, and dogs, which has
been reviewed (Ismailov et al., 1970). Mice given Trichodesma
alkaloids subcutaneously at 0.5 mg/kg develop paresis of the hind
limbs within 12 - 17 days. Opisthotonus and clonic convulsions are
also seen. Doses of 10 - 15 mg/kg of alkaloids produces death in
all animals within 2 - 6 h, as the result of respiratory
depression. Higher doses produce immediate death.
6.4.4 Effects on other organs
Right ventricular hypertrophy, secondary to the primary effects
on the pulmonary arteries, and the resultant pulmonary hypertension
in animals treated with PAs or PA-containing plants have been dealt
with in section 6.4.2. Lalich & Merkow (1961) reported myocarditis
consisting of focal oedema and infiltration with a minimal number
of lymphocytes and mononuclear cells in some rats fed Crotalaria
spectabilis seeds mixed with the diet at a concentration of
0.13 - 2 g/kg. Treated groups consisted of 11 - 24 animals each;
there were 12 controls. The changes were seen in all groups of
animals, but the maximum number of rats (10 out of an unspecified
number of the group) showing these changes was in the group that
received 0.5 g/kg diet for 20 - 31 days. Generally, there was a
close correlation with the presence of pulmonary arteritis.
Renal changes have been described by a number of investigators.
Hayashi & Lalich (1967) observed mild to moderate changes in renal
glomeruli consisting of necrosis, capillary thrombosis, and
degenerative changes in the epithelial and mesangial cells,
thickening of interlobular arteries, and arterial thrombosis in
suckling, male Sprague Dawley rats given a single sc dose of
monocrotaline at 120 mg/kg body weight. Renal changes were seen,
to some extent, in all animals surviving for 41 - 47 days.
Carstens & Allen (1970) studied the effects of feeding
Crotalaria spectabilis seed on the rat kidney. Fifty male Sprague
Dawley rats were fed a diet containing ground Crotalaria
spectabilis seed at 0.2 - 0.8 g/kg for 8 months. The seeds were
estimated to contain approximately 3.5 g monocrotaline/kg; 10
animals served as controls. Renal changes were seen in 33/50
PA-treated rats. In 22 rats, over 75% of the glomeruli were
hyalinized and capsules thickened. In the less severely affected
kidneys, the glomerular basement membrane was thickened and
homogeneous deposits were seen in mesangial areas. Afferent
arterioles and interlobular arteries were markedly thickened. In
the most severely affected vessels, the internal elastic lamina was
necrosed and the larger arteries showed fibrinoid necrosis.
Renal tubular megalocytosis was the dominant lesion described
by Hooper (1974) in mice. Nine male white mice, 10 weeks of age,
were fed Senecio jacobaea, which contained a concentration of
alkaloids (jaconine, jacobine, and seneciphylline) of 2.7 g/kg and
a concentration of N-oxide of 0.9 g/kg, mixed with the diet. The
S. jacobaea was given at 100 g/kg diet for 9 weeks, before being
raised to 200 g/kg diet. Five animals served as controls. The
animals were killed from 63 to 193 days after the start of the
study. All treated animals, except 2 killed on day 63, showed
changes. The large cells occurred in both the proximal tubules and
the loop of Henle. Similar cells were seen in the alveolar and
bronchiolar epithelium. No glomerular lesions were described. The
author mentioned having seen the above changes in rats given
repeated sublethal injections of fulvine and spectabiline. On the
other hand, Kurozumi et al. (1983) observed glomerular lesions in
rats given a single injection of monocrotaline.
A variety of renal lesions has been observed in pigs, a common
pathological feature being renal megalocytosis, which was observed
in pigs poisoned by at least 4 different plant genera containing a
variety of toxic alkaloids (Harding et al., 1964; Peckham et al.,
1974) and has also been observed in wild pigs grazing in areas rich
in PA-containing plants in northern Australia (Hooper, 1978).
McGrath et al. (1975) described glomerular lesions in pigs given
Crotalaria spectabilis seed daily for 43 days. Severe renal
lesions comprising tubular dilatation, megalocytosis, and necrosis
of tubular epithelial cells with casts in the lumen, interstitial
and periglomerular fibrosis, and glomerular hyalinization were
reported by Hooper & Scanlan (1977) in pigs fed Crotalaria retusa
seeds containing monocrotaline. Renal megalocytosis has also been
reported in C. retusa poisoning in horses, sheep, and mice poisoned
by S. jacobaea but not by H. europaeum, and in vervet monkeys with
chronic retrorsine poisoning (Van der Watt et al., 1972).
Lesions have been reported in the stomach and intestines in
field and experimental animals after poisoning with pyrrolizidine
alkaloids, but are difficult to identify as specific PA injury.
Hooper (1975c) conducted studies on sheep, rats, and mice. In the
study on sheep, 12 male cross-bred lambs, 7 - 8 weeks of age, were
newly weaned on to a standard commercial calf grower diet.
Lasiocarpine was administered at the rate of 15 - 20 mg/kg body
weight every 2 - 4 days. Each animal was killed when in terminal
coma. Survival time ranged from 4 to 17 days. In the rat study,
young Wistar-Furth rats (sex not stated) weighing 150 - 200 g were
used. In one group of 11 rats, each animal received an ip
injection of lasiocarpine at the rate of 40 mg/kg body weight.
Three animals received isotonic saline. Animals were killed or
died 2 - 6 days after the injection. A second group of 13 rats
received a dose of 35 mg lasiocarpine/kg body weight; 4 control
animals received saline. All rats received a second injection
48 h later. They were killed 3 - 60 days after the second
administration of lasiocarpine. In the mouse study, 3 mature male
white mice received 6 injections each of lasiocarpine at the rate
of 45 mg/kg body weight followed by 4 injections of 90 mg/kg body
weight at 48-h intervals. There was one control animal.
All animals showed characteristic hepatic lesions. Sheep also
showed severe oedema, haemorrhage, and epithelial necrosis in the
gall bladder; lesions were also found in the central nervous system
and occasionally in the kidney. All animals showed severe
intestinal atrophy. There was inhibition of crypt cell mitosis
leading to mitotic irregularities, abnormal large cells and
syncitial cells, especially in the duodenum of sheep, and severe
villous atrophy with ulceration. Lesions in the intestines were
similar to those caused by radiation and radiomimetic agents. It
was suggested that the local intestinal radiomimetic effect was due
to local exposure to the pyrrole metabolite of lasiocarpine after
excretion through the bile duct. It was proposed that a more
conspicuous and rapid development of duodenal megalocytosis was due
to very rapid turnover of cells in the duodenum.
Other probably secondary effects included haemolysis in sheep
in association with advanced liver disease and high liver-copper
levels (Bull et al., 1956), anaemias and disturbance in iron
metabolism and haematopoiesis (Schoental & Magee, 1959; Schoental,
1963; Peckham et al., 1974; Hooper & Scanlan, 1977) (section
6.4.11), pancreatic oedema and fibrosis (Bras & Hill, 1956;
Schoental & Magee, 1959), cerebral oedema, haemorrhage, and
congestion in the rat brain (Davidson, 1935; Rosenfield & Beath,
1945).
Tumours in the different organs have been dealt with separately
under carcinogenesis (section 6.4.8).
6.4.5 Teratogenicity
The teratogenic potential of PAs was demonstrated by Green &
Christie (1961) who produced a variety of dose-related fetal
abnormalities in the rat, with a single intraperitoneal injection
of heliotrine administered during the second week of gestation.
The dosages ranged from 15 to 300 mg/kg maternal body weight.
Litters exposed to a dose of less than 50 mg did not show any
abnormalities, but abnormalities were observed in litters exposed
to higher doses, and increased in frequency and severity with
increasing dose. The abnormalities included retardation of
development, musculoskeletal defects, especially hypoplasia of the
lower jaw, cleft palate, and other abnormalities. Doses above 200 mg
resulted in the intrauterine death or resorption of many fetuses.
Similar studies were performed by Peterson & Jago (1980) who
compared the effects of heliotrine with its metabolic pyrrole
derivative dehydroheliotridine (DHH), when administered in a single
ip injection to rats on the 14th day of gestation. Heliotrine was
administered at 200 mg/kg body weight and DHH at 30 - 90 mg/kg, 14
days after conception. Effects on embryos, evaluated on the 20th
day, showed that both heliotrine and DHH retarded growth and were
teratogenic, but that the effects of a 40 mg/kg dose of DHH were
equivalent to those of 200 mg/kg heliotrine, i.e., the metabolite
was 2.5 times as effective on a molar basis. DHH produced a number
of skeletal abnormalities including retarded ossification,
distorted ribs, long bones, cleft palate, and feet defects. At
higher doses, growth almost ceased in many tissues and the fetuses
were very immature. However, the embryonic liver parenchyma did
not show the antimitotic effects of DHH.
The teratogenic properties of heliotrine were also demonstrated
in Drosophila larvae fed low levels of the alkaloid (Brink, 1982).
6.4.6 Fetotoxicity
The subject of fetotoxicity has been reviewed by Mattocks
(1986). Sundareson (1942) demonstrated the ability of
pyrrolizidine alkaloids to cross the rat placenta. Twice weekly
injections of the PA, starting at, or after, the 12th day of
gestation, resulted in premature delivery of some litters and many
were born dead. The same author showed that the alkaloids
themselves and not just the pyrroles formed in the dams' livers,
could pass the placental barrier by injecting senecionine into
19-day-old rat fetuses in utero, which produced the characteristic
toxic lesions in the dams. The fetuses were also found to be more
resistant to the lethal effects of the PA than the mother rats.
When 4 fetuses were each administered 1.25 mg of PA, representing
about 200 - 400 mg/kg body weight, which is much higher than the
LD50 for an adult rat, 3 of them were still alive after 2 days.
Green & Christie (1961) did not find any liver damage in fetuses
from pregnant rats given teratogenic doses of heliotrine. Only
mild liver damage was found in the embryo rats whose mothers had
been injected with PAs (heliotrine, lasiocarpine, retrorsine, or
monocrotaline) (Bhattacharya, 1965). In contrast, Schoental (1959)
demonstrated that lasiocarpine and retrorsine, when administered to
lactating rats produced little effect on the mothers, but produced
acute liver lesions in the suckling infants. The lesions were most
severe in 3- to 7-week-old animals. It was suggested that the
infants were affected by the milk from the lactating mothers, which
possibly contained the metabolic products of the PAs.
It would seem from the above studies that the embryo is
relatively more resistant to the toxic effects of PAs in utero than
it is after birth. Mattocks & White (1973) postulated that this
could be due to the low capacity for the metabolic activation of
PAs of the embryo liver, as they had shown that the ability of
liver enzymes to convert retrorsine to toxic metabolites was low in
rats, immediately after birth, but picked up rapidly afterwards.
The susceptibilities of rats of various ages to the hepatotoxic
effects of the PAs was proportional to their capacity to form and
retain the pyrrolic metabolites. Twenty-day-old rats were found to
be more sensitive than older animals.
The effects of fulvine administration on pregnant rats between
9 and 12 days of gestation were studied by Persaud & Hoyte (1974).
Dose-related fetal resorptions were observed, but no hepatic
lesions were seen in the fetuses. On the other hand, Newberne
(1968) observed damage, in both the maternal and fetal livers, when
lasiocarpine was administered to pregnant rats. Acute liver
necrosis was observed in the livers of mothers as well as fetuses
in animals that had received 100 mg lasiocarpine/kg body weight on
day 13 of gestation. However, in animals that received 2 doses of
35 mg/kg body weight on days 13 and 17 of pregnancy, liver necrosis
was seen in the fetal liver but not in that of the mother. It is
not known why lasiocarpine acts differently from other alkaloids
and has a greater effect on the fetal liver. Mattocks (1986) has
postulated the possibility that fetotoxicity was caused chiefly by
toxic metabolites formed in the maternal liver, and that a greater
proportion of such metabolites reached the fetus from lasiocarpine
than from other PAs.
6.4.7 Mutagenicity
A number of PAs that have been shown to be powerful dose
dependent mutagens in Drosophila melanogaster have been listed by
Mattocks (1986). All the compounds are hepatotoxic though the
degree of mutagenicity is not necessarily proportional. Table 12
provides a summary of the mutagenicity tests on different PAs,
related compounds and plant extracts. Clark (1959) demonstrated
the mutagenic effect of heliotrine in Drosophila, in which a
considerable increase in sex-linked recessive lethals was produced,
apparently by interfering with the maturation of germ cells, so
that as soon as the available spermatozoa were used, the males were
no longer capable of breeding. The cell damage was irreversible.
The mutagenic effect of feeding Drosophila males for 24 h with a
medium containing 10-3 mol monocrotaline was comparable to about
1000 R of X rays (Clark, 1976). The Basc test with Drosophila
melanogaster is considered a highly sensitive mutagenicity test
for PAs (Candrian et al., 1984a).
Seneciphylline and senkirkine, known to occur in animal feeds
and medicinal herbs, respectively, were tested for their ability to
produce sex-linked recessive lethals in males of Drosophila
melanogaster using the Basc (3-day feeding method) by Candrian et
al. (1984a). Seneciphylline was found to be mutagenic at
concentrations of 10-5, 10-4, and 10-3 mol, which produced 3.8%
(983 chromosomes tested), 9% (708 chromosomes tested), and 15.3%
(327 chromosomes tested) sex-linked recessive lethals,
respectively. Senkirkine (10-5 mol) was found to produce 4.4% sex-
linked recessive lethals (2541 chromosomes tested) against 0.17%
maximum sensitivity in the late spermatid stage of spermatogenesis
indicating that PAs act as indirect mutagens. Flies fed with milk
from lactating rats given an oral dose of 25 mg seneciphylline/kg
showed 1.2% sex-linked recessive lethals (1477 chromosomes tested)
compared with 0.3% (1533 chromosomes tested) in controls.
Table 12. Mutagenicity tests on pyrrolizidine alkaloids,
related compounds, and source plants
---------------------------------------------------------
Compound or material Type of testa Responseb
---------------------------------------------------------
Clivorine A +
HPC +
Echimidine D +
Echinatine D +
Fulvine D +
Heliotrine D +
P +
F +
CC +
A +
B +
TM +
CM/CC +
Integerrimine D +
Jacobine D ±
P +
Lasiocarpine D +
P +
F +
A +
HPC +
CM/CC +
TM +
Ligularidine A +
Lindelofine A 0
Lycopsamine A 0
Monocrotaline D +
P +
CC +
B +
A 0
HPC +
CT +
Petasitenine (fukinotoxin) A +
HPC +
CM +
---------------------------------------------------------
Table 12. (contd.)
---------------------------------------------------------
Compound or material Type of testa Responseb
---------------------------------------------------------
Platyphylline D 0
Retrorsine A +
D +
CT +
Rosmarinine CT 0
Senecionine D +
A 0
Seneciphylline A 0
P +
D +
Senkirkine A +
HPC +
CM/CC +
D +
Supinine D ±
P +
Mixed alkaloids from
Senecio jacobaea A 0
Senecio numorensis
spp. fuchsii (extract) CM +
A 0
A +
Senecio jacobaea (extract) A +
Senecio longilobus (extract) A 0
Symphytum officinale
(comfrey extract) A 0
Retronecine bis-p-chloro- P +
benzoate
Synthanecine A bis-N-ethyl- CT +
carbamate
Retronecine A 0
Heliotridine D 0
Viridofloric acid A 0
---------------------------------------------------------
Table 12. (contd.)
---------------------------------------------------------
Compound or material Type of testa Responseb
---------------------------------------------------------
Heliotric (heliotrinic) acid D ±
HPC +
Dehydroretronecine CT +
A ±
SCE +
Dehydroheliotridine CM +
Pyrrole HPC 0
2,3-Bishydroxymethyl-1- CT +
methylpyrrole A ±
SCE +
2-Hydroxylmethyl-1-methyl- A 0
pyrrole SCE ±
3-Hydroxylmethyl-1-methyl- A +
pyrrole SCE ±
---------------------------------------------------------
a A = Salmonella ("Ames") test.
B = Other bacterial tests.
CC = Clastogenic activity in cultured cells.
CM = Mutagenicity in cultered mammalian cells.
CT = Cell transformation test.
D = Mutagenicity in Drosophila.
F = Tests in fungus (Aspergillus nidulans).
HPC = Hepatocyte primary culture/DNA repair test.
P = Chromosomal aberrations in plant cells.
SCE = Sister chromatid exchange.
TM = Transplacental micronucleus test.
b + = active.
± = marginally active.
0 = inactive.
Mutagenic properties of 7 PAs extracted from plants to
Salmonella typhimurium TA100 have been demonstrated by a modified
Ames method by Yamanaka et al. (1979). The PAs were clivorine,
fukinotoxin, heliotrine, lasiocarpine, ligularidine, LXC201, and
senkirkine. Pre-incubation of these alkaloids with liver S9 mix
and bacteria in liquid medium was essential for demonstration of
the property. PAs in the heliotridine and otonecine family were
mutagens, while retronecine bases were inactive. Monocrotaline and
heliotrine were not active mutagens to Escherichia coli WP2, even
though they were quite cytotoxic (Green & Muriel, 1975). They were
active in repair deficient strains. Retrorsine was active in
inducing mutations on the Ames Salmonella/microsome assay (Wehner
et al., 1979). Extracts from medicinal plants and noxious weeds
were mutagenic towards Salmonella in the Ames assay (Pool, 1982;
White et al., 1983; Koletsky et al., 1978).
From the limited data available, it seems that the carcinogenic
activity of individual alkaloids parallels their mutagenic
behaviour, but not their relative hepatotoxicities (Culvenor &
Jago, 1979).
6.4.7.1 Chromosome damage
Pyrrolizidine alkaloids have been shown to be capable of
damaging chromosomes in plants, fungi, bacteria, tissue cell
cultures, and the fruit fly (Drosophila melanogaster). Literature
on this topic has been reviewed by Bull et al. (1968), McLean
(1970), and Mattocks (1986).
Several PAs are known for their ability to damage the
chromosomes of growing plant cells (Mattocks, 1986). Similar
properties have been demonstrated in leukocyte cultures from the
marsupial (Potorus tridactylus) (Bick & Jackson, 1968; Bick, 1970).
Bick & Culvenor (1971) found dehydroheliotridine, a metabolite of
heliotrine, to be 10 times more active than the alkaloid.
Infusions of Symphytum officinale L., described in Polish
pharmacopoeia as Radix symphyti, are recommended as expectorants,
especially for children. Furmanowa et al. (1983) demonstrated the
mutagenic effects of an alkaloidal fraction and infusion in this
plant in the meristematic cells of the lateral roots of Vicia faba
L. var minor. Lasiocarpine, a proven carcinogen, served as a
positive control.
Chromosome damage by PAs in the hamster lung cell line was
demonstrated by Takanashi et al. (1980). Stoyel & Clark (1980)
used the transplacental micronucleus test in pregnant female mice
and showed the chromosome damaging properties of heliotrine (225 mg/kg
body weight) and lasiocarpine (86 mg/kg) within 20 h of the injection.
The genotoxicity of heliotrine, monocrotaline, seneciphylline,
and senkirkine was studied by Bruggeman & Van der Hoeven (1985)
using the sister-chromatid exchange (SCE) assay in V79 Chinese
hamster cells co-cultured with primary chick embryo hepatocytes.
Exposure to these PAs resulted in the high induction of SCEs, a
more than 5-fold increase in the SCE rate with 2.5 mg
heliotrine/litre, 4-fold with monocrotaline at 5 mg/litre, 8-fold
with seneciphyline at 1.2 mg/litre, and more than 5-fold response
with senkirkine at 2.5 mg/litre. For all compounds, a dose-
response relationship was observed at concentrations that did not
seriously affect survival. PAs are also known to induce DNA repair
in rodent hepatocytes (Green et al., 1981; Mori et al., 1985). DNA
repair synthesis was elicited by 15 alkaloids, including 11 of
unknown carcinogenic potential (Mori et al., 1985).
There are also a few reports of chromosome damage by PAs in
man. Martin et al. (1972) found chromosome damage in the blood
cells of children with veno-occlusive disease, probably caused by
fulvine. It has also been shown by Ord et al. (1985) that
dehydroretronecine, is able to induce SCE in human lymphocytes.
Kraus et al. (1985) studied the PAs senkirkine and tussilagine,
which occur in a medicinal plant Tussilago farfara, for their
ability to induce chromosome damage in human lymphocytes in vitro.
They were not found to enhance the number of chromosome aberrations
up to concentrations of 1000 µmol. However, heliotrine, used for
comparison, induced chromosomal aberrations at concentrations of
100 µmol. In addition, heliotrine was also found to be capable of
damaging unstimulated eg Go-phase lymphocytes.
6.4.8 Carcinogenesis
Carcinogenesis has been reviewed by McLean (1970), IARC (1976,
1983), and Mattocks (1986). A number of purified PAs, purified or
crude extracts of plants containing them in a mixture or the actual
plant, dried and milled, and several PA metabolites or synthetic
analogue compounds have been tested for carcinogenecity. However,
these include only relatively few of the known cytotoxic PAs. Data
relating to some of the representative studies on rats are
summarized in Table 13. Studies on liver tumours found in rats
given PAs and plant materials are summarized in Table 14. All
experimental animal studies, with the exception of one on chickens
(Campbell, 1956) and one on Syrian golden hamsters (Fushimi et al.,
1978), have been carried out on rats.
The liver is the most common organ involved in experimental
studies. Tumours produced are mostly of epithelial origin, but a
significant number are also vascular. Lack of precision and
diversity of terms used to describe similar or identical tumours
makes it difficult to compare the types of carcinogenic effect in
different studies. Some terms have been used interchangeably,
e.g., hepatomas, hepatocellular carcinomas, haemangiogenic and
cholangiogenic tumours; nodular hyperplasia, pre-neoplasma,
neoplastic nodules, and hepatocellular tumours. In most studies,
there are no supporting photomicrographs to draw any inference as
to whether the tumours were malignant. Difficulties in the
interpretation of data have been commented on by Schoental et al.
(1954) and McLean (1970).
Lasiocarpine has produced the largest yield of tumours. In the
studies of Svoboda & Reddy (1972), 16/18 animals surviving for more
than 56 weeks after receiving ip multidoses of lasiocarpine
developed malignant tumours of the liver. Of these, 10 animals had
more than one tumour. Continuous feeding of rats on a regimen
containing lasiocarpine resulted in all animals (24/24) developing
tumours (NCI, 1978). In one study, a single oral administration of
retrorsine (Schoental & Bensted, 1963) to weanling rats resulted in
7 of the 29 animals that survived for more than one year developing
11 tumours of a wide variety, at least of 5 which were malignant.
It is of note that this PA is known to have caused two cases of
human toxicity together with riddelline in two cases, though the
total intake was proportionately lower (Stillman et al., 1977; Fox
et al., 1978; Huxtable, 1980) (Table 15).
Tumours produced covered a very wide range in unrelated tissues
and organs, for example, the pancreas, urinary bladder, pituitary,
bone, retro-peritoneal tissues, and skin, among others.
Hepatocellular carcinoma and haemangiosarcoma were the most common.
Crude extracts of plants or whole plants have also been
demonstrated to produce a variety of tumours. For example, Senecio
longilobus has been shown to produce tumours in a significantly
large proportion of experimental animals (Harris & Chen, 1970).
Hirono et al. (1973, 1976, 1978, 1979b) demonstrated the
carcinogenic properties of a number of plants, used as food in
Japan, in the rat.
A summary of the relevant experimental data follows.
6.4.8.1 Purified alkaloids
Kuhara et al. (1980) administered clivorine in the drinking-
water at a concentration of 0.05 g/litre to 12 rats of both sexes,
continuously for 340 days followed by plain water. There were 20
control rats. All the treated rats survived 440 days. Eight of
the 12 animals developed liver tumours including 2
haemangioendothelial sarcomas and 6 that were described as
"neoplastic nodules". No liver tumours were seen in the control
animals (Table 13).
Schoental (1975) tested the carcinogenic properties of
heliotrine, with and without prior administration of nicotinamide.
Nicotinamide protects against liver necrosis and so may enhance
tumour yeild, as shown by Rakietin et al. (1971), who studied
pancreatic tumours in rats given streptozootocin.
Heliotrine was administered intragastrically to 4 groups of 26
male weanling rats in 1 or 2 doses of 230 mg/kg and 300 mg/kg body
weight; 2 groups also received nicotinamide at 350 - 500 mg/kg body
weight, administered ip 10 - 15 min before, and 2.5 h after
administration of heliotrine, as per the dosing regimen shown in
Table 13. There were 8 controls. All animals administered the
higher dose of heliotrine (300 mg/kg body weight) died within 5
months of the PA administration. The livers showed lesions
characteristic of PA toxicity. No tumours were seen. Only 1 out
of 4 animals receiving heliotrine alone, survived 27 months and it
showed an islet cell adenoma as well as adenoma of pituitary, but
so did 3 of the 8 controls. In the group receiving 230 mg
heliotrine/kg and also treated with nicotinamide, 4 animals died
within 5.5 months (one with a fibrosarcoma and the other in a
moribund condition), chiefly from toxic liver disease, and two more
had to be killed. Of the 6 animals surviving more than 22 months
after heliotrine treatment, islet cell adenoma was seen in 3
together with other tumours as shown in the table. This tumour is
stated to be extremely rare in the animal strain used. Hepatoma
was seen in only one animal. The role of nicotinamide in this
study is not clear.
Table 13. Summary of data on the carcinogenic action of PAs and PA-containing plants in rats (in chronological order)
Material Strain, Route of Other Duration Period of Tumours Tumour Reference
tested number, administration treatment of observation produced incidence
and sex of and dosing treatment in
animals regimen surviving
animals
Alkaloids Wistar rat drinking-water 1 week until death only nodular Schoental
of Senecio (13 males, (0.05 g/litre) (males) hyperplasia et al. (1954)
jacobaea 12 females) 2 weeks of liver
(females);
followed by gap of
7 weeks
9 males, 0.03 g/litre, until
1 female 3 days/week death
(surviving)
Retrorsine 10 males, 0.03 g/litre, until hepatomas 4/14
4 females 3 days/week death
Isatidine 8 males, 0.05 g/litre
14 females followed by
0.03 g/litre, 20 months until death hepatomas 10/22
3 days/week
3 males, as above choline until hepatomas 3/7
4 females (0.5% in death
drinking-
water), 4
days/week
Table 13. (contd.)
Material Strain, Route of Other Duration Period of Tumours Tumour Reference
tested number, administration treatment of observation produced incidence
and sex of and dosing treatment in
animals regimen surviving
animals
Isatidine 2 males, single ip Schoental
(contd.) 3 females injection of et al. (1954)
2 mg in 0.2 ml
tricaprilyn
followed by
skin application 15 months 15 months hepatoma (?) 1/5
tion of 0.5%
solution, 3
days/week
controls not mentioned
(7 males,
7 females)
Retrorsine Porton single 400 r until death hepatomas 5/25 Schoental &
Wistar intragastric radiation Bensted (1963)
weanling dose of 30 mg/kg to 31/50 hepatocellular 1/25
rat body weight surviving
(50 males) for 100 carcinoma
days; 4/13 with
had head metastases
shielded
mammary 2/25
tumours
Table 13. (contd.)
Material Strain, Route of Other Duration Period of Tumours Tumour Reference
tested number, administration treatment of observation produced incidence
and sex of and dosing treatment in
animals regimen surviving
animals
Retrorsine lung carcinoma 1/25
(contd.)
renal 2/25
carcinomas
colonic 1/25
carcinomas
Porton as above as above splenic 1/25 Schoental &
Wistar rat haemangio- Bensted (1963)
(50 males) endothelioma
osteosarcoma 1/25
bone
leukaemia 1/25
"spindle cell" 1/25
tumour (neck)
Table 13. (contd.)
Material Strain, Route of Other Duration Period of Tumours Tumour Reference
tested number, administration treatment of observation produced incidence
and sex of and dosing treatment in
animals regimen surviving
animals
Retrorsine 95 males, single oral dose until death hepatomas 5/29
(contd.) 95 females of 30 mg/kg body
(weanling) weight mammary tumour 1/29
(controls)
lung carcinoma 1/29
splenic 1/29
haemangio-
endothelioma
uterine 1/29
carcinoma
Porton retroperitoneal 1/29 Schoental &
Wistar rat sarcoma Bensted (1963)
(controls)
squamous cell 1/29
carcinoma
(jaw)
Table 13. (contd.)
Material Strain, Route of Other Duration Period of Tumours Tumour Reference
tested number, administration treatment of observation produced incidence
and sex of and dosing treatment in
animals regimen surviving
animals
Retrorsine 6 males no PAs 400 r until death leukaemia 2/6
(contd.) (weanling) radiation
osteosarcoma 1/6
renal adenoma 1/6
10 males single partial until death hepatomas 2/9
(weanling) intragastric hepatectomy
dose (30 mg/kg squamous cell 1/9
body weight) carcinoma
(9 days after (jaw)
partial
hepatectomy)
Table 13. (contd.)
Material Strain, Route of Other Duration Period of Tumours Tumour Reference
tested number, administration treatment of observation produced incidence
and sex of and dosing treatment in
animals regimen surviving
animals
Mixed PAs Randomly single more than 1 pancreatic 1/15 Schoental et al.
from seeds bred from intragastric dose year? islet cell (1970)
of Porton at 500-1500 mg/kg adenomaa
Amsinckia Wistar body weight
intermedia weanling "papillary 1/15
(intermedine rat tumour"a of
medine and (15 males) urinary
lycopsamine) bladder
pituitary 1/15
adenomaa
as above as above pancreatic 1/15 Schoental et al.
islet cell (1970)
adenocarcinoma
exocrine 1/15
pancreatic
adenoma
Leaves and 2 males 10% mixed with 1 month until death pancreatic 1/2
stems of (weanling) diet (period not islet cell
Heliotropium stated) adenoma
supinum L.
Table 13. (contd.)
Material Strain, Route of Other Duration Period of Tumours Tumour Reference
tested number, administration treatment of observation produced incidence
and sex of and dosing treatment in
animals regimen surviving
animals
Heliotropium 6 males single until death pancreatic 1/6
supinum L. intragastric dose (longer islet cell
(contd.) at 200-300 mg/kg than 1 adenoma
body weight of year)
crude alkaloidal
fraction
controls not stated
Senecio Harlan rat 0.75% of diet until death none Harris & Chen
longilobus 50 males, within 131 (1970)
50 females days
50 males, 0.5% of diet until death none Harris & Chen
50 females within 200 (1970)
days
Table 13. (contd.)
Material Strain, Route of Other Duration Period of Tumours Tumour Reference
tested number, administration treatment of observation produced incidence
and sex of and dosing treatment in
animals regimen surviving
animals
Senecio 40 males, 0.5% of diet for 1 year 428-657 liver cell 4/23
longilobus 40 females 1 month days carcinomas
(contd.) alternating with
normal diet for peritoneal 1/23
2 weeks mesothelioma
50 males, 0.5% of diet 54 weeks 217-470 liver cell 16/47
50 females for 1 week days carcinomas
alternating with
normal diet for angiosarcoma 1/47
1 week
controls not stated
(10 males,
10 females)
Lasiocarpine Fischer rat intraperitoneal till 60-76 hepatocellular 10/18 Svoboda & Reddy
(25 males) injection at moribund weeks carcinomas (1972)
7.8 mg/kg body or had
weight, twice palpable
weekly for 4 tumours cholangio- 1/18
weeks, then carcinoma
once a week
for an additional lung adenomas 5/18
52 weeks
Table 13. (contd.)
Material Strain, Route of Other Duration Period of Tumours Tumour Reference
tested number, administration treatment of observation produced incidence
and sex of and dosing treatment in
animals regimen surviving
animals
Lasiocarpine skin squamous 6/18
(contd.) cell
carcinomas
Fischer rat as above ileal 2/18 Svoboda & Reddy
(25 males) adenocarcinoma (1972)
ileal 1/18
adenomyoma
testicular 1/18
interstitial
cell tumour
controls lung adenomas 2/25
(25 males)
Table 13. (contd.)
Material Strain, Route of Other Duration Period of Tumours Tumour Reference
tested number, administration treatment of observation produced incidence
and sex of and dosing treatment in
animals regimen surviving
animals
Retronecine, Porton single sc spinal cord 1/10 Schoental &
hydrochlorine Wistar injection of ependymo- Cavanagh (1972)
newborn rat 300-1000 mg/kg blastoma
(6 males, body weight
6 females)
"pituitary 5/10
tumour"
"mammary 1/10
tumour"
Hydroxy- 5 males single ip brain 1/5 Schoental &
senkirkine (weanling) injection of astrocytoma Cavanagh (1972)
100-300 mg/kg
body weight
Table 13. (contd.)
Material Strain, Route of Other Duration Period of Tumours Tumour Reference
tested number, administration treatment of observation produced incidence
and sex of and dosing treatment in
animals regimen surviving
animals
Heliotropium 5 females 5% of diet fed Schwann cell 1/5
ramosissimum to 1 pregnant tumour of
rat during 1st spinal cord
15 days of
pregnancy and
from 10th day
of parturition
until weaning;
female off-
spring (5) fed
on experimental
diet for 10 days
at 6 months of
age
controls not stated
Table 13. (contd.)
Material Strain, Route of Other Duration Period of Tumours Tumour Reference
tested number, administration treatment of observation produced incidence
and sex of and dosing treatment in
animals regimen surviving
animals
Groups
I II
Petasites ACI rat 4% of diet for until 480 days liver cell Hirono et al.
japonicus Group I: 6 months; death or until adenomas 6/27 4/19 (1973)
(young 12 males, subsequently, moribund
flower 15 females 8% of diet liver 3/27 8/19
stalks) alternating haemangio-
weekly with endotheliomas
normal diet
liver cell 2/27 1/19
carcinomas
Group II: 4% of diet
11 males,
8 females
controls normal diet none
7 females/
8 females
Table 13. (contd.)
Material Strain, Route of Other Duration Period of Tumours Tumour Reference
tested number, administration treatment of observation produced incidence
and sex of and dosing treatment in
animals regimen surviving
animals
Mono- Sprague gastric intubation 72 weeks liver cell 10/42 Newberne &
crotaline Dawley rat weekly of 25 mg/kg carcinomas Rogers (1973)
(50 males) body weight for
4 weeks, then
8 mg/kg body
weight for
38 weeks
50 males as above, with liver cell 14/33
a diet deficient carcinomas
in lipotropes
Heliotrine Porton intragastric Schoental (1975)
Wistar
weanling
rat
4 males 300 mg/kg body until death none
weight; repeated (5 months)
after 3 weeks to
2 surviving rats
Table 13. (contd.)
Material Strain, Route of Other Duration Period of Tumours Tumour Reference
tested number, administration treatment of observation produced incidence
and sex of and dosing treatment in
animals regimen surviving
animals
Heliotrine 6 males 300 mg/kg body nicotinamide until death none Schoental (1975)
(contd.) weight; repeated (500 mg/kg (5 months)
after 3 weeks body weight),
ip, before
and after
each heliotrine
administration
4 males 230 mg/kg body 27 months pancreatic 1/1
weight; repeated islet cell
to 2/4 surviving adenoma
after 5 days
pituitary 1/1
adenoma
Table 13. (contd.)
Material Strain, Route of Other Duration Period of Tumours Tumour Reference
tested number, administration treatment of observation produced incidence
and sex of and dosing treatment in
animals regimen surviving
animals
Heliotrine 12 males 230 mg/kg body nicotinamide until death fibrosarcoma 1/8
(contd.) weight; repeated (350 mg/kg or killed of jaw
6.5 days later body weight), when moribund
ip, before, up to 27.5 pancreatic 3/6
plus 2 doses months islet cell
after each adenomasb
heliotrine
administration hepatomab 1/6
urinary 1/6
bladder
papillomab
12 males testicular 1/6
interstitial
cell tumourb
2 males ip injection nicotinamide 19-27.5 pituitary 1/2 Schoental (1975)
at 350 mg/kg months adenoma
body weight
Table 13. (contd.)
Material Strain, Route of Other Duration Period of Tumours Tumour Reference
tested number, administration treatment of observation produced incidence
and sex of and dosing treatment in
animals regimen surviving
animals
Heliotrine controls no treatment 19-27.5 pituitary 3/6
(contd.) (6 males) months adenomas
Tussilago ACI rat 32% in diet for 600 days 600 days haemangio- 8/12 Hirono et al.
farfara (6 males, 4 days, then 16% endothelioma (1976)
(coltsfoot) 6 females) until end of
(preblooming (1.5 months study liver cell 1/12
flowers) old) adenomac
hepatocellular 1/12
carcinomac
bladder 1/12
papillomac
Table 13. (contd.)
Material Strain, Route of Other Duration Period of Tumours Tumour Reference
tested number, administration treatment of observation produced incidence
and sex of and dosing treatment in
animals regimen surviving
animals
Tussilago 5 males, 8% in diet 600 days 600 days liver 1/9
farfara 5 females haemangio-
(contd.) endothelioma
6 males, 4% in diet 600 days 600 days none Hirono et al.
5 females (1976)
controls none 600 days 600 days none
(8 males,
8 females)
Dehydro- Sprague sc injection partial 12 months 10 months rhabdomyo- 36/60 Allen et al.
retronecine Dawley rat bi-weekly for 4 hepatectomy sarcomas (5 (1975)
Group I: months at on 15 with
75 males 20 mg/kg body animals metastases)
weight, followed after 4
by 10 mg/kg body months
weight for 8
months
Table 13. (contd.)
Material Strain, Route of Other Duration Period of Tumours Tumour Reference
tested number, administration treatment of observation produced incidence
and sex of and dosing treatment in
animals regimen surviving
animals
Monocrotaline Group II: sc injection partial 12 months 10 months rhabdomyo- 2/60
75 males bi-weekly at hepatectomy sarcomas
5 mg/kg body on 15
weight for 12 animals hepatocellular 2/60
months after 4 carcinomas
months
acute myeloid 2/60
leukaemia
pulmonary 2/60
adenomas
controls sc injection partial 12 months 10 months none mentioned Allen et al.
(50 males) bi-weekly, hepatectomy (1975)
0.1 mol on 5 animals
phosphate after 4
buffer, pH7 months
Table 13. (contd.)
Material Strain, Route of Other Duration Period of Tumours Tumour Reference
tested number, administration treatment of observation produced incidence
and sex of and dosing treatment in
animals regimen surviving
animals
Monocrotaline Sprague sc injection on 12 months any tumours 17/60 Shumaker et al.
(contd.) Dawley rat alternate weeks (several (1976)
(60 males) (5 mg/kg body animals had
weight) more than 1
tumour)
pulmonary 11/60
carcinomas
hepatocellular 5/60
carcinomas
acute myeloid 3/60
leukaemia
rhabdomyo- 4/60
sarcomas
adrenal 8/60
adenomas
kidney adenoma 1/60
Table 13. (contd.)
Material Strain, Route of Other Duration Period of Tumours Tumour Reference
tested number, administration treatment of observation produced incidence
and sex of and dosing treatment in
animals regimen surviving
animals
Dehydro- 60 males sc injection on 12 months 12 months rhabdomyo- 39/60
retronecine alternate weeks until sarcomas at
at 20 mg/kg body moribund site of
weight followed injection
by 10 mg/kg
body weight,
alternate weeks
for 8 months
controls adrenal 2/45
(45 males) adenomas
(same group
as above)
Mono- Sprague single sc pancreatic 16/23 Hayashi et al.
crotaline Dawley rat injection of insulinomas (1977)
(80 males) 40 mg/kg body
weight
Petasitenine ACI rat drinking-water 45 days 72 days none Hirono et al.
(3 males) (0.05% solution) (1977)
(1 month
old)
Table 13. (contd.)
Material Strain, Route of Other Duration Period of Tumours Tumour Reference
tested number, administration treatment of observation produced incidence
and sex of and dosing treatment in
animals regimen surviving
animals
Petasitenine 5 males, 0.01% solution until up to 16 haemangio- 5/10d
(contd.) 6 females death months endothelial
or sarcomas
moribund
liver adenomas 5/10d
controls none fibrosarcoma 1/10 Hirono et al.
(10 males, (subcutaneous) (1977)
9 females)
Lasiocarpine Fischer rat mixed with 55 weeks 59 weeks liver 9/20 Rao & Reddy
(20 males) feed at a angiosarcomas (1978)
concentration of
50 mg/kg hepatocellular 7/20
carcinomas
malignant 1/20e
adnexal tumour
of skin
malignant 1/20e
lymphoma
Table 13. (contd.)
Material Strain, Route of Other Duration Period of Tumours Tumour Reference
tested number, administration treatment of observation produced incidence
and sex of and dosing treatment in
animals regimen surviving
animals
Lasiocarpine controls none
(contd.) (10 males)
Petasites ddN mice 4% of diet as 480 days 480 days lung adenomas 24/39 Fushimi et al.
japonicus 24 males dried flower (1978)
(flower 21 females stalks lung 6/39
stalks) adenocarcinoma
liver 4/39
reticullum
cell sarcoma
liver 1/39
haemangio-
endothelial
sarcoma
thymoma 1/39
leukemia 2/39
Table 13. (contd.)
Material Strain, Route of Other Duration Period of Tumours Tumour Reference
tested number, administration treatment of observation produced incidence
and sex of and dosing treatment in
animals regimen surviving
animals
Petasites control nil 480 days 480 days lung 1g
japonicus 23 males kidney
(contd.) 27 females haemangio-
endothelial
sarcoma 1g
spleen
haemangioma 1g
Swiss 4% diet as 480 days 480 days lung 5/26 Fushimi et al.
strain mice dried flower adenoma (1978)
20 males stalks
20 females leukemia 1/26
controls nil 480 days 480 days liver 1g
23 males haemangio-
20 females endothelioma
breast 1g
carcinoma
Table 13. (contd.)
Material Strain, Route of Other Duration Period of Tumours Tumour Reference
tested number, administration treatment of observation produced incidence
and sex of and dosing treatment in
animals regimen surviving
animals
Petasites lung adenoma 3g
japonicus
(contd.)
leukemia 2g
C57BL/6 4% diet as 480 days 480 days no tumours
mice dried flower
20 males stalks
20 females
controls none 480 days 480 days lung adenoma 1g
20 males
20 females
Syrian 4% diet as 480 days 480 days adrenal 1/25 Fushimi et al.
golden flower stalks cortical (1978)
hamsters adenoma &
13 males breast
17 females carcinoma
Table 13. (contd.)
Material Strain, Route of Other Duration Period of Tumours Tumour Reference
tested number, administration treatment of observation produced incidence
and sex of and dosing treatment in
animals regimen surviving
animals
Petasites controls none 480 days 480 days no tumours
japonicus 12 males
(contd.) 9 females
Symphytum ACI rat 33% of diet as 480 days until death liver adenomas 5/19 Hirono et al.
officinale (1 - 1.5 leaves or moribund (1979b)
(leaves or months old)
root) urinary 1/19
Group I.1: bladder
11 males, papilloma
8 females
urinary 2/19
bladder
carcinomas
(rats with 5/19
tumours)
Table 13. (contd.)
Material Strain, Route of Other Duration Period of Tumours Tumour Reference
tested number, administration treatment of observation produced incidence
and sex of and dosing treatment in
animals regimen surviving
animals
Symphytum Group I.2: 33% of diet as 600 days until death liver adenomas 11/19
officinale 10 males, leaves or moribund
(contd.) 10 females
urinary 2/19
bladder
papillomas
(rats with 11/19
tumours)
Group II: 16% of diet as 600 days until death liver adenomas 7/21 Hirono et al.
11 males, leaves or moribund (1979b)
10 females
haemangio- 1/21
endothelial
sarcoma
Table 13. (contd.)
Material Strain, Route of Other Duration Period of Tumours Tumour Reference
tested number, administration treatment of observation produced incidence
and sex of and dosing treatment in
animals regimen surviving
animals
Symphytum urinary 2/21
officinale bladder
(contd.) papillomas
urinary 1/21
bladder
carcinoma
lymphatic 1/21
leukaemia
colonic 1/21
adenoma
pituitary 1/21
adenoma
(rats with 7/21
tumours)
Table 13. (contd.)
Material Strain, Route of Other Duration Period of Tumours Tumour Reference
tested number, administration treatment of observation produced incidence
and sex of and dosing treatment in
animals regimen surviving
animals
Symphytum Group III: 8% of diet as 600 days until death liver adenoma 1/25 Hirono et al.
officinale 14 males, leaves or moribund (1979b)
(contd.) 14 females
Group IV: 8% of diet as until liver adenomas 19/22
12 males, root death
12 females
urinary 2/19
bladder
papilloma
(rats with 19/22
tumours)
Group V: 4% of diet as until until death liver adenomas 16/42
24 males, root reduced by death or moribund
24 females stages to basal
diet after 180 urinary 1/42
days bladder
carcinoma
Table 13. (contd.)
Material Strain, Route of Other Duration Period of Tumours Tumour Reference
tested number, administration treatment of observation produced incidence
and sex of and dosing treatment in
animals regimen surviving
animals
Symphytum adrenal 2/42
officinale cortical
(contd.) adenomas
(rats with 16/42
tumours)
Group VI: 2% of diet as 280 days liver adenomas 10/23
12 males, root for 190
12 females days reduced by
stages to basal
diet
Group VII: 1% of diet as until liver adenomas 16/24 Hirono et al.
15 males, root for 275 death (1979b)
15 females days and
subsequently a liver 4/24
basal diet haemangio-
alternating sarcomas
with 0.5% at
3-week intervals cholangio- 1/24
carcinoma
Table 13. (contd.)
Material Strain, Route of Other Duration Period of Tumours Tumour Reference
tested number, administration treatment of observation produced incidence
and sex of and dosing treatment in
animals regimen surviving
animals
Symphytum adrenal 1/24
officinale cortical
(contd.) adenomas
(rats with 17/24
tumours)
Group VIII: 0.5% of diet as entire liver adenomas 8/30
15 males, root study
15 females
liver 9/30
haemangio-
sarcomas
pituitary 1/30
adenoma
uterine 1/30
adrenocarcinoma
Table 13. (contd.)
Material Strain, Route of Other Duration Period of Tumours Tumour Reference
tested number, administration treatment of observation produced incidence
and sex of and dosing treatment in
animals regimen surviving
animals
Symphytum gonadal 1/30 Hirono et al.
officinale stromal tumour (1979b)
(contd.)
(rats with 10/30
tumours)
controls none till the urinary 1
(65 males, end bladder
65 females) papilloma
subcutaneous 1
fibrosarcoma
caecal adenoma 1
mammary 1
fibroadenoma
retroperitoneal 1
teratoma
Table 13. (contd.)
Material Strain, Route of Other Duration Period of Tumours Tumour Reference
tested number, administration treatment of observation produced incidence
and sex of and dosing treatment in
animals regimen surviving
animals
Senkirkine ACI rat ip injection of 4 weeks, 650 days liver adenomas 9/20 Hirono et al.
(20 males) 22 mg/kg body 52 weeks or till (1979a,b)
weight, twice moribund myeloid 1/20
weekly, then leukaemia
once weekly
testis
interstitial 1/20
tumour
Symphytine 20 males ip injection of 4 weeks, 650 days liver adenoma 1/20
22 mg/kg body 52 weeks or till
weight, twice death or liver 3/20
weekly, then moribund haemangio-
once weekly sarcomas
controls myeloid 1/20
(20 males) leukemia
Table 13. (contd.)
Material Strain, Route of Other Duration Period of Tumours Tumour Reference
tested number, administration treatment of observation produced incidence
and sex of and dosing treatment in
animals regimen surviving
animals
Symphytine fibroma-soft 1/20
(contd.) tissues
testis- 1/20
interstitial
tumour
Clivorine ACI rat drinking-water 340 days 480 days liver 2/12 Kuhara et al.
(6 males, (0.005%) haemangio- (1980)
6 females) sarcomas
ACI rat drinking-water hepatic 6/12
(6 males, (0.005%) neoplastic
6 females) nodules
testicular 3/12
interstitial
cell tumour
Table 13. (contd.)
Material Strain, Route of Other Duration Period of Tumours Tumour Reference
tested number, administration treatment of observation produced incidence
and sex of and dosing treatment in
animals regimen surviving
animals
Clivorine 10 males, pituitary 1/20
(contd.) 10 females adenoma
testicular 3/20f Kuhara et al.
interstitial
cell tumour
adreno- 1/20f
cortical
adenoma
pancreatic 1/20
aunar cell
adenoma
Table 13. (contd.)
Material Strain, Route of Other Duration Period of Tumours Tumour Reference
tested number, administration treatment of observation produced incidence
and sex of and dosing treatment in
animals regimen surviving
animals
Crude Sprague intragastric 104 weeks 114 weeks liver tumours 13/40 (2 Habs et al.
alkaloidal Dawley rat dose of 8 mg/kg (all types) males, 11 (1982)
extract (20 males, body weight, 5 females)
from 20 females) times per week
Senecio other tumours 11/40
numorensis (all types) (5 males,
fuchsii 6 females)
20 males, intragastric 104 weeks 114 weeks liver tumours 34/40
20 females dose of 40 mg/kg (all types) (5 males,
body weight, 5 29 females)
times per week
other tumours 10/40
(all types) (7 males,
3 females)
controls liver tumour 1/40
(20 males, (male)
20 females)
other tumours 10/40
(all types) (4 males,
6 females)
Table 13. (contd.)
Material Strain, Route of Other Duration Period of Tumours Tumour Reference
tested number, administration treatment of observation produced incidence
and sex of and dosing treatment in
animals regimen surviving
animals
Farfugium AC1 rat diet (20%) 480 days till death liver 6/29 Hirono et al.
japonicum (15 males, or end of haemangio- (1983)
(leaves 14 females) study sarcomas
and
stalks) liver adenomas 7/29
adrenalcortical 7/29
adenomas
adrenal 1/29
phaeochromo-
cytoma
urinary 2/29
bladder
papillomas
ACI rat diet (20%) testicular 2/29
(15 males, interstitial
14 females) tumours
ileal 1/29
adenocarcinoma
Table 13. (contd.)
Material Strain, Route of Other Duration Period of Tumours Tumour Reference
tested number, administration treatment of observation produced incidence
and sex of and dosing treatment in
animals regimen surviving
Senecio 15 males, diet (8%) none Hirono et al
cannabifolius 15 females survived (1983)
(leaves and > 240 days
stalks)
14 males, diet (4%) none
14 females survived
> 240 days
12 males, diet (1%) 480 days till dead liver 1/23
12 females haemangio-
sarcoma
liver adenomas 13/23
adrenal 5/23
cortical
adenomas
urinary 1/23
bladder
papilloma
Table 13. (contd.)
Material Strain, Route of Other Duration Period of Tumours Tumour Reference
tested number, administration treatment of observation produced incidence
and sex of and dosing treatment in
animals regimen surviving
animals
Senecio testicular 1/23
cannabifolius interstitial
(contd.) tumour
12 males, diet (0.2%) 480 days till death liver 8/24
12 females haemangio-
sarcomas
liver cell 3/24
adenomas
adreno- 3/24
cortical
adenomas
adrenal 1/24 Hirono et al.
phaeochromo- (1983)
cytoma
testicular 2/24
interstitial
tumours
pituitary 1/24
adenoma
caecal 1/24
fibrosarcoma
Table 13. (contd.)
Material Strain, Route of Other Duration Period of Tumours Tumour Reference
tested number, administration treatment of observation produced incidence
and sex of and dosing treatment in
animals regimen surviving
animals
Senecio controls basal diet 560 days cortical 3/49
cannabifolius (25 males, adenomas of
(contd.) 24 females adrenal
controls basal diet testicular 3/49 Hirono et al.
(25 males, interstitial (1983)
24 females) tumours
pituitary 2/49
adenomas
a These tumours were present in the same animal.
b Each coexisted in one animal (seen in animals surviving 22 - 27.5 months).
c Together with haemangioendothelioma of the liver.
d Two animals had both tumours.
e Found in the same animal with angiosarcoma.
f In the same animal.
g No data available on number of surviving animals in the control groups.
Note: Figures in column 2 indicate the number of animals used at the start of the study. In column 8, animals developing a tumour out of
the number surviving up to the end of study/or dying with tumour are indicated. Animals developing more than one tumour are
indicated separately in a footnote. Different terms have been used in different studies to describe the same tumour in the liver,
e.g., haemangiosarcoma, angiosarcoma, haemangioendothelioma, haemangioendothelial sarcoma, and haemangiogenic sarcoma.
Table 14. Liver tumours found in rats given PAs and plant materials (condensed results from various authors)a
Alkaloid or plant Alkaloid type Route of Number of Number and types of liver Reference
given Necine Type of administration rats at tumours found
ester autopsy
Clivorine otonecine macrocyclic oral 12 2 haemangiosarcomas Kuhara et al. (1980)
diester 6 neoplastic nodules
Lasiocarpine heliotridine "open" ip 18 11 hepatocellular carcinomas Svoboda & Reddy
diester oral 20 9 angiosarcomas (1972);
7 hepatocellular carcinomas Rao & Reddy (1978)
Monocrotaline retronecine macrocyclic oral 75 24 hepatocellular carcinomas Newberne & Rogers
diester (1973)
Petasitenine otonecine macrocyclic oral 11 5 haemangiosarcomas Hirono et al. (1977)
diester 5 adenomas
Retrorsine retronecine macrocyclic various 14 4 "hepatomas" Schoental et al.
diester (1954);
Schoental & Head
(1957)
Isatidine retronecine macrocyclic various 7 4 "hepatomas" and Schoental et al.
diester "nodular hyperplasia" (1954);
Schoental & Head
(1957)
Senecionine retronecine macrocyclic oral 80 19 hepatocellular tumours Habs et al. (1982)
(+ fuchsisenecionine) (platynecine) diester, 16 cholangiogenic tumours
monoester 12 "haemangiogenic" tumours
Table 14 (cont'd)
Alkaloid or plant Alkaloid type Route of Number of Number and types of liver Reference
given Necine Type of administration rats at tumours found
ester autopsy
Senkirkine otonecine macrocyclic ip 20 9 adenomas Hirono et al.
diester (1979a)
Symphytine retronecine "open" ip 20 1 adenoma Hirono et al.
diester 3 haemangiosarcomas (1979a)
Senecio longilobus macrocyclic oral 47 16 hepatocellular carcinomas Harris & Chen
(seneciphylline, (retronecine) diester 1 angiosarcoma (1970)
retrorsine, etc.)
Petasites japonicus macrocyclic oral 46 11 haemangiosarcomas Hirono et al.
(petasitenine) (otonecine) diester 10 adenomas (1973)
3 hepatocellular carcinomas
Tussilago farfara macrocyclic oral 12 8 haemangiosarcomas Hirono et al.
(senkirkine) (otonecine) diester (1976)
Symphytum officinale monoester + oral 175 81 adenomas Hirono et al.
(various) (retronecine) "open" 3 haemangiosarcomas (1978)
diester
Farfugium japonicum senkirkine oral 29 7 adenomas Hirono et al.
6 haemangioangiosarcomas (1983)
Senecio cannabifolius petasitenine oral 21 16 adenomas Hirono et al.
9 haemangioangiosarcomas (1983)
a From: Mattocks (1986).
Svoboda & Reddy and their group have carried out 2 studies
using lasiocarpine (Svoboda & Reddy, 1972, 1974; Rao & Reddy,
1978). Svoboda & Reddy (1972, 1974) gave repeated ip injections to
25 rats at a dose of 7.8 mg/kg body weight (0.1 LD50) for 56 weeks
as per regimen shown in Table 13. Three rats died of acute liver
necrosis in the initial 4 weeks. Eighteen rats survived 56 weeks,
by which time each animal had received an average cumulative dose
of 125 mg lasiocarpine. Of these, 16 animals developed a variety
of tumours 60 - 76 weeks after the beginning of the study (Table
13). Ten of the 16 animals had more than 1 tumour. Hepatocellular
carcinoma was the most common. The squamous cell carcinoma of the
skin was found to be transplantable. When the same PA, mixed with
the diet at the rate of 50 mg/kg (Rao & Reddy, 1978) (Table 13),
was administered to 20 rats for 55 weeks, 17 animals developed
tumours. Angiosarcoma of the liver emerged as the most common
tumour (9/20 animals), even though hepatocellular carcinomas were
also frequently seen (7/20 animals); squamous cell carcinoma of the
skin was not found, but there was a malignant adnexal tumour of the
skin. The average cumulative dose of the alkaloid was estimated to
be 190 - 200 mg per rat.
Monocrotaline has been studied for its carcinogenic activity in
rats by Schoental & Head (1955), Newberne & Rogers (1973), Allen et
al. (1975), Shumaker et al. (1976), and Hayashi et al. (1977),
using different routes of administration.
Allen et al. (1975) studied the long-term effects on rats of
repeated sc injections of monocrotaline or its major detectable
metabolite, dehydroretronecine. Male Sprague Dawley rats were
given biweekly injections of monocrotaline, at 5 mg/kg body weight
(75 animals) for 12 months, or dehydroretronecine at 20 mg/kg body
weight (75 animals) for 4 months followed by 10 mg/kg body weight
for 8 months. Fifty control animals received phosphate buffer
(Table 13). Partial hepatectomy was performed on 15 animals in
each of the treated groups and 5 in the control group. They were
observed for 10 months following cessation of the injections. Of
the 60 animals surviving in each of the treated groups, those
receiving monocrotaline showed rhabdomyosarcoma at the injection
site (2 animals), hepatocellular carcinoma (2 animals), acute
myelocytic leukaemia (2 animals), and pulmonary adenoma (2
animals). In the group receiving dehydroretronecine, 36 animals
developed rhabdomyosarcomas, and 5 of these animals developed
metastases. None of the control group developed tumours. Tissues
obtained from partial hepatectomies showed that both compounds
caused inhibition of mitotic division in regenerating liver. The
results of the study illustrate the dual alkylating and antimitotic
properties of these agents, commented on by Culvenor et al. (1969).
In a similar study by Shumaker et al. (1976), rats were
administered monocrotaline at 5 mg/kg body weight on alternate
weeks or its metabolite dehydroretronecine at 20 mg/kg body weight,
on alternate weeks for 4 months, followed by a dose of 10 mg/kg on
alternate weeks in the succeeding 8 months (Table 13). Of the 60
rats receiving monocrotaline, 17 developed one or more tumours, but
not until after the treatment was discontinued. The time interval
was not stated. The most common tumour was carcinoma of the lung
(11 animals) followed by hepatocellular carcinoma (5 animals). A
wide variety of other tumours was also seen. A notable feature was
that the metabolite dehydroretronecine did not produce any tumours
by systemic action, but only at the site of injection, where
significant numbers of rhabdomyosarcomas (39/60 animals) were seen.
The marked difference in tumour sites is explained by the fact that
the parent alkaloid monocrotaline has to be metabolized before it
becomes a carcinogen. For this reason, the tumours are distributed
in several organs of the body, whereas dehydroretronecine is itself
carcinogenic and so acts at the site of injection. When
monocrotaline was delivered in a higher dose, but as a single
subcutaneous injection (40 mg/kg body weight) (Hayashi et al.,
1977) to 40 rats there were no malignant tumours but only adenomas
of the islets in the pancreas in 16/23 surviving animals. The
results of the above studies indicate that monocrotaline is
tumorigenic, but the type of tumour and the malignancy both depend
on the route of administration and the dosage used.
Hirono et al. (1977) studied petasitenine, the pure alkaloid
isolated from the flower stalks of the plant, Petasites japonicus
Maxim, which has been found to be carcinogenic for rats (Hirono et
al., 1973). Two groups of ACI rats of both sexes, Group I of 3
animals and Group II of 11 animals, were given the alkaloid in in
the drinking-water, at concentrations of 0.5 g/kg and 0.1 g/kg,
respectively (Table 13). There were 19 controls. All 3 animals in
group I died within 72 days showing marked hepatocellular damage;
no tumours were seen. In Group II, 10 out of 11 rats survived for
more than 160 days. Eight of the 10 animals developed tumours -
liver cell adenomas (5) and haemangiosarcomas (5) (Table 10). Two
animals had both types of tumours. The authors concluded that the
carcinogenicity of the plant was due to petasitenine.
Retrorsine and its N-oxide (isatidine) have been used in
several studies. Schoental et al. (1954) administered retrorsine
at a concentration of 3 mg/litre in the drinking-water to 14 rats
and isatidine at concentrations of 5 mg/litre followed by 3 mg/litre
to 22 rats until death. Twenty-five rats were administered the
alkaloid mixture from Senecio jacobaea Lin at a concentration of
500 mg/litre followed by 300 mg/litre in the drinking-water. Dosing
regimens are shown in Table 13. One group of 7 animals receiving
isatidine received supplementary 0.5% choline in the drinking-water
and another group of 5 animals received isatidine 2 mg as a single ip
administration in 0.2 ml of tricaprilyn, followed by skin application
of alkalids as 0.5% solution for 3 days/week.
The animals receiving mixed alkaloids of the plant showed
extensive liver damage followed by marked nodular hyperplasia; no
tumours developed. The nodules were earlier interpreted as
hepatomas, but later only as an early stage in the progression from
hyperplasia to neoplasia.
The retrorsine group showed extensive liver damage associated
with cirrhosis and nodular hyperplasia. In 4 rats, they were
interpreted as hepatomas.
In the isatidine group, 10 out of the 22 rats developed
hepatomas. Tumours were present in 3 out of 7 animals receiving
isatidine plus choline indicating that the latter had no protective
role. One out of the 5 animals receiving the alkaloid ip and then
through dermal application developed a tumour, which was also
interpreted as a hepatoma.
In another study (Schoental & Bensted, 1963), 95 weanling rats
were administered a single dose of retrorsine at 30 mg/kg body
weight, by stomach tube (Group II). One comparable group of 50
weanling rats (Group I) received 400 r radiation in animals
surviving 100 days after retrorsine administration (31/50). A
third group (Group III) of 6 animals received 400 r radiation
alone. Another group of 10 weanling rats received the PA, 9 days
after partial hepatectomy (Group IV) (Table 13). The additional
treatments were given to study whether they would act as
co-carcinogens and induce neoplasia in hepatocytes, which are known to
show injurious effects for long periods following PA treatment. In
Group I, 19 of the 50 animals receiving a single dose of the PA
died before radiation could be given. Of the 31 remaining animals
that received radiation after the PA, 25 survived 12 months. Among
these, 19 tumours of a wide variety were seen (Table 13). Of the 6
tumours in the liver, only 1 was malignant, having metastasized.
Most of the other tumours were malignant, including those of the
breast, one of which had also metastasized.
In Group II, 29 out of 95 animals that had received one dose of
PA and no radiation survived for more than a year, with a mean
survival time of 23 months. Among these, 7 animals developed
tumours of a wide variety (Table 13). Five tumours in the liver
were benign. Most of the others were malignant. Two tumours, a
cystic tumour of the breast and a carcinoma of the uterus, were
present in one animal. Tumours seen in Groups III and IV, which
were found in animals surviving 12 months or more after the start
of the study, are shown in the Table 13. The 2 tumours of the
liver in Group IV were also benign. The number of control animals,
if any, were not indicated.
The authors concluded that the above studies did not provide
definite evidence of synergistic action in the carcinogenicity of
retrorsine by whole body radiation or partial hepatectomy.
Hirono et al. (1979a) studied the carcinogenic properties of
senkirkine extracted from the dried milled buds of Tussilago
farfara (coltsfoot) and symphytine extracted from dried milled
roots of Symphytum officinale (comfrey). Both Tussilago farfara
(Hirono et al., 1976) and Symphytum officinale (Hirono et al.,
1978) had earlier been demonstrated to have carcinogenic
properties.
Sixty inbred ACI strain male rats were divided into 3 groups of
20 animals each and received repeated ip injections of senkirkine
at 22 mg/kg body weight or symphytine at 13 mg/kg body weight as
per schedule given in Table 13. All animals treated with
senkirkine survived 290 days. Nine out of 20 rats developed liver
adenomas mostly after 350 - 450 days from start of the study.
Cirrhosis of liver was frequently observed. Of the symphytine
group, all animals survived 330 days after the start of the study.
Three animals developed haemangioendothelial sarcoma and one,
liver adenoma. The sarcomas were noted at least 518 days after the
start of the study.
Schoental & Cavanagh (1972) used two alkaloids, retronecine and
hydroxysenkirkine isolated from Crotalaria laburnifolia, which was
injected ip in single doses ranging from 100 to 300 mg/kg body
weight in 5 weanling Porton Wistar rats (Table 13). One animal
that had received the 300 mg/kg dose developed astrocytoma of the
brain after 14 1/2 months. Retronecine hydrochloride was
administered in doses ranging from 300 to 1000 mg/kg body weight by
single subcutaneous injection to 10 new-born rats. One male rat,
which was found to be paraplegic 6.6 months after receiving a dose
of 600 mg PA/kg, was also found to have ependymoblastoma of the
spinal cord. Among the litter mates of this rat, 1 male that had
received a dose of 1000 mg/kg died. The remaining 6 females and 2
males were killed within 22 months of being dosed. Of the females,
5 had pituitary tumours and 1 had a mammary tumour (type not
stated). Two males did not show any significant abnormalities.
One group of 5, 6-month-old female rats, born to dams that had
been fed on a diet containing 50 g dried and powdered Heliotropium
ramosissimum/kg diet, a tribal remedy, used during pregnancy and
parturition, were then themselves fed on the same diet at 6 months
of age, as per the regimen indicated in Table 13. One female rat
was found to be paraplegic at 7 months of age. A tumour, possibly
of Schwann or satellite cell origin, was found.
6.4.8.2 Plant materials
A number of plant materials have been tested for their
carcinogenicity by administering either a mixture of extracted
alkaloids (Cook et al., 1950; Schoental et al., 1954) or, more
often, the dried and milled plant mixed with the diet. The only
study in which the plant alone was fed was that of Campbell (1956),
who produced tumours in chickens by feeding dried and milled
Senecio jacobaea plant. It is notable that the plants tested are
almost all those that have been reported to be used as herbal
medicines and/or food, some of which have been reported to cause
human toxicity.
Schoental et al. (1970) used mixed PAs (intermedine and
lycopsamine) extracted from seeds of Amsinckia intermedia, known to
cause livestock losses in the USA, and leaves and stems from
Heliotropium supinum L., known to be used by women in East Africa
as a herbal medicine, after childbirth. The dosing regimen and
mode of administration are indicated in Table 13. Of the 15 male
weanling rats that had received a single treatment with the
Amsinckia PAs and survived for more than 1 year, 3 rats showed one
adenoma, one adenocarcinoma of the islet cells, and one adenoma of
the exocrine pancreas, respectively (Table 13). In addition, one
of the animals also developed a pituitary adenoma and papillary
tumour of the urinary bladder. Eight animals received the
treatment with Heliotropium supinum L. (Table 13). One out of the
2 weanling rats fed on the plant with the diet and one out of the 6
animals that received a single intragastric dose of the crude
alkaloid fraction developed islet cell adenoma of the pancreas.
The number of control animals, if any, used in the study is not
stated.
Harris & Chen (1970) tested the carcinogenicity of Senecio
longilobus, which has been associated with cases of human toxicity
(Stillman et al., 1977; Huxtable, 1980; Fox et al., 1978). Harlan
rats were divided into 4 groups (equal numbers of both sexes) and
fed diets containing dried and powdered stems and leaves of the
plant in the proportion of 5 -7.5 g/kg. The number of animals in
each group and the feeding regimen are shown in Table 13.
Continuous feeding did not produce any tumours, presumably because
of the comparatively low survival rate of animals. Significant
results were obtained when the animals were fed contaminated diet
alternating with normal diet (Group IV). Of the 100 animals fed on
this regimen for 54 weeks, 47 survived for more than 200 days.
Seventeen of these animals developed malignant tumours in the
livers - hepatocellular carcinomas in 16 and an angiosarcoma in 1
animal after a minimum feeding period of 217 days. In Group III,
fed a contaminated diet for 1 year, 23 rats lived for more than 200
days. Four rats (3 males and 1 female) developed hepatocellular
carcinomas and one, a peritoneal mesothelioma, after a minimum
feeding period of more than 428 days. The tumour-bearing animals
were predominantly male. The authors emphasized the relative
rarity of liver tumours in the strain of animals used. Results
demonstrated that S. longilobus is carcinogenic for rats.
For a study of Schoental & Cavanagh (1972), using dried and
powdered Heliotropium ramosissimum, see section 6.4.8.1.
Hirono et al. tested the carcinogenic effects of 3 widely-used
herbs containing PAs. Hirono et al. (1973) studied the possible
carcinogenic effects of the young flower stalks of Petasites
japonicus Maxim, which has long been used in Japan as food or
herbal remedy as well as the PA, petasitenine, isolated from it.
The flower stalks of the plant were dried, milled, and fed to young
ACI rats of both sexes mixed with the basal diet in the proportion
of 40 - 80 g/kg, as indicated in the feeding regimen in Table 13,
until the animals were moribund or dead. One group of 27 rats was
fed the plant mixed in the proportion of 40 g/kg diet for 6 months
followed by 80 g/kg diet on alternate weeks. The second group of
19 rats was fed a contaminated diet (40 g/kg) continually. Three
animals in Group I died of pneumonia. Eleven out of the remaining
24 rats in this group developed liver tumours after 15 - 16 months
of feeding. There were 2 hepatocellular carcinomas, 6 liver cell
adenomas, and 3 haemangiosarcomas, of which 2 had metastasized. In
Group II, 2 animals died from non-tumorous causes. Of the
remaining 17, 8 developed haemangiosarcomas, 4 liver cell adenomas
and 1 had a hepatocellular carcinoma. The incidence of
haemangiosarcoma was statistically higher in Group II.
In a similar study on mice and hamsters (Fushimi et al. (1978),
groups comprising 20 - 24 male and 20 - 21 female 6-week-old ddN,
Swiss, and C57BL/6 mice, and 13 male and 17 female hamsters, were
fed a diet combining 4% young, dried, and milled flower stalks of
Petasites japonicus Maxim for 480 days. All surviving animals were
killed at the end of the study. Lung adenomas and adenocarcinomas
were found in 30/39 surviving male and female ddN mice combined
(compared with 1/50 in the respective controls) in addition to
other tumours (Table 13). No significant differences in tumour
incidence were observed between treated Swiss and C57BL/6 mice and
hamsters, and the corresponding controls. No data were given on
the tumour incidence according to sex, in treated and control
animals or on survival in the controls.
In studies on rats (Hirono et al., 1976), the dried and
powdered flower buds of Tussilago farfara (coltsfoot) were mixed
with the diet. Forty-nine inbred ACI strain rats were divided into
4 groups and fed diets containing 40 - 320 g T. farfara/kg for up
to 600 days. The number of animals in each group and the dosage
and feeding regimen are given in Table 13. Two groups receiving a
diet containing 80 - 320 g/kg on a continual basis developed
tumours in the liver of the same types as animals in the studies
using Petasites japonicus, e.g., haemangioendotheliomas, liver
cell adenomas, and hepatocellular carcinomas (Table 13). All 12
animals in Group I survived for more than 380 days after the start
of the study. Of these, 8 (5 males, 3 females) developed
haemangioendothelial sarcoma of the liver. In addition, 3 of the 8
rats developed simultaneously hepatocellular adenoma, hepatocellular
carcinoma, or urinary bladder papilloma. In Group II receiving
coltsfoot at 80 g/kg in the diet, 9 out of 10 animals that survived
for more than 420 days developed a haemangioendothelial sarcoma.
Hirono et al. (1978) fed Symphytum officinale, similarly dried
and milled, to the ACI strain of inbred rats of both sexes at
different levels ranging from 80 to 330 g/kg as leaves or 5 - 40 g/kg
as root, for 280 days or more. Eight groups of animals of
19 - 48 animals each were used on different regimens of feeding.
The feeding regimen, the number of animals in each group, and the
duration of treatment are given in Table 13. Sixty-five males and
64 females served as controls. Tumours were induced in all groups
receiving leaves or roots. The most common tumour was liver
adenoma. Haemangiosarcomas were observed but infrequently (3
animals in the whole study). No carcinomas of the liver were seen,
but there was a wide variety of other tumours. It is noteworthy
that, in Group VII, 14 of 15 animals fed the lowest dose of 10 g/kg
for 275 days followed by 5 g/kg or basal diet alternating every 3
weeks, developed tumours, 2 of which were malignant (haemangiosarcomas).
In the control group, single animals each had papilloma of urinary
bladder, caecal adenoma, subcutaneous fibrosarcoma, mammary
fibroadenoma, or retroperitoneal teratoma. The livers of animals that
did not have the tumours showed other features commonly encountered in
animals administered PAs, e.g., megalocytosis, liver cirrhosis,
hyperplastic nodules, etc., suggesting that they were induced by the
PAs contained in the plant. The authors concluded that the
carcinogenic activity of this plant was weaker than that observed in
animals fed Petasites (coltsfoot). Hirono et al. (1979b) have
summarized the above studies on PAs found in edible plants in Japan.
Habs (1982) and Habs et al. (1982) tested a crude alkaloid
extract from the plant Senecio numorensis sp. fuchsii containing
fuchsisenecionine (500 g/kg) and senecionine (10 g/kg). The
extract was administered intragastrically in 2 doses of 8 mg and
40 mg/kg body weight, respectively, to 2 groups of rats of both sexes,
comprising 40 animals each, 5 times a week for 104 weeks (Table
13). A large, dose-related number of liver tumours was produced,
originating in the liver cell and the sinusoidal system, and
predominantly affecting the female animals. The tumour incidence
was higher in Group II (dose, 40 mg/kg) with 34 tumours compared
with 13 in Group I. In Group I (dose, 8 mg/kg), 2 tumours were
found in 20 males compared with 11 tumours among 20 female rats.
Similarly, in Group II, only 6 tumours were found in 20 males
compared with 29 among 20 female rats. The tumours included 19 of
"hepatocellular origin", 16 of "cholangigenic origin", and 12 of
"haemangiogenic origin" (Table 14). Besides the liver tumours, 12
males and 9 females in the treated groups and 4 males and 6 females
in the control group developed a variety of extra-hepatic tumours.
Senecionine is known to be hepatotoxic and capable of being
converted in the rat liver into cytotoxic metabolites.
Fuchsisenecionine is a saturated PA, not previously known to be
cytotoxic. It is therefore likely that senecionine was the
hepatotoxic component and that perhaps the two PAs acted
synergistically with each other, though there is no actual evidence
for synergism. However, this needs confirmation. Moreover, there
appeared to have been other unknown components in the mixture
tested (Mattocks, 1986).
Hirono et al. (1983) studied the carcinogenicity of 2 more
plants (Farfugium japonicum and Senecio cannabifolius) of the tribe
Senecioneae in the family Compositae, the leaves and stalks of
which are used in Japan as human food. Fresh leaves and stalks of
the plants were dried, milled, and mixed with the basal diet.
Inbred strain ACI rats of both sexes, with preponderance of
females, 1.5 months old, were divided into 6 groups. They were fed
diets containing various proportions of the dried plant materials,
as indicated in Table 13. The study was terminated at 480 days,
except for one group, which was studied for 560 days. Besides the
groups shown in the tables, 2 more groups of 30 and 28 animals of
equal numbers of both sexes were fed 8% and 4% of Senecio
cannabifolius, respectively. None of these animals survived more
than 177 days and all died of hepatotoxicity. A wide range of
tumours was observed, mostly in the liver, as shown in Table 13,
the most common being haemangiosarcomas, which were not encountered
in the control group.
The carcinogenicity of Farfugium japonicum is considered to be
due to senkirkine and petasitenine. Senecio cannabifolius contains
senecicannabine, a new macrocyclic PA, seneciphylline, and
jacozine. It is probable that the carcinogenicity of Senecio
cannabifolius is due to these PAs (Hirono, et al., 1983).
Hayes et al. (1985) studied the biological mechanisms by which
PAs initiate carcinogenesis in male Fischer 344 rats. Lasiocarpine
(single or double injection of up to 80 µmol/kg body weight) and
senecionine (single or double injection of up to 160 µmol/kg) were
inactive as initiators of Y-GT-positive nodules in rats exposed to
2-acetylaminofluorene and partial hepatectomy. Administration of
lasiocarpine or senecionine 12 h after partial hepatectomy resulted
in the development of very few nodules. Lasiocarpine given in a
single or double dose (up to 80 µmol/kg) delayed hepatic
regeneration by at least 8 weeks after partial hepatectomy, and
pretreatment with this PA reduced the initiating capacity of
diethylnitrosamine and N-nitrosomethylurea in rats subsequently
selected with 2-acetylaminofluorene and partial hepatectomy.
Resistant nodules selected with lasiocarpine also had the typical
resistant nodule phenotype (positive for Y-GT and epoxide
hydrolase) and also lacked PA-induced megalocytosis. Lasiocarpine
treatment also resulted in small regenerative nodules that were
distinct from resistant nodules, because they were negative for
Y-GT and epoxide hydrolase.
6.4.8.3 Pyrrolizidine alkaloid metabolites and analogous synthetic
compounds
The subject has been reviewed by Mattocks (1986). The
pyrrolizidine alkaloids are converted into pyrrolic esters in the
hepatocytes. These may then be hydrolysed into pyrrolic alcohols,
which are more water soluble and less active than the esters. The
esters may be widely distributed throughout the body. Some of
these compounds and their analogues have been tested for
carcinogenicity.
(a) Pyrrolic esters
(i) Dehydromonocrotaline (monocrotaline pyrrole)
Mattocks & Cabral (1979) tested dehydromonocrotaline on the
skin of mice. In their first study on male BALB/c mice, they
applied the compound on the back at 1- to 2-week intervals.
Thirty-three applications of 1 µmol did not produce any skin
tumours in 16 mice, but 2 developed lung adenomas; no tumours
occurred in 14 control mice treated with the solvent (acetone). In
the second study (Mattocks & Cabral, 1982), 11 female LACA mice
each received 47 applications of 2.5 µmol dehydromonocrotaline; one
animal developed a malignant skin tumour. A second batch of 10
mice was given similar treatment and subsequently received 61
twice-weekly applications of croton oil at the same site. Half of
these animals developed tumours. In the control group of 10 mice,
only 1 tumour was seen. The results of these studies indicate that
dehydromonocrotaline requires the action of a promoter to manifest
carcinogenic potential.
(ii) Dehydroretrorsine
This supposed pyrrolic metabolite of retrorsine was applied to
the skin of male BALB/c mice at 1- to 2-week intervals; 33
treatments of 0.5 or 1 µmol failed to produce tumours in 15 mice
that survived for up to 60 weeks (Mattocks & Cabral, 1979).
(iii) 1-Methyl-2,3-bistrimethylacetoxymethylpyrrole
Mattocks & Cabral (1979, 1982) made 2 studies on this compound.
In the second study, which was more significant, 22 female LACA
mice received 47 dermal applications of 0.5 µmol each. This caused
marked skin damage with ulceration and scarring. Malignant skin
tumours developed in 19 out of 21 surviving animals. Two
hydrolysis products of this ester, pivalic acid and 1-methyl-2,3-
bishydroxymethylpyrrole, were similarly tested (Mattocks, 1986).
Tumours were produced, though fewer.
The results of the above studies suggest that the intact
pyrrolic ester is a carcinogen.
(b) Pyrrolic alcohols
Studies have been conducted using dehydroretronecine
(retronecine pyrrole) and dehydroheliotridine, which are secondary
metabolites of monocrotaline and heliotridine-based PAs,
respectively. Originally they were regarded as (+)- and (-)-
forms, respectively, of dihydro-7-hydroxy-1-hydroxy-methyl-5H-
pyrrolizine. Kadzierski & Buhler (1985, 1986) showed that the
metabolite from monocrotaline is racemic and concluded that the
product from all heliotridine and retro-necine esters is the same
(±)- form.
Johnson et al. (1978) painted the skin on the back of 16 female
Swiss mice with dehydroretronecine, each dose equalling 20 mg/kg
body weight or about 5 µmol per mouse, once a week for 4 weeks and
then twice more after 6 months. Six mice developed skin tumours.
Subcutaneous injections of the same dose yielded tumours in 13 out
of 21 mice. Twenty-eight out of 55 mice given both topical
applications and sc injections developed skin tumours.
When the same study was repeated on 34 BALB/c mice, only one
skin tumour developed (Mattocks & Cabral, 1982). When 17 animals
were given the same dose at more frequent intervals, e.g., 65
weekly doses, no tumours developed.
Similar results were obtained in a study by Shumaker et al.
(1976), already desecribed in section 6.4.8.1, in which 39/60 rats
given repeated subcutaneous injections of dehydro-retronecine
developed rhabdomyosarcomas at the site of injection. The compound
appears to be a direct-acting carcinogen for rats and mice, though
the susceptibility of various strains of mice varies.
Peterson et al. (1983) gave 9 ip injections (60 - 76.5 mg/kg
body weight) of dehydroheliotridine to rats over a 32-week period.
A large variety of tumours was produced. It was concluded that
this metabolite may be responsible for the carcinogenicity of its
parent PA.
6.4.8.4 Molecular structure and carcinogenic activity
Mattocks (1986) has reviewed the present position concerning
the relationship between molecular structure and carcinogenic
activity. Data available at present are not adequate for any
strict correlation to be established between the molecular
structure of PAs and the types of tumours produced by them in the
rat. However, the common determinants in the molecular structure
of all carcinogenic PAs are that they are macrocyclic or "open"
diesters, in which the amino-alcohol moiety is retronecine,
heliotridine, or otonecine. These are all esters of unsaturated
necines and are capable of being metabolized to pyrrolic esters in
the mammalian liver. Studies on pyrrolic esters, the toxic
metabolic product of PAs, have yielded equivocal results.
Monocrotaline has been shown to bind covalently to DNA, which is
associated with the carcinogenic activity of the pyrrolizidine
alkaloids (Robertson, 1982). Dehydromonocrotaline, the primary
metabolite of monocrotaline has been found to be an incomplete
dermal carcinogen (Hooson & Grasso, 1976; Mattocks & Cabral,
1982), whereas the synthetic compound 1-methyl-2,3-
bistrimethylacetoxymethylpyrrole, which is chemically similar to
dehydromonocrotaline, is clearly carcinogenic (Mattocks & Cabral,
1982). Dehydroretronecine (DHR), a second metabolite of
monocrotaline and possibly other retronecine-based PAs, is also
carcinogenic for the skin and is considered a proximate
carcinogenic metabolite, since, unlike monocrotaline, it acts at
the site of application directly and not at remote sites.
6.4.9 Antimitotic activity
Literature on this phenomenon has been reviewed by Jago (1969),
McLean (1970), and Mattocks (1986). The most characteristic
feature of the chronic hepatotoxicity of the PAs is the presence in
the liver of megalocytes (Bull & Dick, 1959; McLean, 1970), which
are generally enlarged hepatocytes containing large, hyperchromatic
nuclei (section 6.4.1.5). These appear to be the result of a
combined action of PAs on the hepatocytes, a stimulus to regenerate
following parenchymal cell injury, and the powerful antimitotic
action of the pyrrole metabolites of the PAs (Mattocks, 1986; Jago,
1969). This property has served as the basis for using a PA
(indicine- N-oxide) as a chemotherapeutic agent for cancer (Letendre
et al., 1981, 1984). Peterson (1965) showed that the number of
mitoses following partial hepatectomy was reduced to 50% or less of
normal values by prior administration of hepatotoxic alkaloids, and
that the effect was dose dependent. The hepatocytes seemed to
continue to grow without dividing. The effect can be produced by a
single sublethal dose of the alkaloids (Schoental & Magee, 1957) or
can be a cumulative effect of small doses (Bull & Dick, 1959). The
lesion appears within a few weeks and may persist for the lifetime of
the animal (Mattocks, 1986). It was characteristically described in
the liver of the rat, but has also been reported in a number of other
animals, e.g., mouse, sheep, horse, and pig, and in some other organs,
e.g., kidney and lung (McLean, 1970). It has not been observed in
human livers (Tandon, H.D. et al., 1978; Mattocks, 1986), but it has
been observed that cultured human fetal liver cells become enlarged
when exposed to PAs (Armstrong et al., 1972) indicating a
susceptibility to the antimitotic effect of PAs. The lesion is not
specific for PAs but has been reported to be produced by a number of
toxins (McLean, 1970), including semisynthetic derivatives of PAs but
not non-hepatotoxic PAs, such as platyphyline (Jago, 1970). The
ultrastructural features of megalocytes are controversial.
However, consistent with other functional and metabolic features,
the cells show morphological characters suggesting increased
metabolic activity with increased exchange of material between the
nucleus and cytoplasm.
This unique reaction of the hepatocytes to PA has been used by
Jago (1970) to develop a method for assessing hepato-toxicity and
by Culvenor et al. (1976a) for screening 62 PAs for acute and
chronic hepatotoxicity and pneumotoxicity.
Mattocks (1986) suggested a scheme for the antimitotic action
of PAs in vivo, consistent with observations of the phenomenon in
animals. The PA is irreversibly metabolized by the hepatic
microsomes into a pyrrolic ester, which can be hydrolysed to a
pyrrolic alcohol. The latter is the agent that inhibits mitosis.
The more reactive primary metabolite may also do this, by reacting
with tissue constituents to give products identical to pyrrolic
alcohols, inhibiting mitosis. On the other hand, it can also
produce acute injury to the cells, which stimulates regeneration.
Thus administration of the PA or the pyrrolic ester can induce
megalocytosis to a much greater extent than a secondary metabolite
alone.
Antimitotic activity does not seem to be directly related to
inhibition of DNA synthesis, since the latter recovers within a
week while mitotic inhibition continues for up to a period of 4
weeks (Peterson, 1965; Armstrong & Zuckerman, 1970). Mattocks &
Legg (1980) have shown that the level of DNA synthesis is reduced
in cells that do not divide, but is not totally inhibited. Samuel
& Jago (1975) investigated the position in the cell cycle of the
antimitotic action of lasiocarpine and of its pyrrolic metabolite,
dehydroheliotridine. Their studies indicated that the alkaloid
acts during the late S or early G2 phase of the cell cycle.
6.4.10 Immunosuppression
Dehydroheliotridine (DHH), a pyrrolic metabolite, has a
significant immunosuppressant activity on the primary response in
young mice; when injected ip shortly before the antigenic stimulus
(Percy & Pierce, 1971). The secondary response to antigenic
stimuli; as measured by the reduction in the number of 7S and 19S
specific antibody-synthesizing cells of the spleen, was suppressed
when DHH was administered at the time of secondary stimulus, but
not when it was given 24 or 36 h after the antigenic stimulus. It
was suggested that dehydroheliotridine selectively destroys or
inactivates cells involved in the initial stages of antigen
recognition and processing.
6.4.11 Effects on mineral metabolism
Aberrations of mineral metabolism have been observed in several
species of animals. The most notable among them relate to
haemolysis and copper metabolism. Anaemia has been reported to
occur in rats following PA poisoning (Schoental & Magee, 1959;
Schoental, 1963) and the kidney and liver show haemosiderosis
(Hayashi & Lalich, 1967). Besides the haemolysis, PAs have been
found to exert a direct inhibitory effect on haematopoiesis in the
livers of new-born rats (Sundaresan, 1942). Disturbances of iron
metabolism and haematopoiesis have also been demonstrated in rats
fed Senecio jacobaea and supplementary copper (Swick et al., 1982d)
and they have been found to develop raised copper levels in the
liver and spleen, when fed on this plant (Swick et al., 1982b).
Miranda et al. (1979) reported elevated levels of iron in the liver
and spleen and of copper in the liver in rats fed on tansy ragwort.
Studies with radioactive iron also indicated a specific inhibitory
effect of PAs on haematopoiesis. High copper levels in the liver
associated with haemoglobinuria have been reported in sheep grazing
on heliotrope (Bull et al., 1956), signs of disease closely
resembling those of chronic copper poisoning. St. George-Grambauer
& Rac (1962) reported a similar outbreak of fatal jaundice due to
haemolytic crisis of chronic copper poisoning in sheep that had
grazed Echium plantagineum over 2 or more seasons. The
pathological changes in the liver were indistinguishable from those
of Heliotropium, and the livers had a high copper content. Studies
of Miranda et al. (1981b) indicate that dietary copper can enhance
PA hepatotoxicity in rats. It has been suggested that the
hepatotoxic effects of some PAs may interfere with the excretion of
copper (Bull & Dick, 1959; Farrington & Gallagher, 1960). Similar
effects have been observed in pigs fed Senecio (Harding et al.,
1964). White et al. (1984) did not observe any rise in hepatic
copper levels in sheep fed Senecio jacobaea.
6.4.12 Methods for the assessment of chronic hepatotoxicity and
pneumotoxicity
Pyrrolizidine alkaloids produce acute as well as chronic liver
damage. The acute effect is seen as extensive necrosis (Schoental
& Magee, 1957), while the chronic effect in rats is manifested
characteristically by the presence of greatly enlarged parenchymal
cells (Schoental & Magee, 1957; Bull & Dick, 1959), which persist
long after a single exposure (Schoental & Magee, 1959). The latter
effect of megalocytosis may manifest without the liver cells going
through the process of necrosis (Schoental & Magee, 1959). This
property has been used by Jago (1970) to develop a method for the
assessment of the relative chronic hepatotoxicity of different
alkaloids. It has since been used by other investigators (Culvenor
et al., 1976a). It consists of the intraperitoneal administration
of a single dose of between 0.025 and 3.2 µmol of the alkaloid per
kg body weight in 0.2 ml aqueous solution to 14-day-old suckling
hooded Wistar rats of both sexes. The litters are randomized among
the mothers, one day before the administration of the toxin, and
then weaned at 28 days. Animals are killed 4 weeks after the
injection. The relative acute toxicity is indicated by the dose
levels that cause death within approximately a week and chronic
hepatotoxicity by those that produce hepatic megalocytosis within 4
weeks of the injection. With this method, it is not only possible
to evaluate the hepatotoxicity of a given alkaloid but also to
compare the hepatotoxic effects of different compounds in relation
to each other on a molar basis. Chronic effects on the lungs can
also be assessed by the same method. Culvenor et al. (1976a) found
this method satisfactory for compounds of medium to high
hepatotoxicity but failed to detect toxicity in certain compounds
of known, low hepatotoxicity.
6.5 Effects on Wild-Life
6.5.1 Deer
There is very little information on the consumption of
PA-containing plants by non-domesticated animals, birds, and other
wild-life. In one instance, the deaths of white-tailed deer in
coastal marshes in Louisiana in 1967, was ascribed to the
consumption of Crotalaria and/or Heliotropium species in a period
of feed scarcity (Seger et al., 1969). The animals had thin and
watery blood, abnormal bone marrow, and serious atrophy of cardiac
and mesenteric adipose tissue. In a 9-year-old doe, the liver was
dull and somewhat granular and evinced megalocytosis of the
hepatocytes. In another 4-year-old animal, the liver appeared
normal but with microscopic evidence of early megalocytosis, with
considerable vacuolization in the centrilobular hepatocytes.
Plants of the genera Crotalaria and Heliotropium were abundant and
there were some Senecio species. There were signs of ingestion of
the plants by the deer.
Senecio jacobaea (tansy ragwort) has been fed experimentally to
black-tailed deer (Odocoileus hemionus columbianus) in Oregon to
determine their susceptibility to poisoning (Dean & Winward, 1974).
The ragwort was given ad libitum together with different levels of
basal ration (85% alfalfa, 10% barley, 5% molasses) to captive
deer. The ragwort was eaten, only when the basal ration was
inadequate. One group, not given any basal ration for 6 days,
began eating ragwort and consumed 5.4 kg dry weight per animal in
42 days. This represented 24% of the animal body weight. The
animals did not show any toxic signs, and blood levels of SGOT and
bilirubin were normal.
6.5.2 Fish
The effects of S. jacobaea alkaloids on rainbow trout (Salmo
gairdneri) fingerlings has been investigated (Hendricks et al.,
1981). Duplicate groups of 80 fingerlings were fed for up to 12
months on diets containing 20 or 100 mg alkaloid/kg. The alkaloid
comprised 91% jacobine, 3% jacazine, 2.5% senecionine, and 2.5%
seneciphylline. The 100 mg/kg diet resulted in severe growth
depression and mortality, which began at 3 - 4 months. Both levels
of PAs produced severe hepatic lesions. The livers from these fish
were shrivelled, mottled yellow or whitish in colour, nodular,
fibrous, and sometimes haemorrhagic. Microscopically, there was
megalocytosis, severe fibrosis, and bile-duct proliferation.
