
INTERNATIONAL PROGRAMME ON CHEMICAL SAFETY
ENVIRONMENTAL HEALTH CRITERIA 7
PHOTOCHEMICAL OXIDANTS
This report contains the collective views of an international group of
experts and does not necessarily represent the decisions or the stated
policy of either the World Health Organization or the United Nations
Environment Programme.
Published under the joint sponsorship of
the United Nations Environment Programme
and the World Health Organization
World Health Organization
Geneva, 1979
ISBN 92 4 154067 2
(c) World Health Organization 1979
Publications of the World Health Organization enjoy copyright
protection in accordance with the provisions of Protocol 2 of the
Universal Copyright Convention. For rights of reproduction or
translation of WHO publications, in part or in toto, application
should be made to the Office of Publications, World Health
Organization, Geneva, Switzerland. The World Health Organization
welcomes such applications.
The designations employed and the presentation of the material in
this publication do not imply the expression of any opinion whatsoever
on the part of the Secretariat of the World Health Organization
concerning the legal status of any country, territory, city or area or
of its authorities, or concerning the delimitation of its frontiers or
boundaries.
The mention of specific companies or of certain manufacturers'
products does not imply that they are endorsed or recommended by the
World Health Organization in preference to others of a similar nature
that are not mentioned. Errors and omissions excepted, the names of
proprietary products are distinguished by initial capital letters.
CONTENTS
ENVIRONMENTAL HEALTH CRITERIA FOR PHOTOCHEMICAL OXIDANTS
1. SUMMARY AND RECOMMENDATIONS FOR FURTHER RESEARCH AND OTHER ACTION
1.1. Summary
1.1.1. Chemistry and analytical methods
1.1.2. Sources of photochemical oxidants and their
precursors
1.1.3. Environmental concentrations and exposures
1.1.4. Effects on experimental animals
1.1.5. Effects on man
1.1.5.1 Controlled exposures
1.1.5.2 Industrial exposure
1.1.5.3 Community exposure
1.1.6. Evaluation of health risks
1.2. Recommendations for further research and other action
1.2.1. Health effects research
1.2.2. Photochemical oxidant control
2. CHEMISTRY AND ANALYTICAL METHODS
2.1. Chemical and physical properties
2.1.1. Ozone
2.1.2. Peroxyacylnitrates
2.1.3. Other oxidants
2.2. Atmospheric chemistry
2.3. Measurement of photochemical oxidant concentrations
2.3.1. Sampling
2.3.2. Analytical methods
2.3.2.1 Ozone
2.3.2.2 Total oxidants
2.3.2.3 Peroxyacetylnitrate
3. SOURCES OF PHOTOCHEMICAL OXIDANTS AND THEIR PRECURSORS
3.1. Natural sources
3.2. Man-made sources of oxidant precursors
3.3. Indoor sources
3.4. Oxidant-precursor relationships
4. ENVIRONMENTAL CONCENTRATIONS AND EXPOSURES
4.1. Background concentrations
4.2. Rural areas
4.3. Urban areas
4.4. Indoor concentrations
5. EFFECTS ON EXPERIMENTAL ANIMALS
5.1. Absorption of ozone
5.2. Effects on the respiratory system
5.2.1. Morphological changes
5.2.1.1 Short-term exposure (24 h or less)
5.2.1.2 Prolonged and repeated exposures
5.2.2. Functional changes
5.2.2.1 Short-term exposure (24 h or less)
5.2.2.2 Prolonged and repeated exposures
5.2.3. Biochemical changes
5.2.3.1 Effects indicating possible mechanisms
of action
5.2.3.2 Biochemical effects at the subcellular
level
5.2.3.3 Extracellular effects
5.2.4. Carcinogenicity
5.2.5. Tolerance to ozone
5.2.6. Effects on the host defence system
5.2.7. Interaction of ozone with bronchoactive and other
chemicals
5.3. Systemic reactions and other effects
5.3.1. Effects on growth
5.3.2. Haematological effects
5.3.2.1 Short-term exposure (24 h or less)
5.3.2.2 Prolonged and repeated exposures
5.3.3. Effects on reproduction
5.3.4. Behavioural and related changes
5.3.4.1 Short-term exposure (24 h or less)
5.3.4.2 Prolonged and repeated exposures
5.3.5. Miscellaneous systemic reactions to lung damage
5.4. Mutagenicity
5.5. Summary table
6. EFFECTS ON MAN
6.1. Controlled exposures
6.1.1. In vitro effects on human tissues
6.1.2. Sensory effects
6.1.3. Effects on respiratory function
6.1.3.1 Exposure to ozone
6.1.3.2 Exposure to mixtures of ozone and other
air pollutants
6.1.3.3 Exposure to peroxyacetylnitrate alone or
in combination with carbon monoxide
6.1.3.4 Exposure to irradiated automobile
exhaust
6.1.3.5 Exposure to ambient air containing
elevated concentrations of oxidants
6.1.4. Changes in electroencephalograms
6.1.5. Chromosomal effects
6.2. Industrial exposure
6.3. Community exposure
6.3.1. Mortality
6.3.2. Annoyance and irritation
6.3.3. Athletic performance
6.3.4. Effects on children
6.3.5. Effects on the incidence of acute respiratory and
cardiovascular diseases
6.3.6. Effects on the prevalence of chronic respiratory
diseases and on pulmonary function
6.3.7. Effects on patients with pre-existing diseases
6.3.7.1 Asthma
6.3.7.2 Chronic respiratory diseases
6.3.8. Cancer
6.3.9. Motor vehicle accidents
6.4. Summary tables
7. EVALUATION OF HEALTH RISKS FROM EXPOSURE TO PHOTOCHEMICAL
OXIDANTS
7.1. Exposure conditions
7.2. Exposure-effect relationships
7.2.1. Animal data
7.2.2. Controlled human exposures
7.2.3. Industrial exposure
7.2.4. Community exposure
7.3. Guidelines on exposure limits
REFERENCES
NOTE TO READERS OF THE CRITERIA DOCUMENTS
While every effort has been made to present information in the
criteria documents as accurately as possible without unduly delaying
their publication, mistakes might have occurred and are likely to
occur in the future. In the interest of all users of the environmental
health criteria documents, readers are kindly requested to communicate
any errors found to the Division of Environmental Health, World Health
Organization, Geneva, Switzerland, in order that they may be included
in corrigenda which will appear in subsequent volumes.
* * *
In addition, experts in any particular field dealt with in the
criteria documents are kindly requested to make available to the WHO
Secretariat any important published information that may have
inadvertently been omitted and which may change the evaluation of
health risks from exposure to the environmental agent under
examination, so that the information may be considered in the event of
updating and re-evaluating the conclusions contained in the criteria
documents.
WHO TASK GROUP ON ENVIRONMENTAL HEALTH CRITERIA FOR PHOTOCHEMICAL
OXIDANTS
Tokyo, 30 August-3 September 1976
Participants
Members
Dr K. Biersteker, Medical Research Division, Municipal Health
Department, Rotterdam, Netherlands (Chairman)
Professor K. A. Bustueva, Department of Community Hygiene, Central
Institute for Advanced Medical Training, Moscow, USSR
(Vice-Chairman)
Dr R. G. Derwent, Environmental and Medical Sciences Division, Atomic
Energy Research Establishment, Harwell, England (Rapporteur)
Dr D. E. Gardner, Biomedical Research Branch, Clinical Studies
Division, Health Effects Research Laboratory, Environmental
Protection Agency, Research Triangle Park, NC, USA
Dr J. Jager, Centre of General and Environmental Hygiene, Institute of
Hygiene and Epidemiology, Prague, Czechoslovakia
Dr G. von Nieding, Laboratory for Respiration and Circulation,
Bethanien Hospital, Moers, Federal Republic of Germany
Mr E. A. Schuck, US Environmental Protection Agency, Environmental
Monitoring and Support Laboratory, Las Vegas, NV, USA
Professor N. Yamaki, Faculty of Engineering, Saitama University,
Saitama, Japan
Observers
Dr J. Kagawa, Department of Medicine, Tokai University, Kanagawa,
Japan
Professor K. Maeda, Department of Medicine, Tokyo University, Tokyo,
Japan
Dr T. Nakajima, Division of Environmental Health Research, Osaka
Prefectural Institute of Public Health, Osaka, Japan
Dr T. Okita, Department of Community Environmental Sciences, The
Institute of Public Health, Tokyo, Japan
Dr H. Watanabe, Hyogo Prefectural Institute of Public Health, Kobe,
Japan
Dr N. Yamate, First Section of Environmental Chemistry, National
Institute of Hygienic Sciences, Tokyo, Japan
Secretariat
Dr G. Freeman, Department of Medical Sciences, Stanford Research
Institute, Menlo Park, CA, USA (Temporary Adviser)
Dr Y. Hasegawa, Medical Officer, Control of Environmental Pollution
and Hazards, World Health Organization, Geneva, Switzerland
(Secretary)
Mr G. Ozolins, Scientist, Control of Environmental Pollution and
Hazards, World Health Organization, Geneva, Switzerland
Professor C. M. Shy, Institute for Environmental Studies and
Department of Epidemiology, School of Public Health, University
of North Carolina, Chapel Hill, NC, USA (Temporary Adviser)
Dr T. Suzuki, The Institute of Public Health, Tokyo, Japan (Temporary
Adviser)
Professor T. Toyama, Department of Preventive Medicine, Keio
University, Tokyo, Japan (Temporary Adviser)
ENVIRONMENTAL HEALTH CRITERIA FOR PHOTOCHEMICAL OXIDANTS
A WHO Task Group on Environmental Health Criteria for
Photochemical Oxidants met in Tokyo from 30 August to 3 September
1976. Dr Y. Hasegawa, Medical Officer, Control of Environmental
Pollution and Hazards, Division of Environmental Health, WHO, opened
the meeting on behalf of the Director-General and expressed the
appreciation of the Organization to the Government of Japan for acting
as host to the meeting. The Task Group reviewed and revised the second
draft criteria document and made an evaluation of the health risks
from exposure to photochemical oxidants.
The first and second drafts of the criteria document were prepared
by Professor Carl M. Shy of the Department of Epidemiology, School of
Public Health, University of North Carolina, Chapel Hill, NC, USA, and
Dr Donald E. Gardner, Chief, Biomedical Research Branch, Clinical
Studies Division, Health Effects Research Laboratory, US Environmental
Protection Agency, Research Triangle Park, NC, USA. The comments upon
which the second draft was based were received from the national focal
points for the WHO Environmental Health Criteria Programme in
Bulgaria, Canada, Czechoslovakia, the Federal Republic of Germany,
Japan, New Zealand, Poland, Sweden, the USA, and the USSR; and from
the Food and Agriculture Organization of the United Nations (FAO),
Rome, the World Meteorological Organization (WMO), Geneva, and the
International Union of Pure and Applied Chemistry (IUPAC). The
collaboration of these national institutions and international
organizations is gratefully acknowledged.
The Secretariat also wishes to acknowledge the most valuable
collaboration in the final phases of the preparation of this document,
of Professor Shy, Dr Gardner, Dr R. G. Derwent of the Environmental
and Medical Sciences Division, Atomic Energy Research Establishment,
Harwell, England, and Professor K. Schaffner of the Institute of
Radiation Chemistry at the Max-Planck-Institute for Carbon Research,
Mulheim an der Ruhr, Federal Republic of Germany.
This document is based primarily on original publications listed
in the reference section. Much valuable information may also be found
in other published criteria documents (US Department of Health,
Education and Welfare, 1970; North Atlantic Treaty Organization, 1974;
National Academy of Sciences, 1977).
Because biological knowledge concerning many components of
photochemical air pollution is limited, the Task Group agreed that a
definition of photochemical oxidants should be given early in the
criteria document.
Photochemical oxidants can be formed as the result of the
sunlight-induced oxidation of precursor pollutants emitted into the
atmosphere. These precursor compounds include the oxides of nitrogen
and a variety of hydrocarbons with different chemical reactivities
with respect to the formation of photochemical oxidants. The principal
oxidants are ozone, nitrogen dioxide, and the peroxyacylnitrates.
However, until recently, measurement methods specific for each of
these oxidants were not available and the most commonly employed
methods were affected, to some extent, by interference from other
atmospheric pollutants. Thus, when such studies are being considered,
it is important to know whether some correction has been made for this
interference, particularly in studies related to health effects in
man.
Although many other ingredients have been identified in
photochemical air pollution, there is little information available at
the moment concerning their biological significance, and they have not
been referred to in this document. The biological significance of
nitrogen dioxide has been reviewed and evaluated in another WHO
environmental health criteria document (World Health Organization,
1977).
Details of the WHO Environmental Health Criteria Programme
including some terms frequently used in the documents may be found in
the general introduction to the Environmental Health Criteria
Programme published together with the environmental health criteria
document on mercury (Environmental Health Criteria 1--Mercury, Geneva,
World Health Organization, 1976).a
The following conversion factors have been used in the present
documentb:
carbon monoxide (CO) 1 ppm = 1150 µg/m3
nitric oxide (NO) 1 ppm = 1230 µg/m3
nitrogen dioxide (NO2) 1 ppm = 1880 µg/m3
nitrous oxide (N2O) 1 ppm = 1800 µg/m3
ozone (O3) 1 ppm = 2000 µg/m3
peroxyacetylnitrate (PAN) 1 ppm = 5000 µg/m3
sulfur dioxide (SO2) 1 ppm = 2600 µg/m3
a Reprints available from the Division of Environmental Health,
World Health Organization, 1211 Geneva 27, Switzerland.
b When converting values expressed in ppm to µg/m3, the numbers
have been rounded up to 2 or, exceptionally 3 significant figures
and, in most cases, concentrations higher than 10 000 µg/m3, have
been expressed in mg/m3.
1. SUMMARY AND RECOMMENDATIONS FOR FURTHER RESEARCH AND OTHER ACTION
1.1 Summary
1.1.1 Chemistry and analytical methods
In the context of this report, photochemical oxidants are
understood to include ozone, nitrogen dioxide, and peroxyacylnitrates.
Many other compounds have been proposed as components of photochemical
air pollution but, as little information is available concerning their
biological significance, these substances have not been discussed in
this document. As nitrogen dioxide is an important air pollutant in
its own right, it is the subject of a separate document (World Health
Organization, 1977). Thus this report deals mainly with ozone and
"oxidants" as measured by the neutral buffered potassium iodide method
(NBKI).
Ozone and peroxyacylnitrates can be measured specifically by
chemiluminescent reactions and by gas chromatography in conjunction
with electron-capture detectors. These methods are highly sensitive
and are not subject to interference from other atmospheric pollutants.
To obtain the most reproducible data, sampling manifolds should be
made entirely of teflon or glass as oxidants in the inlet stream may
react with plastics or metal.
The terms "oxidant" or "total oxidant" are used to describe the
oxidizing property of sampled air as determined by its reaction with
neutral phosphate-buffered potassium iodide. Nitrogen dioxide in
sampled air enhances the reaction with potassium iodide, while sulfur
dioxide inhibits it. The terms "corrected oxidant" or "adjusted
oxidant" indicate that measurements have been corrected for the
presence of nitrogen dioxide and sulfur dioxide. Interference from
these substances is not entirely eliminated by the current systems
used for removing them from the inlet stream. The accuracy of the
analytical procedure for oxidant measurements also depends on the pH
of the buffer solution, reagent concentrations, and other variables.
1.1.2 Sources of photochemical oxidants and their precursors
Ozone, a natural constituent of the stratosphere formed by the
photolysis of molecular oxygen, can be transported by atmospheric
circulation into the lower atmosphere. Natural hydrocarbons including
terpenes from trees and vegetation are also subject to photochemical
reactions producing oxidants. These two processes are the natural
sources of background ozone concentrations.
Ozone and peroxyacylnitrates are formed in the lower atmosphere by
reactions between oxides of nitrogen and an array of photochemically
reactive hydrocarbons. The chemical structure and reactivity of each
organic hydrocarbon determines its importance in the formation of
oxidants. Motor vehicles, space heating, power plants, and industrial
processes are major sources of these oxidant precursors.
A balance between oxidizing and reducing agents would be
maintained in the atmosphere, thus avoiding accumulation of ozone and
other photochemical oxidants, if it were not for the photochemical
degradation of hydrocarbons into peroxy radicals. Peroxy radicals
rapidly convert nitric oxide to nitrogen dioxide, thus shifting the
equilibrium towards ozone production during daylight. At night,
emissions of nitric oxide into the atmosphere serve as a sink for
ozone.
Welding and the manufacture of hydrogen peroxide are the main
sources of occupational exposure to ozone. The use of ultraviolet
lamps, electrostatic precipitators, or photocopying machines may also
generate ozone.
1.1.3 Environmental concentrations and exposures
Since significant concentrations of oxidants in urban areas are
generally restricted to a period of 4-6 h per day, oxidant or ozone
data are most often reported in terms of maximum 1-h concentrations or
in terms of the number of hours or days recorded with hourly
concentrations exceeding a specified value. In isolated places far
removed from sources of pollution, maximum hourly ozone concentrations
of 100 µg/m3 (0.05 ppm) have been recorded. Transport of oxidants
from urban areas for distances of 100-700 kilometres appears to be a
widespread phenomenon, and 1-h ozone concentrations of 120 µg/m3
(0.06 ppm) or more have been observed in rural areas. In some large
cities, maximum 1-h oxidant concentrations exceed 200 µg/m3 (0.1 ppm)
on 5-30% of days, while in Los Angeles it is commonplace for maximum
1-h oxidant values to exceed 200 µg/m3 (0.1 ppm) on most days of the
month between May and October.
Diurnal patterns in oxidant levels are an important feature of the
urban environment and result from hourly changes in solar radiation
and pollutant emission intensity. Maximum hourly ozone levels
frequently occur around noon and are often preceded by peak
concentrations of nitrogen dioxide. Concentrations of
peroxyacetylnitrate are typically between 1/50th and 1/100th of those
of ozone and, in general, closely follow temporal variations in ozone
levels.
On a seasonal basis, oxidant concentrations tend to increase
during the high temperature season, and the frequency of days on which
oxidant concentrations exceed 200 µg/m3 (0.1 ppm) is greatest during
this period.
Oxidant concentrations indoors tend to be lower than those
outdoors, and are reduced by destructive reaction on material
surfaces. They are also reduced by activities that generate nitric
oxide such as smoking and cooking.
1.1.4 Effects on experimental animals
Ozone concentrations of 2000 µg/m3 (1.0 ppm) or less with
exposure periods of up to 24 h produced numerous morphological changes
in the lung parenchyma in several animal species. With prolonged
exposure (6-10 months), pulmonary damage such as emphysema,
atelectasis, focal necrosis, bronchopneumonia, and fibrosis has been
reported. The degree of morphological injury seems to be proportional
to the product of the concentration and the duration of exposure.
Disturbances in respiratory functions have been noted in
experimental animals exposed for 2-5 h to ozone concentrations of
520-2000 µg/m3 (0.26-1.0 ppm).
Biochemical studies that have been conducted to clarify the
mechanisms of ozone toxicity at subcellular level have mainly been
based on two hypotheses: (a) that oxidation of sulfhydryl groups by
ozone causes changes in metabolism that result in toxic effects; and
(b) that ozone reacts with unsaturated lipids to produce lipid
peroxidation and consequent cell damage. However, the subcellular
toxic action of ozone is still not fully understood. In other
biochemical studies, changes have been reported in the mitochondrial
oxygen consumption, the activities of lysosomal and microsomal
enzymes, and in the synthesis of nucleic acids. Studies on the
induction of oedema by lung histamine and on the effects of ozone on
the surface active substance have been inconclusive.
In small rodents, "tolerance" to oedematigenous effects caused by
exposure to ozone concentrations of 2000-8000 µg/m3 (1-4 ppm) has
been obtained by pre-exposure to an ozone concentration of at least
600 µg/m3 (0.3 ppm). However, this did not seem to provide protection
against effects that impair the phagocytic activity of macrophages.
Resistance to artificially-induced respiratory infection was
reduced in several animal species by 3-4 h exposure to ozone
concentrations of 160-800 µg/m3 (0.08-0.40 ppm). The effect of the
ozone was further enhanced by a third stressor such as cold or
exercise. Various mechanisms have been proposed for the enhanced
infectivity including inactivation of a protective factor that favours
survival of alveolar macrophages, inactivation of alveolar macrophage
secretory enzymes, depression of bactericidal activity, and reduction
in the phagocytic activity of alveolar macrophages.
It has been shown that exposure of pregnant mice to ozone at
200-400 µg/m3 (0.1-0.2 ppm) for 7 h per day, for 15 days, increased
neonatal mortality and that exposure of mice to ozone at 400 µg/m3
(0.2 ppm) for 6 h and rats to 1000 µg/m3 (0.5 ppm) for 45 min
resulted in significant losses in motor activity. However, it is not
clear whether these effects are due to the direct action of ozone or
oxidizing agents or whether they are secondary reactions to damage in
the respiratory system caused by ozone.
Data concerning other extrapulmonary effects and the
carcinogenicity and mutagenicity of ozone are inadequate.
Effects produced by exposure to various mixtures of ozone and
other air pollutants, and ambient air containing elevated
concentrations of oxidants are mainly similar to those seen from
exposure to ozone alone. However, one study reported that a single
exposure to a mixture of ozone and nitrogen dioxide produced an
additive effect in reducing resistance to respiratory infection in
mice, and that repeated exposure might give rise to a synergistic
effect. Some effects such as those on growth have only been reported
with exposure to mixtures of pollutants. A deficiency of vitamin E has
also been reported to enhance the toxic effects of ozone.
1.1.5 Effects on man
1.1.5.1 Controlled exposures
A large number of sensory effects in man have been studied under
controlled conditions. The odour threshold for ozone has been shown to
be 15-40 µg/m3 (0.008-0.02 ppm) and the lowest oxidant concentration
producing eye irritation has been suggested to be 200 µg/m3
(0.1 ppm). Various measures of visual perception were affected by a
3-h exposure to ozone concentrations of 400-1000 µg/m3 (0.2-0.5 ppm).
Controlled exposure of healthy male subjects to ozone
concentrations ranging from 200 to 2000 µg/m3 (0.1-1.0 ppm) has been
reported to cause increased airway resistance and decreased
ventilatory performance. Effects at the lower end of this dose-range
were elicited when test subjects carried out intermittent light
exercise during a 2-h exposure period. One investigator failed to
observe changes in airway resistance at an ozone exposure of
500 µg/m3 (0.25 ppm) for 2 h. Thus, not all investigators were in
agreement concerning the lowest experimental ozone exposures that
affect airway resistance; however, three investigators found increased
airway resistance at an ozone concentration of 740 µg/m3 (0.37 ppm).
A 2-h exposure to a combination of ozone at 50 µg/m3 (0.025 ppm),
nitrogen dioxide at 100 µg/m3 (0.05 ppm) and sulfur dioxide at
260 µg/m3 (0.1 ppm) did not have any effect on airway resistance.
However, this combined exposure did enhance the bronchoconstrictor
effect of acetylcholine. A combination of an ozone concentration of
740 µg/m3 (0.37 ppm) and a sulfur dioxide concentration of 960 µg/m3
(0.37 ppm) had a potentiated effect on the impairment of ventilatory
performance compared with the effects of the same concentration of
each gas administered singly. In other studies in which various
mixtures of ozone and other air pollutants were used, sulfur dioxide
seemed to potentiate the effect of ozone more than nitrogen dioxide.
Studies were also performed on volunteer patients with chronic
pulmonary disease. The respiratory function of these patients showed
an improvement when they breathed filtered air for 40 h or more
compared with unfiltered ambient air with an oxidant concentration of
about 400 µg/m3 (0.2 ppm).
Exposures to a peroxyacetylnitrate concentration of 1350 µg/m3
(0.27 ppm) caused minor changes in variables that reflect
cardiorespiratory and temperature regulation.
1.1.5.2 Industrial exposure
Several cases of severe ozone intoxication have been reported in
welders using inert gas-shielded, consumable electrodes which greatly
increased the ultraviolet irradiation of the work area. At ozone
concentrations of 600-1600 µg/m3 (0.3-0.8 ppm), an increasing number
of welders complained of chest constriction and irritation of the
throat, while acute symptoms disappeared when ozone levels were
reduced to 500 µg/m3 (0.25 ppm) or less.
There are very few studies on long-term industrial exposure to
ozone and in most of them the exposure-response relationship has
either not been well evaluated or has been confounded by other
coexistent pollutants.
1.1.5.3 Community exposure
So far, no evidence has been obtained to indicate an association
between peak oxidant concentrations and variations in the daily
mortality rate of the general population. On the other hand, the
association of oxidant levels with eye and respiratory irritation has
been well documented, and in one study made on student nurses in Los
Angeles, a significant increase in the frequency of cough, eye, and
chest discomfort, and headache was demonstrated when maximum hourly
oxidant concentrations reached 100-580 µg/m3 (0.05-0.29 ppm). Hourly
oxidant levels were also correlated with decreased performance in high
school cross-country runners, and the estimate of the lowest
concentration at which this effect occurred was an hourly oxidant
concentration of 240 µg/m3 (0.12 ppm).
Effects of oxidants on children have been extensively studied. A
correlation was detected between the decreases in airway conductance
and ventilatory performance of school children and increase in ozone
levels over a range up to 560 µg/m3 (0.28 ppm); other pollutants
monitored at the same time included nitric oxide, nitrogen dioxide,
sulfur dioxide, and particulate matter. Combinations of these
pollutants may have been responsible for the observed effects. An
attempt was also made to relate the rates of illness in school
children during an influenza epidemic to the pollution gradient which
existed during the season of peak oxidant concentrations, but there
was no significant association. In Japan, a variety of respiratory and
systemic symptoms were reported among school children on several smog-
alert days. The systemic symptoms appeared to be attributable to a
psychosomatic response among the students.
In a study on the incidence of acute respiratory diseases, peak
concentrations of oxidants and mean concentrations of sulfur dioxide
and nitrogen dioxide were found to be correlated with acute episodes
of pharyngitis, bronchitis, and upper respiratory infections among
college students in the Los Angeles Basin. However, there was no
association between the admissions to a hospital in Los Angeles for
cardiovascular conditions and oxidant concentrations.
A few reports are available on the effects of oxidants on patients
with pre-existing diseases. One study suggested a relationship between
the proportion of asthmatics who experienced asthma attacks and daily
peak oxidant levels, but results were confounded by concomitant
seasonal changes.
Studies on the effect of long-term exposure to photochemical
oxidants are relatively few. To date, urban differences in lung cancer
mortality rates in Californian cities do not suggest an influence of
oxidant exposure on lung cancer risk. Similarly, studies have not
revealed any relationships between the prevalence of chronic
respiratory disease and geographical differences in oxidant
concentrations.
As in all studies of urban populations, epidemiological studies of
oxidant exposure cannot yield results on health effects attributable
only to oxidants, since photochemical air pollution typically consists
of ozone, nitrogen dioxide, peroxyacylnitrates, nitrate and sulfate
particulates, and other components. In general, however, observed
health effects were found to be more closely correlated with ozone
levels than with levels of other pollutants.
1.1.6 Evaluation of health risks
Although it is known that ozone is only one of a number of
photochemical oxidants and that there are many other components of
photochemical air pollution, it is the only substance for which a
health protection guideline can be given, based on existing exposure-
effect data.
From controlled human and community exposure studies, ozone
concentrations at which the first adverse effects in man appear have
been reported to be 200-500 µg/m3 (0.1-0.25 ppm). Experimental
studies on animals support these estimates.
The Task Group agreed that a 1-h exposure to ozone of
100-200 µg/m3 (0.05-0.10 ppm) (measured by chemiluminescence) should
be used as a guideline for the protection of public health and that a
safety factor could not be applied because of the relatively high
natural concentrations of ozone.
The Group also considered that a maximum 1-h oxidant concentration
of 120 µg/m3 (0.06 ppm) (measured by the NBKI method), which was
recommended as the long-term goal by the WHO Expert Committee in 1972
and is approximately equal to the highest natural background level of
oxidants, would be the best estimate of the exposure limit for
oxidants for the general population.
In response to the question of whether the proposed guideline was
realistic in view of natural exposure levels and the long-distance
transport of ozone, the Group expressed the view that every effort
should, nevertheless, be made to develop control strategies for
achieving the proposed guideline or at least for not exceeding it more
than once a month.
1.2 Recommendations for Further Research and Other Action
1.2.1 Health effects research
The WHO Task Group was particularly concerned with the potential
for enhanced biological response of combined or sequential exposure of
human populations to nitrogen dioxide and ozone. The recommendations
listed take into account this concern as well as some other gaps in
knowledge concerning the health effects of photochemical oxidants.
(a) The following information should be obtained by means of carefully
controlled exposure studies on human volunteers:
i. data on the lowest concentration of ozone at which various
lung function variables are affected;
ii. effects of sequential exposure to nitrogen dioxide and ozone;
iii. effects of ozone pre-exposure on the sensitivity of airways
to bronchoconstrictor agents;
iv. effects on airways of combined exposure to: ozone and sulfur
dioxide; ozone and tobacco smoke; ozone and increased
temperature or other stressors.
(b) Epidemiological studies should be conducted to evaluate:
i. effects of oxidant exposure on the susceptibility of human
populations to respiratory infections;
ii. comparative effects of exposure of urban populations to
combined nitrogen dioxide and ozone (oxidants) versus
exposure of rural populations to ozone alone. Measurements of
lung function and other variables shown to be affected by
photochemical oxidant and nitrogen dioxide peaks may be used
for these studies.
(c) Experimental animal studies should be conducted to evaluate:
i. effects of intermittent exposure to ozone, mimicking ambient
air exposures;
ii. effects of the joint action of ozone and other pollutants
and/or other environmental stressors;
iii. mechanism of tolerance to oxidants;
iv. carcinogenic, cocarcinogenic, and mutagenic effects of ozone;
v. effects of ozone exposure on humoral and cellular immunity.
1.2.2 Photochemical oxidant control
In order to reduce exposure of the general population to
photochemical oxidants, the ratio of reactive hydrocarbons to oxides
of nitrogen must be carefully controlled as well as their absolute
levels. Unilateral or unbalanced control may result in higher levels
of ozone and/or nitrogen dioxide. The Task Group recommended that to
achieve a balanced control of both reactive hydrocarbons and oxides of
nitrogen, appropriate laboratory and field studies should be conducted
to evaluate the effects that both groups of compounds may have on the
control of photochemical oxidants.
2. CHEMISTRY AND ANALYTICAL METHODS
2.1 Chemical and Physical Properties
2.1.1 Ozone
Ozone is one of the strongest oxidizing agents; only fluorine,
atomic oxygen, and oxygen fluoride (OF2) have higher redox
potentials. Ozone is an important constituent of the upper atmosphere.
Although it is present in only small concentrations (a few parts per
million), ozone is responsible for shielding the earth from
ultraviolet radiation (UV-B) that is biologically harmful. Formation
of ozone occurs predominantly at altitudes above 30 km where solar UV
radiation with wavelengths of less than 242 nm slowly dissociates
molecular oxygen (O2) into oxygen atoms (O). These oxygen atoms
rapidly combine with molecular oxygen to form ozone. Ozone strongly
absorbs solar radiation in the wavelength region of 240-320 nm. It is
this absorption that shields the earth from harmful UV radiation (see
for example National Academy of Sciences, 1977).
Some of the physical properties of ozone, the most abundant
ubiquitous atmospheric oxidant, are listed in Table 1.
Table 1. Physical properties of ozonea
Chemical formula O3
Physical state at NTPb colourless gas
Relative molecular mass 48.0
Melting point - 192.7°C
Boiling point - 111.9°C
Density relative to air 1.658
Vapour density
at 0°C. 101 kPa (760 mmHg) 2.14 g/litre
at 25°C. 101 kPa (760 mmHg) 1.96 g/litre
Solubility at 0°C, 101 kPa (760 mmHg) 0.494 ml/100 ml water
a From: US Department of Health, Education and Welfare (1970).
b NPT = normal temperature and pressure. i.e. 25°C and 101 kPa
(760 mmHg).
The absorption of electromagnetic radiation by ozone in the
ultraviolet and infrared regions is used in analytical methods.
2.1.2 Peroxyacylnitrates
Photochemical processes produce other oxidizing species besides
ozone. These include peroxyacylnitrates, which have the following
general structure:
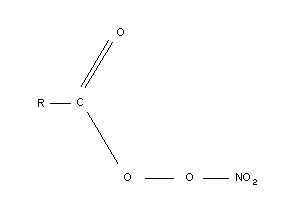 This class of compounds includes:
R = CH3: peroxyacetylnitrate (PAN)
R = C2H5: peroxyproprionylnitrate (PPN)
R = C6H5: peroxybenzoylnitrate (PBzN)
Although each of these species has received some attention, monitoring
data are available only for peroxyacetylnitrate. The physical
properties of this species are described in Table 2.
Table 2. Physical properties of peroxyacetylnitratea
O
"
"
Chemical formula CH3COONO2
Physical state at NTP colourless liquid
Relative molecular mass 121
Boiling point No true boiling point, compound
decomposes before boiling
Vapour pressure at room About 2 kPa (15 mmHg)
temperature
a From: US Department of Health, Education and Welfare (1970).
Peroxyacylnitrates have two characteristics that help in their
detection at low concentrations i.e., absorption in the infrared
region of the spectrum and electron-capturing ability (Stephens,
1969). The second of these characteristics is exploited in the
electron-capture detector which, when used in conjunction with gas
chromatography, provides the basis of an accepted method for the
measurement of peroxyacetylnitrate levels in air.
2.1.3 Other oxidants
Hydrogen peroxide has been identified as a potential photochemical
oxidant. However, it is an extremely difficult substance to detect
specifically in the atmosphere and, at present, it is not possible to
assess its significance as a photochemical air pollutant.
2.2 Atmospheric Chemistry
There are no significant primary emissions of ozone into the
atmosphere and all the ozone found has been formed by chemical
reactions that occur in the air.
In the upper atmosphere, ozone is mainly formed by the action of
solar radiation on molecular oxygena:
O2 + radiation (lambda < 175 nm) --> O(3P) + O(1D) (1)
O2 + radiation (lambda < 242 nm) --> 2 O(3P) (2)
O(3P) + O2 + M --> O3 + M (3)
In the lower atmosphere, ozone-producing processes involve
absorption of solar radiation by nitrogen dioxide:
NO2 + radiation (lambda < 430 nm) ka NO + O(3P) (4)
->
O(3P) + O2 + M --> O3 + M (3)
O3 + NO kb NO2 + O2 (5)
->
a O(3P) is the symbol for atomic oxygen in its lowest energy state
("triplet oxygen"); O(1D) represents the next higher energy state
of atomic oxygen ("singlet oxygen"); M is another molecule that
must be present for the reaction to take place ("third body"),
usually oxygen or nitrogen. Square brackets e.g. [NO] represent
the concentration of the chemical species inside the brackets.
Thus, the mechanism of ozone production during the sunlight
irradiation of polluted air is simple in outline (ozone formation by
the interaction of molecular oxygen with the photoproducts of nitrogen
dioxide) but complex in detail. It is based on reactions (3)-(5) which
give the following expression for the ozone concentration:
ka[NO2]
O3 approx. (6)
kb[NO]
In polluted atmospheres, the most readily observed sink for ozone
involves the emission of nitric oxide. At night-time, the equilibrium
expressed by equation (6) is displaced by the rapid reaction (5), and
continuous nitric oxide emissions rapidly reduce the ozone
concentration to undetectable levels. This atmospheric chemical
process is supplemented by the destruction of ozone at ground level by
contact with soil and vegetation surfaces (Regener & Aldaz, 1969).
In a hydrogen-free atmosphere, a fairly even balance between
oxidizing and reducing agents would be maintained. However, peroxy
radicals (RO2) produced by the photochemical degradation of
hydrocarbons have the important property of reacting with nitric oxide
thereby converting it to nitrogen dioxide. The significance of any
process resulting in the conversion of nitric oxide to nitrogen
dioxide is that during daylight the equilibrium expressed by equation
(6) shifts in favour of ozone production.
There are no significant primary emissions into the atmosphere of
peroxyacylnitrates all of which are formed by atmospheric chemical
reactions of the general type:
RCO(O2) + NO2 --> RCO(O2)NO2 (7)
2.3 Measurement of Photochemical Oxidant Concentrations
2.3.1 Sampling
Ozone is highly reactive with most materials including plastics,
metals, and fabrics. The most reproducible ambient concentration data
have been obtained using sampling manifolds made entirely of teflon or
glass.
As with oxides of nitrogen, residence time in these sampling
manifolds requires specific consideration when sampling air containing
nitric oxide, nitrogen dioxide, and ozone during daylight. Since the
equilibrium shown in equation (6) is disturbed inside the sampling
manifold, ozone concentrations can be underestimated if sampling times
exceed 10 seconds (Butcher & Ruff, 1971).
When measuring ozone, the site of sampling should be selected with
extreme care as exhaust gases from motor vehicles and central heating
appliances readily remove ozone.
2.3.2 Analytical methods
For measurement purposes, oxidants are generally divided into
three categories: ozone, total oxidants, and peroxyacylnitrates. Ozone
and peroxyacylnitrates can be measured specifically while total
oxidants are usually determined as a class of compounds.
The reagent employed to measure the oxidizing property of
photochemical oxidants is a solution of neutral-buffered potassium
iodide (NBKI). This reagent reacts with ozone, nitrogen dioxide, and
peroxyacylnitrates. Reducing agents such as sulfur dioxide have an
inhibiting effect on the reagent solution and must be removed, from
the inlet stream. To this extent, reaction with the potassium iodide
reagent is a measure of the net oxidizing capacity of a sample of
ambient air.
The terms "oxidant" or "total oxidant" are used to describe the
net oxidizing capacity of the sampled air as determined by reaction
with NBKI. The terms "corrected oxidant" or "adjusted oxidant"
indicate that measurements have been corrected for the presence of
reducing agents (sulfur dioxide) or other oxidizing agents (nitrogen
dioxide) in the air.
Long-term averaging values are usually meaningless for
photochemical oxidants and attention is directed to 1-h values
(section 4.3). Thus, the use of continuous instruments with automatic
data collection systems has an advantage. Manual methods are available
for the determination of photochemical oxidants and these may be
automated to a certain extent.
2.3.2.1 Ozone
Continuous ozone analysers are usually based on the
chemiluminescent reaction of ozone with ethylene (Nederbragt et al.,
1965; Warren & Babcock, 1970). A chemiluminescence method based on the
reaction of ozone with certain dyes (Regener, 1964) has been further
developed in the Netherlands (Guicherit, 1975). These techniques are
not subject to atmospheric interference, are highly sensitive
(2 µg/m3 or 0.001 ppm), and perform well under field conditions. The
methods are not absolute and hence some form of calibration involving
potassium iodide or UV absorption spectroscopy is necessary.
