
INTERNATIONAL PROGRAMME ON CHEMICAL SAFETY
ENVIRONMENTAL HEALTH CRITERIA 9
DDT and its Derivatives
This report contains the collective views of an international group of
experts and does not necessarily represent the decisions or the stated
policy of either the World Health Organization or the United Nations
Environment Programme
Published under the joint sponsorship of
the United Nations Environment Programme
and the World Health Organization
World Health Organization
Geneva, 1979
ISBN 92 4 154069 9
(c) World Health Organization 1979
Publications of the World Health Organization enjoy copyright
protection in accordance with the provisions of Protocol 2 of the
Universal Copyright Convention. For rights of reproduction or
translation of WHO publications, in part or in toto, application
should be made to the Office of Publications, World Health
Organization, Geneva, Switzerland. The World Health Organization
welcomes such applications.
The designations employed and the presentation of the material in
this publication do not imply the expression of any opinion whatsoever
on the part of the Secretariat of the World Health Organization
concerning the legal status of any country, territory, city or area or
of its authorities, or concerning the delimitation of its frontiers or
boundaries.
The mention of specific companies or of certain manufacturers'
products does not imply that they are endorsed or recommended by the
World Health Organization in preference to others of a similar nature
that are not mentioned. Errors and omissions excepted, the names of
proprietary products are distinguished by initial capital letters.
CONTENTS
ENVIRONMENTAL HEALTH CRITERIA FOR DDT AND ITS DERIVATIVES
1. SUMMARY AND RECOMMENDATIONS FOR FURTHER STUDIES
1.1. Summary
1.1.1. Properties and analytical methods
1.1.2. Production and uses
1.1.3. Environmental concentrations and exposures
1.1.4. Metabolism
1.1.5. Experimental studies of the effects of DDT
1.1.6. Clinical and epidemiological studies on the effects
of DDT
1.1.7. Dosage-effect relationships
1.1.8. Evaluation of risk
1.2. Recommendations for further studies
1.2.1. Fate in the environment
1.2.2. Monitoring of exposure and effects
1.2.3. Carcinogenicity
1.2.4. Mutagenicity
2. PROPERTIES AND ANALYTICAL METHODS
2.1. Physical and chemical properties of DDT and certain related
compounds
2.1.1. Properties of DDT
2.1.2. Properties of DDT analogues
2.1.3. Formulations of commercial or technical DDT
2.2. Analytical procedures
2.2.1. Statistical criteria for assessing analytical
methods
2.2.2. Limit of analytical detection
2.2.3. Confirmation of the identity of trace residues of
DDT-type compounds
2.2.4. Sampling and extraction
2.2.5. Clean-up procedures
2.2.6. Quantification
2.2.6.1 Determination of DDT-type compounds
2.2.6.2 Determination of p,p'-DDA in urine
2.2.6.3 Method of reporting results
2.2.7. Validation of analytical methods for DDT-type
compounds
2.2.8. Analytical methods for the evaluation of the
biochemical effects of p,p'-DDT and its
analogues
3. SOURCES OF ENVIRONMENTAL POLLUTION
3.1. Discovery and introduction
3.2. Production and use
3.3. Changing patterns of use
4. ENVIRONMENTAL TRANSPORT AND DISTRIBUTION
4.1. Local drift in air
4.2. Distant drift in air
4.3. Distribution in water
4.4. Bioaccumulation of DDT and its degradation in the
environment
5. ENVIRONMENTAL EXPOSURE LEVELS
5.1. Exposure of the general population
5.1.1. DDT in air
5.1.2. DDT in water
5.1.3. DDT in food
5.1.4. Miscellaneous sources
5.1.5. Relative importance of different sources
5.2. Exposure of infants and young children
5.3. Occupational exposure
6. METABOLISM OF DDT
6.1. Uptake
6.1.1. Uptake by inhalation
6.1.2. Uptake from the gastrointestinal tract
6.1.3. Uptake from the skin
6.2. Distribution and storage
6.2.1. Human studies
6.2.1.1 Studies of volunteers
6.2.1.2 Studies of occupationally exposed workers
6.2.1.3 Studies of the general population
6.2.2. Animal studies
6.3. Elimination
6.3.1. Human studies
6.3.1.1 Studies of volunteers
6.3.1.2 Studies of occupationally exposed workers
6.3.1.3 Studies of the general population
6.3.2. Animal studies
6.4. Biotransformation
7. EXPERIMENTAL STUDIES ON THE EFFECTS OF DDT
7.1. Animal studies
7.1.1. Haemopoietic system and immunology
7.1.2. Nervous system and behaviour
7.1.2.1 Cause of death
7.1.2.2 Treatment of poisoning in animals
7.1.3. Renal system
7.1.4. Gastrointestinal tract, liver, and enzymes
7.1.4.1 Liver
7.1.4.2 Microsomal enzymes of the liver
7.1.4.3 Enzymes of intermediary metabolism
7.1.5. Cardiovascular system
7.1.6. Respiratory system
7.1.7. Reproductive system
7.1.8. Endocrine organs
7.1.9. Carcinogenicity
7.1.10. Mutagenicity
7.1.11. Teratogenicity
7.2. Acquisition of tolerance to DDT
7.3. Factors influencing DDT toxicity
7.3.1. Dosage-effect
7.3.1.1 Dosage-effect of DDT
7.3.1.2 Dosage-effect of metabolites and
o,p'-DDT
7.3.2. Age and sex
7.3.3. Nutrition
7.3.4. Species
7.3.5. Other factors
7.4. Human studies
8. EFFECTS OF DDT ON MAN: EPIDEMIOLOGICAL AND CLINICAL STUDIES
8.1. Retrospective studies of DDT-exposed populations
8.1.1. Epidemiological surveillance of persons
occupationally exposed to DDT
8.1.2. Epidemiology of DDT poisoning in the general
population: accidents and suicides
8.1.3. Epidemiology of DDT poisoning in infants and young
children
8.2. Clinical and epidemiological studies of the effects of DDT
on specific organs and systems
8.2.1. Haemopoietic system and immunology
8.2.2. Nervous system
8.2.3. Renal system
8.2.4. Gastrointestinal system
8.2.5. Liver
8.2.5.1 Liver enzymes
8.2.5.2 Other biochemical observations
8.2.6. Cardiovascular system
8.2.7. Reproduction
8.2.8. Endocrine organs
8.2.9. Carcinogenicity
8.2.10. Mutagenicity
8.3. Factors influencing DDT toxicity
8.4. Treatment of poisoning in man
9. EVALUATION OF HEALTH RISKS TO MAN FROM EXPOSURE TO DDT AND
RELATED COMPOUNDS
9.1. Relative contributions of food, water, air, and
miscellaneous sources to total intake
9.1.1. Adult members of the general population
9.1.2. Infants and children
9.1.3. Occupational groups
9.2. Effects of exposure
9.3. Carcinogenicity and mutagenicity
9.4. Effects on microsomal enzymes
9.5. Reproduction and teratogenicity
9.6. Immunosuppression
9.7. Nutritional effects and other factors
9.8. Dosage-effect relationships
9.9. Recommendations on levels of exposure
REFERENCES
ANNEX
REFERENCES
NOTE TO READERS OF THE CRITERIA DOCUMENTS
While every effort has been made to present information in the
criteria documents as accurately as possible without unduly delaying
their publication, mistakes might have occurred and are likely to
occur in the future. In the interest of all users of the environmental
health criteria documents, readers are kindly requested to communicate
any errors found to the Division of Environmental Health, World Health
Organization, Geneva, Switzerland, in order that they may be included
in corrigenda which will appear in subsequent volumes.
In addition, experts in any particular field dealt with in the
criteria documents are kindly requested to make available to the WHO
Secretariat any important published information that may have
inadvertently been omitted and which may change the evaluation of
health risks from exposure to the environmental agent under
examination, so that the information may be considered in the event of
updating and re-evaluation of the conclusions contained in the
criteria documents.
WHO TASK GROUP ON ENVIRONMENTAL HEALTH CRITERIA FOR DDT AND ITS
DERIVATIVES
Participantsa
Members
Dr A. Curley, Toxic Effects Branch, Environmental Toxicology Division,
EPA Health Effects Research Laboratory, Research Triangle Park,
NC, USA
Dr L. Fishbein, National Center for Toxicological Research (US Food &
Drug Administration), Jefferson, AR, USA (Rapporteur)
Dr K. Gheorghiev, Department of Toxicology, Institute of Hygiene &
Occupational Health, Sofia, Bulgaria
Professor F. Korte, Association for Radiation & Environmental
Research, Neuherberg, Munich, Federal Republic of Germany
Professor B. Paccagnella, Second Institute of Hygiene (University of
Padua) Verona, Italy (Chairman)
Professor M. Roberfroid, Department of Biochemistry, Catholic
University of Louvain, Brussels, Belgium
Dr Y. Shirasu, Institute of Environmental Toxicology, Tokyo, Japan
Dr E. M. B. Smith, Department of Health & Social Security, London,
England
Dr D.C. Villeneuve, Biochemical Toxicology Section, Environmental
Health Directorate, Department of National Health & Welfare,
Ottawa, Ontario, Canada (Vice-Chairman)
Representatives of other organizations
Dr M. Stilon de Piro, Occupational Safety & Health Branch,
International Labour Office, Geneva, Switzerland
Mrs M-Th. van der Venne, Health & Safety Directorate, Commission of
the European Communities, Luxembourg
a Invited but unable to attend:
Secretariat
Dr J. F. Copplestone, Medical Officer, Pesticide Development & Safe
Use, Division of Vector Biology & Control, World Health
Organization, Geneva, Switzerland
Dr Y. Hasegawa, Medical Officer, Control of Environmental Pollution &
Hazards, Division of Environmental Health, World Health
Organization, Geneva, Switzerland
Professor W. J. Hayes, Jr, Department of Biochemistry, Vanderbilt
University, Nashville, TE, USA (Temporary Adviser)
Dr M. Vandekar, Medical Officer Pesticide Development & Safe Use,
Division of Vector Biology & Control, World Health Organization,
Geneva (Secretary)
Dr V. B. Vouk, Chief, Control of Environmental Pollution & Hazards,
Division of Environmental Health, World Health Organization,
Geneva
Dr S. Jensen, Swedish Environmental Analytical Laboratory,
Wallenberg Laboratory, Stockholm, Sweden
Professor R. Truhaut, Toxicological Research Centre, René
Descartes University, Paris, France
ENVIRONMENTAL HEALTH CRITERIA FOR DDT AND ITS DERIVATIVES
A WHO Task Group on Environmental Health Criteria for DDT and its
Derivatives met in Geneva from 8-14 November 1977. Dr V. B. Vouk,
Chief of the Control of Environmental Pollution and Hazards Unit
opened the meeting on behalf of the Director-General. The Task Group
reviewed and revised the second draft criteria document and made an
evaluation of the health risks from exposure to DDT and its
derivatives.
Dr W. J. Hayes, Jr and Dr J. Robinson, Sittingbourne Research
Centre, Kent, England, assisted the Secretariat in preparing the first
and second drafts of the DDT criteria document. Comments on which the
second draft was based were received from the national focal points
for the WHO Environmental Health Criteria Programme in Australia,
Belgium, Canada, Finland, France, Greece, Israel, New Zealand,
Pakistan, and USA and from the International Agency for Research on
Cancer (IARC), the International Labour Office (ILO), the
International Union of Biological Sciences (IUBS), the International
Union of Pure and Applied Chemistry (IUPAC), the United Nations
Industrial Development Organization (UNIDO), and from the United
Nations Environmental Programme International Register of Potentially
Toxic Chemicals.
Comments were also received from Dr V. Benes, Czechoslovakia,
Dr S. Gabor, Romania, and Dr P.M. Newberne, USA.
Two subgroups reviewed the major part of the second draft
(sections 2 to 6 and 7 to 8, respectively) and their comments were
accepted as those of the whole group. Sections 1 and 9 were redrafted
and approved at the plenary sessions.
The collaboration of these national institutions, international
organizations, WHO collaborating centres, and individual experts is
gratefully acknowledged. Without their assistance this document would
not have been completed. The Secretariat wishes to thank, in
particular, Dr W. J. Hayes, Jr for his help in all phases of
preparation of the document.
This document is based primarily on original publications listed
in the reference section. However, several comprehensive reviews on
the health effects of DDT have also been used including publications
by the US Environmental Protection Agency (1975), Müller (1959), and
Mrak (1969).
Although the ecological aspects of DDT, including its possible
accumulation in some components of the food chain, its metabolism in
microorganisms and plants, as well as its effects on terrestrial and
aquatic ecosystems are, no doubt, of great interest and importance,
this document is concerned mainly with the discussion of its
metabolism and effects in experimental animals and man that have
direct implications for human health.
Details of the WHO Environmental Health Criteria Programme
including some of the terms frequently used in the documents may be
found in the general introduction to the Environmental Health Criteria
Programme published together with the environmental health criteria
document on mercury (Environmental Health Criteria 1 -- Mercury, World
Health Organization, 1976), now also available as a reprint.
1. SUMMARY AND RECOMMENDATIONS FOR FURTHER STUDIES
1.1 Summary
1.1.1 Properties and analytical methods
DDT which is an acronym for dichlorodiphenyltrichloroethane is the
prototype of broad action, persistent insecticides. It is stable under
most environmental conditions and is resistant to complete breakdown
by the enzymes present in soil microorganisms and higher organisms.
Some of its metabolites, notably 1,1'-(2,2-dichlor-ethenylidene)-
bis[4-chlorobenzene] (DDE), have a stability equal to, or greater than
that of the parent compound. The persistence of DDT and DDE in the
environment is mainly due to the fact that they are soluble in fat and
virtually insoluble in water.
Two techniques have played a major role in the quantitative
analysis of DDT-type compounds. The original Schechter-Haller
colorimetric method introduced in 1945 was modified in 1953 making it
possible to measure both DDT and DDE in the same sample. A second more
reliable and versatile method for the simultaneous analysis of DDT,
DDE and a number of other organochlorine insecticides began to be used
extensively in about 1962. This consisted of gas-liquid chromatography
with destructive and non-destructive detector systems using
multicolumns for the separation of mixtures. Both methods require
understanding and care in the selection, extraction, clean-up, and
subsequent analysis of samples. Later, gas-liquid chromatography was
combined with mass spectrometry, which added a dimension of mass for
each component of a mixture and provided a more reliable technique for
confirmatory analyses.
Analytical methods, their execution, and the reported results have
not been satisfactory in a number of papers. However, good agreement
has been achieved by analysing paired aliquots by the colorimetric and
gas chromatographic method. In the majority of cases, analytical
errors involving human samples have been small compared with the real
differences, either between individual samples or between groups of
samples drawn from populations with substantially different histories
of exposure. This situation is different with environmental samples
where misinterpretation can occur more often.
1.1.2 Production and uses
Synthesis of DDT was reported in 1874 but its effectiveness as an
insecticide was not discovered until 1939. Because of limited
supplies, most of the compound produced in the world was devoted first
to protection of military areas and personnel, mainly against malaria,
typhus, and certain other vectorborne diseases. Even in 1944, only
4366 tonnes of DDT were produced in the United States of America. The
following year, production reached 15 079 tonnes and, on 31 August
1945, DDT was released for commercial sale. Widespread agricultural
use dates from 1946 in the USA and slightly later in most other
countries.
In the USA, use increased until 1959 (35 771 tonnes) and then
declined gradually so that only 13 724 tonnes were used in 1969.
However, because of the export market, production continued to
increase until 1963 (81 154 tonnes) and then this too gradually
decreased. Unfortunately, there does not appear to be a continuous
record of world production of DDT but according to figures supplied to
the Organization for Economic Cooperation and Development (OECD),
worldwide production in 1974 was 60 000 tonnes. It is known that DDT
has been manufactured in many parts of the world including the
developing countries. However, at present there is only one factory in
the USA, one in France, and one in India.
The ban on the use of DDT and certain other organochlorine
insecticides in Sweden from 1 January 1970 was based on a number of
ecological considerations. More recently a number of other developed
countries have restricted or banned the use of DDT except when it is
needed for the protection of health. However, DDT is still used
extensively for both agriculture and vector control in some tropical
countries. If DDT were not used, vast populations would again be
condemned to the ravages of endemic and epidemic malaria. Substitution
of malathion or propoxur for DDT would increase the cost of malaria
control by approximately 3.4- or 8.5-fold, respectively, and these
increases could not be supported by some countries without decreasing
the coverage of their control programmes.
1.1.3 Environmental concentrations and exposures
When sprayed, some DDT always fails to adhere to the target for
which it is intended and drifts away. Vaporization from treated fields
can be detected for more than six months after application. Most of it
settles in the same area with an almost straight-line, inverse
relationship to the logarithm of the distance from the source.
However, some drift is worldwide. Traces of DDT have been recovered
from dust known to have drifted over 1000 km and in water melted from
Antarctic snow. With rare exceptions, the concentration of DDT in air
in nonagricultural areas has been in the range of <1 to 2.36 × 10-6 ng/m3.
In agricultural communities, concentrations have ranged from 1 to 22 ×
10-6 mg/m3. In communities with anti-mosquito fogging programmes,
concentrations of DDT may be much higher, 8.5 × 10-3 mg/m3 being
the highest level recorded.
Although very difficult to measure, concentrations of DDT in
rainwater have usually been of the same order of magnitude
(1.8 × 10-5 to 6.6 × 10-5 mg/litre) in both agricultural areas and
very remote nonagricultural areas, suggesting that the compound is
rather evenly distributed in the air. Presumably because of dust, a
maximum concentration of 4 × 10-4 mg/litre was found in rainwater in
an urban area. Concentrations of DDT in surface water depend on the
soil as well as on rain. The concentrations in the USA are said to
have reached a peak in 1966 and then dropped sharply. The highest
level ever detected in potable water (2 × 10-2 mg/litre) was
reported in 1960. In recent years, all concentrations have been
<1 × 10-3 mg/litre and average concentrations have been similar to
those for rainwater.
Because of drift, DDT concentrations of 0.10-0.90 mg/kg found in
the soil of pastures and other fields not treated with insecticide
were only a little less than those in the soil of cultivated fields
that had been treated with DDT for 10 years or more (0.75 to
2.03 mg/kg). Most of the compound was in the upper 2.5 cm of soil. Due
to evaporation, the total residue of DDT in soils treated for 10 or
more years is of the same order of magnitude as that found soon after
a single application at the same annual rate.
Daily intake of DDT from food has been measured in several
countries. In the USA during 1953-54, average daily intakes of DDT and
of total DDTa were 0.184 and 0.286 mg/man, respectively, most of
which originated from foods of animal origin. Ten years later,
following restrictions with regard to the application of DDT to
livestock, their barns and forage, and to crops eaten directly by
people, the same investigators found daily intakes of 0.038 and 0.087
mg/man for DDT and total DDT, respectively. The so-called Market
Basket Survey showed a gradual decrease in daily intake of DDT to
0.015 mg/man in 1970. Intake in Canada and the United Kingdom was
slightly less for comparable periods. In many countries of Europe and
in other countries with similar diets, the intake of DDT has been
judged to be about the same because of the similarity of diet and of
measurements of the compound in staple foods and other important items
of the diet. There is a need to measure total intake of DDT with food
in some countries where this has not been estimated. Vegetarians
generally consume less DDT than people who include meat in their diet,
and local practices, including the practices of individual farming
families, may greatly influence the DDT intake of the persons
involved. Extensive use of DDT in the home may contribute moderately
to intake but whether this increased intake is via food is unclear.
With few exceptions, the highest average concentration of DDT in
the air to which workers are exposed (about 7 mg/m3) is that
associated with spraying the inside of houses as for malaria control.
However, concentrations as high as 104 mg/m3 have been reported in
a Total DDT is a term used to include both DDT and its metabolites
DDE and TDE (DDD).
places where DDT was prepared and packed. Almost all of the DDT in the
air of workplaces is in the form of aerosols. Because of particle size
and other factors, the amount of DDT that workers may inhale is far
less than the amount reaching unclothed portions of their skin. This
is probably important even though DDT is less easily absorbed through
the skin than many other organochlorine insecticides.
More knowledge concerning exposure of workers has been gained from
measurements of the storage of DDT in the body and its excretion than
from environmental measurements. Studies on volunteers have made it
practical to determine intake from either storage or excretion values.
In making these studies on workers, advantage has been taken of groups
employed full time in the manufacture, formulation, or application of
DDT and of others who were in contact with the material
intermittently, sometimes only for a few hours per day and for a few
weeks per year. In the literature, full-time exposure has been
referred to as "heavy" but usually without any intention of implying
that it was excessive or harmful. In fact, improved occupational
safety and health measures have made it possible to reduce the rate of
absorption associated with occupational exposure.
1.1.4 Metabolism
DDT is absorbed after inhalation and ingestion, the latter being
the more important route of absorption. Absorption of large doses is
facilitated by solution in animal or vegetable fat; absorption of
small doses, such as those found in the residues of food, is virtually
complete and is facilitated by the presence of fat in food. Even in
solution, DDT is poorly absorbed through the skin.
Most of the known facts concerning the distribution, storage, and
excretion of DDT have been demonstrated in man as well as in animals.
The compound is stored preferentially in fat, and its storage in
organs and other tissues following repeated intake is proportional to
the neutral fat content of the tissues. However, uptake of DDT by fat
is slow, thus much more is distributed to other tissues following a
single, large dose and much more to adipose tissue following many
small doses. In spite of the affinity of DDT for adipose tissues, most
of the DDT-related compounds in blood are carried by proteins, less
than 1% being carried in the tiny droplets of fat normally present in
the blood.
Following repeated doses, storage in adipose tissue increases
rapidly at first and then more gradually until a steady state is
reached. In each species, the height of the plateau is proportional to
the dosage;a however, storage is relatively less at higher dosages
because excretion is relatively greater. In man, the time necessary to
reach storage equilibrium is at least one year. There is a gradual
reduction in the amount of DDT stored in the tissues, if exposure to
the compound is discontinued.
Like most species, man converts some DDT to DDE, which is stored
even more avidly than the parent compound. A small amount of 1,1'-(2,2
dichloroethylidene) bis [4-chlorobenzene] (TDE, DDD) an intermediate
in the formation of the main excretory product 2,2-bis(4-chlorophenyl)-
acetic acid (DDA), may also be found in tissues. A number of other
metabolites have been demonstrated in animals but not detected in man.
Technical DDT is more readily excreted and less readily stored than
p,p'-DDT because it contains 15-20% of o,p'-DDT.
DDT dosage-effect relationships have been measured in man by
studying storage and excretion in the general population and in
volunteers. Studies of total diets in the general population revealed
intakes ranging from about 0.02 to 0.20 mg/man per day, in different
subpopulations. In studies on volunteers, dosage was administered
under supervision at the rates of 3.5 and 35 mg/man per day. The
steady-state level of storage in the fat of the volunteers who
received 3.5 mg/man per day was about 50 mg/kg while that of those
receiving 35 mg/man per day was about 300 mg/kg. In recent years, the
concentrations of DDT and of DDT-related compounds stored in adipose
tissues in most populations have averaged <5, and <15 mg/kg,
respectively. Higher values have been found where DDT was used
extensively and without restriction in agriculture or was added
directly to staple foods to control insects. In England and some other
countries where cool weather and a short growing season help to
control insects, the average concentrations of DDT and total DDT
stored in adipose tissues have been <2 and <5 mg/kg, respectively.
In any country, the nonoccupational exposure to DDT and, thus, the
concentrations stored, may vary between subpopulations.
a The Task Group agreed that, for the purposes of this document,
the term dosage should apply to any rate or ratio involving a
dose, e.g., mg/kg, or (mg/kg)/day.
Most reports of the concentrations of total DDT in the blood of
the general population of different countries lie within the range
0.01 to 0.07 mg/litre. The highest single value reported was
0.336 mg/litre and the highest average value was 0.136 mg/litre. The
concentrations of DDT in the blood and other tissues of the fetus or
newborn are lower than in corresponding tissues of the mother.
Concentrations of DDT in human milk have usually been reported to
be in the range of 0.01 to 0.10 mg/litre with the concentration of DDT
plus its metabolites, especially DDE, about twice as high. However, in
a few countries, average values for total DDT ranging from 1 to
5 mg/litre have been reported, the highest value observed being
12.21 mg/litre.
The average concentration of DDA in the urine of the general
public is 0.014 mg/litre, only slightly less than the lowest
concentration detectable by earlier analytical methods.
Occupational exposure commonly produces average concentrations of
DDT and total DDT stored in fat ranging from 50-175 mg/kg and 100 to
300 mg/kg, respectively. The highest values recorded for DDT and total
DDT in a healthy worker, whose exposure was not measured, were 648 and
1131 mg/kg, respectively. Typical concentrations of DDT and total DDT
in the serum or plasma of workers with substantial exposures have
ranged from 0.14 to 0.57 mg/litre and 0.35 to 1.36 mg/litre,
respectively. The concentration of DDA in the urine of substantially
exposed workers has been in the range of 0.5 to 3.0 mg/litre.
Concentrations of DDT or its derivatives in fat, serum, or urine may
be used to estimate the dose, if exposure has been prolonged and
essentially steady. The ranges of storage and excretion, just
mentioned, were measured in workers who were found to have absorbed a
total dosage ranging from 0.25 to 0.5 (mg/kg)/day.
Animal studies indicate that the concentration in serum most
accurately reflects the concentration in the brain, the critical
tissue. In the rat, a level in the brain of 25 mg/kg is not usually
fatal although higher levels tend to be.
1.1.5 Experimental studies of the effects of DDT
The toxicity of a single dose is affected by the solvent vehicle
and representative median lethal dosage (LD50) values for the rat
are 250 mg/kg, for oral administration in oil, and 250-500 mg/kg or
3000 mg/kg for dermal administration in oil, or powder, respectively.
Large doses of DDT produce vomiting in man and other species that
can vomit and this can modify the amount absorbed.
The main effect of DDT is on the nervous system. All parts, both
central and peripheral, are affected to some degree. In animals,
single or repeated doses can produce hyperexcitability, tremor,
ataxia, and finally epileptiform convulsions. Ataxia may be
demonstrated by functional tests in animals that have received daily
dosages too small to produce noticeable clinical effects. Death is
usually due to respiratory failure at the convulsive stage of
poisoning. In some species, DDT sensitizes the heart to arrhythmia,
which is made worse by epinephrine of endogenous or exogenous origin,
and these animals die in ventricular fibrillation.
It appears that the mechanism of the toxic action of DDT is
associated with its effect on the membranes in the nervous system. In
vitro concentrations as low as 10-8 mol/litre change the movement
of both sodium and potassium ions through the axonal membrane, and
this movement is involved in the transmission of nervous impulses.
Other evidence of nervous system effects are changes in the
concentrations of 4-(2-amino-1-hydroxyethyl)-1,2-benzenediol
(norepinephrine) and other neurotransmitters in poisoned animals.
Apart from the nervous system, the liver is the only other organ
significantly affected by DDT. Potentially fatal doses of the compound
cause focal necrosis of liver cells in several species. These lesions
heal by autolysis and phagocytic action in animals that survive. A
distinct form of liver cell change reflecting stimulation of
microsomal enzymes is for all practical purposes confined to rodents.
DDT induces microsomal enzymes in all species tested, but only in some
rodents does the endoplasmic reticulum increase so much that the
entire liver cell enlarges and granules that are normally scattered
throughout the cytoplasm are displaced to the margin of the cell.
These changes are accompanied by a moderate increase in fat droplets
some of which become surrounded by whorls of endoplasmic reticulum to
form so-called lipospheres. These characteristic changes have been
observed by electron micrography as early as four days after
administration of DDT, and may have occurred even earlier.
If DDT is fed for long periods at dietary levels ranging from
2 mg/kg upwards for mice or 5 mg/kg upwards for rats, the changes in
the liver progress from hypertrophy, margination, and lipospheres in
isolated, centrolobular hepatocytes to the formation of nodules of
affected cells. The first change has been observed within 4 days of
administration, the earliest time of observation. With administration
of dosages corresponding to those that people may encounter,
changes in the livers of susceptible rodents require the entire
lifetime of the animal to develop fully. At first, the nodules are
microscopic in size, but some may become more than a centimetre in
diameter, particularly in mice, and show almost complete loss of
lobular architecture. The same series of changes can be produced in
rodents by other inducers of microsomal enzymes, including
phenobarbital. Although there is persuasive evidence that these
multinodular tumours of mice associated with changes in the
endoplasmic reticulum are carcinomas, there is equally convincing
evidence that they are not and the views of some highly qualified
pathologists in this matter remain diametrically opposed. More
important than the question of classification is the fact that the
entire continuum of changes from the prompt response in isolated cells
to the eventual formation of tumours is peculiar to some rodents, and
does not occur in other animals in which the endoplasmic reticulum
does not respond morphologically in the same way.
A number of enzymes of intermediate metabolism are either
stimulated or moderately inhibited by toxic doses of DDT; the
possibility that these changes are the result rather than the cause of
poisoning has not been excluded.
Levels of DDT as high as 200 mg/kg of food that do not produce any
sign of poisoning, have not produced any adverse effects on fertility,
gestation, viability, and lactation, and on the health of the progeny
of rats and mice. Reproduction was normal in dogs receiving a dosage
of 10 mg/kg body weight per day, which is approximately equivalent to
a dietary level of 500 mg/kg for this species.
No teratogenic effects of DDT have been observed in multigeneration
studies of reproduction in several animal species.
There is some uncertainty concerning the effects of DDT on the
immune system; where an effect has been observed, it has been of a
probiotic nature.
Except for the weak estrogenic properties of o,p'-DDT, the
endocrine related effects of DDT and its analogues are confined to the
adrenals and even these effects are now considered to be mainly
secondary to microsomal enzyme induction in the liver.
DDT has not been found to be mutagenic in bacterial test systems,
either without or with metabolic activation. The evidence from
mammalian test systems, in vitro and vivo is inconclusive.
No specific antidote for DDT poisoning is known, but sedatives
(especially phenobarbital) and ionic calcium are useful for treating
poisoning in dogs and monkeys. Glucose or other ready sources of
energy are also helpful in treatment.
1.1.6 Clinical and epidemiological studies on the effects of DDT
Mild poisoning was produced in one volunteer who ingested 750 mg
DDT in oil in order to study its effects. All other poisoning of human
subjects by DDT has been the result of accidental or suicidal
ingestion. No systemic poisoning has resulted from occupational
exposure to DDT, but a few workers have developed rashes or irritation
of the eyes, nose, and throat associated with dust. Most of the very
few fatal cases have involved children who drank solutions of the
compound and whose clinical courses were dominated by solvent
poisoning.
Signs of DDT poisoning in man are entirely similar to those
observed in animals. In addition, persons poisoned have experienced a
prickling sensation of the tongue and around the mouth and nose,
reduction of tactile sense, paraesthesia of the extremities, nausea,
dizziness, confusion, headache, malaise, and restlessness. In most
patients, all signs and symptoms (including vomiting) probably
involved the nervous system; a few had temporary jaundice indicating
liver injury. In the majority of survivors, recovery was well advanced
in 24 hours but a few required a week or more. Three men still had
some weakness and ataxia in their hands five weeks after ingesting an
amount estimated to be as high as 20 000 mg of DDT per person.
Stimulation of microsomal enzymes of the liver has resulted from
full-time occupational exposure and from the therapeutic use of DDT in
the treatment of familial, nonhaemolytic, unconjugated jaundice.
The only demonstrated effects of DDT on the general population are
the storage of the compound and some of its derivatives in the tissues
and their excretion in urine and milk. No confirmed ill-effects of DDT
have been reported in babies, even in communities where the highest
concentrations of the compound in human milk have been observed.
Careful investigation of the largest available groups of workers
who have been exposed for as long as 25 years to significantly higher
levels of DDT than the general population, has not revealed any
evidence that DDT causes cancer in man. The total number of people in
the world who have had many years of full-time occupational exposure
to DDT is smaller than might be supposed. This makes the detection of
any effect with a low incidence difficult. While it has been
recognized that some human carcinogens have been detected only after
comparatively long periods of exposure, it is also known that others
(e.g., 2-naphthylamine) have been detected through their occurrence in
high incidence in small groups following exposure for periods of much
less than 25 years.
1.1.7 Dosage-effect relationships
Dosage-effect relationships for DDT in man have been observed in
connection with acute poisoning, excretion, and storage, and the
induction of microsomal enzymes has been observed at a dosage of
0.25 (mg/kg)/day but not at lower dosages. The dosage of 0.25 (mg/kg)/
day to which workers have been exposed for 25 years is of the same
order of magnitude as the dosage that causes an increase in the
incidence of tumours in male mice of a susceptible strain but not
in females of any strain. (See section 1.2.4). This same dosage is
lower than the nonobserved effect levels for rats, dogs, and monkeys
and far less than the dosage at which rats, mice, and dogs continue to
reproduce successfully for generations.
1.1.8 Evaluation of risk
Food represents the major source of intake of DDT for the general
population. The average intake of DDT from all sources is unlikely to
exceed 0.05 (mg/man)/day. Occupational exposure to DDT is mainly
respiratory and dermal. However, much of what is inhaled is deposited
in the upper respiratory tract and subsequently ingested. The effects
of dermal exposure are minimal because the compound is poorly absorbed
through the skin; the excellent safety record, never matched by any
other insecticide used in antimalaria campaigns, other vector control
programmes, and agriculture is due mainly to this fact. The number of
people throughout the world currently engaged in the manufacture of
DDT is small and, wherever safety and health protection measures are
good, occupational exposure of this group is minimal. Formulators and
applicators are also groups that are occupationally exposed to DDT but
there is no evidence to suggest that their intake is significantly
higher than that of workers engaged in the manufacture of DDT.
No adverse effects have been described in man at repeated dosage
of 1.5 (mg/kg)/day. The large number of measurements that have been
made on samples from human populations do not throw any real light on
the question of maximum-tolerated doses or concentrations, apart from
highlighting the fact that the levels found in volunteers and workers
which were higher than those in the general population were not
associated with any adverse effects.
In the light of currently available information, there is no
evidence that DDT is carcinogenic in man. Liver tumours are produced
in mice and possibly in rats by DDT, DDE, and TDE but there is
disagreement on the significance of these tumours. Studies in
in vitro bacterial test systems have not shown any evidence that
either DDT or DDE is mutagenic. The evidence from mammalian test
systems, both in vitro and in vivo, is inconclusive.
DDT induces microsomal mixed function oxidases in many animal
species and causes marked morphological changes in the liver of some
rodents. At the present time, it is very difficult to assess the
biological significance of this effect for men, since the intake by
members of the general population is much lower than the smallest
dally dosage required to produce such an effect in man and animals.
In both man and animals, there, is no indication that DDT affects
reproduction or produces teratogenic effects although it has been
shown to be embryotoxic in high doses.
DDT appears to have a depressant effect on the immune system
although the evidence is by no means conclusive.
Animal studies indicate that nutritional status influences the
toxicity of DDT. In man, nutritional status will have a similar effect
to that in animals. However, the possibility that starvation in man
could precipitate toxic manifestations is regarded as unlikely as the
stored levels do not approach those found in laboratory animals.
The information derived from human exposure is insufficient to
construct a comprehensive picture of the dosage-effect relationships
for man except in connection with storage and excretion of the
compound and its metabolites.
1.2 Recommendations for Further Studies
DDT is the first synthetic pesticide to which many people have
been exposed to a measurable degree for a period of many years. It has
already been the subject of an enormous amount of scientific study,
but of course there is still more to be learned. The following
recommendations are considered to have special implications for human
health. Much other important biomedical research such as the
continuing use of DDT as a tool for studying the nervous system has
been excluded from this document.
1.2.1 Fate in the environment
There is a serious gap in knowledge of the circulation and fate of
DDT and its analogues in the environment as a whole. Because this is
directly connected with the assessment of future exposure pathways for
man, the behaviour and fate of DDT in the environment should be
studied more extensively. Great progress has been made recently in
demonstrating the breakdown of DDT to carbon dioxide and hydrochloric
acid under laboratory conditions similar to those found in the upper
atmosphere. There is a need for further study of this phenomenon in
the laboratory, especially using DDT labelled with 14C and 3H.
There is an even greater need for seeking quantitative information on
the rate at which photomineralization may occur in nature and the
factors that influence this rate.
1.2.2 Monitoring of exposure and effects
There are fairly accurate estimates of the daily intake of DDT in
several developed countries. In other countries where DDT is most
likely to be used continuously, the daily exposure of the general
population to DDT in food should be monitored, especially if there are
indications that the conditional acceptable daily intake (ADI) of
0.005 (mg/kg)/day might be exceeded. For comparison, monitoring
programmes should be continued in countries where figures are
available for earlier years.
Extensive information is available on the occurrence of DDT and
its metabolites in human fat, blood, and milk. Continued, but limited
monitoring is justified in order to learn the rate at which
concentrations decline following a progressive reduction in the use of
the compound. More extensive monitoring is justified in countries
where base data are not available and where the use of the insecticide
is essential. Particular attention should be given to people with
substantial occupational exposure. If values are found that are not
consistent with the dietary and occupational history of each group,
the cause of the variation should be sought. If values are
unexpectedly high, some improper use may be discovered. If values are
unexpectedly low, some modifying factor such as previously
unrecognized intake of phenobarbital may be revealed.
Clinical studies should be made on any person or group found to
have exceptional levels of storage or excretion in the hope of
learning whether the insecticide has had any influence on their health
or, conversely, to learn whether their nutritional state or the
presence of chronic disease has interacted in any way with storage and
excretion. Obviously such studies must be adequately controlled if the
results are to be of use.
1.2.3 Carcinogenicity
Attention should be turned from the narrow question of the
tumorigenicity of DDT in the liver of mice and rats to the broader
question of the basis for this action of DDT and phenobarbital. A far
wider range of inducers should be studied, keeping in mind that some
inducers may have other important properties related to the initiation
of tumours. Compounds belonging to several classes (e.g., an
organochlorine insecticide, phenobarbital, a pyrethrin, etc.) should
be studied in several species (including one nonrodent) to determine
the dosage-response relationships for: (a) microsomal enzyme activity;
(b) typical morphological changes in the endoplasmic reticulum of
hepatocytes; and (c) liver tumours. These studies together with
continuing epidemiological investigations of the effects of the same
classes of compounds on people should make it easier to extrapolate
from tumorigenesis in animals to the problem of cancer in man.
1.2.4 Mutagenicity
There is satisfactory evidence that DDT is not mutagenic in
bacterial systems, without and with metabolic activation. The evidence
derived from mammalian test systems, both in vitro and in vivo is
inconclusive and should be clarified. Methods of mutagenicity testing
are advancing rapidly, and shorter and possibly more sensitive
mammalian tests are becoming available. A fresh evaluation of the
mutagenicity of DDT in animals would facilitate further assessment of
its significance for man.
2. PROPERTIES AND ANALYTICAL METHODS
2.1 Physical and Chemical Properties of DDT and Certain Related
Compounds
2.1.1 Properties of DDT
The term DDT is generally understood throughout the world and
refers to 1,1'-(2,2,2-trichloroethylidene)-bis(4-chlorobenzene)
( p,p'-DDT). The structure of DDT permits several different isomeric
forms, an example of which is 1-chloro-2[2,2,2-trichloro-1-(4-
chlorophenyl)ethyl]benzene ( o,p'-DDT). The term DDT is also applied
to commercial products consisting predominantly of p,p'-DDT together
with some o,p'-DDT and smaller amounts of other compounds. A typical
example of technical DDT had the following composition: p,p'-DDT,
77.1%; o,p'-DDT, 14.9%; p,p'-TDE, 0.3%; o,p'-TDE, 0.1%; p,p'-DDE,
4.0%; o,p'-DDE, 0.1%; and unidentified compounds, 3.5%.
All isomers of the compound DDT are white, crystalline, tasteless,
almost odourless solids with the empirical formula C14H9Cl5 and a
relative molecular mass of 354.5. The melting range of p,p'-DDT is
108.5-109.0°C and its vapour pressure is 2.53 × 10-5 Pa
(1.9 × 10-7 mm Hg) at 20°C. DDT is soluble in organic solvents as
follows (g/100 ml): benzene, 106; cyclohexanone, 100; chloroform, 96;
petroleum solvents, 4-10; ethanol, 1.5. It is highly insoluble in water.
p,p'-DDT is dehydrochlorinated to form DDE (see Table 1) at
temperatures above the melting point, especially in the presence of
catalysts or light. Solutions in organic solvents are
dehydrochlorinated by alkali or organic bases. Otherwise, DDT
formulations are highly stable. The compound is also relatively
resistant to breakdown by the enzymes found in soil and higher
organisms, and DDE is even more resistant. Under simulated atmospheric
conditions, both DDT and DDE decompose to form carbon dioxide and
hydrochloric acid.
2.1.2 Properties of DDT analogues
The chemical structure of some of the analogues of DDT is shown in
Table 1. The structure of the o,p'- and m,p'-compounds can be inferred
from those of the p,p'-isomers. The table is confined to
compounds that occur in commercial DDT, metabolites formed from them,
Table 1. Structure of p,p'-DDT and its analogues of the form:
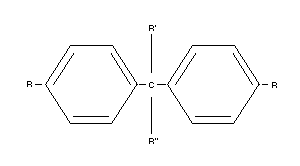 (many of the compounds also exist as o,p'-isomers and other isomers)
Name DDT Chemical name R R' R"
and its major
metabolites
DDT 1,1'-(2,2,2-trichloroethylidene)- -Cl -H -CCl3
bis[4-chlorobenzene]
DDEa 1,1'-(2,2-dichloroethenylidene)- -Cl None =CCl2
bis[4-chlorobenzene]
TDE(DDD)a,b 1,1'-(2,2-dichloroethylidene)- -Cl -H -CHCl2
bis[4-chlorobenzene]
DDMUa 1,1'-(2-chloroethenyldene)- -Cl None =CHCl
bis[4-chlorobenzene]-
DDMSa 1,1'-(2-chtoroethylidene)- -Cl -H -CH2Cl
bis[4-chlorobenzene]
DDNUa 1,1'-bis(4-chlorophenyl)ethylene -Cl None =CH2
DDOHa 2,2-bis(4-chlorophenyl)ethanol -Cl -H -CH2OH
DDAa 2,2-bis(4-chlorophenyl)- -Cl -H -C(O)OH
acetic acid
Table 1 (Cont'd)
Name DDT Chemical name R R' R"
and its major
metabolites
Some related insecticides
NO2
Bulan(R) 2-nitro-1,1 -bis- -Cl -H '
(4-chlorophenyl)butane -CHC2H5
NO2
Prolan(R) 2-nitro-1,1-bis- -Cl -H '
(4-chlorophenylpropane -CHCH2
DMC 4-chloro-alpha[-(4-chlorophenyl)- -Cl -OH -CH3
alpha-(methyl)benzenemethanol
dicocol 4-chloro-alpha-(4-chlorophenyl)-alpha- -Cl -OH -CCl3
(Kelthane(R)) (trichloromethyl)benzenemethanol
chlorobenzilatec ethyl 4-chloro-alpha-(4-chlorophenyl)- -Cl -OH -C(O)OC2H5
alpha-hydroxybenzeneacetate
chloropropopylatc 1-methylethyl 4-chloro-alpha- -Cl -OH -C(O)OCH(CH3)2
(4-chlorophenyl)-aplha-hydroxy-
benzeneacetate
methoxychlorc 1,1'-(2,2,2-trichloroethylidene)- -OCH3 -H -CCl3
bis[4-methoxybenzene]
Table 1 (Cont'd)
Name DDT Chemical name R R' R"
and its major
metabolites
Perthane(R) 1,1'-(2,2-dichloroethylidene)- -C2H5 -H -CHCl2
bis[4-ethylbenzene]
DFDT 1,1'-(2,2,2-trichloroethylidene)- -F -H -CCl3
bis[4-fluorobenzene]
a Recognized metabolite of DDT in the rat.
b As an insecticide, this compound has the ISO approved name of TDE, and it has been sold
under the name Rothane(R); in metabolic studies the same compound has been referred to as
DDD; as a drug, it is called mitotane.
c Common name approved by the International Organization for Standardization (ISO).
and analogues that have had some use as insecticides. It must be
emphasized that even the commercially-available insecticidal analogues
have strikingly different properties. Especially remarkable is the
slow metabolism and marked storage of DDT and its metabolite DDE and
the rapid metabolism and negligible storage of methoxychlor.
No attempt has been made to include in Table 1 the wide range of
compounds that have been synthesized and studied in connexion with
structure-activity relationships, often with the hope of emphasizing
the good properties of DDT and reducing its undesirable properties.
For a more extensive consideration of analogues, see Metcalf (1955).
The formation of metabolites is considered in section 6.4.
2.1.3 Formulations of commercial or technical DDT
Technical DDT has been formulated in almost every conceivable form
including solutions in xylene or petroleum distillates, emulsifiable
concentrates, water-wettable powders, granules, aerosols, smoke
candles, charges for vaporizers, and lotions. Aerosols and other
household formulations are often combined with synergized pyrethrins.
When used as a drug, DDT is called clofenotane (INN) or Dicophane
(British Pharmacopoeia), Klorfenoton (Swedish Pharmacopoeia),
Chlorophenothane (United States Pharmacopoeia). For research or
reference it has been designated OMS 0016 and Ent. 1,506. DDT has been
sold under a variety of tradenames, including: Anofex(R), Cezarex(R),
Dinocide(R), Gesarol(R), Guesapon(R), Guesarol(R), Gyron(R),
Ixodex(R), Neocid(R), Neocidol(R), and Zerdane(R).
2.2 Analytical Procedures
Analytical procedures for determining residues of DDT-type
compounds in environmental samples involve several steps including
collection and extraction of the DDT-type compounds; removal of
coextractives by appropriate clean-up methods; and quantification of
p,p'-DDT and its analogues by a suitable technique. Each of these
major steps is discussed later. It is appropriate, however, first to
outline briefly the statistical criteria used to assess analytical
methods, the estimation of the lower limit of detection of a method,
and the procedure for confirming the chemical identities of the
components measured.
2.2.1 Statistical criteria for assessing analytical methods
The overall reliability of an analytical method can be assessed
using two criteria, namely, reproducibility and systematic error (or
bias). Reproducibility is both conceptually and practically the
simpler criterion; it may be defined as "the quantitative expression
of the random error associated with operators working in different
laboratories, each obtaining single results on identical test material
when applying the same method" (Institute of Petroleum, 1968). It may
be quantitatively specified in various ways, and it is important to
pay attention to the statistic (range standards, deviation, etc.) used
in a particular study to represent the random error of an analytical
method. If the results are normally distributed then the most
efficient statistic measuring reproducibility is the standard
deviation of a set of results (Nalimov, 1963; Youden & Steiner, 1975;
Davies & Goldsmith, 1976). Care should always be taken to ensure that
a particular statistic or statistical technique is appropriate for a
given set of results, by, for example, testing for outliers or, if
there are sufficient results, examining the distribution of the
results, before characterizing the reproducibility of an analytical
method.
There are two subdivisions of the reproducibility criterion,
namely, replication i.e., two or more results, obtained by the same
operator in a given laboratory using the same apparatus for successive
determinations on identical test material, within a short period of
time on the same day; and repeatability i.e., a quantitative
expression of the random error associated in the long run with a
single operator in a given laboratory obtaining successive results
with the same apparatus under constant operating conditions on
identical test material (Institute of Petroleum, 1968).
Reproducibility is, in turn, a subdivision of the random error in the
analysis of identical test material in different laboratories using
different techniques or variations of a particular method. Examples of
the assessment of the random errors found in the determination of
p,p'-DDT and related compounds are given below.
The systematic error of a method is the deviation of the
experimental results from the "true" values; such systematic error
causes the differences between the nominal value and the
experimentally-determined values to have predominantly the same sign
(as opposed to the random errors, where the results are equally likely
to be greater than or less than the true mean). Nominal or "true"
values are available only in the case of fortified (spiked) samples,
but whether such fortified samples are representative of actual
(environmentally incurred) contamination is open to doubt in the case
of some types of material. A rapid nonparametric test of systematic
error can be made using the sign-test or the Wilcoxan signed ranks
test (Conover, 1971).
It is common practice to calculate the "recovery" factor for an
analytical method, i.e., the ratio of the mean observed value to the
nominal value (usually expressed as a percentage), but the statistical
significance of the "recovery" factor should always be assessed. The
ratio of the mean deviation to the standard error of the mean
deviation is an appropriate method of testing the null-hypothesis that
the difference between the nominal value and the observed mean is not
statistically significant.
The term "total error" has been proposed for a function
incorporating both the systematic error and the reproducibility
(McFarren et al., 1970), and these authors also suggested three
classes of total error corresponding to excellent methods, acceptable
methods, and methods that are judged unacceptable. It is pertinent
that McFarren et al. concluded that the total errors in one study of
the determination of DDT-type compounds in water were unacceptable
(>50% for p,p'-DDT, and p,p'-DDE, 24.0-53.6% for o,p'-DDT).
2.2.2 Limit of analytical detection
All analytical determinations have a lower limit corresponding to
that quantity (Delta g) of p,p'-DDT (or a related compound) which
produces a response (Delta r) that cannot be distinguished from
response (Delta r) produced when no p,p'-DDT is present. The
response Delta r is generated by the materials, reagents, and
instruments, used in the procedure, for example, the small voltage
generated by electronic equipment, or the small absorption in a
spectrophotometric method. These responses are known as "blank" or
"noise", and their size depends on the presence of interfering
components in the test material, the purity of the reagents, the
cleanliness of the apparatus, and the design of electronic equipment
(amplifiers, etc.). The concept of limit of detection is a statistical
one and is related to the random variation of the response generated
by a blank or control (Sutherland, 1965; Skogerboe & Grant, 1970;
Kaiser, 1973). Currie (1968) has defined three limiting levels for use
in analytical chemistry: "the net signal level" above which an
observed signal may be reliably recognized; "the true net signal
level" which may, a priori, be expected to lead to detection; and
"the quantifiable level" at which the measurement precision is
sufficient for the quantity present to be estimated satisfactorily. In
many types of sample, the limit of detection is of rather academic
interest as the concentrations of DDT-type compounds in samples are an
order of magnitude greater than the limit of detection of a sensitive
detector such as the electron-capture detector. However, in analyses
of air and drinking-water, for example, it may be necessary to
ascertain the limit of detection by a suitable statistical procedure.
Lower limits of detectability (ng.g-1) suggested in the US EPA
Manual of Analytical Methods (Thompson, 1974) for DDT-type compounds
using gas-liquid chromatography are:
o,p'-DDE Adipose tissue, 10; serum, 1
p,p'-DDE Adipose tissue, 20; serum, 1
o,p'-DDT)
p,p'-TDE) Adipose tissue, 20; serum, 2
p,p'-DDT)
2.2.3 Confirmation of the identity of trace residues of DDT-type
compounds
Confirmation of the identity of p,p'-DDT and related compounds
in many types of environmental samples is not easy when the apparent
amounts present are in the microgram and submicrogram range, since the
classical procedures for the identification of organic compounds
require the use of milligrams of the purified compound. A definition
of chemical identity appropriate to trace analysis has been proposed
(Robinson et al., 1966), a definition that may need revision in
relation to the combined use of gas-liquid chromatography and mass-
spectrometry. Chemical derivatization techniques for DDT-type
compounds have been reviewed by Cochrane & Chau (1971). Other
techniques, that are used routinely in the confirmation of the
identity of DDT-type compounds include: determination of gas-liquid
chromatographic retention times using polar and nonpolar stationary
phases; thin-layer chromatography; paper chromatography; p-values;
infrared microtechniques; carbon skeleton chromatography; conversion
into dichlorobenzophenones; nuclear magnetic resonance; and X-ray
diffraction. Tables of the relative retention times (aldrin = 1.00) of
DDT-type compounds using nine liquid phases (Thompson et al., 1975)
and three mixed liquid phases (Suzuki et al., 1975); gas-liquid
chromatographic retention times for DDT-type compounds are also
summarized by Yermakov (1972). Relative thin-layer chromatographic
Rf values ( p,p'-DDE = 1.00) on alumina using three solvent systems
were reported by Thomas et al. (1968). Extraction p-values for
DDT-type compounds using seven solvent systems are given in the US EPA
Manual (Thompson 1974). Identification of DDT and its metabolites by a
microinfrared technique has been studied by Sierwiski & Helrich (1967)
and reference infrared spectra have been published (Chen et al.,
1972). Asai et al. (1971) used carbon skeleton chromatography to
differentiate polychlorinated biphenyls from DDT-type compounds. The
conversion of DDT-type compounds into the corresponding benzophenones
may be used to identify them in the presence of polychlorinated
biphenyls (Miles, 1972), but this technique has its drawbacks as
p,p'-DDT, p,p'-TDE and p,p'-DDE all give the same p,p'-
dichlorobenzophenone. The use of high resolution nuclear magnetic
resonance spectroscopy (with a time averaging computer to increase
sensitivity), for the confirmation of the presence of p,p'-DDT and
p,p'-DDE in human adipose tissue (total concentration about
13 mg/kg) was studied by Biros (1970).
The combination of gas-liquid chromatography with mass-
spectrometry is a powerful tool for the confirmation of identity of
trace residues of DDT-type compounds. For example, Gordon & Frigerio
(1972) used mass fragmentography and claimed identification of 10 pg
p,p'-DDT; Schaeffer (1974) identified DDT-type compounds in fish
using a fast scan mass-spectrometer to give a multiple mass spectrum
for each of the overlapping peaks.
2.2.4 Sampling and extraction
Before discussing different kinds of samples, it must be noted
that valuable information on many analytical problems may be found in
the US EPA Manual (Thompson, 1974) and the FDA Manual (McMahon &
Sawyer, 1977).
DDT-type compounds may be present in the air in vapour form or
adsorbed on particulate matter. Glass-fibre filters are suitable for
trapping the particulate matter (Stanley et al., 1971; Beyermann &
Eckrich, 1974) and DDT in the vapour form may be trapped using
impingers of the Greenburg-Smith type and a suitable nonvolatile
solvent using ethylene glycol. Miles et al. (1970) reported a trapping
efficiency of more than 99% for DDT. A three-trap system in series
comprising a column containing glass cloth, followed by an impinger
with hexylene glycol, and finally an alumina column was used by
Stanley et al. (1971). They estimated that the collection efficiency
(based on material balance studies) of this system for a mixture of
aerosol and vapour was about 60% for p,p'-DDE, 95% for p,p'-TDE,
and 100% for p,p'-DDT. Beyermann & Eckrich (1974) used glass wool
for the trapping of aerosols, and a stainless-steel net coated with
polyethylene glycol for trapping vapours. Trapping of organochlorine
insecticides in the vapour phase using support-bonded silicones on
various types of Chromosorb was considered to be quantitative by Aue &
Teli (1971): DDT-type compounds were not examined but the trapping of
lindane, aldrin, and heptachlor was considered satisfactory. A cross-
linked polystyrene resin, Chromosorb 102, was used by Thomas & Serber
(1974). These workers reported a collection efficiency of 98% for
o,p'-DDT at a concentration of 15 ng/m3. Herzel & Lahmann (1973)
used silica gel as the support for various liquid phases and concluded
that polyethylene glycol was the best absorbent for DDT-type compounds
in the air. Absolute calibration of the collection efficiency of
aerosols and atmospheres for DDT-related compounds is difficult, but
the studies that have been made indicate that impingers with ethylene
glycol or hexylene glycol are probably the most efficient. The
simplest empirical test of the efficiency of the trapping system is to
use two (or more) impingers in series and analyse the liquid
absorbents from the impingers separately. The ratio of the amounts
present in the first and second impingers should be higher than 10:1.
Detailed instructions for the sampling and analysis of air for
pesticides (including DDT-type compounds) by a method that has been
subjected to interlaboratory study are given in the US EPA Manual
(Thompson, 1974).
For the determination of DDT-type compounds in water an
uncontaminated container may be filled, but if the concentrations of
DDT are likely to be extremely low (as would be expected in most
potable waters), then drawing the water through a suitable trapping
device may he more appropriate; this method also gives a time-weighted
average concentration and can be used in an automated monitoring
procedure.
Benzene was used by Pionke et al. (1968) as the solvent to extract
p,p'-DDT and p,p'-TDE from fortified samples of distilled water
and lake waters. The levels of fortification were high (µg/litre) and
the recoveries were: p,p'-DDT, 96.1% ± 1.02%; p,p'-TDE 97.3% ± 0.89%.
Thus, at concentrations of the order of 0.001 mg/litre, the
benzene extraction method gives excellent recoveries (total errors,
5.9% and 4.5%, respectively). A continuous flow method based
on liquid-liquid partition was developed by Ahling & Jensen (1970);
water is passed through a column containing Chromosorb W coated with
undecane plus a macrogol Carbowax 4000 monostearate. The best
collection efficiencies were found when the two liquid absorbents were
used at concentrations of 10% and 30%, respectively, on the solid
support. At concentrations in the ng/litre range, the optimum recovery
of known amounts of DDT-type compounds added to water were obtained
with 1.5 g coated Chromosorb W per litre of water. Ahnhoff & Josefson
(1974) used continuous flow liquid-liquid partition between water and
cyclohexane (in three extractors in series). The extraction
efficiencies from water containing 5 ng p,p'-TDE or 7.6 ng p,p'-DDT
per litre were greater at a flow rate of 2 litre/h than at
5 litre/h being 90% and 98% for p,p'-DDT, and p,p'-TDE,
respectively, with 92% and 93%, respectively, of the recovered
compounds being found in the first extractor. A method using a solid
adsorbent, a macroreticular resin, XAD-4, has been studied by Musty &
Nickless (1974); at a flow rate of 8 ml/min through a column
containing 2 g XAD-4, satisfactory recoveries of p,p'-DDT and three
related compounds were obtained at concentrations of 2-10 ng-litre.
Carbon is a very efficient adsorber of p,p'-DDT and p,p'-TDE from
water (Rosen & Middleton, 1959), but desorption from charcoal is
difficult; furthermore, the recoveries are not very satisfactory and
this is attributed to chemical changes catalysed by carbon in contact
with water rather than inefficient desorption (Eichelberger &
Lichtenberg, 1971). Another solid adsorbent is polyethylene (Beyermann
& Eckrich, 1973), but in the case of river water variable results were
obtained because of the effects of other solutes. Taylor & Bogacka
(1968) used petroleum ether to extract DDT from water; following a
clean-up with acetonitrile partition and Florisil the overall recovery
(using thin-layer chromatography) was incomplete (about 66%). The
total error of this procedure is unacceptable. Benzene was used as the
extraction solvent by Djatlovitskaja et al. (1972), and a general
review of the determination of organochlorine insecticides (and other
insecticides) in water has been published by Novikova (1973).
The need for scrupulous cleanliness in glassware and purity of
reagents cannot be overstressed in the case of the determination of
DDT-type compounds in air and water as the residues are usually so
small; the use of electronic equipment with a low noise characteristic
and constant checking of the response of detectors are also necessary.
A major difficulty in the determination of organochlorine
insecticides in soils is the initial extraction of the residues
because of a combination of factors, including the wide variation in
soil types. Chiba (1969) emphasized the lack of precision in defining
soil types. In his review article, he concluded that the most
effective solvent systems for extraction of these compounds were
mixtures of n-hexane/acetone (1:1 v/v) or chloroform/methanol
(1:1 v/v), and that the moisture content of the soil should be at
least 5%. The results of a study of the determination of
organochlorine insecticides in three types of soil have been
summarized by Woolson & Kearney (1969). Twelve laboratories
participated using various extraction procedures. In one type of soil
(a silty clay loam) that was fortified with p,p'-DDT at a
concentration of 5 mg/kg, the amounts found in the different
laboratories varied between 1.60 and 5.48 mg/kg. The results of 3 of
the laboratories were discarded by Woolson & Kearney (1969), and the
mean recovery of the other 9 laboratories was 79.3%, with a standard
deviation of 42.2%. Wetting of the soil before extraction was
considered to improve the recoveries, Soxhlet extraction appeared to
be preferable to shaking with the solvent, and hexane/acetone
(1:1 v/v) or hexane/isopropanol (3:1 v/v) appeared better extractants
than other solvents.
A further collaborative study of the determination of organochlo-
rine insecticides in 3 types of soil was reviewed by Woolson (1974).
Although 12 laboratories participated, only 7 completed the study. All
the laboratories used the same extraction, clean-up and quantification
procedures, including premoistening of the soil with ammonium chloride
solution at 0.2 mol/litre. The recoveries of p,p'-DDE, p,p'-TDE,
o,p'-DDT and p,p'-DDT were higher than 80%, with standard errors
of 8-18%. These results are much more consistent than those of the
previous study, and Woolson recommended that premoistening of the soil
with ammonium chloride solution (0.2 mol/litre) followed by extraction
with hexane/acetone (1:1 v/v) should be adopted for the determination
of chlorinated hydrocarbon insecticides in soil.
Several solvents have been used for the extraction of DDT-type
compounds from nonfatty foods such as vegetables, fruit, and cereals;
acetonitrile, alone or mixed with water is used in the method of the
Association of Official Analytical Chemists (AOAC) depending on the
water content of the sample (Horwitz, 1975); Cieleszky et al. (1970)
recommend Soxhlet extraction with diethyl ether.
Maceration of vegetables with a mixture of acetone and hexane was
used by Sissons et al., 1968; the same mixed solvent was used for
cereals, and root vegetables by Abbott et al., 1969 who used
propan-2-ol in the case of fruit and green vegetables. Whiting et al.
(1968), and Skrentny & Dorough (1971) considered a mixture of methanol
and chloroform to be the most efficient extraction solvent for use
with a macerator or a Soxhlet extractor. The acetonitrile extraction
technique was examined by Zerber et al. (1971) for the extraction of
DDT-type compounds from cereal products and feeding stuffs. Diethyl
ether was used by Kucinski (1972) for extraction from canned vegetable
products.
Extraction methods for fat-containing foods are given in the AOAC
Method (Horwitz, 1975), the solvent used, methanol or petroleum ether,
being dependent on the type of sample. Soxhlet extraction using
petroleum ether is recommended by the Federal Health Office of the
Federal Republic of Germany (Anon., 1974); Cieleszky et al. (1970)
also recommended petroleum ether as a suitable solvent.
Smart et al. (1974) compared acetonitrile and dimethyl formamide
as extraction solvents for apples, carrots, potatoes, and vegetables;
a third solvent dimethylsulfoxide was compared with these two solvents
for butter, cheese, and eggs.
Three body tissues or fluids have been analysed in many surveys,
namely adipose tissue, blood (or serum), and mother's milk. The US EPA
Manual (Thompson, 1974) recommends grinding a sample of adipose tissue
with anhydrous sodium sulfate before extraction with petroleum ether;
carbon tetrachloride has been used as a solvent (Mattson et al.,
1953), but it is not appropriate if the final quantification step
involves gas-liquid chromatography and a halogen sensitive detector.
Extraction of DDT-type compounds from blood or serum using hexane has
been described (Dale et al., 1966b), but this extraction procedure is
inefficient, probably as a result of binding by serum proteins. Dale
et al. (1970) investigated a procedure in which the serum was treated
with 97% formic acid before extraction with hexane; experiments using
14C-DDT indicated that extraction with hexane alone gave results
some 40% lower (based on 14C activity) than when the serum was first
treated with formic acid. A modified procedure in which whole blood
was treated with 60% sulfuric acid, and then extracted with a
hexane/acetone (9:1) mixture has been reported by Stretz & Starr
(1973). Samples of blood spiked at 4 different levels with p,p'-DDT
were analysed by 11 different laboratories; considerable discrepancies
were found between laboratories and a further study of the method was
considered desirable. Griffith & Blanke (1974) also investigated the
sulfuric acid method, but a microcoulometric detector was used instead
of an electron-capture detector. According to these workers,
consistent recoveries of p,p'-DDT, p,p'-TDE and p,p'-DDE were
obtained in their laboratory but reproducibility between laboratories
was not studied.
The US EPA Manual (Thompson, 1974) method for the extraction of
DDT-type compounds from human milk involves an acetonitrile/hexane
type extraction. A method for extraction from cow's milk, that makes
use of the stability of p,p'-DDT and its derivatives in the presence
of concentrated sulfuric acid, has been published by Coha & Nedic
(1970); the mixture of milk and concentrated sulfuric acid is
extracted with hexane. Prouty & Cromartie (1970) studied the
recoveries of 14C-DDT in each of the 5 major stages of a method for
determining this compound in the tissues of quail; Soxhlet extraction
with hexane for 6 h, of muscle, liver, heart or brain, after grinding
with sodium sulfate gave recoveries of DDT-type compounds (as 14C-
activity) of 93-105%. Jonczyk (1970) used hexane to extract DDT-type
compounds from blood and reported recoveries of 67-91%. Acetone was
used as the extracting solvent for DDT-type compounds in the adipose
tissue and brain of partridges (Jonczyk et al., 1970). Wood's method,
using dimethyl sulfoxide, was used by Stec & Juszkiewicz (1972), and
was found to give results for DDT-type compounds that compared
favourably with other methods of analysis of animal tissue, eggs, and
milk.
All methods of extracting DDT also result in the removal of
lipids, if they are present. Regardless of the method of extraction,
the results of most analyses have been reported in terms of fresh or
wet weight of samples no matter whether their lipid content was
extremely low (water and urine), low (soils and most vegetables),
intermediate (milk and many tissues), or high (adipose tissue). In
some instances, samples such as adipose tissue, milk, and, to a lesser
degree, other animal tissues known to contain lipids have been
reported in terms of the concentration of pesticide in extractable
lipid. Because DDT and DDE are known to have a marked affinity for
neutral fat, it was originally supposed that reporting in terms of
lipid would reduce variability within any set of samples by excluding
the influence of connective (and sometimes lymphatic) tissue, which
forms a part of each sample. It appears that variation, as measured by
the coefficient of variation, may not be reduced by this kind of
reporting (Casarett et al., 1968). However, there may be other reasons
for reporting on a lipid basis and it is absolutely essential that the
method of reporting be specified.
2.2.5 Clean-up procedures
The extraction procedures remove not only the DDT-type compounds
from the samples analysed but also coextractives to a greater or
lesser degree according to the type of sample. A number of procedures
have been developed that reduce the amounts of the coextractives
relative to that of DDT-type compounds. If interest is confined solely
to DDT-type compounds, then their stability in the presence of
concentrated sulfuric acid is a very useful clean-up procedure
(Mattson et al., 1953; Czegledi-Janko & Cieleszky, 1968; Murphy,
1972). Usually, however, the concentrations of other compounds are
also of interest and this procedure cannot be used if these compounds
are not stable in concentrated sulfuric acid. Two general clean-up
procedures, used in sequence, are appropriate in these circumstances,
namely, liquid-liquid partition followed by liquid-solid partition.
Liquid-liquid partition systems such as hexane/acetonitrile,
hexane/dimethyl formamide, or hexane/dimethyl sulfoxide are the ones
most commonly used. The liquid-solid partition systems generally
consist of Florisil, silica gel, or alumina as the solid phase, and
hexane or mixtures of hexane and various proportions of a polar
solvent (e.g., diethyl ether) as the mobile phase. Separation of
DDT-type compounds from triglycerides in fat-containing tissues is
achieved with considerable efficiency by liquid-liquid partitions, the
hexane/dimethyl formamide or hexane/dimethyl sulfoxide systems being
generally more efficient than hexane/acetonitrile. Separation of
DDT-type compounds from other organochlorine compounds (e.g., aldrin,
dieldrin, polychlorinated aromatics) or from steroids is not very
efficient using liquid-liquid systems, and the liquid-solid partition
systems should be used in these cases. Separation of DDT-type
compounds from polychlorinated biphenyls is particularly difficult
and, as some of these compounds have similar liquid chromatographic
retention times to those of the various DDT-type compounds, the
analysis of samples containing both classes of compounds requires
considerable care. Detailed clean-up procedures are described by de
Faubert Maunder et al. (1964), Cieleszky et al. (1970), Anon. (1974),
Thompson (1974), and by Horwitz (1975).
The separation of DDT-type compounds from polychlorinated
biphenyls, by liquid-solid (silical gel) partition is discussed by
Armour & Burke (1970), Snyder & Reinert (1971), and Masumoto (1972);
the last-mentioned investigator concluded that a number of factors
required careful control if satisfactory separation of DDT-type
compounds from polychlorinated biphenyls (PCBs) were to be achieved,
in particular, the degree of activation of the silicic acid (irregular
distribution of water molecules onto the silicic acid particles was
also probably important). He found that separation of p,p'-DDE from
four Arochlors was incomplete. A collaborative study of the separation
of DDT-type compounds from PCBs using the Armour & Burke silicic acid
column procedure has been reported (Sawyer, 1973).
Florisil and coconut charcoal have also been investigated for the
separation of PCBs from DDT-related compounds (Reynolds, 1969;
Benvenue & Ogata, 1970; and Stijve & Cardinale, 1974). Another
procedure that has been developed for the separation of DDT-PCB
mixtures is the oxidation of p,p'-DDE to p,p'-dichlorobenzophenone
(Miles, 1972). A method for the determination of p,p'-DDT and a
particular PCB isomer that has similar retention time to that of
p,p'-DDT is based on an empirical relation for p-values (Zelinski et
al., 1973).
2.2.6 Quantitation
2.2.6.1 Determination of DDT-type compounds
Two techniques have played a major role in the quantification of
DDT-type compounds, one is a colorimetric method, the other (now the
most widely used) is gas-liquid chromatography with a halogen-
sensitive detector.
The colorimetric procedure of Schechter-Hailer is described in
detail in the Handbook of the Deutsche Forschungsgemeinschaft (1969),
and by Cieleszky et al. (1970) and Horwitz (1975).
Gas-liquid chromatography is essentially a further-method of
separation of compounds, and, although it is the most effective of the
separation procedures (apart possibly from high pressure liquid-liquid
chromatography) it must be realized that it has its limitations,
particularly if used with a highly sensitive but nonselective detector
such as the electron-capture detector. The basic principles of
gas-liquid chromatography are described by Dal Nogare & Juvet (1962)
and Yermakov (1972), for example, and need not be discussed here.
However, attention is drawn to three aspects of the performance of a
column in the gas-liquid chromatographic separation of DDT-type
compounds from other compounds. First, the DDT-type compounds should
not undergo any thermal degradation or other chemical change; second,
the performance of the column should be assessed by the number of
theoretical plates; and third, the performance of the column as regards
ability to separate p,p'-DDT and related compounds from other
compounds that have similar retention times for a particular liquid
phase must be investigated. Transport of p,p'-DDT and analogues
through a column without chemical change is dependent upon the absence
of reactive centres in the column. This can be attained by using an
inactive solid support (by coating the reactive sites with a silane,
if necessary) and ensuring that the surface of the solid support is
completely covered by an inert liquid phase. The performance of a
column should be checked at regular intervals; the US EPA Manual
(Thompson, 1974) suggests a mixture containing five DDT-type compounds
plus eight other organochlorine insecticides for this purpose. Change
in peak shape (i.e., departure from symmetrical peaks) should always
be regarded as a warning sign. In the case of serious doubt about the
stability of p,p'-DDT or an analogue (which may manifest itself as
a change in the retention time relative to that of aldrin or dieldrin
for example), it is suggested that a fraction collection technique be
used and the identity of the component leaving the column at a
particular retention time with that injected confirmed.
Methods of calculating the number of theoretical plates and of
separation factors are given in standard texts. According to the US
EPA Manual, a column of 2 m length should have about 3000 theoretical
plates. The factors that control the performance of gas-liquid
chromatographic columns are discussed in detail by Scott (1970).
Convenient summaries of the retention times (absolute or relative) of
DDT-type compounds, together with those of other pesticides, using
various column conditions have been published (Yermakov, 1972; Zweig &
Sherma, 1972). Retention times of 51 pesticides relative to that of
aldrin using six stationary phases at three temperatures with an
electron-capture detector have been reported by Thompson et al.
(1975); estimates of the relative retention times at other
temperatures were derived from the relationship between relative
retention time and temperature for each liquid phase. The relative
times of DDT-type compounds and other pesticides on eight stationary
phases were determined by Thompson et al. (1969a). Nonpesticidal
organochlorine compounds that have retention times similar to those of
DDT-related compounds include the polychlorinated biphenyls and
polychloronaphthalenes.
Examples of the close similarity between the relative retention
times of DDT-related compounds and those of various PCB isomers have
been published (Bagley et al., 1970; Richardson et al., 1971; Stijve &
Cardinale, 1974). Goerlitz & Law (1972) demonstrated that there are
also similarities between the relative retention times of DDT-type
compounds and those of various isomers of polychlorinated
naphthalenes. Examples of similarities between the relative retention
times of DDT and its analogues and various components in
polychlorinated biphenyls and polychlorinated naphthalenes was also
reported by Griffith & Blanke (1974). Problems arise in the case of
mixtures of toxaphene and DDT-related compounds (Cahill et al., 1970).
The effects of severe infection loading on column performance,
peak configuration, and conversion of p,p'-DDT into p,p'-TDE, were
studied by Thompson et al. (1969b). These investigators found that the
columns they used could be maintained and restored to full or nearly
full performance capacity by daily changing of the glass injection
port insert and the glass-wool plug at the column inlet.
Two different types of detection systems are most frequently used
for the quantification of p,p'-DDT and analogues after elution from
gas-liquid chromatographic columns, namely, electrochemical detectors
and electron-capture detectors. Two types of electrochemical detector
have been developed, the microcoulometric detector and the micro-
electrolytic conductivity detector, that are considerably more
sensitive to p,p'-DDT and its analogues than the original electro-
chemical detectors. Giuffrida & Ives (1969) described modifications and
improvements in microcoulometric gas chromatography and, in the case
of DDT-type compounds in carrots, they obtained responses from their
microcoulometer that were approximately one-fifth of those given by a
particular electron-capture detector; Griffith & Blanke (1974)
described a microcoulometric method for the determination of
p,p'-DDT, p,p'-TDE and p,p'-DDE in blood. According to Dolan
& Hall (1973), the microelectrolytic conductivity detector can be used
for the selective determination of organochlorine pesticides in the
presence of polychlorinated biphenyls. However, the relative
selectivity in regard to p,p'-DDE does not appear to be as great as
that for other organochlorine insecticides.
The electron-capture detector produces an extremely sensitive
response to organochlorine compounds, but its response is not,
unfortunately, very selective and many other classes of compounds have
electron affinity in the vapour phase. A general review of the
principles and characteristics of the electron-capture detector has
been published by Pellizari (1974). The detector may be used under
direct current or pulse sampling conditions; anomalous responses
obtained in the direct current mode of operation are not present under
pulse sampling conditions (Lovelock, 1963). A major limitation of the
electron-capture detector is that its response is linear over a very
limited range only, but a new mode of operation in which the linearity
extends over about four orders of magnitude of response has been
described by Maggs et al. (1971). For this the detector current is
held constant while the frequency of the applied pulses is varied. The
response of electron-capture detectors is liable to change
significantly during use, and these detectors should be recalibrated
at regular intervals, preferably at least once per day with single
standard injections at frequent intervals between injections of
extracts from the samples under investigation. It is of interest that
Mendoza (1971) reported a significant difference in the response of a
gas-liquid chromatograph to p,p'-DDT when injections were made at
fast and slow rates.
The combination of mass-spectrometry with gas-liquid chromato-
graphy was mentioned in section 2.2.4 as a means of confirming the
identity of residues of DDT-type compounds. This combination of
instruments can also be used to quantify the amounts present. The
total ion current corresponding to mass fragments of appropriate mass
number (m/e; in the case of p,p'-DDE, 316, 318, 246 and 248) is
measured and the amount present is calculated from the calibration of
the instrument, preferably by an internal standard. Palmer & Kolmodin-
Hedman (1972) determined the concentration of p,p'-DDE in human
plasma by mass-fragmentography using the ion current corresponding to
m/e 216 and 218; the results showed an excellent agreement with the
values obtained using an electron-capture detector.
Semiquantitative methods of the determination of p,p'-DDT and
its analogues include paper chromatography and thin-layer chromato-
graphy; these methods, which are more appropriately used for the
confirmation of identity of residues, are described by McKinley (1963)
and Sherma (1973) respectively. Bishara et al. (1972a) have given the
tlc Rf values for p,p'-DDT and 14 related compounds using 33 solvent
systems.
2.2.6.2 Determination of p,p'-DDA in urine
A major metabolite of p,p'-DDT, excreted in the urine, is bis-
(4-chlorophenyl)acetic acid ( p,p'-DDA). A method has been developed
by Cranmer et al. (1969), based upon the extraction of p,p'-DDA from
acidified urine, conversion into the methyl ester, Florisil column
clean-up, and gas-liquid chromatographic analysis of the ester. A
detailed outline of this procedure is given by Thompson (1974). The
sensitivity of response of the methyl ester is not high, the limit of
detection being about 2 ng. Cranmer & Copeland (1973) used the
2-chloroethanol ester, the response of an electron-capture detector
being about 3.7 times greater than that of the methylester (i.e.,
limit of detection about 0.5 ng). An advantage of this ester is that
it is well separated from p,p'-DDT, whereas the methyl ester has a
retention time similar to that of p,p'-DDE. The retention time of
the pentafluorobenzyl ester compound is about twice that of p,p'-DDT
and the response of an electron-capture detector is considerably
greater than that for the 2-chloroethanol derivative (Johnson, 1973).
2.2.6.3 Method of reporting results
Francis Galton (1879) pointed out that many vital effects are
distributed logarithmically; his paper was followed by a technical one
(McAlister, 1879) presenting the mathematics. Apparently Robinson &
Hunter (1966) were first to point out that this principle applies to
the storage of pesticides so that the geometric mean is a more
appropriate parameter than the arithmetic mean for expressing
insecticide content. In recent years, increasing use has been made of
the geometric mean as recorded by footnotes in Table 9. Few of the
arithmetic means that have been reported are so much in error that
they should be discarded. As Galton (1879) noted, the difference
between the arithmetic and geometric mean is small if the range of the
values averaged is narrow.
2.2.7 Validation of analytical methods for DDT-type compounds
Ideally, an analytical method should be accurate and highly
precise. A study of the accuracy and precision of art analytical
method must be made in order to assess the relationship between the
actual amount present and the results obtained in practice; such a
study may be called the validation of the procedure. There are two
major types of validation procedure. The first type requires the use
of radio-labelled molecules of p,p'-DDT, e.g., 14C p,p'-DDT.
Prouty & Cromartie (1970) determined the 14C-activity in muscle,
liver, heart, and brain of quail that had been given 14C-labelled
p,p'-DDT. Four of the steps in the analytical procedure were studied
and it was concluded that the major discrepancies occurred during the
elution and concentration steps from Florisil columns and from zones
of thin-layer chromatography plates. Chiba & Morley (1968) used 14C-
labelled p,p'-DDT in a study of soil analysis.
The other validation procedure, which has been studied more
intensively, involves the comparison of the results of analyses with
the values for samples fortified with accurately known amounts of
p,p'-DDT or analogues. There are several variations of this
validation procedure, all comparing the fortified (nominal) value with
the results of different methods in the same laboratory, with the
results of a specified method carried out in different laboratories,
or with the results of different methods in different laboratories.
Versino et al. (1971) compared 8 clean-up procedures for test
mixtures containing 10 pesticides. Two extracts, from apples and
lettuce, were fortified (spiked) with p,p'-DDT at 0.2 mg/litre,
p,p'-TDE at 0.1 mg/litre, and o,p'-DDT at 0.1 mg/litre plus
dieldrin and 7 organophosphate insecticides. The recoveries for the 8
column clean-up procedures ranged from 89-100% for p,p'-DDT, 85-101%
for p,p'-TDE, and 95-100% for o,p'-DDT. It should be noted that
this investigation did not include a study of extraction efficiency
and that the extracts contained only small amounts of lipids. In
studies by Smart et al. (1974), samples were analysed of milk, butter,
cheese, eggs, apples, potatoes, carrots, and cabbages, fortified with
p,p'-DDT, p,p'-TDE, and p,p'-DDE (and 5 other organochlorine
insecticides) at concentrations corresponding to those suggested as
limits by the Codex Alimentarius Commission. Five replicate analyses
of each sample were made, using up to 4 procedures, the final step of
each analytical procedure being gas-liquid chromatography/electron-
capture detection, with 3 different stationary phases. These workers
concluded that there were no gross discrepancies in their results.
However, they did not use the concept of "total error" (see above) in
the discussion of their results, and the total errors for the
determination of the 3 DDT-type compounds, calculated from their
results, were in the range of 12% to 95%.
Results have been reported (Carr, 1970) of a collaborative study
by 10 laboratories of the AOAC method for the determination of 4
DDT-type compounds in samples of butterfat fortified by the addition
of these 4 compounds at 2 different concentrations. The mean recoveries
varied from 86-108%, and the coefficients of variation between
laboratories were in the range 7-28%. The total errors for the 2
levels of fortification respectively were: p,p'-DDT, 22 and 14%;
p,p'-TDE, 17 and 30%; p,p'-DDE, 17 and 36%; and o,p'-DDT, 30 and
43%.
Carr (1971) gives the results of a collaborative study by 8
laboratories of the analysis of fish samples fortified (2
concentrations) with p,p'-DDT, p,p'-TDE and p,p'-DDE, and 3 other
organochlorine insecticides. On the basis of a ranking technique
(Youden & Steiner, 1975), the results of 2 laboratories (Nos. 2 and 8)
for the 6 compounds were rejected as outliers. However, if the results
for the 3 DDT-type compounds are considered, then laboratories 7 and 8
gave results that are outliers. The total errors at the 2
concentrations for the 6 laboratories not rejected by Carr were:
p,p'-DDT, 15 and 36%; p,p'-TDE, 32 and 32%; p,p'-DDE, 26 and 39%.
Sawyer (1973) reported a collaborative study by 9 laboratories of
samples of red salmon fortified with 3 DDT-type compounds and PCBs.
The analyses were carried out without and with silicic acid column
separation of PCBs from DDT-type compounds. The total errors without
and with silicic acid separation, were 34% and 43% respectively for
p,p'-DDT, 50 and 47% for p,p'-TDE and 35 and 36% for p,p'-DDE.
Very similar results were obtained with another type of PCB/DDT
mixture.
Samples of soil, fortified with 4 DDT-type compounds plus 6 other
organochlorine insecticides were analysed in 7 laboratories (Woolson,
1974); the total errors (all collaborators) were: p,p'-DDT, 17-33%;
p,p'-TDE, 19-37%, p,p'-DDE, 18-42%; o,p'-DDT, 28-56%.
Fifteen laboratories collaborated in a study of the determination
of p,p'-DDE in eggs, and p,p'-DDT, p,p'-DDE and o,p'-DDT in
kale (Finsterwalder, 1976). Five laboratories analysed eggs by the
AOAC method, and the total error was 21.1%; the total error for 10
laboratories using a modification of the AOAC method was 30.4%. The
results of one laboratory for analyses of kale were rejected as
outliers, and the total errors for the other 14 laboratories were:
p,p'-DDT, 19.7%; p,p'-DDE, 18.8%; o,p'-DDT, 17.4%.
An international collaborative programme on methods of analysis of
organochlorine insecticides has been sponsored by the Organization for
Economic Cooperation and Development (OECD), and the results of 2
studies have been published. In the first (Holden, 1970), a solution
in hexane of 6 organochlorine compounds, 3 being DDT-related
compounds, was analysed by 17 laboratories in 11 countries. The total
errors for p,p'-DDT, p,p'-TDE and p,p'-DDE were in the range
14-21%, the major component in this total error being the standard
deviation of results from different laboratories, probably indicating
errors of calibration. In the second study (Holden, 1973), a sample of
corn oil fortified with 4 DDT-type compounds and 3 other
organochlorine insecticides was analysed in 19 laboratories in 10
countries: the mean total error for the 4 DDT-type compounds (after
excluding 3 outliers) was 35%.
Some of the results of this collaborative validation study are
very satisfactory, but many of them, as judged by the criterion of
total error, are unsatisfactory, even when outliers are rejected, and
they illustrate the need for scrupulous care by analysts: the use of
clean glassware, chemicals of established purity, and constant
checking of the performance of liquid-solid partition columns and of
instruments.
2.2.8 Analytical methods for the evaluation of the biochemical effects
of p,p'-DDT and its analogues
The increased activity of enzymes, particularly in the hepatic
endoplasmic reticulum, if the exposure to p,p'-DDT and its analogues
is sufficiently great, has been recognized in recent years.
Measurement of such changes in enzyme activity may be made by studying
changes in the rates of metabolism of certain drugs (such as
antipyrine), but methods that do not require the administration of a
drug (and the collection of blood samples if the plasma half-life is
used as the biochemical parameter) are preferable. Two methods, in
which the concentration of either 6-ß-hydroxycortisol or D-glucaric
acid in the urine is measured have been developed. A procedure for the
determination of 6-ß-hydroxycortisol in urine has been described by
Kuntzman et al. (1968); and one for D-glucaric acid by Marsh (1963)
(see section 8.2.5.1).
These enzyme induction changes are not specific for DDT-type
compounds.
3. SOURCES OF ENVIRONMENTAL POLLUTION
3.1 Discovery and Introduction
DDT does not occur naturally. It was first synthesized by Zeidler
as reported in 1874. However, it was not put to any use until its
insecticidal properties were discovered by Paul Müller in 1939. The
Swiss patent was issued in 1942.
As an example of the speed with which DDT was developed and used,
the first sample sent to the USA arrived there in September 1942. This
sample was tested for effectiveness and safety. The results were so
encouraging that manufacture was given high priority. At first, the
entire production was used for the protection of troops against
malaria, typhus, certain other vector-borne diseases, or against
biting flies or other insects that are merely pests. As the supply
increased, DDT was used in the USA for control of malaria in military
areas, that is in the vicinity of military installations, ports, and
transportation centres. As a result of this effort, mosquito
transmission of malaria was brought to an end in the USA in 1953, even
though military personnel and other infected persons from the tropics
continued to reintroduce the disease extensively as late as 1972 and
to a lesser degree thereafter.
3.2 Production and use
The revolution in the control of malaria and typhus among allied
troops and among certain civilian populations during World War II was
accomplished with relatively little DDT. Far greater amounts were
required for the control of agricultural and forest pests and this
became possible when the compound was released in the USA for
commercial use on 31 August 1945. Civilian use in other countries
became possible a little later, first, largely on the basis of
importation and gradually on the basis of local manufacture.
Unfortunately, there is apparently no record of world production of
DDT. Production and use in the USA is shown in Table 2.
Quantities of DDT and related compounds used in or sold for
agricultural purposes in 1970 were as follows (metric tonnes):
Australia (about 1000); Austria (20.5); Botswana (2.0); Canada
(287.0); Columbia (980.0); Czechoslovakia (270.0); Democratic
Kampuchea (46.8); Egypt (3457.0); El Salvador (466.0); Federal
Republic of Germany (152.0); Finland (6.1); Ghana (0.3); Guatemala
(380.0); Hungary (20.6); Iceland (0.3); Israel (10.0); Italy (2178.0);
Japan (401.0); Kuwait (0.2); Madagascar (176.0); Ryukyu Islands
(Japan) (0.3); Sri Lanka (16.6); Sudan (269.0); Upper Volta (1.5); and
Uruguay (5.0) (FAO, 1972). These values total 10 146.2 metric tonnes.
Thus, at least until very recently, the use of DDT was extensive on a
worldwide basis but varied greatly from one country to another.
Table 2. Metric tonnes of DDT produced and used in the USAa
Year Production Use
1944 4 366
1945 15 079
1946 20 220
1947 21 534
1948 9 181
1949 19 822
1950 35 448
1951 48 144
1952 45 327
1953 38 268 28 349
1954 44 088 20 465
1955 56 760 28 032
1956 62 441 34 194
1957 56 136 32 205
1958 65 920 30 255
1959 71 097 35 771
1960 74 471 31 818
1961 77 763 29 061
1962 75 764 30 502
1963 81 154 27 744
1964 56 113 22 925
1965 63 859 24 034
1966 64 115 20 685
1967 46 906 18 260
1968 63 231 14 848
1969 55 839 13 724
1970 26 860 11 316
a Based on data from US Tariff Commission (Hayes, 1975).
3.3 Changing Patterns of Use
Before 1945, all of the DDT produced in the USA for example, was
used or allocated by the military services for various medical and
public health uses. Early in 1945, it became available tor rather
extensive experimental work in agriculture, and it was commercially
available in limited quantities early in the autumn of the same year
(US Department of Agriculture, 1945a,b). The results were so
spectacular that use increased until 1959. In response to a demand for
exports, production continued to increase to about 1963. Even before
then some restrictions were placed on its use, mainly to minimize
residues in food and in the feed of animals that produce milk and
meat. Among the first of these restrictions was that on its use on
dairy cattle or in dairy barns (USDA, 1949). Another important factor
reducing the use of DDT was the increasing resistance of pests. One of
the first species to be affected was the house-fly; because of its
abundance and widespread distribution, its resistance was bound to be
noticed by the public, generally. Although many pests of public health
importance became resistant to DDT in some or all of their range,
resistance among vectors of malaria was less marked. Because malaria
control constitutes such a large segment of vector control, the use of
DDT for vector control has tended to remain stable, while its use in
agriculture has continued to decline, especially in temperate
climates.
The ban on the use of DDT and certain other organochlorine
insecticides in Sweden from 1 January 1970 was based on a number of
ecological considerations.
Government agencies of some other countries attempted to justify
severe restrictions on the use of DDT by alleging that it was a threat
to human health. This was in response to ecologists who considered
that the widespread occurrence of DDT in the environment was
inherently bad and was the direct cause of injury to certain fish and
birds. However, this did not prevent the same agencies from making a
proviso that DDT might be used, if needed, to combat any future threat
from vector-borne disease within their boundaries.
Of course, before the restrictions were put into effect, it had
already been possible to eradicate malaria in the USA and Italy, for
example, and to control for the first time an epidemic of typhus in
Italy and Germany (Simmons, 1959). Apparently, there have never been
accurate figures on the proportion of the compound used for
agriculture and for public health even in those countries that have
recorded their total use of the compound.
As the situation now stands, DDT is still used extensively, both
in agriculture and for vector control, in some tropical countries.
Apparently, information is not available on how much of the
agricultural use involves food protection or how much loss of food
production would result if the use of DDT were discontinued. The
picture with malaria control is clear. Substitution of malathion or
propoxur for DDT would increase the cost of malaria control by
approximately 3.4- and 8.5-fold, respectively, and this increase could
not be supported. If DDT were not used, vast populations in the
malarious areas of the world would be condemned to the frightening
ravages of endemic and epidemic malaria (WHO, 1971).
4. ENVIRONMENTAL TRANSPORT AND DISTRIBUTION
Because DDT has been sprayed on people, domestic animals,
buildings, agricultural crops, and forests, it is not surprising that
it is now distributed widely in the environment. During the
application of any spray or dust to a field, it is often possible to
observe drift of the particulate material. This is especially true if
the application is made by aircraft or by ground equipment that shoots
the spray to the top of orchard trees. If the application is made to
forests, it is very likely that at least part of the spray will fall
directly on streams or lakes. Following application, redistribution is
inevitable. As discussed more fully in the following sections, some
DDT in soil enters the air by evaporation or on wind-blown dust. Even
if watercourses are avoided initially, some insecticide will be washed
into them by rains, mainly in conjunction with soil particles.
4.1 Local Drift in Air
The fact that there is drip is implicit from measurements of DDT
on surfaces just after application. Wilson et al. (1946b) recovered
only 7.5% of the nominal dose from plants; this proportion is typical
but ignores material that may have fallen between plants or between
leaves and come to rest on the ground. Of greater interest are
measurements of total recovery made by means of absorbent targets laid
out in advance. As might be expected, recovery of aerosols is less
than recovery of sprays when other conditions of application are
similar. Only 12.5% was recovered at the centre of swaths following
application of a thermal aerosol by aircraft and recovery decreased at
increasing distances from the centre (Hess & Keener, 1947). In another
study, recovery was 8% for a thermal aerosol and 46% for a spray
(Scudder & Tarzwell, 1950). In a different situation, the average
seasonal recovery for DDT applied as a thermal aerosol ranged from 10
to 12%, while that for spray ranged from 56 to 76% (Tarzwell, 1950).
However, somewhat lower recoveries of DDT sprays have frequently been
reported: 30% (Hoffman & Merkel, 1948), 39% (Hoffman & Surber, 1948),
and 27% (Surber & Friddle, 1949).
Most DDT particles that miss the target for which they are
intended would be expected to fall in the general area. Studies of
residues in soil and in wildlife indicate that this is true. The
logarithm of the concentration of DDT-related compounds in soil
samples collected in a desert area downwind from an area of intensive
agriculture showed an almost straight line inverse relationship to the
logarithm of the distance from the source. The soil levels were about
1 mg/kg at 10 m and about 0.001 mg/kg at 100 000 m from the point of
application. The concentrations of DDT in the tissues of wildlife were
proportional to its concentrations in the soils of their habitats
(Laubscher et al., 1971). Where cultivated fields to which DDT has
been applied for years are interspersed among pastures and other
fields where DDT is not used, the uncultivated soils contain only a
little less DDT than the cultivated ones. In one study, it was found
that most of the DDT was in the top 2.5 cm, but samples for comparison
were taken to a depth of 7.5 cm; the average concentrations were 0.75
to 2.03 mg/kg in cultivated soil and 0.10 to 0.91 mg/kg in
uncultivated sod (US Department of Agriculture, 1966). These
concentrations may be compared (by mathematical calculation alone) to
1.0 mg/kg, the concentration resulting from mixing DDT into soil
uniformly and with no loss whatever following application at the rate
of 1.12 kg/ha, the standard specific gravity (1.47) of soil and a
depth of 7.6 cm being assumed. The range of specific gravities for
most agricultural soils is 0.4 to 2.0 and the corresponding depths
yielding 1 mg/kg are 28 to 5.60 cm. It is of interest that the
residues in cultivated soft were of the order of magnitude that would
be expected from a single application, even though the average
cumulative rate of application during the last 10 years (11.2 kg/ha)
had been 10 times as great.
4.2 Distant Drift in Air
Dust bearing DDT at a concentration of 0.6 mg/kg has been observed
about 1600 km from its area of origin. Other pesticides were also
present in this dust which had settled out on a recently rain-rinsed
roof. The cloud of dust was so dense and unusual that its progress was
reported in newspapers, as the storm that mobilized it in Texas
carried it at least as far as Ohio, where the sample was collected
(Cohen & Pinkerton, 1966).
Dust collected on the island of Barbados by means of nylon nets
treated with 50% aqueous glycerine was assumed to have been blown from
Africa, a distance of over 4850 km. Samples of the dust contained
p,p'-DDT most frequently and in greatest concentration, but the
concentration of all pesticides was only 0.001 to 0.164 mg/kg with an
average of 0.041 mg/kg (Risebrough et al., 1968).
DDT evaporates from sprays and dusts at the time of application
and at any time that dust bearing the compound is mobilized by the
wind. Furthermore, DDT can be detected over treated fields for more
than six months after application, and there is a concentration
gradient from the soil upward (Willis et al., 1971). Under these
circumstances, it is obvious that DDT is transported in the form of a
vapour as well as by means of dust. However, if samples are collected
where no application of DDT has been made, identification of the
origin of the vapour or measurement of the distance it may have
travelled is even more difficult than with dust.
Evidence that DDT in one form or another has travelled great
distances and is, in fact, worldwide in its distribution has been
deduced from finding it in the rainwater of remote, nonagricultural
places (Tarrant & Tatton, 1968) or in water melted from Antarctic snow
(0.00004 mg/litre) (Peterie, 1969). In such remote places as
Eskdalemuir in Scotland and Lerwick in the Shetland Islands, the
average concentrations of p,p'-DDT in rainwater (0.000030 and
0.000046 mg/litre, respectively) were not greatly different from the
averages (0.000018-0.000066 mg/litre) found in widely separated
agricultural areas, suggesting that the compound is rather evenly
distributed in the air (Tarrant & Tatton, 1968).
4.3 Distribution in Water
DDT has a strong tendency to adsorb on surfaces. Most DDT that
enters water is already firmly attached to soil particles, and remains
attached. It was shown very early that, if DDT does find its way into
clear water, it is gradually lost by adsorption on surfaces (Carollo,
1945). The sediments in water tend to move downstream and eventually
to enter estuaries. Of the various chlorinated hydrocarbon
insecticides, DDT and its metabolites are the ones most commonly
found, but the residues tend to be low (Butler, 1969). In fact, in an
estuary associated with the Mississippi River, the levels of
pesticides decreased strikingly from the early 1960s to the late 1960s
(Rowe et al., 1971).
4.4 Bioaccumulation of DDT and Its Degradation in the Environment
Many studies of DDT and related compounds in the environment have
focused on organisms and locations in which concentrations of DDT have
been observed to increase. Concentration in living organisms may be
the result of adsorption from water, of the filtering out of algae or
detritus bearing the compound, or of biological magnification in the
strict sense, that is progressive accumulation in different steps of a
food chain. Although the mechanisms are poorly understood, observation
has shown that residues do reach an equilibrium and sometimes decline.
For example, where DDT was applied to cotton at a cumulative rate of
11.2 kg/ha so that a residue of over 10 mg/kg in the soil would be
expected after many years of continual use, the actual residues ranged
from 0.75 to 2.03 mg/kg (USDA, 1966). An example of decreased residues
was seen in the grebes of Clear Lake (Rudd & Herman, 1972).
Evidence is accumulating that the disintegration of DDT may be
rapid in some situations. Under biologically active, anaerobic
conditions as little as 1% of DDT remained after 12 weeks of
incubation (Hill & McCarty, 1967; Guenzi & Beard, 1968). Probably more
important is the disintegration of DDT under the influence of
ultraviolet light. Hartley (1969) pointed out that much of any
pesticide vapour escaping to 50 metres or more above the ground will
ascend even higher by eddy diffusion and eventually reach the
photochemically active ionosphere. The rapid destruction of DDT by
ultraviolet light under laboratory conditions has been demonstrated
(Mosier et al., 1969; Plimmer et al., 1970; Miller & Narang, 1970;
Plimmer & Klingebiel, 1973; Crosby & Moilanen, 1977). Gab et al.
(1975, 1977) offered evidence that DDT and DDE are converted to carbon
dioxide and hydrochloric acid; the destruction was so complete that
they characterized it as photomineralization. In spite of very real
progress in understanding the fate of DDT in the environment (see
Annex), much more work will be required before a quantitative balance
can be measured between addition of the compound and its
disintegration.
5. ENVIRONMENTAL EXPOSURE LEVELS
5.1. Exposure of the General Population
5.1.1 DDT in air
In spite of the generalization in section 4.2 that DDT is rather
evenly mixed in the air, some increase in concentration may be noted
in connexion with the time and place of application. The highest
concentration of insecticide found in the air of communities with
anti-mosquito fogging programmes was 0.0085 mg/m3 (Tabor, 1966).
Concentrations one or two orders of magnitude greater have been
reported for several insecticides in the breathing zone of orchard
spraymen, and values of 1.2-0.26 mg/m3 have been found at distances
of 500-5000 m from ground spraying (Belonozko et al., 1967). In six
small communities in an agricultural area in the USA, DDT was found in
concentrations ranging from 1 × 10-6 to 22 × 10-6 mg/m3 (Tabor,
1966). Substantially higher values (0.002-0.05 mg/m3) were reported
for centres of population in the USSR (Belonozko & Kucak, 1974). In an
urban location in a generally nonagricultural area of the USA, the
highest concentration found was 2.36 × 10-6 mg/m3 (Antommaria et
al., 1965). The combined concentrations of DDT, dieldrin, and lindane
in the Munich area of the Federal Republic of Germany in 1971 were
even lower, only exceptionally rising as high as 1 × 10-6 mg/m3
(Weil et al., 1973).
With few exceptions, the highest average concentration of DDT in
air to which workers are exposed regularly (7.1 mg/m3) is that
associated with spraying the interior of houses (Wolfe et al., 1959).
However, concentrations ranging from 2 to 104 mg/m3 have been
reported in places where DDT dust was prepared and packed (Medved' et
al., 1975).
5.1.2 DDT in water
Under agricultural conditions, the concentration of DDT in water
may be high. For example, 0.01 mg/litre was found in the runoff from
melting snow from fields where sugar-beets had been grown (Medved' et
al., 1975).
The highest level at which DDT has been found in rainwater in an
urban area during a period of a month is 0.0004 mg/litre (Abbott et
al., 1965). The highest concentration reported in potable water
(0.02 mg/litre) occurred some years ago (Middleton & Lichtenberg,
1960). In a much more comprehensive study made a few years later, the
highest concentration of any insecticide was found (0.00012 mg/litre),
but this was dieldrin and not DDT (Weaver et al., 1965). Many samples
did not contain detectable insecticide of any kind. A study of surface
waters in the USA during the years 1964-1968 indicated that the
residues reached a peak in 1966 and then dropped sharply in 1967 and
1968, in spite of improved analytical methods. The highest value for a
DDT-related compound in those years was 0.00084 mg/litre (Lichtenberg
et al., 1970). By 1971, the concentration in the Federal Republic of
Germany was even lower, averaging 0.00001 mg/litre and never going as
high as 0.001 mg/litre (Weil et al., 1973). The average values for
total DDT in drinking water in Czechoslovakia were 0.000011 and
0.000015 mg/litre in 1972 and 1973, respectively (Hruska & Kociánová,
1975). DDT was not detected (<0.0000000166 mg/litre) in tap water in
a recent survey carried out in Ottawa, Canada (McNeil et al., 1977).
5.1.3 DDT in food
Residues of DDT were measured as early as 1945 (before the
compound was available commercially) on apples to which it had been
applied experimentally for the control of the coddling moth (Harman,
1946). Apparently, the earliest effort to learn how much DDT the
average man obtains from his daily food was that of Walker et al.
(1954), who reported that the amount of insecticide in restaurant
meals in Wenatchee, Washington, USA indicated an average intake of
0.184 and 0.102 mg/man per day for DDT and DDE, respectively. Soon,
other studies revealed similar levels of DDT intake for persons who
ate an ordinary range of foods but who lived in different parts of USA
(Hayes et al., 1956; Durham et al., 1965b). Most of the DDT was in
food of animal origin, and persons who abstained from eating meat but
obtained the food they ate from regular, commercial sources received
an average of only 0.041 and 0.027 mg/man per day of DDT and DDE,
respectively (Haynes et al., 1958). The difference did not depend,
however, on meat per se, for no DDT and only traces of DDE were
found in the meat and other products obtained from Arctic wildlife
that constituted much of the diet of Eskimos (Durham et al., 1961).
Following restrictions on the application of DDT to livestock, to
their barns, and to the forage crops on which they fed, there was a
gradual decrease in residues in animal products used as human foods.
Restrictions on the use of DDT on crops eaten directly by man resulted
in reduced residues in vegetable foods. Complete meals collected
mainly from the same restaurants in Wenatchee and analysed in the same
laboratory indicated that, by 1964, DDT intake was only 0.038 mg/man
per day (Durham et al., 1965b) compared with 0.184 mg/man per day
reported by Walker et al., 11 years earlier. Thus, intake had been
reduced to less than one-fourth. A further reduction to about one-
eighth of the 1953-54 values was indicated by the nationwide study by
the US Food and Drug Administration, usually called the Market Basket
Survey. Intakes of DDT indicated by this study for the succeeding
years 1965-70, were 0.031, 0.041, 0.026, 0.019, 0.016, and
0.015 mg/man per day, respectively (Duggan & Weatherwax, 1967; Duggan,
1968; Duggan & Lipscomb, 1969; Duggan & Corneliusen, 1972).
The widespread shipment of food in the USA tends to explain the
fact that food residues are generally similar in different parts of
the country. However, small differences do exist and may be accounted
for by the fact that, on average, meat is not shipped as far as
vegetables. Much of the feed for livestock is produced on the same
farm or at least in the same area in which the animals are raised.
Whatever the reason, the coefficients of correlation between latitude
and human intake were -0.63 and -0.59 for the sampling periods 1966-67
and 1967-68, respectively (Hayes, 1975).
Early studies (Swackhamer, 1965) indicated that both the frequency
and concentration of residues were slightly less in Canada than in the
USA. More recent studies in Canada similar to the Market Basket Survey
indicated total DDT dietary intakes of 0.018, 0.011, 0.011, 0.007 and
0.007 mg/man per day for the years 1969 to 1973, respectively (Smith,
1971; Smith et al., 1972, 1973, 1975).
The analysis of whole meals collected in south-east England during
1965 and 1966 gave results similar to those obtained during the same
period in the USA; the calculated daily intakes in England were 0.030
and 0.025 mg/man per day for DDT and DDE, respectively (McGill &
Robinson, 1968).
There may be considerable differences in intake in different parts
of the same country. Hruska & Kociánová (1975) reported that the
average intake of DDT plus DDE in 1972 was 0.002 mg/man per day in
southern Bohemia and was 0.099 mg/man per day in Slovakia. Striking
differences may exist between urban and rural areas (Almeida et al.,
1975).
Values for total DDT-related compounds in regular food in the USSR
are not available, but the separate values for DDT and DDE in daily
diets from different regions shown in Table 3 suggest that the total
may be higher than in the UK and USA and that the amount of DDE may
exceed the amount of DDT. Both high total values and an unusually high
proportion of DDE would be consistent with extremely high total values
a few years earlier and recent discontinuation of the use of DDT. In
fact, in the Soviet Union, DDT has been eliminated from the list of
pesticides recommended for use in agriculture since 1970. During the
period 1966-69, 0.8% of food samples contained residues as high as
5.1 mg/kg, and 4.2% contained residues over 1.0 mg/kg.
Although total intake of DDT from food has not been measured in
some parts of the world, worldwide measurements of storage of DDT and
its metabolites in human body fat indicate that the extremes of total
exposure have varied by a factor of about 10, but that total exposure
for most populations has varied by a factor of no more than 3 (see
Table 9).
Table 3. Residues of DDT and DDE in daily cooked diets from
different areas of the USSR during 1971/72a
Area No. of Range of residues of
daily
diets DDT DDE
examined (mg/diet)b (mg/diet)b
North 121 0.00006-0.0103 0.0001-0.0093
West 191 traces-0.094 traces-0.039
South-east 62 traces-0.15 traces-0.330
South I 184 0.02-0.260 0.020-0.5
South II 51 traces-0.052 traces-0.132
a From: Medved' et al. (1975).
b Equivalent to mg/man per day.
It is important to note that local practice may result in high
residues in the food of one or more families even though residues are
low in commercially produced food available in the same area. Thus
Durham et al. (1965b) reported that the average DDT intake from
household meals in Wenatchee was 0.314 mg/man per day at the same time
that restaurant meals in that town contributed only 0.038 mg/man per
day. The difference was largely the result of very high residues in
some eggs eaten by local families; the chickens foraged near orchards,
which had been treated with DDT. The restaurants used commercially
available eggs and not those produced locally. In a similar way,
practices peculiar to one country may account for high residues in
some of their food. For example, very high residues of DDT were
reported in some samples of staple foods in India (Sharangapani &
Pingale, 1954). Dale et al. (1965) suggested that the high levels of
DDT that they found in some Indians might be the result of direct
addition of the compound to staple food to prevent insect infestation,
even though the practice did not have government approval.
5.1.4 Miscellaneous sources
It has been suggested that there is a positive correlation between
the use of household insecticides and the concentration of DDT in
house dust, on the one hand, and the storage of DDT in people on the
other (Deichmann & Radomski, 1968; Radomski et al., 1968; Davies et
al., 1969b, 1975; Edmundson et al., 1970b). However, another study of
dust in 16 urban households, 4 farm households, and 8 households in
which at least one member was a pesticide formulator, failed to reveal
a statistically significant correlation between the levels of various
pesticides in dust and in the serum of people living in the homes.
There were striking individual examples of workers whose homes
contained high concentrations of the compounds they used
professionally and other examples in which there was circumstantial
evidence relating household dust residues to body burden (Starr et
al., 1974).
There can be no doubt that insecticides used in the household or
introduced on the clothing of workers are important sources of intake
of DDT in some instances. It is not clear whether the relevant
absorption involves mainly the inhalation of dust, the contamination
of food within the home, or even dermal absorption.
5.1.5 Relative importance of different sources
It has been estimated (Campbell et al., 1965) that over 90% of the
DDT stored in the general population is derived from food. About 1965,
intake in the USA was approximately 0.04 mg/man per day from food,
less than 0.000046 mg/man per day from water, less than 0.00006 mg/man
per day from urban air and less than 0.0005 mg/man per day from air in
small agricultural communities. The reason for the qualification "less
than" is that the intakes were calculated from the highest
concentrations reported in drinking-water and air because no average
values were established.
Other investigators (Durham et al., 1965b; Morgan & Roan, 1970;
Medved' et al., 1975) concluded independently that ordinary food is
the major source of DDT and related compounds for the general
population. Intake from ordinary food is a base to which other kinds
of intake -- including that from exceptional food -- may be added. An
example of such an addition -- eating eggs from chickens allowed to
run loose in DDT-treated orchards has already been discussed in
section 5.1.3.
5.2 Exposure of Infants and Young Children
Babies tend to be born with slightly lower blood levels of DDT
than are found in their mothers (O'Leary et al., 1970b; Schvartsman et
al., 1974, see also section 6.2.2). This simply indicates that the
placenta excludes some but not all of the DDT available to it. During
the first 10 or 15 years of life, DDT storage levels rise to the adult
population level (Hayes et al., 1958; Hunter et al., 1963; Wassermann
et al., 1965, 1967; Robinson et al., 1965; Davies et al., 1968, 1969a;
Watson et al., 1970).
It has been known for a long time that human milk may contain a
higher concentration of DDT than cow's milk in the same country (Egan
et al., 1965; Quinby et al., 1965b; Ritcey et al., 1972; Olszyna-
Marzys et al., 1973). So far, there is no evidence that this small
difference is of any significance for breast-fed compared with bottle-
fed babies. This is even true in places where the concentration of DDT
in human milk is comparatively high (see section 6.3.1.4).
On the average, the incidence and levels of residues in
commercially prepared food for babies in the USA are lower than those
in raw agricultural products, other processed foods, or samples
examined in the Market Basket Survey (Lipscomb, 1968).
The only DDT exposures of infants that are known to have injured
them in any way are those involving direct access to formulations that
they ate or drank. Such tragic accidents often involved formulations
transferred to unlabelled food or beverage containers. Frequently, the
container was left where a child could reach it easily. Occasionally,
formulations have been stored with food or have ever been handed to a
child as food, or "empty" containers that still contain enough
formulation to kill a child have been carelessly discarded (Hayes,
1975, 1976a).
5.3 Occupational Exposure
In general, annual exposure to DDT is greatest among manufacturers
and formulators, moderate, among those applying it for agricultural
purposes, less among the general population, and least among special
groups whose location or practices minimize their exposure. However,
for brief intervals the exposure during agricultural application may
exceed anything that good industrial practice permits. This
distinction is of great importance in connexion with the more toxic
organic phosphorus compounds, a single heavy exposure to which may
result in poisoning, but is of no known importance in connexion with
DDT, the acute toxicity of which is much less. However DDT has a
greater tendency to storage in the body.
Occupational exposure to DDT is reflected quantitatively by the
concentration of DDT and DDE in blood and fat and by the concentration
of DDA in urine. These aspects of occupational exposure are considered
later. This section outlines measurements that have been made of the
actual degree of exposure under different circumstances.
The most striking result is that the occupational exposure through
areas of skin that are frequently unclothed (face, hands, forearms,
neck, and "V" of chest) is far greater than total respiratory
exposure. Results for DDT are shown in Table 4 (based on measurements
made by methods described by Durham & Wolfe (1962, 1963)). If a
workman has bare feet or legs or does not wear a shirt, the contrast
between respiratory and dermal exposure will be even greater than that
shown in Table 4 which is based on "standard" clothing.
Table 4. Measured respiratory and dermal exposure of workers
to DDT under actual conditions of work
Activity Respiratory Dermal Reference
(mg/kg) (mg/kg)
Indoor house spraying 3.4a 1755 Wolfe et al., 1959
Outdoor house spraying 0.11 243 Wolfe et al., 1959
Spraying forests 4-92b 212b Wassermann
et al., 1960
a Measurement by respirator pad technique.
b Calculated from values given in the original paper.
It has been reported that DDT and a number of other pesticides
persist, for days or even years after last use, on the hands of
workers exposed to them and that at least a part of the material can
be removed for analysis by rinsing the hands in hexane. Evidence that
the residues represented unabsorbed pesticides and not excretion of
stored material included the fact that no correlation was found
between residues on the hands and in the sera of individuals and that
no residues could be found on the hands of a farmer who used
rubberized gloves while working with pesticides and who washed
afterwards with strong cleansing agents (Kazen et al., 1974). The fact
that urinary excretion of DDA may be increased for several days after
a single occupational exposure to DDT (Wolfe et al., 1970) is evidence
that absorption continues for a week or so but not for longer periods.
Furthermore, Wolfe et al. (1970) found that the rate of excretion,
even when detectable, was small compared to the excretion of parathion
metabolite in workers exposed simultaneously to DDT and parathion at a
ratio of 1.0 to 0.25. This emphasizes the minimal dermal absorption of
DDT.
6. METABOLISM OF DDT
6.1 Uptake
6.1.1 Uptake by inhalation
Most DDT dust is of such large particle size (>250 µm) that any
that is inhaled is deposited in the upper respiratory tract and is
eventually swallowed (Hayes, 1975). Toxicity data indicate that
respiratory exposure is of no special importance.
6.1.2 Uptake from the gastrointestinal tract
A review of the early literature indicates that absorption of DDT
from the gastrointestinal tract is slow. Whereas intravenous injection
at the rate of 50 mg/kg produces convulsions in rats in 20 min,
convulsions occur only after 2 h when DDT is administered orally at
two or more times the LD50 value. The onset of convulsions is
delayed for about 6 h when DDT is given to rats orally at
approximately the LD50 value (Dale et al., 1963).
Early studies based on toxicity indicated that DDT, dissolved in
animal or vegetable fats, was absorbed from the gastrointestinal tract
about 1.5 to 10 times more effectively than undissolved DDT. There was
also evidence that large doses of the compound in the gastrointestinal
tract were poorly absorbed from nonabsorbable solvent (Hayes, 1959).
However, in connexion with small repeated doses, the presence or kind
of solvent made little difference; apparently the occurrence of bile
in the intestine and the presence of some fat in the diet were
sufficient to promote absorption of the compound. At high dosage
levels, less 14C-DDT was absorbed and stored in organs and a higher
proportion was excreted in the faeces following oral administration
than after intraperitoneal administration (40% versus 0.9%) (Bishara
et al., 1972b).
Rothe et al. (1957) reported that after giving radioactive DDT to
rats by stomach tube, 41-57% of it was recorded in lymph drained from
the animal by means of a cannula. Less than 0.1% of the activity was
found in the urine, 7.4-37.1% was found in the faeces or in the
intestinal contents when the animals were killed, and 19%-67% of the
activity was found in the carcass. The total dose accounted for
analytically varied from 89% to 118%, thus recovery was complete
within the accuracy of the method. Of the administered DDT not found
in faeces and intestinal contents, 47%-65% was found in the lymph. The
animals that withstood the operation best had peak lymph flows of
nearly 6 ml/h. In these animals, DDT was absorbed at rates as high as
381 µg/h; the rate of absorption reached a maximum within 2-3 h of
intubation and was markedly reduced by the fourth hour. Fifty per cent
of the DDT-derived material found in the lymph was absorbed in the
first 2.5-7 h, and 95% was absorbed within 18 h. Because the lymphatic
duct in the rat is not a single vessel, Rothe et al. (1957) were
unable to exclude the possibility that some, or all, of the DDT that
they later recovered from the carcasses of their animals had been
transported to the general circulation by collateral lymph vessels
rather than by the hepatoportal system. Thus, at least half, and
perhaps all absorption of DDT is by way of the lymph. However, in
studies on rats by Heath & Vandekor (1964), only a small proportion of
dieldrin was absorbed by the lymph. The reason for the marked
difference in the absorption of those organochlorine insecticides is
unknown.
6.1.3 Uptake from the skin
Undissolved DDT is so poorly absorbed through the skin that its
toxicity by this route is difficult to measure. Even dissolved DDT is
poorly absorbed by the skin as indicated by low toxicity (see
Table 5).
Table 5. Acute oral and dermal LD50 of DDT for animalsa
Species Formulation Oral Dermal
(mg/kg) (mg/kg)
Rat Water suspension or powder 500-2500 1 000 000
Oil solution 113-450 250-3000
Mouse Water suspension or powder 300-1600 375 000
Oil solution 100-800 250-500
Guineapig Water suspension or powder 2000 1 500 000
Oil solution 250-560 1000
Rabbit Water suspension or powder 275 375 000
Oil solution 300-1770 300-2820
Cat Water suspension or powder
Oil solution 100-410
Dog Water suspension or powder
Oil solution >300
a From: Hayes (1959).
6.2 Distribution and Storage
6.2.1 Human studies
6.2.1.1 Studies of volunteers
In a study of volunteers who received technical DDT at rates of 0,
3.5, and 35 mg/man per day, the average intakes resulting from dosing
and from traces of DDT in food were 0.0025, 0.05, and 0.5 mg/kg per
day (Hayes et al., 1956). The storage of DDT was proportional to
dosage, but there was an unexplained difference in the storage of the
p,p'-isomer and of technical DDT. For example, following dosing for
12 months, pure p,p'-DDT was stored in fat at an average
concentration of 340 mg/kg, but the sum of isomers from technical
material was stored at an average of only 234 mg/kg. The difference
was statistically significant for the 3.5 mg/man per day dosages given
for 3-6 and for 7-18 months. The difference was significant for the
35 mg/man per day doses after 7-18 months of dosing but not after only
3-6 months.
Men who ate p,p'-DDT showed a definite increase in the absolute
amount of DDE stored. After 6 months at a dosage of 35 mg/man per day,
8 men showed an average concentration of DDE stored in fat of 33 mg/kg
compared with 12 mg/kg for the same individuals at the beginning of
the investigation. There was a further increase in DDE storage as
exposure progressed. However, DDT was stored in so much greater
concentration that the relative storage of DDE decreased sharply.
Thus, after 6 months at a dosage of 35 mg/man per day, 8 men stored
only 14% of their total DDT-derived material in the form of DDE
compared with 65% for the same person at the beginning of the
investigation.
The storage of DDE by men who ate technical DDT presented a
different picture. Until 18 months after exposure, there was no clear
evidence that these men stored any more DDE after exposure than they
did before. However, at 18 months, the only 3 samples available showed
DDE concentrations ranging from 28 to 85 mg/kg, all substantially
above general population levels. Thus, both the total amount stored
and the rate at which DDT converted to DDE served to distinguish the
metabolism of p,p'-DDT and the sum of isomers present in technical
DDT in man (Hayes et al., 1956). A more rapid excretion was
demonstrated for o,p'-DDT by Morgan & Roan (1972).
In a second study (Hayes et al., 1971), volunteers received the
same doses used in the first study. Again, storage of DDT was
proportional to dosage. Although, in this instance also, the storage
of technical DDT was less than that of p,p'-DDT, the difference was
not statistically significant. The real but very gradual accumulation
of DDE was confirmed.
A steady state of storage was approached later in the second study
(18.8-21.5 months) than in the earlier one (about 12 months). However,
although the second study was superior in that more men were studied
for a longer period, it was inferior in that dosage was less regular.
Because of this, it seems impossible to decide whether 12 months or
21.5 months is a more valid estimate of the time necessary for people
to approach a steady state of storage when intake is uninterrupted and
unvarying in amount. It is interesting that the storage levels
eventually reached at the same dosage in the 2 studies were
statistically indistinguishable in most instances (see Table 6). In
the one instance in which a statistical difference existed, the
greater storage by men in the second study may have been explained by
the fact that some of them inadvertently received higher doses than
intended.
Table 6. Storage of DDT in volunteers
Type of DDT Added Concentration of DDTa Significance
dosage First studyb Second studyc of difference
(mg/man 11 months or more 21.5 months (P)
per day) (mg/kg) (mg/kg)
Technical 0 8-17 (12.5 ± 4.5) 16-30 (22.0 ± 2.9) > 0.1
3.5 26-33 (23.8 ± 1.4) 59-76 (50.2 ± 5.6) <0.025
35 101-367 (234 ± 21.4) 105-619 (281 ± 79.5) >0.4
Recrystallized 35 216-466 (340 ± 36.4) 129-659 (325 ± 62.2) >0.2
a Range, mean, and standard error.
b Hayes et al., 1956,
c Hayes et al., 1971.
There was a slow decrease in the levels of fat-stored DDT after
dosing ceased. The concentration remaining following 25.5 months of
recovery was from 32% to 35% of the maximum stored for those who had
received 35 mg/man per day but 66% for those who had received only
3.5 mg/man per day, indicating slower loss at lower storage levels
(Hayes et al., 1971).
Morgan & Roan (1971) fed volunteers not only technical DDT but
also p,p'-DDE and p,p'-TDE. They found that DDE was stored more
tenaciously than the other compounds in man, the order being p,p'-DDE
> p,p'-DDT > o,p'-DDT > p,p'-TDE. The slow metabolism of DDT to
DDE was confirmed. It was noted that p,p'-DDT was lost from storage
in adipose tissue much more slowly in man than in the monkey, dog, or
rat.
Less than 18% of p,p'-DDT and p,p'-DDE is carried in human
erythrocytes. In plasma of ordinary fat content, less than 1% of all
DDT-related compounds is carried by the chylomicrons. Instead, these
compounds are carried by proteins and are undetectable in plasma from
which protein has been precipitated. Following ultracentrifuging,
p,p'-DDT and p,p'-DDE are found mainly in the triglyceride-rich,
low density, and very low density lipoproteins. Following continuous
electrophoresis, these compounds are found mainly in association with
plasma albumin and alpha-globulins (Morgan et al., 1972).
6.2.1.2 Studies of occupationally exposed workers
The highest reported storage of DDT and related compounds remains
that of a healthy worker whose fat contained DDT and DDE (as DDT) at
concentrations of 648 and 483 mg/kg, respectively (Hayes et al.,
1956). Laws et al. (1967) reported considerably lower storage values
among the most exposed persons in a DDT manufacturing plant (see
Table 7).
An important point evident from the table is that, whereas almost
all investigations of workers are said to have been carried out on
"heavily exposed" populations, some of the groups studied had absorbed
little more DDT than is absorbed by the general population --
especially the general population of some tropical countries.
A different situation is indicated in a report by Genina et al.
(1969) who used a total chloride method to analyse samples of blood
from controls and from persons with occupational exposure to DDT,
polychloropinene, and HCH. Whereas the highest average concentration
of total DDT-related material per se in the serum of a worker in the
USA was 2.7 mg/litre (Laws et al., 1967), Genina et al. (1969)
reported organochlorine compounds as high as 38.4 mg/litre in the
blood of a pilot and values as high as 195 mg/litre in the blood of
warehousemen. This concentration is about 20 times the highest value
found by the same authors in their control group (see Table 8). The
factor of 20 is not remarkable, but (especially in view of the fact
that polychloropinene and HCH are excreted more readily than DDT and
DDE) values as high as 0.2 mg/litre in the controls are unexpected.
Table 7. Average concentration of the sum of the isomers of DDT and DDE in fat and serum
and of DDA in the urine of workers engaged in the manufacture, formulation, or
use of DDT
Tissue No. of DDT DDE DDA Total as Estimated Reference
men (mg/kg) (mg/kg) (mg/kg) DDT exposure
(mg/kg) (mg/man
per day)
fat 1 648 437 1131 Hayes et al., 1956
urine 10 0.57 14 Ortelee, 1958
urine 16 1.7 30 Ortelee, 1958
urine 13 2.9 42 Ortelee, 1958
fat 3 51 44 98 3.6 Laws et al., 1967
fat 12 74 50 130 6.2 Laws et al., 1967
fat 20 161 91 263 18 Laws et al., 1967
serum 3 0.2113 0.1968 0.5412 6.3 Laws et al., 1967
serum 12 0.1420 0.1454 0.3584 8.4 Laws et al., 1967
serum 20 0.3020 0.2719 0.7371 17.5 Laws et al., 1967
urine 3 0.0165 0.0203 0.41 0.5629 Laws et al., 1967
urine 12 0.0145 0.0222 0.6 0.7911 Laws et al., 1967
urine 20 0.0145 0.0271 1.27 1.6296 Laws et al., 1967
fat 18 5.2-45.2 Gracheva, 1969
urine 136 0.402 Perini & Ghezzo, 1970
urine 110 0.142 Perini & Ghezzo, 1970
urine 290a 0.061 Perini & Ghezzo, 1970
plasma 16 0.0513 0.0722 0.1321 Wassermann et al., 1970c
serum 4 <0.087 <0.072 Edmundson et al., 1970a
urine 0.080 Edmundson et al., 1970a
serum 18 0.573 0.506 Poland et al., 1970
serum 5 0.004a 0.021a Clifford & Well, 1972
serum 10 0.022 0.055 Clifford & Well, 1972
serum 21 0.021a 0.013a Keil et al., 1972
blood 44 0.761 WHO, 1973
blood 100 1.273 WHO, 1973
Table 7 (Cont'd)
Tissue No. of DDT DDE DDA Total as Estimated Reference
men (mg/kg) (mg/kg) (mg/kg) DDT exposure
(mg/kg) (mg/man
per day)
serum 21 0.300 0.379 0.681 Almeida et al., 1974
serum 25 0.225 0.308 0.504 Almeida et al., 1974
serum 18 0.345 0.257 0.602 Almeida et al., 1974
serum 56 0.004a,b 0.052a,b Morgan & Roan, 1974
serum 32 0.002b 0.026b Morgan & Roan, 1974
serum 32 0.004b 0.047b Morgan & Roan, 1974
serum 32 0.009b 0.075b Morgan & Roan, 1974
serum 31 0.052b 0.222b Morgan & Roan, 1974
plasma 25 1.030 Rabello et al., 1975
plasma 8 0.240 Rabello et al., 1975
plasma 23 0.0389 Richardson et al., 1975
a Control group.
b Approximately equal groups arranged by degree of storage.
Table 8. Percentage of workers with blood organochlorine compound content falling in certain
ranges of concentrationa
Subject group Range of concentrations
0 0.2-0.9 1.0-3.0 4-9 10-50 50
(mg/litre) (mg/litre) (mg/litre) (mg/litre) (mg/litre) (mg/litre)
Control group (47) 21.3 44.7 23.4 10.6 0 0
Pilots (134)
group A 19.1 35.3 25.0 14.7 5.9 0
group B 22.9 27.1 40.6 7.4 2.0 0
Technicians (133)
group A 26.2 16.4 39.3 8.2 9.9 0
group B 39.4 31.2 25.7 3.7 0 0
Agricultural 13.0 43.5 31.0 7.0 2.0 3.5
workers (55)
a From: Genina et al. (1969).
NB: Group A gives the results of investigations at the time of work, group B before work
or a few months after termination. Agricultural workers were studied only during work.
The first evidence that a part of the DDT absorbed by man is
metabolized to DDE was obtained from the analysis of fat from a DDT
plant worker (Mattson et al., 1953).
6.2.1.3 Studies of the general population
DDT in fat. Table 9 summarizes the results of measurements of
DDT and related materials in the body fat of people without
occupational exposure. Several broad generalizations can be made from
the table and from what is known about residues of DDT in food in
different countries. DDT storage in man corresponds with exposure; in
general, exposure tends to be greater in warm climates where there is
a greater need for insecticides. This climatic dependence may be
observed even within a single country (Hayes, 1975). Where
measurements have been made during a sufficiently long period, storage
has decreased as the use of DDT, especially that leading to residues
in food, has decreased.
The method of DDT analysis shifted in about 1962 from the
Schecter-Haller colorimetric method to the gas chromatographic method.
However, as noted by Hayes (1975), this made little difference to the
overall results. Thus the absolute decrease of total DDT storage and
the relative increase of DDE storage observed in some countries is
real.
Circumstances peculiar to some subpopulations explain their
unusual storage of DDT. Thus, persons living in one of the contiguous
states of the USA stored significantly less DDT than other persons in
the state because they did not eat meat (Hayes et al., 1958). Even
less storage of DDT was observed in Eskimos whose diet contained an
unusually high proportion of meat obtained from wildlife in which no
DDT and almost no DDE could be detected (Durham et al., 1961).
No significant difference was found in the concentration of DDT
stored in the fat of different parts of the body (Hayes et al., 1958;
Casarett et al., 1968).
DDE in fat. The accumulation of DDE relative to total DDT-related
compounds is best illustrated in man. Of the total DDT stored
in the fat of workers exposed to technical DDT (about 4% DDE) for
11-19 years, only 38% was in the form of DDE, and, of course, some of
that DDE came from their diets which included meat (Laws et al.,
1967). In India, where many people avoid meat but may consume milk,
cheese, and eggs, 34-41% of total DDT was DDE (Dale et al., 1965). In
the USA, during a time when DDT residues in food were decreasing, the
proportion of total DDT in the form of DDE increased from about 60% in
1955 to about 80% in 1970; during the same interval the concentration
of total DDT in body fat decreased from about 15 mg/kg to slightly
Table 9. Concentration of DDT-derived material in body fat of the general population
Country Year No. of Method of DDTa DDE as Total as DDE as Reference
samples analysis (mg/kg) DDT DDT DDT
(mg/kg) (mg/kg) (% of
total)
North America
Canada 1959-1960 62 colour 1.6 3.3 4.9 67 Read & McKinley, 1961
Canada 1966 47 GLCb and ELC 1.09 2.96 4.39 67 Brown, 1967
Canada 1967-1968 51 GLC 1.56 4.16 5.86 71 Kadis et al., 1970
Canada 1969 4.85 Ritcey et al., 1973
Canada GLC 5.83 Brown & Chow, 1975
USA 1942 10 colour NDc NDc NDc Hayes et al., 1958
USA 1950 75 colour 5.3 -- 5.3 -- Laug et al., 1951
USA 1955 49 colour 7.4 12.5 19.9 63 Hayes et al., 1956
USA 1954-1956 61 colour 4.9 6.8 11.7 58 Hayes et al., 1958
USA 1956 36 colour 5.5 10.1 15.6 65 Hayes et al.. 1971
USA 1961-1962 130 colour 4.0 8.7 12.7 69 Quinby et al., 1965a
USA 1961-1962 28d GLC 2.4 4.3 6.7 64 Dale & Quinby, 1963
USA 1962-1963 282 GLC 2.9 8.2 11.1 74 Hoffman et al., 1964
USA 1964 64 GLC 2.5 5.1 7.6 67 Zavon et al., 1965
USA 1964 25 GLC 2.3 8.0 10.3 77 Hayes et al., 1965
USA 1964-1965 18 GLC 9.0 Schafer & Campbell, 1966
USA 1964-1965 42 GLC 3.1 7.5 10.6 71 Radomski et al., 1968
USA 1962-1966 994 GLC 2.6 7.8 10.4 75 Hoffman et al., 1967
USA 1964-1965 12 GLC 3.79 7.7 11.5 67 Davies et al.. 1965
USA 1965-1967 17 GLC 3.1 5.5 56 Davies et al., 1968
USA 90 GLC 6.1 8.4 73 Davies et al., 1968
USA 17 GLC 4.6 7.8 59 Davies et al., 1968
USA 35 GLC 12.0 16.7 72 Davies et al., 1968
USA -- 42 GLC 3.13e 7.43 10.56 70 Fiserova-Bergerova et
al., 1967
Table 9 (Cont'd)
Country Year No. of Method of DDTa DDE as Total as DDE as Reference
samples analysis (mg/kg) DDT DDT DDT
(mg/kg) (mg/kg) (% of
total)
USA -- 30e GLC 1.33 5.17 6.51 79 Casarett et al., 1968
USA 29f GLC 1.35 4.91 6.31 78 Casarett et al., 1968
USA 30g GLC 1.16 4.99 6.17 81 Casarett et al., 1968
USA 1966-1968 70 GLC 1.54 5.15 6.69 77 Morgan & Roan, 1970
USA 1967 733 GLC 1.34 4.74 6.22 77 Yobs, 1969 (unpublished
data)
USA 1968 3104 GLC 1.56 5.96 7.67 77 Yobs, 1969 (unpublished
data)
USA 1967-1971 103 GLC 1.5 5.6 7.1 79 Warnick, 1972
USA 1970 200 GLC 1.9 8.0 9.9 81 Wyllie et al., 1972
USA 1969-1972 221 23.18 Burns, 1974
USA 1970 1412 7.87h,i Kutz et al., 1974
South America
Argentina 1967 37 GLC 5.5 6.5 13.2 -- Wassermann et al., 1968b
Brazil 1969-1970 38 GLC 1.4 2.7 4.1 Wassermann et al., 1972b
Venezuela 1964 38 GLC 2.9 7.4 10.3 72 Dale, 1971 (unpublished
data)
Europe
Austria 6.33 Pesendorfer et al., 1973
Belgium 20 GLC 1.2 2.1 3.3 64 Maes & Heyndrickx, 1966
Bulgaria 55 GLC 3.8 5.8 10.6n 57 Kaloyanova et al., 1972
Bulgaria 1971-1976 191 GLC 14.7 78 Rizov, 1977
Czechoslovakia 1963-1964 229 colour 5.5 4.1 9.6 43 Halacka et al., 1965
Denmark 1965 18 GLC 0.6 2.7 3.3 82 Weihe, 1966
Denmark 1972-1973 78 GLC 4.1 4.7 87 Kraul & Karloq, 1976
Table 9 (Cont'd)
Country Year No. of Method of DDTa DDE as Total as DDE as Reference
samples analysis (mg/kg) DDT DDT DDT
(mg/kg) (mg/kg) (% of
total)
Finland 1972-1974 73 GLC 2.5 Hattula et al., 1976
France 1961 10 colour 1.7 3.5 5.2 67 Hayes et al., 1963
German Democratic 1966-1967 100 GLC and TLC 3.7 9.47 13.1 71 Engst et al., 1967
Republic
German Democratic 34j 1.8 5.1 6.9 Engst et al., 1970
Republic
Germany, Federal 1958-1959 60 colour 1.0 1.3 2.3 57 Maier-Bode, 1960
Republic of
Germany, Federal 1970 20 GLC 1.1 2.5 3.6 69 Acker & Schulte, 1970
Republic of
Germany, Federal 9.8 Acker & Schulte, 1971
Republic of
Germany, Federal 4.24 Acker & Schulte, 1974
Republic of
Germany, Federal 4.77 Acker & Schulte, 1974
Republic of
Germany, Federal 5.42 Acker & Schulte, 1974
Republic of
Germany, Federal 8.36 Acker & Schulte, 1974
Republic of
Germany, Federal 7.8 Acker & Schulte, 1974
Republic of
Hungary 1960 48 colour 5.7 6.0 12.4 48 Denés, 1962
Italy 1965 9 GLC 1.8 3.2 5.0 63 Kanitz & Castello, 1966
Italy 1965-1966 18 GLC 2.58 8.28 10.86 76 Paccagnella et al., 1967
Italy 1966 22 GLC and TLC 4.69 10.69 15.48 68 Del Vecchio & Leoni, 1967
Italy 1970? 31 GLC 3.38 13.37 16.75 80 Prati & Del Dot, 1971
Italy 1970? 52 GLC 2.14a 8.46a 10.60a 80 Prati et al., 1972
Italy 1970? 33 GLC 0.84a 6.64a 7.48a 89 Prati et al., 1972
Table 9 (Cont'd)
Country Year No. of Method of DDTa DDE as Total as DDE as Reference
samples analysis (mg/kg) DDT DDT DDT
(mg/kg) (mg/kg) (% of
total)
Netherlands 1964 20 colour 1.6 6.1 7.7 79 Wit, 1964
Netherlands -- 11 GLC 0.32 1.89 2.22 86 deVleiger et al., 1968
Norway 56 GLC 3.2 71 Bjerk, 1972
Poland 1965 72 colour 13.4 Bronisz st al., 1967
Poland 65 colour 23.5 62 Bronisz et al.. 1969
Poland 1970 70 GLC 11.4 Juszkiewicz & Stec, 1971
Poland 1972 15 GLC 5.23 68 Bojanowska et al., 1973
Romania 1965 137 -- 13.4 8.3 21.7 39 Aizicovici et al., 1968
Romania 1972-1973 GLC 0.68 1.73 2.41 71 Ciupe, 1976
Spain 1966 41 -- 6.5 9.2 15.7 59 Unpublished data cited
by Wassermann et al., 1968
Switzerland 1.9-16.3 Zimmerli & Marek, 1973
United Kingdom 1961-1962 131 colour -- -- 2.2 -- Hunter et al., 1963
United Kingdom 1963-1964 66 GLC 1.1 2.2 3.3h 67 Egan et al., 1965
United Kingdom 1964 100 GLC -- -- 3.9h -- Robinson et al., 1965
United Kingdom 1964 44 GLC -- -- 4.0h -- Robinson & Hunter, 1966
United Kingdom 1965 101 GLC 1.13 1.72 2.85 60 Cassidy et al.. 1967
United Kingdom 1965-1967 248 GLC 0.78 2.22 3.00 74 Abbott et al., 1968
United Kingdom 1969-1971 201 GLC 0.5 1.8 2.5 72 Abbott et al., 1972
USSR 41 TLC 4.33 3.73 Vas'kovskaja & Komarova.
1967
USSR 197 TLC 5.3- 3.2- Vas'kovskaja, 1969
7.57 7.1
Africa
Kenya 5.4 Wassermann et al., 1972a
Nigeria 1967 43 GLC 5.4 3.1 8.8 Wassermann et al., 1968b
Nigeria 1969 41 GLC 2.1 2.8 6.5 57 Wassermann et al., 1972d
Table 9 (Cont'd)
Country Year No. of Method of DDTa DDE as Total as DDE as Reference
samples analysis (mg/kg) DDT DDT DDT
(mg/kg) (mg/kg) (% of
total)
Africa
South Africa 5.94 43 Wassermann et al., 1970b
South Africa 7.16 Wassermann et al., 1970b
Uganda 2.9 Wassermann et al., 1974a
Asia
India (Delhi area, 1964 67 colour 17 10 26 39 Dale et al., 1965
civilian)
India (other 1964 16 colour 8 5 13 37 Dale et al., 1965
cities, military)
India 94 GLC 13.8h 42 Ramachandran et al., 1974
Israel 1963-1964 254 colour 8.5 10.7 19.2 56 Wassermann et al., 1965
Israel 1965-1966 71 colour 4.6 Wassermann et al., 1967
Israel 1965-1966 133 colour 8.2 Wassermann et al., 1967
Israel 1967-1971 63 GLC 3.0 10.6 14.4 74 Wassermann et al., 1974b
Japan 1968-1969 241 GLC 0.6 1.8 2.4 75 Curlay et al., 1973
Japan 1969-1970 74 6.92 Nishimoto et al., 1970
Japan 1970 4.499 Suzuki et al., 1973
Japan 1971 2.694 Suzuki et al., 1973
Japan 1971 30 12.895 Kasai st al., 1972
Japan 1972 4.001 Suzuki et al., 1973
Japan 1972 42 5.992 Kasai et al., 1972
Japan 1973 6.44 Kawanishi et al., 1973
Japan 1974 6.87 Inoue et al., 1974
Pakistan 60 25.0 Mughal & Rahman, 1973
Thailand 1969-1970 77 12.6 Wassermann et al., 1972c
Oceania
Australia 1965 53 GLC 0.77h 1.03h 1.81h 57 Bick, 1967
Australia 1965-1966 46 colour 3.6 6.6 10.2 64 Wassermann et al., 1968a
Australia 1965-1966 12 GLC 3.0 7.1 10.5 68 Wassermann et al., 1968a
Australia 1971 75 GLC 4.94 Brady & Slyali, 1972
Table 9 (Cont'd)
Country Year No. of Method of DDTa DDE as Total as DDE as Reference
samples analysis (mg/kg) DDT DDT DDT
(mg/kg) (mg/kg) (% of
total)
Oceania
New Zealand 1966 52 GLC 1.6 4.2 5.8 72 Brewerton & McGrath, 1967
New Zealand 1965-1969 254 GLC 3.5 11.0 14.6 75 Copplestone et al., 1973
a p,p'-DDT and o,p'-DDT only. Total as DDT includes TDE (DDD) and other forms when given, which are not shown in this table.
b Gas-liquid chromatography.
c Not detected,
d These 28 samples were also tested for DDT and DDE content by a colorimetric method, and the results are included in the
130 samples listed above.
e Perirenal fat.
f Mesenteric fat.
g Panniculus fat.
h Geometric mean.
i Lipid basis.
j Infants and children.
less than 10 mg/kg (see Table 9). Thus, a low proportion of DDE
indicates a relatively high intake of DDT, a relatively low intake of
DDE residues (e.g. in food), and relatively few years for the
metabolism of stored DDT to DDE.
DDT in blood. The finding of DDT and related compounds in blood
(usually serum) of the general population of different countries is
shown in Table 10. Compared to body fat, blood has been analysed for
DDT in fewer countries and for fewer years. Some of the same
differences between populations and time periods observed in connexion
with DDT in fat have been detected in connexion with DDT in blood, but
the differences are less marked.
There is a small but statistically significant decrease in the
concentrations of DDT-related compounds in the plasma of women 1-6
days postpartum compared with the same women early in pregnancy. Most
of the decrease seems to occur during about the last 10 days before
delivery (Curley & Kimbrough, 1969). In a similar way, the
concentration of DDT-related compounds in various tissues of women at
the time of Caesarean section or normal delivery is less than in
nonpregnant women in the same community (Polishuk et al., 1970).
The concentration of p,p'-DDE is remarkably constant throughout
the day, but minor increases in it and in p,p'-DDT may occur after a
meal (Radamski et al., 1971a).
In so far as can be judged from the bar graphs presented by
Griffith & Blanke (1975), the concentrations of DDT-related materials
they found in postmortem blood were similar to the concentrations
reported in Table 10 for fresh blood except that postmortem blood
contained more TDE (DDD).
DDT and related compounds in other tissues. DDT has been found
in some samples of all human organs. Results reported in the USA and
USSR are shown in Tables 11 and 12, respectively. Results in Table 11
are based on the wet weight of the tissues. Results in Table 12,
presumably, are based on the weight of extractable lipid, but the
paper does not specify this; whatever the basis, the paper reports
concentrations of DDT and DDE in the range of 10.0-35.0 mg/kg in the
heart muscle, liver, and kidneys of 2 persons who had had direct
contact with pesticides. Although concentrations of chlorinated
compounds in liver, kidney, brain, and spleen of persons from Ferrara
Province, Italy, were generally higher than those in the USA (Prati et
al., 1972), the differences were not great.
Table 10. Concentration mg/litre of DDT-related compounds in the blood of members of the general population
Country Year No. of p,p'-DDT o,p'-DDT p,p'-DDE o,p'-DDE p,p- o,p- Total DOT DDE Reference
samples TDE TDE equivalent as %
(DDD) (DDD) total
North America
Canada 0.032 Brown & Chow, 1975
USA (Atlanta) 1965 10 0.0119 0.0013 0.0257 0.0418 68.6 Dale et al., 1966b
USA (Atlanta) 1966 10m 0.0746 Dale et al., 1967
USA (Atlanta) 1966 10f 0.0360 Dale et al., 1967
USA (Louisiana) 1966-1967 53a 0.00182 0.00182 0.00265 0.00265 0.00062 0.00062 0.00501 59.0 Selby et al., 1969
USA (4 states) 1967 64 0.00335 0.00044 0.00837 0.00096 0.00032 0.00032 0.01425 78.0 Yobs, 1969
(unpublished data)
USA (3 states) 1968 106 0.00342 0.00006 0.00927 0.00000 0.00004 0.00009 0.01397 73.4 Yobs, 1969
(unpublished data)
USA (Idaho) 1967-1968 1000 0.0047 0.0220 0.0002 0.02940 83.5 Watson et al., 1970
USA (Atlanta) 1969 30b,c 0.0046 0.0011 0.0062 0.0003 0.0014 0.0144 50.0 Curley et al., 1969
USA 1968 5d 0.0050 Curley & Kimbrough, 1969
USA 1968 10d 0.0205 Curley & Kimbrough, 1969
USA (Florida) 45e 0.0108 O'Leary et al., 1970b
USA (Florida) 107g 0.0152 O'Leary et al., 1970b
USA(Florida) 1970 26 0.03169 Radomski et al., 1971a
South America
Argentina 1970 20h 0.01934 Radomski et al., 1971b
18i 0.01327 Radomski et al., 1971b
19j 0.00869 Radomski et al., 1971b
Brazil 1973? 15f 0.0189 0.0237 0.0453 Schvartsman et al., 1974
Brazil 1973? 15c 0.0118 0.0104 0.0234 Schvartsman et al., 1974
Brazil 1974? 30 0.145 0.026 0.155 0.336 Almeida et al., 1975
Brazil 1974? 11 0.083 0.117 0.194 Almeida et al., 1975
Brazil 1974? 20 0.086 0.121 0.212 Almeida et al., 1975
Table 10 (Cont'd)
Country Year No. of p,p'-DDT o,p'-DDT p,p'-DDE o,p'-DDE p,p- o,p- Total DOT DDE Reference
samples TDE TDE equivalent as %
(DDD) (DDD) total
Europe
Bulgaria 1971-1976 171 0.039a Rizov, 1977
Hungary 1967-1968 120 0.034 Czeglédi-Janko, 1969
Poland 0.172d Jonczyk, 1970
Poland 1972 15 0.030 63 Bojanowska et al., 1973
Switzerland 13 0.0209 Zimmerli & Marek, 1973
Asia
Israel 1975 29 0.0133 0.0112 0.0195 0.0112 0.0087 0.0072 0.0740 47 Polishuk et al., 1977
Japan 1970 10 0.011 Tokutsu et al., 1970
Japan 1971 0.005 Kojima et al., 1971
Japan 1971 138 0.0183 Kasai et al., 1972
Japan 1971 0.0093 Yamagishi et al., 1972
Japan 1971 0.0285 Kaku, 1973
Japan 1972 0.001-0.078 Study Group, 1972
Japan 37 0.0437d Nawa, 1973
Japank 0.1358 Hara et al., 1973
Japanc 0.0210 Hara et al., 1973
0.0779 Abe et al., 1974
Oceania
Australia 52 0.0172 Slyali, 1972
Australia 47 0.0169 Ouw & Shandar, 1974
a Geometric mean. d Maximal value. g Black women. j 1-5 years old.
b Mean of positive values only. e White women. h Adults. k Maternal blood.
c Cord blood from term infants. f Female. i 6-11 years old. m Mile.
Table 11. Average concentrationsb of organochlorine insecticides in various tissues from autopsies
of 44 members of the general populationa
Tissue No. Lipid DDT DDE TDE Heptachlor Dieldrin Total + SEc
analysed content (mg/kg) (mg/kg) (DDD) epoxide (mg/kg) (mg/kg)
(%) (mg/kg) (mg/kg)
Perirenal fat 30 55.7 1.33 4.64 0.0110 0.0220 0.0300 6.03 ± 5.30
Mesenteric fat 29 54.2 1.35 4.40 0.0470 0.0320 0.0630 5.89 ± 4.98
Panniculus fat 30 60.6 1.16 4.48 0.0180 0.0270 0.0270 5.71 ± 5.25
Bone marrow 19 20.6 0.411 2.08 0.0760 0.0040 0.620 2.63 ± 2.21
Lymph noded 11 8.6 0.892 1.38 0.0100 0.0001 0.0190 2.30 ± 4.52
Adrenal 18 10.5 0.125 0.875 0.0570 0.0012 0.0060 1.06 ± 1.31
Kidney 38 3.2 0.0827 0.209 0.0022 0.0009 0.0056 0.300 ± 0.651
Liver 42 2.1 0.0467 0.200 0.0326 0.0019 0.0037 0.285 ± 0.369
Brain 32 7.9 0.0105 0.0831 0.0020 0.0002 0.0031 0.989 ± 0.171
Gonad 36 1.3 0.0150 0.0688 0.0015 0.0001 0.0021 0.0875 ± 0.103
Lung 25 0.7 0.0147 0.0585 0.0009 0.0003 0.0022 0.0766 ± 0.125
Spleen 27 0.6 0.0112 0.0305 0.0031 trace 0.0021 0.0469 ± 0.074
a From: Cassarett et al. (1968).
b Wet tissue basis.
c SE = standard error of the mean.
d Tracheobronchial lymph nodes.
Table 12. Average DDT and DDE contents in certain human organsa
Organ Content % of positive DDT DDE
limits observations (mg/kg) (mg/kg)
Heart muscle 1.0-12.0 77.0 3.69 4.05
Liver 1.0-20.0 85.5 4.62 4.02
Kidney 1.0-12.0 71.0 2.99 3.44
Spleen 2.0-20.0 57.0 2.86 2.86
Pancreas 2.0-15.0 75.0 3.0 2.62
Adrenal 2.0-10.0 84.3 3.84 3.7
Thyroid 2.0-8.0 59.0 1.85 1.85
a From: Vas'kovskaja (1969).
DDT and related compounds in the tissues of infants. The
transfer of DDT to the fetus was observed by Deniés (1962) and
confirmed by many others (Curley et al., 1969; Zavon et al., 1969;
Komarova, 1970; O'Leary et al., 1970a,b,c). Typical findings are shown
in Table 13. It appears that the placenta is partially protective; the
concentrations of DDT and DDE are lower in cord blood than in
corresponding maternal blood (O'Leary et al., 1970b; Schvartsman et
al., 1974). The report by Engst et al. (1970) that infants lose a part
of their DDT stores during the first few months of life, as a result
of rapid growth, is not necessarily contradictory to the observation
that at birth they have less than their mothers, but it must be said
that the values reported by Engst et al. (1970) were relatively high
for infants (see also section 5.2).
Storage in relation to disease. A review (Hayes, 1975) indicates
that there is no agreement in the literature regarding the effect of
health on the storage of chlorinated hydrocarbon insecticides. Some
investigators have not found any difference in the concentration of
DDT in adipose tissue taken by biopsy or during minor elective surgery
in contrast to that taken at autopsy. Some investigators, who used
only autopsy samples, found no relationship between storage of
chlorinated hydrocarbon insecticides and the cause of death. Others,
of whom the first was Deichmann (Deichmann & Radomski, 1968; Radomski
et al., 1968), have reported that storage was 1.7-7.6 times greater in
persons dying of cirrhosis, atherosclerosis, hypertension, idiopathic
amyloidosis, and certain forms of cancer. Weight loss was not really
ruled out in these studies. Its importance was emphasized by Casarett
et al. (1968), who found that disease did not influence concentrations
on a wet weight basis but only on a lipid basis; samples with the
highest levels of DDT on a lipid basis came from persons who not only
had cancer but who were emaciated and had widespread abnormality of
Table 13. The range and mean of measurable concentrations of various organochlorines (mg/kg) in different tissues from stillborn infantsa
Tissues p,p'-DDT o,p'-DDT p,p'-DDE o,p'-DDE p,p'-TDE alpha-HCH ß-HCH gamma-HCH Heptachlor Dieldrin
received (DDD)
10 measurable 3 4 8 0 6 3 6 3 4 3
adipose concentration
range 0.16-2.15 0.35-11.47 0.16-3.19 -- 0.23-14.17 0.09-0.24 0.14-0.44 0.09-0.14 0.07-0.51 0.09-0.35
mean 0.88 3.39 1.22 -- 3.17 0.14 0.26 0.11 0.32 0.24
SE ± 0.63 2.70 0.38 -- 2.22 0.05 0.05 0.02 0.10 0.08
8 measurable 1 1 2 0 2 0 1 1 0 1
spinal concentration
cord range 0.47 0.47 0.30-1.16 -- 0.31-0.70 -- 0.17 0.10 -- 0.09
mean -- -- 0.73 -- 0.51 -- -- -- -- --
SE ± -- -- 0.43 -- 0.20 -- -- -- -- --
8 measurable 3 1 4 0 3 3 1 1 1 2
brain concentration
range 0.28-0.99 0.84 0.25-1.47 -- 0.20-1.22 0.04-0.49 3.81 0.06 0.13 0.84-0.86
mean 0.56 -- 0.65 -- 0.64 0.19 -- -- -- 0.05
SE ± 0.22 -- 0.28 -- 0.30 0.15 -- -- -- 0.01
9 measurable 3 2 6 0 3 2 3 3 2 1
adrenals concentration
range 1.28-1.65 0.36-1.05 0.13-1.96 -- 0.91-1.45 0.40-0.57 0.12-0.71 0.20-0.53 0.46-1.00 0.92
mean 1.48 0.71 1.05 -- 1.11 0.49 0.37 0.33 0.73 --
SE ± 0.11 0.35 0.28 -- 0.17 0.09 0.18 0.10 0.27 --
10 measurable 4 0 6 0 5 5 3 3 3 2
lungs concentration
range 0.57-1.01 -- 0.25-1.35 -- 0.31-1.05 0.07-0.69 0.05-0.18 0.05-0.25 0.08-0.31 0.27-0.72
mean 0.79 -- 0.85 -- 0.75 0.25 0.12 0.12 0.17 0.50
SE ± 0.11 -- 0.22 -- 0.13 0.11 0.04 0.07 0.07 0.23
Table 13 (Cont'd)
Tissues p,p'-DDT o,p'-DDT p,p'-DDE o,p'-DDE p,p'-TDE alpha-HCH ß-HCH gamma-HCH Heptachlor Dieldrin
received (DDD)
10 measurable 4 2 4 0 4 3 3 3 4 3
heart concentration
range 1.04-4.17 0.57-0.68 1.27-4.79 -- 1.02-5.82 0.23-0.52 0.15-0.31 0.19-0.27 0.30-1.56 0.08-1.02
mean 2.17 0.63 2.74 -- 3.27 0.33 0.22 0.22 0.80 0.49
SE ± 0.69 0.06 0.74 -- 1.10 0.09 0.05 0.03 0.30 0.28
10 measurable 5 3 6 0 6 3 4 4 3 2
liver concentration
range 0.15-1.59 0.22-3.42 0.22-2.45 -- 0.19-2.14 0.21-0.32 0.03-0.20 0.05-0.36 0.03-1.67 0.16-0.22
mean 0.79 1.32 0.98 -- 1.01 0.25 0.11 0.24 0.68 0.19
SE ± 0.24 1.05 0.34 -- 0.29 0.04 0.04 0.07 0.50 0.03
9 measurable 4 3 6 0 5 4 5 4 3 3
kidney concentration
range 0.62-7.60 0.29-2.07 0.11-9.78 -- 0.48-9.12 0.11-1.45 0.06-0.61 0.11-0.69 0.19-1.14 0.06-0.50
mean 3.71 1.38 3.57 -- 3.84 0.82 0.29 0.39 0.70 0.34
SE ± 1.70 0.55 1.72 -- 1.87 0.32 0.12 0.14 0.28 0.14
8 measurable 3 2 3 1 3 1 1 2 5 3
spleen concentration
range 0.48-1.04 0.45-2.94 0.60-1.05 0.29 0.18-0.91 0.21 0.16 0.17-0.18 0.10-0.52 0.18-0.51
mean 0.80 1.70 0.86 -- 0.56 -- -- 0.18 0.35 0.31
SE ± 0.17 1.25 0.13 -- 0.21 -- -- 0.005 0.08 0.10
3 measurable 1 0 1 1 0 0 0 0 0 0
pancreas concentration
range 0.49 -- 0.23 0.08
mean -- -- -- -- -- -- -- -- -- --
SE ± -- -- -- -- -- -- -- -- -- --
a From: Curley et al. (1969).
the liver. Factors other than emaciation may be important in some
conditions. For example, Oloffs et al. (1974) found that the DDT
concentration in fat was not influenced by cirrhosis, but that the DDT
concentrations in liver were significantly higher in cirrhotic livers
with severe fatty infiltration and significantly lower in those with
marked fibrosis or bile stasis.
That increased storage of DDT is a result and not a cause of the
diseases in which it sometimes occurs is shown by the fact that
persons with extensive occupational exposure average 10 times more
storage than the highest values reported in connexion with disease.
However, they do not exhibit any predisposition to the diseases in
question, and these diseases have shown no age-specific increase in
incidence related to the introduction and use of insecticides.
6.2.2 Animal studies
A detailed review of the literature (Hayes, 1959) shows that a
number of facts about the distribution and storage of DDT were
established early either by single, classical papers (now fully
confirmed) or by correlation of contributions from several
laboratories. The major results may be summarized as follows:
(a) DDT is stored in all tissues. Storage of the compound in
blood, liver, kidney, heart, and the central nervous system
was reported by Smith & Stohlman (1944).
(b) Higher concentrations of DDT are usually found in adipose
tissue than in other tissues (Ofner & Calvery, 1945).
(c) Rats store DDT in their fat at all accurately measurable
dietary levels including the unintended residues in standard
laboratory feeds.
(d) Following repeated doses, storage in the fat increases rapidly
at first and then more gradually until a peak or plateau is
reached (Laug et al., 1950). It was recognized that repeated
doses at a moderate rate could result in greater total storage
of DDT in the fat than a single dose at the highest rate that
can be tolerated or even a single dose at a rate that
frequently is fatal.
(e) By plotting animal data published no later than 1950, it is
possible to show that, when other factors are kept constant,
the equilibrium storage of DDT in each tissue varies directly
with the daily dosage.
(f) However (with the apparent exception of the dog) storage in
the fat, and perhaps in other tissues, is less extensive in
relation to dosage at higher dietary levels (Fig. 1).
(many of the compounds also exist as o,p'-isomers and other isomers)
Name DDT Chemical name R R' R"
and its major
metabolites
DDT 1,1'-(2,2,2-trichloroethylidene)- -Cl -H -CCl3
bis[4-chlorobenzene]
DDEa 1,1'-(2,2-dichloroethenylidene)- -Cl None =CCl2
bis[4-chlorobenzene]
TDE(DDD)a,b 1,1'-(2,2-dichloroethylidene)- -Cl -H -CHCl2
bis[4-chlorobenzene]
DDMUa 1,1'-(2-chloroethenyldene)- -Cl None =CHCl
bis[4-chlorobenzene]-
DDMSa 1,1'-(2-chtoroethylidene)- -Cl -H -CH2Cl
bis[4-chlorobenzene]
DDNUa 1,1'-bis(4-chlorophenyl)ethylene -Cl None =CH2
DDOHa 2,2-bis(4-chlorophenyl)ethanol -Cl -H -CH2OH
DDAa 2,2-bis(4-chlorophenyl)- -Cl -H -C(O)OH
acetic acid
Table 1 (Cont'd)
Name DDT Chemical name R R' R"
and its major
metabolites
Some related insecticides
NO2
Bulan(R) 2-nitro-1,1 -bis- -Cl -H '
(4-chlorophenyl)butane -CHC2H5
NO2
Prolan(R) 2-nitro-1,1-bis- -Cl -H '
(4-chlorophenylpropane -CHCH2
DMC 4-chloro-alpha[-(4-chlorophenyl)- -Cl -OH -CH3
alpha-(methyl)benzenemethanol
dicocol 4-chloro-alpha-(4-chlorophenyl)-alpha- -Cl -OH -CCl3
(Kelthane(R)) (trichloromethyl)benzenemethanol
chlorobenzilatec ethyl 4-chloro-alpha-(4-chlorophenyl)- -Cl -OH -C(O)OC2H5
alpha-hydroxybenzeneacetate
chloropropopylatc 1-methylethyl 4-chloro-alpha- -Cl -OH -C(O)OCH(CH3)2
(4-chlorophenyl)-aplha-hydroxy-
benzeneacetate
methoxychlorc 1,1'-(2,2,2-trichloroethylidene)- -OCH3 -H -CCl3
bis[4-methoxybenzene]
Table 1 (Cont'd)
Name DDT Chemical name R R' R"
and its major
metabolites
Perthane(R) 1,1'-(2,2-dichloroethylidene)- -C2H5 -H -CHCl2
bis[4-ethylbenzene]
DFDT 1,1'-(2,2,2-trichloroethylidene)- -F -H -CCl3
bis[4-fluorobenzene]
a Recognized metabolite of DDT in the rat.
b As an insecticide, this compound has the ISO approved name of TDE, and it has been sold
under the name Rothane(R); in metabolic studies the same compound has been referred to as
DDD; as a drug, it is called mitotane.
c Common name approved by the International Organization for Standardization (ISO).
and analogues that have had some use as insecticides. It must be
emphasized that even the commercially-available insecticidal analogues
have strikingly different properties. Especially remarkable is the
slow metabolism and marked storage of DDT and its metabolite DDE and
the rapid metabolism and negligible storage of methoxychlor.
No attempt has been made to include in Table 1 the wide range of
compounds that have been synthesized and studied in connexion with
structure-activity relationships, often with the hope of emphasizing
the good properties of DDT and reducing its undesirable properties.
For a more extensive consideration of analogues, see Metcalf (1955).
The formation of metabolites is considered in section 6.4.
2.1.3 Formulations of commercial or technical DDT
Technical DDT has been formulated in almost every conceivable form
including solutions in xylene or petroleum distillates, emulsifiable
concentrates, water-wettable powders, granules, aerosols, smoke
candles, charges for vaporizers, and lotions. Aerosols and other
household formulations are often combined with synergized pyrethrins.
When used as a drug, DDT is called clofenotane (INN) or Dicophane
(British Pharmacopoeia), Klorfenoton (Swedish Pharmacopoeia),
Chlorophenothane (United States Pharmacopoeia). For research or
reference it has been designated OMS 0016 and Ent. 1,506. DDT has been
sold under a variety of tradenames, including: Anofex(R), Cezarex(R),
Dinocide(R), Gesarol(R), Guesapon(R), Guesarol(R), Gyron(R),
Ixodex(R), Neocid(R), Neocidol(R), and Zerdane(R).
2.2 Analytical Procedures
Analytical procedures for determining residues of DDT-type
compounds in environmental samples involve several steps including
collection and extraction of the DDT-type compounds; removal of
coextractives by appropriate clean-up methods; and quantification of
p,p'-DDT and its analogues by a suitable technique. Each of these
major steps is discussed later. It is appropriate, however, first to
outline briefly the statistical criteria used to assess analytical
methods, the estimation of the lower limit of detection of a method,
and the procedure for confirming the chemical identities of the
components measured.
2.2.1 Statistical criteria for assessing analytical methods
The overall reliability of an analytical method can be assessed
using two criteria, namely, reproducibility and systematic error (or
bias). Reproducibility is both conceptually and practically the
simpler criterion; it may be defined as "the quantitative expression
of the random error associated with operators working in different
laboratories, each obtaining single results on identical test material
when applying the same method" (Institute of Petroleum, 1968). It may
be quantitatively specified in various ways, and it is important to
pay attention to the statistic (range standards, deviation, etc.) used
in a particular study to represent the random error of an analytical
method. If the results are normally distributed then the most
efficient statistic measuring reproducibility is the standard
deviation of a set of results (Nalimov, 1963; Youden & Steiner, 1975;
Davies & Goldsmith, 1976). Care should always be taken to ensure that
a particular statistic or statistical technique is appropriate for a
given set of results, by, for example, testing for outliers or, if
there are sufficient results, examining the distribution of the
results, before characterizing the reproducibility of an analytical
method.
There are two subdivisions of the reproducibility criterion,
namely, replication i.e., two or more results, obtained by the same
operator in a given laboratory using the same apparatus for successive
determinations on identical test material, within a short period of
time on the same day; and repeatability i.e., a quantitative
expression of the random error associated in the long run with a
single operator in a given laboratory obtaining successive results
with the same apparatus under constant operating conditions on
identical test material (Institute of Petroleum, 1968).
Reproducibility is, in turn, a subdivision of the random error in the
analysis of identical test material in different laboratories using
different techniques or variations of a particular method. Examples of
the assessment of the random errors found in the determination of
p,p'-DDT and related compounds are given below.
The systematic error of a method is the deviation of the
experimental results from the "true" values; such systematic error
causes the differences between the nominal value and the
experimentally-determined values to have predominantly the same sign
(as opposed to the random errors, where the results are equally likely
to be greater than or less than the true mean). Nominal or "true"
values are available only in the case of fortified (spiked) samples,
but whether such fortified samples are representative of actual
(environmentally incurred) contamination is open to doubt in the case
of some types of material. A rapid nonparametric test of systematic
error can be made using the sign-test or the Wilcoxan signed ranks
test (Conover, 1971).
It is common practice to calculate the "recovery" factor for an
analytical method, i.e., the ratio of the mean observed value to the
nominal value (usually expressed as a percentage), but the statistical
significance of the "recovery" factor should always be assessed. The
ratio of the mean deviation to the standard error of the mean
deviation is an appropriate method of testing the null-hypothesis that
the difference between the nominal value and the observed mean is not
statistically significant.
The term "total error" has been proposed for a function
incorporating both the systematic error and the reproducibility
(McFarren et al., 1970), and these authors also suggested three
classes of total error corresponding to excellent methods, acceptable
methods, and methods that are judged unacceptable. It is pertinent
that McFarren et al. concluded that the total errors in one study of
the determination of DDT-type compounds in water were unacceptable
(>50% for p,p'-DDT, and p,p'-DDE, 24.0-53.6% for o,p'-DDT).
2.2.2 Limit of analytical detection
All analytical determinations have a lower limit corresponding to
that quantity (Delta g) of p,p'-DDT (or a related compound) which
produces a response (Delta r) that cannot be distinguished from
response (Delta r) produced when no p,p'-DDT is present. The
response Delta r is generated by the materials, reagents, and
instruments, used in the procedure, for example, the small voltage
generated by electronic equipment, or the small absorption in a
spectrophotometric method. These responses are known as "blank" or
"noise", and their size depends on the presence of interfering
components in the test material, the purity of the reagents, the
cleanliness of the apparatus, and the design of electronic equipment
(amplifiers, etc.). The concept of limit of detection is a statistical
one and is related to the random variation of the response generated
by a blank or control (Sutherland, 1965; Skogerboe & Grant, 1970;
Kaiser, 1973). Currie (1968) has defined three limiting levels for use
in analytical chemistry: "the net signal level" above which an
observed signal may be reliably recognized; "the true net signal
level" which may, a priori, be expected to lead to detection; and
"the quantifiable level" at which the measurement precision is
sufficient for the quantity present to be estimated satisfactorily. In
many types of sample, the limit of detection is of rather academic
interest as the concentrations of DDT-type compounds in samples are an
order of magnitude greater than the limit of detection of a sensitive
detector such as the electron-capture detector. However, in analyses
of air and drinking-water, for example, it may be necessary to
ascertain the limit of detection by a suitable statistical procedure.
Lower limits of detectability (ng.g-1) suggested in the US EPA
Manual of Analytical Methods (Thompson, 1974) for DDT-type compounds
using gas-liquid chromatography are:
o,p'-DDE Adipose tissue, 10; serum, 1
p,p'-DDE Adipose tissue, 20; serum, 1
o,p'-DDT)
p,p'-TDE) Adipose tissue, 20; serum, 2
p,p'-DDT)
2.2.3 Confirmation of the identity of trace residues of DDT-type
compounds
Confirmation of the identity of p,p'-DDT and related compounds
in many types of environmental samples is not easy when the apparent
amounts present are in the microgram and submicrogram range, since the
classical procedures for the identification of organic compounds
require the use of milligrams of the purified compound. A definition
of chemical identity appropriate to trace analysis has been proposed
(Robinson et al., 1966), a definition that may need revision in
relation to the combined use of gas-liquid chromatography and mass-
spectrometry. Chemical derivatization techniques for DDT-type
compounds have been reviewed by Cochrane & Chau (1971). Other
techniques, that are used routinely in the confirmation of the
identity of DDT-type compounds include: determination of gas-liquid
chromatographic retention times using polar and nonpolar stationary
phases; thin-layer chromatography; paper chromatography; p-values;
infrared microtechniques; carbon skeleton chromatography; conversion
into dichlorobenzophenones; nuclear magnetic resonance; and X-ray
diffraction. Tables of the relative retention times (aldrin = 1.00) of
DDT-type compounds using nine liquid phases (Thompson et al., 1975)
and three mixed liquid phases (Suzuki et al., 1975); gas-liquid
chromatographic retention times for DDT-type compounds are also
summarized by Yermakov (1972). Relative thin-layer chromatographic
Rf values ( p,p'-DDE = 1.00) on alumina using three solvent systems
were reported by Thomas et al. (1968). Extraction p-values for
DDT-type compounds using seven solvent systems are given in the US EPA
Manual (Thompson 1974). Identification of DDT and its metabolites by a
microinfrared technique has been studied by Sierwiski & Helrich (1967)
and reference infrared spectra have been published (Chen et al.,
1972). Asai et al. (1971) used carbon skeleton chromatography to
differentiate polychlorinated biphenyls from DDT-type compounds. The
conversion of DDT-type compounds into the corresponding benzophenones
may be used to identify them in the presence of polychlorinated
biphenyls (Miles, 1972), but this technique has its drawbacks as
p,p'-DDT, p,p'-TDE and p,p'-DDE all give the same p,p'-
dichlorobenzophenone. The use of high resolution nuclear magnetic
resonance spectroscopy (with a time averaging computer to increase
sensitivity), for the confirmation of the presence of p,p'-DDT and
p,p'-DDE in human adipose tissue (total concentration about
13 mg/kg) was studied by Biros (1970).
The combination of gas-liquid chromatography with mass-
spectrometry is a powerful tool for the confirmation of identity of
trace residues of DDT-type compounds. For example, Gordon & Frigerio
(1972) used mass fragmentography and claimed identification of 10 pg
p,p'-DDT; Schaeffer (1974) identified DDT-type compounds in fish
using a fast scan mass-spectrometer to give a multiple mass spectrum
for each of the overlapping peaks.
2.2.4 Sampling and extraction
Before discussing different kinds of samples, it must be noted
that valuable information on many analytical problems may be found in
the US EPA Manual (Thompson, 1974) and the FDA Manual (McMahon &
Sawyer, 1977).
DDT-type compounds may be present in the air in vapour form or
adsorbed on particulate matter. Glass-fibre filters are suitable for
trapping the particulate matter (Stanley et al., 1971; Beyermann &
Eckrich, 1974) and DDT in the vapour form may be trapped using
impingers of the Greenburg-Smith type and a suitable nonvolatile
solvent using ethylene glycol. Miles et al. (1970) reported a trapping
efficiency of more than 99% for DDT. A three-trap system in series
comprising a column containing glass cloth, followed by an impinger
with hexylene glycol, and finally an alumina column was used by
Stanley et al. (1971). They estimated that the collection efficiency
(based on material balance studies) of this system for a mixture of
aerosol and vapour was about 60% for p,p'-DDE, 95% for p,p'-TDE,
and 100% for p,p'-DDT. Beyermann & Eckrich (1974) used glass wool
for the trapping of aerosols, and a stainless-steel net coated with
polyethylene glycol for trapping vapours. Trapping of organochlorine
insecticides in the vapour phase using support-bonded silicones on
various types of Chromosorb was considered to be quantitative by Aue &
Teli (1971): DDT-type compounds were not examined but the trapping of
lindane, aldrin, and heptachlor was considered satisfactory. A cross-
linked polystyrene resin, Chromosorb 102, was used by Thomas & Serber
(1974). These workers reported a collection efficiency of 98% for
o,p'-DDT at a concentration of 15 ng/m3. Herzel & Lahmann (1973)
used silica gel as the support for various liquid phases and concluded
that polyethylene glycol was the best absorbent for DDT-type compounds
in the air. Absolute calibration of the collection efficiency of
aerosols and atmospheres for DDT-related compounds is difficult, but
the studies that have been made indicate that impingers with ethylene
glycol or hexylene glycol are probably the most efficient. The
simplest empirical test of the efficiency of the trapping system is to
use two (or more) impingers in series and analyse the liquid
absorbents from the impingers separately. The ratio of the amounts
present in the first and second impingers should be higher than 10:1.
Detailed instructions for the sampling and analysis of air for
pesticides (including DDT-type compounds) by a method that has been
subjected to interlaboratory study are given in the US EPA Manual
(Thompson, 1974).
For the determination of DDT-type compounds in water an
uncontaminated container may be filled, but if the concentrations of
DDT are likely to be extremely low (as would be expected in most
potable waters), then drawing the water through a suitable trapping
device may he more appropriate; this method also gives a time-weighted
average concentration and can be used in an automated monitoring
procedure.
Benzene was used by Pionke et al. (1968) as the solvent to extract
p,p'-DDT and p,p'-TDE from fortified samples of distilled water
and lake waters. The levels of fortification were high (µg/litre) and
the recoveries were: p,p'-DDT, 96.1% ± 1.02%; p,p'-TDE 97.3% ± 0.89%.
Thus, at concentrations of the order of 0.001 mg/litre, the
benzene extraction method gives excellent recoveries (total errors,
5.9% and 4.5%, respectively). A continuous flow method based
on liquid-liquid partition was developed by Ahling & Jensen (1970);
water is passed through a column containing Chromosorb W coated with
undecane plus a macrogol Carbowax 4000 monostearate. The best
collection efficiencies were found when the two liquid absorbents were
used at concentrations of 10% and 30%, respectively, on the solid
support. At concentrations in the ng/litre range, the optimum recovery
of known amounts of DDT-type compounds added to water were obtained
with 1.5 g coated Chromosorb W per litre of water. Ahnhoff & Josefson
(1974) used continuous flow liquid-liquid partition between water and
cyclohexane (in three extractors in series). The extraction
efficiencies from water containing 5 ng p,p'-TDE or 7.6 ng p,p'-DDT
per litre were greater at a flow rate of 2 litre/h than at
5 litre/h being 90% and 98% for p,p'-DDT, and p,p'-TDE,
respectively, with 92% and 93%, respectively, of the recovered
compounds being found in the first extractor. A method using a solid
adsorbent, a macroreticular resin, XAD-4, has been studied by Musty &
Nickless (1974); at a flow rate of 8 ml/min through a column
containing 2 g XAD-4, satisfactory recoveries of p,p'-DDT and three
related compounds were obtained at concentrations of 2-10 ng-litre.
Carbon is a very efficient adsorber of p,p'-DDT and p,p'-TDE from
water (Rosen & Middleton, 1959), but desorption from charcoal is
difficult; furthermore, the recoveries are not very satisfactory and
this is attributed to chemical changes catalysed by carbon in contact
with water rather than inefficient desorption (Eichelberger &
Lichtenberg, 1971). Another solid adsorbent is polyethylene (Beyermann
& Eckrich, 1973), but in the case of river water variable results were
obtained because of the effects of other solutes. Taylor & Bogacka
(1968) used petroleum ether to extract DDT from water; following a
clean-up with acetonitrile partition and Florisil the overall recovery
(using thin-layer chromatography) was incomplete (about 66%). The
total error of this procedure is unacceptable. Benzene was used as the
extraction solvent by Djatlovitskaja et al. (1972), and a general
review of the determination of organochlorine insecticides (and other
insecticides) in water has been published by Novikova (1973).
The need for scrupulous cleanliness in glassware and purity of
reagents cannot be overstressed in the case of the determination of
DDT-type compounds in air and water as the residues are usually so
small; the use of electronic equipment with a low noise characteristic
and constant checking of the response of detectors are also necessary.
A major difficulty in the determination of organochlorine
insecticides in soils is the initial extraction of the residues
because of a combination of factors, including the wide variation in
soil types. Chiba (1969) emphasized the lack of precision in defining
soil types. In his review article, he concluded that the most
effective solvent systems for extraction of these compounds were
mixtures of n-hexane/acetone (1:1 v/v) or chloroform/methanol
(1:1 v/v), and that the moisture content of the soil should be at
least 5%. The results of a study of the determination of
organochlorine insecticides in three types of soil have been
summarized by Woolson & Kearney (1969). Twelve laboratories
participated using various extraction procedures. In one type of soil
(a silty clay loam) that was fortified with p,p'-DDT at a
concentration of 5 mg/kg, the amounts found in the different
laboratories varied between 1.60 and 5.48 mg/kg. The results of 3 of
the laboratories were discarded by Woolson & Kearney (1969), and the
mean recovery of the other 9 laboratories was 79.3%, with a standard
deviation of 42.2%. Wetting of the soil before extraction was
considered to improve the recoveries, Soxhlet extraction appeared to
be preferable to shaking with the solvent, and hexane/acetone
(1:1 v/v) or hexane/isopropanol (3:1 v/v) appeared better extractants
than other solvents.
A further collaborative study of the determination of organochlo-
rine insecticides in 3 types of soil was reviewed by Woolson (1974).
Although 12 laboratories participated, only 7 completed the study. All
the laboratories used the same extraction, clean-up and quantification
procedures, including premoistening of the soil with ammonium chloride
solution at 0.2 mol/litre. The recoveries of p,p'-DDE, p,p'-TDE,
o,p'-DDT and p,p'-DDT were higher than 80%, with standard errors
of 8-18%. These results are much more consistent than those of the
previous study, and Woolson recommended that premoistening of the soil
with ammonium chloride solution (0.2 mol/litre) followed by extraction
with hexane/acetone (1:1 v/v) should be adopted for the determination
of chlorinated hydrocarbon insecticides in soil.
Several solvents have been used for the extraction of DDT-type
compounds from nonfatty foods such as vegetables, fruit, and cereals;
acetonitrile, alone or mixed with water is used in the method of the
Association of Official Analytical Chemists (AOAC) depending on the
water content of the sample (Horwitz, 1975); Cieleszky et al. (1970)
recommend Soxhlet extraction with diethyl ether.
Maceration of vegetables with a mixture of acetone and hexane was
used by Sissons et al., 1968; the same mixed solvent was used for
cereals, and root vegetables by Abbott et al., 1969 who used
propan-2-ol in the case of fruit and green vegetables. Whiting et al.
(1968), and Skrentny & Dorough (1971) considered a mixture of methanol
and chloroform to be the most efficient extraction solvent for use
with a macerator or a Soxhlet extractor. The acetonitrile extraction
technique was examined by Zerber et al. (1971) for the extraction of
DDT-type compounds from cereal products and feeding stuffs. Diethyl
ether was used by Kucinski (1972) for extraction from canned vegetable
products.
Extraction methods for fat-containing foods are given in the AOAC
Method (Horwitz, 1975), the solvent used, methanol or petroleum ether,
being dependent on the type of sample. Soxhlet extraction using
petroleum ether is recommended by the Federal Health Office of the
Federal Republic of Germany (Anon., 1974); Cieleszky et al. (1970)
also recommended petroleum ether as a suitable solvent.
Smart et al. (1974) compared acetonitrile and dimethyl formamide
as extraction solvents for apples, carrots, potatoes, and vegetables;
a third solvent dimethylsulfoxide was compared with these two solvents
for butter, cheese, and eggs.
Three body tissues or fluids have been analysed in many surveys,
namely adipose tissue, blood (or serum), and mother's milk. The US EPA
Manual (Thompson, 1974) recommends grinding a sample of adipose tissue
with anhydrous sodium sulfate before extraction with petroleum ether;
carbon tetrachloride has been used as a solvent (Mattson et al.,
1953), but it is not appropriate if the final quantification step
involves gas-liquid chromatography and a halogen sensitive detector.
Extraction of DDT-type compounds from blood or serum using hexane has
been described (Dale et al., 1966b), but this extraction procedure is
inefficient, probably as a result of binding by serum proteins. Dale
et al. (1970) investigated a procedure in which the serum was treated
with 97% formic acid before extraction with hexane; experiments using
14C-DDT indicated that extraction with hexane alone gave results
some 40% lower (based on 14C activity) than when the serum was first
treated with formic acid. A modified procedure in which whole blood
was treated with 60% sulfuric acid, and then extracted with a
hexane/acetone (9:1) mixture has been reported by Stretz & Starr
(1973). Samples of blood spiked at 4 different levels with p,p'-DDT
were analysed by 11 different laboratories; considerable discrepancies
were found between laboratories and a further study of the method was
considered desirable. Griffith & Blanke (1974) also investigated the
sulfuric acid method, but a microcoulometric detector was used instead
of an electron-capture detector. According to these workers,
consistent recoveries of p,p'-DDT, p,p'-TDE and p,p'-DDE were
obtained in their laboratory but reproducibility between laboratories
was not studied.
The US EPA Manual (Thompson, 1974) method for the extraction of
DDT-type compounds from human milk involves an acetonitrile/hexane
type extraction. A method for extraction from cow's milk, that makes
use of the stability of p,p'-DDT and its derivatives in the presence
of concentrated sulfuric acid, has been published by Coha & Nedic
(1970); the mixture of milk and concentrated sulfuric acid is
extracted with hexane. Prouty & Cromartie (1970) studied the
recoveries of 14C-DDT in each of the 5 major stages of a method for
determining this compound in the tissues of quail; Soxhlet extraction
with hexane for 6 h, of muscle, liver, heart or brain, after grinding
with sodium sulfate gave recoveries of DDT-type compounds (as 14C-
activity) of 93-105%. Jonczyk (1970) used hexane to extract DDT-type
compounds from blood and reported recoveries of 67-91%. Acetone was
used as the extracting solvent for DDT-type compounds in the adipose
tissue and brain of partridges (Jonczyk et al., 1970). Wood's method,
using dimethyl sulfoxide, was used by Stec & Juszkiewicz (1972), and
was found to give results for DDT-type compounds that compared
favourably with other methods of analysis of animal tissue, eggs, and
milk.
All methods of extracting DDT also result in the removal of
lipids, if they are present. Regardless of the method of extraction,
the results of most analyses have been reported in terms of fresh or
wet weight of samples no matter whether their lipid content was
extremely low (water and urine), low (soils and most vegetables),
intermediate (milk and many tissues), or high (adipose tissue). In
some instances, samples such as adipose tissue, milk, and, to a lesser
degree, other animal tissues known to contain lipids have been
reported in terms of the concentration of pesticide in extractable
lipid. Because DDT and DDE are known to have a marked affinity for
neutral fat, it was originally supposed that reporting in terms of
lipid would reduce variability within any set of samples by excluding
the influence of connective (and sometimes lymphatic) tissue, which
forms a part of each sample. It appears that variation, as measured by
the coefficient of variation, may not be reduced by this kind of
reporting (Casarett et al., 1968). However, there may be other reasons
for reporting on a lipid basis and it is absolutely essential that the
method of reporting be specified.
2.2.5 Clean-up procedures
The extraction procedures remove not only the DDT-type compounds
from the samples analysed but also coextractives to a greater or
lesser degree according to the type of sample. A number of procedures
have been developed that reduce the amounts of the coextractives
relative to that of DDT-type compounds. If interest is confined solely
to DDT-type compounds, then their stability in the presence of
concentrated sulfuric acid is a very useful clean-up procedure
(Mattson et al., 1953; Czegledi-Janko & Cieleszky, 1968; Murphy,
1972). Usually, however, the concentrations of other compounds are
also of interest and this procedure cannot be used if these compounds
are not stable in concentrated sulfuric acid. Two general clean-up
procedures, used in sequence, are appropriate in these circumstances,
namely, liquid-liquid partition followed by liquid-solid partition.
Liquid-liquid partition systems such as hexane/acetonitrile,
hexane/dimethyl formamide, or hexane/dimethyl sulfoxide are the ones
most commonly used. The liquid-solid partition systems generally
consist of Florisil, silica gel, or alumina as the solid phase, and
hexane or mixtures of hexane and various proportions of a polar
solvent (e.g., diethyl ether) as the mobile phase. Separation of
DDT-type compounds from triglycerides in fat-containing tissues is
achieved with considerable efficiency by liquid-liquid partitions, the
hexane/dimethyl formamide or hexane/dimethyl sulfoxide systems being
generally more efficient than hexane/acetonitrile. Separation of
DDT-type compounds from other organochlorine compounds (e.g., aldrin,
dieldrin, polychlorinated aromatics) or from steroids is not very
efficient using liquid-liquid systems, and the liquid-solid partition
systems should be used in these cases. Separation of DDT-type
compounds from polychlorinated biphenyls is particularly difficult
and, as some of these compounds have similar liquid chromatographic
retention times to those of the various DDT-type compounds, the
analysis of samples containing both classes of compounds requires
considerable care. Detailed clean-up procedures are described by de
Faubert Maunder et al. (1964), Cieleszky et al. (1970), Anon. (1974),
Thompson (1974), and by Horwitz (1975).
The separation of DDT-type compounds from polychlorinated
biphenyls, by liquid-solid (silical gel) partition is discussed by
Armour & Burke (1970), Snyder & Reinert (1971), and Masumoto (1972);
the last-mentioned investigator concluded that a number of factors
required careful control if satisfactory separation of DDT-type
compounds from polychlorinated biphenyls (PCBs) were to be achieved,
in particular, the degree of activation of the silicic acid (irregular
distribution of water molecules onto the silicic acid particles was
also probably important). He found that separation of p,p'-DDE from
four Arochlors was incomplete. A collaborative study of the separation
of DDT-type compounds from PCBs using the Armour & Burke silicic acid
column procedure has been reported (Sawyer, 1973).
Florisil and coconut charcoal have also been investigated for the
separation of PCBs from DDT-related compounds (Reynolds, 1969;
Benvenue & Ogata, 1970; and Stijve & Cardinale, 1974). Another
procedure that has been developed for the separation of DDT-PCB
mixtures is the oxidation of p,p'-DDE to p,p'-dichlorobenzophenone
(Miles, 1972). A method for the determination of p,p'-DDT and a
particular PCB isomer that has similar retention time to that of
p,p'-DDT is based on an empirical relation for p-values (Zelinski et
al., 1973).
2.2.6 Quantitation
2.2.6.1 Determination of DDT-type compounds
Two techniques have played a major role in the quantification of
DDT-type compounds, one is a colorimetric method, the other (now the
most widely used) is gas-liquid chromatography with a halogen-
sensitive detector.
The colorimetric procedure of Schechter-Hailer is described in
detail in the Handbook of the Deutsche Forschungsgemeinschaft (1969),
and by Cieleszky et al. (1970) and Horwitz (1975).
Gas-liquid chromatography is essentially a further-method of
separation of compounds, and, although it is the most effective of the
separation procedures (apart possibly from high pressure liquid-liquid
chromatography) it must be realized that it has its limitations,
particularly if used with a highly sensitive but nonselective detector
such as the electron-capture detector. The basic principles of
gas-liquid chromatography are described by Dal Nogare & Juvet (1962)
and Yermakov (1972), for example, and need not be discussed here.
However, attention is drawn to three aspects of the performance of a
column in the gas-liquid chromatographic separation of DDT-type
compounds from other compounds. First, the DDT-type compounds should
not undergo any thermal degradation or other chemical change; second,
the performance of the column should be assessed by the number of
theoretical plates; and third, the performance of the column as regards
ability to separate p,p'-DDT and related compounds from other
compounds that have similar retention times for a particular liquid
phase must be investigated. Transport of p,p'-DDT and analogues
through a column without chemical change is dependent upon the absence
of reactive centres in the column. This can be attained by using an
inactive solid support (by coating the reactive sites with a silane,
if necessary) and ensuring that the surface of the solid support is
completely covered by an inert liquid phase. The performance of a
column should be checked at regular intervals; the US EPA Manual
(Thompson, 1974) suggests a mixture containing five DDT-type compounds
plus eight other organochlorine insecticides for this purpose. Change
in peak shape (i.e., departure from symmetrical peaks) should always
be regarded as a warning sign. In the case of serious doubt about the
stability of p,p'-DDT or an analogue (which may manifest itself as
a change in the retention time relative to that of aldrin or dieldrin
for example), it is suggested that a fraction collection technique be
used and the identity of the component leaving the column at a
particular retention time with that injected confirmed.
Methods of calculating the number of theoretical plates and of
separation factors are given in standard texts. According to the US
EPA Manual, a column of 2 m length should have about 3000 theoretical
plates. The factors that control the performance of gas-liquid
chromatographic columns are discussed in detail by Scott (1970).
Convenient summaries of the retention times (absolute or relative) of
DDT-type compounds, together with those of other pesticides, using
various column conditions have been published (Yermakov, 1972; Zweig &
Sherma, 1972). Retention times of 51 pesticides relative to that of
aldrin using six stationary phases at three temperatures with an
electron-capture detector have been reported by Thompson et al.
(1975); estimates of the relative retention times at other
temperatures were derived from the relationship between relative
retention time and temperature for each liquid phase. The relative
times of DDT-type compounds and other pesticides on eight stationary
phases were determined by Thompson et al. (1969a). Nonpesticidal
organochlorine compounds that have retention times similar to those of
DDT-related compounds include the polychlorinated biphenyls and
polychloronaphthalenes.
Examples of the close similarity between the relative retention
times of DDT-related compounds and those of various PCB isomers have
been published (Bagley et al., 1970; Richardson et al., 1971; Stijve &
Cardinale, 1974). Goerlitz & Law (1972) demonstrated that there are
also similarities between the relative retention times of DDT-type
compounds and those of various isomers of polychlorinated
naphthalenes. Examples of similarities between the relative retention
times of DDT and its analogues and various components in
polychlorinated biphenyls and polychlorinated naphthalenes was also
reported by Griffith & Blanke (1974). Problems arise in the case of
mixtures of toxaphene and DDT-related compounds (Cahill et al., 1970).
The effects of severe infection loading on column performance,
peak configuration, and conversion of p,p'-DDT into p,p'-TDE, were
studied by Thompson et al. (1969b). These investigators found that the
columns they used could be maintained and restored to full or nearly
full performance capacity by daily changing of the glass injection
port insert and the glass-wool plug at the column inlet.
Two different types of detection systems are most frequently used
for the quantification of p,p'-DDT and analogues after elution from
gas-liquid chromatographic columns, namely, electrochemical detectors
and electron-capture detectors. Two types of electrochemical detector
have been developed, the microcoulometric detector and the micro-
electrolytic conductivity detector, that are considerably more
sensitive to p,p'-DDT and its analogues than the original electro-
chemical detectors. Giuffrida & Ives (1969) described modifications and
improvements in microcoulometric gas chromatography and, in the case
of DDT-type compounds in carrots, they obtained responses from their
microcoulometer that were approximately one-fifth of those given by a
particular electron-capture detector; Griffith & Blanke (1974)
described a microcoulometric method for the determination of
p,p'-DDT, p,p'-TDE and p,p'-DDE in blood. According to Dolan
& Hall (1973), the microelectrolytic conductivity detector can be used
for the selective determination of organochlorine pesticides in the
presence of polychlorinated biphenyls. However, the relative
selectivity in regard to p,p'-DDE does not appear to be as great as
that for other organochlorine insecticides.
The electron-capture detector produces an extremely sensitive
response to organochlorine compounds, but its response is not,
unfortunately, very selective and many other classes of compounds have
electron affinity in the vapour phase. A general review of the
principles and characteristics of the electron-capture detector has
been published by Pellizari (1974). The detector may be used under
direct current or pulse sampling conditions; anomalous responses
obtained in the direct current mode of operation are not present under
pulse sampling conditions (Lovelock, 1963). A major limitation of the
electron-capture detector is that its response is linear over a very
limited range only, but a new mode of operation in which the linearity
extends over about four orders of magnitude of response has been
described by Maggs et al. (1971). For this the detector current is
held constant while the frequency of the applied pulses is varied. The
response of electron-capture detectors is liable to change
significantly during use, and these detectors should be recalibrated
at regular intervals, preferably at least once per day with single
standard injections at frequent intervals between injections of
extracts from the samples under investigation. It is of interest that
Mendoza (1971) reported a significant difference in the response of a
gas-liquid chromatograph to p,p'-DDT when injections were made at
fast and slow rates.
The combination of mass-spectrometry with gas-liquid chromato-
graphy was mentioned in section 2.2.4 as a means of confirming the
identity of residues of DDT-type compounds. This combination of
instruments can also be used to quantify the amounts present. The
total ion current corresponding to mass fragments of appropriate mass
number (m/e; in the case of p,p'-DDE, 316, 318, 246 and 248) is
measured and the amount present is calculated from the calibration of
the instrument, preferably by an internal standard. Palmer & Kolmodin-
Hedman (1972) determined the concentration of p,p'-DDE in human
plasma by mass-fragmentography using the ion current corresponding to
m/e 216 and 218; the results showed an excellent agreement with the
values obtained using an electron-capture detector.
Semiquantitative methods of the determination of p,p'-DDT and
its analogues include paper chromatography and thin-layer chromato-
graphy; these methods, which are more appropriately used for the
confirmation of identity of residues, are described by McKinley (1963)
and Sherma (1973) respectively. Bishara et al. (1972a) have given the
tlc Rf values for p,p'-DDT and 14 related compounds using 33 solvent
systems.
2.2.6.2 Determination of p,p'-DDA in urine
A major metabolite of p,p'-DDT, excreted in the urine, is bis-
(4-chlorophenyl)acetic acid ( p,p'-DDA). A method has been developed
by Cranmer et al. (1969), based upon the extraction of p,p'-DDA from
acidified urine, conversion into the methyl ester, Florisil column
clean-up, and gas-liquid chromatographic analysis of the ester. A
detailed outline of this procedure is given by Thompson (1974). The
sensitivity of response of the methyl ester is not high, the limit of
detection being about 2 ng. Cranmer & Copeland (1973) used the
2-chloroethanol ester, the response of an electron-capture detector
being about 3.7 times greater than that of the methylester (i.e.,
limit of detection about 0.5 ng). An advantage of this ester is that
it is well separated from p,p'-DDT, whereas the methyl ester has a
retention time similar to that of p,p'-DDE. The retention time of
the pentafluorobenzyl ester compound is about twice that of p,p'-DDT
and the response of an electron-capture detector is considerably
greater than that for the 2-chloroethanol derivative (Johnson, 1973).
2.2.6.3 Method of reporting results
Francis Galton (1879) pointed out that many vital effects are
distributed logarithmically; his paper was followed by a technical one
(McAlister, 1879) presenting the mathematics. Apparently Robinson &
Hunter (1966) were first to point out that this principle applies to
the storage of pesticides so that the geometric mean is a more
appropriate parameter than the arithmetic mean for expressing
insecticide content. In recent years, increasing use has been made of
the geometric mean as recorded by footnotes in Table 9. Few of the
arithmetic means that have been reported are so much in error that
they should be discarded. As Galton (1879) noted, the difference
between the arithmetic and geometric mean is small if the range of the
values averaged is narrow.
2.2.7 Validation of analytical methods for DDT-type compounds
Ideally, an analytical method should be accurate and highly
precise. A study of the accuracy and precision of art analytical
method must be made in order to assess the relationship between the
actual amount present and the results obtained in practice; such a
study may be called the validation of the procedure. There are two
major types of validation procedure. The first type requires the use
of radio-labelled molecules of p,p'-DDT, e.g., 14C p,p'-DDT.
Prouty & Cromartie (1970) determined the 14C-activity in muscle,
liver, heart, and brain of quail that had been given 14C-labelled
p,p'-DDT. Four of the steps in the analytical procedure were studied
and it was concluded that the major discrepancies occurred during the
elution and concentration steps from Florisil columns and from zones
of thin-layer chromatography plates. Chiba & Morley (1968) used 14C-
labelled p,p'-DDT in a study of soil analysis.
The other validation procedure, which has been studied more
intensively, involves the comparison of the results of analyses with
the values for samples fortified with accurately known amounts of
p,p'-DDT or analogues. There are several variations of this
validation procedure, all comparing the fortified (nominal) value with
the results of different methods in the same laboratory, with the
results of a specified method carried out in different laboratories,
or with the results of different methods in different laboratories.
Versino et al. (1971) compared 8 clean-up procedures for test
mixtures containing 10 pesticides. Two extracts, from apples and
lettuce, were fortified (spiked) with p,p'-DDT at 0.2 mg/litre,
p,p'-TDE at 0.1 mg/litre, and o,p'-DDT at 0.1 mg/litre plus
dieldrin and 7 organophosphate insecticides. The recoveries for the 8
column clean-up procedures ranged from 89-100% for p,p'-DDT, 85-101%
for p,p'-TDE, and 95-100% for o,p'-DDT. It should be noted that
this investigation did not include a study of extraction efficiency
and that the extracts contained only small amounts of lipids. In
studies by Smart et al. (1974), samples were analysed of milk, butter,
cheese, eggs, apples, potatoes, carrots, and cabbages, fortified with
p,p'-DDT, p,p'-TDE, and p,p'-DDE (and 5 other organochlorine
insecticides) at concentrations corresponding to those suggested as
limits by the Codex Alimentarius Commission. Five replicate analyses
of each sample were made, using up to 4 procedures, the final step of
each analytical procedure being gas-liquid chromatography/electron-
capture detection, with 3 different stationary phases. These workers
concluded that there were no gross discrepancies in their results.
However, they did not use the concept of "total error" (see above) in
the discussion of their results, and the total errors for the
determination of the 3 DDT-type compounds, calculated from their
results, were in the range of 12% to 95%.
Results have been reported (Carr, 1970) of a collaborative study
by 10 laboratories of the AOAC method for the determination of 4
DDT-type compounds in samples of butterfat fortified by the addition
of these 4 compounds at 2 different concentrations. The mean recoveries
varied from 86-108%, and the coefficients of variation between
laboratories were in the range 7-28%. The total errors for the 2
levels of fortification respectively were: p,p'-DDT, 22 and 14%;
p,p'-TDE, 17 and 30%; p,p'-DDE, 17 and 36%; and o,p'-DDT, 30 and
43%.
Carr (1971) gives the results of a collaborative study by 8
laboratories of the analysis of fish samples fortified (2
concentrations) with p,p'-DDT, p,p'-TDE and p,p'-DDE, and 3 other
organochlorine insecticides. On the basis of a ranking technique
(Youden & Steiner, 1975), the results of 2 laboratories (Nos. 2 and 8)
for the 6 compounds were rejected as outliers. However, if the results
for the 3 DDT-type compounds are considered, then laboratories 7 and 8
gave results that are outliers. The total errors at the 2
concentrations for the 6 laboratories not rejected by Carr were:
p,p'-DDT, 15 and 36%; p,p'-TDE, 32 and 32%; p,p'-DDE, 26 and 39%.
Sawyer (1973) reported a collaborative study by 9 laboratories of
samples of red salmon fortified with 3 DDT-type compounds and PCBs.
The analyses were carried out without and with silicic acid column
separation of PCBs from DDT-type compounds. The total errors without
and with silicic acid separation, were 34% and 43% respectively for
p,p'-DDT, 50 and 47% for p,p'-TDE and 35 and 36% for p,p'-DDE.
Very similar results were obtained with another type of PCB/DDT
mixture.
Samples of soil, fortified with 4 DDT-type compounds plus 6 other
organochlorine insecticides were analysed in 7 laboratories (Woolson,
1974); the total errors (all collaborators) were: p,p'-DDT, 17-33%;
p,p'-TDE, 19-37%, p,p'-DDE, 18-42%; o,p'-DDT, 28-56%.
Fifteen laboratories collaborated in a study of the determination
of p,p'-DDE in eggs, and p,p'-DDT, p,p'-DDE and o,p'-DDT in
kale (Finsterwalder, 1976). Five laboratories analysed eggs by the
AOAC method, and the total error was 21.1%; the total error for 10
laboratories using a modification of the AOAC method was 30.4%. The
results of one laboratory for analyses of kale were rejected as
outliers, and the total errors for the other 14 laboratories were:
p,p'-DDT, 19.7%; p,p'-DDE, 18.8%; o,p'-DDT, 17.4%.
An international collaborative programme on methods of analysis of
organochlorine insecticides has been sponsored by the Organization for
Economic Cooperation and Development (OECD), and the results of 2
studies have been published. In the first (Holden, 1970), a solution
in hexane of 6 organochlorine compounds, 3 being DDT-related
compounds, was analysed by 17 laboratories in 11 countries. The total
errors for p,p'-DDT, p,p'-TDE and p,p'-DDE were in the range
14-21%, the major component in this total error being the standard
deviation of results from different laboratories, probably indicating
errors of calibration. In the second study (Holden, 1973), a sample of
corn oil fortified with 4 DDT-type compounds and 3 other
organochlorine insecticides was analysed in 19 laboratories in 10
countries: the mean total error for the 4 DDT-type compounds (after
excluding 3 outliers) was 35%.
Some of the results of this collaborative validation study are
very satisfactory, but many of them, as judged by the criterion of
total error, are unsatisfactory, even when outliers are rejected, and
they illustrate the need for scrupulous care by analysts: the use of
clean glassware, chemicals of established purity, and constant
checking of the performance of liquid-solid partition columns and of
instruments.
2.2.8 Analytical methods for the evaluation of the biochemical effects
of p,p'-DDT and its analogues
The increased activity of enzymes, particularly in the hepatic
endoplasmic reticulum, if the exposure to p,p'-DDT and its analogues
is sufficiently great, has been recognized in recent years.
Measurement of such changes in enzyme activity may be made by studying
changes in the rates of metabolism of certain drugs (such as
antipyrine), but methods that do not require the administration of a
drug (and the collection of blood samples if the plasma half-life is
used as the biochemical parameter) are preferable. Two methods, in
which the concentration of either 6-ß-hydroxycortisol or D-glucaric
acid in the urine is measured have been developed. A procedure for the
determination of 6-ß-hydroxycortisol in urine has been described by
Kuntzman et al. (1968); and one for D-glucaric acid by Marsh (1963)
(see section 8.2.5.1).
These enzyme induction changes are not specific for DDT-type
compounds.
3. SOURCES OF ENVIRONMENTAL POLLUTION
3.1 Discovery and Introduction
DDT does not occur naturally. It was first synthesized by Zeidler
as reported in 1874. However, it was not put to any use until its
insecticidal properties were discovered by Paul Müller in 1939. The
Swiss patent was issued in 1942.
As an example of the speed with which DDT was developed and used,
the first sample sent to the USA arrived there in September 1942. This
sample was tested for effectiveness and safety. The results were so
encouraging that manufacture was given high priority. At first, the
entire production was used for the protection of troops against
malaria, typhus, certain other vector-borne diseases, or against
biting flies or other insects that are merely pests. As the supply
increased, DDT was used in the USA for control of malaria in military
areas, that is in the vicinity of military installations, ports, and
transportation centres. As a result of this effort, mosquito
transmission of malaria was brought to an end in the USA in 1953, even
though military personnel and other infected persons from the tropics
continued to reintroduce the disease extensively as late as 1972 and
to a lesser degree thereafter.
3.2 Production and use
The revolution in the control of malaria and typhus among allied
troops and among certain civilian populations during World War II was
accomplished with relatively little DDT. Far greater amounts were
required for the control of agricultural and forest pests and this
became possible when the compound was released in the USA for
commercial use on 31 August 1945. Civilian use in other countries
became possible a little later, first, largely on the basis of
importation and gradually on the basis of local manufacture.
Unfortunately, there is apparently no record of world production of
DDT. Production and use in the USA is shown in Table 2.
Quantities of DDT and related compounds used in or sold for
agricultural purposes in 1970 were as follows (metric tonnes):
Australia (about 1000); Austria (20.5); Botswana (2.0); Canada
(287.0); Columbia (980.0); Czechoslovakia (270.0); Democratic
Kampuchea (46.8); Egypt (3457.0); El Salvador (466.0); Federal
Republic of Germany (152.0); Finland (6.1); Ghana (0.3); Guatemala
(380.0); Hungary (20.6); Iceland (0.3); Israel (10.0); Italy (2178.0);
Japan (401.0); Kuwait (0.2); Madagascar (176.0); Ryukyu Islands
(Japan) (0.3); Sri Lanka (16.6); Sudan (269.0); Upper Volta (1.5); and
Uruguay (5.0) (FAO, 1972). These values total 10 146.2 metric tonnes.
Thus, at least until very recently, the use of DDT was extensive on a
worldwide basis but varied greatly from one country to another.
Table 2. Metric tonnes of DDT produced and used in the USAa
Year Production Use
1944 4 366
1945 15 079
1946 20 220
1947 21 534
1948 9 181
1949 19 822
1950 35 448
1951 48 144
1952 45 327
1953 38 268 28 349
1954 44 088 20 465
1955 56 760 28 032
1956 62 441 34 194
1957 56 136 32 205
1958 65 920 30 255
1959 71 097 35 771
1960 74 471 31 818
1961 77 763 29 061
1962 75 764 30 502
1963 81 154 27 744
1964 56 113 22 925
1965 63 859 24 034
1966 64 115 20 685
1967 46 906 18 260
1968 63 231 14 848
1969 55 839 13 724
1970 26 860 11 316
a Based on data from US Tariff Commission (Hayes, 1975).
3.3 Changing Patterns of Use
Before 1945, all of the DDT produced in the USA for example, was
used or allocated by the military services for various medical and
public health uses. Early in 1945, it became available tor rather
extensive experimental work in agriculture, and it was commercially
available in limited quantities early in the autumn of the same year
(US Department of Agriculture, 1945a,b). The results were so
spectacular that use increased until 1959. In response to a demand for
exports, production continued to increase to about 1963. Even before
then some restrictions were placed on its use, mainly to minimize
residues in food and in the feed of animals that produce milk and
meat. Among the first of these restrictions was that on its use on
dairy cattle or in dairy barns (USDA, 1949). Another important factor
reducing the use of DDT was the increasing resistance of pests. One of
the first species to be affected was the house-fly; because of its
abundance and widespread distribution, its resistance was bound to be
noticed by the public, generally. Although many pests of public health
importance became resistant to DDT in some or all of their range,
resistance among vectors of malaria was less marked. Because malaria
control constitutes such a large segment of vector control, the use of
DDT for vector control has tended to remain stable, while its use in
agriculture has continued to decline, especially in temperate
climates.
The ban on the use of DDT and certain other organochlorine
insecticides in Sweden from 1 January 1970 was based on a number of
ecological considerations.
Government agencies of some other countries attempted to justify
severe restrictions on the use of DDT by alleging that it was a threat
to human health. This was in response to ecologists who considered
that the widespread occurrence of DDT in the environment was
inherently bad and was the direct cause of injury to certain fish and
birds. However, this did not prevent the same agencies from making a
proviso that DDT might be used, if needed, to combat any future threat
from vector-borne disease within their boundaries.
Of course, before the restrictions were put into effect, it had
already been possible to eradicate malaria in the USA and Italy, for
example, and to control for the first time an epidemic of typhus in
Italy and Germany (Simmons, 1959). Apparently, there have never been
accurate figures on the proportion of the compound used for
agriculture and for public health even in those countries that have
recorded their total use of the compound.
As the situation now stands, DDT is still used extensively, both
in agriculture and for vector control, in some tropical countries.
Apparently, information is not available on how much of the
agricultural use involves food protection or how much loss of food
production would result if the use of DDT were discontinued. The
picture with malaria control is clear. Substitution of malathion or
propoxur for DDT would increase the cost of malaria control by
approximately 3.4- and 8.5-fold, respectively, and this increase could
not be supported. If DDT were not used, vast populations in the
malarious areas of the world would be condemned to the frightening
ravages of endemic and epidemic malaria (WHO, 1971).
4. ENVIRONMENTAL TRANSPORT AND DISTRIBUTION
Because DDT has been sprayed on people, domestic animals,
buildings, agricultural crops, and forests, it is not surprising that
it is now distributed widely in the environment. During the
application of any spray or dust to a field, it is often possible to
observe drift of the particulate material. This is especially true if
the application is made by aircraft or by ground equipment that shoots
the spray to the top of orchard trees. If the application is made to
forests, it is very likely that at least part of the spray will fall
directly on streams or lakes. Following application, redistribution is
inevitable. As discussed more fully in the following sections, some
DDT in soil enters the air by evaporation or on wind-blown dust. Even
if watercourses are avoided initially, some insecticide will be washed
into them by rains, mainly in conjunction with soil particles.
4.1 Local Drift in Air
The fact that there is drip is implicit from measurements of DDT
on surfaces just after application. Wilson et al. (1946b) recovered
only 7.5% of the nominal dose from plants; this proportion is typical
but ignores material that may have fallen between plants or between
leaves and come to rest on the ground. Of greater interest are
measurements of total recovery made by means of absorbent targets laid
out in advance. As might be expected, recovery of aerosols is less
than recovery of sprays when other conditions of application are
similar. Only 12.5% was recovered at the centre of swaths following
application of a thermal aerosol by aircraft and recovery decreased at
increasing distances from the centre (Hess & Keener, 1947). In another
study, recovery was 8% for a thermal aerosol and 46% for a spray
(Scudder & Tarzwell, 1950). In a different situation, the average
seasonal recovery for DDT applied as a thermal aerosol ranged from 10
to 12%, while that for spray ranged from 56 to 76% (Tarzwell, 1950).
However, somewhat lower recoveries of DDT sprays have frequently been
reported: 30% (Hoffman & Merkel, 1948), 39% (Hoffman & Surber, 1948),
and 27% (Surber & Friddle, 1949).
Most DDT particles that miss the target for which they are
intended would be expected to fall in the general area. Studies of
residues in soil and in wildlife indicate that this is true. The
logarithm of the concentration of DDT-related compounds in soil
samples collected in a desert area downwind from an area of intensive
agriculture showed an almost straight line inverse relationship to the
logarithm of the distance from the source. The soil levels were about
1 mg/kg at 10 m and about 0.001 mg/kg at 100 000 m from the point of
application. The concentrations of DDT in the tissues of wildlife were
proportional to its concentrations in the soils of their habitats
(Laubscher et al., 1971). Where cultivated fields to which DDT has
been applied for years are interspersed among pastures and other
fields where DDT is not used, the uncultivated soils contain only a
little less DDT than the cultivated ones. In one study, it was found
that most of the DDT was in the top 2.5 cm, but samples for comparison
were taken to a depth of 7.5 cm; the average concentrations were 0.75
to 2.03 mg/kg in cultivated soil and 0.10 to 0.91 mg/kg in
uncultivated sod (US Department of Agriculture, 1966). These
concentrations may be compared (by mathematical calculation alone) to
1.0 mg/kg, the concentration resulting from mixing DDT into soil
uniformly and with no loss whatever following application at the rate
of 1.12 kg/ha, the standard specific gravity (1.47) of soil and a
depth of 7.6 cm being assumed. The range of specific gravities for
most agricultural soils is 0.4 to 2.0 and the corresponding depths
yielding 1 mg/kg are 28 to 5.60 cm. It is of interest that the
residues in cultivated soft were of the order of magnitude that would
be expected from a single application, even though the average
cumulative rate of application during the last 10 years (11.2 kg/ha)
had been 10 times as great.
4.2 Distant Drift in Air
Dust bearing DDT at a concentration of 0.6 mg/kg has been observed
about 1600 km from its area of origin. Other pesticides were also
present in this dust which had settled out on a recently rain-rinsed
roof. The cloud of dust was so dense and unusual that its progress was
reported in newspapers, as the storm that mobilized it in Texas
carried it at least as far as Ohio, where the sample was collected
(Cohen & Pinkerton, 1966).
Dust collected on the island of Barbados by means of nylon nets
treated with 50% aqueous glycerine was assumed to have been blown from
Africa, a distance of over 4850 km. Samples of the dust contained
p,p'-DDT most frequently and in greatest concentration, but the
concentration of all pesticides was only 0.001 to 0.164 mg/kg with an
average of 0.041 mg/kg (Risebrough et al., 1968).
DDT evaporates from sprays and dusts at the time of application
and at any time that dust bearing the compound is mobilized by the
wind. Furthermore, DDT can be detected over treated fields for more
than six months after application, and there is a concentration
gradient from the soil upward (Willis et al., 1971). Under these
circumstances, it is obvious that DDT is transported in the form of a
vapour as well as by means of dust. However, if samples are collected
where no application of DDT has been made, identification of the
origin of the vapour or measurement of the distance it may have
travelled is even more difficult than with dust.
Evidence that DDT in one form or another has travelled great
distances and is, in fact, worldwide in its distribution has been
deduced from finding it in the rainwater of remote, nonagricultural
places (Tarrant & Tatton, 1968) or in water melted from Antarctic snow
(0.00004 mg/litre) (Peterie, 1969). In such remote places as
Eskdalemuir in Scotland and Lerwick in the Shetland Islands, the
average concentrations of p,p'-DDT in rainwater (0.000030 and
0.000046 mg/litre, respectively) were not greatly different from the
averages (0.000018-0.000066 mg/litre) found in widely separated
agricultural areas, suggesting that the compound is rather evenly
distributed in the air (Tarrant & Tatton, 1968).
4.3 Distribution in Water
DDT has a strong tendency to adsorb on surfaces. Most DDT that
enters water is already firmly attached to soil particles, and remains
attached. It was shown very early that, if DDT does find its way into
clear water, it is gradually lost by adsorption on surfaces (Carollo,
1945). The sediments in water tend to move downstream and eventually
to enter estuaries. Of the various chlorinated hydrocarbon
insecticides, DDT and its metabolites are the ones most commonly
found, but the residues tend to be low (Butler, 1969). In fact, in an
estuary associated with the Mississippi River, the levels of
pesticides decreased strikingly from the early 1960s to the late 1960s
(Rowe et al., 1971).
4.4 Bioaccumulation of DDT and Its Degradation in the Environment
Many studies of DDT and related compounds in the environment have
focused on organisms and locations in which concentrations of DDT have
been observed to increase. Concentration in living organisms may be
the result of adsorption from water, of the filtering out of algae or
detritus bearing the compound, or of biological magnification in the
strict sense, that is progressive accumulation in different steps of a
food chain. Although the mechanisms are poorly understood, observation
has shown that residues do reach an equilibrium and sometimes decline.
For example, where DDT was applied to cotton at a cumulative rate of
11.2 kg/ha so that a residue of over 10 mg/kg in the soil would be
expected after many years of continual use, the actual residues ranged
from 0.75 to 2.03 mg/kg (USDA, 1966). An example of decreased residues
was seen in the grebes of Clear Lake (Rudd & Herman, 1972).
Evidence is accumulating that the disintegration of DDT may be
rapid in some situations. Under biologically active, anaerobic
conditions as little as 1% of DDT remained after 12 weeks of
incubation (Hill & McCarty, 1967; Guenzi & Beard, 1968). Probably more
important is the disintegration of DDT under the influence of
ultraviolet light. Hartley (1969) pointed out that much of any
pesticide vapour escaping to 50 metres or more above the ground will
ascend even higher by eddy diffusion and eventually reach the
photochemically active ionosphere. The rapid destruction of DDT by
ultraviolet light under laboratory conditions has been demonstrated
(Mosier et al., 1969; Plimmer et al., 1970; Miller & Narang, 1970;
Plimmer & Klingebiel, 1973; Crosby & Moilanen, 1977). Gab et al.
(1975, 1977) offered evidence that DDT and DDE are converted to carbon
dioxide and hydrochloric acid; the destruction was so complete that
they characterized it as photomineralization. In spite of very real
progress in understanding the fate of DDT in the environment (see
Annex), much more work will be required before a quantitative balance
can be measured between addition of the compound and its
disintegration.
5. ENVIRONMENTAL EXPOSURE LEVELS
5.1. Exposure of the General Population
5.1.1 DDT in air
In spite of the generalization in section 4.2 that DDT is rather
evenly mixed in the air, some increase in concentration may be noted
in connexion with the time and place of application. The highest
concentration of insecticide found in the air of communities with
anti-mosquito fogging programmes was 0.0085 mg/m3 (Tabor, 1966).
Concentrations one or two orders of magnitude greater have been
reported for several insecticides in the breathing zone of orchard
spraymen, and values of 1.2-0.26 mg/m3 have been found at distances
of 500-5000 m from ground spraying (Belonozko et al., 1967). In six
small communities in an agricultural area in the USA, DDT was found in
concentrations ranging from 1 × 10-6 to 22 × 10-6 mg/m3 (Tabor,
1966). Substantially higher values (0.002-0.05 mg/m3) were reported
for centres of population in the USSR (Belonozko & Kucak, 1974). In an
urban location in a generally nonagricultural area of the USA, the
highest concentration found was 2.36 × 10-6 mg/m3 (Antommaria et
al., 1965). The combined concentrations of DDT, dieldrin, and lindane
in the Munich area of the Federal Republic of Germany in 1971 were
even lower, only exceptionally rising as high as 1 × 10-6 mg/m3
(Weil et al., 1973).
With few exceptions, the highest average concentration of DDT in
air to which workers are exposed regularly (7.1 mg/m3) is that
associated with spraying the interior of houses (Wolfe et al., 1959).
However, concentrations ranging from 2 to 104 mg/m3 have been
reported in places where DDT dust was prepared and packed (Medved' et
al., 1975).
5.1.2 DDT in water
Under agricultural conditions, the concentration of DDT in water
may be high. For example, 0.01 mg/litre was found in the runoff from
melting snow from fields where sugar-beets had been grown (Medved' et
al., 1975).
The highest level at which DDT has been found in rainwater in an
urban area during a period of a month is 0.0004 mg/litre (Abbott et
al., 1965). The highest concentration reported in potable water
(0.02 mg/litre) occurred some years ago (Middleton & Lichtenberg,
1960). In a much more comprehensive study made a few years later, the
highest concentration of any insecticide was found (0.00012 mg/litre),
but this was dieldrin and not DDT (Weaver et al., 1965). Many samples
did not contain detectable insecticide of any kind. A study of surface
waters in the USA during the years 1964-1968 indicated that the
residues reached a peak in 1966 and then dropped sharply in 1967 and
1968, in spite of improved analytical methods. The highest value for a
DDT-related compound in those years was 0.00084 mg/litre (Lichtenberg
et al., 1970). By 1971, the concentration in the Federal Republic of
Germany was even lower, averaging 0.00001 mg/litre and never going as
high as 0.001 mg/litre (Weil et al., 1973). The average values for
total DDT in drinking water in Czechoslovakia were 0.000011 and
0.000015 mg/litre in 1972 and 1973, respectively (Hruska & Kociánová,
1975). DDT was not detected (<0.0000000166 mg/litre) in tap water in
a recent survey carried out in Ottawa, Canada (McNeil et al., 1977).
5.1.3 DDT in food
Residues of DDT were measured as early as 1945 (before the
compound was available commercially) on apples to which it had been
applied experimentally for the control of the coddling moth (Harman,
1946). Apparently, the earliest effort to learn how much DDT the
average man obtains from his daily food was that of Walker et al.
(1954), who reported that the amount of insecticide in restaurant
meals in Wenatchee, Washington, USA indicated an average intake of
0.184 and 0.102 mg/man per day for DDT and DDE, respectively. Soon,
other studies revealed similar levels of DDT intake for persons who
ate an ordinary range of foods but who lived in different parts of USA
(Hayes et al., 1956; Durham et al., 1965b). Most of the DDT was in
food of animal origin, and persons who abstained from eating meat but
obtained the food they ate from regular, commercial sources received
an average of only 0.041 and 0.027 mg/man per day of DDT and DDE,
respectively (Haynes et al., 1958). The difference did not depend,
however, on meat per se, for no DDT and only traces of DDE were
found in the meat and other products obtained from Arctic wildlife
that constituted much of the diet of Eskimos (Durham et al., 1961).
Following restrictions on the application of DDT to livestock, to
their barns, and to the forage crops on which they fed, there was a
gradual decrease in residues in animal products used as human foods.
Restrictions on the use of DDT on crops eaten directly by man resulted
in reduced residues in vegetable foods. Complete meals collected
mainly from the same restaurants in Wenatchee and analysed in the same
laboratory indicated that, by 1964, DDT intake was only 0.038 mg/man
per day (Durham et al., 1965b) compared with 0.184 mg/man per day
reported by Walker et al., 11 years earlier. Thus, intake had been
reduced to less than one-fourth. A further reduction to about one-
eighth of the 1953-54 values was indicated by the nationwide study by
the US Food and Drug Administration, usually called the Market Basket
Survey. Intakes of DDT indicated by this study for the succeeding
years 1965-70, were 0.031, 0.041, 0.026, 0.019, 0.016, and
0.015 mg/man per day, respectively (Duggan & Weatherwax, 1967; Duggan,
1968; Duggan & Lipscomb, 1969; Duggan & Corneliusen, 1972).
The widespread shipment of food in the USA tends to explain the
fact that food residues are generally similar in different parts of
the country. However, small differences do exist and may be accounted
for by the fact that, on average, meat is not shipped as far as
vegetables. Much of the feed for livestock is produced on the same
farm or at least in the same area in which the animals are raised.
Whatever the reason, the coefficients of correlation between latitude
and human intake were -0.63 and -0.59 for the sampling periods 1966-67
and 1967-68, respectively (Hayes, 1975).
Early studies (Swackhamer, 1965) indicated that both the frequency
and concentration of residues were slightly less in Canada than in the
USA. More recent studies in Canada similar to the Market Basket Survey
indicated total DDT dietary intakes of 0.018, 0.011, 0.011, 0.007 and
0.007 mg/man per day for the years 1969 to 1973, respectively (Smith,
1971; Smith et al., 1972, 1973, 1975).
The analysis of whole meals collected in south-east England during
1965 and 1966 gave results similar to those obtained during the same
period in the USA; the calculated daily intakes in England were 0.030
and 0.025 mg/man per day for DDT and DDE, respectively (McGill &
Robinson, 1968).
There may be considerable differences in intake in different parts
of the same country. Hruska & Kociánová (1975) reported that the
average intake of DDT plus DDE in 1972 was 0.002 mg/man per day in
southern Bohemia and was 0.099 mg/man per day in Slovakia. Striking
differences may exist between urban and rural areas (Almeida et al.,
1975).
Values for total DDT-related compounds in regular food in the USSR
are not available, but the separate values for DDT and DDE in daily
diets from different regions shown in Table 3 suggest that the total
may be higher than in the UK and USA and that the amount of DDE may
exceed the amount of DDT. Both high total values and an unusually high
proportion of DDE would be consistent with extremely high total values
a few years earlier and recent discontinuation of the use of DDT. In
fact, in the Soviet Union, DDT has been eliminated from the list of
pesticides recommended for use in agriculture since 1970. During the
period 1966-69, 0.8% of food samples contained residues as high as
5.1 mg/kg, and 4.2% contained residues over 1.0 mg/kg.
Although total intake of DDT from food has not been measured in
some parts of the world, worldwide measurements of storage of DDT and
its metabolites in human body fat indicate that the extremes of total
exposure have varied by a factor of about 10, but that total exposure
for most populations has varied by a factor of no more than 3 (see
Table 9).
Table 3. Residues of DDT and DDE in daily cooked diets from
different areas of the USSR during 1971/72a
Area No. of Range of residues of
daily
diets DDT DDE
examined (mg/diet)b (mg/diet)b
North 121 0.00006-0.0103 0.0001-0.0093
West 191 traces-0.094 traces-0.039
South-east 62 traces-0.15 traces-0.330
South I 184 0.02-0.260 0.020-0.5
South II 51 traces-0.052 traces-0.132
a From: Medved' et al. (1975).
b Equivalent to mg/man per day.
It is important to note that local practice may result in high
residues in the food of one or more families even though residues are
low in commercially produced food available in the same area. Thus
Durham et al. (1965b) reported that the average DDT intake from
household meals in Wenatchee was 0.314 mg/man per day at the same time
that restaurant meals in that town contributed only 0.038 mg/man per
day. The difference was largely the result of very high residues in
some eggs eaten by local families; the chickens foraged near orchards,
which had been treated with DDT. The restaurants used commercially
available eggs and not those produced locally. In a similar way,
practices peculiar to one country may account for high residues in
some of their food. For example, very high residues of DDT were
reported in some samples of staple foods in India (Sharangapani &
Pingale, 1954). Dale et al. (1965) suggested that the high levels of
DDT that they found in some Indians might be the result of direct
addition of the compound to staple food to prevent insect infestation,
even though the practice did not have government approval.
5.1.4 Miscellaneous sources
It has been suggested that there is a positive correlation between
the use of household insecticides and the concentration of DDT in
house dust, on the one hand, and the storage of DDT in people on the
other (Deichmann & Radomski, 1968; Radomski et al., 1968; Davies et
al., 1969b, 1975; Edmundson et al., 1970b). However, another study of
dust in 16 urban households, 4 farm households, and 8 households in
which at least one member was a pesticide formulator, failed to reveal
a statistically significant correlation between the levels of various
pesticides in dust and in the serum of people living in the homes.
There were striking individual examples of workers whose homes
contained high concentrations of the compounds they used
professionally and other examples in which there was circumstantial
evidence relating household dust residues to body burden (Starr et
al., 1974).
There can be no doubt that insecticides used in the household or
introduced on the clothing of workers are important sources of intake
of DDT in some instances. It is not clear whether the relevant
absorption involves mainly the inhalation of dust, the contamination
of food within the home, or even dermal absorption.
5.1.5 Relative importance of different sources
It has been estimated (Campbell et al., 1965) that over 90% of the
DDT stored in the general population is derived from food. About 1965,
intake in the USA was approximately 0.04 mg/man per day from food,
less than 0.000046 mg/man per day from water, less than 0.00006 mg/man
per day from urban air and less than 0.0005 mg/man per day from air in
small agricultural communities. The reason for the qualification "less
than" is that the intakes were calculated from the highest
concentrations reported in drinking-water and air because no average
values were established.
Other investigators (Durham et al., 1965b; Morgan & Roan, 1970;
Medved' et al., 1975) concluded independently that ordinary food is
the major source of DDT and related compounds for the general
population. Intake from ordinary food is a base to which other kinds
of intake -- including that from exceptional food -- may be added. An
example of such an addition -- eating eggs from chickens allowed to
run loose in DDT-treated orchards has already been discussed in
section 5.1.3.
5.2 Exposure of Infants and Young Children
Babies tend to be born with slightly lower blood levels of DDT
than are found in their mothers (O'Leary et al., 1970b; Schvartsman et
al., 1974, see also section 6.2.2). This simply indicates that the
placenta excludes some but not all of the DDT available to it. During
the first 10 or 15 years of life, DDT storage levels rise to the adult
population level (Hayes et al., 1958; Hunter et al., 1963; Wassermann
et al., 1965, 1967; Robinson et al., 1965; Davies et al., 1968, 1969a;
Watson et al., 1970).
It has been known for a long time that human milk may contain a
higher concentration of DDT than cow's milk in the same country (Egan
et al., 1965; Quinby et al., 1965b; Ritcey et al., 1972; Olszyna-
Marzys et al., 1973). So far, there is no evidence that this small
difference is of any significance for breast-fed compared with bottle-
fed babies. This is even true in places where the concentration of DDT
in human milk is comparatively high (see section 6.3.1.4).
On the average, the incidence and levels of residues in
commercially prepared food for babies in the USA are lower than those
in raw agricultural products, other processed foods, or samples
examined in the Market Basket Survey (Lipscomb, 1968).
The only DDT exposures of infants that are known to have injured
them in any way are those involving direct access to formulations that
they ate or drank. Such tragic accidents often involved formulations
transferred to unlabelled food or beverage containers. Frequently, the
container was left where a child could reach it easily. Occasionally,
formulations have been stored with food or have ever been handed to a
child as food, or "empty" containers that still contain enough
formulation to kill a child have been carelessly discarded (Hayes,
1975, 1976a).
5.3 Occupational Exposure
In general, annual exposure to DDT is greatest among manufacturers
and formulators, moderate, among those applying it for agricultural
purposes, less among the general population, and least among special
groups whose location or practices minimize their exposure. However,
for brief intervals the exposure during agricultural application may
exceed anything that good industrial practice permits. This
distinction is of great importance in connexion with the more toxic
organic phosphorus compounds, a single heavy exposure to which may
result in poisoning, but is of no known importance in connexion with
DDT, the acute toxicity of which is much less. However DDT has a
greater tendency to storage in the body.
Occupational exposure to DDT is reflected quantitatively by the
concentration of DDT and DDE in blood and fat and by the concentration
of DDA in urine. These aspects of occupational exposure are considered
later. This section outlines measurements that have been made of the
actual degree of exposure under different circumstances.
The most striking result is that the occupational exposure through
areas of skin that are frequently unclothed (face, hands, forearms,
neck, and "V" of chest) is far greater than total respiratory
exposure. Results for DDT are shown in Table 4 (based on measurements
made by methods described by Durham & Wolfe (1962, 1963)). If a
workman has bare feet or legs or does not wear a shirt, the contrast
between respiratory and dermal exposure will be even greater than that
shown in Table 4 which is based on "standard" clothing.
Table 4. Measured respiratory and dermal exposure of workers
to DDT under actual conditions of work
Activity Respiratory Dermal Reference
(mg/kg) (mg/kg)
Indoor house spraying 3.4a 1755 Wolfe et al., 1959
Outdoor house spraying 0.11 243 Wolfe et al., 1959
Spraying forests 4-92b 212b Wassermann
et al., 1960
a Measurement by respirator pad technique.
b Calculated from values given in the original paper.
It has been reported that DDT and a number of other pesticides
persist, for days or even years after last use, on the hands of
workers exposed to them and that at least a part of the material can
be removed for analysis by rinsing the hands in hexane. Evidence that
the residues represented unabsorbed pesticides and not excretion of
stored material included the fact that no correlation was found
between residues on the hands and in the sera of individuals and that
no residues could be found on the hands of a farmer who used
rubberized gloves while working with pesticides and who washed
afterwards with strong cleansing agents (Kazen et al., 1974). The fact
that urinary excretion of DDA may be increased for several days after
a single occupational exposure to DDT (Wolfe et al., 1970) is evidence
that absorption continues for a week or so but not for longer periods.
Furthermore, Wolfe et al. (1970) found that the rate of excretion,
even when detectable, was small compared to the excretion of parathion
metabolite in workers exposed simultaneously to DDT and parathion at a
ratio of 1.0 to 0.25. This emphasizes the minimal dermal absorption of
DDT.
6. METABOLISM OF DDT
6.1 Uptake
6.1.1 Uptake by inhalation
Most DDT dust is of such large particle size (>250 µm) that any
that is inhaled is deposited in the upper respiratory tract and is
eventually swallowed (Hayes, 1975). Toxicity data indicate that
respiratory exposure is of no special importance.
6.1.2 Uptake from the gastrointestinal tract
A review of the early literature indicates that absorption of DDT
from the gastrointestinal tract is slow. Whereas intravenous injection
at the rate of 50 mg/kg produces convulsions in rats in 20 min,
convulsions occur only after 2 h when DDT is administered orally at
two or more times the LD50 value. The onset of convulsions is
delayed for about 6 h when DDT is given to rats orally at
approximately the LD50 value (Dale et al., 1963).
Early studies based on toxicity indicated that DDT, dissolved in
animal or vegetable fats, was absorbed from the gastrointestinal tract
about 1.5 to 10 times more effectively than undissolved DDT. There was
also evidence that large doses of the compound in the gastrointestinal
tract were poorly absorbed from nonabsorbable solvent (Hayes, 1959).
However, in connexion with small repeated doses, the presence or kind
of solvent made little difference; apparently the occurrence of bile
in the intestine and the presence of some fat in the diet were
sufficient to promote absorption of the compound. At high dosage
levels, less 14C-DDT was absorbed and stored in organs and a higher
proportion was excreted in the faeces following oral administration
than after intraperitoneal administration (40% versus 0.9%) (Bishara
et al., 1972b).
Rothe et al. (1957) reported that after giving radioactive DDT to
rats by stomach tube, 41-57% of it was recorded in lymph drained from
the animal by means of a cannula. Less than 0.1% of the activity was
found in the urine, 7.4-37.1% was found in the faeces or in the
intestinal contents when the animals were killed, and 19%-67% of the
activity was found in the carcass. The total dose accounted for
analytically varied from 89% to 118%, thus recovery was complete
within the accuracy of the method. Of the administered DDT not found
in faeces and intestinal contents, 47%-65% was found in the lymph. The
animals that withstood the operation best had peak lymph flows of
nearly 6 ml/h. In these animals, DDT was absorbed at rates as high as
381 µg/h; the rate of absorption reached a maximum within 2-3 h of
intubation and was markedly reduced by the fourth hour. Fifty per cent
of the DDT-derived material found in the lymph was absorbed in the
first 2.5-7 h, and 95% was absorbed within 18 h. Because the lymphatic
duct in the rat is not a single vessel, Rothe et al. (1957) were
unable to exclude the possibility that some, or all, of the DDT that
they later recovered from the carcasses of their animals had been
transported to the general circulation by collateral lymph vessels
rather than by the hepatoportal system. Thus, at least half, and
perhaps all absorption of DDT is by way of the lymph. However, in
studies on rats by Heath & Vandekor (1964), only a small proportion of
dieldrin was absorbed by the lymph. The reason for the marked
difference in the absorption of those organochlorine insecticides is
unknown.
6.1.3 Uptake from the skin
Undissolved DDT is so poorly absorbed through the skin that its
toxicity by this route is difficult to measure. Even dissolved DDT is
poorly absorbed by the skin as indicated by low toxicity (see
Table 5).
Table 5. Acute oral and dermal LD50 of DDT for animalsa
Species Formulation Oral Dermal
(mg/kg) (mg/kg)
Rat Water suspension or powder 500-2500 1 000 000
Oil solution 113-450 250-3000
Mouse Water suspension or powder 300-1600 375 000
Oil solution 100-800 250-500
Guineapig Water suspension or powder 2000 1 500 000
Oil solution 250-560 1000
Rabbit Water suspension or powder 275 375 000
Oil solution 300-1770 300-2820
Cat Water suspension or powder
Oil solution 100-410
Dog Water suspension or powder
Oil solution >300
a From: Hayes (1959).
6.2 Distribution and Storage
6.2.1 Human studies
6.2.1.1 Studies of volunteers
In a study of volunteers who received technical DDT at rates of 0,
3.5, and 35 mg/man per day, the average intakes resulting from dosing
and from traces of DDT in food were 0.0025, 0.05, and 0.5 mg/kg per
day (Hayes et al., 1956). The storage of DDT was proportional to
dosage, but there was an unexplained difference in the storage of the
p,p'-isomer and of technical DDT. For example, following dosing for
12 months, pure p,p'-DDT was stored in fat at an average
concentration of 340 mg/kg, but the sum of isomers from technical
material was stored at an average of only 234 mg/kg. The difference
was statistically significant for the 3.5 mg/man per day dosages given
for 3-6 and for 7-18 months. The difference was significant for the
35 mg/man per day doses after 7-18 months of dosing but not after only
3-6 months.
Men who ate p,p'-DDT showed a definite increase in the absolute
amount of DDE stored. After 6 months at a dosage of 35 mg/man per day,
8 men showed an average concentration of DDE stored in fat of 33 mg/kg
compared with 12 mg/kg for the same individuals at the beginning of
the investigation. There was a further increase in DDE storage as
exposure progressed. However, DDT was stored in so much greater
concentration that the relative storage of DDE decreased sharply.
Thus, after 6 months at a dosage of 35 mg/man per day, 8 men stored
only 14% of their total DDT-derived material in the form of DDE
compared with 65% for the same person at the beginning of the
investigation.
The storage of DDE by men who ate technical DDT presented a
different picture. Until 18 months after exposure, there was no clear
evidence that these men stored any more DDE after exposure than they
did before. However, at 18 months, the only 3 samples available showed
DDE concentrations ranging from 28 to 85 mg/kg, all substantially
above general population levels. Thus, both the total amount stored
and the rate at which DDT converted to DDE served to distinguish the
metabolism of p,p'-DDT and the sum of isomers present in technical
DDT in man (Hayes et al., 1956). A more rapid excretion was
demonstrated for o,p'-DDT by Morgan & Roan (1972).
In a second study (Hayes et al., 1971), volunteers received the
same doses used in the first study. Again, storage of DDT was
proportional to dosage. Although, in this instance also, the storage
of technical DDT was less than that of p,p'-DDT, the difference was
not statistically significant. The real but very gradual accumulation
of DDE was confirmed.
A steady state of storage was approached later in the second study
(18.8-21.5 months) than in the earlier one (about 12 months). However,
although the second study was superior in that more men were studied
for a longer period, it was inferior in that dosage was less regular.
Because of this, it seems impossible to decide whether 12 months or
21.5 months is a more valid estimate of the time necessary for people
to approach a steady state of storage when intake is uninterrupted and
unvarying in amount. It is interesting that the storage levels
eventually reached at the same dosage in the 2 studies were
statistically indistinguishable in most instances (see Table 6). In
the one instance in which a statistical difference existed, the
greater storage by men in the second study may have been explained by
the fact that some of them inadvertently received higher doses than
intended.