Characteristic veno-occlusive changes were seen in the centrilobular
and hepatic veins, which, in the case of fish receiving the 100 mg/kg
diet, appeared after only 2 months on the diet.
6.5.3 Insects
There are no reports of the toxicity of PAs for insects, but
there is substantial literature on the use of PAs by certain insect
families that have evolved with the ability to store the alkaloids
as defensive chemicals and to convert them into pheromones and
other signalling chemicals (see recent reviews by Brown (1984) and
Boppré (1986), and an earlier complementary paper by Edgar (1982)).
In some species, such as moths of the family Arctiidae, the larvae
feed on PA-containing plants. In other families, such as Nymphalid
butterflies of the sub-families Danainae and Ithomiinae, the larvae
of most species live on other plants, but the adult males seek out
PA-containing species and contrive to ingest alkaloids from
wilting, dead, or damaged plant material or from nectar. The
alkaloids so acquired have a functional role as defensive chemicals
against predators and, in some species, are also converted into
pheromones and other signalling chemicals involved in mating. The
alkaloid derivatives may be pyrrolic compounds related to
dehydroretronecine or derivatives of the esterifying acids. In one
Arctiid genus, Creatonotus, the alkaloids have a morphogenetic or
hormonal effect, determining the size of the pheromone-disseminating
organ. Thus, for some insect species, PA-containing plants may be
necessary for survival.
7. EFFECTS ON MAN
The toxic effects of pyrrolizidine alkaloids are principally on
the liver. The toxic disease, produced by consuming PAs derived
from certain plants, is called veno-occlusive disease (VOD), the
pivotal and pathognomonic lesion being the occlusion of the central
and sublobular hepatic veins in the liver. The larger hepatic vein
tributaries are characteristically unaffected in contrast with the
findings in Budd-Chiari syndrome (Bras, 1973).
7.1 Clinical Features of Veno-Occlusive Disease (VOD)
There are several good clinical accounts of the disease, mostly
in the earlier reports from Jamaica, in children (Jelliffe et al.,
1954a,b), and adults (Stuart & Bras, 1955), which have been
summarized by Stuart & Bras (1957). Maksudov (1952) has described
the clinical features among children in outbreaks in the USSR,
where it was called toxic hepatitis with ascites, and Ismailov
(1948a,b), Mnushkin (1949, 1952), and Zheltova (1952) have
described them among adults. Srivastava et al. (1978) also
described the clinical findings among children in a large outbreak.
Children seem to be particularly vulnerable as is evident from the
report of Stuart & Bras (1957) and that of Mohabbat et al. (1976)
concerning a large outbreak, though the disease is rare before the
age of one or two years. Frequently, more than one member of the
family becomes affected (Stuart & Bras, 1957; Mohabbat et al.,
1976; Tandon et al., 1976; Arora et al., 1981) within days or weeks
of each other.
The disease generally has an acute onset, characterized by
rapidly developing and progressing symptoms of upper abdominal
discomfort, dragging pain in right hypochondrium, ascites, and
sometimes oliguria and oedema of the feet. Nausea and vomiting may
be present. Jaundice and fever are rare. There is generally
gross, tender, smooth hepatomegaly often accompanied by massive
pleural effusion, and sometimes slight splenomegaly and minimal
ankle oedema. Liver function tests may show only mild disturbance.
The acute disease is associated with high mortality and a subacute
or chronic onset may lead to cirrhosis. Death often occurs after
oesophageal haemorrhage.
Stuart & Bras (1957) summarized the clinical data of 84
patients ranging in age from 6 months to 53 years. The highest
incidence occurred between the ages of 1 and 3 years (39%), and 26%
of patients belonged to the 3- to 6-year age group. Thus, children
up to 6 years accounted for 65% of total cases. Although the VOD
was relatively uncommon at the 2 extremes of age, early infancy and
adult life, mortality was highest in these groups, being 60% and
54%, respectively. Hepatomegaly and some degree of ascites were
invariably present in acute cases; in 48 out of 64 patients,
ascites was acute enough to require paracentesis. In 38 patients,
hepatomegaly was grossly severe, reaching more than half way down
to the umbilicus. Jaundice was relatively uncommon. Among the
liver function tests, the most significant and consistent changes
were found in the serum-cholinesterase (t = 2.67, 0.02 > P > 0.01)
and serum-albumin levels (t = 2.82, 0.01 > P). Mortality rates
associated with signs of parenchymal liver damage were 74% with
clinical jaundice or high levels of serum-bilirubin, 62% with
diminished serum-cholinesterase, and 58% with considerable anorexia
and apathy. The mortality rates among cases with acute, subacute,
or chronic disease were reported to be 27%, 17%, and 57%,
respectively. Death was mostly due to hepatocellular failure (71%)
in the acute phase and haemetemesis in the chronic phase (75%).
More recent publications (Lyford et al., 1976; McGee et al., 1976;
Tandon, B.N. et al., 1977; Datta et al., 1978a,b) also describe the
haemodynamic data of the hepatic blood flow and the results of
portovenographic studies that suggest outflow tract obstruction in
the liver at the post-sinusoidal level, and irregularity and
obstruction/distortion of the hepatic venous radicles,
respectively.
The clinical course of the disease has been shown schematically
by Stuart & Bras (1957) (Fig. 12). However, the temporal
relationships in the different phases of the disease are not
precise, and their account, at best, represents a trend.
 The onset of the disease may be sudden (acute) or insidious
(subacute or chronic). The acute disease may recover completely or
result in death. A few patients may go on to the subacute phase,
with almost none or very few symptoms, but a persistent
hepatomegaly. The patient may subsequently recover completely, or
may, after or without apparent clinical improvement, go on to the
chronic phase of disease, mostly ending up in cirrhosis. Some
patients with the acute disease may go on to the subacute phase,
even after clinical recovery, or as postulated by the authors,
after a latent period of several years, progress to cirrhosis.
Braginskii & Bobokhadzaev (1965) related an experience
concerning the evolution of this disease that was similar to that
observed in the USSR. About 50 - 60% of cases made a full recovery
and 35% made a partial recovery with continuing hepatomegaly.
About 2% of cases developed persistent ascites with "loss of
working capacity". It has been suggested that these cases may
develop cirrhosis.
7.2 Salient Pathological Features of Veno-Occlusive Disease
The pathological features of the disease at different stages
have been described in detail (Terekhov, 1939, 1952; Dolinskaya,
1952; Bras et al., 1954; Bras & Watler, 1955; Bras & Hill, 1956;
Stuart & Bras, 1957; Stirling et al., 1962; Aikat et al., 1978;
Tandon, B.N. et al., 1978; Tandon, H.D. et al., 1978). The
following description of Bras & Watler (1955) characterizes the
morphological changes at different stages of the disease described
by most investigators.
Morphological features of the liver at autopsy in 19 patients
from Jamaica who died at various stages of VOD have been described.
The ages ranged from 10 months to 45 years. Fourteen patients were
below the age of 14 years. Of the 14 patients, 9 had developed
cirrhosis, 3 died of acute disease, and 7 had various levels of
fibrosis superimposed over acute VOD. In the acute stages, there
was acute centrilobular congestion. The centrilobular and
sublobular veins showed different degrees of thickening of the wall
and occlusion of the lumen, mainly due to subintimal swelling
composed of loose reticular tissues and a few cells including
endothelial cells, occasional histocytes, lymphocytes, and
polymorphs, suggesting an acute exudative process. In addition,
there was a small amount of fibrin. Organization of this exudate
gradually led to collagenization and thickening of the wall. The
centrilobular congestion resulted in compression and even
disappearance of liver cell cords. The reticular framework of the
liver lobule was frequently preserved but was ruptured at places.
Clearly recognizable thrombi occurred sporadically but were not
commonly seen. The portal veins and hepatic arteries were normal.
Stirling et al. (1962) in their description of the early lesion
of VOD of the liver also emphasized that thrombosis of the hepatic
veins was not an important histogenetic factor in the evolution of
the lesion. In the later stages of the disease, hepatic venous
occlusion was chronically established. Perivenous reticular
collapse and the resultant condensation and reduplication of the
reticular framework resulted in non-portal fibrosis and later
cirrhosis. Macroscopically, the cirrhosis looked like Laennec's
cirrhosis. The cirrhotic process, which was non-portal to begin
with, involved portal tracts which also became incorporated in the
connective tissue septa. The presence of oesophageal varices was a
common finding. Extra-hepatic collateral vessels and congestive
splenomegaly were frequently seen. Within the liver, intrahepatic
collateral circulation was established with the coalescence of
sinusoids. The larger hepatic veins and inferior vena cava did not
show any thrombi or other pathological change. The subintimal
swelling observed in the hepatic veins was not seen in any other
vessels in the body. The authors concluded that acute VOD
gradually leads to Laennec's cirrhosis, but, in the initial phases,
it is non-portal.
No clear dose or temporal relationships between the liver and
parenchymal and vascular changes in the liver lobules are evident
from the available human case reports.
It is notable that megalocytosis, which is the hallmark of
chronic PA toxicity in experimental animals and a morphological
manifestation of the antimitotic effect of PAs, has not been
observed in human subjects (Tandon, H.D. et al., 1978) (section
6.4.9).
Tandon H.D. (personal communication) has analysed all published
and unpublished observations including liver biopsies and autopsies
derived from 3 outbreaks of VOD of which two occurred in India
(Tandon, B.N. et al., 1976, 1977; Tandon, R.K. et al., 1976;
Tandon, H.D. et al., 1977) and one occurred in Afghanistan (Tandon,
B.N. et al., 1978; Tandon, H.D. et al., 1978). He commented on the
frequency with which the characteristic veno-occlusion may not be
seen in the needle biopsy in the acute phase of the disease, though
it is invariably observed in the autopsy material. Haemorrhages
may persist for up to 1 year after subsidence of acute symptoms.
There is no significant inflammation accompanying the veno-
occlusive changes. Cirrhosis was reported to have developed within
3 months in one patient, after an initial biopsy had shown no
fibrosis (Stuart & Bras, 1957).
Brooks et al (1970) made an ultrastructural study of the liver
in VOD in Jamaican children. They found extensive damage in the
sinusoidal epithelium resulting in entra-vasation of red cells in
Disse's space and between hepatocytes. The closed structure of the
vessels, the absence of fenestrations, the existence of a basement
membrane, and the presence of collagen in the wall contribute to
the resistance to cellular debris and erythrocytes which track up
Disse's space and tend to narrow the lumen of the sinusoid where it
enters the vein. Fibrin may be present in this location and
occasionally in the sinusoids, but not within hepatic veins
themselves. The venous block, therefore, does not appear to be the
result of thrombosis.
Pancreatic changes similar to those in Kwashiorkor were stated
to be commonly observed (Bras & Hill, 1956; Stuart & Bras, 1957).
7.3 Human Case Reports of Veno-Occlusive Disease
Available data on human cases are summarized in Table 15, with
the countries they have been reported from in alphabetical order.
The first report of this disease in man and its relationship with
consumption of wheat flour contaminated by seeds of a plant of the
genus Senecio was made by Albertjin in 1918 in a report made to the
government of South Africa, as quoted by Wilmot & Robertson (1920),
who gave the first account of this disease in scientific literature
from that country. It is possible that it may have existed even
earlier, as its occurrence in farm animals had been described since
the beginning of the century in veterinary literature (Bull et al.,
1968). Willmot & Robertson (1920) recorded the occurrence of
'bread poisoning' in South Africa for a period of about 10 years,
during which 80 cases had been observed, mostly resulting in death.
The detailed account of clinical data and pathological findings in
11 cases was consistent with what is known as VOD today. The flour
from which the bread was prepared was found to be contaminated with
the flower heads and seeds of Senecio spp.
Isolated case reports are available in the literature (Hashem,
1939; Wurm, 1939) describing changes in childrens' liver
characteristic of veno-occlusive disease; however, in these
studies, no mention was made of possible etiological agents.
A pattern of disease described in the Soviet literature as
dystrophy of the liver, and later as toxic hepatitis with ascites
has been known in the Central Asian republics of the USSR
especially Uzbekistan and Tadjikistan for a long time (Terekhov,
1952). The local inhabitants called the disease, characterized by
rapidly filling ascites, the "camel-belly" syndrome and are
reported to have long distrusted a weed with fine seeds, known
locally as "Kharmyk", as a source of the disease (Dubrovinskii,
1952) (it is notable that, in the Afghan outbreak, which occurred
close to the area in Uzbekistan where the disease had been known,
the toxic seeds were known as "Charmak" among the local
population). The "camel-belly" disease was associated with the use
of bread with a bitter taste (presumably caused by the mixing and
grinding of toxic seeds with the wheat grains). Two waves of
outbreaks occurred, the first between 1931-35 (Terekhov, 1939) and
the second between 1945 - 46 (Dubrovinskii, 1947; Ismailov,
1948a,b). In the first wave of outbreaks, approximately 1000 -
1500 subjects were estimated to have been affected with an overall
mortality rate of 13 - 15%. The age of the affected subjects
ranged from 3 to 50 years. In the second wave of outbreaks, 60 -
70% of the population in agricultural areas were estimated to have
been affected (Ismailov, 1948a,b). Domestic animals also suffered
from the disease (Dubrovinskii, 1947). During the second wave of
outbreaks, the disease was found to have been caused by
contamination of food crops with seeds of Heliotropium lasiocarpum
(Dubrovinskii, 1947; Khanin, 1948). The etiology was confirmed by
studies on a variety of experimental animals using the suspect seed
(Khanin, 1948; Kampanzev, 1952). Pathological features of the
disease in children have been described by Dolinskaya (1952) and in
all age groups by Terekhov (1939, 1952). First an acute form of
the disease was recognized, followed by hepatic cirrhosis, which
was found to result as a consequence of the initial disease
(Ismailov, 1948a,b; Savvina, 1952). The disease has since been
largely eradicated by the agricultural and public health measures
taken.
Table 15. Pyrrolizidine alkaloid poisoning and human reports of veno-occlusive disease (VOD)
(in alphabetical order of the countries reported from)
Country/ Age Suspected Name of Alkaloid Alkaloid Nature of Outcome Reference
number group vehicle of plant concentration lesion (cirrhosis/
cases intoxication and intake died)
Afghanistan
8000 <14-80 contaminated Heliotropium mainly 1.32-1.49 g/kg all stages; many died Tandon &
(approximately) years wheat flour popovii heliotrine, (in the mostly acute Tandon (1975);
gillianum in addition seeds) daily to subacute Mohabbat
to 1 or 2 intake, 2 mg; et al. (1976)
alkaloids total
similar to consumption,
lasiocarpine up to 1.46 g
(75 - 100%
as N-oxides)
Barbados
3 2-4 herbal Crotalaria not analysed acute lesions no details Stuart & Bras
years infusion retusa (1956)
China, People's Republic of
2 49-57 herbal Gynura not analysed acute lesions died Hou (1980)
years infusion segetum
(Compositae)
Ecuador
1 35 years herbal Crotalaria not analysed acute lesions recovered Lyford et al.
infusion juncea (1976)
Table 15 (cont'd)
Country/ Age Suspected Name of Alkaloid Alkaloid Nature of Outcome Reference
number group vehicle of plant concentration lesion (cirrhosis/
cases intoxication and intake died)
Egypt
3 not known Hashem (1939)
59 1-12 plant seed not acute lesions cirrhosis Safouh &
years decoction identified in one Shehata (1965)
in 17
Federal Republic of Germany
8 75-116 not known acute lesions all died Wurm (1939)
days (alimentary
toxaemia)
Hong Kong
4 23-28 herbal Heliotropium pyrrolizidine herbs-alkaloid acute VOD 1 died Kumana et al.
years infusion lasiocarpum alkaloids base 0.42 g/kg; (1983, 1985);
N-oxides Culvenor et al.
1.4 g/kg; daily (1986)
intake, 12 mg
base, 18 mg
N-oxide; total
intake, 570 -
1350 mg up to
development of
symptoms in 3
patients over
19-45 days,
1300 mg up to
Table 15 (cont'd)
Country/ Age Suspected Name of Alkaloid Alkaloid Nature of Outcome Reference
number group vehicle of plant concentration lesion (cirrhosis/
cases intoxication and intake died)
Hong Kong (cont'd)
death in 1
patient. 630 mg
in one case who
remained
asymptomatic.
India
2 25 and herbal not unknown acute recovered Gupta et al.
35 years decoction identified (1963)
and pills
25 12-38 possibly not not analysed acute 12 died; Tandon, R.K.
years 6 contaminated identified lesions; 1 cirrhosis et al. (1976);
patients cereal cirrhosis Tandon, H.D.
studied) in one et al. (1977)
108 3-60+ contaminated Crotalaria crotananine 0.81 g/kg various up to 63% Tandon, B.N.
cereal nana and stages of died et al.
crotaburmine disease (1976, 1977)a
Siddiqui
et al.
(1978a,b)
Table 15 (cont'd)
Country/ Age Suspected Name of Alkaloid Alkaloid Nature of Outcome Reference
number group vehicle of plant concentration lesion (cirrhosis/
cases intoxication and intake died)
India (cont'd)
35 1- > 25 contaminated Crotalaria as above 5.3 g/kg seed acute as above Krishnamachari
years cereal (0 - 1.9% of et al.
seeds in (1977)a;
cereal); daily Siddiqui et al.
intake, 40 mg (1978a,b)
6 20-70 herbal Heliotropium heliotrine 12 - 20 g/kg; acute 3 died Aikat et al.
medicine in eichwaldii as N-oxide total intake, lesions; (2 with (1978);
4 cases by 3 4 g and 10 g cirrhosis fulminant Datta et al.
patients in patients who in one disease) (1978a,b)
died of organizing
fulminant thrombi in
disease hepatic veins
in 4 cases
Iraq
9 3-10 possibly possibly not acute 1 died Al-Hasany &
years wheat flour Senecio identified Mohamed (1970)
South Africa
11 11-19 wheat flour Senecio not centrilobular "majority Wilmot &
(only 5 years as bread ilicifolius; identified haemorrhages; died" Robertson
described) S. burchelli senecionine? central vein (1920)
"dilated"
Table 15 (cont'd)
Country/ Age Suspected Name of Alkaloid Alkaloid Nature of Outcome Reference
number group vehicle of plant concentration lesion (cirrhosis/
cases intoxication and intake died)
South Africa (cont'd)
12 wheat not not centrilobular Selzer & Parker
flour identified; identified haemorrhages; (1951)
"possibly organizing
Senecio" thrombi in
hepatic veins;
3 cases of VOD
12 5-30 herbal not none found "acute" in 3 died of Stein &
months medicines identified in herbal 5 cases; disease; Isaacson (1962)
admitted in medicines "subacute" no follow-up Stein (1957)
4 cases; in 7 cases; on 5
food not no detail
analysed
United Kingdom
1 26 years herbal Maté-Paraguay identified "trace acute with died McGee et al.
infusion tea only as amounts" veno-occlusion (1976)
pyrrolizidine going on to
alkaloids chronic with
fibrosis
1 13 years herbal Symphytum not analysed not known "thrombotic" Weston et al.
infusion officinale variant of (1987)
veno-occlusive
disease
Table 15 (cont'd)
Country/ Age Suspected Name of Alkaloid Alkaloid Nature of Outcome Reference
number group vehicle of plant concentration lesion (cirrhosis/
cases intoxication and intake died)
USA
1 2 months herbal Senecio riddelline 15 g/kg as acute lesions died Fox et al.
infusion longilobus and N-oxides alkaloids; (1978)
of retrorsine, total intake,
seneciphylline, 66 mg
and senecionine
1 6 months herbal Senecio as above 3 g free acute lesions cirrhosis Stillman et al.
infusion longilobus alkaloid plus (1977);
10.5 g N-oxide Huxtable (1980)
per kg; total
intake,
70-147 mg of
combined
alkaloid
and N-oxide
1 49 years herbal Symphytum sp. symphytum 14.1 µg/kg acute lesions recovered Ridker et al.
infusion pyrrolizidine body weight after (1985);
alkaloids per day; total short Huxtable et al.
intake, 85 mg surgery (1986)
USSR
1000 - 1500 3-50 contamination Heliotropium heliotrine, acute lesions 13-15% Mirochnik
years of wheat lasiocarpum lasiocarpine died; (1938);
flour cirrhosis Terekhov
in many (1939)
Table 15 (cont'd)
Country/ Age Suspected Name of Alkaloid Alkaloid Nature of Outcome Reference
number group vehicle of plant concentration lesion (cirrhosis/
cases intoxication and intake died)
USSR (cont'd)
"large contamination Heliotropium heliotrine Dubrovinskii
number" of wheat lasiocarpium lasiocarpine (1947);
flour Ismailov
(1948a,b)
Venezuela
1 5 years "earth and not acute lesions died Grases & Beker
plant eating identified (1972)
habits"
West Indies
11 14 months not known, acute to data Jelliffe et al.
to possibly cirrhosis unclear; (1954b)
9 years herbal cirrhosis
infusions in some
5 23 months not known, not 1 Bras et al.
to 18 possibility identified cirrhosis (1954)
years of "bush
teas"
4 16-45 history of not acute to 3 died Stuart & Bras
years herbal identified acute-on- (1955)
infusion in chronic
one case
Table 15 (cont'd)
Country/ Age Suspected Name of Alkaloid Alkaloid Nature of Outcome Reference
number group vehicle of plant concentration lesion (cirrhosis/
cases intoxication and intake died)
West Indies (cont'd)
84 0.5-53 probably not 11% had Stuart & Bras
years "bush teas" identified; cirrhosis; (1957)
(Senecio and 27% died
Crotalaria)
23 possibly not stated not analysed acute to Bras & Hill
bush teas "cirrhosis" (1956)
(no breakup)
23 1-50 no precise no analysis cirrhosis Bras et al.
years identification; (1961)
"Crotalaria
fulva and
possibly
other toxic
factors"
3 "children" "toxic not acute died Stirling et al.
substances identified (1962)
in herbal
remedies"
a Pertains to the same outbreak.
Selzer & Parker (1951), from South Africa, described 12 cases,
10 of whom came from 3 families that had eaten bread made of flour
of "imperfectly winnowed wheat". Five cases who were autopsied
showed characteristic occlusion of the central vein of the liver
lobules.
Attention on veno-occlusive disease as an entity followed a
spate of reports, mostly from Jamaica, on the occurrence of this
disease, mainly among children (Bras et al., 1954, 1961; Jelliffe
et al., 1954a,b; Bras & Watler, 1955; Bras & Hill, 1956; Stirling
et al., 1962) but also among adults (Stuart & Bras, 1955, 1957).
The youngest patient reported was a 3-month-old infant (Stein &
Isaacson, 1962), but the disease was stated even to have been
observed in the new-born (Stein, 1957). Very often, several
members of the family were affected by disease, which presented
primarily as rapidly filling ascites, within a matter of days,
sometimes accompanied by fever, and leading to hepatic failure. It
was considered an important cause of cirrhosis among children in
Jamaica (Jelliffe et al., 1954a,b). On the basis of evidence of an
almost identical disease occurring among grazing animals (Hill,
1960), and a prevalent practice among the Jamaicans of using herbal
infusions for treating a variety of ailments as a home remedy,
herbs, notably of the Senecio and Crotalaria groups, were suspected
of being a contributory factor (Bras & Hill, 1956; Bras et al.,
1957, 1961; Stuart & Bras, 1957).
Hill (1960) gave a comprehensive account of current knowledge
up to that time of the world-wide distribution of seneciosis in man
and animals in which mention is made of this disease only in Egypt,
the Federal Republic of Germany, South Africa, and the West Indies.
Pyrrolizidine alkaloids were identified as the active element in
the toxic factor in the plants.
However, there are earlier reports of this disease from
elsewhere. Bras (1973) cited a number of reports from Europe from
1905 to 1949, including reports by Wurm (1939) and Teilum (1949),
of an entity in infants and children that was called Endophlebitis
hepatica obliterans. He reported having studied some tissue
sections of liver in Austrian children aged 7 months - 1 year, who
had died with clinical and histological patterns of VOD. Some of
these conformed to the Budd-Chiari syndrome whereas others were
consistent with VOD. The case of a 36-year-old woman reported by
Teilum (1949) does not, however, fall in the usual category of
cases accepted as veno-occlusive, since thrombotic changes were
also seen in the larger hepatic veins and several, in the
peripheral parts of the body, as well as the portal vein.
A comprehensive account of all available published reports of
this disease to date from different parts of the world is given in
Table 15. A clear distinction between the acute and chronic
effects of exposure is not always possible from the published data,
since, in most patients, the history of onset of disease with
intake of the toxic substance is not generally volunteered or
available, and so a precise temporal relationship is difficult to
establish. However, this information is available in a reasonably
accurate form in more recent reports (Stillman et al., 1977; Datta
et al., 1978a,b; Fox et al., 1978; Kumana et al., 1985).
Gupta et al. (1963) from India described 2 cases who had drunk
some herbal infusions for the treatment of skin disorders. The
liver biopsies confirmed changes typical of acute VOD. However,
the herb was not identified and no analysis was made of the
infusion for alkaloids.
The cases of 59 children in Egypt who had symptoms of rapidly
developing abdominal distension and hepatomegaly were reported by
Safouh & Shehata (1965). Their ages ranged between 1 and 12 years,
47 being below 4 years of age. A dietary survey carried out on 17
patients indicated ingestion of drinks made by boiling some common
plant seeds, but these were not identified. Wedge biopsies of
liver were performed in 6 patients and 16 postmortem examinations
were made. In all the autopsy cases, there was thrombotic
obliteration of the main hepatic veins and their ostia. The
central and sublobular veins were not uniformly thickened as they
were frequently dilated or disrupted and some contained fresh
thrombi. There was centrilobular necrosis of the liver lobules.
One patient developed decompensated cirrhosis in 3 years. The
authors observed that the clinical and pathological features
closely resembled those of VOD in Jamaica, but thrombosis was an
unusual feature of the latter disease.
Al-Hasany & Mohamed (1970) described a short outbreak in Iraq,
occurring during a 3-month period, affecting 9 children, all except
one being below 12 years of age, and belonging to 3 Bedouin
families living outside Baghdad. One of the children died.
Autopsy of this case and biopsies on the others showed changes
characteristic of VOD. Poisoning through the wheat flour
contaminated by seeds of some PA-containing plant was suspected,
but no analysis of the food was carried out.
Three of the largest outbreaks of the disease have been
reported from South Asia, two from the same site in central India
(Tandon, B.N. et al., 1976, 1977; Tandon, R.K. et al., 1976;
Krishnamachari et al., 1977; Tandon, H.D. et al., 1977) and one
from North-West Afghanistan (Tandon & Tandon, 1975; Mohabbat et
al., 1976; Tandon, B.N. et al., 1978; Tandon, H.D. et al., 1978).
The first Indian outbreak was reported by Tandon, B.N. et al.
(1976) and Tandon, R.K. et al. (1976) and Tandon, B.N. et al.
(1977) and Tandon, H.D. et al. (1977). It occurred in a group of 5
tribal villages in central India in 1972 - 73. The epidemiology of
the outbreak was later described by Arora et al. (1981). Out of a
total population of 2060 in these villages, 71 households with 366
members were investigated. Among these, 39 cases had developed and
19 had died before commencement of the investigations. The
incidence rate was 1.1% and the case fatality rate was 50%. All
cases occurred among 20 households. In many households, several
members were affected. In one household, 4 out of 5 cases died.
Six sick patients were investigated in detail with repeated liver
biopsies. One patient died 17 months after onset and was
autopsied. The clinical onset was characteristic of disease.
Haemodynamic and radiographic studies suggested a combined post and
perisinusoidal block. Liver biopsy studies showed features
characteristic of acute centrilobular haemorrhagic necrosis with
progressive fibrosis, hepatic veno-occlusion, and non-portal
cirrhosis in one case who survived 17 months after the acute onset.
The etiological factor of this outbreak was not established, though
dietary contamination with pyrrolizidine alkaloids was considered.
A second outbreak occurred at the same site in 1975 and was
studied and reported independently by Krishnamachari et al. (1977)
and Tandon, B.N. et al. (1976, 1977). A total population of 486
was affected, 67 cases were reported, of whom 28 (46%) had died.
There was a strong family history (Tandon, B.N. et al., 1976). In
a later survey, 108 patients were studied and the mortality rate
was estimated to be 63% (Tandon, B.N. et al., 1977). This time the
etiological factor was identified as the plant Crotalaria nana
Burm, which had been growing in the fields of millet (Panicum
miliare), their staple food crop. The seeds of this plant became
mixed with the cereal grain during harvesting. The toxic seeds
contained pyrrolizidine alkaloids that were identified as a
macrocyclic ester closely similar to monocrotaline. The total
alkaloid content was estimated to be 5.3 g/kg of seed, expressed as
monocrotaline. The levels of contamination of the millet with
seeds were reported to be 0 - 3.4 g/kg in the unaffected and
0 - 19 g/kg in the affected households (Krishnamachari et al., 1977).
A precise identification could not be made, but the same material,
independently studied by Siddiqui et al. (1978a,b), were reported
to contain 2 new alkaloids, cronaburmine and crotananine. The seed
contained an alkaloid level of 26 g/kg. The levels of
contamination of the millet were up to 20 g/kg and the amount of
alkaloid ingested was estimated to be up to 40 mg per day.
The largest outbreak reported to date occurred in the Gulran
district of Herat Province in northwest Afghanistan, close to the
border of the USSR. Tandon & Tandon (1975) identified the plant as
being causative factor of the disease, which was surveyed and
reported in detail by Mohabbat et al. (1976). The outbreak, was
estimated to have affected a population of approximately 35 000 in
98 villages. Examination of 7200 inhabitants of the affected
villages showed evidence of disease in 22.6%, which was more
serious in 15%. Thus, it was estimated that approximately 8000
subjects suffered from the disease including 5000 who were
seriously affected. All age groups were affected, but 46% of
subjects were below 14 years of age. However, no sign of disease
was found in children below 2 years of age. A detailed report
concerning the pathological material obtained from 14 liver
biopsies and 8 autopsies was made by Tandon, B.N. et al. (1978) and
Tandon, H.D. et al. (1978). The time interval between the onset of
symptoms and the biopsy/autopsy was not indicated. Pathological
findings were characteristic, ranging from acute disease with
characteristic veno-occlusion to non-portal cirrhosis, which was
observed in 5 of the above 22 cases. The outbreak was ascribed to
massive contamination of wheat, the staple food crop, following 2
years of drought, with the seeds of Heliotropium popovii H. Riedl
subsp. gillianum H. Riedl, which had been growing profusely among
the wheat crop. The seeds contained pyrrolizidine alkaloids at
concentrations reported by 2 laboratories to be 7.2 and 13.2 -
14.9 g/kg, identified as mainly as the N-oxide of heliotrine (74%)
(Mattocks, personal communication), and one or two other compounds
similar in character to lasiocarpine. Samples of wheat from
several villages contained an average of 40 seeds/kg, i.e., 0.03%
by weight. It was estimated that an adult consumed at least 700 g
flour/day, containing approximately 2 mg alkaloid (based on a mean
of the seed analyses). There is some uncertainty about the
estimate, since Mohabbat et al. (1976) also stated that the samples
of the wheat flour were assayed and contained 0.186 - 0.50%
alkaloid. This analysis and the 0.72% result for alkaloid in the
H. popovii seed were from the same laboratory in Kabul (R.N.
Srivastava, personal communication), and together, imply 13 - 36%
seed in the wheat. If correctly reported, the result for the flour
conflicts with the estimated proportion of the seed in the wheat
and can scarcely have been representative.
Tandon & Tandon (1975) stated in their report of the survey
during which the causative factor of the Afghan outbreak was
discovered, that such cases had always been observed by Government
physicians posted in this area in the past, sometimes in
significant numbers, but neither the population nor the physicians
remembered that the disease had occurred in the form of an
outbreak.
There was no mention of VOD in the hepatic lesions observed by
Sobin et al. (1969) among the 121 specimens of liver obtained at
medico-legal autopsies or by needle biopsies at Kabul, though 6.6%
of the 89 autopsy specimens showed "acute passive congestion with
necrosis". The ages of these subjects was not stated and the
authors did not discuss the cause of the lesion.
Following these outbreaks, a number of isolated cases have been
described following the use of herbal medicines. McGee et al.
(1976) reported a case from the United Kingdom of a 26-year-old
woman who had consumed herbal tea containing pyrrolizidine
alkaloids. The one sample examined contained only trace amounts of
alkaloids. Neither the plant nor the alkaloids were further
characterized. The patient had been taking very large quantities
of the tea for about 2 years and it is possible that some of the
earlier batches may have been more heavily contaminated. Maté or
Paraguay tea ( Ilex species), which she was drinking, is stated to
be a popular drink in Brazil and is not believed to contain
pyrrolizidine alkaloids. It has been stated (Huxtable, 1980) that
possibly she ingested the pyrrolizidine alkaloids from some other
unidentified source. The clinical course of the disease progressed
rapidly. Three biopsies carried out within one month and the
autopsy showed characteristic changes including centrizonal
fibrosis, but no cirrhosis. It is notable that some involvement of
muscular pulmonary arteries was also seen.
Lyford et al. (1976) reported the case of a 35-year-old woman
from Ecuador, who had ingested herbal tea prepared from Crotalaria
juncea. She had consumed 1 - 2 litres of this infusion daily for 6
weeks, but no qualitative or quantitative analysis for
pyrrolizidine alkaloids was made. She had had arthralgias for 3
years, for which she had received treatment with indomethacin and
phenylbutazone. The liver biopsy showed characteristic changes of
acute VOD from which she recovered completely as proved by a repeat
biopsy carried out one year later.
The occurrence of acute disease was reported in 2 infants in
Arizona in the USA, aged 6 months and 2 months, respectively,
following ingestion of infusions of a herb, locally called the
Gordolobo Yerba by the Mexican-American population and identified
as Senecio longilobus (Stillman et al., 1977; Fox et al., 1978;
Huxtable, 1980). The plant from which this infusion had been
prepared was found to contain pyrrolizidine alkaloids identified as
riddelline and N-oxides of retrorsine, seneciphylline, and
senecionine (Huxtable, 1980), in a concentration of 3 g free
alkaloid and 10.5 g N-oxides/kg (Stillman et al., 1977). It was
estimated that, during a period of 2 weeks, the 6-month-old infant
received a total dose of between 70 and 147 mg of combined alkaloid
and N-oxide derivative. The liver biopsy showed characteristic
features of acute disease, which had progressed to extensive
central, portal, and sinusoidal fibrosis (Stillman et al., 1977).
The child subsequently developed cirrhosis over the next 8-month
period. The 2-month-old infant was administered an infusion of the
same herb for 4 days, after which he became progressively more ill
and stuporous (Fox et al., 1978). On admission he was diagnosed as
a case of Reye's syndrome, but subsequently developed jaundice and
possibly ascites and died. The sample of herb contained a
concentration of alkaloids of 15 g/kg. It was estimated that the
infant had received a total of 66 mg of mixed alkaloids over the
4-day period. At autopsy, extensive centrilobular haemorrhagic
necrosis of the liver was seen, which is characteristic of the
acute disease. However, no occlusive lesions of the central vein
of the lobules were described, and no obstructive lesions were seen
in the larger hepatic veins or inferior vena cava. No mention was
made of ascites. The basal ganglia showed kernicterus.
Datta et al. (1978 a,b) reported 6 cases that occurred between
1974 and 1977. All of the patients had taken herbal medicines,
identified in 1 case as Heliotropium eichwaldii, which contained
N-oxides of heliotrine. Two patients took the herb as an extract
of the whole plant, which contained an alkaloid concentration of
20 g/kg, for 20 and 50 days, respectively, and developed symptoms
after a time lag of 45 and 90 days, respectively. They were both
estimated to have consumed 200 mg of heliotrine per day, the total
alkaloid intake being 4 g and 10 g, respectively. They had taken
the herb for treatment of epilepsy, and were admitted with acute
onset of symptoms of abdominal pain, ascites, jaundice, hepatic
encephalopathy, and gastrointestinal bleeding, which suggested
fulminant viral hepatitis. They died within 2 - 12 weeks of the
onset of symptoms. Only a brief description of the main autopsy
findings in the liver was given, which indicated that there were
changes characteristic of acute veno-occlusive disease of liver,
including marked centrilobular haemorrhagic necrosis of the liver
lobules, and occlusive lesions of the central and sublobular veins.
It is interesting to note that both patients had also been on long-
term anticonvulsant phenobarbitone therapy. The remaining 4
patients had a chronic insidious onset of disease suggesting
cirrhosis of the liver in 3, and alcoholic liver disease in one.
One of the former had been taking some indigenous powder,
presumably prepared from a herb, the indication and nature of which
are not known. The patient died from hepatic encephalopathy. No
detailed description of the autopsy findings was given, but it was
stated that the central and sublobular veins of the liver showed
chronic occlusive changes. The inferior vena cava and large
hepatic veins were patent. There was non-portal cirrhosis. A
notable feature was that the arsenic levels in the liver tissue
were high (500 µg/kg; normal, 1 µg/kg). There is no mention of
arsenic in the report on the analysis of the indigenous powder
taken by the patient. Two of the remaining 3 patients with chronic
disease, had taken herbal medicine for vitiligo, and one for
diabetes mellitus. The herb was identified as Heliotropium
eichwaldii in the case of one of the vitiligo patients, who had
taken it for 10 days and in whom the onset of symptoms occurred
within 10 days. The herb was taken in the form of seed with an
alkaloid content of 12 g/kg. The daily intake was estimated to be
500 mg of alkaloid and the total intake, 5 g. This patient was
admitted with a clinical diagnosis of cirrhosis of the liver. The
liver biopsy showed changes characteristic of acute VOD. Follow-up
data are not known. The herb taken by the other 2 patients was not
identified. The diabetic patient was being treated with oral
hypoglycaemic drugs and was also known to be an alcoholic. The
indigenous powder being taken by him contained a high concentration
of arsenic (5 mg/kg). The results of haemo-dynamic studies
suggested hepatic venous outflow tract obstruction of the
intrahepatic post-sinusoidal type in the smallest hepatic veins.
Liver biopsy showed characteristic centrilobular haemorrhages.
Central veins could not be recognized. There were mild changes in
the liver cells, but no alcoholic hyaline was seen. The liver
biopsy of the third patient showed characteristic features of acute
disease with veno-occlusion.
Two cases have been reported from China (Hou et al., 1980).
Both were adults who were taken ill after taking medicinal
infusions prepared from Gynura segetum of the family Compositae
(tribe, Senecioneae). The presenting symptoms and the cause of
disease, as well as the pathological findings, were characteristic,
except that one patient had jaundice and also portal vein
thrombosis, which is not a usual feature of the disease. No
chemical analysis of the plant was made and the alkaloid was not
precisely identified. Furthermore, the total intake of alkaloid
was not calculated. It has been stated that this was the first
report of such a case, but that it was possible that the disease
might occur among adults more frequently without being reported.
Ghanem & Hershko (1981) reported 3 cases of Arabs, one 3-year-
old child and 2 adults, who were diagnosed as having VOD of the
liver, on the basis of the clinical findings and morphological
features of liver biopsies. One more patient had occlusive lesions
of both the small and large hepatic vein radicles. They were among
29 patients with clinical features of hepatic vein thrombosis (HVT)
found on a retrospective analysis of data from 9 major hospitals in
Israel. Of these patients, 15 were Jews and 14 were Arabs.
Notable features were that all Jewish patients were adults, whereas
the majority of Arab patients were children below 10 years of age
and primary HVT was 2.4 times more common among the latter. No
analysis of the diet was made for PAs and their etiological role
was suspected only on a presumptive basis. A survey of stored
wheat grain in 9 villages showed that 2 samples were contaminated
with seeds of Lolium, belonging to the Graminae family, which were
found to contain 2 PAs (loline and norloline). However, these PAs
are not known to be hepatotoxic. The authors argued that, even
though in a classical case of VOD there should not be thrombosis of
the larger hepatic vein radicles, the difference in the anatomical
appearance of VOD and that of primary HVT of the near-east type is
not due to a different etiological agent but rather to a difference
in the dose and rate of absorption of the ingested toxic compounds.
A further report of the disease from Hong Kong, by Kumana et
al. (1985), described it in 4 young Chinese women with psoriasis
who took infusions of a herbal remedy, the toxic component of which
has since been identified as Heliotropium lasiocarpum (Culvenor et
al., 1986). They developed symptoms 19 - 45 days after starting
the herbal treatment, and were examined 61 - 68 days after its
initiation. The condition of patient No. 2, who continued taking
the herb for 16 days after the onset of symptoms, deteriorated and
she died of hepatic failure and was autopsied. The liver biopsies
and autopsy confirmed the presence of acute disease in all
patients. Patient No. 4 stopped taking the herb after 21 days, on
account of a new rash. When assessed 77 days later, she had mild
hepatomegaly only. A detailed analysis of the alkaloid content was
carried out for each case. The pyrrolizidine alkaloids were
quantified as if senecionine based. The herb contained 0.42 g
alkaloid/kg and 1.4 g N-oxide/kg. The daily intakes of alkaloid
base and N-oxide were estimated to be 12 ± 1 mg and 18 ± 4 mg,
respectively. The respective cumulative doses of alkaloid (base
and N-oxide) consumed by patients Nos 1, 2, and 3, up to onset of
symptoms, were calculated to be 1350 mg over 45 days, 900 mg over
30 days, and 570 over 19 days, respectively. Patient No. 4 who had
irrefutable histological evidence of disease but did not develop
symptomatic disease, must have consumed 630 mg over 21 days.
Patient No. 2, who died, was estimated to have taken a total amount
of 1380 mg alkaloid over 46 days. The estimated cumulative intake
per kg body weight before the development of symptoms for patients
Nos. 1, 2, 3, and 4 was 26, 15, 12, and 15 mg/kg, respectively. It
should be noted that, in patient No. 2, who died, the cumulative
dose until the onset of symptoms was the same as in patient No. 4,
who was asymptomatic. Moreover, the total intake by patient No. 2,
was 23 mg/kg, which was lower than the intake by patient No. 1, who
survived. The authors compared the intake data of their patients
with those of the Arizona children reported by Stillman et al.
(1977) and Fox et al. (1978). The 6-month-old baby, who survived
but developed cirrhosis, and the 2-month-old baby, who died, are
estimated by comparison to have taken cumulative doses of 12 - 25
and 11 mg/kg, respectively. The above data suggest marked
variation in susceptibility among individual subjects. It is also
known from experimental animal studies that the young and new-born
animals are particularly vulnerable (Jago, 1970).
A case reported from the USA is ascribed to the consumption of
comfrey ( Symphytum sp.) powder, sold as a digestive aid (Ridker et
al., 1985). A 49-year-old woman presented with classical symptoms
and signs of VOD. The haemodynamic data showed a hepatic vein
wedge pressure of 3.07 kPa (23 mmHg) with a sinusoidal pressure of
2.27 kPa (17 mmHg). Hepatic venograms showed near obliteration of
the smaller radicles of the hepatic veins during balloon distension
of one of the intrahepatic venous tributaries, and there was extra-
vasation of the dye into the hepatic parenchyma. A porta-caval
shunt was carried out and the operative findings confirmed the
presence of a post-sinusoidal block. The liver biopsy showed
marked centrilobular necrosis and congestion with dilatation of the
central veins and sinusoids, consistent with hepatic venous outflow
tract obstruction. According to the clinical history, the patient
had been a heavy consumer of food supplements. Apart from several
vitamins and minerals, she had been drinking 3 cups of camomile tea
per week and for 6 months before admission had consumed 1 g/day of
a commercially available herbal tea. For 4 months before
admission, she had taken 2 capsules of "comfrey-pepsin" pills with
each meal. The herbal tea and the pills were analysed for PAs.
Pyrrolizidine alkaloids and their N-oxides were found, but the
compounds were not precisely identified. On the basis of the
analysis of the PA content, the patient was estimated to have
consumed a total of at least 85 mg of PA (Huxtable et al., 1986)
(14.1 µg/kg body weight per day, Ridker et al., 1985). The authors
emphasized that the total PA consumption was relatively low. It
was possible that the patient had other sources of exposure and
probably she had been consuming PA-containing supplements for
longer than the periods stated by her in the clinical history.
The latest is the report of a 13 year old boy from the U.K. who
is stated to have developed symptoms of toxicity following
administration of herbal tea prepared from comfrey leaf (Symphytum
officinale) for treatment of inflammatory bowel disease for two or
three years (Weston et al., 1987). The exact quantity of leaves
consumed and frequency of administration were not known. The liver
biopsy is stated to have shown a "thrombotic variant" of veno-
occlusive disease, though the inferior vena cava and the major
hepatic veins were patent on Doppler ultrasound and percutaneous
phlebography. He had earlier been treated with predinisolone
and sulphasalazine. The case is unusual in so far as the
thrombosis of the central veins of the liver lobules, which is not
a usual feature of veno-occlusive disease of the liver.
7.4 VOD and Cirrhosis of the Liver
Jelliffe et al. (1954b) were the first to draw attention to VOD
being an important cause of cirrhosis among Jamaican children.
Prior to this, Hashem (1939), while reviewing the records of all
cases of cirrhosis admitted to a children's hospital in Egypt since
1933, described 3 cases of a special type of cirrhosis that was
rare in adults. The clinical features and pathological findings
were similar to those in cases of VOD. They speculated that the
cause was some metabolic toxins of gastrointestinal origin. Royes
(1948) suggested that the cirrhosis in the Jamaican children was
very like the disease described from India and Egypt.
The clinical and pathological features of 100 cases of VOD
among Jamaican children were described by Bras et al. (1954).
Sixty-five of the cases were below 12 years of age. None of the
100 patients had cirrhosis initially, but 5 showed occlusive
lesions in hepatic veins and features of non-portal fibrosis. Four
of these 5 cases later developed cirrhosis. The authors concluded
that VOD contributed to a substantial number of all cases of
cirrhosis in this age group. Stuart & Bras (1957) studied 84
patients with VOD including 64 acute, 6 subacute, and 14 chronic
cases. Twenty-three patients were followed up, some for up to 5
years. Autopsy performed on 21/26 cases showed cirrhosis in 11
cases. Notable features were that 1 of the 6 cases of acute
disease described in detail, developed cirrhosis. Of the 2 cases
with chronic disease, 1 developed cirrhosis within 3 months of a
liver biopsy for acute disease, at which time the liver had shown
hepatic venous occlusive lesions but no fibrosis. Autopsy findings
were described by Bras & Watler (1955) in 19 patients aged 10
months - 45 years in different stages of the disease. Nine
patients had cirrhosis that was non-portal to begin with but
finally became indistinguishable from Laennec's portal cirrhosis.
Rhodes (1957) studied the pattern of liver disease among Jamaican
children. A total of 193 liver biopsies was studied derived from
39 children who had one biopsy and 59 who had more than one at
intervals of 1 week - 3 years. Of the 14 autopsies on cases of
VOD, 12 had cirrhosis. A notable feature was that the disease
could occur asymptomatically with hepatomegaly. The autopsy
material from the University College Hospital, Jamaica was analysed
by Bras et al. (1961). Of the 1560 autopsies, 28.5% cases
concerned infants of less than one year old, mostly from poor,
predominantly black families. Cirrhosis was seen in 77 autopsies.
Approximately 30% of these 77 cases were diagnosed as post-VOD.
The authors postulated that they might have resulted from the
ingestion of Crotalaria fulva or some other toxic substances.
In a follow-up study of 61 patients who developed the disease
in an outbreak in the USSR in 1958, 28 developed "Hepatoleinal
syndrome" (Braginski & Bobokhadzaev, 1965). Two of these cases, in
whom the disease in the initial stages was not particularly severe,
developed cirrhosis within 4 years.
Tandon, H.D. (personal communication) has analysed the
pathological data derived from the Afghan and Indian outbreaks on
the basis of 61 liver biopsies and 17 autopsies, including repeat
biopsy studies on 15 patients who were followed-up for 1 month - 3
years after onset in the Indian outbreaks. Three of the 11
patients, followed up for 16 months or longer for persistent
clinical evidence of disease, ended up with cirrhosis and 2 more
had marked fibrosis with equivocal changes of cirrhosis in the
biopsy. Notable features of the study were that the disease
progressed to cirrhosis in patients who were put on a normal diet,
free from alkaloids, after appearance of symptoms of acute disease
and did not receive any subsequent exposure. Impact of alcohol
intake was excluded. There was a poor correlation between the
clinical and pathological severity of disease. Centrilobular
haemorrhages, which are a sign of acute disease, were seen to
persist for over one year in patients, some of whom were apparently
well. At needle biopsy, characteristic hepatic venous occlusions
were not seen in many patients in the acute phase of the disease,
though they were seen in all livers at autopsy and most of them
showed persistent centrilobular haemorrhages. Biopsy findings in
cirrhotic livers were often not histopathologically characteristic
for any specific form of cirrhosis. Features that might suggest
the veno-occlusive etiology of cirrhosis at biopsy included
paraseptal dilatation of sinusoids and persistent haemorrhages or
haemosiderin in the septa.
In studies by Aikat et al. (1978) and Datta et al. (1978a,b), 6
cases of VOD were reported following ingestion of herbal medicines
containing PAs. Four of the patients had symptoms of chronic
disease and one of them developed non-portal cirrhosis.
One of the 2 infants from Arizona, USA, who suffered from VOD
following the administration of PA-containing herbal medicine,
developed cirrhosis during the 8-month period following the
appearance of acute symptoms (Stillman et al., 1977; Fox et al.,
1978; Huxtable, 1980). Huxtable (1980) mentioned the death from
cirrhosis of liver of a 62-year-old woman, who had consumed the
same herb as the 2 infants for 6 months prior to her death.
However, there was no confirmation of the diagnosis of cirrhosis.
7.5 Differences Between VOD and Indian Childhood Cirrhosis (ICC)
A type of cirrhosis of liver, peculiar to the people of Indian
origin, Indian Childhood Cirrhosis (ICC), has been ascribed to PA
toxic etiology (Bras et al., 1954; Rhodes, 1957), owing to the
observation of occlusive changes in the central and sublobular
veins of the liver, by some investigators (Radhakrishna Rao, 1935;
Prabhu, 1940; Jelliffe et al., 1957; Ramalingaswami & Nayak, 1969),
though this is not a characteristic feature of the disease. The
presence of copper-positive granules in the hepatocytes (Salaspuro
& Sipponen, 1976) has added to such a conjecture (Tanner &
Portmann, 1981), because of the reported aberration of copper
metabolism in experimental animals exposed to PAs (section 6.4.11).
However, ICC and VOD are clinically and pathologically distinct.
ICC, confined to infants and children, often affects siblings.
Jaundice is a common sign and hepatosplenomegaly is a common
feature. The disease is almost invariably fatal due to rapidly
developing hepatocellular failure. Liver parenchymal changes are
characterized by marked ballooning degeneration of hepatocytes,
prominent deposits of alcoholic hyaline, severe cholestasis, and
aggressive, pericellular fibrosis (Nayak et al., 1969), all
features not characteristic of VOD. Moreover, occlusive changes in
the hepatic veins are very rare and were not observed by any member
of the liver diseases subcommittee of the Indian Council of Medical
Research (1955), who made a critical study of the disease.
7.6 Chronic Lung Disease
Heath et al. (1975) reported the case of a 19-year-old African
man who had died of congestive cardiac failure, and who was
suspected of having ingested a herbal remedy containing the seeds
of Crotalaria laburnoides. Histopathological examination of the
lungs showed characteristic vascular changes of severe primary
pulmonary hypertension. Powdered seeds of the plant were fed in
the diet to Wistar albino rats for 60 days (Table 11).
Characteristic features of pulmonary hypertension including
hypertensive vascular changes in the lung and right ventricular
hypertrophy of the heart were produced in the animals showing that
the seeds contained an agent capable of inducing pulmonary
hypertension in rats. Apart from this indirect evidence, there was
no proof of such a causal relationship with the pulmonary
hypertensive disease in the patient.
A brief mention is made by McGee et al. (1976) of finding
changes "somewhat similar but rather more mature" (than those seen
in the hepatic veins) involving some branches of the pulmonary
artery in the lower lobe of the left lung, in a case of veno-
occlusive disease of the liver caused by ingestion of PA-containing
herbal teas. The alkaloids were not further characterized. The
changes were also stated to be similar to those produced in
experimental animals by PAs. Apart from these 2 cases, there is no
mention of involvement of the pulmonary arterial system in any of
the case reports available.
The possibility that diet-mediated agents might induce
pulmonary hypertension in man has been discussed at length by
Fishman (1974). A parallel was drawn with the epidemic of
pulmonary hypertension that occurred in Austria, the Federal
Republic of Germany, and Switzerland between 1966 and 1968 (Kay et
al., 1971b), which was suspected of being caused by Aminorex, a
compound that resembles epinephrine and amphetamine in chemical
structure. Although the etiological role of Aminorex could not be
conclusively proved on epidemiological or experimental grounds,
there continues to be a suspicion that agents taken by mouth can
evoke pulmonary hypertension in man. Levine et al. (1973) reported
the cases of 3 children aged 5 1/2 - 13 years, and 11 months,
respectively, with portal hypertension, who developed progressive
pulmonary hypertension resulting in cor pulmonale and death. In
all 3 cases, there was evidence of extra-hepatic portal vein
obstruction confirmed at autopsy, and the symptoms and signs of
portal obstruction had appeared in early childhood. They developed
symptoms of cardio-respiratory failure. Studies of pulmonary and
cardiovascular function including haemodynamic studies of pulmonary
circulation in 2 of the children suggested pulmonary vascular
obstructive disease. At autopsy, no primary parenchymal lung
disease was found. There were vascular changes of advanced
pulmonary hypertension (plexiform lesions), but no evidence of
thromboembolism was found. No factor responsible for pulmonary
vasoconstriction was identified. In one case, the liver was stated
to show coarse nodularity at autopsy, but the microscopic
examination showed only patchy areas of portal fibrosis and
regeneration. In the other 2 cases, there were only non-specific
changes, with fibrosis in one. However, centrilobular congestion
was present in 2 cases. It is possible that some metabolites of
toxic agents, such as PAs, which are metabolized in the liver,
might have blocked a metabolic pathway that ordinarily exerts a
pulmonary antihypertensive effect, or that, by damaging the liver,
vasoactive substances, such as histamine, serotonin, and
catecholamines, might escape metabolic pathways to reach the lungs
and injure the pulmonary vessels. However, no such agents were
identified.
Kay et al. (1971b) made a plea that, on the basis of the
experimental data available, including the ability of several
agents to produce pulmonary hypertension in experimental animals,
and, in spite of the fact that pulmonary vascular disease has never
been demonstrated in human cases of veno-occlusive disease, careful
enquiries should be made on the possibility of patients presenting
with unexplained pulmonary hypertension having ingested a plant
product. A similar plea was made by Heath et al. (1975).
7.7 Trichodesma Poisoning
The disease "Ozhalanger encephalitis", which occurred in the
Samarkand region of Uzbekistan, USSR in the period 1942 - 51, is
believed to have been caused by contamination of food grain with
the seeds of Trichodesma incanum (Shtenberg & Orlova, 1955;
Ismailov et al., 1970), which contain 1.5 - 3.1% alkaloids, mainly
trichodesmine and incanine (Yunusov & Plekhanova, 1959). Clinical
features of the disease have been described by Ismailov et al.
(1970). This outbreak differed from the others described above in
that the primary symptomatology was extra-hepatic. Over 200
patients were affected, not including children below the age of 10,
or the breast-fed infants of the affected mothers. Following
exposure, there was a latent period of about 10 days, then vertigo
and recurring headaches developed leading to nausea and vomiting.
This was followed by generalized malaise, which progressed to
delirium and loss of consciousness. Physical signs included
pathological reflexes in 59% of the patients and paresis of the
extremities and the facial nerve. Death was stated to have been
caused mostly by respiratory depression. Of the 200 patients
affected, 44 died. Autopsy findings were relatively non-specific
degenerative and necrotic lesions scattered in several organs
including the central nervous system. No report of such a disease
is available from outside the USSR.
7.8 Relationship Between Dose Level and Toxic Effects
In some recent human case reports, the PAs consumed have ben
identified and estimates made of the daily and total intakes (Table
16). The relationship between these intake levels and the known
toxicity of the alkaloids in rats has been discussed by Culvenor
(1983) and Mattocks (1986).
Discussion of the relationship between the dose level and toxic
effects in human cases is complicated, because the poisoning is
generally due to a mixture of alkaloids found in naturally-
occurring plant products, consumed as herbal remedies or food, and
different plant species. There are wide differences in the acute
toxicities of the alkaloids, which are the best available measure
of comparative effects due to long-term intake as well.
Furthermore, estimates of intake can, at best, be approximate.
When a large population is affected through the contamination of a
food crop, as happened in the Afghan and Indian outbreaks (section
7.3), no precise estimates are possible regarding the extent of
contamination in different households, the amount of the
contaminated grain consumed, the length of exposure resulting in
the appearance of symptoms or signs of toxicity, or death, in the
cases studied. There may also be various other contributing
factors that are not apparent, e.g., food or cooking habits, on
account of which no conclusive generalizations regarding the
causative role of the toxic agent can be made. Reports on chemical
analysis for the toxic agent and, hence, the amounts ingested, may
not always be reliable (refer to the controversy in the Afghan
outbreak in section 7.3). In cases, such as the one reported by
Ridker et al. (1985), when the total dose of PAs estimated to have
been received by the patient before the disease developed, was only
a fraction of that of other alkaloids causing episodes of human
toxicity (Table 16), it is not certain whether that was the only
toxic alkaloid agent that the patient was exposed to, or whether
there were other contributing factors, particularly as the patient
was stated to be a heavy consumer of herbs, vitamins, and natural
food supplements.
In the known instances of human toxicity the principal
alkaloids involved are heliotrine from Heliotropium, echimidine and
related alkaloids from Symphytum, riddelline and retrosine from
Senecio longilobus, and crotananine and cronaburmine from
Crotalaria nana (Table 16). Approximate acute toxicity values
(LD50 in rats) for these alkaloid mixtures are 300, 500, and
50 mg/kg, respectively. For the mixture from C. nana, for which
there are no experimental data, the acute toxicity was assumed to
be similar to that of monocrotaline, 100 mg/kg. These relativities
need to be taken into account in discussing dose-effect
relationships for the PAs as a group. This has been done by
discussing first the dose estimates for heliotrine, since it was
the main alkaloid in 1 epidemic and in 6 case reports (Table 16).
Then in discussing the other alkaloids, reference is made to a
heliotrine-equivalent dose as well as the actual dose. The
heliotrine equivalent is:
LD50 of heliotrine
actual dose x ----------------------------------
LD50 of alkaloid mixture concerned
The estimated daily intake in poisoning by heliotrine ranges from
0.033 mg/kg body weight in the Afghan epidemic (Mohabbat et al.,
1976), which after a period of about 180 days and a total intake of
about 6 mg/kg caused fatalities, to 3.3 mg/kg, which led, in 2
cases in India (Datta et al., 1978a,b), to death after 20 and 50
days and total intakes of 67 and 167 mg/kg, respectively. In
between are 4 cases in Hong Kong with estimated daily intakes
ranging from 0.49 to 0.71 mg/kg (Kumana et al., 1983, 1985;
Culvenor et al., 1986). Three cases were non-fatal at total doses
of 11 - 27 mg/kg body weight and one was fatal at a total dose of
23 mg/kg. These heliotrine cases imply that daily intakes are
cumulative down to 0.033 mg/kg and may be fairly rapidly fatal
above 0.5 mg/kg. Above a total dose of 6 - 15 mg/kg, VOD may
become evident and sometimes fatal.
In the 2 cases due to riddelline and retrorsine in Senecio
longilobus (Stillman et al., 1977; Fox et al., 1978; Huxtable,
1980), the estimated daily intakes were 0.8 - 0.17 and 3 mg/kg body
weight (equivalent to 3 and 1 mg heliotrine/kg, respectively, and
the total doses were 12 - 25 and 12 mg/kg (equivalent to 72 - 150
and 72 mg heliotrine/kg, respectively). These levels are
comparable with the highest reported intakes of heliotrine and, in
infants, led to the rapid development of VOD and, in one case,
death.
In the epidemic due to mixed crotananine and cronaburmine in
Crotalaria nana (Tandon, B.N. et al., 1976; Tandon, R.K. et al.,
1976; Krishnamachari et al., 1977), the estimated daily intake of
0.66 mg/kg and the total intake of 40 mg/kg (equivalent to 2 and
120 mg heliotrine/kg, respectively) also corresponded to the
highest intake of heliotrine.
In the case of Symphytum poisoning (Ridker et al., 1985), the
estimated daily intake of echimidine and related alkaloids was
0.015 mg/kg and the total dose 1.7 mg/kg (equivalent to 0.009 and
1.0 mg/kg heliotrine, respectively). The dose levels are lower in
equivalent terms than the lowest estimates in cases due to
heliotrine by a factor of about 4 for daily intake and 6 for total
intake. The estimates were based on questioning of the patient and
assay of the material concerned. It seems prudent to conclude that
a daily intake of pyrrolizidine alkaloid as low as the equivalent
of 0.01 mg/kg heliotrine may lead to disease in humans.
Table 16. Estimated intakes of PAs in human beings
-----------------------------------------------------------------------------------------
Principal Age of Daily Period Total dose Toxic Reference
alkaloids(s)a subject intake (days) mg mg/kg effectc
(years) (mg/kg)b
-----------------------------------------------------------------------------------------
1. Heliotrine variousd 0.033 180e 360 6e VOD, Mohabbat et al.
death (1976)
2. Heliotrine (a) 20 3.3 20 4000 67 death Datta et al.
(b) 23 3.3 50 10000 167 death (1978a,b)
3. Riddelline, 0.5 0.8-1.7b 14 70-147 12-25 VOD Stillman et al.
retrorsine (1977); Huxtable
(1980)
4. Riddelline, 0.17 3.0b 4 66 12 death Fox et al. (1978)
retrorsine
5. Crotananine, variousd 0.66 c. 60e 2400 40 VOD, Tandon, B.N. et al.
crotaburmine death (1976); Tandon,
R.K. et al. (1976);
Krishnamachari et
al. (1977)
6. Heliotrine (a) 28 0.59 45 1350 27 VOD Kumana et al.
(b) 26 0.49 46 1380 23 death (1983, 1985);
(c) 23 0.60 19 570 11 VOD Culvenor et al.
(d) 27 0.71 21 630 15 VOD (1986)
7. Echimidine 49 0.015 120 94 1.7 VOD Ridker et al.
(1985)
-----------------------------------------------------------------------------------------
a The principal alkaloid is recorded as the free base, even if there was evidence of its
presence in the plant mainly as N-oxide.
b Calculated for a 60-kg adult, unless definite information available. The 0.5-year
infant was said to be 6 kg, and
c When mentioned, death was a consequence of severe liver damage.
d Epidemic.
e Estimate based on unpublished information available to the Task Group.
There is substantial overlap between intake rates and total
intakes for fatal and non-fatal poisoning. This presumably
reflects the influences of a number of factors, such as individual
sensitivity, age, nutritional status, and general health, but it is
also due to the progressive nature of pyrrolizidine toxicity and
the effects of time. In the epidemics, in which some people died,
only an estimated average intake is available and some who were
alive at the time of investigation may have died later. Comparing
the total intakes for human toxicity with the total doses up to
death observed in the long-term administration of PAs to rats,
1.2 - 10.9 times the LD50 dose, equivalent to 360 - 3270 mg
heliotrine/kg (Table 10, section 6.4.1.5), it is evident that human
beings are more susceptible to the acute and chronic effects of the
alkaloids than rats, sometimes markedly so.
These considerations of the toxic effects in human beings of
various intake levels could provide a basis for some assessment of
the likely hazard from other types of exposure to PAs. For
example, the consumption of comfrey root tea, estimated by Roitman
(1981) to contain 8.5 mg alkaloid per cup, at the rate of 3 cups
per day, or the ingestion of comfrey leaf at the rate of one leaf
per day, could lead to alkaloid ingestion rates of 0.40 and
0.016 mg/kg. These rates are respectively, much greater than, and
equal to, the lowest daily rate causing veno-occlusive disease. Lower
levels of exposure arising from such sources as the consumption of
milk from cows eating PA-containing plants or of honey derived from
such plants, seems unlikely, in practice, to cause acute or
subacute liver disease. However, care should be exercised. In an
experimental situation in which cows were fed Senecio jacobaea,
the milk was reported to contain up to 0.84 mg alkaloid/litre. A
30-kg child drinking 0.5 litre/day of this milk could ingest
0.014 mg/kg alkaloid, equivalent to 0.028 mg heliotrine/kg (assuming
an LD50 of 150 mg/kg for S. jacobaea alkaloid). This is above the
lowest daily rate leading to veno-occlusive disease and the lowest
estimated total toxic dose would be achieved in 36 days. This
level of contamination of milk is undoubtedly extreme and there is
no knowledge of any contamination of commercial milk supplies.
Honey derived from Echium plantagineum has been reported to contain
up to 1 mg alkaloid/kg (Culvenor et al., 1981). A 30-kg child
consuming 30 g/day of honey (a high consumption rate) would ingest
0.001 mg alkaloid/kg body weight. The lowest estimated total toxic
dose (1.7 mg comfrey alkaloid/kg, very similar to Echium alkaloid)
would be achieved in 1700 days. Although it seems likely that
consumption of contaminated milk and honey would lead to acute or
subacute liver disease, the possibility remains that they may
contribute to chronic liver disease or liver tumours.
The possibility of carcinogenic effects due to long-term
exposure to PA-containing plants has been discussed by Culvenor
(1983). Some of the PAs involved in instances of human poisoning
have been found to be carcinogenic in experimental animals (Table
13). Data from some of the significant experimental studies were
summarized by Culvenor (1983) with approximate estimates of PA
dosages administered to rats in terms of mg/kg body weight per day
(Table 17). The dose rates that were carcinogenic for rats (Table
17) ranged from 2 to 6 mg/kg per day for an initial period and
0.2 - 3 mg/kg per day for a remaining period of about 12 months,
except in one study in which a dose of 10 mg/kg per day was used.
It can be seen that, in all except two instances of human
poisonings summarized in Table 16, the estimated daily rates of
intake ranging from 0.015 to 3.3 mg/kg body weight per day are
within close range of those known to induce tumours in rats. In
other reports, the consumption rates are above and below this
range.
Epidemiological studies to assess the carcinogenic role of PAs
for man are not available. In countries with a high incidence of
primary liver cancer, it is possible that PAs may have an additive
effect with those attributed to aflatoxin (Newberne & Rogers, 1973)
and hepatitis B virus. The total evidence now available warrants
long-term studies of the survivors of poisoning outbreaks,
especially where a substantial number of people were affected, as
in the Afghanistan outbreak.
7.9 Pyrrolizidine Alkaloids as a Chemotherapeutic Agent for Cancer
The PA, indicine N-oxide derived from Heliotropium indicum, a
widely used indigenous drug in Ayurvedic medicine, has been found
to have an antitumour activity and has been used in clinical trials
as a chemotherapeutic agent for leukaemia (Letendre et al., 1981,
1984; Cook et al., 1983) and solid tumours (Kovach et al., 1979a,b;
Nichols et al., 1981; Ohnuma et al., 1982; Taylor et al., 1983).
Dosing schedules typically were 5 consecutive intravenous doses of
0.15 - 3 g/m2 body surface area (approximately 2.5 - 5 mg/kg body
weight) repeated at 4- or 6-week intervals (Kovach et al., 1979a;
Letendre et al., 1981; Ohnuma et al., 1982). Hepatic toxicity, as
judged by increased SGOP levels, was infrequent and mild. However,
subsequent trials with this agent have indicated more serious
hepatotoxicity. In a more recent report by the same workers
(Letendre et al., 1984), 5 out of 22 cases of refractory acute
leukaemia, treated with indicine N-oxide, had severe
hepatotoxicity, presumably induced by the drug. One of these
patients had been treated for 18 months with methyl-testosterone, 4
months prior to receiving indicine N-oxide. Symptoms of severe
hepatocellular failure appeared in 3 patients after the initial
course of treatment. This occurred after 4 daily doses of 3 g/m2
surface area in one patient and after 5 daily doses of 3.75 g/m2
surface area in 2 patients. Two other patients who had received
3 g/m2 surface area daily for 5 days developed hepatocellular failure
after the second course of treatment, one at 3.3 g/m2 and the other
at 3.75 g/m2 surface area, daily, for 5 days. In each patient, the
onset of hepatic disease was rapid and the course was downhill.
Livers of 4 of these patients examined at post-mortem showed severe
centrilobular vascular congestion with necrosis of parenchymal
cells, and, in one patient, a few sublobular veins were found to be
occluded.
Miser et al. (1982) reported severe hepatotoxicity in 4 of 45
children treated with indicine N-oxide for refractory leukaemia or
advanced solid tumours. Similarly, Cook et al. (1983) reported the
case of a 5-year-old child with acute myeloid leukaemia who
developed severe hepatic failure within 3 days of starting the
treatment. Autopsy showed massive hepatic necrosis.
However, it should be noted that no hepatic failure was
reported in more than 100 adults with solid tumours, treated with
the same agent (Kovach et al., 1979a,b; Nichols et al., 1981;
Taylor et al., 1983). No hepatotoxic effects were reported by
Ohnuma et al. (1982) among 37 patients who received this drug for
solid tumours. The major toxic effect was myelosuppression (Kovach
et al., 1979b).
Table 17. Rates of administration of PAs leading to tumours in ratsa
------------------------------------------------------------------------------------------
Alkaloid Dosing schedule Approximate Number of Reference
equivalent rateb rats
(mg/kg per day) developing
tumours
------------------------------------------------------------------------------------------
Lasiocarpine (a) 7 mg/kg diet, 24 months 0.70 23/24 Nat. Cancer
Institute
(1978)
(b) 15 mg/kg diet, 24 months 1.50 24/24 Nat. Cancer
Institute
(1978)
(c) 7.8 mg/kg, ip, 2/week for 2.2 for 4 weeks, 16/18 Svoboda &
4 weeks and 1/week for then 1.1 for Reddy (1972)
52 weeks 52 weeks
(d) 50 mg/kg diet, 55 weeks 5.0 18/20 Rao & Reddy
(1978)
(e) 0.39 mg/kg, ip, 3/week, 0.2 2/7 Culvenor &
to death Jago (1979)
Monocrotaline (a) 25 mg/kg, ip, 1/week for 3.5 for 4 weeks, 10/50 Newberne &
4 weeks, and 8 mg/kg, then 1.1 for Rogers (1973)
for 38 weeks 38 weeks
(b) 5 mg/kg sc, once per 0.36 43/60 Shumaker et
2 weeks for 52 weeks al. (1976)
Retrorsine (a) 30 mg/kg, ip, single dose - 7/29 Schoental &
Bensted (1963)
(b) 30 mg/litre in water, 1.3 4/14 Schoental et
3 days/week, to death al. (1954)
(c) 30 - 50 mg N-oxide/litre 1.3 - 2.0 10/22 Schoental et
in water, 3 days/week al. (1954)
for 20 months
------------------------------------------------------------------------------------------
Table 17. (contd)
------------------------------------------------------------------------------------------
Alkaloid Dosing schedule Approximate Number of Reference
equivalent rateb rats
(mg/kg per day) developing
tumours
------------------------------------------------------------------------------------------
Petasitenine 0.1 g/litre in water, 10 8/10 Hirono et
up to 16 months al. (1977)
Senkirkine 22 mg/kg, ip, 2/week for 6 for 4 weeks, 11/24 Hirono et
4 weeks and 1/week for then 3 for al. (1979a)
52 weeks 25 weeks
Symphytine 13 mg/kg, ip, 2/week for 3.7 for 4 weeks, 5/24 Hirono et
4 weeks and 1/week for then 1.9 for al. (1979a)
52 weeks 52 weeks
------------------------------------------------------------------------------------------
a From: Culvenor (1983).
b Where necessary, estimates assume a daily rat food intake of 100 g/kg body weight, and
water intake 100 ml/kg body weight. Injected doses are given pro rata, for daily
administration.
7.10 Prevention of Poisoning in Man
At present, prevention of poisoning can be achieved only by
reducing or eliminating ingestion of the alkaloids. The two
effective procedures are control of PA-containing plants in
agricultural areas and educational programmes directed to the
populations at risk.
The control of plant populations for this purpose has been
carried out only in Uzbekistan, USSR, following the epidemics of
human disease due to contamination of grain by seeds of
Heliotropium lasiocarpum and Trichodesma incanum. The following
measures were introduced and have been effective in preventing
further outbreaks:
1. A state standard was set for the quality of seed grain, which
must be certified by a State Seed Inspectorate. Current
standards prohibit the sowing of wheat, rye, barley, or oats
contaminated by seed of Heliotropium lasiocarpum or Trichodesma
incanum.
2. A state standard was set for the quality of grain stored for
food. The limits for Heliotropium lasiocarpum and
Trichodesma incanum seeds are 0.2% and zero, respectively.
3. Agricultural (agritechnical) measures to ensure minimum
contamination of crops and harvested grain, including
specification of the most suitable methods and timing of
cultivation, use of clean seed for sowing, weeding of crops
prior to maturing of the grain (towards the end of May), and
mechanical cleaning of grain.
4. Methods for monitoring levels of contamination of flour, bread,
and similar products.
5. Publication of educational booklets describing the biological,
environmental, and morphological characteristics of the toxic
weeds, their pathways of distribution and the causes of the
toxicoses experienced.
6. Promotion of weed control by governmental authorities and
provision of legislation to enforce the control measures.
In other countries, the control of some PA-containing weeds in
crops is practised by cultivation and herbicide treatment, in order
to maximize yield and the general quality of the grain. In
pastures, animal management and herbicide treatments are used to
increase pastures and reduce poisoning of animals. Specific
treatment methods differ according to the plant species and the
circumstances. General references were not available to the Task
Group.
In Australia, where Heliotropium europaeum and Echium
plantagineum are widespread weeds in wheat-growing areas but where
normal agricultural practices prevent all but occasional minor
contamination, relevant tolerance standards for wheat delivered at
storage silos are not specific. Heliotropium europaeum seed is
rarely seen and would form part of the "unmillable material" the
seed component of which can be up to 1% of the volume of the wheat.
Seed of Echium plantagineum is occasionally seen in delivered grain
at levels of up to 10 seeds per half litre, the tolerance level for
this seed fraction being 50 seeds per half litre.
8. BIOLOGICAL CONTROL
Biological control methods have been investigated for several
PA-containing plant species, notably Senecio jacobaea, Heliotropium
europaeum, Echium plantagineum, and Trichodesma incanum. The
effectiveness of such methods is variable and good results may be
confined to certain regions where favourable conditions exist. For
example, in control programmes against S. jacobaea in Australia,
Canada, New Zealand, and the USA, using 3 insect species, results
varied from virtually nil to nearly 100% control (Julien 1982).
The effects of the introduction of the cinnabar moth ( Tyria
jacobaea L.) on S. jacobaea in these countries have been summarized
as in Table 18.
Table 18. Results of the attempted control of Senecio
jacobaea (ragwort) with the cinnabar moth
------------------------------------------------------------
Country or region Result
------------------------------------------------------------
Australia Establishment precluded by predation,
parasitism and disease
Western Canada Moth populations stabilized below that
required for control
Eastern Canada Establishment and subsequent notable
reductions in ragwort levels
New Zealand Marginal establishment, moth population
limited by predation and parasitism,
little impact on ragwort
USA Widespread establishment, ragwort levels
sometimes reduced at localities near the
limits of its distribution
------------------------------------------------------------
Several agents are being tested in Australia for the control of
H. europaeum and one species, a flee beetle Longitarsus albineus,
has been released (Julien, 1982; Delfosse, 1985). There are good
prospects in this country for the biological control of Echium
plantagineum and 2 other Echium spp., with 8 insect species
approved for release, when legal restrictions are lifted (Delfosse
& Cullen, 1985a,b). Preliminary studies have been made on the
biological control of Amsinckia and other Senecio species (e.g.,
Pantone et al., 1985).
Given adequate funding, PA-containing plants are a suitable
target for biological control.
9. EVALUATION OF HUMAN HEALTH RISKS AND EFFECTS ON THE ENVIRONMENT
9.1 Human Exposure Conditions
9.1.1 Reported sources of human exposure
The two main sources of exposure of human beings to toxic PAs
that have led to major outbreaks of poisoning with high mortalities
as well as to individual cases in several countries are:
(a) the contamination of cereal grains, such as wheat and
millet, with the seed or other parts of plants containing
alkaloids; and
(b) the consumption for medicinal or dietary purposes of herbs
containing the alkaloids, either as the plant itself or as
infusions.
Consumption of contaminated grain is more likely to occur in
regions where food is in short supply, and particularly when
drought favours infestation of the grain crop by PA-containing
weeds. A qualitative field test for detecting the presence of
toxic pyrrolizidine alkaloids in plant materials, using simple
laboratory methods, is now available (section 2.2.2.5).
9.1.2 Plant species involved
Plant species containing toxic PAs occur throughout the world
and are known in 47 genera in 6 plant families. As many as 6000
species are potentially PA-containing. The most important genera
responsible for human and animal disease are Senecio and other
genera of the tribe Senecioneae (family Compositae), Crotalaria
(family Leguminosae) and Heliotropium, Trichodesma, and other
genera of the family Boraginaceae (sections 3.1 and 3.2).
Approximately 150 different toxic PAs have been isolated from
about 360 plant species that have been investigated and contain
this type of alkaloid. Of these, about 12 have been involved in
instances of human toxicity (section 3.1). The molecular
structures of almost all of these alkaloids are known and the main
outlines of structure-toxicity relationships have been established
(section 2.1).
The alkaloids may occur in all plant parts and are often
present as the N-oxide derivatives, which are also toxic when
ingested orally. Alkaloid contents vary from low (0.1 g/kg dry
weight) to very high (40 g/kg up to the maximum recorded of
180 g/kg in Senecio riddelli). Levels vary with stage of growth,
locality, and other circumstances. In some, but not all, species,
the alkaloid is partly decomposed during the drying or storage of
the plant. The decomposition is largely enzymic and once the plant
material is dry, the alkaloid is fairly stable.
9.1.3 Modes and pathways of exposure
9.1.3.1 Contamination of grain crops
Large outbreaks of poisoning have occurred through
contamination of wheat crops in Afghanistan, India, and the USSR.
In particular, 3 species of Boraginaceae (Heliotropium lasiocarpum,
H. popovii, and H. europaeum) are well adapted to vigorous growth
under the climatic conditions in which wheat is usually grown.
Contamination can be effectively controlled in wheat produced using
modern harvesting techniques and grain seed that is inspected and
controlled for weed seed contamination, but control of
contamination is more difficult where these conditions cannot be
met. Contamination of staple food grain is of particular concern,
since entire populations are exposed, and control may not be
possible, if the people are not aware of the hazard that
PA-containing weeds present.
9.1.3.2 Herbal medicines
Herbal preparations containing PAs are used as tonics,
treatments, preventatives, and food supplements. Such usages are
so widespread that they are nearly universal. Many are
traditional, while others reflect a rejection of, or lack of access
to, standard health care services (section 3.3.2).
Veno-occlusive disease was first recognized as a clinical
entity in Jamaica as a result of the medicinal use of PA-containing
herbs prepared from Crotalaria. Crotalaria-containing herbs have
also been responsible for human poisonings in Barbados, Equador,
and other locations in the West Indies. Heliotropium herbs have
been reported to cause poisoning in Hong Kong and India.
Symphytum- and Senecio-containing herbs have given rise to case
reports in the USA. Other reports of PA poisoning are known in
which the herbs used were not botanically identified. PA-poisoning
has been associated with both home-prepared and commercially
available herbs, the latter including prescriptions by herbalists
(Weston et al., 1987).
Various other genera of PA-containing plants in the families
Boraginaceae, Compositae, and Leguminosae are also widely used as
herbs. No case reports are available for these genera.
Reported cases of PA poisoning due to the use of the herbs are
geographically widespread, but few in number. However, the scale
on which PA-containing plants are used as herbs, the typically
delayed effects of long-term exposure, and the difficulties of
diagnosis led the Task Group to conclude that there is every
indication of under-reporting of intoxications from the use of such
herbs. Symphytum root preparations, in particular, represent a
hazard, and certain user groups are routinely exposed to levels of
Symphytum alkaloids that are higher than those at which
intoxications have been reported.
The risks associated with the use of PA-containing herbs are
accentuated by the difficulties of controlling this use.
9.1.3.3 PA-containing plants used as food and beverages
Some PA-containing plants are used for food or the making of
beverages in many countries, including developed countries. The
following species are known to be used (though many other plants
are also probably used in this way): Cacalia yatabei, Symphytum
species, Ligularia dentata, Petasites japonicus, Senecio burchellii,
S. inadequidens, S. pierotti, Syneilesis palmata, Crotalaria
anagyrodies, C. brevidens, C. juncea, C. laburnifolia, C. pumila,
C. recta, and C. retusa. No information is available on
the extent to which the different types are consumed (section 3.3.3).
9.1.3.4 Other foods contaminated by PAs
Several species of Boraginaceae are nectar and pollen sources
for bees. Echium plantagineum, in particular, is a widespread weed
in some countries and a substantial source of honey containing a
low level of alkaloid. Senecio species are also visited by bees
and yield alkaloid-containing honey, though Senecio-derived honey
is not known to be produced in quantity for sale. Thus, some
regional and local populations are exposed to a low-level intake
through the presence of PAs in honey, and surveillance may be
desirable in countries producing honey (section 3.3.4).
Under experimental conditions, PAs are transmitted from the
feed of dairy cows and goats into the milk. Some PA-containing
species, such as Senecio jacobaea, S. lautus, and Echium plantagineum,
are weeds in dairy pastures in some countries and are eaten by cattle
under certain conditions. There are no published reports of alkaloid
in milk supplies for human consumption (section 3.3.5).
The Task Group was not aware of any reported cases of
pyrrolizidine toxicity that had been ascribed to either honey or
dairy products.
No information was available to the Task Group on the possible
presence of alkaloids or their metabolites in meat from animals
that had consumed PA-containing plants shortly before slaughter.
The results of metabolic studies in rats have indicated that the
alkaloid is rapidly cleared from the body and, therefore, the
levels of PAs in meat are expected to be very low. However, there
is no information on the possibility of alkaloid accumulating in
storage sites.
9.1.4 Levels of intake
Reliable estimates of levels of intake of PAs, especially in
outbreaks of disease caused by the contamination of cereal crops
with the seeds of toxic plants, are extremely difficult to make.
Sampling of the contaminated grain may not be strictly
representative, since the extent of the contamination may vary in
different sites and households, as is evident from the estimates of
PA intake in the Indian and Afghan outbreaks reported in section
7.3. Furthermore, no accurate record is possible of the amount of
contaminated food consumed over an uncertain length of time. No
records of the levels of toxic PA intake are available in the
earlier reports of human toxicity. Where available, estimates of
intake in outbreaks caused by the contamination of staple food
crops have been made on the basis of random sampling of the
contaminated grain in food stores, and rough estimates of daily
consumption by average adults. Food-on-the-plate analyses have not
been made. The estimated lengths of exposure, and hence the amount
of total intake, are also approximate.
The contamination of cereal grains with the seed of
PA-containing plants has caused major epidemics of human poisoning,
though, in the two instances where estimates of alkaloid intake are
available, the intakes were lower than in some exposures due to the
use of herbal medicines. The estimated intakes are summarized in
Table 16. In an outbreak in India, millet contaminated with
Crotalaria nana seed had an average alkaloid content of 0.5 g/kg,
and the estimated daily intake by the population was 0.66 mg/kg
body weight. In a larger outbreak in Afghanistan, due to the seeds
of Heliotropium popovii in wheat, the level of contamination was
probably variable; representative samples of wheat contained
alkaloid at 0.04 g/kg. The estimated daily intake was 0.033 mg/kg
body weight. These intakes, sustained for periods of approximately
2 and 6 months, respectively, resulted in typical acute and
subacute veno-occlusive disease.
The highest intake rates have been associated with the use or
misuse of medicinal herbs and have resulted in acute liver damage
and death. In two occasions, the consumption of Heliotropium
eichwaldii as a treatment for epilepsy led to an estimated intake
of 3.3 mg/kg body weight daily for 20 or 50 days, and the use of
extracts of Senecio longilobus as medicine for young children led
to estimated intakes of 3 and 0.8 - 1.7 mg/kg body weight. The
highest intake led to extensive liver necrosis. However, it is
possible that, in the above case of poisoning by Heliotropium
eichwaldii, the toxicity was enhanced due to simultaneous
administration of phenobarbitone, which has a potentiating effect
on the microsomal enzymes in the liver cells that convert the PAs
to toxic metabolites.
The use of Heliotropium lasiocarpum as a component of a herbal
treatment for psoriasis involved somewhat lower daily intake rates
of 0.49 - 0.71 mg/kg body weight in 4 patients, who, after periods
of 19 - 46 days, developed veno-occlusive disease. The patient
with the longest intake period and a total intake of 1.4 g alkaloid
or 23 mg/kg body weight died.
The lowest ingestion rate leading to a case of veno-occlusive
disease was also due to medicinal herbal treatment or, more
specifically, to the use of a digestive aid containing a
preparation of comfrey root. Commercial herb and food supplement
preparations containing comfrey leaf or root are on sale in many
countries. Limited assays of one comfrey-pepsin preparation
prepared from comfrey root indicated a PA content of 2.9 g/kg.
Another preparation made from comfrey leaf contained up to 0.27 g
alkaloid/kg. The consumption of these preparations led to an
estimated daily intake of 0.015 mg/kg body weight. Veno-occlusive
disease was diagnosed after a 4- to 6-month period.
The consumption of Symphytum officinale (comfrey) and S. x
uplandicum (Russian comfrey) in the form of food, infusions, or
other preparations is widespread, though the full extent cannot be
estimated. A high level of consumption as salad appears to be
about 5 - 6 leaves per day and consumption as comfrey tea probably
reaches a similar level. Limited assays indicate that the average
alkaloid content of the leaf is about 1 mg/leaf, the concentration
being higher in the younger, smaller leaves. The alkaloid intake
from comfrey leaves could therefore vary from a low value, up to
about 6 mg/day, or 0.1 mg/kg body weight for an adult, an intake
within the range producing veno-occlusive disease. However, the
Task Group noted that some people claim to have consumed comfrey at
such a rate without suffering any disease.
Overall, the estimates of intake of PAs by human beings (Table
16) indicate that ingestion rates above 0.015 mg/kg body weight for
the mixture of echimidine and related alkaloids in comfrey may lead
to acute or subacute liver disease. If expressed in terms of
equivalent doses of heliotrine (section 7.8), the estimated total
doses in the known outbreaks or cases of veno-occlusive disease
range from 1 to 167 mg/kg body weight. There is little real
difference in the ranges of estimated total doses in non-fatal
cases (1 - 120 mg/kg body weight) and those leading to death
(6 - 167 mg/kg). These figures, when compared with the total
lethal dose of several PAs in rats, i.e., 1.2 - 10.9 times the LD50
dose (equivalent to 360 - 3270 mg heliotrine/kg) (Table 10), would
seem to indicate that man is markedly more sensitive than the rat
to the toxic effects of PAs with regard to the development of acute
and chronic effects on the liver. It should be noted that these
estimates are based on limited raw data and a number of
assumptions, and so are of uncertain reliability.
The dose estimates indicate strongly that the effects of PAs in
human beings are cumulative at very low intake rates. Lower rates
of intake of PAs may lead to chronic forms of intoxication, though,
at present, there is no evidence on which the degree of risk in
these circumstances can be evaluated. The information available on
dose-response relationships is very limited, but the data support
the conclusion that even low rates of intake of PAs over a period
of time may present a health risk and that exposure should be
minimized wherever possible.
There has not been any systematic monitoring of PAs in cereal
grains, food products, and herbal medicines. Analytical surveys of
these materials are feasible, but it would be difficult to design
surveys that would give direct estimates of the dietary intake of
PAs.
9.2 Acute Effects of Exposure
9.2.1 Acute liver disease
All cases of human intoxication in reported accounts have been
in the acute phase of the disease, the dominant symptom being
rapidly filling ascites. The disease can affect large
subpopulations and, in one study, up to 22.6% of the population was
affected.
Children appear to be the most vulnerable group and mortality
can be high at the extremes of age. The liver is the principal
target organ. In the acute stage of the disease, the liver shows a
characteristic centrilobular haemorrhagic necrosis, which in man is
accompanied by occlusion of the hepatic veins. However,
characteristic veno-occlusive lesions, seen in the central veins of
hepatic lobules, may not always be evident in the needle biopsy
examination of the liver, but are always apparent on examination of
the autopsy material.
9.3 Chronic Effects of Exposure
9.3.1 Cirrhosis of the liver
There is evidence that the administration of a single dose of
PA to experimental animals or a single acute episode of illness in
man, following brief consumption of PA-containing herbs or
PA-contaminated food, may lead to progressive chronic liver disease
resulting in cirrhosis. Cirrhosis may also be a consequence of
long-term low-dose administration of PAs to experimental animals
and possibly also of long-term low intake of PAs by human beings,
though there is no proof of the latter. Cirrhosis resulting from
the toxic effects of PAs in the advanced stage, may not be
distinguishable from that resulting from other causes (sections
6.4.1.5 and 7.4). The Task Group did not find any evidence
suggesting that PAs are a causative factor of the specific disease,
Indian Childhood Cirrhosis (section 7.5).
9.3.2 Mutagenicity and teratogenicity
Several PAs, PA-derivatives, and related compounds have been
shown to produce chromosome aberrations in plants and several cell
culture systems, mutagenic effects ( Salmonella ("Ame's"), sister
chromatid exchanges, and other tests), and teratogenic and
fetotoxic effects in experimental animals (sections 6.4.5, 6.4.6,
6.4.7). Chromosomal aberrations have been reported in the blood
cells of children suffering from veno-occlusive disease, believed
to have been caused by fulvine. The Task Group was not aware of
data on the teratogenic/fetotoxic effects of PAs on human beings
and was unable to evaluate the potential for these effects in PA
exposure.
9.3.3 Cancer of the liver
A relatively large number of people have been exposed, in the
past, to PAs and have suffered acute and chronic toxic effects.
However, no information is available on the long-term follow-up of
these populations, to ascertain whether this type of exposure could
have resulted in an increased incidence of liver cancer or other
types of cancer. Because of this lack of knowledge, it is not
possible, at present, to make an evaluation of the cancer risk due
to PAs. However, various PAs have been shown to be carcinogenic
for experimental animals, which implies that a potential cancer
risk for human beings should be seriously considered.
Of several PAs evaluated for carcinogenicity by IARC (1976,
1983), there is "sufficient or limited evidence" for the
carcinogenicity in experimental animals (IARC, 1976) of
monocrotaline, retrorsine, isatidine, lasiocarpine, petasitenine,
senkirkine, and of extracts of the PA-containing plants Petasites
japonicum, Tussilago farara, Symphytum officinale, Senecio
longilobus, Senecio numorensis, Farfugium japonicum, and Senecio
cannabifolius. These studies were carried out mainly on rats, with
few studies on mice or hamsters (section 6.4.8). The
carcinogenicity data obtained with other PAs are difficult to
evaluate, because of the limited number of treated animals and the
lack of adequate numbers as controls. The main target organ is the
liver, where liver cell tumours and haemangioendothelial sarcomas
were observed. In some instances, tumours in extra-hepatic tissues
(lung, pancreas, intestine) were also observed, namely with
monocrotaline, retrorsine, and lasiocarpine. Some PAs, for
example, retrorsine, have been shown to be carcinogenic after a
single dose. The pyrrolic metabolites have also been shown to be
carcinogenic for rats.