Specific determination of ozone may be accomplished by ultraviolet
absorption spectroscopy in the 200-300 nm wavelength range (Hodgeson,
1972). The advantages of this method are that it is highly sensitive,
does not require cylinders of explosive gases, and that it gives an
absolute measurement (De More & Patapoff, 1976). Very high
concentrations of certain hydrocarbons or mercury vapour may cause
interference.
The dihydroacridine method is a specific, inexpensive, manual
method for ozone determination in which samples are taken every
30 min. Interference effects can be eliminated by parallel sampling
with an identical system fitted with an ozone scrubber. This is the
only suitable method for short-term ozone measurements when a
chemiluminescent or UV absorption instrument is not available (World
Health Organization, 1976).
2.3.2.2 Total oxidants
Total oxidants are generally measured using acid- or neutral-
buffered solutions of potassium iodide (Byers & Saltzman, 1958; US
Department of Health, Education and Welfare, 1970; World Health
Organization, 1976). There are indications that the accuracy of the
method depends on the pH of the buffer solution, the concentration of
the reagent, and other variables. In addition to these drawbacks, the
method is basically unspecific. Any other oxidizing or reducing
species can cause interference. Such interference results when
sampling air containing sulfur dioxide, chlorine, nitrogen dioxide, or
peroxides. Nitrogen dioxide and sulfur dioxide interfere most by
giving erroneously high and low values, respectively.
Interference from sulfur dioxide can be eliminated by passing the
air sample through glass fibre filters impregnated with a suitable
material such as chromium trioxide. Humidity often renders these
scrubbers ineffective because under such conditions they oxidize
nitric oxide to nitrogen dioxide, thus increasing nitrogen dioxide
interference. As there is also ozone loss after extended use, these
scrubbers are not entirely satisfactory (World Health Organization,
1976).
Interference from nitrogen dioxide is more difficult to eliminate.
Some form of adjustment can be made to the total oxidant reading if
continuous nitrogen dioxide measurements are also available. This
correction depends to some extent on experimental conditions and, in
view of the problems previously noted with measurements of oxides of
nitrogen, may not be easy to make.
Other methods are available for the determination of oxidants but
none of them is commonly used. They include the ferrous ammonium
sulphate, alkali potassium iodide, and phenolphthalein methods (World
Health Organization, 1976).
2.3.2.3 Peroxyacetylnitrate
The infrared absorption and electron-capturing properties of
peroxyacetylnitrate have been used for its measurement in simulated
and real atmospheres (Stephens, 1969). Electron-capture detectors
preceded by gas chromatographic separation offer a limit of detection
of 0.5 µg/m3 (0.0001 ppm) for the automatic determination of ambient
concentrations of peroxyacetylnitrate. Water vapours may interfere
with the passage of peroxyacetylnitrate along the chromatographic
column (Farwell & Rasmussen, 1976) and trace contaminants in cylinder
gases can be a problem because of their slow accumulation on
chromatography columns used for continuous measurements.
Peroxyacetylnitrate can also be measured by the hydrolysis of
peroxyacetylnitrate solution to give nitrite ions that can be
determined colorimetrically. However, this method suffers from
interference by nitrogen dioxide (Konno & Okita, 1974; Stephens,
1969).
3. SOURCES OF PHOTOCHEMICAL OXIDANTS AND THEIR PRECURSORS
3.1 Natural Sources
As mentioned previously, ozone is a natural constituent of the
upper atmosphere. A small amount of ozone, which is formed by the
photolysis of molecular oxygen, is carried by atmospheric circulation
into the lower atmosphere (section 4). Natural sources of ozone are
associated with the passage of cold fronts (Ripperton et al., 1971)
and atmospheric electrical phenomena (US Department of Health,
Education & Welfare, 1970).
The photochemical oxidation of natural hydrocarbons including
terpenes from trees and other vegetation takes place during daylight
hours (Rasmussen, 1972; Went, 1966). Generally, these processes are
difficult to study because of the low concentrations of the
hydrocarbons and their short atmospheric residence. However, hazes
often associated with certain forest regions may well be explained by
photochemical aerosol production from natural airborne hydrocarbons
(Grimsrud et al., 1975).
3.2 Man-made Sources of Oxidant Precursors
Emissions of oxides of nitrogen from man-made activities include
important contributions from both stationary and mobile sources (World
Health Organization, 1977).
By comparison, the position with regard to hydrocarbon precursors
is much more complex. In this instance, the term "hydrocarbon" refers
to a class of pollutants that contain carbon atoms and produce
oxidants under irradiation in the presence of oxides of nitrogen. This
is a very wide definition that covers many hundreds of different
organic compounds emitted into the atmosphere by man-made processes.
Not all organic compounds play an equal role in oxidant formation.
The relative importance of each organic compound in this process
depends on its chemical structure and reactivity. The chemical
structure determines the number of nitric oxide --> nitrogen dioxide
conversions involved in the atmospheric degradation of each organic
compound and ozone may be produced at each of these steps (Calvert,
1976; Demerjian et al., 1974). Reactivity requires special attention
because the time scale for ozone or peroxyacetylnitrate production is
related to the time scale for hydrocarbon degradation. For the
so-called "highly reactive" hydrocarbons this may be 1 h or less. It
may take up to 3 h for the less reactive hydrocarbons and require
several days for the so-called unreactive hydrocarbons. Even in the
presence of reactive hydrocarbons, ozone production may only become
significant 10 km downwind from a source, and peak ozone
concentrations may be observed over 60 km downwind (White et al.,
1976). Thus, the relationship between hydrocarbon precursors and
observed oxidant concentrations in large urban areas may be obscured.
There may be marked differences in the nature of the oxidants in air
sampled during the early morning when peak concentrations of oxidant
precursors occur and in that sampled after midday when ozone
concentrations are elevated.
In view of this complexity, it is clearly necessary to have some
form of rational assessment of hydrocarbon reactivity (Darnall et al.,
1976). Complete inventories of individual substances are only
available for gasoline-engine exhaust emissions; for the storage,
distribution, and use of organic substances such as petroleum
products; and for specific industrial processes. An example of total
emissions of oxides of nitrogen and hydrocarbons is given in Table 3.
These figures are presented merely to show the wide diversity of the
processes responsible for oxidant precursor emissions; it is not
sufficient to consider motor vehicles as the only important source.
Table 3. Total emissions of hydrocarbons and oxides of nitrogen
in the Federal Republic of Germany in 1971 in thousands
of tonnesa
Source Total emission (103 tonnes)
Hydrocarbons Oxides of nitrogen
Domestic heating 173 117
Traffic exhaust 325 308
Power plants 14 373
Industrial combustion 53 470
Industrial processes 955 30
a From: Federal Republic of Germany, Ministry of Internal
Affairs (1974).
3.3 Indoor Sources
The use of ultraviolet lamps, electrostatic precipitators,
photocopying machines, and odour control equipment can lead to
increases in indoor concentrations of ozone. Welding and the
manufacture of hydrogen peroxide are important indoor sources of ozone
in industry and pose problems in occupational health (section 6.2).
However, other indoor activities such as smoking and cooking with
gas stoves tend to produce elevated nitric oxide concentrations that
destroy ozone and peroxyacylnitrates (Schuck & Stephens, 1969).
3.4 Oxidant-precursor Relationships
While potential synergistic health effects of oxidants and
nitrogen dioxide are recognized, programmes for the control of these
two pollutants are usually developed independently of one another.
From a control point of view, however, such an approach is not
justified because of the complexity of the photochemical reaction
system. The only predictable way to control the formation of
photochemical oxidants is to reduce the initial components in
incremental steps. Decreasing the primary emission of oxides of
nitrogen without reducing hydrocarbon emissions will lead to an
increase in oxidant levels.
A further example of the uncoordinated approach to air pollution
abatement can be illustrated by examination of the current methods
applied to hydrocarbon control. These methods have focused on
increasing combustion efficiency, an action which does reduce the
hydrocarbon concentrations in exhaust but has the side effect of
causing an increase in atmospheric concentrations of nitrogen dioxide.
As expected, changing the ratio of oxidant precursors has a complex
effect on the formation of photochemical oxidants. For example, the
effect of this change in ratio in the south coast air basin of
California has been to produce substantial reductions in the
concentrations of oxidants in Los Angeles town centre. However, the
reductions become smaller at downwind sites and at 80 kilometres
downwind, an increase in maximum daily values was observed
(Dimitriades, 1976).
4. ENVIRONMENTAL CONCENTRATIONS AND EXPOSURES
4.1 Background Concentrations
Ozone concentrations in places far removed from sources of
pollution show fairly constant values with some seasonal variations
interspersed with irregular maxima due to specific meteorological
events. Monthly mean concentrations of ozone, which vary considerably
with both latitude and the month of the year, are illustrated in
Fig. 1. The reported values range from 10 to 80 µg/m3
(0.005-0.04 ppm).
Many observations indicate that hourly values range from 10 to
100 µg/m3 (0.005-0.05 ppm) (Berry, 1964; Haagen-Smit, 1952; US
Department of Health, Education and Welfare, 1970). However, higher
values have been observed on isolated occasions. In a study at Chalk
River, Ontario, Canada, a maximum value was observed of 120 µg/m3
(0.06 ppm) for 4 h (US Department of Health, Education and Welfare,
1970).
4.2 Rural Areas
Polluted air masses from urban and industrial areas can affect
suburban and rural areas in the direction of the prevailing wind for
considerable distances. Elevated oxidant concentrations have been
measured in a number of downwind rural locations where local sources
of oxidant precursors were insignificant. It has been suggested that
long-distance atmospheric transport might be responsible for many
cases of high oxidant concentrations found over rural areas and some
specific examples are given in Table 4.
A Midwest study in the USA in 1974 showed elevated ozone levels
over an extensive rural area (radius of over 240 km) due to the
combined effects of a number of urban areas. High ozone levels from
one particular urban area extended as far as 48-80 kilometres downwind
(US Environmental Protection Agency, 1976).
Observations of a 1-h concentration of 120 µg/m3 (0.06 ppm) in
rural areas can generally be associated with the transport of man-made
oxidants from distant sources.
This class of compounds includes:
R = CH3: peroxyacetylnitrate (PAN)
R = C2H5: peroxyproprionylnitrate (PPN)
R = C6H5: peroxybenzoylnitrate (PBzN)
Although each of these species has received some attention, monitoring
data are available only for peroxyacetylnitrate. The physical
properties of this species are described in Table 2.
Table 2. Physical properties of peroxyacetylnitratea
O
"
"
Chemical formula CH3COONO2
Physical state at NTP colourless liquid
Relative molecular mass 121
Boiling point No true boiling point, compound
decomposes before boiling
Vapour pressure at room About 2 kPa (15 mmHg)
temperature
a From: US Department of Health, Education and Welfare (1970).
Peroxyacylnitrates have two characteristics that help in their
detection at low concentrations i.e., absorption in the infrared
region of the spectrum and electron-capturing ability (Stephens,
1969). The second of these characteristics is exploited in the
electron-capture detector which, when used in conjunction with gas
chromatography, provides the basis of an accepted method for the
measurement of peroxyacetylnitrate levels in air.
2.1.3 Other oxidants
Hydrogen peroxide has been identified as a potential photochemical
oxidant. However, it is an extremely difficult substance to detect
specifically in the atmosphere and, at present, it is not possible to
assess its significance as a photochemical air pollutant.
2.2 Atmospheric Chemistry
There are no significant primary emissions of ozone into the
atmosphere and all the ozone found has been formed by chemical
reactions that occur in the air.
In the upper atmosphere, ozone is mainly formed by the action of
solar radiation on molecular oxygena:
O2 + radiation (lambda < 175 nm) --> O(3P) + O(1D) (1)
O2 + radiation (lambda < 242 nm) --> 2 O(3P) (2)
O(3P) + O2 + M --> O3 + M (3)
In the lower atmosphere, ozone-producing processes involve
absorption of solar radiation by nitrogen dioxide:
NO2 + radiation (lambda < 430 nm) ka NO + O(3P) (4)
->
O(3P) + O2 + M --> O3 + M (3)
O3 + NO kb NO2 + O2 (5)
->
a O(3P) is the symbol for atomic oxygen in its lowest energy state
("triplet oxygen"); O(1D) represents the next higher energy state
of atomic oxygen ("singlet oxygen"); M is another molecule that
must be present for the reaction to take place ("third body"),
usually oxygen or nitrogen. Square brackets e.g. [NO] represent
the concentration of the chemical species inside the brackets.
Thus, the mechanism of ozone production during the sunlight
irradiation of polluted air is simple in outline (ozone formation by
the interaction of molecular oxygen with the photoproducts of nitrogen
dioxide) but complex in detail. It is based on reactions (3)-(5) which
give the following expression for the ozone concentration:
ka[NO2]
O3 approx. (6)
kb[NO]
In polluted atmospheres, the most readily observed sink for ozone
involves the emission of nitric oxide. At night-time, the equilibrium
expressed by equation (6) is displaced by the rapid reaction (5), and
continuous nitric oxide emissions rapidly reduce the ozone
concentration to undetectable levels. This atmospheric chemical
process is supplemented by the destruction of ozone at ground level by
contact with soil and vegetation surfaces (Regener & Aldaz, 1969).
In a hydrogen-free atmosphere, a fairly even balance between
oxidizing and reducing agents would be maintained. However, peroxy
radicals (RO2) produced by the photochemical degradation of
hydrocarbons have the important property of reacting with nitric oxide
thereby converting it to nitrogen dioxide. The significance of any
process resulting in the conversion of nitric oxide to nitrogen
dioxide is that during daylight the equilibrium expressed by equation
(6) shifts in favour of ozone production.
There are no significant primary emissions into the atmosphere of
peroxyacylnitrates all of which are formed by atmospheric chemical
reactions of the general type:
RCO(O2) + NO2 --> RCO(O2)NO2 (7)
2.3 Measurement of Photochemical Oxidant Concentrations
2.3.1 Sampling
Ozone is highly reactive with most materials including plastics,
metals, and fabrics. The most reproducible ambient concentration data
have been obtained using sampling manifolds made entirely of teflon or
glass.
As with oxides of nitrogen, residence time in these sampling
manifolds requires specific consideration when sampling air containing
nitric oxide, nitrogen dioxide, and ozone during daylight. Since the
equilibrium shown in equation (6) is disturbed inside the sampling
manifold, ozone concentrations can be underestimated if sampling times
exceed 10 seconds (Butcher & Ruff, 1971).
When measuring ozone, the site of sampling should be selected with
extreme care as exhaust gases from motor vehicles and central heating
appliances readily remove ozone.
2.3.2 Analytical methods
For measurement purposes, oxidants are generally divided into
three categories: ozone, total oxidants, and peroxyacylnitrates. Ozone
and peroxyacylnitrates can be measured specifically while total
oxidants are usually determined as a class of compounds.
The reagent employed to measure the oxidizing property of
photochemical oxidants is a solution of neutral-buffered potassium
iodide (NBKI). This reagent reacts with ozone, nitrogen dioxide, and
peroxyacylnitrates. Reducing agents such as sulfur dioxide have an
inhibiting effect on the reagent solution and must be removed, from
the inlet stream. To this extent, reaction with the potassium iodide
reagent is a measure of the net oxidizing capacity of a sample of
ambient air.
The terms "oxidant" or "total oxidant" are used to describe the
net oxidizing capacity of the sampled air as determined by reaction
with NBKI. The terms "corrected oxidant" or "adjusted oxidant"
indicate that measurements have been corrected for the presence of
reducing agents (sulfur dioxide) or other oxidizing agents (nitrogen
dioxide) in the air.
Long-term averaging values are usually meaningless for
photochemical oxidants and attention is directed to 1-h values
(section 4.3). Thus, the use of continuous instruments with automatic
data collection systems has an advantage. Manual methods are available
for the determination of photochemical oxidants and these may be
automated to a certain extent.
2.3.2.1 Ozone
Continuous ozone analysers are usually based on the
chemiluminescent reaction of ozone with ethylene (Nederbragt et al.,
1965; Warren & Babcock, 1970). A chemiluminescence method based on the
reaction of ozone with certain dyes (Regener, 1964) has been further
developed in the Netherlands (Guicherit, 1975). These techniques are
not subject to atmospheric interference, are highly sensitive
(2 µg/m3 or 0.001 ppm), and perform well under field conditions. The
methods are not absolute and hence some form of calibration involving
potassium iodide or UV absorption spectroscopy is necessary.
Specific determination of ozone may be accomplished by ultraviolet
absorption spectroscopy in the 200-300 nm wavelength range (Hodgeson,
1972). The advantages of this method are that it is highly sensitive,
does not require cylinders of explosive gases, and that it gives an
absolute measurement (De More & Patapoff, 1976). Very high
concentrations of certain hydrocarbons or mercury vapour may cause
interference.
The dihydroacridine method is a specific, inexpensive, manual
method for ozone determination in which samples are taken every
30 min. Interference effects can be eliminated by parallel sampling
with an identical system fitted with an ozone scrubber. This is the
only suitable method for short-term ozone measurements when a
chemiluminescent or UV absorption instrument is not available (World
Health Organization, 1976).
2.3.2.2 Total oxidants
Total oxidants are generally measured using acid- or neutral-
buffered solutions of potassium iodide (Byers & Saltzman, 1958; US
Department of Health, Education and Welfare, 1970; World Health
Organization, 1976). There are indications that the accuracy of the
method depends on the pH of the buffer solution, the concentration of
the reagent, and other variables. In addition to these drawbacks, the
method is basically unspecific. Any other oxidizing or reducing
species can cause interference. Such interference results when
sampling air containing sulfur dioxide, chlorine, nitrogen dioxide, or
peroxides. Nitrogen dioxide and sulfur dioxide interfere most by
giving erroneously high and low values, respectively.
Interference from sulfur dioxide can be eliminated by passing the
air sample through glass fibre filters impregnated with a suitable
material such as chromium trioxide. Humidity often renders these
scrubbers ineffective because under such conditions they oxidize
nitric oxide to nitrogen dioxide, thus increasing nitrogen dioxide
interference. As there is also ozone loss after extended use, these
scrubbers are not entirely satisfactory (World Health Organization,
1976).
Interference from nitrogen dioxide is more difficult to eliminate.
Some form of adjustment can be made to the total oxidant reading if
continuous nitrogen dioxide measurements are also available. This
correction depends to some extent on experimental conditions and, in
view of the problems previously noted with measurements of oxides of
nitrogen, may not be easy to make.
Other methods are available for the determination of oxidants but
none of them is commonly used. They include the ferrous ammonium
sulphate, alkali potassium iodide, and phenolphthalein methods (World
Health Organization, 1976).
2.3.2.3 Peroxyacetylnitrate
The infrared absorption and electron-capturing properties of
peroxyacetylnitrate have been used for its measurement in simulated
and real atmospheres (Stephens, 1969). Electron-capture detectors
preceded by gas chromatographic separation offer a limit of detection
of 0.5 µg/m3 (0.0001 ppm) for the automatic determination of ambient
concentrations of peroxyacetylnitrate. Water vapours may interfere
with the passage of peroxyacetylnitrate along the chromatographic
column (Farwell & Rasmussen, 1976) and trace contaminants in cylinder
gases can be a problem because of their slow accumulation on
chromatography columns used for continuous measurements.
Peroxyacetylnitrate can also be measured by the hydrolysis of
peroxyacetylnitrate solution to give nitrite ions that can be
determined colorimetrically. However, this method suffers from
interference by nitrogen dioxide (Konno & Okita, 1974; Stephens,
1969).
3. SOURCES OF PHOTOCHEMICAL OXIDANTS AND THEIR PRECURSORS
3.1 Natural Sources
As mentioned previously, ozone is a natural constituent of the
upper atmosphere. A small amount of ozone, which is formed by the
photolysis of molecular oxygen, is carried by atmospheric circulation
into the lower atmosphere (section 4). Natural sources of ozone are
associated with the passage of cold fronts (Ripperton et al., 1971)
and atmospheric electrical phenomena (US Department of Health,
Education & Welfare, 1970).
The photochemical oxidation of natural hydrocarbons including
terpenes from trees and other vegetation takes place during daylight
hours (Rasmussen, 1972; Went, 1966). Generally, these processes are
difficult to study because of the low concentrations of the
hydrocarbons and their short atmospheric residence. However, hazes
often associated with certain forest regions may well be explained by
photochemical aerosol production from natural airborne hydrocarbons
(Grimsrud et al., 1975).
3.2 Man-made Sources of Oxidant Precursors
Emissions of oxides of nitrogen from man-made activities include
important contributions from both stationary and mobile sources (World
Health Organization, 1977).
By comparison, the position with regard to hydrocarbon precursors
is much more complex. In this instance, the term "hydrocarbon" refers
to a class of pollutants that contain carbon atoms and produce
oxidants under irradiation in the presence of oxides of nitrogen. This
is a very wide definition that covers many hundreds of different
organic compounds emitted into the atmosphere by man-made processes.
Not all organic compounds play an equal role in oxidant formation.
The relative importance of each organic compound in this process
depends on its chemical structure and reactivity. The chemical
structure determines the number of nitric oxide --> nitrogen dioxide
conversions involved in the atmospheric degradation of each organic
compound and ozone may be produced at each of these steps (Calvert,
1976; Demerjian et al., 1974). Reactivity requires special attention
because the time scale for ozone or peroxyacetylnitrate production is
related to the time scale for hydrocarbon degradation. For the
so-called "highly reactive" hydrocarbons this may be 1 h or less. It
may take up to 3 h for the less reactive hydrocarbons and require
several days for the so-called unreactive hydrocarbons. Even in the
presence of reactive hydrocarbons, ozone production may only become
significant 10 km downwind from a source, and peak ozone
concentrations may be observed over 60 km downwind (White et al.,
1976). Thus, the relationship between hydrocarbon precursors and
observed oxidant concentrations in large urban areas may be obscured.
There may be marked differences in the nature of the oxidants in air
sampled during the early morning when peak concentrations of oxidant
precursors occur and in that sampled after midday when ozone
concentrations are elevated.
In view of this complexity, it is clearly necessary to have some
form of rational assessment of hydrocarbon reactivity (Darnall et al.,
1976). Complete inventories of individual substances are only
available for gasoline-engine exhaust emissions; for the storage,
distribution, and use of organic substances such as petroleum
products; and for specific industrial processes. An example of total
emissions of oxides of nitrogen and hydrocarbons is given in Table 3.
These figures are presented merely to show the wide diversity of the
processes responsible for oxidant precursor emissions; it is not
sufficient to consider motor vehicles as the only important source.
Table 3. Total emissions of hydrocarbons and oxides of nitrogen
in the Federal Republic of Germany in 1971 in thousands
of tonnesa
Source Total emission (103 tonnes)
Hydrocarbons Oxides of nitrogen
Domestic heating 173 117
Traffic exhaust 325 308
Power plants 14 373
Industrial combustion 53 470
Industrial processes 955 30
a From: Federal Republic of Germany, Ministry of Internal
Affairs (1974).
3.3 Indoor Sources
The use of ultraviolet lamps, electrostatic precipitators,
photocopying machines, and odour control equipment can lead to
increases in indoor concentrations of ozone. Welding and the
manufacture of hydrogen peroxide are important indoor sources of ozone
in industry and pose problems in occupational health (section 6.2).
However, other indoor activities such as smoking and cooking with
gas stoves tend to produce elevated nitric oxide concentrations that
destroy ozone and peroxyacylnitrates (Schuck & Stephens, 1969).
3.4 Oxidant-precursor Relationships
While potential synergistic health effects of oxidants and
nitrogen dioxide are recognized, programmes for the control of these
two pollutants are usually developed independently of one another.
From a control point of view, however, such an approach is not
justified because of the complexity of the photochemical reaction
system. The only predictable way to control the formation of
photochemical oxidants is to reduce the initial components in
incremental steps. Decreasing the primary emission of oxides of
nitrogen without reducing hydrocarbon emissions will lead to an
increase in oxidant levels.
A further example of the uncoordinated approach to air pollution
abatement can be illustrated by examination of the current methods
applied to hydrocarbon control. These methods have focused on
increasing combustion efficiency, an action which does reduce the
hydrocarbon concentrations in exhaust but has the side effect of
causing an increase in atmospheric concentrations of nitrogen dioxide.
As expected, changing the ratio of oxidant precursors has a complex
effect on the formation of photochemical oxidants. For example, the
effect of this change in ratio in the south coast air basin of
California has been to produce substantial reductions in the
concentrations of oxidants in Los Angeles town centre. However, the
reductions become smaller at downwind sites and at 80 kilometres
downwind, an increase in maximum daily values was observed
(Dimitriades, 1976).
4. ENVIRONMENTAL CONCENTRATIONS AND EXPOSURES
4.1 Background Concentrations
Ozone concentrations in places far removed from sources of
pollution show fairly constant values with some seasonal variations
interspersed with irregular maxima due to specific meteorological
events. Monthly mean concentrations of ozone, which vary considerably
with both latitude and the month of the year, are illustrated in
Fig. 1. The reported values range from 10 to 80 µg/m3
(0.005-0.04 ppm).
Many observations indicate that hourly values range from 10 to
100 µg/m3 (0.005-0.05 ppm) (Berry, 1964; Haagen-Smit, 1952; US
Department of Health, Education and Welfare, 1970). However, higher
values have been observed on isolated occasions. In a study at Chalk
River, Ontario, Canada, a maximum value was observed of 120 µg/m3
(0.06 ppm) for 4 h (US Department of Health, Education and Welfare,
1970).
4.2 Rural Areas
Polluted air masses from urban and industrial areas can affect
suburban and rural areas in the direction of the prevailing wind for
considerable distances. Elevated oxidant concentrations have been
measured in a number of downwind rural locations where local sources
of oxidant precursors were insignificant. It has been suggested that
long-distance atmospheric transport might be responsible for many
cases of high oxidant concentrations found over rural areas and some
specific examples are given in Table 4.
A Midwest study in the USA in 1974 showed elevated ozone levels
over an extensive rural area (radius of over 240 km) due to the
combined effects of a number of urban areas. High ozone levels from
one particular urban area extended as far as 48-80 kilometres downwind
(US Environmental Protection Agency, 1976).
Observations of a 1-h concentration of 120 µg/m3 (0.06 ppm) in
rural areas can generally be associated with the transport of man-made
oxidants from distant sources.
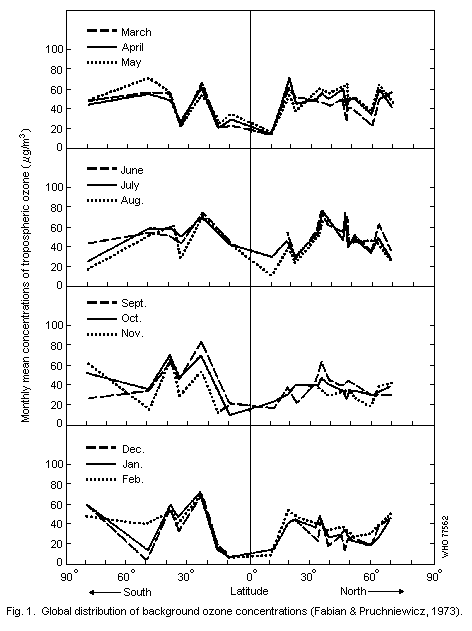 Table 4. Long-distance transport of photochemical oxidants
Rural region Possible source Trajectory Reference
length
(km)
Mineral King Valley, Fresno, California < 100 Miller et al. (1972)
California, USA
Garrett County, New York, Philadelphia, > 100 US Environmental
Maryland, USA Baltimore, Washington DC Protection Agency
or Pittsburgh (1973)
Southern Eire Continental Europe 100-700 Cox et al. (1975)
& Southern UK
New York State, USA Buffalo, New York 100-300 Stasiuk & Coffey (1974)
Tochigi and Gunma Tokyo < 100 Environment Agency
Prefectures, Japan (1976)
Midwest, USA St. Louis > 150 White et al. (1976)
4.3 Urban Areas
Since photochemical oxidants are the products of sunlight-induced
photochemical reactions, elevated concentrations of oxidants in urban
areas are generally restricted to a 4- to 6-h period within a day,
representing only 15-25% of the 24-h interval. For this reason, the
reporting of oxidant or ozone data as daily, monthly, or yearly means
can be misleading when evaluating trends or comparing oxidant
concentrations in different cities. Thus, oxidant or ozone data are
usually reported in terms of highest 1-h concentrations or in terms of
the number of days with hourly concentrations exceeding a specified
value or the number of hours when a given range of concentrations
occurred within a year. However, they may also be given as
instantaneous or five minute peak concentrations or frequency
distributions.
As shown in Table 5, the highest 1-h concentrations at 8 locations
were of the order of 300-800 µg/m3 (0.15-0.40 ppm). It is important
to recognize that the data presented are only for one site in each of
the cities and do not necessarily represent the maximum levels
occurring in these urban areas or provide a good indication of human
exposure levels. For this reason, frequency distributions or reporting
of the number of days or hours when a given concentration was exceeded
are helpful. Such data are presented in Tables 6, 7, and 8 for
selected monitoring stations in Tokyo, Washington DC, and Delft,
respectively, to illustrate the distribution of the concentrations
recorded at these monitoring stations. These tables are not intended
to indicate long-term trends since meteorological patterns that
greatly influence the ambient concentrations of oxidants can vary
considerably from year to year.
The data for Tokyo show both the number of days when a given
concentration was exceeded and the total number of hours in which
concentrations falling within the specified ranges were observed. As
shown in Table 6, an hourly concentration of 200 µg/m3 (0.1 ppm) was
exceeded on 10-30 of days in these years, and in approximately 1-4% of
all the hours in the year.
In Washington, DC, a concentration of 200 µg/m3 (0.1 ppm) was
exceeded, on average, on about 5% of the days, with most of the days
having maximum 1-h concentrations of about 100 µg/m3 (0.05 ppm).
The data for Delft, Netherlands, show the number of hours in the
year when given concentrations were observed. More than 90% of the
hours exhibited concentrations of less than 100 µg/m3 (0.05 ppm).
However, in 1971 and 1973, there were 48 and 30 hours, respectively,
when concentrations exceeded 200 µg/m3 (0.1 ppm).
Table 5. Highest 1-h concentrations of ozone or total oxidants observed at
selected sites in 1974
City Concentration Method
µg/m3 (ppm)
Bonn, Federal Republic of Germany1 290 (0.145) chemiluminescence
Eindhoven, Netherlands2 420 (0.210) chemiluminescence
London, UK3a 294 (0.147) chemiluminescence
Los Angeles, USA4 548 (0.274) NBKI
Osaka, Japan5 320 (0.160) NBKI
Riverside, USA4 744 (0.372) NBKI
Tokyo, Japan5 380 (0.190) NBKI
Washington, USA4 312 (0.156) chemiluminescence
From: 1 Becker & Schurath (1975).
2 Guicherit (1975).
3 Ball (1976).
4 US Environmental Protection Agency (1976).
5 Environment Agency (1975b).
a Data for 1975.
Table 6. Number of days and hours when hourly oxidant concentrations were in the
range of indicated levels at a National Air Sampling Nelwork Station,
Tokyo, Japana
Concentrationb Number of days (hours)
µg/m3 (ppm) 1971 1972 1973 1974
0-100 (0 -0.05) 113 (7032) 185 (7863) 60 (7248) 184 (8122)
120-180 (0.06-0.09) 135 (798) 111 (379) 199 (1050) 134 (495)
200-280 (0.10-0.14) 60 (251) 31 (81) 73 (298) 40 (107)
300-380 (0.15-0.19) 18 (42) 8 (14) 21 (59) 5 (10)
400-480 (0.20-0.24) 6 (8) 1 (2) 7 (14) 0 (0)
>500 (>0.25) 0 (0) 0 (0) 1 (2) 0 (0)
Total 332 (8131) 336 (8339) 361 (8671) 363 (8734)
a From: Tokyo Metropolitan Government (1971-1974) (unpublished data).
b Measured by NBKI method.
Table 7. Number of days when at least one hourly oxidant
concentration was in the range of indicated levels in
Washington, DC, USAa
Concentrationb Number of days
µg/m3 (ppm) 1970 1971 1972
0-100 (0 -0.05) 72 155 134
120-200 (0.06-0.10) 85 127 39
220-300 (0.11-0.15) 8 17 6
>300 (>0.15) 2 0 0
Total 167 299 179
a From: US Environmental Protection Agency (1964-1973).
b Measured by the NBKI method.
Table 8. Number of hours when hourly ozone concentrations were in
the range of indicated levels in Delft, Netherlandsa
Concentrationb Number of hours
µg/m3 (ppm) 1971 1972 1973 1974
0-100 (0 -0.05) 7799 8370 8364 6907
120-150 (0.06 -0.075) 647 325 267 401
152-200 (0.076-0.10) 130 34 81 39
>200 (>0.10) 48 8 30 8
Total 8624 8737 8742 7355
a From: Guicherit (1975).
b Measured by the chemiluminescence method.
When the maximum concentration for a given averaging time, e.g.,
1 h, is known, maximum values for other averaging times can be
estimated from the averaging time-concentration model of Larsen
(1974). The concentrations for various averaging periods, calculated
from the base data of 1-h concentrations of 300 and 800 µg/m3, are
shown in Table 9.
Table 9. Estimated ozone concentrations for various
averaging periodsa
Averaging period Maximum concentration (µg/m3)
1-h (base data) 300 800
3-h 190-215 435-520
1 day 85-120 225-310
1 year 15-30 35-80
a Adapted from: Larsen (1969).
These calculations are based on geometric standard deviations
reported for several cities in the USA (Larsen, 1969).
Caution should be exercised when applying Larsen's model. Although
it is not applicable to averaging times of less than 1 h, the model is
generally accurate when using observed annual means to predict
concentrations between 1 h and 1 day. For periods longer than 1 day it
is applicable only if the observed data closely follow a log-normal
distribution.
Seasonal and diurnal variations in oxidant values are important
characteristics of the urban pattern of environmental concentrations
of this group of pollutants. These temporal variations result from:
(a) variations in oxidant precursors; (b) variations in atmospheric
transport and dilution of pollutants, and (c) variations in
meteorological conditions and other atmospheric variables involved in
the photochemical reaction process. Because of diurnal variations in
intensity of both solar ultraviolet radiation and of precursor
emission rates, maximum daily ozone concentrations frequently occur
around noon. However, such maxima have also been observed during the
morning or afternoon hours, mostly in suburban areas.
Examples of diurnal patterns of oxidants or ozone and nitrogen
dioxide concentrations are shown in Figs. 2, 3, and 4. The close
relationship between oxidants and nitrogen dioxide quite frequently
leads to oxidant peaks following nitrogen dioxide peaks. However, this
is not always, as shown in Fig. 3.
Table 4. Long-distance transport of photochemical oxidants
Rural region Possible source Trajectory Reference
length
(km)
Mineral King Valley, Fresno, California < 100 Miller et al. (1972)
California, USA
Garrett County, New York, Philadelphia, > 100 US Environmental
Maryland, USA Baltimore, Washington DC Protection Agency
or Pittsburgh (1973)
Southern Eire Continental Europe 100-700 Cox et al. (1975)
& Southern UK
New York State, USA Buffalo, New York 100-300 Stasiuk & Coffey (1974)
Tochigi and Gunma Tokyo < 100 Environment Agency
Prefectures, Japan (1976)
Midwest, USA St. Louis > 150 White et al. (1976)
4.3 Urban Areas
Since photochemical oxidants are the products of sunlight-induced
photochemical reactions, elevated concentrations of oxidants in urban
areas are generally restricted to a 4- to 6-h period within a day,
representing only 15-25% of the 24-h interval. For this reason, the
reporting of oxidant or ozone data as daily, monthly, or yearly means
can be misleading when evaluating trends or comparing oxidant
concentrations in different cities. Thus, oxidant or ozone data are
usually reported in terms of highest 1-h concentrations or in terms of
the number of days with hourly concentrations exceeding a specified
value or the number of hours when a given range of concentrations
occurred within a year. However, they may also be given as
instantaneous or five minute peak concentrations or frequency
distributions.
As shown in Table 5, the highest 1-h concentrations at 8 locations
were of the order of 300-800 µg/m3 (0.15-0.40 ppm). It is important
to recognize that the data presented are only for one site in each of
the cities and do not necessarily represent the maximum levels
occurring in these urban areas or provide a good indication of human
exposure levels. For this reason, frequency distributions or reporting
of the number of days or hours when a given concentration was exceeded
are helpful. Such data are presented in Tables 6, 7, and 8 for
selected monitoring stations in Tokyo, Washington DC, and Delft,
respectively, to illustrate the distribution of the concentrations
recorded at these monitoring stations. These tables are not intended
to indicate long-term trends since meteorological patterns that
greatly influence the ambient concentrations of oxidants can vary
considerably from year to year.
The data for Tokyo show both the number of days when a given
concentration was exceeded and the total number of hours in which
concentrations falling within the specified ranges were observed. As
shown in Table 6, an hourly concentration of 200 µg/m3 (0.1 ppm) was
exceeded on 10-30 of days in these years, and in approximately 1-4% of
all the hours in the year.
In Washington, DC, a concentration of 200 µg/m3 (0.1 ppm) was
exceeded, on average, on about 5% of the days, with most of the days
having maximum 1-h concentrations of about 100 µg/m3 (0.05 ppm).
The data for Delft, Netherlands, show the number of hours in the
year when given concentrations were observed. More than 90% of the
hours exhibited concentrations of less than 100 µg/m3 (0.05 ppm).
However, in 1971 and 1973, there were 48 and 30 hours, respectively,
when concentrations exceeded 200 µg/m3 (0.1 ppm).
Table 5. Highest 1-h concentrations of ozone or total oxidants observed at
selected sites in 1974
City Concentration Method
µg/m3 (ppm)
Bonn, Federal Republic of Germany1 290 (0.145) chemiluminescence
Eindhoven, Netherlands2 420 (0.210) chemiluminescence
London, UK3a 294 (0.147) chemiluminescence
Los Angeles, USA4 548 (0.274) NBKI
Osaka, Japan5 320 (0.160) NBKI
Riverside, USA4 744 (0.372) NBKI
Tokyo, Japan5 380 (0.190) NBKI
Washington, USA4 312 (0.156) chemiluminescence
From: 1 Becker & Schurath (1975).
2 Guicherit (1975).
3 Ball (1976).
4 US Environmental Protection Agency (1976).
5 Environment Agency (1975b).
a Data for 1975.
Table 6. Number of days and hours when hourly oxidant concentrations were in the
range of indicated levels at a National Air Sampling Nelwork Station,
Tokyo, Japana
Concentrationb Number of days (hours)
µg/m3 (ppm) 1971 1972 1973 1974
0-100 (0 -0.05) 113 (7032) 185 (7863) 60 (7248) 184 (8122)
120-180 (0.06-0.09) 135 (798) 111 (379) 199 (1050) 134 (495)
200-280 (0.10-0.14) 60 (251) 31 (81) 73 (298) 40 (107)
300-380 (0.15-0.19) 18 (42) 8 (14) 21 (59) 5 (10)
400-480 (0.20-0.24) 6 (8) 1 (2) 7 (14) 0 (0)
>500 (>0.25) 0 (0) 0 (0) 1 (2) 0 (0)
Total 332 (8131) 336 (8339) 361 (8671) 363 (8734)
a From: Tokyo Metropolitan Government (1971-1974) (unpublished data).
b Measured by NBKI method.