Table 6. Storage of DDT in volunteers
Type of DDT Added Concentration of DDTa Significance
dosage First studyb Second studyc of difference
(mg/man 11 months or more 21.5 months (P)
per day) (mg/kg) (mg/kg)
Technical 0 8-17 (12.5 ± 4.5) 16-30 (22.0 ± 2.9) > 0.1
3.5 26-33 (23.8 ± 1.4) 59-76 (50.2 ± 5.6) <0.025
35 101-367 (234 ± 21.4) 105-619 (281 ± 79.5) >0.4
Recrystallized 35 216-466 (340 ± 36.4) 129-659 (325 ± 62.2) >0.2
a Range, mean, and standard error.
b Hayes et al., 1956,
c Hayes et al., 1971.
There was a slow decrease in the levels of fat-stored DDT after
dosing ceased. The concentration remaining following 25.5 months of
recovery was from 32% to 35% of the maximum stored for those who had
received 35 mg/man per day but 66% for those who had received only
3.5 mg/man per day, indicating slower loss at lower storage levels
(Hayes et al., 1971).
Morgan & Roan (1971) fed volunteers not only technical DDT but
also p,p'-DDE and p,p'-TDE. They found that DDE was stored more
tenaciously than the other compounds in man, the order being p,p'-DDE
> p,p'-DDT > o,p'-DDT > p,p'-TDE. The slow metabolism of DDT to
DDE was confirmed. It was noted that p,p'-DDT was lost from storage
in adipose tissue much more slowly in man than in the monkey, dog, or
rat.
Less than 18% of p,p'-DDT and p,p'-DDE is carried in human
erythrocytes. In plasma of ordinary fat content, less than 1% of all
DDT-related compounds is carried by the chylomicrons. Instead, these
compounds are carried by proteins and are undetectable in plasma from
which protein has been precipitated. Following ultracentrifuging,
p,p'-DDT and p,p'-DDE are found mainly in the triglyceride-rich,
low density, and very low density lipoproteins. Following continuous
electrophoresis, these compounds are found mainly in association with
plasma albumin and alpha-globulins (Morgan et al., 1972).
6.2.1.2 Studies of occupationally exposed workers
The highest reported storage of DDT and related compounds remains
that of a healthy worker whose fat contained DDT and DDE (as DDT) at
concentrations of 648 and 483 mg/kg, respectively (Hayes et al.,
1956). Laws et al. (1967) reported considerably lower storage values
among the most exposed persons in a DDT manufacturing plant (see
Table 7).
An important point evident from the table is that, whereas almost
all investigations of workers are said to have been carried out on
"heavily exposed" populations, some of the groups studied had absorbed
little more DDT than is absorbed by the general population --
especially the general population of some tropical countries.
A different situation is indicated in a report by Genina et al.
(1969) who used a total chloride method to analyse samples of blood
from controls and from persons with occupational exposure to DDT,
polychloropinene, and HCH. Whereas the highest average concentration
of total DDT-related material per se in the serum of a worker in the
USA was 2.7 mg/litre (Laws et al., 1967), Genina et al. (1969)
reported organochlorine compounds as high as 38.4 mg/litre in the
blood of a pilot and values as high as 195 mg/litre in the blood of
warehousemen. This concentration is about 20 times the highest value
found by the same authors in their control group (see Table 8). The
factor of 20 is not remarkable, but (especially in view of the fact
that polychloropinene and HCH are excreted more readily than DDT and
DDE) values as high as 0.2 mg/litre in the controls are unexpected.
Table 7. Average concentration of the sum of the isomers of DDT and DDE in fat and serum
and of DDA in the urine of workers engaged in the manufacture, formulation, or
use of DDT
Tissue No. of DDT DDE DDA Total as Estimated Reference
men (mg/kg) (mg/kg) (mg/kg) DDT exposure
(mg/kg) (mg/man
per day)
fat 1 648 437 1131 Hayes et al., 1956
urine 10 0.57 14 Ortelee, 1958
urine 16 1.7 30 Ortelee, 1958
urine 13 2.9 42 Ortelee, 1958
fat 3 51 44 98 3.6 Laws et al., 1967
fat 12 74 50 130 6.2 Laws et al., 1967
fat 20 161 91 263 18 Laws et al., 1967
serum 3 0.2113 0.1968 0.5412 6.3 Laws et al., 1967
serum 12 0.1420 0.1454 0.3584 8.4 Laws et al., 1967
serum 20 0.3020 0.2719 0.7371 17.5 Laws et al., 1967
urine 3 0.0165 0.0203 0.41 0.5629 Laws et al., 1967
urine 12 0.0145 0.0222 0.6 0.7911 Laws et al., 1967
urine 20 0.0145 0.0271 1.27 1.6296 Laws et al., 1967
fat 18 5.2-45.2 Gracheva, 1969
urine 136 0.402 Perini & Ghezzo, 1970
urine 110 0.142 Perini & Ghezzo, 1970
urine 290a 0.061 Perini & Ghezzo, 1970
plasma 16 0.0513 0.0722 0.1321 Wassermann et al., 1970c
serum 4 <0.087 <0.072 Edmundson et al., 1970a
urine 0.080 Edmundson et al., 1970a
serum 18 0.573 0.506 Poland et al., 1970
serum 5 0.004a 0.021a Clifford & Well, 1972
serum 10 0.022 0.055 Clifford & Well, 1972
serum 21 0.021a 0.013a Keil et al., 1972
blood 44 0.761 WHO, 1973
blood 100 1.273 WHO, 1973
Table 7 (Cont'd)
Tissue No. of DDT DDE DDA Total as Estimated Reference
men (mg/kg) (mg/kg) (mg/kg) DDT exposure
(mg/kg) (mg/man
per day)
serum 21 0.300 0.379 0.681 Almeida et al., 1974
serum 25 0.225 0.308 0.504 Almeida et al., 1974
serum 18 0.345 0.257 0.602 Almeida et al., 1974
serum 56 0.004a,b 0.052a,b Morgan & Roan, 1974
serum 32 0.002b 0.026b Morgan & Roan, 1974
serum 32 0.004b 0.047b Morgan & Roan, 1974
serum 32 0.009b 0.075b Morgan & Roan, 1974
serum 31 0.052b 0.222b Morgan & Roan, 1974
plasma 25 1.030 Rabello et al., 1975
plasma 8 0.240 Rabello et al., 1975
plasma 23 0.0389 Richardson et al., 1975
a Control group.
b Approximately equal groups arranged by degree of storage.
Table 8. Percentage of workers with blood organochlorine compound content falling in certain
ranges of concentrationa
Subject group Range of concentrations
0 0.2-0.9 1.0-3.0 4-9 10-50 50
(mg/litre) (mg/litre) (mg/litre) (mg/litre) (mg/litre) (mg/litre)
Control group (47) 21.3 44.7 23.4 10.6 0 0
Pilots (134)
group A 19.1 35.3 25.0 14.7 5.9 0
group B 22.9 27.1 40.6 7.4 2.0 0
Technicians (133)
group A 26.2 16.4 39.3 8.2 9.9 0
group B 39.4 31.2 25.7 3.7 0 0
Agricultural 13.0 43.5 31.0 7.0 2.0 3.5
workers (55)
a From: Genina et al. (1969).
NB: Group A gives the results of investigations at the time of work, group B before work
or a few months after termination. Agricultural workers were studied only during work.
The first evidence that a part of the DDT absorbed by man is
metabolized to DDE was obtained from the analysis of fat from a DDT
plant worker (Mattson et al., 1953).
6.2.1.3 Studies of the general population
DDT in fat. Table 9 summarizes the results of measurements of
DDT and related materials in the body fat of people without
occupational exposure. Several broad generalizations can be made from
the table and from what is known about residues of DDT in food in
different countries. DDT storage in man corresponds with exposure; in
general, exposure tends to be greater in warm climates where there is
a greater need for insecticides. This climatic dependence may be
observed even within a single country (Hayes, 1975). Where
measurements have been made during a sufficiently long period, storage
has decreased as the use of DDT, especially that leading to residues
in food, has decreased.
The method of DDT analysis shifted in about 1962 from the
Schecter-Haller colorimetric method to the gas chromatographic method.
However, as noted by Hayes (1975), this made little difference to the
overall results. Thus the absolute decrease of total DDT storage and
the relative increase of DDE storage observed in some countries is
real.
Circumstances peculiar to some subpopulations explain their
unusual storage of DDT. Thus, persons living in one of the contiguous
states of the USA stored significantly less DDT than other persons in
the state because they did not eat meat (Hayes et al., 1958). Even
less storage of DDT was observed in Eskimos whose diet contained an
unusually high proportion of meat obtained from wildlife in which no
DDT and almost no DDE could be detected (Durham et al., 1961).
No significant difference was found in the concentration of DDT
stored in the fat of different parts of the body (Hayes et al., 1958;
Casarett et al., 1968).
DDE in fat. The accumulation of DDE relative to total DDT-related
compounds is best illustrated in man. Of the total DDT stored
in the fat of workers exposed to technical DDT (about 4% DDE) for
11-19 years, only 38% was in the form of DDE, and, of course, some of
that DDE came from their diets which included meat (Laws et al.,
1967). In India, where many people avoid meat but may consume milk,
cheese, and eggs, 34-41% of total DDT was DDE (Dale et al., 1965). In
the USA, during a time when DDT residues in food were decreasing, the
proportion of total DDT in the form of DDE increased from about 60% in
1955 to about 80% in 1970; during the same interval the concentration
of total DDT in body fat decreased from about 15 mg/kg to slightly
Table 9. Concentration of DDT-derived material in body fat of the general population
Country Year No. of Method of DDTa DDE as Total as DDE as Reference
samples analysis (mg/kg) DDT DDT DDT
(mg/kg) (mg/kg) (% of
total)
North America
Canada 1959-1960 62 colour 1.6 3.3 4.9 67 Read & McKinley, 1961
Canada 1966 47 GLCb and ELC 1.09 2.96 4.39 67 Brown, 1967
Canada 1967-1968 51 GLC 1.56 4.16 5.86 71 Kadis et al., 1970
Canada 1969 4.85 Ritcey et al., 1973
Canada GLC 5.83 Brown & Chow, 1975
USA 1942 10 colour NDc NDc NDc Hayes et al., 1958
USA 1950 75 colour 5.3 -- 5.3 -- Laug et al., 1951
USA 1955 49 colour 7.4 12.5 19.9 63 Hayes et al., 1956
USA 1954-1956 61 colour 4.9 6.8 11.7 58 Hayes et al., 1958
USA 1956 36 colour 5.5 10.1 15.6 65 Hayes et al.. 1971
USA 1961-1962 130 colour 4.0 8.7 12.7 69 Quinby et al., 1965a
USA 1961-1962 28d GLC 2.4 4.3 6.7 64 Dale & Quinby, 1963
USA 1962-1963 282 GLC 2.9 8.2 11.1 74 Hoffman et al., 1964
USA 1964 64 GLC 2.5 5.1 7.6 67 Zavon et al., 1965
USA 1964 25 GLC 2.3 8.0 10.3 77 Hayes et al., 1965
USA 1964-1965 18 GLC 9.0 Schafer & Campbell, 1966
USA 1964-1965 42 GLC 3.1 7.5 10.6 71 Radomski et al., 1968
USA 1962-1966 994 GLC 2.6 7.8 10.4 75 Hoffman et al., 1967
USA 1964-1965 12 GLC 3.79 7.7 11.5 67 Davies et al.. 1965
USA 1965-1967 17 GLC 3.1 5.5 56 Davies et al., 1968
USA 90 GLC 6.1 8.4 73 Davies et al., 1968
USA 17 GLC 4.6 7.8 59 Davies et al., 1968
USA 35 GLC 12.0 16.7 72 Davies et al., 1968
USA -- 42 GLC 3.13e 7.43 10.56 70 Fiserova-Bergerova et
al., 1967
Table 9 (Cont'd)
Country Year No. of Method of DDTa DDE as Total as DDE as Reference
samples analysis (mg/kg) DDT DDT DDT
(mg/kg) (mg/kg) (% of
total)
USA -- 30e GLC 1.33 5.17 6.51 79 Casarett et al., 1968
USA 29f GLC 1.35 4.91 6.31 78 Casarett et al., 1968
USA 30g GLC 1.16 4.99 6.17 81 Casarett et al., 1968
USA 1966-1968 70 GLC 1.54 5.15 6.69 77 Morgan & Roan, 1970
USA 1967 733 GLC 1.34 4.74 6.22 77 Yobs, 1969 (unpublished
data)
USA 1968 3104 GLC 1.56 5.96 7.67 77 Yobs, 1969 (unpublished
data)
USA 1967-1971 103 GLC 1.5 5.6 7.1 79 Warnick, 1972
USA 1970 200 GLC 1.9 8.0 9.9 81 Wyllie et al., 1972
USA 1969-1972 221 23.18 Burns, 1974
USA 1970 1412 7.87h,i Kutz et al., 1974
South America
Argentina 1967 37 GLC 5.5 6.5 13.2 -- Wassermann et al., 1968b
Brazil 1969-1970 38 GLC 1.4 2.7 4.1 Wassermann et al., 1972b
Venezuela 1964 38 GLC 2.9 7.4 10.3 72 Dale, 1971 (unpublished
data)
Europe
Austria 6.33 Pesendorfer et al., 1973
Belgium 20 GLC 1.2 2.1 3.3 64 Maes & Heyndrickx, 1966
Bulgaria 55 GLC 3.8 5.8 10.6n 57 Kaloyanova et al., 1972
Bulgaria 1971-1976 191 GLC 14.7 78 Rizov, 1977
Czechoslovakia 1963-1964 229 colour 5.5 4.1 9.6 43 Halacka et al., 1965
Denmark 1965 18 GLC 0.6 2.7 3.3 82 Weihe, 1966
Denmark 1972-1973 78 GLC 4.1 4.7 87 Kraul & Karloq, 1976
Table 9 (Cont'd)
Country Year No. of Method of DDTa DDE as Total as DDE as Reference
samples analysis (mg/kg) DDT DDT DDT
(mg/kg) (mg/kg) (% of
total)
Finland 1972-1974 73 GLC 2.5 Hattula et al., 1976
France 1961 10 colour 1.7 3.5 5.2 67 Hayes et al., 1963
German Democratic 1966-1967 100 GLC and TLC 3.7 9.47 13.1 71 Engst et al., 1967
Republic
German Democratic 34j 1.8 5.1 6.9 Engst et al., 1970
Republic
Germany, Federal 1958-1959 60 colour 1.0 1.3 2.3 57 Maier-Bode, 1960
Republic of
Germany, Federal 1970 20 GLC 1.1 2.5 3.6 69 Acker & Schulte, 1970
Republic of
Germany, Federal 9.8 Acker & Schulte, 1971
Republic of
Germany, Federal 4.24 Acker & Schulte, 1974
Republic of
Germany, Federal 4.77 Acker & Schulte, 1974
Republic of
Germany, Federal 5.42 Acker & Schulte, 1974
Republic of
Germany, Federal 8.36 Acker & Schulte, 1974
Republic of
Germany, Federal 7.8 Acker & Schulte, 1974
Republic of
Hungary 1960 48 colour 5.7 6.0 12.4 48 Denés, 1962
Italy 1965 9 GLC 1.8 3.2 5.0 63 Kanitz & Castello, 1966
Italy 1965-1966 18 GLC 2.58 8.28 10.86 76 Paccagnella et al., 1967
Italy 1966 22 GLC and TLC 4.69 10.69 15.48 68 Del Vecchio & Leoni, 1967
Italy 1970? 31 GLC 3.38 13.37 16.75 80 Prati & Del Dot, 1971
Italy 1970? 52 GLC 2.14a 8.46a 10.60a 80 Prati et al., 1972
Italy 1970? 33 GLC 0.84a 6.64a 7.48a 89 Prati et al., 1972
Table 9 (Cont'd)
Country Year No. of Method of DDTa DDE as Total as DDE as Reference
samples analysis (mg/kg) DDT DDT DDT
(mg/kg) (mg/kg) (% of
total)
Netherlands 1964 20 colour 1.6 6.1 7.7 79 Wit, 1964
Netherlands -- 11 GLC 0.32 1.89 2.22 86 deVleiger et al., 1968
Norway 56 GLC 3.2 71 Bjerk, 1972
Poland 1965 72 colour 13.4 Bronisz st al., 1967
Poland 65 colour 23.5 62 Bronisz et al.. 1969
Poland 1970 70 GLC 11.4 Juszkiewicz & Stec, 1971
Poland 1972 15 GLC 5.23 68 Bojanowska et al., 1973
Romania 1965 137 -- 13.4 8.3 21.7 39 Aizicovici et al., 1968
Romania 1972-1973 GLC 0.68 1.73 2.41 71 Ciupe, 1976
Spain 1966 41 -- 6.5 9.2 15.7 59 Unpublished data cited
by Wassermann et al., 1968
Switzerland 1.9-16.3 Zimmerli & Marek, 1973
United Kingdom 1961-1962 131 colour -- -- 2.2 -- Hunter et al., 1963
United Kingdom 1963-1964 66 GLC 1.1 2.2 3.3h 67 Egan et al., 1965
United Kingdom 1964 100 GLC -- -- 3.9h -- Robinson et al., 1965
United Kingdom 1964 44 GLC -- -- 4.0h -- Robinson & Hunter, 1966
United Kingdom 1965 101 GLC 1.13 1.72 2.85 60 Cassidy et al.. 1967
United Kingdom 1965-1967 248 GLC 0.78 2.22 3.00 74 Abbott et al., 1968
United Kingdom 1969-1971 201 GLC 0.5 1.8 2.5 72 Abbott et al., 1972
USSR 41 TLC 4.33 3.73 Vas'kovskaja & Komarova.
1967
USSR 197 TLC 5.3- 3.2- Vas'kovskaja, 1969
7.57 7.1
Africa
Kenya 5.4 Wassermann et al., 1972a
Nigeria 1967 43 GLC 5.4 3.1 8.8 Wassermann et al., 1968b
Nigeria 1969 41 GLC 2.1 2.8 6.5 57 Wassermann et al., 1972d
Table 9 (Cont'd)
Country Year No. of Method of DDTa DDE as Total as DDE as Reference
samples analysis (mg/kg) DDT DDT DDT
(mg/kg) (mg/kg) (% of
total)
Africa
South Africa 5.94 43 Wassermann et al., 1970b
South Africa 7.16 Wassermann et al., 1970b
Uganda 2.9 Wassermann et al., 1974a
Asia
India (Delhi area, 1964 67 colour 17 10 26 39 Dale et al., 1965
civilian)
India (other 1964 16 colour 8 5 13 37 Dale et al., 1965
cities, military)
India 94 GLC 13.8h 42 Ramachandran et al., 1974
Israel 1963-1964 254 colour 8.5 10.7 19.2 56 Wassermann et al., 1965
Israel 1965-1966 71 colour 4.6 Wassermann et al., 1967
Israel 1965-1966 133 colour 8.2 Wassermann et al., 1967
Israel 1967-1971 63 GLC 3.0 10.6 14.4 74 Wassermann et al., 1974b
Japan 1968-1969 241 GLC 0.6 1.8 2.4 75 Curlay et al., 1973
Japan 1969-1970 74 6.92 Nishimoto et al., 1970
Japan 1970 4.499 Suzuki et al., 1973
Japan 1971 2.694 Suzuki et al., 1973
Japan 1971 30 12.895 Kasai st al., 1972
Japan 1972 4.001 Suzuki et al., 1973
Japan 1972 42 5.992 Kasai et al., 1972
Japan 1973 6.44 Kawanishi et al., 1973
Japan 1974 6.87 Inoue et al., 1974
Pakistan 60 25.0 Mughal & Rahman, 1973
Thailand 1969-1970 77 12.6 Wassermann et al., 1972c
Oceania
Australia 1965 53 GLC 0.77h 1.03h 1.81h 57 Bick, 1967
Australia 1965-1966 46 colour 3.6 6.6 10.2 64 Wassermann et al., 1968a
Australia 1965-1966 12 GLC 3.0 7.1 10.5 68 Wassermann et al., 1968a
Australia 1971 75 GLC 4.94 Brady & Slyali, 1972
Table 9 (Cont'd)
Country Year No. of Method of DDTa DDE as Total as DDE as Reference
samples analysis (mg/kg) DDT DDT DDT
(mg/kg) (mg/kg) (% of
total)
Oceania
New Zealand 1966 52 GLC 1.6 4.2 5.8 72 Brewerton & McGrath, 1967
New Zealand 1965-1969 254 GLC 3.5 11.0 14.6 75 Copplestone et al., 1973
a p,p'-DDT and o,p'-DDT only. Total as DDT includes TDE (DDD) and other forms when given, which are not shown in this table.
b Gas-liquid chromatography.
c Not detected,
d These 28 samples were also tested for DDT and DDE content by a colorimetric method, and the results are included in the
130 samples listed above.
e Perirenal fat.
f Mesenteric fat.
g Panniculus fat.
h Geometric mean.
i Lipid basis.
j Infants and children.
less than 10 mg/kg (see Table 9). Thus, a low proportion of DDE
indicates a relatively high intake of DDT, a relatively low intake of
DDE residues (e.g. in food), and relatively few years for the
metabolism of stored DDT to DDE.
DDT in blood. The finding of DDT and related compounds in blood
(usually serum) of the general population of different countries is
shown in Table 10. Compared to body fat, blood has been analysed for
DDT in fewer countries and for fewer years. Some of the same
differences between populations and time periods observed in connexion
with DDT in fat have been detected in connexion with DDT in blood, but
the differences are less marked.
There is a small but statistically significant decrease in the
concentrations of DDT-related compounds in the plasma of women 1-6
days postpartum compared with the same women early in pregnancy. Most
of the decrease seems to occur during about the last 10 days before
delivery (Curley & Kimbrough, 1969). In a similar way, the
concentration of DDT-related compounds in various tissues of women at
the time of Caesarean section or normal delivery is less than in
nonpregnant women in the same community (Polishuk et al., 1970).
The concentration of p,p'-DDE is remarkably constant throughout
the day, but minor increases in it and in p,p'-DDT may occur after a
meal (Radamski et al., 1971a).
In so far as can be judged from the bar graphs presented by
Griffith & Blanke (1975), the concentrations of DDT-related materials
they found in postmortem blood were similar to the concentrations
reported in Table 10 for fresh blood except that postmortem blood
contained more TDE (DDD).
DDT and related compounds in other tissues. DDT has been found
in some samples of all human organs. Results reported in the USA and
USSR are shown in Tables 11 and 12, respectively. Results in Table 11
are based on the wet weight of the tissues. Results in Table 12,
presumably, are based on the weight of extractable lipid, but the
paper does not specify this; whatever the basis, the paper reports
concentrations of DDT and DDE in the range of 10.0-35.0 mg/kg in the
heart muscle, liver, and kidneys of 2 persons who had had direct
contact with pesticides. Although concentrations of chlorinated
compounds in liver, kidney, brain, and spleen of persons from Ferrara
Province, Italy, were generally higher than those in the USA (Prati et
al., 1972), the differences were not great.
Table 10. Concentration mg/litre of DDT-related compounds in the blood of members of the general population
Country Year No. of p,p'-DDT o,p'-DDT p,p'-DDE o,p'-DDE p,p- o,p- Total DOT DDE Reference
samples TDE TDE equivalent as %
(DDD) (DDD) total
North America
Canada 0.032 Brown & Chow, 1975
USA (Atlanta) 1965 10 0.0119 0.0013 0.0257 0.0418 68.6 Dale et al., 1966b
USA (Atlanta) 1966 10m 0.0746 Dale et al., 1967
USA (Atlanta) 1966 10f 0.0360 Dale et al., 1967
USA (Louisiana) 1966-1967 53a 0.00182 0.00182 0.00265 0.00265 0.00062 0.00062 0.00501 59.0 Selby et al., 1969
USA (4 states) 1967 64 0.00335 0.00044 0.00837 0.00096 0.00032 0.00032 0.01425 78.0 Yobs, 1969
(unpublished data)
USA (3 states) 1968 106 0.00342 0.00006 0.00927 0.00000 0.00004 0.00009 0.01397 73.4 Yobs, 1969
(unpublished data)
USA (Idaho) 1967-1968 1000 0.0047 0.0220 0.0002 0.02940 83.5 Watson et al., 1970
USA (Atlanta) 1969 30b,c 0.0046 0.0011 0.0062 0.0003 0.0014 0.0144 50.0 Curley et al., 1969
USA 1968 5d 0.0050 Curley & Kimbrough, 1969
USA 1968 10d 0.0205 Curley & Kimbrough, 1969
USA (Florida) 45e 0.0108 O'Leary et al., 1970b
USA (Florida) 107g 0.0152 O'Leary et al., 1970b
USA(Florida) 1970 26 0.03169 Radomski et al., 1971a
South America
Argentina 1970 20h 0.01934 Radomski et al., 1971b
18i 0.01327 Radomski et al., 1971b
19j 0.00869 Radomski et al., 1971b
Brazil 1973? 15f 0.0189 0.0237 0.0453 Schvartsman et al., 1974
Brazil 1973? 15c 0.0118 0.0104 0.0234 Schvartsman et al., 1974
Brazil 1974? 30 0.145 0.026 0.155 0.336 Almeida et al., 1975
Brazil 1974? 11 0.083 0.117 0.194 Almeida et al., 1975
Brazil 1974? 20 0.086 0.121 0.212 Almeida et al., 1975
Table 10 (Cont'd)
Country Year No. of p,p'-DDT o,p'-DDT p,p'-DDE o,p'-DDE p,p- o,p- Total DOT DDE Reference
samples TDE TDE equivalent as %
(DDD) (DDD) total
Europe
Bulgaria 1971-1976 171 0.039a Rizov, 1977
Hungary 1967-1968 120 0.034 Czeglédi-Janko, 1969
Poland 0.172d Jonczyk, 1970
Poland 1972 15 0.030 63 Bojanowska et al., 1973
Switzerland 13 0.0209 Zimmerli & Marek, 1973
Asia
Israel 1975 29 0.0133 0.0112 0.0195 0.0112 0.0087 0.0072 0.0740 47 Polishuk et al., 1977
Japan 1970 10 0.011 Tokutsu et al., 1970
Japan 1971 0.005 Kojima et al., 1971
Japan 1971 138 0.0183 Kasai et al., 1972
Japan 1971 0.0093 Yamagishi et al., 1972
Japan 1971 0.0285 Kaku, 1973
Japan 1972 0.001-0.078 Study Group, 1972
Japan 37 0.0437d Nawa, 1973
Japank 0.1358 Hara et al., 1973
Japanc 0.0210 Hara et al., 1973
0.0779 Abe et al., 1974
Oceania
Australia 52 0.0172 Slyali, 1972
Australia 47 0.0169 Ouw & Shandar, 1974
a Geometric mean. d Maximal value. g Black women. j 1-5 years old.
b Mean of positive values only. e White women. h Adults. k Maternal blood.
c Cord blood from term infants. f Female. i 6-11 years old. m Mile.
Table 11. Average concentrationsb of organochlorine insecticides in various tissues from autopsies
of 44 members of the general populationa
Tissue No. Lipid DDT DDE TDE Heptachlor Dieldrin Total + SEc
analysed content (mg/kg) (mg/kg) (DDD) epoxide (mg/kg) (mg/kg)
(%) (mg/kg) (mg/kg)
Perirenal fat 30 55.7 1.33 4.64 0.0110 0.0220 0.0300 6.03 ± 5.30
Mesenteric fat 29 54.2 1.35 4.40 0.0470 0.0320 0.0630 5.89 ± 4.98
Panniculus fat 30 60.6 1.16 4.48 0.0180 0.0270 0.0270 5.71 ± 5.25
Bone marrow 19 20.6 0.411 2.08 0.0760 0.0040 0.620 2.63 ± 2.21
Lymph noded 11 8.6 0.892 1.38 0.0100 0.0001 0.0190 2.30 ± 4.52
Adrenal 18 10.5 0.125 0.875 0.0570 0.0012 0.0060 1.06 ± 1.31
Kidney 38 3.2 0.0827 0.209 0.0022 0.0009 0.0056 0.300 ± 0.651
Liver 42 2.1 0.0467 0.200 0.0326 0.0019 0.0037 0.285 ± 0.369
Brain 32 7.9 0.0105 0.0831 0.0020 0.0002 0.0031 0.989 ± 0.171
Gonad 36 1.3 0.0150 0.0688 0.0015 0.0001 0.0021 0.0875 ± 0.103
Lung 25 0.7 0.0147 0.0585 0.0009 0.0003 0.0022 0.0766 ± 0.125
Spleen 27 0.6 0.0112 0.0305 0.0031 trace 0.0021 0.0469 ± 0.074
a From: Cassarett et al. (1968).
b Wet tissue basis.
c SE = standard error of the mean.
d Tracheobronchial lymph nodes.
Table 12. Average DDT and DDE contents in certain human organsa
Organ Content % of positive DDT DDE
limits observations (mg/kg) (mg/kg)
Heart muscle 1.0-12.0 77.0 3.69 4.05
Liver 1.0-20.0 85.5 4.62 4.02
Kidney 1.0-12.0 71.0 2.99 3.44
Spleen 2.0-20.0 57.0 2.86 2.86
Pancreas 2.0-15.0 75.0 3.0 2.62
Adrenal 2.0-10.0 84.3 3.84 3.7
Thyroid 2.0-8.0 59.0 1.85 1.85
a From: Vas'kovskaja (1969).
DDT and related compounds in the tissues of infants. The
transfer of DDT to the fetus was observed by Deniés (1962) and
confirmed by many others (Curley et al., 1969; Zavon et al., 1969;
Komarova, 1970; O'Leary et al., 1970a,b,c). Typical findings are shown
in Table 13. It appears that the placenta is partially protective; the
concentrations of DDT and DDE are lower in cord blood than in
corresponding maternal blood (O'Leary et al., 1970b; Schvartsman et
al., 1974). The report by Engst et al. (1970) that infants lose a part
of their DDT stores during the first few months of life, as a result
of rapid growth, is not necessarily contradictory to the observation
that at birth they have less than their mothers, but it must be said
that the values reported by Engst et al. (1970) were relatively high
for infants (see also section 5.2).
Storage in relation to disease. A review (Hayes, 1975) indicates
that there is no agreement in the literature regarding the effect of
health on the storage of chlorinated hydrocarbon insecticides. Some
investigators have not found any difference in the concentration of
DDT in adipose tissue taken by biopsy or during minor elective surgery
in contrast to that taken at autopsy. Some investigators, who used
only autopsy samples, found no relationship between storage of
chlorinated hydrocarbon insecticides and the cause of death. Others,
of whom the first was Deichmann (Deichmann & Radomski, 1968; Radomski
et al., 1968), have reported that storage was 1.7-7.6 times greater in
persons dying of cirrhosis, atherosclerosis, hypertension, idiopathic
amyloidosis, and certain forms of cancer. Weight loss was not really
ruled out in these studies. Its importance was emphasized by Casarett
et al. (1968), who found that disease did not influence concentrations
on a wet weight basis but only on a lipid basis; samples with the
highest levels of DDT on a lipid basis came from persons who not only
had cancer but who were emaciated and had widespread abnormality of
Table 13. The range and mean of measurable concentrations of various organochlorines (mg/kg) in different tissues from stillborn infantsa
Tissues p,p'-DDT o,p'-DDT p,p'-DDE o,p'-DDE p,p'-TDE alpha-HCH ß-HCH gamma-HCH Heptachlor Dieldrin
received (DDD)
10 measurable 3 4 8 0 6 3 6 3 4 3
adipose concentration
range 0.16-2.15 0.35-11.47 0.16-3.19 -- 0.23-14.17 0.09-0.24 0.14-0.44 0.09-0.14 0.07-0.51 0.09-0.35
mean 0.88 3.39 1.22 -- 3.17 0.14 0.26 0.11 0.32 0.24
SE ± 0.63 2.70 0.38 -- 2.22 0.05 0.05 0.02 0.10 0.08
8 measurable 1 1 2 0 2 0 1 1 0 1
spinal concentration
cord range 0.47 0.47 0.30-1.16 -- 0.31-0.70 -- 0.17 0.10 -- 0.09
mean -- -- 0.73 -- 0.51 -- -- -- -- --
SE ± -- -- 0.43 -- 0.20 -- -- -- -- --
8 measurable 3 1 4 0 3 3 1 1 1 2
brain concentration
range 0.28-0.99 0.84 0.25-1.47 -- 0.20-1.22 0.04-0.49 3.81 0.06 0.13 0.84-0.86
mean 0.56 -- 0.65 -- 0.64 0.19 -- -- -- 0.05
SE ± 0.22 -- 0.28 -- 0.30 0.15 -- -- -- 0.01
9 measurable 3 2 6 0 3 2 3 3 2 1
adrenals concentration
range 1.28-1.65 0.36-1.05 0.13-1.96 -- 0.91-1.45 0.40-0.57 0.12-0.71 0.20-0.53 0.46-1.00 0.92
mean 1.48 0.71 1.05 -- 1.11 0.49 0.37 0.33 0.73 --
SE ± 0.11 0.35 0.28 -- 0.17 0.09 0.18 0.10 0.27 --
10 measurable 4 0 6 0 5 5 3 3 3 2
lungs concentration
range 0.57-1.01 -- 0.25-1.35 -- 0.31-1.05 0.07-0.69 0.05-0.18 0.05-0.25 0.08-0.31 0.27-0.72
mean 0.79 -- 0.85 -- 0.75 0.25 0.12 0.12 0.17 0.50
SE ± 0.11 -- 0.22 -- 0.13 0.11 0.04 0.07 0.07 0.23
Table 13 (Cont'd)
Tissues p,p'-DDT o,p'-DDT p,p'-DDE o,p'-DDE p,p'-TDE alpha-HCH ß-HCH gamma-HCH Heptachlor Dieldrin
received (DDD)
10 measurable 4 2 4 0 4 3 3 3 4 3
heart concentration
range 1.04-4.17 0.57-0.68 1.27-4.79 -- 1.02-5.82 0.23-0.52 0.15-0.31 0.19-0.27 0.30-1.56 0.08-1.02
mean 2.17 0.63 2.74 -- 3.27 0.33 0.22 0.22 0.80 0.49
SE ± 0.69 0.06 0.74 -- 1.10 0.09 0.05 0.03 0.30 0.28
10 measurable 5 3 6 0 6 3 4 4 3 2
liver concentration
range 0.15-1.59 0.22-3.42 0.22-2.45 -- 0.19-2.14 0.21-0.32 0.03-0.20 0.05-0.36 0.03-1.67 0.16-0.22
mean 0.79 1.32 0.98 -- 1.01 0.25 0.11 0.24 0.68 0.19
SE ± 0.24 1.05 0.34 -- 0.29 0.04 0.04 0.07 0.50 0.03
9 measurable 4 3 6 0 5 4 5 4 3 3
kidney concentration
range 0.62-7.60 0.29-2.07 0.11-9.78 -- 0.48-9.12 0.11-1.45 0.06-0.61 0.11-0.69 0.19-1.14 0.06-0.50
mean 3.71 1.38 3.57 -- 3.84 0.82 0.29 0.39 0.70 0.34
SE ± 1.70 0.55 1.72 -- 1.87 0.32 0.12 0.14 0.28 0.14
8 measurable 3 2 3 1 3 1 1 2 5 3
spleen concentration
range 0.48-1.04 0.45-2.94 0.60-1.05 0.29 0.18-0.91 0.21 0.16 0.17-0.18 0.10-0.52 0.18-0.51
mean 0.80 1.70 0.86 -- 0.56 -- -- 0.18 0.35 0.31
SE ± 0.17 1.25 0.13 -- 0.21 -- -- 0.005 0.08 0.10
3 measurable 1 0 1 1 0 0 0 0 0 0
pancreas concentration
range 0.49 -- 0.23 0.08
mean -- -- -- -- -- -- -- -- -- --
SE ± -- -- -- -- -- -- -- -- -- --
a From: Curley et al. (1969).
the liver. Factors other than emaciation may be important in some
conditions. For example, Oloffs et al. (1974) found that the DDT
concentration in fat was not influenced by cirrhosis, but that the DDT
concentrations in liver were significantly higher in cirrhotic livers
with severe fatty infiltration and significantly lower in those with
marked fibrosis or bile stasis.
That increased storage of DDT is a result and not a cause of the
diseases in which it sometimes occurs is shown by the fact that
persons with extensive occupational exposure average 10 times more
storage than the highest values reported in connexion with disease.
However, they do not exhibit any predisposition to the diseases in
question, and these diseases have shown no age-specific increase in
incidence related to the introduction and use of insecticides.
6.2.2 Animal studies
A detailed review of the literature (Hayes, 1959) shows that a
number of facts about the distribution and storage of DDT were
established early either by single, classical papers (now fully
confirmed) or by correlation of contributions from several
laboratories. The major results may be summarized as follows:
(a) DDT is stored in all tissues. Storage of the compound in
blood, liver, kidney, heart, and the central nervous system
was reported by Smith & Stohlman (1944).
(b) Higher concentrations of DDT are usually found in adipose
tissue than in other tissues (Ofner & Calvery, 1945).
(c) Rats store DDT in their fat at all accurately measurable
dietary levels including the unintended residues in standard
laboratory feeds.
(d) Following repeated doses, storage in the fat increases rapidly
at first and then more gradually until a peak or plateau is
reached (Laug et al., 1950). It was recognized that repeated
doses at a moderate rate could result in greater total storage
of DDT in the fat than a single dose at the highest rate that
can be tolerated or even a single dose at a rate that
frequently is fatal.
(e) By plotting animal data published no later than 1950, it is
possible to show that, when other factors are kept constant,
the equilibrium storage of DDT in each tissue varies directly
with the daily dosage.
(f) However (with the apparent exception of the dog) storage in
the fat, and perhaps in other tissues, is less extensive in
relation to dosage at higher dietary levels (Fig. 1).
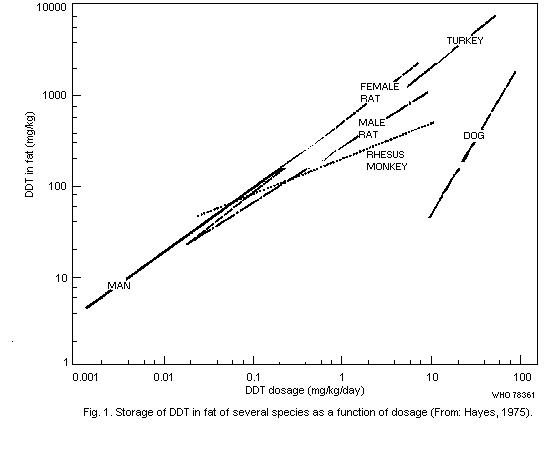 (g) The rat, apparently, tends to lose a part of the DDT it has
stored in fat at a peak level, reached in about 6 months, even
though the same diet is continued.
(h) There is a measurable, although not great, difference between
the storage pattern of different species (Fig. 1).
(i) At higher dosage levels but not at ordinary residue levels,
the female rat consistently stores more DDT in its fat than
the male, when offered the same diet (Fig. 1). The difference
is only accounted for in part by the greater food intake of
the female and must depend to some extent on more rapid
biotransformation in the male. Other species show little or no
sex difference.
(j) The amount of DDT stored in the tissues gradually diminishes
if exposure to the compound is discontinued or reduced.
It is interesting to note that, even in the early studies, there
was satisfactory agreement between different authors and, in fact,
between different laboratories. Later studies have amplified some of
the findings.
Adams et al. (1974) observed that about the same concentrations of
DDT and related compounds are stored by male rats and by females that
reproduce successfully. The lower storage in mated females probably is
accounted for by transfer to the young via the placenta and the milk.
However, other factors may be involved; the disposal of the increased
DDT taken in by the female rat as a result of her high food intake
during lactation has not really been accounted for.
When DDT, some of its analogues, and several other organochlorine
insecticides were fed to male and female rats for 4 generations, there
was little variation in storage of the materials, from one generation
to another, and no evidence of a continuing increase in succeeding
generations (Adams et al., 1974).
Distribution to the fetus
In animals, the concentrations of DDT in the blood and other
tissues of the fetus are lower than those in the corresponding tissues
of the mother (Dedek & Schmidt, 1972). The same is true in man (see
section 5.2).
Interaction with other compounds
There was no evidence from studies on rats that dieldrin
influenced the storage of DDT and its metabolites, even though DDT
caused a marked reduction in storage of dieldrin (Street, 1964).
However, Deichmann et al. (1971a) reported that the administration of
capsules of aldrin (which is metabolized to dieldrin) to male and
female dogs that had reached a steady state of DDT storage caused a
rapid and progressive increase in the concentration of DDT, DDE, and
TDE (DDD) in their blood and fat. The dosage of DDT was 12 (mg/kg)day
and that of aldrin was 0.3 (mg/kg)day. Control dogs that continued to
receive DDT but never received aldrin maintained a plateau of DDT
storage. It is not clear whether the different results in rats and
dogs were due to species, to some unrecognized difference between
dieldrin and aldrin, to the fact that the rats received lower dosages,
or to some unidentified factor.
Studies of the distribution of DDT in various lipid fractions that
are based on tissue extracts obtained with one or more organic
solvents, such as those of Kuz'minskaja et al. (1972), are difficult
to interpret because there is no way of determining how much of the
material was initially associated with protein.
DDE
DDE constitutes about 4% of technical DDT. Most species convert
some of the DDT they ingest to DDE. Finally, most species, including
man, store DDE more tenaciously than they do DDT, the greater part of
which is metabolized by a different pathway to DDA and excreted more
rapidly. The result is that DDE, expressed as a percentage of total
DDT-related compounds, increases in individuals after DDT intake
decreases, and also increases in successive steps of the food chain.
Apparently, the rhesus monkey is an exception. Monkeys store DDE
when it is fed to them but, when feeding stops, the rate of loss of
DDE stored in fat is more rapid than that of DDT (Durham et al.,
1963). Whether it is relative inability to form DDE, unusual ability
to excrete it, or a combination of both that accounts for the fact
that little or no DDE can be found in monkeys fed DDT is not entirely
clear.
6.3 Elimination
6.3.1 Human studies
6.3.1.1 Studies of volunteers
DDA is the main urinary metabolite of DDT. In man, it was found
first in a volunteer by Neal et al. (1946), who reported that,
following ingestion of 770 mg of p,p'-DDT, excretion rose sharply to
4.0 mg/day during the second 24-h period, decreased rapidly on the
third and fourth days, decreased gradually thereafter, but was still
above baseline on the fourteenth day. Judging from a graph, the
highest concentration was about 2.6 mg/kg.
Much later studies in volunteers, who received smaller but
repeated doses, showed that a steady state of excretion was reached
after about 6-8 months. During a 56-week period of continued dosing
after equilibrium was fully established, the concentration of DDA
associated with technical DDT at the rate of 35 mg/man per day varied
from 0.18 to 9.21 mg/kg and averaged 2.98 mg/kg, corresponding values
for p,p'-DDT were 0.40-6.27 mg/kg with a mean of 1.88 mg/kg. Thus,
technical DDT was excreted more effectively and stored in man less
than p,p'-DDT. During the latter half of the dosing period, it was
possible, in the 2 groups receiving recrystallized and technical DDT
at the rate of 35 mg/man per day, to account for an average of 13% and
16%, respectively, of the daily dose in terms of urinary DDA. The
excretion of DDA was relatively constant in each individual, but
marked differences were observed between men receiving the same dose.
For example, over the period of 56 weeks, the highest rate measured
for one man was 0.16 mg/h while the lowest rate for another in the
same group was 0.15 mg/h. Their mean rates during this period were
0.089 and 0.269 mg/h, respectively. The difference was highly
significant (P < 0.001) (Hayes et al., 1971).
6.3.1.2 Studies of occupationally exposed workers
Among workers whose DDT intake was estimated to be about 35 mg/man
per day, Ortelee (1958) reported that the concentration of DDA in
urine ranged from 0.12 to 7.56 mg/litre and averaged 1.71 mg/litre.
Among workers whose exposure was about half as high, Laws et al.
(1967) found concentrations from 0.01 to 2.67 mg/litre with a mean of
0.97 mg/litre.
Continuous sampling of a DDT-formulating plant worker's urine
showed that excretion of DDA increased promptly, when exposure began
on each of 5 consecutive work days and often continued to increase
after exposure, sometimes reaching a peak about midnight before
decreasing rapidly. On the sixth day, when there was no occupational
exposure to DDT, the excretion of DDA continued until a very low level
was reached. The highest concentration of DDA reported in this study
was 0.68 mg/litre (Wolfe & Armstrong, 1971).
6.3.1.3 Studies of the general population
Urine. Cranmer et al. (1969) developed a method for analysing
DDA in urine that, for the first time, made it possible to measure the
compound in every sample. The range found for a small sample of the
population of Florida, USA, was 0.008-0.019 mg/litre, and the mean
(0.014 mg/litre was slightly less than 0.02 mg/litre, the lowest
concentration detectable by earlier methods. Results for general
population studies are shown in Table 14 together with results for
various groups of workers and volunteers.
Table 14. Urinary excretion of DDA by people in the USA with various degrees of exposure to DDTa
Exposure Year No. of DDA excretion (mg/kg) Reference
samples
Range Mean
General population 1954 8 <0.05 -- Hayes et al., 1956
General population 1957 8 <0.02-0.07 -- Hayes et al., 1971
General population 1962 23 <0.02-0.18 -- Durham et al., 1965b
General population 1968 11 0.008-0.019 0.014 Cranmer et al., 1969
Environmentalb 1962 13 <0.02-0.11 -- Durham et al., 1965a
Subjects applying DDT 1962 11 <0.02-0.17 -- Durham et al., 1965a
Formulators 1957 40 0.12-7.56 1.71 Ortelee, 1958
Makers and formulators 1966 35 <0.01-2.67 0.97 Laws et al., 1967
Volunteers given 1953-1954 2 0.10-0.42b 0.21c Hayes et al., 1956
3.5 mg/day orally
Volunteers given 1957-1958 6 0.06-1.98a 0.23b Hayes et al., 1971
3.5 mg/day orally
Volunteers given 1953-1954 6 0.69-9.67b 2.46c Hayes et al., 1956
35 mg/day orally
Volunteers given 1957-1958 6 0.18-9.21c 3.09d Hayes et al., 1971
35 mg/day orally
a Slightly modified from Hayes (1975).
b Residents living within 500 feet of agricultural application.
c Based on all samples after thirty-fifth week of dosage.
d Based on all samples from the thirty-fifth to the ninety-third week after dosage started.
In the general population the urine contained not only DDA but
also neutral compounds; the average concentrations reported by Cueto &
Biros (1967) were: p,p'-DDT, 0.0007 mg/litre and p,p'-DDE,
0.0156 mg/litre. Men with full-time occupational exposure to DDT
excreted much more DDA but showed only a statistically insignificant
increase in the excretion of DDT and DDE.
These values for the excretion of DDA, DDT, and DDE by different,
small groups of people showed an average concentration of
0.0358 mg/litre of DDT-related material. Although the DDT intakes of
these particular groups were not measured, the urinary excretion is of
such an order of magnitude that it may account for the excretion of
all the absorbed DDT.
Milk. As far as the mother is concerned, the secretion of a
compound in milk is a form of excretion. For the infant, the milk is
an important, if not the sole source of intake of the compound in
question. The concentrations of DDT and DDE in milk reported from
different countries are listed in Table 15. Especially high values
have been reported from Guatemala for 1970 (see Table 16) and from the
USSR (Damaskin, 1965). However, additional and more numerous samples
taken only four years later in the same and other communities in
Guatemala revealed entirely different results. The highest single
value observed for total DDT in 1974 was 5.69 mg/litre. The range of
average values for different locations was 0.04-0.86 mg/litre. The
means for total DDT for the three communities studied earlier were: La
Bomba, 0.59 mg/litre; El Rosario, 0.28 mg/litre; and Cerro Colorado,
0.47 mg/litre (Winter et al., 1976). The authors recognized the
importance of the agricultural uses of DDT as a potential source of
the compound in human milk. However, they attributed the change
between 1970 and 1974, almost exclusively, to the substitution of
propoxur for DDT in residential spraying to combat malaria.
The medical importance of DDT in human milk depends entirely on
the dosage of the compound received by babies. The highest
concentration of p,p'-DDT ever reported in a single sample of milk
and the highest average value from one community (see Table 16) would
determine maximum and average intakes of 1.06 and 0.32 (mg/kg) day for
newborn babies, assuming an intake of 0.6 litre per day and a weight
of 3.36 kg. This average intake is of the same order of magnitude as
that encountered by workers who make and formulate DDT. The
corresponding maximum and average intakes of total DDT-related
material for infants in Guatemala would be 2.18 and 0.73 (mg/kg) day,
values not strictly comparable to those of the workers because the
intake of the babies includes so much more DDE, a less toxic compound.
Neither of the papers cited concerning DDT in human milk in Guatemala
mentioned any indication of injury to babies. Absence of injury to
babies would be predicted from studies of the most heavily exposed
workers and also from studies regarding the effect of age on the
susceptibility of animals to DDT (see section 7.3.2).
Table 15. Concentration of DDT-derived material in human milk
Country Year No. of Method DDT DDE as DDT Total as DDE as Reference
samples of (mg/litre) (mg/litre) (mg/litre) DDT
analysis (% of
total)
North America
Canada 1967-1968 147 GLC 0.032 0.097 0.139 -- Ritcey et al., 1972
Canada 15 GLC 0.006-0.032 -- 0.019-0.035 -- Musial et al., 1974
USA 1950 32 colour -- 0.13 -- Laug et al., 1951
USA 1960-1961 10 colour 0.08 0.04 0.12 33 Quinby et al., 1965b
USA 1962 6 colour 0-0.12a 0.025a 0.37b -- West, 1964
USA 1968 ? GLC 0.026 0.047 0.078 60 Cudey & Kimbrough, 1969
USA 1970 53 GLC 0.022 0.083 0.101 80 Kroger, 1972
USA 1970-1971 101 GLC -- -- 0.17 Wilson et al., 1973
USA 1971-1972 40 GLC 0.126 Savage et al., 1973
USA 1973-1974 57 GLC 0.092 0.260 0.344 76 Strassmann & Kutz, 1977
USA 1974 38 GLC 0.447 Woodard et al., 1976
USA 1974 14 GLC 0.075 Woodard et al., 1976
USA 1975 7 GLC 0.323 Woodard et al., 1976
Europe
Belgium 1968 20 GLC 0.05 -- -- -- Heyndrickx & Maes, 1969
Czechoslovakia 1968 -- -- 0.101 -- -- -- Hruska, 1969
Czechoslovakia 393 TLC 0.097 0.112 0.209 54 Suvak, 1970
England 1963-1964 19 GLC 0.05 0.08 0.13 62 Egan et al., 1965
France 1971-1972 3.24c Luquet et al., 1974
France 1972? 3.24c Luquet et al., 1974
German Democratic 1970? 0.569 Adamovic et al., 1971
Republic
German Democratic 1969 57 0.23 Engst & Knoll, 1972
Republic
German Democratic 1970 18 0.16 Engst & Knoll, 1972
Republic
Table 15 (Cont'd)
Country Year No. of Method DDT DDE as DDT Total as DDE as Reference
samples of (mg/litre) (mg/litre) (mg/litre) DDT
analysis (% of
total)
German Democratic 1971 96 0.32 Knoll & Jayarman, 1973a
Republic 1973b
Germany, Federal 1970? 43 GLC 0.031 0.090 0.121 74 Acker & Schulte, 1970
Republic of
Germany, Federal 1971? 0.121 Pfeilsticker, 1973
Republic of
Hungary 1963 10 colour 0.13-0.26a -- -- -- Denés, 1964
Italy 1965? 2 GLC 0.001 0.055 0.056 Kanitz & Castello, 1966
Netherlands 1969 50 GLC 0.9c 1.8c 2.7c 66 Tuinstra, 1971
Poland 1966 26 colour 0.27 -- -- 62 Bronisz & Ochynski, 1968
Poland 1967 25 colour 0.40 -- -- 58 Bronisz & Ochynski, 1968
Poland 1970? 40 GLC 0.08 0.19 0.28 71 Kontek et al., 1971
Portugal 1972 168 GLC 0.326 Graca et al., 1975
Romania 1968? 100 colour 0.054-0.749 0.026-8.30 0.080-9.05 -- Unterman & Sirghie, 1969
Sweden 1967? -- -- 0.117 -- Lotroth, 1968
Sweden 1967-1969 22 GLC 0.039 0.076 0.115 63 Westoo et al., 1970
USSR 1964 16 colour 1.22-4.88 -- -- -- Damaskin, 1968
USSR 1968? 4505 -- 0.1-1.0 -- -- -- Gracheva, 1969
USSR 1969? -- -- 0.09 -- 0.14 -- Gracheva, 1970
USSR 1967? 370 GLC 0.1 -- -- Komarova, 1970
Asia
Israel 1975 29 GLC 0.02 0.03 0.07 44 Polishuk et al., 1977
Japan 1970? 10 0.071 Tokutsu et al., 1970
Japan 1970? 5 0.160 Takeshita & Inuyama. 1970
Japan 1970? 10 0.120 Takeshita & Inuyama. 1970
Japan 1971? 0.04 Kojima et al., 1971
Japan 1971? 0.04 Kojima et al., 1971
Table 15 (Cont'd)
Country Year No. of Method DDT DDE as DDT Total as DDE as Reference
samples of (mg/litre) (mg/litre) (mg/litre) DDT
analysis (% of
total)
Japan 1971? 59 0.019-0.105 Kato et al., 1971
Japan 1971? 14 0.047 Sugaya et al., 1971
Japan 1971 43 GLC 0.095 0.084 0.179 47 Hidaka et al., 1972
Japan 1971 454 GLC 0.0607 Hayashi, 1972a, 1972b
Japan 1971-1972 398 0.0626 Hayashi, 1972a, 1972b
Japan 1971-1972 398 0.0562 -- -- -- Anonymous, 1972
Japan 1971 0.044 Yamagishi et al., 1972
Japan 30 2.0a Mizoguchi et al., 1972
Japan 54 0.035 Taira et al., 1972
Japan 1971-1972 5 0.027 Nagai, 1972
Japan 1971-1972 5 0.037 Nagai, 1972
Japan 1971-1972 5 0.016 Nagai, 1972
Japan 1971-1972 5 0.037 Nagal, 1972
Japan 30 0.033 Oura et al., 1972
Japan 1971-1972 123 0.105 Kawai et al., 1973
Japan 1971-1972 0.038-0.075 Kamata, 1973
Japan 1970 3.780c Suzuki et al., 1973
Japan 1971 3.592c Suzuki et al., 1973
Japan 1972 3.822c Suzuki et al., 1973
Japan 1973 0.0854 Kamata, 1974
Oceania
Australia 1970 1 GLC -- -- 0.014d Newton & Greene, 1972
0.007e
0.066f
67 GLC 0.036 0.105 0.141
Table 15 (Cont'd)
Country Year No. of Method DDT DDE as DDT Total as DDE as Reference
samples of (mg/litre) (mg/litre) (mg/litre) DDT
analysis (% of
total)
Australia
(Brisbane) 1971-1972 20 GLC 0.288 Miller & Fox, 1973
(Mareeba) 1971-1972 20 GLC 0.415
Australia 45 GLC 0.064 Siyali, 1973
Australia 22 GLC 0.010 0.068 0.078 Stacy & Thomas, 1975
Papua New Guinea 1972 16 GLC
(Kar Kar Island) 0.002 0.002 0.004 50 Hornabrook et al., 1972
Papua New Guinea 1972 19 GLC
(Sepik district) 0.008 0.007 0.015 47 Hornabrook et al., 1972
a Range of values for milk containing 4% fat c Concentration in milkfat. e At middle of feeding, 1.2% fat.
containing 3.3-6.6 ppm.
b Maximal value. d At beginning of feeding, 1.8% fat. f At end of feeding, 5.1% fat.
Table 16. Ranges, means, and standard errors of the concentrations
of organochlorine insecticides in the milk of women in
three towns in Guatemalaa
Compound La Bomba El Rosario Cerro Colorado
1970 1970 1971
n = 10 n = 27 n = 9
p.p'-DDT 0.23-4,95 0.16-2.24 0.49-5.94
(mg/litre) (1.00 ± 0.38) (0.77 ± 0.10) (1.78 ± 0.56)
p.p'-DDE 0.12-6.36 0.28-3,10 0.60-6.13
(mg/litre) (1.02 ± 0.58) (0.99 ± 0.14) (2.10 ± 0.61)
p,p'-TDE(DDD) trb-0.16 0.01-0.09 0.05-0.11
(mg/litre) (0.03 ± 0.02) (0.02 ± 0.004) (0.07 ± 0.01)
o,p'-DDT tr-0.29 0.01-0.18 0.06-0.22
(mg/litre) (0.09 ± 0.03) (0.06 ± 0.01) (0.12 ± 0.02)
total as DDT 0.41-11.50 0.34-4.97 1.571-12.21
(mg/litre) (2.15 ± 1.05) (1.84 ± 0.24) (4.07 ± 1.11)
total HCH 0.01-0.10 tr-0.07 0-0.06
(mg/litre) (0.03 ± 0.01) (0.007 ± 0.003) (0.02 ± 0.01)
heptachlor epoxide 0-0.02 tr-0.01 tr
(mg/litre) (0.003 ± 0.002) (0.007 ± 0.0004)
dieldrin tr tr-0.01
(mg/litre) (0.002 ± 0.0005)
a From: Olszyna-Marzys et al. (1973).
b tr = trace.
The slightly greater secretion of DDT and much greater secretion
of various isomers of HCH by urban mothers (compared to rural mothers)
in Japan was attributed to their greater intake of cow's milk (Takano,
1972).
Other routes of excretion. DDT, DDE, and dieldrin are excreted in
the bile; the concentrations for five men without special exposure
varied as follows: p,p'- and o,p'-DDT combined, 0.0000-0.0009 mg/litre;
p,p'-DDE, 0.0005-0.0056 mg/litre; and dieldrin, 0.0000-0.0005 mg/litre.
Higher levels were found in the bile of one pest control operator
(Paschal et al., 1974).
6.3.2 Animal studies
When large doses of DDT are ingested, some of the compound is not
absorbed and is passed in an unaltered state in the faeces. Only
traces of unaltered DDT may be found in the faeces when exposure is by
any route other than oral. However, true faecal excretion of DDT
metabolites was established very early (Wasicky & Unti, 1945; Judah,
1949). In the rat, faecal excretion of DDT exceeded urinary excretion,
irrespective of the route of administration (Hayes, 1965). In man, the
ratio is obviously different. Although the excretion of DDT-related
material in the faeces of man receiving 35 mg/man per day has been
reported using colorimetry (Hayes et al., 1956), this result has never
been confirmed by gas chromatography, even in connexion with workers
whose exposure was heavy and prolonged. Either DDT metabolites are not
excreted by man in the faeces to any great degree, or they are
excreted in one or more forms that differ from those already
demonstrated in rats.
The bile appears to be the principal source of DDT metabolites in
the faeces of rats. When the bile duct was cannulated before
intravenous injection of radioactive DDT, 65% of the dose was
recovered in the bile, 2% in the urine, and only 0.3% in the faeces
(Jensen et al., 1957), and the possibility of some contamination of
the faeces by urine could not be excluded.
The different routes of excretion are not unrelated. Burns et al.
(1957) found that there was an increase in urinary excretion of
radioactive material following ligation of the bile duct in rats fed
radioactive DDT. This is an indirect confirmation of the finding by
Jensen and his colleagues that most of the metabolites in bile consist
of DDA or compounds closely related to it. Although an enterohepatic
circulation of the metabolites of DDT has not been directly proved, it
seems likely that such a circulation exists, as has been demonstrated
for 1,1'-(2,2-dichloroethylidene)-bis[4-ethylbenzene] (Perthane).
Demonstration of the excretion of DDT in milk was first reported
by Woodard et al. (1945) in connexion with a dog fed the compound at
the rate of 80 (mg/kg) day. Within a short time, excretion of DDT in
milk was reported in rats, goats, and cows, and soon afterwards, in
women (Laug et al., 1951). Telford & Guthrie (1945) showed that rats
fed a diet containing DDT at 1000 mg/kg produced milk that was toxic
to their young.
Since the early laboratory studies, the presence of DDT has been
demonstrated repeatedly in the milk of cows. A review (Hayes, 1959)
showed that cows fed substantial, but nontoxic, residues of DDT
commonly excrete 10% or slightly more of the total dose in their milk,
and amounts slightly over 30% have been observed.
Further information on the excretion of DDT in human milk is given
in section 6.3.1.3. It is of interest to repeat here, however, that
lactating women in the general population are apparently in negative
DDT balance. That is, they excrete more DDT in their milk each day
than they acquire in their food. The difference is small and would not
be expected to have much effect on their total body burden of DDT.
Wilson et al. (1946a) showed that DDT was secreted from the skin
of a cow maintained on an oral dosage of about 53 (mg/kg) day.
DDA. Because DDA is the main form in which DDT is excreted, it
might be expected that, following its direct administration, DDA would
be excreted relatively efficiently, and this is true. It was found
very early that, during the first few days after oral dosing, rabbits
excreted DDA in the urine approximately 15 times faster than animals
given DDT at an equivalent dosage. Although the rate of DDA excretion
increased somewhat, the rate of excretion associated with DDT
increased more rapidly so that the values differed by a factor of only
five after the twentieth day of feeding (Smith et al., 1946).
6.4 Biotransformation
The chemical nature of the chief metabolite excreted in the urine
was first elucidated by White & Sweeney (1945). Rabbits were given DDT
(melting point 107-108°C) at a rate of 100 (mg/kg) day for 6 days per
week, and their urine was collected. It contained a considerable
amount of organic chloride, whereas normal rabbit urine did not. Using
the organic chloride test to evaluate different methods of extraction,
the authors were able to isolate a crystalline material containing
25.37% chlorine and melting at 166-166.5°C. The crystals were shown to
be DDA (see Table 1). The product obtained from the urine was
identical to that synthesized from glyoxylic acid and chlorobenzene
and with a compound obtained through the chemical degradation of DDT.
Identity of the 3 compounds and, therefore, their true chemical
nature, was established by the determination of melting points, mixed
melting points, elementary analysis, and X-ray powder diffraction
patterns, as well as by demonstrating the similarity of the
decarboxylation products of the three original materials. Only 80-85%
of the total organic chloride of the rabbit urine was found to be
soluble in alkali and in bicarbonate. For this and other reasons it
was considered possible that DDA was not the only chlorinated organic
compound present.
Later work by many authors has confirmed that DDA is the major
urinary metabolite of DDT in all mammals including man. It may be
added that, in spite of great strides in analytical chemistry, the
nature of other urinary metabolites has not been elucidated fully.
The fact that DDE is stored in tissue was first demonstrated by
Pearce et al. (1952) in connexion with human fat. The authors pointed
out that they did not know whether the compound resulted from partial
degradation of DDT residues on plants or whether the DDE was formed
during the process of digestion or after absorption. It is now known
that some food contains DDE but that man is capable of forming the
product from DDT. The exact mechanism of the biotransformation of DDT
to DDE remains in doubt.
Pearce et al. (1952) established the identity of DDE by comparing
the colorimetric and column chromatographic behaviour of the residue
with those of a chemical standard. A second paper from the same
laboratory (Mattson et al., 1953) added further details confirming the
identity of the compound. Later investigations have confirmed the
identity of DDE by infrared spectrometry and by gas chromatography.
That portion of the metabolism of DDT that leads to DDA has been
clearly elucidated by Peterson & Robinson (1964), who gave evidence
for the sequence of changes shown in Fig. 2. Organ perfusion studies
have indicated that the liver is capable of the biotransformation of
DDT, DDE, TDE, DDMU, and DDMS, while the kidney transforms DDMS, DDNU,
and DDOH (Datta & Nelson, 1970). Cultures of embryonic lung cells are
capable of metabolizing DDT to DDA via DDD (North & Menzer, 1973).
When DDA was discovered, it was postulated, on chemical grounds,
that DDE was a step in its formation (White & Sweeney, 1945); however,
rats that produced both DDE and DDA from DDT were incapable, according
to Peterson & Robinson (1964), of forming DDA when fed preformed DDE.
This finding was contradicted by Datta (1970) and by Datta & Nelson
(1970) who claimed that 14C- p,p'-DDE was converted by rats to
p,p'-DDMU, which then underwent further metabolism to p,p'-DDA via
the route shown in Fig. 2. Datta suggested that the predominance of
detoxication via DDE or TDE (DDD) might depend on physiological
response or the amount of toxicant used. Whatever the reason, the fact
remains that DDE is stored more tenaciously than DDT.
Rhesus monkeys fed either technical or p,p'-DDT store little or
no DDE, although they are fully capable of storing DDE when it is fed
preformed (Durham et al., 1963).
The way in which DDE was lost from storage was not clearly
understood for a long time. In man (Cueto & Biros, 1967), seal, and
guillemot (Jansson et al., 1975) part of it is excreted unchanged, but
the fact that its elimination is promoted by the induction of
microsomal enzymes (see section 7.1.4.2) strongly suggests that it
undergoes metabolism, conjugation, or both. That metabolism does occur
was first demonstrated by identification of 2 hydroxylated derivatives
of DDE in the faeces of wild seals and guillemots and in the bile of
seals (Jansson et al., 1975). When p,p'-DDE was fed to rats, the
same metabolites and one other were isolated from the faeces, and,
within the first 6 days, the metabolites accounted for about 5% of the
dose (Sundström et al., 1975). Later a fourth hydroxylated derivative
was identified from the faeces of rats fed p,p'-DDE. The compounds
(g) The rat, apparently, tends to lose a part of the DDT it has
stored in fat at a peak level, reached in about 6 months, even
though the same diet is continued.
(h) There is a measurable, although not great, difference between
the storage pattern of different species (Fig. 1).
(i) At higher dosage levels but not at ordinary residue levels,
the female rat consistently stores more DDT in its fat than
the male, when offered the same diet (Fig. 1). The difference
is only accounted for in part by the greater food intake of
the female and must depend to some extent on more rapid
biotransformation in the male. Other species show little or no
sex difference.
(j) The amount of DDT stored in the tissues gradually diminishes
if exposure to the compound is discontinued or reduced.
It is interesting to note that, even in the early studies, there
was satisfactory agreement between different authors and, in fact,
between different laboratories. Later studies have amplified some of
the findings.
Adams et al. (1974) observed that about the same concentrations of
DDT and related compounds are stored by male rats and by females that
reproduce successfully. The lower storage in mated females probably is
accounted for by transfer to the young via the placenta and the milk.
However, other factors may be involved; the disposal of the increased
DDT taken in by the female rat as a result of her high food intake
during lactation has not really been accounted for.
When DDT, some of its analogues, and several other organochlorine
insecticides were fed to male and female rats for 4 generations, there
was little variation in storage of the materials, from one generation
to another, and no evidence of a continuing increase in succeeding
generations (Adams et al., 1974).
Distribution to the fetus
In animals, the concentrations of DDT in the blood and other
tissues of the fetus are lower than those in the corresponding tissues
of the mother (Dedek & Schmidt, 1972). The same is true in man (see
section 5.2).
Interaction with other compounds
There was no evidence from studies on rats that dieldrin
influenced the storage of DDT and its metabolites, even though DDT
caused a marked reduction in storage of dieldrin (Street, 1964).
However, Deichmann et al. (1971a) reported that the administration of
capsules of aldrin (which is metabolized to dieldrin) to male and
female dogs that had reached a steady state of DDT storage caused a
rapid and progressive increase in the concentration of DDT, DDE, and
TDE (DDD) in their blood and fat. The dosage of DDT was 12 (mg/kg)day
and that of aldrin was 0.3 (mg/kg)day. Control dogs that continued to
receive DDT but never received aldrin maintained a plateau of DDT
storage. It is not clear whether the different results in rats and
dogs were due to species, to some unrecognized difference between
dieldrin and aldrin, to the fact that the rats received lower dosages,
or to some unidentified factor.
Studies of the distribution of DDT in various lipid fractions that
are based on tissue extracts obtained with one or more organic
solvents, such as those of Kuz'minskaja et al. (1972), are difficult
to interpret because there is no way of determining how much of the
material was initially associated with protein.
DDE
DDE constitutes about 4% of technical DDT. Most species convert
some of the DDT they ingest to DDE. Finally, most species, including
man, store DDE more tenaciously than they do DDT, the greater part of
which is metabolized by a different pathway to DDA and excreted more
rapidly. The result is that DDE, expressed as a percentage of total
DDT-related compounds, increases in individuals after DDT intake
decreases, and also increases in successive steps of the food chain.
Apparently, the rhesus monkey is an exception. Monkeys store DDE
when it is fed to them but, when feeding stops, the rate of loss of
DDE stored in fat is more rapid than that of DDT (Durham et al.,
1963). Whether it is relative inability to form DDE, unusual ability
to excrete it, or a combination of both that accounts for the fact
that little or no DDE can be found in monkeys fed DDT is not entirely
clear.
6.3 Elimination
6.3.1 Human studies
6.3.1.1 Studies of volunteers
DDA is the main urinary metabolite of DDT. In man, it was found
first in a volunteer by Neal et al. (1946), who reported that,
following ingestion of 770 mg of p,p'-DDT, excretion rose sharply to
4.0 mg/day during the second 24-h period, decreased rapidly on the
third and fourth days, decreased gradually thereafter, but was still
above baseline on the fourteenth day. Judging from a graph, the
highest concentration was about 2.6 mg/kg.
Much later studies in volunteers, who received smaller but
repeated doses, showed that a steady state of excretion was reached
after about 6-8 months. During a 56-week period of continued dosing
after equilibrium was fully established, the concentration of DDA
associated with technical DDT at the rate of 35 mg/man per day varied
from 0.18 to 9.21 mg/kg and averaged 2.98 mg/kg, corresponding values
for p,p'-DDT were 0.40-6.27 mg/kg with a mean of 1.88 mg/kg. Thus,
technical DDT was excreted more effectively and stored in man less
than p,p'-DDT. During the latter half of the dosing period, it was
possible, in the 2 groups receiving recrystallized and technical DDT
at the rate of 35 mg/man per day, to account for an average of 13% and
16%, respectively, of the daily dose in terms of urinary DDA. The
excretion of DDA was relatively constant in each individual, but
marked differences were observed between men receiving the same dose.
For example, over the period of 56 weeks, the highest rate measured
for one man was 0.16 mg/h while the lowest rate for another in the
same group was 0.15 mg/h. Their mean rates during this period were
0.089 and 0.269 mg/h, respectively. The difference was highly
significant (P < 0.001) (Hayes et al., 1971).
6.3.1.2 Studies of occupationally exposed workers
Among workers whose DDT intake was estimated to be about 35 mg/man
per day, Ortelee (1958) reported that the concentration of DDA in
urine ranged from 0.12 to 7.56 mg/litre and averaged 1.71 mg/litre.
Among workers whose exposure was about half as high, Laws et al.
(1967) found concentrations from 0.01 to 2.67 mg/litre with a mean of
0.97 mg/litre.
Continuous sampling of a DDT-formulating plant worker's urine
showed that excretion of DDA increased promptly, when exposure began
on each of 5 consecutive work days and often continued to increase
after exposure, sometimes reaching a peak about midnight before
decreasing rapidly. On the sixth day, when there was no occupational
exposure to DDT, the excretion of DDA continued until a very low level
was reached. The highest concentration of DDA reported in this study
was 0.68 mg/litre (Wolfe & Armstrong, 1971).
6.3.1.3 Studies of the general population
Urine. Cranmer et al. (1969) developed a method for analysing
DDA in urine that, for the first time, made it possible to measure the
compound in every sample. The range found for a small sample of the
population of Florida, USA, was 0.008-0.019 mg/litre, and the mean
(0.014 mg/litre was slightly less than 0.02 mg/litre, the lowest
concentration detectable by earlier methods. Results for general
population studies are shown in Table 14 together with results for
various groups of workers and volunteers.
Table 14. Urinary excretion of DDA by people in the USA with various degrees of exposure to DDTa
Exposure Year No. of DDA excretion (mg/kg) Reference
samples
Range Mean
General population 1954 8 <0.05 -- Hayes et al., 1956
General population 1957 8 <0.02-0.07 -- Hayes et al., 1971
General population 1962 23 <0.02-0.18 -- Durham et al., 1965b
General population 1968 11 0.008-0.019 0.014 Cranmer et al., 1969
Environmentalb 1962 13 <0.02-0.11 -- Durham et al., 1965a
Subjects applying DDT 1962 11 <0.02-0.17 -- Durham et al., 1965a
Formulators 1957 40 0.12-7.56 1.71 Ortelee, 1958
Makers and formulators 1966 35 <0.01-2.67 0.97 Laws et al., 1967
Volunteers given 1953-1954 2 0.10-0.42b 0.21c Hayes et al., 1956
3.5 mg/day orally
Volunteers given 1957-1958 6 0.06-1.98a 0.23b Hayes et al., 1971
3.5 mg/day orally
Volunteers given 1953-1954 6 0.69-9.67b 2.46c Hayes et al., 1956
35 mg/day orally
Volunteers given 1957-1958 6 0.18-9.21c 3.09d Hayes et al., 1971
35 mg/day orally
a Slightly modified from Hayes (1975).
b Residents living within 500 feet of agricultural application.
c Based on all samples after thirty-fifth week of dosage.
d Based on all samples from the thirty-fifth to the ninety-third week after dosage started.
In the general population the urine contained not only DDA but
also neutral compounds; the average concentrations reported by Cueto &
Biros (1967) were: p,p'-DDT, 0.0007 mg/litre and p,p'-DDE,
0.0156 mg/litre. Men with full-time occupational exposure to DDT
excreted much more DDA but showed only a statistically insignificant
increase in the excretion of DDT and DDE.
These values for the excretion of DDA, DDT, and DDE by different,
small groups of people showed an average concentration of
0.0358 mg/litre of DDT-related material. Although the DDT intakes of
these particular groups were not measured, the urinary excretion is of
such an order of magnitude that it may account for the excretion of
all the absorbed DDT.
Milk. As far as the mother is concerned, the secretion of a
compound in milk is a form of excretion. For the infant, the milk is
an important, if not the sole source of intake of the compound in
question. The concentrations of DDT and DDE in milk reported from
different countries are listed in Table 15. Especially high values
have been reported from Guatemala for 1970 (see Table 16) and from the
USSR (Damaskin, 1965). However, additional and more numerous samples
taken only four years later in the same and other communities in
Guatemala revealed entirely different results. The highest single
value observed for total DDT in 1974 was 5.69 mg/litre. The range of
average values for different locations was 0.04-0.86 mg/litre. The
means for total DDT for the three communities studied earlier were: La
Bomba, 0.59 mg/litre; El Rosario, 0.28 mg/litre; and Cerro Colorado,
0.47 mg/litre (Winter et al., 1976). The authors recognized the
importance of the agricultural uses of DDT as a potential source of
the compound in human milk. However, they attributed the change
between 1970 and 1974, almost exclusively, to the substitution of
propoxur for DDT in residential spraying to combat malaria.
The medical importance of DDT in human milk depends entirely on
the dosage of the compound received by babies. The highest
concentration of p,p'-DDT ever reported in a single sample of milk
and the highest average value from one community (see Table 16) would
determine maximum and average intakes of 1.06 and 0.32 (mg/kg) day for
newborn babies, assuming an intake of 0.6 litre per day and a weight
of 3.36 kg. This average intake is of the same order of magnitude as
that encountered by workers who make and formulate DDT. The
corresponding maximum and average intakes of total DDT-related
material for infants in Guatemala would be 2.18 and 0.73 (mg/kg) day,
values not strictly comparable to those of the workers because the
intake of the babies includes so much more DDE, a less toxic compound.
Neither of the papers cited concerning DDT in human milk in Guatemala
mentioned any indication of injury to babies. Absence of injury to
babies would be predicted from studies of the most heavily exposed
workers and also from studies regarding the effect of age on the
susceptibility of animals to DDT (see section 7.3.2).
Table 15. Concentration of DDT-derived material in human milk
Country Year No. of Method DDT DDE as DDT Total as DDE as Reference
samples of (mg/litre) (mg/litre) (mg/litre) DDT
analysis (% of
total)
North America
Canada 1967-1968 147 GLC 0.032 0.097 0.139 -- Ritcey et al., 1972
Canada 15 GLC 0.006-0.032 -- 0.019-0.035 -- Musial et al., 1974
USA 1950 32 colour -- 0.13 -- Laug et al., 1951
USA 1960-1961 10 colour 0.08 0.04 0.12 33 Quinby et al., 1965b
USA 1962 6 colour 0-0.12a 0.025a 0.37b -- West, 1964
USA 1968 ? GLC 0.026 0.047 0.078 60 Cudey & Kimbrough, 1969
USA 1970 53 GLC 0.022 0.083 0.101 80 Kroger, 1972
USA 1970-1971 101 GLC -- -- 0.17 Wilson et al., 1973
USA 1971-1972 40 GLC 0.126 Savage et al., 1973
USA 1973-1974 57 GLC 0.092 0.260 0.344 76 Strassmann & Kutz, 1977
USA 1974 38 GLC 0.447 Woodard et al., 1976
USA 1974 14 GLC 0.075 Woodard et al., 1976
USA 1975 7 GLC 0.323 Woodard et al., 1976
Europe
Belgium 1968 20 GLC 0.05 -- -- -- Heyndrickx & Maes, 1969
Czechoslovakia 1968 -- -- 0.101 -- -- -- Hruska, 1969
Czechoslovakia 393 TLC 0.097 0.112 0.209 54 Suvak, 1970
England 1963-1964 19 GLC 0.05 0.08 0.13 62 Egan et al., 1965
France 1971-1972 3.24c Luquet et al., 1974
France 1972? 3.24c Luquet et al., 1974
German Democratic 1970? 0.569 Adamovic et al., 1971
Republic
German Democratic 1969 57 0.23 Engst & Knoll, 1972
Republic
German Democratic 1970 18 0.16 Engst & Knoll, 1972
Republic
Table 15 (Cont'd)
Country Year No. of Method DDT DDE as DDT Total as DDE as Reference
samples of (mg/litre) (mg/litre) (mg/litre) DDT
analysis (% of
total)
German Democratic 1971 96 0.32 Knoll & Jayarman, 1973a
Republic 1973b
Germany, Federal 1970? 43 GLC 0.031 0.090 0.121 74 Acker & Schulte, 1970
Republic of
Germany, Federal 1971? 0.121 Pfeilsticker, 1973
Republic of
Hungary 1963 10 colour 0.13-0.26a -- -- -- Denés, 1964
Italy 1965? 2 GLC 0.001 0.055 0.056 Kanitz & Castello, 1966
Netherlands 1969 50 GLC 0.9c 1.8c 2.7c 66 Tuinstra, 1971
Poland 1966 26 colour 0.27 -- -- 62 Bronisz & Ochynski, 1968
Poland 1967 25 colour 0.40 -- -- 58 Bronisz & Ochynski, 1968
Poland 1970? 40 GLC 0.08 0.19 0.28 71 Kontek et al., 1971
Portugal 1972 168 GLC 0.326 Graca et al., 1975
Romania 1968? 100 colour 0.054-0.749 0.026-8.30 0.080-9.05 -- Unterman & Sirghie, 1969
Sweden 1967? -- -- 0.117 -- Lotroth, 1968
Sweden 1967-1969 22 GLC 0.039 0.076 0.115 63 Westoo et al., 1970
USSR 1964 16 colour 1.22-4.88 -- -- -- Damaskin, 1968
USSR 1968? 4505 -- 0.1-1.0 -- -- -- Gracheva, 1969
USSR 1969? -- -- 0.09 -- 0.14 -- Gracheva, 1970
USSR 1967? 370 GLC 0.1 -- -- Komarova, 1970
Asia
Israel 1975 29 GLC 0.02 0.03 0.07 44 Polishuk et al., 1977
Japan 1970? 10 0.071 Tokutsu et al., 1970
Japan 1970? 5 0.160 Takeshita & Inuyama. 1970
Japan 1970? 10 0.120 Takeshita & Inuyama. 1970
Japan 1971? 0.04 Kojima et al., 1971
Japan 1971? 0.04 Kojima et al., 1971
Table 15 (Cont'd)
Country Year No. of Method DDT DDE as DDT Total as DDE as Reference
samples of (mg/litre) (mg/litre) (mg/litre) DDT
analysis (% of
total)
Japan 1971? 59 0.019-0.105 Kato et al., 1971
Japan 1971? 14 0.047 Sugaya et al., 1971
Japan 1971 43 GLC 0.095 0.084 0.179 47 Hidaka et al., 1972
Japan 1971 454 GLC 0.0607 Hayashi, 1972a, 1972b
Japan 1971-1972 398 0.0626 Hayashi, 1972a, 1972b
Japan 1971-1972 398 0.0562 -- -- -- Anonymous, 1972
Japan 1971 0.044 Yamagishi et al., 1972
Japan 30 2.0a Mizoguchi et al., 1972
Japan 54 0.035 Taira et al., 1972
Japan 1971-1972 5 0.027 Nagai, 1972
Japan 1971-1972 5 0.037 Nagai, 1972
Japan 1971-1972 5 0.016 Nagai, 1972
Japan 1971-1972 5 0.037 Nagal, 1972
Japan 30 0.033 Oura et al., 1972
Japan 1971-1972 123 0.105 Kawai et al., 1973
Japan 1971-1972 0.038-0.075 Kamata, 1973
Japan 1970 3.780c Suzuki et al., 1973
Japan 1971 3.592c Suzuki et al., 1973
Japan 1972 3.822c Suzuki et al., 1973
Japan 1973 0.0854 Kamata, 1974
Oceania
Australia 1970 1 GLC -- -- 0.014d Newton & Greene, 1972
0.007e
0.066f
67 GLC 0.036 0.105 0.141
Table 15 (Cont'd)
Country Year No. of Method DDT DDE as DDT Total as DDE as Reference
samples of (mg/litre) (mg/litre) (mg/litre) DDT
analysis (% of
total)
Australia
(Brisbane) 1971-1972 20 GLC 0.288 Miller & Fox, 1973
(Mareeba) 1971-1972 20 GLC 0.415
Australia 45 GLC 0.064 Siyali, 1973
Australia 22 GLC 0.010 0.068 0.078 Stacy & Thomas, 1975
Papua New Guinea 1972 16 GLC
(Kar Kar Island) 0.002 0.002 0.004 50 Hornabrook et al., 1972
Papua New Guinea 1972 19 GLC
(Sepik district) 0.008 0.007 0.015 47 Hornabrook et al., 1972
a Range of values for milk containing 4% fat c Concentration in milkfat. e At middle of feeding, 1.2% fat.
containing 3.3-6.6 ppm.
b Maximal value. d At beginning of feeding, 1.8% fat. f At end of feeding, 5.1% fat.
Table 16. Ranges, means, and standard errors of the concentrations
of organochlorine insecticides in the milk of women in
three towns in Guatemalaa
Compound La Bomba El Rosario Cerro Colorado
1970 1970 1971
n = 10 n = 27 n = 9
p.p'-DDT 0.23-4,95 0.16-2.24 0.49-5.94
(mg/litre) (1.00 ± 0.38) (0.77 ± 0.10) (1.78 ± 0.56)
p.p'-DDE 0.12-6.36 0.28-3,10 0.60-6.13
(mg/litre) (1.02 ± 0.58) (0.99 ± 0.14) (2.10 ± 0.61)
p,p'-TDE(DDD) trb-0.16 0.01-0.09 0.05-0.11
(mg/litre) (0.03 ± 0.02) (0.02 ± 0.004) (0.07 ± 0.01)
o,p'-DDT tr-0.29 0.01-0.18 0.06-0.22
(mg/litre) (0.09 ± 0.03) (0.06 ± 0.01) (0.12 ± 0.02)
total as DDT 0.41-11.50 0.34-4.97 1.571-12.21
(mg/litre) (2.15 ± 1.05) (1.84 ± 0.24) (4.07 ± 1.11)
total HCH 0.01-0.10 tr-0.07 0-0.06
(mg/litre) (0.03 ± 0.01) (0.007 ± 0.003) (0.02 ± 0.01)
heptachlor epoxide 0-0.02 tr-0.01 tr
(mg/litre) (0.003 ± 0.002) (0.007 ± 0.0004)
dieldrin tr tr-0.01
(mg/litre) (0.002 ± 0.0005)
a From: Olszyna-Marzys et al. (1973).
b tr = trace.
The slightly greater secretion of DDT and much greater secretion
of various isomers of HCH by urban mothers (compared to rural mothers)
in Japan was attributed to their greater intake of cow's milk (Takano,
1972).
Other routes of excretion. DDT, DDE, and dieldrin are excreted in
the bile; the concentrations for five men without special exposure
varied as follows: p,p'- and o,p'-DDT combined, 0.0000-0.0009 mg/litre;
p,p'-DDE, 0.0005-0.0056 mg/litre; and dieldrin, 0.0000-0.0005 mg/litre.
Higher levels were found in the bile of one pest control operator
(Paschal et al., 1974).
6.3.2 Animal studies
When large doses of DDT are ingested, some of the compound is not
absorbed and is passed in an unaltered state in the faeces. Only
traces of unaltered DDT may be found in the faeces when exposure is by
any route other than oral. However, true faecal excretion of DDT
metabolites was established very early (Wasicky & Unti, 1945; Judah,
1949). In the rat, faecal excretion of DDT exceeded urinary excretion,
irrespective of the route of administration (Hayes, 1965). In man, the
ratio is obviously different. Although the excretion of DDT-related
material in the faeces of man receiving 35 mg/man per day has been
reported using colorimetry (Hayes et al., 1956), this result has never
been confirmed by gas chromatography, even in connexion with workers
whose exposure was heavy and prolonged. Either DDT metabolites are not
excreted by man in the faeces to any great degree, or they are
excreted in one or more forms that differ from those already
demonstrated in rats.
The bile appears to be the principal source of DDT metabolites in
the faeces of rats. When the bile duct was cannulated before
intravenous injection of radioactive DDT, 65% of the dose was
recovered in the bile, 2% in the urine, and only 0.3% in the faeces
(Jensen et al., 1957), and the possibility of some contamination of
the faeces by urine could not be excluded.
The different routes of excretion are not unrelated. Burns et al.
(1957) found that there was an increase in urinary excretion of
radioactive material following ligation of the bile duct in rats fed
radioactive DDT. This is an indirect confirmation of the finding by
Jensen and his colleagues that most of the metabolites in bile consist
of DDA or compounds closely related to it. Although an enterohepatic
circulation of the metabolites of DDT has not been directly proved, it
seems likely that such a circulation exists, as has been demonstrated
for 1,1'-(2,2-dichloroethylidene)-bis[4-ethylbenzene] (Perthane).
Demonstration of the excretion of DDT in milk was first reported
by Woodard et al. (1945) in connexion with a dog fed the compound at
the rate of 80 (mg/kg) day. Within a short time, excretion of DDT in
milk was reported in rats, goats, and cows, and soon afterwards, in
women (Laug et al., 1951). Telford & Guthrie (1945) showed that rats
fed a diet containing DDT at 1000 mg/kg produced milk that was toxic
to their young.
Since the early laboratory studies, the presence of DDT has been
demonstrated repeatedly in the milk of cows. A review (Hayes, 1959)
showed that cows fed substantial, but nontoxic, residues of DDT
commonly excrete 10% or slightly more of the total dose in their milk,
and amounts slightly over 30% have been observed.
Further information on the excretion of DDT in human milk is given
in section 6.3.1.3. It is of interest to repeat here, however, that
lactating women in the general population are apparently in negative
DDT balance. That is, they excrete more DDT in their milk each day
than they acquire in their food. The difference is small and would not
be expected to have much effect on their total body burden of DDT.
Wilson et al. (1946a) showed that DDT was secreted from the skin
of a cow maintained on an oral dosage of about 53 (mg/kg) day.
DDA. Because DDA is the main form in which DDT is excreted, it
might be expected that, following its direct administration, DDA would
be excreted relatively efficiently, and this is true. It was found
very early that, during the first few days after oral dosing, rabbits
excreted DDA in the urine approximately 15 times faster than animals
given DDT at an equivalent dosage. Although the rate of DDA excretion
increased somewhat, the rate of excretion associated with DDT
increased more rapidly so that the values differed by a factor of only
five after the twentieth day of feeding (Smith et al., 1946).
6.4 Biotransformation
The chemical nature of the chief metabolite excreted in the urine
was first elucidated by White & Sweeney (1945). Rabbits were given DDT
(melting point 107-108°C) at a rate of 100 (mg/kg) day for 6 days per
week, and their urine was collected. It contained a considerable
amount of organic chloride, whereas normal rabbit urine did not. Using
the organic chloride test to evaluate different methods of extraction,
the authors were able to isolate a crystalline material containing
25.37% chlorine and melting at 166-166.5°C. The crystals were shown to
be DDA (see Table 1). The product obtained from the urine was
identical to that synthesized from glyoxylic acid and chlorobenzene
and with a compound obtained through the chemical degradation of DDT.
Identity of the 3 compounds and, therefore, their true chemical
nature, was established by the determination of melting points, mixed
melting points, elementary analysis, and X-ray powder diffraction
patterns, as well as by demonstrating the similarity of the
decarboxylation products of the three original materials. Only 80-85%
of the total organic chloride of the rabbit urine was found to be
soluble in alkali and in bicarbonate. For this and other reasons it
was considered possible that DDA was not the only chlorinated organic
compound present.
Later work by many authors has confirmed that DDA is the major
urinary metabolite of DDT in all mammals including man. It may be
added that, in spite of great strides in analytical chemistry, the
nature of other urinary metabolites has not been elucidated fully.
The fact that DDE is stored in tissue was first demonstrated by
Pearce et al. (1952) in connexion with human fat. The authors pointed
out that they did not know whether the compound resulted from partial
degradation of DDT residues on plants or whether the DDE was formed
during the process of digestion or after absorption. It is now known
that some food contains DDE but that man is capable of forming the
product from DDT. The exact mechanism of the biotransformation of DDT
to DDE remains in doubt.
Pearce et al. (1952) established the identity of DDE by comparing
the colorimetric and column chromatographic behaviour of the residue
with those of a chemical standard. A second paper from the same
laboratory (Mattson et al., 1953) added further details confirming the
identity of the compound. Later investigations have confirmed the
identity of DDE by infrared spectrometry and by gas chromatography.
That portion of the metabolism of DDT that leads to DDA has been
clearly elucidated by Peterson & Robinson (1964), who gave evidence
for the sequence of changes shown in Fig. 2. Organ perfusion studies
have indicated that the liver is capable of the biotransformation of
DDT, DDE, TDE, DDMU, and DDMS, while the kidney transforms DDMS, DDNU,
and DDOH (Datta & Nelson, 1970). Cultures of embryonic lung cells are
capable of metabolizing DDT to DDA via DDD (North & Menzer, 1973).
When DDA was discovered, it was postulated, on chemical grounds,
that DDE was a step in its formation (White & Sweeney, 1945); however,
rats that produced both DDE and DDA from DDT were incapable, according
to Peterson & Robinson (1964), of forming DDA when fed preformed DDE.
This finding was contradicted by Datta (1970) and by Datta & Nelson
(1970) who claimed that 14C- p,p'-DDE was converted by rats to
p,p'-DDMU, which then underwent further metabolism to p,p'-DDA via
the route shown in Fig. 2. Datta suggested that the predominance of
detoxication via DDE or TDE (DDD) might depend on physiological
response or the amount of toxicant used. Whatever the reason, the fact
remains that DDE is stored more tenaciously than DDT.
Rhesus monkeys fed either technical or p,p'-DDT store little or
no DDE, although they are fully capable of storing DDE when it is fed
preformed (Durham et al., 1963).
The way in which DDE was lost from storage was not clearly
understood for a long time. In man (Cueto & Biros, 1967), seal, and
guillemot (Jansson et al., 1975) part of it is excreted unchanged, but
the fact that its elimination is promoted by the induction of
microsomal enzymes (see section 7.1.4.2) strongly suggests that it
undergoes metabolism, conjugation, or both. That metabolism does occur
was first demonstrated by identification of 2 hydroxylated derivatives
of DDE in the faeces of wild seals and guillemots and in the bile of
seals (Jansson et al., 1975). When p,p'-DDE was fed to rats, the
same metabolites and one other were isolated from the faeces, and,
within the first 6 days, the metabolites accounted for about 5% of the
dose (Sundström et al., 1975). Later a fourth hydroxylated derivative
was identified from the faeces of rats fed p,p'-DDE. The compounds
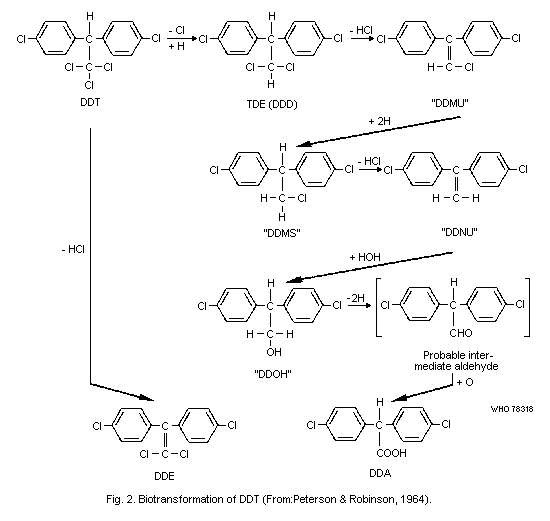 are m-hydroxy- p,p'-DDE [1,1-dichloro-2-( p-chloro- m-hydroxyphenyl)-
2-( p-chlorophenyl)-ethylene, the major metabolite], o-hydroxy- p,p'-DDE,
p-hydroxy- m,p'-DDE (the product of an NIH shift), and p-hydroxy-
p'-DDE. A scheme (Fig. 3) involving m,p-epoxy- p,p'-DDE and o,m-epoxy-
p,p'-DDE was proposed for the formation of these metabolites as
well as a fifth metabolite (Sundström, 1977). Neither the fifth
metabolite nor the hypothetical intermediate have been isolated.
DDE is metabolized not only to easily excretable phenols but also
to m-methylsulfone- p,p'-DDE. In the blubber of seals from the
Baltic, this compound was found in a concentration of 4 mg/kg along
with DDE (138 mg/kg), TDE (DDD) (10 mg/kg), DDT (78 mg/kg) and various
PCB's and their metabolites (150 mg/kg) (Jensen & Jansson, 1976).
The conversion of o,p'-DDT to p,p'-DDT has been reported
(Klein et al., 1965; French & Jefferies, 1969), but, when the
possibility was reinvestigated using 14C- o,p'-DDT, no conversion
could be detected (Cranmer, 1972). The chromatographic peak closely
resembling that of p,p'-DDT observed in the earlier studies
undoubtedly was due to the presence of a metabolite of o,p'-DDT,
which may explain the more rapid metabolism of the o,p'-isomers that
has been observed in rat, man, and perhaps other species. The more
rapid excretion of o,p'-DDT is explained, at least in part, by the
observed ring-hydroxylation of the parent compound in rats (Feil et
al., 1973) and chickens (Feil et al., 1975) and of preformed o,p'-TDE
(DDD) in rats (Reif & Sinsheimer, 1975) and in man (Reif et al.,
1974). At least 13 metabolites were detected in rats and 15 in
chickens. Ring-hydroxylation, which has not been observed with p,p'-
DDT or p,p'-TDE, was present in all species. There were, however,
some species differences. For example, o,p'-DDE and three
hydroxylated o,p'-DDE's were found in the excreta of chickens but
not in the excreta of rats. In 2 patients with adrenal carcinoma for
which they were receiving o,p'-TDE at a rate of 2000 mg/day, as much
as 46-56% of the daily intake was recovered in the urine following
acid hydrolysis. Just over half of the recovered material was in the
form of o,p'-DDA, but the remainder was in the form of hydroxylated
derivatives, specifically m-hydroxy, p-hydroxy-, m-hydroxy- p-
methoxy-, and p-hydroxy- m-methoxy- o,p'-DDA. Some other
hydroxylated compounds were found in trace amounts. All hydroxylation
had occurred on the ring that had its chlorine in the ortho position
(Reif et al., 1974). When the metabolism of a single 100 mg oral dose
of 14C- o,p'-TDE was studied in rats, averages of 7.1 and 87.8% of
the activity were recovered in the urine and faeces, respectively,
within 8 days (Reif & Sinsheimer, 1975). The high recovery indicated
rapid excretion with little storage.
are m-hydroxy- p,p'-DDE [1,1-dichloro-2-( p-chloro- m-hydroxyphenyl)-
2-( p-chlorophenyl)-ethylene, the major metabolite], o-hydroxy- p,p'-DDE,
p-hydroxy- m,p'-DDE (the product of an NIH shift), and p-hydroxy-
p'-DDE. A scheme (Fig. 3) involving m,p-epoxy- p,p'-DDE and o,m-epoxy-
p,p'-DDE was proposed for the formation of these metabolites as
well as a fifth metabolite (Sundström, 1977). Neither the fifth
metabolite nor the hypothetical intermediate have been isolated.
DDE is metabolized not only to easily excretable phenols but also
to m-methylsulfone- p,p'-DDE. In the blubber of seals from the
Baltic, this compound was found in a concentration of 4 mg/kg along
with DDE (138 mg/kg), TDE (DDD) (10 mg/kg), DDT (78 mg/kg) and various
PCB's and their metabolites (150 mg/kg) (Jensen & Jansson, 1976).
The conversion of o,p'-DDT to p,p'-DDT has been reported
(Klein et al., 1965; French & Jefferies, 1969), but, when the
possibility was reinvestigated using 14C- o,p'-DDT, no conversion
could be detected (Cranmer, 1972). The chromatographic peak closely
resembling that of p,p'-DDT observed in the earlier studies
undoubtedly was due to the presence of a metabolite of o,p'-DDT,
which may explain the more rapid metabolism of the o,p'-isomers that
has been observed in rat, man, and perhaps other species. The more
rapid excretion of o,p'-DDT is explained, at least in part, by the
observed ring-hydroxylation of the parent compound in rats (Feil et
al., 1973) and chickens (Feil et al., 1975) and of preformed o,p'-TDE
(DDD) in rats (Reif & Sinsheimer, 1975) and in man (Reif et al.,
1974). At least 13 metabolites were detected in rats and 15 in
chickens. Ring-hydroxylation, which has not been observed with p,p'-
DDT or p,p'-TDE, was present in all species. There were, however,
some species differences. For example, o,p'-DDE and three
hydroxylated o,p'-DDE's were found in the excreta of chickens but
not in the excreta of rats. In 2 patients with adrenal carcinoma for
which they were receiving o,p'-TDE at a rate of 2000 mg/day, as much
as 46-56% of the daily intake was recovered in the urine following
acid hydrolysis. Just over half of the recovered material was in the
form of o,p'-DDA, but the remainder was in the form of hydroxylated
derivatives, specifically m-hydroxy, p-hydroxy-, m-hydroxy- p-
methoxy-, and p-hydroxy- m-methoxy- o,p'-DDA. Some other
hydroxylated compounds were found in trace amounts. All hydroxylation
had occurred on the ring that had its chlorine in the ortho position
(Reif et al., 1974). When the metabolism of a single 100 mg oral dose
of 14C- o,p'-TDE was studied in rats, averages of 7.1 and 87.8% of
the activity were recovered in the urine and faeces, respectively,
within 8 days (Reif & Sinsheimer, 1975). The high recovery indicated
rapid excretion with little storage.
 The compound identified by Peterson & Robinson (1964) as a
"probable intermediate aldehyde" was later synthesized and shown to be
highly labile (McKinney et al., 1969), confirming the guess by
Peterson & Robinson that it was unlikely to accumulate in tissues in
measurable amounts.
Of the compounds shown in Fig. 2 and 3, only DDT, TDE (DDD), DDE,
and DDA are commonly reported in the tissues or excreta of animals,
including man. The symptomatology produced when the metabolites are
administered directly is discussed in section 7.1.1, while uptake,
distribution, and elimination of the compounds are discussed in
sections 6.1.2, 6.2.2, and 6.3.2, respectively.
Although microorganisms, plants, insects, and birds produce many
of the same metabolites that are found in mammals, there are
interesting differences. Nearly 20 derivatives (including mammalian
metabolites) have been identified, and the chemical structure of
several more is still unknown. Some aspects of nonmammalian, as well
as mammalian metabolism have been reviewed (Menzie, 1969; Klein &
Korte, 1970; Fishbein, 1974; Schroeder & Dorozalska, 1975). The
metabolism of microorganisms and plants, as well as that of domestic
animals, may influence the composition of DDT-derived residues in
human food, but there is no evidence that these residues contain a
significant amount of any compound not formed from DDT by human
metabolism.
7. EXPERIMENTAL STUDIES ON THE EFFECTS OF DDT
7.1 Animal Studies
7.1.1 Haemopoietic system and immunology
Many early reports reviewed by Hayes (1959) indicated that large
doses of DDT might not have any effect on the blood or that they might
produce a moderate leukocytosis and a decrease in haemoglobin, with or
without a decrease in the concentration of red cells. The leukocytosis
probably is secondary to stimulation of the sympathetic nervous
system, while the loss of haemoglobin may be nutritional in origin.
Later studies have confirmed the early results. A range of
haematoiogical variables remained unchanged in squirrel monkeys dosed
orally at rates of 0, 0.05, 0.5, 5, and 50 (mg/kg)/day, even though
the highest dosage was fatal within 14 weeks (Cranmer et al., 1972).
Immunology. Inasmuch as some compounds are antibiotic, it is
logical that some may be probiotic, that is, that they either reduce
resistance to infection or increase the virulence of an infecting
organism (Hayes, 1975). Far fewer studies have been made of probiosis
than of antibiosis. A number of papers have reported one or other
possibly probiotic property of DDT, but some of the reports could not
be confirmed, and others have not been retested.
It has been claimed that a change in the phagocytic activity of
white blood cells is an indication of early intoxication by DDT
(Kun'ev, 1965). However, Kaliser (1968) did not find any statistical
difference in in vitro or in vivo phagocytosis of control rats and
those receiving DDT by stomach tube at a rate of 0.25 (mg/kg)/day for
31 days. The highest rate at which men who make and formulate DDT in
the USA now absorb the compound is about 0.25 (mg/kg)/day.
Rats receiving an aqueous suspension of p,p'-DDT of unstated
stability at a concentration of 200 mg/litre as their only source of
water for over 30 days were reported to develop a lower titre of
antibodies to ovalbumen (Wassermann et al., 1969). Rabbits responded
to the same treatment with a statistically significant reduction of
antibody titre against Salmonella and a reduction in antibody titre
against sheep red blood cells that was not statistically significant
(Wassermann et al., 1971). Both the rats and rabbits showed a decrease
in at least one globulin fraction of the blood.
Other reports of changes in immunohaematological indices are those
of Semenceva (1968) and Fridman (1970).
One group of investigators has shown clearly that what at first
appeared to be an immunological response really involved a quite
different, predictable effect. Briefly, it was shown that guineapigs
sensitized to diphtheria toxoid were less susceptible to anaphylaxis
in response to a challenge dose of the toxoid if they were pretreated
with DDT at a dosage of only 1-20 mg/kg. Direct measurement of
antitoxin production indicated little or no difference between
protected and unprotected animals. Furthermore, some protection was
given by DDT administered for only 3 days prior to the induction of
anaphylaxis (Gabliks et al., 1973, 1975). Further studies showed that
DDT treatment reduced the histamine levels in the lungs of both
immunized and nonimmunized animals. The number of detectable mast
cells was also reduced; this was true whether the count was made in
tissues from guineapigs dosed systemically with DDT or in the lungs
and mesenteric tissue taken from untreated animals and exposed to DDT
in vitro at concentrations ranging from 10 to 45 mg/litre. These
results indicate that the protection offered by DDT was the result of
a reduction of the amount of histamine available for sudden release in
response to a challenge dose of toxoid (Askari & Gabliks, 1973).
Regardless of exposure to DDT, immunization leads to an increase in
detectable mast cells (Gabliks et al., 1975).
7.1.2 Nervous system and behaviour
DDT intoxication in animals was well described by Domenjoz (1944).
The first perceptible effect is abnormal susceptibility to fear, with
violent reaction to normally subthreshold stimuli. There is definite
motor unrest and increased frequency of spontaneous movements. As
poisoning increases, hyperirritability, like that seen in strychnine
poisoning develops, but convulsions do not appear at this time. A fine
tremor, recognizable at first only as a terror reaction, is later
present as all intention tremor in connexion with voluntary movement.
Then it is present intermittently without observable cause, and
finally it is present as a coarse tremor without interruption for as
long as several days. Spontaneous movement is limited, and food intake
stops so that surviving animals lose weight. In the later stages,
especially in some species, there are attacks of epileptiform, tonic-
clonic convulsions with opisthotonos.
All the signs are strengthened by external stimuli and become
manifest at first through external stimuli. At all stages, the animals
show normal position and labyrinth reflexes. The picture of poisoning
in mammals recalls the disturbances of movement and tone that are
known in human pathology as the amyostatic syndrome.
Symptoms appear several hours after oral administration of the
compound, and death follows after 24-72 h. The latent period after
intravenous administration at about the LD50 level is approximately
5 min; signs of poisoning reach a maximum level in about 30 min, and
survivors are symptom-free in 18 to 24 h. Animals that survive recover
completely.
In addition to the features of poisoning already mentioned,
Cameron & Burgess (1945) noticed that as rats, guineapigs, and rabbits
become sick, they become cold to the touch and show ruffled fur. Some
show diarrhoea. These authors found that muscular tremors were
preceded by muscular weakness that first occurred in the back and
later in the hind legs. The front legs were relatively spared so that
animals showing marked weakness of the hind quarters could still drag
themselves about. However, several authors have found that the tremor
characteristic of DDT poisoning generally starts in the muscles of the
face, including the eyelids, and spreads caudally with variable
severity until all the muscles are affected. Furthermore, although
weakness of hind quarters has been seen by others, it is not a common
finding.
Like tremor, coldness of the skin and ruffling of the fur probably
represent an indication of disturbed thermal regulation. Apparently it
was not until the work of Hrdina et al. (1975) that an increase of
almost 3°C in body temperature was reported in rats following a fatal
(600 mg/kg) oral dosage of DDT.
Although there is a general similarity in the clinical effects of
DDT in all vertebrate species, some characteristic differences exist.
Cats show greater extensor rigidity and opisthotonos than other
laboratory animals. The stiffness appears first in the distal part of
the extremities and later extends to the proximal part and to the
trunk. Poisoned cats show marked pilomotor activity. Convulsions in
them may become almost continuous. Convulsions are also prominent in
dogs as is ataxia. Tremors are so pronounced in rats that it may be
difficult to detect clonic convulsions in them. Rats poisoned by DDT
show a reddish colour about the eyes just as they do when ill from
many other causes. The colour has been attributed to excessive
secretion of a porphyrin by the Harderian glands.
Poisoning produced by repeated doses of DDT differs from that
produced by a single dose only in so far as the animal may be
gradually debilitated, especially by malnutrition. If food intake is
maintained, tremor may last for weeks, or even intermittently, for
months. If the animals survive a short time after dosing stops,
recovery is complete. However, food intake may be interfered with in
at least two ways. Tremor and more severe signs may interfere
mechanically with eating. Animals offered food containing high
concentrations of DDT often eat little or nothing and lose weight
rapidly. However, the same animals will show excellent appetites when
offered the same kind of food without DDT just after refusing the
major portion of the daily ration of contaminated food. Unlike
dieldrin and some other compounds, DDT seems to have little effect on
appetite as mediated by the central nervous system; it has a great
deal to do with taste.
Animals that have suffered severe weight loss as a result of DDT
poisoning may die partly as a result of general debility. In some
colonies, at least, they have become prey to secondary infection.
In summary, it may be said that animals that die as the result of
repeated large doses of DDT and small animals that die as a
complication of starvation following many somewhat smaller doses of
DDT show the same signs as those seen in animals killed by one or a
few large doses. Even though severely ill, animals that survive a few
days after the last of many doses of DDT recover.
Of samples that may be collected from a living animal, the
concentration of DDT in serum most accurately reflects its
concentration in the brain, the critical tissue. In the rat, levels of
25 mg/kg (wet weight) in the brain are not usually fatal: higher
levels tend to be fatal regardless of whether absorption followed one
or many doses (Dale et al., 1963; Hayes & Dale, 1964). As reviewed by
Hayes (1975), the danger level is approximately the same in several
species of birds.
Behavioural changes may be demonstrated in animals receiving DDT,
daily, at rates too low to produce illness. Khairy (1959) was able to
detect ataxia in the form of changes in gait in rats that had been fed
DDT at dietary levels of 100 mg/kg or more for 21 or more days. Gait
was recorded by smearing the hind paws of the animals with vaseline,
which then recorded their tracks on paper. Gait was recorded in terms
of the tangent, that is the ratio of the width and length of step. At
a body weight dosage of about 5 (mg/kg)/day the ratio was less than
normal, a change the author attributed to an exaggeration of the
stretch reflex. At dosages of about 10, 20, and 30 (mg/kg)/day, the
ratio was progressively greater than the normal as a result of
broadening of the gait and shortening of the steps. These same dosage
levels did not affect problem-solving behaviour or speed of
locomotion. The experimental animals were generally less reactive to
stress than normal ones. Thus, the author attributed hyperirritability
of rats poisoned by DDT to exaggerated motor responses.
The major toxic action of DDT is clearly on the nervous system,
and it requires an intact organism for full expression. The fact that
DDT causes a myotonic response in muscle and substitution of a train
of spikes for the normal diphasic electroneurogram (Eyzaguirre &
Lilienthal, 1949) is in marked contrast with the absence of detectable
injury or, in fact, any response in other isolated tissues. As early
as 1945, Lewis & Richards (1945) found DDT to be inert when it was
applied to tissue cultures of heart, kidney, stomach, intestine,
liver, and muscle from 7 to 9-day chick embryos, and of brain and
spleen from a one-day rat. The physiology of the cells including the
mytoses of fibroblasts was normal. The migration and extension of the
various cells was unchanged. The authors stated that "living
fibrilloblasts, as they moved about in the cultures, sometimes touched
or even migrated over DDT crystals without appreciable injury to
themselves during a period of several days". Some observations were
carried out for periods as long as 21 days.
In spite of the importance of the nervous system, a detailed
review of early literature indicates that, although the presence of
some specialized nervous function may be necessary for the
manifestation of DDT poisoning, the mere occurrence of specialized
nerve fibres in certain protozoa or the occurrence of a rather complex
nervous system in molluscs is not sufficient to render these forms
susceptible. Just as there is no explanation for the effect of DDT on
susceptible species, so there is no explanation for the fact that
certain species and even entire phyla are inherently resistant to the
compound.
A review (Hayes, 1959) of literature on the effects of DDT on the
nervous system reveals that all major parts, both central and
peripheral, are affected. Whereas effects on specific portions,
notably the cerebellum and the motor cortex, have been viewed as of
greatest importance, it probably is more accurate to emphasize the
interaction of functions, all modified to some degree.
There is reason to think that the mechanism of action of DDT is
its action on membranes in the nervous system, especially axonal
membranes. Certainly action on membranes is a fundamental property of
the compound. In fact the potassium conductance induced by valinomycin
at 10-6 mol/litre in a synthetic lecithin-decane membrane is
reversed by DDT at 3 × 10-6 mol/litre (Hilton & O'Brien, 1970).
Attention was focused quite early on the effects of DDT on axonal
membranes. Using the giant axons of the cockroach, Narahashi &
Yamasaki (1960) showed that DDT prolonged the recovery phase of the
action potential. They concluded that it slows the efflux of potassium
ions from the axon. Later, using the voltage clamp technique and giant
axons of the lobster, Narahashi & Haas (1967) showed that DDT, at a
concentration of 5 × 10-4 mol/litre of bathing medium, prolonged the
flow of sodium ions as well as interfering with the flow of potassioum
ions: in other words DDT delayed shutting of the Na+ gate and
prevented full opening of the K+ gate.
Na+-, K+-, and Mg2+-adenosine triphosphatase (EC 3.6.1.3) is
involved in ion transport in the nervous system. Matsumura & Patil
(1969) showed that a preparation of this enzyme from a nerve ending
fraction of the rabbit brain was inhibited by DDT at concentrations as
low as 10-8 mol/litre. There was a good correlation between the
degree of its inhibition by analogues of DDT and their toxicity to
mosquito larvae. A similar enzyme that binds 14C-DDT has been
isolated from the synapses of rat brain (Bratowski & Matsumura, 1972).
Both the electrophysiological changes and the enzyme inhibition
exhibit a negative temperature coefficient, an important feature of
DDT poisoning in insects but not in mammals (Hoffman & Lendle, 1948;
Deichmann et al., 1950).
At a supralethal dosage of 600 mg/kg, DDT caused a marked decrease
in the concentration of cortical and striatal acetylcholine and of
brainstem norepinephrine in rats and a significant increase in
brainstem 5-hydroxyindoleacetic acid. All of the neurotoxic signs of
poisoning were blocked by p-chlorophenylalanine, while other
inhibitors blocked one or other, but not all of the effects. It was
concluded that changes in the metabolism of 5-hydroxytryptamine and
norepinephrine might be responsible for DDT-induced hyperthermia while
acetylcholine might be related to tremors and convulsions (Hrdina et
al., 1973). These and related matters have been reviewed in great
detail by Hrdina et al. (1975). Apparently studies have not been made
at a range of dosages that would make it possible to know whether
these changes are a result or a cause of poisoning; the possible
therapeutic effect of p-chlorophenylalanine has also not been
explored.
It was reported by Haikina & Silina (1971) that administration of
DDT to rats at only one-fifth of the LD50 for the 2 days increased
the amount of 5-oxyindoleacetic acid excreted in urine by 188%. This
indicates a change in the metabolism of serotonin, but its
significance is not clear.
One sensitive measure of brain activity is the electroencephalo-
gram (EEG). Farkas et al. (1969) found that the EEG wave frequency
increased considerably in resting rats that had received
20 (mg/kg)/day of DDT as a result of dietary intake. Rats that had
received either 5 or 10 (mg/kg)/day did not exhibit this change while
at rest, but even those receiving 5 (mg/kg)/day exhibited this change
when exposed to a rhythmic light stimulus. The EEG may become abnormal
only a minute or two after administration of a large dose of DDT;
4 stages of the electrical activity culminating in generalized
seizures have been described by Joy (1973). Phenobarbital, but not
phenytoin or trimethadione, was effective in stopping the seizures.
7.1.2.1 Cause of death
Death from DDT poisoning is usually the result of respiratory
arrest. The heart continues to beat to the end and in some instances
continues a little while after respiration stops. Deichmann et al.
(1950) found that the onset of hyperirritability in rats was
accompanied by an increase in the frequency and amplitude of
respiration. Later, with the occurrence of tremors, the depth of
respiration frequently returned to a more normal level, but the rate
remained high. In some animals, respiration stopped suddenly after a
deep inspiration during a tonic convulsion. In other animals, the rate
and amplitude decreased progressively and finally ceased without any
terminal spasm. Animals that die of respiratory failure caused by DDT
do so after a relatively long period of muscular activity that leaves
them exhausted.
It was shown by Philips & Gilman (1946) and Philips et al. (1946)
that the hearts of dogs given large intravenous doses of DDT were
sensitized to epinephrine. This was true not only of injected
epinephrine but also of the compound released by the adrenal glands
during a seizure. Stimulated in this way, the sensitized hearts of
dogs developed an irreversible, fatal ventricular fibrillation.
However, the hearts of monkeys were able to recover from fibrillation
and resume normal rhythm. It is not clear how important sensitization
of the myocardium is when DDT is administered by other routes, but
ventricular fibrillation may be the cause of death in animals that die
suddenly, soon after onset of poisoning.
7.1.2.2 Treatment of poisoning in animals
Studies on the treatment of poisoning will be discussed in this
section since all the more successful studies of treatment of animals
poisoned by DDT involve the nervous system. Smith and Stohlman (1944)
noted the possibility that narcotics in general, may exhibit an
antagonism to DDT. Rats survived on a diet containing DDT at a
concentration of 1000 mg/kg for 90 days when they received
cyclohexanone in the same diet at the rate of 2000 mg/kg, but were
uniformly killed in a shorter period when they received DDT at the
same rate without cyclohexanone. Later, it was shown that
cyclohexanone offers no protection when used as a solvent for single
massive doses of DDT (Deichmann et al., 1950).
Smith & Stohlman (1945) showed that, when given as required after
the onset of illness, urethane and, to a lesser extent, phenytoin
sodium protected rats from poisoning. Sodium amobarbital gave slight
benefit, sodium phenobarbital a doubtful benefit, and paraldehyde no
protection at all. All drugs were given intraperitoneally except
paraldehyde, which was given by stomach tube. The mortality of rats
treated with urethane was 12.5% and that of their controls was 80%. A
total dosage of 1.2-2.5 mg/kg spread over a period of 1-3 days, was
found most satisfactory. Phenytoin sodium gave a mortality of 46.7%,
compared with 96.7% for the controls. The smallest effective dosage
was 200-250 mg/kg, a value very close to the LD50 which, under the
conditions of the test was 300 mg/kg.
Lauger et al. (1945a, b) also found that sodium phenobarbital was
of questionable value in treating rats poisoned by DDT. However,
completely different results were seen in larger animals.
Phenobarbital was, by far, the most outstanding remedy tested by
Philips & Gilman (1946). In a dosage well below the anaesthetic level,
it not only prevented death in many instances but also controlled
tremor and convulsions. Signs of illness were more readily controlled
in dogs and cats than in monkeys, which required nearly a full
anaesthetic dosage before tremors completely disappeared.
Magnesium sulfate did not reduce mortality in poisoned dogs and
cats although it did control tremors and convulsions briefly. Sodium
bromide was entirely ineffective. Mortality was reduced with urethane,
but a full anaesthetic dosage was required to control tremor and
convulsion. Similarly, sodium barbital and sodium pentobarbital
controlled symptoms only when given in full anaesthetic doses and,
even then, did not greatly reduce mortality. Phenytoin, when given to
rats before they received DDT, reduced the lethal action without
showing a notable effect on the signs of poisoning; phenytoin was not
effective in cats.
Vaz et al. (1945) were, apparently, the first to note the
antidotal effect of calcium in DDT poisoning. Dogs were given DDT
orally as a 10% oily solution at a daily dosage of 100 mg/kg until
signs of intoxication appeared. The same dosage could then be repeated
to produce intense symptomatology from which the animals would recover
spontaneously in 12-24 h. For the actual tests, a larger challenge
dosage of DDT (150 to 200 mg/kg) was used. Each dose of calcium
gluconate (30 ml of a 10% solution) was injected intravenously into
dogs weighing 8-18 kg. Dogs that were injected with calcium gluconate
dally for 4 days and, challenged with a large dose of DDT on the
fourth day, did not develop any symptoms or only slight ones. Dogs
receiving a single dose of calcium gluconate showed symptoms of short
duration and survived following a dosage of DDT large enough to kill 2
controls.
Cats poisoned by the intravenous injection of a soya-lecithin-corn
oil emulsion of DDT were studied by Koster (1947). A comparison was
made of several aspects of intoxication including number of
convulsions, general severity (tremors, prostration, dyspnoea),
duration, and mortality. Both calcium gluconate and sodium gluconate
reduced mortality but not severity. Gluconic acid increased the
survival time, reduced mortality, but did not reduce the number or
severity of the convulsions. Calcium chloride reduced convulsions, but
not mortality or tremors. Molecular equivalent doses of the candidate
antidotes were used. Gluconic acid and its two salts were effective
against an LD95 dosage of DDT. The life-saving capacity of calcium
gluconate at a rate of 40 mg/kg was confirmed by Judah (1949) even
though he found normal blood calcium values in most poisoned but
unmedicated animals. One animal showed a high calcium value, and
Cameron & Burgess (1945) reported a similar result. It has been
suggested that increased blood calcium may be associated with acidosis
caused by the accumulation of lactate.
Thus, calcium has an antidotal action against DDT in intact
animals of several species. The suppression by calcium of the effect
of DDT on the isolated nerve and muscle of the rat has been
demonstrated (Eyzaguirre & Lilienthal, 1949). The hypothesis has been
advanced (Welsh & Gordon, 1946; Gordon & Welsh, 1948) that certain
neurotoxins, including DDT, act by delaying the restoration of calcium
ions to a surface complex, following breaking of the chelate linkage
of calcium ions to surface polar groups by an initial exciting
impulse. This action of the neurotoxin is conceived as depending
largely on its physical rather than on its chemical properties. The
hypothesis is helpful in explaining the fact that a wide variety of
chemically unrelated compounds produce repetitive responses in
excitable tissue and also the fact that many compounds that show a
high toxicity for arthropods and mammals are fat-soluble and
relatively inert chemically. It has been pointed out that this
hypothesis postulates a very localized action of calcium at the nerve-
cell membrane; the hypothesis is not inconsistent with the finding
that the blood calcium of poisoned animals may be unchanged or even
increased.
Having observed the effect of DDT on the metabolism of glucose and
glycogen, Lauger et al. (1945a, b) investigated the use of glucose as
an antidote. All of 10 dogs given 2000 mg of DDT per kilogram body
weight orally in the form of an oil solution died within 8-24 h. Five
of 10 dogs treated with one or more, 20 ml doses of 20% glucose
survived the same dosage of DDT. The glucose was given intravenously
in most instances.
Koster (1947) found that glucose given before or after an LD33
dosage reduced convulsions and mortality and, when given before the
poison, reduced tremors, prostration, and dyspnoea in cats. Glucose,
unlike gluconic acid and its sodium and calcium salts, was ineffective
against an LD95 dosage except to increase the time of survival.
Insulin, given intra-muscularly 16-25 min before DDT, increased the
survival time and the severity of poisoning but did not affect
mortality or convulsions. When given 53-130 min before DDT, insulin
reduced convulsions in animals which died but increased convulsions,
tremors, and other disorders in the survivors.
The failure of amino and sulfhydryl compounds to influence the
action of DDT was noted by Von Oettingen & Sharpless, 1946). Likewise,
the addition of 0.2% choline chloride to the diet of rats receiving
repeated doses of DDT had no effect on the accumulation of lipids in
their liver (Sarett & Jandorf, 1947).
In summary, it would appear that sedatives, ionic calcium, and
glucose or other ready sources of energy are useful in treating
poisoning by DDT. Dogs and cats can be treated somewhat more
successfully than rats, perhaps because the metabolism of larger
animals is slower.
Although respiration may be temporarily restored in animals
poisoned by DDT, studies have not been made to determine whether
respiratory arrest in this condition is truly reversible as it is in
poisoning by certain organic phosphorus insecticides.
7.1.3 Renal system
No dysfunction of the renal system attributable to DDT has been
found even in animals receiving dosages sufficient to cause
dysfunction of the nervous system or striking morphological changes of
the liver. It is true that mild to moderate morphological changes have
been reported in the kidneys of animals that have received massive
single doses or repeated doses; for example, fatty degeneration,
necrosis, and calcification (Lillie et al., 1947; Stohlman & Lillie,
1948) or slight brown pigmentation of the convoluted tubular
epithelium (Fitzhugh & Nelson, 1947). However, it sometimes has
happened that a complete absence of change in the kidney has been
reported in connexion with other studies carried out in the same
laboratories (Lillie & Smith, 1944; Nelson et al., 1944).
7.1.4 Gastrointestinal tract, liver, and enzymes
Large doses of DDT produce vomiting in species that can vomit.
Only doses that produce rather severe poisoning lead to anorexia.
7.1.4.1 Liver
Large doses of DDT cause focal necrosis of liver cells in several
species (Lillie & Smith, 1944; Nelson et al., 1944; Cameron & Burgess,
1945; Lillie et al., 1947; Deichmann et al., 1950; Ortega et al.,
1956). At least some investigators (Cameron & Burgess, 1945) have
considered that the liver lesions produced by large dosages are
sufficient to account for death. All have agreed that necrotic lesions
occur only in connexion with potentially fatal dosages.
A very different kind of liver change is produced in some rodents
but not in other animals by small or moderate dosages of DDT. The
biochemical aspects of these changes are discussed in section 7.1.4.2,
while the morphological aspects are discussed in section 7.1.9.
7.1.4.2 Microsomal enzymes of the liver
All enzymes can be inhibited in vitro and many of them can be
inhibited in vivo. The toxic action of a number of compounds is
clearly the result of their inhibition of one or more enzymes. So far,
only a few enzymes or enzyme systems are known to be induced by
chemicals; the outstanding example is the induction of microsomal,
mixed-function enzymes of the liver and some other organs that are
produced in greater quantity in response to certain hormones and other
normal constituents of the body (Conney, 1967), some foods
(Wattenberg, 1971), or a wide range of drugs or other foreign
chemicals. Such induction requires intact cells and does not occur
when the inducer is brought in contact with the purified enzymes in
vitro. Microsomal enzymes were first recognized and studied in the
liver, but they are now known to exist, generally in lower
concentrations, in other tissues.
Microsomal enzymes are known to be associated with oxidation ( N-,
O-, and S-dealkylation, deamination, epoxidation, disulfuration,
hydroxylation) of both rings and side chains, oxidation of both
nitrogen and sulfur, reduction of nitro groups and of azo compounds,
hydrolysis, and conjugation. Most of the changes produced by
microsomal enzymes render oil-soluble compounds more water-soluble
and, therefore, more easily excreted. Mainly for this reason, most
biotransformations promoted by microsomal enzymes are true
detoxications. However, some of the reactions promoted by this system
of enzymes render specific compounds more toxic.
In the rat, DDT has been shown to promote the biotransformation of
hexobarbital (Hart & Fouts, 1963), phenazone (Hart & Fouts, 1963;
Kinoshita et al., 1966), p-nitrobenzoic acid (Hart & Fouts, 1963),
aniline (Hart & Fouts, 1963), dieldrin (Street, 1964; Street et al.,
1966; Street & Chadwick, 1967; Pearl & Kupfer, 1971), o-ethyl o-4-
nitrophenyl phenylphosphonothioate (EPN) (Kinoshita et al., 1966),
p-nitroanisole (Kinoshita et al., 1966; Vainio, 1974), methyprylon
(Datta & Nelson, 1968), meprobamate (Datta & Nelson, 1968),
chlordizepoxide (Datta & Nelson, 1968), aldrin (Gillett, 1968),
lindane (Chadwick et al., 1971b), phenylbutazone (Welch & Harrison,
1966), pentobarbital (Fredricks et al., 1974), 3,4-benzpyrene (Vainio,
1974), and p-nitrophenol. The biotransformation of p-nitrophenol
involves conjugation by the microsomal enzyme uridinediphospho-
glucuronyltransferase, and its demonstration requires activation of
the microsomes, for example by trypsin (Vainio, 1974, 1975; Rantanen et
al., 1975).
In the mouse, DDT has been shown to promote the biotransformation
of pentobarbital (Gabliks & Maltby-Askari, 1970).
In the guineapig, DDT promoted the metabolism of dieldrin
(Wagstaff & Street, 1970).
DDT in the squirrel monkey promoted the metabolism of EPN and
p-nitroanisole; the first required a DDT dosage of 5.0 (mg/kg)/day, but
the latter required only 0.5 (mg/kg)/day (Cranmer et al., 1972). DDT
did not promote the metabolism of 14C-DDT in squirrel monkeys
(Chadwick et al., 1971a) but did promote the metabolism of
phenylbutazone in the dog (Welch & Harrison, 1966) and the metabolism
of estradiol in the pigeon (Peakall, 1970).
In the chicken, DDT failed to affect N-demethylase or the
concentration of cytochrome P-450, and reduced aniline hydroxylase
activity. The influences of dosage, duration of dosing species, and
reproductive state on microsomal enzymes in birds are poorly
understood (Sell et al., 1971).
o,p'-TDE has been shown to promote the metabolism of
pentobarbital in the mouse (Gabliks & Maltby-Askari, 1970),
phenobarbital in the rat (Straw et al., 1965), and cortisol in the
guineapig (Kupfer et al., 1964).
The metabolism of DDT is promoted by DDT itself in the hamster but
not in the mouse (Gingell & Wallcave, 1974).
In rats, the metabolism of DDT is promoted by phenytoin (Cranmer,
1970). The metabolism of DDT and DDE in cows is promoted by
phenobarbital (Alary et al., 1971; Fries et al., 1971).
DDE, whether fed directly or produced metabolically from DDT,
appears to be more important than DDT in inducing microsomal enzymes.
The tissue level of DDE necessary for enzyme induction is lower in the
rat than in the quail (and presumably other birds). Thus Bunyan et al.
(1972), using residues in the heart as an index, found a maximum
increase in cyto-chrome P-450 per gram of liver and a maximum activity
of aniline hydroxylase levels at DDE levels of approximately 3 mg/kg
in rats and 40 mg/kg in quail. However, at any given dietary level,
higher tissue levels were reached by quail than by rats so that the
dosage responses of the two were similar.
Different inducers may activate different enzymes and, therefore,
different metabolic pathways. Pretreatment of rats with lindane caused
them to metabolize a single dose of radioactive lindane 2.5 times more
efficiently than controls, whereas pretreatment with DDT caused a
3.5-fold increase in the metabolism of lindane. Furthermore, the DDT
pretreatment was followed by a different proportion of radioactive
metabolites with a predominance of tetrachlorophenols, especially
2,3,4,5-tetrachlorophenol (Chadwick et al., 1971b).
The enzymes of weanling rats are more subject to induction than
those of adult rats, but there is no evidence of a lag-period in
induction in adults (Chadwick et al., 1975).
7.1.4.3 Enzymes of intermediary metabolism
As discussed in section 7.1.2, there is some reason to think that
DDT acts by influencing an enzyme critical to the function of
neurones. It is certainly clear that many of the side-effects of DDT
are the result of its induction of microsomal enzymes (see sections
7.1.4.2 and 7.1.9). In addition, DDT has been shown in vitro and
sometimes in vivo to influence some enzymes of intermediary
metabolism and other miscellaneous enzymes. So far, evidence is
lacking that the degree of this inhibition in the intact organism is
sufficient to have any influence on function.
The hyperglycaemia observed during much of the early part of acute
poisoning may be associated with an increase in 4 gluconeogenic
enzymes (pyruvate carboxylase (EC 6.4.1.1), phosphoenolpyruvate
carboxykinase (EC 4.1.1.32), fructose 1,6-diphosphatase, and glucose
6-phosphatase (EC 3.1.3.9)). Increases in these enzymes in the renal
cortex of rats, observed after a single dose at a rate as low as
100 mg/kg, were greater at a dose of 600 mg/kg. The reaction occurred
in both adrenalectomized and normal rats (Kacew & Singhal, 1972).
Similar responses were observed in the same enzymes in the liver
following a single dose at the rate of 100 mg/kg or more or following
45 daily doses at rates of 5 or 25 (mg/kg)/day. The changes are not
mediated through a release of corticosteroids from the adrenal glands
(Kacew & Singhal, 1973). The same authors (Hrdina et al., 1975;
Singhal & Kacew, 1976) have reviewed the extensive evidence
contributed mainly by themselves indicating that the changes in
glucose homeostasis are mediated by stimulation of the cyclic
adenosine monophosphate (AMP)-adenylate cyclase system in the liver
and in the kidney cortex by p,p'-DDT and a number of other organic
chlorine pesticides. However, the smallest single dosage of p,p'-DDT
that produced a statistically significant change in the enzymes or in
the production of cyclic AMP was 180 mg/kg and o,p'-DDT was as
effective as the p,p'-isomer; these findings indicate that the
carbohydrate changes are results not causes of poisoning.
DDT and some other compounds induce increased activity of
D-glucuronolacetone dehydrogenase (EC 1.1.1.70) in the supernatant
fraction of rat liver, and this is consistent with evidence of
increased D-glucaric acid in urine after dog treatment (Marselos &
Hanninen, 1974). Urinary excretion of L-ascorbic acid is also
increased by DDT, but apparently this is not caused by an increase in
an enzyme producing this acid but rather by an increase in several
microsomal and cytosol enzymes that contribute to an increase in free
glucuronic acid from which L-ascorbic acid is formed (Rantanen et al.,
1975).
A review (Hayes, 1959) of early literature indicates that high
concentrations of DDT inhibit phosphatidase (EC 3.1.1.4), muscle
phosphatases, carbonic anhydrase (EC 4.2.1.1), oxalacetic carboxylase
(EC 4.1.1.3), and increase the activity of cytochrome oxidase
(EC 1.9.3.1) and succinc dehydrogenase (EC 1.3.99.1). However, none of
these changes with the possible exception of inhibition of carbonic
anhydrase could be shown to have any connexion with the toxic action
of DDT or even with its side-effects. Neal et al. (1944) reported a
small but consistent increase in the volume of urine excreted in 24 h
when dogs were dosed orally or by insufflation at the rate of 100
(mg/kg)/day. No other change in the urine and no change in kidney
function was demonstrated. The possibility that increased urinary
output is related to the inhibition of carbonic anhydrase (Torda &
Wolff, 1949) may deserve attention. However, re-examination of data
from volunteers receiving 3.5 or 35 mg/man per day did not indicate
any increase in urinary volume compared with controls (Hayes et al.,
1971).
It has been claimed (Keller, 1952) that DDT inhibits carbonic
anhydrase in bovine erthrocytes. However, the method was criticized by
Dvorchik et al. (1971), who found in vitro inhibition only by
concentrations of DDT, unlikely to be survived by living animals. Far
more attention has been given to inhibition of carbonic anhydrase in
the shell gland of birds than in erythrocytes, and it has been
suggested (Bitman et al., 1970; Peakall, 1970) that inhibition of the
shell gland enzyme is an explanation for eggshell thinning in certain
birds. However, the same criticism holds for the shell gland; there is
no evidence that the degree of inhibition reported interferes with
function.
On the other hand, many enzymes including plasma amylase, aldolase
(EC 4.1.2.13), glutamic-pyruvic transaminase (EC 2.6.1.2), and
isocitric dehydrogenase (EC 1.1.1.41) were not changed in squirrel
monkeys given dosages ranging from 0.05 to 50 (mg/kg)/day; the highest
dosage proved fatal within 14 weeks (Cranmer et al., 1972).
7.1.5 Cardiovascular system
Most dogs, killed by a single dose of DDT, die of ventricular
fibrillation, and the same is true of some cats, monkeys, and rabbits
(Philips & Gilman, 1946). At any given dosage of DDT, ventricular
fibrillation is more likely to occur if the animal receives exogenous
epinephrine or is stimulated in such a way that the compound is
released by the adrenals. Besides fibrillation, dogs may exhibit
extrasystoles and changes in the T-wave (Philips et al., 1946).
Monkeys differ from dogs in that the DDT-sensitized heart is able to
recover from fibrillation and resume a normal rhythm (Philips et al.,
1946).
Thus, DDT not only sensitizes the myocardium in a way similar to
that of halogenated hydrocarbon solvents, but, through its action on
the central nervous system, produces the stimulus that increases the
likelihood of fibrillation.
There is no evidence that repeated, tolerated doses of DDT
sensitize the heart. Rats were fed DDT at a dietary level of about
10 (mg/kg)/day for 8 months, during which time they received weekly,
intraperitoneal doses of vasopressin, a compound that causes a
temporary myocardial ischaemia. Electrocardiograms did not show any
significant increase in cardiac arrhythmias in the DDT-fed rats
compared with controls. Intravenous noradrenalin given at the end of
the 8-month period did not produce a greater incidence of arrhythmias
in the DDT-fed rats. The same results were obtained in rabbits treated
in essentially the same way (Jeyaratnam & Forshaw, 1974).
TDE. The main action of TDE, and especially of o,p'-TDE, is on
the liver with secondary effects on the adrenals in dogs and perhaps
in other species. However, Cueto (1970) showed that at a dosage of
50 (mg/kg)/day for 14 days, o,p'-TDE caused a gradually progressive
hypotensive failure in dogs injected with epinephrine or
norepinephrine, while leaving the cardioaccelerator and immediate
pressor response to these drugs unchanged. The hypotensive failure was
associated with weakening of the contractile force of the heart and
with a reduction of plasma volume. The latter may have been caused by
a loss of fluid from the intravascular compartment and was not caused
by a release of histamine. The hypotensive state could be prevented to
a significant degree by pretreatment with prednisolone.
7.1.6 Respiratory system
The effects of DDT on the respiratory system are secondary to
effects on the nervous system and are discussed in section 7.1.2.1.
7.1.7 Reproductive system
It was shown very early (Burlington & Linderman, 1950) that DDT
produces a striking inhibition of testicular growth and secondary
sexual characteristics of cockerels, when injected subcutaneously in
dosages as high as 300 (mg/kg)/day. Changes in the testis involve the
tubules, and not the interstitial tissues, and they have been
attributed to an estrogen-like action of DDT.
It must be noted that the action of DDT on the testis of the
chicken is dosage-related. Before the problem of residues became
evident, DDT was used extensively for control of lice and common mites
on chickens without any adverse effects on egg production or other
aspects of reproduction. Many rats would be killed the first day if
they were given the dosage of DDT that has been shown to affect the
testis in cockerels. The report that, under special conditions, DDT
has it gonadotoxic effect (Rybakova, 1968) is of questionable
significance in view of the results of multigeneration tests in rats,
mice, and dogs.
Ottoboni (1969) found that female rats reproduced normally when
fed DDT for two generations at dietary levels as high as 200 mg/kg
(about 10 (mg/kg)/day, except during lactation when intake was
increased about 3-fold). In fact, at a dietary level of 20 mg/kg, the
dams had a significantly longer reproductive life span (14.55 months)
than did their littermate controls (8.91 months); the number of
females becoming pregnant after the age of 17 months and the number of
successful pregnancies after that age differed significantly between
the two groups (Ottoboni, 1972).
In a study focused mainly on DDT in milk, the ability of rats to
reproduce at a dietary level of 200 mg/kg was confirmed, and the
ability of dams, injected intraperitoneally at levels as high as
100 (mg/kg)/day, to rear their young was demonstrated (Hayes, 1976b).
A six-generation test of reproduction in mice did not show any
effect of DDT at a dietary level of 25 mg/kg on fertility, gestation,
viability, lactation, and survival. A dietary level of 100 mg/kg
produced a slight reduction in lactation and survival in some
generations but not all, and the effect was not progressive. A level
of 250 mg/kg was distinctly injurious to reproduction (Keplinger et
al., 1970). The dietary concentrations used equalled dosages of 3.33,
13.3 and 33.2 (mg/kg)/day in nonpregnant, non-lactating, adult female
mice. The intake is much higher in both young and lactating mice. The
authors concluded that their study provided no obvious reason for
continuing reproduction tests for more than three generations.
Four female dogs of unstated age that had previously received DDT
at the rate of 12 (mg/kg)/day, 5 days a week, for 14 months were mated
when they went into heat. The males involved had been fed aldrin
(0.15 (mg/kg)/day) plus DDT (60(mg/kg)/day) for 14 months prior to
breeding but not during breeding. Two of the females went into heat
but failed to become pregnant, and one failed to come into heat during
12 months after feeding stopped. Four of 6 pups born to the fourth
female died within one week of birth; the other 2 were weaned
successfully even though only 2 posterior mammae of the mother were
functional (Deichmann et al., 1971b). A 3-generation study failed to
confirm any of the injuries suggested by the study of 4 dogs. In the
3-generation study, male and female dogs were fed technical DDT from
weaning at rates of 0, 1, 5, and 10 (mg/kg)/day. Observations were
made on 135 adult females, 63 adult males and 650 pups. There were no
statistically significant differences between controls and DDT-treated
dogs in length of gestation, fertility, success of pregnancy, litter
size, lactation ability of the dams, viability at birth, survival to
weaning, sex distribution, growth of pups, morbidity, mortality,
organ/body weight ratios, or gross histological abnormalities in all
the animals studied. The only clear difference was that DDT-treated
females had their first estrous 2 or 3 months earlier than the control
animals. There was a slight increase in liver/body weight ratio in
some DDT-treated animals but the difference was not statistically
significant, dosage related, or associated with any histological
change (Ottoboni et al., 1977).
o,p'-DDT. Intraperitoneal injection of technical DDT at a dosage
as low as 5 mg/kg or of o,p'-DDT at 1 mg/kg caused a significant
increase in the weight of the uterus of normal, immature female rats
or of ovariectomized adult females. Stimulation caused by p,p'-DDT
was much less. Treatment of rats with DDT, especially o,p'-DDT, 2 h
before injection of estradiol-17-6,7-3H inhibited uptake of the
hormone by the uterus in vivo, possibly by competition for binding
sites. Isomers of TDE and DDE do not influence uterine weight or the
binding of estradiol (Welch et al., 1969). It seems unlikely that
metabolic activation of o,p'-DDT is necessary, as is true of o,p'-
methoxychlor. The binding and estrogenic activity of DDT analogues in
rats is only about 1/10 000 as great as that of diethylstilbesterol
(Nelson, 1973).
A considerably smaller dosage of o,p'-DDT resulting from a
dietary level of 10 mg/kg for 2-9 months did not have any effect on
reproduction in ewes (Wrenn et al., 197 lb). In a similar way, dietary
levels of o,p'-DDT as high as 40 mg/kg, giving a dosage level of
about 2.1 (mg/kg)/day in rats, failed to interfere with reproduction
and lactation in these animals although dosage was continued
throughout 2 pregnancies (Wrenn et al., 1971a).
The report (Heinrichs et al., 1971) that o,p'-DDT significantly
advances puberty, induces persistent vaginal estrus after a period of
normal estrus cycles, and causes other reproductive abnormalities in
female rats would appear at first to be inconsistent with the lack of
effect of technical DDT or of o,p'-DDT on reproduction cited above.
The same is true of other effects of o,p'-DDT demonstrated by the
same investigators (Gellert et al., 1972). The abnormal effects were
obtained initially by injecting 1 mg of the o,p'-DDT subcutaneously
on the second, third, and fourth days of life (counting the day of
birth as zero). Because rat pups on the third day weigh about 12 g or
less each, it follows that the subcutaneous dosage was about
83.3 (mg/kg)/day or more, that is about 40 times greater than the
highest oral dosage of o,p'-isomer fed to breeding rats and about
105 times greater than the levels ingested by the general population
with their food.
Because of its estrogenic properties, DDT was considered as a
possible cause of abortion in dairy cattle, but no evidence of a
relationship was found (Macklin & Bibelin, 1971).
7.1.8 Endocrine organs
Except for the weak estrogenic properties of o,p'-DDT, the
endocrine-related effects of DDT and its analogues are confined to the
adrenals, and even these effects of 2 isomers of TDE are now
considered to be mainly secondary to the induction of microsomal
enzymes of the liver in most species.
TDE. TDE (DDD) is an insecticide in its own right as well as a
metabolite of DDT. The compound is used as a drug to control different
forms of adrenal overproduction of corticoids in man (see section
8.2.8). This therapy was originally based on the demonstration that
DDD (Nelson & Woodard, 1948, 1949) and especially o,p'-TDE (Cueto &
Brown, 1958) caused gross atrophy of the adrenals and degeneration of
the cells of its inner cortex in dogs. This is true even though it was
originally reported (Nelson & Woodard, 1948, 1949) that TDE produced
almost no detectable damage to the adrenals of rats, mice, rabbits,
and monkeys, and the finding was confirmed and extended by other
investigators to other species, including man (Zimmerman et al.,
1956). In the dog, o,p'-TDE produced gross atrophy of the adrenals,
when administered at a dosage of only 4 (mg/kg)/day. The dosage of
technical grade TDE required to produce the same effect was
50-200 (mg/kg)/day (Cueto & Brown, 1958). However, in spite of its
exceptional susceptibility, there is a definite threshold below which
the dog does not respond. About 15% of technical DDT is o,p'-isomer,
much of which is gradually metabolized to o,p'-TDE ( o,p'-DDD). Yet
dogs remained healthy and reproduced normally in a 3-generation study
involving dosages of technical DDT as high as 10 (mg/kg)/day (see
section 7.1.7).
It has recently been shown that, following massive dosage
(60 mg/kg, administered intravenously), all of the isomers of TDE
inhibited ACTH-induced steroid production in the dog, but the
inhibition reached 50% of the control only 27 min after dosing with
the m,p'-isomer compared with 87 min with the o,p'-isomer and
4-18 h with the p,p'-isomer. There was marked temporal correlation
between the percentage inhibition of adenocorticotropic hormone
(ACTH)-induced steroid production, the disruption of normal cellular
structure of the fascicular and reticular zones of the adrenal cortex,
and the severity of the damage to mitochondria in these zones caused
by the 3 isomers (Hart et al., 1973). The effectiveness of m,p'-TDE
for treating metastatic adrenocortical carcinoma had already been
demonstrated (Nichols et al., 1961), but it cannot be said that its
value for this purpose has been compared adequately with that of
o,p'-TDE. Furthermore, no effort has apparently been made to compare
the effect of small daily doses of the 2 isomers in dogs.
Like other organochlorine insecticides, o,p'-TDE stimulates
hepatic microsomal oxygenation of both drugs and steroids and,
according to a very thorough review by Kupfer (1967), this may explain
much of its action on corticoid metabolism in a wide range of species.
Increased breakdown is indicated by increased excretion of polar
metabolites, while nonpolar metabolites remain stable or even decrease
-- a finding recently encountered in human patients (Hellman et al.,
1973). However, the demonstrated effect on corticoid metabolism fails
to explain why o,p'- and m,p'-TDE are unique in their overall
effects on the adrenals, including their ability to produce
adrenocortical atrophy in the dog. Other powerful inducers of
microsomal enzymes lack these effects. It is clear that a reduction of
steroid production accompanies atrophy of the adrenals of the dog. The
review already cited (Kupfer, 1967) considers: (a) reduced steroid
production in species other than the dog, including the possibility
that such reduction is secondary to inhibition of glucose-6-phosphate-
dehydrogenase (EC 1.1.1.49) activity in the adrenals and (b) blockage
of steroid action by asteroid metabolite formed under the influence of
DDD. However, the existence of these effects, much less their
importance, remains obscure.
o,p'-DDT. Oral administration of o,p'-DDT to dogs at a rate of
50 (mg/kg)/day stimulated the microsomal enzymes of the liver as
indicated by increase in liver size, total protein, microsomal
protein, and cytochrome P-450 concentration and by direct measurements
of enzyme activity. These changes in the liver were accompanied,
initially, by an increase in the size of the adrenals and of the cells
of the zona fasciculata; these cells became vacuolated and devoid of
acidophilic cytoplasm, and their nuclei became hyperchromatic and
often peripheral in position. Synthesis of corticosteroids by the
adrenal was not blocked (Copeland & Cranmer, 1974). Thus the effect of
a substantial dosage of o,p'-DDT was quite different from that of
o,p'-TDE (DDE), although part of the metabolism of o,p'-DDT must
be by the same route.
7.1.9 Carcinogenicity
There is no doubt that DDT and a number of other organochlorine
insecticides cause marked changes in the liver in various rodents and
that these changes progress to tumour formation in some species,
notably the mouse. There is serious disagreement as to whether the
mouse tumours are malignant. Regardless of their nature, there is
virtual certainty that they are peculiar to rodents and, therefore,
interpretation of their significance for man or useful animals is
difficult.
Evidence for the carcinogenicity of DDT and its metabolites has
been reviewed by the International Agency for Research on Cancer
(IARC, 1974). Most of the experimental results are summarized in Table
17. The conclusions of the IARC were that: (a) the hepatocarcino-
genicity of DDT administered by the oral route has been demonstrated in
several strains of mice, and shows a dosage-response relationship; (b)
a dietary level of 2 mg/kg (above 0.3 (mg/kg)/day) produces a
significant increase in hepatomas in male but not in female CFl mice
and not in either sex of BALB/c mice; (c) increased incidence of
tumours has been reported in some other organs of mice but not
confirmed in multigeneration studies using a wide range of dosages;
(d) evidence for the carcinogenicity of DDT in rats is not convincing
and is negative in hamsters even at the higher dietary levels that
they tolerate in comparison with rats and mice; (e) negative results
in dogs and monkeys are inconclusive because of the small groups
studied and the short duration of treatment; (f) liver cell tumour
induction in trout is inconclusive because of a lack in control of the
diet; and (g) the carcinogenicity of p,p'-DDE is similar to that of
DDT, but TDE produces a significant incidence of lung tumours.
Actually, the number of dogs and monkeys was not small compared
with similar studies on other chemicals. In an investigation on
monkeys, dosing at lower levels was continued for 7.5 years. In both
dogs and monkeys, dosages sufficient to cause liver damage, death, and
neurological indications of DDT poisoning were included in the
protocols (see Table 17). Apparently no new studies on dogs and
monkeys have been reported. On the other hand, a new study on rats
(Rossi et al., 1977) has given definite evidence of the tumorigenicity
of DDT and phenobarbital, confirming the conclusion of Fitzhugh &
Nelson (1947) which was based on less extensive data.
In mice, liver tumours similar to those caused by DDT (see
Table 17) have been reported in connexion with DDE and DDD (Tomatis &
Turusov, 1975), chlorobenzilate, HCH, aldrin, dieldrin, mirex, and
terpene polychlorinates (Tomatis et al., 1973). Similar tumours have
been caused by the important drug, phenobarbital (Wright et al., 1972;
Peraino et al., 1973; Thorpe & Walker, 1973; Ponomarkov et al., 1976).
TDE also caused lung tumours (Tomatis et al., 1974a).
The IARC review did not discuss the controversy over the nature of
the tumours induced by DDT in the livers of some rodents, and it did
not consider the relationship of these tumours to the induction of
microsomal enzymes. The following paragraphs are concerned with these
matters and specifically with those inducers that behave like DDT. The
biochemical pattern of induction of mixed function oxidase enzymes is
similar for DDT and phenobarbital but distinctly different for
3-methylcholanthrene (Vainio, 1975). Thus 3-methylcholanthrene and
compounds like it must be excluded from the discussion. Similarly, the
high degree of correlation between the ability of compounds to induce
parenchymal liver tumours in mice and their ability to induce tumours
in the liver and other organs of rats and hamsters cannot be accepted
uncritically. As demonstrated in a study by Tomatis et al. (1973),
this correlation is extremely good for compounds that are, or are
suspected of being, carcinogens in man. However, the correlation is
poor for organochlorine insecticides. In fact, the only one of these
compounds that has increased the incidence of a tumour in another
species is DDT, which induced liver tumours in rats in one experiment.
The early morphological response of the rodent liver to DDT is
similar to its response to moderate dosages of HCH, chlordane,
dieldrin, camphechlor (Toxaphene) (Lehman, 1952; Ortega et al., 1956),
and the important drug, phenobarbital (Wright et al., 1972; Thorpe &
Walker, 1973). The earliest change involves so much increase in the
smooth endoplasmic reticulum of individual liver cells that they
enlarge, and the large granules that are ordinarily scattered
throughout the cytoplasm are displaced to the periphery of the
affected cell. Quite early, some of the endoplasmic reticulum forms
whorls that may have fat droplets as their centres -- thus justifying
the term "lipospheres", applied to them by Ortega et al. (1956).
Others have referred to these inclusions as "hyaline oxyphil masses"
(Lillie & Smith, 1944) or "lamellar bodies" (Ito et al., 1973). These
changes are accompanied by some increase in fat droplets, not all of
which become surrounded by endoplasmic reticulum. This combination of
changes (hypertrophy, margination, and lipospheres) is characteristic
of rodents and of compounds that induce microsomal enzymes. Certain
other changes have been reported but not confirmed. These include
enlargement and morphological changes of the mitochondria (Obuchowska
& Pawlowska-Tochman, 1973; Watari, 1973), increased numbers of primary
lysosomes, and atrophy of the Golgi body (Watari, 1973), none of which
were found by Ortega (1966).
Table 17. Effect on various animals of repeated oral doses of DDT
Dosage Species Duration Results Reference
Range Method and Animals Mortality Other
(mg/kg)/day concentration per test (%)
(No.)
41-80 800 mg/kg in diet rata 2 years 36 males, 24 females Increased mortality, Fitzhugh & Nelson, 1947
typical liver changes,
liver tumours
46 mg/kg then mouseb 18 months 36 males, 36 females Hepatomas in 51 and Innes et al., 1969
140 mg/kg in diet 21% of males and females
respectively compared with
18 and 0.6% of controls
3200 mg/kg in diet dog 39-49 10 100 Liver damage, no Lehman, 1965
months tumours
5000 mg/kg in diet monkey 70 days 1 male 100 Fatal poisoning Durham et al., 1963
21-40 400 mg/kg in diet rata 2 years 24 males, 12 females Increased mortality, Fitzhugh & Nelson, 1947
typical liver changes
500 mg/kg in diet rat 2.9 years 37 males, 35 females Liver tumours in 45% Rossi et al., 1977
250 mg/kg in diet mousec 2 103 males, 90 females Risk of liver tumour Tomatis et al., 1972
generations increased 3.7 and 18.5
times in males and females,
respectively
250 mg/kg in diet mousec 2 31 males, 121 females Liver tumours in 48 Terracini et al., 1973
generations and 59% of males and
females, respectively
2000 mg/kg in diet dog 39-49 4 25 Minor liver damage Lehman, 1965
months but no tumours
Table 17 (Cont'd)
Dosage Species Duration Results Reference
Range Method and Animals Mortality Other
(mg/kg)/day concentration per test (%)
(No.)
11-20 100 mg/kg in diet moused 2 years 100 males, 100 females Hepatomas increased Fitzhugh, 1970
in females of one strain
but no increase in
hepatocarcinomas
100 mg/kg in diet mousec 2 years 30 males, 30 females Risk of liver tumours Walker et al., 1973
increased 4.4 times
100 mg/kg in diet mousec 2 years 30 males, 3 females Risk of liver tumours Thorpe & Walker, 1973
increased 3.3 and 4.2 times
in males and females
respectively
5-10 50 mg/kg in diet mousec 2 127 males, 104 females Risk of liver tumours Tomatis et al., 1972
generations increased 2.45 and 3.46
times in males and females,
respectively
50 mg/kg in diet mousec 2 years 30 males, 30 females Risk of liver tumours Walker et al., 1973
increased 2.9 times
400 mg/kg in diet dog 39-49 2 0 No effect Lehman, 1965
months
Table 17 (Cont'd)
Dosage Species Duration Results Reference
Range Method and Animals Mortality Other
(mg/kg)/day concentration per test (%)
(No.)
2.6-5 20 mg/kg in diet mousee 2 48 males, 128 females No increase in tumours Terracini et al., 1973
generations
200 mg/kg in diet monkey 3-7.5 5 males, 5 females No toxic effect Durham et al., 1963
years
1.26-2.5 10 mg/kg in diet mousec 2 104 males, 124 females Risk of liver tumour Tomatis et al., 1972
generations increased 2.26 and 2.46
times in males and females,
respectively
0.626-1.25 25 mg/kg in diet rat 2 years No clinical effect; Treon & Cleveland, 1955
males survived
longer than controls
50 mg/kg in diet monkey 1.6 years 4 males, 1 female No toxic effect Durham et al., 1963
0.3126- 10 mg/kg in diet rat 2 years Typical liver changes; Fitzhugh, 1948
0.625 no effect on
reproduction
12.5 mg/kg in diet rat 2 years No effect Treon & Cleveland, 1955
2.8-3.0 mg/kg in mousee 5 683 Tumours in 28.7% in Tarjan & Kemeny, 1969
diet generations controlsf
0.15626- 2 mg/kg in diet mousee 2 124 males, 111 females Risk of liver tumour Tomatis et al., 1972
0.3125 generations doubled in males,
unchanged in females
2 mg/kg in diet mousee 2 58 males, 135 females No increase in tumours Terracini et al., 1973
generations
Table 17 (Cont'd)
Dosage Species Duration Results Reference
Range Method and Animals Mortality Other
(mg/kg)/day concentration per test (%)
(No.)
0.078126- 2.5 mg/kg in diet rat 2 years No effect Treon & Cleveland, 1955
0.15625 5 mg/kg in diet monkey 1.4-7.5 5 males No toxic effect Durham et al., 1963
years
a Osborne-Mendel. e BALB/c mice.
b Both (C57BL/6 × C3H/Anf) FI and (C57BL/6 × AKR) FI mice. f Lung carcinoma in 16.9% to 1.2% in controls; lymphomas 4.8% compared to
c CFI mice. 1.0% in controls; leukaemias 12.4% and 2.6%; other tumours 5.8% and 1.0%,
d BALB/cJ and C3HeB/Fe J. respectively.
The characteristic changes develop promptly. An increase in smooth
endoplasmic reticulum and the appearance of lamellar structures have
been seen as early as 4 and 7 days, respectively, after the beginning
of dosing (Wright et al., 1972).
Although microsomal enzymes may be induced in other species,
morphological changes in the liver, as viewed by the light microscope
are not the same (Laug et al., 1950; Lehman, 1952; Ortega et al.,
1956), or occur to lesser degree as viewed by the electron microscope
(Wright et al., 1972).
The changes in liver cells that characterize the induction of
microsomal enzymes in rodents are distinct from the focal necrosis
that may be produced with about the same ease in the livers of rodents
or other species by fatal or near fatal dosages of organochlorine
insecticides. The necrotic lesions do not progress because, if such
high dosages are continued the animal dies, and, if dosing is stopped
and the animal survives, the necrotic cells are removed by autolysis
and phagocytic action and the lesions usually heal without scarring
(Cameron & Burgess, 1945). Nevertheless, some scarring was found by
Lillie & Smith (1944) and Lillie et al. (1947).
At least in the early stages, the changes in liver cells that
characterize induction of microsomal enzymes in rodents are reversible
(Fitzhugh & Nelson, 1947; Ortega et al., 1956; Wright et al., 1972).
The reversibility does not depend on cell removal but simply on
reversion of the physiological and morphological condition of the
cells to their original condition.
Of course, reversibility is incompatible with progression, but
whether observed irreversibility will be associated with progression
must be determined directly in each instance. In the following
paragraphs, the question of progression is discussed only after
consideration of the problem of irreversibility in general.
If dosing with organochlorine insecticides or other inducers is
continued long enough and at a sufficiently high level, the liver
changes become irreversible, if for no other reason than that the
remaining life span of the animals is too short to permit excretion of
the inducing chemical or complete reversion of the liver cells to
their original state. The stage at which this shift to irreversibility
occurs remains unknown, but it seems very likely that dosages
sufficient to produce irreversible morphological change also exceed
the physiological adaptability of the liver. The important distinction
between adaptation and injury in relation to enzyme induction has been
studied using dieldrin.
Although some persons have tended to view even moderate
enlargement of the liver or of individual liver cells as an injury,
the evidence is strong that these changes are usually adaptive and
beneficial to the organism, when they are the result of an increase of
smooth endoplasmic reticulum and an associated increase in the
activity of liver microsomal enzymes (Barka & Popper, 1967). However,
it is obvious that any stimulus or effect may be harmful, if
excessive. Hutterer et al. (1968) demonstrated that a distinction may
be drawn between adaptation to dieldrin and decompensation resulting
from excessive doses of it. Some of these authors, including Popper,
showed that the same distinction could be drawn in connexion with
other sources of potential liver injury.
It was found that daily intraperitoneal administration of dieldrin
to rats at the rate of 2 mg/kg produced enlargement of the liver,
hypertrophy of the smooth endoplasmic reticulum, increases in
microsomal protein and P-450 haemoprotein, and associated increases in
the activity of microsomal enzymes; however, normal activity of other
enzymes not derived from the microsomes was maintained. The activity
of microsomal enzymes per mole of available P-450 haemoprotein
remained unchanged. The highest level of activity of the processing
enzymes was reached after 14 days, after which the new steady state
was maintained. Rats that had received dieldrin at a rate of 2 mg/kg
per day for 28 days were more tolerant to dieldrin than normal rats,
as shown by the fact that they survived 25 consecutive daily doses at
the rate of 5 mg/kg, a dosage that produced 70% mortality in
previously untreated rats. In spite of the ability of the rats,
pretreated with a moderate dosage of dieldrin, to survive a large
dosage, their livers showed definite indications of decompensation in
response to the high dosage. Although the smooth endoplasmic reticulum
remained hypertrophic and the microsomal protein and P-450
haemoprotein concentrations remained elevated, the enzyme activities
per mole of available P-450 haemoprotein decreased, as did the
activity of some enzymes not associated with microsomes. Much of the
excess smooth endoplasmic reticulum formed tightly packed clusters of
tubular membranes with no glycogen and little hyaloplasm, and some of
the mitochondrial membranes were injured. It was suggested that the
phase of decompensation represented by hypertrophic but hypoactive
smooth endoplasmic reticulum might serve as a sensitive criterion of
toxic injury before microscopic changes of a clearly harmful sort
become recognizable (Hutterer et al., 1968).
Other studies indicating not only the presence of adaptive change
over a range of dosages but also the failure of adaptation and onset
of injury at sufficiently high dosage levels have been reported for
butylated hydroxy-toluene (Gilbert & Golberg, 1967) and for DDT
(Hoffman et al., 1970). Hoffman and his colleagues found that, when
DDT was fed to male weanling rats for only 14 days at dietary
concentrations of 0.5 to 2048 mg/kg, concentrations of 0.5 and 2 mg/kg
had no effect on the O-demethylation reaction used as a test, but
concentrations of 4-750 mg/kg produced increases in the rate of
metabolism, proportional to the log of dosage. Extrapolation of this
portion of the dosage response curve to the abscissa provided a
calculated no-effect level of 3.27 mg/kg equivalent to about
0.327 (mg/kg)/day. This is in reasonable agreement with other
estimates of the threshold for induction of various enzymes in the
rat, including some studies involving longer administration of DDT.
These estimates, expressed as (mg/kg)/day, are approximately 0.05
(Kinoshita et al., 1966; Street et al., 1969), 0.5 (Schwabe &
Wendling, 1967) and 0.125 (Gillett, 1968). All of the estimates are of
the same order of magnitude as the 0.25 (mg/kg)/day known to be
effective in man (Laws et al., 1967; Poland et al., 1970), but all are
over 100 times greater than the highest dosage received by members of
the general population during the late 1960s (Duggan, 1968).
Increasing the dietary level to more than 750 mg/kg did not produce
any further increase in enzyme activity. Intake of less than 128 mg/kg
did not produce any increase in liver weight, within the period of
observation; increase was proportional to dosage within the range of
128 to 512 mg/kg and was submaximal at intakes above 512 mg/kg.
Some other compounds, notably phenobarbital, produced
morphological changes in the liver similar to those produced by some
organochlorine insecticides (Wright et al., 1972; Thorpe & Walker,
1973). It seems possible that sufficiently high doses of
phenobarbital, for example, may lead to a failure of adaptation and to
levels of enzyme activity that do not correspond to dosage.
As indicated above, the earliest morphological changes caused by
enzyme inducers in the rodent liver involve separate cells in the
centrolobular area. If the dosage is sufficiently high and prolonged,
nodules consisting entirely of hypertrophied cells may appear. At
first, these microscopic nodules are distinguishable only by pattern;
they have no bounding membrane and they do not compress or change in
any other detectable way the smaller liver cells that surround them.
Some nodules may become large enough to be seen without a microscope,
and a few may exceed 1 cm in diameter. In these large nodules, there
is almost complete loss of lobular architecture. Nodules apparently
were first described by Fitzhugh & Nelson (1947) who felt that they
could be regarded as adenomas or as low grade hepatic cell carcinomas.
The use of the second term is not clear because neither mitoses,
tissue invasion, nor metastasis was observed. Although Ortega et al.
(1956) reported small nodules in the livers of treated rats, and
although they examined tissue loaned by Fitzhugh & Nelson's
laboratory, they were entirely unimpressed by the lesions, referring
to them as "focal incongruities".
Almost 3 decades after the first study, there is no more agreement
than is reflected in the preceding paragraph. The views of some
pathologists remain diametrically opposed. This is true even though
the finding of: (a) pulmonary metastases of hepatic cells in mice that
had received DDT (Tomatis et al., 1972; Walker et al., 1973), ß-HCH,
gamma-HCH, dieldrin, or phenobarbital (Thorpe & Walker, 1973); or (b)
progression of liver enlargement beginning 12 weeks after cessation of
ingestion of alpha-HCH by mice for 24 or 36 weeks (Nagasaki et al.,
1974) or progressive increase in the size of liver nodules after DDT
feeding was stopped (Tomatis et al., 1974b; Tomatis & Turusov, 1975);
or even (c) in HCH-exposed mice the time-pattern of increase in liver
weight (as reflected by body weight), which gained momentum only after
a delay of 4 weeks but showed a further acceleration in the thirteenth
week, in spite of decreased food consumption (Tomii et al., 1972),
would appear to establish, without question, that at least some of the
liver changes produced by these compounds in rodents are malignant.
Of course, the reason for disagreement is that the tumours
produced by DDT, other organochlorine insecticides, and phenobarbital
differ in their biochemistry and are not malignant in the classical
sense. Specifically, (a) they do not actively invade tissues; (b)
their "metastases" do not grow; (c) they produce little shortening of
life span; and (d) mice receiving 5.5 (mg/kg)/day as a result of
dietary intake of DDT show a decrease in the success of
transplantation and a significant increase in survival in mice in
which tumours grew following inoculation with an otherwise uniformly
transplantable and uniformly fatal ependymoma (Laws, 1971).
Although the displacement of liver cells to the lung occasionally
seen after prolonged dosage with DDT is usually referred to as
metastasis, it might better be called embolism because the lesion does
not progress and, therefore, lacks the clinical significance of a real
metastasis. Because it does not grow, the lesion is hard to find. A
number of investigators have failed to mention liver cells in the
spleen, lymph nodes, or lungs, and some have stated specifically that
they were not found (Nagasaki, 1973; Rossi et al., 1977).
Perhaps the most illuminating study of the liver changes caused by
DDT is that of Kuwabara & Takayama (1974). They used fluorinyl
acetamide (2,7-FAA) as a positive control in their studies of DDT and
HCH. The 3 compounds were given at dietary concentrations of 250, 250,
and 600 mg/kg, respectively. The lesion caused by 2,7-FAA differed
from those caused by either of the other compounds in 3 ways: (a) it
started as hyper-plastic nodules rather than as isolated cell changes;
(b) the final lesion was hepatocellular carcinoma in contrast with the
adenoma caused by DDT or HCH; and (c) alpha-fetoprotein was formed,
which did not occur with DDT or HCH. Other workers have also failed to
find alpha-fetoprotein in mice treated with an organochlorine
insecticide (Hanada et al., 1973).
It must be emphasized that the organochlorine insecticides and
phenobarbital do not produce, in other animals, the early, visible
changes in the endoplasmic reticulum that are so characteristic of
some rodents and that progress to tumour formation in rodents. That
these compounds do not lead to tumour formation in other animals might
have been predicted by the fact that they do not cause the early
changes, characterized by hypertrophy, margination, and lipospheres.
All available evidence indicates that man does not appear to be
susceptible to the tumorigenic action of the organochlorine
insecticides and phenobarbital. No increase in the occurrence of
tumours has been found in heavily-exposed populations. This includes
groups of workers who manufacture and formulate DDT and dieldrin and
who have been examined carefully for tumours (Laws et al., 1967;
Jager, 1970).
Finally, a study based on a complete tumour registry did not
indicate any increase of tumours attributable to phenobarbital among
men and women who received heavy, essentially lifelong dosing with
this drug for the control of epilepsy (Clemmesen et al., 1974).
In summary, in spite of disagreement about the interpretation of
the liver cell changes, there is general agreement about their
development and appearance. The change that can be detected first and
can be produced by the smallest effective dosage involves the
endoplasmic reticulum. The initial change is reversible, but, even
more important, it is peculiar to rodents. There is no evidence that
anything from the first increase in endoplasmic reticulum to the final
development of a highly nodular liver with occasional displacement of
cells to the lung has any bearing on the health of man or other
animals in which the endoplasmic reticulum does not respond in this
way.
7.1.10 Mutagenicity
DDT has been tested in a number of ways for possible mutational
effects. Shirasu et al. (1976) listed DDT as a negative chemical in
microbial mutagenicity screening studies on 166 pesticides. The test
system consisted of rec-assay using H 17 Rec+ and M 45 Rec-strains
of Bacillus subtilis and reversion assays without metabolic
activation using auzotrophic strains of Escherichia coli (WP 2) and
Salmonella typhimurium (Ames series). Further studies by the same
authors, with metabolic activation, failed to reveal mutagenicity of
DDT (Shirasu et al., 1977). McCann et al. (1975) and McCann & Ames
(1976), reported negative results on DDE in Salmonella typhimurium
testing with metabolic activation.
At a dosage of 105 mg/kg, DDT did not produce any increase in
dominant lethals in mice (Epstein & Shafner, 1968). Concentrations of
10 mg/kg or higher produced chromosome breaks and exchange figures in
a marsupial somatic cell line (Palmer et al., 1972). Saturated
solutions produced chromosome breaks in the root tips of onion and
other plants (Vaarama, 1947). A slight mutagenic effect in mammals has
been reported by Markarian (1966). Deletions plus gaps were reported
to be more common in the chromosomes of mice that had received DDT.
An unconventional test for mutagenicity involved examination of
explants of pulmonary tissue from embryonic mice whose dams had been
fed dietary concentrations of DDT of 10 and 50 mg/kg. An increase in
diffuse hyperplasia and focal proliferation was observed, but a
dosage-response relationship was not clear. Some of the embryos were
allowed to live and the experiment was repeated in subsequent
generations. There was no continuing progression of the reported
changes in succeeding generations (Sabad et al., 1972).
7.1.11 Teratogenicity
When p,p'-DDT was administered to pregnant mice at a rate of
1 mg/kg on days 10, 12, and 17 of gestation, it was not teratogenic
but did alter the gonads and decrease the fertility of the young,
especially the females (McLachlan & Dixon, 1972). A single dose at the
rate of 25 mg/kg or repeated doses of 2.5 (mg/kg) day given during
pregnancy may be embryotoxic but not teratogenic to mice (Schmidt,
1973). The reason why one or a few doses during pregnancy may be
embryotoxic although the same dosage is harmless, when administered
during the entire reproductive period, is of theoretical but not
practical importance.
Teratogenic effects of DDT have not been seen in studies of
reproduction including those for 2 generations in rats, 6 generations
in mice, and 3 generations in dogs (see section 7.1.7).
7.2 Acquisition of Tolerance to DDT
Because DDT stimulates microsomal, mixed-function enzymes and the
action of these enzymes on DDT is one of detoxication, it might be
expected that some tolerance might develop. Such tolerance has been
demonstrated in the case of dieldrin; rats that received this compound
for 28 days at a rate of 2 mg/kg survived 25 additional days at a rate
of 5 mg/kg, a dosage that killed 70% of previously untreated rats
(Hutterer et al., 1968). Apparently the possibility of tolerance to
DDT has not been explored.
7.3 Factors Influencing DDT Toxicity
7.3.1 Dosage effect
7.3.1.1 Dosage-effect of DDT
Table 5 summarizes the acute oral and dermal toxicity of DDT in
common laboratory animals, and Table 18 summarizes the subcutaneous
intravenous and intraperitoneal toxicity. Both tables are condensed
from an earlier review (Hayes, 1959) that gives references and
additional details. It may be concluded that dissolved DDT is absorbed
through all portals. Absorption of DDT powder through the skin is
negligible. It is frequently impossible to put enough DDT dust on the
skin of animals to kill them, so that an LD50 value for this
formulation cannot be determined by the dermal route. Although
formulation is important in determining the toxicity of DDT by other
routes, the difference is not so great as it is in connexion with skin
exposure. DDT is about 4 times more toxic when given intravenously
than when given orally, and about 40 times more toxic when given
intravenously than when given dermally.
Table 18. Acute subcutaneous, intravenous, and intraperitoneal LD50 of DDT
in common laboratory animalsa
Species Formulation Subcutaneous Intravenous Intraperitoneal
(mg/kg) (mg/kg) (mg/kg)
Rat Water suspension or powder >2000
Oil solution 200-1500 47 80-200
Mouse Water suspension or powder 1000-1500
Oil solution 300
Guineapig Water suspension or powder
Oil solution 900 150
Rabbit Water suspension or powder
Oil solution 250->3200 30-41 <2100
Cat Water suspension or powder
Oil solution <650 32
Dog Water suspension or powder
Oil solution 68
Monkey Water suspension or powder
Oil solution 55
a From: Hayes (1959).
In general, DDT appears to be more toxic as a solution in
vegetable oil or animal fat than when given in some petroleum
fraction. Petroleum may act as a laxative. The heavier fractions are
never absorbed and DDT dissolved in such fractions has to be extracted
from the solvent in order to show toxicity.
In summary, DDT is a compound of moderately acute toxicity.
Compared with other organochlorine insecticides of equal or greater
toxicity, it is remarkable in being only slightly absorbed by the
skin.
The effects of repeated doses of DDT are summarized in Table 17.
The 90-dose oral LD50 of technical DDT in rats is
46.0 (mg/kg)/day (Gaines, 1969). The chronicity index is 5.4. Thus the
compound has only a moderate tendency to cause cumulative effects, and
this limited tendency is fully explained by the accumulation of DDT
itself in tissues as a result of continuing intake. In fact, this
accumulations, which is strictly dosage dependent, is detectable at
all measurable levels of intake. The relationship in man is shown in
Fig. 4 (p. 138).
If storage is considered undesirable per se, then DDT is without
a no-injurious-effect level. However, the same may be said for all
compounds that are absorbed, for the presence of all of them in the
bodies of exposed organisms -- perhaps at very low levels -- may be
assumed; failure to demonstrate low levels of storage does not depend
on physiology but only on the limitations of analytical chemistry and
on the lack of persistence of chemists.
A number of papers have reported no-effect levels for DDT within
the variables investigated, namely: rat, 0.05 mg/kg (Lehman, 1965);
dog, 8 mg/kg (Lehman, 1965); and monkey, 2.2-5.54 mg/kg (Durham et
al., 1963).
There remain reports of effects in animals at the lowest dosages
investigated. For example, decreased serum albumin and increased ß-
and gamma-globulins in the blood of rats and rabbits maintained on a
dosage of 0.2 (mg/kg)/day for 3-11 months was reported by Kagan et al.
(1969).
In studies carried out in rats and dogs, toxicity, as measured by
the maximum no-effect level, was seldom very different from the
corresponding value resulting from 90 days of exposure at the same
dosage range. The largest factor of difference observed when 33
chemicals were investigated in rats was 12 (20 for minimal effect
level), and for half of them the factor was 2 or less. In 21,
rat-to-dog comparisons of long-term toxicity, in no instance was the
dog more sensitive than the rat (Weil & McCollister, 1963). Although
LD50 studies offer a poor basis for predicting long-term toxicity,
the lowest dosage that will produce a minimum effect, when administered
for the lifetime of rats, can be predicted with reasonable success
from a test lasting only 7 days and with good success from a 90-day
study (Weil et al., 1969). These results offer some perspective for
judging the ultimate effect of exposure to compounds that have been
commercially available for less than one human lifetime.
Summary of long-term toxicity studies. The lowest dosages that
have been studied in animals are of the same order of magnitude as
those encountered by men who make or formulate DDT and, therefore,
hundreds of times greater than the dosages encountered by the general
population. The animal studies have been continued long after a steady
state of storage has been achieved. From the results it can be
concluded that bioaccumulation sufficient to produce neurotoxicity or
other clinical effects, including a reduction of the life span, can
occur only at dosage levels substantially higher than those
encountered by the most heavily exposed workers. DDT dosages
encountered by workers produced a small but detectable increase in
liver changes (hypertrophy, margination, and liposphere formation) in
some groups of mice and rats. The same changes occurred in low
incidence in control mice and rats but not in other animals (see
section 7.1.9).
7.3.1.2 Dosage-effect of metabolites and o,p'-DDT
Acute oral LD50 values of DDT metabolites commonly found in
tissues of excreta are shown in Table 19. Readily absorbable
formulations of the metabolites are less toxic than the most
absorbable preparations of the parent compounds (see for example
Table 5).
Table 19. Oral LD50 values of metabolites of DDT
Compound Species LD50 Reference
(mg/kg)
DDE rat, male 880 Gaines, 1960
DDE rat, female 1240 Gaines, 1960
ODE mouse 700 von Oettingen & Sharpless, 1946
DDE mouse 1000 Domenjoz, 1946a,b
TDE(DDD) rat, male >4000 Gaines, 1969
DDA rat 1900 Smith et al., 1946
DDA rat, male 740 Gaines, 1960
DDA rat, female 600 Gaines, 1960
DDA mouse 720 von Oettingen & Sharpless, 1946
DDA mouse 590 Domenjoz, 1946a,b
DDA. Rats tolerate higher tissue levels of DDA than of DDT.
Eighteen hours after intravenous injection of DDA at a rate of
100 mg/kg, tissue levels were still higher than those usually found in
animals, fatally poisoned by DDT (Judah, 1949).
DDA produces less injury to the liver than DDT but produces
greater damage to the kidney especially at high intravenous dosages
(Lillie et al., 1947). This is consistent with the finding of Spicer
et al. (1947) that, following administration of DDT, DDA constitutes a
higher proportion of DDT-related compounds in the kidney (25%) than in
any other tissue, e.g., 12% in the liver, 10% in the brain, and even
less in other tissues.
o,p'-DDT. At an oral dosage of 150 mg/kg, p,p'-DDT produces
severe illness in all rats and kills about half of them, but o,p'-DDT
at the same dosage does not produce illness even though the
concentrations of the 2 compounds in the brain at various intervals
after dosing are about the same. At a dosage of 3000 mg/kg, o,p'-DDT
produces mild to moderate illness, and the concentration in the brain
is 5-9 times the concentration of p,p'-DDT necessary to produce
similar symptoms. Thus, p,p'-DDT appears to be inherently more toxic
than the o,p'-isomer (Dale et al., 1966a).
7.3.2 Age and sex
Young animals eat more than adults in relationship to their body
weight. For this and other reasons, they are often more susceptible
than adults to poison in food. However, young animals are inherently
less susceptible to certain compounds. There is no evidence that DDT
is more toxic to the young than to the adults of any species,
including man. In the rat, the young are less susceptible than adults
to a single dose and about equally susceptible to repeated doses as
shown in Table 20. According to Henderson & Woolley (1969), the
relative insusceptibility of the young is associated with relatively
poor absorption of DDT by the central nervous system and by the less
inherent susceptibility of the young brain to DDT already absorbed by
it. Further studies by the same authors (Henderson & Woolley, 1970)
showed that fatal poisoning of both 10- and 60-day-old rats involved
hyperexcitability and intense tremor followed by prostration and
eventual respiratory failure. However, in the adult rat, DDT caused
convulsions, an increase in respiration and heart rate, and a lethal
increase in body temperature (40-42°C) prior to death, but the body
temperature of the immature rat decreased during acute intoxication by
DDT. The authors suggested that, whereas DDT is a direct depressant of
respiration in both young and old rats, the additional toxic responses
manifested by seizures and hyperthermia accounted for the increased
lethality of DDT in mature animals.
There is virtually no sex difference in the acute toxicity of DDT
to rats; the LD50 was 113 and 118 in males and females, respectively
(Gaines, 1960). When DDT is fed to rats at ordinary dietary levels,
the 2 sexes store it equally. However, at higher dosages, females
store more of the compound; the difference is explained mainly by the
lower activity of the liver microsomal enzymes in female rats and,
only in part, by the relatively higher food intake of the females.
Table 20. Effect of age on the toxicity of DDT to rats
Number Age LD50 Reference
of doses (mg/kg)a
1 newborn 4000 Lu et al.. 1965
1 newborn 2356 Harrison, 1975
1 10 days 728 Henderson & Woolley, 1969
1 14-16 days 437.8 Lu et al., 1965
1 weanling 355.2 Lu et al,, 1965
1 2 months 250 Henderson & Woolley, 1969
1 3-4 months 194.5 Lu et al., 1965
1 middle-aged 235.8 Lu et al,, 1965
1 adult 225 Harrison, 1975
4 preweaning 279.2 Lu et al., 1965
4 adult 285.6 Lu et al., 1965
a Total intake one or more doses.
7.3.3 Nutrition
Fat. Nutrition influences the toxicity of DDT in connexion with
both fat and protein. Fatness influences the amount that can be stored
inactively in the body, and it is of importance in mitigating acute
poisoning. This action of fatness or "good condition" has been noted
in connexion with mammals (Spicer et al., 1947) and fish (Hoffmann &
Surber, 1948). In contrast, laboratory animals are slightly more
susceptible to repeated large doses administered as part of a diet
containing a moderate proportion of fat (5% or more) than as part of a
very low fat diet (0.5%) (Sauberlich & Baumann, 1947). This difference
is thought to be associated with absorption from the gastrointestinal
tract.
Rats that have stored large amounts of DDT in the fat may suffer
tremors, if starvation or some other cause leads to a mobilization of
their fat (Fitzhugh & Nelson, 1947). If DDT intake is stopped when
starvation begins, and, if the concentration of DDT is measured only
once following the interval of starvation, the results may be erratic,
reflecting, to a greater or lesser degree, both mobilization of fat
and excretion of metabolites as in studies reported by Deichmann et
al. (1972).
However, in nature, starvation is more often partial than
complete, and, if the original diet contains enough DDT to cause
substantial storage, whatever food may be found in a period of
scarcity is also likely to be contaminated. The initial effect of the
mobilization of fat is to increase the concentration of DDT in the
remaining fat and in other tissues. Excretion is increased in response
to the increased tissue levels but may not be fast enough to prevent
the accumulation of a toxic concentration in the brain. If intake of
DDT is stopped, the increased rate of excretion eventually leads to
reduced storage (Dale et al., 1962). These findings in rats have been
confirmed, in regard to both the initial increase in the concentration
of DDT (Dedek & Schmidt, 1972; Stenberg & Diky, 1973) and the later
reduction (Brodeur & Lambert, 1973). Similar findings have been
reported in birds (Adamczyk, 1971).
The effect of fat mobilization on the toxicity of DDT is the same
whether it is caused by withholding food or by disease that causes
partial refusal of food (Hayes, 1975).
It is highly unlikely that poisoning by DDT will be precipitated
in man by starvation because: (a) very few subjects store the compound
in concentrations as high as those required to demonstrate the
phenomenon in rats; and (b) the metabolic rate in man is so much
slower than that in rats that elimination of DDT in man would
counterbalance that produced by its mobilization.
Lipids. The association of lipids with the function of
microsomal enzymes is generally recognized as is the fact that DDT
induces these enzymes. Therefore, it might have been expected that DDT
and essential fatty acids would interact. Tinsley & Lowry (1972) found
that the growth of female rats receiving p,p'-DDT at a dietary level
of 150 mg/kg was depressed, if they received a diet deficient in
essential fatty acids, but was slightly stimulated, if they received
the same diet supplemented with these acids. Another variable
influenced by the same factors was the ratio of various liver lipids.
The changes in fatty acid composition were related to the
proliferation of hepatic smooth and endoplasmic reticulum; it was
suggested that DDT influenced essential fatty acid metabolism by
increasing the demand for them.
In contrast, a variety of diets (containing fats that may occur in
the human diet and that were in approximately the same proportion as
fats in the typical human food in the USA) had little or no influence
on the storage of DDT and a wide range of pesticides fed to rats for 4
generations in a combination of rates 200 times those found in the
Market Basket Study of food in the USA (Adams et al., 1974).
Ascorbic acid. In squirrel monkeys (and presumably in other
species) only 2 days on an ascorbic acid-deficient diet impaired both
the induction of O-demethylase and the stimulation of the glucuronic
acid system by DDT (5 mg/monkey/day) (Chadwick et al., 1971a). In
guineapigs, maintenance of induction of microsomal enzymes required a
higher dietary level of ascorbic acid than prevention of scurvy
(Wagstaff, 1971).
Protein. Smith & Stohlman (1945) found only slightly greater
mortality and liver pathology in rats fed DDT at 500 mg/kg in a diet
containing protein at 80 g/kg than in one containing 280 g/kg. The
finding that low dietary protein predisposes to DDT poisoning has been
confirmed (Sauberlich & Baumann, 1947; Boyd & DeCastro, 1968, 1970;
Boyd & Krijnen, 1969); however, even zero protein intake increased
toxicity only 4-fold, the smallest factor observed in comparable
studies of 9 pesticides. The effect of protein deficiency on toxicity
may involve a crippling of the microsomal enzymes of the liver or it
may act synergistically with compounds that cause anorexia. Rats fed a
diet containing casein at 810 g/kg exhibited evidence of renal
overload and were more susceptible to DDT (Boyd & DeCastro, 1970).
7.3.4 Species
The acute toxicity and metabolism of DDT were studied in mice and
hamsters because of the marked difference in their susceptibility to
liver tumours induced by DDT. The central nervous systems of the 2
species are equally sensitive, the concentration of DDT in their
brains at death being similar. However, after an oral dosage of
500 mg/kg, the DDT concentration in the mouse brain was twice that in
the hamster. This cannot be explained by a difference in absorption,
metabolism, or excretion but apparently is due to a difference, in the
permeability of the blood/brain barrier in the 2 species. When animals
receive DDT at a dietary level of 250 mg/kg for 6 weeks, the residues
in fat and liver were 7/8 times higher in the mouse, a fact only
partially explained by the greater food intake of the mouse relative
to body weight. Although urinary excretion of 14C-DDT was similar in
previously unexposed hamsters and mice, this excretion was stimulated
in the hamster but little affected in the mouse by previous dietary
exposure to DDT (Gingell & Wallcave, 1974).
Mice also differ from rats in the hormonal regulation of the basic
activity of hepatic microsomal mixed-function enzymes as well as in
the response of these enzymes to inducers (Chhabra & Fouts, 1974).
7.3.5 Other factors
A number of other factors are known to influence the toxicity of
some compounds, and the degree of difference may be very great in
isolated instances. Factors that have been reviewed elsewhere (Hayes,
1975) include (in addition to those listed above) interaction of
compounds, strain, individual differences, isolation and crowding,
other social and psychological factors, temperature, pressure and
altitude, light and other radiation, circadian and other rhythms,
seasonal differences, and relative humidity. None of these additional
factors is known to have an important effect on the survival of
animals receiving DDT. The possibility of the interaction of DDT with
aldrin, pyrethrin, piperonyl butoxide, malathion, dichloropheno-
xyacetic acid (2,4-D), and a number of food additives has been
explored systematically without finding anything but simple additive
results (Fitzhugh, 1966). However, pyrethrins, especially synergized
pyrethrins, have an additive and perhaps synergistic effect on the
changes in liver morphology associated with repeated doses of DDT in
rats (Kimbrough et al., 1968).
7.4 Human Studies
Oral exposure. Table 21 summarizes the effects of one or a few
carefully measured oral doses of DDT. The results are consistent with
those in accidents reported by Garrett (1947) and Hsieh (1954) in
which it was possible to estimate accurately the amount ingested. It
may be concluded that a single dose at the rate of 10 mg/kg produced
illness in some but not all subjects even though no vomiting occurred.
In general, smaller doses did not produce illness, although a dosage
of 6 mg/kg produced perspiration, headache, and nausea in a man who
was sickly and who was hungry at the time of eating. Persons who were
made sick by 10 mg/kg did not have convulsions, but convulsions
occurred frequently when the dosage level was 16 mg/kg or greater
(Hsieh, 1954). Rarely, a dosage as high as 20 mg/kg might be taken
without apparent effect (MacCormack, 1945). Dosages at least as high
as 285 mg/kg have been taken without fatal result (Garrett, 1947).
However, large doses lead to prompt vomiting, so that the amount
actually retained cannot be determined accurately.
It has been noted, in the course of tests with volunteers, that
dilute colloidal aqueous suspensions of DDT are odourless and
tasteless (Domenjoz, 1946a; Hoffman & Lendle, 1948). Saturated
alcoholic solutions of DDT have a weak aromatic taste or rather odour.
Some people find these solutions slightly anaesthetic to the tongue
(Hoffman & Lendle, 1948). The taste of DDT in vegetable oil is so
slight that many persons could not identify capsules containing 0,
3.5, and 35 mg of DDT when they were presented separately but could
arrange them in proper order when one of each was available for
comparison (Hayes, personal communication, 1977).
The possible clinical effects of many repeated doses of DDT were
first explored by Fennah (1945). Because of his interest in predicting
the results of indiscriminate use, he expressed the exposures in terms
of environmental levels rather than in dosage units. The exposures
were clearly higher than those ordinarily encountered. In one test,
lasting a total of 11.5 months, Fennah daily inhaled 100 mg of pure
DDT and drank water dusted at the rate of 3240 mg/m2. Much of the
inhaled dust must have been deposited in the upper respiratory tract
and swallowed. Later, for one month, Fennah ate food all of which had
been sprayed at the rate of 2160 mg/m2 after it had been served. No
ill-effect of any kind was observed.
Table 21. Summary of the effects of one or a few oral doses of DDT on volunteers
Dose (mg) and Result Reference
formulation
250 × 9, No effect. Domenjoz, 1946a
suspension
1500, butter No effect, but lice killed when fed 6 MacCormack, 1945
solution and 12 h after dose.
500, oil No effect. Neal et al., 1946
solution
700, oil No effect. Neal et al., 1946
solution
250, suspension None except slight disturbance of Velbinger, 1947a,b
sensitivity of mouth.
250, oil Variable hyperesthesia of mouth, Velbinger, 1947a,b
solution
500, oil Variable hyperesthesia of mouth. Velbinger, 1947a,b
solution
750, oil Disturbance of sensitivity of lower part of Velbinger, 1947a,b
solution face; uncertain gait; peak reaction (6 h after
ingestion) characterized by malaise, cold moist
skin, and hypersensitivity to contact;
reflexes normal.
1000, oil Same as above; no joint pains, fatigue, fear, Velbinger, 1947a,b
solution or difficulty in seeing or hearing.
1500, oil Prickling of tongue and around mouth and nose Velbinger, 1947a,b
solution beginning 2.5 h after dose; disturbance of
equilibrium; dizziness; confusion; tremor
of extremities; peak reaction (10 h after
ingestion) characterized by severe malaise,
headache, and fatigue; delayed vomiting;
almost complete recovery in 24 h.
Some later studies on volunteers have been designed to explore the
details of storage and excretion of DDT in man and to search for
possible effects of dosages considered to be safe. In the first of
these studies, men were given 0, 3.5, and 35 (mg/man)/day. These
administered dosages, plus DDT measured in the men's food, resulted in
dosage levels of 0.0021-0.0034, 0.038-0.063, and 0.36-0.61
(mg/kg)/day, respectively, the exact value depending on the weight of
each individual. Six volunteers received the highest dosage of
technical DDT for 12 months, and 3 received it for 18 months. A
smaller number of men ingested the lower dosage of technical DDT or
one of the dosages of p,p'-DDT for 12 or 18 months. No volunteer
complained of any symptoms or showed, by the tests used, any sign of
illness that did not have an easily recognizable cause clearly
unrelated to the exposure to DDT. At intervals, the men were given a
systems review, physical examination, and a variety of laboratory
tests. Particular attention was given to the neurological examination
and liver function tests, because the major effects of DDT in animals
involve the nervous system and the liver (Hayes et al., 1956). The
same result was obtained in a second study in which the same dosages
were given for 21 months and the volunteers were observed for a
minimum of 27 additional months (Hayes et al., 1971). Information on
storage and excretion gathered in these studies has already been
discussed in sections 6.2.1.1 and 6.3.1.1.
Recently, DDT has been used on an experimental basis at dosage
rates varying from 0.3 to 3 (mg/kg)/day for periods up to 7 months in
an attempt to decrease serum bilirubin levels in selected patients
with jaundice. No side-effects were observed. No improvement was noted
in patients with jaundice based on cirrhosis who did not have any
demonstrated liver enzymes deficiency. However, in a patient with
familial, nonhaemolytic, unconjugated jaundice based on a deficienty
of glucuronyl-transferase, treatment with DDT rapidly reduced the
plasma bilirubin level to the normal range and relieved the patient of
nausea and malaise from which he had suffered intermittently. The
liver function tests as well as other laboratory findings remained
normal. The improvement was maintained during the 6 months that DDT
was administered, and had persisted for 7 additional months at the
time the report was written. In this case, a dosage of 1.5 (mg/kg)/day
produced a steady rise in plasma levels of p,p'-DDT from an initial
level of 0.005 mg/litre to a maximum of 1.33 mg/litre at the end of
treatment. At this time, the concentration in body fat was 203 mg/kg.
Plasma levels fell slowly after dosing was stopped (Thompson et al.,
1969). The highest dally intake in this series was 6 times greater
than the highest level administered in earlier studies of volunteers
and about 7500 times greater than the DDT intake of the general
population. The highest value for p,p'-DDT in serum observed in the
entire series was 1.330 mg/litre compared with 0.996 mg/litre, the
highest value reported by Laws et al. (1967) for formulating plant
workers.
Dermal exposure. Depending on dosage, oral administration of DDT
to volunteers either did not produce any illness or produced only
brief poisoning similar to that seen in experimental animals. The oral
dosage necessary to produce any clinical effect was almost always
10 mg/kg or more. However, in 2 studies involving only 3 subjects in
all, experimental dermal exposure to DDT was followed by fatigue,
aching of the limbs, anxiety, or irritability, and other subjective
complaints. Recovery was delayed for a month or more (Case, 1945;
Wigglesworth, 1945). In neither study was there an independent
control. Although the dosage was unmeasured, the amounts of DDT
absorbed must have been much smaller than those involved in the oral
tests. One of the studies involved self-experimentation by one man. A
similar but somewhat more severe test on 6 volunteers did not produce
any toxic or irritant effects at all (Dangerfield, 1946). In view of
all other experiments and extensive practical experience, it must be
concluded that the illnesses reported by Wigglesworth and by Case were
unrelated to DDT.
With the exceptions just mentioned, dermal exposure to DDT has not
been associated with any illness or, usually, with any irritation
(Wasicky & Unti, 1944; Draize et al., 1944; Cameron & Burgess, 1945;
Fennah, 1945; Dangerfield, 1946; Chin & T'Ant, 1946; Domenjoz, 1946a;
Haag et al., 1948). In fact, Hoffman & Lendle (1948) reported that
even subcutaneous injection of colloidal suspensions of DDT in saline
in concentrations up to 30 mg/litre did not cause irritation.
Zein-el-Dine (1946) reported that DDT-impregnated clothing caused a
slight, transient dermatitis, but the method of impregnation was not
stated and the absence of solvent was not guaranteed. In other more
thorough studies DDT-impregnated clothing was found to be non-
irritating (Cameron & Burgess, 1945; Domenjoz, 1946a).
Small pads impregnated with different formulations of DDT were
applied to the inner surface of the forearm of 32 volunteers whose
cutaneous sensation had previously been measured for a period of 5
weeks. Pads impregnated with all the elements of the formulation
except DDT were applied to the corresponding position of the other arm
as a control. Powdered DDT and a solution of DDT at 50 g/litre showed
little effect. Solutions in olive oil and petrolaturn at 100 g/litre
and 200 g/litre did not show any remarkable effect on sensation of
pain, cold, or heat but reduced tactile sensation in most cases so
that the minimum pressure that could arouse the tactile sensation was
1-2.5 g/cm2 higher than for the control (Chim & T'Ant, 1946).
Respiratory exposure. Neal et al. (1944) reported almost
continuous daily exposures to aerosols sufficient to leave a white
deposit of DDT on the nasal vibrissae of the volunteers. This exposure
produced moderate irritation of the nose, throat, and eyes. Except for
this irritation during exposure, there were no symptoms, and
laboratory tests and physical examination, including neurological
evaluation, failed to reveal any significant changes. The studies by
Fennah (1945), which involved both respiratory and oral exposure, did
not produce any detectable ill-effects.
8. EFFECTS OF DDT ON MAN-EPIDEMIOLOGICAL AND CLINICAL STUDIES
8.1 Retrospective Studies on DDT-Exposed Populations
8.1.1 Epidemiological surveillance of persons occupationally exposed
to DDT
The safety record of DDT is phenomenally good. It has been used
for mass delousing in such a way that the bodies and inner clothing of
thousands of people of all ages and states of health have been
liberally dusted with the compound. By necessity, the persons applying
the DDT work in a cloud of the material. Other subjects have sprayed
the interior of hundreds of millions of homes in tropical and
subtropical countries under conditions involving (Wolfe et al., 1959)
extensive dermal and respiratory exposure. A smaller number of men
have made or formulated DDT for many years. Extensive experience and
numerous medical studies of groups of workers have been reviewed
(Hayes, 1959). Dermatitis was commonly observed among men who used DDT
solutions. The rashes were clearly due to the solvent, especially
kerosene. As often happens with rashes caused by petroleum
distillates, they were most severe in men when they first started work
and cleared in a few days unless contamination was exceptionally
severe. A smaller number of workers experienced mild narcotic effects
(vertigo and nausea) from solvents when working in confined spaces.
Gil & Miron (1949) reported that some persons suffered temporary
irritability, fatigue, and other ill-defined symptoms after exposure
to the dusty atmosphere of a delousing station, but the relation of
these atypical findings to DDT was not clear. With these exceptions
due largely to solvents, no illnesses clearly attributable to the
formulations, much less to DDT, were revealed by the early studies.
Ortelee (1958) carried out clinical and laboratory examinations of
40 workers, all of whom were exposed to DDT and some of whom were
exposed to a number of other pesticides. The men had been employed at
this work with heavy exposure for 0.4 to 6.5 years and with slightly
less exposure for as much as eight years. Exposure was so intense
that, during working hours, many of the men were coated with a heavy
layer of concentrated DDT dust. By comparing their excretion of DDA
with that of volunteers given known doses of DDT, it was possible to
estimate that the average dosages of 3 groups of the workers with
different degrees of occupational exposure were 14, 30, and 42 mg/man
per day, respectively. With the exception of the excretion of DDA and
the occurrence of a few cases of minor irritation of the skin and
eyes, no correlation was found between any abnormality and exposure to
the insecticide. Since very large doses of DDT injure the nervous
system and liver of experimental animals, special attention was given
to a complete neurological examination and to laboratory tests for
liver function. Although a few abnormalities were revealed, none was
detected in relation to DDT.
Thirty-five men employed from 11 to 19 years in a plant that had
produced DDT continuously and exclusively since 1947 and, at the time
of the study, was producing 2722 metric tonnes per month were studied
by Laws et al. (1967). Findings from medical histories, physical
examinations, routine clinical laboratory tests, and chest X-ray films
did not reveal any ill-effects attributable to exposure to DDT. The
overall range of storage of the sum of isomers and metabolites of DDT
in the men's fat was 38-647 mg/kg compared with an average of 8 mg/kg
for the general population. Based on their storage of DDT in fat and
excretion of DDA in urine, it was estimated that the average daily
intake of DDT by the 20 men with high occupational exposure was
17.5-18 mg/man per day compared with an average of 0.028 mg/man per
day for members of the general population. There was significant
correlation ( r = +0.64) between the concentration of total DDT-related
material in the fat and the serum of the workers. The average
concentration in fat was 338 times higher than that in serum -- a
factor about 3 times greater than that for people without occupational
exposure. Compared to members of the general population, the workers
were found to store a smaller proportion of DDT-related material in
the form of DDE; the difference was shown to be related chiefly to
intensity rather than to duration of exposure. DDE is relatively a
much less important and DDA a much more important excretory product in
occupationally-exposed men compared with men in the general
population. A further study of the same men involved in DDT production
is discussed in section 8.2.5.
By far the largest number of heavily-exposed workers whose health
has been investigated are those associated with malaria control in
Brazil and India (WHO, 1973). In Brazil, periodic clinical
examinations were made of 202 spraymen exposed to DDT for 6 or more
years, 77 spraymen exposed for 13 years ending in 1959, and 406
controls. In the first examination carried out in 1971, minor
differences between exposed and unexposed groups were observed in some
neurological tests, but this result was not confirmed by the second
examination in the same year nor in subsequent examinations. During a
3-year period, a survey of illnesses requiring medical care during the
6 months preceding each periodic medical examination failed to
demonstrate any differences between exposed and control groups. A
relatively small number of analyses indicated that the concentration
of DDT in the blood of spraymen was about three times higher than that
of controls.
In India, the blood levels of 144 spraymen were 7.5-15 times
higher than those of the controls and were at least as high as those
reported for workers who make and formulate DDT elsewhere (see
Table 7). When the spraymen were examined, the only differences from
the controls were that knee reflexes were brisker, slight tremor was
more often present, and a timed Romberg test was more poorly performed
by the spraymen. The positive results led to the selection of 20 men
for re-examination by a neurologist who concluded that the differences
found initially were not real or that the tests had returned to normal
within the few months between the 2 examinations. The signs were not
dosage-related, since they were not correlated with serum levels of
DDT.
It has been known for several years that substantial doses of DDT
and several other organochlorine insecticides stimulate the microsomal
enzymes of the liver. This property of DDT was put to practical use in
treating a patient with familial, nonhaemolytic, unconjugated
jaundice, as described earlier. It was, therefore, entirely expected
that persons with sufficient occupational exposure to a variety of
pesticides would be able to metabolize a test drug (phenazone) more
rapidly on the average than persons without occupational exposure were
able to do. However, the change was not one of significantly
increasing the fastest normal rate but of bringing all the workers up
to a high level. There was no indication that the change had any
effect on the workers' health (Laws et al., 1967, 1973; Kolmodin et
al., 1969; Poland et al., 1970).
In addition to the studies already mentioned regarding workers
with extensive storage, and excretion of DDT as a result of heavy
exposure to DDT, studies have also been made of a larger number of
workers with lesser storage and excretion following lesser exposure to
DDT but greater exposure to other insecticides. Further studies (Long
et al., 1969; Morgan & Roan, 1969, 1974; Warnick & Carter, 1972;
Sandifer et al., 1972; Embry et al., 1972; Tsutsui et al., 1974; Ouw &
Shandar, 1974) have failed to reveal effects of clinical significance
among workers with prolonged, moderate exposure not only to
organochlorine but also to organophosphorus and other types of
insecticides. Small but statistically significant differences have
appeared in the medical history or clinical laboratory results of some
of these workers compared with the controls, but in no instance have
the differences been of any medical importance, and dosage-response
relationships have been unclear or absent. In several instances, the
statistically significant differences have been opposite in different
groups of workers; for example, creatinine phosphokinase activity was
lower than that of controls in subjects applying the insecticide but
higher in operators. Seasonal variations present one year were lacking
the next. The possibility of adaptive change (other than enzyme
induction) has been suggested (Tocci et al., 1969), but this, like the
reality of the changes, remains unproved.
In some instances statistically significant differences have been
found between workers and controls selected from the general
population in connexion with parameters that have no known biochemical
relationship to DDT and for which another explanation has not been
excluded. For example, Keil et al., (1972) reported significant linear
correlations between serum vitamin A and plasma DDT, TDE, and DDE
levels.
There are a few reports of acute illness among workers attributed
to exposure to mixtures of DDT and other materials. In so far as the
dosage was very large, as in certain accidents that have occurred to
individuals or groups in the general populations (see section 8.2.2),
one would expect similar results. However, in at least one instance,
headache, dizziness, nausea, vomiting, pain and numbness of the limbs,
and general weakness beginning 1-1.5 h after entering a treated field
(Kolyada & Mikhal'Chenkova, 1973) suggested food poisoning or
hysteria.
Finally, there are studies of workers exposed to DDT and various
other pesticides that are reported to have produced a variety of
subjective and even objective medical findings. Interpretation of
these reports is difficult because: (a) the findings do not resemble
those of poisoned animals or of persons poisoned as a result of
accident or suicide; and (b) the papers fail to report how the medical
findings and the absenteeism of the pesticide workers compares with
those of workers of comparable age, sex, and exertion who are not
exposed to chemicals. The fact that the workers in question were
exposed to mixtures of pesticides is not in itself an explanation
because studies on many workers who were exposed to mixtures have not
revealed any consistent differences between exposed subjects and
unexposed controls. However, an explanation may lie in the degree of
exposure. Reports of very high levels of organochlorine compounds in
blood samples and of DDT in milk samples from populations in which
illness was found are discussed in sections 6.2.1.2 and 6.3.1.2.
The reports under discussion tend to fall in 2 categories, those
involving general debility and those involving a single organ or
system. Conditions representative of general debility include
dermatitis, subtle blood changes, general weakness, palpitations,
functional angiospasm, headache, dizziness, diminished appetite,
vomiting, lower abdominal pain, chronic gastritis, benign chronic
hepatitis, isomnia, a sympathetic vascular/asthenic syndrome,
vegetative dystonia, and confusion (Kostiuk & Mukhtrova, 1970; Bezugli
et al., 1973).
Organs, systems, or functions that have been studied with the
exclusion of other organs, systems, or functions of the same workers
include: the respiratory system (Boiko & Krasniuk, 1969), liver
(Bezuglyi & Kaskevich, 1969), stomach (Krasniuk & Platonova, 1969;
Platonova, 1970), kidneys (Krasniuk et al., 1968), adrenals (Bakseyev,
1973), skin (Karimov, 1969, 1970), and labour and the puerperium
(Komarova, 1970; Nikitina, 1974). An indication that the difficulties
under discussion are not serious is their reversal or prophylaxis by
means of diet. Lescenko & Polonskaia (1969) described in detail two
dietary supplements composed of ordinary foods plus sea-kale and a
selection of vitamins and trace metals. Organochlorine-exposed workers
who received these diet products showed a normalization of protein
metabolism manifested by an increase in total serum protein, improved
lipid metabolism, and enriched vitamin and trace element supplies in
the organism. All of these effects led to an improvement in the
detoxifying function of the liver, which was viewed as the most
frequent site of adverse effects of exposure to organochlorine
compounds.
8.1.2 Epidemiology of DDT poisoning in the general population:
accidents and suicides
The only demonstrated effects of DDT on the general population are
the storage of the compound and some of its derivatives in the tissues
and their excretion in urine and milk. The facts were reviewed in
sections 6.2.13 and 6.3.1.2. Briefly, DDT and some of its derivatives
are found in all or nearly all persons in the population. The
concentration is higher in tissues that have a high neutral fat
content. Thus, for members of the general public the concentration of
DDT-related compounds in adipose tissue is 100 or more times greater
than the concentration in plasma (Laws et al., 1967). However, in
spite of this great difference, sufficiently sensitive methods have
demonstrated DDT in all tissues including the fetus and in all body
fluids including human milk. These relationships are exactly what
would be predicted from what is known of the storage of drugs and
other compounds. Actual chemical demonstration of the distribution of
DDT has been established for several years. Thus, its occurrence was
first reported in human tissue (Howell, 1948), in tissue of the
general population (Laug et al., 1951) in human milk (Laug et al.,
1951), and in the human fetus (Denés, 1962).
There is extensive evidence that the mount of DDT and related
material in the general diet in the USA has decreased as the use of
DDT in that country has decreased, especially its use on forage.
During the early 1950s, total DDT-related intake was approximately
0.265 mg/man per day and that for DDT was 0.163 mg/man per day (Walker
et al., 1954). The average intake of DDT-related compounds based on a
very large number of samples collected in different parts of the
country during 1964-67 was 0.063 mg/man per day and that for DDT was
0.028 mg/man per day (Duggan, 1968). With decreased use of DDT, a
gradual decrease in the storage of DDT and related material in human
fat would be expected. Because only a few samples of fat were
collected in the early studies of human tissue, there is some
statistical uncertainty as to whether the decrease in storage that has
been observed is real or whether it merely reflects variation due to
sampling. In any event, by 1968, the average storage level of total
DDT-equivalent material in fat was 7.67 mg/kg and that for DDT was
1.46 mg/kg. These averages were based on just over 3000 samples
collected during the first half of 1968. The number of samples
involved in this particular study was greater than the sum of all of
the samples used in early studies. The best available values for
concentrations in serum are 0.0294 mg/litre for total DDT-equivalent
and 0.0047 mg/litre for p,p'-DDT.
Cases of accidental and suicidal poisoning in which the effects
were clearly caused by DDT are summarized in Table 22. All of these
cases involved ingestion. The signs and symptoms of poisoning were
entirely consistent with those observed in volunteers, except that the
spectrum of effects was broader because some of the accidental and
suicidal doses were very high. A few persons have apparently been
killed by uncomplicated DDT poisoning, but none of these cases was
reported in detail. Death has been caused much more frequently by the
ingestion of solutions of DDT, but in most instances the signs and
symptoms were predominantly or exclusively those of poisoning by the
solvent (Hayes, 1959). This does not mean that the toxicity of the
solvent always predominates. For example, the recurrent convulsions in
a case reported by Cunningham & Hill (1952), though more
characteristic of poisoning by one of the cyclodienes, was certainly
not typical of solvent poisoning. A 2-year-old child drank an unknown
quantity of fly spray of which 5% was DDT, but the nature of the other
active ingredients or the solvent was unknown. About 1 h after taking
the material, the child became unconscious and had a generalized,
sustained convulsion. Convulsions were present when the child was
hospitalized 2 h after taking the poison, but the fits were controlled
by barbiturates and other sedatives. Convulsions reoccurred on the
fourth day and again on the twenty-first day but ceased each time
following renewal of treatment. On the twelfth day, it was noted that
the patient was deaf. Hearing began to improve about the twenty-fourth
day and was normal, as were other neurological and psychic findings,
when the patient was seen about 2.5 months after the accident.
Clinical effects of one toxicant may be modified by combining it
with another. For example, prolonged illness would not be expected
from ingestion of DDT at a rate of 27 mg/kg. However, when DDT and
lindane were ingested in a suicidal attempt at dosages thought to be
27 mg/kg and 18 mg/kg, respectively, clinical remission of convulsions
and liver involvement was delayed until the twentieth day, and the EEG
did not return to normal until the thirty-ninth day (Eskenasy, 1972).
There have not been any accidents of suicided involving raspatory
or dermal exposure to DDT leading to recognized signs and symptoms of
poisoning, even though sufficient respiratory exposure to aerosols or
sufficient dermal exposure to solutions can cause poisoning in
animals; the difference is certainly one of dosage.
It has been alleged that DDT causes or contributes to a wide
variety of diseases of man and animals not previously recognized as
being associated with any chemical. Such diseases include
cardiovascular disease, cancer, atypical pneumonia, retrolental
fibroplasia, poliomyelitis, hepatitis, and "neuropsychiatric
manifestations" (Biskind & Beiber, 1949; Biskind, 1952, 1953; and
others). Without exception the causes of these diseases were unknown
or at least unproved at the time of the allegation. Needless to say,
Table 22. Summary of the effects of the accidental or suicidal ingestion of DDT
Individual dose Results and reference
(mg) Formulation
Number of persons
300-4500 Onset in 1 h; vomiting; restlessness; headache; heart weak and
in food slow; recovery next day (Mulhens, 1946).
1 man
unknown dose Onset in 2-2.5 h; all subjects weak and giddy; 4 subjects vomited;
in tarts 2 subjects hospitalized; one subject confused, uncoordinated, weak;
25 man one subject with palpitations and numbness of hands; recovery in
24-48 h (Mackerras & West, 1946).
5000-6000 Onset 2-3 h; throbbing headache; dizziness; incoordination;
in pancakes paraesthesia of extremities; urge to defaecate; wide nonreacting
3 men pupils; reduced vision; dysarthria; facial weakness; tremor; ataxic
gait; reduced sensitivity to touch; reduced reflexes; positive Romberg;
slightly low blood pressure and persistent irregular heart action;
partial recovery in 2-3 days, but slight jaundice appeared 4-5 days
after ingestion and lasted 3-4 days; all subjects normal 19 days after
poisoning except for irregular heart action in one subject (Naevested,
1947).
2000 No illness (Naevested, 1947).
in pancakes
2 men
up to 20 000 Onset in 30-60 min in those most severely affected; men first
in bread seen 2-3 h after ingestion; in spite of severe early vomiting that
28 men reduced the effective dose, severity of illness and especially intensity of
numbness and paralysis of extremities proportional to amount of DDT
ingested; all but 8 men recovered in 48 h; 5 others fully recovered in 2
weeks, but 3 men still had some weakness and ataxia of the hands
5 weeks after ingestion (Garrett, 1947, 1950, unpublished data).
unknown dose Onset about 3.5 h after ingestion; total of about 85 cases of which 37
in flour were hospitalized; symptoms mild and similar to those in earlier
about 100 women outbreaks except for gastrointestinal disturbance in most severe cases
including abdominal pain and diarrhoea as well as nausea; most
subjects fully recovered in 24 h (Jude & Girard, 1949).
unknown dose Symptoms in established cases similar to those reported earlier
14 cases (Francone et al., 1952).
Table 22 (Cont'd)
Individual dose Results and reference
(mg) Formulation
Number of persons
286-1716 With the exception of one man who was already sick when he received
in meatballs a dosage of 6 mg/kg, poisoning did not occur at dosages of
8 cases, 11 5.1-10.3 mg/kg. Ingestion of 16.3-120.5 mg/kg produced excessive
exposed perspiration, nausea, vomiting, convulsions, headache, increased
salivation, tremors, tachycardia, and cyanosis of the lips. Onset
varied from 2-6 h depending on dosage. Recovery required as much
as 2 days (Hsieh, 1954).
unknown dose Death 13 h after suicidal ingestion (Committee on Pesticides, 1951).
1 case
unknown dose Twenty-two separate cases, including 15 attempted suicides; some
22 unrelated cases complicated by solvents; 3 deaths (Committee on Pesticides, 1951).
the charge that DDT predisposes to poliomyelitis was dropped after the
disease was controlled through the use of vaccines. Unfortunately,
there is no immediate possibility of controlling cardiovascular
disease, cancer, or many of the less common conditions in man that
have been ascribed to DDT. In the meantime, such irresponsible claims
could produce great harm and, if taken seriously, even interfere with
the scientific search for true causes and realistic means of
preventing the conditions in question.
8.1.3 Epidemiology of DDT poisoning in infants and young children
Nothing is known fundamentally to distinguish the epidemiology of
DDT poisoning among children from that among adults. In both
instances, poisoning has never been confirmed, except where the dosage
was large, usually as the result of an accident and usually involving
gross carelessness. Probably a larger number of cases have occurred in
infants simply because they are more likely to eat and drink
formulations that they find in unlabelled containers frequently
originally intended for food. However, as far as DDT is concerned, all
the large outbreaks of poisoning have involved adults under military
conditions, thus children were not exposed. As might be expected, some
deaths of adults but none of children has apparently involved suicide.
Most, if not all, cases in adults were uncomplicated poisoning by DDT,
but several cases in children involved the drinking of solutions so
that the signs and symptoms were actually caused by the solvent
(Reingold & Lasky, 1947).
8.2 Clinical and Epidemiological Studies of the Effects of DDT on
Specific Organs and Systems
8.2.1 Haemopoietic system and immunology
In acute poisoning, a slight decrease in haemaglobin and a
moderate leukocytosis without any constant deviation in the
differential white count have been observed in volunteers (Velbinger,
1947a,b). These findings are considered secondary to the neurological
effects.
There is a strong tendency to blame blood dyscrasias, other
manifestations of "hypersensitivity", and, in fact, many diseases of
unknown cause on any new chemical that gains widespread attention. DDT
was no exception. A review of the early literature (Hayes, 1959)
indicates that blood dyscrasias and an unbelievable range of other
diseases were, in fact, blamed on DDT. Only a circumstantial
relationship was ever established between these diseases and exposure
to DDT, and this is true of the small number of reports of blood
dyscrasias (Murray et al., 1973) or angioneurotic oedema (Vanat &
Vanat, 1971) that have appeared recently. Later, fewer new reports
appeared linking DDT to diseases of unknown cause, although the use of
DDT increased greatly. It is true that available tests do not make it
possible to exclude a particular compound as a cause of an isolated
case of blood dyscrasia. However, it is noteworthy that the rate at
which these disorders occur has remained essentially unchanged since
before DDT was introduced (Hayes, 1975).
8.2.2 Nervous system
The effects of carefully measured doses of DDT that proved to be
just above the minimum toxic level are best described from studies of
volunteers (see section 7.4). Similar early signs and symptoms have
been encountered in cases of accidental poisoning that frequently
progressed to more severe illness as described in section 8.1.2.
Briefly, the earliest symptom of poisoning by DDT is
hyperaesthesia of the mouth and lower part of the face. This is
followed by paraesthesia of the same area and of the tongue and then
by dizziness, an objective disturbance of equilibrium, paraesthesia
and tremor of the extremities, confusion, malaise, headache, fatigue,
and delayed vomiting. The vomiting is probably of central origin and
not due to local irritation. Convulsions occur only in severe
poisoning.
Onset may be as soon as 30 min after ingestion of a large dose or
as late as 6 h after smaller but still toxic doses. Recovery from mild
poisoning is essentially complete in 24 h, but recovery from severe
poisoning requires several days. In two instances, there was some
residual weakness and ataxia of the hands, 5 weeks after ingestion.
Electroencephalograms were obtained from 73 workers exposed to
DDT, HCH, and chlorobenzilate for periods ranging from 7 months to 20
years. Just over 78% of the records were normal and 21.9% were
abnormal. The most severe changes involved persons exposed to the 3
compounds for 1-2 years; less severe changes were seen with either
shorter or longer exposure. The changes were not correlated with age,
the range and mean of age for those judged abnormal being almost
identical to these values for persons considered normal. Some of the
records showed bitemporal sharp waves with shifting lateralization
combined with low voltage theta activity. Other records showed spike
complexes, paroxymal discharges composed of slow and sharp waves most
pronounced anteriorly, and low voltage rhythmic spikes posteriorly.
None of the persons examined showed any abnormal clinical neurological
finding (Israeli & Mayersdorf, 1973; Mayersdorf & Israeli, 1974). The
incidence of abnormal electroencephalograms in the general population
is 9.0% or 9.2%, according to other investigators cited by Israeli &
Mayersdorf. Czegledi-Janko & Avar (1970) considered that nonspecific
EEG abnormalities occurred in 10-20% of the general population.
The frequency and degree of olfactory disorders, especially in the
ability to detect peppermint and acetic acid in an olfactory analyser,
were reported to be greater among persons exposed to pesticides, and
to increase with duration of exposure (Salihodzaev & Ferstat, 1972).
Whether any of the persons exposed to pesticides experienced any
clinical difficulty or social inconvenience associated with olfactory
sensation is not clear.
8.2.3 Renal system
There is no indication of renal damage in people, accidentally
poisoned by DDT, or in workers heavily exposed to it.
8.2.4 Gastrointestinal system
Except for vomiting, which probably is of central origin, the
gastrointestinal system has not been affected in acute poisoning.
8.2.5 Liver
Involvement of the liver has been mentioned in only a small
proportion of cases of accidental poisoning by DDT. In 3 men who ate
pancakes made with DDT and thus ingested 5000-6000 mg each, slight
jaundice appeared after 4-5 days and lasted 3-4 days (Naevested,
1947). Hepatic involvement and convulsions were reported in an
unsuccessful suicide attempt by ingesting DDT and lindane (Eskenasy,
1972).
Laws et al. (1973) made a detailed study of the liver function of
31 men who had made and formulated DDT and who had been the subjects
of an earlier study (see section 8.1.1). Judging from their excretion
and storage, the men's exposure was equivalent to oral intakes of DDT
at rates ranging from 3.6 to 18 mg/man per day for periods ranging
from 16 to 25 years and averaging 21 years. All tests were in the
normal range for total protein, albumin, total bilirubin, thymol
turbidity, and retention of sulfobromophthalein sodium (BSP). One man
had mild elevations in levels of both alkaline phosphatase
(EC 3.1.3.1) (16 units) and serum glutamic pyruvic transaminase
(EC 2.6.1.2) SGPT (42 units). Another man had an alkaline phosphatase
concentration of 14 units, while a third man had an SGPT level of 49
units. The alpha-fetoprotein test was negative for all 20 of the men
tested.
8.2.5.1 Liver enzymes
The induction of human microsomal enzymes of the liver by various
drugs was well known when Kolmodin et al. (1969) demonstrated this
effect in workers exposed to a variety of pesticides, including DDT.
Later, Poland et al. (1970) showed that workers who made and
formulated DDT and absorbed it at an average rate of about
0.25 (mg/kg)/day metabolized phenylbutazone more rapidly on the
average than controls and excreted more 6ß-hydroxycortisol.
Occupational exposure increased the drug-metabolizing ability of some
workers, so that they all metabolized test drugs with the efficiency
of those members of the general population who were most efficient in
this respect. The concentration of p,p'-DDT in the serum of the
workers studied by Poland averaged 0.573 mg/litre. In other workers
with less exposure to DDT, as indicated by average serum levels of
0.052 mg/litre, there was no increase in the urinary excretion of
D-glucaric acid, which is increased by a number of exogenous and
endogenous substances that induce microsomal enzymes (Morgan & Roan,
1974).
Thompson et al. (1969) demonstrated, in a different way, the
induction of microsomal enzymes by using DDT at a dosage of
1.5 (mg/kg)/day for 6 months in the successful treatment of
unconjugated hyperbilirubinaemia. In a similar way, Rappolt (1970)
used DDT to promote metabolism of an overdose of phenobarbital. It is
of interest that the levels of DDE in the serum of some workers
studied by Morgan & Roan (1974) approached those of workers studied by
Poland et al. (1970). The lack of induction in one group and its
presence in the other suggests that enzymes are induced in man more
readily by DDT than by DDE.
DDT promotes its own metabolism in some species of laboratory
animals. That the same is true in man is indicated by the fact that
storage of DDT is relatively less at higher dosages (see Fig. 4).
However, the metabolism and subsequent excretion of DDT can be
promoted even more by other inducing agents. Patients who received
phenobarbital or, more especially, phenytoin stored much less DDT than
other persons with similar exposure to DDT (Davies et al., 1969a;
The compound identified by Peterson & Robinson (1964) as a
"probable intermediate aldehyde" was later synthesized and shown to be
highly labile (McKinney et al., 1969), confirming the guess by
Peterson & Robinson that it was unlikely to accumulate in tissues in
measurable amounts.
Of the compounds shown in Fig. 2 and 3, only DDT, TDE (DDD), DDE,
and DDA are commonly reported in the tissues or excreta of animals,
including man. The symptomatology produced when the metabolites are
administered directly is discussed in section 7.1.1, while uptake,
distribution, and elimination of the compounds are discussed in
sections 6.1.2, 6.2.2, and 6.3.2, respectively.
Although microorganisms, plants, insects, and birds produce many
of the same metabolites that are found in mammals, there are
interesting differences. Nearly 20 derivatives (including mammalian
metabolites) have been identified, and the chemical structure of
several more is still unknown. Some aspects of nonmammalian, as well
as mammalian metabolism have been reviewed (Menzie, 1969; Klein &
Korte, 1970; Fishbein, 1974; Schroeder & Dorozalska, 1975). The
metabolism of microorganisms and plants, as well as that of domestic
animals, may influence the composition of DDT-derived residues in
human food, but there is no evidence that these residues contain a
significant amount of any compound not formed from DDT by human
metabolism.
7. EXPERIMENTAL STUDIES ON THE EFFECTS OF DDT
7.1 Animal Studies
7.1.1 Haemopoietic system and immunology
Many early reports reviewed by Hayes (1959) indicated that large
doses of DDT might not have any effect on the blood or that they might
produce a moderate leukocytosis and a decrease in haemoglobin, with or
without a decrease in the concentration of red cells. The leukocytosis
probably is secondary to stimulation of the sympathetic nervous
system, while the loss of haemoglobin may be nutritional in origin.
Later studies have confirmed the early results. A range of
haematoiogical variables remained unchanged in squirrel monkeys dosed
orally at rates of 0, 0.05, 0.5, 5, and 50 (mg/kg)/day, even though
the highest dosage was fatal within 14 weeks (Cranmer et al., 1972).
Immunology. Inasmuch as some compounds are antibiotic, it is
logical that some may be probiotic, that is, that they either reduce
resistance to infection or increase the virulence of an infecting
organism (Hayes, 1975). Far fewer studies have been made of probiosis
than of antibiosis. A number of papers have reported one or other
possibly probiotic property of DDT, but some of the reports could not
be confirmed, and others have not been retested.
It has been claimed that a change in the phagocytic activity of
white blood cells is an indication of early intoxication by DDT
(Kun'ev, 1965). However, Kaliser (1968) did not find any statistical
difference in in vitro or in vivo phagocytosis of control rats and
those receiving DDT by stomach tube at a rate of 0.25 (mg/kg)/day for
31 days. The highest rate at which men who make and formulate DDT in
the USA now absorb the compound is about 0.25 (mg/kg)/day.
Rats receiving an aqueous suspension of p,p'-DDT of unstated
stability at a concentration of 200 mg/litre as their only source of
water for over 30 days were reported to develop a lower titre of
antibodies to ovalbumen (Wassermann et al., 1969). Rabbits responded
to the same treatment with a statistically significant reduction of
antibody titre against Salmonella and a reduction in antibody titre
against sheep red blood cells that was not statistically significant
(Wassermann et al., 1971). Both the rats and rabbits showed a decrease
in at least one globulin fraction of the blood.
Other reports of changes in immunohaematological indices are those
of Semenceva (1968) and Fridman (1970).
One group of investigators has shown clearly that what at first
appeared to be an immunological response really involved a quite
different, predictable effect. Briefly, it was shown that guineapigs
sensitized to diphtheria toxoid were less susceptible to anaphylaxis
in response to a challenge dose of the toxoid if they were pretreated
with DDT at a dosage of only 1-20 mg/kg. Direct measurement of
antitoxin production indicated little or no difference between
protected and unprotected animals. Furthermore, some protection was
given by DDT administered for only 3 days prior to the induction of
anaphylaxis (Gabliks et al., 1973, 1975). Further studies showed that
DDT treatment reduced the histamine levels in the lungs of both
immunized and nonimmunized animals. The number of detectable mast
cells was also reduced; this was true whether the count was made in
tissues from guineapigs dosed systemically with DDT or in the lungs
and mesenteric tissue taken from untreated animals and exposed to DDT
in vitro at concentrations ranging from 10 to 45 mg/litre. These
results indicate that the protection offered by DDT was the result of
a reduction of the amount of histamine available for sudden release in
response to a challenge dose of toxoid (Askari & Gabliks, 1973).
Regardless of exposure to DDT, immunization leads to an increase in
detectable mast cells (Gabliks et al., 1975).
7.1.2 Nervous system and behaviour
DDT intoxication in animals was well described by Domenjoz (1944).
The first perceptible effect is abnormal susceptibility to fear, with
violent reaction to normally subthreshold stimuli. There is definite
motor unrest and increased frequency of spontaneous movements. As
poisoning increases, hyperirritability, like that seen in strychnine
poisoning develops, but convulsions do not appear at this time. A fine
tremor, recognizable at first only as a terror reaction, is later
present as all intention tremor in connexion with voluntary movement.
Then it is present intermittently without observable cause, and
finally it is present as a coarse tremor without interruption for as
long as several days. Spontaneous movement is limited, and food intake
stops so that surviving animals lose weight. In the later stages,
especially in some species, there are attacks of epileptiform, tonic-
clonic convulsions with opisthotonos.
All the signs are strengthened by external stimuli and become
manifest at first through external stimuli. At all stages, the animals
show normal position and labyrinth reflexes. The picture of poisoning
in mammals recalls the disturbances of movement and tone that are
known in human pathology as the amyostatic syndrome.
Symptoms appear several hours after oral administration of the
compound, and death follows after 24-72 h. The latent period after
intravenous administration at about the LD50 level is approximately
5 min; signs of poisoning reach a maximum level in about 30 min, and
survivors are symptom-free in 18 to 24 h. Animals that survive recover
completely.
In addition to the features of poisoning already mentioned,
Cameron & Burgess (1945) noticed that as rats, guineapigs, and rabbits
become sick, they become cold to the touch and show ruffled fur. Some
show diarrhoea. These authors found that muscular tremors were
preceded by muscular weakness that first occurred in the back and
later in the hind legs. The front legs were relatively spared so that
animals showing marked weakness of the hind quarters could still drag
themselves about. However, several authors have found that the tremor
characteristic of DDT poisoning generally starts in the muscles of the
face, including the eyelids, and spreads caudally with variable
severity until all the muscles are affected. Furthermore, although
weakness of hind quarters has been seen by others, it is not a common
finding.
Like tremor, coldness of the skin and ruffling of the fur probably
represent an indication of disturbed thermal regulation. Apparently it
was not until the work of Hrdina et al. (1975) that an increase of
almost 3°C in body temperature was reported in rats following a fatal
(600 mg/kg) oral dosage of DDT.
Although there is a general similarity in the clinical effects of
DDT in all vertebrate species, some characteristic differences exist.
Cats show greater extensor rigidity and opisthotonos than other
laboratory animals. The stiffness appears first in the distal part of
the extremities and later extends to the proximal part and to the
trunk. Poisoned cats show marked pilomotor activity. Convulsions in
them may become almost continuous. Convulsions are also prominent in
dogs as is ataxia. Tremors are so pronounced in rats that it may be
difficult to detect clonic convulsions in them. Rats poisoned by DDT
show a reddish colour about the eyes just as they do when ill from
many other causes. The colour has been attributed to excessive
secretion of a porphyrin by the Harderian glands.
Poisoning produced by repeated doses of DDT differs from that
produced by a single dose only in so far as the animal may be
gradually debilitated, especially by malnutrition. If food intake is
maintained, tremor may last for weeks, or even intermittently, for
months. If the animals survive a short time after dosing stops,
recovery is complete. However, food intake may be interfered with in
at least two ways. Tremor and more severe signs may interfere
mechanically with eating. Animals offered food containing high
concentrations of DDT often eat little or nothing and lose weight
rapidly. However, the same animals will show excellent appetites when
offered the same kind of food without DDT just after refusing the
major portion of the daily ration of contaminated food. Unlike
dieldrin and some other compounds, DDT seems to have little effect on
appetite as mediated by the central nervous system; it has a great
deal to do with taste.
Animals that have suffered severe weight loss as a result of DDT
poisoning may die partly as a result of general debility. In some
colonies, at least, they have become prey to secondary infection.
In summary, it may be said that animals that die as the result of
repeated large doses of DDT and small animals that die as a
complication of starvation following many somewhat smaller doses of
DDT show the same signs as those seen in animals killed by one or a
few large doses. Even though severely ill, animals that survive a few
days after the last of many doses of DDT recover.
Of samples that may be collected from a living animal, the
concentration of DDT in serum most accurately reflects its
concentration in the brain, the critical tissue. In the rat, levels of
25 mg/kg (wet weight) in the brain are not usually fatal: higher
levels tend to be fatal regardless of whether absorption followed one
or many doses (Dale et al., 1963; Hayes & Dale, 1964). As reviewed by
Hayes (1975), the danger level is approximately the same in several
species of birds.
Behavioural changes may be demonstrated in animals receiving DDT,
daily, at rates too low to produce illness. Khairy (1959) was able to
detect ataxia in the form of changes in gait in rats that had been fed
DDT at dietary levels of 100 mg/kg or more for 21 or more days. Gait
was recorded by smearing the hind paws of the animals with vaseline,
which then recorded their tracks on paper. Gait was recorded in terms
of the tangent, that is the ratio of the width and length of step. At
a body weight dosage of about 5 (mg/kg)/day the ratio was less than
normal, a change the author attributed to an exaggeration of the
stretch reflex. At dosages of about 10, 20, and 30 (mg/kg)/day, the
ratio was progressively greater than the normal as a result of
broadening of the gait and shortening of the steps. These same dosage
levels did not affect problem-solving behaviour or speed of
locomotion. The experimental animals were generally less reactive to
stress than normal ones. Thus, the author attributed hyperirritability
of rats poisoned by DDT to exaggerated motor responses.
The major toxic action of DDT is clearly on the nervous system,
and it requires an intact organism for full expression. The fact that
DDT causes a myotonic response in muscle and substitution of a train
of spikes for the normal diphasic electroneurogram (Eyzaguirre &
Lilienthal, 1949) is in marked contrast with the absence of detectable
injury or, in fact, any response in other isolated tissues. As early
as 1945, Lewis & Richards (1945) found DDT to be inert when it was
applied to tissue cultures of heart, kidney, stomach, intestine,
liver, and muscle from 7 to 9-day chick embryos, and of brain and
spleen from a one-day rat. The physiology of the cells including the
mytoses of fibroblasts was normal. The migration and extension of the
various cells was unchanged. The authors stated that "living
fibrilloblasts, as they moved about in the cultures, sometimes touched
or even migrated over DDT crystals without appreciable injury to
themselves during a period of several days". Some observations were
carried out for periods as long as 21 days.
In spite of the importance of the nervous system, a detailed
review of early literature indicates that, although the presence of
some specialized nervous function may be necessary for the
manifestation of DDT poisoning, the mere occurrence of specialized
nerve fibres in certain protozoa or the occurrence of a rather complex
nervous system in molluscs is not sufficient to render these forms
susceptible. Just as there is no explanation for the effect of DDT on
susceptible species, so there is no explanation for the fact that
certain species and even entire phyla are inherently resistant to the
compound.
A review (Hayes, 1959) of literature on the effects of DDT on the
nervous system reveals that all major parts, both central and
peripheral, are affected. Whereas effects on specific portions,
notably the cerebellum and the motor cortex, have been viewed as of
greatest importance, it probably is more accurate to emphasize the
interaction of functions, all modified to some degree.
There is reason to think that the mechanism of action of DDT is
its action on membranes in the nervous system, especially axonal
membranes. Certainly action on membranes is a fundamental property of
the compound. In fact the potassium conductance induced by valinomycin
at 10-6 mol/litre in a synthetic lecithin-decane membrane is
reversed by DDT at 3 × 10-6 mol/litre (Hilton & O'Brien, 1970).
Attention was focused quite early on the effects of DDT on axonal
membranes. Using the giant axons of the cockroach, Narahashi &
Yamasaki (1960) showed that DDT prolonged the recovery phase of the
action potential. They concluded that it slows the efflux of potassium
ions from the axon. Later, using the voltage clamp technique and giant
axons of the lobster, Narahashi & Haas (1967) showed that DDT, at a
concentration of 5 × 10-4 mol/litre of bathing medium, prolonged the
flow of sodium ions as well as interfering with the flow of potassioum
ions: in other words DDT delayed shutting of the Na+ gate and
prevented full opening of the K+ gate.
Na+-, K+-, and Mg2+-adenosine triphosphatase (EC 3.6.1.3) is
involved in ion transport in the nervous system. Matsumura & Patil
(1969) showed that a preparation of this enzyme from a nerve ending
fraction of the rabbit brain was inhibited by DDT at concentrations as
low as 10-8 mol/litre. There was a good correlation between the
degree of its inhibition by analogues of DDT and their toxicity to
mosquito larvae. A similar enzyme that binds 14C-DDT has been
isolated from the synapses of rat brain (Bratowski & Matsumura, 1972).
Both the electrophysiological changes and the enzyme inhibition
exhibit a negative temperature coefficient, an important feature of
DDT poisoning in insects but not in mammals (Hoffman & Lendle, 1948;
Deichmann et al., 1950).
At a supralethal dosage of 600 mg/kg, DDT caused a marked decrease
in the concentration of cortical and striatal acetylcholine and of
brainstem norepinephrine in rats and a significant increase in
brainstem 5-hydroxyindoleacetic acid. All of the neurotoxic signs of
poisoning were blocked by p-chlorophenylalanine, while other
inhibitors blocked one or other, but not all of the effects. It was
concluded that changes in the metabolism of 5-hydroxytryptamine and
norepinephrine might be responsible for DDT-induced hyperthermia while
acetylcholine might be related to tremors and convulsions (Hrdina et
al., 1973). These and related matters have been reviewed in great
detail by Hrdina et al. (1975). Apparently studies have not been made
at a range of dosages that would make it possible to know whether
these changes are a result or a cause of poisoning; the possible
therapeutic effect of p-chlorophenylalanine has also not been
explored.
It was reported by Haikina & Silina (1971) that administration of
DDT to rats at only one-fifth of the LD50 for the 2 days increased
the amount of 5-oxyindoleacetic acid excreted in urine by 188%. This
indicates a change in the metabolism of serotonin, but its
significance is not clear.
One sensitive measure of brain activity is the electroencephalo-
gram (EEG). Farkas et al. (1969) found that the EEG wave frequency
increased considerably in resting rats that had received
20 (mg/kg)/day of DDT as a result of dietary intake. Rats that had
received either 5 or 10 (mg/kg)/day did not exhibit this change while
at rest, but even those receiving 5 (mg/kg)/day exhibited this change
when exposed to a rhythmic light stimulus. The EEG may become abnormal
only a minute or two after administration of a large dose of DDT;
4 stages of the electrical activity culminating in generalized
seizures have been described by Joy (1973). Phenobarbital, but not
phenytoin or trimethadione, was effective in stopping the seizures.
7.1.2.1 Cause of death
Death from DDT poisoning is usually the result of respiratory
arrest. The heart continues to beat to the end and in some instances
continues a little while after respiration stops. Deichmann et al.