It may be recalled that several of the PAs involved in human
poisoning include the above compounds. It is notable that the dose
rates that have been effective in inducing tumours in rats, mostly
equivalent to 0.2 - 3 mg/kg body weight per day (Table 17), are
roughly similar in magnitude to estimated intake rates (0.49 -
3.3 mg/kg body weight per day) (Table 16) in several episodes of human
toxicity. Comparison of the total intakes resulting in human
toxicity with the total doses to death observed in the chronic
toxicity studies on rats indicates that human beings are more
susceptible (section 7.8) and suggests that human beings may
survive for sufficient time to develop cancer after only a brief
exposure at this level or a longer exposure at a markedly lower
level. A more quantitative assessment is not possible on the basis
of the available information, and the Task Group stressed the need
for appropriate epidemiological studies.
9.3.4 Effects on other organs
Substantiated reports of PA-induced extra-hepatic injury in man
are limited to Trichodesma intoxication, in which symptoms and
signs were predominantly neurological. The range of organs
affected by other PAs in experimental and farm animals suggests
that exposure of human beings to other PAs may also carry the
potential for extra-hepatic injury.
There are extensive reports of experimental studies in which
PAs have been demonstrated to produce the characteristic vascular
changes of primary pulmonary hypertension and consequent right
ventricular hypertrophy of the heart in rats and non-human primates
(section 6.4.2). Susceptibility is age dependent, weanling rats
being more vulnerable than older animals. There is only
circumstantial evidence of PA-induced pulmonary vascular disease in
one patient (section 7.6), but judging by the experimental evidence
available, it is possible that human beings may be susceptible to
PA-induced cardiopulmonary changes.
In the opinion of the Task Group, the neurological involvement
which is a dominant feature in PA-intoxicated horses and is also
seen in cows and sheep, cannot be explained solely as a consequence
of liver damage. Central nervous system lesions have been
demonstrated in sheep, pigs, and rats. Distribution studies of the
radiolabelled metabolite, 3H-dehydroretronecine, show increasing
accumulation of radioactivity in the brain with time.
Trichodesma alkaloids, structurally related to monocrotaline,
are neurotoxic agents. Trichodesma toxicosis in man has been
reported only from the USSR, together with several studies on
experimental animals. Detailed reports on the pathological
findings were not available to the Task Group, but the information
available indicated that the central nervous system was the primary
target organ (sections 6.4.3 and 7.7).
Stomach and intestinal lesions have been shown in PA-exposed
sheep, mice, cows, and rats. Distribution studies with
radiolabelled pyrroles showed a high retention of radioactivity in
the stomach, consistent with the acid-sensitive nature of the
pyrroles. In rats, pyrrolic metabolites are secreted in high
concentrations in the bile.
Kidney changes following to PA administration have been shown
in mice, pigs, horses, sheep, and monkeys. Pyrrolizidine
metabolites have been found covalently bound to kidney DNA in rats.
Urinary excretion is a major route of excretion of metabolic
products of PAs in rats.
There is no evidence of involvement of organs other than the
liver and central nervous system ascribed primarily to PA toxicity
in any of the published human case reports. It is possible that
under some circumstances, other major organ systems may also be at
risk. As bioactivation of PAs has been demonstrated only in the
liver, the risk of damage should be expected to be lower in the
organs.
9.4 Effects on the Environment
9.4.1 Agriculture
In some countries, PA-containing weeds densely cover areas of
up to thousands of square kilometres. Their adverse effects
include the covering of pastures, additional costs in agricultural
production, and the poisoning of farm animals. The toxicity of PAs
for farm animals, including sheep, cattle, horses, pigs, goats, and
poultry, which has been the inspiration for much of the investigation
of PA toxicity. In Australia, for example, Heliotropium europaeum
and Echium plantagineum cause the death of thousands of animals
annually (section 6.2).
9.4.2 Wild-life
By contrast, little is known about the consumption of PA-containing
plants by wild-life, or of their individual sensitivities. The death
of deer in Louisiana has been ascribed to eating Heliotropium or
Crotalaria species, and an experimental study has shown that the
rainbow trout (Salmo gairdneri) is sensitive to Senecio jacobaea
alkaloids (sections 6.5.1 and 6.5.2).
There is no information on the effects of the alkaloids on
field rodents or other seed-eating mammals and birds that might be
expected to consume seeds of PA-containing plants and to suffer
toxic effects.
9.4.3 Insects
Many species of insects, such as some moths of the family
Arctidae and butterflies of the sub-families Danainae and
Ithominae, have become dependent on PA-containing plants, using the
alkaloids as defensive chemicals and derivatives of them as
pheromones and other signalling chemicals. Thus, complete
elimination of PA-containing plants in a region might lead to a
marked reduction in the local population of insects of this type
(section 6.5.3).
9.4.4 Soil and water
There have not been any studies on the fate of PAs when the
plants in which they occur wilt and age. If alkaloid is leached
into soil or water, it is probably readily degraded by microorganisms
since, as a base and ester, it is subject to oxidative and hydrolytic
reactions.
REFERENCES
AFZELIUS, B.A. & SCHOENTAL, R. (1967) The ultrastructure of
enlarged hepatocytes induced in rats with a single oral dose of
retrorsine, pyrrolizidine (Senecio) alkaloids. J. ultra-struct.
Res., 20: 328-345.
AIKAT, B.K., BHUSNURMATH, S.R., DATTA, D.V., & CHHUTTANI, P.N.
(1978) Veno-occlusive disease in north-west India. Indian J.
Pathol. Microbiol., 21: 203-211.
AL-HASANY, M. & MOHAMED, A.S. (1970) Veno-occlusive disease of the
liver in Iraq. Arch. dis. Child., 45: 722-724.
ALLEN, J.R. & CARSTENS, L.A. (1968) Veno-occlusive disease in
rhesus monkeys. Am. J. vet. Res., 29: 1681-1694.
ALLEN, J.R. & CARSTENS, L.A. (1970) Pulmonary vascular occlusions
initiated by endothelial lysis in monocrotaline-intoxicated rats.
Exp. mol. Pathol.., 13: 159-171.
ALLEN, J.R. & CARSTENS, L.A. (1971) Monocrotaline induced Budd-
Chiari syndrome in monkeys. Am. J. dig. Dis., 16: 111-121.
ALLEN, J.R. & CHESNEY, C.F. (1972) Effect of age on development of
cor pulmonale in non-human primates following pyrrolizidine
alkaloid intoxication. Exp. mol. Pathol., 17: 220-232.
ALLEN, J.R., CHILDS, G.R., & CRAVENS, W.W. (1960) Crotalaria
spectabilis toxicity in chickens. Proc. Soc. Exp. Biol. Med., 104:
434-436.
ALLEN, J.R., LALICH, J.J., & SCHMUTTLE, S.M. (1963) Crotalaria
spectabilis induced cirrhosis in turkeys. Lab. Invest., 12: 512-517.
ALLEN, J.R., CARSTENS, L.A., & OLSON, B.E. (1967) Veno-occlusive
disease in Macaca speciosa monkeys. Am. J. Pathol., 50: 653-667.
ALLEN, J.R., CARSTENS, L.A., & KATAGIRI, G.J. (1969) Hepatic veins
of monkeys with veno-occlusive disease-sequential ultrastructural
changes. Arch. Pathol., 87: 279-289.
ALLEN, J.R., CHESNEY, C.F., & FRAZEE, W.J. (1972) Modifications of
pyrrolizidine alkaloids intoxication resulting from altered
microsomal enzymes. Toxicol. appl. Pharmacol., 23: 470-479.
ALLEN, J.R., HSU, I.-C., & CARSTENS, L.A. (1975) Dehydroretronecine
induced rhabdomyosarcomas in rats. Cancer Res., 35: 997-1002.
AMES, M.M. & POWIS, G. (1978) Determination of indicine N-oxide
and indicine in plasma and urine by electron-capture gas-liquid
chromatography. J. Chromatogr., 166: 519-526.
ARMSTRONG, S.J. & ZUCKERMAN, A.J. (1970) Production of pyrroles
from pyrrolizidine alkaloids by human embryo tissue. Nature
(Lond.), 228: 569-570.
ARMSTRONG, S.J., ZUCKERMAN, A.J., & BIRD, R.G. (1972) Induction of
morphological changes in human embryo liver cells by the
pyrrolizidine alkaloid lasiocarpine. Br. J. exp. Pathol., 53:
145-149.
ARORA, R.R., PYARELAL, GHOSH, T.K., MATHUR, K.K., & TANDON, B.N.
(1981) Epidemiology of veno-occlusive disease in tribal population
of Madhya Pradesh and Bihar. J. commun. Dis. (India), 13: 147-151.
ARSECULERATNE, S.N., GUNATILAKA, A.A.L., & PANABOKKE, R.G. (1981)
Studies on medicinal plants of Sri Lanka: occurrence of
pyrrolizidine alkaloids and hepatotoxic properties in some
traditional medicinal herbs. J. Ethnopharmacol., 4: 159-177.
ASADA, Y., FURUYA, T., & MURAKAMI, N. (1981) Pyrrolizidine
alkaloids from Ligularia japonica. Planta Med., 42: 202-203.
ASADA, Y., SHIRAISHI, M., TAKEUCHI, T., OSAWA, Y., & FURUYA, T.
(1985) Pyrrolizidine alkaloids from Crassocephalum crepidioides.
Planta Med., 51: 539-540.
BARNES, J.M., MAGEE, P.N., & SCHOENTAL, R. (1964) Lesions in the
lungs and livers of rats poisoned with pyrrolizidine alkaloids
fulvine and its N-oxide. J. Pathol. Bacteriol., 88: 521-531.
BERRY, D.M. & BRAS, G. (1957) Venous occlusion of the liver in
Crotalaria and Senecio poisoning. North Am. Vet., 38: 323-327.
BHATTACHARYA, K.J. (1965) Foetal and neonatal responses to
hepatotoxic agents. J. Pathol. Bacteriol., 90: 151-161.
BICCHI, C., D'AMATO, A., & CAPPELLETTI, E. (1985) Determination
of pyrrolizidine alkaloids in Senecio inaequidens D.C. by
capillary gas chromatography. J. Chromatogr., 349: 23-29.
BICK, Y.A.E. (1970) Comparison of the effects of LSD, heliotrine,
and X-irradiation on chromosome breakage and the effects of LSD on
the rats of cell division. Nature (Lond.), 226: 1165-1167.
BICK, Y.A.E. & CULVENOR, C.C.J. (1971) Effects of dehydro-
heliotridine, a metabolite of pyrrolizidine alkaloids on chromosome
structure and cell division in cultures of animal cells. Cytobios,
3: 245-255.
BICK, Y.A.E. & JACKSON, W.D. (1968) Effects of the pyrrolizidine
alkaloid heliotrine on cell division and chromosome breakage in
cultures of leucocytes from the marsupial Potorus tridactylus.
Aust. J. biol. Sci., 21: 469-481.
BICK, Y.A.E., CULVENOR, C.C.J., & JAGO, M.V. (1975) Comparative
effects of pyrrolizidine alkaloids and related compounds on
leukocyte cultures from Potorus tridactylus. Cytobios, 14: 151-160.
BIRECKA, H., FROHLICH, M.W., HULL, L., & CHASKES, M.J. (1980)
Pyrrolizidine alkaloids of Heliotropium from Mexico and adjacent
USA. Phytochemistry, 19: 421-426.
BIRECKA, H., CATALFAMO, J.L., & EISEN, R.N. (1981) A sensitive
method for detection and quantitative determination of
pyrrolizidine alkaloids. Phytochemistry, 20: 343-344.
BLACK, D.N. & JAGO, M.V. (1970) Interaction of
dehydroheliotridine, a metabolite of heliotridine based
pyrrolizidine alkaloids, with natural and heat denatured RNA.
Biochem. J., 118: 347-353.
BOHLMANN, F., KLOSE, W., & NIKISCH, K. (1979) [Synthesis of
dehydroheliotridine and 3-oxy-dehydroheliotridine.] Tetrahedr.
Lett., 1979: 3699-3702 (in German).
BOHLMANN, F., ZDERO, C., JAKUPOVIC, J., GRENZ, M., CASTRO, V.,
KING, R.M., ROBINSON, H., & VINCENT, L.P.D. (1986) Further
pyrrolizidine alkaloids and furoeremophilanes from Senecio species.
Phytochemistry, 15: 1151-1159.
BOPPRE, M. (1986) Insects pharmacophagously utilizing defensive
plant chemicals (pyrrolizidine alkaloids). Naturwiss enschaften, 73:
17-26.
BRAGINSKII, B.M. & BOBOKHADZAEV, I. (1965) [Hepatosplenomegaly
against the background of heliotropic toxicosis.] Sov. Med., 28:
57-60 (in Russian).
BRAS, G. (1973) Aspects of hepatic vascular diseases. In: Gall,
E.A. & Mostofi, F.K., ed. The liver: International Academy of
Pathology monograph, Baltimore, Maryland, Williams and Wilkins,
pp. 406-530.
BRAS, G. & HILL, K.R. (1956) Veno-occlusive disease of the liver -
essential pathology. Lancet, 2: 161-163.
BRAS, G. & WATLER, D.C. (1955) Further observations on the
morphology of veno-occlusive disease of the liver in Jamaica. West
Indian med. J., 4: 201-211.
BRAS, G., JELLIFFE, D.B., & STUART, K.L. (1954) Veno-occlusive
disease of the liver with nonportal type of cirrhosis occurring in
Jamaica. Arch. Pathol., 57: 285-300.
BRAS, G., BERRY, D.M., & GYORGI, P. (1957) Plants as etiological
factor in veno-occlusive disease of liver. Lancet, 1: 960-962.
BRAS, G., BROOKS, S.E.H., & WATLER, D.C. (1961) Cirrhosis of liver
in Jamaica. J. Pathol. Bacteriol., 82: 503-512.
BRAUCHLI, J., LUTHY, J., ZWEIFEL, U., & SCHLATTER, C. (1982)
Pyrrolizidine alkaloids from Symphytum officinale L. and their
percutaneous absorption in rats. Experientia (Basel), 38:
1085-1087.
BRIGGS, L.H., CAMBIE, R.C., CANDY, B.J., O'DONOVAN, G.M., RUSSELL,
R.A., & SEELYE, R.N. (1965) Alkaloids of New Zealand Senecio
species. Part II: senkirkine. J. Chem. Soc., C1965: 2492-2498.
BRINK, N.G. (1982) Somatic and teratogenic effects induced by
heliotrine in Drosophilia. Mutat. Res., 104: 105-111.
BROOKS, W.E.H., MILLER, C.G., MCKENZIE, K., AUDRETSCH, J.J., &
BRAS, G. (1970) Acute veno-occlusive disease of the liver. Fine
structure in Jamaican children. Arch. Pathol., 89: 507-529.
BROWN, K.S. (1984) Chemical ecology of dehydropyrrolizidine
alkaloids in adult Ithomiinae (Lepidoptera: Nymphalidae). Rev.
Bras. Biol., 44: 435-440.
BRUGGEMAN, I.M. & VAN DER HOEVEN, J.C.M. (1985) Induction of SCEs
by some pyrrolizidine alkaloids in V79 Chinese hamster cells co-
cultured with chick embryo hepatocytes. Mutat. Res., 142: 209-212.
BRUNER, L.H., CARPENTER, L.J., HAMLOW, P., & ROTH, R.A. (1986)
Effect of a mixed-function oxidase inducer and inhibitor on
monocrotaline pyrrole pneumotoxicity. Toxicol. appl. Pharmacol., 85:
416-427.
BUCKMASTER, G.W., CHEEKE, P.R., ARSCOTT, G.H., DICKINSON, E.D.,
PIERSON, M.L., & SHULL, L.R. (1977) Response of Japanese quail to
dietary and injected pyrrolizidine (Senecio) alkaloid. J. anim.
Sci., 45(6): 1322-1325.
BULL, L.B. (1955) The histological evidence of liver damage from
pyrrolizidine alkaloids. Aust. vet. J., 31: 33-40.
BULL, L.B. & DICK, A.T. (1959) The chronic pathological effects on
the liver of the rat of the pyrrolizidine alkaloids heliotrine,
lasiocarpine, and their N-oxides. J. Pathol. Bacteriol., 78: 483-502.
BULL, L.B. & DICK, A.T. (1960) The function of total dose in the
production of chronic lethal disease in rats by periodic injections
of the pyrrolizidine alkaloid heliotrine. Aust. J. exp. Biol. med.
Sci., 38: 515.
BULL, L.B., DICK, A.T., KEAST, J.C., & EDGAR, G. (1956) An
experimental investigation of the hepatotoxic and other effects on
sheep of consumption of Heliotropium europaeum L. Heliotropium
poisoning of sheep. Aust. J. agric. Res., 7: 281-332.
BULL, L.B., DICK, A.T., & MCKENZIE, J.S. (1958) The acute effects
of heliotrine and lasiocarpine and their N-oxides on the rat. J.
Pathol. Bacteriol., 75: 17-25.
BULL, L.B., CULVENOR, C.C.J., & DICK, A.T. (1968) The
pyrrolizidine alkaloids, Amsterdam, North Holland Publishing Co.
BURGUERA, J.A., EDDS, G.T., & OSUNA, O. (1983) Influence of
selenium on aflatoxin B1 or Crotalaria toxicity in turkey poults.
Am. J. vet. Res., 44: 1714-1717.
BURNS, J. (1972) The heart and pulmonary arteries in rat fed on
Senecio jacobaea. J. Pathol., 106: 187-194.
BUTLER, W.H., MATTOCKS, A.R., & BARNES, J.M. (1970) Lesions in the
liver and lungs of rats given pyrrole derivatives of pyrrolizidine
alkaloids. J. Pathol., 100: 169-175.
CAMPBELL, J.G. (1956) An investigation of the hepatotoxic effects
in the fowl of ragwort ( Senecio jacobaea Linn) with special
reference to the induction of liver tumours with seneciphylline.
Proc. R. Soc. Edinburgh, B66: 111-130.
CAMPBELL, J.G. (1957a) Studies on the influence of sex hormones on
the avian liver. II. Acute liver damage in the male fowl and the
protective effect of oestrogen, as determined by a liver function
test. J. Endocrinol., 15: 346-350.
CAMPBELL, J.G. (1957b) Studies on the influence of sex hormones on
avian liver. III. Oestrogen-induced regeneration of the chronically
damaged liver. J. Endocrinol., 15: 351-354.
CANDRIAN, U., LUTHY, J., GRAF, U., & SCHLATTER, CH. (1984a)
Mutagenic activity of the pyrrolizidine alkaloids seneciphylline
and senkirkine in Drosophila and their transfer into rat milk.
Food chem. Toxicol., 22: 223-225.
CANDRIAN, U., LUTHY, J., SCHMID, P., SCHLATTER, CH., & GALLASZ, E.
(1984b) Stability of pyrrolizidine alkaloids in hay and silage. J.
agric. food Chem., 32: 935-937.
CANDRIAN, U., LUTHY, J., & SCHLATTER, CH. (1985) In vivo binding
of retronecine-labelled (3H), seneciphylline, and 3H-senecionine to
DNA of rat liver, lung and kidney. Chem.-biol. Interact., 54: 57-69.
CARILLO, L. & AVIADO, D.M. (1969) Monocrotaline induced pulmonary
hypertension and p-chlorophenylalanine. Lab. Invest., 20: 213-218.
CARSTENS, L.A. & ALLEN, J.R. (1970) Arterial degeneration and
glomerular hyalinization in the kidney of monocrotaline intoxicated
rats. Am. J. Pathol., 60: 75-92.
CHALMERS, A.H., CULVENOR, C.C.J., & SMITH, L.W. (1965)
Characterization of pyrrolizidine alkaloids by gas, thin layer, and
paper chromatography. J. Chromatogr., 20: 270-277.
CHEEKE, P.R. & GORMAN, G.R. (1974) Influence of dietary protein
and sulphur amino-acid levels on the toxicity of Senecio jacobaea
(tansy ragwort) to rats. Nutr. Rep. Int., 9: 197-207.
CHEEKE, P.R. & PIERSON-GOEGER, M.L. (1983) Toxicity of Senecio
jacobaea and pyrrolizidine alkaloids in various laboratory animals
and avian species. Toxicol. Lett., 18: 343-349.
CHEN, K.K. (1945) Pharmacology. Hepatotoxic alkaloids. Ann. Rev.
Physiol., 7: 695-697.
CHEN, K.K., HARRIS, P.N., & ROSE, C.L. (1940) The action and
toxicity of platyphylline and seneciphylline. J. Pharmacol. exp.
Ther., 68: 130-140.
CHESNEY, C.F. & ALLEN, J.R. (1973a) Resistance of the guinea pig
to pyrrolizidine alkaloid intoxication. Toxicol. appl. Pharmacol.,
26: 385-392.
CHESNEY, C.F. & ALLEN, J.R. (1973b) Endocardial fibrosis
associated with monocrotaline induced pulmonary hypertension in
non-human primates (Macaca arctoides). Am. J. vet. Res., 34:
1577-1581.
CHESNEY, C.F., HSU, I.C., & ALLEN, J.R. (1974) Modifications of
the in vitro metabolism of the hepatotoxic pyrrolizidine alkaloid,
monocrotaline. Res. Commun. chem. Pathol. Pharmacol., 8: 567-570.
CHOPRA, R.N., ed. (1933) Indigenous drugs of India, Calcutta, The
Art Press.
CLARK, A.M. (1959) Mutagenic activity of the alkaloid heliotrine
in Drosophila. Nature (Lond.), 183: 731-732.
CLARK, A.M. (1976) Naturally occurring mutagens. Mutat. Res., 32:
361-174.
COOK, B.A., SINNHUBER, J.R., THOMAS, P.J., OLSON, T.A., SILVERMAN,
T.A., JONES, R., WHITEHEAD, V.M., & ROYMANN, F.B. (1983) Hepatic
failure secondary to indicine N-oxide toxicity. A pediatric
oncology group study. Cancer, 52: 61-63.
COOK, J.W., DUFFY, E., & SCHOENTAL, R. (1950) Primary liver
tumours in rats following feeding with alkaloids of Senecio
jacobaea. Br. J. Cancer, 4: 405-410.
CRAIG, A.M., SHEGGEBY, G., & WICKS, C.E. (1984) Large scale
extraction of pyrrolizidine alkaloids from tansy ragwort (Senecio
jacobaea). Vet. hum. Toxicol., 26: 108-111.
CULVENOR, C.C.J. (1968) Tumour inhibitory activity of
pyrrolizidine alkaloids. J. pharm. Sci., 57: 1112-1117.
CULVENOR, C.C.J. (1978) Pyrrolizidine alkaloids: occurrence and
systematic importance in angiosperms. Bot. Notiser, 131: 473-486.
CULVENOR, C.C.J. (1980) Alkaloids and human disease. In: Smith,
R.L. & Bababunmi, E.A., ed. Toxicology in the tropics, London,
Taylor & Francis Ltd., pp. 124-141.
CULVENOR, C.C.J. (1983) Estimated intakes of pyrrolizidine
alkaloids by humans. A comparison with dose rates causing tumours
in rats. J. Toxicol. environ. Health, 11: 625-635.
CULVENOR, C.C.J. (1985) Pyrrolizidine alkaloids: some aspects of the
Australian involvement. Trends pharmacol. Sci., 6: 18-22.
CULVENOR, C.C.J. & JAGO, M.V. (1979) Carcinogenic plant products
and DNA. In: Grover, P.L., ed. Chemical carcinogens and DNA, Boca
Raton, Florida, CRC Press, Vol. 1, 161 pp.
CULVENOR, C.C.J., DOWNING, D.T., EDGAR, J.A., & JAGO, M.V. (1969)
Pyrrolizidine alkaloids as alkylating and antimitotic agents. N.Y.
Acad. Sci., 163: 837-847.
CULVENOR, C.C.J., EDGAR, J.A., SMITH, L.W., & TWEEDDALE, H.J.
(1970a) Dihydropyrrolizines. III. Preparation and reactions of
derivatives related to pyrrolizidine alkaloids. Aust. J. Chem., 23:
1853-1867.
CULVENOR, C.C.J., EDGAR, J.A., SMITH, L.W., & TWEEDDALE, H.J.
(1970b) Dehydrolizines. IV. Manganese dioxide oxidation of
1,2-dihydropyrrolizidines. Aust. J. Chem., 23: 1869-1879.
CULVENOR, C.C.J., EDGAR, J.A., JAGO, M.V., OUTTERIDGE, A., PETERSON
J.E., & SMITH, L.W. (1976a) Hepato- and pneumotoxicity of
pyrrolizidine alkaloids and derivatives in relation to molecular
structure. Chem.-biol. Interact., 12: 299-324.
CULVENOR, C.C.J., EDGAR, J.A., SMITH, L.W., & HIRONO, I. (1976b)
The occurrence of senkirkine in Tussilago farfara. Aust. J. Chem.,
29: 229-233.
CULVENOR, C.C.J., CLARKE, M., EDGAR, J.A., FRAHN, J.L., JAGO, M.V.,
PETERSON, J.E., & SMITH, L.W. (1980a) Structure and toxicity of
the alkaloids of Russian comfrey ( Symphytum x uplandicum Nyman), a
medicinal herb and item of human diet. Experientia (Basel), 36:
377-389.
CULVENOR, C.C.J., EDGAR, J.A., FRAHN, J.L., & SMITH, L.W. (1980b)
The alkaloids of Symphytum x uplandicum (Russian comfrey). Aust.
J. Chem., 33: 1105-1113.
CULVENOR, C.C.J., EDGAR, J.A., & SMITH, L.W. (1981) Pyrrolizidine
alkaloids in honey from Echium plantagineum L. J. agric. food
Chem., 29: 958-960.
CULVENOR, C.C.J., JAGO, M.V., PETERSON, J.E., SMITH, L.W., PAYNE,
A.L, CAMPBELL, D.G., EDGAR, J.A., & FRAHN, J.L. (1984) Toxicity of
Echium plantagineum (Paterson's curse). I. Marginal toxic effects
of Merino wethers from long-term feeding. Aust. J. agric. Res., 35:
293-304.
CULVENOR, C.C.J., EDGAR, J.A., SMITH, L.W., KUMANA, C.R., & LIN,
H.J. (1986) Heliotropium lasiocarpum Fisch and Mey identified as
cause of veno-occlusive disease due to a herbal tea. Lancet, 1: 978.
DANN, A.T. (1960) Detection of N-oxides of the pyrrolizidine
alkaloids. Nature, 4730: 1051.
DANNINGER, T., HAGEMANN, U., SCHMIDT, V., & SCHOENHOEFER, P.S.
(1983) Toxicity of pyrrolizidine alkaloid-containing medicinal
plants. Pharm. Ztg, 128: 289-303.
DATTA, D.V., KHUROO, M.S., MATTOCKS, A.R., AIKAT, B.K., &
CHHUTTANI, P.N. (1978a) Veno-occlusive disease of liver due to
Heliotropium plant used as medicinal herb (report of six cases with
review of literature). J. Assoc. Phys. India, 26(5): 383-393.
DATTA, D.V., KHUROO, M.S., MATTOCKS, A.R., AIKAT, B.K., &
CHHUTTANNI, P.N. (1978b) Herbal medicines and veno-occlusive
disease in India. Postgrad. med. J., 54: 511-515.
DAVIDSON, C.S. (1963) Plants and fungi as etiologic agents of
cirrhosis. New Engl. J. Med., 268: 1072-1073.
DAVIDSON, J. (1935) The action of retrorsine on rat's liver. J.
Pathol. Bacteriol., 40: 285-295.
DEAGEN, J.T. & DEINZER, M.L. (1977) Improvement in the extraction
of pyrrolizidine alkaloids. Lloydia, 40: 395-397.
DEAN, R.E. & WINWARD, A.H. (1974) An investigation into the
possibility of tansy ragwort poisoning of black-tailed deer. J.
wildl. Dis., 10: 166-169
DEINZER, M.L., THOMSON, P.A., BURGETT, D.M., & ISAACSON, D.L.
(1977) Pyrrolizidine alkaloids: their occurrence in honey from
tansy ragwort (S. jacobaea). Science, 195: 497-499.
DEINZER, M.L., THOMSON, P.A., GRIFFIN, D., & DICKINSON, E. (1978)
Sensitive analytical method for pyrrolizidine alkaloids. The mass
spectra of retronecine derivatives. Biomed. mass Spectrom., 5:
175-179.
DELFOSSE, E.S. (1985) Re-evaluation of the biological control
program for Heliotropium europaeum in Australia. In: Delfosse,
E.S., ed. Proceedings of the VI International Symposium on
Biological Control of Weeds, 19-25 August 1984, Vancouver, Canada,
Ottawa Agriculture Canada, pp. 735-42.
DELFOSSE, E.S. & CULLEN, J.M. (1985a) CSIRO division of Entomology
Submission to the enquiries into biological control of Echium
plantagineum L., Paterson's curse/Salvation Jane. Plant Prot. Q.,
1: 24-40.
DELFOSSE, E.S. & CULLEN, J.M. (1985b) Echium plantagineum:
catalyst for conflict and change in Australia. In: Delfosse, E.S.,
ed. Proceedings of the VI International Symposium on Biological
Control of Weeds, 19-25 August, 1984, Vancouver, Canada, Ottawa
Agriculture Canada pp. 249-292.
DELORME, P., JAY, M., & FERRY, S. (1977) Phytochemical inventory
in indigenous Boraginaceae: study of the alkaloids and polyphenolic
compounds (anthocyanic and flavonoid compounds). Plant. Méd.
Phytothér., 11: 5-11. (in french)
DE WAAL, H.L. & VAN TWISK, P. (1964) The chemical composition of
four Senecio species from Kruger National Park. Koedoe (South
Africa), 7: 40-42.
DICK, A.T., DANN, A.T., BULL, L.B., & CULVENOR, C.C.J. (1963)
Vitamin B12 and the detoxication of hepatotoxic pyrrolizidine
alkaloids in rumen liquor. Nature (Lond.), 197: 207-208.
DICKINSON, J.O. (1980) Release of pyrrolizidine alkaloids into
milk. Proc. West. Pharmacol. Soc., 23: 377-379.
DICKINSON, J.O., COOKE, M.P., KING, R.R., & MOHAMED, P.A. (1976)
Milk transfer of pyrrolizidine alkaloids in cattle. J. Am. Vet.
Med. Assoc., 169: 1192-1196.
DIMENNA, G.P., KRICK, T.P., & SEGALL, H.J. (1980) Rapid high
performance liquid chromatography isolation of monoesters,
diesters, and macrocyclic diesters and pyrrolizidine alkaloids from
Senecio jacobaea and Amsinckia intermedia. J. Chromatogr., 192:
474-478.
DOLINSKAYA, K.N. (1952) [Pathomorphology of heliotropic hepatic
dystrophy in children.] In: Milenkov, S.M. & Kizhaikin, Y., ed.
[Collection of scientific papers on Toxic Hepatitis with Ascites,]
Tashkent, Publishing House of the University of Central Asia,
pp. 165-172 (in Russian).
DREIFUSS, P.A. (1984) A study of the mass spectra of
pyrrolizidine alkaloids by negative ion chemical ionization and
ms/ms: the identification of a monoester pyrrolizidine alkaloid in
Eupatorium rugosum. Diss. Abstr. Int. B., 45:'1183-1184.
DRIVER, H.E. & MATTOCKS, A.R. (1984) The toxic effects in rats of
some synthanecine carbamate and phosphate esters analogous to
hepatotoxic pyrrolizidine alkaloids. Chem.-biol. Interact., 51:
201-218.
DUBROVINSKII, S.B. (1946) [About the alimentary toxicosis caused
by heliotrope.] J. Sov. Prot. Health, 6: 17-21 (in Russian).
DUBROVINSKII, S.B. (1952) [The etiology of toxic hepatitis with
ascites.] In: Millenkov, S.M. & Kizhaikin, Y., ed. [Collection of
scientific papers on Toxic Hepatitis with Ascites, Tashkent, USSR,]
Tashkent, Publishing House of the University of Central Asia,
pp. 9-25 (in Russian).
EASTMAN, D.F. & SEGALL, H.J. (1982) A new pyrrolizidine alkaloid
metabolite, 19-hydroxysenecionine, isolated from in vitro mouse
hepatic microsomes using high performance liquid chromatography.
Drug Metab. Disp., 10: 696-699.
EASTMAN, D.F., DIMENNA, G.P., & SEGALL, H.J. (1982) Covalent
binding of two pyrrolizidine alkaloids, senecionine, and
seneciphylline to hepatic macromolecules and their distribution,
excretion and transfer into milk of lactating mice. Drug Metab.
Disp., 10: 236-240.
EDGAR, J.A. (1982) Pyrrolizidine alkaloids sequestered by Solomon
Island Danaine butterflies. The feeding preferences of the Danainae
and Ithomiinae. J. Zool. (Lond.), 196: 385-399.
EDGAR, J.A. (1985) Gas chromatography of pyrrolizidine alkaloids.
In: Seawright, A.A., Hegarty, M.P., James, L.F., & Keeler, R.F.,
ed. Plant toxicology. Proceedings of the Australia - USA Poisonous
Plants Symposium, Brisbane, Australia, 14-18 May 1984, Brisbane,
Queensland Poisonous Plants Committee, pp. 227-234.
EISENSTEIN, D. & HUXTABLE, R.J. (1979) Approaches to the treatment
of pyrrolizidine toxicosis. In: Cheeke, P.R., ed. Proceedings of
the Symposium on Pyrrolizidine (Senecio) Alkaloids: Toxicity,
Metabolism, and Poisonous Plant Control Measures, Corvallis,
Oregon, USA, 23-24 February 1979, Oregon, Nutrition Research
Institute, pp. 109-113.
EL DAREER, S.M., TILLERY, K.F., LLOYD, H.H., & HILL, D.L. (1982)
Disposition of indicine N-oxide in mice and monkeys. Cancer Treat.
Rep., 66: 183-186.
EMMEL, M.W. (1948) Crotalaria poisoning in cattle. J. Am. Vet.
Med. Assoc., 113: 164.
EMMEL, M.W., SANDERS, D.A., & HENLEY, W.W. (1935) Crotalaria
spectabilis seed poisoning in swine. J. Am. Vet. Med. Assoc., 86:
43-49.
EVANS, J.V., PENG, A., & NIELSEN, C.J. (1979) The gas
chromatographic mass spectrometric analysis of the new anti-tumour
drug indicine-N-oxide utilizing a novel reaction accompanying
trimethysilylation. Biomed. mass Spectrom., 6: 38-43.
EVANS, J.V., DALEY, S.K., MCCLUSKY, G.A., & NIELSEN, C.J. (1980)
Direct quantitative analysis of indicine N-oxide in cancer patient
samples by gas chromatography using the internal standard
heliotrine N-oxide including a mass spectral comparison of their
trimethylsilyl derivatives. Biomed. mass Spectrom., 7: 65-73.
FARRINGTON, K.J. & GALLAGHER, C.H. (1960) Complexes of copper with
some pyrrolizidine alkaloids and with some of their esterifying
acids. Aust. J. biol. Sci., 13: 600-603.
FISH, M.S., SWEELEY, C.C., JOHNSON, N.M., LAWRENCE, E.P., &
HORNING, E.C. (1956) Chemical and enzymic rearrangements of
N,N-dimethylaminoacid oxides. Biochem. Biophys. Acta, 21: 196-197.
FISHMAN, A.P. (1974) Dietary pulmonary hypertension. Circ. Res.,
35: 657-660.
FORSYTH, A.A. (1968) British poisonous plants, London, Her
Majesty's Stationery Office.
FOX, D.W., HART, M.C., BERGESON, P.S., JARRETT, P.B., STILLMAN,
A.E., & HUXTABLE, R.J. (1978) Pyrrolizidine (Senecio)
intoxication mimicking Reye's syndrome. J. Paediatr., 93: 980-982.
FRAHN, J.L., CULVENOR, C.C.J., & MILLS, J.A. (1980) Preparative
separation of the pyrrolizidine alkaloids, intermedine and
lycopsamine, as their borate complexes. J. Chromatogr., 195:
379-383.
FURMANOWA, M., GUZEWSKA, J., & BELDOWSKA, B. (1983) Mutagenic
effects of aqueous extracts of Symphytum officinale L. and of its
alkaloidal fractions. J. appl. Toxicol., 3: 127-130.
FURUYA, T. & HIKICHI, M. (1971) Alkaloids and triterpenoids of
Symphytum officinale. Phytochemistry, 10: 2217-2220.
FUSHIMI, K., KATO, K., KATO, T., MATSUBARA, N., & HIRONO, I. (1978)
Carcinogenicity of flower stalks of Petasites japonicus Maxim in
mice and Syrian golden hamsters. Toxicol. Lett., 1: 291-294.
GADELLA, T.W.J., KLIPHUIS, E., & HUIZING, H.J. (1983) Cyto- and
chemotaxonomical studies on the sections officinalia and coerulea
of the genus Symphytum. Bot. Helv., 93: 169-192.
GANEY, P.E., FINK, G.D., & ROTH, R.A. (1985) The effect of dietary
restriction and altered sodium intake on the cardiopulmonary
toxicity of monocrotaline pyrrole. Toxicol. appl. Pharmacol., 78:
55-62.
GARDINER, M.R., ROYCE, R., & BOKOR, A. (1965) Studies on
Crotalaria crispata, a newly recognized cause of Kimberley horse
disease. J. Pathol. Bacteriol., 89: 43-55.
GHANEM, J. & HERSHKO, C. (1981) Veno-occlusive disease and primary
hepatic vein thrombosis in Israeli Arabs. Isr. J. med. Sci., 17:
339-347.
GHODSI, F. & WILL, J.A. (1981) Changes in pulmonary structure and
function induced by monocrotaline intoxication. Am. J. Physiol.,
240: H149-H155.
GILRUTH, J.A. (1903) Hepatic cirrhosis affecting horses and cattle
(so-called "Winton disease"), Wellington, New Zealand Department
of Agriculture, pp. 228-279 (11th annual report).
GOEGER, D.E., CHEEKE, P.R., SCHMITZ, J.A., & BUHLER, D.R. (1982)
Toxicity of tansy ragwort (Senecio jacobaea) to goats. Am. J. vet.
Res., 43: 252-254.
GOEGER, D.E., CHEEKE, P.R., RAMSDELL, H.S., NICHOLSON, S.S., &
BUHLER, D.R. (1983) Comparison of the toxicities of Senecio
jacobaea, Senecio vulgaris and Senecio glabellus in rats.
Toxicol. Lett., 15: 19-23.
GONZALEZ, A.G., DE LA FUENTE, G., REINA, M., & LOYOLA, L.A. (1986a)
Pyrrolizidine alkaloids from Senecio phillipicus and Senecio
illinitus. Planta Med., 52: 160.
GONZALEZ, E., GARCIA, R., LEMUS, I., & ERAZO, S. (1986b)
Pharmacological studies on senecionine, a pyrrolizidine alkaloid
from Senecio fistulosus Poepp. Ex less. (hualtata). An. R. Acad.
Farm., 52(1): 123-132.
GRASES, P.J. & BEKER, S.G. (1972) Veno-occlusive disease of the
liver. A case from Venezuela. Am. J. Med., 53: 511-516.
GRAY, A.I., NIC, A.N., TSAOIR, E., & WATERMAN, P.G. (1983)
Hepatotoxic alkaloids and allantoin in Symphytum tuberosum. J.
Pharm. Pharmacol., 35(Suppl): 13.
GREEN, C.R. & CHRISTIE, G.S. (1961) Malformations in foetal rats
induced by the pyrrolizidine alkaloid, heliotrine. Br. J. exp.
Pathol., 42: 369-378.
GREEN, M.H.L. & MURIEL, W.J. (1975) Use of repair-deficient
strains of Escherichia coli and liver microsomes to detect and
characterise DNA damage caused by the pyrrolizidine alkaloids
heliotrine and monocrotaline. Mutat. Res., 28: 331-336.
GREEN, C.E., SEGALL, H.J., & BYARD, J.L. (1981) Metabolism,
cytotoxicity, and genotoxicity of the pyrrolizidine alkaloid
senecionine in primary cultures of rat hepatocytes. Toxicol. appl.
Pharmacol., 60: 176-185.
GUENGERICH, F.P. (1977) Separation and purification of multiple
forms of microsomal cytochrome P-450. J. biol. Chem., 252:
3970-3979.
GUENGERICH, F.P. & MITCHELL, M.B. (1980) Metabolic activation of
model pyrroles by cytochrome P-450. Drug Metab. Disp., 8: 34-38.
GUIDUGLI, F.H., PESTCHANKER, M.J., DESALMERON, M.S.A., & GIORDANO,
O.S. (1986) 1-hydroxyplatyphyllide, a norsequiterpene lactone
from Senecio gilliesiano. Phytochemistry, 25: 1923-1926.
GUNER, N. (1986) Alkaloids of Heliotropium suaveolens. J. nat.
Prod., 49: 369.
GUPTA, P.S., GUPTA, G.D., & SHARMA, M.L. (1963) Veno-occlusive
disease of the liver. Br. med. J., 1: 1184-1186.
HABS, H. (1982) Senecio numorensis fuchsii. Carcinogenic and
mutagenic activity of an alkaloidal extract from a phytotherapeutic
drug. Dtsch. Apoth. Ztg, 122: 799-804.
HABS, H., HABS, M., MARQUARDT, H., RODER, E., SCHMAHL, D., &
WIEDENFELD, H. (1982) Carcinogenic and mutagenic activity of an
alkaloidal extract of Senecio numorensis spp. fuchsii.
Arzneimittelforsch, 32: 144-148.
HAGGLUND, K.M., L'EMPEREUR, K.M., ROBY, M.R., & STERMITZ, F.R.
(1985) Latifoline and latifoline N-oxide from Hackelia
floribunda, the western false forget-me-not. J. nat. Prod., 48:
638-639.
HAMMOUDA, F.M., RIZK, A.M., ISMAIL, S.I., ATTEYA, S.Z., GHALEB,
H.A., MADKOUR, M.K., POWAND, A.E., & WOOD, G. (1984) Poisonous
plants contaminating edible ones and toxic substances in plant
foods. Part 3: pyrrolizidine alkaloids from Heliotropium digynum
Forssk (= H. luteum, Poir). Pharmazie, 39: 703-705.
HARDING, J.D.J., LEWIS, G., DONE, J.T., & ALLCROFT, R. (1964)
Experimental poisoning by Senecio jacobaea in pigs. Pathol. Vet.,
1: 204-220.
HARRIS, C., REDDY, J., CHIGA, M., & SVOBODA, D. (1969)
Polyribosomal disaggregation by lasiocarpine. Biophys. Acta 180:
587-589.
HARRIS, P.N. & CHEN, K.K. (1970) Development of hepatic tumours in
rats following ingestion of Senecio longilobus. Cancer Res., 30:
2881-2886.
HARRIS, P.N., ROSE, C.L., & CHEN, K.K. (1957) Hepatotoxic and
pharmacological properties of heliotrine. Arch. Pathol., 64:
152-157.
HARTMANN, T. & ZIMMER, M. (1986) Organ-specific distribution and
accumulation of pyrrolizidine alkaloids during the life history of
2 annual Senecio species. J. plant Physiol., 122: 67-80.
HASHEM, M. (1939) Etiology and pathology of types of liver
cirrhosis in Egyptian children. J. Egypt. Med. Assoc., 22: 319-354.
HAYASHI, Y. (1966) Excretion and alteration of monocrotaline in
rats after a subcutaneous injection (abstract). Fed. Proc., 25: 688.
HAYASHI, Y. & LALICH, J.J. (1967) Renal and pulmonary alterations
induced by a single injection of monocrotaline. Proc. Soc. Exp.
Biol. Med., 124: 392-396.
HAYASHI, Y. & LALICH, J.J. (1968) Protective effect of
mercaptoethylamine and cysteine against monocrotaline intoxication
in rats. Toxicol. appl. Pharmacol., 12: 36-43.
HAYASHI, Y., HUSSA, J.F., & LALICH, J.J. (1967) Cor pulmonale in
rats. Lab. Invest., 16: 875-881.
HAYASHI, Y., KATO, M., OTSUKA, H. (1979) Inhibitory effects of
diet reduction on Monocrotaline intoxication in rats. Toxicol.
Lett., 3: 151-155.
HAYASHI, Y., SHIMADA, M., & KATAYAMA, H. (1977) Experimental
insulinoma in rats after a single administration of monocrotaline.
Toxicol. Lett., 1: 41-44.
HAYASHI, Y., KOKUBO, T., TAKAHASHI, M., FURUKAWA, F., OTSUKA, H., &
HASHIMOTO, K. (1984) Correlative morphological and biochemical
studies on monocrotaline-induced pulmonary alterations in rats.
Toxicol. Lett., 21: 65-77.
HAYES, M.A., ROBERTS, E., & FARBER, E. (1985) Initiation and
selection of resistant hepatocyte nodules in rats given the
pyrrolizidine alkaloids lasiocarpine and senecionine. Cancer Res.,
45: 3726-3734.
HEATH, D., SHABA, J., WILLIAMS, A., SMITH, P., & KOMBE, A. (1975)
A pulmonary hypertension-producing plant from Tanzania. Thorax, 30:
399-404.
HENDRICKS, J.D., SINNHUBER, R.O., HENDERSON, M.D., & BUHLER, D.R.
(1981) Liver and kidney pathology in rainbow trout (Salmo
gairdneri) exposed to dietary pyrrolizidine (Senecio) alkaloids.
Exp. mol. Pathol., 35: 170.183.
HILL, K.R. (1960) Worldwide distribution of seneciosis in man and
animals. Proc. R. Soc. Med., 53: 281-282.
HILLIKER, K.S. & ROTH, R.A. (1984) Alteration of monocrotaline
pyrrole-induced cardiopulmonary effects in rats by hydrallazine,
dexamethasone or sulphinpyrazone. Br. J. Pharmacol., 82: 375-380.
HILLIKER, K.S., BELL, T.G., & ROTH, R.A. (1983) Monocrotaline
pyrrole-induced pulmonary hypertension in fawn-hooded rats with
platelet storage pool deficiency: 5 hydroxytryptamine uptake by
isolated, perfused lungs. Thromb. Haemostasis (Stuttgart), 50:
844-847.
HILLIKER, K.S., BELL, T.G., LORIMER, D., & ROTH, R.A. (1984)
Effects of thrombocytopaenia in monocrotaline pyrrole-induced
pulmonary hypertension. Am. J. Physiol., 246: H747-H753.
HIRONO, I., SHIMIZU, M., FUSHIMI, K., MORI, H., & KATO, K. (1973)
Carcinogenic activity of Petasites japonicus Maxim, a kind of
coltsfoot. Gann Monogr. Cancer Res., 64: 527-528.
HIRONO, I., MORI, H., & CULVENOR, C.C.J. (1976) Carcinogenic
activity of coltsfoot, Tussilago farfara L. Gann Monogr. Cancer
Res., 67: 125-129.
HIRONO, I., MORI, H., YAMADA, K., HIRATA, Y., HAGA, M., TATEMATSU,
H., & KANIE, S. (1977) Carcinogenic activity of petasitenine, a
new pyrrolizidine alkaloid isolated from Petasites japonicus
Maxim. J. Natl Cancer Inst., 58: 1155-1157.
HIRONO, I., MORI, H., & HAGA, M. (1978) Carcinogenic activity of
Symphytum officinale. J. Natl Cancer Inst., 61: 865-869.
HIRONO, I., HAGA, M., FUJII, M., MATSUURA, S., MATSUBARA, N.,
NAKAYAMA, M., FURUYA, T., HIKICHI, M., TAKANASHI, H., UCHIDA, E.,
HOSAKA, S., & UENO, I. (1979a) Induction of hepatic tumours in
rats by senkirkine and symphytine. J. Natl Cancer Inst., 63: 469-472.
HIRONO, I., MORI, H., HAGA, M., FUJII, M., YAMADA, K., TAKANASHI,
H., UCHIDA, E., HOSAKA, S., UENO, I., MATSUSHIMA, I., UMEZAVA, K.,
& SHIRAI, A. (1979b) Edible plants containing carcinogenic
pyrrolizidine alkaloids in Japan. In: Miller, B.C. ET AL., ed.
Naturally occurring carcinogens, mutagens, and modulators of
carcinogenesis, Baltimore, Maryland, University Park Press, pp.
79-87.
HIRONO, I., UENO, I., AISO, S., YAMAJI, T., & HAGA, M. (1983)
Carcinogenic activity of Farfagium japonicum and Senecio
cannabifolius. Cancer Lett., 20: 191-198.
HOET, P., ASTIGUETA, M., & FRISQUE, A.M. (1981) Phytochemical
study of Crotalaria nitens. Rev. Latinoam. Quim., 12: 34-35.
HOOPER, P.T. (1972) Spongy degeneration in the brain in relation
to hepatic disease and ammonia toxicity in domestic animals. Vet.
Res., 90: 37-38.
HOOPER, P.T. (1974) The pathology of Senecio jacobaea poisoning of
mice. J. Pathol., 113: 227-230.
HOOPER, P.T. (1975a) Spongy degeneration in the central nervous
system of domestic animals. Part I: morphology. Acta neuropathol.
(Berlin), 31: 325-334.
HOOPER, P.T. (1975b) Spongy degeneration in the central nervous
system of domestic animals. Part III: occurrence and pathogenesis -
hepatocerebral disease caused by hyper-ammonaemia. Acta
neuropathol. (Berlin), 31: 343-351.
HOOPER, P.T. (1975c) Experimental acute gastro-intestinal disease
caused by the pyrrolizidine alkaloid, lasiocarpine. J. comp.
Pathol., 85: 341-349.
HOOPER, P.T. (1978) Pyrrolizidine alkaloid poisoning-pathology
with particular reference to differences in animal and plant
species. In: Keeler, R.F., Van Kampen, K.R., & James, L.F., ed.
Effects of poisonous plants on livestock, New York, Academic
Press, pp. 161-176.
HOOPER, P.T. & SCANLAN, W.A. (1977) Crotalaria retusa poisoning in
pigs and poultry. Aust. vet. J., 53: 109-114.
HOOPER, P.T., BEST, S.M., & MURRAY, D.R. (1974) Hyper-ammonaemia
and spongy degeneration of the brain in sheep affected with hepatic
necrosis. Res. vet. Sci., 16: 216-222.
HOOSON, J. & GRASSO, P. (1976) Cytotoxic and carcinogenic response
to monocrotaline pyrrole. J. Pathol., 118: 121-127.
HOU JINQUI, XIA YUDIN, YU ZHANSHI, AN YAN, & TANG YIXAI (1980)
[Veno-occlusive disease of the liver with report of 2 cases.] Chung
Hua Nei ko Tsa Chih, 19: 187-191 (in Chinese).
HSU, I.C., ALLEN, J.R., & CHESNEY, C.F. (1973) Identification and
toxicological effects of dehydroretronecine, a metabolite of
monocrotaline. Proc. Soc. Exp. Biol. Med., 144: 834-838.
HSU, I.C., SHUMAKER, R.C., & ALLEN, J.R. (1974) Tissue
distribution of tritium labelled dehydroretronecine. Chem.-biol.
Interact., 8: 163.
HUIZING, H.J., DE BOER, R., & MALINGRE, T.M. (1981) Preparative
ion-pair high performance liquid chromatography and gas
chromatography of pyrrolizidine alkaloids from comfrey. J.
Chromatogr., 214: 257-262.
HURLEY, J.V. & JAGO, M.V. (1975) Pulmonary oedema in rats given
dehydromonocrotaline: a topographic and electron-microscopic
study. J. Pathol., 117: 23-32.
HUXTABLE, R.J. (1980) Herbal teas and toxins: novel aspects of
pyrrolizidine poisoning in the United States. Perspect. Biol. Med.,
24: 1-14.
HUXTABLE, R., PAPLANUS, S., & LAUGHARN, J. (1977) The prevention
of monocrotaline induced right ventricular hypertrophy. Chest, 71:
308-310.
HUXTABLE, R.J., CIARAMITARO, D., & EISENSTEIN, D. (1978) The
effect of a pyrrolizidine alkaloid, monocrotaline and a pyrrole,
dehydroretronecine, on the biochemical functions of the pulmonary
endothelium. Mol. Pharmacol., 14: 1189-1203.
HUXTABLE, R.J., LUTHY, J., & ZWEIFEL, V. (1986) Toxicity of
comfrey-pepsin preparations: letters to the editor. New Engl. J.
Med., 315: 1095.
IARC (1976) Pyrrolizidine alkaloids. In: Some naturally occurring
substances, Lyons, International Agency for Research on Cancer,
pp. 265-342 (IARC Monograph on the Evaluation of Carcinogenic Risk
of Chemicals to Man, Vol. 10).
IARC (1983) Some food additives, feed additives and naturally
occurring substances, Lyons, International Agency for Research on
Cancer, pp. 207-245 (IARC Monograph on the Evaluation of
Carcinogenic Risk of Chemicals to Humans, Vol. 31).
INDIAN COUNCIL OF MEDICAL RESEARCH (1955) Infantile cirrhosis of
the liver in India. Indian J. med. Res., 43: 723-747.
ISMAILOV, N.I. (1948a) [On clinical features, etiology and
pathogenesis of heliotropic dystrophy (toxic hepatitis with
ascites).] Clin. Med., 8: 28 (in Russian).
ISMAILOV, N.I. (1948b) [Heliotropic toxicosis (toxic hepatitis
with ascites),] Tashkent, Academy of Sciences of Uzbekistan,
pp. 1-120 (in Russian).
ISMAILOV, N.I., MADZHIDOV, N.M., MAGRUPOV, A.I., MAKHKAMOV, G.M., &
MUKMINOVA, S.G. (1970) [Clinical signs, diagnosis and treatment of
Trichodesma toxicosis (alimentary toxic encephalopathy),]
Tashkent, Meditsina, p. 85 (in Russian).
JAGO, M.V. (1969) The development of the hepatic megalocytosis
of chronic pyrrolizidine alkaloid poisoning. Am. J. Pathol., 56:
405-422.
JAGO, M.V. (1970) A method for the assessment of the chronic
hepatotoxicity of pyrrolizidine alkaloids. Aust. J. exp. Biol. med.
Sci., 48: 93-103.
JAGO, M.V. (1971) Factors affecting the chronic hepatotoxicity
of pyrrolizidine alkaloids. J. Pathol., 105: 1-11.
JAGO, M.V., LANIGAN, G.W., BINGLEY, J.B., PIEREY, D.W.T., WHITTEN,
J.H., & TITCHEN, D.A. (1969) Excretion of the pyrrolizidine
alkaloid heliotrine in the urine and bile of sheep. J. Pathol., 98:
115-128.
JAGO, M.V., EDGAR, J.A., SMITH, L.W., & CULVENOR, C.C.J. (1970)
Metabolic conversion of heliotrine based pyrrolizidine alkaloids to
dehydroheliotridine. Mol. Pharmacol., 6: 402-406.
JELLIFFE, D.B., BRAS, G., & STUART, K.L. (1954a) Veno-occlusive
disease of the liver. Paediatrics, 14: 334-339.
JELLIFFE, D.B., BRAS, G., & STUART, K.L. (1954b) The clinical
picture of veno-occlusive disease of the liver in Jamaican
children. Ann. trop. Med. Parasitol., 48: 386-396.
JELLIFFE, D.B., BRAS, G., & MUKHERJEE, K.L. (1957) Veno-occlusive
disease of the liver and Indian childhood cirrhosis. Arch. dis.
Child., 32: 369-385.
JOHNSON, A.E. (1976) Changes in calves and rats consuming milk
from cows fed chronic lethal doses of Senecio jacobaea (tansy
ragwort). Am. J. vet. Res., 37: 107-110.
JOHNSON, A.E. (1979) Toxicity of tansy ragwort to cattle. In:
Cheeke, P.R., ed. Proceedings of the Symposium on Pyrrolizidine
(Senecio) Alkaloids: Toxicity, Metabolism, and Poisonous Plant
Control Measures, Corvallis, Oregon, USA, 23-24 February 1979,
Oregon, Nutrition Research Institute, pp. 129-134.
JOHNSON, A.E. (1982) Failure of mineral-vitamin supplements to
prevent tansy ragwort (Senecio jacobaea) toxicosis in cattle. Am.
J. vet. Res., 43: 718-723.
JOHNSON, A.E. & MOLYNEUX, R.J. (1984) Toxicity of thread leaf
groundsel (Senecio douglasii var. longilobus) to cattle. Am. J.
vet. Res., 45: 26-31.
JOHNSON, A.E., MOLYNEUX, R.J., & STUART, L.D. (1985) Toxicity of
Riddel's groundsel (Senecio riddelli) to cattle. Am. J. vet. Res.,
46: 577-582.
JOHNSON, W.D. (1981) Mechanism of in vitro acute toxicity of
dehydromonocrotaline a metabolite of pyrrolizidine alkaloid
monocrotaline. Toxicologist, 1: 107-108.
JOHNSON, W.D., ROBERTSON, K.A., POUNDS, J.G., & ALLEN, J.R. (1978)
Dehydroretronecine-induced skin tumours in mice. J. Natl Cancer
Inst., 61: 85-89.
JULIEN, M.H., ed. (1982) Biological control of weeds. A world
catalog of agents and their target weeds, Curepe, Trinidad and
Toabago, Commonwealth Institute of Biological Control.
JUNEJA, T.R., GUPTA, R.L., & SAMANTA, S. (1984) Activation of
monocrotaline, fulvine and their derivatives to toxic pyrroles by
some thiols. Toxicol. Lett., 21: 185-189.
KAMPANZEV, N.N. (1952) [Experimental heliotropic hepatitis.] In:
Milenkov, S.M. & Kizhaikin, Y., ed. [Collection of scientific
papers on Toxic Hepatitis with Ascites,] Tashkent, Publishing
House of the University of Central Asia, pp. 165-172 (in Russian).
KAY, J.M. & HEATH, D. (1966) Observation on the pulmonary arteries
and heart weight of rats fed on Crotalaria spectabilis seeds. J.
Pathol., 92: 385-394.
KAY, J.M. & HEATH, D. (1969) Crotalaria spectabilis: the pulmonary
hypertension plant, Springfield, Illinois, Charles C. Thomas, p.
38.
KAY, J.M., HARRIS, P., & HEATH, D. (1967a) Pulmonary hypertension
produced in rats by ingestion of Crotalaria spectabilis seeds.
Thorax, 22: 176-179.
KAY, J.M., GILLUND, T.D., & HEATH, D. (1967b) Mast cells in the
lungs of rats fed on Crotalaria spectabilis seeds. Am. J. Pathol.,
51: 1031-1044.
KAY, J.M., HEATH, D., SMITH, P., BRAS, G., & SUMMERELL, J. (1971a)
Fulvine and pulmonary circulation. Thorax, 26: 249-261.
KAY, J.M., SMITH, P., & HEATH, D. (1971b) Aminorex and the
pulmonary circulation. Thorax, 26: 262-270.
KAY, J.M., KEANE, P.M., & SUYAMA, K.L. (1985) Pulmonary
hypertension induced in rats by monocrotaline and chronic hypoxia
is reduced by p-chlorophenylalanine. Respiration, 47: 48-56.
KEDZIERSKI, B. & BUHLER, D.R. (1985) Configuration of necine
pyrroles - toxic metabolites of pyrrolizidine alkaloids. Toxicol.
Lett., 25: 115-119.
KEDZIERSKI, B. & BUHLER, D.R. (1986) The formation of 6,7-di-
hydro-7-hydroxy-1-hydroxymethyl-5H-pyrrolizine, a metabolite of
pyrrolizidine alkaloids. Chem.-biol. Interact., 57: 217-222.
KHANIN, M.N. (1948) [The etiology of toxic hepatitis with
ascites.] Arch. Pathol. USSR, 1: 42-47 (in Russian).
KIM, H.L. & JONES, L.P. (1982) Protective effects of butylated
anisole, ethoxyquin and disulfiran on acute pyrrolizidine alkaloid
poisoning in mice. Res. Comm. chem. Pathol. Pharmacol., 36: 341-344.
KIRFEL, A., WILL, G., WIEDENFELD, H. & RODER, E. (1980)
(1aR,6bR,10R,11R)-9,15-dioxo-10-hydroxy-10,11,13-trimethyl-1a,2,
3,6b-tetrabydro-5H-pyrrolizino-[1a,6b,6a,c],8-dioxa-15-cis-tride
cene,C18H25NO5. Cryst. Styruct. Comm., 9: 353-361.
KNIGHT, A.P., KIMBERLING, C.V., STERMITZ, F.R., & ROBY, M.R. (1984)
Cynoglossum officinale (hound's tongue) - a cause of pyrrolizidine
alkaloid poisoning in horses. J. Am. Vet. Med. Assoc., 185: 647-650.
KOEKEMOER, M.J. & WARREN, F.L. (1951) The occurrence and
preparation of the N -oxides. An improved method of extraction of
the Senecio alkaloids. J. Chem. Soc., C1951: pp. 66-68.
KOLETSKY, A., OYASU, R., & REDDY, J.K. (1978) Mutagenicity of the
pyrrolizidine (Senecio) alkaloid, lasiocarpine in the
salmonella/microsome test. Lab. Invest., 38: 352 (Abstract).
KOVACH, J.S., MOERTEL, C.G., & HAHN, R.G. (1979a) A phase 1 study
of indicine- N-oxide. Proc. Am. Assoc. Cancer Res., 20: 357.
KOVACH, J.S., AMES, M.M., POWIS, G., MOERTEL, C.G., HAHN, R.G., &
CREAGAN E.T. (1979b) Toxicity and pharmacokinetics of a
pyrrolizidine alkaloid, indicine- N-oxide, in humans. Cancer Res.,
39: 4540-4544.
KRAUS, C., ABEL, G., & SCHIMMER, O. (1985) [Studies on the
chromosome damaging effect of some pyrrolizidine alkaloids in human
lymphocytes in vitro.] Planta Med., 51: 89-91 (in German).
KRISHNAMACHARI, K.A.V.R., BHAT, R.V., KRISHNAMURTHY, D.,
KRISHNASWAMY, K., & NAGARAJAN (1977) Aetiopathogenesis of endemic
ascites in Sarguja district of Madhya Pradesh. Indian J. med. Res.,
65: 672-678.
KUHARA, K., TAKANASHI, H., HIRONO, I., FURUYA, T., & ASADA, Y.
(1980) Carcinogenic activity of clivorine, a pyrrolizidine
alkaloid isolated from Ligularia dentata. Cancer Lett., 10: 117-122.
KUMANA, C.R., NG, M., LIN, H.J., KO, W., WU, P.C., & TODD, D.
(1983) Hepatic veno-occlusive disease due to toxic alkaloid herbal
tea - letter to the editor. Lancet, 10 December: 1360-1361.
KUMANA, C.R., NG, M., LIN, H.J., KO, W., WU, P.C., & TODD, D.
(1985) Herbal tea induced hepatic veno-occlusive disease:
quantification of toxic alkaloid exposure in adults. Gut, 26:
101-104.
KUROZUMI, T., TANAKA, K., KIDO, M., & SHOYAMA, Y. (1983)
Monocrotaline-induced renal lesions. Exp. mol. Pathol., 39: 377-386.
LAFRANCONI, W.M. & HUXTABLE, R.J. (1983) Changes in angiotensin-
converting enzyme activity in lungs damaged by the pyrrolizidine
alkaloid, monocrotaline. Thorax, 38: 307-309.
LAFRANCONI, W.M. & HUXTABLE, R.J. (1984) Hepatic metabolism and
pulmonary toxicity of monocrotaline using isolated perfused liver
and lung. Biochem. Pharmacol., 33: 2479-2484.
LAFRANCONI, W.M., DUHAMEL, R.C., BRENDEL, K., & HUXTABLE,R.J.
(1984) Differentiation of the cardiac and pulmonary toxicity of
monocrotaline, a pyrrolizidine alkaloid. Biochem. Pharmacol., 33:
191-197.
LAFRANCONI, W.M., OHNUMA, S., & HUXTABLE, R.S. (1985) Biliary
excretion of novel pneumotoxic metabolites of the pyrrolizidine
alkaloid monocrotaline. Toxicon, 23: 903-992.
LALICH, J.J. & EHRHART, L.A. (1962) Monocrotaline induced
pulmonary arteritis in rats. J. atheroscler. Res., 2: 482-492.
LALICH, J.J. & MERKOW, L. (1961) Pulmonary arteritis produced in
rats by feeding Crotalaria spectabilis. Lab. Invest., 10: 744-750.
LANCET (1984) Pyrrolizidine alkaloids (editorial). Lancet, 1:
201-202.
LANGLEBEN, D. & REID, L.M. (1985) Effect of methylprednisolone on
monocrotaline-induced pulmonary vascular disease and right
ventricular hypertrophy. Lab. Invest., 52: 298-303.
LANIGAN, G.W. (1971) Metabolism of pyrrolizidine alkaloids in the
ovine rumen. III. The competitive relationship between heliotrine
metabolism and methanogenesis in rumen fluid in vitro. Aust. J.
agric. Res., 22: 123-130.
LANIGAN, G.W. (1972) Metabolism of pyrrolizidine alkaloids in
ovine rumen. IV. Effects of chloral hydrate and halogenated
methanes on rumen methanogenesis and alkaloid metabolism in
fistulated sheep. Aust. J. agric. Res., 23: 1085-1091.
LANIGAN, G.W. & SMITH, L.W. (1970a) Metabolism of pyrrolizidine
alkaloids in the ovine rumen. I. Formation of 7 alpha-hydroxy-
1alpha-methyl-8alpha-pyrrolizidine from heliotrine and
lasiocarpine. Aust. J. agric. Res., 21: 493-500.
LANIGAN, G.W. & SMITH, L.W. (1970b) Metabolism of pyrrolizidine
alkaloids in the ovine rumen. II. Formation of 7alpha-hydroxy-
1alpha-methyl-8alpha-pyrrolizidine from heliotrine and
lasiocarpine. Aust. J. agric. Res., 22: 123-130.
LANIGAN, G.W. & WHITTEM, J.H. (1970) Cobalt pellets and
Heliotropium europaeum poisoning in penned sheep. Aust. vet. J.,
46: 17-21.
LANIGAN, G.W., PAYNE, A.L., & PETERSON, J.E. (1978) Anti-
methanogenic drugs and Heliotropium europaeum poisoning in penned
sheep. Aust. J. Agric. Res., 29: 1281-1292.
LAWS, L. (1968) Toxicity of Crotalaria mucronata to sheep. Aust.
vet. J., 44: 453-455.
LETENDRE, L., SMITHSON, W.A., GILCHRIST, G.S., BURGERT, E.O., Jr,
HOGLAND, C.H., AMES, M.M., POWIS, G., & KOVACH, J.S. (1981)
Activity of indicine- N-oxide in refractory acute leukemia. Cancer,
47: 437-441.
LETENDRE, L., LUDWIG, J., PERRAULT, J., SMITHSON, W.A., & KOVACH,
J.S. (1984) Hepatocellular toxicity during the treatment of
refractory acute leukemia with indicine- N-oxide. Cancer, 54:
1256-1259.
LEVINE, O.R., HARRIS, R.C., BLANCE, W.A., & MELLINS, R.B. (1973)
Progressive pulmonary hypertension in children with portal
hypertension. J. Pediatr., 83: 964-972.
LIN, J.J., LIU, C., & SVOBODA, D.J. (1974) Long-term effects of
aflatoxin B1 and vocal hepatitis on marmoset liver: a preliminary
report. Lab. Invest., 30: 267-278.
LUTHY, J., ZWEIFEL, U., SCHLATTER, C., & BENN, M.H. (1980)
[Pyrrolizidine alkaloids in coltsfoot ( Tussilago farfara L.) of
various sources.] Mitt. Geb. Lebensm. Hyg., 71: 73-80 (in German
with English summary).
LUTHY, J., ZWEIFEL, U., KARLHUBER, B., & SCHLATTER, C. (1981)
Pyrrolizidine alkaloids of Senecio alpinus L. and their detection
in feedstuffs. J. agric. food Chem., 29: 302-305.
LUTHY, J., HEIM, TH., & SCHLATTER, CH. (1983) Transfer of [3H]
pyrrolizidine alkaloids from Senecio vulgaris L. and metabolites
into rat milk and tissues. Toxicol. Lett., 17: 283-288.
LUTHY, J., BRAUCHLI, J., ZWEIFEL, U., SCHMID, P., & SCHLATTER, CH.
(1984) [Pyrrolizidine alkaloid in arzneipflanzen der Boraginaceen:
Borago officinalis L. and Pulmonaria officinalis L.] Pharm. Acta
Helv., 59: 242-246 (in German).
LYFORD, C.L., VERGAVA, G.G., & MOELLER, D.D. (1976) Hepatic veno-
occlusive disease originating in Ecuador. Gastro-enterology, 70:
105-108.
MCCOMISH, M., BODEK, I., & BRAUFMAN, A.R. (1980) Quantitation of
the antineoplastic agent indicine- N-oxide in human plasma by
differential pulse polarography. J. pharmacol. Sci., 69: 727-729.
MCCOY, J.W., ROBY, M.R., & STERMITZ, F.R. (1983) Analysis of plant
alkaloid mixtures by ammonia chemical ionization mass spectroscopy.
J. nat. Prod., 46: 894-900.
MCGEE, J.O'D., PATRICK, R.S., WOOD, C.B., & BLUMGART, L.H. (1976)
A case of veno-occlusive disease of the liver in Britain associated
with herbal tea consumption. J. clin. Pathol., 29: 788-794.
MCGRATH, J.P.M., DUNCAN, J.R., & MUNNELL, J.F. (1975) Crotalaria
spectabilis toxicity in swine: characterization of the renal
glomerular lesion. J. comp. Pathol., 85(2): 185-194.
MCLEAN, E.K. (1969) The early sinusoidal lesion in experimental
veno-occlusive disease. Br. J. exp. Pathol., 50: 223-229.
MCLEAN, E.K. (1970) The toxic actions of pyrrolizidine (Senecio)
alkaloids. Pharmacol. Rev., 22: 429-483.
MCLEAN, E.K. (1974) Senecio and other plants as liver poisons.
Isr. J. med. Sci., 10: 436-440.
MCLEAN, E.K. & HILL, K.R. (1969) Portal hypertension in acute
experimental veno-occulsive disease of the liver in rats. Br. J.
exp. Pathol., 50: 37-41.
MCLEAN, E.K., BRAS, G., & GYORGY, P. (1964) Veno-occlusive lesions
in livers of rats fed Crotalaria fulva. Br. J. exp. Pathol., 45:
242-247.
MAKSUDOV, A.M. (1952) [Heliotropic toxic hepatitis with ascites.]
In: Milenkov, S.M. & Kizhaikin, Y., ed. [Collection of scientific
papers on Toxic Hepatitis with Ascites,] Tashkent, Publishing House
of the University of Central Asia, pp. 103-116 (in Russian).
MARTIN, P.A., THORBURNE, M.K., HUTCHINSON, S., BRAS, G., & MILLER,
C.G. (1972) Preliminary findings of chromosome studies in rats and
humans with veno-occlusive disease. Br. J. exp. Pathol., 53, 374-380.
MATTOCKS, A.R. (1961) Extraction of heat-labile alkaloids from
plants. Nature (Lond.), 191: 1281-1282.
MATTOCKS, A.R. (1967a) Spectrophotometric determination of
unsaturated pyrrolizidine alkaloids. Anal. Chem., 39: 443-447.
MATTOCKS, A.R. (1967b) Detection of pyrrolizidine alkaloids on
thin-layer chromatograms. J. Chromatogr., 27: 505-508.
MATTOCKS, A.R. (1968a) Toxicity of pyrrolizidine alkaloids. Nature
(Lond.), 217: 723-728.
MATTOCKS, A.R. (1968b) Spectrophotometric determination of
pyrrolizidine alkaloids: some improvements. Anal. Chem., 40: 1749.
MATTOCKS, A.R. (1969) Dehydropyrrolizine derivatives from
unsaturated pyrrolizidine alkaloids. J. Chem. Soc., C1969:
1155-1162.
MATTOCKS, A.R. (1971a) Synthetic compounds with toxic properties
similar to those of pyrrolizidine alkaloids and their pyrrolic
metabolites. Nature (Lond.), 232: 476.
MATTOCKS, A.R. (1971b) The occurrence and analysis of
pyrrolizidine alkaloid N-oxides. Xenobiotica, 1: 451-453.
MATTOCKS, A.R. (1971c) Hepatotoxic effects due to pyrrolizidine
alkaloid N-oxides. Xenobiotica, 1: 563-565.
MATTOCKS, A.R. (1971d) A field test for N-oxides of unsaturated
pyrrolizidine alkaloids. Trop. Sci., 13: 65-70.
MATTOCKS, A.R. (1972a) Toxicity and metabolism of Senecio
alkaloids. In: Harborne, J.B., ed. Phytochemical ecology, London,
New York, Academic Press, pp. 179-200.
MATTOCKS, A.R. (1972b) Acute hepatotoxicity and pyrrolic
metabolites in rats dosed with pyrrolizidine alkaloids. Chem.-biol.
Interact., 5: 227-242.
MATTOCKS, A.R. (1973) Mechanisms of pyrrolizidine alkaloid
toxicity. Pharmacology and the future of man. In: Proceedings of
the 5th International Congress on Pharmacology, San Francisco,
1972, Basel, Karger, Vol. 2, pp. 114-123.
MATTOCKS, A.R. (1977) Tissue distribution of radioactivity in rats
given tritiated analogues of hepatotoxic pyrrolizidine alkaloids.
Xenobiotica, 7: 665-670.
MATTOCKS, A.R. (1980) Toxic pyrrolizidine alkaloids in comfrey.
Lancet, 22 November: 1136-1137.
MATTOCKS, A.R. (1981a) Liver cell enlargement in rats given
hydroxymethyl pyrroles analogous to pyrrolizidine alkaloid
metabolites, followed later by the hepatotoxin dimethylnitrosamine.
Toxicol. Lett., 8: 201-205.
MATTOCKS, A.R. (1981b) Relation of structural features to pyrrolic
metabolites in livers of rats given pyrrolizidine alkaloids and
derivatives. Chem.-biol. Interact., 35: 301-310.
MATTOCKS, A.R. (1981c) A simple preparation of dehydroretronecine
using potassium nitrosodisulphonate. Chem. Ind., 7: 251.
MATTOCKS, A.R. (1982) Hydrolysis and hepatotoxicity of retronecine
diesters. Toxicol. Lett., 14: 111-116.
MATTOCKS, A.R. (1986) Chemistry and toxicology of pyrrolizidine
alkaloids, London, New York, Academic Press.
MATTOCKS, A.R. & BIRD, I. (1983) Pyrrolic and N-oxide metabolites
formed from pyrrolizidine alkaloids by hepatic microsomes in vitro:
relevance to in vivo hepatotoxicity. Chem.-biol. Interact., 43:
209-222.
MATTOCKS, A.R. & CABRAL, J.R.P. (1979) Effects of some pyrrolic
and dehydropyrrolizine esters on mouse skin: a preliminary study.
Tumori, 65: 289-293.
MATTOCKS, A.R. & CABRAL, J.R.P. (1982) Carcinogenicity of some
pyrrolizidine alkaloid metabolites and analogues. Cancer Lett., 17:
61-66.
MATTOCKS, A.R. & DRIVER, H.E. (1983) A comparison of the
pneumotoxicity of some pyrrolic esters and similar compounds
analogous to pyrrolizidine alkaloid metabolites given intravenously
to rats. Toxicology, 27: 159-177.
MATTOCKS, A.R. & DRIVER, H.E. (1987) Toxic actions of senaetnine,
a new pyrrolizidine alkaloid, in rats. Toxicol. Lett., 38: 315-319.
MATTOCKS, A.R. & JUKES, R. (1987) New improved field test for
toxic pyrrolizidine alkaloids. J. nat. Prod., 50: 161-166.
MATTOCKS, A.R. & LEGG, R.F. (1980) Antimitotic activity of
dehydroretronecine, a pyrrolizidine alkaloid metabolite, and some
analogous compounds in a rat liver parenchymal cell line. Chem.-
biol. Interact., 30: 325-336.
MATTOCKS, A.R. & WHITE, I.N.H. (1970) Estimation of metabolites
of pyrrolizidine alkaloids in animal tissues. Anal. Biochem., 38:
529-535.
MATTOCKS, A.R. & WHITE, I.N.H. (1971a) The conversion of
pyrrolizidine alkaloids to dihydropyrrolizine derivatives by rat-
liver microsomes in vitro. Chem.-biol. Interact., 3: 383-396.
MATTOCKS, A.R. & WHITE, I.N.H. (1971b) Pyrrolic metabolites from
non-toxic pyrrolizidine alkaloids. Nat. new Biol., 231: 114-115.
MATTOCKS, A.R. & WHITE, I.N.H. (1973) Toxic effects and pyrrolic
metabolites in the liver of young rats given the pyrrolizidine
alkaloid retrorsine. Chem.-biol. Interact., 6: 297-306.
MATTOCKS, A.R. & WHITE, I.N.H. (1976) The distribution of
[3H]-synthanecine A bis- n-ethylcarbamate and its metabolites
in the rat. Chem.-biol. Interact., 15: 173-184.
MEYRICK, B.O. & REID, L.M. (1979) Development of pulmonary
arterial changes in rats fed Crotalaria spectabilis. Am. J.
Pathol., 94: 37-50.
MEYRICK, B.O. & REID, L.M. (1982) Crotalaria-induced pulmonary
hypertension: uptake of 3H-thymidine by the cells of the pulmonary
circulation and alveolar walls. Am. J. Pathol., 106: 84-94.
MILKOWSKY, A.S. (1985) The synthesis and anti-tumour activity of
monocyclic analogs of indicine- N-oxide. Diss. Abstr. Int., 45(3):
844.
MILLER, W.C., RICE, D.L., KREUSEL, R.G., & BEDROSSIAN, C.W.M.
(1978) Monocrotaline model of non-cardiogenic pulmonary edema in
dogs. J. appl. Physiol., 45: 962-965.
MIRANDA, C.L., BUHLER, D.R., & CHEEKE, P.R. (1979) The effect of
tansy ragwort consumption on mineral metabolism in the rat. In:
Cheeke, P.R., ed. Proceedings of the Symposium on Pyrrolizidine
(Senecio) Alkaloids: Toxicity, Metabolism, and Poisonous Plant
Control Measures, Corvallis, Oregon, USA, 23-24 February 1979,
Oregon, Nutrition Research Institute, pp. 61-64.
MIRANDA, C.L., CARPENTER, H.M., CHEEKE, P.R., & BUHLER, D.R.
(1981a) Effect of ethoxyquin on the toxicity of pyrrolizidine
alkaloid monocrotaline and on hepatic long metabolism in mice.
Chem.-biol. Interact., 37: 95-107.
MIRANDA, C.L., HENDERSON, M.C., & BUHLER, D.R. (1981b) Dietary
copper enhances the hepatotoxicity of Senecio jacobaea in rats.
Toxicol. appl. Pharmacol., 60(3): 418-423.
MIRANDA, C.L., REED, R.L., CHEEKE, P.R., & BUHLER, D.R. (1981c)
Protective effects of butylated hydroxyanisole against the acute
toxicity of monocrotaline in mice. Toxicol. appl. Pharmacol., 59:
424-430.
MIRANDA, C.L., BUHLER, D.R., RAMSDELL, H.S., CHEEKE, P.R., &
SCHMITZ, J.A. (1982a) Modifications of chronic hepatotoxicity of
pyrrolizidine (Senecio) alkaloids by butylated hydroxyanisole
and cysteine. Toxicol. Lett., 10: 177-182.
MIRANDA, C.L., HENDERSON, M.C., BUHLER, D.R., & SCHMITZ, J.A.
(1982b) Comparative effects of antioxidants on the toxicity of
mixed pyrrolizidine alkaloids from Senecio jacobaea in mice. J.
Toxicol. environ. Health, 9(5-6): 933-939.
MIRANDA, C.L., HENDERSON, M.C., REED, R.L., SCHMITZ, J.A., &
BUHLER, D.R. (1982c) Protective action of zinc against
pyrrolizidine alkaloid-induced hepatotoxicity in rats. J. Toxicol.
environ. Health, 9: 359-366.
MIROCHNIK, M.F. (1938) [Clinical course and etiopathogenesis of
toxic hepatites with ascites.] Medical Institute, scientific
papers of the second therapeutic clinic, Vol I, Tashkent, Medical
Literature Publishers.
MISER, J.S., MISER, A.W., SMITHSON, W.A., COCCIA, P.F., AMES, M.M.,
DAVIS, D.M., HUGHES, C.S., & GILCHRIST, G.S. (1982) A phase I
trial of indicine- N-oxide in childhood malignancy. Proc. Am. Soc.
Clin. Oncol., 1: 137.
MNUSHKIN, A.S. (1949) [Some materials to clinical features and
pathogenesis of toxic hepatitis with ascites.] Sov. Med., 1: 8-9
(in Russian).
MNUSHKIN, A.S. (1952) [The clinical features, pathogenesis and
treatment of toxic hepatitis with ascites.] In: Milenkov, S.M. &
Kizhaikin, Y., ed. [Collection of scientific papers on Toxic
Hepatitis with Ascites,] Tashkent, Publishing House of the
University of Central Asia, pp. 91-98 (in Russian).
MOHABBAT, O., SRIVASTAVA, R.N., YOUNOS, M.S., SEDIQ, G.G., MENZAD,
A.A., & ARAM, G.N. (1976) An outbreak of hepatic veno-occlusive
disease in north-western Afghanistan. Lancet, 7 August: 269-271.
MOLTENI, A., WARD, W.F., TS'AO, C.-S., PORT, C.D., & SOLLIDAY, N.H.
(1984) Monocrotaline-induced pulmonary endothelial dysfunction in
rats. Proc. Soc. Exp. Biol. Med., 176: 88-94.
MOLYNEUX, R.J. & ROITMAN, J.N. (1980) Specific detection of
pyrrolizidine alkaloids on thin-layer chromatograms. J.
Chromatogr., 195: 412-415.
MOLYNEUX, R.J., JOHNSON, A.E., ROITMAN, J.N., & BENSON, M.E. (1979)
Chemistry of toxic range plants. Determination of pyrrolizidine
alkaloid content and composition in Senecio species by nuclear
magnetic resonance spectroscopy. J. agric. food Chem., 27: 494-499.
MORI, H., SUGIE, S., YOSHIMI, N., ASADA, Y., FURUYA, T., &
WILLIAMS, G.M. (1985) Genotoxicity of a variety of pyrrolizidine
alkaloids in the hepatocyte primary culture-DNA repair test using
rat, mouse, and hamster hepatocytes. Cancer Res., 45: 3125-3129.
MUNIER, R. (1953) Separation of alkaloids from their N-oxides by
paper chromatography. Bull. Soc. Chim. Biol., 35: 1225.
NARDI, N.B., GIMMLER, M.C., & LEITE, B.G. (1980) Antimitotic
action of integerrimine in rats. Rev. Bras. Genet., 3: 387-392.
NATIONAL CANCER INSTITUTE (1978) Bioassay of lasiocarpine for
possible carcinogenecity, Bethesda, Maryland, National Cancer
Institute, US Department of Health, Education and Welfare (Report
of Carcinogenesis Testing Programme, Division of Cancer Cause and
Prevention).
NAYAK, N.C., SAGREIYA, K., & RAMALINGASWAMI, V. (1969) Indian
childhood cirrhosis - the nature and significance of cytoplasmic
hyaline of hepatocytes. Arch. Pathol., 88: 631-637.
NEWBERNE, P.M. (1968) The influence of a low isotrope diet on
response of maternal and fetal rats to lasiocarpine. Cancer Res.,
28: 2327-2337.
NEWBERNE, P.M. & ROGERS, A.E. (1973) Nutrition, monocrotaline and
aflatoxin B1 in liver carcinogenesis. Plant Food Man, 1: 23-31.
NEWBERNE, P.M., WILSON, R., & ROGERS, A.E. (1971) Effects of a
low-lipotrope diet on the response of young male rats to the
pyrrolizidine alkaloid monocrotaline. Toxicol. appl. Pharmacol., 18:
387-397.
NEWBERNE, P.M., CHAN, W.C., & ROGERS, A.E. (1974) Influence of
light, riboflavin and carotene on the response of rats to the acute
toxicity of aflatoxin and monocrotaline. Toxicol. appl. Pharmacol.,
28: 200-208.
NICHOLS, W.C., MOERTL, C.G., RUBIN, J., SCHULT, A.J., & BRITTEL,
J.C. (1981) Phase II trial of indicine- N-oxide (NSC132319) in
patients with advanced colorectal carcinoma. Cancer Treat. Rep., 65:
337-339.
NIWA, H., ISHIWATA, H., & YAMADA, K. (1985) Isolation of
petasitenine, a carcinogenic pyrrolizidine alkaloid from Farfugium
japonicum. J. nat. Prod., 48: 10083-1004.
NOLAN, J.P., SCHEIG, R.L., & KLATSKIN, G. (1966) Delayed hepatitis
and cirrhosis in weanling rats following a single small dose of the
Senecio alkaloid, lasiocarpine. Am. J. Pathol., 49: 129-151.
OHNUMA, T., SRIDHAR, K.S., RATNER, L.H., & HOLLAND, J.F. (1982)
Phase I study of indicine- N-oxide in patients with advanced cancer.
Cancer Treat. Rep., 66(7): 1509-1515.
OHTSUBO, K., ITO, Y., SAITO, M., FURUYA, T., & HIKICHI, M. (1977)
Hypertrophy of pulmonary arteries and arterioles with cor pulmonale
in rats induced by seneciphylline, a pyrrolizidine alkaloid.
Experientia (Basel), 33: 498-499.
OLSON, J.W., HACKER, A.D., ALTIERE, R.J., & GILLESPIE, M.N. (1984)
Polyamines and the development of monocrotaline-induced pulmonary
hypertension. Am. J. Physiol., 247: H682-H685.
OLSON, J.W., ATKINSON, J.E., HACKER, A.D., ALTIERE, R.J., &
GILLESPIE, M.N. (1985) Suppression of polyamine biosynthesis
prevents monocrotaline induced pulmonary oedema and arterial medial
thickening. Toxicol. appl. Pharmacol., 81: 91-99.
ORD, M.J., HERBERT, A., & MATTOCKS, A.R. (1985) The ability of
bifunctional and monofunctional pyrrole compounds to induce sister
chromatid exchange (SCE) in human lymphocytes and mutations in
Salmonella typhimurium. Mutat. Res., 149: 485-493.
PANTONE, D.J., BROWN, S.M., & WOMERSLEY, C. (1985) Biological
control of fiddleneck. California Agric., 39: 4-9.
PECKHAM, J.C., SANGSTER, L.P., & JONES, O.H. (1974) Crotalaria
spectabilis poisoning in swine. J. Am. Vet. Med. Assoc., 165:
633-638.
PEDERSEN, E. (1975) Pyrrolizidine alkaloids in Danish species of
the family Boraginaceae. Arch. Pharm. Chem. Sci. Ed., 3: 55-64.
PERCY, J.J. & PIERCE, A.E. (1971) Immunosuppressive activity of
the pyrrolizidine alkaloid metabolite dehydroheliotridine.
Immunology, 21: 273-280.
PERSAUD, J.V. & HOYTE, D.A. (1974) Pregnancy and progeny in rats
treated with the pyrrolizidine alkaloid fulvine. Exp. Pathol., 9:
59-63.
PESTCHANKER, M.J. & GIORDANO, O.S. (1986) Pyrrolizidine alkaloids
from five Senecio species. J. nat. Prod., 49: 722-723.
PESTCHANKER, M.J., ASCHERI, M.S., & GIORDANO, O.S. (1985a)
Uspallatine, a pyrrolizidine alkaloid from Senecio uspallatensis.
Phytochemistry, 24: 1622-1624.
PESTCHANKER, M.J., ASCHERI, M.S., & GIORDANO, O.S. (1985b)
Pyrrolizidine alkaloids from Senecio subulatus and S. glandulosus.
Planta Med., 51: 165-166.
PETERSON, J.E. (1965) Effects of pyrrolizidine alkaloid
lasiocarpine N -oxide on nuclear and cell division in the liver of
rats. J. Pathol. Bacteriol., 89: 153-171.
PETERSON, J.E. & CULVENOR, C.C.J. (1983) Plant and fungal toxins.
In: Keeler, R.F. & Tu, A.T., ed. Handbook of natural toxins, New
York, Marcel Dekker, Vol. 1, pp. 637-681.
PETERSON, J.E. & JAGO, M.V. (1980) Comparison of the toxic effects
of dehydroheliotridine and heliotrine in pregnant rats and their
embryos. J. Pathol., 131: 339-355.
PETERSON, J.E. & JAGO, M.V. (1984) Toxicity of Echium plantagineum
(Paterson's curse): pyrrolizidine alkaloid poisoning in rats. Aust.
J. agric. Res., 35: 305-316.
PETERSON, J.E., SAMUEL, A., & JAGO, M.V. (1972) Pathological
effects of dehydroheliotridine, a metabolite of heliotridine-based
pyrrolizidine alkaloids in the young rat. J. Pathol., 107: 107-189.
PETERSON, J.E., JAGO, M.V., REDDY, J.K., & JARRETT, R.G. (1983)
Neoplasia and chronic disease associated with the prolonged
administration of dehydroheliotridine to rats. J. Natl Cancer
Inst., 70: 381-386.
PETRY, T.W., BOWDEN, G.P., HUXTABLE, R.J., & SIPES, I.G. (1984)
Characterization of hepatic DNA damage induced in rats by the
pyrrolizidine alkaloid monocrotaline. Cancer Res., 44: 1505-1509.
PETRY, T.W., BOWDEN, G.P., BUHLER, D.R., & SIPES, I.G. (1986)
Genotoxicity of the pyrrolizidine alkaloid jacobine in rats.
Toxicol. Lett., 32: 275-281.
PIERCY, P.L. & RUSOFF, L.L. (1946) Crotalaria spectabilis
poisoning in Louisiana livestock. J. Am. Vet. Med. Assoc., 108:
69-73.
PIERSON, M.L., CHEEKE, P.R., & DICKINSON, E.O. (1977) Resistance
of the rabbit to dietary pyrrolizidine (Senecio) alkaloid. Res.
Commun. chem. Pathol. Pharmacol., 16: 561-564.
PIETERS, L.A.C. & VLIETINCK, A.J. (1985) Quantitative 'H Fourier
transform nuclear magnetic resonance spectroscopic analysis of
mixtures of pyrrolizidine alkaloids from Senecio vulgaris.
Fresenius' Z. Anal. Chem., 321: 355-358.
PIETERS, L.A.C. & VLIETINCK, A.J. (1986) Comparison of high-
performance liquid chromatography with 1H nuclear magnetic
resonance spectrometry for the quantitative analysis of
pyrrolizidine alkaloids from Senecio vulgaris. J. liq. Chromatogr.,
9: 745-755.
PLESTINA, R. & STONER, H.B. (1972) Pulmonary oedema in rats given
monocrotaline pyrrole. J. Pathol., 106: 235-249.
POOL, B.L. (1982) Genotoxic activity of an alkaloidal extract of
Senecio nemorensis spp. fuchsii in Salmonella typhimurium and
Escherichia coli systems (letter to the editor). Toxicology, 24:
351-355.
POWIS, G., AMES, M.M., & KOVACH, J.S. (1979) Metabolic conversion
of indicine- N-oxide to indicine in rabbits and humans. Cancer
Res., 39: 3564-3570.
PRABHU, M.B. (1940) Infantile cirrhosis of liver. Indian J.
Pediatr., 7: 121-134.
RADHAKRISHNA RAO, M.V. (1935) Histopathology of the liver in
infantile biliary cirrhosis. Indian J. med. Res., 23: 69-90.
RAJAGOPALAN, T.R. & NEGI, R.K.S. (1985) Alkaloids from Doronicum
pardalianches Linn. Indian J. Chem., 24B, 882.