Table 7. Number of days when at least one hourly oxidant
concentration was in the range of indicated levels in
Washington, DC, USAa
Concentrationb Number of days
µg/m3 (ppm) 1970 1971 1972
0-100 (0 -0.05) 72 155 134
120-200 (0.06-0.10) 85 127 39
220-300 (0.11-0.15) 8 17 6
>300 (>0.15) 2 0 0
Total 167 299 179
a From: US Environmental Protection Agency (1964-1973).
b Measured by the NBKI method.
Table 8. Number of hours when hourly ozone concentrations were in
the range of indicated levels in Delft, Netherlandsa
Concentrationb Number of hours
µg/m3 (ppm) 1971 1972 1973 1974
0-100 (0 -0.05) 7799 8370 8364 6907
120-150 (0.06 -0.075) 647 325 267 401
152-200 (0.076-0.10) 130 34 81 39
>200 (>0.10) 48 8 30 8
Total 8624 8737 8742 7355
a From: Guicherit (1975).
b Measured by the chemiluminescence method.
When the maximum concentration for a given averaging time, e.g.,
1 h, is known, maximum values for other averaging times can be
estimated from the averaging time-concentration model of Larsen
(1974). The concentrations for various averaging periods, calculated
from the base data of 1-h concentrations of 300 and 800 µg/m3, are
shown in Table 9.
Table 9. Estimated ozone concentrations for various
averaging periodsa
Averaging period Maximum concentration (µg/m3)
1-h (base data) 300 800
3-h 190-215 435-520
1 day 85-120 225-310
1 year 15-30 35-80
a Adapted from: Larsen (1969).
These calculations are based on geometric standard deviations
reported for several cities in the USA (Larsen, 1969).
Caution should be exercised when applying Larsen's model. Although
it is not applicable to averaging times of less than 1 h, the model is
generally accurate when using observed annual means to predict
concentrations between 1 h and 1 day. For periods longer than 1 day it
is applicable only if the observed data closely follow a log-normal
distribution.
Seasonal and diurnal variations in oxidant values are important
characteristics of the urban pattern of environmental concentrations
of this group of pollutants. These temporal variations result from:
(a) variations in oxidant precursors; (b) variations in atmospheric
transport and dilution of pollutants, and (c) variations in
meteorological conditions and other atmospheric variables involved in
the photochemical reaction process. Because of diurnal variations in
intensity of both solar ultraviolet radiation and of precursor
emission rates, maximum daily ozone concentrations frequently occur
around noon. However, such maxima have also been observed during the
morning or afternoon hours, mostly in suburban areas.
Examples of diurnal patterns of oxidants or ozone and nitrogen
dioxide concentrations are shown in Figs. 2, 3, and 4. The close
relationship between oxidants and nitrogen dioxide quite frequently
leads to oxidant peaks following nitrogen dioxide peaks. However, this
is not always, as shown in Fig. 3.
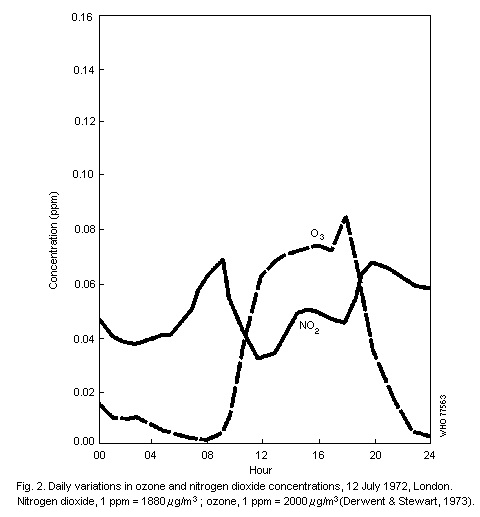
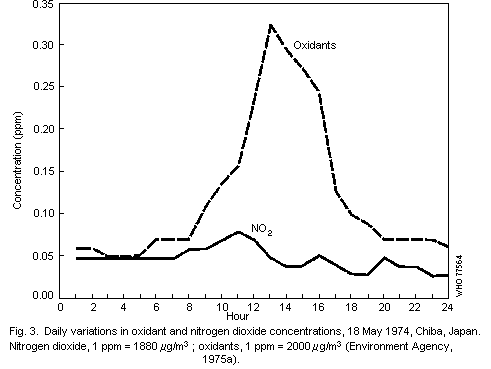
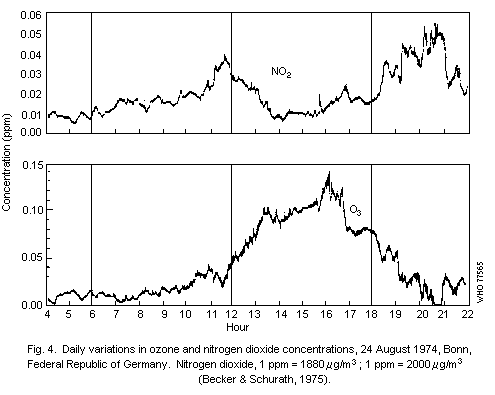 Seasonal variations in oxidant concentrations are manifested by
increases in the diurnal maxima and by increases in the number of days
per month that exhibit elevated oxidant values. For example, Fig. 5
shows the number of days per month in which the oxidant concentration
in Los Angeles, USA, equalled or exceeded 200 µg/m3 (0.1 ppm).
Similarly, Fig. 6 shows the number of days per month in Delft,
Netherlands, that also exhibited ozone concentrations equal to or
above 200 µg/m3 (0.1 ppm).
Peroxyacetylnitrate is generally formed simultaneously with ozone.
However, comparatively few measurements have been made of
peroxyacetylnitrate in the ambient atmosphere. The ratio of
peroxyacetylnitrate to ozone observed at a maximum concentration of
peroxyacetylnitrate was about 1:100 in rural England (Sandalls et al.,
1974) and about 1:50 in Delft, Netherlands (Nieboer & Van Ham, 1976).
Variations in concentrations of peroxyacetylnitrate often follow those
of ozone, as shown in Fig. 7 (Sandalls et al., 1974). However, this is
not always the case (Stephens, 1976).
4.4 Indoor Concentrations
Oxidant concentrations inside buildings tend to be lower than those
outdoors because of destructive reactions that occur on most surfaces
(Mueller et al., 1973; Sabersky et al., 1973). However, certain indoor
sources of ozone (section 3.3) may increase indoor concentrations.
Indoor concentrations at places of work are discussed in section 6.2.
Seasonal variations in oxidant concentrations are manifested by
increases in the diurnal maxima and by increases in the number of days
per month that exhibit elevated oxidant values. For example, Fig. 5
shows the number of days per month in which the oxidant concentration
in Los Angeles, USA, equalled or exceeded 200 µg/m3 (0.1 ppm).
Similarly, Fig. 6 shows the number of days per month in Delft,
Netherlands, that also exhibited ozone concentrations equal to or
above 200 µg/m3 (0.1 ppm).
Peroxyacetylnitrate is generally formed simultaneously with ozone.
However, comparatively few measurements have been made of
peroxyacetylnitrate in the ambient atmosphere. The ratio of
peroxyacetylnitrate to ozone observed at a maximum concentration of
peroxyacetylnitrate was about 1:100 in rural England (Sandalls et al.,
1974) and about 1:50 in Delft, Netherlands (Nieboer & Van Ham, 1976).
Variations in concentrations of peroxyacetylnitrate often follow those
of ozone, as shown in Fig. 7 (Sandalls et al., 1974). However, this is
not always the case (Stephens, 1976).
4.4 Indoor Concentrations
Oxidant concentrations inside buildings tend to be lower than those
outdoors because of destructive reactions that occur on most surfaces
(Mueller et al., 1973; Sabersky et al., 1973). However, certain indoor
sources of ozone (section 3.3) may increase indoor concentrations.
Indoor concentrations at places of work are discussed in section 6.2.
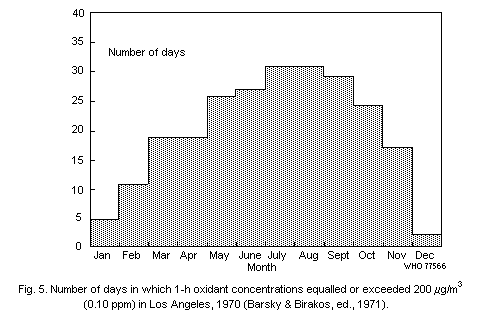
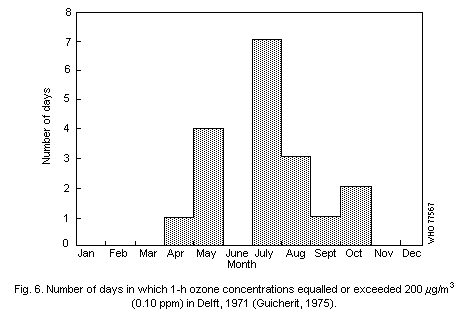
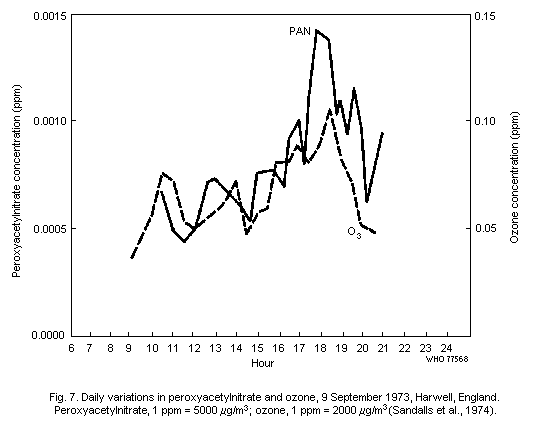 5. EFFECTS ON EXPERIMENTAL ANIMALS
There is considerable evidence to show that even short exposure to
high concentrations of ozone may endanger the health of experimental
animals. In reviewing this evidence, emphasis has been placed on
studies in which animals were exposed to concentrations of oxidants of
2000 µg/m3 (1.0 ppm) or less, since these studies are more relevant
for predicting the health risk to man. However, some experiments
conducted at higher concentrations have also been discussed when it
was considered that they would contribute to a better understanding of
the mechanism of the biological action of oxidants.
5.1 Absorption of Ozone
A number of factors can influence the transport and removal of
ozone in the upper airways such as: (a) nasal morphology; (b) route,
rate, and depth of breathing; and (c) biochemical composition and
amount of mucus. The decomposition of ozone within the upper airways
may protect the lower part of the respiratory tract against the
irritant gas. Various attempts to determine or model the respiratory
absorption of ozone in the upper airways have been made (McJilton et
al., 1972; Vaughan et al., 1969; Yokoyama & Frank, 1972). This work
has recently been reviewed by Miller (1977) who developed a
mathematical model for the transport and removal of ozone in the
respiratory tract of guineapigs, rabbits, and man, and predicted that
whatever the initial concentration, the respiratory bronchioles would
receive the highest dose of ozone. This agrees well with various
experimental studies in animals. For all three species the
relationship between respiratory bronchiolar concentration and the
inhaled ozone concentration at the tracheal level is linear on a
log-log scale at concentrations greater than 100 µg/m3 (0.05 ppm),
the respiratory bronchiolar dose for rabbits being 80% of that for man
and twice that for guineapigs.
5.2 Effects on the Respiratory System
5.2.1 Morphological changes
5.2.1.1 Short-term exposure (24 h or less)
The primary target of ozone is the respiratory tract and
particularly the pulmonary parenchyma. In small laboratory animals,
exposure to ozone at acutely toxic concentrations results in pulmonary
oedema, haemorrhage, and death. The LD50 is about 12 mg/m3 (6.0 ppm).
At lower concentrations in the range of 400-2000 µg/m3 (0.2-1.0 ppm),
ozone causes numerous changes in both the epithelial and endothelial
cells of the lung and ultrastructural effect indicate that the primary
lesions are in the epithelial lining of the terminal bronchioles and
the proximal alveoli.
The sequence of degeneration, desquamation, and destruction of
type I alveolar cells in rats following exposure to an ozone
concentration of 400 µg/m3 (0.2 ppm) for 2 h was demonstrated in
electron microscopic studies by Stephens et al. (1974). The type II
epithelial cells appeared to be more resistant. Freeman et al. (1974)
also reported significant histological changes in type I epithelial
cells after 4 h exposure to a concentration of 1800 µg/m3. The loss
of ciliated epithelium throughout the upper respiratory tract,
swelling and denudation of type I cells, erythrocyte lysis within
alveolar capillaries, and breakdown of capillary endothelium has been
reported in cats exposed to ozone concentrations of 520, 1000, and
2000 µg/m3 (0.26, 0.5, and 1.0 ppm) for 4.7-6.6 h (Boatman et al.,
1974). These effects appeared to be dose-related. Similar effects were
noted by Bils (1970) in mice after a 7-h exposure to ozone at
concentrations of 1200 and 2600 µg/m3 (0.6 and 1.3 ppm).
Cell renewal rate within the alveoli was studied by Evans et al.
(1971) by injecting tritiated thymidine into 18 to 20-month-old mice
before exposing them for 6 h to ozone concentrations of 1000, 2400,
5000, or 7000 µg/m3 (0.5, 1.2, 2.5, and 3.5 ppm). Immediately
following exposure, the number of labelled alveolar cells (those
synthesizing DNA) was significantly lower in all exposed groups
compared with controls.
Similar morphological studies have also been performed using
complex mixtures containing oxidants. Ultrastructural alterations
consisting of disrupted cytoplasm and abnormal mitochondria were seen
in the alveolar tissue of mice exposed for 2-3 h to Los Angeles air
that had a total oxidant concentration of 800 µg/m3 (0.4 ppm) (Bils,
1966). Exposure of mice for 3 h to an irradiated synthetic atmosphere
that contained propylene, nitric oxide, carbon monoxide, and water
vapour and simulated a heavy smog, gave rise to a similar pattern of
ultrastructural changes (Bils & Romanovsky, 1967). Rats continuously
exposed for 24 h to a mixture of ozone at a concentration of
500 µg/m3 (0.25 ppm) and nitrogen dioxide at a concentration of
4700 µg/m3 (2.5 ppm) exhibited increases in the numbers of alveolar
macrophages and of free cells in the lung resembling desquamated type
I cells, and hypertrophy of the epithelium were noted in rats
continuously exposed for 24 h to a mixture of ozone at a concentration
of 500 µg/m3 (0.25 ppm) and nitrogen dioxide at a concentration of
4700 µg/m3 (2.5 ppm) (Freeman et al., 1974).
5.2.1.2 Prolonged and repeated exposures
Prolonged exposure to low levels of ozone causes more extensive
and irreparable damage to the lung than the oedematigenous and acute
inflammatory reactions observed following short exposure to high
concentrations. Emphysema, atelectasis, focal necrosis, broncho-
pneumonia, and fibrosis have been reported, often accompanied by a
variety of cellular alterations. The degree of morphological injury
appears to be proportional to the concentration and time of exposure.
Freeman et al. (1974) described a number of pathomorphological
changes in rats continuously exposed to ozone at concentrations of
1100 and 1800 µg/m3 (0.54 and 0.88 ppm) for as long as 6 months.
After 48 h of exposure to the lower concentration, an influx of
macrophages and an increase in mitotic figures were observed, and the
alveolar ducts became demarcated by hypertrophic alveolar epithelium.
Most of these changes became more obvious with increasing length of
exposure with the exception of the terminal bronchioles which appeared
to recover and return to normal. After exposure to the higher
concentration of 1800 µg/m3 (0.88 ppm) for 48 h, the bronchiolar
epithelium exhibited both metaplasia and fibrosis. Adenoma-like
structures containing large numbers of macrophages and fibrotic
lesions appeared after 6 days and after 3 weeks of exposure, half of
the rats had died and were found to have emphysema-like lesions.
Using scanning electron microscopy, Ikematsu et al. (1976) found
that continuous exposure to an ozone concentration of 2000 µg/m3
(1.0 ppm) for 10 days resulted not only in desquamation of epithelial
cells but also in the appearance of inflammatory cells in the tonsils
of rabbits. Similar pathological effects were observed with
intermittent exposure indicating that the animals were unable to
recover sufficiently during the periods of exposure to clean air.
Emphysematous and vascular lesions in the lung of the rabbit described
by P'an et al. (1972), were the result of repeated exposure to
800 µg/m3 (0.4 ppm) for 6 h per day, 5 days per week, for 10 months.
Stokinger et al. (1957) also reported fibrotic changes and chronic
bronchial and bronchiolar emphysema in the lungs of mice, rats,
guineapigs, and hamsters exposed to an ozone concentration of
2000 µg/m3 (1.0 ppm) for 6 h per day, 5 days per week, for 433 days.
However, under the same conditions of exposure, the effects in dogs
were limited to the trachea and large bronchi. When dogs were exposed
to ozone concentrations ranging from 2000 to 6000 µg/m3 (1-3 ppm) for
8, 16, or 24 h daily for 18 months, the morphological damage was
roughly proportional to the product of the concentration and the time
of exposure, the epithelial lining of the terminal airways and
proximal alveoli being most adversely affected (Freeman et al., 1973;
Stephens et al., 1973). Similarly, Castleman et al. (1973a) found that
the walls of the terminal airways and the interalveolar septa of rat
lung were most affected by continuous exposure to an ozone
concentration of 1600 µg/m3 for 7 days.
Intermittent exposure for 8 h per day, for 7 days to an ozone
concentration of 400 µg/m3 (0.2 ppm) produced damage to the
respiratory bronchioles in bonnet monkeys. In rats fed a normal diet,
similar effects were seen at the same concentration of ozone, but, in
vitamin E-deficient rats, an equivalent effect was caused by ozone at
200 µg/m3 (0.1 ppm) (Dungworth et al., 1975; Dungworth, 1976).
Loosli et al. (1972) measured the cell turnover rate in mice and
found it to be significantly higher in animals exposed to synthetic
photochemical air pollution for 8-12 months than in control animals
breathing filtered air. The synthetic air pollution contained ozone at
600-840 µg/m3 (0.30-42 ppm), carbon monoxide at 3.5-12 mg/m3
(3-10 ppm), nitrogen dioxide at 1300-1600 µg/m3 (0.70-0.85 ppm), and
sulfur dioxide at 5700-6000 µg/m3 (2.2-2.3 ppm). The effects were
similar to those seen with exposure to pure ozone.
Rats exposed for 1 month to a mixture of ozone at 1800 µg/m3
(0.9 ppm) and nitrogen dioxide at 1700 µg/m3 (0.9 ppm), developed
enlarged alveolar spaces (Freeman et al., 1974). In rats exposed for
2 weeks to a combination of ozone at 500 µg/m3 (0.25 ppm), and
nitrogen dioxide at 4700 µg/m3 (2.5 ppm), the bronchiolar epithelium
became cuboidal. However, after 6 months of exposure, the tissue
appeared to be normal (Freeman et al., 1974).
Slightly inflammatory and proliferative changes were observed by
Nakajima et al. (1972) in the bronchial membranes of mice exposed to
irradiated auto exhaust that contained oxidant concentrations of
200-300 µg/m3 (0.1-0.15 ppm), for 2-3 h per day, 5 days per week, for
30 days.
5.2.2 Functional changes
5.2.2.1 Short-term exposure (24 h or less)
The first abnormal sign observed in various animal species during
exposure to ozone is an irregular respiratory pattern. This usually
appears within the first few minutes of exposure and, in most cases,
the animal returns to normal, when allowed to recover in the clean
air.
Functional changes in the respiratory system have been observed in
several species of animals at ozone concentrations of less than
2000 µg/m3 (1.0 ppm). Yokoyama (1972a) studied the ventilatory
function of guinea-pigs before, during, and after a 2-h exposure to an
ozone concentration of 1000 µg/m3 (0.5 ppm). He reported increases in
the frequency of respiration and airway resistance, but a decrease in
tidal volume. In another investigation, Yokoyama (1973) exposed only
the right lung of rabbits to ozone at 2000 µg/m3 (1 ppm) for
3 h and used the left lung as a control. The exposed lung of rabbits
killed 1 and 3 days after exposure, had a reduced vital capacity.
However, the reduction in vital capacity in those killed 7 days after
exposure was only slight and was not significant. Other studies on
lung function in guineapigs exposed to ozone for 2 h indicated that
pulmonary flow resistance was not altered by exposure to
concentrations of 700 and 1400 µg/m3 (0.34 and 0.68 ppm), but that it
increased significantly at exposure levels of 2000 and 2700 µg/m3
(1.08 and 1.35 ppm) (Murphy et al., 1964a).
Scheel et al. (1959) exposed rats to an ozone concentration of
4000 µg/m3 (2 ppm) for 3 h. Decreases in minute ventilation, tidal
volume, and oxygen uptake, that occurred immediately after exposure,
reached minimum recorded values 8 h later. All measurements returned
to normal levels 20 h after exposure.
When cats were exposed for an average of 4.6 h to ozone
concentrations of 520, 1000, and 2000 µg/m3 (0.26, 0.5, and 1 ppm),
Watanabe et al. (1973b) found that pulmonary flow resistance increased
with increasing ozone concentrations. Dynamic compliance was reduced,
but to a lesser extent, and vital capacity was unaffected. The
proportion of animals that showed a reduction in diffusion capacity
appeared to increase with increasing ozone concentrations.
There are a few studies in which animals were exposed to ambient
air and irradiated auto exhaust containing high concentrations of
oxidants, including those of Swann & Balchum (1966) who measured the
total expiratory flow resistance in guineapigs on days of unusual
weather and smog conditions in Los Angeles. When the resistance was
compared with routine monthly measurements on the same animals,
significant increases were found at oxidant levels of approximately
600 µg/m3 (0.30 ppm) or more. Substantial increases in resistance
were also observed, when relatively high concentrations of nitrogen
dioxide (1700 µg/m3; 0.92 ppm), carbon monoxide (30 mg/m3;
26 ppm), and hydrocarbons (16 ppm) were present, but the oxidant level
(80 µg/m3; 0.04 ppm) was relatively low.
The effects on guineapigs of a 4-h exposure to diluted,
irradiated, or nonirradiated exhaust atmospheres were reported by
Murphy et al. (1963). Marked, rapid increases in total expiratory flow
resistance accompanied by a decrease in respiratory rate and a small
increase in tidal volume occurred during exposure to irradiated
exhaust. The reaction to nonirradiated exhaust was comparatively
slight. Ranges of concentrations of the main pollutants in the
exhaust-contaminated air used in this study were: for irradiated
exhaust gases: total oxidants, 660-1640 µg/m3 (0.33-0.82 ppm);
nitrogen dioxide, 800-10 000 µg/m3 (0.43-5.5 ppm); and carbon
monoxide, 39-360 mg/m3 (34-310 ppm): for nonirradiated exhaust gases:
total oxidants, less than 40 µg/m3 (0.02 ppm); nitrogen dioxide,
710-3000 µg/m3 (0.38-1.58 ppm); and carbon monoxide, 98-345 mg/m3
(85-300 ppm). In addition, increased levels of formaldehyde, acrolein,
and olefin were present.
5.2.2.2 Prolonged and repeated exposures
The few available pulmonary function studies on animals exposed to
ozone for extended periods of time include a study by Bartlett et al.
(1975) who reported that continuous exposure of rats to an ozone
concentration of 400 µg/m3 (0.2 ppm) for 30 days caused a 16%
increase in lung volume and an increase in alveolar dimension. A
reduction in lung elasticity that was also reported was possibly an
effect of ozone on collagen. Yokoyama (1974) exposed rabbits to an
ozone concentration of 4000 µg/ma (2 ppm), for 6 h per day, for 3-4
days, and found that the pulmonary flow resistance was greater, and
compliance lower, than in the controls. In animals exposed to
2000 µg/m3 (1 ppm), for 6 h per day, for 7-8 days, the values were
between those of the controls and of the group treated with
4000 µg/m3 (2 ppm).
5.2.3 Biochemical changes
5.2.3.1 Effects indicating possible mechanisms of action
The actual mechanism of ozone toxicity at the subcellular level is
still obscure. Studies of the biochemical effect of ozone have mainly
been based on two hypotheses: (a) that ozone interacts with readily
oxidizable substances thus altering the course of metabolism and
producing a toxic effect; and (b) that ozone interacts with
unsaturated lipids to produce lipid peroxidation and consequent cell
damage.
Sulfhydryl systems appear in the cell not only as reducing
substances but also as functional constituents of a variety of enzymes
and proteins. Ozone is capable of oxidizing these substances causing
inactivation of enzymes and alterations in the structure and function
of the cell membrane.
In studies on ozone oxidation of glutathione in vitro, Mudd et
al. (1969) showed that both fast and slow oxidation occurred. It has
also been shown that the oxidation of reduced glutathione (GSH)
results in oxidized glutathione (GSSG), though some of the sulfhydryl
groups form higher oxidation products that are not reduced by the
reductases available in the cells (Menzel, 1971). Mountain (1963)
demonstrated in vivo oxidation of reduced glutathione and a decrease
in the sulfur-containing enzyme succinic dehydrogenase (1.3.99.1)
activity in the lungs of mice. King (1961) also found a decrease in
sulfhydryl content and of enzymatic function of partially purified
glyceraldehyde-3-phosphate dehydrogenase (1.2.1.12) in rat lungs
following exposure to an ozone concentration of 2400 µg/m3 (1.2 ppm)
for 4 weeks.
When rats were exposed to an ozone concentration of 4000 µg/m3
(2.0 ppm) for 4-8h, the concentrations of both the protein and
nonprotein sulfhydryls in the lungs decreased. The activities of lung
enzymes containing sulfhydryl also decreased including those of
glucose-6-phosphate dehydrogenase (1.1.1.49), glutathione reductase
(1.6.4.2), and cytochrome c reductase related to succinate and
reduced nicotinamide adenine dinucleotide (NADH) (DeLucia et al.,
1972). Expanding these studies, DeLucia et al. (1975) showed that the
magnitude of the decrease in the nonprotein sulfhydryl groups in rats
was dependent on the duration of exposure and the concentration of
ozone. Significant decreases were not observed at an ozone
concentration of 1600 µg/m3 (0.8 ppm) for 24 h.
Further studies have shown that exposure to ozone tends to
increase the activity of enzymes that protect against intracellular
oxidation. When rats were continuously exposed to an ozone
concentration of 1500 µg/m3 (0.75 ppm) for 1, 3, 10, or 29 days, it
was noted that the activities of glutathione peroxidase (1.11.1.9),
glutathione reductase (1.6.4.2), glucose-6-phosphate dehydrogenase,
6-phosphogluconate dehydrogenase (1.1.1.43), and pyruvate kinase
(2.7.1.40) were lower than those in the controls after 1 day but
higher after, 3, 10, and 29 days of exposure (Chow & Tappel, 1973). In
another study by the same investigators, rats exposed to 400 µg/m3
(0.2 ppm) continuously for 8 days or intermittently (8 h per day) for
7 days showed significantly increased glutathione peroxidase activity
in the lung. Fukase et al. (1975a, 1975b) exposed mice to ozone
concentrations of 400, 1000, or 2000 µg/m3 (0.2, 0.5 or 1.0 ppm) for
4 h per day, for 30 days, and found progressive increases in the
levels of glutathione and vitamin C in the lung with increasing ozone
concentration. The authors also reported significant increases in the
activities of glutathione peroxidase, glutathione reductase, and
glucose-6-phosphate dehydrogenase in the lungs of mice. Exposure of
both rhesus and bonnet monkeys to levels of ozone ranging from 400 to
1600 µg/m3 (0.2-0.8 ppm) for 8 h per day for 7 days, resulted in
increased activities of succinate oxidase and glutathione peroxidase
in the lungs. Linear regression analysis showed a significant
correlation between ozone concentration and the augmentation in
activity of these enzymes (Dungworth et al., 1975).
Many investigators have attributed the biological effect of ozone
to lipid peroxidation (Fournier, 1973; Goldstein et al., 1969; Menzel,
1970; Roehm et al., 1971b). Oxidation of unsaturated fatty acids by
ozone has been demonstrated both in vivo and in vitro. The
mechanism of such action is based on the proclivity of ozone to react
with the ethylene groups of the acid to form peroxides. Their
decomposition results in the further formation of free radicals
capable of initiating peroxidation of other unsaturated fatty acids.
The breakdown products (peroxides, carbonyl compounds) may themselves
be cytotoxic.
Evidence of lung lipid peroxidation during ozone exposure was
suggested by Goldstein et al. (1969) who found conjugated diene bonds
in an extract of lungs of mice exposed to 800-1400 µg/m3
(0.4-0.7 ppm) for 4 h. Another index of lipid peroxidation is an
increase in malonaldehyde. In studies by Chow & Tappel (1972), the
malonaldehyde concentration increased in the lungs of rats exposed
continuously to 1400-1600 µg/m3 (0.7-0.8 ppm) for 5 and 7 days.
Further evidence of the role of peroxidation in ozone toxicity is
the fact that animals deficient in vitamin E are more susceptible to
ozone. This is discussed in detail in section 5.2.7.
5.2.3.2 Biochemical effects at the subcellular level
In morphological and ultrastructural studies, swelling and
degenerative changes in lung mitochondria have frequently been
reported following ozone exposure. Mitochondrial functions are
critical to the cellular terminal substrate oxidation and energy
production. These organelles in lung cells may be the target for ozone
since many mitochondrial enzyme activities are sulfhydryl-dependent
and mitochondrial membranes contain abundant unsaturated
phospholipids. It has therefore been suggested that the lung
mitochondria may be a sensitive test system for detecting and
evaluating ozone toxicity.
Mustafa et al. (1973) found a 45% increase in pulmonary
mitochondrial oxygen consumption in rats continuously exposed to
1600 µg/m3 (0.8 ppm) for 10-20 days. A 3-fold increase in the number
of type II cells was also reported. These cells are rich in
mitochondria and may have been responsible for the increase in oxygen
consumption. A 17% increase in oxygen consumption was noted with
continuous exposure to an ozone concentration of 400 µg/m3 (0.2 ppm)
for 7 days. This effect appeared to be dose-related.
Lysosomes are vitally important in the intra- and possibly
extracellular destruction of inhaled matter. The hydrolytic enzyme
system of lysosomes in the alveolar machrophage is crucial to maintain
the sterility of the lung against inhaled microbes. Any inactivation
of these enzymes would be expected to increase the risk of respiratory
disease.
Hurst et al. (1970) reported that, when rabbits were exposed for
3 h to an ozone concentration of 500 µg/m3 (0.25 ppm), there was a
reduction in the activity of lysosomal hydrolases, i.e., acid
phosphatase (3.1.3.2), lysozyme (3.2.1.17), and beta-glucuronidase
(3.2.1.31). In vitro exposure of rabbit alveolar macrophages
produced a similar decrease in lysosomal hydrolases (Hurst & Coffin,
1971).
Ozone may also induce increases in the concentrations of these
enzymes which may be related to eventual chronic lung disease.
When the specific activities of a number of lysosomal hydrolases
were measured in whole lung homogenates of rats, there was a
significant increase in activities after the animals had been
continuously exposed to an ozone concentration of 1400-1600 µg/m3
(0.7-0.8 ppm) for 5-7 days (Dillard et al., 1972). This increase could
be attributed to an inflammatory reaction induced by the ozone (Coffin
et al., 1968b).
Castleman et al. (1973b) exposed rats continuously for 7 days to
ozone concentrations of 1400-1600 µg/m3 (0.7-0.8 ppm). Using histo-
and cytochemical techniques, they observed increased acid phosphatase
activity but no change in beta-glucuronidase activity.
One of the structural alterations reported following exposure to
ozone is a change in the appearance of the endoplasmic reticulum.
Biochemical evidence of the effects of ozone on microsomal enzymes was
reported by Palmer et al. (1971, 1972). After a 3-h exposure to ozone
at 1500 µg/m3 (0.75 ppm), the lung tissue of hamsters and the
tracheobronchial mucosa of rabbits showed reductions of 33% and 53%,
respectively, in the activity of benzopyrene hydroxylase (1.14.14.2),
a mixed function oxidase that depends on cytochrome P-450 and is
located in the endoplasmic reticulum. Similar results were obtained by
Goldstein et al. (1975) who exposed rabbits for 90 rain to
2000 µg/m3 (1.0 ppm) and demonstrated a decrease in the rabbit lung
cytochrome P-450 concentration. It is of interest that the maximum
effect was observed a few days after exposure.
Very little information is available describing alterations in
nucleic acids related to ozone exposure. Most of the studies have been
conducted at much higher concentrations than those found in the
environment and have yielded conflicting results. Since DNA synthesis,
cell division, and growth are closely linked, further studies
clarifying the potential hazard would be of value.
In studies on mice exposed to an ozone concentration of
5000 µg/m3 (2.5 ppm) for 2 h per day, for 120 days, Werthamer et al.
(1974) noted reductions in both DNA and RNA syntheses and a
concomitant increase in protein synthesis. Evans et al. (1971) exposed
aging mice for 6 h to 1000-7000 µg/m3 (0.5-3.5 ppm) and found that,
regardless of the ozone concentration, there was inhibition of DNA
synthesis. The authors believed that this indicated a reduction in the
ability of the alveoli to act as a source of new cells and to maintain
the integrity of lung tissue during ozone exposure.
5.2.3.3 Extracellular effects
Since the primary cause of death from high concentrations of ozone
is pulmonary oedema, investigators have attempted to determine the
role of histamine in the pulmonary toxicity of ozone. The lung is rich
in histamine -- containing mast cells and among the many effects of
histamine are oedematigenous alterations in vascular capillaries.
Oedema, whatever the cause, reduces the number of alveoli
participating in gas exchange, and produces conditions favourable for
bacterial growth. The exact concentration of ozone required to produce
oedema depends on the animal species. It is generally believed that
gross oedema is probably not elicited in any species exposed to ozone
at the concentrations found in the ambient air.
Alpert et al. (1971a) used a radio labelled albumen technique to
detect the presence of pulmonary oedema in rats. A significant
increase in albumen levels in pulmonary lavage fluid appeared after
6 h exposure to 1000 µg/m3 (0.5 ppm).
The available data on lung histamine are conflicting. Dixon &
Mountain (1965) reported that, following a single exposure of mice to
an ozone concentration of 2000 µg/m3 (1.0 ppm) for 5 h, there was a
release of histamine from the lungs that persisted for at least 4
days. Pretreatment with an antihistamine (promethazine) reduced the
amount of oedema following exposure to a sublethal dose of ozone. It
should be noted that the antihistamine used is a phenothiazine
derivative the action of which might stabilize membranes and trap free
radicals. In contrast, Easton & Murphy (1967) were unable to
demonstrate any reduction of lung histamine in guineapigs exposed to
ozone concentrations of 10-12 mg/m3 (5-6 ppm) for 2 h. Cronin & Giri
(1974) also failed to observe any differences in the lung histamine
level in rats exposed to an ozone concentration of 8000 µg/m3
(4.0 ppm) for 4 h, although pulmonary oedema was evident.
Surface tension is an important contributor to the elastic
properties of the lung. Any alteration of the normal surface tension
in the alveoli may be implicated in the development of chronic lung
disease. Consequently, several investigators have examined the effect
of ozone on pulmonary surface activity. Gardner et al. (1971) exposed
rabbits to ozone levels as high as 20 mg/m3 (10 ppm) for 2.5 h and
then isolated the surface active material by pulmonary lavage. They
found that ozone did not alter the surface tension of this material
and that in vitro exposure did not affect the properties of
dipalmitoyl lecithin, a principal component of this surface active
substance. Similar results were reported by Huber et al. (1971) who
showed that a 3-h exposure of rabbits to ozone at 10 mg/m3 (5 ppm)
did not alter surface activity in the lavaged alveolar lining material
nor in extracts of the whole lung. On the other hand Yokoyama (1972a)
reported that in vitro exposure of guineapig lung extracts to ozone
concentrations of 1000 to 24000 µg/m3 (0.5-12 ppm) for 25-60 min
resulted in a rapid increase in surface tension. However, he, too, did
not find any change when dipalmitoyl lecithin was exposed to ozone.
The effect of ozone on the appearance of lung tissue lipids in
saline lavage fluid was studied by Kyei-Aboagye et al. (1973). They
proposed that ozone affected the lung by decreasing lecithin formation
while simultaneously stimulating the release of surfactant lecithins
(palmitoyl and oleyl).
In the lung, there is a ground substance between the basement
membrane of the alveolar epithelium and the capillary endothelium. Any
destruction of the integrity of this substance could affect the
elasticity of the lung. Buell et al. (1965) fractionated the lung
tissue of rabbits, after a 1-h exposure to an ozone concentration of
2000 µg/m3 (1.0 ppm), into soluble, lipid, and protein fractions. The
isolation of aldehydes and ketones from the protein fraction indicated
structural changes. It was suggested that, once these compounds were
formed, they might affect the intra-and intermolecular crosslinking of
elastic protein molecules which would in turn cause a reduction in the
elasticity of the lung.
5.2.4 Carcinogenicity
The possibility that ozone might be carcinogenic has been studied
in experimental animals.
Exposure to ozonized gasoline with ozone concentrations of
2000-7600 µg/m3 (1.0-3.8 ppm) for 52 weeks caused an increased
incidence of lung tumours in strain A mice (350 in each experimental
group). After 40 weeks, tumours were found in 21% of animals in the
control group and in 63% in the test group. After 52 weeks, the
incidence of tumour-bearing animals in the control group exposed to
washed air was 41% compared with 80% in the test group (Kotin & Falk,
1956). Additional studies using the same atmosphere of ozonized
gasoline were conducted on C57BL mice (405 in each experimental group)
(Kotin et al., 1958). After 92 weeks, the incidence of tumour-bearing
animals in the control group was 1.6% compared with 9.6% in the
exposed group.
Although these studies indicate that ozone may be tumorigenic
further work is necessary to confirm these results.
A number of studies including that of Penha et al. (1972) have not
been considered in this document because information concerning the
numbers of animals tested, control groups, etc. was inadequate.
5.2.5 Tolerance to ozone
The term "tolerance" refers to the fact that exposure to a
nonlethal dose of a specific toxic substance protects the host against
subsequent exposure to higher dose of the same chemical or of
different agents with similar toxicological properties (cross-
tolerance). Stokinger et al. (1956) reported such a protective effect
for ozone. Tolerance to ozone has been reviewed by Fairchild (1967)
and has also been reported by many other investigators (Henschler,
1960; Matzen, 1957; Mendenhall & Stokinger, 1959), In small rodents,
tolerance can be initiated by a concentration as low as 600 µg/m3
(0.3 ppm), maximum protection being obtained with concentrations
within the range of 2000-8000 µg/m3 (1-4 ppm) (Stokinger & Scheel,
1962). A single exposure to ozone can also induce a cross-tolerance
against subsequent lethal doses of X-ray irradiation (Hattori et al.,
1963), nitrogen dioxide, hydrogen peroxide, carbonic dichloride
(phosgene), ethanone (ketene), nitrosyl chloride, or hydrogen sulfide
(Fairchild, 1967).