(1950) found that the onset of hyperirritability in rats was
accompanied by an increase in the frequency and amplitude of
respiration. Later, with the occurrence of tremors, the depth of
respiration frequently returned to a more normal level, but the rate
remained high. In some animals, respiration stopped suddenly after a
deep inspiration during a tonic convulsion. In other animals, the rate
and amplitude decreased progressively and finally ceased without any
terminal spasm. Animals that die of respiratory failure caused by DDT
do so after a relatively long period of muscular activity that leaves
them exhausted.
It was shown by Philips & Gilman (1946) and Philips et al. (1946)
that the hearts of dogs given large intravenous doses of DDT were
sensitized to epinephrine. This was true not only of injected
epinephrine but also of the compound released by the adrenal glands
during a seizure. Stimulated in this way, the sensitized hearts of
dogs developed an irreversible, fatal ventricular fibrillation.
However, the hearts of monkeys were able to recover from fibrillation
and resume normal rhythm. It is not clear how important sensitization
of the myocardium is when DDT is administered by other routes, but
ventricular fibrillation may be the cause of death in animals that die
suddenly, soon after onset of poisoning.
7.1.2.2 Treatment of poisoning in animals
Studies on the treatment of poisoning will be discussed in this
section since all the more successful studies of treatment of animals
poisoned by DDT involve the nervous system. Smith and Stohlman (1944)
noted the possibility that narcotics in general, may exhibit an
antagonism to DDT. Rats survived on a diet containing DDT at a
concentration of 1000 mg/kg for 90 days when they received
cyclohexanone in the same diet at the rate of 2000 mg/kg, but were
uniformly killed in a shorter period when they received DDT at the
same rate without cyclohexanone. Later, it was shown that
cyclohexanone offers no protection when used as a solvent for single
massive doses of DDT (Deichmann et al., 1950).
Smith & Stohlman (1945) showed that, when given as required after
the onset of illness, urethane and, to a lesser extent, phenytoin
sodium protected rats from poisoning. Sodium amobarbital gave slight
benefit, sodium phenobarbital a doubtful benefit, and paraldehyde no
protection at all. All drugs were given intraperitoneally except
paraldehyde, which was given by stomach tube. The mortality of rats
treated with urethane was 12.5% and that of their controls was 80%. A
total dosage of 1.2-2.5 mg/kg spread over a period of 1-3 days, was
found most satisfactory. Phenytoin sodium gave a mortality of 46.7%,
compared with 96.7% for the controls. The smallest effective dosage
was 200-250 mg/kg, a value very close to the LD50 which, under the
conditions of the test was 300 mg/kg.
Lauger et al. (1945a, b) also found that sodium phenobarbital was
of questionable value in treating rats poisoned by DDT. However,
completely different results were seen in larger animals.
Phenobarbital was, by far, the most outstanding remedy tested by
Philips & Gilman (1946). In a dosage well below the anaesthetic level,
it not only prevented death in many instances but also controlled
tremor and convulsions. Signs of illness were more readily controlled
in dogs and cats than in monkeys, which required nearly a full
anaesthetic dosage before tremors completely disappeared.
Magnesium sulfate did not reduce mortality in poisoned dogs and
cats although it did control tremors and convulsions briefly. Sodium
bromide was entirely ineffective. Mortality was reduced with urethane,
but a full anaesthetic dosage was required to control tremor and
convulsion. Similarly, sodium barbital and sodium pentobarbital
controlled symptoms only when given in full anaesthetic doses and,
even then, did not greatly reduce mortality. Phenytoin, when given to
rats before they received DDT, reduced the lethal action without
showing a notable effect on the signs of poisoning; phenytoin was not
effective in cats.
Vaz et al. (1945) were, apparently, the first to note the
antidotal effect of calcium in DDT poisoning. Dogs were given DDT
orally as a 10% oily solution at a daily dosage of 100 mg/kg until
signs of intoxication appeared. The same dosage could then be repeated
to produce intense symptomatology from which the animals would recover
spontaneously in 12-24 h. For the actual tests, a larger challenge
dosage of DDT (150 to 200 mg/kg) was used. Each dose of calcium
gluconate (30 ml of a 10% solution) was injected intravenously into
dogs weighing 8-18 kg. Dogs that were injected with calcium gluconate
dally for 4 days and, challenged with a large dose of DDT on the
fourth day, did not develop any symptoms or only slight ones. Dogs
receiving a single dose of calcium gluconate showed symptoms of short
duration and survived following a dosage of DDT large enough to kill 2
controls.
Cats poisoned by the intravenous injection of a soya-lecithin-corn
oil emulsion of DDT were studied by Koster (1947). A comparison was
made of several aspects of intoxication including number of
convulsions, general severity (tremors, prostration, dyspnoea),
duration, and mortality. Both calcium gluconate and sodium gluconate
reduced mortality but not severity. Gluconic acid increased the
survival time, reduced mortality, but did not reduce the number or
severity of the convulsions. Calcium chloride reduced convulsions, but
not mortality or tremors. Molecular equivalent doses of the candidate
antidotes were used. Gluconic acid and its two salts were effective
against an LD95 dosage of DDT. The life-saving capacity of calcium
gluconate at a rate of 40 mg/kg was confirmed by Judah (1949) even
though he found normal blood calcium values in most poisoned but
unmedicated animals. One animal showed a high calcium value, and
Cameron & Burgess (1945) reported a similar result. It has been
suggested that increased blood calcium may be associated with acidosis
caused by the accumulation of lactate.
Thus, calcium has an antidotal action against DDT in intact
animals of several species. The suppression by calcium of the effect
of DDT on the isolated nerve and muscle of the rat has been
demonstrated (Eyzaguirre & Lilienthal, 1949). The hypothesis has been
advanced (Welsh & Gordon, 1946; Gordon & Welsh, 1948) that certain
neurotoxins, including DDT, act by delaying the restoration of calcium
ions to a surface complex, following breaking of the chelate linkage
of calcium ions to surface polar groups by an initial exciting
impulse. This action of the neurotoxin is conceived as depending
largely on its physical rather than on its chemical properties. The
hypothesis is helpful in explaining the fact that a wide variety of
chemically unrelated compounds produce repetitive responses in
excitable tissue and also the fact that many compounds that show a
high toxicity for arthropods and mammals are fat-soluble and
relatively inert chemically. It has been pointed out that this
hypothesis postulates a very localized action of calcium at the nerve-
cell membrane; the hypothesis is not inconsistent with the finding
that the blood calcium of poisoned animals may be unchanged or even
increased.
Having observed the effect of DDT on the metabolism of glucose and
glycogen, Lauger et al. (1945a, b) investigated the use of glucose as
an antidote. All of 10 dogs given 2000 mg of DDT per kilogram body
weight orally in the form of an oil solution died within 8-24 h. Five
of 10 dogs treated with one or more, 20 ml doses of 20% glucose
survived the same dosage of DDT. The glucose was given intravenously
in most instances.
Koster (1947) found that glucose given before or after an LD33
dosage reduced convulsions and mortality and, when given before the
poison, reduced tremors, prostration, and dyspnoea in cats. Glucose,
unlike gluconic acid and its sodium and calcium salts, was ineffective
against an LD95 dosage except to increase the time of survival.
Insulin, given intra-muscularly 16-25 min before DDT, increased the
survival time and the severity of poisoning but did not affect
mortality or convulsions. When given 53-130 min before DDT, insulin
reduced convulsions in animals which died but increased convulsions,
tremors, and other disorders in the survivors.
The failure of amino and sulfhydryl compounds to influence the
action of DDT was noted by Von Oettingen & Sharpless, 1946). Likewise,
the addition of 0.2% choline chloride to the diet of rats receiving
repeated doses of DDT had no effect on the accumulation of lipids in
their liver (Sarett & Jandorf, 1947).
In summary, it would appear that sedatives, ionic calcium, and
glucose or other ready sources of energy are useful in treating
poisoning by DDT. Dogs and cats can be treated somewhat more
successfully than rats, perhaps because the metabolism of larger
animals is slower.
Although respiration may be temporarily restored in animals
poisoned by DDT, studies have not been made to determine whether
respiratory arrest in this condition is truly reversible as it is in
poisoning by certain organic phosphorus insecticides.
7.1.3 Renal system
No dysfunction of the renal system attributable to DDT has been
found even in animals receiving dosages sufficient to cause
dysfunction of the nervous system or striking morphological changes of
the liver. It is true that mild to moderate morphological changes have
been reported in the kidneys of animals that have received massive
single doses or repeated doses; for example, fatty degeneration,
necrosis, and calcification (Lillie et al., 1947; Stohlman & Lillie,
1948) or slight brown pigmentation of the convoluted tubular
epithelium (Fitzhugh & Nelson, 1947). However, it sometimes has
happened that a complete absence of change in the kidney has been
reported in connexion with other studies carried out in the same
laboratories (Lillie & Smith, 1944; Nelson et al., 1944).
7.1.4 Gastrointestinal tract, liver, and enzymes
Large doses of DDT produce vomiting in species that can vomit.
Only doses that produce rather severe poisoning lead to anorexia.
7.1.4.1 Liver
Large doses of DDT cause focal necrosis of liver cells in several
species (Lillie & Smith, 1944; Nelson et al., 1944; Cameron & Burgess,
1945; Lillie et al., 1947; Deichmann et al., 1950; Ortega et al.,
1956). At least some investigators (Cameron & Burgess, 1945) have
considered that the liver lesions produced by large dosages are
sufficient to account for death. All have agreed that necrotic lesions
occur only in connexion with potentially fatal dosages.
A very different kind of liver change is produced in some rodents
but not in other animals by small or moderate dosages of DDT. The
biochemical aspects of these changes are discussed in section 7.1.4.2,
while the morphological aspects are discussed in section 7.1.9.
7.1.4.2 Microsomal enzymes of the liver
All enzymes can be inhibited in vitro and many of them can be
inhibited in vivo. The toxic action of a number of compounds is
clearly the result of their inhibition of one or more enzymes. So far,
only a few enzymes or enzyme systems are known to be induced by
chemicals; the outstanding example is the induction of microsomal,
mixed-function enzymes of the liver and some other organs that are
produced in greater quantity in response to certain hormones and other
normal constituents of the body (Conney, 1967), some foods
(Wattenberg, 1971), or a wide range of drugs or other foreign
chemicals. Such induction requires intact cells and does not occur
when the inducer is brought in contact with the purified enzymes in
vitro. Microsomal enzymes were first recognized and studied in the
liver, but they are now known to exist, generally in lower
concentrations, in other tissues.
Microsomal enzymes are known to be associated with oxidation ( N-,
O-, and S-dealkylation, deamination, epoxidation, disulfuration,
hydroxylation) of both rings and side chains, oxidation of both
nitrogen and sulfur, reduction of nitro groups and of azo compounds,
hydrolysis, and conjugation. Most of the changes produced by
microsomal enzymes render oil-soluble compounds more water-soluble
and, therefore, more easily excreted. Mainly for this reason, most
biotransformations promoted by microsomal enzymes are true
detoxications. However, some of the reactions promoted by this system
of enzymes render specific compounds more toxic.
In the rat, DDT has been shown to promote the biotransformation of
hexobarbital (Hart & Fouts, 1963), phenazone (Hart & Fouts, 1963;
Kinoshita et al., 1966), p-nitrobenzoic acid (Hart & Fouts, 1963),
aniline (Hart & Fouts, 1963), dieldrin (Street, 1964; Street et al.,
1966; Street & Chadwick, 1967; Pearl & Kupfer, 1971), o-ethyl o-4-
nitrophenyl phenylphosphonothioate (EPN) (Kinoshita et al., 1966),
p-nitroanisole (Kinoshita et al., 1966; Vainio, 1974), methyprylon
(Datta & Nelson, 1968), meprobamate (Datta & Nelson, 1968),
chlordizepoxide (Datta & Nelson, 1968), aldrin (Gillett, 1968),
lindane (Chadwick et al., 1971b), phenylbutazone (Welch & Harrison,
1966), pentobarbital (Fredricks et al., 1974), 3,4-benzpyrene (Vainio,
1974), and p-nitrophenol. The biotransformation of p-nitrophenol
involves conjugation by the microsomal enzyme uridinediphospho-
glucuronyltransferase, and its demonstration requires activation of
the microsomes, for example by trypsin (Vainio, 1974, 1975; Rantanen et
al., 1975).
In the mouse, DDT has been shown to promote the biotransformation
of pentobarbital (Gabliks & Maltby-Askari, 1970).
In the guineapig, DDT promoted the metabolism of dieldrin
(Wagstaff & Street, 1970).
DDT in the squirrel monkey promoted the metabolism of EPN and
p-nitroanisole; the first required a DDT dosage of 5.0 (mg/kg)/day, but
the latter required only 0.5 (mg/kg)/day (Cranmer et al., 1972). DDT
did not promote the metabolism of 14C-DDT in squirrel monkeys
(Chadwick et al., 1971a) but did promote the metabolism of
phenylbutazone in the dog (Welch & Harrison, 1966) and the metabolism
of estradiol in the pigeon (Peakall, 1970).
In the chicken, DDT failed to affect N-demethylase or the
concentration of cytochrome P-450, and reduced aniline hydroxylase
activity. The influences of dosage, duration of dosing species, and
reproductive state on microsomal enzymes in birds are poorly
understood (Sell et al., 1971).
o,p'-TDE has been shown to promote the metabolism of
pentobarbital in the mouse (Gabliks & Maltby-Askari, 1970),
phenobarbital in the rat (Straw et al., 1965), and cortisol in the
guineapig (Kupfer et al., 1964).
The metabolism of DDT is promoted by DDT itself in the hamster but
not in the mouse (Gingell & Wallcave, 1974).
In rats, the metabolism of DDT is promoted by phenytoin (Cranmer,
1970). The metabolism of DDT and DDE in cows is promoted by
phenobarbital (Alary et al., 1971; Fries et al., 1971).
DDE, whether fed directly or produced metabolically from DDT,
appears to be more important than DDT in inducing microsomal enzymes.
The tissue level of DDE necessary for enzyme induction is lower in the
rat than in the quail (and presumably other birds). Thus Bunyan et al.
(1972), using residues in the heart as an index, found a maximum
increase in cyto-chrome P-450 per gram of liver and a maximum activity
of aniline hydroxylase levels at DDE levels of approximately 3 mg/kg
in rats and 40 mg/kg in quail. However, at any given dietary level,
higher tissue levels were reached by quail than by rats so that the
dosage responses of the two were similar.
Different inducers may activate different enzymes and, therefore,
different metabolic pathways. Pretreatment of rats with lindane caused
them to metabolize a single dose of radioactive lindane 2.5 times more
efficiently than controls, whereas pretreatment with DDT caused a
3.5-fold increase in the metabolism of lindane. Furthermore, the DDT
pretreatment was followed by a different proportion of radioactive
metabolites with a predominance of tetrachlorophenols, especially
2,3,4,5-tetrachlorophenol (Chadwick et al., 1971b).
The enzymes of weanling rats are more subject to induction than
those of adult rats, but there is no evidence of a lag-period in
induction in adults (Chadwick et al., 1975).
7.1.4.3 Enzymes of intermediary metabolism
As discussed in section 7.1.2, there is some reason to think that
DDT acts by influencing an enzyme critical to the function of
neurones. It is certainly clear that many of the side-effects of DDT
are the result of its induction of microsomal enzymes (see sections
7.1.4.2 and 7.1.9). In addition, DDT has been shown in vitro and
sometimes in vivo to influence some enzymes of intermediary
metabolism and other miscellaneous enzymes. So far, evidence is
lacking that the degree of this inhibition in the intact organism is
sufficient to have any influence on function.
The hyperglycaemia observed during much of the early part of acute
poisoning may be associated with an increase in 4 gluconeogenic
enzymes (pyruvate carboxylase (EC 6.4.1.1), phosphoenolpyruvate
carboxykinase (EC 4.1.1.32), fructose 1,6-diphosphatase, and glucose
6-phosphatase (EC 3.1.3.9)). Increases in these enzymes in the renal
cortex of rats, observed after a single dose at a rate as low as
100 mg/kg, were greater at a dose of 600 mg/kg. The reaction occurred
in both adrenalectomized and normal rats (Kacew & Singhal, 1972).
Similar responses were observed in the same enzymes in the liver
following a single dose at the rate of 100 mg/kg or more or following
45 daily doses at rates of 5 or 25 (mg/kg)/day. The changes are not
mediated through a release of corticosteroids from the adrenal glands
(Kacew & Singhal, 1973). The same authors (Hrdina et al., 1975;
Singhal & Kacew, 1976) have reviewed the extensive evidence
contributed mainly by themselves indicating that the changes in
glucose homeostasis are mediated by stimulation of the cyclic
adenosine monophosphate (AMP)-adenylate cyclase system in the liver
and in the kidney cortex by p,p'-DDT and a number of other organic
chlorine pesticides. However, the smallest single dosage of p,p'-DDT
that produced a statistically significant change in the enzymes or in
the production of cyclic AMP was 180 mg/kg and o,p'-DDT was as
effective as the p,p'-isomer; these findings indicate that the
carbohydrate changes are results not causes of poisoning.
DDT and some other compounds induce increased activity of
D-glucuronolacetone dehydrogenase (EC 1.1.1.70) in the supernatant
fraction of rat liver, and this is consistent with evidence of
increased D-glucaric acid in urine after dog treatment (Marselos &
Hanninen, 1974). Urinary excretion of L-ascorbic acid is also
increased by DDT, but apparently this is not caused by an increase in
an enzyme producing this acid but rather by an increase in several
microsomal and cytosol enzymes that contribute to an increase in free
glucuronic acid from which L-ascorbic acid is formed (Rantanen et al.,
1975).
A review (Hayes, 1959) of early literature indicates that high
concentrations of DDT inhibit phosphatidase (EC 3.1.1.4), muscle
phosphatases, carbonic anhydrase (EC 4.2.1.1), oxalacetic carboxylase
(EC 4.1.1.3), and increase the activity of cytochrome oxidase
(EC 1.9.3.1) and succinc dehydrogenase (EC 1.3.99.1). However, none of
these changes with the possible exception of inhibition of carbonic
anhydrase could be shown to have any connexion with the toxic action
of DDT or even with its side-effects. Neal et al. (1944) reported a
small but consistent increase in the volume of urine excreted in 24 h
when dogs were dosed orally or by insufflation at the rate of 100
(mg/kg)/day. No other change in the urine and no change in kidney
function was demonstrated. The possibility that increased urinary
output is related to the inhibition of carbonic anhydrase (Torda &
Wolff, 1949) may deserve attention. However, re-examination of data
from volunteers receiving 3.5 or 35 mg/man per day did not indicate
any increase in urinary volume compared with controls (Hayes et al.,
1971).
It has been claimed (Keller, 1952) that DDT inhibits carbonic
anhydrase in bovine erthrocytes. However, the method was criticized by
Dvorchik et al. (1971), who found in vitro inhibition only by
concentrations of DDT, unlikely to be survived by living animals. Far
more attention has been given to inhibition of carbonic anhydrase in
the shell gland of birds than in erythrocytes, and it has been
suggested (Bitman et al., 1970; Peakall, 1970) that inhibition of the
shell gland enzyme is an explanation for eggshell thinning in certain
birds. However, the same criticism holds for the shell gland; there is
no evidence that the degree of inhibition reported interferes with
function.
On the other hand, many enzymes including plasma amylase, aldolase
(EC 4.1.2.13), glutamic-pyruvic transaminase (EC 2.6.1.2), and
isocitric dehydrogenase (EC 1.1.1.41) were not changed in squirrel
monkeys given dosages ranging from 0.05 to 50 (mg/kg)/day; the highest
dosage proved fatal within 14 weeks (Cranmer et al., 1972).
7.1.5 Cardiovascular system
Most dogs, killed by a single dose of DDT, die of ventricular
fibrillation, and the same is true of some cats, monkeys, and rabbits
(Philips & Gilman, 1946). At any given dosage of DDT, ventricular
fibrillation is more likely to occur if the animal receives exogenous
epinephrine or is stimulated in such a way that the compound is
released by the adrenals. Besides fibrillation, dogs may exhibit
extrasystoles and changes in the T-wave (Philips et al., 1946).
Monkeys differ from dogs in that the DDT-sensitized heart is able to
recover from fibrillation and resume a normal rhythm (Philips et al.,
1946).
Thus, DDT not only sensitizes the myocardium in a way similar to
that of halogenated hydrocarbon solvents, but, through its action on
the central nervous system, produces the stimulus that increases the
likelihood of fibrillation.
There is no evidence that repeated, tolerated doses of DDT
sensitize the heart. Rats were fed DDT at a dietary level of about
10 (mg/kg)/day for 8 months, during which time they received weekly,
intraperitoneal doses of vasopressin, a compound that causes a
temporary myocardial ischaemia. Electrocardiograms did not show any
significant increase in cardiac arrhythmias in the DDT-fed rats
compared with controls. Intravenous noradrenalin given at the end of
the 8-month period did not produce a greater incidence of arrhythmias
in the DDT-fed rats. The same results were obtained in rabbits treated
in essentially the same way (Jeyaratnam & Forshaw, 1974).
TDE. The main action of TDE, and especially of o,p'-TDE, is on
the liver with secondary effects on the adrenals in dogs and perhaps
in other species. However, Cueto (1970) showed that at a dosage of
50 (mg/kg)/day for 14 days, o,p'-TDE caused a gradually progressive
hypotensive failure in dogs injected with epinephrine or
norepinephrine, while leaving the cardioaccelerator and immediate
pressor response to these drugs unchanged. The hypotensive failure was
associated with weakening of the contractile force of the heart and
with a reduction of plasma volume. The latter may have been caused by
a loss of fluid from the intravascular compartment and was not caused
by a release of histamine. The hypotensive state could be prevented to
a significant degree by pretreatment with prednisolone.
7.1.6 Respiratory system
The effects of DDT on the respiratory system are secondary to
effects on the nervous system and are discussed in section 7.1.2.1.
7.1.7 Reproductive system
It was shown very early (Burlington & Linderman, 1950) that DDT
produces a striking inhibition of testicular growth and secondary
sexual characteristics of cockerels, when injected subcutaneously in
dosages as high as 300 (mg/kg)/day. Changes in the testis involve the
tubules, and not the interstitial tissues, and they have been
attributed to an estrogen-like action of DDT.
It must be noted that the action of DDT on the testis of the
chicken is dosage-related. Before the problem of residues became
evident, DDT was used extensively for control of lice and common mites
on chickens without any adverse effects on egg production or other
aspects of reproduction. Many rats would be killed the first day if
they were given the dosage of DDT that has been shown to affect the
testis in cockerels. The report that, under special conditions, DDT
has it gonadotoxic effect (Rybakova, 1968) is of questionable
significance in view of the results of multigeneration tests in rats,
mice, and dogs.
Ottoboni (1969) found that female rats reproduced normally when
fed DDT for two generations at dietary levels as high as 200 mg/kg
(about 10 (mg/kg)/day, except during lactation when intake was
increased about 3-fold). In fact, at a dietary level of 20 mg/kg, the
dams had a significantly longer reproductive life span (14.55 months)
than did their littermate controls (8.91 months); the number of
females becoming pregnant after the age of 17 months and the number of
successful pregnancies after that age differed significantly between
the two groups (Ottoboni, 1972).
In a study focused mainly on DDT in milk, the ability of rats to
reproduce at a dietary level of 200 mg/kg was confirmed, and the
ability of dams, injected intraperitoneally at levels as high as
100 (mg/kg)/day, to rear their young was demonstrated (Hayes, 1976b).
A six-generation test of reproduction in mice did not show any
effect of DDT at a dietary level of 25 mg/kg on fertility, gestation,
viability, lactation, and survival. A dietary level of 100 mg/kg
produced a slight reduction in lactation and survival in some
generations but not all, and the effect was not progressive. A level
of 250 mg/kg was distinctly injurious to reproduction (Keplinger et
al., 1970). The dietary concentrations used equalled dosages of 3.33,
13.3 and 33.2 (mg/kg)/day in nonpregnant, non-lactating, adult female
mice. The intake is much higher in both young and lactating mice. The
authors concluded that their study provided no obvious reason for
continuing reproduction tests for more than three generations.
Four female dogs of unstated age that had previously received DDT
at the rate of 12 (mg/kg)/day, 5 days a week, for 14 months were mated
when they went into heat. The males involved had been fed aldrin
(0.15 (mg/kg)/day) plus DDT (60(mg/kg)/day) for 14 months prior to
breeding but not during breeding. Two of the females went into heat
but failed to become pregnant, and one failed to come into heat during
12 months after feeding stopped. Four of 6 pups born to the fourth
female died within one week of birth; the other 2 were weaned
successfully even though only 2 posterior mammae of the mother were
functional (Deichmann et al., 1971b). A 3-generation study failed to
confirm any of the injuries suggested by the study of 4 dogs. In the
3-generation study, male and female dogs were fed technical DDT from
weaning at rates of 0, 1, 5, and 10 (mg/kg)/day. Observations were
made on 135 adult females, 63 adult males and 650 pups. There were no
statistically significant differences between controls and DDT-treated
dogs in length of gestation, fertility, success of pregnancy, litter
size, lactation ability of the dams, viability at birth, survival to
weaning, sex distribution, growth of pups, morbidity, mortality,
organ/body weight ratios, or gross histological abnormalities in all
the animals studied. The only clear difference was that DDT-treated
females had their first estrous 2 or 3 months earlier than the control
animals. There was a slight increase in liver/body weight ratio in
some DDT-treated animals but the difference was not statistically
significant, dosage related, or associated with any histological
change (Ottoboni et al., 1977).
o,p'-DDT. Intraperitoneal injection of technical DDT at a dosage
as low as 5 mg/kg or of o,p'-DDT at 1 mg/kg caused a significant
increase in the weight of the uterus of normal, immature female rats
or of ovariectomized adult females. Stimulation caused by p,p'-DDT
was much less. Treatment of rats with DDT, especially o,p'-DDT, 2 h
before injection of estradiol-17-6,7-3H inhibited uptake of the
hormone by the uterus in vivo, possibly by competition for binding
sites. Isomers of TDE and DDE do not influence uterine weight or the
binding of estradiol (Welch et al., 1969). It seems unlikely that
metabolic activation of o,p'-DDT is necessary, as is true of o,p'-
methoxychlor. The binding and estrogenic activity of DDT analogues in
rats is only about 1/10 000 as great as that of diethylstilbesterol
(Nelson, 1973).
A considerably smaller dosage of o,p'-DDT resulting from a
dietary level of 10 mg/kg for 2-9 months did not have any effect on
reproduction in ewes (Wrenn et al., 197 lb). In a similar way, dietary
levels of o,p'-DDT as high as 40 mg/kg, giving a dosage level of
about 2.1 (mg/kg)/day in rats, failed to interfere with reproduction
and lactation in these animals although dosage was continued
throughout 2 pregnancies (Wrenn et al., 1971a).
The report (Heinrichs et al., 1971) that o,p'-DDT significantly
advances puberty, induces persistent vaginal estrus after a period of
normal estrus cycles, and causes other reproductive abnormalities in
female rats would appear at first to be inconsistent with the lack of
effect of technical DDT or of o,p'-DDT on reproduction cited above.
The same is true of other effects of o,p'-DDT demonstrated by the
same investigators (Gellert et al., 1972). The abnormal effects were
obtained initially by injecting 1 mg of the o,p'-DDT subcutaneously
on the second, third, and fourth days of life (counting the day of
birth as zero). Because rat pups on the third day weigh about 12 g or
less each, it follows that the subcutaneous dosage was about
83.3 (mg/kg)/day or more, that is about 40 times greater than the
highest oral dosage of o,p'-isomer fed to breeding rats and about
105 times greater than the levels ingested by the general population
with their food.
Because of its estrogenic properties, DDT was considered as a
possible cause of abortion in dairy cattle, but no evidence of a
relationship was found (Macklin & Bibelin, 1971).
7.1.8 Endocrine organs
Except for the weak estrogenic properties of o,p'-DDT, the
endocrine-related effects of DDT and its analogues are confined to the
adrenals, and even these effects of 2 isomers of TDE are now
considered to be mainly secondary to the induction of microsomal
enzymes of the liver in most species.
TDE. TDE (DDD) is an insecticide in its own right as well as a
metabolite of DDT. The compound is used as a drug to control different
forms of adrenal overproduction of corticoids in man (see section
8.2.8). This therapy was originally based on the demonstration that
DDD (Nelson & Woodard, 1948, 1949) and especially o,p'-TDE (Cueto &
Brown, 1958) caused gross atrophy of the adrenals and degeneration of
the cells of its inner cortex in dogs. This is true even though it was
originally reported (Nelson & Woodard, 1948, 1949) that TDE produced
almost no detectable damage to the adrenals of rats, mice, rabbits,
and monkeys, and the finding was confirmed and extended by other
investigators to other species, including man (Zimmerman et al.,
1956). In the dog, o,p'-TDE produced gross atrophy of the adrenals,
when administered at a dosage of only 4 (mg/kg)/day. The dosage of
technical grade TDE required to produce the same effect was
50-200 (mg/kg)/day (Cueto & Brown, 1958). However, in spite of its
exceptional susceptibility, there is a definite threshold below which
the dog does not respond. About 15% of technical DDT is o,p'-isomer,
much of which is gradually metabolized to o,p'-TDE ( o,p'-DDD). Yet
dogs remained healthy and reproduced normally in a 3-generation study
involving dosages of technical DDT as high as 10 (mg/kg)/day (see
section 7.1.7).
It has recently been shown that, following massive dosage
(60 mg/kg, administered intravenously), all of the isomers of TDE
inhibited ACTH-induced steroid production in the dog, but the
inhibition reached 50% of the control only 27 min after dosing with
the m,p'-isomer compared with 87 min with the o,p'-isomer and
4-18 h with the p,p'-isomer. There was marked temporal correlation
between the percentage inhibition of adenocorticotropic hormone
(ACTH)-induced steroid production, the disruption of normal cellular
structure of the fascicular and reticular zones of the adrenal cortex,
and the severity of the damage to mitochondria in these zones caused
by the 3 isomers (Hart et al., 1973). The effectiveness of m,p'-TDE
for treating metastatic adrenocortical carcinoma had already been
demonstrated (Nichols et al., 1961), but it cannot be said that its
value for this purpose has been compared adequately with that of
o,p'-TDE. Furthermore, no effort has apparently been made to compare
the effect of small daily doses of the 2 isomers in dogs.
Like other organochlorine insecticides, o,p'-TDE stimulates
hepatic microsomal oxygenation of both drugs and steroids and,
according to a very thorough review by Kupfer (1967), this may explain
much of its action on corticoid metabolism in a wide range of species.
Increased breakdown is indicated by increased excretion of polar
metabolites, while nonpolar metabolites remain stable or even decrease
-- a finding recently encountered in human patients (Hellman et al.,
1973). However, the demonstrated effect on corticoid metabolism fails
to explain why o,p'- and m,p'-TDE are unique in their overall
effects on the adrenals, including their ability to produce
adrenocortical atrophy in the dog. Other powerful inducers of
microsomal enzymes lack these effects. It is clear that a reduction of
steroid production accompanies atrophy of the adrenals of the dog. The
review already cited (Kupfer, 1967) considers: (a) reduced steroid
production in species other than the dog, including the possibility
that such reduction is secondary to inhibition of glucose-6-phosphate-
dehydrogenase (EC 1.1.1.49) activity in the adrenals and (b) blockage
of steroid action by asteroid metabolite formed under the influence of
DDD. However, the existence of these effects, much less their
importance, remains obscure.
o,p'-DDT. Oral administration of o,p'-DDT to dogs at a rate of
50 (mg/kg)/day stimulated the microsomal enzymes of the liver as
indicated by increase in liver size, total protein, microsomal
protein, and cytochrome P-450 concentration and by direct measurements
of enzyme activity. These changes in the liver were accompanied,
initially, by an increase in the size of the adrenals and of the cells
of the zona fasciculata; these cells became vacuolated and devoid of
acidophilic cytoplasm, and their nuclei became hyperchromatic and
often peripheral in position. Synthesis of corticosteroids by the
adrenal was not blocked (Copeland & Cranmer, 1974). Thus the effect of
a substantial dosage of o,p'-DDT was quite different from that of
o,p'-TDE (DDE), although part of the metabolism of o,p'-DDT must
be by the same route.
7.1.9 Carcinogenicity
There is no doubt that DDT and a number of other organochlorine
insecticides cause marked changes in the liver in various rodents and
that these changes progress to tumour formation in some species,
notably the mouse. There is serious disagreement as to whether the
mouse tumours are malignant. Regardless of their nature, there is
virtual certainty that they are peculiar to rodents and, therefore,
interpretation of their significance for man or useful animals is
difficult.
Evidence for the carcinogenicity of DDT and its metabolites has
been reviewed by the International Agency for Research on Cancer
(IARC, 1974). Most of the experimental results are summarized in Table
17. The conclusions of the IARC were that: (a) the hepatocarcino-
genicity of DDT administered by the oral route has been demonstrated in
several strains of mice, and shows a dosage-response relationship; (b)
a dietary level of 2 mg/kg (above 0.3 (mg/kg)/day) produces a
significant increase in hepatomas in male but not in female CFl mice
and not in either sex of BALB/c mice; (c) increased incidence of
tumours has been reported in some other organs of mice but not
confirmed in multigeneration studies using a wide range of dosages;
(d) evidence for the carcinogenicity of DDT in rats is not convincing
and is negative in hamsters even at the higher dietary levels that
they tolerate in comparison with rats and mice; (e) negative results
in dogs and monkeys are inconclusive because of the small groups
studied and the short duration of treatment; (f) liver cell tumour
induction in trout is inconclusive because of a lack in control of the
diet; and (g) the carcinogenicity of p,p'-DDE is similar to that of
DDT, but TDE produces a significant incidence of lung tumours.
Actually, the number of dogs and monkeys was not small compared
with similar studies on other chemicals. In an investigation on
monkeys, dosing at lower levels was continued for 7.5 years. In both
dogs and monkeys, dosages sufficient to cause liver damage, death, and
neurological indications of DDT poisoning were included in the
protocols (see Table 17). Apparently no new studies on dogs and
monkeys have been reported. On the other hand, a new study on rats
(Rossi et al., 1977) has given definite evidence of the tumorigenicity
of DDT and phenobarbital, confirming the conclusion of Fitzhugh &
Nelson (1947) which was based on less extensive data.
In mice, liver tumours similar to those caused by DDT (see
Table 17) have been reported in connexion with DDE and DDD (Tomatis &
Turusov, 1975), chlorobenzilate, HCH, aldrin, dieldrin, mirex, and
terpene polychlorinates (Tomatis et al., 1973). Similar tumours have
been caused by the important drug, phenobarbital (Wright et al., 1972;
Peraino et al., 1973; Thorpe & Walker, 1973; Ponomarkov et al., 1976).
TDE also caused lung tumours (Tomatis et al., 1974a).
The IARC review did not discuss the controversy over the nature of
the tumours induced by DDT in the livers of some rodents, and it did
not consider the relationship of these tumours to the induction of
microsomal enzymes. The following paragraphs are concerned with these
matters and specifically with those inducers that behave like DDT. The
biochemical pattern of induction of mixed function oxidase enzymes is
similar for DDT and phenobarbital but distinctly different for
3-methylcholanthrene (Vainio, 1975). Thus 3-methylcholanthrene and
compounds like it must be excluded from the discussion. Similarly, the
high degree of correlation between the ability of compounds to induce
parenchymal liver tumours in mice and their ability to induce tumours
in the liver and other organs of rats and hamsters cannot be accepted
uncritically. As demonstrated in a study by Tomatis et al. (1973),
this correlation is extremely good for compounds that are, or are
suspected of being, carcinogens in man. However, the correlation is
poor for organochlorine insecticides. In fact, the only one of these
compounds that has increased the incidence of a tumour in another
species is DDT, which induced liver tumours in rats in one experiment.
The early morphological response of the rodent liver to DDT is
similar to its response to moderate dosages of HCH, chlordane,
dieldrin, camphechlor (Toxaphene) (Lehman, 1952; Ortega et al., 1956),
and the important drug, phenobarbital (Wright et al., 1972; Thorpe &
Walker, 1973). The earliest change involves so much increase in the
smooth endoplasmic reticulum of individual liver cells that they
enlarge, and the large granules that are ordinarily scattered
throughout the cytoplasm are displaced to the periphery of the
affected cell. Quite early, some of the endoplasmic reticulum forms
whorls that may have fat droplets as their centres -- thus justifying
the term "lipospheres", applied to them by Ortega et al. (1956).
Others have referred to these inclusions as "hyaline oxyphil masses"
(Lillie & Smith, 1944) or "lamellar bodies" (Ito et al., 1973). These
changes are accompanied by some increase in fat droplets, not all of
which become surrounded by endoplasmic reticulum. This combination of
changes (hypertrophy, margination, and lipospheres) is characteristic
of rodents and of compounds that induce microsomal enzymes. Certain
other changes have been reported but not confirmed. These include
enlargement and morphological changes of the mitochondria (Obuchowska
& Pawlowska-Tochman, 1973; Watari, 1973), increased numbers of primary
lysosomes, and atrophy of the Golgi body (Watari, 1973), none of which
were found by Ortega (1966).
Table 17. Effect on various animals of repeated oral doses of DDT
Dosage Species Duration Results Reference
Range Method and Animals Mortality Other
(mg/kg)/day concentration per test (%)
(No.)
41-80 800 mg/kg in diet rata 2 years 36 males, 24 females Increased mortality, Fitzhugh & Nelson, 1947
typical liver changes,
liver tumours
46 mg/kg then mouseb 18 months 36 males, 36 females Hepatomas in 51 and Innes et al., 1969
140 mg/kg in diet 21% of males and females
respectively compared with
18 and 0.6% of controls
3200 mg/kg in diet dog 39-49 10 100 Liver damage, no Lehman, 1965
months tumours
5000 mg/kg in diet monkey 70 days 1 male 100 Fatal poisoning Durham et al., 1963
21-40 400 mg/kg in diet rata 2 years 24 males, 12 females Increased mortality, Fitzhugh & Nelson, 1947
typical liver changes
500 mg/kg in diet rat 2.9 years 37 males, 35 females Liver tumours in 45% Rossi et al., 1977
250 mg/kg in diet mousec 2 103 males, 90 females Risk of liver tumour Tomatis et al., 1972
generations increased 3.7 and 18.5
times in males and females,
respectively
250 mg/kg in diet mousec 2 31 males, 121 females Liver tumours in 48 Terracini et al., 1973
generations and 59% of males and
females, respectively
2000 mg/kg in diet dog 39-49 4 25 Minor liver damage Lehman, 1965
months but no tumours
Table 17 (Cont'd)
Dosage Species Duration Results Reference
Range Method and Animals Mortality Other
(mg/kg)/day concentration per test (%)
(No.)
11-20 100 mg/kg in diet moused 2 years 100 males, 100 females Hepatomas increased Fitzhugh, 1970
in females of one strain
but no increase in
hepatocarcinomas
100 mg/kg in diet mousec 2 years 30 males, 30 females Risk of liver tumours Walker et al., 1973
increased 4.4 times
100 mg/kg in diet mousec 2 years 30 males, 3 females Risk of liver tumours Thorpe & Walker, 1973
increased 3.3 and 4.2 times
in males and females
respectively
5-10 50 mg/kg in diet mousec 2 127 males, 104 females Risk of liver tumours Tomatis et al., 1972
generations increased 2.45 and 3.46
times in males and females,
respectively
50 mg/kg in diet mousec 2 years 30 males, 30 females Risk of liver tumours Walker et al., 1973
increased 2.9 times
400 mg/kg in diet dog 39-49 2 0 No effect Lehman, 1965
months
Table 17 (Cont'd)
Dosage Species Duration Results Reference
Range Method and Animals Mortality Other
(mg/kg)/day concentration per test (%)
(No.)
2.6-5 20 mg/kg in diet mousee 2 48 males, 128 females No increase in tumours Terracini et al., 1973
generations
200 mg/kg in diet monkey 3-7.5 5 males, 5 females No toxic effect Durham et al., 1963
years
1.26-2.5 10 mg/kg in diet mousec 2 104 males, 124 females Risk of liver tumour Tomatis et al., 1972
generations increased 2.26 and 2.46
times in males and females,
respectively
0.626-1.25 25 mg/kg in diet rat 2 years No clinical effect; Treon & Cleveland, 1955
males survived
longer than controls
50 mg/kg in diet monkey 1.6 years 4 males, 1 female No toxic effect Durham et al., 1963
0.3126- 10 mg/kg in diet rat 2 years Typical liver changes; Fitzhugh, 1948
0.625 no effect on
reproduction
12.5 mg/kg in diet rat 2 years No effect Treon & Cleveland, 1955
2.8-3.0 mg/kg in mousee 5 683 Tumours in 28.7% in Tarjan & Kemeny, 1969
diet generations controlsf
0.15626- 2 mg/kg in diet mousee 2 124 males, 111 females Risk of liver tumour Tomatis et al., 1972
0.3125 generations doubled in males,
unchanged in females
2 mg/kg in diet mousee 2 58 males, 135 females No increase in tumours Terracini et al., 1973
generations
Table 17 (Cont'd)
Dosage Species Duration Results Reference
Range Method and Animals Mortality Other
(mg/kg)/day concentration per test (%)
(No.)
0.078126- 2.5 mg/kg in diet rat 2 years No effect Treon & Cleveland, 1955
0.15625 5 mg/kg in diet monkey 1.4-7.5 5 males No toxic effect Durham et al., 1963
years
a Osborne-Mendel. e BALB/c mice.
b Both (C57BL/6 × C3H/Anf) FI and (C57BL/6 × AKR) FI mice. f Lung carcinoma in 16.9% to 1.2% in controls; lymphomas 4.8% compared to
c CFI mice. 1.0% in controls; leukaemias 12.4% and 2.6%; other tumours 5.8% and 1.0%,
d BALB/cJ and C3HeB/Fe J. respectively.
The characteristic changes develop promptly. An increase in smooth
endoplasmic reticulum and the appearance of lamellar structures have
been seen as early as 4 and 7 days, respectively, after the beginning
of dosing (Wright et al., 1972).
Although microsomal enzymes may be induced in other species,
morphological changes in the liver, as viewed by the light microscope
are not the same (Laug et al., 1950; Lehman, 1952; Ortega et al.,
1956), or occur to lesser degree as viewed by the electron microscope
(Wright et al., 1972).
The changes in liver cells that characterize the induction of
microsomal enzymes in rodents are distinct from the focal necrosis
that may be produced with about the same ease in the livers of rodents
or other species by fatal or near fatal dosages of organochlorine
insecticides. The necrotic lesions do not progress because, if such
high dosages are continued the animal dies, and, if dosing is stopped
and the animal survives, the necrotic cells are removed by autolysis
and phagocytic action and the lesions usually heal without scarring
(Cameron & Burgess, 1945). Nevertheless, some scarring was found by
Lillie & Smith (1944) and Lillie et al. (1947).
At least in the early stages, the changes in liver cells that
characterize induction of microsomal enzymes in rodents are reversible
(Fitzhugh & Nelson, 1947; Ortega et al., 1956; Wright et al., 1972).
The reversibility does not depend on cell removal but simply on
reversion of the physiological and morphological condition of the
cells to their original condition.
Of course, reversibility is incompatible with progression, but
whether observed irreversibility will be associated with progression
must be determined directly in each instance. In the following
paragraphs, the question of progression is discussed only after
consideration of the problem of irreversibility in general.
If dosing with organochlorine insecticides or other inducers is
continued long enough and at a sufficiently high level, the liver
changes become irreversible, if for no other reason than that the
remaining life span of the animals is too short to permit excretion of
the inducing chemical or complete reversion of the liver cells to
their original state. The stage at which this shift to irreversibility
occurs remains unknown, but it seems very likely that dosages
sufficient to produce irreversible morphological change also exceed
the physiological adaptability of the liver. The important distinction
between adaptation and injury in relation to enzyme induction has been
studied using dieldrin.
Although some persons have tended to view even moderate
enlargement of the liver or of individual liver cells as an injury,
the evidence is strong that these changes are usually adaptive and
beneficial to the organism, when they are the result of an increase of
smooth endoplasmic reticulum and an associated increase in the
activity of liver microsomal enzymes (Barka & Popper, 1967). However,
it is obvious that any stimulus or effect may be harmful, if
excessive. Hutterer et al. (1968) demonstrated that a distinction may
be drawn between adaptation to dieldrin and decompensation resulting
from excessive doses of it. Some of these authors, including Popper,
showed that the same distinction could be drawn in connexion with
other sources of potential liver injury.
It was found that daily intraperitoneal administration of dieldrin
to rats at the rate of 2 mg/kg produced enlargement of the liver,
hypertrophy of the smooth endoplasmic reticulum, increases in
microsomal protein and P-450 haemoprotein, and associated increases in
the activity of microsomal enzymes; however, normal activity of other
enzymes not derived from the microsomes was maintained. The activity
of microsomal enzymes per mole of available P-450 haemoprotein
remained unchanged. The highest level of activity of the processing
enzymes was reached after 14 days, after which the new steady state
was maintained. Rats that had received dieldrin at a rate of 2 mg/kg
per day for 28 days were more tolerant to dieldrin than normal rats,
as shown by the fact that they survived 25 consecutive daily doses at
the rate of 5 mg/kg, a dosage that produced 70% mortality in
previously untreated rats. In spite of the ability of the rats,
pretreated with a moderate dosage of dieldrin, to survive a large
dosage, their livers showed definite indications of decompensation in
response to the high dosage. Although the smooth endoplasmic reticulum
remained hypertrophic and the microsomal protein and P-450
haemoprotein concentrations remained elevated, the enzyme activities
per mole of available P-450 haemoprotein decreased, as did the
activity of some enzymes not associated with microsomes. Much of the
excess smooth endoplasmic reticulum formed tightly packed clusters of
tubular membranes with no glycogen and little hyaloplasm, and some of
the mitochondrial membranes were injured. It was suggested that the
phase of decompensation represented by hypertrophic but hypoactive
smooth endoplasmic reticulum might serve as a sensitive criterion of
toxic injury before microscopic changes of a clearly harmful sort
become recognizable (Hutterer et al., 1968).
Other studies indicating not only the presence of adaptive change
over a range of dosages but also the failure of adaptation and onset
of injury at sufficiently high dosage levels have been reported for
butylated hydroxy-toluene (Gilbert & Golberg, 1967) and for DDT
(Hoffman et al., 1970). Hoffman and his colleagues found that, when
DDT was fed to male weanling rats for only 14 days at dietary
concentrations of 0.5 to 2048 mg/kg, concentrations of 0.5 and 2 mg/kg
had no effect on the O-demethylation reaction used as a test, but
concentrations of 4-750 mg/kg produced increases in the rate of
metabolism, proportional to the log of dosage. Extrapolation of this
portion of the dosage response curve to the abscissa provided a
calculated no-effect level of 3.27 mg/kg equivalent to about
0.327 (mg/kg)/day. This is in reasonable agreement with other
estimates of the threshold for induction of various enzymes in the
rat, including some studies involving longer administration of DDT.
These estimates, expressed as (mg/kg)/day, are approximately 0.05
(Kinoshita et al., 1966; Street et al., 1969), 0.5 (Schwabe &
Wendling, 1967) and 0.125 (Gillett, 1968). All of the estimates are of
the same order of magnitude as the 0.25 (mg/kg)/day known to be
effective in man (Laws et al., 1967; Poland et al., 1970), but all are
over 100 times greater than the highest dosage received by members of
the general population during the late 1960s (Duggan, 1968).
Increasing the dietary level to more than 750 mg/kg did not produce
any further increase in enzyme activity. Intake of less than 128 mg/kg
did not produce any increase in liver weight, within the period of
observation; increase was proportional to dosage within the range of
128 to 512 mg/kg and was submaximal at intakes above 512 mg/kg.
Some other compounds, notably phenobarbital, produced
morphological changes in the liver similar to those produced by some
organochlorine insecticides (Wright et al., 1972; Thorpe & Walker,
1973). It seems possible that sufficiently high doses of
phenobarbital, for example, may lead to a failure of adaptation and to
levels of enzyme activity that do not correspond to dosage.
As indicated above, the earliest morphological changes caused by
enzyme inducers in the rodent liver involve separate cells in the
centrolobular area. If the dosage is sufficiently high and prolonged,
nodules consisting entirely of hypertrophied cells may appear. At
first, these microscopic nodules are distinguishable only by pattern;
they have no bounding membrane and they do not compress or change in
any other detectable way the smaller liver cells that surround them.
Some nodules may become large enough to be seen without a microscope,
and a few may exceed 1 cm in diameter. In these large nodules, there
is almost complete loss of lobular architecture. Nodules apparently
were first described by Fitzhugh & Nelson (1947) who felt that they
could be regarded as adenomas or as low grade hepatic cell carcinomas.
The use of the second term is not clear because neither mitoses,
tissue invasion, nor metastasis was observed. Although Ortega et al.
(1956) reported small nodules in the livers of treated rats, and
although they examined tissue loaned by Fitzhugh & Nelson's
laboratory, they were entirely unimpressed by the lesions, referring
to them as "focal incongruities".
Almost 3 decades after the first study, there is no more agreement
than is reflected in the preceding paragraph. The views of some
pathologists remain diametrically opposed. This is true even though
the finding of: (a) pulmonary metastases of hepatic cells in mice that
had received DDT (Tomatis et al., 1972; Walker et al., 1973), ß-HCH,
gamma-HCH, dieldrin, or phenobarbital (Thorpe & Walker, 1973); or (b)
progression of liver enlargement beginning 12 weeks after cessation of
ingestion of alpha-HCH by mice for 24 or 36 weeks (Nagasaki et al.,
1974) or progressive increase in the size of liver nodules after DDT
feeding was stopped (Tomatis et al., 1974b; Tomatis & Turusov, 1975);
or even (c) in HCH-exposed mice the time-pattern of increase in liver
weight (as reflected by body weight), which gained momentum only after
a delay of 4 weeks but showed a further acceleration in the thirteenth
week, in spite of decreased food consumption (Tomii et al., 1972),
would appear to establish, without question, that at least some of the
liver changes produced by these compounds in rodents are malignant.
Of course, the reason for disagreement is that the tumours
produced by DDT, other organochlorine insecticides, and phenobarbital
differ in their biochemistry and are not malignant in the classical
sense. Specifically, (a) they do not actively invade tissues; (b)
their "metastases" do not grow; (c) they produce little shortening of
life span; and (d) mice receiving 5.5 (mg/kg)/day as a result of
dietary intake of DDT show a decrease in the success of
transplantation and a significant increase in survival in mice in
which tumours grew following inoculation with an otherwise uniformly
transplantable and uniformly fatal ependymoma (Laws, 1971).
Although the displacement of liver cells to the lung occasionally
seen after prolonged dosage with DDT is usually referred to as
metastasis, it might better be called embolism because the lesion does
not progress and, therefore, lacks the clinical significance of a real
metastasis. Because it does not grow, the lesion is hard to find. A
number of investigators have failed to mention liver cells in the
spleen, lymph nodes, or lungs, and some have stated specifically that
they were not found (Nagasaki, 1973; Rossi et al., 1977).
Perhaps the most illuminating study of the liver changes caused by
DDT is that of Kuwabara & Takayama (1974). They used fluorinyl
acetamide (2,7-FAA) as a positive control in their studies of DDT and
HCH. The 3 compounds were given at dietary concentrations of 250, 250,
and 600 mg/kg, respectively. The lesion caused by 2,7-FAA differed
from those caused by either of the other compounds in 3 ways: (a) it
started as hyper-plastic nodules rather than as isolated cell changes;
(b) the final lesion was hepatocellular carcinoma in contrast with the
adenoma caused by DDT or HCH; and (c) alpha-fetoprotein was formed,
which did not occur with DDT or HCH. Other workers have also failed to
find alpha-fetoprotein in mice treated with an organochlorine
insecticide (Hanada et al., 1973).
It must be emphasized that the organochlorine insecticides and
phenobarbital do not produce, in other animals, the early, visible
changes in the endoplasmic reticulum that are so characteristic of
some rodents and that progress to tumour formation in rodents. That
these compounds do not lead to tumour formation in other animals might
have been predicted by the fact that they do not cause the early
changes, characterized by hypertrophy, margination, and lipospheres.
All available evidence indicates that man does not appear to be
susceptible to the tumorigenic action of the organochlorine
insecticides and phenobarbital. No increase in the occurrence of
tumours has been found in heavily-exposed populations. This includes
groups of workers who manufacture and formulate DDT and dieldrin and
who have been examined carefully for tumours (Laws et al., 1967;
Jager, 1970).
Finally, a study based on a complete tumour registry did not
indicate any increase of tumours attributable to phenobarbital among
men and women who received heavy, essentially lifelong dosing with
this drug for the control of epilepsy (Clemmesen et al., 1974).
In summary, in spite of disagreement about the interpretation of
the liver cell changes, there is general agreement about their
development and appearance. The change that can be detected first and
can be produced by the smallest effective dosage involves the
endoplasmic reticulum. The initial change is reversible, but, even
more important, it is peculiar to rodents. There is no evidence that
anything from the first increase in endoplasmic reticulum to the final
development of a highly nodular liver with occasional displacement of
cells to the lung has any bearing on the health of man or other
animals in which the endoplasmic reticulum does not respond in this
way.
7.1.10 Mutagenicity
DDT has been tested in a number of ways for possible mutational
effects. Shirasu et al. (1976) listed DDT as a negative chemical in
microbial mutagenicity screening studies on 166 pesticides. The test
system consisted of rec-assay using H 17 Rec+ and M 45 Rec-strains
of Bacillus subtilis and reversion assays without metabolic
activation using auzotrophic strains of Escherichia coli (WP 2) and
Salmonella typhimurium (Ames series). Further studies by the same
authors, with metabolic activation, failed to reveal mutagenicity of
DDT (Shirasu et al., 1977). McCann et al. (1975) and McCann & Ames
(1976), reported negative results on DDE in Salmonella typhimurium
testing with metabolic activation.
At a dosage of 105 mg/kg, DDT did not produce any increase in
dominant lethals in mice (Epstein & Shafner, 1968). Concentrations of
10 mg/kg or higher produced chromosome breaks and exchange figures in
a marsupial somatic cell line (Palmer et al., 1972). Saturated
solutions produced chromosome breaks in the root tips of onion and
other plants (Vaarama, 1947). A slight mutagenic effect in mammals has
been reported by Markarian (1966). Deletions plus gaps were reported
to be more common in the chromosomes of mice that had received DDT.
An unconventional test for mutagenicity involved examination of
explants of pulmonary tissue from embryonic mice whose dams had been
fed dietary concentrations of DDT of 10 and 50 mg/kg. An increase in
diffuse hyperplasia and focal proliferation was observed, but a
dosage-response relationship was not clear. Some of the embryos were
allowed to live and the experiment was repeated in subsequent
generations. There was no continuing progression of the reported
changes in succeeding generations (Sabad et al., 1972).
7.1.11 Teratogenicity
When p,p'-DDT was administered to pregnant mice at a rate of
1 mg/kg on days 10, 12, and 17 of gestation, it was not teratogenic
but did alter the gonads and decrease the fertility of the young,
especially the females (McLachlan & Dixon, 1972). A single dose at the
rate of 25 mg/kg or repeated doses of 2.5 (mg/kg) day given during
pregnancy may be embryotoxic but not teratogenic to mice (Schmidt,
1973). The reason why one or a few doses during pregnancy may be
embryotoxic although the same dosage is harmless, when administered
during the entire reproductive period, is of theoretical but not
practical importance.
Teratogenic effects of DDT have not been seen in studies of
reproduction including those for 2 generations in rats, 6 generations
in mice, and 3 generations in dogs (see section 7.1.7).
7.2 Acquisition of Tolerance to DDT
Because DDT stimulates microsomal, mixed-function enzymes and the
action of these enzymes on DDT is one of detoxication, it might be
expected that some tolerance might develop. Such tolerance has been
demonstrated in the case of dieldrin; rats that received this compound
for 28 days at a rate of 2 mg/kg survived 25 additional days at a rate
of 5 mg/kg, a dosage that killed 70% of previously untreated rats
(Hutterer et al., 1968). Apparently the possibility of tolerance to
DDT has not been explored.
7.3 Factors Influencing DDT Toxicity
7.3.1 Dosage effect
7.3.1.1 Dosage-effect of DDT
Table 5 summarizes the acute oral and dermal toxicity of DDT in
common laboratory animals, and Table 18 summarizes the subcutaneous
intravenous and intraperitoneal toxicity. Both tables are condensed
from an earlier review (Hayes, 1959) that gives references and
additional details. It may be concluded that dissolved DDT is absorbed
through all portals. Absorption of DDT powder through the skin is
negligible. It is frequently impossible to put enough DDT dust on the
skin of animals to kill them, so that an LD50 value for this
formulation cannot be determined by the dermal route. Although
formulation is important in determining the toxicity of DDT by other
routes, the difference is not so great as it is in connexion with skin
exposure. DDT is about 4 times more toxic when given intravenously
than when given orally, and about 40 times more toxic when given
intravenously than when given dermally.
Table 18. Acute subcutaneous, intravenous, and intraperitoneal LD50 of DDT
in common laboratory animalsa
Species Formulation Subcutaneous Intravenous Intraperitoneal
(mg/kg) (mg/kg) (mg/kg)
Rat Water suspension or powder >2000
Oil solution 200-1500 47 80-200
Mouse Water suspension or powder 1000-1500
Oil solution 300
Guineapig Water suspension or powder
Oil solution 900 150
Rabbit Water suspension or powder
Oil solution 250->3200 30-41 <2100
Cat Water suspension or powder
Oil solution <650 32
Dog Water suspension or powder
Oil solution 68
Monkey Water suspension or powder
Oil solution 55
a From: Hayes (1959).
In general, DDT appears to be more toxic as a solution in
vegetable oil or animal fat than when given in some petroleum
fraction. Petroleum may act as a laxative. The heavier fractions are
never absorbed and DDT dissolved in such fractions has to be extracted
from the solvent in order to show toxicity.
In summary, DDT is a compound of moderately acute toxicity.
Compared with other organochlorine insecticides of equal or greater
toxicity, it is remarkable in being only slightly absorbed by the
skin.
The effects of repeated doses of DDT are summarized in Table 17.
The 90-dose oral LD50 of technical DDT in rats is
46.0 (mg/kg)/day (Gaines, 1969). The chronicity index is 5.4. Thus the
compound has only a moderate tendency to cause cumulative effects, and
this limited tendency is fully explained by the accumulation of DDT
itself in tissues as a result of continuing intake. In fact, this
accumulations, which is strictly dosage dependent, is detectable at
all measurable levels of intake. The relationship in man is shown in
Fig. 4 (p. 138).
If storage is considered undesirable per se, then DDT is without
a no-injurious-effect level. However, the same may be said for all
compounds that are absorbed, for the presence of all of them in the
bodies of exposed organisms -- perhaps at very low levels -- may be
assumed; failure to demonstrate low levels of storage does not depend
on physiology but only on the limitations of analytical chemistry and
on the lack of persistence of chemists.
A number of papers have reported no-effect levels for DDT within
the variables investigated, namely: rat, 0.05 mg/kg (Lehman, 1965);
dog, 8 mg/kg (Lehman, 1965); and monkey, 2.2-5.54 mg/kg (Durham et
al., 1963).
There remain reports of effects in animals at the lowest dosages
investigated. For example, decreased serum albumin and increased ß-
and gamma-globulins in the blood of rats and rabbits maintained on a
dosage of 0.2 (mg/kg)/day for 3-11 months was reported by Kagan et al.
(1969).
In studies carried out in rats and dogs, toxicity, as measured by
the maximum no-effect level, was seldom very different from the
corresponding value resulting from 90 days of exposure at the same
dosage range. The largest factor of difference observed when 33
chemicals were investigated in rats was 12 (20 for minimal effect
level), and for half of them the factor was 2 or less. In 21,
rat-to-dog comparisons of long-term toxicity, in no instance was the
dog more sensitive than the rat (Weil & McCollister, 1963). Although
LD50 studies offer a poor basis for predicting long-term toxicity,
the lowest dosage that will produce a minimum effect, when administered
for the lifetime of rats, can be predicted with reasonable success
from a test lasting only 7 days and with good success from a 90-day
study (Weil et al., 1969). These results offer some perspective for
judging the ultimate effect of exposure to compounds that have been
commercially available for less than one human lifetime.
Summary of long-term toxicity studies. The lowest dosages that
have been studied in animals are of the same order of magnitude as
those encountered by men who make or formulate DDT and, therefore,
hundreds of times greater than the dosages encountered by the general
population. The animal studies have been continued long after a steady
state of storage has been achieved. From the results it can be
concluded that bioaccumulation sufficient to produce neurotoxicity or
other clinical effects, including a reduction of the life span, can
occur only at dosage levels substantially higher than those
encountered by the most heavily exposed workers. DDT dosages
encountered by workers produced a small but detectable increase in
liver changes (hypertrophy, margination, and liposphere formation) in
some groups of mice and rats. The same changes occurred in low
incidence in control mice and rats but not in other animals (see
section 7.1.9).
7.3.1.2 Dosage-effect of metabolites and o,p'-DDT
Acute oral LD50 values of DDT metabolites commonly found in
tissues of excreta are shown in Table 19. Readily absorbable
formulations of the metabolites are less toxic than the most
absorbable preparations of the parent compounds (see for example
Table 5).
Table 19. Oral LD50 values of metabolites of DDT
Compound Species LD50 Reference
(mg/kg)
DDE rat, male 880 Gaines, 1960
DDE rat, female 1240 Gaines, 1960
ODE mouse 700 von Oettingen & Sharpless, 1946
DDE mouse 1000 Domenjoz, 1946a,b
TDE(DDD) rat, male >4000 Gaines, 1969
DDA rat 1900 Smith et al., 1946
DDA rat, male 740 Gaines, 1960
DDA rat, female 600 Gaines, 1960
DDA mouse 720 von Oettingen & Sharpless, 1946
DDA mouse 590 Domenjoz, 1946a,b
DDA. Rats tolerate higher tissue levels of DDA than of DDT.
Eighteen hours after intravenous injection of DDA at a rate of
100 mg/kg, tissue levels were still higher than those usually found in
animals, fatally poisoned by DDT (Judah, 1949).
DDA produces less injury to the liver than DDT but produces
greater damage to the kidney especially at high intravenous dosages
(Lillie et al., 1947). This is consistent with the finding of Spicer
et al. (1947) that, following administration of DDT, DDA constitutes a
higher proportion of DDT-related compounds in the kidney (25%) than in
any other tissue, e.g., 12% in the liver, 10% in the brain, and even
less in other tissues.
o,p'-DDT. At an oral dosage of 150 mg/kg, p,p'-DDT produces
severe illness in all rats and kills about half of them, but o,p'-DDT
at the same dosage does not produce illness even though the
concentrations of the 2 compounds in the brain at various intervals
after dosing are about the same. At a dosage of 3000 mg/kg, o,p'-DDT
produces mild to moderate illness, and the concentration in the brain
is 5-9 times the concentration of p,p'-DDT necessary to produce
similar symptoms. Thus, p,p'-DDT appears to be inherently more toxic
than the o,p'-isomer (Dale et al., 1966a).
7.3.2 Age and sex
Young animals eat more than adults in relationship to their body
weight. For this and other reasons, they are often more susceptible
than adults to poison in food. However, young animals are inherently
less susceptible to certain compounds. There is no evidence that DDT
is more toxic to the young than to the adults of any species,
including man. In the rat, the young are less susceptible than adults
to a single dose and about equally susceptible to repeated doses as
shown in Table 20. According to Henderson & Woolley (1969), the
relative insusceptibility of the young is associated with relatively
poor absorption of DDT by the central nervous system and by the less
inherent susceptibility of the young brain to DDT already absorbed by
it. Further studies by the same authors (Henderson & Woolley, 1970)
showed that fatal poisoning of both 10- and 60-day-old rats involved
hyperexcitability and intense tremor followed by prostration and
eventual respiratory failure. However, in the adult rat, DDT caused
convulsions, an increase in respiration and heart rate, and a lethal
increase in body temperature (40-42°C) prior to death, but the body
temperature of the immature rat decreased during acute intoxication by
DDT. The authors suggested that, whereas DDT is a direct depressant of
respiration in both young and old rats, the additional toxic responses
manifested by seizures and hyperthermia accounted for the increased
lethality of DDT in mature animals.
There is virtually no sex difference in the acute toxicity of DDT
to rats; the LD50 was 113 and 118 in males and females, respectively
(Gaines, 1960). When DDT is fed to rats at ordinary dietary levels,
the 2 sexes store it equally. However, at higher dosages, females
store more of the compound; the difference is explained mainly by the
lower activity of the liver microsomal enzymes in female rats and,
only in part, by the relatively higher food intake of the females.
Table 20. Effect of age on the toxicity of DDT to rats
Number Age LD50 Reference
of doses (mg/kg)a
1 newborn 4000 Lu et al.. 1965
1 newborn 2356 Harrison, 1975
1 10 days 728 Henderson & Woolley, 1969
1 14-16 days 437.8 Lu et al., 1965
1 weanling 355.2 Lu et al,, 1965
1 2 months 250 Henderson & Woolley, 1969
1 3-4 months 194.5 Lu et al., 1965
1 middle-aged 235.8 Lu et al,, 1965
1 adult 225 Harrison, 1975
4 preweaning 279.2 Lu et al., 1965
4 adult 285.6 Lu et al., 1965
a Total intake one or more doses.
7.3.3 Nutrition
Fat. Nutrition influences the toxicity of DDT in connexion with
both fat and protein. Fatness influences the amount that can be stored
inactively in the body, and it is of importance in mitigating acute
poisoning. This action of fatness or "good condition" has been noted
in connexion with mammals (Spicer et al., 1947) and fish (Hoffmann &
Surber, 1948). In contrast, laboratory animals are slightly more
susceptible to repeated large doses administered as part of a diet
containing a moderate proportion of fat (5% or more) than as part of a
very low fat diet (0.5%) (Sauberlich & Baumann, 1947). This difference
is thought to be associated with absorption from the gastrointestinal
tract.
Rats that have stored large amounts of DDT in the fat may suffer
tremors, if starvation or some other cause leads to a mobilization of
their fat (Fitzhugh & Nelson, 1947). If DDT intake is stopped when
starvation begins, and, if the concentration of DDT is measured only
once following the interval of starvation, the results may be erratic,
reflecting, to a greater or lesser degree, both mobilization of fat
and excretion of metabolites as in studies reported by Deichmann et
al. (1972).
However, in nature, starvation is more often partial than
complete, and, if the original diet contains enough DDT to cause
substantial storage, whatever food may be found in a period of
scarcity is also likely to be contaminated. The initial effect of the
mobilization of fat is to increase the concentration of DDT in the
remaining fat and in other tissues. Excretion is increased in response
to the increased tissue levels but may not be fast enough to prevent
the accumulation of a toxic concentration in the brain. If intake of
DDT is stopped, the increased rate of excretion eventually leads to
reduced storage (Dale et al., 1962). These findings in rats have been
confirmed, in regard to both the initial increase in the concentration
of DDT (Dedek & Schmidt, 1972; Stenberg & Diky, 1973) and the later
reduction (Brodeur & Lambert, 1973). Similar findings have been
reported in birds (Adamczyk, 1971).
The effect of fat mobilization on the toxicity of DDT is the same
whether it is caused by withholding food or by disease that causes
partial refusal of food (Hayes, 1975).
It is highly unlikely that poisoning by DDT will be precipitated
in man by starvation because: (a) very few subjects store the compound
in concentrations as high as those required to demonstrate the
phenomenon in rats; and (b) the metabolic rate in man is so much
slower than that in rats that elimination of DDT in man would
counterbalance that produced by its mobilization.
Lipids. The association of lipids with the function of
microsomal enzymes is generally recognized as is the fact that DDT
induces these enzymes. Therefore, it might have been expected that DDT
and essential fatty acids would interact. Tinsley & Lowry (1972) found
that the growth of female rats receiving p,p'-DDT at a dietary level
of 150 mg/kg was depressed, if they received a diet deficient in
essential fatty acids, but was slightly stimulated, if they received
the same diet supplemented with these acids. Another variable
influenced by the same factors was the ratio of various liver lipids.
The changes in fatty acid composition were related to the
proliferation of hepatic smooth and endoplasmic reticulum; it was
suggested that DDT influenced essential fatty acid metabolism by
increasing the demand for them.
In contrast, a variety of diets (containing fats that may occur in
the human diet and that were in approximately the same proportion as
fats in the typical human food in the USA) had little or no influence
on the storage of DDT and a wide range of pesticides fed to rats for 4
generations in a combination of rates 200 times those found in the
Market Basket Study of food in the USA (Adams et al., 1974).
Ascorbic acid. In squirrel monkeys (and presumably in other
species) only 2 days on an ascorbic acid-deficient diet impaired both
the induction of O-demethylase and the stimulation of the glucuronic
acid system by DDT (5 mg/monkey/day) (Chadwick et al., 1971a). In
guineapigs, maintenance of induction of microsomal enzymes required a
higher dietary level of ascorbic acid than prevention of scurvy
(Wagstaff, 1971).
Protein. Smith & Stohlman (1945) found only slightly greater
mortality and liver pathology in rats fed DDT at 500 mg/kg in a diet
containing protein at 80 g/kg than in one containing 280 g/kg. The
finding that low dietary protein predisposes to DDT poisoning has been
confirmed (Sauberlich & Baumann, 1947; Boyd & DeCastro, 1968, 1970;
Boyd & Krijnen, 1969); however, even zero protein intake increased
toxicity only 4-fold, the smallest factor observed in comparable
studies of 9 pesticides. The effect of protein deficiency on toxicity
may involve a crippling of the microsomal enzymes of the liver or it
may act synergistically with compounds that cause anorexia. Rats fed a
diet containing casein at 810 g/kg exhibited evidence of renal
overload and were more susceptible to DDT (Boyd & DeCastro, 1970).
7.3.4 Species
The acute toxicity and metabolism of DDT were studied in mice and
hamsters because of the marked difference in their susceptibility to
liver tumours induced by DDT. The central nervous systems of the 2
species are equally sensitive, the concentration of DDT in their
brains at death being similar. However, after an oral dosage of
500 mg/kg, the DDT concentration in the mouse brain was twice that in
the hamster. This cannot be explained by a difference in absorption,
metabolism, or excretion but apparently is due to a difference, in the
permeability of the blood/brain barrier in the 2 species. When animals
receive DDT at a dietary level of 250 mg/kg for 6 weeks, the residues
in fat and liver were 7/8 times higher in the mouse, a fact only
partially explained by the greater food intake of the mouse relative
to body weight. Although urinary excretion of 14C-DDT was similar in
previously unexposed hamsters and mice, this excretion was stimulated
in the hamster but little affected in the mouse by previous dietary
exposure to DDT (Gingell & Wallcave, 1974).
Mice also differ from rats in the hormonal regulation of the basic
activity of hepatic microsomal mixed-function enzymes as well as in
the response of these enzymes to inducers (Chhabra & Fouts, 1974).
7.3.5 Other factors
A number of other factors are known to influence the toxicity of
some compounds, and the degree of difference may be very great in
isolated instances. Factors that have been reviewed elsewhere (Hayes,
1975) include (in addition to those listed above) interaction of
compounds, strain, individual differences, isolation and crowding,
other social and psychological factors, temperature, pressure and
altitude, light and other radiation, circadian and other rhythms,
seasonal differences, and relative humidity. None of these additional
factors is known to have an important effect on the survival of
animals receiving DDT. The possibility of the interaction of DDT with
aldrin, pyrethrin, piperonyl butoxide, malathion, dichloropheno-
xyacetic acid (2,4-D), and a number of food additives has been
explored systematically without finding anything but simple additive
results (Fitzhugh, 1966). However, pyrethrins, especially synergized
pyrethrins, have an additive and perhaps synergistic effect on the
changes in liver morphology associated with repeated doses of DDT in
rats (Kimbrough et al., 1968).
7.4 Human Studies
Oral exposure. Table 21 summarizes the effects of one or a few
carefully measured oral doses of DDT. The results are consistent with
those in accidents reported by Garrett (1947) and Hsieh (1954) in
which it was possible to estimate accurately the amount ingested. It
may be concluded that a single dose at the rate of 10 mg/kg produced
illness in some but not all subjects even though no vomiting occurred.
In general, smaller doses did not produce illness, although a dosage
of 6 mg/kg produced perspiration, headache, and nausea in a man who
was sickly and who was hungry at the time of eating. Persons who were
made sick by 10 mg/kg did not have convulsions, but convulsions
occurred frequently when the dosage level was 16 mg/kg or greater
(Hsieh, 1954). Rarely, a dosage as high as 20 mg/kg might be taken
without apparent effect (MacCormack, 1945). Dosages at least as high
as 285 mg/kg have been taken without fatal result (Garrett, 1947).
However, large doses lead to prompt vomiting, so that the amount
actually retained cannot be determined accurately.
It has been noted, in the course of tests with volunteers, that
dilute colloidal aqueous suspensions of DDT are odourless and
tasteless (Domenjoz, 1946a; Hoffman & Lendle, 1948). Saturated
alcoholic solutions of DDT have a weak aromatic taste or rather odour.
Some people find these solutions slightly anaesthetic to the tongue
(Hoffman & Lendle, 1948). The taste of DDT in vegetable oil is so
slight that many persons could not identify capsules containing 0,
3.5, and 35 mg of DDT when they were presented separately but could
arrange them in proper order when one of each was available for
comparison (Hayes, personal communication, 1977).
The possible clinical effects of many repeated doses of DDT were
first explored by Fennah (1945). Because of his interest in predicting
the results of indiscriminate use, he expressed the exposures in terms
of environmental levels rather than in dosage units. The exposures
were clearly higher than those ordinarily encountered. In one test,
lasting a total of 11.5 months, Fennah daily inhaled 100 mg of pure
DDT and drank water dusted at the rate of 3240 mg/m2. Much of the
inhaled dust must have been deposited in the upper respiratory tract
and swallowed. Later, for one month, Fennah ate food all of which had
been sprayed at the rate of 2160 mg/m2 after it had been served. No
ill-effect of any kind was observed.
Table 21. Summary of the effects of one or a few oral doses of DDT on volunteers
Dose (mg) and Result Reference
formulation
250 × 9, No effect. Domenjoz, 1946a
suspension
1500, butter No effect, but lice killed when fed 6 MacCormack, 1945
solution and 12 h after dose.
500, oil No effect. Neal et al., 1946
solution
700, oil No effect. Neal et al., 1946
solution
250, suspension None except slight disturbance of Velbinger, 1947a,b
sensitivity of mouth.
250, oil Variable hyperesthesia of mouth, Velbinger, 1947a,b
solution
500, oil Variable hyperesthesia of mouth. Velbinger, 1947a,b
solution
750, oil Disturbance of sensitivity of lower part of Velbinger, 1947a,b
solution face; uncertain gait; peak reaction (6 h after
ingestion) characterized by malaise, cold moist
skin, and hypersensitivity to contact;
reflexes normal.
1000, oil Same as above; no joint pains, fatigue, fear, Velbinger, 1947a,b
solution or difficulty in seeing or hearing.
1500, oil Prickling of tongue and around mouth and nose Velbinger, 1947a,b
solution beginning 2.5 h after dose; disturbance of
equilibrium; dizziness; confusion; tremor
of extremities; peak reaction (10 h after
ingestion) characterized by severe malaise,
headache, and fatigue; delayed vomiting;
almost complete recovery in 24 h.
Some later studies on volunteers have been designed to explore the
details of storage and excretion of DDT in man and to search for
possible effects of dosages considered to be safe. In the first of
these studies, men were given 0, 3.5, and 35 (mg/man)/day. These
administered dosages, plus DDT measured in the men's food, resulted in
dosage levels of 0.0021-0.0034, 0.038-0.063, and 0.36-0.61
(mg/kg)/day, respectively, the exact value depending on the weight of
each individual. Six volunteers received the highest dosage of
technical DDT for 12 months, and 3 received it for 18 months. A
smaller number of men ingested the lower dosage of technical DDT or
one of the dosages of p,p'-DDT for 12 or 18 months. No volunteer
complained of any symptoms or showed, by the tests used, any sign of
illness that did not have an easily recognizable cause clearly
unrelated to the exposure to DDT. At intervals, the men were given a
systems review, physical examination, and a variety of laboratory
tests. Particular attention was given to the neurological examination
and liver function tests, because the major effects of DDT in animals
involve the nervous system and the liver (Hayes et al., 1956). The
same result was obtained in a second study in which the same dosages
were given for 21 months and the volunteers were observed for a
minimum of 27 additional months (Hayes et al., 1971). Information on
storage and excretion gathered in these studies has already been
discussed in sections 6.2.1.1 and 6.3.1.1.
Recently, DDT has been used on an experimental basis at dosage
rates varying from 0.3 to 3 (mg/kg)/day for periods up to 7 months in
an attempt to decrease serum bilirubin levels in selected patients
with jaundice. No side-effects were observed. No improvement was noted
in patients with jaundice based on cirrhosis who did not have any
demonstrated liver enzymes deficiency. However, in a patient with
familial, nonhaemolytic, unconjugated jaundice based on a deficienty
of glucuronyl-transferase, treatment with DDT rapidly reduced the
plasma bilirubin level to the normal range and relieved the patient of
nausea and malaise from which he had suffered intermittently. The
liver function tests as well as other laboratory findings remained
normal. The improvement was maintained during the 6 months that DDT
was administered, and had persisted for 7 additional months at the
time the report was written. In this case, a dosage of 1.5 (mg/kg)/day
produced a steady rise in plasma levels of p,p'-DDT from an initial
level of 0.005 mg/litre to a maximum of 1.33 mg/litre at the end of
treatment. At this time, the concentration in body fat was 203 mg/kg.
Plasma levels fell slowly after dosing was stopped (Thompson et al.,
1969). The highest dally intake in this series was 6 times greater
than the highest level administered in earlier studies of volunteers
and about 7500 times greater than the DDT intake of the general
population. The highest value for p,p'-DDT in serum observed in the
entire series was 1.330 mg/litre compared with 0.996 mg/litre, the
highest value reported by Laws et al. (1967) for formulating plant
workers.
Dermal exposure. Depending on dosage, oral administration of DDT
to volunteers either did not produce any illness or produced only
brief poisoning similar to that seen in experimental animals. The oral
dosage necessary to produce any clinical effect was almost always
10 mg/kg or more. However, in 2 studies involving only 3 subjects in
all, experimental dermal exposure to DDT was followed by fatigue,
aching of the limbs, anxiety, or irritability, and other subjective
complaints. Recovery was delayed for a month or more (Case, 1945;
Wigglesworth, 1945). In neither study was there an independent
control. Although the dosage was unmeasured, the amounts of DDT
absorbed must have been much smaller than those involved in the oral
tests. One of the studies involved self-experimentation by one man. A
similar but somewhat more severe test on 6 volunteers did not produce
any toxic or irritant effects at all (Dangerfield, 1946). In view of
all other experiments and extensive practical experience, it must be
concluded that the illnesses reported by Wigglesworth and by Case were
unrelated to DDT.
With the exceptions just mentioned, dermal exposure to DDT has not
been associated with any illness or, usually, with any irritation
(Wasicky & Unti, 1944; Draize et al., 1944; Cameron & Burgess, 1945;
Fennah, 1945; Dangerfield, 1946; Chin & T'Ant, 1946; Domenjoz, 1946a;
Haag et al., 1948). In fact, Hoffman & Lendle (1948) reported that
even subcutaneous injection of colloidal suspensions of DDT in saline
in concentrations up to 30 mg/litre did not cause irritation.
Zein-el-Dine (1946) reported that DDT-impregnated clothing caused a
slight, transient dermatitis, but the method of impregnation was not
stated and the absence of solvent was not guaranteed. In other more
thorough studies DDT-impregnated clothing was found to be non-
irritating (Cameron & Burgess, 1945; Domenjoz, 1946a).
Small pads impregnated with different formulations of DDT were
applied to the inner surface of the forearm of 32 volunteers whose
cutaneous sensation had previously been measured for a period of 5
weeks. Pads impregnated with all the elements of the formulation
except DDT were applied to the corresponding position of the other arm
as a control. Powdered DDT and a solution of DDT at 50 g/litre showed
little effect. Solutions in olive oil and petrolaturn at 100 g/litre
and 200 g/litre did not show any remarkable effect on sensation of
pain, cold, or heat but reduced tactile sensation in most cases so
that the minimum pressure that could arouse the tactile sensation was
1-2.5 g/cm2 higher than for the control (Chim & T'Ant, 1946).
Respiratory exposure. Neal et al. (1944) reported almost
continuous daily exposures to aerosols sufficient to leave a white
deposit of DDT on the nasal vibrissae of the volunteers. This exposure
produced moderate irritation of the nose, throat, and eyes. Except for
this irritation during exposure, there were no symptoms, and
laboratory tests and physical examination, including neurological
evaluation, failed to reveal any significant changes. The studies by
Fennah (1945), which involved both respiratory and oral exposure, did
not produce any detectable ill-effects.
8. EFFECTS OF DDT ON MAN-EPIDEMIOLOGICAL AND CLINICAL STUDIES
8.1 Retrospective Studies on DDT-Exposed Populations
8.1.1 Epidemiological surveillance of persons occupationally exposed
to DDT
The safety record of DDT is phenomenally good. It has been used
for mass delousing in such a way that the bodies and inner clothing of
thousands of people of all ages and states of health have been
liberally dusted with the compound. By necessity, the persons applying
the DDT work in a cloud of the material. Other subjects have sprayed
the interior of hundreds of millions of homes in tropical and
subtropical countries under conditions involving (Wolfe et al., 1959)
extensive dermal and respiratory exposure. A smaller number of men
have made or formulated DDT for many years. Extensive experience and
numerous medical studies of groups of workers have been reviewed
(Hayes, 1959). Dermatitis was commonly observed among men who used DDT
solutions. The rashes were clearly due to the solvent, especially
kerosene. As often happens with rashes caused by petroleum
distillates, they were most severe in men when they first started work
and cleared in a few days unless contamination was exceptionally
severe. A smaller number of workers experienced mild narcotic effects
(vertigo and nausea) from solvents when working in confined spaces.
Gil & Miron (1949) reported that some persons suffered temporary
irritability, fatigue, and other ill-defined symptoms after exposure
to the dusty atmosphere of a delousing station, but the relation of
these atypical findings to DDT was not clear. With these exceptions
due largely to solvents, no illnesses clearly attributable to the
formulations, much less to DDT, were revealed by the early studies.
Ortelee (1958) carried out clinical and laboratory examinations of
40 workers, all of whom were exposed to DDT and some of whom were
exposed to a number of other pesticides. The men had been employed at
this work with heavy exposure for 0.4 to 6.5 years and with slightly
less exposure for as much as eight years. Exposure was so intense
that, during working hours, many of the men were coated with a heavy
layer of concentrated DDT dust. By comparing their excretion of DDA
with that of volunteers given known doses of DDT, it was possible to
estimate that the average dosages of 3 groups of the workers with
different degrees of occupational exposure were 14, 30, and 42 mg/man
per day, respectively. With the exception of the excretion of DDA and
the occurrence of a few cases of minor irritation of the skin and
eyes, no correlation was found between any abnormality and exposure to
the insecticide. Since very large doses of DDT injure the nervous
system and liver of experimental animals, special attention was given
to a complete neurological examination and to laboratory tests for
liver function. Although a few abnormalities were revealed, none was
detected in relation to DDT.
Thirty-five men employed from 11 to 19 years in a plant that had
produced DDT continuously and exclusively since 1947 and, at the time
of the study, was producing 2722 metric tonnes per month were studied
by Laws et al. (1967). Findings from medical histories, physical
examinations, routine clinical laboratory tests, and chest X-ray films
did not reveal any ill-effects attributable to exposure to DDT. The
overall range of storage of the sum of isomers and metabolites of DDT
in the men's fat was 38-647 mg/kg compared with an average of 8 mg/kg
for the general population. Based on their storage of DDT in fat and
excretion of DDA in urine, it was estimated that the average daily
intake of DDT by the 20 men with high occupational exposure was
17.5-18 mg/man per day compared with an average of 0.028 mg/man per
day for members of the general population. There was significant
correlation ( r = +0.64) between the concentration of total DDT-related
material in the fat and the serum of the workers. The average
concentration in fat was 338 times higher than that in serum -- a
factor about 3 times greater than that for people without occupational
exposure. Compared to members of the general population, the workers
were found to store a smaller proportion of DDT-related material in
the form of DDE; the difference was shown to be related chiefly to
intensity rather than to duration of exposure. DDE is relatively a
much less important and DDA a much more important excretory product in
occupationally-exposed men compared with men in the general
population. A further study of the same men involved in DDT production
is discussed in section 8.2.5.
By far the largest number of heavily-exposed workers whose health
has been investigated are those associated with malaria control in
Brazil and India (WHO, 1973). In Brazil, periodic clinical
examinations were made of 202 spraymen exposed to DDT for 6 or more
years, 77 spraymen exposed for 13 years ending in 1959, and 406
controls. In the first examination carried out in 1971, minor
differences between exposed and unexposed groups were observed in some
neurological tests, but this result was not confirmed by the second
examination in the same year nor in subsequent examinations. During a
3-year period, a survey of illnesses requiring medical care during the
6 months preceding each periodic medical examination failed to
demonstrate any differences between exposed and control groups. A
relatively small number of analyses indicated that the concentration
of DDT in the blood of spraymen was about three times higher than that
of controls.
In India, the blood levels of 144 spraymen were 7.5-15 times
higher than those of the controls and were at least as high as those
reported for workers who make and formulate DDT elsewhere (see
Table 7). When the spraymen were examined, the only differences from
the controls were that knee reflexes were brisker, slight tremor was
more often present, and a timed Romberg test was more poorly performed
by the spraymen. The positive results led to the selection of 20 men
for re-examination by a neurologist who concluded that the differences
found initially were not real or that the tests had returned to normal
within the few months between the 2 examinations. The signs were not
dosage-related, since they were not correlated with serum levels of
DDT.
It has been known for several years that substantial doses of DDT
and several other organochlorine insecticides stimulate the microsomal
enzymes of the liver. This property of DDT was put to practical use in
treating a patient with familial, nonhaemolytic, unconjugated
jaundice, as described earlier. It was, therefore, entirely expected
that persons with sufficient occupational exposure to a variety of
pesticides would be able to metabolize a test drug (phenazone) more
rapidly on the average than persons without occupational exposure were
able to do. However, the change was not one of significantly
increasing the fastest normal rate but of bringing all the workers up
to a high level. There was no indication that the change had any
effect on the workers' health (Laws et al., 1967, 1973; Kolmodin et
al., 1969; Poland et al., 1970).
In addition to the studies already mentioned regarding workers
with extensive storage, and excretion of DDT as a result of heavy
exposure to DDT, studies have also been made of a larger number of
workers with lesser storage and excretion following lesser exposure to
DDT but greater exposure to other insecticides. Further studies (Long
et al., 1969; Morgan & Roan, 1969, 1974; Warnick & Carter, 1972;
Sandifer et al., 1972; Embry et al., 1972; Tsutsui et al., 1974; Ouw &
Shandar, 1974) have failed to reveal effects of clinical significance
among workers with prolonged, moderate exposure not only to
organochlorine but also to organophosphorus and other types of
insecticides. Small but statistically significant differences have
appeared in the medical history or clinical laboratory results of some
of these workers compared with the controls, but in no instance have
the differences been of any medical importance, and dosage-response
relationships have been unclear or absent. In several instances, the
statistically significant differences have been opposite in different
groups of workers; for example, creatinine phosphokinase activity was
lower than that of controls in subjects applying the insecticide but
higher in operators. Seasonal variations present one year were lacking
the next. The possibility of adaptive change (other than enzyme
induction) has been suggested (Tocci et al., 1969), but this, like the
reality of the changes, remains unproved.
In some instances statistically significant differences have been
found between workers and controls selected from the general
population in connexion with parameters that have no known biochemical
relationship to DDT and for which another explanation has not been
excluded. For example, Keil et al., (1972) reported significant linear
correlations between serum vitamin A and plasma DDT, TDE, and DDE
levels.
There are a few reports of acute illness among workers attributed
to exposure to mixtures of DDT and other materials. In so far as the
dosage was very large, as in certain accidents that have occurred to
individuals or groups in the general populations (see section 8.2.2),
one would expect similar results. However, in at least one instance,
headache, dizziness, nausea, vomiting, pain and numbness of the limbs,
and general weakness beginning 1-1.5 h after entering a treated field
(Kolyada & Mikhal'Chenkova, 1973) suggested food poisoning or
hysteria.
Finally, there are studies of workers exposed to DDT and various
other pesticides that are reported to have produced a variety of
subjective and even objective medical findings. Interpretation of
these reports is difficult because: (a) the findings do not resemble
those of poisoned animals or of persons poisoned as a result of
accident or suicide; and (b) the papers fail to report how the medical
findings and the absenteeism of the pesticide workers compares with
those of workers of comparable age, sex, and exertion who are not
exposed to chemicals. The fact that the workers in question were
exposed to mixtures of pesticides is not in itself an explanation
because studies on many workers who were exposed to mixtures have not
revealed any consistent differences between exposed subjects and
unexposed controls. However, an explanation may lie in the degree of
exposure. Reports of very high levels of organochlorine compounds in
blood samples and of DDT in milk samples from populations in which
illness was found are discussed in sections 6.2.1.2 and 6.3.1.2.
The reports under discussion tend to fall in 2 categories, those
involving general debility and those involving a single organ or
system. Conditions representative of general debility include
dermatitis, subtle blood changes, general weakness, palpitations,
functional angiospasm, headache, dizziness, diminished appetite,
vomiting, lower abdominal pain, chronic gastritis, benign chronic
hepatitis, isomnia, a sympathetic vascular/asthenic syndrome,
vegetative dystonia, and confusion (Kostiuk & Mukhtrova, 1970; Bezugli
et al., 1973).
Organs, systems, or functions that have been studied with the
exclusion of other organs, systems, or functions of the same workers
include: the respiratory system (Boiko & Krasniuk, 1969), liver
(Bezuglyi & Kaskevich, 1969), stomach (Krasniuk & Platonova, 1969;
Platonova, 1970), kidneys (Krasniuk et al., 1968), adrenals (Bakseyev,
1973), skin (Karimov, 1969, 1970), and labour and the puerperium
(Komarova, 1970; Nikitina, 1974). An indication that the difficulties
under discussion are not serious is their reversal or prophylaxis by
means of diet. Lescenko & Polonskaia (1969) described in detail two
dietary supplements composed of ordinary foods plus sea-kale and a
selection of vitamins and trace metals. Organochlorine-exposed workers
who received these diet products showed a normalization of protein
metabolism manifested by an increase in total serum protein, improved
lipid metabolism, and enriched vitamin and trace element supplies in
the organism. All of these effects led to an improvement in the
detoxifying function of the liver, which was viewed as the most
frequent site of adverse effects of exposure to organochlorine
compounds.
8.1.2 Epidemiology of DDT poisoning in the general population:
accidents and suicides
The only demonstrated effects of DDT on the general population are
the storage of the compound and some of its derivatives in the tissues
and their excretion in urine and milk. The facts were reviewed in
sections 6.2.13 and 6.3.1.2. Briefly, DDT and some of its derivatives
are found in all or nearly all persons in the population. The
concentration is higher in tissues that have a high neutral fat
content. Thus, for members of the general public the concentration of
DDT-related compounds in adipose tissue is 100 or more times greater
than the concentration in plasma (Laws et al., 1967). However, in
spite of this great difference, sufficiently sensitive methods have
demonstrated DDT in all tissues including the fetus and in all body
fluids including human milk. These relationships are exactly what
would be predicted from what is known of the storage of drugs and
other compounds. Actual chemical demonstration of the distribution of
DDT has been established for several years. Thus, its occurrence was
first reported in human tissue (Howell, 1948), in tissue of the
general population (Laug et al., 1951) in human milk (Laug et al.,
1951), and in the human fetus (Denés, 1962).
There is extensive evidence that the mount of DDT and related
material in the general diet in the USA has decreased as the use of
DDT in that country has decreased, especially its use on forage.
During the early 1950s, total DDT-related intake was approximately
0.265 mg/man per day and that for DDT was 0.163 mg/man per day (Walker
et al., 1954). The average intake of DDT-related compounds based on a
very large number of samples collected in different parts of the
country during 1964-67 was 0.063 mg/man per day and that for DDT was
0.028 mg/man per day (Duggan, 1968). With decreased use of DDT, a
gradual decrease in the storage of DDT and related material in human
fat would be expected. Because only a few samples of fat were
collected in the early studies of human tissue, there is some
statistical uncertainty as to whether the decrease in storage that has
been observed is real or whether it merely reflects variation due to
sampling. In any event, by 1968, the average storage level of total
DDT-equivalent material in fat was 7.67 mg/kg and that for DDT was
1.46 mg/kg. These averages were based on just over 3000 samples
collected during the first half of 1968. The number of samples
involved in this particular study was greater than the sum of all of
the samples used in early studies. The best available values for
concentrations in serum are 0.0294 mg/litre for total DDT-equivalent
and 0.0047 mg/litre for p,p'-DDT.
Cases of accidental and suicidal poisoning in which the effects
were clearly caused by DDT are summarized in Table 22. All of these
cases involved ingestion. The signs and symptoms of poisoning were
entirely consistent with those observed in volunteers, except that the
spectrum of effects was broader because some of the accidental and
suicidal doses were very high. A few persons have apparently been
killed by uncomplicated DDT poisoning, but none of these cases was
reported in detail. Death has been caused much more frequently by the
ingestion of solutions of DDT, but in most instances the signs and
symptoms were predominantly or exclusively those of poisoning by the
solvent (Hayes, 1959). This does not mean that the toxicity of the
solvent always predominates. For example, the recurrent convulsions in
a case reported by Cunningham & Hill (1952), though more
characteristic of poisoning by one of the cyclodienes, was certainly
not typical of solvent poisoning. A 2-year-old child drank an unknown
quantity of fly spray of which 5% was DDT, but the nature of the other
active ingredients or the solvent was unknown. About 1 h after taking
the material, the child became unconscious and had a generalized,
sustained convulsion. Convulsions were present when the child was
hospitalized 2 h after taking the poison, but the fits were controlled
by barbiturates and other sedatives. Convulsions reoccurred on the
fourth day and again on the twenty-first day but ceased each time
following renewal of treatment. On the twelfth day, it was noted that
the patient was deaf. Hearing began to improve about the twenty-fourth
day and was normal, as were other neurological and psychic findings,
when the patient was seen about 2.5 months after the accident.
Clinical effects of one toxicant may be modified by combining it
with another. For example, prolonged illness would not be expected
from ingestion of DDT at a rate of 27 mg/kg. However, when DDT and
lindane were ingested in a suicidal attempt at dosages thought to be
27 mg/kg and 18 mg/kg, respectively, clinical remission of convulsions
and liver involvement was delayed until the twentieth day, and the EEG
did not return to normal until the thirty-ninth day (Eskenasy, 1972).
There have not been any accidents of suicided involving raspatory
or dermal exposure to DDT leading to recognized signs and symptoms of
poisoning, even though sufficient respiratory exposure to aerosols or
sufficient dermal exposure to solutions can cause poisoning in
animals; the difference is certainly one of dosage.
It has been alleged that DDT causes or contributes to a wide
variety of diseases of man and animals not previously recognized as
being associated with any chemical. Such diseases include
cardiovascular disease, cancer, atypical pneumonia, retrolental
fibroplasia, poliomyelitis, hepatitis, and "neuropsychiatric
manifestations" (Biskind & Beiber, 1949; Biskind, 1952, 1953; and
others). Without exception the causes of these diseases were unknown
or at least unproved at the time of the allegation. Needless to say,
Table 22. Summary of the effects of the accidental or suicidal ingestion of DDT
Individual dose Results and reference
(mg) Formulation
Number of persons
300-4500 Onset in 1 h; vomiting; restlessness; headache; heart weak and
in food slow; recovery next day (Mulhens, 1946).
1 man
unknown dose Onset in 2-2.5 h; all subjects weak and giddy; 4 subjects vomited;
in tarts 2 subjects hospitalized; one subject confused, uncoordinated, weak;
25 man one subject with palpitations and numbness of hands; recovery in
24-48 h (Mackerras & West, 1946).
5000-6000 Onset 2-3 h; throbbing headache; dizziness; incoordination;
in pancakes paraesthesia of extremities; urge to defaecate; wide nonreacting
3 men pupils; reduced vision; dysarthria; facial weakness; tremor; ataxic
gait; reduced sensitivity to touch; reduced reflexes; positive Romberg;
slightly low blood pressure and persistent irregular heart action;
partial recovery in 2-3 days, but slight jaundice appeared 4-5 days
after ingestion and lasted 3-4 days; all subjects normal 19 days after
poisoning except for irregular heart action in one subject (Naevested,
1947).
2000 No illness (Naevested, 1947).
in pancakes
2 men
up to 20 000 Onset in 30-60 min in those most severely affected; men first
in bread seen 2-3 h after ingestion; in spite of severe early vomiting that
28 men reduced the effective dose, severity of illness and especially intensity of
numbness and paralysis of extremities proportional to amount of DDT
ingested; all but 8 men recovered in 48 h; 5 others fully recovered in 2
weeks, but 3 men still had some weakness and ataxia of the hands
5 weeks after ingestion (Garrett, 1947, 1950, unpublished data).
unknown dose Onset about 3.5 h after ingestion; total of about 85 cases of which 37
in flour were hospitalized; symptoms mild and similar to those in earlier
about 100 women outbreaks except for gastrointestinal disturbance in most severe cases
including abdominal pain and diarrhoea as well as nausea; most
subjects fully recovered in 24 h (Jude & Girard, 1949).
unknown dose Symptoms in established cases similar to those reported earlier
14 cases (Francone et al., 1952).
Table 22 (Cont'd)
Individual dose Results and reference
(mg) Formulation
Number of persons
286-1716 With the exception of one man who was already sick when he received
in meatballs a dosage of 6 mg/kg, poisoning did not occur at dosages of
8 cases, 11 5.1-10.3 mg/kg. Ingestion of 16.3-120.5 mg/kg produced excessive
exposed perspiration, nausea, vomiting, convulsions, headache, increased
salivation, tremors, tachycardia, and cyanosis of the lips. Onset
varied from 2-6 h depending on dosage. Recovery required as much
as 2 days (Hsieh, 1954).
unknown dose Death 13 h after suicidal ingestion (Committee on Pesticides, 1951).
1 case
unknown dose Twenty-two separate cases, including 15 attempted suicides; some
22 unrelated cases complicated by solvents; 3 deaths (Committee on Pesticides, 1951).
the charge that DDT predisposes to poliomyelitis was dropped after the
disease was controlled through the use of vaccines. Unfortunately,
there is no immediate possibility of controlling cardiovascular
disease, cancer, or many of the less common conditions in man that
have been ascribed to DDT. In the meantime, such irresponsible claims
could produce great harm and, if taken seriously, even interfere with
the scientific search for true causes and realistic means of
preventing the conditions in question.
8.1.3 Epidemiology of DDT poisoning in infants and young children
Nothing is known fundamentally to distinguish the epidemiology of
DDT poisoning among children from that among adults. In both
instances, poisoning has never been confirmed, except where the dosage
was large, usually as the result of an accident and usually involving
gross carelessness. Probably a larger number of cases have occurred in
infants simply because they are more likely to eat and drink
formulations that they find in unlabelled containers frequently
originally intended for food. However, as far as DDT is concerned, all
the large outbreaks of poisoning have involved adults under military
conditions, thus children were not exposed. As might be expected, some
deaths of adults but none of children has apparently involved suicide.
Most, if not all, cases in adults were uncomplicated poisoning by DDT,
but several cases in children involved the drinking of solutions so
that the signs and symptoms were actually caused by the solvent
(Reingold & Lasky, 1947).
8.2 Clinical and Epidemiological Studies of the Effects of DDT on
Specific Organs and Systems
8.2.1 Haemopoietic system and immunology
In acute poisoning, a slight decrease in haemaglobin and a
moderate leukocytosis without any constant deviation in the
differential white count have been observed in volunteers (Velbinger,
1947a,b). These findings are considered secondary to the neurological
effects.
There is a strong tendency to blame blood dyscrasias, other
manifestations of "hypersensitivity", and, in fact, many diseases of
unknown cause on any new chemical that gains widespread attention. DDT
was no exception. A review of the early literature (Hayes, 1959)
indicates that blood dyscrasias and an unbelievable range of other
diseases were, in fact, blamed on DDT. Only a circumstantial
relationship was ever established between these diseases and exposure
to DDT, and this is true of the small number of reports of blood
dyscrasias (Murray et al., 1973) or angioneurotic oedema (Vanat &
Vanat, 1971) that have appeared recently. Later, fewer new reports
appeared linking DDT to diseases of unknown cause, although the use of
DDT increased greatly. It is true that available tests do not make it
possible to exclude a particular compound as a cause of an isolated
case of blood dyscrasia. However, it is noteworthy that the rate at
which these disorders occur has remained essentially unchanged since
before DDT was introduced (Hayes, 1975).
8.2.2 Nervous system
The effects of carefully measured doses of DDT that proved to be
just above the minimum toxic level are best described from studies of
volunteers (see section 7.4). Similar early signs and symptoms have
been encountered in cases of accidental poisoning that frequently
progressed to more severe illness as described in section 8.1.2.
Briefly, the earliest symptom of poisoning by DDT is
hyperaesthesia of the mouth and lower part of the face. This is
followed by paraesthesia of the same area and of the tongue and then
by dizziness, an objective disturbance of equilibrium, paraesthesia
and tremor of the extremities, confusion, malaise, headache, fatigue,
and delayed vomiting. The vomiting is probably of central origin and
not due to local irritation. Convulsions occur only in severe
poisoning.
Onset may be as soon as 30 min after ingestion of a large dose or
as late as 6 h after smaller but still toxic doses. Recovery from mild
poisoning is essentially complete in 24 h, but recovery from severe
poisoning requires several days. In two instances, there was some
residual weakness and ataxia of the hands, 5 weeks after ingestion.
Electroencephalograms were obtained from 73 workers exposed to
DDT, HCH, and chlorobenzilate for periods ranging from 7 months to 20
years. Just over 78% of the records were normal and 21.9% were
abnormal. The most severe changes involved persons exposed to the 3
compounds for 1-2 years; less severe changes were seen with either
shorter or longer exposure. The changes were not correlated with age,
the range and mean of age for those judged abnormal being almost
identical to these values for persons considered normal. Some of the
records showed bitemporal sharp waves with shifting lateralization
combined with low voltage theta activity. Other records showed spike
complexes, paroxymal discharges composed of slow and sharp waves most
pronounced anteriorly, and low voltage rhythmic spikes posteriorly.
None of the persons examined showed any abnormal clinical neurological
finding (Israeli & Mayersdorf, 1973; Mayersdorf & Israeli, 1974). The
incidence of abnormal electroencephalograms in the general population
is 9.0% or 9.2%, according to other investigators cited by Israeli &
Mayersdorf. Czegledi-Janko & Avar (1970) considered that nonspecific
EEG abnormalities occurred in 10-20% of the general population.
The frequency and degree of olfactory disorders, especially in the
ability to detect peppermint and acetic acid in an olfactory analyser,
were reported to be greater among persons exposed to pesticides, and
to increase with duration of exposure (Salihodzaev & Ferstat, 1972).
Whether any of the persons exposed to pesticides experienced any
clinical difficulty or social inconvenience associated with olfactory
sensation is not clear.
8.2.3 Renal system
There is no indication of renal damage in people, accidentally
poisoned by DDT, or in workers heavily exposed to it.
8.2.4 Gastrointestinal system
Except for vomiting, which probably is of central origin, the
gastrointestinal system has not been affected in acute poisoning.
8.2.5 Liver
Involvement of the liver has been mentioned in only a small
proportion of cases of accidental poisoning by DDT. In 3 men who ate
pancakes made with DDT and thus ingested 5000-6000 mg each, slight
jaundice appeared after 4-5 days and lasted 3-4 days (Naevested,
1947). Hepatic involvement and convulsions were reported in an
unsuccessful suicide attempt by ingesting DDT and lindane (Eskenasy,
1972).
Laws et al. (1973) made a detailed study of the liver function of
31 men who had made and formulated DDT and who had been the subjects
of an earlier study (see section 8.1.1). Judging from their excretion
and storage, the men's exposure was equivalent to oral intakes of DDT
at rates ranging from 3.6 to 18 mg/man per day for periods ranging
from 16 to 25 years and averaging 21 years. All tests were in the
normal range for total protein, albumin, total bilirubin, thymol
turbidity, and retention of sulfobromophthalein sodium (BSP). One man
had mild elevations in levels of both alkaline phosphatase
(EC 3.1.3.1) (16 units) and serum glutamic pyruvic transaminase
(EC 2.6.1.2) SGPT (42 units). Another man had an alkaline phosphatase
concentration of 14 units, while a third man had an SGPT level of 49
units. The alpha-fetoprotein test was negative for all 20 of the men
tested.
8.2.5.1 Liver enzymes
The induction of human microsomal enzymes of the liver by various
drugs was well known when Kolmodin et al. (1969) demonstrated this
effect in workers exposed to a variety of pesticides, including DDT.
Later, Poland et al. (1970) showed that workers who made and
formulated DDT and absorbed it at an average rate of about
0.25 (mg/kg)/day metabolized phenylbutazone more rapidly on the
average than controls and excreted more 6ß-hydroxycortisol.
Occupational exposure increased the drug-metabolizing ability of some
workers, so that they all metabolized test drugs with the efficiency
of those members of the general population who were most efficient in
this respect. The concentration of p,p'-DDT in the serum of the
workers studied by Poland averaged 0.573 mg/litre. In other workers
with less exposure to DDT, as indicated by average serum levels of
0.052 mg/litre, there was no increase in the urinary excretion of
D-glucaric acid, which is increased by a number of exogenous and
endogenous substances that induce microsomal enzymes (Morgan & Roan,
1974).
Thompson et al. (1969) demonstrated, in a different way, the
induction of microsomal enzymes by using DDT at a dosage of
1.5 (mg/kg)/day for 6 months in the successful treatment of
unconjugated hyperbilirubinaemia. In a similar way, Rappolt (1970)
used DDT to promote metabolism of an overdose of phenobarbital. It is
of interest that the levels of DDE in the serum of some workers
studied by Morgan & Roan (1974) approached those of workers studied by
Poland et al. (1970). The lack of induction in one group and its
presence in the other suggests that enzymes are induced in man more
readily by DDT than by DDE.
DDT promotes its own metabolism in some species of laboratory
animals. That the same is true in man is indicated by the fact that
storage of DDT is relatively less at higher dosages (see Fig. 4).
However, the metabolism and subsequent excretion of DDT can be
promoted even more by other inducing agents. Patients who received
phenobarbital or, more especially, phenytoin stored much less DDT than
other persons with similar exposure to DDT (Davies et al., 1969a;
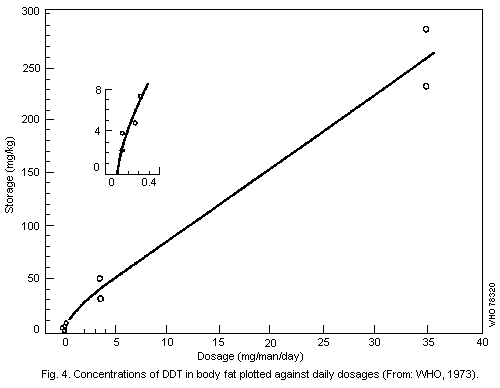 Edmundson et al., 1970b; Watson et al., 1972). This result concerning
phenytoin was confirmed by McQueen et al. (1972) who also showed that
other drugs produced a smaller but still highly significant reduction
in DDT storage. Establishment of a reduced equilibrium appeared to
require about 2 months. Within this period, the regression of the
level of DDT plus DDE on duration of treatment with phenytoin was
highly significant ( P < 0.001).
At the end of 9 months' treatment, the body fat of nonepileptic
volunteers given phenytoin at a rate of 300 mg/man per day contained
an average of 25% of the DDT and 39% of the DDE concentrations
originally present before administration of the drug (Davies et al.,
1971).
The same was true of workers whose exposure was greater than that
of the general population. Maintenance doses of phenobarbital,
phenytoin, or a combination of the two kept the storage levels of
several organochlorine insecticides in epileptic workers as low as, or
lower than levels in the general population (Schoor, 1970; Kwalick,
1971).
8.2.5.2 Other biochemical observations
A positive linear correlation has been reported for the
concentrations of vitamin A and of DDT-related compounds in the serum
of men with at least 5 years of occupational exposure to DDT. However,
the workers' DDT levels were little higher than those of persons in
the general population (see Table 7), and their vitamin A levels were
within normal limits (Keil et al., 1972). Perhaps they were better fed
than the controls.
Compared to 86 unexposed workers, the serum total cholesterol
values of 206 workers in a chemical plant where unidentified
organochlorine insecticides were made and formulated were higher in
workers who were less than 25 years old, lower in those between 25-34
years and 35-44 years, and higher in those who were 45 years old or
more. The differences were significant only for the oldest groups
(Wassermann et al., 1970a).
8.2.6 Cardiovascular system
The small amount of knowledge concerning the effect of DDT on the
human heart fails to show whether cardiac arrhythmia might be a
possible cause of death in acute poisoning, as is true in some species
of laboratory animals. Palpitations, tachycardia, and irregular heart
action have been noted in some, but not all cases of acute poisoning
(Mackerras & West, 1946; Naevested, 1947; Hsieh, 1954).
8.2.7 Reproduction
There is no indication that DDT has influenced reproduction except
to increase it as an indirect result of disease control, especially
malaria control.
After Laws et al. (1967) had completed their study, Wilcox (1967)
found that the 36 most heavily exposed workers involved had fathered
58 children before they began working at the DDT factory and 93
children afterwards.
O'Leary et al. (1970c) did not find any significant relationship
between abortion and blood levels of DDT-related compounds.
8.2.8 Endocrine organs
Average protein-bound iodine (PBI)levels of 0.0542 and
0.0693 mg/litre, respectively, were reported in the sera of 42 workers
occupationally-exposed to organochlorine insecticides and in 51
workers who were not exposed. The difference was statistically
significant even though all values fell within the normal range of
0.04-0.08 mg/litre (Wassermann, D. et al., 1971). It was not recorded
whether the workers involved were from the same factory as those with
10 or more years of occupational exposure whose plasma DDT levels were
reported by Wassermann et al. (1970c) (see Table 7). The small
difference in PBI levels is difficult to evaluate. It was the view of
Clifford & Weil (1972) that there was not any evidence that
occupational exposure had had an effect on human endocrine organs.
TDE. Following the demonstration (discussed in section 7.1.8)
that TDE caused atrophy of a part of the adrenal cortex of dogs,
o,p'-TDE, and to a lesser degree m,p'-TDE, have been used in man,
under the name of mitotane, in the hope of controlling excessive
cortical secretion or of reducing the size of adrenal tumors. The
underlying condition may be hyperplasia or adrenocortical carcinoma.
The dosage given has varied from 7 to 285 (mg/kg)/day, but a dosage of
approximately 100 (mg/kg)/day for many weeks has been necessary to
produce any benefit in man (Bergenstal et al., 1960; Wallace et al.,
1961; Gallagher et al., 1962; Verdon et al., 1962; Bledsoe et al.,
1964; and Southern et al., 1966a,b).
The effects of idiopathic hyperplasia may be controlled; in fact a
state of adrenal insufficiency may be produced (Canlorbe et al., 1971;
Sizonenko et al., 1974).
o,p'-TDE may also give symptomatic relief of excessive
adrenocortical activity secondary to a tumour that produces ACTH
(Carey et al., 1973).
A favourable response was produced in about one-fourth to one-half
of patients with inoperable adrenocortical carcinoma (Canlorbe et al.,
1971; Hoffman & Mattox, 1972; Lubitz et al., 1973; Montgomery &
Struck, 1973). In fact, an occasional cure, involving complete
regression of metastases, was produced by chemotherapy including
o,p'-TDE (Perevodchikova et al., 1972; Schick, 1973). More commonly,
symptoms were relieved and life was prolonged by little more than 7-8
months (Canlorbe et al., 1971; Hoffman & Mattox, 1972; Lubitz et al.,
1973). The success of treatment was often indicated early on by a
reduction in steroid excretion (Hoffman & Mattox, 1972; Lubitz et al.,
1973).
The large dosage of o,p'-TDE necessary to produce clinical
benefit often produced general lassitude, anorexia, nausea, vomiting,
diarrhoea, and dermatitis (Naruse et al., 1970; Hoffman & Mattox,
1972; Nitshke & Link, 1972; Perevodcikova et al., 1972; Lubitz et al.,
1973). Apathy ranged from mild dulling of interest to profound
psychotic depression (Hoffman & Mattox, 1972). More rarely,
gynaecomastia, haematuria, leukopenia, and thrombocytopenia have been
reported (Luton et al., 1972; Perevodcikova et al., 1972). The
symptoms disappeared soon after administration of the drug ceased or
when the dosage was reduced (Perevodcikova et al., 1972).
Even large, therapeutic doses of o,p'-TDE did not cause
histological alterations in the adrenals in man (Wallace et al.,
1961). Furthermore, dosages in the therapeutic range (specifically
those between 110 and 140 (mg/kg)/day did not produce any detectable
injury to the liver, kidney, or bone marrow. All patients treated in
this way experienced significant anorexia and nausea, and some showed
central nervous system depression varying from lethargy to somnolence.
These toxic effects cleared when dosing was discontinued (Bergenstal
et al., 1960).
Kupfer (1967) reviewed extensive literature that indicated that
the effect in man and other species, except the dog, is caused by
stimulation of corticoid metabolism by massive doses of o,p'-TDE and
not by any direct effect on the adrenal. Southern et al. (1966a,b)
agreed that the effect was predominantly extra-adrenal in man, when
the drug was first given, but offered evidence that adrenal secretion
of cortisol was eventually reduced. However, even if therapeutic doses
eventually have a direct effect on the adrenal, doses encountered by
workers exposed to technical DDT do not (Clifford & Weil, 1972; Morgan
& Roan, 1973).
8.2.9 Carcinogenicity
Laws et al. (1967) did not find any case of cancer or blood
dyscrasia among the 35 heavily exposed workers in a DDT factory nor
did the medical records of 63 men who had worked there for more than 5
years reveal these diseases. Two men were employed who had a history
of successfully treated cancer before they came to work, but no
employee had contracted cancer during the 19 years that the plant had
been in operation; during this period, the work force varied from 111
to 135.
In the USA, the total death rates for cancer of the liver and its
biliary passages (classified individually as "primary", "secondary",
and "not stated whether primary or secondary") lead to the conclusion
that there has been a significant, almost constant decrease in the
total rate of liver cancer deaths from 8.8 in 1930 to 8.4 in 1944
(when DDT was introduced) to 5.6 in 1972. This almost constant decline
in total liver cancer death rates for the past 42 years offers no
evidence of any increase in liver cancer deaths since the introduction
of the first organochlorine pesticide into the environment. The
decrease in liver cancer deaths is even more significant in light of
the increasing life span of the general population in the USA, which
has resulted in an increased percentage of the population at risk from
cancer over these years. In spite of the limitations inherent in the
interpretation of such data, this record is a reminder that, more than
30 years after the introduction of DDT, there is no evidence,
whatsoever, that DDT is carcinogenic in man (Deichmann & MacDonald,
1976, 1977).
In the USA, the incidence of cancer is lower in rural counties
than in metropolitan areas in general (Mason et al., 1975).
It is sometimes implied that epidemiological evidence is useless
for revealing the carcinogenicity of a material for man unless it
involves large numbers of people who have been exposed to the material
for most or all of a lifetime. The fact is that some human carcinogens
have been detected through their occurrence in high incidence in small
groups for periods of much less than 25 years. What was commonly
considered the first recognition of chemical carcinogenesis in man
depended on the observations made by a single surgeon (Pott, 1775) in
a small fraction of his patients. Such was the intensity of the
exposure of the apprentices of chimney sweepers that cancer of the
scrotum often appeared at puberty. The editor responsible for
compiling the writings of Pott (1790) added a footnote indicating that
he had seen such a cancer in "an infant under eight years of age". It
must be understood that boys did not usually become apprentice chimney
sweepers before they were four-years-old. In connection with tumours
of the bladder mainly caused by ß-naphthylamine but to a lesser degree
by other aromatic amines, Hueper (1942) reviewed cases in which the
time from the first exposure to recognition of symptoms was 8-41,
9-28, and 2-35 years; in one series of 83 cases, 71% of the tumours
appeared from 1 to 15 years after exposure. The same author cited
reports (p. 104) of cases of malignant epitheliomas in persons exposed
to pitch for 18, 24, 24, and 36 months, respectively. Kleinfeld (1967)
reported 50-76% incidence of bladder cancer among several groups of
workers. He also noted a sharp drop in incidence of this condition
following decrease -- but not discontinuation -- of occupational
exposure to ß-naphthylamine.
8.2.10 Mutagenicity
Evidence regarding the mutagenic activity of DDT and its
significance in man is uncertain partly because the chromosomal
changes that are examined are sensitive to viral infections and
chemotherapy. The latter may not be recognized at the time of sampling
and may not have been shown to injure health through a mutagenic
mechanism.
Comparing samples collected in winter and during the peak season
of pesticide application, a slight increase in chromatid breaks was
reported in the cultured lymphocytes of workers exposed to a wide
variety of insecticides said to include DDT, although this was claimed
at a time when the use of DDT was banned. A somewhat larger increase
was reported for men exposed mainly to herbicides (Yoder et al.,
1973). In another study, lymphocytes cultured from workers with an
average DDT plasma level of 0.999 mg/litre showed significantly more
chromosomal and chromatid aberrations than cells cultured from
controls with an average plasma level of 0.275 mg/litre. The
difference was not significant in other comparisons in which the
average plasma levels were 1.030 versus 0.380 mg/litre and 0.240
versus 0.030 mg/litre, respectively (Rabello et al., 1975).
Examination of all of the data presented by the authors suggests a
simple dosage-effect relationship was present, with a detectable
effect starting somewhere between 0.2 and 0.4 mg/litre and increasing
at levels higher than 0.4 mg/litre.
8.3 Factors Influencing DDT Toxicity
There is no evidence that any factor except dosage is of practical
importance in determining DDT toxicity in man. Factors that have been
considered as possibly affecting asymptomatic storage of DDT include
age, sex, and race. Differences observed in connexion with these
factors are small, medically insignificant, and probably secondary to
dosage (Hayes, 1975).
Storage has also been reported to be greater in the tissues of
people with certain diseases (Deichmann & Radomski, 1968; Raclomski et
al., 1968; Vas'kovskaja, 1969; Dacre & Jennings, 1970; Jonczyk et al.,
1974). Again, the reported differences are small, and the highest
values for the samples in question are small compared with those found
in healthy workers (Hayes, 1975). Furthermore, a number of authors
have reported a similar range of storage in persons undergoing minor,
elective surgery and in those who have died from various causes (Hayes
et al., 1958; Dale et al., 1965; Robinson et al., 1965; Wassermann et
al., 1965). Some authors (Hunter et al., 1963; Robinson et al., 1965;
Hoffman et al., 1967; Hoffman, 1968; Morgan & Roan, 1970) have failed
to find any relationship between storage of insecticides and the cause
of death. Where a relationship was found, there was often the
possibility that the higher values were found in diseases that
involved some degree of wasting prior to death. Casarett et al. (1968)
found that higher values occurred in persons who had 3 characteristics
in common: emaciation, cancer, and widespread abnormality of the
liver.
A slightly greater storage of DDT and DDE that was reported in
persons who underwent splenectomy for hepatosplenic schistosomiasis
compared with those operated on for other conditions, mainly hernia
was statistically significant. No such difference was observed in
connexion with dieldrin, ß-HCH, or heptachlor epoxide (Wassermann et
al., 1975). Whereas it was speculated that the increased storage of
DDT and DDE might have been the result of a reduction in metabolism,
secondary to liver injury, the possibility of greater exposure as a
result of greater use of DDT in irrigated areas was not excluded.
8.4 Treatment of Poisoning in Man
No useful guidance regarding treatment has been obtained from the
very few cases of DDT poisoning that have occurred. Animal studies
indicate that sedatives, ionic calcium, and glucose or another ready
source of energy would be useful. On the basis of experience in
treating people poisoned by different convulsive poisons, it seems
likely that diazepam would be beneficial.
9. EVALUATION OF HEALTH RISKS TO MAN FROM EXPOSURE TO DDT AND RELATED
COMPOUNDS
9.1 Relative Contributions of Food, Water, Air, and Miscellaneous
Sources to Total Intake
9.1.1 Adult members of the general population
Food represents the major source of intake of DDT in the general
population. It has been estimated (section 5.1.5) that over 90% of the
DDT stored in the general population is derived from food. Around
1965, when the use of DDT was at its peak, intake in the USA was
approximately 0.04 mg/man per day from food, less than 0.000046 mg/man
per day from water, less than 0.00006 mg/man per day from urban air
and less than 0.0005 mg/man per day from the air in small agricultural
communities. The reason for the qualification "less than", is that the
intakes were calculated from the highest concentrations reported in
drinking-water and air.
Although total intake of DDT from food has not been measured in
some parts of the world, worldwide measurements of the storage of DDT
and its metabolites in human body fat indicate that the extremes of
total exposure have varied by a factor of about 10, but that total
exposure for most populations has varied by a factor of no more than 3
(see Table 9).
DDT in the dust in a house (as indicated either by a history of
extensive household application of insecticides or by the finding of
relatively high levels of DDT in house dust) can contribute to the
storage of DDT-related compounds in persons living in the house
(section 5.1.4). However, although the contribution of house dust to
DDT intake has been established, it is not quite clear how this
contribution occurs. Some of the dust may contaminate food in the
process of preparation and some may be inhaled and later swallowed
after deposition in the upper airway. It is difficult to believe that
enough DDT is present in such houses in the form of vapour or
respirable dust to cause a substantial increase in the total exposure
of the inhabitants, but no critical study has been made of this
matter. Clearly, some DDT will enter by the respiratory route.
9.1.2 Infants and children
At birth, infants tend to have slightly lower levels of DDT than
adults in the same population. This is because the placenta offers
partial protection against the passage of DDT and related compounds.
Although human milk tends to have a somewhat higher concentration
of DDT than cow's milk (see section 6.3.1.3), the difference, if any,
that this makes to the rate at which breast-fed and bottle-fed babies
store the compound has not been established. It is possible that the
conditional acceptable daily intake (ADI) might be exceeded in an
infant wholly fed on breast milk. However, the ADI is calculated on
the basis of lifetime exposure, and short-term variations can be
regarded as not having any significance.
The only really important way in which the exposure of infants and
children differs from that of adults in the same community involves
accidental exposure (see section 8.1.3).
9.1.3 Occupational groups
Occupational exposure (section 5.3) to DDT is initially almost
exclusively through the respiratory and dermal routes. However, the
particles of many insecticidal dusts, wettable powders, and sprays are
too large to reach the lower respiratory tract. As a result, most of
the particles inhaled are deposited in the upper respiratory tract,
carried to the pharynx by ciliary action, and eventually swallowed
(section 6.1.1).
Although dermal exposure to DDT is high under some occupational
situations, the effect is minimal because the compound is so poorly
absorbed through the skin (section 6.1.3). The excellent safety record
of DDT, never matched by any other insecticide used in antimalaria
campaigns, other vector control programmes, and agriculture, is based
mainly on its poor absorption through the skin.
The number of people with full-time occupational exposure to DDT
alone is small. For example, at the time of one study, the only
factory making the compound in the USA produced 2722 metric tonnes per
month using a work force of about 145. Following the recommendations
of an Expert Committee, the World Health Organization studied spraymen
who had applied only DDT for 5 years or more. Only 272 suitable
subjects could be located in Brazil and only 144 in India. The
concentrations of DDT and its derivatives in the blood of the
preliminary and main study groups in India were 0.761 and
1.272 mg/litre, respectively. The blood levels of spraymen in Brazil
were about 3 times those of the controls.
The absorbed dosage of the men who made and formulated DDT for 10
years or more was about 18 mg/man per day (see section 8.1.1). The
exposure of other workers, notably those applying DDT for agricultural
purposes has usually been an order of magnitude less (see Table 7).
9.2 Effects of Exposure
No adverse effects have been described at repeated dosages of
1.5 (mg/kg)/day or less (see section 7.4). The large number of
measurements that have been made on samples from human populations
have not made it possible to define a maximum dosage that man can
absorb without any adverse effect but have highlighted the finding
that the high levels found in volunteers and workers were harmless for
at least 25 years.
Table 23 summarizes the clinical aspects of DDT in man.
Table 23. Dosage-effects of DDT in man
Single dose
(mg/kg) Observation
Unknown Fatal
16-286 Prompt vomiting at higher doses
(all poisoned, convulsions in some)
6-10 Moderate poisoning
Repeated
exposure
(mg/kg)/day
1.5 Administered as therapy for 6 monthsa
0.5 Administered to volunteers for 21 monthsa
0.5 Exposure of workers for 6.5 yearsa
0.25 Exposure of workers for 25 yearsa
0.0025 Intake of population in the USA, 1953-54a
0.0002 Intake of population in the USA, 1969-70a
a Without any adverse effect.
In considering the safety of workers who are employed in the DDT
industry, it is useful to consider the results of animal experiments.
Rats withstand a daily dosage at least 10 times that of these workers
without any detectable clinical effect (section 7, Table 17), although
minimal reversible tissue changes may be present. Dogs and monkeys
also withstand a daily dosage 10 times higher than that of the
workers, but they do not show the tissue changes, which seem to be
peculiar to some rodents. Because workers have tolerated high dosages
of DDT for over a fifth of a lifetime without detectable harm and
since animals withstand larger dosages for an entire lifetime without
injury, there is good reason to predict the continuing safety of the
workers.
The experience already gained from workers can be used to predict
the future safety of the general population in relation to DDT. Many
workers have now been exposed to DDT for much more than one-fifth of
their life span. Since they have not suffered detectable harm, it
seems most unlikely that the general population will be harmed by
dosages 200-1250 times smaller than those to which the workers are
exposed. It has been shown for at least 2 animal species that toxicity
resulting from a lifetime of exposure is seldom very different from
toxicity resulting from 90 days of exposure at the same dosage rate.
The largest factor of difference observed when 33 chemicals were
investigated was 20, and, for half of them, the factor was 2 or less
(see section 7.3.1.1). Ninety days constitute about one-eighth of the
lifespan of a rat, and this is less than the fraction of the human
life span that has been studied so far.
9.3 Carcinogenicity and Mutagenicity
Liver tumours are produced in mice by many chlorinated compounds,
including DDT. There is also evidence to suggest that DDT metabolites
DDE and TDE (DDD) produce hepatic tumours in mice and that TDE also
produces lung tumours. Information on the tumorigenicity of DDT in
rats (see section 7.1.9) is conflicting; some studies report tumour
formation while other studies report negative data. Carcinogenicity
studies in the hamster were negative (see section 7.1.9). The
occurrence of tumours in some rodents only, casts doubt on the
significance of the phenomenon and on extrapolation of the findings to
man.
Studies on the incidence of all cancers reported in those parts of
some countries with known high agricultural use of DDT in the 1950s
and early 1960s have not demonstrated any trends in any type of cancer
associated with the use of DDT in relation to these areas (see section
8.2.9).
The question as to whether DDT is carcinogenic in man has not been
answered unequivocally. Although the cross-sectional epidemiological
studies on workers exposed to DDT and the observation studies on
volunteers are limited, there is not any currently available evidence
to suggest that DDT is tumorigenic or carcinogenic in man (see section
8.2.9).
Recent studies on in vitro bacterial test systems with or
without metabolic activation have not shown any evidence that either
DDT or DDE is mutagenic (see section 8.2.10). The evidence for the
mutagenicity of DDT in mammalian test systems is inconclusive.
9.4 Effects on Microsomal Enzymes
There is no doubt that exposure to DDT results in the induction of
microsomal mixed function oxidases and causes marked morphological
changes in the liver of some rodents (see section 7.1.9). In some
rodents, notably the mouse, these morphological changes have been
related to tumorigenicity. Microsomal enzymes are also induced by DDT
in other species, but the liver does not show the same morphological
changes.
The only effect for which something approaching a threshold has
been demonstrated is the induction of microsomal enzymes in workers in
association with an average serum value of 0.573 mg/litre for p,p'-
DDT but not in workers with serum levels as high as 0.052 mg/litre, a
value essentially identical to the highest reported for the general
population. Furthermore, although some groups of workers experienced
an increase in their average enzyme activity, no person exceeded the
range of activity found in normal people in the general population.
DDT will not induce liver microsomal enzymes in the general
population because their intake of the compound is so much less than
the smallest dosage capable of producing this effect in animals or man
(see sections 7.1.4.2 and 8.2.5.1).
9.5 Reproduction and Teratogenicity
Effects on reproduction in mammals have been studied in the mouse,
the rat, and the dog (see section 7.1.7). In the mouse, a multi-
generation study at dietary levels of DDT of 25, 100, and 250 mg/kg
showed effects on fertility and reproduction only at the highest
level, equivalent to 33 (mg/kg)/day. In the rat, normal reproduction
was maintained at a dietary level of 200 mg/kg. In the dog, dietary
intake at dosages up to 10 (mg/kg)/day did not produce any effects on
reproduction other than earlier estrus in the DDT-treated females.
In man, there is no indication that DDT affects reproduction (see
section 8.2.7); no impairment of fertility was observed in a study of
men occupationally-exposed for more than 10 years to a measured
average daily intake in the region of 18 mg/man per day (equivalent to
0.25 (mg/kg)/day).
Studies in the mouse, the rat, and the dog have not shown any
evidence of teratogenicity. In the mouse, dosage at the rate of
1 mg/kg was not teratogenic; a single dosage of 25 mg/kg or repeated
doses at the rate of 2.5 mg/kg were embrytoxic but not teratogenic.
9.6 Immunosuppression
DDT appears to have a depressant effect on the immune system
although the evidence is by no means conclusive. Rats and rabbits
receiving DDT in aqueous suspension at a concentration of 200 mg/litre
showed a depression in antibody formation and decrease in at least one
globulin fraction of the blood. Rats receiving a dosage of
0.25 (mg/kg)/day by gavage did not show any changes in the phagocytic
activity of the white blood cells. In the guineapig, dosages of
1-20 mg/kg did not have any effects on antitoxin production but
produced a reduction in tissue histamine levels (see section 7.1.1).
9.7 Nutritional Effects and Other Factors
Animal studies indicate that nutritional status influences the
toxicity of DDT (see section 7.3.3). The preferential storage of DDT
in fat can mitigate the effect of acute poisoning. If rats that have
stored large amounts of DDT are starved, they may suffer toxic effects
due to mobilization of fat and DDT.
In man, nutritional status will have a similar effect to that
found in other animals. However, the possibility that starvation in
man could precipitate toxic manifestations is regarded as unlikely as
the stored levels do not approach those found in laboratory animals
and the lower metabolic rate of man results in slower mobilization. In
fact, severe weight loss sometimes does cause some increase in storage
of DDT in connexion with certain wasting diseases; however, people
with full-time occupational exposure to DDT average 10 times more
storage than the highest values reported in connection with disease
but do not exhibit predisposition to the diseases in question (see
section 6.2.1.3).
Although young animals are often more susceptible to toxic
chemicals than adults, there is no evidence that DDT is more toxic to
young animals of any species including man. In fact, in the rat, the
young are less susceptible to a single dose than the adults (see
section 7.3.2, Table 20).
9.8 Dosage-Effect Relationships
Dosage-effect relationships for DDT in man have been observed in
connection with acute poisoning (see Table 23), excretion, and storage
(see Fig. 4), and the induction of microsomal enzymes, which has been
observed at a dosage of 0.25 (mg/kg)/day but not at lower dosages. The
dosage of 0.25 (mg/kg)/day to which workers have been exposed for 25
years is of the same order of magnitude that causes an increase in
tumours in male mice of a susceptible strain but not in females of any
strain (see section 7.1.9). As shown in Table 24, this dosage in
workers is less than the no-effect levels for rats, dogs, and monkeys
and far less than the dosage at which rats, mice, and dogs
successfully reproduce for generations. The equilibrium levels of DDT
and its metabolites found in the blood and fat of people with
full-time occupational exposure and the much lower levels found in the
general population have not been associated with any adverse effects.
9.9 Recommendations on Levels of Exposure
The data from intake, exposure, and levels in populations supports
the current conditional ADI for DDT, which affords a considerable
margin of safety.
If the total intake of DDT from food and other sources rises above
0.005 (mg/kg)/day (the conditional ADI) then the situation should be
investigated.
Table 24. Dosage-effect of DDT in animals
Single Route Observation
(mg/kg)
3000 dermal LD50 of powder in adult rat
2356-4000 oral LD50 of oil solution in newborn rat
250-500 dermal LD50 of oil solution in adult rat
250 oral LD50 of oil solution in adult rat
Repeated
Exposure
(mg/kg)/day
300 subcutaneous inhibition of testicular growth in cockerels
41-80 oral increased mortality in rats, 2-year study
41-80 oral 100% mortality in dogs in 39-49 months
41-80 oral 100% mortality in monkeys, 70 days
21-40 oral 25% mortality in dogs in 39-49 months
33.2 oral harmful to reproduction in mice
13.3 oral slight reduction in lactation and survival of some but not all
generations of mice (6 generation test)
10 oral no harmful effect on reproduction in dog (3 generation test)
10 oral no harmful effect on reproduction in rat (2 generation test)
5-10 oral no-effect level in dog, 2 generations
2.6-5 oral no-effect level in monkey, 7.5 years
0.63-1.25 oral no-effect level in rat, 2 year test
0.16-0.31 oral risk of liver tumours doubled in male mice but no effect in
female
0.3 oral no-effect level for induction of microsomal enzymes in rats
The concentration of DDT in the air in industrial, agricultural,
or disease control areas should not exceed 1 mg/m3 on a time-
weighted basis (40 h per week). Several countries have their own
standards that range from 0.1 to 1 mg/m3, which seem to afford an
acceptable margin of safety.
There is ample reason to predict the continuing safety of workers
producing and using DDT. No harmful effect has ever been reported in
vector control operators who have applied DDT during the last 3
decades in public health programmes. Nevertheless, as in the case of
any chemical, occupational safety and health measures should always be
applied to ensure that contact with DDT by workers is kept to a
minimum.
The only index of exposure of DDT or its metabolites is the
analysis of these compounds in tissues or excreta. For most purposes
it is best to sample serum or plasma. In subjects with relatively
constant, prolonged exposure, concentrations of DDT and its
metabolites in the blood are in equilibrium with those in all other
tissues, including adipose tissue. In subjects who have accidentally
received a single large dose, the concentration in the brain is
reflected more accurately by a serum sample than by a fat sample.
REFERENCES
ABBOTT, D.C., HARRISON, R. B., TATTON, J. O'G., & THOMPSON, J. (1965)
Organochlorine pesticides in the atmospheric environment. Nature
(Lond.), 208: 1317-1318.
ABBOTT, D.C., GOULDING, R., & TATTON, J. O'G. (1968) Organochlorine
pesticide residues in human fat in Great Britain, Br. med. J.,
3: 146-149.
ABBOTT, D.C., HOLMES, D.C., & TATTON, J. O'G. (1969) Pesticide
residues in the total diet in England and Wales, 1966-67. J. Sci.
Food Agric., 20: 245-249.
ABBOTT, D. C, COLLINS, G. B., & GOULDING, R. (1972) Organochlorine
pesticide residues in human fat in the United Kingdom, 1969-71.
Br. med. J., 2: 553-556.
ABE, J., INOUE, Y., & TAKAMATSU, M. (1974) [On the residual amounts of
PCB and organochlorine pesticides in human blood.] Jpn J. Hyg.,
29: 93 (in Japanese).
ACKER, L. & SCHULTE, E. (1970) [The occurrence of polychlorinated
biphenyls and hexachlorobenzene along chlorinated insecticides in
human milk and human adipose tissue.] Naturwissenschaften,
57: 497 (in German).
ACKER, L. & SCHULTE, E. (1971) [Organochlorine compounds in the human
body.] Umschau, 71: 848 (in German).
ACKER, L. & SCHULTE, E. (1974) [Organochlorine compounds in human
fat.] Naturwissenschaften, 61: 32 (in German).
ADAMCZYK, E. (1971) [Translocation of DDT in chickens under the
influence of hunger.] Med. Weter, 27: 103-105 (in Polish).
ADAMOVIC, V., SOKIC, B., & PETROVIC, O. (1971) [On factors influencing
the occurrence of organochlorine insecticides in newborns.]
Ernaehrungsforschung, 16: 579-585 (in German).
ADAMS, M., COON, F. B., & POLING, C. E. (1974) Insecticides in the
tissues of four generations of rats fed different dietary fats
containing a mixture of chlorinated hydrocarbon insecticides.
J. agric. food Chem. 22: 69-75.
AHLING, B. & JENSEN, S. (1970) Reversed liquid-liquid partition in
determination of PCBs and chlorinated hydrocarbon insecticides in
water. Anal. Chem, 42: 1485-1486.
AHNHOFF, M. & JOSEFSON, B. (1974) Simple apparatus for on-site
continuous liquid-liquid extraction of organic compounds from
natural waters. Anal. Chem., 46: 658-663.
AIZICOVICI et al., Travaux Scientifiques de l'Institut d'Hygiene de
Jassy, 1960-1966, p. 142 (Quoted by: Wasserman, M., Sofoluwe,
G. O., Wasserman, D., Groner, Y., & Lazarovitch, S. (1968) Storage
of organochlorine insecticides in body fat of people in Nigeria.
In: Proceedings of the Lagos International Seminar on
Occupational Health for Developing Countries, Nigeria, 1-6 April
1968.
ALARY, J. G., GUAY, P., & BRODEUR, J. (1971) Effect of phenobarbital
on the metabolism of DDT in the rat and in the bovine. Toxicol.
appl. Pharmacol., 18: 457-468.
ALMEIDA, W. F., PIGATI, P., GAETA, R., & UNGARO, M. T. S. (1975) DDT
residues in human blood serum in Brazil. Environ. Qual. Saf.,
3 (Suppl.): 586-588.
ANON. (1972) [BHC and DDT residues in human milk on the decline in
Japan.] Noyaku Bijin, 56: 458 (in Japanese).
ANON. (1974) [Research methods for determination of chlorinated
hydrocarbon pesticide residues in or on food of animal origin.]
Bundesgesundheitsblatt, 17 (18): 269-276 (in German).
ANTOMMARIA, P., CORN, M., & DeMAIO, L. (1965) Airborne particulates in
Pittsburgh: Association with p,p'-DDT. Science, 150: 1476-1477.
ARMOUR, J. A. & BURKE, J. A. (1970) Separating polychlorinated
biphenyls from DDT and its analogues. J. Assoc. Off. Anal. Chem.,
53: 761-768.
ASAI, R. I., GUNTHER, F. A., WESTLAKE, W. E., & IWATA, Y. (1971)
Differentiation of PCBs from DDT by carbon skeleton chromatography.
J. agric. food. Chem., 19: 39-398.
ASKARI, E. M. & GABLIKS, J. (1973) DDT and immunological responses.
II. Altered histamine levels and anaphylactic shock in guineapigs.
Arch. environ. Health, 26: 309-312.
AUE, W. A. & TELI, P. M. (1971) Sampling air pollutants with support-
bonded chromatographic phases. J. Chromatogr., 62: 15-27.
BAGLEY, G. E., REICHEL, W. L., & CROMARTIE, E. (1970) Identification
of PCBs in two bald eagles by combined gas-liquid chromatography-
mass spectrometry. J. Assoc. Off. Anal. Chem., 53: 251-261.
BAKSEYEV, N. S. (1973) [Prophylaxis of pathology of the fetus and
newborn child (experience of the Ukrainian SSR).) Vestn. Akad.
Med. Nauk (SSSR), 28: 69-75 (in Russian).
BARKA, T. & POPPER, H. (1967) Liver enlargement and drug toxicity.
Medicine, 46: 103-117.
BELONOZKO, G. A. & KUCAK, JU. A. (1974) [Hygienic features of the
content of pesticides in the atmosphere.] In: Medved, L. E., ed.
Hygiene of application and toxicology of pesticides and the
clinical features of pesticides poisoning. A collection of
papers, Issue No. 5, pp. 63-74 Kiev. Vniigintox (in Russian).
BELONOZKO, G. A., VEVCHURKO, S. N., KUCHAK, U. A., TOVSTENKO, A. E., &
SOROKENA, L. V. (1967) [The spread of pesticides by air currents
in the environment in relation to method of application.] In:
Medved, L. E., ed. Hygiene and toxicology of pesticides and the
clinical features of pesticide poisoning. A collection of papers,
pp. 24-35 Kiev, Vniigintox (in Russian).
BENVENUE, A. & OGATA, J. N. (1970) Note on the use of florisil
adsorbent for separation of PCBs from chlorinated pesticides.
J. Chromatogr., 63: 142-144.
BERGENSTAL, D. M., HERTZ, R., LIPSETT, M. B., & MOY, R. H. (1960)
Chemotherapy of adrenocortical cancer with o,p'-DDD. Ann.
intern. Med., 53: 672-682.
BEYERMANN, K. & ECKRICH, W. (1973) [Adsorption of traces of
insecticides from water on polyethylene.] Z. anal Chem.,
265 (1): 1-4 (in German).
BEYERMANN, K. & ECKRICH, W. (1974) [Differentiation of the aerosol-
bound and gaseous fractions of insecticides in air.] Z. anal.
Chem., 269 (4): 279-284 (in German).
BEZUGLYI, V. P. & KASKEVICH, L. M. (1969) [Some indices of the
functional state of the liver in flying personnel engaged in
aerial chemical operations.] Gig. Tr. Prof. Zabol., 13 (10):
52-53 (in Russian).
BEZUGLYI, V. P., ODINTSOVA, I. L. R., & GORSKAYA, N. Z. (1973)
[Morphological composition of the blood in persons working with a
complex of organochlorine and organophosphorus pesticides.]
Vrach. Delox, 11: 150-154 (in Russian).
BICK, M. (1967) Chlorinated hydrocarbon residues in human body fat.
Med. J. Aust., 1: 1127-1129.
BIROS, F. J. (1970) Analysis for p,p'-DDT and p,p'-DDE pesticide
residues by NMR spectroscopy. J. Assoc. Off. Anal Chem.,
53: 733-736.
BISHARA, R. H., BORN, G. S., & CHRISTIAN, J. E. (1972a) Thin-layer
chromatography of DDT and some related compounds on aluminium
oxide chromatoplates. J. Chromatography, 64: 135-145.
BISHARA, R. H., BORN, G. S., & CHRISTIAN, J. E. (1972b) Radiotracer
distribution and excretion study of chlorophenothane in rats.
J. pharm. Sci, 61: 1912-1916.
BISKIND, M. S. (1952) [The new insecticides and the public health.]
Harefuah, 44: 9-13 (in Hebrew).
BISKIND, M. S. (1953) Public health aspects of the new insecticides.
Am. J. dig. Dis., 20: 331-341.
BISKIND, M. S. & BIEBER, I. (1949) DDT poisoning -- A new syndrome
with neuropsychiatric manifestations. Am. J. Psychother.,
3: 261-270.
BITMAN, J., CECIL, H. C., & FRIES, G. F. (1970) DDT-induced inhibition
of avian shell gland carbonic anhydrase. A mechanism for thin
eggshells. Science, 168: 594-596.
BJERK, J. E. (1972) [DDT and polychlorinated biphenyl residues in
human material from Norway.] Tidsskr, Nor. Laegeforen.,
92: 15-19, 37-38 (in Norwegian).
BLEDSOE, T., ROLAND, D. P., HEY, R. L., & LIDDLE, G. W. (1964) An
effect of o,p'-DDD on extra-adrenal metabolism of cortisol in
man. J. clin. Endocrinol. Metab., 24: 1303-1311.
BOIKO, V. G. & KRASNYUK, E. P. (1969) [Clinical characteristics of the
pathology of respiratory tracts of persons working with DDT.]
Z. ushn. nos. gorl. Bolezn., 29: 35-38 (in Russian).
BOJANOWSKA, A., JONCZYK, W., TRACZYK, Z., & KLAWE, Z. (1973) [DDT and
alpha-BHC content of blood and fatty tissue of subjects not
professionally exposed to these compounds.] Pol. Tyg. Lek.,
28: 1999-2001 (in Polish).
BOYD, E. M. & DECASTRO, E. S. (1968) Protein-deficient diet and DDT
toxicity. Bull. World Health Organ., 38: 141-150.
BOYD, E. M. & DECASTRO, E. S. (1970) Toxicity of dicophane (DDT) in
relation to dietary protein intake. Ind. Med., Surg.,
39: 229-236.
BOYD, E. M. & KRIJNEN, C. J. (1969) Dietary protein and DDT toxicity.
Bull. environ. Contam. Toxicol., 4: 256-261.
BRADY, M. N. & SIYALl, D. S. (1972) Hexachlorobenzene in human fat.
Med. J. Aust., 1: 158-161.
BRATOWSKI, T. A. & MATSUMURA, F. (1972) Properties of a brain
adenosine triphosphatase sensitive to DDT. J. econ. Entomol.,
65: 1238-1245.
BREWERTON, H. V. & MCGRATH, H. J. W. (1967) Insecticides in human fat
in New Zealand. N.Z.J. Sci., 10: 486-492.
BRODEUR, J. & LAMBERT, G. (1973) Further studies on the effect of
starvation and microsomal enzyme induction on the mobilization of
DDT residues in rats. Toxicol. appl. Pharmacol., 25: 488.
BRONISZ, H. & OCHYNSKI, J. (1968) [DDT and DDE content in the milk of
women.] Biul. Inst. Ochr. Rosl., 41: 99-102 (in Polish).
BRONISZ, H., RUSIECKI, W., OCHYNSKI, J., & BERNARD, E. (1967) [DDT in
adipose tissue of Polish population.] Diss. Pharm. Pharmacol.,
19: 309-314 (in Polish).
BRONISZ, H., OCHYSNSKI, J., & WLODZIMIERA, L. (1969) [Contents of DDT
in the adipose tissue of the Polish population.] Ann. Univ.
Mariae Curies -- Sklodwska. sect. D. (Med.), 24: 93-99 (in
Polish).
BROWN, J. R. (1967) Organochlorine pesticide residues in human depot
fat. Can. Med. Assoc, J., 97: 367-373.
BROWN, J. R. & CHOW, L. Y. (1975) Comparative study of DDT and its
derivatives in human blood samples in Norfolk County and Holland
Marsh, Ontario. Bull. environ. Contam. Toxicol., 13: 483-488.
BUNYAN, P. J., TOWNSHEND, M. G., & TAYLOR, A. (1972) Pesticide induced
changes in hepatic microsomal enzyme systems. Some effects of 1,1-
di( p-chlorophenyl)-2,2,2-trichloroethane (DDT) and 1,1-di( p-
chlorophenyl)-2,2-dichloroethylene (DDE) in the rat and Japanese
quail. Chem. Biol. Interactions, 5: 13-26.
BURLINGTON, H. & LINDERMAN, V. F. (1950) Effect of DDT on testes and
secondary sex characteristics of White Leghorn cockerels. Proc.
Soc. Exp. Biol., 74: 48-51.
BURNS, E. C., DAHM, P. A., & LINDQUIST, D. A. (1957) Secretion of DDT
metabolites in the bile of rats. J. Pharmacol. exp. Ther.,
121: 55-62.
BURNS, J. E. (1974) Organochlorine pesticide and polychlorinated
biphenyl residues in biopsied human adipose tissue -- Texas
1969-1972. Pestic. Monit. J., 7: 122-126.
BUTLER, P. A. (1969) Monitoring pesticide pollution. Biol. Sci.,
19: 889-891.
CAHILL, W. P., ESTESEN, B. J., & WARE, G. W. (1970) Determination of
DDT in presence of Toxaphene (Camphechlor) residues. Bull,
environ. Contam. Toxicol., 5: 260-262.
CAMERON, G. R. & BURGESS, F. (1945) The toxicity of 2,2-bis-( p-
chlorophenyl)-1,1,1-trichloroethane (DDT). Br. med. J.,
1: 865-871.
CAMPBELL, J. E., RICHARDSON, L. A., & SCHAFER, M. L. (1965)
Insecticide residues in the human diet. Arch. environ. Health,
10: 831-836.
CANLORBE, P., BADER, J. C., & JOB, J. C. (1971) Diagnostic problems
arising from a virilizing tumour of the adrenal cortex. (Case
report -- Literature review.) Sem. Hop., 47: 2255-2270.
CAREY, R. M., ORTH, D. N., & HARTMANN, W. H. (1973) Malignant melanoma
with ectopic production of adrenocorticotrophic hormone:
palliative treatment with inhibitors of adrenal steroid
biosynthesis. J. clin. Endocrinol. Metab., 36: 482-487.
CAROLLO, J. A. (1945) The removal of DDT from water supplies. J. Am.
Water Works Assoc., 37: 1310-1317.
CARR, R. L. (1970) Collaborative study of the Mills method of multiple
chlorinated pesticide residues in butter fat. J. Assoc. Off. Anal
Chem., 53: 152-154.
CARR, R. L. (1971) Collaborative study of a method of multiple
chlorinated pesticide residues in fish. J. Assoc. Off. Anal.
Chem., 54: 525-527.
CASARETT, L. J., FRYER, G. C., YAUGER, W. L., & KLEMMER, H. W. (1968)
Organochlorine pesticide residues in human tissues-Hawaii. Arch.
environ. Health, 17: 306-311.
CASE, R. A. M. (1945) Toxic effects of 2,2-bis(p-chlorophenyl)-1,1,1-
trichlorethane (DDT) in man. Br. med. J., 2: 842-845.
CASSIDY, W., FISHER, A. J., PEDEN, J. D., & PARRY-JONES, A. (1967)
Organochlorine pesticide residues in human fats from Somerset.
Mon. Bull. Minist. Health Lab. Serv. (Lond.), 26 (1): 2-6.
CHADWICK, R. W., CRANMER, M. F., & PEOPLES, A. J. (1971a) Metabolic
alterations in the squirrel monkey induced by DDT administration
and ascorbic acid deficiency. Toxicol. appl. Pharmacol.,
20: 308-318.
CHADWICK, R. W., CRANMER, M. F., & PEOPLES, A. J. (1971b) Comparative
stimulation of gamma-HCH metabolism by pre-treatment of rats with
gamma-HCH, DDT, and DDT + gamma-YCH. Toxicol. appl. Pharmacol.,
18: 685-695.
CHADWICK, R. W, LINKO, R. S., FREAL, J. J., & ROBBINS, A. L. (1975)
The effect of age and long-term low-level DDT exposure on the
response to enzyme induction in the rat. Toxicol. appl.
Pharmacol., 31: 469-480.
CHHABRA, R. S. & FOUTS, J. R. (1974) Stimulation of hepatic drug
metabolizing enzymes by DDT, polycyclic hydrocarbons or
phenobarbital in adrenalectomized or castrated mice. Toxicol.
appl. Pharmacol., 28: 465-476.
CHEN, JO-YUN, T. & DORITY, R. W. (1972) Infrared studies of DDT,
structurally related halogenated pesticides, and some metabolites.
J. Assoc. Off. Anal Chem., 55: 15-31.
CHIBA, M. (1969) Factors affecting the extraction of organochlorine
insecticides from soil. Residue Rev., 30: 63-113.
CHIBA, M. & MORLEY, H. V. (1968) Losses of pesticides during sample
preparation. J. Assoc. Off. Anal Chem., 51: 55-62.
CHIN, Y. & T'ANT, C. (1946) The effect of DDT on cutaneous sensation
in man. Science, 103: 654.
CIELESZKY, V., DENES, A., KNOLL, R., ENGST, R., & KOTARSKY, A. (1970)
[Methods for performing residue analysis of plant protection and
pest control agents in foodstuffs. II. Method for identifying and
the semiquantitative determination of residues of chlorinated
hydrocarbons in different food and in soil.] Nahrung,
14: 643-666 (in German).
CIUPE, R. (1976) [Determination of HCH, p,p'-DDT, p,p'-DDE in foods
and in the adipose tissue.] Igiena, 25: 113-117 (in Rumanian).
CLEMMESEN, J., FUGELSANG-FREDERICKSEN, V., & PLUM, C. M. (1974) Are
anticonvulsants oncogenic? Lancet, 1: 705-707.
CLIFFORD, N. J. & WEIL, J. (1972) Cortisol metabolism in persons
occupationally exposed to DDT. Arch. environ. Health,
24: 145-147.
COCHRANE, W. P. & CHAU, A. S. Y. (1971) Chemical derivitization
techniques for confirmation of organochlorine residue identity.
Pesticide identification at the residue level. Adv. Chem. Ser.,
140: 11-26.
COHA, F. & NEDIC, M. (1970) Improved gas-liquid chromatographic method
for analysis of DDT and its metabolites in milk. Anal. Lett.,
3: 419-421.
COHEN, J. M. & PINKERTON, C. (1966) Widespread translocation of
pesticides by air transport and rainout. Adv. Chem. Ser.,
60: 163-176.
COMMITTEE ON PESTICIDES (1951) Pharmacologic and toxicologic aspects
of DDT (chlorophenothane U.S.P.). J. Am. Med. Assoc.,
145: 728-733.
CONNEY, A. H. (1967) Pharmacological implications of microsomal enzyme
induction. Pharmacol. Rev., 19: 317-366.
CONOVER, W. J. (1971) Practical non-parametric statistics, New York,
Wiley & Sons, 462 pp.
COPELAND, F. M. & CRANMER, M. F. (1974) Effects of o,p'-DDT on the
adrenal gland and hepatic microsomal enzyme system of the beagle
dog. Toxicol. appl. Pharmacol., 27: 1-10.
COPPLESTONE, J. F., HUNNEGO, J. N., & HARRISON, D. L. (1973)
Insecticides in adult New Zealanders -- A five year study. N.Z.J.
Sci., 16: 27-39.
CRANMER, M. F. (1970) Effect of diphenylhydantoin on storage in the
rat. Toxicol. appl. Pharmacol., 17: 315.
CRANMER, M. F. (1972) Absence of conversion of o,p'-DDT to p,p'-DDT
in the rat. Bull. environ. Contam. Toxicol., 7: 121-124.
CRANMER, M. F. & COPELAND, M. F. (1973) Electron-capture gas-liquid
chromatographic analysis of DDA: utilization of 2-chloroethanol
derivative. Bull. environ. Contam. Toxicol., 9: 186-192.
CRANMER, M. F., CARROLL, J. J., & COPELAND, M. F. (1969) Determination
of DDT and metabolites, including DDA, in human urine by gas
chromatography. Bull. environ. Contam. Toxicol., 4: 214-223.
CRANMER, M. F., PEOPLES, A., & CHADWICK, R. (1972) Biochemical effects
of repeated administration of p,p'-DDT on the squirrel monkey.
Toxicol. appl. Pharmacol., 21: 98-101.
CROSBY, D. G. & MOILANEN, K. W. (1977). Vapor-phase photodecomposition
of DDT. Chemosphere, No. 4, pp 167-172.
CUETO, C., JR (1970) Cardiovascular effects of o,p'-DDD. Ind. Med.
Surg., 39: 31-32.
CUETO, C., JR & BIROS, F. J. (1967) Chlorinated insecticides and
related materials in human urine. Toxicol. appl. Pharmacol.,
10: 261-269.
CUETO, C., JR & BROWN, J. H. U. (1958) Biological studies on an
adrenocorticolytic agent and the isolation of the active
components. Endocrinology, 62: 334-339.
CUNNINGHAM, R. E. & HILL, F. S. (1952) Convulsions and deafness
following ingestion of DDT. Pediatrics, 9: 745-747.
CURLEY, A. & KIMBROUGH, R. (1969) Chlorinated hydrocarbon insecticides
in plasma and milk of pregnant and lactating women. Arch.
environ. Health, 18: 156-164.
CURLEY, A., COPELAND, M. F., & KIMBROUGH, R. D. (1969) Chlorinated
hydrocarbon insecticides in organs of stillborn and blood of
newborn babies. Arch. environ. Health, 19: 628-632.
CURLEY, A., BURSE, W. W., JENNINGS, R. W., & VILLANUEVA, E. C. (1973)
Chlorinated hydrocarbon pesticides and related compounds in
adipose tissue from people of Japan. Nature (Lond.),
242: 338-340.
CURRIE, L. A. (1968) Limits for qualitative detection and quantitative
determination. Anal. Chem., 40: 586-593.
CZEGLEDI-JANKO, G. (1969) [Residues of chlorinated hydrocarbons in the
blood of inhabitants of Budapest in 1967-1968 with particular
reference to lindane.] Z. Ges. Hyg. Ihre Grenzgeb., 15: 755-761
(in German).
CZEGLEDI-JANKO, G. & AVAR, P. (1970) Occupational exposure to lindane:
Clinical and laboratory findings. Br. J. ind. Med., 27: 283-286.
CZEGLEDI-JANKO, G. & CIELESZKY, V. (1968) A one-step extraction and
clean-up procedure for gas-liquid chromatographic determination of
some organochlorine pesticide residues in blood. Analyst,
93: 445-452.
DACRE, J. C. & JENNINGS R. W. (1970) Organochlorine insecticides in
normal and carcinogenic human lung tissues. Toxicol. appl.
Pharmacol., 17: 277.
DALE, W. E. & QUINBY, G. E. (1963) Chlorinated insecticides in the
body fat of people in the United States. Science, 142: 593-595.
DALE, W. E., GAINES, T. B., & HAYES, W. J., JR (1962) Storage and
excretion of DDT in starved rats. Toxicol. appl. Pharmacol.,
4: 89-106.
DALE, W. E., GAINES, T. B., HAYES, W. J., JR, & PEARCE, G. W. (1963)
Poisoning by DDT: Relation between clinical signs and
concentration in rat brain. Science, 142: 1474-1476.
DALE, W. E., COPELAND, M. F., & HAYES, W. J., JR (1965) Chlorinated
insecticides in the body fat of people in India. Bull. World
Health Organ., 33: 471-477.
DALE, W. E., COPELAND, M. F., PEARCE, G. W., & MILES, J. W. (1966a)
Concentration of o,p'-DDT in rat brain at various intervals
after dosing. Arch. int. Pharmacodyn. Ther., 162: 40-43.
DALE, W. E., CURLEY, A., & CUETO, C., JR (1966b) Hexane extractable
chlorinated pesticides in human blood. Life Sci., 5: 47-54.
DALE, W. E., CURLEY, A., & HAYES, W. J., JR (1967) Determination of
chlorinated insecticides in human blood. Ind. Med. Surg.,
36: 275-280.
DALE, W. E., MILES, J. W, & GAINES, T. B. (1970) Quantitative method
for determination of DDT and DDT metabolites in blood serum.
J. Assoc. Off. Anal. Chem., 53: 1287-1292.
DAL NOGARE, S. & JUVET, R. S. (1962) Gas-liquid chromatography, New
York, Interscience, 450 pp.
DAMASKIN, V. I. (1965) [The extent of the accumulation of DDT in the
human body in connection with its assimilation with food, and its
toxic effect.] Gig. i Sanit., 30 (10): 109-111 (in Russian).
DANGERFIELD, W. G. (1946) Toxicity of DDT to man. Br. med. J.,
1: 27.
DATTA, P. R. (1970) In vitro detoxification of p,p'-DDT via p,p'-
DDE to p,p'-DDA in rats. Ind. Med. Surg., 39: 190-194.
DATTA, P. R. & NELSON, M. J. (1968) Enhanced metabolism of
methyprylon, meprobamate, and chlordiazepoxide hydrochloride after
chronic feeding of a low dietary level of DDT to male and female
rats. Toxicol. appl. Pharmacol., 13: 346-352.
DATTA, P. R. & NELSON, M. F. (1970) p,p'-DDT detoxication by
isolated perfused rat liver and kidney. Ind. Med. Surg,
39: 195-198.
DAVIES, J. E., WELKE, J. O., & RADOMSKI, J. L. (1965) Epidemiological
aspects of the use of pesticides in the South. J. occup. Med.,
7: 612-618.
DAVIES, J. E., EDMUNDSON, W. F., SCHNEIDER, N. J., & CASSADY, J. C.
(1968) Problems of prevalence of pesticide residues in humans.
Pestic. Monit. J., 2: 80-85.
DAVIES, J. E., EDMUNDSON, W. R., CARTER, C. H., & BARQUET, A. (1969a)
Effect of anticonvulsant drugs on dicophane (DDT) residues in man.
Lancet, 2: 7-9.
DAVIES, J. E., EDMUNDSON, W. F., MACEO, A., BARQUET, A., & CASSADY, J.
(1969b) An epidemiologic application of the study of DDE levels in
whole blood. Am. J. Public Health, 59: 435-441.
DAVIES, J. E., EDMUNDSON, W. F., MACEO, A., IRVIN, G. L., III,
CASSADY, J., & BARQUET, A. (1971) Reduction of pesticide residues
in human adipose tissue with diphenylhydantoin. Food Cosmet.
Toxicol., 9: 413-423.
DAVIES, J. E., EDMUNDSON, W. F., & RAFEONELLI, A. (1975) The role of
house dust in human DDT pollution. Am. J. Public Health,
65: 53-57.
DAVIES, O. L. & GOLDSMITH, P. L. (1976) Statistical methods in
research and production, 4th ed., London, Longmans, 478 pp.
DEDEK, W. & SCHMIDT, R. (1972) Studies on the transplacental transport
and metabolism of 3H- and 14C-labeled DDT in pregnant mice
under hunger stress. Pharmazie, 27: 294-297.
DE FAUBERT MAUNDER, M. J., EGAN, H., GODLY, E. W., HAMMOND, E. W.,
ROBUM, J., & THOMSON, J. (1964) Clean-up of animal fats and dairy
products for the analysis of chlorinated pesticide residues.
Analyst, 89: 168-174.
DEICHMANN, W. B. & MACDONALD, W. E. (1976) Liver cancer deaths in the
continental USA from 1930 to 1972. Am. Ind. Hyg. Assoc. J.,
37: 495-498.
DEICHMANN, W. B. & MACDONALD, W. E. (1977) Organochlorine pesticides
and liver cancer deaths in the United States, 1930-1972.
Ecotoxicol. environ. Saf. 1: 89-110.
DEICHMANN, W. B. & RADOMSKI, J. L. (1968) Retention of pesticides in
human adipose tissue -- Preliminary report. Ind. Med. Surg.,
37: 218-219.
DEICHMANN, W. B., WITHERUP, S., & KITZMILLER, K. V. (1950) The
toxicity of DDT, Cincinnati, Kettering Laboratory of Applied
Physiology, 233 pp.
DEICHMANN, W. B., MACDONALD, W. E., & CUBIT, D. A. (1971a) DDT tissue
retention: Sudden rise induced by the addition of aldrin to a
fixed DDT intake. Science, 172: 275-276.
DEICHMANN, W. B., MACDONALD, W. E., BEASLEY, A. G., & CUBIT, D.
(1971b) Subnormal reproduction in beagle dogs induced by DDT and
aldrin. Ind. Med. Surg. 40 (2): 10-20.
DEICHMANN, W. B., MACDONALD, W. E., CUBIT, D. A., & BEASLEY, A. G.
(1972) Effects of starvation in rats with elevated DDT and
dieldrin levels. Int. Arch. Arbeitsmed., 29: 233-252.
DEL VECCHIO, V. & LEONI, V. (1967) [Study on the amount of chlorinated
insecticides in biological materials. II. Chlorinated insecticides
in adipose tissue in certain groups of the Italian population.]
Nuovi Ann. Ig. Microbiol., 18: 107-128 (in Italian).
DENES, A. (1962) [Problems of food chemistry concerning residues of
chlorinated hydrocarbons.] Nahrung, 6: 48-56 (in German).
DENES, A. (1964) Investigation of chlorinated hydrocarbon residues in
animal and vegetable fats. In: 1963 Year Book, Budapest,
Hungary, Institute of Nutrition.
DEUTSCHE FORSCHUNGSGEMEINSCHAFT (1969) [Residue analysis of plant
protection agents.] Weinheim, Verlag Chemie, 400 pp. (in German).
DE VLIEGER, M., ROBINSON, J., BALDWIN, M. K., CRABTREE, A. N., & VAN
DUK, M. C. (1968) The organochlorine insecticide content of human
tissue. Arch. environ. Health, 17: 759-767.
DOLAN, J. W. & HALL, R. C. (1973) Enhancement of the sensitivity and
selectivity of the Coulson electrolytic conductivity detector to
chlorinated hydrocarbon pesticides. Anal. Chem., 45: 2198-2204.
DOMENJOZ, R. (1944) [Experimental findings with a new insecticide
(Neocid, Geigy), a contribution to the theory contact-toxicity
action.] Schweiz. Med. Wochenschr., 74: 952-958 (in German).
DOMENJOZ, R. (1946a) [On the biological action of a DDT-derivative.]
Arch. Int. Pharmacodyn. Ther., 73: 128-146 (in German).
DOMENJOZ, R. (1946b) [Biological action of some DDT derivatives.]
Helv. Chem. Acta., 29: 1317-1322 (in French).
DRAIZE, J. H., NELSON, A. A., & CALVERY, H. O. (1944) The percutaneous
absorption of DDT (2,2-bis-(p-chlorophenyl)-1,1,1-trichloroethane)
in laboratory animals. J. Pharmacol. exp. Ther., 82: 159-166.
DUGGAN, R. E. (1968) Residues in food and feed. Pesticide residue
levels in food in the United States from July 1, 1963 to June 30,
1967. Pestic. Monit. J., 2: 2-46.
DUGGAN, R. E. & CORNELIUSSEN, P. E. (1972) Dietary intake of pesticide
chemicals in the United States (III), June 1968-April 1970.
Pestic. Monit. J., 5: 331-341.
DUGGAN, R. E. & LIPSCOMB, C. Q. (1969) Dietary intake of pesticide
chemicals in the United States (II), June 1966-April 1968.
Pestic. Monit. J., 2: 153-162.
DUGGAN, R. E. & WEATHERWAX, J. R. (1967) Dietary intake of pesticide
chemicals: Calculated daily consumption of pesticides with foods
are discussed and compared with currently accepted values.
Science, 157: 1006-1010.
DURHAM, W. F. & WOLFE, H. R. (1962) Measurement of the exposure of
workers to pesticides. Bull. World Health Organ. 26: 75-91.
DURHAM, W. F. & WOLFE, H. R. (1963) An additional note regarding
measurement of the exposure of workers to pesticides. Bull. World
Health Organ., 29: 279-281.
DURHAM, W. F., ARMSTRONG, J. F., UPHOLT, W. M., & HELLER, C. (1961)
Insecticide-content of diet and body fat of Alaskan natives.
Science, 134: 1880- 1881.
DURHAM, W. F., ORTEGA, P., & HAYES, W. J., JR (1963) The effect of
various dietary levels of DDT on liver function, cell morphology,
and DDT storage in the rhesus monkey. Arch. int. Pharmacodyn.
Ther., 141: 111-129.
DURHAM, W. F., ARMSTRONG, J. F., & QUNBY, G. E. (1965a) DDA excretion
levels. Arch. environ. Health, 11: 76-79.
DURHAM, W. F., ARMSTRONG, J. F., & QUINBY, G. E. (1965b) DDT and DDE
content of complete prepared meals. Arch. environ. Health,
11: 641-647.
DVORCHIK, B. H., ISTIN, M., & MAREN, T. H. (1971) Does DDT inhibit
carbonic anhydrase? Science, 172: 728-729.
DJATLOVlTSKAJA, F. G., GLADENKO, Y. F., & KRUCININA, A. A. (1972)
[Determination of organochlorine insecticides in reservoir water.]
Probl. anal Khim., 2: 43-36 (in Russian).
EDMUNDSON, W. F., DAVIES, J. E., & CRANMER, M. (1970a) DDT and DDE in
blood and urine of men exposed to 3 per cent DDT aerosol. Public
Health Rep., 85: 457-463.
EDMUNDSON, W. F., DAVIES, J. E., MACEO, A., & MORGADE, C. (1970b) Drug
and environmental effects on DDT residues in human blood. South.
med. J., 63: 1440-1441.
EGAN, H., GOULDING, R., ROBURN, J., & TATTON, J. O'G. (1965)
Organochlorine pesticide residues in human fat and human milk.
Br. med. J., 2: 66-69.
EICHELBERGER, J. W. & LICHTENBERG, J. J. (1971) Carbon adsorption for
recovery of pesticides (from water). J. Am. Water Works Assoc.,
63 (1): 25-27.
EMBRY, T. L., MORGAN, D. P., & ROAN, C. C. (1972) Search for
abnormalities of heme synthesis and sympathoadrenal activity in
workers regularly exposed to pesticides. J. occup. Med.,
14: 918-921.
ENGST, R. & KNOLL, R. (1972) DDT and DDE residues in human milk.
Nahrung, 16: 130-131.
ENGST, R., KNOLL, R., & NICKEL, B. (1967) [On the accumulation of
chlorinated hydrocarbons particularly of DDT and its metabolite
DDE in human fat.] Pharmazie, 22: 654-661 (in German).
ENGST, R., KNOLL, R., & NICKEL, B. (1970) [Occurrence of DDT and DDE
in fatty tissues and in organs of infants.] Pharmazie,
24: 673-676 (in German).
EPSTEIN, S.S. & SHAFNER, H. (1968) Chemical mutagens in the human
environment. Nature (Lond.), 219: 385-387.
ESKENASY, J. J. (1972) Status epilepticus by
dichlorodiphenyltrichloroethane and hexachlorocyclohexane
poisoning. Rev. Roum. Neurol., 9: 435-442.
EYZAGUIRRE, C. & LILIENTHAL, J. L., JR (1949) Veratrinic effects of
pentamethylenetetrazol (Metrazol) and 2,2-bis-( p-chlorophenyl)-
1,1,1-trichloroethane (DDT) on mammalian neuromuscular function.
Proc. Soc. Exp. Biol., 70: 272-275.
FAO (1972) Production yearbook-1971, Rome, Food and Agriculture
Organization, Vol. 25, pp. 499-537.
FAO/WHO (1973) 1972 Evaluations of some pesticide residues in food,
Geneva, WHO, 587 pp. (WHO Pest. Res. Series No. 2).
FARKAS, I., DESI, I., & KEMENY, T. (1969) [Effect of orally consumed
DDT on the rest and load EEG curves.] Egeszsegtudomany,
13 (2): 195-201 (in Hungarian).
FEIL, V. J., LAMOUREUX, C. H., STYRVOKY, E., ZAYLSKIE, R. G., THACKER,
E. J., & HOLMAN, G. M. (1973) Metabolism of o,p'-DDT in rats.
J. agric. food Chem, 21: 1072-1078.
FEIL, V. J., LAMOUREUX, C. H., & ZAYLSKIE, R. G. (1975) Metabolism of
o,p'-DDT in chickens. J. agric. food Chem., 23: 382-388.
FENNAH, R. G. (1945) Preliminary tests with DDT against insect pests
of food-crops in the Lesser Antilles. Trop. Agric., 22: 222-226.
FINSTERWALDER, C. E. (1976) Collaborative study of an extension of the
Mills et al. method for the determination of pesticide residues in
food. J. Assoc. Off. Anal. Chem, 59: 169-171.
FISEROVA-BERGEROVA, V., RADOMSKI, J. L., DAVIES, J. E., & DAVIES, J.
H. (1967) Levels of chlorinated hydrocarbon pesticides in human
tissues. Ind. Med. Surg., 36: 65-70.
FISHBEIN, L. F. (1974) Chromatographic and biological aspects of DDT
and its metabolites. J. Chromatogr., 98: 177-251.
FITZHUGH, O. G. (1948) Use of DDT insecticides on food products. Ind.
Eng. Chem., 40: 704-705.
FITZHUGH, O. G. (1966) Problems related to the use of pesticides.
Can. Med. Assoc. J., 94: 598-604.
FITZHUGH, O. G. (1970) A summary of a carcinogenic study of DDT in
mice from Food and Drug Administration, USA. In: FAO/ WHO 1969
evaluations of some pesticide residues in foods, Geneva. WHO,
pp. 61-64 (WHO/Food Add./70.38).
FITZHUGH, O. G. & NELSON, A. A. (1947) The chronic oral toxicity of
DDT (2,2-bis- p-chlorophenyl- 1,1,1-trichloroethane).
J. Pharmacol., 89: 18-30.
FRANCONE, M.P., MARLANI, F. H., & DEMARE, C. (1952) [Clinical signs of
poisoning by DDT.] Rev. Asoc. Méd. Argent., 66: 56-59 (in
Spanish).
FREDERICKS, C. M., LARSON, R. E., & ASTON, R. (1974) Effect of chronic
DDT exposure on pentobarbital tolerance and intolerance in the
rat. Toxicol. appl. Pharmacol., 27: 99-107.
FRENCH, M. C. & JEFFERIES, D. J. (1969) Degradation and disappearance
of ortho-, para-isomer of technical DDT in living and dead arian
tissues. Science, 165: 914-916.
FRIDMAN, G. I. (1970) [Effect of 7-trichlorofon and DDT on some
specific and non-specific characteristics of the
immunobiological and general reactivity of the organism (e.g.
problems of toxicological reactions of low intensity)] In:
[Questions of pesticide hygiene and toxicology.] Moscow,
Medicina, pp. 139-145 (in Russian).
FRIES, G. F., MARROW, G. S., JR, LESTER, J. W., & GORDON, C. H. (1971)
Effect of microsomal enzyme inducing drugs on DDT and dieldrin
elimination from cows. J. dairy Sci., 54: 364-368.
GÄB, S., NITZ, S., PARLAR, H., & KORTE, F. (1975) Photomineralisation
of certain aromatic xenobiotica. Chemosphere, No. 4,
pp. 251-256.
GÄB, S., SCHMITZER, J., THAMM, H. W., PARLAR, H. & KORTE, F. (1977)
Photomineralisation rate of organic compounds adsorbed on
particulate matter. Nature (Lond.), 270: 331-333.
GABLIKS, J. & MALTBY-ASKARI, E. (1970) The effect of chlorinated
hydrocarbons on drug metabolism in mice. Ind. Med. Surg.,
39: 347-350.
GABLIKS, J., ASKARI, E. M., & YOLEN, N. (1973) DDT and immunological
responses. I. Serum antibodies and anaphylactic shock in guinea
pigs. Arch. environ. Health, 26: 305-308.
GABLIKS, J., AL-ZUBAIDY, T., & ASKARI, E. (1975) DDT and immunological
responses. III. Reduced anaphylaxis and mast cell populations in
rats fed DDT. Arch. environ. Health, 30: 81-84.
GAINES, T. B. (1960) The acute toxicity of pesticides to rats.
Toxicol. appl. Pharmacol., 2: 88-99.
GAMES, T. B. (1969) Acute toxicity of pesticides. Toxicol. appl.
Pharmacol, 14: 515-534.
GALLAGHER, T. F., FUKUSHIMA, D. K., & HELLMANN, L. (1962) The effect
of ortho-, para-DDD on steroid borne metabolites in adrenocortical
carcinoma. Metab. clin. Exper., 11: 1155-1161.
GALTON, F. (1879) The geometric mean, in vital and social statistics.
Proc. R. Soc. Lond. B, 29: 365-367.
GARRETT, R. M. (1947) Toxicity of DDT for man. J. Med. Assoc.
Alabama, 17: 74-76.
GELLERT, R. J., HEINRICHS, W. L., & SWERDLOFF, R. S. (1972) DDT
homologues: Estrogen like effects on the vagina, uterus, and
pituitary of the rat. Endocrinology, 91: 1095-1100.
GENINA, S. A., SVETLAJA, E. N., & KOMAROVA, L. I. (1969) [Blood levels
of organic chlorine pesticides and some haematological indicators
in people employed in applying pesticides from the air.] In:
Medved', M. E., ed. The hygiene of application and the toxicology
of pesticides and the clinical features of pesticide poisoning. A
collection of papers, Issue No. 7, pp. 492-496, Kiev,
Vniigintoks (in Russian).
GIL G. P. & MIRON, B. F. (1949) [Investigation on toxicity of DDT to
man.] Med. Colon., 14: 459-470 (in Spanish).
GILBERT, D. & GOLBERG, L. (1967) BHT oxidase. A liver-microsomal
enzyme induced by the treatment of rats with butylated
hydroxytoluene. Food Cosmet. Toxicol., 5: 481-890.
GILLETT, J. W. (1968) No effect level of DDT in induction of
microsomal epoxidation. J. agric. food Chem., 16: 295-297.
GINGELL, R. & WALLCAVE, L. (1974) Species differences in the acute
toxicity and tissue distribution of DDT in mice and hamsters.
Toxicol. appl. Pharmacol., 28: 385-394.
GIUFFRIDA, L. & IVES, N. F. (1969) Microcoulomeric gas chromatography:
Improvement in instrumentation. J. Assoc. Off. Anal. Chem.,
52: 541-545.
GOERLITZ, D. F. & LAW, L. M. (1972) Chlorinated naphthalenes in
pesticide analysis. Bull. environ. Contain. Toxicol.,
7: 243-251.
GORDON, A. E. & FRIGERIO, A. (1972) Mass fragmentography as an
application of gas-liquid chromatograph-mass spectrometry in
biological research. J. Chromatogr., 73: 401-417.
GORDON, H. T. & WELSH, J. H. (1948) The role of ions in axon surface
reactions to toxic organic compounds. J. cell comp. Physiol.,
31: 395-420.
GRACA, I., SILVA FERDANANDES, A. M. S, & MOURAO, H. C. (1975)
Pesticides in people -- Organochlorine insecticide residues in
human milk in Portugal. Pestic. Monit. J., 8: 148-156.
GRACHEVA, G. V. (1969) [The possibility of DDT accumulation in the
organism of persons not having occupational contact with it.]
Faktory Vneshn. Sredy ikh Znachen, 1: 125-129 (in Russian).
GRACHEVA, G. V. (1970) [DDT excretion with the milk of nursing mothers
occupationally unexposed to the effect of this insecticide.] Vop.
Pitan., 29: 75-78 (in Russian).
GRIFFITH, F. D., JR & BLANKE, R. V. (1974) Microcoulometric
determination of organochlorine pesticides in human blood.
J. Assoc. Off. Anal Chem., 57: 595-603.
GRIFFITH, F. D., JR & BLANKE, R. V. (1975) Pesticides in people --
Blood organochlorine pesticide levels in Virginia residents.
Pestic. Monit. J., 8: 219-223.
GUENZI, W. D. & BEARD, W. E. (1968) Anaerobic conversion of DDT to DDD
and aerobic stability in soil. Proc. Soil Sci. Soc. Am.,
32: 522-534.
HAAG, H. B, EINNEGAN, J. K., LARSON, P.S., DREYFUSS, M. L., MAIN, R.
J., & RIESE, W. (1948) Comparative chronic toxicity for warm-
blooded animals of 2,2-bis( p-chlorophenyl)-1,1,1-trichloroethane
(DDT) and 2,2-bis-( p-chlorophenyl)-1,1-dichloroethane (DDD).
Ind. Med. Surg., 17: 477-484.
HAJKINA, B. I. & SILINA, V. F. (1971) [The effect of some
organochlorine pesticides on serotonin metabolism.] Farmakol.
Toxikol., 34: 357-359 (in Russian).
HALACKA, K., KAKL, J., & VYMETAL, F. (1965) [A reflexion in human fat
tissue of the extensive use of DDT.] Czech. Hyg., 10: 188-192
(in Czech).
HANADA, M., YUTANI, C., & MIYAJI, T. (1973) Induction of hepatoma in
mice by benzene hexachloride. Gann, 64: 511-513.
HARA, A., OWASAKI, I., NAWA, H, YOSHIOKA, Y., & YOKOO, S. (1973)
[Organochlorine insecticide residues in blood of pregnant women
and in umbilical cord blood.] Prog. Med., 84: 79-80 (in
Japanese).
HARMAN, S. W. (1946) DDT for codling moth control in western New York
in 1945. J. econ. Entomol., 39: 208-210.
HARRISON, R. D. (1975) Comparative toxicity of some selected
pesticides in neonatal and adult rats. Toxicol. appl. Pharmacol.,
32: 443-446.
HART, L. G. & FOUTS, J. R. (1963) Effects of acute and chronic DDT
administration on hepatic microsomal drug metabolism in the rat.
Proc. Soc. Exp. Biol. Med., 114: 388-392.
HART, M. M., REGAN, R. L., & ADAMSON, R. H. (1973) The effect of
isomers of DDD on the ACTH-induced steroid output, histology, and
ultrastructure of the dog adrenal cortex. Toxicol. appl.
Pharmacol., 24: 101-113.
HARTLEY, G. S. (1969) Evaporation of pesticides. In: Gould, R. F., ed.
Pesticidal Formulations Research, Washington, DC, American
Chemical Society, pp. 115-134 (Adv. Chem. Ser. 86).
HATTULA, M. L., IKKALA, J., ISOMÄKI, M., MÄÄTTÄ, K., & ARSTILA, A. U.
(1976) Chlorinated hydrocarbon residues (PCB and DDT) in human
liver, adipose tissue and brain in Finland. Acta Pharmacol.
Toxicol., 39: 545-554.
HAYASHI, M. (1972a) [Pollution of mothers' milk by organochlorine
pesticides.] Jpn J. Public Health, 19: 437-441 (in Japanese).
HAYASHI, M. (1972b) [Pesticide pollution of mothers' milk.] J. Jpn
Med. Assoc., 68: 1281-1286 (in Japanese).
HAYES, W. J., JR (1959) Pharmacology and toxicology of DDT. In:
Müller, P., ed. DDT: The insecticide dichlorodiphenyltri-
chloroethane and its significance, Basel, Birkhäuser Verlag,
Vol. 2, pp. 9-247.
HAYES, W. J., JR (1965) Review of the metabolism of chlorinated
hydrocarbon insecticides especially in mammals. Ann. Rev.
Pharmacol., 5: 27-52.
HAYES, W. J., JR (1975) Toxicology of pesticides, Baltimore,
Williams & Wilkins Co., 580 pp.
HAYES, W. J., JR (1976a) Mortality in 1969 from pesticides, including
aerosols. Arch. environ. Health, 31: 61-72.
HAYES, W. J., JR (1976b) Dosage relationships associated with DDT in
milk. Toxicol. appl. Pharmacol., 38: 19-28.
HAYES, W. J., JR & DALE, W. E. (1964) Concentration of DDT in brain
and other tissues in relationship to symptomatology. Toxicol.
appl. Pharmacol., 6: 349.
HAYES, W. J., JR, DURHAM, W. F., & CUETO, C., JR (1956) The effect of
known repeated oral doses of chlorphenothane (DDT) in man. J. Am.
Med. Assoc., 162: 890-897.
HAYES, W. J., JR, QUINBY, G. E., WALKER, K. C., ELLIOTT, J. W., &
UPHOLD, W. M. (1958) Storage of DDT and DDE in people with
different degrees of exposure to DDT. Am. Med. Assoc. Arch. ind.
Health, 18: 398-496.
HAYES, W. J., JR, DALE, W. E., & LEBRETON, R. (1963) Storage of
insecticides in French people. Nature (Lond.), 199: 1189-1191.
HAYES, W. J., JR, DALE, W. E., & BURSE, V. W. (1965) Chlorinated
hydrocarbon pesticides in the fat of people in New Orleans. Life
Sci., 4: 1611-1615.
HAYES, W. J, JR, DALE, W. E., & PIRKLE, C. I. (1971) Evidence of
safety of long-term, high, oral doses of DDT for man. Arch.
environ. Health, 22: 119-135.
HEATH, D. F. & VANDEKAR, M. (1964) Toxicity and metabolism of dieldrin
in rats. Br. J. ind. Med, 21: 269-279.
HEINRICKS, W. J., GELLERT, R. J., BAKKE, J. L., & LAWRENCE, N. L.
(1971) DDT administered to neonatal rats induces persistent estrus
syndrome. Science, 173: 642-643.
HELLMAN, L., BRADLOW, H. L., & ZUMOFF, B. (1973) Decreased conversion
of androgens to normal 17-ketosteroid metabolites as a result of
treatment with o,p'-DDD. J. clin. Endocrinol. Metab.,
36: 801-803.
HENDERSON, G. L. & WOOLLEY, D. E. (1969) Tissue concentrations of DDT:
Correlation with neurotoxicity in young and adult rats. Proc.
West. Pharmacol. Soc., 12: 58-62.
HENDERSON, G. L. & WOOLLEY, D. E. (1970) Mechanisms of neurotoxic
action of 1,1,1-trichloro-2,2-bis-( p-chlorophenyl)-ethane (DDT)
in immature and adult rats. J. Pharmacol. exp. Ther.,
175: 60-68.
HERZEL, F. & LAHMANN, E. (1973) [Contribution to quantitative
determination of pesticides in the air.] Gesundh.-Ing,
94: 275-279 (in German).
HESS, A.D. & KEENER, G. G., JR (1947) Effect of airplane-distributed
DDT thermal aerosols on fish and fish food organisms. J. Wildl.
Manage., 11: 1-10.
HEYNDRICKX, A. & MAES, R. (1969) The excretion of chlorinated
hydrocarbon insecticides in human mother milk. J. Pharm. Belg.,
9-10: 459-463.
HIDAKA, K., OHE, T., & FUJIWARA, K. (1972) [PCB and organochlorine
pesticides in mother's milk.] Igakuno Ayumi 82: 519-520 (in
Japanese).
HILL, D. W. & MCCARTY, P. L. (1967) Anaerobic degradation of selected
chlorinated hydrocarbon pesticides. J. Water Pollut. Control
Fed., 39: 1259-1277.
HILTON, B. D. & O'BRIEN, R. D. (1970) Antagonism by DDT of the effect
of valinomycin on synthetic membrane. Science, 168: 841-843.
HOFFMANN, C. H. & MERKEL, E. P. (1948) Fluctuations in insect
populations associated with aerial applications of DDT to forests.
J. econ. Entomol., 41: 467-473.
HOFFMANN, C. H. & SURBER, E. W. (1948) Effects of an aerial
application of wettable DDT on fish and fish food organisms in
Back Creek, West Virginia. Trans. Am. Fish. Soc., 75: 48-58.
HOFFMAN, D. G., WORTH, H. M., EMMERSON, J. L., & ANDERSON, R. C.
(1970) Stimulation of hepatic drug-metabolizing enzymes by
chlorphenothane (DDT); the relationship to liver enlargement and
hepatotoxicity in the rat. Toxicol. appl. Pharmacol.,
16: 171-178.
HOFFMAN, D. L. & MATTOX, V. R. (1972) Treatment of adrenocortical
carcinoma with o,p'-DDD. Med. Clin. North Am., 56: 999-1012.
HOFFMAN I. & LENDLE, L. (1948) [On the action of new insecticide
compounds.] Arch. exp. Pharmakol., 205: 223-242 (in German).
HOFFMAN, W. S. (1968) Clinical evaluation of the effects of pesticides
in man. Ind. Med. Surg., 37: 289-292.
HOFFMAN, W. S., FISHBEIN, W. I., & ANDELMAN, M. B. (1964) The
pesticide content of human fat tissue. Arch. environ. Health,
9: 387-394.
HOFFMAN, W. S., ADLER, H., FISHBEIN, W. I., & BAUER, F. C. (1967)
Relation of pesticide concentrations in fat to pathological
changes in tissues. Arch. environ. Health, 15: 758-765.
HOLDEN, A. V. (1970) International co-operative study of
organochlorine pesticide residues in terrestrial and aquatic
wildlife, 1967/68. Pestic. Monit. J., 4: 117-135.
HOLDEN, A. V. (1973) International co-operative study of
organochlorine and mercury residues in wildlife. Pestic. Monit.
J., 7: 37-52.
HORNABROOK, R. W., DYMENT, P. G., GOMES, E. D., & WISEMAN, J. S.
(1972) DDT residues in human milk from New Guinea natives. Med.
J. Aust., 1: 1297-1300.
HORWITZ, W., ed. (1975) Official methods of analysis, Washington,
DC, Association of the Official Analytic Chemists, 1094 pp.
HOWELL, D. E. (1948) A case of DDT storage in human fat. Proc. Okla.
Acad. Sci., 29: 31-32.
HRDINA, P. D., SINGHAL, R. L., PETERS, D. A. V., & LING, G. M. (1973)
Some neurochemical alterations during acute DDT poisoning.
Toxicol. appl. Pharmacol., 25: 276-288.
HRDINA, P. D., SINGHAL, R. L., & LING, G. M. (1975) DDT and related
hydrocarbon insecticides: Pharmacological basis of their toxicity
in mammals. Adv. Pharmacol. Chemother., 12: 31-88.
HRUSHKA, J. (1969) [DDT residues in the milk, butter, and fat of
cattle.] Veterinarstvi, 19 (11): 492-498 (in Czech).
HRUSHKA, J. & KOCIANOVA, M. (1975) [Contamination of the food chain by
chlorinated insecticides in Southern Bohemia.] Czech. Hyg.,
20: 421-428 (in Czech).
HSIEH, H. C. (1954) DDT intoxication in a family in Southern Taiwan.
Am. Med. Assoc. Arch. ind. Hyg., 10: 334-346.
HUEPER, W. C. (1942) Occupational tumors and allied diseases,
Springfield. IL. Thomas, pp. 496-497.
HUNTER, C. G., ROBINSON, J., & RICHARDSON, A. (1963) Chlorinated
insecticide content of human body fat in southern England.
Br. med. J., 1: 221-224.
HUTTERER, F., SCHAFFNER, F., KLION, F. M., & POPPER, H. (1968)
Hypertrophic, hypoactive smooth endoplasmic reticulum: A sensitive
indicator of hepatotoxicity exemplified by dieldrin. Science,
161: 1017-1019.
INNES, J. R. M., ULLAND, B. M., VALERIO, M. G., PETRUCELLI, L.,
FISHBEIN, L., HART, E. R., PALLOTTA, A. J., BATES, R. R., FALK, H.
L., GART, J. J., KLEIN, M., MITCHELL, & PETERS, J. (1969) Bioassay
of pesticides and industrial chemicals for tumorigenicity in mice.
A preliminary note. J. Natl Cancer Inst., 42: 1101-1114.
INOUE, Y., ABE, J., TAKAMATSU, M., & AOKI, N. (1974) [A study of the
qualitative and quantitative ratio of PCB and organochlorine
pesticide residues in the blood and adipose tissue.] Jpn J. Hyg.,
29: 92 (in Japanese).
INSTITUTE OF PETROLEUM (1968) Recommended practice for determining
precision data for methods on petroleum products and lubricants,
London, Institute of Petroleum.
ISRAELI, VON R. & MAYERSDORF, A. (1973) [Pathological changes in the
EEG during work with halogen-containing insecticides.] Zentralbl.
Arbeitsmed., 11: 340-343 (in German).
INTERNATIONAL AGENCY FOR RESEARCH ON CANCER (1974) IARC monographs:
The evaluation of the carcinogenic risk of chemicals to man, some
organochloride pesticides, Lyons, IARC, 241 pp. (IARC
monographs, Vol. 5).
ITO, N., NAGASAKI, H., ARAI, M., SUGIHARA, S., & MAKIURA, S. (1973)
Histological and ultrastructural studies on the
hepatocarcinogenicity of benzene hexachloride in mice. J. Natl
Cancer Inst., 51: 817-826.
JAGER, K. W. (1970) Aldrin, Dieldrin, Endrin, and Telodrin: An
epidemiological and toxicological study of long-term occupational
exposure, New York, Elsevier, 239 pp.
JANSSON, B., JENSEN, S., OLSSON, M., RENBERG, L., SUNDSTRÖM, G., &
VAZ, R. (1975) Identification by GC-MS of phenolic metabolites of
PCB and p,p'-DDE isolated from Baltic guillemot and seal.
Ambio, 4: 93-97.
JENSEN, S. & JANSSON, B. (1976) Anthropogenic substances in seal from
the Baltic: Methyl sulfone metabolites of PCB and DDE. Ambio,
5: 257-260.
JENSEN, J. A., CUETO, C., JR, DALE, W. E., ROTHE, C. F., PEARCE, G.
W., & MATTSON, A. M. (1957) Metabolism of insecticides. DDT
metabolites in feces and bile of rats. J. agric. food Chem.,
5: 919-925.
JEYARATNAM, J. & FORSHAW, J. (1974) A study of the cardiac effects of
DDT in laboratory animals. Bull. World Health Organ.,
51: 531-535.
JOHNSON, L. G. (1973) Formation of pentafluorobenzyl derivatives for
the identification and quantitation of acid and phenolic pesticide
residues. J. Assoc. Off. Anal. Chem., 56: 1503-1505.
JONCZYK, H. (1970) [The content of organochlorine insecticides in the
blood of healthy persons.] Rocz. panstw. Zakl. Hig., 21: 589-593
(in Polish).
JONCZYK, H., DMOCH, J., & BOJANOWSKA, A. (1970) [Determination of
chlorinated hydrocarbons in adipose tissue and brain of
Partridges.) Rocz. panst. Zakl. Hig., 21: 409-415 (in Polish).
JONCZYK, H., RUDOWSKI, W., TRACZYK, Z., & KLAWE, Z. (1974) [DDT and
its metabolites and gamma-HCH in blood, bone marrow, and fatty
tissue in certain hematological syndromes.] Pol. Tyg. Lek.,
29: 1573-1576 (in Polish).
JOY R. M. (1973) Electrical correlates of preconvulsive and convulsive
doses of chlorinated hydrocarbon insecticides in the CNS.
Neuropharmacology, 12: 63-76.
JUDAH, J. D. (1949) Studies on the metabolism and mode of action of
DDT. Br. J. Pharmacol, 4: 120-131.
JUDE, A. & GIRARD, P. (1949) [DDT toxicity -- mass poisoning by
accidental ingestion.] Ann. Med. lég. Criminol., Police Sci. Med.
Soc. Toxicol., 29: 209-213 (in French).
JUSZKIEWlCZ, T. & STEC, J. (1971) [Polichlorinated insecticide
residues in adipose tissue of farmers in the Lublin Province
(Poland).] Pol. Tyg. Lek., 26: 462-464 (in Polish).
KACEW, S. & SINGHAL, R. L. (1972) Role of adrenals in acute effects of
p.p'-DDT renal glucose metabolism. Fed. Proc., 31 (2): 520 Abs
(Abstract No. 1727).
KACEW, S. & SINGHAL, R. L. (1973) Adaptive response of hepatic
carbohydrate metabolism to oral administration of p,p'-1,1,1-
trichloro-2,2-bis-( p-chlorophenyl)-ethane in rats. Biochem.
Pharmacol., 22: 47-57.
KADIS, V. W., BREITKREITZ, W. E., & JONASSON, O. J. (1970) Insecticide
levels in human tissues of Alberta residents. Can. J. Public
Health, 61: 413-416.
KAGAN, Y. S., RODIONOV, G. A., WORONINA, L. Y., VELICHKO, L. S.,
KULAGIN, O. M., & PEREMITINA, A.D. (1969) [Effect of DDT on the
functional and morphological condition of the liver.] Vrach.
Delo, 12: 101-105 (in Russian).
KAISER, H. (1973) Guiding concepts relating to trace analysis. Pure
appl. Chem., 34: 35-61.
KAKU, S. (1973) [An epidemiological study on organochlorine pesticide
contamination. On pesticide residues in plasma of so-called
healthy Japanese in 1971.] J. Kurume Med. Assoc., 36: 307-326
(in Japanese).
KALISER, L. A. (1968) An in vitro and in vivo study of the effect
of DDT on the phagocytic activity of rat white blood cells.
Toxicol. appl. Pharmacol., 12: 353-357.
KALOYANOVA, F., MIHAILOVA, Z., GEORGIV, G., BENCHEV, I., RISOV, V., &
VELICHKOVA, V. (1972) Organochlorine pesticides in the fat of the
general population of Bulgaria. Abstracts of the XVII
International Congress on Occupational Health, Buenos Aires,
p. 75.
KAMATA, T. (1974) [On the present status of environmental pollution by
organochlorine pesticide residues.] Jpn J. Public Health,
21: 163-164 (in Japanese).
KAMATA, T. (1974) [Hygienic studies on pesticide residues: Part 4.
Environmental contamination by organochlorine pesticides.] Med.
J. Hiroshima Univ., 22: 315-325 (in Japanese).
KANITZ, S. & CASTELLO, G. (1966) [On the presence of residues of some
disinfectants in human adipose tissue and in food.] G. Ig. Med.
Prev., 7: 1-19 (in Italian).
KARIMOV, A. M. (1969) [Skin sensitivity to organochlorine
insecticides.] Med. Zh. Uzbekistant (No. 9): 58-60 (in Russian).
KARIMOV, A. M. (1970) [Occupational skin diseases in cotton growers
caused by chemical poisons and measures for their prevention.]
Gig. Tr. Prof. Zabol., 14: 35-37 (in Russian).
KASAI, A., ASANU, A. S., & NAKAMURA, S. (1972) [Studies on
organochlorine pesticide residues in human organs, Part III.]
J. Jpn Assoc. Rural Med., 21: 296-297 (in Japanese).
KATO, K., YAMADA, T., WATANABE, S., WADA, Y., FUKUDA, M., KURODA, M.,
NAKAOKA, S., TAKAHASHI, W., MIYASHIRO, K., MASUAD, J. & YASUKATA,
S. (1971) [Analyses of residual pesticides in vegetables, cows'
milk, and mothers' milk.] Ann. Rep. Kanagawa Prefect. Inst.
Public Health, 21: 85-92 (in Japanese).
KAWAI, Y., HORI, Y., NIGAWA, Y., YAMAMOTO, I., TSUZUKI, T., KITAYAMA,
M., & MORI, K. (1973) [On the pollution of mothers' milk by
pesticides and PCB in Hokkaido.] J. Food Hyg. Soc. Jpn,
14: 302-303 (in Japanese).
KAWANISHI, A., ASANUMA, S., & NAKAMURA, S. (1973) [Studies on
organochlorine pesticide residues in humans. Report 4.] J. Jpn
Assoc. Rural Med., 22: 278-279 (in Japanese).
KAZEN, C., BLOOMER, A., WELCH, R., OUDBIER, A., & PRICE, H. (1974)
Persistence of pesticides on the hands of some occupationally
exposed people. Arch. environ. Health, 29: 315-318.
KEIL, J. E., SANDIFER, S. H., FINKLEA, J. H., & PRIESTER, L. E. (1972)
Serum vitamin A elevation in DDT exposed volunteers. Bull.
environ. Contam. Toxicol., 8: 317-320.
KELLER, H. (1952) [Dichlorodiphenyltrichloroethane (DDT), additional
investigations on its properties and application.]
Naturwissenschaften, 39: 109 (in German).
KEPLINGER, M. L, DEICHMANN, W. B., & SALA, F. (1970) Effect of
combinations of pesticides on reproduction in mice. In: Deichmann,
W. B., ed. Pesticides Symposia, Miami, Halos & Associates,
pp. 125-138.
KHAIRY, M. (1959) Changes in behavior associated with a nervous system
poison (DDT). Q. J. exp. Physiol., 11 (2): 91-94.
KIMBROUGH, R. D., GAINES, T. B., & HAYES, W. J., JR (1968) Combined
effect of DDT, pyrethrum, and piperonyl butoxide on rat liver.
Arch. environ. Health, 16: 333-341.
KINOSHITA, F. K., FRAWLEY, J. P., & DUBOIS, K. P. (1966) Quantitative
measurement of induction of hepatic microsomal enzymes by various
dietary levels of DDT and toxaphene in rats. Toxicol. appl.
Pharmacol, 9: 505-513.
KLEIN, W. & KORTE, F. (1970) [Metabolism of chlorinated hydrocarbons.]
In: Wegler, R., ed. Chemie der Pflanzenschutz-und Schädlings-
bekämpfungsmittel, Band I, Berlin, Springer Verlag, pp. 199-218
(in German).
KLEIN, A. K., LAUG, E. P., KATTA, P. R., & MENDEL, J. L. (1965)
Evidence for the conversion of o,p'-DDT (1,1,1-trichloro-2- o-
chlorophenyl-2- p-chlorophenylethane) to p,p'-DDT (1,1,1-
trichloro-2,2-bis( p-chlorophenyl)ethane) in rats. J. Am. Chem.
Assoc., 87: 2520-2522.
KLEINFELD, M. (1967) Cancer of urinary bladder in a dye plant: A
medical and environmental study. In: Deichmann, W. B., ed.
Bladder Cancer, A Symposium, Birmingham, Al., Aesculapius,
pp. 136-144.
KNIPLING, E. F. (1959) The use of DDT in veterinary medicine. In:
Müller, P., ed. DDT, the insecticide dichlorodiphenyltri-
chloroethane and its significance, Basel, Switzerland, Birkhäuser
Verlag, Vol. II, pp. 505-570.
KNOLL, W. & JAYARAMAN, S. (1973a) [Organochlorine pesticide residues
in human milk.] Z. Gesamt. Hyg., 19: 43-45 (in German).
KNOLL, W. & JAYARAMAN, S. (1973b) [On the contamination of human milk
with chlorinated hydrocarbons.] Nahrung, 17: 599-615 (in German).
KOJIMA, S., SAITO, M., KONNO, H., & OZAWA, K. (1971) [Results of
investigation of organochlorine pesticide residues in mothers'
milk and in the blood of the mothers.] Rep. Akita Prefect. Inst.
Public Health, 16: 65-68 (in Japanese).
KOLMODIN, B., AZARNOFF, D. L., & SJOQVIST, F. (1969) Effect of
environmental factors on drug metabolism: Decreased plasma half-
life of antipyrine in workers exposed to chlorinated hydrocarbon
insecticides. Clin. Pharmacol. Ther, 10: 638-642.
KOLYADA, I. S. & MIKHAL'CHENKOVA, O. F. (1973) [Acute intoxication by
chlorine-containing pesticides.] Gig. Tr. Prof. Zabol., 5: 43
(in Russian).
KOMAROVA, L. I. (1970) [The excretion of DDT in mothers' milk and its
effect on the organism of mother and child.] Pediatr. Akus.
Ginek., 1: 19-20 (in Russian).
KONTEK, M., KUBACKI, S., PARADOWSKI, S., & WIERZCHOWIECKA, B. (1971)
[Chlorinated insecticides in human milk.] Pediatr. Pol.,
46: 183-188 (in Polish).
KOSTER, R. (1947) Differentiation of gluconate, glucose, calcium, and
insulin effects on DDT poisoning in cats. Fed. Proc., 6: 346.
KOSTIUK, O. T. & MUKHTAROVA, N. D. (1970) [Catecholamines and the
functional state of the hypothalamus under the action of a complex
of organochlorine and organophosphorus pesticides.] Gig. Tr.
Prof. Zabol., 14: 35-38 (in Russian).
KRASNIUK, E. P. & PLATONOVA, V. I. (1969) [Functional disorders of the
stomach under prolonged effects of organochlorine chemical
poisons.] Vrach. Delo, No. 9, 99-101 (in Russian).
KRASNIUK, E. P., LOGANOVSKII, N. G., MAKOVSKAYA, E. I., & RAPPOPORT,
M. B. (1968) [Functional and morphological changes in the kidneys
during the effects of DDT on the body.] Soy. Med., 31: 38-42
(in Russian).
KRAUL, I. & KARLOG, O. (1976) Persistent organochlorinated compounds
in human organs collected in Denmark, 1972-1973. Acta Pharmacol.
Toxicol., 38: 38-48.
KROGER, M. (1972) Insecticide residues in human milk. J. Pediatr.,
80: 401-405.
KUCINSKI, A. L. (1972) [On the extraction of DDT and hexachlorane from
some canned foods of animal and vegetable origin.] Vopr. Pitan.,
31 (1): 93 (in Russian).
KUN'EV, V. V. (1965) [Influence of low DDT doses on the phagocytic
state of the blood of albino rats.] Vopr. Pitan., 24: 74-78 (in
Russian).
KUNTZMANN, R., JACOBSON, M., LEVIN, W., & CONNEY, D. H. (1968)
Stimulatory effect of N-phenylbarbitol on cortisol hydroxylation
in man. Biochem. Pharmacol., 17: 565-571.
KUPFER, D. (1967) Effects of some pesticides and related compounds on
steroid function and metabolism. Res. Rev., 19: 11-30.
KUPFER, T., BALAZS, T., & BUYSKE, D. A. (1964) Stimulation by o,p'-
DDD of cortisol metabolism in the guinea pig. Life Sci.,
3: 959-964.
KUTZ, F. W., YOBS, A. R., JOHNSON, W. G., & WIERSMA, G. B. (1974)
Pesticide residues in adipose tissue of the general population of
the United States, FY 1970 survey. Bull. Soc. Pharmacol. Environ.
Pathol., 2: 4-10.
KUWABARA, N. & TAKAYAMA, S. (1974) [Comparison of histogenesis of
liver of mice administered respectively BHC, DDT, and 2,7-FAA.]
Proc. Jpn Cancer Assoc., 33: 50 (in Japanese).
KUZ'MINSKAJA, U. A., KLISENKO, M. A., & HAYKINA, B. I. (1972)
[Peculiarities of the distribution of some pesticides in the lipid
fractions of the liver.] Vopr. Pitan., 31: 48-52 (in Russian).
KWALIK, D. S. (1971) Anticonvulsants and DDT residues. J. Am. Med.
Assoc., 215: 120-121.
LAUBSCHER, J. A., DUTT, G. R., & ROAN, C. C. (1971) Chlorinated
insecticide residues in wildlife and soil as a function of
distance from application. Pestic. Monit. J., 5: 251-258.
LAUG, E. P., NELSON, A. A., FITZHUGH, O. G., &. KUNZE, F. M. (1950)
Liver-cell alteration and DDT storage in the fat of the rat
induced by dietary levels of 1 to 50 ppm of DDT. J. Pharmacol.
98: 268-273.
LAUG, E. P, KUNZE, F. M., & PRICKETT, C. S. (1951) Occurrence of DDT
in human fat and milk. Am. Med. Assoc. Arch. indust. Hyg. occup.
Med., 3: 245-246.
LAÜGER, P., PULVER, R., & MONTIGEL, C. (1945a) [Mode of action of
4,4'-dichlorodiphenyltrichloromethylmetan (DDT-Geigy) in
warmblooded organism.] Experientia (Basel), 1: 120-121 (in
German).
LAÜGER, P., PULVER, R., & MONTIGEL, C. (1945b) [Mode of action of
4,4'-dichlorodiphenyltrichloromethylmetan (DDT-Geigy) in
warmblooded organism.] Helr. Physiol. Acta, 3: 405-415 (in
German).
LAWS, E. R. (1971) Evidence of antitumorigenic effects of DDT. Arch.
environ. Health, 23: 181-183.
LAWS, E. R., JR, CURLEY, A., & BIROS, F. J. (1967) Men with intensive
occupational exposure to DDT. A clinical and chemical study.
Arch. environ. Health, 15: 766-775.
LAWS, E. R, JR, MADDREY, W. C., CURLEY, A., & BURSE, V. W. (1973)
Long-term occupational exposure to DDT. Arch. environ. Health,
27: 318-321.
LEHMAN, A. J. (1952) Chemicals in foods: A report to the Association
of Food and Drug Officials on current developments. Part II,
Pesticides. Section V. Q. Bull. Assoc. Food Drug Off. US,
16: 126-132.
LEHMAN, A. J. (1965) Summaries of pesticide toxicity, Topeka, Ks,
Association of Food and Drug Officials of the United States.
LESCENKO, P. D. & POLONSKAIA, M. N. (1969) [New products in the
prophylactic nutrition of workers in the organochlorine industry.]
Vrach. Delo, No. 8, pp. 106-110 (in Russian).
LEWIS, W. H. & RICHARDS, A. G, JR (1945) Non-toxicity of DDT on cells
in cultures. Science, 102: 330-331.
LICHTENBERG, J. L., EICHELBERGER, J. W., DRESSMAN, R. C., &
LONGBOTTOM, J. E. (1970) Pesticides in surface waters of the
United States -- a 5-year summary, 1964-68. Pestic. Monit. J.,
4: 71-86.
LILLIE, R. D. & SMITH, M. I. (1944) Pathology of experimental
poisoning in cats, rabbits, and rats with 2,2-bis-(para-
chlorphenyl)-1,1,1-trichlorethane. Public Health Rep.,
59: 979-984.
LILLIE, R. D., SMITH, M. I., & STOHLMAN, E. F. (1947) Pathologic
action of DDT and certain of its analogs and derivatives. Arch.
Pathol., 43: 127-142.
LIPSCOMB, G. Q. (1968) Residues in food and feed -- Pesticide residues
in prepared baby foods in the United States. Pestic. Monit. J.,
2: 104-108.
LÖFROTH, G. (1968) Pesticides and catastrophe. New Sci.,
40: 567-568.
LONG, K. L., BEAT, V. B., GOMBART, A. K., SHEETS, R. F., HAMILTON, H.
E., FALABALLA, F., BONDERMAN, D. P., & CHOI, U. Y. (1969) The
epidemiology of pesticides in a rural area. Am. Ind. Hyg. Assoc.
J., 30: 398-304.
LOVELOCK J. E. (1963) Electron absorption detectors and techniques for
use in quantitative and qualitative analysis by gas-liquid
chromatography. Anal. Chem., 35: 474-481.
LU, F. C., JESSUP, D. C., & LAVALÉE, A. (1965) Toxicity of pesticides
to young versus adult rats. Food Cosmet. Toxicol., 3: 591-596.
LUBITZ, J. A., FEEMAN, L., & OKUM, R. (1973) Mitotane use in
inoperable adrenal cortical carcinoma. J. Am. Med. Assoc.,
233: 1109-1112.
LUQUET, F., GOURSAUD, J. G., & CASALIS, J. (1974) Organochlorine
pesticide residues in animal and human milk. Ann. Fals. Expert.
Chim., 67: 217-239.
LUTON, J. P, VALCKE, J. C., REMY, J. M., MATHIEU DE FOSSEY, B., &
BRICAIRE, H. (1972) Gynecomastia after long-term treatment of
Cushing's diseases with o,p'-DDT. Ann Endocrinol.,
33: 290-293.
MACCORMACK, J. D. (1945) Infestation and DDT. Irish J. med. Sci.
6: 627-634.
MACKERRAS, I. M. & WEST, R. F. K. (1946) 'DDT' poisoning in man.
Med. J. Aust., 1: 400-401.
MACKLIN, A. W. & BIBELIN, W. E. (1971) The relation of pesticides to
abortion in dairy cattle. J. Am. Vet. Med. Assoc.,
159: 1743-1748.
MAES, R. & HEYNORICKX, A. (1966) Distribution of organic chlorinated
insecticides in human tissues. Meded. Rijksfac. Landbouwwet.
Gent., 31: 1021-1025.
MAGGS, R. J., JOYNES, P. L., DAVIES, A. J., & LOVELOCK, J. E. (1971)
The electron capture detector -- A new mode of operation. Anal.
Chem., 43: 1966-1971.
MAIER-BODE, H. (1960) [DDT in body fat of man.] Med. Exp, 1: 146-152
(in German).
MARKARIAN, D. S. (1966) [Cytogenetic effects of some chlorine-
containing organic insecticides on mouse bone-marrow cell nuclei.]
Genetika, 1: 132-137 (in Russian).
MARNELOS, M. & HANNINEN, O. (1974) Enhancement of D-glucuronolactone
and acetaldehyde dehydrogenase activities in the rat liver by
inducers of drug metabolism. Biochem. Pharmacol., 23: 1457-1466.
MARSH, C. A. (1963) Metabolism of D-glucuronolactone in the mammalian
system. Biochem. J., 86: 77-86.
MASON, T. J., MCKAY, F. W., HOOVER, F., BLOT, W. J., & FRAUMENI, J. F,
JR (1975) Atlas of cancer mortality for U.S. Counties:
1950-1969, Washington, DC, US Department of Health, Education
and Welfare, Public Health Service (DHEW Publication No. (NH)
75-780).
MASUMOTO, H. T. (1972) Study of the silicic acid procedure of Armour
and Burke for separation of PCBs from DDT and its analogues.
J. Assoc. Off. Anal. Chem., 55: 1092-1100.
MATSUMURA, F. & PATIL, K. C. (1969) Adenosine triphosphatase sensitive
to DDT in synapses of rat brain. Science, 166: 121-122.
MATTSON, A. M., SPILLANE, J. T., BAKER, C., & PEARCE, G. W. (1953)
Determination of DDT and related substances in human fat. Anal.
Chem., 25: 1065-1070.
MAYERSDORF, A. & ISRAELI, R. (1974) Toxic effects of chlorinated
hydrocarbon insecticides on the human electroencephalogram. Arch.
environ. Health, 28: 159-163.
MCALISTER, D. (1879) The law of the geometric mean. Proc. R. Soc.
London B, 29: 367-376.
McCANN, J. & AMES, B. N. (1976) Detection of carcinogens as mutagens
in the Salmonella/microsome test: Assay of 300 chemicals:
Discussion. Proc. Natl Acad. Sci. USA, 73: 950-954.
McCANN, J., CHOL, E., YAMASAKI, E., & AMES, B. N. (1975) Detection of
carcinogens as mutagens in the Salmonella/microsome test: Assay
of 300 chemicals. Proc. Natl Acad. Sci. USA, 72: 5135-5139.
McFARREN, E. F., LISHKA, R. J., & PARKER, J. H. (1970) Criterion for
judging acceptability of analytical methods. Anal. Chem.,
42: 358-365.
McGILL, A. E. J. & ROBINSON, J. (1968) Organochlorine insecticide
residues in complete prepared meals: A 12-month survey in S.E.
England. Food Cosmet. Toxicol., 6: 45-57.
McKINLEY, W. P. (1963) Paper chromatography. In: Zweig, G., ed.
Analytical methods for pesticides, plant growth regulators and
food additives, New York, Academic Press, Vol. 1, pp. 227-252.
McKINNEY, J. D., BOOZIER, E. L., HOPKINS, H. P., & SUGGS, J. E. (1969)
Synthesis and reactions of a proposed DDT metabolite, 2,2'-bis
( p-chlorophenyl)acetaldehyde. Experientia (Basel),
25: 897-898.
McLACHLAN, J. A. & DIXON, R. L. (1972) Gonadal function in mice
exposed prenatally to p,p'-DDT. Toxicol. appl. Pharmacol.,
22: 327.
McMAHON, B. M. & SAWYER, L. D., ed. (1977) Pesticide analytical
manual. Volume I. Methods which detect multiple residues,
Rockville, MD, US Food and Drug Administration.
McNEIL, E. E., OTSON, R., MILES, W. F., & RAJABALEE, F. J. M. (1977)
Determination of chlorinated pesticides in potable water.
J. Chromatogr., 123: 277-286.
McQUEEN, E. G., OWEN, D., & FERRY, D. G. (1972) Effect of phenytoin
and other drugs in reducing serum DDT levels. N.Z. med. J.,
75: 208-211.
MEDVED', L. I., KAGAN, J, S., SPYNU, E. I., DLISENKO, M. A.,
ANTONOVIC, E. A., BEZUGLYJ, V. P., KRYZANOVSKAYJA, M. V., &
POL'CENKO, V. I. (1975) National review on chlorinated
pesticides, Kiev, All-Union Institute of the Ministry of Health
of the USSR for Research on the Hygiene and Toxicology of
Pesticides, Polymers, and Plastics, 74 pp.
MENDOZA, E. E. (1971) Note on the significant difference in gas-liquid
chromatographic responses to insecticide injections at fast and
slow rates. J. Chromatogr. Sci., 9 (12): 753-754.
MENZIE, C. M. (1969) Metabolism of pesticides, Washington, DC, US
Government Printing Office (Special Scientific Report, Wildlife
No. 127).
METCALF, R. L. (1955) Organic insecticides, their chemistry and mode
of action, New York, Intersciences, 392 pp.
MIDDLETON, F. M. & LICHTENBERG, J. J. (1960) Measurements of organic
contaminants in the nation's rivers. Ind. Eng. Chem.,
52: 99A-102A.
MILES, J. R. (1972) Conversion of DDT and its metabolites into
dichlorobanzophenones for analysis in presence of PCBs. J. Assoc.
Off. Anal. Chem., 55: 1039-1041.
MILES, J. W., FETZER, L. E., & PEARCE, G. W. (1970) Collection and
determination of trace quantities of pesticides in air. Environ.
Sci. Technol., 4: 420-425.
MILLER, G. J. & FOX, J. A. (1973) Chlorinated hydrocarbon pesticide
residues in Queensland human milks. Med. J. Aust., 2: 261-264.
MILLER, L. L. & NARANG, R. S. (1970) Induced photolysis of DDT.
Science, 169: 368-370.
MIZOGUCHI, M., YAMAGISHI, T., USHIO, F., FUJIMOTO, C., TAKEBA, K.,
KANI, T., HARUTA, M., YASUHARA, K., & KUBOTA, H. (1972) [On the
residual amount of organochlorine pesticides in human milk in
Tokyo metropolitan area.] Jpn J. public Health, 19: 541-544
(in Japanese).
MONTGOMERY, J. A. & STRUCK, R. F. (1973) The relation of the
metabolism of anticancer agents to their activity. Forschr.
Arzneimittelforsch., 17: 32-304.
MORGAN, D. P. & ROAN, C. C. (1969) Renal function in persons
occupationally exposed to pesticides. Arch. environ. Health,
19: 633-636.
MORGAN, D. P. & ROAN, C. C. (1970) Chlorinated hydrocarbon pesticide
residue in human tissues. Arch. environ. Health, 20: 452-457.
MORGAN, D. P. & ROAN, C. C. (1971) Absorption, storage and metabolic
conversion of ingested DDT and metabolites in man. Arch. environ.
Health, 22: 301-308.
MORGAN, D. P. & ROAN, C. C. (1972) Loss of DDT from storage in human
body fat. Nature (Load.), 238: 221-223.
MORGAN, D. P. & ROAN, C. C. (1973) Adrenocortical function in persons
occupationally exposed to pesticides. J. occup. Med., 15: 26-28.
MORGAN, D. P. & ROAN, C. C. (1974) Liver function in workers having
high tissue stores of chlorinated hydrocarbon pesticides. Arch.
environ. Health, 29: 14-17.
MORGAN, D. P., ROAN, C. C., & PASCHAL, E. H. (1972) Transport of DDT,
DDE, and dieldrin in human blood. Bull environ. Contain.
Toxicol., 8: 321-326.
MOSIER, A. R., GUENZI, W. D., & MILLER, L. L. (1969) Photochemical
decomposition of DDT in a free-radical mechanism. Science,
164: 1083-1085.
MRAK, E. M. (1969) Report of the secretary's commission on pesticides
and their relationship to environmental health, Washington, US
Government Printing Office, 677 pp.
MUGHAL, H. A. & RAHMAN, M. A. (1973) Organochlorine pesticide content
of human adipose tissue in Karachi. Arch. environ. Health, 27:
396-398.
MÜLHENS, K. (1946) [The importance of bis (chlorophenyl)
(trichloromethyl)-methane preparations as insecticides in
combating infectious diseases.] Dtsch. Med. Wochenschr.,
71: 164-169 (in German).
MÜLLER, P., ed. (1959) DDT, the insecticide dichlorodiphenyltri-
chloroethane and its significance. Basel, Switzerland, Birkhäuser
Verlag, Vol. 2.
MURPHY, P. G. (1972) Sulphuric acid for the clean-up of animal tissues
for analysis of acid-stable chlorinated hydrocarbon residues.
J. Assoc. Off. Anal Chem., 55: 360-362.
MURRAY, C. P. F. V., SAUCEDA, F. M., & NAVAREZ, G. M. (1973)
[Environmental contamination and the health of children.] Salud
Publica Mex., 15: 91-100 (in Spanish).
MUSIAL, C. J., HUTZINGER, O., ZITKO, V., & CROCKER, J. (1974) Presence
of PCB, DDE, and DDT in human milk in the provinces of New
Brunswick and Nova Scotia, Canada. Bull. environ. Contam.
Toxicol., 12: 258-267.
MUSTY, P. R. & NICKLESS, G. (1974) Use of Amberlite XAD-4 for
extraction and recovery of chlorinated hydrocarbon insecticides
and PCBs from water. J. Chromatogr., 89: 185-190.
NARVESTED, R. (1947) [Poisoning with DDT powder and some other
incidences of poisoning.] Tidsskr. Norske Laegeforen.,
67: 261-263 (in Norwegian).
NAGAI, I. (1972) [Residues of organochlorine pesticides in mother's
milk in Yamaguchi Prefecture. Annual Report of the Yamaguchi
Prefecture.] Res. Inst. Public Health, 14: 93-94 (in Japanese).
NAGASAKI, H. (1973) [Experimental studies on chronic toxicity of
benzene hexachloride (BHC).] J. Nara Med. Assoc., 24: 1-26
(in Japanese).
NAGASAKI, H., AOI, H., MAKIURA, S., AOKI, Z., ARAI, M., KONISHI, Y., &
ITO, N. (1974) [Characteristic features of hepatoma of mice due to
alpha-BHC and several factors of carcinogenesis.] Proc. Jpn
Cancer Assoc., 33: 78 (in Japanese).
NALIMOV, V. V. (1963) The application of mathematical statistics to
chemical analysis, London, Pergamon Press, 294 pp.
NARAHASHI, T. & HAAS, H. G. (1967) DDT: Interaction with nerve
membrane conductance changes. Science, 157: 1438-1440.
NARAHASHI, T. & YAMASAKI, T. (1960) Mechanism of increase in negative
after-potential by dicophanum (DDT) in the giant axon of the
cockroach. J. Physiol. (Lond.), 152: 122-140.
NARUSE, R., SASAKI, T., YAMOKA, T., MATSUOKA, K., NEGORO, Y., ITASAKA,
Y., URUSHIBATA, K., CHIN, T., KATO, K., KAKO, K., GOTO, Y.,
EGUCHI, Y., YOGO, H., TOMITA, A., & ASAI, M. (1970) [Clinical use
of o,p'-DDD in adrenal cortex cancer.] Horumon Rinsho,
18: 241-244 (in Japanese).
NAWA, H. (1973) [Studies on intoxication by organochlorine pesticides
for agricultural use. Part 2. Examination of concentration of
organochlorine pesticides in sera of healthy people and patients
with various diseases.] J. Okayama Med. Soc., 85: 47-58 (in
Japanese).
NEAL, P. A., VON OETTINGEN, W. F., SMITH, W. W., MALMO, R. B., DUNN,
R. C., MORAN, H. E., SWEENEY, T. R., ARMSTRONG, D. W., & WHITE, W.
C. (1944) Toxicity and potential dangers of aerosols, mists, and
dusting powders containing DDT. Public Health Rep., 177
(Suppl.): 1-32.
NEAL, P. A., SWEENEY, T. R., SPICER, S.S., & VON OETTINGEN, W. F.
(1946) The excretion of DDT (2,2-bis-( p-chlorophenyl)-1,1,1-
trichloroethane) in man, together with clinical observations.
Public Health Rep., 61: 403-409.
NELSON, A. A. & WOODARD, G. (1948) Adrenal cortical atrophy and liver
damage produced in dogs by feeding 2,2-bis-(parachlorophenyl)-1,1-
dichlorethane (DDD). Fed. Proc., 7: 277.
NELSON, A. A. & WOODARD, G. (1949) Severe adrenal cortical atrophy
(cytotoxic) and hepatic damage produced in dogs by feeding 2,2-
bis-(parachlorophenyl)-1,1-dichloroethane (DDD or TDE). Arch.
Pathol., 48: 387-394.
NELSON, A. A., DRAIZE, J. H., WOODARD, G., FITZHUGH, O. G., SMITH, R.
B., JR, & CALVERY, H. O. (1944) Histopathological changes
following administration of DDT in several species of animals.
Public Health Rep., 59: 1009-1020.
NELSON, J. A. (1973) Effects of DDT analogs and polychlorinated
biphenyls (PCB) mixtures on 3H-estradiol binding to rat uterine
receptor. Fed. Proc., 32: 236.
NEWTON, K. O. & GREENE, N. C. (1972) Organochlorine pesticide residue
levels in human milk -- Victoria, Australia, 1970. Pestic. Monit.
J., 6: 4-8.
NICHOLS, J., PRESTLEY, W. E., & NICHOLS, F. (1961) Effects of m,p'-
DDT in a case of adrenal cortical carcinoma. Curr. Ther. Res.,
3: 266-271.
NIKITINA, Y. I. (1974) [Course of labor and puerperium in vineyard
workers and milkmaids in the Crimea.] Gig. Tr. Prof. Zabol,
18: 17-20 (in Russian).
NISHIMOTO, T., UYETA, M., & TAUE, S. (1970) [The accumulation of
organochlorine pesticides in human adipose tissue.] Prog. Med.,
73: 275-277 (in Japanese).
NITSCHKE, U. & LINK, K. (1972) [Clinical and biochemical aspects of
hormone-producing adrenal carcinomas.] Z. Gesamite Inn. Med. Ihre
Grenzgeb., 27: 896-905 (in German).
NORTH, H. H. & MENZER, R. E. (1973) Metabolism of DDT in human
embryonic lung cell cultures. J. agric. food. Chem.,
21: 509-510.
NOVIKOVA, K. F. (1973) [Quantitative assay of organochlorine and
organophosphate pesticide residues in food products, soil, water.]
Zh. Vses. Khim. Obshchest., 18 (5): 562-570 (in Russian).
OBUSCHOWSKA, D. & PAWLOWKA-TOCHMAN, A. (1973) [The effect of the
gamma-isomer of HCH (Lindane) on the ultrastructure of the liver
cell.] Ann. Univ. Mariae Curie-Sklodowska, 28 (9): 63-66 (in
Polish).
OFNER, R. R. & CALVERY, H. O. (1945) Determination of DDT (2,2-bis-
( p-chlorophenyl)-1,1,1-trichlorethane) and its metabolite in
biological materials by use of the Schechter-Haller method.
J. Pharmacol., 85: 363-370.
O'LEARY, J. A., DAVIES, J. E., EDMUNDSON, W. F., & FELDMAN, M. (1970a)
Correlation of prematurity and DDE levels in fetal whole blood.
Am. J. Obstet. Gynecol., 106: 939.
O'LEARY, J. A., DAVIES, J. E., EDMUNDSON, W. F., & REICH, G. A.
(1970b) Transplacental passage of pesticides. Am. J. Obstet.
Gynecol., 107: 65-68.
O'LEARY, J. A., DAVIES, J. E., & FELDMAN, M. (1970c) Spontaneous
abortion and human pesticide residues of DDT and DDE. Am. J.
Obstet. Gynecol., 108: 1291-1292.
OLOFFS, C. P., HARDWICK, D. F., SZETO, S. Y., & MOERMAN, D. G. (1974)
DDT, dieldrin, and heptachlor epoxide in humans with liver
cirrhosis. Clin. Biochem., 7: 297-306.
OLSZYNA-MARZYS, A. E., DE CAMPOS, M., FARVAR, M. T., & THOMAS, M.
(1973) [Residues of chlorinated pesticides in human milk in
Guatemala.] Bol. Of. Sanit. Panam., 74: 93-107 (in Spanish).
ORTEGA, P. (1966) Light and electron microscopy of dichlorodiphenyl-
trichloroethane (DDT) poisoning in the rat liver. Lab. Invest.,
15: 657-679.
ORTEGA, P., HAYES, W. J., JR, DURHAM, W. F., & MATTSON, A. (1956) DDT
in the diet of the rat. Its effect on DDT storage, liver function
and cell morphology, Washington, DC, US Government Printing
Office, 27 pp. (Public Health Monograph No. 43, PHS Publication
No. 484).
ORTELEE, M. F. (1958) Study of men with prolonged intensive
occupational exposure to DDT. Am. Med. Assoc. Arch. indust.
Health, 18: 433-440.
OTTOBONI, A. (1969) Effect of DDT on reproduction in the rat.
Toxicol. appl. Pharmacol., 14: 74-81.
OTTOBONI, A. (1972) Effect of DDT on the reproductive lifespan in the
female rat. Toxicol. appl. Pharmacol., 22: 497-502.
OTTOBONI, A., BISSELL, G. D., & HEXTER, A. C. (1977) Effects of DDT on
reproduction in multiple generations of beagle dogs. Arch.
environ. Contam. Toxicol., 6: 83-101.
OURA, H., KOBATASHI, H., OURA, T., SENDA, I., & KUBOTA, K. (1972) [On
the pollution of human milk by PCB and organochlorine pesticides.]
J. Jpn Assoc. Rural Med., 21: 300-301 (in Japanese).
OUW, K. H. & SHANDAR, A. G. (1974) A health survey of Wee Waa
residents during 1973 aerial spraying season. Med. J. Aust.,
2: 871-873.
PACCAONELLA, B., PRATI, L., & CAVAZZINI, G. (1967) [Organochlorine
insecticides in adipose tissue of residents in Ferrara province.]
Nuovi Ann. Ig. Microbiol., 18: 17-26 (in Italian).
PALMER, K. A, GREEN, S, & LEGATOR, M. S. (1972) Cytogenetic effects of
DDT and derivatives of DDT in a cultured mammalian cell line.
Toxicol. appl. Pharmacol., 22: 355-364.
PALMER, L. & KOLMODIN-HEDMAN, B. (1972) Improved quantitative gas
chromatographic method for analysis of small quantities of
chlorinated hydrocarbon pesticides in human plasma.
J. Chromatogr., 74: 21-30.
PASCHAL, E. H., ROAN, C. C., & MORGAN, D. P. (1974) Evidence of
excretion of chlorinated hydrocarbon pesticides by the human
liver. Bull. environ. Contam. Toxicol., 12: 547-554.
PEAKALL, D. B. (1970) p,p'-DDT: Effect on calcium metabolism and
concentration of estradiol in the blood. Science, 168: 592-594.
PEARCE, G. W., MATTSON, A. M., & HAYES, W. J., JR (1952) Examination
of human fat for presence of DDT. Science, 116: 254-256.
PEARL, W. & KUPFER, D. (1971) Stimulation of dieldrin elimination by
thiouracil derivative of DDT in the rat: enhancement of dieldrin
oxidative metabolism. Chem. biol. Interactions, 4: 91-96.
PELLIZARI, E. D. (1974) Electron capture detection in gas
chromatography. J. Chromatogr., 98: 323-361.
PERAINO, C., FRY, R. J. M., & STAFFELDT, E. (1973) Enhancement of
spontaneous hepatic tumorigenesis in C3H mice by dietary
phenobarbital. J. Natl Cancer Inst., 51: 1349-1350.
PEREVODCIKOVA, N. I., PLATINSKIY, L. V., & KERSTMAN, V. I. (1972) [The
treatment of inoperable forms of malignant tumors of the adrenal
cortex with o,p'-DDD.] Vopr. Onkol., 18: 24-29 (in Russian).
PERINI, G. & GHEZZO, F. (1970) [Dichlorodiphenylacetic acid (DDA)
urinary levels in the rural population of Ferrara Province.]
L'Arcisp. S. Anna di Ferrara, 23: 3-12 (in Italian).
PESENDORFER, H., EICHLER, I., & GLOFKE, E. (1973) [Informative
analyses of organochlorine pesticide and PCB residues in human
adipose tissue (from the area of Vienna).] Wien. Klin.
Wochenschr., 85: 218-222 (in German).
PETERLE, T. J. (1969) DDT in Antarctic snow. Nature (Lond.),
224: 620.
PETERSON, J. E. & ROBINSON, W. H. (1964) Metabolic products of p,p'-
DDT in the rat. Toxicol. appl. Pharmacol, 6: 321-327.
PFEILSTICKER, K. (1973) [Pesticides in baby food.] Monatsschr.
Kinderheilkd., 121: 551-553 (in German).
PHILIPS, F. S. & GILMAN, A. (1946) Studies on the pharmacology of DDT
(2,2-bis-(parachlorophenyl)-1,1,1-trichloroethane). I. The acute
toxicity of DDT following intravenous injection in mammals with
observations on the treatment of acute DDT poisoning.
J. Pharmacol., 86: 213-221.
PHILIPS, F. S., GILMAN, A., & CRESCITELLI, F. N. (1946) Studies on the
pharmacology of DDT (2,2-bis-(parachlorophenyl)-1,1,1-
trichloroethane). II. The sensitization of the myocardium to
sympathetic stimulation during acute DDT intoxication.
J. Pharmacol., 86: 222-228.
PIONKE, H. B., KONRAD, J. C., CHESTER, G., & ARMSTRONG, D.E. (1968)
Extraction of organochlorine and organophosphate insecticides from
lake water. Analyst, 93: 363-367.
PLATONOVA, V. I. (1970) [Disturbances of the functional conditions of
the stomach with prolonged effect on the body of some
organochlorine pesticides.] Gig. Tr. Resp. Mezhved, 3B,
6: 142-147 (in Russian).
PLIMMER, J. R. & KLINGEBIEL, U. I. (1973) PCB-formation. Science,
181: 994-995.
PLIMMER, J. R, KLINGEBIEL, U. I., & HUMMER, B.E. (1970) Photooxidation
of DDT and DDE. Science, 167: 67-69.
POLAND, A., SMITH, D., KUNTZMAN, R., JACOBSON, M., & CONNEY, A. H.
(1970) Effect of intensive occupational exposure to DDT an
phenylbutazone and cortisol metabolism in human subjects. Clin.
Pharmacol. Ther., 11: 724-732.
POLISHUK, Z. W., WASSERMANN, M., WASSERMANN, D., GRONER, Y.,
LAZAROVICI, S., & TOMATIS, L. (1970) Effects of pregnancy on
storage of organochlorine insecticides. Arch. environ. Health,
20: 215-217.
POLISHUK, Z. W., RUN, M., WASSERMANN, M., CUCOS, S., WASSERMANN, D., &
LEMESCH, C. (1977) Organochlorine compounds in human blood plasma
and milk. Pestic. Monit. J., 10: 121-129.
PONOMARKOV, V., TOMATIS, L., & TURUSOV, V. (1976) The effect of long-
term administration of phenobarbitone in CF-1 mice. Cancer Lett.,
1: 165-172.
POTT, P. (1775) Chirurgical observations relative to the cataract,
the polypus of the nose, the cancer of the scrotum, etc. London,
T. F. Carnegy, 208 pp.
POTT, P. (1790) The chirurgical works of Percival Pott, F.R.S.
surgeon to St. Bartholomew's Hospital, A new edition with his
last corrections, London, J. Johnson, 3 vol.
PRATI, L. & DEL DOT, M. (1971) [Research on the levels of accumulation
of synthetic organic chloride pesticides, in human adipose tissues
in the province of Trento.] L'Ig. Mod., 64: 35-43 (in Italian).
PRATI, L., PAVANELLO, R., & GHEZZO, F. (1972) Storage of chlorinated
pesticides in human organs and tissues in Ferrara Province, Italy.
Bull. World Health Organ., 46: 363-369.
PROUTY, R. M. & CROMARTIE, E. (1970) Stepwise recovery of DDT and
dieldrin from tissues of Coturnix japonica (quail) during residue
analysis. Environ. Sci. Technol., 4: 768-769.
QUINBY, G. E., HAYES, W. J., JR, ARMSTRONG, J. F., & DURHAM, W. F.
(1965a) DDT storage in the US population. J. Am. Med. Assoc.,
191: 175-179.
QUINBY, G. E., ARMSTRONG, J. F., & DURHAM, W. F. (1965b) DDT in human
milk. Nature (Lond.), 207: 726-728.
RABELLO, M. N., BECAK, W., ALMEIDA, W. F., PIGATI, P., UNGARO, M. T.,
MURATA, T., & PEREIRA, C. A. B. (1975) Cytogenetic study on
individuals occupationally exposed to DDT. Mutat. Res.,
28: 449-454.
RADOMSKI, J. L., DEICHMANN, W. B., & CLIZER, E. E. (1968) Pesticide
concentrations in the liver, brain, and adipose tissue of terminal
hospital patients. Food Cosmet. Toxicol, 6: 209-220.