RAKIETEN, N., GORDON, B.S., BEATTY, A., COONEY, D.A., DAVIS, R.D.,
& SCHIEN, P.S. (1971) Pancreatic islet cell tumours produced by
the combined action of streptozotocin and nicotinamide. Proc. Soc.
Exp. Biol. Med., 137: 280-283.
RAMALINGASWAMI, V. & NAYAK, N.C. (1969) Liver disease in India.
In: Popper, H. & Schaffner, F., ed. Progress in liver diseases,
New York, London, Grune and Stratton, pp. 222-235.
RAMSDELL, A.S. & BUHLER, D.R. (1981) High performance liquid
chromatographic analysis of pyrrolizidine (Senecio) alkaloids
using a reversed phase styrene-divinylbenzene resin column. J.
Chromatogr., 210: 154-158.
RAO, M.S. & REDDY, J.K. (1978) Malignant neoplasms in rats fed
lasiocarpine. Br. J. Cancer, 37: 289-293.
RAPPAPORT, A.M., KNOBLAUCH, M., SUMMERSKILL, J., & BRAS, G. (1967)
Experimental veno-occlusive disease. Gastroenterology, 54: 164.
RATNOFF, O.D. & MIRICK, G.S. (1949) Influence of sex upon the
lethal effects of an hepatotoxic alkaloid, monocrotaline. Bull.
Johns Hopkins Hosp., 84: 507-25.
RECHICIGL, M., Jr, ed. (1983) CRC Handbook on naturally occurring
food toxicants, Boca Raton, Florida, CRC Press, 266 pp.
RESCH, J.F. & MEINWALD, J. (1982) A revised structure for
acetylheliosupine. Phytochemistry, 21: 2340-2431.
RHODES, K. (1957) Two types of liver disease in Jamaican children.
West Indian med. J., 6: 1-29.
RIDKER, P.M., OHKUMA, S., MCDERMOTT, W.V., TREY, C., & HUXTABLE,
R.J. (1985) Hepatic veno-occlusive disease associated with
consumption of pyrrolizidine alkaloid containing dietary
supplements. Gastroenterology, 88: 1050-1054.
RIZK, A.M., HAMMOUDA, F.M., ISMAIL, S.I., GHALEB, H.A., MADKOUR,
M.K., POHLAND, A.E., & WOOD, G. (1983) Poisonous plants
contaminating edible ones and toxic substances in plant foods. I.
Alkaloids from Senecio desfontainei. Fitoterapia, 54: 115-121.
ROBERTSON, K.A. (1982) Alkylation of N2 in deoxyguanosine by
dehydroretronecine, a carcinogenic metabolite of the pyrrolizidine
alkaloid monocrotaline. Cancer Res., 42: 8-14.
ROBINS, D.J. (1982) The pyrrolizidine alkaloids. In: Herz, W.,
Grisebach, H., & Kirby, G.W., ed. Progress in the chemistry of
organic natural products, Vienna, New York, Springer-Verlag, pp.
115-203.
RODER, E., WEIDENFELD, H., & STENGL, P. (1981) Pyrrolizidine
alkaloids senecionine and retrorsine from Senecio inaequidens.
Planta Med., 41: 412-413.
RODER, E., WIEDENFELD, H., & KNOZINGER-FISCHER (1984a)
Pyrrolizidine alkaloid from Senecio abrotanifolius spp.
abroatanifolius, spp. abrotanifolius, and var. tiroliensis.
Planta Med., 41: 412-413.
RODER, E., WIEDENFELD, H., & BRITZ-KIRSTGEN, R. (1984b)
Pyrrolizidine alkaloids from Senecio cacaliaster. Phytochemistry,
23: 1761-1763.
ROGERS, A.E. & NEWBERNE, P.M. (1971) Lasiocarpine factors
influencing its toxicity and effects in liver cell division.
Toxicol. appl. Pharmacol., 18: 356-366.
ROITMAN, J.N. (1981) Comfrey and liver damage. Lancet, 25 April:
944.
ROITMAN, J.N. (1983) Ingestion of pyrrolizidine alkaloids: a
health hazard of global proportions. In: Finlay, J.W. & Schwass,
D.E., ed. Xenobiotics in foods and feeds, Washington DC, American
Chemical Society, pp. 345-378 (ACS Series).
ROSE, A.L., GARDNER, C.A., MCDONNELL, J.D., & BULL, L.B. (1957a)
Field and experimental investigation of "walk-about" disease of
horses (Kimberley horse disease) in northern Australia: Crotalaria
poisoning in horses. Part II. Aust. vet. J., 33: 49-62.
ROSE, A.L., GARDNER, C.A., MCDONNELL, J.D., & BULL, L.B. (1957b)
Field and experimental investigation of "walk about" disease of
horses (Kimberley horse disease) in northern Australia. Part I.
Aust. vet. J., 33: 25-33.
ROSE, C.L., HARRIS, P.N., & CHEN, K.K. (1959) Some pharmacological
action of supinine and lasiocarpine. J. Pharmacol. exp. Ther., 126:
179-184.
ROSE, E.F. (1972) Senecio species: toxic plants used as food and
medicine in the Transkei. S. Afr. Med. J., 46: 1039-1043.
ROSENFIELD, I. & BEATH, O.A. (1945) Tissue changes induced by
Senecio riddellii. Am. J. clin. Pathol., 15: 407-412.
ROTH, R.A., DOTZLAF, L.A., BARANYI, B., & HOOK, J.B. (1981) Effect
of monocrotaline ingestion on liver, kidney and lung of rat.
Toxicol. appl. Pharmacol., 60: 193-203.
ROYES, K. (1948) Infantile hepatic cirrhosis in Jamaica. Caribb.
med. J., 10: 16-48.
SAFOUH, M. & SHEHATA, A.H. (1965) Hepatic vein occlusion disease
of Egyptian children. J. Pediatr., 67: 415-432.
ST. GEORGE-GRAMBAUER, T.D. & RAC, R. (1962) Hepatogenous chronic
copper poisoning in sheep in south Australia due to consumption of
Echium plantagineum L. (Salvation Jane). Aust. vet. J., 38: 288-293.
SALASPURO, M. & SIPPONEN, P. (1976) Demonstration of an
intracellular copper-binding protein in orcein staining in long
standing cholestatic liver diseases. Gut, 17: 787-790.
SAMUEL, A. & JAGO, M.V. (1975) Localization in the cell cycle of
the antimitotic action of the pyrrolizidine alkaloid, lasiocarpine,
and its metabolite, dehydroheliotridine. Chem.-biol. Interact., 10:
185-197.
SANDERS, D.A., SHEALY, A.L., & EMMEL, M.W. (1936) The pathology of
Crotalaria spectabilis Roth poisoning in cattle. J. Am. Vet. Med.
Assoc., 89: 150-156.
SAVIN, I.G., (1983) [Research of the influence of heliotrin on
the microsomal mono oxygenase systems of the rat liver.] N.E.
Pyrogov 2nd Moscow State Medical Institute. (Thesis in biological
chemistry) (in Russian).
SAVVINA, K.I. (1952) Pathological anatomy of atrophic hepatic
cirrhosis. Arch. Pathol., 14: 65-70.
SCHOENTAL, R. (1959) Liver lesions in young rats suckled by
mothers treated with the pyrrolizidine (Senecio) alkaloids,
lasiocarpine, and retrorsine. J. Pathol. Bacteriol., 77: 485-504.
SCHOENTAL, R. (1961) Herbal medicines and liver disease. J. exp.
med. Sci., 4: 126-135.
SCHOENTAL, R. (1963) Liver disease and 'natural' hepatotoxins.
Bull. World Health Organ., 29: 823-833.
SCHOENTAL, R. (1968) Toxicology and carcinogenic action of
pyrrolizidine alkaloids. Cancer Res., 28: 2237-2246.
SCHOENTAL, R. (1970) Hepatotoxic activity of retrorsine,
senkirkine and hydroxysenkirkine in newborn rats, and the role of
epoxides in carcinogenesis by pyrrolizidine alkaloids and
aflatoxins. Nature (Lond.), 227: 401-402.
SCHOENTAL, R. (1975) Pancreatic islet-cell and other tumours in
rats given heliotrine, a monoester pyrrolizidine alkaloid, and
nicotinamide. Cancer Res., 35: 2020-2024.
SCHOENTAL, R. & BENSTED, J.P.M. (1963) Effects of whole body
irradiation and of partial hepatectomy on the liver lesions induced
in rats by a single dose of retrorsine, a pyrrolizidine (Senecio)
alkaloid. Br. J. Cancer, 17: 242-251.
SCHOENTAL, R. & CAVANAGH, J.B. (1972) Brain and spinal cord
tumours in rats treated with pyrrolizidine alkaloids. J. Natl
Cancer Inst., 49: 665-671.
SCHOENTAL, R. & COADY, A. (1968) The hepatotoxicity of some
Ethiopian and east African plants including some used as
traditional medicines. East Afr. med. J., 45: 577-579.
SCHOENTAL, R. & HEAD, M.A. (1955) Pathological changes in rats as
result of treatment with monocrotaline. Br. J. Cancer, 9: 229-237.
SCHOENTAL, R. & HEAD, M.A. (1957) Progression of liver lesions
produced in rats by temporary treatment with pyrrolizidine
(Senecio) alkaloids, and the effects of betaine and high casein
diet. Br. J. Cancer, 11: 535-544.
SCHOENTAL, R. & MAGEE, P.N. (1957) Chronic liver changes in rats
after a single dose of lasiocarpine, a pyrrolizidine (Senecio)
alkaloid. J. Pathol. Bacteriol., 74: 305-319.
SCHOENTAL, R. & MAGEE, P.N. (1959) Further observation on the
subacute and chronic liver changes rats after a single dose of
various pyrrolizidine (Senecio) alkaloids. J. Pathol. Bacteriol.,
78: 471-482.
SCHOENTAL, R. & MATTOCKS, A.R. (1960) Hepatotoxic activity of
semisynthetic analogues of pyrrolizidine alkaloids. Nature (Lond.),
185: 842-843.
SCHOENTAL, R., HEAD, M.A., & PEACOCK, P.R. (1954) Senecio
alkaloids: primary liver tumours in rats as a result of treatment
with (1) mixture of alkaloids from S. jacobaea Lin, (2)
retrorsine, (3) isatidine. Br. J. Cancer, 8: 458-465.
SCHOENTAL, R., FOWLER, M.E., & COADY, A. (1970) Islet cell tumours
of the pancreas found in rats given pyrrolizidine alkaloids from
Amsinckia intermedia Fisch and Mey and from Heliotropium supinum.
Cancer Res., 30: 2127-2131.
SEGALL, H.J. (1979a) Reversed phase isolation of pyrrolizidine
alkaloids. Liq. Chromatogr., 2: 429-436.
SEGALL, H.J. (1979b) Preparative isolation of pyrrolizidine
alkaloids derived from Senecio vulgaris. Liq. Chromatogr., 2:
1319-1323.
SEGALL, H.J., DALLAS, J.L., & HADDON, W.F. (1984) Two
dihydropyrrolizine alkaloid metabolites isolated from mouse hepatic
microsomes in vitro. Drug Metab. Disp., 12: 68-71.
SEGALL, H.J., WILSON, D.W., DALLAS, J.L., & HADDON, W.F. (1985)
Trans-4-hydroxy-2-hexenol: a reactive metabolite from the
macrocyclic pyrrolizidine alkaloid senecionine. Science, 229: 472-475.
SEGER, C.L., NEWSON, J.D., ROTH, E.E., & HUTCHINSON, W.R. (1969)
Chronic toxic hepatitis in deer from a Louisiana coastal marsh.
Bull. Wildlife Disease Assoc. Proc. Ann. Conf., pp. 295-6.
SELZER, G. & PARKER, R.G.F. (1951) Senecio poisoning exhibiting
as Chiari's syndrome: a report of 12 cases. Am. J. Pathol., 27:
885-907.
SENER, B., TEMIZER, H., TEMIZER, A., & KARAKAYA, A.E. (1986) High
performance liquid chromatographic determination of alkaloids in
Senecio vernalis. J. Pharm. Belg., 41: 115-117.
SHARMA, R.K., KHAJURIA, G.S., & ATAL, C.K. (1965) Thin layer
chromatography of pyrrolizidine alkaloids. J. Chromatogr., 19:
433-434.
SHERLOCK, S. (1968) Diseases of liver and biliary system, 4th
ed., Oxford, Edinburgh, Blackwell Scientific Publishers, 250 pp.
SHTENBERG, A.I. & ORLOVA, N.V. (1955) [The question of the
etiology of the so-called "Ozhalangar Encephalitis".] Vopr. Pitan.,
14: 27-31 (in Russian).
SHULL, L.R., BUCKMASTER, G.W., & CHEEKE, P.R. (1976) Factors
influencing pyrrolizidine (Senecio) alkaloid metabolism: species,
liver sulphydryls and rumen fermentation. J. anim. Sci., 43: 1247-1253.
SHUMAKER, R.C., ROBERTSON, K.A., HSU, I.C., & ALLEN, J.R. (1976)
Neoplastic transformation in tissues of rats exposed to
monocrotaline or dehydroretronecine. J. Natl Cancer Inst., 56: 787-789.
SIDDIQI, M.A., SURI, K.A., SURI, M.P., & ATAL, C.K. (1978a) Novel
pyrrolizidine alkaloid from Crotalaria nana. Phytochemistry, 17:
2143-2144.
SIDDIQI, M.A., SURI, K.A., SURI, O.P., & ATAL, C.K. (1978b) Genus
Crotalaria. Part 34. Cronaburmine, a new pyrrolizidine alkaloid
from Crotalaria nana Burm. Indian J. Chem., 16B: 1132-1133.
SMITH, L.W. & CULVENOR, C.C.J. (1981) Plant sources of
hepatotoxic pyrrolizidine alkaloids. J. nat. Prod., 44: 129-152.
SMITH, T.E., WEISBACH, H., & UDENFRIEND S. (1962) Studies on the
mechanism of monoaminooxidase: metabolism of N,N-dimethyltryptamine
and N,N-dimethyltryptamine- N-oxide. Biochemistry, 1: 137.
SOBIN, L.H., FETRAT, M.E., & ANWER, M.A. (1969) Hepatic lesions in
Afghanistan. Trop. geogr. Med., 21: 27-29.
SRIVASTAVA, R.N., MOHABBAT, O., GHANI, A.R., & ARAM, G.N. (1978)
Veno-occlusive disease of the liver. Indian. Paediatr., 15: 143-146.
SRUNGBOONMEE, S. & MASKASAME, C. (1981) A preliminary study on
the toxicity of Crotalaria juncea to cattle. Sattawaphaet San, 32:
91-107.
STEIN, H. (1957) Veno-occlusive disease of liver in African
children. Br. med. J., 29 June: 1496-1499.
STEIN, H. & ISAACSON, C. (1962) Veno-occlusive disease of the
liver. Br. med. J., 10 February: 372-374.
STENMARK, K.R., MORGANROTH, M.L., REMIGIO, L.K., VOELKEL, N.F.,
MURPHY, R.C., HENSON, P.M., MATHIAS, M.M., & REEVES, J. (1985)
Alveolar inflammation and arachidonate metabolism in monocrotaline-
induced pulmonary hypertension. Am. J. Physiol., 248: H859-H866.
STILLMAN, A.E., HUXTABLE, R.J., CONSROE, P., KOHNEN, P., & SMITH,
S. (1977) Hepatic veno-occlusive disease due to pyrrolizidine
poisoning in Arizona. Gastroenterology, 73: 349-352.
STIRLING, G.A., BRAS, G., & URQUHART, A.E. (1962) The early
lesions in veno-occlusive disease of the liver. Arch. dis. Child.,
37: 535-538.
STOYEL, C. & CLARK, A.M. (1980) The transplacental micronucleus
test. Mutat. Res., 74: 393-398.
STUART, K.L. & BRAS, G. (1955) Clinical observations on veno-
occlusive disease of the liver in Jamaican adults. Br. med. J., 2:
348-352.
STUART, K.L. & BRAS, G. (1956) Veno-occlusive disease of the liver
in Barbados. West Indian med. J., 5: 33-36.
STUART, K.L. & BRAS, G. (1957) Veno-occlusive disease of the
liver. Q. J. Med., 26: 291-315.
STYLES, J., ASHBY, J., & MATTOCKS, A.R. (1980) Evaluation in vitro
of several pyrrolizidine alkaloid carcinogens: observation on the
essential pyrrolic nucleus. Carcinogenesis, 1: 161-164.
SUFFNESS, M. & CORDELL, G.A. (1985) Antitumour alkaloids. In:
Brossi, A., ed. The alkaloids, New York, Academic Press,
pp. 1-347.
SUGITA, T., HYERS, T.M., DAUBER, I.M., WAGNER, W.W., MCMURTRY,
I.F., & REEVES, J.T. (1983a) Lung leak precedes right ventricular
hypertrophy in monocrotaline treated rats. J. appl. Physiol., 54:
371-374.
SUGITA, T., STENMARK, K.R., WAGNER, W.W., HENSON, P.M., HENSON,
J.E., HYERS, T.M., & REEVES, J.T. (1983b) Abnormal alveolar cells
in monocrotaline induced pulmonary hypertension. Exp. lung Res.,
5: 201-215.
SUNDARESON, A.E. (1942) An experimental study of placental
permeability to cirrhogenic poisons. J. Pathol. Bacteriol., 54:
289-298.
SVOBODA, D. & REDDY, J.K. (1972) Malignant tumours in rats given
lasiocarpine. Cancer Res., 32: 908-912.
SVOBODA, D. & REDDY, J.K. (1974) Lasiocarpine induced,
transplantable squamous cell carcinoma of rat skin. J. Natl Cancer
Inst., 53: 1415-1418.
SVOBODA, D. & SOGA, J. (1966) Early effects of pyrrolizidine
alkaloids on the fine structure of rat liver cells. Am. J. Pathol.,
48: 347-373.
SWICK, R.A., CHEEKE, P.R., & BUHLER, D.R. (1979) Factors affecting
the toxicity of dietary tansy ragwort to rats. In: Cheeke, P.R.,
ed. Proceedings of the Symposium on Pyrrolizidine (Senecio)
Alkaloids: Toxicity, Metabolism, and Poisonous Plant Control
Measures, Corvallis, Oregon, USA, 23-24 February 1979, Oregon,
Nutrition Research Institute.
SWICK, R.A., CHEEKE, P.R., GOEGER, D.E., & BUHLER, D.R. (1982a)
Effect of dietary Senecio jacobaea and injected Senecio alkaloids
and monocrotaline on guinea pigs. J. anim. Sci., 55: 1411-1416.
SWICK, R.A., CHEEKE, P.R., & BUHLER, D.R. (1982b) Subcellular
distribution of hepatic copper, zinc and iron and serum
ceruloplasmin in rats intoxicated by oral pyrrolizidine (Senecio)
alkaloids. J. anim. Sci., 55(6): 1425-1430.
SWICK, R.A., CHEEKE, P.R., PATTON, N.M., & BUHLER, D.R. (1982c)
Absorption and excretion of pyrrolizidine (Senecio) alkaloids and
their effects on mineral metabolism in rabbits. J. anim. Sci., 55:
1417-1424.
SWICK, R.A., CHEEKE, P.R., MIRANDA, C.L., & BUHLER, D.R. (1982d)
The effect of consumption of the pyrrolizidine alkaloid containing
plant Senecio jacobaea on iron and copper metabolism in the rat.
J. Toxicol. environ. Health, 10: 757-768.
SWICK, R.A., CHEEKE, P.R., RAMSDELL, H.S., & BUHLER, D.R. (1983)
Effect of sheep rumen fermentation and methane inhibition on the
toxicity of Senecio jacobaea. J. anim. Sci., 56: 645-651.
TAKANASHI, H., UMEDA, M., & HIRONO, I. (1980) Chromosomal
aberrations and mutation in cultured mammalian cells induced by
pyrrolizidine alkaloids. Mutat. Res., 78: 67-77.
TAKEOKA, O., ANGEVINE, M., & LALICH, J. (1962) Stimulation of mast
cells in rat fed various chemicals. Am. J. Pathol., 40: 545-554.
TANDON, B.N., TANDON, H.D., TANDON, R.K., NARENDRANATHAN, M., &
JOSHI, Y.K. (1976) Epidemic of veno-occlusive disease in central
India. Lancet, 7 August: 271-272.
TANDON, B.N., TANDON, H.D., KOSHY, A., NARENDRANATHAN, M., JOSHI,
Y.K., TANDON, R.K., BHARGAVA, S., RAJANI, M., BHATIA, M.L.,
MANCHANDA, S.C., & KASTURI, T.E. (1977) Epidemiological,
clinical, biochemical and haemodynamic study of veno-occlusive
disease of the liver due to Crotalaria alkaloids in India. J. All
Ind. Inst. Med. Sci., 3: 165-175.
TANDON, B.N., TANDON, H.D., & MATTOCKS, A.R. (1978) Study of an
epidemic of veno-occlusive disease in Afghanistan. Indian J. med.
Res., 68: 84-90.
TANDON, H.D. & TANDON, B.N. (1975) Epidemic of liver disease -
Gulran District, Herat Province, Afghanistan, Alexandria, World
Health Organization, Regional Office for the Eastern Mediterranean
(Assignment report No. EM/AFG/OCD/001/RB).
TANDON, H.D., TANDON, B.N., TANDON, R., & NAYAK, N.C. (1977) A
pathological study of the liver in an epidemic outbreak of veno-
occlusive disease. Indian J. med. Res., 65: 679-684.
TANDON, H.D., TANDON, B.N., & MATTOCKS, A.R. (1978) An epidemic of
veno-occlusive disease of the liver in Afghanistan. Am. J.
Gastroenterol., 72: 607-613.
TANDON, R.K., TANDON, B.N., TANDON, H.D., BHATIA, M.L., BHARGAVA,
S., LAL, P., & ARORA, R.R. (1976) Study of an epidemic of veno-
occlusive disease in India. Gut, 17: 849-855.
TANNER, M.S. & PORTMANN, B. (1981) Indian childhood cirrhosis.
Arch. dis. Child., 56: 4-6.
TAYLOR, S., BELT, R.J., HAAS, C.D., & HOOGSTRATEN, B. (1983) Phase
I trial of indicine- N-oxide on two dose schedules. Cancer, 51:
1988-1991.
TEILUM, G. (1949) Endophlebitis hepatica obliterans. Acta pathol.
microbiol., 26: 147-166.
TEMIZER, A., ONAR, A.N., SENER, B., TEMIZER, H., & KARAKAYA, A.E.
(1985) Determination of alkaloids by differential pulse
polarography. I. Senecio alkaloids. J. Pharm. Belg., 40(2): 75-78.
TEREKHOV, G.N. (1939) [Pathomorphology of toxic hepatitis with
ascites.] In: Mirochnik, M.F., ed. [Functional, diagnostic and
pathological changes of toxic hepatitis with ascites,] Tashkent,
State Publishing House of Science Technology and Socio-economic
Literature of Uzbekistan, pp. 145-200 (in Russian).
TEREKHOV, G.N. (1952) [The pathomorphology of alimentary toxic
dystrophy with ascites.] In: Milenkov, S.M. & Kizhaikin, Y., ed.
[Collection of scientific papers on Toxic Hepatitis with Ascites,]
Tashkent, Publishing House of the University of Central Asia, pp.
148-164 (in Russian).
THORPE, E. & FORD, E.J.H. (1968) Development of hepatic lesions in
calves fed with ragwort (Senecio jacobaea). J. comp. Pathol., 78:
195-205.
TITTEL, G., HINZ, H., & WAGNER, H. (1979) Quantitative
determination of pyrrolizidine alkaloids in Symphyti radix by
HPLC. Planta Med., 37: 1-8.
TUCHWEBER, B., KOVAKS, K., JAGO, M.V., & BEAULIEU, T. (1974)
Effect of steroidal and non-steroidal microsomal enzyme inducers on
the hepatotoxicity of pyrrolizidine alkaloids in rats. Res. Commun
chem. Pathol. Pharmacol., 7: 459-480.
TUCKER, A., BRYANT, S.E., FROST, H.H., & MIGALLY, N. (1983)
Chemical sympathectomy and serotonin inhibition reduce
monocrotaline-induced right ventricular hypertrophy in rats. Can.
J. Physiol. Pharmacol., 61: 356-362.
TURNER, J.H. & LALICH, J.J. (1965) Experimental cor pulmonale in
the rat. Arch. Pathol., 79: 409-418.
VALDIVIA, E., SONNAD, J., HAYASHI, Y., & LALICH, J.J. (1967a)
Experimental interstitial pulmonary oedema. Angiology, 18: 378-383.
VALDIVIA, E., LALICH, J., HAYASHI, Y., & SONNAD, J. (1967b)
Alterations in pulmonary alveoli after a single injection of
monocrotaline. Arch. Pathol., 84: 64-76.
VAN DER WATT, J.J., PURCHASE, I.F.H., & TUSTIN, R.C. (1972) The
chronic toxicity of retrorsine, a pyrrolizidine alkaloid, in vervet
monkeys. J. Pathol., 107: 279-287.
VILLARROEL, L.H., TORRES, R.G., NAVARRO, J.M., & FAJARDO, V.M.
(1985) Senecionine and seneciphylline from Senecio patagonicus.
Fitoterapia, 56: 250-251.
VISCONTINI, M. & GILHOF-SCHAUFELBERGER, H. (1971) Synthesis of (±)
dehydroheliotridine. Helv. chim. Acta, 54: 449-456.
WAGENVOORT, C.A., DINGEMANS, K.P., & LOTGERING, G.G. (1974a)
Electron microscopy of pulmonary vasculature after application of
fulvine. Thorax, 29: 511-521.
WAGENVOORT, C.A., WAGENVOORT, N., & DIJK, H.J. (1974b) Effect of
fulvine on pulmonary arteries and veins of the rat. Thorax, 29:
522-529.
WAGNER, H., NEIDHARDT, U., & TITTEL, G. (1981) TLC and HPLC
analysis of pyrrolizidine N-oxide alkaloids of Symphyti radix.
Planta Med., 41: 232-239.
WAKIM, K.G., HARRIS, P.N., & CHEN, K.K. (1946) The effects of
senecionine on the monkey. J. Pharmacol. exp. Ther., 87: 38-45.
WALKER, K.H. & KIRKLAND, P.D. (1981) Senecio lautus toxicity in
cattle. Aust. vet. J., 57: 1-7.
WATT, J.M. & BREYER-BRANDWIJK, M.G. (1962) Medicinal and poisonous
plants of southern and eastern Africa, Edinburgh, London, E. and
S. Livingstone, 584 p.
WEHNER, F.C., THIEL, P.G., & VAN RENSBURG, S.J. (1979)
Mutagenicity of alkaloids in the Salmonella/microsome system.
Mutat. Res., 66: 187-190.
WESTON, C.F.M., COOPER, B.T., DAVIES, J.D., & LEVINE, D.F. (1987)
Veno-occlusive disease of the liver secondary to ingestion of
comfrey. Br. med. J., 295: 183.
WHITE, I.N.H. (1976) The role of liver glutathion in the acute
toxicity of retrorsine to rats. Chem.-biol. Interact., 13: 333-342.
WHITE, I.N.H. (1977) Excretion of pyrrolic metabolites in bile of
rats given retrorsine or the bis- n-ethylcarbamate of synthanecine
A. Chem.-biol. Interact., 16: 169-180.
WHITE, I.N.H. & MATTOCKS, A.R. (1971) Some factors affecting the
conversion of pyrrolizidine alkaloids to N-oxides and to pyrrolic
derivatives in vitro. Xenobiotica, 1: 503-505.
WHITE, I.N.H. & MATTOCKS, A.R. (1972) Reactions of dihydro-
pyrrolizidine with deoxyribonucleic acid in vitro. Biochem.
J., 128: 291-297.
WHITE, I.N.H., MATTOCKS, A.R., & BUTLER, W.H. (1973) The
conversion of the pyrrolizidine alkaloid retrorsine to pyrrolic
derivatives in vivo and in vitro and its acute toxicity to
various animal species. Chem.-biol. Interact., 6: 207-218.
WHITE, R.D., KRUMPERMAN, P.H., CHEEKE, P.R., & BUHLER, D.R. (1983)
An evaluation of acetone extracts from six plants in the Ames
mutagenicity test. Toxicol. Lett., 15: 25-31.
WHITE, R.D., SWICK, R.A., CHEEKE, P.R. (1984) Effect of dietary
copper and molybdenum on tansy ragwort (Senecio Jacobaea) toxicity
in sheep. Am. J. vet. Res., 45: 159-161.
WHO (1980) Inventory of medicinal plants used in the different
countries, Geneva, World Health Organization (Document No.
DPM/80.3).
WICKRAMANAYAKE, P.P., ARBOGAST, B.L., BUHLER, D.R., DEINZER, M.L.,
& BURLINGAME, A.L. (1985) Alkylation of nucleosides and
nucleotides by dehydroretronecine: characterization of covalent
adducts by liquid secondary ion mass spectrometry. J. Am. Chem.
Soc., 107: 2485-2488.
WIEDENFELD, H. (1982) Two pyrrolizidine alkaloids from Gynura
scandens. Phytochemistry, 21: 2767-2768.
WIEDENFELD, H., PASTEWKA, U., STENGL, P., & ROEDER, E. (1981) On
the gas chromatographical determination of the pyrrolizidine
alkaloids of some Senecio species. Planta Med., 41: 124-128.
WIEDENFELD, H., RODER, E., & ANDERS, E. (1985) Pyrrolizidine
alkaloids from seeds of Crotalaria scassellatii. Phytochemistry,
24: 376-378.
WILLIAMS, A.O., EDINGTON, G.M., & OBAKPONOVWE, P.C. (1967)
Hepatocellular carcinoma in infancy and childhood in Ibadan,
western Nigeria. Br. J. Cancer, 21(3): 474-482.
WILMOT, F.C. & ROBERTSON, G.W. (1920) Senecio disease or
cirrhosis of the liver due to Senecio poisoning. Lancet,
23 October: 848-849.
WITTIG, M. & STEPHEN, C. (1964) Modification of microsomal lipid
peroxidation and drug metabolism by cytoplasmic copper. Res.
Commun. Chem. Pathol. Pharmacol., 44: 477-493.
WONG, R.Y. & ROITMAN, J.N. (1984) Structure and absolute
configuration of (+)-doronine-benzene (1:1). Acta Crystallogr.,
Sect. C: Cryst. Struc. Commun., C40: 163-166.
WURM, H. (1939) [A cluster of endophlebilis hepatica obliterans in
the age group of suckling infants.] Klin. Wochenschr., 18: 1527-1531
(in German).
YAMANAKA, H., NAGAO, M., SUGIMURA, T., FURUYA, T., SHIRAI, A., &
MATSUSHIMA, T. (1979) Mutagenicity of pyrrolizidine alkaloids in
the Salmonella/mammalian microsome test. Mutat. Res., 68: 211-216.
YULDASHEVA, L.N. & SULTANOVA, R.G. (1983) [Oxidative reactions in
rat liver tissue under conditions of chronic heliotrine hepatitis.]
Vopr. Med. Khim., 29: 81-85 (in Russian).
YUNUSOV, S.YU. & PLEKHANOVA, N.V. (1959) [The alkaloids of
Trichodesma incanum. The structure of incanine and trichesmine.]
Zh. Obshch. Khim., 29: 677-684 (in Russian).
ZHELTOVA, L.I. (1952) [The clinical course of toxic hepatitis.]
In: Milenkov, S.M. & Kizhaikin, Y., ed. [Collection of scientific
papers on Toxic Hepatitis with Ascites,] Tashkent, Publishing
House of the University of Central Asia, pp. 76-90 (in Russian).
APPENDIX I
Pyrrolizidine Alkaloids and Their Plant Sources
ISOLATIONS OF TOXIC PYRROLIZIDINE ALKALOIDS - (8701081148)
Alkaloid Plant Sources Reference Reference
Location
19-Acetoxysenkirkine Senecio laricifolius H.B.K. Bohlmann et al. (1986) A
6-Acetylanacrotine Crotalaria agatiflora Schweinf. Culvenor & Smith (1972) B281
6-Acetyl-trans-anacrotine Crotalaria agatiflora Schweinf. Culvenor & Smith (1972) B281
Acetylcrotaverrine Crotalaria verrucosa L. O.P. Suri et al. (1976) B339
C. walkeri Arnott K.A. Suri et al. (1976) B340
7-Acetylechinatine Cynoglossum amabile Stapf. and Drummond Culvenor & Smith, unpubl.
Lindelofia spectabilis Lehm. Rao et al. (1974) B62
Symphytum asperum Lepech. Pedersen (1975b) A
S. officinale Linn. Pedersen (1975b) A
Acetylgynuramine Gynura scandens O. Hoffm. Wiedenfeld (1982) C
Acetylheliosupine Cynoglossum officinale L. Pedersen (1970); Resch & Meinwald (1982) B43, A
Myosotis sylvatica Hoffm. Resch & Meinwald (1982) A
Symphytum asperum Lepech. Pedersen (1975) A
S. officinale Linn. Pedersen (1975) A
Acetylindicine Heliotropium indicum L. Mattocks (1967a) B71
7-Acetylintermedine Borago officinale L. Luthy et al. (1984) A
Symphytum aspera Roitman (1981) A
S. officinale Linn. Roder et al. (1982) C
S. x uplandicum Nyman Culvenor et al. (1980a), (1980b) A, A
Alkaloid Plant Sources Reference Reference
Location
Acetyllasiocarpine Heliotropium europaeum L. Culvenor et al. (1975) B69
7-Acetyllycopsamine Amsinckia menziesii (Lehm.) Nels Roitman, (1983a) C
& Macbr.
Anchusa officinalis L. Pedersen (1975); Broch-Due & A, B32
Aasen (1980)
Borago officinale L. Luthy et al. (1984) A
Symphytum aspera Roitman (1981) A
S. officinale Linn. Huizing & Malingre (1981) C
S. x uplandicum Nyman Culvenor et al. (1980a, 1980b) A, A
3a'-Acetyllycopsamine Amsinckia menziesii (Lehm.) Nels. Roitman (1983a C
& Macbr.
7-Acetylmadurensine Crotalaria agatiflora Schweinf. Culvenor & Smith (1972) B281
7-Acetyl-cis-madurensine Crotalaria agatiflora Schweinf. Culvenor & Smith (1972) B281
7-Acetylscorpioidine Myosotis scorpioides L. Resch et al. (1982) C
Acetylseneciphylline Senecio pterophorus DC. Edgar et al. (1976) B246
18-Acetylsenkirkine Senecio illinitus Phill. Gonzalez et al. (1986a) A
S. kirkii Hook. f. ex Kirk Briggs et al. (1965) A
S. tenuifolius Burm. Bhakuni & Gupta (1982) C
Acetylsyneilesine Syneilesis palmata Maxim. Hikichi & Furuya (1976) B279
Amabiline Borago officinale L. Luthy et al. (1984) A
Cynoglossum amabile Stapf. et Drummond Culvenor & Smith (1967) B33
C. glochidiatum Wall. ex Lindl. K.A. Suri et al. (1975a) B36
Eupatorium cannabinum L. Luthy et al. (1984) A
Lindelofia angustifolia (Schrenk) Brand. K.A. Suri et al. (1975a) B36
Alkaloid Plant Sources Reference Reference
Location
Anacrotine Crotalaria agatifolia Schweinf. Culvenor & Smith (1972) B281
C. incana L. Mattocks (1968) B302
C. laburnifolia L. Snehelata et al. (1966); Sawhney B309,
et al. (1967) 291
C. laburnifolia L. subsp. eldomae Crout (1972) B312
C. micans Link. Atal et al. (1966a) B280
C. verrucosa L. Subramanian & Nagarajan (1967) B338
Anadoline Symphytum orientale Ulubelen & Doganca (1971); Culvenor B103,
et al. (1975) 104
S. tuberosum L. Ulubelen & Ocal (1977) B105
Angelylechimidine (or Symphytum asperum Lepech. Gadella et al. (1983) A
isomer) S. x uplandicum Nyman Gadella et al. (1983) A
7-Angelylheliotridine Heliotropium supinum L. Crowley & Culvenor (1959) B83
trachelanthate
7-Angelylheliotridine Heliotropium supinum L. Crowley & Culvenor (1959) B83
viridiflorate
7-Angelylheliotrine Heliotropium digynum Forssk. Hammouda et al. (1984) A
H. eichwaldii Steud ex DC. O.P. Suri et al. (1975) B63
7-Angelyl-9-sarracinyl- Senecio triangularis Hook. Rueger & Benn (1983) C
retronecine
6-Angelyl-trans-anacrotine Crotalaria agatiflora Schweinf. Culvenor & Smith (1972) B281
Asperumine Echium vulgare L. Karimov et al. (1975) B50
Symphytum asperum Lepech. Man'ko et al. (1969); Man'ko & B95, 96
Kotowskii (1970); Man'ko et al. 1970 B97
S. caucasicum Bieb. Man'ko et al. (1972); Mel'kumova B98, 99
et al. 1974
Alkaloid Plant Sources Reference Reference
Location
Axillaridine Crotalaria axillaris Ait. Crout (1968b, 1969) B287, 288
C. scassellatii Chiov Wiedenfeld et al. (1985) A
Axillarine Crotalaria axillaris Ait. Crout (1968b, 1969) B287, 288
C. scassellatii Chiov Wiedenfeld et al. (1985) A
Bisline Senecio othonniformis Fourcade Coucourakis & Gordon-Gray (1970) B224
S. petasitis DC. Gonzalez et al. (1973) B225
Brachyglottine Brachyglottis repanda Forst. et Forst. White, pers. commun.
Carategine Lindelofia tschimganica Akramov et al. (1965) B87
Rindera oblongifolia M. Pop. Akramov et al. (1965) B87
Solenanthus karategenius Lipsky Akramov et al. (1964) B93
Chlorodeoxysceleratine Senecio latifolius DC. (S. sceleratus) Gordon-Gray (1967) B261
Clivorine Ligularia brachyphylla Hand. Mazz. Klasek et al. (1971) B134
L. clivorum Klasek et al. (1967, 1969, 1970); B135,
Birnbaum et al. (1971) 136,
137,
138
L. dentata (A. Gray) Hara Klasek et al. (1971) B134
L. elegans (Cass.) Klasek et al. (1971) B134
Crispatine Crotalaria candicans W. & A. Suri et al. (1982) C
C. crispata F. Muell. ex Benth. Culvenor & Smith (1963) B294
C. lunata Beddome ex Polhill Rothschild et al. (1979) C
C. madurensis R. Wight Habib et al. (1971) B315
Crobarbatine Crotalaria barbata R. Graham ex R. Wight Puri et al. (1973) B289
Walk.-Arn.
Alkaloid Plant Sources Reference Reference
Location
Cromadurine Crotalaria madurensis Wight Rao et al. (1974, 1975b) B62, 316
Cronaburmine Crotalaria nana Burm. Siddiqi et al. (1978b) A
Crosemperine Crotalaria aegyptiaca Benth. Zalkow et al. (1979) B419
C. semperflorens Vent. Atal et al. (1967) B331
Crotaflorine Crotalaria agatiflora Schweinf. Culvenor & Smith (1972) B281
Crotafoline Crotalaria laburnifolia L. subsp. eldomae Crout (1972) B312
Crotalarine Crotalaria burhia Buch-Ham. Ali & Adil (1973); Rao et al. (1975a) B292,
293
Crotaleschenine Crotalaria leschenaultii Suri & Atal (1967); Smith et al. (1988) B313
Crotananine Crotalaria nana Burm. Siddiqi et al. (1978a) A
Crotastriatine Crotalaria pallida Ait. (syn. C. Gandhi et al. (1968); Batra et al. (1975) B323,
mucronata Desv., C. striata DC.) 324
Crotaverrine Crotalaria verrucosa L. O.P. Suri et al. (1976) B339
C. walkeri Arnott K.A. Suri et al. (1976) B340
Cruentine A Senecio cruentus DC. Chu & Chu (1964) B172
Cruentine B Senecio cruentus DC. Chu & Chu (1964) B172
Curassavinine Heliotropium curassavicum Linn. Mohanraj et al. (1982a) C
Cynaustine Borago officinale L. (or amabiline ?) Larson et al. (1984) C
Cynoglossum australe R. Br. Culvenor & Smith, 1967 B33
C. lanceolatum Forsk. Suri et al. 1975a B36
Alkaloid Plant Sources Reference Reference
Location
Deoxyaxillarine Crotalaria scassellatii Chiov Wiedenfeld et al. 1985 A
Diacetyllycopsamine Amsinckia menziesii (Lehm.) Nels. & Macbr. Roitman, 1983a C
Dibenzoylretronecine Caccinea glauca Savi. Siddiqi et al. 1978a B346
Dicrotaline Crotalaria dura J.M. Wood et Evans Marais, 1944; Adams & Van Duuren, 1953a B295, 296
C. globifera E. Mey. Marais, 1944; Adams & Van B295,
Duuren, 1953a 296
N-(Dihydropyrrolizino- Heliotropium europaeum L. Culvenor & Smith, 1969 B68
methyl) heliotrine chloride
15,20-Dihydroxyerucifoline Senecio dolichodoryius Cuatr. Bohlmann et al. 1986 A
Dihydroxytriangularine Alkanna tinctoria Tausch Roder et al. 1984b C
Doronenine Senecio abrotanifolius ssp. Roder et al. 1984a A
abrotanifolius
S. abrotanifolius ssp. abrotanifolius Roder et al. 1984a A
var tiroliensis
S. doronicum L. Roder et al. 1979a, 1980; Kirfel B176, C
et al. 1980 A
Doronine Doronicum macrophyllum Alieva et al. 1976 B122
Senecio abrotanifolius ssp. Roder et al. 1984a A
abrotanifolius
S. abrotanifolius ssp. abrotanifolius Roder et al. 1984a A
var tiroliensis
S. clevelandii E.L. Greene Wong & Roitman, 1984 A
S. othonnae Bieb. Khalilov et al. 1977 B223
Alkaloid Plant Sources Reference Reference
Location
H. suaveolens Bieb. Guner, 1986 A
H. supinum L. Crowley & Culvenor, 1959 B83
Lappula glochidiata Suri et al. 1978 B84
Lindelofia angustifolia (Schrenk) Brand. Rao et al. 1974; Suri et al. 1975a B62, 36
L. spectabilis Lehm. Rao et al. 1974; Suri et al. 1975a B62, 36
L. stylosa (Kar. et Kir.) Brand. Kiyamitdinova et al. 1967 B75
L. tschimganica Akramov et al. 1965 B87
Paracynoglossum imeritinum (Kusn.) M. Pop. Man'ko & Marchenko, 1971b B88
Prestonia sp. Edgar, 1985 A
Rindera austroechinata M. Pop. Akramov et al. 1965 B87
R. baldshuanica Kusnezov Akramov et al. 1965 B87
R. cyclodonta Akramov et al. 1967a B59
R. echinata Regel Men'shikov & Denisova, 1953 B92
R. oblongifolia M. Pop. Akramov et al. 1965 B87
Solenanthus circinnatus Ledeb. Akramov et al. 1964 B93
S. coronatus Kiyamitdinova et al. 1967 B75
S. karateginus Lipsky Akramov et al. 1964 B93
Symphytum asperum Lepech. Man'ko et al. 1970b B97
S. caucasicum Bieb. Man'ko et al. 1972 B98
S. officinale Linn. Man'ko et al. 1970a; Huizing & B101, C
Malingre, 1979
Echiumine Amsinckia hispida (Ruiz et Pav.) Culvenor & Smith, 1966a B30
I.M. Johnston
A. intermedia Fisch et C. Mey. Culvenor & Smith, 1966a B30
A. lycopsoides Lehm. Culvenor & Smith, 1966a B30
Echium plantagineum L. Culvenor, 1956 B48
Emiline Emilia flammea Cass. Kohlmunzer & Tomczyk, 1969; Tomczyk & B123, 124
Kohlmunzer, 1971; Kohlmunzer B125
et al. 1971
Alkaloid Plant Sources Reference Reference
Location
13,19-Epoxyseneciphylline Senecio megaphyllus Green. Bohlmann et al. 1986 A
S. usgorensis Cuatr. Bohlmann et al. 1986 A
13,19-Epoxyspartiodine Senecio megaphyllus Green. Bohlmann et al. 1986 A
Erucifoline Senecio aegypticus L. Klasek et al. 1968a B145
S. erraticus Berthol. subsp. Schroter & Santavy, 1960; Sedmera B179,
barbaraeifolius Krock. et al. 1972 180
S. erucifolius L. Kompis & Santavy, 1962; Sedmera B183,
et al. 1972 180
Europine Heliotropium arbainense Zalkow et al. 1979 B419
H. digynum Forssk. Hammouda et al. 1984 A
H. europaeum L. Culvenor, 1954 B66
H. lasiocarpum Fisch and Mey. Culvenor et al. 1986 A
H. maris-mortui Zalkow et al. 1978 B74
H. rotundifolium Zalkow et al. 1978 B74
H. suaveolens Bieb. Guner, 1986 A
Trichodesma africana Zalkow et al. 1979 B419
Floricaline Cacalia floridana Cava et al. 1968 B119
Floridanine Cacalia floridana Cava et al. 1968 B119
Doronicum macrophyllum Alieva et al. 1976 B122
Senecio aureus L. Roder et al. 1983 C
S. erraticus Berthol. Gaiduk et al. 1974 B177
S. othonnae Bieb. Khalilov & Telezhenetskaya, 1973b B222
Florosenine Cacalia floridana Cava et al. 1968 B119
Senecio aureus L. Roder et al. 1983 C
S. fluviatilis Wallr. Klasek et al. 1973b B185
S. quebradensis Greem. Bohlmann et al. 1986 A
Alkaloid Plant Sources Reference Reference
Location
Fulvine Crotalaria berteroana DC. (C. fulva Roxb.) Schoental, 1963 B297
C. crispata F. Muell. ex Benth. Culvenor & Smith, 1963 B294
C. madurensis R. Wight Atal et al. 1966a; Habib et al. 1971 B280, 315
C. paniculata Willd. Subramanium et al. 1968 B325
Globiferine Crotalaria globifera E. Mey. Brown et al. 1984 C
Grahamine Crotalaria grahamiana R. Wight et Atal et al. 1969 B299
Walk.-Arn.
Grantaline Crotalaria globifera E. Mey Brawn et al., 1984 C
C. virgulata subsp. grantiana (Harv.) Smith & Culvenor, 1984 C
Polhill (C. grantiana Harvey)
Grantianine Crotalaria globifera E. Mey. Brown et al. 1984 C
C. virgulata subsp. grantiana (Harv.) Adams et al. 1942b; Adams & B301
Polhill (C. grantiana Harvey) Gianturco, 1956b; Smith & B259, C
Culvenor, 1984
Gynuramine Gynura scandens O. Hoffm. Wiedenfeld, 1982 A
Heleurine Heliotropium europaeum L. Culvenor, 1954 B66
H. indicum L. Hoque et al. 1976 B72
H. lasiocarpum Fisch and Mey. Culvenor et al. 1986 A
Heliosupine Cynoglossum creticum Zalkow et al. 1979 B419
C. officinale Man'ko & Borisyuk, 1957; Man'ko, 1959; B38, 39
Sykulska, 1961 B40
C. pictum Man'ko & Marchenko, 1971b, 1972b B44, 45
C. viridiflorum Pallas ex Lehm. Man'ko, 1972 B34
Echium vulgare L. Man'ko, 1964 B49
Heliotropium supinum L. Denisova et al. 1953; Crowley & B82, 83
Culvenor, 1959
Alkaloid Plant Sources Reference Reference
Location
Myosotis sylvatica Hoffm. Culvenor & Smith, unpubl.
Paracynoglossum imeritinum (Kusn.) Man'ko & Marchenko, 1971 B88
Symphytum asperum Lepech. Man'ko et al. 1970b B97
S. officinale Linn. Man'ko et al. 1970a B101
Heliotrine Heliotropium acutiflorum Akramov et al. 1968 B51
H. arbainense Zalkow et al. 1979 B419
H. arguzioides Kar. et Kir. Zolotavina, 1963 B53
H. curassavicum Linn. Rajagopalan & Batra, 1977b B56
H. dasycarpum Ledeb. Akramov et al. 1961a B54
H. digynum Forssk. Hammouda et al. 1984 A
H. eichwaldi Steud. ex DC. Gandhi et al. 1966a B60
H. europaeum L. Trautner & Neufeld, 1949; Culvenor B64, 65
et al. 1954
H. indicum L. Hoque et al. 1976 B72
H. lasiocarpum Fisch et C. Mey. Men'shikov, 1932 B73
H. olgae Kiyamitdinova et al. 1967; Sheveleva B75, 76
et al. 1969
H. popovii H. Riedl. subsp. gillianum Mohabbet et al. 1976 B77
H. ramosissimum Habib, 1975; Schoental & B79, 78
Cavanagh, 1972
H. suaveolens Bieb. Guner, 1986 A
H. supinum L. Pandey et al. 1983 C
H. transoxanum Akramov et al. 1968 B51
Heliovinine Heliotropium curassavicum Linn. Mohanraj et al. 1982c C
Heterophylline Parsonsia heterophylla A. Cunn. Edgar et al. 1980 B418
P. spiralis Edgar et al. 1980 B418
18-Hydroxysenkirkine Crotalaria laburnifolia L. subsp. Crout, 1972 B312
19-Hydroxysenkirkine Senecio laricifolius H.B.K. Bohlmann et al. 1986 A
Alkaloid Plant Sources Reference Reference
Location
Incanine Heliotropium olgae Sheveleva et al. 1969 B76
Trichodesma incanum Alph. DC. Yunusov & Plekhanova, 1953, 1957, B110, 111
1959; Tashkhodzhaev et al. 1979a B112, C
Indicine Heliotropium amplexicaule Vahl. Ketterer et al. 1987, (in press)
H. indicum L. Mattocks et al. 1961 B70
Prestonia sp. Edgar, 1985 A
Integerrimine Cacalia hastata L. subsp. orientalis Hayashi et al. 1972 B120
Kitamura
Crotalaria brevidens Benth. var. Suri et al. 1975b B304
intermedia (Kotschy) Polhill
C. brevifolia Sawhney & Atal, 1966 B290
C. incana L. Adams & Van Duuren, 1953b B187
C. pallida Ait. Sawhney et al. 1967 B291
C. tetragona Roxb. Puri et al. 1974 B314
C. zanzibarica Benth. (C. usaramoensis) Culvenor & Smith, 1966b B337
Petasites hybridus L. Luthy et al. 1983 C
Senecio alpinus L. Luthy et al. 1981 A
S. antieuphorbium (L.) Sch. Bip. Rodriguez & Gonzalez, 1969 B152
S. brasiliensis DC. Motidome & Ferreira, 1966a B163
S. brasiliensis Less. var tripartitus Nardi et al. 1980 A
S. durieui Gay Panizo & Rodriguez, 1974 B157
S. erraticus Berthol. subsp. Santavy et al. 1962 B182
barbaraeifolius Krock.
S. faberi Hemsl. Wei et al. 1982 C
S. formosus Munoz Quevedos, 1976 B186
S. glandulosus Don ex Hook. et Arn. Pestchanker et al. 1985b A
S. inaequidens DC. Bicchi et al. 1985 A
Alkaloid Plant Sources Reference Reference
Location
S. incanus L. subsp. carniolicus Klasek et al. 1968b B147
(Willd.) Br.-Bl.
S. integerrimus Manske, 1939a; Roitman et al. 1979 B156, 420
S. kleinia Sch. Bip. Gonzalez & Calero, 1958b B205
S. leucostachys Baker Pestchanker & Giordano, 1986 A
S. magnificus F. Muell. Gellert & Mate, 1964 B216
S. morrisonensis Hayata Lu, Sheng-Teh, 1972 B217
S. nebrodensis L. var sicula Plescia et al. 1976 B218
S. ragonesei Cabr. Pestcharnker & Giordano, 1986 A
S. spathulatus A. Rich. White, 1969 B158
S. squalidus L. Kropman & Warren, 1950; Gonzalez & B265,
Calero, 1958a 205
S. tenuifolius Burm. Bhakuni & Gupta, 1982 C
S. triangularis Hook. Roitman, 1983b C
S. vernalis Walst. et Kit. Sener et al. 1986; Hartmann & A, A
Zimmer, 1986
S. viscosus L. Barger & Blackie, 1936; Santavy et al. B264,
1962 182
S. vulgaris L. Pieters & Vlietinck, 1986 A
Intermedine Amsinckia hispida (Ruiz et Pav.) Culvenor & Smith, 1966a B30
I.M. Johnston
A. intermedia Fisch et C. Mey. Culvenor & Smith, 1966a B30
A. lycopsoides Lehm. Culvenor & Smith, 1966a B30
A. menziesii (Lehm.) Nels. & Macbr. Roitman, 1983 C
Borago officinale L. Luthy et al. 1984 A
Conoclinium coelestinium (L.) DC Herz et al. 1981 C
Eupatorium compositifolium Walt. Herz et al. 1981 C
Symphytum aspera Roitman, 1981 A
S. officinale Linn. Roder et al. 1982 C
S. x uplandicum Nyman Culvenor et al. 1980a, 1980b A, A
Trichodesma africana Zalkow et al. 1979 B419
Alkaloid Plant Sources Reference Reference
Location
Isocromadurine Crotalaria candicans W. and A. Suri et al. 1982 C
C. madurensis R. Wight ]Rao et al. 1975c B317
Isoline Senecio othonniformis Fourcade Coucourakis & Gordon-Gray, 1970; B224
Coucourakis et al. 1972 B225
Jacobine Crassocephalum crepidioides Asada et al. 1985 A
Senecio alpinus L. Luthy et al. 1981 A
S. brasiliensis DC. Adams & Gianturco, 1956e B148
S. cineraria DC. Barger & Blackie, 1937 B167
S. jacobaea L. Manske, 1931; Blackie, 1937; B194, 153
Bradbury & Culvenor, 1954 B198
S. paludosus L. Blackie, 1937; Dorosh & Alekseev, 1960 B153, 227
Alekseev, 1961b B228
Jacoline Crassocephalum crepidioides Asada et al. 1985 A
Senecio alpinus L. Luthy et al. 1981 A
S. jacobaea L. Bradbury & Culvenor, 1954 B198
Jaconine Senecio alpinus L. Luthy et al. 1981 A
S. jacobaea L. Bradbury & Culvenor, 1954 B198
Jacozine Senecio alpinus L. Luthy et al. 1981 A
S. cannabifolius Less Asada et al. 1982 C
S.jacobaea L. Bradbury & Culvenor, 1954; Culvenor, B198,
1964 202
Junceine Crotalaria juncea L. Adams & Gianturco, 1956a, 1956b, 1956c B305,
B306
B307
C. wightiana Grah. ex Wight & Arn. Atal et al. 1966b B329
Alkaloid Plant Sources Reference Reference
Location
Lasiocarpine Heliotropium arbainense Zalkow et al. 1979 B419
H. arborescens L. Carcamo-Marquez, 1961 B52
H. curassavicum Linn. Rajagopalan & Batra, 1977b B56
H. digynum Forssk. Hammouda et al. 1984 A
H. eichwaldii Steud. ex DC. Rao et al. 1974 B62
H. europaeum L. Culvenor et al. 1954 B65
H. indicum Linn. Hoque et al. 1976 B72
H. lasiocarpum Fisch. et C. Mey. Men'shikov, 1932 B73
H. maris mortui Zalkow et al. 1979 B419
H. suaveolens Bieb. Guner, 1986 A
H. supinum L. Pandey et al. 1983 C
Lappula intermedia Man'ko & Vasil'kov, 1968 B85
Symphytum caucasicum Man'ko et al. 1969 B95
S. officinale Linn. Man'ko et al. 1969, 1970a B95, 101
Latifoline Cynoglossum latifolium R. Br. Crowley & Culvenor, 1962 B37
Hackelia floribunda Hagglund et al. 1985 A
Ligudentine Ligularia brachyphylla Hand.-Mazz. Klasek et al. 1971 B134
L. dentata (A. Gray) Hara. Klasek et al. 1971 B134
Ligularidine Ligularia dentata (A. Gray) Hara Hikichi et al. 1979, Asada & B436, C
Furuya, 1984a
Ligularine Ligularia brachyphylla Hand.-Mazz. Klasek et al. 1971 B134
L. dentata (A. Gray) Hara. Klasek et al. 1971 B134
L. elegans Cass. Klasek et al. 1971 B134
Ligularizine Ligularia dentata (A. Gray) Hara. Asada & Furuya, 1984a C
Alkaloid Plant Sources Reference Reference
Location
Lycopsamine Amsinckia hispida (Ruiz et Pav.) Culvenor & Smith, 1966a B30
I.M. Johnston
A. intermedia Fisch. et C. Mey. Culvenor & Smith, 1966a B30
A. lycopsoides Lehm. Culvenor & Smith, 1966a B30
A. menziesii (Lehm.) Nels. & Macbr. Roitman, 1983a C
Anchusa officinalis L. Broch-Due & Aasen, 1980 B32
Borago officinale L. Larson et al. 1984; Luthy et al. 1984 C, A
Eupatorium compositifolium Walt. Herz et al. 1981 C
Heliotropium steudneri Vatke Schneider et al. 1975 B8O
Messerschmidia sibirica Hikichi et al. 1980 C
Parsonsia eucalyptophylla F. Muell. Edgar & Culvenor, 1975 B28
P. straminea (R. Br.) F. Muell. Edgar & Culvenor, 1975 B28
Prestonia sp. Edgar, 1985 A
Symphytum asperum Lepech. Roitman, 1981 A
Symphytum officinale Linn. Huizing & Malingre, 1981 C
Symphytum x uplandicum Nyman Culvenor et al. 1980a, 1980b A, A
Madurensine Crotalaria agatiflora Schweinf. Atal et al. 1966a B280
C. laburnifolia L. subsp. eldomae Crout, 1972 B312
C. madurensis R. Wight Atal et al. 1966a; Mahran et al. 1979 B280, 426
Merenskine Senecio latifolius DC. Bredenkamp et al. 1985 C
Monocrotaline Crotalaria aegyptiaca Benth. Mahran et al. 1979; Zalkow et al. 1979 B426, 419
C. assamica Benth. Crotalaria Research Group, 1974 B286
C. burhia Rao et al. 1975 B293
C. cephalotes Steud. ex A. Rich Pilbeam et al. 1983 C
C. crispata F. Muell. ex Benth. Culvenor & Smith, 1963 B294
C. cunninghamii R. Br. Pilbeam et al. 1983 C
C. grahamiana R. Wight ex Walk.-Arn. Gandhi et al. 1966b; Atal et al. 1969 B298, 299
C. leschenaultii DC. Suri & Atal, 1967 B313
Alkaloid Plant Sources Reference Reference
Location
C. leiloba Bartl. Puri et al. 1974 B314
C. mitchellii Benth. Culvenor et al. 1967b B318
C. mysorensis Roth. Sawhney & Atal, 1968 B319
C. nitens Kunth. Hoet et al. 1981 A
C. novae-hollandiae DC. subsp. Culvenor et al. 1967b B318
lasiophylla (Benth.) A. Lee
C. paulina schrank. Pilbeam et al. 1983 C
C. quinquefolia L. Pilbeam et al. 1983 C
C. recta Steud. ex A. Rich Crout, 1968a B326
C. retusa L. Adams & Rogers, 1939; Culvenor & B327,
Smith, 1957a 328
C. sagittalis L. Willette & Cammarata, 1972 B330
C. spectabilis Roth. Neal et al. 1935; Adams & Rogers, B325,
1939 327
C. stipularia Desv. Puri et al. 1974 B314
Lindelofia spectabilis Lehm. Rao et al. 1974 B62
Monocrotalinine Crotalaria grahamiana Wight et Arn. Rajagopalan & Batra, 1977a B300
Myoscorpine Myosotis scorpioides L. Resch et al. 1982 C
Symphytum officinale Linn. Resch et al. 1982C C
Neoligularidine Ligularia dentata (A. Gray) Hara. Asada & Furuya, 1984a C
Neopetasitenine Ligularia japonica Asada et al. 1981 A
Petasites japonicus Maxim. Yamada et al. 1976a B19
Neosenkirkine Senecio auricola Bourg. Panizo & Rodriguez, 1974 B157
S. grandifolius Less. Bohlmann et al. 1986 A
S. pierotii Asada & Furuya, 1982 C
Neotriangularine Senecio triangularis Hook. Roitman, 1983b C
Alkaloid Plant Sources Reference Reference
Location
Nilgirine Crotalaria pallida Ait. Atal et al. 1968 B322
Onetine Senecio othonnae Bieb. Danilova et al. 1962 B221
Otosenine Cacalia floridana Cava et al. 1968 B119
Doronicum macrophyllum Alieva et al. 1976 B122
D. pardalianches Linn. Rajagopalan & Negi, 1985 A
Emilia flammea Cass. Kohlmunzer & Tomczyk, 1969 B123
Senecio aegypticus L. Klasek et al. 1968a B145
S. aureus L. Resch et al. 1983, Roder et al. 1983 C, C
S. cineraria DC. Habib, 1974 B170
S. desfontainei Druce Haddad et al. 1963; Klasek B173,
et al. 1968a 145
S. erraticus Berthol. Gaiduk et al. 1974 B177
S. erraticus subsp. barbaraeifolius Krock. Santavy, 1958; Schroter & Santavy, 1960 B178, 179
S. fluviatilis Wallr. Klasek et al. 1973b B185
S. jacobaea L. Akramov et al. 1968 B51
S. othonnae Bieb. Zhdanovich & Men'shikov, 1941; B220
Danilova et al. 1962 B221
S. renardii Winkl. Danilova & Konovalova, 1950 B249
S. tomentosus Adams et al. 1956; Schroter & B271,
Santavy, 1960 179
Parsonsine Parsonsia heterophylla A. Cunn. Eggers & Gainsford, 1979; Edgar et al. B417,
1980 418
P. spiralis Edgar et al. 1980 B418
Petasinine Petasites japonicus Maxim. Yamada et al. 1978b B141
Petasitenine Farfugium japonicum Kitam Niwa et al. 1985 A
Petasites japonicus Maxim. Yamada et al. 1976a, 1976b; B19, 139
Furuya et al. 1976 B20
Alkaloid Plant Sources Reference Reference
Location
Retroisosenine Senecio nemorensis L. var. bulgaricus Nghia et al. 1976 B219
(Vel.) Stoj. et Stef.
S. nemorensis L. var subdecurrens Klasek et al. 1980a B422
Retrorsine Crotalaria spartioides DC. Bruemmerhoff & de Waal, 1961 B334
C. zanzibarica Benth. (C. usaramoensis) Culvenor & Smith, 1966b B337
Senecio ambrosioides Adams & Gianturco, 1956e B148
S. ampullaceus Hook. Adams & Govindachari, 1949b; Adams & B149, 151
Looker, 1951; Warren et al. 1950 B150
S. bipinnatisectus Belcher White, 1969 B158
S. brasiliensis DC. Motidome & Ferreira, 1966a B163
S. bupleuroides DC. Sapiro, 1949 B164
S. cineraria DC. Klasek et al. 1975 B171
S. cruentus DC. Asada et al. 1982 C
S. cymbaroides Roitman et al. 1979 B420
S. desfontainei Druce Rizk et al. 1983 A
S. discolor DC. Schoental, 1960; Hennig, 1961 B174, 175
S. douglasii DC. Adams & Govindachari, 1949b; Adams B149,
& Looker, 1951 151
S. eremophilus Richards Adams & Govindachari, 1949b; Adams B149,
& Looker, 1951 151
S. erucifolius L. Ferry & Brazier, 1976 B184
S. filaginoides (H. et A.) DC. Pestchanker & Giordano, 1986 A
S. formosus Munoz Quevedo, 1976 B186
S. gilliesiano Guidugli et al. 1986 A
S. glaberrimus DC. Blackie, 1937 B153
S. glandulosus Don ex Hook. et Arn. Pestchanker et al. 1985b A
S. graminifolius N.J. Jacq. de Waal, 1941 B188
S. griesbachii Motidome & Ferreira, 1966b B190
S. ilicifolius Thunb. de Waal, 1940a, 1940b, 1941; Culvenor B191, 192
& Smith, 1954 B188, 127
Alkaloid Plant Sources Reference Reference
Location
S. inaequidens DC. Roder et al. 1981 A
S. isatideus DC. Blackie, 1937; de Waal, 1939 B153, 193
S. jacobaea L. Ferry & Brazier, 1976 B184
S. latifolius DC. Watt, 1909; Barger et al. 1935 B211, 212
S. longilobus Benth. Adams & Govindachari, 1949b; Adams & B149, 151
Looker, 1951; Warren et al. 1950 B150
S. paucicalyculatus Klatt Pretorius, 1949 B230
S. phillipicus Roegel et Koern Gonzalez et al. 1986a A
S. pterophorus DC. de Waal, 1940b, 1941; Culvenor & B192, 188
Smith, 1954 127
S. quadridentatus Labill. Culvenor & Smith, 1955 B247
S. ragonesei Cabr. Pestchanker & Giordano, 1986 A
S. retrorsus DC. Manske, 1931; de Waal, 1939 B194, 193
S. riddellii Torr. et A. Gray Roitman et al. 1979 B420
S. riddellii Torr. et A. Gray var. Adams & Govindachari, 1949b B149
parksii Cory.
S. ruderalis Harvey Leisegang, 1950 B254
S. seratophiloides Griseb. Pestchanker & Giordano, 1986 A
S. spartioides Roitman et al. 1979 B420
S. subulatus Don ex Hook. et Arn Pestchanker et al. 1985b A
var erectus
S. swaziensis Compton Gordon-Gray et al. 1972; Gordon-Gray B268,
& Wells, 1974 270
S. triangularis Hook. Roitman, 1983b C
S. uspallatensis Pestchanker et al. 1985a A
S. venosus Harvey Blackie, 1937 B153
S. vernalis Waldst. et Kit. Roder et al. 1979b C
S. viminalis Bremek. de Waal & van Twisk, 1964 A
S. vulgaris L. Tschu Shun et al. 1960 B277
S. werneriaefolius Roitman et al. 1979 B420
Alkaloid Plant Sources Reference Reference
Location
Retusamine Crotalaria mitchellii Benth. Culvenor et al. 1967b B318
C. mitchellii Benth. subsp. laevis Culvenor et al. 1967b B318
A. Lee
C. novae-hollandiae DC. subsp. Culvenor et al. 1967b B318
lasiophylla Benth. A. Lee
C. novae-hollandiae DC. subsp. Culvenor et al. 1967b B318
novae-hollandiae
C. retusa L. Culvenor & Smith, 1957a; Wunderlich, B328, C
1962
Riddelliine Crotalaria juncea L. Adams & Gianturco, 1956a B305
Senecio aegypticus L. Klasek et al. 1968a B145
S. ambrosioides Roitman et al. 1979 B420
S. cruentus DC. Asada et al. 1982 C
S. cymbaroides Roitman et al. 1979 B420
S. desfontainei Druce Haddad et al. 1963; Klasek et al. B173,
1968a 145
S. douglassi DC. Adams & Govindachari, 1949b; Adams & B149,
Looker, 1951 151
S. eremophilus Richards Adams & Govindachari, 1949b; Adams & B149,
Looker, 1951 151
S. longilobus Benth. Adams & Govindachari, 1949b; Adams & B149,
Looker, 1951; 151
Warren et al. 1950 B150
S. riddellii Torr. et A. Gray Manske, 1939a; Adams et al. 1942c B156, 252
S. riddellii Torr. et A. Gray var. Adams & Govindachari, 1949b B149
parksii (Cory)
S. spartioides Roitman et al. 1979 B420
S. vernalis Walst. et Kit. Sener et al. 1986 A
S. vulgaris Roitman et al. 1979 B420
Alkaloid Plant Sources Reference Reference
Location
Rinderine Eupatorium altissimum L. Herz et al. 1981 C
E. serotinum Michx. Locock et al. 1966 B131
Prestonia sp. Edgar, 1985 A
Rindera baldshuanica Kusnezov Akramov et al. 1961c B91
Solenanthus turkestanicus Regel Akramov et al. 1962 B94
et Smirnov
(Kusnezov)
Sceleratine Senecio latifolius DC. (S. sceleratus) de Waal & Pretorius, 1941; de Waal B257,
et al. 1963 260
Scorpioidine Myosotis scorpioides L. Resch et al. 1982 C
Sencalenine Senecio cacaliaster (Lam.) Roder et al. 1984b A
Senecicannabine Senecio cannabifolius Less. Asada et al. 1982 C
Senecionine Brachyglottis repanda Forst. et Forst. Mortimer & White, 1967 B117
Caltha biflora Stermitz & Adamovics, 1977 B341
C. leptosepala Stermitz & Adamovics, 1977 B341
Castilleja rhexifolia Rydb. Stermitz & Suess, 1978 B342
Castilleja "rhexifolia aff. miniata" Roby & Stermitz, 1984 C
Crotalaria juncea L. Adams & Gianturco, 1956a B305
C. micans Link. Sethi & Atal, 1964 B284
C. zanzibarica Benth. (C. usaramoensis) Culvenor & Smith, 1966b B337
Emilia sonchifolia DC. Culvenor, unpubl.
Erechtites hieracifolia (L.) Raf. ex DC. Manske, 1939b; Culvenor & Smith, 1954 B126, 127
Gynura segetum (Lour.) Merr. Hua et al. 1983; Liang & Roder, 1984 C, C
Ligularia japonica Asada et al. 1981 A
Petasites hybridus L. Luthy et al. 1983 C
P. laevigatus (Willd.) Reichenb. Massagetov & Kuzovkov, 1953 B142
Senecio aegypticus L. Klasek et al. 1968a; Gharbo & B145,
Habib, 1969 146
Alkaloid Plant Sources Reference Reference
Location
S. alpinus (L.) Scop. Luthy et al. 1981 A
S. ambrosioides Adams & Gianturco, 1956e B148
S. ampullaceus Hook. Adams & Govindachari, 1949b; Adams & B149,
Looker, 1951; 151
Warren et al. 1950 B150
S. argentino Baker (vira-vira Hieron) Pestchanker & Giordano, 1986 A
S. aureus L. Manske, 1936, 1939a B155, 156
S. brasiliensis DC. Fonseca, 1951; Novelli & De Varella, B162,
1945; Adams & Gianturco, 1956e 161
B148
S. carthamoides Greene Adams & Govindachari, 1949b; Adams B149,
& Looker, 1951 151
S. cineraria DC. Barger & Blackie, 1937; Adams & B167,
Govindachari, 1949a; 168
Alekseev et al. 1962a B169
S. congestus (R.Br.) DC. Roder et al. 1982 C
S. cruentus DC. Asada et al. 1982 C
S. cymbaroides Roitman et al. 1979 B420
S. desfontainei Druce Klasek et al. 1968a B145
S. discolor DC. Schoental, 1960; Hennig, 1961 B174, 175
S. douglasii DC. Adams & Govindachari, 1949b; Adams B149,
& Looker, 1951 151
S. eremophilus Richards Adams & Govindachari, 1949b; Adams B149,
& Looker, 1951 151
S. erraticus Berthol. Gaiduk et al. 1974 B177
S. erraticus Berthol. subsp. Santavy, 1958; Schroter & Santavy, B178,
barbaraeifolius Krock. 1960; Kompis et al. 1960 179
B181
S. erucifolius L. Kompis & Santavy, 1962 B183
S. filaginoides (H. et A.) DC. Pestchanker & Giordano, 1986 A
Alkaloid Plant Sources Reference Reference
Location
S. fistulosus Poepp. ex Less. Gonzalez et al. 1986b A
S. fremontii Torr. et A. Gray Adams & Gianturco, 1956e B148
S. gilliesiano Guidugli et al. 1986 A
S. glabellus Turcz. DC. Adams & Van Duuren, 1953b B187
S. ilicifolius Thunb. de Waal, 1940a, 1940b, 1941; Culvenor B191,
& Smith, 1954 192
B188,
127
S. illinitus Phill. Gonzalez et al. 1986a A
S. inaequidens DC. Roder et al. 1981 A
S. integerrimus Nutt. Manske, 1939a B156
S. jacobaea L. Bradbury & Mosbauer, 1956 B199
S. laricifolius H.B.K. Bohlmann et al. 1986 A
S. lautus Forst. f. ex Willd. Culvenor unpubl.
S. leucostachys Baker Pestchanker & Giordano, 1986 A
S. longiflorus Sch. Bip. de Waal & van Twisk, 1964 A
S. magnificus F. Muell. Culvenor, 1962 B215
S. multilobatus MaCoy et al. 1983 A
S. multivenius Benth. in Oerst Bohlmann et al. 1986 A
S. nebrodensis L. var. sicula Plescia et al. 1976 B218
S. nemorensis L. subsp. fuchsii Gmel. Wiedenfeld & Roder, 1979 B423
S. pampeanus Cabrera Novelli, 1958 B229
S. pancicii Degen var arnautorum Jizba et al. 1982 C
(Velen.) Stoj. Stef. et Kit.
S. pancicii Degen var pancicii Jizba et al. 1982 C
S. patagonicus Hook. and Arn. Villarroel et al. 1985 A
S. petasitis DC. Gharbo & Habib, 1969 B146
S. pimpinellifolius H.B.K. Bohlmann et al. 1986 A
S. pseudo-arnica Less. Manske, 1939a B156
S. pterophorus DC. de Waal, 1940b, 1941; Culvenor & B192,
Smith, 1954 188
B127
Alkaloid Plant Sources Reference Reference
Location
S. quadridentatus Labill. Culvenor & Smith, 1955 B247
S. sandrasicus Temizer et al. 1985 A
S. scandens Batra & Rajagopalan, 1977 B256
S. seratophiloides Griseb. Pestchanker & Giordano, 1986 A
S. spartioides Manske, 1939a; Adams & Gianturco, B156,
1957b 262
S. spathulatus A. Rich White, 1969 B158
S. squalidus L. Barger & Blackie, 1936; Kropman B264,
& Warren, 1950 265
S. subalpinus C. Koch Trivedi & Santavy, 1963 B267
S. subulatus Don ex Hook. et Arn Pestchanker et al. 1985b A
var erectus
S. tenuifolius Burm. Bhakuni & Gupta, 1982 C
S. tomentosus Adams et al. 1956 B271
S. triangularis Hook. Kupchan & Suffness, 1967 B272
S. uintahensis Roitman et al. 1979 B420
S. vernalis Waldst. et Kit. Roder et al. 1979b C
S. viminalis Bremek. de Waal & van Twisk, 1964 A
S. viscosus L. Barger & Blackie, 1936; Santavy et al. B264,
1962 182
S. vulgaris L. Grandval & Lajoux, 1895; Barger & B275,
Blackie, 1936; Konovalova & 264
Orekhov, 1937a; Tschu Shun B276
et al. 1960 B277
S. wernariaefolius Roitman et al. 1979 B420
Syneilesis palmata Maxim. Hikichi & Furuya, 1976 B279
Tussilago farfara Rosberger et al. 1981 C
7-Senecioyl-9-(2-hydroxy- Senecio caudatus DC. Bohlmann et al. 1986 A
3-acetylbutyrl)retronecine
Alkaloid Plant Sources Reference Reference
Location
7-Senecioyl-9-(2-hydroxy- Senecio caudatus DC. Bohlmann et al. 1986 A
methyl-2,3-dihydroxy-
butyrylretronecine
7-Senecioyl-9-(2-methyl- Senecio caudatus DC. Bohlmann et al. 1986 A
2,3-dihydroxybutyryl-
retronecine
7-Senecioylretronecine Senecio cacaliaster (Lam.) Roder et al. 1984b C
S. caudatus DC. Bohlmann et al. 1986 A
S. triangularis Hook. Rueger & Benn, 1983a C
S. variabilis Sch. Bip. Bohlmann et al. 1986 A
9-Senecioylretronecine Senecio caudatus DC. Bohlmann et al. 1986 A
S. variabilis Sch. Bip. Bohlmann et al. 1986 A
7-Senecioyl-9-sarracinyl- Senecio cacaliaster Roder et al. 1984b C
retronecine S. caudatus DC. Bohlmann et al. 1986 A
S. triangularis Hook. Rueger & Benn, 1983 C
S. ungeniensis Thell. Bohlmann et al. 1986 A
S. variabilis Sch. Bip. Bohlmann et al. 1986 A
Seneciphylline Adenostyles alliarae Yakhontova et al. 1976 B115
A. glabra Wiedenfeld et al. 1984 C
A. rhombifolius (Willd.) M. Pimen.subsp. Pimenov et al. 1975 B116
platyphylloides
Crotalaria juncea L. Adams & Gianturco, 1956a B305
Erechtites hieracifolia (L.) Raf. ex DC. Manske, 1939a; Culvenor & Smith, 1954 B126, 127
Senecio alpinus (L.) Scop. Klasek et al. 1968b; Luthy et al. 1981 B147, A
S. ambrosioides Adams & Gianturco, 1956e B148
Alkaloid Plant Sources Reference Reference
Location
S. ampullaceus Hook. Adams & Govindachari, 1949b; Adams & B149,
Looker, 1951; Warren et al. 1950 151
B150
S. aquaticus Hill Blackie, 1937; Evans & Evans, 1949 B153, 154
S. borysthenicus Red'ko, 1956; Alekseev, 1961a B159, 160
S. brasiliensis DC. Fonseca, 1951; Novelli & de Varella, B162,
161
1945; Adams & Gianturco, 1956e B148
S. cannabifolius Less. Alekseev, 1964; Asada et al. 1982 B165, C
S. carthamoides Greene Adams & Govindachari, 1949b; Adams & B149,
Looker, 1951 151
S. chrysanthemoides Wali & Handa, 1964 B166
S. cineraria DC. Barger & Blackie, 1937; Adams & B167,
Govindachari, 1949a 168
S. cruentus DC. Asada et al. 1982 C
S. cymbaroides Roitman et al. 1979 B420
S. desfontainei Druce Gharbo & Habib, 1969 B146
S. douglasii DC. Adams & Govindachari, 1949b; Adams & B149,
Looker, 1951 151
S. eremophilus Richards Adams & Govindachari, 1949b; Adams & B149,
Looker, 1951 151
S. erraticus Berthol. subsp. Kompis et al. 1960; Santavy B181,
barbaraeifolius Krock. et al. 1962 182
S. erucifolius L. Kompis & Santavy, 1962 B183
S. fluviatilis Wallr. Klasek et al. 1973b B185
S. fremontii Torr. et A. Gray Adams & Gianturco, 1956e B148
S. grandifolia Glonti, 1958 B189
S. ilicifolius Thunb. de Waal, 1940a, 1940b, 1941; B191, 192
Culvenor & Smith, 1954 B188,
127
Alkaloid Plant Sources Reference Reference
Location
S. incanus L. subsp. carniolus Klasek et al. 1968b B147
(Willd.) Br.-Bl.
S. jacobaea L. Blackie, 1937; Bradbury & B153,
Culvenor, 1954 198
S. krylovii Sapunova & Ban'kovskii, 1968 B207
S. kubensis Grossh. Khalilov & Telezhenetskaya, 1973a B208
S. lampsanoides Khalilov & Damirov, 1974 B210
S. laricifolius H.B.K. Bohlmann et al. 1986 A
S. latifolius DC. Danilova et al. 1960 B213
S. longiflorus Sch. Bip. de Waal & van Twisk, 1964 A
S. longilobus Benth. Adams & Govindachari, 1949b; Adams & B149,
Looker, 1951 151
S. minimus Poir. White, 1969 B158
S. multivenius Benth. in Oerst Bohlmann et al. 1986 A
S. othonnae Bieb. Zhdanovich & Men'shikov, 1941; B220
Danilova et al. 1962 B221
S. palmatus Pall. Alekseev, 1960 B226
S. paludosus L. Blackie, 1937; Dorosh & Alekseev, B153,
1960; 227
Alekseev, 1961b B228
S. pancicii Degen var arnautorum Jizba et al. 1982 C
(Velen.) Stoj. Stef. et Kit.
S. pancicii Degen var panicii Jizba et al. 1982 C
S. patagonicus Hook. and Arn. Villarroel et al. 1985 A
S. paucifolius S.G. Gmel. Alekseev & Ban'kovskii, 1965 B231
S. phillipicusRoegel et Koern Gonzalez et al. 1986a A
S. platyphylloides Somm. et Lev. Murav'eva, 1964b, 1965; Dauksha, 1970 B233, 234
B235
S. platyphyllus (Bieb.) DC. Orekhov, 1935; Konovalova & B236,
Orekhov, 1938; 237
Konovalova, 1951 B238
Alkaloid Plant Sources Reference Reference
Location
S. pojarkovae Chernova & Murav'eva, 1974 B243
S. propinquus Ait. Khalilov et al. 1972 B245
S. pterophorus DC. de Waal, 1940b, 1941; Culvenor & B192,
Smith, 1954 188
B127
S. quadridentatus Labill. Culvenor & Smith, 1955 B247
S. racemosus Khmel, 1961 B248
S. renardii Winkl. Danilova & Konovalova, 1950 B249
S. rhombifolius (Willd.) Sch. Bip. Khalilov & Telezhenetskaya, 1973a B208
S. scandens Batra & Rajagopalan, 1977 B256
S. spartioides Torr. et A. Gray Manske, 1939a; Adams & Gianturco, B156,
1957b 262
S. spathulatus Benn et al. 1979 B263
S. stenocephalus Maxim. Konovalova & Orekhov, 1937b B266
S. subalpinus C. Koch. Trivedi & Santavy, 1963; Klasek B267,
et al. 1968b 147
S. vernalis Walst. et Kit. Sener et al. 1986; Hartmann & A, A
Zimmer, 1986
S. vulgaris L. Barger & Blackie, 1936; Konovalova & B264,
Orenkhov, 1937a; 276
Tschu Shun et al. 1960 B277
Senecivernine Senecio inaequidens Bicchi et al. 1985 A
S. seratophylloides Griseb. Pestchanker & Giordano, 1986 A
S. vernalis Waldst. et Kit. Roder et al. 1979b C
Senkirkine Brachyglottis repanda Forst. et Forst. Mortimer & White, 1967 B117
Crotalaria laburnifolia subsp. eldomae Crout, 1972 B312
Farfugium japonicum Kitam. Furuya et al. 1971 B133
Petasites albus L. Luthy et al. 1983 C
P. hybridus L. Luthy et al. 1983 C
P. japonicus Maxim. Yamada et al. 1978a B140
Alkaloid Plant Sources Reference Reference
Location
P. laevigatus (Willd.) Reichenb. Massagetov & Kuzovkov, 1953 B142
Senecio antieuphorbium (L.) Sch. Bip. Rodriguez & Gonzalez, 1969 B152
S. desfontainei Druce Rizk et al. 1983 A
S. grandifolius Less. Bohlmann et al. 1986 A
S. illinitus Phill. Gonzalez et al. 1986a A
S. jacobaea L. Akramov et al. 1968 B51
S. kirkii Hook. f. ex Kirk. Briggs et al. 1948; Briggs et al. 1965 B203, 204
S. kleinia Sch. Bip. Rodriguez et al. 1967 B206
S. laricifolius H.B.K. Bohlmann et al. 1986 A
S. pierotii Asada & Furuya, 1982 C
S. procerus L. var. procerus Stoj. Jovceva et al. 1978 B244
Stef. et Kit.
S. quebradensis Greenm. Bohlmann et al. 1986 A
S. renardii Winkl. Danilova & Konovalova, 1950; Briggs B249,
et al. 1965 204
S. tenuifolius Burm. Bhakuni & Gupta, 1982 C
S. vernalis Waldst. et Kit. Roder et al. 1979b C
S. uintahensis Roitman et al. 1979 B420
Tussilago farfara Culvenor et al. 1976b; Borka & A, B425,
Onshuus, 1979; Luthy et al, 1980 A
Sincamidine Amsinckia intermedia Fisch. et C. Mey. Culvenor & Smith, 1966a B30
Spartioidine Senecio spartioides Torr. et A. Gray Manske, 1939a; Adams & Gianturco, 1957 B156,
262
S. vulgaris L. Pieters & Vlietinck, 1986 A
Spectabiline Crotalaria spectabilis Roth Culvenor & Smith, 1957b B336
Spiracine Parsonsia spiralis Wall. Edgar et al. 1980 B418
Spiraline Parsonsia spiralis Wall. Edgar et al. 1980 B418
Alkaloid Plant Sources Reference Reference
Location
Spiranine Parsonsia spiralis Wall. Edgar et al. 1980 B418
Supinine Borago officinale L. Luthy et al. 1984 A
Eupatorium cannabinum L. Pederson, 1975a B128
E. serotinum Michx. Locock et al. 1966 B131
E. stoechadosmum Hanse Furuya & Hikichi, 1973 B132
Heliotropium europeum L. Culvenor, 1954 B66
H. indicum L. Hoque et al. 1976 B72
H. supinum L. Men'shikov & Gurevich, 1949; Crowley & B81, 83
Culvenor, 1959
Tournefortia sarmentosa Lam. Crowley & Culvenor, 1955 B107
Trichodesma zeylanicum (Burm. f.) R. Br. O'Kelly & Sargeant, 1961 B113
Swazine Senecio barbellatus DC. Gordon-Gray & Wells, 1974 B270
S. swaziensis Compton Gordon-Gray et al. 1972; Laing & B268,
Sommerville, 1972 269
Symlandine Symphytum asperum Lepech. Roitman, 1981 A
S. officinale Linn. Roder et al. 1982 C
S. tuberosum L. Gray et al. 1983 A
S. x uplandicum Nyman Culvenor et al. 1980a, 1980b A, A
Symphytine Myosotis scorpioides L. Resch et al. 1982 C
Symphytum aspera Roitman, 1981 A
S. officinale Linn. Furuya & Araki, 1968; Furuya & B15, 16
Hikichi, 1971
S. peregrinum Ledeb. Gadella et al. 1983 A
S. x uplandicum Nyman Culvenor et al. 1980a, 1980b A, A
Syneilesine Syneilesis palmata Maxim. Hikichi & Furuya, 1974, 1976 B278, 279
Alkaloid Plant Sources Reference Reference
Location
Triangularine Alkanna tinctoria Tausch Roder et al. 1984b C
Senecio triangularis Hook. Roitman, 1983b C
Trichodesmine Crotalaria globifera E. Mey. Brown et al. 1984 C
C. juncea L. Adams & Gianturco, 1956a B305
C. lunata Beddome ex Polhill Rothschild et al. 1979 C
C. recta Steud ex A. Rich. Crout, 1968a B326
C. wightiana Grah. ex Wight & Arn. Atal et al. 1966b B329
C. tetragona Roxb. Puri et al. 1974 B314
Heliotropium arguzioides Kar. et Kir. Akramov et al. 1961aB54
Trichodesma incanum Alph. DC. Men'shikov & Rubinstein, 1935; B108
Men'shikov, 1936; Yunusov & B109,
Plekhanova, 1957, 1959; B111, 112
Tashkhodzhaev et al. 1979 C
Uluganine Ulugbeckia tschimganica (B.Fedtsch.) Zak. Khasanova et al. 1974 B114
Uplandicine Symphytum x uplandicum Nyman Culvenor et al. 1980a, 1980b A, A
Usaramine Crotalaria brevidens Benth. var. Suri et al. 1975b B304
intermedia (Kotschy) Polhill
C. brevifolia Sawhney et al. 1967 B291
C. incana L. Sawhney & Atal, 1970a B303
C. pallida Ait. Sawhney et al. 1967 B291
C. zanzibarica Benth. (C. usaramoensis) Culvenor & Smith, 1966b B337
Senecio glandulosus Don ex Hook. et Arn. Pestchanker et al. 1985b A
S. seratophylloides Griseb Pestchanker & Giordano, 1986 A
S. vulgaris L. Pieters & Vlietinck, 1986 A
Alkaloid Plant Sources Reference Reference
Location
Uspallatine Senecio argentino Baker (vira-vira Hieron) Pestchanker et al. 1985a A
S. leucostachys Baker Pestchanker & Giordano, 1986 A
S. seratophiloides Griseb Pestchanker & Giordano, 1986 A
S. uspallatensis Pestchanker et al. 1985a A
Yamataimine Cacalia yatabei Maxim. Hikichi et al. 1978 B121
A - References in this publication.
B - References in Smith & Culvenor, J. Nat. Prod., 44, 129-152 (1981), with reference number.
C - References in Mattocks, Chemistry and Toxicology of Pyrrolizidine Alkaloids, 1986.
APPENDIX II
Table 1. Plants containing hepatotoxic pyrrolizidine alkaloids
------------------------------------------------------------------------------------------
Plant Constituent alkaloids Plant part Referencea
------------------------------------------------------------------------------------------
Apocynaceae
Fernaldia pandurata loroquin root B 29
(syn. Urechites karwinsky
Mueller)
Parsonsia eucalyptophylla lycopsamine aeriel B 28
(F. Muell.)
Parsonsia heterophylla parsonsine whole B 417
A. Cunn. heterophylline B 418
Parsonsia spiralis Wall. heterophylline leaf B 418
parsonsine
spiracine
spiranine
spiraline
Parsonsia straminea (R. Br.) lycopsamine aerial B 28
F. Muell.
Parsonsia estonia sp. echinatine A Edgar (1985)
Boraginaceae
Alkanna tinctoria Tausch 7-angelylretronecine C Roder et al.
dihydroxytriangularine (1984b)
triangularine
Amsinckia hispida (Ruiz intermedine whole B 30
et Pav.) M. Johnston lycopsamine
echiumine
Amsinckia intermedia Fisch intermedine whole B 30
et C. Mey lycopsamine
echiumine
sincamidine
echimidine
Amsinckia lycopsoides Lehm. intermedine whole B 30
lycopsamine
echiumine
Amsinckia menziesii (Lehm.) 7-acetyllycopsamine aerial C Roitman (1983a)
Nels and Macbr. 3'-acetyllycopsamine
diacetyllycopsamine
lycopsamine
intermedine
------------------------------------------------------------------------------------------
APPENDIX II Table 1. (contd.)
------------------------------------------------------------------------------------------
Plant Constituent alkaloids Plant part Referencea
------------------------------------------------------------------------------------------
Boraginaceae (contd.)
Anchusa arvensis ( L.) Bieb. echinatine whole B 31
(or diastereoisomer)
Anchusa officinalis L. 7-acetyllycopsamine whole B 31
(or diastereoisomer)
lycopsamine B 32
Asperugo procumbens L. supinine (or whole B 31
diastereoisomer)
lycopsamine (or
diastereoisomer)
Borago officinalis L. lycopsamine aerial, root C Larson et al.
amabiline (1984)
supinine A Luthy et al.
intermedine (1984)
acetylintermedine
acetyllycopsamine
thesinine seed, flower
Cynoglossum amabile Stapf amabiline whole B 33, 34
& Drummond echinatine
Cynoglossum australe R. Br. cynaustine whole B 33
cynaustraline
heliosupine
Cynoglossum creticum heliosupine aerial B 419
echinatine
Cynoglossum glochidiatum amabiline whole B 36
Wall. ex Lindl.
Cynoglossum lanceolatum cynaustraline whole B 36
Forsk cynaustine
Cynoglossum latifolium latifoline aerial B 37
R. Br. 7-angelylretronecine
Cynoglossum officinale L. heliosupine aerial B 38, 39, 40
echinatine root, aerial B 41, 42
acetylheliosupine aerial B 43
7-angelylheliotridine
Cynoglossum pictum Ait. heliosupine root, aerial B 44, 45
echinatine
pictumine aerial B 46
------------------------------------------------------------------------------------------
APPENDIX II Table 1. (contd.)
------------------------------------------------------------------------------------------
Plant Constituent alkaloids Plant part Referencea
------------------------------------------------------------------------------------------
Boraginaceae (contd.)
Cynoglossum viridiflorum viridiflorine root B 47
Pallas ex Lehm. heliosupine B 34
Echium plantagineum L. echiumine aerial B 48
(Echium lycopsis L.) echimidine
Echium vulgare L. heliosupine aerial B 49
asperumine aerial B 50
echinatine (or whole B 31
diastereoisomer)
Hackelia floribunda latifoline A Hagglund et al.