Although the mechanism of tolerance is still not well understood,
several possibilities have been envisaged. Fairchild (1967) observed
that the level of reduced glutathione was maintained in ozone-tolerant
animals but not in the nontolerant group. This could be brought about
either by directly blocking the oxidation of reduced glutathione or
through some enzymatic pathway that would stimulate production of
reduced glutathione in response to a second exposure to ozone.
However, it is possible that the maintenance of these thiols in the
tolerant animal may be a result of the tolerance rather than the
cause. The studies of Chow & Tappel (1972, 1973) provide biochemical
support for this hypothesis. In addition, it has been shown (Mountain
et al., 1960) that the activities of serum alkaline phosphatase
(3.1.3.1), adrenal succinate dehydrogenase (1.3.99.1), and glucose-6-
phosphatase (3.1.3.9) either remain normal or are only slightly
altered in tolerant animals.
Fukase et al. (1975a) reported tolerance in mice to an ozone
concentration of 20-58 mg/m3 (10-29 ppm) after pre-exposure to
concentrations of 400-2000 [µg/m3 (0.2-1.0 ppm) and proposed that the
mechanism involved an increase in reduced glutathione and the
elevation of the activity of the peroxidative metabolic pathway.
Physical swelling of the intra-alveolar septa has also been proposed
by Henschler et al. (1964) as a mechanism of protection against the
oedematigenous effects of ozone.
Unilateral lung exposure models showed that, in order to induce
protection in a particular lung, the tissue must have actually come
into contact with ozone (Alpert & Lewis, 1971; Frank et al., 1970a).
There was no significant crossover effect from contralateral lung,
suggesting that there was no basis for assuming a circulating humoral
factor, and that the phenomenon was purely local. While reduction in
oedema development could be induced by pre-exposure to ozone, no
protection was obtained against the influx of polymorphonuclear
leukocytes into the lung (Gardner et al., 1972). It was also apparent
that tolerance did not influence the number of recoverable cells in
pulmonary lavage, their phagocytic capability, or the loss of
macrophage hydrolytic enzyme activity. This indicated the possibility
that tolerance protects only against pulmonary oedema and not against
other more subtle reactions.
As previously mentioned, tolerance is initiated in small rodents
at approximately 600 µg/m3 (0.3 ppm) (Stokinger & Scheel, 1962).
According to Alpert et al. (1971a) this approximates the lowest level
at which oedema can be demonstrated in rats by means of recovery of
labelled serum albumen via lung lavage. Thus, Coffin & Gardner (1972b)
postulated that it is probably necessary to produce a minimal
oedematigenous response before tolerance develops.
Experiments designed to test the susceptibility to bacterial
infection of "tolerant" animals have been conducted by Coffin &
Gardner (1972b). These studies illustrated that the "tolerant" animals
were only partially protected against the joint effects of ozone and a
viable microorganism. Partial protection was afforded by tolerance
only when the initiating dose of ozone was 600 µg/m3 (0.3 ppm) for
3 h (Coffin & Gardner, 1975). The authors considered that the
tolerance to infection seen at levels of ozone above 600 µg/m3
(0.3 ppm) could be due to the prevention of the formation of oedema
and that tolerance was only partial because tolerance to the action of
ozone on cellular and noncellular specific defence systems could not
be induced. This was shown by Gardner et al. (1972) who reported that
there was no tolerance to ozone damage in the alveolar macrophages,
which are the prime defence against infectious disease in the lower
part of the respiratory tract.
In their study on the effects of ozone on laboratory-induced
allergic respiratory disease in guineapigs, Matsumura et al. (1972)
found that animals that had received pretreatment with ozone at
2000 µg/m3 (1 ppm) for 1 h before a challenging exposure of
4000 µg/m3 (2 ppm) showed only slight respiratory reaction to
acetylcholine inhalation compared with those in which tolerance had
not been induced.
A variety of neurohumoral factors resulting from exposure to ozone
have been described by Fairchild (1963), who reported that
thyroidectomy, adrenalectomy, and hypophysectomy would increase the
resistance of rats to otherwise lethal doses of ozone by preventing
pulmonary oedema.
While the mechanism of tolerance in relation to ozone-induced
oedema is still not well understood, it has been suggested that the
thymus might play a role. Thymectomized mice were unable to develop
tolerance to ozone although the sham-operated animals exhibited
tolerance under the same experimental conditions (Gregory et al.,
1967).
5.2.6 Effects on the host defence system
Animals that are exposed to ozone have an increased susceptibility
to disease-producing biological agents, which can result in an
increased incidence of pulmonary infectious disease and death. Animal
models have been developed by several investigators to examine this
phenomenon experimentally by exposing animals to the pollutants and to
aerosolized viable microorganisms. The high sensitivity of this model
system is probably due to the fact that it reflects a summation of all
the subtle effects that ozone has on the lung. Any alteration in
cellular defence and ciliary activity, oedema, and immunosuppression
would allow the viable organism to multiply and cause disease. In mice
exposed to ozone at concentrations ranging from 2600 to 8800 µg/m3
(1.3 to 4.4 ppm) for 3 h, or 1700 µg/m3 (0.84 ppm) for 4 h, per day,
5 days per week, for 2 weeks, and subsequently challenged with
Klebsiella pneumoniae, resistance to respiratory infection was
significantly reduced (Miller & Ehrlich, 1958). Similar results were
obtained with hamsters. In a study to elucidate a relationship between
the time of exposure to ozone and the time of challenge with the
infectious agent, Purvis et al. (1961) found a decrease in resistance
to infection in mice treated with the bacterial aerosol within 19 h of
exposure to the gas or 27 h before exposure. A subsequent study by
Coffin and co-workers (1968a) showed that the lowest concentration of
ozone necessary to produce this effect in mice was 160 µg/m3
(0.08 ppm) for 3 h; Coffin & Gardner (1972) established a dose-
response relationship.
Further enhancement of toxicity could be demonstrated when a third
interactant such as cold or exercise was added to this infectivity
model. Housing mice at 6°-9°C for 3 h prior to exposure to ozone at
1400-1800 µg/m3 (0.7-0.9 ppm) for 2 h and to the microorganisms
increased the mortality rate compared with that in animals housed at
room temperature (Coffin & Blommer, 1965). Mice subjected to physical
activity while being exposed to ozone at 200-600 µg/m3 (0.1-0.3 ppm)
were less resistant to infectious agents than animals that were at
rest during exposure (Gardner et al., 1974b).
The effects of low concentrations of ozone on mice with induced
silicosis were investigated by Goldstein et al. (1972). Starting at an
ozone concentration of 800 µg/m3 (0.4 ppm) for 4 h, a progressive
decrease in pulmonary bactericidal activity occurred with exposure to
increasing concentrations of ozone. Silicosis itself did not inhibit
bactericidal activity.
Further research has sought to delineate the mechanisms by which
ozone reduces the resistance of animals to bacterial infection. It
appears that a number of factors may be responsible.
The number of organisms initially deposited within the lung does
not play a major role in the enhancement of mortality since the
pulmonary deposition of the microorganisms is less in ozone-treated
animals. However, regardless of the initial deposition, ozone-treated
animals subsequently have more organisms within the lungs, mainly
because of reduced bactericidal ability and the subsequent
multiplication of the inhaled organism (Coffin & Gardner, 1972a).
Goldstein et al. (1971b) found that the magnitude of the increase in
bacterial numbers was correlated with an increase in ozone
concentration up to 5200 µg/m3 (2.58 ppm). In rabbits exposed to
ozone at a concentration of 1000 µg/m3 (0.5 ppm), for 16 h per day,
for 7 months, Friberg et al. (1972) did not find any effect on the
physical removal of inhaled particles or on the number of macrophages
but, under the same conditions, a significant decrease was found in
the clearance of viable Escherichia coli in the lungs of guinea-
pigs.
The effect of ozone on antibacterial activity in the mouse lung
was determined in vivo by investigating the removal of bacteria by
mucociliary activity and by bactericidal activity simultaneously
(Goldstein, 1971a, 1971b). Mice were exposed to various concentrations
or ozone including 1200, 1400, 1600, and 2200 µg/m3 (0.62, 0.70,
0.80, and 1.1 ppm), for 17 h prior to, or 4 h after, infection with
aerosols of radiolabelled Staphylococcus aureus. Inhibition of
pulmonary bactericidal activity was shown at an ozone concentration as
low as 1200 µg/m3 (0.62 ppm) and activity decreased progressively
with increasing levels of ozone. The authors proposed that the
bactericidal defect was due to dysfunction of the alveolar macrophage.
Rabbits exposed to an ozone concentration of 600 µg/m3 (0.3 ppm)
for 3 h showed an impairment of the phagocytic properties of the
pulmonary alveolar macrophages (Coffin et al., 1968b). Furthermore,
exposure to ozone at 10 mg/m3 (5 ppm) for 3 h resulted in a reduction
in the total number of macrophages with a concomitant influx of
polymorphonuclear leukocytes into the lower respiratory tract (Coffin
& Gardner, 1972a). Enzyme activity was reduced in macrophages
recovered by lavage after exposure of rabbits to ozone at levels as
low as 500 µg/m3 (0.25 ppm) for 3 h (Hurst et al., 1970). The enzymes
studied were lysozyme (3.2.1.17), acid phosphatase, and beta-
glucuronidase and since these enzymes are involved in the
intracellular degradation of ingested bacteria, their reduction could
contribute to the poor bactericidal effect seen in the studies
mentioned previously.
Other effects on the alveolar macrophage have also been reported.
For example, alveolar macrophages from rabbits exposed to 4000 µg/m3
(2.0 ppm) for 8 h per day for 7 days exhibited an increased membrane
fragility (Dowell et al., 1970) and a dose-related reduction in
interferon was observed in the alveolar macrophages of rabbits exposed
to ozone concentrations of 2000, 6000, and 10 000 µg/m3 (1, 3, and
5 ppm) for 3 h (Shingu et al., 1972).
Morphological changes seen in rabbit alveolar macrophages after
exposure to ozone at a concentration of 10 mg/m3 (5 ppm) for 3 h
included dilatation of the endoplasmic reticulum and perinuclear
envelope, swelling of the mitochondria, intracellular vacuolization,
cell lysis, and the formation of myelin figures and autophagic
vacuoles (Huber et al., 1971). The ultrastructure of pulmonary
macrophages was also examined in situ in the lung tissue of rats
exposed to ozone at 6000 µg/m3 (3 ppm) for 4 h (Plopper et al.,
1973b). Immediately after exposure, the cells resembled those of
unexposed animals, but 12 h later there were twice as many
macrophages. Many of these had large granular cytoplasmic inclusions
that indicated increased phagocytic activity.
There is evidence that the effect seen on the alveolar macrophage
may be mediated through the noncellular milieu of the lung. Within the
lung, the macrophages are located close to the so-called pulmonary
surfactant contained in the extracellular lining of the epithelial
surface of the lung alveoli. It is possible that ozone might also
react with this lining film, which in turn, might be deleterious to
the cell. Gardner (1971) and Gardner et al. (1971) conducted
experiments on lavage fluid containing this surface-active substance
and found that, when isolated from rabbits exposed to ozone at
20 mg/m3 (10 ppm) for 2.5 h, it could adversely affect the stability
of the alveolar macrophage in vitro. A similar effect was noted when
ozone was bubbled through the lavage fluid in vitro. The effect on
the lavage fluid could be seen at levels as low as 200 µg/m3
(0.1 ppm) for a 2.5-h, in vivo exposure or after only 30-min in
vitro exposure. Since no significant alteration in the surface
tension of the lavage fluid was produced by ozone exposure, it is
suggested that the effect might be on a nonlipid component, possibly a
protein. The activity found in the cell-free pulmonary lavage fluid
has been called the "protective factor".
Ozone may also inactivate some opsinogenic factor within the lung
since Holzman et al. (1968) showed that it has a protein-degrading
property. Exposure of mice and rabbits to a concentration of
10 mg/m3 (5 ppm) for 3 h reduced the activity of active lysozyme,
obtained by bronchopulmonary lavage, by approximately 30%.
Various components of the endogenous defence mechanism of the lung
were studied through a unilateral lung exposure model (Alpert et al.,
1971b). Exposure to ozone at concentrations ranging from 1000 to
6000 µg/m3 (0.5 to 3 ppm) for 3 h decreased cellular viability,
depressed the intracellular hydrolytic enzymes and increased the
absolute number of polymorphonuclear leucocytes in the pulmonary
lavage fluid. All effects were dose-related and were found only in the
lung under treatment and not in the contralateral lung that breathed
ambient air.
Heuter et al. (1966) reported that exposure to an irradiated
automobile exhaust atmosphere for 15 months increased the
susceptibility of mice to pulmonary infection during the latter half
of the animal's lifetime. The oxidant concentrations in the irradiated
exposure, chamber ranged from 400 to 2000 µg/m3 (0.2-1.0 ppm). In a
similar study using irradiated automobile exhaust, enhancement of
mortality compared with that of control animals kept in filtered air
was noted with exposure to total oxidants at 300 µg/m3 (0.15 ppm) and
carbon monoxide at 29 mg/m3 (25 ppm) for 4 h. Coexistent
concentrations of nitrogen dioxide ranged from a trace to 1900 µg/m3
(1.0 ppm) (Coffin & Blommer, 1967).
Goldstein et al. (1974) studied the bactericidal effect when mice
were exposed to a combination of nitrogen dioxide and ozone, and
concluded that the combined pollutants caused bactericidal dysfunction
at concentrations that were approximately the same as the lowest
concentrations that caused similar dysfunction when the animals were
exposed to the gases individually.
Erlich et al. (1977) observed that a single joint exposure of mice
to nitrogen dioxide and ozone resulted in an addition of effects and
that a synergistic action might result from repeated exposures to the
mixture.
The animals were exposed for 3 h to 16 different combinations of
nitrogen dioxide at levels of 0, 2800, 3800, 6600, and 9400 µg/m3
(0, 1.5, 2.0, 3.5, and 5.0 ppm) and ozone at 0, 100, 200, and
1000 µg/m3 (0, 0.05, 0.1, and 0.5 ppm). Within 1 h of termination of
exposure to the pollutants, the mice were infected with Streptococcus
pyogenes. Excess mortality rates due to exposure to the mixture of
the two gases were approximately equivalent to the sum of those
induced by the inhalation of each individual pollutant. In mice
exposed repeatedly for 3 h per day, for 20 days, to a mixture of
nitrogen dioxide and ozone at concentrations of 3800 µg/ma (2.0 ppm)
and 100 µg/m3 (0.05 ppm), respectively, and challenged with
Streptococcus aerosol, the number of deaths was significantly higher
than in the control group. On the other hand, repeated daily exposure
to either nitrogen dioxide or ozone at the above-mentioned
concentrations did not show any major effect on the mortality rate.
The authors considered that this result might suggest a synergistic
action of the two pollutants making them more effective in reducing
resistance to respiratory infection.
5.2.7 Interaction of ozone with bronchoactive and other chemicals
The effects of pre-exposure to ozone on the sensitivity of
guineapigs to inhaled acetylcholine were studied by Matsumura et al.
(1972). Animals pre-exposed to an ozone concentration of 4000 µg/m3
(2 ppm) for 30 min manifested severe difficulty in breathing and many
died. This effect persisted for as long as 2 h after exposure. In a
similar study, Matsumura (1970a) sensitized guineapigs to albumen and
found that repeated 30 min pre-exposures to an ozone concentration of
10 mg/m3 (5 ppm) enhanced the sensitization. An ozone concentration
of 2000 µg/m3 (1 ppm) had no such effect.
Ozone-exposed guineapigs were also more susceptible to the toxic
action of injected histamine (Easton & Murphy, 1967). A 2-h exposure
to ozone at 10 mg/m3 (5 ppm) caused severe lung function changes and
increased mortality. This increased susceptibility to histamine was
detectable for as long as 12 h after the end of the exposure.
In order to study the effects of ozone on susceptibility to
serotonin, Suzuki & Nagaoka (1973) injected the compound into the
abdominal cavity of rats after exposing the animals to ozone at
concentrations ranging from 2000 to 12 000 µg/m3 (1-6 ppm) for 3 h.
Mortality increased with increasing levels of ozone, but fatalities
did not occur in a control group injected with serotonin and breathing
filtered air.
A report by Goldstein et al. (1970) that a deficiency of vitamin E
increased the toxicity of ozone in the rat has been supported by the
studies of Roehm et al. (1971b, 1972) and Menzel et al. (1972). These
investigators observed a significantly shorter 50% lethal time and
more pronounced signs of respiratory distress in ozone-exposed rats
(2000 µg/m3 (1.0 ppm) for 9 days continuously) that were fed vitamin
E-depleted diets in comparison with those fed vitamin E-supplemented
diets. When the ozone concentration was reduced to 1000 µg/m3
(0.5 ppm), pulmonary oedema became evident and mortality rates
increased in animals fed vitamin E-deficient diets after 6 weeks of
exposure. In rats continuously exposed to toxic levels of ozone
ranging from 1400 to 1600 µg/m3 (0.7-0.8 ppm), protection by dietary
vitamin E against lung lipid peroxidation was proportional to the
logarithm of the concentration of the vitamin in the diet (Fletcher &
Tappel, 1973). In further studies by Mustafa (1975), rats were fed a
basal diet containing vitamin E at either 66 mg/kg or 11 mg/kg for 5
weeks, and then exposed continuously to an ozone concentration of
200 µg/m3 (0.1 ppm), for 7 days. Oxygen consumption was measured in
lung homogenate using succinate as a substrate. Animals receiving the
higher concentration of vitamin E (66 mg/kg) were relatively
insensitive to this level of ozone, i.e., there was no significant
increase in oxygen consumption, but those receiving the lower
concentration of vitamin E (11 mg/kg) showed a significant increase in
oxygen consumption.
5.3 Systemic Reactions and other Effects
The number of studies on the effects of ozone on a wide range of
biological phenomena including growth, reproduction, and behaviour is
increasing but many of these studies need further confirmation.
Furthermore, even when a correlation is found between ozone exposure
and effects, it is difficult to decide whether the effects are due to
the direct oxidizing action of ozone or are secondary reactions to
pulmonary injury caused by ozone.
5.3.1 Effects on growth
There does not seem to be any convincing study which shows that
ozone, at the concentrations found in ambient air, has any detrimental
effect on body growth. Nevskaja & Kocetkova (1961) who exposed rats to
a mixture of ozone at 800 µg/m3 (0.4 ppm) and sulfuric acid at
7000 µg/m3 for 5 h per day, 6 days per week, for 100 days, and Loosli
et al. (1972) who exposed mice to synthetic photochemical air
pollution containing ozone at 600-840 µg/m3 (0.30-0.42 ppm) reported
that exposed animals weighed less than the controls. However, Emik et
al. (1971) did not find any significant differences between the growth
of guineapigs breathing ambient air containing oxidants at a mean
concentration of 110 µg/m3 (0.057 ppm) for over two years, and that
of the controls breathing filtered air.
5.3.2 Haematological effects
It is still questionable whether the haematological effects noted
in ozone exposure are due to the direct action of ozone on the
cellular and acellular components of the blood as it passes through
the lung capillaries or whether they are caused by oxidizing
intermediates, such as ozonides or peroxides, that might penetrate the
alveolar basement membrane and enter the pulmonary circulation. A
third possibility would be that these effects are secondary reactions
induced by ozone perhaps causing the release of some mediating
substance, yet to be identified.
5.3.2.1 Short-term exposure (24 h or less)
Short-term exposure to high concentrations of ozone ranging from
6000 to 16 000 µg/m3 (3.0-8.0 ppm) has been reported to cause
increases in neutrophil-lymphocyte ratios in rats (Bobb & Fairchild,
1967), and in the number of erythrocytes and leukocyte indices in mice
(Kusumoto et al., 1976). Within this range of concentrations,
Goldstein et al. (1968) and Goldstein (1973) found a reduction in
acetylcholinesterase (3.1.1.7) in the erythrocytes of mice and
induction of hydrogen peroxide formation in the circulating
erythrocytes of rats and mice. Veninga (1970, unpublished data)a
reported doubling in the number of binucleated lymphocytes and
increased levels of serum glutamic pyruvic transaminase (2.6.1.2) but
no changes in blood catalase (1.11.1.6) in mice exposed for 2 h to an
ozone concentration of 400 µg/m3 (0.2 ppm).
Increased resistance to haemolysis of erythrocytes was reported in
mice exposed to an ozone concentration of 2000 µg/m3 (1 ppm) for
30 min (Mizoguchi et al., 1973). Menzel et al. (1975) presented
evidence that fatty acid ozonides produced Heinz bodies in
erythrocytes in mice exposed to ozone at 1700 µg/m3 (0.85 ppm) for
4 h. Further exposure for 3 days resulted in a decline in the number
of Heinz body positive cells. It is of interest to note that these
bodies were not produced with ozone in the absence of serum containing
unsaturated lipids. The authors postulated that this indicated an
oxidation of the erythrocyte membrane and suggested that fatty acid
ozonides might be the toxic intermediaries. At lower concentrations,
Brinkman et al. (1964) noted increased sphering of erythrocytes of
mice, rabbits, and rats, after an in vitro exposure to ozone at
400 µg/m3 (0.2 ppm) for 1-2 h.
a Veninga, T. S. Ozone-induced alterations in murine blood and liver.
Paper presented at the 2nd International Clean Air Congress, Washington,
DC, 6-11 December 1970 (No. MB-15E).
A 3-h exposure to irradiated automobile exhaust containing an
oxidant concentration of 740-1200 µg/m3 (0.37-0.58 ppm) was found to
increase the number of leukocytes in the blood of mice and reduce the
level of serum alkaline phosphatase (3.1.3.1) (Kusumoto et al., 1976).
5.3.2.2 Prolonged and repeated exposures
Increasing the length of exposure provided further evidence for
the oxidizing effect of ozone on the blood of rabbits and rats. After
continuous exposure for 8 days to ozone at 1600 µg/m3 (0.8 ppm), the
lysozyme activity in the plasma and soluble fraction of lung of rats
significantly increased (Chow et al., 1974). Continuous exposure of
rats to an ozone concentration of 110 µg/m3 (0.06 ppm) for 93 days
resulted in a decrease in blood cholinesterase (3.1.1.8) activity
which returned to normal 12 days after exposure ceased (Eglite, 1968).
More recently, Jegier & P'an (1973) and P'an & Jegier (1972) reported
a rise in serum trypsin protein esterase in rabbits exposed to
800 µg/m3 (0.4 ppm) for 6 h per day, 5 days per week, for 10 months.
Long-term, combined exposure to ozone and carbon monoxide produced
a reduced level of serum glutamic oxaloacetic transaminase (2.6.1.1)
in rabbits. The animals were exposed for 1000 days to urban air
containing oxidants and carbon monoxide at mean concentrations over 2
years of 110 µg/m3 (0.057 ppm) and 2000 µg/m3 (1.7 ppm)
respectively, (Emik et al., 1971).
5.3.3 Effects on reproduction
A few studies have been reported concerning the possible effects
of ozone and photochemical oxidants on reproduction. The data indicate
that the newborn animals may be more susceptible to exposure to
oxidants than the parents.
Brinkman et al. (1964) and Veninga (1967) exposed pregnant mice
for 7 h per day, 5 days per week, for 3 weeks to ozone concentrations
of 200-400 µg/m3 (0.1-0.2 ppm). They found a 4-fold increase in
neonatal mortality. The second author also observed increases in the
incidence of both incisor growth and blepharophimosis in the new born.
Similar studies using irradiated automobile exhaust were performed
by Hueter et al. (1966) who found that mice exposed for 13 months
before, during, and after gestation showed a marked decrease in the
number and frequency of litters, the survival of infants, and the
total number of pups born. Nonirradiated automobile exhaust did not
produce any significant effects. The oxidant concentrations in the
irradiated exposure chamber ranged from 400 to 2000 µg/m3 (0.2 to
1.0 ppm). In a follow-up study, Lewis et al. (1967) found that the
number of female mice that did not become pregnant after mating with
pretreated males was twice that found in mice mated with untreated
controls.
5.3.4 Behavioural and related changes
5.3.4.1 Short-term exposure (24 h or less)
Behavioural changes in response to acute ozone exposures have not
been studied very extensively. The major effect that has been noted is
a reduction in spontaneous activity. Murphy et al. (1964a) and
Konigsberg & Bachman (1970) found significant losses in motor activity
in mice exposed to 400 µg/m3 (0.2 ppm) for 6 h and in rats exposed to
1000 µg/m3 (0.5 ppm) for 45 min, respectively.
Using an evoked response technique, Xintaras et al. (1966)
measured a reduction in the amplitude of response to a flash in the
specific visual cortex and in the superior colliculus in rats exposed
to ozone at 1000-2000 µg/m3 (0.5-1.0 ppm) for 1 h. In studies on
rhesus monkeys, Reynolds & Chaffee (1970) reported increases in both
simple and choice reaction times after a 30-min exposure to an ozone
concentration of 1000 µg/m3 (0.5 ppm).
Gardner et al. (1974) showed that the pentobarbital-induced
sleeping time of mice significantly increased after 2 and 3 exposures
each of 3 h, to an ozone concentration of 2000 µg/m3 (1 ppm). After
the third exposure, there was no change in the sleeping time compared
with the controls unless in subsequent exposure the ozone
concentration was raised to 10 mg/m3 (5 ppm), when the sleeping time
again differed significantly. The authors suggested that ozone might
be deactivating a liver microsomal enzyme that was responsible for the
detoxification of the drug.
5.3.4.2 Prolonged and repeated exposures
Additional studies on the effects of ozone on the behaviour of
animals have been conducted with longer exposures and complex mixtures
of air pollutants. It appears that the reduction of voluntary running
time may be an extremely sensitive indicator of ozone toxicity. The
mechanism of this change remains obscure.
Continuous exposure for 1 week to an ozone concentration of
2000 µg/m3 (1.0 ppm) caused an 84% reduction in voluntary activity in
rats (Fletcher & Tappel, 1973). Decreased running activity has also
been observed in mice exposed continuously to urban air with elevated
oxidant levels and to irradiated motor vehicle exhaust (Emik et al.,
1971; Heuter et al., 1966).
According to Litt et al. (1968), ozone-exposed rats required a
longer time to learn a specific task; learning was unstable and they
exhibited a reduced ability for temporal discrimination. The animals
were exposed intermittently for 2 months to ozone concentrations of
600-1000 µg/m3 (0.3-0.5 ppm). Nevskaja & Kocetkova (1961) exposed
rats to a mixture of ozone at 800 µg/m3 (0.4 ppm) and sulfuric acid
at 7000 µg/m3 for 5 h per day, 6 days per week, for 100 days, and
found that a conditioned reflex was retarded.
5.3.5 Miscellaneous systemic reactions to lung damage
Other changes have been reported which indicate biochemical,
physiological, and structural effects at sites distant from the lung
that are possibly related to the inhalation of ozone. In most of these
studies, the levels of ozone employed greatly exceeded those in the
ambient air. After exposing rabbits to an ozone concentration of
1500 µg/m3 (0.75 ppm) for 4-8 h, Atwal & Wilson (1974) found
histological and ultrastructural changes in the parathyroid glands.
They reported hyperplasia of the chief cells, large numbers of
secretion granules, proliferation and hypertrophy of the rough
endoplasmic reticulum, Golgi complex, mitochondria, lipid bodies, and
free ribosomes. These effects were seen up to 66 h after exposure.
Atwal et al. (1975) expanded their short-term exposure study by
exposing rabbits to 1500 µg/m3 (0.75 ppm) for 48 h and found
morphological changes in the parathyroid gland similar to those that
they had reported for 4-8 h of exposure. The data suggested that ozone
might trigger off an immune reaction which caused inflammatory injury
to the parathyroid gland.
It was demonstrated by Brinkman et al. (1964) that exposure to an
ozone concentration of 400 µg/m3 (0.2 ppm), for 5 h per day, for 3
weeks, resulted in the rupture of nuclear envelopes and the extrusion
of the contents of myocardial muscle fibres in rabbits and mice. This
effect became reversible one month after the exposure.
There are some data which suggest that ozone might accelerate the
aging process. Stokinger (1965) reported premature aging in rabbits
after 1 year of weekly 1-h exposures to ozone. The major changes found
in the exposed animals were premature calcification of the
sternocostal cartilage, severe depletion of body fat, dull cornea,
sagging conjunctivae, and a general appearance of not thriving.
5.4 Mutagenicity
Chromosome aberrations (anaphase bridges) were found in 42% of
root meristem cells of Vicia faba exposed to an ozone concentration
of 8000 mg/m3 (4000 ppm) for 1 h, but none were found in cells
exposed to clean air (Fetner, 1958). When chick embryonic fibroblasts
were exposed to ozone concentrations of 5000-10 000 mg/m3
(2500-5000 ppm) for 24 h, a small number of cells were found with
chromosome bridges in anaphase and telophase and with nuclear
fragments (Sachsenmaier et al., 1965). Using a much lower
concentration, Pace et al. (1969) exposed strain L cells continuously
to an ozone concentration of 8000 µg/m3 (4 ppm) for 30 h, and found a
significant reduction in cell survival compared with that in the
control group. The authors considered that this was due to ozone
interference with mitotic activity.
In vivo exposure studies on mammals by Zelac et al. (1971a,
1971b) are of special interest. Female Chinese hamsters were exposed
to an ozone concentration of 400 µg/m3 (0.2 ppm) for 5 h and the
number of circulating blood lymphocytes with chromosomal breaks was
measured immediately after exposure and 6, and 15.5 days later. A
significant increase in chromosomal breaks compared with pre-exposure
values was still observed 15 days after exposure.
Although further confirmation is required, these data and the
results of studies on chromosomal changes in human tissues (see
section 6.1.1) seem to indicate that ozone might be a mutagenic agent.
5.5 Summary Table
Table 10 is a summary of experimental animal studies that provide
quantitative information useful for the establishment of guidelines
for the protection of public health with respect to exposure to ozone
at concentrations of up to 2000 µg/m3 (1.0 ppm).
Table 10. Experimental animal studies
Local effects on the respiratory system
1. Morphological changes
Ozone Length of exposure
concentration Number
of
µg/m3 (ppm) number h/day Effects Responsea Species animals Reference
of days
1800 (0.88) 180 24 Epithelial injury seen as early as 4 h n.a.b rat n.a. Freeman et al.
after the beginning of exposure; after (1974)
3 weeks half of animals died and
emphysema-like lesions observed.
1600 (0.8) 7 24 Walls and interalveolar septa of terminal -- rat 8 (8) Castleman et al.
airways thickened and infiltrated by (1973a)
mononuclear cells.
1200 (0.6) 1 7 Swelling of epithelial alveolar lining -- mouse 32 (13) Bile (1970)
cells & endothelium cells with
occasional breaks in basement
membrane.
1100 (0.54) 180 24 Progressive changes in the airway -- rat n.a. Freeman et al.
epithelium after 6 days. (1974)
1000 (0.5) 1 6 Immediately after the exposure the -- mouse 16(12) Evans et al.
number of alveolar cells significantly (1971)
decreased.
800 (0.4) 5 per 6 Emphysematous & vascular-type -- rabbit 6 (6) P'an et al.
week x lesions. (1972)
10 month
a Number of animals showing effects/total number of animals; numbers in brackets refer to control groups.
b Not available.
Table 10. Experimental animal studies
Local effects on the respiratory system
1. Morphological changes cont'd.
Ozone Length of exposure
concentration Number
of
µg/m3 (ppm) number h/day Effects Responsea Species animals Reference
of days
520- (0.26 1 4.7-6.6 Dose-related loss of ciliated epithelium. -- cat 14 (3) Boatman et al.
2000 -1.0) (1974)
400 (0.2) 1 2 Degenerative changes in type I cells. -- rat n.a. Stephens et al.
(1974)
2. Functional changes
1400 (0.68) 1 2 No significant increase in flow -- guinea- 10 (10) Murphy et al.
resistance. pig (1964a)
1000 (0.5) 1 2 Increase in airways resistance and -- guinea- 10 (10) Yokoyame
breathing frequency with decrease in pig (1972a)
tidal volume.
520- (0.26 1 4.6 Increased flow resistance. -- cat 10 (4) Watanabe et al.
1000 -0.5) (1973b)
400 (0.2) 30 24 Reduction in lung elasticity; increase in -- rat 44 (44) Bartlett et al.
lung volume and in alveolar (1975)
dimensions.
a Number of animals showing effects/total number of animals; numbers in brackets refer to control groups.
b Not available.
Table 10. Experimental animal studies
Local effects on the respiratory system
3. Biochemical changes
Ozone Length of exposure
concentration Number
of
µg/m3 (ppm) number h/day Effects Responsea Species animals Reference
of days
1500 (0.75) 1 3 Reduction in activity of benzopyrene -- hamster 25 (95) Palmer et al.
hydroxylase (1.14.14.2). & 8 (15) (1971. 1972)
rabbit
1400- (0.7 7 24 Increased acid phosphatase activity. -- rat 14 (12) Castleman et al.
1600 -0.8) (1973b)
1400 (0.7) 5 24 Indication of lipid peroxidation; -- rat 33 (20) Chow & Tappel
increase in lysosomal hydrolase (1972); Dillard
activity. et al. (1972)
1000 (0.5) 1 6 Increased albumen recovery from -- rat 10 (18) Alpert et al.
alveolar spaces. (1971a)
800- (0.4 1 4 Evidence of formation of lipid peroxides n.a.b mouse n.a. Goldstein et al.
1400 -0.7) in the lung. (1969)
500 (0.25) 1 3 Reduced activity of several lysosomal -- rabbit 6 (6) Hurst et al.
hydrolases. (1970)
400 (0.2) 7 24 Increase in pulmonary mitochondrial -- rat 5-8 Mustafa et al.
oxygen consumption. (5-8) (1973)
a Number of animals showing effects/total number of animals; numbers in brackets refer to control groups.
b Not available.
Table 10. Experimental animal studies
Local effects on the respiratory system
4. Effects on the host defence system
Ozone Length of exposure
concentration Number
of
µg/m3 (ppm) number h/day Effects Responsea Species animals Reference
of days
1200- (0.62 1 17 Inhibition of pulmonary bactericidal -- mouse 20 (29) Goldstein et al.
1600 -0.80) activity. (1971b)
1000 (0.5) 210 16 No effect on physical clearance of -- rabbit 8 (8) Friberg et al.
inhaled particles or on the number of (1972)
macrophages.
1000 (0.5) 60 16 Decrease in the clearance of viable -- guinea- 18 (18) Friberg et al.
Escherichia coli. pig (1972)
800 (0.4) 1 4 Inhibition of bactericidal activity; no -- mouse 38 (37) Goldstein et al.
additive role of the induced silicosis. (1972)
600 (0.3) 1 3 Impairment of phagocytic properties of -- rabbit n.a.b Coffin et al.
pulmonary alveolar macrophages. (1968b)
500 (0.25) 1 3 Diminished enzyme activities of alveolar -- rabbit 6 (6) Hurst et al.
(1970)
macrophages.
160 (0.08) 1 3 Increased susceptibility to 15/40 mouse 40 (40) Coffin et al.
Streptococcus. (6/40) (1968a)
a Number of animals showing effects/total number of animals; numbers in brackets refer to control groups.
b Not available.
Table 10. Experimental animal studies--continued
II. Systemic reactions and other effects
1. Haematological effects
Ozone Length of exposure
concentration Number
of
µg/m3 (ppm) number h/day Effects Responsea Species animals Reference
of days
1700 (0.85) 1 4 Formation of Heinz bodies in red cells. n.a.b mouse n.a. Menzel et al.
(1975)
1600 (0.8) 8 24 Increase of lysozyme activity in plasma -- rat 8 (8) Chow et al.
and soluble fraction of lung. (1974)
800 (0.4) 5 per 6 increase in serum trypsin protein -- rabbit 6 (6) P'an & Jegier
week x esterase. (1972); Jegier
10 months & P'an (1973)
600 (0.3) 1 1 Inhibition of acetylcholine esterase -- ox (in -- P'an & Jegier
(3.1.1.7) activity. vitro) (1970)
400 (0.2) 1 1-2 Increased sphering of red blood cells. -- mouse, -- Brinkman et al.
rabbit, (1964)
rat (in
vitro)
400 (0.2) 1 2 Doubling in number of binucleated -- mouse n.a. Veninga (1970,
lymphocytes. unpublished)
110 (0.06) 93 24 Decrease in blood choline esterase -- rat 15 (15) Eglite (1968)
activity.
a Number of animals showing effects/total number of animals; numbers in brackets refer to control groups.
b Not available.
Table 10. Experimental animal studies--continued
II. Systemic reactions and other effects
2. Effects on reproduction
Ozone Length of exposure
concentration Number
of
µg/m3 (ppm) number h/day Effects Responsea Species animals Reference
of days
400 (0.2) 5 per 7 Increase in neonatal mortality. n.a. mouse n.a. Veninga (1967)
week x
gestation
period +
1st 3
weeks of
life
200- (0.1 5 per 7 Increase in neonatal mortality. n.a. mouse n.a. Brinkman et al.
400 -0.2) week x (1964)
3 weeks
1000- (0.5 1 1 Decrease in the amplitude of evoked -- rat 3 (3) Xintaras et al.
2000 -1.0) response to flash. (1966)
a Number of animals showing effects/total number of animals; numbers in brackets refer to control groups.
b Not available.
Table 10. Experimental animal studies--continued
II. Systemic reactions and other effects
3. Behavioural changes
Ozone Length of exposure
concentration Number
of
µg/m3 (ppm) number h/day Effects Responsea Species animals Reference
of days
1000 (0.5) 1 0.5 increase in simple and choice reaction -- rhesus 4 (4) Reynolds &
time. monkey Chaffee (1970)
1000 (0.5) 1 0.75 Significantly reduced motor activity. -- rat 12 (12) Konigsberg &
Bachman
(1970)
600- (0.3 60 intermittently Increase in time to learn specific tasks. -- rat 6 (6) Litt et al.
1000 -0.5) (variable (1966)
intervals)
400 (0.2) 1 6 Reduction in spontaneous running -- mouse 9 (9) Murphy et al.
activity. (1964a)
4. Miscellaneous extrapulmonary changes
1500 (0.75) 1-2 4-8, 24 Histological changes in parathyroid -- rabbit 16 (16) Atwal & Wilson
gland. (1974); Atwal
et al. (1975)
400 (0.2) 21 5 Structural changes in myocardial -- rabbit n.a. Brinkman et al.
muscle fibres. & mouse (1964)
400 (0.2) 1 5 Increase in chromosomal breaks in -- hamster 8 (4) Zelac et al.
circulating lymphocytes. (1971a,b)
a Number of animals showing effects/total number of animals; numbers in brackets refer to control groups.
b Not available.