RADOMSKI, J. L., DEICHMANN, W. B., REY, A. A., & MERKIN, T. (1971a)
Human pesticide blood levels as a measure of body burden and
pesticide exposure. Toxicol. appl. Pharmacol., 20: 175-185.
RADOMSKI, J. L, ASTOLFI, E., DEICHMANN, W. B., & REY, A. A. (1971b)
Blood levels of organochlorine pesticides in Argentina:
Occupationally and nonoccupationally exposed adults, children and
newborn infants. Toxicol. appl. Pharmacol., 20: 186-193.
RAMACHANDRAN, M., SHARMA, M. I. D., SHARMA, S.C., MATHUR, P.S.,
ARAVINDAKSHAN, A., & EDWARD, G. J. (1974) DDT and its metabolites
in the body fat of Indians. J. commun. Dis., 6: 256-259.
RANTANEN, T., MARSELOS, M., & PUHAKAINEN, E. (1975) Effects of 1,1,1-
trichloro-2,2-bis-( p-chlorophenyl)ethane (DDT) administration on
the glucuronic acid pathway in the rat liver. Environ. Qual.
Saf., 3 (Suppl.): 494-499.
RAPPOLT, R. T. (1970) Use of oral DDT in three human barbiturate
intoxications: CNS arousal and/or hepatic enzyme induction by
reciprocal detoxicants. Ind. Med. Surg., 39: 319.
READ, S. T. & MCKINLEY, W. P. (1961) DDT and DDE content of human fat.
A survey. Arch. environ. Health, 5: 209-211.
REIF, V. D. & SINSHEIMER, J. E. (1975) Metabolism of 1-( o-
chlorophenyl)-1-( p-chlorophenyl)-2-2-dichloroethane ( o,p'-DDD)
in rats Drug Metab. Dispos., 3: 15-25.
REIF, V. D., SINSHEIMER, J. E., WARD, J. C., & SCHTEINGART, D. E. J.
(1974). Aromatic hydroxylation and alkyl oxidation in metabolism
of mitotane ( o,p'-DDD) in humans. Pharmacol. Sci.,
63: 1730-1736.
REINGOLD, I. M. & LASKY, I. I. (1947) Acute fatal poisoning following
ingestion of a solution of DDT. Ann. intern. Med., 26: 945-947.
REYNOLDS, L. M. (1969) Polychlorinated biphenyls (PCBs) and their
interference with pesticide residue analysis. Bull. environ.
Contam. Toxicol., 4: 128-143.
RICHARDSON, A., ROBINSON, J., CRABTREE, N., & BALDWIN, M. K. (1971)
Residues in polychlorobiphenyls in biological samples. Pestic.
Monit. J., 4: 169-176.
RICHARDSON, J. A., KEIL, J. E., & SANDIFER, S. H. (1975) Catecholamine
metabolism in humans exposed to pesticides. Environ. Res.,
9: 290-294.
RISEBROUGH, R. W., HUGGETT, R. J., GRIFFIN, J. J., & GOLDBERG, E. D.
(1968) Pesticides: Transatlantic movements in the northeast
trades. Science, 159: 1233-1235.
RITCEY, W. R., SAVARY, G., & McCULLY, J. A. (1972) Organochlorine
insecticide residues in human milk, evaporated milk, and some milk
substitutes in Canada. Can. J. public Health, 63: 125-132.
RITCEY, W. R., SAVARY, G., & McCULLY, J. A. (1973) Organochlorine
insecticide residues in human adipose tissue of Canadians. Can.
J. public Health, 64: 380-386.
RIZOV, N. A. (1977) [Organochlorine pesticides in the fat and blood
of the general population of Bulgaria and methods for their
determination.] Dissertation. Sofia, Inst. Hygiene and
Occupational Health, Academy of Medicine, 117 pp. (in Bulgarian).
ROBINSON, J., RICHARDSON, A., & ELGAR, K. E. (1966) Chemical identity
in ultramicroanalysis, New York, American Chemical Society
(abstracts).
ROBINSON, J., RICHARDSON, A., HUNTER, C. G., CRABTREE, A. N., & REES,
H. J. (1965) Organochlorine insecticide content of human adipose
tissue in Southeastern England. Br. J. ind. Med., 22: 220-229.
ROSEN, A. A. & MIDDLETON, F. M. (1959) Chlorinated insecticides in
surface waters. Anal. Chem., 31: 1729-1732.
ROSSI, R., RAVERA, M., REPETTI, G., & SANTI, L. (1977) Long-term
administration of DDT or phenobarbital-Na in Wistar rats. Int. J.
Cancer, 19: 179-185.
ROTHE, C. F., MATTSON, A. M., NUESLEIN, R. M., & HAYES, W. J., JR
(1957) Metabolism of chlorophenothane (DDT): Intestinal lymphatic
absorption. Am. Med. Assoc. Arch. ind. Health, 16: 82-86.
ROWE, D. R., CANTER, L. W., SNYDER, P. J., & MASON, J. W. (1971)
Dieldrin and endrin concentrations in a Lousiana estuary. Pestic.
Monit. J., 4: 177-183.
RUDD, R. L. & HERMAN, S. G. (1972) Ecosystemic transferal of
pesticides residues in an aquatic environment. In: Matsumura, F.,
Boush, G. M., Misato, T., ed. Environmental toxicology of
pesticides, Part VII, New York, Academic Press.
RYBAKOVA, M. N. (1968) [The effect of certain pesticides on the
hypophysis and its gonadotrophic function.] Gig. i Sanit.,
33: 27-31 (in Russian).
SABAD, L. M., KOLESNICENKO, T. S., & NIKONOVA, T. V. (1972) [On a
possible blastomogenicity of DDT.] Vopr. Pitan., 30: 63-66
(in Russian).
SALIHODZAEV, S. S. & FERSTAT, V. N. (1972) [Condition of the olfactory
analyzer under the effect of organochlorine and organophosphorus
pesticides.] Gig. i Sanit., 37: 95-96 (in Russian).
SANDIFER, S. H., KEIL, J. E., FINKLEA, J. F., & GADSDEN, R. H. (1972)
Pesticide effects on occupationally exposed workers: A summary of
four years observation of industry and farm volunteers in South
Carolina. Ind. Med. Surg., 41: 7-12.
SARETT, H. P. & JANDORF, B. J. (1947) Effect of chronic DDT
intoxication in rats on lipids and other constituents of the
liver. J. Pharmacol., 91: 340-344.
SAUBERLICH, H. E. & BAUMANN, C. A. (1947) Effect of dietary variation
upon the toxicity of DDT to rats or mice. Proc. Sec. Exp. Biol.
Med., 66: 642-645.
SAVAGE, E. P., TESSARI, J. D., MALBERG, J. W., WHEELER, H. W., &
BAGBY, J. R. (1973) Organochlorine pesticide residues and
polychlorinated biphenyls in human milk, Colorado 1971-1972.
Pestic. Monit. J., 7: 1-5.
SAWYER, I. D. (1973) Collaborative study of the recovery and gas
chromatographic quantitation of PCBs in chicken fat and PCB-DDT
combinations in fish. J. Assoc. Off. Anal. Chem., 56: 1015-1023.
SCHAEFER, R. G. (1974) [Mass spectrometric identification and
quantification of chlorinated hydrocarbons in fish.] Chem. Ztg.,
98: 241-247 (in German).
SCHAFER, M. L. & CAMPBELL, J. E. (1966) Distribution of pesticide
residues in human body tissues from Montgomery County, Ohio,
Washington, DC, American Chemical Society, pp. 89-98 (Adv. Chem.
Ser. No. 60).
SCHMICK, M. (1973) Survival with adrenal carcinoma. J. Am. Med.
Assoc., 224: 1763.
SCHMIDT, R. (1973) [Effect of 1,1,1-trichloro-2,2-bis( p-
chlorophenyl) ethane (DDT) on the prenatal development of the
mouse, under consideration of distribution of tritium-labeled and
carbon-14-labeled DDT in pregnant mice.] Biol. Runtisch.,
11: 316-317 (in German).
SCHOOR, W. P. (1970) Effect of anticonvulsant drugs on insecticide
residues. Lancet, 2: 520-521.
SCHROEDER, G. & DOROZALSKA. (1975) [Degradation of DDT and its
analogues.] Wiad. Chem., 29: 553-565 (in Polish).
SCHVARTSMAN, S., ALMEIDA, W. F., COSTA VAZ, F. A., CORRADINI, H. B.,
PIGATI, P., GAETA, R., & UNGARO, M. T. (1974) Blood levels of DDT
in nonoccupationally exposed mothers and newborn infants in a city
in Brazil. In: Coulston, F. & Korte, F., ed. EQS Environmental
Quality and Safety, New York, Academic Press, Vol. 3,
pp. 154-156.
SCHWABE, U. & WENDLING, I. (1967) [Acceleration of drug metabolism by
small doses of DDT and other chlorinated hydrocarbon
insecticides.] Arzneim. Forsch., 17: 614-618 (in German).
SCOTT, R. P. W. (1970) Determination of the optimum conditions to
effect a separation by gas chromatography. Adv. Chromatogr.,
9: 193-214.
SCUDDER, H. I. & TARZWELL, C. M. (1950) Effects of DDT mosquito
larviciding on wildlife. IV. The effects on terrestrial insect
population of routine larviciding by airplane. Public Health
Rep., 65: 71-87.
SELBY, L. A., NEWELL, K. W., HAUSER, G. A., & JUNKER, G. (1969)
Comparison of chlorinated hydrocarbon pesticides in maternal blood
and placental tissues. Environ. Res., 2: 247-255.
SELL, J. L., DAVISON, K. L., & PUYEAR, R. L. (1971) Aniline
hydroxylase, N-demethylase, and cytochrome P-450 in liver
microsomes of hens fed DDT and dieldrin. J. agile. food Chem.,
19: 58-60.
SEMENCAEVA, E. M. (1968) [The mechanism of action of DDT and certain
other pesticides on the immunological response of the body.] In:
Medved', L. E., ed. The hygiene of application of the toxicology
of pesticides and the clinical features of pesticides poisoning.
Scientific Conference on the Safe Use & Toxicology of Pesticides,
11-14 June, 1968, Issue No. 6, Kiev, Acad. of Sciences, USSR,
pp. 805-815 (in Russian).
SHARAHGAPANI, M. V. & PINGALE, S. V. (1954) The hazard of DDT-
treatment of potatoes. Bull. Cent. Food Technol. Res. Inst.
Mysore, 4: 57.
SHERMA, J. (1973) Thin-layer chromatography: Recent advances. In:
Zweig, G., ed. Analytical methods for pesticides and plant growth
regulators, New York, Academic Press, Vol. VIII, pp. 3-87.
SHIRASU, Y., MORIYA, M., KATO, K., FURUHASHI, A., & KADA, T. (1976)
Mutagenicity screening of pesticides in the microbial system.
Mutat. Res., 40: 19-30.
SHIRASU, Y., MORIYA, M., KATO, K., LIENARD, F., TEZUKA, H., TERAMOTO,
S., & KADA, T. (1977) Mutagenicity screening on pesticides and
modification products: A basis of carcinogenicity evaluation. In:
Hiatt, H. H., Watson, J. D., & Winsten, J. A., ed. Origins of
human cancer, Book A, Incidence of cancer in humans, Cold Spring
Harbor, NY, Cold Spring Harbor Laboratory, Vol. 4, pp. 267-285.
SIERWISKI, M. & HELRICH, K. (1967) Separation, identification and
measurement of DDT and its metabolites. J. Assoc. Off. Anal.
Chem., 50: 627-633.
SIMMONS, S. W. (1959) The use of DDT insecticides in human medicine.
In: Müller, P., ed. DDT, the insecticide dichlorodiphenyltri-
chloroethane and its significance, Basel, Switzerland, Birkhäuser
Verlag, Vol. II, pp. 251-502.
SINGHAL, R. L. & KACEW, S. (1976) The role of cyclic AMP in
chlorinated hydrocarbon-induced toxicity. Fed. Proc.,
35: 2618-2623.
SISSONS, D. J., TELLING, G. M., & USHER, C. D. (1968) A rapid and
sensitive procedure for routine determination of organochlorine
pesticide residues in vegetables. J. Chromatogr., 33: 435-449.
SIYALI, D. S. (1972) Hexachlorobenzene and other organochlorine
pesticides in human blood. Med. J. Aust, 2: 1063-1066.
SIYALI, D. S. (1973) Polychlorinated hiphenyls, hexachlorobenzene and
other organochlorine pesticides in human milk. Med. J. Aust,
2: 815-818.
SIZONENKO, P. C., DORET, A. M., RIONDEL, A. M., & PAUNIER, L. (1974)
Cushing's syndrome due to bilateral adrenal cortical hyperplasia
in a 13-year-old girl: Successful treatment with o,p'-DDD.
Helv. Paediatr. Acta, 29: 195-202.
SKOGERBOE, R. K. & GRANT, C. L. (1970) Comments on the definitions of
the terms sensitivity and detection limit. Spectrosc. Lett.,
3: 215-220.
SKRENTNY, R. F. & DOROUGH, H. W. (1971) Efficiency of several
extraction procedures in removing organochlorine insecticides from
tobacco. Tobacco Sci., 15: 111-113.
SMART, N. A., HILL A. R. C., & ROUGHAN, P. A. (1974) Chlorinated
hydrocarbon pesticides residues in foodstuffs: Comparison of
methods. J. Assoc. Off. Anal. Chem., 57: 153-164.
SMITH, D. C. (1971) Pesticide residues in the total diet in Canada.
Pestic. Sci., 2: 92-95.
SMITH, D. C., SAUDI, E., & LEDUC, R. (1972) Pesticide residues in the
total diet in Canada. II-1970. Pestic. Sci., 3: 207-210.
SMITH, D. C., LEDUC, R., & CHARBONNEAU, C. (1973) Pesticide residues
in the total diet in Canada. III-1971. Pestic. Sci., 4: 211-214.
SMITH, D. C., LEDUC, R., & TREMBLAY, L. (1975) Pesticide residues in
the total diet in Canada. IV-1972 and 1973. Pestic. Sci.,
6: 75-82.
SMITH, M. I. & STOHLMAN, E. F. (1944) The pharmacologic action of 2,2-
bis-( p-chlorophenyl)-1,1,1-trichlorethane and its estimation in
the tissues and body fluids. Public Health Rep., 59: 984-993.
SMITH, M. I. & STOHLMAN, E. F. (1945) Further studies on the
pharmacologic action of 2,2-bis-( p-chlorophenyl)-1,1,1-
trichloroethane (DDT). Public Health Rep., 60: 289-301.
SMITH, M. I., BAUER, H., STOHLMAN, E. F., & LILLIE, R. D. (1946) The
pharmacologic action of certain analogues and derivatives of DDT.
J. Pharmacol., 88: 359-365.
SNYDER, D. & REINERT, R. (1971) Rapid separation of PCBs from DDT and
its analogues on silica gel. Bull. environ. Contam. Toxicol.,
6: 385-390.
SOUTHERN, A. L., TOCHIMOTO, S., ISURUGI, K., GORCHER, G. G., KRIKUN,
E., & STYPULKOWSKI, W. (1966a) The effect of 2,2-bis-(2-
chlorophenyl-4-chlorophenyl)-1,1,1-dichloroethane ( o,p'-DDD) on
the metabolism of infused cortisol-7-H. Steroids, 7: 11-29.
SOUTHERN, A. L., TOCHIMOTO, S., STROM, L., RATUSCHINI, A., RASS, H., &
GORCHER, G. (1966b) Remission in Cushing's syndrome with o,p'-
DDD. J. clin. Endocrinol. Metab., 26: 268-278.
SPICER, S. S., SWEENEY, T. R., VON OETTINGEN, W. F., LILLIE, R. D., &
NEAL, P. A. (1947) Toxicological observations on goats fed large
doses of DDT. Vet. Med., 42: 289-293.
STACY, G. I. & THOMAS, B. W. (1975) Organochlorine pesticide residues
in human milk, Western Australia-1970-71. Pestic. Monit. J.,
9: 64-66.
STANELY, C. W., BARNEY, J. E., HELTON, M. R., & YOBS, A. R. (1971)
Measurement of atmospheric levels of pesticides. Environ. Sci.
Technol., 5: 431-435.
STARR, M. G., ALDRICH, F. D., McDOUGALL, W. D., III, & MOUNCE, L. M.
(1974) Contribution of household dust to human exposure to
pesticides. Pestic. Monit. J., 8: 209-212.
STEC, J. & JUSZKIEWICZ, T. (1972) [Determination of polychlorinated
insecticide residues in animal products using Woods methods.]
Med. Weter., 28: 634-637 (in Polish).
STENBERG, A. I. & DIKY, V. V. (1973) [Changes in the metabolism of DDT
deposited in the omentum of fasting albino rats.] Vopr. Pitan.,
32; 39-43 (in Russian).
STIJVE, T. & CARDINALE, E. (1974) [Rapid determination of chlorinated
pesticides, PCBs, and a number of phosphated insecticides in fatty
foods.] Mitt. Lebensmittelunters. Hyg., 65: 131-150 (in German).
STOHLMAN, E. F. & LILLIE, R. D. (1948) The effect of DDT on the blood
sugar and of glucose administration on the acute and chronic
poisoning of DDT in rabbits. J. Pharmacol., 93: 351-361.
STRASSMANN, S.C. & KUTZ, F. W. (1977) Insecticide residues in human
milk from Arkansas and Mississippi, 1973-74. Pestic. Monit. J.,
10: 130-133.
STRAW, J. A., WATERS, I. W., & FREQLY, M. J. (1965) Effect of o,p'-
DDD on hepatic metabolism of phenobarbital in rat. Proc. Exp.
Biol. Med., 118: 391-394.
STREET, J. C. (1964) DDT antagonism to dieldrin storage in adipose
tissue of rats. Science, 146: 1580-1581.
STREET, J. C. & CHADWICK, R. W. (1967) Stimulation of dieldrin
metabolism by DDT. Toxicol. appl. Pharmacol., 11: 68-71.
STREET, J. C., WANG, M., & BLAU, A. D. (1966) Drug effects on dieldrin
storage in rat tissue. Bull. environ. Contam. Toxicol., 1: 6-15.
STREET, J. C., MAYER, F. L., & WAGSTAFF, D. J. (1969) Ecological
significance of pesticide interactions. Ind. Med. Surg,
38: 409-414.
STRETZ, P. E. & STARR, H. M. (1973) Determination of chlorinated
pesticides in whole blood. J. Assoc. Off. Anal Chem.,
56: 1173-1177.
STUDY GROUP OF MINISTRY OF HEALTH-WELFARE FOR ORGANOCHLORINE PESTICIDE
RESIDUES IN MOTHERS' MILK (1972) VIII. [Organochlorine pesticide
residues in blood during lactation.] J. Food Hyg. Soc. Jpn,
13: 438-450 (in Japanese).
SUGAYA, T. et al. (1971) [Organochlorine pesticide residues in human
milk.] J. Jpn Assoc. Rural Med., 19: 379-380 (in Japanese).
SUNDSTRÖM, G. (1977). Metabolic hydroxylation of the aromatic rings of
1,1-dichloro-2,2-bis(4-chlorophenyl)ethylene ( p,p'-DDE) by the
rat. J. agric. food Chem., 25: 18-21.
SUNDSTRÖM, G., JANSSON, B., & JENSEN, S. (1975) Structure of phenolic
metabolites of p,p'-DDE in rat, wild seal and guillemot. Nature
(Lond.), 255: 627-628.
SURBER, E. W. & FRIDDLE, D. D. (1949) Relative toxicity of suspension
and oil formulations of DDT to native fishes in Black Creek, West
Virginia. Trans. Am. Fish. Soc., 76: 315-321.
SUTHERLAND, G. L. (1965) Residue analytical limits of detectability.
Residue Rev., 10: 85-96.
SUVAK, L. N. (1970) [DDT in mothers' milk.] Zdravoochr. Kosinev,
13: 19-21 (in Russian).
SUZUKI, M., YAMATO, Y., & WATANABE, T. (1975) Gas chromatographic
resolution of organochlorine insecticides on OV-1/OV-17,
OV-210/OV-17 and OV-225/OV-17 mixed phase systems. J. Assoc. Off.
Anal. Chem., 58: 297-300.
SUZUKI, Y., SUGAYA, H., & SASAKI, S. (1973) [Results of 3 years'
tracing investigation of organochlorine pesticide residues in
humans.] J. Jpn Assoc. Med., 22: 276-277 (in Japanese).
SWACKHAMER, A.B. (1965) Report on pesticide residues in restaurant
meals in Canada. Pestic. Prog., 3: 108-114.
TABOR, E. C. (1966) Pesticides in urban atmospheres. Contamination of
urban air through the use of insecticides. Trans. NY Acad. Sci.,
28: 569-578.
TAIRA, M., HASHIMOTO, S., & SHIMOHIRA, I. (1972) [Organochlorine
pesticide residues in human breast milk.] Bull. Hyogo Prefect.
Public Health Lab., 7: 19-23 (in Japanese).
TAKANO, H. (1972) [Results of inspection of pesticide residues in
mothers' milk and market milk.] Annu. Rep. Iwate Inst. of Public
Health, 15: 88-91 (in Japanese).
TAKESHITA, T. & INUYAMA, Y. (1970) [Organochlorine residues in
mothers' milk and blood.] Annu. Rep. Shimane Prefect. Inst.
Public Health, 12: 27-28 (in Japanese).
TARJAN, R. & KEMENY, T. (1969) Multigeneration studies on DDT in mice.
Food Cosmet. Toxicol., 7: 215-222.
TARRANT, K. R. & TATTON, J. O'G. (1968) Organochlorine pesticides in
rainwater in the British Isles. Nature (Lond.), 219: 725-727.
TARZWELL, C. M. (1950) Effects of DDT mosquito larviciding on
wildlife. V. Effects on fishes of the routine manual and airplane
application of DDT and other mosquito larvicides. Public Health
Rep., 65: 231-255.
TAYLOR, R. & BOGACKA, T. (1968) [Determination of DDT, methoxychlor or
lindane in water by extraction and thin-layer chromatography.]
Chem. Anal., 13: 227-234 (in Polish).
TELFORD, H. S. & GUTHRIE, J. E. (1945) Transmission of the toxicity of
DDT through the milk of white rats and goats. Science, 102: 647.
TERRACINI, B., TESTA, M. C., CABRAL, J. R., & DAY, N. (1973) The
effects of long-term feeding of DDT to BALB/c mice. Int. J.
Cancer, 11: 747-764.
THOMAS, E. J., BURKE, J. A., & LAWRENCE, J. H. (1968) Thin-layer
chromatography; relative migration date (R-TDE) of chlorinated
pesticides. J. Chromatogr, 35: 119-121.
THOMAS, T. C. & SERBER, J. N. (1974) Chromosorb 102, an efficient
medium for trapping pesticides from air. Bull. environ. Contam.
Toxicol., 12: 17-25.
THOMPSON, J. F., ed. (1974) Analysis of pesticide residues in human
and environmental samples, a compilation of methods selected for
use in pesticide monitoring programs, Research Triangle Park,
NC, US Environmental Protection Agency, 1 vol.
THOMPSON, J. F., WALKER, A. C., & MOSEMAN, R. F. (1969a) Evaluation of
8 gas chromatographic columns for chlorinated pesticides.
J. Assoc. Off. Anal. Chem., 52: 1263-1277.
THOMPSON, J. F., WALKER, A. C., & MOSEMAN, R. F. (1969b) Study of the
performance of gas-chromatographic columns under severe injection
loading. J. Assoc. Off. Anal. Chem., 52: 1251-1262.
THOMPSON, J. F., MANN, J. B., APODACA, A. O., & KANTOR, E. J. (1975)
Relative retention ratios of 95 pesticides and metabolites on nine
gas-liquid chromatographic columns over a temperature range of
170-204°C on two detection modes. J. Assoc. Off. Anal. Chem., 58:
1037-1050.
THOMPSON, R. P. H., PILCHER, C. W. T., ROBINSON, J., STATHERS, G. M.,
MCLEAN, A. E. M., & WILLIAMS, R. (1969) Treatment of unconjugated
jaundice with dicophane. Lancet, 2: 4-6.
THORPE, E. & WALKER, A. I. T. (1973) The toxicology of dieldrin
(HEOD). II. Comparative long-term oral toxicity studies in mice
with dieldrin, DDT, phenobarbitone, ß-BHC, and gamma-BHC. Food
Cosmet. Toxicol., 11: 433-442.
TINSLEY, I. J. & LOWRY, R. R. (1972) An interaction of DDT in the
metabolism of essential fatty acids. Lipids, 7: 182-185.
TOCCI, P.M., MANN, J. B., DAVIES, J. E., & EDMUNDSON, W. F. (1969)
Biochemical differences found in persons chronically exposed to
high levels of pesticides. Ind. Med. Surg., 38: 188-195.
TOKUTSU, K., KOYAMA, T., & TOKOYAMA, T. (1970) [Pesticide residues in
mothers' milk and plasma in Wakayama Prefecture.] Annu. Rep.
Wakayama Prefect. Inst. Public Health, 19: 59-62 (in Japanese).
TOMATIS, L. & TURUSOV, V. (1975) Studies on the carcinogenicity of
DDT. GANN Monograph Cancer Res., 17: 219-241.
TOMATIS, L., TUROSOV, V., DAY, N., & CHARLES, R. T. (1972) The effect
of long-term exposure to DDT on CF-1 mice. Int. J. Cancer,
10: 489-506.
TOMATIS, L., PARTENSKY, C., & MONTESANO. R. (1973) The predictive
value of mouse liver tumor induction in carcinogenicity testing --
A literature survey. Int. J. Cancer, 12: 1-20.
TOMATIS, L., TURUSOV, V., CHARLES, R. T., & BOICCHI, M. (1974a).
Effect of long-term exposure to 1,1-dichloro-2,2-bis( p-
chlorophenyl)ethylene, 1,1-dichloro-2,2-bis( p-chlorophenyl)
ethane, and to the two chemicals combined on CF-1 mice. J. Natl
Cancer Inst., 52: 883-891.
TOMATIS, L., TURUSOV, V., CHARLES, R. T., BOICCHI, M., & GATI, F.
(1974b) Liver tumors in CF-1 mice exposed for limited periods to
technical DDT. Z. Krebs. Forsch., 82: 25-35.
TOMII, S., NAGASAKI, H., & MEGA, Y. (1972) [Studies on BHC as a
carcinogen.] Jpn. J. Hyg., 27: 113 (in Japanese).
TORDA, C. & WOLFF, H. G. (1949) Effect of convulsant and
anticonvulsant agents on the activity of carbonic anhydrase.
J. Pharmacol. exp. Ther., 95: 444-447.
TREON, J. F. & CLEVELAND, F. P. (1955) Toxicity of certain chlorinated
hydrocarbon insecticides for laboratory animals with special
reference to aldrin and dieldrin. J. agric. food Chem.,
3: 402-408.
TSUTSUI, J., KATO, T., & NISHIKAWA, T. (1974) [Results of health
survey on persons engaging in pesticide application in Suzuka
area. Mie Prefecture.] J. Jpn. Assoc. Rural Med., 23: 518-521
(in Japanese).
TUINSTRA, L. G. M. TH. (1971) [Organochlorine insecticide residues in
human milk in the Leiden Region.] Ned. Melk-Zuiveltijdschr.,
25: 24-32 (in Dutch).
UNTERMAN, H. W. & SIRGHIE, E. (1969) [Presence of organochlorines in
human body.] 18: 221-226 (in Romanian).
US DEPT OF AGRICULTURE, AGRICULTURAL RESEARCH ADMINISTRATION (1949)
USDA entomologists recommend substitute insecticide for DDT to
control insects on dairy cattle and in dairy barns. News
Release, March 24.
U.S. DEPT OF AGRICULTURE, AGRICULTURAL RESEARCH SERVICE (1966)
Monitoring agricultural pesticide residues. A preliminary report
of studies on soil, sediment and water in Mississippi River
delta, Washington, DC, US Government Printing Office.
US DEPT OF AGRICULTURE, BUREAU OF ENTOMOLOGY AND PLANT QUARANTINE
(1945a) Release of February 13, 1945.
US DEPT OF AGRICULTURE, BUREAU OF ENTOMOLOGY AND PLANT QUARANTINE
(1945b) Outlook for supplies of DDT insecticides. Release of
August 6, 1945.
US DEPT OF HEALTH EDUCATION AND WELFARE (1969) Manual for evaluating
public drinking water supplies. A manual of practice recommended
by the Public Health Service, Washington, DC, US Government
Printing Office, I (PHS Pub. No. 1820).
US ENVIRONMENTAL PROTECTION AGENCY (1975) DDT. A review of scientific
and economic aspects of the decision to ban its use as a
pesticide, Washington US EPA, US Government Printing Office,
300 pp. (EPA-504-1-75-022).
VAARAMA, A. (1947) The influence of DDT pesticides upon plant mitosis.
Hereditas, 33: 191-219.
VAINO, H. (1974) Enhancement of hepatic microsomal drug oxidation and
glucuronidation in rat by 1,1,1-trichloro-2,2-bis( p-
chlorophenyl)ethane (DDT). Chem. biol. Interactions, 9: 7-14.
VAINIO, H. (1975) Stimulation of microsomal drug-metabolizing enzymes
in rat liver by 1,1,1-trichloro-2,2-bis( p-chlorophenyl)ethane
(DDT), pregnenolone-16alpha-carbonitrile (PEN), and
polychlorinated biphenyls (PCBs). Environ. Qual. Saf.,
3 (Suppl.): 486-490.
VANAT, S. V. & VANAT, I. M. (1971) [Contribution to the toxic-allergic
reaction induced by DDT.] Klin. Med. (Mosk.), 49: 126-127
(in Russian).
VAS'KOVSKAJA & KOMAROVA (1967) Quoted by: Kagan et al., Res. Rev, 27:
43-79.
VAS'KOVSKAJA, L. F. (1969) [Accumulation of certain chloroorganic
insecticides in the bodies of experimental animals and humans.]
In: Medved', L. E., ed. The hygiene of application and the
toxicology of pesticides and the clinical features of pesticide
poisoning. A collection of papers. Issue No. 7, Kiev,
Vniggintox, pp. 503-796 (in Russian).
VAZ A., PEREIRA, R. S., & MALHETRO, D. M. (1945) Calcium in prevention
and treatment of experimental DDT poisoning. Science,
101: 434-436.
VELBINGER, H. H. (1947a) [On the question of DDT toxicity to man.]
Dtsch. Gesundheitwes., 2: 355-358 (in German).
VELBINGER, H. H. (1947b) [Contribution to the toxicology of "DDT"-
agent, dichlorodiphenyl-trichloromethylmethane.] Pharmazie,
2: 268-274 (in German).
VERDON, T. A., JR, BRUTON, J., HERMAN, R. H., & BEISEL, W. R. (1962)
Clinical and chemical response of functioning adrenal cortical
carcinoma to ortho-, para-DDD. Metab. Clin. Exp., 11: 226-234.
VERSINO, B., VENNE, M-TH VAN DER, & VISSERS, H. (1971) Comparison of
some clean-up columns for residue analysis of chlorine and
phosphorus containing pesticides. J. Assoc. Off. Anal Chem.,
54: 147-149.
VON OETTINGEN, W. F. & SHARPLESS, N. E. (1946) The toxicity and toxic
manifestations of 2,2-bis-( p-chlorophenyl)-1,1,1-trichloroethane
(DDT) as influenced by chemical changes in the molecule. A
contribution to the relation between chemical constitution and
toxicological action. J. Pharmacol. exp. Ther., 88: 400-413.
WAGSTAFF, D. J. (1971) Ascorbic acid deficiency and induction of
hepatic microsomal hydroxylative enzymes by organochlorine
pesticides. Toxicol. appl. Pharmacol., 19: 10-19.
WAGSTAFF, D. J. & STREET, J. C. (1970) Dieldrin-DDT interactions in
guinea pigs. Toxicol. appl. Pharmacol., 17: 276.
WALKER, A. I. T., THORPE, E., & STEVENSON, D. E. (1973) The toxicology
of dieldrin (HEOD). I. Long-term oral toxicity studies in mice.
Food Cosmet. Toxicol., 11: 415-432.
WALKER, K. C., GOETTE, M. B., & BATCHELOR, G. S. (1954) Pesticide
residues in foods: Dichlorodiphenyltrichloroethane and
dichlorodiphenyldichloroethylene content of prepared meals.
J. agric. food. Chem., 2: 1034-1037.
WALLACE, Z. E., SILVERSTEIN, J. N., VILLADOLID, L. S., WEISENFELD, S.
(1961) Cushing's syndrome due to adrenocortical hyperplasia. New
Engl. J. Med., 265: 1088-1093.
WARNICK, S. L. (1972) Organochlorine pesticide levels in human serum
and adipose tissue, Utah-Fiscal years 1967-71. Pestic. Monit. J.,
6: 9-13.
WARNICK, S. L. & CARTER, J. E. (1972) Some findings in a study of
workers occupationally exposed to pesticides. Arch. environ.
Health, 25: 265-270.
WASICKY, R. & UNTI, O. (1944) [Dichloro-diphenyl-trichloroethanol
(DDT) does not have any effect on the larvae of "Culicides".]
Arch. Hig. S. Paulo, 9: 87-102 (in Portuguese).
WASICKY, R. & UNTI, O. (1945) [Dichlorodiphenyltrichloroethane (DDT),
additional investigations on its properties and application.]
Arch. Hig. S. Paulo, 10: 49-64 (in Portuguese).
WASSERMANN, D., WASSERMANN, M., DJAVAHERIN, H., GORIN, I., BARISH, M.,
& TAVOR, R. (1971) Effect of organochlorine insecticides on serum
PBI level in occupationally exposed people. Bull. environ.
Contam. Toxicol., 6: 85-88.
WASSERMANN, M., ILIESCU, S., MANDRIC, G., & HORVATH, P. (1960) Toxic
hazards during DDT- and BHC-spraying of forests against Lymantria
monacha. Arch. ind. Health, 21: 503-508.
WASSERMANN, M., GON, M., WASSERMANN, D., & ZELLERMAYER, L. (1965) DDT
and DDE in the body fat of people in Israel. Arch. environ.
Health, 11: 375-379.
WASSERMANN, M., WASSERMANN, D., ZELLERMAYER, L., & GON, M. (1967)
Pesticides in people. Storage of DDT in the people of Israel.
Pestic. Monit. J., 1: 15-20.
WASSERMANN, M., CURNOW, D. H., FORTE, P. N., & GRONER, Y. (1968a)
Storage of organochlorine pesticide in the body of people in
Western Australia. Ind. Med. Surg., 37: 295-300.
WASSERMANN, M., SOFOLUWE, G. I., WASSERMANN, D., GRONERS, I., &
LAZAROVITCH, S. (1968a) Storage of organochlorine insecticides in
the body fat of people in Nigeria. In: Wasserman, H. & Sofoluwe,
G. O., ed. The Lagos International Seminar on Occupational Health
for Developing Countries, 1-6 April 1968, pp. 188-202.
WASSERMANN, M., WASSERMANN, D., GERSHON, Z., & ZELLERMAYER, L. (1969)
Effect of organochlorine insecticides on body defence systems.
Ann. NY Acad. Sci., 160: 393-401.
WASSERMANN, M., WASSERMANN, D., ARONOVSKI, I., IVRIANI, I., &
ROSENFELD, D. (1970a) The effects of organochlorine insecticides
on serum cholesterol level in people occupationally exposed. Bull
environ. Contam. Toxicol., 5: 368-372.
WASSERMANN, M., WASSERMANN, D., LAZAROVICI, S., COETZEE, A. M., &
TOMATIS, L. (1970b) Present state of the organochlorine
insecticides in the general populations of South Africa. South
Afr. med. J., 44: 646-648.
WASSERMANN, M., WASSERMANN, D., & IVRIANI, I. (1970c) Organochlorine
insecticides in the plasma of occupationally exposed workers. In:
Deichmann, W. B., Radomski, J. L., & Penalver, R. A., ed.
Pesticides Symposia, Miami, Florida, Halos. pp. 311-314.
WASSERMANN, M., WASSERMANN, D., KEDAR, E., & DJAVAHERIAN, M. (1971)
Immunological and detoxication interactions in p,p'-DDT fed
rabbits. Bull. environ. Contam. Toxicol., 6: 426-534.
WASSERMANN, M., ROGOFF, M. G., TOMATIS, L., & DAY, N. E. (1972a)
Storage of organochlorine insecticides in the adipose tissue of
people in Kenya. Ann. Soc. Belg. Med. Trop., 52 (6): 509-514.
WASSERMANN, M., NOGUEIRA, D. P., TOMATIS, L., ATHIE, E., WASSERMANN,
D., KJAVAHERIAN, M., & GUTTEL, C. (1972b) Storage of
organochlorine insecticides in people of Sao Paulo, Brazil. Ind.
Med. Surg., 41: (3): 22-25.
WASSERMANN, M., TRISHNANANDA, M., TOMATIS, L., DAY, N. E, WASSERMANN,
D., RUNGPITARANGSI, V., CHAMSAKOL, V., DJAVAHERIAN, M., & CUCUS,
S. (1972c) Storage of organochlorine insecticides in the adipose
tissue of people from Thailand. Southeast Asian J. trop. Med.
Public Health, 3: 280-285.
WASSERMANN, M., SOFOLUWE, G. O., TOMATIS, L., DAY, N. E., WASSERMANN,
D., & LAZAROVICI, S. (1972d) Storage of organochlorine
insecticides in people in Nigeria. Environ. Physiol. Biochem.,
2: 59-67.
WASSERMANN, M., TOMATIS, L., WASSERMANN, D., DAY, N. E., &
DJAVAHERIAN, M. (1974a) Storage of organochlorine insecticides in
adipose tissue of Ugandans. Bull. environ. Contam. Toxicol.,
12: 501-508.
WASSERMANN, M., TOMATIS, L., WASSERMANN, D., DAY, N. E., GRONER, Y.,
LAZAROVICI, S., & ROSENFELD, D. (1974b) Epidemiology of
organochlorine insecticides in the adipose tissue of Israelis.
Pestic. Monit. J., 8: 1-7.
WASSERMANN, M., PRATA, A., TOMATIS, L., WASSERMANN, D., IVRIANI, I., &
OLIVIERA, V. (1975) Storage of organochlorine insecticides in
hepato-splenic schistosomiasis. Rev. Soc. Bras. Mad. Trop.,
8: 271-273.
WATARI, N. (1973) [Ultrastructural alterations of the mouse liver
after the prolonged administration of BHC.] J. Jpn. Electron
Microsc. Soc., 5: 1410-1420; 1449-1456 (in Japanese).
WATSON, M., BENSON, W. W., & GABICA, J. (1970) Serum organochlorine
pesticide levels in people in Southern Idaho. Pestic. Monit. J.,
4: 47-50.
WATSON, M., GABICA, J., & BENSON, W. W. (1972) Serum organochlorine
pesticides in mentally retarded patients on differing drug
regimens. Clin. Pharmacol. Ther., 13: 186-192.
WATTENBERG, L. W. (1971) Studies of polycyclic hydrocarbon
hydroxylases of the intestine possibly related to cancer. Effect
of diet on benzypyrene hydroxylase activity. Cancer, 28: 99-102.
WEAVER, L., GUNNERSON, C. G., BREIDENBACH, A. W., & LICHTENBERG, J. J.
(1965) Chlorinated hydrocarbon pesticides in major US river
basins. Public Health Rep., 80: 481-493.
WEIHE, M. (1966) [Chlorinated insecticides in the body fat of people
in Denmark.] Ugeskr. Laeg., 128: 881-882 (in Danish).
WEIL, C. S. & McCOLLISTER, D. D. (1963) Relationship between short-
and long-term feeding studies in designing an effective toxicity
test. J. agric. food Chem., 11: 486-491.
WEIL, C. S., WOODSIDE, M. D., BERNARD, J. R., & CARPENTER, C. P.
(1969) Relationship between single-peroral, one-week, and ninety-
day rat feeding studies. Toxicol. appl. Pharmacol., 14: 426-431.
WEIL, L., QUENTIN, K. E., & RONICKE, G. (1973) Pesticide levels in air
dust particles in the Federal Republic of Germany. In: The
Technical University of Munich and the German Society for
Research, Commission on Research on Air Pollution, Notice VIII,
23 pp.
WELCH, R. M. & HARRISON, Y. (1966) Reduced drug toxicity following
insecticide treatment. Pharmacologist, 8: 217.
WELCH, R. M., LEVIN, W., & CONNEY, A. H. (1969) Estrogenic action of
DDT and its analogs. Toxicol. appl. Pharmacol., 14: 358-367.
WELCH, J. H. & GORDON, H. T. (1946) The mode of action of DDT. Fed.
Proc., 5: 1.
WEST, I. (1964) Pesticides as contaminants. Arch. environ. Health,
9: 626-631.
WESTOO, G., NOREN, K., & ANDERSSON, M. (1970) [Levels of
organochlorine pesticides and polychlorinated biphenyls in
margarine, vegetable oils, and some foods of animal origin on the
Swedish market in 1967-1969.] Vaar foeda, 22: 9-31 (in Swedish).
WHITE, W. C. & SWEENEY, T. R. (1945) The metabolism of 2,2-bis-( p-
chlorophenyl-1,1,1-trichloroethane (DDT). I. A metabolite from
rabbit urine di-( p-chlorophenyl)-acetic acid; its isolation,
identification, and synthesis. Public Health Rep., 60: 66-71.
WHITING, F. M., STULL, J. W., BROWN, W. H., MILLBROTH, M., & WARE, G.
W. (1968) Comparison of extraction methods for analysis of DDT,
DDE and TDE, in alfalfa hay. J. dairy Sci., 51: 1039-1041.
WHO (1971) Appendix 14: The place of DDT in operations against malaria
and other vector-borne diseases. Off. Rec. World Health Organ.,
190: 176-182.
WHO (1973) WHO Technical Report Series No. 513 (Safe use of
pesticides) 54 pp.
WIGGLESWORTH, V. B. (1945) A case of DDT poisoning in man. Br. med.
J., 1: 517.
WILCOX, A. R. (1967) USPH investigation. DDT health effects. Inter-
Office Correspondence to M. V. Anthony, Stauffer Chemical Co.
WILLIS, G. H., PARR, J. F., & SMITH, S. (1971) Volatilization of soil-
applied DDT and DDD from flooded and nonflooded plots. Pestic
Monit. J., 4: 204-208.
WILSON, D. J., LOCKER, D. J., RITZEN, C. A., WATSON, J. T., &
SCHAFFNER, W. (1973) DDT concentrations in human milk. Am. J.
Dis. Child., 125: 814-817.
WILSON, H. F., ALLEN, N. N., BOHSTEDT, G., BETHEIL, J., & LARDY, H. A.
(1946a) Feeding experiments with DDT-treated pea vine silage with
special reference to dairy cows, sheep, and laboratory animals.
J. econ. Entomol., 36 (6): 801-806.
WILSON, H. F., SRIVASTAVA, A. S., HULL, W. B., BETHEIL, J., & LARDY,
H. A. (1946b) DDT residues on pea vines and canned peas from
fields treated with DDT dusts. J. econ. Entomol., 39
(6): 806-809.
WINTER, M., THOMAS, M., WERNICK, S., LEVIN, S., & FARVAR, M. T. (1976)
Analysis of pesticide residues in 290 samples of Guatemalan
mother's milk. Bull. environ. Contam. Toxicol., 16: 652-657.
WOLFE, H. R. & ARMSTRONG, J. F. (1971) Exposure of formulating plant
workers to DDT. Arch. environ. Health, 23: 169-176.
WOLFE, H. R., WALKER, K. C., ELLIOTT, J. W., & DURHAM, W. F. (1959)
Evaluation of the health hazards involved in house-spraying with
DDT. Bull. Worm Health Organ., 20: 1-14.
WOLFE, H. R., DURHAM, W. F, & ARMSTRONG, J. F. (1970) Urinary
excretion of insecticide metabolites: excretion of para-
nitrophenol and DDA as indicators of exposure to parathion and
DDT. Arch. envrion. Health, 21: 711-716.
WOODARD, G., OFNER, R. R., & MONTGOMERY, C. M. (1945) Accumulation of
DDT in the body fat and its appearance in the milk of dogs.
Science, 102: 177-178.
WOODARD, B. T., FERGUSON, B. B., & WILSON, D. J. (1976) DDT levels in
milk of rural indigent blacks, Research Triangle Park, NC, Health
Effects Research Laboratory, US Environmental Protection Agency,
16 pp. (EPA 600/1-76-032).
WOOLSON E. A. (1974) Extraction of chlorinated hydrocarbon
insecticides from soil. J. Off. Anal. Chem, 57: 604-609.
WOOLSON E. A. & KEARNEY, P. C. (1969) Survey of chlorinated
insecticide analyses in soils. J. Assoc. Off. Anal. Chem.,
52: 1202-1206.
WRENN, T. R., WEYANT, J. R., FRIES, G. F., & BITMAN, J. (1971a) Effect
of several dietary levels of o,p'-DDT on reproduction and
lactation in the rat. Bull. environ. Contam. Toxicol.,
6: 471-479.
WRENN, T., RANDALL, J., WEYANT, R., FRIES, G. F., & BITMAN, J. (1971b)
Influence of dietary o,p'-DDT on reproduction and lactation of
ewes. J. animal Sci., 33: 1288-1292.
WRIGHT, A. S., POTTER, D., WOODER, M. F., DONNINGER, C., & GREENLAND,
R. D. (1972) Effects of dieldrin on mammalian hepatocytes, Food
Cosmet. Toxicol., 10: 311-322.
WYLLIE, J., GABICA, J., & BENSON, W. W. (1972) Comparative
organochlorine pesticide residues in serum and biopsied lipoid
tissue: A survey of 200 persons in southern Idaho -- 1970.
Pestic. Monit. J., 6: 84-88.
YAMAGISHI, T, TAKEBA, K., FUGIMOTO, C., MORIMOTO, K., & HARUTA, M.
(1972) [On the organochlorine pesticide residues in mothers' body
and her fetus' body.] Rep. Tokyo Public Health Stud., 50: 44-45
(in Japanese).
YERMAKOV, V. V. (1972) [Gas chromatographic methods for determining
pesticides in biological material.] Moscow, Izdatelstvo Nauka (in
Russian).
YODER, J. M., WATSON, M., & BENSON, W. W. (1973) Lymphocyte chromosome
analysis of agricultural workers during extensive occupational
exposure to pesticides. Mutat. Res., 21: 335-340.
YOUDEN, W. J. & STEINER, E. H. (1975) Statistical Manual of the
Association of Official Analytical Chemists, Washington, DC,
AOAC.
ZAVON, M. R., HINE, C. H., & PARKER, K. D. (1965) Chlorinated
hydrocarbon insecticides in human body fat in the United States.
J. Assoc. Off. Anal. Chem., 193: 837-839.
ZAVON, M. R., TYE, R., & LATORRE, E. (1969) Chlorinated hydrocarbon
insecticide content of the neonate. Ann. NY Acad. Sci.,
160: 196-200.
ZEINE-EL-DINE, K. (1946) The insecticide DDT. J. Egypt Med. Assoc.,
29: 38-54.
ZELINSKI, S. G., TASHIRO, J., & WORTHEN, L. R. (1973) A gas-
chromatographic method of quantifying DDT in the presence of
interference from PCBs. J. Chromatogr., 84: 67-73.
ZERBER, J., KURASIAK, K., & CIELIEZAK, A. (1971) [Determination of
chlorinated hydrocarbon residues in cereal products and feeds.]
Chem. Anal. 16: 1061-1066 (in Polish).
ZIMMERLI, B. & MAREK, B. (1973) [The pesticide burden of the Swiss
population (analyses of prepared meals, human fat, human serum,
cigarettes, and cosmetics.)] Mitt. Lebensmitt. Hyg., 64: 459-479
(in German).
ZIMMERMAN, B., BLOCH, H. S., WILLIAMS, W. L., HITCHCOCK, C. R., &
HOEISCHER, B. (1956) Effects of DDD (1,1,dichloro-2,2-bis( p-
chloro-phenyl)-ethane) on human adrenal. Attempted to use adrenal
destructive agent in treatment of disseminated mammary and
prostatic cancer. Cancer, 9: 940-948.
ZWEIG, G. & SHERMA, J. (1972) Analytical Methods for Pesticides and
Plant Growth Regulators, New York, Academic Press, Vol 6,
765 pp.
ANNEX
TRANSFORMATION OF P,P'-DDT IN THE ENVIRONMENTa
1 Abiotic Transformations
Since 1969, the photolysis of p,p'-DDT (Annex Fig. 1, formula
II) and of its known primary environmental degradation product, DDE
(1,1-dichloro-2,2-bis [ p-chlorophenyl]ethylene; Annex Fig. 1,
formula VII) has been studied by irradiation in methanol at 260 nm
(Plimmer et al., 1970). The products formed were DDMU (1-chloro-2,2-
bis [ p-chlorophenyl] ethylene; Annex Fig. 1, Formula III),
dichlorobenzophenone (Annex Fig 1, formula IV) and dichlorobiphenyl
(Annex Fig. 1, Formula V). The formation of the last compounds
proceeded via dichlorobenzophenone as an intermediate. The detection
of the chlorinated biphenyl raised the question as to whether DDT
might be a source of PCBs in the environment. Upon investigation of
the reaction pathways leading to these substances, it was shown that
DDE is converted to 3,6-dichlorofluoroenone (Annex Fig. 1, formula VI;
Plimmer & Klingebiel, 1969) which is photooxidized to 3,3'-dichloro-
biphenyl-2-carboxylic acid. Subsequent decarboxylation of this acid
could yield traces of 3,3'-dichlorobiphenyl; the decarbonylation of
another photolysis product of DDT, trichlorobenzophenone, could yield
traces of trichlorobiphenyl, demonstrating that the formation of PCBs
with more than 2 chlorine atoms was also possible (Plimmer &
Klingebiel, 1973).
Irradiation studies with substances in organic solvents are not
necessarily predictive for the environment. However, studies with DDT
vapour in sunlight confirmed the results obtained with dissolved
substances. The proposed pathways of DDT photolysis under
environmental conditions is shown in Annex Fig. 1 (Moilanen & Crosby,
1973).
In 1972, DDE was irradiated in solvents, in the solid state, and
in the gaseous phase, with UV-light of various wavelengths. The
results are shown in Annex Fig. 2. Besides the known photoproducts IV
and III, a "trichlorinated DDMU" (Annex Fig. 2, formula VIII) and 2
compounds with longer side chains (Annex Fig 2, formula IX and X) were
identified; these 2 substances, however, were formed only upon
irradiation in a solvent and originated from the reaction with the
solvent (Kerner et al., 1972).
a Prepared by Dr F. Korte at the request of the Task Group.
Edmundson et al., 1970b; Watson et al., 1972). This result concerning
phenytoin was confirmed by McQueen et al. (1972) who also showed that
other drugs produced a smaller but still highly significant reduction
in DDT storage. Establishment of a reduced equilibrium appeared to
require about 2 months. Within this period, the regression of the
level of DDT plus DDE on duration of treatment with phenytoin was
highly significant ( P < 0.001).
At the end of 9 months' treatment, the body fat of nonepileptic
volunteers given phenytoin at a rate of 300 mg/man per day contained
an average of 25% of the DDT and 39% of the DDE concentrations
originally present before administration of the drug (Davies et al.,
1971).
The same was true of workers whose exposure was greater than that
of the general population. Maintenance doses of phenobarbital,
phenytoin, or a combination of the two kept the storage levels of
several organochlorine insecticides in epileptic workers as low as, or
lower than levels in the general population (Schoor, 1970; Kwalick,
1971).
8.2.5.2 Other biochemical observations
A positive linear correlation has been reported for the
concentrations of vitamin A and of DDT-related compounds in the serum
of men with at least 5 years of occupational exposure to DDT. However,
the workers' DDT levels were little higher than those of persons in
the general population (see Table 7), and their vitamin A levels were
within normal limits (Keil et al., 1972). Perhaps they were better fed
than the controls.
Compared to 86 unexposed workers, the serum total cholesterol
values of 206 workers in a chemical plant where unidentified
organochlorine insecticides were made and formulated were higher in
workers who were less than 25 years old, lower in those between 25-34
years and 35-44 years, and higher in those who were 45 years old or
more. The differences were significant only for the oldest groups
(Wassermann et al., 1970a).
8.2.6 Cardiovascular system
The small amount of knowledge concerning the effect of DDT on the
human heart fails to show whether cardiac arrhythmia might be a
possible cause of death in acute poisoning, as is true in some species
of laboratory animals. Palpitations, tachycardia, and irregular heart
action have been noted in some, but not all cases of acute poisoning
(Mackerras & West, 1946; Naevested, 1947; Hsieh, 1954).
8.2.7 Reproduction
There is no indication that DDT has influenced reproduction except
to increase it as an indirect result of disease control, especially
malaria control.
After Laws et al. (1967) had completed their study, Wilcox (1967)
found that the 36 most heavily exposed workers involved had fathered
58 children before they began working at the DDT factory and 93
children afterwards.
O'Leary et al. (1970c) did not find any significant relationship
between abortion and blood levels of DDT-related compounds.
8.2.8 Endocrine organs
Average protein-bound iodine (PBI)levels of 0.0542 and
0.0693 mg/litre, respectively, were reported in the sera of 42 workers
occupationally-exposed to organochlorine insecticides and in 51
workers who were not exposed. The difference was statistically
significant even though all values fell within the normal range of
0.04-0.08 mg/litre (Wassermann, D. et al., 1971). It was not recorded
whether the workers involved were from the same factory as those with
10 or more years of occupational exposure whose plasma DDT levels were
reported by Wassermann et al. (1970c) (see Table 7). The small
difference in PBI levels is difficult to evaluate. It was the view of
Clifford & Weil (1972) that there was not any evidence that
occupational exposure had had an effect on human endocrine organs.
TDE. Following the demonstration (discussed in section 7.1.8)
that TDE caused atrophy of a part of the adrenal cortex of dogs,
o,p'-TDE, and to a lesser degree m,p'-TDE, have been used in man,
under the name of mitotane, in the hope of controlling excessive
cortical secretion or of reducing the size of adrenal tumors. The
underlying condition may be hyperplasia or adrenocortical carcinoma.
The dosage given has varied from 7 to 285 (mg/kg)/day, but a dosage of
approximately 100 (mg/kg)/day for many weeks has been necessary to
produce any benefit in man (Bergenstal et al., 1960; Wallace et al.,
1961; Gallagher et al., 1962; Verdon et al., 1962; Bledsoe et al.,
1964; and Southern et al., 1966a,b).
The effects of idiopathic hyperplasia may be controlled; in fact a
state of adrenal insufficiency may be produced (Canlorbe et al., 1971;
Sizonenko et al., 1974).
o,p'-TDE may also give symptomatic relief of excessive
adrenocortical activity secondary to a tumour that produces ACTH
(Carey et al., 1973).
A favourable response was produced in about one-fourth to one-half
of patients with inoperable adrenocortical carcinoma (Canlorbe et al.,
1971; Hoffman & Mattox, 1972; Lubitz et al., 1973; Montgomery &
Struck, 1973). In fact, an occasional cure, involving complete
regression of metastases, was produced by chemotherapy including
o,p'-TDE (Perevodchikova et al., 1972; Schick, 1973). More commonly,
symptoms were relieved and life was prolonged by little more than 7-8
months (Canlorbe et al., 1971; Hoffman & Mattox, 1972; Lubitz et al.,
1973). The success of treatment was often indicated early on by a
reduction in steroid excretion (Hoffman & Mattox, 1972; Lubitz et al.,
1973).
The large dosage of o,p'-TDE necessary to produce clinical
benefit often produced general lassitude, anorexia, nausea, vomiting,
diarrhoea, and dermatitis (Naruse et al., 1970; Hoffman & Mattox,
1972; Nitshke & Link, 1972; Perevodcikova et al., 1972; Lubitz et al.,
1973). Apathy ranged from mild dulling of interest to profound
psychotic depression (Hoffman & Mattox, 1972). More rarely,
gynaecomastia, haematuria, leukopenia, and thrombocytopenia have been
reported (Luton et al., 1972; Perevodcikova et al., 1972). The
symptoms disappeared soon after administration of the drug ceased or
when the dosage was reduced (Perevodcikova et al., 1972).
Even large, therapeutic doses of o,p'-TDE did not cause
histological alterations in the adrenals in man (Wallace et al.,
1961). Furthermore, dosages in the therapeutic range (specifically
those between 110 and 140 (mg/kg)/day did not produce any detectable
injury to the liver, kidney, or bone marrow. All patients treated in
this way experienced significant anorexia and nausea, and some showed
central nervous system depression varying from lethargy to somnolence.
These toxic effects cleared when dosing was discontinued (Bergenstal
et al., 1960).
Kupfer (1967) reviewed extensive literature that indicated that
the effect in man and other species, except the dog, is caused by
stimulation of corticoid metabolism by massive doses of o,p'-TDE and
not by any direct effect on the adrenal. Southern et al. (1966a,b)
agreed that the effect was predominantly extra-adrenal in man, when
the drug was first given, but offered evidence that adrenal secretion
of cortisol was eventually reduced. However, even if therapeutic doses
eventually have a direct effect on the adrenal, doses encountered by
workers exposed to technical DDT do not (Clifford & Weil, 1972; Morgan
& Roan, 1973).
8.2.9 Carcinogenicity
Laws et al. (1967) did not find any case of cancer or blood
dyscrasia among the 35 heavily exposed workers in a DDT factory nor
did the medical records of 63 men who had worked there for more than 5
years reveal these diseases. Two men were employed who had a history
of successfully treated cancer before they came to work, but no
employee had contracted cancer during the 19 years that the plant had
been in operation; during this period, the work force varied from 111
to 135.
In the USA, the total death rates for cancer of the liver and its
biliary passages (classified individually as "primary", "secondary",
and "not stated whether primary or secondary") lead to the conclusion
that there has been a significant, almost constant decrease in the
total rate of liver cancer deaths from 8.8 in 1930 to 8.4 in 1944
(when DDT was introduced) to 5.6 in 1972. This almost constant decline
in total liver cancer death rates for the past 42 years offers no
evidence of any increase in liver cancer deaths since the introduction
of the first organochlorine pesticide into the environment. The
decrease in liver cancer deaths is even more significant in light of
the increasing life span of the general population in the USA, which
has resulted in an increased percentage of the population at risk from
cancer over these years. In spite of the limitations inherent in the
interpretation of such data, this record is a reminder that, more than
30 years after the introduction of DDT, there is no evidence,
whatsoever, that DDT is carcinogenic in man (Deichmann & MacDonald,
1976, 1977).
In the USA, the incidence of cancer is lower in rural counties
than in metropolitan areas in general (Mason et al., 1975).
It is sometimes implied that epidemiological evidence is useless
for revealing the carcinogenicity of a material for man unless it
involves large numbers of people who have been exposed to the material
for most or all of a lifetime. The fact is that some human carcinogens
have been detected through their occurrence in high incidence in small
groups for periods of much less than 25 years. What was commonly
considered the first recognition of chemical carcinogenesis in man
depended on the observations made by a single surgeon (Pott, 1775) in
a small fraction of his patients. Such was the intensity of the
exposure of the apprentices of chimney sweepers that cancer of the
scrotum often appeared at puberty. The editor responsible for
compiling the writings of Pott (1790) added a footnote indicating that
he had seen such a cancer in "an infant under eight years of age". It
must be understood that boys did not usually become apprentice chimney
sweepers before they were four-years-old. In connection with tumours
of the bladder mainly caused by ß-naphthylamine but to a lesser degree
by other aromatic amines, Hueper (1942) reviewed cases in which the
time from the first exposure to recognition of symptoms was 8-41,
9-28, and 2-35 years; in one series of 83 cases, 71% of the tumours
appeared from 1 to 15 years after exposure. The same author cited
reports (p. 104) of cases of malignant epitheliomas in persons exposed
to pitch for 18, 24, 24, and 36 months, respectively. Kleinfeld (1967)
reported 50-76% incidence of bladder cancer among several groups of
workers. He also noted a sharp drop in incidence of this condition
following decrease -- but not discontinuation -- of occupational
exposure to ß-naphthylamine.
8.2.10 Mutagenicity
Evidence regarding the mutagenic activity of DDT and its
significance in man is uncertain partly because the chromosomal
changes that are examined are sensitive to viral infections and
chemotherapy. The latter may not be recognized at the time of sampling
and may not have been shown to injure health through a mutagenic
mechanism.
Comparing samples collected in winter and during the peak season
of pesticide application, a slight increase in chromatid breaks was
reported in the cultured lymphocytes of workers exposed to a wide
variety of insecticides said to include DDT, although this was claimed
at a time when the use of DDT was banned. A somewhat larger increase
was reported for men exposed mainly to herbicides (Yoder et al.,
1973). In another study, lymphocytes cultured from workers with an
average DDT plasma level of 0.999 mg/litre showed significantly more
chromosomal and chromatid aberrations than cells cultured from
controls with an average plasma level of 0.275 mg/litre. The
difference was not significant in other comparisons in which the
average plasma levels were 1.030 versus 0.380 mg/litre and 0.240
versus 0.030 mg/litre, respectively (Rabello et al., 1975).
Examination of all of the data presented by the authors suggests a
simple dosage-effect relationship was present, with a detectable
effect starting somewhere between 0.2 and 0.4 mg/litre and increasing
at levels higher than 0.4 mg/litre.
8.3 Factors Influencing DDT Toxicity
There is no evidence that any factor except dosage is of practical
importance in determining DDT toxicity in man. Factors that have been
considered as possibly affecting asymptomatic storage of DDT include
age, sex, and race. Differences observed in connexion with these
factors are small, medically insignificant, and probably secondary to
dosage (Hayes, 1975).
Storage has also been reported to be greater in the tissues of
people with certain diseases (Deichmann & Radomski, 1968; Raclomski et
al., 1968; Vas'kovskaja, 1969; Dacre & Jennings, 1970; Jonczyk et al.,
1974). Again, the reported differences are small, and the highest
values for the samples in question are small compared with those found
in healthy workers (Hayes, 1975). Furthermore, a number of authors
have reported a similar range of storage in persons undergoing minor,
elective surgery and in those who have died from various causes (Hayes
et al., 1958; Dale et al., 1965; Robinson et al., 1965; Wassermann et
al., 1965). Some authors (Hunter et al., 1963; Robinson et al., 1965;
Hoffman et al., 1967; Hoffman, 1968; Morgan & Roan, 1970) have failed
to find any relationship between storage of insecticides and the cause
of death. Where a relationship was found, there was often the
possibility that the higher values were found in diseases that
involved some degree of wasting prior to death. Casarett et al. (1968)
found that higher values occurred in persons who had 3 characteristics
in common: emaciation, cancer, and widespread abnormality of the
liver.
A slightly greater storage of DDT and DDE that was reported in
persons who underwent splenectomy for hepatosplenic schistosomiasis
compared with those operated on for other conditions, mainly hernia
was statistically significant. No such difference was observed in
connexion with dieldrin, ß-HCH, or heptachlor epoxide (Wassermann et
al., 1975). Whereas it was speculated that the increased storage of
DDT and DDE might have been the result of a reduction in metabolism,
secondary to liver injury, the possibility of greater exposure as a
result of greater use of DDT in irrigated areas was not excluded.
8.4 Treatment of Poisoning in Man
No useful guidance regarding treatment has been obtained from the
very few cases of DDT poisoning that have occurred. Animal studies
indicate that sedatives, ionic calcium, and glucose or another ready
source of energy would be useful. On the basis of experience in
treating people poisoned by different convulsive poisons, it seems
likely that diazepam would be beneficial.
9. EVALUATION OF HEALTH RISKS TO MAN FROM EXPOSURE TO DDT AND RELATED
COMPOUNDS
9.1 Relative Contributions of Food, Water, Air, and Miscellaneous
Sources to Total Intake
9.1.1 Adult members of the general population
Food represents the major source of intake of DDT in the general
population. It has been estimated (section 5.1.5) that over 90% of the
DDT stored in the general population is derived from food. Around
1965, when the use of DDT was at its peak, intake in the USA was
approximately 0.04 mg/man per day from food, less than 0.000046 mg/man
per day from water, less than 0.00006 mg/man per day from urban air
and less than 0.0005 mg/man per day from the air in small agricultural
communities. The reason for the qualification "less than", is that the
intakes were calculated from the highest concentrations reported in
drinking-water and air.
Although total intake of DDT from food has not been measured in
some parts of the world, worldwide measurements of the storage of DDT
and its metabolites in human body fat indicate that the extremes of
total exposure have varied by a factor of about 10, but that total
exposure for most populations has varied by a factor of no more than 3
(see Table 9).
DDT in the dust in a house (as indicated either by a history of
extensive household application of insecticides or by the finding of
relatively high levels of DDT in house dust) can contribute to the
storage of DDT-related compounds in persons living in the house
(section 5.1.4). However, although the contribution of house dust to
DDT intake has been established, it is not quite clear how this
contribution occurs. Some of the dust may contaminate food in the
process of preparation and some may be inhaled and later swallowed
after deposition in the upper airway. It is difficult to believe that
enough DDT is present in such houses in the form of vapour or
respirable dust to cause a substantial increase in the total exposure
of the inhabitants, but no critical study has been made of this
matter. Clearly, some DDT will enter by the respiratory route.
9.1.2 Infants and children
At birth, infants tend to have slightly lower levels of DDT than
adults in the same population. This is because the placenta offers
partial protection against the passage of DDT and related compounds.
Although human milk tends to have a somewhat higher concentration
of DDT than cow's milk (see section 6.3.1.3), the difference, if any,
that this makes to the rate at which breast-fed and bottle-fed babies
store the compound has not been established. It is possible that the
conditional acceptable daily intake (ADI) might be exceeded in an
infant wholly fed on breast milk. However, the ADI is calculated on
the basis of lifetime exposure, and short-term variations can be
regarded as not having any significance.
The only really important way in which the exposure of infants and
children differs from that of adults in the same community involves
accidental exposure (see section 8.1.3).
9.1.3 Occupational groups
Occupational exposure (section 5.3) to DDT is initially almost
exclusively through the respiratory and dermal routes. However, the
particles of many insecticidal dusts, wettable powders, and sprays are
too large to reach the lower respiratory tract. As a result, most of
the particles inhaled are deposited in the upper respiratory tract,
carried to the pharynx by ciliary action, and eventually swallowed
(section 6.1.1).
Although dermal exposure to DDT is high under some occupational
situations, the effect is minimal because the compound is so poorly
absorbed through the skin (section 6.1.3). The excellent safety record
of DDT, never matched by any other insecticide used in antimalaria
campaigns, other vector control programmes, and agriculture, is based
mainly on its poor absorption through the skin.
The number of people with full-time occupational exposure to DDT
alone is small. For example, at the time of one study, the only
factory making the compound in the USA produced 2722 metric tonnes per
month using a work force of about 145. Following the recommendations
of an Expert Committee, the World Health Organization studied spraymen
who had applied only DDT for 5 years or more. Only 272 suitable
subjects could be located in Brazil and only 144 in India. The
concentrations of DDT and its derivatives in the blood of the
preliminary and main study groups in India were 0.761 and
1.272 mg/litre, respectively. The blood levels of spraymen in Brazil
were about 3 times those of the controls.
The absorbed dosage of the men who made and formulated DDT for 10
years or more was about 18 mg/man per day (see section 8.1.1). The
exposure of other workers, notably those applying DDT for agricultural
purposes has usually been an order of magnitude less (see Table 7).
9.2 Effects of Exposure
No adverse effects have been described at repeated dosages of
1.5 (mg/kg)/day or less (see section 7.4). The large number of
measurements that have been made on samples from human populations
have not made it possible to define a maximum dosage that man can
absorb without any adverse effect but have highlighted the finding
that the high levels found in volunteers and workers were harmless for
at least 25 years.
Table 23 summarizes the clinical aspects of DDT in man.
Table 23. Dosage-effects of DDT in man
Single dose
(mg/kg) Observation
Unknown Fatal
16-286 Prompt vomiting at higher doses
(all poisoned, convulsions in some)
6-10 Moderate poisoning
Repeated
exposure
(mg/kg)/day
1.5 Administered as therapy for 6 monthsa
0.5 Administered to volunteers for 21 monthsa
0.5 Exposure of workers for 6.5 yearsa
0.25 Exposure of workers for 25 yearsa
0.0025 Intake of population in the USA, 1953-54a
0.0002 Intake of population in the USA, 1969-70a
a Without any adverse effect.
In considering the safety of workers who are employed in the DDT
industry, it is useful to consider the results of animal experiments.
Rats withstand a daily dosage at least 10 times that of these workers
without any detectable clinical effect (section 7, Table 17), although
minimal reversible tissue changes may be present. Dogs and monkeys
also withstand a daily dosage 10 times higher than that of the
workers, but they do not show the tissue changes, which seem to be
peculiar to some rodents. Because workers have tolerated high dosages
of DDT for over a fifth of a lifetime without detectable harm and
since animals withstand larger dosages for an entire lifetime without
injury, there is good reason to predict the continuing safety of the
workers.
The experience already gained from workers can be used to predict
the future safety of the general population in relation to DDT. Many
workers have now been exposed to DDT for much more than one-fifth of
their life span. Since they have not suffered detectable harm, it
seems most unlikely that the general population will be harmed by
dosages 200-1250 times smaller than those to which the workers are
exposed. It has been shown for at least 2 animal species that toxicity
resulting from a lifetime of exposure is seldom very different from
toxicity resulting from 90 days of exposure at the same dosage rate.
The largest factor of difference observed when 33 chemicals were
investigated was 20, and, for half of them, the factor was 2 or less
(see section 7.3.1.1). Ninety days constitute about one-eighth of the
lifespan of a rat, and this is less than the fraction of the human
life span that has been studied so far.
9.3 Carcinogenicity and Mutagenicity
Liver tumours are produced in mice by many chlorinated compounds,
including DDT. There is also evidence to suggest that DDT metabolites
DDE and TDE (DDD) produce hepatic tumours in mice and that TDE also
produces lung tumours. Information on the tumorigenicity of DDT in
rats (see section 7.1.9) is conflicting; some studies report tumour
formation while other studies report negative data. Carcinogenicity
studies in the hamster were negative (see section 7.1.9). The
occurrence of tumours in some rodents only, casts doubt on the
significance of the phenomenon and on extrapolation of the findings to
man.
Studies on the incidence of all cancers reported in those parts of
some countries with known high agricultural use of DDT in the 1950s
and early 1960s have not demonstrated any trends in any type of cancer
associated with the use of DDT in relation to these areas (see section
8.2.9).
The question as to whether DDT is carcinogenic in man has not been
answered unequivocally. Although the cross-sectional epidemiological
studies on workers exposed to DDT and the observation studies on
volunteers are limited, there is not any currently available evidence
to suggest that DDT is tumorigenic or carcinogenic in man (see section
8.2.9).
Recent studies on in vitro bacterial test systems with or
without metabolic activation have not shown any evidence that either
DDT or DDE is mutagenic (see section 8.2.10). The evidence for the
mutagenicity of DDT in mammalian test systems is inconclusive.
9.4 Effects on Microsomal Enzymes
There is no doubt that exposure to DDT results in the induction of
microsomal mixed function oxidases and causes marked morphological
changes in the liver of some rodents (see section 7.1.9). In some
rodents, notably the mouse, these morphological changes have been
related to tumorigenicity. Microsomal enzymes are also induced by DDT
in other species, but the liver does not show the same morphological
changes.
The only effect for which something approaching a threshold has
been demonstrated is the induction of microsomal enzymes in workers in
association with an average serum value of 0.573 mg/litre for p,p'-
DDT but not in workers with serum levels as high as 0.052 mg/litre, a
value essentially identical to the highest reported for the general
population. Furthermore, although some groups of workers experienced
an increase in their average enzyme activity, no person exceeded the
range of activity found in normal people in the general population.
DDT will not induce liver microsomal enzymes in the general
population because their intake of the compound is so much less than
the smallest dosage capable of producing this effect in animals or man
(see sections 7.1.4.2 and 8.2.5.1).
9.5 Reproduction and Teratogenicity
Effects on reproduction in mammals have been studied in the mouse,
the rat, and the dog (see section 7.1.7). In the mouse, a multi-
generation study at dietary levels of DDT of 25, 100, and 250 mg/kg
showed effects on fertility and reproduction only at the highest
level, equivalent to 33 (mg/kg)/day. In the rat, normal reproduction
was maintained at a dietary level of 200 mg/kg. In the dog, dietary
intake at dosages up to 10 (mg/kg)/day did not produce any effects on
reproduction other than earlier estrus in the DDT-treated females.
In man, there is no indication that DDT affects reproduction (see
section 8.2.7); no impairment of fertility was observed in a study of
men occupationally-exposed for more than 10 years to a measured
average daily intake in the region of 18 mg/man per day (equivalent to
0.25 (mg/kg)/day).
Studies in the mouse, the rat, and the dog have not shown any
evidence of teratogenicity. In the mouse, dosage at the rate of
1 mg/kg was not teratogenic; a single dosage of 25 mg/kg or repeated
doses at the rate of 2.5 mg/kg were embrytoxic but not teratogenic.
9.6 Immunosuppression
DDT appears to have a depressant effect on the immune system
although the evidence is by no means conclusive. Rats and rabbits
receiving DDT in aqueous suspension at a concentration of 200 mg/litre
showed a depression in antibody formation and decrease in at least one
globulin fraction of the blood. Rats receiving a dosage of
0.25 (mg/kg)/day by gavage did not show any changes in the phagocytic
activity of the white blood cells. In the guineapig, dosages of
1-20 mg/kg did not have any effects on antitoxin production but
produced a reduction in tissue histamine levels (see section 7.1.1).
9.7 Nutritional Effects and Other Factors
Animal studies indicate that nutritional status influences the
toxicity of DDT (see section 7.3.3). The preferential storage of DDT
in fat can mitigate the effect of acute poisoning. If rats that have
stored large amounts of DDT are starved, they may suffer toxic effects
due to mobilization of fat and DDT.
In man, nutritional status will have a similar effect to that
found in other animals. However, the possibility that starvation in
man could precipitate toxic manifestations is regarded as unlikely as
the stored levels do not approach those found in laboratory animals
and the lower metabolic rate of man results in slower mobilization. In
fact, severe weight loss sometimes does cause some increase in storage
of DDT in connexion with certain wasting diseases; however, people
with full-time occupational exposure to DDT average 10 times more
storage than the highest values reported in connection with disease
but do not exhibit predisposition to the diseases in question (see
section 6.2.1.3).
Although young animals are often more susceptible to toxic
chemicals than adults, there is no evidence that DDT is more toxic to
young animals of any species including man. In fact, in the rat, the
young are less susceptible to a single dose than the adults (see
section 7.3.2, Table 20).
9.8 Dosage-Effect Relationships
Dosage-effect relationships for DDT in man have been observed in
connection with acute poisoning (see Table 23), excretion, and storage
(see Fig. 4), and the induction of microsomal enzymes, which has been
observed at a dosage of 0.25 (mg/kg)/day but not at lower dosages. The
dosage of 0.25 (mg/kg)/day to which workers have been exposed for 25
years is of the same order of magnitude that causes an increase in
tumours in male mice of a susceptible strain but not in females of any
strain (see section 7.1.9). As shown in Table 24, this dosage in
workers is less than the no-effect levels for rats, dogs, and monkeys
and far less than the dosage at which rats, mice, and dogs
successfully reproduce for generations. The equilibrium levels of DDT
and its metabolites found in the blood and fat of people with
full-time occupational exposure and the much lower levels found in the
general population have not been associated with any adverse effects.
9.9 Recommendations on Levels of Exposure
The data from intake, exposure, and levels in populations supports
the current conditional ADI for DDT, which affords a considerable
margin of safety.
If the total intake of DDT from food and other sources rises above
0.005 (mg/kg)/day (the conditional ADI) then the situation should be
investigated.
Table 24. Dosage-effect of DDT in animals
Single Route Observation
(mg/kg)
3000 dermal LD50 of powder in adult rat
2356-4000 oral LD50 of oil solution in newborn rat
250-500 dermal LD50 of oil solution in adult rat
250 oral LD50 of oil solution in adult rat
Repeated
Exposure
(mg/kg)/day
300 subcutaneous inhibition of testicular growth in cockerels
41-80 oral increased mortality in rats, 2-year study
41-80 oral 100% mortality in dogs in 39-49 months
41-80 oral 100% mortality in monkeys, 70 days
21-40 oral 25% mortality in dogs in 39-49 months
33.2 oral harmful to reproduction in mice
13.3 oral slight reduction in lactation and survival of some but not all
generations of mice (6 generation test)
10 oral no harmful effect on reproduction in dog (3 generation test)
10 oral no harmful effect on reproduction in rat (2 generation test)
5-10 oral no-effect level in dog, 2 generations
2.6-5 oral no-effect level in monkey, 7.5 years
0.63-1.25 oral no-effect level in rat, 2 year test
0.16-0.31 oral risk of liver tumours doubled in male mice but no effect in
female
0.3 oral no-effect level for induction of microsomal enzymes in rats
The concentration of DDT in the air in industrial, agricultural,
or disease control areas should not exceed 1 mg/m3 on a time-
weighted basis (40 h per week). Several countries have their own
standards that range from 0.1 to 1 mg/m3, which seem to afford an
acceptable margin of safety.
There is ample reason to predict the continuing safety of workers
producing and using DDT. No harmful effect has ever been reported in
vector control operators who have applied DDT during the last 3
decades in public health programmes. Nevertheless, as in the case of
any chemical, occupational safety and health measures should always be
applied to ensure that contact with DDT by workers is kept to a
minimum.
The only index of exposure of DDT or its metabolites is the
analysis of these compounds in tissues or excreta. For most purposes
it is best to sample serum or plasma. In subjects with relatively
constant, prolonged exposure, concentrations of DDT and its
metabolites in the blood are in equilibrium with those in all other
tissues, including adipose tissue. In subjects who have accidentally
received a single large dose, the concentration in the brain is
reflected more accurately by a serum sample than by a fat sample.
REFERENCES
ABBOTT, D.C., HARRISON, R. B., TATTON, J. O'G., & THOMPSON, J. (1965)
Organochlorine pesticides in the atmospheric environment. Nature
(Lond.), 208: 1317-1318.
ABBOTT, D.C., GOULDING, R., & TATTON, J. O'G. (1968) Organochlorine
pesticide residues in human fat in Great Britain, Br. med. J.,
3: 146-149.
ABBOTT, D.C., HOLMES, D.C., & TATTON, J. O'G. (1969) Pesticide
residues in the total diet in England and Wales, 1966-67. J. Sci.
Food Agric., 20: 245-249.
ABBOTT, D. C, COLLINS, G. B., & GOULDING, R. (1972) Organochlorine
pesticide residues in human fat in the United Kingdom, 1969-71.
Br. med. J., 2: 553-556.
ABE, J., INOUE, Y., & TAKAMATSU, M. (1974) [On the residual amounts of
PCB and organochlorine pesticides in human blood.] Jpn J. Hyg.,
29: 93 (in Japanese).
ACKER, L. & SCHULTE, E. (1970) [The occurrence of polychlorinated
biphenyls and hexachlorobenzene along chlorinated insecticides in
human milk and human adipose tissue.] Naturwissenschaften,
57: 497 (in German).
ACKER, L. & SCHULTE, E. (1971) [Organochlorine compounds in the human
body.] Umschau, 71: 848 (in German).
ACKER, L. & SCHULTE, E. (1974) [Organochlorine compounds in human
fat.] Naturwissenschaften, 61: 32 (in German).
ADAMCZYK, E. (1971) [Translocation of DDT in chickens under the
influence of hunger.] Med. Weter, 27: 103-105 (in Polish).
ADAMOVIC, V., SOKIC, B., & PETROVIC, O. (1971) [On factors influencing
the occurrence of organochlorine insecticides in newborns.]
Ernaehrungsforschung, 16: 579-585 (in German).
ADAMS, M., COON, F. B., & POLING, C. E. (1974) Insecticides in the
tissues of four generations of rats fed different dietary fats
containing a mixture of chlorinated hydrocarbon insecticides.
J. agric. food Chem. 22: 69-75.
AHLING, B. & JENSEN, S. (1970) Reversed liquid-liquid partition in
determination of PCBs and chlorinated hydrocarbon insecticides in
water. Anal. Chem, 42: 1485-1486.
AHNHOFF, M. & JOSEFSON, B. (1974) Simple apparatus for on-site
continuous liquid-liquid extraction of organic compounds from
natural waters. Anal. Chem., 46: 658-663.
AIZICOVICI et al., Travaux Scientifiques de l'Institut d'Hygiene de
Jassy, 1960-1966, p. 142 (Quoted by: Wasserman, M., Sofoluwe,
G. O., Wasserman, D., Groner, Y., & Lazarovitch, S. (1968) Storage
of organochlorine insecticides in body fat of people in Nigeria.
In: Proceedings of the Lagos International Seminar on
Occupational Health for Developing Countries, Nigeria, 1-6 April
1968.
ALARY, J. G., GUAY, P., & BRODEUR, J. (1971) Effect of phenobarbital
on the metabolism of DDT in the rat and in the bovine. Toxicol.
appl. Pharmacol., 18: 457-468.
ALMEIDA, W. F., PIGATI, P., GAETA, R., & UNGARO, M. T. S. (1975) DDT
residues in human blood serum in Brazil. Environ. Qual. Saf.,
3 (Suppl.): 586-588.
ANON. (1972) [BHC and DDT residues in human milk on the decline in
Japan.] Noyaku Bijin, 56: 458 (in Japanese).
ANON. (1974) [Research methods for determination of chlorinated
hydrocarbon pesticide residues in or on food of animal origin.]
Bundesgesundheitsblatt, 17 (18): 269-276 (in German).
ANTOMMARIA, P., CORN, M., & DeMAIO, L. (1965) Airborne particulates in
Pittsburgh: Association with p,p'-DDT. Science, 150: 1476-1477.
ARMOUR, J. A. & BURKE, J. A. (1970) Separating polychlorinated
biphenyls from DDT and its analogues. J. Assoc. Off. Anal. Chem.,
53: 761-768.
ASAI, R. I., GUNTHER, F. A., WESTLAKE, W. E., & IWATA, Y. (1971)
Differentiation of PCBs from DDT by carbon skeleton chromatography.
J. agric. food. Chem., 19: 39-398.
ASKARI, E. M. & GABLIKS, J. (1973) DDT and immunological responses.
II. Altered histamine levels and anaphylactic shock in guineapigs.
Arch. environ. Health, 26: 309-312.
AUE, W. A. & TELI, P. M. (1971) Sampling air pollutants with support-
bonded chromatographic phases. J. Chromatogr., 62: 15-27.
BAGLEY, G. E., REICHEL, W. L., & CROMARTIE, E. (1970) Identification
of PCBs in two bald eagles by combined gas-liquid chromatography-
mass spectrometry. J. Assoc. Off. Anal. Chem., 53: 251-261.
BAKSEYEV, N. S. (1973) [Prophylaxis of pathology of the fetus and
newborn child (experience of the Ukrainian SSR).) Vestn. Akad.
Med. Nauk (SSSR), 28: 69-75 (in Russian).
BARKA, T. & POPPER, H. (1967) Liver enlargement and drug toxicity.
Medicine, 46: 103-117.
BELONOZKO, G. A. & KUCAK, JU. A. (1974) [Hygienic features of the
content of pesticides in the atmosphere.] In: Medved, L. E., ed.
Hygiene of application and toxicology of pesticides and the
clinical features of pesticides poisoning. A collection of
papers, Issue No. 5, pp. 63-74 Kiev. Vniigintox (in Russian).
BELONOZKO, G. A., VEVCHURKO, S. N., KUCHAK, U. A., TOVSTENKO, A. E., &
SOROKENA, L. V. (1967) [The spread of pesticides by air currents
in the environment in relation to method of application.] In:
Medved, L. E., ed. Hygiene and toxicology of pesticides and the
clinical features of pesticide poisoning. A collection of papers,
pp. 24-35 Kiev, Vniigintox (in Russian).
BENVENUE, A. & OGATA, J. N. (1970) Note on the use of florisil
adsorbent for separation of PCBs from chlorinated pesticides.
J. Chromatogr., 63: 142-144.
BERGENSTAL, D. M., HERTZ, R., LIPSETT, M. B., & MOY, R. H. (1960)
Chemotherapy of adrenocortical cancer with o,p'-DDD. Ann.
intern. Med., 53: 672-682.
BEYERMANN, K. & ECKRICH, W. (1973) [Adsorption of traces of
insecticides from water on polyethylene.] Z. anal Chem.,
265 (1): 1-4 (in German).
BEYERMANN, K. & ECKRICH, W. (1974) [Differentiation of the aerosol-
bound and gaseous fractions of insecticides in air.] Z. anal.
Chem., 269 (4): 279-284 (in German).
BEZUGLYI, V. P. & KASKEVICH, L. M. (1969) [Some indices of the
functional state of the liver in flying personnel engaged in
aerial chemical operations.] Gig. Tr. Prof. Zabol., 13 (10):
52-53 (in Russian).
BEZUGLYI, V. P., ODINTSOVA, I. L. R., & GORSKAYA, N. Z. (1973)
[Morphological composition of the blood in persons working with a
complex of organochlorine and organophosphorus pesticides.]
Vrach. Delox, 11: 150-154 (in Russian).
BICK, M. (1967) Chlorinated hydrocarbon residues in human body fat.
Med. J. Aust., 1: 1127-1129.
BIROS, F. J. (1970) Analysis for p,p'-DDT and p,p'-DDE pesticide
residues by NMR spectroscopy. J. Assoc. Off. Anal Chem.,
53: 733-736.
BISHARA, R. H., BORN, G. S., & CHRISTIAN, J. E. (1972a) Thin-layer
chromatography of DDT and some related compounds on aluminium
oxide chromatoplates. J. Chromatography, 64: 135-145.
BISHARA, R. H., BORN, G. S., & CHRISTIAN, J. E. (1972b) Radiotracer
distribution and excretion study of chlorophenothane in rats.
J. pharm. Sci, 61: 1912-1916.
BISKIND, M. S. (1952) [The new insecticides and the public health.]
Harefuah, 44: 9-13 (in Hebrew).
BISKIND, M. S. (1953) Public health aspects of the new insecticides.
Am. J. dig. Dis., 20: 331-341.
BISKIND, M. S. & BIEBER, I. (1949) DDT poisoning -- A new syndrome
with neuropsychiatric manifestations. Am. J. Psychother.,
3: 261-270.
BITMAN, J., CECIL, H. C., & FRIES, G. F. (1970) DDT-induced inhibition
of avian shell gland carbonic anhydrase. A mechanism for thin
eggshells. Science, 168: 594-596.
BJERK, J. E. (1972) [DDT and polychlorinated biphenyl residues in
human material from Norway.] Tidsskr, Nor. Laegeforen.,
92: 15-19, 37-38 (in Norwegian).
BLEDSOE, T., ROLAND, D. P., HEY, R. L., & LIDDLE, G. W. (1964) An
effect of o,p'-DDD on extra-adrenal metabolism of cortisol in
man. J. clin. Endocrinol. Metab., 24: 1303-1311.
BOIKO, V. G. & KRASNYUK, E. P. (1969) [Clinical characteristics of the
pathology of respiratory tracts of persons working with DDT.]
Z. ushn. nos. gorl. Bolezn., 29: 35-38 (in Russian).
BOJANOWSKA, A., JONCZYK, W., TRACZYK, Z., & KLAWE, Z. (1973) [DDT and
alpha-BHC content of blood and fatty tissue of subjects not
professionally exposed to these compounds.] Pol. Tyg. Lek.,
28: 1999-2001 (in Polish).
BOYD, E. M. & DECASTRO, E. S. (1968) Protein-deficient diet and DDT
toxicity. Bull. World Health Organ., 38: 141-150.
BOYD, E. M. & DECASTRO, E. S. (1970) Toxicity of dicophane (DDT) in
relation to dietary protein intake. Ind. Med., Surg.,
39: 229-236.
BOYD, E. M. & KRIJNEN, C. J. (1969) Dietary protein and DDT toxicity.
Bull. environ. Contam. Toxicol., 4: 256-261.
BRADY, M. N. & SIYALl, D. S. (1972) Hexachlorobenzene in human fat.
Med. J. Aust., 1: 158-161.
BRATOWSKI, T. A. & MATSUMURA, F. (1972) Properties of a brain
adenosine triphosphatase sensitive to DDT. J. econ. Entomol.,
65: 1238-1245.
BREWERTON, H. V. & MCGRATH, H. J. W. (1967) Insecticides in human fat
in New Zealand. N.Z.J. Sci., 10: 486-492.
BRODEUR, J. & LAMBERT, G. (1973) Further studies on the effect of
starvation and microsomal enzyme induction on the mobilization of
DDT residues in rats. Toxicol. appl. Pharmacol., 25: 488.
BRONISZ, H. & OCHYNSKI, J. (1968) [DDT and DDE content in the milk of
women.] Biul. Inst. Ochr. Rosl., 41: 99-102 (in Polish).
BRONISZ, H., RUSIECKI, W., OCHYNSKI, J., & BERNARD, E. (1967) [DDT in
adipose tissue of Polish population.] Diss. Pharm. Pharmacol.,
19: 309-314 (in Polish).
BRONISZ, H., OCHYSNSKI, J., & WLODZIMIERA, L. (1969) [Contents of DDT
in the adipose tissue of the Polish population.] Ann. Univ.
Mariae Curies -- Sklodwska. sect. D. (Med.), 24: 93-99 (in
Polish).
BROWN, J. R. (1967) Organochlorine pesticide residues in human depot
fat. Can. Med. Assoc, J., 97: 367-373.
BROWN, J. R. & CHOW, L. Y. (1975) Comparative study of DDT and its
derivatives in human blood samples in Norfolk County and Holland
Marsh, Ontario. Bull. environ. Contam. Toxicol., 13: 483-488.
BUNYAN, P. J., TOWNSHEND, M. G., & TAYLOR, A. (1972) Pesticide induced
changes in hepatic microsomal enzyme systems. Some effects of 1,1-
di( p-chlorophenyl)-2,2,2-trichloroethane (DDT) and 1,1-di( p-
chlorophenyl)-2,2-dichloroethylene (DDE) in the rat and Japanese
quail. Chem. Biol. Interactions, 5: 13-26.
BURLINGTON, H. & LINDERMAN, V. F. (1950) Effect of DDT on testes and
secondary sex characteristics of White Leghorn cockerels. Proc.
Soc. Exp. Biol., 74: 48-51.
BURNS, E. C., DAHM, P. A., & LINDQUIST, D. A. (1957) Secretion of DDT
metabolites in the bile of rats. J. Pharmacol. exp. Ther.,
121: 55-62.
BURNS, J. E. (1974) Organochlorine pesticide and polychlorinated
biphenyl residues in biopsied human adipose tissue -- Texas
1969-1972. Pestic. Monit. J., 7: 122-126.
BUTLER, P. A. (1969) Monitoring pesticide pollution. Biol. Sci.,
19: 889-891.
CAHILL, W. P., ESTESEN, B. J., & WARE, G. W. (1970) Determination of
DDT in presence of Toxaphene (Camphechlor) residues. Bull,
environ. Contam. Toxicol., 5: 260-262.
CAMERON, G. R. & BURGESS, F. (1945) The toxicity of 2,2-bis-( p-
chlorophenyl)-1,1,1-trichloroethane (DDT). Br. med. J.,
1: 865-871.
CAMPBELL, J. E., RICHARDSON, L. A., & SCHAFER, M. L. (1965)
Insecticide residues in the human diet. Arch. environ. Health,
10: 831-836.
CANLORBE, P., BADER, J. C., & JOB, J. C. (1971) Diagnostic problems
arising from a virilizing tumour of the adrenal cortex. (Case
report -- Literature review.) Sem. Hop., 47: 2255-2270.
CAREY, R. M., ORTH, D. N., & HARTMANN, W. H. (1973) Malignant melanoma
with ectopic production of adrenocorticotrophic hormone:
palliative treatment with inhibitors of adrenal steroid
biosynthesis. J. clin. Endocrinol. Metab., 36: 482-487.
CAROLLO, J. A. (1945) The removal of DDT from water supplies. J. Am.
Water Works Assoc., 37: 1310-1317.
CARR, R. L. (1970) Collaborative study of the Mills method of multiple
chlorinated pesticide residues in butter fat. J. Assoc. Off. Anal
Chem., 53: 152-154.
CARR, R. L. (1971) Collaborative study of a method of multiple
chlorinated pesticide residues in fish. J. Assoc. Off. Anal.
Chem., 54: 525-527.
CASARETT, L. J., FRYER, G. C., YAUGER, W. L., & KLEMMER, H. W. (1968)
Organochlorine pesticide residues in human tissues-Hawaii. Arch.
environ. Health, 17: 306-311.
CASE, R. A. M. (1945) Toxic effects of 2,2-bis(p-chlorophenyl)-1,1,1-
trichlorethane (DDT) in man. Br. med. J., 2: 842-845.
CASSIDY, W., FISHER, A. J., PEDEN, J. D., & PARRY-JONES, A. (1967)
Organochlorine pesticide residues in human fats from Somerset.
Mon. Bull. Minist. Health Lab. Serv. (Lond.), 26 (1): 2-6.
CHADWICK, R. W., CRANMER, M. F., & PEOPLES, A. J. (1971a) Metabolic
alterations in the squirrel monkey induced by DDT administration
and ascorbic acid deficiency. Toxicol. appl. Pharmacol.,
20: 308-318.
CHADWICK, R. W., CRANMER, M. F., & PEOPLES, A. J. (1971b) Comparative
stimulation of gamma-HCH metabolism by pre-treatment of rats with
gamma-HCH, DDT, and DDT + gamma-YCH. Toxicol. appl. Pharmacol.,
18: 685-695.
CHADWICK, R. W, LINKO, R. S., FREAL, J. J., & ROBBINS, A. L. (1975)
The effect of age and long-term low-level DDT exposure on the
response to enzyme induction in the rat. Toxicol. appl.
Pharmacol., 31: 469-480.
CHHABRA, R. S. & FOUTS, J. R. (1974) Stimulation of hepatic drug
metabolizing enzymes by DDT, polycyclic hydrocarbons or
phenobarbital in adrenalectomized or castrated mice. Toxicol.
appl. Pharmacol., 28: 465-476.
CHEN, JO-YUN, T. & DORITY, R. W. (1972) Infrared studies of DDT,
structurally related halogenated pesticides, and some metabolites.
J. Assoc. Off. Anal Chem., 55: 15-31.
CHIBA, M. (1969) Factors affecting the extraction of organochlorine
insecticides from soil. Residue Rev., 30: 63-113.
CHIBA, M. & MORLEY, H. V. (1968) Losses of pesticides during sample
preparation. J. Assoc. Off. Anal Chem., 51: 55-62.
CHIN, Y. & T'ANT, C. (1946) The effect of DDT on cutaneous sensation
in man. Science, 103: 654.
CIELESZKY, V., DENES, A., KNOLL, R., ENGST, R., & KOTARSKY, A. (1970)
[Methods for performing residue analysis of plant protection and
pest control agents in foodstuffs. II. Method for identifying and
the semiquantitative determination of residues of chlorinated
hydrocarbons in different food and in soil.] Nahrung,
14: 643-666 (in German).
CIUPE, R. (1976) [Determination of HCH, p,p'-DDT, p,p'-DDE in foods
and in the adipose tissue.] Igiena, 25: 113-117 (in Rumanian).
CLEMMESEN, J., FUGELSANG-FREDERICKSEN, V., & PLUM, C. M. (1974) Are
anticonvulsants oncogenic? Lancet, 1: 705-707.
CLIFFORD, N. J. & WEIL, J. (1972) Cortisol metabolism in persons
occupationally exposed to DDT. Arch. environ. Health,
24: 145-147.
COCHRANE, W. P. & CHAU, A. S. Y. (1971) Chemical derivitization
techniques for confirmation of organochlorine residue identity.
Pesticide identification at the residue level. Adv. Chem. Ser.,
140: 11-26.
COHA, F. & NEDIC, M. (1970) Improved gas-liquid chromatographic method
for analysis of DDT and its metabolites in milk. Anal. Lett.,
3: 419-421.
COHEN, J. M. & PINKERTON, C. (1966) Widespread translocation of
pesticides by air transport and rainout. Adv. Chem. Ser.,
60: 163-176.
COMMITTEE ON PESTICIDES (1951) Pharmacologic and toxicologic aspects
of DDT (chlorophenothane U.S.P.). J. Am. Med. Assoc.,
145: 728-733.
CONNEY, A. H. (1967) Pharmacological implications of microsomal enzyme
induction. Pharmacol. Rev., 19: 317-366.
CONOVER, W. J. (1971) Practical non-parametric statistics, New York,
Wiley & Sons, 462 pp.
COPELAND, F. M. & CRANMER, M. F. (1974) Effects of o,p'-DDT on the
adrenal gland and hepatic microsomal enzyme system of the beagle
dog. Toxicol. appl. Pharmacol., 27: 1-10.
COPPLESTONE, J. F., HUNNEGO, J. N., & HARRISON, D. L. (1973)
Insecticides in adult New Zealanders -- A five year study. N.Z.J.
Sci., 16: 27-39.
CRANMER, M. F. (1970) Effect of diphenylhydantoin on storage in the
rat. Toxicol. appl. Pharmacol., 17: 315.
CRANMER, M. F. (1972) Absence of conversion of o,p'-DDT to p,p'-DDT
in the rat. Bull. environ. Contam. Toxicol., 7: 121-124.
CRANMER, M. F. & COPELAND, M. F. (1973) Electron-capture gas-liquid
chromatographic analysis of DDA: utilization of 2-chloroethanol
derivative. Bull. environ. Contam. Toxicol., 9: 186-192.
CRANMER, M. F., CARROLL, J. J., & COPELAND, M. F. (1969) Determination
of DDT and metabolites, including DDA, in human urine by gas
chromatography. Bull. environ. Contam. Toxicol., 4: 214-223.
CRANMER, M. F., PEOPLES, A., & CHADWICK, R. (1972) Biochemical effects
of repeated administration of p,p'-DDT on the squirrel monkey.
Toxicol. appl. Pharmacol., 21: 98-101.
CROSBY, D. G. & MOILANEN, K. W. (1977). Vapor-phase photodecomposition
of DDT. Chemosphere, No. 4, pp 167-172.
CUETO, C., JR (1970) Cardiovascular effects of o,p'-DDD. Ind. Med.
Surg., 39: 31-32.
CUETO, C., JR & BIROS, F. J. (1967) Chlorinated insecticides and
related materials in human urine. Toxicol. appl. Pharmacol.,
10: 261-269.
CUETO, C., JR & BROWN, J. H. U. (1958) Biological studies on an
adrenocorticolytic agent and the isolation of the active
components. Endocrinology, 62: 334-339.
CUNNINGHAM, R. E. & HILL, F. S. (1952) Convulsions and deafness
following ingestion of DDT. Pediatrics, 9: 745-747.
CURLEY, A. & KIMBROUGH, R. (1969) Chlorinated hydrocarbon insecticides
in plasma and milk of pregnant and lactating women. Arch.
environ. Health, 18: 156-164.
CURLEY, A., COPELAND, M. F., & KIMBROUGH, R. D. (1969) Chlorinated
hydrocarbon insecticides in organs of stillborn and blood of
newborn babies. Arch. environ. Health, 19: 628-632.
CURLEY, A., BURSE, W. W., JENNINGS, R. W., & VILLANUEVA, E. C. (1973)
Chlorinated hydrocarbon pesticides and related compounds in
adipose tissue from people of Japan. Nature (Lond.),
242: 338-340.
CURRIE, L. A. (1968) Limits for qualitative detection and quantitative
determination. Anal. Chem., 40: 586-593.
CZEGLEDI-JANKO, G. (1969) [Residues of chlorinated hydrocarbons in the
blood of inhabitants of Budapest in 1967-1968 with particular
reference to lindane.] Z. Ges. Hyg. Ihre Grenzgeb., 15: 755-761
(in German).
CZEGLEDI-JANKO, G. & AVAR, P. (1970) Occupational exposure to lindane:
Clinical and laboratory findings. Br. J. ind. Med., 27: 283-286.
CZEGLEDI-JANKO, G. & CIELESZKY, V. (1968) A one-step extraction and
clean-up procedure for gas-liquid chromatographic determination of
some organochlorine pesticide residues in blood. Analyst,
93: 445-452.
DACRE, J. C. & JENNINGS R. W. (1970) Organochlorine insecticides in
normal and carcinogenic human lung tissues. Toxicol. appl.
Pharmacol., 17: 277.
DALE, W. E. & QUINBY, G. E. (1963) Chlorinated insecticides in the
body fat of people in the United States. Science, 142: 593-595.
DALE, W. E., GAINES, T. B., & HAYES, W. J., JR (1962) Storage and
excretion of DDT in starved rats. Toxicol. appl. Pharmacol.,
4: 89-106.
DALE, W. E., GAINES, T. B., HAYES, W. J., JR, & PEARCE, G. W. (1963)
Poisoning by DDT: Relation between clinical signs and
concentration in rat brain. Science, 142: 1474-1476.
DALE, W. E., COPELAND, M. F., & HAYES, W. J., JR (1965) Chlorinated
insecticides in the body fat of people in India. Bull. World
Health Organ., 33: 471-477.
DALE, W. E., COPELAND, M. F., PEARCE, G. W., & MILES, J. W. (1966a)
Concentration of o,p'-DDT in rat brain at various intervals
after dosing. Arch. int. Pharmacodyn. Ther., 162: 40-43.
DALE, W. E., CURLEY, A., & CUETO, C., JR (1966b) Hexane extractable
chlorinated pesticides in human blood. Life Sci., 5: 47-54.
DALE, W. E., CURLEY, A., & HAYES, W. J., JR (1967) Determination of
chlorinated insecticides in human blood. Ind. Med. Surg.,
36: 275-280.
DALE, W. E., MILES, J. W, & GAINES, T. B. (1970) Quantitative method
for determination of DDT and DDT metabolites in blood serum.
J. Assoc. Off. Anal. Chem., 53: 1287-1292.
DAL NOGARE, S. & JUVET, R. S. (1962) Gas-liquid chromatography, New
York, Interscience, 450 pp.
DAMASKIN, V. I. (1965) [The extent of the accumulation of DDT in the
human body in connection with its assimilation with food, and its
toxic effect.] Gig. i Sanit., 30 (10): 109-111 (in Russian).
DANGERFIELD, W. G. (1946) Toxicity of DDT to man. Br. med. J.,
1: 27.
DATTA, P. R. (1970) In vitro detoxification of p,p'-DDT via p,p'-
DDE to p,p'-DDA in rats. Ind. Med. Surg., 39: 190-194.
DATTA, P. R. & NELSON, M. J. (1968) Enhanced metabolism of
methyprylon, meprobamate, and chlordiazepoxide hydrochloride after
chronic feeding of a low dietary level of DDT to male and female
rats. Toxicol. appl. Pharmacol., 13: 346-352.
DATTA, P. R. & NELSON, M. F. (1970) p,p'-DDT detoxication by
isolated perfused rat liver and kidney. Ind. Med. Surg,
39: 195-198.
DAVIES, J. E., WELKE, J. O., & RADOMSKI, J. L. (1965) Epidemiological
aspects of the use of pesticides in the South. J. occup. Med.,
7: 612-618.
DAVIES, J. E., EDMUNDSON, W. F., SCHNEIDER, N. J., & CASSADY, J. C.
(1968) Problems of prevalence of pesticide residues in humans.
Pestic. Monit. J., 2: 80-85.
DAVIES, J. E., EDMUNDSON, W. R., CARTER, C. H., & BARQUET, A. (1969a)
Effect of anticonvulsant drugs on dicophane (DDT) residues in man.
Lancet, 2: 7-9.
DAVIES, J. E., EDMUNDSON, W. F., MACEO, A., BARQUET, A., & CASSADY, J.
(1969b) An epidemiologic application of the study of DDE levels in
whole blood. Am. J. Public Health, 59: 435-441.
DAVIES, J. E., EDMUNDSON, W. F., MACEO, A., IRVIN, G. L., III,
CASSADY, J., & BARQUET, A. (1971) Reduction of pesticide residues
in human adipose tissue with diphenylhydantoin. Food Cosmet.
Toxicol., 9: 413-423.
DAVIES, J. E., EDMUNDSON, W. F., & RAFEONELLI, A. (1975) The role of
house dust in human DDT pollution. Am. J. Public Health,
65: 53-57.
DAVIES, O. L. & GOLDSMITH, P. L. (1976) Statistical methods in
research and production, 4th ed., London, Longmans, 478 pp.
DEDEK, W. & SCHMIDT, R. (1972) Studies on the transplacental transport
and metabolism of 3H- and 14C-labeled DDT in pregnant mice
under hunger stress. Pharmazie, 27: 294-297.
DE FAUBERT MAUNDER, M. J., EGAN, H., GODLY, E. W., HAMMOND, E. W.,
ROBUM, J., & THOMSON, J. (1964) Clean-up of animal fats and dairy
products for the analysis of chlorinated pesticide residues.
Analyst, 89: 168-174.
DEICHMANN, W. B. & MACDONALD, W. E. (1976) Liver cancer deaths in the
continental USA from 1930 to 1972. Am. Ind. Hyg. Assoc. J.,
37: 495-498.
DEICHMANN, W. B. & MACDONALD, W. E. (1977) Organochlorine pesticides
and liver cancer deaths in the United States, 1930-1972.
Ecotoxicol. environ. Saf. 1: 89-110.
DEICHMANN, W. B. & RADOMSKI, J. L. (1968) Retention of pesticides in
human adipose tissue -- Preliminary report. Ind. Med. Surg.,
37: 218-219.
DEICHMANN, W. B., WITHERUP, S., & KITZMILLER, K. V. (1950) The
toxicity of DDT, Cincinnati, Kettering Laboratory of Applied
Physiology, 233 pp.
DEICHMANN, W. B., MACDONALD, W. E., & CUBIT, D. A. (1971a) DDT tissue
retention: Sudden rise induced by the addition of aldrin to a
fixed DDT intake. Science, 172: 275-276.
DEICHMANN, W. B., MACDONALD, W. E., BEASLEY, A. G., & CUBIT, D.
(1971b) Subnormal reproduction in beagle dogs induced by DDT and
aldrin. Ind. Med. Surg. 40 (2): 10-20.
DEICHMANN, W. B., MACDONALD, W. E., CUBIT, D. A., & BEASLEY, A. G.
(1972) Effects of starvation in rats with elevated DDT and
dieldrin levels. Int. Arch. Arbeitsmed., 29: 233-252.
DEL VECCHIO, V. & LEONI, V. (1967) [Study on the amount of chlorinated
insecticides in biological materials. II. Chlorinated insecticides
in adipose tissue in certain groups of the Italian population.]
Nuovi Ann. Ig. Microbiol., 18: 107-128 (in Italian).
DENES, A. (1962) [Problems of food chemistry concerning residues of
chlorinated hydrocarbons.] Nahrung, 6: 48-56 (in German).
DENES, A. (1964) Investigation of chlorinated hydrocarbon residues in
animal and vegetable fats. In: 1963 Year Book, Budapest,
Hungary, Institute of Nutrition.
DEUTSCHE FORSCHUNGSGEMEINSCHAFT (1969) [Residue analysis of plant
protection agents.] Weinheim, Verlag Chemie, 400 pp. (in German).
DE VLIEGER, M., ROBINSON, J., BALDWIN, M. K., CRABTREE, A. N., & VAN
DUK, M. C. (1968) The organochlorine insecticide content of human
tissue. Arch. environ. Health, 17: 759-767.
DOLAN, J. W. & HALL, R. C. (1973) Enhancement of the sensitivity and
selectivity of the Coulson electrolytic conductivity detector to
chlorinated hydrocarbon pesticides. Anal. Chem., 45: 2198-2204.
DOMENJOZ, R. (1944) [Experimental findings with a new insecticide
(Neocid, Geigy), a contribution to the theory contact-toxicity
action.] Schweiz. Med. Wochenschr., 74: 952-958 (in German).
DOMENJOZ, R. (1946a) [On the biological action of a DDT-derivative.]
Arch. Int. Pharmacodyn. Ther., 73: 128-146 (in German).
DOMENJOZ, R. (1946b) [Biological action of some DDT derivatives.]
Helv. Chem. Acta., 29: 1317-1322 (in French).
DRAIZE, J. H., NELSON, A. A., & CALVERY, H. O. (1944) The percutaneous
absorption of DDT (2,2-bis-(p-chlorophenyl)-1,1,1-trichloroethane)
in laboratory animals. J. Pharmacol. exp. Ther., 82: 159-166.
DUGGAN, R. E. (1968) Residues in food and feed. Pesticide residue
levels in food in the United States from July 1, 1963 to June 30,
1967. Pestic. Monit. J., 2: 2-46.
DUGGAN, R. E. & CORNELIUSSEN, P. E. (1972) Dietary intake of pesticide
chemicals in the United States (III), June 1968-April 1970.
Pestic. Monit. J., 5: 331-341.
DUGGAN, R. E. & LIPSCOMB, C. Q. (1969) Dietary intake of pesticide
chemicals in the United States (II), June 1966-April 1968.
Pestic. Monit. J., 2: 153-162.
DUGGAN, R. E. & WEATHERWAX, J. R. (1967) Dietary intake of pesticide
chemicals: Calculated daily consumption of pesticides with foods
are discussed and compared with currently accepted values.
Science, 157: 1006-1010.
DURHAM, W. F. & WOLFE, H. R. (1962) Measurement of the exposure of
workers to pesticides. Bull. World Health Organ. 26: 75-91.
DURHAM, W. F. & WOLFE, H. R. (1963) An additional note regarding
measurement of the exposure of workers to pesticides. Bull. World
Health Organ., 29: 279-281.
DURHAM, W. F., ARMSTRONG, J. F., UPHOLT, W. M., & HELLER, C. (1961)
Insecticide-content of diet and body fat of Alaskan natives.
Science, 134: 1880- 1881.
DURHAM, W. F., ORTEGA, P., & HAYES, W. J., JR (1963) The effect of
various dietary levels of DDT on liver function, cell morphology,
and DDT storage in the rhesus monkey. Arch. int. Pharmacodyn.
Ther., 141: 111-129.
DURHAM, W. F., ARMSTRONG, J. F., & QUNBY, G. E. (1965a) DDA excretion
levels. Arch. environ. Health, 11: 76-79.
DURHAM, W. F., ARMSTRONG, J. F., & QUINBY, G. E. (1965b) DDT and DDE
content of complete prepared meals. Arch. environ. Health,
11: 641-647.
DVORCHIK, B. H., ISTIN, M., & MAREN, T. H. (1971) Does DDT inhibit
carbonic anhydrase? Science, 172: 728-729.
DJATLOVlTSKAJA, F. G., GLADENKO, Y. F., & KRUCININA, A. A. (1972)
[Determination of organochlorine insecticides in reservoir water.]
Probl. anal Khim., 2: 43-36 (in Russian).
EDMUNDSON, W. F., DAVIES, J. E., & CRANMER, M. (1970a) DDT and DDE in
blood and urine of men exposed to 3 per cent DDT aerosol. Public
Health Rep., 85: 457-463.
EDMUNDSON, W. F., DAVIES, J. E., MACEO, A., & MORGADE, C. (1970b) Drug
and environmental effects on DDT residues in human blood. South.
med. J., 63: 1440-1441.
EGAN, H., GOULDING, R., ROBURN, J., & TATTON, J. O'G. (1965)
Organochlorine pesticide residues in human fat and human milk.
Br. med. J., 2: 66-69.
EICHELBERGER, J. W. & LICHTENBERG, J. J. (1971) Carbon adsorption for
recovery of pesticides (from water). J. Am. Water Works Assoc.,
63 (1): 25-27.
EMBRY, T. L., MORGAN, D. P., & ROAN, C. C. (1972) Search for
abnormalities of heme synthesis and sympathoadrenal activity in
workers regularly exposed to pesticides. J. occup. Med.,
14: 918-921.
ENGST, R. & KNOLL, R. (1972) DDT and DDE residues in human milk.
Nahrung, 16: 130-131.
ENGST, R., KNOLL, R., & NICKEL, B. (1967) [On the accumulation of
chlorinated hydrocarbons particularly of DDT and its metabolite
DDE in human fat.] Pharmazie, 22: 654-661 (in German).
ENGST, R., KNOLL, R., & NICKEL, B. (1970) [Occurrence of DDT and DDE
in fatty tissues and in organs of infants.] Pharmazie,
24: 673-676 (in German).
EPSTEIN, S.S. & SHAFNER, H. (1968) Chemical mutagens in the human
environment. Nature (Lond.), 219: 385-387.
ESKENASY, J. J. (1972) Status epilepticus by
dichlorodiphenyltrichloroethane and hexachlorocyclohexane
poisoning. Rev. Roum. Neurol., 9: 435-442.
EYZAGUIRRE, C. & LILIENTHAL, J. L., JR (1949) Veratrinic effects of
pentamethylenetetrazol (Metrazol) and 2,2-bis-( p-chlorophenyl)-
1,1,1-trichloroethane (DDT) on mammalian neuromuscular function.
Proc. Soc. Exp. Biol., 70: 272-275.
FAO (1972) Production yearbook-1971, Rome, Food and Agriculture
Organization, Vol. 25, pp. 499-537.
FAO/WHO (1973) 1972 Evaluations of some pesticide residues in food,
Geneva, WHO, 587 pp. (WHO Pest. Res. Series No. 2).
FARKAS, I., DESI, I., & KEMENY, T. (1969) [Effect of orally consumed
DDT on the rest and load EEG curves.] Egeszsegtudomany,
13 (2): 195-201 (in Hungarian).
FEIL, V. J., LAMOUREUX, C. H., STYRVOKY, E., ZAYLSKIE, R. G., THACKER,
E. J., & HOLMAN, G. M. (1973) Metabolism of o,p'-DDT in rats.
J. agric. food Chem, 21: 1072-1078.
FEIL, V. J., LAMOUREUX, C. H., & ZAYLSKIE, R. G. (1975) Metabolism of
o,p'-DDT in chickens. J. agric. food Chem., 23: 382-388.
FENNAH, R. G. (1945) Preliminary tests with DDT against insect pests
of food-crops in the Lesser Antilles. Trop. Agric., 22: 222-226.
FINSTERWALDER, C. E. (1976) Collaborative study of an extension of the
Mills et al. method for the determination of pesticide residues in
food. J. Assoc. Off. Anal. Chem, 59: 169-171.
FISEROVA-BERGEROVA, V., RADOMSKI, J. L., DAVIES, J. E., & DAVIES, J.
H. (1967) Levels of chlorinated hydrocarbon pesticides in human
tissues. Ind. Med. Surg., 36: 65-70.
FISHBEIN, L. F. (1974) Chromatographic and biological aspects of DDT
and its metabolites. J. Chromatogr., 98: 177-251.
FITZHUGH, O. G. (1948) Use of DDT insecticides on food products. Ind.
Eng. Chem., 40: 704-705.
FITZHUGH, O. G. (1966) Problems related to the use of pesticides.
Can. Med. Assoc. J., 94: 598-604.
FITZHUGH, O. G. (1970) A summary of a carcinogenic study of DDT in
mice from Food and Drug Administration, USA. In: FAO/ WHO 1969
evaluations of some pesticide residues in foods, Geneva. WHO,
pp. 61-64 (WHO/Food Add./70.38).
FITZHUGH, O. G. & NELSON, A. A. (1947) The chronic oral toxicity of
DDT (2,2-bis- p-chlorophenyl- 1,1,1-trichloroethane).
J. Pharmacol., 89: 18-30.
FRANCONE, M.P., MARLANI, F. H., & DEMARE, C. (1952) [Clinical signs of
poisoning by DDT.] Rev. Asoc. Méd. Argent., 66: 56-59 (in
Spanish).
FREDERICKS, C. M., LARSON, R. E., & ASTON, R. (1974) Effect of chronic
DDT exposure on pentobarbital tolerance and intolerance in the
rat. Toxicol. appl. Pharmacol., 27: 99-107.
FRENCH, M. C. & JEFFERIES, D. J. (1969) Degradation and disappearance
of ortho-, para-isomer of technical DDT in living and dead arian
tissues. Science, 165: 914-916.
FRIDMAN, G. I. (1970) [Effect of 7-trichlorofon and DDT on some
specific and non-specific characteristics of the
immunobiological and general reactivity of the organism (e.g.
problems of toxicological reactions of low intensity)] In:
[Questions of pesticide hygiene and toxicology.] Moscow,
Medicina, pp. 139-145 (in Russian).
FRIES, G. F., MARROW, G. S., JR, LESTER, J. W., & GORDON, C. H. (1971)
Effect of microsomal enzyme inducing drugs on DDT and dieldrin
elimination from cows. J. dairy Sci., 54: 364-368.
GÄB, S., NITZ, S., PARLAR, H., & KORTE, F. (1975) Photomineralisation
of certain aromatic xenobiotica. Chemosphere, No. 4,
pp. 251-256.
GÄB, S., SCHMITZER, J., THAMM, H. W., PARLAR, H. & KORTE, F. (1977)
Photomineralisation rate of organic compounds adsorbed on
particulate matter. Nature (Lond.), 270: 331-333.
GABLIKS, J. & MALTBY-ASKARI, E. (1970) The effect of chlorinated
hydrocarbons on drug metabolism in mice. Ind. Med. Surg.,
39: 347-350.
GABLIKS, J., ASKARI, E. M., & YOLEN, N. (1973) DDT and immunological
responses. I. Serum antibodies and anaphylactic shock in guinea
pigs. Arch. environ. Health, 26: 305-308.
GABLIKS, J., AL-ZUBAIDY, T., & ASKARI, E. (1975) DDT and immunological
responses. III. Reduced anaphylaxis and mast cell populations in
rats fed DDT. Arch. environ. Health, 30: 81-84.
GAINES, T. B. (1960) The acute toxicity of pesticides to rats.
Toxicol. appl. Pharmacol., 2: 88-99.
GAMES, T. B. (1969) Acute toxicity of pesticides. Toxicol. appl.
Pharmacol, 14: 515-534.
GALLAGHER, T. F., FUKUSHIMA, D. K., & HELLMANN, L. (1962) The effect
of ortho-, para-DDD on steroid borne metabolites in adrenocortical
carcinoma. Metab. clin. Exper., 11: 1155-1161.
GALTON, F. (1879) The geometric mean, in vital and social statistics.
Proc. R. Soc. Lond. B, 29: 365-367.
GARRETT, R. M. (1947) Toxicity of DDT for man. J. Med. Assoc.
Alabama, 17: 74-76.
GELLERT, R. J., HEINRICHS, W. L., & SWERDLOFF, R. S. (1972) DDT
homologues: Estrogen like effects on the vagina, uterus, and
pituitary of the rat. Endocrinology, 91: 1095-1100.
GENINA, S. A., SVETLAJA, E. N., & KOMAROVA, L. I. (1969) [Blood levels
of organic chlorine pesticides and some haematological indicators
in people employed in applying pesticides from the air.] In:
Medved', M. E., ed. The hygiene of application and the toxicology
of pesticides and the clinical features of pesticide poisoning. A
collection of papers, Issue No. 7, pp. 492-496, Kiev,
Vniigintoks (in Russian).
GIL G. P. & MIRON, B. F. (1949) [Investigation on toxicity of DDT to
man.] Med. Colon., 14: 459-470 (in Spanish).
GILBERT, D. & GOLBERG, L. (1967) BHT oxidase. A liver-microsomal
enzyme induced by the treatment of rats with butylated
hydroxytoluene. Food Cosmet. Toxicol., 5: 481-890.
GILLETT, J. W. (1968) No effect level of DDT in induction of
microsomal epoxidation. J. agric. food Chem., 16: 295-297.
GINGELL, R. & WALLCAVE, L. (1974) Species differences in the acute
toxicity and tissue distribution of DDT in mice and hamsters.
Toxicol. appl. Pharmacol., 28: 385-394.
GIUFFRIDA, L. & IVES, N. F. (1969) Microcoulomeric gas chromatography:
Improvement in instrumentation. J. Assoc. Off. Anal. Chem.,
52: 541-545.
GOERLITZ, D. F. & LAW, L. M. (1972) Chlorinated naphthalenes in
pesticide analysis. Bull. environ. Contain. Toxicol.,
7: 243-251.
GORDON, A. E. & FRIGERIO, A. (1972) Mass fragmentography as an
application of gas-liquid chromatograph-mass spectrometry in
biological research. J. Chromatogr., 73: 401-417.
GORDON, H. T. & WELSH, J. H. (1948) The role of ions in axon surface
reactions to toxic organic compounds. J. cell comp. Physiol.,
31: 395-420.
GRACA, I., SILVA FERDANANDES, A. M. S, & MOURAO, H. C. (1975)
Pesticides in people -- Organochlorine insecticide residues in
human milk in Portugal. Pestic. Monit. J., 8: 148-156.
GRACHEVA, G. V. (1969) [The possibility of DDT accumulation in the
organism of persons not having occupational contact with it.]
Faktory Vneshn. Sredy ikh Znachen, 1: 125-129 (in Russian).
GRACHEVA, G. V. (1970) [DDT excretion with the milk of nursing mothers
occupationally unexposed to the effect of this insecticide.] Vop.
Pitan., 29: 75-78 (in Russian).
GRIFFITH, F. D., JR & BLANKE, R. V. (1974) Microcoulometric
determination of organochlorine pesticides in human blood.
J. Assoc. Off. Anal Chem., 57: 595-603.
GRIFFITH, F. D., JR & BLANKE, R. V. (1975) Pesticides in people --
Blood organochlorine pesticide levels in Virginia residents.
Pestic. Monit. J., 8: 219-223.
GUENZI, W. D. & BEARD, W. E. (1968) Anaerobic conversion of DDT to DDD
and aerobic stability in soil. Proc. Soil Sci. Soc. Am.,
32: 522-534.
HAAG, H. B, EINNEGAN, J. K., LARSON, P.S., DREYFUSS, M. L., MAIN, R.
J., & RIESE, W. (1948) Comparative chronic toxicity for warm-
blooded animals of 2,2-bis( p-chlorophenyl)-1,1,1-trichloroethane
(DDT) and 2,2-bis-( p-chlorophenyl)-1,1-dichloroethane (DDD).
Ind. Med. Surg., 17: 477-484.
HAJKINA, B. I. & SILINA, V. F. (1971) [The effect of some
organochlorine pesticides on serotonin metabolism.] Farmakol.
Toxikol., 34: 357-359 (in Russian).
HALACKA, K., KAKL, J., & VYMETAL, F. (1965) [A reflexion in human fat
tissue of the extensive use of DDT.] Czech. Hyg., 10: 188-192
(in Czech).
HANADA, M., YUTANI, C., & MIYAJI, T. (1973) Induction of hepatoma in
mice by benzene hexachloride. Gann, 64: 511-513.
HARA, A., OWASAKI, I., NAWA, H, YOSHIOKA, Y., & YOKOO, S. (1973)
[Organochlorine insecticide residues in blood of pregnant women
and in umbilical cord blood.] Prog. Med., 84: 79-80 (in
Japanese).
HARMAN, S. W. (1946) DDT for codling moth control in western New York
in 1945. J. econ. Entomol., 39: 208-210.
HARRISON, R. D. (1975) Comparative toxicity of some selected
pesticides in neonatal and adult rats. Toxicol. appl. Pharmacol.,
32: 443-446.
HART, L. G. & FOUTS, J. R. (1963) Effects of acute and chronic DDT
administration on hepatic microsomal drug metabolism in the rat.
Proc. Soc. Exp. Biol. Med., 114: 388-392.
HART, M. M., REGAN, R. L., & ADAMSON, R. H. (1973) The effect of
isomers of DDD on the ACTH-induced steroid output, histology, and
ultrastructure of the dog adrenal cortex. Toxicol. appl.
Pharmacol., 24: 101-113.
HARTLEY, G. S. (1969) Evaporation of pesticides. In: Gould, R. F., ed.
Pesticidal Formulations Research, Washington, DC, American
Chemical Society, pp. 115-134 (Adv. Chem. Ser. 86).
HATTULA, M. L., IKKALA, J., ISOMÄKI, M., MÄÄTTÄ, K., & ARSTILA, A. U.
(1976) Chlorinated hydrocarbon residues (PCB and DDT) in human
liver, adipose tissue and brain in Finland. Acta Pharmacol.
Toxicol., 39: 545-554.
HAYASHI, M. (1972a) [Pollution of mothers' milk by organochlorine
pesticides.] Jpn J. Public Health, 19: 437-441 (in Japanese).
HAYASHI, M. (1972b) [Pesticide pollution of mothers' milk.] J. Jpn
Med. Assoc., 68: 1281-1286 (in Japanese).
HAYES, W. J., JR (1959) Pharmacology and toxicology of DDT. In:
Müller, P., ed. DDT: The insecticide dichlorodiphenyltri-
chloroethane and its significance, Basel, Birkhäuser Verlag,
Vol. 2, pp. 9-247.
HAYES, W. J., JR (1965) Review of the metabolism of chlorinated
hydrocarbon insecticides especially in mammals. Ann. Rev.
Pharmacol., 5: 27-52.
HAYES, W. J., JR (1975) Toxicology of pesticides, Baltimore,
Williams & Wilkins Co., 580 pp.
HAYES, W. J., JR (1976a) Mortality in 1969 from pesticides, including
aerosols. Arch. environ. Health, 31: 61-72.
HAYES, W. J., JR (1976b) Dosage relationships associated with DDT in
milk. Toxicol. appl. Pharmacol., 38: 19-28.
HAYES, W. J., JR & DALE, W. E. (1964) Concentration of DDT in brain
and other tissues in relationship to symptomatology. Toxicol.
appl. Pharmacol., 6: 349.
HAYES, W. J., JR, DURHAM, W. F., & CUETO, C., JR (1956) The effect of
known repeated oral doses of chlorphenothane (DDT) in man. J. Am.
Med. Assoc., 162: 890-897.
HAYES, W. J., JR, QUINBY, G. E., WALKER, K. C., ELLIOTT, J. W., &
UPHOLD, W. M. (1958) Storage of DDT and DDE in people with
different degrees of exposure to DDT. Am. Med. Assoc. Arch. ind.
Health, 18: 398-496.
HAYES, W. J., JR, DALE, W. E., & LEBRETON, R. (1963) Storage of
insecticides in French people. Nature (Lond.), 199: 1189-1191.
HAYES, W. J., JR, DALE, W. E., & BURSE, V. W. (1965) Chlorinated
hydrocarbon pesticides in the fat of people in New Orleans. Life
Sci., 4: 1611-1615.
HAYES, W. J, JR, DALE, W. E., & PIRKLE, C. I. (1971) Evidence of
safety of long-term, high, oral doses of DDT for man. Arch.
environ. Health, 22: 119-135.
HEATH, D. F. & VANDEKAR, M. (1964) Toxicity and metabolism of dieldrin
in rats. Br. J. ind. Med, 21: 269-279.
HEINRICKS, W. J., GELLERT, R. J., BAKKE, J. L., & LAWRENCE, N. L.
(1971) DDT administered to neonatal rats induces persistent estrus
syndrome. Science, 173: 642-643.
HELLMAN, L., BRADLOW, H. L., & ZUMOFF, B. (1973) Decreased conversion
of androgens to normal 17-ketosteroid metabolites as a result of
treatment with o,p'-DDD. J. clin. Endocrinol. Metab.,
36: 801-803.
HENDERSON, G. L. & WOOLLEY, D. E. (1969) Tissue concentrations of DDT:
Correlation with neurotoxicity in young and adult rats. Proc.
West. Pharmacol. Soc., 12: 58-62.
HENDERSON, G. L. & WOOLLEY, D. E. (1970) Mechanisms of neurotoxic
action of 1,1,1-trichloro-2,2-bis-( p-chlorophenyl)-ethane (DDT)
in immature and adult rats. J. Pharmacol. exp. Ther.,
175: 60-68.
HERZEL, F. & LAHMANN, E. (1973) [Contribution to quantitative
determination of pesticides in the air.] Gesundh.-Ing,
94: 275-279 (in German).
HESS, A.D. & KEENER, G. G., JR (1947) Effect of airplane-distributed
DDT thermal aerosols on fish and fish food organisms. J. Wildl.
Manage., 11: 1-10.
HEYNDRICKX, A. & MAES, R. (1969) The excretion of chlorinated
hydrocarbon insecticides in human mother milk. J. Pharm. Belg.,
9-10: 459-463.
HIDAKA, K., OHE, T., & FUJIWARA, K. (1972) [PCB and organochlorine
pesticides in mother's milk.] Igakuno Ayumi 82: 519-520 (in
Japanese).
HILL, D. W. & MCCARTY, P. L. (1967) Anaerobic degradation of selected
chlorinated hydrocarbon pesticides. J. Water Pollut. Control
Fed., 39: 1259-1277.
HILTON, B. D. & O'BRIEN, R. D. (1970) Antagonism by DDT of the effect
of valinomycin on synthetic membrane. Science, 168: 841-843.
HOFFMANN, C. H. & MERKEL, E. P. (1948) Fluctuations in insect
populations associated with aerial applications of DDT to forests.
J. econ. Entomol., 41: 467-473.
HOFFMANN, C. H. & SURBER, E. W. (1948) Effects of an aerial
application of wettable DDT on fish and fish food organisms in
Back Creek, West Virginia. Trans. Am. Fish. Soc., 75: 48-58.
HOFFMAN, D. G., WORTH, H. M., EMMERSON, J. L., & ANDERSON, R. C.
(1970) Stimulation of hepatic drug-metabolizing enzymes by
chlorphenothane (DDT); the relationship to liver enlargement and
hepatotoxicity in the rat. Toxicol. appl. Pharmacol.,
16: 171-178.
HOFFMAN, D. L. & MATTOX, V. R. (1972) Treatment of adrenocortical
carcinoma with o,p'-DDD. Med. Clin. North Am., 56: 999-1012.
HOFFMAN I. & LENDLE, L. (1948) [On the action of new insecticide
compounds.] Arch. exp. Pharmakol., 205: 223-242 (in German).
HOFFMAN, W. S. (1968) Clinical evaluation of the effects of pesticides
in man. Ind. Med. Surg., 37: 289-292.
HOFFMAN, W. S., FISHBEIN, W. I., & ANDELMAN, M. B. (1964) The
pesticide content of human fat tissue. Arch. environ. Health,
9: 387-394.
HOFFMAN, W. S., ADLER, H., FISHBEIN, W. I., & BAUER, F. C. (1967)
Relation of pesticide concentrations in fat to pathological
changes in tissues. Arch. environ. Health, 15: 758-765.
HOLDEN, A. V. (1970) International co-operative study of
organochlorine pesticide residues in terrestrial and aquatic
wildlife, 1967/68. Pestic. Monit. J., 4: 117-135.
HOLDEN, A. V. (1973) International co-operative study of
organochlorine and mercury residues in wildlife. Pestic. Monit.
J., 7: 37-52.
HORNABROOK, R. W., DYMENT, P. G., GOMES, E. D., & WISEMAN, J. S.
(1972) DDT residues in human milk from New Guinea natives. Med.
J. Aust., 1: 1297-1300.
HORWITZ, W., ed. (1975) Official methods of analysis, Washington,
DC, Association of the Official Analytic Chemists, 1094 pp.
HOWELL, D. E. (1948) A case of DDT storage in human fat. Proc. Okla.
Acad. Sci., 29: 31-32.
HRDINA, P. D., SINGHAL, R. L., PETERS, D. A. V., & LING, G. M. (1973)
Some neurochemical alterations during acute DDT poisoning.
Toxicol. appl. Pharmacol., 25: 276-288.
HRDINA, P. D., SINGHAL, R. L., & LING, G. M. (1975) DDT and related
hydrocarbon insecticides: Pharmacological basis of their toxicity
in mammals. Adv. Pharmacol. Chemother., 12: 31-88.
HRUSHKA, J. (1969) [DDT residues in the milk, butter, and fat of
cattle.] Veterinarstvi, 19 (11): 492-498 (in Czech).
HRUSHKA, J. & KOCIANOVA, M. (1975) [Contamination of the food chain by
chlorinated insecticides in Southern Bohemia.] Czech. Hyg.,
20: 421-428 (in Czech).
HSIEH, H. C. (1954) DDT intoxication in a family in Southern Taiwan.
Am. Med. Assoc. Arch. ind. Hyg., 10: 334-346.
HUEPER, W. C. (1942) Occupational tumors and allied diseases,
Springfield. IL. Thomas, pp. 496-497.
HUNTER, C. G., ROBINSON, J., & RICHARDSON, A. (1963) Chlorinated
insecticide content of human body fat in southern England.
Br. med. J., 1: 221-224.
HUTTERER, F., SCHAFFNER, F., KLION, F. M., & POPPER, H. (1968)
Hypertrophic, hypoactive smooth endoplasmic reticulum: A sensitive
indicator of hepatotoxicity exemplified by dieldrin. Science,
161: 1017-1019.
INNES, J. R. M., ULLAND, B. M., VALERIO, M. G., PETRUCELLI, L.,
FISHBEIN, L., HART, E. R., PALLOTTA, A. J., BATES, R. R., FALK, H.
L., GART, J. J., KLEIN, M., MITCHELL, & PETERS, J. (1969) Bioassay
of pesticides and industrial chemicals for tumorigenicity in mice.
A preliminary note. J. Natl Cancer Inst., 42: 1101-1114.
INOUE, Y., ABE, J., TAKAMATSU, M., & AOKI, N. (1974) [A study of the
qualitative and quantitative ratio of PCB and organochlorine
pesticide residues in the blood and adipose tissue.] Jpn J. Hyg.,
29: 92 (in Japanese).
INSTITUTE OF PETROLEUM (1968) Recommended practice for determining
precision data for methods on petroleum products and lubricants,
London, Institute of Petroleum.
ISRAELI, VON R. & MAYERSDORF, A. (1973) [Pathological changes in the
EEG during work with halogen-containing insecticides.] Zentralbl.
Arbeitsmed., 11: 340-343 (in German).
INTERNATIONAL AGENCY FOR RESEARCH ON CANCER (1974) IARC monographs:
The evaluation of the carcinogenic risk of chemicals to man, some
organochloride pesticides, Lyons, IARC, 241 pp. (IARC
monographs, Vol. 5).
ITO, N., NAGASAKI, H., ARAI, M., SUGIHARA, S., & MAKIURA, S. (1973)
Histological and ultrastructural studies on the
hepatocarcinogenicity of benzene hexachloride in mice. J. Natl
Cancer Inst., 51: 817-826.
JAGER, K. W. (1970) Aldrin, Dieldrin, Endrin, and Telodrin: An
epidemiological and toxicological study of long-term occupational
exposure, New York, Elsevier, 239 pp.
JANSSON, B., JENSEN, S., OLSSON, M., RENBERG, L., SUNDSTRÖM, G., &
VAZ, R. (1975) Identification by GC-MS of phenolic metabolites of
PCB and p,p'-DDE isolated from Baltic guillemot and seal.
Ambio, 4: 93-97.
JENSEN, S. & JANSSON, B. (1976) Anthropogenic substances in seal from
the Baltic: Methyl sulfone metabolites of PCB and DDE. Ambio,
5: 257-260.
JENSEN, J. A., CUETO, C., JR, DALE, W. E., ROTHE, C. F., PEARCE, G.
W., & MATTSON, A. M. (1957) Metabolism of insecticides. DDT
metabolites in feces and bile of rats. J. agric. food Chem.,
5: 919-925.
JEYARATNAM, J. & FORSHAW, J. (1974) A study of the cardiac effects of
DDT in laboratory animals. Bull. World Health Organ.,
51: 531-535.
JOHNSON, L. G. (1973) Formation of pentafluorobenzyl derivatives for
the identification and quantitation of acid and phenolic pesticide
residues. J. Assoc. Off. Anal. Chem., 56: 1503-1505.
JONCZYK, H. (1970) [The content of organochlorine insecticides in the
blood of healthy persons.] Rocz. panstw. Zakl. Hig., 21: 589-593
(in Polish).
JONCZYK, H., DMOCH, J., & BOJANOWSKA, A. (1970) [Determination of
chlorinated hydrocarbons in adipose tissue and brain of
Partridges.) Rocz. panst. Zakl. Hig., 21: 409-415 (in Polish).
JONCZYK, H., RUDOWSKI, W., TRACZYK, Z., & KLAWE, Z. (1974) [DDT and
its metabolites and gamma-HCH in blood, bone marrow, and fatty
tissue in certain hematological syndromes.] Pol. Tyg. Lek.,
29: 1573-1576 (in Polish).
JOY R. M. (1973) Electrical correlates of preconvulsive and convulsive
doses of chlorinated hydrocarbon insecticides in the CNS.
Neuropharmacology, 12: 63-76.
JUDAH, J. D. (1949) Studies on the metabolism and mode of action of
DDT. Br. J. Pharmacol, 4: 120-131.
JUDE, A. & GIRARD, P. (1949) [DDT toxicity -- mass poisoning by
accidental ingestion.] Ann. Med. lég. Criminol., Police Sci. Med.
Soc. Toxicol., 29: 209-213 (in French).
JUSZKIEWlCZ, T. & STEC, J. (1971) [Polichlorinated insecticide
residues in adipose tissue of farmers in the Lublin Province
(Poland).] Pol. Tyg. Lek., 26: 462-464 (in Polish).
KACEW, S. & SINGHAL, R. L. (1972) Role of adrenals in acute effects of
p.p'-DDT renal glucose metabolism. Fed. Proc., 31 (2): 520 Abs
(Abstract No. 1727).
KACEW, S. & SINGHAL, R. L. (1973) Adaptive response of hepatic
carbohydrate metabolism to oral administration of p,p'-1,1,1-
trichloro-2,2-bis-( p-chlorophenyl)-ethane in rats. Biochem.
Pharmacol., 22: 47-57.
KADIS, V. W., BREITKREITZ, W. E., & JONASSON, O. J. (1970) Insecticide
levels in human tissues of Alberta residents. Can. J. Public
Health, 61: 413-416.
KAGAN, Y. S., RODIONOV, G. A., WORONINA, L. Y., VELICHKO, L. S.,
KULAGIN, O. M., & PEREMITINA, A.D. (1969) [Effect of DDT on the
functional and morphological condition of the liver.] Vrach.
Delo, 12: 101-105 (in Russian).
KAISER, H. (1973) Guiding concepts relating to trace analysis. Pure
appl. Chem., 34: 35-61.
KAKU, S. (1973) [An epidemiological study on organochlorine pesticide
contamination. On pesticide residues in plasma of so-called
healthy Japanese in 1971.] J. Kurume Med. Assoc., 36: 307-326
(in Japanese).
KALISER, L. A. (1968) An in vitro and in vivo study of the effect
of DDT on the phagocytic activity of rat white blood cells.
Toxicol. appl. Pharmacol., 12: 353-357.
KALOYANOVA, F., MIHAILOVA, Z., GEORGIV, G., BENCHEV, I., RISOV, V., &
VELICHKOVA, V. (1972) Organochlorine pesticides in the fat of the
general population of Bulgaria. Abstracts of the XVII
International Congress on Occupational Health, Buenos Aires,
p. 75.
KAMATA, T. (1974) [On the present status of environmental pollution by
organochlorine pesticide residues.] Jpn J. Public Health,
21: 163-164 (in Japanese).
KAMATA, T. (1974) [Hygienic studies on pesticide residues: Part 4.
Environmental contamination by organochlorine pesticides.] Med.
J. Hiroshima Univ., 22: 315-325 (in Japanese).
KANITZ, S. & CASTELLO, G. (1966) [On the presence of residues of some
disinfectants in human adipose tissue and in food.] G. Ig. Med.
Prev., 7: 1-19 (in Italian).
KARIMOV, A. M. (1969) [Skin sensitivity to organochlorine
insecticides.] Med. Zh. Uzbekistant (No. 9): 58-60 (in Russian).
KARIMOV, A. M. (1970) [Occupational skin diseases in cotton growers
caused by chemical poisons and measures for their prevention.]
Gig. Tr. Prof. Zabol., 14: 35-37 (in Russian).
KASAI, A., ASANU, A. S., & NAKAMURA, S. (1972) [Studies on
organochlorine pesticide residues in human organs, Part III.]
J. Jpn Assoc. Rural Med., 21: 296-297 (in Japanese).
KATO, K., YAMADA, T., WATANABE, S., WADA, Y., FUKUDA, M., KURODA, M.,
NAKAOKA, S., TAKAHASHI, W., MIYASHIRO, K., MASUAD, J. & YASUKATA,
S. (1971) [Analyses of residual pesticides in vegetables, cows'
milk, and mothers' milk.] Ann. Rep. Kanagawa Prefect. Inst.
Public Health, 21: 85-92 (in Japanese).
KAWAI, Y., HORI, Y., NIGAWA, Y., YAMAMOTO, I., TSUZUKI, T., KITAYAMA,
M., & MORI, K. (1973) [On the pollution of mothers' milk by
pesticides and PCB in Hokkaido.] J. Food Hyg. Soc. Jpn,
14: 302-303 (in Japanese).
KAWANISHI, A., ASANUMA, S., & NAKAMURA, S. (1973) [Studies on
organochlorine pesticide residues in humans. Report 4.] J. Jpn
Assoc. Rural Med., 22: 278-279 (in Japanese).
KAZEN, C., BLOOMER, A., WELCH, R., OUDBIER, A., & PRICE, H. (1974)
Persistence of pesticides on the hands of some occupationally
exposed people. Arch. environ. Health, 29: 315-318.
KEIL, J. E., SANDIFER, S. H., FINKLEA, J. H., & PRIESTER, L. E. (1972)
Serum vitamin A elevation in DDT exposed volunteers. Bull.
environ. Contam. Toxicol., 8: 317-320.
KELLER, H. (1952) [Dichlorodiphenyltrichloroethane (DDT), additional
investigations on its properties and application.]
Naturwissenschaften, 39: 109 (in German).
KEPLINGER, M. L, DEICHMANN, W. B., & SALA, F. (1970) Effect of
combinations of pesticides on reproduction in mice. In: Deichmann,
W. B., ed. Pesticides Symposia, Miami, Halos & Associates,
pp. 125-138.
KHAIRY, M. (1959) Changes in behavior associated with a nervous system
poison (DDT). Q. J. exp. Physiol., 11 (2): 91-94.
KIMBROUGH, R. D., GAINES, T. B., & HAYES, W. J., JR (1968) Combined
effect of DDT, pyrethrum, and piperonyl butoxide on rat liver.
Arch. environ. Health, 16: 333-341.
KINOSHITA, F. K., FRAWLEY, J. P., & DUBOIS, K. P. (1966) Quantitative
measurement of induction of hepatic microsomal enzymes by various
dietary levels of DDT and toxaphene in rats. Toxicol. appl.
Pharmacol, 9: 505-513.
KLEIN, W. & KORTE, F. (1970) [Metabolism of chlorinated hydrocarbons.]
In: Wegler, R., ed. Chemie der Pflanzenschutz-und Schädlings-
bekämpfungsmittel, Band I, Berlin, Springer Verlag, pp. 199-218
(in German).
KLEIN, A. K., LAUG, E. P., KATTA, P. R., & MENDEL, J. L. (1965)
Evidence for the conversion of o,p'-DDT (1,1,1-trichloro-2- o-
chlorophenyl-2- p-chlorophenylethane) to p,p'-DDT (1,1,1-
trichloro-2,2-bis( p-chlorophenyl)ethane) in rats. J. Am. Chem.
Assoc., 87: 2520-2522.
KLEINFELD, M. (1967) Cancer of urinary bladder in a dye plant: A
medical and environmental study. In: Deichmann, W. B., ed.
Bladder Cancer, A Symposium, Birmingham, Al., Aesculapius,
pp. 136-144.
KNIPLING, E. F. (1959) The use of DDT in veterinary medicine. In:
Müller, P., ed. DDT, the insecticide dichlorodiphenyltri-
chloroethane and its significance, Basel, Switzerland, Birkhäuser
Verlag, Vol. II, pp. 505-570.
KNOLL, W. & JAYARAMAN, S. (1973a) [Organochlorine pesticide residues
in human milk.] Z. Gesamt. Hyg., 19: 43-45 (in German).
KNOLL, W. & JAYARAMAN, S. (1973b) [On the contamination of human milk
with chlorinated hydrocarbons.] Nahrung, 17: 599-615 (in German).
KOJIMA, S., SAITO, M., KONNO, H., & OZAWA, K. (1971) [Results of
investigation of organochlorine pesticide residues in mothers'
milk and in the blood of the mothers.] Rep. Akita Prefect. Inst.
Public Health, 16: 65-68 (in Japanese).
KOLMODIN, B., AZARNOFF, D. L., & SJOQVIST, F. (1969) Effect of
environmental factors on drug metabolism: Decreased plasma half-
life of antipyrine in workers exposed to chlorinated hydrocarbon
insecticides. Clin. Pharmacol. Ther, 10: 638-642.
KOLYADA, I. S. & MIKHAL'CHENKOVA, O. F. (1973) [Acute intoxication by
chlorine-containing pesticides.] Gig. Tr. Prof. Zabol., 5: 43
(in Russian).
KOMAROVA, L. I. (1970) [The excretion of DDT in mothers' milk and its
effect on the organism of mother and child.] Pediatr. Akus.
Ginek., 1: 19-20 (in Russian).
KONTEK, M., KUBACKI, S., PARADOWSKI, S., & WIERZCHOWIECKA, B. (1971)
[Chlorinated insecticides in human milk.] Pediatr. Pol.,
46: 183-188 (in Polish).
KOSTER, R. (1947) Differentiation of gluconate, glucose, calcium, and
insulin effects on DDT poisoning in cats. Fed. Proc., 6: 346.
KOSTIUK, O. T. & MUKHTAROVA, N. D. (1970) [Catecholamines and the
functional state of the hypothalamus under the action of a complex
of organochlorine and organophosphorus pesticides.] Gig. Tr.
Prof. Zabol., 14: 35-38 (in Russian).
KRASNIUK, E. P. & PLATONOVA, V. I. (1969) [Functional disorders of the
stomach under prolonged effects of organochlorine chemical
poisons.] Vrach. Delo, No. 9, 99-101 (in Russian).
KRASNIUK, E. P., LOGANOVSKII, N. G., MAKOVSKAYA, E. I., & RAPPOPORT,
M. B. (1968) [Functional and morphological changes in the kidneys
during the effects of DDT on the body.] Soy. Med., 31: 38-42
(in Russian).
KRAUL, I. & KARLOG, O. (1976) Persistent organochlorinated compounds
in human organs collected in Denmark, 1972-1973. Acta Pharmacol.
Toxicol., 38: 38-48.
KROGER, M. (1972) Insecticide residues in human milk. J. Pediatr.,
80: 401-405.
KUCINSKI, A. L. (1972) [On the extraction of DDT and hexachlorane from
some canned foods of animal and vegetable origin.] Vopr. Pitan.,
31 (1): 93 (in Russian).
KUN'EV, V. V. (1965) [Influence of low DDT doses on the phagocytic
state of the blood of albino rats.] Vopr. Pitan., 24: 74-78 (in
Russian).
KUNTZMANN, R., JACOBSON, M., LEVIN, W., & CONNEY, D. H. (1968)
Stimulatory effect of N-phenylbarbitol on cortisol hydroxylation
in man. Biochem. Pharmacol., 17: 565-571.
KUPFER, D. (1967) Effects of some pesticides and related compounds on
steroid function and metabolism. Res. Rev., 19: 11-30.
KUPFER, T., BALAZS, T., & BUYSKE, D. A. (1964) Stimulation by o,p'-
DDD of cortisol metabolism in the guinea pig. Life Sci.,
3: 959-964.
KUTZ, F. W., YOBS, A. R., JOHNSON, W. G., & WIERSMA, G. B. (1974)
Pesticide residues in adipose tissue of the general population of
the United States, FY 1970 survey. Bull. Soc. Pharmacol. Environ.
Pathol., 2: 4-10.
KUWABARA, N. & TAKAYAMA, S. (1974) [Comparison of histogenesis of
liver of mice administered respectively BHC, DDT, and 2,7-FAA.]
Proc. Jpn Cancer Assoc., 33: 50 (in Japanese).
KUZ'MINSKAJA, U. A., KLISENKO, M. A., & HAYKINA, B. I. (1972)
[Peculiarities of the distribution of some pesticides in the lipid
fractions of the liver.] Vopr. Pitan., 31: 48-52 (in Russian).
KWALIK, D. S. (1971) Anticonvulsants and DDT residues. J. Am. Med.
Assoc., 215: 120-121.
LAUBSCHER, J. A., DUTT, G. R., & ROAN, C. C. (1971) Chlorinated
insecticide residues in wildlife and soil as a function of
distance from application. Pestic. Monit. J., 5: 251-258.
LAUG, E. P., NELSON, A. A., FITZHUGH, O. G., &. KUNZE, F. M. (1950)
Liver-cell alteration and DDT storage in the fat of the rat
induced by dietary levels of 1 to 50 ppm of DDT. J. Pharmacol.
98: 268-273.
LAUG, E. P, KUNZE, F. M., & PRICKETT, C. S. (1951) Occurrence of DDT
in human fat and milk. Am. Med. Assoc. Arch. indust. Hyg. occup.
Med., 3: 245-246.
LAÜGER, P., PULVER, R., & MONTIGEL, C. (1945a) [Mode of action of
4,4'-dichlorodiphenyltrichloromethylmetan (DDT-Geigy) in
warmblooded organism.] Experientia (Basel), 1: 120-121 (in
German).
LAÜGER, P., PULVER, R., & MONTIGEL, C. (1945b) [Mode of action of
4,4'-dichlorodiphenyltrichloromethylmetan (DDT-Geigy) in
warmblooded organism.] Helr. Physiol. Acta, 3: 405-415 (in
German).
LAWS, E. R. (1971) Evidence of antitumorigenic effects of DDT. Arch.
environ. Health, 23: 181-183.
LAWS, E. R., JR, CURLEY, A., & BIROS, F. J. (1967) Men with intensive
occupational exposure to DDT. A clinical and chemical study.
Arch. environ. Health, 15: 766-775.
LAWS, E. R, JR, MADDREY, W. C., CURLEY, A., & BURSE, V. W. (1973)
Long-term occupational exposure to DDT. Arch. environ. Health,
27: 318-321.
LEHMAN, A. J. (1952) Chemicals in foods: A report to the Association
of Food and Drug Officials on current developments. Part II,
Pesticides. Section V. Q. Bull. Assoc. Food Drug Off. US,
16: 126-132.
LEHMAN, A. J. (1965) Summaries of pesticide toxicity, Topeka, Ks,
Association of Food and Drug Officials of the United States.
LESCENKO, P. D. & POLONSKAIA, M. N. (1969) [New products in the
prophylactic nutrition of workers in the organochlorine industry.]
Vrach. Delo, No. 8, pp. 106-110 (in Russian).
LEWIS, W. H. & RICHARDS, A. G, JR (1945) Non-toxicity of DDT on cells
in cultures. Science, 102: 330-331.
LICHTENBERG, J. L., EICHELBERGER, J. W., DRESSMAN, R. C., &
LONGBOTTOM, J. E. (1970) Pesticides in surface waters of the
United States -- a 5-year summary, 1964-68. Pestic. Monit. J.,
4: 71-86.
LILLIE, R. D. & SMITH, M. I. (1944) Pathology of experimental
poisoning in cats, rabbits, and rats with 2,2-bis-(para-
chlorphenyl)-1,1,1-trichlorethane. Public Health Rep.,
59: 979-984.
LILLIE, R. D., SMITH, M. I., & STOHLMAN, E. F. (1947) Pathologic
action of DDT and certain of its analogs and derivatives. Arch.
Pathol., 43: 127-142.
LIPSCOMB, G. Q. (1968) Residues in food and feed -- Pesticide residues
in prepared baby foods in the United States. Pestic. Monit. J.,
2: 104-108.
LÖFROTH, G. (1968) Pesticides and catastrophe. New Sci.,
40: 567-568.
LONG, K. L., BEAT, V. B., GOMBART, A. K., SHEETS, R. F., HAMILTON, H.
E., FALABALLA, F., BONDERMAN, D. P., & CHOI, U. Y. (1969) The
epidemiology of pesticides in a rural area. Am. Ind. Hyg. Assoc.
J., 30: 398-304.
LOVELOCK J. E. (1963) Electron absorption detectors and techniques for
use in quantitative and qualitative analysis by gas-liquid
chromatography. Anal. Chem., 35: 474-481.
LU, F. C., JESSUP, D. C., & LAVALÉE, A. (1965) Toxicity of pesticides
to young versus adult rats. Food Cosmet. Toxicol., 3: 591-596.
LUBITZ, J. A., FEEMAN, L., & OKUM, R. (1973) Mitotane use in
inoperable adrenal cortical carcinoma. J. Am. Med. Assoc.,
233: 1109-1112.
LUQUET, F., GOURSAUD, J. G., & CASALIS, J. (1974) Organochlorine
pesticide residues in animal and human milk. Ann. Fals. Expert.
Chim., 67: 217-239.
LUTON, J. P, VALCKE, J. C., REMY, J. M., MATHIEU DE FOSSEY, B., &
BRICAIRE, H. (1972) Gynecomastia after long-term treatment of
Cushing's diseases with o,p'-DDT. Ann Endocrinol.,
33: 290-293.
MACCORMACK, J. D. (1945) Infestation and DDT. Irish J. med. Sci.
6: 627-634.
MACKERRAS, I. M. & WEST, R. F. K. (1946) 'DDT' poisoning in man.
Med. J. Aust., 1: 400-401.
MACKLIN, A. W. & BIBELIN, W. E. (1971) The relation of pesticides to
abortion in dairy cattle. J. Am. Vet. Med. Assoc.,
159: 1743-1748.
MAES, R. & HEYNORICKX, A. (1966) Distribution of organic chlorinated
insecticides in human tissues. Meded. Rijksfac. Landbouwwet.
Gent., 31: 1021-1025.
MAGGS, R. J., JOYNES, P. L., DAVIES, A. J., & LOVELOCK, J. E. (1971)
The electron capture detector -- A new mode of operation. Anal.
Chem., 43: 1966-1971.
MAIER-BODE, H. (1960) [DDT in body fat of man.] Med. Exp, 1: 146-152
(in German).
MARKARIAN, D. S. (1966) [Cytogenetic effects of some chlorine-
containing organic insecticides on mouse bone-marrow cell nuclei.]
Genetika, 1: 132-137 (in Russian).
MARNELOS, M. & HANNINEN, O. (1974) Enhancement of D-glucuronolactone
and acetaldehyde dehydrogenase activities in the rat liver by
inducers of drug metabolism. Biochem. Pharmacol., 23: 1457-1466.
MARSH, C. A. (1963) Metabolism of D-glucuronolactone in the mammalian
system. Biochem. J., 86: 77-86.
MASON, T. J., MCKAY, F. W., HOOVER, F., BLOT, W. J., & FRAUMENI, J. F,
JR (1975) Atlas of cancer mortality for U.S. Counties:
1950-1969, Washington, DC, US Department of Health, Education
and Welfare, Public Health Service (DHEW Publication No. (NH)
75-780).
MASUMOTO, H. T. (1972) Study of the silicic acid procedure of Armour
and Burke for separation of PCBs from DDT and its analogues.
J. Assoc. Off. Anal. Chem., 55: 1092-1100.
MATSUMURA, F. & PATIL, K. C. (1969) Adenosine triphosphatase sensitive
to DDT in synapses of rat brain. Science, 166: 121-122.
MATTSON, A. M., SPILLANE, J. T., BAKER, C., & PEARCE, G. W. (1953)
Determination of DDT and related substances in human fat. Anal.
Chem., 25: 1065-1070.
MAYERSDORF, A. & ISRAELI, R. (1974) Toxic effects of chlorinated
hydrocarbon insecticides on the human electroencephalogram. Arch.
environ. Health, 28: 159-163.
MCALISTER, D. (1879) The law of the geometric mean. Proc. R. Soc.
London B, 29: 367-376.
McCANN, J. & AMES, B. N. (1976) Detection of carcinogens as mutagens
in the Salmonella/microsome test: Assay of 300 chemicals:
Discussion. Proc. Natl Acad. Sci. USA, 73: 950-954.
McCANN, J., CHOL, E., YAMASAKI, E., & AMES, B. N. (1975) Detection of
carcinogens as mutagens in the Salmonella/microsome test: Assay
of 300 chemicals. Proc. Natl Acad. Sci. USA, 72: 5135-5139.
McFARREN, E. F., LISHKA, R. J., & PARKER, J. H. (1970) Criterion for
judging acceptability of analytical methods. Anal. Chem.,
42: 358-365.
McGILL, A. E. J. & ROBINSON, J. (1968) Organochlorine insecticide
residues in complete prepared meals: A 12-month survey in S.E.
England. Food Cosmet. Toxicol., 6: 45-57.
McKINLEY, W. P. (1963) Paper chromatography. In: Zweig, G., ed.
Analytical methods for pesticides, plant growth regulators and
food additives, New York, Academic Press, Vol. 1, pp. 227-252.
McKINNEY, J. D., BOOZIER, E. L., HOPKINS, H. P., & SUGGS, J. E. (1969)
Synthesis and reactions of a proposed DDT metabolite, 2,2'-bis
( p-chlorophenyl)acetaldehyde. Experientia (Basel),
25: 897-898.
McLACHLAN, J. A. & DIXON, R. L. (1972) Gonadal function in mice
exposed prenatally to p,p'-DDT. Toxicol. appl. Pharmacol.,
22: 327.
McMAHON, B. M. & SAWYER, L. D., ed. (1977) Pesticide analytical
manual. Volume I. Methods which detect multiple residues,
Rockville, MD, US Food and Drug Administration.
McNEIL, E. E., OTSON, R., MILES, W. F., & RAJABALEE, F. J. M. (1977)
Determination of chlorinated pesticides in potable water.
J. Chromatogr., 123: 277-286.
McQUEEN, E. G., OWEN, D., & FERRY, D. G. (1972) Effect of phenytoin
and other drugs in reducing serum DDT levels. N.Z. med. J.,
75: 208-211.
MEDVED', L. I., KAGAN, J, S., SPYNU, E. I., DLISENKO, M. A.,
ANTONOVIC, E. A., BEZUGLYJ, V. P., KRYZANOVSKAYJA, M. V., &
POL'CENKO, V. I. (1975) National review on chlorinated
pesticides, Kiev, All-Union Institute of the Ministry of Health
of the USSR for Research on the Hygiene and Toxicology of
Pesticides, Polymers, and Plastics, 74 pp.
MENDOZA, E. E. (1971) Note on the significant difference in gas-liquid
chromatographic responses to insecticide injections at fast and
slow rates. J. Chromatogr. Sci., 9 (12): 753-754.
MENZIE, C. M. (1969) Metabolism of pesticides, Washington, DC, US
Government Printing Office (Special Scientific Report, Wildlife
No. 127).
METCALF, R. L. (1955) Organic insecticides, their chemistry and mode
of action, New York, Intersciences, 392 pp.
MIDDLETON, F. M. & LICHTENBERG, J. J. (1960) Measurements of organic
contaminants in the nation's rivers. Ind. Eng. Chem.,
52: 99A-102A.
MILES, J. R. (1972) Conversion of DDT and its metabolites into
dichlorobanzophenones for analysis in presence of PCBs. J. Assoc.
Off. Anal. Chem., 55: 1039-1041.
MILES, J. W., FETZER, L. E., & PEARCE, G. W. (1970) Collection and
determination of trace quantities of pesticides in air. Environ.
Sci. Technol., 4: 420-425.
MILLER, G. J. & FOX, J. A. (1973) Chlorinated hydrocarbon pesticide
residues in Queensland human milks. Med. J. Aust., 2: 261-264.
MILLER, L. L. & NARANG, R. S. (1970) Induced photolysis of DDT.
Science, 169: 368-370.
MIZOGUCHI, M., YAMAGISHI, T., USHIO, F., FUJIMOTO, C., TAKEBA, K.,
KANI, T., HARUTA, M., YASUHARA, K., & KUBOTA, H. (1972) [On the
residual amount of organochlorine pesticides in human milk in
Tokyo metropolitan area.] Jpn J. public Health, 19: 541-544
(in Japanese).
MONTGOMERY, J. A. & STRUCK, R. F. (1973) The relation of the
metabolism of anticancer agents to their activity. Forschr.
Arzneimittelforsch., 17: 32-304.
MORGAN, D. P. & ROAN, C. C. (1969) Renal function in persons
occupationally exposed to pesticides. Arch. environ. Health,
19: 633-636.
MORGAN, D. P. & ROAN, C. C. (1970) Chlorinated hydrocarbon pesticide
residue in human tissues. Arch. environ. Health, 20: 452-457.
MORGAN, D. P. & ROAN, C. C. (1971) Absorption, storage and metabolic
conversion of ingested DDT and metabolites in man. Arch. environ.
Health, 22: 301-308.
MORGAN, D. P. & ROAN, C. C. (1972) Loss of DDT from storage in human
body fat. Nature (Load.), 238: 221-223.
MORGAN, D. P. & ROAN, C. C. (1973) Adrenocortical function in persons
occupationally exposed to pesticides. J. occup. Med., 15: 26-28.
MORGAN, D. P. & ROAN, C. C. (1974) Liver function in workers having
high tissue stores of chlorinated hydrocarbon pesticides. Arch.
environ. Health, 29: 14-17.
MORGAN, D. P., ROAN, C. C., & PASCHAL, E. H. (1972) Transport of DDT,
DDE, and dieldrin in human blood. Bull environ. Contain.
Toxicol., 8: 321-326.
MOSIER, A. R., GUENZI, W. D., & MILLER, L. L. (1969) Photochemical
decomposition of DDT in a free-radical mechanism. Science,
164: 1083-1085.
MRAK, E. M. (1969) Report of the secretary's commission on pesticides
and their relationship to environmental health, Washington, US
Government Printing Office, 677 pp.
MUGHAL, H. A. & RAHMAN, M. A. (1973) Organochlorine pesticide content
of human adipose tissue in Karachi. Arch. environ. Health, 27:
396-398.
MÜLHENS, K. (1946) [The importance of bis (chlorophenyl)
(trichloromethyl)-methane preparations as insecticides in
combating infectious diseases.] Dtsch. Med. Wochenschr.,
71: 164-169 (in German).
MÜLLER, P., ed. (1959) DDT, the insecticide dichlorodiphenyltri-
chloroethane and its significance. Basel, Switzerland, Birkhäuser
Verlag, Vol. 2.
MURPHY, P. G. (1972) Sulphuric acid for the clean-up of animal tissues
for analysis of acid-stable chlorinated hydrocarbon residues.
J. Assoc. Off. Anal Chem., 55: 360-362.
MURRAY, C. P. F. V., SAUCEDA, F. M., & NAVAREZ, G. M. (1973)
[Environmental contamination and the health of children.] Salud
Publica Mex., 15: 91-100 (in Spanish).
MUSIAL, C. J., HUTZINGER, O., ZITKO, V., & CROCKER, J. (1974) Presence
of PCB, DDE, and DDT in human milk in the provinces of New
Brunswick and Nova Scotia, Canada. Bull. environ. Contam.
Toxicol., 12: 258-267.
MUSTY, P. R. & NICKLESS, G. (1974) Use of Amberlite XAD-4 for
extraction and recovery of chlorinated hydrocarbon insecticides
and PCBs from water. J. Chromatogr., 89: 185-190.
NARVESTED, R. (1947) [Poisoning with DDT powder and some other
incidences of poisoning.] Tidsskr. Norske Laegeforen.,
67: 261-263 (in Norwegian).
NAGAI, I. (1972) [Residues of organochlorine pesticides in mother's
milk in Yamaguchi Prefecture. Annual Report of the Yamaguchi
Prefecture.] Res. Inst. Public Health, 14: 93-94 (in Japanese).
NAGASAKI, H. (1973) [Experimental studies on chronic toxicity of
benzene hexachloride (BHC).] J. Nara Med. Assoc., 24: 1-26
(in Japanese).
NAGASAKI, H., AOI, H., MAKIURA, S., AOKI, Z., ARAI, M., KONISHI, Y., &
ITO, N. (1974) [Characteristic features of hepatoma of mice due to
alpha-BHC and several factors of carcinogenesis.] Proc. Jpn
Cancer Assoc., 33: 78 (in Japanese).
NALIMOV, V. V. (1963) The application of mathematical statistics to
chemical analysis, London, Pergamon Press, 294 pp.
NARAHASHI, T. & HAAS, H. G. (1967) DDT: Interaction with nerve
membrane conductance changes. Science, 157: 1438-1440.
NARAHASHI, T. & YAMASAKI, T. (1960) Mechanism of increase in negative
after-potential by dicophanum (DDT) in the giant axon of the
cockroach. J. Physiol. (Lond.), 152: 122-140.
NARUSE, R., SASAKI, T., YAMOKA, T., MATSUOKA, K., NEGORO, Y., ITASAKA,
Y., URUSHIBATA, K., CHIN, T., KATO, K., KAKO, K., GOTO, Y.,
EGUCHI, Y., YOGO, H., TOMITA, A., & ASAI, M. (1970) [Clinical use
of o,p'-DDD in adrenal cortex cancer.] Horumon Rinsho,
18: 241-244 (in Japanese).
NAWA, H. (1973) [Studies on intoxication by organochlorine pesticides
for agricultural use. Part 2. Examination of concentration of
organochlorine pesticides in sera of healthy people and patients
with various diseases.] J. Okayama Med. Soc., 85: 47-58 (in
Japanese).
NEAL, P. A., VON OETTINGEN, W. F., SMITH, W. W., MALMO, R. B., DUNN,
R. C., MORAN, H. E., SWEENEY, T. R., ARMSTRONG, D. W., & WHITE, W.
C. (1944) Toxicity and potential dangers of aerosols, mists, and
dusting powders containing DDT. Public Health Rep., 177
(Suppl.): 1-32.
NEAL, P. A., SWEENEY, T. R., SPICER, S.S., & VON OETTINGEN, W. F.
(1946) The excretion of DDT (2,2-bis-( p-chlorophenyl)-1,1,1-
trichloroethane) in man, together with clinical observations.
Public Health Rep., 61: 403-409.
NELSON, A. A. & WOODARD, G. (1948) Adrenal cortical atrophy and liver
damage produced in dogs by feeding 2,2-bis-(parachlorophenyl)-1,1-
dichlorethane (DDD). Fed. Proc., 7: 277.
NELSON, A. A. & WOODARD, G. (1949) Severe adrenal cortical atrophy
(cytotoxic) and hepatic damage produced in dogs by feeding 2,2-
bis-(parachlorophenyl)-1,1-dichloroethane (DDD or TDE). Arch.
Pathol., 48: 387-394.
NELSON, A. A., DRAIZE, J. H., WOODARD, G., FITZHUGH, O. G., SMITH, R.
B., JR, & CALVERY, H. O. (1944) Histopathological changes
following administration of DDT in several species of animals.
Public Health Rep., 59: 1009-1020.
NELSON, J. A. (1973) Effects of DDT analogs and polychlorinated
biphenyls (PCB) mixtures on 3H-estradiol binding to rat uterine
receptor. Fed. Proc., 32: 236.
NEWTON, K. O. & GREENE, N. C. (1972) Organochlorine pesticide residue
levels in human milk -- Victoria, Australia, 1970. Pestic. Monit.
J., 6: 4-8.
NICHOLS, J., PRESTLEY, W. E., & NICHOLS, F. (1961) Effects of m,p'-
DDT in a case of adrenal cortical carcinoma. Curr. Ther. Res.,
3: 266-271.
NIKITINA, Y. I. (1974) [Course of labor and puerperium in vineyard
workers and milkmaids in the Crimea.] Gig. Tr. Prof. Zabol,
18: 17-20 (in Russian).
NISHIMOTO, T., UYETA, M., & TAUE, S. (1970) [The accumulation of
organochlorine pesticides in human adipose tissue.] Prog. Med.,
73: 275-277 (in Japanese).
NITSCHKE, U. & LINK, K. (1972) [Clinical and biochemical aspects of
hormone-producing adrenal carcinomas.] Z. Gesamite Inn. Med. Ihre
Grenzgeb., 27: 896-905 (in German).
NORTH, H. H. & MENZER, R. E. (1973) Metabolism of DDT in human
embryonic lung cell cultures. J. agric. food. Chem.,
21: 509-510.
NOVIKOVA, K. F. (1973) [Quantitative assay of organochlorine and
organophosphate pesticide residues in food products, soil, water.]
Zh. Vses. Khim. Obshchest., 18 (5): 562-570 (in Russian).
OBUSCHOWSKA, D. & PAWLOWKA-TOCHMAN, A. (1973) [The effect of the
gamma-isomer of HCH (Lindane) on the ultrastructure of the liver
cell.] Ann. Univ. Mariae Curie-Sklodowska, 28 (9): 63-66 (in
Polish).
OFNER, R. R. & CALVERY, H. O. (1945) Determination of DDT (2,2-bis-
( p-chlorophenyl)-1,1,1-trichlorethane) and its metabolite in
biological materials by use of the Schechter-Haller method.
J. Pharmacol., 85: 363-370.
O'LEARY, J. A., DAVIES, J. E., EDMUNDSON, W. F., & FELDMAN, M. (1970a)
Correlation of prematurity and DDE levels in fetal whole blood.
Am. J. Obstet. Gynecol., 106: 939.
O'LEARY, J. A., DAVIES, J. E., EDMUNDSON, W. F., & REICH, G. A.
(1970b) Transplacental passage of pesticides. Am. J. Obstet.
Gynecol., 107: 65-68.
O'LEARY, J. A., DAVIES, J. E., & FELDMAN, M. (1970c) Spontaneous
abortion and human pesticide residues of DDT and DDE. Am. J.
Obstet. Gynecol., 108: 1291-1292.
OLOFFS, C. P., HARDWICK, D. F., SZETO, S. Y., & MOERMAN, D. G. (1974)
DDT, dieldrin, and heptachlor epoxide in humans with liver
cirrhosis. Clin. Biochem., 7: 297-306.
OLSZYNA-MARZYS, A. E., DE CAMPOS, M., FARVAR, M. T., & THOMAS, M.
(1973) [Residues of chlorinated pesticides in human milk in
Guatemala.] Bol. Of. Sanit. Panam., 74: 93-107 (in Spanish).
ORTEGA, P. (1966) Light and electron microscopy of dichlorodiphenyl-
trichloroethane (DDT) poisoning in the rat liver. Lab. Invest.,
15: 657-679.
ORTEGA, P., HAYES, W. J., JR, DURHAM, W. F., & MATTSON, A. (1956) DDT
in the diet of the rat. Its effect on DDT storage, liver function
and cell morphology, Washington, DC, US Government Printing
Office, 27 pp. (Public Health Monograph No. 43, PHS Publication
No. 484).
ORTELEE, M. F. (1958) Study of men with prolonged intensive
occupational exposure to DDT. Am. Med. Assoc. Arch. indust.
Health, 18: 433-440.
OTTOBONI, A. (1969) Effect of DDT on reproduction in the rat.
Toxicol. appl. Pharmacol., 14: 74-81.
OTTOBONI, A. (1972) Effect of DDT on the reproductive lifespan in the
female rat. Toxicol. appl. Pharmacol., 22: 497-502.
OTTOBONI, A., BISSELL, G. D., & HEXTER, A. C. (1977) Effects of DDT on
reproduction in multiple generations of beagle dogs. Arch.
environ. Contam. Toxicol., 6: 83-101.
OURA, H., KOBATASHI, H., OURA, T., SENDA, I., & KUBOTA, K. (1972) [On
the pollution of human milk by PCB and organochlorine pesticides.]
J. Jpn Assoc. Rural Med., 21: 300-301 (in Japanese).
OUW, K. H. & SHANDAR, A. G. (1974) A health survey of Wee Waa
residents during 1973 aerial spraying season. Med. J. Aust.,
2: 871-873.
PACCAONELLA, B., PRATI, L., & CAVAZZINI, G. (1967) [Organochlorine
insecticides in adipose tissue of residents in Ferrara province.]
Nuovi Ann. Ig. Microbiol., 18: 17-26 (in Italian).
PALMER, K. A, GREEN, S, & LEGATOR, M. S. (1972) Cytogenetic effects of
DDT and derivatives of DDT in a cultured mammalian cell line.
Toxicol. appl. Pharmacol., 22: 355-364.
PALMER, L. & KOLMODIN-HEDMAN, B. (1972) Improved quantitative gas
chromatographic method for analysis of small quantities of
chlorinated hydrocarbon pesticides in human plasma.
J. Chromatogr., 74: 21-30.
PASCHAL, E. H., ROAN, C. C., & MORGAN, D. P. (1974) Evidence of
excretion of chlorinated hydrocarbon pesticides by the human
liver. Bull. environ. Contam. Toxicol., 12: 547-554.
PEAKALL, D. B. (1970) p,p'-DDT: Effect on calcium metabolism and
concentration of estradiol in the blood. Science, 168: 592-594.
PEARCE, G. W., MATTSON, A. M., & HAYES, W. J., JR (1952) Examination
of human fat for presence of DDT. Science, 116: 254-256.
PEARL, W. & KUPFER, D. (1971) Stimulation of dieldrin elimination by
thiouracil derivative of DDT in the rat: enhancement of dieldrin
oxidative metabolism. Chem. biol. Interactions, 4: 91-96.
PELLIZARI, E. D. (1974) Electron capture detection in gas
chromatography. J. Chromatogr., 98: 323-361.
PERAINO, C., FRY, R. J. M., & STAFFELDT, E. (1973) Enhancement of
spontaneous hepatic tumorigenesis in C3H mice by dietary
phenobarbital. J. Natl Cancer Inst., 51: 1349-1350.
PEREVODCIKOVA, N. I., PLATINSKIY, L. V., & KERSTMAN, V. I. (1972) [The
treatment of inoperable forms of malignant tumors of the adrenal
cortex with o,p'-DDD.] Vopr. Onkol., 18: 24-29 (in Russian).
PERINI, G. & GHEZZO, F. (1970) [Dichlorodiphenylacetic acid (DDA)
urinary levels in the rural population of Ferrara Province.]
L'Arcisp. S. Anna di Ferrara, 23: 3-12 (in Italian).
PESENDORFER, H., EICHLER, I., & GLOFKE, E. (1973) [Informative
analyses of organochlorine pesticide and PCB residues in human
adipose tissue (from the area of Vienna).] Wien. Klin.
Wochenschr., 85: 218-222 (in German).
PETERLE, T. J. (1969) DDT in Antarctic snow. Nature (Lond.),
224: 620.
PETERSON, J. E. & ROBINSON, W. H. (1964) Metabolic products of p,p'-
DDT in the rat. Toxicol. appl. Pharmacol, 6: 321-327.
PFEILSTICKER, K. (1973) [Pesticides in baby food.] Monatsschr.
Kinderheilkd., 121: 551-553 (in German).
PHILIPS, F. S. & GILMAN, A. (1946) Studies on the pharmacology of DDT
(2,2-bis-(parachlorophenyl)-1,1,1-trichloroethane). I. The acute
toxicity of DDT following intravenous injection in mammals with
observations on the treatment of acute DDT poisoning.
J. Pharmacol., 86: 213-221.
PHILIPS, F. S., GILMAN, A., & CRESCITELLI, F. N. (1946) Studies on the
pharmacology of DDT (2,2-bis-(parachlorophenyl)-1,1,1-
trichloroethane). II. The sensitization of the myocardium to
sympathetic stimulation during acute DDT intoxication.
J. Pharmacol., 86: 222-228.
PIONKE, H. B., KONRAD, J. C., CHESTER, G., & ARMSTRONG, D.E. (1968)
Extraction of organochlorine and organophosphate insecticides from
lake water. Analyst, 93: 363-367.
PLATONOVA, V. I. (1970) [Disturbances of the functional conditions of
the stomach with prolonged effect on the body of some
organochlorine pesticides.] Gig. Tr. Resp. Mezhved, 3B,
6: 142-147 (in Russian).
PLIMMER, J. R. & KLINGEBIEL, U. I. (1973) PCB-formation. Science,
181: 994-995.
PLIMMER, J. R, KLINGEBIEL, U. I., & HUMMER, B.E. (1970) Photooxidation
of DDT and DDE. Science, 167: 67-69.
POLAND, A., SMITH, D., KUNTZMAN, R., JACOBSON, M., & CONNEY, A. H.
(1970) Effect of intensive occupational exposure to DDT an
phenylbutazone and cortisol metabolism in human subjects. Clin.
Pharmacol. Ther., 11: 724-732.
POLISHUK, Z. W., WASSERMANN, M., WASSERMANN, D., GRONER, Y.,
LAZAROVICI, S., & TOMATIS, L. (1970) Effects of pregnancy on
storage of organochlorine insecticides. Arch. environ. Health,
20: 215-217.
POLISHUK, Z. W., RUN, M., WASSERMANN, M., CUCOS, S., WASSERMANN, D., &
LEMESCH, C. (1977) Organochlorine compounds in human blood plasma
and milk. Pestic. Monit. J., 10: 121-129.
PONOMARKOV, V., TOMATIS, L., & TURUSOV, V. (1976) The effect of long-
term administration of phenobarbitone in CF-1 mice. Cancer Lett.,
1: 165-172.
POTT, P. (1775) Chirurgical observations relative to the cataract,
the polypus of the nose, the cancer of the scrotum, etc. London,
T. F. Carnegy, 208 pp.
POTT, P. (1790) The chirurgical works of Percival Pott, F.R.S.
surgeon to St. Bartholomew's Hospital, A new edition with his
last corrections, London, J. Johnson, 3 vol.
PRATI, L. & DEL DOT, M. (1971) [Research on the levels of accumulation
of synthetic organic chloride pesticides, in human adipose tissues
in the province of Trento.] L'Ig. Mod., 64: 35-43 (in Italian).
PRATI, L., PAVANELLO, R., & GHEZZO, F. (1972) Storage of chlorinated
pesticides in human organs and tissues in Ferrara Province, Italy.
Bull. World Health Organ., 46: 363-369.
PROUTY, R. M. & CROMARTIE, E. (1970) Stepwise recovery of DDT and
dieldrin from tissues of Coturnix japonica (quail) during residue
analysis. Environ. Sci. Technol., 4: 768-769.
QUINBY, G. E., HAYES, W. J., JR, ARMSTRONG, J. F., & DURHAM, W. F.
(1965a) DDT storage in the US population. J. Am. Med. Assoc.,
191: 175-179.
QUINBY, G. E., ARMSTRONG, J. F., & DURHAM, W. F. (1965b) DDT in human
milk. Nature (Lond.), 207: 726-728.
RABELLO, M. N., BECAK, W., ALMEIDA, W. F., PIGATI, P., UNGARO, M. T.,
MURATA, T., & PEREIRA, C. A. B. (1975) Cytogenetic study on
individuals occupationally exposed to DDT. Mutat. Res.,
28: 449-454.
RADOMSKI, J. L., DEICHMANN, W. B., & CLIZER, E. E. (1968) Pesticide
concentrations in the liver, brain, and adipose tissue of terminal
hospital patients. Food Cosmet. Toxicol, 6: 209-220.
RADOMSKI, J. L., DEICHMANN, W. B., REY, A. A., & MERKIN, T. (1971a)
Human pesticide blood levels as a measure of body burden and
pesticide exposure. Toxicol. appl. Pharmacol., 20: 175-185.
RADOMSKI, J. L, ASTOLFI, E., DEICHMANN, W. B., & REY, A. A. (1971b)
Blood levels of organochlorine pesticides in Argentina:
Occupationally and nonoccupationally exposed adults, children and
newborn infants. Toxicol. appl. Pharmacol., 20: 186-193.
RAMACHANDRAN, M., SHARMA, M. I. D., SHARMA, S.C., MATHUR, P.S.,
ARAVINDAKSHAN, A., & EDWARD, G. J. (1974) DDT and its metabolites
in the body fat of Indians. J. commun. Dis., 6: 256-259.
RANTANEN, T., MARSELOS, M., & PUHAKAINEN, E. (1975) Effects of 1,1,1-
trichloro-2,2-bis-( p-chlorophenyl)ethane (DDT) administration on
the glucuronic acid pathway in the rat liver. Environ. Qual.
Saf., 3 (Suppl.): 494-499.
RAPPOLT, R. T. (1970) Use of oral DDT in three human barbiturate
intoxications: CNS arousal and/or hepatic enzyme induction by
reciprocal detoxicants. Ind. Med. Surg., 39: 319.
READ, S. T. & MCKINLEY, W. P. (1961) DDT and DDE content of human fat.
A survey. Arch. environ. Health, 5: 209-211.
REIF, V. D. & SINSHEIMER, J. E. (1975) Metabolism of 1-( o-
chlorophenyl)-1-( p-chlorophenyl)-2-2-dichloroethane ( o,p'-DDD)
in rats Drug Metab. Dispos., 3: 15-25.
REIF, V. D., SINSHEIMER, J. E., WARD, J. C., & SCHTEINGART, D. E. J.
(1974). Aromatic hydroxylation and alkyl oxidation in metabolism
of mitotane ( o,p'-DDD) in humans. Pharmacol. Sci.,
63: 1730-1736.
REINGOLD, I. M. & LASKY, I. I. (1947) Acute fatal poisoning following
ingestion of a solution of DDT. Ann. intern. Med., 26: 945-947.
REYNOLDS, L. M. (1969) Polychlorinated biphenyls (PCBs) and their
interference with pesticide residue analysis. Bull. environ.
Contam. Toxicol., 4: 128-143.
RICHARDSON, A., ROBINSON, J., CRABTREE, N., & BALDWIN, M. K. (1971)
Residues in polychlorobiphenyls in biological samples. Pestic.
Monit. J., 4: 169-176.
RICHARDSON, J. A., KEIL, J. E., & SANDIFER, S. H. (1975) Catecholamine
metabolism in humans exposed to pesticides. Environ. Res.,
9: 290-294.
RISEBROUGH, R. W., HUGGETT, R. J., GRIFFIN, J. J., & GOLDBERG, E. D.
(1968) Pesticides: Transatlantic movements in the northeast
trades. Science, 159: 1233-1235.
RITCEY, W. R., SAVARY, G., & McCULLY, J. A. (1972) Organochlorine
insecticide residues in human milk, evaporated milk, and some milk
substitutes in Canada. Can. J. public Health, 63: 125-132.
RITCEY, W. R., SAVARY, G., & McCULLY, J. A. (1973) Organochlorine
insecticide residues in human adipose tissue of Canadians. Can.
J. public Health, 64: 380-386.
RIZOV, N. A. (1977) [Organochlorine pesticides in the fat and blood
of the general population of Bulgaria and methods for their
determination.] Dissertation. Sofia, Inst. Hygiene and
Occupational Health, Academy of Medicine, 117 pp. (in Bulgarian).
ROBINSON, J., RICHARDSON, A., & ELGAR, K. E. (1966) Chemical identity
in ultramicroanalysis, New York, American Chemical Society
(abstracts).
ROBINSON, J., RICHARDSON, A., HUNTER, C. G., CRABTREE, A. N., & REES,
H. J. (1965) Organochlorine insecticide content of human adipose
tissue in Southeastern England. Br. J. ind. Med., 22: 220-229.
ROSEN, A. A. & MIDDLETON, F. M. (1959) Chlorinated insecticides in
surface waters. Anal. Chem., 31: 1729-1732.
ROSSI, R., RAVERA, M., REPETTI, G., & SANTI, L. (1977) Long-term
administration of DDT or phenobarbital-Na in Wistar rats. Int. J.
Cancer, 19: 179-185.
ROTHE, C. F., MATTSON, A. M., NUESLEIN, R. M., & HAYES, W. J., JR
(1957) Metabolism of chlorophenothane (DDT): Intestinal lymphatic
absorption. Am. Med. Assoc. Arch. ind. Health, 16: 82-86.
ROWE, D. R., CANTER, L. W., SNYDER, P. J., & MASON, J. W. (1971)
Dieldrin and endrin concentrations in a Lousiana estuary. Pestic.
Monit. J., 4: 177-183.
RUDD, R. L. & HERMAN, S. G. (1972) Ecosystemic transferal of
pesticides residues in an aquatic environment. In: Matsumura, F.,
Boush, G. M., Misato, T., ed. Environmental toxicology of
pesticides, Part VII, New York, Academic Press.
RYBAKOVA, M. N. (1968) [The effect of certain pesticides on the
hypophysis and its gonadotrophic function.] Gig. i Sanit.,
33: 27-31 (in Russian).
SABAD, L. M., KOLESNICENKO, T. S., & NIKONOVA, T. V. (1972) [On a
possible blastomogenicity of DDT.] Vopr. Pitan., 30: 63-66
(in Russian).
SALIHODZAEV, S. S. & FERSTAT, V. N. (1972) [Condition of the olfactory
analyzer under the effect of organochlorine and organophosphorus
pesticides.] Gig. i Sanit., 37: 95-96 (in Russian).
SANDIFER, S. H., KEIL, J. E., FINKLEA, J. F., & GADSDEN, R. H. (1972)
Pesticide effects on occupationally exposed workers: A summary of
four years observation of industry and farm volunteers in South
Carolina. Ind. Med. Surg., 41: 7-12.
SARETT, H. P. & JANDORF, B. J. (1947) Effect of chronic DDT
intoxication in rats on lipids and other constituents of the
liver. J. Pharmacol., 91: 340-344.
SAUBERLICH, H. E. & BAUMANN, C. A. (1947) Effect of dietary variation
upon the toxicity of DDT to rats or mice. Proc. Sec. Exp. Biol.
Med., 66: 642-645.
SAVAGE, E. P., TESSARI, J. D., MALBERG, J. W., WHEELER, H. W., &
BAGBY, J. R. (1973) Organochlorine pesticide residues and
polychlorinated biphenyls in human milk, Colorado 1971-1972.
Pestic. Monit. J., 7: 1-5.
SAWYER, I. D. (1973) Collaborative study of the recovery and gas
chromatographic quantitation of PCBs in chicken fat and PCB-DDT
combinations in fish. J. Assoc. Off. Anal. Chem., 56: 1015-1023.
SCHAEFER, R. G. (1974) [Mass spectrometric identification and
quantification of chlorinated hydrocarbons in fish.] Chem. Ztg.,
98: 241-247 (in German).
SCHAFER, M. L. & CAMPBELL, J. E. (1966) Distribution of pesticide
residues in human body tissues from Montgomery County, Ohio,
Washington, DC, American Chemical Society, pp. 89-98 (Adv. Chem.
Ser. No. 60).
SCHMICK, M. (1973) Survival with adrenal carcinoma. J. Am. Med.
Assoc., 224: 1763.
SCHMIDT, R. (1973) [Effect of 1,1,1-trichloro-2,2-bis( p-
chlorophenyl) ethane (DDT) on the prenatal development of the
mouse, under consideration of distribution of tritium-labeled and
carbon-14-labeled DDT in pregnant mice.] Biol. Runtisch.,
11: 316-317 (in German).
SCHOOR, W. P. (1970) Effect of anticonvulsant drugs on insecticide
residues. Lancet, 2: 520-521.
SCHROEDER, G. & DOROZALSKA. (1975) [Degradation of DDT and its
analogues.] Wiad. Chem., 29: 553-565 (in Polish).
SCHVARTSMAN, S., ALMEIDA, W. F., COSTA VAZ, F. A., CORRADINI, H. B.,
PIGATI, P., GAETA, R., & UNGARO, M. T. (1974) Blood levels of DDT
in nonoccupationally exposed mothers and newborn infants in a city
in Brazil. In: Coulston, F. & Korte, F., ed. EQS Environmental
Quality and Safety, New York, Academic Press, Vol. 3,
pp. 154-156.
SCHWABE, U. & WENDLING, I. (1967) [Acceleration of drug metabolism by
small doses of DDT and other chlorinated hydrocarbon
insecticides.] Arzneim. Forsch., 17: 614-618 (in German).
SCOTT, R. P. W. (1970) Determination of the optimum conditions to
effect a separation by gas chromatography. Adv. Chromatogr.,
9: 193-214.
SCUDDER, H. I. & TARZWELL, C. M. (1950) Effects of DDT mosquito
larviciding on wildlife. IV. The effects on terrestrial insect
population of routine larviciding by airplane. Public Health
Rep., 65: 71-87.
SELBY, L. A., NEWELL, K. W., HAUSER, G. A., & JUNKER, G. (1969)
Comparison of chlorinated hydrocarbon pesticides in maternal blood
and placental tissues. Environ. Res., 2: 247-255.
SELL, J. L., DAVISON, K. L., & PUYEAR, R. L. (1971) Aniline
hydroxylase, N-demethylase, and cytochrome P-450 in liver
microsomes of hens fed DDT and dieldrin. J. agile. food Chem.,
19: 58-60.
SEMENCAEVA, E. M. (1968) [The mechanism of action of DDT and certain
other pesticides on the immunological response of the body.] In:
Medved', L. E., ed. The hygiene of application of the toxicology
of pesticides and the clinical features of pesticides poisoning.
Scientific Conference on the Safe Use & Toxicology of Pesticides,
11-14 June, 1968, Issue No. 6, Kiev, Acad. of Sciences, USSR,
pp. 805-815 (in Russian).
SHARAHGAPANI, M. V. & PINGALE, S. V. (1954) The hazard of DDT-
treatment of potatoes. Bull. Cent. Food Technol. Res. Inst.
Mysore, 4: 57.
SHERMA, J. (1973) Thin-layer chromatography: Recent advances. In:
Zweig, G., ed. Analytical methods for pesticides and plant growth
regulators, New York, Academic Press, Vol. VIII, pp. 3-87.
SHIRASU, Y., MORIYA, M., KATO, K., FURUHASHI, A., & KADA, T. (1976)
Mutagenicity screening of pesticides in the microbial system.
Mutat. Res., 40: 19-30.
SHIRASU, Y., MORIYA, M., KATO, K., LIENARD, F., TEZUKA, H., TERAMOTO,
S., & KADA, T. (1977) Mutagenicity screening on pesticides and
modification products: A basis of carcinogenicity evaluation. In:
Hiatt, H. H., Watson, J. D., & Winsten, J. A., ed. Origins of
human cancer, Book A, Incidence of cancer in humans, Cold Spring
Harbor, NY, Cold Spring Harbor Laboratory, Vol. 4, pp. 267-285.
SIERWISKI, M. & HELRICH, K. (1967) Separation, identification and
measurement of DDT and its metabolites. J. Assoc. Off. Anal.
Chem., 50: 627-633.
SIMMONS, S. W. (1959) The use of DDT insecticides in human medicine.
In: Müller, P., ed. DDT, the insecticide dichlorodiphenyltri-
chloroethane and its significance, Basel, Switzerland, Birkhäuser
Verlag, Vol. II, pp. 251-502.
SINGHAL, R. L. & KACEW, S. (1976) The role of cyclic AMP in
chlorinated hydrocarbon-induced toxicity. Fed. Proc.,
35: 2618-2623.
SISSONS, D. J., TELLING, G. M., & USHER, C. D. (1968) A rapid and
sensitive procedure for routine determination of organochlorine
pesticide residues in vegetables. J. Chromatogr., 33: 435-449.
SIYALI, D. S. (1972) Hexachlorobenzene and other organochlorine
pesticides in human blood. Med. J. Aust, 2: 1063-1066.
SIYALI, D. S. (1973) Polychlorinated hiphenyls, hexachlorobenzene and
other organochlorine pesticides in human milk. Med. J. Aust,
2: 815-818.
SIZONENKO, P. C., DORET, A. M., RIONDEL, A. M., & PAUNIER, L. (1974)
Cushing's syndrome due to bilateral adrenal cortical hyperplasia
in a 13-year-old girl: Successful treatment with o,p'-DDD.
Helv. Paediatr. Acta, 29: 195-202.
SKOGERBOE, R. K. & GRANT, C. L. (1970) Comments on the definitions of
the terms sensitivity and detection limit. Spectrosc. Lett.,
3: 215-220.
SKRENTNY, R. F. & DOROUGH, H. W. (1971) Efficiency of several
extraction procedures in removing organochlorine insecticides from
tobacco. Tobacco Sci., 15: 111-113.
SMART, N. A., HILL A. R. C., & ROUGHAN, P. A. (1974) Chlorinated
hydrocarbon pesticides residues in foodstuffs: Comparison of
methods. J. Assoc. Off. Anal. Chem., 57: 153-164.
SMITH, D. C. (1971) Pesticide residues in the total diet in Canada.
Pestic. Sci., 2: 92-95.
SMITH, D. C., SAUDI, E., & LEDUC, R. (1972) Pesticide residues in the
total diet in Canada. II-1970. Pestic. Sci., 3: 207-210.
SMITH, D. C., LEDUC, R., & CHARBONNEAU, C. (1973) Pesticide residues
in the total diet in Canada. III-1971. Pestic. Sci., 4: 211-214.
SMITH, D. C., LEDUC, R., & TREMBLAY, L. (1975) Pesticide residues in
the total diet in Canada. IV-1972 and 1973. Pestic. Sci.,
6: 75-82.
SMITH, M. I. & STOHLMAN, E. F. (1944) The pharmacologic action of 2,2-
bis-( p-chlorophenyl)-1,1,1-trichlorethane and its estimation in
the tissues and body fluids. Public Health Rep., 59: 984-993.
SMITH, M. I. & STOHLMAN, E. F. (1945) Further studies on the
pharmacologic action of 2,2-bis-( p-chlorophenyl)-1,1,1-
trichloroethane (DDT). Public Health Rep., 60: 289-301.
SMITH, M. I., BAUER, H., STOHLMAN, E. F., & LILLIE, R. D. (1946) The
pharmacologic action of certain analogues and derivatives of DDT.
J. Pharmacol., 88: 359-365.
SNYDER, D. & REINERT, R. (1971) Rapid separation of PCBs from DDT and
its analogues on silica gel. Bull. environ. Contam. Toxicol.,
6: 385-390.
SOUTHERN, A. L., TOCHIMOTO, S., ISURUGI, K., GORCHER, G. G., KRIKUN,
E., & STYPULKOWSKI, W. (1966a) The effect of 2,2-bis-(2-
chlorophenyl-4-chlorophenyl)-1,1,1-dichloroethane ( o,p'-DDD) on
the metabolism of infused cortisol-7-H. Steroids, 7: 11-29.
SOUTHERN, A. L., TOCHIMOTO, S., STROM, L., RATUSCHINI, A., RASS, H., &
GORCHER, G. (1966b) Remission in Cushing's syndrome with o,p'-
DDD. J. clin. Endocrinol. Metab., 26: 268-278.
SPICER, S. S., SWEENEY, T. R., VON OETTINGEN, W. F., LILLIE, R. D., &
NEAL, P. A. (1947) Toxicological observations on goats fed large
doses of DDT. Vet. Med., 42: 289-293.
STACY, G. I. & THOMAS, B. W. (1975) Organochlorine pesticide residues
in human milk, Western Australia-1970-71. Pestic. Monit. J.,
9: 64-66.
STANELY, C. W., BARNEY, J. E., HELTON, M. R., & YOBS, A. R. (1971)
Measurement of atmospheric levels of pesticides. Environ. Sci.
Technol., 5: 431-435.
STARR, M. G., ALDRICH, F. D., McDOUGALL, W. D., III, & MOUNCE, L. M.
(1974) Contribution of household dust to human exposure to
pesticides. Pestic. Monit. J., 8: 209-212.
STEC, J. & JUSZKIEWICZ, T. (1972) [Determination of polychlorinated
insecticide residues in animal products using Woods methods.]
Med. Weter., 28: 634-637 (in Polish).
STENBERG, A. I. & DIKY, V. V. (1973) [Changes in the metabolism of DDT
deposited in the omentum of fasting albino rats.] Vopr. Pitan.,
32; 39-43 (in Russian).
STIJVE, T. & CARDINALE, E. (1974) [Rapid determination of chlorinated
pesticides, PCBs, and a number of phosphated insecticides in fatty
foods.] Mitt. Lebensmittelunters. Hyg., 65: 131-150 (in German).
STOHLMAN, E. F. & LILLIE, R. D. (1948) The effect of DDT on the blood
sugar and of glucose administration on the acute and chronic
poisoning of DDT in rabbits. J. Pharmacol., 93: 351-361.
STRASSMANN, S.C. & KUTZ, F. W. (1977) Insecticide residues in human
milk from Arkansas and Mississippi, 1973-74. Pestic. Monit. J.,
10: 130-133.
STRAW, J. A., WATERS, I. W., & FREQLY, M. J. (1965) Effect of o,p'-
DDD on hepatic metabolism of phenobarbital in rat. Proc. Exp.
Biol. Med., 118: 391-394.
STREET, J. C. (1964) DDT antagonism to dieldrin storage in adipose
tissue of rats. Science, 146: 1580-1581.
STREET, J. C. & CHADWICK, R. W. (1967) Stimulation of dieldrin
metabolism by DDT. Toxicol. appl. Pharmacol., 11: 68-71.
STREET, J. C., WANG, M., & BLAU, A. D. (1966) Drug effects on dieldrin
storage in rat tissue. Bull. environ. Contam. Toxicol., 1: 6-15.
STREET, J. C., MAYER, F. L., & WAGSTAFF, D. J. (1969) Ecological
significance of pesticide interactions. Ind. Med. Surg,
38: 409-414.
STRETZ, P. E. & STARR, H. M. (1973) Determination of chlorinated
pesticides in whole blood. J. Assoc. Off. Anal Chem.,
56: 1173-1177.
STUDY GROUP OF MINISTRY OF HEALTH-WELFARE FOR ORGANOCHLORINE PESTICIDE
RESIDUES IN MOTHERS' MILK (1972) VIII. [Organochlorine pesticide
residues in blood during lactation.] J. Food Hyg. Soc. Jpn,
13: 438-450 (in Japanese).
SUGAYA, T. et al. (1971) [Organochlorine pesticide residues in human
milk.] J. Jpn Assoc. Rural Med., 19: 379-380 (in Japanese).
SUNDSTRÖM, G. (1977). Metabolic hydroxylation of the aromatic rings of
1,1-dichloro-2,2-bis(4-chlorophenyl)ethylene ( p,p'-DDE) by the
rat. J. agric. food Chem., 25: 18-21.
SUNDSTRÖM, G., JANSSON, B., & JENSEN, S. (1975) Structure of phenolic
metabolites of p,p'-DDE in rat, wild seal and guillemot. Nature
(Lond.), 255: 627-628.
SURBER, E. W. & FRIDDLE, D. D. (1949) Relative toxicity of suspension
and oil formulations of DDT to native fishes in Black Creek, West
Virginia. Trans. Am. Fish. Soc., 76: 315-321.
SUTHERLAND, G. L. (1965) Residue analytical limits of detectability.
Residue Rev., 10: 85-96.
SUVAK, L. N. (1970) [DDT in mothers' milk.] Zdravoochr. Kosinev,
13: 19-21 (in Russian).
SUZUKI, M., YAMATO, Y., & WATANABE, T. (1975) Gas chromatographic
resolution of organochlorine insecticides on OV-1/OV-17,
OV-210/OV-17 and OV-225/OV-17 mixed phase systems. J. Assoc. Off.
Anal. Chem., 58: 297-300.
SUZUKI, Y., SUGAYA, H., & SASAKI, S. (1973) [Results of 3 years'
tracing investigation of organochlorine pesticide residues in
humans.] J. Jpn Assoc. Med., 22: 276-277 (in Japanese).
SWACKHAMER, A.B. (1965) Report on pesticide residues in restaurant
meals in Canada. Pestic. Prog., 3: 108-114.
TABOR, E. C. (1966) Pesticides in urban atmospheres. Contamination of
urban air through the use of insecticides. Trans. NY Acad. Sci.,
28: 569-578.
TAIRA, M., HASHIMOTO, S., & SHIMOHIRA, I. (1972) [Organochlorine
pesticide residues in human breast milk.] Bull. Hyogo Prefect.
Public Health Lab., 7: 19-23 (in Japanese).
TAKANO, H. (1972) [Results of inspection of pesticide residues in
mothers' milk and market milk.] Annu. Rep. Iwate Inst. of Public
Health, 15: 88-91 (in Japanese).
TAKESHITA, T. & INUYAMA, Y. (1970) [Organochlorine residues in
mothers' milk and blood.] Annu. Rep. Shimane Prefect. Inst.
Public Health, 12: 27-28 (in Japanese).
TARJAN, R. & KEMENY, T. (1969) Multigeneration studies on DDT in mice.
Food Cosmet. Toxicol., 7: 215-222.
TARRANT, K. R. & TATTON, J. O'G. (1968) Organochlorine pesticides in
rainwater in the British Isles. Nature (Lond.), 219: 725-727.
TARZWELL, C. M. (1950) Effects of DDT mosquito larviciding on
wildlife. V. Effects on fishes of the routine manual and airplane
application of DDT and other mosquito larvicides. Public Health
Rep., 65: 231-255.
TAYLOR, R. & BOGACKA, T. (1968) [Determination of DDT, methoxychlor or
lindane in water by extraction and thin-layer chromatography.]
Chem. Anal., 13: 227-234 (in Polish).
TELFORD, H. S. & GUTHRIE, J. E. (1945) Transmission of the toxicity of
DDT through the milk of white rats and goats. Science, 102: 647.
TERRACINI, B., TESTA, M. C., CABRAL, J. R., & DAY, N. (1973) The
effects of long-term feeding of DDT to BALB/c mice. Int. J.
Cancer, 11: 747-764.
THOMAS, E. J., BURKE, J. A., & LAWRENCE, J. H. (1968) Thin-layer
chromatography; relative migration date (R-TDE) of chlorinated
pesticides. J. Chromatogr, 35: 119-121.
THOMAS, T. C. & SERBER, J. N. (1974) Chromosorb 102, an efficient
medium for trapping pesticides from air. Bull. environ. Contam.
Toxicol., 12: 17-25.
THOMPSON, J. F., ed. (1974) Analysis of pesticide residues in human
and environmental samples, a compilation of methods selected for
use in pesticide monitoring programs, Research Triangle Park,
NC, US Environmental Protection Agency, 1 vol.
THOMPSON, J. F., WALKER, A. C., & MOSEMAN, R. F. (1969a) Evaluation of
8 gas chromatographic columns for chlorinated pesticides.
J. Assoc. Off. Anal. Chem., 52: 1263-1277.
THOMPSON, J. F., WALKER, A. C., & MOSEMAN, R. F. (1969b) Study of the
performance of gas-chromatographic columns under severe injection
loading. J. Assoc. Off. Anal. Chem., 52: 1251-1262.
THOMPSON, J. F., MANN, J. B., APODACA, A. O., & KANTOR, E. J. (1975)
Relative retention ratios of 95 pesticides and metabolites on nine
gas-liquid chromatographic columns over a temperature range of
170-204°C on two detection modes. J. Assoc. Off. Anal. Chem., 58:
1037-1050.
THOMPSON, R. P. H., PILCHER, C. W. T., ROBINSON, J., STATHERS, G. M.,
MCLEAN, A. E. M., & WILLIAMS, R. (1969) Treatment of unconjugated
jaundice with dicophane. Lancet, 2: 4-6.
THORPE, E. & WALKER, A. I. T. (1973) The toxicology of dieldrin
(HEOD). II. Comparative long-term oral toxicity studies in mice
with dieldrin, DDT, phenobarbitone, ß-BHC, and gamma-BHC. Food
Cosmet. Toxicol., 11: 433-442.
TINSLEY, I. J. & LOWRY, R. R. (1972) An interaction of DDT in the
metabolism of essential fatty acids. Lipids, 7: 182-185.
TOCCI, P.M., MANN, J. B., DAVIES, J. E., & EDMUNDSON, W. F. (1969)
Biochemical differences found in persons chronically exposed to
high levels of pesticides. Ind. Med. Surg., 38: 188-195.
TOKUTSU, K., KOYAMA, T., & TOKOYAMA, T. (1970) [Pesticide residues in
mothers' milk and plasma in Wakayama Prefecture.] Annu. Rep.
Wakayama Prefect. Inst. Public Health, 19: 59-62 (in Japanese).
TOMATIS, L. & TURUSOV, V. (1975) Studies on the carcinogenicity of
DDT. GANN Monograph Cancer Res., 17: 219-241.
TOMATIS, L., TUROSOV, V., DAY, N., & CHARLES, R. T. (1972) The effect
of long-term exposure to DDT on CF-1 mice. Int. J. Cancer,
10: 489-506.
TOMATIS, L., PARTENSKY, C., & MONTESANO. R. (1973) The predictive
value of mouse liver tumor induction in carcinogenicity testing --
A literature survey. Int. J. Cancer, 12: 1-20.
TOMATIS, L., TURUSOV, V., CHARLES, R. T., & BOICCHI, M. (1974a).
Effect of long-term exposure to 1,1-dichloro-2,2-bis( p-
chlorophenyl)ethylene, 1,1-dichloro-2,2-bis( p-chlorophenyl)
ethane, and to the two chemicals combined on CF-1 mice. J. Natl
Cancer Inst., 52: 883-891.
TOMATIS, L., TURUSOV, V., CHARLES, R. T., BOICCHI, M., & GATI, F.
(1974b) Liver tumors in CF-1 mice exposed for limited periods to
technical DDT. Z. Krebs. Forsch., 82: 25-35.
TOMII, S., NAGASAKI, H., & MEGA, Y. (1972) [Studies on BHC as a
carcinogen.] Jpn. J. Hyg., 27: 113 (in Japanese).
TORDA, C. & WOLFF, H. G. (1949) Effect of convulsant and
anticonvulsant agents on the activity of carbonic anhydrase.
J. Pharmacol. exp. Ther., 95: 444-447.
TREON, J. F. & CLEVELAND, F. P. (1955) Toxicity of certain chlorinated
hydrocarbon insecticides for laboratory animals with special
reference to aldrin and dieldrin. J. agric. food Chem.,
3: 402-408.
TSUTSUI, J., KATO, T., & NISHIKAWA, T. (1974) [Results of health
survey on persons engaging in pesticide application in Suzuka
area. Mie Prefecture.] J. Jpn. Assoc. Rural Med., 23: 518-521
(in Japanese).
TUINSTRA, L. G. M. TH. (1971) [Organochlorine insecticide residues in
human milk in the Leiden Region.] Ned. Melk-Zuiveltijdschr.,
25: 24-32 (in Dutch).
UNTERMAN, H. W. & SIRGHIE, E. (1969) [Presence of organochlorines in
human body.] 18: 221-226 (in Romanian).
US DEPT OF AGRICULTURE, AGRICULTURAL RESEARCH ADMINISTRATION (1949)
USDA entomologists recommend substitute insecticide for DDT to
control insects on dairy cattle and in dairy barns. News
Release, March 24.
U.S. DEPT OF AGRICULTURE, AGRICULTURAL RESEARCH SERVICE (1966)
Monitoring agricultural pesticide residues. A preliminary report
of studies on soil, sediment and water in Mississippi River
delta, Washington, DC, US Government Printing Office.
US DEPT OF AGRICULTURE, BUREAU OF ENTOMOLOGY AND PLANT QUARANTINE
(1945a) Release of February 13, 1945.
US DEPT OF AGRICULTURE, BUREAU OF ENTOMOLOGY AND PLANT QUARANTINE
(1945b) Outlook for supplies of DDT insecticides. Release of
August 6, 1945.
US DEPT OF HEALTH EDUCATION AND WELFARE (1969) Manual for evaluating
public drinking water supplies. A manual of practice recommended
by the Public Health Service, Washington, DC, US Government
Printing Office, I (PHS Pub. No. 1820).
US ENVIRONMENTAL PROTECTION AGENCY (1975) DDT. A review of scientific
and economic aspects of the decision to ban its use as a
pesticide, Washington US EPA, US Government Printing Office,
300 pp. (EPA-504-1-75-022).
VAARAMA, A. (1947) The influence of DDT pesticides upon plant mitosis.
Hereditas, 33: 191-219.
VAINO, H. (1974) Enhancement of hepatic microsomal drug oxidation and
glucuronidation in rat by 1,1,1-trichloro-2,2-bis( p-
chlorophenyl)ethane (DDT). Chem. biol. Interactions, 9: 7-14.
VAINIO, H. (1975) Stimulation of microsomal drug-metabolizing enzymes
in rat liver by 1,1,1-trichloro-2,2-bis( p-chlorophenyl)ethane
(DDT), pregnenolone-16alpha-carbonitrile (PEN), and
polychlorinated biphenyls (PCBs). Environ. Qual. Saf.,
3 (Suppl.): 486-490.
VANAT, S. V. & VANAT, I. M. (1971) [Contribution to the toxic-allergic
reaction induced by DDT.] Klin. Med. (Mosk.), 49: 126-127
(in Russian).
VAS'KOVSKAJA & KOMAROVA (1967) Quoted by: Kagan et al., Res. Rev, 27:
43-79.
VAS'KOVSKAJA, L. F. (1969) [Accumulation of certain chloroorganic
insecticides in the bodies of experimental animals and humans.]
In: Medved', L. E., ed. The hygiene of application and the
toxicology of pesticides and the clinical features of pesticide
poisoning. A collection of papers. Issue No. 7, Kiev,
Vniggintox, pp. 503-796 (in Russian).
VAZ A., PEREIRA, R. S., & MALHETRO, D. M. (1945) Calcium in prevention
and treatment of experimental DDT poisoning. Science,
101: 434-436.
VELBINGER, H. H. (1947a) [On the question of DDT toxicity to man.]
Dtsch. Gesundheitwes., 2: 355-358 (in German).
VELBINGER, H. H. (1947b) [Contribution to the toxicology of "DDT"-
agent, dichlorodiphenyl-trichloromethylmethane.] Pharmazie,
2: 268-274 (in German).
VERDON, T. A., JR, BRUTON, J., HERMAN, R. H., & BEISEL, W. R. (1962)
Clinical and chemical response of functioning adrenal cortical
carcinoma to ortho-, para-DDD. Metab. Clin. Exp., 11: 226-234.
VERSINO, B., VENNE, M-TH VAN DER, & VISSERS, H. (1971) Comparison of
some clean-up columns for residue analysis of chlorine and
phosphorus containing pesticides. J. Assoc. Off. Anal Chem.,
54: 147-149.
VON OETTINGEN, W. F. & SHARPLESS, N. E. (1946) The toxicity and toxic
manifestations of 2,2-bis-( p-chlorophenyl)-1,1,1-trichloroethane
(DDT) as influenced by chemical changes in the molecule. A
contribution to the relation between chemical constitution and
toxicological action. J. Pharmacol. exp. Ther., 88: 400-413.
WAGSTAFF, D. J. (1971) Ascorbic acid deficiency and induction of
hepatic microsomal hydroxylative enzymes by organochlorine
pesticides. Toxicol. appl. Pharmacol., 19: 10-19.
WAGSTAFF, D. J. & STREET, J. C. (1970) Dieldrin-DDT interactions in
guinea pigs. Toxicol. appl. Pharmacol., 17: 276.
WALKER, A. I. T., THORPE, E., & STEVENSON, D. E. (1973) The toxicology
of dieldrin (HEOD). I. Long-term oral toxicity studies in mice.
Food Cosmet. Toxicol., 11: 415-432.
WALKER, K. C., GOETTE, M. B., & BATCHELOR, G. S. (1954) Pesticide
residues in foods: Dichlorodiphenyltrichloroethane and
dichlorodiphenyldichloroethylene content of prepared meals.
J. agric. food. Chem., 2: 1034-1037.
WALLACE, Z. E., SILVERSTEIN, J. N., VILLADOLID, L. S., WEISENFELD, S.
(1961) Cushing's syndrome due to adrenocortical hyperplasia. New
Engl. J. Med., 265: 1088-1093.
WARNICK, S. L. (1972) Organochlorine pesticide levels in human serum
and adipose tissue, Utah-Fiscal years 1967-71. Pestic. Monit. J.,
6: 9-13.
WARNICK, S. L. & CARTER, J. E. (1972) Some findings in a study of
workers occupationally exposed to pesticides. Arch. environ.
Health, 25: 265-270.
WASICKY, R. & UNTI, O. (1944) [Dichloro-diphenyl-trichloroethanol
(DDT) does not have any effect on the larvae of "Culicides".]
Arch. Hig. S. Paulo, 9: 87-102 (in Portuguese).
WASICKY, R. & UNTI, O. (1945) [Dichlorodiphenyltrichloroethane (DDT),
additional investigations on its properties and application.]
Arch. Hig. S. Paulo, 10: 49-64 (in Portuguese).
WASSERMANN, D., WASSERMANN, M., DJAVAHERIN, H., GORIN, I., BARISH, M.,
& TAVOR, R. (1971) Effect of organochlorine insecticides on serum
PBI level in occupationally exposed people. Bull. environ.
Contam. Toxicol., 6: 85-88.
WASSERMANN, M., ILIESCU, S., MANDRIC, G., & HORVATH, P. (1960) Toxic
hazards during DDT- and BHC-spraying of forests against Lymantria
monacha. Arch. ind. Health, 21: 503-508.
WASSERMANN, M., GON, M., WASSERMANN, D., & ZELLERMAYER, L. (1965) DDT
and DDE in the body fat of people in Israel. Arch. environ.
Health, 11: 375-379.
WASSERMANN, M., WASSERMANN, D., ZELLERMAYER, L., & GON, M. (1967)
Pesticides in people. Storage of DDT in the people of Israel.
Pestic. Monit. J., 1: 15-20.
WASSERMANN, M., CURNOW, D. H., FORTE, P. N., & GRONER, Y. (1968a)
Storage of organochlorine pesticide in the body of people in
Western Australia. Ind. Med. Surg., 37: 295-300.
WASSERMANN, M., SOFOLUWE, G. I., WASSERMANN, D., GRONERS, I., &
LAZAROVITCH, S. (1968a) Storage of organochlorine insecticides in
the body fat of people in Nigeria. In: Wasserman, H. & Sofoluwe,
G. O., ed. The Lagos International Seminar on Occupational Health
for Developing Countries, 1-6 April 1968, pp. 188-202.
WASSERMANN, M., WASSERMANN, D., GERSHON, Z., & ZELLERMAYER, L. (1969)
Effect of organochlorine insecticides on body defence systems.
Ann. NY Acad. Sci., 160: 393-401.
WASSERMANN, M., WASSERMANN, D., ARONOVSKI, I., IVRIANI, I., &
ROSENFELD, D. (1970a) The effects of organochlorine insecticides
on serum cholesterol level in people occupationally exposed. Bull
environ. Contam. Toxicol., 5: 368-372.
WASSERMANN, M., WASSERMANN, D., LAZAROVICI, S., COETZEE, A. M., &
TOMATIS, L. (1970b) Present state of the organochlorine
insecticides in the general populations of South Africa. South
Afr. med. J., 44: 646-648.
WASSERMANN, M., WASSERMANN, D., & IVRIANI, I. (1970c) Organochlorine
insecticides in the plasma of occupationally exposed workers. In:
Deichmann, W. B., Radomski, J. L., & Penalver, R. A., ed.
Pesticides Symposia, Miami, Florida, Halos. pp. 311-314.
WASSERMANN, M., WASSERMANN, D., KEDAR, E., & DJAVAHERIAN, M. (1971)
Immunological and detoxication interactions in p,p'-DDT fed
rabbits. Bull. environ. Contam. Toxicol., 6: 426-534.
WASSERMANN, M., ROGOFF, M. G., TOMATIS, L., & DAY, N. E. (1972a)
Storage of organochlorine insecticides in the adipose tissue of
people in Kenya. Ann. Soc. Belg. Med. Trop., 52 (6): 509-514.
WASSERMANN, M., NOGUEIRA, D. P., TOMATIS, L., ATHIE, E., WASSERMANN,
D., KJAVAHERIAN, M., & GUTTEL, C. (1972b) Storage of
organochlorine insecticides in people of Sao Paulo, Brazil. Ind.
Med. Surg., 41: (3): 22-25.
WASSERMANN, M., TRISHNANANDA, M., TOMATIS, L., DAY, N. E, WASSERMANN,
D., RUNGPITARANGSI, V., CHAMSAKOL, V., DJAVAHERIAN, M., & CUCUS,
S. (1972c) Storage of organochlorine insecticides in the adipose
tissue of people from Thailand. Southeast Asian J. trop. Med.
Public Health, 3: 280-285.
WASSERMANN, M., SOFOLUWE, G. O., TOMATIS, L., DAY, N. E., WASSERMANN,
D., & LAZAROVICI, S. (1972d) Storage of organochlorine
insecticides in people in Nigeria. Environ. Physiol. Biochem.,
2: 59-67.
WASSERMANN, M., TOMATIS, L., WASSERMANN, D., DAY, N. E., &
DJAVAHERIAN, M. (1974a) Storage of organochlorine insecticides in
adipose tissue of Ugandans. Bull. environ. Contam. Toxicol.,
12: 501-508.
WASSERMANN, M., TOMATIS, L., WASSERMANN, D., DAY, N. E., GRONER, Y.,
LAZAROVICI, S., & ROSENFELD, D. (1974b) Epidemiology of
organochlorine insecticides in the adipose tissue of Israelis.
Pestic. Monit. J., 8: 1-7.
WASSERMANN, M., PRATA, A., TOMATIS, L., WASSERMANN, D., IVRIANI, I., &
OLIVIERA, V. (1975) Storage of organochlorine insecticides in
hepato-splenic schistosomiasis. Rev. Soc. Bras. Mad. Trop.,
8: 271-273.
WATARI, N. (1973) [Ultrastructural alterations of the mouse liver
after the prolonged administration of BHC.] J. Jpn. Electron
Microsc. Soc., 5: 1410-1420; 1449-1456 (in Japanese).
WATSON, M., BENSON, W. W., & GABICA, J. (1970) Serum organochlorine
pesticide levels in people in Southern Idaho. Pestic. Monit. J.,
4: 47-50.
WATSON, M., GABICA, J., & BENSON, W. W. (1972) Serum organochlorine
pesticides in mentally retarded patients on differing drug
regimens. Clin. Pharmacol. Ther., 13: 186-192.
WATTENBERG, L. W. (1971) Studies of polycyclic hydrocarbon
hydroxylases of the intestine possibly related to cancer. Effect
of diet on benzypyrene hydroxylase activity. Cancer, 28: 99-102.
WEAVER, L., GUNNERSON, C. G., BREIDENBACH, A. W., & LICHTENBERG, J. J.
(1965) Chlorinated hydrocarbon pesticides in major US river
basins. Public Health Rep., 80: 481-493.
WEIHE, M. (1966) [Chlorinated insecticides in the body fat of people
in Denmark.] Ugeskr. Laeg., 128: 881-882 (in Danish).
WEIL, C. S. & McCOLLISTER, D. D. (1963) Relationship between short-
and long-term feeding studies in designing an effective toxicity
test. J. agric. food Chem., 11: 486-491.
WEIL, C. S., WOODSIDE, M. D., BERNARD, J. R., & CARPENTER, C. P.
(1969) Relationship between single-peroral, one-week, and ninety-
day rat feeding studies. Toxicol. appl. Pharmacol., 14: 426-431.
WEIL, L., QUENTIN, K. E., & RONICKE, G. (1973) Pesticide levels in air
dust particles in the Federal Republic of Germany. In: The
Technical University of Munich and the German Society for
Research, Commission on Research on Air Pollution, Notice VIII,
23 pp.
WELCH, R. M. & HARRISON, Y. (1966) Reduced drug toxicity following
insecticide treatment. Pharmacologist, 8: 217.
WELCH, R. M., LEVIN, W., & CONNEY, A. H. (1969) Estrogenic action of
DDT and its analogs. Toxicol. appl. Pharmacol., 14: 358-367.
WELCH, J. H. & GORDON, H. T. (1946) The mode of action of DDT. Fed.
Proc., 5: 1.
WEST, I. (1964) Pesticides as contaminants. Arch. environ. Health,
9: 626-631.
WESTOO, G., NOREN, K., & ANDERSSON, M. (1970) [Levels of
organochlorine pesticides and polychlorinated biphenyls in
margarine, vegetable oils, and some foods of animal origin on the
Swedish market in 1967-1969.] Vaar foeda, 22: 9-31 (in Swedish).
WHITE, W. C. & SWEENEY, T. R. (1945) The metabolism of 2,2-bis-( p-
chlorophenyl-1,1,1-trichloroethane (DDT). I. A metabolite from
rabbit urine di-( p-chlorophenyl)-acetic acid; its isolation,
identification, and synthesis. Public Health Rep., 60: 66-71.
WHITING, F. M., STULL, J. W., BROWN, W. H., MILLBROTH, M., & WARE, G.
W. (1968) Comparison of extraction methods for analysis of DDT,
DDE and TDE, in alfalfa hay. J. dairy Sci., 51: 1039-1041.
WHO (1971) Appendix 14: The place of DDT in operations against malaria
and other vector-borne diseases. Off. Rec. World Health Organ.,
190: 176-182.
WHO (1973) WHO Technical Report Series No. 513 (Safe use of
pesticides) 54 pp.
WIGGLESWORTH, V. B. (1945) A case of DDT poisoning in man. Br. med.
J., 1: 517.
WILCOX, A. R. (1967) USPH investigation. DDT health effects. Inter-
Office Correspondence to M. V. Anthony, Stauffer Chemical Co.
WILLIS, G. H., PARR, J. F., & SMITH, S. (1971) Volatilization of soil-
applied DDT and DDD from flooded and nonflooded plots. Pestic
Monit. J., 4: 204-208.
WILSON, D. J., LOCKER, D. J., RITZEN, C. A., WATSON, J. T., &
SCHAFFNER, W. (1973) DDT concentrations in human milk. Am. J.
Dis. Child., 125: 814-817.
WILSON, H. F., ALLEN, N. N., BOHSTEDT, G., BETHEIL, J., & LARDY, H. A.
(1946a) Feeding experiments with DDT-treated pea vine silage with
special reference to dairy cows, sheep, and laboratory animals.
J. econ. Entomol., 36 (6): 801-806.
WILSON, H. F., SRIVASTAVA, A. S., HULL, W. B., BETHEIL, J., & LARDY,
H. A. (1946b) DDT residues on pea vines and canned peas from
fields treated with DDT dusts. J. econ. Entomol., 39
(6): 806-809.
WINTER, M., THOMAS, M., WERNICK, S., LEVIN, S., & FARVAR, M. T. (1976)
Analysis of pesticide residues in 290 samples of Guatemalan
mother's milk. Bull. environ. Contam. Toxicol., 16: 652-657.
WOLFE, H. R. & ARMSTRONG, J. F. (1971) Exposure of formulating plant
workers to DDT. Arch. environ. Health, 23: 169-176.
WOLFE, H. R., WALKER, K. C., ELLIOTT, J. W., & DURHAM, W. F. (1959)
Evaluation of the health hazards involved in house-spraying with
DDT. Bull. Worm Health Organ., 20: 1-14.
WOLFE, H. R., DURHAM, W. F, & ARMSTRONG, J. F. (1970) Urinary
excretion of insecticide metabolites: excretion of para-
nitrophenol and DDA as indicators of exposure to parathion and
DDT. Arch. envrion. Health, 21: 711-716.
WOODARD, G., OFNER, R. R., & MONTGOMERY, C. M. (1945) Accumulation of
DDT in the body fat and its appearance in the milk of dogs.
Science, 102: 177-178.
WOODARD, B. T., FERGUSON, B. B., & WILSON, D. J. (1976) DDT levels in
milk of rural indigent blacks, Research Triangle Park, NC, Health
Effects Research Laboratory, US Environmental Protection Agency,
16 pp. (EPA 600/1-76-032).
WOOLSON E. A. (1974) Extraction of chlorinated hydrocarbon
insecticides from soil. J. Off. Anal. Chem, 57: 604-609.
WOOLSON E. A. & KEARNEY, P. C. (1969) Survey of chlorinated
insecticide analyses in soils. J. Assoc. Off. Anal. Chem.,
52: 1202-1206.
WRENN, T. R., WEYANT, J. R., FRIES, G. F., & BITMAN, J. (1971a) Effect
of several dietary levels of o,p'-DDT on reproduction and
lactation in the rat. Bull. environ. Contam. Toxicol.,
6: 471-479.
WRENN, T., RANDALL, J., WEYANT, R., FRIES, G. F., & BITMAN, J. (1971b)
Influence of dietary o,p'-DDT on reproduction and lactation of
ewes. J. animal Sci., 33: 1288-1292.
WRIGHT, A. S., POTTER, D., WOODER, M. F., DONNINGER, C., & GREENLAND,
R. D. (1972) Effects of dieldrin on mammalian hepatocytes, Food
Cosmet. Toxicol., 10: 311-322.
WYLLIE, J., GABICA, J., & BENSON, W. W. (1972) Comparative
organochlorine pesticide residues in serum and biopsied lipoid
tissue: A survey of 200 persons in southern Idaho -- 1970.
Pestic. Monit. J., 6: 84-88.
YAMAGISHI, T, TAKEBA, K., FUGIMOTO, C., MORIMOTO, K., & HARUTA, M.
(1972) [On the organochlorine pesticide residues in mothers' body
and her fetus' body.] Rep. Tokyo Public Health Stud., 50: 44-45
(in Japanese).
YERMAKOV, V. V. (1972) [Gas chromatographic methods for determining
pesticides in biological material.] Moscow, Izdatelstvo Nauka (in
Russian).
YODER, J. M., WATSON, M., & BENSON, W. W. (1973) Lymphocyte chromosome
analysis of agricultural workers during extensive occupational
exposure to pesticides. Mutat. Res., 21: 335-340.
YOUDEN, W. J. & STEINER, E. H. (1975) Statistical Manual of the
Association of Official Analytical Chemists, Washington, DC,
AOAC.
ZAVON, M. R., HINE, C. H., & PARKER, K. D. (1965) Chlorinated
hydrocarbon insecticides in human body fat in the United States.
J. Assoc. Off. Anal. Chem., 193: 837-839.
ZAVON, M. R., TYE, R., & LATORRE, E. (1969) Chlorinated hydrocarbon
insecticide content of the neonate. Ann. NY Acad. Sci.,
160: 196-200.
ZEINE-EL-DINE, K. (1946) The insecticide DDT. J. Egypt Med. Assoc.,
29: 38-54.
ZELINSKI, S. G., TASHIRO, J., & WORTHEN, L. R. (1973) A gas-
chromatographic method of quantifying DDT in the presence of
interference from PCBs. J. Chromatogr., 84: 67-73.
ZERBER, J., KURASIAK, K., & CIELIEZAK, A. (1971) [Determination of
chlorinated hydrocarbon residues in cereal products and feeds.]
Chem. Anal. 16: 1061-1066 (in Polish).
ZIMMERLI, B. & MAREK, B. (1973) [The pesticide burden of the Swiss
population (analyses of prepared meals, human fat, human serum,
cigarettes, and cosmetics.)] Mitt. Lebensmitt. Hyg., 64: 459-479
(in German).
ZIMMERMAN, B., BLOCH, H. S., WILLIAMS, W. L., HITCHCOCK, C. R., &
HOEISCHER, B. (1956) Effects of DDD (1,1,dichloro-2,2-bis( p-
chloro-phenyl)-ethane) on human adrenal. Attempted to use adrenal
destructive agent in treatment of disseminated mammary and
prostatic cancer. Cancer, 9: 940-948.
ZWEIG, G. & SHERMA, J. (1972) Analytical Methods for Pesticides and
Plant Growth Regulators, New York, Academic Press, Vol 6,
765 pp.
ANNEX
TRANSFORMATION OF P,P'-DDT IN THE ENVIRONMENTa
1 Abiotic Transformations
Since 1969, the photolysis of p,p'-DDT (Annex Fig. 1, formula
II) and of its known primary environmental degradation product, DDE
(1,1-dichloro-2,2-bis [ p-chlorophenyl]ethylene; Annex Fig. 1,
formula VII) has been studied by irradiation in methanol at 260 nm
(Plimmer et al., 1970). The products formed were DDMU (1-chloro-2,2-
bis [ p-chlorophenyl] ethylene; Annex Fig. 1, Formula III),
dichlorobenzophenone (Annex Fig 1, formula IV) and dichlorobiphenyl
(Annex Fig. 1, Formula V). The formation of the last compounds
proceeded via dichlorobenzophenone as an intermediate. The detection
of the chlorinated biphenyl raised the question as to whether DDT
might be a source of PCBs in the environment. Upon investigation of
the reaction pathways leading to these substances, it was shown that
DDE is converted to 3,6-dichlorofluoroenone (Annex Fig. 1, formula VI;
Plimmer & Klingebiel, 1969) which is photooxidized to 3,3'-dichloro-
biphenyl-2-carboxylic acid. Subsequent decarboxylation of this acid
could yield traces of 3,3'-dichlorobiphenyl; the decarbonylation of
another photolysis product of DDT, trichlorobenzophenone, could yield
traces of trichlorobiphenyl, demonstrating that the formation of PCBs
with more than 2 chlorine atoms was also possible (Plimmer &
Klingebiel, 1973).
Irradiation studies with substances in organic solvents are not
necessarily predictive for the environment. However, studies with DDT
vapour in sunlight confirmed the results obtained with dissolved
substances. The proposed pathways of DDT photolysis under
environmental conditions is shown in Annex Fig. 1 (Moilanen & Crosby,
1973).
In 1972, DDE was irradiated in solvents, in the solid state, and
in the gaseous phase, with UV-light of various wavelengths. The
results are shown in Annex Fig. 2. Besides the known photoproducts IV
and III, a "trichlorinated DDMU" (Annex Fig. 2, formula VIII) and 2
compounds with longer side chains (Annex Fig 2, formula IX and X) were
identified; these 2 substances, however, were formed only upon
irradiation in a solvent and originated from the reaction with the
solvent (Kerner et al., 1972).
a Prepared by Dr F. Korte at the request of the Task Group.
 In a recent study on the photoisomerization and photodegradation
of DDE under simulated natural conditions (inert solvents, a good
hydrogen donor solvent, UV-light similar or equal 300 nm), the
compound VIII (Annex Fig. 2) with the 3 phenyl-bound chlorine atoms
was detected and characterized as a mixture of the E- and Z-isomers
which were separated and isolated. Both isomers were also found in
natural samples like tobacco and pine needles. DDMU was also detected
in these studies and 2 substances so far unknown, a tetrachlorinated
phenanthrene and a tetra-ring-chlorinated diphenylethylene; the
formation of tri- and tetrachlorobiphenyls was confirmed (Göthe et
al., 1976).
The behaviour of compounds in their adsorbed form is equally as
significant environmentally as their photochemical behaviour in the
gaseous and solid states.
Irradiation of DDE, adsorbed on silicagel, with wavelengths
> 230 nm, resulted in the formation of dichlorobenzophenone and its
trichlorinated analogue. Irradiation of DDT and DDE in the solid form
in an oxygen stream with wavelengths > 230 nm, resulted in partial
mineralization to give carbon dioxide and hydrochloric acid (Gab et
al., 1975).
The results presented here show that a large number of DDT-derived
chlorinated compounds must be included, when considering the possible
effects of DDT residues in the ecosphere.
2 Biotransformations Other Than Mammalian Metabolism
2.1 Birds
Two main pathways of DDT metabolism exist in mammals i.e.,
dehydrochlorination to DDE (Annex Fig. 3, formula VII) and stepwise
degradation to DDA (bis-[ p-chlorophenyl] acetic acid) via TDE (DDD)
(1,1-dichloro-2,2-bis [ p-chlorophenyl] ethane; Annex Fig. 3,
formula I). However, in birds, the pathway varies with species, and
data from studies on the administration of chronic and acute dosages
of DDT to pigeons, quail, and blackbirds show that DDE is the primary
metabolic product in the first 2 species, and TDE (DDD) in the third
(Bailey et al., 1972). The TDE-path-way does exist in the pigeon as a
minor pathway but, in contrast to mammals, only as far as DDMU (Annex
Fig. 3, formula III).
Thus, DDA, the degradation product of DDMU excreted by mammals, is
not formed in the pigeon (Bunyan et al., 1966; Bailey et al., 1969).
When its precursors in mammals, DDMS (1-chloro-2,2-bis[ p-
chlorophenyl] ethane; Annex Fig. 3, formula XI) and DDN U (1,1-bis
[ p-chlorophenyl] ethylene; Annex Fig. 3, formula XII) are
administered to the pigeon, they are rapidly converted: DDMS is
converted to DDMU, and DDNU is metabolized quickly and excreted as
DDNS (1,1-bis [ p-chlorophenyl] ethane; Annex Fig. 3, formula XIII),
a metabolite that was not found in mammals (Bailey et al., 1972). The
metabolic pathways for DDT in the pigeon are shown in Annex Fig. 3.
2.2 Insects
Investigations on the detoxication mechanism of DDT in insects are
interesting as regards the problem of resistance. In general, the
phenomenon of insect resistance is related to detoxication of the
insecticide by metabolization to nontoxic compounds. The metabolic
pathways of DDT in insects are many and depend on species and even on
strains.
Annex Fig. 4 shows only the major metabolic products.
The first conversion product identified in resistant houseflies
was DDE. This conversion is catalyzed by the enzyme DDT-dehydro-
chlorinase (EC 4.5.1.1) which had already been isolated in the pure
form in the 1950s. Further insect metabolites of DDT are DDD (isolated
for example from Stomoxys calcitrans), DDA (isolated from example
from Quiscalus quiscula, Heliothis virescens and Coleomegilla
maculata) and dichlorobenzophenone (from Leucophae). The
detoxiation of DDT in Triatoma infestans, Drosophila melanogaster,
Culex tarsalis, and other species is performed by hydroxylation and
results in kelthane (Annex Fig. 4, formula XV), a substance which is a
commercial acaricide. A great number of unidentified and water-soluble
conversion products of DDT was observed in many species as reviewed by
Klein & Korte (1970).
In a recent study on the photoisomerization and photodegradation
of DDE under simulated natural conditions (inert solvents, a good
hydrogen donor solvent, UV-light similar or equal 300 nm), the
compound VIII (Annex Fig. 2) with the 3 phenyl-bound chlorine atoms
was detected and characterized as a mixture of the E- and Z-isomers
which were separated and isolated. Both isomers were also found in
natural samples like tobacco and pine needles. DDMU was also detected
in these studies and 2 substances so far unknown, a tetrachlorinated
phenanthrene and a tetra-ring-chlorinated diphenylethylene; the
formation of tri- and tetrachlorobiphenyls was confirmed (Göthe et
al., 1976).
The behaviour of compounds in their adsorbed form is equally as
significant environmentally as their photochemical behaviour in the
gaseous and solid states.
Irradiation of DDE, adsorbed on silicagel, with wavelengths
> 230 nm, resulted in the formation of dichlorobenzophenone and its
trichlorinated analogue. Irradiation of DDT and DDE in the solid form
in an oxygen stream with wavelengths > 230 nm, resulted in partial
mineralization to give carbon dioxide and hydrochloric acid (Gab et
al., 1975).
The results presented here show that a large number of DDT-derived
chlorinated compounds must be included, when considering the possible
effects of DDT residues in the ecosphere.
2 Biotransformations Other Than Mammalian Metabolism
2.1 Birds
Two main pathways of DDT metabolism exist in mammals i.e.,
dehydrochlorination to DDE (Annex Fig. 3, formula VII) and stepwise
degradation to DDA (bis-[ p-chlorophenyl] acetic acid) via TDE (DDD)
(1,1-dichloro-2,2-bis [ p-chlorophenyl] ethane; Annex Fig. 3,
formula I). However, in birds, the pathway varies with species, and
data from studies on the administration of chronic and acute dosages
of DDT to pigeons, quail, and blackbirds show that DDE is the primary
metabolic product in the first 2 species, and TDE (DDD) in the third
(Bailey et al., 1972). The TDE-path-way does exist in the pigeon as a
minor pathway but, in contrast to mammals, only as far as DDMU (Annex
Fig. 3, formula III).
Thus, DDA, the degradation product of DDMU excreted by mammals, is
not formed in the pigeon (Bunyan et al., 1966; Bailey et al., 1969).
When its precursors in mammals, DDMS (1-chloro-2,2-bis[ p-
chlorophenyl] ethane; Annex Fig. 3, formula XI) and DDN U (1,1-bis
[ p-chlorophenyl] ethylene; Annex Fig. 3, formula XII) are
administered to the pigeon, they are rapidly converted: DDMS is
converted to DDMU, and DDNU is metabolized quickly and excreted as
DDNS (1,1-bis [ p-chlorophenyl] ethane; Annex Fig. 3, formula XIII),
a metabolite that was not found in mammals (Bailey et al., 1972). The
metabolic pathways for DDT in the pigeon are shown in Annex Fig. 3.