(1985)
Heliotropium acutiflorum heliotrine aerial B 51
Heliotropium arbainense heliotrine aerial B 419
europine
lasiocarpine
Heliotropium arborescens L. lasiocarpine aerial B 52
(Heliotropium peruvianum L.)
Heliotropium arguzioides heliotrine aerial B 53
Kar. et Kir. trichodesmine aerial, root B 54, 55
Heliotropium curassavicum heliotrine whole B 56
Linn. lasiocarpine
angelylheliotridine
curassavine aerial B 57
heliovicine
trachelanthamidine B 58
acetylcurassavine aerial C Mohanraj et al.
heliocurassavinine (1982)
heliocurassavine
heliocoromandaline
heliocurassavicine
curassanecine
curassavinine
coromandalinine
heliovinine
Heliotropium dasycarpum heliotrine aerial, root B 54
Ledeb. seed B 59
------------------------------------------------------------------------------------------
APPENDIX II Table 1. (contd.)
------------------------------------------------------------------------------------------
Plant Constituent alkaloids Plant part Referencea
------------------------------------------------------------------------------------------
Boraginaceae (contd.)
Heliotropium digynum heliotrine A Hammouda et al.
(Heliotropium luteum) lasiocarpine (1984)
europine
angelylheliotrine
Heliotropium eichwaldi heliotrine whole B 60, 61
Steud. ex DC. lasiocarpine aerial B 62
7-angelylheliotrine aerial B 63
Heliotropium europaeum heliotrine whole B 64, 65
lasiocarpine
europine whole B 66, 67
supinine
heleurine
N-dihydropyrrolizino- whole B 68
methyl-
heliotrine chloride
acetyllasiocarpine whole B 69
Heliotropium indicum L. indicine aerial B 70
acetylindicine aerial B 71
indicinine
echinatine aerial B 72
supinine
heleurine
lasiocarpine
Heliotropium lasiocarpum heliotrine aerial B 73
Fisch. et Mey. lasiocarpine
europine A Culvenor et al.
heleurine (1986)
Heliotropium maris-mortui europine aerial B 74
lasiocarpine aerial B 419
Heliotropium olgae heliotrine aerial, root B 75
Heliotropium popovii H. heliotrine seed B 77
Riedl. subsp. gillianum
H. Riedl.
Heliotropium ramosissimum heliotrine aerial B 78, 79
(Lehm.) Dec. (syn. heleurine
Heliotropium persicum L., supinine
Heliotropium undulatum) lasiocarpine
Heliotropium rotundifolium europine aerial B 74
------------------------------------------------------------------------------------------
APPENDIX II Table 1. (contd.)
------------------------------------------------------------------------------------------
Plant Constituent alkaloids Plant part Referencea
------------------------------------------------------------------------------------------
Boraginaceae (contd.)
Heliotropium steudneri Vatke lycopsamine leaf B 80
Heliotropium suaveolens Bieb. heliotrine aerial A Guner (1986)
lasiocarpine
europine
echinatine
Heliotropium supinum L. supinine root B 81
heliosupine root B 82
echinatine whole B 83
7-angelylheliotridine
7-angelylheliotridine
viridiflorate
7-angelylheliotridine
trachelanthate
heliotrine seed, leaf C Pandey et al.
lasiocarpine (1983)
Heliotropium transoxanum heliotrine aerial B 51
Lappula glochidiata echinatine aerial B 84
Lappula intermedia lasiocarpine aerial B 85
Lindelofia angustifolia echinatine aerial B 62, 36
(Schrenk) Brand. amabiline
Lindelofia spectabilis echinatine aerial B 62, 63
Lehm. 7-acetylechinatine
monocrotaline
Lindelofia stylosa viridiflorine aerial B 86
(Kar. et Kir.) Brand echinatine seed B 75
lindelofine
Lindelofia tschimganica carategine aerial B 87
echinatine
viridiflorine
Lithospermum officinale L. acetylechimidinyl- whole B 31
retronecine
(or diastereoisomer)
Messerschmidia sibirica lycopsamine whole C Hikichi et al.
angelylretronecine (1980)
------------------------------------------------------------------------------------------
APPENDIX II Table 1. (contd.)
------------------------------------------------------------------------------------------
Plant Constituent alkaloids Plant part Referencea
------------------------------------------------------------------------------------------
Boraginaceae (contd.)
Myosotis scorpioides L. 7-acetylscorpioidine aerial C Resch et al.
(syn. Myosotis scorpioidine (1982)
palustris L.) symphytine
myoscorpine
Paracynoglossum imeritinum heliosupine aerial, root B 88
(Kusn.) M. Pop. echinatine B 89, 90
Rindera austroechinata echinatine whole, seed B 87
M. Pop.
Rindera baldshuanica rinderine aerial B 91
Kusnezov echinatine aerial B 87
trachelanthamine
turkestanine
Rindera cyclodonta Bge. echinatine aerial B 59
Rindera echinata Regel echinatine aerial B 92
trachelanthamine aerial B 59
Rindera oblongifolia carategine aerial B 87
M. Pop. echinatine
turkestanine
Solenanthus circinnatus echinatine seed, aerial, B 93
Ledeb. root
Solenanthus coronatus echinatine aerial B 75
Solenanthus karateginius carategine aerial B 93
Lipsky echinatine
Solenanthus turkestanicus rinderine aerial B 94
turkestanine
Symphytum asperum Lepech. asperumine aerial, root B 95, 96
echinatine aerial, root B 97
acetylheliosupine whole B 31
(or diastereoisomer)
7-acetyllycopsamine leaf, root C Roitman (1981)
intermedine
symlandine
7-acetylintermedine
symphytine
lycopsamine
echimidine
angelylechimidine root A Gadella et al.
(or diastereoisomer) (1983)
------------------------------------------------------------------------------------------
APPENDIX II Table 1. (contd.)
------------------------------------------------------------------------------------------
Plant Constituent alkaloids Plant part Referencea
------------------------------------------------------------------------------------------
Boraginaceae (contd.)
Symphytum caucasicum Bieb. lasiocarpine aerial, root B 95
asperumine aerial, root B 98, 99
echinatine
echimidine
Symphytum officinale Linn. symphytine root B 15, 16
echimidine B 100
lasiocarpine aerial, root B 95
heliosupine aerial, root B 101
viridiflorine root
echinatine root
acetylechimidine B 31
(or diastereoisomer)
7-acetyllycopsamine aerial, root A Huizing et al.
lycopsamine (1981)
intermedine
7-acetylintermedine
symlandine C Roder et al.
myoscorpine root (1982a)
C Resch et al.
(1982)
Symphytum orientale anadoline whole B 102, 103, 104
symphytine
echimidine
Symphytum peregrinum Ledeb. echimidine root A Gadella et al.
symphytine (1983)
Symphytum tuberosum L. echimidine whole B 105
anadoline
symlandine root A Gray et al.
(1983)
Symphytum x uplandicum Nyman lycopsamine aerial B 31, 17, 106
intermedine
uplandicine
7-acetyllycopsamine
7-acetylintermedine
echimidine
symphytine
symlandine
angelylechimidine root A Gadella et al.
(or diastereoisomer) (1983)
Tournefortia sarmentosa Lam. supinine leaf, stem B 107
------------------------------------------------------------------------------------------
APPENDIX II Table 1. (contd.)
------------------------------------------------------------------------------------------
Plant Constituent alkaloids Plant part Referencea
------------------------------------------------------------------------------------------
Boraginaceae (contd.)
Trichodesma africana intermedine aerial B 419
europine
Trichodesma incanum Alph. trichodesmine aerial B 108, 109
DC. incanine seed, aerial, B 110, 111, 112
root
Trichodesma zeylanicum supinine seed B 113
(Burm. f) R. Br.
Ulugbekia tschimganica uluganine B 114
Compositae
Adenostyles alliariae platyphylline root B 115
seneciphylline
Adenostyles glabra seneciphylline C Wiedenfeld et al.
(1984)
Adenostyles rhombifolius platyphylline aerial B 116
(Willd.) M. Pimen. ssp. seneciphylline
platyphylloides
Brachyglottis repanda senecionine aerial B 117
Forst. et Forst. senkirkine
brachyglottine B 118
Cacalia floridana (= Senecio otosenine aerial B 119
floridanus Sch. Bip.) florosenine
floridanine
floricaline
Cacalia hastata L. subsp. integerrimine root B 120
orientalis Kitamura
Cacalia yatabei Maxim yamataimine root B 121
Conoclinium coelestinium intermedine C Herz et al. (1981)
(L.) DC
Crassocephalum crepidioides jacobine aerial A Asada et al.
jacoline (1985)
Doronicum macrophyllum otosenine root B 122
floridanine
doronine
------------------------------------------------------------------------------------------
APPENDIX II Table 1. (contd.)
------------------------------------------------------------------------------------------
Plant Constituent alkaloids Plant part Referencea
------------------------------------------------------------------------------------------
Compositae (contd.)
Doronicum pardalianches otosenine root A Rajagopalan & Negi
Linn. (1985)
Echinacea angustifolia DC. tussilagine whole C
isotussilagine
Echinacea purpurea M. tussilagine whole C
isotussilagine
Emilia flammea Cass. otosenine aerial, root B 123, 124, 125
emiline
Erechtites hieracifolia (L.) senecionine aerial B 126, 127
Raf. ex DC. seneciphylline
Eupatorium altissimum L. rinderine C Herz et al. (1981)
angelylheliotridine
Eupatorium cannabinum L. echinatine aerial B 128
supinine
amabiline A Luthy et al.
(1984)
Eupatorium compositifolium intermedine C Herz et al. (1981)
Walt. lycopsamine
Eupatorium maculatum L. echinatine root B 129
trachelanthamidine
Eupatorium purpureum probably echinatine aerial B 130
Eupatorium serotinum Michx. supinine aerial B 131
rinderine
Eupatorium stoechadosmum lindelofine root B 132
Hance supinine
Farfugium japonicum Kitam. senkirkine root, leaf B 133
farfugine whole C Niwa et al. (1983)
petasitenine whole A Niwa et al. (1985)
Gynura scandens O. Hoffm. gynuramine C Wiedenfeld (1982)
acetylgynuramine
Gynura segetum (Lour.) Merr. senecionine C Liang & Roder
(1984)
------------------------------------------------------------------------------------------
APPENDIX II Table 1. (contd.)
------------------------------------------------------------------------------------------
Plant Constituent alkaloids Plant part Referencea
------------------------------------------------------------------------------------------
Compositae (contd.)
Ligularia brachyphylla clivorine aerial B 134
Hand.-Mazz. ligularine
ligudentine
Ligularia clivorum clivorine aerial B 135, 136,
137, 138
Ligularia dentata clivorine aerial B 134
(A. Gray) Hara ligularine
ligudentine
ligularidine whole B 436
ligularinine aerial, root C Asada & Furuya
ligularizine (1984a)
neoligularidine
Ligularia elegans (Cass.) clivorine aerial B 134
[syn. Ligularia macrophylla ligularine
(Ledeb. DC.)]
Ligularia japonica senecionine root A Asada et al.
neopetasitenine (1981)
platyphylline
Petasites albus L. senkirkine aerial C Luthy et al.
(1983)
Petasites hybridus L. senecionine aerial C Luthy et al.
integerrimine (1983)
senkirkine
Petasites japonicus Maxim. petasitenine aerial B 20, 19, 139
(fukinotoxin)
neopetasitenine
senkirkine stem B 140
petasinine aerial B 141
petasinoside
Petasites laevigatus (Willd.) platyphylline aerial B 142
Reichenb. [syn. Nardosmia senkirkine (renardine) aerial B 143, 144
laevigata (Willd.) DC.] senecionine
Senecio abrotanifolius doronine aerial A Roder et al.
ssp. abrotanifolius doronenine (1984a)
bulgarsenine
Senecio abrotanifolius doronine aerial A Roder et al.
ssp. abrotanifolius var. doronenine (1984a)
tiroliensis bulgarsenine
------------------------------------------------------------------------------------------
APPENDIX II Table 1. (contd.)
------------------------------------------------------------------------------------------
Plant Constituent alkaloids Plant part Referencea
------------------------------------------------------------------------------------------
Senecio aegypticus L. senecionine whole B 145, 146
otosenine aerial
riddelliine
erucifoline
Senecio alpinus (L.) Scop. seneciphylline aerial B 147
jacozine
jacobine whole A Luthy et al.
integerrimine (1981)
jacoline
senecionine
jaconine
Senecio ambrosioides retrorsine whole B 148
(= Senecio brasiliensis seneciphylline
Less.) senecionine
riddelliine whole B 420
Senecio ampullaceus Hook. senecionine whole B 149, 150, 151
seneciphylline
retrorsine
Senecio antieuphorbium integerrimine aerial B 152
(L.) Sch. Bip. senkirkine
Senecio aquaticus Hill seneciphylline aerial B 153, 154
Senecio aureus L. senecionine aerial B 155, 156
otosenine aerial C Resch et al.
floridanine (1983)
florosenine C Roder et al.
(1983)
Senecio auricola Bourg. neosenkirkine aerial B 157
Senecio barbellatus DC. swazine B 270
retrorsine
Senecio bipinnatisectus retrorsine aerial, root B 158
Belcher (syn.
Erechtites atkinsoniae)
Senecio borysthenicus seneciphylline aerial, root B 159, 160
(= Senecio prealtus
Berthol.)
Senecio brasiliensis senecionine leaf B 161, 162
DC. (syn. seneciphylline B 148, 163
Senecio ambrosioides) jacobine
integerrimine
retrorsine
------------------------------------------------------------------------------------------
APPENDIX II Table 1. (contd.)
------------------------------------------------------------------------------------------
Plant Constituent alkaloids Plant part Referencea
------------------------------------------------------------------------------------------
Compositae (contd.)
Senecio brasiliensis Less integerrimine A Nardi et al.
var tripartitus (1980)
Senecio bupleuroides DC. retrorsine aerial B 164
Senecio cacaliaster (Lam.) sencalenine A Roder et al.
bulgarsenine (1984b)
7-senecioylretrone-
cine
7-senecioyl-9-sarra-
cinoylretronecine
Senecio cannabifolius Less. seneciphylline aerial B 165
senecicannabine aerial, root C Asada et al.
jacozine (1982a)
Senecio carthamoides Greene senecionine whole B 149, 151
seneciphylline
Senecio caudatus DC. 7-senecioylretrone- aerial A Bohlmann et al.
cine (1986)
9-senecioylretrone-
cine
7-senecioyl-9-sarra-
cinylretronecine
7-senecioyl-9-(2-
methyl-2,3-dihyroxy-
butyryl)retronecine
7-senecioyl-9-(2-
methy-2-hydroxy-3-
acetoxybutyryl)
retronecine
retronecine
2-senecioyl-(-)-
macronecine
9-senecioyl-(-)-
macronecine
senecicaudatine-9-
senecioate
senecidaudatine-9-
isovalerate
norsenecicaudatine-
9-sencioate
senecicaudatinal
semiacetal
Senecio chrysanthemoides seneciphylline B 166
------------------------------------------------------------------------------------------
APPENDIX II Table 1. (contd.)
------------------------------------------------------------------------------------------
Plant Constituent alkaloids Plant part Referencea
------------------------------------------------------------------------------------------
Compositae (contd.)
Senecio cineraria DC. jacobine aerial B 153, 167
senecionine seed B 168
seneciphylline aerial B 169
otosenine aerial B 170
retrorsine aerial B 171
Senecio congestus (R. Br.) senecionine C Roder et al.
DC. [syn. Senecio palustris neoplatyphylline (1982b)
(L.) Hooker, Senecio platyphylline
tubicaulis Mansfeld)
Senecio cruentus DC. senecionine C Asada et al.
seneciphylline (1982b)
retrorsine
riddelliine
Senecio cymbaroides senecionine whole B 420
seneciphylline
riddelliine
retrorsine
Senecio desfonainei Druce senecionine aerial B 173, 145
otosenine
riddelliine
seneciphylline aerial B 146
retrorsine A Rizk et al. (1983)
senkirkine
angelylretronecine
Senecio discolor DC. retrorsine leaf B 174
senecionine aerial B 175
Senecio dolichodoryius 15,20-dihyroxyeruci- aerial A Bohlmann et al.
Cuatr. foline (1986)
Senecio doronicum L. bulgarsenine leaf B 176
doronenine
Senecio douglasii DC. retrorsine whole B 149, 150, 151
riddelliine
seneciphylline
senecionine
Senecio durieui Gay integerrimine whole B 157
Senecio eremophilus senecionine aerial B 149, 150, 151
Richards seneciphylline
retrorsine
riddelliine
------------------------------------------------------------------------------------------
APPENDIX II Table 1. (contd.)
------------------------------------------------------------------------------------------
Plant Constituent alkaloids Plant part Referencea
------------------------------------------------------------------------------------------
Compositae (contd.)
Senecio erraticus Berthol. senecionine aerial B 177
otosenine
floridanine
Senecio erraticus Berthol. senecionine B 178, 179, 180
subsp. barbaraeifolius Krock otosenine
erucifoline
seneciphylline leaf B 181
integerrimine aerial B 182
Senecio erucifolius L. senecionine aerial B 153
seneciphylline aerial B 183, 180
erucifoline
retrorsine aerial B 184
Senecio faberi Hemsl. integerrimine C Wei et al. (1982)
Senecio filaginoides senecionine root A Pestchanker &
(H. et A.) DC. retrorsine Giordano (1986)
Senecio fistulosus senecionine A Gonzalez et al.
Poepp. ex Less. (1986b)
Senecio fluviatilis Wallr. seneciphylline aerial B 185
otosenine
florosenine
Senecio formosus integerrimine aerial B 186
retrorsine
Senecio fremontii Torr. seneciphylline whole B 148
et A. Gray senecionine
Senecio gilliesiano senecionine root A Guidugli et al.
retrorsine (1986)
Senecio glabellus senecionine whole B 187
(Turcz.) DC.
Senecio glaberrimus DC. retrorsine aerial B 153
Senecio glandulosus integerrimine root A Pestchanker et al.
Don ex Hook. et Arn. retrorsine (1985b)
usaramine
Senecio graminifolius retrorsine aerial B 188
N.J. Jacq. graminifoline
------------------------------------------------------------------------------------------
APPENDIX II Table 1. (contd.)
------------------------------------------------------------------------------------------
Plant Constituent alkaloids Plant part Referencea
------------------------------------------------------------------------------------------
Compositae (contd.)
Senecio grandifolia Jaqu. platyphylline root, leaf, B 189
seneciphylline stem
Senecio grandifolius Less. senkirkine aerial A Bohlmann et al.
neosenkirkine (1986)
Senecio griesbachii Baker retrorsine aerial B 190
Senecio ilicifolius Thunb. senecionine aerial B 191, 192, 188, 127
seneciphylline
retrorsine
Senecio illinitus Phill. senkirkine aerial A Gonzalez et al.
O-acetylsenkirkine (1986a)
senecionine
Senecio inaequidens DC. retrorsine A Roder et al.
senecionine (1981)
senecivernine aerial A Bicchi et al.
integerrimine (1985)
Senecio incanus L. seneciphylline aerial B 147
subsp. carniolicus integerrimine
(Willd.) Br.-Bl.
Senecio integerrimus Nutt. integerrimine aerial B 156
senecionine
neoplatyphylline whole B 420
platyphylline
Senecio isatideus DC. retrorsine aerial B 153, 193
Senecio jacobaea L. seneciphylline aerial B 194, 195
senecionine B 196, 197
jacobine B 153, 197, 198
jaconine B 199, 200, 201
jacozine
otosenine aerial B 51
senkirkine
retrorsine aerial B 184
Senecio kirkii Hook. f. senkirkine bark, leaf B 203
ex Kirk O-acetylsenkirkine leaf B 204
Senecio kleinia Sch. Bip. integerrimine stem B 205
senkirkine stem B 206
Senecio krylovii seneciphylline aerial B 207
------------------------------------------------------------------------------------------
APPENDIX II Table 1. (contd.)
------------------------------------------------------------------------------------------
Plant Constituent alkaloids Plant part Referencea
------------------------------------------------------------------------------------------
Compositae (contd.)
Senecio kubensis Grossh. seneciphylline aerial B 208
Senecio lampsanoides seneciphylline aerial, root B 209, 210
Senecio laricifolius senecionine aerial A Bohlmann et al.
H.B.K. seneciphylline (1986)
senkirkine
19-hydroxysenkirkine
19-acetoxysenkirkine
Senecio latifolius DC. retrorsine aerial B 211, 212
(syn. Senecio sceleratus seneciphylline aerial B 213
Schweikerdt) platyphylline
sceleratine aerial B 260
chlorodeoxysceleratine aerial B 261;
(merenskine) C Bredenkamp et al.
(1985)
Senecio leucostachys Baker senecionine root A Pestchanker &
Giordano (1986)
Senecio longilobus Benth. seneciphylline whole B 156, 214, 150
retrorsine B 151
riddelliine whole B 149
Senecio magnificus F. Muell. senecionine aerial B 215
integerrimine B 216
Senecio megaphyllus Green. 13,19-epoxyseneci- aerial A Bohlmann et al.
phylline (1986)
13,19-epoxysparti-
oidine
Senecio minimus Poir seneciphylline aerial B 158
------------------------------------------------------------------------------------------
APPENDIX II Table 1. (contd.)
------------------------------------------------------------------------------------------
Plant Constituent alkaloids Plant part Referencea
------------------------------------------------------------------------------------------
Compositae (contd.)
Senecio morrisonensis Hayata integerrimine whole B 217
Senecio multilobatus senecionine A McCoy et al.
(1983)
Senecio multivenius seneciphylline aerial A Bohlmann et al.
Benth. in Oerst. senecionine (1986)
Senecio nebrodensis L. integerrimine whole B 218
var sicula senecionine
Senecio nemorensis L. bulgarsenine leaf B 219
var bulgaricus retrosisosenine
(Vel) Stoj. nemorensine
Senecio nemorensis L. ssp. fuchsisenecionine B 362, 363, 364, 365
fuchsii Gmel.
senecionine B 423
Senecio nemorensis L. nemorensine aerial, root B 371
subdecurrens Griseb. retroisosenine aerial B 422
bulgarsenine
Senecio othonnae Bieb. otosenine aerial, root B 220
onetine root B 221
seneciphylline
floridanine aerial, root B 222
doronine aerial B 223
Senecio othonniformis bisline aerial B 22, 225
Fourcade isoline
Senecio palmatus Pall. seneciphylline root B 226
Senecio paludosus L. seneciphylline root, aerial B 153, 227, 228
Senecio pampeanus Cabrera senecionine aerial B 229
Senecio pancicii Degen senecionine whole C Jizba et al.
var arnautorum (Velen.) seneciphylline (1982)
Stoj., Stef. et Kit.
Senecio pancicii Degen senecionine whole C Jizba et al.
var pancicii (1982)
Senecio patagonicus senecionine aerial A Villaroel et al.
Hook. and Arn. seneciphylline (1985)
------------------------------------------------------------------------------------------
APPENDIX II Table 1. (contd.)
------------------------------------------------------------------------------------------
Plant Constituent alkaloids Plant part Referencea
------------------------------------------------------------------------------------------
Compositae (contd.)
Senecio paucicalyculatus paucicaline whole B 230
(Klatt.) retrorsine
Senecio paucifolius seneciphylline B 231
S.G. Gmel.
Senecio petasitis DC. senecionine leaf B 146
bisline (?) aerial B 232
Senecio phillipicus retrorsine aerial A Gonzalez et al.
Rogel et Koern. (1986a)
Senecio pierotii neosenkirkine aerial, root C Asada & Furuya
senkirkine (1982)
Senecio pimpinellifolius senecionine aerial A Bohlmann et al.
H.B.K. (1986)
Senecio platyphylloides platyphylline root B 233, 234, 235
Somm. et Lev. seneciphylline
Senecio platyphyllus platyphylline root, aerial B 236, 237, 238
(Bieb.) DC. seneciphylline leaf B 239
neoplatyphylline root B 240, 241
sarracine root B 242
Senecio pojarkovae sarracine root B 243
seneciphylline
Senecio procerus L. senkirkine aerial, root B 244
var procerus procerine
Stoj. Stef. et Kit.
Senecio propinquus Ait. seneciphylline aerial, root B 209, 245
Senecio pseudo-arnica Less. senecionine aerial B 156
Senecio pterophorus senecionine aerial B 192, 188, 127
seneciphylline
retrorsine
rosmarinine
acetylseneciphylline
Senecio quadridentatus senecionine aerial B 247
Labill. (syn. Erechtites seneciphylline
quadridentata DC.) retrorsine
------------------------------------------------------------------------------------------
APPENDIX II Table 1. (contd.)
------------------------------------------------------------------------------------------
Plant Constituent alkaloids Plant part Referencea
------------------------------------------------------------------------------------------
Compositae (contd.)
Senecio quebradensis senkirkine aerial A Bohlmann et al.
Greenm. florosenine (1986)
Senecio racemosus DC. seneciphylline root B 248
Senecio renardii Winkl. seneciphylline aerial B 249, 250
senkirkine (renardine)
otosenine
Senecio retrorsus DC. retrorsine aerial B 194, 193
Senecio rhombifolius sarracine root B 251, 233
(Willd.) Sch. Bip. platyphylline aerial, root B 208
seneciphylline
neoplatyphylline
Senecio riddellii riddelliine aerial B 156
Torr. et A. Gray whole B 252, 253
retrorsine B 420
Senecio riddellii retrorsine whole B 149
Torr. et A. Gray riddelliine
var. parksii (Cory)
Senecio ruderalis Harvey retrorsine aerial B 254
Senecio ruwenzoriensis ruwenine whole B 255
S. Moore ruzorine
Senecio sandrasicus senecionine A Temizer et al.
(1985)
Senecio scandens senecionine whole B 256
seneciphylline
Senecio seratophiloides senecionine root A Pestchanker &
Griseb. senecivernine Giordano (1986)
usaramine
retrorsine
uspallatine
Senecio spartioides seneciphylline aerial B 156, 262
Torr. et A. Gray senecionine
spartiodine
riddelliine whole B 420
retrorsine
------------------------------------------------------------------------------------------
APPENDIX II Table 1. (contd.)
------------------------------------------------------------------------------------------
Plant Constituent alkaloids Plant part Referencea
------------------------------------------------------------------------------------------
Compositae (contd.)
Senecio spathulatus senecionine aerial, root B 158
A. Rich. integerrimine
seneciphylline B 263
Senecio squalidus L. senecionine aerial B 153, 264, 265
integerrimine B 205
Senecio stenocephalus seneciphylline aerial B 266
Maxim.
Senecio subalpinus senecionine leaf B 267
C. Koch. seneciphylline aerial B 147
integerrimine
Senecio subulatus dihydroretrosine root A Pestchanker et
Don ex Hook. retrorsine al. (1985b)
et Arn. var. erectus senecionine
Senecio swaziensis retrorsine aerial B 268, 269, 270
Compton swazine
Senecio tenuifolius senecionine aerial C Bhakuni & Gupta
Burm. integerrimine (1982)
senkirkine
o-acetylsenkirkine
Senecio tomentosus senecionine aerial B 271, 179
otosenine
(tomentosine)
Senecio triangularis senecionine aerial B 272
Hook. integerrimine C Roitman (1983b)
platyphylline
rosmarinine
retrorsine
triangularine
neotriangularine
7-angelylretronecine whole C Rueger & Benn
7-senecioylretrone- (1973)
cine
7-angelyl-9-sarra-
cinylretronecine
7-senecioyl-9-sarra-
cinylretronecine
Senecio uintahensis senkirkine whole B 420
senecionine
------------------------------------------------------------------------------------------
APPENDIX II Table 1. (contd.)
------------------------------------------------------------------------------------------
Plant Constituent alkaloids Plant part Referencea
------------------------------------------------------------------------------------------
Compositae (contd.)
Senecio umgeniensis 7-senecioyl-9-sarra- aerial A Bohlmann et al.
Thell. cinylretronecine (1986)
Senecio usgorensis 13,19-epoxyseneci- aerial A Bohlmann et al.
Cuatr. phylline (1986)
Senecio uspallatensis retrorsine root A Pestchanker et
uspallatine al. (1985a)
Senecio variabilis Sch. 7-senecioylretrone- aerial, root A Bohlmann et al.
Bip. cine (1986)
9-senecioylretrone-
cine
7-senecioyl-9-sarra-
cinylretronecine
Senecio venosus Harvey retrorsine aerial B 153
Senecio vernalis Walst. retrorsine aerial B 273, 274
et Kit. senecionine
senkirkine
senecivernine
integerrimine A Sener et al.
seneciphylline (1986)
riddelliine
retronecine
Senecio viscosus L. senecionine aerial B 264, 153
integerrimine aerial B 182
Senecio vulgaris L. senecionine aerial B 275, 264, 153
seneciphylline aerial B 155, 276, 277
retrorsine
riddelliine whole B 420
integerrimine A Pieters &
spartioidine Vlietinck (1986)
usaramine
Senecio senecionine whole B 420
werneriaefolius retrorsine
Syneilesis palmata syneilesine aerial, root B 278, 279
Maxim. acetylsyneilesine
senecionine
------------------------------------------------------------------------------------------
APPENDIX II Table 1. (contd.)
------------------------------------------------------------------------------------------
Plant Constituent alkaloids Plant part Referencea
------------------------------------------------------------------------------------------
Compositae (contd.)
Tussilago farfara L. senkirkine flower B 18, 425
leaf, stem C Rosberger et al.
(1981)
senecionine leaf, stem A Luthy et al.
(1980)
tussilagine C Roder et al.
(1981b)
Leguminosae
Crotalaria aegyptica crosemperine B 419
Benth. monocrotaline
7beta-hydroxy-1- B 426
methylene-8alpha-
pyrrolizidine
Crotalaria agatiflora maduraensine aerial B 280
Schweinf. anacrotine aerial B 281
7-acetylmadurensine
6-acetylanacrotine
7-acetyl-cis-
madurensine
6-acetyl-trans-
anacrotine
crotaflorine
6-angelyl-trans-
anacrotine
Crotalaria assamica Benth. monocrotaline B 285, 286
Crotalaria axillaris Ait. axillarine seed B 287, 288
axillaridine
Crotalaria barbata R. Graham crobarbatine seed B 289
ex R. Wight et Walk.-Arn.
Crotalaria berteroana DC. fulvine aerial B 297
(syn. Crotalaria fulva Roxb.)
Crotalaria brevidens integerrimine seed B 304
Benth. var. intermedia usaramine
(Kotschy) Polhill(syn.
Crotalaria intermedia
Kotschy)
Crotalaria breviflora DC. integerrimine seed B 290, 291
usaramine
------------------------------------------------------------------------------------------
APPENDIX II Table 1. (contd.)
------------------------------------------------------------------------------------------
Plant Constituent alkaloids Plant part Referencea
------------------------------------------------------------------------------------------
Leguminosae (contd.)
Crotalaria burhia Ham. crotalarine aerial B 292, 293
ex Benth. monocrotaline
Crotalaria candicans crocandine seed B 380
W. and A. isocorcandine
isocromadurine seed C Suri et al.
crispatine (1982)
turneforcidine
cropodine seed C Haksar et al.
(1982)
Crotalaria cephalotes Steud. monocrotaline seed C Pilbeam et al.
ex A. Rich (1983)
Crotalaria crispata monocrotaline whole B 294
F. Muell. ex Benth. fulvine
crispatine
Crotalaria cunninghamii monocrotaline seed C Pilbeam et al.
R. Br. (1983)
Crotalaria dura dicrotaline aerial B 295, 296
J.M. Wood et Evans
Crotalaria fulva Roxb.
(see Crotalaria
berteroana DC.)
Crotalaria globifera E. Mey dicrotaline aerial B 295, 296
globiferine seed C Brown et al.
grantianine (1984)
grantaline
trichodesmine
Crotalaria grahamiana monocrotaline seed B 298
Wight & Arn. grahamine seed B 299
monocrotalinine whole B 300
Crotalaria incana L. integerrimine seed B 187
anacrotine aerial B 302
usaramine seed B 303
Crotalaria juncea L. senecionine seed B 305, 306, 307
seneciphylline
riddelliine
trichodesmine
junceine
------------------------------------------------------------------------------------------
APPENDIX II Table 1. (contd.)
------------------------------------------------------------------------------------------
Plant Constituent alkaloids Plant part Referencea
------------------------------------------------------------------------------------------
Leguminosae (contd.)
Crotalaria laburnifolia L. anacrotine seed B 308, 309, 310,
(crotalaburnine) 291, 311
Crotalaria laburnifolia L. madurensine aerial B 312
subsp. eldomae anacrotine
senkirkine
hydroxysenkirkine
crotafoline
Crotalaria leschenaultii monocrotaline seed B 313
Crotalaria leiloba Bartl. monocrotaline seed B 314
(syn. Crotalaria
ferruginea Wall.)
Crotalaria madurensis madurensine seed, flower, B 280
R. Wight leaf
crispatine aerial B 315
fulvine
cromadurine seed B 62, 316
isocromadurine seed B 317
Crotalaria micans Link. 1-methylenepyrroliz- seed B 282, 283
(syn. Crotalaria anagyroides idine seed B 284
Humb. et al.) senecionine B 280
anacrotine
Crotalaria mitchellii Benth. monocrotaline whole B 318
retusamine whole
Crotalaria mitchellii Benth. retusamine whole B 318
subsp. laevis A. Lee,
published as
"sp. aff. mitchellii")
Crotalaria mysorensis Roth. monocrotaline seed B 319
Crotalaria nana Burm. crotananine seed B 320
cronaburmine seed B 427
Crotalaria nitens Kunth. monocrotaline A Hoet et al.
(1981)
Crotalaria novae-hollandiae monocrotaline whole B 318
DC. subsp. lasiophylla retusamine
(Benth) A. Lee
------------------------------------------------------------------------------------------
APPENDIX II Table 1. (contd.)
------------------------------------------------------------------------------------------
Plant Constituent alkaloids Plant part Referencea
------------------------------------------------------------------------------------------
Leguminosae (contd.)
Crotalaria novae-hollandiae retusamine seed B 318
DC. subsp. novae-hollandiae
(syn. Crotalaria
crassipes Hook.)
Crotalaria pallida Ait. usaramine seed B 291
(syn. Crotalaria mucronata, nilgirine seed B 322
Crotalaria striata) crotastriatine seed B 323, 324
Crotalaria paniculata Willd. fulvine seed B 325
Crotalaria paulina Schrank monocrotaline seed C Pilbeam et al.
(1983)
Crotalaria quinquefolia L. monocrotaline seed C Pilbeam et al.
(1983)
Crotalaria recta Steud. monocrotaline aerial B 326
ex A. Rich
Crotalaria retusa L. monocrotaline seed B 327
retusine seed, aerial B 328
retusamine
retronecine
Crotalaria sagittalis L. monocrotaline seed B 330
Crotalaria scassellatii axillaridine seed A Wiedenfeld et
Chiov. axillarine al. (1985)
deoxyaxillarine
Crotalaria semperflorens crosemperine seed B 331
Vent.
Crotalaria spartioides DC. retrorsine aerial B 334
Crotalaria spectabilis Roth. monocrotaline seed B 335, 227
(syn. Crotalaria sericea spectabiline seed, whole B 336
Retz)
Crotalaria stipularia Desv. monocrotaline seed B 314
Crotalaria tetragona Roxb. integerrimine seed B 314
trichodesmine
Crotalaria verrucosa L. anacrotine seed B 338
crotaverrine seed B 339
acetylcrotaverrine
------------------------------------------------------------------------------------------
APPENDIX II Table 1. (contd.)
------------------------------------------------------------------------------------------
Plant Constituent alkaloids Plant part Referencea
------------------------------------------------------------------------------------------
Leguminosae (contd.)
Crotalaria virgulata grantianine seed B 301, 259
subsp. grantiana (Harv.) grantaline C Smith & Culvenor
Polhill (syn. Crotalaria 1-hydroxymethyl- (1984)
grantiana Harv.) 1beta,2beta-epoxy-
pyrrolizidine
Crotalaria walkeri Arn. crotaverrine seed B 340
acetylcrotaverrine
Crotalaria wightiana Grah. junceine seed B 329
ex Wight & Arn. (syn. trichodesmine
Crotalaria rubiginosa Willd.
var. wightiana J.G. Baker)
Crotalaria zanzibarica integerrimine seed B 187
Benth. (syn. Crotalaria usaramine seed B 337
usaramoensis E.G. Baker) senecionine
retrorsine
Ranunculaceae
Caltha biflora DC. senecionine aerial B 341
Caltha leptosepala DC. senecionine aerial, root B 341
Scrophulariaceae
Castilleja rhexifolia senecionine B 342
Rydb. sarracine C Roby & Stermitz
indicine or isomer (1984)
------------------------------------------------------------------------------------------
a A = References in the reference list of this document.
B = References in Smith & Culvenor (1981), J. nat. Prod., 44: 129-152 (with reference
number).
C = References in Mattocks (1986), Chemistry and toxicology of pyrrolizidine alkaloids.
APPENDIX II
Table 2. Plants containing known alkaloids that are non-hepatotoxic (aminoalcohols and esters)
------------------------------------------------------------------------------------------
Plant Constituent alkaloids Plant part Referencea
------------------------------------------------------------------------------------------
A. Families in which hepatotoxic alkaloids also occur
Apocynaceae
Alafia multiflora alafine seed B 343
Anodendron affine Druce alloanodendrine aerial B 344, 345
anodendrin
Boraginaceae
Caccinea glauca Savi 7,9-dibenzoylretrone- fl B 346
cine
Ehretia aspera Willd. ehretinine leaf A Suri et al.
(1980)
Heliotropium angiospermum 1-hydroxymethyl- whole C Birecka et al.
Murray 1beta,2beta-epoxy- (1983)
pyrrolizidine
Heliotropium ovalifolium heliofoline whole C Mohanraj et al.
Forsk retronecine (1981)
Heliotropium spathulatum acetylcurassavine aerial A Birecka et al.
Rydb. curassavine (1980)
Heliotropium strigosum Willd. strigosine aerial B 347
Lindelofia macrostyla (Bunge) lindelofine aerial B 348
M. Pop. (syn. Lindelofia lindelofamine
anchusoides,Paracaryum
heliocarpum Kern.)
Lindelofia olgae (Regel et viridiflorine aerial B 94
Smirnov) Brand
Lindelofia pterocarpa (Rupr.) viridiflorine aerial B 93
M. Pop.
Macrotomia echioides Boiss. macrotomine aerial B 350
Paracaryum himalayense viridiflorine aerial B 93
(Klotsch) C.B. Clark
Tournefortia sibirica L. turneforcine aerial B 351
Trachelanthus hissoricus viridiflorine leaf B 352
Lipsky trachelanthamine
------------------------------------------------------------------------------------------
APPENDIX II Table 2. (contd.)
------------------------------------------------------------------------------------------
Plant Constituent alkaloids Plant part Referencea
------------------------------------------------------------------------------------------
Boraginaceae (contd.)
Trachelanthus korolkovii trachelanthamine aerial B 353, 354, 355, 94
(Lipsky) B. Fedtsch.
Celastraceae
Bhesa archboldiana 9-angelylretronecine bk B 356
(Merr. & Perry) Ding Hou
[syn. Kurrimia archboldiana
(Merr. & Perry)]
Compositae
Adenostyles rhombifolius sarracine aerial B 116
(Willd.) M. Pimen. ssp.
rhombifolia chemovar.
sarracinifera
Adenostyles rhombifolius platyphylline aerial B 116
(Willd.) M. Pimen. ssp.
rhombifolia chemovar.
platyphyllinifera
Cacalia hastata L. hastacine root B 357, 241
Cacalia robusta hastacine B 358
Senecio amphibolus macrophylline aerial B 359
Senecio angulatus L. angularine whole B 360
rosmarinine
Senecio aronicoides hygrophylline whole B 420
Senecio brachypodus DC. rosmarinine aerial, root B 361
Senecio francheti Winkl. sarracine aerial B 352
franchetine
Senecio glastifolius sarracine A Mortimer & White
(1975)
Senecio hygrophyllus R.A. platyphylline aerial B 366, 361
Dyer et C.A. Smith (syn. rosmarinine
Senecio adnatus DC.) hygrophylline
Senecio macrophyllus Bieb. macrophylline aerial B 368
Senecio mikanioides Otto. ex sarracine aerial B 155, 369, 370
Walp.
------------------------------------------------------------------------------------------
APPENDIX II Table 2. (contd.)
------------------------------------------------------------------------------------------
Plant Constituent alkaloids Plant part Referencea
------------------------------------------------------------------------------------------
Compositae (contd.)
Senecio nemorensis L. ssp. nemorensine aerial B 371
fuchsii var. nova (Zlatnik)
Senecio nemorensis L. ssp. nemorensine aerial B 371
jaquinianus (Rchb.) Durand
Senicio ovirensis ssp. angelylheliotridine A Roder et al.
gaudinil (1980)
Senecio pauciligulatus rosmarinine aerial B 361
Dyer et Sm.
Senecio rivularis DC. 7-angelylheliotridine aerial B 182, 135
Senecio rosmarinifolius Linn. rosmarinine aerial B 191, 192, 188
Senecio salignus DC. 7-angelylheliotridine aerial A Bohlmann et al.
(1986)
Senecio sarracenius L. sarracine aerial B 153, 372, 373
Senecio schvetsovii Korsh macrophylline aerial B 231
Senecio sylvaticus L. silvasenecine aerial B 362, 153
sarracine aerial B 374
Senecio taiwanensis Hayata rosmarinine aerial B 217
Senecio tournefortii Lap. platyphylline aerial B 375
Leguminosae
Crotalaria albida Heyne ex croalbidine aerial B 376, 377
Roth (syn. Crotalaria montana
Roxb.)
Crotalaria aridicola Domin. 1-methoxymethyl-1,2- aerial B 378
dehydropyrrolizidine
7beta-hydroxy-1-
methoxymethyl-1,2-
dehydropyrrolizidine
7beta-acetoxy-1- whole B 379
methoxymethyl-1,2-
dehydropyrrolizidine
Crotalaria damarensis Engl. 1-methylenepyrrolizi- whole, seed B 381, 283
dine
------------------------------------------------------------------------------------------
APPENDIX II Table 2. (contd.)
------------------------------------------------------------------------------------------
Plant Constituent alkaloids Plant part Referencea
------------------------------------------------------------------------------------------
Leguminosae (contd.)
Crotalaria goreensis Guill. 7beta-hydroxy-1- aerial, seed B 382
et Perr. methylene-8beta-
pyrrolizidine
7beta-hydroxy-1-
methylene-8alpha-
pyrrolizidine
Crotalaria grandistipulata 1-methylenepyrrolizi- seed B 434
Harms dine
Crotalaria lachnophora 1-methylenepyrrolizi- seed B 434
A. Rich dine
Crotalaria maypurensis Humb 7beta-hydroxy-1- aerial B 383
et al. methylene-8beta-
pyrrolizidine
7beta-hydroxy-1-
methylene-8alpha-
pyrrolizidine
Crotalaria medicaginea Lam. 1-methoxymethyl-1,2- whole, seed B 378
dehydropyrrolizidine
7beta-hydroxy-1-
methoxymethyl-1,2-
dehydropyrrolizidine
1alpha-methoxymethyl- whole, seed
1beta,2beta-
epoxypyrrolizidine
7alpha-hydroxy-1- seed
methoxymethyl-1,2-
dehydropyrrolizidine
1alpha-hydroxymethyl- aerial
1beta,2beta-
epoxypyrrolizidine
Crotalaria natalitia Meissner 1-methylenepyrrolizi- seed B 434
dine
Crotalaria podocarpa DC. 7-hydroxy-1-methylene- seed B 435
pyrrolizidine
Crotalaria stolzii (Bak. f.) 1-methylenepyrrolizi- seed B 434
Milne-Redh. ex Polhill dine
Ranunculacae laburnum L. laburnine seed B 387, 388, 389
1-hydroxymethyl-7-e B 390
hydroxypyrrolizidin
------------------------------------------------------------------------------------------
APPENDIX II Table 2. (contd.)
------------------------------------------------------------------------------------------
Plant Constituent alkaloids Plant part Referencea
------------------------------------------------------------------------------------------
Scrophulariacea
Castilleja "rhexifolia aff. sarracine C Roby & Stermitz
miniata" 7-angelylplatynecine (1984)
8-angelylplatynecine
B. Families in which hepatotoxic alkaloids are not known to occur
Orchidaceae
Chysis bractescens Lindl. 1alpha-methoxycar- whole B 391, 392
bonyl-8beta-
pyrrolizidine
1alpha-ethoxycar-
bonyl-8beta-
pyrrolizidine
Doritis pulcherrima phalaenopsine La or T whole B 393
(syn. Phalaenopsis esmerelda)
Hammarbya paludosa (L.) O.K. paludosine whole B 394
hammarbine whole B 395
Kingiella taenialis (Lindl.) phalaenopsine La whole B 396
Rolfe
Liparis auriculata (Blume) auriculine whole B 397
Liparis bicallosa Schltr. laburnine whole B 397
malaxine whole B 398, 399
Liparis hachijoensis Nakai laburnine whole B 397
malaxine whole B 398
Liparis keitaoensis Hay. keitaoine whole B 395
keitine
Liparis kumokiri F. Maekawa kumokirine whole B 400, 398
Liparis loeselii (L.) L.C. auriculine whole B 394
Rich
Liparis nervosa Lindl. nervosine whole B 398, 401
Malaxis congesta comb. nov. malaxin whole B 402
(Rchb. f.)
Malaxis grandifolia Schltr. grandifoline whole B 403
Phalaenopsis amabilis Bl. phalaenopsine T whole B 404, 405, 393
Phalaenopsis amboinensis phalaenopsine La whole B 393
------------------------------------------------------------------------------------------
APPENDIX II Table 2. (contd.)
------------------------------------------------------------------------------------------
Plant Constituent alkaloids Plant part Referencea
------------------------------------------------------------------------------------------
Orchidaceae (contd.)
Phalaenopsis aphrodite phalaenopsine T whole B 393
Phalaenopsis cornu-cervi cornucervine whole B 404, 393
Rchb. f.
Phalaenopsis equestris phalaenopsin ls whole B 393
Rchb. f. phalaenopsin T
Phalaenopsis fimbriata phalaenopsine T whole B 393
Phalaenopsis hieroglyfica phalaenopsine T or La whole B 393
Phalaenopsis lueddemanniana phalaenopsine T or La whole B 393
Phalaenopsis mannii Rchb. f. phalaenopsine La whole B 393
Phalaenopsis sanderiana phalaenopsine La whole B 393
Rchb. f. phalaenopsine T
Phalaenopsis schilleriana phalaenopsine La whole B 393
Phalaenopsis stuartiana phalaenopsine La whole B 393
Rchb. f. phalaenopsine T
Phalaenopsis sumatrana phalaenopsis La whole B 393
Phalaenopsis violacea phalaenopsis La or T whole B 393
Vanda cristata Lindl. acetyllaburnine whole B 407
Vanda helvola Bl. laburnine whole B 408
acetyllaburnine
Vanda hindsii Lindl. acetyllaburnine whole B 408
Vanda luzonica Loher acetyllaburnine whole B 408
Vandopsis gigantea Pfitz. laburnine whole B 408
lindelofidine
acetyllaburnine
acetyllindelofidine
Vandopsis lissochiloides laburnine whole B 408
Pfitz. lindelofidine
acetyllaburnine
acetyllindelofidine
------------------------------------------------------------------------------------------
APPENDIX II Table 2. (contd.)
------------------------------------------------------------------------------------------
Plant Constituent alkaloids Plant part Referencea
------------------------------------------------------------------------------------------
Rhizophoraceae
Cassipourea gummiflua Tulasne cassipourine stem, leaf B 409
var. verticellata Lewis bk B 410
Santalaceae
Thesium minkwitzianum thesine aerial B 411
B. Fedtsch. thesinine B 412, 413
thesinicine
isoretronecanol root
Sapotaceae
Mimusops elengi L. 1-hydroxymethyl- B 414
pyrrolizidine
tiglate
Planchonella anteridifera planchonelline leaf B 415
(C.T. White et W.D. Francis) tiglyllaburnine
H.J. Lamb benzoyllaburnine
Planchonella thyrsoidea C.T. planchonelline leaf B 415
White ex F.S. Walker tiglyllaburnine
benzoyllaburnine
Planchonella sp. (NGF 24722) trans-beta-thio- leaf B 416
acrylyl-(-)-iso-
retronecanol
tiglylisoretronecanol
------------------------------------------------------------------------------------------
a A = References in the reference list of this document.
B = References in Smith & Culvenor (1981), J. nat. Prod., 44: 129-152 (with reference
number).
C = References in Mattocks (1986), Chemistry and toxicology of pyrrolizidine alkaloids.
The onset of the disease may be sudden (acute) or insidious
(subacute or chronic). The acute disease may recover completely or
result in death. A few patients may go on to the subacute phase,
with almost none or very few symptoms, but a persistent
hepatomegaly. The patient may subsequently recover completely, or
may, after or without apparent clinical improvement, go on to the
chronic phase of disease, mostly ending up in cirrhosis. Some
patients with the acute disease may go on to the subacute phase,
even after clinical recovery, or as postulated by the authors,
after a latent period of several years, progress to cirrhosis.
Braginskii & Bobokhadzaev (1965) related an experience
concerning the evolution of this disease that was similar to that
observed in the USSR. About 50 - 60% of cases made a full recovery
and 35% made a partial recovery with continuing hepatomegaly.
About 2% of cases developed persistent ascites with "loss of
working capacity". It has been suggested that these cases may
develop cirrhosis.
7.2 Salient Pathological Features of Veno-Occlusive Disease
The pathological features of the disease at different stages
have been described in detail (Terekhov, 1939, 1952; Dolinskaya,
1952; Bras et al., 1954; Bras & Watler, 1955; Bras & Hill, 1956;
Stuart & Bras, 1957; Stirling et al., 1962; Aikat et al., 1978;
Tandon, B.N. et al., 1978; Tandon, H.D. et al., 1978). The
following description of Bras & Watler (1955) characterizes the
morphological changes at different stages of the disease described
by most investigators.
Morphological features of the liver at autopsy in 19 patients
from Jamaica who died at various stages of VOD have been described.
The ages ranged from 10 months to 45 years. Fourteen patients were
below the age of 14 years. Of the 14 patients, 9 had developed
cirrhosis, 3 died of acute disease, and 7 had various levels of
fibrosis superimposed over acute VOD. In the acute stages, there
was acute centrilobular congestion. The centrilobular and
sublobular veins showed different degrees of thickening of the wall
and occlusion of the lumen, mainly due to subintimal swelling
composed of loose reticular tissues and a few cells including
endothelial cells, occasional histocytes, lymphocytes, and
polymorphs, suggesting an acute exudative process. In addition,
there was a small amount of fibrin. Organization of this exudate
gradually led to collagenization and thickening of the wall. The
centrilobular congestion resulted in compression and even
disappearance of liver cell cords. The reticular framework of the
liver lobule was frequently preserved but was ruptured at places.
Clearly recognizable thrombi occurred sporadically but were not
commonly seen. The portal veins and hepatic arteries were normal.
Stirling et al. (1962) in their description of the early lesion
of VOD of the liver also emphasized that thrombosis of the hepatic
veins was not an important histogenetic factor in the evolution of
the lesion. In the later stages of the disease, hepatic venous
occlusion was chronically established. Perivenous reticular
collapse and the resultant condensation and reduplication of the
reticular framework resulted in non-portal fibrosis and later
cirrhosis. Macroscopically, the cirrhosis looked like Laennec's
cirrhosis. The cirrhotic process, which was non-portal to begin
with, involved portal tracts which also became incorporated in the
connective tissue septa. The presence of oesophageal varices was a
common finding. Extra-hepatic collateral vessels and congestive
splenomegaly were frequently seen. Within the liver, intrahepatic
collateral circulation was established with the coalescence of
sinusoids. The larger hepatic veins and inferior vena cava did not
show any thrombi or other pathological change. The subintimal
swelling observed in the hepatic veins was not seen in any other
vessels in the body. The authors concluded that acute VOD
gradually leads to Laennec's cirrhosis, but, in the initial phases,
it is non-portal.
No clear dose or temporal relationships between the liver and
parenchymal and vascular changes in the liver lobules are evident
from the available human case reports.
It is notable that megalocytosis, which is the hallmark of
chronic PA toxicity in experimental animals and a morphological
manifestation of the antimitotic effect of PAs, has not been
observed in human subjects (Tandon, H.D. et al., 1978) (section
6.4.9).
Tandon H.D. (personal communication) has analysed all published
and unpublished observations including liver biopsies and autopsies
derived from 3 outbreaks of VOD of which two occurred in India
(Tandon, B.N. et al., 1976, 1977; Tandon, R.K. et al., 1976;
Tandon, H.D. et al., 1977) and one occurred in Afghanistan (Tandon,
B.N. et al., 1978; Tandon, H.D. et al., 1978). He commented on the
frequency with which the characteristic veno-occlusion may not be
seen in the needle biopsy in the acute phase of the disease, though
it is invariably observed in the autopsy material. Haemorrhages
may persist for up to 1 year after subsidence of acute symptoms.
There is no significant inflammation accompanying the veno-
occlusive changes. Cirrhosis was reported to have developed within
3 months in one patient, after an initial biopsy had shown no
fibrosis (Stuart & Bras, 1957).
Brooks et al (1970) made an ultrastructural study of the liver
in VOD in Jamaican children. They found extensive damage in the
sinusoidal epithelium resulting in entra-vasation of red cells in
Disse's space and between hepatocytes. The closed structure of the
vessels, the absence of fenestrations, the existence of a basement
membrane, and the presence of collagen in the wall contribute to
the resistance to cellular debris and erythrocytes which track up
Disse's space and tend to narrow the lumen of the sinusoid where it
enters the vein. Fibrin may be present in this location and
occasionally in the sinusoids, but not within hepatic veins
themselves. The venous block, therefore, does not appear to be the
result of thrombosis.
Pancreatic changes similar to those in Kwashiorkor were stated
to be commonly observed (Bras & Hill, 1956; Stuart & Bras, 1957).
7.3 Human Case Reports of Veno-Occlusive Disease
Available data on human cases are summarized in Table 15, with
the countries they have been reported from in alphabetical order.
The first report of this disease in man and its relationship with
consumption of wheat flour contaminated by seeds of a plant of the
genus Senecio was made by Albertjin in 1918 in a report made to the
government of South Africa, as quoted by Wilmot & Robertson (1920),
who gave the first account of this disease in scientific literature
from that country. It is possible that it may have existed even
earlier, as its occurrence in farm animals had been described since
the beginning of the century in veterinary literature (Bull et al.,
1968). Willmot & Robertson (1920) recorded the occurrence of
'bread poisoning' in South Africa for a period of about 10 years,
during which 80 cases had been observed, mostly resulting in death.
The detailed account of clinical data and pathological findings in
11 cases was consistent with what is known as VOD today. The flour
from which the bread was prepared was found to be contaminated with
the flower heads and seeds of Senecio spp.
Isolated case reports are available in the literature (Hashem,
1939; Wurm, 1939) describing changes in childrens' liver
characteristic of veno-occlusive disease; however, in these
studies, no mention was made of possible etiological agents.
A pattern of disease described in the Soviet literature as
dystrophy of the liver, and later as toxic hepatitis with ascites
has been known in the Central Asian republics of the USSR
especially Uzbekistan and Tadjikistan for a long time (Terekhov,
1952). The local inhabitants called the disease, characterized by
rapidly filling ascites, the "camel-belly" syndrome and are
reported to have long distrusted a weed with fine seeds, known
locally as "Kharmyk", as a source of the disease (Dubrovinskii,
1952) (it is notable that, in the Afghan outbreak, which occurred
close to the area in Uzbekistan where the disease had been known,
the toxic seeds were known as "Charmak" among the local
population). The "camel-belly" disease was associated with the use
of bread with a bitter taste (presumably caused by the mixing and
grinding of toxic seeds with the wheat grains). Two waves of
outbreaks occurred, the first between 1931-35 (Terekhov, 1939) and
the second between 1945 - 46 (Dubrovinskii, 1947; Ismailov,
1948a,b). In the first wave of outbreaks, approximately 1000 -
1500 subjects were estimated to have been affected with an overall
mortality rate of 13 - 15%. The age of the affected subjects
ranged from 3 to 50 years. In the second wave of outbreaks, 60 -
70% of the population in agricultural areas were estimated to have
been affected (Ismailov, 1948a,b). Domestic animals also suffered
from the disease (Dubrovinskii, 1947). During the second wave of
outbreaks, the disease was found to have been caused by
contamination of food crops with seeds of Heliotropium lasiocarpum
(Dubrovinskii, 1947; Khanin, 1948). The etiology was confirmed by
studies on a variety of experimental animals using the suspect seed
(Khanin, 1948; Kampanzev, 1952). Pathological features of the
disease in children have been described by Dolinskaya (1952) and in
all age groups by Terekhov (1939, 1952). First an acute form of
the disease was recognized, followed by hepatic cirrhosis, which
was found to result as a consequence of the initial disease
(Ismailov, 1948a,b; Savvina, 1952). The disease has since been
largely eradicated by the agricultural and public health measures
taken.
Table 15. Pyrrolizidine alkaloid poisoning and human reports of veno-occlusive disease (VOD)
(in alphabetical order of the countries reported from)
Country/ Age Suspected Name of Alkaloid Alkaloid Nature of Outcome Reference
number group vehicle of plant concentration lesion (cirrhosis/
cases intoxication and intake died)
Afghanistan
8000 <14-80 contaminated Heliotropium mainly 1.32-1.49 g/kg all stages; many died Tandon &
(approximately) years wheat flour popovii heliotrine, (in the mostly acute Tandon (1975);
gillianum in addition seeds) daily to subacute Mohabbat
to 1 or 2 intake, 2 mg; et al. (1976)
alkaloids total
similar to consumption,
lasiocarpine up to 1.46 g
(75 - 100%
as N-oxides)
Barbados
3 2-4 herbal Crotalaria not analysed acute lesions no details Stuart & Bras
years infusion retusa (1956)
China, People's Republic of
2 49-57 herbal Gynura not analysed acute lesions died Hou (1980)
years infusion segetum
(Compositae)
Ecuador
1 35 years herbal Crotalaria not analysed acute lesions recovered Lyford et al.
infusion juncea (1976)
Table 15 (cont'd)
Country/ Age Suspected Name of Alkaloid Alkaloid Nature of Outcome Reference
number group vehicle of plant concentration lesion (cirrhosis/
cases intoxication and intake died)
Egypt
3 not known Hashem (1939)
59 1-12 plant seed not acute lesions cirrhosis Safouh &
years decoction identified in one Shehata (1965)
in 17
Federal Republic of Germany
8 75-116 not known acute lesions all died Wurm (1939)
days (alimentary
toxaemia)
Hong Kong
4 23-28 herbal Heliotropium pyrrolizidine herbs-alkaloid acute VOD 1 died Kumana et al.
years infusion lasiocarpum alkaloids base 0.42 g/kg; (1983, 1985);
N-oxides Culvenor et al.
1.4 g/kg; daily (1986)
intake, 12 mg
base, 18 mg
N-oxide; total
intake, 570 -
1350 mg up to
development of
symptoms in 3
patients over
19-45 days,
1300 mg up to
Table 15 (cont'd)
Country/ Age Suspected Name of Alkaloid Alkaloid Nature of Outcome Reference
number group vehicle of plant concentration lesion (cirrhosis/
cases intoxication and intake died)
Hong Kong (cont'd)
death in 1
patient. 630 mg
in one case who
remained
asymptomatic.
India
2 25 and herbal not unknown acute recovered Gupta et al.
35 years decoction identified (1963)
and pills
25 12-38 possibly not not analysed acute 12 died; Tandon, R.K.
years 6 contaminated identified lesions; 1 cirrhosis et al. (1976);
patients cereal cirrhosis Tandon, H.D.
studied) in one et al. (1977)
108 3-60+ contaminated Crotalaria crotananine 0.81 g/kg various up to 63% Tandon, B.N.
cereal nana and stages of died et al.
crotaburmine disease (1976, 1977)a
Siddiqui
et al.
(1978a,b)
Table 15 (cont'd)
Country/ Age Suspected Name of Alkaloid Alkaloid Nature of Outcome Reference
number group vehicle of plant concentration lesion (cirrhosis/
cases intoxication and intake died)
India (cont'd)
35 1- > 25 contaminated Crotalaria as above 5.3 g/kg seed acute as above Krishnamachari
years cereal (0 - 1.9% of et al.
seeds in (1977)a;
cereal); daily Siddiqui et al.
intake, 40 mg (1978a,b)
6 20-70 herbal Heliotropium heliotrine 12 - 20 g/kg; acute 3 died Aikat et al.
medicine in eichwaldii as N-oxide total intake, lesions; (2 with (1978);
4 cases by 3 4 g and 10 g cirrhosis fulminant Datta et al.
patients in patients who in one disease) (1978a,b)
died of organizing
fulminant thrombi in
disease hepatic veins
in 4 cases
Iraq
9 3-10 possibly possibly not acute 1 died Al-Hasany &
years wheat flour Senecio identified Mohamed (1970)
South Africa
11 11-19 wheat flour Senecio not centrilobular "majority Wilmot &
(only 5 years as bread ilicifolius; identified haemorrhages; died" Robertson
described) S. burchelli senecionine? central vein (1920)
"dilated"
Table 15 (cont'd)
Country/ Age Suspected Name of Alkaloid Alkaloid Nature of Outcome Reference
number group vehicle of plant concentration lesion (cirrhosis/
cases intoxication and intake died)
South Africa (cont'd)
12 wheat not not centrilobular Selzer & Parker
flour identified; identified haemorrhages; (1951)
"possibly organizing
Senecio" thrombi in
hepatic veins;
3 cases of VOD
12 5-30 herbal not none found "acute" in 3 died of Stein &
months medicines identified in herbal 5 cases; disease; Isaacson (1962)
admitted in medicines "subacute" no follow-up Stein (1957)
4 cases; in 7 cases; on 5
food not no detail
analysed
United Kingdom
1 26 years herbal Maté-Paraguay identified "trace acute with died McGee et al.
infusion tea only as amounts" veno-occlusion (1976)
pyrrolizidine going on to
alkaloids chronic with
fibrosis
1 13 years herbal Symphytum not analysed not known "thrombotic" Weston et al.
infusion officinale variant of (1987)
veno-occlusive
disease
Table 15 (cont'd)
Country/ Age Suspected Name of Alkaloid Alkaloid Nature of Outcome Reference
number group vehicle of plant concentration lesion (cirrhosis/
cases intoxication and intake died)
USA
1 2 months herbal Senecio riddelline 15 g/kg as acute lesions died Fox et al.
infusion longilobus and N-oxides alkaloids; (1978)
of retrorsine, total intake,
seneciphylline, 66 mg
and senecionine
1 6 months herbal Senecio as above 3 g free acute lesions cirrhosis Stillman et al.
infusion longilobus alkaloid plus (1977);
10.5 g N-oxide Huxtable (1980)
per kg; total
intake,
70-147 mg of
combined
alkaloid
and N-oxide
1 49 years herbal Symphytum sp. symphytum 14.1 µg/kg acute lesions recovered Ridker et al.
infusion pyrrolizidine body weight after (1985);
alkaloids per day; total short Huxtable et al.
intake, 85 mg surgery (1986)
USSR
1000 - 1500 3-50 contamination Heliotropium heliotrine, acute lesions 13-15% Mirochnik
years of wheat lasiocarpum lasiocarpine died; (1938);
flour cirrhosis Terekhov
in many (1939)
Table 15 (cont'd)
Country/ Age Suspected Name of Alkaloid Alkaloid Nature of Outcome Reference
number group vehicle of plant concentration lesion (cirrhosis/
cases intoxication and intake died)
USSR (cont'd)
"large contamination Heliotropium heliotrine Dubrovinskii
number" of wheat lasiocarpium lasiocarpine (1947);
flour Ismailov
(1948a,b)
Venezuela
1 5 years "earth and not acute lesions died Grases & Beker
plant eating identified (1972)
habits"
West Indies
11 14 months not known, acute to data Jelliffe et al.
to possibly cirrhosis unclear; (1954b)
9 years herbal cirrhosis
infusions in some
5 23 months not known, not 1 Bras et al.
to 18 possibility identified cirrhosis (1954)
years of "bush
teas"
4 16-45 history of not acute to 3 died Stuart & Bras
years herbal identified acute-on- (1955)
infusion in chronic
one case
Table 15 (cont'd)
Country/ Age Suspected Name of Alkaloid Alkaloid Nature of Outcome Reference
number group vehicle of plant concentration lesion (cirrhosis/
cases intoxication and intake died)
West Indies (cont'd)
84 0.5-53 probably not 11% had Stuart & Bras
years "bush teas" identified; cirrhosis; (1957)
(Senecio and 27% died
Crotalaria)
23 possibly not stated not analysed acute to Bras & Hill
bush teas "cirrhosis" (1956)
(no breakup)
23 1-50 no precise no analysis cirrhosis Bras et al.
years identification; (1961)
"Crotalaria
fulva and
possibly
other toxic
factors"
3 "children" "toxic not acute died Stirling et al.
substances identified (1962)
in herbal
remedies"
a Pertains to the same outbreak.
Selzer & Parker (1951), from South Africa, described 12 cases,
10 of whom came from 3 families that had eaten bread made of flour
of "imperfectly winnowed wheat". Five cases who were autopsied
showed characteristic occlusion of the central vein of the liver
lobules.
Attention on veno-occlusive disease as an entity followed a
spate of reports, mostly from Jamaica, on the occurrence of this
disease, mainly among children (Bras et al., 1954, 1961; Jelliffe
et al., 1954a,b; Bras & Watler, 1955; Bras & Hill, 1956; Stirling
et al., 1962) but also among adults (Stuart & Bras, 1955, 1957).
The youngest patient reported was a 3-month-old infant (Stein &
Isaacson, 1962), but the disease was stated even to have been
observed in the new-born (Stein, 1957). Very often, several
members of the family were affected by disease, which presented
primarily as rapidly filling ascites, within a matter of days,
sometimes accompanied by fever, and leading to hepatic failure. It
was considered an important cause of cirrhosis among children in
Jamaica (Jelliffe et al., 1954a,b). On the basis of evidence of an
almost identical disease occurring among grazing animals (Hill,
1960), and a prevalent practice among the Jamaicans of using herbal
infusions for treating a variety of ailments as a home remedy,
herbs, notably of the Senecio and Crotalaria groups, were suspected
of being a contributory factor (Bras & Hill, 1956; Bras et al.,
1957, 1961; Stuart & Bras, 1957).
Hill (1960) gave a comprehensive account of current knowledge
up to that time of the world-wide distribution of seneciosis in man
and animals in which mention is made of this disease only in Egypt,
the Federal Republic of Germany, South Africa, and the West Indies.
Pyrrolizidine alkaloids were identified as the active element in
the toxic factor in the plants.
However, there are earlier reports of this disease from
elsewhere. Bras (1973) cited a number of reports from Europe from
1905 to 1949, including reports by Wurm (1939) and Teilum (1949),
of an entity in infants and children that was called Endophlebitis
hepatica obliterans. He reported having studied some tissue
sections of liver in Austrian children aged 7 months - 1 year, who
had died with clinical and histological patterns of VOD. Some of
these conformed to the Budd-Chiari syndrome whereas others were
consistent with VOD. The case of a 36-year-old woman reported by
Teilum (1949) does not, however, fall in the usual category of
cases accepted as veno-occlusive, since thrombotic changes were
also seen in the larger hepatic veins and several, in the
peripheral parts of the body, as well as the portal vein.
A comprehensive account of all available published reports of
this disease to date from different parts of the world is given in
Table 15. A clear distinction between the acute and chronic
effects of exposure is not always possible from the published data,
since, in most patients, the history of onset of disease with
intake of the toxic substance is not generally volunteered or
available, and so a precise temporal relationship is difficult to
establish. However, this information is available in a reasonably
accurate form in more recent reports (Stillman et al., 1977; Datta
et al., 1978a,b; Fox et al., 1978; Kumana et al., 1985).
Gupta et al. (1963) from India described 2 cases who had drunk
some herbal infusions for the treatment of skin disorders. The
liver biopsies confirmed changes typical of acute VOD. However,
the herb was not identified and no analysis was made of the
infusion for alkaloids.
The cases of 59 children in Egypt who had symptoms of rapidly
developing abdominal distension and hepatomegaly were reported by
Safouh & Shehata (1965). Their ages ranged between 1 and 12 years,
47 being below 4 years of age. A dietary survey carried out on 17
patients indicated ingestion of drinks made by boiling some common
plant seeds, but these were not identified. Wedge biopsies of
liver were performed in 6 patients and 16 postmortem examinations
were made. In all the autopsy cases, there was thrombotic
obliteration of the main hepatic veins and their ostia. The
central and sublobular veins were not uniformly thickened as they
were frequently dilated or disrupted and some contained fresh
thrombi. There was centrilobular necrosis of the liver lobules.
One patient developed decompensated cirrhosis in 3 years. The
authors observed that the clinical and pathological features
closely resembled those of VOD in Jamaica, but thrombosis was an
unusual feature of the latter disease.
Al-Hasany & Mohamed (1970) described a short outbreak in Iraq,
occurring during a 3-month period, affecting 9 children, all except
one being below 12 years of age, and belonging to 3 Bedouin
families living outside Baghdad. One of the children died.
Autopsy of this case and biopsies on the others showed changes
characteristic of VOD. Poisoning through the wheat flour
contaminated by seeds of some PA-containing plant was suspected,
but no analysis of the food was carried out.
Three of the largest outbreaks of the disease have been
reported from South Asia, two from the same site in central India
(Tandon, B.N. et al., 1976, 1977; Tandon, R.K. et al., 1976;
Krishnamachari et al., 1977; Tandon, H.D. et al., 1977) and one
from North-West Afghanistan (Tandon & Tandon, 1975; Mohabbat et
al., 1976; Tandon, B.N. et al., 1978; Tandon, H.D. et al., 1978).
The first Indian outbreak was reported by Tandon, B.N. et al.
(1976) and Tandon, R.K. et al. (1976) and Tandon, B.N. et al.
(1977) and Tandon, H.D. et al. (1977). It occurred in a group of 5
tribal villages in central India in 1972 - 73. The epidemiology of
the outbreak was later described by Arora et al. (1981). Out of a
total population of 2060 in these villages, 71 households with 366
members were investigated. Among these, 39 cases had developed and
19 had died before commencement of the investigations. The
incidence rate was 1.1% and the case fatality rate was 50%. All
cases occurred among 20 households. In many households, several
members were affected. In one household, 4 out of 5 cases died.
Six sick patients were investigated in detail with repeated liver
biopsies. One patient died 17 months after onset and was
autopsied. The clinical onset was characteristic of disease.
Haemodynamic and radiographic studies suggested a combined post and
perisinusoidal block. Liver biopsy studies showed features
characteristic of acute centrilobular haemorrhagic necrosis with
progressive fibrosis, hepatic veno-occlusion, and non-portal
cirrhosis in one case who survived 17 months after the acute onset.
The etiological factor of this outbreak was not established, though
dietary contamination with pyrrolizidine alkaloids was considered.
A second outbreak occurred at the same site in 1975 and was
studied and reported independently by Krishnamachari et al. (1977)
and Tandon, B.N. et al. (1976, 1977). A total population of 486
was affected, 67 cases were reported, of whom 28 (46%) had died.
There was a strong family history (Tandon, B.N. et al., 1976). In
a later survey, 108 patients were studied and the mortality rate
was estimated to be 63% (Tandon, B.N. et al., 1977). This time the
etiological factor was identified as the plant Crotalaria nana
Burm, which had been growing in the fields of millet (Panicum
miliare), their staple food crop. The seeds of this plant became
mixed with the cereal grain during harvesting. The toxic seeds
contained pyrrolizidine alkaloids that were identified as a
macrocyclic ester closely similar to monocrotaline. The total
alkaloid content was estimated to be 5.3 g/kg of seed, expressed as
monocrotaline. The levels of contamination of the millet with
seeds were reported to be 0 - 3.4 g/kg in the unaffected and
0 - 19 g/kg in the affected households (Krishnamachari et al., 1977).
A precise identification could not be made, but the same material,
independently studied by Siddiqui et al. (1978a,b), were reported
to contain 2 new alkaloids, cronaburmine and crotananine. The seed
contained an alkaloid level of 26 g/kg. The levels of
contamination of the millet were up to 20 g/kg and the amount of
alkaloid ingested was estimated to be up to 40 mg per day.
The largest outbreak reported to date occurred in the Gulran
district of Herat Province in northwest Afghanistan, close to the
border of the USSR. Tandon & Tandon (1975) identified the plant as
being causative factor of the disease, which was surveyed and
reported in detail by Mohabbat et al. (1976). The outbreak, was
estimated to have affected a population of approximately 35 000 in
98 villages. Examination of 7200 inhabitants of the affected
villages showed evidence of disease in 22.6%, which was more
serious in 15%. Thus, it was estimated that approximately 8000
subjects suffered from the disease including 5000 who were
seriously affected. All age groups were affected, but 46% of
subjects were below 14 years of age. However, no sign of disease
was found in children below 2 years of age. A detailed report
concerning the pathological material obtained from 14 liver
biopsies and 8 autopsies was made by Tandon, B.N. et al. (1978) and
Tandon, H.D. et al. (1978). The time interval between the onset of
symptoms and the biopsy/autopsy was not indicated. Pathological
findings were characteristic, ranging from acute disease with
characteristic veno-occlusion to non-portal cirrhosis, which was
observed in 5 of the above 22 cases. The outbreak was ascribed to
massive contamination of wheat, the staple food crop, following 2
years of drought, with the seeds of Heliotropium popovii H. Riedl
subsp. gillianum H. Riedl, which had been growing profusely among
the wheat crop. The seeds contained pyrrolizidine alkaloids at
concentrations reported by 2 laboratories to be 7.2 and 13.2 -
14.9 g/kg, identified as mainly as the N-oxide of heliotrine (74%)
(Mattocks, personal communication), and one or two other compounds
similar in character to lasiocarpine. Samples of wheat from
several villages contained an average of 40 seeds/kg, i.e., 0.03%
by weight. It was estimated that an adult consumed at least 700 g
flour/day, containing approximately 2 mg alkaloid (based on a mean
of the seed analyses). There is some uncertainty about the
estimate, since Mohabbat et al. (1976) also stated that the samples
of the wheat flour were assayed and contained 0.186 - 0.50%
alkaloid. This analysis and the 0.72% result for alkaloid in the
H. popovii seed were from the same laboratory in Kabul (R.N.
Srivastava, personal communication), and together, imply 13 - 36%
seed in the wheat. If correctly reported, the result for the flour
conflicts with the estimated proportion of the seed in the wheat
and can scarcely have been representative.
Tandon & Tandon (1975) stated in their report of the survey
during which the causative factor of the Afghan outbreak was
discovered, that such cases had always been observed by Government
physicians posted in this area in the past, sometimes in
significant numbers, but neither the population nor the physicians
remembered that the disease had occurred in the form of an
outbreak.
There was no mention of VOD in the hepatic lesions observed by
Sobin et al. (1969) among the 121 specimens of liver obtained at
medico-legal autopsies or by needle biopsies at Kabul, though 6.6%
of the 89 autopsy specimens showed "acute passive congestion with
necrosis". The ages of these subjects was not stated and the
authors did not discuss the cause of the lesion.
Following these outbreaks, a number of isolated cases have been
described following the use of herbal medicines. McGee et al.
(1976) reported a case from the United Kingdom of a 26-year-old
woman who had consumed herbal tea containing pyrrolizidine
alkaloids. The one sample examined contained only trace amounts of
alkaloids. Neither the plant nor the alkaloids were further
characterized. The patient had been taking very large quantities
of the tea for about 2 years and it is possible that some of the
earlier batches may have been more heavily contaminated. Maté or
Paraguay tea ( Ilex species), which she was drinking, is stated to
be a popular drink in Brazil and is not believed to contain
pyrrolizidine alkaloids. It has been stated (Huxtable, 1980) that
possibly she ingested the pyrrolizidine alkaloids from some other
unidentified source. The clinical course of the disease progressed
rapidly. Three biopsies carried out within one month and the
autopsy showed characteristic changes including centrizonal
fibrosis, but no cirrhosis. It is notable that some involvement of
muscular pulmonary arteries was also seen.
Lyford et al. (1976) reported the case of a 35-year-old woman
from Ecuador, who had ingested herbal tea prepared from Crotalaria
juncea. She had consumed 1 - 2 litres of this infusion daily for 6
weeks, but no qualitative or quantitative analysis for
pyrrolizidine alkaloids was made. She had had arthralgias for 3
years, for which she had received treatment with indomethacin and
phenylbutazone. The liver biopsy showed characteristic changes of
acute VOD from which she recovered completely as proved by a repeat
biopsy carried out one year later.
The occurrence of acute disease was reported in 2 infants in
Arizona in the USA, aged 6 months and 2 months, respectively,
following ingestion of infusions of a herb, locally called the
Gordolobo Yerba by the Mexican-American population and identified
as Senecio longilobus (Stillman et al., 1977; Fox et al., 1978;
Huxtable, 1980). The plant from which this infusion had been
prepared was found to contain pyrrolizidine alkaloids identified as
riddelline and N-oxides of retrorsine, seneciphylline, and
senecionine (Huxtable, 1980), in a concentration of 3 g free
alkaloid and 10.5 g N-oxides/kg (Stillman et al., 1977). It was
estimated that, during a period of 2 weeks, the 6-month-old infant
received a total dose of between 70 and 147 mg of combined alkaloid
and N-oxide derivative. The liver biopsy showed characteristic
features of acute disease, which had progressed to extensive
central, portal, and sinusoidal fibrosis (Stillman et al., 1977).
The child subsequently developed cirrhosis over the next 8-month
period. The 2-month-old infant was administered an infusion of the
same herb for 4 days, after which he became progressively more ill
and stuporous (Fox et al., 1978). On admission he was diagnosed as
a case of Reye's syndrome, but subsequently developed jaundice and
possibly ascites and died. The sample of herb contained a
concentration of alkaloids of 15 g/kg. It was estimated that the
infant had received a total of 66 mg of mixed alkaloids over the
4-day period. At autopsy, extensive centrilobular haemorrhagic
necrosis of the liver was seen, which is characteristic of the
acute disease. However, no occlusive lesions of the central vein
of the lobules were described, and no obstructive lesions were seen
in the larger hepatic veins or inferior vena cava. No mention was
made of ascites. The basal ganglia showed kernicterus.
Datta et al. (1978 a,b) reported 6 cases that occurred between
1974 and 1977. All of the patients had taken herbal medicines,
identified in 1 case as Heliotropium eichwaldii, which contained
N-oxides of heliotrine. Two patients took the herb as an extract
of the whole plant, which contained an alkaloid concentration of
20 g/kg, for 20 and 50 days, respectively, and developed symptoms
after a time lag of 45 and 90 days, respectively. They were both
estimated to have consumed 200 mg of heliotrine per day, the total
alkaloid intake being 4 g and 10 g, respectively. They had taken
the herb for treatment of epilepsy, and were admitted with acute
onset of symptoms of abdominal pain, ascites, jaundice, hepatic
encephalopathy, and gastrointestinal bleeding, which suggested
fulminant viral hepatitis. They died within 2 - 12 weeks of the
onset of symptoms. Only a brief description of the main autopsy
findings in the liver was given, which indicated that there were
changes characteristic of acute veno-occlusive disease of liver,
including marked centrilobular haemorrhagic necrosis of the liver
lobules, and occlusive lesions of the central and sublobular veins.
It is interesting to note that both patients had also been on long-
term anticonvulsant phenobarbitone therapy. The remaining 4
patients had a chronic insidious onset of disease suggesting
cirrhosis of the liver in 3, and alcoholic liver disease in one.
One of the former had been taking some indigenous powder,
presumably prepared from a herb, the indication and nature of which
are not known. The patient died from hepatic encephalopathy. No
detailed description of the autopsy findings was given, but it was
stated that the central and sublobular veins of the liver showed
chronic occlusive changes. The inferior vena cava and large
hepatic veins were patent. There was non-portal cirrhosis. A
notable feature was that the arsenic levels in the liver tissue
were high (500 µg/kg; normal, 1 µg/kg). There is no mention of
arsenic in the report on the analysis of the indigenous powder
taken by the patient. Two of the remaining 3 patients with chronic
disease, had taken herbal medicine for vitiligo, and one for
diabetes mellitus. The herb was identified as Heliotropium
eichwaldii in the case of one of the vitiligo patients, who had
taken it for 10 days and in whom the onset of symptoms occurred
within 10 days. The herb was taken in the form of seed with an
alkaloid content of 12 g/kg. The daily intake was estimated to be
500 mg of alkaloid and the total intake, 5 g. This patient was
admitted with a clinical diagnosis of cirrhosis of the liver. The
liver biopsy showed changes characteristic of acute VOD. Follow-up
data are not known. The herb taken by the other 2 patients was not
identified. The diabetic patient was being treated with oral
hypoglycaemic drugs and was also known to be an alcoholic. The
indigenous powder being taken by him contained a high concentration
of arsenic (5 mg/kg). The results of haemo-dynamic studies
suggested hepatic venous outflow tract obstruction of the
intrahepatic post-sinusoidal type in the smallest hepatic veins.
Liver biopsy showed characteristic centrilobular haemorrhages.
Central veins could not be recognized. There were mild changes in
the liver cells, but no alcoholic hyaline was seen. The liver
biopsy of the third patient showed characteristic features of acute
disease with veno-occlusion.
Two cases have been reported from China (Hou et al., 1980).
Both were adults who were taken ill after taking medicinal
infusions prepared from Gynura segetum of the family Compositae
(tribe, Senecioneae). The presenting symptoms and the cause of
disease, as well as the pathological findings, were characteristic,
except that one patient had jaundice and also portal vein
thrombosis, which is not a usual feature of the disease. No
chemical analysis of the plant was made and the alkaloid was not
precisely identified. Furthermore, the total intake of alkaloid
was not calculated. It has been stated that this was the first
report of such a case, but that it was possible that the disease
might occur among adults more frequently without being reported.
Ghanem & Hershko (1981) reported 3 cases of Arabs, one 3-year-
old child and 2 adults, who were diagnosed as having VOD of the
liver, on the basis of the clinical findings and morphological
features of liver biopsies. One more patient had occlusive lesions
of both the small and large hepatic vein radicles. They were among
29 patients with clinical features of hepatic vein thrombosis (HVT)
found on a retrospective analysis of data from 9 major hospitals in
Israel. Of these patients, 15 were Jews and 14 were Arabs.
Notable features were that all Jewish patients were adults, whereas
the majority of Arab patients were children below 10 years of age
and primary HVT was 2.4 times more common among the latter. No
analysis of the diet was made for PAs and their etiological role
was suspected only on a presumptive basis. A survey of stored
wheat grain in 9 villages showed that 2 samples were contaminated
with seeds of Lolium, belonging to the Graminae family, which were
found to contain 2 PAs (loline and norloline). However, these PAs
are not known to be hepatotoxic. The authors argued that, even
though in a classical case of VOD there should not be thrombosis of
the larger hepatic vein radicles, the difference in the anatomical
appearance of VOD and that of primary HVT of the near-east type is
not due to a different etiological agent but rather to a difference
in the dose and rate of absorption of the ingested toxic compounds.
A further report of the disease from Hong Kong, by Kumana et
al. (1985), described it in 4 young Chinese women with psoriasis
who took infusions of a herbal remedy, the toxic component of which
has since been identified as Heliotropium lasiocarpum (Culvenor et
al., 1986). They developed symptoms 19 - 45 days after starting
the herbal treatment, and were examined 61 - 68 days after its
initiation. The condition of patient No. 2, who continued taking
the herb for 16 days after the onset of symptoms, deteriorated and
she died of hepatic failure and was autopsied. The liver biopsies
and autopsy confirmed the presence of acute disease in all
patients. Patient No. 4 stopped taking the herb after 21 days, on
account of a new rash. When assessed 77 days later, she had mild
hepatomegaly only. A detailed analysis of the alkaloid content was
carried out for each case. The pyrrolizidine alkaloids were
quantified as if senecionine based. The herb contained 0.42 g
alkaloid/kg and 1.4 g N-oxide/kg. The daily intakes of alkaloid
base and N-oxide were estimated to be 12 ± 1 mg and 18 ± 4 mg,
respectively. The respective cumulative doses of alkaloid (base
and N-oxide) consumed by patients Nos 1, 2, and 3, up to onset of
symptoms, were calculated to be 1350 mg over 45 days, 900 mg over
30 days, and 570 over 19 days, respectively. Patient No. 4 who had
irrefutable histological evidence of disease but did not develop
symptomatic disease, must have consumed 630 mg over 21 days.
Patient No. 2, who died, was estimated to have taken a total amount
of 1380 mg alkaloid over 46 days. The estimated cumulative intake
per kg body weight before the development of symptoms for patients
Nos. 1, 2, 3, and 4 was 26, 15, 12, and 15 mg/kg, respectively. It
should be noted that, in patient No. 2, who died, the cumulative
dose until the onset of symptoms was the same as in patient No. 4,
who was asymptomatic. Moreover, the total intake by patient No. 2,
was 23 mg/kg, which was lower than the intake by patient No. 1, who
survived. The authors compared the intake data of their patients
with those of the Arizona children reported by Stillman et al.
(1977) and Fox et al. (1978). The 6-month-old baby, who survived
but developed cirrhosis, and the 2-month-old baby, who died, are
estimated by comparison to have taken cumulative doses of 12 - 25
and 11 mg/kg, respectively. The above data suggest marked
variation in susceptibility among individual subjects. It is also
known from experimental animal studies that the young and new-born
animals are particularly vulnerable (Jago, 1970).
A case reported from the USA is ascribed to the consumption of
comfrey ( Symphytum sp.) powder, sold as a digestive aid (Ridker et
al., 1985). A 49-year-old woman presented with classical symptoms
and signs of VOD. The haemodynamic data showed a hepatic vein
wedge pressure of 3.07 kPa (23 mmHg) with a sinusoidal pressure of
2.27 kPa (17 mmHg). Hepatic venograms showed near obliteration of
the smaller radicles of the hepatic veins during balloon distension
of one of the intrahepatic venous tributaries, and there was extra-
vasation of the dye into the hepatic parenchyma. A porta-caval
shunt was carried out and the operative findings confirmed the
presence of a post-sinusoidal block. The liver biopsy showed
marked centrilobular necrosis and congestion with dilatation of the
central veins and sinusoids, consistent with hepatic venous outflow
tract obstruction. According to the clinical history, the patient
had been a heavy consumer of food supplements. Apart from several
vitamins and minerals, she had been drinking 3 cups of camomile tea
per week and for 6 months before admission had consumed 1 g/day of
a commercially available herbal tea. For 4 months before
admission, she had taken 2 capsules of "comfrey-pepsin" pills with
each meal. The herbal tea and the pills were analysed for PAs.
Pyrrolizidine alkaloids and their N-oxides were found, but the
compounds were not precisely identified. On the basis of the
analysis of the PA content, the patient was estimated to have
consumed a total of at least 85 mg of PA (Huxtable et al., 1986)
(14.1 µg/kg body weight per day, Ridker et al., 1985). The authors
emphasized that the total PA consumption was relatively low. It
was possible that the patient had other sources of exposure and
probably she had been consuming PA-containing supplements for
longer than the periods stated by her in the clinical history.
The latest is the report of a 13 year old boy from the U.K. who
is stated to have developed symptoms of toxicity following
administration of herbal tea prepared from comfrey leaf (Symphytum
officinale) for treatment of inflammatory bowel disease for two or
three years (Weston et al., 1987). The exact quantity of leaves
consumed and frequency of administration were not known. The liver
biopsy is stated to have shown a "thrombotic variant" of veno-
occlusive disease, though the inferior vena cava and the major
hepatic veins were patent on Doppler ultrasound and percutaneous
phlebography. He had earlier been treated with predinisolone
and sulphasalazine. The case is unusual in so far as the
thrombosis of the central veins of the liver lobules, which is not
a usual feature of veno-occlusive disease of the liver.
7.4 VOD and Cirrhosis of the Liver
Jelliffe et al. (1954b) were the first to draw attention to VOD
being an important cause of cirrhosis among Jamaican children.
Prior to this, Hashem (1939), while reviewing the records of all
cases of cirrhosis admitted to a children's hospital in Egypt since
1933, described 3 cases of a special type of cirrhosis that was
rare in adults. The clinical features and pathological findings
were similar to those in cases of VOD. They speculated that the
cause was some metabolic toxins of gastrointestinal origin. Royes
(1948) suggested that the cirrhosis in the Jamaican children was
very like the disease described from India and Egypt.
The clinical and pathological features of 100 cases of VOD
among Jamaican children were described by Bras et al. (1954).
Sixty-five of the cases were below 12 years of age. None of the
100 patients had cirrhosis initially, but 5 showed occlusive
lesions in hepatic veins and features of non-portal fibrosis. Four
of these 5 cases later developed cirrhosis. The authors concluded
that VOD contributed to a substantial number of all cases of
cirrhosis in this age group. Stuart & Bras (1957) studied 84
patients with VOD including 64 acute, 6 subacute, and 14 chronic
cases. Twenty-three patients were followed up, some for up to 5
years. Autopsy performed on 21/26 cases showed cirrhosis in 11
cases. Notable features were that 1 of the 6 cases of acute
disease described in detail, developed cirrhosis. Of the 2 cases
with chronic disease, 1 developed cirrhosis within 3 months of a
liver biopsy for acute disease, at which time the liver had shown
hepatic venous occlusive lesions but no fibrosis. Autopsy findings
were described by Bras & Watler (1955) in 19 patients aged 10
months - 45 years in different stages of the disease. Nine
patients had cirrhosis that was non-portal to begin with but
finally became indistinguishable from Laennec's portal cirrhosis.
Rhodes (1957) studied the pattern of liver disease among Jamaican
children. A total of 193 liver biopsies was studied derived from
39 children who had one biopsy and 59 who had more than one at
intervals of 1 week - 3 years. Of the 14 autopsies on cases of
VOD, 12 had cirrhosis. A notable feature was that the disease
could occur asymptomatically with hepatomegaly. The autopsy
material from the University College Hospital, Jamaica was analysed
by Bras et al. (1961). Of the 1560 autopsies, 28.5% cases
concerned infants of less than one year old, mostly from poor,
predominantly black families. Cirrhosis was seen in 77 autopsies.
Approximately 30% of these 77 cases were diagnosed as post-VOD.
The authors postulated that they might have resulted from the
ingestion of Crotalaria fulva or some other toxic substances.
In a follow-up study of 61 patients who developed the disease
in an outbreak in the USSR in 1958, 28 developed "Hepatoleinal
syndrome" (Braginski & Bobokhadzaev, 1965). Two of these cases, in
whom the disease in the initial stages was not particularly severe,
developed cirrhosis within 4 years.
Tandon, H.D. (personal communication) has analysed the
pathological data derived from the Afghan and Indian outbreaks on
the basis of 61 liver biopsies and 17 autopsies, including repeat
biopsy studies on 15 patients who were followed-up for 1 month - 3
years after onset in the Indian outbreaks. Three of the 11
patients, followed up for 16 months or longer for persistent
clinical evidence of disease, ended up with cirrhosis and 2 more
had marked fibrosis with equivocal changes of cirrhosis in the
biopsy. Notable features of the study were that the disease
progressed to cirrhosis in patients who were put on a normal diet,
free from alkaloids, after appearance of symptoms of acute disease
and did not receive any subsequent exposure. Impact of alcohol
intake was excluded. There was a poor correlation between the
clinical and pathological severity of disease. Centrilobular
haemorrhages, which are a sign of acute disease, were seen to
persist for over one year in patients, some of whom were apparently
well. At needle biopsy, characteristic hepatic venous occlusions
were not seen in many patients in the acute phase of the disease,
though they were seen in all livers at autopsy and most of them
showed persistent centrilobular haemorrhages. Biopsy findings in
cirrhotic livers were often not histopathologically characteristic
for any specific form of cirrhosis. Features that might suggest
the veno-occlusive etiology of cirrhosis at biopsy included
paraseptal dilatation of sinusoids and persistent haemorrhages or
haemosiderin in the septa.