6. EFFECTS ON MAN
6.1 Controlled Exposures
A considerable number of studies have been performed, under
controlled conditions, on the effects of ozone on both healthy
subjects and patients, with their consent. Some of these studies have
provided useful information for the evaluation of exposure-effect
relationships. A few in vitro studies using human tissues have also
helped to clarify the mechanisms of the biological actions of ozone.
There have been few human studies on other oxidants.
6.1.1 In vitro effects on human tissues
Brinkman et al. (1964) found that in vitro exposure of human red
blood cells to an ozone concentration of 0.5 mg/m3 (0.25 ppm) for
30 min accelerated sphering of these cells by X-ray irradiation,
compared with unexposed cells. Chromosome breakages in human cell
(epidermoid carcinoma cell) cultures exposed to an ozone concentration
of 16 mg/m3 (8 ppm) for 5 or 10 min were equivalent to those produced
by X-rays (200R, 250 kV) (Fetner, 1962).
The production of interferon was suppressed when human tonsil
lymphocytes were exposed in vitro to an ozone concentration of
10 mg/m3 (5 ppm) for 3 h (Watanabe et al., 1973a).
Goldstein (1976) measured the combined effects of ozone at
4-82 mg/m3 (2-41 ppm) and nitrogen dioxide at 6.8-190 mg/m3
(3.6-102 ppm) on human red cells, in vitro, and found that the
absolute and relative concentrations of the pollutants as well as the
sequence of administration could affect the interaction. In general,
the effects of the two pollutants on the variables measured (osmotic
fragility, acetylcholin-esterase (3.1.1.7) activity, lipid
peroxidation, reduced glutathione, and methaemoglobin levels) were
additive. At lower pollutant doses, a synergistic effect on the
increase in lipid peroxides was reported. Ozone also potentiated the
formation of methaemoglobin due to the action of nitrogen dioxide.
6.1.2 Sensory effects
The effects of ozone or oxidants on sensory organs have been
studied in terms of eye irritation, changes in visual parameters, and
olfactory thresholds.
Eye irritation in two groups of 20 female telephone company
employees working in identical adjacent rooms was evaluated by
Richardson & Middleton (1957, 1958) in relation to oxidant
concentrations from May to November 1956. Activated-carbon and dummy
air-filter media were switched periodically between the two rooms so
that the groups were alternately exposed to test and control
conditions. In all cases, differences in eye irritation between the
activated-carbon filtered and nonfiltered test conditions were highly
significant (p < 0.01). The scatter diagram (Fig. 8) suggests the
existence of a threshold for eye irritation at an oxidant
concentration of approximately 200 µg/m3 (0.10 ppm).
5. EFFECTS ON EXPERIMENTAL ANIMALS
There is considerable evidence to show that even short exposure to
high concentrations of ozone may endanger the health of experimental
animals. In reviewing this evidence, emphasis has been placed on
studies in which animals were exposed to concentrations of oxidants of
2000 µg/m3 (1.0 ppm) or less, since these studies are more relevant
for predicting the health risk to man. However, some experiments
conducted at higher concentrations have also been discussed when it
was considered that they would contribute to a better understanding of
the mechanism of the biological action of oxidants.
5.1 Absorption of Ozone
A number of factors can influence the transport and removal of
ozone in the upper airways such as: (a) nasal morphology; (b) route,
rate, and depth of breathing; and (c) biochemical composition and
amount of mucus. The decomposition of ozone within the upper airways
may protect the lower part of the respiratory tract against the
irritant gas. Various attempts to determine or model the respiratory
absorption of ozone in the upper airways have been made (McJilton et
al., 1972; Vaughan et al., 1969; Yokoyama & Frank, 1972). This work
has recently been reviewed by Miller (1977) who developed a
mathematical model for the transport and removal of ozone in the
respiratory tract of guineapigs, rabbits, and man, and predicted that
whatever the initial concentration, the respiratory bronchioles would
receive the highest dose of ozone. This agrees well with various
experimental studies in animals. For all three species the
relationship between respiratory bronchiolar concentration and the
inhaled ozone concentration at the tracheal level is linear on a
log-log scale at concentrations greater than 100 µg/m3 (0.05 ppm),
the respiratory bronchiolar dose for rabbits being 80% of that for man
and twice that for guineapigs.
5.2 Effects on the Respiratory System
5.2.1 Morphological changes
5.2.1.1 Short-term exposure (24 h or less)
The primary target of ozone is the respiratory tract and
particularly the pulmonary parenchyma. In small laboratory animals,
exposure to ozone at acutely toxic concentrations results in pulmonary
oedema, haemorrhage, and death. The LD50 is about 12 mg/m3 (6.0 ppm).
At lower concentrations in the range of 400-2000 µg/m3 (0.2-1.0 ppm),
ozone causes numerous changes in both the epithelial and endothelial
cells of the lung and ultrastructural effect indicate that the primary
lesions are in the epithelial lining of the terminal bronchioles and
the proximal alveoli.
The sequence of degeneration, desquamation, and destruction of
type I alveolar cells in rats following exposure to an ozone
concentration of 400 µg/m3 (0.2 ppm) for 2 h was demonstrated in
electron microscopic studies by Stephens et al. (1974). The type II
epithelial cells appeared to be more resistant. Freeman et al. (1974)
also reported significant histological changes in type I epithelial
cells after 4 h exposure to a concentration of 1800 µg/m3. The loss
of ciliated epithelium throughout the upper respiratory tract,
swelling and denudation of type I cells, erythrocyte lysis within
alveolar capillaries, and breakdown of capillary endothelium has been
reported in cats exposed to ozone concentrations of 520, 1000, and
2000 µg/m3 (0.26, 0.5, and 1.0 ppm) for 4.7-6.6 h (Boatman et al.,
1974). These effects appeared to be dose-related. Similar effects were
noted by Bils (1970) in mice after a 7-h exposure to ozone at
concentrations of 1200 and 2600 µg/m3 (0.6 and 1.3 ppm).
Cell renewal rate within the alveoli was studied by Evans et al.
(1971) by injecting tritiated thymidine into 18 to 20-month-old mice
before exposing them for 6 h to ozone concentrations of 1000, 2400,
5000, or 7000 µg/m3 (0.5, 1.2, 2.5, and 3.5 ppm). Immediately
following exposure, the number of labelled alveolar cells (those
synthesizing DNA) was significantly lower in all exposed groups
compared with controls.
Similar morphological studies have also been performed using
complex mixtures containing oxidants. Ultrastructural alterations
consisting of disrupted cytoplasm and abnormal mitochondria were seen
in the alveolar tissue of mice exposed for 2-3 h to Los Angeles air
that had a total oxidant concentration of 800 µg/m3 (0.4 ppm) (Bils,
1966). Exposure of mice for 3 h to an irradiated synthetic atmosphere
that contained propylene, nitric oxide, carbon monoxide, and water
vapour and simulated a heavy smog, gave rise to a similar pattern of
ultrastructural changes (Bils & Romanovsky, 1967). Rats continuously
exposed for 24 h to a mixture of ozone at a concentration of
500 µg/m3 (0.25 ppm) and nitrogen dioxide at a concentration of
4700 µg/m3 (2.5 ppm) exhibited increases in the numbers of alveolar
macrophages and of free cells in the lung resembling desquamated type
I cells, and hypertrophy of the epithelium were noted in rats
continuously exposed for 24 h to a mixture of ozone at a concentration
of 500 µg/m3 (0.25 ppm) and nitrogen dioxide at a concentration of
4700 µg/m3 (2.5 ppm) (Freeman et al., 1974).
5.2.1.2 Prolonged and repeated exposures
Prolonged exposure to low levels of ozone causes more extensive
and irreparable damage to the lung than the oedematigenous and acute
inflammatory reactions observed following short exposure to high
concentrations. Emphysema, atelectasis, focal necrosis, broncho-
pneumonia, and fibrosis have been reported, often accompanied by a
variety of cellular alterations. The degree of morphological injury
appears to be proportional to the concentration and time of exposure.
Freeman et al. (1974) described a number of pathomorphological
changes in rats continuously exposed to ozone at concentrations of
1100 and 1800 µg/m3 (0.54 and 0.88 ppm) for as long as 6 months.
After 48 h of exposure to the lower concentration, an influx of
macrophages and an increase in mitotic figures were observed, and the
alveolar ducts became demarcated by hypertrophic alveolar epithelium.
Most of these changes became more obvious with increasing length of
exposure with the exception of the terminal bronchioles which appeared
to recover and return to normal. After exposure to the higher
concentration of 1800 µg/m3 (0.88 ppm) for 48 h, the bronchiolar
epithelium exhibited both metaplasia and fibrosis. Adenoma-like
structures containing large numbers of macrophages and fibrotic
lesions appeared after 6 days and after 3 weeks of exposure, half of
the rats had died and were found to have emphysema-like lesions.
Using scanning electron microscopy, Ikematsu et al. (1976) found
that continuous exposure to an ozone concentration of 2000 µg/m3
(1.0 ppm) for 10 days resulted not only in desquamation of epithelial
cells but also in the appearance of inflammatory cells in the tonsils
of rabbits. Similar pathological effects were observed with
intermittent exposure indicating that the animals were unable to
recover sufficiently during the periods of exposure to clean air.
Emphysematous and vascular lesions in the lung of the rabbit described
by P'an et al. (1972), were the result of repeated exposure to
800 µg/m3 (0.4 ppm) for 6 h per day, 5 days per week, for 10 months.
Stokinger et al. (1957) also reported fibrotic changes and chronic
bronchial and bronchiolar emphysema in the lungs of mice, rats,
guineapigs, and hamsters exposed to an ozone concentration of
2000 µg/m3 (1.0 ppm) for 6 h per day, 5 days per week, for 433 days.
However, under the same conditions of exposure, the effects in dogs
were limited to the trachea and large bronchi. When dogs were exposed
to ozone concentrations ranging from 2000 to 6000 µg/m3 (1-3 ppm) for
8, 16, or 24 h daily for 18 months, the morphological damage was
roughly proportional to the product of the concentration and the time
of exposure, the epithelial lining of the terminal airways and
proximal alveoli being most adversely affected (Freeman et al., 1973;
Stephens et al., 1973). Similarly, Castleman et al. (1973a) found that
the walls of the terminal airways and the interalveolar septa of rat
lung were most affected by continuous exposure to an ozone
concentration of 1600 µg/m3 for 7 days.
Intermittent exposure for 8 h per day, for 7 days to an ozone
concentration of 400 µg/m3 (0.2 ppm) produced damage to the
respiratory bronchioles in bonnet monkeys. In rats fed a normal diet,
similar effects were seen at the same concentration of ozone, but, in
vitamin E-deficient rats, an equivalent effect was caused by ozone at
200 µg/m3 (0.1 ppm) (Dungworth et al., 1975; Dungworth, 1976).
Loosli et al. (1972) measured the cell turnover rate in mice and
found it to be significantly higher in animals exposed to synthetic
photochemical air pollution for 8-12 months than in control animals
breathing filtered air. The synthetic air pollution contained ozone at
600-840 µg/m3 (0.30-42 ppm), carbon monoxide at 3.5-12 mg/m3
(3-10 ppm), nitrogen dioxide at 1300-1600 µg/m3 (0.70-0.85 ppm), and
sulfur dioxide at 5700-6000 µg/m3 (2.2-2.3 ppm). The effects were
similar to those seen with exposure to pure ozone.
Rats exposed for 1 month to a mixture of ozone at 1800 µg/m3
(0.9 ppm) and nitrogen dioxide at 1700 µg/m3 (0.9 ppm), developed
enlarged alveolar spaces (Freeman et al., 1974). In rats exposed for
2 weeks to a combination of ozone at 500 µg/m3 (0.25 ppm), and
nitrogen dioxide at 4700 µg/m3 (2.5 ppm), the bronchiolar epithelium
became cuboidal. However, after 6 months of exposure, the tissue
appeared to be normal (Freeman et al., 1974).
Slightly inflammatory and proliferative changes were observed by
Nakajima et al. (1972) in the bronchial membranes of mice exposed to
irradiated auto exhaust that contained oxidant concentrations of
200-300 µg/m3 (0.1-0.15 ppm), for 2-3 h per day, 5 days per week, for
30 days.
5.2.2 Functional changes
5.2.2.1 Short-term exposure (24 h or less)
The first abnormal sign observed in various animal species during
exposure to ozone is an irregular respiratory pattern. This usually
appears within the first few minutes of exposure and, in most cases,
the animal returns to normal, when allowed to recover in the clean
air.
Functional changes in the respiratory system have been observed in
several species of animals at ozone concentrations of less than
2000 µg/m3 (1.0 ppm). Yokoyama (1972a) studied the ventilatory
function of guinea-pigs before, during, and after a 2-h exposure to an
ozone concentration of 1000 µg/m3 (0.5 ppm). He reported increases in
the frequency of respiration and airway resistance, but a decrease in
tidal volume. In another investigation, Yokoyama (1973) exposed only
the right lung of rabbits to ozone at 2000 µg/m3 (1 ppm) for
3 h and used the left lung as a control. The exposed lung of rabbits
killed 1 and 3 days after exposure, had a reduced vital capacity.
However, the reduction in vital capacity in those killed 7 days after
exposure was only slight and was not significant. Other studies on
lung function in guineapigs exposed to ozone for 2 h indicated that
pulmonary flow resistance was not altered by exposure to
concentrations of 700 and 1400 µg/m3 (0.34 and 0.68 ppm), but that it
increased significantly at exposure levels of 2000 and 2700 µg/m3
(1.08 and 1.35 ppm) (Murphy et al., 1964a).
Scheel et al. (1959) exposed rats to an ozone concentration of
4000 µg/m3 (2 ppm) for 3 h. Decreases in minute ventilation, tidal
volume, and oxygen uptake, that occurred immediately after exposure,
reached minimum recorded values 8 h later. All measurements returned
to normal levels 20 h after exposure.
When cats were exposed for an average of 4.6 h to ozone
concentrations of 520, 1000, and 2000 µg/m3 (0.26, 0.5, and 1 ppm),
Watanabe et al. (1973b) found that pulmonary flow resistance increased
with increasing ozone concentrations. Dynamic compliance was reduced,
but to a lesser extent, and vital capacity was unaffected. The
proportion of animals that showed a reduction in diffusion capacity
appeared to increase with increasing ozone concentrations.
There are a few studies in which animals were exposed to ambient
air and irradiated auto exhaust containing high concentrations of
oxidants, including those of Swann & Balchum (1966) who measured the
total expiratory flow resistance in guineapigs on days of unusual
weather and smog conditions in Los Angeles. When the resistance was
compared with routine monthly measurements on the same animals,
significant increases were found at oxidant levels of approximately
600 µg/m3 (0.30 ppm) or more. Substantial increases in resistance
were also observed, when relatively high concentrations of nitrogen
dioxide (1700 µg/m3; 0.92 ppm), carbon monoxide (30 mg/m3;
26 ppm), and hydrocarbons (16 ppm) were present, but the oxidant level
(80 µg/m3; 0.04 ppm) was relatively low.
The effects on guineapigs of a 4-h exposure to diluted,
irradiated, or nonirradiated exhaust atmospheres were reported by
Murphy et al. (1963). Marked, rapid increases in total expiratory flow
resistance accompanied by a decrease in respiratory rate and a small
increase in tidal volume occurred during exposure to irradiated
exhaust. The reaction to nonirradiated exhaust was comparatively
slight. Ranges of concentrations of the main pollutants in the
exhaust-contaminated air used in this study were: for irradiated
exhaust gases: total oxidants, 660-1640 µg/m3 (0.33-0.82 ppm);
nitrogen dioxide, 800-10 000 µg/m3 (0.43-5.5 ppm); and carbon
monoxide, 39-360 mg/m3 (34-310 ppm): for nonirradiated exhaust gases:
total oxidants, less than 40 µg/m3 (0.02 ppm); nitrogen dioxide,
710-3000 µg/m3 (0.38-1.58 ppm); and carbon monoxide, 98-345 mg/m3
(85-300 ppm). In addition, increased levels of formaldehyde, acrolein,
and olefin were present.
5.2.2.2 Prolonged and repeated exposures
The few available pulmonary function studies on animals exposed to
ozone for extended periods of time include a study by Bartlett et al.
(1975) who reported that continuous exposure of rats to an ozone
concentration of 400 µg/m3 (0.2 ppm) for 30 days caused a 16%
increase in lung volume and an increase in alveolar dimension. A
reduction in lung elasticity that was also reported was possibly an
effect of ozone on collagen. Yokoyama (1974) exposed rabbits to an
ozone concentration of 4000 µg/ma (2 ppm), for 6 h per day, for 3-4
days, and found that the pulmonary flow resistance was greater, and
compliance lower, than in the controls. In animals exposed to
2000 µg/m3 (1 ppm), for 6 h per day, for 7-8 days, the values were
between those of the controls and of the group treated with
4000 µg/m3 (2 ppm).
5.2.3 Biochemical changes
5.2.3.1 Effects indicating possible mechanisms of action
The actual mechanism of ozone toxicity at the subcellular level is
still obscure. Studies of the biochemical effect of ozone have mainly
been based on two hypotheses: (a) that ozone interacts with readily
oxidizable substances thus altering the course of metabolism and
producing a toxic effect; and (b) that ozone interacts with
unsaturated lipids to produce lipid peroxidation and consequent cell
damage.
Sulfhydryl systems appear in the cell not only as reducing
substances but also as functional constituents of a variety of enzymes
and proteins. Ozone is capable of oxidizing these substances causing
inactivation of enzymes and alterations in the structure and function
of the cell membrane.
In studies on ozone oxidation of glutathione in vitro, Mudd et
al. (1969) showed that both fast and slow oxidation occurred. It has
also been shown that the oxidation of reduced glutathione (GSH)
results in oxidized glutathione (GSSG), though some of the sulfhydryl
groups form higher oxidation products that are not reduced by the
reductases available in the cells (Menzel, 1971). Mountain (1963)
demonstrated in vivo oxidation of reduced glutathione and a decrease
in the sulfur-containing enzyme succinic dehydrogenase (1.3.99.1)
activity in the lungs of mice. King (1961) also found a decrease in
sulfhydryl content and of enzymatic function of partially purified
glyceraldehyde-3-phosphate dehydrogenase (1.2.1.12) in rat lungs
following exposure to an ozone concentration of 2400 µg/m3 (1.2 ppm)
for 4 weeks.
When rats were exposed to an ozone concentration of 4000 µg/m3
(2.0 ppm) for 4-8h, the concentrations of both the protein and
nonprotein sulfhydryls in the lungs decreased. The activities of lung
enzymes containing sulfhydryl also decreased including those of
glucose-6-phosphate dehydrogenase (1.1.1.49), glutathione reductase
(1.6.4.2), and cytochrome c reductase related to succinate and
reduced nicotinamide adenine dinucleotide (NADH) (DeLucia et al.,
1972). Expanding these studies, DeLucia et al. (1975) showed that the
magnitude of the decrease in the nonprotein sulfhydryl groups in rats
was dependent on the duration of exposure and the concentration of
ozone. Significant decreases were not observed at an ozone
concentration of 1600 µg/m3 (0.8 ppm) for 24 h.
Further studies have shown that exposure to ozone tends to
increase the activity of enzymes that protect against intracellular
oxidation. When rats were continuously exposed to an ozone
concentration of 1500 µg/m3 (0.75 ppm) for 1, 3, 10, or 29 days, it
was noted that the activities of glutathione peroxidase (1.11.1.9),
glutathione reductase (1.6.4.2), glucose-6-phosphate dehydrogenase,
6-phosphogluconate dehydrogenase (1.1.1.43), and pyruvate kinase
(2.7.1.40) were lower than those in the controls after 1 day but
higher after, 3, 10, and 29 days of exposure (Chow & Tappel, 1973). In
another study by the same investigators, rats exposed to 400 µg/m3
(0.2 ppm) continuously for 8 days or intermittently (8 h per day) for
7 days showed significantly increased glutathione peroxidase activity
in the lung. Fukase et al. (1975a, 1975b) exposed mice to ozone
concentrations of 400, 1000, or 2000 µg/m3 (0.2, 0.5 or 1.0 ppm) for
4 h per day, for 30 days, and found progressive increases in the
levels of glutathione and vitamin C in the lung with increasing ozone
concentration. The authors also reported significant increases in the
activities of glutathione peroxidase, glutathione reductase, and
glucose-6-phosphate dehydrogenase in the lungs of mice. Exposure of
both rhesus and bonnet monkeys to levels of ozone ranging from 400 to
1600 µg/m3 (0.2-0.8 ppm) for 8 h per day for 7 days, resulted in
increased activities of succinate oxidase and glutathione peroxidase
in the lungs. Linear regression analysis showed a significant
correlation between ozone concentration and the augmentation in
activity of these enzymes (Dungworth et al., 1975).
Many investigators have attributed the biological effect of ozone
to lipid peroxidation (Fournier, 1973; Goldstein et al., 1969; Menzel,
1970; Roehm et al., 1971b). Oxidation of unsaturated fatty acids by
ozone has been demonstrated both in vivo and in vitro. The
mechanism of such action is based on the proclivity of ozone to react
with the ethylene groups of the acid to form peroxides. Their
decomposition results in the further formation of free radicals
capable of initiating peroxidation of other unsaturated fatty acids.
The breakdown products (peroxides, carbonyl compounds) may themselves
be cytotoxic.
Evidence of lung lipid peroxidation during ozone exposure was
suggested by Goldstein et al. (1969) who found conjugated diene bonds
in an extract of lungs of mice exposed to 800-1400 µg/m3
(0.4-0.7 ppm) for 4 h. Another index of lipid peroxidation is an
increase in malonaldehyde. In studies by Chow & Tappel (1972), the
malonaldehyde concentration increased in the lungs of rats exposed
continuously to 1400-1600 µg/m3 (0.7-0.8 ppm) for 5 and 7 days.
Further evidence of the role of peroxidation in ozone toxicity is
the fact that animals deficient in vitamin E are more susceptible to
ozone. This is discussed in detail in section 5.2.7.
5.2.3.2 Biochemical effects at the subcellular level
In morphological and ultrastructural studies, swelling and
degenerative changes in lung mitochondria have frequently been
reported following ozone exposure. Mitochondrial functions are
critical to the cellular terminal substrate oxidation and energy
production. These organelles in lung cells may be the target for ozone
since many mitochondrial enzyme activities are sulfhydryl-dependent
and mitochondrial membranes contain abundant unsaturated
phospholipids. It has therefore been suggested that the lung
mitochondria may be a sensitive test system for detecting and
evaluating ozone toxicity.
Mustafa et al. (1973) found a 45% increase in pulmonary
mitochondrial oxygen consumption in rats continuously exposed to
1600 µg/m3 (0.8 ppm) for 10-20 days. A 3-fold increase in the number
of type II cells was also reported. These cells are rich in
mitochondria and may have been responsible for the increase in oxygen
consumption. A 17% increase in oxygen consumption was noted with
continuous exposure to an ozone concentration of 400 µg/m3 (0.2 ppm)
for 7 days. This effect appeared to be dose-related.
Lysosomes are vitally important in the intra- and possibly
extracellular destruction of inhaled matter. The hydrolytic enzyme
system of lysosomes in the alveolar machrophage is crucial to maintain
the sterility of the lung against inhaled microbes. Any inactivation
of these enzymes would be expected to increase the risk of respiratory
disease.
Hurst et al. (1970) reported that, when rabbits were exposed for
3 h to an ozone concentration of 500 µg/m3 (0.25 ppm), there was a
reduction in the activity of lysosomal hydrolases, i.e., acid
phosphatase (3.1.3.2), lysozyme (3.2.1.17), and beta-glucuronidase
(3.2.1.31). In vitro exposure of rabbit alveolar macrophages
produced a similar decrease in lysosomal hydrolases (Hurst & Coffin,
1971).
Ozone may also induce increases in the concentrations of these
enzymes which may be related to eventual chronic lung disease.
When the specific activities of a number of lysosomal hydrolases
were measured in whole lung homogenates of rats, there was a
significant increase in activities after the animals had been
continuously exposed to an ozone concentration of 1400-1600 µg/m3
(0.7-0.8 ppm) for 5-7 days (Dillard et al., 1972). This increase could
be attributed to an inflammatory reaction induced by the ozone (Coffin
et al., 1968b).
Castleman et al. (1973b) exposed rats continuously for 7 days to
ozone concentrations of 1400-1600 µg/m3 (0.7-0.8 ppm). Using histo-
and cytochemical techniques, they observed increased acid phosphatase
activity but no change in beta-glucuronidase activity.
One of the structural alterations reported following exposure to
ozone is a change in the appearance of the endoplasmic reticulum.
Biochemical evidence of the effects of ozone on microsomal enzymes was
reported by Palmer et al. (1971, 1972). After a 3-h exposure to ozone
at 1500 µg/m3 (0.75 ppm), the lung tissue of hamsters and the
tracheobronchial mucosa of rabbits showed reductions of 33% and 53%,
respectively, in the activity of benzopyrene hydroxylase (1.14.14.2),
a mixed function oxidase that depends on cytochrome P-450 and is
located in the endoplasmic reticulum. Similar results were obtained by
Goldstein et al. (1975) who exposed rabbits for 90 rain to
2000 µg/m3 (1.0 ppm) and demonstrated a decrease in the rabbit lung
cytochrome P-450 concentration. It is of interest that the maximum
effect was observed a few days after exposure.
Very little information is available describing alterations in
nucleic acids related to ozone exposure. Most of the studies have been
conducted at much higher concentrations than those found in the
environment and have yielded conflicting results. Since DNA synthesis,
cell division, and growth are closely linked, further studies
clarifying the potential hazard would be of value.
In studies on mice exposed to an ozone concentration of
5000 µg/m3 (2.5 ppm) for 2 h per day, for 120 days, Werthamer et al.
(1974) noted reductions in both DNA and RNA syntheses and a
concomitant increase in protein synthesis. Evans et al. (1971) exposed
aging mice for 6 h to 1000-7000 µg/m3 (0.5-3.5 ppm) and found that,
regardless of the ozone concentration, there was inhibition of DNA
synthesis. The authors believed that this indicated a reduction in the
ability of the alveoli to act as a source of new cells and to maintain
the integrity of lung tissue during ozone exposure.
5.2.3.3 Extracellular effects
Since the primary cause of death from high concentrations of ozone
is pulmonary oedema, investigators have attempted to determine the
role of histamine in the pulmonary toxicity of ozone. The lung is rich
in histamine -- containing mast cells and among the many effects of
histamine are oedematigenous alterations in vascular capillaries.
Oedema, whatever the cause, reduces the number of alveoli
participating in gas exchange, and produces conditions favourable for
bacterial growth. The exact concentration of ozone required to produce
oedema depends on the animal species. It is generally believed that
gross oedema is probably not elicited in any species exposed to ozone
at the concentrations found in the ambient air.
Alpert et al. (1971a) used a radio labelled albumen technique to
detect the presence of pulmonary oedema in rats. A significant
increase in albumen levels in pulmonary lavage fluid appeared after
6 h exposure to 1000 µg/m3 (0.5 ppm).
The available data on lung histamine are conflicting. Dixon &
Mountain (1965) reported that, following a single exposure of mice to
an ozone concentration of 2000 µg/m3 (1.0 ppm) for 5 h, there was a
release of histamine from the lungs that persisted for at least 4
days. Pretreatment with an antihistamine (promethazine) reduced the
amount of oedema following exposure to a sublethal dose of ozone. It
should be noted that the antihistamine used is a phenothiazine
derivative the action of which might stabilize membranes and trap free
radicals. In contrast, Easton & Murphy (1967) were unable to
demonstrate any reduction of lung histamine in guineapigs exposed to
ozone concentrations of 10-12 mg/m3 (5-6 ppm) for 2 h. Cronin & Giri
(1974) also failed to observe any differences in the lung histamine
level in rats exposed to an ozone concentration of 8000 µg/m3
(4.0 ppm) for 4 h, although pulmonary oedema was evident.
Surface tension is an important contributor to the elastic
properties of the lung. Any alteration of the normal surface tension
in the alveoli may be implicated in the development of chronic lung
disease. Consequently, several investigators have examined the effect
of ozone on pulmonary surface activity. Gardner et al. (1971) exposed
rabbits to ozone levels as high as 20 mg/m3 (10 ppm) for 2.5 h and
then isolated the surface active material by pulmonary lavage. They
found that ozone did not alter the surface tension of this material
and that in vitro exposure did not affect the properties of
dipalmitoyl lecithin, a principal component of this surface active
substance. Similar results were reported by Huber et al. (1971) who
showed that a 3-h exposure of rabbits to ozone at 10 mg/m3 (5 ppm)
did not alter surface activity in the lavaged alveolar lining material
nor in extracts of the whole lung. On the other hand Yokoyama (1972a)
reported that in vitro exposure of guineapig lung extracts to ozone
concentrations of 1000 to 24000 µg/m3 (0.5-12 ppm) for 25-60 min
resulted in a rapid increase in surface tension. However, he, too, did
not find any change when dipalmitoyl lecithin was exposed to ozone.
The effect of ozone on the appearance of lung tissue lipids in
saline lavage fluid was studied by Kyei-Aboagye et al. (1973). They
proposed that ozone affected the lung by decreasing lecithin formation
while simultaneously stimulating the release of surfactant lecithins
(palmitoyl and oleyl).
In the lung, there is a ground substance between the basement
membrane of the alveolar epithelium and the capillary endothelium. Any
destruction of the integrity of this substance could affect the
elasticity of the lung. Buell et al. (1965) fractionated the lung
tissue of rabbits, after a 1-h exposure to an ozone concentration of
2000 µg/m3 (1.0 ppm), into soluble, lipid, and protein fractions. The
isolation of aldehydes and ketones from the protein fraction indicated
structural changes. It was suggested that, once these compounds were
formed, they might affect the intra-and intermolecular crosslinking of
elastic protein molecules which would in turn cause a reduction in the
elasticity of the lung.
5.2.4 Carcinogenicity
The possibility that ozone might be carcinogenic has been studied
in experimental animals.
Exposure to ozonized gasoline with ozone concentrations of
2000-7600 µg/m3 (1.0-3.8 ppm) for 52 weeks caused an increased
incidence of lung tumours in strain A mice (350 in each experimental
group). After 40 weeks, tumours were found in 21% of animals in the
control group and in 63% in the test group. After 52 weeks, the
incidence of tumour-bearing animals in the control group exposed to
washed air was 41% compared with 80% in the test group (Kotin & Falk,
1956). Additional studies using the same atmosphere of ozonized
gasoline were conducted on C57BL mice (405 in each experimental group)
(Kotin et al., 1958). After 92 weeks, the incidence of tumour-bearing
animals in the control group was 1.6% compared with 9.6% in the
exposed group.
Although these studies indicate that ozone may be tumorigenic
further work is necessary to confirm these results.
A number of studies including that of Penha et al. (1972) have not
been considered in this document because information concerning the
numbers of animals tested, control groups, etc. was inadequate.
5.2.5 Tolerance to ozone
The term "tolerance" refers to the fact that exposure to a
nonlethal dose of a specific toxic substance protects the host against
subsequent exposure to higher dose of the same chemical or of
different agents with similar toxicological properties (cross-
tolerance). Stokinger et al. (1956) reported such a protective effect
for ozone. Tolerance to ozone has been reviewed by Fairchild (1967)
and has also been reported by many other investigators (Henschler,
1960; Matzen, 1957; Mendenhall & Stokinger, 1959), In small rodents,
tolerance can be initiated by a concentration as low as 600 µg/m3
(0.3 ppm), maximum protection being obtained with concentrations
within the range of 2000-8000 µg/m3 (1-4 ppm) (Stokinger & Scheel,
1962). A single exposure to ozone can also induce a cross-tolerance
against subsequent lethal doses of X-ray irradiation (Hattori et al.,
1963), nitrogen dioxide, hydrogen peroxide, carbonic dichloride
(phosgene), ethanone (ketene), nitrosyl chloride, or hydrogen sulfide
(Fairchild, 1967).
Although the mechanism of tolerance is still not well understood,
several possibilities have been envisaged. Fairchild (1967) observed
that the level of reduced glutathione was maintained in ozone-tolerant
animals but not in the nontolerant group. This could be brought about
either by directly blocking the oxidation of reduced glutathione or
through some enzymatic pathway that would stimulate production of
reduced glutathione in response to a second exposure to ozone.
However, it is possible that the maintenance of these thiols in the
tolerant animal may be a result of the tolerance rather than the
cause. The studies of Chow & Tappel (1972, 1973) provide biochemical
support for this hypothesis. In addition, it has been shown (Mountain
et al., 1960) that the activities of serum alkaline phosphatase
(3.1.3.1), adrenal succinate dehydrogenase (1.3.99.1), and glucose-6-
phosphatase (3.1.3.9) either remain normal or are only slightly
altered in tolerant animals.
Fukase et al. (1975a) reported tolerance in mice to an ozone
concentration of 20-58 mg/m3 (10-29 ppm) after pre-exposure to
concentrations of 400-2000 [µg/m3 (0.2-1.0 ppm) and proposed that the
mechanism involved an increase in reduced glutathione and the
elevation of the activity of the peroxidative metabolic pathway.
Physical swelling of the intra-alveolar septa has also been proposed
by Henschler et al. (1964) as a mechanism of protection against the
oedematigenous effects of ozone.
Unilateral lung exposure models showed that, in order to induce
protection in a particular lung, the tissue must have actually come
into contact with ozone (Alpert & Lewis, 1971; Frank et al., 1970a).
There was no significant crossover effect from contralateral lung,
suggesting that there was no basis for assuming a circulating humoral
factor, and that the phenomenon was purely local. While reduction in
oedema development could be induced by pre-exposure to ozone, no
protection was obtained against the influx of polymorphonuclear
leukocytes into the lung (Gardner et al., 1972). It was also apparent
that tolerance did not influence the number of recoverable cells in
pulmonary lavage, their phagocytic capability, or the loss of
macrophage hydrolytic enzyme activity. This indicated the possibility
that tolerance protects only against pulmonary oedema and not against
other more subtle reactions.
As previously mentioned, tolerance is initiated in small rodents
at approximately 600 µg/m3 (0.3 ppm) (Stokinger & Scheel, 1962).
According to Alpert et al. (1971a) this approximates the lowest level
at which oedema can be demonstrated in rats by means of recovery of
labelled serum albumen via lung lavage. Thus, Coffin & Gardner (1972b)
postulated that it is probably necessary to produce a minimal
oedematigenous response before tolerance develops.
Experiments designed to test the susceptibility to bacterial
infection of "tolerant" animals have been conducted by Coffin &
Gardner (1972b). These studies illustrated that the "tolerant" animals
were only partially protected against the joint effects of ozone and a
viable microorganism. Partial protection was afforded by tolerance
only when the initiating dose of ozone was 600 µg/m3 (0.3 ppm) for
3 h (Coffin & Gardner, 1975). The authors considered that the
tolerance to infection seen at levels of ozone above 600 µg/m3
(0.3 ppm) could be due to the prevention of the formation of oedema
and that tolerance was only partial because tolerance to the action of
ozone on cellular and noncellular specific defence systems could not
be induced. This was shown by Gardner et al. (1972) who reported that
there was no tolerance to ozone damage in the alveolar macrophages,
which are the prime defence against infectious disease in the lower
part of the respiratory tract.
In their study on the effects of ozone on laboratory-induced
allergic respiratory disease in guineapigs, Matsumura et al. (1972)
found that animals that had received pretreatment with ozone at
2000 µg/m3 (1 ppm) for 1 h before a challenging exposure of
4000 µg/m3 (2 ppm) showed only slight respiratory reaction to
acetylcholine inhalation compared with those in which tolerance had
not been induced.
A variety of neurohumoral factors resulting from exposure to ozone
have been described by Fairchild (1963), who reported that
thyroidectomy, adrenalectomy, and hypophysectomy would increase the
resistance of rats to otherwise lethal doses of ozone by preventing
pulmonary oedema.
While the mechanism of tolerance in relation to ozone-induced
oedema is still not well understood, it has been suggested that the
thymus might play a role. Thymectomized mice were unable to develop
tolerance to ozone although the sham-operated animals exhibited
tolerance under the same experimental conditions (Gregory et al.,
1967).
5.2.6 Effects on the host defence system
Animals that are exposed to ozone have an increased susceptibility
to disease-producing biological agents, which can result in an
increased incidence of pulmonary infectious disease and death. Animal
models have been developed by several investigators to examine this
phenomenon experimentally by exposing animals to the pollutants and to
aerosolized viable microorganisms. The high sensitivity of this model
system is probably due to the fact that it reflects a summation of all
the subtle effects that ozone has on the lung. Any alteration in
cellular defence and ciliary activity, oedema, and immunosuppression
would allow the viable organism to multiply and cause disease. In mice
exposed to ozone at concentrations ranging from 2600 to 8800 µg/m3
(1.3 to 4.4 ppm) for 3 h, or 1700 µg/m3 (0.84 ppm) for 4 h, per day,
5 days per week, for 2 weeks, and subsequently challenged with
Klebsiella pneumoniae, resistance to respiratory infection was
significantly reduced (Miller & Ehrlich, 1958). Similar results were
obtained with hamsters. In a study to elucidate a relationship between
the time of exposure to ozone and the time of challenge with the
infectious agent, Purvis et al. (1961) found a decrease in resistance
to infection in mice treated with the bacterial aerosol within 19 h of
exposure to the gas or 27 h before exposure. A subsequent study by
Coffin and co-workers (1968a) showed that the lowest concentration of
ozone necessary to produce this effect in mice was 160 µg/m3
(0.08 ppm) for 3 h; Coffin & Gardner (1972) established a dose-
response relationship.
Further enhancement of toxicity could be demonstrated when a third
interactant such as cold or exercise was added to this infectivity
model. Housing mice at 6°-9°C for 3 h prior to exposure to ozone at
1400-1800 µg/m3 (0.7-0.9 ppm) for 2 h and to the microorganisms
increased the mortality rate compared with that in animals housed at
room temperature (Coffin & Blommer, 1965). Mice subjected to physical
activity while being exposed to ozone at 200-600 µg/m3 (0.1-0.3 ppm)
were less resistant to infectious agents than animals that were at
rest during exposure (Gardner et al., 1974b).
The effects of low concentrations of ozone on mice with induced
silicosis were investigated by Goldstein et al. (1972). Starting at an
ozone concentration of 800 µg/m3 (0.4 ppm) for 4 h, a progressive
decrease in pulmonary bactericidal activity occurred with exposure to
increasing concentrations of ozone. Silicosis itself did not inhibit
bactericidal activity.
Further research has sought to delineate the mechanisms by which
ozone reduces the resistance of animals to bacterial infection. It
appears that a number of factors may be responsible.
The number of organisms initially deposited within the lung does
not play a major role in the enhancement of mortality since the
pulmonary deposition of the microorganisms is less in ozone-treated
animals. However, regardless of the initial deposition, ozone-treated
animals subsequently have more organisms within the lungs, mainly
because of reduced bactericidal ability and the subsequent
multiplication of the inhaled organism (Coffin & Gardner, 1972a).