2.2 Insects
Investigations on the detoxication mechanism of DDT in insects are
interesting as regards the problem of resistance. In general, the
phenomenon of insect resistance is related to detoxication of the
insecticide by metabolization to nontoxic compounds. The metabolic
pathways of DDT in insects are many and depend on species and even on
strains.
Annex Fig. 4 shows only the major metabolic products.
The first conversion product identified in resistant houseflies
was DDE. This conversion is catalyzed by the enzyme DDT-dehydro-
chlorinase (EC 4.5.1.1) which had already been isolated in the pure
form in the 1950s. Further insect metabolites of DDT are DDD (isolated
for example from Stomoxys calcitrans), DDA (isolated from example
from Quiscalus quiscula, Heliothis virescens and Coleomegilla
maculata) and dichlorobenzophenone (from Leucophae). The
detoxiation of DDT in Triatoma infestans, Drosophila melanogaster,
Culex tarsalis, and other species is performed by hydroxylation and
results in kelthane (Annex Fig. 4, formula XV), a substance which is a
commercial acaricide. A great number of unidentified and water-soluble
conversion products of DDT was observed in many species as reviewed by
Klein & Korte (1970).
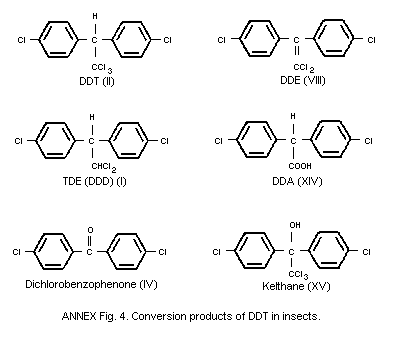 Although TDE (DDD) has not been found as a DDT-metabolite in
Culex tarsalis, it has been concluded from differences between
susceptible and resistant strains that it is an intermediate in the
further degradation of DDT. After application of TDE 14C to this
insect, DDMU and DDDOH (1,1-bis[ p-chlorophenyl]-2,2-dichloroethanol;
Annex Fig. 5, formula XVI) were found as major metabolites;
furthermore, 3 polar compounds were chromatographically identical with
DDA, DBH (dichlorobenzhydrole; Annex Fig. 5, formula XVIII) and PCBA
( p-chlorobenzoic acid; Annex Fig. 5, formula XVIII), which were also
observed after application of DDMU-14C (Plapp et al., 1965). The
occurrence of PCBA indicates the complete breakdown of one of the 2
rings of DDT and thus the possibility of a complete biological
degradation of the whole molecule.
2.3 Higher plants
Although the transformation of DDT in higher plants is rather
limited (2% in spinach within 18 days, 5% in cabbage within 14 weeks),
it must not be neglected since a considerable part of the DDT used on
a worldwide basis is applied, intentionally or unintentionally, to
plants. The conversion products that have been identified (Annex
Fig. 6) are DDE, TDE (DDD), DDMU, DDA, conjugates of DDA, and a
conjugate of DBH (Zimmer & Klein, 1972), which means that the
metabolites in plants are not chemically different from those in other
organisms.
In a study of the accumulation and distribution of p,p'-DDT in
an apple orchard, DDT residues in or on the roots and leaves of the
herbage and the roots, bark, leaves, and fruit of the trees were
recorded for an orchard sprayed annually (Stringer et al., 1975).
During 13 years, there were increasing amounts of DDE, TDE, and DDMU
in relation to DDT, in the bark of apple trees indicating some
breakdown on the bark (< 10%). DDE and TDE were also observed after
application of p,p'-DDT to cotton (Nash et al., 1977). These 2
substances seem to be common conversion products of DDT in plants. The
o,p'-DDT observed in the last 2 experiments seems to be an impurity
of the DDT rather than a metabolite.
2.4 Microorganisms and soil
The most common metabolic reaction of DDT in microorganisms is
reductive dechlorination resulting in the formation of TDE. This
reaction has been demonstrated in Escherichia coli (in rat
intestine), Aerobacter aerogenes, Proteus vulgaris, and in yeasts.
In contrast to metabolism in higher animals, dechlorination by
microorganisms is anaerobic and is catalysed by reduced cytochrome
oxidase (EC 1.9.3.1). Fe (II)-cytochrome oxidase isolated from
Aerobacter converts DDT to TDE in vitro (Klein & Korte, 1970). The
conversion of DDT to TDE (DDD) in bodies of water (Miskus et al.,
1965) and in other reducing environments characteristic of dead and
Although TDE (DDD) has not been found as a DDT-metabolite in
Culex tarsalis, it has been concluded from differences between
susceptible and resistant strains that it is an intermediate in the
further degradation of DDT. After application of TDE 14C to this
insect, DDMU and DDDOH (1,1-bis[ p-chlorophenyl]-2,2-dichloroethanol;
Annex Fig. 5, formula XVI) were found as major metabolites;
furthermore, 3 polar compounds were chromatographically identical with
DDA, DBH (dichlorobenzhydrole; Annex Fig. 5, formula XVIII) and PCBA
( p-chlorobenzoic acid; Annex Fig. 5, formula XVIII), which were also
observed after application of DDMU-14C (Plapp et al., 1965). The
occurrence of PCBA indicates the complete breakdown of one of the 2
rings of DDT and thus the possibility of a complete biological
degradation of the whole molecule.
2.3 Higher plants
Although the transformation of DDT in higher plants is rather
limited (2% in spinach within 18 days, 5% in cabbage within 14 weeks),
it must not be neglected since a considerable part of the DDT used on
a worldwide basis is applied, intentionally or unintentionally, to
plants. The conversion products that have been identified (Annex
Fig. 6) are DDE, TDE (DDD), DDMU, DDA, conjugates of DDA, and a
conjugate of DBH (Zimmer & Klein, 1972), which means that the
metabolites in plants are not chemically different from those in other
organisms.
In a study of the accumulation and distribution of p,p'-DDT in
an apple orchard, DDT residues in or on the roots and leaves of the
herbage and the roots, bark, leaves, and fruit of the trees were
recorded for an orchard sprayed annually (Stringer et al., 1975).
During 13 years, there were increasing amounts of DDE, TDE, and DDMU
in relation to DDT, in the bark of apple trees indicating some
breakdown on the bark (< 10%). DDE and TDE were also observed after
application of p,p'-DDT to cotton (Nash et al., 1977). These 2
substances seem to be common conversion products of DDT in plants. The
o,p'-DDT observed in the last 2 experiments seems to be an impurity
of the DDT rather than a metabolite.
2.4 Microorganisms and soil
The most common metabolic reaction of DDT in microorganisms is
reductive dechlorination resulting in the formation of TDE. This
reaction has been demonstrated in Escherichia coli (in rat
intestine), Aerobacter aerogenes, Proteus vulgaris, and in yeasts.
In contrast to metabolism in higher animals, dechlorination by
microorganisms is anaerobic and is catalysed by reduced cytochrome
oxidase (EC 1.9.3.1). Fe (II)-cytochrome oxidase isolated from
Aerobacter converts DDT to TDE in vitro (Klein & Korte, 1970). The
conversion of DDT to TDE (DDD) in bodies of water (Miskus et al.,
1965) and in other reducing environments characteristic of dead and
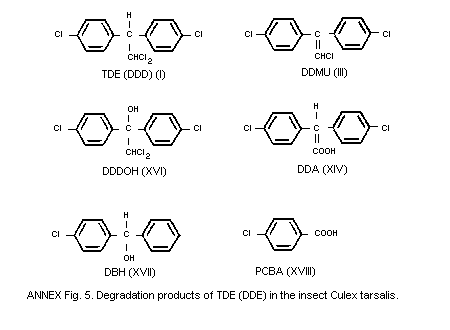
 decaying matter (Zoro et al., 1974) is mediated by reduced iron
porphyrins and is not an essential part of cell metabolism. These
findings have considerable environmental significance since most
living material contains iron porphyrins bound with protein in complex
molecules. The porphyrins are released after decay of the organic
substances, and may then be regarded as widespread environmental
agents that convert, on a larger scale, the persistent DDT to the less
persistent TDE. TDE is susceptible to further abiotic or biotic
degradation.
However, the formation of DDE and DDA from DDT by microorganisms
is also possible. For instance, both DDE and TDE were isolated from
Serratia marcescens and Alkaligenes faecalis (Stenersen, 1965) and
DDA was isolated from microbial cultures obtained from agricultural
soil (Patil et al., 1970).
In a model experiment with anaerobic activated sludge and p,p'-
DDT-14C, TDE, p,p'-dichlorobenzophenone, DDMU and a so far unknown
metabolite, DDCN (bis[ p-chlorophenyl] acetonitrile), were detected
as conversion products. The last of these substances, a minor
conversion product, was also found in the sediment layer of the Lake
Mälaren in Sweden (0.2 mg/kg dry weight). DDCN is formed via TDE or
DDE, but directly from DDT (Jensen et al., 1972).
The question of "bound residues" in soil, which is now under
discussion for a number of "non-persistent" pesticides, especially
those that are anilin-derived, seems also to be relevant for
"persistent" substances such as DDT, although the percentage of bound
residues is less than for less persistent pesticides. The formation of
25% of bound DDT-residues within 28 days (Lichtenstein et al., 1977)
justifies a reassessment of the persistence of DDT in soil. Further
information should be obtained concerning the nature and the potential
biological activity of the compounds that are bound.
3 Conclusion
A multitude of conversion products are formed from DDT under
environmental conditions. Nearly 20 of these (including mammalian
metabolites) have been identified so far, but the chemical structure
of a number of other compounds is still unknown. Very little is known
of the toxicological properties of these conversion products with the
exception of major products such as DDE and TDE. This should be
remembered when the unwanted effects of DDT in the environment are
evaluated. However, there is even less information concerning the fate
in the environment of many other pesticides including those that are
used as DDT-substitutes.
REFERENCES
BAILEY, S., BUNYAN, P. J., RENNISON, B. D., & TAYLOR, A. (1969) The
metabolism of 1,1-di( p-chlorophenyl)-2,2,2-trichlorethane and
1,1-di( p-chlorophenyl)-2,2-dichloroethane in the pigeon.
Toxicol. appl. Pharmacol., 14: 13-22.
BAILEY, S., BUNYAN, P. J., TAYLOR, A., & TOLWORTH, U. K. (1972) The
metabolism of p,p'-DDT in some avian species. Environ. Qual.
Saf., 1: 244.
BUNYAN, P. J., PAGE, J. M. J., & TAYLOR, A. (1966) In vitro
metabolism of p,p'-DDT in pigeon liver. Nature (Lond.),
210: 1048-1049.
GAB S., NITZ, S., PARLAR, H., & KORTE, F. (1975) [Contributions on
ecological chemistry CV. Photomineralization of certain aromatic
xenobiotica.] Chemosphere, 4: 251-256 (in German).
GOTHE, R., WACHTMEISTER, C. A., AKERMARK, B., BAECKSTROM, P., JANSSON,
B., & JENSEN, S. (1976) Photo-isomerisation and photo-degradation
of DDE. Tetrahedron Lett., pp. 4051-4504.
JENSEN, S., GOTHE, R., & KINDSTEDT, M.O. (1972) Bis- ( p-chlorophenyl-
acetonitrile (DDCN), a new DDT derivative formed in anaerobic
digested sewage sludge and lake sediment. Nature (Lond.),
240: 421-422.
KERNER, I., KLEIN, W., & KORTE, F. (1972) [Contributions on ecological
chemistry -- XXXIII. Photochemical reactions of 1.1-dichloro-2
( p,p'-dichlorophenyl) ethylene (DDE).] Tetrahedron Lett.,
28: 1575-1578 (in German).
KLEIN, A. K, LAUG, E. P., DATTA, P. R., WATTS, J. O., & COHEN, J. T.
(1964) Metabolites: Reductive dechlorination of DDT to DDD and
isomeric transformation of o,p'-DDT to p,p'-DDT in vitro. J.
Assoc. Off. Agric. Chem., 47: 1129-1145.
KLEIN, W. & KORTE, F. (1970) [Review: Metabolism of organochlorine
compounds.] In: Wegler, R., ed. [The chemistry of plant
protection and pest control agents.] Volume 1, pp. 199-218.
Berlin-Heidelberg-New York, Springer Verlag (in German).
LOCHTENSTEIN, E. P., KATAN, J., & ANDEREGG, B. N. (1977) Binding of
"persistent" and "nonpersistent" 14C-labelled insecticides in an
agricultural soil. J. agric. food Chem., 25: 43-47.
MISKUS, R. P., BLAIR, D., & CASIDA, J. E. (1965) Conversion of DDT to
DDD by bovine rumen fluid, lake water and reduced porphyries.
J. agric. food Chem., 13: 481-482.
MOILANEN, K. W. & CROSBY, D. G. (1973) DDT, an unrecognized source of
polychlorinated biphenyls. Science, 180: 578-579.
NASH, R. G., BEALL, M. L., & HARRIS, W. G. (1977) Toxaphene and 1,1,1-
trichloro-2,2-bis ( p-chlorophenyl) ethane (DDT) losses from
cotton in an agroecosystem chamber. J. agric. food Chem.
25: 336-341.
PATIL, K. C., MATSUMURA, F., & BOUSH, G. M. (1970) Degradation of
endrin, aldrin, and DDT by soil microorganisms. Appl. Microbiol.,
19: 879-881.
PLAPP, F. W., CHAPMAN, G. A., & MORGAN, J. W. (1965) DDT resistance in
Culex tarsalis Coquillett; Cross resistance to related compounds
and metabolic fate of a C14 labelled DDT analog. J. econ.
Entomol., 58: 1064-1069.
PLIMMER, J. R. & KLINGEBIEL, U. I. (1969) A photocyclization reaction
of dichloro-2,2-bis-( p-chlorophenyl) ethylene (DDE). Chem.
Commun., p. 648.
PLIMMER, J. R. & KLINGEBIEL, U. I. (1973) PCB-formation. Science,
181: (4104): 994-995.
PLIMMER, J. R., KLINGEBIEL, U. I., & HUMMER, B. E. (1970) Photo-
oxidation of DDT and DDE. Science, 167: 67-69.
STENERSEN, J. H. V. (1965) DDT metabolism in resistant and susceptible
flies and in bacteria, Nature (Lond.), 707: 660-661.
STRINGER, A., PICKARD, J. A., & LYON, C. H. (1965) Accumulation and
distribution of p,p'-DDT and related compounds in an apple
orchard. II. Residues in trees and herbage. Pestic. Sci.,
6: 223-232.
ZIMMER, M. & KLEIN, W. (1972) [Contributions on ecological chemistry
XXXVII. Behaviour of residues and transformations of p,p'-DDT-
14C and its analogues p,p'-DDT-14C and p,p'-DDD-14C in
higher plants.] Chemosphere, 1: 3-6 (in German).
ZORO, J. A., HUNTER, J. M., EGLINTON, G., & WARE, G. C. (1974)
Degradation of p,p'-DDT in reducing environments. Nature
(Lond.), 247: 235-236.
decaying matter (Zoro et al., 1974) is mediated by reduced iron
porphyrins and is not an essential part of cell metabolism. These
findings have considerable environmental significance since most
living material contains iron porphyrins bound with protein in complex
molecules. The porphyrins are released after decay of the organic
substances, and may then be regarded as widespread environmental
agents that convert, on a larger scale, the persistent DDT to the less
persistent TDE. TDE is susceptible to further abiotic or biotic
degradation.
However, the formation of DDE and DDA from DDT by microorganisms
is also possible. For instance, both DDE and TDE were isolated from
Serratia marcescens and Alkaligenes faecalis (Stenersen, 1965) and
DDA was isolated from microbial cultures obtained from agricultural
soil (Patil et al., 1970).
In a model experiment with anaerobic activated sludge and p,p'-
DDT-14C, TDE, p,p'-dichlorobenzophenone, DDMU and a so far unknown
metabolite, DDCN (bis[ p-chlorophenyl] acetonitrile), were detected
as conversion products. The last of these substances, a minor
conversion product, was also found in the sediment layer of the Lake
Mälaren in Sweden (0.2 mg/kg dry weight). DDCN is formed via TDE or
DDE, but directly from DDT (Jensen et al., 1972).
The question of "bound residues" in soil, which is now under
discussion for a number of "non-persistent" pesticides, especially
those that are anilin-derived, seems also to be relevant for
"persistent" substances such as DDT, although the percentage of bound
residues is less than for less persistent pesticides. The formation of
25% of bound DDT-residues within 28 days (Lichtenstein et al., 1977)
justifies a reassessment of the persistence of DDT in soil. Further
information should be obtained concerning the nature and the potential
biological activity of the compounds that are bound.
3 Conclusion
A multitude of conversion products are formed from DDT under
environmental conditions. Nearly 20 of these (including mammalian
metabolites) have been identified so far, but the chemical structure
of a number of other compounds is still unknown. Very little is known
of the toxicological properties of these conversion products with the
exception of major products such as DDE and TDE. This should be
remembered when the unwanted effects of DDT in the environment are
evaluated. However, there is even less information concerning the fate
in the environment of many other pesticides including those that are
used as DDT-substitutes.
REFERENCES
BAILEY, S., BUNYAN, P. J., RENNISON, B. D., & TAYLOR, A. (1969) The
metabolism of 1,1-di( p-chlorophenyl)-2,2,2-trichlorethane and
1,1-di( p-chlorophenyl)-2,2-dichloroethane in the pigeon.
Toxicol. appl. Pharmacol., 14: 13-22.
BAILEY, S., BUNYAN, P. J., TAYLOR, A., & TOLWORTH, U. K. (1972) The
metabolism of p,p'-DDT in some avian species. Environ. Qual.
Saf., 1: 244.
BUNYAN, P. J., PAGE, J. M. J., & TAYLOR, A. (1966) In vitro
metabolism of p,p'-DDT in pigeon liver. Nature (Lond.),
210: 1048-1049.
GAB S., NITZ, S., PARLAR, H., & KORTE, F. (1975) [Contributions on
ecological chemistry CV. Photomineralization of certain aromatic
xenobiotica.] Chemosphere, 4: 251-256 (in German).
GOTHE, R., WACHTMEISTER, C. A., AKERMARK, B., BAECKSTROM, P., JANSSON,
B., & JENSEN, S. (1976) Photo-isomerisation and photo-degradation
of DDE. Tetrahedron Lett., pp. 4051-4504.
JENSEN, S., GOTHE, R., & KINDSTEDT, M.O. (1972) Bis- ( p-chlorophenyl-
acetonitrile (DDCN), a new DDT derivative formed in anaerobic
digested sewage sludge and lake sediment. Nature (Lond.),
240: 421-422.
KERNER, I., KLEIN, W., & KORTE, F. (1972) [Contributions on ecological
chemistry -- XXXIII. Photochemical reactions of 1.1-dichloro-2
( p,p'-dichlorophenyl) ethylene (DDE).] Tetrahedron Lett.,
28: 1575-1578 (in German).
KLEIN, A. K, LAUG, E. P., DATTA, P. R., WATTS, J. O., & COHEN, J. T.
(1964) Metabolites: Reductive dechlorination of DDT to DDD and
isomeric transformation of o,p'-DDT to p,p'-DDT in vitro. J.
Assoc. Off. Agric. Chem., 47: 1129-1145.
KLEIN, W. & KORTE, F. (1970) [Review: Metabolism of organochlorine
compounds.] In: Wegler, R., ed. [The chemistry of plant
protection and pest control agents.] Volume 1, pp. 199-218.
Berlin-Heidelberg-New York, Springer Verlag (in German).
LOCHTENSTEIN, E. P., KATAN, J., & ANDEREGG, B. N. (1977) Binding of
"persistent" and "nonpersistent" 14C-labelled insecticides in an
agricultural soil. J. agric. food Chem., 25: 43-47.
MISKUS, R. P., BLAIR, D., & CASIDA, J. E. (1965) Conversion of DDT to
DDD by bovine rumen fluid, lake water and reduced porphyries.
J. agric. food Chem., 13: 481-482.
MOILANEN, K. W. & CROSBY, D. G. (1973) DDT, an unrecognized source of
polychlorinated biphenyls. Science, 180: 578-579.
NASH, R. G., BEALL, M. L., & HARRIS, W. G. (1977) Toxaphene and 1,1,1-
trichloro-2,2-bis ( p-chlorophenyl) ethane (DDT) losses from
cotton in an agroecosystem chamber. J. agric. food Chem.
25: 336-341.
PATIL, K. C., MATSUMURA, F., & BOUSH, G. M. (1970) Degradation of
endrin, aldrin, and DDT by soil microorganisms. Appl. Microbiol.,
19: 879-881.
PLAPP, F. W., CHAPMAN, G. A., & MORGAN, J. W. (1965) DDT resistance in
Culex tarsalis Coquillett; Cross resistance to related compounds
and metabolic fate of a C14 labelled DDT analog. J. econ.
Entomol., 58: 1064-1069.
PLIMMER, J. R. & KLINGEBIEL, U. I. (1969) A photocyclization reaction
of dichloro-2,2-bis-( p-chlorophenyl) ethylene (DDE). Chem.
Commun., p. 648.
PLIMMER, J. R. & KLINGEBIEL, U. I. (1973) PCB-formation. Science,
181: (4104): 994-995.
PLIMMER, J. R., KLINGEBIEL, U. I., & HUMMER, B. E. (1970) Photo-
oxidation of DDT and DDE. Science, 167: 67-69.
STENERSEN, J. H. V. (1965) DDT metabolism in resistant and susceptible
flies and in bacteria, Nature (Lond.), 707: 660-661.
STRINGER, A., PICKARD, J. A., & LYON, C. H. (1965) Accumulation and
distribution of p,p'-DDT and related compounds in an apple
orchard. II. Residues in trees and herbage. Pestic. Sci.,
6: 223-232.
ZIMMER, M. & KLEIN, W. (1972) [Contributions on ecological chemistry
XXXVII. Behaviour of residues and transformations of p,p'-DDT-
14C and its analogues p,p'-DDT-14C and p,p'-DDD-14C in
higher plants.] Chemosphere, 1: 3-6 (in German).
ZORO, J. A., HUNTER, J. M., EGLINTON, G., & WARE, G. C. (1974)
Degradation of p,p'-DDT in reducing environments. Nature
(Lond.), 247: 235-236.
 (many of the compounds also exist as o,p'-isomers and other isomers)
Name DDT Chemical name R R' R"
and its major
metabolites
DDT 1,1'-(2,2,2-trichloroethylidene)- -Cl -H -CCl3
bis[4-chlorobenzene]
DDEa 1,1'-(2,2-dichloroethenylidene)- -Cl None =CCl2
bis[4-chlorobenzene]
TDE(DDD)a,b 1,1'-(2,2-dichloroethylidene)- -Cl -H -CHCl2
bis[4-chlorobenzene]
DDMUa 1,1'-(2-chloroethenyldene)- -Cl None =CHCl
bis[4-chlorobenzene]-
DDMSa 1,1'-(2-chtoroethylidene)- -Cl -H -CH2Cl
bis[4-chlorobenzene]
DDNUa 1,1'-bis(4-chlorophenyl)ethylene -Cl None =CH2
DDOHa 2,2-bis(4-chlorophenyl)ethanol -Cl -H -CH2OH
DDAa 2,2-bis(4-chlorophenyl)- -Cl -H -C(O)OH
acetic acid
Table 1 (Cont'd)
Name DDT Chemical name R R' R"
and its major
metabolites
Some related insecticides
NO2
Bulan(R) 2-nitro-1,1 -bis- -Cl -H '
(4-chlorophenyl)butane -CHC2H5
NO2
Prolan(R) 2-nitro-1,1-bis- -Cl -H '
(4-chlorophenylpropane -CHCH2
DMC 4-chloro-alpha[-(4-chlorophenyl)- -Cl -OH -CH3
alpha-(methyl)benzenemethanol
dicocol 4-chloro-alpha-(4-chlorophenyl)-alpha- -Cl -OH -CCl3
(Kelthane(R)) (trichloromethyl)benzenemethanol
chlorobenzilatec ethyl 4-chloro-alpha-(4-chlorophenyl)- -Cl -OH -C(O)OC2H5
alpha-hydroxybenzeneacetate
chloropropopylatc 1-methylethyl 4-chloro-alpha- -Cl -OH -C(O)OCH(CH3)2
(4-chlorophenyl)-aplha-hydroxy-
benzeneacetate
methoxychlorc 1,1'-(2,2,2-trichloroethylidene)- -OCH3 -H -CCl3
bis[4-methoxybenzene]
Table 1 (Cont'd)
Name DDT Chemical name R R' R"
and its major
metabolites
Perthane(R) 1,1'-(2,2-dichloroethylidene)- -C2H5 -H -CHCl2
bis[4-ethylbenzene]
DFDT 1,1'-(2,2,2-trichloroethylidene)- -F -H -CCl3
bis[4-fluorobenzene]
a Recognized metabolite of DDT in the rat.
b As an insecticide, this compound has the ISO approved name of TDE, and it has been sold
under the name Rothane(R); in metabolic studies the same compound has been referred to as
DDD; as a drug, it is called mitotane.
c Common name approved by the International Organization for Standardization (ISO).
and analogues that have had some use as insecticides. It must be
emphasized that even the commercially-available insecticidal analogues
have strikingly different properties. Especially remarkable is the
slow metabolism and marked storage of DDT and its metabolite DDE and
the rapid metabolism and negligible storage of methoxychlor.
No attempt has been made to include in Table 1 the wide range of
compounds that have been synthesized and studied in connexion with
structure-activity relationships, often with the hope of emphasizing
the good properties of DDT and reducing its undesirable properties.
For a more extensive consideration of analogues, see Metcalf (1955).
The formation of metabolites is considered in section 6.4.
2.1.3 Formulations of commercial or technical DDT
Technical DDT has been formulated in almost every conceivable form
including solutions in xylene or petroleum distillates, emulsifiable
concentrates, water-wettable powders, granules, aerosols, smoke
candles, charges for vaporizers, and lotions. Aerosols and other
household formulations are often combined with synergized pyrethrins.
When used as a drug, DDT is called clofenotane (INN) or Dicophane
(British Pharmacopoeia), Klorfenoton (Swedish Pharmacopoeia),
Chlorophenothane (United States Pharmacopoeia). For research or
reference it has been designated OMS 0016 and Ent. 1,506. DDT has been
sold under a variety of tradenames, including: Anofex(R), Cezarex(R),
Dinocide(R), Gesarol(R), Guesapon(R), Guesarol(R), Gyron(R),
Ixodex(R), Neocid(R), Neocidol(R), and Zerdane(R).
2.2 Analytical Procedures
Analytical procedures for determining residues of DDT-type
compounds in environmental samples involve several steps including
collection and extraction of the DDT-type compounds; removal of
coextractives by appropriate clean-up methods; and quantification of
p,p'-DDT and its analogues by a suitable technique. Each of these
major steps is discussed later. It is appropriate, however, first to
outline briefly the statistical criteria used to assess analytical
methods, the estimation of the lower limit of detection of a method,
and the procedure for confirming the chemical identities of the
components measured.
2.2.1 Statistical criteria for assessing analytical methods
The overall reliability of an analytical method can be assessed
using two criteria, namely, reproducibility and systematic error (or
bias). Reproducibility is both conceptually and practically the
simpler criterion; it may be defined as "the quantitative expression
of the random error associated with operators working in different
laboratories, each obtaining single results on identical test material
when applying the same method" (Institute of Petroleum, 1968). It may
be quantitatively specified in various ways, and it is important to
pay attention to the statistic (range standards, deviation, etc.) used
in a particular study to represent the random error of an analytical
method. If the results are normally distributed then the most
efficient statistic measuring reproducibility is the standard
deviation of a set of results (Nalimov, 1963; Youden & Steiner, 1975;
Davies & Goldsmith, 1976). Care should always be taken to ensure that
a particular statistic or statistical technique is appropriate for a
given set of results, by, for example, testing for outliers or, if
there are sufficient results, examining the distribution of the
results, before characterizing the reproducibility of an analytical
method.
There are two subdivisions of the reproducibility criterion,
namely, replication i.e., two or more results, obtained by the same
operator in a given laboratory using the same apparatus for successive
determinations on identical test material, within a short period of
time on the same day; and repeatability i.e., a quantitative
expression of the random error associated in the long run with a
single operator in a given laboratory obtaining successive results
with the same apparatus under constant operating conditions on
identical test material (Institute of Petroleum, 1968).
Reproducibility is, in turn, a subdivision of the random error in the
analysis of identical test material in different laboratories using
different techniques or variations of a particular method. Examples of
the assessment of the random errors found in the determination of
p,p'-DDT and related compounds are given below.
The systematic error of a method is the deviation of the
experimental results from the "true" values; such systematic error
causes the differences between the nominal value and the
experimentally-determined values to have predominantly the same sign
(as opposed to the random errors, where the results are equally likely
to be greater than or less than the true mean). Nominal or "true"
values are available only in the case of fortified (spiked) samples,
but whether such fortified samples are representative of actual
(environmentally incurred) contamination is open to doubt in the case
of some types of material. A rapid nonparametric test of systematic
error can be made using the sign-test or the Wilcoxan signed ranks
test (Conover, 1971).
It is common practice to calculate the "recovery" factor for an
analytical method, i.e., the ratio of the mean observed value to the
nominal value (usually expressed as a percentage), but the statistical
significance of the "recovery" factor should always be assessed. The
ratio of the mean deviation to the standard error of the mean
deviation is an appropriate method of testing the null-hypothesis that
the difference between the nominal value and the observed mean is not
statistically significant.
The term "total error" has been proposed for a function
incorporating both the systematic error and the reproducibility
(McFarren et al., 1970), and these authors also suggested three
classes of total error corresponding to excellent methods, acceptable
methods, and methods that are judged unacceptable. It is pertinent
that McFarren et al. concluded that the total errors in one study of
the determination of DDT-type compounds in water were unacceptable
(>50% for p,p'-DDT, and p,p'-DDE, 24.0-53.6% for o,p'-DDT).
2.2.2 Limit of analytical detection
All analytical determinations have a lower limit corresponding to
that quantity (Delta g) of p,p'-DDT (or a related compound) which
produces a response (Delta r) that cannot be distinguished from
response (Delta r) produced when no p,p'-DDT is present. The
response Delta r is generated by the materials, reagents, and
instruments, used in the procedure, for example, the small voltage
generated by electronic equipment, or the small absorption in a
spectrophotometric method. These responses are known as "blank" or
"noise", and their size depends on the presence of interfering
components in the test material, the purity of the reagents, the
cleanliness of the apparatus, and the design of electronic equipment
(amplifiers, etc.). The concept of limit of detection is a statistical
one and is related to the random variation of the response generated
by a blank or control (Sutherland, 1965; Skogerboe & Grant, 1970;
Kaiser, 1973). Currie (1968) has defined three limiting levels for use
in analytical chemistry: "the net signal level" above which an
observed signal may be reliably recognized; "the true net signal
level" which may, a priori, be expected to lead to detection; and
"the quantifiable level" at which the measurement precision is
sufficient for the quantity present to be estimated satisfactorily. In
many types of sample, the limit of detection is of rather academic
interest as the concentrations of DDT-type compounds in samples are an
order of magnitude greater than the limit of detection of a sensitive
detector such as the electron-capture detector. However, in analyses
of air and drinking-water, for example, it may be necessary to
ascertain the limit of detection by a suitable statistical procedure.
Lower limits of detectability (ng.g-1) suggested in the US EPA
Manual of Analytical Methods (Thompson, 1974) for DDT-type compounds
using gas-liquid chromatography are:
o,p'-DDE Adipose tissue, 10; serum, 1
p,p'-DDE Adipose tissue, 20; serum, 1
o,p'-DDT)
p,p'-TDE) Adipose tissue, 20; serum, 2
p,p'-DDT)
2.2.3 Confirmation of the identity of trace residues of DDT-type
compounds
Confirmation of the identity of p,p'-DDT and related compounds
in many types of environmental samples is not easy when the apparent
amounts present are in the microgram and submicrogram range, since the
classical procedures for the identification of organic compounds
require the use of milligrams of the purified compound. A definition
of chemical identity appropriate to trace analysis has been proposed
(Robinson et al., 1966), a definition that may need revision in
relation to the combined use of gas-liquid chromatography and mass-
spectrometry. Chemical derivatization techniques for DDT-type
compounds have been reviewed by Cochrane & Chau (1971). Other
techniques, that are used routinely in the confirmation of the
identity of DDT-type compounds include: determination of gas-liquid
chromatographic retention times using polar and nonpolar stationary
phases; thin-layer chromatography; paper chromatography; p-values;
infrared microtechniques; carbon skeleton chromatography; conversion
into dichlorobenzophenones; nuclear magnetic resonance; and X-ray
diffraction. Tables of the relative retention times (aldrin = 1.00) of
DDT-type compounds using nine liquid phases (Thompson et al., 1975)
and three mixed liquid phases (Suzuki et al., 1975); gas-liquid
chromatographic retention times for DDT-type compounds are also
summarized by Yermakov (1972). Relative thin-layer chromatographic
Rf values ( p,p'-DDE = 1.00) on alumina using three solvent systems
were reported by Thomas et al. (1968). Extraction p-values for
DDT-type compounds using seven solvent systems are given in the US EPA
Manual (Thompson 1974). Identification of DDT and its metabolites by a
microinfrared technique has been studied by Sierwiski & Helrich (1967)
and reference infrared spectra have been published (Chen et al.,
1972). Asai et al. (1971) used carbon skeleton chromatography to
differentiate polychlorinated biphenyls from DDT-type compounds. The
conversion of DDT-type compounds into the corresponding benzophenones
may be used to identify them in the presence of polychlorinated
biphenyls (Miles, 1972), but this technique has its drawbacks as
p,p'-DDT, p,p'-TDE and p,p'-DDE all give the same p,p'-
dichlorobenzophenone. The use of high resolution nuclear magnetic
resonance spectroscopy (with a time averaging computer to increase
sensitivity), for the confirmation of the presence of p,p'-DDT and
p,p'-DDE in human adipose tissue (total concentration about
13 mg/kg) was studied by Biros (1970).
The combination of gas-liquid chromatography with mass-
spectrometry is a powerful tool for the confirmation of identity of
trace residues of DDT-type compounds. For example, Gordon & Frigerio
(1972) used mass fragmentography and claimed identification of 10 pg
p,p'-DDT; Schaeffer (1974) identified DDT-type compounds in fish
using a fast scan mass-spectrometer to give a multiple mass spectrum
for each of the overlapping peaks.
2.2.4 Sampling and extraction
Before discussing different kinds of samples, it must be noted
that valuable information on many analytical problems may be found in
the US EPA Manual (Thompson, 1974) and the FDA Manual (McMahon &
Sawyer, 1977).
DDT-type compounds may be present in the air in vapour form or
adsorbed on particulate matter. Glass-fibre filters are suitable for
trapping the particulate matter (Stanley et al., 1971; Beyermann &
Eckrich, 1974) and DDT in the vapour form may be trapped using
impingers of the Greenburg-Smith type and a suitable nonvolatile
solvent using ethylene glycol. Miles et al. (1970) reported a trapping
efficiency of more than 99% for DDT. A three-trap system in series
comprising a column containing glass cloth, followed by an impinger
with hexylene glycol, and finally an alumina column was used by
Stanley et al. (1971). They estimated that the collection efficiency
(based on material balance studies) of this system for a mixture of
aerosol and vapour was about 60% for p,p'-DDE, 95% for p,p'-TDE,
and 100% for p,p'-DDT. Beyermann & Eckrich (1974) used glass wool
for the trapping of aerosols, and a stainless-steel net coated with
polyethylene glycol for trapping vapours. Trapping of organochlorine
insecticides in the vapour phase using support-bonded silicones on
various types of Chromosorb was considered to be quantitative by Aue &
Teli (1971): DDT-type compounds were not examined but the trapping of
lindane, aldrin, and heptachlor was considered satisfactory. A cross-
linked polystyrene resin, Chromosorb 102, was used by Thomas & Serber
(1974). These workers reported a collection efficiency of 98% for
o,p'-DDT at a concentration of 15 ng/m3. Herzel & Lahmann (1973)
used silica gel as the support for various liquid phases and concluded
that polyethylene glycol was the best absorbent for DDT-type compounds
in the air. Absolute calibration of the collection efficiency of
aerosols and atmospheres for DDT-related compounds is difficult, but
the studies that have been made indicate that impingers with ethylene
glycol or hexylene glycol are probably the most efficient. The
simplest empirical test of the efficiency of the trapping system is to
use two (or more) impingers in series and analyse the liquid
absorbents from the impingers separately. The ratio of the amounts
present in the first and second impingers should be higher than 10:1.
Detailed instructions for the sampling and analysis of air for
pesticides (including DDT-type compounds) by a method that has been
subjected to interlaboratory study are given in the US EPA Manual
(Thompson, 1974).
For the determination of DDT-type compounds in water an
uncontaminated container may be filled, but if the concentrations of
DDT are likely to be extremely low (as would be expected in most
potable waters), then drawing the water through a suitable trapping
device may he more appropriate; this method also gives a time-weighted
average concentration and can be used in an automated monitoring
procedure.
Benzene was used by Pionke et al. (1968) as the solvent to extract
p,p'-DDT and p,p'-TDE from fortified samples of distilled water
and lake waters. The levels of fortification were high (µg/litre) and
the recoveries were: p,p'-DDT, 96.1% ± 1.02%; p,p'-TDE 97.3% ± 0.89%.
Thus, at concentrations of the order of 0.001 mg/litre, the
benzene extraction method gives excellent recoveries (total errors,
5.9% and 4.5%, respectively). A continuous flow method based
on liquid-liquid partition was developed by Ahling & Jensen (1970);
water is passed through a column containing Chromosorb W coated with
undecane plus a macrogol Carbowax 4000 monostearate. The best
collection efficiencies were found when the two liquid absorbents were
used at concentrations of 10% and 30%, respectively, on the solid
support. At concentrations in the ng/litre range, the optimum recovery
of known amounts of DDT-type compounds added to water were obtained
with 1.5 g coated Chromosorb W per litre of water. Ahnhoff & Josefson
(1974) used continuous flow liquid-liquid partition between water and
cyclohexane (in three extractors in series). The extraction
efficiencies from water containing 5 ng p,p'-TDE or 7.6 ng p,p'-DDT
per litre were greater at a flow rate of 2 litre/h than at
5 litre/h being 90% and 98% for p,p'-DDT, and p,p'-TDE,
respectively, with 92% and 93%, respectively, of the recovered
compounds being found in the first extractor. A method using a solid
adsorbent, a macroreticular resin, XAD-4, has been studied by Musty &
Nickless (1974); at a flow rate of 8 ml/min through a column
containing 2 g XAD-4, satisfactory recoveries of p,p'-DDT and three
related compounds were obtained at concentrations of 2-10 ng-litre.
Carbon is a very efficient adsorber of p,p'-DDT and p,p'-TDE from
water (Rosen & Middleton, 1959), but desorption from charcoal is
difficult; furthermore, the recoveries are not very satisfactory and
this is attributed to chemical changes catalysed by carbon in contact
with water rather than inefficient desorption (Eichelberger &
Lichtenberg, 1971). Another solid adsorbent is polyethylene (Beyermann
& Eckrich, 1973), but in the case of river water variable results were
obtained because of the effects of other solutes. Taylor & Bogacka
(1968) used petroleum ether to extract DDT from water; following a
clean-up with acetonitrile partition and Florisil the overall recovery
(using thin-layer chromatography) was incomplete (about 66%). The
total error of this procedure is unacceptable. Benzene was used as the
extraction solvent by Djatlovitskaja et al. (1972), and a general
review of the determination of organochlorine insecticides (and other
insecticides) in water has been published by Novikova (1973).
The need for scrupulous cleanliness in glassware and purity of
reagents cannot be overstressed in the case of the determination of
DDT-type compounds in air and water as the residues are usually so
small; the use of electronic equipment with a low noise characteristic
and constant checking of the response of detectors are also necessary.
A major difficulty in the determination of organochlorine
insecticides in soils is the initial extraction of the residues
because of a combination of factors, including the wide variation in
soil types. Chiba (1969) emphasized the lack of precision in defining
soil types. In his review article, he concluded that the most
effective solvent systems for extraction of these compounds were
mixtures of n-hexane/acetone (1:1 v/v) or chloroform/methanol
(1:1 v/v), and that the moisture content of the soil should be at
least 5%. The results of a study of the determination of
organochlorine insecticides in three types of soil have been
summarized by Woolson & Kearney (1969). Twelve laboratories
participated using various extraction procedures. In one type of soil
(a silty clay loam) that was fortified with p,p'-DDT at a
concentration of 5 mg/kg, the amounts found in the different
laboratories varied between 1.60 and 5.48 mg/kg. The results of 3 of
the laboratories were discarded by Woolson & Kearney (1969), and the
mean recovery of the other 9 laboratories was 79.3%, with a standard
deviation of 42.2%. Wetting of the soil before extraction was
considered to improve the recoveries, Soxhlet extraction appeared to
be preferable to shaking with the solvent, and hexane/acetone
(1:1 v/v) or hexane/isopropanol (3:1 v/v) appeared better extractants
than other solvents.
A further collaborative study of the determination of organochlo-
rine insecticides in 3 types of soil was reviewed by Woolson (1974).
Although 12 laboratories participated, only 7 completed the study. All
the laboratories used the same extraction, clean-up and quantification
procedures, including premoistening of the soil with ammonium chloride
solution at 0.2 mol/litre. The recoveries of p,p'-DDE, p,p'-TDE,
o,p'-DDT and p,p'-DDT were higher than 80%, with standard errors
of 8-18%. These results are much more consistent than those of the
previous study, and Woolson recommended that premoistening of the soil
with ammonium chloride solution (0.2 mol/litre) followed by extraction
with hexane/acetone (1:1 v/v) should be adopted for the determination
of chlorinated hydrocarbon insecticides in soil.
Several solvents have been used for the extraction of DDT-type
compounds from nonfatty foods such as vegetables, fruit, and cereals;
acetonitrile, alone or mixed with water is used in the method of the
Association of Official Analytical Chemists (AOAC) depending on the
water content of the sample (Horwitz, 1975); Cieleszky et al. (1970)
recommend Soxhlet extraction with diethyl ether.
Maceration of vegetables with a mixture of acetone and hexane was
used by Sissons et al., 1968; the same mixed solvent was used for
cereals, and root vegetables by Abbott et al., 1969 who used
propan-2-ol in the case of fruit and green vegetables. Whiting et al.
(1968), and Skrentny & Dorough (1971) considered a mixture of methanol
and chloroform to be the most efficient extraction solvent for use
with a macerator or a Soxhlet extractor. The acetonitrile extraction
technique was examined by Zerber et al. (1971) for the extraction of
DDT-type compounds from cereal products and feeding stuffs. Diethyl
ether was used by Kucinski (1972) for extraction from canned vegetable
products.
Extraction methods for fat-containing foods are given in the AOAC
Method (Horwitz, 1975), the solvent used, methanol or petroleum ether,
being dependent on the type of sample. Soxhlet extraction using
petroleum ether is recommended by the Federal Health Office of the
Federal Republic of Germany (Anon., 1974); Cieleszky et al. (1970)
also recommended petroleum ether as a suitable solvent.
Smart et al. (1974) compared acetonitrile and dimethyl formamide
as extraction solvents for apples, carrots, potatoes, and vegetables;
a third solvent dimethylsulfoxide was compared with these two solvents
for butter, cheese, and eggs.
Three body tissues or fluids have been analysed in many surveys,
namely adipose tissue, blood (or serum), and mother's milk. The US EPA
Manual (Thompson, 1974) recommends grinding a sample of adipose tissue
with anhydrous sodium sulfate before extraction with petroleum ether;
carbon tetrachloride has been used as a solvent (Mattson et al.,
1953), but it is not appropriate if the final quantification step
involves gas-liquid chromatography and a halogen sensitive detector.
Extraction of DDT-type compounds from blood or serum using hexane has
been described (Dale et al., 1966b), but this extraction procedure is
inefficient, probably as a result of binding by serum proteins. Dale
et al. (1970) investigated a procedure in which the serum was treated
with 97% formic acid before extraction with hexane; experiments using
14C-DDT indicated that extraction with hexane alone gave results
some 40% lower (based on 14C activity) than when the serum was first
treated with formic acid. A modified procedure in which whole blood
was treated with 60% sulfuric acid, and then extracted with a
hexane/acetone (9:1) mixture has been reported by Stretz & Starr
(1973). Samples of blood spiked at 4 different levels with p,p'-DDT
were analysed by 11 different laboratories; considerable discrepancies
were found between laboratories and a further study of the method was
considered desirable. Griffith & Blanke (1974) also investigated the
sulfuric acid method, but a microcoulometric detector was used instead
of an electron-capture detector. According to these workers,
consistent recoveries of p,p'-DDT, p,p'-TDE and p,p'-DDE were
obtained in their laboratory but reproducibility between laboratories
was not studied.
The US EPA Manual (Thompson, 1974) method for the extraction of
DDT-type compounds from human milk involves an acetonitrile/hexane
type extraction. A method for extraction from cow's milk, that makes
use of the stability of p,p'-DDT and its derivatives in the presence
of concentrated sulfuric acid, has been published by Coha & Nedic
(1970); the mixture of milk and concentrated sulfuric acid is
extracted with hexane. Prouty & Cromartie (1970) studied the
recoveries of 14C-DDT in each of the 5 major stages of a method for
determining this compound in the tissues of quail; Soxhlet extraction
with hexane for 6 h, of muscle, liver, heart or brain, after grinding
with sodium sulfate gave recoveries of DDT-type compounds (as 14C-
activity) of 93-105%. Jonczyk (1970) used hexane to extract DDT-type
compounds from blood and reported recoveries of 67-91%. Acetone was
used as the extracting solvent for DDT-type compounds in the adipose
tissue and brain of partridges (Jonczyk et al., 1970). Wood's method,
using dimethyl sulfoxide, was used by Stec & Juszkiewicz (1972), and
was found to give results for DDT-type compounds that compared
favourably with other methods of analysis of animal tissue, eggs, and
milk.
All methods of extracting DDT also result in the removal of
lipids, if they are present. Regardless of the method of extraction,
the results of most analyses have been reported in terms of fresh or
wet weight of samples no matter whether their lipid content was
extremely low (water and urine), low (soils and most vegetables),
intermediate (milk and many tissues), or high (adipose tissue). In
some instances, samples such as adipose tissue, milk, and, to a lesser
degree, other animal tissues known to contain lipids have been
reported in terms of the concentration of pesticide in extractable
lipid. Because DDT and DDE are known to have a marked affinity for
neutral fat, it was originally supposed that reporting in terms of
lipid would reduce variability within any set of samples by excluding
the influence of connective (and sometimes lymphatic) tissue, which
forms a part of each sample. It appears that variation, as measured by
the coefficient of variation, may not be reduced by this kind of
reporting (Casarett et al., 1968). However, there may be other reasons
for reporting on a lipid basis and it is absolutely essential that the
method of reporting be specified.
2.2.5 Clean-up procedures
The extraction procedures remove not only the DDT-type compounds
from the samples analysed but also coextractives to a greater or
lesser degree according to the type of sample. A number of procedures
have been developed that reduce the amounts of the coextractives
relative to that of DDT-type compounds. If interest is confined solely
to DDT-type compounds, then their stability in the presence of
concentrated sulfuric acid is a very useful clean-up procedure
(Mattson et al., 1953; Czegledi-Janko & Cieleszky, 1968; Murphy,
1972). Usually, however, the concentrations of other compounds are
also of interest and this procedure cannot be used if these compounds
are not stable in concentrated sulfuric acid. Two general clean-up
procedures, used in sequence, are appropriate in these circumstances,
namely, liquid-liquid partition followed by liquid-solid partition.
Liquid-liquid partition systems such as hexane/acetonitrile,
hexane/dimethyl formamide, or hexane/dimethyl sulfoxide are the ones
most commonly used. The liquid-solid partition systems generally
consist of Florisil, silica gel, or alumina as the solid phase, and
hexane or mixtures of hexane and various proportions of a polar
solvent (e.g., diethyl ether) as the mobile phase. Separation of
DDT-type compounds from triglycerides in fat-containing tissues is
achieved with considerable efficiency by liquid-liquid partitions, the
hexane/dimethyl formamide or hexane/dimethyl sulfoxide systems being
generally more efficient than hexane/acetonitrile. Separation of
DDT-type compounds from other organochlorine compounds (e.g., aldrin,
dieldrin, polychlorinated aromatics) or from steroids is not very
efficient using liquid-liquid systems, and the liquid-solid partition
systems should be used in these cases. Separation of DDT-type
compounds from polychlorinated biphenyls is particularly difficult
and, as some of these compounds have similar liquid chromatographic
retention times to those of the various DDT-type compounds, the
analysis of samples containing both classes of compounds requires
considerable care. Detailed clean-up procedures are described by de
Faubert Maunder et al. (1964), Cieleszky et al. (1970), Anon. (1974),
Thompson (1974), and by Horwitz (1975).
The separation of DDT-type compounds from polychlorinated
biphenyls, by liquid-solid (silical gel) partition is discussed by
Armour & Burke (1970), Snyder & Reinert (1971), and Masumoto (1972);
the last-mentioned investigator concluded that a number of factors
required careful control if satisfactory separation of DDT-type
compounds from polychlorinated biphenyls (PCBs) were to be achieved,
in particular, the degree of activation of the silicic acid (irregular
distribution of water molecules onto the silicic acid particles was
also probably important). He found that separation of p,p'-DDE from
four Arochlors was incomplete. A collaborative study of the separation
of DDT-type compounds from PCBs using the Armour & Burke silicic acid
column procedure has been reported (Sawyer, 1973).
Florisil and coconut charcoal have also been investigated for the
separation of PCBs from DDT-related compounds (Reynolds, 1969;
Benvenue & Ogata, 1970; and Stijve & Cardinale, 1974). Another
procedure that has been developed for the separation of DDT-PCB
mixtures is the oxidation of p,p'-DDE to p,p'-dichlorobenzophenone
(Miles, 1972). A method for the determination of p,p'-DDT and a
particular PCB isomer that has similar retention time to that of
p,p'-DDT is based on an empirical relation for p-values (Zelinski et
al., 1973).
2.2.6 Quantitation
2.2.6.1 Determination of DDT-type compounds
Two techniques have played a major role in the quantification of
DDT-type compounds, one is a colorimetric method, the other (now the
most widely used) is gas-liquid chromatography with a halogen-
sensitive detector.
The colorimetric procedure of Schechter-Hailer is described in
detail in the Handbook of the Deutsche Forschungsgemeinschaft (1969),
and by Cieleszky et al. (1970) and Horwitz (1975).
Gas-liquid chromatography is essentially a further-method of
separation of compounds, and, although it is the most effective of the
separation procedures (apart possibly from high pressure liquid-liquid
chromatography) it must be realized that it has its limitations,
particularly if used with a highly sensitive but nonselective detector
such as the electron-capture detector. The basic principles of
gas-liquid chromatography are described by Dal Nogare & Juvet (1962)
and Yermakov (1972), for example, and need not be discussed here.
However, attention is drawn to three aspects of the performance of a
column in the gas-liquid chromatographic separation of DDT-type
compounds from other compounds. First, the DDT-type compounds should
not undergo any thermal degradation or other chemical change; second,
the performance of the column should be assessed by the number of
theoretical plates; and third, the performance of the column as regards
ability to separate p,p'-DDT and related compounds from other
compounds that have similar retention times for a particular liquid
phase must be investigated. Transport of p,p'-DDT and analogues
through a column without chemical change is dependent upon the absence
of reactive centres in the column. This can be attained by using an
inactive solid support (by coating the reactive sites with a silane,
if necessary) and ensuring that the surface of the solid support is
completely covered by an inert liquid phase. The performance of a
column should be checked at regular intervals; the US EPA Manual
(Thompson, 1974) suggests a mixture containing five DDT-type compounds
plus eight other organochlorine insecticides for this purpose. Change
in peak shape (i.e., departure from symmetrical peaks) should always
be regarded as a warning sign. In the case of serious doubt about the
stability of p,p'-DDT or an analogue (which may manifest itself as
a change in the retention time relative to that of aldrin or dieldrin
for example), it is suggested that a fraction collection technique be
used and the identity of the component leaving the column at a
particular retention time with that injected confirmed.
Methods of calculating the number of theoretical plates and of
separation factors are given in standard texts. According to the US
EPA Manual, a column of 2 m length should have about 3000 theoretical
plates. The factors that control the performance of gas-liquid
chromatographic columns are discussed in detail by Scott (1970).
Convenient summaries of the retention times (absolute or relative) of
DDT-type compounds, together with those of other pesticides, using
various column conditions have been published (Yermakov, 1972; Zweig &
Sherma, 1972). Retention times of 51 pesticides relative to that of
aldrin using six stationary phases at three temperatures with an
electron-capture detector have been reported by Thompson et al.
(1975); estimates of the relative retention times at other
temperatures were derived from the relationship between relative
retention time and temperature for each liquid phase. The relative
times of DDT-type compounds and other pesticides on eight stationary
phases were determined by Thompson et al. (1969a). Nonpesticidal
organochlorine compounds that have retention times similar to those of
DDT-related compounds include the polychlorinated biphenyls and
polychloronaphthalenes.
Examples of the close similarity between the relative retention
times of DDT-related compounds and those of various PCB isomers have
been published (Bagley et al., 1970; Richardson et al., 1971; Stijve &
Cardinale, 1974). Goerlitz & Law (1972) demonstrated that there are
also similarities between the relative retention times of DDT-type
compounds and those of various isomers of polychlorinated
naphthalenes. Examples of similarities between the relative retention
times of DDT and its analogues and various components in
polychlorinated biphenyls and polychlorinated naphthalenes was also
reported by Griffith & Blanke (1974). Problems arise in the case of
mixtures of toxaphene and DDT-related compounds (Cahill et al., 1970).
The effects of severe infection loading on column performance,
peak configuration, and conversion of p,p'-DDT into p,p'-TDE, were
studied by Thompson et al. (1969b). These investigators found that the
columns they used could be maintained and restored to full or nearly
full performance capacity by daily changing of the glass injection
port insert and the glass-wool plug at the column inlet.
Two different types of detection systems are most frequently used
for the quantification of p,p'-DDT and analogues after elution from
gas-liquid chromatographic columns, namely, electrochemical detectors
and electron-capture detectors. Two types of electrochemical detector
have been developed, the microcoulometric detector and the micro-
electrolytic conductivity detector, that are considerably more
sensitive to p,p'-DDT and its analogues than the original electro-
chemical detectors. Giuffrida & Ives (1969) described modifications and
improvements in microcoulometric gas chromatography and, in the case
of DDT-type compounds in carrots, they obtained responses from their
microcoulometer that were approximately one-fifth of those given by a
particular electron-capture detector; Griffith & Blanke (1974)
described a microcoulometric method for the determination of
p,p'-DDT, p,p'-TDE and p,p'-DDE in blood. According to Dolan
& Hall (1973), the microelectrolytic conductivity detector can be used
for the selective determination of organochlorine pesticides in the
presence of polychlorinated biphenyls. However, the relative
selectivity in regard to p,p'-DDE does not appear to be as great as
that for other organochlorine insecticides.
The electron-capture detector produces an extremely sensitive
response to organochlorine compounds, but its response is not,
unfortunately, very selective and many other classes of compounds have
electron affinity in the vapour phase. A general review of the
principles and characteristics of the electron-capture detector has
been published by Pellizari (1974). The detector may be used under
direct current or pulse sampling conditions; anomalous responses
obtained in the direct current mode of operation are not present under
pulse sampling conditions (Lovelock, 1963). A major limitation of the
electron-capture detector is that its response is linear over a very
limited range only, but a new mode of operation in which the linearity
extends over about four orders of magnitude of response has been
described by Maggs et al. (1971). For this the detector current is
held constant while the frequency of the applied pulses is varied. The
response of electron-capture detectors is liable to change
significantly during use, and these detectors should be recalibrated
at regular intervals, preferably at least once per day with single
standard injections at frequent intervals between injections of
extracts from the samples under investigation. It is of interest that
Mendoza (1971) reported a significant difference in the response of a
gas-liquid chromatograph to p,p'-DDT when injections were made at
fast and slow rates.
The combination of mass-spectrometry with gas-liquid chromato-
graphy was mentioned in section 2.2.4 as a means of confirming the
identity of residues of DDT-type compounds. This combination of
instruments can also be used to quantify the amounts present. The
total ion current corresponding to mass fragments of appropriate mass
number (m/e; in the case of p,p'-DDE, 316, 318, 246 and 248) is
measured and the amount present is calculated from the calibration of
the instrument, preferably by an internal standard. Palmer & Kolmodin-
Hedman (1972) determined the concentration of p,p'-DDE in human
plasma by mass-fragmentography using the ion current corresponding to
m/e 216 and 218; the results showed an excellent agreement with the
values obtained using an electron-capture detector.
Semiquantitative methods of the determination of p,p'-DDT and
its analogues include paper chromatography and thin-layer chromato-
graphy; these methods, which are more appropriately used for the
confirmation of identity of residues, are described by McKinley (1963)
and Sherma (1973) respectively. Bishara et al. (1972a) have given the
tlc Rf values for p,p'-DDT and 14 related compounds using 33 solvent
systems.
2.2.6.2 Determination of p,p'-DDA in urine
A major metabolite of p,p'-DDT, excreted in the urine, is bis-
(4-chlorophenyl)acetic acid ( p,p'-DDA). A method has been developed
by Cranmer et al. (1969), based upon the extraction of p,p'-DDA from
acidified urine, conversion into the methyl ester, Florisil column
clean-up, and gas-liquid chromatographic analysis of the ester. A
detailed outline of this procedure is given by Thompson (1974). The
sensitivity of response of the methyl ester is not high, the limit of
detection being about 2 ng. Cranmer & Copeland (1973) used the
2-chloroethanol ester, the response of an electron-capture detector
being about 3.7 times greater than that of the methylester (i.e.,
limit of detection about 0.5 ng). An advantage of this ester is that
it is well separated from p,p'-DDT, whereas the methyl ester has a
retention time similar to that of p,p'-DDE. The retention time of
the pentafluorobenzyl ester compound is about twice that of p,p'-DDT
and the response of an electron-capture detector is considerably
greater than that for the 2-chloroethanol derivative (Johnson, 1973).
2.2.6.3 Method of reporting results
Francis Galton (1879) pointed out that many vital effects are
distributed logarithmically; his paper was followed by a technical one
(McAlister, 1879) presenting the mathematics. Apparently Robinson &
Hunter (1966) were first to point out that this principle applies to
the storage of pesticides so that the geometric mean is a more
appropriate parameter than the arithmetic mean for expressing
insecticide content. In recent years, increasing use has been made of
the geometric mean as recorded by footnotes in Table 9. Few of the
arithmetic means that have been reported are so much in error that
they should be discarded. As Galton (1879) noted, the difference
between the arithmetic and geometric mean is small if the range of the
values averaged is narrow.
2.2.7 Validation of analytical methods for DDT-type compounds
Ideally, an analytical method should be accurate and highly
precise. A study of the accuracy and precision of art analytical
method must be made in order to assess the relationship between the
actual amount present and the results obtained in practice; such a
study may be called the validation of the procedure. There are two
major types of validation procedure. The first type requires the use
of radio-labelled molecules of p,p'-DDT, e.g., 14C p,p'-DDT.
Prouty & Cromartie (1970) determined the 14C-activity in muscle,
liver, heart, and brain of quail that had been given 14C-labelled
p,p'-DDT. Four of the steps in the analytical procedure were studied
and it was concluded that the major discrepancies occurred during the
elution and concentration steps from Florisil columns and from zones
of thin-layer chromatography plates. Chiba & Morley (1968) used 14C-
labelled p,p'-DDT in a study of soil analysis.
The other validation procedure, which has been studied more
intensively, involves the comparison of the results of analyses with
the values for samples fortified with accurately known amounts of
p,p'-DDT or analogues. There are several variations of this
validation procedure, all comparing the fortified (nominal) value with
the results of different methods in the same laboratory, with the
results of a specified method carried out in different laboratories,
or with the results of different methods in different laboratories.
Versino et al. (1971) compared 8 clean-up procedures for test
mixtures containing 10 pesticides. Two extracts, from apples and
lettuce, were fortified (spiked) with p,p'-DDT at 0.2 mg/litre,
p,p'-TDE at 0.1 mg/litre, and o,p'-DDT at 0.1 mg/litre plus
dieldrin and 7 organophosphate insecticides. The recoveries for the 8
column clean-up procedures ranged from 89-100% for p,p'-DDT, 85-101%
for p,p'-TDE, and 95-100% for o,p'-DDT. It should be noted that
this investigation did not include a study of extraction efficiency
and that the extracts contained only small amounts of lipids. In
studies by Smart et al. (1974), samples were analysed of milk, butter,
cheese, eggs, apples, potatoes, carrots, and cabbages, fortified with
p,p'-DDT, p,p'-TDE, and p,p'-DDE (and 5 other organochlorine
insecticides) at concentrations corresponding to those suggested as
limits by the Codex Alimentarius Commission. Five replicate analyses
of each sample were made, using up to 4 procedures, the final step of
each analytical procedure being gas-liquid chromatography/electron-
capture detection, with 3 different stationary phases. These workers
concluded that there were no gross discrepancies in their results.
However, they did not use the concept of "total error" (see above) in
the discussion of their results, and the total errors for the
determination of the 3 DDT-type compounds, calculated from their
results, were in the range of 12% to 95%.
Results have been reported (Carr, 1970) of a collaborative study
by 10 laboratories of the AOAC method for the determination of 4
DDT-type compounds in samples of butterfat fortified by the addition
of these 4 compounds at 2 different concentrations. The mean recoveries
varied from 86-108%, and the coefficients of variation between
laboratories were in the range 7-28%. The total errors for the 2
levels of fortification respectively were: p,p'-DDT, 22 and 14%;
p,p'-TDE, 17 and 30%; p,p'-DDE, 17 and 36%; and o,p'-DDT, 30 and
43%.
Carr (1971) gives the results of a collaborative study by 8
laboratories of the analysis of fish samples fortified (2
concentrations) with p,p'-DDT, p,p'-TDE and p,p'-DDE, and 3 other
organochlorine insecticides. On the basis of a ranking technique
(Youden & Steiner, 1975), the results of 2 laboratories (Nos. 2 and 8)
for the 6 compounds were rejected as outliers. However, if the results
for the 3 DDT-type compounds are considered, then laboratories 7 and 8
gave results that are outliers. The total errors at the 2
concentrations for the 6 laboratories not rejected by Carr were:
p,p'-DDT, 15 and 36%; p,p'-TDE, 32 and 32%; p,p'-DDE, 26 and 39%.
Sawyer (1973) reported a collaborative study by 9 laboratories of
samples of red salmon fortified with 3 DDT-type compounds and PCBs.
The analyses were carried out without and with silicic acid column
separation of PCBs from DDT-type compounds. The total errors without
and with silicic acid separation, were 34% and 43% respectively for
p,p'-DDT, 50 and 47% for p,p'-TDE and 35 and 36% for p,p'-DDE.
Very similar results were obtained with another type of PCB/DDT
mixture.
Samples of soil, fortified with 4 DDT-type compounds plus 6 other
organochlorine insecticides were analysed in 7 laboratories (Woolson,
1974); the total errors (all collaborators) were: p,p'-DDT, 17-33%;
p,p'-TDE, 19-37%, p,p'-DDE, 18-42%; o,p'-DDT, 28-56%.
Fifteen laboratories collaborated in a study of the determination
of p,p'-DDE in eggs, and p,p'-DDT, p,p'-DDE and o,p'-DDT in
kale (Finsterwalder, 1976). Five laboratories analysed eggs by the
AOAC method, and the total error was 21.1%; the total error for 10
laboratories using a modification of the AOAC method was 30.4%. The
results of one laboratory for analyses of kale were rejected as
outliers, and the total errors for the other 14 laboratories were:
p,p'-DDT, 19.7%; p,p'-DDE, 18.8%; o,p'-DDT, 17.4%.
An international collaborative programme on methods of analysis of
organochlorine insecticides has been sponsored by the Organization for
Economic Cooperation and Development (OECD), and the results of 2
studies have been published. In the first (Holden, 1970), a solution
in hexane of 6 organochlorine compounds, 3 being DDT-related
compounds, was analysed by 17 laboratories in 11 countries. The total
errors for p,p'-DDT, p,p'-TDE and p,p'-DDE were in the range
14-21%, the major component in this total error being the standard
deviation of results from different laboratories, probably indicating
errors of calibration. In the second study (Holden, 1973), a sample of
corn oil fortified with 4 DDT-type compounds and 3 other
organochlorine insecticides was analysed in 19 laboratories in 10
countries: the mean total error for the 4 DDT-type compounds (after
excluding 3 outliers) was 35%.
Some of the results of this collaborative validation study are
very satisfactory, but many of them, as judged by the criterion of
total error, are unsatisfactory, even when outliers are rejected, and
they illustrate the need for scrupulous care by analysts: the use of
clean glassware, chemicals of established purity, and constant
checking of the performance of liquid-solid partition columns and of
instruments.
2.2.8 Analytical methods for the evaluation of the biochemical effects
of p,p'-DDT and its analogues
The increased activity of enzymes, particularly in the hepatic
endoplasmic reticulum, if the exposure to p,p'-DDT and its analogues
is sufficiently great, has been recognized in recent years.
Measurement of such changes in enzyme activity may be made by studying
changes in the rates of metabolism of certain drugs (such as
antipyrine), but methods that do not require the administration of a
drug (and the collection of blood samples if the plasma half-life is
used as the biochemical parameter) are preferable. Two methods, in
which the concentration of either 6-ß-hydroxycortisol or D-glucaric
acid in the urine is measured have been developed. A procedure for the
determination of 6-ß-hydroxycortisol in urine has been described by
Kuntzman et al. (1968); and one for D-glucaric acid by Marsh (1963)
(see section 8.2.5.1).
These enzyme induction changes are not specific for DDT-type
compounds.
3. SOURCES OF ENVIRONMENTAL POLLUTION
3.1 Discovery and Introduction
DDT does not occur naturally. It was first synthesized by Zeidler
as reported in 1874. However, it was not put to any use until its
insecticidal properties were discovered by Paul Müller in 1939. The
Swiss patent was issued in 1942.
As an example of the speed with which DDT was developed and used,
the first sample sent to the USA arrived there in September 1942. This
sample was tested for effectiveness and safety. The results were so
encouraging that manufacture was given high priority. At first, the
entire production was used for the protection of troops against
malaria, typhus, certain other vector-borne diseases, or against
biting flies or other insects that are merely pests. As the supply
increased, DDT was used in the USA for control of malaria in military
areas, that is in the vicinity of military installations, ports, and
transportation centres. As a result of this effort, mosquito
transmission of malaria was brought to an end in the USA in 1953, even
though military personnel and other infected persons from the tropics
continued to reintroduce the disease extensively as late as 1972 and
to a lesser degree thereafter.
3.2 Production and use
The revolution in the control of malaria and typhus among allied
troops and among certain civilian populations during World War II was
accomplished with relatively little DDT. Far greater amounts were
required for the control of agricultural and forest pests and this
became possible when the compound was released in the USA for
commercial use on 31 August 1945. Civilian use in other countries
became possible a little later, first, largely on the basis of
importation and gradually on the basis of local manufacture.
Unfortunately, there is apparently no record of world production of
DDT. Production and use in the USA is shown in Table 2.
Quantities of DDT and related compounds used in or sold for
agricultural purposes in 1970 were as follows (metric tonnes):
Australia (about 1000); Austria (20.5); Botswana (2.0); Canada
(287.0); Columbia (980.0); Czechoslovakia (270.0); Democratic
Kampuchea (46.8); Egypt (3457.0); El Salvador (466.0); Federal
Republic of Germany (152.0); Finland (6.1); Ghana (0.3); Guatemala
(380.0); Hungary (20.6); Iceland (0.3); Israel (10.0); Italy (2178.0);
Japan (401.0); Kuwait (0.2); Madagascar (176.0); Ryukyu Islands
(Japan) (0.3); Sri Lanka (16.6); Sudan (269.0); Upper Volta (1.5); and
Uruguay (5.0) (FAO, 1972). These values total 10 146.2 metric tonnes.
Thus, at least until very recently, the use of DDT was extensive on a
worldwide basis but varied greatly from one country to another.
Table 2. Metric tonnes of DDT produced and used in the USAa
Year Production Use
1944 4 366
1945 15 079
1946 20 220
1947 21 534
1948 9 181
1949 19 822
1950 35 448
1951 48 144
1952 45 327
1953 38 268 28 349
1954 44 088 20 465
1955 56 760 28 032
1956 62 441 34 194
1957 56 136 32 205
1958 65 920 30 255
1959 71 097 35 771
1960 74 471 31 818
1961 77 763 29 061
1962 75 764 30 502
1963 81 154 27 744
1964 56 113 22 925
1965 63 859 24 034
1966 64 115 20 685
1967 46 906 18 260
1968 63 231 14 848
1969 55 839 13 724
1970 26 860 11 316
a Based on data from US Tariff Commission (Hayes, 1975).
3.3 Changing Patterns of Use
Before 1945, all of the DDT produced in the USA for example, was
used or allocated by the military services for various medical and
public health uses. Early in 1945, it became available tor rather
extensive experimental work in agriculture, and it was commercially
available in limited quantities early in the autumn of the same year
(US Department of Agriculture, 1945a,b). The results were so
spectacular that use increased until 1959. In response to a demand for
exports, production continued to increase to about 1963. Even before
then some restrictions were placed on its use, mainly to minimize
residues in food and in the feed of animals that produce milk and
meat. Among the first of these restrictions was that on its use on
dairy cattle or in dairy barns (USDA, 1949). Another important factor
reducing the use of DDT was the increasing resistance of pests. One of
the first species to be affected was the house-fly; because of its
abundance and widespread distribution, its resistance was bound to be
noticed by the public, generally. Although many pests of public health
importance became resistant to DDT in some or all of their range,
resistance among vectors of malaria was less marked. Because malaria
control constitutes such a large segment of vector control, the use of
DDT for vector control has tended to remain stable, while its use in
agriculture has continued to decline, especially in temperate
climates.
The ban on the use of DDT and certain other organochlorine
insecticides in Sweden from 1 January 1970 was based on a number of
ecological considerations.
Government agencies of some other countries attempted to justify
severe restrictions on the use of DDT by alleging that it was a threat
to human health. This was in response to ecologists who considered
that the widespread occurrence of DDT in the environment was
inherently bad and was the direct cause of injury to certain fish and
birds. However, this did not prevent the same agencies from making a
proviso that DDT might be used, if needed, to combat any future threat
from vector-borne disease within their boundaries.
Of course, before the restrictions were put into effect, it had
already been possible to eradicate malaria in the USA and Italy, for
example, and to control for the first time an epidemic of typhus in
Italy and Germany (Simmons, 1959). Apparently, there have never been
accurate figures on the proportion of the compound used for
agriculture and for public health even in those countries that have
recorded their total use of the compound.
As the situation now stands, DDT is still used extensively, both
in agriculture and for vector control, in some tropical countries.
Apparently, information is not available on how much of the
agricultural use involves food protection or how much loss of food
production would result if the use of DDT were discontinued. The
picture with malaria control is clear. Substitution of malathion or
propoxur for DDT would increase the cost of malaria control by
approximately 3.4- and 8.5-fold, respectively, and this increase could
not be supported. If DDT were not used, vast populations in the
malarious areas of the world would be condemned to the frightening
ravages of endemic and epidemic malaria (WHO, 1971).
4. ENVIRONMENTAL TRANSPORT AND DISTRIBUTION
Because DDT has been sprayed on people, domestic animals,
buildings, agricultural crops, and forests, it is not surprising that
it is now distributed widely in the environment. During the
application of any spray or dust to a field, it is often possible to
observe drift of the particulate material. This is especially true if
the application is made by aircraft or by ground equipment that shoots
the spray to the top of orchard trees. If the application is made to
forests, it is very likely that at least part of the spray will fall
directly on streams or lakes. Following application, redistribution is
inevitable. As discussed more fully in the following sections, some
DDT in soil enters the air by evaporation or on wind-blown dust. Even
if watercourses are avoided initially, some insecticide will be washed
into them by rains, mainly in conjunction with soil particles.
4.1 Local Drift in Air
The fact that there is drip is implicit from measurements of DDT
on surfaces just after application. Wilson et al. (1946b) recovered
only 7.5% of the nominal dose from plants; this proportion is typical
but ignores material that may have fallen between plants or between
leaves and come to rest on the ground. Of greater interest are
measurements of total recovery made by means of absorbent targets laid
out in advance. As might be expected, recovery of aerosols is less
than recovery of sprays when other conditions of application are
similar. Only 12.5% was recovered at the centre of swaths following
application of a thermal aerosol by aircraft and recovery decreased at
increasing distances from the centre (Hess & Keener, 1947). In another
study, recovery was 8% for a thermal aerosol and 46% for a spray
(Scudder & Tarzwell, 1950). In a different situation, the average
seasonal recovery for DDT applied as a thermal aerosol ranged from 10
to 12%, while that for spray ranged from 56 to 76% (Tarzwell, 1950).
However, somewhat lower recoveries of DDT sprays have frequently been
reported: 30% (Hoffman & Merkel, 1948), 39% (Hoffman & Surber, 1948),
and 27% (Surber & Friddle, 1949).
Most DDT particles that miss the target for which they are
intended would be expected to fall in the general area. Studies of
residues in soil and in wildlife indicate that this is true. The
logarithm of the concentration of DDT-related compounds in soil
samples collected in a desert area downwind from an area of intensive
agriculture showed an almost straight line inverse relationship to the
logarithm of the distance from the source. The soil levels were about
1 mg/kg at 10 m and about 0.001 mg/kg at 100 000 m from the point of
application. The concentrations of DDT in the tissues of wildlife were
proportional to its concentrations in the soils of their habitats
(Laubscher et al., 1971). Where cultivated fields to which DDT has
been applied for years are interspersed among pastures and other
fields where DDT is not used, the uncultivated soils contain only a
little less DDT than the cultivated ones. In one study, it was found
that most of the DDT was in the top 2.5 cm, but samples for comparison
were taken to a depth of 7.5 cm; the average concentrations were 0.75
to 2.03 mg/kg in cultivated soil and 0.10 to 0.91 mg/kg in
uncultivated sod (US Department of Agriculture, 1966). These
concentrations may be compared (by mathematical calculation alone) to
1.0 mg/kg, the concentration resulting from mixing DDT into soil
uniformly and with no loss whatever following application at the rate
of 1.12 kg/ha, the standard specific gravity (1.47) of soil and a
depth of 7.6 cm being assumed. The range of specific gravities for
most agricultural soils is 0.4 to 2.0 and the corresponding depths
yielding 1 mg/kg are 28 to 5.60 cm. It is of interest that the
residues in cultivated soft were of the order of magnitude that would
be expected from a single application, even though the average
cumulative rate of application during the last 10 years (11.2 kg/ha)
had been 10 times as great.
4.2 Distant Drift in Air
Dust bearing DDT at a concentration of 0.6 mg/kg has been observed
about 1600 km from its area of origin. Other pesticides were also
present in this dust which had settled out on a recently rain-rinsed
roof. The cloud of dust was so dense and unusual that its progress was
reported in newspapers, as the storm that mobilized it in Texas
carried it at least as far as Ohio, where the sample was collected
(Cohen & Pinkerton, 1966).
Dust collected on the island of Barbados by means of nylon nets
treated with 50% aqueous glycerine was assumed to have been blown from
Africa, a distance of over 4850 km. Samples of the dust contained
p,p'-DDT most frequently and in greatest concentration, but the
concentration of all pesticides was only 0.001 to 0.164 mg/kg with an
average of 0.041 mg/kg (Risebrough et al., 1968).
DDT evaporates from sprays and dusts at the time of application
and at any time that dust bearing the compound is mobilized by the
wind. Furthermore, DDT can be detected over treated fields for more
than six months after application, and there is a concentration
gradient from the soil upward (Willis et al., 1971). Under these
circumstances, it is obvious that DDT is transported in the form of a
vapour as well as by means of dust. However, if samples are collected
where no application of DDT has been made, identification of the
origin of the vapour or measurement of the distance it may have
travelled is even more difficult than with dust.
Evidence that DDT in one form or another has travelled great
distances and is, in fact, worldwide in its distribution has been
deduced from finding it in the rainwater of remote, nonagricultural
places (Tarrant & Tatton, 1968) or in water melted from Antarctic snow
(0.00004 mg/litre) (Peterie, 1969). In such remote places as
Eskdalemuir in Scotland and Lerwick in the Shetland Islands, the
average concentrations of p,p'-DDT in rainwater (0.000030 and
0.000046 mg/litre, respectively) were not greatly different from the
averages (0.000018-0.000066 mg/litre) found in widely separated
agricultural areas, suggesting that the compound is rather evenly
distributed in the air (Tarrant & Tatton, 1968).
4.3 Distribution in Water
DDT has a strong tendency to adsorb on surfaces. Most DDT that
enters water is already firmly attached to soil particles, and remains
attached. It was shown very early that, if DDT does find its way into
clear water, it is gradually lost by adsorption on surfaces (Carollo,
1945). The sediments in water tend to move downstream and eventually
to enter estuaries. Of the various chlorinated hydrocarbon
insecticides, DDT and its metabolites are the ones most commonly
found, but the residues tend to be low (Butler, 1969). In fact, in an
estuary associated with the Mississippi River, the levels of
pesticides decreased strikingly from the early 1960s to the late 1960s
(Rowe et al., 1971).
4.4 Bioaccumulation of DDT and Its Degradation in the Environment
Many studies of DDT and related compounds in the environment have
focused on organisms and locations in which concentrations of DDT have
been observed to increase. Concentration in living organisms may be
the result of adsorption from water, of the filtering out of algae or
detritus bearing the compound, or of biological magnification in the
strict sense, that is progressive accumulation in different steps of a
food chain. Although the mechanisms are poorly understood, observation
has shown that residues do reach an equilibrium and sometimes decline.
For example, where DDT was applied to cotton at a cumulative rate of
11.2 kg/ha so that a residue of over 10 mg/kg in the soil would be
expected after many years of continual use, the actual residues ranged
from 0.75 to 2.03 mg/kg (USDA, 1966). An example of decreased residues
was seen in the grebes of Clear Lake (Rudd & Herman, 1972).
Evidence is accumulating that the disintegration of DDT may be
rapid in some situations. Under biologically active, anaerobic
conditions as little as 1% of DDT remained after 12 weeks of
incubation (Hill & McCarty, 1967; Guenzi & Beard, 1968). Probably more
important is the disintegration of DDT under the influence of
ultraviolet light. Hartley (1969) pointed out that much of any
pesticide vapour escaping to 50 metres or more above the ground will
ascend even higher by eddy diffusion and eventually reach the
photochemically active ionosphere. The rapid destruction of DDT by
ultraviolet light under laboratory conditions has been demonstrated
(Mosier et al., 1969; Plimmer et al., 1970; Miller & Narang, 1970;
Plimmer & Klingebiel, 1973; Crosby & Moilanen, 1977). Gab et al.
(1975, 1977) offered evidence that DDT and DDE are converted to carbon
dioxide and hydrochloric acid; the destruction was so complete that
they characterized it as photomineralization. In spite of very real
progress in understanding the fate of DDT in the environment (see
Annex), much more work will be required before a quantitative balance
can be measured between addition of the compound and its
disintegration.
5. ENVIRONMENTAL EXPOSURE LEVELS
5.1. Exposure of the General Population
5.1.1 DDT in air
In spite of the generalization in section 4.2 that DDT is rather
evenly mixed in the air, some increase in concentration may be noted
in connexion with the time and place of application. The highest
concentration of insecticide found in the air of communities with
anti-mosquito fogging programmes was 0.0085 mg/m3 (Tabor, 1966).
Concentrations one or two orders of magnitude greater have been
reported for several insecticides in the breathing zone of orchard
spraymen, and values of 1.2-0.26 mg/m3 have been found at distances
of 500-5000 m from ground spraying (Belonozko et al., 1967). In six
small communities in an agricultural area in the USA, DDT was found in
concentrations ranging from 1 × 10-6 to 22 × 10-6 mg/m3 (Tabor,
1966). Substantially higher values (0.002-0.05 mg/m3) were reported
for centres of population in the USSR (Belonozko & Kucak, 1974). In an
urban location in a generally nonagricultural area of the USA, the
highest concentration found was 2.36 × 10-6 mg/m3 (Antommaria et
al., 1965). The combined concentrations of DDT, dieldrin, and lindane
in the Munich area of the Federal Republic of Germany in 1971 were
even lower, only exceptionally rising as high as 1 × 10-6 mg/m3
(Weil et al., 1973).
With few exceptions, the highest average concentration of DDT in
air to which workers are exposed regularly (7.1 mg/m3) is that
associated with spraying the interior of houses (Wolfe et al., 1959).
However, concentrations ranging from 2 to 104 mg/m3 have been
reported in places where DDT dust was prepared and packed (Medved' et
al., 1975).
5.1.2 DDT in water
Under agricultural conditions, the concentration of DDT in water
may be high. For example, 0.01 mg/litre was found in the runoff from
melting snow from fields where sugar-beets had been grown (Medved' et
al., 1975).
The highest level at which DDT has been found in rainwater in an
urban area during a period of a month is 0.0004 mg/litre (Abbott et
al., 1965). The highest concentration reported in potable water
(0.02 mg/litre) occurred some years ago (Middleton & Lichtenberg,
1960). In a much more comprehensive study made a few years later, the
highest concentration of any insecticide was found (0.00012 mg/litre),
but this was dieldrin and not DDT (Weaver et al., 1965). Many samples
did not contain detectable insecticide of any kind. A study of surface
waters in the USA during the years 1964-1968 indicated that the
residues reached a peak in 1966 and then dropped sharply in 1967 and
1968, in spite of improved analytical methods. The highest value for a
DDT-related compound in those years was 0.00084 mg/litre (Lichtenberg
et al., 1970). By 1971, the concentration in the Federal Republic of
Germany was even lower, averaging 0.00001 mg/litre and never going as
high as 0.001 mg/litre (Weil et al., 1973). The average values for
total DDT in drinking water in Czechoslovakia were 0.000011 and
0.000015 mg/litre in 1972 and 1973, respectively (Hruska & Kociánová,
1975). DDT was not detected (<0.0000000166 mg/litre) in tap water in
a recent survey carried out in Ottawa, Canada (McNeil et al., 1977).
5.1.3 DDT in food
Residues of DDT were measured as early as 1945 (before the
compound was available commercially) on apples to which it had been
applied experimentally for the control of the coddling moth (Harman,
1946). Apparently, the earliest effort to learn how much DDT the
average man obtains from his daily food was that of Walker et al.
(1954), who reported that the amount of insecticide in restaurant
meals in Wenatchee, Washington, USA indicated an average intake of
0.184 and 0.102 mg/man per day for DDT and DDE, respectively. Soon,
other studies revealed similar levels of DDT intake for persons who
ate an ordinary range of foods but who lived in different parts of USA
(Hayes et al., 1956; Durham et al., 1965b). Most of the DDT was in
food of animal origin, and persons who abstained from eating meat but
obtained the food they ate from regular, commercial sources received
an average of only 0.041 and 0.027 mg/man per day of DDT and DDE,
respectively (Haynes et al., 1958). The difference did not depend,
however, on meat per se, for no DDT and only traces of DDE were
found in the meat and other products obtained from Arctic wildlife
that constituted much of the diet of Eskimos (Durham et al., 1961).
Following restrictions on the application of DDT to livestock, to
their barns, and to the forage crops on which they fed, there was a
gradual decrease in residues in animal products used as human foods.
Restrictions on the use of DDT on crops eaten directly by man resulted
in reduced residues in vegetable foods. Complete meals collected
mainly from the same restaurants in Wenatchee and analysed in the same
laboratory indicated that, by 1964, DDT intake was only 0.038 mg/man
per day (Durham et al., 1965b) compared with 0.184 mg/man per day
reported by Walker et al., 11 years earlier. Thus, intake had been
reduced to less than one-fourth. A further reduction to about one-
eighth of the 1953-54 values was indicated by the nationwide study by
the US Food and Drug Administration, usually called the Market Basket
Survey. Intakes of DDT indicated by this study for the succeeding
years 1965-70, were 0.031, 0.041, 0.026, 0.019, 0.016, and
0.015 mg/man per day, respectively (Duggan & Weatherwax, 1967; Duggan,
1968; Duggan & Lipscomb, 1969; Duggan & Corneliusen, 1972).
The widespread shipment of food in the USA tends to explain the
fact that food residues are generally similar in different parts of
the country. However, small differences do exist and may be accounted
for by the fact that, on average, meat is not shipped as far as
vegetables. Much of the feed for livestock is produced on the same
farm or at least in the same area in which the animals are raised.
Whatever the reason, the coefficients of correlation between latitude
and human intake were -0.63 and -0.59 for the sampling periods 1966-67
and 1967-68, respectively (Hayes, 1975).
Early studies (Swackhamer, 1965) indicated that both the frequency
and concentration of residues were slightly less in Canada than in the
USA. More recent studies in Canada similar to the Market Basket Survey
indicated total DDT dietary intakes of 0.018, 0.011, 0.011, 0.007 and
0.007 mg/man per day for the years 1969 to 1973, respectively (Smith,
1971; Smith et al., 1972, 1973, 1975).
The analysis of whole meals collected in south-east England during
1965 and 1966 gave results similar to those obtained during the same
period in the USA; the calculated daily intakes in England were 0.030
and 0.025 mg/man per day for DDT and DDE, respectively (McGill &
Robinson, 1968).
There may be considerable differences in intake in different parts
of the same country. Hruska & Kociánová (1975) reported that the
average intake of DDT plus DDE in 1972 was 0.002 mg/man per day in
southern Bohemia and was 0.099 mg/man per day in Slovakia. Striking
differences may exist between urban and rural areas (Almeida et al.,
1975).
Values for total DDT-related compounds in regular food in the USSR
are not available, but the separate values for DDT and DDE in daily
diets from different regions shown in Table 3 suggest that the total
may be higher than in the UK and USA and that the amount of DDE may
exceed the amount of DDT. Both high total values and an unusually high
proportion of DDE would be consistent with extremely high total values
a few years earlier and recent discontinuation of the use of DDT. In
fact, in the Soviet Union, DDT has been eliminated from the list of
pesticides recommended for use in agriculture since 1970. During the
period 1966-69, 0.8% of food samples contained residues as high as
5.1 mg/kg, and 4.2% contained residues over 1.0 mg/kg.
Although total intake of DDT from food has not been measured in
some parts of the world, worldwide measurements of storage of DDT and
its metabolites in human body fat indicate that the extremes of total
exposure have varied by a factor of about 10, but that total exposure
for most populations has varied by a factor of no more than 3 (see
Table 9).
Table 3. Residues of DDT and DDE in daily cooked diets from
different areas of the USSR during 1971/72a
Area No. of Range of residues of
daily
diets DDT DDE
examined (mg/diet)b (mg/diet)b
North 121 0.00006-0.0103 0.0001-0.0093
West 191 traces-0.094 traces-0.039
South-east 62 traces-0.15 traces-0.330
South I 184 0.02-0.260 0.020-0.5
South II 51 traces-0.052 traces-0.132
a From: Medved' et al. (1975).
b Equivalent to mg/man per day.
It is important to note that local practice may result in high
residues in the food of one or more families even though residues are
low in commercially produced food available in the same area. Thus
Durham et al. (1965b) reported that the average DDT intake from
household meals in Wenatchee was 0.314 mg/man per day at the same time
that restaurant meals in that town contributed only 0.038 mg/man per
day. The difference was largely the result of very high residues in
some eggs eaten by local families; the chickens foraged near orchards,
which had been treated with DDT. The restaurants used commercially
available eggs and not those produced locally. In a similar way,
practices peculiar to one country may account for high residues in
some of their food. For example, very high residues of DDT were
reported in some samples of staple foods in India (Sharangapani &
Pingale, 1954). Dale et al. (1965) suggested that the high levels of
DDT that they found in some Indians might be the result of direct
addition of the compound to staple food to prevent insect infestation,
even though the practice did not have government approval.
5.1.4 Miscellaneous sources
It has been suggested that there is a positive correlation between
the use of household insecticides and the concentration of DDT in
house dust, on the one hand, and the storage of DDT in people on the
other (Deichmann & Radomski, 1968; Radomski et al., 1968; Davies et
al., 1969b, 1975; Edmundson et al., 1970b). However, another study of
dust in 16 urban households, 4 farm households, and 8 households in
which at least one member was a pesticide formulator, failed to reveal
a statistically significant correlation between the levels of various
pesticides in dust and in the serum of people living in the homes.
There were striking individual examples of workers whose homes
contained high concentrations of the compounds they used
professionally and other examples in which there was circumstantial
evidence relating household dust residues to body burden (Starr et
al., 1974).
There can be no doubt that insecticides used in the household or
introduced on the clothing of workers are important sources of intake
of DDT in some instances. It is not clear whether the relevant
absorption involves mainly the inhalation of dust, the contamination
of food within the home, or even dermal absorption.
5.1.5 Relative importance of different sources
It has been estimated (Campbell et al., 1965) that over 90% of the
DDT stored in the general population is derived from food. About 1965,
intake in the USA was approximately 0.04 mg/man per day from food,
less than 0.000046 mg/man per day from water, less than 0.00006 mg/man
per day from urban air and less than 0.0005 mg/man per day from air in
small agricultural communities. The reason for the qualification "less
than" is that the intakes were calculated from the highest
concentrations reported in drinking-water and air because no average
values were established.
Other investigators (Durham et al., 1965b; Morgan & Roan, 1970;
Medved' et al., 1975) concluded independently that ordinary food is
the major source of DDT and related compounds for the general
population. Intake from ordinary food is a base to which other kinds
of intake -- including that from exceptional food -- may be added. An
example of such an addition -- eating eggs from chickens allowed to
run loose in DDT-treated orchards has already been discussed in
section 5.1.3.
5.2 Exposure of Infants and Young Children
Babies tend to be born with slightly lower blood levels of DDT
than are found in their mothers (O'Leary et al., 1970b; Schvartsman et
al., 1974, see also section 6.2.2). This simply indicates that the
placenta excludes some but not all of the DDT available to it. During
the first 10 or 15 years of life, DDT storage levels rise to the adult
population level (Hayes et al., 1958; Hunter et al., 1963; Wassermann
et al., 1965, 1967; Robinson et al., 1965; Davies et al., 1968, 1969a;
Watson et al., 1970).
It has been known for a long time that human milk may contain a
higher concentration of DDT than cow's milk in the same country (Egan
et al., 1965; Quinby et al., 1965b; Ritcey et al., 1972; Olszyna-
Marzys et al., 1973). So far, there is no evidence that this small
difference is of any significance for breast-fed compared with bottle-
fed babies. This is even true in places where the concentration of DDT
in human milk is comparatively high (see section 6.3.1.4).
On the average, the incidence and levels of residues in
commercially prepared food for babies in the USA are lower than those
in raw agricultural products, other processed foods, or samples
examined in the Market Basket Survey (Lipscomb, 1968).
The only DDT exposures of infants that are known to have injured
them in any way are those involving direct access to formulations that
they ate or drank. Such tragic accidents often involved formulations
transferred to unlabelled food or beverage containers. Frequently, the
container was left where a child could reach it easily. Occasionally,
formulations have been stored with food or have ever been handed to a
child as food, or "empty" containers that still contain enough
formulation to kill a child have been carelessly discarded (Hayes,
1975, 1976a).
5.3 Occupational Exposure
In general, annual exposure to DDT is greatest among manufacturers
and formulators, moderate, among those applying it for agricultural
purposes, less among the general population, and least among special
groups whose location or practices minimize their exposure. However,
for brief intervals the exposure during agricultural application may
exceed anything that good industrial practice permits. This
distinction is of great importance in connexion with the more toxic
organic phosphorus compounds, a single heavy exposure to which may
result in poisoning, but is of no known importance in connexion with
DDT, the acute toxicity of which is much less. However DDT has a
greater tendency to storage in the body.
Occupational exposure to DDT is reflected quantitatively by the
concentration of DDT and DDE in blood and fat and by the concentration
of DDA in urine. These aspects of occupational exposure are considered
later. This section outlines measurements that have been made of the
actual degree of exposure under different circumstances.
The most striking result is that the occupational exposure through
areas of skin that are frequently unclothed (face, hands, forearms,
neck, and "V" of chest) is far greater than total respiratory
exposure. Results for DDT are shown in Table 4 (based on measurements
made by methods described by Durham & Wolfe (1962, 1963)). If a
workman has bare feet or legs or does not wear a shirt, the contrast
between respiratory and dermal exposure will be even greater than that
shown in Table 4 which is based on "standard" clothing.
Table 4. Measured respiratory and dermal exposure of workers
to DDT under actual conditions of work
Activity Respiratory Dermal Reference
(mg/kg) (mg/kg)
Indoor house spraying 3.4a 1755 Wolfe et al., 1959
Outdoor house spraying 0.11 243 Wolfe et al., 1959
Spraying forests 4-92b 212b Wassermann
et al., 1960
a Measurement by respirator pad technique.
b Calculated from values given in the original paper.
It has been reported that DDT and a number of other pesticides
persist, for days or even years after last use, on the hands of
workers exposed to them and that at least a part of the material can
be removed for analysis by rinsing the hands in hexane. Evidence that
the residues represented unabsorbed pesticides and not excretion of
stored material included the fact that no correlation was found
between residues on the hands and in the sera of individuals and that
no residues could be found on the hands of a farmer who used
rubberized gloves while working with pesticides and who washed
afterwards with strong cleansing agents (Kazen et al., 1974). The fact
that urinary excretion of DDA may be increased for several days after
a single occupational exposure to DDT (Wolfe et al., 1970) is evidence
that absorption continues for a week or so but not for longer periods.
Furthermore, Wolfe et al. (1970) found that the rate of excretion,
even when detectable, was small compared to the excretion of parathion
metabolite in workers exposed simultaneously to DDT and parathion at a
ratio of 1.0 to 0.25. This emphasizes the minimal dermal absorption of
DDT.
6. METABOLISM OF DDT
6.1 Uptake
6.1.1 Uptake by inhalation
Most DDT dust is of such large particle size (>250 µm) that any
that is inhaled is deposited in the upper respiratory tract and is
eventually swallowed (Hayes, 1975). Toxicity data indicate that
respiratory exposure is of no special importance.
6.1.2 Uptake from the gastrointestinal tract
A review of the early literature indicates that absorption of DDT
from the gastrointestinal tract is slow. Whereas intravenous injection
at the rate of 50 mg/kg produces convulsions in rats in 20 min,
convulsions occur only after 2 h when DDT is administered orally at
two or more times the LD50 value. The onset of convulsions is
delayed for about 6 h when DDT is given to rats orally at
approximately the LD50 value (Dale et al., 1963).
Early studies based on toxicity indicated that DDT, dissolved in
animal or vegetable fats, was absorbed from the gastrointestinal tract
about 1.5 to 10 times more effectively than undissolved DDT. There was
also evidence that large doses of the compound in the gastrointestinal
tract were poorly absorbed from nonabsorbable solvent (Hayes, 1959).
However, in connexion with small repeated doses, the presence or kind
of solvent made little difference; apparently the occurrence of bile
in the intestine and the presence of some fat in the diet were
sufficient to promote absorption of the compound. At high dosage
levels, less 14C-DDT was absorbed and stored in organs and a higher
proportion was excreted in the faeces following oral administration
than after intraperitoneal administration (40% versus 0.9%) (Bishara
et al., 1972b).
Rothe et al. (1957) reported that after giving radioactive DDT to
rats by stomach tube, 41-57% of it was recorded in lymph drained from
the animal by means of a cannula. Less than 0.1% of the activity was
found in the urine, 7.4-37.1% was found in the faeces or in the
intestinal contents when the animals were killed, and 19%-67% of the
activity was found in the carcass. The total dose accounted for
analytically varied from 89% to 118%, thus recovery was complete
within the accuracy of the method. Of the administered DDT not found
in faeces and intestinal contents, 47%-65% was found in the lymph. The
animals that withstood the operation best had peak lymph flows of
nearly 6 ml/h. In these animals, DDT was absorbed at rates as high as
381 µg/h; the rate of absorption reached a maximum within 2-3 h of
intubation and was markedly reduced by the fourth hour. Fifty per cent
of the DDT-derived material found in the lymph was absorbed in the
first 2.5-7 h, and 95% was absorbed within 18 h. Because the lymphatic
duct in the rat is not a single vessel, Rothe et al. (1957) were
unable to exclude the possibility that some, or all, of the DDT that
they later recovered from the carcasses of their animals had been
transported to the general circulation by collateral lymph vessels
rather than by the hepatoportal system. Thus, at least half, and
perhaps all absorption of DDT is by way of the lymph. However, in
studies on rats by Heath & Vandekor (1964), only a small proportion of
dieldrin was absorbed by the lymph. The reason for the marked
difference in the absorption of those organochlorine insecticides is
unknown.
6.1.3 Uptake from the skin
Undissolved DDT is so poorly absorbed through the skin that its
toxicity by this route is difficult to measure. Even dissolved DDT is
poorly absorbed by the skin as indicated by low toxicity (see
Table 5).
Table 5. Acute oral and dermal LD50 of DDT for animalsa
Species Formulation Oral Dermal
(mg/kg) (mg/kg)
Rat Water suspension or powder 500-2500 1 000 000
Oil solution 113-450 250-3000
Mouse Water suspension or powder 300-1600 375 000
Oil solution 100-800 250-500
Guineapig Water suspension or powder 2000 1 500 000
Oil solution 250-560 1000
Rabbit Water suspension or powder 275 375 000
Oil solution 300-1770 300-2820
Cat Water suspension or powder
Oil solution 100-410
Dog Water suspension or powder
Oil solution >300
a From: Hayes (1959).
6.2 Distribution and Storage
6.2.1 Human studies
6.2.1.1 Studies of volunteers
In a study of volunteers who received technical DDT at rates of 0,
3.5, and 35 mg/man per day, the average intakes resulting from dosing
and from traces of DDT in food were 0.0025, 0.05, and 0.5 mg/kg per
day (Hayes et al., 1956). The storage of DDT was proportional to
dosage, but there was an unexplained difference in the storage of the
p,p'-isomer and of technical DDT. For example, following dosing for
12 months, pure p,p'-DDT was stored in fat at an average
concentration of 340 mg/kg, but the sum of isomers from technical
material was stored at an average of only 234 mg/kg. The difference
was statistically significant for the 3.5 mg/man per day dosages given
for 3-6 and for 7-18 months. The difference was significant for the
35 mg/man per day doses after 7-18 months of dosing but not after only
3-6 months.
Men who ate p,p'-DDT showed a definite increase in the absolute
amount of DDE stored. After 6 months at a dosage of 35 mg/man per day,
8 men showed an average concentration of DDE stored in fat of 33 mg/kg
compared with 12 mg/kg for the same individuals at the beginning of
the investigation. There was a further increase in DDE storage as
exposure progressed. However, DDT was stored in so much greater
concentration that the relative storage of DDE decreased sharply.
Thus, after 6 months at a dosage of 35 mg/man per day, 8 men stored
only 14% of their total DDT-derived material in the form of DDE
compared with 65% for the same person at the beginning of the
investigation.
The storage of DDE by men who ate technical DDT presented a
different picture. Until 18 months after exposure, there was no clear
evidence that these men stored any more DDE after exposure than they
did before. However, at 18 months, the only 3 samples available showed
DDE concentrations ranging from 28 to 85 mg/kg, all substantially
above general population levels. Thus, both the total amount stored
and the rate at which DDT converted to DDE served to distinguish the
metabolism of p,p'-DDT and the sum of isomers present in technical
DDT in man (Hayes et al., 1956). A more rapid excretion was
demonstrated for o,p'-DDT by Morgan & Roan (1972).
In a second study (Hayes et al., 1971), volunteers received the
same doses used in the first study. Again, storage of DDT was
proportional to dosage. Although, in this instance also, the storage
of technical DDT was less than that of p,p'-DDT, the difference was
not statistically significant. The real but very gradual accumulation
of DDE was confirmed.
A steady state of storage was approached later in the second study
(18.8-21.5 months) than in the earlier one (about 12 months). However,
although the second study was superior in that more men were studied
for a longer period, it was inferior in that dosage was less regular.
Because of this, it seems impossible to decide whether 12 months or
21.5 months is a more valid estimate of the time necessary for people
to approach a steady state of storage when intake is uninterrupted and
unvarying in amount. It is interesting that the storage levels
eventually reached at the same dosage in the 2 studies were
statistically indistinguishable in most instances (see Table 6). In
the one instance in which a statistical difference existed, the
greater storage by men in the second study may have been explained by
the fact that some of them inadvertently received higher doses than
intended.
Table 6. Storage of DDT in volunteers
Type of DDT Added Concentration of DDTa Significance
dosage First studyb Second studyc of difference
(mg/man 11 months or more 21.5 months (P)
per day) (mg/kg) (mg/kg)
Technical 0 8-17 (12.5 ± 4.5) 16-30 (22.0 ± 2.9) > 0.1
3.5 26-33 (23.8 ± 1.4) 59-76 (50.2 ± 5.6) <0.025
35 101-367 (234 ± 21.4) 105-619 (281 ± 79.5) >0.4
Recrystallized 35 216-466 (340 ± 36.4) 129-659 (325 ± 62.2) >0.2
a Range, mean, and standard error.
b Hayes et al., 1956,
c Hayes et al., 1971.
There was a slow decrease in the levels of fat-stored DDT after
dosing ceased. The concentration remaining following 25.5 months of
recovery was from 32% to 35% of the maximum stored for those who had
received 35 mg/man per day but 66% for those who had received only
3.5 mg/man per day, indicating slower loss at lower storage levels
(Hayes et al., 1971).
Morgan & Roan (1971) fed volunteers not only technical DDT but
also p,p'-DDE and p,p'-TDE. They found that DDE was stored more
tenaciously than the other compounds in man, the order being p,p'-DDE
> p,p'-DDT > o,p'-DDT > p,p'-TDE. The slow metabolism of DDT to
DDE was confirmed. It was noted that p,p'-DDT was lost from storage
in adipose tissue much more slowly in man than in the monkey, dog, or
rat.
Less than 18% of p,p'-DDT and p,p'-DDE is carried in human
erythrocytes. In plasma of ordinary fat content, less than 1% of all
DDT-related compounds is carried by the chylomicrons. Instead, these
compounds are carried by proteins and are undetectable in plasma from
which protein has been precipitated. Following ultracentrifuging,
p,p'-DDT and p,p'-DDE are found mainly in the triglyceride-rich,
low density, and very low density lipoproteins. Following continuous
electrophoresis, these compounds are found mainly in association with
plasma albumin and alpha-globulins (Morgan et al., 1972).
6.2.1.2 Studies of occupationally exposed workers
The highest reported storage of DDT and related compounds remains
that of a healthy worker whose fat contained DDT and DDE (as DDT) at
concentrations of 648 and 483 mg/kg, respectively (Hayes et al.,
1956). Laws et al. (1967) reported considerably lower storage values
among the most exposed persons in a DDT manufacturing plant (see
Table 7).
An important point evident from the table is that, whereas almost
all investigations of workers are said to have been carried out on
"heavily exposed" populations, some of the groups studied had absorbed
little more DDT than is absorbed by the general population --
especially the general population of some tropical countries.
A different situation is indicated in a report by Genina et al.
(1969) who used a total chloride method to analyse samples of blood
from controls and from persons with occupational exposure to DDT,
polychloropinene, and HCH. Whereas the highest average concentration
of total DDT-related material per se in the serum of a worker in the
USA was 2.7 mg/litre (Laws et al., 1967), Genina et al. (1969)
reported organochlorine compounds as high as 38.4 mg/litre in the
blood of a pilot and values as high as 195 mg/litre in the blood of
warehousemen. This concentration is about 20 times the highest value
found by the same authors in their control group (see Table 8). The
factor of 20 is not remarkable, but (especially in view of the fact
that polychloropinene and HCH are excreted more readily than DDT and
DDE) values as high as 0.2 mg/litre in the controls are unexpected.
Table 7. Average concentration of the sum of the isomers of DDT and DDE in fat and serum
and of DDA in the urine of workers engaged in the manufacture, formulation, or
use of DDT
Tissue No. of DDT DDE DDA Total as Estimated Reference
men (mg/kg) (mg/kg) (mg/kg) DDT exposure
(mg/kg) (mg/man
per day)
fat 1 648 437 1131 Hayes et al., 1956
urine 10 0.57 14 Ortelee, 1958
urine 16 1.7 30 Ortelee, 1958
urine 13 2.9 42 Ortelee, 1958
fat 3 51 44 98 3.6 Laws et al., 1967
fat 12 74 50 130 6.2 Laws et al., 1967
fat 20 161 91 263 18 Laws et al., 1967
serum 3 0.2113 0.1968 0.5412 6.3 Laws et al., 1967
serum 12 0.1420 0.1454 0.3584 8.4 Laws et al., 1967
serum 20 0.3020 0.2719 0.7371 17.5 Laws et al., 1967
urine 3 0.0165 0.0203 0.41 0.5629 Laws et al., 1967
urine 12 0.0145 0.0222 0.6 0.7911 Laws et al., 1967
urine 20 0.0145 0.0271 1.27 1.6296 Laws et al., 1967
fat 18 5.2-45.2 Gracheva, 1969
urine 136 0.402 Perini & Ghezzo, 1970
urine 110 0.142 Perini & Ghezzo, 1970
urine 290a 0.061 Perini & Ghezzo, 1970
plasma 16 0.0513 0.0722 0.1321 Wassermann et al., 1970c
serum 4 <0.087 <0.072 Edmundson et al., 1970a
urine 0.080 Edmundson et al., 1970a
serum 18 0.573 0.506 Poland et al., 1970
serum 5 0.004a 0.021a Clifford & Well, 1972
serum 10 0.022 0.055 Clifford & Well, 1972
serum 21 0.021a 0.013a Keil et al., 1972
blood 44 0.761 WHO, 1973
blood 100 1.273 WHO, 1973
Table 7 (Cont'd)
Tissue No. of DDT DDE DDA Total as Estimated Reference
men (mg/kg) (mg/kg) (mg/kg) DDT exposure
(mg/kg) (mg/man
per day)
serum 21 0.300 0.379 0.681 Almeida et al., 1974
serum 25 0.225 0.308 0.504 Almeida et al., 1974
serum 18 0.345 0.257 0.602 Almeida et al., 1974
serum 56 0.004a,b 0.052a,b Morgan & Roan, 1974
serum 32 0.002b 0.026b Morgan & Roan, 1974
serum 32 0.004b 0.047b Morgan & Roan, 1974
serum 32 0.009b 0.075b Morgan & Roan, 1974
serum 31 0.052b 0.222b Morgan & Roan, 1974
plasma 25 1.030 Rabello et al., 1975
plasma 8 0.240 Rabello et al., 1975
plasma 23 0.0389 Richardson et al., 1975
a Control group.
b Approximately equal groups arranged by degree of storage.
Table 8. Percentage of workers with blood organochlorine compound content falling in certain
ranges of concentrationa
Subject group Range of concentrations
0 0.2-0.9 1.0-3.0 4-9 10-50 50
(mg/litre) (mg/litre) (mg/litre) (mg/litre) (mg/litre) (mg/litre)
Control group (47) 21.3 44.7 23.4 10.6 0 0
Pilots (134)
group A 19.1 35.3 25.0 14.7 5.9 0
group B 22.9 27.1 40.6 7.4 2.0 0
Technicians (133)
group A 26.2 16.4 39.3 8.2 9.9 0
group B 39.4 31.2 25.7 3.7 0 0
Agricultural 13.0 43.5 31.0 7.0 2.0 3.5
workers (55)
a From: Genina et al. (1969).
NB: Group A gives the results of investigations at the time of work, group B before work
or a few months after termination. Agricultural workers were studied only during work.
The first evidence that a part of the DDT absorbed by man is
metabolized to DDE was obtained from the analysis of fat from a DDT
plant worker (Mattson et al., 1953).
6.2.1.3 Studies of the general population
DDT in fat. Table 9 summarizes the results of measurements of
DDT and related materials in the body fat of people without
occupational exposure. Several broad generalizations can be made from
the table and from what is known about residues of DDT in food in
different countries. DDT storage in man corresponds with exposure; in
general, exposure tends to be greater in warm climates where there is
a greater need for insecticides. This climatic dependence may be
observed even within a single country (Hayes, 1975). Where
measurements have been made during a sufficiently long period, storage
has decreased as the use of DDT, especially that leading to residues
in food, has decreased.
The method of DDT analysis shifted in about 1962 from the
Schecter-Haller colorimetric method to the gas chromatographic method.
However, as noted by Hayes (1975), this made little difference to the
overall results. Thus the absolute decrease of total DDT storage and
the relative increase of DDE storage observed in some countries is
real.
Circumstances peculiar to some subpopulations explain their
unusual storage of DDT. Thus, persons living in one of the contiguous
states of the USA stored significantly less DDT than other persons in
the state because they did not eat meat (Hayes et al., 1958). Even
less storage of DDT was observed in Eskimos whose diet contained an
unusually high proportion of meat obtained from wildlife in which no
DDT and almost no DDE could be detected (Durham et al., 1961).
No significant difference was found in the concentration of DDT
stored in the fat of different parts of the body (Hayes et al., 1958;
Casarett et al., 1968).
DDE in fat. The accumulation of DDE relative to total DDT-related
compounds is best illustrated in man. Of the total DDT stored
in the fat of workers exposed to technical DDT (about 4% DDE) for
11-19 years, only 38% was in the form of DDE, and, of course, some of
that DDE came from their diets which included meat (Laws et al.,
1967). In India, where many people avoid meat but may consume milk,
cheese, and eggs, 34-41% of total DDT was DDE (Dale et al., 1965). In
the USA, during a time when DDT residues in food were decreasing, the
proportion of total DDT in the form of DDE increased from about 60% in
1955 to about 80% in 1970; during the same interval the concentration
of total DDT in body fat decreased from about 15 mg/kg to slightly
Table 9. Concentration of DDT-derived material in body fat of the general population
Country Year No. of Method of DDTa DDE as Total as DDE as Reference
samples analysis (mg/kg) DDT DDT DDT
(mg/kg) (mg/kg) (% of
total)
North America
Canada 1959-1960 62 colour 1.6 3.3 4.9 67 Read & McKinley, 1961
Canada 1966 47 GLCb and ELC 1.09 2.96 4.39 67 Brown, 1967
Canada 1967-1968 51 GLC 1.56 4.16 5.86 71 Kadis et al., 1970
Canada 1969 4.85 Ritcey et al., 1973
Canada GLC 5.83 Brown & Chow, 1975
USA 1942 10 colour NDc NDc NDc Hayes et al., 1958
USA 1950 75 colour 5.3 -- 5.3 -- Laug et al., 1951
USA 1955 49 colour 7.4 12.5 19.9 63 Hayes et al., 1956
USA 1954-1956 61 colour 4.9 6.8 11.7 58 Hayes et al., 1958
USA 1956 36 colour 5.5 10.1 15.6 65 Hayes et al.. 1971
USA 1961-1962 130 colour 4.0 8.7 12.7 69 Quinby et al., 1965a
USA 1961-1962 28d GLC 2.4 4.3 6.7 64 Dale & Quinby, 1963
USA 1962-1963 282 GLC 2.9 8.2 11.1 74 Hoffman et al., 1964
USA 1964 64 GLC 2.5 5.1 7.6 67 Zavon et al., 1965
USA 1964 25 GLC 2.3 8.0 10.3 77 Hayes et al., 1965
USA 1964-1965 18 GLC 9.0 Schafer & Campbell, 1966
USA 1964-1965 42 GLC 3.1 7.5 10.6 71 Radomski et al., 1968
USA 1962-1966 994 GLC 2.6 7.8 10.4 75 Hoffman et al., 1967
USA 1964-1965 12 GLC 3.79 7.7 11.5 67 Davies et al.. 1965
USA 1965-1967 17 GLC 3.1 5.5 56 Davies et al., 1968
USA 90 GLC 6.1 8.4 73 Davies et al., 1968
USA 17 GLC 4.6 7.8 59 Davies et al., 1968
USA 35 GLC 12.0 16.7 72 Davies et al., 1968
USA -- 42 GLC 3.13e 7.43 10.56 70 Fiserova-Bergerova et
al., 1967
Table 9 (Cont'd)
Country Year No. of Method of DDTa DDE as Total as DDE as Reference
samples analysis (mg/kg) DDT DDT DDT
(mg/kg) (mg/kg) (% of
total)
USA -- 30e GLC 1.33 5.17 6.51 79 Casarett et al., 1968
USA 29f GLC 1.35 4.91 6.31 78 Casarett et al., 1968
USA 30g GLC 1.16 4.99 6.17 81 Casarett et al., 1968
USA 1966-1968 70 GLC 1.54 5.15 6.69 77 Morgan & Roan, 1970
USA 1967 733 GLC 1.34 4.74 6.22 77 Yobs, 1969 (unpublished
data)
USA 1968 3104 GLC 1.56 5.96 7.67 77 Yobs, 1969 (unpublished
data)
USA 1967-1971 103 GLC 1.5 5.6 7.1 79 Warnick, 1972
USA 1970 200 GLC 1.9 8.0 9.9 81 Wyllie et al., 1972
USA 1969-1972 221 23.18 Burns, 1974
USA 1970 1412 7.87h,i Kutz et al., 1974
South America
Argentina 1967 37 GLC 5.5 6.5 13.2 -- Wassermann et al., 1968b
Brazil 1969-1970 38 GLC 1.4 2.7 4.1 Wassermann et al., 1972b
Venezuela 1964 38 GLC 2.9 7.4 10.3 72 Dale, 1971 (unpublished
data)
Europe
Austria 6.33 Pesendorfer et al., 1973
Belgium 20 GLC 1.2 2.1 3.3 64 Maes & Heyndrickx, 1966
Bulgaria 55 GLC 3.8 5.8 10.6n 57 Kaloyanova et al., 1972
Bulgaria 1971-1976 191 GLC 14.7 78 Rizov, 1977
Czechoslovakia 1963-1964 229 colour 5.5 4.1 9.6 43 Halacka et al., 1965
Denmark 1965 18 GLC 0.6 2.7 3.3 82 Weihe, 1966
Denmark 1972-1973 78 GLC 4.1 4.7 87 Kraul & Karloq, 1976
Table 9 (Cont'd)
Country Year No. of Method of DDTa DDE as Total as DDE as Reference
samples analysis (mg/kg) DDT DDT DDT
(mg/kg) (mg/kg) (% of
total)
Finland 1972-1974 73 GLC 2.5 Hattula et al., 1976
France 1961 10 colour 1.7 3.5 5.2 67 Hayes et al., 1963
German Democratic 1966-1967 100 GLC and TLC 3.7 9.47 13.1 71 Engst et al., 1967
Republic
German Democratic 34j 1.8 5.1 6.9 Engst et al., 1970
Republic
Germany, Federal 1958-1959 60 colour 1.0 1.3 2.3 57 Maier-Bode, 1960
Republic of
Germany, Federal 1970 20 GLC 1.1 2.5 3.6 69 Acker & Schulte, 1970
Republic of
Germany, Federal 9.8 Acker & Schulte, 1971
Republic of
Germany, Federal 4.24 Acker & Schulte, 1974
Republic of
Germany, Federal 4.77 Acker & Schulte, 1974
Republic of
Germany, Federal 5.42 Acker & Schulte, 1974
Republic of
Germany, Federal 8.36 Acker & Schulte, 1974
Republic of
Germany, Federal 7.8 Acker & Schulte, 1974
Republic of
Hungary 1960 48 colour 5.7 6.0 12.4 48 Denés, 1962
Italy 1965 9 GLC 1.8 3.2 5.0 63 Kanitz & Castello, 1966
Italy 1965-1966 18 GLC 2.58 8.28 10.86 76 Paccagnella et al., 1967
Italy 1966 22 GLC and TLC 4.69 10.69 15.48 68 Del Vecchio & Leoni, 1967
Italy 1970? 31 GLC 3.38 13.37 16.75 80 Prati & Del Dot, 1971
Italy 1970? 52 GLC 2.14a 8.46a 10.60a 80 Prati et al., 1972
Italy 1970? 33 GLC 0.84a 6.64a 7.48a 89 Prati et al., 1972
Table 9 (Cont'd)
Country Year No. of Method of DDTa DDE as Total as DDE as Reference
samples analysis (mg/kg) DDT DDT DDT
(mg/kg) (mg/kg) (% of
total)
Netherlands 1964 20 colour 1.6 6.1 7.7 79 Wit, 1964
Netherlands -- 11 GLC 0.32 1.89 2.22 86 deVleiger et al., 1968
Norway 56 GLC 3.2 71 Bjerk, 1972
Poland 1965 72 colour 13.4 Bronisz st al., 1967
Poland 65 colour 23.5 62 Bronisz et al.. 1969
Poland 1970 70 GLC 11.4 Juszkiewicz & Stec, 1971
Poland 1972 15 GLC 5.23 68 Bojanowska et al., 1973
Romania 1965 137 -- 13.4 8.3 21.7 39 Aizicovici et al., 1968
Romania 1972-1973 GLC 0.68 1.73 2.41 71 Ciupe, 1976
Spain 1966 41 -- 6.5 9.2 15.7 59 Unpublished data cited
by Wassermann et al., 1968
Switzerland 1.9-16.3 Zimmerli & Marek, 1973
United Kingdom 1961-1962 131 colour -- -- 2.2 -- Hunter et al., 1963
United Kingdom 1963-1964 66 GLC 1.1 2.2 3.3h 67 Egan et al., 1965
United Kingdom 1964 100 GLC -- -- 3.9h -- Robinson et al., 1965
United Kingdom 1964 44 GLC -- -- 4.0h -- Robinson & Hunter, 1966
United Kingdom 1965 101 GLC 1.13 1.72 2.85 60 Cassidy et al.. 1967
United Kingdom 1965-1967 248 GLC 0.78 2.22 3.00 74 Abbott et al., 1968
United Kingdom 1969-1971 201 GLC 0.5 1.8 2.5 72 Abbott et al., 1972
USSR 41 TLC 4.33 3.73 Vas'kovskaja & Komarova.
1967
USSR 197 TLC 5.3- 3.2- Vas'kovskaja, 1969
7.57 7.1
Africa
Kenya 5.4 Wassermann et al., 1972a
Nigeria 1967 43 GLC 5.4 3.1 8.8 Wassermann et al., 1968b
Nigeria 1969 41 GLC 2.1 2.8 6.5 57 Wassermann et al., 1972d
Table 9 (Cont'd)
Country Year No. of Method of DDTa DDE as Total as DDE as Reference
samples analysis (mg/kg) DDT DDT DDT
(mg/kg) (mg/kg) (% of
total)
Africa
South Africa 5.94 43 Wassermann et al., 1970b
South Africa 7.16 Wassermann et al., 1970b
Uganda 2.9 Wassermann et al., 1974a
Asia
India (Delhi area, 1964 67 colour 17 10 26 39 Dale et al., 1965
civilian)
India (other 1964 16 colour 8 5 13 37 Dale et al., 1965
cities, military)
India 94 GLC 13.8h 42 Ramachandran et al., 1974
Israel 1963-1964 254 colour 8.5 10.7 19.2 56 Wassermann et al., 1965
Israel 1965-1966 71 colour 4.6 Wassermann et al., 1967
Israel 1965-1966 133 colour 8.2 Wassermann et al., 1967
Israel 1967-1971 63 GLC 3.0 10.6 14.4 74 Wassermann et al., 1974b
Japan 1968-1969 241 GLC 0.6 1.8 2.4 75 Curlay et al., 1973
Japan 1969-1970 74 6.92 Nishimoto et al., 1970
Japan 1970 4.499 Suzuki et al., 1973
Japan 1971 2.694 Suzuki et al., 1973
Japan 1971 30 12.895 Kasai st al., 1972
Japan 1972 4.001 Suzuki et al., 1973
Japan 1972 42 5.992 Kasai et al., 1972
Japan 1973 6.44 Kawanishi et al., 1973
Japan 1974 6.87 Inoue et al., 1974
Pakistan 60 25.0 Mughal & Rahman, 1973
Thailand 1969-1970 77 12.6 Wassermann et al., 1972c
Oceania
Australia 1965 53 GLC 0.77h 1.03h 1.81h 57 Bick, 1967
Australia 1965-1966 46 colour 3.6 6.6 10.2 64 Wassermann et al., 1968a
Australia 1965-1966 12 GLC 3.0 7.1 10.5 68 Wassermann et al., 1968a
Australia 1971 75 GLC 4.94 Brady & Slyali, 1972
Table 9 (Cont'd)
Country Year No. of Method of DDTa DDE as Total as DDE as Reference
samples analysis (mg/kg) DDT DDT DDT
(mg/kg) (mg/kg) (% of
total)
Oceania
New Zealand 1966 52 GLC 1.6 4.2 5.8 72 Brewerton & McGrath, 1967
New Zealand 1965-1969 254 GLC 3.5 11.0 14.6 75 Copplestone et al., 1973
a p,p'-DDT and o,p'-DDT only. Total as DDT includes TDE (DDD) and other forms when given, which are not shown in this table.
b Gas-liquid chromatography.
c Not detected,
d These 28 samples were also tested for DDT and DDE content by a colorimetric method, and the results are included in the
130 samples listed above.
e Perirenal fat.
f Mesenteric fat.
g Panniculus fat.
h Geometric mean.
i Lipid basis.
j Infants and children.
less than 10 mg/kg (see Table 9). Thus, a low proportion of DDE
indicates a relatively high intake of DDT, a relatively low intake of
DDE residues (e.g. in food), and relatively few years for the
metabolism of stored DDT to DDE.
DDT in blood. The finding of DDT and related compounds in blood
(usually serum) of the general population of different countries is
shown in Table 10. Compared to body fat, blood has been analysed for
DDT in fewer countries and for fewer years. Some of the same
differences between populations and time periods observed in connexion
with DDT in fat have been detected in connexion with DDT in blood, but
the differences are less marked.
There is a small but statistically significant decrease in the
concentrations of DDT-related compounds in the plasma of women 1-6
days postpartum compared with the same women early in pregnancy. Most
of the decrease seems to occur during about the last 10 days before
delivery (Curley & Kimbrough, 1969). In a similar way, the
concentration of DDT-related compounds in various tissues of women at
the time of Caesarean section or normal delivery is less than in
nonpregnant women in the same community (Polishuk et al., 1970).
The concentration of p,p'-DDE is remarkably constant throughout
the day, but minor increases in it and in p,p'-DDT may occur after a
meal (Radamski et al., 1971a).
In so far as can be judged from the bar graphs presented by
Griffith & Blanke (1975), the concentrations of DDT-related materials
they found in postmortem blood were similar to the concentrations
reported in Table 10 for fresh blood except that postmortem blood
contained more TDE (DDD).
DDT and related compounds in other tissues. DDT has been found
in some samples of all human organs. Results reported in the USA and
USSR are shown in Tables 11 and 12, respectively. Results in Table 11
are based on the wet weight of the tissues. Results in Table 12,
presumably, are based on the weight of extractable lipid, but the
paper does not specify this; whatever the basis, the paper reports
concentrations of DDT and DDE in the range of 10.0-35.0 mg/kg in the
heart muscle, liver, and kidneys of 2 persons who had had direct
contact with pesticides. Although concentrations of chlorinated
compounds in liver, kidney, brain, and spleen of persons from Ferrara
Province, Italy, were generally higher than those in the USA (Prati et
al., 1972), the differences were not great.
Table 10. Concentration mg/litre of DDT-related compounds in the blood of members of the general population
Country Year No. of p,p'-DDT o,p'-DDT p,p'-DDE o,p'-DDE p,p- o,p- Total DOT DDE Reference
samples TDE TDE equivalent as %
(DDD) (DDD) total
North America
Canada 0.032 Brown & Chow, 1975
USA (Atlanta) 1965 10 0.0119 0.0013 0.0257 0.0418 68.6 Dale et al., 1966b
USA (Atlanta) 1966 10m 0.0746 Dale et al., 1967
USA (Atlanta) 1966 10f 0.0360 Dale et al., 1967
USA (Louisiana) 1966-1967 53a 0.00182 0.00182 0.00265 0.00265 0.00062 0.00062 0.00501 59.0 Selby et al., 1969
USA (4 states) 1967 64 0.00335 0.00044 0.00837 0.00096 0.00032 0.00032 0.01425 78.0 Yobs, 1969
(unpublished data)
USA (3 states) 1968 106 0.00342 0.00006 0.00927 0.00000 0.00004 0.00009 0.01397 73.4 Yobs, 1969
(unpublished data)
USA (Idaho) 1967-1968 1000 0.0047 0.0220 0.0002 0.02940 83.5 Watson et al., 1970
USA (Atlanta) 1969 30b,c 0.0046 0.0011 0.0062 0.0003 0.0014 0.0144 50.0 Curley et al., 1969
USA 1968 5d 0.0050 Curley & Kimbrough, 1969
USA 1968 10d 0.0205 Curley & Kimbrough, 1969
USA (Florida) 45e 0.0108 O'Leary et al., 1970b
USA (Florida) 107g 0.0152 O'Leary et al., 1970b
USA(Florida) 1970 26 0.03169 Radomski et al., 1971a
South America
Argentina 1970 20h 0.01934 Radomski et al., 1971b
18i 0.01327 Radomski et al., 1971b
19j 0.00869 Radomski et al., 1971b
Brazil 1973? 15f 0.0189 0.0237 0.0453 Schvartsman et al., 1974
Brazil 1973? 15c 0.0118 0.0104 0.0234 Schvartsman et al., 1974
Brazil 1974? 30 0.145 0.026 0.155 0.336 Almeida et al., 1975
Brazil 1974? 11 0.083 0.117 0.194 Almeida et al., 1975
Brazil 1974? 20 0.086 0.121 0.212 Almeida et al., 1975
Table 10 (Cont'd)
Country Year No. of p,p'-DDT o,p'-DDT p,p'-DDE o,p'-DDE p,p- o,p- Total DOT DDE Reference
samples TDE TDE equivalent as %
(DDD) (DDD) total
Europe
Bulgaria 1971-1976 171 0.039a Rizov, 1977
Hungary 1967-1968 120 0.034 Czeglédi-Janko, 1969
Poland 0.172d Jonczyk, 1970
Poland 1972 15 0.030 63 Bojanowska et al., 1973
Switzerland 13 0.0209 Zimmerli & Marek, 1973
Asia
Israel 1975 29 0.0133 0.0112 0.0195 0.0112 0.0087 0.0072 0.0740 47 Polishuk et al., 1977
Japan 1970 10 0.011 Tokutsu et al., 1970
Japan 1971 0.005 Kojima et al., 1971
Japan 1971 138 0.0183 Kasai et al., 1972
Japan 1971 0.0093 Yamagishi et al., 1972
Japan 1971 0.0285 Kaku, 1973
Japan 1972 0.001-0.078 Study Group, 1972
Japan 37 0.0437d Nawa, 1973
Japank 0.1358 Hara et al., 1973
Japanc 0.0210 Hara et al., 1973
0.0779 Abe et al., 1974
Oceania
Australia 52 0.0172 Slyali, 1972
Australia 47 0.0169 Ouw & Shandar, 1974
a Geometric mean. d Maximal value. g Black women. j 1-5 years old.
b Mean of positive values only. e White women. h Adults. k Maternal blood.
c Cord blood from term infants. f Female. i 6-11 years old. m Mile.
Table 11. Average concentrationsb of organochlorine insecticides in various tissues from autopsies
of 44 members of the general populationa
Tissue No. Lipid DDT DDE TDE Heptachlor Dieldrin Total + SEc
analysed content (mg/kg) (mg/kg) (DDD) epoxide (mg/kg) (mg/kg)
(%) (mg/kg) (mg/kg)
Perirenal fat 30 55.7 1.33 4.64 0.0110 0.0220 0.0300 6.03 ± 5.30
Mesenteric fat 29 54.2 1.35 4.40 0.0470 0.0320 0.0630 5.89 ± 4.98
Panniculus fat 30 60.6 1.16 4.48 0.0180 0.0270 0.0270 5.71 ± 5.25
Bone marrow 19 20.6 0.411 2.08 0.0760 0.0040 0.620 2.63 ± 2.21
Lymph noded 11 8.6 0.892 1.38 0.0100 0.0001 0.0190 2.30 ± 4.52
Adrenal 18 10.5 0.125 0.875 0.0570 0.0012 0.0060 1.06 ± 1.31
Kidney 38 3.2 0.0827 0.209 0.0022 0.0009 0.0056 0.300 ± 0.651
Liver 42 2.1 0.0467 0.200 0.0326 0.0019 0.0037 0.285 ± 0.369
Brain 32 7.9 0.0105 0.0831 0.0020 0.0002 0.0031 0.989 ± 0.171
Gonad 36 1.3 0.0150 0.0688 0.0015 0.0001 0.0021 0.0875 ± 0.103
Lung 25 0.7 0.0147 0.0585 0.0009 0.0003 0.0022 0.0766 ± 0.125
Spleen 27 0.6 0.0112 0.0305 0.0031 trace 0.0021 0.0469 ± 0.074
a From: Cassarett et al. (1968).
b Wet tissue basis.
c SE = standard error of the mean.
d Tracheobronchial lymph nodes.
Table 12. Average DDT and DDE contents in certain human organsa
Organ Content % of positive DDT DDE
limits observations (mg/kg) (mg/kg)
Heart muscle 1.0-12.0 77.0 3.69 4.05
Liver 1.0-20.0 85.5 4.62 4.02
Kidney 1.0-12.0 71.0 2.99 3.44
Spleen 2.0-20.0 57.0 2.86 2.86
Pancreas 2.0-15.0 75.0 3.0 2.62
Adrenal 2.0-10.0 84.3 3.84 3.7
Thyroid 2.0-8.0 59.0 1.85 1.85
a From: Vas'kovskaja (1969).
DDT and related compounds in the tissues of infants. The
transfer of DDT to the fetus was observed by Deniés (1962) and
confirmed by many others (Curley et al., 1969; Zavon et al., 1969;
Komarova, 1970; O'Leary et al., 1970a,b,c). Typical findings are shown
in Table 13. It appears that the placenta is partially protective; the
concentrations of DDT and DDE are lower in cord blood than in
corresponding maternal blood (O'Leary et al., 1970b; Schvartsman et
al., 1974). The report by Engst et al. (1970) that infants lose a part
of their DDT stores during the first few months of life, as a result
of rapid growth, is not necessarily contradictory to the observation
that at birth they have less than their mothers, but it must be said
that the values reported by Engst et al. (1970) were relatively high
for infants (see also section 5.2).
Storage in relation to disease. A review (Hayes, 1975) indicates
that there is no agreement in the literature regarding the effect of
health on the storage of chlorinated hydrocarbon insecticides. Some
investigators have not found any difference in the concentration of
DDT in adipose tissue taken by biopsy or during minor elective surgery
in contrast to that taken at autopsy. Some investigators, who used
only autopsy samples, found no relationship between storage of
chlorinated hydrocarbon insecticides and the cause of death. Others,
of whom the first was Deichmann (Deichmann & Radomski, 1968; Radomski
et al., 1968), have reported that storage was 1.7-7.6 times greater in
persons dying of cirrhosis, atherosclerosis, hypertension, idiopathic
amyloidosis, and certain forms of cancer. Weight loss was not really
ruled out in these studies. Its importance was emphasized by Casarett
et al. (1968), who found that disease did not influence concentrations
on a wet weight basis but only on a lipid basis; samples with the
highest levels of DDT on a lipid basis came from persons who not only
had cancer but who were emaciated and had widespread abnormality of
Table 13. The range and mean of measurable concentrations of various organochlorines (mg/kg) in different tissues from stillborn infantsa
Tissues p,p'-DDT o,p'-DDT p,p'-DDE o,p'-DDE p,p'-TDE alpha-HCH ß-HCH gamma-HCH Heptachlor Dieldrin
received (DDD)
10 measurable 3 4 8 0 6 3 6 3 4 3
adipose concentration
range 0.16-2.15 0.35-11.47 0.16-3.19 -- 0.23-14.17 0.09-0.24 0.14-0.44 0.09-0.14 0.07-0.51 0.09-0.35
mean 0.88 3.39 1.22 -- 3.17 0.14 0.26 0.11 0.32 0.24
SE ± 0.63 2.70 0.38 -- 2.22 0.05 0.05 0.02 0.10 0.08
8 measurable 1 1 2 0 2 0 1 1 0 1
spinal concentration
cord range 0.47 0.47 0.30-1.16 -- 0.31-0.70 -- 0.17 0.10 -- 0.09
mean -- -- 0.73 -- 0.51 -- -- -- -- --
SE ± -- -- 0.43 -- 0.20 -- -- -- -- --
8 measurable 3 1 4 0 3 3 1 1 1 2
brain concentration
range 0.28-0.99 0.84 0.25-1.47 -- 0.20-1.22 0.04-0.49 3.81 0.06 0.13 0.84-0.86
mean 0.56 -- 0.65 -- 0.64 0.19 -- -- -- 0.05
SE ± 0.22 -- 0.28 -- 0.30 0.15 -- -- -- 0.01
9 measurable 3 2 6 0 3 2 3 3 2 1
adrenals concentration
range 1.28-1.65 0.36-1.05 0.13-1.96 -- 0.91-1.45 0.40-0.57 0.12-0.71 0.20-0.53 0.46-1.00 0.92
mean 1.48 0.71 1.05 -- 1.11 0.49 0.37 0.33 0.73 --
SE ± 0.11 0.35 0.28 -- 0.17 0.09 0.18 0.10 0.27 --
10 measurable 4 0 6 0 5 5 3 3 3 2
lungs concentration
range 0.57-1.01 -- 0.25-1.35 -- 0.31-1.05 0.07-0.69 0.05-0.18 0.05-0.25 0.08-0.31 0.27-0.72
mean 0.79 -- 0.85 -- 0.75 0.25 0.12 0.12 0.17 0.50
SE ± 0.11 -- 0.22 -- 0.13 0.11 0.04 0.07 0.07 0.23
Table 13 (Cont'd)
Tissues p,p'-DDT o,p'-DDT p,p'-DDE o,p'-DDE p,p'-TDE alpha-HCH ß-HCH gamma-HCH Heptachlor Dieldrin
received (DDD)
10 measurable 4 2 4 0 4 3 3 3 4 3
heart concentration
range 1.04-4.17 0.57-0.68 1.27-4.79 -- 1.02-5.82 0.23-0.52 0.15-0.31 0.19-0.27 0.30-1.56 0.08-1.02
mean 2.17 0.63 2.74 -- 3.27 0.33 0.22 0.22 0.80 0.49
SE ± 0.69 0.06 0.74 -- 1.10 0.09 0.05 0.03 0.30 0.28
10 measurable 5 3 6 0 6 3 4 4 3 2
liver concentration
range 0.15-1.59 0.22-3.42 0.22-2.45 -- 0.19-2.14 0.21-0.32 0.03-0.20 0.05-0.36 0.03-1.67 0.16-0.22
mean 0.79 1.32 0.98 -- 1.01 0.25 0.11 0.24 0.68 0.19
SE ± 0.24 1.05 0.34 -- 0.29 0.04 0.04 0.07 0.50 0.03
9 measurable 4 3 6 0 5 4 5 4 3 3
kidney concentration
range 0.62-7.60 0.29-2.07 0.11-9.78 -- 0.48-9.12 0.11-1.45 0.06-0.61 0.11-0.69 0.19-1.14 0.06-0.50
mean 3.71 1.38 3.57 -- 3.84 0.82 0.29 0.39 0.70 0.34
SE ± 1.70 0.55 1.72 -- 1.87 0.32 0.12 0.14 0.28 0.14
8 measurable 3 2 3 1 3 1 1 2 5 3
spleen concentration
range 0.48-1.04 0.45-2.94 0.60-1.05 0.29 0.18-0.91 0.21 0.16 0.17-0.18 0.10-0.52 0.18-0.51
mean 0.80 1.70 0.86 -- 0.56 -- -- 0.18 0.35 0.31
SE ± 0.17 1.25 0.13 -- 0.21 -- -- 0.005 0.08 0.10
3 measurable 1 0 1 1 0 0 0 0 0 0
pancreas concentration
range 0.49 -- 0.23 0.08
mean -- -- -- -- -- -- -- -- -- --
SE ± -- -- -- -- -- -- -- -- -- --
a From: Curley et al. (1969).
the liver. Factors other than emaciation may be important in some
conditions. For example, Oloffs et al. (1974) found that the DDT
concentration in fat was not influenced by cirrhosis, but that the DDT
concentrations in liver were significantly higher in cirrhotic livers
with severe fatty infiltration and significantly lower in those with
marked fibrosis or bile stasis.
That increased storage of DDT is a result and not a cause of the
diseases in which it sometimes occurs is shown by the fact that
persons with extensive occupational exposure average 10 times more
storage than the highest values reported in connexion with disease.
However, they do not exhibit any predisposition to the diseases in
question, and these diseases have shown no age-specific increase in
incidence related to the introduction and use of insecticides.
6.2.2 Animal studies
A detailed review of the literature (Hayes, 1959) shows that a
number of facts about the distribution and storage of DDT were
established early either by single, classical papers (now fully
confirmed) or by correlation of contributions from several
laboratories. The major results may be summarized as follows:
(a) DDT is stored in all tissues. Storage of the compound in
blood, liver, kidney, heart, and the central nervous system
was reported by Smith & Stohlman (1944).
(b) Higher concentrations of DDT are usually found in adipose
tissue than in other tissues (Ofner & Calvery, 1945).
(c) Rats store DDT in their fat at all accurately measurable
dietary levels including the unintended residues in standard
laboratory feeds.
(d) Following repeated doses, storage in the fat increases rapidly
at first and then more gradually until a peak or plateau is
reached (Laug et al., 1950). It was recognized that repeated
doses at a moderate rate could result in greater total storage
of DDT in the fat than a single dose at the highest rate that
can be tolerated or even a single dose at a rate that
frequently is fatal.
(e) By plotting animal data published no later than 1950, it is
possible to show that, when other factors are kept constant,
the equilibrium storage of DDT in each tissue varies directly
with the daily dosage.
(f) However (with the apparent exception of the dog) storage in
the fat, and perhaps in other tissues, is less extensive in
relation to dosage at higher dietary levels (Fig. 1).
(many of the compounds also exist as o,p'-isomers and other isomers)
Name DDT Chemical name R R' R"
and its major
metabolites
DDT 1,1'-(2,2,2-trichloroethylidene)- -Cl -H -CCl3
bis[4-chlorobenzene]
DDEa 1,1'-(2,2-dichloroethenylidene)- -Cl None =CCl2
bis[4-chlorobenzene]
TDE(DDD)a,b 1,1'-(2,2-dichloroethylidene)- -Cl -H -CHCl2
bis[4-chlorobenzene]
DDMUa 1,1'-(2-chloroethenyldene)- -Cl None =CHCl
bis[4-chlorobenzene]-
DDMSa 1,1'-(2-chtoroethylidene)- -Cl -H -CH2Cl
bis[4-chlorobenzene]
DDNUa 1,1'-bis(4-chlorophenyl)ethylene -Cl None =CH2
DDOHa 2,2-bis(4-chlorophenyl)ethanol -Cl -H -CH2OH
DDAa 2,2-bis(4-chlorophenyl)- -Cl -H -C(O)OH
acetic acid
Table 1 (Cont'd)
Name DDT Chemical name R R' R"
and its major
metabolites
Some related insecticides
NO2
Bulan(R) 2-nitro-1,1 -bis- -Cl -H '
(4-chlorophenyl)butane -CHC2H5
NO2
Prolan(R) 2-nitro-1,1-bis- -Cl -H '
(4-chlorophenylpropane -CHCH2
DMC 4-chloro-alpha[-(4-chlorophenyl)- -Cl -OH -CH3
alpha-(methyl)benzenemethanol
dicocol 4-chloro-alpha-(4-chlorophenyl)-alpha- -Cl -OH -CCl3
(Kelthane(R)) (trichloromethyl)benzenemethanol
chlorobenzilatec ethyl 4-chloro-alpha-(4-chlorophenyl)- -Cl -OH -C(O)OC2H5
alpha-hydroxybenzeneacetate
chloropropopylatc 1-methylethyl 4-chloro-alpha- -Cl -OH -C(O)OCH(CH3)2
(4-chlorophenyl)-aplha-hydroxy-
benzeneacetate
methoxychlorc 1,1'-(2,2,2-trichloroethylidene)- -OCH3 -H -CCl3
bis[4-methoxybenzene]
Table 1 (Cont'd)
Name DDT Chemical name R R' R"
and its major
metabolites
Perthane(R) 1,1'-(2,2-dichloroethylidene)- -C2H5 -H -CHCl2
bis[4-ethylbenzene]
DFDT 1,1'-(2,2,2-trichloroethylidene)- -F -H -CCl3
bis[4-fluorobenzene]
a Recognized metabolite of DDT in the rat.
b As an insecticide, this compound has the ISO approved name of TDE, and it has been sold
under the name Rothane(R); in metabolic studies the same compound has been referred to as
DDD; as a drug, it is called mitotane.
c Common name approved by the International Organization for Standardization (ISO).
and analogues that have had some use as insecticides. It must be
emphasized that even the commercially-available insecticidal analogues
have strikingly different properties. Especially remarkable is the
slow metabolism and marked storage of DDT and its metabolite DDE and
the rapid metabolism and negligible storage of methoxychlor.
No attempt has been made to include in Table 1 the wide range of
compounds that have been synthesized and studied in connexion with
structure-activity relationships, often with the hope of emphasizing
the good properties of DDT and reducing its undesirable properties.
For a more extensive consideration of analogues, see Metcalf (1955).
The formation of metabolites is considered in section 6.4.
2.1.3 Formulations of commercial or technical DDT
Technical DDT has been formulated in almost every conceivable form
including solutions in xylene or petroleum distillates, emulsifiable
concentrates, water-wettable powders, granules, aerosols, smoke
candles, charges for vaporizers, and lotions. Aerosols and other
household formulations are often combined with synergized pyrethrins.
When used as a drug, DDT is called clofenotane (INN) or Dicophane
(British Pharmacopoeia), Klorfenoton (Swedish Pharmacopoeia),
Chlorophenothane (United States Pharmacopoeia). For research or
reference it has been designated OMS 0016 and Ent. 1,506. DDT has been
sold under a variety of tradenames, including: Anofex(R), Cezarex(R),
Dinocide(R), Gesarol(R), Guesapon(R), Guesarol(R), Gyron(R),
Ixodex(R), Neocid(R), Neocidol(R), and Zerdane(R).
2.2 Analytical Procedures
Analytical procedures for determining residues of DDT-type
compounds in environmental samples involve several steps including
collection and extraction of the DDT-type compounds; removal of
coextractives by appropriate clean-up methods; and quantification of
p,p'-DDT and its analogues by a suitable technique. Each of these
major steps is discussed later. It is appropriate, however, first to
outline briefly the statistical criteria used to assess analytical
methods, the estimation of the lower limit of detection of a method,
and the procedure for confirming the chemical identities of the
components measured.
2.2.1 Statistical criteria for assessing analytical methods
The overall reliability of an analytical method can be assessed
using two criteria, namely, reproducibility and systematic error (or
bias). Reproducibility is both conceptually and practically the
simpler criterion; it may be defined as "the quantitative expression
of the random error associated with operators working in different
laboratories, each obtaining single results on identical test material
when applying the same method" (Institute of Petroleum, 1968). It may
be quantitatively specified in various ways, and it is important to
pay attention to the statistic (range standards, deviation, etc.) used
in a particular study to represent the random error of an analytical
method. If the results are normally distributed then the most
efficient statistic measuring reproducibility is the standard
deviation of a set of results (Nalimov, 1963; Youden & Steiner, 1975;
Davies & Goldsmith, 1976). Care should always be taken to ensure that
a particular statistic or statistical technique is appropriate for a
given set of results, by, for example, testing for outliers or, if
there are sufficient results, examining the distribution of the
results, before characterizing the reproducibility of an analytical
method.
There are two subdivisions of the reproducibility criterion,
namely, replication i.e., two or more results, obtained by the same
operator in a given laboratory using the same apparatus for successive
determinations on identical test material, within a short period of
time on the same day; and repeatability i.e., a quantitative
expression of the random error associated in the long run with a
single operator in a given laboratory obtaining successive results
with the same apparatus under constant operating conditions on
identical test material (Institute of Petroleum, 1968).
Reproducibility is, in turn, a subdivision of the random error in the
analysis of identical test material in different laboratories using
different techniques or variations of a particular method. Examples of
the assessment of the random errors found in the determination of
p,p'-DDT and related compounds are given below.
The systematic error of a method is the deviation of the
experimental results from the "true" values; such systematic error
causes the differences between the nominal value and the
experimentally-determined values to have predominantly the same sign
(as opposed to the random errors, where the results are equally likely
to be greater than or less than the true mean). Nominal or "true"
values are available only in the case of fortified (spiked) samples,
but whether such fortified samples are representative of actual
(environmentally incurred) contamination is open to doubt in the case
of some types of material. A rapid nonparametric test of systematic
error can be made using the sign-test or the Wilcoxan signed ranks
test (Conover, 1971).
It is common practice to calculate the "recovery" factor for an
analytical method, i.e., the ratio of the mean observed value to the
nominal value (usually expressed as a percentage), but the statistical
significance of the "recovery" factor should always be assessed. The
ratio of the mean deviation to the standard error of the mean
deviation is an appropriate method of testing the null-hypothesis that
the difference between the nominal value and the observed mean is not
statistically significant.
The term "total error" has been proposed for a function
incorporating both the systematic error and the reproducibility
(McFarren et al., 1970), and these authors also suggested three
classes of total error corresponding to excellent methods, acceptable
methods, and methods that are judged unacceptable. It is pertinent
that McFarren et al. concluded that the total errors in one study of
the determination of DDT-type compounds in water were unacceptable
(>50% for p,p'-DDT, and p,p'-DDE, 24.0-53.6% for o,p'-DDT).
2.2.2 Limit of analytical detection
All analytical determinations have a lower limit corresponding to
that quantity (Delta g) of p,p'-DDT (or a related compound) which
produces a response (Delta r) that cannot be distinguished from
response (Delta r) produced when no p,p'-DDT is present. The
response Delta r is generated by the materials, reagents, and
instruments, used in the procedure, for example, the small voltage
generated by electronic equipment, or the small absorption in a
spectrophotometric method. These responses are known as "blank" or
"noise", and their size depends on the presence of interfering
components in the test material, the purity of the reagents, the
cleanliness of the apparatus, and the design of electronic equipment
(amplifiers, etc.). The concept of limit of detection is a statistical
one and is related to the random variation of the response generated
by a blank or control (Sutherland, 1965; Skogerboe & Grant, 1970;
Kaiser, 1973). Currie (1968) has defined three limiting levels for use
in analytical chemistry: "the net signal level" above which an
observed signal may be reliably recognized; "the true net signal
level" which may, a priori, be expected to lead to detection; and
"the quantifiable level" at which the measurement precision is
sufficient for the quantity present to be estimated satisfactorily. In
many types of sample, the limit of detection is of rather academic
interest as the concentrations of DDT-type compounds in samples are an
order of magnitude greater than the limit of detection of a sensitive
detector such as the electron-capture detector. However, in analyses
of air and drinking-water, for example, it may be necessary to
ascertain the limit of detection by a suitable statistical procedure.
Lower limits of detectability (ng.g-1) suggested in the US EPA
Manual of Analytical Methods (Thompson, 1974) for DDT-type compounds
using gas-liquid chromatography are:
o,p'-DDE Adipose tissue, 10; serum, 1
p,p'-DDE Adipose tissue, 20; serum, 1
o,p'-DDT)
p,p'-TDE) Adipose tissue, 20; serum, 2
p,p'-DDT)
2.2.3 Confirmation of the identity of trace residues of DDT-type
compounds
Confirmation of the identity of p,p'-DDT and related compounds
in many types of environmental samples is not easy when the apparent
amounts present are in the microgram and submicrogram range, since the
classical procedures for the identification of organic compounds
require the use of milligrams of the purified compound. A definition
of chemical identity appropriate to trace analysis has been proposed
(Robinson et al., 1966), a definition that may need revision in
relation to the combined use of gas-liquid chromatography and mass-
spectrometry. Chemical derivatization techniques for DDT-type
compounds have been reviewed by Cochrane & Chau (1971). Other
techniques, that are used routinely in the confirmation of the
identity of DDT-type compounds include: determination of gas-liquid
chromatographic retention times using polar and nonpolar stationary
phases; thin-layer chromatography; paper chromatography; p-values;
infrared microtechniques; carbon skeleton chromatography; conversion
into dichlorobenzophenones; nuclear magnetic resonance; and X-ray
diffraction. Tables of the relative retention times (aldrin = 1.00) of
DDT-type compounds using nine liquid phases (Thompson et al., 1975)
and three mixed liquid phases (Suzuki et al., 1975); gas-liquid
chromatographic retention times for DDT-type compounds are also
summarized by Yermakov (1972). Relative thin-layer chromatographic
Rf values ( p,p'-DDE = 1.00) on alumina using three solvent systems
were reported by Thomas et al. (1968). Extraction p-values for
DDT-type compounds using seven solvent systems are given in the US EPA
Manual (Thompson 1974). Identification of DDT and its metabolites by a
microinfrared technique has been studied by Sierwiski & Helrich (1967)
and reference infrared spectra have been published (Chen et al.,
1972). Asai et al. (1971) used carbon skeleton chromatography to
differentiate polychlorinated biphenyls from DDT-type compounds. The
conversion of DDT-type compounds into the corresponding benzophenones
may be used to identify them in the presence of polychlorinated
biphenyls (Miles, 1972), but this technique has its drawbacks as
p,p'-DDT, p,p'-TDE and p,p'-DDE all give the same p,p'-
dichlorobenzophenone. The use of high resolution nuclear magnetic
resonance spectroscopy (with a time averaging computer to increase
sensitivity), for the confirmation of the presence of p,p'-DDT and
p,p'-DDE in human adipose tissue (total concentration about
13 mg/kg) was studied by Biros (1970).
The combination of gas-liquid chromatography with mass-
spectrometry is a powerful tool for the confirmation of identity of
trace residues of DDT-type compounds. For example, Gordon & Frigerio
(1972) used mass fragmentography and claimed identification of 10 pg
p,p'-DDT; Schaeffer (1974) identified DDT-type compounds in fish
using a fast scan mass-spectrometer to give a multiple mass spectrum
for each of the overlapping peaks.
2.2.4 Sampling and extraction
Before discussing different kinds of samples, it must be noted
that valuable information on many analytical problems may be found in
the US EPA Manual (Thompson, 1974) and the FDA Manual (McMahon &
Sawyer, 1977).
DDT-type compounds may be present in the air in vapour form or
adsorbed on particulate matter. Glass-fibre filters are suitable for
trapping the particulate matter (Stanley et al., 1971; Beyermann &
Eckrich, 1974) and DDT in the vapour form may be trapped using
impingers of the Greenburg-Smith type and a suitable nonvolatile
solvent using ethylene glycol. Miles et al. (1970) reported a trapping
efficiency of more than 99% for DDT. A three-trap system in series
comprising a column containing glass cloth, followed by an impinger
with hexylene glycol, and finally an alumina column was used by
Stanley et al. (1971). They estimated that the collection efficiency
(based on material balance studies) of this system for a mixture of
aerosol and vapour was about 60% for p,p'-DDE, 95% for p,p'-TDE,
and 100% for p,p'-DDT. Beyermann & Eckrich (1974) used glass wool
for the trapping of aerosols, and a stainless-steel net coated with
polyethylene glycol for trapping vapours. Trapping of organochlorine
insecticides in the vapour phase using support-bonded silicones on
various types of Chromosorb was considered to be quantitative by Aue &
Teli (1971): DDT-type compounds were not examined but the trapping of
lindane, aldrin, and heptachlor was considered satisfactory. A cross-
linked polystyrene resin, Chromosorb 102, was used by Thomas & Serber
(1974). These workers reported a collection efficiency of 98% for
o,p'-DDT at a concentration of 15 ng/m3. Herzel & Lahmann (1973)
used silica gel as the support for various liquid phases and concluded
that polyethylene glycol was the best absorbent for DDT-type compounds
in the air. Absolute calibration of the collection efficiency of
aerosols and atmospheres for DDT-related compounds is difficult, but
the studies that have been made indicate that impingers with ethylene
glycol or hexylene glycol are probably the most efficient. The
simplest empirical test of the efficiency of the trapping system is to
use two (or more) impingers in series and analyse the liquid
absorbents from the impingers separately. The ratio of the amounts
present in the first and second impingers should be higher than 10:1.
Detailed instructions for the sampling and analysis of air for
pesticides (including DDT-type compounds) by a method that has been
subjected to interlaboratory study are given in the US EPA Manual
(Thompson, 1974).
For the determination of DDT-type compounds in water an
uncontaminated container may be filled, but if the concentrations of
DDT are likely to be extremely low (as would be expected in most
potable waters), then drawing the water through a suitable trapping
device may he more appropriate; this method also gives a time-weighted
average concentration and can be used in an automated monitoring
procedure.
Benzene was used by Pionke et al. (1968) as the solvent to extract
p,p'-DDT and p,p'-TDE from fortified samples of distilled water
and lake waters. The levels of fortification were high (µg/litre) and
the recoveries were: p,p'-DDT, 96.1% ± 1.02%; p,p'-TDE 97.3% ± 0.89%.
Thus, at concentrations of the order of 0.001 mg/litre, the
benzene extraction method gives excellent recoveries (total errors,
5.9% and 4.5%, respectively). A continuous flow method based
on liquid-liquid partition was developed by Ahling & Jensen (1970);
water is passed through a column containing Chromosorb W coated with
undecane plus a macrogol Carbowax 4000 monostearate. The best
collection efficiencies were found when the two liquid absorbents were
used at concentrations of 10% and 30%, respectively, on the solid
support. At concentrations in the ng/litre range, the optimum recovery
of known amounts of DDT-type compounds added to water were obtained
with 1.5 g coated Chromosorb W per litre of water. Ahnhoff & Josefson
(1974) used continuous flow liquid-liquid partition between water and
cyclohexane (in three extractors in series). The extraction
efficiencies from water containing 5 ng p,p'-TDE or 7.6 ng p,p'-DDT
per litre were greater at a flow rate of 2 litre/h than at
5 litre/h being 90% and 98% for p,p'-DDT, and p,p'-TDE,
respectively, with 92% and 93%, respectively, of the recovered
compounds being found in the first extractor. A method using a solid
adsorbent, a macroreticular resin, XAD-4, has been studied by Musty &
Nickless (1974); at a flow rate of 8 ml/min through a column
containing 2 g XAD-4, satisfactory recoveries of p,p'-DDT and three
related compounds were obtained at concentrations of 2-10 ng-litre.
Carbon is a very efficient adsorber of p,p'-DDT and p,p'-TDE from
water (Rosen & Middleton, 1959), but desorption from charcoal is
difficult; furthermore, the recoveries are not very satisfactory and
this is attributed to chemical changes catalysed by carbon in contact
with water rather than inefficient desorption (Eichelberger &
Lichtenberg, 1971). Another solid adsorbent is polyethylene (Beyermann
& Eckrich, 1973), but in the case of river water variable results were
obtained because of the effects of other solutes. Taylor & Bogacka
(1968) used petroleum ether to extract DDT from water; following a
clean-up with acetonitrile partition and Florisil the overall recovery
(using thin-layer chromatography) was incomplete (about 66%). The
total error of this procedure is unacceptable. Benzene was used as the
extraction solvent by Djatlovitskaja et al. (1972), and a general
review of the determination of organochlorine insecticides (and other
insecticides) in water has been published by Novikova (1973).
The need for scrupulous cleanliness in glassware and purity of
reagents cannot be overstressed in the case of the determination of
DDT-type compounds in air and water as the residues are usually so
small; the use of electronic equipment with a low noise characteristic
and constant checking of the response of detectors are also necessary.
A major difficulty in the determination of organochlorine
insecticides in soils is the initial extraction of the residues
because of a combination of factors, including the wide variation in
soil types. Chiba (1969) emphasized the lack of precision in defining
soil types. In his review article, he concluded that the most
effective solvent systems for extraction of these compounds were
mixtures of n-hexane/acetone (1:1 v/v) or chloroform/methanol
(1:1 v/v), and that the moisture content of the soil should be at
least 5%. The results of a study of the determination of
organochlorine insecticides in three types of soil have been
summarized by Woolson & Kearney (1969). Twelve laboratories
participated using various extraction procedures. In one type of soil
(a silty clay loam) that was fortified with p,p'-DDT at a
concentration of 5 mg/kg, the amounts found in the different
laboratories varied between 1.60 and 5.48 mg/kg. The results of 3 of
the laboratories were discarded by Woolson & Kearney (1969), and the
mean recovery of the other 9 laboratories was 79.3%, with a standard
deviation of 42.2%. Wetting of the soil before extraction was
considered to improve the recoveries, Soxhlet extraction appeared to
be preferable to shaking with the solvent, and hexane/acetone
(1:1 v/v) or hexane/isopropanol (3:1 v/v) appeared better extractants
than other solvents.
A further collaborative study of the determination of organochlo-
rine insecticides in 3 types of soil was reviewed by Woolson (1974).
Although 12 laboratories participated, only 7 completed the study. All
the laboratories used the same extraction, clean-up and quantification
procedures, including premoistening of the soil with ammonium chloride
solution at 0.2 mol/litre. The recoveries of p,p'-DDE, p,p'-TDE,
o,p'-DDT and p,p'-DDT were higher than 80%, with standard errors
of 8-18%. These results are much more consistent than those of the
previous study, and Woolson recommended that premoistening of the soil
with ammonium chloride solution (0.2 mol/litre) followed by extraction
with hexane/acetone (1:1 v/v) should be adopted for the determination
of chlorinated hydrocarbon insecticides in soil.
Several solvents have been used for the extraction of DDT-type
compounds from nonfatty foods such as vegetables, fruit, and cereals;
acetonitrile, alone or mixed with water is used in the method of the
Association of Official Analytical Chemists (AOAC) depending on the
water content of the sample (Horwitz, 1975); Cieleszky et al. (1970)
recommend Soxhlet extraction with diethyl ether.
Maceration of vegetables with a mixture of acetone and hexane was
used by Sissons et al., 1968; the same mixed solvent was used for
cereals, and root vegetables by Abbott et al., 1969 who used
propan-2-ol in the case of fruit and green vegetables. Whiting et al.
(1968), and Skrentny & Dorough (1971) considered a mixture of methanol
and chloroform to be the most efficient extraction solvent for use
with a macerator or a Soxhlet extractor. The acetonitrile extraction
technique was examined by Zerber et al. (1971) for the extraction of
DDT-type compounds from cereal products and feeding stuffs. Diethyl
ether was used by Kucinski (1972) for extraction from canned vegetable
products.
Extraction methods for fat-containing foods are given in the AOAC
Method (Horwitz, 1975), the solvent used, methanol or petroleum ether,
being dependent on the type of sample. Soxhlet extraction using
petroleum ether is recommended by the Federal Health Office of the
Federal Republic of Germany (Anon., 1974); Cieleszky et al. (1970)
also recommended petroleum ether as a suitable solvent.
Smart et al. (1974) compared acetonitrile and dimethyl formamide
as extraction solvents for apples, carrots, potatoes, and vegetables;
a third solvent dimethylsulfoxide was compared with these two solvents
for butter, cheese, and eggs.
Three body tissues or fluids have been analysed in many surveys,
namely adipose tissue, blood (or serum), and mother's milk. The US EPA
Manual (Thompson, 1974) recommends grinding a sample of adipose tissue
with anhydrous sodium sulfate before extraction with petroleum ether;
carbon tetrachloride has been used as a solvent (Mattson et al.,
1953), but it is not appropriate if the final quantification step
involves gas-liquid chromatography and a halogen sensitive detector.
Extraction of DDT-type compounds from blood or serum using hexane has
been described (Dale et al., 1966b), but this extraction procedure is
inefficient, probably as a result of binding by serum proteins. Dale
et al. (1970) investigated a procedure in which the serum was treated
with 97% formic acid before extraction with hexane; experiments using
14C-DDT indicated that extraction with hexane alone gave results
some 40% lower (based on 14C activity) than when the serum was first
treated with formic acid. A modified procedure in which whole blood
was treated with 60% sulfuric acid, and then extracted with a
hexane/acetone (9:1) mixture has been reported by Stretz & Starr
(1973). Samples of blood spiked at 4 different levels with p,p'-DDT
were analysed by 11 different laboratories; considerable discrepancies
were found between laboratories and a further study of the method was
considered desirable. Griffith & Blanke (1974) also investigated the
sulfuric acid method, but a microcoulometric detector was used instead
of an electron-capture detector. According to these workers,
consistent recoveries of p,p'-DDT, p,p'-TDE and p,p'-DDE were
obtained in their laboratory but reproducibility between laboratories
was not studied.
The US EPA Manual (Thompson, 1974) method for the extraction of
DDT-type compounds from human milk involves an acetonitrile/hexane
type extraction. A method for extraction from cow's milk, that makes
use of the stability of p,p'-DDT and its derivatives in the presence
of concentrated sulfuric acid, has been published by Coha & Nedic
(1970); the mixture of milk and concentrated sulfuric acid is
extracted with hexane. Prouty & Cromartie (1970) studied the
recoveries of 14C-DDT in each of the 5 major stages of a method for
determining this compound in the tissues of quail; Soxhlet extraction
with hexane for 6 h, of muscle, liver, heart or brain, after grinding
with sodium sulfate gave recoveries of DDT-type compounds (as 14C-
activity) of 93-105%. Jonczyk (1970) used hexane to extract DDT-type
compounds from blood and reported recoveries of 67-91%. Acetone was
used as the extracting solvent for DDT-type compounds in the adipose
tissue and brain of partridges (Jonczyk et al., 1970). Wood's method,
using dimethyl sulfoxide, was used by Stec & Juszkiewicz (1972), and
was found to give results for DDT-type compounds that compared
favourably with other methods of analysis of animal tissue, eggs, and
milk.
All methods of extracting DDT also result in the removal of
lipids, if they are present. Regardless of the method of extraction,
the results of most analyses have been reported in terms of fresh or
wet weight of samples no matter whether their lipid content was
extremely low (water and urine), low (soils and most vegetables),
intermediate (milk and many tissues), or high (adipose tissue). In
some instances, samples such as adipose tissue, milk, and, to a lesser
degree, other animal tissues known to contain lipids have been
reported in terms of the concentration of pesticide in extractable
lipid. Because DDT and DDE are known to have a marked affinity for
neutral fat, it was originally supposed that reporting in terms of
lipid would reduce variability within any set of samples by excluding
the influence of connective (and sometimes lymphatic) tissue, which
forms a part of each sample. It appears that variation, as measured by
the coefficient of variation, may not be reduced by this kind of
reporting (Casarett et al., 1968). However, there may be other reasons
for reporting on a lipid basis and it is absolutely essential that the
method of reporting be specified.
2.2.5 Clean-up procedures
The extraction procedures remove not only the DDT-type compounds
from the samples analysed but also coextractives to a greater or
lesser degree according to the type of sample. A number of procedures
have been developed that reduce the amounts of the coextractives
relative to that of DDT-type compounds. If interest is confined solely
to DDT-type compounds, then their stability in the presence of
concentrated sulfuric acid is a very useful clean-up procedure
(Mattson et al., 1953; Czegledi-Janko & Cieleszky, 1968; Murphy,
1972). Usually, however, the concentrations of other compounds are
also of interest and this procedure cannot be used if these compounds
are not stable in concentrated sulfuric acid. Two general clean-up
procedures, used in sequence, are appropriate in these circumstances,
namely, liquid-liquid partition followed by liquid-solid partition.
Liquid-liquid partition systems such as hexane/acetonitrile,
hexane/dimethyl formamide, or hexane/dimethyl sulfoxide are the ones
most commonly used. The liquid-solid partition systems generally
consist of Florisil, silica gel, or alumina as the solid phase, and
hexane or mixtures of hexane and various proportions of a polar
solvent (e.g., diethyl ether) as the mobile phase. Separation of
DDT-type compounds from triglycerides in fat-containing tissues is
achieved with considerable efficiency by liquid-liquid partitions, the
hexane/dimethyl formamide or hexane/dimethyl sulfoxide systems being
generally more efficient than hexane/acetonitrile. Separation of
DDT-type compounds from other organochlorine compounds (e.g., aldrin,
dieldrin, polychlorinated aromatics) or from steroids is not very
efficient using liquid-liquid systems, and the liquid-solid partition
systems should be used in these cases. Separation of DDT-type
compounds from polychlorinated biphenyls is particularly difficult
and, as some of these compounds have similar liquid chromatographic
retention times to those of the various DDT-type compounds, the
analysis of samples containing both classes of compounds requires
considerable care. Detailed clean-up procedures are described by de
Faubert Maunder et al. (1964), Cieleszky et al. (1970), Anon. (1974),
Thompson (1974), and by Horwitz (1975).
The separation of DDT-type compounds from polychlorinated
biphenyls, by liquid-solid (silical gel) partition is discussed by
Armour & Burke (1970), Snyder & Reinert (1971), and Masumoto (1972);
the last-mentioned investigator concluded that a number of factors
required careful control if satisfactory separation of DDT-type
compounds from polychlorinated biphenyls (PCBs) were to be achieved,
in particular, the degree of activation of the silicic acid (irregular
distribution of water molecules onto the silicic acid particles was
also probably important). He found that separation of p,p'-DDE from
four Arochlors was incomplete. A collaborative study of the separation
of DDT-type compounds from PCBs using the Armour & Burke silicic acid
column procedure has been reported (Sawyer, 1973).
Florisil and coconut charcoal have also been investigated for the
separation of PCBs from DDT-related compounds (Reynolds, 1969;
Benvenue & Ogata, 1970; and Stijve & Cardinale, 1974). Another
procedure that has been developed for the separation of DDT-PCB
mixtures is the oxidation of p,p'-DDE to p,p'-dichlorobenzophenone
(Miles, 1972). A method for the determination of p,p'-DDT and a
particular PCB isomer that has similar retention time to that of
p,p'-DDT is based on an empirical relation for p-values (Zelinski et
al., 1973).
2.2.6 Quantitation
2.2.6.1 Determination of DDT-type compounds
Two techniques have played a major role in the quantification of
DDT-type compounds, one is a colorimetric method, the other (now the
most widely used) is gas-liquid chromatography with a halogen-
sensitive detector.
The colorimetric procedure of Schechter-Hailer is described in
detail in the Handbook of the Deutsche Forschungsgemeinschaft (1969),
and by Cieleszky et al. (1970) and Horwitz (1975).
Gas-liquid chromatography is essentially a further-method of
separation of compounds, and, although it is the most effective of the
separation procedures (apart possibly from high pressure liquid-liquid
chromatography) it must be realized that it has its limitations,
particularly if used with a highly sensitive but nonselective detector
such as the electron-capture detector. The basic principles of
gas-liquid chromatography are described by Dal Nogare & Juvet (1962)
and Yermakov (1972), for example, and need not be discussed here.
However, attention is drawn to three aspects of the performance of a
column in the gas-liquid chromatographic separation of DDT-type
compounds from other compounds. First, the DDT-type compounds should
not undergo any thermal degradation or other chemical change; second,
the performance of the column should be assessed by the number of
theoretical plates; and third, the performance of the column as regards
ability to separate p,p'-DDT and related compounds from other
compounds that have similar retention times for a particular liquid
phase must be investigated. Transport of p,p'-DDT and analogues
through a column without chemical change is dependent upon the absence
of reactive centres in the column. This can be attained by using an
inactive solid support (by coating the reactive sites with a silane,
if necessary) and ensuring that the surface of the solid support is
completely covered by an inert liquid phase. The performance of a
column should be checked at regular intervals; the US EPA Manual
(Thompson, 1974) suggests a mixture containing five DDT-type compounds
plus eight other organochlorine insecticides for this purpose. Change
in peak shape (i.e., departure from symmetrical peaks) should always
be regarded as a warning sign. In the case of serious doubt about the
stability of p,p'-DDT or an analogue (which may manifest itself as
a change in the retention time relative to that of aldrin or dieldrin
for example), it is suggested that a fraction collection technique be
used and the identity of the component leaving the column at a
particular retention time with that injected confirmed.
Methods of calculating the number of theoretical plates and of
separation factors are given in standard texts. According to the US
EPA Manual, a column of 2 m length should have about 3000 theoretical
plates. The factors that control the performance of gas-liquid
chromatographic columns are discussed in detail by Scott (1970).
Convenient summaries of the retention times (absolute or relative) of
DDT-type compounds, together with those of other pesticides, using
various column conditions have been published (Yermakov, 1972; Zweig &
Sherma, 1972). Retention times of 51 pesticides relative to that of
aldrin using six stationary phases at three temperatures with an
electron-capture detector have been reported by Thompson et al.
(1975); estimates of the relative retention times at other
temperatures were derived from the relationship between relative
retention time and temperature for each liquid phase. The relative
times of DDT-type compounds and other pesticides on eight stationary
phases were determined by Thompson et al. (1969a). Nonpesticidal
organochlorine compounds that have retention times similar to those of
DDT-related compounds include the polychlorinated biphenyls and
polychloronaphthalenes.
Examples of the close similarity between the relative retention
times of DDT-related compounds and those of various PCB isomers have
been published (Bagley et al., 1970; Richardson et al., 1971; Stijve &
Cardinale, 1974). Goerlitz & Law (1972) demonstrated that there are
also similarities between the relative retention times of DDT-type
compounds and those of various isomers of polychlorinated
naphthalenes. Examples of similarities between the relative retention
times of DDT and its analogues and various components in
polychlorinated biphenyls and polychlorinated naphthalenes was also
reported by Griffith & Blanke (1974). Problems arise in the case of
mixtures of toxaphene and DDT-related compounds (Cahill et al., 1970).
The effects of severe infection loading on column performance,
peak configuration, and conversion of p,p'-DDT into p,p'-TDE, were
studied by Thompson et al. (1969b). These investigators found that the
columns they used could be maintained and restored to full or nearly
full performance capacity by daily changing of the glass injection
port insert and the glass-wool plug at the column inlet.
Two different types of detection systems are most frequently used
for the quantification of p,p'-DDT and analogues after elution from
gas-liquid chromatographic columns, namely, electrochemical detectors
and electron-capture detectors. Two types of electrochemical detector
have been developed, the microcoulometric detector and the micro-
electrolytic conductivity detector, that are considerably more
sensitive to p,p'-DDT and its analogues than the original electro-
chemical detectors. Giuffrida & Ives (1969) described modifications and
improvements in microcoulometric gas chromatography and, in the case
of DDT-type compounds in carrots, they obtained responses from their
microcoulometer that were approximately one-fifth of those given by a
particular electron-capture detector; Griffith & Blanke (1974)
described a microcoulometric method for the determination of
p,p'-DDT, p,p'-TDE and p,p'-DDE in blood. According to Dolan
& Hall (1973), the microelectrolytic conductivity detector can be used
for the selective determination of organochlorine pesticides in the
presence of polychlorinated biphenyls. However, the relative
selectivity in regard to p,p'-DDE does not appear to be as great as
that for other organochlorine insecticides.
The electron-capture detector produces an extremely sensitive
response to organochlorine compounds, but its response is not,
unfortunately, very selective and many other classes of compounds have
electron affinity in the vapour phase. A general review of the
principles and characteristics of the electron-capture detector has
been published by Pellizari (1974). The detector may be used under
direct current or pulse sampling conditions; anomalous responses
obtained in the direct current mode of operation are not present under
pulse sampling conditions (Lovelock, 1963). A major limitation of the
electron-capture detector is that its response is linear over a very
limited range only, but a new mode of operation in which the linearity
extends over about four orders of magnitude of response has been
described by Maggs et al. (1971). For this the detector current is
held constant while the frequency of the applied pulses is varied. The
response of electron-capture detectors is liable to change
significantly during use, and these detectors should be recalibrated
at regular intervals, preferably at least once per day with single
standard injections at frequent intervals between injections of
extracts from the samples under investigation. It is of interest that
Mendoza (1971) reported a significant difference in the response of a
gas-liquid chromatograph to p,p'-DDT when injections were made at
fast and slow rates.
The combination of mass-spectrometry with gas-liquid chromato-
graphy was mentioned in section 2.2.4 as a means of confirming the
identity of residues of DDT-type compounds. This combination of
instruments can also be used to quantify the amounts present. The
total ion current corresponding to mass fragments of appropriate mass
number (m/e; in the case of p,p'-DDE, 316, 318, 246 and 248) is
measured and the amount present is calculated from the calibration of
the instrument, preferably by an internal standard. Palmer & Kolmodin-
Hedman (1972) determined the concentration of p,p'-DDE in human
plasma by mass-fragmentography using the ion current corresponding to
m/e 216 and 218; the results showed an excellent agreement with the
values obtained using an electron-capture detector.
Semiquantitative methods of the determination of p,p'-DDT and
its analogues include paper chromatography and thin-layer chromato-
graphy; these methods, which are more appropriately used for the
confirmation of identity of residues, are described by McKinley (1963)
and Sherma (1973) respectively. Bishara et al. (1972a) have given the
tlc Rf values for p,p'-DDT and 14 related compounds using 33 solvent
systems.
2.2.6.2 Determination of p,p'-DDA in urine
A major metabolite of p,p'-DDT, excreted in the urine, is bis-
(4-chlorophenyl)acetic acid ( p,p'-DDA). A method has been developed
by Cranmer et al. (1969), based upon the extraction of p,p'-DDA from
acidified urine, conversion into the methyl ester, Florisil column
clean-up, and gas-liquid chromatographic analysis of the ester. A
detailed outline of this procedure is given by Thompson (1974). The
sensitivity of response of the methyl ester is not high, the limit of
detection being about 2 ng. Cranmer & Copeland (1973) used the
2-chloroethanol ester, the response of an electron-capture detector
being about 3.7 times greater than that of the methylester (i.e.,
limit of detection about 0.5 ng). An advantage of this ester is that
it is well separated from p,p'-DDT, whereas the methyl ester has a
retention time similar to that of p,p'-DDE. The retention time of
the pentafluorobenzyl ester compound is about twice that of p,p'-DDT
and the response of an electron-capture detector is considerably
greater than that for the 2-chloroethanol derivative (Johnson, 1973).
2.2.6.3 Method of reporting results
Francis Galton (1879) pointed out that many vital effects are
distributed logarithmically; his paper was followed by a technical one
(McAlister, 1879) presenting the mathematics. Apparently Robinson &
Hunter (1966) were first to point out that this principle applies to
the storage of pesticides so that the geometric mean is a more
appropriate parameter than the arithmetic mean for expressing
insecticide content. In recent years, increasing use has been made of
the geometric mean as recorded by footnotes in Table 9. Few of the
arithmetic means that have been reported are so much in error that
they should be discarded. As Galton (1879) noted, the difference
between the arithmetic and geometric mean is small if the range of the
values averaged is narrow.
2.2.7 Validation of analytical methods for DDT-type compounds
Ideally, an analytical method should be accurate and highly
precise. A study of the accuracy and precision of art analytical
method must be made in order to assess the relationship between the
actual amount present and the results obtained in practice; such a
study may be called the validation of the procedure. There are two
major types of validation procedure. The first type requires the use
of radio-labelled molecules of p,p'-DDT, e.g., 14C p,p'-DDT.
Prouty & Cromartie (1970) determined the 14C-activity in muscle,
liver, heart, and brain of quail that had been given 14C-labelled
p,p'-DDT. Four of the steps in the analytical procedure were studied
and it was concluded that the major discrepancies occurred during the
elution and concentration steps from Florisil columns and from zones
of thin-layer chromatography plates. Chiba & Morley (1968) used 14C-
labelled p,p'-DDT in a study of soil analysis.
The other validation procedure, which has been studied more
intensively, involves the comparison of the results of analyses with
the values for samples fortified with accurately known amounts of
p,p'-DDT or analogues. There are several variations of this
validation procedure, all comparing the fortified (nominal) value with
the results of different methods in the same laboratory, with the
results of a specified method carried out in different laboratories,
or with the results of different methods in different laboratories.
Versino et al. (1971) compared 8 clean-up procedures for test
mixtures containing 10 pesticides. Two extracts, from apples and
lettuce, were fortified (spiked) with p,p'-DDT at 0.2 mg/litre,
p,p'-TDE at 0.1 mg/litre, and o,p'-DDT at 0.1 mg/litre plus
dieldrin and 7 organophosphate insecticides. The recoveries for the 8
column clean-up procedures ranged from 89-100% for p,p'-DDT, 85-101%
for p,p'-TDE, and 95-100% for o,p'-DDT. It should be noted that
this investigation did not include a study of extraction efficiency
and that the extracts contained only small amounts of lipids. In
studies by Smart et al. (1974), samples were analysed of milk, butter,
cheese, eggs, apples, potatoes, carrots, and cabbages, fortified with
p,p'-DDT, p,p'-TDE, and p,p'-DDE (and 5 other organochlorine
insecticides) at concentrations corresponding to those suggested as
limits by the Codex Alimentarius Commission. Five replicate analyses
of each sample were made, using up to 4 procedures, the final step of
each analytical procedure being gas-liquid chromatography/electron-
capture detection, with 3 different stationary phases. These workers
concluded that there were no gross discrepancies in their results.
However, they did not use the concept of "total error" (see above) in
the discussion of their results, and the total errors for the
determination of the 3 DDT-type compounds, calculated from their
results, were in the range of 12% to 95%.
Results have been reported (Carr, 1970) of a collaborative study
by 10 laboratories of the AOAC method for the determination of 4
DDT-type compounds in samples of butterfat fortified by the addition
of these 4 compounds at 2 different concentrations. The mean recoveries
varied from 86-108%, and the coefficients of variation between
laboratories were in the range 7-28%. The total errors for the 2
levels of fortification respectively were: p,p'-DDT, 22 and 14%;
p,p'-TDE, 17 and 30%; p,p'-DDE, 17 and 36%; and o,p'-DDT, 30 and
43%.
Carr (1971) gives the results of a collaborative study by 8
laboratories of the analysis of fish samples fortified (2
concentrations) with p,p'-DDT, p,p'-TDE and p,p'-DDE, and 3 other
organochlorine insecticides. On the basis of a ranking technique
(Youden & Steiner, 1975), the results of 2 laboratories (Nos. 2 and 8)
for the 6 compounds were rejected as outliers. However, if the results
for the 3 DDT-type compounds are considered, then laboratories 7 and 8
gave results that are outliers. The total errors at the 2
concentrations for the 6 laboratories not rejected by Carr were:
p,p'-DDT, 15 and 36%; p,p'-TDE, 32 and 32%; p,p'-DDE, 26 and 39%.
Sawyer (1973) reported a collaborative study by 9 laboratories of
samples of red salmon fortified with 3 DDT-type compounds and PCBs.
The analyses were carried out without and with silicic acid column
separation of PCBs from DDT-type compounds. The total errors without
and with silicic acid separation, were 34% and 43% respectively for
p,p'-DDT, 50 and 47% for p,p'-TDE and 35 and 36% for p,p'-DDE.
Very similar results were obtained with another type of PCB/DDT
mixture.
Samples of soil, fortified with 4 DDT-type compounds plus 6 other
organochlorine insecticides were analysed in 7 laboratories (Woolson,
1974); the total errors (all collaborators) were: p,p'-DDT, 17-33%;
p,p'-TDE, 19-37%, p,p'-DDE, 18-42%; o,p'-DDT, 28-56%.
Fifteen laboratories collaborated in a study of the determination
of p,p'-DDE in eggs, and p,p'-DDT, p,p'-DDE and o,p'-DDT in
kale (Finsterwalder, 1976). Five laboratories analysed eggs by the
AOAC method, and the total error was 21.1%; the total error for 10
laboratories using a modification of the AOAC method was 30.4%. The
results of one laboratory for analyses of kale were rejected as
outliers, and the total errors for the other 14 laboratories were:
p,p'-DDT, 19.7%; p,p'-DDE, 18.8%; o,p'-DDT, 17.4%.
An international collaborative programme on methods of analysis of
organochlorine insecticides has been sponsored by the Organization for
Economic Cooperation and Development (OECD), and the results of 2
studies have been published. In the first (Holden, 1970), a solution
in hexane of 6 organochlorine compounds, 3 being DDT-related
compounds, was analysed by 17 laboratories in 11 countries. The total
errors for p,p'-DDT, p,p'-TDE and p,p'-DDE were in the range
14-21%, the major component in this total error being the standard
deviation of results from different laboratories, probably indicating
errors of calibration. In the second study (Holden, 1973), a sample of
corn oil fortified with 4 DDT-type compounds and 3 other
organochlorine insecticides was analysed in 19 laboratories in 10
countries: the mean total error for the 4 DDT-type compounds (after
excluding 3 outliers) was 35%.
Some of the results of this collaborative validation study are
very satisfactory, but many of them, as judged by the criterion of
total error, are unsatisfactory, even when outliers are rejected, and
they illustrate the need for scrupulous care by analysts: the use of
clean glassware, chemicals of established purity, and constant
checking of the performance of liquid-solid partition columns and of
instruments.
2.2.8 Analytical methods for the evaluation of the biochemical effects
of p,p'-DDT and its analogues
The increased activity of enzymes, particularly in the hepatic
endoplasmic reticulum, if the exposure to p,p'-DDT and its analogues
is sufficiently great, has been recognized in recent years.
Measurement of such changes in enzyme activity may be made by studying
changes in the rates of metabolism of certain drugs (such as
antipyrine), but methods that do not require the administration of a
drug (and the collection of blood samples if the plasma half-life is
used as the biochemical parameter) are preferable. Two methods, in
which the concentration of either 6-ß-hydroxycortisol or D-glucaric
acid in the urine is measured have been developed. A procedure for the
determination of 6-ß-hydroxycortisol in urine has been described by
Kuntzman et al. (1968); and one for D-glucaric acid by Marsh (1963)
(see section 8.2.5.1).
These enzyme induction changes are not specific for DDT-type
compounds.
3. SOURCES OF ENVIRONMENTAL POLLUTION
3.1 Discovery and Introduction
DDT does not occur naturally. It was first synthesized by Zeidler
as reported in 1874. However, it was not put to any use until its
insecticidal properties were discovered by Paul Müller in 1939. The
Swiss patent was issued in 1942.
As an example of the speed with which DDT was developed and used,
the first sample sent to the USA arrived there in September 1942. This
sample was tested for effectiveness and safety. The results were so
encouraging that manufacture was given high priority. At first, the
entire production was used for the protection of troops against
malaria, typhus, certain other vector-borne diseases, or against
biting flies or other insects that are merely pests. As the supply
increased, DDT was used in the USA for control of malaria in military
areas, that is in the vicinity of military installations, ports, and
transportation centres. As a result of this effort, mosquito
transmission of malaria was brought to an end in the USA in 1953, even
though military personnel and other infected persons from the tropics
continued to reintroduce the disease extensively as late as 1972 and
to a lesser degree thereafter.
3.2 Production and use
The revolution in the control of malaria and typhus among allied
troops and among certain civilian populations during World War II was
accomplished with relatively little DDT. Far greater amounts were
required for the control of agricultural and forest pests and this
became possible when the compound was released in the USA for
commercial use on 31 August 1945. Civilian use in other countries
became possible a little later, first, largely on the basis of
importation and gradually on the basis of local manufacture.
Unfortunately, there is apparently no record of world production of
DDT. Production and use in the USA is shown in Table 2.
Quantities of DDT and related compounds used in or sold for
agricultural purposes in 1970 were as follows (metric tonnes):
Australia (about 1000); Austria (20.5); Botswana (2.0); Canada
(287.0); Columbia (980.0); Czechoslovakia (270.0); Democratic
Kampuchea (46.8); Egypt (3457.0); El Salvador (466.0); Federal
Republic of Germany (152.0); Finland (6.1); Ghana (0.3); Guatemala
(380.0); Hungary (20.6); Iceland (0.3); Israel (10.0); Italy (2178.0);
Japan (401.0); Kuwait (0.2); Madagascar (176.0); Ryukyu Islands
(Japan) (0.3); Sri Lanka (16.6); Sudan (269.0); Upper Volta (1.5); and
Uruguay (5.0) (FAO, 1972). These values total 10 146.2 metric tonnes.
Thus, at least until very recently, the use of DDT was extensive on a
worldwide basis but varied greatly from one country to another.
Table 2. Metric tonnes of DDT produced and used in the USAa
Year Production Use
1944 4 366
1945 15 079
1946 20 220
1947 21 534
1948 9 181
1949 19 822
1950 35 448
1951 48 144
1952 45 327
1953 38 268 28 349
1954 44 088 20 465
1955 56 760 28 032
1956 62 441 34 194
1957 56 136 32 205
1958 65 920 30 255
1959 71 097 35 771
1960 74 471 31 818
1961 77 763 29 061
1962 75 764 30 502
1963 81 154 27 744
1964 56 113 22 925
1965 63 859 24 034
1966 64 115 20 685
1967 46 906 18 260
1968 63 231 14 848
1969 55 839 13 724
1970 26 860 11 316
a Based on data from US Tariff Commission (Hayes, 1975).
3.3 Changing Patterns of Use
Before 1945, all of the DDT produced in the USA for example, was
used or allocated by the military services for various medical and
public health uses. Early in 1945, it became available tor rather
extensive experimental work in agriculture, and it was commercially
available in limited quantities early in the autumn of the same year
(US Department of Agriculture, 1945a,b). The results were so
spectacular that use increased until 1959. In response to a demand for
exports, production continued to increase to about 1963. Even before
then some restrictions were placed on its use, mainly to minimize
residues in food and in the feed of animals that produce milk and
meat. Among the first of these restrictions was that on its use on
dairy cattle or in dairy barns (USDA, 1949). Another important factor
reducing the use of DDT was the increasing resistance of pests. One of
the first species to be affected was the house-fly; because of its
abundance and widespread distribution, its resistance was bound to be
noticed by the public, generally. Although many pests of public health
importance became resistant to DDT in some or all of their range,
resistance among vectors of malaria was less marked. Because malaria
control constitutes such a large segment of vector control, the use of
DDT for vector control has tended to remain stable, while its use in
agriculture has continued to decline, especially in temperate
climates.
The ban on the use of DDT and certain other organochlorine
insecticides in Sweden from 1 January 1970 was based on a number of
ecological considerations.
Government agencies of some other countries attempted to justify
severe restrictions on the use of DDT by alleging that it was a threat
to human health. This was in response to ecologists who considered
that the widespread occurrence of DDT in the environment was
inherently bad and was the direct cause of injury to certain fish and
birds. However, this did not prevent the same agencies from making a
proviso that DDT might be used, if needed, to combat any future threat
from vector-borne disease within their boundaries.
Of course, before the restrictions were put into effect, it had
already been possible to eradicate malaria in the USA and Italy, for
example, and to control for the first time an epidemic of typhus in
Italy and Germany (Simmons, 1959). Apparently, there have never been
accurate figures on the proportion of the compound used for
agriculture and for public health even in those countries that have
recorded their total use of the compound.
As the situation now stands, DDT is still used extensively, both
in agriculture and for vector control, in some tropical countries.
Apparently, information is not available on how much of the
agricultural use involves food protection or how much loss of food
production would result if the use of DDT were discontinued. The
picture with malaria control is clear. Substitution of malathion or
propoxur for DDT would increase the cost of malaria control by
approximately 3.4- and 8.5-fold, respectively, and this increase could
not be supported. If DDT were not used, vast populations in the
malarious areas of the world would be condemned to the frightening
ravages of endemic and epidemic malaria (WHO, 1971).
4. ENVIRONMENTAL TRANSPORT AND DISTRIBUTION
Because DDT has been sprayed on people, domestic animals,
buildings, agricultural crops, and forests, it is not surprising that
it is now distributed widely in the environment. During the
application of any spray or dust to a field, it is often possible to
observe drift of the particulate material. This is especially true if
the application is made by aircraft or by ground equipment that shoots
the spray to the top of orchard trees. If the application is made to
forests, it is very likely that at least part of the spray will fall
directly on streams or lakes. Following application, redistribution is
inevitable. As discussed more fully in the following sections, some
DDT in soil enters the air by evaporation or on wind-blown dust. Even
if watercourses are avoided initially, some insecticide will be washed
into them by rains, mainly in conjunction with soil particles.
4.1 Local Drift in Air
The fact that there is drip is implicit from measurements of DDT
on surfaces just after application. Wilson et al. (1946b) recovered
only 7.5% of the nominal dose from plants; this proportion is typical
but ignores material that may have fallen between plants or between
leaves and come to rest on the ground. Of greater interest are
measurements of total recovery made by means of absorbent targets laid
out in advance. As might be expected, recovery of aerosols is less
than recovery of sprays when other conditions of application are
similar. Only 12.5% was recovered at the centre of swaths following
application of a thermal aerosol by aircraft and recovery decreased at
increasing distances from the centre (Hess & Keener, 1947). In another
study, recovery was 8% for a thermal aerosol and 46% for a spray
(Scudder & Tarzwell, 1950). In a different situation, the average
seasonal recovery for DDT applied as a thermal aerosol ranged from 10
to 12%, while that for spray ranged from 56 to 76% (Tarzwell, 1950).
However, somewhat lower recoveries of DDT sprays have frequently been
reported: 30% (Hoffman & Merkel, 1948), 39% (Hoffman & Surber, 1948),
and 27% (Surber & Friddle, 1949).
Most DDT particles that miss the target for which they are
intended would be expected to fall in the general area. Studies of
residues in soil and in wildlife indicate that this is true. The
logarithm of the concentration of DDT-related compounds in soil
samples collected in a desert area downwind from an area of intensive
agriculture showed an almost straight line inverse relationship to the
logarithm of the distance from the source. The soil levels were about
1 mg/kg at 10 m and about 0.001 mg/kg at 100 000 m from the point of
application. The concentrations of DDT in the tissues of wildlife were
proportional to its concentrations in the soils of their habitats
(Laubscher et al., 1971). Where cultivated fields to which DDT has
been applied for years are interspersed among pastures and other
fields where DDT is not used, the uncultivated soils contain only a
little less DDT than the cultivated ones. In one study, it was found
that most of the DDT was in the top 2.5 cm, but samples for comparison
were taken to a depth of 7.5 cm; the average concentrations were 0.75
to 2.03 mg/kg in cultivated soil and 0.10 to 0.91 mg/kg in
uncultivated sod (US Department of Agriculture, 1966). These
concentrations may be compared (by mathematical calculation alone) to
1.0 mg/kg, the concentration resulting from mixing DDT into soil
uniformly and with no loss whatever following application at the rate
of 1.12 kg/ha, the standard specific gravity (1.47) of soil and a
depth of 7.6 cm being assumed. The range of specific gravities for
most agricultural soils is 0.4 to 2.0 and the corresponding depths
yielding 1 mg/kg are 28 to 5.60 cm. It is of interest that the
residues in cultivated soft were of the order of magnitude that would
be expected from a single application, even though the average
cumulative rate of application during the last 10 years (11.2 kg/ha)
had been 10 times as great.
4.2 Distant Drift in Air
Dust bearing DDT at a concentration of 0.6 mg/kg has been observed
about 1600 km from its area of origin. Other pesticides were also
present in this dust which had settled out on a recently rain-rinsed
roof. The cloud of dust was so dense and unusual that its progress was
reported in newspapers, as the storm that mobilized it in Texas
carried it at least as far as Ohio, where the sample was collected
(Cohen & Pinkerton, 1966).
Dust collected on the island of Barbados by means of nylon nets
treated with 50% aqueous glycerine was assumed to have been blown from
Africa, a distance of over 4850 km. Samples of the dust contained
p,p'-DDT most frequently and in greatest concentration, but the
concentration of all pesticides was only 0.001 to 0.164 mg/kg with an
average of 0.041 mg/kg (Risebrough et al., 1968).
DDT evaporates from sprays and dusts at the time of application
and at any time that dust bearing the compound is mobilized by the
wind. Furthermore, DDT can be detected over treated fields for more
than six months after application, and there is a concentration
gradient from the soil upward (Willis et al., 1971). Under these
circumstances, it is obvious that DDT is transported in the form of a
vapour as well as by means of dust. However, if samples are collected
where no application of DDT has been made, identification of the
origin of the vapour or measurement of the distance it may have
travelled is even more difficult than with dust.
Evidence that DDT in one form or another has travelled great
distances and is, in fact, worldwide in its distribution has been
deduced from finding it in the rainwater of remote, nonagricultural
places (Tarrant & Tatton, 1968) or in water melted from Antarctic snow
(0.00004 mg/litre) (Peterie, 1969). In such remote places as
Eskdalemuir in Scotland and Lerwick in the Shetland Islands, the
average concentrations of p,p'-DDT in rainwater (0.000030 and
0.000046 mg/litre, respectively) were not greatly different from the
averages (0.000018-0.000066 mg/litre) found in widely separated
agricultural areas, suggesting that the compound is rather evenly
distributed in the air (Tarrant & Tatton, 1968).
4.3 Distribution in Water
DDT has a strong tendency to adsorb on surfaces. Most DDT that
enters water is already firmly attached to soil particles, and remains
attached. It was shown very early that, if DDT does find its way into
clear water, it is gradually lost by adsorption on surfaces (Carollo,
1945). The sediments in water tend to move downstream and eventually
to enter estuaries. Of the various chlorinated hydrocarbon
insecticides, DDT and its metabolites are the ones most commonly
found, but the residues tend to be low (Butler, 1969). In fact, in an
estuary associated with the Mississippi River, the levels of
pesticides decreased strikingly from the early 1960s to the late 1960s
(Rowe et al., 1971).
4.4 Bioaccumulation of DDT and Its Degradation in the Environment
Many studies of DDT and related compounds in the environment have
focused on organisms and locations in which concentrations of DDT have
been observed to increase. Concentration in living organisms may be
the result of adsorption from water, of the filtering out of algae or
detritus bearing the compound, or of biological magnification in the
strict sense, that is progressive accumulation in different steps of a
food chain. Although the mechanisms are poorly understood, observation
has shown that residues do reach an equilibrium and sometimes decline.
For example, where DDT was applied to cotton at a cumulative rate of
11.2 kg/ha so that a residue of over 10 mg/kg in the soil would be
expected after many years of continual use, the actual residues ranged
from 0.75 to 2.03 mg/kg (USDA, 1966). An example of decreased residues
was seen in the grebes of Clear Lake (Rudd & Herman, 1972).
Evidence is accumulating that the disintegration of DDT may be
rapid in some situations. Under biologically active, anaerobic
conditions as little as 1% of DDT remained after 12 weeks of
incubation (Hill & McCarty, 1967; Guenzi & Beard, 1968). Probably more
important is the disintegration of DDT under the influence of
ultraviolet light. Hartley (1969) pointed out that much of any
pesticide vapour escaping to 50 metres or more above the ground will
ascend even higher by eddy diffusion and eventually reach the
photochemically active ionosphere. The rapid destruction of DDT by
ultraviolet light under laboratory conditions has been demonstrated
(Mosier et al., 1969; Plimmer et al., 1970; Miller & Narang, 1970;
Plimmer & Klingebiel, 1973; Crosby & Moilanen, 1977). Gab et al.
(1975, 1977) offered evidence that DDT and DDE are converted to carbon
dioxide and hydrochloric acid; the destruction was so complete that
they characterized it as photomineralization. In spite of very real
progress in understanding the fate of DDT in the environment (see
Annex), much more work will be required before a quantitative balance
can be measured between addition of the compound and its
disintegration.
5. ENVIRONMENTAL EXPOSURE LEVELS
5.1. Exposure of the General Population
5.1.1 DDT in air
In spite of the generalization in section 4.2 that DDT is rather
evenly mixed in the air, some increase in concentration may be noted
in connexion with the time and place of application. The highest
concentration of insecticide found in the air of communities with
anti-mosquito fogging programmes was 0.0085 mg/m3 (Tabor, 1966).
Concentrations one or two orders of magnitude greater have been
reported for several insecticides in the breathing zone of orchard
spraymen, and values of 1.2-0.26 mg/m3 have been found at distances
of 500-5000 m from ground spraying (Belonozko et al., 1967). In six
small communities in an agricultural area in the USA, DDT was found in
concentrations ranging from 1 × 10-6 to 22 × 10-6 mg/m3 (Tabor,
1966). Substantially higher values (0.002-0.05 mg/m3) were reported
for centres of population in the USSR (Belonozko & Kucak, 1974). In an
urban location in a generally nonagricultural area of the USA, the
highest concentration found was 2.36 × 10-6 mg/m3 (Antommaria et
al., 1965). The combined concentrations of DDT, dieldrin, and lindane
in the Munich area of the Federal Republic of Germany in 1971 were
even lower, only exceptionally rising as high as 1 × 10-6 mg/m3
(Weil et al., 1973).
With few exceptions, the highest average concentration of DDT in
air to which workers are exposed regularly (7.1 mg/m3) is that
associated with spraying the interior of houses (Wolfe et al., 1959).
However, concentrations ranging from 2 to 104 mg/m3 have been
reported in places where DDT dust was prepared and packed (Medved' et
al., 1975).
5.1.2 DDT in water
Under agricultural conditions, the concentration of DDT in water
may be high. For example, 0.01 mg/litre was found in the runoff from
melting snow from fields where sugar-beets had been grown (Medved' et
al., 1975).
The highest level at which DDT has been found in rainwater in an
urban area during a period of a month is 0.0004 mg/litre (Abbott et
al., 1965). The highest concentration reported in potable water
(0.02 mg/litre) occurred some years ago (Middleton & Lichtenberg,
1960). In a much more comprehensive study made a few years later, the
highest concentration of any insecticide was found (0.00012 mg/litre),
but this was dieldrin and not DDT (Weaver et al., 1965). Many samples
did not contain detectable insecticide of any kind. A study of surface
waters in the USA during the years 1964-1968 indicated that the
residues reached a peak in 1966 and then dropped sharply in 1967 and
1968, in spite of improved analytical methods. The highest value for a
DDT-related compound in those years was 0.00084 mg/litre (Lichtenberg
et al., 1970). By 1971, the concentration in the Federal Republic of
Germany was even lower, averaging 0.00001 mg/litre and never going as
high as 0.001 mg/litre (Weil et al., 1973). The average values for
total DDT in drinking water in Czechoslovakia were 0.000011 and
0.000015 mg/litre in 1972 and 1973, respectively (Hruska & Kociánová,
1975). DDT was not detected (<0.0000000166 mg/litre) in tap water in
a recent survey carried out in Ottawa, Canada (McNeil et al., 1977).
5.1.3 DDT in food
Residues of DDT were measured as early as 1945 (before the
compound was available commercially) on apples to which it had been
applied experimentally for the control of the coddling moth (Harman,
1946). Apparently, the earliest effort to learn how much DDT the
average man obtains from his daily food was that of Walker et al.
(1954), who reported that the amount of insecticide in restaurant
meals in Wenatchee, Washington, USA indicated an average intake of
0.184 and 0.102 mg/man per day for DDT and DDE, respectively. Soon,
other studies revealed similar levels of DDT intake for persons who
ate an ordinary range of foods but who lived in different parts of USA
(Hayes et al., 1956; Durham et al., 1965b). Most of the DDT was in
food of animal origin, and persons who abstained from eating meat but
obtained the food they ate from regular, commercial sources received
an average of only 0.041 and 0.027 mg/man per day of DDT and DDE,
respectively (Haynes et al., 1958). The difference did not depend,
however, on meat per se, for no DDT and only traces of DDE were
found in the meat and other products obtained from Arctic wildlife
that constituted much of the diet of Eskimos (Durham et al., 1961).
Following restrictions on the application of DDT to livestock, to
their barns, and to the forage crops on which they fed, there was a
gradual decrease in residues in animal products used as human foods.
Restrictions on the use of DDT on crops eaten directly by man resulted
in reduced residues in vegetable foods. Complete meals collected
mainly from the same restaurants in Wenatchee and analysed in the same
laboratory indicated that, by 1964, DDT intake was only 0.038 mg/man
per day (Durham et al., 1965b) compared with 0.184 mg/man per day
reported by Walker et al., 11 years earlier. Thus, intake had been
reduced to less than one-fourth. A further reduction to about one-
eighth of the 1953-54 values was indicated by the nationwide study by
the US Food and Drug Administration, usually called the Market Basket
Survey. Intakes of DDT indicated by this study for the succeeding
years 1965-70, were 0.031, 0.041, 0.026, 0.019, 0.016, and
0.015 mg/man per day, respectively (Duggan & Weatherwax, 1967; Duggan,
1968; Duggan & Lipscomb, 1969; Duggan & Corneliusen, 1972).
The widespread shipment of food in the USA tends to explain the
fact that food residues are generally similar in different parts of
the country. However, small differences do exist and may be accounted
for by the fact that, on average, meat is not shipped as far as
vegetables. Much of the feed for livestock is produced on the same
farm or at least in the same area in which the animals are raised.
Whatever the reason, the coefficients of correlation between latitude
and human intake were -0.63 and -0.59 for the sampling periods 1966-67
and 1967-68, respectively (Hayes, 1975).
Early studies (Swackhamer, 1965) indicated that both the frequency
and concentration of residues were slightly less in Canada than in the
USA. More recent studies in Canada similar to the Market Basket Survey
indicated total DDT dietary intakes of 0.018, 0.011, 0.011, 0.007 and
0.007 mg/man per day for the years 1969 to 1973, respectively (Smith,
1971; Smith et al., 1972, 1973, 1975).
The analysis of whole meals collected in south-east England during
1965 and 1966 gave results similar to those obtained during the same
period in the USA; the calculated daily intakes in England were 0.030
and 0.025 mg/man per day for DDT and DDE, respectively (McGill &
Robinson, 1968).
There may be considerable differences in intake in different parts
of the same country. Hruska & Kociánová (1975) reported that the
average intake of DDT plus DDE in 1972 was 0.002 mg/man per day in
southern Bohemia and was 0.099 mg/man per day in Slovakia. Striking
differences may exist between urban and rural areas (Almeida et al.,
1975).
Values for total DDT-related compounds in regular food in the USSR
are not available, but the separate values for DDT and DDE in daily
diets from different regions shown in Table 3 suggest that the total
may be higher than in the UK and USA and that the amount of DDE may
exceed the amount of DDT. Both high total values and an unusually high
proportion of DDE would be consistent with extremely high total values
a few years earlier and recent discontinuation of the use of DDT. In
fact, in the Soviet Union, DDT has been eliminated from the list of
pesticides recommended for use in agriculture since 1970. During the
period 1966-69, 0.8% of food samples contained residues as high as
5.1 mg/kg, and 4.2% contained residues over 1.0 mg/kg.
Although total intake of DDT from food has not been measured in
some parts of the world, worldwide measurements of storage of DDT and
its metabolites in human body fat indicate that the extremes of total
exposure have varied by a factor of about 10, but that total exposure
for most populations has varied by a factor of no more than 3 (see
Table 9).
Table 3. Residues of DDT and DDE in daily cooked diets from
different areas of the USSR during 1971/72a
Area No. of Range of residues of
daily
diets DDT DDE
examined (mg/diet)b (mg/diet)b
North 121 0.00006-0.0103 0.0001-0.0093
West 191 traces-0.094 traces-0.039
South-east 62 traces-0.15 traces-0.330
South I 184 0.02-0.260 0.020-0.5
South II 51 traces-0.052 traces-0.132
a From: Medved' et al. (1975).
b Equivalent to mg/man per day.
It is important to note that local practice may result in high
residues in the food of one or more families even though residues are
low in commercially produced food available in the same area. Thus
Durham et al. (1965b) reported that the average DDT intake from
household meals in Wenatchee was 0.314 mg/man per day at the same time
that restaurant meals in that town contributed only 0.038 mg/man per
day. The difference was largely the result of very high residues in
some eggs eaten by local families; the chickens foraged near orchards,
which had been treated with DDT. The restaurants used commercially
available eggs and not those produced locally. In a similar way,
practices peculiar to one country may account for high residues in
some of their food. For example, very high residues of DDT were
reported in some samples of staple foods in India (Sharangapani &
Pingale, 1954). Dale et al. (1965) suggested that the high levels of
DDT that they found in some Indians might be the result of direct
addition of the compound to staple food to prevent insect infestation,
even though the practice did not have government approval.
5.1.4 Miscellaneous sources
It has been suggested that there is a positive correlation between
the use of household insecticides and the concentration of DDT in
house dust, on the one hand, and the storage of DDT in people on the
other (Deichmann & Radomski, 1968; Radomski et al., 1968; Davies et
al., 1969b, 1975; Edmundson et al., 1970b). However, another study of
dust in 16 urban households, 4 farm households, and 8 households in
which at least one member was a pesticide formulator, failed to reveal
a statistically significant correlation between the levels of various
pesticides in dust and in the serum of people living in the homes.
There were striking individual examples of workers whose homes
contained high concentrations of the compounds they used
professionally and other examples in which there was circumstantial
evidence relating household dust residues to body burden (Starr et
al., 1974).
There can be no doubt that insecticides used in the household or
introduced on the clothing of workers are important sources of intake
of DDT in some instances. It is not clear whether the relevant
absorption involves mainly the inhalation of dust, the contamination
of food within the home, or even dermal absorption.
5.1.5 Relative importance of different sources
It has been estimated (Campbell et al., 1965) that over 90% of the
DDT stored in the general population is derived from food. About 1965,
intake in the USA was approximately 0.04 mg/man per day from food,
less than 0.000046 mg/man per day from water, less than 0.00006 mg/man
per day from urban air and less than 0.0005 mg/man per day from air in
small agricultural communities. The reason for the qualification "less
than" is that the intakes were calculated from the highest
concentrations reported in drinking-water and air because no average
values were established.
Other investigators (Durham et al., 1965b; Morgan & Roan, 1970;
Medved' et al., 1975) concluded independently that ordinary food is
the major source of DDT and related compounds for the general
population. Intake from ordinary food is a base to which other kinds
of intake -- including that from exceptional food -- may be added. An
example of such an addition -- eating eggs from chickens allowed to
run loose in DDT-treated orchards has already been discussed in
section 5.1.3.
5.2 Exposure of Infants and Young Children
Babies tend to be born with slightly lower blood levels of DDT
than are found in their mothers (O'Leary et al., 1970b; Schvartsman et
al., 1974, see also section 6.2.2). This simply indicates that the
placenta excludes some but not all of the DDT available to it. During
the first 10 or 15 years of life, DDT storage levels rise to the adult
population level (Hayes et al., 1958; Hunter et al., 1963; Wassermann
et al., 1965, 1967; Robinson et al., 1965; Davies et al., 1968, 1969a;
Watson et al., 1970).
It has been known for a long time that human milk may contain a
higher concentration of DDT than cow's milk in the same country (Egan
et al., 1965; Quinby et al., 1965b; Ritcey et al., 1972; Olszyna-
Marzys et al., 1973). So far, there is no evidence that this small
difference is of any significance for breast-fed compared with bottle-
fed babies. This is even true in places where the concentration of DDT
in human milk is comparatively high (see section 6.3.1.4).
On the average, the incidence and levels of residues in
commercially prepared food for babies in the USA are lower than those
in raw agricultural products, other processed foods, or samples
examined in the Market Basket Survey (Lipscomb, 1968).
The only DDT exposures of infants that are known to have injured
them in any way are those involving direct access to formulations that
they ate or drank. Such tragic accidents often involved formulations
transferred to unlabelled food or beverage containers. Frequently, the
container was left where a child could reach it easily. Occasionally,
formulations have been stored with food or have ever been handed to a
child as food, or "empty" containers that still contain enough
formulation to kill a child have been carelessly discarded (Hayes,
1975, 1976a).
5.3 Occupational Exposure
In general, annual exposure to DDT is greatest among manufacturers
and formulators, moderate, among those applying it for agricultural
purposes, less among the general population, and least among special
groups whose location or practices minimize their exposure. However,
for brief intervals the exposure during agricultural application may
exceed anything that good industrial practice permits. This
distinction is of great importance in connexion with the more toxic
organic phosphorus compounds, a single heavy exposure to which may
result in poisoning, but is of no known importance in connexion with
DDT, the acute toxicity of which is much less. However DDT has a
greater tendency to storage in the body.
Occupational exposure to DDT is reflected quantitatively by the
concentration of DDT and DDE in blood and fat and by the concentration
of DDA in urine. These aspects of occupational exposure are considered
later. This section outlines measurements that have been made of the
actual degree of exposure under different circumstances.
The most striking result is that the occupational exposure through
areas of skin that are frequently unclothed (face, hands, forearms,
neck, and "V" of chest) is far greater than total respiratory
exposure. Results for DDT are shown in Table 4 (based on measurements
made by methods described by Durham & Wolfe (1962, 1963)). If a
workman has bare feet or legs or does not wear a shirt, the contrast
between respiratory and dermal exposure will be even greater than that
shown in Table 4 which is based on "standard" clothing.
Table 4. Measured respiratory and dermal exposure of workers
to DDT under actual conditions of work
Activity Respiratory Dermal Reference
(mg/kg) (mg/kg)
Indoor house spraying 3.4a 1755 Wolfe et al., 1959
Outdoor house spraying 0.11 243 Wolfe et al., 1959
Spraying forests 4-92b 212b Wassermann
et al., 1960
a Measurement by respirator pad technique.
b Calculated from values given in the original paper.
It has been reported that DDT and a number of other pesticides
persist, for days or even years after last use, on the hands of
workers exposed to them and that at least a part of the material can
be removed for analysis by rinsing the hands in hexane. Evidence that
the residues represented unabsorbed pesticides and not excretion of
stored material included the fact that no correlation was found
between residues on the hands and in the sera of individuals and that
no residues could be found on the hands of a farmer who used
rubberized gloves while working with pesticides and who washed
afterwards with strong cleansing agents (Kazen et al., 1974). The fact
that urinary excretion of DDA may be increased for several days after
a single occupational exposure to DDT (Wolfe et al., 1970) is evidence
that absorption continues for a week or so but not for longer periods.
Furthermore, Wolfe et al. (1970) found that the rate of excretion,
even when detectable, was small compared to the excretion of parathion
metabolite in workers exposed simultaneously to DDT and parathion at a
ratio of 1.0 to 0.25. This emphasizes the minimal dermal absorption of
DDT.
6. METABOLISM OF DDT
6.1 Uptake
6.1.1 Uptake by inhalation
Most DDT dust is of such large particle size (>250 µm) that any
that is inhaled is deposited in the upper respiratory tract and is
eventually swallowed (Hayes, 1975). Toxicity data indicate that
respiratory exposure is of no special importance.
6.1.2 Uptake from the gastrointestinal tract
A review of the early literature indicates that absorption of DDT
from the gastrointestinal tract is slow. Whereas intravenous injection
at the rate of 50 mg/kg produces convulsions in rats in 20 min,
convulsions occur only after 2 h when DDT is administered orally at
two or more times the LD50 value. The onset of convulsions is
delayed for about 6 h when DDT is given to rats orally at
approximately the LD50 value (Dale et al., 1963).
Early studies based on toxicity indicated that DDT, dissolved in
animal or vegetable fats, was absorbed from the gastrointestinal tract
about 1.5 to 10 times more effectively than undissolved DDT. There was
also evidence that large doses of the compound in the gastrointestinal
tract were poorly absorbed from nonabsorbable solvent (Hayes, 1959).
However, in connexion with small repeated doses, the presence or kind
of solvent made little difference; apparently the occurrence of bile
in the intestine and the presence of some fat in the diet were
sufficient to promote absorption of the compound. At high dosage
levels, less 14C-DDT was absorbed and stored in organs and a higher
proportion was excreted in the faeces following oral administration
than after intraperitoneal administration (40% versus 0.9%) (Bishara
et al., 1972b).
Rothe et al. (1957) reported that after giving radioactive DDT to
rats by stomach tube, 41-57% of it was recorded in lymph drained from
the animal by means of a cannula. Less than 0.1% of the activity was
found in the urine, 7.4-37.1% was found in the faeces or in the
intestinal contents when the animals were killed, and 19%-67% of the
activity was found in the carcass. The total dose accounted for
analytically varied from 89% to 118%, thus recovery was complete
within the accuracy of the method. Of the administered DDT not found
in faeces and intestinal contents, 47%-65% was found in the lymph. The
animals that withstood the operation best had peak lymph flows of
nearly 6 ml/h. In these animals, DDT was absorbed at rates as high as
381 µg/h; the rate of absorption reached a maximum within 2-3 h of
intubation and was markedly reduced by the fourth hour. Fifty per cent
of the DDT-derived material found in the lymph was absorbed in the
first 2.5-7 h, and 95% was absorbed within 18 h. Because the lymphatic
duct in the rat is not a single vessel, Rothe et al. (1957) were
unable to exclude the possibility that some, or all, of the DDT that
they later recovered from the carcasses of their animals had been
transported to the general circulation by collateral lymph vessels
rather than by the hepatoportal system. Thus, at least half, and
perhaps all absorption of DDT is by way of the lymph. However, in
studies on rats by Heath & Vandekor (1964), only a small proportion of
dieldrin was absorbed by the lymph. The reason for the marked
difference in the absorption of those organochlorine insecticides is
unknown.
6.1.3 Uptake from the skin
Undissolved DDT is so poorly absorbed through the skin that its
toxicity by this route is difficult to measure. Even dissolved DDT is
poorly absorbed by the skin as indicated by low toxicity (see
Table 5).
Table 5. Acute oral and dermal LD50 of DDT for animalsa
Species Formulation Oral Dermal
(mg/kg) (mg/kg)
Rat Water suspension or powder 500-2500 1 000 000
Oil solution 113-450 250-3000
Mouse Water suspension or powder 300-1600 375 000
Oil solution 100-800 250-500
Guineapig Water suspension or powder 2000 1 500 000
Oil solution 250-560 1000
Rabbit Water suspension or powder 275 375 000
Oil solution 300-1770 300-2820
Cat Water suspension or powder
Oil solution 100-410
Dog Water suspension or powder
Oil solution >300
a From: Hayes (1959).
6.2 Distribution and Storage
6.2.1 Human studies
6.2.1.1 Studies of volunteers
In a study of volunteers who received technical DDT at rates of 0,
3.5, and 35 mg/man per day, the average intakes resulting from dosing
and from traces of DDT in food were 0.0025, 0.05, and 0.5 mg/kg per
day (Hayes et al., 1956). The storage of DDT was proportional to
dosage, but there was an unexplained difference in the storage of the
p,p'-isomer and of technical DDT. For example, following dosing for
12 months, pure p,p'-DDT was stored in fat at an average
concentration of 340 mg/kg, but the sum of isomers from technical
material was stored at an average of only 234 mg/kg. The difference
was statistically significant for the 3.5 mg/man per day dosages given
for 3-6 and for 7-18 months. The difference was significant for the
35 mg/man per day doses after 7-18 months of dosing but not after only
3-6 months.
Men who ate p,p'-DDT showed a definite increase in the absolute
amount of DDE stored. After 6 months at a dosage of 35 mg/man per day,
8 men showed an average concentration of DDE stored in fat of 33 mg/kg
compared with 12 mg/kg for the same individuals at the beginning of
the investigation. There was a further increase in DDE storage as
exposure progressed. However, DDT was stored in so much greater
concentration that the relative storage of DDE decreased sharply.
Thus, after 6 months at a dosage of 35 mg/man per day, 8 men stored
only 14% of their total DDT-derived material in the form of DDE
compared with 65% for the same person at the beginning of the
investigation.
The storage of DDE by men who ate technical DDT presented a
different picture. Until 18 months after exposure, there was no clear
evidence that these men stored any more DDE after exposure than they
did before. However, at 18 months, the only 3 samples available showed
DDE concentrations ranging from 28 to 85 mg/kg, all substantially
above general population levels. Thus, both the total amount stored
and the rate at which DDT converted to DDE served to distinguish the
metabolism of p,p'-DDT and the sum of isomers present in technical
DDT in man (Hayes et al., 1956). A more rapid excretion was
demonstrated for o,p'-DDT by Morgan & Roan (1972).
In a second study (Hayes et al., 1971), volunteers received the
same doses used in the first study. Again, storage of DDT was
proportional to dosage. Although, in this instance also, the storage
of technical DDT was less than that of p,p'-DDT, the difference was
not statistically significant. The real but very gradual accumulation
of DDE was confirmed.
A steady state of storage was approached later in the second study
(18.8-21.5 months) than in the earlier one (about 12 months). However,
although the second study was superior in that more men were studied
for a longer period, it was inferior in that dosage was less regular.
Because of this, it seems impossible to decide whether 12 months or
21.5 months is a more valid estimate of the time necessary for people
to approach a steady state of storage when intake is uninterrupted and
unvarying in amount. It is interesting that the storage levels
eventually reached at the same dosage in the 2 studies were
statistically indistinguishable in most instances (see Table 6). In
the one instance in which a statistical difference existed, the
greater storage by men in the second study may have been explained by
the fact that some of them inadvertently received higher doses than
intended.
Table 6. Storage of DDT in volunteers
Type of DDT Added Concentration of DDTa Significance
dosage First studyb Second studyc of difference
(mg/man 11 months or more 21.5 months (P)
per day) (mg/kg) (mg/kg)
Technical 0 8-17 (12.5 ± 4.5) 16-30 (22.0 ± 2.9) > 0.1
3.5 26-33 (23.8 ± 1.4) 59-76 (50.2 ± 5.6) <0.025
35 101-367 (234 ± 21.4) 105-619 (281 ± 79.5) >0.4
Recrystallized 35 216-466 (340 ± 36.4) 129-659 (325 ± 62.2) >0.2
a Range, mean, and standard error.
b Hayes et al., 1956,
c Hayes et al., 1971.
There was a slow decrease in the levels of fat-stored DDT after
dosing ceased. The concentration remaining following 25.5 months of
recovery was from 32% to 35% of the maximum stored for those who had
received 35 mg/man per day but 66% for those who had received only
3.5 mg/man per day, indicating slower loss at lower storage levels
(Hayes et al., 1971).
Morgan & Roan (1971) fed volunteers not only technical DDT but
also p,p'-DDE and p,p'-TDE. They found that DDE was stored more
tenaciously than the other compounds in man, the order being p,p'-DDE
> p,p'-DDT > o,p'-DDT > p,p'-TDE. The slow metabolism of DDT to
DDE was confirmed. It was noted that p,p'-DDT was lost from storage
in adipose tissue much more slowly in man than in the monkey, dog, or
rat.
Less than 18% of p,p'-DDT and p,p'-DDE is carried in human
erythrocytes. In plasma of ordinary fat content, less than 1% of all
DDT-related compounds is carried by the chylomicrons. Instead, these
compounds are carried by proteins and are undetectable in plasma from
which protein has been precipitated. Following ultracentrifuging,
p,p'-DDT and p,p'-DDE are found mainly in the triglyceride-rich,
low density, and very low density lipoproteins. Following continuous
electrophoresis, these compounds are found mainly in association with
plasma albumin and alpha-globulins (Morgan et al., 1972).
6.2.1.2 Studies of occupationally exposed workers
The highest reported storage of DDT and related compounds remains
that of a healthy worker whose fat contained DDT and DDE (as DDT) at
concentrations of 648 and 483 mg/kg, respectively (Hayes et al.,
1956). Laws et al. (1967) reported considerably lower storage values
among the most exposed persons in a DDT manufacturing plant (see
Table 7).
An important point evident from the table is that, whereas almost
all investigations of workers are said to have been carried out on
"heavily exposed" populations, some of the groups studied had absorbed
little more DDT than is absorbed by the general population --
especially the general population of some tropical countries.
A different situation is indicated in a report by Genina et al.
(1969) who used a total chloride method to analyse samples of blood
from controls and from persons with occupational exposure to DDT,
polychloropinene, and HCH. Whereas the highest average concentration
of total DDT-related material per se in the serum of a worker in the
USA was 2.7 mg/litre (Laws et al., 1967), Genina et al. (1969)
reported organochlorine compounds as high as 38.4 mg/litre in the
blood of a pilot and values as high as 195 mg/litre in the blood of
warehousemen. This concentration is about 20 times the highest value
found by the same authors in their control group (see Table 8). The
factor of 20 is not remarkable, but (especially in view of the fact
that polychloropinene and HCH are excreted more readily than DDT and
DDE) values as high as 0.2 mg/litre in the controls are unexpected.
Table 7. Average concentration of the sum of the isomers of DDT and DDE in fat and serum
and of DDA in the urine of workers engaged in the manufacture, formulation, or
use of DDT
Tissue No. of DDT DDE DDA Total as Estimated Reference
men (mg/kg) (mg/kg) (mg/kg) DDT exposure
(mg/kg) (mg/man
per day)
fat 1 648 437 1131 Hayes et al., 1956
urine 10 0.57 14 Ortelee, 1958
urine 16 1.7 30 Ortelee, 1958
urine 13 2.9 42 Ortelee, 1958
fat 3 51 44 98 3.6 Laws et al., 1967
fat 12 74 50 130 6.2 Laws et al., 1967
fat 20 161 91 263 18 Laws et al., 1967
serum 3 0.2113 0.1968 0.5412 6.3 Laws et al., 1967
serum 12 0.1420 0.1454 0.3584 8.4 Laws et al., 1967
serum 20 0.3020 0.2719 0.7371 17.5 Laws et al., 1967
urine 3 0.0165 0.0203 0.41 0.5629 Laws et al., 1967
urine 12 0.0145 0.0222 0.6 0.7911 Laws et al., 1967
urine 20 0.0145 0.0271 1.27 1.6296 Laws et al., 1967
fat 18 5.2-45.2 Gracheva, 1969
urine 136 0.402 Perini & Ghezzo, 1970
urine 110 0.142 Perini & Ghezzo, 1970
urine 290a 0.061 Perini & Ghezzo, 1970
plasma 16 0.0513 0.0722 0.1321 Wassermann et al., 1970c
serum 4 <0.087 <0.072 Edmundson et al., 1970a
urine 0.080 Edmundson et al., 1970a
serum 18 0.573 0.506 Poland et al., 1970
serum 5 0.004a 0.021a Clifford & Well, 1972
serum 10 0.022 0.055 Clifford & Well, 1972
serum 21 0.021a 0.013a Keil et al., 1972
blood 44 0.761 WHO, 1973
blood 100 1.273 WHO, 1973
Table 7 (Cont'd)
Tissue No. of DDT DDE DDA Total as Estimated Reference
men (mg/kg) (mg/kg) (mg/kg) DDT exposure
(mg/kg) (mg/man
per day)
serum 21 0.300 0.379 0.681 Almeida et al., 1974
serum 25 0.225 0.308 0.504 Almeida et al., 1974
serum 18 0.345 0.257 0.602 Almeida et al., 1974
serum 56 0.004a,b 0.052a,b Morgan & Roan, 1974
serum 32 0.002b 0.026b Morgan & Roan, 1974
serum 32 0.004b 0.047b Morgan & Roan, 1974
serum 32 0.009b 0.075b Morgan & Roan, 1974
serum 31 0.052b 0.222b Morgan & Roan, 1974
plasma 25 1.030 Rabello et al., 1975
plasma 8 0.240 Rabello et al., 1975
plasma 23 0.0389 Richardson et al., 1975
a Control group.
b Approximately equal groups arranged by degree of storage.
Table 8. Percentage of workers with blood organochlorine compound content falling in certain
ranges of concentrationa
Subject group Range of concentrations
0 0.2-0.9 1.0-3.0 4-9 10-50 50
(mg/litre) (mg/litre) (mg/litre) (mg/litre) (mg/litre) (mg/litre)
Control group (47) 21.3 44.7 23.4 10.6 0 0
Pilots (134)
group A 19.1 35.3 25.0 14.7 5.9 0
group B 22.9 27.1 40.6 7.4 2.0 0
Technicians (133)
group A 26.2 16.4 39.3 8.2 9.9 0
group B 39.4 31.2 25.7 3.7 0 0
Agricultural 13.0 43.5 31.0 7.0 2.0 3.5
workers (55)
a From: Genina et al. (1969).
NB: Group A gives the results of investigations at the time of work, group B before work
or a few months after termination. Agricultural workers were studied only during work.
The first evidence that a part of the DDT absorbed by man is
metabolized to DDE was obtained from the analysis of fat from a DDT
plant worker (Mattson et al., 1953).
6.2.1.3 Studies of the general population
DDT in fat. Table 9 summarizes the results of measurements of
DDT and related materials in the body fat of people without
occupational exposure. Several broad generalizations can be made from
the table and from what is known about residues of DDT in food in
different countries. DDT storage in man corresponds with exposure; in
general, exposure tends to be greater in warm climates where there is
a greater need for insecticides. This climatic dependence may be
observed even within a single country (Hayes, 1975). Where
measurements have been made during a sufficiently long period, storage
has decreased as the use of DDT, especially that leading to residues
in food, has decreased.
The method of DDT analysis shifted in about 1962 from the
Schecter-Haller colorimetric method to the gas chromatographic method.
However, as noted by Hayes (1975), this made little difference to the
overall results. Thus the absolute decrease of total DDT storage and
the relative increase of DDE storage observed in some countries is
real.
Circumstances peculiar to some subpopulations explain their
unusual storage of DDT. Thus, persons living in one of the contiguous
states of the USA stored significantly less DDT than other persons in
the state because they did not eat meat (Hayes et al., 1958). Even
less storage of DDT was observed in Eskimos whose diet contained an
unusually high proportion of meat obtained from wildlife in which no
DDT and almost no DDE could be detected (Durham et al., 1961).
No significant difference was found in the concentration of DDT
stored in the fat of different parts of the body (Hayes et al., 1958;
Casarett et al., 1968).
DDE in fat. The accumulation of DDE relative to total DDT-related
compounds is best illustrated in man. Of the total DDT stored
in the fat of workers exposed to technical DDT (about 4% DDE) for
11-19 years, only 38% was in the form of DDE, and, of course, some of
that DDE came from their diets which included meat (Laws et al.,
1967). In India, where many people avoid meat but may consume milk,
cheese, and eggs, 34-41% of total DDT was DDE (Dale et al., 1965). In
the USA, during a time when DDT residues in food were decreasing, the
proportion of total DDT in the form of DDE increased from about 60% in
1955 to about 80% in 1970; during the same interval the concentration
of total DDT in body fat decreased from about 15 mg/kg to slightly
Table 9. Concentration of DDT-derived material in body fat of the general population
Country Year No. of Method of DDTa DDE as Total as DDE as Reference
samples analysis (mg/kg) DDT DDT DDT
(mg/kg) (mg/kg) (% of
total)
North America
Canada 1959-1960 62 colour 1.6 3.3 4.9 67 Read & McKinley, 1961
Canada 1966 47 GLCb and ELC 1.09 2.96 4.39 67 Brown, 1967
Canada 1967-1968 51 GLC 1.56 4.16 5.86 71 Kadis et al., 1970
Canada 1969 4.85 Ritcey et al., 1973
Canada GLC 5.83 Brown & Chow, 1975
USA 1942 10 colour NDc NDc NDc Hayes et al., 1958
USA 1950 75 colour 5.3 -- 5.3 -- Laug et al., 1951
USA 1955 49 colour 7.4 12.5 19.9 63 Hayes et al., 1956
USA 1954-1956 61 colour 4.9 6.8 11.7 58 Hayes et al., 1958
USA 1956 36 colour 5.5 10.1 15.6 65 Hayes et al.. 1971
USA 1961-1962 130 colour 4.0 8.7 12.7 69 Quinby et al., 1965a
USA 1961-1962 28d GLC 2.4 4.3 6.7 64 Dale & Quinby, 1963
USA 1962-1963 282 GLC 2.9 8.2 11.1 74 Hoffman et al., 1964
USA 1964 64 GLC 2.5 5.1 7.6 67 Zavon et al., 1965
USA 1964 25 GLC 2.3 8.0 10.3 77 Hayes et al., 1965
USA 1964-1965 18 GLC 9.0 Schafer & Campbell, 1966
USA 1964-1965 42 GLC 3.1 7.5 10.6 71 Radomski et al., 1968
USA 1962-1966 994 GLC 2.6 7.8 10.4 75 Hoffman et al., 1967
USA 1964-1965 12 GLC 3.79 7.7 11.5 67 Davies et al.. 1965
USA 1965-1967 17 GLC 3.1 5.5 56 Davies et al., 1968
USA 90 GLC 6.1 8.4 73 Davies et al., 1968
USA 17 GLC 4.6 7.8 59 Davies et al., 1968
USA 35 GLC 12.0 16.7 72 Davies et al., 1968
USA -- 42 GLC 3.13e 7.43 10.56 70 Fiserova-Bergerova et
al., 1967
Table 9 (Cont'd)
Country Year No. of Method of DDTa DDE as Total as DDE as Reference
samples analysis (mg/kg) DDT DDT DDT
(mg/kg) (mg/kg) (% of
total)
USA -- 30e GLC 1.33 5.17 6.51 79 Casarett et al., 1968
USA 29f GLC 1.35 4.91 6.31 78 Casarett et al., 1968
USA 30g GLC 1.16 4.99 6.17 81 Casarett et al., 1968
USA 1966-1968 70 GLC 1.54 5.15 6.69 77 Morgan & Roan, 1970
USA 1967 733 GLC 1.34 4.74 6.22 77 Yobs, 1969 (unpublished
data)
USA 1968 3104 GLC 1.56 5.96 7.67 77 Yobs, 1969 (unpublished
data)
USA 1967-1971 103 GLC 1.5 5.6 7.1 79 Warnick, 1972
USA 1970 200 GLC 1.9 8.0 9.9 81 Wyllie et al., 1972
USA 1969-1972 221 23.18 Burns, 1974
USA 1970 1412 7.87h,i Kutz et al., 1974
South America
Argentina 1967 37 GLC 5.5 6.5 13.2 -- Wassermann et al., 1968b
Brazil 1969-1970 38 GLC 1.4 2.7 4.1 Wassermann et al., 1972b
Venezuela 1964 38 GLC 2.9 7.4 10.3 72 Dale, 1971 (unpublished
data)
Europe
Austria 6.33 Pesendorfer et al., 1973
Belgium 20 GLC 1.2 2.1 3.3 64 Maes & Heyndrickx, 1966
Bulgaria 55 GLC 3.8 5.8 10.6n 57 Kaloyanova et al., 1972
Bulgaria 1971-1976 191 GLC 14.7 78 Rizov, 1977
Czechoslovakia 1963-1964 229 colour 5.5 4.1 9.6 43 Halacka et al., 1965
Denmark 1965 18 GLC 0.6 2.7 3.3 82 Weihe, 1966
Denmark 1972-1973 78 GLC 4.1 4.7 87 Kraul & Karloq, 1976
Table 9 (Cont'd)
Country Year No. of Method of DDTa DDE as Total as DDE as Reference
samples analysis (mg/kg) DDT DDT DDT
(mg/kg) (mg/kg) (% of
total)
Finland 1972-1974 73 GLC 2.5 Hattula et al., 1976
France 1961 10 colour 1.7 3.5 5.2 67 Hayes et al., 1963
German Democratic 1966-1967 100 GLC and TLC 3.7 9.47 13.1 71 Engst et al., 1967
Republic
German Democratic 34j 1.8 5.1 6.9 Engst et al., 1970
Republic
Germany, Federal 1958-1959 60 colour 1.0 1.3 2.3 57 Maier-Bode, 1960
Republic of
Germany, Federal 1970 20 GLC 1.1 2.5 3.6 69 Acker & Schulte, 1970
Republic of
Germany, Federal 9.8 Acker & Schulte, 1971
Republic of
Germany, Federal 4.24 Acker & Schulte, 1974
Republic of
Germany, Federal 4.77 Acker & Schulte, 1974
Republic of
Germany, Federal 5.42 Acker & Schulte, 1974
Republic of
Germany, Federal 8.36 Acker & Schulte, 1974
Republic of
Germany, Federal 7.8 Acker & Schulte, 1974
Republic of
Hungary 1960 48 colour 5.7 6.0 12.4 48 Denés, 1962
Italy 1965 9 GLC 1.8 3.2 5.0 63 Kanitz & Castello, 1966
Italy 1965-1966 18 GLC 2.58 8.28 10.86 76 Paccagnella et al., 1967
Italy 1966 22 GLC and TLC 4.69 10.69 15.48 68 Del Vecchio & Leoni, 1967
Italy 1970? 31 GLC 3.38 13.37 16.75 80 Prati & Del Dot, 1971
Italy 1970? 52 GLC 2.14a 8.46a 10.60a 80 Prati et al., 1972
Italy 1970? 33 GLC 0.84a 6.64a 7.48a 89 Prati et al., 1972
Table 9 (Cont'd)
Country Year No. of Method of DDTa DDE as Total as DDE as Reference
samples analysis (mg/kg) DDT DDT DDT
(mg/kg) (mg/kg) (% of
total)
Netherlands 1964 20 colour 1.6 6.1 7.7 79 Wit, 1964
Netherlands -- 11 GLC 0.32 1.89 2.22 86 deVleiger et al., 1968
Norway 56 GLC 3.2 71 Bjerk, 1972
Poland 1965 72 colour 13.4 Bronisz st al., 1967
Poland 65 colour 23.5 62 Bronisz et al.. 1969
Poland 1970 70 GLC 11.4 Juszkiewicz & Stec, 1971
Poland 1972 15 GLC 5.23 68 Bojanowska et al., 1973
Romania 1965 137 -- 13.4 8.3 21.7 39 Aizicovici et al., 1968
Romania 1972-1973 GLC 0.68 1.73 2.41 71 Ciupe, 1976
Spain 1966 41 -- 6.5 9.2 15.7 59 Unpublished data cited
by Wassermann et al., 1968
Switzerland 1.9-16.3 Zimmerli & Marek, 1973
United Kingdom 1961-1962 131 colour -- -- 2.2 -- Hunter et al., 1963
United Kingdom 1963-1964 66 GLC 1.1 2.2 3.3h 67 Egan et al., 1965
United Kingdom 1964 100 GLC -- -- 3.9h -- Robinson et al., 1965
United Kingdom 1964 44 GLC -- -- 4.0h -- Robinson & Hunter, 1966
United Kingdom 1965 101 GLC 1.13 1.72 2.85 60 Cassidy et al.. 1967
United Kingdom 1965-1967 248 GLC 0.78 2.22 3.00 74 Abbott et al., 1968
United Kingdom 1969-1971 201 GLC 0.5 1.8 2.5 72 Abbott et al., 1972
USSR 41 TLC 4.33 3.73 Vas'kovskaja & Komarova.