In studies by Aikat et al. (1978) and Datta et al. (1978a,b), 6
cases of VOD were reported following ingestion of herbal medicines
containing PAs. Four of the patients had symptoms of chronic
disease and one of them developed non-portal cirrhosis.
One of the 2 infants from Arizona, USA, who suffered from VOD
following the administration of PA-containing herbal medicine,
developed cirrhosis during the 8-month period following the
appearance of acute symptoms (Stillman et al., 1977; Fox et al.,
1978; Huxtable, 1980). Huxtable (1980) mentioned the death from
cirrhosis of liver of a 62-year-old woman, who had consumed the
same herb as the 2 infants for 6 months prior to her death.
However, there was no confirmation of the diagnosis of cirrhosis.
7.5 Differences Between VOD and Indian Childhood Cirrhosis (ICC)
A type of cirrhosis of liver, peculiar to the people of Indian
origin, Indian Childhood Cirrhosis (ICC), has been ascribed to PA
toxic etiology (Bras et al., 1954; Rhodes, 1957), owing to the
observation of occlusive changes in the central and sublobular
veins of the liver, by some investigators (Radhakrishna Rao, 1935;
Prabhu, 1940; Jelliffe et al., 1957; Ramalingaswami & Nayak, 1969),
though this is not a characteristic feature of the disease. The
presence of copper-positive granules in the hepatocytes (Salaspuro
& Sipponen, 1976) has added to such a conjecture (Tanner &
Portmann, 1981), because of the reported aberration of copper
metabolism in experimental animals exposed to PAs (section 6.4.11).
However, ICC and VOD are clinically and pathologically distinct.
ICC, confined to infants and children, often affects siblings.
Jaundice is a common sign and hepatosplenomegaly is a common
feature. The disease is almost invariably fatal due to rapidly
developing hepatocellular failure. Liver parenchymal changes are
characterized by marked ballooning degeneration of hepatocytes,
prominent deposits of alcoholic hyaline, severe cholestasis, and
aggressive, pericellular fibrosis (Nayak et al., 1969), all
features not characteristic of VOD. Moreover, occlusive changes in
the hepatic veins are very rare and were not observed by any member
of the liver diseases subcommittee of the Indian Council of Medical
Research (1955), who made a critical study of the disease.
7.6 Chronic Lung Disease
Heath et al. (1975) reported the case of a 19-year-old African
man who had died of congestive cardiac failure, and who was
suspected of having ingested a herbal remedy containing the seeds
of Crotalaria laburnoides. Histopathological examination of the
lungs showed characteristic vascular changes of severe primary
pulmonary hypertension. Powdered seeds of the plant were fed in
the diet to Wistar albino rats for 60 days (Table 11).
Characteristic features of pulmonary hypertension including
hypertensive vascular changes in the lung and right ventricular
hypertrophy of the heart were produced in the animals showing that
the seeds contained an agent capable of inducing pulmonary
hypertension in rats. Apart from this indirect evidence, there was
no proof of such a causal relationship with the pulmonary
hypertensive disease in the patient.
A brief mention is made by McGee et al. (1976) of finding
changes "somewhat similar but rather more mature" (than those seen
in the hepatic veins) involving some branches of the pulmonary
artery in the lower lobe of the left lung, in a case of veno-
occlusive disease of the liver caused by ingestion of PA-containing
herbal teas. The alkaloids were not further characterized. The
changes were also stated to be similar to those produced in
experimental animals by PAs. Apart from these 2 cases, there is no
mention of involvement of the pulmonary arterial system in any of
the case reports available.
The possibility that diet-mediated agents might induce
pulmonary hypertension in man has been discussed at length by
Fishman (1974). A parallel was drawn with the epidemic of
pulmonary hypertension that occurred in Austria, the Federal
Republic of Germany, and Switzerland between 1966 and 1968 (Kay et
al., 1971b), which was suspected of being caused by Aminorex, a
compound that resembles epinephrine and amphetamine in chemical
structure. Although the etiological role of Aminorex could not be
conclusively proved on epidemiological or experimental grounds,
there continues to be a suspicion that agents taken by mouth can
evoke pulmonary hypertension in man. Levine et al. (1973) reported
the cases of 3 children aged 5 1/2 - 13 years, and 11 months,
respectively, with portal hypertension, who developed progressive
pulmonary hypertension resulting in cor pulmonale and death. In
all 3 cases, there was evidence of extra-hepatic portal vein
obstruction confirmed at autopsy, and the symptoms and signs of
portal obstruction had appeared in early childhood. They developed
symptoms of cardio-respiratory failure. Studies of pulmonary and
cardiovascular function including haemodynamic studies of pulmonary
circulation in 2 of the children suggested pulmonary vascular
obstructive disease. At autopsy, no primary parenchymal lung
disease was found. There were vascular changes of advanced
pulmonary hypertension (plexiform lesions), but no evidence of
thromboembolism was found. No factor responsible for pulmonary
vasoconstriction was identified. In one case, the liver was stated
to show coarse nodularity at autopsy, but the microscopic
examination showed only patchy areas of portal fibrosis and
regeneration. In the other 2 cases, there were only non-specific
changes, with fibrosis in one. However, centrilobular congestion
was present in 2 cases. It is possible that some metabolites of
toxic agents, such as PAs, which are metabolized in the liver,
might have blocked a metabolic pathway that ordinarily exerts a
pulmonary antihypertensive effect, or that, by damaging the liver,
vasoactive substances, such as histamine, serotonin, and
catecholamines, might escape metabolic pathways to reach the lungs
and injure the pulmonary vessels. However, no such agents were
identified.
Kay et al. (1971b) made a plea that, on the basis of the
experimental data available, including the ability of several
agents to produce pulmonary hypertension in experimental animals,
and, in spite of the fact that pulmonary vascular disease has never
been demonstrated in human cases of veno-occlusive disease, careful
enquiries should be made on the possibility of patients presenting
with unexplained pulmonary hypertension having ingested a plant
product. A similar plea was made by Heath et al. (1975).
7.7 Trichodesma Poisoning
The disease "Ozhalanger encephalitis", which occurred in the
Samarkand region of Uzbekistan, USSR in the period 1942 - 51, is
believed to have been caused by contamination of food grain with
the seeds of Trichodesma incanum (Shtenberg & Orlova, 1955;
Ismailov et al., 1970), which contain 1.5 - 3.1% alkaloids, mainly
trichodesmine and incanine (Yunusov & Plekhanova, 1959). Clinical
features of the disease have been described by Ismailov et al.
(1970). This outbreak differed from the others described above in
that the primary symptomatology was extra-hepatic. Over 200
patients were affected, not including children below the age of 10,
or the breast-fed infants of the affected mothers. Following
exposure, there was a latent period of about 10 days, then vertigo
and recurring headaches developed leading to nausea and vomiting.
This was followed by generalized malaise, which progressed to
delirium and loss of consciousness. Physical signs included
pathological reflexes in 59% of the patients and paresis of the
extremities and the facial nerve. Death was stated to have been
caused mostly by respiratory depression. Of the 200 patients
affected, 44 died. Autopsy findings were relatively non-specific
degenerative and necrotic lesions scattered in several organs
including the central nervous system. No report of such a disease
is available from outside the USSR.
7.8 Relationship Between Dose Level and Toxic Effects
In some recent human case reports, the PAs consumed have ben
identified and estimates made of the daily and total intakes (Table
16). The relationship between these intake levels and the known
toxicity of the alkaloids in rats has been discussed by Culvenor
(1983) and Mattocks (1986).
Discussion of the relationship between the dose level and toxic
effects in human cases is complicated, because the poisoning is
generally due to a mixture of alkaloids found in naturally-
occurring plant products, consumed as herbal remedies or food, and
different plant species. There are wide differences in the acute
toxicities of the alkaloids, which are the best available measure
of comparative effects due to long-term intake as well.
Furthermore, estimates of intake can, at best, be approximate.
When a large population is affected through the contamination of a
food crop, as happened in the Afghan and Indian outbreaks (section
7.3), no precise estimates are possible regarding the extent of
contamination in different households, the amount of the
contaminated grain consumed, the length of exposure resulting in
the appearance of symptoms or signs of toxicity, or death, in the
cases studied. There may also be various other contributing
factors that are not apparent, e.g., food or cooking habits, on
account of which no conclusive generalizations regarding the
causative role of the toxic agent can be made. Reports on chemical
analysis for the toxic agent and, hence, the amounts ingested, may
not always be reliable (refer to the controversy in the Afghan
outbreak in section 7.3). In cases, such as the one reported by
Ridker et al. (1985), when the total dose of PAs estimated to have
been received by the patient before the disease developed, was only
a fraction of that of other alkaloids causing episodes of human
toxicity (Table 16), it is not certain whether that was the only
toxic alkaloid agent that the patient was exposed to, or whether
there were other contributing factors, particularly as the patient
was stated to be a heavy consumer of herbs, vitamins, and natural
food supplements.
In the known instances of human toxicity the principal
alkaloids involved are heliotrine from Heliotropium, echimidine and
related alkaloids from Symphytum, riddelline and retrosine from
Senecio longilobus, and crotananine and cronaburmine from
Crotalaria nana (Table 16). Approximate acute toxicity values
(LD50 in rats) for these alkaloid mixtures are 300, 500, and
50 mg/kg, respectively. For the mixture from C. nana, for which
there are no experimental data, the acute toxicity was assumed to
be similar to that of monocrotaline, 100 mg/kg. These relativities
need to be taken into account in discussing dose-effect
relationships for the PAs as a group. This has been done by
discussing first the dose estimates for heliotrine, since it was
the main alkaloid in 1 epidemic and in 6 case reports (Table 16).
Then in discussing the other alkaloids, reference is made to a
heliotrine-equivalent dose as well as the actual dose. The
heliotrine equivalent is:
LD50 of heliotrine
actual dose x ----------------------------------
LD50 of alkaloid mixture concerned
The estimated daily intake in poisoning by heliotrine ranges from
0.033 mg/kg body weight in the Afghan epidemic (Mohabbat et al.,
1976), which after a period of about 180 days and a total intake of
about 6 mg/kg caused fatalities, to 3.3 mg/kg, which led, in 2
cases in India (Datta et al., 1978a,b), to death after 20 and 50
days and total intakes of 67 and 167 mg/kg, respectively. In
between are 4 cases in Hong Kong with estimated daily intakes
ranging from 0.49 to 0.71 mg/kg (Kumana et al., 1983, 1985;
Culvenor et al., 1986). Three cases were non-fatal at total doses
of 11 - 27 mg/kg body weight and one was fatal at a total dose of
23 mg/kg. These heliotrine cases imply that daily intakes are
cumulative down to 0.033 mg/kg and may be fairly rapidly fatal
above 0.5 mg/kg. Above a total dose of 6 - 15 mg/kg, VOD may
become evident and sometimes fatal.
In the 2 cases due to riddelline and retrorsine in Senecio
longilobus (Stillman et al., 1977; Fox et al., 1978; Huxtable,
1980), the estimated daily intakes were 0.8 - 0.17 and 3 mg/kg body
weight (equivalent to 3 and 1 mg heliotrine/kg, respectively, and
the total doses were 12 - 25 and 12 mg/kg (equivalent to 72 - 150
and 72 mg heliotrine/kg, respectively). These levels are
comparable with the highest reported intakes of heliotrine and, in
infants, led to the rapid development of VOD and, in one case,
death.
In the epidemic due to mixed crotananine and cronaburmine in
Crotalaria nana (Tandon, B.N. et al., 1976; Tandon, R.K. et al.,
1976; Krishnamachari et al., 1977), the estimated daily intake of
0.66 mg/kg and the total intake of 40 mg/kg (equivalent to 2 and
120 mg heliotrine/kg, respectively) also corresponded to the
highest intake of heliotrine.
In the case of Symphytum poisoning (Ridker et al., 1985), the
estimated daily intake of echimidine and related alkaloids was
0.015 mg/kg and the total dose 1.7 mg/kg (equivalent to 0.009 and
1.0 mg/kg heliotrine, respectively). The dose levels are lower in
equivalent terms than the lowest estimates in cases due to
heliotrine by a factor of about 4 for daily intake and 6 for total
intake. The estimates were based on questioning of the patient and
assay of the material concerned. It seems prudent to conclude that
a daily intake of pyrrolizidine alkaloid as low as the equivalent
of 0.01 mg/kg heliotrine may lead to disease in humans.
Table 16. Estimated intakes of PAs in human beings
-----------------------------------------------------------------------------------------
Principal Age of Daily Period Total dose Toxic Reference
alkaloids(s)a subject intake (days) mg mg/kg effectc
(years) (mg/kg)b
-----------------------------------------------------------------------------------------
1. Heliotrine variousd 0.033 180e 360 6e VOD, Mohabbat et al.
death (1976)
2. Heliotrine (a) 20 3.3 20 4000 67 death Datta et al.
(b) 23 3.3 50 10000 167 death (1978a,b)
3. Riddelline, 0.5 0.8-1.7b 14 70-147 12-25 VOD Stillman et al.
retrorsine (1977); Huxtable
(1980)
4. Riddelline, 0.17 3.0b 4 66 12 death Fox et al. (1978)
retrorsine
5. Crotananine, variousd 0.66 c. 60e 2400 40 VOD, Tandon, B.N. et al.
crotaburmine death (1976); Tandon,
R.K. et al. (1976);
Krishnamachari et
al. (1977)
6. Heliotrine (a) 28 0.59 45 1350 27 VOD Kumana et al.
(b) 26 0.49 46 1380 23 death (1983, 1985);
(c) 23 0.60 19 570 11 VOD Culvenor et al.
(d) 27 0.71 21 630 15 VOD (1986)
7. Echimidine 49 0.015 120 94 1.7 VOD Ridker et al.
(1985)
-----------------------------------------------------------------------------------------
a The principal alkaloid is recorded as the free base, even if there was evidence of its
presence in the plant mainly as N-oxide.
b Calculated for a 60-kg adult, unless definite information available. The 0.5-year
infant was said to be 6 kg, and
c When mentioned, death was a consequence of severe liver damage.
d Epidemic.
e Estimate based on unpublished information available to the Task Group.
There is substantial overlap between intake rates and total
intakes for fatal and non-fatal poisoning. This presumably
reflects the influences of a number of factors, such as individual
sensitivity, age, nutritional status, and general health, but it is
also due to the progressive nature of pyrrolizidine toxicity and
the effects of time. In the epidemics, in which some people died,
only an estimated average intake is available and some who were
alive at the time of investigation may have died later. Comparing
the total intakes for human toxicity with the total doses up to
death observed in the long-term administration of PAs to rats,
1.2 - 10.9 times the LD50 dose, equivalent to 360 - 3270 mg
heliotrine/kg (Table 10, section 6.4.1.5), it is evident that human
beings are more susceptible to the acute and chronic effects of the
alkaloids than rats, sometimes markedly so.
These considerations of the toxic effects in human beings of
various intake levels could provide a basis for some assessment of
the likely hazard from other types of exposure to PAs. For
example, the consumption of comfrey root tea, estimated by Roitman
(1981) to contain 8.5 mg alkaloid per cup, at the rate of 3 cups
per day, or the ingestion of comfrey leaf at the rate of one leaf
per day, could lead to alkaloid ingestion rates of 0.40 and
0.016 mg/kg. These rates are respectively, much greater than, and
equal to, the lowest daily rate causing veno-occlusive disease. Lower
levels of exposure arising from such sources as the consumption of
milk from cows eating PA-containing plants or of honey derived from
such plants, seems unlikely, in practice, to cause acute or
subacute liver disease. However, care should be exercised. In an
experimental situation in which cows were fed Senecio jacobaea,
the milk was reported to contain up to 0.84 mg alkaloid/litre. A
30-kg child drinking 0.5 litre/day of this milk could ingest
0.014 mg/kg alkaloid, equivalent to 0.028 mg heliotrine/kg (assuming
an LD50 of 150 mg/kg for S. jacobaea alkaloid). This is above the
lowest daily rate leading to veno-occlusive disease and the lowest
estimated total toxic dose would be achieved in 36 days. This
level of contamination of milk is undoubtedly extreme and there is
no knowledge of any contamination of commercial milk supplies.
Honey derived from Echium plantagineum has been reported to contain
up to 1 mg alkaloid/kg (Culvenor et al., 1981). A 30-kg child
consuming 30 g/day of honey (a high consumption rate) would ingest
0.001 mg alkaloid/kg body weight. The lowest estimated total toxic
dose (1.7 mg comfrey alkaloid/kg, very similar to Echium alkaloid)
would be achieved in 1700 days. Although it seems likely that
consumption of contaminated milk and honey would lead to acute or
subacute liver disease, the possibility remains that they may
contribute to chronic liver disease or liver tumours.
The possibility of carcinogenic effects due to long-term
exposure to PA-containing plants has been discussed by Culvenor
(1983). Some of the PAs involved in instances of human poisoning
have been found to be carcinogenic in experimental animals (Table
13). Data from some of the significant experimental studies were
summarized by Culvenor (1983) with approximate estimates of PA
dosages administered to rats in terms of mg/kg body weight per day
(Table 17). The dose rates that were carcinogenic for rats (Table
17) ranged from 2 to 6 mg/kg per day for an initial period and
0.2 - 3 mg/kg per day for a remaining period of about 12 months,
except in one study in which a dose of 10 mg/kg per day was used.
It can be seen that, in all except two instances of human
poisonings summarized in Table 16, the estimated daily rates of
intake ranging from 0.015 to 3.3 mg/kg body weight per day are
within close range of those known to induce tumours in rats. In
other reports, the consumption rates are above and below this
range.
Epidemiological studies to assess the carcinogenic role of PAs
for man are not available. In countries with a high incidence of
primary liver cancer, it is possible that PAs may have an additive
effect with those attributed to aflatoxin (Newberne & Rogers, 1973)
and hepatitis B virus. The total evidence now available warrants
long-term studies of the survivors of poisoning outbreaks,
especially where a substantial number of people were affected, as
in the Afghanistan outbreak.
7.9 Pyrrolizidine Alkaloids as a Chemotherapeutic Agent for Cancer
The PA, indicine N-oxide derived from Heliotropium indicum, a
widely used indigenous drug in Ayurvedic medicine, has been found
to have an antitumour activity and has been used in clinical trials
as a chemotherapeutic agent for leukaemia (Letendre et al., 1981,
1984; Cook et al., 1983) and solid tumours (Kovach et al., 1979a,b;
Nichols et al., 1981; Ohnuma et al., 1982; Taylor et al., 1983).
Dosing schedules typically were 5 consecutive intravenous doses of
0.15 - 3 g/m2 body surface area (approximately 2.5 - 5 mg/kg body
weight) repeated at 4- or 6-week intervals (Kovach et al., 1979a;
Letendre et al., 1981; Ohnuma et al., 1982). Hepatic toxicity, as
judged by increased SGOP levels, was infrequent and mild. However,
subsequent trials with this agent have indicated more serious
hepatotoxicity. In a more recent report by the same workers
(Letendre et al., 1984), 5 out of 22 cases of refractory acute
leukaemia, treated with indicine N-oxide, had severe
hepatotoxicity, presumably induced by the drug. One of these
patients had been treated for 18 months with methyl-testosterone, 4
months prior to receiving indicine N-oxide. Symptoms of severe
hepatocellular failure appeared in 3 patients after the initial
course of treatment. This occurred after 4 daily doses of 3 g/m2
surface area in one patient and after 5 daily doses of 3.75 g/m2
surface area in 2 patients. Two other patients who had received
3 g/m2 surface area daily for 5 days developed hepatocellular failure
after the second course of treatment, one at 3.3 g/m2 and the other
at 3.75 g/m2 surface area, daily, for 5 days. In each patient, the
onset of hepatic disease was rapid and the course was downhill.
Livers of 4 of these patients examined at post-mortem showed severe
centrilobular vascular congestion with necrosis of parenchymal
cells, and, in one patient, a few sublobular veins were found to be
occluded.
Miser et al. (1982) reported severe hepatotoxicity in 4 of 45
children treated with indicine N-oxide for refractory leukaemia or
advanced solid tumours. Similarly, Cook et al. (1983) reported the
case of a 5-year-old child with acute myeloid leukaemia who
developed severe hepatic failure within 3 days of starting the
treatment. Autopsy showed massive hepatic necrosis.
However, it should be noted that no hepatic failure was
reported in more than 100 adults with solid tumours, treated with
the same agent (Kovach et al., 1979a,b; Nichols et al., 1981;
Taylor et al., 1983). No hepatotoxic effects were reported by
Ohnuma et al. (1982) among 37 patients who received this drug for
solid tumours. The major toxic effect was myelosuppression (Kovach
et al., 1979b).
Table 17. Rates of administration of PAs leading to tumours in ratsa
------------------------------------------------------------------------------------------
Alkaloid Dosing schedule Approximate Number of Reference
equivalent rateb rats
(mg/kg per day) developing
tumours
------------------------------------------------------------------------------------------
Lasiocarpine (a) 7 mg/kg diet, 24 months 0.70 23/24 Nat. Cancer
Institute
(1978)
(b) 15 mg/kg diet, 24 months 1.50 24/24 Nat. Cancer
Institute
(1978)
(c) 7.8 mg/kg, ip, 2/week for 2.2 for 4 weeks, 16/18 Svoboda &
4 weeks and 1/week for then 1.1 for Reddy (1972)
52 weeks 52 weeks
(d) 50 mg/kg diet, 55 weeks 5.0 18/20 Rao & Reddy
(1978)
(e) 0.39 mg/kg, ip, 3/week, 0.2 2/7 Culvenor &
to death Jago (1979)
Monocrotaline (a) 25 mg/kg, ip, 1/week for 3.5 for 4 weeks, 10/50 Newberne &
4 weeks, and 8 mg/kg, then 1.1 for Rogers (1973)
for 38 weeks 38 weeks
(b) 5 mg/kg sc, once per 0.36 43/60 Shumaker et
2 weeks for 52 weeks al. (1976)
Retrorsine (a) 30 mg/kg, ip, single dose - 7/29 Schoental &
Bensted (1963)
(b) 30 mg/litre in water, 1.3 4/14 Schoental et
3 days/week, to death al. (1954)
(c) 30 - 50 mg N-oxide/litre 1.3 - 2.0 10/22 Schoental et
in water, 3 days/week al. (1954)
for 20 months
------------------------------------------------------------------------------------------
Table 17. (contd)
------------------------------------------------------------------------------------------
Alkaloid Dosing schedule Approximate Number of Reference
equivalent rateb rats
(mg/kg per day) developing
tumours
------------------------------------------------------------------------------------------
Petasitenine 0.1 g/litre in water, 10 8/10 Hirono et
up to 16 months al. (1977)
Senkirkine 22 mg/kg, ip, 2/week for 6 for 4 weeks, 11/24 Hirono et
4 weeks and 1/week for then 3 for al. (1979a)
52 weeks 25 weeks
Symphytine 13 mg/kg, ip, 2/week for 3.7 for 4 weeks, 5/24 Hirono et
4 weeks and 1/week for then 1.9 for al. (1979a)
52 weeks 52 weeks
------------------------------------------------------------------------------------------
a From: Culvenor (1983).
b Where necessary, estimates assume a daily rat food intake of 100 g/kg body weight, and
water intake 100 ml/kg body weight. Injected doses are given pro rata, for daily
administration.
7.10 Prevention of Poisoning in Man
At present, prevention of poisoning can be achieved only by
reducing or eliminating ingestion of the alkaloids. The two
effective procedures are control of PA-containing plants in
agricultural areas and educational programmes directed to the
populations at risk.
The control of plant populations for this purpose has been
carried out only in Uzbekistan, USSR, following the epidemics of
human disease due to contamination of grain by seeds of
Heliotropium lasiocarpum and Trichodesma incanum. The following
measures were introduced and have been effective in preventing
further outbreaks:
1. A state standard was set for the quality of seed grain, which
must be certified by a State Seed Inspectorate. Current
standards prohibit the sowing of wheat, rye, barley, or oats
contaminated by seed of Heliotropium lasiocarpum or Trichodesma
incanum.
2. A state standard was set for the quality of grain stored for
food. The limits for Heliotropium lasiocarpum and
Trichodesma incanum seeds are 0.2% and zero, respectively.
3. Agricultural (agritechnical) measures to ensure minimum
contamination of crops and harvested grain, including
specification of the most suitable methods and timing of
cultivation, use of clean seed for sowing, weeding of crops
prior to maturing of the grain (towards the end of May), and
mechanical cleaning of grain.
4. Methods for monitoring levels of contamination of flour, bread,
and similar products.
5. Publication of educational booklets describing the biological,
environmental, and morphological characteristics of the toxic
weeds, their pathways of distribution and the causes of the
toxicoses experienced.
6. Promotion of weed control by governmental authorities and
provision of legislation to enforce the control measures.
In other countries, the control of some PA-containing weeds in
crops is practised by cultivation and herbicide treatment, in order
to maximize yield and the general quality of the grain. In
pastures, animal management and herbicide treatments are used to
increase pastures and reduce poisoning of animals. Specific
treatment methods differ according to the plant species and the
circumstances. General references were not available to the Task
Group.
In Australia, where Heliotropium europaeum and Echium
plantagineum are widespread weeds in wheat-growing areas but where
normal agricultural practices prevent all but occasional minor
contamination, relevant tolerance standards for wheat delivered at
storage silos are not specific. Heliotropium europaeum seed is
rarely seen and would form part of the "unmillable material" the
seed component of which can be up to 1% of the volume of the wheat.
Seed of Echium plantagineum is occasionally seen in delivered grain
at levels of up to 10 seeds per half litre, the tolerance level for
this seed fraction being 50 seeds per half litre.
8. BIOLOGICAL CONTROL
Biological control methods have been investigated for several
PA-containing plant species, notably Senecio jacobaea, Heliotropium
europaeum, Echium plantagineum, and Trichodesma incanum. The
effectiveness of such methods is variable and good results may be
confined to certain regions where favourable conditions exist. For
example, in control programmes against S. jacobaea in Australia,
Canada, New Zealand, and the USA, using 3 insect species, results
varied from virtually nil to nearly 100% control (Julien 1982).
The effects of the introduction of the cinnabar moth ( Tyria
jacobaea L.) on S. jacobaea in these countries have been summarized
as in Table 18.
Table 18. Results of the attempted control of Senecio
jacobaea (ragwort) with the cinnabar moth
------------------------------------------------------------
Country or region Result
------------------------------------------------------------
Australia Establishment precluded by predation,
parasitism and disease
Western Canada Moth populations stabilized below that
required for control
Eastern Canada Establishment and subsequent notable
reductions in ragwort levels
New Zealand Marginal establishment, moth population
limited by predation and parasitism,
little impact on ragwort
USA Widespread establishment, ragwort levels
sometimes reduced at localities near the
limits of its distribution
------------------------------------------------------------
Several agents are being tested in Australia for the control of
H. europaeum and one species, a flee beetle Longitarsus albineus,
has been released (Julien, 1982; Delfosse, 1985). There are good
prospects in this country for the biological control of Echium
plantagineum and 2 other Echium spp., with 8 insect species
approved for release, when legal restrictions are lifted (Delfosse
& Cullen, 1985a,b). Preliminary studies have been made on the
biological control of Amsinckia and other Senecio species (e.g.,
Pantone et al., 1985).
Given adequate funding, PA-containing plants are a suitable
target for biological control.
9. EVALUATION OF HUMAN HEALTH RISKS AND EFFECTS ON THE ENVIRONMENT
9.1 Human Exposure Conditions
9.1.1 Reported sources of human exposure
The two main sources of exposure of human beings to toxic PAs
that have led to major outbreaks of poisoning with high mortalities
as well as to individual cases in several countries are:
(a) the contamination of cereal grains, such as wheat and
millet, with the seed or other parts of plants containing
alkaloids; and
(b) the consumption for medicinal or dietary purposes of herbs
containing the alkaloids, either as the plant itself or as
infusions.
Consumption of contaminated grain is more likely to occur in
regions where food is in short supply, and particularly when
drought favours infestation of the grain crop by PA-containing
weeds. A qualitative field test for detecting the presence of
toxic pyrrolizidine alkaloids in plant materials, using simple
laboratory methods, is now available (section 2.2.2.5).
9.1.2 Plant species involved
Plant species containing toxic PAs occur throughout the world
and are known in 47 genera in 6 plant families. As many as 6000
species are potentially PA-containing. The most important genera
responsible for human and animal disease are Senecio and other
genera of the tribe Senecioneae (family Compositae), Crotalaria
(family Leguminosae) and Heliotropium, Trichodesma, and other
genera of the family Boraginaceae (sections 3.1 and 3.2).
Approximately 150 different toxic PAs have been isolated from
about 360 plant species that have been investigated and contain
this type of alkaloid. Of these, about 12 have been involved in
instances of human toxicity (section 3.1). The molecular
structures of almost all of these alkaloids are known and the main
outlines of structure-toxicity relationships have been established
(section 2.1).
The alkaloids may occur in all plant parts and are often
present as the N-oxide derivatives, which are also toxic when
ingested orally. Alkaloid contents vary from low (0.1 g/kg dry
weight) to very high (40 g/kg up to the maximum recorded of
180 g/kg in Senecio riddelli). Levels vary with stage of growth,
locality, and other circumstances. In some, but not all, species,
the alkaloid is partly decomposed during the drying or storage of
the plant. The decomposition is largely enzymic and once the plant
material is dry, the alkaloid is fairly stable.
9.1.3 Modes and pathways of exposure
9.1.3.1 Contamination of grain crops
Large outbreaks of poisoning have occurred through
contamination of wheat crops in Afghanistan, India, and the USSR.
In particular, 3 species of Boraginaceae (Heliotropium lasiocarpum,
H. popovii, and H. europaeum) are well adapted to vigorous growth
under the climatic conditions in which wheat is usually grown.
Contamination can be effectively controlled in wheat produced using
modern harvesting techniques and grain seed that is inspected and
controlled for weed seed contamination, but control of
contamination is more difficult where these conditions cannot be
met. Contamination of staple food grain is of particular concern,
since entire populations are exposed, and control may not be
possible, if the people are not aware of the hazard that
PA-containing weeds present.
9.1.3.2 Herbal medicines
Herbal preparations containing PAs are used as tonics,
treatments, preventatives, and food supplements. Such usages are
so widespread that they are nearly universal. Many are
traditional, while others reflect a rejection of, or lack of access
to, standard health care services (section 3.3.2).
Veno-occlusive disease was first recognized as a clinical
entity in Jamaica as a result of the medicinal use of PA-containing
herbs prepared from Crotalaria. Crotalaria-containing herbs have
also been responsible for human poisonings in Barbados, Equador,
and other locations in the West Indies. Heliotropium herbs have
been reported to cause poisoning in Hong Kong and India.
Symphytum- and Senecio-containing herbs have given rise to case
reports in the USA. Other reports of PA poisoning are known in
which the herbs used were not botanically identified. PA-poisoning
has been associated with both home-prepared and commercially
available herbs, the latter including prescriptions by herbalists
(Weston et al., 1987).
Various other genera of PA-containing plants in the families
Boraginaceae, Compositae, and Leguminosae are also widely used as
herbs. No case reports are available for these genera.
Reported cases of PA poisoning due to the use of the herbs are
geographically widespread, but few in number. However, the scale
on which PA-containing plants are used as herbs, the typically
delayed effects of long-term exposure, and the difficulties of
diagnosis led the Task Group to conclude that there is every
indication of under-reporting of intoxications from the use of such
herbs. Symphytum root preparations, in particular, represent a
hazard, and certain user groups are routinely exposed to levels of
Symphytum alkaloids that are higher than those at which
intoxications have been reported.
The risks associated with the use of PA-containing herbs are
accentuated by the difficulties of controlling this use.
9.1.3.3 PA-containing plants used as food and beverages
Some PA-containing plants are used for food or the making of
beverages in many countries, including developed countries. The
following species are known to be used (though many other plants
are also probably used in this way): Cacalia yatabei, Symphytum
species, Ligularia dentata, Petasites japonicus, Senecio burchellii,
S. inadequidens, S. pierotti, Syneilesis palmata, Crotalaria
anagyrodies, C. brevidens, C. juncea, C. laburnifolia, C. pumila,
C. recta, and C. retusa. No information is available on
the extent to which the different types are consumed (section 3.3.3).
9.1.3.4 Other foods contaminated by PAs
Several species of Boraginaceae are nectar and pollen sources
for bees. Echium plantagineum, in particular, is a widespread weed
in some countries and a substantial source of honey containing a
low level of alkaloid. Senecio species are also visited by bees
and yield alkaloid-containing honey, though Senecio-derived honey
is not known to be produced in quantity for sale. Thus, some
regional and local populations are exposed to a low-level intake
through the presence of PAs in honey, and surveillance may be
desirable in countries producing honey (section 3.3.4).
Under experimental conditions, PAs are transmitted from the
feed of dairy cows and goats into the milk. Some PA-containing
species, such as Senecio jacobaea, S. lautus, and Echium plantagineum,
are weeds in dairy pastures in some countries and are eaten by cattle
under certain conditions. There are no published reports of alkaloid
in milk supplies for human consumption (section 3.3.5).
The Task Group was not aware of any reported cases of
pyrrolizidine toxicity that had been ascribed to either honey or
dairy products.
No information was available to the Task Group on the possible
presence of alkaloids or their metabolites in meat from animals
that had consumed PA-containing plants shortly before slaughter.
The results of metabolic studies in rats have indicated that the
alkaloid is rapidly cleared from the body and, therefore, the
levels of PAs in meat are expected to be very low. However, there
is no information on the possibility of alkaloid accumulating in
storage sites.
9.1.4 Levels of intake
Reliable estimates of levels of intake of PAs, especially in
outbreaks of disease caused by the contamination of cereal crops
with the seeds of toxic plants, are extremely difficult to make.
Sampling of the contaminated grain may not be strictly
representative, since the extent of the contamination may vary in
different sites and households, as is evident from the estimates of
PA intake in the Indian and Afghan outbreaks reported in section
7.3. Furthermore, no accurate record is possible of the amount of
contaminated food consumed over an uncertain length of time. No
records of the levels of toxic PA intake are available in the
earlier reports of human toxicity. Where available, estimates of
intake in outbreaks caused by the contamination of staple food
crops have been made on the basis of random sampling of the
contaminated grain in food stores, and rough estimates of daily
consumption by average adults. Food-on-the-plate analyses have not
been made. The estimated lengths of exposure, and hence the amount
of total intake, are also approximate.
The contamination of cereal grains with the seed of
PA-containing plants has caused major epidemics of human poisoning,
though, in the two instances where estimates of alkaloid intake are
available, the intakes were lower than in some exposures due to the
use of herbal medicines. The estimated intakes are summarized in
Table 16. In an outbreak in India, millet contaminated with
Crotalaria nana seed had an average alkaloid content of 0.5 g/kg,
and the estimated daily intake by the population was 0.66 mg/kg
body weight. In a larger outbreak in Afghanistan, due to the seeds
of Heliotropium popovii in wheat, the level of contamination was
probably variable; representative samples of wheat contained
alkaloid at 0.04 g/kg. The estimated daily intake was 0.033 mg/kg
body weight. These intakes, sustained for periods of approximately
2 and 6 months, respectively, resulted in typical acute and
subacute veno-occlusive disease.
The highest intake rates have been associated with the use or
misuse of medicinal herbs and have resulted in acute liver damage
and death. In two occasions, the consumption of Heliotropium
eichwaldii as a treatment for epilepsy led to an estimated intake
of 3.3 mg/kg body weight daily for 20 or 50 days, and the use of
extracts of Senecio longilobus as medicine for young children led
to estimated intakes of 3 and 0.8 - 1.7 mg/kg body weight. The
highest intake led to extensive liver necrosis. However, it is
possible that, in the above case of poisoning by Heliotropium
eichwaldii, the toxicity was enhanced due to simultaneous
administration of phenobarbitone, which has a potentiating effect
on the microsomal enzymes in the liver cells that convert the PAs
to toxic metabolites.
The use of Heliotropium lasiocarpum as a component of a herbal
treatment for psoriasis involved somewhat lower daily intake rates
of 0.49 - 0.71 mg/kg body weight in 4 patients, who, after periods
of 19 - 46 days, developed veno-occlusive disease. The patient
with the longest intake period and a total intake of 1.4 g alkaloid
or 23 mg/kg body weight died.
The lowest ingestion rate leading to a case of veno-occlusive
disease was also due to medicinal herbal treatment or, more
specifically, to the use of a digestive aid containing a
preparation of comfrey root. Commercial herb and food supplement
preparations containing comfrey leaf or root are on sale in many
countries. Limited assays of one comfrey-pepsin preparation
prepared from comfrey root indicated a PA content of 2.9 g/kg.
Another preparation made from comfrey leaf contained up to 0.27 g
alkaloid/kg. The consumption of these preparations led to an
estimated daily intake of 0.015 mg/kg body weight. Veno-occlusive
disease was diagnosed after a 4- to 6-month period.
The consumption of Symphytum officinale (comfrey) and S. x
uplandicum (Russian comfrey) in the form of food, infusions, or
other preparations is widespread, though the full extent cannot be
estimated. A high level of consumption as salad appears to be
about 5 - 6 leaves per day and consumption as comfrey tea probably
reaches a similar level. Limited assays indicate that the average
alkaloid content of the leaf is about 1 mg/leaf, the concentration
being higher in the younger, smaller leaves. The alkaloid intake
from comfrey leaves could therefore vary from a low value, up to
about 6 mg/day, or 0.1 mg/kg body weight for an adult, an intake
within the range producing veno-occlusive disease. However, the
Task Group noted that some people claim to have consumed comfrey at
such a rate without suffering any disease.
Overall, the estimates of intake of PAs by human beings (Table
16) indicate that ingestion rates above 0.015 mg/kg body weight for
the mixture of echimidine and related alkaloids in comfrey may lead
to acute or subacute liver disease. If expressed in terms of
equivalent doses of heliotrine (section 7.8), the estimated total
doses in the known outbreaks or cases of veno-occlusive disease
range from 1 to 167 mg/kg body weight. There is little real
difference in the ranges of estimated total doses in non-fatal
cases (1 - 120 mg/kg body weight) and those leading to death
(6 - 167 mg/kg). These figures, when compared with the total
lethal dose of several PAs in rats, i.e., 1.2 - 10.9 times the LD50
dose (equivalent to 360 - 3270 mg heliotrine/kg) (Table 10), would
seem to indicate that man is markedly more sensitive than the rat
to the toxic effects of PAs with regard to the development of acute
and chronic effects on the liver. It should be noted that these
estimates are based on limited raw data and a number of
assumptions, and so are of uncertain reliability.
The dose estimates indicate strongly that the effects of PAs in
human beings are cumulative at very low intake rates. Lower rates
of intake of PAs may lead to chronic forms of intoxication, though,
at present, there is no evidence on which the degree of risk in
these circumstances can be evaluated. The information available on
dose-response relationships is very limited, but the data support
the conclusion that even low rates of intake of PAs over a period
of time may present a health risk and that exposure should be
minimized wherever possible.
There has not been any systematic monitoring of PAs in cereal
grains, food products, and herbal medicines. Analytical surveys of
these materials are feasible, but it would be difficult to design
surveys that would give direct estimates of the dietary intake of
PAs.
9.2 Acute Effects of Exposure
9.2.1 Acute liver disease
All cases of human intoxication in reported accounts have been
in the acute phase of the disease, the dominant symptom being
rapidly filling ascites. The disease can affect large
subpopulations and, in one study, up to 22.6% of the population was
affected.
Children appear to be the most vulnerable group and mortality
can be high at the extremes of age. The liver is the principal
target organ. In the acute stage of the disease, the liver shows a
characteristic centrilobular haemorrhagic necrosis, which in man is
accompanied by occlusion of the hepatic veins. However,
characteristic veno-occlusive lesions, seen in the central veins of
hepatic lobules, may not always be evident in the needle biopsy
examination of the liver, but are always apparent on examination of
the autopsy material.
9.3 Chronic Effects of Exposure
9.3.1 Cirrhosis of the liver
There is evidence that the administration of a single dose of
PA to experimental animals or a single acute episode of illness in
man, following brief consumption of PA-containing herbs or
PA-contaminated food, may lead to progressive chronic liver disease
resulting in cirrhosis. Cirrhosis may also be a consequence of
long-term low-dose administration of PAs to experimental animals
and possibly also of long-term low intake of PAs by human beings,
though there is no proof of the latter. Cirrhosis resulting from
the toxic effects of PAs in the advanced stage, may not be
distinguishable from that resulting from other causes (sections
6.4.1.5 and 7.4). The Task Group did not find any evidence
suggesting that PAs are a causative factor of the specific disease,
Indian Childhood Cirrhosis (section 7.5).
9.3.2 Mutagenicity and teratogenicity
Several PAs, PA-derivatives, and related compounds have been
shown to produce chromosome aberrations in plants and several cell
culture systems, mutagenic effects ( Salmonella ("Ame's"), sister
chromatid exchanges, and other tests), and teratogenic and
fetotoxic effects in experimental animals (sections 6.4.5, 6.4.6,
6.4.7). Chromosomal aberrations have been reported in the blood
cells of children suffering from veno-occlusive disease, believed
to have been caused by fulvine. The Task Group was not aware of
data on the teratogenic/fetotoxic effects of PAs on human beings
and was unable to evaluate the potential for these effects in PA
exposure.
9.3.3 Cancer of the liver
A relatively large number of people have been exposed, in the
past, to PAs and have suffered acute and chronic toxic effects.
However, no information is available on the long-term follow-up of
these populations, to ascertain whether this type of exposure could
have resulted in an increased incidence of liver cancer or other
types of cancer. Because of this lack of knowledge, it is not
possible, at present, to make an evaluation of the cancer risk due
to PAs. However, various PAs have been shown to be carcinogenic
for experimental animals, which implies that a potential cancer
risk for human beings should be seriously considered.
Of several PAs evaluated for carcinogenicity by IARC (1976,
1983), there is "sufficient or limited evidence" for the
carcinogenicity in experimental animals (IARC, 1976) of
monocrotaline, retrorsine, isatidine, lasiocarpine, petasitenine,
senkirkine, and of extracts of the PA-containing plants Petasites
japonicum, Tussilago farara, Symphytum officinale, Senecio
longilobus, Senecio numorensis, Farfugium japonicum, and Senecio
cannabifolius. These studies were carried out mainly on rats, with
few studies on mice or hamsters (section 6.4.8). The
carcinogenicity data obtained with other PAs are difficult to
evaluate, because of the limited number of treated animals and the
lack of adequate numbers as controls. The main target organ is the
liver, where liver cell tumours and haemangioendothelial sarcomas
were observed. In some instances, tumours in extra-hepatic tissues
(lung, pancreas, intestine) were also observed, namely with
monocrotaline, retrorsine, and lasiocarpine. Some PAs, for
example, retrorsine, have been shown to be carcinogenic after a
single dose. The pyrrolic metabolites have also been shown to be
carcinogenic for rats.
It may be recalled that several of the PAs involved in human
poisoning include the above compounds. It is notable that the dose
rates that have been effective in inducing tumours in rats, mostly
equivalent to 0.2 - 3 mg/kg body weight per day (Table 17), are
roughly similar in magnitude to estimated intake rates (0.49 -
3.3 mg/kg body weight per day) (Table 16) in several episodes of human
toxicity. Comparison of the total intakes resulting in human
toxicity with the total doses to death observed in the chronic
toxicity studies on rats indicates that human beings are more
susceptible (section 7.8) and suggests that human beings may
survive for sufficient time to develop cancer after only a brief
exposure at this level or a longer exposure at a markedly lower
level. A more quantitative assessment is not possible on the basis
of the available information, and the Task Group stressed the need
for appropriate epidemiological studies.
9.3.4 Effects on other organs
Substantiated reports of PA-induced extra-hepatic injury in man
are limited to Trichodesma intoxication, in which symptoms and
signs were predominantly neurological. The range of organs
affected by other PAs in experimental and farm animals suggests
that exposure of human beings to other PAs may also carry the
potential for extra-hepatic injury.
There are extensive reports of experimental studies in which
PAs have been demonstrated to produce the characteristic vascular
changes of primary pulmonary hypertension and consequent right
ventricular hypertrophy of the heart in rats and non-human primates
(section 6.4.2). Susceptibility is age dependent, weanling rats
being more vulnerable than older animals. There is only
circumstantial evidence of PA-induced pulmonary vascular disease in
one patient (section 7.6), but judging by the experimental evidence
available, it is possible that human beings may be susceptible to
PA-induced cardiopulmonary changes.
In the opinion of the Task Group, the neurological involvement
which is a dominant feature in PA-intoxicated horses and is also
seen in cows and sheep, cannot be explained solely as a consequence
of liver damage. Central nervous system lesions have been
demonstrated in sheep, pigs, and rats. Distribution studies of the
radiolabelled metabolite, 3H-dehydroretronecine, show increasing
accumulation of radioactivity in the brain with time.
Trichodesma alkaloids, structurally related to monocrotaline,
are neurotoxic agents. Trichodesma toxicosis in man has been
reported only from the USSR, together with several studies on
experimental animals. Detailed reports on the pathological
findings were not available to the Task Group, but the information
available indicated that the central nervous system was the primary
target organ (sections 6.4.3 and 7.7).
Stomach and intestinal lesions have been shown in PA-exposed
sheep, mice, cows, and rats. Distribution studies with
radiolabelled pyrroles showed a high retention of radioactivity in
the stomach, consistent with the acid-sensitive nature of the
pyrroles. In rats, pyrrolic metabolites are secreted in high
concentrations in the bile.
Kidney changes following to PA administration have been shown
in mice, pigs, horses, sheep, and monkeys. Pyrrolizidine
metabolites have been found covalently bound to kidney DNA in rats.
Urinary excretion is a major route of excretion of metabolic
products of PAs in rats.
There is no evidence of involvement of organs other than the
liver and central nervous system ascribed primarily to PA toxicity
in any of the published human case reports. It is possible that
under some circumstances, other major organ systems may also be at
risk. As bioactivation of PAs has been demonstrated only in the
liver, the risk of damage should be expected to be lower in the
organs.
9.4 Effects on the Environment
9.4.1 Agriculture
In some countries, PA-containing weeds densely cover areas of
up to thousands of square kilometres. Their adverse effects
include the covering of pastures, additional costs in agricultural
production, and the poisoning of farm animals. The toxicity of PAs
for farm animals, including sheep, cattle, horses, pigs, goats, and
poultry, which has been the inspiration for much of the investigation
of PA toxicity. In Australia, for example, Heliotropium europaeum
and Echium plantagineum cause the death of thousands of animals
annually (section 6.2).
9.4.2 Wild-life
By contrast, little is known about the consumption of PA-containing
plants by wild-life, or of their individual sensitivities. The death
of deer in Louisiana has been ascribed to eating Heliotropium or
Crotalaria species, and an experimental study has shown that the
rainbow trout (Salmo gairdneri) is sensitive to Senecio jacobaea
alkaloids (sections 6.5.1 and 6.5.2).
There is no information on the effects of the alkaloids on
field rodents or other seed-eating mammals and birds that might be
expected to consume seeds of PA-containing plants and to suffer
toxic effects.
9.4.3 Insects
Many species of insects, such as some moths of the family
Arctidae and butterflies of the sub-families Danainae and
Ithominae, have become dependent on PA-containing plants, using the
alkaloids as defensive chemicals and derivatives of them as
pheromones and other signalling chemicals. Thus, complete
elimination of PA-containing plants in a region might lead to a
marked reduction in the local population of insects of this type
(section 6.5.3).
9.4.4 Soil and water
There have not been any studies on the fate of PAs when the
plants in which they occur wilt and age. If alkaloid is leached
into soil or water, it is probably readily degraded by microorganisms
since, as a base and ester, it is subject to oxidative and hydrolytic
reactions.
REFERENCES
AFZELIUS, B.A. & SCHOENTAL, R. (1967) The ultrastructure of
enlarged hepatocytes induced in rats with a single oral dose of
retrorsine, pyrrolizidine (Senecio) alkaloids. J. ultra-struct.
Res., 20: 328-345.
AIKAT, B.K., BHUSNURMATH, S.R., DATTA, D.V., & CHHUTTANI, P.N.
(1978) Veno-occlusive disease in north-west India. Indian J.
Pathol. Microbiol., 21: 203-211.
AL-HASANY, M. & MOHAMED, A.S. (1970) Veno-occlusive disease of the
liver in Iraq. Arch. dis. Child., 45: 722-724.
ALLEN, J.R. & CARSTENS, L.A. (1968) Veno-occlusive disease in
rhesus monkeys. Am. J. vet. Res., 29: 1681-1694.
ALLEN, J.R. & CARSTENS, L.A. (1970) Pulmonary vascular occlusions
initiated by endothelial lysis in monocrotaline-intoxicated rats.
Exp. mol. Pathol.., 13: 159-171.
ALLEN, J.R. & CARSTENS, L.A. (1971) Monocrotaline induced Budd-
Chiari syndrome in monkeys. Am. J. dig. Dis., 16: 111-121.
ALLEN, J.R. & CHESNEY, C.F. (1972) Effect of age on development of
cor pulmonale in non-human primates following pyrrolizidine
alkaloid intoxication. Exp. mol. Pathol., 17: 220-232.
ALLEN, J.R., CHILDS, G.R., & CRAVENS, W.W. (1960) Crotalaria
spectabilis toxicity in chickens. Proc. Soc. Exp. Biol. Med., 104:
434-436.
ALLEN, J.R., LALICH, J.J., & SCHMUTTLE, S.M. (1963) Crotalaria
spectabilis induced cirrhosis in turkeys. Lab. Invest., 12: 512-517.
ALLEN, J.R., CARSTENS, L.A., & OLSON, B.E. (1967) Veno-occlusive
disease in Macaca speciosa monkeys. Am. J. Pathol., 50: 653-667.
ALLEN, J.R., CARSTENS, L.A., & KATAGIRI, G.J. (1969) Hepatic veins
of monkeys with veno-occlusive disease-sequential ultrastructural
changes. Arch. Pathol., 87: 279-289.
ALLEN, J.R., CHESNEY, C.F., & FRAZEE, W.J. (1972) Modifications of
pyrrolizidine alkaloids intoxication resulting from altered
microsomal enzymes. Toxicol. appl. Pharmacol., 23: 470-479.
ALLEN, J.R., HSU, I.-C., & CARSTENS, L.A. (1975) Dehydroretronecine
induced rhabdomyosarcomas in rats. Cancer Res., 35: 997-1002.
AMES, M.M. & POWIS, G. (1978) Determination of indicine N-oxide
and indicine in plasma and urine by electron-capture gas-liquid
chromatography. J. Chromatogr., 166: 519-526.
ARMSTRONG, S.J. & ZUCKERMAN, A.J. (1970) Production of pyrroles
from pyrrolizidine alkaloids by human embryo tissue. Nature
(Lond.), 228: 569-570.
ARMSTRONG, S.J., ZUCKERMAN, A.J., & BIRD, R.G. (1972) Induction of
morphological changes in human embryo liver cells by the
pyrrolizidine alkaloid lasiocarpine. Br. J. exp. Pathol., 53:
145-149.
ARORA, R.R., PYARELAL, GHOSH, T.K., MATHUR, K.K., & TANDON, B.N.
(1981) Epidemiology of veno-occlusive disease in tribal population
of Madhya Pradesh and Bihar. J. commun. Dis. (India), 13: 147-151.
ARSECULERATNE, S.N., GUNATILAKA, A.A.L., & PANABOKKE, R.G. (1981)
Studies on medicinal plants of Sri Lanka: occurrence of
pyrrolizidine alkaloids and hepatotoxic properties in some
traditional medicinal herbs. J. Ethnopharmacol., 4: 159-177.
ASADA, Y., FURUYA, T., & MURAKAMI, N. (1981) Pyrrolizidine
alkaloids from Ligularia japonica. Planta Med., 42: 202-203.
ASADA, Y., SHIRAISHI, M., TAKEUCHI, T., OSAWA, Y., & FURUYA, T.
(1985) Pyrrolizidine alkaloids from Crassocephalum crepidioides.
Planta Med., 51: 539-540.
BARNES, J.M., MAGEE, P.N., & SCHOENTAL, R. (1964) Lesions in the
lungs and livers of rats poisoned with pyrrolizidine alkaloids
fulvine and its N-oxide. J. Pathol. Bacteriol., 88: 521-531.
BERRY, D.M. & BRAS, G. (1957) Venous occlusion of the liver in
Crotalaria and Senecio poisoning. North Am. Vet., 38: 323-327.
BHATTACHARYA, K.J. (1965) Foetal and neonatal responses to
hepatotoxic agents. J. Pathol. Bacteriol., 90: 151-161.
BICCHI, C., D'AMATO, A., & CAPPELLETTI, E. (1985) Determination
of pyrrolizidine alkaloids in Senecio inaequidens D.C. by
capillary gas chromatography. J. Chromatogr., 349: 23-29.
BICK, Y.A.E. (1970) Comparison of the effects of LSD, heliotrine,
and X-irradiation on chromosome breakage and the effects of LSD on
the rats of cell division. Nature (Lond.), 226: 1165-1167.
BICK, Y.A.E. & CULVENOR, C.C.J. (1971) Effects of dehydro-
heliotridine, a metabolite of pyrrolizidine alkaloids on chromosome
structure and cell division in cultures of animal cells. Cytobios,
3: 245-255.
BICK, Y.A.E. & JACKSON, W.D. (1968) Effects of the pyrrolizidine
alkaloid heliotrine on cell division and chromosome breakage in
cultures of leucocytes from the marsupial Potorus tridactylus.
Aust. J. biol. Sci., 21: 469-481.
BICK, Y.A.E., CULVENOR, C.C.J., & JAGO, M.V. (1975) Comparative
effects of pyrrolizidine alkaloids and related compounds on
leukocyte cultures from Potorus tridactylus. Cytobios, 14: 151-160.
BIRECKA, H., FROHLICH, M.W., HULL, L., & CHASKES, M.J. (1980)
Pyrrolizidine alkaloids of Heliotropium from Mexico and adjacent
USA. Phytochemistry, 19: 421-426.
BIRECKA, H., CATALFAMO, J.L., & EISEN, R.N. (1981) A sensitive
method for detection and quantitative determination of
pyrrolizidine alkaloids. Phytochemistry, 20: 343-344.
BLACK, D.N. & JAGO, M.V. (1970) Interaction of
dehydroheliotridine, a metabolite of heliotridine based
pyrrolizidine alkaloids, with natural and heat denatured RNA.
Biochem. J., 118: 347-353.
BOHLMANN, F., KLOSE, W., & NIKISCH, K. (1979) [Synthesis of
dehydroheliotridine and 3-oxy-dehydroheliotridine.] Tetrahedr.
Lett., 1979: 3699-3702 (in German).
BOHLMANN, F., ZDERO, C., JAKUPOVIC, J., GRENZ, M., CASTRO, V.,
KING, R.M., ROBINSON, H., & VINCENT, L.P.D. (1986) Further
pyrrolizidine alkaloids and furoeremophilanes from Senecio species.
Phytochemistry, 15: 1151-1159.
BOPPRE, M. (1986) Insects pharmacophagously utilizing defensive
plant chemicals (pyrrolizidine alkaloids). Naturwiss enschaften, 73:
17-26.
BRAGINSKII, B.M. & BOBOKHADZAEV, I. (1965) [Hepatosplenomegaly
against the background of heliotropic toxicosis.] Sov. Med., 28:
57-60 (in Russian).
BRAS, G. (1973) Aspects of hepatic vascular diseases. In: Gall,
E.A. & Mostofi, F.K., ed. The liver: International Academy of
Pathology monograph, Baltimore, Maryland, Williams and Wilkins,
pp. 406-530.
BRAS, G. & HILL, K.R. (1956) Veno-occlusive disease of the liver -
essential pathology. Lancet, 2: 161-163.
BRAS, G. & WATLER, D.C. (1955) Further observations on the
morphology of veno-occlusive disease of the liver in Jamaica. West
Indian med. J., 4: 201-211.
BRAS, G., JELLIFFE, D.B., & STUART, K.L. (1954) Veno-occlusive
disease of the liver with nonportal type of cirrhosis occurring in
Jamaica. Arch. Pathol., 57: 285-300.
BRAS, G., BERRY, D.M., & GYORGI, P. (1957) Plants as etiological
factor in veno-occlusive disease of liver. Lancet, 1: 960-962.
BRAS, G., BROOKS, S.E.H., & WATLER, D.C. (1961) Cirrhosis of liver
in Jamaica. J. Pathol. Bacteriol., 82: 503-512.
BRAUCHLI, J., LUTHY, J., ZWEIFEL, U., & SCHLATTER, C. (1982)
Pyrrolizidine alkaloids from Symphytum officinale L. and their
percutaneous absorption in rats. Experientia (Basel), 38:
1085-1087.
BRIGGS, L.H., CAMBIE, R.C., CANDY, B.J., O'DONOVAN, G.M., RUSSELL,
R.A., & SEELYE, R.N. (1965) Alkaloids of New Zealand Senecio
species. Part II: senkirkine. J. Chem. Soc., C1965: 2492-2498.
BRINK, N.G. (1982) Somatic and teratogenic effects induced by
heliotrine in Drosophilia. Mutat. Res., 104: 105-111.
BROOKS, W.E.H., MILLER, C.G., MCKENZIE, K., AUDRETSCH, J.J., &
BRAS, G. (1970) Acute veno-occlusive disease of the liver. Fine
structure in Jamaican children. Arch. Pathol., 89: 507-529.
BROWN, K.S. (1984) Chemical ecology of dehydropyrrolizidine
alkaloids in adult Ithomiinae (Lepidoptera: Nymphalidae). Rev.
Bras. Biol., 44: 435-440.
BRUGGEMAN, I.M. & VAN DER HOEVEN, J.C.M. (1985) Induction of SCEs
by some pyrrolizidine alkaloids in V79 Chinese hamster cells co-
cultured with chick embryo hepatocytes. Mutat. Res., 142: 209-212.
BRUNER, L.H., CARPENTER, L.J., HAMLOW, P., & ROTH, R.A. (1986)
Effect of a mixed-function oxidase inducer and inhibitor on
monocrotaline pyrrole pneumotoxicity. Toxicol. appl. Pharmacol., 85:
416-427.
BUCKMASTER, G.W., CHEEKE, P.R., ARSCOTT, G.H., DICKINSON, E.D.,
PIERSON, M.L., & SHULL, L.R. (1977) Response of Japanese quail to
dietary and injected pyrrolizidine (Senecio) alkaloid. J. anim.
Sci., 45(6): 1322-1325.
BULL, L.B. (1955) The histological evidence of liver damage from
pyrrolizidine alkaloids. Aust. vet. J., 31: 33-40.
BULL, L.B. & DICK, A.T. (1959) The chronic pathological effects on
the liver of the rat of the pyrrolizidine alkaloids heliotrine,
lasiocarpine, and their N-oxides. J. Pathol. Bacteriol., 78: 483-502.
BULL, L.B. & DICK, A.T. (1960) The function of total dose in the
production of chronic lethal disease in rats by periodic injections
of the pyrrolizidine alkaloid heliotrine. Aust. J. exp. Biol. med.
Sci., 38: 515.
BULL, L.B., DICK, A.T., KEAST, J.C., & EDGAR, G. (1956) An
experimental investigation of the hepatotoxic and other effects on
sheep of consumption of Heliotropium europaeum L. Heliotropium
poisoning of sheep. Aust. J. agric. Res., 7: 281-332.
BULL, L.B., DICK, A.T., & MCKENZIE, J.S. (1958) The acute effects
of heliotrine and lasiocarpine and their N-oxides on the rat. J.
Pathol. Bacteriol., 75: 17-25.
BULL, L.B., CULVENOR, C.C.J., & DICK, A.T. (1968) The
pyrrolizidine alkaloids, Amsterdam, North Holland Publishing Co.
BURGUERA, J.A., EDDS, G.T., & OSUNA, O. (1983) Influence of
selenium on aflatoxin B1 or Crotalaria toxicity in turkey poults.
Am. J. vet. Res., 44: 1714-1717.
BURNS, J. (1972) The heart and pulmonary arteries in rat fed on
Senecio jacobaea. J. Pathol., 106: 187-194.
BUTLER, W.H., MATTOCKS, A.R., & BARNES, J.M. (1970) Lesions in the
liver and lungs of rats given pyrrole derivatives of pyrrolizidine
alkaloids. J. Pathol., 100: 169-175.
CAMPBELL, J.G. (1956) An investigation of the hepatotoxic effects
in the fowl of ragwort ( Senecio jacobaea Linn) with special
reference to the induction of liver tumours with seneciphylline.
Proc. R. Soc. Edinburgh, B66: 111-130.
CAMPBELL, J.G. (1957a) Studies on the influence of sex hormones on
the avian liver. II. Acute liver damage in the male fowl and the
protective effect of oestrogen, as determined by a liver function
test. J. Endocrinol., 15: 346-350.
CAMPBELL, J.G. (1957b) Studies on the influence of sex hormones on
avian liver. III. Oestrogen-induced regeneration of the chronically
damaged liver. J. Endocrinol., 15: 351-354.
CANDRIAN, U., LUTHY, J., GRAF, U., & SCHLATTER, CH. (1984a)
Mutagenic activity of the pyrrolizidine alkaloids seneciphylline
and senkirkine in Drosophila and their transfer into rat milk.
Food chem. Toxicol., 22: 223-225.
CANDRIAN, U., LUTHY, J., SCHMID, P., SCHLATTER, CH., & GALLASZ, E.
(1984b) Stability of pyrrolizidine alkaloids in hay and silage. J.
agric. food Chem., 32: 935-937.
CANDRIAN, U., LUTHY, J., & SCHLATTER, CH. (1985) In vivo binding
of retronecine-labelled (3H), seneciphylline, and 3H-senecionine to
DNA of rat liver, lung and kidney. Chem.-biol. Interact., 54: 57-69.
CARILLO, L. & AVIADO, D.M. (1969) Monocrotaline induced pulmonary
hypertension and p-chlorophenylalanine. Lab. Invest., 20: 213-218.
CARSTENS, L.A. & ALLEN, J.R. (1970) Arterial degeneration and
glomerular hyalinization in the kidney of monocrotaline intoxicated
rats. Am. J. Pathol., 60: 75-92.
CHALMERS, A.H., CULVENOR, C.C.J., & SMITH, L.W. (1965)
Characterization of pyrrolizidine alkaloids by gas, thin layer, and
paper chromatography. J. Chromatogr., 20: 270-277.
CHEEKE, P.R. & GORMAN, G.R. (1974) Influence of dietary protein
and sulphur amino-acid levels on the toxicity of Senecio jacobaea
(tansy ragwort) to rats. Nutr. Rep. Int., 9: 197-207.
CHEEKE, P.R. & PIERSON-GOEGER, M.L. (1983) Toxicity of Senecio
jacobaea and pyrrolizidine alkaloids in various laboratory animals
and avian species. Toxicol. Lett., 18: 343-349.
CHEN, K.K. (1945) Pharmacology. Hepatotoxic alkaloids. Ann. Rev.
Physiol., 7: 695-697.
CHEN, K.K., HARRIS, P.N., & ROSE, C.L. (1940) The action and
toxicity of platyphylline and seneciphylline. J. Pharmacol. exp.
Ther., 68: 130-140.
CHESNEY, C.F. & ALLEN, J.R. (1973a) Resistance of the guinea pig
to pyrrolizidine alkaloid intoxication. Toxicol. appl. Pharmacol.,
26: 385-392.
CHESNEY, C.F. & ALLEN, J.R. (1973b) Endocardial fibrosis
associated with monocrotaline induced pulmonary hypertension in
non-human primates (Macaca arctoides). Am. J. vet. Res., 34:
1577-1581.
CHESNEY, C.F., HSU, I.C., & ALLEN, J.R. (1974) Modifications of
the in vitro metabolism of the hepatotoxic pyrrolizidine alkaloid,
monocrotaline. Res. Commun. chem. Pathol. Pharmacol., 8: 567-570.
CHOPRA, R.N., ed. (1933) Indigenous drugs of India, Calcutta, The
Art Press.
CLARK, A.M. (1959) Mutagenic activity of the alkaloid heliotrine
in Drosophila. Nature (Lond.), 183: 731-732.
CLARK, A.M. (1976) Naturally occurring mutagens. Mutat. Res., 32:
361-174.
COOK, B.A., SINNHUBER, J.R., THOMAS, P.J., OLSON, T.A., SILVERMAN,
T.A., JONES, R., WHITEHEAD, V.M., & ROYMANN, F.B. (1983) Hepatic
failure secondary to indicine N-oxide toxicity. A pediatric
oncology group study. Cancer, 52: 61-63.
COOK, J.W., DUFFY, E., & SCHOENTAL, R. (1950) Primary liver
tumours in rats following feeding with alkaloids of Senecio
jacobaea. Br. J. Cancer, 4: 405-410.
CRAIG, A.M., SHEGGEBY, G., & WICKS, C.E. (1984) Large scale
extraction of pyrrolizidine alkaloids from tansy ragwort (Senecio
jacobaea). Vet. hum. Toxicol., 26: 108-111.
CULVENOR, C.C.J. (1968) Tumour inhibitory activity of
pyrrolizidine alkaloids. J. pharm. Sci., 57: 1112-1117.
CULVENOR, C.C.J. (1978) Pyrrolizidine alkaloids: occurrence and
systematic importance in angiosperms. Bot. Notiser, 131: 473-486.
CULVENOR, C.C.J. (1980) Alkaloids and human disease. In: Smith,
R.L. & Bababunmi, E.A., ed. Toxicology in the tropics, London,
Taylor & Francis Ltd., pp. 124-141.
CULVENOR, C.C.J. (1983) Estimated intakes of pyrrolizidine
alkaloids by humans. A comparison with dose rates causing tumours
in rats. J. Toxicol. environ. Health, 11: 625-635.
CULVENOR, C.C.J. (1985) Pyrrolizidine alkaloids: some aspects of the
Australian involvement. Trends pharmacol. Sci., 6: 18-22.
CULVENOR, C.C.J. & JAGO, M.V. (1979) Carcinogenic plant products
and DNA. In: Grover, P.L., ed. Chemical carcinogens and DNA, Boca
Raton, Florida, CRC Press, Vol. 1, 161 pp.
CULVENOR, C.C.J., DOWNING, D.T., EDGAR, J.A., & JAGO, M.V. (1969)
Pyrrolizidine alkaloids as alkylating and antimitotic agents. N.Y.
Acad. Sci., 163: 837-847.
CULVENOR, C.C.J., EDGAR, J.A., SMITH, L.W., & TWEEDDALE, H.J.
(1970a) Dihydropyrrolizines. III. Preparation and reactions of
derivatives related to pyrrolizidine alkaloids. Aust. J. Chem., 23:
1853-1867.
CULVENOR, C.C.J., EDGAR, J.A., SMITH, L.W., & TWEEDDALE, H.J.
(1970b) Dehydrolizines. IV. Manganese dioxide oxidation of
1,2-dihydropyrrolizidines. Aust. J. Chem., 23: 1869-1879.
CULVENOR, C.C.J., EDGAR, J.A., JAGO, M.V., OUTTERIDGE, A., PETERSON
J.E., & SMITH, L.W. (1976a) Hepato- and pneumotoxicity of
pyrrolizidine alkaloids and derivatives in relation to molecular
structure. Chem.-biol. Interact., 12: 299-324.
CULVENOR, C.C.J., EDGAR, J.A., SMITH, L.W., & HIRONO, I. (1976b)
The occurrence of senkirkine in Tussilago farfara. Aust. J. Chem.,
29: 229-233.
CULVENOR, C.C.J., CLARKE, M., EDGAR, J.A., FRAHN, J.L., JAGO, M.V.,
PETERSON, J.E., & SMITH, L.W. (1980a) Structure and toxicity of
the alkaloids of Russian comfrey ( Symphytum x uplandicum Nyman), a
medicinal herb and item of human diet. Experientia (Basel), 36:
377-389.
CULVENOR, C.C.J., EDGAR, J.A., FRAHN, J.L., & SMITH, L.W. (1980b)
The alkaloids of Symphytum x uplandicum (Russian comfrey). Aust.
J. Chem., 33: 1105-1113.
CULVENOR, C.C.J., EDGAR, J.A., & SMITH, L.W. (1981) Pyrrolizidine
alkaloids in honey from Echium plantagineum L. J. agric. food
Chem., 29: 958-960.
CULVENOR, C.C.J., JAGO, M.V., PETERSON, J.E., SMITH, L.W., PAYNE,
A.L, CAMPBELL, D.G., EDGAR, J.A., & FRAHN, J.L. (1984) Toxicity of
Echium plantagineum (Paterson's curse). I. Marginal toxic effects
of Merino wethers from long-term feeding. Aust. J. agric. Res., 35:
293-304.
CULVENOR, C.C.J., EDGAR, J.A., SMITH, L.W., KUMANA, C.R., & LIN,
H.J. (1986) Heliotropium lasiocarpum Fisch and Mey identified as
cause of veno-occlusive disease due to a herbal tea. Lancet, 1: 978.
DANN, A.T. (1960) Detection of N-oxides of the pyrrolizidine
alkaloids. Nature, 4730: 1051.
DANNINGER, T., HAGEMANN, U., SCHMIDT, V., & SCHOENHOEFER, P.S.
(1983) Toxicity of pyrrolizidine alkaloid-containing medicinal
plants. Pharm. Ztg, 128: 289-303.
DATTA, D.V., KHUROO, M.S., MATTOCKS, A.R., AIKAT, B.K., &
CHHUTTANI, P.N. (1978a) Veno-occlusive disease of liver due to
Heliotropium plant used as medicinal herb (report of six cases with
review of literature). J. Assoc. Phys. India, 26(5): 383-393.
DATTA, D.V., KHUROO, M.S., MATTOCKS, A.R., AIKAT, B.K., &
CHHUTTANNI, P.N. (1978b) Herbal medicines and veno-occlusive
disease in India. Postgrad. med. J., 54: 511-515.
DAVIDSON, C.S. (1963) Plants and fungi as etiologic agents of
cirrhosis. New Engl. J. Med., 268: 1072-1073.
DAVIDSON, J. (1935) The action of retrorsine on rat's liver. J.
Pathol. Bacteriol., 40: 285-295.
DEAGEN, J.T. & DEINZER, M.L. (1977) Improvement in the extraction
of pyrrolizidine alkaloids. Lloydia, 40: 395-397.
DEAN, R.E. & WINWARD, A.H. (1974) An investigation into the
possibility of tansy ragwort poisoning of black-tailed deer. J.
wildl. Dis., 10: 166-169
DEINZER, M.L., THOMSON, P.A., BURGETT, D.M., & ISAACSON, D.L.
(1977) Pyrrolizidine alkaloids: their occurrence in honey from
tansy ragwort (S. jacobaea). Science, 195: 497-499.
DEINZER, M.L., THOMSON, P.A., GRIFFIN, D., & DICKINSON, E. (1978)
Sensitive analytical method for pyrrolizidine alkaloids. The mass
spectra of retronecine derivatives. Biomed. mass Spectrom., 5:
175-179.
DELFOSSE, E.S. (1985) Re-evaluation of the biological control
program for Heliotropium europaeum in Australia. In: Delfosse,
E.S., ed. Proceedings of the VI International Symposium on
Biological Control of Weeds, 19-25 August 1984, Vancouver, Canada,
Ottawa Agriculture Canada, pp. 735-42.
DELFOSSE, E.S. & CULLEN, J.M. (1985a) CSIRO division of Entomology
Submission to the enquiries into biological control of Echium
plantagineum L., Paterson's curse/Salvation Jane. Plant Prot. Q.,
1: 24-40.
DELFOSSE, E.S. & CULLEN, J.M. (1985b) Echium plantagineum:
catalyst for conflict and change in Australia. In: Delfosse, E.S.,
ed. Proceedings of the VI International Symposium on Biological
Control of Weeds, 19-25 August, 1984, Vancouver, Canada, Ottawa
Agriculture Canada pp. 249-292.
DELORME, P., JAY, M., & FERRY, S. (1977) Phytochemical inventory
in indigenous Boraginaceae: study of the alkaloids and polyphenolic
compounds (anthocyanic and flavonoid compounds). Plant. Méd.
Phytothér., 11: 5-11. (in french)
DE WAAL, H.L. & VAN TWISK, P. (1964) The chemical composition of
four Senecio species from Kruger National Park. Koedoe (South
Africa), 7: 40-42.
DICK, A.T., DANN, A.T., BULL, L.B., & CULVENOR, C.C.J. (1963)
Vitamin B12 and the detoxication of hepatotoxic pyrrolizidine
alkaloids in rumen liquor. Nature (Lond.), 197: 207-208.
DICKINSON, J.O. (1980) Release of pyrrolizidine alkaloids into
milk. Proc. West. Pharmacol. Soc., 23: 377-379.
DICKINSON, J.O., COOKE, M.P., KING, R.R., & MOHAMED, P.A. (1976)
Milk transfer of pyrrolizidine alkaloids in cattle. J. Am. Vet.
Med. Assoc., 169: 1192-1196.
DIMENNA, G.P., KRICK, T.P., & SEGALL, H.J. (1980) Rapid high
performance liquid chromatography isolation of monoesters,
diesters, and macrocyclic diesters and pyrrolizidine alkaloids from
Senecio jacobaea and Amsinckia intermedia. J. Chromatogr., 192:
474-478.
DOLINSKAYA, K.N. (1952) [Pathomorphology of heliotropic hepatic
dystrophy in children.] In: Milenkov, S.M. & Kizhaikin, Y., ed.
[Collection of scientific papers on Toxic Hepatitis with Ascites,]
Tashkent, Publishing House of the University of Central Asia,
pp. 165-172 (in Russian).
DREIFUSS, P.A. (1984) A study of the mass spectra of
pyrrolizidine alkaloids by negative ion chemical ionization and
ms/ms: the identification of a monoester pyrrolizidine alkaloid in
Eupatorium rugosum. Diss. Abstr. Int. B., 45:'1183-1184.
DRIVER, H.E. & MATTOCKS, A.R. (1984) The toxic effects in rats of
some synthanecine carbamate and phosphate esters analogous to
hepatotoxic pyrrolizidine alkaloids. Chem.-biol. Interact., 51:
201-218.
DUBROVINSKII, S.B. (1946) [About the alimentary toxicosis caused
by heliotrope.] J. Sov. Prot. Health, 6: 17-21 (in Russian).
DUBROVINSKII, S.B. (1952) [The etiology of toxic hepatitis with
ascites.] In: Millenkov, S.M. & Kizhaikin, Y., ed. [Collection of
scientific papers on Toxic Hepatitis with Ascites, Tashkent, USSR,]
Tashkent, Publishing House of the University of Central Asia,
pp. 9-25 (in Russian).
EASTMAN, D.F. & SEGALL, H.J. (1982) A new pyrrolizidine alkaloid
metabolite, 19-hydroxysenecionine, isolated from in vitro mouse
hepatic microsomes using high performance liquid chromatography.
Drug Metab. Disp., 10: 696-699.
EASTMAN, D.F., DIMENNA, G.P., & SEGALL, H.J. (1982) Covalent
binding of two pyrrolizidine alkaloids, senecionine, and
seneciphylline to hepatic macromolecules and their distribution,
excretion and transfer into milk of lactating mice. Drug Metab.
Disp., 10: 236-240.
EDGAR, J.A. (1982) Pyrrolizidine alkaloids sequestered by Solomon
Island Danaine butterflies. The feeding preferences of the Danainae
and Ithomiinae. J. Zool. (Lond.), 196: 385-399.
EDGAR, J.A. (1985) Gas chromatography of pyrrolizidine alkaloids.
In: Seawright, A.A., Hegarty, M.P., James, L.F., & Keeler, R.F.,
ed. Plant toxicology. Proceedings of the Australia - USA Poisonous
Plants Symposium, Brisbane, Australia, 14-18 May 1984, Brisbane,
Queensland Poisonous Plants Committee, pp. 227-234.
EISENSTEIN, D. & HUXTABLE, R.J. (1979) Approaches to the treatment
of pyrrolizidine toxicosis. In: Cheeke, P.R., ed. Proceedings of
the Symposium on Pyrrolizidine (Senecio) Alkaloids: Toxicity,
Metabolism, and Poisonous Plant Control Measures, Corvallis,
Oregon, USA, 23-24 February 1979, Oregon, Nutrition Research
Institute, pp. 109-113.
EL DAREER, S.M., TILLERY, K.F., LLOYD, H.H., & HILL, D.L. (1982)
Disposition of indicine N-oxide in mice and monkeys. Cancer Treat.
Rep., 66: 183-186.
EMMEL, M.W. (1948) Crotalaria poisoning in cattle. J. Am. Vet.
Med. Assoc., 113: 164.
EMMEL, M.W., SANDERS, D.A., & HENLEY, W.W. (1935) Crotalaria
spectabilis seed poisoning in swine. J. Am. Vet. Med. Assoc., 86:
43-49.
EVANS, J.V., PENG, A., & NIELSEN, C.J. (1979) The gas
chromatographic mass spectrometric analysis of the new anti-tumour
drug indicine-N-oxide utilizing a novel reaction accompanying
trimethysilylation. Biomed. mass Spectrom., 6: 38-43.
EVANS, J.V., DALEY, S.K., MCCLUSKY, G.A., & NIELSEN, C.J. (1980)
Direct quantitative analysis of indicine N-oxide in cancer patient
samples by gas chromatography using the internal standard
heliotrine N-oxide including a mass spectral comparison of their
trimethylsilyl derivatives. Biomed. mass Spectrom., 7: 65-73.
FARRINGTON, K.J. & GALLAGHER, C.H. (1960) Complexes of copper with
some pyrrolizidine alkaloids and with some of their esterifying
acids. Aust. J. biol. Sci., 13: 600-603.
FISH, M.S., SWEELEY, C.C., JOHNSON, N.M., LAWRENCE, E.P., &
HORNING, E.C. (1956) Chemical and enzymic rearrangements of
N,N-dimethylaminoacid oxides. Biochem. Biophys. Acta, 21: 196-197.
FISHMAN, A.P. (1974) Dietary pulmonary hypertension. Circ. Res.,
35: 657-660.
FORSYTH, A.A. (1968) British poisonous plants, London, Her
Majesty's Stationery Office.
FOX, D.W., HART, M.C., BERGESON, P.S., JARRETT, P.B., STILLMAN,
A.E., & HUXTABLE, R.J. (1978) Pyrrolizidine (Senecio)
intoxication mimicking Reye's syndrome. J. Paediatr., 93: 980-982.
FRAHN, J.L., CULVENOR, C.C.J., & MILLS, J.A. (1980) Preparative
separation of the pyrrolizidine alkaloids, intermedine and
lycopsamine, as their borate complexes. J. Chromatogr., 195:
379-383.
FURMANOWA, M., GUZEWSKA, J., & BELDOWSKA, B. (1983) Mutagenic
effects of aqueous extracts of Symphytum officinale L. and of its
alkaloidal fractions. J. appl. Toxicol., 3: 127-130.
FURUYA, T. & HIKICHI, M. (1971) Alkaloids and triterpenoids of
Symphytum officinale. Phytochemistry, 10: 2217-2220.
FUSHIMI, K., KATO, K., KATO, T., MATSUBARA, N., & HIRONO, I. (1978)
Carcinogenicity of flower stalks of Petasites japonicus Maxim in
mice and Syrian golden hamsters. Toxicol. Lett., 1: 291-294.
GADELLA, T.W.J., KLIPHUIS, E., & HUIZING, H.J. (1983) Cyto- and
chemotaxonomical studies on the sections officinalia and coerulea
of the genus Symphytum. Bot. Helv., 93: 169-192.
GANEY, P.E., FINK, G.D., & ROTH, R.A. (1985) The effect of dietary
restriction and altered sodium intake on the cardiopulmonary
toxicity of monocrotaline pyrrole. Toxicol. appl. Pharmacol., 78:
55-62.
GARDINER, M.R., ROYCE, R., & BOKOR, A. (1965) Studies on
Crotalaria crispata, a newly recognized cause of Kimberley horse
disease. J. Pathol. Bacteriol., 89: 43-55.
GHANEM, J. & HERSHKO, C. (1981) Veno-occlusive disease and primary
hepatic vein thrombosis in Israeli Arabs. Isr. J. med. Sci., 17:
339-347.
GHODSI, F. & WILL, J.A. (1981) Changes in pulmonary structure and
function induced by monocrotaline intoxication. Am. J. Physiol.,
240: H149-H155.
GILRUTH, J.A. (1903) Hepatic cirrhosis affecting horses and cattle
(so-called "Winton disease"), Wellington, New Zealand Department
of Agriculture, pp. 228-279 (11th annual report).
GOEGER, D.E., CHEEKE, P.R., SCHMITZ, J.A., & BUHLER, D.R. (1982)
Toxicity of tansy ragwort (Senecio jacobaea) to goats. Am. J. vet.
Res., 43: 252-254.
GOEGER, D.E., CHEEKE, P.R., RAMSDELL, H.S., NICHOLSON, S.S., &
BUHLER, D.R. (1983) Comparison of the toxicities of Senecio
jacobaea, Senecio vulgaris and Senecio glabellus in rats.
Toxicol. Lett., 15: 19-23.
GONZALEZ, A.G., DE LA FUENTE, G., REINA, M., & LOYOLA, L.A. (1986a)
Pyrrolizidine alkaloids from Senecio phillipicus and Senecio
illinitus. Planta Med., 52: 160.
GONZALEZ, E., GARCIA, R., LEMUS, I., & ERAZO, S. (1986b)
Pharmacological studies on senecionine, a pyrrolizidine alkaloid
from Senecio fistulosus Poepp. Ex less. (hualtata). An. R. Acad.
Farm., 52(1): 123-132.
GRASES, P.J. & BEKER, S.G. (1972) Veno-occlusive disease of the
liver. A case from Venezuela. Am. J. Med., 53: 511-516.
GRAY, A.I., NIC, A.N., TSAOIR, E., & WATERMAN, P.G. (1983)
Hepatotoxic alkaloids and allantoin in Symphytum tuberosum. J.
Pharm. Pharmacol., 35(Suppl): 13.
GREEN, C.R. & CHRISTIE, G.S. (1961) Malformations in foetal rats
induced by the pyrrolizidine alkaloid, heliotrine. Br. J. exp.
Pathol., 42: 369-378.
GREEN, M.H.L. & MURIEL, W.J. (1975) Use of repair-deficient
strains of Escherichia coli and liver microsomes to detect and
characterise DNA damage caused by the pyrrolizidine alkaloids
heliotrine and monocrotaline. Mutat. Res., 28: 331-336.
GREEN, C.E., SEGALL, H.J., & BYARD, J.L. (1981) Metabolism,
cytotoxicity, and genotoxicity of the pyrrolizidine alkaloid
senecionine in primary cultures of rat hepatocytes. Toxicol. appl.
Pharmacol., 60: 176-185.
GUENGERICH, F.P. (1977) Separation and purification of multiple
forms of microsomal cytochrome P-450. J. biol. Chem., 252:
3970-3979.
GUENGERICH, F.P. & MITCHELL, M.B. (1980) Metabolic activation of
model pyrroles by cytochrome P-450. Drug Metab. Disp., 8: 34-38.
GUIDUGLI, F.H., PESTCHANKER, M.J., DESALMERON, M.S.A., & GIORDANO,
O.S. (1986) 1-hydroxyplatyphyllide, a norsequiterpene lactone
from Senecio gilliesiano. Phytochemistry, 25: 1923-1926.
GUNER, N. (1986) Alkaloids of Heliotropium suaveolens. J. nat.
Prod., 49: 369.
GUPTA, P.S., GUPTA, G.D., & SHARMA, M.L. (1963) Veno-occlusive
disease of the liver. Br. med. J., 1: 1184-1186.
HABS, H. (1982) Senecio numorensis fuchsii. Carcinogenic and
mutagenic activity of an alkaloidal extract from a phytotherapeutic
drug. Dtsch. Apoth. Ztg, 122: 799-804.
HABS, H., HABS, M., MARQUARDT, H., RODER, E., SCHMAHL, D., &
WIEDENFELD, H. (1982) Carcinogenic and mutagenic activity of an
alkaloidal extract of Senecio numorensis spp. fuchsii.
Arzneimittelforsch, 32: 144-148.
HAGGLUND, K.M., L'EMPEREUR, K.M., ROBY, M.R., & STERMITZ, F.R.
(1985) Latifoline and latifoline N-oxide from Hackelia
floribunda, the western false forget-me-not. J. nat. Prod., 48:
638-639.
HAMMOUDA, F.M., RIZK, A.M., ISMAIL, S.I., ATTEYA, S.Z., GHALEB,
H.A., MADKOUR, M.K., POWAND, A.E., & WOOD, G. (1984) Poisonous
plants contaminating edible ones and toxic substances in plant
foods. Part 3: pyrrolizidine alkaloids from Heliotropium digynum
Forssk (= H. luteum, Poir). Pharmazie, 39: 703-705.
HARDING, J.D.J., LEWIS, G., DONE, J.T., & ALLCROFT, R. (1964)
Experimental poisoning by Senecio jacobaea in pigs. Pathol. Vet.,
1: 204-220.
HARRIS, C., REDDY, J., CHIGA, M., & SVOBODA, D. (1969)
Polyribosomal disaggregation by lasiocarpine. Biophys. Acta 180:
587-589.
HARRIS, P.N. & CHEN, K.K. (1970) Development of hepatic tumours in
rats following ingestion of Senecio longilobus. Cancer Res., 30:
2881-2886.
HARRIS, P.N., ROSE, C.L., & CHEN, K.K. (1957) Hepatotoxic and
pharmacological properties of heliotrine. Arch. Pathol., 64:
152-157.
HARTMANN, T. & ZIMMER, M. (1986) Organ-specific distribution and
accumulation of pyrrolizidine alkaloids during the life history of
2 annual Senecio species. J. plant Physiol., 122: 67-80.
HASHEM, M. (1939) Etiology and pathology of types of liver
cirrhosis in Egyptian children. J. Egypt. Med. Assoc., 22: 319-354.
HAYASHI, Y. (1966) Excretion and alteration of monocrotaline in
rats after a subcutaneous injection (abstract). Fed. Proc., 25: 688.
HAYASHI, Y. & LALICH, J.J. (1967) Renal and pulmonary alterations
induced by a single injection of monocrotaline. Proc. Soc. Exp.
Biol. Med., 124: 392-396.
HAYASHI, Y. & LALICH, J.J. (1968) Protective effect of
mercaptoethylamine and cysteine against monocrotaline intoxication
in rats. Toxicol. appl. Pharmacol., 12: 36-43.
HAYASHI, Y., HUSSA, J.F., & LALICH, J.J. (1967) Cor pulmonale in
rats. Lab. Invest., 16: 875-881.
HAYASHI, Y., KATO, M., OTSUKA, H. (1979) Inhibitory effects of
diet reduction on Monocrotaline intoxication in rats. Toxicol.
Lett., 3: 151-155.
HAYASHI, Y., SHIMADA, M., & KATAYAMA, H. (1977) Experimental
insulinoma in rats after a single administration of monocrotaline.
Toxicol. Lett., 1: 41-44.
HAYASHI, Y., KOKUBO, T., TAKAHASHI, M., FURUKAWA, F., OTSUKA, H., &
HASHIMOTO, K. (1984) Correlative morphological and biochemical
studies on monocrotaline-induced pulmonary alterations in rats.
Toxicol. Lett., 21: 65-77.
HAYES, M.A., ROBERTS, E., & FARBER, E. (1985) Initiation and
selection of resistant hepatocyte nodules in rats given the
pyrrolizidine alkaloids lasiocarpine and senecionine. Cancer Res.,
45: 3726-3734.
HEATH, D., SHABA, J., WILLIAMS, A., SMITH, P., & KOMBE, A. (1975)
A pulmonary hypertension-producing plant from Tanzania. Thorax, 30:
399-404.
HENDRICKS, J.D., SINNHUBER, R.O., HENDERSON, M.D., & BUHLER, D.R.
(1981) Liver and kidney pathology in rainbow trout (Salmo
gairdneri) exposed to dietary pyrrolizidine (Senecio) alkaloids.
Exp. mol. Pathol., 35: 170.183.
HILL, K.R. (1960) Worldwide distribution of seneciosis in man and
animals. Proc. R. Soc. Med., 53: 281-282.
HILLIKER, K.S. & ROTH, R.A. (1984) Alteration of monocrotaline
pyrrole-induced cardiopulmonary effects in rats by hydrallazine,
dexamethasone or sulphinpyrazone. Br. J. Pharmacol., 82: 375-380.
HILLIKER, K.S., BELL, T.G., & ROTH, R.A. (1983) Monocrotaline
pyrrole-induced pulmonary hypertension in fawn-hooded rats with
platelet storage pool deficiency: 5 hydroxytryptamine uptake by
isolated, perfused lungs. Thromb. Haemostasis (Stuttgart), 50:
844-847.
HILLIKER, K.S., BELL, T.G., LORIMER, D., & ROTH, R.A. (1984)
Effects of thrombocytopaenia in monocrotaline pyrrole-induced
pulmonary hypertension. Am. J. Physiol., 246: H747-H753.
HIRONO, I., SHIMIZU, M., FUSHIMI, K., MORI, H., & KATO, K. (1973)
Carcinogenic activity of Petasites japonicus Maxim, a kind of
coltsfoot. Gann Monogr. Cancer Res., 64: 527-528.
HIRONO, I., MORI, H., & CULVENOR, C.C.J. (1976) Carcinogenic
activity of coltsfoot, Tussilago farfara L. Gann Monogr. Cancer
Res., 67: 125-129.
HIRONO, I., MORI, H., YAMADA, K., HIRATA, Y., HAGA, M., TATEMATSU,
H., & KANIE, S. (1977) Carcinogenic activity of petasitenine, a
new pyrrolizidine alkaloid isolated from Petasites japonicus
Maxim. J. Natl Cancer Inst., 58: 1155-1157.
HIRONO, I., MORI, H., & HAGA, M. (1978) Carcinogenic activity of
Symphytum officinale. J. Natl Cancer Inst., 61: 865-869.
HIRONO, I., HAGA, M., FUJII, M., MATSUURA, S., MATSUBARA, N.,
NAKAYAMA, M., FURUYA, T., HIKICHI, M., TAKANASHI, H., UCHIDA, E.,
HOSAKA, S., & UENO, I. (1979a) Induction of hepatic tumours in
rats by senkirkine and symphytine. J. Natl Cancer Inst., 63: 469-472.
HIRONO, I., MORI, H., HAGA, M., FUJII, M., YAMADA, K., TAKANASHI,
H., UCHIDA, E., HOSAKA, S., UENO, I., MATSUSHIMA, I., UMEZAVA, K.,
& SHIRAI, A. (1979b) Edible plants containing carcinogenic
pyrrolizidine alkaloids in Japan. In: Miller, B.C. ET AL., ed.
Naturally occurring carcinogens, mutagens, and modulators of
carcinogenesis, Baltimore, Maryland, University Park Press, pp.
79-87.
HIRONO, I., UENO, I., AISO, S., YAMAJI, T., & HAGA, M. (1983)
Carcinogenic activity of Farfagium japonicum and Senecio
cannabifolius. Cancer Lett., 20: 191-198.
HOET, P., ASTIGUETA, M., & FRISQUE, A.M. (1981) Phytochemical
study of Crotalaria nitens. Rev. Latinoam. Quim., 12: 34-35.
HOOPER, P.T. (1972) Spongy degeneration in the brain in relation
to hepatic disease and ammonia toxicity in domestic animals. Vet.