Goldstein et al. (1971b) found that the magnitude of the increase in
bacterial numbers was correlated with an increase in ozone
concentration up to 5200 µg/m3 (2.58 ppm). In rabbits exposed to
ozone at a concentration of 1000 µg/m3 (0.5 ppm), for 16 h per day,
for 7 months, Friberg et al. (1972) did not find any effect on the
physical removal of inhaled particles or on the number of macrophages
but, under the same conditions, a significant decrease was found in
the clearance of viable Escherichia coli in the lungs of guinea-
pigs.
The effect of ozone on antibacterial activity in the mouse lung
was determined in vivo by investigating the removal of bacteria by
mucociliary activity and by bactericidal activity simultaneously
(Goldstein, 1971a, 1971b). Mice were exposed to various concentrations
or ozone including 1200, 1400, 1600, and 2200 µg/m3 (0.62, 0.70,
0.80, and 1.1 ppm), for 17 h prior to, or 4 h after, infection with
aerosols of radiolabelled Staphylococcus aureus. Inhibition of
pulmonary bactericidal activity was shown at an ozone concentration as
low as 1200 µg/m3 (0.62 ppm) and activity decreased progressively
with increasing levels of ozone. The authors proposed that the
bactericidal defect was due to dysfunction of the alveolar macrophage.
Rabbits exposed to an ozone concentration of 600 µg/m3 (0.3 ppm)
for 3 h showed an impairment of the phagocytic properties of the
pulmonary alveolar macrophages (Coffin et al., 1968b). Furthermore,
exposure to ozone at 10 mg/m3 (5 ppm) for 3 h resulted in a reduction
in the total number of macrophages with a concomitant influx of
polymorphonuclear leukocytes into the lower respiratory tract (Coffin
& Gardner, 1972a). Enzyme activity was reduced in macrophages
recovered by lavage after exposure of rabbits to ozone at levels as
low as 500 µg/m3 (0.25 ppm) for 3 h (Hurst et al., 1970). The enzymes
studied were lysozyme (3.2.1.17), acid phosphatase, and beta-
glucuronidase and since these enzymes are involved in the
intracellular degradation of ingested bacteria, their reduction could
contribute to the poor bactericidal effect seen in the studies
mentioned previously.
Other effects on the alveolar macrophage have also been reported.
For example, alveolar macrophages from rabbits exposed to 4000 µg/m3
(2.0 ppm) for 8 h per day for 7 days exhibited an increased membrane
fragility (Dowell et al., 1970) and a dose-related reduction in
interferon was observed in the alveolar macrophages of rabbits exposed
to ozone concentrations of 2000, 6000, and 10 000 µg/m3 (1, 3, and
5 ppm) for 3 h (Shingu et al., 1972).
Morphological changes seen in rabbit alveolar macrophages after
exposure to ozone at a concentration of 10 mg/m3 (5 ppm) for 3 h
included dilatation of the endoplasmic reticulum and perinuclear
envelope, swelling of the mitochondria, intracellular vacuolization,
cell lysis, and the formation of myelin figures and autophagic
vacuoles (Huber et al., 1971). The ultrastructure of pulmonary
macrophages was also examined in situ in the lung tissue of rats
exposed to ozone at 6000 µg/m3 (3 ppm) for 4 h (Plopper et al.,
1973b). Immediately after exposure, the cells resembled those of
unexposed animals, but 12 h later there were twice as many
macrophages. Many of these had large granular cytoplasmic inclusions
that indicated increased phagocytic activity.
There is evidence that the effect seen on the alveolar macrophage
may be mediated through the noncellular milieu of the lung. Within the
lung, the macrophages are located close to the so-called pulmonary
surfactant contained in the extracellular lining of the epithelial
surface of the lung alveoli. It is possible that ozone might also
react with this lining film, which in turn, might be deleterious to
the cell. Gardner (1971) and Gardner et al. (1971) conducted
experiments on lavage fluid containing this surface-active substance
and found that, when isolated from rabbits exposed to ozone at
20 mg/m3 (10 ppm) for 2.5 h, it could adversely affect the stability
of the alveolar macrophage in vitro. A similar effect was noted when
ozone was bubbled through the lavage fluid in vitro. The effect on
the lavage fluid could be seen at levels as low as 200 µg/m3
(0.1 ppm) for a 2.5-h, in vivo exposure or after only 30-min in
vitro exposure. Since no significant alteration in the surface
tension of the lavage fluid was produced by ozone exposure, it is
suggested that the effect might be on a nonlipid component, possibly a
protein. The activity found in the cell-free pulmonary lavage fluid
has been called the "protective factor".
Ozone may also inactivate some opsinogenic factor within the lung
since Holzman et al. (1968) showed that it has a protein-degrading
property. Exposure of mice and rabbits to a concentration of
10 mg/m3 (5 ppm) for 3 h reduced the activity of active lysozyme,
obtained by bronchopulmonary lavage, by approximately 30%.
Various components of the endogenous defence mechanism of the lung
were studied through a unilateral lung exposure model (Alpert et al.,
1971b). Exposure to ozone at concentrations ranging from 1000 to
6000 µg/m3 (0.5 to 3 ppm) for 3 h decreased cellular viability,
depressed the intracellular hydrolytic enzymes and increased the
absolute number of polymorphonuclear leucocytes in the pulmonary
lavage fluid. All effects were dose-related and were found only in the
lung under treatment and not in the contralateral lung that breathed
ambient air.
Heuter et al. (1966) reported that exposure to an irradiated
automobile exhaust atmosphere for 15 months increased the
susceptibility of mice to pulmonary infection during the latter half
of the animal's lifetime. The oxidant concentrations in the irradiated
exposure, chamber ranged from 400 to 2000 µg/m3 (0.2-1.0 ppm). In a
similar study using irradiated automobile exhaust, enhancement of
mortality compared with that of control animals kept in filtered air
was noted with exposure to total oxidants at 300 µg/m3 (0.15 ppm) and
carbon monoxide at 29 mg/m3 (25 ppm) for 4 h. Coexistent
concentrations of nitrogen dioxide ranged from a trace to 1900 µg/m3
(1.0 ppm) (Coffin & Blommer, 1967).
Goldstein et al. (1974) studied the bactericidal effect when mice
were exposed to a combination of nitrogen dioxide and ozone, and
concluded that the combined pollutants caused bactericidal dysfunction
at concentrations that were approximately the same as the lowest
concentrations that caused similar dysfunction when the animals were
exposed to the gases individually.
Erlich et al. (1977) observed that a single joint exposure of mice
to nitrogen dioxide and ozone resulted in an addition of effects and
that a synergistic action might result from repeated exposures to the
mixture.
The animals were exposed for 3 h to 16 different combinations of
nitrogen dioxide at levels of 0, 2800, 3800, 6600, and 9400 µg/m3
(0, 1.5, 2.0, 3.5, and 5.0 ppm) and ozone at 0, 100, 200, and
1000 µg/m3 (0, 0.05, 0.1, and 0.5 ppm). Within 1 h of termination of
exposure to the pollutants, the mice were infected with Streptococcus
pyogenes. Excess mortality rates due to exposure to the mixture of
the two gases were approximately equivalent to the sum of those
induced by the inhalation of each individual pollutant. In mice
exposed repeatedly for 3 h per day, for 20 days, to a mixture of
nitrogen dioxide and ozone at concentrations of 3800 µg/ma (2.0 ppm)
and 100 µg/m3 (0.05 ppm), respectively, and challenged with
Streptococcus aerosol, the number of deaths was significantly higher
than in the control group. On the other hand, repeated daily exposure
to either nitrogen dioxide or ozone at the above-mentioned
concentrations did not show any major effect on the mortality rate.
The authors considered that this result might suggest a synergistic
action of the two pollutants making them more effective in reducing
resistance to respiratory infection.
5.2.7 Interaction of ozone with bronchoactive and other chemicals
The effects of pre-exposure to ozone on the sensitivity of
guineapigs to inhaled acetylcholine were studied by Matsumura et al.
(1972). Animals pre-exposed to an ozone concentration of 4000 µg/m3
(2 ppm) for 30 min manifested severe difficulty in breathing and many
died. This effect persisted for as long as 2 h after exposure. In a
similar study, Matsumura (1970a) sensitized guineapigs to albumen and
found that repeated 30 min pre-exposures to an ozone concentration of
10 mg/m3 (5 ppm) enhanced the sensitization. An ozone concentration
of 2000 µg/m3 (1 ppm) had no such effect.
Ozone-exposed guineapigs were also more susceptible to the toxic
action of injected histamine (Easton & Murphy, 1967). A 2-h exposure
to ozone at 10 mg/m3 (5 ppm) caused severe lung function changes and
increased mortality. This increased susceptibility to histamine was
detectable for as long as 12 h after the end of the exposure.
In order to study the effects of ozone on susceptibility to
serotonin, Suzuki & Nagaoka (1973) injected the compound into the
abdominal cavity of rats after exposing the animals to ozone at
concentrations ranging from 2000 to 12 000 µg/m3 (1-6 ppm) for 3 h.
Mortality increased with increasing levels of ozone, but fatalities
did not occur in a control group injected with serotonin and breathing
filtered air.
A report by Goldstein et al. (1970) that a deficiency of vitamin E
increased the toxicity of ozone in the rat has been supported by the
studies of Roehm et al. (1971b, 1972) and Menzel et al. (1972). These
investigators observed a significantly shorter 50% lethal time and
more pronounced signs of respiratory distress in ozone-exposed rats
(2000 µg/m3 (1.0 ppm) for 9 days continuously) that were fed vitamin
E-depleted diets in comparison with those fed vitamin E-supplemented
diets. When the ozone concentration was reduced to 1000 µg/m3
(0.5 ppm), pulmonary oedema became evident and mortality rates
increased in animals fed vitamin E-deficient diets after 6 weeks of
exposure. In rats continuously exposed to toxic levels of ozone
ranging from 1400 to 1600 µg/m3 (0.7-0.8 ppm), protection by dietary
vitamin E against lung lipid peroxidation was proportional to the
logarithm of the concentration of the vitamin in the diet (Fletcher &
Tappel, 1973). In further studies by Mustafa (1975), rats were fed a
basal diet containing vitamin E at either 66 mg/kg or 11 mg/kg for 5
weeks, and then exposed continuously to an ozone concentration of
200 µg/m3 (0.1 ppm), for 7 days. Oxygen consumption was measured in
lung homogenate using succinate as a substrate. Animals receiving the
higher concentration of vitamin E (66 mg/kg) were relatively
insensitive to this level of ozone, i.e., there was no significant
increase in oxygen consumption, but those receiving the lower
concentration of vitamin E (11 mg/kg) showed a significant increase in
oxygen consumption.
5.3 Systemic Reactions and other Effects
The number of studies on the effects of ozone on a wide range of
biological phenomena including growth, reproduction, and behaviour is
increasing but many of these studies need further confirmation.
Furthermore, even when a correlation is found between ozone exposure
and effects, it is difficult to decide whether the effects are due to
the direct oxidizing action of ozone or are secondary reactions to
pulmonary injury caused by ozone.
5.3.1 Effects on growth
There does not seem to be any convincing study which shows that
ozone, at the concentrations found in ambient air, has any detrimental
effect on body growth. Nevskaja & Kocetkova (1961) who exposed rats to
a mixture of ozone at 800 µg/m3 (0.4 ppm) and sulfuric acid at
7000 µg/m3 for 5 h per day, 6 days per week, for 100 days, and Loosli
et al. (1972) who exposed mice to synthetic photochemical air
pollution containing ozone at 600-840 µg/m3 (0.30-0.42 ppm) reported
that exposed animals weighed less than the controls. However, Emik et
al. (1971) did not find any significant differences between the growth
of guineapigs breathing ambient air containing oxidants at a mean
concentration of 110 µg/m3 (0.057 ppm) for over two years, and that
of the controls breathing filtered air.
5.3.2 Haematological effects
It is still questionable whether the haematological effects noted
in ozone exposure are due to the direct action of ozone on the
cellular and acellular components of the blood as it passes through
the lung capillaries or whether they are caused by oxidizing
intermediates, such as ozonides or peroxides, that might penetrate the
alveolar basement membrane and enter the pulmonary circulation. A
third possibility would be that these effects are secondary reactions
induced by ozone perhaps causing the release of some mediating
substance, yet to be identified.
5.3.2.1 Short-term exposure (24 h or less)
Short-term exposure to high concentrations of ozone ranging from
6000 to 16 000 µg/m3 (3.0-8.0 ppm) has been reported to cause
increases in neutrophil-lymphocyte ratios in rats (Bobb & Fairchild,
1967), and in the number of erythrocytes and leukocyte indices in mice
(Kusumoto et al., 1976). Within this range of concentrations,
Goldstein et al. (1968) and Goldstein (1973) found a reduction in
acetylcholinesterase (3.1.1.7) in the erythrocytes of mice and
induction of hydrogen peroxide formation in the circulating
erythrocytes of rats and mice. Veninga (1970, unpublished data)a
reported doubling in the number of binucleated lymphocytes and
increased levels of serum glutamic pyruvic transaminase (2.6.1.2) but
no changes in blood catalase (1.11.1.6) in mice exposed for 2 h to an
ozone concentration of 400 µg/m3 (0.2 ppm).
Increased resistance to haemolysis of erythrocytes was reported in
mice exposed to an ozone concentration of 2000 µg/m3 (1 ppm) for
30 min (Mizoguchi et al., 1973). Menzel et al. (1975) presented
evidence that fatty acid ozonides produced Heinz bodies in
erythrocytes in mice exposed to ozone at 1700 µg/m3 (0.85 ppm) for
4 h. Further exposure for 3 days resulted in a decline in the number
of Heinz body positive cells. It is of interest to note that these
bodies were not produced with ozone in the absence of serum containing
unsaturated lipids. The authors postulated that this indicated an
oxidation of the erythrocyte membrane and suggested that fatty acid
ozonides might be the toxic intermediaries. At lower concentrations,
Brinkman et al. (1964) noted increased sphering of erythrocytes of
mice, rabbits, and rats, after an in vitro exposure to ozone at
400 µg/m3 (0.2 ppm) for 1-2 h.
a Veninga, T. S. Ozone-induced alterations in murine blood and liver.
Paper presented at the 2nd International Clean Air Congress, Washington,
DC, 6-11 December 1970 (No. MB-15E).
A 3-h exposure to irradiated automobile exhaust containing an
oxidant concentration of 740-1200 µg/m3 (0.37-0.58 ppm) was found to
increase the number of leukocytes in the blood of mice and reduce the
level of serum alkaline phosphatase (3.1.3.1) (Kusumoto et al., 1976).
5.3.2.2 Prolonged and repeated exposures
Increasing the length of exposure provided further evidence for
the oxidizing effect of ozone on the blood of rabbits and rats. After
continuous exposure for 8 days to ozone at 1600 µg/m3 (0.8 ppm), the
lysozyme activity in the plasma and soluble fraction of lung of rats
significantly increased (Chow et al., 1974). Continuous exposure of
rats to an ozone concentration of 110 µg/m3 (0.06 ppm) for 93 days
resulted in a decrease in blood cholinesterase (3.1.1.8) activity
which returned to normal 12 days after exposure ceased (Eglite, 1968).
More recently, Jegier & P'an (1973) and P'an & Jegier (1972) reported
a rise in serum trypsin protein esterase in rabbits exposed to
800 µg/m3 (0.4 ppm) for 6 h per day, 5 days per week, for 10 months.
Long-term, combined exposure to ozone and carbon monoxide produced
a reduced level of serum glutamic oxaloacetic transaminase (2.6.1.1)
in rabbits. The animals were exposed for 1000 days to urban air
containing oxidants and carbon monoxide at mean concentrations over 2
years of 110 µg/m3 (0.057 ppm) and 2000 µg/m3 (1.7 ppm)
respectively, (Emik et al., 1971).
5.3.3 Effects on reproduction
A few studies have been reported concerning the possible effects
of ozone and photochemical oxidants on reproduction. The data indicate
that the newborn animals may be more susceptible to exposure to
oxidants than the parents.
Brinkman et al. (1964) and Veninga (1967) exposed pregnant mice
for 7 h per day, 5 days per week, for 3 weeks to ozone concentrations
of 200-400 µg/m3 (0.1-0.2 ppm). They found a 4-fold increase in
neonatal mortality. The second author also observed increases in the
incidence of both incisor growth and blepharophimosis in the new born.
Similar studies using irradiated automobile exhaust were performed
by Hueter et al. (1966) who found that mice exposed for 13 months
before, during, and after gestation showed a marked decrease in the
number and frequency of litters, the survival of infants, and the
total number of pups born. Nonirradiated automobile exhaust did not
produce any significant effects. The oxidant concentrations in the
irradiated exposure chamber ranged from 400 to 2000 µg/m3 (0.2 to
1.0 ppm). In a follow-up study, Lewis et al. (1967) found that the
number of female mice that did not become pregnant after mating with
pretreated males was twice that found in mice mated with untreated
controls.
5.3.4 Behavioural and related changes
5.3.4.1 Short-term exposure (24 h or less)
Behavioural changes in response to acute ozone exposures have not
been studied very extensively. The major effect that has been noted is
a reduction in spontaneous activity. Murphy et al. (1964a) and
Konigsberg & Bachman (1970) found significant losses in motor activity
in mice exposed to 400 µg/m3 (0.2 ppm) for 6 h and in rats exposed to
1000 µg/m3 (0.5 ppm) for 45 min, respectively.
Using an evoked response technique, Xintaras et al. (1966)
measured a reduction in the amplitude of response to a flash in the
specific visual cortex and in the superior colliculus in rats exposed
to ozone at 1000-2000 µg/m3 (0.5-1.0 ppm) for 1 h. In studies on
rhesus monkeys, Reynolds & Chaffee (1970) reported increases in both
simple and choice reaction times after a 30-min exposure to an ozone
concentration of 1000 µg/m3 (0.5 ppm).
Gardner et al. (1974) showed that the pentobarbital-induced
sleeping time of mice significantly increased after 2 and 3 exposures
each of 3 h, to an ozone concentration of 2000 µg/m3 (1 ppm). After
the third exposure, there was no change in the sleeping time compared
with the controls unless in subsequent exposure the ozone
concentration was raised to 10 mg/m3 (5 ppm), when the sleeping time
again differed significantly. The authors suggested that ozone might
be deactivating a liver microsomal enzyme that was responsible for the
detoxification of the drug.
5.3.4.2 Prolonged and repeated exposures
Additional studies on the effects of ozone on the behaviour of
animals have been conducted with longer exposures and complex mixtures
of air pollutants. It appears that the reduction of voluntary running
time may be an extremely sensitive indicator of ozone toxicity. The
mechanism of this change remains obscure.
Continuous exposure for 1 week to an ozone concentration of
2000 µg/m3 (1.0 ppm) caused an 84% reduction in voluntary activity in
rats (Fletcher & Tappel, 1973). Decreased running activity has also
been observed in mice exposed continuously to urban air with elevated
oxidant levels and to irradiated motor vehicle exhaust (Emik et al.,
1971; Heuter et al., 1966).
According to Litt et al. (1968), ozone-exposed rats required a
longer time to learn a specific task; learning was unstable and they
exhibited a reduced ability for temporal discrimination. The animals
were exposed intermittently for 2 months to ozone concentrations of
600-1000 µg/m3 (0.3-0.5 ppm). Nevskaja & Kocetkova (1961) exposed
rats to a mixture of ozone at 800 µg/m3 (0.4 ppm) and sulfuric acid
at 7000 µg/m3 for 5 h per day, 6 days per week, for 100 days, and
found that a conditioned reflex was retarded.
5.3.5 Miscellaneous systemic reactions to lung damage
Other changes have been reported which indicate biochemical,
physiological, and structural effects at sites distant from the lung
that are possibly related to the inhalation of ozone. In most of these
studies, the levels of ozone employed greatly exceeded those in the
ambient air. After exposing rabbits to an ozone concentration of
1500 µg/m3 (0.75 ppm) for 4-8 h, Atwal & Wilson (1974) found
histological and ultrastructural changes in the parathyroid glands.
They reported hyperplasia of the chief cells, large numbers of
secretion granules, proliferation and hypertrophy of the rough
endoplasmic reticulum, Golgi complex, mitochondria, lipid bodies, and
free ribosomes. These effects were seen up to 66 h after exposure.
Atwal et al. (1975) expanded their short-term exposure study by
exposing rabbits to 1500 µg/m3 (0.75 ppm) for 48 h and found
morphological changes in the parathyroid gland similar to those that
they had reported for 4-8 h of exposure. The data suggested that ozone
might trigger off an immune reaction which caused inflammatory injury
to the parathyroid gland.
It was demonstrated by Brinkman et al. (1964) that exposure to an
ozone concentration of 400 µg/m3 (0.2 ppm), for 5 h per day, for 3
weeks, resulted in the rupture of nuclear envelopes and the extrusion
of the contents of myocardial muscle fibres in rabbits and mice. This
effect became reversible one month after the exposure.
There are some data which suggest that ozone might accelerate the
aging process. Stokinger (1965) reported premature aging in rabbits
after 1 year of weekly 1-h exposures to ozone. The major changes found
in the exposed animals were premature calcification of the
sternocostal cartilage, severe depletion of body fat, dull cornea,
sagging conjunctivae, and a general appearance of not thriving.
5.4 Mutagenicity
Chromosome aberrations (anaphase bridges) were found in 42% of
root meristem cells of Vicia faba exposed to an ozone concentration
of 8000 mg/m3 (4000 ppm) for 1 h, but none were found in cells
exposed to clean air (Fetner, 1958). When chick embryonic fibroblasts
were exposed to ozone concentrations of 5000-10 000 mg/m3
(2500-5000 ppm) for 24 h, a small number of cells were found with
chromosome bridges in anaphase and telophase and with nuclear
fragments (Sachsenmaier et al., 1965). Using a much lower
concentration, Pace et al. (1969) exposed strain L cells continuously
to an ozone concentration of 8000 µg/m3 (4 ppm) for 30 h, and found a
significant reduction in cell survival compared with that in the
control group. The authors considered that this was due to ozone
interference with mitotic activity.
In vivo exposure studies on mammals by Zelac et al. (1971a,
1971b) are of special interest. Female Chinese hamsters were exposed
to an ozone concentration of 400 µg/m3 (0.2 ppm) for 5 h and the
number of circulating blood lymphocytes with chromosomal breaks was
measured immediately after exposure and 6, and 15.5 days later. A
significant increase in chromosomal breaks compared with pre-exposure
values was still observed 15 days after exposure.
Although further confirmation is required, these data and the
results of studies on chromosomal changes in human tissues (see
section 6.1.1) seem to indicate that ozone might be a mutagenic agent.
5.5 Summary Table
Table 10 is a summary of experimental animal studies that provide
quantitative information useful for the establishment of guidelines
for the protection of public health with respect to exposure to ozone
at concentrations of up to 2000 µg/m3 (1.0 ppm).
Table 10. Experimental animal studies
Local effects on the respiratory system
1. Morphological changes
Ozone Length of exposure
concentration Number
of
µg/m3 (ppm) number h/day Effects Responsea Species animals Reference
of days
1800 (0.88) 180 24 Epithelial injury seen as early as 4 h n.a.b rat n.a. Freeman et al.
after the beginning of exposure; after (1974)
3 weeks half of animals died and
emphysema-like lesions observed.
1600 (0.8) 7 24 Walls and interalveolar septa of terminal -- rat 8 (8) Castleman et al.
airways thickened and infiltrated by (1973a)
mononuclear cells.
1200 (0.6) 1 7 Swelling of epithelial alveolar lining -- mouse 32 (13) Bile (1970)
cells & endothelium cells with
occasional breaks in basement
membrane.
1100 (0.54) 180 24 Progressive changes in the airway -- rat n.a. Freeman et al.
epithelium after 6 days. (1974)
1000 (0.5) 1 6 Immediately after the exposure the -- mouse 16(12) Evans et al.
number of alveolar cells significantly (1971)
decreased.
800 (0.4) 5 per 6 Emphysematous & vascular-type -- rabbit 6 (6) P'an et al.
week x lesions. (1972)
10 month
a Number of animals showing effects/total number of animals; numbers in brackets refer to control groups.
b Not available.
Table 10. Experimental animal studies
Local effects on the respiratory system
1. Morphological changes cont'd.
Ozone Length of exposure
concentration Number
of
µg/m3 (ppm) number h/day Effects Responsea Species animals Reference
of days
520- (0.26 1 4.7-6.6 Dose-related loss of ciliated epithelium. -- cat 14 (3) Boatman et al.
2000 -1.0) (1974)
400 (0.2) 1 2 Degenerative changes in type I cells. -- rat n.a. Stephens et al.
(1974)
2. Functional changes
1400 (0.68) 1 2 No significant increase in flow -- guinea- 10 (10) Murphy et al.
resistance. pig (1964a)
1000 (0.5) 1 2 Increase in airways resistance and -- guinea- 10 (10) Yokoyame
breathing frequency with decrease in pig (1972a)
tidal volume.
520- (0.26 1 4.6 Increased flow resistance. -- cat 10 (4) Watanabe et al.
1000 -0.5) (1973b)
400 (0.2) 30 24 Reduction in lung elasticity; increase in -- rat 44 (44) Bartlett et al.
lung volume and in alveolar (1975)
dimensions.
a Number of animals showing effects/total number of animals; numbers in brackets refer to control groups.
b Not available.
Table 10. Experimental animal studies
Local effects on the respiratory system
3. Biochemical changes
Ozone Length of exposure
concentration Number
of
µg/m3 (ppm) number h/day Effects Responsea Species animals Reference
of days
1500 (0.75) 1 3 Reduction in activity of benzopyrene -- hamster 25 (95) Palmer et al.
hydroxylase (1.14.14.2). & 8 (15) (1971. 1972)
rabbit
1400- (0.7 7 24 Increased acid phosphatase activity. -- rat 14 (12) Castleman et al.
1600 -0.8) (1973b)
1400 (0.7) 5 24 Indication of lipid peroxidation; -- rat 33 (20) Chow & Tappel
increase in lysosomal hydrolase (1972); Dillard
activity. et al. (1972)
1000 (0.5) 1 6 Increased albumen recovery from -- rat 10 (18) Alpert et al.
alveolar spaces. (1971a)
800- (0.4 1 4 Evidence of formation of lipid peroxides n.a.b mouse n.a. Goldstein et al.
1400 -0.7) in the lung. (1969)
500 (0.25) 1 3 Reduced activity of several lysosomal -- rabbit 6 (6) Hurst et al.
hydrolases. (1970)
400 (0.2) 7 24 Increase in pulmonary mitochondrial -- rat 5-8 Mustafa et al.
oxygen consumption. (5-8) (1973)
a Number of animals showing effects/total number of animals; numbers in brackets refer to control groups.
b Not available.
Table 10. Experimental animal studies
Local effects on the respiratory system
4. Effects on the host defence system
Ozone Length of exposure
concentration Number
of
µg/m3 (ppm) number h/day Effects Responsea Species animals Reference
of days
1200- (0.62 1 17 Inhibition of pulmonary bactericidal -- mouse 20 (29) Goldstein et al.
1600 -0.80) activity. (1971b)
1000 (0.5) 210 16 No effect on physical clearance of -- rabbit 8 (8) Friberg et al.
inhaled particles or on the number of (1972)
macrophages.
1000 (0.5) 60 16 Decrease in the clearance of viable -- guinea- 18 (18) Friberg et al.
Escherichia coli. pig (1972)
800 (0.4) 1 4 Inhibition of bactericidal activity; no -- mouse 38 (37) Goldstein et al.
additive role of the induced silicosis. (1972)
600 (0.3) 1 3 Impairment of phagocytic properties of -- rabbit n.a.b Coffin et al.
pulmonary alveolar macrophages. (1968b)
500 (0.25) 1 3 Diminished enzyme activities of alveolar -- rabbit 6 (6) Hurst et al.
(1970)
macrophages.
160 (0.08) 1 3 Increased susceptibility to 15/40 mouse 40 (40) Coffin et al.
Streptococcus. (6/40) (1968a)
a Number of animals showing effects/total number of animals; numbers in brackets refer to control groups.
b Not available.
Table 10. Experimental animal studies--continued
II. Systemic reactions and other effects
1. Haematological effects
Ozone Length of exposure
concentration Number
of
µg/m3 (ppm) number h/day Effects Responsea Species animals Reference
of days
1700 (0.85) 1 4 Formation of Heinz bodies in red cells. n.a.b mouse n.a. Menzel et al.
(1975)
1600 (0.8) 8 24 Increase of lysozyme activity in plasma -- rat 8 (8) Chow et al.
and soluble fraction of lung. (1974)
800 (0.4) 5 per 6 increase in serum trypsin protein -- rabbit 6 (6) P'an & Jegier
week x esterase. (1972); Jegier
10 months & P'an (1973)
600 (0.3) 1 1 Inhibition of acetylcholine esterase -- ox (in -- P'an & Jegier
(3.1.1.7) activity. vitro) (1970)
400 (0.2) 1 1-2 Increased sphering of red blood cells. -- mouse, -- Brinkman et al.
rabbit, (1964)
rat (in
vitro)
400 (0.2) 1 2 Doubling in number of binucleated -- mouse n.a. Veninga (1970,
lymphocytes. unpublished)
110 (0.06) 93 24 Decrease in blood choline esterase -- rat 15 (15) Eglite (1968)
activity.
a Number of animals showing effects/total number of animals; numbers in brackets refer to control groups.
b Not available.
Table 10. Experimental animal studies--continued
II. Systemic reactions and other effects
2. Effects on reproduction
Ozone Length of exposure
concentration Number
of
µg/m3 (ppm) number h/day Effects Responsea Species animals Reference
of days
400 (0.2) 5 per 7 Increase in neonatal mortality. n.a. mouse n.a. Veninga (1967)
week x
gestation
period +
1st 3
weeks of
life
200- (0.1 5 per 7 Increase in neonatal mortality. n.a. mouse n.a. Brinkman et al.
400 -0.2) week x (1964)
3 weeks
1000- (0.5 1 1 Decrease in the amplitude of evoked -- rat 3 (3) Xintaras et al.
2000 -1.0) response to flash. (1966)
a Number of animals showing effects/total number of animals; numbers in brackets refer to control groups.
b Not available.
Table 10. Experimental animal studies--continued
II. Systemic reactions and other effects
3. Behavioural changes
Ozone Length of exposure
concentration Number
of
µg/m3 (ppm) number h/day Effects Responsea Species animals Reference
of days
1000 (0.5) 1 0.5 increase in simple and choice reaction -- rhesus 4 (4) Reynolds &
time. monkey Chaffee (1970)
1000 (0.5) 1 0.75 Significantly reduced motor activity. -- rat 12 (12) Konigsberg &
Bachman
(1970)
600- (0.3 60 intermittently Increase in time to learn specific tasks. -- rat 6 (6) Litt et al.
1000 -0.5) (variable (1966)
intervals)
400 (0.2) 1 6 Reduction in spontaneous running -- mouse 9 (9) Murphy et al.
activity. (1964a)
4. Miscellaneous extrapulmonary changes
1500 (0.75) 1-2 4-8, 24 Histological changes in parathyroid -- rabbit 16 (16) Atwal & Wilson
gland. (1974); Atwal
et al. (1975)
400 (0.2) 21 5 Structural changes in myocardial -- rabbit n.a. Brinkman et al.
muscle fibres. & mouse (1964)
400 (0.2) 1 5 Increase in chromosomal breaks in -- hamster 8 (4) Zelac et al.
circulating lymphocytes. (1971a,b)
a Number of animals showing effects/total number of animals; numbers in brackets refer to control groups.
b Not available.
6. EFFECTS ON MAN
6.1 Controlled Exposures
A considerable number of studies have been performed, under
controlled conditions, on the effects of ozone on both healthy
subjects and patients, with their consent. Some of these studies have
provided useful information for the evaluation of exposure-effect
relationships. A few in vitro studies using human tissues have also
helped to clarify the mechanisms of the biological actions of ozone.
There have been few human studies on other oxidants.
6.1.1 In vitro effects on human tissues
Brinkman et al. (1964) found that in vitro exposure of human red
blood cells to an ozone concentration of 0.5 mg/m3 (0.25 ppm) for
30 min accelerated sphering of these cells by X-ray irradiation,
compared with unexposed cells. Chromosome breakages in human cell
(epidermoid carcinoma cell) cultures exposed to an ozone concentration
of 16 mg/m3 (8 ppm) for 5 or 10 min were equivalent to those produced
by X-rays (200R, 250 kV) (Fetner, 1962).
The production of interferon was suppressed when human tonsil
lymphocytes were exposed in vitro to an ozone concentration of
10 mg/m3 (5 ppm) for 3 h (Watanabe et al., 1973a).
Goldstein (1976) measured the combined effects of ozone at
4-82 mg/m3 (2-41 ppm) and nitrogen dioxide at 6.8-190 mg/m3
(3.6-102 ppm) on human red cells, in vitro, and found that the
absolute and relative concentrations of the pollutants as well as the
sequence of administration could affect the interaction. In general,
the effects of the two pollutants on the variables measured (osmotic
fragility, acetylcholin-esterase (3.1.1.7) activity, lipid
peroxidation, reduced glutathione, and methaemoglobin levels) were
additive. At lower pollutant doses, a synergistic effect on the
increase in lipid peroxides was reported. Ozone also potentiated the
formation of methaemoglobin due to the action of nitrogen dioxide.
6.1.2 Sensory effects
The effects of ozone or oxidants on sensory organs have been
studied in terms of eye irritation, changes in visual parameters, and
olfactory thresholds.
Eye irritation in two groups of 20 female telephone company
employees working in identical adjacent rooms was evaluated by
Richardson & Middleton (1957, 1958) in relation to oxidant
concentrations from May to November 1956. Activated-carbon and dummy
air-filter media were switched periodically between the two rooms so
that the groups were alternately exposed to test and control
conditions. In all cases, differences in eye irritation between the
activated-carbon filtered and nonfiltered test conditions were highly
significant (p < 0.01). The scatter diagram (Fig. 8) suggests the
existence of a threshold for eye irritation at an oxidant
concentration of approximately 200 µg/m3 (0.10 ppm).
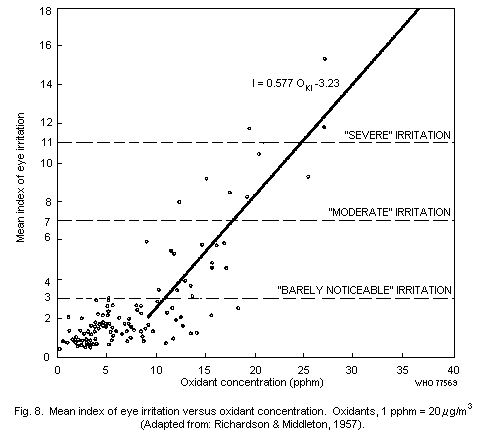 The index of eye irritation increased progressively as oxidant
concentrations exceeded this value. No significant correlations
between eye irritation and concentrations of nitrogen dioxide or
suspended particulates were observed. However, in interpreting these
results, care must be exercised in drawing conclusions regarding
cause-effect relationships, as other controlled exposure studies have
shown that ozone is not an eye irritant. By comparison,
peroxyacylnitrates, acrolein, and peroxybenzoyl-nitrates have all been
shown to be strong eye irritants (Heuss & Glasson, 1968; Schuck &
Doyle, 1959; Stephens et al., 1961). Each of these compounds is a
product of the photochemical reaction system and thus is highly
correlated in time with measured levels of oxidants or ozone.
Lagerwerff (1963) measured the effect of exposure to ozone on
visual parameters in 22 male and 6 female volunteers. The subjects
were exposed to ozone at 400, 700, and 1000 µg/m3 (0.20, 0.35, and
0.50 ppm) for 3 h, and again for 6 h, with 10 days rest between
exposures. Visual acuity, depth perception, lateral and vertical
phoria, divergence and convergence, near vision, and peripheral,
colour, and night vision were tested. Considerable decreases in visual
acuity in scotopic and mesopic ranges, increases in peripheral vision,
changes in extra ocular muscle balance, and decrease in night vision
were observed in the majority of subjects. A few subjects also
developed a nonproductive cough at the highest ozone concentration,
and several complained of difficulties with mental concentration at
these levels.
Studies of the olfactory threshold in 10-14 male volunteer test
subjects exposed for 30 min to a series of different ozone
concentrations were performed by Henschler et al. (1960). The lowest
ozone concentration used, 40 µg/m3 (0.02 ppm), was recognized
immediately by 9 out of 10 subjects. The subjects reported that the
odour diminished rapidly, and that it was no longer perceptible within
´-12 min. When exposed to an ozone concentration of 100 µg/m3
(0.05 ppm), 13 out of 14 subjects indicated that the odour was
considerably stronger and that it lasted longer (2-30 min, with an
average duration of 13 min). In a study by Eglite (1968), it was found
that the threshold of smell for ozone was 15 µg/m3 (0.008 ppm) for
the most sensitive subject in a group of 20 persons.
6.1.3 Effects on respiratory function
6.1.3.1 Exposure to ozone
A considerable number of controlled studies on exposure to ozone
have been reported. However, with one exception, all these studies
were concerned with short-term exposures of less than 6 h.
A highly significant fall in steady state diffusion capacity and
0.75-second forced expiratory volume (FEVo.75) was noted in each of 16
test replicates on 10 men and 1 woman aged 20-45 years, exposed to
ozone concentrations of 1200-1600 µg/m3 (0.6 to 0.8 ppm) for 2 h
(Young et al., 1964).
Goldsmith & Nadel (1969) exposed 4 healthy males, for 1 h, to
ozone concentrations of 200, 800, 1200, and 2000 µg/m3 (0.1, 0.4,
0.6, and 1.0 ppm). Consistent increases in airway resistance (Raw)
were demonstrated only with exposure to 2000 µg/m3 (1 ppm). Lower
concentrations caused an increase in Raw in some subjects, but a
clear dose-effect relationship could not be established at levels
below 2000 µg/m3 (1 ppm). When exposed to an ozone concentration of
1500 µg/m3 (0.75 ppm) for 2 h, 10 healthy males exhibited significant
increases in Raw, reductions in maximum static elastic recoil
pressure of the lung, and a fall in maximum flow at 50% of vital
capacity (Bates et al., 1972). Exercise on a bicycle ergometer during
ozone exposure accentuated these pulmonary function changes, and most
subjects complained of substernal soreness and cough at the end of a
2-h period. These observations were extended by Hazucha et al. (1973)
to include an ozone concentration of 740 µg/m3 (0.37 ppm) for 2 h and
subjects were intermittently exercised during exposure. After exposure
to 740 and 1500 µg/m3 (0.37 and 0.75 ppm) for 2 h, both smokers and
non-smokers (6 subjects per group) revealed significant decreases in
forced vital capacity (FVC), one-second forced expiratory volume
(FEV1.0), mid-maximal expiratory flow rate (MMFR), and maximum
expiratory flow rate at 50% of vital capacity (MEFR 50%), but the
effects were greater at 1500 µg/m3 (0.75 ppm). These effects were
closely related to the changes measured in the closing volume and
indicated an early effect in the small airways.