1967
USSR 197 TLC 5.3- 3.2- Vas'kovskaja, 1969
7.57 7.1
Africa
Kenya 5.4 Wassermann et al., 1972a
Nigeria 1967 43 GLC 5.4 3.1 8.8 Wassermann et al., 1968b
Nigeria 1969 41 GLC 2.1 2.8 6.5 57 Wassermann et al., 1972d
Table 9 (Cont'd)
Country Year No. of Method of DDTa DDE as Total as DDE as Reference
samples analysis (mg/kg) DDT DDT DDT
(mg/kg) (mg/kg) (% of
total)
Africa
South Africa 5.94 43 Wassermann et al., 1970b
South Africa 7.16 Wassermann et al., 1970b
Uganda 2.9 Wassermann et al., 1974a
Asia
India (Delhi area, 1964 67 colour 17 10 26 39 Dale et al., 1965
civilian)
India (other 1964 16 colour 8 5 13 37 Dale et al., 1965
cities, military)
India 94 GLC 13.8h 42 Ramachandran et al., 1974
Israel 1963-1964 254 colour 8.5 10.7 19.2 56 Wassermann et al., 1965
Israel 1965-1966 71 colour 4.6 Wassermann et al., 1967
Israel 1965-1966 133 colour 8.2 Wassermann et al., 1967
Israel 1967-1971 63 GLC 3.0 10.6 14.4 74 Wassermann et al., 1974b
Japan 1968-1969 241 GLC 0.6 1.8 2.4 75 Curlay et al., 1973
Japan 1969-1970 74 6.92 Nishimoto et al., 1970
Japan 1970 4.499 Suzuki et al., 1973
Japan 1971 2.694 Suzuki et al., 1973
Japan 1971 30 12.895 Kasai st al., 1972
Japan 1972 4.001 Suzuki et al., 1973
Japan 1972 42 5.992 Kasai et al., 1972
Japan 1973 6.44 Kawanishi et al., 1973
Japan 1974 6.87 Inoue et al., 1974
Pakistan 60 25.0 Mughal & Rahman, 1973
Thailand 1969-1970 77 12.6 Wassermann et al., 1972c
Oceania
Australia 1965 53 GLC 0.77h 1.03h 1.81h 57 Bick, 1967
Australia 1965-1966 46 colour 3.6 6.6 10.2 64 Wassermann et al., 1968a
Australia 1965-1966 12 GLC 3.0 7.1 10.5 68 Wassermann et al., 1968a
Australia 1971 75 GLC 4.94 Brady & Slyali, 1972
Table 9 (Cont'd)
Country Year No. of Method of DDTa DDE as Total as DDE as Reference
samples analysis (mg/kg) DDT DDT DDT
(mg/kg) (mg/kg) (% of
total)
Oceania
New Zealand 1966 52 GLC 1.6 4.2 5.8 72 Brewerton & McGrath, 1967
New Zealand 1965-1969 254 GLC 3.5 11.0 14.6 75 Copplestone et al., 1973
a p,p'-DDT and o,p'-DDT only. Total as DDT includes TDE (DDD) and other forms when given, which are not shown in this table.
b Gas-liquid chromatography.
c Not detected,
d These 28 samples were also tested for DDT and DDE content by a colorimetric method, and the results are included in the
130 samples listed above.
e Perirenal fat.
f Mesenteric fat.
g Panniculus fat.
h Geometric mean.
i Lipid basis.
j Infants and children.
less than 10 mg/kg (see Table 9). Thus, a low proportion of DDE
indicates a relatively high intake of DDT, a relatively low intake of
DDE residues (e.g. in food), and relatively few years for the
metabolism of stored DDT to DDE.
DDT in blood. The finding of DDT and related compounds in blood
(usually serum) of the general population of different countries is
shown in Table 10. Compared to body fat, blood has been analysed for
DDT in fewer countries and for fewer years. Some of the same
differences between populations and time periods observed in connexion
with DDT in fat have been detected in connexion with DDT in blood, but
the differences are less marked.
There is a small but statistically significant decrease in the
concentrations of DDT-related compounds in the plasma of women 1-6
days postpartum compared with the same women early in pregnancy. Most
of the decrease seems to occur during about the last 10 days before
delivery (Curley & Kimbrough, 1969). In a similar way, the
concentration of DDT-related compounds in various tissues of women at
the time of Caesarean section or normal delivery is less than in
nonpregnant women in the same community (Polishuk et al., 1970).
The concentration of p,p'-DDE is remarkably constant throughout
the day, but minor increases in it and in p,p'-DDT may occur after a
meal (Radamski et al., 1971a).
In so far as can be judged from the bar graphs presented by
Griffith & Blanke (1975), the concentrations of DDT-related materials
they found in postmortem blood were similar to the concentrations
reported in Table 10 for fresh blood except that postmortem blood
contained more TDE (DDD).
DDT and related compounds in other tissues. DDT has been found
in some samples of all human organs. Results reported in the USA and
USSR are shown in Tables 11 and 12, respectively. Results in Table 11
are based on the wet weight of the tissues. Results in Table 12,
presumably, are based on the weight of extractable lipid, but the
paper does not specify this; whatever the basis, the paper reports
concentrations of DDT and DDE in the range of 10.0-35.0 mg/kg in the
heart muscle, liver, and kidneys of 2 persons who had had direct
contact with pesticides. Although concentrations of chlorinated
compounds in liver, kidney, brain, and spleen of persons from Ferrara
Province, Italy, were generally higher than those in the USA (Prati et
al., 1972), the differences were not great.
Table 10. Concentration mg/litre of DDT-related compounds in the blood of members of the general population
Country Year No. of p,p'-DDT o,p'-DDT p,p'-DDE o,p'-DDE p,p- o,p- Total DOT DDE Reference
samples TDE TDE equivalent as %
(DDD) (DDD) total
North America
Canada 0.032 Brown & Chow, 1975
USA (Atlanta) 1965 10 0.0119 0.0013 0.0257 0.0418 68.6 Dale et al., 1966b
USA (Atlanta) 1966 10m 0.0746 Dale et al., 1967
USA (Atlanta) 1966 10f 0.0360 Dale et al., 1967
USA (Louisiana) 1966-1967 53a 0.00182 0.00182 0.00265 0.00265 0.00062 0.00062 0.00501 59.0 Selby et al., 1969
USA (4 states) 1967 64 0.00335 0.00044 0.00837 0.00096 0.00032 0.00032 0.01425 78.0 Yobs, 1969
(unpublished data)
USA (3 states) 1968 106 0.00342 0.00006 0.00927 0.00000 0.00004 0.00009 0.01397 73.4 Yobs, 1969
(unpublished data)
USA (Idaho) 1967-1968 1000 0.0047 0.0220 0.0002 0.02940 83.5 Watson et al., 1970
USA (Atlanta) 1969 30b,c 0.0046 0.0011 0.0062 0.0003 0.0014 0.0144 50.0 Curley et al., 1969
USA 1968 5d 0.0050 Curley & Kimbrough, 1969
USA 1968 10d 0.0205 Curley & Kimbrough, 1969
USA (Florida) 45e 0.0108 O'Leary et al., 1970b
USA (Florida) 107g 0.0152 O'Leary et al., 1970b
USA(Florida) 1970 26 0.03169 Radomski et al., 1971a
South America
Argentina 1970 20h 0.01934 Radomski et al., 1971b
18i 0.01327 Radomski et al., 1971b
19j 0.00869 Radomski et al., 1971b
Brazil 1973? 15f 0.0189 0.0237 0.0453 Schvartsman et al., 1974
Brazil 1973? 15c 0.0118 0.0104 0.0234 Schvartsman et al., 1974
Brazil 1974? 30 0.145 0.026 0.155 0.336 Almeida et al., 1975
Brazil 1974? 11 0.083 0.117 0.194 Almeida et al., 1975
Brazil 1974? 20 0.086 0.121 0.212 Almeida et al., 1975
Table 10 (Cont'd)
Country Year No. of p,p'-DDT o,p'-DDT p,p'-DDE o,p'-DDE p,p- o,p- Total DOT DDE Reference
samples TDE TDE equivalent as %
(DDD) (DDD) total
Europe
Bulgaria 1971-1976 171 0.039a Rizov, 1977
Hungary 1967-1968 120 0.034 Czeglédi-Janko, 1969
Poland 0.172d Jonczyk, 1970
Poland 1972 15 0.030 63 Bojanowska et al., 1973
Switzerland 13 0.0209 Zimmerli & Marek, 1973
Asia
Israel 1975 29 0.0133 0.0112 0.0195 0.0112 0.0087 0.0072 0.0740 47 Polishuk et al., 1977
Japan 1970 10 0.011 Tokutsu et al., 1970
Japan 1971 0.005 Kojima et al., 1971
Japan 1971 138 0.0183 Kasai et al., 1972
Japan 1971 0.0093 Yamagishi et al., 1972
Japan 1971 0.0285 Kaku, 1973
Japan 1972 0.001-0.078 Study Group, 1972
Japan 37 0.0437d Nawa, 1973
Japank 0.1358 Hara et al., 1973
Japanc 0.0210 Hara et al., 1973
0.0779 Abe et al., 1974
Oceania
Australia 52 0.0172 Slyali, 1972
Australia 47 0.0169 Ouw & Shandar, 1974
a Geometric mean. d Maximal value. g Black women. j 1-5 years old.
b Mean of positive values only. e White women. h Adults. k Maternal blood.
c Cord blood from term infants. f Female. i 6-11 years old. m Mile.
Table 11. Average concentrationsb of organochlorine insecticides in various tissues from autopsies
of 44 members of the general populationa
Tissue No. Lipid DDT DDE TDE Heptachlor Dieldrin Total + SEc
analysed content (mg/kg) (mg/kg) (DDD) epoxide (mg/kg) (mg/kg)
(%) (mg/kg) (mg/kg)
Perirenal fat 30 55.7 1.33 4.64 0.0110 0.0220 0.0300 6.03 ± 5.30
Mesenteric fat 29 54.2 1.35 4.40 0.0470 0.0320 0.0630 5.89 ± 4.98
Panniculus fat 30 60.6 1.16 4.48 0.0180 0.0270 0.0270 5.71 ± 5.25
Bone marrow 19 20.6 0.411 2.08 0.0760 0.0040 0.620 2.63 ± 2.21
Lymph noded 11 8.6 0.892 1.38 0.0100 0.0001 0.0190 2.30 ± 4.52
Adrenal 18 10.5 0.125 0.875 0.0570 0.0012 0.0060 1.06 ± 1.31
Kidney 38 3.2 0.0827 0.209 0.0022 0.0009 0.0056 0.300 ± 0.651
Liver 42 2.1 0.0467 0.200 0.0326 0.0019 0.0037 0.285 ± 0.369
Brain 32 7.9 0.0105 0.0831 0.0020 0.0002 0.0031 0.989 ± 0.171
Gonad 36 1.3 0.0150 0.0688 0.0015 0.0001 0.0021 0.0875 ± 0.103
Lung 25 0.7 0.0147 0.0585 0.0009 0.0003 0.0022 0.0766 ± 0.125
Spleen 27 0.6 0.0112 0.0305 0.0031 trace 0.0021 0.0469 ± 0.074
a From: Cassarett et al. (1968).
b Wet tissue basis.
c SE = standard error of the mean.
d Tracheobronchial lymph nodes.
Table 12. Average DDT and DDE contents in certain human organsa
Organ Content % of positive DDT DDE
limits observations (mg/kg) (mg/kg)
Heart muscle 1.0-12.0 77.0 3.69 4.05
Liver 1.0-20.0 85.5 4.62 4.02
Kidney 1.0-12.0 71.0 2.99 3.44
Spleen 2.0-20.0 57.0 2.86 2.86
Pancreas 2.0-15.0 75.0 3.0 2.62
Adrenal 2.0-10.0 84.3 3.84 3.7
Thyroid 2.0-8.0 59.0 1.85 1.85
a From: Vas'kovskaja (1969).
DDT and related compounds in the tissues of infants. The
transfer of DDT to the fetus was observed by Deniés (1962) and
confirmed by many others (Curley et al., 1969; Zavon et al., 1969;
Komarova, 1970; O'Leary et al., 1970a,b,c). Typical findings are shown
in Table 13. It appears that the placenta is partially protective; the
concentrations of DDT and DDE are lower in cord blood than in
corresponding maternal blood (O'Leary et al., 1970b; Schvartsman et
al., 1974). The report by Engst et al. (1970) that infants lose a part
of their DDT stores during the first few months of life, as a result
of rapid growth, is not necessarily contradictory to the observation
that at birth they have less than their mothers, but it must be said
that the values reported by Engst et al. (1970) were relatively high
for infants (see also section 5.2).
Storage in relation to disease. A review (Hayes, 1975) indicates
that there is no agreement in the literature regarding the effect of
health on the storage of chlorinated hydrocarbon insecticides. Some
investigators have not found any difference in the concentration of
DDT in adipose tissue taken by biopsy or during minor elective surgery
in contrast to that taken at autopsy. Some investigators, who used
only autopsy samples, found no relationship between storage of
chlorinated hydrocarbon insecticides and the cause of death. Others,
of whom the first was Deichmann (Deichmann & Radomski, 1968; Radomski
et al., 1968), have reported that storage was 1.7-7.6 times greater in
persons dying of cirrhosis, atherosclerosis, hypertension, idiopathic
amyloidosis, and certain forms of cancer. Weight loss was not really
ruled out in these studies. Its importance was emphasized by Casarett
et al. (1968), who found that disease did not influence concentrations
on a wet weight basis but only on a lipid basis; samples with the
highest levels of DDT on a lipid basis came from persons who not only
had cancer but who were emaciated and had widespread abnormality of
Table 13. The range and mean of measurable concentrations of various organochlorines (mg/kg) in different tissues from stillborn infantsa
Tissues p,p'-DDT o,p'-DDT p,p'-DDE o,p'-DDE p,p'-TDE alpha-HCH ß-HCH gamma-HCH Heptachlor Dieldrin
received (DDD)
10 measurable 3 4 8 0 6 3 6 3 4 3
adipose concentration
range 0.16-2.15 0.35-11.47 0.16-3.19 -- 0.23-14.17 0.09-0.24 0.14-0.44 0.09-0.14 0.07-0.51 0.09-0.35
mean 0.88 3.39 1.22 -- 3.17 0.14 0.26 0.11 0.32 0.24
SE ± 0.63 2.70 0.38 -- 2.22 0.05 0.05 0.02 0.10 0.08
8 measurable 1 1 2 0 2 0 1 1 0 1
spinal concentration
cord range 0.47 0.47 0.30-1.16 -- 0.31-0.70 -- 0.17 0.10 -- 0.09
mean -- -- 0.73 -- 0.51 -- -- -- -- --
SE ± -- -- 0.43 -- 0.20 -- -- -- -- --
8 measurable 3 1 4 0 3 3 1 1 1 2
brain concentration
range 0.28-0.99 0.84 0.25-1.47 -- 0.20-1.22 0.04-0.49 3.81 0.06 0.13 0.84-0.86
mean 0.56 -- 0.65 -- 0.64 0.19 -- -- -- 0.05
SE ± 0.22 -- 0.28 -- 0.30 0.15 -- -- -- 0.01
9 measurable 3 2 6 0 3 2 3 3 2 1
adrenals concentration
range 1.28-1.65 0.36-1.05 0.13-1.96 -- 0.91-1.45 0.40-0.57 0.12-0.71 0.20-0.53 0.46-1.00 0.92
mean 1.48 0.71 1.05 -- 1.11 0.49 0.37 0.33 0.73 --
SE ± 0.11 0.35 0.28 -- 0.17 0.09 0.18 0.10 0.27 --
10 measurable 4 0 6 0 5 5 3 3 3 2
lungs concentration
range 0.57-1.01 -- 0.25-1.35 -- 0.31-1.05 0.07-0.69 0.05-0.18 0.05-0.25 0.08-0.31 0.27-0.72
mean 0.79 -- 0.85 -- 0.75 0.25 0.12 0.12 0.17 0.50
SE ± 0.11 -- 0.22 -- 0.13 0.11 0.04 0.07 0.07 0.23
Table 13 (Cont'd)
Tissues p,p'-DDT o,p'-DDT p,p'-DDE o,p'-DDE p,p'-TDE alpha-HCH ß-HCH gamma-HCH Heptachlor Dieldrin
received (DDD)
10 measurable 4 2 4 0 4 3 3 3 4 3
heart concentration
range 1.04-4.17 0.57-0.68 1.27-4.79 -- 1.02-5.82 0.23-0.52 0.15-0.31 0.19-0.27 0.30-1.56 0.08-1.02
mean 2.17 0.63 2.74 -- 3.27 0.33 0.22 0.22 0.80 0.49
SE ± 0.69 0.06 0.74 -- 1.10 0.09 0.05 0.03 0.30 0.28
10 measurable 5 3 6 0 6 3 4 4 3 2
liver concentration
range 0.15-1.59 0.22-3.42 0.22-2.45 -- 0.19-2.14 0.21-0.32 0.03-0.20 0.05-0.36 0.03-1.67 0.16-0.22
mean 0.79 1.32 0.98 -- 1.01 0.25 0.11 0.24 0.68 0.19
SE ± 0.24 1.05 0.34 -- 0.29 0.04 0.04 0.07 0.50 0.03
9 measurable 4 3 6 0 5 4 5 4 3 3
kidney concentration
range 0.62-7.60 0.29-2.07 0.11-9.78 -- 0.48-9.12 0.11-1.45 0.06-0.61 0.11-0.69 0.19-1.14 0.06-0.50
mean 3.71 1.38 3.57 -- 3.84 0.82 0.29 0.39 0.70 0.34
SE ± 1.70 0.55 1.72 -- 1.87 0.32 0.12 0.14 0.28 0.14
8 measurable 3 2 3 1 3 1 1 2 5 3
spleen concentration
range 0.48-1.04 0.45-2.94 0.60-1.05 0.29 0.18-0.91 0.21 0.16 0.17-0.18 0.10-0.52 0.18-0.51
mean 0.80 1.70 0.86 -- 0.56 -- -- 0.18 0.35 0.31
SE ± 0.17 1.25 0.13 -- 0.21 -- -- 0.005 0.08 0.10
3 measurable 1 0 1 1 0 0 0 0 0 0
pancreas concentration
range 0.49 -- 0.23 0.08
mean -- -- -- -- -- -- -- -- -- --
SE ± -- -- -- -- -- -- -- -- -- --
a From: Curley et al. (1969).
the liver. Factors other than emaciation may be important in some
conditions. For example, Oloffs et al. (1974) found that the DDT
concentration in fat was not influenced by cirrhosis, but that the DDT
concentrations in liver were significantly higher in cirrhotic livers
with severe fatty infiltration and significantly lower in those with
marked fibrosis or bile stasis.
That increased storage of DDT is a result and not a cause of the
diseases in which it sometimes occurs is shown by the fact that
persons with extensive occupational exposure average 10 times more
storage than the highest values reported in connexion with disease.
However, they do not exhibit any predisposition to the diseases in
question, and these diseases have shown no age-specific increase in
incidence related to the introduction and use of insecticides.
6.2.2 Animal studies
A detailed review of the literature (Hayes, 1959) shows that a
number of facts about the distribution and storage of DDT were
established early either by single, classical papers (now fully
confirmed) or by correlation of contributions from several
laboratories. The major results may be summarized as follows:
(a) DDT is stored in all tissues. Storage of the compound in
blood, liver, kidney, heart, and the central nervous system
was reported by Smith & Stohlman (1944).
(b) Higher concentrations of DDT are usually found in adipose
tissue than in other tissues (Ofner & Calvery, 1945).
(c) Rats store DDT in their fat at all accurately measurable
dietary levels including the unintended residues in standard
laboratory feeds.
(d) Following repeated doses, storage in the fat increases rapidly
at first and then more gradually until a peak or plateau is
reached (Laug et al., 1950). It was recognized that repeated
doses at a moderate rate could result in greater total storage
of DDT in the fat than a single dose at the highest rate that
can be tolerated or even a single dose at a rate that
frequently is fatal.
(e) By plotting animal data published no later than 1950, it is
possible to show that, when other factors are kept constant,
the equilibrium storage of DDT in each tissue varies directly
with the daily dosage.
(f) However (with the apparent exception of the dog) storage in
the fat, and perhaps in other tissues, is less extensive in
relation to dosage at higher dietary levels (Fig. 1).
 (g) The rat, apparently, tends to lose a part of the DDT it has
stored in fat at a peak level, reached in about 6 months, even
though the same diet is continued.
(h) There is a measurable, although not great, difference between
the storage pattern of different species (Fig. 1).
(i) At higher dosage levels but not at ordinary residue levels,
the female rat consistently stores more DDT in its fat than
the male, when offered the same diet (Fig. 1). The difference
is only accounted for in part by the greater food intake of
the female and must depend to some extent on more rapid
biotransformation in the male. Other species show little or no
sex difference.
(j) The amount of DDT stored in the tissues gradually diminishes
if exposure to the compound is discontinued or reduced.
It is interesting to note that, even in the early studies, there
was satisfactory agreement between different authors and, in fact,
between different laboratories. Later studies have amplified some of
the findings.
Adams et al. (1974) observed that about the same concentrations of
DDT and related compounds are stored by male rats and by females that
reproduce successfully. The lower storage in mated females probably is
accounted for by transfer to the young via the placenta and the milk.
However, other factors may be involved; the disposal of the increased
DDT taken in by the female rat as a result of her high food intake
during lactation has not really been accounted for.
When DDT, some of its analogues, and several other organochlorine
insecticides were fed to male and female rats for 4 generations, there
was little variation in storage of the materials, from one generation
to another, and no evidence of a continuing increase in succeeding
generations (Adams et al., 1974).
Distribution to the fetus
In animals, the concentrations of DDT in the blood and other
tissues of the fetus are lower than those in the corresponding tissues
of the mother (Dedek & Schmidt, 1972). The same is true in man (see
section 5.2).
Interaction with other compounds
There was no evidence from studies on rats that dieldrin
influenced the storage of DDT and its metabolites, even though DDT
caused a marked reduction in storage of dieldrin (Street, 1964).
However, Deichmann et al. (1971a) reported that the administration of
capsules of aldrin (which is metabolized to dieldrin) to male and
female dogs that had reached a steady state of DDT storage caused a
rapid and progressive increase in the concentration of DDT, DDE, and
TDE (DDD) in their blood and fat. The dosage of DDT was 12 (mg/kg)day
and that of aldrin was 0.3 (mg/kg)day. Control dogs that continued to
receive DDT but never received aldrin maintained a plateau of DDT
storage. It is not clear whether the different results in rats and
dogs were due to species, to some unrecognized difference between
dieldrin and aldrin, to the fact that the rats received lower dosages,
or to some unidentified factor.
Studies of the distribution of DDT in various lipid fractions that
are based on tissue extracts obtained with one or more organic
solvents, such as those of Kuz'minskaja et al. (1972), are difficult
to interpret because there is no way of determining how much of the
material was initially associated with protein.
DDE
DDE constitutes about 4% of technical DDT. Most species convert
some of the DDT they ingest to DDE. Finally, most species, including
man, store DDE more tenaciously than they do DDT, the greater part of
which is metabolized by a different pathway to DDA and excreted more
rapidly. The result is that DDE, expressed as a percentage of total
DDT-related compounds, increases in individuals after DDT intake
decreases, and also increases in successive steps of the food chain.
Apparently, the rhesus monkey is an exception. Monkeys store DDE
when it is fed to them but, when feeding stops, the rate of loss of
DDE stored in fat is more rapid than that of DDT (Durham et al.,
1963). Whether it is relative inability to form DDE, unusual ability
to excrete it, or a combination of both that accounts for the fact
that little or no DDE can be found in monkeys fed DDT is not entirely
clear.
6.3 Elimination
6.3.1 Human studies
6.3.1.1 Studies of volunteers
DDA is the main urinary metabolite of DDT. In man, it was found
first in a volunteer by Neal et al. (1946), who reported that,
following ingestion of 770 mg of p,p'-DDT, excretion rose sharply to
4.0 mg/day during the second 24-h period, decreased rapidly on the
third and fourth days, decreased gradually thereafter, but was still
above baseline on the fourteenth day. Judging from a graph, the
highest concentration was about 2.6 mg/kg.
Much later studies in volunteers, who received smaller but
repeated doses, showed that a steady state of excretion was reached
after about 6-8 months. During a 56-week period of continued dosing
after equilibrium was fully established, the concentration of DDA
associated with technical DDT at the rate of 35 mg/man per day varied
from 0.18 to 9.21 mg/kg and averaged 2.98 mg/kg, corresponding values
for p,p'-DDT were 0.40-6.27 mg/kg with a mean of 1.88 mg/kg. Thus,
technical DDT was excreted more effectively and stored in man less
than p,p'-DDT. During the latter half of the dosing period, it was
possible, in the 2 groups receiving recrystallized and technical DDT
at the rate of 35 mg/man per day, to account for an average of 13% and
16%, respectively, of the daily dose in terms of urinary DDA. The
excretion of DDA was relatively constant in each individual, but
marked differences were observed between men receiving the same dose.
For example, over the period of 56 weeks, the highest rate measured
for one man was 0.16 mg/h while the lowest rate for another in the
same group was 0.15 mg/h. Their mean rates during this period were
0.089 and 0.269 mg/h, respectively. The difference was highly
significant (P < 0.001) (Hayes et al., 1971).
6.3.1.2 Studies of occupationally exposed workers
Among workers whose DDT intake was estimated to be about 35 mg/man
per day, Ortelee (1958) reported that the concentration of DDA in
urine ranged from 0.12 to 7.56 mg/litre and averaged 1.71 mg/litre.
Among workers whose exposure was about half as high, Laws et al.
(1967) found concentrations from 0.01 to 2.67 mg/litre with a mean of
0.97 mg/litre.
Continuous sampling of a DDT-formulating plant worker's urine
showed that excretion of DDA increased promptly, when exposure began
on each of 5 consecutive work days and often continued to increase
after exposure, sometimes reaching a peak about midnight before
decreasing rapidly. On the sixth day, when there was no occupational
exposure to DDT, the excretion of DDA continued until a very low level
was reached. The highest concentration of DDA reported in this study
was 0.68 mg/litre (Wolfe & Armstrong, 1971).
6.3.1.3 Studies of the general population
Urine. Cranmer et al. (1969) developed a method for analysing
DDA in urine that, for the first time, made it possible to measure the
compound in every sample. The range found for a small sample of the
population of Florida, USA, was 0.008-0.019 mg/litre, and the mean
(0.014 mg/litre was slightly less than 0.02 mg/litre, the lowest
concentration detectable by earlier methods. Results for general
population studies are shown in Table 14 together with results for
various groups of workers and volunteers.
Table 14. Urinary excretion of DDA by people in the USA with various degrees of exposure to DDTa
Exposure Year No. of DDA excretion (mg/kg) Reference
samples
Range Mean
General population 1954 8 <0.05 -- Hayes et al., 1956
General population 1957 8 <0.02-0.07 -- Hayes et al., 1971
General population 1962 23 <0.02-0.18 -- Durham et al., 1965b
General population 1968 11 0.008-0.019 0.014 Cranmer et al., 1969
Environmentalb 1962 13 <0.02-0.11 -- Durham et al., 1965a
Subjects applying DDT 1962 11 <0.02-0.17 -- Durham et al., 1965a
Formulators 1957 40 0.12-7.56 1.71 Ortelee, 1958
Makers and formulators 1966 35 <0.01-2.67 0.97 Laws et al., 1967
Volunteers given 1953-1954 2 0.10-0.42b 0.21c Hayes et al., 1956
3.5 mg/day orally
Volunteers given 1957-1958 6 0.06-1.98a 0.23b Hayes et al., 1971
3.5 mg/day orally
Volunteers given 1953-1954 6 0.69-9.67b 2.46c Hayes et al., 1956
35 mg/day orally
Volunteers given 1957-1958 6 0.18-9.21c 3.09d Hayes et al., 1971
35 mg/day orally
a Slightly modified from Hayes (1975).
b Residents living within 500 feet of agricultural application.
c Based on all samples after thirty-fifth week of dosage.
d Based on all samples from the thirty-fifth to the ninety-third week after dosage started.
In the general population the urine contained not only DDA but
also neutral compounds; the average concentrations reported by Cueto &
Biros (1967) were: p,p'-DDT, 0.0007 mg/litre and p,p'-DDE,
0.0156 mg/litre. Men with full-time occupational exposure to DDT
excreted much more DDA but showed only a statistically insignificant
increase in the excretion of DDT and DDE.
These values for the excretion of DDA, DDT, and DDE by different,
small groups of people showed an average concentration of
0.0358 mg/litre of DDT-related material. Although the DDT intakes of
these particular groups were not measured, the urinary excretion is of
such an order of magnitude that it may account for the excretion of
all the absorbed DDT.
Milk. As far as the mother is concerned, the secretion of a
compound in milk is a form of excretion. For the infant, the milk is
an important, if not the sole source of intake of the compound in
question. The concentrations of DDT and DDE in milk reported from
different countries are listed in Table 15. Especially high values
have been reported from Guatemala for 1970 (see Table 16) and from the
USSR (Damaskin, 1965). However, additional and more numerous samples
taken only four years later in the same and other communities in
Guatemala revealed entirely different results. The highest single
value observed for total DDT in 1974 was 5.69 mg/litre. The range of
average values for different locations was 0.04-0.86 mg/litre. The
means for total DDT for the three communities studied earlier were: La
Bomba, 0.59 mg/litre; El Rosario, 0.28 mg/litre; and Cerro Colorado,
0.47 mg/litre (Winter et al., 1976). The authors recognized the
importance of the agricultural uses of DDT as a potential source of
the compound in human milk. However, they attributed the change
between 1970 and 1974, almost exclusively, to the substitution of
propoxur for DDT in residential spraying to combat malaria.
The medical importance of DDT in human milk depends entirely on
the dosage of the compound received by babies. The highest
concentration of p,p'-DDT ever reported in a single sample of milk
and the highest average value from one community (see Table 16) would
determine maximum and average intakes of 1.06 and 0.32 (mg/kg) day for
newborn babies, assuming an intake of 0.6 litre per day and a weight
of 3.36 kg. This average intake is of the same order of magnitude as
that encountered by workers who make and formulate DDT. The
corresponding maximum and average intakes of total DDT-related
material for infants in Guatemala would be 2.18 and 0.73 (mg/kg) day,
values not strictly comparable to those of the workers because the
intake of the babies includes so much more DDE, a less toxic compound.
Neither of the papers cited concerning DDT in human milk in Guatemala
mentioned any indication of injury to babies. Absence of injury to
babies would be predicted from studies of the most heavily exposed
workers and also from studies regarding the effect of age on the
susceptibility of animals to DDT (see section 7.3.2).
Table 15. Concentration of DDT-derived material in human milk
Country Year No. of Method DDT DDE as DDT Total as DDE as Reference
samples of (mg/litre) (mg/litre) (mg/litre) DDT
analysis (% of
total)
North America
Canada 1967-1968 147 GLC 0.032 0.097 0.139 -- Ritcey et al., 1972
Canada 15 GLC 0.006-0.032 -- 0.019-0.035 -- Musial et al., 1974
USA 1950 32 colour -- 0.13 -- Laug et al., 1951
USA 1960-1961 10 colour 0.08 0.04 0.12 33 Quinby et al., 1965b
USA 1962 6 colour 0-0.12a 0.025a 0.37b -- West, 1964
USA 1968 ? GLC 0.026 0.047 0.078 60 Cudey & Kimbrough, 1969
USA 1970 53 GLC 0.022 0.083 0.101 80 Kroger, 1972
USA 1970-1971 101 GLC -- -- 0.17 Wilson et al., 1973
USA 1971-1972 40 GLC 0.126 Savage et al., 1973
USA 1973-1974 57 GLC 0.092 0.260 0.344 76 Strassmann & Kutz, 1977
USA 1974 38 GLC 0.447 Woodard et al., 1976
USA 1974 14 GLC 0.075 Woodard et al., 1976
USA 1975 7 GLC 0.323 Woodard et al., 1976
Europe
Belgium 1968 20 GLC 0.05 -- -- -- Heyndrickx & Maes, 1969
Czechoslovakia 1968 -- -- 0.101 -- -- -- Hruska, 1969
Czechoslovakia 393 TLC 0.097 0.112 0.209 54 Suvak, 1970
England 1963-1964 19 GLC 0.05 0.08 0.13 62 Egan et al., 1965
France 1971-1972 3.24c Luquet et al., 1974
France 1972? 3.24c Luquet et al., 1974
German Democratic 1970? 0.569 Adamovic et al., 1971
Republic
German Democratic 1969 57 0.23 Engst & Knoll, 1972
Republic
German Democratic 1970 18 0.16 Engst & Knoll, 1972
Republic
Table 15 (Cont'd)
Country Year No. of Method DDT DDE as DDT Total as DDE as Reference
samples of (mg/litre) (mg/litre) (mg/litre) DDT
analysis (% of
total)
German Democratic 1971 96 0.32 Knoll & Jayarman, 1973a
Republic 1973b
Germany, Federal 1970? 43 GLC 0.031 0.090 0.121 74 Acker & Schulte, 1970
Republic of
Germany, Federal 1971? 0.121 Pfeilsticker, 1973
Republic of
Hungary 1963 10 colour 0.13-0.26a -- -- -- Denés, 1964
Italy 1965? 2 GLC 0.001 0.055 0.056 Kanitz & Castello, 1966
Netherlands 1969 50 GLC 0.9c 1.8c 2.7c 66 Tuinstra, 1971
Poland 1966 26 colour 0.27 -- -- 62 Bronisz & Ochynski, 1968
Poland 1967 25 colour 0.40 -- -- 58 Bronisz & Ochynski, 1968
Poland 1970? 40 GLC 0.08 0.19 0.28 71 Kontek et al., 1971
Portugal 1972 168 GLC 0.326 Graca et al., 1975
Romania 1968? 100 colour 0.054-0.749 0.026-8.30 0.080-9.05 -- Unterman & Sirghie, 1969
Sweden 1967? -- -- 0.117 -- Lotroth, 1968
Sweden 1967-1969 22 GLC 0.039 0.076 0.115 63 Westoo et al., 1970
USSR 1964 16 colour 1.22-4.88 -- -- -- Damaskin, 1968
USSR 1968? 4505 -- 0.1-1.0 -- -- -- Gracheva, 1969
USSR 1969? -- -- 0.09 -- 0.14 -- Gracheva, 1970
USSR 1967? 370 GLC 0.1 -- -- Komarova, 1970
Asia
Israel 1975 29 GLC 0.02 0.03 0.07 44 Polishuk et al., 1977
Japan 1970? 10 0.071 Tokutsu et al., 1970
Japan 1970? 5 0.160 Takeshita & Inuyama. 1970
Japan 1970? 10 0.120 Takeshita & Inuyama. 1970
Japan 1971? 0.04 Kojima et al., 1971
Japan 1971? 0.04 Kojima et al., 1971
Table 15 (Cont'd)
Country Year No. of Method DDT DDE as DDT Total as DDE as Reference
samples of (mg/litre) (mg/litre) (mg/litre) DDT
analysis (% of
total)
Japan 1971? 59 0.019-0.105 Kato et al., 1971
Japan 1971? 14 0.047 Sugaya et al., 1971
Japan 1971 43 GLC 0.095 0.084 0.179 47 Hidaka et al., 1972
Japan 1971 454 GLC 0.0607 Hayashi, 1972a, 1972b
Japan 1971-1972 398 0.0626 Hayashi, 1972a, 1972b
Japan 1971-1972 398 0.0562 -- -- -- Anonymous, 1972
Japan 1971 0.044 Yamagishi et al., 1972
Japan 30 2.0a Mizoguchi et al., 1972
Japan 54 0.035 Taira et al., 1972
Japan 1971-1972 5 0.027 Nagai, 1972
Japan 1971-1972 5 0.037 Nagai, 1972
Japan 1971-1972 5 0.016 Nagai, 1972
Japan 1971-1972 5 0.037 Nagal, 1972
Japan 30 0.033 Oura et al., 1972
Japan 1971-1972 123 0.105 Kawai et al., 1973
Japan 1971-1972 0.038-0.075 Kamata, 1973
Japan 1970 3.780c Suzuki et al., 1973
Japan 1971 3.592c Suzuki et al., 1973
Japan 1972 3.822c Suzuki et al., 1973
Japan 1973 0.0854 Kamata, 1974
Oceania
Australia 1970 1 GLC -- -- 0.014d Newton & Greene, 1972
0.007e
0.066f
67 GLC 0.036 0.105 0.141
Table 15 (Cont'd)
Country Year No. of Method DDT DDE as DDT Total as DDE as Reference
samples of (mg/litre) (mg/litre) (mg/litre) DDT
analysis (% of
total)
Australia
(Brisbane) 1971-1972 20 GLC 0.288 Miller & Fox, 1973
(Mareeba) 1971-1972 20 GLC 0.415
Australia 45 GLC 0.064 Siyali, 1973
Australia 22 GLC 0.010 0.068 0.078 Stacy & Thomas, 1975
Papua New Guinea 1972 16 GLC
(Kar Kar Island) 0.002 0.002 0.004 50 Hornabrook et al., 1972
Papua New Guinea 1972 19 GLC
(Sepik district) 0.008 0.007 0.015 47 Hornabrook et al., 1972
a Range of values for milk containing 4% fat c Concentration in milkfat. e At middle of feeding, 1.2% fat.
containing 3.3-6.6 ppm.
b Maximal value. d At beginning of feeding, 1.8% fat. f At end of feeding, 5.1% fat.
Table 16. Ranges, means, and standard errors of the concentrations
of organochlorine insecticides in the milk of women in
three towns in Guatemalaa
Compound La Bomba El Rosario Cerro Colorado
1970 1970 1971
n = 10 n = 27 n = 9
p.p'-DDT 0.23-4,95 0.16-2.24 0.49-5.94
(mg/litre) (1.00 ± 0.38) (0.77 ± 0.10) (1.78 ± 0.56)
p.p'-DDE 0.12-6.36 0.28-3,10 0.60-6.13
(mg/litre) (1.02 ± 0.58) (0.99 ± 0.14) (2.10 ± 0.61)
p,p'-TDE(DDD) trb-0.16 0.01-0.09 0.05-0.11
(mg/litre) (0.03 ± 0.02) (0.02 ± 0.004) (0.07 ± 0.01)
o,p'-DDT tr-0.29 0.01-0.18 0.06-0.22
(mg/litre) (0.09 ± 0.03) (0.06 ± 0.01) (0.12 ± 0.02)
total as DDT 0.41-11.50 0.34-4.97 1.571-12.21
(mg/litre) (2.15 ± 1.05) (1.84 ± 0.24) (4.07 ± 1.11)
total HCH 0.01-0.10 tr-0.07 0-0.06
(mg/litre) (0.03 ± 0.01) (0.007 ± 0.003) (0.02 ± 0.01)
heptachlor epoxide 0-0.02 tr-0.01 tr
(mg/litre) (0.003 ± 0.002) (0.007 ± 0.0004)
dieldrin tr tr-0.01
(mg/litre) (0.002 ± 0.0005)
a From: Olszyna-Marzys et al. (1973).
b tr = trace.
The slightly greater secretion of DDT and much greater secretion
of various isomers of HCH by urban mothers (compared to rural mothers)
in Japan was attributed to their greater intake of cow's milk (Takano,
1972).
Other routes of excretion. DDT, DDE, and dieldrin are excreted in
the bile; the concentrations for five men without special exposure
varied as follows: p,p'- and o,p'-DDT combined, 0.0000-0.0009 mg/litre;
p,p'-DDE, 0.0005-0.0056 mg/litre; and dieldrin, 0.0000-0.0005 mg/litre.
Higher levels were found in the bile of one pest control operator
(Paschal et al., 1974).
6.3.2 Animal studies
When large doses of DDT are ingested, some of the compound is not
absorbed and is passed in an unaltered state in the faeces. Only
traces of unaltered DDT may be found in the faeces when exposure is by
any route other than oral. However, true faecal excretion of DDT
metabolites was established very early (Wasicky & Unti, 1945; Judah,
1949). In the rat, faecal excretion of DDT exceeded urinary excretion,
irrespective of the route of administration (Hayes, 1965). In man, the
ratio is obviously different. Although the excretion of DDT-related
material in the faeces of man receiving 35 mg/man per day has been
reported using colorimetry (Hayes et al., 1956), this result has never
been confirmed by gas chromatography, even in connexion with workers
whose exposure was heavy and prolonged. Either DDT metabolites are not
excreted by man in the faeces to any great degree, or they are
excreted in one or more forms that differ from those already
demonstrated in rats.
The bile appears to be the principal source of DDT metabolites in
the faeces of rats. When the bile duct was cannulated before
intravenous injection of radioactive DDT, 65% of the dose was
recovered in the bile, 2% in the urine, and only 0.3% in the faeces
(Jensen et al., 1957), and the possibility of some contamination of
the faeces by urine could not be excluded.
The different routes of excretion are not unrelated. Burns et al.
(1957) found that there was an increase in urinary excretion of
radioactive material following ligation of the bile duct in rats fed
radioactive DDT. This is an indirect confirmation of the finding by
Jensen and his colleagues that most of the metabolites in bile consist
of DDA or compounds closely related to it. Although an enterohepatic
circulation of the metabolites of DDT has not been directly proved, it
seems likely that such a circulation exists, as has been demonstrated
for 1,1'-(2,2-dichloroethylidene)-bis[4-ethylbenzene] (Perthane).
Demonstration of the excretion of DDT in milk was first reported
by Woodard et al. (1945) in connexion with a dog fed the compound at
the rate of 80 (mg/kg) day. Within a short time, excretion of DDT in
milk was reported in rats, goats, and cows, and soon afterwards, in
women (Laug et al., 1951). Telford & Guthrie (1945) showed that rats
fed a diet containing DDT at 1000 mg/kg produced milk that was toxic
to their young.
Since the early laboratory studies, the presence of DDT has been
demonstrated repeatedly in the milk of cows. A review (Hayes, 1959)
showed that cows fed substantial, but nontoxic, residues of DDT
commonly excrete 10% or slightly more of the total dose in their milk,
and amounts slightly over 30% have been observed.
Further information on the excretion of DDT in human milk is given
in section 6.3.1.3. It is of interest to repeat here, however, that
lactating women in the general population are apparently in negative
DDT balance. That is, they excrete more DDT in their milk each day
than they acquire in their food. The difference is small and would not
be expected to have much effect on their total body burden of DDT.
Wilson et al. (1946a) showed that DDT was secreted from the skin
of a cow maintained on an oral dosage of about 53 (mg/kg) day.
DDA. Because DDA is the main form in which DDT is excreted, it
might be expected that, following its direct administration, DDA would
be excreted relatively efficiently, and this is true. It was found
very early that, during the first few days after oral dosing, rabbits
excreted DDA in the urine approximately 15 times faster than animals
given DDT at an equivalent dosage. Although the rate of DDA excretion
increased somewhat, the rate of excretion associated with DDT
increased more rapidly so that the values differed by a factor of only
five after the twentieth day of feeding (Smith et al., 1946).
6.4 Biotransformation
The chemical nature of the chief metabolite excreted in the urine
was first elucidated by White & Sweeney (1945). Rabbits were given DDT
(melting point 107-108°C) at a rate of 100 (mg/kg) day for 6 days per
week, and their urine was collected. It contained a considerable
amount of organic chloride, whereas normal rabbit urine did not. Using
the organic chloride test to evaluate different methods of extraction,
the authors were able to isolate a crystalline material containing
25.37% chlorine and melting at 166-166.5°C. The crystals were shown to
be DDA (see Table 1). The product obtained from the urine was
identical to that synthesized from glyoxylic acid and chlorobenzene
and with a compound obtained through the chemical degradation of DDT.
Identity of the 3 compounds and, therefore, their true chemical
nature, was established by the determination of melting points, mixed
melting points, elementary analysis, and X-ray powder diffraction
patterns, as well as by demonstrating the similarity of the
decarboxylation products of the three original materials. Only 80-85%
of the total organic chloride of the rabbit urine was found to be
soluble in alkali and in bicarbonate. For this and other reasons it
was considered possible that DDA was not the only chlorinated organic
compound present.
Later work by many authors has confirmed that DDA is the major
urinary metabolite of DDT in all mammals including man. It may be
added that, in spite of great strides in analytical chemistry, the
nature of other urinary metabolites has not been elucidated fully.
The fact that DDE is stored in tissue was first demonstrated by
Pearce et al. (1952) in connexion with human fat. The authors pointed
out that they did not know whether the compound resulted from partial
degradation of DDT residues on plants or whether the DDE was formed
during the process of digestion or after absorption. It is now known
that some food contains DDE but that man is capable of forming the
product from DDT. The exact mechanism of the biotransformation of DDT
to DDE remains in doubt.
Pearce et al. (1952) established the identity of DDE by comparing
the colorimetric and column chromatographic behaviour of the residue
with those of a chemical standard. A second paper from the same
laboratory (Mattson et al., 1953) added further details confirming the
identity of the compound. Later investigations have confirmed the
identity of DDE by infrared spectrometry and by gas chromatography.
That portion of the metabolism of DDT that leads to DDA has been
clearly elucidated by Peterson & Robinson (1964), who gave evidence
for the sequence of changes shown in Fig. 2. Organ perfusion studies
have indicated that the liver is capable of the biotransformation of
DDT, DDE, TDE, DDMU, and DDMS, while the kidney transforms DDMS, DDNU,
and DDOH (Datta & Nelson, 1970). Cultures of embryonic lung cells are
capable of metabolizing DDT to DDA via DDD (North & Menzer, 1973).
When DDA was discovered, it was postulated, on chemical grounds,
that DDE was a step in its formation (White & Sweeney, 1945); however,
rats that produced both DDE and DDA from DDT were incapable, according
to Peterson & Robinson (1964), of forming DDA when fed preformed DDE.
This finding was contradicted by Datta (1970) and by Datta & Nelson
(1970) who claimed that 14C- p,p'-DDE was converted by rats to
p,p'-DDMU, which then underwent further metabolism to p,p'-DDA via
the route shown in Fig. 2. Datta suggested that the predominance of
detoxication via DDE or TDE (DDD) might depend on physiological
response or the amount of toxicant used. Whatever the reason, the fact
remains that DDE is stored more tenaciously than DDT.
Rhesus monkeys fed either technical or p,p'-DDT store little or
no DDE, although they are fully capable of storing DDE when it is fed
preformed (Durham et al., 1963).
The way in which DDE was lost from storage was not clearly
understood for a long time. In man (Cueto & Biros, 1967), seal, and
guillemot (Jansson et al., 1975) part of it is excreted unchanged, but
the fact that its elimination is promoted by the induction of
microsomal enzymes (see section 7.1.4.2) strongly suggests that it
undergoes metabolism, conjugation, or both. That metabolism does occur
was first demonstrated by identification of 2 hydroxylated derivatives
of DDE in the faeces of wild seals and guillemots and in the bile of
seals (Jansson et al., 1975). When p,p'-DDE was fed to rats, the
same metabolites and one other were isolated from the faeces, and,
within the first 6 days, the metabolites accounted for about 5% of the
dose (Sundström et al., 1975). Later a fourth hydroxylated derivative
was identified from the faeces of rats fed p,p'-DDE. The compounds
(g) The rat, apparently, tends to lose a part of the DDT it has
stored in fat at a peak level, reached in about 6 months, even
though the same diet is continued.
(h) There is a measurable, although not great, difference between
the storage pattern of different species (Fig. 1).
(i) At higher dosage levels but not at ordinary residue levels,
the female rat consistently stores more DDT in its fat than
the male, when offered the same diet (Fig. 1). The difference
is only accounted for in part by the greater food intake of
the female and must depend to some extent on more rapid
biotransformation in the male. Other species show little or no
sex difference.
(j) The amount of DDT stored in the tissues gradually diminishes
if exposure to the compound is discontinued or reduced.
It is interesting to note that, even in the early studies, there
was satisfactory agreement between different authors and, in fact,
between different laboratories. Later studies have amplified some of
the findings.
Adams et al. (1974) observed that about the same concentrations of
DDT and related compounds are stored by male rats and by females that
reproduce successfully. The lower storage in mated females probably is
accounted for by transfer to the young via the placenta and the milk.
However, other factors may be involved; the disposal of the increased
DDT taken in by the female rat as a result of her high food intake
during lactation has not really been accounted for.
When DDT, some of its analogues, and several other organochlorine
insecticides were fed to male and female rats for 4 generations, there
was little variation in storage of the materials, from one generation
to another, and no evidence of a continuing increase in succeeding
generations (Adams et al., 1974).
Distribution to the fetus
In animals, the concentrations of DDT in the blood and other
tissues of the fetus are lower than those in the corresponding tissues
of the mother (Dedek & Schmidt, 1972). The same is true in man (see
section 5.2).
Interaction with other compounds
There was no evidence from studies on rats that dieldrin
influenced the storage of DDT and its metabolites, even though DDT
caused a marked reduction in storage of dieldrin (Street, 1964).
However, Deichmann et al. (1971a) reported that the administration of
capsules of aldrin (which is metabolized to dieldrin) to male and
female dogs that had reached a steady state of DDT storage caused a
rapid and progressive increase in the concentration of DDT, DDE, and
TDE (DDD) in their blood and fat. The dosage of DDT was 12 (mg/kg)day
and that of aldrin was 0.3 (mg/kg)day. Control dogs that continued to
receive DDT but never received aldrin maintained a plateau of DDT
storage. It is not clear whether the different results in rats and
dogs were due to species, to some unrecognized difference between
dieldrin and aldrin, to the fact that the rats received lower dosages,
or to some unidentified factor.
Studies of the distribution of DDT in various lipid fractions that
are based on tissue extracts obtained with one or more organic
solvents, such as those of Kuz'minskaja et al. (1972), are difficult
to interpret because there is no way of determining how much of the
material was initially associated with protein.
DDE
DDE constitutes about 4% of technical DDT. Most species convert
some of the DDT they ingest to DDE. Finally, most species, including
man, store DDE more tenaciously than they do DDT, the greater part of
which is metabolized by a different pathway to DDA and excreted more
rapidly. The result is that DDE, expressed as a percentage of total
DDT-related compounds, increases in individuals after DDT intake
decreases, and also increases in successive steps of the food chain.
Apparently, the rhesus monkey is an exception. Monkeys store DDE
when it is fed to them but, when feeding stops, the rate of loss of
DDE stored in fat is more rapid than that of DDT (Durham et al.,
1963). Whether it is relative inability to form DDE, unusual ability
to excrete it, or a combination of both that accounts for the fact
that little or no DDE can be found in monkeys fed DDT is not entirely
clear.
6.3 Elimination
6.3.1 Human studies
6.3.1.1 Studies of volunteers
DDA is the main urinary metabolite of DDT. In man, it was found
first in a volunteer by Neal et al. (1946), who reported that,
following ingestion of 770 mg of p,p'-DDT, excretion rose sharply to
4.0 mg/day during the second 24-h period, decreased rapidly on the
third and fourth days, decreased gradually thereafter, but was still
above baseline on the fourteenth day. Judging from a graph, the
highest concentration was about 2.6 mg/kg.
Much later studies in volunteers, who received smaller but
repeated doses, showed that a steady state of excretion was reached
after about 6-8 months. During a 56-week period of continued dosing
after equilibrium was fully established, the concentration of DDA
associated with technical DDT at the rate of 35 mg/man per day varied
from 0.18 to 9.21 mg/kg and averaged 2.98 mg/kg, corresponding values
for p,p'-DDT were 0.40-6.27 mg/kg with a mean of 1.88 mg/kg. Thus,
technical DDT was excreted more effectively and stored in man less
than p,p'-DDT. During the latter half of the dosing period, it was
possible, in the 2 groups receiving recrystallized and technical DDT
at the rate of 35 mg/man per day, to account for an average of 13% and
16%, respectively, of the daily dose in terms of urinary DDA. The
excretion of DDA was relatively constant in each individual, but
marked differences were observed between men receiving the same dose.
For example, over the period of 56 weeks, the highest rate measured
for one man was 0.16 mg/h while the lowest rate for another in the
same group was 0.15 mg/h. Their mean rates during this period were
0.089 and 0.269 mg/h, respectively. The difference was highly
significant (P < 0.001) (Hayes et al., 1971).
6.3.1.2 Studies of occupationally exposed workers
Among workers whose DDT intake was estimated to be about 35 mg/man
per day, Ortelee (1958) reported that the concentration of DDA in
urine ranged from 0.12 to 7.56 mg/litre and averaged 1.71 mg/litre.
Among workers whose exposure was about half as high, Laws et al.
(1967) found concentrations from 0.01 to 2.67 mg/litre with a mean of
0.97 mg/litre.
Continuous sampling of a DDT-formulating plant worker's urine
showed that excretion of DDA increased promptly, when exposure began
on each of 5 consecutive work days and often continued to increase
after exposure, sometimes reaching a peak about midnight before
decreasing rapidly. On the sixth day, when there was no occupational
exposure to DDT, the excretion of DDA continued until a very low level
was reached. The highest concentration of DDA reported in this study
was 0.68 mg/litre (Wolfe & Armstrong, 1971).
6.3.1.3 Studies of the general population
Urine. Cranmer et al. (1969) developed a method for analysing
DDA in urine that, for the first time, made it possible to measure the
compound in every sample. The range found for a small sample of the
population of Florida, USA, was 0.008-0.019 mg/litre, and the mean
(0.014 mg/litre was slightly less than 0.02 mg/litre, the lowest
concentration detectable by earlier methods. Results for general
population studies are shown in Table 14 together with results for
various groups of workers and volunteers.
Table 14. Urinary excretion of DDA by people in the USA with various degrees of exposure to DDTa
Exposure Year No. of DDA excretion (mg/kg) Reference
samples
Range Mean
General population 1954 8 <0.05 -- Hayes et al., 1956
General population 1957 8 <0.02-0.07 -- Hayes et al., 1971
General population 1962 23 <0.02-0.18 -- Durham et al., 1965b
General population 1968 11 0.008-0.019 0.014 Cranmer et al., 1969
Environmentalb 1962 13 <0.02-0.11 -- Durham et al., 1965a
Subjects applying DDT 1962 11 <0.02-0.17 -- Durham et al., 1965a
Formulators 1957 40 0.12-7.56 1.71 Ortelee, 1958
Makers and formulators 1966 35 <0.01-2.67 0.97 Laws et al., 1967
Volunteers given 1953-1954 2 0.10-0.42b 0.21c Hayes et al., 1956
3.5 mg/day orally
Volunteers given 1957-1958 6 0.06-1.98a 0.23b Hayes et al., 1971
3.5 mg/day orally
Volunteers given 1953-1954 6 0.69-9.67b 2.46c Hayes et al., 1956
35 mg/day orally
Volunteers given 1957-1958 6 0.18-9.21c 3.09d Hayes et al., 1971
35 mg/day orally
a Slightly modified from Hayes (1975).
b Residents living within 500 feet of agricultural application.
c Based on all samples after thirty-fifth week of dosage.
d Based on all samples from the thirty-fifth to the ninety-third week after dosage started.
In the general population the urine contained not only DDA but
also neutral compounds; the average concentrations reported by Cueto &
Biros (1967) were: p,p'-DDT, 0.0007 mg/litre and p,p'-DDE,
0.0156 mg/litre. Men with full-time occupational exposure to DDT
excreted much more DDA but showed only a statistically insignificant
increase in the excretion of DDT and DDE.
These values for the excretion of DDA, DDT, and DDE by different,
small groups of people showed an average concentration of
0.0358 mg/litre of DDT-related material. Although the DDT intakes of
these particular groups were not measured, the urinary excretion is of
such an order of magnitude that it may account for the excretion of
all the absorbed DDT.
Milk. As far as the mother is concerned, the secretion of a
compound in milk is a form of excretion. For the infant, the milk is
an important, if not the sole source of intake of the compound in
question. The concentrations of DDT and DDE in milk reported from
different countries are listed in Table 15. Especially high values
have been reported from Guatemala for 1970 (see Table 16) and from the
USSR (Damaskin, 1965). However, additional and more numerous samples
taken only four years later in the same and other communities in
Guatemala revealed entirely different results. The highest single
value observed for total DDT in 1974 was 5.69 mg/litre. The range of
average values for different locations was 0.04-0.86 mg/litre. The
means for total DDT for the three communities studied earlier were: La
Bomba, 0.59 mg/litre; El Rosario, 0.28 mg/litre; and Cerro Colorado,
0.47 mg/litre (Winter et al., 1976). The authors recognized the
importance of the agricultural uses of DDT as a potential source of
the compound in human milk. However, they attributed the change
between 1970 and 1974, almost exclusively, to the substitution of
propoxur for DDT in residential spraying to combat malaria.
The medical importance of DDT in human milk depends entirely on
the dosage of the compound received by babies. The highest
concentration of p,p'-DDT ever reported in a single sample of milk
and the highest average value from one community (see Table 16) would
determine maximum and average intakes of 1.06 and 0.32 (mg/kg) day for
newborn babies, assuming an intake of 0.6 litre per day and a weight
of 3.36 kg. This average intake is of the same order of magnitude as
that encountered by workers who make and formulate DDT. The
corresponding maximum and average intakes of total DDT-related
material for infants in Guatemala would be 2.18 and 0.73 (mg/kg) day,
values not strictly comparable to those of the workers because the
intake of the babies includes so much more DDE, a less toxic compound.
Neither of the papers cited concerning DDT in human milk in Guatemala
mentioned any indication of injury to babies. Absence of injury to
babies would be predicted from studies of the most heavily exposed
workers and also from studies regarding the effect of age on the
susceptibility of animals to DDT (see section 7.3.2).
Table 15. Concentration of DDT-derived material in human milk
Country Year No. of Method DDT DDE as DDT Total as DDE as Reference
samples of (mg/litre) (mg/litre) (mg/litre) DDT
analysis (% of
total)
North America
Canada 1967-1968 147 GLC 0.032 0.097 0.139 -- Ritcey et al., 1972
Canada 15 GLC 0.006-0.032 -- 0.019-0.035 -- Musial et al., 1974
USA 1950 32 colour -- 0.13 -- Laug et al., 1951
USA 1960-1961 10 colour 0.08 0.04 0.12 33 Quinby et al., 1965b
USA 1962 6 colour 0-0.12a 0.025a 0.37b -- West, 1964
USA 1968 ? GLC 0.026 0.047 0.078 60 Cudey & Kimbrough, 1969
USA 1970 53 GLC 0.022 0.083 0.101 80 Kroger, 1972
USA 1970-1971 101 GLC -- -- 0.17 Wilson et al., 1973
USA 1971-1972 40 GLC 0.126 Savage et al., 1973
USA 1973-1974 57 GLC 0.092 0.260 0.344 76 Strassmann & Kutz, 1977
USA 1974 38 GLC 0.447 Woodard et al., 1976
USA 1974 14 GLC 0.075 Woodard et al., 1976
USA 1975 7 GLC 0.323 Woodard et al., 1976
Europe
Belgium 1968 20 GLC 0.05 -- -- -- Heyndrickx & Maes, 1969
Czechoslovakia 1968 -- -- 0.101 -- -- -- Hruska, 1969
Czechoslovakia 393 TLC 0.097 0.112 0.209 54 Suvak, 1970
England 1963-1964 19 GLC 0.05 0.08 0.13 62 Egan et al., 1965
France 1971-1972 3.24c Luquet et al., 1974
France 1972? 3.24c Luquet et al., 1974
German Democratic 1970? 0.569 Adamovic et al., 1971
Republic
German Democratic 1969 57 0.23 Engst & Knoll, 1972
Republic
German Democratic 1970 18 0.16 Engst & Knoll, 1972
Republic
Table 15 (Cont'd)
Country Year No. of Method DDT DDE as DDT Total as DDE as Reference
samples of (mg/litre) (mg/litre) (mg/litre) DDT
analysis (% of
total)
German Democratic 1971 96 0.32 Knoll & Jayarman, 1973a
Republic 1973b
Germany, Federal 1970? 43 GLC 0.031 0.090 0.121 74 Acker & Schulte, 1970
Republic of
Germany, Federal 1971? 0.121 Pfeilsticker, 1973
Republic of
Hungary 1963 10 colour 0.13-0.26a -- -- -- Denés, 1964
Italy 1965? 2 GLC 0.001 0.055 0.056 Kanitz & Castello, 1966
Netherlands 1969 50 GLC 0.9c 1.8c 2.7c 66 Tuinstra, 1971
Poland 1966 26 colour 0.27 -- -- 62 Bronisz & Ochynski, 1968
Poland 1967 25 colour 0.40 -- -- 58 Bronisz & Ochynski, 1968
Poland 1970? 40 GLC 0.08 0.19 0.28 71 Kontek et al., 1971
Portugal 1972 168 GLC 0.326 Graca et al., 1975
Romania 1968? 100 colour 0.054-0.749 0.026-8.30 0.080-9.05 -- Unterman & Sirghie, 1969
Sweden 1967? -- -- 0.117 -- Lotroth, 1968
Sweden 1967-1969 22 GLC 0.039 0.076 0.115 63 Westoo et al., 1970
USSR 1964 16 colour 1.22-4.88 -- -- -- Damaskin, 1968
USSR 1968? 4505 -- 0.1-1.0 -- -- -- Gracheva, 1969
USSR 1969? -- -- 0.09 -- 0.14 -- Gracheva, 1970
USSR 1967? 370 GLC 0.1 -- -- Komarova, 1970
Asia
Israel 1975 29 GLC 0.02 0.03 0.07 44 Polishuk et al., 1977
Japan 1970? 10 0.071 Tokutsu et al., 1970
Japan 1970? 5 0.160 Takeshita & Inuyama. 1970
Japan 1970? 10 0.120 Takeshita & Inuyama. 1970
Japan 1971? 0.04 Kojima et al., 1971
Japan 1971? 0.04 Kojima et al., 1971
Table 15 (Cont'd)
Country Year No. of Method DDT DDE as DDT Total as DDE as Reference
samples of (mg/litre) (mg/litre) (mg/litre) DDT
analysis (% of
total)
Japan 1971? 59 0.019-0.105 Kato et al., 1971
Japan 1971? 14 0.047 Sugaya et al., 1971
Japan 1971 43 GLC 0.095 0.084 0.179 47 Hidaka et al., 1972
Japan 1971 454 GLC 0.0607 Hayashi, 1972a, 1972b
Japan 1971-1972 398 0.0626 Hayashi, 1972a, 1972b
Japan 1971-1972 398 0.0562 -- -- -- Anonymous, 1972
Japan 1971 0.044 Yamagishi et al., 1972
Japan 30 2.0a Mizoguchi et al., 1972
Japan 54 0.035 Taira et al., 1972
Japan 1971-1972 5 0.027 Nagai, 1972
Japan 1971-1972 5 0.037 Nagai, 1972
Japan 1971-1972 5 0.016 Nagai, 1972
Japan 1971-1972 5 0.037 Nagal, 1972
Japan 30 0.033 Oura et al., 1972
Japan 1971-1972 123 0.105 Kawai et al., 1973
Japan 1971-1972 0.038-0.075 Kamata, 1973
Japan 1970 3.780c Suzuki et al., 1973
Japan 1971 3.592c Suzuki et al., 1973
Japan 1972 3.822c Suzuki et al., 1973
Japan 1973 0.0854 Kamata, 1974
Oceania
Australia 1970 1 GLC -- -- 0.014d Newton & Greene, 1972
0.007e
0.066f
67 GLC 0.036 0.105 0.141
Table 15 (Cont'd)
Country Year No. of Method DDT DDE as DDT Total as DDE as Reference
samples of (mg/litre) (mg/litre) (mg/litre) DDT
analysis (% of
total)
Australia
(Brisbane) 1971-1972 20 GLC 0.288 Miller & Fox, 1973
(Mareeba) 1971-1972 20 GLC 0.415
Australia 45 GLC 0.064 Siyali, 1973
Australia 22 GLC 0.010 0.068 0.078 Stacy & Thomas, 1975
Papua New Guinea 1972 16 GLC
(Kar Kar Island) 0.002 0.002 0.004 50 Hornabrook et al., 1972
Papua New Guinea 1972 19 GLC
(Sepik district) 0.008 0.007 0.015 47 Hornabrook et al., 1972
a Range of values for milk containing 4% fat c Concentration in milkfat. e At middle of feeding, 1.2% fat.
containing 3.3-6.6 ppm.
b Maximal value. d At beginning of feeding, 1.8% fat. f At end of feeding, 5.1% fat.
Table 16. Ranges, means, and standard errors of the concentrations
of organochlorine insecticides in the milk of women in
three towns in Guatemalaa
Compound La Bomba El Rosario Cerro Colorado
1970 1970 1971
n = 10 n = 27 n = 9
p.p'-DDT 0.23-4,95 0.16-2.24 0.49-5.94
(mg/litre) (1.00 ± 0.38) (0.77 ± 0.10) (1.78 ± 0.56)
p.p'-DDE 0.12-6.36 0.28-3,10 0.60-6.13
(mg/litre) (1.02 ± 0.58) (0.99 ± 0.14) (2.10 ± 0.61)
p,p'-TDE(DDD) trb-0.16 0.01-0.09 0.05-0.11
(mg/litre) (0.03 ± 0.02) (0.02 ± 0.004) (0.07 ± 0.01)
o,p'-DDT tr-0.29 0.01-0.18 0.06-0.22
(mg/litre) (0.09 ± 0.03) (0.06 ± 0.01) (0.12 ± 0.02)
total as DDT 0.41-11.50 0.34-4.97 1.571-12.21
(mg/litre) (2.15 ± 1.05) (1.84 ± 0.24) (4.07 ± 1.11)
total HCH 0.01-0.10 tr-0.07 0-0.06
(mg/litre) (0.03 ± 0.01) (0.007 ± 0.003) (0.02 ± 0.01)
heptachlor epoxide 0-0.02 tr-0.01 tr
(mg/litre) (0.003 ± 0.002) (0.007 ± 0.0004)
dieldrin tr tr-0.01
(mg/litre) (0.002 ± 0.0005)
a From: Olszyna-Marzys et al. (1973).
b tr = trace.
The slightly greater secretion of DDT and much greater secretion
of various isomers of HCH by urban mothers (compared to rural mothers)
in Japan was attributed to their greater intake of cow's milk (Takano,
1972).
Other routes of excretion. DDT, DDE, and dieldrin are excreted in
the bile; the concentrations for five men without special exposure
varied as follows: p,p'- and o,p'-DDT combined, 0.0000-0.0009 mg/litre;
p,p'-DDE, 0.0005-0.0056 mg/litre; and dieldrin, 0.0000-0.0005 mg/litre.
Higher levels were found in the bile of one pest control operator
(Paschal et al., 1974).
6.3.2 Animal studies
When large doses of DDT are ingested, some of the compound is not
absorbed and is passed in an unaltered state in the faeces. Only
traces of unaltered DDT may be found in the faeces when exposure is by
any route other than oral. However, true faecal excretion of DDT
metabolites was established very early (Wasicky & Unti, 1945; Judah,
1949). In the rat, faecal excretion of DDT exceeded urinary excretion,
irrespective of the route of administration (Hayes, 1965). In man, the
ratio is obviously different. Although the excretion of DDT-related
material in the faeces of man receiving 35 mg/man per day has been
reported using colorimetry (Hayes et al., 1956), this result has never
been confirmed by gas chromatography, even in connexion with workers
whose exposure was heavy and prolonged. Either DDT metabolites are not
excreted by man in the faeces to any great degree, or they are
excreted in one or more forms that differ from those already
demonstrated in rats.
The bile appears to be the principal source of DDT metabolites in
the faeces of rats. When the bile duct was cannulated before
intravenous injection of radioactive DDT, 65% of the dose was
recovered in the bile, 2% in the urine, and only 0.3% in the faeces
(Jensen et al., 1957), and the possibility of some contamination of
the faeces by urine could not be excluded.
The different routes of excretion are not unrelated. Burns et al.
(1957) found that there was an increase in urinary excretion of
radioactive material following ligation of the bile duct in rats fed
radioactive DDT. This is an indirect confirmation of the finding by
Jensen and his colleagues that most of the metabolites in bile consist
of DDA or compounds closely related to it. Although an enterohepatic
circulation of the metabolites of DDT has not been directly proved, it
seems likely that such a circulation exists, as has been demonstrated
for 1,1'-(2,2-dichloroethylidene)-bis[4-ethylbenzene] (Perthane).
Demonstration of the excretion of DDT in milk was first reported
by Woodard et al. (1945) in connexion with a dog fed the compound at
the rate of 80 (mg/kg) day. Within a short time, excretion of DDT in
milk was reported in rats, goats, and cows, and soon afterwards, in
women (Laug et al., 1951). Telford & Guthrie (1945) showed that rats
fed a diet containing DDT at 1000 mg/kg produced milk that was toxic
to their young.
Since the early laboratory studies, the presence of DDT has been
demonstrated repeatedly in the milk of cows. A review (Hayes, 1959)
showed that cows fed substantial, but nontoxic, residues of DDT
commonly excrete 10% or slightly more of the total dose in their milk,
and amounts slightly over 30% have been observed.
Further information on the excretion of DDT in human milk is given
in section 6.3.1.3. It is of interest to repeat here, however, that
lactating women in the general population are apparently in negative
DDT balance. That is, they excrete more DDT in their milk each day
than they acquire in their food. The difference is small and would not
be expected to have much effect on their total body burden of DDT.
Wilson et al. (1946a) showed that DDT was secreted from the skin
of a cow maintained on an oral dosage of about 53 (mg/kg) day.
DDA. Because DDA is the main form in which DDT is excreted, it
might be expected that, following its direct administration, DDA would
be excreted relatively efficiently, and this is true. It was found
very early that, during the first few days after oral dosing, rabbits
excreted DDA in the urine approximately 15 times faster than animals
given DDT at an equivalent dosage. Although the rate of DDA excretion
increased somewhat, the rate of excretion associated with DDT
increased more rapidly so that the values differed by a factor of only
five after the twentieth day of feeding (Smith et al., 1946).
6.4 Biotransformation
The chemical nature of the chief metabolite excreted in the urine
was first elucidated by White & Sweeney (1945). Rabbits were given DDT
(melting point 107-108°C) at a rate of 100 (mg/kg) day for 6 days per
week, and their urine was collected. It contained a considerable
amount of organic chloride, whereas normal rabbit urine did not. Using
the organic chloride test to evaluate different methods of extraction,
the authors were able to isolate a crystalline material containing
25.37% chlorine and melting at 166-166.5°C. The crystals were shown to
be DDA (see Table 1). The product obtained from the urine was
identical to that synthesized from glyoxylic acid and chlorobenzene
and with a compound obtained through the chemical degradation of DDT.
Identity of the 3 compounds and, therefore, their true chemical
nature, was established by the determination of melting points, mixed
melting points, elementary analysis, and X-ray powder diffraction
patterns, as well as by demonstrating the similarity of the
decarboxylation products of the three original materials. Only 80-85%
of the total organic chloride of the rabbit urine was found to be
soluble in alkali and in bicarbonate. For this and other reasons it
was considered possible that DDA was not the only chlorinated organic
compound present.
Later work by many authors has confirmed that DDA is the major
urinary metabolite of DDT in all mammals including man. It may be
added that, in spite of great strides in analytical chemistry, the
nature of other urinary metabolites has not been elucidated fully.
The fact that DDE is stored in tissue was first demonstrated by
Pearce et al. (1952) in connexion with human fat. The authors pointed
out that they did not know whether the compound resulted from partial
degradation of DDT residues on plants or whether the DDE was formed
during the process of digestion or after absorption. It is now known
that some food contains DDE but that man is capable of forming the
product from DDT. The exact mechanism of the biotransformation of DDT
to DDE remains in doubt.
Pearce et al. (1952) established the identity of DDE by comparing
the colorimetric and column chromatographic behaviour of the residue
with those of a chemical standard. A second paper from the same
laboratory (Mattson et al., 1953) added further details confirming the
identity of the compound. Later investigations have confirmed the
identity of DDE by infrared spectrometry and by gas chromatography.
That portion of the metabolism of DDT that leads to DDA has been
clearly elucidated by Peterson & Robinson (1964), who gave evidence
for the sequence of changes shown in Fig. 2. Organ perfusion studies
have indicated that the liver is capable of the biotransformation of
DDT, DDE, TDE, DDMU, and DDMS, while the kidney transforms DDMS, DDNU,
and DDOH (Datta & Nelson, 1970). Cultures of embryonic lung cells are
capable of metabolizing DDT to DDA via DDD (North & Menzer, 1973).
When DDA was discovered, it was postulated, on chemical grounds,
that DDE was a step in its formation (White & Sweeney, 1945); however,
rats that produced both DDE and DDA from DDT were incapable, according
to Peterson & Robinson (1964), of forming DDA when fed preformed DDE.
This finding was contradicted by Datta (1970) and by Datta & Nelson
(1970) who claimed that 14C- p,p'-DDE was converted by rats to
p,p'-DDMU, which then underwent further metabolism to p,p'-DDA via
the route shown in Fig. 2. Datta suggested that the predominance of
detoxication via DDE or TDE (DDD) might depend on physiological
response or the amount of toxicant used. Whatever the reason, the fact
remains that DDE is stored more tenaciously than DDT.
Rhesus monkeys fed either technical or p,p'-DDT store little or
no DDE, although they are fully capable of storing DDE when it is fed
preformed (Durham et al., 1963).
The way in which DDE was lost from storage was not clearly
understood for a long time. In man (Cueto & Biros, 1967), seal, and
guillemot (Jansson et al., 1975) part of it is excreted unchanged, but
the fact that its elimination is promoted by the induction of
microsomal enzymes (see section 7.1.4.2) strongly suggests that it
undergoes metabolism, conjugation, or both. That metabolism does occur
was first demonstrated by identification of 2 hydroxylated derivatives
of DDE in the faeces of wild seals and guillemots and in the bile of
seals (Jansson et al., 1975). When p,p'-DDE was fed to rats, the
same metabolites and one other were isolated from the faeces, and,
within the first 6 days, the metabolites accounted for about 5% of the
dose (Sundström et al., 1975). Later a fourth hydroxylated derivative
was identified from the faeces of rats fed p,p'-DDE. The compounds
 are m-hydroxy- p,p'-DDE [1,1-dichloro-2-( p-chloro- m-hydroxyphenyl)-
2-( p-chlorophenyl)-ethylene, the major metabolite], o-hydroxy- p,p'-DDE,
p-hydroxy- m,p'-DDE (the product of an NIH shift), and p-hydroxy-
p'-DDE. A scheme (Fig. 3) involving m,p-epoxy- p,p'-DDE and o,m-epoxy-
p,p'-DDE was proposed for the formation of these metabolites as
well as a fifth metabolite (Sundström, 1977). Neither the fifth
metabolite nor the hypothetical intermediate have been isolated.
DDE is metabolized not only to easily excretable phenols but also
to m-methylsulfone- p,p'-DDE. In the blubber of seals from the
Baltic, this compound was found in a concentration of 4 mg/kg along
with DDE (138 mg/kg), TDE (DDD) (10 mg/kg), DDT (78 mg/kg) and various
PCB's and their metabolites (150 mg/kg) (Jensen & Jansson, 1976).
The conversion of o,p'-DDT to p,p'-DDT has been reported
(Klein et al., 1965; French & Jefferies, 1969), but, when the
possibility was reinvestigated using 14C- o,p'-DDT, no conversion
could be detected (Cranmer, 1972). The chromatographic peak closely
resembling that of p,p'-DDT observed in the earlier studies
undoubtedly was due to the presence of a metabolite of o,p'-DDT,
which may explain the more rapid metabolism of the o,p'-isomers that
has been observed in rat, man, and perhaps other species. The more
rapid excretion of o,p'-DDT is explained, at least in part, by the
observed ring-hydroxylation of the parent compound in rats (Feil et
al., 1973) and chickens (Feil et al., 1975) and of preformed o,p'-TDE
(DDD) in rats (Reif & Sinsheimer, 1975) and in man (Reif et al.,
1974). At least 13 metabolites were detected in rats and 15 in
chickens. Ring-hydroxylation, which has not been observed with p,p'-
DDT or p,p'-TDE, was present in all species. There were, however,
some species differences. For example, o,p'-DDE and three
hydroxylated o,p'-DDE's were found in the excreta of chickens but
not in the excreta of rats. In 2 patients with adrenal carcinoma for
which they were receiving o,p'-TDE at a rate of 2000 mg/day, as much
as 46-56% of the daily intake was recovered in the urine following
acid hydrolysis. Just over half of the recovered material was in the
form of o,p'-DDA, but the remainder was in the form of hydroxylated
derivatives, specifically m-hydroxy, p-hydroxy-, m-hydroxy- p-
methoxy-, and p-hydroxy- m-methoxy- o,p'-DDA. Some other
hydroxylated compounds were found in trace amounts. All hydroxylation
had occurred on the ring that had its chlorine in the ortho position
(Reif et al., 1974). When the metabolism of a single 100 mg oral dose
of 14C- o,p'-TDE was studied in rats, averages of 7.1 and 87.8% of
the activity were recovered in the urine and faeces, respectively,
within 8 days (Reif & Sinsheimer, 1975). The high recovery indicated
rapid excretion with little storage.
are m-hydroxy- p,p'-DDE [1,1-dichloro-2-( p-chloro- m-hydroxyphenyl)-
2-( p-chlorophenyl)-ethylene, the major metabolite], o-hydroxy- p,p'-DDE,
p-hydroxy- m,p'-DDE (the product of an NIH shift), and p-hydroxy-
p'-DDE. A scheme (Fig. 3) involving m,p-epoxy- p,p'-DDE and o,m-epoxy-
p,p'-DDE was proposed for the formation of these metabolites as
well as a fifth metabolite (Sundström, 1977). Neither the fifth
metabolite nor the hypothetical intermediate have been isolated.
DDE is metabolized not only to easily excretable phenols but also
to m-methylsulfone- p,p'-DDE. In the blubber of seals from the
Baltic, this compound was found in a concentration of 4 mg/kg along
with DDE (138 mg/kg), TDE (DDD) (10 mg/kg), DDT (78 mg/kg) and various
PCB's and their metabolites (150 mg/kg) (Jensen & Jansson, 1976).
The conversion of o,p'-DDT to p,p'-DDT has been reported
(Klein et al., 1965; French & Jefferies, 1969), but, when the
possibility was reinvestigated using 14C- o,p'-DDT, no conversion
could be detected (Cranmer, 1972). The chromatographic peak closely
resembling that of p,p'-DDT observed in the earlier studies
undoubtedly was due to the presence of a metabolite of o,p'-DDT,
which may explain the more rapid metabolism of the o,p'-isomers that
has been observed in rat, man, and perhaps other species. The more
rapid excretion of o,p'-DDT is explained, at least in part, by the
observed ring-hydroxylation of the parent compound in rats (Feil et
al., 1973) and chickens (Feil et al., 1975) and of preformed o,p'-TDE
(DDD) in rats (Reif & Sinsheimer, 1975) and in man (Reif et al.,
1974). At least 13 metabolites were detected in rats and 15 in
chickens. Ring-hydroxylation, which has not been observed with p,p'-
DDT or p,p'-TDE, was present in all species. There were, however,
some species differences. For example, o,p'-DDE and three
hydroxylated o,p'-DDE's were found in the excreta of chickens but
not in the excreta of rats. In 2 patients with adrenal carcinoma for
which they were receiving o,p'-TDE at a rate of 2000 mg/day, as much
as 46-56% of the daily intake was recovered in the urine following
acid hydrolysis. Just over half of the recovered material was in the
form of o,p'-DDA, but the remainder was in the form of hydroxylated
derivatives, specifically m-hydroxy, p-hydroxy-, m-hydroxy- p-
methoxy-, and p-hydroxy- m-methoxy- o,p'-DDA. Some other
hydroxylated compounds were found in trace amounts. All hydroxylation
had occurred on the ring that had its chlorine in the ortho position
(Reif et al., 1974). When the metabolism of a single 100 mg oral dose
of 14C- o,p'-TDE was studied in rats, averages of 7.1 and 87.8% of
the activity were recovered in the urine and faeces, respectively,
within 8 days (Reif & Sinsheimer, 1975). The high recovery indicated
rapid excretion with little storage.
 The compound identified by Peterson & Robinson (1964) as a
"probable intermediate aldehyde" was later synthesized and shown to be
highly labile (McKinney et al., 1969), confirming the guess by
Peterson & Robinson that it was unlikely to accumulate in tissues in
measurable amounts.
Of the compounds shown in Fig. 2 and 3, only DDT, TDE (DDD), DDE,
and DDA are commonly reported in the tissues or excreta of animals,
including man. The symptomatology produced when the metabolites are
administered directly is discussed in section 7.1.1, while uptake,
distribution, and elimination of the compounds are discussed in
sections 6.1.2, 6.2.2, and 6.3.2, respectively.
Although microorganisms, plants, insects, and birds produce many
of the same metabolites that are found in mammals, there are
interesting differences. Nearly 20 derivatives (including mammalian
metabolites) have been identified, and the chemical structure of
several more is still unknown. Some aspects of nonmammalian, as well
as mammalian metabolism have been reviewed (Menzie, 1969; Klein &
Korte, 1970; Fishbein, 1974; Schroeder & Dorozalska, 1975). The
metabolism of microorganisms and plants, as well as that of domestic
animals, may influence the composition of DDT-derived residues in
human food, but there is no evidence that these residues contain a
significant amount of any compound not formed from DDT by human
metabolism.
7. EXPERIMENTAL STUDIES ON THE EFFECTS OF DDT
7.1 Animal Studies
7.1.1 Haemopoietic system and immunology
Many early reports reviewed by Hayes (1959) indicated that large
doses of DDT might not have any effect on the blood or that they might
produce a moderate leukocytosis and a decrease in haemoglobin, with or
without a decrease in the concentration of red cells. The leukocytosis
probably is secondary to stimulation of the sympathetic nervous
system, while the loss of haemoglobin may be nutritional in origin.
Later studies have confirmed the early results. A range of
haematoiogical variables remained unchanged in squirrel monkeys dosed
orally at rates of 0, 0.05, 0.5, 5, and 50 (mg/kg)/day, even though
the highest dosage was fatal within 14 weeks (Cranmer et al., 1972).
Immunology. Inasmuch as some compounds are antibiotic, it is
logical that some may be probiotic, that is, that they either reduce
resistance to infection or increase the virulence of an infecting
organism (Hayes, 1975). Far fewer studies have been made of probiosis
than of antibiosis. A number of papers have reported one or other
possibly probiotic property of DDT, but some of the reports could not
be confirmed, and others have not been retested.
It has been claimed that a change in the phagocytic activity of
white blood cells is an indication of early intoxication by DDT
(Kun'ev, 1965). However, Kaliser (1968) did not find any statistical
difference in in vitro or in vivo phagocytosis of control rats and
those receiving DDT by stomach tube at a rate of 0.25 (mg/kg)/day for
31 days. The highest rate at which men who make and formulate DDT in
the USA now absorb the compound is about 0.25 (mg/kg)/day.
Rats receiving an aqueous suspension of p,p'-DDT of unstated
stability at a concentration of 200 mg/litre as their only source of
water for over 30 days were reported to develop a lower titre of
antibodies to ovalbumen (Wassermann et al., 1969). Rabbits responded
to the same treatment with a statistically significant reduction of
antibody titre against Salmonella and a reduction in antibody titre
against sheep red blood cells that was not statistically significant
(Wassermann et al., 1971). Both the rats and rabbits showed a decrease
in at least one globulin fraction of the blood.
Other reports of changes in immunohaematological indices are those
of Semenceva (1968) and Fridman (1970).
One group of investigators has shown clearly that what at first
appeared to be an immunological response really involved a quite
different, predictable effect. Briefly, it was shown that guineapigs
sensitized to diphtheria toxoid were less susceptible to anaphylaxis
in response to a challenge dose of the toxoid if they were pretreated
with DDT at a dosage of only 1-20 mg/kg. Direct measurement of
antitoxin production indicated little or no difference between
protected and unprotected animals. Furthermore, some protection was
given by DDT administered for only 3 days prior to the induction of
anaphylaxis (Gabliks et al., 1973, 1975). Further studies showed that
DDT treatment reduced the histamine levels in the lungs of both
immunized and nonimmunized animals. The number of detectable mast
cells was also reduced; this was true whether the count was made in
tissues from guineapigs dosed systemically with DDT or in the lungs
and mesenteric tissue taken from untreated animals and exposed to DDT
in vitro at concentrations ranging from 10 to 45 mg/litre. These
results indicate that the protection offered by DDT was the result of
a reduction of the amount of histamine available for sudden release in
response to a challenge dose of toxoid (Askari & Gabliks, 1973).
Regardless of exposure to DDT, immunization leads to an increase in
detectable mast cells (Gabliks et al., 1975).
7.1.2 Nervous system and behaviour
DDT intoxication in animals was well described by Domenjoz (1944).
The first perceptible effect is abnormal susceptibility to fear, with
violent reaction to normally subthreshold stimuli. There is definite
motor unrest and increased frequency of spontaneous movements. As
poisoning increases, hyperirritability, like that seen in strychnine
poisoning develops, but convulsions do not appear at this time. A fine
tremor, recognizable at first only as a terror reaction, is later
present as all intention tremor in connexion with voluntary movement.
Then it is present intermittently without observable cause, and
finally it is present as a coarse tremor without interruption for as
long as several days. Spontaneous movement is limited, and food intake
stops so that surviving animals lose weight. In the later stages,
especially in some species, there are attacks of epileptiform, tonic-
clonic convulsions with opisthotonos.
All the signs are strengthened by external stimuli and become
manifest at first through external stimuli. At all stages, the animals
show normal position and labyrinth reflexes. The picture of poisoning
in mammals recalls the disturbances of movement and tone that are
known in human pathology as the amyostatic syndrome.
Symptoms appear several hours after oral administration of the
compound, and death follows after 24-72 h. The latent period after
intravenous administration at about the LD50 level is approximately
5 min; signs of poisoning reach a maximum level in about 30 min, and
survivors are symptom-free in 18 to 24 h. Animals that survive recover
completely.
In addition to the features of poisoning already mentioned,
Cameron & Burgess (1945) noticed that as rats, guineapigs, and rabbits
become sick, they become cold to the touch and show ruffled fur. Some
show diarrhoea. These authors found that muscular tremors were
preceded by muscular weakness that first occurred in the back and
later in the hind legs. The front legs were relatively spared so that
animals showing marked weakness of the hind quarters could still drag
themselves about. However, several authors have found that the tremor
characteristic of DDT poisoning generally starts in the muscles of the
face, including the eyelids, and spreads caudally with variable
severity until all the muscles are affected. Furthermore, although
weakness of hind quarters has been seen by others, it is not a common
finding.
Like tremor, coldness of the skin and ruffling of the fur probably
represent an indication of disturbed thermal regulation. Apparently it
was not until the work of Hrdina et al. (1975) that an increase of
almost 3°C in body temperature was reported in rats following a fatal
(600 mg/kg) oral dosage of DDT.
Although there is a general similarity in the clinical effects of
DDT in all vertebrate species, some characteristic differences exist.
Cats show greater extensor rigidity and opisthotonos than other
laboratory animals. The stiffness appears first in the distal part of
the extremities and later extends to the proximal part and to the
trunk. Poisoned cats show marked pilomotor activity. Convulsions in
them may become almost continuous. Convulsions are also prominent in
dogs as is ataxia. Tremors are so pronounced in rats that it may be
difficult to detect clonic convulsions in them. Rats poisoned by DDT
show a reddish colour about the eyes just as they do when ill from
many other causes. The colour has been attributed to excessive
secretion of a porphyrin by the Harderian glands.
Poisoning produced by repeated doses of DDT differs from that
produced by a single dose only in so far as the animal may be
gradually debilitated, especially by malnutrition. If food intake is
maintained, tremor may last for weeks, or even intermittently, for
months. If the animals survive a short time after dosing stops,
recovery is complete. However, food intake may be interfered with in
at least two ways. Tremor and more severe signs may interfere
mechanically with eating. Animals offered food containing high
concentrations of DDT often eat little or nothing and lose weight
rapidly. However, the same animals will show excellent appetites when
offered the same kind of food without DDT just after refusing the
major portion of the daily ration of contaminated food. Unlike
dieldrin and some other compounds, DDT seems to have little effect on
appetite as mediated by the central nervous system; it has a great
deal to do with taste.
Animals that have suffered severe weight loss as a result of DDT
poisoning may die partly as a result of general debility. In some
colonies, at least, they have become prey to secondary infection.
In summary, it may be said that animals that die as the result of
repeated large doses of DDT and small animals that die as a
complication of starvation following many somewhat smaller doses of
DDT show the same signs as those seen in animals killed by one or a
few large doses. Even though severely ill, animals that survive a few
days after the last of many doses of DDT recover.
Of samples that may be collected from a living animal, the
concentration of DDT in serum most accurately reflects its
concentration in the brain, the critical tissue. In the rat, levels of
25 mg/kg (wet weight) in the brain are not usually fatal: higher
levels tend to be fatal regardless of whether absorption followed one
or many doses (Dale et al., 1963; Hayes & Dale, 1964). As reviewed by
Hayes (1975), the danger level is approximately the same in several
species of birds.
Behavioural changes may be demonstrated in animals receiving DDT,
daily, at rates too low to produce illness. Khairy (1959) was able to
detect ataxia in the form of changes in gait in rats that had been fed
DDT at dietary levels of 100 mg/kg or more for 21 or more days. Gait
was recorded by smearing the hind paws of the animals with vaseline,
which then recorded their tracks on paper. Gait was recorded in terms
of the tangent, that is the ratio of the width and length of step. At
a body weight dosage of about 5 (mg/kg)/day the ratio was less than
normal, a change the author attributed to an exaggeration of the
stretch reflex. At dosages of about 10, 20, and 30 (mg/kg)/day, the
ratio was progressively greater than the normal as a result of
broadening of the gait and shortening of the steps. These same dosage
levels did not affect problem-solving behaviour or speed of
locomotion. The experimental animals were generally less reactive to
stress than normal ones. Thus, the author attributed hyperirritability
of rats poisoned by DDT to exaggerated motor responses.
The major toxic action of DDT is clearly on the nervous system,
and it requires an intact organism for full expression. The fact that
DDT causes a myotonic response in muscle and substitution of a train
of spikes for the normal diphasic electroneurogram (Eyzaguirre &
Lilienthal, 1949) is in marked contrast with the absence of detectable
injury or, in fact, any response in other isolated tissues. As early
as 1945, Lewis & Richards (1945) found DDT to be inert when it was
applied to tissue cultures of heart, kidney, stomach, intestine,
liver, and muscle from 7 to 9-day chick embryos, and of brain and
spleen from a one-day rat. The physiology of the cells including the
mytoses of fibroblasts was normal. The migration and extension of the
various cells was unchanged. The authors stated that "living
fibrilloblasts, as they moved about in the cultures, sometimes touched
or even migrated over DDT crystals without appreciable injury to
themselves during a period of several days". Some observations were
carried out for periods as long as 21 days.
In spite of the importance of the nervous system, a detailed
review of early literature indicates that, although the presence of
some specialized nervous function may be necessary for the
manifestation of DDT poisoning, the mere occurrence of specialized
nerve fibres in certain protozoa or the occurrence of a rather complex
nervous system in molluscs is not sufficient to render these forms
susceptible. Just as there is no explanation for the effect of DDT on
susceptible species, so there is no explanation for the fact that
certain species and even entire phyla are inherently resistant to the
compound.
A review (Hayes, 1959) of literature on the effects of DDT on the
nervous system reveals that all major parts, both central and
peripheral, are affected. Whereas effects on specific portions,
notably the cerebellum and the motor cortex, have been viewed as of
greatest importance, it probably is more accurate to emphasize the
interaction of functions, all modified to some degree.
There is reason to think that the mechanism of action of DDT is
its action on membranes in the nervous system, especially axonal
membranes. Certainly action on membranes is a fundamental property of
the compound. In fact the potassium conductance induced by valinomycin
at 10-6 mol/litre in a synthetic lecithin-decane membrane is
reversed by DDT at 3 × 10-6 mol/litre (Hilton & O'Brien, 1970).
Attention was focused quite early on the effects of DDT on axonal
membranes. Using the giant axons of the cockroach, Narahashi &
Yamasaki (1960) showed that DDT prolonged the recovery phase of the
action potential. They concluded that it slows the efflux of potassium
ions from the axon. Later, using the voltage clamp technique and giant
axons of the lobster, Narahashi & Haas (1967) showed that DDT, at a
concentration of 5 × 10-4 mol/litre of bathing medium, prolonged the
flow of sodium ions as well as interfering with the flow of potassioum
ions: in other words DDT delayed shutting of the Na+ gate and
prevented full opening of the K+ gate.
Na+-, K+-, and Mg2+-adenosine triphosphatase (EC 3.6.1.3) is
involved in ion transport in the nervous system. Matsumura & Patil
(1969) showed that a preparation of this enzyme from a nerve ending
fraction of the rabbit brain was inhibited by DDT at concentrations as
low as 10-8 mol/litre. There was a good correlation between the
degree of its inhibition by analogues of DDT and their toxicity to
mosquito larvae. A similar enzyme that binds 14C-DDT has been
isolated from the synapses of rat brain (Bratowski & Matsumura, 1972).
Both the electrophysiological changes and the enzyme inhibition
exhibit a negative temperature coefficient, an important feature of
DDT poisoning in insects but not in mammals (Hoffman & Lendle, 1948;
Deichmann et al., 1950).
At a supralethal dosage of 600 mg/kg, DDT caused a marked decrease
in the concentration of cortical and striatal acetylcholine and of
brainstem norepinephrine in rats and a significant increase in
brainstem 5-hydroxyindoleacetic acid. All of the neurotoxic signs of
poisoning were blocked by p-chlorophenylalanine, while other
inhibitors blocked one or other, but not all of the effects. It was
concluded that changes in the metabolism of 5-hydroxytryptamine and
norepinephrine might be responsible for DDT-induced hyperthermia while
acetylcholine might be related to tremors and convulsions (Hrdina et
al., 1973). These and related matters have been reviewed in great
detail by Hrdina et al. (1975). Apparently studies have not been made
at a range of dosages that would make it possible to know whether
these changes are a result or a cause of poisoning; the possible
therapeutic effect of p-chlorophenylalanine has also not been
explored.
It was reported by Haikina & Silina (1971) that administration of
DDT to rats at only one-fifth of the LD50 for the 2 days increased
the amount of 5-oxyindoleacetic acid excreted in urine by 188%. This
indicates a change in the metabolism of serotonin, but its
significance is not clear.
One sensitive measure of brain activity is the electroencephalo-
gram (EEG). Farkas et al. (1969) found that the EEG wave frequency
increased considerably in resting rats that had received
20 (mg/kg)/day of DDT as a result of dietary intake. Rats that had
received either 5 or 10 (mg/kg)/day did not exhibit this change while
at rest, but even those receiving 5 (mg/kg)/day exhibited this change
when exposed to a rhythmic light stimulus. The EEG may become abnormal
only a minute or two after administration of a large dose of DDT;
4 stages of the electrical activity culminating in generalized
seizures have been described by Joy (1973). Phenobarbital, but not
phenytoin or trimethadione, was effective in stopping the seizures.
7.1.2.1 Cause of death
Death from DDT poisoning is usually the result of respiratory
arrest. The heart continues to beat to the end and in some instances
continues a little while after respiration stops. Deichmann et al.
(1950) found that the onset of hyperirritability in rats was
accompanied by an increase in the frequency and amplitude of
respiration. Later, with the occurrence of tremors, the depth of
respiration frequently returned to a more normal level, but the rate
remained high. In some animals, respiration stopped suddenly after a
deep inspiration during a tonic convulsion. In other animals, the rate
and amplitude decreased progressively and finally ceased without any
terminal spasm. Animals that die of respiratory failure caused by DDT
do so after a relatively long period of muscular activity that leaves
them exhausted.
It was shown by Philips & Gilman (1946) and Philips et al. (1946)
that the hearts of dogs given large intravenous doses of DDT were
sensitized to epinephrine. This was true not only of injected
epinephrine but also of the compound released by the adrenal glands
during a seizure. Stimulated in this way, the sensitized hearts of
dogs developed an irreversible, fatal ventricular fibrillation.
However, the hearts of monkeys were able to recover from fibrillation
and resume normal rhythm. It is not clear how important sensitization
of the myocardium is when DDT is administered by other routes, but
ventricular fibrillation may be the cause of death in animals that die
suddenly, soon after onset of poisoning.
7.1.2.2 Treatment of poisoning in animals
Studies on the treatment of poisoning will be discussed in this
section since all the more successful studies of treatment of animals
poisoned by DDT involve the nervous system. Smith and Stohlman (1944)
noted the possibility that narcotics in general, may exhibit an
antagonism to DDT. Rats survived on a diet containing DDT at a
concentration of 1000 mg/kg for 90 days when they received
cyclohexanone in the same diet at the rate of 2000 mg/kg, but were
uniformly killed in a shorter period when they received DDT at the
same rate without cyclohexanone. Later, it was shown that
cyclohexanone offers no protection when used as a solvent for single
massive doses of DDT (Deichmann et al., 1950).
Smith & Stohlman (1945) showed that, when given as required after
the onset of illness, urethane and, to a lesser extent, phenytoin
sodium protected rats from poisoning. Sodium amobarbital gave slight
benefit, sodium phenobarbital a doubtful benefit, and paraldehyde no
protection at all. All drugs were given intraperitoneally except
paraldehyde, which was given by stomach tube. The mortality of rats
treated with urethane was 12.5% and that of their controls was 80%. A
total dosage of 1.2-2.5 mg/kg spread over a period of 1-3 days, was
found most satisfactory. Phenytoin sodium gave a mortality of 46.7%,
compared with 96.7% for the controls. The smallest effective dosage
was 200-250 mg/kg, a value very close to the LD50 which, under the
conditions of the test was 300 mg/kg.
Lauger et al. (1945a, b) also found that sodium phenobarbital was
of questionable value in treating rats poisoned by DDT. However,
completely different results were seen in larger animals.
Phenobarbital was, by far, the most outstanding remedy tested by
Philips & Gilman (1946). In a dosage well below the anaesthetic level,
it not only prevented death in many instances but also controlled
tremor and convulsions. Signs of illness were more readily controlled
in dogs and cats than in monkeys, which required nearly a full
anaesthetic dosage before tremors completely disappeared.
Magnesium sulfate did not reduce mortality in poisoned dogs and
cats although it did control tremors and convulsions briefly. Sodium
bromide was entirely ineffective. Mortality was reduced with urethane,
but a full anaesthetic dosage was required to control tremor and
convulsion. Similarly, sodium barbital and sodium pentobarbital
controlled symptoms only when given in full anaesthetic doses and,
even then, did not greatly reduce mortality. Phenytoin, when given to
rats before they received DDT, reduced the lethal action without
showing a notable effect on the signs of poisoning; phenytoin was not
effective in cats.
Vaz et al. (1945) were, apparently, the first to note the
antidotal effect of calcium in DDT poisoning. Dogs were given DDT
orally as a 10% oily solution at a daily dosage of 100 mg/kg until
signs of intoxication appeared. The same dosage could then be repeated
to produce intense symptomatology from which the animals would recover
spontaneously in 12-24 h. For the actual tests, a larger challenge
dosage of DDT (150 to 200 mg/kg) was used. Each dose of calcium
gluconate (30 ml of a 10% solution) was injected intravenously into
dogs weighing 8-18 kg. Dogs that were injected with calcium gluconate
dally for 4 days and, challenged with a large dose of DDT on the
fourth day, did not develop any symptoms or only slight ones. Dogs
receiving a single dose of calcium gluconate showed symptoms of short
duration and survived following a dosage of DDT large enough to kill 2
controls.
Cats poisoned by the intravenous injection of a soya-lecithin-corn
oil emulsion of DDT were studied by Koster (1947). A comparison was
made of several aspects of intoxication including number of
convulsions, general severity (tremors, prostration, dyspnoea),
duration, and mortality. Both calcium gluconate and sodium gluconate
reduced mortality but not severity. Gluconic acid increased the
survival time, reduced mortality, but did not reduce the number or
severity of the convulsions. Calcium chloride reduced convulsions, but
not mortality or tremors. Molecular equivalent doses of the candidate
antidotes were used. Gluconic acid and its two salts were effective
against an LD95 dosage of DDT. The life-saving capacity of calcium
gluconate at a rate of 40 mg/kg was confirmed by Judah (1949) even
though he found normal blood calcium values in most poisoned but
unmedicated animals. One animal showed a high calcium value, and
Cameron & Burgess (1945) reported a similar result. It has been
suggested that increased blood calcium may be associated with acidosis
caused by the accumulation of lactate.
Thus, calcium has an antidotal action against DDT in intact
animals of several species. The suppression by calcium of the effect
of DDT on the isolated nerve and muscle of the rat has been
demonstrated (Eyzaguirre & Lilienthal, 1949). The hypothesis has been
advanced (Welsh & Gordon, 1946; Gordon & Welsh, 1948) that certain
neurotoxins, including DDT, act by delaying the restoration of calcium
ions to a surface complex, following breaking of the chelate linkage
of calcium ions to surface polar groups by an initial exciting
impulse. This action of the neurotoxin is conceived as depending
largely on its physical rather than on its chemical properties. The
hypothesis is helpful in explaining the fact that a wide variety of
chemically unrelated compounds produce repetitive responses in
excitable tissue and also the fact that many compounds that show a
high toxicity for arthropods and mammals are fat-soluble and
relatively inert chemically. It has been pointed out that this
hypothesis postulates a very localized action of calcium at the nerve-
cell membrane; the hypothesis is not inconsistent with the finding
that the blood calcium of poisoned animals may be unchanged or even
increased.
Having observed the effect of DDT on the metabolism of glucose and
glycogen, Lauger et al. (1945a, b) investigated the use of glucose as
an antidote. All of 10 dogs given 2000 mg of DDT per kilogram body
weight orally in the form of an oil solution died within 8-24 h. Five
of 10 dogs treated with one or more, 20 ml doses of 20% glucose
survived the same dosage of DDT. The glucose was given intravenously
in most instances.
Koster (1947) found that glucose given before or after an LD33
dosage reduced convulsions and mortality and, when given before the
poison, reduced tremors, prostration, and dyspnoea in cats. Glucose,
unlike gluconic acid and its sodium and calcium salts, was ineffective
against an LD95 dosage except to increase the time of survival.
Insulin, given intra-muscularly 16-25 min before DDT, increased the
survival time and the severity of poisoning but did not affect
mortality or convulsions. When given 53-130 min before DDT, insulin
reduced convulsions in animals which died but increased convulsions,
tremors, and other disorders in the survivors.
The failure of amino and sulfhydryl compounds to influence the
action of DDT was noted by Von Oettingen & Sharpless, 1946). Likewise,
the addition of 0.2% choline chloride to the diet of rats receiving
repeated doses of DDT had no effect on the accumulation of lipids in
their liver (Sarett & Jandorf, 1947).
In summary, it would appear that sedatives, ionic calcium, and
glucose or other ready sources of energy are useful in treating
poisoning by DDT. Dogs and cats can be treated somewhat more
successfully than rats, perhaps because the metabolism of larger
animals is slower.
Although respiration may be temporarily restored in animals
poisoned by DDT, studies have not been made to determine whether
respiratory arrest in this condition is truly reversible as it is in
poisoning by certain organic phosphorus insecticides.
7.1.3 Renal system
No dysfunction of the renal system attributable to DDT has been
found even in animals receiving dosages sufficient to cause
dysfunction of the nervous system or striking morphological changes of
the liver. It is true that mild to moderate morphological changes have
been reported in the kidneys of animals that have received massive
single doses or repeated doses; for example, fatty degeneration,
necrosis, and calcification (Lillie et al., 1947; Stohlman & Lillie,
1948) or slight brown pigmentation of the convoluted tubular
epithelium (Fitzhugh & Nelson, 1947). However, it sometimes has
happened that a complete absence of change in the kidney has been
reported in connexion with other studies carried out in the same
laboratories (Lillie & Smith, 1944; Nelson et al., 1944).
7.1.4 Gastrointestinal tract, liver, and enzymes
Large doses of DDT produce vomiting in species that can vomit.
Only doses that produce rather severe poisoning lead to anorexia.
7.1.4.1 Liver
Large doses of DDT cause focal necrosis of liver cells in several
species (Lillie & Smith, 1944; Nelson et al., 1944; Cameron & Burgess,
1945; Lillie et al., 1947; Deichmann et al., 1950; Ortega et al.,
1956). At least some investigators (Cameron & Burgess, 1945) have
considered that the liver lesions produced by large dosages are
sufficient to account for death. All have agreed that necrotic lesions
occur only in connexion with potentially fatal dosages.
A very different kind of liver change is produced in some rodents
but not in other animals by small or moderate dosages of DDT. The
biochemical aspects of these changes are discussed in section 7.1.4.2,
while the morphological aspects are discussed in section 7.1.9.
7.1.4.2 Microsomal enzymes of the liver
All enzymes can be inhibited in vitro and many of them can be
inhibited in vivo. The toxic action of a number of compounds is
clearly the result of their inhibition of one or more enzymes. So far,
only a few enzymes or enzyme systems are known to be induced by
chemicals; the outstanding example is the induction of microsomal,
mixed-function enzymes of the liver and some other organs that are
produced in greater quantity in response to certain hormones and other
normal constituents of the body (Conney, 1967), some foods
(Wattenberg, 1971), or a wide range of drugs or other foreign
chemicals. Such induction requires intact cells and does not occur
when the inducer is brought in contact with the purified enzymes in
vitro. Microsomal enzymes were first recognized and studied in the
liver, but they are now known to exist, generally in lower
concentrations, in other tissues.
Microsomal enzymes are known to be associated with oxidation ( N-,
O-, and S-dealkylation, deamination, epoxidation, disulfuration,
hydroxylation) of both rings and side chains, oxidation of both
nitrogen and sulfur, reduction of nitro groups and of azo compounds,
hydrolysis, and conjugation. Most of the changes produced by
microsomal enzymes render oil-soluble compounds more water-soluble
and, therefore, more easily excreted. Mainly for this reason, most
biotransformations promoted by microsomal enzymes are true
detoxications. However, some of the reactions promoted by this system
of enzymes render specific compounds more toxic.
In the rat, DDT has been shown to promote the biotransformation of
hexobarbital (Hart & Fouts, 1963), phenazone (Hart & Fouts, 1963;
Kinoshita et al., 1966), p-nitrobenzoic acid (Hart & Fouts, 1963),
aniline (Hart & Fouts, 1963), dieldrin (Street, 1964; Street et al.,
1966; Street & Chadwick, 1967; Pearl & Kupfer, 1971), o-ethyl o-4-
nitrophenyl phenylphosphonothioate (EPN) (Kinoshita et al., 1966),
p-nitroanisole (Kinoshita et al., 1966; Vainio, 1974), methyprylon
(Datta & Nelson, 1968), meprobamate (Datta & Nelson, 1968),
chlordizepoxide (Datta & Nelson, 1968), aldrin (Gillett, 1968),
lindane (Chadwick et al., 1971b), phenylbutazone (Welch & Harrison,
1966), pentobarbital (Fredricks et al., 1974), 3,4-benzpyrene (Vainio,
1974), and p-nitrophenol. The biotransformation of p-nitrophenol
involves conjugation by the microsomal enzyme uridinediphospho-
glucuronyltransferase, and its demonstration requires activation of
the microsomes, for example by trypsin (Vainio, 1974, 1975; Rantanen et
al., 1975).
In the mouse, DDT has been shown to promote the biotransformation
of pentobarbital (Gabliks & Maltby-Askari, 1970).
In the guineapig, DDT promoted the metabolism of dieldrin
(Wagstaff & Street, 1970).
DDT in the squirrel monkey promoted the metabolism of EPN and
p-nitroanisole; the first required a DDT dosage of 5.0 (mg/kg)/day, but
the latter required only 0.5 (mg/kg)/day (Cranmer et al., 1972). DDT
did not promote the metabolism of 14C-DDT in squirrel monkeys
(Chadwick et al., 1971a) but did promote the metabolism of
phenylbutazone in the dog (Welch & Harrison, 1966) and the metabolism
of estradiol in the pigeon (Peakall, 1970).
In the chicken, DDT failed to affect N-demethylase or the
concentration of cytochrome P-450, and reduced aniline hydroxylase
activity. The influences of dosage, duration of dosing species, and
reproductive state on microsomal enzymes in birds are poorly
understood (Sell et al., 1971).
o,p'-TDE has been shown to promote the metabolism of
pentobarbital in the mouse (Gabliks & Maltby-Askari, 1970),
phenobarbital in the rat (Straw et al., 1965), and cortisol in the
guineapig (Kupfer et al., 1964).
The metabolism of DDT is promoted by DDT itself in the hamster but
not in the mouse (Gingell & Wallcave, 1974).
In rats, the metabolism of DDT is promoted by phenytoin (Cranmer,
1970). The metabolism of DDT and DDE in cows is promoted by
phenobarbital (Alary et al., 1971; Fries et al., 1971).
DDE, whether fed directly or produced metabolically from DDT,
appears to be more important than DDT in inducing microsomal enzymes.
The tissue level of DDE necessary for enzyme induction is lower in the
rat than in the quail (and presumably other birds). Thus Bunyan et al.
(1972), using residues in the heart as an index, found a maximum
increase in cyto-chrome P-450 per gram of liver and a maximum activity
of aniline hydroxylase levels at DDE levels of approximately 3 mg/kg
in rats and 40 mg/kg in quail. However, at any given dietary level,
higher tissue levels were reached by quail than by rats so that the
dosage responses of the two were similar.
Different inducers may activate different enzymes and, therefore,
different metabolic pathways. Pretreatment of rats with lindane caused
them to metabolize a single dose of radioactive lindane 2.5 times more
efficiently than controls, whereas pretreatment with DDT caused a
3.5-fold increase in the metabolism of lindane. Furthermore, the DDT
pretreatment was followed by a different proportion of radioactive
metabolites with a predominance of tetrachlorophenols, especially
2,3,4,5-tetrachlorophenol (Chadwick et al., 1971b).
The enzymes of weanling rats are more subject to induction than
those of adult rats, but there is no evidence of a lag-period in
induction in adults (Chadwick et al., 1975).
7.1.4.3 Enzymes of intermediary metabolism
As discussed in section 7.1.2, there is some reason to think that
DDT acts by influencing an enzyme critical to the function of
neurones. It is certainly clear that many of the side-effects of DDT
are the result of its induction of microsomal enzymes (see sections
7.1.4.2 and 7.1.9). In addition, DDT has been shown in vitro and
sometimes in vivo to influence some enzymes of intermediary
metabolism and other miscellaneous enzymes. So far, evidence is
lacking that the degree of this inhibition in the intact organism is
sufficient to have any influence on function.
The hyperglycaemia observed during much of the early part of acute
poisoning may be associated with an increase in 4 gluconeogenic
enzymes (pyruvate carboxylase (EC 6.4.1.1), phosphoenolpyruvate
carboxykinase (EC 4.1.1.32), fructose 1,6-diphosphatase, and glucose
6-phosphatase (EC 3.1.3.9)). Increases in these enzymes in the renal
cortex of rats, observed after a single dose at a rate as low as
100 mg/kg, were greater at a dose of 600 mg/kg. The reaction occurred
in both adrenalectomized and normal rats (Kacew & Singhal, 1972).
Similar responses were observed in the same enzymes in the liver
following a single dose at the rate of 100 mg/kg or more or following
45 daily doses at rates of 5 or 25 (mg/kg)/day. The changes are not
mediated through a release of corticosteroids from the adrenal glands
(Kacew & Singhal, 1973). The same authors (Hrdina et al., 1975;
Singhal & Kacew, 1976) have reviewed the extensive evidence
contributed mainly by themselves indicating that the changes in
glucose homeostasis are mediated by stimulation of the cyclic
adenosine monophosphate (AMP)-adenylate cyclase system in the liver
and in the kidney cortex by p,p'-DDT and a number of other organic
chlorine pesticides. However, the smallest single dosage of p,p'-DDT
that produced a statistically significant change in the enzymes or in
the production of cyclic AMP was 180 mg/kg and o,p'-DDT was as
effective as the p,p'-isomer; these findings indicate that the
carbohydrate changes are results not causes of poisoning.
DDT and some other compounds induce increased activity of
D-glucuronolacetone dehydrogenase (EC 1.1.1.70) in the supernatant
fraction of rat liver, and this is consistent with evidence of
increased D-glucaric acid in urine after dog treatment (Marselos &
Hanninen, 1974). Urinary excretion of L-ascorbic acid is also
increased by DDT, but apparently this is not caused by an increase in
an enzyme producing this acid but rather by an increase in several
microsomal and cytosol enzymes that contribute to an increase in free
glucuronic acid from which L-ascorbic acid is formed (Rantanen et al.,
1975).
A review (Hayes, 1959) of early literature indicates that high
concentrations of DDT inhibit phosphatidase (EC 3.1.1.4), muscle
phosphatases, carbonic anhydrase (EC 4.2.1.1), oxalacetic carboxylase
(EC 4.1.1.3), and increase the activity of cytochrome oxidase
(EC 1.9.3.1) and succinc dehydrogenase (EC 1.3.99.1). However, none of
these changes with the possible exception of inhibition of carbonic
anhydrase could be shown to have any connexion with the toxic action
of DDT or even with its side-effects. Neal et al. (1944) reported a
small but consistent increase in the volume of urine excreted in 24 h
when dogs were dosed orally or by insufflation at the rate of 100
(mg/kg)/day. No other change in the urine and no change in kidney
function was demonstrated. The possibility that increased urinary
output is related to the inhibition of carbonic anhydrase (Torda &
Wolff, 1949) may deserve attention. However, re-examination of data
from volunteers receiving 3.5 or 35 mg/man per day did not indicate
any increase in urinary volume compared with controls (Hayes et al.,
1971).
It has been claimed (Keller, 1952) that DDT inhibits carbonic
anhydrase in bovine erthrocytes. However, the method was criticized by
Dvorchik et al. (1971), who found in vitro inhibition only by
concentrations of DDT, unlikely to be survived by living animals. Far
more attention has been given to inhibition of carbonic anhydrase in
the shell gland of birds than in erythrocytes, and it has been
suggested (Bitman et al., 1970; Peakall, 1970) that inhibition of the
shell gland enzyme is an explanation for eggshell thinning in certain
birds. However, the same criticism holds for the shell gland; there is
no evidence that the degree of inhibition reported interferes with
function.
On the other hand, many enzymes including plasma amylase, aldolase
(EC 4.1.2.13), glutamic-pyruvic transaminase (EC 2.6.1.2), and
isocitric dehydrogenase (EC 1.1.1.41) were not changed in squirrel
monkeys given dosages ranging from 0.05 to 50 (mg/kg)/day; the highest
dosage proved fatal within 14 weeks (Cranmer et al., 1972).
7.1.5 Cardiovascular system
Most dogs, killed by a single dose of DDT, die of ventricular
fibrillation, and the same is true of some cats, monkeys, and rabbits
(Philips & Gilman, 1946). At any given dosage of DDT, ventricular
fibrillation is more likely to occur if the animal receives exogenous
epinephrine or is stimulated in such a way that the compound is
released by the adrenals. Besides fibrillation, dogs may exhibit
extrasystoles and changes in the T-wave (Philips et al., 1946).
Monkeys differ from dogs in that the DDT-sensitized heart is able to
recover from fibrillation and resume a normal rhythm (Philips et al.,
1946).
Thus, DDT not only sensitizes the myocardium in a way similar to
that of halogenated hydrocarbon solvents, but, through its action on
the central nervous system, produces the stimulus that increases the
likelihood of fibrillation.
There is no evidence that repeated, tolerated doses of DDT
sensitize the heart. Rats were fed DDT at a dietary level of about
10 (mg/kg)/day for 8 months, during which time they received weekly,
intraperitoneal doses of vasopressin, a compound that causes a
temporary myocardial ischaemia. Electrocardiograms did not show any
significant increase in cardiac arrhythmias in the DDT-fed rats
compared with controls. Intravenous noradrenalin given at the end of
the 8-month period did not produce a greater incidence of arrhythmias
in the DDT-fed rats. The same results were obtained in rabbits treated
in essentially the same way (Jeyaratnam & Forshaw, 1974).
TDE. The main action of TDE, and especially of o,p'-TDE, is on
the liver with secondary effects on the adrenals in dogs and perhaps
in other species. However, Cueto (1970) showed that at a dosage of
50 (mg/kg)/day for 14 days, o,p'-TDE caused a gradually progressive
hypotensive failure in dogs injected with epinephrine or
norepinephrine, while leaving the cardioaccelerator and immediate
pressor response to these drugs unchanged. The hypotensive failure was
associated with weakening of the contractile force of the heart and
with a reduction of plasma volume. The latter may have been caused by
a loss of fluid from the intravascular compartment and was not caused
by a release of histamine. The hypotensive state could be prevented to
a significant degree by pretreatment with prednisolone.
7.1.6 Respiratory system
The effects of DDT on the respiratory system are secondary to
effects on the nervous system and are discussed in section 7.1.2.1.
7.1.7 Reproductive system
It was shown very early (Burlington & Linderman, 1950) that DDT
produces a striking inhibition of testicular growth and secondary
sexual characteristics of cockerels, when injected subcutaneously in
dosages as high as 300 (mg/kg)/day. Changes in the testis involve the
tubules, and not the interstitial tissues, and they have been
attributed to an estrogen-like action of DDT.
It must be noted that the action of DDT on the testis of the
chicken is dosage-related. Before the problem of residues became
evident, DDT was used extensively for control of lice and common mites
on chickens without any adverse effects on egg production or other
aspects of reproduction. Many rats would be killed the first day if
they were given the dosage of DDT that has been shown to affect the
testis in cockerels. The report that, under special conditions, DDT
has it gonadotoxic effect (Rybakova, 1968) is of questionable
significance in view of the results of multigeneration tests in rats,
mice, and dogs.
Ottoboni (1969) found that female rats reproduced normally when
fed DDT for two generations at dietary levels as high as 200 mg/kg
(about 10 (mg/kg)/day, except during lactation when intake was
increased about 3-fold). In fact, at a dietary level of 20 mg/kg, the
dams had a significantly longer reproductive life span (14.55 months)
than did their littermate controls (8.91 months); the number of
females becoming pregnant after the age of 17 months and the number of
successful pregnancies after that age differed significantly between
the two groups (Ottoboni, 1972).
In a study focused mainly on DDT in milk, the ability of rats to
reproduce at a dietary level of 200 mg/kg was confirmed, and the
ability of dams, injected intraperitoneally at levels as high as
100 (mg/kg)/day, to rear their young was demonstrated (Hayes, 1976b).
A six-generation test of reproduction in mice did not show any
effect of DDT at a dietary level of 25 mg/kg on fertility, gestation,
viability, lactation, and survival. A dietary level of 100 mg/kg
produced a slight reduction in lactation and survival in some
generations but not all, and the effect was not progressive. A level
of 250 mg/kg was distinctly injurious to reproduction (Keplinger et
al., 1970). The dietary concentrations used equalled dosages of 3.33,
13.3 and 33.2 (mg/kg)/day in nonpregnant, non-lactating, adult female
mice. The intake is much higher in both young and lactating mice. The
authors concluded that their study provided no obvious reason for
continuing reproduction tests for more than three generations.
Four female dogs of unstated age that had previously received DDT
at the rate of 12 (mg/kg)/day, 5 days a week, for 14 months were mated
when they went into heat. The males involved had been fed aldrin
(0.15 (mg/kg)/day) plus DDT (60(mg/kg)/day) for 14 months prior to
breeding but not during breeding. Two of the females went into heat
but failed to become pregnant, and one failed to come into heat during
12 months after feeding stopped. Four of 6 pups born to the fourth
female died within one week of birth; the other 2 were weaned
successfully even though only 2 posterior mammae of the mother were
functional (Deichmann et al., 1971b). A 3-generation study failed to
confirm any of the injuries suggested by the study of 4 dogs. In the
3-generation study, male and female dogs were fed technical DDT from
weaning at rates of 0, 1, 5, and 10 (mg/kg)/day. Observations were
made on 135 adult females, 63 adult males and 650 pups. There were no
statistically significant differences between controls and DDT-treated
dogs in length of gestation, fertility, success of pregnancy, litter
size, lactation ability of the dams, viability at birth, survival to
weaning, sex distribution, growth of pups, morbidity, mortality,
organ/body weight ratios, or gross histological abnormalities in all
the animals studied. The only clear difference was that DDT-treated
females had their first estrous 2 or 3 months earlier than the control
animals. There was a slight increase in liver/body weight ratio in
some DDT-treated animals but the difference was not statistically
significant, dosage related, or associated with any histological
change (Ottoboni et al., 1977).
o,p'-DDT. Intraperitoneal injection of technical DDT at a dosage
as low as 5 mg/kg or of o,p'-DDT at 1 mg/kg caused a significant
increase in the weight of the uterus of normal, immature female rats
or of ovariectomized adult females. Stimulation caused by p,p'-DDT
was much less. Treatment of rats with DDT, especially o,p'-DDT, 2 h
before injection of estradiol-17-6,7-3H inhibited uptake of the
hormone by the uterus in vivo, possibly by competition for binding
sites. Isomers of TDE and DDE do not influence uterine weight or the
binding of estradiol (Welch et al., 1969). It seems unlikely that
metabolic activation of o,p'-DDT is necessary, as is true of o,p'-
methoxychlor. The binding and estrogenic activity of DDT analogues in
rats is only about 1/10 000 as great as that of diethylstilbesterol
(Nelson, 1973).
A considerably smaller dosage of o,p'-DDT resulting from a
dietary level of 10 mg/kg for 2-9 months did not have any effect on
reproduction in ewes (Wrenn et al., 197 lb). In a similar way, dietary
levels of o,p'-DDT as high as 40 mg/kg, giving a dosage level of
about 2.1 (mg/kg)/day in rats, failed to interfere with reproduction
and lactation in these animals although dosage was continued
throughout 2 pregnancies (Wrenn et al., 1971a).
The report (Heinrichs et al., 1971) that o,p'-DDT significantly
advances puberty, induces persistent vaginal estrus after a period of
normal estrus cycles, and causes other reproductive abnormalities in
female rats would appear at first to be inconsistent with the lack of
effect of technical DDT or of o,p'-DDT on reproduction cited above.
The same is true of other effects of o,p'-DDT demonstrated by the
same investigators (Gellert et al., 1972). The abnormal effects were
obtained initially by injecting 1 mg of the o,p'-DDT subcutaneously
on the second, third, and fourth days of life (counting the day of
birth as zero). Because rat pups on the third day weigh about 12 g or
less each, it follows that the subcutaneous dosage was about
83.3 (mg/kg)/day or more, that is about 40 times greater than the
highest oral dosage of o,p'-isomer fed to breeding rats and about
105 times greater than the levels ingested by the general population
with their food.
Because of its estrogenic properties, DDT was considered as a
possible cause of abortion in dairy cattle, but no evidence of a
relationship was found (Macklin & Bibelin, 1971).
7.1.8 Endocrine organs
Except for the weak estrogenic properties of o,p'-DDT, the
endocrine-related effects of DDT and its analogues are confined to the
adrenals, and even these effects of 2 isomers of TDE are now
considered to be mainly secondary to the induction of microsomal
enzymes of the liver in most species.
TDE. TDE (DDD) is an insecticide in its own right as well as a
metabolite of DDT. The compound is used as a drug to control different
forms of adrenal overproduction of corticoids in man (see section
8.2.8). This therapy was originally based on the demonstration that
DDD (Nelson & Woodard, 1948, 1949) and especially o,p'-TDE (Cueto &
Brown, 1958) caused gross atrophy of the adrenals and degeneration of
the cells of its inner cortex in dogs. This is true even though it was
originally reported (Nelson & Woodard, 1948, 1949) that TDE produced
almost no detectable damage to the adrenals of rats, mice, rabbits,
and monkeys, and the finding was confirmed and extended by other
investigators to other species, including man (Zimmerman et al.,
1956). In the dog, o,p'-TDE produced gross atrophy of the adrenals,
when administered at a dosage of only 4 (mg/kg)/day. The dosage of
technical grade TDE required to produce the same effect was
50-200 (mg/kg)/day (Cueto & Brown, 1958). However, in spite of its
exceptional susceptibility, there is a definite threshold below which
the dog does not respond. About 15% of technical DDT is o,p'-isomer,
much of which is gradually metabolized to o,p'-TDE ( o,p'-DDD). Yet
dogs remained healthy and reproduced normally in a 3-generation study
involving dosages of technical DDT as high as 10 (mg/kg)/day (see
section 7.1.7).
It has recently been shown that, following massive dosage
(60 mg/kg, administered intravenously), all of the isomers of TDE
inhibited ACTH-induced steroid production in the dog, but the
inhibition reached 50% of the control only 27 min after dosing with
the m,p'-isomer compared with 87 min with the o,p'-isomer and
4-18 h with the p,p'-isomer. There was marked temporal correlation
between the percentage inhibition of adenocorticotropic hormone
(ACTH)-induced steroid production, the disruption of normal cellular
structure of the fascicular and reticular zones of the adrenal cortex,
and the severity of the damage to mitochondria in these zones caused
by the 3 isomers (Hart et al., 1973). The effectiveness of m,p'-TDE
for treating metastatic adrenocortical carcinoma had already been
demonstrated (Nichols et al., 1961), but it cannot be said that its
value for this purpose has been compared adequately with that of
o,p'-TDE. Furthermore, no effort has apparently been made to compare
the effect of small daily doses of the 2 isomers in dogs.
Like other organochlorine insecticides, o,p'-TDE stimulates
hepatic microsomal oxygenation of both drugs and steroids and,
according to a very thorough review by Kupfer (1967), this may explain
much of its action on corticoid metabolism in a wide range of species.
Increased breakdown is indicated by increased excretion of polar
metabolites, while nonpolar metabolites remain stable or even decrease
-- a finding recently encountered in human patients (Hellman et al.,
1973). However, the demonstrated effect on corticoid metabolism fails
to explain why o,p'- and m,p'-TDE are unique in their overall
effects on the adrenals, including their ability to produce
adrenocortical atrophy in the dog. Other powerful inducers of
microsomal enzymes lack these effects. It is clear that a reduction of
steroid production accompanies atrophy of the adrenals of the dog. The
review already cited (Kupfer, 1967) considers: (a) reduced steroid
production in species other than the dog, including the possibility
that such reduction is secondary to inhibition of glucose-6-phosphate-
dehydrogenase (EC 1.1.1.49) activity in the adrenals and (b) blockage
of steroid action by asteroid metabolite formed under the influence of
DDD. However, the existence of these effects, much less their
importance, remains obscure.
o,p'-DDT. Oral administration of o,p'-DDT to dogs at a rate of
50 (mg/kg)/day stimulated the microsomal enzymes of the liver as
indicated by increase in liver size, total protein, microsomal
protein, and cytochrome P-450 concentration and by direct measurements
of enzyme activity. These changes in the liver were accompanied,
initially, by an increase in the size of the adrenals and of the cells
of the zona fasciculata; these cells became vacuolated and devoid of
acidophilic cytoplasm, and their nuclei became hyperchromatic and
often peripheral in position. Synthesis of corticosteroids by the
adrenal was not blocked (Copeland & Cranmer, 1974). Thus the effect of
a substantial dosage of o,p'-DDT was quite different from that of
o,p'-TDE (DDE), although part of the metabolism of o,p'-DDT must
be by the same route.
7.1.9 Carcinogenicity
There is no doubt that DDT and a number of other organochlorine
insecticides cause marked changes in the liver in various rodents and
that these changes progress to tumour formation in some species,
notably the mouse. There is serious disagreement as to whether the
mouse tumours are malignant. Regardless of their nature, there is
virtual certainty that they are peculiar to rodents and, therefore,
interpretation of their significance for man or useful animals is
difficult.
Evidence for the carcinogenicity of DDT and its metabolites has
been reviewed by the International Agency for Research on Cancer
(IARC, 1974). Most of the experimental results are summarized in Table
17. The conclusions of the IARC were that: (a) the hepatocarcino-
genicity of DDT administered by the oral route has been demonstrated in
several strains of mice, and shows a dosage-response relationship; (b)
a dietary level of 2 mg/kg (above 0.3 (mg/kg)/day) produces a
significant increase in hepatomas in male but not in female CFl mice
and not in either sex of BALB/c mice; (c) increased incidence of
tumours has been reported in some other organs of mice but not
confirmed in multigeneration studies using a wide range of dosages;
(d) evidence for the carcinogenicity of DDT in rats is not convincing
and is negative in hamsters even at the higher dietary levels that
they tolerate in comparison with rats and mice; (e) negative results
in dogs and monkeys are inconclusive because of the small groups
studied and the short duration of treatment; (f) liver cell tumour
induction in trout is inconclusive because of a lack in control of the
diet; and (g) the carcinogenicity of p,p'-DDE is similar to that of
DDT, but TDE produces a significant incidence of lung tumours.
Actually, the number of dogs and monkeys was not small compared
with similar studies on other chemicals. In an investigation on
monkeys, dosing at lower levels was continued for 7.5 years. In both
dogs and monkeys, dosages sufficient to cause liver damage, death, and
neurological indications of DDT poisoning were included in the
protocols (see Table 17). Apparently no new studies on dogs and
monkeys have been reported. On the other hand, a new study on rats
(Rossi et al., 1977) has given definite evidence of the tumorigenicity
of DDT and phenobarbital, confirming the conclusion of Fitzhugh &
Nelson (1947) which was based on less extensive data.
In mice, liver tumours similar to those caused by DDT (see
Table 17) have been reported in connexion with DDE and DDD (Tomatis &
Turusov, 1975), chlorobenzilate, HCH, aldrin, dieldrin, mirex, and
terpene polychlorinates (Tomatis et al., 1973). Similar tumours have
been caused by the important drug, phenobarbital (Wright et al., 1972;
Peraino et al., 1973; Thorpe & Walker, 1973; Ponomarkov et al., 1976).
TDE also caused lung tumours (Tomatis et al., 1974a).
The IARC review did not discuss the controversy over the nature of
the tumours induced by DDT in the livers of some rodents, and it did
not consider the relationship of these tumours to the induction of
microsomal enzymes. The following paragraphs are concerned with these
matters and specifically with those inducers that behave like DDT. The
biochemical pattern of induction of mixed function oxidase enzymes is
similar for DDT and phenobarbital but distinctly different for
3-methylcholanthrene (Vainio, 1975). Thus 3-methylcholanthrene and
compounds like it must be excluded from the discussion. Similarly, the
high degree of correlation between the ability of compounds to induce
parenchymal liver tumours in mice and their ability to induce tumours
in the liver and other organs of rats and hamsters cannot be accepted
uncritically. As demonstrated in a study by Tomatis et al. (1973),
this correlation is extremely good for compounds that are, or are
suspected of being, carcinogens in man. However, the correlation is
poor for organochlorine insecticides. In fact, the only one of these
compounds that has increased the incidence of a tumour in another
species is DDT, which induced liver tumours in rats in one experiment.
The early morphological response of the rodent liver to DDT is
similar to its response to moderate dosages of HCH, chlordane,
dieldrin, camphechlor (Toxaphene) (Lehman, 1952; Ortega et al., 1956),
and the important drug, phenobarbital (Wright et al., 1972; Thorpe &
Walker, 1973). The earliest change involves so much increase in the
smooth endoplasmic reticulum of individual liver cells that they
enlarge, and the large granules that are ordinarily scattered
throughout the cytoplasm are displaced to the periphery of the
affected cell. Quite early, some of the endoplasmic reticulum forms
whorls that may have fat droplets as their centres -- thus justifying
the term "lipospheres", applied to them by Ortega et al. (1956).
Others have referred to these inclusions as "hyaline oxyphil masses"
(Lillie & Smith, 1944) or "lamellar bodies" (Ito et al., 1973). These
changes are accompanied by some increase in fat droplets, not all of
which become surrounded by endoplasmic reticulum. This combination of
changes (hypertrophy, margination, and lipospheres) is characteristic
of rodents and of compounds that induce microsomal enzymes. Certain
other changes have been reported but not confirmed. These include
enlargement and morphological changes of the mitochondria (Obuchowska
& Pawlowska-Tochman, 1973; Watari, 1973), increased numbers of primary
lysosomes, and atrophy of the Golgi body (Watari, 1973), none of which
were found by Ortega (1966).
Table 17. Effect on various animals of repeated oral doses of DDT
Dosage Species Duration Results Reference
Range Method and Animals Mortality Other
(mg/kg)/day concentration per test (%)
(No.)
41-80 800 mg/kg in diet rata 2 years 36 males, 24 females Increased mortality, Fitzhugh & Nelson, 1947
typical liver changes,
liver tumours
46 mg/kg then mouseb 18 months 36 males, 36 females Hepatomas in 51 and Innes et al., 1969
140 mg/kg in diet 21% of males and females
respectively compared with
18 and 0.6% of controls
3200 mg/kg in diet dog 39-49 10 100 Liver damage, no Lehman, 1965
months tumours
5000 mg/kg in diet monkey 70 days 1 male 100 Fatal poisoning Durham et al., 1963
21-40 400 mg/kg in diet rata 2 years 24 males, 12 females Increased mortality, Fitzhugh & Nelson, 1947
typical liver changes
500 mg/kg in diet rat 2.9 years 37 males, 35 females Liver tumours in 45% Rossi et al., 1977
250 mg/kg in diet mousec 2 103 males, 90 females Risk of liver tumour Tomatis et al., 1972
generations increased 3.7 and 18.5
times in males and females,
respectively
250 mg/kg in diet mousec 2 31 males, 121 females Liver tumours in 48 Terracini et al., 1973
generations and 59% of males and
females, respectively
2000 mg/kg in diet dog 39-49 4 25 Minor liver damage Lehman, 1965
months but no tumours
Table 17 (Cont'd)
Dosage Species Duration Results Reference
Range Method and Animals Mortality Other
(mg/kg)/day concentration per test (%)
(No.)
11-20 100 mg/kg in diet moused 2 years 100 males, 100 females Hepatomas increased Fitzhugh, 1970
in females of one strain
but no increase in
hepatocarcinomas
100 mg/kg in diet mousec 2 years 30 males, 30 females Risk of liver tumours Walker et al., 1973
increased 4.4 times
100 mg/kg in diet mousec 2 years 30 males, 3 females Risk of liver tumours Thorpe & Walker, 1973
increased 3.3 and 4.2 times
in males and females
respectively
5-10 50 mg/kg in diet mousec 2 127 males, 104 females Risk of liver tumours Tomatis et al., 1972
generations increased 2.45 and 3.46
times in males and females,
respectively
50 mg/kg in diet mousec 2 years 30 males, 30 females Risk of liver tumours Walker et al., 1973
increased 2.9 times
400 mg/kg in diet dog 39-49 2 0 No effect Lehman, 1965
months
Table 17 (Cont'd)
Dosage Species Duration Results Reference
Range Method and Animals Mortality Other
(mg/kg)/day concentration per test (%)
(No.)
2.6-5 20 mg/kg in diet mousee 2 48 males, 128 females No increase in tumours Terracini et al., 1973
generations
200 mg/kg in diet monkey 3-7.5 5 males, 5 females No toxic effect Durham et al., 1963
years
1.26-2.5 10 mg/kg in diet mousec 2 104 males, 124 females Risk of liver tumour Tomatis et al., 1972
generations increased 2.26 and 2.46
times in males and females,
respectively
0.626-1.25 25 mg/kg in diet rat 2 years No clinical effect; Treon & Cleveland, 1955
males survived
longer than controls
50 mg/kg in diet monkey 1.6 years 4 males, 1 female No toxic effect Durham et al., 1963
0.3126- 10 mg/kg in diet rat 2 years Typical liver changes; Fitzhugh, 1948
0.625 no effect on
reproduction
12.5 mg/kg in diet rat 2 years No effect Treon & Cleveland, 1955
2.8-3.0 mg/kg in mousee 5 683 Tumours in 28.7% in Tarjan & Kemeny, 1969
diet generations controlsf
0.15626- 2 mg/kg in diet mousee 2 124 males, 111 females Risk of liver tumour Tomatis et al., 1972
0.3125 generations doubled in males,
unchanged in females
2 mg/kg in diet mousee 2 58 males, 135 females No increase in tumours Terracini et al., 1973
generations
Table 17 (Cont'd)
Dosage Species Duration Results Reference
Range Method and Animals Mortality Other
(mg/kg)/day concentration per test (%)
(No.)
0.078126- 2.5 mg/kg in diet rat 2 years No effect Treon & Cleveland, 1955
0.15625 5 mg/kg in diet monkey 1.4-7.5 5 males No toxic effect Durham et al., 1963
years
a Osborne-Mendel. e BALB/c mice.
b Both (C57BL/6 × C3H/Anf) FI and (C57BL/6 × AKR) FI mice. f Lung carcinoma in 16.9% to 1.2% in controls; lymphomas 4.8% compared to
c CFI mice. 1.0% in controls; leukaemias 12.4% and 2.6%; other tumours 5.8% and 1.0%,
d BALB/cJ and C3HeB/Fe J. respectively.
The characteristic changes develop promptly. An increase in smooth
endoplasmic reticulum and the appearance of lamellar structures have
been seen as early as 4 and 7 days, respectively, after the beginning
of dosing (Wright et al., 1972).
Although microsomal enzymes may be induced in other species,
morphological changes in the liver, as viewed by the light microscope
are not the same (Laug et al., 1950; Lehman, 1952; Ortega et al.,
1956), or occur to lesser degree as viewed by the electron microscope
(Wright et al., 1972).
The changes in liver cells that characterize the induction of
microsomal enzymes in rodents are distinct from the focal necrosis
that may be produced with about the same ease in the livers of rodents
or other species by fatal or near fatal dosages of organochlorine
insecticides. The necrotic lesions do not progress because, if such
high dosages are continued the animal dies, and, if dosing is stopped
and the animal survives, the necrotic cells are removed by autolysis
and phagocytic action and the lesions usually heal without scarring
(Cameron & Burgess, 1945). Nevertheless, some scarring was found by
Lillie & Smith (1944) and Lillie et al. (1947).
At least in the early stages, the changes in liver cells that
characterize induction of microsomal enzymes in rodents are reversible
(Fitzhugh & Nelson, 1947; Ortega et al., 1956; Wright et al., 1972).
The reversibility does not depend on cell removal but simply on
reversion of the physiological and morphological condition of the
cells to their original condition.
Of course, reversibility is incompatible with progression, but
whether observed irreversibility will be associated with progression
must be determined directly in each instance. In the following
paragraphs, the question of progression is discussed only after
consideration of the problem of irreversibility in general.
If dosing with organochlorine insecticides or other inducers is
continued long enough and at a sufficiently high level, the liver
changes become irreversible, if for no other reason than that the
remaining life span of the animals is too short to permit excretion of
the inducing chemical or complete reversion of the liver cells to
their original state. The stage at which this shift to irreversibility
occurs remains unknown, but it seems very likely that dosages
sufficient to produce irreversible morphological change also exceed
the physiological adaptability of the liver. The important distinction
between adaptation and injury in relation to enzyme induction has been
studied using dieldrin.
Although some persons have tended to view even moderate
enlargement of the liver or of individual liver cells as an injury,
the evidence is strong that these changes are usually adaptive and
beneficial to the organism, when they are the result of an increase of
smooth endoplasmic reticulum and an associated increase in the
activity of liver microsomal enzymes (Barka & Popper, 1967). However,
it is obvious that any stimulus or effect may be harmful, if
excessive. Hutterer et al. (1968) demonstrated that a distinction may
be drawn between adaptation to dieldrin and decompensation resulting
from excessive doses of it. Some of these authors, including Popper,
showed that the same distinction could be drawn in connexion with
other sources of potential liver injury.
It was found that daily intraperitoneal administration of dieldrin
to rats at the rate of 2 mg/kg produced enlargement of the liver,
hypertrophy of the smooth endoplasmic reticulum, increases in
microsomal protein and P-450 haemoprotein, and associated increases in
the activity of microsomal enzymes; however, normal activity of other
enzymes not derived from the microsomes was maintained. The activity
of microsomal enzymes per mole of available P-450 haemoprotein
remained unchanged. The highest level of activity of the processing
enzymes was reached after 14 days, after which the new steady state
was maintained. Rats that had received dieldrin at a rate of 2 mg/kg
per day for 28 days were more tolerant to dieldrin than normal rats,
as shown by the fact that they survived 25 consecutive daily doses at
the rate of 5 mg/kg, a dosage that produced 70% mortality in
previously untreated rats. In spite of the ability of the rats,
pretreated with a moderate dosage of dieldrin, to survive a large
dosage, their livers showed definite indications of decompensation in
response to the high dosage. Although the smooth endoplasmic reticulum
remained hypertrophic and the microsomal protein and P-450
haemoprotein concentrations remained elevated, the enzyme activities
per mole of available P-450 haemoprotein decreased, as did the
activity of some enzymes not associated with microsomes. Much of the
excess smooth endoplasmic reticulum formed tightly packed clusters of
tubular membranes with no glycogen and little hyaloplasm, and some of
the mitochondrial membranes were injured. It was suggested that the
phase of decompensation represented by hypertrophic but hypoactive
smooth endoplasmic reticulum might serve as a sensitive criterion of
toxic injury before microscopic changes of a clearly harmful sort
become recognizable (Hutterer et al., 1968).
Other studies indicating not only the presence of adaptive change
over a range of dosages but also the failure of adaptation and onset
of injury at sufficiently high dosage levels have been reported for
butylated hydroxy-toluene (Gilbert & Golberg, 1967) and for DDT
(Hoffman et al., 1970). Hoffman and his colleagues found that, when
DDT was fed to male weanling rats for only 14 days at dietary
concentrations of 0.5 to 2048 mg/kg, concentrations of 0.5 and 2 mg/kg
had no effect on the O-demethylation reaction used as a test, but
concentrations of 4-750 mg/kg produced increases in the rate of
metabolism, proportional to the log of dosage. Extrapolation of this
portion of the dosage response curve to the abscissa provided a
calculated no-effect level of 3.27 mg/kg equivalent to about
0.327 (mg/kg)/day. This is in reasonable agreement with other
estimates of the threshold for induction of various enzymes in the
rat, including some studies involving longer administration of DDT.
These estimates, expressed as (mg/kg)/day, are approximately 0.05
(Kinoshita et al., 1966; Street et al., 1969), 0.5 (Schwabe &
Wendling, 1967) and 0.125 (Gillett, 1968). All of the estimates are of
the same order of magnitude as the 0.25 (mg/kg)/day known to be
effective in man (Laws et al., 1967; Poland et al., 1970), but all are
over 100 times greater than the highest dosage received by members of
the general population during the late 1960s (Duggan, 1968).
Increasing the dietary level to more than 750 mg/kg did not produce
any further increase in enzyme activity. Intake of less than 128 mg/kg
did not produce any increase in liver weight, within the period of
observation; increase was proportional to dosage within the range of
128 to 512 mg/kg and was submaximal at intakes above 512 mg/kg.
Some other compounds, notably phenobarbital, produced
morphological changes in the liver similar to those produced by some
organochlorine insecticides (Wright et al., 1972; Thorpe & Walker,
1973). It seems possible that sufficiently high doses of
phenobarbital, for example, may lead to a failure of adaptation and to
levels of enzyme activity that do not correspond to dosage.
As indicated above, the earliest morphological changes caused by
enzyme inducers in the rodent liver involve separate cells in the
centrolobular area. If the dosage is sufficiently high and prolonged,
nodules consisting entirely of hypertrophied cells may appear. At
first, these microscopic nodules are distinguishable only by pattern;
they have no bounding membrane and they do not compress or change in
any other detectable way the smaller liver cells that surround them.
Some nodules may become large enough to be seen without a microscope,
and a few may exceed 1 cm in diameter. In these large nodules, there
is almost complete loss of lobular architecture. Nodules apparently
were first described by Fitzhugh & Nelson (1947) who felt that they
could be regarded as adenomas or as low grade hepatic cell carcinomas.
The use of the second term is not clear because neither mitoses,
tissue invasion, nor metastasis was observed. Although Ortega et al.
(1956) reported small nodules in the livers of treated rats, and
although they examined tissue loaned by Fitzhugh & Nelson's
laboratory, they were entirely unimpressed by the lesions, referring
to them as "focal incongruities".
Almost 3 decades after the first study, there is no more agreement
than is reflected in the preceding paragraph. The views of some
pathologists remain diametrically opposed. This is true even though
the finding of: (a) pulmonary metastases of hepatic cells in mice that
had received DDT (Tomatis et al., 1972; Walker et al., 1973), ß-HCH,
gamma-HCH, dieldrin, or phenobarbital (Thorpe & Walker, 1973); or (b)
progression of liver enlargement beginning 12 weeks after cessation of
ingestion of alpha-HCH by mice for 24 or 36 weeks (Nagasaki et al.,
1974) or progressive increase in the size of liver nodules after DDT
feeding was stopped (Tomatis et al., 1974b; Tomatis & Turusov, 1975);
or even (c) in HCH-exposed mice the time-pattern of increase in liver
weight (as reflected by body weight), which gained momentum only after
a delay of 4 weeks but showed a further acceleration in the thirteenth
week, in spite of decreased food consumption (Tomii et al., 1972),
would appear to establish, without question, that at least some of the
liver changes produced by these compounds in rodents are malignant.
Of course, the reason for disagreement is that the tumours
produced by DDT, other organochlorine insecticides, and phenobarbital
differ in their biochemistry and are not malignant in the classical
sense. Specifically, (a) they do not actively invade tissues; (b)
their "metastases" do not grow; (c) they produce little shortening of
life span; and (d) mice receiving 5.5 (mg/kg)/day as a result of
dietary intake of DDT show a decrease in the success of
transplantation and a significant increase in survival in mice in
which tumours grew following inoculation with an otherwise uniformly
transplantable and uniformly fatal ependymoma (Laws, 1971).
Although the displacement of liver cells to the lung occasionally
seen after prolonged dosage with DDT is usually referred to as
metastasis, it might better be called embolism because the lesion does
not progress and, therefore, lacks the clinical significance of a real
metastasis. Because it does not grow, the lesion is hard to find. A
number of investigators have failed to mention liver cells in the
spleen, lymph nodes, or lungs, and some have stated specifically that
they were not found (Nagasaki, 1973; Rossi et al., 1977).
Perhaps the most illuminating study of the liver changes caused by
DDT is that of Kuwabara & Takayama (1974). They used fluorinyl
acetamide (2,7-FAA) as a positive control in their studies of DDT and
HCH. The 3 compounds were given at dietary concentrations of 250, 250,
and 600 mg/kg, respectively. The lesion caused by 2,7-FAA differed
from those caused by either of the other compounds in 3 ways: (a) it
started as hyper-plastic nodules rather than as isolated cell changes;
(b) the final lesion was hepatocellular carcinoma in contrast with the
adenoma caused by DDT or HCH; and (c) alpha-fetoprotein was formed,
which did not occur with DDT or HCH. Other workers have also failed to
find alpha-fetoprotein in mice treated with an organochlorine
insecticide (Hanada et al., 1973).
It must be emphasized that the organochlorine insecticides and
phenobarbital do not produce, in other animals, the early, visible
changes in the endoplasmic reticulum that are so characteristic of
some rodents and that progress to tumour formation in rodents. That
these compounds do not lead to tumour formation in other animals might
have been predicted by the fact that they do not cause the early
changes, characterized by hypertrophy, margination, and lipospheres.
All available evidence indicates that man does not appear to be
susceptible to the tumorigenic action of the organochlorine
insecticides and phenobarbital. No increase in the occurrence of
tumours has been found in heavily-exposed populations. This includes
groups of workers who manufacture and formulate DDT and dieldrin and
who have been examined carefully for tumours (Laws et al., 1967;
Jager, 1970).
Finally, a study based on a complete tumour registry did not
indicate any increase of tumours attributable to phenobarbital among
men and women who received heavy, essentially lifelong dosing with
this drug for the control of epilepsy (Clemmesen et al., 1974).
In summary, in spite of disagreement about the interpretation of
the liver cell changes, there is general agreement about their
development and appearance. The change that can be detected first and
can be produced by the smallest effective dosage involves the
endoplasmic reticulum. The initial change is reversible, but, even
more important, it is peculiar to rodents. There is no evidence that
anything from the first increase in endoplasmic reticulum to the final
development of a highly nodular liver with occasional displacement of
cells to the lung has any bearing on the health of man or other
animals in which the endoplasmic reticulum does not respond in this
way.
7.1.10 Mutagenicity
DDT has been tested in a number of ways for possible mutational
effects. Shirasu et al. (1976) listed DDT as a negative chemical in
microbial mutagenicity screening studies on 166 pesticides. The test
system consisted of rec-assay using H 17 Rec+ and M 45 Rec-strains
of Bacillus subtilis and reversion assays without metabolic
activation using auzotrophic strains of Escherichia coli (WP 2) and
Salmonella typhimurium (Ames series). Further studies by the same
authors, with metabolic activation, failed to reveal mutagenicity of
DDT (Shirasu et al., 1977). McCann et al. (1975) and McCann & Ames
(1976), reported negative results on DDE in Salmonella typhimurium
testing with metabolic activation.
At a dosage of 105 mg/kg, DDT did not produce any increase in
dominant lethals in mice (Epstein & Shafner, 1968). Concentrations of
10 mg/kg or higher produced chromosome breaks and exchange figures in
a marsupial somatic cell line (Palmer et al., 1972). Saturated
solutions produced chromosome breaks in the root tips of onion and
other plants (Vaarama, 1947). A slight mutagenic effect in mammals has
been reported by Markarian (1966). Deletions plus gaps were reported
to be more common in the chromosomes of mice that had received DDT.
An unconventional test for mutagenicity involved examination of
explants of pulmonary tissue from embryonic mice whose dams had been
fed dietary concentrations of DDT of 10 and 50 mg/kg. An increase in
diffuse hyperplasia and focal proliferation was observed, but a
dosage-response relationship was not clear. Some of the embryos were
allowed to live and the experiment was repeated in subsequent
generations. There was no continuing progression of the reported
changes in succeeding generations (Sabad et al., 1972).
7.1.11 Teratogenicity
When p,p'-DDT was administered to pregnant mice at a rate of
1 mg/kg on days 10, 12, and 17 of gestation, it was not teratogenic
but did alter the gonads and decrease the fertility of the young,
especially the females (McLachlan & Dixon, 1972). A single dose at the
rate of 25 mg/kg or repeated doses of 2.5 (mg/kg) day given during
pregnancy may be embryotoxic but not teratogenic to mice (Schmidt,
1973). The reason why one or a few doses during pregnancy may be
embryotoxic although the same dosage is harmless, when administered
during the entire reproductive period, is of theoretical but not
practical importance.
Teratogenic effects of DDT have not been seen in studies of
reproduction including those for 2 generations in rats, 6 generations
in mice, and 3 generations in dogs (see section 7.1.7).
7.2 Acquisition of Tolerance to DDT
Because DDT stimulates microsomal, mixed-function enzymes and the
action of these enzymes on DDT is one of detoxication, it might be
expected that some tolerance might develop. Such tolerance has been
demonstrated in the case of dieldrin; rats that received this compound
for 28 days at a rate of 2 mg/kg survived 25 additional days at a rate
of 5 mg/kg, a dosage that killed 70% of previously untreated rats
(Hutterer et al., 1968). Apparently the possibility of tolerance to
DDT has not been explored.
7.3 Factors Influencing DDT Toxicity
7.3.1 Dosage effect
7.3.1.1 Dosage-effect of DDT
Table 5 summarizes the acute oral and dermal toxicity of DDT in
common laboratory animals, and Table 18 summarizes the subcutaneous
intravenous and intraperitoneal toxicity. Both tables are condensed
from an earlier review (Hayes, 1959) that gives references and
additional details. It may be concluded that dissolved DDT is absorbed
through all portals. Absorption of DDT powder through the skin is
negligible. It is frequently impossible to put enough DDT dust on the
skin of animals to kill them, so that an LD50 value for this
formulation cannot be determined by the dermal route. Although
formulation is important in determining the toxicity of DDT by other
routes, the difference is not so great as it is in connexion with skin
exposure. DDT is about 4 times more toxic when given intravenously
than when given orally, and about 40 times more toxic when given
intravenously than when given dermally.
Table 18. Acute subcutaneous, intravenous, and intraperitoneal LD50 of DDT
in common laboratory animalsa
Species Formulation Subcutaneous Intravenous Intraperitoneal
(mg/kg) (mg/kg) (mg/kg)
Rat Water suspension or powder >2000
Oil solution 200-1500 47 80-200
Mouse Water suspension or powder 1000-1500
Oil solution 300
Guineapig Water suspension or powder
Oil solution 900 150
Rabbit Water suspension or powder
Oil solution 250->3200 30-41 <2100
Cat Water suspension or powder
Oil solution <650 32
Dog Water suspension or powder
Oil solution 68
Monkey Water suspension or powder
Oil solution 55
a From: Hayes (1959).
In general, DDT appears to be more toxic as a solution in
vegetable oil or animal fat than when given in some petroleum
fraction. Petroleum may act as a laxative. The heavier fractions are
never absorbed and DDT dissolved in such fractions has to be extracted
from the solvent in order to show toxicity.
In summary, DDT is a compound of moderately acute toxicity.
Compared with other organochlorine insecticides of equal or greater
toxicity, it is remarkable in being only slightly absorbed by the
skin.
The effects of repeated doses of DDT are summarized in Table 17.
The 90-dose oral LD50 of technical DDT in rats is
46.0 (mg/kg)/day (Gaines, 1969). The chronicity index is 5.4. Thus the
compound has only a moderate tendency to cause cumulative effects, and
this limited tendency is fully explained by the accumulation of DDT
itself in tissues as a result of continuing intake. In fact, this
accumulations, which is strictly dosage dependent, is detectable at
all measurable levels of intake. The relationship in man is shown in
Fig. 4 (p. 138).
If storage is considered undesirable per se, then DDT is without
a no-injurious-effect level. However, the same may be said for all
compounds that are absorbed, for the presence of all of them in the
bodies of exposed organisms -- perhaps at very low levels -- may be
assumed; failure to demonstrate low levels of storage does not depend
on physiology but only on the limitations of analytical chemistry and
on the lack of persistence of chemists.
A number of papers have reported no-effect levels for DDT within
the variables investigated, namely: rat, 0.05 mg/kg (Lehman, 1965);
dog, 8 mg/kg (Lehman, 1965); and monkey, 2.2-5.54 mg/kg (Durham et
al., 1963).
There remain reports of effects in animals at the lowest dosages
investigated. For example, decreased serum albumin and increased ß-
and gamma-globulins in the blood of rats and rabbits maintained on a
dosage of 0.2 (mg/kg)/day for 3-11 months was reported by Kagan et al.
(1969).
In studies carried out in rats and dogs, toxicity, as measured by
the maximum no-effect level, was seldom very different from the
corresponding value resulting from 90 days of exposure at the same
dosage range. The largest factor of difference observed when 33
chemicals were investigated in rats was 12 (20 for minimal effect
level), and for half of them the factor was 2 or less. In 21,
rat-to-dog comparisons of long-term toxicity, in no instance was the
dog more sensitive than the rat (Weil & McCollister, 1963). Although
LD50 studies offer a poor basis for predicting long-term toxicity,
the lowest dosage that will produce a minimum effect, when administered
for the lifetime of rats, can be predicted with reasonable success
from a test lasting only 7 days and with good success from a 90-day
study (Weil et al., 1969). These results offer some perspective for
judging the ultimate effect of exposure to compounds that have been
commercially available for less than one human lifetime.
Summary of long-term toxicity studies. The lowest dosages that
have been studied in animals are of the same order of magnitude as
those encountered by men who make or formulate DDT and, therefore,
hundreds of times greater than the dosages encountered by the general
population. The animal studies have been continued long after a steady
state of storage has been achieved. From the results it can be
concluded that bioaccumulation sufficient to produce neurotoxicity or
other clinical effects, including a reduction of the life span, can
occur only at dosage levels substantially higher than those
encountered by the most heavily exposed workers. DDT dosages
encountered by workers produced a small but detectable increase in
liver changes (hypertrophy, margination, and liposphere formation) in
some groups of mice and rats. The same changes occurred in low
incidence in control mice and rats but not in other animals (see
section 7.1.9).
7.3.1.2 Dosage-effect of metabolites and o,p'-DDT
Acute oral LD50 values of DDT metabolites commonly found in
tissues of excreta are shown in Table 19. Readily absorbable
formulations of the metabolites are less toxic than the most
absorbable preparations of the parent compounds (see for example
Table 5).
Table 19. Oral LD50 values of metabolites of DDT
Compound Species LD50 Reference
(mg/kg)
DDE rat, male 880 Gaines, 1960
DDE rat, female 1240 Gaines, 1960
ODE mouse 700 von Oettingen & Sharpless, 1946
DDE mouse 1000 Domenjoz, 1946a,b
TDE(DDD) rat, male >4000 Gaines, 1969
DDA rat 1900 Smith et al., 1946
DDA rat, male 740 Gaines, 1960
DDA rat, female 600 Gaines, 1960
DDA mouse 720 von Oettingen & Sharpless, 1946
DDA mouse 590 Domenjoz, 1946a,b
DDA. Rats tolerate higher tissue levels of DDA than of DDT.
Eighteen hours after intravenous injection of DDA at a rate of
100 mg/kg, tissue levels were still higher than those usually found in
animals, fatally poisoned by DDT (Judah, 1949).
DDA produces less injury to the liver than DDT but produces
greater damage to the kidney especially at high intravenous dosages
(Lillie et al., 1947). This is consistent with the finding of Spicer
et al. (1947) that, following administration of DDT, DDA constitutes a
higher proportion of DDT-related compounds in the kidney (25%) than in
any other tissue, e.g., 12% in the liver, 10% in the brain, and even
less in other tissues.
o,p'-DDT. At an oral dosage of 150 mg/kg, p,p'-DDT produces
severe illness in all rats and kills about half of them, but o,p'-DDT
at the same dosage does not produce illness even though the
concentrations of the 2 compounds in the brain at various intervals
after dosing are about the same. At a dosage of 3000 mg/kg, o,p'-DDT
produces mild to moderate illness, and the concentration in the brain
is 5-9 times the concentration of p,p'-DDT necessary to produce
similar symptoms. Thus, p,p'-DDT appears to be inherently more toxic
than the o,p'-isomer (Dale et al., 1966a).
7.3.2 Age and sex
Young animals eat more than adults in relationship to their body
weight. For this and other reasons, they are often more susceptible
than adults to poison in food. However, young animals are inherently
less susceptible to certain compounds. There is no evidence that DDT
is more toxic to the young than to the adults of any species,
including man. In the rat, the young are less susceptible than adults
to a single dose and about equally susceptible to repeated doses as
shown in Table 20. According to Henderson & Woolley (1969), the
relative insusceptibility of the young is associated with relatively
poor absorption of DDT by the central nervous system and by the less
inherent susceptibility of the young brain to DDT already absorbed by
it. Further studies by the same authors (Henderson & Woolley, 1970)
showed that fatal poisoning of both 10- and 60-day-old rats involved
hyperexcitability and intense tremor followed by prostration and
eventual respiratory failure. However, in the adult rat, DDT caused
convulsions, an increase in respiration and heart rate, and a lethal
increase in body temperature (40-42°C) prior to death, but the body
temperature of the immature rat decreased during acute intoxication by
DDT. The authors suggested that, whereas DDT is a direct depressant of
respiration in both young and old rats, the additional toxic responses
manifested by seizures and hyperthermia accounted for the increased
lethality of DDT in mature animals.
There is virtually no sex difference in the acute toxicity of DDT
to rats; the LD50 was 113 and 118 in males and females, respectively
(Gaines, 1960). When DDT is fed to rats at ordinary dietary levels,
the 2 sexes store it equally. However, at higher dosages, females
store more of the compound; the difference is explained mainly by the
lower activity of the liver microsomal enzymes in female rats and,
only in part, by the relatively higher food intake of the females.
Table 20. Effect of age on the toxicity of DDT to rats
Number Age LD50 Reference
of doses (mg/kg)a
1 newborn 4000 Lu et al.. 1965
1 newborn 2356 Harrison, 1975
1 10 days 728 Henderson & Woolley, 1969
1 14-16 days 437.8 Lu et al., 1965
1 weanling 355.2 Lu et al,, 1965
1 2 months 250 Henderson & Woolley, 1969
1 3-4 months 194.5 Lu et al., 1965
1 middle-aged 235.8 Lu et al,, 1965
1 adult 225 Harrison, 1975
4 preweaning 279.2 Lu et al., 1965
4 adult 285.6 Lu et al., 1965
a Total intake one or more doses.
7.3.3 Nutrition
Fat. Nutrition influences the toxicity of DDT in connexion with
both fat and protein. Fatness influences the amount that can be stored
inactively in the body, and it is of importance in mitigating acute
poisoning. This action of fatness or "good condition" has been noted
in connexion with mammals (Spicer et al., 1947) and fish (Hoffmann &
Surber, 1948). In contrast, laboratory animals are slightly more
susceptible to repeated large doses administered as part of a diet
containing a moderate proportion of fat (5% or more) than as part of a
very low fat diet (0.5%) (Sauberlich & Baumann, 1947). This difference
is thought to be associated with absorption from the gastrointestinal
tract.
Rats that have stored large amounts of DDT in the fat may suffer
tremors, if starvation or some other cause leads to a mobilization of
their fat (Fitzhugh & Nelson, 1947). If DDT intake is stopped when
starvation begins, and, if the concentration of DDT is measured only
once following the interval of starvation, the results may be erratic,
reflecting, to a greater or lesser degree, both mobilization of fat
and excretion of metabolites as in studies reported by Deichmann et
al. (1972).
However, in nature, starvation is more often partial than
complete, and, if the original diet contains enough DDT to cause
substantial storage, whatever food may be found in a period of
scarcity is also likely to be contaminated. The initial effect of the
mobilization of fat is to increase the concentration of DDT in the
remaining fat and in other tissues. Excretion is increased in response
to the increased tissue levels but may not be fast enough to prevent
the accumulation of a toxic concentration in the brain. If intake of
DDT is stopped, the increased rate of excretion eventually leads to
reduced storage (Dale et al., 1962). These findings in rats have been
confirmed, in regard to both the initial increase in the concentration
of DDT (Dedek & Schmidt, 1972; Stenberg & Diky, 1973) and the later
reduction (Brodeur & Lambert, 1973). Similar findings have been
reported in birds (Adamczyk, 1971).
The effect of fat mobilization on the toxicity of DDT is the same
whether it is caused by withholding food or by disease that causes
partial refusal of food (Hayes, 1975).
It is highly unlikely that poisoning by DDT will be precipitated
in man by starvation because: (a) very few subjects store the compound
in concentrations as high as those required to demonstrate the
phenomenon in rats; and (b) the metabolic rate in man is so much
slower than that in rats that elimination of DDT in man would
counterbalance that produced by its mobilization.
Lipids. The association of lipids with the function of
microsomal enzymes is generally recognized as is the fact that DDT
induces these enzymes. Therefore, it might have been expected that DDT
and essential fatty acids would interact. Tinsley & Lowry (1972) found
that the growth of female rats receiving p,p'-DDT at a dietary level
of 150 mg/kg was depressed, if they received a diet deficient in
essential fatty acids, but was slightly stimulated, if they received
the same diet supplemented with these acids. Another variable
influenced by the same factors was the ratio of various liver lipids.
The changes in fatty acid composition were related to the
proliferation of hepatic smooth and endoplasmic reticulum; it was
suggested that DDT influenced essential fatty acid metabolism by
increasing the demand for them.
In contrast, a variety of diets (containing fats that may occur in
the human diet and that were in approximately the same proportion as
fats in the typical human food in the USA) had little or no influence
on the storage of DDT and a wide range of pesticides fed to rats for 4
generations in a combination of rates 200 times those found in the
Market Basket Study of food in the USA (Adams et al., 1974).
Ascorbic acid. In squirrel monkeys (and presumably in other
species) only 2 days on an ascorbic acid-deficient diet impaired both
the induction of O-demethylase and the stimulation of the glucuronic
acid system by DDT (5 mg/monkey/day) (Chadwick et al., 1971a). In
guineapigs, maintenance of induction of microsomal enzymes required a
higher dietary level of ascorbic acid than prevention of scurvy
(Wagstaff, 1971).
Protein. Smith & Stohlman (1945) found only slightly greater
mortality and liver pathology in rats fed DDT at 500 mg/kg in a diet
containing protein at 80 g/kg than in one containing 280 g/kg. The
finding that low dietary protein predisposes to DDT poisoning has been
confirmed (Sauberlich & Baumann, 1947; Boyd & DeCastro, 1968, 1970;
Boyd & Krijnen, 1969); however, even zero protein intake increased
toxicity only 4-fold, the smallest factor observed in comparable
studies of 9 pesticides. The effect of protein deficiency on toxicity
may involve a crippling of the microsomal enzymes of the liver or it
may act synergistically with compounds that cause anorexia. Rats fed a
diet containing casein at 810 g/kg exhibited evidence of renal
overload and were more susceptible to DDT (Boyd & DeCastro, 1970).
7.3.4 Species
The acute toxicity and metabolism of DDT were studied in mice and
hamsters because of the marked difference in their susceptibility to
liver tumours induced by DDT. The central nervous systems of the 2
species are equally sensitive, the concentration of DDT in their
brains at death being similar. However, after an oral dosage of
500 mg/kg, the DDT concentration in the mouse brain was twice that in
the hamster. This cannot be explained by a difference in absorption,
metabolism, or excretion but apparently is due to a difference, in the
permeability of the blood/brain barrier in the 2 species. When animals
receive DDT at a dietary level of 250 mg/kg for 6 weeks, the residues
in fat and liver were 7/8 times higher in the mouse, a fact only
partially explained by the greater food intake of the mouse relative
to body weight. Although urinary excretion of 14C-DDT was similar in
previously unexposed hamsters and mice, this excretion was stimulated
in the hamster but little affected in the mouse by previous dietary
exposure to DDT (Gingell & Wallcave, 1974).
Mice also differ from rats in the hormonal regulation of the basic
activity of hepatic microsomal mixed-function enzymes as well as in
the response of these enzymes to inducers (Chhabra & Fouts, 1974).
7.3.5 Other factors
A number of other factors are known to influence the toxicity of
some compounds, and the degree of difference may be very great in
isolated instances. Factors that have been reviewed elsewhere (Hayes,
1975) include (in addition to those listed above) interaction of
compounds, strain, individual differences, isolation and crowding,
other social and psychological factors, temperature, pressure and
altitude, light and other radiation, circadian and other rhythms,
seasonal differences, and relative humidity. None of these additional
factors is known to have an important effect on the survival of
animals receiving DDT. The possibility of the interaction of DDT with
aldrin, pyrethrin, piperonyl butoxide, malathion, dichloropheno-
xyacetic acid (2,4-D), and a number of food additives has been
explored systematically without finding anything but simple additive
results (Fitzhugh, 1966). However, pyrethrins, especially synergized
pyrethrins, have an additive and perhaps synergistic effect on the
changes in liver morphology associated with repeated doses of DDT in
rats (Kimbrough et al., 1968).
7.4 Human Studies
Oral exposure. Table 21 summarizes the effects of one or a few
carefully measured oral doses of DDT. The results are consistent with
those in accidents reported by Garrett (1947) and Hsieh (1954) in
which it was possible to estimate accurately the amount ingested. It
may be concluded that a single dose at the rate of 10 mg/kg produced
illness in some but not all subjects even though no vomiting occurred.
In general, smaller doses did not produce illness, although a dosage
of 6 mg/kg produced perspiration, headache, and nausea in a man who
was sickly and who was hungry at the time of eating. Persons who were
made sick by 10 mg/kg did not have convulsions, but convulsions
occurred frequently when the dosage level was 16 mg/kg or greater
(Hsieh, 1954). Rarely, a dosage as high as 20 mg/kg might be taken
without apparent effect (MacCormack, 1945). Dosages at least as high
as 285 mg/kg have been taken without fatal result (Garrett, 1947).
However, large doses lead to prompt vomiting, so that the amount
actually retained cannot be determined accurately.
It has been noted, in the course of tests with volunteers, that
dilute colloidal aqueous suspensions of DDT are odourless and
tasteless (Domenjoz, 1946a; Hoffman & Lendle, 1948). Saturated
alcoholic solutions of DDT have a weak aromatic taste or rather odour.
Some people find these solutions slightly anaesthetic to the tongue
(Hoffman & Lendle, 1948). The taste of DDT in vegetable oil is so
slight that many persons could not identify capsules containing 0,
3.5, and 35 mg of DDT when they were presented separately but could
arrange them in proper order when one of each was available for
comparison (Hayes, personal communication, 1977).
The possible clinical effects of many repeated doses of DDT were
first explored by Fennah (1945). Because of his interest in predicting
the results of indiscriminate use, he expressed the exposures in terms
of environmental levels rather than in dosage units. The exposures
were clearly higher than those ordinarily encountered. In one test,
lasting a total of 11.5 months, Fennah daily inhaled 100 mg of pure
DDT and drank water dusted at the rate of 3240 mg/m2. Much of the
inhaled dust must have been deposited in the upper respiratory tract
and swallowed. Later, for one month, Fennah ate food all of which had
been sprayed at the rate of 2160 mg/m2 after it had been served. No
ill-effect of any kind was observed.
Table 21. Summary of the effects of one or a few oral doses of DDT on volunteers
Dose (mg) and Result Reference
formulation
250 × 9, No effect. Domenjoz, 1946a
suspension
1500, butter No effect, but lice killed when fed 6 MacCormack, 1945
solution and 12 h after dose.
500, oil No effect. Neal et al., 1946
solution
700, oil No effect. Neal et al., 1946
solution
250, suspension None except slight disturbance of Velbinger, 1947a,b
sensitivity of mouth.
250, oil Variable hyperesthesia of mouth, Velbinger, 1947a,b
solution
500, oil Variable hyperesthesia of mouth. Velbinger, 1947a,b
solution
750, oil Disturbance of sensitivity of lower part of Velbinger, 1947a,b
solution face; uncertain gait; peak reaction (6 h after
ingestion) characterized by malaise, cold moist
skin, and hypersensitivity to contact;
reflexes normal.
1000, oil Same as above; no joint pains, fatigue, fear, Velbinger, 1947a,b
solution or difficulty in seeing or hearing.
1500, oil Prickling of tongue and around mouth and nose Velbinger, 1947a,b
solution beginning 2.5 h after dose; disturbance of
equilibrium; dizziness; confusion; tremor
of extremities; peak reaction (10 h after
ingestion) characterized by severe malaise,
headache, and fatigue; delayed vomiting;
almost complete recovery in 24 h.
Some later studies on volunteers have been designed to explore the
details of storage and excretion of DDT in man and to search for
possible effects of dosages considered to be safe. In the first of
these studies, men were given 0, 3.5, and 35 (mg/man)/day. These
administered dosages, plus DDT measured in the men's food, resulted in
dosage levels of 0.0021-0.0034, 0.038-0.063, and 0.36-0.61
(mg/kg)/day, respectively, the exact value depending on the weight of
each individual. Six volunteers received the highest dosage of
technical DDT for 12 months, and 3 received it for 18 months. A
smaller number of men ingested the lower dosage of technical DDT or
one of the dosages of p,p'-DDT for 12 or 18 months. No volunteer
complained of any symptoms or showed, by the tests used, any sign of
illness that did not have an easily recognizable cause clearly
unrelated to the exposure to DDT. At intervals, the men were given a
systems review, physical examination, and a variety of laboratory
tests. Particular attention was given to the neurological examination
and liver function tests, because the major effects of DDT in animals
involve the nervous system and the liver (Hayes et al., 1956). The
same result was obtained in a second study in which the same dosages
were given for 21 months and the volunteers were observed for a
minimum of 27 additional months (Hayes et al., 1971). Information on
storage and excretion gathered in these studies has already been
discussed in sections 6.2.1.1 and 6.3.1.1.
Recently, DDT has been used on an experimental basis at dosage
rates varying from 0.3 to 3 (mg/kg)/day for periods up to 7 months in
an attempt to decrease serum bilirubin levels in selected patients
with jaundice. No side-effects were observed. No improvement was noted
in patients with jaundice based on cirrhosis who did not have any
demonstrated liver enzymes deficiency. However, in a patient with
familial, nonhaemolytic, unconjugated jaundice based on a deficienty
of glucuronyl-transferase, treatment with DDT rapidly reduced the
plasma bilirubin level to the normal range and relieved the patient of
nausea and malaise from which he had suffered intermittently. The
liver function tests as well as other laboratory findings remained
normal. The improvement was maintained during the 6 months that DDT
was administered, and had persisted for 7 additional months at the
time the report was written. In this case, a dosage of 1.5 (mg/kg)/day
produced a steady rise in plasma levels of p,p'-DDT from an initial
level of 0.005 mg/litre to a maximum of 1.33 mg/litre at the end of
treatment. At this time, the concentration in body fat was 203 mg/kg.
Plasma levels fell slowly after dosing was stopped (Thompson et al.,
1969). The highest dally intake in this series was 6 times greater
than the highest level administered in earlier studies of volunteers
and about 7500 times greater than the DDT intake of the general
population. The highest value for p,p'-DDT in serum observed in the
entire series was 1.330 mg/litre compared with 0.996 mg/litre, the
highest value reported by Laws et al. (1967) for formulating plant
workers.
Dermal exposure. Depending on dosage, oral administration of DDT
to volunteers either did not produce any illness or produced only
brief poisoning similar to that seen in experimental animals. The oral
dosage necessary to produce any clinical effect was almost always
10 mg/kg or more. However, in 2 studies involving only 3 subjects in
all, experimental dermal exposure to DDT was followed by fatigue,
aching of the limbs, anxiety, or irritability, and other subjective
complaints. Recovery was delayed for a month or more (Case, 1945;
Wigglesworth, 1945). In neither study was there an independent
control. Although the dosage was unmeasured, the amounts of DDT
absorbed must have been much smaller than those involved in the oral
tests. One of the studies involved self-experimentation by one man. A
similar but somewhat more severe test on 6 volunteers did not produce
any toxic or irritant effects at all (Dangerfield, 1946). In view of
all other experiments and extensive practical experience, it must be
concluded that the illnesses reported by Wigglesworth and by Case were
unrelated to DDT.
With the exceptions just mentioned, dermal exposure to DDT has not
been associated with any illness or, usually, with any irritation
(Wasicky & Unti, 1944; Draize et al., 1944; Cameron & Burgess, 1945;
Fennah, 1945; Dangerfield, 1946; Chin & T'Ant, 1946; Domenjoz, 1946a;
Haag et al., 1948). In fact, Hoffman & Lendle (1948) reported that
even subcutaneous injection of colloidal suspensions of DDT in saline
in concentrations up to 30 mg/litre did not cause irritation.
Zein-el-Dine (1946) reported that DDT-impregnated clothing caused a
slight, transient dermatitis, but the method of impregnation was not
stated and the absence of solvent was not guaranteed. In other more
thorough studies DDT-impregnated clothing was found to be non-
irritating (Cameron & Burgess, 1945; Domenjoz, 1946a).
Small pads impregnated with different formulations of DDT were
applied to the inner surface of the forearm of 32 volunteers whose
cutaneous sensation had previously been measured for a period of 5
weeks. Pads impregnated with all the elements of the formulation
except DDT were applied to the corresponding position of the other arm
as a control. Powdered DDT and a solution of DDT at 50 g/litre showed
little effect. Solutions in olive oil and petrolaturn at 100 g/litre
and 200 g/litre did not show any remarkable effect on sensation of
pain, cold, or heat but reduced tactile sensation in most cases so
that the minimum pressure that could arouse the tactile sensation was
1-2.5 g/cm2 higher than for the control (Chim & T'Ant, 1946).
Respiratory exposure. Neal et al. (1944) reported almost
continuous daily exposures to aerosols sufficient to leave a white
deposit of DDT on the nasal vibrissae of the volunteers. This exposure
produced moderate irritation of the nose, throat, and eyes. Except for
this irritation during exposure, there were no symptoms, and
laboratory tests and physical examination, including neurological
evaluation, failed to reveal any significant changes. The studies by
Fennah (1945), which involved both respiratory and oral exposure, did
not produce any detectable ill-effects.
8. EFFECTS OF DDT ON MAN-EPIDEMIOLOGICAL AND CLINICAL STUDIES
8.1 Retrospective Studies on DDT-Exposed Populations
8.1.1 Epidemiological surveillance of persons occupationally exposed
to DDT
The safety record of DDT is phenomenally good. It has been used
for mass delousing in such a way that the bodies and inner clothing of
thousands of people of all ages and states of health have been
liberally dusted with the compound. By necessity, the persons applying
the DDT work in a cloud of the material. Other subjects have sprayed
the interior of hundreds of millions of homes in tropical and
subtropical countries under conditions involving (Wolfe et al., 1959)
extensive dermal and respiratory exposure. A smaller number of men
have made or formulated DDT for many years. Extensive experience and
numerous medical studies of groups of workers have been reviewed
(Hayes, 1959). Dermatitis was commonly observed among men who used DDT
solutions. The rashes were clearly due to the solvent, especially
kerosene. As often happens with rashes caused by petroleum
distillates, they were most severe in men when they first started work
and cleared in a few days unless contamination was exceptionally
severe. A smaller number of workers experienced mild narcotic effects
(vertigo and nausea) from solvents when working in confined spaces.
Gil & Miron (1949) reported that some persons suffered temporary
irritability, fatigue, and other ill-defined symptoms after exposure
to the dusty atmosphere of a delousing station, but the relation of
these atypical findings to DDT was not clear. With these exceptions
due largely to solvents, no illnesses clearly attributable to the
formulations, much less to DDT, were revealed by the early studies.
Ortelee (1958) carried out clinical and laboratory examinations of
40 workers, all of whom were exposed to DDT and some of whom were
exposed to a number of other pesticides. The men had been employed at
this work with heavy exposure for 0.4 to 6.5 years and with slightly
less exposure for as much as eight years. Exposure was so intense
that, during working hours, many of the men were coated with a heavy
layer of concentrated DDT dust. By comparing their excretion of DDA
with that of volunteers given known doses of DDT, it was possible to
estimate that the average dosages of 3 groups of the workers with
different degrees of occupational exposure were 14, 30, and 42 mg/man
per day, respectively. With the exception of the excretion of DDA and
the occurrence of a few cases of minor irritation of the skin and
eyes, no correlation was found between any abnormality and exposure to
the insecticide. Since very large doses of DDT injure the nervous
system and liver of experimental animals, special attention was given
to a complete neurological examination and to laboratory tests for
liver function. Although a few abnormalities were revealed, none was
detected in relation to DDT.
Thirty-five men employed from 11 to 19 years in a plant that had
produced DDT continuously and exclusively since 1947 and, at the time
of the study, was producing 2722 metric tonnes per month were studied
by Laws et al. (1967). Findings from medical histories, physical
examinations, routine clinical laboratory tests, and chest X-ray films
did not reveal any ill-effects attributable to exposure to DDT. The
overall range of storage of the sum of isomers and metabolites of DDT
in the men's fat was 38-647 mg/kg compared with an average of 8 mg/kg
for the general population. Based on their storage of DDT in fat and
excretion of DDA in urine, it was estimated that the average daily
intake of DDT by the 20 men with high occupational exposure was
17.5-18 mg/man per day compared with an average of 0.028 mg/man per
day for members of the general population. There was significant
correlation ( r = +0.64) between the concentration of total DDT-related
material in the fat and the serum of the workers. The average
concentration in fat was 338 times higher than that in serum -- a
factor about 3 times greater than that for people without occupational
exposure. Compared to members of the general population, the workers
were found to store a smaller proportion of DDT-related material in
the form of DDE; the difference was shown to be related chiefly to
intensity rather than to duration of exposure. DDE is relatively a
much less important and DDA a much more important excretory product in
occupationally-exposed men compared with men in the general
population. A further study of the same men involved in DDT production
is discussed in section 8.2.5.
By far the largest number of heavily-exposed workers whose health
has been investigated are those associated with malaria control in
Brazil and India (WHO, 1973). In Brazil, periodic clinical
examinations were made of 202 spraymen exposed to DDT for 6 or more
years, 77 spraymen exposed for 13 years ending in 1959, and 406
controls. In the first examination carried out in 1971, minor
differences between exposed and unexposed groups were observed in some
neurological tests, but this result was not confirmed by the second
examination in the same year nor in subsequent examinations. During a
3-year period, a survey of illnesses requiring medical care during the
6 months preceding each periodic medical examination failed to
demonstrate any differences between exposed and control groups. A
relatively small number of analyses indicated that the concentration
of DDT in the blood of spraymen was about three times higher than that
of controls.
In India, the blood levels of 144 spraymen were 7.5-15 times
higher than those of the controls and were at least as high as those
reported for workers who make and formulate DDT elsewhere (see
Table 7). When the spraymen were examined, the only differences from
the controls were that knee reflexes were brisker, slight tremor was
more often present, and a timed Romberg test was more poorly performed
by the spraymen. The positive results led to the selection of 20 men
for re-examination by a neurologist who concluded that the differences
found initially were not real or that the tests had returned to normal
within the few months between the 2 examinations. The signs were not
dosage-related, since they were not correlated with serum levels of
DDT.
It has been known for several years that substantial doses of DDT
and several other organochlorine insecticides stimulate the microsomal
enzymes of the liver. This property of DDT was put to practical use in
treating a patient with familial, nonhaemolytic, unconjugated
jaundice, as described earlier. It was, therefore, entirely expected
that persons with sufficient occupational exposure to a variety of
pesticides would be able to metabolize a test drug (phenazone) more
rapidly on the average than persons without occupational exposure were
able to do. However, the change was not one of significantly
increasing the fastest normal rate but of bringing all the workers up
to a high level. There was no indication that the change had any
effect on the workers' health (Laws et al., 1967, 1973; Kolmodin et
al., 1969; Poland et al., 1970).
In addition to the studies already mentioned regarding workers
with extensive storage, and excretion of DDT as a result of heavy
exposure to DDT, studies have also been made of a larger number of
workers with lesser storage and excretion following lesser exposure to
DDT but greater exposure to other insecticides. Further studies (Long
et al., 1969; Morgan & Roan, 1969, 1974; Warnick & Carter, 1972;
Sandifer et al., 1972; Embry et al., 1972; Tsutsui et al., 1974; Ouw &
Shandar, 1974) have failed to reveal effects of clinical significance
among workers with prolonged, moderate exposure not only to
organochlorine but also to organophosphorus and other types of
insecticides. Small but statistically significant differences have
appeared in the medical history or clinical laboratory results of some
of these workers compared with the controls, but in no instance have
the differences been of any medical importance, and dosage-response
relationships have been unclear or absent. In several instances, the
statistically significant differences have been opposite in different
groups of workers; for example, creatinine phosphokinase activity was
lower than that of controls in subjects applying the insecticide but
higher in operators. Seasonal variations present one year were lacking
the next. The possibility of adaptive change (other than enzyme
induction) has been suggested (Tocci et al., 1969), but this, like the
reality of the changes, remains unproved.
In some instances statistically significant differences have been
found between workers and controls selected from the general
population in connexion with parameters that have no known biochemical
relationship to DDT and for which another explanation has not been
excluded. For example, Keil et al., (1972) reported significant linear
correlations between serum vitamin A and plasma DDT, TDE, and DDE
levels.
There are a few reports of acute illness among workers attributed
to exposure to mixtures of DDT and other materials. In so far as the
dosage was very large, as in certain accidents that have occurred to
individuals or groups in the general populations (see section 8.2.2),
one would expect similar results. However, in at least one instance,
headache, dizziness, nausea, vomiting, pain and numbness of the limbs,
and general weakness beginning 1-1.5 h after entering a treated field
(Kolyada & Mikhal'Chenkova, 1973) suggested food poisoning or
hysteria.
Finally, there are studies of workers exposed to DDT and various
other pesticides that are reported to have produced a variety of
subjective and even objective medical findings. Interpretation of
these reports is difficult because: (a) the findings do not resemble
those of poisoned animals or of persons poisoned as a result of
accident or suicide; and (b) the papers fail to report how the medical
findings and the absenteeism of the pesticide workers compares with
those of workers of comparable age, sex, and exertion who are not
exposed to chemicals. The fact that the workers in question were
exposed to mixtures of pesticides is not in itself an explanation
because studies on many workers who were exposed to mixtures have not
revealed any consistent differences between exposed subjects and
unexposed controls. However, an explanation may lie in the degree of
exposure. Reports of very high levels of organochlorine compounds in
blood samples and of DDT in milk samples from populations in which
illness was found are discussed in sections 6.2.1.2 and 6.3.1.2.
The reports under discussion tend to fall in 2 categories, those
involving general debility and those involving a single organ or
system. Conditions representative of general debility include
dermatitis, subtle blood changes, general weakness, palpitations,
functional angiospasm, headache, dizziness, diminished appetite,
vomiting, lower abdominal pain, chronic gastritis, benign chronic
hepatitis, isomnia, a sympathetic vascular/asthenic syndrome,
vegetative dystonia, and confusion (Kostiuk & Mukhtrova, 1970; Bezugli
et al., 1973).
Organs, systems, or functions that have been studied with the
exclusion of other organs, systems, or functions of the same workers
include: the respiratory system (Boiko & Krasniuk, 1969), liver
(Bezuglyi & Kaskevich, 1969), stomach (Krasniuk & Platonova, 1969;
Platonova, 1970), kidneys (Krasniuk et al., 1968), adrenals (Bakseyev,
1973), skin (Karimov, 1969, 1970), and labour and the puerperium
(Komarova, 1970; Nikitina, 1974). An indication that the difficulties
under discussion are not serious is their reversal or prophylaxis by
means of diet. Lescenko & Polonskaia (1969) described in detail two
dietary supplements composed of ordinary foods plus sea-kale and a
selection of vitamins and trace metals. Organochlorine-exposed workers
who received these diet products showed a normalization of protein
metabolism manifested by an increase in total serum protein, improved
lipid metabolism, and enriched vitamin and trace element supplies in
the organism. All of these effects led to an improvement in the
detoxifying function of the liver, which was viewed as the most
frequent site of adverse effects of exposure to organochlorine
compounds.
8.1.2 Epidemiology of DDT poisoning in the general population:
accidents and suicides
The only demonstrated effects of DDT on the general population are
the storage of the compound and some of its derivatives in the tissues
and their excretion in urine and milk. The facts were reviewed in
sections 6.2.13 and 6.3.1.2. Briefly, DDT and some of its derivatives
are found in all or nearly all persons in the population. The
concentration is higher in tissues that have a high neutral fat
content. Thus, for members of the general public the concentration of
DDT-related compounds in adipose tissue is 100 or more times greater
than the concentration in plasma (Laws et al., 1967). However, in
spite of this great difference, sufficiently sensitive methods have
demonstrated DDT in all tissues including the fetus and in all body
fluids including human milk. These relationships are exactly what
would be predicted from what is known of the storage of drugs and
other compounds. Actual chemical demonstration of the distribution of
DDT has been established for several years. Thus, its occurrence was
first reported in human tissue (Howell, 1948), in tissue of the
general population (Laug et al., 1951) in human milk (Laug et al.,
1951), and in the human fetus (Denés, 1962).
There is extensive evidence that the mount of DDT and related
material in the general diet in the USA has decreased as the use of
DDT in that country has decreased, especially its use on forage.
During the early 1950s, total DDT-related intake was approximately
0.265 mg/man per day and that for DDT was 0.163 mg/man per day (Walker
et al., 1954). The average intake of DDT-related compounds based on a
very large number of samples collected in different parts of the
country during 1964-67 was 0.063 mg/man per day and that for DDT was
0.028 mg/man per day (Duggan, 1968). With decreased use of DDT, a
gradual decrease in the storage of DDT and related material in human
fat would be expected. Because only a few samples of fat were
collected in the early studies of human tissue, there is some
statistical uncertainty as to whether the decrease in storage that has
been observed is real or whether it merely reflects variation due to
sampling. In any event, by 1968, the average storage level of total
DDT-equivalent material in fat was 7.67 mg/kg and that for DDT was
1.46 mg/kg. These averages were based on just over 3000 samples
collected during the first half of 1968. The number of samples
involved in this particular study was greater than the sum of all of
the samples used in early studies. The best available values for
concentrations in serum are 0.0294 mg/litre for total DDT-equivalent
and 0.0047 mg/litre for p,p'-DDT.
Cases of accidental and suicidal poisoning in which the effects
were clearly caused by DDT are summarized in Table 22. All of these
cases involved ingestion. The signs and symptoms of poisoning were
entirely consistent with those observed in volunteers, except that the
spectrum of effects was broader because some of the accidental and
suicidal doses were very high. A few persons have apparently been
killed by uncomplicated DDT poisoning, but none of these cases was
reported in detail. Death has been caused much more frequently by the
ingestion of solutions of DDT, but in most instances the signs and
symptoms were predominantly or exclusively those of poisoning by the
solvent (Hayes, 1959). This does not mean that the toxicity of the
solvent always predominates. For example, the recurrent convulsions in
a case reported by Cunningham & Hill (1952), though more
characteristic of poisoning by one of the cyclodienes, was certainly
not typical of solvent poisoning. A 2-year-old child drank an unknown
quantity of fly spray of which 5% was DDT, but the nature of the other
active ingredients or the solvent was unknown. About 1 h after taking
the material, the child became unconscious and had a generalized,
sustained convulsion. Convulsions were present when the child was
hospitalized 2 h after taking the poison, but the fits were controlled
by barbiturates and other sedatives. Convulsions reoccurred on the
fourth day and again on the twenty-first day but ceased each time
following renewal of treatment. On the twelfth day, it was noted that
the patient was deaf. Hearing began to improve about the twenty-fourth
day and was normal, as were other neurological and psychic findings,
when the patient was seen about 2.5 months after the accident.
Clinical effects of one toxicant may be modified by combining it
with another. For example, prolonged illness would not be expected
from ingestion of DDT at a rate of 27 mg/kg. However, when DDT and
lindane were ingested in a suicidal attempt at dosages thought to be
27 mg/kg and 18 mg/kg, respectively, clinical remission of convulsions
and liver involvement was delayed until the twentieth day, and the EEG
did not return to normal until the thirty-ninth day (Eskenasy, 1972).
There have not been any accidents of suicided involving raspatory
or dermal exposure to DDT leading to recognized signs and symptoms of
poisoning, even though sufficient respiratory exposure to aerosols or
sufficient dermal exposure to solutions can cause poisoning in
animals; the difference is certainly one of dosage.
It has been alleged that DDT causes or contributes to a wide
variety of diseases of man and animals not previously recognized as
being associated with any chemical. Such diseases include
cardiovascular disease, cancer, atypical pneumonia, retrolental
fibroplasia, poliomyelitis, hepatitis, and "neuropsychiatric
manifestations" (Biskind & Beiber, 1949; Biskind, 1952, 1953; and
others). Without exception the causes of these diseases were unknown
or at least unproved at the time of the allegation. Needless to say,
Table 22. Summary of the effects of the accidental or suicidal ingestion of DDT
Individual dose Results and reference
(mg) Formulation
Number of persons
300-4500 Onset in 1 h; vomiting; restlessness; headache; heart weak and
in food slow; recovery next day (Mulhens, 1946).
1 man
unknown dose Onset in 2-2.5 h; all subjects weak and giddy; 4 subjects vomited;
in tarts 2 subjects hospitalized; one subject confused, uncoordinated, weak;
25 man one subject with palpitations and numbness of hands; recovery in
24-48 h (Mackerras & West, 1946).
5000-6000 Onset 2-3 h; throbbing headache; dizziness; incoordination;
in pancakes paraesthesia of extremities; urge to defaecate; wide nonreacting
3 men pupils; reduced vision; dysarthria; facial weakness; tremor; ataxic
gait; reduced sensitivity to touch; reduced reflexes; positive Romberg;
slightly low blood pressure and persistent irregular heart action;
partial recovery in 2-3 days, but slight jaundice appeared 4-5 days
after ingestion and lasted 3-4 days; all subjects normal 19 days after
poisoning except for irregular heart action in one subject (Naevested,
1947).
2000 No illness (Naevested, 1947).
in pancakes
2 men
up to 20 000 Onset in 30-60 min in those most severely affected; men first
in bread seen 2-3 h after ingestion; in spite of severe early vomiting that
28 men reduced the effective dose, severity of illness and especially intensity of
numbness and paralysis of extremities proportional to amount of DDT
ingested; all but 8 men recovered in 48 h; 5 others fully recovered in 2
weeks, but 3 men still had some weakness and ataxia of the hands
5 weeks after ingestion (Garrett, 1947, 1950, unpublished data).
unknown dose Onset about 3.5 h after ingestion; total of about 85 cases of which 37
in flour were hospitalized; symptoms mild and similar to those in earlier
about 100 women outbreaks except for gastrointestinal disturbance in most severe cases
including abdominal pain and diarrhoea as well as nausea; most
subjects fully recovered in 24 h (Jude & Girard, 1949).
unknown dose Symptoms in established cases similar to those reported earlier
14 cases (Francone et al., 1952).
Table 22 (Cont'd)
Individual dose Results and reference
(mg) Formulation
Number of persons
286-1716 With the exception of one man who was already sick when he received
in meatballs a dosage of 6 mg/kg, poisoning did not occur at dosages of
8 cases, 11 5.1-10.3 mg/kg. Ingestion of 16.3-120.5 mg/kg produced excessive
exposed perspiration, nausea, vomiting, convulsions, headache, increased
salivation, tremors, tachycardia, and cyanosis of the lips. Onset
varied from 2-6 h depending on dosage. Recovery required as much
as 2 days (Hsieh, 1954).
unknown dose Death 13 h after suicidal ingestion (Committee on Pesticides, 1951).
1 case
unknown dose Twenty-two separate cases, including 15 attempted suicides; some
22 unrelated cases complicated by solvents; 3 deaths (Committee on Pesticides, 1951).
the charge that DDT predisposes to poliomyelitis was dropped after the
disease was controlled through the use of vaccines. Unfortunately,
there is no immediate possibility of controlling cardiovascular
disease, cancer, or many of the less common conditions in man that
have been ascribed to DDT. In the meantime, such irresponsible claims
could produce great harm and, if taken seriously, even interfere with
the scientific search for true causes and realistic means of
preventing the conditions in question.
8.1.3 Epidemiology of DDT poisoning in infants and young children
Nothing is known fundamentally to distinguish the epidemiology of
DDT poisoning among children from that among adults. In both
instances, poisoning has never been confirmed, except where the dosage
was large, usually as the result of an accident and usually involving
gross carelessness. Probably a larger number of cases have occurred in
infants simply because they are more likely to eat and drink
formulations that they find in unlabelled containers frequently
originally intended for food. However, as far as DDT is concerned, all
the large outbreaks of poisoning have involved adults under military
conditions, thus children were not exposed. As might be expected, some
deaths of adults but none of children has apparently involved suicide.
Most, if not all, cases in adults were uncomplicated poisoning by DDT,
but several cases in children involved the drinking of solutions so
that the signs and symptoms were actually caused by the solvent
(Reingold & Lasky, 1947).
8.2 Clinical and Epidemiological Studies of the Effects of DDT on
Specific Organs and Systems
8.2.1 Haemopoietic system and immunology
In acute poisoning, a slight decrease in haemaglobin and a
moderate leukocytosis without any constant deviation in the
differential white count have been observed in volunteers (Velbinger,
1947a,b). These findings are considered secondary to the neurological
effects.
There is a strong tendency to blame blood dyscrasias, other
manifestations of "hypersensitivity", and, in fact, many diseases of
unknown cause on any new chemical that gains widespread attention. DDT
was no exception. A review of the early literature (Hayes, 1959)
indicates that blood dyscrasias and an unbelievable range of other
diseases were, in fact, blamed on DDT. Only a circumstantial
relationship was ever established between these diseases and exposure
to DDT, and this is true of the small number of reports of blood
dyscrasias (Murray et al., 1973) or angioneurotic oedema (Vanat &
Vanat, 1971) that have appeared recently. Later, fewer new reports
appeared linking DDT to diseases of unknown cause, although the use of
DDT increased greatly. It is true that available tests do not make it
possible to exclude a particular compound as a cause of an isolated
case of blood dyscrasia. However, it is noteworthy that the rate at
which these disorders occur has remained essentially unchanged since
before DDT was introduced (Hayes, 1975).
8.2.2 Nervous system
The effects of carefully measured doses of DDT that proved to be
just above the minimum toxic level are best described from studies of
volunteers (see section 7.4). Similar early signs and symptoms have
been encountered in cases of accidental poisoning that frequently
progressed to more severe illness as described in section 8.1.2.
Briefly, the earliest symptom of poisoning by DDT is
hyperaesthesia of the mouth and lower part of the face. This is
followed by paraesthesia of the same area and of the tongue and then
by dizziness, an objective disturbance of equilibrium, paraesthesia
and tremor of the extremities, confusion, malaise, headache, fatigue,
and delayed vomiting. The vomiting is probably of central origin and
not due to local irritation. Convulsions occur only in severe
poisoning.
Onset may be as soon as 30 min after ingestion of a large dose or
as late as 6 h after smaller but still toxic doses. Recovery from mild
poisoning is essentially complete in 24 h, but recovery from severe
poisoning requires several days. In two instances, there was some
residual weakness and ataxia of the hands, 5 weeks after ingestion.
Electroencephalograms were obtained from 73 workers exposed to
DDT, HCH, and chlorobenzilate for periods ranging from 7 months to 20
years. Just over 78% of the records were normal and 21.9% were
abnormal. The most severe changes involved persons exposed to the 3
compounds for 1-2 years; less severe changes were seen with either
shorter or longer exposure. The changes were not correlated with age,
the range and mean of age for those judged abnormal being almost
identical to these values for persons considered normal. Some of the
records showed bitemporal sharp waves with shifting lateralization
combined with low voltage theta activity. Other records showed spike
complexes, paroxymal discharges composed of slow and sharp waves most
pronounced anteriorly, and low voltage rhythmic spikes posteriorly.
None of the persons examined showed any abnormal clinical neurological
finding (Israeli & Mayersdorf, 1973; Mayersdorf & Israeli, 1974). The
incidence of abnormal electroencephalograms in the general population
is 9.0% or 9.2%, according to other investigators cited by Israeli &
Mayersdorf. Czegledi-Janko & Avar (1970) considered that nonspecific
EEG abnormalities occurred in 10-20% of the general population.
The frequency and degree of olfactory disorders, especially in the
ability to detect peppermint and acetic acid in an olfactory analyser,
were reported to be greater among persons exposed to pesticides, and
to increase with duration of exposure (Salihodzaev & Ferstat, 1972).
Whether any of the persons exposed to pesticides experienced any
clinical difficulty or social inconvenience associated with olfactory
sensation is not clear.
8.2.3 Renal system
There is no indication of renal damage in people, accidentally
poisoned by DDT, or in workers heavily exposed to it.
8.2.4 Gastrointestinal system
Except for vomiting, which probably is of central origin, the
gastrointestinal system has not been affected in acute poisoning.
8.2.5 Liver
Involvement of the liver has been mentioned in only a small
proportion of cases of accidental poisoning by DDT. In 3 men who ate
pancakes made with DDT and thus ingested 5000-6000 mg each, slight
jaundice appeared after 4-5 days and lasted 3-4 days (Naevested,
1947). Hepatic involvement and convulsions were reported in an
unsuccessful suicide attempt by ingesting DDT and lindane (Eskenasy,
1972).
Laws et al. (1973) made a detailed study of the liver function of
31 men who had made and formulated DDT and who had been the subjects
of an earlier study (see section 8.1.1). Judging from their excretion
and storage, the men's exposure was equivalent to oral intakes of DDT
at rates ranging from 3.6 to 18 mg/man per day for periods ranging
from 16 to 25 years and averaging 21 years. All tests were in the
normal range for total protein, albumin, total bilirubin, thymol
turbidity, and retention of sulfobromophthalein sodium (BSP). One man
had mild elevations in levels of both alkaline phosphatase
(EC 3.1.3.1) (16 units) and serum glutamic pyruvic transaminase
(EC 2.6.1.2) SGPT (42 units). Another man had an alkaline phosphatase
concentration of 14 units, while a third man had an SGPT level of 49
units. The alpha-fetoprotein test was negative for all 20 of the men
tested.
8.2.5.1 Liver enzymes
The induction of human microsomal enzymes of the liver by various
drugs was well known when Kolmodin et al. (1969) demonstrated this
effect in workers exposed to a variety of pesticides, including DDT.
Later, Poland et al. (1970) showed that workers who made and
formulated DDT and absorbed it at an average rate of about
0.25 (mg/kg)/day metabolized phenylbutazone more rapidly on the
average than controls and excreted more 6ß-hydroxycortisol.
Occupational exposure increased the drug-metabolizing ability of some
workers, so that they all metabolized test drugs with the efficiency
of those members of the general population who were most efficient in
this respect. The concentration of p,p'-DDT in the serum of the
workers studied by Poland averaged 0.573 mg/litre. In other workers
with less exposure to DDT, as indicated by average serum levels of
0.052 mg/litre, there was no increase in the urinary excretion of
D-glucaric acid, which is increased by a number of exogenous and
endogenous substances that induce microsomal enzymes (Morgan & Roan,
1974).
Thompson et al. (1969) demonstrated, in a different way, the
induction of microsomal enzymes by using DDT at a dosage of
1.5 (mg/kg)/day for 6 months in the successful treatment of
unconjugated hyperbilirubinaemia. In a similar way, Rappolt (1970)
used DDT to promote metabolism of an overdose of phenobarbital. It is
of interest that the levels of DDE in the serum of some workers
studied by Morgan & Roan (1974) approached those of workers studied by
Poland et al. (1970). The lack of induction in one group and its
presence in the other suggests that enzymes are induced in man more
readily by DDT than by DDE.
DDT promotes its own metabolism in some species of laboratory
animals. That the same is true in man is indicated by the fact that
storage of DDT is relatively less at higher dosages (see Fig. 4).
However, the metabolism and subsequent excretion of DDT can be
promoted even more by other inducing agents. Patients who received
phenobarbital or, more especially, phenytoin stored much less DDT than
other persons with similar exposure to DDT (Davies et al., 1969a;
The compound identified by Peterson & Robinson (1964) as a
"probable intermediate aldehyde" was later synthesized and shown to be
highly labile (McKinney et al., 1969), confirming the guess by
Peterson & Robinson that it was unlikely to accumulate in tissues in
measurable amounts.
Of the compounds shown in Fig. 2 and 3, only DDT, TDE (DDD), DDE,
and DDA are commonly reported in the tissues or excreta of animals,
including man. The symptomatology produced when the metabolites are
administered directly is discussed in section 7.1.1, while uptake,
distribution, and elimination of the compounds are discussed in
sections 6.1.2, 6.2.2, and 6.3.2, respectively.
Although microorganisms, plants, insects, and birds produce many
of the same metabolites that are found in mammals, there are
interesting differences. Nearly 20 derivatives (including mammalian
metabolites) have been identified, and the chemical structure of
several more is still unknown. Some aspects of nonmammalian, as well
as mammalian metabolism have been reviewed (Menzie, 1969; Klein &
Korte, 1970; Fishbein, 1974; Schroeder & Dorozalska, 1975). The
metabolism of microorganisms and plants, as well as that of domestic
animals, may influence the composition of DDT-derived residues in
human food, but there is no evidence that these residues contain a
significant amount of any compound not formed from DDT by human
metabolism.
7. EXPERIMENTAL STUDIES ON THE EFFECTS OF DDT
7.1 Animal Studies
7.1.1 Haemopoietic system and immunology
Many early reports reviewed by Hayes (1959) indicated that large
doses of DDT might not have any effect on the blood or that they might
produce a moderate leukocytosis and a decrease in haemoglobin, with or
without a decrease in the concentration of red cells. The leukocytosis
probably is secondary to stimulation of the sympathetic nervous
system, while the loss of haemoglobin may be nutritional in origin.
Later studies have confirmed the early results. A range of
haematoiogical variables remained unchanged in squirrel monkeys dosed
orally at rates of 0, 0.05, 0.5, 5, and 50 (mg/kg)/day, even though
the highest dosage was fatal within 14 weeks (Cranmer et al., 1972).
Immunology. Inasmuch as some compounds are antibiotic, it is
logical that some may be probiotic, that is, that they either reduce
resistance to infection or increase the virulence of an infecting
organism (Hayes, 1975). Far fewer studies have been made of probiosis
than of antibiosis. A number of papers have reported one or other
possibly probiotic property of DDT, but some of the reports could not
be confirmed, and others have not been retested.
It has been claimed that a change in the phagocytic activity of
white blood cells is an indication of early intoxication by DDT
(Kun'ev, 1965). However, Kaliser (1968) did not find any statistical
difference in in vitro or in vivo phagocytosis of control rats and
those receiving DDT by stomach tube at a rate of 0.25 (mg/kg)/day for
31 days. The highest rate at which men who make and formulate DDT in
the USA now absorb the compound is about 0.25 (mg/kg)/day.
Rats receiving an aqueous suspension of p,p'-DDT of unstated
stability at a concentration of 200 mg/litre as their only source of
water for over 30 days were reported to develop a lower titre of
antibodies to ovalbumen (Wassermann et al., 1969). Rabbits responded
to the same treatment with a statistically significant reduction of
antibody titre against Salmonella and a reduction in antibody titre
against sheep red blood cells that was not statistically significant
(Wassermann et al., 1971). Both the rats and rabbits showed a decrease
in at least one globulin fraction of the blood.
Other reports of changes in immunohaematological indices are those
of Semenceva (1968) and Fridman (1970).
One group of investigators has shown clearly that what at first
appeared to be an immunological response really involved a quite
different, predictable effect. Briefly, it was shown that guineapigs
sensitized to diphtheria toxoid were less susceptible to anaphylaxis
in response to a challenge dose of the toxoid if they were pretreated
with DDT at a dosage of only 1-20 mg/kg. Direct measurement of
antitoxin production indicated little or no difference between
protected and unprotected animals. Furthermore, some protection was
given by DDT administered for only 3 days prior to the induction of
anaphylaxis (Gabliks et al., 1973, 1975). Further studies showed that
DDT treatment reduced the histamine levels in the lungs of both
immunized and nonimmunized animals. The number of detectable mast
cells was also reduced; this was true whether the count was made in
tissues from guineapigs dosed systemically with DDT or in the lungs
and mesenteric tissue taken from untreated animals and exposed to DDT
in vitro at concentrations ranging from 10 to 45 mg/litre. These
results indicate that the protection offered by DDT was the result of
a reduction of the amount of histamine available for sudden release in
response to a challenge dose of toxoid (Askari & Gabliks, 1973).
Regardless of exposure to DDT, immunization leads to an increase in
detectable mast cells (Gabliks et al., 1975).
7.1.2 Nervous system and behaviour
DDT intoxication in animals was well described by Domenjoz (1944).
The first perceptible effect is abnormal susceptibility to fear, with
violent reaction to normally subthreshold stimuli. There is definite
motor unrest and increased frequency of spontaneous movements. As
poisoning increases, hyperirritability, like that seen in strychnine
poisoning develops, but convulsions do not appear at this time. A fine
tremor, recognizable at first only as a terror reaction, is later
present as all intention tremor in connexion with voluntary movement.
Then it is present intermittently without observable cause, and
finally it is present as a coarse tremor without interruption for as
long as several days. Spontaneous movement is limited, and food intake
stops so that surviving animals lose weight. In the later stages,
especially in some species, there are attacks of epileptiform, tonic-
clonic convulsions with opisthotonos.
All the signs are strengthened by external stimuli and become
manifest at first through external stimuli. At all stages, the animals
show normal position and labyrinth reflexes. The picture of poisoning
in mammals recalls the disturbances of movement and tone that are
known in human pathology as the amyostatic syndrome.
Symptoms appear several hours after oral administration of the
compound, and death follows after 24-72 h. The latent period after
intravenous administration at about the LD50 level is approximately
5 min; signs of poisoning reach a maximum level in about 30 min, and
survivors are symptom-free in 18 to 24 h. Animals that survive recover
completely.
In addition to the features of poisoning already mentioned,
Cameron & Burgess (1945) noticed that as rats, guineapigs, and rabbits
become sick, they become cold to the touch and show ruffled fur. Some
show diarrhoea. These authors found that muscular tremors were
preceded by muscular weakness that first occurred in the back and
later in the hind legs. The front legs were relatively spared so that
animals showing marked weakness of the hind quarters could still drag
themselves about. However, several authors have found that the tremor
characteristic of DDT poisoning generally starts in the muscles of the
face, including the eyelids, and spreads caudally with variable
severity until all the muscles are affected. Furthermore, although
weakness of hind quarters has been seen by others, it is not a common
finding.
Like tremor, coldness of the skin and ruffling of the fur probably
represent an indication of disturbed thermal regulation. Apparently it
was not until the work of Hrdina et al. (1975) that an increase of
almost 3°C in body temperature was reported in rats following a fatal
(600 mg/kg) oral dosage of DDT.
Although there is a general similarity in the clinical effects of
DDT in all vertebrate species, some characteristic differences exist.
Cats show greater extensor rigidity and opisthotonos than other
laboratory animals. The stiffness appears first in the distal part of
the extremities and later extends to the proximal part and to the
trunk. Poisoned cats show marked pilomotor activity. Convulsions in
them may become almost continuous. Convulsions are also prominent in
dogs as is ataxia. Tremors are so pronounced in rats that it may be
difficult to detect clonic convulsions in them. Rats poisoned by DDT
show a reddish colour about the eyes just as they do when ill from
many other causes. The colour has been attributed to excessive
secretion of a porphyrin by the Harderian glands.
Poisoning produced by repeated doses of DDT differs from that
produced by a single dose only in so far as the animal may be
gradually debilitated, especially by malnutrition. If food intake is
maintained, tremor may last for weeks, or even intermittently, for
months. If the animals survive a short time after dosing stops,
recovery is complete. However, food intake may be interfered with in
at least two ways. Tremor and more severe signs may interfere
mechanically with eating. Animals offered food containing high
concentrations of DDT often eat little or nothing and lose weight
rapidly. However, the same animals will show excellent appetites when
offered the same kind of food without DDT just after refusing the
major portion of the daily ration of contaminated food. Unlike
dieldrin and some other compounds, DDT seems to have little effect on
appetite as mediated by the central nervous system; it has a great
deal to do with taste.
Animals that have suffered severe weight loss as a result of DDT
poisoning may die partly as a result of general debility. In some
colonies, at least, they have become prey to secondary infection.
In summary, it may be said that animals that die as the result of
repeated large doses of DDT and small animals that die as a
complication of starvation following many somewhat smaller doses of
DDT show the same signs as those seen in animals killed by one or a
few large doses. Even though severely ill, animals that survive a few
days after the last of many doses of DDT recover.
Of samples that may be collected from a living animal, the
concentration of DDT in serum most accurately reflects its
concentration in the brain, the critical tissue. In the rat, levels of
25 mg/kg (wet weight) in the brain are not usually fatal: higher
levels tend to be fatal regardless of whether absorption followed one
or many doses (Dale et al., 1963; Hayes & Dale, 1964). As reviewed by
Hayes (1975), the danger level is approximately the same in several
species of birds.
Behavioural changes may be demonstrated in animals receiving DDT,
daily, at rates too low to produce illness. Khairy (1959) was able to
detect ataxia in the form of changes in gait in rats that had been fed
DDT at dietary levels of 100 mg/kg or more for 21 or more days. Gait
was recorded by smearing the hind paws of the animals with vaseline,
which then recorded their tracks on paper. Gait was recorded in terms
of the tangent, that is the ratio of the width and length of step. At
a body weight dosage of about 5 (mg/kg)/day the ratio was less than
normal, a change the author attributed to an exaggeration of the
stretch reflex. At dosages of about 10, 20, and 30 (mg/kg)/day, the
ratio was progressively greater than the normal as a result of
broadening of the gait and shortening of the steps. These same dosage
levels did not affect problem-solving behaviour or speed of
locomotion. The experimental animals were generally less reactive to
stress than normal ones. Thus, the author attributed hyperirritability
of rats poisoned by DDT to exaggerated motor responses.
The major toxic action of DDT is clearly on the nervous system,
and it requires an intact organism for full expression. The fact that
DDT causes a myotonic response in muscle and substitution of a train
of spikes for the normal diphasic electroneurogram (Eyzaguirre &
Lilienthal, 1949) is in marked contrast with the absence of detectable
injury or, in fact, any response in other isolated tissues. As early
as 1945, Lewis & Richards (1945) found DDT to be inert when it was
applied to tissue cultures of heart, kidney, stomach, intestine,
liver, and muscle from 7 to 9-day chick embryos, and of brain and
spleen from a one-day rat. The physiology of the cells including the
mytoses of fibroblasts was normal. The migration and extension of the
various cells was unchanged. The authors stated that "living
fibrilloblasts, as they moved about in the cultures, sometimes touched
or even migrated over DDT crystals without appreciable injury to
themselves during a period of several days". Some observations were
carried out for periods as long as 21 days.
In spite of the importance of the nervous system, a detailed
review of early literature indicates that, although the presence of
some specialized nervous function may be necessary for the
manifestation of DDT poisoning, the mere occurrence of specialized
nerve fibres in certain protozoa or the occurrence of a rather complex
nervous system in molluscs is not sufficient to render these forms
susceptible. Just as there is no explanation for the effect of DDT on
susceptible species, so there is no explanation for the fact that
certain species and even entire phyla are inherently resistant to the
compound.
A review (Hayes, 1959) of literature on the effects of DDT on the
nervous system reveals that all major parts, both central and
peripheral, are affected. Whereas effects on specific portions,
notably the cerebellum and the motor cortex, have been viewed as of
greatest importance, it probably is more accurate to emphasize the
interaction of functions, all modified to some degree.
There is reason to think that the mechanism of action of DDT is
its action on membranes in the nervous system, especially axonal
membranes. Certainly action on membranes is a fundamental property of
the compound. In fact the potassium conductance induced by valinomycin
at 10-6 mol/litre in a synthetic lecithin-decane membrane is
reversed by DDT at 3 × 10-6 mol/litre (Hilton & O'Brien, 1970).
Attention was focused quite early on the effects of DDT on axonal
membranes. Using the giant axons of the cockroach, Narahashi &
Yamasaki (1960) showed that DDT prolonged the recovery phase of the
action potential. They concluded that it slows the efflux of potassium
ions from the axon. Later, using the voltage clamp technique and giant
axons of the lobster, Narahashi & Haas (1967) showed that DDT, at a
concentration of 5 × 10-4 mol/litre of bathing medium, prolonged the
flow of sodium ions as well as interfering with the flow of potassioum
ions: in other words DDT delayed shutting of the Na+ gate and
prevented full opening of the K+ gate.
Na+-, K+-, and Mg2+-adenosine triphosphatase (EC 3.6.1.3) is
involved in ion transport in the nervous system. Matsumura & Patil
(1969) showed that a preparation of this enzyme from a nerve ending
fraction of the rabbit brain was inhibited by DDT at concentrations as
low as 10-8 mol/litre. There was a good correlation between the
degree of its inhibition by analogues of DDT and their toxicity to
mosquito larvae. A similar enzyme that binds 14C-DDT has been
isolated from the synapses of rat brain (Bratowski & Matsumura, 1972).
Both the electrophysiological changes and the enzyme inhibition
exhibit a negative temperature coefficient, an important feature of
DDT poisoning in insects but not in mammals (Hoffman & Lendle, 1948;
Deichmann et al., 1950).
At a supralethal dosage of 600 mg/kg, DDT caused a marked decrease
in the concentration of cortical and striatal acetylcholine and of
brainstem norepinephrine in rats and a significant increase in
brainstem 5-hydroxyindoleacetic acid. All of the neurotoxic signs of
poisoning were blocked by p-chlorophenylalanine, while other
inhibitors blocked one or other, but not all of the effects. It was
concluded that changes in the metabolism of 5-hydroxytryptamine and
norepinephrine might be responsible for DDT-induced hyperthermia while
acetylcholine might be related to tremors and convulsions (Hrdina et
al., 1973). These and related matters have been reviewed in great
detail by Hrdina et al. (1975). Apparently studies have not been made
at a range of dosages that would make it possible to know whether
these changes are a result or a cause of poisoning; the possible
therapeutic effect of p-chlorophenylalanine has also not been
explored.
It was reported by Haikina & Silina (1971) that administration of
DDT to rats at only one-fifth of the LD50 for the 2 days increased
the amount of 5-oxyindoleacetic acid excreted in urine by 188%. This
indicates a change in the metabolism of serotonin, but its
significance is not clear.
One sensitive measure of brain activity is the electroencephalo-
gram (EEG). Farkas et al. (1969) found that the EEG wave frequency
increased considerably in resting rats that had received
20 (mg/kg)/day of DDT as a result of dietary intake. Rats that had
received either 5 or 10 (mg/kg)/day did not exhibit this change while
at rest, but even those receiving 5 (mg/kg)/day exhibited this change
when exposed to a rhythmic light stimulus. The EEG may become abnormal
only a minute or two after administration of a large dose of DDT;
4 stages of the electrical activity culminating in generalized
seizures have been described by Joy (1973). Phenobarbital, but not
phenytoin or trimethadione, was effective in stopping the seizures.
7.1.2.1 Cause of death
Death from DDT poisoning is usually the result of respiratory
arrest. The heart continues to beat to the end and in some instances
continues a little while after respiration stops. Deichmann et al.
(1950) found that the onset of hyperirritability in rats was
accompanied by an increase in the frequency and amplitude of
respiration. Later, with the occurrence of tremors, the depth of
respiration frequently returned to a more normal level, but the rate
remained high. In some animals, respiration stopped suddenly after a
deep inspiration during a tonic convulsion. In other animals, the rate
and amplitude decreased progressively and finally ceased without any
terminal spasm. Animals that die of respiratory failure caused by DDT
do so after a relatively long period of muscular activity that leaves
them exhausted.
It was shown by Philips & Gilman (1946) and Philips et al. (1946)
that the hearts of dogs given large intravenous doses of DDT were
sensitized to epinephrine. This was true not only of injected
epinephrine but also of the compound released by the adrenal glands
during a seizure. Stimulated in this way, the sensitized hearts of
dogs developed an irreversible, fatal ventricular fibrillation.
However, the hearts of monkeys were able to recover from fibrillation
and resume normal rhythm. It is not clear how important sensitization
of the myocardium is when DDT is administered by other routes, but
ventricular fibrillation may be the cause of death in animals that die
suddenly, soon after onset of poisoning.
7.1.2.2 Treatment of poisoning in animals
Studies on the treatment of poisoning will be discussed in this
section since all the more successful studies of treatment of animals
poisoned by DDT involve the nervous system. Smith and Stohlman (1944)
noted the possibility that narcotics in general, may exhibit an
antagonism to DDT. Rats survived on a diet containing DDT at a
concentration of 1000 mg/kg for 90 days when they received
cyclohexanone in the same diet at the rate of 2000 mg/kg, but were
uniformly killed in a shorter period when they received DDT at the
same rate without cyclohexanone. Later, it was shown that
cyclohexanone offers no protection when used as a solvent for single
massive doses of DDT (Deichmann et al., 1950).
Smith & Stohlman (1945) showed that, when given as required after
the onset of illness, urethane and, to a lesser extent, phenytoin
sodium protected rats from poisoning. Sodium amobarbital gave slight
benefit, sodium phenobarbital a doubtful benefit, and paraldehyde no
protection at all. All drugs were given intraperitoneally except
paraldehyde, which was given by stomach tube. The mortality of rats
treated with urethane was 12.5% and that of their controls was 80%. A
total dosage of 1.2-2.5 mg/kg spread over a period of 1-3 days, was
found most satisfactory. Phenytoin sodium gave a mortality of 46.7%,
compared with 96.7% for the controls. The smallest effective dosage
was 200-250 mg/kg, a value very close to the LD50 which, under the
conditions of the test was 300 mg/kg.
Lauger et al. (1945a, b) also found that sodium phenobarbital was
of questionable value in treating rats poisoned by DDT. However,
completely different results were seen in larger animals.
Phenobarbital was, by far, the most outstanding remedy tested by
Philips & Gilman (1946). In a dosage well below the anaesthetic level,
it not only prevented death in many instances but also controlled
tremor and convulsions. Signs of illness were more readily controlled
in dogs and cats than in monkeys, which required nearly a full
anaesthetic dosage before tremors completely disappeared.
Magnesium sulfate did not reduce mortality in poisoned dogs and
cats although it did control tremors and convulsions briefly. Sodium
bromide was entirely ineffective. Mortality was reduced with urethane,
but a full anaesthetic dosage was required to control tremor and
convulsion. Similarly, sodium barbital and sodium pentobarbital
controlled symptoms only when given in full anaesthetic doses and,
even then, did not greatly reduce mortality. Phenytoin, when given to
rats before they received DDT, reduced the lethal action without
showing a notable effect on the signs of poisoning; phenytoin was not
effective in cats.
Vaz et al. (1945) were, apparently, the first to note the
antidotal effect of calcium in DDT poisoning. Dogs were given DDT
orally as a 10% oily solution at a daily dosage of 100 mg/kg until
signs of intoxication appeared. The same dosage could then be repeated
to produce intense symptomatology from which the animals would recover
spontaneously in 12-24 h. For the actual tests, a larger challenge
dosage of DDT (150 to 200 mg/kg) was used. Each dose of calcium
gluconate (30 ml of a 10% solution) was injected intravenously into
dogs weighing 8-18 kg. Dogs that were injected with calcium gluconate
dally for 4 days and, challenged with a large dose of DDT on the
fourth day, did not develop any symptoms or only slight ones. Dogs
receiving a single dose of calcium gluconate showed symptoms of short
duration and survived following a dosage of DDT large enough to kill 2
controls.
Cats poisoned by the intravenous injection of a soya-lecithin-corn
oil emulsion of DDT were studied by Koster (1947). A comparison was
made of several aspects of intoxication including number of
convulsions, general severity (tremors, prostration, dyspnoea),
duration, and mortality. Both calcium gluconate and sodium gluconate
reduced mortality but not severity. Gluconic acid increased the
survival time, reduced mortality, but did not reduce the number or
severity of the convulsions. Calcium chloride reduced convulsions, but
not mortality or tremors. Molecular equivalent doses of the candidate
antidotes were used. Gluconic acid and its two salts were effective
against an LD95 dosage of DDT. The life-saving capacity of calcium
gluconate at a rate of 40 mg/kg was confirmed by Judah (1949) even
though he found normal blood calcium values in most poisoned but
unmedicated animals. One animal showed a high calcium value, and
Cameron & Burgess (1945) reported a similar result. It has been
suggested that increased blood calcium may be associated with acidosis
caused by the accumulation of lactate.
Thus, calcium has an antidotal action against DDT in intact
animals of several species. The suppression by calcium of the effect
of DDT on the isolated nerve and muscle of the rat has been
demonstrated (Eyzaguirre & Lilienthal, 1949). The hypothesis has been
advanced (Welsh & Gordon, 1946; Gordon & Welsh, 1948) that certain
neurotoxins, including DDT, act by delaying the restoration of calcium
ions to a surface complex, following breaking of the chelate linkage
of calcium ions to surface polar groups by an initial exciting
impulse. This action of the neurotoxin is conceived as depending
largely on its physical rather than on its chemical properties. The
hypothesis is helpful in explaining the fact that a wide variety of
chemically unrelated compounds produce repetitive responses in
excitable tissue and also the fact that many compounds that show a
high toxicity for arthropods and mammals are fat-soluble and
relatively inert chemically. It has been pointed out that this
hypothesis postulates a very localized action of calcium at the nerve-
cell membrane; the hypothesis is not inconsistent with the finding
that the blood calcium of poisoned animals may be unchanged or even
increased.
Having observed the effect of DDT on the metabolism of glucose and
glycogen, Lauger et al. (1945a, b) investigated the use of glucose as
an antidote. All of 10 dogs given 2000 mg of DDT per kilogram body
weight orally in the form of an oil solution died within 8-24 h. Five
of 10 dogs treated with one or more, 20 ml doses of 20% glucose
survived the same dosage of DDT. The glucose was given intravenously
in most instances.
Koster (1947) found that glucose given before or after an LD33
dosage reduced convulsions and mortality and, when given before the
poison, reduced tremors, prostration, and dyspnoea in cats. Glucose,
unlike gluconic acid and its sodium and calcium salts, was ineffective
against an LD95 dosage except to increase the time of survival.
Insulin, given intra-muscularly 16-25 min before DDT, increased the
survival time and the severity of poisoning but did not affect
mortality or convulsions. When given 53-130 min before DDT, insulin
reduced convulsions in animals which died but increased convulsions,
tremors, and other disorders in the survivors.
The failure of amino and sulfhydryl compounds to influence the
action of DDT was noted by Von Oettingen & Sharpless, 1946). Likewise,
the addition of 0.2% choline chloride to the diet of rats receiving
repeated doses of DDT had no effect on the accumulation of lipids in
their liver (Sarett & Jandorf, 1947).
In summary, it would appear that sedatives, ionic calcium, and
glucose or other ready sources of energy are useful in treating
poisoning by DDT. Dogs and cats can be treated somewhat more
successfully than rats, perhaps because the metabolism of larger
animals is slower.
Although respiration may be temporarily restored in animals
poisoned by DDT, studies have not been made to determine whether
respiratory arrest in this condition is truly reversible as it is in
poisoning by certain organic phosphorus insecticides.
7.1.3 Renal system
No dysfunction of the renal system attributable to DDT has been
found even in animals receiving dosages sufficient to cause
dysfunction of the nervous system or striking morphological changes of
the liver. It is true that mild to moderate morphological changes have
been reported in the kidneys of animals that have received massive
single doses or repeated doses; for example, fatty degeneration,
necrosis, and calcification (Lillie et al., 1947; Stohlman & Lillie,
1948) or slight brown pigmentation of the convoluted tubular
epithelium (Fitzhugh & Nelson, 1947). However, it sometimes has
happened that a complete absence of change in the kidney has been
reported in connexion with other studies carried out in the same
laboratories (Lillie & Smith, 1944; Nelson et al., 1944).
7.1.4 Gastrointestinal tract, liver, and enzymes
Large doses of DDT produce vomiting in species that can vomit.
Only doses that produce rather severe poisoning lead to anorexia.
7.1.4.1 Liver
Large doses of DDT cause focal necrosis of liver cells in several
species (Lillie & Smith, 1944; Nelson et al., 1944; Cameron & Burgess,
1945; Lillie et al., 1947; Deichmann et al., 1950; Ortega et al.,
1956). At least some investigators (Cameron & Burgess, 1945) have
considered that the liver lesions produced by large dosages are
sufficient to account for death. All have agreed that necrotic lesions
occur only in connexion with potentially fatal dosages.
A very different kind of liver change is produced in some rodents
but not in other animals by small or moderate dosages of DDT. The
biochemical aspects of these changes are discussed in section 7.1.4.2,
while the morphological aspects are discussed in section 7.1.9.
7.1.4.2 Microsomal enzymes of the liver
All enzymes can be inhibited in vitro and many of them can be
inhibited in vivo. The toxic action of a number of compounds is
clearly the result of their inhibition of one or more enzymes. So far,
only a few enzymes or enzyme systems are known to be induced by
chemicals; the outstanding example is the induction of microsomal,
mixed-function enzymes of the liver and some other organs that are
produced in greater quantity in response to certain hormones and other
normal constituents of the body (Conney, 1967), some foods
(Wattenberg, 1971), or a wide range of drugs or other foreign
chemicals. Such induction requires intact cells and does not occur
when the inducer is brought in contact with the purified enzymes in
vitro. Microsomal enzymes were first recognized and studied in the
liver, but they are now known to exist, generally in lower
concentrations, in other tissues.
Microsomal enzymes are known to be associated with oxidation ( N-,
O-, and S-dealkylation, deamination, epoxidation, disulfuration,
hydroxylation) of both rings and side chains, oxidation of both
nitrogen and sulfur, reduction of nitro groups and of azo compounds,
hydrolysis, and conjugation. Most of the changes produced by
microsomal enzymes render oil-soluble compounds more water-soluble
and, therefore, more easily excreted. Mainly for this reason, most
biotransformations promoted by microsomal enzymes are true
detoxications. However, some of the reactions promoted by this system
of enzymes render specific compounds more toxic.
In the rat, DDT has been shown to promote the biotransformation of
hexobarbital (Hart & Fouts, 1963), phenazone (Hart & Fouts, 1963;
Kinoshita et al., 1966), p-nitrobenzoic acid (Hart & Fouts, 1963),
aniline (Hart & Fouts, 1963), dieldrin (Street, 1964; Street et al.,
1966; Street & Chadwick, 1967; Pearl & Kupfer, 1971), o-ethyl o-4-
nitrophenyl phenylphosphonothioate (EPN) (Kinoshita et al., 1966),
p-nitroanisole (Kinoshita et al., 1966; Vainio, 1974), methyprylon
(Datta & Nelson, 1968), meprobamate (Datta & Nelson, 1968),
chlordizepoxide (Datta & Nelson, 1968), aldrin (Gillett, 1968),
lindane (Chadwick et al., 1971b), phenylbutazone (Welch & Harrison,
1966), pentobarbital (Fredricks et al., 1974), 3,4-benzpyrene (Vainio,
1974), and p-nitrophenol. The biotransformation of p-nitrophenol
involves conjugation by the microsomal enzyme uridinediphospho-
glucuronyltransferase, and its demonstration requires activation of
the microsomes, for example by trypsin (Vainio, 1974, 1975; Rantanen et
al., 1975).
In the mouse, DDT has been shown to promote the biotransformation
of pentobarbital (Gabliks & Maltby-Askari, 1970).
In the guineapig, DDT promoted the metabolism of dieldrin
(Wagstaff & Street, 1970).
DDT in the squirrel monkey promoted the metabolism of EPN and
p-nitroanisole; the first required a DDT dosage of 5.0 (mg/kg)/day, but
the latter required only 0.5 (mg/kg)/day (Cranmer et al., 1972). DDT
did not promote the metabolism of 14C-DDT in squirrel monkeys
(Chadwick et al., 1971a) but did promote the metabolism of
phenylbutazone in the dog (Welch & Harrison, 1966) and the metabolism
of estradiol in the pigeon (Peakall, 1970).
In the chicken, DDT failed to affect N-demethylase or the
concentration of cytochrome P-450, and reduced aniline hydroxylase
activity. The influences of dosage, duration of dosing species, and
reproductive state on microsomal enzymes in birds are poorly
understood (Sell et al., 1971).
o,p'-TDE has been shown to promote the metabolism of
pentobarbital in the mouse (Gabliks & Maltby-Askari, 1970),
phenobarbital in the rat (Straw et al., 1965), and cortisol in the
guineapig (Kupfer et al., 1964).
The metabolism of DDT is promoted by DDT itself in the hamster but
not in the mouse (Gingell & Wallcave, 1974).
In rats, the metabolism of DDT is promoted by phenytoin (Cranmer,
1970). The metabolism of DDT and DDE in cows is promoted by
phenobarbital (Alary et al., 1971; Fries et al., 1971).
DDE, whether fed directly or produced metabolically from DDT,
appears to be more important than DDT in inducing microsomal enzymes.
The tissue level of DDE necessary for enzyme induction is lower in the
rat than in the quail (and presumably other birds). Thus Bunyan et al.
(1972), using residues in the heart as an index, found a maximum
increase in cyto-chrome P-450 per gram of liver and a maximum activity
of aniline hydroxylase levels at DDE levels of approximately 3 mg/kg
in rats and 40 mg/kg in quail. However, at any given dietary level,
higher tissue levels were reached by quail than by rats so that the
dosage responses of the two were similar.
Different inducers may activate different enzymes and, therefore,
different metabolic pathways. Pretreatment of rats with lindane caused
them to metabolize a single dose of radioactive lindane 2.5 times more
efficiently than controls, whereas pretreatment with DDT caused a
3.5-fold increase in the metabolism of lindane. Furthermore, the DDT
pretreatment was followed by a different proportion of radioactive
metabolites with a predominance of tetrachlorophenols, especially
2,3,4,5-tetrachlorophenol (Chadwick et al., 1971b).
The enzymes of weanling rats are more subject to induction than
those of adult rats, but there is no evidence of a lag-period in
induction in adults (Chadwick et al., 1975).
7.1.4.3 Enzymes of intermediary metabolism
As discussed in section 7.1.2, there is some reason to think that
DDT acts by influencing an enzyme critical to the function of
neurones. It is certainly clear that many of the side-effects of DDT
are the result of its induction of microsomal enzymes (see sections
7.1.4.2 and 7.1.9). In addition, DDT has been shown in vitro and
sometimes in vivo to influence some enzymes of intermediary
metabolism and other miscellaneous enzymes. So far, evidence is
lacking that the degree of this inhibition in the intact organism is
sufficient to have any influence on function.
The hyperglycaemia observed during much of the early part of acute
poisoning may be associated with an increase in 4 gluconeogenic
enzymes (pyruvate carboxylase (EC 6.4.1.1), phosphoenolpyruvate
carboxykinase (EC 4.1.1.32), fructose 1,6-diphosphatase, and glucose
6-phosphatase (EC 3.1.3.9)). Increases in these enzymes in the renal
cortex of rats, observed after a single dose at a rate as low as
100 mg/kg, were greater at a dose of 600 mg/kg. The reaction occurred
in both adrenalectomized and normal rats (Kacew & Singhal, 1972).
Similar responses were observed in the same enzymes in the liver
following a single dose at the rate of 100 mg/kg or more or following
45 daily doses at rates of 5 or 25 (mg/kg)/day. The changes are not
mediated through a release of corticosteroids from the adrenal glands
(Kacew & Singhal, 1973). The same authors (Hrdina et al., 1975;
Singhal & Kacew, 1976) have reviewed the extensive evidence
contributed mainly by themselves indicating that the changes in
glucose homeostasis are mediated by stimulation of the cyclic
adenosine monophosphate (AMP)-adenylate cyclase system in the liver
and in the kidney cortex by p,p'-DDT and a number of other organic
chlorine pesticides. However, the smallest single dosage of p,p'-DDT
that produced a statistically significant change in the enzymes or in
the production of cyclic AMP was 180 mg/kg and o,p'-DDT was as
effective as the p,p'-isomer; these findings indicate that the
carbohydrate changes are results not causes of poisoning.
DDT and some other compounds induce increased activity of
D-glucuronolacetone dehydrogenase (EC 1.1.1.70) in the supernatant
fraction of rat liver, and this is consistent with evidence of
increased D-glucaric acid in urine after dog treatment (Marselos &
Hanninen, 1974). Urinary excretion of L-ascorbic acid is also
increased by DDT, but apparently this is not caused by an increase in
an enzyme producing this acid but rather by an increase in several
microsomal and cytosol enzymes that contribute to an increase in free
glucuronic acid from which L-ascorbic acid is formed (Rantanen et al.,
1975).
A review (Hayes, 1959) of early literature indicates that high
concentrations of DDT inhibit phosphatidase (EC 3.1.1.4), muscle
phosphatases, carbonic anhydrase (EC 4.2.1.1), oxalacetic carboxylase
(EC 4.1.1.3), and increase the activity of cytochrome oxidase
(EC 1.9.3.1) and succinc dehydrogenase (EC 1.3.99.1). However, none of
these changes with the possible exception of inhibition of carbonic
anhydrase could be shown to have any connexion with the toxic action
of DDT or even with its side-effects. Neal et al. (1944) reported a
small but consistent increase in the volume of urine excreted in 24 h
when dogs were dosed orally or by insufflation at the rate of 100
(mg/kg)/day. No other change in the urine and no change in kidney
function was demonstrated. The possibility that increased urinary
output is related to the inhibition of carbonic anhydrase (Torda &
Wolff, 1949) may deserve attention. However, re-examination of data
from volunteers receiving 3.5 or 35 mg/man per day did not indicate
any increase in urinary volume compared with controls (Hayes et al.,
1971).
It has been claimed (Keller, 1952) that DDT inhibits carbonic
anhydrase in bovine erthrocytes. However, the method was criticized by
Dvorchik et al. (1971), who found in vitro inhibition only by
concentrations of DDT, unlikely to be survived by living animals. Far
more attention has been given to inhibition of carbonic anhydrase in
the shell gland of birds than in erythrocytes, and it has been
suggested (Bitman et al., 1970; Peakall, 1970) that inhibition of the
shell gland enzyme is an explanation for eggshell thinning in certain
birds. However, the same criticism holds for the shell gland; there is
no evidence that the degree of inhibition reported interferes with
function.
On the other hand, many enzymes including plasma amylase, aldolase
(EC 4.1.2.13), glutamic-pyruvic transaminase (EC 2.6.1.2), and
isocitric dehydrogenase (EC 1.1.1.41) were not changed in squirrel
monkeys given dosages ranging from 0.05 to 50 (mg/kg)/day; the highest
dosage proved fatal within 14 weeks (Cranmer et al., 1972).
7.1.5 Cardiovascular system
Most dogs, killed by a single dose of DDT, die of ventricular
fibrillation, and the same is true of some cats, monkeys, and rabbits
(Philips & Gilman, 1946). At any given dosage of DDT, ventricular
fibrillation is more likely to occur if the animal receives exogenous
epinephrine or is stimulated in such a way that the compound is
released by the adrenals. Besides fibrillation, dogs may exhibit
extrasystoles and changes in the T-wave (Philips et al., 1946).
Monkeys differ from dogs in that the DDT-sensitized heart is able to
recover from fibrillation and resume a normal rhythm (Philips et al.,
1946).
Thus, DDT not only sensitizes the myocardium in a way similar to
that of halogenated hydrocarbon solvents, but, through its action on
the central nervous system, produces the stimulus that increases the
likelihood of fibrillation.