Res., 90: 37-38.
HOOPER, P.T. (1974) The pathology of Senecio jacobaea poisoning of
mice. J. Pathol., 113: 227-230.
HOOPER, P.T. (1975a) Spongy degeneration in the central nervous
system of domestic animals. Part I: morphology. Acta neuropathol.
(Berlin), 31: 325-334.
HOOPER, P.T. (1975b) Spongy degeneration in the central nervous
system of domestic animals. Part III: occurrence and pathogenesis -
hepatocerebral disease caused by hyper-ammonaemia. Acta
neuropathol. (Berlin), 31: 343-351.
HOOPER, P.T. (1975c) Experimental acute gastro-intestinal disease
caused by the pyrrolizidine alkaloid, lasiocarpine. J. comp.
Pathol., 85: 341-349.
HOOPER, P.T. (1978) Pyrrolizidine alkaloid poisoning-pathology
with particular reference to differences in animal and plant
species. In: Keeler, R.F., Van Kampen, K.R., & James, L.F., ed.
Effects of poisonous plants on livestock, New York, Academic
Press, pp. 161-176.
HOOPER, P.T. & SCANLAN, W.A. (1977) Crotalaria retusa poisoning in
pigs and poultry. Aust. vet. J., 53: 109-114.
HOOPER, P.T., BEST, S.M., & MURRAY, D.R. (1974) Hyper-ammonaemia
and spongy degeneration of the brain in sheep affected with hepatic
necrosis. Res. vet. Sci., 16: 216-222.
HOOSON, J. & GRASSO, P. (1976) Cytotoxic and carcinogenic response
to monocrotaline pyrrole. J. Pathol., 118: 121-127.
HOU JINQUI, XIA YUDIN, YU ZHANSHI, AN YAN, & TANG YIXAI (1980)
[Veno-occlusive disease of the liver with report of 2 cases.] Chung
Hua Nei ko Tsa Chih, 19: 187-191 (in Chinese).
HSU, I.C., ALLEN, J.R., & CHESNEY, C.F. (1973) Identification and
toxicological effects of dehydroretronecine, a metabolite of
monocrotaline. Proc. Soc. Exp. Biol. Med., 144: 834-838.
HSU, I.C., SHUMAKER, R.C., & ALLEN, J.R. (1974) Tissue
distribution of tritium labelled dehydroretronecine. Chem.-biol.
Interact., 8: 163.
HUIZING, H.J., DE BOER, R., & MALINGRE, T.M. (1981) Preparative
ion-pair high performance liquid chromatography and gas
chromatography of pyrrolizidine alkaloids from comfrey. J.
Chromatogr., 214: 257-262.
HURLEY, J.V. & JAGO, M.V. (1975) Pulmonary oedema in rats given
dehydromonocrotaline: a topographic and electron-microscopic
study. J. Pathol., 117: 23-32.
HUXTABLE, R.J. (1980) Herbal teas and toxins: novel aspects of
pyrrolizidine poisoning in the United States. Perspect. Biol. Med.,
24: 1-14.
HUXTABLE, R., PAPLANUS, S., & LAUGHARN, J. (1977) The prevention
of monocrotaline induced right ventricular hypertrophy. Chest, 71:
308-310.
HUXTABLE, R.J., CIARAMITARO, D., & EISENSTEIN, D. (1978) The
effect of a pyrrolizidine alkaloid, monocrotaline and a pyrrole,
dehydroretronecine, on the biochemical functions of the pulmonary
endothelium. Mol. Pharmacol., 14: 1189-1203.
HUXTABLE, R.J., LUTHY, J., & ZWEIFEL, V. (1986) Toxicity of
comfrey-pepsin preparations: letters to the editor. New Engl. J.
Med., 315: 1095.
IARC (1976) Pyrrolizidine alkaloids. In: Some naturally occurring
substances, Lyons, International Agency for Research on Cancer,
pp. 265-342 (IARC Monograph on the Evaluation of Carcinogenic Risk
of Chemicals to Man, Vol. 10).
IARC (1983) Some food additives, feed additives and naturally
occurring substances, Lyons, International Agency for Research on
Cancer, pp. 207-245 (IARC Monograph on the Evaluation of
Carcinogenic Risk of Chemicals to Humans, Vol. 31).
INDIAN COUNCIL OF MEDICAL RESEARCH (1955) Infantile cirrhosis of
the liver in India. Indian J. med. Res., 43: 723-747.
ISMAILOV, N.I. (1948a) [On clinical features, etiology and
pathogenesis of heliotropic dystrophy (toxic hepatitis with
ascites).] Clin. Med., 8: 28 (in Russian).
ISMAILOV, N.I. (1948b) [Heliotropic toxicosis (toxic hepatitis
with ascites),] Tashkent, Academy of Sciences of Uzbekistan,
pp. 1-120 (in Russian).
ISMAILOV, N.I., MADZHIDOV, N.M., MAGRUPOV, A.I., MAKHKAMOV, G.M., &
MUKMINOVA, S.G. (1970) [Clinical signs, diagnosis and treatment of
Trichodesma toxicosis (alimentary toxic encephalopathy),]
Tashkent, Meditsina, p. 85 (in Russian).
JAGO, M.V. (1969) The development of the hepatic megalocytosis
of chronic pyrrolizidine alkaloid poisoning. Am. J. Pathol., 56:
405-422.
JAGO, M.V. (1970) A method for the assessment of the chronic
hepatotoxicity of pyrrolizidine alkaloids. Aust. J. exp. Biol. med.
Sci., 48: 93-103.
JAGO, M.V. (1971) Factors affecting the chronic hepatotoxicity
of pyrrolizidine alkaloids. J. Pathol., 105: 1-11.
JAGO, M.V., LANIGAN, G.W., BINGLEY, J.B., PIEREY, D.W.T., WHITTEN,
J.H., & TITCHEN, D.A. (1969) Excretion of the pyrrolizidine
alkaloid heliotrine in the urine and bile of sheep. J. Pathol., 98:
115-128.
JAGO, M.V., EDGAR, J.A., SMITH, L.W., & CULVENOR, C.C.J. (1970)
Metabolic conversion of heliotrine based pyrrolizidine alkaloids to
dehydroheliotridine. Mol. Pharmacol., 6: 402-406.
JELLIFFE, D.B., BRAS, G., & STUART, K.L. (1954a) Veno-occlusive
disease of the liver. Paediatrics, 14: 334-339.
JELLIFFE, D.B., BRAS, G., & STUART, K.L. (1954b) The clinical
picture of veno-occlusive disease of the liver in Jamaican
children. Ann. trop. Med. Parasitol., 48: 386-396.
JELLIFFE, D.B., BRAS, G., & MUKHERJEE, K.L. (1957) Veno-occlusive
disease of the liver and Indian childhood cirrhosis. Arch. dis.
Child., 32: 369-385.
JOHNSON, A.E. (1976) Changes in calves and rats consuming milk
from cows fed chronic lethal doses of Senecio jacobaea (tansy
ragwort). Am. J. vet. Res., 37: 107-110.
JOHNSON, A.E. (1979) Toxicity of tansy ragwort to cattle. In:
Cheeke, P.R., ed. Proceedings of the Symposium on Pyrrolizidine
(Senecio) Alkaloids: Toxicity, Metabolism, and Poisonous Plant
Control Measures, Corvallis, Oregon, USA, 23-24 February 1979,
Oregon, Nutrition Research Institute, pp. 129-134.
JOHNSON, A.E. (1982) Failure of mineral-vitamin supplements to
prevent tansy ragwort (Senecio jacobaea) toxicosis in cattle. Am.
J. vet. Res., 43: 718-723.
JOHNSON, A.E. & MOLYNEUX, R.J. (1984) Toxicity of thread leaf
groundsel (Senecio douglasii var. longilobus) to cattle. Am. J.
vet. Res., 45: 26-31.
JOHNSON, A.E., MOLYNEUX, R.J., & STUART, L.D. (1985) Toxicity of
Riddel's groundsel (Senecio riddelli) to cattle. Am. J. vet. Res.,
46: 577-582.
JOHNSON, W.D. (1981) Mechanism of in vitro acute toxicity of
dehydromonocrotaline a metabolite of pyrrolizidine alkaloid
monocrotaline. Toxicologist, 1: 107-108.
JOHNSON, W.D., ROBERTSON, K.A., POUNDS, J.G., & ALLEN, J.R. (1978)
Dehydroretronecine-induced skin tumours in mice. J. Natl Cancer
Inst., 61: 85-89.
JULIEN, M.H., ed. (1982) Biological control of weeds. A world
catalog of agents and their target weeds, Curepe, Trinidad and
Toabago, Commonwealth Institute of Biological Control.
JUNEJA, T.R., GUPTA, R.L., & SAMANTA, S. (1984) Activation of
monocrotaline, fulvine and their derivatives to toxic pyrroles by
some thiols. Toxicol. Lett., 21: 185-189.
KAMPANZEV, N.N. (1952) [Experimental heliotropic hepatitis.] In:
Milenkov, S.M. & Kizhaikin, Y., ed. [Collection of scientific
papers on Toxic Hepatitis with Ascites,] Tashkent, Publishing
House of the University of Central Asia, pp. 165-172 (in Russian).
KAY, J.M. & HEATH, D. (1966) Observation on the pulmonary arteries
and heart weight of rats fed on Crotalaria spectabilis seeds. J.
Pathol., 92: 385-394.
KAY, J.M. & HEATH, D. (1969) Crotalaria spectabilis: the pulmonary
hypertension plant, Springfield, Illinois, Charles C. Thomas, p.
38.
KAY, J.M., HARRIS, P., & HEATH, D. (1967a) Pulmonary hypertension
produced in rats by ingestion of Crotalaria spectabilis seeds.
Thorax, 22: 176-179.
KAY, J.M., GILLUND, T.D., & HEATH, D. (1967b) Mast cells in the
lungs of rats fed on Crotalaria spectabilis seeds. Am. J. Pathol.,
51: 1031-1044.
KAY, J.M., HEATH, D., SMITH, P., BRAS, G., & SUMMERELL, J. (1971a)
Fulvine and pulmonary circulation. Thorax, 26: 249-261.
KAY, J.M., SMITH, P., & HEATH, D. (1971b) Aminorex and the
pulmonary circulation. Thorax, 26: 262-270.
KAY, J.M., KEANE, P.M., & SUYAMA, K.L. (1985) Pulmonary
hypertension induced in rats by monocrotaline and chronic hypoxia
is reduced by p-chlorophenylalanine. Respiration, 47: 48-56.
KEDZIERSKI, B. & BUHLER, D.R. (1985) Configuration of necine
pyrroles - toxic metabolites of pyrrolizidine alkaloids. Toxicol.
Lett., 25: 115-119.
KEDZIERSKI, B. & BUHLER, D.R. (1986) The formation of 6,7-di-
hydro-7-hydroxy-1-hydroxymethyl-5H-pyrrolizine, a metabolite of
pyrrolizidine alkaloids. Chem.-biol. Interact., 57: 217-222.
KHANIN, M.N. (1948) [The etiology of toxic hepatitis with
ascites.] Arch. Pathol. USSR, 1: 42-47 (in Russian).
KIM, H.L. & JONES, L.P. (1982) Protective effects of butylated
anisole, ethoxyquin and disulfiran on acute pyrrolizidine alkaloid
poisoning in mice. Res. Comm. chem. Pathol. Pharmacol., 36: 341-344.
KIRFEL, A., WILL, G., WIEDENFELD, H. & RODER, E. (1980)
(1aR,6bR,10R,11R)-9,15-dioxo-10-hydroxy-10,11,13-trimethyl-1a,2,
3,6b-tetrabydro-5H-pyrrolizino-[1a,6b,6a,c],8-dioxa-15-cis-tride
cene,C18H25NO5. Cryst. Styruct. Comm., 9: 353-361.
KNIGHT, A.P., KIMBERLING, C.V., STERMITZ, F.R., & ROBY, M.R. (1984)
Cynoglossum officinale (hound's tongue) - a cause of pyrrolizidine
alkaloid poisoning in horses. J. Am. Vet. Med. Assoc., 185: 647-650.
KOEKEMOER, M.J. & WARREN, F.L. (1951) The occurrence and
preparation of the N -oxides. An improved method of extraction of
the Senecio alkaloids. J. Chem. Soc., C1951: pp. 66-68.
KOLETSKY, A., OYASU, R., & REDDY, J.K. (1978) Mutagenicity of the
pyrrolizidine (Senecio) alkaloid, lasiocarpine in the
salmonella/microsome test. Lab. Invest., 38: 352 (Abstract).
KOVACH, J.S., MOERTEL, C.G., & HAHN, R.G. (1979a) A phase 1 study
of indicine- N-oxide. Proc. Am. Assoc. Cancer Res., 20: 357.
KOVACH, J.S., AMES, M.M., POWIS, G., MOERTEL, C.G., HAHN, R.G., &
CREAGAN E.T. (1979b) Toxicity and pharmacokinetics of a
pyrrolizidine alkaloid, indicine- N-oxide, in humans. Cancer Res.,
39: 4540-4544.
KRAUS, C., ABEL, G., & SCHIMMER, O. (1985) [Studies on the
chromosome damaging effect of some pyrrolizidine alkaloids in human
lymphocytes in vitro.] Planta Med., 51: 89-91 (in German).
KRISHNAMACHARI, K.A.V.R., BHAT, R.V., KRISHNAMURTHY, D.,
KRISHNASWAMY, K., & NAGARAJAN (1977) Aetiopathogenesis of endemic
ascites in Sarguja district of Madhya Pradesh. Indian J. med. Res.,
65: 672-678.
KUHARA, K., TAKANASHI, H., HIRONO, I., FURUYA, T., & ASADA, Y.
(1980) Carcinogenic activity of clivorine, a pyrrolizidine
alkaloid isolated from Ligularia dentata. Cancer Lett., 10: 117-122.
KUMANA, C.R., NG, M., LIN, H.J., KO, W., WU, P.C., & TODD, D.
(1983) Hepatic veno-occlusive disease due to toxic alkaloid herbal
tea - letter to the editor. Lancet, 10 December: 1360-1361.
KUMANA, C.R., NG, M., LIN, H.J., KO, W., WU, P.C., & TODD, D.
(1985) Herbal tea induced hepatic veno-occlusive disease:
quantification of toxic alkaloid exposure in adults. Gut, 26:
101-104.
KUROZUMI, T., TANAKA, K., KIDO, M., & SHOYAMA, Y. (1983)
Monocrotaline-induced renal lesions. Exp. mol. Pathol., 39: 377-386.
LAFRANCONI, W.M. & HUXTABLE, R.J. (1983) Changes in angiotensin-
converting enzyme activity in lungs damaged by the pyrrolizidine
alkaloid, monocrotaline. Thorax, 38: 307-309.
LAFRANCONI, W.M. & HUXTABLE, R.J. (1984) Hepatic metabolism and
pulmonary toxicity of monocrotaline using isolated perfused liver
and lung. Biochem. Pharmacol., 33: 2479-2484.
LAFRANCONI, W.M., DUHAMEL, R.C., BRENDEL, K., & HUXTABLE,R.J.
(1984) Differentiation of the cardiac and pulmonary toxicity of
monocrotaline, a pyrrolizidine alkaloid. Biochem. Pharmacol., 33:
191-197.
LAFRANCONI, W.M., OHNUMA, S., & HUXTABLE, R.S. (1985) Biliary
excretion of novel pneumotoxic metabolites of the pyrrolizidine
alkaloid monocrotaline. Toxicon, 23: 903-992.
LALICH, J.J. & EHRHART, L.A. (1962) Monocrotaline induced
pulmonary arteritis in rats. J. atheroscler. Res., 2: 482-492.
LALICH, J.J. & MERKOW, L. (1961) Pulmonary arteritis produced in
rats by feeding Crotalaria spectabilis. Lab. Invest., 10: 744-750.
LANCET (1984) Pyrrolizidine alkaloids (editorial). Lancet, 1:
201-202.
LANGLEBEN, D. & REID, L.M. (1985) Effect of methylprednisolone on
monocrotaline-induced pulmonary vascular disease and right
ventricular hypertrophy. Lab. Invest., 52: 298-303.
LANIGAN, G.W. (1971) Metabolism of pyrrolizidine alkaloids in the
ovine rumen. III. The competitive relationship between heliotrine
metabolism and methanogenesis in rumen fluid in vitro. Aust. J.
agric. Res., 22: 123-130.
LANIGAN, G.W. (1972) Metabolism of pyrrolizidine alkaloids in
ovine rumen. IV. Effects of chloral hydrate and halogenated
methanes on rumen methanogenesis and alkaloid metabolism in
fistulated sheep. Aust. J. agric. Res., 23: 1085-1091.
LANIGAN, G.W. & SMITH, L.W. (1970a) Metabolism of pyrrolizidine
alkaloids in the ovine rumen. I. Formation of 7 alpha-hydroxy-
1alpha-methyl-8alpha-pyrrolizidine from heliotrine and
lasiocarpine. Aust. J. agric. Res., 21: 493-500.
LANIGAN, G.W. & SMITH, L.W. (1970b) Metabolism of pyrrolizidine
alkaloids in the ovine rumen. II. Formation of 7alpha-hydroxy-
1alpha-methyl-8alpha-pyrrolizidine from heliotrine and
lasiocarpine. Aust. J. agric. Res., 22: 123-130.
LANIGAN, G.W. & WHITTEM, J.H. (1970) Cobalt pellets and
Heliotropium europaeum poisoning in penned sheep. Aust. vet. J.,
46: 17-21.
LANIGAN, G.W., PAYNE, A.L., & PETERSON, J.E. (1978) Anti-
methanogenic drugs and Heliotropium europaeum poisoning in penned
sheep. Aust. J. Agric. Res., 29: 1281-1292.
LAWS, L. (1968) Toxicity of Crotalaria mucronata to sheep. Aust.
vet. J., 44: 453-455.
LETENDRE, L., SMITHSON, W.A., GILCHRIST, G.S., BURGERT, E.O., Jr,
HOGLAND, C.H., AMES, M.M., POWIS, G., & KOVACH, J.S. (1981)
Activity of indicine- N-oxide in refractory acute leukemia. Cancer,
47: 437-441.
LETENDRE, L., LUDWIG, J., PERRAULT, J., SMITHSON, W.A., & KOVACH,
J.S. (1984) Hepatocellular toxicity during the treatment of
refractory acute leukemia with indicine- N-oxide. Cancer, 54:
1256-1259.
LEVINE, O.R., HARRIS, R.C., BLANCE, W.A., & MELLINS, R.B. (1973)
Progressive pulmonary hypertension in children with portal
hypertension. J. Pediatr., 83: 964-972.
LIN, J.J., LIU, C., & SVOBODA, D.J. (1974) Long-term effects of
aflatoxin B1 and vocal hepatitis on marmoset liver: a preliminary
report. Lab. Invest., 30: 267-278.
LUTHY, J., ZWEIFEL, U., SCHLATTER, C., & BENN, M.H. (1980)
[Pyrrolizidine alkaloids in coltsfoot ( Tussilago farfara L.) of
various sources.] Mitt. Geb. Lebensm. Hyg., 71: 73-80 (in German
with English summary).
LUTHY, J., ZWEIFEL, U., KARLHUBER, B., & SCHLATTER, C. (1981)
Pyrrolizidine alkaloids of Senecio alpinus L. and their detection
in feedstuffs. J. agric. food Chem., 29: 302-305.
LUTHY, J., HEIM, TH., & SCHLATTER, CH. (1983) Transfer of [3H]
pyrrolizidine alkaloids from Senecio vulgaris L. and metabolites
into rat milk and tissues. Toxicol. Lett., 17: 283-288.
LUTHY, J., BRAUCHLI, J., ZWEIFEL, U., SCHMID, P., & SCHLATTER, CH.
(1984) [Pyrrolizidine alkaloid in arzneipflanzen der Boraginaceen:
Borago officinalis L. and Pulmonaria officinalis L.] Pharm. Acta
Helv., 59: 242-246 (in German).
LYFORD, C.L., VERGAVA, G.G., & MOELLER, D.D. (1976) Hepatic veno-
occlusive disease originating in Ecuador. Gastro-enterology, 70:
105-108.
MCCOMISH, M., BODEK, I., & BRAUFMAN, A.R. (1980) Quantitation of
the antineoplastic agent indicine- N-oxide in human plasma by
differential pulse polarography. J. pharmacol. Sci., 69: 727-729.
MCCOY, J.W., ROBY, M.R., & STERMITZ, F.R. (1983) Analysis of plant
alkaloid mixtures by ammonia chemical ionization mass spectroscopy.
J. nat. Prod., 46: 894-900.
MCGEE, J.O'D., PATRICK, R.S., WOOD, C.B., & BLUMGART, L.H. (1976)
A case of veno-occlusive disease of the liver in Britain associated
with herbal tea consumption. J. clin. Pathol., 29: 788-794.
MCGRATH, J.P.M., DUNCAN, J.R., & MUNNELL, J.F. (1975) Crotalaria
spectabilis toxicity in swine: characterization of the renal
glomerular lesion. J. comp. Pathol., 85(2): 185-194.
MCLEAN, E.K. (1969) The early sinusoidal lesion in experimental
veno-occlusive disease. Br. J. exp. Pathol., 50: 223-229.
MCLEAN, E.K. (1970) The toxic actions of pyrrolizidine (Senecio)
alkaloids. Pharmacol. Rev., 22: 429-483.
MCLEAN, E.K. (1974) Senecio and other plants as liver poisons.
Isr. J. med. Sci., 10: 436-440.
MCLEAN, E.K. & HILL, K.R. (1969) Portal hypertension in acute
experimental veno-occulsive disease of the liver in rats. Br. J.
exp. Pathol., 50: 37-41.
MCLEAN, E.K., BRAS, G., & GYORGY, P. (1964) Veno-occlusive lesions
in livers of rats fed Crotalaria fulva. Br. J. exp. Pathol., 45:
242-247.
MAKSUDOV, A.M. (1952) [Heliotropic toxic hepatitis with ascites.]
In: Milenkov, S.M. & Kizhaikin, Y., ed. [Collection of scientific
papers on Toxic Hepatitis with Ascites,] Tashkent, Publishing House
of the University of Central Asia, pp. 103-116 (in Russian).
MARTIN, P.A., THORBURNE, M.K., HUTCHINSON, S., BRAS, G., & MILLER,
C.G. (1972) Preliminary findings of chromosome studies in rats and
humans with veno-occlusive disease. Br. J. exp. Pathol., 53, 374-380.
MATTOCKS, A.R. (1961) Extraction of heat-labile alkaloids from
plants. Nature (Lond.), 191: 1281-1282.
MATTOCKS, A.R. (1967a) Spectrophotometric determination of
unsaturated pyrrolizidine alkaloids. Anal. Chem., 39: 443-447.
MATTOCKS, A.R. (1967b) Detection of pyrrolizidine alkaloids on
thin-layer chromatograms. J. Chromatogr., 27: 505-508.
MATTOCKS, A.R. (1968a) Toxicity of pyrrolizidine alkaloids. Nature
(Lond.), 217: 723-728.
MATTOCKS, A.R. (1968b) Spectrophotometric determination of
pyrrolizidine alkaloids: some improvements. Anal. Chem., 40: 1749.
MATTOCKS, A.R. (1969) Dehydropyrrolizine derivatives from
unsaturated pyrrolizidine alkaloids. J. Chem. Soc., C1969:
1155-1162.
MATTOCKS, A.R. (1971a) Synthetic compounds with toxic properties
similar to those of pyrrolizidine alkaloids and their pyrrolic
metabolites. Nature (Lond.), 232: 476.
MATTOCKS, A.R. (1971b) The occurrence and analysis of
pyrrolizidine alkaloid N-oxides. Xenobiotica, 1: 451-453.
MATTOCKS, A.R. (1971c) Hepatotoxic effects due to pyrrolizidine
alkaloid N-oxides. Xenobiotica, 1: 563-565.
MATTOCKS, A.R. (1971d) A field test for N-oxides of unsaturated
pyrrolizidine alkaloids. Trop. Sci., 13: 65-70.
MATTOCKS, A.R. (1972a) Toxicity and metabolism of Senecio
alkaloids. In: Harborne, J.B., ed. Phytochemical ecology, London,
New York, Academic Press, pp. 179-200.
MATTOCKS, A.R. (1972b) Acute hepatotoxicity and pyrrolic
metabolites in rats dosed with pyrrolizidine alkaloids. Chem.-biol.
Interact., 5: 227-242.
MATTOCKS, A.R. (1973) Mechanisms of pyrrolizidine alkaloid
toxicity. Pharmacology and the future of man. In: Proceedings of
the 5th International Congress on Pharmacology, San Francisco,
1972, Basel, Karger, Vol. 2, pp. 114-123.
MATTOCKS, A.R. (1977) Tissue distribution of radioactivity in rats
given tritiated analogues of hepatotoxic pyrrolizidine alkaloids.
Xenobiotica, 7: 665-670.
MATTOCKS, A.R. (1980) Toxic pyrrolizidine alkaloids in comfrey.
Lancet, 22 November: 1136-1137.
MATTOCKS, A.R. (1981a) Liver cell enlargement in rats given
hydroxymethyl pyrroles analogous to pyrrolizidine alkaloid
metabolites, followed later by the hepatotoxin dimethylnitrosamine.
Toxicol. Lett., 8: 201-205.
MATTOCKS, A.R. (1981b) Relation of structural features to pyrrolic
metabolites in livers of rats given pyrrolizidine alkaloids and
derivatives. Chem.-biol. Interact., 35: 301-310.
MATTOCKS, A.R. (1981c) A simple preparation of dehydroretronecine
using potassium nitrosodisulphonate. Chem. Ind., 7: 251.
MATTOCKS, A.R. (1982) Hydrolysis and hepatotoxicity of retronecine
diesters. Toxicol. Lett., 14: 111-116.
MATTOCKS, A.R. (1986) Chemistry and toxicology of pyrrolizidine
alkaloids, London, New York, Academic Press.
MATTOCKS, A.R. & BIRD, I. (1983) Pyrrolic and N-oxide metabolites
formed from pyrrolizidine alkaloids by hepatic microsomes in vitro:
relevance to in vivo hepatotoxicity. Chem.-biol. Interact., 43:
209-222.
MATTOCKS, A.R. & CABRAL, J.R.P. (1979) Effects of some pyrrolic
and dehydropyrrolizine esters on mouse skin: a preliminary study.
Tumori, 65: 289-293.
MATTOCKS, A.R. & CABRAL, J.R.P. (1982) Carcinogenicity of some
pyrrolizidine alkaloid metabolites and analogues. Cancer Lett., 17:
61-66.
MATTOCKS, A.R. & DRIVER, H.E. (1983) A comparison of the
pneumotoxicity of some pyrrolic esters and similar compounds
analogous to pyrrolizidine alkaloid metabolites given intravenously
to rats. Toxicology, 27: 159-177.
MATTOCKS, A.R. & DRIVER, H.E. (1987) Toxic actions of senaetnine,
a new pyrrolizidine alkaloid, in rats. Toxicol. Lett., 38: 315-319.
MATTOCKS, A.R. & JUKES, R. (1987) New improved field test for
toxic pyrrolizidine alkaloids. J. nat. Prod., 50: 161-166.
MATTOCKS, A.R. & LEGG, R.F. (1980) Antimitotic activity of
dehydroretronecine, a pyrrolizidine alkaloid metabolite, and some
analogous compounds in a rat liver parenchymal cell line. Chem.-
biol. Interact., 30: 325-336.
MATTOCKS, A.R. & WHITE, I.N.H. (1970) Estimation of metabolites
of pyrrolizidine alkaloids in animal tissues. Anal. Biochem., 38:
529-535.
MATTOCKS, A.R. & WHITE, I.N.H. (1971a) The conversion of
pyrrolizidine alkaloids to dihydropyrrolizine derivatives by rat-
liver microsomes in vitro. Chem.-biol. Interact., 3: 383-396.
MATTOCKS, A.R. & WHITE, I.N.H. (1971b) Pyrrolic metabolites from
non-toxic pyrrolizidine alkaloids. Nat. new Biol., 231: 114-115.
MATTOCKS, A.R. & WHITE, I.N.H. (1973) Toxic effects and pyrrolic
metabolites in the liver of young rats given the pyrrolizidine
alkaloid retrorsine. Chem.-biol. Interact., 6: 297-306.
MATTOCKS, A.R. & WHITE, I.N.H. (1976) The distribution of
[3H]-synthanecine A bis- n-ethylcarbamate and its metabolites
in the rat. Chem.-biol. Interact., 15: 173-184.
MEYRICK, B.O. & REID, L.M. (1979) Development of pulmonary
arterial changes in rats fed Crotalaria spectabilis. Am. J.
Pathol., 94: 37-50.
MEYRICK, B.O. & REID, L.M. (1982) Crotalaria-induced pulmonary
hypertension: uptake of 3H-thymidine by the cells of the pulmonary
circulation and alveolar walls. Am. J. Pathol., 106: 84-94.
MILKOWSKY, A.S. (1985) The synthesis and anti-tumour activity of
monocyclic analogs of indicine- N-oxide. Diss. Abstr. Int., 45(3):
844.
MILLER, W.C., RICE, D.L., KREUSEL, R.G., & BEDROSSIAN, C.W.M.
(1978) Monocrotaline model of non-cardiogenic pulmonary edema in
dogs. J. appl. Physiol., 45: 962-965.
MIRANDA, C.L., BUHLER, D.R., & CHEEKE, P.R. (1979) The effect of
tansy ragwort consumption on mineral metabolism in the rat. In:
Cheeke, P.R., ed. Proceedings of the Symposium on Pyrrolizidine
(Senecio) Alkaloids: Toxicity, Metabolism, and Poisonous Plant
Control Measures, Corvallis, Oregon, USA, 23-24 February 1979,
Oregon, Nutrition Research Institute, pp. 61-64.
MIRANDA, C.L., CARPENTER, H.M., CHEEKE, P.R., & BUHLER, D.R.
(1981a) Effect of ethoxyquin on the toxicity of pyrrolizidine
alkaloid monocrotaline and on hepatic long metabolism in mice.
Chem.-biol. Interact., 37: 95-107.
MIRANDA, C.L., HENDERSON, M.C., & BUHLER, D.R. (1981b) Dietary
copper enhances the hepatotoxicity of Senecio jacobaea in rats.
Toxicol. appl. Pharmacol., 60(3): 418-423.
MIRANDA, C.L., REED, R.L., CHEEKE, P.R., & BUHLER, D.R. (1981c)
Protective effects of butylated hydroxyanisole against the acute
toxicity of monocrotaline in mice. Toxicol. appl. Pharmacol., 59:
424-430.
MIRANDA, C.L., BUHLER, D.R., RAMSDELL, H.S., CHEEKE, P.R., &
SCHMITZ, J.A. (1982a) Modifications of chronic hepatotoxicity of
pyrrolizidine (Senecio) alkaloids by butylated hydroxyanisole
and cysteine. Toxicol. Lett., 10: 177-182.
MIRANDA, C.L., HENDERSON, M.C., BUHLER, D.R., & SCHMITZ, J.A.
(1982b) Comparative effects of antioxidants on the toxicity of
mixed pyrrolizidine alkaloids from Senecio jacobaea in mice. J.
Toxicol. environ. Health, 9(5-6): 933-939.
MIRANDA, C.L., HENDERSON, M.C., REED, R.L., SCHMITZ, J.A., &
BUHLER, D.R. (1982c) Protective action of zinc against
pyrrolizidine alkaloid-induced hepatotoxicity in rats. J. Toxicol.
environ. Health, 9: 359-366.
MIROCHNIK, M.F. (1938) [Clinical course and etiopathogenesis of
toxic hepatites with ascites.] Medical Institute, scientific
papers of the second therapeutic clinic, Vol I, Tashkent, Medical
Literature Publishers.
MISER, J.S., MISER, A.W., SMITHSON, W.A., COCCIA, P.F., AMES, M.M.,
DAVIS, D.M., HUGHES, C.S., & GILCHRIST, G.S. (1982) A phase I
trial of indicine- N-oxide in childhood malignancy. Proc. Am. Soc.
Clin. Oncol., 1: 137.
MNUSHKIN, A.S. (1949) [Some materials to clinical features and
pathogenesis of toxic hepatitis with ascites.] Sov. Med., 1: 8-9
(in Russian).
MNUSHKIN, A.S. (1952) [The clinical features, pathogenesis and
treatment of toxic hepatitis with ascites.] In: Milenkov, S.M. &
Kizhaikin, Y., ed. [Collection of scientific papers on Toxic
Hepatitis with Ascites,] Tashkent, Publishing House of the
University of Central Asia, pp. 91-98 (in Russian).
MOHABBAT, O., SRIVASTAVA, R.N., YOUNOS, M.S., SEDIQ, G.G., MENZAD,
A.A., & ARAM, G.N. (1976) An outbreak of hepatic veno-occlusive
disease in north-western Afghanistan. Lancet, 7 August: 269-271.
MOLTENI, A., WARD, W.F., TS'AO, C.-S., PORT, C.D., & SOLLIDAY, N.H.
(1984) Monocrotaline-induced pulmonary endothelial dysfunction in
rats. Proc. Soc. Exp. Biol. Med., 176: 88-94.
MOLYNEUX, R.J. & ROITMAN, J.N. (1980) Specific detection of
pyrrolizidine alkaloids on thin-layer chromatograms. J.
Chromatogr., 195: 412-415.
MOLYNEUX, R.J., JOHNSON, A.E., ROITMAN, J.N., & BENSON, M.E. (1979)
Chemistry of toxic range plants. Determination of pyrrolizidine
alkaloid content and composition in Senecio species by nuclear
magnetic resonance spectroscopy. J. agric. food Chem., 27: 494-499.
MORI, H., SUGIE, S., YOSHIMI, N., ASADA, Y., FURUYA, T., &
WILLIAMS, G.M. (1985) Genotoxicity of a variety of pyrrolizidine
alkaloids in the hepatocyte primary culture-DNA repair test using
rat, mouse, and hamster hepatocytes. Cancer Res., 45: 3125-3129.
MUNIER, R. (1953) Separation of alkaloids from their N-oxides by
paper chromatography. Bull. Soc. Chim. Biol., 35: 1225.
NARDI, N.B., GIMMLER, M.C., & LEITE, B.G. (1980) Antimitotic
action of integerrimine in rats. Rev. Bras. Genet., 3: 387-392.
NATIONAL CANCER INSTITUTE (1978) Bioassay of lasiocarpine for
possible carcinogenecity, Bethesda, Maryland, National Cancer
Institute, US Department of Health, Education and Welfare (Report
of Carcinogenesis Testing Programme, Division of Cancer Cause and
Prevention).
NAYAK, N.C., SAGREIYA, K., & RAMALINGASWAMI, V. (1969) Indian
childhood cirrhosis - the nature and significance of cytoplasmic
hyaline of hepatocytes. Arch. Pathol., 88: 631-637.
NEWBERNE, P.M. (1968) The influence of a low isotrope diet on
response of maternal and fetal rats to lasiocarpine. Cancer Res.,
28: 2327-2337.
NEWBERNE, P.M. & ROGERS, A.E. (1973) Nutrition, monocrotaline and
aflatoxin B1 in liver carcinogenesis. Plant Food Man, 1: 23-31.
NEWBERNE, P.M., WILSON, R., & ROGERS, A.E. (1971) Effects of a
low-lipotrope diet on the response of young male rats to the
pyrrolizidine alkaloid monocrotaline. Toxicol. appl. Pharmacol., 18:
387-397.
NEWBERNE, P.M., CHAN, W.C., & ROGERS, A.E. (1974) Influence of
light, riboflavin and carotene on the response of rats to the acute
toxicity of aflatoxin and monocrotaline. Toxicol. appl. Pharmacol.,
28: 200-208.
NICHOLS, W.C., MOERTL, C.G., RUBIN, J., SCHULT, A.J., & BRITTEL,
J.C. (1981) Phase II trial of indicine- N-oxide (NSC132319) in
patients with advanced colorectal carcinoma. Cancer Treat. Rep., 65:
337-339.
NIWA, H., ISHIWATA, H., & YAMADA, K. (1985) Isolation of
petasitenine, a carcinogenic pyrrolizidine alkaloid from Farfugium
japonicum. J. nat. Prod., 48: 10083-1004.
NOLAN, J.P., SCHEIG, R.L., & KLATSKIN, G. (1966) Delayed hepatitis
and cirrhosis in weanling rats following a single small dose of the
Senecio alkaloid, lasiocarpine. Am. J. Pathol., 49: 129-151.
OHNUMA, T., SRIDHAR, K.S., RATNER, L.H., & HOLLAND, J.F. (1982)
Phase I study of indicine- N-oxide in patients with advanced cancer.
Cancer Treat. Rep., 66(7): 1509-1515.
OHTSUBO, K., ITO, Y., SAITO, M., FURUYA, T., & HIKICHI, M. (1977)
Hypertrophy of pulmonary arteries and arterioles with cor pulmonale
in rats induced by seneciphylline, a pyrrolizidine alkaloid.
Experientia (Basel), 33: 498-499.
OLSON, J.W., HACKER, A.D., ALTIERE, R.J., & GILLESPIE, M.N. (1984)
Polyamines and the development of monocrotaline-induced pulmonary
hypertension. Am. J. Physiol., 247: H682-H685.
OLSON, J.W., ATKINSON, J.E., HACKER, A.D., ALTIERE, R.J., &
GILLESPIE, M.N. (1985) Suppression of polyamine biosynthesis
prevents monocrotaline induced pulmonary oedema and arterial medial
thickening. Toxicol. appl. Pharmacol., 81: 91-99.
ORD, M.J., HERBERT, A., & MATTOCKS, A.R. (1985) The ability of
bifunctional and monofunctional pyrrole compounds to induce sister
chromatid exchange (SCE) in human lymphocytes and mutations in
Salmonella typhimurium. Mutat. Res., 149: 485-493.
PANTONE, D.J., BROWN, S.M., & WOMERSLEY, C. (1985) Biological
control of fiddleneck. California Agric., 39: 4-9.
PECKHAM, J.C., SANGSTER, L.P., & JONES, O.H. (1974) Crotalaria
spectabilis poisoning in swine. J. Am. Vet. Med. Assoc., 165:
633-638.
PEDERSEN, E. (1975) Pyrrolizidine alkaloids in Danish species of
the family Boraginaceae. Arch. Pharm. Chem. Sci. Ed., 3: 55-64.
PERCY, J.J. & PIERCE, A.E. (1971) Immunosuppressive activity of
the pyrrolizidine alkaloid metabolite dehydroheliotridine.
Immunology, 21: 273-280.
PERSAUD, J.V. & HOYTE, D.A. (1974) Pregnancy and progeny in rats
treated with the pyrrolizidine alkaloid fulvine. Exp. Pathol., 9:
59-63.
PESTCHANKER, M.J. & GIORDANO, O.S. (1986) Pyrrolizidine alkaloids
from five Senecio species. J. nat. Prod., 49: 722-723.
PESTCHANKER, M.J., ASCHERI, M.S., & GIORDANO, O.S. (1985a)
Uspallatine, a pyrrolizidine alkaloid from Senecio uspallatensis.
Phytochemistry, 24: 1622-1624.
PESTCHANKER, M.J., ASCHERI, M.S., & GIORDANO, O.S. (1985b)
Pyrrolizidine alkaloids from Senecio subulatus and S. glandulosus.
Planta Med., 51: 165-166.
PETERSON, J.E. (1965) Effects of pyrrolizidine alkaloid
lasiocarpine N -oxide on nuclear and cell division in the liver of
rats. J. Pathol. Bacteriol., 89: 153-171.
PETERSON, J.E. & CULVENOR, C.C.J. (1983) Plant and fungal toxins.
In: Keeler, R.F. & Tu, A.T., ed. Handbook of natural toxins, New
York, Marcel Dekker, Vol. 1, pp. 637-681.
PETERSON, J.E. & JAGO, M.V. (1980) Comparison of the toxic effects
of dehydroheliotridine and heliotrine in pregnant rats and their
embryos. J. Pathol., 131: 339-355.
PETERSON, J.E. & JAGO, M.V. (1984) Toxicity of Echium plantagineum
(Paterson's curse): pyrrolizidine alkaloid poisoning in rats. Aust.
J. agric. Res., 35: 305-316.
PETERSON, J.E., SAMUEL, A., & JAGO, M.V. (1972) Pathological
effects of dehydroheliotridine, a metabolite of heliotridine-based
pyrrolizidine alkaloids in the young rat. J. Pathol., 107: 107-189.
PETERSON, J.E., JAGO, M.V., REDDY, J.K., & JARRETT, R.G. (1983)
Neoplasia and chronic disease associated with the prolonged
administration of dehydroheliotridine to rats. J. Natl Cancer
Inst., 70: 381-386.
PETRY, T.W., BOWDEN, G.P., HUXTABLE, R.J., & SIPES, I.G. (1984)
Characterization of hepatic DNA damage induced in rats by the
pyrrolizidine alkaloid monocrotaline. Cancer Res., 44: 1505-1509.
PETRY, T.W., BOWDEN, G.P., BUHLER, D.R., & SIPES, I.G. (1986)
Genotoxicity of the pyrrolizidine alkaloid jacobine in rats.
Toxicol. Lett., 32: 275-281.
PIERCY, P.L. & RUSOFF, L.L. (1946) Crotalaria spectabilis
poisoning in Louisiana livestock. J. Am. Vet. Med. Assoc., 108:
69-73.
PIERSON, M.L., CHEEKE, P.R., & DICKINSON, E.O. (1977) Resistance
of the rabbit to dietary pyrrolizidine (Senecio) alkaloid. Res.
Commun. chem. Pathol. Pharmacol., 16: 561-564.
PIETERS, L.A.C. & VLIETINCK, A.J. (1985) Quantitative 'H Fourier
transform nuclear magnetic resonance spectroscopic analysis of
mixtures of pyrrolizidine alkaloids from Senecio vulgaris.
Fresenius' Z. Anal. Chem., 321: 355-358.
PIETERS, L.A.C. & VLIETINCK, A.J. (1986) Comparison of high-
performance liquid chromatography with 1H nuclear magnetic
resonance spectrometry for the quantitative analysis of
pyrrolizidine alkaloids from Senecio vulgaris. J. liq. Chromatogr.,
9: 745-755.
PLESTINA, R. & STONER, H.B. (1972) Pulmonary oedema in rats given
monocrotaline pyrrole. J. Pathol., 106: 235-249.
POOL, B.L. (1982) Genotoxic activity of an alkaloidal extract of
Senecio nemorensis spp. fuchsii in Salmonella typhimurium and
Escherichia coli systems (letter to the editor). Toxicology, 24:
351-355.
POWIS, G., AMES, M.M., & KOVACH, J.S. (1979) Metabolic conversion
of indicine- N-oxide to indicine in rabbits and humans. Cancer
Res., 39: 3564-3570.
PRABHU, M.B. (1940) Infantile cirrhosis of liver. Indian J.
Pediatr., 7: 121-134.
RADHAKRISHNA RAO, M.V. (1935) Histopathology of the liver in
infantile biliary cirrhosis. Indian J. med. Res., 23: 69-90.
RAJAGOPALAN, T.R. & NEGI, R.K.S. (1985) Alkaloids from Doronicum
pardalianches Linn. Indian J. Chem., 24B, 882.
RAKIETEN, N., GORDON, B.S., BEATTY, A., COONEY, D.A., DAVIS, R.D.,
& SCHIEN, P.S. (1971) Pancreatic islet cell tumours produced by
the combined action of streptozotocin and nicotinamide. Proc. Soc.
Exp. Biol. Med., 137: 280-283.
RAMALINGASWAMI, V. & NAYAK, N.C. (1969) Liver disease in India.
In: Popper, H. & Schaffner, F., ed. Progress in liver diseases,
New York, London, Grune and Stratton, pp. 222-235.
RAMSDELL, A.S. & BUHLER, D.R. (1981) High performance liquid
chromatographic analysis of pyrrolizidine (Senecio) alkaloids
using a reversed phase styrene-divinylbenzene resin column. J.
Chromatogr., 210: 154-158.
RAO, M.S. & REDDY, J.K. (1978) Malignant neoplasms in rats fed
lasiocarpine. Br. J. Cancer, 37: 289-293.
RAPPAPORT, A.M., KNOBLAUCH, M., SUMMERSKILL, J., & BRAS, G. (1967)
Experimental veno-occlusive disease. Gastroenterology, 54: 164.
RATNOFF, O.D. & MIRICK, G.S. (1949) Influence of sex upon the
lethal effects of an hepatotoxic alkaloid, monocrotaline. Bull.
Johns Hopkins Hosp., 84: 507-25.
RECHICIGL, M., Jr, ed. (1983) CRC Handbook on naturally occurring
food toxicants, Boca Raton, Florida, CRC Press, 266 pp.
RESCH, J.F. & MEINWALD, J. (1982) A revised structure for
acetylheliosupine. Phytochemistry, 21: 2340-2431.
RHODES, K. (1957) Two types of liver disease in Jamaican children.
West Indian med. J., 6: 1-29.
RIDKER, P.M., OHKUMA, S., MCDERMOTT, W.V., TREY, C., & HUXTABLE,
R.J. (1985) Hepatic veno-occlusive disease associated with
consumption of pyrrolizidine alkaloid containing dietary
supplements. Gastroenterology, 88: 1050-1054.
RIZK, A.M., HAMMOUDA, F.M., ISMAIL, S.I., GHALEB, H.A., MADKOUR,
M.K., POHLAND, A.E., & WOOD, G. (1983) Poisonous plants
contaminating edible ones and toxic substances in plant foods. I.
Alkaloids from Senecio desfontainei. Fitoterapia, 54: 115-121.
ROBERTSON, K.A. (1982) Alkylation of N2 in deoxyguanosine by
dehydroretronecine, a carcinogenic metabolite of the pyrrolizidine
alkaloid monocrotaline. Cancer Res., 42: 8-14.
ROBINS, D.J. (1982) The pyrrolizidine alkaloids. In: Herz, W.,
Grisebach, H., & Kirby, G.W., ed. Progress in the chemistry of
organic natural products, Vienna, New York, Springer-Verlag, pp.
115-203.
RODER, E., WEIDENFELD, H., & STENGL, P. (1981) Pyrrolizidine
alkaloids senecionine and retrorsine from Senecio inaequidens.
Planta Med., 41: 412-413.
RODER, E., WIEDENFELD, H., & KNOZINGER-FISCHER (1984a)
Pyrrolizidine alkaloid from Senecio abrotanifolius spp.
abroatanifolius, spp. abrotanifolius, and var. tiroliensis.
Planta Med., 41: 412-413.
RODER, E., WIEDENFELD, H., & BRITZ-KIRSTGEN, R. (1984b)
Pyrrolizidine alkaloids from Senecio cacaliaster. Phytochemistry,
23: 1761-1763.
ROGERS, A.E. & NEWBERNE, P.M. (1971) Lasiocarpine factors
influencing its toxicity and effects in liver cell division.
Toxicol. appl. Pharmacol., 18: 356-366.
ROITMAN, J.N. (1981) Comfrey and liver damage. Lancet, 25 April:
944.
ROITMAN, J.N. (1983) Ingestion of pyrrolizidine alkaloids: a
health hazard of global proportions. In: Finlay, J.W. & Schwass,
D.E., ed. Xenobiotics in foods and feeds, Washington DC, American
Chemical Society, pp. 345-378 (ACS Series).
ROSE, A.L., GARDNER, C.A., MCDONNELL, J.D., & BULL, L.B. (1957a)
Field and experimental investigation of "walk-about" disease of
horses (Kimberley horse disease) in northern Australia: Crotalaria
poisoning in horses. Part II. Aust. vet. J., 33: 49-62.
ROSE, A.L., GARDNER, C.A., MCDONNELL, J.D., & BULL, L.B. (1957b)
Field and experimental investigation of "walk about" disease of
horses (Kimberley horse disease) in northern Australia. Part I.
Aust. vet. J., 33: 25-33.
ROSE, C.L., HARRIS, P.N., & CHEN, K.K. (1959) Some pharmacological
action of supinine and lasiocarpine. J. Pharmacol. exp. Ther., 126:
179-184.
ROSE, E.F. (1972) Senecio species: toxic plants used as food and
medicine in the Transkei. S. Afr. Med. J., 46: 1039-1043.
ROSENFIELD, I. & BEATH, O.A. (1945) Tissue changes induced by
Senecio riddellii. Am. J. clin. Pathol., 15: 407-412.
ROTH, R.A., DOTZLAF, L.A., BARANYI, B., & HOOK, J.B. (1981) Effect
of monocrotaline ingestion on liver, kidney and lung of rat.
Toxicol. appl. Pharmacol., 60: 193-203.
ROYES, K. (1948) Infantile hepatic cirrhosis in Jamaica. Caribb.
med. J., 10: 16-48.
SAFOUH, M. & SHEHATA, A.H. (1965) Hepatic vein occlusion disease
of Egyptian children. J. Pediatr., 67: 415-432.
ST. GEORGE-GRAMBAUER, T.D. & RAC, R. (1962) Hepatogenous chronic
copper poisoning in sheep in south Australia due to consumption of
Echium plantagineum L. (Salvation Jane). Aust. vet. J., 38: 288-293.
SALASPURO, M. & SIPPONEN, P. (1976) Demonstration of an
intracellular copper-binding protein in orcein staining in long
standing cholestatic liver diseases. Gut, 17: 787-790.
SAMUEL, A. & JAGO, M.V. (1975) Localization in the cell cycle of
the antimitotic action of the pyrrolizidine alkaloid, lasiocarpine,
and its metabolite, dehydroheliotridine. Chem.-biol. Interact., 10:
185-197.
SANDERS, D.A., SHEALY, A.L., & EMMEL, M.W. (1936) The pathology of
Crotalaria spectabilis Roth poisoning in cattle. J. Am. Vet. Med.
Assoc., 89: 150-156.
SAVIN, I.G., (1983) [Research of the influence of heliotrin on
the microsomal mono oxygenase systems of the rat liver.] N.E.
Pyrogov 2nd Moscow State Medical Institute. (Thesis in biological
chemistry) (in Russian).
SAVVINA, K.I. (1952) Pathological anatomy of atrophic hepatic
cirrhosis. Arch. Pathol., 14: 65-70.
SCHOENTAL, R. (1959) Liver lesions in young rats suckled by
mothers treated with the pyrrolizidine (Senecio) alkaloids,
lasiocarpine, and retrorsine. J. Pathol. Bacteriol., 77: 485-504.
SCHOENTAL, R. (1961) Herbal medicines and liver disease. J. exp.
med. Sci., 4: 126-135.
SCHOENTAL, R. (1963) Liver disease and 'natural' hepatotoxins.
Bull. World Health Organ., 29: 823-833.
SCHOENTAL, R. (1968) Toxicology and carcinogenic action of
pyrrolizidine alkaloids. Cancer Res., 28: 2237-2246.
SCHOENTAL, R. (1970) Hepatotoxic activity of retrorsine,
senkirkine and hydroxysenkirkine in newborn rats, and the role of
epoxides in carcinogenesis by pyrrolizidine alkaloids and
aflatoxins. Nature (Lond.), 227: 401-402.
SCHOENTAL, R. (1975) Pancreatic islet-cell and other tumours in
rats given heliotrine, a monoester pyrrolizidine alkaloid, and
nicotinamide. Cancer Res., 35: 2020-2024.
SCHOENTAL, R. & BENSTED, J.P.M. (1963) Effects of whole body
irradiation and of partial hepatectomy on the liver lesions induced
in rats by a single dose of retrorsine, a pyrrolizidine (Senecio)
alkaloid. Br. J. Cancer, 17: 242-251.
SCHOENTAL, R. & CAVANAGH, J.B. (1972) Brain and spinal cord
tumours in rats treated with pyrrolizidine alkaloids. J. Natl
Cancer Inst., 49: 665-671.
SCHOENTAL, R. & COADY, A. (1968) The hepatotoxicity of some
Ethiopian and east African plants including some used as
traditional medicines. East Afr. med. J., 45: 577-579.
SCHOENTAL, R. & HEAD, M.A. (1955) Pathological changes in rats as
result of treatment with monocrotaline. Br. J. Cancer, 9: 229-237.
SCHOENTAL, R. & HEAD, M.A. (1957) Progression of liver lesions
produced in rats by temporary treatment with pyrrolizidine
(Senecio) alkaloids, and the effects of betaine and high casein
diet. Br. J. Cancer, 11: 535-544.
SCHOENTAL, R. & MAGEE, P.N. (1957) Chronic liver changes in rats
after a single dose of lasiocarpine, a pyrrolizidine (Senecio)
alkaloid. J. Pathol. Bacteriol., 74: 305-319.
SCHOENTAL, R. & MAGEE, P.N. (1959) Further observation on the
subacute and chronic liver changes rats after a single dose of
various pyrrolizidine (Senecio) alkaloids. J. Pathol. Bacteriol.,
78: 471-482.
SCHOENTAL, R. & MATTOCKS, A.R. (1960) Hepatotoxic activity of
semisynthetic analogues of pyrrolizidine alkaloids. Nature (Lond.),
185: 842-843.
SCHOENTAL, R., HEAD, M.A., & PEACOCK, P.R. (1954) Senecio
alkaloids: primary liver tumours in rats as a result of treatment
with (1) mixture of alkaloids from S. jacobaea Lin, (2)
retrorsine, (3) isatidine. Br. J. Cancer, 8: 458-465.
SCHOENTAL, R., FOWLER, M.E., & COADY, A. (1970) Islet cell tumours
of the pancreas found in rats given pyrrolizidine alkaloids from
Amsinckia intermedia Fisch and Mey and from Heliotropium supinum.
Cancer Res., 30: 2127-2131.
SEGALL, H.J. (1979a) Reversed phase isolation of pyrrolizidine
alkaloids. Liq. Chromatogr., 2: 429-436.
SEGALL, H.J. (1979b) Preparative isolation of pyrrolizidine
alkaloids derived from Senecio vulgaris. Liq. Chromatogr., 2:
1319-1323.
SEGALL, H.J., DALLAS, J.L., & HADDON, W.F. (1984) Two
dihydropyrrolizine alkaloid metabolites isolated from mouse hepatic
microsomes in vitro. Drug Metab. Disp., 12: 68-71.
SEGALL, H.J., WILSON, D.W., DALLAS, J.L., & HADDON, W.F. (1985)
Trans-4-hydroxy-2-hexenol: a reactive metabolite from the
macrocyclic pyrrolizidine alkaloid senecionine. Science, 229: 472-475.
SEGER, C.L., NEWSON, J.D., ROTH, E.E., & HUTCHINSON, W.R. (1969)
Chronic toxic hepatitis in deer from a Louisiana coastal marsh.
Bull. Wildlife Disease Assoc. Proc. Ann. Conf., pp. 295-6.
SELZER, G. & PARKER, R.G.F. (1951) Senecio poisoning exhibiting
as Chiari's syndrome: a report of 12 cases. Am. J. Pathol., 27:
885-907.
SENER, B., TEMIZER, H., TEMIZER, A., & KARAKAYA, A.E. (1986) High
performance liquid chromatographic determination of alkaloids in
Senecio vernalis. J. Pharm. Belg., 41: 115-117.
SHARMA, R.K., KHAJURIA, G.S., & ATAL, C.K. (1965) Thin layer
chromatography of pyrrolizidine alkaloids. J. Chromatogr., 19:
433-434.
SHERLOCK, S. (1968) Diseases of liver and biliary system, 4th
ed., Oxford, Edinburgh, Blackwell Scientific Publishers, 250 pp.
SHTENBERG, A.I. & ORLOVA, N.V. (1955) [The question of the
etiology of the so-called "Ozhalangar Encephalitis".] Vopr. Pitan.,
14: 27-31 (in Russian).
SHULL, L.R., BUCKMASTER, G.W., & CHEEKE, P.R. (1976) Factors
influencing pyrrolizidine (Senecio) alkaloid metabolism: species,
liver sulphydryls and rumen fermentation. J. anim. Sci., 43: 1247-1253.
SHUMAKER, R.C., ROBERTSON, K.A., HSU, I.C., & ALLEN, J.R. (1976)
Neoplastic transformation in tissues of rats exposed to
monocrotaline or dehydroretronecine. J. Natl Cancer Inst., 56: 787-789.
SIDDIQI, M.A., SURI, K.A., SURI, M.P., & ATAL, C.K. (1978a) Novel
pyrrolizidine alkaloid from Crotalaria nana. Phytochemistry, 17:
2143-2144.
SIDDIQI, M.A., SURI, K.A., SURI, O.P., & ATAL, C.K. (1978b) Genus
Crotalaria. Part 34. Cronaburmine, a new pyrrolizidine alkaloid
from Crotalaria nana Burm. Indian J. Chem., 16B: 1132-1133.
SMITH, L.W. & CULVENOR, C.C.J. (1981) Plant sources of
hepatotoxic pyrrolizidine alkaloids. J. nat. Prod., 44: 129-152.
SMITH, T.E., WEISBACH, H., & UDENFRIEND S. (1962) Studies on the
mechanism of monoaminooxidase: metabolism of N,N-dimethyltryptamine
and N,N-dimethyltryptamine- N-oxide. Biochemistry, 1: 137.
SOBIN, L.H., FETRAT, M.E., & ANWER, M.A. (1969) Hepatic lesions in
Afghanistan. Trop. geogr. Med., 21: 27-29.
SRIVASTAVA, R.N., MOHABBAT, O., GHANI, A.R., & ARAM, G.N. (1978)
Veno-occlusive disease of the liver. Indian. Paediatr., 15: 143-146.
SRUNGBOONMEE, S. & MASKASAME, C. (1981) A preliminary study on
the toxicity of Crotalaria juncea to cattle. Sattawaphaet San, 32:
91-107.
STEIN, H. (1957) Veno-occlusive disease of liver in African
children. Br. med. J., 29 June: 1496-1499.
STEIN, H. & ISAACSON, C. (1962) Veno-occlusive disease of the
liver. Br. med. J., 10 February: 372-374.
STENMARK, K.R., MORGANROTH, M.L., REMIGIO, L.K., VOELKEL, N.F.,
MURPHY, R.C., HENSON, P.M., MATHIAS, M.M., & REEVES, J. (1985)
Alveolar inflammation and arachidonate metabolism in monocrotaline-
induced pulmonary hypertension. Am. J. Physiol., 248: H859-H866.
STILLMAN, A.E., HUXTABLE, R.J., CONSROE, P., KOHNEN, P., & SMITH,
S. (1977) Hepatic veno-occlusive disease due to pyrrolizidine
poisoning in Arizona. Gastroenterology, 73: 349-352.
STIRLING, G.A., BRAS, G., & URQUHART, A.E. (1962) The early
lesions in veno-occlusive disease of the liver. Arch. dis. Child.,
37: 535-538.
STOYEL, C. & CLARK, A.M. (1980) The transplacental micronucleus
test. Mutat. Res., 74: 393-398.
STUART, K.L. & BRAS, G. (1955) Clinical observations on veno-
occlusive disease of the liver in Jamaican adults. Br. med. J., 2:
348-352.
STUART, K.L. & BRAS, G. (1956) Veno-occlusive disease of the liver
in Barbados. West Indian med. J., 5: 33-36.
STUART, K.L. & BRAS, G. (1957) Veno-occlusive disease of the
liver. Q. J. Med., 26: 291-315.
STYLES, J., ASHBY, J., & MATTOCKS, A.R. (1980) Evaluation in vitro
of several pyrrolizidine alkaloid carcinogens: observation on the
essential pyrrolic nucleus. Carcinogenesis, 1: 161-164.
SUFFNESS, M. & CORDELL, G.A. (1985) Antitumour alkaloids. In:
Brossi, A., ed. The alkaloids, New York, Academic Press,
pp. 1-347.
SUGITA, T., HYERS, T.M., DAUBER, I.M., WAGNER, W.W., MCMURTRY,
I.F., & REEVES, J.T. (1983a) Lung leak precedes right ventricular
hypertrophy in monocrotaline treated rats. J. appl. Physiol., 54:
371-374.
SUGITA, T., STENMARK, K.R., WAGNER, W.W., HENSON, P.M., HENSON,
J.E., HYERS, T.M., & REEVES, J.T. (1983b) Abnormal alveolar cells
in monocrotaline induced pulmonary hypertension. Exp. lung Res.,
5: 201-215.
SUNDARESON, A.E. (1942) An experimental study of placental
permeability to cirrhogenic poisons. J. Pathol. Bacteriol., 54:
289-298.
SVOBODA, D. & REDDY, J.K. (1972) Malignant tumours in rats given
lasiocarpine. Cancer Res., 32: 908-912.
SVOBODA, D. & REDDY, J.K. (1974) Lasiocarpine induced,
transplantable squamous cell carcinoma of rat skin. J. Natl Cancer
Inst., 53: 1415-1418.
SVOBODA, D. & SOGA, J. (1966) Early effects of pyrrolizidine
alkaloids on the fine structure of rat liver cells. Am. J. Pathol.,
48: 347-373.
SWICK, R.A., CHEEKE, P.R., & BUHLER, D.R. (1979) Factors affecting
the toxicity of dietary tansy ragwort to rats. In: Cheeke, P.R.,
ed. Proceedings of the Symposium on Pyrrolizidine (Senecio)
Alkaloids: Toxicity, Metabolism, and Poisonous Plant Control
Measures, Corvallis, Oregon, USA, 23-24 February 1979, Oregon,
Nutrition Research Institute.
SWICK, R.A., CHEEKE, P.R., GOEGER, D.E., & BUHLER, D.R. (1982a)
Effect of dietary Senecio jacobaea and injected Senecio alkaloids
and monocrotaline on guinea pigs. J. anim. Sci., 55: 1411-1416.
SWICK, R.A., CHEEKE, P.R., & BUHLER, D.R. (1982b) Subcellular
distribution of hepatic copper, zinc and iron and serum
ceruloplasmin in rats intoxicated by oral pyrrolizidine (Senecio)
alkaloids. J. anim. Sci., 55(6): 1425-1430.
SWICK, R.A., CHEEKE, P.R., PATTON, N.M., & BUHLER, D.R. (1982c)
Absorption and excretion of pyrrolizidine (Senecio) alkaloids and
their effects on mineral metabolism in rabbits. J. anim. Sci., 55:
1417-1424.
SWICK, R.A., CHEEKE, P.R., MIRANDA, C.L., & BUHLER, D.R. (1982d)
The effect of consumption of the pyrrolizidine alkaloid containing
plant Senecio jacobaea on iron and copper metabolism in the rat.
J. Toxicol. environ. Health, 10: 757-768.
SWICK, R.A., CHEEKE, P.R., RAMSDELL, H.S., & BUHLER, D.R. (1983)
Effect of sheep rumen fermentation and methane inhibition on the
toxicity of Senecio jacobaea. J. anim. Sci., 56: 645-651.
TAKANASHI, H., UMEDA, M., & HIRONO, I. (1980) Chromosomal
aberrations and mutation in cultured mammalian cells induced by
pyrrolizidine alkaloids. Mutat. Res., 78: 67-77.
TAKEOKA, O., ANGEVINE, M., & LALICH, J. (1962) Stimulation of mast
cells in rat fed various chemicals. Am. J. Pathol., 40: 545-554.
TANDON, B.N., TANDON, H.D., TANDON, R.K., NARENDRANATHAN, M., &
JOSHI, Y.K. (1976) Epidemic of veno-occlusive disease in central
India. Lancet, 7 August: 271-272.
TANDON, B.N., TANDON, H.D., KOSHY, A., NARENDRANATHAN, M., JOSHI,
Y.K., TANDON, R.K., BHARGAVA, S., RAJANI, M., BHATIA, M.L.,
MANCHANDA, S.C., & KASTURI, T.E. (1977) Epidemiological,
clinical, biochemical and haemodynamic study of veno-occlusive
disease of the liver due to Crotalaria alkaloids in India. J. All
Ind. Inst. Med. Sci., 3: 165-175.
TANDON, B.N., TANDON, H.D., & MATTOCKS, A.R. (1978) Study of an
epidemic of veno-occlusive disease in Afghanistan. Indian J. med.
Res., 68: 84-90.
TANDON, H.D. & TANDON, B.N. (1975) Epidemic of liver disease -
Gulran District, Herat Province, Afghanistan, Alexandria, World
Health Organization, Regional Office for the Eastern Mediterranean
(Assignment report No. EM/AFG/OCD/001/RB).
TANDON, H.D., TANDON, B.N., TANDON, R., & NAYAK, N.C. (1977) A
pathological study of the liver in an epidemic outbreak of veno-
occlusive disease. Indian J. med. Res., 65: 679-684.
TANDON, H.D., TANDON, B.N., & MATTOCKS, A.R. (1978) An epidemic of
veno-occlusive disease of the liver in Afghanistan. Am. J.
Gastroenterol., 72: 607-613.
TANDON, R.K., TANDON, B.N., TANDON, H.D., BHATIA, M.L., BHARGAVA,
S., LAL, P., & ARORA, R.R. (1976) Study of an epidemic of veno-
occlusive disease in India. Gut, 17: 849-855.
TANNER, M.S. & PORTMANN, B. (1981) Indian childhood cirrhosis.
Arch. dis. Child., 56: 4-6.
TAYLOR, S., BELT, R.J., HAAS, C.D., & HOOGSTRATEN, B. (1983) Phase
I trial of indicine- N-oxide on two dose schedules. Cancer, 51:
1988-1991.
TEILUM, G. (1949) Endophlebitis hepatica obliterans. Acta pathol.
microbiol., 26: 147-166.
TEMIZER, A., ONAR, A.N., SENER, B., TEMIZER, H., & KARAKAYA, A.E.
(1985) Determination of alkaloids by differential pulse
polarography. I. Senecio alkaloids. J. Pharm. Belg., 40(2): 75-78.
TEREKHOV, G.N. (1939) [Pathomorphology of toxic hepatitis with
ascites.] In: Mirochnik, M.F., ed. [Functional, diagnostic and
pathological changes of toxic hepatitis with ascites,] Tashkent,
State Publishing House of Science Technology and Socio-economic
Literature of Uzbekistan, pp. 145-200 (in Russian).
TEREKHOV, G.N. (1952) [The pathomorphology of alimentary toxic
dystrophy with ascites.] In: Milenkov, S.M. & Kizhaikin, Y., ed.
[Collection of scientific papers on Toxic Hepatitis with Ascites,]
Tashkent, Publishing House of the University of Central Asia, pp.
148-164 (in Russian).
THORPE, E. & FORD, E.J.H. (1968) Development of hepatic lesions in
calves fed with ragwort (Senecio jacobaea). J. comp. Pathol., 78:
195-205.
TITTEL, G., HINZ, H., & WAGNER, H. (1979) Quantitative
determination of pyrrolizidine alkaloids in Symphyti radix by
HPLC. Planta Med., 37: 1-8.
TUCHWEBER, B., KOVAKS, K., JAGO, M.V., & BEAULIEU, T. (1974)
Effect of steroidal and non-steroidal microsomal enzyme inducers on
the hepatotoxicity of pyrrolizidine alkaloids in rats. Res. Commun
chem. Pathol. Pharmacol., 7: 459-480.
TUCKER, A., BRYANT, S.E., FROST, H.H., & MIGALLY, N. (1983)
Chemical sympathectomy and serotonin inhibition reduce
monocrotaline-induced right ventricular hypertrophy in rats. Can.
J. Physiol. Pharmacol., 61: 356-362.
TURNER, J.H. & LALICH, J.J. (1965) Experimental cor pulmonale in
the rat. Arch. Pathol., 79: 409-418.
VALDIVIA, E., SONNAD, J., HAYASHI, Y., & LALICH, J.J. (1967a)
Experimental interstitial pulmonary oedema. Angiology, 18: 378-383.
VALDIVIA, E., LALICH, J., HAYASHI, Y., & SONNAD, J. (1967b)
Alterations in pulmonary alveoli after a single injection of
monocrotaline. Arch. Pathol., 84: 64-76.
VAN DER WATT, J.J., PURCHASE, I.F.H., & TUSTIN, R.C. (1972) The
chronic toxicity of retrorsine, a pyrrolizidine alkaloid, in vervet
monkeys. J. Pathol., 107: 279-287.
VILLARROEL, L.H., TORRES, R.G., NAVARRO, J.M., & FAJARDO, V.M.
(1985) Senecionine and seneciphylline from Senecio patagonicus.
Fitoterapia, 56: 250-251.
VISCONTINI, M. & GILHOF-SCHAUFELBERGER, H. (1971) Synthesis of (±)
dehydroheliotridine. Helv. chim. Acta, 54: 449-456.
WAGENVOORT, C.A., DINGEMANS, K.P., & LOTGERING, G.G. (1974a)
Electron microscopy of pulmonary vasculature after application of
fulvine. Thorax, 29: 511-521.
WAGENVOORT, C.A., WAGENVOORT, N., & DIJK, H.J. (1974b) Effect of
fulvine on pulmonary arteries and veins of the rat. Thorax, 29:
522-529.
WAGNER, H., NEIDHARDT, U., & TITTEL, G. (1981) TLC and HPLC
analysis of pyrrolizidine N-oxide alkaloids of Symphyti radix.
Planta Med., 41: 232-239.
WAKIM, K.G., HARRIS, P.N., & CHEN, K.K. (1946) The effects of
senecionine on the monkey. J. Pharmacol. exp. Ther., 87: 38-45.
WALKER, K.H. & KIRKLAND, P.D. (1981) Senecio lautus toxicity in
cattle. Aust. vet. J., 57: 1-7.
WATT, J.M. & BREYER-BRANDWIJK, M.G. (1962) Medicinal and poisonous
plants of southern and eastern Africa, Edinburgh, London, E. and
S. Livingstone, 584 p.
WEHNER, F.C., THIEL, P.G., & VAN RENSBURG, S.J. (1979)
Mutagenicity of alkaloids in the Salmonella/microsome system.
Mutat. Res., 66: 187-190.
WESTON, C.F.M., COOPER, B.T., DAVIES, J.D., & LEVINE, D.F. (1987)
Veno-occlusive disease of the liver secondary to ingestion of
comfrey. Br. med. J., 295: 183.
WHITE, I.N.H. (1976) The role of liver glutathion in the acute
toxicity of retrorsine to rats. Chem.-biol. Interact., 13: 333-342.
WHITE, I.N.H. (1977) Excretion of pyrrolic metabolites in bile of
rats given retrorsine or the bis- n-ethylcarbamate of synthanecine
A. Chem.-biol. Interact., 16: 169-180.
WHITE, I.N.H. & MATTOCKS, A.R. (1971) Some factors affecting the
conversion of pyrrolizidine alkaloids to N-oxides and to pyrrolic
derivatives in vitro. Xenobiotica, 1: 503-505.
WHITE, I.N.H. & MATTOCKS, A.R. (1972) Reactions of dihydro-
pyrrolizidine with deoxyribonucleic acid in vitro. Biochem.
J., 128: 291-297.
WHITE, I.N.H., MATTOCKS, A.R., & BUTLER, W.H. (1973) The
conversion of the pyrrolizidine alkaloid retrorsine to pyrrolic
derivatives in vivo and in vitro and its acute toxicity to
various animal species. Chem.-biol. Interact., 6: 207-218.
WHITE, R.D., KRUMPERMAN, P.H., CHEEKE, P.R., & BUHLER, D.R. (1983)
An evaluation of acetone extracts from six plants in the Ames
mutagenicity test. Toxicol. Lett., 15: 25-31.
WHITE, R.D., SWICK, R.A., CHEEKE, P.R. (1984) Effect of dietary
copper and molybdenum on tansy ragwort (Senecio Jacobaea) toxicity
in sheep. Am. J. vet. Res., 45: 159-161.
WHO (1980) Inventory of medicinal plants used in the different
countries, Geneva, World Health Organization (Document No.
DPM/80.3).
WICKRAMANAYAKE, P.P., ARBOGAST, B.L., BUHLER, D.R., DEINZER, M.L.,
& BURLINGAME, A.L. (1985) Alkylation of nucleosides and
nucleotides by dehydroretronecine: characterization of covalent
adducts by liquid secondary ion mass spectrometry. J. Am. Chem.
Soc., 107: 2485-2488.
WIEDENFELD, H. (1982) Two pyrrolizidine alkaloids from Gynura
scandens. Phytochemistry, 21: 2767-2768.
WIEDENFELD, H., PASTEWKA, U., STENGL, P., & ROEDER, E. (1981) On
the gas chromatographical determination of the pyrrolizidine
alkaloids of some Senecio species. Planta Med., 41: 124-128.
WIEDENFELD, H., RODER, E., & ANDERS, E. (1985) Pyrrolizidine
alkaloids from seeds of Crotalaria scassellatii. Phytochemistry,
24: 376-378.
WILLIAMS, A.O., EDINGTON, G.M., & OBAKPONOVWE, P.C. (1967)
Hepatocellular carcinoma in infancy and childhood in Ibadan,
western Nigeria. Br. J. Cancer, 21(3): 474-482.
WILMOT, F.C. & ROBERTSON, G.W. (1920) Senecio disease or
cirrhosis of the liver due to Senecio poisoning. Lancet,
23 October: 848-849.
WITTIG, M. & STEPHEN, C. (1964) Modification of microsomal lipid
peroxidation and drug metabolism by cytoplasmic copper. Res.
Commun. Chem. Pathol. Pharmacol., 44: 477-493.
WONG, R.Y. & ROITMAN, J.N. (1984) Structure and absolute
configuration of (+)-doronine-benzene (1:1). Acta Crystallogr.,
Sect. C: Cryst. Struc. Commun., C40: 163-166.
WURM, H. (1939) [A cluster of endophlebilis hepatica obliterans in
the age group of suckling infants.] Klin. Wochenschr., 18: 1527-1531
(in German).
YAMANAKA, H., NAGAO, M., SUGIMURA, T., FURUYA, T., SHIRAI, A., &
MATSUSHIMA, T. (1979) Mutagenicity of pyrrolizidine alkaloids in
the Salmonella/mammalian microsome test. Mutat. Res., 68: 211-216.
YULDASHEVA, L.N. & SULTANOVA, R.G. (1983) [Oxidative reactions in
rat liver tissue under conditions of chronic heliotrine hepatitis.]
Vopr. Med. Khim., 29: 81-85 (in Russian).
YUNUSOV, S.YU. & PLEKHANOVA, N.V. (1959) [The alkaloids of
Trichodesma incanum. The structure of incanine and trichesmine.]
Zh. Obshch. Khim., 29: 677-684 (in Russian).
ZHELTOVA, L.I. (1952) [The clinical course of toxic hepatitis.]
In: Milenkov, S.M. & Kizhaikin, Y., ed. [Collection of scientific
papers on Toxic Hepatitis with Ascites,] Tashkent, Publishing
House of the University of Central Asia, pp. 76-90 (in Russian).
APPENDIX I
Pyrrolizidine Alkaloids and Their Plant Sources
ISOLATIONS OF TOXIC PYRROLIZIDINE ALKALOIDS - (8701081148)
Alkaloid Plant Sources Reference Reference
Location
19-Acetoxysenkirkine Senecio laricifolius H.B.K. Bohlmann et al. (1986) A
6-Acetylanacrotine Crotalaria agatiflora Schweinf. Culvenor & Smith (1972) B281
6-Acetyl-trans-anacrotine Crotalaria agatiflora Schweinf. Culvenor & Smith (1972) B281
Acetylcrotaverrine Crotalaria verrucosa L. O.P. Suri et al. (1976) B339
C. walkeri Arnott K.A. Suri et al. (1976) B340
7-Acetylechinatine Cynoglossum amabile Stapf. and Drummond Culvenor & Smith, unpubl.
Lindelofia spectabilis Lehm. Rao et al. (1974) B62
Symphytum asperum Lepech. Pedersen (1975b) A
S. officinale Linn. Pedersen (1975b) A
Acetylgynuramine Gynura scandens O. Hoffm. Wiedenfeld (1982) C
Acetylheliosupine Cynoglossum officinale L. Pedersen (1970); Resch & Meinwald (1982) B43, A
Myosotis sylvatica Hoffm. Resch & Meinwald (1982) A
Symphytum asperum Lepech. Pedersen (1975) A
S. officinale Linn. Pedersen (1975) A
Acetylindicine Heliotropium indicum L. Mattocks (1967a) B71
7-Acetylintermedine Borago officinale L. Luthy et al. (1984) A
Symphytum aspera Roitman (1981) A
S. officinale Linn. Roder et al. (1982) C
S. x uplandicum Nyman Culvenor et al. (1980a), (1980b) A, A
Alkaloid Plant Sources Reference Reference
Location
Acetyllasiocarpine Heliotropium europaeum L. Culvenor et al. (1975) B69
7-Acetyllycopsamine Amsinckia menziesii (Lehm.) Nels Roitman, (1983a) C
& Macbr.
Anchusa officinalis L. Pedersen (1975); Broch-Due & A, B32
Aasen (1980)
Borago officinale L. Luthy et al. (1984) A
Symphytum aspera Roitman (1981) A
S. officinale Linn. Huizing & Malingre (1981) C
S. x uplandicum Nyman Culvenor et al. (1980a, 1980b) A, A
3a'-Acetyllycopsamine Amsinckia menziesii (Lehm.) Nels. Roitman (1983a C
& Macbr.
7-Acetylmadurensine Crotalaria agatiflora Schweinf. Culvenor & Smith (1972) B281
7-Acetyl-cis-madurensine Crotalaria agatiflora Schweinf. Culvenor & Smith (1972) B281
7-Acetylscorpioidine Myosotis scorpioides L. Resch et al. (1982) C
Acetylseneciphylline Senecio pterophorus DC. Edgar et al. (1976) B246
18-Acetylsenkirkine Senecio illinitus Phill. Gonzalez et al. (1986a) A
S. kirkii Hook. f. ex Kirk Briggs et al. (1965) A
S. tenuifolius Burm. Bhakuni & Gupta (1982) C
Acetylsyneilesine Syneilesis palmata Maxim. Hikichi & Furuya (1976) B279
Amabiline Borago officinale L. Luthy et al. (1984) A
Cynoglossum amabile Stapf. et Drummond Culvenor & Smith (1967) B33
C. glochidiatum Wall. ex Lindl. K.A. Suri et al. (1975a) B36
Eupatorium cannabinum L. Luthy et al. (1984) A
Lindelofia angustifolia (Schrenk) Brand. K.A. Suri et al. (1975a) B36
Alkaloid Plant Sources Reference Reference
Location
Anacrotine Crotalaria agatifolia Schweinf. Culvenor & Smith (1972) B281
C. incana L. Mattocks (1968) B302
C. laburnifolia L. Snehelata et al. (1966); Sawhney B309,
et al. (1967) 291
C. laburnifolia L. subsp. eldomae Crout (1972) B312
C. micans Link. Atal et al. (1966a) B280
C. verrucosa L. Subramanian & Nagarajan (1967) B338
Anadoline Symphytum orientale Ulubelen & Doganca (1971); Culvenor B103,
et al. (1975) 104
S. tuberosum L. Ulubelen & Ocal (1977) B105
Angelylechimidine (or Symphytum asperum Lepech. Gadella et al. (1983) A
isomer) S. x uplandicum Nyman Gadella et al. (1983) A
7-Angelylheliotridine Heliotropium supinum L. Crowley & Culvenor (1959) B83
trachelanthate
7-Angelylheliotridine Heliotropium supinum L. Crowley & Culvenor (1959) B83
viridiflorate
7-Angelylheliotrine Heliotropium digynum Forssk. Hammouda et al. (1984) A
H. eichwaldii Steud ex DC. O.P. Suri et al. (1975) B63
7-Angelyl-9-sarracinyl- Senecio triangularis Hook. Rueger & Benn (1983) C
retronecine
6-Angelyl-trans-anacrotine Crotalaria agatiflora Schweinf. Culvenor & Smith (1972) B281
Asperumine Echium vulgare L. Karimov et al. (1975) B50
Symphytum asperum Lepech. Man'ko et al. (1969); Man'ko & B95, 96
Kotowskii (1970); Man'ko et al. 1970 B97
S. caucasicum Bieb. Man'ko et al. (1972); Mel'kumova B98, 99
et al. 1974
Alkaloid Plant Sources Reference Reference
Location
Axillaridine Crotalaria axillaris Ait. Crout (1968b, 1969) B287, 288
C. scassellatii Chiov Wiedenfeld et al. (1985) A
Axillarine Crotalaria axillaris Ait. Crout (1968b, 1969) B287, 288
C. scassellatii Chiov Wiedenfeld et al. (1985) A
Bisline Senecio othonniformis Fourcade Coucourakis & Gordon-Gray (1970) B224
S. petasitis DC. Gonzalez et al. (1973) B225
Brachyglottine Brachyglottis repanda Forst. et Forst. White, pers. commun.
Carategine Lindelofia tschimganica Akramov et al. (1965) B87
Rindera oblongifolia M. Pop. Akramov et al. (1965) B87
Solenanthus karategenius Lipsky Akramov et al. (1964) B93
Chlorodeoxysceleratine Senecio latifolius DC. (S. sceleratus) Gordon-Gray (1967) B261
Clivorine Ligularia brachyphylla Hand. Mazz. Klasek et al. (1971) B134
L. clivorum Klasek et al. (1967, 1969, 1970); B135,
Birnbaum et al. (1971) 136,
137,
138
L. dentata (A. Gray) Hara Klasek et al. (1971) B134
L. elegans (Cass.) Klasek et al. (1971) B134
Crispatine Crotalaria candicans W. & A. Suri et al. (1982) C
C. crispata F. Muell. ex Benth. Culvenor & Smith (1963) B294
C. lunata Beddome ex Polhill Rothschild et al. (1979) C
C. madurensis R. Wight Habib et al. (1971) B315
Crobarbatine Crotalaria barbata R. Graham ex R. Wight Puri et al. (1973) B289
Walk.-Arn.
Alkaloid Plant Sources Reference Reference
Location
Cromadurine Crotalaria madurensis Wight Rao et al. (1974, 1975b) B62, 316
Cronaburmine Crotalaria nana Burm. Siddiqi et al. (1978b) A
Crosemperine Crotalaria aegyptiaca Benth. Zalkow et al. (1979) B419
C. semperflorens Vent. Atal et al. (1967) B331
Crotaflorine Crotalaria agatiflora Schweinf. Culvenor & Smith (1972) B281
Crotafoline Crotalaria laburnifolia L. subsp. eldomae Crout (1972) B312
Crotalarine Crotalaria burhia Buch-Ham. Ali & Adil (1973); Rao et al. (1975a) B292,
293
Crotaleschenine Crotalaria leschenaultii Suri & Atal (1967); Smith et al. (1988) B313
Crotananine Crotalaria nana Burm. Siddiqi et al. (1978a) A
Crotastriatine Crotalaria pallida Ait. (syn. C. Gandhi et al. (1968); Batra et al. (1975) B323,
mucronata Desv., C. striata DC.) 324
Crotaverrine Crotalaria verrucosa L. O.P. Suri et al. (1976) B339
C. walkeri Arnott K.A. Suri et al. (1976) B340
Cruentine A Senecio cruentus DC. Chu & Chu (1964) B172
Cruentine B Senecio cruentus DC. Chu & Chu (1964) B172
Curassavinine Heliotropium curassavicum Linn. Mohanraj et al. (1982a) C
Cynaustine Borago officinale L. (or amabiline ?) Larson et al. (1984) C
Cynoglossum australe R. Br. Culvenor & Smith, 1967 B33
C. lanceolatum Forsk. Suri et al. 1975a B36
Alkaloid Plant Sources Reference Reference
Location
Deoxyaxillarine Crotalaria scassellatii Chiov Wiedenfeld et al. 1985 A
Diacetyllycopsamine Amsinckia menziesii (Lehm.) Nels. & Macbr. Roitman, 1983a C
Dibenzoylretronecine Caccinea glauca Savi. Siddiqi et al. 1978a B346
Dicrotaline Crotalaria dura J.M. Wood et Evans Marais, 1944; Adams & Van Duuren, 1953a B295, 296
C. globifera E. Mey. Marais, 1944; Adams & Van B295,
Duuren, 1953a 296
N-(Dihydropyrrolizino- Heliotropium europaeum L. Culvenor & Smith, 1969 B68
methyl) heliotrine chloride
15,20-Dihydroxyerucifoline Senecio dolichodoryius Cuatr. Bohlmann et al. 1986 A
Dihydroxytriangularine Alkanna tinctoria Tausch Roder et al. 1984b C
Doronenine Senecio abrotanifolius ssp. Roder et al. 1984a A
abrotanifolius
S. abrotanifolius ssp. abrotanifolius Roder et al. 1984a A
var tiroliensis
S. doronicum L. Roder et al. 1979a, 1980; Kirfel B176, C
et al. 1980 A
Doronine Doronicum macrophyllum Alieva et al. 1976 B122
Senecio abrotanifolius ssp. Roder et al. 1984a A
abrotanifolius
S. abrotanifolius ssp. abrotanifolius Roder et al. 1984a A
var tiroliensis
S. clevelandii E.L. Greene Wong & Roitman, 1984 A
S. othonnae Bieb. Khalilov et al. 1977 B223
Alkaloid Plant Sources Reference Reference
Location
H. suaveolens Bieb. Guner, 1986 A
H. supinum L. Crowley & Culvenor, 1959 B83
Lappula glochidiata Suri et al. 1978 B84
Lindelofia angustifolia (Schrenk) Brand. Rao et al. 1974; Suri et al. 1975a B62, 36
L. spectabilis Lehm. Rao et al. 1974; Suri et al. 1975a B62, 36
L. stylosa (Kar. et Kir.) Brand. Kiyamitdinova et al. 1967 B75
L. tschimganica Akramov et al. 1965 B87
Paracynoglossum imeritinum (Kusn.) M. Pop. Man'ko & Marchenko, 1971b B88
Prestonia sp. Edgar, 1985 A
Rindera austroechinata M. Pop. Akramov et al. 1965 B87
R. baldshuanica Kusnezov Akramov et al. 1965 B87
R. cyclodonta Akramov et al. 1967a B59
R. echinata Regel Men'shikov & Denisova, 1953 B92
R. oblongifolia M. Pop. Akramov et al. 1965 B87
Solenanthus circinnatus Ledeb. Akramov et al. 1964 B93
S. coronatus Kiyamitdinova et al. 1967 B75
S. karateginus Lipsky Akramov et al. 1964 B93
Symphytum asperum Lepech. Man'ko et al. 1970b B97
S. caucasicum Bieb. Man'ko et al. 1972 B98
S. officinale Linn. Man'ko et al. 1970a; Huizing & B101, C
Malingre, 1979
Echiumine Amsinckia hispida (Ruiz et Pav.) Culvenor & Smith, 1966a B30
I.M. Johnston
A. intermedia Fisch et C. Mey. Culvenor & Smith, 1966a B30
A. lycopsoides Lehm. Culvenor & Smith, 1966a B30
Echium plantagineum L. Culvenor, 1956 B48
Emiline Emilia flammea Cass. Kohlmunzer & Tomczyk, 1969; Tomczyk & B123, 124
Kohlmunzer, 1971; Kohlmunzer B125
et al. 1971
Alkaloid Plant Sources Reference Reference
Location
13,19-Epoxyseneciphylline Senecio megaphyllus Green. Bohlmann et al. 1986 A
S. usgorensis Cuatr. Bohlmann et al. 1986 A
13,19-Epoxyspartiodine Senecio megaphyllus Green. Bohlmann et al. 1986 A
Erucifoline Senecio aegypticus L. Klasek et al. 1968a B145
S. erraticus Berthol. subsp. Schroter & Santavy, 1960; Sedmera B179,
barbaraeifolius Krock. et al. 1972 180
S. erucifolius L. Kompis & Santavy, 1962; Sedmera B183,
et al. 1972 180
Europine Heliotropium arbainense Zalkow et al. 1979 B419
H. digynum Forssk. Hammouda et al. 1984 A
H. europaeum L. Culvenor, 1954 B66
H. lasiocarpum Fisch and Mey. Culvenor et al. 1986 A
H. maris-mortui Zalkow et al. 1978 B74
H. rotundifolium Zalkow et al. 1978 B74
H. suaveolens Bieb. Guner, 1986 A
Trichodesma africana Zalkow et al. 1979 B419
Floricaline Cacalia floridana Cava et al. 1968 B119
Floridanine Cacalia floridana Cava et al. 1968 B119
Doronicum macrophyllum Alieva et al. 1976 B122
Senecio aureus L. Roder et al. 1983 C
S. erraticus Berthol. Gaiduk et al. 1974 B177
S. othonnae Bieb. Khalilov & Telezhenetskaya, 1973b B222
Florosenine Cacalia floridana Cava et al. 1968 B119
Senecio aureus L. Roder et al. 1983 C
S. fluviatilis Wallr. Klasek et al. 1973b B185
S. quebradensis Greem. Bohlmann et al. 1986 A
Alkaloid Plant Sources Reference Reference
Location
Fulvine Crotalaria berteroana DC. (C. fulva Roxb.) Schoental, 1963 B297
C. crispata F. Muell. ex Benth. Culvenor & Smith, 1963 B294
C. madurensis R. Wight Atal et al. 1966a; Habib et al. 1971 B280, 315
C. paniculata Willd. Subramanium et al. 1968 B325
Globiferine Crotalaria globifera E. Mey. Brown et al. 1984 C
Grahamine Crotalaria grahamiana R. Wight et Atal et al. 1969 B299
Walk.-Arn.
Grantaline Crotalaria globifera E. Mey Brawn et al., 1984 C
C. virgulata subsp. grantiana (Harv.) Smith & Culvenor, 1984 C
Polhill (C. grantiana Harvey)
Grantianine Crotalaria globifera E. Mey. Brown et al. 1984 C
C. virgulata subsp. grantiana (Harv.) Adams et al. 1942b; Adams & B301
Polhill (C. grantiana Harvey) Gianturco, 1956b; Smith & B259, C
Culvenor, 1984
Gynuramine Gynura scandens O. Hoffm. Wiedenfeld, 1982 A
Heleurine Heliotropium europaeum L. Culvenor, 1954 B66
H. indicum L. Hoque et al. 1976 B72
H. lasiocarpum Fisch and Mey. Culvenor et al. 1986 A
Heliosupine Cynoglossum creticum Zalkow et al. 1979 B419
C. officinale Man'ko & Borisyuk, 1957; Man'ko, 1959; B38, 39
Sykulska, 1961 B40
C. pictum Man'ko & Marchenko, 1971b, 1972b B44, 45
C. viridiflorum Pallas ex Lehm. Man'ko, 1972 B34
Echium vulgare L. Man'ko, 1964 B49
Heliotropium supinum L. Denisova et al. 1953; Crowley & B82, 83
Culvenor, 1959
Alkaloid Plant Sources Reference Reference
Location
Myosotis sylvatica Hoffm. Culvenor & Smith, unpubl.