Seven healthy males, exposed to an ozone concentration of
1000 µg/m3 (0.50 ppm) for 2 h while performing intermittent light
exercise, showed decreases in lung function measurements and oxidative
changes in erythrocytes; 3 subjects had symptoms such as cough,
substernal discomfort, and malaise. When 2 healthy and 3 sensitive
subjects (those with a prestudy history of cough, chest discomfort, or
wheezing associated with allergy or air pollution exposure, but with
normal base line pulmonary function studies) were exposed to an ozone
concentration of 740 µg/m3 (0.37 ppm) for 2 h under the same
conditions of exercise, there was a significant increase in the total
respiratory resistance compared with the pre-exposure resistance.
Oxidative biochemical changes were detected in the blood of this
group, but were not as severe as in those exposed to an ozone
concentration of 1000 µg/m3 (0.5 ppm). Three healthy and 3 sensitive
subjects exposed to 500 µg/m3 (0.25 ppm) for 2 h did not show any
consistent physiological changes attributable to exposure (Hackney et
al., 1975). All the subjects of the above study were southern
Californians. In order to study possible adaptation of these subjects
to long-term ambient ozone exposure, the same investigators compared
the effects of ozone exposure on southern Californians with those on
Canadians and found that the latter were more responsive (Hackney et
al., 1977).
Ohmori (1974) found increased breathing frequency and volume in 4
healthy males who were exposed under exercise to ozone concentrations
of 200-500 µg/m3 (0.1-0.25 ppm) for 30 min. Four normal male subjects
exposed for 5 min to a combination of ozone at 1800 µg/m3 (0.9 ppm)
and light exercise showed a highly significant decrease in specific
airway conductance (Kagawa & Toyama, 1975a). Folinsbee and co-workers
(1975) tested the reaction of 28 normal adults (20 males, 8 females)
exposed to ozone at concentrations of 740, 1000, or 1500 µg/m3 (0.37,
0.50, or 0.75 ppm) for 2h: the subjects were either at rest or
exercising intermittently with sufficient intensity to increase lung
ventilation by a factor of 2.5. There was a gradation of complaints
depending on the ozone concentration. Under exercise, increase in
respiratory frequency was closely correlated with the total dose of
ozone and, at any given concentration, the effect was greater with the
subject who had exercised. Statistically significant decreases were
found in FVC at ozone concentrations of 1000 µg/m3 (0.50 ppm) or
more. Decrease in MEFR occurred in 50% of subjects at concentrations
of 740 µg/m3 (0.37 ppm) or more.
The effects of ozone on pulmonary function were studied in 22
young, healthy, male, nonsmokers who were exposed to 800 µg/m3
(0.4 ppm) for 2 and 4 h. Subjects were seated during the exposure
except for 2 exercise periods of 15 min (bicycle), beginning after 1
and 3 h of exposure, respectively. Significant changes in FVC, MMFR
and Raw occurred after 2 h of exposure. Borderline effects were
reported for FEV1.0, V50 (expiratory flow rate at 50% FVC) and V25
(expiratory flow at 25% FVC). After 4 h of exposure, changes in all of
these lung function variables became statistically significant (Rummo
et al., 1975, unpublished data).a In studies by Kerr et al. (1975),
20 lightly exercising subjects (19 males and 1 female) were exposed to
an ozone concentration of 1000 µg/m3 (0.5 ppm) for 6 h. Subjects,
particularly nonsmokers, commonly reported dry cough and chest
discomfort. Significant changes were produced in airway conductance,
pulmonary resistance, and forced expiratory volumes, but not in
diffusing capacity.
a Rummo, N.J., Knelson, I. H., Lassiter, S., Cram, J. J., & House,
D. (1975). Effects of ozone on pulmonary function in healthy young
men. Research Triangle Park, NC, US Environmental Protection Agency,
17 pp. (In-house Technical Report).
Changes in the pulmonary function of 11 male subjects, aged 24-38
years, exposed to an ozone concentration of 200 µg/m3 (0.1 ppm) for
2 h, were compared with a 1-h pre- and post-control period without
ozone and with a control series without ozone. Under test conditions,
which included intermittent light exercise, a significant increase in
Raw and in the alveolar to arterial oxygen pressure difference
(AaDO2) was observed in 7 out of 11 subjects (yon Nieding et al.,
1977).
In an attempt to study the effects of long-term repeated exposures
to ozone, Bennet (1962) exposed 2 groups of 6 healthy males to
400 µg/m3 (0.2 ppm) or 1000 µg/m3 (0.5 ppm) respectively, for 3 h
per day, 6 days per week for 12 weeks. The 400 µg/m3 (0.2 ppm)
exposure group did not experience any symptoms or changes in the
forced expiratory volume. While the second group was also
asymptomatic, the FEV1.0 showed a significant decrease during the last
few weeks of exposure.
6.1.3.2 Exposure to mixtures of ozone and other air pollutants
Bates & Hazucha (1973) demonstrated a synergistic action of ozone
at 740 µg/m3 (0.37) and sulfur dioxide at 960 µg/m3 (0.37 ppm). With
4 normal subjects engaged intermittently in light exercise, a 2-h
exposure to sulfur dioxide, only, at 960 µg/m3 (0.37 ppm) did not
produce any changes in FVC, FEV1.0, MMFR, and MEFR 50%, while exposure
to an ozone concentration of 740 µg/m3 (0.37 ppm) caused a 10-15%
reduction in these lung function variables. However, exposure to a
combination of these gases at the same concentrations resulted in a
20-45% decline in lung function measurements and this effect was even
greater than the changes caused by a 2-h exposure to an ozone
concentration of 1500 µg/m3 (0.75 ppm). On the other hand, addition
of nitrogen dioxide at a concentration of 560 µg/m3 (0.30 ppm) to
ozone at 500 µg/m3 (0.25 ppm) did not result in any decrement in the
lung functions of 3 healthy and 3 sensitive (section 6.1.3.1) subjects
(Hackney et al., 1975a).
In a series of studies already mentioned in section 6.1.3.1, von
Nieding et al. (1977) found that the effects of exposure to an ozone
concentration of 200 µg/m3 (0.1 ppm) were not enhanced by combination
with nitrogen dioxide at 9400 µg/m3 (5 ppm), or by combination with
nitrogen dioxide at 9400 µg/m3 (5 ppm) and sulfur dioxide at
13 000 µg/m3 (5 ppm), though recovery time was delayed in the last
experiment. Exposure for 2 h to a combination of ozone at 50 µg/m3
(0.025 ppm), nitrogen dioxide at 100 µg/m3 (0.05 ppm), and sulfur
dioxide at 260 µg/m3 (0.1 ppm) did not have any effect on Raw, or on
AaDO2. However, there was a dose-dependent increase in the
sensitivity to acetylcholine of the bronchial tree compared with the
controls. This effect was observed by measuring the increase in Raw
after inhalation of 2% acetylcholine alone and in combination with the
previously mentioned mixture of gases at the same concentrations. The
effect became more pronounced when the acetylcholine was combined with
ozone at 200 µg/m3 (0.1 ppm), nitrogen dioxide at 9400 µg/m3
(5 ppm), and sulfur dioxide at 13 000 µg/m3 (5 ppm).
6.1.3.3 Exposure to peroxyacetylnitrate alone or in combination with
carbon monoxide
Thirty-two male college students were exposed to a peroxyacetyl-
nitrate concentration of 1500 µg/m3 (0.3 ppm) for 5 min while
exercising on a bicycle ergometer (Smith, 1965). A statistically
significant increase in oxygen uptake was observed during exercise, in
comparison with that observed when filtered air was breathed. MEFR was
significantly decreased by exposure to this gas during the recovery
phase following exercise. However, peroxyacetylnitrate did not have
any effect when the subjects were at rest.
The metabolic, temperature, and cardiorespiratory reactions of 20
healthy males (10 smokers and 10 nonsmokers) were monitored while
working to their maximum and breathing filtered air or 3 different gas
mixtures at 25 ± 0.5°C and a relative humidity of 20 ± 2% (Raven et
al., 1974). The mixtures were carbon monoxide in filtered air at
57 500 µg/m3 (50 ppm), peroxyacetylnitrate in filtered air at
1350 µg/m3 (0.27 ppm), and a combination of the two. Maximum aerobic
capacity did not decrease significantly in either group during
exercise and exposure to any of the pollutant gas mixtures compared
with filtered air. For both smokers and nonsmokers exposure to
peroxyacetylnitrate and carbon monoxide alone or in combination, while
exercising to maximum aerobic capacity, produced minor alterations in
cardiorespiratory and temperature regulating variables.
6.1.3.4 Exposure to irradiated automobile exhaust
Fourteen college students were exposed on 2 occasions to
irradiated automobile exhaust and measurements were made of reaction
time, vital capacity, and submaximum work performance on the bicycle
ergometer. The individuals were exposed to a mixture of the following
gases: carbon monoxide, carbon dioxide, nitric oxide, nitrogen
dioxide, hydrocarbons, aldehydes, formaldehyde, and oxidants (Table
12, section IID). It appeared that exposure to this mixture of
pollutants had little effect on the types of human motor performance
chosen for this study (Holland et al., 1968).
6.1.3.5 Exposure to ambient air containing elevated concentrations of
oxidants
The effect of oxidant air pollution on patients with chronic
obstructive lung disease was studied by Motley et al. (1959). Twenty
normal individuals and 46 patients with chronic lung disease were
evaluated by measuring lung volumes, forced expiratory volumes, and
nitrogen washout before and after removal from ambient Los Angeles air
into a room with clean filtered air. No significant changes were
detected in the pulmonary function of normal subjects or in patients
with chronic lung disease who entered the room on nonsmoggy days.
However an improvement in lung function, particularly a decrease in
residual lung volume and nitrogen washout time, occurred among
patients who entered the filtered room on smoggy days and remained for
40 or more hours. Simultaneous measurements of oxidants were not
obtained at the study site, but during the study, ambient hourly
oxidant concentrations at nearby monitoring stations ranged from
400-1400 µg/m3 (0.2-0.7 ppm). Subjects with chronic lung disease, who
remained in the filtered air for only 18-20 h, did not experience any
significant improvement in lung function.
In a similar study, Balchum (1973) observed the pulmonary function
of 15 patients with moderately severe chronic obstructive lung
disease, who spent one week in a room without air filtration and a
second week in clean filtered air. During the first week, when
unfiltered ambient air was drawn into the room, 1-h oxidant
concentrations averaged 220 µg/m3 (0.11 ppm) and ranged up to
400 µg/m3 (0.2 ppm). During the second week, when air filters were
activated, oxidant exposures ranged from 40 to 60 µg/m3 (0.02 to
0.03 ppm). Comparison of the effects of unfiltered and filtered air
revealed a decrease in airway resistance and an increase in the
arterial pO2 during the week of filtered air breathing. Changes were
observed both at rest and during exercise in about 75% of the
subjects. Decreases in airway resistance first became apparent after
breathing filtered air for 48 h.
6.1.4 Changes in electroencephalograms
Changes (decrease in the alpha rhythms) in electroencephalograms
were studied by Eglite (1968) in relation to exposure to ozone for
3 min. All 3 healthy subjects studied showed changes when exposed to
an ozone concentration of 20 µg/m3 (0.01 ppm) and 1 subject reacted
at a concentration at 10 µg/m3 (0.005 ppm).
6.1.5 Chromosomal effects
In a recent study by Metz et al (1975), 6 human volunteers were
exposed to an ozone concentration of 1 mg/m3 (0.5 ppm) for 6-10 h,
and the circulating lymphocytes were examined for chromosomal changes.
Although no chromosome aberrations were found, there was a significant
increase in the number of minor chromosomal abnormalities (chromatid
deletions) compared with pre-exposed lymphocytes.
6.2 Industrial Exposure
Several studies on the effects of industrial exposure have been
reported, but in most of them, the effects of ozone have been
confounded by the coexistence of other pollutants and a threshold
concentration has not been determined.
Kleinfield et al. (1957) reported several cases of severe ozone
intoxication in welders using an inert gas-shielded consumable
electrode which greatly increased the ultraviolet radiation. Ozone was
measured at the breathing zone in 3 plants using this welding
technique. No complaints or clinical findings were associated with
ozone concentrations of 500 µg/m3 (0.25 ppm) or less. At
concentrations of 600-1600 µg/m3 (0.3-0.8 ppm), an increasing number
of welders complained of chest constriction and irritation of the
throat.
Similar findings using this welding technique were reported by
Challen et al. (1958), when 11 out of 14 workers complained of
respiratory symptoms at ozone concentrations in the range of
1600-3400 µg/m3 (0.8-1.7 ppm). Symptoms disappeared when ozone levels
were reduced to 400 µg/m3 (0.2 ppm). Young et al. (1963) failed to
detect any significant changes in lung function tests (vital capacity,
functional residual capacity, MMFR, FEV0.75, or diffusing capacity) in
7 men engaged in argon-shielded electric arc welding when ozone
concentrations were 400-600 µg/m3 (0.2-0.3 ppm).
Kudrjavceva (1963) described a complex of symptoms that included
headache, weakness, increased muscular excitability, and decreased
memory among workers engaged in the manufacture of hydrogen peroxide
and exposed to ozone concentrations ranging from 500-800 µg/m3
(0.25-0.40 ppm) for 7-10 years. The investigator suggested that these
reactions might be due to prolonged exposure to ozone under working
conditions. Increased prevalence of bronchitis and emphysema,
accompanied by decreased expiratory flow rate, was also reported in
workers involved in the manufacture of hydrogen peroxide by Nevskaja &
Diterihs (1957). Ozone concentrations of 80-1000 µg/m3
(0.04-0.50 ppm) were measured; sulfuric acid aerosol was also present.
Control groups were not included in either of these studies.
In several countries, occupational exposure limits for ozone have
been set as maximum, time-weighted averages for an 8-h workday or 40-h
week (ILO, 1977). In some countries, the exposure limit is 100 µg/m3
(0.05 ppm), in others, 200 µg/m3 (0.1 ppm). In addition, a short-term
exposure limit of 600 µg/m3 (0.3 ppm), for periods up to 15 min, has
been tentatively proposed by the USA provided that there are not more
than 4 such periods per day with at least 60 min between each period,
and that the daily time-weighted average is not exceeded. The German
Democratic Republic has set a limit of 200 µg/m3 for exposure periods
not exceeding 30 min and the USSR has proposed an 8-h mean value of
100 µg/m3 (0.05 ppm).
6.3 Community Exposure
Epidemiological studies on the association between human health
effects and exposure to photochemical oxidants have largely been
carried out in the Los Angeles air basin. For most of these studies,
investigators made use of measurements of photochemical oxidants
obtained from a network of air monitoring stations operated by the Los
Angeles County Air Pollution Control District. Oxidants were measured
by the unbuffered potassium iodide method which yields oxidant values
15-25% lower than the values obtained with the 1 or 2% neutral-
buffered potassium iodide method more commonly used in regions outside
the Los Angeles air basin.
As in all studies on urban populations, the observed health
effects of photochemical oxidant exposure cannot be attributed only to
oxidants. Photochemical smog typically consists of ozone, nitrogen
dioxide, peroxyacylnitrates and other nitrate compounds, sulfates,
other particulate aerosols, and reducing agents. In combination, these
pollutants may have an independent, additive, or synergistic effect on
human health. In general, however, ozone appears to be the most
biologically active pollutant and the correlations between health
effects and pollutant exposure were found when results concerning
ozone, rather than other identified pollutants, were statistically
analysed. Since controlled exposure studies in man and animals confirm
the greater biological reactivity of ozone compared with other
components of photochemical smog, it seems reasonable to conclude, for
the purpose of developing health protection guidelines, that ozone is
the principal agent responsible for the exposure-response
relationships observed in epidemiological studies on photochemical
oxidants.
6.3.1 Mortality
Studies conducted by the California State Department of Public
Health (1955, 1956) over the periods July-November 1953 and 1954, and
July-December 1955 revealed that, during these months, the daily
mortality rate of Los Angeles residents, aged 65 or over, was strongly
influenced by a heat wave but was not consistently altered either by
variations in oxidant concentrations or by the occurrence of smog-
alert days (ozone concentrations of 600 µg/m3 (0.3 ppm) or more).
No significant correlations were found by Massey et al. (1961,
unpublished data) between daily mortality rates and daily oxidant
levels in an analysis of two areas of Los Angeles County, selected for
similarities in temperature and for differences in air pollution
levels (values not given). Hechter & Goldsmith (1961) also failed to
find a significant correlation between monthly mortality rates due to
cardiorespiratory diseases and pollutant levels (monthly means of
daily maxima that ranged from 80 to 400 µg/m3; 0.04-0.2 ppm) in Los
Angeles County for the years 1956-58. The authors made Fourier curve
analyses of the data in order to remove the major effect of season of
year on mortality rate and pollutant levels. An association between
respiratory and cardiac deaths and Los Angeles smog episodes with 1-h
concentrations exceeding 400 µg/m3 (0.2 ppm) was observed by Mills
(1957) but he failed to take into account seasonal fluctuations in
deaths and pollutants. However, it is possible that the method of
adjustment for seasonal effects might mask a real effect of pollutant
concentrations on mortality. Therefore no conclusive statement can be
made concerning the lack of an association between mortality rate and
short-term variations in oxidant levels.
A model of daytime wind flow over Los Angeles was constructed by
Mahoney (1971) to divide the city into 5 wind zones each about
10 kilometres in width and representing distance downwind along the
path of air flow. The mortality rate of the white population, adjusted
for age, sex, and income level, from noncancerous respiratory diseases
during 1961 increased in successive downwind zones affected by the Los
Angeles sea breeze. The adjusted mortality rate in the wind zone
immediately adjacent to the Pacific Ocean was 53 per 100 000, while
mortality in the afferent wind zone most remote from the ocean was
111 per 100 000. The author suggested that this geographical
difference in mortality might be consistent with an effect of
temperature, humidity, or oxidant air pollution.
6.3.2 Annoyance and irritation
In Los Angeles, 75% of the population complained that they were
"bothered" by air pollution either at home or at work compared with
24% in San Francisco and 22% in the "rest of the State". Eye
complaints, which were relatively high (33%) in Los Angeles County and
in the "rest of the State", were low (14%) in the San Francisco Bay
area. However, fewer cases of hayfever and sinus trouble were reported
in Los Angeles County compared with other areas (Hausknecht, 1960).
The association of oxidant levels with eye irritation was
investigated (Renzetti & Gobran, 1957) in a panel of office and
factory workers and a second panel of scientists residing in the Los
Angeles Basin. The data demonstrated increasing eye irritation with
increasing maximum instantaneous oxidant concentrations over a range
of values from 100 to 900 µg/m3 (0.05-0.45 ppm).
Daily symptoms associated with eye and respiratory irritation were
studied by Hammer et al. (1974) in a group of Los Angeles student
nurses in relation to daily oxidant levels. Headache, eye discomfort,
cough, and chest discomfort were all found to be related to daily
maximum hourly oxidant concentrations. On days when maximum hourly
concentrations were 800-1000 µg/m3 (0.40-0.50 ppm), students reported
48% more cough and 100% more chest discomfort compared with days when
oxidant levels were below the US air quality standard of 160 µg/m3
(0.80 ppm). Using "hockey stick" functions,a the threshold levels
have been determined as maximum 1-h concentrations of 100 µg/m3
(0.05 ppm) for headache, 300 µg/m3 (0.15 ppm) for eye irritation,
530 µg/m3 (0.27 ppm) for cough, and 580 µg/m3 (0.29 ppm) for chest
discomfort. Exposure-response relationships are shown in Table 11.
6.3.3 Athletic performance
The effect of oxidant concentrations on athletic performance was
studied in Los Angeles by Wayne et al. (1967) in 21 competitive team
events for High school cross-country runners from 1959-64. Since
running times tended to improve throughout the season, team
performance at a meeting was evaluated by determining the percentage
of individual athletes who failed to improve when their running time
was compared with that of their previous performance on the same
course. Oxidant levels in the hour before the meeting, which ranged
from below 100 µg/m3 (0.05 ppm) to 600 µg/m3 (0.3 ppm), were highly
correlated ( r=0.88) with decreased performance, as shown in Fig. 9.
Consistently high correlations were obtained when the observations
were divided into the periods 1959-1961 ( r=0.945) and 1962-64
( r=0.945). Correlations with other pollutants (carbon monoxide and
particulates) and with meteorological variables (temperature, relative
humidity, wind velocity, wind direction) were considerably lower and
usually not significant. The authors speculated that the observed
oxidant-athletic performance relationship could be due to a direct
effect on oxygen use or to respiratory discomfort associated with
exercise in an atmosphere containing a high concentration of oxidants.
A statistical test for threshold values, based on segmental regression
analysis ("hockey stick" functions) applied to these data (Barth et
al., 1971; Hasselblad et al., 1976) gave a threshold estimate of
240 µg/m3 (0.12 ppm) (1-h value) with a 95% confidence interval of
134 to 326 µg/m3 (0.067-0.163 ppm).
a The term hockey stick function has been used by Hammer et al.
(1974) and Hasselblad et al. (1976) to describe a function
consisting of a curve with zero slope up to a certain point and
increasing monotonically from that point.
Table 11. Relative increase in headache, eye discomfort, cough, and chest discomfort in relation to photochemical oxidant
exposure of student nurses in Los Angelesa
Relative increase in symptom
Daily maximum 1-h oxidant Headache Eye discomfort Cough Chest discomfort
level
µg/m3 (ppm) Simple Adjustedb Simple Adjustedb Simple Adjustedb Simple Adjustedb
<160 (<0.08) 1.00 (16.4)c 1.00 (10.6) 1.00 (8.9) 1.00 (5.2) 1.00 (13.0) 1.00 (9.5) 1.00 (3.5) 1.00 (1.8)
180 (0.09) 0.97 1.00 1.01 1.07 1.02 1.07 0.91 1.05
200-280 (0.10-0.14) 0.95 1.03 0.96 1.13 0.91 0.99 0.97 1.00
300-380 (0.15-0.19) 0.95 1.07 1.12 1.33 0.95 1.02 1.05 0.94
400-480 (0.20-0.24) 0.95 1.08 1.37 1.77 0.89 0.96 0.89 0.89
500-580 (0.25-0.29) 1.01 1.07 1.67 2.15 0.95 1.01 1.03 1.11
600-780 (0.30-0.39) 1.03 1.26 2.37 3.42 1.16 1.23 1.17 1.27
800-1000 (0.40-0.50) 1.02 1.41 3.93 6.11 1.48 1.77 2.00 3.22
a From: Hammer et al. (1974).
b Excludes all days when the symptom was reported in conjunction with either "feverish", "chilly", or "temperature".
c Bracketed figure gives baseline symptom rate as mean daily percentage of symptom reported.
The index of eye irritation increased progressively as oxidant
concentrations exceeded this value. No significant correlations
between eye irritation and concentrations of nitrogen dioxide or
suspended particulates were observed. However, in interpreting these
results, care must be exercised in drawing conclusions regarding
cause-effect relationships, as other controlled exposure studies have
shown that ozone is not an eye irritant. By comparison,
peroxyacylnitrates, acrolein, and peroxybenzoyl-nitrates have all been
shown to be strong eye irritants (Heuss & Glasson, 1968; Schuck &
Doyle, 1959; Stephens et al., 1961). Each of these compounds is a
product of the photochemical reaction system and thus is highly
correlated in time with measured levels of oxidants or ozone.
Lagerwerff (1963) measured the effect of exposure to ozone on
visual parameters in 22 male and 6 female volunteers. The subjects
were exposed to ozone at 400, 700, and 1000 µg/m3 (0.20, 0.35, and
0.50 ppm) for 3 h, and again for 6 h, with 10 days rest between
exposures. Visual acuity, depth perception, lateral and vertical
phoria, divergence and convergence, near vision, and peripheral,
colour, and night vision were tested. Considerable decreases in visual
acuity in scotopic and mesopic ranges, increases in peripheral vision,
changes in extra ocular muscle balance, and decrease in night vision
were observed in the majority of subjects. A few subjects also
developed a nonproductive cough at the highest ozone concentration,
and several complained of difficulties with mental concentration at
these levels.
Studies of the olfactory threshold in 10-14 male volunteer test
subjects exposed for 30 min to a series of different ozone
concentrations were performed by Henschler et al. (1960). The lowest
ozone concentration used, 40 µg/m3 (0.02 ppm), was recognized
immediately by 9 out of 10 subjects. The subjects reported that the
odour diminished rapidly, and that it was no longer perceptible within
´-12 min. When exposed to an ozone concentration of 100 µg/m3
(0.05 ppm), 13 out of 14 subjects indicated that the odour was
considerably stronger and that it lasted longer (2-30 min, with an
average duration of 13 min). In a study by Eglite (1968), it was found
that the threshold of smell for ozone was 15 µg/m3 (0.008 ppm) for
the most sensitive subject in a group of 20 persons.
6.1.3 Effects on respiratory function
6.1.3.1 Exposure to ozone
A considerable number of controlled studies on exposure to ozone
have been reported. However, with one exception, all these studies
were concerned with short-term exposures of less than 6 h.
A highly significant fall in steady state diffusion capacity and
0.75-second forced expiratory volume (FEVo.75) was noted in each of 16
test replicates on 10 men and 1 woman aged 20-45 years, exposed to
ozone concentrations of 1200-1600 µg/m3 (0.6 to 0.8 ppm) for 2 h
(Young et al., 1964).
Goldsmith & Nadel (1969) exposed 4 healthy males, for 1 h, to
ozone concentrations of 200, 800, 1200, and 2000 µg/m3 (0.1, 0.4,
0.6, and 1.0 ppm). Consistent increases in airway resistance (Raw)
were demonstrated only with exposure to 2000 µg/m3 (1 ppm). Lower
concentrations caused an increase in Raw in some subjects, but a
clear dose-effect relationship could not be established at levels
below 2000 µg/m3 (1 ppm). When exposed to an ozone concentration of
1500 µg/m3 (0.75 ppm) for 2 h, 10 healthy males exhibited significant
increases in Raw, reductions in maximum static elastic recoil
pressure of the lung, and a fall in maximum flow at 50% of vital
capacity (Bates et al., 1972). Exercise on a bicycle ergometer during
ozone exposure accentuated these pulmonary function changes, and most
subjects complained of substernal soreness and cough at the end of a
2-h period. These observations were extended by Hazucha et al. (1973)
to include an ozone concentration of 740 µg/m3 (0.37 ppm) for 2 h and
subjects were intermittently exercised during exposure. After exposure
to 740 and 1500 µg/m3 (0.37 and 0.75 ppm) for 2 h, both smokers and
non-smokers (6 subjects per group) revealed significant decreases in
forced vital capacity (FVC), one-second forced expiratory volume
(FEV1.0), mid-maximal expiratory flow rate (MMFR), and maximum
expiratory flow rate at 50% of vital capacity (MEFR 50%), but the
effects were greater at 1500 µg/m3 (0.75 ppm). These effects were
closely related to the changes measured in the closing volume and
indicated an early effect in the small airways.
Seven healthy males, exposed to an ozone concentration of
1000 µg/m3 (0.50 ppm) for 2 h while performing intermittent light
exercise, showed decreases in lung function measurements and oxidative
changes in erythrocytes; 3 subjects had symptoms such as cough,
substernal discomfort, and malaise. When 2 healthy and 3 sensitive
subjects (those with a prestudy history of cough, chest discomfort, or
wheezing associated with allergy or air pollution exposure, but with
normal base line pulmonary function studies) were exposed to an ozone
concentration of 740 µg/m3 (0.37 ppm) for 2 h under the same
conditions of exercise, there was a significant increase in the total
respiratory resistance compared with the pre-exposure resistance.
Oxidative biochemical changes were detected in the blood of this
group, but were not as severe as in those exposed to an ozone
concentration of 1000 µg/m3 (0.5 ppm). Three healthy and 3 sensitive
subjects exposed to 500 µg/m3 (0.25 ppm) for 2 h did not show any
consistent physiological changes attributable to exposure (Hackney et
al., 1975). All the subjects of the above study were southern
Californians. In order to study possible adaptation of these subjects
to long-term ambient ozone exposure, the same investigators compared
the effects of ozone exposure on southern Californians with those on
Canadians and found that the latter were more responsive (Hackney et
al., 1977).
Ohmori (1974) found increased breathing frequency and volume in 4
healthy males who were exposed under exercise to ozone concentrations
of 200-500 µg/m3 (0.1-0.25 ppm) for 30 min. Four normal male subjects
exposed for 5 min to a combination of ozone at 1800 µg/m3 (0.9 ppm)
and light exercise showed a highly significant decrease in specific
airway conductance (Kagawa & Toyama, 1975a). Folinsbee and co-workers
(1975) tested the reaction of 28 normal adults (20 males, 8 females)
exposed to ozone at concentrations of 740, 1000, or 1500 µg/m3 (0.37,
0.50, or 0.75 ppm) for 2h: the subjects were either at rest or
exercising intermittently with sufficient intensity to increase lung
ventilation by a factor of 2.5. There was a gradation of complaints
depending on the ozone concentration. Under exercise, increase in
respiratory frequency was closely correlated with the total dose of
ozone and, at any given concentration, the effect was greater with the
subject who had exercised. Statistically significant decreases were
found in FVC at ozone concentrations of 1000 µg/m3 (0.50 ppm) or
more. Decrease in MEFR occurred in 50% of subjects at concentrations
of 740 µg/m3 (0.37 ppm) or more.
The effects of ozone on pulmonary function were studied in 22
young, healthy, male, nonsmokers who were exposed to 800 µg/m3
(0.4 ppm) for 2 and 4 h. Subjects were seated during the exposure
except for 2 exercise periods of 15 min (bicycle), beginning after 1
and 3 h of exposure, respectively. Significant changes in FVC, MMFR
and Raw occurred after 2 h of exposure. Borderline effects were
reported for FEV1.0, V50 (expiratory flow rate at 50% FVC) and V25
(expiratory flow at 25% FVC). After 4 h of exposure, changes in all of
these lung function variables became statistically significant (Rummo
et al., 1975, unpublished data).a In studies by Kerr et al. (1975),
20 lightly exercising subjects (19 males and 1 female) were exposed to
an ozone concentration of 1000 µg/m3 (0.5 ppm) for 6 h. Subjects,
particularly nonsmokers, commonly reported dry cough and chest
discomfort. Significant changes were produced in airway conductance,
pulmonary resistance, and forced expiratory volumes, but not in
diffusing capacity.
a Rummo, N.J., Knelson, I. H., Lassiter, S., Cram, J. J., & House,
D. (1975). Effects of ozone on pulmonary function in healthy young
men. Research Triangle Park, NC, US Environmental Protection Agency,
17 pp. (In-house Technical Report).
Changes in the pulmonary function of 11 male subjects, aged 24-38
years, exposed to an ozone concentration of 200 µg/m3 (0.1 ppm) for
2 h, were compared with a 1-h pre- and post-control period without
ozone and with a control series without ozone. Under test conditions,
which included intermittent light exercise, a significant increase in
Raw and in the alveolar to arterial oxygen pressure difference
(AaDO2) was observed in 7 out of 11 subjects (yon Nieding et al.,
1977).
In an attempt to study the effects of long-term repeated exposures
to ozone, Bennet (1962) exposed 2 groups of 6 healthy males to
400 µg/m3 (0.2 ppm) or 1000 µg/m3 (0.5 ppm) respectively, for 3 h
per day, 6 days per week for 12 weeks. The 400 µg/m3 (0.2 ppm)
exposure group did not experience any symptoms or changes in the
forced expiratory volume. While the second group was also
asymptomatic, the FEV1.0 showed a significant decrease during the last
few weeks of exposure.
6.1.3.2 Exposure to mixtures of ozone and other air pollutants
Bates & Hazucha (1973) demonstrated a synergistic action of ozone
at 740 µg/m3 (0.37) and sulfur dioxide at 960 µg/m3 (0.37 ppm). With
4 normal subjects engaged intermittently in light exercise, a 2-h
exposure to sulfur dioxide, only, at 960 µg/m3 (0.37 ppm) did not
produce any changes in FVC, FEV1.0, MMFR, and MEFR 50%, while exposure
to an ozone concentration of 740 µg/m3 (0.37 ppm) caused a 10-15%
reduction in these lung function variables. However, exposure to a
combination of these gases at the same concentrations resulted in a
20-45% decline in lung function measurements and this effect was even
greater than the changes caused by a 2-h exposure to an ozone
concentration of 1500 µg/m3 (0.75 ppm). On the other hand, addition
of nitrogen dioxide at a concentration of 560 µg/m3 (0.30 ppm) to
ozone at 500 µg/m3 (0.25 ppm) did not result in any decrement in the
lung functions of 3 healthy and 3 sensitive (section 6.1.3.1) subjects
(Hackney et al., 1975a).
In a series of studies already mentioned in section 6.1.3.1, von
Nieding et al. (1977) found that the effects of exposure to an ozone
concentration of 200 µg/m3 (0.1 ppm) were not enhanced by combination
with nitrogen dioxide at 9400 µg/m3 (5 ppm), or by combination with
nitrogen dioxide at 9400 µg/m3 (5 ppm) and sulfur dioxide at
13 000 µg/m3 (5 ppm), though recovery time was delayed in the last
experiment. Exposure for 2 h to a combination of ozone at 50 µg/m3
(0.025 ppm), nitrogen dioxide at 100 µg/m3 (0.05 ppm), and sulfur
dioxide at 260 µg/m3 (0.1 ppm) did not have any effect on Raw, or on
AaDO2. However, there was a dose-dependent increase in the
sensitivity to acetylcholine of the bronchial tree compared with the
controls. This effect was observed by measuring the increase in Raw
after inhalation of 2% acetylcholine alone and in combination with the
previously mentioned mixture of gases at the same concentrations. The
effect became more pronounced when the acetylcholine was combined with
ozone at 200 µg/m3 (0.1 ppm), nitrogen dioxide at 9400 µg/m3
(5 ppm), and sulfur dioxide at 13 000 µg/m3 (5 ppm).
6.1.3.3 Exposure to peroxyacetylnitrate alone or in combination with
carbon monoxide
Thirty-two male college students were exposed to a peroxyacetyl-
nitrate concentration of 1500 µg/m3 (0.3 ppm) for 5 min while
exercising on a bicycle ergometer (Smith, 1965). A statistically
significant increase in oxygen uptake was observed during exercise, in
comparison with that observed when filtered air was breathed. MEFR was
significantly decreased by exposure to this gas during the recovery
phase following exercise. However, peroxyacetylnitrate did not have
any effect when the subjects were at rest.
The metabolic, temperature, and cardiorespiratory reactions of 20
healthy males (10 smokers and 10 nonsmokers) were monitored while
working to their maximum and breathing filtered air or 3 different gas
mixtures at 25 ± 0.5°C and a relative humidity of 20 ± 2% (Raven et
al., 1974). The mixtures were carbon monoxide in filtered air at
57 500 µg/m3 (50 ppm), peroxyacetylnitrate in filtered air at
1350 µg/m3 (0.27 ppm), and a combination of the two. Maximum aerobic
capacity did not decrease significantly in either group during
exercise and exposure to any of the pollutant gas mixtures compared
with filtered air. For both smokers and nonsmokers exposure to
peroxyacetylnitrate and carbon monoxide alone or in combination, while
exercising to maximum aerobic capacity, produced minor alterations in
cardiorespiratory and temperature regulating variables.
6.1.3.4 Exposure to irradiated automobile exhaust
Fourteen college students were exposed on 2 occasions to
irradiated automobile exhaust and measurements were made of reaction
time, vital capacity, and submaximum work performance on the bicycle
ergometer. The individuals were exposed to a mixture of the following
gases: carbon monoxide, carbon dioxide, nitric oxide, nitrogen
dioxide, hydrocarbons, aldehydes, formaldehyde, and oxidants (Table
12, section IID). It appeared that exposure to this mixture of
pollutants had little effect on the types of human motor performance
chosen for this study (Holland et al., 1968).
6.1.3.5 Exposure to ambient air containing elevated concentrations of
oxidants
The effect of oxidant air pollution on patients with chronic
obstructive lung disease was studied by Motley et al. (1959). Twenty
normal individuals and 46 patients with chronic lung disease were
evaluated by measuring lung volumes, forced expiratory volumes, and
nitrogen washout before and after removal from ambient Los Angeles air
into a room with clean filtered air. No significant changes were
detected in the pulmonary function of normal subjects or in patients
with chronic lung disease who entered the room on nonsmoggy days.
However an improvement in lung function, particularly a decrease in
residual lung volume and nitrogen washout time, occurred among
patients who entered the filtered room on smoggy days and remained for
40 or more hours. Simultaneous measurements of oxidants were not
obtained at the study site, but during the study, ambient hourly
oxidant concentrations at nearby monitoring stations ranged from
400-1400 µg/m3 (0.2-0.7 ppm). Subjects with chronic lung disease, who
remained in the filtered air for only 18-20 h, did not experience any
significant improvement in lung function.
In a similar study, Balchum (1973) observed the pulmonary function
of 15 patients with moderately severe chronic obstructive lung
disease, who spent one week in a room without air filtration and a
second week in clean filtered air. During the first week, when
unfiltered ambient air was drawn into the room, 1-h oxidant
concentrations averaged 220 µg/m3 (0.11 ppm) and ranged up to
400 µg/m3 (0.2 ppm). During the second week, when air filters were
activated, oxidant exposures ranged from 40 to 60 µg/m3 (0.02 to
0.03 ppm). Comparison of the effects of unfiltered and filtered air
revealed a decrease in airway resistance and an increase in the
arterial pO2 during the week of filtered air breathing. Changes were
observed both at rest and during exercise in about 75% of the
subjects. Decreases in airway resistance first became apparent after
breathing filtered air for 48 h.
6.1.4 Changes in electroencephalograms
Changes (decrease in the alpha rhythms) in electroencephalograms
were studied by Eglite (1968) in relation to exposure to ozone for
3 min. All 3 healthy subjects studied showed changes when exposed to
an ozone concentration of 20 µg/m3 (0.01 ppm) and 1 subject reacted
at a concentration at 10 µg/m3 (0.005 ppm).
6.1.5 Chromosomal effects
In a recent study by Metz et al (1975), 6 human volunteers were
exposed to an ozone concentration of 1 mg/m3 (0.5 ppm) for 6-10 h,
and the circulating lymphocytes were examined for chromosomal changes.
Although no chromosome aberrations were found, there was a significant
increase in the number of minor chromosomal abnormalities (chromatid
deletions) compared with pre-exposed lymphocytes.
6.2 Industrial Exposure
Several studies on the effects of industrial exposure have been
reported, but in most of them, the effects of ozone have been
confounded by the coexistence of other pollutants and a threshold
concentration has not been determined.