There is no evidence that repeated, tolerated doses of DDT
sensitize the heart. Rats were fed DDT at a dietary level of about
10 (mg/kg)/day for 8 months, during which time they received weekly,
intraperitoneal doses of vasopressin, a compound that causes a
temporary myocardial ischaemia. Electrocardiograms did not show any
significant increase in cardiac arrhythmias in the DDT-fed rats
compared with controls. Intravenous noradrenalin given at the end of
the 8-month period did not produce a greater incidence of arrhythmias
in the DDT-fed rats. The same results were obtained in rabbits treated
in essentially the same way (Jeyaratnam & Forshaw, 1974).
TDE. The main action of TDE, and especially of o,p'-TDE, is on
the liver with secondary effects on the adrenals in dogs and perhaps
in other species. However, Cueto (1970) showed that at a dosage of
50 (mg/kg)/day for 14 days, o,p'-TDE caused a gradually progressive
hypotensive failure in dogs injected with epinephrine or
norepinephrine, while leaving the cardioaccelerator and immediate
pressor response to these drugs unchanged. The hypotensive failure was
associated with weakening of the contractile force of the heart and
with a reduction of plasma volume. The latter may have been caused by
a loss of fluid from the intravascular compartment and was not caused
by a release of histamine. The hypotensive state could be prevented to
a significant degree by pretreatment with prednisolone.
7.1.6 Respiratory system
The effects of DDT on the respiratory system are secondary to
effects on the nervous system and are discussed in section 7.1.2.1.
7.1.7 Reproductive system
It was shown very early (Burlington & Linderman, 1950) that DDT
produces a striking inhibition of testicular growth and secondary
sexual characteristics of cockerels, when injected subcutaneously in
dosages as high as 300 (mg/kg)/day. Changes in the testis involve the
tubules, and not the interstitial tissues, and they have been
attributed to an estrogen-like action of DDT.
It must be noted that the action of DDT on the testis of the
chicken is dosage-related. Before the problem of residues became
evident, DDT was used extensively for control of lice and common mites
on chickens without any adverse effects on egg production or other
aspects of reproduction. Many rats would be killed the first day if
they were given the dosage of DDT that has been shown to affect the
testis in cockerels. The report that, under special conditions, DDT
has it gonadotoxic effect (Rybakova, 1968) is of questionable
significance in view of the results of multigeneration tests in rats,
mice, and dogs.
Ottoboni (1969) found that female rats reproduced normally when
fed DDT for two generations at dietary levels as high as 200 mg/kg
(about 10 (mg/kg)/day, except during lactation when intake was
increased about 3-fold). In fact, at a dietary level of 20 mg/kg, the
dams had a significantly longer reproductive life span (14.55 months)
than did their littermate controls (8.91 months); the number of
females becoming pregnant after the age of 17 months and the number of
successful pregnancies after that age differed significantly between
the two groups (Ottoboni, 1972).
In a study focused mainly on DDT in milk, the ability of rats to
reproduce at a dietary level of 200 mg/kg was confirmed, and the
ability of dams, injected intraperitoneally at levels as high as
100 (mg/kg)/day, to rear their young was demonstrated (Hayes, 1976b).
A six-generation test of reproduction in mice did not show any
effect of DDT at a dietary level of 25 mg/kg on fertility, gestation,
viability, lactation, and survival. A dietary level of 100 mg/kg
produced a slight reduction in lactation and survival in some
generations but not all, and the effect was not progressive. A level
of 250 mg/kg was distinctly injurious to reproduction (Keplinger et
al., 1970). The dietary concentrations used equalled dosages of 3.33,
13.3 and 33.2 (mg/kg)/day in nonpregnant, non-lactating, adult female
mice. The intake is much higher in both young and lactating mice. The
authors concluded that their study provided no obvious reason for
continuing reproduction tests for more than three generations.
Four female dogs of unstated age that had previously received DDT
at the rate of 12 (mg/kg)/day, 5 days a week, for 14 months were mated
when they went into heat. The males involved had been fed aldrin
(0.15 (mg/kg)/day) plus DDT (60(mg/kg)/day) for 14 months prior to
breeding but not during breeding. Two of the females went into heat
but failed to become pregnant, and one failed to come into heat during
12 months after feeding stopped. Four of 6 pups born to the fourth
female died within one week of birth; the other 2 were weaned
successfully even though only 2 posterior mammae of the mother were
functional (Deichmann et al., 1971b). A 3-generation study failed to
confirm any of the injuries suggested by the study of 4 dogs. In the
3-generation study, male and female dogs were fed technical DDT from
weaning at rates of 0, 1, 5, and 10 (mg/kg)/day. Observations were
made on 135 adult females, 63 adult males and 650 pups. There were no
statistically significant differences between controls and DDT-treated
dogs in length of gestation, fertility, success of pregnancy, litter
size, lactation ability of the dams, viability at birth, survival to
weaning, sex distribution, growth of pups, morbidity, mortality,
organ/body weight ratios, or gross histological abnormalities in all
the animals studied. The only clear difference was that DDT-treated
females had their first estrous 2 or 3 months earlier than the control
animals. There was a slight increase in liver/body weight ratio in
some DDT-treated animals but the difference was not statistically
significant, dosage related, or associated with any histological
change (Ottoboni et al., 1977).
o,p'-DDT. Intraperitoneal injection of technical DDT at a dosage
as low as 5 mg/kg or of o,p'-DDT at 1 mg/kg caused a significant
increase in the weight of the uterus of normal, immature female rats
or of ovariectomized adult females. Stimulation caused by p,p'-DDT
was much less. Treatment of rats with DDT, especially o,p'-DDT, 2 h
before injection of estradiol-17-6,7-3H inhibited uptake of the
hormone by the uterus in vivo, possibly by competition for binding
sites. Isomers of TDE and DDE do not influence uterine weight or the
binding of estradiol (Welch et al., 1969). It seems unlikely that
metabolic activation of o,p'-DDT is necessary, as is true of o,p'-
methoxychlor. The binding and estrogenic activity of DDT analogues in
rats is only about 1/10 000 as great as that of diethylstilbesterol
(Nelson, 1973).
A considerably smaller dosage of o,p'-DDT resulting from a
dietary level of 10 mg/kg for 2-9 months did not have any effect on
reproduction in ewes (Wrenn et al., 197 lb). In a similar way, dietary
levels of o,p'-DDT as high as 40 mg/kg, giving a dosage level of
about 2.1 (mg/kg)/day in rats, failed to interfere with reproduction
and lactation in these animals although dosage was continued
throughout 2 pregnancies (Wrenn et al., 1971a).
The report (Heinrichs et al., 1971) that o,p'-DDT significantly
advances puberty, induces persistent vaginal estrus after a period of
normal estrus cycles, and causes other reproductive abnormalities in
female rats would appear at first to be inconsistent with the lack of
effect of technical DDT or of o,p'-DDT on reproduction cited above.
The same is true of other effects of o,p'-DDT demonstrated by the
same investigators (Gellert et al., 1972). The abnormal effects were
obtained initially by injecting 1 mg of the o,p'-DDT subcutaneously
on the second, third, and fourth days of life (counting the day of
birth as zero). Because rat pups on the third day weigh about 12 g or
less each, it follows that the subcutaneous dosage was about
83.3 (mg/kg)/day or more, that is about 40 times greater than the
highest oral dosage of o,p'-isomer fed to breeding rats and about
105 times greater than the levels ingested by the general population
with their food.
Because of its estrogenic properties, DDT was considered as a
possible cause of abortion in dairy cattle, but no evidence of a
relationship was found (Macklin & Bibelin, 1971).
7.1.8 Endocrine organs
Except for the weak estrogenic properties of o,p'-DDT, the
endocrine-related effects of DDT and its analogues are confined to the
adrenals, and even these effects of 2 isomers of TDE are now
considered to be mainly secondary to the induction of microsomal
enzymes of the liver in most species.
TDE. TDE (DDD) is an insecticide in its own right as well as a
metabolite of DDT. The compound is used as a drug to control different
forms of adrenal overproduction of corticoids in man (see section
8.2.8). This therapy was originally based on the demonstration that
DDD (Nelson & Woodard, 1948, 1949) and especially o,p'-TDE (Cueto &
Brown, 1958) caused gross atrophy of the adrenals and degeneration of
the cells of its inner cortex in dogs. This is true even though it was
originally reported (Nelson & Woodard, 1948, 1949) that TDE produced
almost no detectable damage to the adrenals of rats, mice, rabbits,
and monkeys, and the finding was confirmed and extended by other
investigators to other species, including man (Zimmerman et al.,
1956). In the dog, o,p'-TDE produced gross atrophy of the adrenals,
when administered at a dosage of only 4 (mg/kg)/day. The dosage of
technical grade TDE required to produce the same effect was
50-200 (mg/kg)/day (Cueto & Brown, 1958). However, in spite of its
exceptional susceptibility, there is a definite threshold below which
the dog does not respond. About 15% of technical DDT is o,p'-isomer,
much of which is gradually metabolized to o,p'-TDE ( o,p'-DDD). Yet
dogs remained healthy and reproduced normally in a 3-generation study
involving dosages of technical DDT as high as 10 (mg/kg)/day (see
section 7.1.7).
It has recently been shown that, following massive dosage
(60 mg/kg, administered intravenously), all of the isomers of TDE
inhibited ACTH-induced steroid production in the dog, but the
inhibition reached 50% of the control only 27 min after dosing with
the m,p'-isomer compared with 87 min with the o,p'-isomer and
4-18 h with the p,p'-isomer. There was marked temporal correlation
between the percentage inhibition of adenocorticotropic hormone
(ACTH)-induced steroid production, the disruption of normal cellular
structure of the fascicular and reticular zones of the adrenal cortex,
and the severity of the damage to mitochondria in these zones caused
by the 3 isomers (Hart et al., 1973). The effectiveness of m,p'-TDE
for treating metastatic adrenocortical carcinoma had already been
demonstrated (Nichols et al., 1961), but it cannot be said that its
value for this purpose has been compared adequately with that of
o,p'-TDE. Furthermore, no effort has apparently been made to compare
the effect of small daily doses of the 2 isomers in dogs.
Like other organochlorine insecticides, o,p'-TDE stimulates
hepatic microsomal oxygenation of both drugs and steroids and,
according to a very thorough review by Kupfer (1967), this may explain
much of its action on corticoid metabolism in a wide range of species.
Increased breakdown is indicated by increased excretion of polar
metabolites, while nonpolar metabolites remain stable or even decrease
-- a finding recently encountered in human patients (Hellman et al.,
1973). However, the demonstrated effect on corticoid metabolism fails
to explain why o,p'- and m,p'-TDE are unique in their overall
effects on the adrenals, including their ability to produce
adrenocortical atrophy in the dog. Other powerful inducers of
microsomal enzymes lack these effects. It is clear that a reduction of
steroid production accompanies atrophy of the adrenals of the dog. The
review already cited (Kupfer, 1967) considers: (a) reduced steroid
production in species other than the dog, including the possibility
that such reduction is secondary to inhibition of glucose-6-phosphate-
dehydrogenase (EC 1.1.1.49) activity in the adrenals and (b) blockage
of steroid action by asteroid metabolite formed under the influence of
DDD. However, the existence of these effects, much less their
importance, remains obscure.
o,p'-DDT. Oral administration of o,p'-DDT to dogs at a rate of
50 (mg/kg)/day stimulated the microsomal enzymes of the liver as
indicated by increase in liver size, total protein, microsomal
protein, and cytochrome P-450 concentration and by direct measurements
of enzyme activity. These changes in the liver were accompanied,
initially, by an increase in the size of the adrenals and of the cells
of the zona fasciculata; these cells became vacuolated and devoid of
acidophilic cytoplasm, and their nuclei became hyperchromatic and
often peripheral in position. Synthesis of corticosteroids by the
adrenal was not blocked (Copeland & Cranmer, 1974). Thus the effect of
a substantial dosage of o,p'-DDT was quite different from that of
o,p'-TDE (DDE), although part of the metabolism of o,p'-DDT must
be by the same route.
7.1.9 Carcinogenicity
There is no doubt that DDT and a number of other organochlorine
insecticides cause marked changes in the liver in various rodents and
that these changes progress to tumour formation in some species,
notably the mouse. There is serious disagreement as to whether the
mouse tumours are malignant. Regardless of their nature, there is
virtual certainty that they are peculiar to rodents and, therefore,
interpretation of their significance for man or useful animals is
difficult.
Evidence for the carcinogenicity of DDT and its metabolites has
been reviewed by the International Agency for Research on Cancer
(IARC, 1974). Most of the experimental results are summarized in Table
17. The conclusions of the IARC were that: (a) the hepatocarcino-
genicity of DDT administered by the oral route has been demonstrated in
several strains of mice, and shows a dosage-response relationship; (b)
a dietary level of 2 mg/kg (above 0.3 (mg/kg)/day) produces a
significant increase in hepatomas in male but not in female CFl mice
and not in either sex of BALB/c mice; (c) increased incidence of
tumours has been reported in some other organs of mice but not
confirmed in multigeneration studies using a wide range of dosages;
(d) evidence for the carcinogenicity of DDT in rats is not convincing
and is negative in hamsters even at the higher dietary levels that
they tolerate in comparison with rats and mice; (e) negative results
in dogs and monkeys are inconclusive because of the small groups
studied and the short duration of treatment; (f) liver cell tumour
induction in trout is inconclusive because of a lack in control of the
diet; and (g) the carcinogenicity of p,p'-DDE is similar to that of
DDT, but TDE produces a significant incidence of lung tumours.
Actually, the number of dogs and monkeys was not small compared
with similar studies on other chemicals. In an investigation on
monkeys, dosing at lower levels was continued for 7.5 years. In both
dogs and monkeys, dosages sufficient to cause liver damage, death, and
neurological indications of DDT poisoning were included in the
protocols (see Table 17). Apparently no new studies on dogs and
monkeys have been reported. On the other hand, a new study on rats
(Rossi et al., 1977) has given definite evidence of the tumorigenicity
of DDT and phenobarbital, confirming the conclusion of Fitzhugh &
Nelson (1947) which was based on less extensive data.
In mice, liver tumours similar to those caused by DDT (see
Table 17) have been reported in connexion with DDE and DDD (Tomatis &
Turusov, 1975), chlorobenzilate, HCH, aldrin, dieldrin, mirex, and
terpene polychlorinates (Tomatis et al., 1973). Similar tumours have
been caused by the important drug, phenobarbital (Wright et al., 1972;
Peraino et al., 1973; Thorpe & Walker, 1973; Ponomarkov et al., 1976).
TDE also caused lung tumours (Tomatis et al., 1974a).
The IARC review did not discuss the controversy over the nature of
the tumours induced by DDT in the livers of some rodents, and it did
not consider the relationship of these tumours to the induction of
microsomal enzymes. The following paragraphs are concerned with these
matters and specifically with those inducers that behave like DDT. The
biochemical pattern of induction of mixed function oxidase enzymes is
similar for DDT and phenobarbital but distinctly different for
3-methylcholanthrene (Vainio, 1975). Thus 3-methylcholanthrene and
compounds like it must be excluded from the discussion. Similarly, the
high degree of correlation between the ability of compounds to induce
parenchymal liver tumours in mice and their ability to induce tumours
in the liver and other organs of rats and hamsters cannot be accepted
uncritically. As demonstrated in a study by Tomatis et al. (1973),
this correlation is extremely good for compounds that are, or are
suspected of being, carcinogens in man. However, the correlation is
poor for organochlorine insecticides. In fact, the only one of these
compounds that has increased the incidence of a tumour in another
species is DDT, which induced liver tumours in rats in one experiment.
The early morphological response of the rodent liver to DDT is
similar to its response to moderate dosages of HCH, chlordane,
dieldrin, camphechlor (Toxaphene) (Lehman, 1952; Ortega et al., 1956),
and the important drug, phenobarbital (Wright et al., 1972; Thorpe &
Walker, 1973). The earliest change involves so much increase in the
smooth endoplasmic reticulum of individual liver cells that they
enlarge, and the large granules that are ordinarily scattered
throughout the cytoplasm are displaced to the periphery of the
affected cell. Quite early, some of the endoplasmic reticulum forms
whorls that may have fat droplets as their centres -- thus justifying
the term "lipospheres", applied to them by Ortega et al. (1956).
Others have referred to these inclusions as "hyaline oxyphil masses"
(Lillie & Smith, 1944) or "lamellar bodies" (Ito et al., 1973). These
changes are accompanied by some increase in fat droplets, not all of
which become surrounded by endoplasmic reticulum. This combination of
changes (hypertrophy, margination, and lipospheres) is characteristic
of rodents and of compounds that induce microsomal enzymes. Certain
other changes have been reported but not confirmed. These include
enlargement and morphological changes of the mitochondria (Obuchowska
& Pawlowska-Tochman, 1973; Watari, 1973), increased numbers of primary
lysosomes, and atrophy of the Golgi body (Watari, 1973), none of which
were found by Ortega (1966).
Table 17. Effect on various animals of repeated oral doses of DDT
Dosage Species Duration Results Reference
Range Method and Animals Mortality Other
(mg/kg)/day concentration per test (%)
(No.)
41-80 800 mg/kg in diet rata 2 years 36 males, 24 females Increased mortality, Fitzhugh & Nelson, 1947
typical liver changes,
liver tumours
46 mg/kg then mouseb 18 months 36 males, 36 females Hepatomas in 51 and Innes et al., 1969
140 mg/kg in diet 21% of males and females
respectively compared with
18 and 0.6% of controls
3200 mg/kg in diet dog 39-49 10 100 Liver damage, no Lehman, 1965
months tumours
5000 mg/kg in diet monkey 70 days 1 male 100 Fatal poisoning Durham et al., 1963
21-40 400 mg/kg in diet rata 2 years 24 males, 12 females Increased mortality, Fitzhugh & Nelson, 1947
typical liver changes
500 mg/kg in diet rat 2.9 years 37 males, 35 females Liver tumours in 45% Rossi et al., 1977
250 mg/kg in diet mousec 2 103 males, 90 females Risk of liver tumour Tomatis et al., 1972
generations increased 3.7 and 18.5
times in males and females,
respectively
250 mg/kg in diet mousec 2 31 males, 121 females Liver tumours in 48 Terracini et al., 1973
generations and 59% of males and
females, respectively
2000 mg/kg in diet dog 39-49 4 25 Minor liver damage Lehman, 1965
months but no tumours
Table 17 (Cont'd)
Dosage Species Duration Results Reference
Range Method and Animals Mortality Other
(mg/kg)/day concentration per test (%)
(No.)
11-20 100 mg/kg in diet moused 2 years 100 males, 100 females Hepatomas increased Fitzhugh, 1970
in females of one strain
but no increase in
hepatocarcinomas
100 mg/kg in diet mousec 2 years 30 males, 30 females Risk of liver tumours Walker et al., 1973
increased 4.4 times
100 mg/kg in diet mousec 2 years 30 males, 3 females Risk of liver tumours Thorpe & Walker, 1973
increased 3.3 and 4.2 times
in males and females
respectively
5-10 50 mg/kg in diet mousec 2 127 males, 104 females Risk of liver tumours Tomatis et al., 1972
generations increased 2.45 and 3.46
times in males and females,
respectively
50 mg/kg in diet mousec 2 years 30 males, 30 females Risk of liver tumours Walker et al., 1973
increased 2.9 times
400 mg/kg in diet dog 39-49 2 0 No effect Lehman, 1965
months
Table 17 (Cont'd)
Dosage Species Duration Results Reference
Range Method and Animals Mortality Other
(mg/kg)/day concentration per test (%)
(No.)
2.6-5 20 mg/kg in diet mousee 2 48 males, 128 females No increase in tumours Terracini et al., 1973
generations
200 mg/kg in diet monkey 3-7.5 5 males, 5 females No toxic effect Durham et al., 1963
years
1.26-2.5 10 mg/kg in diet mousec 2 104 males, 124 females Risk of liver tumour Tomatis et al., 1972
generations increased 2.26 and 2.46
times in males and females,
respectively
0.626-1.25 25 mg/kg in diet rat 2 years No clinical effect; Treon & Cleveland, 1955
males survived
longer than controls
50 mg/kg in diet monkey 1.6 years 4 males, 1 female No toxic effect Durham et al., 1963
0.3126- 10 mg/kg in diet rat 2 years Typical liver changes; Fitzhugh, 1948
0.625 no effect on
reproduction
12.5 mg/kg in diet rat 2 years No effect Treon & Cleveland, 1955
2.8-3.0 mg/kg in mousee 5 683 Tumours in 28.7% in Tarjan & Kemeny, 1969
diet generations controlsf
0.15626- 2 mg/kg in diet mousee 2 124 males, 111 females Risk of liver tumour Tomatis et al., 1972
0.3125 generations doubled in males,
unchanged in females
2 mg/kg in diet mousee 2 58 males, 135 females No increase in tumours Terracini et al., 1973
generations
Table 17 (Cont'd)
Dosage Species Duration Results Reference
Range Method and Animals Mortality Other
(mg/kg)/day concentration per test (%)
(No.)
0.078126- 2.5 mg/kg in diet rat 2 years No effect Treon & Cleveland, 1955
0.15625 5 mg/kg in diet monkey 1.4-7.5 5 males No toxic effect Durham et al., 1963
years
a Osborne-Mendel. e BALB/c mice.
b Both (C57BL/6 × C3H/Anf) FI and (C57BL/6 × AKR) FI mice. f Lung carcinoma in 16.9% to 1.2% in controls; lymphomas 4.8% compared to
c CFI mice. 1.0% in controls; leukaemias 12.4% and 2.6%; other tumours 5.8% and 1.0%,
d BALB/cJ and C3HeB/Fe J. respectively.
The characteristic changes develop promptly. An increase in smooth
endoplasmic reticulum and the appearance of lamellar structures have
been seen as early as 4 and 7 days, respectively, after the beginning
of dosing (Wright et al., 1972).
Although microsomal enzymes may be induced in other species,
morphological changes in the liver, as viewed by the light microscope
are not the same (Laug et al., 1950; Lehman, 1952; Ortega et al.,
1956), or occur to lesser degree as viewed by the electron microscope
(Wright et al., 1972).
The changes in liver cells that characterize the induction of
microsomal enzymes in rodents are distinct from the focal necrosis
that may be produced with about the same ease in the livers of rodents
or other species by fatal or near fatal dosages of organochlorine
insecticides. The necrotic lesions do not progress because, if such
high dosages are continued the animal dies, and, if dosing is stopped
and the animal survives, the necrotic cells are removed by autolysis
and phagocytic action and the lesions usually heal without scarring
(Cameron & Burgess, 1945). Nevertheless, some scarring was found by
Lillie & Smith (1944) and Lillie et al. (1947).
At least in the early stages, the changes in liver cells that
characterize induction of microsomal enzymes in rodents are reversible
(Fitzhugh & Nelson, 1947; Ortega et al., 1956; Wright et al., 1972).
The reversibility does not depend on cell removal but simply on
reversion of the physiological and morphological condition of the
cells to their original condition.
Of course, reversibility is incompatible with progression, but
whether observed irreversibility will be associated with progression
must be determined directly in each instance. In the following
paragraphs, the question of progression is discussed only after
consideration of the problem of irreversibility in general.
If dosing with organochlorine insecticides or other inducers is
continued long enough and at a sufficiently high level, the liver
changes become irreversible, if for no other reason than that the
remaining life span of the animals is too short to permit excretion of
the inducing chemical or complete reversion of the liver cells to
their original state. The stage at which this shift to irreversibility
occurs remains unknown, but it seems very likely that dosages
sufficient to produce irreversible morphological change also exceed
the physiological adaptability of the liver. The important distinction
between adaptation and injury in relation to enzyme induction has been
studied using dieldrin.
Although some persons have tended to view even moderate
enlargement of the liver or of individual liver cells as an injury,
the evidence is strong that these changes are usually adaptive and
beneficial to the organism, when they are the result of an increase of
smooth endoplasmic reticulum and an associated increase in the
activity of liver microsomal enzymes (Barka & Popper, 1967). However,
it is obvious that any stimulus or effect may be harmful, if
excessive. Hutterer et al. (1968) demonstrated that a distinction may
be drawn between adaptation to dieldrin and decompensation resulting
from excessive doses of it. Some of these authors, including Popper,
showed that the same distinction could be drawn in connexion with
other sources of potential liver injury.
It was found that daily intraperitoneal administration of dieldrin
to rats at the rate of 2 mg/kg produced enlargement of the liver,
hypertrophy of the smooth endoplasmic reticulum, increases in
microsomal protein and P-450 haemoprotein, and associated increases in
the activity of microsomal enzymes; however, normal activity of other
enzymes not derived from the microsomes was maintained. The activity
of microsomal enzymes per mole of available P-450 haemoprotein
remained unchanged. The highest level of activity of the processing
enzymes was reached after 14 days, after which the new steady state
was maintained. Rats that had received dieldrin at a rate of 2 mg/kg
per day for 28 days were more tolerant to dieldrin than normal rats,
as shown by the fact that they survived 25 consecutive daily doses at
the rate of 5 mg/kg, a dosage that produced 70% mortality in
previously untreated rats. In spite of the ability of the rats,
pretreated with a moderate dosage of dieldrin, to survive a large
dosage, their livers showed definite indications of decompensation in
response to the high dosage. Although the smooth endoplasmic reticulum
remained hypertrophic and the microsomal protein and P-450
haemoprotein concentrations remained elevated, the enzyme activities
per mole of available P-450 haemoprotein decreased, as did the
activity of some enzymes not associated with microsomes. Much of the
excess smooth endoplasmic reticulum formed tightly packed clusters of
tubular membranes with no glycogen and little hyaloplasm, and some of
the mitochondrial membranes were injured. It was suggested that the
phase of decompensation represented by hypertrophic but hypoactive
smooth endoplasmic reticulum might serve as a sensitive criterion of
toxic injury before microscopic changes of a clearly harmful sort
become recognizable (Hutterer et al., 1968).
Other studies indicating not only the presence of adaptive change
over a range of dosages but also the failure of adaptation and onset
of injury at sufficiently high dosage levels have been reported for
butylated hydroxy-toluene (Gilbert & Golberg, 1967) and for DDT
(Hoffman et al., 1970). Hoffman and his colleagues found that, when
DDT was fed to male weanling rats for only 14 days at dietary
concentrations of 0.5 to 2048 mg/kg, concentrations of 0.5 and 2 mg/kg
had no effect on the O-demethylation reaction used as a test, but
concentrations of 4-750 mg/kg produced increases in the rate of
metabolism, proportional to the log of dosage. Extrapolation of this
portion of the dosage response curve to the abscissa provided a
calculated no-effect level of 3.27 mg/kg equivalent to about
0.327 (mg/kg)/day. This is in reasonable agreement with other
estimates of the threshold for induction of various enzymes in the
rat, including some studies involving longer administration of DDT.
These estimates, expressed as (mg/kg)/day, are approximately 0.05
(Kinoshita et al., 1966; Street et al., 1969), 0.5 (Schwabe &
Wendling, 1967) and 0.125 (Gillett, 1968). All of the estimates are of
the same order of magnitude as the 0.25 (mg/kg)/day known to be
effective in man (Laws et al., 1967; Poland et al., 1970), but all are
over 100 times greater than the highest dosage received by members of
the general population during the late 1960s (Duggan, 1968).
Increasing the dietary level to more than 750 mg/kg did not produce
any further increase in enzyme activity. Intake of less than 128 mg/kg
did not produce any increase in liver weight, within the period of
observation; increase was proportional to dosage within the range of
128 to 512 mg/kg and was submaximal at intakes above 512 mg/kg.
Some other compounds, notably phenobarbital, produced
morphological changes in the liver similar to those produced by some
organochlorine insecticides (Wright et al., 1972; Thorpe & Walker,
1973). It seems possible that sufficiently high doses of
phenobarbital, for example, may lead to a failure of adaptation and to
levels of enzyme activity that do not correspond to dosage.
As indicated above, the earliest morphological changes caused by
enzyme inducers in the rodent liver involve separate cells in the
centrolobular area. If the dosage is sufficiently high and prolonged,
nodules consisting entirely of hypertrophied cells may appear. At
first, these microscopic nodules are distinguishable only by pattern;
they have no bounding membrane and they do not compress or change in
any other detectable way the smaller liver cells that surround them.
Some nodules may become large enough to be seen without a microscope,
and a few may exceed 1 cm in diameter. In these large nodules, there
is almost complete loss of lobular architecture. Nodules apparently
were first described by Fitzhugh & Nelson (1947) who felt that they
could be regarded as adenomas or as low grade hepatic cell carcinomas.
The use of the second term is not clear because neither mitoses,
tissue invasion, nor metastasis was observed. Although Ortega et al.
(1956) reported small nodules in the livers of treated rats, and
although they examined tissue loaned by Fitzhugh & Nelson's
laboratory, they were entirely unimpressed by the lesions, referring
to them as "focal incongruities".
Almost 3 decades after the first study, there is no more agreement
than is reflected in the preceding paragraph. The views of some
pathologists remain diametrically opposed. This is true even though
the finding of: (a) pulmonary metastases of hepatic cells in mice that
had received DDT (Tomatis et al., 1972; Walker et al., 1973), ß-HCH,
gamma-HCH, dieldrin, or phenobarbital (Thorpe & Walker, 1973); or (b)
progression of liver enlargement beginning 12 weeks after cessation of
ingestion of alpha-HCH by mice for 24 or 36 weeks (Nagasaki et al.,
1974) or progressive increase in the size of liver nodules after DDT
feeding was stopped (Tomatis et al., 1974b; Tomatis & Turusov, 1975);
or even (c) in HCH-exposed mice the time-pattern of increase in liver
weight (as reflected by body weight), which gained momentum only after
a delay of 4 weeks but showed a further acceleration in the thirteenth
week, in spite of decreased food consumption (Tomii et al., 1972),
would appear to establish, without question, that at least some of the
liver changes produced by these compounds in rodents are malignant.
Of course, the reason for disagreement is that the tumours
produced by DDT, other organochlorine insecticides, and phenobarbital
differ in their biochemistry and are not malignant in the classical
sense. Specifically, (a) they do not actively invade tissues; (b)
their "metastases" do not grow; (c) they produce little shortening of
life span; and (d) mice receiving 5.5 (mg/kg)/day as a result of
dietary intake of DDT show a decrease in the success of
transplantation and a significant increase in survival in mice in
which tumours grew following inoculation with an otherwise uniformly
transplantable and uniformly fatal ependymoma (Laws, 1971).
Although the displacement of liver cells to the lung occasionally
seen after prolonged dosage with DDT is usually referred to as
metastasis, it might better be called embolism because the lesion does
not progress and, therefore, lacks the clinical significance of a real
metastasis. Because it does not grow, the lesion is hard to find. A
number of investigators have failed to mention liver cells in the
spleen, lymph nodes, or lungs, and some have stated specifically that
they were not found (Nagasaki, 1973; Rossi et al., 1977).
Perhaps the most illuminating study of the liver changes caused by
DDT is that of Kuwabara & Takayama (1974). They used fluorinyl
acetamide (2,7-FAA) as a positive control in their studies of DDT and
HCH. The 3 compounds were given at dietary concentrations of 250, 250,
and 600 mg/kg, respectively. The lesion caused by 2,7-FAA differed
from those caused by either of the other compounds in 3 ways: (a) it
started as hyper-plastic nodules rather than as isolated cell changes;
(b) the final lesion was hepatocellular carcinoma in contrast with the
adenoma caused by DDT or HCH; and (c) alpha-fetoprotein was formed,
which did not occur with DDT or HCH. Other workers have also failed to
find alpha-fetoprotein in mice treated with an organochlorine
insecticide (Hanada et al., 1973).
It must be emphasized that the organochlorine insecticides and
phenobarbital do not produce, in other animals, the early, visible
changes in the endoplasmic reticulum that are so characteristic of
some rodents and that progress to tumour formation in rodents. That
these compounds do not lead to tumour formation in other animals might
have been predicted by the fact that they do not cause the early
changes, characterized by hypertrophy, margination, and lipospheres.
All available evidence indicates that man does not appear to be
susceptible to the tumorigenic action of the organochlorine
insecticides and phenobarbital. No increase in the occurrence of
tumours has been found in heavily-exposed populations. This includes
groups of workers who manufacture and formulate DDT and dieldrin and
who have been examined carefully for tumours (Laws et al., 1967;
Jager, 1970).
Finally, a study based on a complete tumour registry did not
indicate any increase of tumours attributable to phenobarbital among
men and women who received heavy, essentially lifelong dosing with
this drug for the control of epilepsy (Clemmesen et al., 1974).
In summary, in spite of disagreement about the interpretation of
the liver cell changes, there is general agreement about their
development and appearance. The change that can be detected first and
can be produced by the smallest effective dosage involves the
endoplasmic reticulum. The initial change is reversible, but, even
more important, it is peculiar to rodents. There is no evidence that
anything from the first increase in endoplasmic reticulum to the final
development of a highly nodular liver with occasional displacement of
cells to the lung has any bearing on the health of man or other
animals in which the endoplasmic reticulum does not respond in this
way.
7.1.10 Mutagenicity
DDT has been tested in a number of ways for possible mutational
effects. Shirasu et al. (1976) listed DDT as a negative chemical in
microbial mutagenicity screening studies on 166 pesticides. The test
system consisted of rec-assay using H 17 Rec+ and M 45 Rec-strains
of Bacillus subtilis and reversion assays without metabolic
activation using auzotrophic strains of Escherichia coli (WP 2) and
Salmonella typhimurium (Ames series). Further studies by the same
authors, with metabolic activation, failed to reveal mutagenicity of
DDT (Shirasu et al., 1977). McCann et al. (1975) and McCann & Ames
(1976), reported negative results on DDE in Salmonella typhimurium
testing with metabolic activation.
At a dosage of 105 mg/kg, DDT did not produce any increase in
dominant lethals in mice (Epstein & Shafner, 1968). Concentrations of
10 mg/kg or higher produced chromosome breaks and exchange figures in
a marsupial somatic cell line (Palmer et al., 1972). Saturated
solutions produced chromosome breaks in the root tips of onion and
other plants (Vaarama, 1947). A slight mutagenic effect in mammals has
been reported by Markarian (1966). Deletions plus gaps were reported
to be more common in the chromosomes of mice that had received DDT.
An unconventional test for mutagenicity involved examination of
explants of pulmonary tissue from embryonic mice whose dams had been
fed dietary concentrations of DDT of 10 and 50 mg/kg. An increase in
diffuse hyperplasia and focal proliferation was observed, but a
dosage-response relationship was not clear. Some of the embryos were
allowed to live and the experiment was repeated in subsequent
generations. There was no continuing progression of the reported
changes in succeeding generations (Sabad et al., 1972).
7.1.11 Teratogenicity
When p,p'-DDT was administered to pregnant mice at a rate of
1 mg/kg on days 10, 12, and 17 of gestation, it was not teratogenic
but did alter the gonads and decrease the fertility of the young,
especially the females (McLachlan & Dixon, 1972). A single dose at the
rate of 25 mg/kg or repeated doses of 2.5 (mg/kg) day given during
pregnancy may be embryotoxic but not teratogenic to mice (Schmidt,
1973). The reason why one or a few doses during pregnancy may be
embryotoxic although the same dosage is harmless, when administered
during the entire reproductive period, is of theoretical but not
practical importance.
Teratogenic effects of DDT have not been seen in studies of
reproduction including those for 2 generations in rats, 6 generations
in mice, and 3 generations in dogs (see section 7.1.7).
7.2 Acquisition of Tolerance to DDT
Because DDT stimulates microsomal, mixed-function enzymes and the
action of these enzymes on DDT is one of detoxication, it might be
expected that some tolerance might develop. Such tolerance has been
demonstrated in the case of dieldrin; rats that received this compound
for 28 days at a rate of 2 mg/kg survived 25 additional days at a rate
of 5 mg/kg, a dosage that killed 70% of previously untreated rats
(Hutterer et al., 1968). Apparently the possibility of tolerance to
DDT has not been explored.
7.3 Factors Influencing DDT Toxicity
7.3.1 Dosage effect
7.3.1.1 Dosage-effect of DDT
Table 5 summarizes the acute oral and dermal toxicity of DDT in
common laboratory animals, and Table 18 summarizes the subcutaneous
intravenous and intraperitoneal toxicity. Both tables are condensed
from an earlier review (Hayes, 1959) that gives references and
additional details. It may be concluded that dissolved DDT is absorbed
through all portals. Absorption of DDT powder through the skin is
negligible. It is frequently impossible to put enough DDT dust on the
skin of animals to kill them, so that an LD50 value for this
formulation cannot be determined by the dermal route. Although
formulation is important in determining the toxicity of DDT by other
routes, the difference is not so great as it is in connexion with skin
exposure. DDT is about 4 times more toxic when given intravenously
than when given orally, and about 40 times more toxic when given
intravenously than when given dermally.
Table 18. Acute subcutaneous, intravenous, and intraperitoneal LD50 of DDT
in common laboratory animalsa
Species Formulation Subcutaneous Intravenous Intraperitoneal
(mg/kg) (mg/kg) (mg/kg)
Rat Water suspension or powder >2000
Oil solution 200-1500 47 80-200
Mouse Water suspension or powder 1000-1500
Oil solution 300
Guineapig Water suspension or powder
Oil solution 900 150
Rabbit Water suspension or powder
Oil solution 250->3200 30-41 <2100
Cat Water suspension or powder
Oil solution <650 32
Dog Water suspension or powder
Oil solution 68
Monkey Water suspension or powder
Oil solution 55
a From: Hayes (1959).
In general, DDT appears to be more toxic as a solution in
vegetable oil or animal fat than when given in some petroleum
fraction. Petroleum may act as a laxative. The heavier fractions are
never absorbed and DDT dissolved in such fractions has to be extracted
from the solvent in order to show toxicity.
In summary, DDT is a compound of moderately acute toxicity.
Compared with other organochlorine insecticides of equal or greater
toxicity, it is remarkable in being only slightly absorbed by the
skin.
The effects of repeated doses of DDT are summarized in Table 17.
The 90-dose oral LD50 of technical DDT in rats is
46.0 (mg/kg)/day (Gaines, 1969). The chronicity index is 5.4. Thus the
compound has only a moderate tendency to cause cumulative effects, and
this limited tendency is fully explained by the accumulation of DDT
itself in tissues as a result of continuing intake. In fact, this
accumulations, which is strictly dosage dependent, is detectable at
all measurable levels of intake. The relationship in man is shown in
Fig. 4 (p. 138).
If storage is considered undesirable per se, then DDT is without
a no-injurious-effect level. However, the same may be said for all
compounds that are absorbed, for the presence of all of them in the
bodies of exposed organisms -- perhaps at very low levels -- may be
assumed; failure to demonstrate low levels of storage does not depend
on physiology but only on the limitations of analytical chemistry and
on the lack of persistence of chemists.
A number of papers have reported no-effect levels for DDT within
the variables investigated, namely: rat, 0.05 mg/kg (Lehman, 1965);
dog, 8 mg/kg (Lehman, 1965); and monkey, 2.2-5.54 mg/kg (Durham et
al., 1963).
There remain reports of effects in animals at the lowest dosages
investigated. For example, decreased serum albumin and increased ß-
and gamma-globulins in the blood of rats and rabbits maintained on a
dosage of 0.2 (mg/kg)/day for 3-11 months was reported by Kagan et al.
(1969).
In studies carried out in rats and dogs, toxicity, as measured by
the maximum no-effect level, was seldom very different from the
corresponding value resulting from 90 days of exposure at the same
dosage range. The largest factor of difference observed when 33
chemicals were investigated in rats was 12 (20 for minimal effect
level), and for half of them the factor was 2 or less. In 21,
rat-to-dog comparisons of long-term toxicity, in no instance was the
dog more sensitive than the rat (Weil & McCollister, 1963). Although
LD50 studies offer a poor basis for predicting long-term toxicity,
the lowest dosage that will produce a minimum effect, when administered
for the lifetime of rats, can be predicted with reasonable success
from a test lasting only 7 days and with good success from a 90-day
study (Weil et al., 1969). These results offer some perspective for
judging the ultimate effect of exposure to compounds that have been
commercially available for less than one human lifetime.
Summary of long-term toxicity studies. The lowest dosages that
have been studied in animals are of the same order of magnitude as
those encountered by men who make or formulate DDT and, therefore,
hundreds of times greater than the dosages encountered by the general
population. The animal studies have been continued long after a steady
state of storage has been achieved. From the results it can be
concluded that bioaccumulation sufficient to produce neurotoxicity or
other clinical effects, including a reduction of the life span, can
occur only at dosage levels substantially higher than those
encountered by the most heavily exposed workers. DDT dosages
encountered by workers produced a small but detectable increase in
liver changes (hypertrophy, margination, and liposphere formation) in
some groups of mice and rats. The same changes occurred in low
incidence in control mice and rats but not in other animals (see
section 7.1.9).
7.3.1.2 Dosage-effect of metabolites and o,p'-DDT
Acute oral LD50 values of DDT metabolites commonly found in
tissues of excreta are shown in Table 19. Readily absorbable
formulations of the metabolites are less toxic than the most
absorbable preparations of the parent compounds (see for example
Table 5).
Table 19. Oral LD50 values of metabolites of DDT
Compound Species LD50 Reference
(mg/kg)
DDE rat, male 880 Gaines, 1960
DDE rat, female 1240 Gaines, 1960
ODE mouse 700 von Oettingen & Sharpless, 1946
DDE mouse 1000 Domenjoz, 1946a,b
TDE(DDD) rat, male >4000 Gaines, 1969
DDA rat 1900 Smith et al., 1946
DDA rat, male 740 Gaines, 1960
DDA rat, female 600 Gaines, 1960
DDA mouse 720 von Oettingen & Sharpless, 1946
DDA mouse 590 Domenjoz, 1946a,b
DDA. Rats tolerate higher tissue levels of DDA than of DDT.
Eighteen hours after intravenous injection of DDA at a rate of
100 mg/kg, tissue levels were still higher than those usually found in
animals, fatally poisoned by DDT (Judah, 1949).
DDA produces less injury to the liver than DDT but produces
greater damage to the kidney especially at high intravenous dosages
(Lillie et al., 1947). This is consistent with the finding of Spicer
et al. (1947) that, following administration of DDT, DDA constitutes a
higher proportion of DDT-related compounds in the kidney (25%) than in
any other tissue, e.g., 12% in the liver, 10% in the brain, and even
less in other tissues.
o,p'-DDT. At an oral dosage of 150 mg/kg, p,p'-DDT produces
severe illness in all rats and kills about half of them, but o,p'-DDT
at the same dosage does not produce illness even though the
concentrations of the 2 compounds in the brain at various intervals
after dosing are about the same. At a dosage of 3000 mg/kg, o,p'-DDT
produces mild to moderate illness, and the concentration in the brain
is 5-9 times the concentration of p,p'-DDT necessary to produce
similar symptoms. Thus, p,p'-DDT appears to be inherently more toxic
than the o,p'-isomer (Dale et al., 1966a).
7.3.2 Age and sex
Young animals eat more than adults in relationship to their body
weight. For this and other reasons, they are often more susceptible
than adults to poison in food. However, young animals are inherently
less susceptible to certain compounds. There is no evidence that DDT
is more toxic to the young than to the adults of any species,
including man. In the rat, the young are less susceptible than adults
to a single dose and about equally susceptible to repeated doses as
shown in Table 20. According to Henderson & Woolley (1969), the
relative insusceptibility of the young is associated with relatively
poor absorption of DDT by the central nervous system and by the less
inherent susceptibility of the young brain to DDT already absorbed by
it. Further studies by the same authors (Henderson & Woolley, 1970)
showed that fatal poisoning of both 10- and 60-day-old rats involved
hyperexcitability and intense tremor followed by prostration and
eventual respiratory failure. However, in the adult rat, DDT caused
convulsions, an increase in respiration and heart rate, and a lethal
increase in body temperature (40-42°C) prior to death, but the body
temperature of the immature rat decreased during acute intoxication by
DDT. The authors suggested that, whereas DDT is a direct depressant of
respiration in both young and old rats, the additional toxic responses
manifested by seizures and hyperthermia accounted for the increased
lethality of DDT in mature animals.
There is virtually no sex difference in the acute toxicity of DDT
to rats; the LD50 was 113 and 118 in males and females, respectively
(Gaines, 1960). When DDT is fed to rats at ordinary dietary levels,
the 2 sexes store it equally. However, at higher dosages, females
store more of the compound; the difference is explained mainly by the
lower activity of the liver microsomal enzymes in female rats and,
only in part, by the relatively higher food intake of the females.
Table 20. Effect of age on the toxicity of DDT to rats
Number Age LD50 Reference
of doses (mg/kg)a
1 newborn 4000 Lu et al.. 1965
1 newborn 2356 Harrison, 1975
1 10 days 728 Henderson & Woolley, 1969
1 14-16 days 437.8 Lu et al., 1965
1 weanling 355.2 Lu et al,, 1965
1 2 months 250 Henderson & Woolley, 1969
1 3-4 months 194.5 Lu et al., 1965
1 middle-aged 235.8 Lu et al,, 1965
1 adult 225 Harrison, 1975
4 preweaning 279.2 Lu et al., 1965
4 adult 285.6 Lu et al., 1965
a Total intake one or more doses.
7.3.3 Nutrition
Fat. Nutrition influences the toxicity of DDT in connexion with
both fat and protein. Fatness influences the amount that can be stored
inactively in the body, and it is of importance in mitigating acute
poisoning. This action of fatness or "good condition" has been noted
in connexion with mammals (Spicer et al., 1947) and fish (Hoffmann &
Surber, 1948). In contrast, laboratory animals are slightly more
susceptible to repeated large doses administered as part of a diet
containing a moderate proportion of fat (5% or more) than as part of a
very low fat diet (0.5%) (Sauberlich & Baumann, 1947). This difference
is thought to be associated with absorption from the gastrointestinal
tract.
Rats that have stored large amounts of DDT in the fat may suffer
tremors, if starvation or some other cause leads to a mobilization of
their fat (Fitzhugh & Nelson, 1947). If DDT intake is stopped when
starvation begins, and, if the concentration of DDT is measured only
once following the interval of starvation, the results may be erratic,
reflecting, to a greater or lesser degree, both mobilization of fat
and excretion of metabolites as in studies reported by Deichmann et
al. (1972).
However, in nature, starvation is more often partial than
complete, and, if the original diet contains enough DDT to cause
substantial storage, whatever food may be found in a period of
scarcity is also likely to be contaminated. The initial effect of the
mobilization of fat is to increase the concentration of DDT in the
remaining fat and in other tissues. Excretion is increased in response
to the increased tissue levels but may not be fast enough to prevent
the accumulation of a toxic concentration in the brain. If intake of
DDT is stopped, the increased rate of excretion eventually leads to
reduced storage (Dale et al., 1962). These findings in rats have been
confirmed, in regard to both the initial increase in the concentration
of DDT (Dedek & Schmidt, 1972; Stenberg & Diky, 1973) and the later
reduction (Brodeur & Lambert, 1973). Similar findings have been
reported in birds (Adamczyk, 1971).
The effect of fat mobilization on the toxicity of DDT is the same
whether it is caused by withholding food or by disease that causes
partial refusal of food (Hayes, 1975).
It is highly unlikely that poisoning by DDT will be precipitated
in man by starvation because: (a) very few subjects store the compound
in concentrations as high as those required to demonstrate the
phenomenon in rats; and (b) the metabolic rate in man is so much
slower than that in rats that elimination of DDT in man would
counterbalance that produced by its mobilization.
Lipids. The association of lipids with the function of
microsomal enzymes is generally recognized as is the fact that DDT
induces these enzymes. Therefore, it might have been expected that DDT
and essential fatty acids would interact. Tinsley & Lowry (1972) found
that the growth of female rats receiving p,p'-DDT at a dietary level
of 150 mg/kg was depressed, if they received a diet deficient in
essential fatty acids, but was slightly stimulated, if they received
the same diet supplemented with these acids. Another variable
influenced by the same factors was the ratio of various liver lipids.
The changes in fatty acid composition were related to the
proliferation of hepatic smooth and endoplasmic reticulum; it was
suggested that DDT influenced essential fatty acid metabolism by
increasing the demand for them.
In contrast, a variety of diets (containing fats that may occur in
the human diet and that were in approximately the same proportion as
fats in the typical human food in the USA) had little or no influence
on the storage of DDT and a wide range of pesticides fed to rats for 4
generations in a combination of rates 200 times those found in the
Market Basket Study of food in the USA (Adams et al., 1974).
Ascorbic acid. In squirrel monkeys (and presumably in other
species) only 2 days on an ascorbic acid-deficient diet impaired both
the induction of O-demethylase and the stimulation of the glucuronic
acid system by DDT (5 mg/monkey/day) (Chadwick et al., 1971a). In
guineapigs, maintenance of induction of microsomal enzymes required a
higher dietary level of ascorbic acid than prevention of scurvy
(Wagstaff, 1971).
Protein. Smith & Stohlman (1945) found only slightly greater
mortality and liver pathology in rats fed DDT at 500 mg/kg in a diet
containing protein at 80 g/kg than in one containing 280 g/kg. The
finding that low dietary protein predisposes to DDT poisoning has been
confirmed (Sauberlich & Baumann, 1947; Boyd & DeCastro, 1968, 1970;
Boyd & Krijnen, 1969); however, even zero protein intake increased
toxicity only 4-fold, the smallest factor observed in comparable
studies of 9 pesticides. The effect of protein deficiency on toxicity
may involve a crippling of the microsomal enzymes of the liver or it
may act synergistically with compounds that cause anorexia. Rats fed a
diet containing casein at 810 g/kg exhibited evidence of renal
overload and were more susceptible to DDT (Boyd & DeCastro, 1970).
7.3.4 Species
The acute toxicity and metabolism of DDT were studied in mice and
hamsters because of the marked difference in their susceptibility to
liver tumours induced by DDT. The central nervous systems of the 2
species are equally sensitive, the concentration of DDT in their
brains at death being similar. However, after an oral dosage of
500 mg/kg, the DDT concentration in the mouse brain was twice that in
the hamster. This cannot be explained by a difference in absorption,
metabolism, or excretion but apparently is due to a difference, in the
permeability of the blood/brain barrier in the 2 species. When animals
receive DDT at a dietary level of 250 mg/kg for 6 weeks, the residues
in fat and liver were 7/8 times higher in the mouse, a fact only
partially explained by the greater food intake of the mouse relative
to body weight. Although urinary excretion of 14C-DDT was similar in
previously unexposed hamsters and mice, this excretion was stimulated
in the hamster but little affected in the mouse by previous dietary
exposure to DDT (Gingell & Wallcave, 1974).
Mice also differ from rats in the hormonal regulation of the basic
activity of hepatic microsomal mixed-function enzymes as well as in
the response of these enzymes to inducers (Chhabra & Fouts, 1974).
7.3.5 Other factors
A number of other factors are known to influence the toxicity of
some compounds, and the degree of difference may be very great in
isolated instances. Factors that have been reviewed elsewhere (Hayes,
1975) include (in addition to those listed above) interaction of
compounds, strain, individual differences, isolation and crowding,
other social and psychological factors, temperature, pressure and
altitude, light and other radiation, circadian and other rhythms,
seasonal differences, and relative humidity. None of these additional
factors is known to have an important effect on the survival of
animals receiving DDT. The possibility of the interaction of DDT with
aldrin, pyrethrin, piperonyl butoxide, malathion, dichloropheno-
xyacetic acid (2,4-D), and a number of food additives has been
explored systematically without finding anything but simple additive
results (Fitzhugh, 1966). However, pyrethrins, especially synergized
pyrethrins, have an additive and perhaps synergistic effect on the
changes in liver morphology associated with repeated doses of DDT in
rats (Kimbrough et al., 1968).
7.4 Human Studies
Oral exposure. Table 21 summarizes the effects of one or a few
carefully measured oral doses of DDT. The results are consistent with
those in accidents reported by Garrett (1947) and Hsieh (1954) in
which it was possible to estimate accurately the amount ingested. It
may be concluded that a single dose at the rate of 10 mg/kg produced
illness in some but not all subjects even though no vomiting occurred.
In general, smaller doses did not produce illness, although a dosage
of 6 mg/kg produced perspiration, headache, and nausea in a man who
was sickly and who was hungry at the time of eating. Persons who were
made sick by 10 mg/kg did not have convulsions, but convulsions
occurred frequently when the dosage level was 16 mg/kg or greater
(Hsieh, 1954). Rarely, a dosage as high as 20 mg/kg might be taken
without apparent effect (MacCormack, 1945). Dosages at least as high
as 285 mg/kg have been taken without fatal result (Garrett, 1947).
However, large doses lead to prompt vomiting, so that the amount
actually retained cannot be determined accurately.
It has been noted, in the course of tests with volunteers, that
dilute colloidal aqueous suspensions of DDT are odourless and
tasteless (Domenjoz, 1946a; Hoffman & Lendle, 1948). Saturated
alcoholic solutions of DDT have a weak aromatic taste or rather odour.
Some people find these solutions slightly anaesthetic to the tongue
(Hoffman & Lendle, 1948). The taste of DDT in vegetable oil is so
slight that many persons could not identify capsules containing 0,
3.5, and 35 mg of DDT when they were presented separately but could
arrange them in proper order when one of each was available for
comparison (Hayes, personal communication, 1977).
The possible clinical effects of many repeated doses of DDT were
first explored by Fennah (1945). Because of his interest in predicting
the results of indiscriminate use, he expressed the exposures in terms
of environmental levels rather than in dosage units. The exposures
were clearly higher than those ordinarily encountered. In one test,
lasting a total of 11.5 months, Fennah daily inhaled 100 mg of pure
DDT and drank water dusted at the rate of 3240 mg/m2. Much of the
inhaled dust must have been deposited in the upper respiratory tract
and swallowed. Later, for one month, Fennah ate food all of which had
been sprayed at the rate of 2160 mg/m2 after it had been served. No
ill-effect of any kind was observed.
Table 21. Summary of the effects of one or a few oral doses of DDT on volunteers
Dose (mg) and Result Reference
formulation
250 × 9, No effect. Domenjoz, 1946a
suspension
1500, butter No effect, but lice killed when fed 6 MacCormack, 1945
solution and 12 h after dose.
500, oil No effect. Neal et al., 1946
solution
700, oil No effect. Neal et al., 1946
solution
250, suspension None except slight disturbance of Velbinger, 1947a,b
sensitivity of mouth.
250, oil Variable hyperesthesia of mouth, Velbinger, 1947a,b
solution
500, oil Variable hyperesthesia of mouth. Velbinger, 1947a,b
solution
750, oil Disturbance of sensitivity of lower part of Velbinger, 1947a,b
solution face; uncertain gait; peak reaction (6 h after
ingestion) characterized by malaise, cold moist
skin, and hypersensitivity to contact;
reflexes normal.
1000, oil Same as above; no joint pains, fatigue, fear, Velbinger, 1947a,b
solution or difficulty in seeing or hearing.
1500, oil Prickling of tongue and around mouth and nose Velbinger, 1947a,b
solution beginning 2.5 h after dose; disturbance of
equilibrium; dizziness; confusion; tremor
of extremities; peak reaction (10 h after
ingestion) characterized by severe malaise,
headache, and fatigue; delayed vomiting;
almost complete recovery in 24 h.
Some later studies on volunteers have been designed to explore the
details of storage and excretion of DDT in man and to search for
possible effects of dosages considered to be safe. In the first of
these studies, men were given 0, 3.5, and 35 (mg/man)/day. These
administered dosages, plus DDT measured in the men's food, resulted in
dosage levels of 0.0021-0.0034, 0.038-0.063, and 0.36-0.61
(mg/kg)/day, respectively, the exact value depending on the weight of
each individual. Six volunteers received the highest dosage of
technical DDT for 12 months, and 3 received it for 18 months. A
smaller number of men ingested the lower dosage of technical DDT or
one of the dosages of p,p'-DDT for 12 or 18 months. No volunteer
complained of any symptoms or showed, by the tests used, any sign of
illness that did not have an easily recognizable cause clearly
unrelated to the exposure to DDT. At intervals, the men were given a
systems review, physical examination, and a variety of laboratory
tests. Particular attention was given to the neurological examination
and liver function tests, because the major effects of DDT in animals
involve the nervous system and the liver (Hayes et al., 1956). The
same result was obtained in a second study in which the same dosages
were given for 21 months and the volunteers were observed for a
minimum of 27 additional months (Hayes et al., 1971). Information on
storage and excretion gathered in these studies has already been
discussed in sections 6.2.1.1 and 6.3.1.1.
Recently, DDT has been used on an experimental basis at dosage
rates varying from 0.3 to 3 (mg/kg)/day for periods up to 7 months in
an attempt to decrease serum bilirubin levels in selected patients
with jaundice. No side-effects were observed. No improvement was noted
in patients with jaundice based on cirrhosis who did not have any
demonstrated liver enzymes deficiency. However, in a patient with
familial, nonhaemolytic, unconjugated jaundice based on a deficienty
of glucuronyl-transferase, treatment with DDT rapidly reduced the
plasma bilirubin level to the normal range and relieved the patient of
nausea and malaise from which he had suffered intermittently. The
liver function tests as well as other laboratory findings remained
normal. The improvement was maintained during the 6 months that DDT
was administered, and had persisted for 7 additional months at the
time the report was written. In this case, a dosage of 1.5 (mg/kg)/day
produced a steady rise in plasma levels of p,p'-DDT from an initial
level of 0.005 mg/litre to a maximum of 1.33 mg/litre at the end of
treatment. At this time, the concentration in body fat was 203 mg/kg.
Plasma levels fell slowly after dosing was stopped (Thompson et al.,
1969). The highest dally intake in this series was 6 times greater
than the highest level administered in earlier studies of volunteers
and about 7500 times greater than the DDT intake of the general
population. The highest value for p,p'-DDT in serum observed in the
entire series was 1.330 mg/litre compared with 0.996 mg/litre, the
highest value reported by Laws et al. (1967) for formulating plant
workers.
Dermal exposure. Depending on dosage, oral administration of DDT
to volunteers either did not produce any illness or produced only
brief poisoning similar to that seen in experimental animals. The oral
dosage necessary to produce any clinical effect was almost always
10 mg/kg or more. However, in 2 studies involving only 3 subjects in
all, experimental dermal exposure to DDT was followed by fatigue,
aching of the limbs, anxiety, or irritability, and other subjective
complaints. Recovery was delayed for a month or more (Case, 1945;
Wigglesworth, 1945). In neither study was there an independent
control. Although the dosage was unmeasured, the amounts of DDT
absorbed must have been much smaller than those involved in the oral
tests. One of the studies involved self-experimentation by one man. A
similar but somewhat more severe test on 6 volunteers did not produce
any toxic or irritant effects at all (Dangerfield, 1946). In view of
all other experiments and extensive practical experience, it must be
concluded that the illnesses reported by Wigglesworth and by Case were
unrelated to DDT.
With the exceptions just mentioned, dermal exposure to DDT has not
been associated with any illness or, usually, with any irritation
(Wasicky & Unti, 1944; Draize et al., 1944; Cameron & Burgess, 1945;
Fennah, 1945; Dangerfield, 1946; Chin & T'Ant, 1946; Domenjoz, 1946a;
Haag et al., 1948). In fact, Hoffman & Lendle (1948) reported that
even subcutaneous injection of colloidal suspensions of DDT in saline
in concentrations up to 30 mg/litre did not cause irritation.
Zein-el-Dine (1946) reported that DDT-impregnated clothing caused a
slight, transient dermatitis, but the method of impregnation was not
stated and the absence of solvent was not guaranteed. In other more
thorough studies DDT-impregnated clothing was found to be non-
irritating (Cameron & Burgess, 1945; Domenjoz, 1946a).
Small pads impregnated with different formulations of DDT were
applied to the inner surface of the forearm of 32 volunteers whose
cutaneous sensation had previously been measured for a period of 5
weeks. Pads impregnated with all the elements of the formulation
except DDT were applied to the corresponding position of the other arm
as a control. Powdered DDT and a solution of DDT at 50 g/litre showed
little effect. Solutions in olive oil and petrolaturn at 100 g/litre
and 200 g/litre did not show any remarkable effect on sensation of
pain, cold, or heat but reduced tactile sensation in most cases so
that the minimum pressure that could arouse the tactile sensation was
1-2.5 g/cm2 higher than for the control (Chim & T'Ant, 1946).
Respiratory exposure. Neal et al. (1944) reported almost
continuous daily exposures to aerosols sufficient to leave a white
deposit of DDT on the nasal vibrissae of the volunteers. This exposure
produced moderate irritation of the nose, throat, and eyes. Except for
this irritation during exposure, there were no symptoms, and
laboratory tests and physical examination, including neurological
evaluation, failed to reveal any significant changes. The studies by
Fennah (1945), which involved both respiratory and oral exposure, did
not produce any detectable ill-effects.
8. EFFECTS OF DDT ON MAN-EPIDEMIOLOGICAL AND CLINICAL STUDIES
8.1 Retrospective Studies on DDT-Exposed Populations
8.1.1 Epidemiological surveillance of persons occupationally exposed
to DDT
The safety record of DDT is phenomenally good. It has been used
for mass delousing in such a way that the bodies and inner clothing of
thousands of people of all ages and states of health have been
liberally dusted with the compound. By necessity, the persons applying
the DDT work in a cloud of the material. Other subjects have sprayed
the interior of hundreds of millions of homes in tropical and
subtropical countries under conditions involving (Wolfe et al., 1959)
extensive dermal and respiratory exposure. A smaller number of men
have made or formulated DDT for many years. Extensive experience and
numerous medical studies of groups of workers have been reviewed
(Hayes, 1959). Dermatitis was commonly observed among men who used DDT
solutions. The rashes were clearly due to the solvent, especially
kerosene. As often happens with rashes caused by petroleum
distillates, they were most severe in men when they first started work
and cleared in a few days unless contamination was exceptionally
severe. A smaller number of workers experienced mild narcotic effects
(vertigo and nausea) from solvents when working in confined spaces.
Gil & Miron (1949) reported that some persons suffered temporary
irritability, fatigue, and other ill-defined symptoms after exposure
to the dusty atmosphere of a delousing station, but the relation of
these atypical findings to DDT was not clear. With these exceptions
due largely to solvents, no illnesses clearly attributable to the
formulations, much less to DDT, were revealed by the early studies.
Ortelee (1958) carried out clinical and laboratory examinations of
40 workers, all of whom were exposed to DDT and some of whom were
exposed to a number of other pesticides. The men had been employed at
this work with heavy exposure for 0.4 to 6.5 years and with slightly
less exposure for as much as eight years. Exposure was so intense
that, during working hours, many of the men were coated with a heavy
layer of concentrated DDT dust. By comparing their excretion of DDA
with that of volunteers given known doses of DDT, it was possible to
estimate that the average dosages of 3 groups of the workers with
different degrees of occupational exposure were 14, 30, and 42 mg/man
per day, respectively. With the exception of the excretion of DDA and
the occurrence of a few cases of minor irritation of the skin and
eyes, no correlation was found between any abnormality and exposure to
the insecticide. Since very large doses of DDT injure the nervous
system and liver of experimental animals, special attention was given
to a complete neurological examination and to laboratory tests for
liver function. Although a few abnormalities were revealed, none was
detected in relation to DDT.
Thirty-five men employed from 11 to 19 years in a plant that had
produced DDT continuously and exclusively since 1947 and, at the time
of the study, was producing 2722 metric tonnes per month were studied
by Laws et al. (1967). Findings from medical histories, physical
examinations, routine clinical laboratory tests, and chest X-ray films
did not reveal any ill-effects attributable to exposure to DDT. The
overall range of storage of the sum of isomers and metabolites of DDT
in the men's fat was 38-647 mg/kg compared with an average of 8 mg/kg
for the general population. Based on their storage of DDT in fat and
excretion of DDA in urine, it was estimated that the average daily
intake of DDT by the 20 men with high occupational exposure was
17.5-18 mg/man per day compared with an average of 0.028 mg/man per
day for members of the general population. There was significant
correlation ( r = +0.64) between the concentration of total DDT-related
material in the fat and the serum of the workers. The average
concentration in fat was 338 times higher than that in serum -- a
factor about 3 times greater than that for people without occupational
exposure. Compared to members of the general population, the workers
were found to store a smaller proportion of DDT-related material in
the form of DDE; the difference was shown to be related chiefly to
intensity rather than to duration of exposure. DDE is relatively a
much less important and DDA a much more important excretory product in
occupationally-exposed men compared with men in the general
population. A further study of the same men involved in DDT production
is discussed in section 8.2.5.
By far the largest number of heavily-exposed workers whose health
has been investigated are those associated with malaria control in
Brazil and India (WHO, 1973). In Brazil, periodic clinical
examinations were made of 202 spraymen exposed to DDT for 6 or more
years, 77 spraymen exposed for 13 years ending in 1959, and 406
controls. In the first examination carried out in 1971, minor
differences between exposed and unexposed groups were observed in some
neurological tests, but this result was not confirmed by the second
examination in the same year nor in subsequent examinations. During a
3-year period, a survey of illnesses requiring medical care during the
6 months preceding each periodic medical examination failed to
demonstrate any differences between exposed and control groups. A
relatively small number of analyses indicated that the concentration
of DDT in the blood of spraymen was about three times higher than that
of controls.
In India, the blood levels of 144 spraymen were 7.5-15 times
higher than those of the controls and were at least as high as those
reported for workers who make and formulate DDT elsewhere (see
Table 7). When the spraymen were examined, the only differences from
the controls were that knee reflexes were brisker, slight tremor was
more often present, and a timed Romberg test was more poorly performed
by the spraymen. The positive results led to the selection of 20 men
for re-examination by a neurologist who concluded that the differences
found initially were not real or that the tests had returned to normal
within the few months between the 2 examinations. The signs were not
dosage-related, since they were not correlated with serum levels of
DDT.
It has been known for several years that substantial doses of DDT
and several other organochlorine insecticides stimulate the microsomal
enzymes of the liver. This property of DDT was put to practical use in
treating a patient with familial, nonhaemolytic, unconjugated
jaundice, as described earlier. It was, therefore, entirely expected
that persons with sufficient occupational exposure to a variety of
pesticides would be able to metabolize a test drug (phenazone) more
rapidly on the average than persons without occupational exposure were
able to do. However, the change was not one of significantly
increasing the fastest normal rate but of bringing all the workers up
to a high level. There was no indication that the change had any
effect on the workers' health (Laws et al., 1967, 1973; Kolmodin et
al., 1969; Poland et al., 1970).
In addition to the studies already mentioned regarding workers
with extensive storage, and excretion of DDT as a result of heavy
exposure to DDT, studies have also been made of a larger number of
workers with lesser storage and excretion following lesser exposure to
DDT but greater exposure to other insecticides. Further studies (Long
et al., 1969; Morgan & Roan, 1969, 1974; Warnick & Carter, 1972;
Sandifer et al., 1972; Embry et al., 1972; Tsutsui et al., 1974; Ouw &
Shandar, 1974) have failed to reveal effects of clinical significance
among workers with prolonged, moderate exposure not only to
organochlorine but also to organophosphorus and other types of
insecticides. Small but statistically significant differences have
appeared in the medical history or clinical laboratory results of some
of these workers compared with the controls, but in no instance have
the differences been of any medical importance, and dosage-response
relationships have been unclear or absent. In several instances, the
statistically significant differences have been opposite in different
groups of workers; for example, creatinine phosphokinase activity was
lower than that of controls in subjects applying the insecticide but
higher in operators. Seasonal variations present one year were lacking
the next. The possibility of adaptive change (other than enzyme
induction) has been suggested (Tocci et al., 1969), but this, like the
reality of the changes, remains unproved.
In some instances statistically significant differences have been
found between workers and controls selected from the general
population in connexion with parameters that have no known biochemical
relationship to DDT and for which another explanation has not been
excluded. For example, Keil et al., (1972) reported significant linear
correlations between serum vitamin A and plasma DDT, TDE, and DDE
levels.
There are a few reports of acute illness among workers attributed
to exposure to mixtures of DDT and other materials. In so far as the
dosage was very large, as in certain accidents that have occurred to
individuals or groups in the general populations (see section 8.2.2),
one would expect similar results. However, in at least one instance,
headache, dizziness, nausea, vomiting, pain and numbness of the limbs,
and general weakness beginning 1-1.5 h after entering a treated field
(Kolyada & Mikhal'Chenkova, 1973) suggested food poisoning or
hysteria.
Finally, there are studies of workers exposed to DDT and various
other pesticides that are reported to have produced a variety of
subjective and even objective medical findings. Interpretation of
these reports is difficult because: (a) the findings do not resemble
those of poisoned animals or of persons poisoned as a result of
accident or suicide; and (b) the papers fail to report how the medical
findings and the absenteeism of the pesticide workers compares with
those of workers of comparable age, sex, and exertion who are not
exposed to chemicals. The fact that the workers in question were
exposed to mixtures of pesticides is not in itself an explanation
because studies on many workers who were exposed to mixtures have not
revealed any consistent differences between exposed subjects and
unexposed controls. However, an explanation may lie in the degree of
exposure. Reports of very high levels of organochlorine compounds in
blood samples and of DDT in milk samples from populations in which
illness was found are discussed in sections 6.2.1.2 and 6.3.1.2.
The reports under discussion tend to fall in 2 categories, those
involving general debility and those involving a single organ or
system. Conditions representative of general debility include
dermatitis, subtle blood changes, general weakness, palpitations,
functional angiospasm, headache, dizziness, diminished appetite,
vomiting, lower abdominal pain, chronic gastritis, benign chronic
hepatitis, isomnia, a sympathetic vascular/asthenic syndrome,
vegetative dystonia, and confusion (Kostiuk & Mukhtrova, 1970; Bezugli
et al., 1973).
Organs, systems, or functions that have been studied with the
exclusion of other organs, systems, or functions of the same workers
include: the respiratory system (Boiko & Krasniuk, 1969), liver
(Bezuglyi & Kaskevich, 1969), stomach (Krasniuk & Platonova, 1969;
Platonova, 1970), kidneys (Krasniuk et al., 1968), adrenals (Bakseyev,
1973), skin (Karimov, 1969, 1970), and labour and the puerperium
(Komarova, 1970; Nikitina, 1974). An indication that the difficulties
under discussion are not serious is their reversal or prophylaxis by
means of diet. Lescenko & Polonskaia (1969) described in detail two
dietary supplements composed of ordinary foods plus sea-kale and a
selection of vitamins and trace metals. Organochlorine-exposed workers
who received these diet products showed a normalization of protein
metabolism manifested by an increase in total serum protein, improved
lipid metabolism, and enriched vitamin and trace element supplies in
the organism. All of these effects led to an improvement in the
detoxifying function of the liver, which was viewed as the most
frequent site of adverse effects of exposure to organochlorine
compounds.
8.1.2 Epidemiology of DDT poisoning in the general population:
accidents and suicides
The only demonstrated effects of DDT on the general population are
the storage of the compound and some of its derivatives in the tissues
and their excretion in urine and milk. The facts were reviewed in
sections 6.2.13 and 6.3.1.2. Briefly, DDT and some of its derivatives
are found in all or nearly all persons in the population. The
concentration is higher in tissues that have a high neutral fat
content. Thus, for members of the general public the concentration of
DDT-related compounds in adipose tissue is 100 or more times greater
than the concentration in plasma (Laws et al., 1967). However, in
spite of this great difference, sufficiently sensitive methods have
demonstrated DDT in all tissues including the fetus and in all body
fluids including human milk. These relationships are exactly what
would be predicted from what is known of the storage of drugs and
other compounds. Actual chemical demonstration of the distribution of
DDT has been established for several years. Thus, its occurrence was
first reported in human tissue (Howell, 1948), in tissue of the
general population (Laug et al., 1951) in human milk (Laug et al.,
1951), and in the human fetus (Denés, 1962).
There is extensive evidence that the mount of DDT and related
material in the general diet in the USA has decreased as the use of
DDT in that country has decreased, especially its use on forage.
During the early 1950s, total DDT-related intake was approximately
0.265 mg/man per day and that for DDT was 0.163 mg/man per day (Walker
et al., 1954). The average intake of DDT-related compounds based on a
very large number of samples collected in different parts of the
country during 1964-67 was 0.063 mg/man per day and that for DDT was
0.028 mg/man per day (Duggan, 1968). With decreased use of DDT, a
gradual decrease in the storage of DDT and related material in human
fat would be expected. Because only a few samples of fat were
collected in the early studies of human tissue, there is some
statistical uncertainty as to whether the decrease in storage that has
been observed is real or whether it merely reflects variation due to
sampling. In any event, by 1968, the average storage level of total
DDT-equivalent material in fat was 7.67 mg/kg and that for DDT was
1.46 mg/kg. These averages were based on just over 3000 samples
collected during the first half of 1968. The number of samples
involved in this particular study was greater than the sum of all of
the samples used in early studies. The best available values for
concentrations in serum are 0.0294 mg/litre for total DDT-equivalent
and 0.0047 mg/litre for p,p'-DDT.
Cases of accidental and suicidal poisoning in which the effects
were clearly caused by DDT are summarized in Table 22. All of these
cases involved ingestion. The signs and symptoms of poisoning were
entirely consistent with those observed in volunteers, except that the
spectrum of effects was broader because some of the accidental and
suicidal doses were very high. A few persons have apparently been
killed by uncomplicated DDT poisoning, but none of these cases was
reported in detail. Death has been caused much more frequently by the
ingestion of solutions of DDT, but in most instances the signs and
symptoms were predominantly or exclusively those of poisoning by the
solvent (Hayes, 1959). This does not mean that the toxicity of the
solvent always predominates. For example, the recurrent convulsions in
a case reported by Cunningham & Hill (1952), though more
characteristic of poisoning by one of the cyclodienes, was certainly
not typical of solvent poisoning. A 2-year-old child drank an unknown
quantity of fly spray of which 5% was DDT, but the nature of the other
active ingredients or the solvent was unknown. About 1 h after taking
the material, the child became unconscious and had a generalized,
sustained convulsion. Convulsions were present when the child was
hospitalized 2 h after taking the poison, but the fits were controlled
by barbiturates and other sedatives. Convulsions reoccurred on the
fourth day and again on the twenty-first day but ceased each time
following renewal of treatment. On the twelfth day, it was noted that
the patient was deaf. Hearing began to improve about the twenty-fourth
day and was normal, as were other neurological and psychic findings,
when the patient was seen about 2.5 months after the accident.
Clinical effects of one toxicant may be modified by combining it
with another. For example, prolonged illness would not be expected
from ingestion of DDT at a rate of 27 mg/kg. However, when DDT and
lindane were ingested in a suicidal attempt at dosages thought to be
27 mg/kg and 18 mg/kg, respectively, clinical remission of convulsions
and liver involvement was delayed until the twentieth day, and the EEG
did not return to normal until the thirty-ninth day (Eskenasy, 1972).
There have not been any accidents of suicided involving raspatory
or dermal exposure to DDT leading to recognized signs and symptoms of
poisoning, even though sufficient respiratory exposure to aerosols or
sufficient dermal exposure to solutions can cause poisoning in
animals; the difference is certainly one of dosage.
It has been alleged that DDT causes or contributes to a wide
variety of diseases of man and animals not previously recognized as
being associated with any chemical. Such diseases include
cardiovascular disease, cancer, atypical pneumonia, retrolental
fibroplasia, poliomyelitis, hepatitis, and "neuropsychiatric
manifestations" (Biskind & Beiber, 1949; Biskind, 1952, 1953; and
others). Without exception the causes of these diseases were unknown
or at least unproved at the time of the allegation. Needless to say,
Table 22. Summary of the effects of the accidental or suicidal ingestion of DDT
Individual dose Results and reference
(mg) Formulation
Number of persons
300-4500 Onset in 1 h; vomiting; restlessness; headache; heart weak and
in food slow; recovery next day (Mulhens, 1946).
1 man
unknown dose Onset in 2-2.5 h; all subjects weak and giddy; 4 subjects vomited;
in tarts 2 subjects hospitalized; one subject confused, uncoordinated, weak;
25 man one subject with palpitations and numbness of hands; recovery in
24-48 h (Mackerras & West, 1946).
5000-6000 Onset 2-3 h; throbbing headache; dizziness; incoordination;
in pancakes paraesthesia of extremities; urge to defaecate; wide nonreacting
3 men pupils; reduced vision; dysarthria; facial weakness; tremor; ataxic
gait; reduced sensitivity to touch; reduced reflexes; positive Romberg;
slightly low blood pressure and persistent irregular heart action;
partial recovery in 2-3 days, but slight jaundice appeared 4-5 days
after ingestion and lasted 3-4 days; all subjects normal 19 days after
poisoning except for irregular heart action in one subject (Naevested,
1947).
2000 No illness (Naevested, 1947).
in pancakes
2 men
up to 20 000 Onset in 30-60 min in those most severely affected; men first
in bread seen 2-3 h after ingestion; in spite of severe early vomiting that
28 men reduced the effective dose, severity of illness and especially intensity of
numbness and paralysis of extremities proportional to amount of DDT
ingested; all but 8 men recovered in 48 h; 5 others fully recovered in 2
weeks, but 3 men still had some weakness and ataxia of the hands
5 weeks after ingestion (Garrett, 1947, 1950, unpublished data).
unknown dose Onset about 3.5 h after ingestion; total of about 85 cases of which 37
in flour were hospitalized; symptoms mild and similar to those in earlier
about 100 women outbreaks except for gastrointestinal disturbance in most severe cases
including abdominal pain and diarrhoea as well as nausea; most
subjects fully recovered in 24 h (Jude & Girard, 1949).
unknown dose Symptoms in established cases similar to those reported earlier
14 cases (Francone et al., 1952).
Table 22 (Cont'd)
Individual dose Results and reference
(mg) Formulation
Number of persons
286-1716 With the exception of one man who was already sick when he received
in meatballs a dosage of 6 mg/kg, poisoning did not occur at dosages of
8 cases, 11 5.1-10.3 mg/kg. Ingestion of 16.3-120.5 mg/kg produced excessive
exposed perspiration, nausea, vomiting, convulsions, headache, increased
salivation, tremors, tachycardia, and cyanosis of the lips. Onset
varied from 2-6 h depending on dosage. Recovery required as much
as 2 days (Hsieh, 1954).
unknown dose Death 13 h after suicidal ingestion (Committee on Pesticides, 1951).
1 case
unknown dose Twenty-two separate cases, including 15 attempted suicides; some
22 unrelated cases complicated by solvents; 3 deaths (Committee on Pesticides, 1951).
the charge that DDT predisposes to poliomyelitis was dropped after the
disease was controlled through the use of vaccines. Unfortunately,
there is no immediate possibility of controlling cardiovascular
disease, cancer, or many of the less common conditions in man that
have been ascribed to DDT. In the meantime, such irresponsible claims
could produce great harm and, if taken seriously, even interfere with
the scientific search for true causes and realistic means of
preventing the conditions in question.
8.1.3 Epidemiology of DDT poisoning in infants and young children
Nothing is known fundamentally to distinguish the epidemiology of
DDT poisoning among children from that among adults. In both
instances, poisoning has never been confirmed, except where the dosage
was large, usually as the result of an accident and usually involving
gross carelessness. Probably a larger number of cases have occurred in
infants simply because they are more likely to eat and drink
formulations that they find in unlabelled containers frequently
originally intended for food. However, as far as DDT is concerned, all
the large outbreaks of poisoning have involved adults under military
conditions, thus children were not exposed. As might be expected, some
deaths of adults but none of children has apparently involved suicide.
Most, if not all, cases in adults were uncomplicated poisoning by DDT,
but several cases in children involved the drinking of solutions so
that the signs and symptoms were actually caused by the solvent
(Reingold & Lasky, 1947).
8.2 Clinical and Epidemiological Studies of the Effects of DDT on
Specific Organs and Systems
8.2.1 Haemopoietic system and immunology
In acute poisoning, a slight decrease in haemaglobin and a
moderate leukocytosis without any constant deviation in the
differential white count have been observed in volunteers (Velbinger,
1947a,b). These findings are considered secondary to the neurological
effects.
There is a strong tendency to blame blood dyscrasias, other
manifestations of "hypersensitivity", and, in fact, many diseases of
unknown cause on any new chemical that gains widespread attention. DDT
was no exception. A review of the early literature (Hayes, 1959)
indicates that blood dyscrasias and an unbelievable range of other
diseases were, in fact, blamed on DDT. Only a circumstantial
relationship was ever established between these diseases and exposure
to DDT, and this is true of the small number of reports of blood
dyscrasias (Murray et al., 1973) or angioneurotic oedema (Vanat &
Vanat, 1971) that have appeared recently. Later, fewer new reports
appeared linking DDT to diseases of unknown cause, although the use of
DDT increased greatly. It is true that available tests do not make it
possible to exclude a particular compound as a cause of an isolated
case of blood dyscrasia. However, it is noteworthy that the rate at
which these disorders occur has remained essentially unchanged since
before DDT was introduced (Hayes, 1975).
8.2.2 Nervous system
The effects of carefully measured doses of DDT that proved to be
just above the minimum toxic level are best described from studies of
volunteers (see section 7.4). Similar early signs and symptoms have
been encountered in cases of accidental poisoning that frequently
progressed to more severe illness as described in section 8.1.2.
Briefly, the earliest symptom of poisoning by DDT is
hyperaesthesia of the mouth and lower part of the face. This is
followed by paraesthesia of the same area and of the tongue and then
by dizziness, an objective disturbance of equilibrium, paraesthesia
and tremor of the extremities, confusion, malaise, headache, fatigue,
and delayed vomiting. The vomiting is probably of central origin and
not due to local irritation. Convulsions occur only in severe
poisoning.
Onset may be as soon as 30 min after ingestion of a large dose or
as late as 6 h after smaller but still toxic doses. Recovery from mild
poisoning is essentially complete in 24 h, but recovery from severe
poisoning requires several days. In two instances, there was some
residual weakness and ataxia of the hands, 5 weeks after ingestion.
Electroencephalograms were obtained from 73 workers exposed to
DDT, HCH, and chlorobenzilate for periods ranging from 7 months to 20
years. Just over 78% of the records were normal and 21.9% were
abnormal. The most severe changes involved persons exposed to the 3
compounds for 1-2 years; less severe changes were seen with either
shorter or longer exposure. The changes were not correlated with age,
the range and mean of age for those judged abnormal being almost
identical to these values for persons considered normal. Some of the
records showed bitemporal sharp waves with shifting lateralization
combined with low voltage theta activity. Other records showed spike
complexes, paroxymal discharges composed of slow and sharp waves most
pronounced anteriorly, and low voltage rhythmic spikes posteriorly.
None of the persons examined showed any abnormal clinical neurological
finding (Israeli & Mayersdorf, 1973; Mayersdorf & Israeli, 1974). The
incidence of abnormal electroencephalograms in the general population
is 9.0% or 9.2%, according to other investigators cited by Israeli &
Mayersdorf. Czegledi-Janko & Avar (1970) considered that nonspecific
EEG abnormalities occurred in 10-20% of the general population.
The frequency and degree of olfactory disorders, especially in the
ability to detect peppermint and acetic acid in an olfactory analyser,
were reported to be greater among persons exposed to pesticides, and
to increase with duration of exposure (Salihodzaev & Ferstat, 1972).
Whether any of the persons exposed to pesticides experienced any
clinical difficulty or social inconvenience associated with olfactory
sensation is not clear.
8.2.3 Renal system
There is no indication of renal damage in people, accidentally
poisoned by DDT, or in workers heavily exposed to it.
8.2.4 Gastrointestinal system
Except for vomiting, which probably is of central origin, the
gastrointestinal system has not been affected in acute poisoning.
8.2.5 Liver
Involvement of the liver has been mentioned in only a small
proportion of cases of accidental poisoning by DDT. In 3 men who ate
pancakes made with DDT and thus ingested 5000-6000 mg each, slight
jaundice appeared after 4-5 days and lasted 3-4 days (Naevested,
1947). Hepatic involvement and convulsions were reported in an
unsuccessful suicide attempt by ingesting DDT and lindane (Eskenasy,
1972).
Laws et al. (1973) made a detailed study of the liver function of
31 men who had made and formulated DDT and who had been the subjects
of an earlier study (see section 8.1.1). Judging from their excretion
and storage, the men's exposure was equivalent to oral intakes of DDT
at rates ranging from 3.6 to 18 mg/man per day for periods ranging
from 16 to 25 years and averaging 21 years. All tests were in the
normal range for total protein, albumin, total bilirubin, thymol
turbidity, and retention of sulfobromophthalein sodium (BSP). One man
had mild elevations in levels of both alkaline phosphatase
(EC 3.1.3.1) (16 units) and serum glutamic pyruvic transaminase
(EC 2.6.1.2) SGPT (42 units). Another man had an alkaline phosphatase
concentration of 14 units, while a third man had an SGPT level of 49
units. The alpha-fetoprotein test was negative for all 20 of the men
tested.
8.2.5.1 Liver enzymes
The induction of human microsomal enzymes of the liver by various
drugs was well known when Kolmodin et al. (1969) demonstrated this
effect in workers exposed to a variety of pesticides, including DDT.
Later, Poland et al. (1970) showed that workers who made and
formulated DDT and absorbed it at an average rate of about
0.25 (mg/kg)/day metabolized phenylbutazone more rapidly on the
average than controls and excreted more 6ß-hydroxycortisol.
Occupational exposure increased the drug-metabolizing ability of some
workers, so that they all metabolized test drugs with the efficiency
of those members of the general population who were most efficient in
this respect. The concentration of p,p'-DDT in the serum of the
workers studied by Poland averaged 0.573 mg/litre. In other workers
with less exposure to DDT, as indicated by average serum levels of
0.052 mg/litre, there was no increase in the urinary excretion of
D-glucaric acid, which is increased by a number of exogenous and
endogenous substances that induce microsomal enzymes (Morgan & Roan,
1974).
Thompson et al. (1969) demonstrated, in a different way, the
induction of microsomal enzymes by using DDT at a dosage of
1.5 (mg/kg)/day for 6 months in the successful treatment of
unconjugated hyperbilirubinaemia. In a similar way, Rappolt (1970)
used DDT to promote metabolism of an overdose of phenobarbital. It is
of interest that the levels of DDE in the serum of some workers
studied by Morgan & Roan (1974) approached those of workers studied by
Poland et al. (1970). The lack of induction in one group and its
presence in the other suggests that enzymes are induced in man more
readily by DDT than by DDE.
DDT promotes its own metabolism in some species of laboratory
animals. That the same is true in man is indicated by the fact that
storage of DDT is relatively less at higher dosages (see Fig. 4).
However, the metabolism and subsequent excretion of DDT can be
promoted even more by other inducing agents. Patients who received
phenobarbital or, more especially, phenytoin stored much less DDT than
other persons with similar exposure to DDT (Davies et al., 1969a;
 Edmundson et al., 1970b; Watson et al., 1972). This result concerning
phenytoin was confirmed by McQueen et al. (1972) who also showed that
other drugs produced a smaller but still highly significant reduction
in DDT storage. Establishment of a reduced equilibrium appeared to
require about 2 months. Within this period, the regression of the
level of DDT plus DDE on duration of treatment with phenytoin was
highly significant ( P < 0.001).
At the end of 9 months' treatment, the body fat of nonepileptic
volunteers given phenytoin at a rate of 300 mg/man per day contained
an average of 25% of the DDT and 39% of the DDE concentrations
originally present before administration of the drug (Davies et al.,
1971).
The same was true of workers whose exposure was greater than that
of the general population. Maintenance doses of phenobarbital,
phenytoin, or a combination of the two kept the storage levels of
several organochlorine insecticides in epileptic workers as low as, or
lower than levels in the general population (Schoor, 1970; Kwalick,
1971).
8.2.5.2 Other biochemical observations
A positive linear correlation has been reported for the
concentrations of vitamin A and of DDT-related compounds in the serum
of men with at least 5 years of occupational exposure to DDT. However,
the workers' DDT levels were little higher than those of persons in
the general population (see Table 7), and their vitamin A levels were
within normal limits (Keil et al., 1972). Perhaps they were better fed
than the controls.
Compared to 86 unexposed workers, the serum total cholesterol
values of 206 workers in a chemical plant where unidentified
organochlorine insecticides were made and formulated were higher in
workers who were less than 25 years old, lower in those between 25-34
years and 35-44 years, and higher in those who were 45 years old or
more. The differences were significant only for the oldest groups
(Wassermann et al., 1970a).
8.2.6 Cardiovascular system
The small amount of knowledge concerning the effect of DDT on the
human heart fails to show whether cardiac arrhythmia might be a
possible cause of death in acute poisoning, as is true in some species
of laboratory animals. Palpitations, tachycardia, and irregular heart
action have been noted in some, but not all cases of acute poisoning
(Mackerras & West, 1946; Naevested, 1947; Hsieh, 1954).
8.2.7 Reproduction
There is no indication that DDT has influenced reproduction except
to increase it as an indirect result of disease control, especially
malaria control.
After Laws et al. (1967) had completed their study, Wilcox (1967)
found that the 36 most heavily exposed workers involved had fathered
58 children before they began working at the DDT factory and 93
children afterwards.
O'Leary et al. (1970c) did not find any significant relationship
between abortion and blood levels of DDT-related compounds.
8.2.8 Endocrine organs
Average protein-bound iodine (PBI)levels of 0.0542 and
0.0693 mg/litre, respectively, were reported in the sera of 42 workers
occupationally-exposed to organochlorine insecticides and in 51
workers who were not exposed. The difference was statistically
significant even though all values fell within the normal range of
0.04-0.08 mg/litre (Wassermann, D. et al., 1971). It was not recorded
whether the workers involved were from the same factory as those with
10 or more years of occupational exposure whose plasma DDT levels were
reported by Wassermann et al. (1970c) (see Table 7). The small
difference in PBI levels is difficult to evaluate. It was the view of
Clifford & Weil (1972) that there was not any evidence that
occupational exposure had had an effect on human endocrine organs.
TDE. Following the demonstration (discussed in section 7.1.8)
that TDE caused atrophy of a part of the adrenal cortex of dogs,
o,p'-TDE, and to a lesser degree m,p'-TDE, have been used in man,
under the name of mitotane, in the hope of controlling excessive
cortical secretion or of reducing the size of adrenal tumors. The
underlying condition may be hyperplasia or adrenocortical carcinoma.
The dosage given has varied from 7 to 285 (mg/kg)/day, but a dosage of
approximately 100 (mg/kg)/day for many weeks has been necessary to
produce any benefit in man (Bergenstal et al., 1960; Wallace et al.,
1961; Gallagher et al., 1962; Verdon et al., 1962; Bledsoe et al.,
1964; and Southern et al., 1966a,b).
The effects of idiopathic hyperplasia may be controlled; in fact a
state of adrenal insufficiency may be produced (Canlorbe et al., 1971;
Sizonenko et al., 1974).
o,p'-TDE may also give symptomatic relief of excessive
adrenocortical activity secondary to a tumour that produces ACTH
(Carey et al., 1973).
A favourable response was produced in about one-fourth to one-half
of patients with inoperable adrenocortical carcinoma (Canlorbe et al.,
1971; Hoffman & Mattox, 1972; Lubitz et al., 1973; Montgomery &
Struck, 1973). In fact, an occasional cure, involving complete
regression of metastases, was produced by chemotherapy including
o,p'-TDE (Perevodchikova et al., 1972; Schick, 1973). More commonly,
symptoms were relieved and life was prolonged by little more than 7-8
months (Canlorbe et al., 1971; Hoffman & Mattox, 1972; Lubitz et al.,
1973). The success of treatment was often indicated early on by a
reduction in steroid excretion (Hoffman & Mattox, 1972; Lubitz et al.,
1973).
The large dosage of o,p'-TDE necessary to produce clinical
benefit often produced general lassitude, anorexia, nausea, vomiting,
diarrhoea, and dermatitis (Naruse et al., 1970; Hoffman & Mattox,
1972; Nitshke & Link, 1972; Perevodcikova et al., 1972; Lubitz et al.,
1973). Apathy ranged from mild dulling of interest to profound
psychotic depression (Hoffman & Mattox, 1972). More rarely,
gynaecomastia, haematuria, leukopenia, and thrombocytopenia have been
reported (Luton et al., 1972; Perevodcikova et al., 1972). The
symptoms disappeared soon after administration of the drug ceased or
when the dosage was reduced (Perevodcikova et al., 1972).
Even large, therapeutic doses of o,p'-TDE did not cause
histological alterations in the adrenals in man (Wallace et al.,
1961). Furthermore, dosages in the therapeutic range (specifically
those between 110 and 140 (mg/kg)/day did not produce any detectable
injury to the liver, kidney, or bone marrow. All patients treated in
this way experienced significant anorexia and nausea, and some showed
central nervous system depression varying from lethargy to somnolence.
These toxic effects cleared when dosing was discontinued (Bergenstal
et al., 1960).
Kupfer (1967) reviewed extensive literature that indicated that
the effect in man and other species, except the dog, is caused by
stimulation of corticoid metabolism by massive doses of o,p'-TDE and
not by any direct effect on the adrenal. Southern et al. (1966a,b)
agreed that the effect was predominantly extra-adrenal in man, when
the drug was first given, but offered evidence that adrenal secretion
of cortisol was eventually reduced. However, even if therapeutic doses
eventually have a direct effect on the adrenal, doses encountered by
workers exposed to technical DDT do not (Clifford & Weil, 1972; Morgan
& Roan, 1973).
8.2.9 Carcinogenicity
Laws et al. (1967) did not find any case of cancer or blood
dyscrasia among the 35 heavily exposed workers in a DDT factory nor
did the medical records of 63 men who had worked there for more than 5
years reveal these diseases. Two men were employed who had a history
of successfully treated cancer before they came to work, but no
employee had contracted cancer during the 19 years that the plant had
been in operation; during this period, the work force varied from 111
to 135.
In the USA, the total death rates for cancer of the liver and its
biliary passages (classified individually as "primary", "secondary",
and "not stated whether primary or secondary") lead to the conclusion
that there has been a significant, almost constant decrease in the
total rate of liver cancer deaths from 8.8 in 1930 to 8.4 in 1944
(when DDT was introduced) to 5.6 in 1972. This almost constant decline
in total liver cancer death rates for the past 42 years offers no
evidence of any increase in liver cancer deaths since the introduction
of the first organochlorine pesticide into the environment. The
decrease in liver cancer deaths is even more significant in light of
the increasing life span of the general population in the USA, which
has resulted in an increased percentage of the population at risk from
cancer over these years. In spite of the limitations inherent in the
interpretation of such data, this record is a reminder that, more than
30 years after the introduction of DDT, there is no evidence,
whatsoever, that DDT is carcinogenic in man (Deichmann & MacDonald,
1976, 1977).
In the USA, the incidence of cancer is lower in rural counties
than in metropolitan areas in general (Mason et al., 1975).
It is sometimes implied that epidemiological evidence is useless
for revealing the carcinogenicity of a material for man unless it
involves large numbers of people who have been exposed to the material
for most or all of a lifetime. The fact is that some human carcinogens
have been detected through their occurrence in high incidence in small
groups for periods of much less than 25 years. What was commonly
considered the first recognition of chemical carcinogenesis in man
depended on the observations made by a single surgeon (Pott, 1775) in
a small fraction of his patients. Such was the intensity of the
exposure of the apprentices of chimney sweepers that cancer of the
scrotum often appeared at puberty. The editor responsible for
compiling the writings of Pott (1790) added a footnote indicating that
he had seen such a cancer in "an infant under eight years of age". It
must be understood that boys did not usually become apprentice chimney
sweepers before they were four-years-old. In connection with tumours
of the bladder mainly caused by ß-naphthylamine but to a lesser degree
by other aromatic amines, Hueper (1942) reviewed cases in which the
time from the first exposure to recognition of symptoms was 8-41,
9-28, and 2-35 years; in one series of 83 cases, 71% of the tumours
appeared from 1 to 15 years after exposure. The same author cited
reports (p. 104) of cases of malignant epitheliomas in persons exposed
to pitch for 18, 24, 24, and 36 months, respectively. Kleinfeld (1967)
reported 50-76% incidence of bladder cancer among several groups of
workers. He also noted a sharp drop in incidence of this condition
following decrease -- but not discontinuation -- of occupational
exposure to ß-naphthylamine.
8.2.10 Mutagenicity
Evidence regarding the mutagenic activity of DDT and its
significance in man is uncertain partly because the chromosomal
changes that are examined are sensitive to viral infections and
chemotherapy. The latter may not be recognized at the time of sampling
and may not have been shown to injure health through a mutagenic
mechanism.
Comparing samples collected in winter and during the peak season
of pesticide application, a slight increase in chromatid breaks was
reported in the cultured lymphocytes of workers exposed to a wide
variety of insecticides said to include DDT, although this was claimed
at a time when the use of DDT was banned. A somewhat larger increase
was reported for men exposed mainly to herbicides (Yoder et al.,
1973). In another study, lymphocytes cultured from workers with an
average DDT plasma level of 0.999 mg/litre showed significantly more
chromosomal and chromatid aberrations than cells cultured from
controls with an average plasma level of 0.275 mg/litre. The
difference was not significant in other comparisons in which the
average plasma levels were 1.030 versus 0.380 mg/litre and 0.240
versus 0.030 mg/litre, respectively (Rabello et al., 1975).
Examination of all of the data presented by the authors suggests a
simple dosage-effect relationship was present, with a detectable
effect starting somewhere between 0.2 and 0.4 mg/litre and increasing
at levels higher than 0.4 mg/litre.
8.3 Factors Influencing DDT Toxicity
There is no evidence that any factor except dosage is of practical
importance in determining DDT toxicity in man. Factors that have been
considered as possibly affecting asymptomatic storage of DDT include
age, sex, and race. Differences observed in connexion with these
factors are small, medically insignificant, and probably secondary to
dosage (Hayes, 1975).
Storage has also been reported to be greater in the tissues of
people with certain diseases (Deichmann & Radomski, 1968; Raclomski et
al., 1968; Vas'kovskaja, 1969; Dacre & Jennings, 1970; Jonczyk et al.,
1974). Again, the reported differences are small, and the highest
values for the samples in question are small compared with those found
in healthy workers (Hayes, 1975). Furthermore, a number of authors
have reported a similar range of storage in persons undergoing minor,
elective surgery and in those who have died from various causes (Hayes
et al., 1958; Dale et al., 1965; Robinson et al., 1965; Wassermann et
al., 1965). Some authors (Hunter et al., 1963; Robinson et al., 1965;
Hoffman et al., 1967; Hoffman, 1968; Morgan & Roan, 1970) have failed
to find any relationship between storage of insecticides and the cause
of death. Where a relationship was found, there was often the
possibility that the higher values were found in diseases that
involved some degree of wasting prior to death. Casarett et al. (1968)
found that higher values occurred in persons who had 3 characteristics
in common: emaciation, cancer, and widespread abnormality of the
liver.
A slightly greater storage of DDT and DDE that was reported in
persons who underwent splenectomy for hepatosplenic schistosomiasis
compared with those operated on for other conditions, mainly hernia
was statistically significant. No such difference was observed in
connexion with dieldrin, ß-HCH, or heptachlor epoxide (Wassermann et
al., 1975). Whereas it was speculated that the increased storage of
DDT and DDE might have been the result of a reduction in metabolism,
secondary to liver injury, the possibility of greater exposure as a
result of greater use of DDT in irrigated areas was not excluded.
8.4 Treatment of Poisoning in Man
No useful guidance regarding treatment has been obtained from the
very few cases of DDT poisoning that have occurred. Animal studies
indicate that sedatives, ionic calcium, and glucose or another ready
source of energy would be useful. On the basis of experience in
treating people poisoned by different convulsive poisons, it seems
likely that diazepam would be beneficial.
9. EVALUATION OF HEALTH RISKS TO MAN FROM EXPOSURE TO DDT AND RELATED
COMPOUNDS
9.1 Relative Contributions of Food, Water, Air, and Miscellaneous
Sources to Total Intake
9.1.1 Adult members of the general population
Food represents the major source of intake of DDT in the general
population. It has been estimated (section 5.1.5) that over 90% of the
DDT stored in the general population is derived from food. Around
1965, when the use of DDT was at its peak, intake in the USA was
approximately 0.04 mg/man per day from food, less than 0.000046 mg/man
per day from water, less than 0.00006 mg/man per day from urban air
and less than 0.0005 mg/man per day from the air in small agricultural
communities. The reason for the qualification "less than", is that the
intakes were calculated from the highest concentrations reported in
drinking-water and air.
Although total intake of DDT from food has not been measured in
some parts of the world, worldwide measurements of the storage of DDT
and its metabolites in human body fat indicate that the extremes of
total exposure have varied by a factor of about 10, but that total
exposure for most populations has varied by a factor of no more than 3
(see Table 9).
DDT in the dust in a house (as indicated either by a history of
extensive household application of insecticides or by the finding of
relatively high levels of DDT in house dust) can contribute to the
storage of DDT-related compounds in persons living in the house
(section 5.1.4). However, although the contribution of house dust to
DDT intake has been established, it is not quite clear how this
contribution occurs. Some of the dust may contaminate food in the
process of preparation and some may be inhaled and later swallowed
after deposition in the upper airway. It is difficult to believe that
enough DDT is present in such houses in the form of vapour or
respirable dust to cause a substantial increase in the total exposure
of the inhabitants, but no critical study has been made of this
matter. Clearly, some DDT will enter by the respiratory route.
9.1.2 Infants and children
At birth, infants tend to have slightly lower levels of DDT than
adults in the same population. This is because the placenta offers
partial protection against the passage of DDT and related compounds.
Although human milk tends to have a somewhat higher concentration
of DDT than cow's milk (see section 6.3.1.3), the difference, if any,
that this makes to the rate at which breast-fed and bottle-fed babies
store the compound has not been established. It is possible that the
conditional acceptable daily intake (ADI) might be exceeded in an
infant wholly fed on breast milk. However, the ADI is calculated on
the basis of lifetime exposure, and short-term variations can be
regarded as not having any significance.
The only really important way in which the exposure of infants and
children differs from that of adults in the same community involves
accidental exposure (see section 8.1.3).
9.1.3 Occupational groups
Occupational exposure (section 5.3) to DDT is initially almost
exclusively through the respiratory and dermal routes. However, the
particles of many insecticidal dusts, wettable powders, and sprays are
too large to reach the lower respiratory tract. As a result, most of
the particles inhaled are deposited in the upper respiratory tract,
carried to the pharynx by ciliary action, and eventually swallowed
(section 6.1.1).
Although dermal exposure to DDT is high under some occupational
situations, the effect is minimal because the compound is so poorly
absorbed through the skin (section 6.1.3). The excellent safety record
of DDT, never matched by any other insecticide used in antimalaria
campaigns, other vector control programmes, and agriculture, is based
mainly on its poor absorption through the skin.
The number of people with full-time occupational exposure to DDT
alone is small. For example, at the time of one study, the only
factory making the compound in the USA produced 2722 metric tonnes per
month using a work force of about 145. Following the recommendations
of an Expert Committee, the World Health Organization studied spraymen
who had applied only DDT for 5 years or more. Only 272 suitable
subjects could be located in Brazil and only 144 in India. The
concentrations of DDT and its derivatives in the blood of the
preliminary and main study groups in India were 0.761 and
1.272 mg/litre, respectively. The blood levels of spraymen in Brazil
were about 3 times those of the controls.
The absorbed dosage of the men who made and formulated DDT for 10
years or more was about 18 mg/man per day (see section 8.1.1). The
exposure of other workers, notably those applying DDT for agricultural
purposes has usually been an order of magnitude less (see Table 7).
9.2 Effects of Exposure
No adverse effects have been described at repeated dosages of
1.5 (mg/kg)/day or less (see section 7.4). The large number of
measurements that have been made on samples from human populations
have not made it possible to define a maximum dosage that man can
absorb without any adverse effect but have highlighted the finding
that the high levels found in volunteers and workers were harmless for
at least 25 years.
Table 23 summarizes the clinical aspects of DDT in man.
Table 23. Dosage-effects of DDT in man
Single dose
(mg/kg) Observation
Unknown Fatal
16-286 Prompt vomiting at higher doses
(all poisoned, convulsions in some)
6-10 Moderate poisoning
Repeated
exposure
(mg/kg)/day
1.5 Administered as therapy for 6 monthsa
0.5 Administered to volunteers for 21 monthsa
0.5 Exposure of workers for 6.5 yearsa
0.25 Exposure of workers for 25 yearsa
0.0025 Intake of population in the USA, 1953-54a
0.0002 Intake of population in the USA, 1969-70a
a Without any adverse effect.
In considering the safety of workers who are employed in the DDT
industry, it is useful to consider the results of animal experiments.
Rats withstand a daily dosage at least 10 times that of these workers
without any detectable clinical effect (section 7, Table 17), although
minimal reversible tissue changes may be present. Dogs and monkeys
also withstand a daily dosage 10 times higher than that of the
workers, but they do not show the tissue changes, which seem to be
peculiar to some rodents. Because workers have tolerated high dosages
of DDT for over a fifth of a lifetime without detectable harm and
since animals withstand larger dosages for an entire lifetime without
injury, there is good reason to predict the continuing safety of the
workers.
The experience already gained from workers can be used to predict
the future safety of the general population in relation to DDT. Many
workers have now been exposed to DDT for much more than one-fifth of
their life span. Since they have not suffered detectable harm, it
seems most unlikely that the general population will be harmed by
dosages 200-1250 times smaller than those to which the workers are
exposed. It has been shown for at least 2 animal species that toxicity
resulting from a lifetime of exposure is seldom very different from
toxicity resulting from 90 days of exposure at the same dosage rate.
The largest factor of difference observed when 33 chemicals were
investigated was 20, and, for half of them, the factor was 2 or less
(see section 7.3.1.1). Ninety days constitute about one-eighth of the
lifespan of a rat, and this is less than the fraction of the human
life span that has been studied so far.
9.3 Carcinogenicity and Mutagenicity
Liver tumours are produced in mice by many chlorinated compounds,
including DDT. There is also evidence to suggest that DDT metabolites
DDE and TDE (DDD) produce hepatic tumours in mice and that TDE also
produces lung tumours. Information on the tumorigenicity of DDT in
rats (see section 7.1.9) is conflicting; some studies report tumour
formation while other studies report negative data. Carcinogenicity
studies in the hamster were negative (see section 7.1.9). The
occurrence of tumours in some rodents only, casts doubt on the
significance of the phenomenon and on extrapolation of the findings to
man.
Studies on the incidence of all cancers reported in those parts of
some countries with known high agricultural use of DDT in the 1950s
and early 1960s have not demonstrated any trends in any type of cancer
associated with the use of DDT in relation to these areas (see section
8.2.9).
The question as to whether DDT is carcinogenic in man has not been
answered unequivocally. Although the cross-sectional epidemiological
studies on workers exposed to DDT and the observation studies on
volunteers are limited, there is not any currently available evidence
to suggest that DDT is tumorigenic or carcinogenic in man (see section
8.2.9).
Recent studies on in vitro bacterial test systems with or
without metabolic activation have not shown any evidence that either
DDT or DDE is mutagenic (see section 8.2.10). The evidence for the
mutagenicity of DDT in mammalian test systems is inconclusive.
9.4 Effects on Microsomal Enzymes
There is no doubt that exposure to DDT results in the induction of
microsomal mixed function oxidases and causes marked morphological
changes in the liver of some rodents (see section 7.1.9). In some
rodents, notably the mouse, these morphological changes have been
related to tumorigenicity. Microsomal enzymes are also induced by DDT
in other species, but the liver does not show the same morphological
changes.
The only effect for which something approaching a threshold has
been demonstrated is the induction of microsomal enzymes in workers in
association with an average serum value of 0.573 mg/litre for p,p'-
DDT but not in workers with serum levels as high as 0.052 mg/litre, a
value essentially identical to the highest reported for the general
population. Furthermore, although some groups of workers experienced
an increase in their average enzyme activity, no person exceeded the
range of activity found in normal people in the general population.
DDT will not induce liver microsomal enzymes in the general
population because their intake of the compound is so much less than
the smallest dosage capable of producing this effect in animals or man
(see sections 7.1.4.2 and 8.2.5.1).
9.5 Reproduction and Teratogenicity
Effects on reproduction in mammals have been studied in the mouse,
the rat, and the dog (see section 7.1.7). In the mouse, a multi-
generation study at dietary levels of DDT of 25, 100, and 250 mg/kg
showed effects on fertility and reproduction only at the highest
level, equivalent to 33 (mg/kg)/day. In the rat, normal reproduction
was maintained at a dietary level of 200 mg/kg. In the dog, dietary
intake at dosages up to 10 (mg/kg)/day did not produce any effects on
reproduction other than earlier estrus in the DDT-treated females.
In man, there is no indication that DDT affects reproduction (see
section 8.2.7); no impairment of fertility was observed in a study of
men occupationally-exposed for more than 10 years to a measured
average daily intake in the region of 18 mg/man per day (equivalent to
0.25 (mg/kg)/day).
Studies in the mouse, the rat, and the dog have not shown any
evidence of teratogenicity. In the mouse, dosage at the rate of
1 mg/kg was not teratogenic; a single dosage of 25 mg/kg or repeated
doses at the rate of 2.5 mg/kg were embrytoxic but not teratogenic.
9.6 Immunosuppression
DDT appears to have a depressant effect on the immune system
although the evidence is by no means conclusive. Rats and rabbits
receiving DDT in aqueous suspension at a concentration of 200 mg/litre
showed a depression in antibody formation and decrease in at least one
globulin fraction of the blood. Rats receiving a dosage of
0.25 (mg/kg)/day by gavage did not show any changes in the phagocytic
activity of the white blood cells. In the guineapig, dosages of
1-20 mg/kg did not have any effects on antitoxin production but
produced a reduction in tissue histamine levels (see section 7.1.1).
9.7 Nutritional Effects and Other Factors
Animal studies indicate that nutritional status influences the
toxicity of DDT (see section 7.3.3). The preferential storage of DDT
in fat can mitigate the effect of acute poisoning. If rats that have
stored large amounts of DDT are starved, they may suffer toxic effects
due to mobilization of fat and DDT.
In man, nutritional status will have a similar effect to that
found in other animals. However, the possibility that starvation in
man could precipitate toxic manifestations is regarded as unlikely as
the stored levels do not approach those found in laboratory animals
and the lower metabolic rate of man results in slower mobilization. In
fact, severe weight loss sometimes does cause some increase in storage
of DDT in connexion with certain wasting diseases; however, people
with full-time occupational exposure to DDT average 10 times more
storage than the highest values reported in connection with disease
but do not exhibit predisposition to the diseases in question (see
section 6.2.1.3).
Although young animals are often more susceptible to toxic
chemicals than adults, there is no evidence that DDT is more toxic to
young animals of any species including man. In fact, in the rat, the
young are less susceptible to a single dose than the adults (see
section 7.3.2, Table 20).
9.8 Dosage-Effect Relationships
Dosage-effect relationships for DDT in man have been observed in
connection with acute poisoning (see Table 23), excretion, and storage
(see Fig. 4), and the induction of microsomal enzymes, which has been
observed at a dosage of 0.25 (mg/kg)/day but not at lower dosages. The
dosage of 0.25 (mg/kg)/day to which workers have been exposed for 25
years is of the same order of magnitude that causes an increase in
tumours in male mice of a susceptible strain but not in females of any
strain (see section 7.1.9). As shown in Table 24, this dosage in
workers is less than the no-effect levels for rats, dogs, and monkeys
and far less than the dosage at which rats, mice, and dogs
successfully reproduce for generations. The equilibrium levels of DDT
and its metabolites found in the blood and fat of people with
full-time occupational exposure and the much lower levels found in the
general population have not been associated with any adverse effects.
9.9 Recommendations on Levels of Exposure
The data from intake, exposure, and levels in populations supports
the current conditional ADI for DDT, which affords a considerable
margin of safety.
If the total intake of DDT from food and other sources rises above
0.005 (mg/kg)/day (the conditional ADI) then the situation should be
investigated.
Table 24. Dosage-effect of DDT in animals
Single Route Observation
(mg/kg)
3000 dermal LD50 of powder in adult rat
2356-4000 oral LD50 of oil solution in newborn rat
250-500 dermal LD50 of oil solution in adult rat
250 oral LD50 of oil solution in adult rat
Repeated
Exposure
(mg/kg)/day
300 subcutaneous inhibition of testicular growth in cockerels
41-80 oral increased mortality in rats, 2-year study
41-80 oral 100% mortality in dogs in 39-49 months
41-80 oral 100% mortality in monkeys, 70 days
21-40 oral 25% mortality in dogs in 39-49 months
33.2 oral harmful to reproduction in mice
13.3 oral slight reduction in lactation and survival of some but not all
generations of mice (6 generation test)
10 oral no harmful effect on reproduction in dog (3 generation test)
10 oral no harmful effect on reproduction in rat (2 generation test)
5-10 oral no-effect level in dog, 2 generations
2.6-5 oral no-effect level in monkey, 7.5 years
0.63-1.25 oral no-effect level in rat, 2 year test
0.16-0.31 oral risk of liver tumours doubled in male mice but no effect in
female
0.3 oral no-effect level for induction of microsomal enzymes in rats
The concentration of DDT in the air in industrial, agricultural,
or disease control areas should not exceed 1 mg/m3 on a time-
weighted basis (40 h per week). Several countries have their own
standards that range from 0.1 to 1 mg/m3, which seem to afford an
acceptable margin of safety.
There is ample reason to predict the continuing safety of workers
producing and using DDT. No harmful effect has ever been reported in
vector control operators who have applied DDT during the last 3
decades in public health programmes. Nevertheless, as in the case of
any chemical, occupational safety and health measures should always be
applied to ensure that contact with DDT by workers is kept to a
minimum.
The only index of exposure of DDT or its metabolites is the
analysis of these compounds in tissues or excreta. For most purposes
it is best to sample serum or plasma. In subjects with relatively
constant, prolonged exposure, concentrations of DDT and its
metabolites in the blood are in equilibrium with those in all other
tissues, including adipose tissue. In subjects who have accidentally
received a single large dose, the concentration in the brain is
reflected more accurately by a serum sample than by a fat sample.
REFERENCES
ABBOTT, D.C., HARRISON, R. B., TATTON, J. O'G., & THOMPSON, J. (1965)
Organochlorine pesticides in the atmospheric environment. Nature
(Lond.), 208: 1317-1318.
ABBOTT, D.C., GOULDING, R., & TATTON, J. O'G. (1968) Organochlorine
pesticide residues in human fat in Great Britain, Br. med. J.,
3: 146-149.
ABBOTT, D.C., HOLMES, D.C., & TATTON, J. O'G. (1969) Pesticide
residues in the total diet in England and Wales, 1966-67. J. Sci.
Food Agric., 20: 245-249.
ABBOTT, D. C, COLLINS, G. B., & GOULDING, R. (1972) Organochlorine
pesticide residues in human fat in the United Kingdom, 1969-71.
Br. med. J., 2: 553-556.
ABE, J., INOUE, Y., & TAKAMATSU, M. (1974) [On the residual amounts of
PCB and organochlorine pesticides in human blood.] Jpn J. Hyg.,
29: 93 (in Japanese).
ACKER, L. & SCHULTE, E. (1970) [The occurrence of polychlorinated
biphenyls and hexachlorobenzene along chlorinated insecticides in
human milk and human adipose tissue.] Naturwissenschaften,
57: 497 (in German).
ACKER, L. & SCHULTE, E. (1971) [Organochlorine compounds in the human
body.] Umschau, 71: 848 (in German).
ACKER, L. & SCHULTE, E. (1974) [Organochlorine compounds in human
fat.] Naturwissenschaften, 61: 32 (in German).
ADAMCZYK, E. (1971) [Translocation of DDT in chickens under the
influence of hunger.] Med. Weter, 27: 103-105 (in Polish).
ADAMOVIC, V., SOKIC, B., & PETROVIC, O. (1971) [On factors influencing
the occurrence of organochlorine insecticides in newborns.]
Ernaehrungsforschung, 16: 579-585 (in German).
ADAMS, M., COON, F. B., & POLING, C. E. (1974) Insecticides in the
tissues of four generations of rats fed different dietary fats
containing a mixture of chlorinated hydrocarbon insecticides.
J. agric. food Chem. 22: 69-75.
AHLING, B. & JENSEN, S. (1970) Reversed liquid-liquid partition in
determination of PCBs and chlorinated hydrocarbon insecticides in
water. Anal. Chem, 42: 1485-1486.
AHNHOFF, M. & JOSEFSON, B. (1974) Simple apparatus for on-site
continuous liquid-liquid extraction of organic compounds from
natural waters. Anal. Chem., 46: 658-663.
AIZICOVICI et al., Travaux Scientifiques de l'Institut d'Hygiene de
Jassy, 1960-1966, p. 142 (Quoted by: Wasserman, M., Sofoluwe,
G. O., Wasserman, D., Groner, Y., & Lazarovitch, S. (1968) Storage
of organochlorine insecticides in body fat of people in Nigeria.
In: Proceedings of the Lagos International Seminar on
Occupational Health for Developing Countries, Nigeria, 1-6 April
1968.
ALARY, J. G., GUAY, P., & BRODEUR, J. (1971) Effect of phenobarbital
on the metabolism of DDT in the rat and in the bovine. Toxicol.
appl. Pharmacol., 18: 457-468.
ALMEIDA, W. F., PIGATI, P., GAETA, R., & UNGARO, M. T. S. (1975) DDT
residues in human blood serum in Brazil. Environ. Qual. Saf.,
3 (Suppl.): 586-588.
ANON. (1972) [BHC and DDT residues in human milk on the decline in
Japan.] Noyaku Bijin, 56: 458 (in Japanese).
ANON. (1974) [Research methods for determination of chlorinated
hydrocarbon pesticide residues in or on food of animal origin.]
Bundesgesundheitsblatt, 17 (18): 269-276 (in German).
ANTOMMARIA, P., CORN, M., & DeMAIO, L. (1965) Airborne particulates in
Pittsburgh: Association with p,p'-DDT. Science, 150: 1476-1477.
ARMOUR, J. A. & BURKE, J. A. (1970) Separating polychlorinated
biphenyls from DDT and its analogues. J. Assoc. Off. Anal. Chem.,
53: 761-768.
ASAI, R. I., GUNTHER, F. A., WESTLAKE, W. E., & IWATA, Y. (1971)
Differentiation of PCBs from DDT by carbon skeleton chromatography.
J. agric. food. Chem., 19: 39-398.
ASKARI, E. M. & GABLIKS, J. (1973) DDT and immunological responses.
II. Altered histamine levels and anaphylactic shock in guineapigs.
Arch. environ. Health, 26: 309-312.
AUE, W. A. & TELI, P. M. (1971) Sampling air pollutants with support-
bonded chromatographic phases. J. Chromatogr., 62: 15-27.
BAGLEY, G. E., REICHEL, W. L., & CROMARTIE, E. (1970) Identification
of PCBs in two bald eagles by combined gas-liquid chromatography-
mass spectrometry. J. Assoc. Off. Anal. Chem., 53: 251-261.
BAKSEYEV, N. S. (1973) [Prophylaxis of pathology of the fetus and
newborn child (experience of the Ukrainian SSR).) Vestn. Akad.
Med. Nauk (SSSR), 28: 69-75 (in Russian).
BARKA, T. & POPPER, H. (1967) Liver enlargement and drug toxicity.
Medicine, 46: 103-117.
BELONOZKO, G. A. & KUCAK, JU. A. (1974) [Hygienic features of the
content of pesticides in the atmosphere.] In: Medved, L. E., ed.
Hygiene of application and toxicology of pesticides and the
clinical features of pesticides poisoning. A collection of
papers, Issue No. 5, pp. 63-74 Kiev. Vniigintox (in Russian).
BELONOZKO, G. A., VEVCHURKO, S. N., KUCHAK, U. A., TOVSTENKO, A. E., &
SOROKENA, L. V. (1967) [The spread of pesticides by air currents
in the environment in relation to method of application.] In:
Medved, L. E., ed. Hygiene and toxicology of pesticides and the
clinical features of pesticide poisoning. A collection of papers,
pp. 24-35 Kiev, Vniigintox (in Russian).
BENVENUE, A. & OGATA, J. N. (1970) Note on the use of florisil
adsorbent for separation of PCBs from chlorinated pesticides.
J. Chromatogr., 63: 142-144.
BERGENSTAL, D. M., HERTZ, R., LIPSETT, M. B., & MOY, R. H. (1960)
Chemotherapy of adrenocortical cancer with o,p'-DDD. Ann.
intern. Med., 53: 672-682.
BEYERMANN, K. & ECKRICH, W. (1973) [Adsorption of traces of
insecticides from water on polyethylene.] Z. anal Chem.,
265 (1): 1-4 (in German).
BEYERMANN, K. & ECKRICH, W. (1974) [Differentiation of the aerosol-
bound and gaseous fractions of insecticides in air.] Z. anal.
Chem., 269 (4): 279-284 (in German).
BEZUGLYI, V. P. & KASKEVICH, L. M. (1969) [Some indices of the
functional state of the liver in flying personnel engaged in
aerial chemical operations.] Gig. Tr. Prof. Zabol., 13 (10):
52-53 (in Russian).
BEZUGLYI, V. P., ODINTSOVA, I. L. R., & GORSKAYA, N. Z. (1973)
[Morphological composition of the blood in persons working with a
complex of organochlorine and organophosphorus pesticides.]
Vrach. Delox, 11: 150-154 (in Russian).
BICK, M. (1967) Chlorinated hydrocarbon residues in human body fat.
Med. J. Aust., 1: 1127-1129.
BIROS, F. J. (1970) Analysis for p,p'-DDT and p,p'-DDE pesticide
residues by NMR spectroscopy. J. Assoc. Off. Anal Chem.,
53: 733-736.
BISHARA, R. H., BORN, G. S., & CHRISTIAN, J. E. (1972a) Thin-layer
chromatography of DDT and some related compounds on aluminium
oxide chromatoplates. J. Chromatography, 64: 135-145.
BISHARA, R. H., BORN, G. S., & CHRISTIAN, J. E. (1972b) Radiotracer
distribution and excretion study of chlorophenothane in rats.
J. pharm. Sci, 61: 1912-1916.
BISKIND, M. S. (1952) [The new insecticides and the public health.]
Harefuah, 44: 9-13 (in Hebrew).
BISKIND, M. S. (1953) Public health aspects of the new insecticides.
Am. J. dig. Dis., 20: 331-341.
BISKIND, M. S. & BIEBER, I. (1949) DDT poisoning -- A new syndrome
with neuropsychiatric manifestations. Am. J. Psychother.,
3: 261-270.
BITMAN, J., CECIL, H. C., & FRIES, G. F. (1970) DDT-induced inhibition
of avian shell gland carbonic anhydrase. A mechanism for thin
eggshells. Science, 168: 594-596.
BJERK, J. E. (1972) [DDT and polychlorinated biphenyl residues in
human material from Norway.] Tidsskr, Nor. Laegeforen.,
92: 15-19, 37-38 (in Norwegian).
BLEDSOE, T., ROLAND, D. P., HEY, R. L., & LIDDLE, G. W. (1964) An
effect of o,p'-DDD on extra-adrenal metabolism of cortisol in
man. J. clin. Endocrinol. Metab., 24: 1303-1311.
BOIKO, V. G. & KRASNYUK, E. P. (1969) [Clinical characteristics of the
pathology of respiratory tracts of persons working with DDT.]
Z. ushn. nos. gorl. Bolezn., 29: 35-38 (in Russian).
BOJANOWSKA, A., JONCZYK, W., TRACZYK, Z., & KLAWE, Z. (1973) [DDT and
alpha-BHC content of blood and fatty tissue of subjects not
professionally exposed to these compounds.] Pol. Tyg. Lek.,
28: 1999-2001 (in Polish).
BOYD, E. M. & DECASTRO, E. S. (1968) Protein-deficient diet and DDT
toxicity. Bull. World Health Organ., 38: 141-150.
BOYD, E. M. & DECASTRO, E. S. (1970) Toxicity of dicophane (DDT) in
relation to dietary protein intake. Ind. Med., Surg.,
39: 229-236.
BOYD, E. M. & KRIJNEN, C. J. (1969) Dietary protein and DDT toxicity.
Bull. environ. Contam. Toxicol., 4: 256-261.
BRADY, M. N. & SIYALl, D. S. (1972) Hexachlorobenzene in human fat.
Med. J. Aust., 1: 158-161.
BRATOWSKI, T. A. & MATSUMURA, F. (1972) Properties of a brain
adenosine triphosphatase sensitive to DDT. J. econ. Entomol.,
65: 1238-1245.
BREWERTON, H. V. & MCGRATH, H. J. W. (1967) Insecticides in human fat
in New Zealand. N.Z.J. Sci., 10: 486-492.
BRODEUR, J. & LAMBERT, G. (1973) Further studies on the effect of
starvation and microsomal enzyme induction on the mobilization of
DDT residues in rats. Toxicol. appl. Pharmacol., 25: 488.
BRONISZ, H. & OCHYNSKI, J. (1968) [DDT and DDE content in the milk of
women.] Biul. Inst. Ochr. Rosl., 41: 99-102 (in Polish).
BRONISZ, H., RUSIECKI, W., OCHYNSKI, J., & BERNARD, E. (1967) [DDT in
adipose tissue of Polish population.] Diss. Pharm. Pharmacol.,
19: 309-314 (in Polish).
BRONISZ, H., OCHYSNSKI, J., & WLODZIMIERA, L. (1969) [Contents of DDT
in the adipose tissue of the Polish population.] Ann. Univ.
Mariae Curies -- Sklodwska. sect. D. (Med.), 24: 93-99 (in
Polish).
BROWN, J. R. (1967) Organochlorine pesticide residues in human depot
fat. Can. Med. Assoc, J., 97: 367-373.
BROWN, J. R. & CHOW, L. Y. (1975) Comparative study of DDT and its
derivatives in human blood samples in Norfolk County and Holland
Marsh, Ontario. Bull. environ. Contam. Toxicol., 13: 483-488.
BUNYAN, P. J., TOWNSHEND, M. G., & TAYLOR, A. (1972) Pesticide induced
changes in hepatic microsomal enzyme systems. Some effects of 1,1-
di( p-chlorophenyl)-2,2,2-trichloroethane (DDT) and 1,1-di( p-
chlorophenyl)-2,2-dichloroethylene (DDE) in the rat and Japanese
quail. Chem. Biol. Interactions, 5: 13-26.
BURLINGTON, H. & LINDERMAN, V. F. (1950) Effect of DDT on testes and
secondary sex characteristics of White Leghorn cockerels. Proc.
Soc. Exp. Biol., 74: 48-51.
BURNS, E. C., DAHM, P. A., & LINDQUIST, D. A. (1957) Secretion of DDT
metabolites in the bile of rats. J. Pharmacol. exp. Ther.,
121: 55-62.
BURNS, J. E. (1974) Organochlorine pesticide and polychlorinated
biphenyl residues in biopsied human adipose tissue -- Texas
1969-1972. Pestic. Monit. J., 7: 122-126.
BUTLER, P. A. (1969) Monitoring pesticide pollution. Biol. Sci.,
19: 889-891.
CAHILL, W. P., ESTESEN, B. J., & WARE, G. W. (1970) Determination of
DDT in presence of Toxaphene (Camphechlor) residues. Bull,
environ. Contam. Toxicol., 5: 260-262.
CAMERON, G. R. & BURGESS, F. (1945) The toxicity of 2,2-bis-( p-
chlorophenyl)-1,1,1-trichloroethane (DDT). Br. med. J.,
1: 865-871.
CAMPBELL, J. E., RICHARDSON, L. A., & SCHAFER, M. L. (1965)
Insecticide residues in the human diet. Arch. environ. Health,
10: 831-836.
CANLORBE, P., BADER, J. C., & JOB, J. C. (1971) Diagnostic problems
arising from a virilizing tumour of the adrenal cortex. (Case
report -- Literature review.) Sem. Hop., 47: 2255-2270.
CAREY, R. M., ORTH, D. N., & HARTMANN, W. H. (1973) Malignant melanoma
with ectopic production of adrenocorticotrophic hormone:
palliative treatment with inhibitors of adrenal steroid
biosynthesis. J. clin. Endocrinol. Metab., 36: 482-487.
CAROLLO, J. A. (1945) The removal of DDT from water supplies. J. Am.
Water Works Assoc., 37: 1310-1317.
CARR, R. L. (1970) Collaborative study of the Mills method of multiple
chlorinated pesticide residues in butter fat. J. Assoc. Off. Anal
Chem., 53: 152-154.
CARR, R. L. (1971) Collaborative study of a method of multiple
chlorinated pesticide residues in fish. J. Assoc. Off. Anal.
Chem., 54: 525-527.
CASARETT, L. J., FRYER, G. C., YAUGER, W. L., & KLEMMER, H. W. (1968)
Organochlorine pesticide residues in human tissues-Hawaii. Arch.
environ. Health, 17: 306-311.
CASE, R. A. M. (1945) Toxic effects of 2,2-bis(p-chlorophenyl)-1,1,1-
trichlorethane (DDT) in man. Br. med. J., 2: 842-845.
CASSIDY, W., FISHER, A. J., PEDEN, J. D., & PARRY-JONES, A. (1967)
Organochlorine pesticide residues in human fats from Somerset.
Mon. Bull. Minist. Health Lab. Serv. (Lond.), 26 (1): 2-6.
CHADWICK, R. W., CRANMER, M. F., & PEOPLES, A. J. (1971a) Metabolic
alterations in the squirrel monkey induced by DDT administration
and ascorbic acid deficiency. Toxicol. appl. Pharmacol.,
20: 308-318.
CHADWICK, R. W., CRANMER, M. F., & PEOPLES, A. J. (1971b) Comparative
stimulation of gamma-HCH metabolism by pre-treatment of rats with
gamma-HCH, DDT, and DDT + gamma-YCH. Toxicol. appl. Pharmacol.,
18: 685-695.
CHADWICK, R. W, LINKO, R. S., FREAL, J. J., & ROBBINS, A. L. (1975)
The effect of age and long-term low-level DDT exposure on the
response to enzyme induction in the rat. Toxicol. appl.
Pharmacol., 31: 469-480.
CHHABRA, R. S. & FOUTS, J. R. (1974) Stimulation of hepatic drug
metabolizing enzymes by DDT, polycyclic hydrocarbons or
phenobarbital in adrenalectomized or castrated mice. Toxicol.
appl. Pharmacol., 28: 465-476.
CHEN, JO-YUN, T. & DORITY, R. W. (1972) Infrared studies of DDT,
structurally related halogenated pesticides, and some metabolites.
J. Assoc. Off. Anal Chem., 55: 15-31.
CHIBA, M. (1969) Factors affecting the extraction of organochlorine
insecticides from soil. Residue Rev., 30: 63-113.
CHIBA, M. & MORLEY, H. V. (1968) Losses of pesticides during sample
preparation. J. Assoc. Off. Anal Chem., 51: 55-62.
CHIN, Y. & T'ANT, C. (1946) The effect of DDT on cutaneous sensation
in man. Science, 103: 654.
CIELESZKY, V., DENES, A., KNOLL, R., ENGST, R., & KOTARSKY, A. (1970)
[Methods for performing residue analysis of plant protection and
pest control agents in foodstuffs. II. Method for identifying and
the semiquantitative determination of residues of chlorinated
hydrocarbons in different food and in soil.] Nahrung,
14: 643-666 (in German).
CIUPE, R. (1976) [Determination of HCH, p,p'-DDT, p,p'-DDE in foods
and in the adipose tissue.] Igiena, 25: 113-117 (in Rumanian).
CLEMMESEN, J., FUGELSANG-FREDERICKSEN, V., & PLUM, C. M. (1974) Are
anticonvulsants oncogenic? Lancet, 1: 705-707.
CLIFFORD, N. J. & WEIL, J. (1972) Cortisol metabolism in persons
occupationally exposed to DDT. Arch. environ. Health,
24: 145-147.
COCHRANE, W. P. & CHAU, A. S. Y. (1971) Chemical derivitization
techniques for confirmation of organochlorine residue identity.
Pesticide identification at the residue level. Adv. Chem. Ser.,
140: 11-26.
COHA, F. & NEDIC, M. (1970) Improved gas-liquid chromatographic method
for analysis of DDT and its metabolites in milk. Anal. Lett.,
3: 419-421.
COHEN, J. M. & PINKERTON, C. (1966) Widespread translocation of
pesticides by air transport and rainout. Adv. Chem. Ser.,
60: 163-176.
COMMITTEE ON PESTICIDES (1951) Pharmacologic and toxicologic aspects
of DDT (chlorophenothane U.S.P.). J. Am. Med. Assoc.,
145: 728-733.
CONNEY, A. H. (1967) Pharmacological implications of microsomal enzyme
induction. Pharmacol. Rev., 19: 317-366.
CONOVER, W. J. (1971) Practical non-parametric statistics, New York,
Wiley & Sons, 462 pp.
COPELAND, F. M. & CRANMER, M. F. (1974) Effects of o,p'-DDT on the
adrenal gland and hepatic microsomal enzyme system of the beagle
dog. Toxicol. appl. Pharmacol., 27: 1-10.
COPPLESTONE, J. F., HUNNEGO, J. N., & HARRISON, D. L. (1973)
Insecticides in adult New Zealanders -- A five year study. N.Z.J.
Sci., 16: 27-39.
CRANMER, M. F. (1970) Effect of diphenylhydantoin on storage in the
rat. Toxicol. appl. Pharmacol., 17: 315.
CRANMER, M. F. (1972) Absence of conversion of o,p'-DDT to p,p'-DDT
in the rat. Bull. environ. Contam. Toxicol., 7: 121-124.
CRANMER, M. F. & COPELAND, M. F. (1973) Electron-capture gas-liquid
chromatographic analysis of DDA: utilization of 2-chloroethanol
derivative. Bull. environ. Contam. Toxicol., 9: 186-192.
CRANMER, M. F., CARROLL, J. J., & COPELAND, M. F. (1969) Determination
of DDT and metabolites, including DDA, in human urine by gas
chromatography. Bull. environ. Contam. Toxicol., 4: 214-223.
CRANMER, M. F., PEOPLES, A., & CHADWICK, R. (1972) Biochemical effects
of repeated administration of p,p'-DDT on the squirrel monkey.
Toxicol. appl. Pharmacol., 21: 98-101.
CROSBY, D. G. & MOILANEN, K. W. (1977). Vapor-phase photodecomposition
of DDT. Chemosphere, No. 4, pp 167-172.
CUETO, C., JR (1970) Cardiovascular effects of o,p'-DDD. Ind. Med.
Surg., 39: 31-32.
CUETO, C., JR & BIROS, F. J. (1967) Chlorinated insecticides and
related materials in human urine. Toxicol. appl. Pharmacol.,
10: 261-269.
CUETO, C., JR & BROWN, J. H. U. (1958) Biological studies on an
adrenocorticolytic agent and the isolation of the active
components. Endocrinology, 62: 334-339.
CUNNINGHAM, R. E. & HILL, F. S. (1952) Convulsions and deafness
following ingestion of DDT. Pediatrics, 9: 745-747.
CURLEY, A. & KIMBROUGH, R. (1969) Chlorinated hydrocarbon insecticides
in plasma and milk of pregnant and lactating women. Arch.
environ. Health, 18: 156-164.
CURLEY, A., COPELAND, M. F., & KIMBROUGH, R. D. (1969) Chlorinated
hydrocarbon insecticides in organs of stillborn and blood of
newborn babies. Arch. environ. Health, 19: 628-632.
CURLEY, A., BURSE, W. W., JENNINGS, R. W., & VILLANUEVA, E. C. (1973)
Chlorinated hydrocarbon pesticides and related compounds in
adipose tissue from people of Japan. Nature (Lond.),
242: 338-340.
CURRIE, L. A. (1968) Limits for qualitative detection and quantitative
determination. Anal. Chem., 40: 586-593.
CZEGLEDI-JANKO, G. (1969) [Residues of chlorinated hydrocarbons in the
blood of inhabitants of Budapest in 1967-1968 with particular
reference to lindane.] Z. Ges. Hyg. Ihre Grenzgeb., 15: 755-761
(in German).
CZEGLEDI-JANKO, G. & AVAR, P. (1970) Occupational exposure to lindane:
Clinical and laboratory findings. Br. J. ind. Med., 27: 283-286.
CZEGLEDI-JANKO, G. & CIELESZKY, V. (1968) A one-step extraction and
clean-up procedure for gas-liquid chromatographic determination of
some organochlorine pesticide residues in blood. Analyst,
93: 445-452.
DACRE, J. C. & JENNINGS R. W. (1970) Organochlorine insecticides in
normal and carcinogenic human lung tissues. Toxicol. appl.
Pharmacol., 17: 277.
DALE, W. E. & QUINBY, G. E. (1963) Chlorinated insecticides in the
body fat of people in the United States. Science, 142: 593-595.
DALE, W. E., GAINES, T. B., & HAYES, W. J., JR (1962) Storage and
excretion of DDT in starved rats. Toxicol. appl. Pharmacol.,
4: 89-106.
DALE, W. E., GAINES, T. B., HAYES, W. J., JR, & PEARCE, G. W. (1963)
Poisoning by DDT: Relation between clinical signs and
concentration in rat brain. Science, 142: 1474-1476.
DALE, W. E., COPELAND, M. F., & HAYES, W. J., JR (1965) Chlorinated
insecticides in the body fat of people in India. Bull. World
Health Organ., 33: 471-477.
DALE, W. E., COPELAND, M. F., PEARCE, G. W., & MILES, J. W. (1966a)
Concentration of o,p'-DDT in rat brain at various intervals
after dosing. Arch. int. Pharmacodyn. Ther., 162: 40-43.
DALE, W. E., CURLEY, A., & CUETO, C., JR (1966b) Hexane extractable
chlorinated pesticides in human blood. Life Sci., 5: 47-54.
DALE, W. E., CURLEY, A., & HAYES, W. J., JR (1967) Determination of
chlorinated insecticides in human blood. Ind. Med. Surg.,
36: 275-280.
DALE, W. E., MILES, J. W, & GAINES, T. B. (1970) Quantitative method
for determination of DDT and DDT metabolites in blood serum.
J. Assoc. Off. Anal. Chem., 53: 1287-1292.
DAL NOGARE, S. & JUVET, R. S. (1962) Gas-liquid chromatography, New
York, Interscience, 450 pp.
DAMASKIN, V. I. (1965) [The extent of the accumulation of DDT in the
human body in connection with its assimilation with food, and its
toxic effect.] Gig. i Sanit., 30 (10): 109-111 (in Russian).
DANGERFIELD, W. G. (1946) Toxicity of DDT to man. Br. med. J.,
1: 27.
DATTA, P. R. (1970) In vitro detoxification of p,p'-DDT via p,p'-
DDE to p,p'-DDA in rats. Ind. Med. Surg., 39: 190-194.
DATTA, P. R. & NELSON, M. J. (1968) Enhanced metabolism of
methyprylon, meprobamate, and chlordiazepoxide hydrochloride after
chronic feeding of a low dietary level of DDT to male and female
rats. Toxicol. appl. Pharmacol., 13: 346-352.
DATTA, P. R. & NELSON, M. F. (1970) p,p'-DDT detoxication by
isolated perfused rat liver and kidney. Ind. Med. Surg,
39: 195-198.
DAVIES, J. E., WELKE, J. O., & RADOMSKI, J. L. (1965) Epidemiological
aspects of the use of pesticides in the South. J. occup. Med.,
7: 612-618.
DAVIES, J. E., EDMUNDSON, W. F., SCHNEIDER, N. J., & CASSADY, J. C.
(1968) Problems of prevalence of pesticide residues in humans.
Pestic. Monit. J., 2: 80-85.
DAVIES, J. E., EDMUNDSON, W. R., CARTER, C. H., & BARQUET, A. (1969a)
Effect of anticonvulsant drugs on dicophane (DDT) residues in man.
Lancet, 2: 7-9.
DAVIES, J. E., EDMUNDSON, W. F., MACEO, A., BARQUET, A., & CASSADY, J.
(1969b) An epidemiologic application of the study of DDE levels in
whole blood. Am. J. Public Health, 59: 435-441.
DAVIES, J. E., EDMUNDSON, W. F., MACEO, A., IRVIN, G. L., III,
CASSADY, J., & BARQUET, A. (1971) Reduction of pesticide residues
in human adipose tissue with diphenylhydantoin. Food Cosmet.
Toxicol., 9: 413-423.
DAVIES, J. E., EDMUNDSON, W. F., & RAFEONELLI, A. (1975) The role of
house dust in human DDT pollution. Am. J. Public Health,
65: 53-57.
DAVIES, O. L. & GOLDSMITH, P. L. (1976) Statistical methods in
research and production, 4th ed., London, Longmans, 478 pp.
DEDEK, W. & SCHMIDT, R. (1972) Studies on the transplacental transport
and metabolism of 3H- and 14C-labeled DDT in pregnant mice
under hunger stress. Pharmazie, 27: 294-297.
DE FAUBERT MAUNDER, M. J., EGAN, H., GODLY, E. W., HAMMOND, E. W.,
ROBUM, J., & THOMSON, J. (1964) Clean-up of animal fats and dairy
products for the analysis of chlorinated pesticide residues.
Analyst, 89: 168-174.
DEICHMANN, W. B. & MACDONALD, W. E. (1976) Liver cancer deaths in the
continental USA from 1930 to 1972. Am. Ind. Hyg. Assoc. J.,
37: 495-498.
DEICHMANN, W. B. & MACDONALD, W. E. (1977) Organochlorine pesticides
and liver cancer deaths in the United States, 1930-1972.
Ecotoxicol. environ. Saf. 1: 89-110.
DEICHMANN, W. B. & RADOMSKI, J. L. (1968) Retention of pesticides in
human adipose tissue -- Preliminary report. Ind. Med. Surg.,
37: 218-219.
DEICHMANN, W. B., WITHERUP, S., & KITZMILLER, K. V. (1950) The
toxicity of DDT, Cincinnati, Kettering Laboratory of Applied
Physiology, 233 pp.
DEICHMANN, W. B., MACDONALD, W. E., & CUBIT, D. A. (1971a) DDT tissue
retention: Sudden rise induced by the addition of aldrin to a
fixed DDT intake. Science, 172: 275-276.
DEICHMANN, W. B., MACDONALD, W. E., BEASLEY, A. G., & CUBIT, D.
(1971b) Subnormal reproduction in beagle dogs induced by DDT and
aldrin. Ind. Med. Surg. 40 (2): 10-20.
DEICHMANN, W. B., MACDONALD, W. E., CUBIT, D. A., & BEASLEY, A. G.
(1972) Effects of starvation in rats with elevated DDT and
dieldrin levels. Int. Arch. Arbeitsmed., 29: 233-252.
DEL VECCHIO, V. & LEONI, V. (1967) [Study on the amount of chlorinated
insecticides in biological materials. II. Chlorinated insecticides
in adipose tissue in certain groups of the Italian population.]
Nuovi Ann. Ig. Microbiol., 18: 107-128 (in Italian).
DENES, A. (1962) [Problems of food chemistry concerning residues of
chlorinated hydrocarbons.] Nahrung, 6: 48-56 (in German).
DENES, A. (1964) Investigation of chlorinated hydrocarbon residues in
animal and vegetable fats. In: 1963 Year Book, Budapest,
Hungary, Institute of Nutrition.
DEUTSCHE FORSCHUNGSGEMEINSCHAFT (1969) [Residue analysis of plant
protection agents.] Weinheim, Verlag Chemie, 400 pp. (in German).
DE VLIEGER, M., ROBINSON, J., BALDWIN, M. K., CRABTREE, A. N., & VAN
DUK, M. C. (1968) The organochlorine insecticide content of human
tissue. Arch. environ. Health, 17: 759-767.
DOLAN, J. W. & HALL, R. C. (1973) Enhancement of the sensitivity and
selectivity of the Coulson electrolytic conductivity detector to
chlorinated hydrocarbon pesticides. Anal. Chem., 45: 2198-2204.
DOMENJOZ, R. (1944) [Experimental findings with a new insecticide
(Neocid, Geigy), a contribution to the theory contact-toxicity
action.] Schweiz. Med. Wochenschr., 74: 952-958 (in German).
DOMENJOZ, R. (1946a) [On the biological action of a DDT-derivative.]
Arch. Int. Pharmacodyn. Ther., 73: 128-146 (in German).
DOMENJOZ, R. (1946b) [Biological action of some DDT derivatives.]
Helv. Chem. Acta., 29: 1317-1322 (in French).
DRAIZE, J. H., NELSON, A. A., & CALVERY, H. O. (1944) The percutaneous
absorption of DDT (2,2-bis-(p-chlorophenyl)-1,1,1-trichloroethane)
in laboratory animals. J. Pharmacol. exp. Ther., 82: 159-166.
DUGGAN, R. E. (1968) Residues in food and feed. Pesticide residue
levels in food in the United States from July 1, 1963 to June 30,
1967. Pestic. Monit. J., 2: 2-46.
DUGGAN, R. E. & CORNELIUSSEN, P. E. (1972) Dietary intake of pesticide
chemicals in the United States (III), June 1968-April 1970.
Pestic. Monit. J., 5: 331-341.
DUGGAN, R. E. & LIPSCOMB, C. Q. (1969) Dietary intake of pesticide
chemicals in the United States (II), June 1966-April 1968.
Pestic. Monit. J., 2: 153-162.
DUGGAN, R. E. & WEATHERWAX, J. R. (1967) Dietary intake of pesticide
chemicals: Calculated daily consumption of pesticides with foods
are discussed and compared with currently accepted values.
Science, 157: 1006-1010.
DURHAM, W. F. & WOLFE, H. R. (1962) Measurement of the exposure of
workers to pesticides. Bull. World Health Organ. 26: 75-91.
DURHAM, W. F. & WOLFE, H. R. (1963) An additional note regarding
measurement of the exposure of workers to pesticides. Bull. World
Health Organ., 29: 279-281.
DURHAM, W. F., ARMSTRONG, J. F., UPHOLT, W. M., & HELLER, C. (1961)
Insecticide-content of diet and body fat of Alaskan natives.
Science, 134: 1880- 1881.
DURHAM, W. F., ORTEGA, P., & HAYES, W. J., JR (1963) The effect of
various dietary levels of DDT on liver function, cell morphology,
and DDT storage in the rhesus monkey. Arch. int. Pharmacodyn.
Ther., 141: 111-129.
DURHAM, W. F., ARMSTRONG, J. F., & QUNBY, G. E. (1965a) DDA excretion
levels. Arch. environ. Health, 11: 76-79.
DURHAM, W. F., ARMSTRONG, J. F., & QUINBY, G. E. (1965b) DDT and DDE
content of complete prepared meals. Arch. environ. Health,
11: 641-647.
DVORCHIK, B. H., ISTIN, M., & MAREN, T. H. (1971) Does DDT inhibit
carbonic anhydrase? Science, 172: 728-729.
DJATLOVlTSKAJA, F. G., GLADENKO, Y. F., & KRUCININA, A. A. (1972)
[Determination of organochlorine insecticides in reservoir water.]
Probl. anal Khim., 2: 43-36 (in Russian).
EDMUNDSON, W. F., DAVIES, J. E., & CRANMER, M. (1970a) DDT and DDE in
blood and urine of men exposed to 3 per cent DDT aerosol. Public
Health Rep., 85: 457-463.
EDMUNDSON, W. F., DAVIES, J. E., MACEO, A., & MORGADE, C. (1970b) Drug
and environmental effects on DDT residues in human blood. South.
med. J., 63: 1440-1441.
EGAN, H., GOULDING, R., ROBURN, J., & TATTON, J. O'G. (1965)
Organochlorine pesticide residues in human fat and human milk.
Br. med. J., 2: 66-69.
EICHELBERGER, J. W. & LICHTENBERG, J. J. (1971) Carbon adsorption for
recovery of pesticides (from water). J. Am. Water Works Assoc.,
63 (1): 25-27.
EMBRY, T. L., MORGAN, D. P., & ROAN, C. C. (1972) Search for
abnormalities of heme synthesis and sympathoadrenal activity in
workers regularly exposed to pesticides. J. occup. Med.,
14: 918-921.
ENGST, R. & KNOLL, R. (1972) DDT and DDE residues in human milk.
Nahrung, 16: 130-131.
ENGST, R., KNOLL, R., & NICKEL, B. (1967) [On the accumulation of
chlorinated hydrocarbons particularly of DDT and its metabolite
DDE in human fat.] Pharmazie, 22: 654-661 (in German).
ENGST, R., KNOLL, R., & NICKEL, B. (1970) [Occurrence of DDT and DDE
in fatty tissues and in organs of infants.] Pharmazie,
24: 673-676 (in German).
EPSTEIN, S.S. & SHAFNER, H. (1968) Chemical mutagens in the human
environment. Nature (Lond.), 219: 385-387.
ESKENASY, J. J. (1972) Status epilepticus by
dichlorodiphenyltrichloroethane and hexachlorocyclohexane
poisoning. Rev. Roum. Neurol., 9: 435-442.
EYZAGUIRRE, C. & LILIENTHAL, J. L., JR (1949) Veratrinic effects of
pentamethylenetetrazol (Metrazol) and 2,2-bis-( p-chlorophenyl)-
1,1,1-trichloroethane (DDT) on mammalian neuromuscular function.
Proc. Soc. Exp. Biol., 70: 272-275.
FAO (1972) Production yearbook-1971, Rome, Food and Agriculture
Organization, Vol. 25, pp. 499-537.
FAO/WHO (1973) 1972 Evaluations of some pesticide residues in food,
Geneva, WHO, 587 pp. (WHO Pest. Res. Series No. 2).
FARKAS, I., DESI, I., & KEMENY, T. (1969) [Effect of orally consumed
DDT on the rest and load EEG curves.] Egeszsegtudomany,
13 (2): 195-201 (in Hungarian).
FEIL, V. J., LAMOUREUX, C. H., STYRVOKY, E., ZAYLSKIE, R. G., THACKER,
E. J., & HOLMAN, G. M. (1973) Metabolism of o,p'-DDT in rats.
J. agric. food Chem, 21: 1072-1078.
FEIL, V. J., LAMOUREUX, C. H., & ZAYLSKIE, R. G. (1975) Metabolism of
o,p'-DDT in chickens. J. agric. food Chem., 23: 382-388.
FENNAH, R. G. (1945) Preliminary tests with DDT against insect pests
of food-crops in the Lesser Antilles. Trop. Agric., 22: 222-226.
FINSTERWALDER, C. E. (1976) Collaborative study of an extension of the
Mills et al. method for the determination of pesticide residues in
food. J. Assoc. Off. Anal. Chem, 59: 169-171.
FISEROVA-BERGEROVA, V., RADOMSKI, J. L., DAVIES, J. E., & DAVIES, J.
H. (1967) Levels of chlorinated hydrocarbon pesticides in human
tissues. Ind. Med. Surg., 36: 65-70.
FISHBEIN, L. F. (1974) Chromatographic and biological aspects of DDT
and its metabolites. J. Chromatogr., 98: 177-251.
FITZHUGH, O. G. (1948) Use of DDT insecticides on food products. Ind.
Eng. Chem., 40: 704-705.
FITZHUGH, O. G. (1966) Problems related to the use of pesticides.
Can. Med. Assoc. J., 94: 598-604.
FITZHUGH, O. G. (1970) A summary of a carcinogenic study of DDT in
mice from Food and Drug Administration, USA. In: FAO/ WHO 1969
evaluations of some pesticide residues in foods, Geneva. WHO,
pp. 61-64 (WHO/Food Add./70.38).
FITZHUGH, O. G. & NELSON, A. A. (1947) The chronic oral toxicity of
DDT (2,2-bis- p-chlorophenyl- 1,1,1-trichloroethane).
J. Pharmacol., 89: 18-30.
FRANCONE, M.P., MARLANI, F. H., & DEMARE, C. (1952) [Clinical signs of
poisoning by DDT.] Rev. Asoc. Méd. Argent., 66: 56-59 (in
Spanish).
FREDERICKS, C. M., LARSON, R. E., & ASTON, R. (1974) Effect of chronic
DDT exposure on pentobarbital tolerance and intolerance in the
rat. Toxicol. appl. Pharmacol., 27: 99-107.
FRENCH, M. C. & JEFFERIES, D. J. (1969) Degradation and disappearance
of ortho-, para-isomer of technical DDT in living and dead arian
tissues. Science, 165: 914-916.
FRIDMAN, G. I. (1970) [Effect of 7-trichlorofon and DDT on some
specific and non-specific characteristics of the
immunobiological and general reactivity of the organism (e.g.
problems of toxicological reactions of low intensity)] In:
[Questions of pesticide hygiene and toxicology.] Moscow,
Medicina, pp. 139-145 (in Russian).
FRIES, G. F., MARROW, G. S., JR, LESTER, J. W., & GORDON, C. H. (1971)
Effect of microsomal enzyme inducing drugs on DDT and dieldrin
elimination from cows. J. dairy Sci., 54: 364-368.
GÄB, S., NITZ, S., PARLAR, H., & KORTE, F. (1975) Photomineralisation
of certain aromatic xenobiotica. Chemosphere, No. 4,
pp. 251-256.
GÄB, S., SCHMITZER, J., THAMM, H. W., PARLAR, H. & KORTE, F. (1977)
Photomineralisation rate of organic compounds adsorbed on
particulate matter. Nature (Lond.), 270: 331-333.
GABLIKS, J. & MALTBY-ASKARI, E. (1970) The effect of chlorinated
hydrocarbons on drug metabolism in mice. Ind. Med. Surg.,
39: 347-350.
GABLIKS, J., ASKARI, E. M., & YOLEN, N. (1973) DDT and immunological
responses. I. Serum antibodies and anaphylactic shock in guinea
pigs. Arch. environ. Health, 26: 305-308.
GABLIKS, J., AL-ZUBAIDY, T., & ASKARI, E. (1975) DDT and immunological
responses. III. Reduced anaphylaxis and mast cell populations in
rats fed DDT. Arch. environ. Health, 30: 81-84.
GAINES, T. B. (1960) The acute toxicity of pesticides to rats.
Toxicol. appl. Pharmacol., 2: 88-99.
GAMES, T. B. (1969) Acute toxicity of pesticides. Toxicol. appl.
Pharmacol, 14: 515-534.
GALLAGHER, T. F., FUKUSHIMA, D. K., & HELLMANN, L. (1962) The effect
of ortho-, para-DDD on steroid borne metabolites in adrenocortical
carcinoma. Metab. clin. Exper., 11: 1155-1161.
GALTON, F. (1879) The geometric mean, in vital and social statistics.
Proc. R. Soc. Lond. B, 29: 365-367.
GARRETT, R. M. (1947) Toxicity of DDT for man. J. Med. Assoc.
Alabama, 17: 74-76.
GELLERT, R. J., HEINRICHS, W. L., & SWERDLOFF, R. S. (1972) DDT
homologues: Estrogen like effects on the vagina, uterus, and
pituitary of the rat. Endocrinology, 91: 1095-1100.
GENINA, S. A., SVETLAJA, E. N., & KOMAROVA, L. I. (1969) [Blood levels
of organic chlorine pesticides and some haematological indicators
in people employed in applying pesticides from the air.] In:
Medved', M. E., ed. The hygiene of application and the toxicology
of pesticides and the clinical features of pesticide poisoning. A
collection of papers, Issue No. 7, pp. 492-496, Kiev,
Vniigintoks (in Russian).
GIL G. P. & MIRON, B. F. (1949) [Investigation on toxicity of DDT to
man.] Med. Colon., 14: 459-470 (in Spanish).
GILBERT, D. & GOLBERG, L. (1967) BHT oxidase. A liver-microsomal
enzyme induced by the treatment of rats with butylated
hydroxytoluene. Food Cosmet. Toxicol., 5: 481-890.
GILLETT, J. W. (1968) No effect level of DDT in induction of
microsomal epoxidation. J. agric. food Chem., 16: 295-297.
GINGELL, R. & WALLCAVE, L. (1974) Species differences in the acute
toxicity and tissue distribution of DDT in mice and hamsters.
Toxicol. appl. Pharmacol., 28: 385-394.
GIUFFRIDA, L. & IVES, N. F. (1969) Microcoulomeric gas chromatography:
Improvement in instrumentation. J. Assoc. Off. Anal. Chem.,
52: 541-545.
GOERLITZ, D. F. & LAW, L. M. (1972) Chlorinated naphthalenes in
pesticide analysis. Bull. environ. Contain. Toxicol.,
7: 243-251.
GORDON, A. E. & FRIGERIO, A. (1972) Mass fragmentography as an
application of gas-liquid chromatograph-mass spectrometry in
biological research. J. Chromatogr., 73: 401-417.
GORDON, H. T. & WELSH, J. H. (1948) The role of ions in axon surface
reactions to toxic organic compounds. J. cell comp. Physiol.,
31: 395-420.
GRACA, I., SILVA FERDANANDES, A. M. S, & MOURAO, H. C. (1975)
Pesticides in people -- Organochlorine insecticide residues in
human milk in Portugal. Pestic. Monit. J., 8: 148-156.
GRACHEVA, G. V. (1969) [The possibility of DDT accumulation in the
organism of persons not having occupational contact with it.]
Faktory Vneshn. Sredy ikh Znachen, 1: 125-129 (in Russian).
GRACHEVA, G. V. (1970) [DDT excretion with the milk of nursing mothers
occupationally unexposed to the effect of this insecticide.] Vop.
Pitan., 29: 75-78 (in Russian).
GRIFFITH, F. D., JR & BLANKE, R. V. (1974) Microcoulometric
determination of organochlorine pesticides in human blood.
J. Assoc. Off. Anal Chem., 57: 595-603.
GRIFFITH, F. D., JR & BLANKE, R. V. (1975) Pesticides in people --
Blood organochlorine pesticide levels in Virginia residents.
Pestic. Monit. J., 8: 219-223.
GUENZI, W. D. & BEARD, W. E. (1968) Anaerobic conversion of DDT to DDD
and aerobic stability in soil. Proc. Soil Sci. Soc. Am.,
32: 522-534.
HAAG, H. B, EINNEGAN, J. K., LARSON, P.S., DREYFUSS, M. L., MAIN, R.
J., & RIESE, W. (1948) Comparative chronic toxicity for warm-
blooded animals of 2,2-bis( p-chlorophenyl)-1,1,1-trichloroethane
(DDT) and 2,2-bis-( p-chlorophenyl)-1,1-dichloroethane (DDD).
Ind. Med. Surg., 17: 477-484.
HAJKINA, B. I. & SILINA, V. F. (1971) [The effect of some
organochlorine pesticides on serotonin metabolism.] Farmakol.
Toxikol., 34: 357-359 (in Russian).
HALACKA, K., KAKL, J., & VYMETAL, F. (1965) [A reflexion in human fat
tissue of the extensive use of DDT.] Czech. Hyg., 10: 188-192
(in Czech).
HANADA, M., YUTANI, C., & MIYAJI, T. (1973) Induction of hepatoma in
mice by benzene hexachloride. Gann, 64: 511-513.
HARA, A., OWASAKI, I., NAWA, H, YOSHIOKA, Y., & YOKOO, S. (1973)
[Organochlorine insecticide residues in blood of pregnant women
and in umbilical cord blood.] Prog. Med., 84: 79-80 (in
Japanese).
HARMAN, S. W. (1946) DDT for codling moth control in western New York
in 1945. J. econ. Entomol., 39: 208-210.
HARRISON, R. D. (1975) Comparative toxicity of some selected
pesticides in neonatal and adult rats. Toxicol. appl. Pharmacol.,
32: 443-446.
HART, L. G. & FOUTS, J. R. (1963) Effects of acute and chronic DDT
administration on hepatic microsomal drug metabolism in the rat.
Proc. Soc. Exp. Biol. Med., 114: 388-392.
HART, M. M., REGAN, R. L., & ADAMSON, R. H. (1973) The effect of
isomers of DDD on the ACTH-induced steroid output, histology, and
ultrastructure of the dog adrenal cortex. Toxicol. appl.
Pharmacol., 24: 101-113.
HARTLEY, G. S. (1969) Evaporation of pesticides. In: Gould, R. F., ed.
Pesticidal Formulations Research, Washington, DC, American
Chemical Society, pp. 115-134 (Adv. Chem. Ser. 86).
HATTULA, M. L., IKKALA, J., ISOMÄKI, M., MÄÄTTÄ, K., & ARSTILA, A. U.
(1976) Chlorinated hydrocarbon residues (PCB and DDT) in human
liver, adipose tissue and brain in Finland. Acta Pharmacol.
Toxicol., 39: 545-554.
HAYASHI, M. (1972a) [Pollution of mothers' milk by organochlorine
pesticides.] Jpn J. Public Health, 19: 437-441 (in Japanese).
HAYASHI, M. (1972b) [Pesticide pollution of mothers' milk.] J. Jpn
Med. Assoc., 68: 1281-1286 (in Japanese).
HAYES, W. J., JR (1959) Pharmacology and toxicology of DDT. In:
Müller, P., ed. DDT: The insecticide dichlorodiphenyltri-
chloroethane and its significance, Basel, Birkhäuser Verlag,
Vol. 2, pp. 9-247.
HAYES, W. J., JR (1965) Review of the metabolism of chlorinated
hydrocarbon insecticides especially in mammals. Ann. Rev.
Pharmacol., 5: 27-52.
HAYES, W. J., JR (1975) Toxicology of pesticides, Baltimore,
Williams & Wilkins Co., 580 pp.
HAYES, W. J., JR (1976a) Mortality in 1969 from pesticides, including
aerosols. Arch. environ. Health, 31: 61-72.
HAYES, W. J., JR (1976b) Dosage relationships associated with DDT in
milk. Toxicol. appl. Pharmacol., 38: 19-28.
HAYES, W. J., JR & DALE, W. E. (1964) Concentration of DDT in brain
and other tissues in relationship to symptomatology. Toxicol.
appl. Pharmacol., 6: 349.
HAYES, W. J., JR, DURHAM, W. F., & CUETO, C., JR (1956) The effect of
known repeated oral doses of chlorphenothane (DDT) in man. J. Am.
Med. Assoc., 162: 890-897.
HAYES, W. J., JR, QUINBY, G. E., WALKER, K. C., ELLIOTT, J. W., &
UPHOLD, W. M. (1958) Storage of DDT and DDE in people with
different degrees of exposure to DDT. Am. Med. Assoc. Arch. ind.
Health, 18: 398-496.
HAYES, W. J., JR, DALE, W. E., & LEBRETON, R. (1963) Storage of
insecticides in French people. Nature (Lond.), 199: 1189-1191.
HAYES, W. J., JR, DALE, W. E., & BURSE, V. W. (1965) Chlorinated
hydrocarbon pesticides in the fat of people in New Orleans. Life
Sci., 4: 1611-1615.
HAYES, W. J, JR, DALE, W. E., & PIRKLE, C. I. (1971) Evidence of
safety of long-term, high, oral doses of DDT for man. Arch.
environ. Health, 22: 119-135.
HEATH, D. F. & VANDEKAR, M. (1964) Toxicity and metabolism of dieldrin
in rats. Br. J. ind. Med, 21: 269-279.
HEINRICKS, W. J., GELLERT, R. J., BAKKE, J. L., & LAWRENCE, N. L.
(1971) DDT administered to neonatal rats induces persistent estrus
syndrome. Science, 173: 642-643.
HELLMAN, L., BRADLOW, H. L., & ZUMOFF, B. (1973) Decreased conversion
of androgens to normal 17-ketosteroid metabolites as a result of
treatment with o,p'-DDD. J. clin. Endocrinol. Metab.,
36: 801-803.
HENDERSON, G. L. & WOOLLEY, D. E. (1969) Tissue concentrations of DDT:
Correlation with neurotoxicity in young and adult rats. Proc.
West. Pharmacol. Soc., 12: 58-62.
HENDERSON, G. L. & WOOLLEY, D. E. (1970) Mechanisms of neurotoxic
action of 1,1,1-trichloro-2,2-bis-( p-chlorophenyl)-ethane (DDT)
in immature and adult rats. J. Pharmacol. exp. Ther.,
175: 60-68.
HERZEL, F. & LAHMANN, E. (1973) [Contribution to quantitative
determination of pesticides in the air.] Gesundh.-Ing,
94: 275-279 (in German).
HESS, A.D. & KEENER, G. G., JR (1947) Effect of airplane-distributed
DDT thermal aerosols on fish and fish food organisms. J. Wildl.
Manage., 11: 1-10.
HEYNDRICKX, A. & MAES, R. (1969) The excretion of chlorinated
hydrocarbon insecticides in human mother milk. J. Pharm. Belg.,
9-10: 459-463.
HIDAKA, K., OHE, T., & FUJIWARA, K. (1972) [PCB and organochlorine
pesticides in mother's milk.] Igakuno Ayumi 82: 519-520 (in
Japanese).
HILL, D. W. & MCCARTY, P. L. (1967) Anaerobic degradation of selected
chlorinated hydrocarbon pesticides. J. Water Pollut. Control
Fed., 39: 1259-1277.
HILTON, B. D. & O'BRIEN, R. D. (1970) Antagonism by DDT of the effect
of valinomycin on synthetic membrane. Science, 168: 841-843.
HOFFMANN, C. H. & MERKEL, E. P. (1948) Fluctuations in insect
populations associated with aerial applications of DDT to forests.
J. econ. Entomol., 41: 467-473.
HOFFMANN, C. H. & SURBER, E. W. (1948) Effects of an aerial
application of wettable DDT on fish and fish food organisms in
Back Creek, West Virginia. Trans. Am. Fish. Soc., 75: 48-58.
HOFFMAN, D. G., WORTH, H. M., EMMERSON, J. L., & ANDERSON, R. C.
(1970) Stimulation of hepatic drug-metabolizing enzymes by
chlorphenothane (DDT); the relationship to liver enlargement and
hepatotoxicity in the rat. Toxicol. appl. Pharmacol.,
16: 171-178.
HOFFMAN, D. L. & MATTOX, V. R. (1972) Treatment of adrenocortical
carcinoma with o,p'-DDD. Med. Clin. North Am., 56: 999-1012.
HOFFMAN I. & LENDLE, L. (1948) [On the action of new insecticide
compounds.] Arch. exp. Pharmakol., 205: 223-242 (in German).
HOFFMAN, W. S. (1968) Clinical evaluation of the effects of pesticides
in man. Ind. Med. Surg., 37: 289-292.
HOFFMAN, W. S., FISHBEIN, W. I., & ANDELMAN, M. B. (1964) The
pesticide content of human fat tissue. Arch. environ. Health,
9: 387-394.
HOFFMAN, W. S., ADLER, H., FISHBEIN, W. I., & BAUER, F. C. (1967)
Relation of pesticide concentrations in fat to pathological
changes in tissues. Arch. environ. Health, 15: 758-765.
HOLDEN, A. V. (1970) International co-operative study of
organochlorine pesticide residues in terrestrial and aquatic
wildlife, 1967/68. Pestic. Monit. J., 4: 117-135.
HOLDEN, A. V. (1973) International co-operative study of
organochlorine and mercury residues in wildlife. Pestic. Monit.
J., 7: 37-52.
HORNABROOK, R. W., DYMENT, P. G., GOMES, E. D., & WISEMAN, J. S.
(1972) DDT residues in human milk from New Guinea natives. Med.
J. Aust., 1: 1297-1300.
HORWITZ, W., ed. (1975) Official methods of analysis, Washington,
DC, Association of the Official Analytic Chemists, 1094 pp.
HOWELL, D. E. (1948) A case of DDT storage in human fat. Proc. Okla.
Acad. Sci., 29: 31-32.
HRDINA, P. D., SINGHAL, R. L., PETERS, D. A. V., & LING, G. M. (1973)
Some neurochemical alterations during acute DDT poisoning.
Toxicol. appl. Pharmacol., 25: 276-288.
HRDINA, P. D., SINGHAL, R. L., & LING, G. M. (1975) DDT and related
hydrocarbon insecticides: Pharmacological basis of their toxicity
in mammals. Adv. Pharmacol. Chemother., 12: 31-88.
HRUSHKA, J. (1969) [DDT residues in the milk, butter, and fat of
cattle.] Veterinarstvi, 19 (11): 492-498 (in Czech).
HRUSHKA, J. & KOCIANOVA, M. (1975) [Contamination of the food chain by
chlorinated insecticides in Southern Bohemia.] Czech. Hyg.,
20: 421-428 (in Czech).
HSIEH, H. C. (1954) DDT intoxication in a family in Southern Taiwan.
Am. Med. Assoc. Arch. ind. Hyg., 10: 334-346.
HUEPER, W. C. (1942) Occupational tumors and allied diseases,
Springfield. IL. Thomas, pp. 496-497.
HUNTER, C. G., ROBINSON, J., & RICHARDSON, A. (1963) Chlorinated
insecticide content of human body fat in southern England.
Br. med. J., 1: 221-224.
HUTTERER, F., SCHAFFNER, F., KLION, F. M., & POPPER, H. (1968)
Hypertrophic, hypoactive smooth endoplasmic reticulum: A sensitive
indicator of hepatotoxicity exemplified by dieldrin. Science,
161: 1017-1019.
INNES, J. R. M., ULLAND, B. M., VALERIO, M. G., PETRUCELLI, L.,
FISHBEIN, L., HART, E. R., PALLOTTA, A. J., BATES, R. R., FALK, H.
L., GART, J. J., KLEIN, M., MITCHELL, & PETERS, J. (1969) Bioassay
of pesticides and industrial chemicals for tumorigenicity in mice.
A preliminary note. J. Natl Cancer Inst., 42: 1101-1114.
INOUE, Y., ABE, J., TAKAMATSU, M., & AOKI, N. (1974) [A study of the
qualitative and quantitative ratio of PCB and organochlorine
pesticide residues in the blood and adipose tissue.] Jpn J. Hyg.,
29: 92 (in Japanese).
INSTITUTE OF PETROLEUM (1968) Recommended practice for determining
precision data for methods on petroleum products and lubricants,
London, Institute of Petroleum.
ISRAELI, VON R. & MAYERSDORF, A. (1973) [Pathological changes in the
EEG during work with halogen-containing insecticides.] Zentralbl.
Arbeitsmed., 11: 340-343 (in German).
INTERNATIONAL AGENCY FOR RESEARCH ON CANCER (1974) IARC monographs:
The evaluation of the carcinogenic risk of chemicals to man, some
organochloride pesticides, Lyons, IARC, 241 pp. (IARC
monographs, Vol. 5).
ITO, N., NAGASAKI, H., ARAI, M., SUGIHARA, S., & MAKIURA, S. (1973)
Histological and ultrastructural studies on the
hepatocarcinogenicity of benzene hexachloride in mice. J. Natl
Cancer Inst., 51: 817-826.
JAGER, K. W. (1970) Aldrin, Dieldrin, Endrin, and Telodrin: An
epidemiological and toxicological study of long-term occupational
exposure, New York, Elsevier, 239 pp.
JANSSON, B., JENSEN, S., OLSSON, M., RENBERG, L., SUNDSTRÖM, G., &
VAZ, R. (1975) Identification by GC-MS of phenolic metabolites of
PCB and p,p'-DDE isolated from Baltic guillemot and seal.
Ambio, 4: 93-97.
JENSEN, S. & JANSSON, B. (1976) Anthropogenic substances in seal from
the Baltic: Methyl sulfone metabolites of PCB and DDE. Ambio,
5: 257-260.
JENSEN, J. A., CUETO, C., JR, DALE, W. E., ROTHE, C. F., PEARCE, G.
W., & MATTSON, A. M. (1957) Metabolism of insecticides. DDT
metabolites in feces and bile of rats. J. agric. food Chem.,
5: 919-925.
JEYARATNAM, J. & FORSHAW, J. (1974) A study of the cardiac effects of
DDT in laboratory animals. Bull. World Health Organ.,
51: 531-535.
JOHNSON, L. G. (1973) Formation of pentafluorobenzyl derivatives for
the identification and quantitation of acid and phenolic pesticide
residues. J. Assoc. Off. Anal. Chem., 56: 1503-1505.
JONCZYK, H. (1970) [The content of organochlorine insecticides in the
blood of healthy persons.] Rocz. panstw. Zakl. Hig., 21: 589-593
(in Polish).
JONCZYK, H., DMOCH, J., & BOJANOWSKA, A. (1970) [Determination of
chlorinated hydrocarbons in adipose tissue and brain of
Partridges.) Rocz. panst. Zakl. Hig., 21: 409-415 (in Polish).
JONCZYK, H., RUDOWSKI, W., TRACZYK, Z., & KLAWE, Z. (1974) [DDT and
its metabolites and gamma-HCH in blood, bone marrow, and fatty
tissue in certain hematological syndromes.] Pol. Tyg. Lek.,
29: 1573-1576 (in Polish).
JOY R. M. (1973) Electrical correlates of preconvulsive and convulsive
doses of chlorinated hydrocarbon insecticides in the CNS.
Neuropharmacology, 12: 63-76.
JUDAH, J. D. (1949) Studies on the metabolism and mode of action of
DDT. Br. J. Pharmacol, 4: 120-131.
JUDE, A. & GIRARD, P. (1949) [DDT toxicity -- mass poisoning by
accidental ingestion.] Ann. Med. lég. Criminol., Police Sci. Med.
Soc. Toxicol., 29: 209-213 (in French).
JUSZKIEWlCZ, T. & STEC, J. (1971) [Polichlorinated insecticide
residues in adipose tissue of farmers in the Lublin Province
(Poland).] Pol. Tyg. Lek., 26: 462-464 (in Polish).
KACEW, S. & SINGHAL, R. L. (1972) Role of adrenals in acute effects of
p.p'-DDT renal glucose metabolism. Fed. Proc., 31 (2): 520 Abs
(Abstract No. 1727).
KACEW, S. & SINGHAL, R. L. (1973) Adaptive response of hepatic
carbohydrate metabolism to oral administration of p,p'-1,1,1-
trichloro-2,2-bis-( p-chlorophenyl)-ethane in rats. Biochem.
Pharmacol., 22: 47-57.
KADIS, V. W., BREITKREITZ, W. E., & JONASSON, O. J. (1970) Insecticide
levels in human tissues of Alberta residents. Can. J. Public
Health, 61: 413-416.
KAGAN, Y. S., RODIONOV, G. A., WORONINA, L. Y., VELICHKO, L. S.,
KULAGIN, O. M., & PEREMITINA, A.D. (1969) [Effect of DDT on the
functional and morphological condition of the liver.] Vrach.
Delo, 12: 101-105 (in Russian).
KAISER, H. (1973) Guiding concepts relating to trace analysis. Pure
appl. Chem., 34: 35-61.
KAKU, S. (1973) [An epidemiological study on organochlorine pesticide
contamination. On pesticide residues in plasma of so-called
healthy Japanese in 1971.] J. Kurume Med. Assoc., 36: 307-326
(in Japanese).
KALISER, L. A. (1968) An in vitro and in vivo study of the effect
of DDT on the phagocytic activity of rat white blood cells.
Toxicol. appl. Pharmacol., 12: 353-357.
KALOYANOVA, F., MIHAILOVA, Z., GEORGIV, G., BENCHEV, I., RISOV, V., &
VELICHKOVA, V. (1972) Organochlorine pesticides in the fat of the
general population of Bulgaria. Abstracts of the XVII
International Congress on Occupational Health, Buenos Aires,
p. 75.
KAMATA, T. (1974) [On the present status of environmental pollution by
organochlorine pesticide residues.] Jpn J. Public Health,
21: 163-164 (in Japanese).
KAMATA, T. (1974) [Hygienic studies on pesticide residues: Part 4.
Environmental contamination by organochlorine pesticides.] Med.
J. Hiroshima Univ., 22: 315-325 (in Japanese).
KANITZ, S. & CASTELLO, G. (1966) [On the presence of residues of some
disinfectants in human adipose tissue and in food.] G. Ig. Med.
Prev., 7: 1-19 (in Italian).
KARIMOV, A. M. (1969) [Skin sensitivity to organochlorine
insecticides.] Med. Zh. Uzbekistant (No. 9): 58-60 (in Russian).
KARIMOV, A. M. (1970) [Occupational skin diseases in cotton growers
caused by chemical poisons and measures for their prevention.]
Gig. Tr. Prof. Zabol., 14: 35-37 (in Russian).
KASAI, A., ASANU, A. S., & NAKAMURA, S. (1972) [Studies on
organochlorine pesticide residues in human organs, Part III.]
J. Jpn Assoc. Rural Med., 21: 296-297 (in Japanese).
KATO, K., YAMADA, T., WATANABE, S., WADA, Y., FUKUDA, M., KURODA, M.,
NAKAOKA, S., TAKAHASHI, W., MIYASHIRO, K., MASUAD, J. & YASUKATA,
S. (1971) [Analyses of residual pesticides in vegetables, cows'
milk, and mothers' milk.] Ann. Rep. Kanagawa Prefect. Inst.
Public Health, 21: 85-92 (in Japanese).
KAWAI, Y., HORI, Y., NIGAWA, Y., YAMAMOTO, I., TSUZUKI, T., KITAYAMA,
M., & MORI, K. (1973) [On the pollution of mothers' milk by
pesticides and PCB in Hokkaido.] J. Food Hyg. Soc. Jpn,
14: 302-303 (in Japanese).
KAWANISHI, A., ASANUMA, S., & NAKAMURA, S. (1973) [Studies on
organochlorine pesticide residues in humans. Report 4.] J. Jpn
Assoc. Rural Med., 22: 278-279 (in Japanese).
KAZEN, C., BLOOMER, A., WELCH, R., OUDBIER, A., & PRICE, H. (1974)
Persistence of pesticides on the hands of some occupationally
exposed people. Arch. environ. Health, 29: 315-318.
KEIL, J. E., SANDIFER, S. H., FINKLEA, J. H., & PRIESTER, L. E. (1972)
Serum vitamin A elevation in DDT exposed volunteers. Bull.
environ. Contam. Toxicol., 8: 317-320.
KELLER, H. (1952) [Dichlorodiphenyltrichloroethane (DDT), additional
investigations on its properties and application.]
Naturwissenschaften, 39: 109 (in German).
KEPLINGER, M. L, DEICHMANN, W. B., & SALA, F. (1970) Effect of
combinations of pesticides on reproduction in mice. In: Deichmann,
W. B., ed. Pesticides Symposia, Miami, Halos & Associates,
pp. 125-138.
KHAIRY, M. (1959) Changes in behavior associated with a nervous system
poison (DDT). Q. J. exp. Physiol., 11 (2): 91-94.
KIMBROUGH, R. D., GAINES, T. B., & HAYES, W. J., JR (1968) Combined
effect of DDT, pyrethrum, and piperonyl butoxide on rat liver.
Arch. environ. Health, 16: 333-341.
KINOSHITA, F. K., FRAWLEY, J. P., & DUBOIS, K. P. (1966) Quantitative
measurement of induction of hepatic microsomal enzymes by various
dietary levels of DDT and toxaphene in rats. Toxicol. appl.
Pharmacol, 9: 505-513.
KLEIN, W. & KORTE, F. (1970) [Metabolism of chlorinated hydrocarbons.]
In: Wegler, R., ed. Chemie der Pflanzenschutz-und Schädlings-
bekämpfungsmittel, Band I, Berlin, Springer Verlag, pp. 199-218
(in German).
KLEIN, A. K., LAUG, E. P., KATTA, P. R., & MENDEL, J. L. (1965)
Evidence for the conversion of o,p'-DDT (1,1,1-trichloro-2- o-
chlorophenyl-2- p-chlorophenylethane) to p,p'-DDT (1,1,1-
trichloro-2,2-bis( p-chlorophenyl)ethane) in rats. J. Am. Chem.
Assoc., 87: 2520-2522.
KLEINFELD, M. (1967) Cancer of urinary bladder in a dye plant: A
medical and environmental study. In: Deichmann, W. B., ed.
Bladder Cancer, A Symposium, Birmingham, Al., Aesculapius,
pp. 136-144.
KNIPLING, E. F. (1959) The use of DDT in veterinary medicine. In:
Müller, P., ed. DDT, the insecticide dichlorodiphenyltri-
chloroethane and its significance, Basel, Switzerland, Birkhäuser
Verlag, Vol. II, pp. 505-570.
KNOLL, W. & JAYARAMAN, S. (1973a) [Organochlorine pesticide residues
in human milk.] Z. Gesamt. Hyg., 19: 43-45 (in German).
KNOLL, W. & JAYARAMAN, S. (1973b) [On the contamination of human milk
with chlorinated hydrocarbons.] Nahrung, 17: 599-615 (in German).
KOJIMA, S., SAITO, M., KONNO, H., & OZAWA, K. (1971) [Results of
investigation of organochlorine pesticide residues in mothers'
milk and in the blood of the mothers.] Rep. Akita Prefect. Inst.
Public Health, 16: 65-68 (in Japanese).
KOLMODIN, B., AZARNOFF, D. L., & SJOQVIST, F. (1969) Effect of
environmental factors on drug metabolism: Decreased plasma half-
life of antipyrine in workers exposed to chlorinated hydrocarbon
insecticides. Clin. Pharmacol. Ther, 10: 638-642.
KOLYADA, I. S. & MIKHAL'CHENKOVA, O. F. (1973) [Acute intoxication by
chlorine-containing pesticides.] Gig. Tr. Prof. Zabol., 5: 43
(in Russian).
KOMAROVA, L. I. (1970) [The excretion of DDT in mothers' milk and its
effect on the organism of mother and child.] Pediatr. Akus.
Ginek., 1: 19-20 (in Russian).
KONTEK, M., KUBACKI, S., PARADOWSKI, S., & WIERZCHOWIECKA, B. (1971)
[Chlorinated insecticides in human milk.] Pediatr. Pol.,
46: 183-188 (in Polish).
KOSTER, R. (1947) Differentiation of gluconate, glucose, calcium, and
insulin effects on DDT poisoning in cats. Fed. Proc., 6: 346.
KOSTIUK, O. T. & MUKHTAROVA, N. D. (1970) [Catecholamines and the
functional state of the hypothalamus under the action of a complex
of organochlorine and organophosphorus pesticides.] Gig. Tr.
Prof. Zabol., 14: 35-38 (in Russian).
KRASNIUK, E. P. & PLATONOVA, V. I. (1969) [Functional disorders of the
stomach under prolonged effects of organochlorine chemical
poisons.] Vrach. Delo, No. 9, 99-101 (in Russian).
KRASNIUK, E. P., LOGANOVSKII, N. G., MAKOVSKAYA, E. I., & RAPPOPORT,
M. B. (1968) [Functional and morphological changes in the kidneys
during the effects of DDT on the body.] Soy. Med., 31: 38-42
(in Russian).
KRAUL, I. & KARLOG, O. (1976) Persistent organochlorinated compounds
in human organs collected in Denmark, 1972-1973. Acta Pharmacol.
Toxicol., 38: 38-48.
KROGER, M. (1972) Insecticide residues in human milk. J. Pediatr.,
80: 401-405.
KUCINSKI, A. L. (1972) [On the extraction of DDT and hexachlorane from
some canned foods of animal and vegetable origin.] Vopr. Pitan.,
31 (1): 93 (in Russian).
KUN'EV, V. V. (1965) [Influence of low DDT doses on the phagocytic
state of the blood of albino rats.] Vopr. Pitan., 24: 74-78 (in
Russian).
KUNTZMANN, R., JACOBSON, M., LEVIN, W., & CONNEY, D. H. (1968)
Stimulatory effect of N-phenylbarbitol on cortisol hydroxylation
in man. Biochem. Pharmacol., 17: 565-571.
KUPFER, D. (1967) Effects of some pesticides and related compounds on
steroid function and metabolism. Res. Rev., 19: 11-30.
KUPFER, T., BALAZS, T., & BUYSKE, D. A. (1964) Stimulation by o,p'-
DDD of cortisol metabolism in the guinea pig. Life Sci.,
3: 959-964.
KUTZ, F. W., YOBS, A. R., JOHNSON, W. G., & WIERSMA, G. B. (1974)
Pesticide residues in adipose tissue of the general population of
the United States, FY 1970 survey. Bull. Soc. Pharmacol. Environ.
Pathol., 2: 4-10.
KUWABARA, N. & TAKAYAMA, S. (1974) [Comparison of histogenesis of
liver of mice administered respectively BHC, DDT, and 2,7-FAA.]
Proc. Jpn Cancer Assoc., 33: 50 (in Japanese).
KUZ'MINSKAJA, U. A., KLISENKO, M. A., & HAYKINA, B. I. (1972)
[Peculiarities of the distribution of some pesticides in the lipid
fractions of the liver.] Vopr. Pitan., 31: 48-52 (in Russian).
KWALIK, D. S. (1971) Anticonvulsants and DDT residues. J. Am. Med.
Assoc., 215: 120-121.
LAUBSCHER, J. A., DUTT, G. R., & ROAN, C. C. (1971) Chlorinated
insecticide residues in wildlife and soil as a function of
distance from application. Pestic. Monit. J., 5: 251-258.
LAUG, E. P., NELSON, A. A., FITZHUGH, O. G., &. KUNZE, F. M. (1950)
Liver-cell alteration and DDT storage in the fat of the rat
induced by dietary levels of 1 to 50 ppm of DDT. J. Pharmacol.
98: 268-273.
LAUG, E. P, KUNZE, F. M., & PRICKETT, C. S. (1951) Occurrence of DDT
in human fat and milk. Am. Med. Assoc. Arch. indust. Hyg. occup.
Med., 3: 245-246.
LAÜGER, P., PULVER, R., & MONTIGEL, C. (1945a) [Mode of action of
4,4'-dichlorodiphenyltrichloromethylmetan (DDT-Geigy) in
warmblooded organism.] Experientia (Basel), 1: 120-121 (in
German).
LAÜGER, P., PULVER, R., & MONTIGEL, C. (1945b) [Mode of action of
4,4'-dichlorodiphenyltrichloromethylmetan (DDT-Geigy) in
warmblooded organism.] Helr. Physiol. Acta, 3: 405-415 (in
German).
LAWS, E. R. (1971) Evidence of antitumorigenic effects of DDT. Arch.
environ. Health, 23: 181-183.
LAWS, E. R., JR, CURLEY, A., & BIROS, F. J. (1967) Men with intensive
occupational exposure to DDT. A clinical and chemical study.
Arch. environ. Health, 15: 766-775.
LAWS, E. R, JR, MADDREY, W. C., CURLEY, A., & BURSE, V. W. (1973)
Long-term occupational exposure to DDT. Arch. environ. Health,
27: 318-321.
LEHMAN, A. J. (1952) Chemicals in foods: A report to the Association
of Food and Drug Officials on current developments. Part II,
Pesticides. Section V. Q. Bull. Assoc. Food Drug Off. US,
16: 126-132.
LEHMAN, A. J. (1965) Summaries of pesticide toxicity, Topeka, Ks,
Association of Food and Drug Officials of the United States.
LESCENKO, P. D. & POLONSKAIA, M. N. (1969) [New products in the
prophylactic nutrition of workers in the organochlorine industry.]
Vrach. Delo, No. 8, pp. 106-110 (in Russian).
LEWIS, W. H. & RICHARDS, A. G, JR (1945) Non-toxicity of DDT on cells
in cultures. Science, 102: 330-331.
LICHTENBERG, J. L., EICHELBERGER, J. W., DRESSMAN, R. C., &
LONGBOTTOM, J. E. (1970) Pesticides in surface waters of the
United States -- a 5-year summary, 1964-68. Pestic. Monit. J.,
4: 71-86.
LILLIE, R. D. & SMITH, M. I. (1944) Pathology of experimental
poisoning in cats, rabbits, and rats with 2,2-bis-(para-
chlorphenyl)-1,1,1-trichlorethane. Public Health Rep.,
59: 979-984.
LILLIE, R. D., SMITH, M. I., & STOHLMAN, E. F. (1947) Pathologic
action of DDT and certain of its analogs and derivatives. Arch.
Pathol., 43: 127-142.
LIPSCOMB, G. Q. (1968) Residues in food and feed -- Pesticide residues
in prepared baby foods in the United States. Pestic. Monit. J.,
2: 104-108.
LÖFROTH, G. (1968) Pesticides and catastrophe. New Sci.,
40: 567-568.
LONG, K. L., BEAT, V. B., GOMBART, A. K., SHEETS, R. F., HAMILTON, H.
E., FALABALLA, F., BONDERMAN, D. P., & CHOI, U. Y. (1969) The
epidemiology of pesticides in a rural area. Am. Ind. Hyg. Assoc.
J., 30: 398-304.
LOVELOCK J. E. (1963) Electron absorption detectors and techniques for
use in quantitative and qualitative analysis by gas-liquid
chromatography. Anal. Chem., 35: 474-481.
LU, F. C., JESSUP, D. C., & LAVALÉE, A. (1965) Toxicity of pesticides
to young versus adult rats. Food Cosmet. Toxicol., 3: 591-596.
LUBITZ, J. A., FEEMAN, L., & OKUM, R. (1973) Mitotane use in
inoperable adrenal cortical carcinoma. J. Am. Med. Assoc.,
233: 1109-1112.
LUQUET, F., GOURSAUD, J. G., & CASALIS, J. (1974) Organochlorine
pesticide residues in animal and human milk. Ann. Fals. Expert.
Chim., 67: 217-239.
LUTON, J. P, VALCKE, J. C., REMY, J. M., MATHIEU DE FOSSEY, B., &
BRICAIRE, H. (1972) Gynecomastia after long-term treatment of
Cushing's diseases with o,p'-DDT. Ann Endocrinol.,
33: 290-293.
MACCORMACK, J. D. (1945) Infestation and DDT. Irish J. med. Sci.
6: 627-634.
MACKERRAS, I. M. & WEST, R. F. K. (1946) 'DDT' poisoning in man.
Med. J. Aust., 1: 400-401.
MACKLIN, A. W. & BIBELIN, W. E. (1971) The relation of pesticides to
abortion in dairy cattle. J. Am. Vet. Med. Assoc.,
159: 1743-1748.
MAES, R. & HEYNORICKX, A. (1966) Distribution of organic chlorinated
insecticides in human tissues. Meded. Rijksfac. Landbouwwet.
Gent., 31: 1021-1025.
MAGGS, R. J., JOYNES, P. L., DAVIES, A. J., & LOVELOCK, J. E. (1971)
The electron capture detector -- A new mode of operation. Anal.
Chem., 43: 1966-1971.
MAIER-BODE, H. (1960) [DDT in body fat of man.] Med. Exp, 1: 146-152
(in German).
MARKARIAN, D. S. (1966) [Cytogenetic effects of some chlorine-
containing organic insecticides on mouse bone-marrow cell nuclei.]
Genetika, 1: 132-137 (in Russian).
MARNELOS, M. & HANNINEN, O. (1974) Enhancement of D-glucuronolactone
and acetaldehyde dehydrogenase activities in the rat liver by
inducers of drug metabolism. Biochem. Pharmacol., 23: 1457-1466.
MARSH, C. A. (1963) Metabolism of D-glucuronolactone in the mammalian
system. Biochem. J., 86: 77-86.
MASON, T. J., MCKAY, F. W., HOOVER, F., BLOT, W. J., & FRAUMENI, J. F,
JR (1975) Atlas of cancer mortality for U.S. Counties:
1950-1969, Washington, DC, US Department of Health, Education
and Welfare, Public Health Service (DHEW Publication No. (NH)
75-780).
MASUMOTO, H. T. (1972) Study of the silicic acid procedure of Armour
and Burke for separation of PCBs from DDT and its analogues.
J. Assoc. Off. Anal. Chem., 55: 1092-1100.
MATSUMURA, F. & PATIL, K. C. (1969) Adenosine triphosphatase sensitive
to DDT in synapses of rat brain. Science, 166: 121-122.
MATTSON, A. M., SPILLANE, J. T., BAKER, C., & PEARCE, G. W. (1953)
Determination of DDT and related substances in human fat. Anal.
Chem., 25: 1065-1070.
MAYERSDORF, A. & ISRAELI, R. (1974) Toxic effects of chlorinated
hydrocarbon insecticides on the human electroencephalogram. Arch.
environ. Health, 28: 159-163.
MCALISTER, D. (1879) The law of the geometric mean. Proc. R. Soc.
London B, 29: 367-376.
McCANN, J. & AMES, B. N. (1976) Detection of carcinogens as mutagens
in the Salmonella/microsome test: Assay of 300 chemicals:
Discussion. Proc. Natl Acad. Sci. USA, 73: 950-954.
McCANN, J., CHOL, E., YAMASAKI, E., & AMES, B. N. (1975) Detection of
carcinogens as mutagens in the Salmonella/microsome test: Assay
of 300 chemicals. Proc. Natl Acad. Sci. USA, 72: 5135-5139.
McFARREN, E. F., LISHKA, R. J., & PARKER, J. H. (1970) Criterion for
judging acceptability of analytical methods. Anal. Chem.,
42: 358-365.
McGILL, A. E. J. & ROBINSON, J. (1968) Organochlorine insecticide
residues in complete prepared meals: A 12-month survey in S.E.
England. Food Cosmet. Toxicol., 6: 45-57.
McKINLEY, W. P. (1963) Paper chromatography. In: Zweig, G., ed.
Analytical methods for pesticides, plant growth regulators and
food additives, New York, Academic Press, Vol. 1, pp. 227-252.
McKINNEY, J. D., BOOZIER, E. L., HOPKINS, H. P., & SUGGS, J. E. (1969)
Synthesis and reactions of a proposed DDT metabolite, 2,2'-bis
( p-chlorophenyl)acetaldehyde. Experientia (Basel),
25: 897-898.
McLACHLAN, J. A. & DIXON, R. L. (1972) Gonadal function in mice
exposed prenatally to p,p'-DDT. Toxicol. appl. Pharmacol.,
22: 327.
McMAHON, B. M. & SAWYER, L. D., ed. (1977) Pesticide analytical
manual. Volume I. Methods which detect multiple residues,
Rockville, MD, US Food and Drug Administration.
McNEIL, E. E., OTSON, R., MILES, W. F., & RAJABALEE, F. J. M. (1977)
Determination of chlorinated pesticides in potable water.
J. Chromatogr., 123: 277-286.
McQUEEN, E. G., OWEN, D., & FERRY, D. G. (1972) Effect of phenytoin
and other drugs in reducing serum DDT levels. N.Z. med. J.,
75: 208-211.
MEDVED', L. I., KAGAN, J, S., SPYNU, E. I., DLISENKO, M. A.,
ANTONOVIC, E. A., BEZUGLYJ, V. P., KRYZANOVSKAYJA, M. V., &
POL'CENKO, V. I. (1975) National review on chlorinated
pesticides, Kiev, All-Union Institute of the Ministry of Health
of the USSR for Research on the Hygiene and Toxicology of
Pesticides, Polymers, and Plastics, 74 pp.
MENDOZA, E. E. (1971) Note on the significant difference in gas-liquid
chromatographic responses to insecticide injections at fast and
slow rates. J. Chromatogr. Sci., 9 (12): 753-754.
MENZIE, C. M. (1969) Metabolism of pesticides, Washington, DC, US
Government Printing Office (Special Scientific Report, Wildlife
No. 127).
METCALF, R. L. (1955) Organic insecticides, their chemistry and mode
of action, New York, Intersciences, 392 pp.
MIDDLETON, F. M. & LICHTENBERG, J. J. (1960) Measurements of organic
contaminants in the nation's rivers. Ind. Eng. Chem.,
52: 99A-102A.
MILES, J. R. (1972) Conversion of DDT and its metabolites into
dichlorobanzophenones for analysis in presence of PCBs. J. Assoc.
Off. Anal. Chem., 55: 1039-1041.
MILES, J. W., FETZER, L. E., & PEARCE, G. W. (1970) Collection and
determination of trace quantities of pesticides in air. Environ.
Sci. Technol., 4: 420-425.
MILLER, G. J. & FOX, J. A. (1973) Chlorinated hydrocarbon pesticide
residues in Queensland human milks. Med. J. Aust., 2: 261-264.
MILLER, L. L. & NARANG, R. S. (1970) Induced photolysis of DDT.
Science, 169: 368-370.
MIZOGUCHI, M., YAMAGISHI, T., USHIO, F., FUJIMOTO, C., TAKEBA, K.,
KANI, T., HARUTA, M., YASUHARA, K., & KUBOTA, H. (1972) [On the
residual amount of organochlorine pesticides in human milk in
Tokyo metropolitan area.] Jpn J. public Health, 19: 541-544
(in Japanese).
MONTGOMERY, J. A. & STRUCK, R. F. (1973) The relation of the
metabolism of anticancer agents to their activity. Forschr.
Arzneimittelforsch., 17: 32-304.
MORGAN, D. P. & ROAN, C. C. (1969) Renal function in persons
occupationally exposed to pesticides. Arch. environ. Health,
19: 633-636.
MORGAN, D. P. & ROAN, C. C. (1970) Chlorinated hydrocarbon pesticide
residue in human tissues. Arch. environ. Health, 20: 452-457.
MORGAN, D. P. & ROAN, C. C. (1971) Absorption, storage and metabolic
conversion of ingested DDT and metabolites in man. Arch. environ.
Health, 22: 301-308.
MORGAN, D. P. & ROAN, C. C. (1972) Loss of DDT from storage in human
body fat. Nature (Load.), 238: 221-223.
MORGAN, D. P. & ROAN, C. C. (1973) Adrenocortical function in persons
occupationally exposed to pesticides. J. occup. Med., 15: 26-28.
MORGAN, D. P. & ROAN, C. C. (1974) Liver function in workers having
high tissue stores of chlorinated hydrocarbon pesticides. Arch.
environ. Health, 29: 14-17.
MORGAN, D. P., ROAN, C. C., & PASCHAL, E. H. (1972) Transport of DDT,
DDE, and dieldrin in human blood. Bull environ. Contain.
Toxicol., 8: 321-326.
MOSIER, A. R., GUENZI, W. D., & MILLER, L. L. (1969) Photochemical
decomposition of DDT in a free-radical mechanism. Science,
164: 1083-1085.
MRAK, E. M. (1969) Report of the secretary's commission on pesticides
and their relationship to environmental health, Washington, US
Government Printing Office, 677 pp.
MUGHAL, H. A. & RAHMAN, M. A. (1973) Organochlorine pesticide content
of human adipose tissue in Karachi. Arch. environ. Health, 27:
396-398.
MÜLHENS, K. (1946) [The importance of bis (chlorophenyl)
(trichloromethyl)-methane preparations as insecticides in
combating infectious diseases.] Dtsch. Med. Wochenschr.,
71: 164-169 (in German).
MÜLLER, P., ed. (1959) DDT, the insecticide dichlorodiphenyltri-
chloroethane and its significance. Basel, Switzerland, Birkhäuser
Verlag, Vol. 2.
MURPHY, P. G. (1972) Sulphuric acid for the clean-up of animal tissues
for analysis of acid-stable chlorinated hydrocarbon residues.
J. Assoc. Off. Anal Chem., 55: 360-362.
MURRAY, C. P. F. V., SAUCEDA, F. M., & NAVAREZ, G. M. (1973)
[Environmental contamination and the health of children.] Salud
Publica Mex., 15: 91-100 (in Spanish).
MUSIAL, C. J., HUTZINGER, O., ZITKO, V., & CROCKER, J. (1974) Presence
of PCB, DDE, and DDT in human milk in the provinces of New
Brunswick and Nova Scotia, Canada. Bull. environ. Contam.
Toxicol., 12: 258-267.
MUSTY, P. R. & NICKLESS, G. (1974) Use of Amberlite XAD-4 for
extraction and recovery of chlorinated hydrocarbon insecticides
and PCBs from water. J. Chromatogr., 89: 185-190.
NARVESTED, R. (1947) [Poisoning with DDT powder and some other
incidences of poisoning.] Tidsskr. Norske Laegeforen.,
67: 261-263 (in Norwegian).
NAGAI, I. (1972) [Residues of organochlorine pesticides in mother's
milk in Yamaguchi Prefecture. Annual Report of the Yamaguchi
Prefecture.] Res. Inst. Public Health, 14: 93-94 (in Japanese).
NAGASAKI, H. (1973) [Experimental studies on chronic toxicity of
benzene hexachloride (BHC).] J. Nara Med. Assoc., 24: 1-26
(in Japanese).
NAGASAKI, H., AOI, H., MAKIURA, S., AOKI, Z., ARAI, M., KONISHI, Y., &
ITO, N. (1974) [Characteristic features of hepatoma of mice due to
alpha-BHC and several factors of carcinogenesis.] Proc. Jpn
Cancer Assoc., 33: 78 (in Japanese).
NALIMOV, V. V. (1963) The application of mathematical statistics to
chemical analysis, London, Pergamon Press, 294 pp.
NARAHASHI, T. & HAAS, H. G. (1967) DDT: Interaction with nerve
membrane conductance changes. Science, 157: 1438-1440.
NARAHASHI, T. & YAMASAKI, T. (1960) Mechanism of increase in negative
after-potential by dicophanum (DDT) in the giant axon of the
cockroach. J. Physiol. (Lond.), 152: 122-140.
NARUSE, R., SASAKI, T., YAMOKA, T., MATSUOKA, K., NEGORO, Y., ITASAKA,
Y., URUSHIBATA, K., CHIN, T., KATO, K., KAKO, K., GOTO, Y.,
EGUCHI, Y., YOGO, H., TOMITA, A., & ASAI, M. (1970) [Clinical use
of o,p'-DDD in adrenal cortex cancer.] Horumon Rinsho,
18: 241-244 (in Japanese).
NAWA, H. (1973) [Studies on intoxication by organochlorine pesticides
for agricultural use. Part 2. Examination of concentration of
organochlorine pesticides in sera of healthy people and patients
with various diseases.] J. Okayama Med. Soc., 85: 47-58 (in
Japanese).
NEAL, P. A., VON OETTINGEN, W. F., SMITH, W. W., MALMO, R. B., DUNN,
R. C., MORAN, H. E., SWEENEY, T. R., ARMSTRONG, D. W., & WHITE, W.
C. (1944) Toxicity and potential dangers of aerosols, mists, and
dusting powders containing DDT. Public Health Rep., 177
(Suppl.): 1-32.
NEAL, P. A., SWEENEY, T. R., SPICER, S.S., & VON OETTINGEN, W. F.
(1946) The excretion of DDT (2,2-bis-( p-chlorophenyl)-1,1,1-
trichloroethane) in man, together with clinical observations.
Public Health Rep., 61: 403-409.
NELSON, A. A. & WOODARD, G. (1948) Adrenal cortical atrophy and liver
damage produced in dogs by feeding 2,2-bis-(parachlorophenyl)-1,1-
dichlorethane (DDD). Fed. Proc., 7: 277.
NELSON, A. A. & WOODARD, G. (1949) Severe adrenal cortical atrophy
(cytotoxic) and hepatic damage produced in dogs by feeding 2,2-
bis-(parachlorophenyl)-1,1-dichloroethane (DDD or TDE). Arch.
Pathol., 48: 387-394.
NELSON, A. A., DRAIZE, J. H., WOODARD, G., FITZHUGH, O. G., SMITH, R.
B., JR, & CALVERY, H. O. (1944) Histopathological changes
following administration of DDT in several species of animals.
Public Health Rep., 59: 1009-1020.
NELSON, J. A. (1973) Effects of DDT analogs and polychlorinated
biphenyls (PCB) mixtures on 3H-estradiol binding to rat uterine
receptor. Fed. Proc., 32: 236.
NEWTON, K. O. & GREENE, N. C. (1972) Organochlorine pesticide residue
levels in human milk -- Victoria, Australia, 1970. Pestic. Monit.
J., 6: 4-8.
NICHOLS, J., PRESTLEY, W. E., & NICHOLS, F. (1961) Effects of m,p'-
DDT in a case of adrenal cortical carcinoma. Curr. Ther. Res.,
3: 266-271.
NIKITINA, Y. I. (1974) [Course of labor and puerperium in vineyard
workers and milkmaids in the Crimea.] Gig. Tr. Prof. Zabol,
18: 17-20 (in Russian).
NISHIMOTO, T., UYETA, M., & TAUE, S. (1970) [The accumulation of
organochlorine pesticides in human adipose tissue.] Prog. Med.,
73: 275-277 (in Japanese).
NITSCHKE, U. & LINK, K. (1972) [Clinical and biochemical aspects of
hormone-producing adrenal carcinomas.] Z. Gesamite Inn. Med. Ihre
Grenzgeb., 27: 896-905 (in German).
NORTH, H. H. & MENZER, R. E. (1973) Metabolism of DDT in human
embryonic lung cell cultures. J. agric. food. Chem.,
21: 509-510.
NOVIKOVA, K. F. (1973) [Quantitative assay of organochlorine and
organophosphate pesticide residues in food products, soil, water.]
Zh. Vses. Khim. Obshchest., 18 (5): 562-570 (in Russian).
OBUSCHOWSKA, D. & PAWLOWKA-TOCHMAN, A. (1973) [The effect of the
gamma-isomer of HCH (Lindane) on the ultrastructure of the liver
cell.] Ann. Univ. Mariae Curie-Sklodowska, 28 (9): 63-66 (in
Polish).
OFNER, R. R. & CALVERY, H. O. (1945) Determination of DDT (2,2-bis-
( p-chlorophenyl)-1,1,1-trichlorethane) and its metabolite in
biological materials by use of the Schechter-Haller method.
J. Pharmacol., 85: 363-370.
O'LEARY, J. A., DAVIES, J. E., EDMUNDSON, W. F., & FELDMAN, M. (1970a)
Correlation of prematurity and DDE levels in fetal whole blood.
Am. J. Obstet. Gynecol., 106: 939.
O'LEARY, J. A., DAVIES, J. E., EDMUNDSON, W. F., & REICH, G. A.
(1970b) Transplacental passage of pesticides. Am. J. Obstet.
Gynecol., 107: 65-68.
O'LEARY, J. A., DAVIES, J. E., & FELDMAN, M. (1970c) Spontaneous
abortion and human pesticide residues of DDT and DDE. Am. J.
Obstet. Gynecol., 108: 1291-1292.
OLOFFS, C. P., HARDWICK, D. F., SZETO, S. Y., & MOERMAN, D. G. (1974)
DDT, dieldrin, and heptachlor epoxide in humans with liver
cirrhosis. Clin. Biochem., 7: 297-306.
OLSZYNA-MARZYS, A. E., DE CAMPOS, M., FARVAR, M. T., & THOMAS, M.
(1973) [Residues of chlorinated pesticides in human milk in
Guatemala.] Bol. Of. Sanit. Panam., 74: 93-107 (in Spanish).
ORTEGA, P. (1966) Light and electron microscopy of dichlorodiphenyl-
trichloroethane (DDT) poisoning in the rat liver. Lab. Invest.,
15: 657-679.
ORTEGA, P., HAYES, W. J., JR, DURHAM, W. F., & MATTSON, A. (1956) DDT
in the diet of the rat. Its effect on DDT storage, liver function
and cell morphology, Washington, DC, US Government Printing
Office, 27 pp. (Public Health Monograph No. 43, PHS Publication
No. 484).
ORTELEE, M. F. (1958) Study of men with prolonged intensive
occupational exposure to DDT. Am. Med. Assoc. Arch. indust.
Health, 18: 433-440.
OTTOBONI, A. (1969) Effect of DDT on reproduction in the rat.
Toxicol. appl. Pharmacol., 14: 74-81.
OTTOBONI, A. (1972) Effect of DDT on the reproductive lifespan in the
female rat. Toxicol. appl. Pharmacol., 22: 497-502.
OTTOBONI, A., BISSELL, G. D., & HEXTER, A. C. (1977) Effects of DDT on
reproduction in multiple generations of beagle dogs. Arch.
environ. Contam. Toxicol., 6: 83-101.
OURA, H., KOBATASHI, H., OURA, T., SENDA, I., & KUBOTA, K. (1972) [On
the pollution of human milk by PCB and organochlorine pesticides.]
J. Jpn Assoc. Rural Med., 21: 300-301 (in Japanese).
OUW, K. H. & SHANDAR, A. G. (1974) A health survey of Wee Waa
residents during 1973 aerial spraying season. Med. J. Aust.,
2: 871-873.
PACCAONELLA, B., PRATI, L., & CAVAZZINI, G. (1967) [Organochlorine
insecticides in adipose tissue of residents in Ferrara province.]
Nuovi Ann. Ig. Microbiol., 18: 17-26 (in Italian).
PALMER, K. A, GREEN, S, & LEGATOR, M. S. (1972) Cytogenetic effects of
DDT and derivatives of DDT in a cultured mammalian cell line.
Toxicol. appl. Pharmacol., 22: 355-364.
PALMER, L. & KOLMODIN-HEDMAN, B. (1972) Improved quantitative gas
chromatographic method for analysis of small quantities of
chlorinated hydrocarbon pesticides in human plasma.
J. Chromatogr., 74: 21-30.
PASCHAL, E. H., ROAN, C. C., & MORGAN, D. P. (1974) Evidence of
excretion of chlorinated hydrocarbon pesticides by the human
liver. Bull. environ. Contam. Toxicol., 12: 547-554.
PEAKALL, D. B. (1970) p,p'-DDT: Effect on calcium metabolism and
concentration of estradiol in the blood. Science, 168: 592-594.
PEARCE, G. W., MATTSON, A. M., & HAYES, W. J., JR (1952) Examination
of human fat for presence of DDT. Science, 116: 254-256.
PEARL, W. & KUPFER, D. (1971) Stimulation of dieldrin elimination by
thiouracil derivative of DDT in the rat: enhancement of dieldrin
oxidative metabolism. Chem. biol. Interactions, 4: 91-96.
PELLIZARI, E. D. (1974) Electron capture detection in gas
chromatography. J. Chromatogr., 98: 323-361.
PERAINO, C., FRY, R. J. M., & STAFFELDT, E. (1973) Enhancement of
spontaneous hepatic tumorigenesis in C3H mice by dietary
phenobarbital. J. Natl Cancer Inst., 51: 1349-1350.
PEREVODCIKOVA, N. I., PLATINSKIY, L. V., & KERSTMAN, V. I. (1972) [The
treatment of inoperable forms of malignant tumors of the adrenal
cortex with o,p'-DDD.] Vopr. Onkol., 18: 24-29 (in Russian).
PERINI, G. & GHEZZO, F. (1970) [Dichlorodiphenylacetic acid (DDA)
urinary levels in the rural population of Ferrara Province.]
L'Arcisp. S. Anna di Ferrara, 23: 3-12 (in Italian).
PESENDORFER, H., EICHLER, I., & GLOFKE, E. (1973) [Informative
analyses of organochlorine pesticide and PCB residues in human
adipose tissue (from the area of Vienna).] Wien. Klin.
Wochenschr., 85: 218-222 (in German).
PETERLE, T. J. (1969) DDT in Antarctic snow. Nature (Lond.),
224: 620.
PETERSON, J. E. & ROBINSON, W. H. (1964) Metabolic products of p,p'-
DDT in the rat. Toxicol. appl. Pharmacol, 6: 321-327.
PFEILSTICKER, K. (1973) [Pesticides in baby food.] Monatsschr.
Kinderheilkd., 121: 551-553 (in German).
PHILIPS, F. S. & GILMAN, A. (1946) Studies on the pharmacology of DDT
(2,2-bis-(parachlorophenyl)-1,1,1-trichloroethane). I. The acute
toxicity of DDT following intravenous injection in mammals with
observations on the treatment of acute DDT poisoning.
J. Pharmacol., 86: 213-221.
PHILIPS, F. S., GILMAN, A., & CRESCITELLI, F. N. (1946) Studies on the
pharmacology of DDT (2,2-bis-(parachlorophenyl)-1,1,1-
trichloroethane). II. The sensitization of the myocardium to
sympathetic stimulation during acute DDT intoxication.
J. Pharmacol., 86: 222-228.
PIONKE, H. B., KONRAD, J. C., CHESTER, G., & ARMSTRONG, D.E. (1968)
Extraction of organochlorine and organophosphate insecticides from
lake water. Analyst, 93: 363-367.
PLATONOVA, V. I. (1970) [Disturbances of the functional conditions of
the stomach with prolonged effect on the body of some
organochlorine pesticides.] Gig. Tr. Resp. Mezhved, 3B,
6: 142-147 (in Russian).
PLIMMER, J. R. & KLINGEBIEL, U. I. (1973) PCB-formation. Science,
181: 994-995.
PLIMMER, J. R, KLINGEBIEL, U. I., & HUMMER, B.E. (1970) Photooxidation
of DDT and DDE. Science, 167: 67-69.
POLAND, A., SMITH, D., KUNTZMAN, R., JACOBSON, M., & CONNEY, A. H.
(1970) Effect of intensive occupational exposure to DDT an
phenylbutazone and cortisol metabolism in human subjects. Clin.
Pharmacol. Ther., 11: 724-732.
POLISHUK, Z. W., WASSERMANN, M., WASSERMANN, D., GRONER, Y.,
LAZAROVICI, S., & TOMATIS, L. (1970) Effects of pregnancy on
storage of organochlorine insecticides. Arch. environ. Health,
20: 215-217.
POLISHUK, Z. W., RUN, M., WASSERMANN, M., CUCOS, S., WASSERMANN, D., &
LEMESCH, C. (1977) Organochlorine compounds in human blood plasma
and milk. Pestic. Monit. J., 10: 121-129.
PONOMARKOV, V., TOMATIS, L., & TURUSOV, V. (1976) The effect of long-
term administration of phenobarbitone in CF-1 mice. Cancer Lett.,
1: 165-172.
POTT, P. (1775) Chirurgical observations relative to the cataract,
the polypus of the nose, the cancer of the scrotum, etc. London,
T. F. Carnegy, 208 pp.
POTT, P. (1790) The chirurgical works of Percival Pott, F.R.S.
surgeon to St. Bartholomew's Hospital, A new edition with his
last corrections, London, J. Johnson, 3 vol.
PRATI, L. & DEL DOT, M. (1971) [Research on the levels of accumulation
of synthetic organic chloride pesticides, in human adipose tissues
in the province of Trento.] L'Ig. Mod., 64: 35-43 (in Italian).
PRATI, L., PAVANELLO, R., & GHEZZO, F. (1972) Storage of chlorinated
pesticides in human organs and tissues in Ferrara Province, Italy.
Bull. World Health Organ., 46: 363-369.
PROUTY, R. M. & CROMARTIE, E. (1970) Stepwise recovery of DDT and
dieldrin from tissues of Coturnix japonica (quail) during residue
analysis. Environ. Sci. Technol., 4: 768-769.
QUINBY, G. E., HAYES, W. J., JR, ARMSTRONG, J. F., & DURHAM, W. F.
(1965a) DDT storage in the US population. J. Am. Med. Assoc.,
191: 175-179.
QUINBY, G. E., ARMSTRONG, J. F., & DURHAM, W. F. (1965b) DDT in human
milk. Nature (Lond.), 207: 726-728.
RABELLO, M. N., BECAK, W., ALMEIDA, W. F., PIGATI, P., UNGARO, M. T.,
MURATA, T., & PEREIRA, C. A. B. (1975) Cytogenetic study on
individuals occupationally exposed to DDT. Mutat. Res.,
28: 449-454.
RADOMSKI, J. L., DEICHMANN, W. B., & CLIZER, E. E. (1968) Pesticide
concentrations in the liver, brain, and adipose tissue of terminal
hospital patients. Food Cosmet. Toxicol, 6: 209-220.
RADOMSKI, J. L., DEICHMANN, W. B., REY, A. A., & MERKIN, T. (1971a)
Human pesticide blood levels as a measure of body burden and
pesticide exposure. Toxicol. appl. Pharmacol., 20: 175-185.
RADOMSKI, J. L, ASTOLFI, E., DEICHMANN, W. B., & REY, A. A. (1971b)
Blood levels of organochlorine pesticides in Argentina:
Occupationally and nonoccupationally exposed adults, children and
newborn infants. Toxicol. appl. Pharmacol., 20: 186-193.
RAMACHANDRAN, M., SHARMA, M. I. D., SHARMA, S.C., MATHUR, P.S.,
ARAVINDAKSHAN, A., & EDWARD, G. J. (1974) DDT and its metabolites
in the body fat of Indians. J. commun. Dis., 6: 256-259.
RANTANEN, T., MARSELOS, M., & PUHAKAINEN, E. (1975) Effects of 1,1,1-
trichloro-2,2-bis-( p-chlorophenyl)ethane (DDT) administration on
the glucuronic acid pathway in the rat liver. Environ. Qual.
Saf., 3 (Suppl.): 494-499.
RAPPOLT, R. T. (1970) Use of oral DDT in three human barbiturate
intoxications: CNS arousal and/or hepatic enzyme induction by
reciprocal detoxicants. Ind. Med. Surg., 39: 319.
READ, S. T. & MCKINLEY, W. P. (1961) DDT and DDE content of human fat.
A survey. Arch. environ. Health, 5: 209-211.
REIF, V. D. & SINSHEIMER, J. E. (1975) Metabolism of 1-( o-
chlorophenyl)-1-( p-chlorophenyl)-2-2-dichloroethane ( o,p'-DDD)
in rats Drug Metab. Dispos., 3: 15-25.
REIF, V. D., SINSHEIMER, J. E., WARD, J. C., & SCHTEINGART, D. E. J.
(1974). Aromatic hydroxylation and alkyl oxidation in metabolism
of mitotane ( o,p'-DDD) in humans. Pharmacol. Sci.,
63: 1730-1736.
REINGOLD, I. M. & LASKY, I. I. (1947) Acute fatal poisoning following
ingestion of a solution of DDT. Ann. intern. Med., 26: 945-947.
REYNOLDS, L. M. (1969) Polychlorinated biphenyls (PCBs) and their
interference with pesticide residue analysis. Bull. environ.
Contam. Toxicol., 4: 128-143.
RICHARDSON, A., ROBINSON, J., CRABTREE, N., & BALDWIN, M. K. (1971)
Residues in polychlorobiphenyls in biological samples. Pestic.
Monit. J., 4: 169-176.
RICHARDSON, J. A., KEIL, J. E., & SANDIFER, S. H. (1975) Catecholamine
metabolism in humans exposed to pesticides. Environ. Res.,
9: 290-294.
RISEBROUGH, R. W., HUGGETT, R. J., GRIFFIN, J. J., & GOLDBERG, E. D.
(1968) Pesticides: Transatlantic movements in the northeast
trades. Science, 159: 1233-1235.
RITCEY, W. R., SAVARY, G., & McCULLY, J. A. (1972) Organochlorine
insecticide residues in human milk, evaporated milk, and some milk
substitutes in Canada. Can. J. public Health, 63: 125-132.
RITCEY, W. R., SAVARY, G., & McCULLY, J. A. (1973) Organochlorine
insecticide residues in human adipose tissue of Canadians. Can.
J. public Health, 64: 380-386.
RIZOV, N. A. (1977) [Organochlorine pesticides in the fat and blood
of the general population of Bulgaria and methods for their
determination.] Dissertation. Sofia, Inst. Hygiene and
Occupational Health, Academy of Medicine, 117 pp. (in Bulgarian).
ROBINSON, J., RICHARDSON, A., & ELGAR, K. E. (1966) Chemical identity
in ultramicroanalysis, New York, American Chemical Society
(abstracts).
ROBINSON, J., RICHARDSON, A., HUNTER, C. G., CRABTREE, A. N., & REES,
H. J. (1965) Organochlorine insecticide content of human adipose
tissue in Southeastern England. Br. J. ind. Med., 22: 220-229.
ROSEN, A. A. & MIDDLETON, F. M. (1959) Chlorinated insecticides in
surface waters. Anal. Chem., 31: 1729-1732.
ROSSI, R., RAVERA, M., REPETTI, G., & SANTI, L. (1977) Long-term
administration of DDT or phenobarbital-Na in Wistar rats. Int. J.
Cancer, 19: 179-185.
ROTHE, C. F., MATTSON, A. M., NUESLEIN, R. M., & HAYES, W. J., JR
(1957) Metabolism of chlorophenothane (DDT): Intestinal lymphatic
absorption. Am. Med. Assoc. Arch. ind. Health, 16: 82-86.
ROWE, D. R., CANTER, L. W., SNYDER, P. J., & MASON, J. W. (1971)
Dieldrin and endrin concentrations in a Lousiana estuary. Pestic.
Monit. J., 4: 177-183.
RUDD, R. L. & HERMAN, S. G. (1972) Ecosystemic transferal of
pesticides residues in an aquatic environment. In: Matsumura, F.,
Boush, G. M., Misato, T., ed. Environmental toxicology of
pesticides, Part VII, New York, Academic Press.
RYBAKOVA, M. N. (1968) [The effect of certain pesticides on the
hypophysis and its gonadotrophic function.] Gig. i Sanit.,
33: 27-31 (in Russian).
SABAD, L. M., KOLESNICENKO, T. S., & NIKONOVA, T. V. (1972) [On a
possible blastomogenicity of DDT.] Vopr. Pitan., 30: 63-66
(in Russian).
SALIHODZAEV, S. S. & FERSTAT, V. N. (1972) [Condition of the olfactory
analyzer under the effect of organochlorine and organophosphorus
pesticides.] Gig. i Sanit., 37: 95-96 (in Russian).
SANDIFER, S. H., KEIL, J. E., FINKLEA, J. F., & GADSDEN, R. H. (1972)
Pesticide effects on occupationally exposed workers: A summary of
four years observation of industry and farm volunteers in South
Carolina. Ind. Med. Surg., 41: 7-12.
SARETT, H. P. & JANDORF, B. J. (1947) Effect of chronic DDT
intoxication in rats on lipids and other constituents of the
liver. J. Pharmacol., 91: 340-344.
SAUBERLICH, H. E. & BAUMANN, C. A. (1947) Effect of dietary variation
upon the toxicity of DDT to rats or mice. Proc. Sec. Exp. Biol.
Med., 66: 642-645.
SAVAGE, E. P., TESSARI, J. D., MALBERG, J. W., WHEELER, H. W., &
BAGBY, J. R. (1973) Organochlorine pesticide residues and
polychlorinated biphenyls in human milk, Colorado 1971-1972.
Pestic. Monit. J., 7: 1-5.
SAWYER, I. D. (1973) Collaborative study of the recovery and gas
chromatographic quantitation of PCBs in chicken fat and PCB-DDT
combinations in fish. J. Assoc. Off. Anal. Chem., 56: 1015-1023.
SCHAEFER, R. G. (1974) [Mass spectrometric identification and
quantification of chlorinated hydrocarbons in fish.] Chem. Ztg.,
98: 241-247 (in German).
SCHAFER, M. L. & CAMPBELL, J. E. (1966) Distribution of pesticide
residues in human body tissues from Montgomery County, Ohio,
Washington, DC, American Chemical Society, pp. 89-98 (Adv. Chem.
Ser. No. 60).
SCHMICK, M. (1973) Survival with adrenal carcinoma. J. Am. Med.
Assoc., 224: 1763.
SCHMIDT, R. (1973) [Effect of 1,1,1-trichloro-2,2-bis( p-
chlorophenyl) ethane (DDT) on the prenatal development of the
mouse, under consideration of distribution of tritium-labeled and
carbon-14-labeled DDT in pregnant mice.] Biol. Runtisch.,
11: 316-317 (in German).
SCHOOR, W. P. (1970) Effect of anticonvulsant drugs on insecticide
residues. Lancet, 2: 520-521.
SCHROEDER, G. & DOROZALSKA. (1975) [Degradation of DDT and its
analogues.] Wiad. Chem., 29: 553-565 (in Polish).
SCHVARTSMAN, S., ALMEIDA, W. F., COSTA VAZ, F. A., CORRADINI, H. B.,
PIGATI, P., GAETA, R., & UNGARO, M. T. (1974) Blood levels of DDT
in nonoccupationally exposed mothers and newborn infants in a city
in Brazil. In: Coulston, F. & Korte, F., ed. EQS Environmental
Quality and Safety, New York, Academic Press, Vol. 3,
pp. 154-156.
SCHWABE, U. & WENDLING, I. (1967) [Acceleration of drug metabolism by
small doses of DDT and other chlorinated hydrocarbon
insecticides.] Arzneim. Forsch., 17: 614-618 (in German).
SCOTT, R. P. W. (1970) Determination of the optimum conditions to
effect a separation by gas chromatography. Adv. Chromatogr.,
9: 193-214.
SCUDDER, H. I. & TARZWELL, C. M. (1950) Effects of DDT mosquito
larviciding on wildlife. IV. The effects on terrestrial insect
population of routine larviciding by airplane. Public Health
Rep., 65: 71-87.
SELBY, L. A., NEWELL, K. W., HAUSER, G. A., & JUNKER, G. (1969)
Comparison of chlorinated hydrocarbon pesticides in maternal blood
and placental tissues. Environ. Res., 2: 247-255.
SELL, J. L., DAVISON, K. L., & PUYEAR, R. L. (1971) Aniline
hydroxylase, N-demethylase, and cytochrome P-450 in liver
microsomes of hens fed DDT and dieldrin. J. agile. food Chem.,
19: 58-60.
SEMENCAEVA, E. M. (1968) [The mechanism of action of DDT and certain
other pesticides on the immunological response of the body.] In:
Medved', L. E., ed. The hygiene of application of the toxicology
of pesticides and the clinical features of pesticides poisoning.
Scientific Conference on the Safe Use & Toxicology of Pesticides,
11-14 June, 1968, Issue No. 6, Kiev, Acad. of Sciences, USSR,
pp. 805-815 (in Russian).
SHARAHGAPANI, M. V. & PINGALE, S. V. (1954) The hazard of DDT-
treatment of potatoes. Bull. Cent. Food Technol. Res. Inst.
Mysore, 4: 57.
SHERMA, J. (1973) Thin-layer chromatography: Recent advances. In:
Zweig, G., ed. Analytical methods for pesticides and plant growth
regulators, New York, Academic Press, Vol. VIII, pp. 3-87.
SHIRASU, Y., MORIYA, M., KATO, K., FURUHASHI, A., & KADA, T. (1976)
Mutagenicity screening of pesticides in the microbial system.
Mutat. Res., 40: 19-30.
SHIRASU, Y., MORIYA, M., KATO, K., LIENARD, F., TEZUKA, H., TERAMOTO,
S., & KADA, T. (1977) Mutagenicity screening on pesticides and
modification products: A basis of carcinogenicity evaluation. In:
Hiatt, H. H., Watson, J. D., & Winsten, J. A., ed. Origins of
human cancer, Book A, Incidence of cancer in humans, Cold Spring
Harbor, NY, Cold Spring Harbor Laboratory, Vol. 4, pp. 267-285.
SIERWISKI, M. & HELRICH, K. (1967) Separation, identification and
measurement of DDT and its metabolites. J. Assoc. Off. Anal.
Chem., 50: 627-633.
SIMMONS, S. W. (1959) The use of DDT insecticides in human medicine.
In: Müller, P., ed. DDT, the insecticide dichlorodiphenyltri-
chloroethane and its significance, Basel, Switzerland, Birkhäuser
Verlag, Vol. II, pp. 251-502.
SINGHAL, R. L. & KACEW, S. (1976) The role of cyclic AMP in
chlorinated hydrocarbon-induced toxicity. Fed. Proc.,
35: 2618-2623.
SISSONS, D. J., TELLING, G. M., & USHER, C. D. (1968) A rapid and
sensitive procedure for routine determination of organochlorine
pesticide residues in vegetables. J. Chromatogr., 33: 435-449.
SIYALI, D. S. (1972) Hexachlorobenzene and other organochlorine
pesticides in human blood. Med. J. Aust, 2: 1063-1066.
SIYALI, D. S. (1973) Polychlorinated hiphenyls, hexachlorobenzene and
other organochlorine pesticides in human milk. Med. J. Aust,
2: 815-818.
SIZONENKO, P. C., DORET, A. M., RIONDEL, A. M., & PAUNIER, L. (1974)
Cushing's syndrome due to bilateral adrenal cortical hyperplasia
in a 13-year-old girl: Successful treatment with o,p'-DDD.
Helv. Paediatr. Acta, 29: 195-202.
SKOGERBOE, R. K. & GRANT, C. L. (1970) Comments on the definitions of
the terms sensitivity and detection limit. Spectrosc. Lett.,
3: 215-220.
SKRENTNY, R. F. & DOROUGH, H. W. (1971) Efficiency of several
extraction procedures in removing organochlorine insecticides from
tobacco. Tobacco Sci., 15: 111-113.
SMART, N. A., HILL A. R. C., & ROUGHAN, P. A. (1974) Chlorinated
hydrocarbon pesticides residues in foodstuffs: Comparison of
methods. J. Assoc. Off. Anal. Chem., 57: 153-164.
SMITH, D. C. (1971) Pesticide residues in the total diet in Canada.
Pestic. Sci., 2: 92-95.
SMITH, D. C., SAUDI, E., & LEDUC, R. (1972) Pesticide residues in the
total diet in Canada. II-1970. Pestic. Sci., 3: 207-210.
SMITH, D. C., LEDUC, R., & CHARBONNEAU, C. (1973) Pesticide residues
in the total diet in Canada. III-1971. Pestic. Sci., 4: 211-214.
SMITH, D. C., LEDUC, R., & TREMBLAY, L. (1975) Pesticide residues in
the total diet in Canada. IV-1972 and 1973. Pestic. Sci.,
6: 75-82.
SMITH, M. I. & STOHLMAN, E. F. (1944) The pharmacologic action of 2,2-
bis-( p-chlorophenyl)-1,1,1-trichlorethane and its estimation in
the tissues and body fluids. Public Health Rep., 59: 984-993.
SMITH, M. I. & STOHLMAN, E. F. (1945) Further studies on the
pharmacologic action of 2,2-bis-( p-chlorophenyl)-1,1,1-
trichloroethane (DDT). Public Health Rep., 60: 289-301.
SMITH, M. I., BAUER, H., STOHLMAN, E. F., & LILLIE, R. D. (1946) The
pharmacologic action of certain analogues and derivatives of DDT.
J. Pharmacol., 88: 359-365.
SNYDER, D. & REINERT, R. (1971) Rapid separation of PCBs from DDT and
its analogues on silica gel. Bull. environ. Contam. Toxicol.,
6: 385-390.
SOUTHERN, A. L., TOCHIMOTO, S., ISURUGI, K., GORCHER, G. G., KRIKUN,
E., & STYPULKOWSKI, W. (1966a) The effect of 2,2-bis-(2-
chlorophenyl-4-chlorophenyl)-1,1,1-dichloroethane ( o,p'-DDD) on
the metabolism of infused cortisol-7-H. Steroids, 7: 11-29.
SOUTHERN, A. L., TOCHIMOTO, S., STROM, L., RATUSCHINI, A., RASS, H., &
GORCHER, G. (1966b) Remission in Cushing's syndrome with o,p'-
DDD. J. clin. Endocrinol. Metab., 26: 268-278.
SPICER, S. S., SWEENEY, T. R., VON OETTINGEN, W. F., LILLIE, R. D., &
NEAL, P. A. (1947) Toxicological observations on goats fed large
doses of DDT. Vet. Med., 42: 289-293.
STACY, G. I. & THOMAS, B. W. (1975) Organochlorine pesticide residues
in human milk, Western Australia-1970-71. Pestic. Monit. J.,
9: 64-66.
STANELY, C. W., BARNEY, J. E., HELTON, M. R., & YOBS, A. R. (1971)
Measurement of atmospheric levels of pesticides. Environ. Sci.
Technol., 5: 431-435.
STARR, M. G., ALDRICH, F. D., McDOUGALL, W. D., III, & MOUNCE, L. M.
(1974) Contribution of household dust to human exposure to
pesticides. Pestic. Monit. J., 8: 209-212.
STEC, J. & JUSZKIEWICZ, T. (1972) [Determination of polychlorinated
insecticide residues in animal products using Woods methods.]
Med. Weter., 28: 634-637 (in Polish).
STENBERG, A. I. & DIKY, V. V. (1973) [Changes in the metabolism of DDT
deposited in the omentum of fasting albino rats.] Vopr. Pitan.,
32; 39-43 (in Russian).
STIJVE, T. & CARDINALE, E. (1974) [Rapid determination of chlorinated
pesticides, PCBs, and a number of phosphated insecticides in fatty
foods.] Mitt. Lebensmittelunters. Hyg., 65: 131-150 (in German).
STOHLMAN, E. F. & LILLIE, R. D. (1948) The effect of DDT on the blood
sugar and of glucose administration on the acute and chronic
poisoning of DDT in rabbits. J. Pharmacol., 93: 351-361.
STRASSMANN, S.C. & KUTZ, F. W. (1977) Insecticide residues in human
milk from Arkansas and Mississippi, 1973-74. Pestic. Monit. J.,
10: 130-133.
STRAW, J. A., WATERS, I. W., & FREQLY, M. J. (1965) Effect of o,p'-
DDD on hepatic metabolism of phenobarbital in rat. Proc. Exp.
Biol. Med., 118: 391-394.
STREET, J. C. (1964) DDT antagonism to dieldrin storage in adipose
tissue of rats. Science, 146: 1580-1581.
STREET, J. C. & CHADWICK, R. W. (1967) Stimulation of dieldrin
metabolism by DDT. Toxicol. appl. Pharmacol., 11: 68-71.
STREET, J. C., WANG, M., & BLAU, A. D. (1966) Drug effects on dieldrin
storage in rat tissue. Bull. environ. Contam. Toxicol., 1: 6-15.
STREET, J. C., MAYER, F. L., & WAGSTAFF, D. J. (1969) Ecological
significance of pesticide interactions. Ind. Med. Surg,
38: 409-414.
STRETZ, P. E. & STARR, H. M. (1973) Determination of chlorinated
pesticides in whole blood. J. Assoc. Off. Anal Chem.,
56: 1173-1177.
STUDY GROUP OF MINISTRY OF HEALTH-WELFARE FOR ORGANOCHLORINE PESTICIDE
RESIDUES IN MOTHERS' MILK (1972) VIII. [Organochlorine pesticide
residues in blood during lactation.] J. Food Hyg. Soc. Jpn,
13: 438-450 (in Japanese).
SUGAYA, T. et al. (1971) [Organochlorine pesticide residues in human
milk.] J. Jpn Assoc. Rural Med., 19: 379-380 (in Japanese).
SUNDSTRÖM, G. (1977). Metabolic hydroxylation of the aromatic rings of
1,1-dichloro-2,2-bis(4-chlorophenyl)ethylene ( p,p'-DDE) by the
rat. J. agric. food Chem., 25: 18-21.
SUNDSTRÖM, G., JANSSON, B., & JENSEN, S. (1975) Structure of phenolic
metabolites of p,p'-DDE in rat, wild seal and guillemot. Nature
(Lond.), 255: 627-628.
SURBER, E. W. & FRIDDLE, D. D. (1949) Relative toxicity of suspension
and oil formulations of DDT to native fishes in Black Creek, West
Virginia. Trans. Am. Fish. Soc., 76: 315-321.
SUTHERLAND, G. L. (1965) Residue analytical limits of detectability.
Residue Rev., 10: 85-96.
SUVAK, L. N. (1970) [DDT in mothers' milk.] Zdravoochr. Kosinev,
13: 19-21 (in Russian).
SUZUKI, M., YAMATO, Y., & WATANABE, T. (1975) Gas chromatographic
resolution of organochlorine insecticides on OV-1/OV-17,
OV-210/OV-17 and OV-225/OV-17 mixed phase systems. J. Assoc. Off.
Anal. Chem., 58: 297-300.
SUZUKI, Y., SUGAYA, H., & SASAKI, S. (1973) [Results of 3 years'
tracing investigation of organochlorine pesticide residues in
humans.] J. Jpn Assoc. Med., 22: 276-277 (in Japanese).
SWACKHAMER, A.B. (1965) Report on pesticide residues in restaurant
meals in Canada. Pestic. Prog., 3: 108-114.
TABOR, E. C. (1966) Pesticides in urban atmospheres. Contamination of
urban air through the use of insecticides. Trans. NY Acad. Sci.,
28: 569-578.
TAIRA, M., HASHIMOTO, S., & SHIMOHIRA, I. (1972) [Organochlorine
pesticide residues in human breast milk.] Bull. Hyogo Prefect.
Public Health Lab., 7: 19-23 (in Japanese).
TAKANO, H. (1972) [Results of inspection of pesticide residues in
mothers' milk and market milk.] Annu. Rep. Iwate Inst. of Public
Health, 15: 88-91 (in Japanese).
TAKESHITA, T. & INUYAMA, Y. (1970) [Organochlorine residues in
mothers' milk and blood.] Annu. Rep. Shimane Prefect. Inst.
Public Health, 12: 27-28 (in Japanese).
TARJAN, R. & KEMENY, T. (1969) Multigeneration studies on DDT in mice.