Paracynoglossum imeritinum (Kusn.) Man'ko & Marchenko, 1971 B88
Symphytum asperum Lepech. Man'ko et al. 1970b B97
S. officinale Linn. Man'ko et al. 1970a B101
Heliotrine Heliotropium acutiflorum Akramov et al. 1968 B51
H. arbainense Zalkow et al. 1979 B419
H. arguzioides Kar. et Kir. Zolotavina, 1963 B53
H. curassavicum Linn. Rajagopalan & Batra, 1977b B56
H. dasycarpum Ledeb. Akramov et al. 1961a B54
H. digynum Forssk. Hammouda et al. 1984 A
H. eichwaldi Steud. ex DC. Gandhi et al. 1966a B60
H. europaeum L. Trautner & Neufeld, 1949; Culvenor B64, 65
et al. 1954
H. indicum L. Hoque et al. 1976 B72
H. lasiocarpum Fisch et C. Mey. Men'shikov, 1932 B73
H. olgae Kiyamitdinova et al. 1967; Sheveleva B75, 76
et al. 1969
H. popovii H. Riedl. subsp. gillianum Mohabbet et al. 1976 B77
H. ramosissimum Habib, 1975; Schoental & B79, 78
Cavanagh, 1972
H. suaveolens Bieb. Guner, 1986 A
H. supinum L. Pandey et al. 1983 C
H. transoxanum Akramov et al. 1968 B51
Heliovinine Heliotropium curassavicum Linn. Mohanraj et al. 1982c C
Heterophylline Parsonsia heterophylla A. Cunn. Edgar et al. 1980 B418
P. spiralis Edgar et al. 1980 B418
18-Hydroxysenkirkine Crotalaria laburnifolia L. subsp. Crout, 1972 B312
19-Hydroxysenkirkine Senecio laricifolius H.B.K. Bohlmann et al. 1986 A
Alkaloid Plant Sources Reference Reference
Location
Incanine Heliotropium olgae Sheveleva et al. 1969 B76
Trichodesma incanum Alph. DC. Yunusov & Plekhanova, 1953, 1957, B110, 111
1959; Tashkhodzhaev et al. 1979a B112, C
Indicine Heliotropium amplexicaule Vahl. Ketterer et al. 1987, (in press)
H. indicum L. Mattocks et al. 1961 B70
Prestonia sp. Edgar, 1985 A
Integerrimine Cacalia hastata L. subsp. orientalis Hayashi et al. 1972 B120
Kitamura
Crotalaria brevidens Benth. var. Suri et al. 1975b B304
intermedia (Kotschy) Polhill
C. brevifolia Sawhney & Atal, 1966 B290
C. incana L. Adams & Van Duuren, 1953b B187
C. pallida Ait. Sawhney et al. 1967 B291
C. tetragona Roxb. Puri et al. 1974 B314
C. zanzibarica Benth. (C. usaramoensis) Culvenor & Smith, 1966b B337
Petasites hybridus L. Luthy et al. 1983 C
Senecio alpinus L. Luthy et al. 1981 A
S. antieuphorbium (L.) Sch. Bip. Rodriguez & Gonzalez, 1969 B152
S. brasiliensis DC. Motidome & Ferreira, 1966a B163
S. brasiliensis Less. var tripartitus Nardi et al. 1980 A
S. durieui Gay Panizo & Rodriguez, 1974 B157
S. erraticus Berthol. subsp. Santavy et al. 1962 B182
barbaraeifolius Krock.
S. faberi Hemsl. Wei et al. 1982 C
S. formosus Munoz Quevedos, 1976 B186
S. glandulosus Don ex Hook. et Arn. Pestchanker et al. 1985b A
S. inaequidens DC. Bicchi et al. 1985 A
Alkaloid Plant Sources Reference Reference
Location
S. incanus L. subsp. carniolicus Klasek et al. 1968b B147
(Willd.) Br.-Bl.
S. integerrimus Manske, 1939a; Roitman et al. 1979 B156, 420
S. kleinia Sch. Bip. Gonzalez & Calero, 1958b B205
S. leucostachys Baker Pestchanker & Giordano, 1986 A
S. magnificus F. Muell. Gellert & Mate, 1964 B216
S. morrisonensis Hayata Lu, Sheng-Teh, 1972 B217
S. nebrodensis L. var sicula Plescia et al. 1976 B218
S. ragonesei Cabr. Pestcharnker & Giordano, 1986 A
S. spathulatus A. Rich. White, 1969 B158
S. squalidus L. Kropman & Warren, 1950; Gonzalez & B265,
Calero, 1958a 205
S. tenuifolius Burm. Bhakuni & Gupta, 1982 C
S. triangularis Hook. Roitman, 1983b C
S. vernalis Walst. et Kit. Sener et al. 1986; Hartmann & A, A
Zimmer, 1986
S. viscosus L. Barger & Blackie, 1936; Santavy et al. B264,
1962 182
S. vulgaris L. Pieters & Vlietinck, 1986 A
Intermedine Amsinckia hispida (Ruiz et Pav.) Culvenor & Smith, 1966a B30
I.M. Johnston
A. intermedia Fisch et C. Mey. Culvenor & Smith, 1966a B30
A. lycopsoides Lehm. Culvenor & Smith, 1966a B30
A. menziesii (Lehm.) Nels. & Macbr. Roitman, 1983 C
Borago officinale L. Luthy et al. 1984 A
Conoclinium coelestinium (L.) DC Herz et al. 1981 C
Eupatorium compositifolium Walt. Herz et al. 1981 C
Symphytum aspera Roitman, 1981 A
S. officinale Linn. Roder et al. 1982 C
S. x uplandicum Nyman Culvenor et al. 1980a, 1980b A, A
Trichodesma africana Zalkow et al. 1979 B419
Alkaloid Plant Sources Reference Reference
Location
Isocromadurine Crotalaria candicans W. and A. Suri et al. 1982 C
C. madurensis R. Wight ]Rao et al. 1975c B317
Isoline Senecio othonniformis Fourcade Coucourakis & Gordon-Gray, 1970; B224
Coucourakis et al. 1972 B225
Jacobine Crassocephalum crepidioides Asada et al. 1985 A
Senecio alpinus L. Luthy et al. 1981 A
S. brasiliensis DC. Adams & Gianturco, 1956e B148
S. cineraria DC. Barger & Blackie, 1937 B167
S. jacobaea L. Manske, 1931; Blackie, 1937; B194, 153
Bradbury & Culvenor, 1954 B198
S. paludosus L. Blackie, 1937; Dorosh & Alekseev, 1960 B153, 227
Alekseev, 1961b B228
Jacoline Crassocephalum crepidioides Asada et al. 1985 A
Senecio alpinus L. Luthy et al. 1981 A
S. jacobaea L. Bradbury & Culvenor, 1954 B198
Jaconine Senecio alpinus L. Luthy et al. 1981 A
S. jacobaea L. Bradbury & Culvenor, 1954 B198
Jacozine Senecio alpinus L. Luthy et al. 1981 A
S. cannabifolius Less Asada et al. 1982 C
S.jacobaea L. Bradbury & Culvenor, 1954; Culvenor, B198,
1964 202
Junceine Crotalaria juncea L. Adams & Gianturco, 1956a, 1956b, 1956c B305,
B306
B307
C. wightiana Grah. ex Wight & Arn. Atal et al. 1966b B329
Alkaloid Plant Sources Reference Reference
Location
Lasiocarpine Heliotropium arbainense Zalkow et al. 1979 B419
H. arborescens L. Carcamo-Marquez, 1961 B52
H. curassavicum Linn. Rajagopalan & Batra, 1977b B56
H. digynum Forssk. Hammouda et al. 1984 A
H. eichwaldii Steud. ex DC. Rao et al. 1974 B62
H. europaeum L. Culvenor et al. 1954 B65
H. indicum Linn. Hoque et al. 1976 B72
H. lasiocarpum Fisch. et C. Mey. Men'shikov, 1932 B73
H. maris mortui Zalkow et al. 1979 B419
H. suaveolens Bieb. Guner, 1986 A
H. supinum L. Pandey et al. 1983 C
Lappula intermedia Man'ko & Vasil'kov, 1968 B85
Symphytum caucasicum Man'ko et al. 1969 B95
S. officinale Linn. Man'ko et al. 1969, 1970a B95, 101
Latifoline Cynoglossum latifolium R. Br. Crowley & Culvenor, 1962 B37
Hackelia floribunda Hagglund et al. 1985 A
Ligudentine Ligularia brachyphylla Hand.-Mazz. Klasek et al. 1971 B134
L. dentata (A. Gray) Hara. Klasek et al. 1971 B134
Ligularidine Ligularia dentata (A. Gray) Hara Hikichi et al. 1979, Asada & B436, C
Furuya, 1984a
Ligularine Ligularia brachyphylla Hand.-Mazz. Klasek et al. 1971 B134
L. dentata (A. Gray) Hara. Klasek et al. 1971 B134
L. elegans Cass. Klasek et al. 1971 B134
Ligularizine Ligularia dentata (A. Gray) Hara. Asada & Furuya, 1984a C
Alkaloid Plant Sources Reference Reference
Location
Lycopsamine Amsinckia hispida (Ruiz et Pav.) Culvenor & Smith, 1966a B30
I.M. Johnston
A. intermedia Fisch. et C. Mey. Culvenor & Smith, 1966a B30
A. lycopsoides Lehm. Culvenor & Smith, 1966a B30
A. menziesii (Lehm.) Nels. & Macbr. Roitman, 1983a C
Anchusa officinalis L. Broch-Due & Aasen, 1980 B32
Borago officinale L. Larson et al. 1984; Luthy et al. 1984 C, A
Eupatorium compositifolium Walt. Herz et al. 1981 C
Heliotropium steudneri Vatke Schneider et al. 1975 B8O
Messerschmidia sibirica Hikichi et al. 1980 C
Parsonsia eucalyptophylla F. Muell. Edgar & Culvenor, 1975 B28
P. straminea (R. Br.) F. Muell. Edgar & Culvenor, 1975 B28
Prestonia sp. Edgar, 1985 A
Symphytum asperum Lepech. Roitman, 1981 A
Symphytum officinale Linn. Huizing & Malingre, 1981 C
Symphytum x uplandicum Nyman Culvenor et al. 1980a, 1980b A, A
Madurensine Crotalaria agatiflora Schweinf. Atal et al. 1966a B280
C. laburnifolia L. subsp. eldomae Crout, 1972 B312
C. madurensis R. Wight Atal et al. 1966a; Mahran et al. 1979 B280, 426
Merenskine Senecio latifolius DC. Bredenkamp et al. 1985 C
Monocrotaline Crotalaria aegyptiaca Benth. Mahran et al. 1979; Zalkow et al. 1979 B426, 419
C. assamica Benth. Crotalaria Research Group, 1974 B286
C. burhia Rao et al. 1975 B293
C. cephalotes Steud. ex A. Rich Pilbeam et al. 1983 C
C. crispata F. Muell. ex Benth. Culvenor & Smith, 1963 B294
C. cunninghamii R. Br. Pilbeam et al. 1983 C
C. grahamiana R. Wight ex Walk.-Arn. Gandhi et al. 1966b; Atal et al. 1969 B298, 299
C. leschenaultii DC. Suri & Atal, 1967 B313
Alkaloid Plant Sources Reference Reference
Location
C. leiloba Bartl. Puri et al. 1974 B314
C. mitchellii Benth. Culvenor et al. 1967b B318
C. mysorensis Roth. Sawhney & Atal, 1968 B319
C. nitens Kunth. Hoet et al. 1981 A
C. novae-hollandiae DC. subsp. Culvenor et al. 1967b B318
lasiophylla (Benth.) A. Lee
C. paulina schrank. Pilbeam et al. 1983 C
C. quinquefolia L. Pilbeam et al. 1983 C
C. recta Steud. ex A. Rich Crout, 1968a B326
C. retusa L. Adams & Rogers, 1939; Culvenor & B327,
Smith, 1957a 328
C. sagittalis L. Willette & Cammarata, 1972 B330
C. spectabilis Roth. Neal et al. 1935; Adams & Rogers, B325,
1939 327
C. stipularia Desv. Puri et al. 1974 B314
Lindelofia spectabilis Lehm. Rao et al. 1974 B62
Monocrotalinine Crotalaria grahamiana Wight et Arn. Rajagopalan & Batra, 1977a B300
Myoscorpine Myosotis scorpioides L. Resch et al. 1982 C
Symphytum officinale Linn. Resch et al. 1982C C
Neoligularidine Ligularia dentata (A. Gray) Hara. Asada & Furuya, 1984a C
Neopetasitenine Ligularia japonica Asada et al. 1981 A
Petasites japonicus Maxim. Yamada et al. 1976a B19
Neosenkirkine Senecio auricola Bourg. Panizo & Rodriguez, 1974 B157
S. grandifolius Less. Bohlmann et al. 1986 A
S. pierotii Asada & Furuya, 1982 C
Neotriangularine Senecio triangularis Hook. Roitman, 1983b C
Alkaloid Plant Sources Reference Reference
Location
Nilgirine Crotalaria pallida Ait. Atal et al. 1968 B322
Onetine Senecio othonnae Bieb. Danilova et al. 1962 B221
Otosenine Cacalia floridana Cava et al. 1968 B119
Doronicum macrophyllum Alieva et al. 1976 B122
D. pardalianches Linn. Rajagopalan & Negi, 1985 A
Emilia flammea Cass. Kohlmunzer & Tomczyk, 1969 B123
Senecio aegypticus L. Klasek et al. 1968a B145
S. aureus L. Resch et al. 1983, Roder et al. 1983 C, C
S. cineraria DC. Habib, 1974 B170
S. desfontainei Druce Haddad et al. 1963; Klasek B173,
et al. 1968a 145
S. erraticus Berthol. Gaiduk et al. 1974 B177
S. erraticus subsp. barbaraeifolius Krock. Santavy, 1958; Schroter & Santavy, 1960 B178, 179
S. fluviatilis Wallr. Klasek et al. 1973b B185
S. jacobaea L. Akramov et al. 1968 B51
S. othonnae Bieb. Zhdanovich & Men'shikov, 1941; B220
Danilova et al. 1962 B221
S. renardii Winkl. Danilova & Konovalova, 1950 B249
S. tomentosus Adams et al. 1956; Schroter & B271,
Santavy, 1960 179
Parsonsine Parsonsia heterophylla A. Cunn. Eggers & Gainsford, 1979; Edgar et al. B417,
1980 418
P. spiralis Edgar et al. 1980 B418
Petasinine Petasites japonicus Maxim. Yamada et al. 1978b B141
Petasitenine Farfugium japonicum Kitam Niwa et al. 1985 A
Petasites japonicus Maxim. Yamada et al. 1976a, 1976b; B19, 139
Furuya et al. 1976 B20
Alkaloid Plant Sources Reference Reference
Location
Retroisosenine Senecio nemorensis L. var. bulgaricus Nghia et al. 1976 B219
(Vel.) Stoj. et Stef.
S. nemorensis L. var subdecurrens Klasek et al. 1980a B422
Retrorsine Crotalaria spartioides DC. Bruemmerhoff & de Waal, 1961 B334
C. zanzibarica Benth. (C. usaramoensis) Culvenor & Smith, 1966b B337
Senecio ambrosioides Adams & Gianturco, 1956e B148
S. ampullaceus Hook. Adams & Govindachari, 1949b; Adams & B149, 151
Looker, 1951; Warren et al. 1950 B150
S. bipinnatisectus Belcher White, 1969 B158
S. brasiliensis DC. Motidome & Ferreira, 1966a B163
S. bupleuroides DC. Sapiro, 1949 B164
S. cineraria DC. Klasek et al. 1975 B171
S. cruentus DC. Asada et al. 1982 C
S. cymbaroides Roitman et al. 1979 B420
S. desfontainei Druce Rizk et al. 1983 A
S. discolor DC. Schoental, 1960; Hennig, 1961 B174, 175
S. douglasii DC. Adams & Govindachari, 1949b; Adams B149,
& Looker, 1951 151
S. eremophilus Richards Adams & Govindachari, 1949b; Adams B149,
& Looker, 1951 151
S. erucifolius L. Ferry & Brazier, 1976 B184
S. filaginoides (H. et A.) DC. Pestchanker & Giordano, 1986 A
S. formosus Munoz Quevedo, 1976 B186
S. gilliesiano Guidugli et al. 1986 A
S. glaberrimus DC. Blackie, 1937 B153
S. glandulosus Don ex Hook. et Arn. Pestchanker et al. 1985b A
S. graminifolius N.J. Jacq. de Waal, 1941 B188
S. griesbachii Motidome & Ferreira, 1966b B190
S. ilicifolius Thunb. de Waal, 1940a, 1940b, 1941; Culvenor B191, 192
& Smith, 1954 B188, 127
Alkaloid Plant Sources Reference Reference
Location
S. inaequidens DC. Roder et al. 1981 A
S. isatideus DC. Blackie, 1937; de Waal, 1939 B153, 193
S. jacobaea L. Ferry & Brazier, 1976 B184
S. latifolius DC. Watt, 1909; Barger et al. 1935 B211, 212
S. longilobus Benth. Adams & Govindachari, 1949b; Adams & B149, 151
Looker, 1951; Warren et al. 1950 B150
S. paucicalyculatus Klatt Pretorius, 1949 B230
S. phillipicus Roegel et Koern Gonzalez et al. 1986a A
S. pterophorus DC. de Waal, 1940b, 1941; Culvenor & B192, 188
Smith, 1954 127
S. quadridentatus Labill. Culvenor & Smith, 1955 B247
S. ragonesei Cabr. Pestchanker & Giordano, 1986 A
S. retrorsus DC. Manske, 1931; de Waal, 1939 B194, 193
S. riddellii Torr. et A. Gray Roitman et al. 1979 B420
S. riddellii Torr. et A. Gray var. Adams & Govindachari, 1949b B149
parksii Cory.
S. ruderalis Harvey Leisegang, 1950 B254
S. seratophiloides Griseb. Pestchanker & Giordano, 1986 A
S. spartioides Roitman et al. 1979 B420
S. subulatus Don ex Hook. et Arn Pestchanker et al. 1985b A
var erectus
S. swaziensis Compton Gordon-Gray et al. 1972; Gordon-Gray B268,
& Wells, 1974 270
S. triangularis Hook. Roitman, 1983b C
S. uspallatensis Pestchanker et al. 1985a A
S. venosus Harvey Blackie, 1937 B153
S. vernalis Waldst. et Kit. Roder et al. 1979b C
S. viminalis Bremek. de Waal & van Twisk, 1964 A
S. vulgaris L. Tschu Shun et al. 1960 B277
S. werneriaefolius Roitman et al. 1979 B420
Alkaloid Plant Sources Reference Reference
Location
Retusamine Crotalaria mitchellii Benth. Culvenor et al. 1967b B318
C. mitchellii Benth. subsp. laevis Culvenor et al. 1967b B318
A. Lee
C. novae-hollandiae DC. subsp. Culvenor et al. 1967b B318
lasiophylla Benth. A. Lee
C. novae-hollandiae DC. subsp. Culvenor et al. 1967b B318
novae-hollandiae
C. retusa L. Culvenor & Smith, 1957a; Wunderlich, B328, C
1962
Riddelliine Crotalaria juncea L. Adams & Gianturco, 1956a B305
Senecio aegypticus L. Klasek et al. 1968a B145
S. ambrosioides Roitman et al. 1979 B420
S. cruentus DC. Asada et al. 1982 C
S. cymbaroides Roitman et al. 1979 B420
S. desfontainei Druce Haddad et al. 1963; Klasek et al. B173,
1968a 145
S. douglassi DC. Adams & Govindachari, 1949b; Adams & B149,
Looker, 1951 151
S. eremophilus Richards Adams & Govindachari, 1949b; Adams & B149,
Looker, 1951 151
S. longilobus Benth. Adams & Govindachari, 1949b; Adams & B149,
Looker, 1951; 151
Warren et al. 1950 B150
S. riddellii Torr. et A. Gray Manske, 1939a; Adams et al. 1942c B156, 252
S. riddellii Torr. et A. Gray var. Adams & Govindachari, 1949b B149
parksii (Cory)
S. spartioides Roitman et al. 1979 B420
S. vernalis Walst. et Kit. Sener et al. 1986 A
S. vulgaris Roitman et al. 1979 B420
Alkaloid Plant Sources Reference Reference
Location
Rinderine Eupatorium altissimum L. Herz et al. 1981 C
E. serotinum Michx. Locock et al. 1966 B131
Prestonia sp. Edgar, 1985 A
Rindera baldshuanica Kusnezov Akramov et al. 1961c B91
Solenanthus turkestanicus Regel Akramov et al. 1962 B94
et Smirnov
(Kusnezov)
Sceleratine Senecio latifolius DC. (S. sceleratus) de Waal & Pretorius, 1941; de Waal B257,
et al. 1963 260
Scorpioidine Myosotis scorpioides L. Resch et al. 1982 C
Sencalenine Senecio cacaliaster (Lam.) Roder et al. 1984b A
Senecicannabine Senecio cannabifolius Less. Asada et al. 1982 C
Senecionine Brachyglottis repanda Forst. et Forst. Mortimer & White, 1967 B117
Caltha biflora Stermitz & Adamovics, 1977 B341
C. leptosepala Stermitz & Adamovics, 1977 B341
Castilleja rhexifolia Rydb. Stermitz & Suess, 1978 B342
Castilleja "rhexifolia aff. miniata" Roby & Stermitz, 1984 C
Crotalaria juncea L. Adams & Gianturco, 1956a B305
C. micans Link. Sethi & Atal, 1964 B284
C. zanzibarica Benth. (C. usaramoensis) Culvenor & Smith, 1966b B337
Emilia sonchifolia DC. Culvenor, unpubl.
Erechtites hieracifolia (L.) Raf. ex DC. Manske, 1939b; Culvenor & Smith, 1954 B126, 127
Gynura segetum (Lour.) Merr. Hua et al. 1983; Liang & Roder, 1984 C, C
Ligularia japonica Asada et al. 1981 A
Petasites hybridus L. Luthy et al. 1983 C
P. laevigatus (Willd.) Reichenb. Massagetov & Kuzovkov, 1953 B142
Senecio aegypticus L. Klasek et al. 1968a; Gharbo & B145,
Habib, 1969 146
Alkaloid Plant Sources Reference Reference
Location
S. alpinus (L.) Scop. Luthy et al. 1981 A
S. ambrosioides Adams & Gianturco, 1956e B148
S. ampullaceus Hook. Adams & Govindachari, 1949b; Adams & B149,
Looker, 1951; 151
Warren et al. 1950 B150
S. argentino Baker (vira-vira Hieron) Pestchanker & Giordano, 1986 A
S. aureus L. Manske, 1936, 1939a B155, 156
S. brasiliensis DC. Fonseca, 1951; Novelli & De Varella, B162,
1945; Adams & Gianturco, 1956e 161
B148
S. carthamoides Greene Adams & Govindachari, 1949b; Adams B149,
& Looker, 1951 151
S. cineraria DC. Barger & Blackie, 1937; Adams & B167,
Govindachari, 1949a; 168
Alekseev et al. 1962a B169
S. congestus (R.Br.) DC. Roder et al. 1982 C
S. cruentus DC. Asada et al. 1982 C
S. cymbaroides Roitman et al. 1979 B420
S. desfontainei Druce Klasek et al. 1968a B145
S. discolor DC. Schoental, 1960; Hennig, 1961 B174, 175
S. douglasii DC. Adams & Govindachari, 1949b; Adams B149,
& Looker, 1951 151
S. eremophilus Richards Adams & Govindachari, 1949b; Adams B149,
& Looker, 1951 151
S. erraticus Berthol. Gaiduk et al. 1974 B177
S. erraticus Berthol. subsp. Santavy, 1958; Schroter & Santavy, B178,
barbaraeifolius Krock. 1960; Kompis et al. 1960 179
B181
S. erucifolius L. Kompis & Santavy, 1962 B183
S. filaginoides (H. et A.) DC. Pestchanker & Giordano, 1986 A
Alkaloid Plant Sources Reference Reference
Location
S. fistulosus Poepp. ex Less. Gonzalez et al. 1986b A
S. fremontii Torr. et A. Gray Adams & Gianturco, 1956e B148
S. gilliesiano Guidugli et al. 1986 A
S. glabellus Turcz. DC. Adams & Van Duuren, 1953b B187
S. ilicifolius Thunb. de Waal, 1940a, 1940b, 1941; Culvenor B191,
& Smith, 1954 192
B188,
127
S. illinitus Phill. Gonzalez et al. 1986a A
S. inaequidens DC. Roder et al. 1981 A
S. integerrimus Nutt. Manske, 1939a B156
S. jacobaea L. Bradbury & Mosbauer, 1956 B199
S. laricifolius H.B.K. Bohlmann et al. 1986 A
S. lautus Forst. f. ex Willd. Culvenor unpubl.
S. leucostachys Baker Pestchanker & Giordano, 1986 A
S. longiflorus Sch. Bip. de Waal & van Twisk, 1964 A
S. magnificus F. Muell. Culvenor, 1962 B215
S. multilobatus MaCoy et al. 1983 A
S. multivenius Benth. in Oerst Bohlmann et al. 1986 A
S. nebrodensis L. var. sicula Plescia et al. 1976 B218
S. nemorensis L. subsp. fuchsii Gmel. Wiedenfeld & Roder, 1979 B423
S. pampeanus Cabrera Novelli, 1958 B229
S. pancicii Degen var arnautorum Jizba et al. 1982 C
(Velen.) Stoj. Stef. et Kit.
S. pancicii Degen var pancicii Jizba et al. 1982 C
S. patagonicus Hook. and Arn. Villarroel et al. 1985 A
S. petasitis DC. Gharbo & Habib, 1969 B146
S. pimpinellifolius H.B.K. Bohlmann et al. 1986 A
S. pseudo-arnica Less. Manske, 1939a B156
S. pterophorus DC. de Waal, 1940b, 1941; Culvenor & B192,
Smith, 1954 188
B127
Alkaloid Plant Sources Reference Reference
Location
S. quadridentatus Labill. Culvenor & Smith, 1955 B247
S. sandrasicus Temizer et al. 1985 A
S. scandens Batra & Rajagopalan, 1977 B256
S. seratophiloides Griseb. Pestchanker & Giordano, 1986 A
S. spartioides Manske, 1939a; Adams & Gianturco, B156,
1957b 262
S. spathulatus A. Rich White, 1969 B158
S. squalidus L. Barger & Blackie, 1936; Kropman B264,
& Warren, 1950 265
S. subalpinus C. Koch Trivedi & Santavy, 1963 B267
S. subulatus Don ex Hook. et Arn Pestchanker et al. 1985b A
var erectus
S. tenuifolius Burm. Bhakuni & Gupta, 1982 C
S. tomentosus Adams et al. 1956 B271
S. triangularis Hook. Kupchan & Suffness, 1967 B272
S. uintahensis Roitman et al. 1979 B420
S. vernalis Waldst. et Kit. Roder et al. 1979b C
S. viminalis Bremek. de Waal & van Twisk, 1964 A
S. viscosus L. Barger & Blackie, 1936; Santavy et al. B264,
1962 182
S. vulgaris L. Grandval & Lajoux, 1895; Barger & B275,
Blackie, 1936; Konovalova & 264
Orekhov, 1937a; Tschu Shun B276
et al. 1960 B277
S. wernariaefolius Roitman et al. 1979 B420
Syneilesis palmata Maxim. Hikichi & Furuya, 1976 B279
Tussilago farfara Rosberger et al. 1981 C
7-Senecioyl-9-(2-hydroxy- Senecio caudatus DC. Bohlmann et al. 1986 A
3-acetylbutyrl)retronecine
Alkaloid Plant Sources Reference Reference
Location
7-Senecioyl-9-(2-hydroxy- Senecio caudatus DC. Bohlmann et al. 1986 A
methyl-2,3-dihydroxy-
butyrylretronecine
7-Senecioyl-9-(2-methyl- Senecio caudatus DC. Bohlmann et al. 1986 A
2,3-dihydroxybutyryl-
retronecine
7-Senecioylretronecine Senecio cacaliaster (Lam.) Roder et al. 1984b C
S. caudatus DC. Bohlmann et al. 1986 A
S. triangularis Hook. Rueger & Benn, 1983a C
S. variabilis Sch. Bip. Bohlmann et al. 1986 A
9-Senecioylretronecine Senecio caudatus DC. Bohlmann et al. 1986 A
S. variabilis Sch. Bip. Bohlmann et al. 1986 A
7-Senecioyl-9-sarracinyl- Senecio cacaliaster Roder et al. 1984b C
retronecine S. caudatus DC. Bohlmann et al. 1986 A
S. triangularis Hook. Rueger & Benn, 1983 C
S. ungeniensis Thell. Bohlmann et al. 1986 A
S. variabilis Sch. Bip. Bohlmann et al. 1986 A
Seneciphylline Adenostyles alliarae Yakhontova et al. 1976 B115
A. glabra Wiedenfeld et al. 1984 C
A. rhombifolius (Willd.) M. Pimen.subsp. Pimenov et al. 1975 B116
platyphylloides
Crotalaria juncea L. Adams & Gianturco, 1956a B305
Erechtites hieracifolia (L.) Raf. ex DC. Manske, 1939a; Culvenor & Smith, 1954 B126, 127
Senecio alpinus (L.) Scop. Klasek et al. 1968b; Luthy et al. 1981 B147, A
S. ambrosioides Adams & Gianturco, 1956e B148
Alkaloid Plant Sources Reference Reference
Location
S. ampullaceus Hook. Adams & Govindachari, 1949b; Adams & B149,
Looker, 1951; Warren et al. 1950 151
B150
S. aquaticus Hill Blackie, 1937; Evans & Evans, 1949 B153, 154
S. borysthenicus Red'ko, 1956; Alekseev, 1961a B159, 160
S. brasiliensis DC. Fonseca, 1951; Novelli & de Varella, B162,
161
1945; Adams & Gianturco, 1956e B148
S. cannabifolius Less. Alekseev, 1964; Asada et al. 1982 B165, C
S. carthamoides Greene Adams & Govindachari, 1949b; Adams & B149,
Looker, 1951 151
S. chrysanthemoides Wali & Handa, 1964 B166
S. cineraria DC. Barger & Blackie, 1937; Adams & B167,
Govindachari, 1949a 168
S. cruentus DC. Asada et al. 1982 C
S. cymbaroides Roitman et al. 1979 B420
S. desfontainei Druce Gharbo & Habib, 1969 B146
S. douglasii DC. Adams & Govindachari, 1949b; Adams & B149,
Looker, 1951 151
S. eremophilus Richards Adams & Govindachari, 1949b; Adams & B149,
Looker, 1951 151
S. erraticus Berthol. subsp. Kompis et al. 1960; Santavy B181,
barbaraeifolius Krock. et al. 1962 182
S. erucifolius L. Kompis & Santavy, 1962 B183
S. fluviatilis Wallr. Klasek et al. 1973b B185
S. fremontii Torr. et A. Gray Adams & Gianturco, 1956e B148
S. grandifolia Glonti, 1958 B189
S. ilicifolius Thunb. de Waal, 1940a, 1940b, 1941; B191, 192
Culvenor & Smith, 1954 B188,
127
Alkaloid Plant Sources Reference Reference
Location
S. incanus L. subsp. carniolus Klasek et al. 1968b B147
(Willd.) Br.-Bl.
S. jacobaea L. Blackie, 1937; Bradbury & B153,
Culvenor, 1954 198
S. krylovii Sapunova & Ban'kovskii, 1968 B207
S. kubensis Grossh. Khalilov & Telezhenetskaya, 1973a B208
S. lampsanoides Khalilov & Damirov, 1974 B210
S. laricifolius H.B.K. Bohlmann et al. 1986 A
S. latifolius DC. Danilova et al. 1960 B213
S. longiflorus Sch. Bip. de Waal & van Twisk, 1964 A
S. longilobus Benth. Adams & Govindachari, 1949b; Adams & B149,
Looker, 1951 151
S. minimus Poir. White, 1969 B158
S. multivenius Benth. in Oerst Bohlmann et al. 1986 A
S. othonnae Bieb. Zhdanovich & Men'shikov, 1941; B220
Danilova et al. 1962 B221
S. palmatus Pall. Alekseev, 1960 B226
S. paludosus L. Blackie, 1937; Dorosh & Alekseev, B153,
1960; 227
Alekseev, 1961b B228
S. pancicii Degen var arnautorum Jizba et al. 1982 C
(Velen.) Stoj. Stef. et Kit.
S. pancicii Degen var panicii Jizba et al. 1982 C
S. patagonicus Hook. and Arn. Villarroel et al. 1985 A
S. paucifolius S.G. Gmel. Alekseev & Ban'kovskii, 1965 B231
S. phillipicusRoegel et Koern Gonzalez et al. 1986a A
S. platyphylloides Somm. et Lev. Murav'eva, 1964b, 1965; Dauksha, 1970 B233, 234
B235
S. platyphyllus (Bieb.) DC. Orekhov, 1935; Konovalova & B236,
Orekhov, 1938; 237
Konovalova, 1951 B238
Alkaloid Plant Sources Reference Reference
Location
S. pojarkovae Chernova & Murav'eva, 1974 B243
S. propinquus Ait. Khalilov et al. 1972 B245
S. pterophorus DC. de Waal, 1940b, 1941; Culvenor & B192,
Smith, 1954 188
B127
S. quadridentatus Labill. Culvenor & Smith, 1955 B247
S. racemosus Khmel, 1961 B248
S. renardii Winkl. Danilova & Konovalova, 1950 B249
S. rhombifolius (Willd.) Sch. Bip. Khalilov & Telezhenetskaya, 1973a B208
S. scandens Batra & Rajagopalan, 1977 B256
S. spartioides Torr. et A. Gray Manske, 1939a; Adams & Gianturco, B156,
1957b 262
S. spathulatus Benn et al. 1979 B263
S. stenocephalus Maxim. Konovalova & Orekhov, 1937b B266
S. subalpinus C. Koch. Trivedi & Santavy, 1963; Klasek B267,
et al. 1968b 147
S. vernalis Walst. et Kit. Sener et al. 1986; Hartmann & A, A
Zimmer, 1986
S. vulgaris L. Barger & Blackie, 1936; Konovalova & B264,
Orenkhov, 1937a; 276
Tschu Shun et al. 1960 B277
Senecivernine Senecio inaequidens Bicchi et al. 1985 A
S. seratophylloides Griseb. Pestchanker & Giordano, 1986 A
S. vernalis Waldst. et Kit. Roder et al. 1979b C
Senkirkine Brachyglottis repanda Forst. et Forst. Mortimer & White, 1967 B117
Crotalaria laburnifolia subsp. eldomae Crout, 1972 B312
Farfugium japonicum Kitam. Furuya et al. 1971 B133
Petasites albus L. Luthy et al. 1983 C
P. hybridus L. Luthy et al. 1983 C
P. japonicus Maxim. Yamada et al. 1978a B140
Alkaloid Plant Sources Reference Reference
Location
P. laevigatus (Willd.) Reichenb. Massagetov & Kuzovkov, 1953 B142
Senecio antieuphorbium (L.) Sch. Bip. Rodriguez & Gonzalez, 1969 B152
S. desfontainei Druce Rizk et al. 1983 A
S. grandifolius Less. Bohlmann et al. 1986 A
S. illinitus Phill. Gonzalez et al. 1986a A
S. jacobaea L. Akramov et al. 1968 B51
S. kirkii Hook. f. ex Kirk. Briggs et al. 1948; Briggs et al. 1965 B203, 204
S. kleinia Sch. Bip. Rodriguez et al. 1967 B206
S. laricifolius H.B.K. Bohlmann et al. 1986 A
S. pierotii Asada & Furuya, 1982 C
S. procerus L. var. procerus Stoj. Jovceva et al. 1978 B244
Stef. et Kit.
S. quebradensis Greenm. Bohlmann et al. 1986 A
S. renardii Winkl. Danilova & Konovalova, 1950; Briggs B249,
et al. 1965 204
S. tenuifolius Burm. Bhakuni & Gupta, 1982 C
S. vernalis Waldst. et Kit. Roder et al. 1979b C
S. uintahensis Roitman et al. 1979 B420
Tussilago farfara Culvenor et al. 1976b; Borka & A, B425,
Onshuus, 1979; Luthy et al, 1980 A
Sincamidine Amsinckia intermedia Fisch. et C. Mey. Culvenor & Smith, 1966a B30
Spartioidine Senecio spartioides Torr. et A. Gray Manske, 1939a; Adams & Gianturco, 1957 B156,
262
S. vulgaris L. Pieters & Vlietinck, 1986 A
Spectabiline Crotalaria spectabilis Roth Culvenor & Smith, 1957b B336
Spiracine Parsonsia spiralis Wall. Edgar et al. 1980 B418
Spiraline Parsonsia spiralis Wall. Edgar et al. 1980 B418
Alkaloid Plant Sources Reference Reference
Location
Spiranine Parsonsia spiralis Wall. Edgar et al. 1980 B418
Supinine Borago officinale L. Luthy et al. 1984 A
Eupatorium cannabinum L. Pederson, 1975a B128
E. serotinum Michx. Locock et al. 1966 B131
E. stoechadosmum Hanse Furuya & Hikichi, 1973 B132
Heliotropium europeum L. Culvenor, 1954 B66
H. indicum L. Hoque et al. 1976 B72
H. supinum L. Men'shikov & Gurevich, 1949; Crowley & B81, 83
Culvenor, 1959
Tournefortia sarmentosa Lam. Crowley & Culvenor, 1955 B107
Trichodesma zeylanicum (Burm. f.) R. Br. O'Kelly & Sargeant, 1961 B113
Swazine Senecio barbellatus DC. Gordon-Gray & Wells, 1974 B270
S. swaziensis Compton Gordon-Gray et al. 1972; Laing & B268,
Sommerville, 1972 269
Symlandine Symphytum asperum Lepech. Roitman, 1981 A
S. officinale Linn. Roder et al. 1982 C
S. tuberosum L. Gray et al. 1983 A
S. x uplandicum Nyman Culvenor et al. 1980a, 1980b A, A
Symphytine Myosotis scorpioides L. Resch et al. 1982 C
Symphytum aspera Roitman, 1981 A
S. officinale Linn. Furuya & Araki, 1968; Furuya & B15, 16
Hikichi, 1971
S. peregrinum Ledeb. Gadella et al. 1983 A
S. x uplandicum Nyman Culvenor et al. 1980a, 1980b A, A
Syneilesine Syneilesis palmata Maxim. Hikichi & Furuya, 1974, 1976 B278, 279
Alkaloid Plant Sources Reference Reference
Location
Triangularine Alkanna tinctoria Tausch Roder et al. 1984b C
Senecio triangularis Hook. Roitman, 1983b C
Trichodesmine Crotalaria globifera E. Mey. Brown et al. 1984 C
C. juncea L. Adams & Gianturco, 1956a B305
C. lunata Beddome ex Polhill Rothschild et al. 1979 C
C. recta Steud ex A. Rich. Crout, 1968a B326
C. wightiana Grah. ex Wight & Arn. Atal et al. 1966b B329
C. tetragona Roxb. Puri et al. 1974 B314
Heliotropium arguzioides Kar. et Kir. Akramov et al. 1961aB54
Trichodesma incanum Alph. DC. Men'shikov & Rubinstein, 1935; B108
Men'shikov, 1936; Yunusov & B109,
Plekhanova, 1957, 1959; B111, 112
Tashkhodzhaev et al. 1979 C
Uluganine Ulugbeckia tschimganica (B.Fedtsch.) Zak. Khasanova et al. 1974 B114
Uplandicine Symphytum x uplandicum Nyman Culvenor et al. 1980a, 1980b A, A
Usaramine Crotalaria brevidens Benth. var. Suri et al. 1975b B304
intermedia (Kotschy) Polhill
C. brevifolia Sawhney et al. 1967 B291
C. incana L. Sawhney & Atal, 1970a B303
C. pallida Ait. Sawhney et al. 1967 B291
C. zanzibarica Benth. (C. usaramoensis) Culvenor & Smith, 1966b B337
Senecio glandulosus Don ex Hook. et Arn. Pestchanker et al. 1985b A
S. seratophylloides Griseb Pestchanker & Giordano, 1986 A
S. vulgaris L. Pieters & Vlietinck, 1986 A
Alkaloid Plant Sources Reference Reference
Location
Uspallatine Senecio argentino Baker (vira-vira Hieron) Pestchanker et al. 1985a A
S. leucostachys Baker Pestchanker & Giordano, 1986 A
S. seratophiloides Griseb Pestchanker & Giordano, 1986 A
S. uspallatensis Pestchanker et al. 1985a A
Yamataimine Cacalia yatabei Maxim. Hikichi et al. 1978 B121
A - References in this publication.
B - References in Smith & Culvenor, J. Nat. Prod., 44, 129-152 (1981), with reference number.
C - References in Mattocks, Chemistry and Toxicology of Pyrrolizidine Alkaloids, 1986.
APPENDIX II
Table 1. Plants containing hepatotoxic pyrrolizidine alkaloids
------------------------------------------------------------------------------------------
Plant Constituent alkaloids Plant part Referencea
------------------------------------------------------------------------------------------
Apocynaceae
Fernaldia pandurata loroquin root B 29
(syn. Urechites karwinsky
Mueller)
Parsonsia eucalyptophylla lycopsamine aeriel B 28
(F. Muell.)
Parsonsia heterophylla parsonsine whole B 417
A. Cunn. heterophylline B 418
Parsonsia spiralis Wall. heterophylline leaf B 418
parsonsine
spiracine
spiranine
spiraline
Parsonsia straminea (R. Br.) lycopsamine aerial B 28
F. Muell.
Parsonsia estonia sp. echinatine A Edgar (1985)
Boraginaceae
Alkanna tinctoria Tausch 7-angelylretronecine C Roder et al.
dihydroxytriangularine (1984b)
triangularine
Amsinckia hispida (Ruiz intermedine whole B 30
et Pav.) M. Johnston lycopsamine
echiumine
Amsinckia intermedia Fisch intermedine whole B 30
et C. Mey lycopsamine
echiumine
sincamidine
echimidine
Amsinckia lycopsoides Lehm. intermedine whole B 30
lycopsamine
echiumine
Amsinckia menziesii (Lehm.) 7-acetyllycopsamine aerial C Roitman (1983a)
Nels and Macbr. 3'-acetyllycopsamine
diacetyllycopsamine
lycopsamine
intermedine
------------------------------------------------------------------------------------------
APPENDIX II Table 1. (contd.)
------------------------------------------------------------------------------------------
Plant Constituent alkaloids Plant part Referencea
------------------------------------------------------------------------------------------
Boraginaceae (contd.)
Anchusa arvensis ( L.) Bieb. echinatine whole B 31
(or diastereoisomer)
Anchusa officinalis L. 7-acetyllycopsamine whole B 31
(or diastereoisomer)
lycopsamine B 32
Asperugo procumbens L. supinine (or whole B 31
diastereoisomer)
lycopsamine (or
diastereoisomer)
Borago officinalis L. lycopsamine aerial, root C Larson et al.
amabiline (1984)
supinine A Luthy et al.
intermedine (1984)
acetylintermedine
acetyllycopsamine
thesinine seed, flower
Cynoglossum amabile Stapf amabiline whole B 33, 34
& Drummond echinatine
Cynoglossum australe R. Br. cynaustine whole B 33
cynaustraline
heliosupine
Cynoglossum creticum heliosupine aerial B 419
echinatine
Cynoglossum glochidiatum amabiline whole B 36
Wall. ex Lindl.
Cynoglossum lanceolatum cynaustraline whole B 36
Forsk cynaustine
Cynoglossum latifolium latifoline aerial B 37
R. Br. 7-angelylretronecine
Cynoglossum officinale L. heliosupine aerial B 38, 39, 40
echinatine root, aerial B 41, 42
acetylheliosupine aerial B 43
7-angelylheliotridine
Cynoglossum pictum Ait. heliosupine root, aerial B 44, 45
echinatine
pictumine aerial B 46
------------------------------------------------------------------------------------------
APPENDIX II Table 1. (contd.)
------------------------------------------------------------------------------------------
Plant Constituent alkaloids Plant part Referencea
------------------------------------------------------------------------------------------
Boraginaceae (contd.)
Cynoglossum viridiflorum viridiflorine root B 47
Pallas ex Lehm. heliosupine B 34
Echium plantagineum L. echiumine aerial B 48
(Echium lycopsis L.) echimidine
Echium vulgare L. heliosupine aerial B 49
asperumine aerial B 50
echinatine (or whole B 31
diastereoisomer)
Hackelia floribunda latifoline A Hagglund et al.
(1985)
Heliotropium acutiflorum heliotrine aerial B 51
Heliotropium arbainense heliotrine aerial B 419
europine
lasiocarpine
Heliotropium arborescens L. lasiocarpine aerial B 52
(Heliotropium peruvianum L.)
Heliotropium arguzioides heliotrine aerial B 53
Kar. et Kir. trichodesmine aerial, root B 54, 55
Heliotropium curassavicum heliotrine whole B 56
Linn. lasiocarpine
angelylheliotridine
curassavine aerial B 57
heliovicine
trachelanthamidine B 58
acetylcurassavine aerial C Mohanraj et al.
heliocurassavinine (1982)
heliocurassavine
heliocoromandaline
heliocurassavicine
curassanecine
curassavinine
coromandalinine
heliovinine
Heliotropium dasycarpum heliotrine aerial, root B 54
Ledeb. seed B 59
------------------------------------------------------------------------------------------
APPENDIX II Table 1. (contd.)
------------------------------------------------------------------------------------------
Plant Constituent alkaloids Plant part Referencea
------------------------------------------------------------------------------------------
Boraginaceae (contd.)
Heliotropium digynum heliotrine A Hammouda et al.
(Heliotropium luteum) lasiocarpine (1984)
europine
angelylheliotrine
Heliotropium eichwaldi heliotrine whole B 60, 61
Steud. ex DC. lasiocarpine aerial B 62
7-angelylheliotrine aerial B 63
Heliotropium europaeum heliotrine whole B 64, 65
lasiocarpine
europine whole B 66, 67
supinine
heleurine
N-dihydropyrrolizino- whole B 68
methyl-
heliotrine chloride
acetyllasiocarpine whole B 69
Heliotropium indicum L. indicine aerial B 70
acetylindicine aerial B 71
indicinine
echinatine aerial B 72
supinine
heleurine
lasiocarpine
Heliotropium lasiocarpum heliotrine aerial B 73
Fisch. et Mey. lasiocarpine
europine A Culvenor et al.
heleurine (1986)
Heliotropium maris-mortui europine aerial B 74
lasiocarpine aerial B 419
Heliotropium olgae heliotrine aerial, root B 75
Heliotropium popovii H. heliotrine seed B 77
Riedl. subsp. gillianum
H. Riedl.
Heliotropium ramosissimum heliotrine aerial B 78, 79
(Lehm.) Dec. (syn. heleurine
Heliotropium persicum L., supinine
Heliotropium undulatum) lasiocarpine
Heliotropium rotundifolium europine aerial B 74
------------------------------------------------------------------------------------------
APPENDIX II Table 1. (contd.)
------------------------------------------------------------------------------------------
Plant Constituent alkaloids Plant part Referencea
------------------------------------------------------------------------------------------
Boraginaceae (contd.)
Heliotropium steudneri Vatke lycopsamine leaf B 80
Heliotropium suaveolens Bieb. heliotrine aerial A Guner (1986)
lasiocarpine
europine
echinatine
Heliotropium supinum L. supinine root B 81
heliosupine root B 82
echinatine whole B 83
7-angelylheliotridine
7-angelylheliotridine
viridiflorate
7-angelylheliotridine
trachelanthate
heliotrine seed, leaf C Pandey et al.
lasiocarpine (1983)
Heliotropium transoxanum heliotrine aerial B 51
Lappula glochidiata echinatine aerial B 84
Lappula intermedia lasiocarpine aerial B 85
Lindelofia angustifolia echinatine aerial B 62, 36
(Schrenk) Brand. amabiline
Lindelofia spectabilis echinatine aerial B 62, 63
Lehm. 7-acetylechinatine
monocrotaline
Lindelofia stylosa viridiflorine aerial B 86
(Kar. et Kir.) Brand echinatine seed B 75
lindelofine
Lindelofia tschimganica carategine aerial B 87
echinatine
viridiflorine
Lithospermum officinale L. acetylechimidinyl- whole B 31
retronecine
(or diastereoisomer)
Messerschmidia sibirica lycopsamine whole C Hikichi et al.
angelylretronecine (1980)
------------------------------------------------------------------------------------------
APPENDIX II Table 1. (contd.)
------------------------------------------------------------------------------------------
Plant Constituent alkaloids Plant part Referencea
------------------------------------------------------------------------------------------
Boraginaceae (contd.)
Myosotis scorpioides L. 7-acetylscorpioidine aerial C Resch et al.
(syn. Myosotis scorpioidine (1982)
palustris L.) symphytine
myoscorpine
Paracynoglossum imeritinum heliosupine aerial, root B 88
(Kusn.) M. Pop. echinatine B 89, 90
Rindera austroechinata echinatine whole, seed B 87
M. Pop.
Rindera baldshuanica rinderine aerial B 91
Kusnezov echinatine aerial B 87
trachelanthamine
turkestanine
Rindera cyclodonta Bge. echinatine aerial B 59
Rindera echinata Regel echinatine aerial B 92
trachelanthamine aerial B 59
Rindera oblongifolia carategine aerial B 87
M. Pop. echinatine
turkestanine
Solenanthus circinnatus echinatine seed, aerial, B 93
Ledeb. root
Solenanthus coronatus echinatine aerial B 75
Solenanthus karateginius carategine aerial B 93
Lipsky echinatine
Solenanthus turkestanicus rinderine aerial B 94
turkestanine
Symphytum asperum Lepech. asperumine aerial, root B 95, 96
echinatine aerial, root B 97
acetylheliosupine whole B 31
(or diastereoisomer)
7-acetyllycopsamine leaf, root C Roitman (1981)
intermedine
symlandine
7-acetylintermedine
symphytine
lycopsamine
echimidine
angelylechimidine root A Gadella et al.
(or diastereoisomer) (1983)
------------------------------------------------------------------------------------------
APPENDIX II Table 1. (contd.)
------------------------------------------------------------------------------------------
Plant Constituent alkaloids Plant part Referencea
------------------------------------------------------------------------------------------
Boraginaceae (contd.)
Symphytum caucasicum Bieb. lasiocarpine aerial, root B 95
asperumine aerial, root B 98, 99
echinatine
echimidine
Symphytum officinale Linn. symphytine root B 15, 16
echimidine B 100
lasiocarpine aerial, root B 95
heliosupine aerial, root B 101
viridiflorine root
echinatine root
acetylechimidine B 31
(or diastereoisomer)
7-acetyllycopsamine aerial, root A Huizing et al.
lycopsamine (1981)
intermedine
7-acetylintermedine
symlandine C Roder et al.
myoscorpine root (1982a)
C Resch et al.
(1982)
Symphytum orientale anadoline whole B 102, 103, 104
symphytine
echimidine
Symphytum peregrinum Ledeb. echimidine root A Gadella et al.
symphytine (1983)
Symphytum tuberosum L. echimidine whole B 105
anadoline
symlandine root A Gray et al.
(1983)
Symphytum x uplandicum Nyman lycopsamine aerial B 31, 17, 106
intermedine
uplandicine
7-acetyllycopsamine
7-acetylintermedine
echimidine
symphytine
symlandine
angelylechimidine root A Gadella et al.
(or diastereoisomer) (1983)
Tournefortia sarmentosa Lam. supinine leaf, stem B 107
------------------------------------------------------------------------------------------
APPENDIX II Table 1. (contd.)
------------------------------------------------------------------------------------------
Plant Constituent alkaloids Plant part Referencea
------------------------------------------------------------------------------------------
Boraginaceae (contd.)
Trichodesma africana intermedine aerial B 419
europine
Trichodesma incanum Alph. trichodesmine aerial B 108, 109
DC. incanine seed, aerial, B 110, 111, 112
root
Trichodesma zeylanicum supinine seed B 113
(Burm. f) R. Br.
Ulugbekia tschimganica uluganine B 114
Compositae
Adenostyles alliariae platyphylline root B 115
seneciphylline
Adenostyles glabra seneciphylline C Wiedenfeld et al.
(1984)
Adenostyles rhombifolius platyphylline aerial B 116
(Willd.) M. Pimen. ssp. seneciphylline
platyphylloides
Brachyglottis repanda senecionine aerial B 117
Forst. et Forst. senkirkine
brachyglottine B 118
Cacalia floridana (= Senecio otosenine aerial B 119
floridanus Sch. Bip.) florosenine
floridanine
floricaline
Cacalia hastata L. subsp. integerrimine root B 120
orientalis Kitamura
Cacalia yatabei Maxim yamataimine root B 121
Conoclinium coelestinium intermedine C Herz et al. (1981)
(L.) DC
Crassocephalum crepidioides jacobine aerial A Asada et al.
jacoline (1985)
Doronicum macrophyllum otosenine root B 122
floridanine
doronine
------------------------------------------------------------------------------------------
APPENDIX II Table 1. (contd.)
------------------------------------------------------------------------------------------
Plant Constituent alkaloids Plant part Referencea
------------------------------------------------------------------------------------------
Compositae (contd.)
Doronicum pardalianches otosenine root A Rajagopalan & Negi
Linn. (1985)
Echinacea angustifolia DC. tussilagine whole C
isotussilagine
Echinacea purpurea M. tussilagine whole C
isotussilagine
Emilia flammea Cass. otosenine aerial, root B 123, 124, 125
emiline
Erechtites hieracifolia (L.) senecionine aerial B 126, 127
Raf. ex DC. seneciphylline
Eupatorium altissimum L. rinderine C Herz et al. (1981)
angelylheliotridine
Eupatorium cannabinum L. echinatine aerial B 128
supinine
amabiline A Luthy et al.
(1984)
Eupatorium compositifolium intermedine C Herz et al. (1981)
Walt. lycopsamine
Eupatorium maculatum L. echinatine root B 129
trachelanthamidine
Eupatorium purpureum probably echinatine aerial B 130
Eupatorium serotinum Michx. supinine aerial B 131
rinderine
Eupatorium stoechadosmum lindelofine root B 132
Hance supinine
Farfugium japonicum Kitam. senkirkine root, leaf B 133
farfugine whole C Niwa et al. (1983)
petasitenine whole A Niwa et al. (1985)
Gynura scandens O. Hoffm. gynuramine C Wiedenfeld (1982)
acetylgynuramine
Gynura segetum (Lour.) Merr. senecionine C Liang & Roder
(1984)
------------------------------------------------------------------------------------------
APPENDIX II Table 1. (contd.)
------------------------------------------------------------------------------------------
Plant Constituent alkaloids Plant part Referencea
------------------------------------------------------------------------------------------
Compositae (contd.)
Ligularia brachyphylla clivorine aerial B 134
Hand.-Mazz. ligularine
ligudentine
Ligularia clivorum clivorine aerial B 135, 136,
137, 138
Ligularia dentata clivorine aerial B 134
(A. Gray) Hara ligularine
ligudentine
ligularidine whole B 436
ligularinine aerial, root C Asada & Furuya
ligularizine (1984a)
neoligularidine
Ligularia elegans (Cass.) clivorine aerial B 134
[syn. Ligularia macrophylla ligularine
(Ledeb. DC.)]
Ligularia japonica senecionine root A Asada et al.
neopetasitenine (1981)
platyphylline
Petasites albus L. senkirkine aerial C Luthy et al.
(1983)
Petasites hybridus L. senecionine aerial C Luthy et al.
integerrimine (1983)
senkirkine
Petasites japonicus Maxim. petasitenine aerial B 20, 19, 139
(fukinotoxin)
neopetasitenine
senkirkine stem B 140
petasinine aerial B 141
petasinoside
Petasites laevigatus (Willd.) platyphylline aerial B 142
Reichenb. [syn. Nardosmia senkirkine (renardine) aerial B 143, 144
laevigata (Willd.) DC.] senecionine
Senecio abrotanifolius doronine aerial A Roder et al.
ssp. abrotanifolius doronenine (1984a)
bulgarsenine
Senecio abrotanifolius doronine aerial A Roder et al.
ssp. abrotanifolius var. doronenine (1984a)
tiroliensis bulgarsenine
------------------------------------------------------------------------------------------
APPENDIX II Table 1. (contd.)
------------------------------------------------------------------------------------------
Plant Constituent alkaloids Plant part Referencea
------------------------------------------------------------------------------------------
Senecio aegypticus L. senecionine whole B 145, 146
otosenine aerial
riddelliine
erucifoline
Senecio alpinus (L.) Scop. seneciphylline aerial B 147
jacozine
jacobine whole A Luthy et al.
integerrimine (1981)
jacoline
senecionine
jaconine
Senecio ambrosioides retrorsine whole B 148
(= Senecio brasiliensis seneciphylline
Less.) senecionine
riddelliine whole B 420
Senecio ampullaceus Hook. senecionine whole B 149, 150, 151
seneciphylline
retrorsine
Senecio antieuphorbium integerrimine aerial B 152
(L.) Sch. Bip. senkirkine
Senecio aquaticus Hill seneciphylline aerial B 153, 154
Senecio aureus L. senecionine aerial B 155, 156
otosenine aerial C Resch et al.
floridanine (1983)
florosenine C Roder et al.
(1983)
Senecio auricola Bourg. neosenkirkine aerial B 157
Senecio barbellatus DC. swazine B 270
retrorsine
Senecio bipinnatisectus retrorsine aerial, root B 158
Belcher (syn.
Erechtites atkinsoniae)
Senecio borysthenicus seneciphylline aerial, root B 159, 160
(= Senecio prealtus
Berthol.)
Senecio brasiliensis senecionine leaf B 161, 162
DC. (syn. seneciphylline B 148, 163
Senecio ambrosioides) jacobine
integerrimine
retrorsine
------------------------------------------------------------------------------------------
APPENDIX II Table 1. (contd.)
------------------------------------------------------------------------------------------
Plant Constituent alkaloids Plant part Referencea
------------------------------------------------------------------------------------------
Compositae (contd.)
Senecio brasiliensis Less integerrimine A Nardi et al.
var tripartitus (1980)
Senecio bupleuroides DC. retrorsine aerial B 164
Senecio cacaliaster (Lam.) sencalenine A Roder et al.
bulgarsenine (1984b)
7-senecioylretrone-
cine
7-senecioyl-9-sarra-
cinoylretronecine
Senecio cannabifolius Less. seneciphylline aerial B 165
senecicannabine aerial, root C Asada et al.
jacozine (1982a)
Senecio carthamoides Greene senecionine whole B 149, 151
seneciphylline
Senecio caudatus DC. 7-senecioylretrone- aerial A Bohlmann et al.
cine (1986)
9-senecioylretrone-
cine
7-senecioyl-9-sarra-
cinylretronecine
7-senecioyl-9-(2-
methyl-2,3-dihyroxy-
butyryl)retronecine
7-senecioyl-9-(2-
methy-2-hydroxy-3-
acetoxybutyryl)
retronecine
retronecine
2-senecioyl-(-)-
macronecine
9-senecioyl-(-)-
macronecine
senecicaudatine-9-
senecioate
senecidaudatine-9-
isovalerate
norsenecicaudatine-
9-sencioate
senecicaudatinal
semiacetal
Senecio chrysanthemoides seneciphylline B 166
------------------------------------------------------------------------------------------
APPENDIX II Table 1. (contd.)
------------------------------------------------------------------------------------------
Plant Constituent alkaloids Plant part Referencea
------------------------------------------------------------------------------------------
Compositae (contd.)
Senecio cineraria DC. jacobine aerial B 153, 167
senecionine seed B 168
seneciphylline aerial B 169
otosenine aerial B 170
retrorsine aerial B 171
Senecio congestus (R. Br.) senecionine C Roder et al.
DC. [syn. Senecio palustris neoplatyphylline (1982b)
(L.) Hooker, Senecio platyphylline
tubicaulis Mansfeld)
Senecio cruentus DC. senecionine C Asada et al.
seneciphylline (1982b)
retrorsine
riddelliine
Senecio cymbaroides senecionine whole B 420
seneciphylline
riddelliine
retrorsine
Senecio desfonainei Druce senecionine aerial B 173, 145
otosenine
riddelliine
seneciphylline aerial B 146
retrorsine A Rizk et al. (1983)
senkirkine
angelylretronecine
Senecio discolor DC. retrorsine leaf B 174
senecionine aerial B 175
Senecio dolichodoryius 15,20-dihyroxyeruci- aerial A Bohlmann et al.
Cuatr. foline (1986)
Senecio doronicum L. bulgarsenine leaf B 176
doronenine
Senecio douglasii DC. retrorsine whole B 149, 150, 151
riddelliine
seneciphylline
senecionine
Senecio durieui Gay integerrimine whole B 157
Senecio eremophilus senecionine aerial B 149, 150, 151
Richards seneciphylline
retrorsine
riddelliine
------------------------------------------------------------------------------------------
APPENDIX II Table 1. (contd.)
------------------------------------------------------------------------------------------
Plant Constituent alkaloids Plant part Referencea
------------------------------------------------------------------------------------------
Compositae (contd.)
Senecio erraticus Berthol. senecionine aerial B 177
otosenine
floridanine
Senecio erraticus Berthol. senecionine B 178, 179, 180
subsp. barbaraeifolius Krock otosenine
erucifoline
seneciphylline leaf B 181
integerrimine aerial B 182
Senecio erucifolius L. senecionine aerial B 153
seneciphylline aerial B 183, 180
erucifoline
retrorsine aerial B 184
Senecio faberi Hemsl. integerrimine C Wei et al. (1982)
Senecio filaginoides senecionine root A Pestchanker &
(H. et A.) DC. retrorsine Giordano (1986)
Senecio fistulosus senecionine A Gonzalez et al.
Poepp. ex Less. (1986b)
Senecio fluviatilis Wallr. seneciphylline aerial B 185
otosenine
florosenine
Senecio formosus integerrimine aerial B 186
retrorsine
Senecio fremontii Torr. seneciphylline whole B 148
et A. Gray senecionine
Senecio gilliesiano senecionine root A Guidugli et al.
retrorsine (1986)
Senecio glabellus senecionine whole B 187
(Turcz.) DC.
Senecio glaberrimus DC. retrorsine aerial B 153
Senecio glandulosus integerrimine root A Pestchanker et al.
Don ex Hook. et Arn. retrorsine (1985b)
usaramine
Senecio graminifolius retrorsine aerial B 188
N.J. Jacq. graminifoline
------------------------------------------------------------------------------------------
APPENDIX II Table 1. (contd.)
------------------------------------------------------------------------------------------
Plant Constituent alkaloids Plant part Referencea
------------------------------------------------------------------------------------------
Compositae (contd.)
Senecio grandifolia Jaqu. platyphylline root, leaf, B 189
seneciphylline stem
Senecio grandifolius Less. senkirkine aerial A Bohlmann et al.
neosenkirkine (1986)
Senecio griesbachii Baker retrorsine aerial B 190
Senecio ilicifolius Thunb. senecionine aerial B 191, 192, 188, 127
seneciphylline
retrorsine
Senecio illinitus Phill. senkirkine aerial A Gonzalez et al.
O-acetylsenkirkine (1986a)
senecionine
Senecio inaequidens DC. retrorsine A Roder et al.
senecionine (1981)
senecivernine aerial A Bicchi et al.
integerrimine (1985)
Senecio incanus L. seneciphylline aerial B 147
subsp. carniolicus integerrimine
(Willd.) Br.-Bl.
Senecio integerrimus Nutt. integerrimine aerial B 156
senecionine
neoplatyphylline whole B 420
platyphylline
Senecio isatideus DC. retrorsine aerial B 153, 193
Senecio jacobaea L. seneciphylline aerial B 194, 195
senecionine B 196, 197
jacobine B 153, 197, 198
jaconine B 199, 200, 201
jacozine
otosenine aerial B 51
senkirkine
retrorsine aerial B 184
Senecio kirkii Hook. f. senkirkine bark, leaf B 203
ex Kirk O-acetylsenkirkine leaf B 204
Senecio kleinia Sch. Bip. integerrimine stem B 205
senkirkine stem B 206
Senecio krylovii seneciphylline aerial B 207
------------------------------------------------------------------------------------------
APPENDIX II Table 1. (contd.)
------------------------------------------------------------------------------------------
Plant Constituent alkaloids Plant part Referencea
------------------------------------------------------------------------------------------
Compositae (contd.)
Senecio kubensis Grossh. seneciphylline aerial B 208
Senecio lampsanoides seneciphylline aerial, root B 209, 210
Senecio laricifolius senecionine aerial A Bohlmann et al.
H.B.K. seneciphylline (1986)
senkirkine
19-hydroxysenkirkine
19-acetoxysenkirkine
Senecio latifolius DC. retrorsine aerial B 211, 212
(syn. Senecio sceleratus seneciphylline aerial B 213
Schweikerdt) platyphylline
sceleratine aerial B 260
chlorodeoxysceleratine aerial B 261;
(merenskine) C Bredenkamp et al.
(1985)
Senecio leucostachys Baker senecionine root A Pestchanker &
Giordano (1986)
Senecio longilobus Benth. seneciphylline whole B 156, 214, 150
retrorsine B 151
riddelliine whole B 149
Senecio magnificus F. Muell. senecionine aerial B 215
integerrimine B 216
Senecio megaphyllus Green. 13,19-epoxyseneci- aerial A Bohlmann et al.
phylline (1986)
13,19-epoxysparti-
oidine
Senecio minimus Poir seneciphylline aerial B 158
------------------------------------------------------------------------------------------
APPENDIX II Table 1. (contd.)
------------------------------------------------------------------------------------------
Plant Constituent alkaloids Plant part Referencea
------------------------------------------------------------------------------------------
Compositae (contd.)
Senecio morrisonensis Hayata integerrimine whole B 217
Senecio multilobatus senecionine A McCoy et al.
(1983)
Senecio multivenius seneciphylline aerial A Bohlmann et al.
Benth. in Oerst. senecionine (1986)
Senecio nebrodensis L. integerrimine whole B 218
var sicula senecionine
Senecio nemorensis L. bulgarsenine leaf B 219
var bulgaricus retrosisosenine
(Vel) Stoj. nemorensine
Senecio nemorensis L. ssp. fuchsisenecionine B 362, 363, 364, 365
fuchsii Gmel.
senecionine B 423
Senecio nemorensis L. nemorensine aerial, root B 371
subdecurrens Griseb. retroisosenine aerial B 422
bulgarsenine
Senecio othonnae Bieb. otosenine aerial, root B 220
onetine root B 221
seneciphylline
floridanine aerial, root B 222
doronine aerial B 223
Senecio othonniformis bisline aerial B 22, 225
Fourcade isoline
Senecio palmatus Pall. seneciphylline root B 226
Senecio paludosus L. seneciphylline root, aerial B 153, 227, 228
Senecio pampeanus Cabrera senecionine aerial B 229
Senecio pancicii Degen senecionine whole C Jizba et al.
var arnautorum (Velen.) seneciphylline (1982)
Stoj., Stef. et Kit.
Senecio pancicii Degen senecionine whole C Jizba et al.
var pancicii (1982)
Senecio patagonicus senecionine aerial A Villaroel et al.
Hook. and Arn. seneciphylline (1985)
------------------------------------------------------------------------------------------
APPENDIX II Table 1. (contd.)
------------------------------------------------------------------------------------------
Plant Constituent alkaloids Plant part Referencea
------------------------------------------------------------------------------------------
Compositae (contd.)
Senecio paucicalyculatus paucicaline whole B 230
(Klatt.) retrorsine
Senecio paucifolius seneciphylline B 231
S.G. Gmel.
Senecio petasitis DC. senecionine leaf B 146
bisline (?) aerial B 232
Senecio phillipicus retrorsine aerial A Gonzalez et al.
Rogel et Koern. (1986a)
Senecio pierotii neosenkirkine aerial, root C Asada & Furuya
senkirkine (1982)
Senecio pimpinellifolius senecionine aerial A Bohlmann et al.
H.B.K. (1986)
Senecio platyphylloides platyphylline root B 233, 234, 235
Somm. et Lev. seneciphylline
Senecio platyphyllus platyphylline root, aerial B 236, 237, 238
(Bieb.) DC. seneciphylline leaf B 239
neoplatyphylline root B 240, 241
sarracine root B 242
Senecio pojarkovae sarracine root B 243
seneciphylline
Senecio procerus L. senkirkine aerial, root B 244
var procerus procerine
Stoj. Stef. et Kit.
Senecio propinquus Ait. seneciphylline aerial, root B 209, 245
Senecio pseudo-arnica Less. senecionine aerial B 156
Senecio pterophorus senecionine aerial B 192, 188, 127
seneciphylline
retrorsine
rosmarinine
acetylseneciphylline
Senecio quadridentatus senecionine aerial B 247
Labill. (syn. Erechtites seneciphylline
quadridentata DC.) retrorsine
------------------------------------------------------------------------------------------
APPENDIX II Table 1. (contd.)
------------------------------------------------------------------------------------------
Plant Constituent alkaloids Plant part Referencea
------------------------------------------------------------------------------------------
Compositae (contd.)
Senecio quebradensis senkirkine aerial A Bohlmann et al.
Greenm. florosenine (1986)
Senecio racemosus DC. seneciphylline root B 248
Senecio renardii Winkl. seneciphylline aerial B 249, 250
senkirkine (renardine)
otosenine
Senecio retrorsus DC. retrorsine aerial B 194, 193
Senecio rhombifolius sarracine root B 251, 233
(Willd.) Sch. Bip. platyphylline aerial, root B 208
seneciphylline
neoplatyphylline
Senecio riddellii riddelliine aerial B 156
Torr. et A. Gray whole B 252, 253
retrorsine B 420
Senecio riddellii retrorsine whole B 149
Torr. et A. Gray riddelliine
var. parksii (Cory)
Senecio ruderalis Harvey retrorsine aerial B 254
Senecio ruwenzoriensis ruwenine whole B 255
S. Moore ruzorine
Senecio sandrasicus senecionine A Temizer et al.
(1985)
Senecio scandens senecionine whole B 256
seneciphylline
Senecio seratophiloides senecionine root A Pestchanker &
Griseb. senecivernine Giordano (1986)
usaramine
retrorsine
uspallatine
Senecio spartioides seneciphylline aerial B 156, 262
Torr. et A. Gray senecionine
spartiodine
riddelliine whole B 420
retrorsine
------------------------------------------------------------------------------------------
APPENDIX II Table 1. (contd.)
------------------------------------------------------------------------------------------
Plant Constituent alkaloids Plant part Referencea
------------------------------------------------------------------------------------------
Compositae (contd.)
Senecio spathulatus senecionine aerial, root B 158
A. Rich. integerrimine
seneciphylline B 263
Senecio squalidus L. senecionine aerial B 153, 264, 265
integerrimine B 205
Senecio stenocephalus seneciphylline aerial B 266
Maxim.
Senecio subalpinus senecionine leaf B 267
C. Koch. seneciphylline aerial B 147
integerrimine
Senecio subulatus dihydroretrosine root A Pestchanker et
Don ex Hook. retrorsine al. (1985b)
et Arn. var. erectus senecionine
Senecio swaziensis retrorsine aerial B 268, 269, 270
Compton swazine
Senecio tenuifolius senecionine aerial C Bhakuni & Gupta
Burm. integerrimine (1982)
senkirkine
o-acetylsenkirkine
Senecio tomentosus senecionine aerial B 271, 179
otosenine
(tomentosine)
Senecio triangularis senecionine aerial B 272
Hook. integerrimine C Roitman (1983b)
platyphylline
rosmarinine
retrorsine
triangularine
neotriangularine
7-angelylretronecine whole C Rueger & Benn
7-senecioylretrone- (1973)
cine
7-angelyl-9-sarra-
cinylretronecine
7-senecioyl-9-sarra-
cinylretronecine
Senecio uintahensis senkirkine whole B 420
senecionine
------------------------------------------------------------------------------------------
APPENDIX II Table 1. (contd.)
------------------------------------------------------------------------------------------
Plant Constituent alkaloids Plant part Referencea
------------------------------------------------------------------------------------------
Compositae (contd.)
Senecio umgeniensis 7-senecioyl-9-sarra- aerial A Bohlmann et al.
Thell. cinylretronecine (1986)
Senecio usgorensis 13,19-epoxyseneci- aerial A Bohlmann et al.
Cuatr. phylline (1986)
Senecio uspallatensis retrorsine root A Pestchanker et
uspallatine al. (1985a)
Senecio variabilis Sch. 7-senecioylretrone- aerial, root A Bohlmann et al.
Bip. cine (1986)
9-senecioylretrone-
cine
7-senecioyl-9-sarra-
cinylretronecine
Senecio venosus Harvey retrorsine aerial B 153
Senecio vernalis Walst. retrorsine aerial B 273, 274
et Kit. senecionine
senkirkine
senecivernine
integerrimine A Sener et al.
seneciphylline (1986)
riddelliine
retronecine
Senecio viscosus L. senecionine aerial B 264, 153
integerrimine aerial B 182
Senecio vulgaris L. senecionine aerial B 275, 264, 153
seneciphylline aerial B 155, 276, 277
retrorsine
riddelliine whole B 420
integerrimine A Pieters &
spartioidine Vlietinck (1986)
usaramine
Senecio senecionine whole B 420
werneriaefolius retrorsine
Syneilesis palmata syneilesine aerial, root B 278, 279
Maxim. acetylsyneilesine
senecionine
------------------------------------------------------------------------------------------
APPENDIX II Table 1. (contd.)
------------------------------------------------------------------------------------------
Plant Constituent alkaloids Plant part Referencea
------------------------------------------------------------------------------------------
Compositae (contd.)
Tussilago farfara L. senkirkine flower B 18, 425
leaf, stem C Rosberger et al.
(1981)
senecionine leaf, stem A Luthy et al.
(1980)
tussilagine C Roder et al.
(1981b)
Leguminosae
Crotalaria aegyptica crosemperine B 419
Benth. monocrotaline
7beta-hydroxy-1- B 426
methylene-8alpha-
pyrrolizidine
Crotalaria agatiflora maduraensine aerial B 280
Schweinf. anacrotine aerial B 281
7-acetylmadurensine
6-acetylanacrotine
7-acetyl-cis-
madurensine
6-acetyl-trans-
anacrotine
crotaflorine
6-angelyl-trans-
anacrotine
Crotalaria assamica Benth. monocrotaline B 285, 286
Crotalaria axillaris Ait. axillarine seed B 287, 288
axillaridine
Crotalaria barbata R. Graham crobarbatine seed B 289
ex R. Wight et Walk.-Arn.
Crotalaria berteroana DC. fulvine aerial B 297
(syn. Crotalaria fulva Roxb.)
Crotalaria brevidens integerrimine seed B 304
Benth. var. intermedia usaramine
(Kotschy) Polhill(syn.
Crotalaria intermedia
Kotschy)
Crotalaria breviflora DC. integerrimine seed B 290, 291
usaramine
------------------------------------------------------------------------------------------
APPENDIX II Table 1. (contd.)
------------------------------------------------------------------------------------------
Plant Constituent alkaloids Plant part Referencea
------------------------------------------------------------------------------------------
Leguminosae (contd.)
Crotalaria burhia Ham. crotalarine aerial B 292, 293
ex Benth. monocrotaline
Crotalaria candicans crocandine seed B 380
W. and A. isocorcandine
isocromadurine seed C Suri et al.
crispatine (1982)
turneforcidine
cropodine seed C Haksar et al.
(1982)
Crotalaria cephalotes Steud. monocrotaline seed C Pilbeam et al.
ex A. Rich (1983)
Crotalaria crispata monocrotaline whole B 294
F. Muell. ex Benth. fulvine
crispatine
Crotalaria cunninghamii monocrotaline seed C Pilbeam et al.
R. Br. (1983)
Crotalaria dura dicrotaline aerial B 295, 296
J.M. Wood et Evans
Crotalaria fulva Roxb.
(see Crotalaria
berteroana DC.)
Crotalaria globifera E. Mey dicrotaline aerial B 295, 296
globiferine seed C Brown et al.
grantianine (1984)
grantaline
trichodesmine
Crotalaria grahamiana monocrotaline seed B 298
Wight & Arn. grahamine seed B 299
monocrotalinine whole B 300
Crotalaria incana L. integerrimine seed B 187
anacrotine aerial B 302
usaramine seed B 303
Crotalaria juncea L. senecionine seed B 305, 306, 307
seneciphylline
riddelliine
trichodesmine
junceine
------------------------------------------------------------------------------------------
APPENDIX II Table 1. (contd.)
------------------------------------------------------------------------------------------
Plant Constituent alkaloids Plant part Referencea
------------------------------------------------------------------------------------------
Leguminosae (contd.)
Crotalaria laburnifolia L. anacrotine seed B 308, 309, 310,
(crotalaburnine) 291, 311
Crotalaria laburnifolia L. madurensine aerial B 312
subsp. eldomae anacrotine
senkirkine
hydroxysenkirkine
crotafoline
Crotalaria leschenaultii monocrotaline seed B 313
Crotalaria leiloba Bartl. monocrotaline seed B 314
(syn. Crotalaria
ferruginea Wall.)
Crotalaria madurensis madurensine seed, flower, B 280
R. Wight leaf
crispatine aerial B 315
fulvine
cromadurine seed B 62, 316
isocromadurine seed B 317
Crotalaria micans Link. 1-methylenepyrroliz- seed B 282, 283
(syn. Crotalaria anagyroides idine seed B 284
Humb. et al.) senecionine B 280
anacrotine
Crotalaria mitchellii Benth. monocrotaline whole B 318
retusamine whole
Crotalaria mitchellii Benth. retusamine whole B 318
subsp. laevis A. Lee,
published as
"sp. aff. mitchellii")
Crotalaria mysorensis Roth. monocrotaline seed B 319
Crotalaria nana Burm. crotananine seed B 320
cronaburmine seed B 427
Crotalaria nitens Kunth. monocrotaline A Hoet et al.
(1981)
Crotalaria novae-hollandiae monocrotaline whole B 318
DC. subsp. lasiophylla retusamine
(Benth) A. Lee
------------------------------------------------------------------------------------------
APPENDIX II Table 1. (contd.)
------------------------------------------------------------------------------------------
Plant Constituent alkaloids Plant part Referencea
------------------------------------------------------------------------------------------
Leguminosae (contd.)
Crotalaria novae-hollandiae retusamine seed B 318
DC. subsp. novae-hollandiae
(syn. Crotalaria
crassipes Hook.)
Crotalaria pallida Ait. usaramine seed B 291
(syn. Crotalaria mucronata, nilgirine seed B 322
Crotalaria striata) crotastriatine seed B 323, 324
Crotalaria paniculata Willd. fulvine seed B 325
Crotalaria paulina Schrank monocrotaline seed C Pilbeam et al.
(1983)
Crotalaria quinquefolia L. monocrotaline seed C Pilbeam et al.
(1983)
Crotalaria recta Steud. monocrotaline aerial B 326
ex A. Rich
Crotalaria retusa L. monocrotaline seed B 327
retusine seed, aerial B 328
retusamine
retronecine
Crotalaria sagittalis L. monocrotaline seed B 330
Crotalaria scassellatii axillaridine seed A Wiedenfeld et
Chiov. axillarine al. (1985)
deoxyaxillarine
Crotalaria semperflorens crosemperine seed B 331
Vent.
Crotalaria spartioides DC. retrorsine aerial B 334
Crotalaria spectabilis Roth. monocrotaline seed B 335, 227
(syn. Crotalaria sericea spectabiline seed, whole B 336
Retz)
Crotalaria stipularia Desv. monocrotaline seed B 314
Crotalaria tetragona Roxb. integerrimine seed B 314
trichodesmine
Crotalaria verrucosa L. anacrotine seed B 338
crotaverrine seed B 339
acetylcrotaverrine
------------------------------------------------------------------------------------------
APPENDIX II Table 1. (contd.)
------------------------------------------------------------------------------------------
Plant Constituent alkaloids Plant part Referencea
------------------------------------------------------------------------------------------
Leguminosae (contd.)
Crotalaria virgulata grantianine seed B 301, 259
subsp. grantiana (Harv.) grantaline C Smith & Culvenor
Polhill (syn. Crotalaria 1-hydroxymethyl- (1984)
grantiana Harv.) 1beta,2beta-epoxy-
pyrrolizidine
Crotalaria walkeri Arn. crotaverrine seed B 340
acetylcrotaverrine
Crotalaria wightiana Grah. junceine seed B 329
ex Wight & Arn. (syn. trichodesmine
Crotalaria rubiginosa Willd.
var. wightiana J.G. Baker)
Crotalaria zanzibarica integerrimine seed B 187
Benth. (syn. Crotalaria usaramine seed B 337
usaramoensis E.G. Baker) senecionine
retrorsine
Ranunculaceae
Caltha biflora DC. senecionine aerial B 341
Caltha leptosepala DC. senecionine aerial, root B 341
Scrophulariaceae
Castilleja rhexifolia senecionine B 342
Rydb. sarracine C Roby & Stermitz
indicine or isomer (1984)
------------------------------------------------------------------------------------------
a A = References in the reference list of this document.
B = References in Smith & Culvenor (1981), J. nat. Prod., 44: 129-152 (with reference
number).
C = References in Mattocks (1986), Chemistry and toxicology of pyrrolizidine alkaloids.
APPENDIX II
Table 2. Plants containing known alkaloids that are non-hepatotoxic (aminoalcohols and esters)
------------------------------------------------------------------------------------------
Plant Constituent alkaloids Plant part Referencea
------------------------------------------------------------------------------------------
A. Families in which hepatotoxic alkaloids also occur
Apocynaceae
Alafia multiflora alafine seed B 343
Anodendron affine Druce alloanodendrine aerial B 344, 345
anodendrin
Boraginaceae
Caccinea glauca Savi 7,9-dibenzoylretrone- fl B 346
cine
Ehretia aspera Willd. ehretinine leaf A Suri et al.
(1980)
Heliotropium angiospermum 1-hydroxymethyl- whole C Birecka et al.
Murray 1beta,2beta-epoxy- (1983)
pyrrolizidine
Heliotropium ovalifolium heliofoline whole C Mohanraj et al.
Forsk retronecine (1981)
Heliotropium spathulatum acetylcurassavine aerial A Birecka et al.
Rydb. curassavine (1980)
Heliotropium strigosum Willd. strigosine aerial B 347
Lindelofia macrostyla (Bunge) lindelofine aerial B 348
M. Pop. (syn. Lindelofia lindelofamine
anchusoides,Paracaryum
heliocarpum Kern.)
Lindelofia olgae (Regel et viridiflorine aerial B 94
Smirnov) Brand
Lindelofia pterocarpa (Rupr.) viridiflorine aerial B 93
M. Pop.
Macrotomia echioides Boiss. macrotomine aerial B 350
Paracaryum himalayense viridiflorine aerial B 93
(Klotsch) C.B. Clark
Tournefortia sibirica L. turneforcine aerial B 351
Trachelanthus hissoricus viridiflorine leaf B 352
Lipsky trachelanthamine
------------------------------------------------------------------------------------------
APPENDIX II Table 2. (contd.)
------------------------------------------------------------------------------------------
Plant Constituent alkaloids Plant part Referencea
------------------------------------------------------------------------------------------
Boraginaceae (contd.)
Trachelanthus korolkovii trachelanthamine aerial B 353, 354, 355, 94
(Lipsky) B. Fedtsch.
Celastraceae
Bhesa archboldiana 9-angelylretronecine bk B 356
(Merr. & Perry) Ding Hou
[syn. Kurrimia archboldiana
(Merr. & Perry)]
Compositae
Adenostyles rhombifolius sarracine aerial B 116
(Willd.) M. Pimen. ssp.
rhombifolia chemovar.
sarracinifera
Adenostyles rhombifolius platyphylline aerial B 116
(Willd.) M. Pimen. ssp.
rhombifolia chemovar.
platyphyllinifera
Cacalia hastata L. hastacine root B 357, 241
Cacalia robusta hastacine B 358
Senecio amphibolus macrophylline aerial B 359
Senecio angulatus L. angularine whole B 360
rosmarinine
Senecio aronicoides hygrophylline whole B 420
Senecio brachypodus DC. rosmarinine aerial, root B 361
Senecio francheti Winkl. sarracine aerial B 352
franchetine
Senecio glastifolius sarracine A Mortimer & White
(1975)
Senecio hygrophyllus R.A. platyphylline aerial B 366, 361
Dyer et C.A. Smith (syn. rosmarinine
Senecio adnatus DC.) hygrophylline
Senecio macrophyllus Bieb. macrophylline aerial B 368
Senecio mikanioides Otto. ex sarracine aerial B 155, 369, 370
Walp.
------------------------------------------------------------------------------------------
APPENDIX II Table 2. (contd.)
------------------------------------------------------------------------------------------
Plant Constituent alkaloids Plant part Referencea
------------------------------------------------------------------------------------------
Compositae (contd.)
Senecio nemorensis L. ssp. nemorensine aerial B 371
fuchsii var. nova (Zlatnik)
Senecio nemorensis L. ssp. nemorensine aerial B 371
jaquinianus (Rchb.) Durand
Senicio ovirensis ssp. angelylheliotridine A Roder et al.
gaudinil (1980)
Senecio pauciligulatus rosmarinine aerial B 361
Dyer et Sm.
Senecio rivularis DC. 7-angelylheliotridine aerial B 182, 135
Senecio rosmarinifolius Linn. rosmarinine aerial B 191, 192, 188
Senecio salignus DC. 7-angelylheliotridine aerial A Bohlmann et al.
(1986)
Senecio sarracenius L. sarracine aerial B 153, 372, 373
Senecio schvetsovii Korsh macrophylline aerial B 231
Senecio sylvaticus L. silvasenecine aerial B 362, 153
sarracine aerial B 374
Senecio taiwanensis Hayata rosmarinine aerial B 217
Senecio tournefortii Lap. platyphylline aerial B 375
Leguminosae
Crotalaria albida Heyne ex croalbidine aerial B 376, 377
Roth (syn. Crotalaria montana
Roxb.)
Crotalaria aridicola Domin. 1-methoxymethyl-1,2- aerial B 378
dehydropyrrolizidine
7beta-hydroxy-1-
methoxymethyl-1,2-
dehydropyrrolizidine
7beta-acetoxy-1- whole B 379
methoxymethyl-1,2-
dehydropyrrolizidine
Crotalaria damarensis Engl. 1-methylenepyrrolizi- whole, seed B 381, 283
dine
------------------------------------------------------------------------------------------
APPENDIX II Table 2. (contd.)
------------------------------------------------------------------------------------------
Plant Constituent alkaloids Plant part Referencea
------------------------------------------------------------------------------------------
Leguminosae (contd.)
Crotalaria goreensis Guill. 7beta-hydroxy-1- aerial, seed B 382
et Perr. methylene-8beta-
pyrrolizidine
7beta-hydroxy-1-
methylene-8alpha-
pyrrolizidine
Crotalaria grandistipulata 1-methylenepyrrolizi- seed B 434
Harms dine
Crotalaria lachnophora 1-methylenepyrrolizi- seed B 434
A. Rich dine
Crotalaria maypurensis Humb 7beta-hydroxy-1- aerial B 383
et al. methylene-8beta-
pyrrolizidine
7beta-hydroxy-1-
methylene-8alpha-
pyrrolizidine
Crotalaria medicaginea Lam. 1-methoxymethyl-1,2- whole, seed B 378
dehydropyrrolizidine
7beta-hydroxy-1-
methoxymethyl-1,2-
dehydropyrrolizidine
1alpha-methoxymethyl- whole, seed
1beta,2beta-
epoxypyrrolizidine
7alpha-hydroxy-1- seed
methoxymethyl-1,2-
dehydropyrrolizidine
1alpha-hydroxymethyl- aerial
1beta,2beta-
epoxypyrrolizidine
Crotalaria natalitia Meissner 1-methylenepyrrolizi- seed B 434
dine
Crotalaria podocarpa DC. 7-hydroxy-1-methylene- seed B 435
pyrrolizidine
Crotalaria stolzii (Bak. f.) 1-methylenepyrrolizi- seed B 434
Milne-Redh. ex Polhill dine
Ranunculacae laburnum L. laburnine seed B 387, 388, 389
1-hydroxymethyl-7-e B 390
hydroxypyrrolizidin
------------------------------------------------------------------------------------------
APPENDIX II Table 2. (contd.)
------------------------------------------------------------------------------------------
Plant Constituent alkaloids Plant part Referencea
------------------------------------------------------------------------------------------
Scrophulariacea
Castilleja "rhexifolia aff. sarracine C Roby & Stermitz
miniata" 7-angelylplatynecine (1984)
8-angelylplatynecine
B. Families in which hepatotoxic alkaloids are not known to occur
Orchidaceae
Chysis bractescens Lindl. 1alpha-methoxycar- whole B 391, 392
bonyl-8beta-
pyrrolizidine
1alpha-ethoxycar-
bonyl-8beta-
pyrrolizidine
Doritis pulcherrima phalaenopsine La or T whole B 393
(syn. Phalaenopsis esmerelda)
Hammarbya paludosa (L.) O.K. paludosine whole B 394
hammarbine whole B 395
Kingiella taenialis (Lindl.) phalaenopsine La whole B 396
Rolfe
Liparis auriculata (Blume) auriculine whole B 397
Liparis bicallosa Schltr. laburnine whole B 397
malaxine whole B 398, 399
Liparis hachijoensis Nakai laburnine whole B 397
malaxine whole B 398
Liparis keitaoensis Hay. keitaoine whole B 395
keitine
Liparis kumokiri F. Maekawa kumokirine whole B 400, 398
Liparis loeselii (L.) L.C. auriculine whole B 394
Rich
Liparis nervosa Lindl. nervosine whole B 398, 401
Malaxis congesta comb. nov. malaxin whole B 402
(Rchb. f.)
Malaxis grandifolia Schltr. grandifoline whole B 403
Phalaenopsis amabilis Bl. phalaenopsine T whole B 404, 405, 393
Phalaenopsis amboinensis phalaenopsine La whole B 393
------------------------------------------------------------------------------------------
APPENDIX II Table 2. (contd.)
------------------------------------------------------------------------------------------
Plant Constituent alkaloids Plant part Referencea
------------------------------------------------------------------------------------------
Orchidaceae (contd.)
Phalaenopsis aphrodite phalaenopsine T whole B 393
Phalaenopsis cornu-cervi cornucervine whole B 404, 393
Rchb. f.
Phalaenopsis equestris phalaenopsin ls whole B 393
Rchb. f. phalaenopsin T
Phalaenopsis fimbriata phalaenopsine T whole B 393
Phalaenopsis hieroglyfica phalaenopsine T or La whole B 393
Phalaenopsis lueddemanniana phalaenopsine T or La whole B 393
Phalaenopsis mannii Rchb. f. phalaenopsine La whole B 393
Phalaenopsis sanderiana phalaenopsine La whole B 393
Rchb. f. phalaenopsine T
Phalaenopsis schilleriana phalaenopsine La whole B 393
Phalaenopsis stuartiana phalaenopsine La whole B 393
Rchb. f. phalaenopsine T
Phalaenopsis sumatrana phalaenopsis La whole B 393
Phalaenopsis violacea phalaenopsis La or T whole B 393
Vanda cristata Lindl. acetyllaburnine whole B 407
Vanda helvola Bl. laburnine whole B 408
acetyllaburnine
Vanda hindsii Lindl. acetyllaburnine whole B 408
Vanda luzonica Loher acetyllaburnine whole B 408
Vandopsis gigantea Pfitz. laburnine whole B 408
lindelofidine
acetyllaburnine
acetyllindelofidine
Vandopsis lissochiloides laburnine whole B 408
Pfitz. lindelofidine
acetyllaburnine
acetyllindelofidine
------------------------------------------------------------------------------------------
APPENDIX II Table 2. (contd.)
------------------------------------------------------------------------------------------
Plant Constituent alkaloids Plant part Referencea
------------------------------------------------------------------------------------------
Rhizophoraceae
Cassipourea gummiflua Tulasne cassipourine stem, leaf B 409
var. verticellata Lewis bk B 410
Santalaceae
Thesium minkwitzianum thesine aerial B 411
B. Fedtsch. thesinine B 412, 413
thesinicine
isoretronecanol root
Sapotaceae
Mimusops elengi L. 1-hydroxymethyl- B 414
pyrrolizidine
tiglate
Planchonella anteridifera planchonelline leaf B 415
(C.T. White et W.D. Francis) tiglyllaburnine
H.J. Lamb benzoyllaburnine
Planchonella thyrsoidea C.T. planchonelline leaf B 415
White ex F.S. Walker tiglyllaburnine
benzoyllaburnine
Planchonella sp. (NGF 24722) trans-beta-thio- leaf B 416
acrylyl-(-)-iso-
retronecanol
tiglylisoretronecanol
------------------------------------------------------------------------------------------
a A = References in the reference list of this document.
B = References in Smith & Culvenor (1981), J. nat. Prod., 44: 129-152 (with reference
number).
C = References in Mattocks (1986), Chemistry and toxicology of pyrrolizidine alkaloids.