Food Cosmet. Toxicol., 7: 215-222.
TARRANT, K. R. & TATTON, J. O'G. (1968) Organochlorine pesticides in
rainwater in the British Isles. Nature (Lond.), 219: 725-727.
TARZWELL, C. M. (1950) Effects of DDT mosquito larviciding on
wildlife. V. Effects on fishes of the routine manual and airplane
application of DDT and other mosquito larvicides. Public Health
Rep., 65: 231-255.
TAYLOR, R. & BOGACKA, T. (1968) [Determination of DDT, methoxychlor or
lindane in water by extraction and thin-layer chromatography.]
Chem. Anal., 13: 227-234 (in Polish).
TELFORD, H. S. & GUTHRIE, J. E. (1945) Transmission of the toxicity of
DDT through the milk of white rats and goats. Science, 102: 647.
TERRACINI, B., TESTA, M. C., CABRAL, J. R., & DAY, N. (1973) The
effects of long-term feeding of DDT to BALB/c mice. Int. J.
Cancer, 11: 747-764.
THOMAS, E. J., BURKE, J. A., & LAWRENCE, J. H. (1968) Thin-layer
chromatography; relative migration date (R-TDE) of chlorinated
pesticides. J. Chromatogr, 35: 119-121.
THOMAS, T. C. & SERBER, J. N. (1974) Chromosorb 102, an efficient
medium for trapping pesticides from air. Bull. environ. Contam.
Toxicol., 12: 17-25.
THOMPSON, J. F., ed. (1974) Analysis of pesticide residues in human
and environmental samples, a compilation of methods selected for
use in pesticide monitoring programs, Research Triangle Park,
NC, US Environmental Protection Agency, 1 vol.
THOMPSON, J. F., WALKER, A. C., & MOSEMAN, R. F. (1969a) Evaluation of
8 gas chromatographic columns for chlorinated pesticides.
J. Assoc. Off. Anal. Chem., 52: 1263-1277.
THOMPSON, J. F., WALKER, A. C., & MOSEMAN, R. F. (1969b) Study of the
performance of gas-chromatographic columns under severe injection
loading. J. Assoc. Off. Anal. Chem., 52: 1251-1262.
THOMPSON, J. F., MANN, J. B., APODACA, A. O., & KANTOR, E. J. (1975)
Relative retention ratios of 95 pesticides and metabolites on nine
gas-liquid chromatographic columns over a temperature range of
170-204°C on two detection modes. J. Assoc. Off. Anal. Chem., 58:
1037-1050.
THOMPSON, R. P. H., PILCHER, C. W. T., ROBINSON, J., STATHERS, G. M.,
MCLEAN, A. E. M., & WILLIAMS, R. (1969) Treatment of unconjugated
jaundice with dicophane. Lancet, 2: 4-6.
THORPE, E. & WALKER, A. I. T. (1973) The toxicology of dieldrin
(HEOD). II. Comparative long-term oral toxicity studies in mice
with dieldrin, DDT, phenobarbitone, ß-BHC, and gamma-BHC. Food
Cosmet. Toxicol., 11: 433-442.
TINSLEY, I. J. & LOWRY, R. R. (1972) An interaction of DDT in the
metabolism of essential fatty acids. Lipids, 7: 182-185.
TOCCI, P.M., MANN, J. B., DAVIES, J. E., & EDMUNDSON, W. F. (1969)
Biochemical differences found in persons chronically exposed to
high levels of pesticides. Ind. Med. Surg., 38: 188-195.
TOKUTSU, K., KOYAMA, T., & TOKOYAMA, T. (1970) [Pesticide residues in
mothers' milk and plasma in Wakayama Prefecture.] Annu. Rep.
Wakayama Prefect. Inst. Public Health, 19: 59-62 (in Japanese).
TOMATIS, L. & TURUSOV, V. (1975) Studies on the carcinogenicity of
DDT. GANN Monograph Cancer Res., 17: 219-241.
TOMATIS, L., TUROSOV, V., DAY, N., & CHARLES, R. T. (1972) The effect
of long-term exposure to DDT on CF-1 mice. Int. J. Cancer,
10: 489-506.
TOMATIS, L., PARTENSKY, C., & MONTESANO. R. (1973) The predictive
value of mouse liver tumor induction in carcinogenicity testing --
A literature survey. Int. J. Cancer, 12: 1-20.
TOMATIS, L., TURUSOV, V., CHARLES, R. T., & BOICCHI, M. (1974a).
Effect of long-term exposure to 1,1-dichloro-2,2-bis( p-
chlorophenyl)ethylene, 1,1-dichloro-2,2-bis( p-chlorophenyl)
ethane, and to the two chemicals combined on CF-1 mice. J. Natl
Cancer Inst., 52: 883-891.
TOMATIS, L., TURUSOV, V., CHARLES, R. T., BOICCHI, M., & GATI, F.
(1974b) Liver tumors in CF-1 mice exposed for limited periods to
technical DDT. Z. Krebs. Forsch., 82: 25-35.
TOMII, S., NAGASAKI, H., & MEGA, Y. (1972) [Studies on BHC as a
carcinogen.] Jpn. J. Hyg., 27: 113 (in Japanese).
TORDA, C. & WOLFF, H. G. (1949) Effect of convulsant and
anticonvulsant agents on the activity of carbonic anhydrase.
J. Pharmacol. exp. Ther., 95: 444-447.
TREON, J. F. & CLEVELAND, F. P. (1955) Toxicity of certain chlorinated
hydrocarbon insecticides for laboratory animals with special
reference to aldrin and dieldrin. J. agric. food Chem.,
3: 402-408.
TSUTSUI, J., KATO, T., & NISHIKAWA, T. (1974) [Results of health
survey on persons engaging in pesticide application in Suzuka
area. Mie Prefecture.] J. Jpn. Assoc. Rural Med., 23: 518-521
(in Japanese).
TUINSTRA, L. G. M. TH. (1971) [Organochlorine insecticide residues in
human milk in the Leiden Region.] Ned. Melk-Zuiveltijdschr.,
25: 24-32 (in Dutch).
UNTERMAN, H. W. & SIRGHIE, E. (1969) [Presence of organochlorines in
human body.] 18: 221-226 (in Romanian).
US DEPT OF AGRICULTURE, AGRICULTURAL RESEARCH ADMINISTRATION (1949)
USDA entomologists recommend substitute insecticide for DDT to
control insects on dairy cattle and in dairy barns. News
Release, March 24.
U.S. DEPT OF AGRICULTURE, AGRICULTURAL RESEARCH SERVICE (1966)
Monitoring agricultural pesticide residues. A preliminary report
of studies on soil, sediment and water in Mississippi River
delta, Washington, DC, US Government Printing Office.
US DEPT OF AGRICULTURE, BUREAU OF ENTOMOLOGY AND PLANT QUARANTINE
(1945a) Release of February 13, 1945.
US DEPT OF AGRICULTURE, BUREAU OF ENTOMOLOGY AND PLANT QUARANTINE
(1945b) Outlook for supplies of DDT insecticides. Release of
August 6, 1945.
US DEPT OF HEALTH EDUCATION AND WELFARE (1969) Manual for evaluating
public drinking water supplies. A manual of practice recommended
by the Public Health Service, Washington, DC, US Government
Printing Office, I (PHS Pub. No. 1820).
US ENVIRONMENTAL PROTECTION AGENCY (1975) DDT. A review of scientific
and economic aspects of the decision to ban its use as a
pesticide, Washington US EPA, US Government Printing Office,
300 pp. (EPA-504-1-75-022).
VAARAMA, A. (1947) The influence of DDT pesticides upon plant mitosis.
Hereditas, 33: 191-219.
VAINO, H. (1974) Enhancement of hepatic microsomal drug oxidation and
glucuronidation in rat by 1,1,1-trichloro-2,2-bis( p-
chlorophenyl)ethane (DDT). Chem. biol. Interactions, 9: 7-14.
VAINIO, H. (1975) Stimulation of microsomal drug-metabolizing enzymes
in rat liver by 1,1,1-trichloro-2,2-bis( p-chlorophenyl)ethane
(DDT), pregnenolone-16alpha-carbonitrile (PEN), and
polychlorinated biphenyls (PCBs). Environ. Qual. Saf.,
3 (Suppl.): 486-490.
VANAT, S. V. & VANAT, I. M. (1971) [Contribution to the toxic-allergic
reaction induced by DDT.] Klin. Med. (Mosk.), 49: 126-127
(in Russian).
VAS'KOVSKAJA & KOMAROVA (1967) Quoted by: Kagan et al., Res. Rev, 27:
43-79.
VAS'KOVSKAJA, L. F. (1969) [Accumulation of certain chloroorganic
insecticides in the bodies of experimental animals and humans.]
In: Medved', L. E., ed. The hygiene of application and the
toxicology of pesticides and the clinical features of pesticide
poisoning. A collection of papers. Issue No. 7, Kiev,
Vniggintox, pp. 503-796 (in Russian).
VAZ A., PEREIRA, R. S., & MALHETRO, D. M. (1945) Calcium in prevention
and treatment of experimental DDT poisoning. Science,
101: 434-436.
VELBINGER, H. H. (1947a) [On the question of DDT toxicity to man.]
Dtsch. Gesundheitwes., 2: 355-358 (in German).
VELBINGER, H. H. (1947b) [Contribution to the toxicology of "DDT"-
agent, dichlorodiphenyl-trichloromethylmethane.] Pharmazie,
2: 268-274 (in German).
VERDON, T. A., JR, BRUTON, J., HERMAN, R. H., & BEISEL, W. R. (1962)
Clinical and chemical response of functioning adrenal cortical
carcinoma to ortho-, para-DDD. Metab. Clin. Exp., 11: 226-234.
VERSINO, B., VENNE, M-TH VAN DER, & VISSERS, H. (1971) Comparison of
some clean-up columns for residue analysis of chlorine and
phosphorus containing pesticides. J. Assoc. Off. Anal Chem.,
54: 147-149.
VON OETTINGEN, W. F. & SHARPLESS, N. E. (1946) The toxicity and toxic
manifestations of 2,2-bis-( p-chlorophenyl)-1,1,1-trichloroethane
(DDT) as influenced by chemical changes in the molecule. A
contribution to the relation between chemical constitution and
toxicological action. J. Pharmacol. exp. Ther., 88: 400-413.
WAGSTAFF, D. J. (1971) Ascorbic acid deficiency and induction of
hepatic microsomal hydroxylative enzymes by organochlorine
pesticides. Toxicol. appl. Pharmacol., 19: 10-19.
WAGSTAFF, D. J. & STREET, J. C. (1970) Dieldrin-DDT interactions in
guinea pigs. Toxicol. appl. Pharmacol., 17: 276.
WALKER, A. I. T., THORPE, E., & STEVENSON, D. E. (1973) The toxicology
of dieldrin (HEOD). I. Long-term oral toxicity studies in mice.
Food Cosmet. Toxicol., 11: 415-432.
WALKER, K. C., GOETTE, M. B., & BATCHELOR, G. S. (1954) Pesticide
residues in foods: Dichlorodiphenyltrichloroethane and
dichlorodiphenyldichloroethylene content of prepared meals.
J. agric. food. Chem., 2: 1034-1037.
WALLACE, Z. E., SILVERSTEIN, J. N., VILLADOLID, L. S., WEISENFELD, S.
(1961) Cushing's syndrome due to adrenocortical hyperplasia. New
Engl. J. Med., 265: 1088-1093.
WARNICK, S. L. (1972) Organochlorine pesticide levels in human serum
and adipose tissue, Utah-Fiscal years 1967-71. Pestic. Monit. J.,
6: 9-13.
WARNICK, S. L. & CARTER, J. E. (1972) Some findings in a study of
workers occupationally exposed to pesticides. Arch. environ.
Health, 25: 265-270.
WASICKY, R. & UNTI, O. (1944) [Dichloro-diphenyl-trichloroethanol
(DDT) does not have any effect on the larvae of "Culicides".]
Arch. Hig. S. Paulo, 9: 87-102 (in Portuguese).
WASICKY, R. & UNTI, O. (1945) [Dichlorodiphenyltrichloroethane (DDT),
additional investigations on its properties and application.]
Arch. Hig. S. Paulo, 10: 49-64 (in Portuguese).
WASSERMANN, D., WASSERMANN, M., DJAVAHERIN, H., GORIN, I., BARISH, M.,
& TAVOR, R. (1971) Effect of organochlorine insecticides on serum
PBI level in occupationally exposed people. Bull. environ.
Contam. Toxicol., 6: 85-88.
WASSERMANN, M., ILIESCU, S., MANDRIC, G., & HORVATH, P. (1960) Toxic
hazards during DDT- and BHC-spraying of forests against Lymantria
monacha. Arch. ind. Health, 21: 503-508.
WASSERMANN, M., GON, M., WASSERMANN, D., & ZELLERMAYER, L. (1965) DDT
and DDE in the body fat of people in Israel. Arch. environ.
Health, 11: 375-379.
WASSERMANN, M., WASSERMANN, D., ZELLERMAYER, L., & GON, M. (1967)
Pesticides in people. Storage of DDT in the people of Israel.
Pestic. Monit. J., 1: 15-20.
WASSERMANN, M., CURNOW, D. H., FORTE, P. N., & GRONER, Y. (1968a)
Storage of organochlorine pesticide in the body of people in
Western Australia. Ind. Med. Surg., 37: 295-300.
WASSERMANN, M., SOFOLUWE, G. I., WASSERMANN, D., GRONERS, I., &
LAZAROVITCH, S. (1968a) Storage of organochlorine insecticides in
the body fat of people in Nigeria. In: Wasserman, H. & Sofoluwe,
G. O., ed. The Lagos International Seminar on Occupational Health
for Developing Countries, 1-6 April 1968, pp. 188-202.
WASSERMANN, M., WASSERMANN, D., GERSHON, Z., & ZELLERMAYER, L. (1969)
Effect of organochlorine insecticides on body defence systems.
Ann. NY Acad. Sci., 160: 393-401.
WASSERMANN, M., WASSERMANN, D., ARONOVSKI, I., IVRIANI, I., &
ROSENFELD, D. (1970a) The effects of organochlorine insecticides
on serum cholesterol level in people occupationally exposed. Bull
environ. Contam. Toxicol., 5: 368-372.
WASSERMANN, M., WASSERMANN, D., LAZAROVICI, S., COETZEE, A. M., &
TOMATIS, L. (1970b) Present state of the organochlorine
insecticides in the general populations of South Africa. South
Afr. med. J., 44: 646-648.
WASSERMANN, M., WASSERMANN, D., & IVRIANI, I. (1970c) Organochlorine
insecticides in the plasma of occupationally exposed workers. In:
Deichmann, W. B., Radomski, J. L., & Penalver, R. A., ed.
Pesticides Symposia, Miami, Florida, Halos. pp. 311-314.
WASSERMANN, M., WASSERMANN, D., KEDAR, E., & DJAVAHERIAN, M. (1971)
Immunological and detoxication interactions in p,p'-DDT fed
rabbits. Bull. environ. Contam. Toxicol., 6: 426-534.
WASSERMANN, M., ROGOFF, M. G., TOMATIS, L., & DAY, N. E. (1972a)
Storage of organochlorine insecticides in the adipose tissue of
people in Kenya. Ann. Soc. Belg. Med. Trop., 52 (6): 509-514.
WASSERMANN, M., NOGUEIRA, D. P., TOMATIS, L., ATHIE, E., WASSERMANN,
D., KJAVAHERIAN, M., & GUTTEL, C. (1972b) Storage of
organochlorine insecticides in people of Sao Paulo, Brazil. Ind.
Med. Surg., 41: (3): 22-25.
WASSERMANN, M., TRISHNANANDA, M., TOMATIS, L., DAY, N. E, WASSERMANN,
D., RUNGPITARANGSI, V., CHAMSAKOL, V., DJAVAHERIAN, M., & CUCUS,
S. (1972c) Storage of organochlorine insecticides in the adipose
tissue of people from Thailand. Southeast Asian J. trop. Med.
Public Health, 3: 280-285.
WASSERMANN, M., SOFOLUWE, G. O., TOMATIS, L., DAY, N. E., WASSERMANN,
D., & LAZAROVICI, S. (1972d) Storage of organochlorine
insecticides in people in Nigeria. Environ. Physiol. Biochem.,
2: 59-67.
WASSERMANN, M., TOMATIS, L., WASSERMANN, D., DAY, N. E., &
DJAVAHERIAN, M. (1974a) Storage of organochlorine insecticides in
adipose tissue of Ugandans. Bull. environ. Contam. Toxicol.,
12: 501-508.
WASSERMANN, M., TOMATIS, L., WASSERMANN, D., DAY, N. E., GRONER, Y.,
LAZAROVICI, S., & ROSENFELD, D. (1974b) Epidemiology of
organochlorine insecticides in the adipose tissue of Israelis.
Pestic. Monit. J., 8: 1-7.
WASSERMANN, M., PRATA, A., TOMATIS, L., WASSERMANN, D., IVRIANI, I., &
OLIVIERA, V. (1975) Storage of organochlorine insecticides in
hepato-splenic schistosomiasis. Rev. Soc. Bras. Mad. Trop.,
8: 271-273.
WATARI, N. (1973) [Ultrastructural alterations of the mouse liver
after the prolonged administration of BHC.] J. Jpn. Electron
Microsc. Soc., 5: 1410-1420; 1449-1456 (in Japanese).
WATSON, M., BENSON, W. W., & GABICA, J. (1970) Serum organochlorine
pesticide levels in people in Southern Idaho. Pestic. Monit. J.,
4: 47-50.
WATSON, M., GABICA, J., & BENSON, W. W. (1972) Serum organochlorine
pesticides in mentally retarded patients on differing drug
regimens. Clin. Pharmacol. Ther., 13: 186-192.
WATTENBERG, L. W. (1971) Studies of polycyclic hydrocarbon
hydroxylases of the intestine possibly related to cancer. Effect
of diet on benzypyrene hydroxylase activity. Cancer, 28: 99-102.
WEAVER, L., GUNNERSON, C. G., BREIDENBACH, A. W., & LICHTENBERG, J. J.
(1965) Chlorinated hydrocarbon pesticides in major US river
basins. Public Health Rep., 80: 481-493.
WEIHE, M. (1966) [Chlorinated insecticides in the body fat of people
in Denmark.] Ugeskr. Laeg., 128: 881-882 (in Danish).
WEIL, C. S. & McCOLLISTER, D. D. (1963) Relationship between short-
and long-term feeding studies in designing an effective toxicity
test. J. agric. food Chem., 11: 486-491.
WEIL, C. S., WOODSIDE, M. D., BERNARD, J. R., & CARPENTER, C. P.
(1969) Relationship between single-peroral, one-week, and ninety-
day rat feeding studies. Toxicol. appl. Pharmacol., 14: 426-431.
WEIL, L., QUENTIN, K. E., & RONICKE, G. (1973) Pesticide levels in air
dust particles in the Federal Republic of Germany. In: The
Technical University of Munich and the German Society for
Research, Commission on Research on Air Pollution, Notice VIII,
23 pp.
WELCH, R. M. & HARRISON, Y. (1966) Reduced drug toxicity following
insecticide treatment. Pharmacologist, 8: 217.
WELCH, R. M., LEVIN, W., & CONNEY, A. H. (1969) Estrogenic action of
DDT and its analogs. Toxicol. appl. Pharmacol., 14: 358-367.
WELCH, J. H. & GORDON, H. T. (1946) The mode of action of DDT. Fed.
Proc., 5: 1.
WEST, I. (1964) Pesticides as contaminants. Arch. environ. Health,
9: 626-631.
WESTOO, G., NOREN, K., & ANDERSSON, M. (1970) [Levels of
organochlorine pesticides and polychlorinated biphenyls in
margarine, vegetable oils, and some foods of animal origin on the
Swedish market in 1967-1969.] Vaar foeda, 22: 9-31 (in Swedish).
WHITE, W. C. & SWEENEY, T. R. (1945) The metabolism of 2,2-bis-( p-
chlorophenyl-1,1,1-trichloroethane (DDT). I. A metabolite from
rabbit urine di-( p-chlorophenyl)-acetic acid; its isolation,
identification, and synthesis. Public Health Rep., 60: 66-71.
WHITING, F. M., STULL, J. W., BROWN, W. H., MILLBROTH, M., & WARE, G.
W. (1968) Comparison of extraction methods for analysis of DDT,
DDE and TDE, in alfalfa hay. J. dairy Sci., 51: 1039-1041.
WHO (1971) Appendix 14: The place of DDT in operations against malaria
and other vector-borne diseases. Off. Rec. World Health Organ.,
190: 176-182.
WHO (1973) WHO Technical Report Series No. 513 (Safe use of
pesticides) 54 pp.
WIGGLESWORTH, V. B. (1945) A case of DDT poisoning in man. Br. med.
J., 1: 517.
WILCOX, A. R. (1967) USPH investigation. DDT health effects. Inter-
Office Correspondence to M. V. Anthony, Stauffer Chemical Co.
WILLIS, G. H., PARR, J. F., & SMITH, S. (1971) Volatilization of soil-
applied DDT and DDD from flooded and nonflooded plots. Pestic
Monit. J., 4: 204-208.
WILSON, D. J., LOCKER, D. J., RITZEN, C. A., WATSON, J. T., &
SCHAFFNER, W. (1973) DDT concentrations in human milk. Am. J.
Dis. Child., 125: 814-817.
WILSON, H. F., ALLEN, N. N., BOHSTEDT, G., BETHEIL, J., & LARDY, H. A.
(1946a) Feeding experiments with DDT-treated pea vine silage with
special reference to dairy cows, sheep, and laboratory animals.
J. econ. Entomol., 36 (6): 801-806.
WILSON, H. F., SRIVASTAVA, A. S., HULL, W. B., BETHEIL, J., & LARDY,
H. A. (1946b) DDT residues on pea vines and canned peas from
fields treated with DDT dusts. J. econ. Entomol., 39
(6): 806-809.
WINTER, M., THOMAS, M., WERNICK, S., LEVIN, S., & FARVAR, M. T. (1976)
Analysis of pesticide residues in 290 samples of Guatemalan
mother's milk. Bull. environ. Contam. Toxicol., 16: 652-657.
WOLFE, H. R. & ARMSTRONG, J. F. (1971) Exposure of formulating plant
workers to DDT. Arch. environ. Health, 23: 169-176.
WOLFE, H. R., WALKER, K. C., ELLIOTT, J. W., & DURHAM, W. F. (1959)
Evaluation of the health hazards involved in house-spraying with
DDT. Bull. Worm Health Organ., 20: 1-14.
WOLFE, H. R., DURHAM, W. F, & ARMSTRONG, J. F. (1970) Urinary
excretion of insecticide metabolites: excretion of para-
nitrophenol and DDA as indicators of exposure to parathion and
DDT. Arch. envrion. Health, 21: 711-716.
WOODARD, G., OFNER, R. R., & MONTGOMERY, C. M. (1945) Accumulation of
DDT in the body fat and its appearance in the milk of dogs.
Science, 102: 177-178.
WOODARD, B. T., FERGUSON, B. B., & WILSON, D. J. (1976) DDT levels in
milk of rural indigent blacks, Research Triangle Park, NC, Health
Effects Research Laboratory, US Environmental Protection Agency,
16 pp. (EPA 600/1-76-032).
WOOLSON E. A. (1974) Extraction of chlorinated hydrocarbon
insecticides from soil. J. Off. Anal. Chem, 57: 604-609.
WOOLSON E. A. & KEARNEY, P. C. (1969) Survey of chlorinated
insecticide analyses in soils. J. Assoc. Off. Anal. Chem.,
52: 1202-1206.
WRENN, T. R., WEYANT, J. R., FRIES, G. F., & BITMAN, J. (1971a) Effect
of several dietary levels of o,p'-DDT on reproduction and
lactation in the rat. Bull. environ. Contam. Toxicol.,
6: 471-479.
WRENN, T., RANDALL, J., WEYANT, R., FRIES, G. F., & BITMAN, J. (1971b)
Influence of dietary o,p'-DDT on reproduction and lactation of
ewes. J. animal Sci., 33: 1288-1292.
WRIGHT, A. S., POTTER, D., WOODER, M. F., DONNINGER, C., & GREENLAND,
R. D. (1972) Effects of dieldrin on mammalian hepatocytes, Food
Cosmet. Toxicol., 10: 311-322.
WYLLIE, J., GABICA, J., & BENSON, W. W. (1972) Comparative
organochlorine pesticide residues in serum and biopsied lipoid
tissue: A survey of 200 persons in southern Idaho -- 1970.
Pestic. Monit. J., 6: 84-88.
YAMAGISHI, T, TAKEBA, K., FUGIMOTO, C., MORIMOTO, K., & HARUTA, M.
(1972) [On the organochlorine pesticide residues in mothers' body
and her fetus' body.] Rep. Tokyo Public Health Stud., 50: 44-45
(in Japanese).
YERMAKOV, V. V. (1972) [Gas chromatographic methods for determining
pesticides in biological material.] Moscow, Izdatelstvo Nauka (in
Russian).
YODER, J. M., WATSON, M., & BENSON, W. W. (1973) Lymphocyte chromosome
analysis of agricultural workers during extensive occupational
exposure to pesticides. Mutat. Res., 21: 335-340.
YOUDEN, W. J. & STEINER, E. H. (1975) Statistical Manual of the
Association of Official Analytical Chemists, Washington, DC,
AOAC.
ZAVON, M. R., HINE, C. H., & PARKER, K. D. (1965) Chlorinated
hydrocarbon insecticides in human body fat in the United States.
J. Assoc. Off. Anal. Chem., 193: 837-839.
ZAVON, M. R., TYE, R., & LATORRE, E. (1969) Chlorinated hydrocarbon
insecticide content of the neonate. Ann. NY Acad. Sci.,
160: 196-200.
ZEINE-EL-DINE, K. (1946) The insecticide DDT. J. Egypt Med. Assoc.,
29: 38-54.
ZELINSKI, S. G., TASHIRO, J., & WORTHEN, L. R. (1973) A gas-
chromatographic method of quantifying DDT in the presence of
interference from PCBs. J. Chromatogr., 84: 67-73.
ZERBER, J., KURASIAK, K., & CIELIEZAK, A. (1971) [Determination of
chlorinated hydrocarbon residues in cereal products and feeds.]
Chem. Anal. 16: 1061-1066 (in Polish).
ZIMMERLI, B. & MAREK, B. (1973) [The pesticide burden of the Swiss
population (analyses of prepared meals, human fat, human serum,
cigarettes, and cosmetics.)] Mitt. Lebensmitt. Hyg., 64: 459-479
(in German).
ZIMMERMAN, B., BLOCH, H. S., WILLIAMS, W. L., HITCHCOCK, C. R., &
HOEISCHER, B. (1956) Effects of DDD (1,1,dichloro-2,2-bis( p-
chloro-phenyl)-ethane) on human adrenal. Attempted to use adrenal
destructive agent in treatment of disseminated mammary and
prostatic cancer. Cancer, 9: 940-948.
ZWEIG, G. & SHERMA, J. (1972) Analytical Methods for Pesticides and
Plant Growth Regulators, New York, Academic Press, Vol 6,
765 pp.
ANNEX
TRANSFORMATION OF P,P'-DDT IN THE ENVIRONMENTa
1 Abiotic Transformations
Since 1969, the photolysis of p,p'-DDT (Annex Fig. 1, formula
II) and of its known primary environmental degradation product, DDE
(1,1-dichloro-2,2-bis [ p-chlorophenyl]ethylene; Annex Fig. 1,
formula VII) has been studied by irradiation in methanol at 260 nm
(Plimmer et al., 1970). The products formed were DDMU (1-chloro-2,2-
bis [ p-chlorophenyl] ethylene; Annex Fig. 1, Formula III),
dichlorobenzophenone (Annex Fig 1, formula IV) and dichlorobiphenyl
(Annex Fig. 1, Formula V). The formation of the last compounds
proceeded via dichlorobenzophenone as an intermediate. The detection
of the chlorinated biphenyl raised the question as to whether DDT
might be a source of PCBs in the environment. Upon investigation of
the reaction pathways leading to these substances, it was shown that
DDE is converted to 3,6-dichlorofluoroenone (Annex Fig. 1, formula VI;
Plimmer & Klingebiel, 1969) which is photooxidized to 3,3'-dichloro-
biphenyl-2-carboxylic acid. Subsequent decarboxylation of this acid
could yield traces of 3,3'-dichlorobiphenyl; the decarbonylation of
another photolysis product of DDT, trichlorobenzophenone, could yield
traces of trichlorobiphenyl, demonstrating that the formation of PCBs
with more than 2 chlorine atoms was also possible (Plimmer &
Klingebiel, 1973).
Irradiation studies with substances in organic solvents are not
necessarily predictive for the environment. However, studies with DDT
vapour in sunlight confirmed the results obtained with dissolved
substances. The proposed pathways of DDT photolysis under
environmental conditions is shown in Annex Fig. 1 (Moilanen & Crosby,
1973).
In 1972, DDE was irradiated in solvents, in the solid state, and
in the gaseous phase, with UV-light of various wavelengths. The
results are shown in Annex Fig. 2. Besides the known photoproducts IV
and III, a "trichlorinated DDMU" (Annex Fig. 2, formula VIII) and 2
compounds with longer side chains (Annex Fig 2, formula IX and X) were
identified; these 2 substances, however, were formed only upon
irradiation in a solvent and originated from the reaction with the
solvent (Kerner et al., 1972).
a Prepared by Dr F. Korte at the request of the Task Group.
Edmundson et al., 1970b; Watson et al., 1972). This result concerning
phenytoin was confirmed by McQueen et al. (1972) who also showed that
other drugs produced a smaller but still highly significant reduction
in DDT storage. Establishment of a reduced equilibrium appeared to
require about 2 months. Within this period, the regression of the
level of DDT plus DDE on duration of treatment with phenytoin was
highly significant ( P < 0.001).
At the end of 9 months' treatment, the body fat of nonepileptic
volunteers given phenytoin at a rate of 300 mg/man per day contained
an average of 25% of the DDT and 39% of the DDE concentrations
originally present before administration of the drug (Davies et al.,
1971).
The same was true of workers whose exposure was greater than that
of the general population. Maintenance doses of phenobarbital,
phenytoin, or a combination of the two kept the storage levels of
several organochlorine insecticides in epileptic workers as low as, or
lower than levels in the general population (Schoor, 1970; Kwalick,
1971).
8.2.5.2 Other biochemical observations
A positive linear correlation has been reported for the
concentrations of vitamin A and of DDT-related compounds in the serum
of men with at least 5 years of occupational exposure to DDT. However,
the workers' DDT levels were little higher than those of persons in
the general population (see Table 7), and their vitamin A levels were
within normal limits (Keil et al., 1972). Perhaps they were better fed
than the controls.
Compared to 86 unexposed workers, the serum total cholesterol
values of 206 workers in a chemical plant where unidentified
organochlorine insecticides were made and formulated were higher in
workers who were less than 25 years old, lower in those between 25-34
years and 35-44 years, and higher in those who were 45 years old or
more. The differences were significant only for the oldest groups
(Wassermann et al., 1970a).
8.2.6 Cardiovascular system
The small amount of knowledge concerning the effect of DDT on the
human heart fails to show whether cardiac arrhythmia might be a
possible cause of death in acute poisoning, as is true in some species
of laboratory animals. Palpitations, tachycardia, and irregular heart
action have been noted in some, but not all cases of acute poisoning
(Mackerras & West, 1946; Naevested, 1947; Hsieh, 1954).
8.2.7 Reproduction
There is no indication that DDT has influenced reproduction except
to increase it as an indirect result of disease control, especially
malaria control.
After Laws et al. (1967) had completed their study, Wilcox (1967)
found that the 36 most heavily exposed workers involved had fathered
58 children before they began working at the DDT factory and 93
children afterwards.
O'Leary et al. (1970c) did not find any significant relationship
between abortion and blood levels of DDT-related compounds.
8.2.8 Endocrine organs
Average protein-bound iodine (PBI)levels of 0.0542 and
0.0693 mg/litre, respectively, were reported in the sera of 42 workers
occupationally-exposed to organochlorine insecticides and in 51
workers who were not exposed. The difference was statistically
significant even though all values fell within the normal range of
0.04-0.08 mg/litre (Wassermann, D. et al., 1971). It was not recorded
whether the workers involved were from the same factory as those with
10 or more years of occupational exposure whose plasma DDT levels were
reported by Wassermann et al. (1970c) (see Table 7). The small
difference in PBI levels is difficult to evaluate. It was the view of
Clifford & Weil (1972) that there was not any evidence that
occupational exposure had had an effect on human endocrine organs.
TDE. Following the demonstration (discussed in section 7.1.8)
that TDE caused atrophy of a part of the adrenal cortex of dogs,
o,p'-TDE, and to a lesser degree m,p'-TDE, have been used in man,
under the name of mitotane, in the hope of controlling excessive
cortical secretion or of reducing the size of adrenal tumors. The
underlying condition may be hyperplasia or adrenocortical carcinoma.
The dosage given has varied from 7 to 285 (mg/kg)/day, but a dosage of
approximately 100 (mg/kg)/day for many weeks has been necessary to
produce any benefit in man (Bergenstal et al., 1960; Wallace et al.,
1961; Gallagher et al., 1962; Verdon et al., 1962; Bledsoe et al.,
1964; and Southern et al., 1966a,b).
The effects of idiopathic hyperplasia may be controlled; in fact a
state of adrenal insufficiency may be produced (Canlorbe et al., 1971;
Sizonenko et al., 1974).
o,p'-TDE may also give symptomatic relief of excessive
adrenocortical activity secondary to a tumour that produces ACTH
(Carey et al., 1973).
A favourable response was produced in about one-fourth to one-half
of patients with inoperable adrenocortical carcinoma (Canlorbe et al.,
1971; Hoffman & Mattox, 1972; Lubitz et al., 1973; Montgomery &
Struck, 1973). In fact, an occasional cure, involving complete
regression of metastases, was produced by chemotherapy including
o,p'-TDE (Perevodchikova et al., 1972; Schick, 1973). More commonly,
symptoms were relieved and life was prolonged by little more than 7-8
months (Canlorbe et al., 1971; Hoffman & Mattox, 1972; Lubitz et al.,
1973). The success of treatment was often indicated early on by a
reduction in steroid excretion (Hoffman & Mattox, 1972; Lubitz et al.,
1973).
The large dosage of o,p'-TDE necessary to produce clinical
benefit often produced general lassitude, anorexia, nausea, vomiting,
diarrhoea, and dermatitis (Naruse et al., 1970; Hoffman & Mattox,
1972; Nitshke & Link, 1972; Perevodcikova et al., 1972; Lubitz et al.,
1973). Apathy ranged from mild dulling of interest to profound
psychotic depression (Hoffman & Mattox, 1972). More rarely,
gynaecomastia, haematuria, leukopenia, and thrombocytopenia have been
reported (Luton et al., 1972; Perevodcikova et al., 1972). The
symptoms disappeared soon after administration of the drug ceased or
when the dosage was reduced (Perevodcikova et al., 1972).
Even large, therapeutic doses of o,p'-TDE did not cause
histological alterations in the adrenals in man (Wallace et al.,
1961). Furthermore, dosages in the therapeutic range (specifically
those between 110 and 140 (mg/kg)/day did not produce any detectable
injury to the liver, kidney, or bone marrow. All patients treated in
this way experienced significant anorexia and nausea, and some showed
central nervous system depression varying from lethargy to somnolence.
These toxic effects cleared when dosing was discontinued (Bergenstal
et al., 1960).
Kupfer (1967) reviewed extensive literature that indicated that
the effect in man and other species, except the dog, is caused by
stimulation of corticoid metabolism by massive doses of o,p'-TDE and
not by any direct effect on the adrenal. Southern et al. (1966a,b)
agreed that the effect was predominantly extra-adrenal in man, when
the drug was first given, but offered evidence that adrenal secretion
of cortisol was eventually reduced. However, even if therapeutic doses
eventually have a direct effect on the adrenal, doses encountered by
workers exposed to technical DDT do not (Clifford & Weil, 1972; Morgan
& Roan, 1973).
8.2.9 Carcinogenicity
Laws et al. (1967) did not find any case of cancer or blood
dyscrasia among the 35 heavily exposed workers in a DDT factory nor
did the medical records of 63 men who had worked there for more than 5
years reveal these diseases. Two men were employed who had a history
of successfully treated cancer before they came to work, but no
employee had contracted cancer during the 19 years that the plant had
been in operation; during this period, the work force varied from 111
to 135.
In the USA, the total death rates for cancer of the liver and its
biliary passages (classified individually as "primary", "secondary",
and "not stated whether primary or secondary") lead to the conclusion
that there has been a significant, almost constant decrease in the
total rate of liver cancer deaths from 8.8 in 1930 to 8.4 in 1944
(when DDT was introduced) to 5.6 in 1972. This almost constant decline
in total liver cancer death rates for the past 42 years offers no
evidence of any increase in liver cancer deaths since the introduction
of the first organochlorine pesticide into the environment. The
decrease in liver cancer deaths is even more significant in light of
the increasing life span of the general population in the USA, which
has resulted in an increased percentage of the population at risk from
cancer over these years. In spite of the limitations inherent in the
interpretation of such data, this record is a reminder that, more than
30 years after the introduction of DDT, there is no evidence,
whatsoever, that DDT is carcinogenic in man (Deichmann & MacDonald,
1976, 1977).
In the USA, the incidence of cancer is lower in rural counties
than in metropolitan areas in general (Mason et al., 1975).
It is sometimes implied that epidemiological evidence is useless
for revealing the carcinogenicity of a material for man unless it
involves large numbers of people who have been exposed to the material
for most or all of a lifetime. The fact is that some human carcinogens
have been detected through their occurrence in high incidence in small
groups for periods of much less than 25 years. What was commonly
considered the first recognition of chemical carcinogenesis in man
depended on the observations made by a single surgeon (Pott, 1775) in
a small fraction of his patients. Such was the intensity of the
exposure of the apprentices of chimney sweepers that cancer of the
scrotum often appeared at puberty. The editor responsible for
compiling the writings of Pott (1790) added a footnote indicating that
he had seen such a cancer in "an infant under eight years of age". It
must be understood that boys did not usually become apprentice chimney
sweepers before they were four-years-old. In connection with tumours
of the bladder mainly caused by ß-naphthylamine but to a lesser degree
by other aromatic amines, Hueper (1942) reviewed cases in which the
time from the first exposure to recognition of symptoms was 8-41,
9-28, and 2-35 years; in one series of 83 cases, 71% of the tumours
appeared from 1 to 15 years after exposure. The same author cited
reports (p. 104) of cases of malignant epitheliomas in persons exposed
to pitch for 18, 24, 24, and 36 months, respectively. Kleinfeld (1967)
reported 50-76% incidence of bladder cancer among several groups of
workers. He also noted a sharp drop in incidence of this condition
following decrease -- but not discontinuation -- of occupational
exposure to ß-naphthylamine.
8.2.10 Mutagenicity
Evidence regarding the mutagenic activity of DDT and its
significance in man is uncertain partly because the chromosomal
changes that are examined are sensitive to viral infections and
chemotherapy. The latter may not be recognized at the time of sampling
and may not have been shown to injure health through a mutagenic
mechanism.
Comparing samples collected in winter and during the peak season
of pesticide application, a slight increase in chromatid breaks was
reported in the cultured lymphocytes of workers exposed to a wide
variety of insecticides said to include DDT, although this was claimed
at a time when the use of DDT was banned. A somewhat larger increase
was reported for men exposed mainly to herbicides (Yoder et al.,
1973). In another study, lymphocytes cultured from workers with an
average DDT plasma level of 0.999 mg/litre showed significantly more
chromosomal and chromatid aberrations than cells cultured from
controls with an average plasma level of 0.275 mg/litre. The
difference was not significant in other comparisons in which the
average plasma levels were 1.030 versus 0.380 mg/litre and 0.240
versus 0.030 mg/litre, respectively (Rabello et al., 1975).
Examination of all of the data presented by the authors suggests a
simple dosage-effect relationship was present, with a detectable
effect starting somewhere between 0.2 and 0.4 mg/litre and increasing
at levels higher than 0.4 mg/litre.
8.3 Factors Influencing DDT Toxicity
There is no evidence that any factor except dosage is of practical
importance in determining DDT toxicity in man. Factors that have been
considered as possibly affecting asymptomatic storage of DDT include
age, sex, and race. Differences observed in connexion with these
factors are small, medically insignificant, and probably secondary to
dosage (Hayes, 1975).
Storage has also been reported to be greater in the tissues of
people with certain diseases (Deichmann & Radomski, 1968; Raclomski et
al., 1968; Vas'kovskaja, 1969; Dacre & Jennings, 1970; Jonczyk et al.,
1974). Again, the reported differences are small, and the highest
values for the samples in question are small compared with those found
in healthy workers (Hayes, 1975). Furthermore, a number of authors
have reported a similar range of storage in persons undergoing minor,
elective surgery and in those who have died from various causes (Hayes
et al., 1958; Dale et al., 1965; Robinson et al., 1965; Wassermann et
al., 1965). Some authors (Hunter et al., 1963; Robinson et al., 1965;
Hoffman et al., 1967; Hoffman, 1968; Morgan & Roan, 1970) have failed
to find any relationship between storage of insecticides and the cause
of death. Where a relationship was found, there was often the
possibility that the higher values were found in diseases that
involved some degree of wasting prior to death. Casarett et al. (1968)
found that higher values occurred in persons who had 3 characteristics
in common: emaciation, cancer, and widespread abnormality of the
liver.
A slightly greater storage of DDT and DDE that was reported in
persons who underwent splenectomy for hepatosplenic schistosomiasis
compared with those operated on for other conditions, mainly hernia
was statistically significant. No such difference was observed in
connexion with dieldrin, ß-HCH, or heptachlor epoxide (Wassermann et
al., 1975). Whereas it was speculated that the increased storage of
DDT and DDE might have been the result of a reduction in metabolism,
secondary to liver injury, the possibility of greater exposure as a
result of greater use of DDT in irrigated areas was not excluded.
8.4 Treatment of Poisoning in Man
No useful guidance regarding treatment has been obtained from the
very few cases of DDT poisoning that have occurred. Animal studies
indicate that sedatives, ionic calcium, and glucose or another ready
source of energy would be useful. On the basis of experience in
treating people poisoned by different convulsive poisons, it seems
likely that diazepam would be beneficial.
9. EVALUATION OF HEALTH RISKS TO MAN FROM EXPOSURE TO DDT AND RELATED
COMPOUNDS
9.1 Relative Contributions of Food, Water, Air, and Miscellaneous
Sources to Total Intake
9.1.1 Adult members of the general population
Food represents the major source of intake of DDT in the general
population. It has been estimated (section 5.1.5) that over 90% of the
DDT stored in the general population is derived from food. Around
1965, when the use of DDT was at its peak, intake in the USA was
approximately 0.04 mg/man per day from food, less than 0.000046 mg/man
per day from water, less than 0.00006 mg/man per day from urban air
and less than 0.0005 mg/man per day from the air in small agricultural
communities. The reason for the qualification "less than", is that the
intakes were calculated from the highest concentrations reported in
drinking-water and air.
Although total intake of DDT from food has not been measured in
some parts of the world, worldwide measurements of the storage of DDT
and its metabolites in human body fat indicate that the extremes of
total exposure have varied by a factor of about 10, but that total
exposure for most populations has varied by a factor of no more than 3
(see Table 9).
DDT in the dust in a house (as indicated either by a history of
extensive household application of insecticides or by the finding of
relatively high levels of DDT in house dust) can contribute to the
storage of DDT-related compounds in persons living in the house
(section 5.1.4). However, although the contribution of house dust to
DDT intake has been established, it is not quite clear how this
contribution occurs. Some of the dust may contaminate food in the
process of preparation and some may be inhaled and later swallowed
after deposition in the upper airway. It is difficult to believe that
enough DDT is present in such houses in the form of vapour or
respirable dust to cause a substantial increase in the total exposure
of the inhabitants, but no critical study has been made of this
matter. Clearly, some DDT will enter by the respiratory route.
9.1.2 Infants and children
At birth, infants tend to have slightly lower levels of DDT than
adults in the same population. This is because the placenta offers
partial protection against the passage of DDT and related compounds.
Although human milk tends to have a somewhat higher concentration
of DDT than cow's milk (see section 6.3.1.3), the difference, if any,
that this makes to the rate at which breast-fed and bottle-fed babies
store the compound has not been established. It is possible that the
conditional acceptable daily intake (ADI) might be exceeded in an
infant wholly fed on breast milk. However, the ADI is calculated on
the basis of lifetime exposure, and short-term variations can be
regarded as not having any significance.
The only really important way in which the exposure of infants and
children differs from that of adults in the same community involves
accidental exposure (see section 8.1.3).
9.1.3 Occupational groups
Occupational exposure (section 5.3) to DDT is initially almost
exclusively through the respiratory and dermal routes. However, the
particles of many insecticidal dusts, wettable powders, and sprays are
too large to reach the lower respiratory tract. As a result, most of
the particles inhaled are deposited in the upper respiratory tract,
carried to the pharynx by ciliary action, and eventually swallowed
(section 6.1.1).
Although dermal exposure to DDT is high under some occupational
situations, the effect is minimal because the compound is so poorly
absorbed through the skin (section 6.1.3). The excellent safety record
of DDT, never matched by any other insecticide used in antimalaria
campaigns, other vector control programmes, and agriculture, is based
mainly on its poor absorption through the skin.
The number of people with full-time occupational exposure to DDT
alone is small. For example, at the time of one study, the only
factory making the compound in the USA produced 2722 metric tonnes per
month using a work force of about 145. Following the recommendations
of an Expert Committee, the World Health Organization studied spraymen
who had applied only DDT for 5 years or more. Only 272 suitable
subjects could be located in Brazil and only 144 in India. The
concentrations of DDT and its derivatives in the blood of the
preliminary and main study groups in India were 0.761 and
1.272 mg/litre, respectively. The blood levels of spraymen in Brazil
were about 3 times those of the controls.
The absorbed dosage of the men who made and formulated DDT for 10
years or more was about 18 mg/man per day (see section 8.1.1). The
exposure of other workers, notably those applying DDT for agricultural
purposes has usually been an order of magnitude less (see Table 7).
9.2 Effects of Exposure
No adverse effects have been described at repeated dosages of
1.5 (mg/kg)/day or less (see section 7.4). The large number of
measurements that have been made on samples from human populations
have not made it possible to define a maximum dosage that man can
absorb without any adverse effect but have highlighted the finding
that the high levels found in volunteers and workers were harmless for
at least 25 years.
Table 23 summarizes the clinical aspects of DDT in man.
Table 23. Dosage-effects of DDT in man
Single dose
(mg/kg) Observation
Unknown Fatal
16-286 Prompt vomiting at higher doses
(all poisoned, convulsions in some)
6-10 Moderate poisoning
Repeated
exposure
(mg/kg)/day
1.5 Administered as therapy for 6 monthsa
0.5 Administered to volunteers for 21 monthsa
0.5 Exposure of workers for 6.5 yearsa
0.25 Exposure of workers for 25 yearsa
0.0025 Intake of population in the USA, 1953-54a
0.0002 Intake of population in the USA, 1969-70a
a Without any adverse effect.
In considering the safety of workers who are employed in the DDT
industry, it is useful to consider the results of animal experiments.
Rats withstand a daily dosage at least 10 times that of these workers
without any detectable clinical effect (section 7, Table 17), although
minimal reversible tissue changes may be present. Dogs and monkeys
also withstand a daily dosage 10 times higher than that of the
workers, but they do not show the tissue changes, which seem to be
peculiar to some rodents. Because workers have tolerated high dosages
of DDT for over a fifth of a lifetime without detectable harm and
since animals withstand larger dosages for an entire lifetime without
injury, there is good reason to predict the continuing safety of the
workers.
The experience already gained from workers can be used to predict
the future safety of the general population in relation to DDT. Many
workers have now been exposed to DDT for much more than one-fifth of
their life span. Since they have not suffered detectable harm, it
seems most unlikely that the general population will be harmed by
dosages 200-1250 times smaller than those to which the workers are
exposed. It has been shown for at least 2 animal species that toxicity
resulting from a lifetime of exposure is seldom very different from
toxicity resulting from 90 days of exposure at the same dosage rate.
The largest factor of difference observed when 33 chemicals were
investigated was 20, and, for half of them, the factor was 2 or less
(see section 7.3.1.1). Ninety days constitute about one-eighth of the
lifespan of a rat, and this is less than the fraction of the human
life span that has been studied so far.
9.3 Carcinogenicity and Mutagenicity
Liver tumours are produced in mice by many chlorinated compounds,
including DDT. There is also evidence to suggest that DDT metabolites
DDE and TDE (DDD) produce hepatic tumours in mice and that TDE also
produces lung tumours. Information on the tumorigenicity of DDT in
rats (see section 7.1.9) is conflicting; some studies report tumour
formation while other studies report negative data. Carcinogenicity
studies in the hamster were negative (see section 7.1.9). The
occurrence of tumours in some rodents only, casts doubt on the
significance of the phenomenon and on extrapolation of the findings to
man.
Studies on the incidence of all cancers reported in those parts of
some countries with known high agricultural use of DDT in the 1950s
and early 1960s have not demonstrated any trends in any type of cancer
associated with the use of DDT in relation to these areas (see section
8.2.9).
The question as to whether DDT is carcinogenic in man has not been
answered unequivocally. Although the cross-sectional epidemiological
studies on workers exposed to DDT and the observation studies on
volunteers are limited, there is not any currently available evidence
to suggest that DDT is tumorigenic or carcinogenic in man (see section
8.2.9).
Recent studies on in vitro bacterial test systems with or
without metabolic activation have not shown any evidence that either
DDT or DDE is mutagenic (see section 8.2.10). The evidence for the
mutagenicity of DDT in mammalian test systems is inconclusive.
9.4 Effects on Microsomal Enzymes
There is no doubt that exposure to DDT results in the induction of
microsomal mixed function oxidases and causes marked morphological
changes in the liver of some rodents (see section 7.1.9). In some
rodents, notably the mouse, these morphological changes have been
related to tumorigenicity. Microsomal enzymes are also induced by DDT
in other species, but the liver does not show the same morphological
changes.
The only effect for which something approaching a threshold has
been demonstrated is the induction of microsomal enzymes in workers in
association with an average serum value of 0.573 mg/litre for p,p'-
DDT but not in workers with serum levels as high as 0.052 mg/litre, a
value essentially identical to the highest reported for the general
population. Furthermore, although some groups of workers experienced
an increase in their average enzyme activity, no person exceeded the
range of activity found in normal people in the general population.
DDT will not induce liver microsomal enzymes in the general
population because their intake of the compound is so much less than
the smallest dosage capable of producing this effect in animals or man
(see sections 7.1.4.2 and 8.2.5.1).
9.5 Reproduction and Teratogenicity
Effects on reproduction in mammals have been studied in the mouse,
the rat, and the dog (see section 7.1.7). In the mouse, a multi-
generation study at dietary levels of DDT of 25, 100, and 250 mg/kg
showed effects on fertility and reproduction only at the highest
level, equivalent to 33 (mg/kg)/day. In the rat, normal reproduction
was maintained at a dietary level of 200 mg/kg. In the dog, dietary
intake at dosages up to 10 (mg/kg)/day did not produce any effects on
reproduction other than earlier estrus in the DDT-treated females.
In man, there is no indication that DDT affects reproduction (see
section 8.2.7); no impairment of fertility was observed in a study of
men occupationally-exposed for more than 10 years to a measured
average daily intake in the region of 18 mg/man per day (equivalent to
0.25 (mg/kg)/day).
Studies in the mouse, the rat, and the dog have not shown any
evidence of teratogenicity. In the mouse, dosage at the rate of
1 mg/kg was not teratogenic; a single dosage of 25 mg/kg or repeated
doses at the rate of 2.5 mg/kg were embrytoxic but not teratogenic.
9.6 Immunosuppression
DDT appears to have a depressant effect on the immune system
although the evidence is by no means conclusive. Rats and rabbits
receiving DDT in aqueous suspension at a concentration of 200 mg/litre
showed a depression in antibody formation and decrease in at least one
globulin fraction of the blood. Rats receiving a dosage of
0.25 (mg/kg)/day by gavage did not show any changes in the phagocytic
activity of the white blood cells. In the guineapig, dosages of
1-20 mg/kg did not have any effects on antitoxin production but
produced a reduction in tissue histamine levels (see section 7.1.1).
9.7 Nutritional Effects and Other Factors
Animal studies indicate that nutritional status influences the
toxicity of DDT (see section 7.3.3). The preferential storage of DDT
in fat can mitigate the effect of acute poisoning. If rats that have
stored large amounts of DDT are starved, they may suffer toxic effects
due to mobilization of fat and DDT.
In man, nutritional status will have a similar effect to that
found in other animals. However, the possibility that starvation in
man could precipitate toxic manifestations is regarded as unlikely as
the stored levels do not approach those found in laboratory animals
and the lower metabolic rate of man results in slower mobilization. In
fact, severe weight loss sometimes does cause some increase in storage
of DDT in connexion with certain wasting diseases; however, people
with full-time occupational exposure to DDT average 10 times more
storage than the highest values reported in connection with disease
but do not exhibit predisposition to the diseases in question (see
section 6.2.1.3).
Although young animals are often more susceptible to toxic
chemicals than adults, there is no evidence that DDT is more toxic to
young animals of any species including man. In fact, in the rat, the
young are less susceptible to a single dose than the adults (see
section 7.3.2, Table 20).
9.8 Dosage-Effect Relationships
Dosage-effect relationships for DDT in man have been observed in
connection with acute poisoning (see Table 23), excretion, and storage
(see Fig. 4), and the induction of microsomal enzymes, which has been
observed at a dosage of 0.25 (mg/kg)/day but not at lower dosages. The
dosage of 0.25 (mg/kg)/day to which workers have been exposed for 25
years is of the same order of magnitude that causes an increase in
tumours in male mice of a susceptible strain but not in females of any
strain (see section 7.1.9). As shown in Table 24, this dosage in
workers is less than the no-effect levels for rats, dogs, and monkeys
and far less than the dosage at which rats, mice, and dogs
successfully reproduce for generations. The equilibrium levels of DDT
and its metabolites found in the blood and fat of people with
full-time occupational exposure and the much lower levels found in the
general population have not been associated with any adverse effects.
9.9 Recommendations on Levels of Exposure
The data from intake, exposure, and levels in populations supports
the current conditional ADI for DDT, which affords a considerable
margin of safety.
If the total intake of DDT from food and other sources rises above
0.005 (mg/kg)/day (the conditional ADI) then the situation should be
investigated.
Table 24. Dosage-effect of DDT in animals
Single Route Observation
(mg/kg)
3000 dermal LD50 of powder in adult rat
2356-4000 oral LD50 of oil solution in newborn rat
250-500 dermal LD50 of oil solution in adult rat
250 oral LD50 of oil solution in adult rat
Repeated
Exposure
(mg/kg)/day
300 subcutaneous inhibition of testicular growth in cockerels
41-80 oral increased mortality in rats, 2-year study
41-80 oral 100% mortality in dogs in 39-49 months
41-80 oral 100% mortality in monkeys, 70 days
21-40 oral 25% mortality in dogs in 39-49 months
33.2 oral harmful to reproduction in mice
13.3 oral slight reduction in lactation and survival of some but not all
generations of mice (6 generation test)
10 oral no harmful effect on reproduction in dog (3 generation test)
10 oral no harmful effect on reproduction in rat (2 generation test)
5-10 oral no-effect level in dog, 2 generations
2.6-5 oral no-effect level in monkey, 7.5 years
0.63-1.25 oral no-effect level in rat, 2 year test
0.16-0.31 oral risk of liver tumours doubled in male mice but no effect in
female
0.3 oral no-effect level for induction of microsomal enzymes in rats
The concentration of DDT in the air in industrial, agricultural,
or disease control areas should not exceed 1 mg/m3 on a time-
weighted basis (40 h per week). Several countries have their own
standards that range from 0.1 to 1 mg/m3, which seem to afford an
acceptable margin of safety.
There is ample reason to predict the continuing safety of workers
producing and using DDT. No harmful effect has ever been reported in
vector control operators who have applied DDT during the last 3
decades in public health programmes. Nevertheless, as in the case of
any chemical, occupational safety and health measures should always be
applied to ensure that contact with DDT by workers is kept to a
minimum.
The only index of exposure of DDT or its metabolites is the
analysis of these compounds in tissues or excreta. For most purposes
it is best to sample serum or plasma. In subjects with relatively
constant, prolonged exposure, concentrations of DDT and its
metabolites in the blood are in equilibrium with those in all other
tissues, including adipose tissue. In subjects who have accidentally
received a single large dose, the concentration in the brain is
reflected more accurately by a serum sample than by a fat sample.
REFERENCES
ABBOTT, D.C., HARRISON, R. B., TATTON, J. O'G., & THOMPSON, J. (1965)
Organochlorine pesticides in the atmospheric environment. Nature
(Lond.), 208: 1317-1318.
ABBOTT, D.C., GOULDING, R., & TATTON, J. O'G. (1968) Organochlorine
pesticide residues in human fat in Great Britain, Br. med. J.,
3: 146-149.
ABBOTT, D.C., HOLMES, D.C., & TATTON, J. O'G. (1969) Pesticide
residues in the total diet in England and Wales, 1966-67. J. Sci.
Food Agric., 20: 245-249.
ABBOTT, D. C, COLLINS, G. B., & GOULDING, R. (1972) Organochlorine
pesticide residues in human fat in the United Kingdom, 1969-71.
Br. med. J., 2: 553-556.
ABE, J., INOUE, Y., & TAKAMATSU, M. (1974) [On the residual amounts of
PCB and organochlorine pesticides in human blood.] Jpn J. Hyg.,
29: 93 (in Japanese).
ACKER, L. & SCHULTE, E. (1970) [The occurrence of polychlorinated
biphenyls and hexachlorobenzene along chlorinated insecticides in
human milk and human adipose tissue.] Naturwissenschaften,
57: 497 (in German).
ACKER, L. & SCHULTE, E. (1971) [Organochlorine compounds in the human
body.] Umschau, 71: 848 (in German).
ACKER, L. & SCHULTE, E. (1974) [Organochlorine compounds in human
fat.] Naturwissenschaften, 61: 32 (in German).
ADAMCZYK, E. (1971) [Translocation of DDT in chickens under the
influence of hunger.] Med. Weter, 27: 103-105 (in Polish).
ADAMOVIC, V., SOKIC, B., & PETROVIC, O. (1971) [On factors influencing
the occurrence of organochlorine insecticides in newborns.]
Ernaehrungsforschung, 16: 579-585 (in German).
ADAMS, M., COON, F. B., & POLING, C. E. (1974) Insecticides in the
tissues of four generations of rats fed different dietary fats
containing a mixture of chlorinated hydrocarbon insecticides.
J. agric. food Chem. 22: 69-75.
AHLING, B. & JENSEN, S. (1970) Reversed liquid-liquid partition in
determination of PCBs and chlorinated hydrocarbon insecticides in
water. Anal. Chem, 42: 1485-1486.
AHNHOFF, M. & JOSEFSON, B. (1974) Simple apparatus for on-site
continuous liquid-liquid extraction of organic compounds from
natural waters. Anal. Chem., 46: 658-663.
AIZICOVICI et al., Travaux Scientifiques de l'Institut d'Hygiene de
Jassy, 1960-1966, p. 142 (Quoted by: Wasserman, M., Sofoluwe,
G. O., Wasserman, D., Groner, Y., & Lazarovitch, S. (1968) Storage
of organochlorine insecticides in body fat of people in Nigeria.
In: Proceedings of the Lagos International Seminar on
Occupational Health for Developing Countries, Nigeria, 1-6 April
1968.
ALARY, J. G., GUAY, P., & BRODEUR, J. (1971) Effect of phenobarbital
on the metabolism of DDT in the rat and in the bovine. Toxicol.
appl. Pharmacol., 18: 457-468.
ALMEIDA, W. F., PIGATI, P., GAETA, R., & UNGARO, M. T. S. (1975) DDT
residues in human blood serum in Brazil. Environ. Qual. Saf.,
3 (Suppl.): 586-588.
ANON. (1972) [BHC and DDT residues in human milk on the decline in
Japan.] Noyaku Bijin, 56: 458 (in Japanese).
ANON. (1974) [Research methods for determination of chlorinated
hydrocarbon pesticide residues in or on food of animal origin.]
Bundesgesundheitsblatt, 17 (18): 269-276 (in German).
ANTOMMARIA, P., CORN, M., & DeMAIO, L. (1965) Airborne particulates in
Pittsburgh: Association with p,p'-DDT. Science, 150: 1476-1477.
ARMOUR, J. A. & BURKE, J. A. (1970) Separating polychlorinated
biphenyls from DDT and its analogues. J. Assoc. Off. Anal. Chem.,
53: 761-768.
ASAI, R. I., GUNTHER, F. A., WESTLAKE, W. E., & IWATA, Y. (1971)
Differentiation of PCBs from DDT by carbon skeleton chromatography.
J. agric. food. Chem., 19: 39-398.
ASKARI, E. M. & GABLIKS, J. (1973) DDT and immunological responses.
II. Altered histamine levels and anaphylactic shock in guineapigs.
Arch. environ. Health, 26: 309-312.
AUE, W. A. & TELI, P. M. (1971) Sampling air pollutants with support-
bonded chromatographic phases. J. Chromatogr., 62: 15-27.
BAGLEY, G. E., REICHEL, W. L., & CROMARTIE, E. (1970) Identification
of PCBs in two bald eagles by combined gas-liquid chromatography-
mass spectrometry. J. Assoc. Off. Anal. Chem., 53: 251-261.
BAKSEYEV, N. S. (1973) [Prophylaxis of pathology of the fetus and
newborn child (experience of the Ukrainian SSR).) Vestn. Akad.
Med. Nauk (SSSR), 28: 69-75 (in Russian).
BARKA, T. & POPPER, H. (1967) Liver enlargement and drug toxicity.
Medicine, 46: 103-117.
BELONOZKO, G. A. & KUCAK, JU. A. (1974) [Hygienic features of the
content of pesticides in the atmosphere.] In: Medved, L. E., ed.
Hygiene of application and toxicology of pesticides and the
clinical features of pesticides poisoning. A collection of
papers, Issue No. 5, pp. 63-74 Kiev. Vniigintox (in Russian).
BELONOZKO, G. A., VEVCHURKO, S. N., KUCHAK, U. A., TOVSTENKO, A. E., &
SOROKENA, L. V. (1967) [The spread of pesticides by air currents
in the environment in relation to method of application.] In:
Medved, L. E., ed. Hygiene and toxicology of pesticides and the
clinical features of pesticide poisoning. A collection of papers,
pp. 24-35 Kiev, Vniigintox (in Russian).
BENVENUE, A. & OGATA, J. N. (1970) Note on the use of florisil
adsorbent for separation of PCBs from chlorinated pesticides.
J. Chromatogr., 63: 142-144.
BERGENSTAL, D. M., HERTZ, R., LIPSETT, M. B., & MOY, R. H. (1960)
Chemotherapy of adrenocortical cancer with o,p'-DDD. Ann.
intern. Med., 53: 672-682.
BEYERMANN, K. & ECKRICH, W. (1973) [Adsorption of traces of
insecticides from water on polyethylene.] Z. anal Chem.,
265 (1): 1-4 (in German).
BEYERMANN, K. & ECKRICH, W. (1974) [Differentiation of the aerosol-
bound and gaseous fractions of insecticides in air.] Z. anal.
Chem., 269 (4): 279-284 (in German).
BEZUGLYI, V. P. & KASKEVICH, L. M. (1969) [Some indices of the
functional state of the liver in flying personnel engaged in
aerial chemical operations.] Gig. Tr. Prof. Zabol., 13 (10):
52-53 (in Russian).
BEZUGLYI, V. P., ODINTSOVA, I. L. R., & GORSKAYA, N. Z. (1973)
[Morphological composition of the blood in persons working with a
complex of organochlorine and organophosphorus pesticides.]
Vrach. Delox, 11: 150-154 (in Russian).
BICK, M. (1967) Chlorinated hydrocarbon residues in human body fat.
Med. J. Aust., 1: 1127-1129.
BIROS, F. J. (1970) Analysis for p,p'-DDT and p,p'-DDE pesticide
residues by NMR spectroscopy. J. Assoc. Off. Anal Chem.,
53: 733-736.
BISHARA, R. H., BORN, G. S., & CHRISTIAN, J. E. (1972a) Thin-layer
chromatography of DDT and some related compounds on aluminium
oxide chromatoplates. J. Chromatography, 64: 135-145.
BISHARA, R. H., BORN, G. S., & CHRISTIAN, J. E. (1972b) Radiotracer
distribution and excretion study of chlorophenothane in rats.
J. pharm. Sci, 61: 1912-1916.
BISKIND, M. S. (1952) [The new insecticides and the public health.]
Harefuah, 44: 9-13 (in Hebrew).
BISKIND, M. S. (1953) Public health aspects of the new insecticides.
Am. J. dig. Dis., 20: 331-341.
BISKIND, M. S. & BIEBER, I. (1949) DDT poisoning -- A new syndrome
with neuropsychiatric manifestations. Am. J. Psychother.,
3: 261-270.
BITMAN, J., CECIL, H. C., & FRIES, G. F. (1970) DDT-induced inhibition
of avian shell gland carbonic anhydrase. A mechanism for thin
eggshells. Science, 168: 594-596.
BJERK, J. E. (1972) [DDT and polychlorinated biphenyl residues in
human material from Norway.] Tidsskr, Nor. Laegeforen.,
92: 15-19, 37-38 (in Norwegian).
BLEDSOE, T., ROLAND, D. P., HEY, R. L., & LIDDLE, G. W. (1964) An
effect of o,p'-DDD on extra-adrenal metabolism of cortisol in
man. J. clin. Endocrinol. Metab., 24: 1303-1311.
BOIKO, V. G. & KRASNYUK, E. P. (1969) [Clinical characteristics of the
pathology of respiratory tracts of persons working with DDT.]
Z. ushn. nos. gorl. Bolezn., 29: 35-38 (in Russian).
BOJANOWSKA, A., JONCZYK, W., TRACZYK, Z., & KLAWE, Z. (1973) [DDT and
alpha-BHC content of blood and fatty tissue of subjects not
professionally exposed to these compounds.] Pol. Tyg. Lek.,
28: 1999-2001 (in Polish).
BOYD, E. M. & DECASTRO, E. S. (1968) Protein-deficient diet and DDT
toxicity. Bull. World Health Organ., 38: 141-150.
BOYD, E. M. & DECASTRO, E. S. (1970) Toxicity of dicophane (DDT) in
relation to dietary protein intake. Ind. Med., Surg.,
39: 229-236.
BOYD, E. M. & KRIJNEN, C. J. (1969) Dietary protein and DDT toxicity.
Bull. environ. Contam. Toxicol., 4: 256-261.
BRADY, M. N. & SIYALl, D. S. (1972) Hexachlorobenzene in human fat.
Med. J. Aust., 1: 158-161.
BRATOWSKI, T. A. & MATSUMURA, F. (1972) Properties of a brain
adenosine triphosphatase sensitive to DDT. J. econ. Entomol.,
65: 1238-1245.
BREWERTON, H. V. & MCGRATH, H. J. W. (1967) Insecticides in human fat
in New Zealand. N.Z.J. Sci., 10: 486-492.
BRODEUR, J. & LAMBERT, G. (1973) Further studies on the effect of
starvation and microsomal enzyme induction on the mobilization of
DDT residues in rats. Toxicol. appl. Pharmacol., 25: 488.
BRONISZ, H. & OCHYNSKI, J. (1968) [DDT and DDE content in the milk of
women.] Biul. Inst. Ochr. Rosl., 41: 99-102 (in Polish).
BRONISZ, H., RUSIECKI, W., OCHYNSKI, J., & BERNARD, E. (1967) [DDT in
adipose tissue of Polish population.] Diss. Pharm. Pharmacol.,
19: 309-314 (in Polish).
BRONISZ, H., OCHYSNSKI, J., & WLODZIMIERA, L. (1969) [Contents of DDT
in the adipose tissue of the Polish population.] Ann. Univ.
Mariae Curies -- Sklodwska. sect. D. (Med.), 24: 93-99 (in
Polish).
BROWN, J. R. (1967) Organochlorine pesticide residues in human depot
fat. Can. Med. Assoc, J., 97: 367-373.
BROWN, J. R. & CHOW, L. Y. (1975) Comparative study of DDT and its
derivatives in human blood samples in Norfolk County and Holland
Marsh, Ontario. Bull. environ. Contam. Toxicol., 13: 483-488.
BUNYAN, P. J., TOWNSHEND, M. G., & TAYLOR, A. (1972) Pesticide induced
changes in hepatic microsomal enzyme systems. Some effects of 1,1-
di( p-chlorophenyl)-2,2,2-trichloroethane (DDT) and 1,1-di( p-
chlorophenyl)-2,2-dichloroethylene (DDE) in the rat and Japanese
quail. Chem. Biol. Interactions, 5: 13-26.
BURLINGTON, H. & LINDERMAN, V. F. (1950) Effect of DDT on testes and
secondary sex characteristics of White Leghorn cockerels. Proc.
Soc. Exp. Biol., 74: 48-51.
BURNS, E. C., DAHM, P. A., & LINDQUIST, D. A. (1957) Secretion of DDT
metabolites in the bile of rats. J. Pharmacol. exp. Ther.,
121: 55-62.
BURNS, J. E. (1974) Organochlorine pesticide and polychlorinated
biphenyl residues in biopsied human adipose tissue -- Texas
1969-1972. Pestic. Monit. J., 7: 122-126.
BUTLER, P. A. (1969) Monitoring pesticide pollution. Biol. Sci.,
19: 889-891.
CAHILL, W. P., ESTESEN, B. J., & WARE, G. W. (1970) Determination of
DDT in presence of Toxaphene (Camphechlor) residues. Bull,
environ. Contam. Toxicol., 5: 260-262.
CAMERON, G. R. & BURGESS, F. (1945) The toxicity of 2,2-bis-( p-
chlorophenyl)-1,1,1-trichloroethane (DDT). Br. med. J.,
1: 865-871.
CAMPBELL, J. E., RICHARDSON, L. A., & SCHAFER, M. L. (1965)
Insecticide residues in the human diet. Arch. environ. Health,
10: 831-836.
CANLORBE, P., BADER, J. C., & JOB, J. C. (1971) Diagnostic problems
arising from a virilizing tumour of the adrenal cortex. (Case
report -- Literature review.) Sem. Hop., 47: 2255-2270.
CAREY, R. M., ORTH, D. N., & HARTMANN, W. H. (1973) Malignant melanoma
with ectopic production of adrenocorticotrophic hormone:
palliative treatment with inhibitors of adrenal steroid
biosynthesis. J. clin. Endocrinol. Metab., 36: 482-487.
CAROLLO, J. A. (1945) The removal of DDT from water supplies. J. Am.
Water Works Assoc., 37: 1310-1317.
CARR, R. L. (1970) Collaborative study of the Mills method of multiple
chlorinated pesticide residues in butter fat. J. Assoc. Off. Anal
Chem., 53: 152-154.
CARR, R. L. (1971) Collaborative study of a method of multiple
chlorinated pesticide residues in fish. J. Assoc. Off. Anal.
Chem., 54: 525-527.
CASARETT, L. J., FRYER, G. C., YAUGER, W. L., & KLEMMER, H. W. (1968)
Organochlorine pesticide residues in human tissues-Hawaii. Arch.
environ. Health, 17: 306-311.
CASE, R. A. M. (1945) Toxic effects of 2,2-bis(p-chlorophenyl)-1,1,1-
trichlorethane (DDT) in man. Br. med. J., 2: 842-845.
CASSIDY, W., FISHER, A. J., PEDEN, J. D., & PARRY-JONES, A. (1967)
Organochlorine pesticide residues in human fats from Somerset.
Mon. Bull. Minist. Health Lab. Serv. (Lond.), 26 (1): 2-6.
CHADWICK, R. W., CRANMER, M. F., & PEOPLES, A. J. (1971a) Metabolic
alterations in the squirrel monkey induced by DDT administration
and ascorbic acid deficiency. Toxicol. appl. Pharmacol.,
20: 308-318.
CHADWICK, R. W., CRANMER, M. F., & PEOPLES, A. J. (1971b) Comparative
stimulation of gamma-HCH metabolism by pre-treatment of rats with
gamma-HCH, DDT, and DDT + gamma-YCH. Toxicol. appl. Pharmacol.,
18: 685-695.
CHADWICK, R. W, LINKO, R. S., FREAL, J. J., & ROBBINS, A. L. (1975)
The effect of age and long-term low-level DDT exposure on the
response to enzyme induction in the rat. Toxicol. appl.
Pharmacol., 31: 469-480.
CHHABRA, R. S. & FOUTS, J. R. (1974) Stimulation of hepatic drug
metabolizing enzymes by DDT, polycyclic hydrocarbons or
phenobarbital in adrenalectomized or castrated mice. Toxicol.
appl. Pharmacol., 28: 465-476.
CHEN, JO-YUN, T. & DORITY, R. W. (1972) Infrared studies of DDT,
structurally related halogenated pesticides, and some metabolites.
J. Assoc. Off. Anal Chem., 55: 15-31.
CHIBA, M. (1969) Factors affecting the extraction of organochlorine
insecticides from soil. Residue Rev., 30: 63-113.
CHIBA, M. & MORLEY, H. V. (1968) Losses of pesticides during sample
preparation. J. Assoc. Off. Anal Chem., 51: 55-62.
CHIN, Y. & T'ANT, C. (1946) The effect of DDT on cutaneous sensation
in man. Science, 103: 654.
CIELESZKY, V., DENES, A., KNOLL, R., ENGST, R., & KOTARSKY, A. (1970)
[Methods for performing residue analysis of plant protection and
pest control agents in foodstuffs. II. Method for identifying and
the semiquantitative determination of residues of chlorinated
hydrocarbons in different food and in soil.] Nahrung,
14: 643-666 (in German).
CIUPE, R. (1976) [Determination of HCH, p,p'-DDT, p,p'-DDE in foods
and in the adipose tissue.] Igiena, 25: 113-117 (in Rumanian).
CLEMMESEN, J., FUGELSANG-FREDERICKSEN, V., & PLUM, C. M. (1974) Are
anticonvulsants oncogenic? Lancet, 1: 705-707.
CLIFFORD, N. J. & WEIL, J. (1972) Cortisol metabolism in persons
occupationally exposed to DDT. Arch. environ. Health,
24: 145-147.
COCHRANE, W. P. & CHAU, A. S. Y. (1971) Chemical derivitization
techniques for confirmation of organochlorine residue identity.
Pesticide identification at the residue level. Adv. Chem. Ser.,
140: 11-26.
COHA, F. & NEDIC, M. (1970) Improved gas-liquid chromatographic method
for analysis of DDT and its metabolites in milk. Anal. Lett.,
3: 419-421.
COHEN, J. M. & PINKERTON, C. (1966) Widespread translocation of
pesticides by air transport and rainout. Adv. Chem. Ser.,
60: 163-176.
COMMITTEE ON PESTICIDES (1951) Pharmacologic and toxicologic aspects
of DDT (chlorophenothane U.S.P.). J. Am. Med. Assoc.,
145: 728-733.
CONNEY, A. H. (1967) Pharmacological implications of microsomal enzyme
induction. Pharmacol. Rev., 19: 317-366.
CONOVER, W. J. (1971) Practical non-parametric statistics, New York,
Wiley & Sons, 462 pp.
COPELAND, F. M. & CRANMER, M. F. (1974) Effects of o,p'-DDT on the
adrenal gland and hepatic microsomal enzyme system of the beagle
dog. Toxicol. appl. Pharmacol., 27: 1-10.
COPPLESTONE, J. F., HUNNEGO, J. N., & HARRISON, D. L. (1973)
Insecticides in adult New Zealanders -- A five year study. N.Z.J.
Sci., 16: 27-39.
CRANMER, M. F. (1970) Effect of diphenylhydantoin on storage in the
rat. Toxicol. appl. Pharmacol., 17: 315.
CRANMER, M. F. (1972) Absence of conversion of o,p'-DDT to p,p'-DDT
in the rat. Bull. environ. Contam. Toxicol., 7: 121-124.
CRANMER, M. F. & COPELAND, M. F. (1973) Electron-capture gas-liquid
chromatographic analysis of DDA: utilization of 2-chloroethanol
derivative. Bull. environ. Contam. Toxicol., 9: 186-192.
CRANMER, M. F., CARROLL, J. J., & COPELAND, M. F. (1969) Determination
of DDT and metabolites, including DDA, in human urine by gas
chromatography. Bull. environ. Contam. Toxicol., 4: 214-223.
CRANMER, M. F., PEOPLES, A., & CHADWICK, R. (1972) Biochemical effects
of repeated administration of p,p'-DDT on the squirrel monkey.
Toxicol. appl. Pharmacol., 21: 98-101.
CROSBY, D. G. & MOILANEN, K. W. (1977). Vapor-phase photodecomposition
of DDT. Chemosphere, No. 4, pp 167-172.
CUETO, C., JR (1970) Cardiovascular effects of o,p'-DDD. Ind. Med.
Surg., 39: 31-32.
CUETO, C., JR & BIROS, F. J. (1967) Chlorinated insecticides and
related materials in human urine. Toxicol. appl. Pharmacol.,
10: 261-269.
CUETO, C., JR & BROWN, J. H. U. (1958) Biological studies on an
adrenocorticolytic agent and the isolation of the active
components. Endocrinology, 62: 334-339.
CUNNINGHAM, R. E. & HILL, F. S. (1952) Convulsions and deafness
following ingestion of DDT. Pediatrics, 9: 745-747.
CURLEY, A. & KIMBROUGH, R. (1969) Chlorinated hydrocarbon insecticides
in plasma and milk of pregnant and lactating women. Arch.
environ. Health, 18: 156-164.
CURLEY, A., COPELAND, M. F., & KIMBROUGH, R. D. (1969) Chlorinated
hydrocarbon insecticides in organs of stillborn and blood of
newborn babies. Arch. environ. Health, 19: 628-632.
CURLEY, A., BURSE, W. W., JENNINGS, R. W., & VILLANUEVA, E. C. (1973)
Chlorinated hydrocarbon pesticides and related compounds in
adipose tissue from people of Japan. Nature (Lond.),
242: 338-340.
CURRIE, L. A. (1968) Limits for qualitative detection and quantitative
determination. Anal. Chem., 40: 586-593.
CZEGLEDI-JANKO, G. (1969) [Residues of chlorinated hydrocarbons in the
blood of inhabitants of Budapest in 1967-1968 with particular
reference to lindane.] Z. Ges. Hyg. Ihre Grenzgeb., 15: 755-761
(in German).
CZEGLEDI-JANKO, G. & AVAR, P. (1970) Occupational exposure to lindane:
Clinical and laboratory findings. Br. J. ind. Med., 27: 283-286.
CZEGLEDI-JANKO, G. & CIELESZKY, V. (1968) A one-step extraction and
clean-up procedure for gas-liquid chromatographic determination of
some organochlorine pesticide residues in blood. Analyst,
93: 445-452.
DACRE, J. C. & JENNINGS R. W. (1970) Organochlorine insecticides in
normal and carcinogenic human lung tissues. Toxicol. appl.
Pharmacol., 17: 277.
DALE, W. E. & QUINBY, G. E. (1963) Chlorinated insecticides in the
body fat of people in the United States. Science, 142: 593-595.
DALE, W. E., GAINES, T. B., & HAYES, W. J., JR (1962) Storage and
excretion of DDT in starved rats. Toxicol. appl. Pharmacol.,
4: 89-106.
DALE, W. E., GAINES, T. B., HAYES, W. J., JR, & PEARCE, G. W. (1963)
Poisoning by DDT: Relation between clinical signs and
concentration in rat brain. Science, 142: 1474-1476.
DALE, W. E., COPELAND, M. F., & HAYES, W. J., JR (1965) Chlorinated
insecticides in the body fat of people in India. Bull. World
Health Organ., 33: 471-477.
DALE, W. E., COPELAND, M. F., PEARCE, G. W., & MILES, J. W. (1966a)
Concentration of o,p'-DDT in rat brain at various intervals
after dosing. Arch. int. Pharmacodyn. Ther., 162: 40-43.
DALE, W. E., CURLEY, A., & CUETO, C., JR (1966b) Hexane extractable
chlorinated pesticides in human blood. Life Sci., 5: 47-54.
DALE, W. E., CURLEY, A., & HAYES, W. J., JR (1967) Determination of
chlorinated insecticides in human blood. Ind. Med. Surg.,
36: 275-280.
DALE, W. E., MILES, J. W, & GAINES, T. B. (1970) Quantitative method
for determination of DDT and DDT metabolites in blood serum.
J. Assoc. Off. Anal. Chem., 53: 1287-1292.
DAL NOGARE, S. & JUVET, R. S. (1962) Gas-liquid chromatography, New
York, Interscience, 450 pp.
DAMASKIN, V. I. (1965) [The extent of the accumulation of DDT in the
human body in connection with its assimilation with food, and its
toxic effect.] Gig. i Sanit., 30 (10): 109-111 (in Russian).
DANGERFIELD, W. G. (1946) Toxicity of DDT to man. Br. med. J.,
1: 27.
DATTA, P. R. (1970) In vitro detoxification of p,p'-DDT via p,p'-
DDE to p,p'-DDA in rats. Ind. Med. Surg., 39: 190-194.
DATTA, P. R. & NELSON, M. J. (1968) Enhanced metabolism of
methyprylon, meprobamate, and chlordiazepoxide hydrochloride after
chronic feeding of a low dietary level of DDT to male and female
rats. Toxicol. appl. Pharmacol., 13: 346-352.
DATTA, P. R. & NELSON, M. F. (1970) p,p'-DDT detoxication by
isolated perfused rat liver and kidney. Ind. Med. Surg,
39: 195-198.
DAVIES, J. E., WELKE, J. O., & RADOMSKI, J. L. (1965) Epidemiological
aspects of the use of pesticides in the South. J. occup. Med.,
7: 612-618.
DAVIES, J. E., EDMUNDSON, W. F., SCHNEIDER, N. J., & CASSADY, J. C.
(1968) Problems of prevalence of pesticide residues in humans.
Pestic. Monit. J., 2: 80-85.
DAVIES, J. E., EDMUNDSON, W. R., CARTER, C. H., & BARQUET, A. (1969a)
Effect of anticonvulsant drugs on dicophane (DDT) residues in man.
Lancet, 2: 7-9.
DAVIES, J. E., EDMUNDSON, W. F., MACEO, A., BARQUET, A., & CASSADY, J.
(1969b) An epidemiologic application of the study of DDE levels in
whole blood. Am. J. Public Health, 59: 435-441.
DAVIES, J. E., EDMUNDSON, W. F., MACEO, A., IRVIN, G. L., III,
CASSADY, J., & BARQUET, A. (1971) Reduction of pesticide residues
in human adipose tissue with diphenylhydantoin. Food Cosmet.
Toxicol., 9: 413-423.
DAVIES, J. E., EDMUNDSON, W. F., & RAFEONELLI, A. (1975) The role of
house dust in human DDT pollution. Am. J. Public Health,
65: 53-57.
DAVIES, O. L. & GOLDSMITH, P. L. (1976) Statistical methods in
research and production, 4th ed., London, Longmans, 478 pp.
DEDEK, W. & SCHMIDT, R. (1972) Studies on the transplacental transport
and metabolism of 3H- and 14C-labeled DDT in pregnant mice
under hunger stress. Pharmazie, 27: 294-297.
DE FAUBERT MAUNDER, M. J., EGAN, H., GODLY, E. W., HAMMOND, E. W.,
ROBUM, J., & THOMSON, J. (1964) Clean-up of animal fats and dairy
products for the analysis of chlorinated pesticide residues.
Analyst, 89: 168-174.
DEICHMANN, W. B. & MACDONALD, W. E. (1976) Liver cancer deaths in the
continental USA from 1930 to 1972. Am. Ind. Hyg. Assoc. J.,
37: 495-498.
DEICHMANN, W. B. & MACDONALD, W. E. (1977) Organochlorine pesticides
and liver cancer deaths in the United States, 1930-1972.
Ecotoxicol. environ. Saf. 1: 89-110.
DEICHMANN, W. B. & RADOMSKI, J. L. (1968) Retention of pesticides in
human adipose tissue -- Preliminary report. Ind. Med. Surg.,
37: 218-219.
DEICHMANN, W. B., WITHERUP, S., & KITZMILLER, K. V. (1950) The
toxicity of DDT, Cincinnati, Kettering Laboratory of Applied
Physiology, 233 pp.
DEICHMANN, W. B., MACDONALD, W. E., & CUBIT, D. A. (1971a) DDT tissue
retention: Sudden rise induced by the addition of aldrin to a
fixed DDT intake. Science, 172: 275-276.
DEICHMANN, W. B., MACDONALD, W. E., BEASLEY, A. G., & CUBIT, D.
(1971b) Subnormal reproduction in beagle dogs induced by DDT and
aldrin. Ind. Med. Surg. 40 (2): 10-20.
DEICHMANN, W. B., MACDONALD, W. E., CUBIT, D. A., & BEASLEY, A. G.
(1972) Effects of starvation in rats with elevated DDT and
dieldrin levels. Int. Arch. Arbeitsmed., 29: 233-252.
DEL VECCHIO, V. & LEONI, V. (1967) [Study on the amount of chlorinated
insecticides in biological materials. II. Chlorinated insecticides
in adipose tissue in certain groups of the Italian population.]
Nuovi Ann. Ig. Microbiol., 18: 107-128 (in Italian).
DENES, A. (1962) [Problems of food chemistry concerning residues of
chlorinated hydrocarbons.] Nahrung, 6: 48-56 (in German).
DENES, A. (1964) Investigation of chlorinated hydrocarbon residues in
animal and vegetable fats. In: 1963 Year Book, Budapest,
Hungary, Institute of Nutrition.
DEUTSCHE FORSCHUNGSGEMEINSCHAFT (1969) [Residue analysis of plant
protection agents.] Weinheim, Verlag Chemie, 400 pp. (in German).
DE VLIEGER, M., ROBINSON, J., BALDWIN, M. K., CRABTREE, A. N., & VAN
DUK, M. C. (1968) The organochlorine insecticide content of human
tissue. Arch. environ. Health, 17: 759-767.
DOLAN, J. W. & HALL, R. C. (1973) Enhancement of the sensitivity and
selectivity of the Coulson electrolytic conductivity detector to
chlorinated hydrocarbon pesticides. Anal. Chem., 45: 2198-2204.
DOMENJOZ, R. (1944) [Experimental findings with a new insecticide
(Neocid, Geigy), a contribution to the theory contact-toxicity
action.] Schweiz. Med. Wochenschr., 74: 952-958 (in German).
DOMENJOZ, R. (1946a) [On the biological action of a DDT-derivative.]
Arch. Int. Pharmacodyn. Ther., 73: 128-146 (in German).
DOMENJOZ, R. (1946b) [Biological action of some DDT derivatives.]
Helv. Chem. Acta., 29: 1317-1322 (in French).
DRAIZE, J. H., NELSON, A. A., & CALVERY, H. O. (1944) The percutaneous
absorption of DDT (2,2-bis-(p-chlorophenyl)-1,1,1-trichloroethane)
in laboratory animals. J. Pharmacol. exp. Ther., 82: 159-166.
DUGGAN, R. E. (1968) Residues in food and feed. Pesticide residue
levels in food in the United States from July 1, 1963 to June 30,
1967. Pestic. Monit. J., 2: 2-46.
DUGGAN, R. E. & CORNELIUSSEN, P. E. (1972) Dietary intake of pesticide
chemicals in the United States (III), June 1968-April 1970.
Pestic. Monit. J., 5: 331-341.
DUGGAN, R. E. & LIPSCOMB, C. Q. (1969) Dietary intake of pesticide
chemicals in the United States (II), June 1966-April 1968.
Pestic. Monit. J., 2: 153-162.
DUGGAN, R. E. & WEATHERWAX, J. R. (1967) Dietary intake of pesticide
chemicals: Calculated daily consumption of pesticides with foods
are discussed and compared with currently accepted values.
Science, 157: 1006-1010.
DURHAM, W. F. & WOLFE, H. R. (1962) Measurement of the exposure of
workers to pesticides. Bull. World Health Organ. 26: 75-91.
DURHAM, W. F. & WOLFE, H. R. (1963) An additional note regarding
measurement of the exposure of workers to pesticides. Bull. World
Health Organ., 29: 279-281.
DURHAM, W. F., ARMSTRONG, J. F., UPHOLT, W. M., & HELLER, C. (1961)
Insecticide-content of diet and body fat of Alaskan natives.
Science, 134: 1880- 1881.
DURHAM, W. F., ORTEGA, P., & HAYES, W. J., JR (1963) The effect of
various dietary levels of DDT on liver function, cell morphology,
and DDT storage in the rhesus monkey. Arch. int. Pharmacodyn.
Ther., 141: 111-129.
DURHAM, W. F., ARMSTRONG, J. F., & QUNBY, G. E. (1965a) DDA excretion
levels. Arch. environ. Health, 11: 76-79.
DURHAM, W. F., ARMSTRONG, J. F., & QUINBY, G. E. (1965b) DDT and DDE
content of complete prepared meals. Arch. environ. Health,
11: 641-647.
DVORCHIK, B. H., ISTIN, M., & MAREN, T. H. (1971) Does DDT inhibit
carbonic anhydrase? Science, 172: 728-729.
DJATLOVlTSKAJA, F. G., GLADENKO, Y. F., & KRUCININA, A. A. (1972)
[Determination of organochlorine insecticides in reservoir water.]
Probl. anal Khim., 2: 43-36 (in Russian).
EDMUNDSON, W. F., DAVIES, J. E., & CRANMER, M. (1970a) DDT and DDE in
blood and urine of men exposed to 3 per cent DDT aerosol. Public
Health Rep., 85: 457-463.
EDMUNDSON, W. F., DAVIES, J. E., MACEO, A., & MORGADE, C. (1970b) Drug
and environmental effects on DDT residues in human blood. South.
med. J., 63: 1440-1441.
EGAN, H., GOULDING, R., ROBURN, J., & TATTON, J. O'G. (1965)
Organochlorine pesticide residues in human fat and human milk.
Br. med. J., 2: 66-69.
EICHELBERGER, J. W. & LICHTENBERG, J. J. (1971) Carbon adsorption for
recovery of pesticides (from water). J. Am. Water Works Assoc.,
63 (1): 25-27.
EMBRY, T. L., MORGAN, D. P., & ROAN, C. C. (1972) Search for
abnormalities of heme synthesis and sympathoadrenal activity in
workers regularly exposed to pesticides. J. occup. Med.,
14: 918-921.
ENGST, R. & KNOLL, R. (1972) DDT and DDE residues in human milk.
Nahrung, 16: 130-131.
ENGST, R., KNOLL, R., & NICKEL, B. (1967) [On the accumulation of
chlorinated hydrocarbons particularly of DDT and its metabolite
DDE in human fat.] Pharmazie, 22: 654-661 (in German).
ENGST, R., KNOLL, R., & NICKEL, B. (1970) [Occurrence of DDT and DDE
in fatty tissues and in organs of infants.] Pharmazie,
24: 673-676 (in German).
EPSTEIN, S.S. & SHAFNER, H. (1968) Chemical mutagens in the human
environment. Nature (Lond.), 219: 385-387.
ESKENASY, J. J. (1972) Status epilepticus by
dichlorodiphenyltrichloroethane and hexachlorocyclohexane
poisoning. Rev. Roum. Neurol., 9: 435-442.
EYZAGUIRRE, C. & LILIENTHAL, J. L., JR (1949) Veratrinic effects of
pentamethylenetetrazol (Metrazol) and 2,2-bis-( p-chlorophenyl)-
1,1,1-trichloroethane (DDT) on mammalian neuromuscular function.
Proc. Soc. Exp. Biol., 70: 272-275.
FAO (1972) Production yearbook-1971, Rome, Food and Agriculture
Organization, Vol. 25, pp. 499-537.
FAO/WHO (1973) 1972 Evaluations of some pesticide residues in food,
Geneva, WHO, 587 pp. (WHO Pest. Res. Series No. 2).
FARKAS, I., DESI, I., & KEMENY, T. (1969) [Effect of orally consumed
DDT on the rest and load EEG curves.] Egeszsegtudomany,
13 (2): 195-201 (in Hungarian).
FEIL, V. J., LAMOUREUX, C. H., STYRVOKY, E., ZAYLSKIE, R. G., THACKER,
E. J., & HOLMAN, G. M. (1973) Metabolism of o,p'-DDT in rats.
J. agric. food Chem, 21: 1072-1078.
FEIL, V. J., LAMOUREUX, C. H., & ZAYLSKIE, R. G. (1975) Metabolism of
o,p'-DDT in chickens. J. agric. food Chem., 23: 382-388.
FENNAH, R. G. (1945) Preliminary tests with DDT against insect pests
of food-crops in the Lesser Antilles. Trop. Agric., 22: 222-226.
FINSTERWALDER, C. E. (1976) Collaborative study of an extension of the
Mills et al. method for the determination of pesticide residues in
food. J. Assoc. Off. Anal. Chem, 59: 169-171.
FISEROVA-BERGEROVA, V., RADOMSKI, J. L., DAVIES, J. E., & DAVIES, J.
H. (1967) Levels of chlorinated hydrocarbon pesticides in human
tissues. Ind. Med. Surg., 36: 65-70.
FISHBEIN, L. F. (1974) Chromatographic and biological aspects of DDT
and its metabolites. J. Chromatogr., 98: 177-251.
FITZHUGH, O. G. (1948) Use of DDT insecticides on food products. Ind.
Eng. Chem., 40: 704-705.
FITZHUGH, O. G. (1966) Problems related to the use of pesticides.
Can. Med. Assoc. J., 94: 598-604.
FITZHUGH, O. G. (1970) A summary of a carcinogenic study of DDT in
mice from Food and Drug Administration, USA. In: FAO/ WHO 1969
evaluations of some pesticide residues in foods, Geneva. WHO,
pp. 61-64 (WHO/Food Add./70.38).
FITZHUGH, O. G. & NELSON, A. A. (1947) The chronic oral toxicity of
DDT (2,2-bis- p-chlorophenyl- 1,1,1-trichloroethane).
J. Pharmacol., 89: 18-30.
FRANCONE, M.P., MARLANI, F. H., & DEMARE, C. (1952) [Clinical signs of
poisoning by DDT.] Rev. Asoc. Méd. Argent., 66: 56-59 (in
Spanish).
FREDERICKS, C. M., LARSON, R. E., & ASTON, R. (1974) Effect of chronic
DDT exposure on pentobarbital tolerance and intolerance in the
rat. Toxicol. appl. Pharmacol., 27: 99-107.
FRENCH, M. C. & JEFFERIES, D. J. (1969) Degradation and disappearance
of ortho-, para-isomer of technical DDT in living and dead arian
tissues. Science, 165: 914-916.
FRIDMAN, G. I. (1970) [Effect of 7-trichlorofon and DDT on some
specific and non-specific characteristics of the
immunobiological and general reactivity of the organism (e.g.
problems of toxicological reactions of low intensity)] In:
[Questions of pesticide hygiene and toxicology.] Moscow,
Medicina, pp. 139-145 (in Russian).
FRIES, G. F., MARROW, G. S., JR, LESTER, J. W., & GORDON, C. H. (1971)
Effect of microsomal enzyme inducing drugs on DDT and dieldrin
elimination from cows. J. dairy Sci., 54: 364-368.
GÄB, S., NITZ, S., PARLAR, H., & KORTE, F. (1975) Photomineralisation
of certain aromatic xenobiotica. Chemosphere, No. 4,
pp. 251-256.
GÄB, S., SCHMITZER, J., THAMM, H. W., PARLAR, H. & KORTE, F. (1977)
Photomineralisation rate of organic compounds adsorbed on
particulate matter. Nature (Lond.), 270: 331-333.
GABLIKS, J. & MALTBY-ASKARI, E. (1970) The effect of chlorinated
hydrocarbons on drug metabolism in mice. Ind. Med. Surg.,
39: 347-350.
GABLIKS, J., ASKARI, E. M., & YOLEN, N. (1973) DDT and immunological
responses. I. Serum antibodies and anaphylactic shock in guinea
pigs. Arch. environ. Health, 26: 305-308.
GABLIKS, J., AL-ZUBAIDY, T., & ASKARI, E. (1975) DDT and immunological
responses. III. Reduced anaphylaxis and mast cell populations in
rats fed DDT. Arch. environ. Health, 30: 81-84.
GAINES, T. B. (1960) The acute toxicity of pesticides to rats.
Toxicol. appl. Pharmacol., 2: 88-99.
GAMES, T. B. (1969) Acute toxicity of pesticides. Toxicol. appl.
Pharmacol, 14: 515-534.
GALLAGHER, T. F., FUKUSHIMA, D. K., & HELLMANN, L. (1962) The effect
of ortho-, para-DDD on steroid borne metabolites in adrenocortical
carcinoma. Metab. clin. Exper., 11: 1155-1161.
GALTON, F. (1879) The geometric mean, in vital and social statistics.
Proc. R. Soc. Lond. B, 29: 365-367.
GARRETT, R. M. (1947) Toxicity of DDT for man. J. Med. Assoc.
Alabama, 17: 74-76.
GELLERT, R. J., HEINRICHS, W. L., & SWERDLOFF, R. S. (1972) DDT
homologues: Estrogen like effects on the vagina, uterus, and
pituitary of the rat. Endocrinology, 91: 1095-1100.
GENINA, S. A., SVETLAJA, E. N., & KOMAROVA, L. I. (1969) [Blood levels
of organic chlorine pesticides and some haematological indicators
in people employed in applying pesticides from the air.] In:
Medved', M. E., ed. The hygiene of application and the toxicology
of pesticides and the clinical features of pesticide poisoning. A
collection of papers, Issue No. 7, pp. 492-496, Kiev,
Vniigintoks (in Russian).
GIL G. P. & MIRON, B. F. (1949) [Investigation on toxicity of DDT to
man.] Med. Colon., 14: 459-470 (in Spanish).
GILBERT, D. & GOLBERG, L. (1967) BHT oxidase. A liver-microsomal
enzyme induced by the treatment of rats with butylated
hydroxytoluene. Food Cosmet. Toxicol., 5: 481-890.
GILLETT, J. W. (1968) No effect level of DDT in induction of
microsomal epoxidation. J. agric. food Chem., 16: 295-297.
GINGELL, R. & WALLCAVE, L. (1974) Species differences in the acute
toxicity and tissue distribution of DDT in mice and hamsters.
Toxicol. appl. Pharmacol., 28: 385-394.
GIUFFRIDA, L. & IVES, N. F. (1969) Microcoulomeric gas chromatography:
Improvement in instrumentation. J. Assoc. Off. Anal. Chem.,
52: 541-545.
GOERLITZ, D. F. & LAW, L. M. (1972) Chlorinated naphthalenes in
pesticide analysis. Bull. environ. Contain. Toxicol.,
7: 243-251.
GORDON, A. E. & FRIGERIO, A. (1972) Mass fragmentography as an
application of gas-liquid chromatograph-mass spectrometry in
biological research. J. Chromatogr., 73: 401-417.
GORDON, H. T. & WELSH, J. H. (1948) The role of ions in axon surface
reactions to toxic organic compounds. J. cell comp. Physiol.,
31: 395-420.
GRACA, I., SILVA FERDANANDES, A. M. S, & MOURAO, H. C. (1975)
Pesticides in people -- Organochlorine insecticide residues in
human milk in Portugal. Pestic. Monit. J., 8: 148-156.
GRACHEVA, G. V. (1969) [The possibility of DDT accumulation in the
organism of persons not having occupational contact with it.]
Faktory Vneshn. Sredy ikh Znachen, 1: 125-129 (in Russian).
GRACHEVA, G. V. (1970) [DDT excretion with the milk of nursing mothers
occupationally unexposed to the effect of this insecticide.] Vop.
Pitan., 29: 75-78 (in Russian).
GRIFFITH, F. D., JR & BLANKE, R. V. (1974) Microcoulometric
determination of organochlorine pesticides in human blood.
J. Assoc. Off. Anal Chem., 57: 595-603.
GRIFFITH, F. D., JR & BLANKE, R. V. (1975) Pesticides in people --
Blood organochlorine pesticide levels in Virginia residents.
Pestic. Monit. J., 8: 219-223.
GUENZI, W. D. & BEARD, W. E. (1968) Anaerobic conversion of DDT to DDD
and aerobic stability in soil. Proc. Soil Sci. Soc. Am.,
32: 522-534.
HAAG, H. B, EINNEGAN, J. K., LARSON, P.S., DREYFUSS, M. L., MAIN, R.
J., & RIESE, W. (1948) Comparative chronic toxicity for warm-
blooded animals of 2,2-bis( p-chlorophenyl)-1,1,1-trichloroethane
(DDT) and 2,2-bis-( p-chlorophenyl)-1,1-dichloroethane (DDD).
Ind. Med. Surg., 17: 477-484.
HAJKINA, B. I. & SILINA, V. F. (1971) [The effect of some
organochlorine pesticides on serotonin metabolism.] Farmakol.
Toxikol., 34: 357-359 (in Russian).
HALACKA, K., KAKL, J., & VYMETAL, F. (1965) [A reflexion in human fat
tissue of the extensive use of DDT.] Czech. Hyg., 10: 188-192
(in Czech).
HANADA, M., YUTANI, C., & MIYAJI, T. (1973) Induction of hepatoma in
mice by benzene hexachloride. Gann, 64: 511-513.
HARA, A., OWASAKI, I., NAWA, H, YOSHIOKA, Y., & YOKOO, S. (1973)
[Organochlorine insecticide residues in blood of pregnant women
and in umbilical cord blood.] Prog. Med., 84: 79-80 (in
Japanese).
HARMAN, S. W. (1946) DDT for codling moth control in western New York
in 1945. J. econ. Entomol., 39: 208-210.
HARRISON, R. D. (1975) Comparative toxicity of some selected
pesticides in neonatal and adult rats. Toxicol. appl. Pharmacol.,
32: 443-446.
HART, L. G. & FOUTS, J. R. (1963) Effects of acute and chronic DDT
administration on hepatic microsomal drug metabolism in the rat.
Proc. Soc. Exp. Biol. Med., 114: 388-392.
HART, M. M., REGAN, R. L., & ADAMSON, R. H. (1973) The effect of
isomers of DDD on the ACTH-induced steroid output, histology, and
ultrastructure of the dog adrenal cortex. Toxicol. appl.
Pharmacol., 24: 101-113.
HARTLEY, G. S. (1969) Evaporation of pesticides. In: Gould, R. F., ed.
Pesticidal Formulations Research, Washington, DC, American
Chemical Society, pp. 115-134 (Adv. Chem. Ser. 86).
HATTULA, M. L., IKKALA, J., ISOMÄKI, M., MÄÄTTÄ, K., & ARSTILA, A. U.
(1976) Chlorinated hydrocarbon residues (PCB and DDT) in human
liver, adipose tissue and brain in Finland. Acta Pharmacol.
Toxicol., 39: 545-554.
HAYASHI, M. (1972a) [Pollution of mothers' milk by organochlorine
pesticides.] Jpn J. Public Health, 19: 437-441 (in Japanese).
HAYASHI, M. (1972b) [Pesticide pollution of mothers' milk.] J. Jpn
Med. Assoc., 68: 1281-1286 (in Japanese).
HAYES, W. J., JR (1959) Pharmacology and toxicology of DDT. In:
Müller, P., ed. DDT: The insecticide dichlorodiphenyltri-
chloroethane and its significance, Basel, Birkhäuser Verlag,
Vol. 2, pp. 9-247.
HAYES, W. J., JR (1965) Review of the metabolism of chlorinated
hydrocarbon insecticides especially in mammals. Ann. Rev.
Pharmacol., 5: 27-52.
HAYES, W. J., JR (1975) Toxicology of pesticides, Baltimore,
Williams & Wilkins Co., 580 pp.
HAYES, W. J., JR (1976a) Mortality in 1969 from pesticides, including
aerosols. Arch. environ. Health, 31: 61-72.
HAYES, W. J., JR (1976b) Dosage relationships associated with DDT in
milk. Toxicol. appl. Pharmacol., 38: 19-28.
HAYES, W. J., JR & DALE, W. E. (1964) Concentration of DDT in brain
and other tissues in relationship to symptomatology. Toxicol.
appl. Pharmacol., 6: 349.
HAYES, W. J., JR, DURHAM, W. F., & CUETO, C., JR (1956) The effect of
known repeated oral doses of chlorphenothane (DDT) in man. J. Am.
Med. Assoc., 162: 890-897.
HAYES, W. J., JR, QUINBY, G. E., WALKER, K. C., ELLIOTT, J. W., &
UPHOLD, W. M. (1958) Storage of DDT and DDE in people with
different degrees of exposure to DDT. Am. Med. Assoc. Arch. ind.
Health, 18: 398-496.
HAYES, W. J., JR, DALE, W. E., & LEBRETON, R. (1963) Storage of
insecticides in French people. Nature (Lond.), 199: 1189-1191.
HAYES, W. J., JR, DALE, W. E., & BURSE, V. W. (1965) Chlorinated
hydrocarbon pesticides in the fat of people in New Orleans. Life
Sci., 4: 1611-1615.
HAYES, W. J, JR, DALE, W. E., & PIRKLE, C. I. (1971) Evidence of
safety of long-term, high, oral doses of DDT for man. Arch.
environ. Health, 22: 119-135.
HEATH, D. F. & VANDEKAR, M. (1964) Toxicity and metabolism of dieldrin
in rats. Br. J. ind. Med, 21: 269-279.
HEINRICKS, W. J., GELLERT, R. J., BAKKE, J. L., & LAWRENCE, N. L.
(1971) DDT administered to neonatal rats induces persistent estrus
syndrome. Science, 173: 642-643.
HELLMAN, L., BRADLOW, H. L., & ZUMOFF, B. (1973) Decreased conversion
of androgens to normal 17-ketosteroid metabolites as a result of
treatment with o,p'-DDD. J. clin. Endocrinol. Metab.,
36: 801-803.
HENDERSON, G. L. & WOOLLEY, D. E. (1969) Tissue concentrations of DDT:
Correlation with neurotoxicity in young and adult rats. Proc.
West. Pharmacol. Soc., 12: 58-62.
HENDERSON, G. L. & WOOLLEY, D. E. (1970) Mechanisms of neurotoxic
action of 1,1,1-trichloro-2,2-bis-( p-chlorophenyl)-ethane (DDT)
in immature and adult rats. J. Pharmacol. exp. Ther.,
175: 60-68.
HERZEL, F. & LAHMANN, E. (1973) [Contribution to quantitative
determination of pesticides in the air.] Gesundh.-Ing,
94: 275-279 (in German).
HESS, A.D. & KEENER, G. G., JR (1947) Effect of airplane-distributed
DDT thermal aerosols on fish and fish food organisms. J. Wildl.
Manage., 11: 1-10.
HEYNDRICKX, A. & MAES, R. (1969) The excretion of chlorinated
hydrocarbon insecticides in human mother milk. J. Pharm. Belg.,
9-10: 459-463.
HIDAKA, K., OHE, T., & FUJIWARA, K. (1972) [PCB and organochlorine
pesticides in mother's milk.] Igakuno Ayumi 82: 519-520 (in
Japanese).
HILL, D. W. & MCCARTY, P. L. (1967) Anaerobic degradation of selected
chlorinated hydrocarbon pesticides. J. Water Pollut. Control
Fed., 39: 1259-1277.
HILTON, B. D. & O'BRIEN, R. D. (1970) Antagonism by DDT of the effect
of valinomycin on synthetic membrane. Science, 168: 841-843.
HOFFMANN, C. H. & MERKEL, E. P. (1948) Fluctuations in insect
populations associated with aerial applications of DDT to forests.
J. econ. Entomol., 41: 467-473.
HOFFMANN, C. H. & SURBER, E. W. (1948) Effects of an aerial
application of wettable DDT on fish and fish food organisms in
Back Creek, West Virginia. Trans. Am. Fish. Soc., 75: 48-58.
HOFFMAN, D. G., WORTH, H. M., EMMERSON, J. L., & ANDERSON, R. C.
(1970) Stimulation of hepatic drug-metabolizing enzymes by
chlorphenothane (DDT); the relationship to liver enlargement and
hepatotoxicity in the rat. Toxicol. appl. Pharmacol.,
16: 171-178.
HOFFMAN, D. L. & MATTOX, V. R. (1972) Treatment of adrenocortical
carcinoma with o,p'-DDD. Med. Clin. North Am., 56: 999-1012.
HOFFMAN I. & LENDLE, L. (1948) [On the action of new insecticide
compounds.] Arch. exp. Pharmakol., 205: 223-242 (in German).
HOFFMAN, W. S. (1968) Clinical evaluation of the effects of pesticides
in man. Ind. Med. Surg., 37: 289-292.
HOFFMAN, W. S., FISHBEIN, W. I., & ANDELMAN, M. B. (1964) The
pesticide content of human fat tissue. Arch. environ. Health,
9: 387-394.
HOFFMAN, W. S., ADLER, H., FISHBEIN, W. I., & BAUER, F. C. (1967)
Relation of pesticide concentrations in fat to pathological
changes in tissues. Arch. environ. Health, 15: 758-765.
HOLDEN, A. V. (1970) International co-operative study of
organochlorine pesticide residues in terrestrial and aquatic
wildlife, 1967/68. Pestic. Monit. J., 4: 117-135.
HOLDEN, A. V. (1973) International co-operative study of
organochlorine and mercury residues in wildlife. Pestic. Monit.
J., 7: 37-52.
HORNABROOK, R. W., DYMENT, P. G., GOMES, E. D., & WISEMAN, J. S.
(1972) DDT residues in human milk from New Guinea natives. Med.
J. Aust., 1: 1297-1300.
HORWITZ, W., ed. (1975) Official methods of analysis, Washington,
DC, Association of the Official Analytic Chemists, 1094 pp.
HOWELL, D. E. (1948) A case of DDT storage in human fat. Proc. Okla.
Acad. Sci., 29: 31-32.
HRDINA, P. D., SINGHAL, R. L., PETERS, D. A. V., & LING, G. M. (1973)
Some neurochemical alterations during acute DDT poisoning.
Toxicol. appl. Pharmacol., 25: 276-288.
HRDINA, P. D., SINGHAL, R. L., & LING, G. M. (1975) DDT and related
hydrocarbon insecticides: Pharmacological basis of their toxicity
in mammals. Adv. Pharmacol. Chemother., 12: 31-88.
HRUSHKA, J. (1969) [DDT residues in the milk, butter, and fat of
cattle.] Veterinarstvi, 19 (11): 492-498 (in Czech).
HRUSHKA, J. & KOCIANOVA, M. (1975) [Contamination of the food chain by
chlorinated insecticides in Southern Bohemia.] Czech. Hyg.,
20: 421-428 (in Czech).
HSIEH, H. C. (1954) DDT intoxication in a family in Southern Taiwan.
Am. Med. Assoc. Arch. ind. Hyg., 10: 334-346.
HUEPER, W. C. (1942) Occupational tumors and allied diseases,
Springfield. IL. Thomas, pp. 496-497.
HUNTER, C. G., ROBINSON, J., & RICHARDSON, A. (1963) Chlorinated
insecticide content of human body fat in southern England.
Br. med. J., 1: 221-224.
HUTTERER, F., SCHAFFNER, F., KLION, F. M., & POPPER, H. (1968)
Hypertrophic, hypoactive smooth endoplasmic reticulum: A sensitive
indicator of hepatotoxicity exemplified by dieldrin. Science,
161: 1017-1019.
INNES, J. R. M., ULLAND, B. M., VALERIO, M. G., PETRUCELLI, L.,
FISHBEIN, L., HART, E. R., PALLOTTA, A. J., BATES, R. R., FALK, H.
L., GART, J. J., KLEIN, M., MITCHELL, & PETERS, J. (1969) Bioassay
of pesticides and industrial chemicals for tumorigenicity in mice.
A preliminary note. J. Natl Cancer Inst., 42: 1101-1114.
INOUE, Y., ABE, J., TAKAMATSU, M., & AOKI, N. (1974) [A study of the
qualitative and quantitative ratio of PCB and organochlorine
pesticide residues in the blood and adipose tissue.] Jpn J. Hyg.,
29: 92 (in Japanese).
INSTITUTE OF PETROLEUM (1968) Recommended practice for determining
precision data for methods on petroleum products and lubricants,
London, Institute of Petroleum.
ISRAELI, VON R. & MAYERSDORF, A. (1973) [Pathological changes in the
EEG during work with halogen-containing insecticides.] Zentralbl.
Arbeitsmed., 11: 340-343 (in German).
INTERNATIONAL AGENCY FOR RESEARCH ON CANCER (1974) IARC monographs:
The evaluation of the carcinogenic risk of chemicals to man, some
organochloride pesticides, Lyons, IARC, 241 pp. (IARC
monographs, Vol. 5).
ITO, N., NAGASAKI, H., ARAI, M., SUGIHARA, S., & MAKIURA, S. (1973)
Histological and ultrastructural studies on the
hepatocarcinogenicity of benzene hexachloride in mice. J. Natl
Cancer Inst., 51: 817-826.
JAGER, K. W. (1970) Aldrin, Dieldrin, Endrin, and Telodrin: An
epidemiological and toxicological study of long-term occupational
exposure, New York, Elsevier, 239 pp.
JANSSON, B., JENSEN, S., OLSSON, M., RENBERG, L., SUNDSTRÖM, G., &
VAZ, R. (1975) Identification by GC-MS of phenolic metabolites of
PCB and p,p'-DDE isolated from Baltic guillemot and seal.
Ambio, 4: 93-97.
JENSEN, S. & JANSSON, B. (1976) Anthropogenic substances in seal from
the Baltic: Methyl sulfone metabolites of PCB and DDE. Ambio,
5: 257-260.
JENSEN, J. A., CUETO, C., JR, DALE, W. E., ROTHE, C. F., PEARCE, G.
W., & MATTSON, A. M. (1957) Metabolism of insecticides. DDT
metabolites in feces and bile of rats. J. agric. food Chem.,
5: 919-925.
JEYARATNAM, J. & FORSHAW, J. (1974) A study of the cardiac effects of
DDT in laboratory animals. Bull. World Health Organ.,
51: 531-535.
JOHNSON, L. G. (1973) Formation of pentafluorobenzyl derivatives for
the identification and quantitation of acid and phenolic pesticide
residues. J. Assoc. Off. Anal. Chem., 56: 1503-1505.
JONCZYK, H. (1970) [The content of organochlorine insecticides in the
blood of healthy persons.] Rocz. panstw. Zakl. Hig., 21: 589-593
(in Polish).
JONCZYK, H., DMOCH, J., & BOJANOWSKA, A. (1970) [Determination of
chlorinated hydrocarbons in adipose tissue and brain of
Partridges.) Rocz. panst. Zakl. Hig., 21: 409-415 (in Polish).
JONCZYK, H., RUDOWSKI, W., TRACZYK, Z., & KLAWE, Z. (1974) [DDT and
its metabolites and gamma-HCH in blood, bone marrow, and fatty
tissue in certain hematological syndromes.] Pol. Tyg. Lek.,
29: 1573-1576 (in Polish).
JOY R. M. (1973) Electrical correlates of preconvulsive and convulsive
doses of chlorinated hydrocarbon insecticides in the CNS.
Neuropharmacology, 12: 63-76.
JUDAH, J. D. (1949) Studies on the metabolism and mode of action of
DDT. Br. J. Pharmacol, 4: 120-131.
JUDE, A. & GIRARD, P. (1949) [DDT toxicity -- mass poisoning by
accidental ingestion.] Ann. Med. lég. Criminol., Police Sci. Med.
Soc. Toxicol., 29: 209-213 (in French).
JUSZKIEWlCZ, T. & STEC, J. (1971) [Polichlorinated insecticide
residues in adipose tissue of farmers in the Lublin Province
(Poland).] Pol. Tyg. Lek., 26: 462-464 (in Polish).
KACEW, S. & SINGHAL, R. L. (1972) Role of adrenals in acute effects of
p.p'-DDT renal glucose metabolism. Fed. Proc., 31 (2): 520 Abs
(Abstract No. 1727).
KACEW, S. & SINGHAL, R. L. (1973) Adaptive response of hepatic
carbohydrate metabolism to oral administration of p,p'-1,1,1-
trichloro-2,2-bis-( p-chlorophenyl)-ethane in rats. Biochem.
Pharmacol., 22: 47-57.
KADIS, V. W., BREITKREITZ, W. E., & JONASSON, O. J. (1970) Insecticide
levels in human tissues of Alberta residents. Can. J. Public
Health, 61: 413-416.
KAGAN, Y. S., RODIONOV, G. A., WORONINA, L. Y., VELICHKO, L. S.,
KULAGIN, O. M., & PEREMITINA, A.D. (1969) [Effect of DDT on the
functional and morphological condition of the liver.] Vrach.
Delo, 12: 101-105 (in Russian).
KAISER, H. (1973) Guiding concepts relating to trace analysis. Pure
appl. Chem., 34: 35-61.
KAKU, S. (1973) [An epidemiological study on organochlorine pesticide
contamination. On pesticide residues in plasma of so-called
healthy Japanese in 1971.] J. Kurume Med. Assoc., 36: 307-326
(in Japanese).
KALISER, L. A. (1968) An in vitro and in vivo study of the effect
of DDT on the phagocytic activity of rat white blood cells.
Toxicol. appl. Pharmacol., 12: 353-357.
KALOYANOVA, F., MIHAILOVA, Z., GEORGIV, G., BENCHEV, I., RISOV, V., &
VELICHKOVA, V. (1972) Organochlorine pesticides in the fat of the
general population of Bulgaria. Abstracts of the XVII
International Congress on Occupational Health, Buenos Aires,
p. 75.
KAMATA, T. (1974) [On the present status of environmental pollution by
organochlorine pesticide residues.] Jpn J. Public Health,
21: 163-164 (in Japanese).
KAMATA, T. (1974) [Hygienic studies on pesticide residues: Part 4.
Environmental contamination by organochlorine pesticides.] Med.
J. Hiroshima Univ., 22: 315-325 (in Japanese).
KANITZ, S. & CASTELLO, G. (1966) [On the presence of residues of some
disinfectants in human adipose tissue and in food.] G. Ig. Med.
Prev., 7: 1-19 (in Italian).
KARIMOV, A. M. (1969) [Skin sensitivity to organochlorine
insecticides.] Med. Zh. Uzbekistant (No. 9): 58-60 (in Russian).
KARIMOV, A. M. (1970) [Occupational skin diseases in cotton growers
caused by chemical poisons and measures for their prevention.]
Gig. Tr. Prof. Zabol., 14: 35-37 (in Russian).
KASAI, A., ASANU, A. S., & NAKAMURA, S. (1972) [Studies on
organochlorine pesticide residues in human organs, Part III.]
J. Jpn Assoc. Rural Med., 21: 296-297 (in Japanese).
KATO, K., YAMADA, T., WATANABE, S., WADA, Y., FUKUDA, M., KURODA, M.,
NAKAOKA, S., TAKAHASHI, W., MIYASHIRO, K., MASUAD, J. & YASUKATA,
S. (1971) [Analyses of residual pesticides in vegetables, cows'
milk, and mothers' milk.] Ann. Rep. Kanagawa Prefect. Inst.
Public Health, 21: 85-92 (in Japanese).
KAWAI, Y., HORI, Y., NIGAWA, Y., YAMAMOTO, I., TSUZUKI, T., KITAYAMA,
M., & MORI, K. (1973) [On the pollution of mothers' milk by
pesticides and PCB in Hokkaido.] J. Food Hyg. Soc. Jpn,
14: 302-303 (in Japanese).
KAWANISHI, A., ASANUMA, S., & NAKAMURA, S. (1973) [Studies on
organochlorine pesticide residues in humans. Report 4.] J. Jpn
Assoc. Rural Med., 22: 278-279 (in Japanese).
KAZEN, C., BLOOMER, A., WELCH, R., OUDBIER, A., & PRICE, H. (1974)
Persistence of pesticides on the hands of some occupationally
exposed people. Arch. environ. Health, 29: 315-318.
KEIL, J. E., SANDIFER, S. H., FINKLEA, J. H., & PRIESTER, L. E. (1972)
Serum vitamin A elevation in DDT exposed volunteers. Bull.
environ. Contam. Toxicol., 8: 317-320.
KELLER, H. (1952) [Dichlorodiphenyltrichloroethane (DDT), additional
investigations on its properties and application.]
Naturwissenschaften, 39: 109 (in German).
KEPLINGER, M. L, DEICHMANN, W. B., & SALA, F. (1970) Effect of
combinations of pesticides on reproduction in mice. In: Deichmann,
W. B., ed. Pesticides Symposia, Miami, Halos & Associates,
pp. 125-138.
KHAIRY, M. (1959) Changes in behavior associated with a nervous system
poison (DDT). Q. J. exp. Physiol., 11 (2): 91-94.
KIMBROUGH, R. D., GAINES, T. B., & HAYES, W. J., JR (1968) Combined
effect of DDT, pyrethrum, and piperonyl butoxide on rat liver.
Arch. environ. Health, 16: 333-341.
KINOSHITA, F. K., FRAWLEY, J. P., & DUBOIS, K. P. (1966) Quantitative
measurement of induction of hepatic microsomal enzymes by various
dietary levels of DDT and toxaphene in rats. Toxicol. appl.
Pharmacol, 9: 505-513.
KLEIN, W. & KORTE, F. (1970) [Metabolism of chlorinated hydrocarbons.]
In: Wegler, R., ed. Chemie der Pflanzenschutz-und Schädlings-
bekämpfungsmittel, Band I, Berlin, Springer Verlag, pp. 199-218
(in German).
KLEIN, A. K., LAUG, E. P., KATTA, P. R., & MENDEL, J. L. (1965)
Evidence for the conversion of o,p'-DDT (1,1,1-trichloro-2- o-
chlorophenyl-2- p-chlorophenylethane) to p,p'-DDT (1,1,1-
trichloro-2,2-bis( p-chlorophenyl)ethane) in rats. J. Am. Chem.
Assoc., 87: 2520-2522.
KLEINFELD, M. (1967) Cancer of urinary bladder in a dye plant: A
medical and environmental study. In: Deichmann, W. B., ed.
Bladder Cancer, A Symposium, Birmingham, Al., Aesculapius,
pp. 136-144.
KNIPLING, E. F. (1959) The use of DDT in veterinary medicine. In:
Müller, P., ed. DDT, the insecticide dichlorodiphenyltri-
chloroethane and its significance, Basel, Switzerland, Birkhäuser
Verlag, Vol. II, pp. 505-570.
KNOLL, W. & JAYARAMAN, S. (1973a) [Organochlorine pesticide residues
in human milk.] Z. Gesamt. Hyg., 19: 43-45 (in German).
KNOLL, W. & JAYARAMAN, S. (1973b) [On the contamination of human milk
with chlorinated hydrocarbons.] Nahrung, 17: 599-615 (in German).
KOJIMA, S., SAITO, M., KONNO, H., & OZAWA, K. (1971) [Results of
investigation of organochlorine pesticide residues in mothers'
milk and in the blood of the mothers.] Rep. Akita Prefect. Inst.
Public Health, 16: 65-68 (in Japanese).
KOLMODIN, B., AZARNOFF, D. L., & SJOQVIST, F. (1969) Effect of
environmental factors on drug metabolism: Decreased plasma half-
life of antipyrine in workers exposed to chlorinated hydrocarbon
insecticides. Clin. Pharmacol. Ther, 10: 638-642.
KOLYADA, I. S. & MIKHAL'CHENKOVA, O. F. (1973) [Acute intoxication by
chlorine-containing pesticides.] Gig. Tr. Prof. Zabol., 5: 43
(in Russian).
KOMAROVA, L. I. (1970) [The excretion of DDT in mothers' milk and its
effect on the organism of mother and child.] Pediatr. Akus.
Ginek., 1: 19-20 (in Russian).
KONTEK, M., KUBACKI, S., PARADOWSKI, S., & WIERZCHOWIECKA, B. (1971)
[Chlorinated insecticides in human milk.] Pediatr. Pol.,
46: 183-188 (in Polish).
KOSTER, R. (1947) Differentiation of gluconate, glucose, calcium, and
insulin effects on DDT poisoning in cats. Fed. Proc., 6: 346.
KOSTIUK, O. T. & MUKHTAROVA, N. D. (1970) [Catecholamines and the
functional state of the hypothalamus under the action of a complex
of organochlorine and organophosphorus pesticides.] Gig. Tr.
Prof. Zabol., 14: 35-38 (in Russian).
KRASNIUK, E. P. & PLATONOVA, V. I. (1969) [Functional disorders of the
stomach under prolonged effects of organochlorine chemical
poisons.] Vrach. Delo, No. 9, 99-101 (in Russian).
KRASNIUK, E. P., LOGANOVSKII, N. G., MAKOVSKAYA, E. I., & RAPPOPORT,
M. B. (1968) [Functional and morphological changes in the kidneys
during the effects of DDT on the body.] Soy. Med., 31: 38-42
(in Russian).
KRAUL, I. & KARLOG, O. (1976) Persistent organochlorinated compounds
in human organs collected in Denmark, 1972-1973. Acta Pharmacol.
Toxicol., 38: 38-48.
KROGER, M. (1972) Insecticide residues in human milk. J. Pediatr.,
80: 401-405.
KUCINSKI, A. L. (1972) [On the extraction of DDT and hexachlorane from
some canned foods of animal and vegetable origin.] Vopr. Pitan.,
31 (1): 93 (in Russian).
KUN'EV, V. V. (1965) [Influence of low DDT doses on the phagocytic
state of the blood of albino rats.] Vopr. Pitan., 24: 74-78 (in
Russian).
KUNTZMANN, R., JACOBSON, M., LEVIN, W., & CONNEY, D. H. (1968)
Stimulatory effect of N-phenylbarbitol on cortisol hydroxylation
in man. Biochem. Pharmacol., 17: 565-571.
KUPFER, D. (1967) Effects of some pesticides and related compounds on
steroid function and metabolism. Res. Rev., 19: 11-30.
KUPFER, T., BALAZS, T., & BUYSKE, D. A. (1964) Stimulation by o,p'-
DDD of cortisol metabolism in the guinea pig. Life Sci.,
3: 959-964.
KUTZ, F. W., YOBS, A. R., JOHNSON, W. G., & WIERSMA, G. B. (1974)
Pesticide residues in adipose tissue of the general population of
the United States, FY 1970 survey. Bull. Soc. Pharmacol. Environ.
Pathol., 2: 4-10.
KUWABARA, N. & TAKAYAMA, S. (1974) [Comparison of histogenesis of
liver of mice administered respectively BHC, DDT, and 2,7-FAA.]
Proc. Jpn Cancer Assoc., 33: 50 (in Japanese).
KUZ'MINSKAJA, U. A., KLISENKO, M. A., & HAYKINA, B. I. (1972)
[Peculiarities of the distribution of some pesticides in the lipid
fractions of the liver.] Vopr. Pitan., 31: 48-52 (in Russian).
KWALIK, D. S. (1971) Anticonvulsants and DDT residues. J. Am. Med.
Assoc., 215: 120-121.
LAUBSCHER, J. A., DUTT, G. R., & ROAN, C. C. (1971) Chlorinated
insecticide residues in wildlife and soil as a function of
distance from application. Pestic. Monit. J., 5: 251-258.
LAUG, E. P., NELSON, A. A., FITZHUGH, O. G., &. KUNZE, F. M. (1950)
Liver-cell alteration and DDT storage in the fat of the rat
induced by dietary levels of 1 to 50 ppm of DDT. J. Pharmacol.
98: 268-273.
LAUG, E. P, KUNZE, F. M., & PRICKETT, C. S. (1951) Occurrence of DDT
in human fat and milk. Am. Med. Assoc. Arch. indust. Hyg. occup.
Med., 3: 245-246.
LAÜGER, P., PULVER, R., & MONTIGEL, C. (1945a) [Mode of action of
4,4'-dichlorodiphenyltrichloromethylmetan (DDT-Geigy) in
warmblooded organism.] Experientia (Basel), 1: 120-121 (in
German).
LAÜGER, P., PULVER, R., & MONTIGEL, C. (1945b) [Mode of action of
4,4'-dichlorodiphenyltrichloromethylmetan (DDT-Geigy) in
warmblooded organism.] Helr. Physiol. Acta, 3: 405-415 (in
German).
LAWS, E. R. (1971) Evidence of antitumorigenic effects of DDT. Arch.
environ. Health, 23: 181-183.
LAWS, E. R., JR, CURLEY, A., & BIROS, F. J. (1967) Men with intensive
occupational exposure to DDT. A clinical and chemical study.
Arch. environ. Health, 15: 766-775.
LAWS, E. R, JR, MADDREY, W. C., CURLEY, A., & BURSE, V. W. (1973)
Long-term occupational exposure to DDT. Arch. environ. Health,
27: 318-321.
LEHMAN, A. J. (1952) Chemicals in foods: A report to the Association
of Food and Drug Officials on current developments. Part II,
Pesticides. Section V. Q. Bull. Assoc. Food Drug Off. US,
16: 126-132.
LEHMAN, A. J. (1965) Summaries of pesticide toxicity, Topeka, Ks,
Association of Food and Drug Officials of the United States.
LESCENKO, P. D. & POLONSKAIA, M. N. (1969) [New products in the
prophylactic nutrition of workers in the organochlorine industry.]
Vrach. Delo, No. 8, pp. 106-110 (in Russian).
LEWIS, W. H. & RICHARDS, A. G, JR (1945) Non-toxicity of DDT on cells
in cultures. Science, 102: 330-331.
LICHTENBERG, J. L., EICHELBERGER, J. W., DRESSMAN, R. C., &
LONGBOTTOM, J. E. (1970) Pesticides in surface waters of the
United States -- a 5-year summary, 1964-68. Pestic. Monit. J.,
4: 71-86.
LILLIE, R. D. & SMITH, M. I. (1944) Pathology of experimental
poisoning in cats, rabbits, and rats with 2,2-bis-(para-
chlorphenyl)-1,1,1-trichlorethane. Public Health Rep.,
59: 979-984.
LILLIE, R. D., SMITH, M. I., & STOHLMAN, E. F. (1947) Pathologic
action of DDT and certain of its analogs and derivatives. Arch.
Pathol., 43: 127-142.
LIPSCOMB, G. Q. (1968) Residues in food and feed -- Pesticide residues
in prepared baby foods in the United States. Pestic. Monit. J.,
2: 104-108.
LÖFROTH, G. (1968) Pesticides and catastrophe. New Sci.,
40: 567-568.
LONG, K. L., BEAT, V. B., GOMBART, A. K., SHEETS, R. F., HAMILTON, H.
E., FALABALLA, F., BONDERMAN, D. P., & CHOI, U. Y. (1969) The
epidemiology of pesticides in a rural area. Am. Ind. Hyg. Assoc.
J., 30: 398-304.
LOVELOCK J. E. (1963) Electron absorption detectors and techniques for
use in quantitative and qualitative analysis by gas-liquid
chromatography. Anal. Chem., 35: 474-481.
LU, F. C., JESSUP, D. C., & LAVALÉE, A. (1965) Toxicity of pesticides
to young versus adult rats. Food Cosmet. Toxicol., 3: 591-596.
LUBITZ, J. A., FEEMAN, L., & OKUM, R. (1973) Mitotane use in
inoperable adrenal cortical carcinoma. J. Am. Med. Assoc.,
233: 1109-1112.
LUQUET, F., GOURSAUD, J. G., & CASALIS, J. (1974) Organochlorine
pesticide residues in animal and human milk. Ann. Fals. Expert.
Chim., 67: 217-239.
LUTON, J. P, VALCKE, J. C., REMY, J. M., MATHIEU DE FOSSEY, B., &
BRICAIRE, H. (1972) Gynecomastia after long-term treatment of
Cushing's diseases with o,p'-DDT. Ann Endocrinol.,
33: 290-293.
MACCORMACK, J. D. (1945) Infestation and DDT. Irish J. med. Sci.
6: 627-634.
MACKERRAS, I. M. & WEST, R. F. K. (1946) 'DDT' poisoning in man.
Med. J. Aust., 1: 400-401.
MACKLIN, A. W. & BIBELIN, W. E. (1971) The relation of pesticides to
abortion in dairy cattle. J. Am. Vet. Med. Assoc.,
159: 1743-1748.
MAES, R. & HEYNORICKX, A. (1966) Distribution of organic chlorinated
insecticides in human tissues. Meded. Rijksfac. Landbouwwet.
Gent., 31: 1021-1025.
MAGGS, R. J., JOYNES, P. L., DAVIES, A. J., & LOVELOCK, J. E. (1971)
The electron capture detector -- A new mode of operation. Anal.
Chem., 43: 1966-1971.
MAIER-BODE, H. (1960) [DDT in body fat of man.] Med. Exp, 1: 146-152
(in German).
MARKARIAN, D. S. (1966) [Cytogenetic effects of some chlorine-
containing organic insecticides on mouse bone-marrow cell nuclei.]
Genetika, 1: 132-137 (in Russian).
MARNELOS, M. & HANNINEN, O. (1974) Enhancement of D-glucuronolactone
and acetaldehyde dehydrogenase activities in the rat liver by
inducers of drug metabolism. Biochem. Pharmacol., 23: 1457-1466.
MARSH, C. A. (1963) Metabolism of D-glucuronolactone in the mammalian
system. Biochem. J., 86: 77-86.
MASON, T. J., MCKAY, F. W., HOOVER, F., BLOT, W. J., & FRAUMENI, J. F,
JR (1975) Atlas of cancer mortality for U.S. Counties:
1950-1969, Washington, DC, US Department of Health, Education
and Welfare, Public Health Service (DHEW Publication No. (NH)
75-780).
MASUMOTO, H. T. (1972) Study of the silicic acid procedure of Armour
and Burke for separation of PCBs from DDT and its analogues.
J. Assoc. Off. Anal. Chem., 55: 1092-1100.
MATSUMURA, F. & PATIL, K. C. (1969) Adenosine triphosphatase sensitive
to DDT in synapses of rat brain. Science, 166: 121-122.
MATTSON, A. M., SPILLANE, J. T., BAKER, C., & PEARCE, G. W. (1953)
Determination of DDT and related substances in human fat. Anal.
Chem., 25: 1065-1070.
MAYERSDORF, A. & ISRAELI, R. (1974) Toxic effects of chlorinated
hydrocarbon insecticides on the human electroencephalogram. Arch.
environ. Health, 28: 159-163.
MCALISTER, D. (1879) The law of the geometric mean. Proc. R. Soc.
London B, 29: 367-376.
McCANN, J. & AMES, B. N. (1976) Detection of carcinogens as mutagens
in the Salmonella/microsome test: Assay of 300 chemicals:
Discussion. Proc. Natl Acad. Sci. USA, 73: 950-954.
McCANN, J., CHOL, E., YAMASAKI, E., & AMES, B. N. (1975) Detection of
carcinogens as mutagens in the Salmonella/microsome test: Assay
of 300 chemicals. Proc. Natl Acad. Sci. USA, 72: 5135-5139.
McFARREN, E. F., LISHKA, R. J., & PARKER, J. H. (1970) Criterion for
judging acceptability of analytical methods. Anal. Chem.,
42: 358-365.
McGILL, A. E. J. & ROBINSON, J. (1968) Organochlorine insecticide
residues in complete prepared meals: A 12-month survey in S.E.
England. Food Cosmet. Toxicol., 6: 45-57.
McKINLEY, W. P. (1963) Paper chromatography. In: Zweig, G., ed.
Analytical methods for pesticides, plant growth regulators and
food additives, New York, Academic Press, Vol. 1, pp. 227-252.
McKINNEY, J. D., BOOZIER, E. L., HOPKINS, H. P., & SUGGS, J. E. (1969)
Synthesis and reactions of a proposed DDT metabolite, 2,2'-bis
( p-chlorophenyl)acetaldehyde. Experientia (Basel),
25: 897-898.
McLACHLAN, J. A. & DIXON, R. L. (1972) Gonadal function in mice
exposed prenatally to p,p'-DDT. Toxicol. appl. Pharmacol.,
22: 327.
McMAHON, B. M. & SAWYER, L. D., ed. (1977) Pesticide analytical
manual. Volume I. Methods which detect multiple residues,
Rockville, MD, US Food and Drug Administration.
McNEIL, E. E., OTSON, R., MILES, W. F., & RAJABALEE, F. J. M. (1977)
Determination of chlorinated pesticides in potable water.
J. Chromatogr., 123: 277-286.
McQUEEN, E. G., OWEN, D., & FERRY, D. G. (1972) Effect of phenytoin
and other drugs in reducing serum DDT levels. N.Z. med. J.,
75: 208-211.
MEDVED', L. I., KAGAN, J, S., SPYNU, E. I., DLISENKO, M. A.,
ANTONOVIC, E. A., BEZUGLYJ, V. P., KRYZANOVSKAYJA, M. V., &
POL'CENKO, V. I. (1975) National review on chlorinated
pesticides, Kiev, All-Union Institute of the Ministry of Health
of the USSR for Research on the Hygiene and Toxicology of
Pesticides, Polymers, and Plastics, 74 pp.
MENDOZA, E. E. (1971) Note on the significant difference in gas-liquid
chromatographic responses to insecticide injections at fast and
slow rates. J. Chromatogr. Sci., 9 (12): 753-754.
MENZIE, C. M. (1969) Metabolism of pesticides, Washington, DC, US
Government Printing Office (Special Scientific Report, Wildlife
No. 127).
METCALF, R. L. (1955) Organic insecticides, their chemistry and mode
of action, New York, Intersciences, 392 pp.
MIDDLETON, F. M. & LICHTENBERG, J. J. (1960) Measurements of organic
contaminants in the nation's rivers. Ind. Eng. Chem.,
52: 99A-102A.
MILES, J. R. (1972) Conversion of DDT and its metabolites into
dichlorobanzophenones for analysis in presence of PCBs. J. Assoc.
Off. Anal. Chem., 55: 1039-1041.
MILES, J. W., FETZER, L. E., & PEARCE, G. W. (1970) Collection and
determination of trace quantities of pesticides in air. Environ.
Sci. Technol., 4: 420-425.
MILLER, G. J. & FOX, J. A. (1973) Chlorinated hydrocarbon pesticide
residues in Queensland human milks. Med. J. Aust., 2: 261-264.
MILLER, L. L. & NARANG, R. S. (1970) Induced photolysis of DDT.
Science, 169: 368-370.
MIZOGUCHI, M., YAMAGISHI, T., USHIO, F., FUJIMOTO, C., TAKEBA, K.,
KANI, T., HARUTA, M., YASUHARA, K., & KUBOTA, H. (1972) [On the
residual amount of organochlorine pesticides in human milk in
Tokyo metropolitan area.] Jpn J. public Health, 19: 541-544
(in Japanese).
MONTGOMERY, J. A. & STRUCK, R. F. (1973) The relation of the
metabolism of anticancer agents to their activity. Forschr.
Arzneimittelforsch., 17: 32-304.
MORGAN, D. P. & ROAN, C. C. (1969) Renal function in persons
occupationally exposed to pesticides. Arch. environ. Health,
19: 633-636.
MORGAN, D. P. & ROAN, C. C. (1970) Chlorinated hydrocarbon pesticide
residue in human tissues. Arch. environ. Health, 20: 452-457.
MORGAN, D. P. & ROAN, C. C. (1971) Absorption, storage and metabolic
conversion of ingested DDT and metabolites in man. Arch. environ.
Health, 22: 301-308.
MORGAN, D. P. & ROAN, C. C. (1972) Loss of DDT from storage in human
body fat. Nature (Load.), 238: 221-223.
MORGAN, D. P. & ROAN, C. C. (1973) Adrenocortical function in persons
occupationally exposed to pesticides. J. occup. Med., 15: 26-28.
MORGAN, D. P. & ROAN, C. C. (1974) Liver function in workers having
high tissue stores of chlorinated hydrocarbon pesticides. Arch.
environ. Health, 29: 14-17.
MORGAN, D. P., ROAN, C. C., & PASCHAL, E. H. (1972) Transport of DDT,
DDE, and dieldrin in human blood. Bull environ. Contain.
Toxicol., 8: 321-326.
MOSIER, A. R., GUENZI, W. D., & MILLER, L. L. (1969) Photochemical
decomposition of DDT in a free-radical mechanism. Science,
164: 1083-1085.
MRAK, E. M. (1969) Report of the secretary's commission on pesticides
and their relationship to environmental health, Washington, US
Government Printing Office, 677 pp.
MUGHAL, H. A. & RAHMAN, M. A. (1973) Organochlorine pesticide content
of human adipose tissue in Karachi. Arch. environ. Health, 27:
396-398.
MÜLHENS, K. (1946) [The importance of bis (chlorophenyl)
(trichloromethyl)-methane preparations as insecticides in
combating infectious diseases.] Dtsch. Med. Wochenschr.,
71: 164-169 (in German).
MÜLLER, P., ed. (1959) DDT, the insecticide dichlorodiphenyltri-
chloroethane and its significance. Basel, Switzerland, Birkhäuser
Verlag, Vol. 2.
MURPHY, P. G. (1972) Sulphuric acid for the clean-up of animal tissues
for analysis of acid-stable chlorinated hydrocarbon residues.
J. Assoc. Off. Anal Chem., 55: 360-362.
MURRAY, C. P. F. V., SAUCEDA, F. M., & NAVAREZ, G. M. (1973)
[Environmental contamination and the health of children.] Salud
Publica Mex., 15: 91-100 (in Spanish).
MUSIAL, C. J., HUTZINGER, O., ZITKO, V., & CROCKER, J. (1974) Presence
of PCB, DDE, and DDT in human milk in the provinces of New
Brunswick and Nova Scotia, Canada. Bull. environ. Contam.
Toxicol., 12: 258-267.
MUSTY, P. R. & NICKLESS, G. (1974) Use of Amberlite XAD-4 for
extraction and recovery of chlorinated hydrocarbon insecticides
and PCBs from water. J. Chromatogr., 89: 185-190.
NARVESTED, R. (1947) [Poisoning with DDT powder and some other
incidences of poisoning.] Tidsskr. Norske Laegeforen.,
67: 261-263 (in Norwegian).
NAGAI, I. (1972) [Residues of organochlorine pesticides in mother's
milk in Yamaguchi Prefecture. Annual Report of the Yamaguchi
Prefecture.] Res. Inst. Public Health, 14: 93-94 (in Japanese).
NAGASAKI, H. (1973) [Experimental studies on chronic toxicity of
benzene hexachloride (BHC).] J. Nara Med. Assoc., 24: 1-26
(in Japanese).
NAGASAKI, H., AOI, H., MAKIURA, S., AOKI, Z., ARAI, M., KONISHI, Y., &
ITO, N. (1974) [Characteristic features of hepatoma of mice due to
alpha-BHC and several factors of carcinogenesis.] Proc. Jpn
Cancer Assoc., 33: 78 (in Japanese).
NALIMOV, V. V. (1963) The application of mathematical statistics to
chemical analysis, London, Pergamon Press, 294 pp.
NARAHASHI, T. & HAAS, H. G. (1967) DDT: Interaction with nerve
membrane conductance changes. Science, 157: 1438-1440.
NARAHASHI, T. & YAMASAKI, T. (1960) Mechanism of increase in negative
after-potential by dicophanum (DDT) in the giant axon of the
cockroach. J. Physiol. (Lond.), 152: 122-140.
NARUSE, R., SASAKI, T., YAMOKA, T., MATSUOKA, K., NEGORO, Y., ITASAKA,
Y., URUSHIBATA, K., CHIN, T., KATO, K., KAKO, K., GOTO, Y.,
EGUCHI, Y., YOGO, H., TOMITA, A., & ASAI, M. (1970) [Clinical use
of o,p'-DDD in adrenal cortex cancer.] Horumon Rinsho,
18: 241-244 (in Japanese).
NAWA, H. (1973) [Studies on intoxication by organochlorine pesticides
for agricultural use. Part 2. Examination of concentration of
organochlorine pesticides in sera of healthy people and patients
with various diseases.] J. Okayama Med. Soc., 85: 47-58 (in
Japanese).
NEAL, P. A., VON OETTINGEN, W. F., SMITH, W. W., MALMO, R. B., DUNN,
R. C., MORAN, H. E., SWEENEY, T. R., ARMSTRONG, D. W., & WHITE, W.
C. (1944) Toxicity and potential dangers of aerosols, mists, and
dusting powders containing DDT. Public Health Rep., 177
(Suppl.): 1-32.
NEAL, P. A., SWEENEY, T. R., SPICER, S.S., & VON OETTINGEN, W. F.
(1946) The excretion of DDT (2,2-bis-( p-chlorophenyl)-1,1,1-
trichloroethane) in man, together with clinical observations.
Public Health Rep., 61: 403-409.
NELSON, A. A. & WOODARD, G. (1948) Adrenal cortical atrophy and liver
damage produced in dogs by feeding 2,2-bis-(parachlorophenyl)-1,1-
dichlorethane (DDD). Fed. Proc., 7: 277.
NELSON, A. A. & WOODARD, G. (1949) Severe adrenal cortical atrophy
(cytotoxic) and hepatic damage produced in dogs by feeding 2,2-
bis-(parachlorophenyl)-1,1-dichloroethane (DDD or TDE). Arch.
Pathol., 48: 387-394.
NELSON, A. A., DRAIZE, J. H., WOODARD, G., FITZHUGH, O. G., SMITH, R.
B., JR, & CALVERY, H. O. (1944) Histopathological changes
following administration of DDT in several species of animals.
Public Health Rep., 59: 1009-1020.
NELSON, J. A. (1973) Effects of DDT analogs and polychlorinated
biphenyls (PCB) mixtures on 3H-estradiol binding to rat uterine
receptor. Fed. Proc., 32: 236.
NEWTON, K. O. & GREENE, N. C. (1972) Organochlorine pesticide residue
levels in human milk -- Victoria, Australia, 1970. Pestic. Monit.
J., 6: 4-8.
NICHOLS, J., PRESTLEY, W. E., & NICHOLS, F. (1961) Effects of m,p'-
DDT in a case of adrenal cortical carcinoma. Curr. Ther. Res.,
3: 266-271.
NIKITINA, Y. I. (1974) [Course of labor and puerperium in vineyard
workers and milkmaids in the Crimea.] Gig. Tr. Prof. Zabol,
18: 17-20 (in Russian).
NISHIMOTO, T., UYETA, M., & TAUE, S. (1970) [The accumulation of
organochlorine pesticides in human adipose tissue.] Prog. Med.,
73: 275-277 (in Japanese).
NITSCHKE, U. & LINK, K. (1972) [Clinical and biochemical aspects of
hormone-producing adrenal carcinomas.] Z. Gesamite Inn. Med. Ihre
Grenzgeb., 27: 896-905 (in German).
NORTH, H. H. & MENZER, R. E. (1973) Metabolism of DDT in human
embryonic lung cell cultures. J. agric. food. Chem.,
21: 509-510.
NOVIKOVA, K. F. (1973) [Quantitative assay of organochlorine and
organophosphate pesticide residues in food products, soil, water.]
Zh. Vses. Khim. Obshchest., 18 (5): 562-570 (in Russian).
OBUSCHOWSKA, D. & PAWLOWKA-TOCHMAN, A. (1973) [The effect of the
gamma-isomer of HCH (Lindane) on the ultrastructure of the liver
cell.] Ann. Univ. Mariae Curie-Sklodowska, 28 (9): 63-66 (in
Polish).
OFNER, R. R. & CALVERY, H. O. (1945) Determination of DDT (2,2-bis-
( p-chlorophenyl)-1,1,1-trichlorethane) and its metabolite in
biological materials by use of the Schechter-Haller method.
J. Pharmacol., 85: 363-370.
O'LEARY, J. A., DAVIES, J. E., EDMUNDSON, W. F., & FELDMAN, M. (1970a)
Correlation of prematurity and DDE levels in fetal whole blood.
Am. J. Obstet. Gynecol., 106: 939.
O'LEARY, J. A., DAVIES, J. E., EDMUNDSON, W. F., & REICH, G. A.
(1970b) Transplacental passage of pesticides. Am. J. Obstet.
Gynecol., 107: 65-68.
O'LEARY, J. A., DAVIES, J. E., & FELDMAN, M. (1970c) Spontaneous
abortion and human pesticide residues of DDT and DDE. Am. J.
Obstet. Gynecol., 108: 1291-1292.
OLOFFS, C. P., HARDWICK, D. F., SZETO, S. Y., & MOERMAN, D. G. (1974)
DDT, dieldrin, and heptachlor epoxide in humans with liver
cirrhosis. Clin. Biochem., 7: 297-306.
OLSZYNA-MARZYS, A. E., DE CAMPOS, M., FARVAR, M. T., & THOMAS, M.
(1973) [Residues of chlorinated pesticides in human milk in
Guatemala.] Bol. Of. Sanit. Panam., 74: 93-107 (in Spanish).
ORTEGA, P. (1966) Light and electron microscopy of dichlorodiphenyl-
trichloroethane (DDT) poisoning in the rat liver. Lab. Invest.,
15: 657-679.
ORTEGA, P., HAYES, W. J., JR, DURHAM, W. F., & MATTSON, A. (1956) DDT
in the diet of the rat. Its effect on DDT storage, liver function
and cell morphology, Washington, DC, US Government Printing
Office, 27 pp. (Public Health Monograph No. 43, PHS Publication
No. 484).
ORTELEE, M. F. (1958) Study of men with prolonged intensive
occupational exposure to DDT. Am. Med. Assoc. Arch. indust.
Health, 18: 433-440.
OTTOBONI, A. (1969) Effect of DDT on reproduction in the rat.
Toxicol. appl. Pharmacol., 14: 74-81.
OTTOBONI, A. (1972) Effect of DDT on the reproductive lifespan in the
female rat. Toxicol. appl. Pharmacol., 22: 497-502.
OTTOBONI, A., BISSELL, G. D., & HEXTER, A. C. (1977) Effects of DDT on
reproduction in multiple generations of beagle dogs. Arch.
environ. Contam. Toxicol., 6: 83-101.
OURA, H., KOBATASHI, H., OURA, T., SENDA, I., & KUBOTA, K. (1972) [On
the pollution of human milk by PCB and organochlorine pesticides.]
J. Jpn Assoc. Rural Med., 21: 300-301 (in Japanese).
OUW, K. H. & SHANDAR, A. G. (1974) A health survey of Wee Waa
residents during 1973 aerial spraying season. Med. J. Aust.,
2: 871-873.
PACCAONELLA, B., PRATI, L., & CAVAZZINI, G. (1967) [Organochlorine
insecticides in adipose tissue of residents in Ferrara province.]
Nuovi Ann. Ig. Microbiol., 18: 17-26 (in Italian).
PALMER, K. A, GREEN, S, & LEGATOR, M. S. (1972) Cytogenetic effects of
DDT and derivatives of DDT in a cultured mammalian cell line.
Toxicol. appl. Pharmacol., 22: 355-364.
PALMER, L. & KOLMODIN-HEDMAN, B. (1972) Improved quantitative gas
chromatographic method for analysis of small quantities of
chlorinated hydrocarbon pesticides in human plasma.
J. Chromatogr., 74: 21-30.
PASCHAL, E. H., ROAN, C. C., & MORGAN, D. P. (1974) Evidence of
excretion of chlorinated hydrocarbon pesticides by the human
liver. Bull. environ. Contam. Toxicol., 12: 547-554.
PEAKALL, D. B. (1970) p,p'-DDT: Effect on calcium metabolism and
concentration of estradiol in the blood. Science, 168: 592-594.
PEARCE, G. W., MATTSON, A. M., & HAYES, W. J., JR (1952) Examination
of human fat for presence of DDT. Science, 116: 254-256.
PEARL, W. & KUPFER, D. (1971) Stimulation of dieldrin elimination by
thiouracil derivative of DDT in the rat: enhancement of dieldrin
oxidative metabolism. Chem. biol. Interactions, 4: 91-96.
PELLIZARI, E. D. (1974) Electron capture detection in gas
chromatography. J. Chromatogr., 98: 323-361.
PERAINO, C., FRY, R. J. M., & STAFFELDT, E. (1973) Enhancement of
spontaneous hepatic tumorigenesis in C3H mice by dietary
phenobarbital. J. Natl Cancer Inst., 51: 1349-1350.
PEREVODCIKOVA, N. I., PLATINSKIY, L. V., & KERSTMAN, V. I. (1972) [The
treatment of inoperable forms of malignant tumors of the adrenal
cortex with o,p'-DDD.] Vopr. Onkol., 18: 24-29 (in Russian).
PERINI, G. & GHEZZO, F. (1970) [Dichlorodiphenylacetic acid (DDA)
urinary levels in the rural population of Ferrara Province.]
L'Arcisp. S. Anna di Ferrara, 23: 3-12 (in Italian).
PESENDORFER, H., EICHLER, I., & GLOFKE, E. (1973) [Informative
analyses of organochlorine pesticide and PCB residues in human
adipose tissue (from the area of Vienna).] Wien. Klin.
Wochenschr., 85: 218-222 (in German).
PETERLE, T. J. (1969) DDT in Antarctic snow. Nature (Lond.),
224: 620.
PETERSON, J. E. & ROBINSON, W. H. (1964) Metabolic products of p,p'-
DDT in the rat. Toxicol. appl. Pharmacol, 6: 321-327.
PFEILSTICKER, K. (1973) [Pesticides in baby food.] Monatsschr.
Kinderheilkd., 121: 551-553 (in German).
PHILIPS, F. S. & GILMAN, A. (1946) Studies on the pharmacology of DDT
(2,2-bis-(parachlorophenyl)-1,1,1-trichloroethane). I. The acute
toxicity of DDT following intravenous injection in mammals with
observations on the treatment of acute DDT poisoning.
J. Pharmacol., 86: 213-221.
PHILIPS, F. S., GILMAN, A., & CRESCITELLI, F. N. (1946) Studies on the
pharmacology of DDT (2,2-bis-(parachlorophenyl)-1,1,1-
trichloroethane). II. The sensitization of the myocardium to
sympathetic stimulation during acute DDT intoxication.
J. Pharmacol., 86: 222-228.
PIONKE, H. B., KONRAD, J. C., CHESTER, G., & ARMSTRONG, D.E. (1968)
Extraction of organochlorine and organophosphate insecticides from
lake water. Analyst, 93: 363-367.
PLATONOVA, V. I. (1970) [Disturbances of the functional conditions of
the stomach with prolonged effect on the body of some
organochlorine pesticides.] Gig. Tr. Resp. Mezhved, 3B,
6: 142-147 (in Russian).
PLIMMER, J. R. & KLINGEBIEL, U. I. (1973) PCB-formation. Science,
181: 994-995.
PLIMMER, J. R, KLINGEBIEL, U. I., & HUMMER, B.E. (1970) Photooxidation
of DDT and DDE. Science, 167: 67-69.
POLAND, A., SMITH, D., KUNTZMAN, R., JACOBSON, M., & CONNEY, A. H.
(1970) Effect of intensive occupational exposure to DDT an
phenylbutazone and cortisol metabolism in human subjects. Clin.
Pharmacol. Ther., 11: 724-732.
POLISHUK, Z. W., WASSERMANN, M., WASSERMANN, D., GRONER, Y.,
LAZAROVICI, S., & TOMATIS, L. (1970) Effects of pregnancy on
storage of organochlorine insecticides. Arch. environ. Health,
20: 215-217.
POLISHUK, Z. W., RUN, M., WASSERMANN, M., CUCOS, S., WASSERMANN, D., &
LEMESCH, C. (1977) Organochlorine compounds in human blood plasma
and milk. Pestic. Monit. J., 10: 121-129.
PONOMARKOV, V., TOMATIS, L., & TURUSOV, V. (1976) The effect of long-
term administration of phenobarbitone in CF-1 mice. Cancer Lett.,
1: 165-172.
POTT, P. (1775) Chirurgical observations relative to the cataract,
the polypus of the nose, the cancer of the scrotum, etc. London,
T. F. Carnegy, 208 pp.
POTT, P. (1790) The chirurgical works of Percival Pott, F.R.S.
surgeon to St. Bartholomew's Hospital, A new edition with his
last corrections, London, J. Johnson, 3 vol.
PRATI, L. & DEL DOT, M. (1971) [Research on the levels of accumulation
of synthetic organic chloride pesticides, in human adipose tissues
in the province of Trento.] L'Ig. Mod., 64: 35-43 (in Italian).
PRATI, L., PAVANELLO, R., & GHEZZO, F. (1972) Storage of chlorinated
pesticides in human organs and tissues in Ferrara Province, Italy.
Bull. World Health Organ., 46: 363-369.
PROUTY, R. M. & CROMARTIE, E. (1970) Stepwise recovery of DDT and
dieldrin from tissues of Coturnix japonica (quail) during residue
analysis. Environ. Sci. Technol., 4: 768-769.
QUINBY, G. E., HAYES, W. J., JR, ARMSTRONG, J. F., & DURHAM, W. F.
(1965a) DDT storage in the US population. J. Am. Med. Assoc.,
191: 175-179.
QUINBY, G. E., ARMSTRONG, J. F., & DURHAM, W. F. (1965b) DDT in human
milk. Nature (Lond.), 207: 726-728.
RABELLO, M. N., BECAK, W., ALMEIDA, W. F., PIGATI, P., UNGARO, M. T.,
MURATA, T., & PEREIRA, C. A. B. (1975) Cytogenetic study on
individuals occupationally exposed to DDT. Mutat. Res.,
28: 449-454.
RADOMSKI, J. L., DEICHMANN, W. B., & CLIZER, E. E. (1968) Pesticide
concentrations in the liver, brain, and adipose tissue of terminal
hospital patients. Food Cosmet. Toxicol, 6: 209-220.
RADOMSKI, J. L., DEICHMANN, W. B., REY, A. A., & MERKIN, T. (1971a)
Human pesticide blood levels as a measure of body burden and
pesticide exposure. Toxicol. appl. Pharmacol., 20: 175-185.
RADOMSKI, J. L, ASTOLFI, E., DEICHMANN, W. B., & REY, A. A. (1971b)
Blood levels of organochlorine pesticides in Argentina:
Occupationally and nonoccupationally exposed adults, children and
newborn infants. Toxicol. appl. Pharmacol., 20: 186-193.
RAMACHANDRAN, M., SHARMA, M. I. D., SHARMA, S.C., MATHUR, P.S.,
ARAVINDAKSHAN, A., & EDWARD, G. J. (1974) DDT and its metabolites
in the body fat of Indians. J. commun. Dis., 6: 256-259.
RANTANEN, T., MARSELOS, M., & PUHAKAINEN, E. (1975) Effects of 1,1,1-
trichloro-2,2-bis-( p-chlorophenyl)ethane (DDT) administration on
the glucuronic acid pathway in the rat liver. Environ. Qual.
Saf., 3 (Suppl.): 494-499.
RAPPOLT, R. T. (1970) Use of oral DDT in three human barbiturate
intoxications: CNS arousal and/or hepatic enzyme induction by
reciprocal detoxicants. Ind. Med. Surg., 39: 319.
READ, S. T. & MCKINLEY, W. P. (1961) DDT and DDE content of human fat.
A survey. Arch. environ. Health, 5: 209-211.
REIF, V. D. & SINSHEIMER, J. E. (1975) Metabolism of 1-( o-
chlorophenyl)-1-( p-chlorophenyl)-2-2-dichloroethane ( o,p'-DDD)
in rats Drug Metab. Dispos., 3: 15-25.
REIF, V. D., SINSHEIMER, J. E., WARD, J. C., & SCHTEINGART, D. E. J.
(1974). Aromatic hydroxylation and alkyl oxidation in metabolism
of mitotane ( o,p'-DDD) in humans. Pharmacol. Sci.,
63: 1730-1736.
REINGOLD, I. M. & LASKY, I. I. (1947) Acute fatal poisoning following
ingestion of a solution of DDT. Ann. intern. Med., 26: 945-947.
REYNOLDS, L. M. (1969) Polychlorinated biphenyls (PCBs) and their
interference with pesticide residue analysis. Bull. environ.
Contam. Toxicol., 4: 128-143.
RICHARDSON, A., ROBINSON, J., CRABTREE, N., & BALDWIN, M. K. (1971)
Residues in polychlorobiphenyls in biological samples. Pestic.
Monit. J., 4: 169-176.
RICHARDSON, J. A., KEIL, J. E., & SANDIFER, S. H. (1975) Catecholamine
metabolism in humans exposed to pesticides. Environ. Res.,
9: 290-294.
RISEBROUGH, R. W., HUGGETT, R. J., GRIFFIN, J. J., & GOLDBERG, E. D.
(1968) Pesticides: Transatlantic movements in the northeast
trades. Science, 159: 1233-1235.
RITCEY, W. R., SAVARY, G., & McCULLY, J. A. (1972) Organochlorine
insecticide residues in human milk, evaporated milk, and some milk
substitutes in Canada. Can. J. public Health, 63: 125-132.
RITCEY, W. R., SAVARY, G., & McCULLY, J. A. (1973) Organochlorine
insecticide residues in human adipose tissue of Canadians. Can.
J. public Health, 64: 380-386.
RIZOV, N. A. (1977) [Organochlorine pesticides in the fat and blood
of the general population of Bulgaria and methods for their
determination.] Dissertation. Sofia, Inst. Hygiene and
Occupational Health, Academy of Medicine, 117 pp. (in Bulgarian).
ROBINSON, J., RICHARDSON, A., & ELGAR, K. E. (1966) Chemical identity
in ultramicroanalysis, New York, American Chemical Society
(abstracts).
ROBINSON, J., RICHARDSON, A., HUNTER, C. G., CRABTREE, A. N., & REES,
H. J. (1965) Organochlorine insecticide content of human adipose
tissue in Southeastern England. Br. J. ind. Med., 22: 220-229.
ROSEN, A. A. & MIDDLETON, F. M. (1959) Chlorinated insecticides in
surface waters. Anal. Chem., 31: 1729-1732.
ROSSI, R., RAVERA, M., REPETTI, G., & SANTI, L. (1977) Long-term
administration of DDT or phenobarbital-Na in Wistar rats. Int. J.
Cancer, 19: 179-185.
ROTHE, C. F., MATTSON, A. M., NUESLEIN, R. M., & HAYES, W. J., JR
(1957) Metabolism of chlorophenothane (DDT): Intestinal lymphatic
absorption. Am. Med. Assoc. Arch. ind. Health, 16: 82-86.
ROWE, D. R., CANTER, L. W., SNYDER, P. J., & MASON, J. W. (1971)
Dieldrin and endrin concentrations in a Lousiana estuary. Pestic.
Monit. J., 4: 177-183.
RUDD, R. L. & HERMAN, S. G. (1972) Ecosystemic transferal of
pesticides residues in an aquatic environment. In: Matsumura, F.,
Boush, G. M., Misato, T., ed. Environmental toxicology of
pesticides, Part VII, New York, Academic Press.
RYBAKOVA, M. N. (1968) [The effect of certain pesticides on the
hypophysis and its gonadotrophic function.] Gig. i Sanit.,
33: 27-31 (in Russian).
SABAD, L. M., KOLESNICENKO, T. S., & NIKONOVA, T. V. (1972) [On a
possible blastomogenicity of DDT.] Vopr. Pitan., 30: 63-66
(in Russian).
SALIHODZAEV, S. S. & FERSTAT, V. N. (1972) [Condition of the olfactory
analyzer under the effect of organochlorine and organophosphorus
pesticides.] Gig. i Sanit., 37: 95-96 (in Russian).
SANDIFER, S. H., KEIL, J. E., FINKLEA, J. F., & GADSDEN, R. H. (1972)
Pesticide effects on occupationally exposed workers: A summary of
four years observation of industry and farm volunteers in South
Carolina. Ind. Med. Surg., 41: 7-12.
SARETT, H. P. & JANDORF, B. J. (1947) Effect of chronic DDT
intoxication in rats on lipids and other constituents of the
liver. J. Pharmacol., 91: 340-344.
SAUBERLICH, H. E. & BAUMANN, C. A. (1947) Effect of dietary variation
upon the toxicity of DDT to rats or mice. Proc. Sec. Exp. Biol.
Med., 66: 642-645.
SAVAGE, E. P., TESSARI, J. D., MALBERG, J. W., WHEELER, H. W., &
BAGBY, J. R. (1973) Organochlorine pesticide residues and
polychlorinated biphenyls in human milk, Colorado 1971-1972.
Pestic. Monit. J., 7: 1-5.
SAWYER, I. D. (1973) Collaborative study of the recovery and gas
chromatographic quantitation of PCBs in chicken fat and PCB-DDT
combinations in fish. J. Assoc. Off. Anal. Chem., 56: 1015-1023.
SCHAEFER, R. G. (1974) [Mass spectrometric identification and
quantification of chlorinated hydrocarbons in fish.] Chem. Ztg.,
98: 241-247 (in German).
SCHAFER, M. L. & CAMPBELL, J. E. (1966) Distribution of pesticide
residues in human body tissues from Montgomery County, Ohio,
Washington, DC, American Chemical Society, pp. 89-98 (Adv. Chem.
Ser. No. 60).
SCHMICK, M. (1973) Survival with adrenal carcinoma. J. Am. Med.
Assoc., 224: 1763.
SCHMIDT, R. (1973) [Effect of 1,1,1-trichloro-2,2-bis( p-
chlorophenyl) ethane (DDT) on the prenatal development of the
mouse, under consideration of distribution of tritium-labeled and
carbon-14-labeled DDT in pregnant mice.] Biol. Runtisch.,
11: 316-317 (in German).
SCHOOR, W. P. (1970) Effect of anticonvulsant drugs on insecticide
residues. Lancet, 2: 520-521.
SCHROEDER, G. & DOROZALSKA. (1975) [Degradation of DDT and its
analogues.] Wiad. Chem., 29: 553-565 (in Polish).
SCHVARTSMAN, S., ALMEIDA, W. F., COSTA VAZ, F. A., CORRADINI, H. B.,
PIGATI, P., GAETA, R., & UNGARO, M. T. (1974) Blood levels of DDT
in nonoccupationally exposed mothers and newborn infants in a city
in Brazil. In: Coulston, F. & Korte, F., ed. EQS Environmental
Quality and Safety, New York, Academic Press, Vol. 3,
pp. 154-156.
SCHWABE, U. & WENDLING, I. (1967) [Acceleration of drug metabolism by
small doses of DDT and other chlorinated hydrocarbon
insecticides.] Arzneim. Forsch., 17: 614-618 (in German).
SCOTT, R. P. W. (1970) Determination of the optimum conditions to
effect a separation by gas chromatography. Adv. Chromatogr.,
9: 193-214.
SCUDDER, H. I. & TARZWELL, C. M. (1950) Effects of DDT mosquito
larviciding on wildlife. IV. The effects on terrestrial insect
population of routine larviciding by airplane. Public Health
Rep., 65: 71-87.
SELBY, L. A., NEWELL, K. W., HAUSER, G. A., & JUNKER, G. (1969)
Comparison of chlorinated hydrocarbon pesticides in maternal blood
and placental tissues. Environ. Res., 2: 247-255.
SELL, J. L., DAVISON, K. L., & PUYEAR, R. L. (1971) Aniline
hydroxylase, N-demethylase, and cytochrome P-450 in liver
microsomes of hens fed DDT and dieldrin. J. agile. food Chem.,
19: 58-60.
SEMENCAEVA, E. M. (1968) [The mechanism of action of DDT and certain
other pesticides on the immunological response of the body.] In:
Medved', L. E., ed. The hygiene of application of the toxicology
of pesticides and the clinical features of pesticides poisoning.
Scientific Conference on the Safe Use & Toxicology of Pesticides,
11-14 June, 1968, Issue No. 6, Kiev, Acad. of Sciences, USSR,
pp. 805-815 (in Russian).
SHARAHGAPANI, M. V. & PINGALE, S. V. (1954) The hazard of DDT-
treatment of potatoes. Bull. Cent. Food Technol. Res. Inst.
Mysore, 4: 57.
SHERMA, J. (1973) Thin-layer chromatography: Recent advances. In:
Zweig, G., ed. Analytical methods for pesticides and plant growth
regulators, New York, Academic Press, Vol. VIII, pp. 3-87.
SHIRASU, Y., MORIYA, M., KATO, K., FURUHASHI, A., & KADA, T. (1976)
Mutagenicity screening of pesticides in the microbial system.
Mutat. Res., 40: 19-30.
SHIRASU, Y., MORIYA, M., KATO, K., LIENARD, F., TEZUKA, H., TERAMOTO,
S., & KADA, T. (1977) Mutagenicity screening on pesticides and
modification products: A basis of carcinogenicity evaluation. In:
Hiatt, H. H., Watson, J. D., & Winsten, J. A., ed. Origins of
human cancer, Book A, Incidence of cancer in humans, Cold Spring
Harbor, NY, Cold Spring Harbor Laboratory, Vol. 4, pp. 267-285.
SIERWISKI, M. & HELRICH, K. (1967) Separation, identification and
measurement of DDT and its metabolites. J. Assoc. Off. Anal.
Chem., 50: 627-633.
SIMMONS, S. W. (1959) The use of DDT insecticides in human medicine.
In: Müller, P., ed. DDT, the insecticide dichlorodiphenyltri-
chloroethane and its significance, Basel, Switzerland, Birkhäuser
Verlag, Vol. II, pp. 251-502.
SINGHAL, R. L. & KACEW, S. (1976) The role of cyclic AMP in
chlorinated hydrocarbon-induced toxicity. Fed. Proc.,
35: 2618-2623.
SISSONS, D. J., TELLING, G. M., & USHER, C. D. (1968) A rapid and
sensitive procedure for routine determination of organochlorine
pesticide residues in vegetables. J. Chromatogr., 33: 435-449.
SIYALI, D. S. (1972) Hexachlorobenzene and other organochlorine
pesticides in human blood. Med. J. Aust, 2: 1063-1066.
SIYALI, D. S. (1973) Polychlorinated hiphenyls, hexachlorobenzene and
other organochlorine pesticides in human milk. Med. J. Aust,
2: 815-818.
SIZONENKO, P. C., DORET, A. M., RIONDEL, A. M., & PAUNIER, L. (1974)
Cushing's syndrome due to bilateral adrenal cortical hyperplasia
in a 13-year-old girl: Successful treatment with o,p'-DDD.
Helv. Paediatr. Acta, 29: 195-202.
SKOGERBOE, R. K. & GRANT, C. L. (1970) Comments on the definitions of
the terms sensitivity and detection limit. Spectrosc. Lett.,
3: 215-220.
SKRENTNY, R. F. & DOROUGH, H. W. (1971) Efficiency of several
extraction procedures in removing organochlorine insecticides from
tobacco. Tobacco Sci., 15: 111-113.
SMART, N. A., HILL A. R. C., & ROUGHAN, P. A. (1974) Chlorinated
hydrocarbon pesticides residues in foodstuffs: Comparison of
methods. J. Assoc. Off. Anal. Chem., 57: 153-164.
SMITH, D. C. (1971) Pesticide residues in the total diet in Canada.
Pestic. Sci., 2: 92-95.
SMITH, D. C., SAUDI, E., & LEDUC, R. (1972) Pesticide residues in the
total diet in Canada. II-1970. Pestic. Sci., 3: 207-210.
SMITH, D. C., LEDUC, R., & CHARBONNEAU, C. (1973) Pesticide residues
in the total diet in Canada. III-1971. Pestic. Sci., 4: 211-214.
SMITH, D. C., LEDUC, R., & TREMBLAY, L. (1975) Pesticide residues in
the total diet in Canada. IV-1972 and 1973. Pestic. Sci.,
6: 75-82.
SMITH, M. I. & STOHLMAN, E. F. (1944) The pharmacologic action of 2,2-
bis-( p-chlorophenyl)-1,1,1-trichlorethane and its estimation in
the tissues and body fluids. Public Health Rep., 59: 984-993.
SMITH, M. I. & STOHLMAN, E. F. (1945) Further studies on the
pharmacologic action of 2,2-bis-( p-chlorophenyl)-1,1,1-
trichloroethane (DDT). Public Health Rep., 60: 289-301.
SMITH, M. I., BAUER, H., STOHLMAN, E. F., & LILLIE, R. D. (1946) The
pharmacologic action of certain analogues and derivatives of DDT.
J. Pharmacol., 88: 359-365.
SNYDER, D. & REINERT, R. (1971) Rapid separation of PCBs from DDT and
its analogues on silica gel. Bull. environ. Contam. Toxicol.,
6: 385-390.
SOUTHERN, A. L., TOCHIMOTO, S., ISURUGI, K., GORCHER, G. G., KRIKUN,
E., & STYPULKOWSKI, W. (1966a) The effect of 2,2-bis-(2-
chlorophenyl-4-chlorophenyl)-1,1,1-dichloroethane ( o,p'-DDD) on
the metabolism of infused cortisol-7-H. Steroids, 7: 11-29.
SOUTHERN, A. L., TOCHIMOTO, S., STROM, L., RATUSCHINI, A., RASS, H., &
GORCHER, G. (1966b) Remission in Cushing's syndrome with o,p'-
DDD. J. clin. Endocrinol. Metab., 26: 268-278.
SPICER, S. S., SWEENEY, T. R., VON OETTINGEN, W. F., LILLIE, R. D., &
NEAL, P. A. (1947) Toxicological observations on goats fed large
doses of DDT. Vet. Med., 42: 289-293.
STACY, G. I. & THOMAS, B. W. (1975) Organochlorine pesticide residues
in human milk, Western Australia-1970-71. Pestic. Monit. J.,
9: 64-66.
STANELY, C. W., BARNEY, J. E., HELTON, M. R., & YOBS, A. R. (1971)
Measurement of atmospheric levels of pesticides. Environ. Sci.
Technol., 5: 431-435.
STARR, M. G., ALDRICH, F. D., McDOUGALL, W. D., III, & MOUNCE, L. M.
(1974) Contribution of household dust to human exposure to
pesticides. Pestic. Monit. J., 8: 209-212.
STEC, J. & JUSZKIEWICZ, T. (1972) [Determination of polychlorinated
insecticide residues in animal products using Woods methods.]
Med. Weter., 28: 634-637 (in Polish).
STENBERG, A. I. & DIKY, V. V. (1973) [Changes in the metabolism of DDT
deposited in the omentum of fasting albino rats.] Vopr. Pitan.,
32; 39-43 (in Russian).
STIJVE, T. & CARDINALE, E. (1974) [Rapid determination of chlorinated
pesticides, PCBs, and a number of phosphated insecticides in fatty
foods.] Mitt. Lebensmittelunters. Hyg., 65: 131-150 (in German).
STOHLMAN, E. F. & LILLIE, R. D. (1948) The effect of DDT on the blood
sugar and of glucose administration on the acute and chronic
poisoning of DDT in rabbits. J. Pharmacol., 93: 351-361.
STRASSMANN, S.C. & KUTZ, F. W. (1977) Insecticide residues in human
milk from Arkansas and Mississippi, 1973-74. Pestic. Monit. J.,
10: 130-133.
STRAW, J. A., WATERS, I. W., & FREQLY, M. J. (1965) Effect of o,p'-
DDD on hepatic metabolism of phenobarbital in rat. Proc. Exp.
Biol. Med., 118: 391-394.
STREET, J. C. (1964) DDT antagonism to dieldrin storage in adipose
tissue of rats. Science, 146: 1580-1581.
STREET, J. C. & CHADWICK, R. W. (1967) Stimulation of dieldrin
metabolism by DDT. Toxicol. appl. Pharmacol., 11: 68-71.
STREET, J. C., WANG, M., & BLAU, A. D. (1966) Drug effects on dieldrin
storage in rat tissue. Bull. environ. Contam. Toxicol., 1: 6-15.
STREET, J. C., MAYER, F. L., & WAGSTAFF, D. J. (1969) Ecological
significance of pesticide interactions. Ind. Med. Surg,
38: 409-414.
STRETZ, P. E. & STARR, H. M. (1973) Determination of chlorinated
pesticides in whole blood. J. Assoc. Off. Anal Chem.,
56: 1173-1177.
STUDY GROUP OF MINISTRY OF HEALTH-WELFARE FOR ORGANOCHLORINE PESTICIDE
RESIDUES IN MOTHERS' MILK (1972) VIII. [Organochlorine pesticide
residues in blood during lactation.] J. Food Hyg. Soc. Jpn,
13: 438-450 (in Japanese).
SUGAYA, T. et al. (1971) [Organochlorine pesticide residues in human
milk.] J. Jpn Assoc. Rural Med., 19: 379-380 (in Japanese).
SUNDSTRÖM, G. (1977). Metabolic hydroxylation of the aromatic rings of
1,1-dichloro-2,2-bis(4-chlorophenyl)ethylene ( p,p'-DDE) by the
rat. J. agric. food Chem., 25: 18-21.
SUNDSTRÖM, G., JANSSON, B., & JENSEN, S. (1975) Structure of phenolic
metabolites of p,p'-DDE in rat, wild seal and guillemot. Nature
(Lond.), 255: 627-628.
SURBER, E. W. & FRIDDLE, D. D. (1949) Relative toxicity of suspension
and oil formulations of DDT to native fishes in Black Creek, West
Virginia. Trans. Am. Fish. Soc., 76: 315-321.
SUTHERLAND, G. L. (1965) Residue analytical limits of detectability.
Residue Rev., 10: 85-96.
SUVAK, L. N. (1970) [DDT in mothers' milk.] Zdravoochr. Kosinev,
13: 19-21 (in Russian).
SUZUKI, M., YAMATO, Y., & WATANABE, T. (1975) Gas chromatographic
resolution of organochlorine insecticides on OV-1/OV-17,
OV-210/OV-17 and OV-225/OV-17 mixed phase systems. J. Assoc. Off.
Anal. Chem., 58: 297-300.
SUZUKI, Y., SUGAYA, H., & SASAKI, S. (1973) [Results of 3 years'
tracing investigation of organochlorine pesticide residues in
humans.] J. Jpn Assoc. Med., 22: 276-277 (in Japanese).
SWACKHAMER, A.B. (1965) Report on pesticide residues in restaurant
meals in Canada. Pestic. Prog., 3: 108-114.
TABOR, E. C. (1966) Pesticides in urban atmospheres. Contamination of
urban air through the use of insecticides. Trans. NY Acad. Sci.,
28: 569-578.
TAIRA, M., HASHIMOTO, S., & SHIMOHIRA, I. (1972) [Organochlorine
pesticide residues in human breast milk.] Bull. Hyogo Prefect.
Public Health Lab., 7: 19-23 (in Japanese).
TAKANO, H. (1972) [Results of inspection of pesticide residues in
mothers' milk and market milk.] Annu. Rep. Iwate Inst. of Public
Health, 15: 88-91 (in Japanese).
TAKESHITA, T. & INUYAMA, Y. (1970) [Organochlorine residues in
mothers' milk and blood.] Annu. Rep. Shimane Prefect. Inst.
Public Health, 12: 27-28 (in Japanese).
TARJAN, R. & KEMENY, T. (1969) Multigeneration studies on DDT in mice.
Food Cosmet. Toxicol., 7: 215-222.
TARRANT, K. R. & TATTON, J. O'G. (1968) Organochlorine pesticides in
rainwater in the British Isles. Nature (Lond.), 219: 725-727.
TARZWELL, C. M. (1950) Effects of DDT mosquito larviciding on
wildlife. V. Effects on fishes of the routine manual and airplane
application of DDT and other mosquito larvicides. Public Health
Rep., 65: 231-255.
TAYLOR, R. & BOGACKA, T. (1968) [Determination of DDT, methoxychlor or
lindane in water by extraction and thin-layer chromatography.]
Chem. Anal., 13: 227-234 (in Polish).
TELFORD, H. S. & GUTHRIE, J. E. (1945) Transmission of the toxicity of
DDT through the milk of white rats and goats. Science, 102: 647.
TERRACINI, B., TESTA, M. C., CABRAL, J. R., & DAY, N. (1973) The
effects of long-term feeding of DDT to BALB/c mice. Int. J.
Cancer, 11: 747-764.
THOMAS, E. J., BURKE, J. A., & LAWRENCE, J. H. (1968) Thin-layer
chromatography; relative migration date (R-TDE) of chlorinated
pesticides. J. Chromatogr, 35: 119-121.
THOMAS, T. C. & SERBER, J. N. (1974) Chromosorb 102, an efficient
medium for trapping pesticides from air. Bull. environ. Contam.
Toxicol., 12: 17-25.
THOMPSON, J. F., ed. (1974) Analysis of pesticide residues in human
and environmental samples, a compilation of methods selected for
use in pesticide monitoring programs, Research Triangle Park,
NC, US Environmental Protection Agency, 1 vol.
THOMPSON, J. F., WALKER, A. C., & MOSEMAN, R. F. (1969a) Evaluation of
8 gas chromatographic columns for chlorinated pesticides.
J. Assoc. Off. Anal. Chem., 52: 1263-1277.
THOMPSON, J. F., WALKER, A. C., & MOSEMAN, R. F. (1969b) Study of the
performance of gas-chromatographic columns under severe injection
loading. J. Assoc. Off. Anal. Chem., 52: 1251-1262.
THOMPSON, J. F., MANN, J. B., APODACA, A. O., & KANTOR, E. J. (1975)
Relative retention ratios of 95 pesticides and metabolites on nine
gas-liquid chromatographic columns over a temperature range of
170-204°C on two detection modes. J. Assoc. Off. Anal. Chem., 58:
1037-1050.
THOMPSON, R. P. H., PILCHER, C. W. T., ROBINSON, J., STATHERS, G. M.,
MCLEAN, A. E. M., & WILLIAMS, R. (1969) Treatment of unconjugated
jaundice with dicophane. Lancet, 2: 4-6.
THORPE, E. & WALKER, A. I. T. (1973) The toxicology of dieldrin
(HEOD). II. Comparative long-term oral toxicity studies in mice
with dieldrin, DDT, phenobarbitone, ß-BHC, and gamma-BHC. Food
Cosmet. Toxicol., 11: 433-442.
TINSLEY, I. J. & LOWRY, R. R. (1972) An interaction of DDT in the
metabolism of essential fatty acids. Lipids, 7: 182-185.
TOCCI, P.M., MANN, J. B., DAVIES, J. E., & EDMUNDSON, W. F. (1969)
Biochemical differences found in persons chronically exposed to
high levels of pesticides. Ind. Med. Surg., 38: 188-195.
TOKUTSU, K., KOYAMA, T., & TOKOYAMA, T. (1970) [Pesticide residues in
mothers' milk and plasma in Wakayama Prefecture.] Annu. Rep.
Wakayama Prefect. Inst. Public Health, 19: 59-62 (in Japanese).
TOMATIS, L. & TURUSOV, V. (1975) Studies on the carcinogenicity of
DDT. GANN Monograph Cancer Res., 17: 219-241.
TOMATIS, L., TUROSOV, V., DAY, N., & CHARLES, R. T. (1972) The effect
of long-term exposure to DDT on CF-1 mice. Int. J. Cancer,
10: 489-506.
TOMATIS, L., PARTENSKY, C., & MONTESANO. R. (1973) The predictive
value of mouse liver tumor induction in carcinogenicity testing --
A literature survey. Int. J. Cancer, 12: 1-20.
TOMATIS, L., TURUSOV, V., CHARLES, R. T., & BOICCHI, M. (1974a).
Effect of long-term exposure to 1,1-dichloro-2,2-bis( p-
chlorophenyl)ethylene, 1,1-dichloro-2,2-bis( p-chlorophenyl)
ethane, and to the two chemicals combined on CF-1 mice. J. Natl
Cancer Inst., 52: 883-891.
TOMATIS, L., TURUSOV, V., CHARLES, R. T., BOICCHI, M., & GATI, F.
(1974b) Liver tumors in CF-1 mice exposed for limited periods to
technical DDT. Z. Krebs. Forsch., 82: 25-35.
TOMII, S., NAGASAKI, H., & MEGA, Y. (1972) [Studies on BHC as a
carcinogen.] Jpn. J. Hyg., 27: 113 (in Japanese).
TORDA, C. & WOLFF, H. G. (1949) Effect of convulsant and
anticonvulsant agents on the activity of carbonic anhydrase.
J. Pharmacol. exp. Ther., 95: 444-447.
TREON, J. F. & CLEVELAND, F. P. (1955) Toxicity of certain chlorinated
hydrocarbon insecticides for laboratory animals with special
reference to aldrin and dieldrin. J. agric. food Chem.,
3: 402-408.
TSUTSUI, J., KATO, T., & NISHIKAWA, T. (1974) [Results of health
survey on persons engaging in pesticide application in Suzuka
area. Mie Prefecture.] J. Jpn. Assoc. Rural Med., 23: 518-521
(in Japanese).
TUINSTRA, L. G. M. TH. (1971) [Organochlorine insecticide residues in
human milk in the Leiden Region.] Ned. Melk-Zuiveltijdschr.,
25: 24-32 (in Dutch).
UNTERMAN, H. W. & SIRGHIE, E. (1969) [Presence of organochlorines in
human body.] 18: 221-226 (in Romanian).
US DEPT OF AGRICULTURE, AGRICULTURAL RESEARCH ADMINISTRATION (1949)
USDA entomologists recommend substitute insecticide for DDT to
control insects on dairy cattle and in dairy barns. News
Release, March 24.
U.S. DEPT OF AGRICULTURE, AGRICULTURAL RESEARCH SERVICE (1966)
Monitoring agricultural pesticide residues. A preliminary report
of studies on soil, sediment and water in Mississippi River
delta, Washington, DC, US Government Printing Office.
US DEPT OF AGRICULTURE, BUREAU OF ENTOMOLOGY AND PLANT QUARANTINE
(1945a) Release of February 13, 1945.
US DEPT OF AGRICULTURE, BUREAU OF ENTOMOLOGY AND PLANT QUARANTINE
(1945b) Outlook for supplies of DDT insecticides. Release of
August 6, 1945.
US DEPT OF HEALTH EDUCATION AND WELFARE (1969) Manual for evaluating
public drinking water supplies. A manual of practice recommended
by the Public Health Service, Washington, DC, US Government
Printing Office, I (PHS Pub. No. 1820).
US ENVIRONMENTAL PROTECTION AGENCY (1975) DDT. A review of scientific
and economic aspects of the decision to ban its use as a
pesticide, Washington US EPA, US Government Printing Office,
300 pp. (EPA-504-1-75-022).
VAARAMA, A. (1947) The influence of DDT pesticides upon plant mitosis.
Hereditas, 33: 191-219.
VAINO, H. (1974) Enhancement of hepatic microsomal drug oxidation and
glucuronidation in rat by 1,1,1-trichloro-2,2-bis( p-
chlorophenyl)ethane (DDT). Chem. biol. Interactions, 9: 7-14.
VAINIO, H. (1975) Stimulation of microsomal drug-metabolizing enzymes
in rat liver by 1,1,1-trichloro-2,2-bis( p-chlorophenyl)ethane
(DDT), pregnenolone-16alpha-carbonitrile (PEN), and
polychlorinated biphenyls (PCBs). Environ. Qual. Saf.,
3 (Suppl.): 486-490.
VANAT, S. V. & VANAT, I. M. (1971) [Contribution to the toxic-allergic
reaction induced by DDT.] Klin. Med. (Mosk.), 49: 126-127
(in Russian).
VAS'KOVSKAJA & KOMAROVA (1967) Quoted by: Kagan et al., Res. Rev, 27:
43-79.
VAS'KOVSKAJA, L. F. (1969) [Accumulation of certain chloroorganic
insecticides in the bodies of experimental animals and humans.]
In: Medved', L. E., ed. The hygiene of application and the
toxicology of pesticides and the clinical features of pesticide
poisoning. A collection of papers. Issue No. 7, Kiev,
Vniggintox, pp. 503-796 (in Russian).
VAZ A., PEREIRA, R. S., & MALHETRO, D. M. (1945) Calcium in prevention
and treatment of experimental DDT poisoning. Science,
101: 434-436.
VELBINGER, H. H. (1947a) [On the question of DDT toxicity to man.]
Dtsch. Gesundheitwes., 2: 355-358 (in German).
VELBINGER, H. H. (1947b) [Contribution to the toxicology of "DDT"-
agent, dichlorodiphenyl-trichloromethylmethane.] Pharmazie,
2: 268-274 (in German).
VERDON, T. A., JR, BRUTON, J., HERMAN, R. H., & BEISEL, W. R. (1962)
Clinical and chemical response of functioning adrenal cortical
carcinoma to ortho-, para-DDD. Metab. Clin. Exp., 11: 226-234.
VERSINO, B., VENNE, M-TH VAN DER, & VISSERS, H. (1971) Comparison of
some clean-up columns for residue analysis of chlorine and
phosphorus containing pesticides. J. Assoc. Off. Anal Chem.,
54: 147-149.
VON OETTINGEN, W. F. & SHARPLESS, N. E. (1946) The toxicity and toxic
manifestations of 2,2-bis-( p-chlorophenyl)-1,1,1-trichloroethane
(DDT) as influenced by chemical changes in the molecule. A
contribution to the relation between chemical constitution and
toxicological action. J. Pharmacol. exp. Ther., 88: 400-413.
WAGSTAFF, D. J. (1971) Ascorbic acid deficiency and induction of
hepatic microsomal hydroxylative enzymes by organochlorine
pesticides. Toxicol. appl. Pharmacol., 19: 10-19.
WAGSTAFF, D. J. & STREET, J. C. (1970) Dieldrin-DDT interactions in
guinea pigs. Toxicol. appl. Pharmacol., 17: 276.
WALKER, A. I. T., THORPE, E., & STEVENSON, D. E. (1973) The toxicology
of dieldrin (HEOD). I. Long-term oral toxicity studies in mice.
Food Cosmet. Toxicol., 11: 415-432.
WALKER, K. C., GOETTE, M. B., & BATCHELOR, G. S. (1954) Pesticide
residues in foods: Dichlorodiphenyltrichloroethane and
dichlorodiphenyldichloroethylene content of prepared meals.
J. agric. food. Chem., 2: 1034-1037.
WALLACE, Z. E., SILVERSTEIN, J. N., VILLADOLID, L. S., WEISENFELD, S.
(1961) Cushing's syndrome due to adrenocortical hyperplasia. New
Engl. J. Med., 265: 1088-1093.
WARNICK, S. L. (1972) Organochlorine pesticide levels in human serum
and adipose tissue, Utah-Fiscal years 1967-71. Pestic. Monit. J.,
6: 9-13.
WARNICK, S. L. & CARTER, J. E. (1972) Some findings in a study of
workers occupationally exposed to pesticides. Arch. environ.
Health, 25: 265-270.
WASICKY, R. & UNTI, O. (1944) [Dichloro-diphenyl-trichloroethanol
(DDT) does not have any effect on the larvae of "Culicides".]
Arch. Hig. S. Paulo, 9: 87-102 (in Portuguese).
WASICKY, R. & UNTI, O. (1945) [Dichlorodiphenyltrichloroethane (DDT),
additional investigations on its properties and application.]
Arch. Hig. S. Paulo, 10: 49-64 (in Portuguese).
WASSERMANN, D., WASSERMANN, M., DJAVAHERIN, H., GORIN, I., BARISH, M.,
& TAVOR, R. (1971) Effect of organochlorine insecticides on serum
PBI level in occupationally exposed people. Bull. environ.
Contam. Toxicol., 6: 85-88.
WASSERMANN, M., ILIESCU, S., MANDRIC, G., & HORVATH, P. (1960) Toxic
hazards during DDT- and BHC-spraying of forests against Lymantria
monacha. Arch. ind. Health, 21: 503-508.
WASSERMANN, M., GON, M., WASSERMANN, D., & ZELLERMAYER, L. (1965) DDT
and DDE in the body fat of people in Israel. Arch. environ.
Health, 11: 375-379.
WASSERMANN, M., WASSERMANN, D., ZELLERMAYER, L., & GON, M. (1967)
Pesticides in people. Storage of DDT in the people of Israel.
Pestic. Monit. J., 1: 15-20.
WASSERMANN, M., CURNOW, D. H., FORTE, P. N., & GRONER, Y. (1968a)
Storage of organochlorine pesticide in the body of people in
Western Australia. Ind. Med. Surg., 37: 295-300.
WASSERMANN, M., SOFOLUWE, G. I., WASSERMANN, D., GRONERS, I., &
LAZAROVITCH, S. (1968a) Storage of organochlorine insecticides in
the body fat of people in Nigeria. In: Wasserman, H. & Sofoluwe,
G. O., ed. The Lagos International Seminar on Occupational Health
for Developing Countries, 1-6 April 1968, pp. 188-202.
WASSERMANN, M., WASSERMANN, D., GERSHON, Z., & ZELLERMAYER, L. (1969)
Effect of organochlorine insecticides on body defence systems.
Ann. NY Acad. Sci., 160: 393-401.
WASSERMANN, M., WASSERMANN, D., ARONOVSKI, I., IVRIANI, I., &
ROSENFELD, D. (1970a) The effects of organochlorine insecticides
on serum cholesterol level in people occupationally exposed. Bull
environ. Contam. Toxicol., 5: 368-372.
WASSERMANN, M., WASSERMANN, D., LAZAROVICI, S., COETZEE, A. M., &
TOMATIS, L. (1970b) Present state of the organochlorine
insecticides in the general populations of South Africa. South
Afr. med. J., 44: 646-648.
WASSERMANN, M., WASSERMANN, D., & IVRIANI, I. (1970c) Organochlorine
insecticides in the plasma of occupationally exposed workers. In:
Deichmann, W. B., Radomski, J. L., & Penalver, R. A., ed.
Pesticides Symposia, Miami, Florida, Halos. pp. 311-314.
WASSERMANN, M., WASSERMANN, D., KEDAR, E., & DJAVAHERIAN, M. (1971)
Immunological and detoxication interactions in p,p'-DDT fed
rabbits. Bull. environ. Contam. Toxicol., 6: 426-534.
WASSERMANN, M., ROGOFF, M. G., TOMATIS, L., & DAY, N. E. (1972a)
Storage of organochlorine insecticides in the adipose tissue of
people in Kenya. Ann. Soc. Belg. Med. Trop., 52 (6): 509-514.
WASSERMANN, M., NOGUEIRA, D. P., TOMATIS, L., ATHIE, E., WASSERMANN,
D., KJAVAHERIAN, M., & GUTTEL, C. (1972b) Storage of
organochlorine insecticides in people of Sao Paulo, Brazil. Ind.
Med. Surg., 41: (3): 22-25.
WASSERMANN, M., TRISHNANANDA, M., TOMATIS, L., DAY, N. E, WASSERMANN,
D., RUNGPITARANGSI, V., CHAMSAKOL, V., DJAVAHERIAN, M., & CUCUS,
S. (1972c) Storage of organochlorine insecticides in the adipose
tissue of people from Thailand. Southeast Asian J. trop. Med.
Public Health, 3: 280-285.
WASSERMANN, M., SOFOLUWE, G. O., TOMATIS, L., DAY, N. E., WASSERMANN,
D., & LAZAROVICI, S. (1972d) Storage of organochlorine
insecticides in people in Nigeria. Environ. Physiol. Biochem.,
2: 59-67.
WASSERMANN, M., TOMATIS, L., WASSERMANN, D., DAY, N. E., &
DJAVAHERIAN, M. (1974a) Storage of organochlorine insecticides in
adipose tissue of Ugandans. Bull. environ. Contam. Toxicol.,
12: 501-508.
WASSERMANN, M., TOMATIS, L., WASSERMANN, D., DAY, N. E., GRONER, Y.,
LAZAROVICI, S., & ROSENFELD, D. (1974b) Epidemiology of
organochlorine insecticides in the adipose tissue of Israelis.
Pestic. Monit. J., 8: 1-7.
WASSERMANN, M., PRATA, A., TOMATIS, L., WASSERMANN, D., IVRIANI, I., &
OLIVIERA, V. (1975) Storage of organochlorine insecticides in
hepato-splenic schistosomiasis. Rev. Soc. Bras. Mad. Trop.,
8: 271-273.
WATARI, N. (1973) [Ultrastructural alterations of the mouse liver
after the prolonged administration of BHC.] J. Jpn. Electron
Microsc. Soc., 5: 1410-1420; 1449-1456 (in Japanese).
WATSON, M., BENSON, W. W., & GABICA, J. (1970) Serum organochlorine
pesticide levels in people in Southern Idaho. Pestic. Monit. J.,
4: 47-50.
WATSON, M., GABICA, J., & BENSON, W. W. (1972) Serum organochlorine
pesticides in mentally retarded patients on differing drug
regimens. Clin. Pharmacol. Ther., 13: 186-192.
WATTENBERG, L. W. (1971) Studies of polycyclic hydrocarbon
hydroxylases of the intestine possibly related to cancer. Effect
of diet on benzypyrene hydroxylase activity. Cancer, 28: 99-102.
WEAVER, L., GUNNERSON, C. G., BREIDENBACH, A. W., & LICHTENBERG, J. J.
(1965) Chlorinated hydrocarbon pesticides in major US river
basins. Public Health Rep., 80: 481-493.
WEIHE, M. (1966) [Chlorinated insecticides in the body fat of people
in Denmark.] Ugeskr. Laeg., 128: 881-882 (in Danish).
WEIL, C. S. & McCOLLISTER, D. D. (1963) Relationship between short-
and long-term feeding studies in designing an effective toxicity
test. J. agric. food Chem., 11: 486-491.
WEIL, C. S., WOODSIDE, M. D., BERNARD, J. R., & CARPENTER, C. P.
(1969) Relationship between single-peroral, one-week, and ninety-
day rat feeding studies. Toxicol. appl. Pharmacol., 14: 426-431.
WEIL, L., QUENTIN, K. E., & RONICKE, G. (1973) Pesticide levels in air
dust particles in the Federal Republic of Germany. In: The
Technical University of Munich and the German Society for
Research, Commission on Research on Air Pollution, Notice VIII,
23 pp.
WELCH, R. M. & HARRISON, Y. (1966) Reduced drug toxicity following
insecticide treatment. Pharmacologist, 8: 217.
WELCH, R. M., LEVIN, W., & CONNEY, A. H. (1969) Estrogenic action of
DDT and its analogs. Toxicol. appl. Pharmacol., 14: 358-367.
WELCH, J. H. & GORDON, H. T. (1946) The mode of action of DDT. Fed.
Proc., 5: 1.
WEST, I. (1964) Pesticides as contaminants. Arch. environ. Health,
9: 626-631.
WESTOO, G., NOREN, K., & ANDERSSON, M. (1970) [Levels of
organochlorine pesticides and polychlorinated biphenyls in
margarine, vegetable oils, and some foods of animal origin on the
Swedish market in 1967-1969.] Vaar foeda, 22: 9-31 (in Swedish).
WHITE, W. C. & SWEENEY, T. R. (1945) The metabolism of 2,2-bis-( p-
chlorophenyl-1,1,1-trichloroethane (DDT). I. A metabolite from
rabbit urine di-( p-chlorophenyl)-acetic acid; its isolation,
identification, and synthesis. Public Health Rep., 60: 66-71.
WHITING, F. M., STULL, J. W., BROWN, W. H., MILLBROTH, M., & WARE, G.
W. (1968) Comparison of extraction methods for analysis of DDT,
DDE and TDE, in alfalfa hay. J. dairy Sci., 51: 1039-1041.
WHO (1971) Appendix 14: The place of DDT in operations against malaria
and other vector-borne diseases. Off. Rec. World Health Organ.,
190: 176-182.
WHO (1973) WHO Technical Report Series No. 513 (Safe use of
pesticides) 54 pp.
WIGGLESWORTH, V. B. (1945) A case of DDT poisoning in man. Br. med.
J., 1: 517.
WILCOX, A. R. (1967) USPH investigation. DDT health effects. Inter-
Office Correspondence to M. V. Anthony, Stauffer Chemical Co.
WILLIS, G. H., PARR, J. F., & SMITH, S. (1971) Volatilization of soil-
applied DDT and DDD from flooded and nonflooded plots. Pestic
Monit. J., 4: 204-208.
WILSON, D. J., LOCKER, D. J., RITZEN, C. A., WATSON, J. T., &
SCHAFFNER, W. (1973) DDT concentrations in human milk. Am. J.
Dis. Child., 125: 814-817.
WILSON, H. F., ALLEN, N. N., BOHSTEDT, G., BETHEIL, J., & LARDY, H. A.
(1946a) Feeding experiments with DDT-treated pea vine silage with
special reference to dairy cows, sheep, and laboratory animals.
J. econ. Entomol., 36 (6): 801-806.
WILSON, H. F., SRIVASTAVA, A. S., HULL, W. B., BETHEIL, J., & LARDY,
H. A. (1946b) DDT residues on pea vines and canned peas from
fields treated with DDT dusts. J. econ. Entomol., 39
(6): 806-809.
WINTER, M., THOMAS, M., WERNICK, S., LEVIN, S., & FARVAR, M. T. (1976)
Analysis of pesticide residues in 290 samples of Guatemalan
mother's milk. Bull. environ. Contam. Toxicol., 16: 652-657.
WOLFE, H. R. & ARMSTRONG, J. F. (1971) Exposure of formulating plant
workers to DDT. Arch. environ. Health, 23: 169-176.
WOLFE, H. R., WALKER, K. C., ELLIOTT, J. W., & DURHAM, W. F. (1959)
Evaluation of the health hazards involved in house-spraying with
DDT. Bull. Worm Health Organ., 20: 1-14.
WOLFE, H. R., DURHAM, W. F, & ARMSTRONG, J. F. (1970) Urinary
excretion of insecticide metabolites: excretion of para-
nitrophenol and DDA as indicators of exposure to parathion and
DDT. Arch. envrion. Health, 21: 711-716.
WOODARD, G., OFNER, R. R., & MONTGOMERY, C. M. (1945) Accumulation of
DDT in the body fat and its appearance in the milk of dogs.
Science, 102: 177-178.
WOODARD, B. T., FERGUSON, B. B., & WILSON, D. J. (1976) DDT levels in
milk of rural indigent blacks, Research Triangle Park, NC, Health
Effects Research Laboratory, US Environmental Protection Agency,
16 pp. (EPA 600/1-76-032).
WOOLSON E. A. (1974) Extraction of chlorinated hydrocarbon
insecticides from soil. J. Off. Anal. Chem, 57: 604-609.
WOOLSON E. A. & KEARNEY, P. C. (1969) Survey of chlorinated
insecticide analyses in soils. J. Assoc. Off. Anal. Chem.,
52: 1202-1206.
WRENN, T. R., WEYANT, J. R., FRIES, G. F., & BITMAN, J. (1971a) Effect
of several dietary levels of o,p'-DDT on reproduction and
lactation in the rat. Bull. environ. Contam. Toxicol.,
6: 471-479.
WRENN, T., RANDALL, J., WEYANT, R., FRIES, G. F., & BITMAN, J. (1971b)
Influence of dietary o,p'-DDT on reproduction and lactation of
ewes. J. animal Sci., 33: 1288-1292.
WRIGHT, A. S., POTTER, D., WOODER, M. F., DONNINGER, C., & GREENLAND,
R. D. (1972) Effects of dieldrin on mammalian hepatocytes, Food
Cosmet. Toxicol., 10: 311-322.
WYLLIE, J., GABICA, J., & BENSON, W. W. (1972) Comparative
organochlorine pesticide residues in serum and biopsied lipoid
tissue: A survey of 200 persons in southern Idaho -- 1970.
Pestic. Monit. J., 6: 84-88.
YAMAGISHI, T, TAKEBA, K., FUGIMOTO, C., MORIMOTO, K., & HARUTA, M.
(1972) [On the organochlorine pesticide residues in mothers' body
and her fetus' body.] Rep. Tokyo Public Health Stud., 50: 44-45
(in Japanese).
YERMAKOV, V. V. (1972) [Gas chromatographic methods for determining
pesticides in biological material.] Moscow, Izdatelstvo Nauka (in
Russian).
YODER, J. M., WATSON, M., & BENSON, W. W. (1973) Lymphocyte chromosome
analysis of agricultural workers during extensive occupational
exposure to pesticides. Mutat. Res., 21: 335-340.
YOUDEN, W. J. & STEINER, E. H. (1975) Statistical Manual of the
Association of Official Analytical Chemists, Washington, DC,
AOAC.
ZAVON, M. R., HINE, C. H., & PARKER, K. D. (1965) Chlorinated
hydrocarbon insecticides in human body fat in the United States.
J. Assoc. Off. Anal. Chem., 193: 837-839.
ZAVON, M. R., TYE, R., & LATORRE, E. (1969) Chlorinated hydrocarbon
insecticide content of the neonate. Ann. NY Acad. Sci.,
160: 196-200.
ZEINE-EL-DINE, K. (1946) The insecticide DDT. J. Egypt Med. Assoc.,
29: 38-54.
ZELINSKI, S. G., TASHIRO, J., & WORTHEN, L. R. (1973) A gas-
chromatographic method of quantifying DDT in the presence of
interference from PCBs. J. Chromatogr., 84: 67-73.
ZERBER, J., KURASIAK, K., & CIELIEZAK, A. (1971) [Determination of
chlorinated hydrocarbon residues in cereal products and feeds.]
Chem. Anal. 16: 1061-1066 (in Polish).
ZIMMERLI, B. & MAREK, B. (1973) [The pesticide burden of the Swiss
population (analyses of prepared meals, human fat, human serum,
cigarettes, and cosmetics.)] Mitt. Lebensmitt. Hyg., 64: 459-479
(in German).
ZIMMERMAN, B., BLOCH, H. S., WILLIAMS, W. L., HITCHCOCK, C. R., &
HOEISCHER, B. (1956) Effects of DDD (1,1,dichloro-2,2-bis( p-
chloro-phenyl)-ethane) on human adrenal. Attempted to use adrenal
destructive agent in treatment of disseminated mammary and
prostatic cancer. Cancer, 9: 940-948.
ZWEIG, G. & SHERMA, J. (1972) Analytical Methods for Pesticides and
Plant Growth Regulators, New York, Academic Press, Vol 6,
765 pp.
ANNEX
TRANSFORMATION OF P,P'-DDT IN THE ENVIRONMENTa
1 Abiotic Transformations
Since 1969, the photolysis of p,p'-DDT (Annex Fig. 1, formula
II) and of its known primary environmental degradation product, DDE
(1,1-dichloro-2,2-bis [ p-chlorophenyl]ethylene; Annex Fig. 1,
formula VII) has been studied by irradiation in methanol at 260 nm
(Plimmer et al., 1970). The products formed were DDMU (1-chloro-2,2-
bis [ p-chlorophenyl] ethylene; Annex Fig. 1, Formula III),
dichlorobenzophenone (Annex Fig 1, formula IV) and dichlorobiphenyl
(Annex Fig. 1, Formula V). The formation of the last compounds
proceeded via dichlorobenzophenone as an intermediate. The detection
of the chlorinated biphenyl raised the question as to whether DDT
might be a source of PCBs in the environment. Upon investigation of
the reaction pathways leading to these substances, it was shown that
DDE is converted to 3,6-dichlorofluoroenone (Annex Fig. 1, formula VI;
Plimmer & Klingebiel, 1969) which is photooxidized to 3,3'-dichloro-
biphenyl-2-carboxylic acid. Subsequent decarboxylation of this acid
could yield traces of 3,3'-dichlorobiphenyl; the decarbonylation of
another photolysis product of DDT, trichlorobenzophenone, could yield
traces of trichlorobiphenyl, demonstrating that the formation of PCBs
with more than 2 chlorine atoms was also possible (Plimmer &
Klingebiel, 1973).
Irradiation studies with substances in organic solvents are not
necessarily predictive for the environment. However, studies with DDT
vapour in sunlight confirmed the results obtained with dissolved
substances. The proposed pathways of DDT photolysis under
environmental conditions is shown in Annex Fig. 1 (Moilanen & Crosby,
1973).
In 1972, DDE was irradiated in solvents, in the solid state, and
in the gaseous phase, with UV-light of various wavelengths. The
results are shown in Annex Fig. 2. Besides the known photoproducts IV
and III, a "trichlorinated DDMU" (Annex Fig. 2, formula VIII) and 2
compounds with longer side chains (Annex Fig 2, formula IX and X) were
identified; these 2 substances, however, were formed only upon
irradiation in a solvent and originated from the reaction with the
solvent (Kerner et al., 1972).
a Prepared by Dr F. Korte at the request of the Task Group.

 In a recent study on the photoisomerization and photodegradation
of DDE under simulated natural conditions (inert solvents, a good
hydrogen donor solvent, UV-light similar or equal 300 nm), the
compound VIII (Annex Fig. 2) with the 3 phenyl-bound chlorine atoms
was detected and characterized as a mixture of the E- and Z-isomers
which were separated and isolated. Both isomers were also found in
natural samples like tobacco and pine needles. DDMU was also detected
in these studies and 2 substances so far unknown, a tetrachlorinated
phenanthrene and a tetra-ring-chlorinated diphenylethylene; the
formation of tri- and tetrachlorobiphenyls was confirmed (Göthe et
al., 1976).
The behaviour of compounds in their adsorbed form is equally as
significant environmentally as their photochemical behaviour in the
gaseous and solid states.
Irradiation of DDE, adsorbed on silicagel, with wavelengths
> 230 nm, resulted in the formation of dichlorobenzophenone and its
trichlorinated analogue. Irradiation of DDT and DDE in the solid form
in an oxygen stream with wavelengths > 230 nm, resulted in partial
mineralization to give carbon dioxide and hydrochloric acid (Gab et
al., 1975).
The results presented here show that a large number of DDT-derived
chlorinated compounds must be included, when considering the possible
effects of DDT residues in the ecosphere.
2 Biotransformations Other Than Mammalian Metabolism
2.1 Birds
Two main pathways of DDT metabolism exist in mammals i.e.,
dehydrochlorination to DDE (Annex Fig. 3, formula VII) and stepwise
degradation to DDA (bis-[ p-chlorophenyl] acetic acid) via TDE (DDD)
(1,1-dichloro-2,2-bis [ p-chlorophenyl] ethane; Annex Fig. 3,
formula I). However, in birds, the pathway varies with species, and
data from studies on the administration of chronic and acute dosages
of DDT to pigeons, quail, and blackbirds show that DDE is the primary
metabolic product in the first 2 species, and TDE (DDD) in the third
(Bailey et al., 1972). The TDE-path-way does exist in the pigeon as a
minor pathway but, in contrast to mammals, only as far as DDMU (Annex
Fig. 3, formula III).
Thus, DDA, the degradation product of DDMU excreted by mammals, is
not formed in the pigeon (Bunyan et al., 1966; Bailey et al., 1969).
When its precursors in mammals, DDMS (1-chloro-2,2-bis[ p-
chlorophenyl] ethane; Annex Fig. 3, formula XI) and DDN U (1,1-bis
[ p-chlorophenyl] ethylene; Annex Fig. 3, formula XII) are
administered to the pigeon, they are rapidly converted: DDMS is
converted to DDMU, and DDNU is metabolized quickly and excreted as
DDNS (1,1-bis [ p-chlorophenyl] ethane; Annex Fig. 3, formula XIII),
a metabolite that was not found in mammals (Bailey et al., 1972). The
metabolic pathways for DDT in the pigeon are shown in Annex Fig. 3.
2.2 Insects
Investigations on the detoxication mechanism of DDT in insects are
interesting as regards the problem of resistance. In general, the
phenomenon of insect resistance is related to detoxication of the
insecticide by metabolization to nontoxic compounds. The metabolic
pathways of DDT in insects are many and depend on species and even on
strains.
Annex Fig. 4 shows only the major metabolic products.
The first conversion product identified in resistant houseflies
was DDE. This conversion is catalyzed by the enzyme DDT-dehydro-
chlorinase (EC 4.5.1.1) which had already been isolated in the pure
form in the 1950s. Further insect metabolites of DDT are DDD (isolated
for example from Stomoxys calcitrans), DDA (isolated from example
from Quiscalus quiscula, Heliothis virescens and Coleomegilla
maculata) and dichlorobenzophenone (from Leucophae). The
detoxiation of DDT in Triatoma infestans, Drosophila melanogaster,
Culex tarsalis, and other species is performed by hydroxylation and
results in kelthane (Annex Fig. 4, formula XV), a substance which is a
commercial acaricide. A great number of unidentified and water-soluble
conversion products of DDT was observed in many species as reviewed by
Klein & Korte (1970).
In a recent study on the photoisomerization and photodegradation
of DDE under simulated natural conditions (inert solvents, a good
hydrogen donor solvent, UV-light similar or equal 300 nm), the
compound VIII (Annex Fig. 2) with the 3 phenyl-bound chlorine atoms
was detected and characterized as a mixture of the E- and Z-isomers
which were separated and isolated. Both isomers were also found in
natural samples like tobacco and pine needles. DDMU was also detected
in these studies and 2 substances so far unknown, a tetrachlorinated
phenanthrene and a tetra-ring-chlorinated diphenylethylene; the
formation of tri- and tetrachlorobiphenyls was confirmed (Göthe et
al., 1976).
The behaviour of compounds in their adsorbed form is equally as
significant environmentally as their photochemical behaviour in the
gaseous and solid states.
Irradiation of DDE, adsorbed on silicagel, with wavelengths
> 230 nm, resulted in the formation of dichlorobenzophenone and its
trichlorinated analogue. Irradiation of DDT and DDE in the solid form
in an oxygen stream with wavelengths > 230 nm, resulted in partial
mineralization to give carbon dioxide and hydrochloric acid (Gab et
al., 1975).
The results presented here show that a large number of DDT-derived
chlorinated compounds must be included, when considering the possible
effects of DDT residues in the ecosphere.
2 Biotransformations Other Than Mammalian Metabolism
2.1 Birds
Two main pathways of DDT metabolism exist in mammals i.e.,
dehydrochlorination to DDE (Annex Fig. 3, formula VII) and stepwise
degradation to DDA (bis-[ p-chlorophenyl] acetic acid) via TDE (DDD)
(1,1-dichloro-2,2-bis [ p-chlorophenyl] ethane; Annex Fig. 3,
formula I). However, in birds, the pathway varies with species, and
data from studies on the administration of chronic and acute dosages
of DDT to pigeons, quail, and blackbirds show that DDE is the primary
metabolic product in the first 2 species, and TDE (DDD) in the third
(Bailey et al., 1972). The TDE-path-way does exist in the pigeon as a
minor pathway but, in contrast to mammals, only as far as DDMU (Annex
Fig. 3, formula III).
Thus, DDA, the degradation product of DDMU excreted by mammals, is
not formed in the pigeon (Bunyan et al., 1966; Bailey et al., 1969).
When its precursors in mammals, DDMS (1-chloro-2,2-bis[ p-
chlorophenyl] ethane; Annex Fig. 3, formula XI) and DDN U (1,1-bis
[ p-chlorophenyl] ethylene; Annex Fig. 3, formula XII) are
administered to the pigeon, they are rapidly converted: DDMS is
converted to DDMU, and DDNU is metabolized quickly and excreted as
DDNS (1,1-bis [ p-chlorophenyl] ethane; Annex Fig. 3, formula XIII),
a metabolite that was not found in mammals (Bailey et al., 1972). The
metabolic pathways for DDT in the pigeon are shown in Annex Fig. 3.
2.2 Insects
Investigations on the detoxication mechanism of DDT in insects are
interesting as regards the problem of resistance. In general, the
phenomenon of insect resistance is related to detoxication of the
insecticide by metabolization to nontoxic compounds. The metabolic
pathways of DDT in insects are many and depend on species and even on
strains.
Annex Fig. 4 shows only the major metabolic products.
The first conversion product identified in resistant houseflies
was DDE. This conversion is catalyzed by the enzyme DDT-dehydro-
chlorinase (EC 4.5.1.1) which had already been isolated in the pure
form in the 1950s. Further insect metabolites of DDT are DDD (isolated
for example from Stomoxys calcitrans), DDA (isolated from example
from Quiscalus quiscula, Heliothis virescens and Coleomegilla
maculata) and dichlorobenzophenone (from Leucophae). The
detoxiation of DDT in Triatoma infestans, Drosophila melanogaster,
Culex tarsalis, and other species is performed by hydroxylation and
results in kelthane (Annex Fig. 4, formula XV), a substance which is a
commercial acaricide. A great number of unidentified and water-soluble
conversion products of DDT was observed in many species as reviewed by
Klein & Korte (1970).

 Although TDE (DDD) has not been found as a DDT-metabolite in
Culex tarsalis, it has been concluded from differences between
susceptible and resistant strains that it is an intermediate in the
further degradation of DDT. After application of TDE 14C to this
insect, DDMU and DDDOH (1,1-bis[ p-chlorophenyl]-2,2-dichloroethanol;
Annex Fig. 5, formula XVI) were found as major metabolites;
furthermore, 3 polar compounds were chromatographically identical with
DDA, DBH (dichlorobenzhydrole; Annex Fig. 5, formula XVIII) and PCBA
( p-chlorobenzoic acid; Annex Fig. 5, formula XVIII), which were also
observed after application of DDMU-14C (Plapp et al., 1965). The
occurrence of PCBA indicates the complete breakdown of one of the 2
rings of DDT and thus the possibility of a complete biological
degradation of the whole molecule.
2.3 Higher plants
Although the transformation of DDT in higher plants is rather
limited (2% in spinach within 18 days, 5% in cabbage within 14 weeks),
it must not be neglected since a considerable part of the DDT used on
a worldwide basis is applied, intentionally or unintentionally, to
plants. The conversion products that have been identified (Annex
Fig. 6) are DDE, TDE (DDD), DDMU, DDA, conjugates of DDA, and a
conjugate of DBH (Zimmer & Klein, 1972), which means that the
metabolites in plants are not chemically different from those in other
organisms.
In a study of the accumulation and distribution of p,p'-DDT in
an apple orchard, DDT residues in or on the roots and leaves of the
herbage and the roots, bark, leaves, and fruit of the trees were
recorded for an orchard sprayed annually (Stringer et al., 1975).
During 13 years, there were increasing amounts of DDE, TDE, and DDMU
in relation to DDT, in the bark of apple trees indicating some
breakdown on the bark (< 10%). DDE and TDE were also observed after
application of p,p'-DDT to cotton (Nash et al., 1977). These 2
substances seem to be common conversion products of DDT in plants. The
o,p'-DDT observed in the last 2 experiments seems to be an impurity
of the DDT rather than a metabolite.
2.4 Microorganisms and soil
The most common metabolic reaction of DDT in microorganisms is
reductive dechlorination resulting in the formation of TDE. This
reaction has been demonstrated in Escherichia coli (in rat
intestine), Aerobacter aerogenes, Proteus vulgaris, and in yeasts.
In contrast to metabolism in higher animals, dechlorination by
microorganisms is anaerobic and is catalysed by reduced cytochrome
oxidase (EC 1.9.3.1). Fe (II)-cytochrome oxidase isolated from
Aerobacter converts DDT to TDE in vitro (Klein & Korte, 1970). The
conversion of DDT to TDE (DDD) in bodies of water (Miskus et al.,
1965) and in other reducing environments characteristic of dead and
Although TDE (DDD) has not been found as a DDT-metabolite in
Culex tarsalis, it has been concluded from differences between
susceptible and resistant strains that it is an intermediate in the
further degradation of DDT. After application of TDE 14C to this
insect, DDMU and DDDOH (1,1-bis[ p-chlorophenyl]-2,2-dichloroethanol;
Annex Fig. 5, formula XVI) were found as major metabolites;
furthermore, 3 polar compounds were chromatographically identical with
DDA, DBH (dichlorobenzhydrole; Annex Fig. 5, formula XVIII) and PCBA
( p-chlorobenzoic acid; Annex Fig. 5, formula XVIII), which were also
observed after application of DDMU-14C (Plapp et al., 1965). The
occurrence of PCBA indicates the complete breakdown of one of the 2
rings of DDT and thus the possibility of a complete biological
degradation of the whole molecule.
2.3 Higher plants
Although the transformation of DDT in higher plants is rather
limited (2% in spinach within 18 days, 5% in cabbage within 14 weeks),
it must not be neglected since a considerable part of the DDT used on
a worldwide basis is applied, intentionally or unintentionally, to
plants. The conversion products that have been identified (Annex
Fig. 6) are DDE, TDE (DDD), DDMU, DDA, conjugates of DDA, and a
conjugate of DBH (Zimmer & Klein, 1972), which means that the
metabolites in plants are not chemically different from those in other
organisms.
In a study of the accumulation and distribution of p,p'-DDT in
an apple orchard, DDT residues in or on the roots and leaves of the
herbage and the roots, bark, leaves, and fruit of the trees were
recorded for an orchard sprayed annually (Stringer et al., 1975).
During 13 years, there were increasing amounts of DDE, TDE, and DDMU
in relation to DDT, in the bark of apple trees indicating some
breakdown on the bark (< 10%). DDE and TDE were also observed after
application of p,p'-DDT to cotton (Nash et al., 1977). These 2
substances seem to be common conversion products of DDT in plants. The
o,p'-DDT observed in the last 2 experiments seems to be an impurity
of the DDT rather than a metabolite.
2.4 Microorganisms and soil
The most common metabolic reaction of DDT in microorganisms is
reductive dechlorination resulting in the formation of TDE. This
reaction has been demonstrated in Escherichia coli (in rat
intestine), Aerobacter aerogenes, Proteus vulgaris, and in yeasts.
In contrast to metabolism in higher animals, dechlorination by
microorganisms is anaerobic and is catalysed by reduced cytochrome
oxidase (EC 1.9.3.1). Fe (II)-cytochrome oxidase isolated from
Aerobacter converts DDT to TDE in vitro (Klein & Korte, 1970). The
conversion of DDT to TDE (DDD) in bodies of water (Miskus et al.,
1965) and in other reducing environments characteristic of dead and

 decaying matter (Zoro et al., 1974) is mediated by reduced iron
porphyrins and is not an essential part of cell metabolism. These
findings have considerable environmental significance since most
living material contains iron porphyrins bound with protein in complex
molecules. The porphyrins are released after decay of the organic
substances, and may then be regarded as widespread environmental
agents that convert, on a larger scale, the persistent DDT to the less
persistent TDE. TDE is susceptible to further abiotic or biotic
degradation.
However, the formation of DDE and DDA from DDT by microorganisms
is also possible. For instance, both DDE and TDE were isolated from
Serratia marcescens and Alkaligenes faecalis (Stenersen, 1965) and
DDA was isolated from microbial cultures obtained from agricultural
soil (Patil et al., 1970).
In a model experiment with anaerobic activated sludge and p,p'-
DDT-14C, TDE, p,p'-dichlorobenzophenone, DDMU and a so far unknown
metabolite, DDCN (bis[ p-chlorophenyl] acetonitrile), were detected
as conversion products. The last of these substances, a minor
conversion product, was also found in the sediment layer of the Lake
Mälaren in Sweden (0.2 mg/kg dry weight). DDCN is formed via TDE or
DDE, but directly from DDT (Jensen et al., 1972).
The question of "bound residues" in soil, which is now under
discussion for a number of "non-persistent" pesticides, especially
those that are anilin-derived, seems also to be relevant for
"persistent" substances such as DDT, although the percentage of bound
residues is less than for less persistent pesticides. The formation of
25% of bound DDT-residues within 28 days (Lichtenstein et al., 1977)
justifies a reassessment of the persistence of DDT in soil. Further
information should be obtained concerning the nature and the potential
biological activity of the compounds that are bound.
3 Conclusion
A multitude of conversion products are formed from DDT under
environmental conditions. Nearly 20 of these (including mammalian
metabolites) have been identified so far, but the chemical structure
of a number of other compounds is still unknown. Very little is known
of the toxicological properties of these conversion products with the
exception of major products such as DDE and TDE. This should be
remembered when the unwanted effects of DDT in the environment are
evaluated. However, there is even less information concerning the fate
in the environment of many other pesticides including those that are
used as DDT-substitutes.
REFERENCES
BAILEY, S., BUNYAN, P. J., RENNISON, B. D., & TAYLOR, A. (1969) The
metabolism of 1,1-di( p-chlorophenyl)-2,2,2-trichlorethane and
1,1-di( p-chlorophenyl)-2,2-dichloroethane in the pigeon.
Toxicol. appl. Pharmacol., 14: 13-22.
BAILEY, S., BUNYAN, P. J., TAYLOR, A., & TOLWORTH, U. K. (1972) The
metabolism of p,p'-DDT in some avian species. Environ. Qual.
Saf., 1: 244.
BUNYAN, P. J., PAGE, J. M. J., & TAYLOR, A. (1966) In vitro
metabolism of p,p'-DDT in pigeon liver. Nature (Lond.),
210: 1048-1049.
GAB S., NITZ, S., PARLAR, H., & KORTE, F. (1975) [Contributions on
ecological chemistry CV. Photomineralization of certain aromatic
xenobiotica.] Chemosphere, 4: 251-256 (in German).
GOTHE, R., WACHTMEISTER, C. A., AKERMARK, B., BAECKSTROM, P., JANSSON,
B., & JENSEN, S. (1976) Photo-isomerisation and photo-degradation
of DDE. Tetrahedron Lett., pp. 4051-4504.
JENSEN, S., GOTHE, R., & KINDSTEDT, M.O. (1972) Bis- ( p-chlorophenyl-
acetonitrile (DDCN), a new DDT derivative formed in anaerobic
digested sewage sludge and lake sediment. Nature (Lond.),
240: 421-422.
KERNER, I., KLEIN, W., & KORTE, F. (1972) [Contributions on ecological
chemistry -- XXXIII. Photochemical reactions of 1.1-dichloro-2
( p,p'-dichlorophenyl) ethylene (DDE).] Tetrahedron Lett.,
28: 1575-1578 (in German).
KLEIN, A. K, LAUG, E. P., DATTA, P. R., WATTS, J. O., & COHEN, J. T.
(1964) Metabolites: Reductive dechlorination of DDT to DDD and
isomeric transformation of o,p'-DDT to p,p'-DDT in vitro. J.
Assoc. Off. Agric. Chem., 47: 1129-1145.
KLEIN, W. & KORTE, F. (1970) [Review: Metabolism of organochlorine
compounds.] In: Wegler, R., ed. [The chemistry of plant
protection and pest control agents.] Volume 1, pp. 199-218.
Berlin-Heidelberg-New York, Springer Verlag (in German).
LOCHTENSTEIN, E. P., KATAN, J., & ANDEREGG, B. N. (1977) Binding of
"persistent" and "nonpersistent" 14C-labelled insecticides in an
agricultural soil. J. agric. food Chem., 25: 43-47.
MISKUS, R. P., BLAIR, D., & CASIDA, J. E. (1965) Conversion of DDT to
DDD by bovine rumen fluid, lake water and reduced porphyries.
J. agric. food Chem., 13: 481-482.
MOILANEN, K. W. & CROSBY, D. G. (1973) DDT, an unrecognized source of
polychlorinated biphenyls. Science, 180: 578-579.
NASH, R. G., BEALL, M. L., & HARRIS, W. G. (1977) Toxaphene and 1,1,1-
trichloro-2,2-bis ( p-chlorophenyl) ethane (DDT) losses from
cotton in an agroecosystem chamber. J. agric. food Chem.
25: 336-341.
PATIL, K. C., MATSUMURA, F., & BOUSH, G. M. (1970) Degradation of
endrin, aldrin, and DDT by soil microorganisms. Appl. Microbiol.,
19: 879-881.
PLAPP, F. W., CHAPMAN, G. A., & MORGAN, J. W. (1965) DDT resistance in
Culex tarsalis Coquillett; Cross resistance to related compounds
and metabolic fate of a C14 labelled DDT analog. J. econ.
Entomol., 58: 1064-1069.
PLIMMER, J. R. & KLINGEBIEL, U. I. (1969) A photocyclization reaction
of dichloro-2,2-bis-( p-chlorophenyl) ethylene (DDE). Chem.
Commun., p. 648.
PLIMMER, J. R. & KLINGEBIEL, U. I. (1973) PCB-formation. Science,
181: (4104): 994-995.
PLIMMER, J. R., KLINGEBIEL, U. I., & HUMMER, B. E. (1970) Photo-
oxidation of DDT and DDE. Science, 167: 67-69.
STENERSEN, J. H. V. (1965) DDT metabolism in resistant and susceptible
flies and in bacteria, Nature (Lond.), 707: 660-661.
STRINGER, A., PICKARD, J. A., & LYON, C. H. (1965) Accumulation and
distribution of p,p'-DDT and related compounds in an apple
orchard. II. Residues in trees and herbage. Pestic. Sci.,
6: 223-232.
ZIMMER, M. & KLEIN, W. (1972) [Contributions on ecological chemistry
XXXVII. Behaviour of residues and transformations of p,p'-DDT-
14C and its analogues p,p'-DDT-14C and p,p'-DDD-14C in
higher plants.] Chemosphere, 1: 3-6 (in German).
ZORO, J. A., HUNTER, J. M., EGLINTON, G., & WARE, G. C. (1974)
Degradation of p,p'-DDT in reducing environments. Nature
(Lond.), 247: 235-236.
decaying matter (Zoro et al., 1974) is mediated by reduced iron
porphyrins and is not an essential part of cell metabolism. These
findings have considerable environmental significance since most
living material contains iron porphyrins bound with protein in complex
molecules. The porphyrins are released after decay of the organic
substances, and may then be regarded as widespread environmental
agents that convert, on a larger scale, the persistent DDT to the less
persistent TDE. TDE is susceptible to further abiotic or biotic
degradation.
However, the formation of DDE and DDA from DDT by microorganisms
is also possible. For instance, both DDE and TDE were isolated from
Serratia marcescens and Alkaligenes faecalis (Stenersen, 1965) and
DDA was isolated from microbial cultures obtained from agricultural
soil (Patil et al., 1970).
In a model experiment with anaerobic activated sludge and p,p'-
DDT-14C, TDE, p,p'-dichlorobenzophenone, DDMU and a so far unknown
metabolite, DDCN (bis[ p-chlorophenyl] acetonitrile), were detected
as conversion products. The last of these substances, a minor
conversion product, was also found in the sediment layer of the Lake
Mälaren in Sweden (0.2 mg/kg dry weight). DDCN is formed via TDE or
DDE, but directly from DDT (Jensen et al., 1972).
The question of "bound residues" in soil, which is now under
discussion for a number of "non-persistent" pesticides, especially
those that are anilin-derived, seems also to be relevant for
"persistent" substances such as DDT, although the percentage of bound
residues is less than for less persistent pesticides. The formation of
25% of bound DDT-residues within 28 days (Lichtenstein et al., 1977)
justifies a reassessment of the persistence of DDT in soil. Further
information should be obtained concerning the nature and the potential
biological activity of the compounds that are bound.
3 Conclusion
A multitude of conversion products are formed from DDT under
environmental conditions. Nearly 20 of these (including mammalian
metabolites) have been identified so far, but the chemical structure
of a number of other compounds is still unknown. Very little is known
of the toxicological properties of these conversion products with the
exception of major products such as DDE and TDE. This should be
remembered when the unwanted effects of DDT in the environment are
evaluated. However, there is even less information concerning the fate
in the environment of many other pesticides including those that are
used as DDT-substitutes.
REFERENCES
BAILEY, S., BUNYAN, P. J., RENNISON, B. D., & TAYLOR, A. (1969) The
metabolism of 1,1-di( p-chlorophenyl)-2,2,2-trichlorethane and
1,1-di( p-chlorophenyl)-2,2-dichloroethane in the pigeon.
Toxicol. appl. Pharmacol., 14: 13-22.
BAILEY, S., BUNYAN, P. J., TAYLOR, A., & TOLWORTH, U. K. (1972) The
metabolism of p,p'-DDT in some avian species. Environ. Qual.
Saf., 1: 244.
BUNYAN, P. J., PAGE, J. M. J., & TAYLOR, A. (1966) In vitro
metabolism of p,p'-DDT in pigeon liver. Nature (Lond.),
210: 1048-1049.
GAB S., NITZ, S., PARLAR, H., & KORTE, F. (1975) [Contributions on
ecological chemistry CV. Photomineralization of certain aromatic
xenobiotica.] Chemosphere, 4: 251-256 (in German).
GOTHE, R., WACHTMEISTER, C. A., AKERMARK, B., BAECKSTROM, P., JANSSON,
B., & JENSEN, S. (1976) Photo-isomerisation and photo-degradation
of DDE. Tetrahedron Lett., pp. 4051-4504.
JENSEN, S., GOTHE, R., & KINDSTEDT, M.O. (1972) Bis- ( p-chlorophenyl-
acetonitrile (DDCN), a new DDT derivative formed in anaerobic
digested sewage sludge and lake sediment. Nature (Lond.),
240: 421-422.
KERNER, I., KLEIN, W., & KORTE, F. (1972) [Contributions on ecological
chemistry -- XXXIII. Photochemical reactions of 1.1-dichloro-2
( p,p'-dichlorophenyl) ethylene (DDE).] Tetrahedron Lett.,
28: 1575-1578 (in German).
KLEIN, A. K, LAUG, E. P., DATTA, P. R., WATTS, J. O., & COHEN, J. T.
(1964) Metabolites: Reductive dechlorination of DDT to DDD and
isomeric transformation of o,p'-DDT to p,p'-DDT in vitro. J.
Assoc. Off. Agric. Chem., 47: 1129-1145.
KLEIN, W. & KORTE, F. (1970) [Review: Metabolism of organochlorine
compounds.] In: Wegler, R., ed. [The chemistry of plant
protection and pest control agents.] Volume 1, pp. 199-218.
Berlin-Heidelberg-New York, Springer Verlag (in German).
LOCHTENSTEIN, E. P., KATAN, J., & ANDEREGG, B. N. (1977) Binding of
"persistent" and "nonpersistent" 14C-labelled insecticides in an
agricultural soil. J. agric. food Chem., 25: 43-47.
MISKUS, R. P., BLAIR, D., & CASIDA, J. E. (1965) Conversion of DDT to
DDD by bovine rumen fluid, lake water and reduced porphyries.
J. agric. food Chem., 13: 481-482.
MOILANEN, K. W. & CROSBY, D. G. (1973) DDT, an unrecognized source of
polychlorinated biphenyls. Science, 180: 578-579.
NASH, R. G., BEALL, M. L., & HARRIS, W. G. (1977) Toxaphene and 1,1,1-
trichloro-2,2-bis ( p-chlorophenyl) ethane (DDT) losses from
cotton in an agroecosystem chamber. J. agric. food Chem.
25: 336-341.
PATIL, K. C., MATSUMURA, F., & BOUSH, G. M. (1970) Degradation of
endrin, aldrin, and DDT by soil microorganisms. Appl. Microbiol.,
19: 879-881.
PLAPP, F. W., CHAPMAN, G. A., & MORGAN, J. W. (1965) DDT resistance in
Culex tarsalis Coquillett; Cross resistance to related compounds
and metabolic fate of a C14 labelled DDT analog. J. econ.
Entomol., 58: 1064-1069.
PLIMMER, J. R. & KLINGEBIEL, U. I. (1969) A photocyclization reaction
of dichloro-2,2-bis-( p-chlorophenyl) ethylene (DDE). Chem.
Commun., p. 648.
PLIMMER, J. R. & KLINGEBIEL, U. I. (1973) PCB-formation. Science,
181: (4104): 994-995.
PLIMMER, J. R., KLINGEBIEL, U. I., & HUMMER, B. E. (1970) Photo-
oxidation of DDT and DDE. Science, 167: 67-69.
STENERSEN, J. H. V. (1965) DDT metabolism in resistant and susceptible
flies and in bacteria, Nature (Lond.), 707: 660-661.
STRINGER, A., PICKARD, J. A., & LYON, C. H. (1965) Accumulation and
distribution of p,p'-DDT and related compounds in an apple
orchard. II. Residues in trees and herbage. Pestic. Sci.,
6: 223-232.
ZIMMER, M. & KLEIN, W. (1972) [Contributions on ecological chemistry
XXXVII. Behaviour of residues and transformations of p,p'-DDT-
14C and its analogues p,p'-DDT-14C and p,p'-DDD-14C in
higher plants.] Chemosphere, 1: 3-6 (in German).
ZORO, J. A., HUNTER, J. M., EGLINTON, G., & WARE, G. C. (1974)
Degradation of p,p'-DDT in reducing environments. Nature
(Lond.), 247: 235-236.