Kleinfield et al. (1957) reported several cases of severe ozone
intoxication in welders using an inert gas-shielded consumable
electrode which greatly increased the ultraviolet radiation. Ozone was
measured at the breathing zone in 3 plants using this welding
technique. No complaints or clinical findings were associated with
ozone concentrations of 500 µg/m3 (0.25 ppm) or less. At
concentrations of 600-1600 µg/m3 (0.3-0.8 ppm), an increasing number
of welders complained of chest constriction and irritation of the
throat.
Similar findings using this welding technique were reported by
Challen et al. (1958), when 11 out of 14 workers complained of
respiratory symptoms at ozone concentrations in the range of
1600-3400 µg/m3 (0.8-1.7 ppm). Symptoms disappeared when ozone levels
were reduced to 400 µg/m3 (0.2 ppm). Young et al. (1963) failed to
detect any significant changes in lung function tests (vital capacity,
functional residual capacity, MMFR, FEV0.75, or diffusing capacity) in
7 men engaged in argon-shielded electric arc welding when ozone
concentrations were 400-600 µg/m3 (0.2-0.3 ppm).
Kudrjavceva (1963) described a complex of symptoms that included
headache, weakness, increased muscular excitability, and decreased
memory among workers engaged in the manufacture of hydrogen peroxide
and exposed to ozone concentrations ranging from 500-800 µg/m3
(0.25-0.40 ppm) for 7-10 years. The investigator suggested that these
reactions might be due to prolonged exposure to ozone under working
conditions. Increased prevalence of bronchitis and emphysema,
accompanied by decreased expiratory flow rate, was also reported in
workers involved in the manufacture of hydrogen peroxide by Nevskaja &
Diterihs (1957). Ozone concentrations of 80-1000 µg/m3
(0.04-0.50 ppm) were measured; sulfuric acid aerosol was also present.
Control groups were not included in either of these studies.
In several countries, occupational exposure limits for ozone have
been set as maximum, time-weighted averages for an 8-h workday or 40-h
week (ILO, 1977). In some countries, the exposure limit is 100 µg/m3
(0.05 ppm), in others, 200 µg/m3 (0.1 ppm). In addition, a short-term
exposure limit of 600 µg/m3 (0.3 ppm), for periods up to 15 min, has
been tentatively proposed by the USA provided that there are not more
than 4 such periods per day with at least 60 min between each period,
and that the daily time-weighted average is not exceeded. The German
Democratic Republic has set a limit of 200 µg/m3 for exposure periods
not exceeding 30 min and the USSR has proposed an 8-h mean value of
100 µg/m3 (0.05 ppm).
6.3 Community Exposure
Epidemiological studies on the association between human health
effects and exposure to photochemical oxidants have largely been
carried out in the Los Angeles air basin. For most of these studies,
investigators made use of measurements of photochemical oxidants
obtained from a network of air monitoring stations operated by the Los
Angeles County Air Pollution Control District. Oxidants were measured
by the unbuffered potassium iodide method which yields oxidant values
15-25% lower than the values obtained with the 1 or 2% neutral-
buffered potassium iodide method more commonly used in regions outside
the Los Angeles air basin.
As in all studies on urban populations, the observed health
effects of photochemical oxidant exposure cannot be attributed only to
oxidants. Photochemical smog typically consists of ozone, nitrogen
dioxide, peroxyacylnitrates and other nitrate compounds, sulfates,
other particulate aerosols, and reducing agents. In combination, these
pollutants may have an independent, additive, or synergistic effect on
human health. In general, however, ozone appears to be the most
biologically active pollutant and the correlations between health
effects and pollutant exposure were found when results concerning
ozone, rather than other identified pollutants, were statistically
analysed. Since controlled exposure studies in man and animals confirm
the greater biological reactivity of ozone compared with other
components of photochemical smog, it seems reasonable to conclude, for
the purpose of developing health protection guidelines, that ozone is
the principal agent responsible for the exposure-response
relationships observed in epidemiological studies on photochemical
oxidants.
6.3.1 Mortality
Studies conducted by the California State Department of Public
Health (1955, 1956) over the periods July-November 1953 and 1954, and
July-December 1955 revealed that, during these months, the daily
mortality rate of Los Angeles residents, aged 65 or over, was strongly
influenced by a heat wave but was not consistently altered either by
variations in oxidant concentrations or by the occurrence of smog-
alert days (ozone concentrations of 600 µg/m3 (0.3 ppm) or more).
No significant correlations were found by Massey et al. (1961,
unpublished data) between daily mortality rates and daily oxidant
levels in an analysis of two areas of Los Angeles County, selected for
similarities in temperature and for differences in air pollution
levels (values not given). Hechter & Goldsmith (1961) also failed to
find a significant correlation between monthly mortality rates due to
cardiorespiratory diseases and pollutant levels (monthly means of
daily maxima that ranged from 80 to 400 µg/m3; 0.04-0.2 ppm) in Los
Angeles County for the years 1956-58. The authors made Fourier curve
analyses of the data in order to remove the major effect of season of
year on mortality rate and pollutant levels. An association between
respiratory and cardiac deaths and Los Angeles smog episodes with 1-h
concentrations exceeding 400 µg/m3 (0.2 ppm) was observed by Mills
(1957) but he failed to take into account seasonal fluctuations in
deaths and pollutants. However, it is possible that the method of
adjustment for seasonal effects might mask a real effect of pollutant
concentrations on mortality. Therefore no conclusive statement can be
made concerning the lack of an association between mortality rate and
short-term variations in oxidant levels.
A model of daytime wind flow over Los Angeles was constructed by
Mahoney (1971) to divide the city into 5 wind zones each about
10 kilometres in width and representing distance downwind along the
path of air flow. The mortality rate of the white population, adjusted
for age, sex, and income level, from noncancerous respiratory diseases
during 1961 increased in successive downwind zones affected by the Los
Angeles sea breeze. The adjusted mortality rate in the wind zone
immediately adjacent to the Pacific Ocean was 53 per 100 000, while
mortality in the afferent wind zone most remote from the ocean was
111 per 100 000. The author suggested that this geographical
difference in mortality might be consistent with an effect of
temperature, humidity, or oxidant air pollution.
6.3.2 Annoyance and irritation
In Los Angeles, 75% of the population complained that they were
"bothered" by air pollution either at home or at work compared with
24% in San Francisco and 22% in the "rest of the State". Eye
complaints, which were relatively high (33%) in Los Angeles County and
in the "rest of the State", were low (14%) in the San Francisco Bay
area. However, fewer cases of hayfever and sinus trouble were reported
in Los Angeles County compared with other areas (Hausknecht, 1960).
The association of oxidant levels with eye irritation was
investigated (Renzetti & Gobran, 1957) in a panel of office and
factory workers and a second panel of scientists residing in the Los
Angeles Basin. The data demonstrated increasing eye irritation with
increasing maximum instantaneous oxidant concentrations over a range
of values from 100 to 900 µg/m3 (0.05-0.45 ppm).
Daily symptoms associated with eye and respiratory irritation were
studied by Hammer et al. (1974) in a group of Los Angeles student
nurses in relation to daily oxidant levels. Headache, eye discomfort,
cough, and chest discomfort were all found to be related to daily
maximum hourly oxidant concentrations. On days when maximum hourly
concentrations were 800-1000 µg/m3 (0.40-0.50 ppm), students reported
48% more cough and 100% more chest discomfort compared with days when
oxidant levels were below the US air quality standard of 160 µg/m3
(0.80 ppm). Using "hockey stick" functions,a the threshold levels
have been determined as maximum 1-h concentrations of 100 µg/m3
(0.05 ppm) for headache, 300 µg/m3 (0.15 ppm) for eye irritation,
530 µg/m3 (0.27 ppm) for cough, and 580 µg/m3 (0.29 ppm) for chest
discomfort. Exposure-response relationships are shown in Table 11.
6.3.3 Athletic performance
The effect of oxidant concentrations on athletic performance was
studied in Los Angeles by Wayne et al. (1967) in 21 competitive team
events for High school cross-country runners from 1959-64. Since
running times tended to improve throughout the season, team
performance at a meeting was evaluated by determining the percentage
of individual athletes who failed to improve when their running time
was compared with that of their previous performance on the same
course. Oxidant levels in the hour before the meeting, which ranged
from below 100 µg/m3 (0.05 ppm) to 600 µg/m3 (0.3 ppm), were highly
correlated ( r=0.88) with decreased performance, as shown in Fig. 9.
Consistently high correlations were obtained when the observations
were divided into the periods 1959-1961 ( r=0.945) and 1962-64
( r=0.945). Correlations with other pollutants (carbon monoxide and
particulates) and with meteorological variables (temperature, relative
humidity, wind velocity, wind direction) were considerably lower and
usually not significant. The authors speculated that the observed
oxidant-athletic performance relationship could be due to a direct
effect on oxygen use or to respiratory discomfort associated with
exercise in an atmosphere containing a high concentration of oxidants.
A statistical test for threshold values, based on segmental regression
analysis ("hockey stick" functions) applied to these data (Barth et
al., 1971; Hasselblad et al., 1976) gave a threshold estimate of
240 µg/m3 (0.12 ppm) (1-h value) with a 95% confidence interval of
134 to 326 µg/m3 (0.067-0.163 ppm).
a The term hockey stick function has been used by Hammer et al.
(1974) and Hasselblad et al. (1976) to describe a function
consisting of a curve with zero slope up to a certain point and
increasing monotonically from that point.
Table 11. Relative increase in headache, eye discomfort, cough, and chest discomfort in relation to photochemical oxidant
exposure of student nurses in Los Angelesa
Relative increase in symptom
Daily maximum 1-h oxidant Headache Eye discomfort Cough Chest discomfort
level
µg/m3 (ppm) Simple Adjustedb Simple Adjustedb Simple Adjustedb Simple Adjustedb
<160 (<0.08) 1.00 (16.4)c 1.00 (10.6) 1.00 (8.9) 1.00 (5.2) 1.00 (13.0) 1.00 (9.5) 1.00 (3.5) 1.00 (1.8)
180 (0.09) 0.97 1.00 1.01 1.07 1.02 1.07 0.91 1.05
200-280 (0.10-0.14) 0.95 1.03 0.96 1.13 0.91 0.99 0.97 1.00
300-380 (0.15-0.19) 0.95 1.07 1.12 1.33 0.95 1.02 1.05 0.94
400-480 (0.20-0.24) 0.95 1.08 1.37 1.77 0.89 0.96 0.89 0.89
500-580 (0.25-0.29) 1.01 1.07 1.67 2.15 0.95 1.01 1.03 1.11
600-780 (0.30-0.39) 1.03 1.26 2.37 3.42 1.16 1.23 1.17 1.27
800-1000 (0.40-0.50) 1.02 1.41 3.93 6.11 1.48 1.77 2.00 3.22
a From: Hammer et al. (1974).
b Excludes all days when the symptom was reported in conjunction with either "feverish", "chilly", or "temperature".
c Bracketed figure gives baseline symptom rate as mean daily percentage of symptom reported.
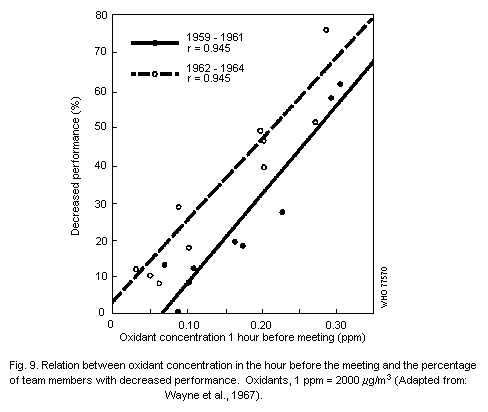 6.3.4 Effects on children
Ventilatory performance, measured twice a month for 11 months by
the Wright Peak Flow Meter, in 78 elementary school children in two
cities in the Los Angeles Basin was unaffected by differences in the
daily mean oxidant levels which were 320 µg/m3 (0.16 ppm) and
220 µg/m3 (0.11 ppm) respectively (McMillan et al., 1969).
Kagawa & Toyama (1975b) and Kagawa et al. (1976) studied the
weekly variations in lung function (airway conductance and ventilatory
performance) of 20, normal, 11-year-old, school children in Tokyo in
relation to variations in temperature and ambient concentrations of
ozone, nitrogen dioxide, nitric oxide, sulfur dioxide, hydrocarbons,
and particulate matter. Students were tested from June 1972 to October
1973. Ozone levels were determined by the ethylene-chemiluminescence
method. Temperature was the factor most highly correlated with
variations in specific airway conductance (negative correlation) and
maximum expiratory flow rate (Vmax) at 25 and 50% FVC (positive
correlation). Significant negative correlations between ozone and
specific airway conductance, and between nitrogen dioxide, nitric
oxide, sulfur dioxide, and particulate matter and Vmax at 25 or 50%
FVC were observed in some children. The ranges of hourly pollutant
levels, at the time of the lung function test (13h00), that were used
for correlation during the study period were: 0-560 µg/m3
(0-0.28 ppm) for ozone, 40-550 µg/m3 (0.02-0.29 ppm) for nitrogen
dioxide, 30-340 µg/m3 (0.01-0.13 ppm) for sulfur dioxide, and
50-490 µg/m3 for particulate matter (Toyama et al., 1977).
The effect of oxidant air pollution exposure on the incidence and
duration of A2 influenza in an epidemic that occurred in 1968-69 was
studied retrospectively by Pearlman et al. (1971) in 3500 elementary
school children from 5 southern California communities. These areas
were selected to represent an exposure gradient for photochemical
oxidants, although no difference in community oxidant exposure was
present at the time (December 1968-January 1969) of the epidemic.
Information from school absenteeism, questionnaires on illness, and
haemagglutination-inhibition titres did not reveal any statistically
significant morbidity differences corresponding to the pollution
gradient that existed during the season of peak oxidant levels.
Several Japanese investigators have reported acute reactions among
school children exposed to moderately elevated concentrations of
oxidants of 200-400 µg/m3 (0.10-0.20 ppm), on smoggy days in a number
of urban areas. Not only eye and respiratory irritation, but systemic
symptoms such as paraesthesia, prostration, and convulsions were
observed. In reviewing these studies the WHO Task Group was aided by
several investigators and observers from Japan. In the opinion of the
Task Group, eye and respiratory effects observed during these episodes
may well have been caused by reported oxidant concentrations. However,
systemic symptoms could not be explained only by the observed oxidant
concentrations, but were more likely to be attributable to individual
psychosomatic reactions among the students. This judgement is
supported by the observation that acute systemic reactions were
observed in only some of the students, most of whom were exercising at
the time of peak oxidant levels. Thus, the students may well have
experienced acute irritation of the pharynx and trachea, enhanced by
their active status. However, the general population in the same
neighbourhood, including young children in primary grades and school
teachers, did not appear to have had these systemic symptoms. These
comments are based on the following studies.
Fujii (1972) reported the acute effects of an episode of
photochemical smog in Osaka, Japan, on 27 August 1971. The oxidant
concentration measured by the NBKI method rose to an hourly value of
360 µg/m3 (0.18 ppm) by 14h00. At this time, the peak ozone
concentration, determined by the chemiluminescent method was
480 µg/m3 (0.24 ppm) and the maximum sulfur dioxide concentration was
180 µg/m3 (0.07 ppm). A total of 249 school children reported
symptoms including pain in the throat, headache, coughing, breathing
difficulties, eye irritation, and numbness in the limbs. On 14
September 1971, maximum hourly ozone concentrations reached 480 µg/m3
(0.24 ppm) and a total of 290 persons complained of similar symptoms.
These data do not provide a basis for estimating symptom frequency in
the general population, because the total population at risk was not
reported.
In an evaluation of the clinical status of 15 high school children
who were hospitalized after the onset of acute symptoms that began
during photochemical smog episodes in Osaka, Japan, Adachi & Nakajima
(1974) stated that "they were astonished by the seriousness of the
conditions which surpassed by far the symptoms previously known in
connection with the relation between photochemical oxidant
concentration and its effect on man in the case of Los Angeles".
Affected students were not only found to have irritation of the eyes
and respiratory tract, but, systemic symptoms such as paraesthesia,
muscle spasms as well as an increased leukocyte count and borderline
elevation of serum alkaline phosphatase (3.1.3.1).
Based upon complaints found in schools that were registered with
local health centres located in Japanese cities, the Japan Public
Health Association (1976) reported a significant increase in
subjective complaints, particularly eye irritation, when photochemical
oxidant concentrations exceeded hourly values of 300 µg/m3
(0.15 ppm), as determined by the 10% NBKI method.
Mikami & Kudo (1973) described various local and systemic symptoms
in 82 school children that were attributed to 3 photochemical air
pollution episodes in Tokyo and Osaka in 1970, 1971, and 1972 with
maximum 1-h concentrations ranging from 300 to 580 µg/m3 (0.15 to
0.29 ppm). A large proportion of cases were reported to have eye
irritation, throat irritation, cough, breathlessness, headache, and
chills. The authors pointed out that many of these symptoms had not
been reported under Los Angeles smog conditions.
6.3.5 Effects on the incidence of acute respiratory and
cardiovascular diseases
In studies on college students in the Los Angeles Basin, Durham
(1974) showed that acute episodes of pharyngitis, bronchitis, and
upper respiratory infections were associated with peak concentrations
of oxidants and mean concentrations of sulfur dioxide and nitrogen
dioxide. Oxidants, sulfur dioxide, and nitrogen dioxide
(concentrations not given) were, in this order, consistently
associated with the various episodes of illness. A comparison of
selected high and low pollution days indicated that photochemical air
pollution might have been responsible for a 16.7% increase in acute
respiratory symptoms seen in Los Angeles schools situated in areas of
highest concentration compared with those in areas of lowest
concentration. The author's method of data presentation did not permit
the estimation of dose-response relationships or of threshold
concentrations.
Brant & Hill (1964) and Brant (1965) did not find any correlation
between admissions for cardiovascular conditions to Los Angeles County
Hospital and oxidant levels (170 µg/m3; 0.08 ppm as a mean value of
daily 7-h measurements for the study periods) either on the day of
admission or for 2 weeks prior to admission, for the period between 8
August and 25 December 1954. However, Sterling et al. (1966, 1967)
found statistically significant but very low correlations between
admissions to Los Angeles hospitals for respiratory episodes during
the period 17 March-26 October 1961 and daily mean levels of oxidants
(74 µg/m3; 0.037 ppm), ozone (84 µg/m3; 0.042 ppm), and carbon
monoxide (11 mg/m3; 9.5 ppm). Because of the limited time period and
the extremely low correlation coefficients, it is difficult to
conclude that a real relationship was demonstrated by these studies.
6.3.6 Effects on the prevalence of chronic respiratory diseases and
on pulmonary function
Deane et al. (1965) conducted a survey on the prevalence of
chronic respiratory disease in outdoor male telephone workers in San
Francisco and Los Angeles. Symptoms of persistent cough and phlegm
were slightly less prevalent in Los Angeles than in San Francisco in
the 40-49-year group but more prevalent in the 50-59-year group (31.4%
compared with 16.3% in San Francisco). These data were adjusted for
cigarette smoking. No differences in pulmonary function test results
were found.
The prevalence of chronic respiratory symptoms and the results of
pulmonary function tests were compared in 2 similar groups of
nonsmoking, adult, male and female Seventh Day Adventists aged 45-64
years residing in Los Angeles and San Diego, respectively. Annual mean
oxidant values (94 µg/m3; 0.047 ppm and 76 µg/m3; 0.038 ppm), were
essentially the same in the 2 cities, but a mean of maximum daily
concentrations in Los Angeles (290 µg/m3; 0.144 ppm) was twice as
high as that in San Diego (148 µg/m3; 0.074 ppm). The survey was
performed when oxidant levels were low and similar in both cities, to
minimize effects attributable to acute oxidant exposures. No
differences were found in symptom prevalence or in several measures of
ventilatory function. Prevalence rates for chronic respiratory disease
were uniformly low (less than 4%) in both groups (Cohen et al., 1972).
6.3.7 Effects on patients with pre-existing diseases
6.3.7.1 Asthma
The association between reported asthma attacks in 137 patients
from Pasadena, California, and oxidant levels during the period 3
September-9 December 1956 was studied by Schoettlin & Landau (1961).
Daily records of the time of onset of asthma attacks were kept by each
patient and collected weekly. The daily number of patients afflicted
with asthma was moderately well correlated (r = 0.37) with concurrent
maximum hourly oxidant readings. Asthma attacks were more weakly
correlated with temperature, relative humidity, and water vapour
pressure than with oxidants. The number of patients having attacks on
days when maximum 1-h oxidant levels were higher than 500 µg/m3
(0.25 ppm) was significantly greater (p = 0.05) than the number of
those having attacks on days with lower oxidant levels. Unfortunately,
the analysis failed to isolate the pronounced seasonal variation of
asthma. In the northern hemisphere, asthma attack rates tend to reach
a peak in October and November (Booth et al., 1965), and decline
sharply in late November and early December. This pattern corresponds
closely to seasonal declines in peak oxidant levels. Hence, it is
possible that observed asthma-oxidant correlations have been secondary
to simultaneous seasonal changes in asthma frequency and oxidant
levels.
6.3.7.2 Chronic respiratory diseases
Studies over 18 months on 25 patients with severe, chronic,
obstructive lung disease at a chronic disease centre in Los Angeles
County showed that the prevailing levels of oxidants, oxidant
precursors, temperature, and relative humidity did not produce any
effects on lung function (Rokaw & Massey, 1962).
In studies on the effects of ambient air pollution exposure on
armed forces veterans with chronic respiratory disease living in the
Domiciliary Unit and Chronic Disease Annex of the Los Angeles Veterans
Administration Center, subjects were evaluated once a week by
pulmonary function tests and respiratory symptom questionnaires
(Schoettlin, 1962). Analysis of variance did not show any
statistically significant effects of air pollution (concentrations not
reported) on respiratory symptoms or function, although maximum
concentrations of oxidant and oxidant precursors consistently
accounted for more of the variation in frequency of symptoms and
clinical signs of disease than maximum temperature, relative humidity,
or pollen counts.
6.3.8 Cancer
A 5-year prospective study of lung cancer from 1958 to 1963 was
conducted by Buell et al. (1967) among 69 160 members of the
California American Legion. Long-term residents of Los Angeles County
had slightly lower age-smoking adjusted lung cancer rates (95.4 per
100 000) than residents of the San Francisco Bay area counties and San
Diego County (102 per 100 000). These urban groups, in turn, had
higher rates than all other California counties (75.5 per 100 000).
Nonsmokers followed the same geographical pattern: 28.1 per 100 000
for Los Angeles, 43.9 per 100 000 for San Francisco and San Diego,
11.2 per 100 000 for all others. Smokers of more than one packet of 20
cigarettes a day in Los Angeles had the highest lung cancer rates,
241.3 per 100 000, compared with San Francisco-San Diego, 226 per
100 000, and with other counties, 137.5 per 100 000. The duration of
exposure necessary to induce lung cancer may have been longer than the
actual ozone exposures in this population study. Thus, it would appear
worthwhile to extend these observations to subsequent years.
6.3.9 Motor vehicle accidents
The association between automobile accidents and days of elevated
oxidant levels (120-480 µg/m3; 0.06-0.24 ppm) in Los Angeles was
studied from August to October in 1963 and 1965. Applying a sign-test
and nonparametric correlation analysis to the data, Ury (1968) found a
statistically significant relationship between oxidant levels and
automobile accidents. Concentrations of carbon monoxide, oxides of
nitrogen, and other pollutants would also be relatively elevated when
oxidants were high and may have contributed to the results reported.
Furthermore, the association of accidents with oxidant levels may be
confounded by the fact that traffic jams produce both more accidents
and a greater output of oxidant precursors.
6.4. Summary Tables
Studies on the health effects of controlled, industrial, and
community exposures that provide quantitative information useful for
establishing guidelines for the protection of public health with
respect to photochemical oxidants are summarized in Tables 12, 13 and
14.
Table 12. Controlled human studies
I. Sensory effects
Ozone concentration Length of Effects Responsea Subjects Reference
exposure
µg/m3 (ppm) days h/day
400 (0.2) 1 3 and 6 Diminution in various measures of Not applicable 22 healthy males, 6 Lagerwerff (1963)
700 (0.35) 1 3 and 6 visual perception; noneffect dose healthy females
1000 (0.5) 1 3 and 6 not determined; no mention of
dose-response relationship.
> 200 (> 0.1) 1 working Increasing eye irritation with Not applicable 20 women office Richardson &
hours (study increase in oxidant exposures; workers Middleton
repeated for no apparent effects below (1957, 1958)
123 days) 200 µg/m3 (0.1 ppm).
40-100 (0.02- Immediate odour perception after 9/10 at 10-14 healthy males Henschler et al.
0.05) beginning of exposure; odour 40 µg/m3 (1960)
perception disappeared in ´-12 13/14 at
min; odour considerably stronger at 100 µg/m3
higher dose.
15-40 (0.008 Odour perception immediately after Not available 20 healthy subjects Eglite (1968)
-0.02) beginning of exposure; the most
sensitive person perceived odour
at the level of 15 µg/m3
(0.008 ppm).
II. Effects on respiratory function
A. Exposure to ozone
Ozone concentration Length of Effects Subjects Reference
exposure
µg/m3 (ppm) days h/day
2000 (1.0) 1 1 Consistent increase in airway 4 healthy males Goldsmith et Nadel
resistance; exposures at 200, 800, (1969)
and 1200 µg/m3 (0.1, 0.4, 0.6 ppm)
caused effect in some subjects,
but no dose-response pattern.
1800 (0.9) 1 5 min Highly significant decrease of airway 4 healthy males Kagawa & Toyama
conductance after inhalation with exercise. (1975a)
1200- (0.6-0.8) 1 2 Significant reduction in diffusing capacity of 10 healthy men and Young et al.
1600 lung and in FEV0.75b; substernal soreness one healthy woman (1964)
present in all subjects.
1000 (0.5) 6/wk 3 Significant decrease in FEV1.0c; no effect at 12 healthy males Bennet (1962)
x 12 400 µg/m3 (0.2 ppm).
wk5
1000 (0.5) 1 6 Significant change in airway conductance 20 healthy subjects Kerr et al. (1975)
and pulmonary resistance (dry cough and (19 men and 1
chest discomfort). woman)
1000 (0.5) 1 2 Decrease in pulmonary function 7 healthy males Hackney et al.
measurements. (1975a)
a Response = number of subjects showing effect described
total number of subjects
Table 12. Controlled human studies--continued
II. Effects on respiratory function
A. Exposure to ozone (cont'd)
Ozone concentration Length of Effects Subjects Reference
exposure
µg/m3 (ppm) days h/day
800 (0.4) 1 2 and 4 After 2-h exposure: Rawd increase, FVCe 22 healthy male Rummo et al.
decrease MMFRf decrease; after 4-h subjects (1975.
exposure: Rawd increase, FVCe decrease. unpublished
MMFRf decrease and additionally FEV1.0c data)
decrease; RVg, FRCh, and TCLi did not
change.
740 & (0.37 & 1 2 Significant decrease in ventilatory function 6-10 healthy males Bates et al.
1500 0.75) and in closing volume under intermittent (1972).
exercise; effect more pronounced at higher Hazucha et al.
dose. (1973)
740, (0.37, 1 2 Dose dependent change of ventilatory 28 healthy subjects Folinsbee et al.
1000 & 0.50 & pattern; significant reduction in MEFRj at (20 males and 8 (1975)
1500 0.75) 50% of vital capacity at 740 µg/m3 females)
(0.37 ppm) or more, under exercise.
740 (0.37) 1 2 Significant increase in total respiratory 2 healthy and 3 Hackney et al.
resistance under intermittent light exercise sensitively males. (1975a)
500 (0.25) 1 2 No consistent changes in lung function. 3 healthy and 3 Hackney et al.
sensitively males (1975a)
200- (0.1- 1 30 min Tendency towards increase in breathing 4 healthy male Ohmori (1974)
500 0.25) frequency and volume under exercise. subjects
200 (0.1) 1 2 Increase of Rawd and AaDO2k in 7 of 11 test 11 healthy male von Neiding et al.
subjects under intermittent light exercise. subjects (1977)
B. Exposure to mixtures of ozone and other air pollutants
Ozone concentration Length of Effects Subjects Reference
exposure
µg/m3 (ppm) days h/day
740 + 960 (0.37 + 0.37 1 2 More pronounced decrease in ventilatory 4 healthy males Bates & Hazucha
sulfur sulfur function end in closing volume than in (1973)
dioxide dioxide single exposure to ozone at 740 µg/m.3
(0.37 ppm).
200 + 9400 (0.1 + 5 1 2 Increases in Rawd and AaDO2k similar 11 healthy male von Nieding et al.
nitrogen nitrogen to those observed with ozone alone at subjects (1977)
dioxide dioxide 200 µg/m3 (0.1 ppm).
200 + 9400 (0.1 + 5 1 2 Increases in Rawd and AaDO2k similar 11 healthy male von Nieding et al.
nitrogen nitrogen to those observed with ozone at subjects (1977)
dioxide + dioxide + 5 200 µg/m3 (0.1 ppm) + nitrogen dioxide
sulfur sulfur at 9400 µg/m3 (5 ppm); recovery time
dioxide dioxide) delayed.
50 + 100 (0.025 + 0.05 1 2 No effect on Rawd and AaDO2k; 11 healthy male von Nieding et al.
dioxide +260 nitrogen sensitivity of the respiratory tract subjects (1977)
sulfur dioxide dioxide + 0.1 to acetylcholine increased.
sulfur dioxide)
C. Exposure to peroxyacetylnitrate
Ozone concentration Length of Effects Subjects Reference
exposure
µg/m3 (ppm) days h/day
1500 (0.3) 1 5 min Increase in oxygen uptake with exercise; 32 college males Smith (1965)
no effect at rest; significant decrease
in MEFRj during the recovery phase
following exercise.
1350 (0.27) 1 42 min Minor changes in cardiorespiratory and 20 healthy male Raven et al.
temperature regulation parameters. subjects (1974)
II. Effects on respiratory function
D. Exposure to irradiated automobile exhaust
Oxidants (0.22 Short term No significant changes in reaction 14 healthy Holland et al.
440-540 -0.27) (no details time, vital capacity, and submaximum subjects(1968)
given) work performance on the bicycle
ergometer.
Carbon (15-29)
monoxide
17-
33 mg/m3
Table 12. Controlled human studies--continued
II. Effects on respiratory function
E. Exposure to ambient air with an elevated concentration of oxidants
Ozone concentration Length of Effects Subjects Reference
exposure
µg/m3 (ppm) days h/day
Carbon (800-1400)
dioxide
1520-
2660 mg/m3
Nitric (0.38-0.58)
oxide
470-710
Nitrogen (0.7-1.0)
dioxide
1300-1900
Hydrocarbons
traces
Aldehydes (0.2-0.7)
Formaldehyde (0.2-0.24)
250-300
400-1400 (0.2-0.7) 2-90 h Improvement in lung function upon 46 patients with Motley et al.
residence in clean filtered room chronic lung (1959)
for 40 h or longer; threshold disease
concentration not determined.
100-460 (0.05-0.23) 21 24 Decrease in airway resistance and 15 patients with Balchum (1973)
increase in arterial partial oxygen moderately severe
pressure during week of residence chronic lung
in clean filtered air; threshold disease
concentration not determined.
Table 12. Controlled human studies
III. Changes in the electroencephalogram
Ozone concentration Length of Effects Responsea Subjects Reference
exposure
µg/m3 (ppm) days h/day
10 (0.005) 1 3 min Decrease in alpha rhythm. 1/3 3 healthy subjects Eglite (1968)
15 (0.008) 1 3 min Decrease in alpha rhythm. 2/3 3 healthy subjects Eglite (1968)
20 (0.01) 1 3 min Decrease in alpha rhythm. 3/3 3 healthy subjects Eglite (1968)
a Response = number of subjects showing effect described
total number of subjects
b FEV0.75 = 0.75 second forced expiratory volume
c FEV1.0 = one second forced expiratory volume
d Raw = airway resistance
e FVC = forced vital capacity
f MMFR = mid-maximal expiratory flow rate
g RV = residual volume
h FRC = functional residual capacity
i TLC = total lung capacity
j MEFR = maximum expiratory flow rate
k AaDO2 = alveolar to arterial oxygen pressure difference
l sensitive (subjects) = those with a prestudy history of cough, chest discomfort, or wheezing associated with
allergy or air pollution exposure, but with normal base-line pulmonary function studies
Table 13. Studies on the effects of industrial exposures
Ozone concentration Averaging Effects Reference
time
µg/m3 (ppm)
1600-3400 (0.8-1.7) 1 h 11 of 14 welders complained of respiratory symptoms; symptoms Challen et al. (1958)
disappeared when ozone levels were reduced to
400 µg/m3 (0.2 ppm); nitrogen dioxide probably present.
600-1600 (0.3-0.8) 1 h Increased frequency of chest tightness and throat irritation among Kleinfeld et al. (1957)
welders; no complaints at 500 µg/m3 (0.25 ppm); welders also
exposed to nitrogen dioxide and particulates but concentration
levels not given.
500-800 (0.25-0.40) long-term Increased frequency of headache, weakness, change in neuromuscular Kudrjavceva (1963)
sensitivity, and decrease in memory among workers manufacturing
hydrogen peroxide and exposed to ozone for 7-10 years; threshold
concentration not determined.
400-600 (0.2-0.3) 1 h No evidence for changes in vital capacity or functional residual Young et al. (1963)
capacity in welders; nitrogen dioxide probably present.
80-1000 (0.04-0.50) long-term Increased prevalence of bronchitis and emphysema in workers Nevskaja & Diterihs
engaged in manufacture of hydrogen peroxide for many years; (1957)
sulfuric acid aerosols also present; threshold concentration not
determined.
Table 14. Studies on the effects of community exposures
Hourly oxidant concentration Effects Subjects Reference
µg/m3 (ppm)
500 and above (0.25 and above) Increased frequency of asthma; possible confounding of 137 patients with Schoettlin
asthma-oxidant association with seasonal effects; other asthma & Landau (1961)
pollutants present but not reported.
approx. 1000 (approx. 0.50) Increased symptoms began at hourly concentrations of 102 student Hammer et al.
(headache) 100 µg/m3 (0.05 ppm), (eye irritation) nurses (1974)
300 µg/m3 (0.15 ppm), (cough) 530 µg/m3 (0.265 ppm).
(chest discomfort) 580 µg/m3 (0.29 ppm); threshold
levels determined by "hockey stick" functions.
240 and above (0.12 and above) Impaired performance determined by failure to improve 116 high school Wayne et al.
running times; threshold determined by "hockey stick" cross-country (1967)
functions. runners
> 300 (0.15) Increased frequency of complaints, particularly eye 7440 school Japan Public
irritation. children Health
Association (1976)
0-560 (0-0.28) Specific airway conductance of sensitive subjects was 20 healthy Kagawa et al.
(range at time significantly decreased with increasing hourly oxidant 11-year-old et al. (1977)
of lung concentrations; temperature, nitric oxide, nitrogen school children
function test) dioxide, sulfur dioxide, and particulate matter also
showed significant correlations with various
respiratory function tests of highly reactive children;
threshold concentration not determined.
7. EVALUATION OF HEALTH RISKS FROM EXPOSURE TO PHOTOCHEMICAL OXIDANTS
There appears to be sufficient information from experimental and
epidemiological studies to justify an attempt to establish guidelines
on the exposure limits for ozone and to review those for "oxidants"
(as measured by the neutral-buffered potassium iodide method (NBKI)),
proposed by a WHO Expert Committee in 1972. The Task Group appreciated
the fact that photochemical air pollution contains other substances
besides ozone, such as nitrogen dioxide, peroxyacetylnitrate, and
possibly many other gaseous and particulate products of atmospheric
photochemical reactions. However, present knowledge about the
composition of photochemical pollution, the concentrations of
individual components, and their possible impact on human health is so
limited that no attempt can be made to estimate exposure limits for
any single compound other than ozone. The Task Group was, of course,
aware that some sensory effects of photochemical air pollution (such
as eye irritation) might be due to a large extent, to these poorly
defined components of the photochemical oxidant mixture. As nitrogen
dioxide is an important air pollutant in its own right, it has been
discussed in a separate criteria document (World Health Organization,
1977).
7.1 Exposure Conditions
Exposure of man to ozone must have occurred for millions of years,
as ozone, present naturally in higher tropospheric layers, is also
found regularly in the lower atmosphere even in completely uninhabited
regions like the Antarctic. These natural concentrations have been
reported to have values ranging from 10 to 100 µg/m3
(0.005-0.05 ppm). It is difficult to determine the proportions of
natural to man-made oxidants (including ozone) that occur in rural
areas in most countries. In general, ozone concentrations greater than
120 µg/m3 (0.06 ppm) are considered to be related to man-made
activities. Ozone may be transported over hundreds of kilometres and
rural populations may be exposed to the pollutant, which earlier was
considered to exist only in Urban areas. A characteristic of such
exposures is that the precursors have vanished and ozone can therefore
persist for days in succession, since it does not come into contact
with other pollutants that act as its scavengers.
In large urban areas with strong sunshine and dense traffic or
other sources of precursors, photochemical air pollution is a daylight
phenomenon with maximum 1-h ozone concentrations sometimes as high as
300-800 µg/m3 (0.15-0.4 ppm), occurring around noon or somewhat
later. Such peak concentrations are preceded by nitrogen dioxide peaks
and accompanied by concurrent rises in peroxyacetylnitrate
concentrations. In contrast to oxides of sulfur and smoke, ozone
exposures are always intermittent, the peak concentrations rarely
lasting for more than 2-3 h. In the low temperature season,
photochemical reactions are much less likely to occur at rates
sufficient to produce large quantities of ozone.
Unless certain industrial technological processes (e.g., welding)
are in operation, ozone concentrations indoors tend to be considerably
lower than those outdoors due to the presence of reactive surfaces,
air conditioning, and indoor smoking.
7.2 Exposure-effect Relationships
Information presented in sections 5 and 6 is sufficient to
evaluate the relationship between exposure and the associated effects,
at least for some biological changes observed in man and experimental
animals.
7.2.1 Animal data
There is a considerable amount of evidence that short-term,
prolonged, or repeated exposure to ozone concentrations ranging from
200 to 400 µg/m3 (0.1-0.2 ppm) can cause a variety of biological
changes in several animal species and that these effects become more
pronounced with higher concentrations and increased exposure time (see
Table 10). These effects are, of course, also influenced by other
factors such as the animal species, the length of the interval between
exposures, the presence of other pollutants, low temperature, and
physical activity.
The host's pulmonary defence mechanisms against infectious
microorganisms are affected in several animal species by exposure to
ozone (section 5.2.6). This may result in rapid multiplication of
infectious microorganisms in situ, causing disease and eventually
death. An increase in mortality, resulting from the joint action of
infectious microorganisms and ozone, has been demonstrated in
artificially infected mice after a 3-h exposure to ozone at 160 µg/m3
(0.08 ppm). These effects were dose-related.
Pathomorphological changes in the respiratory tract of various
animal species, such as the rat, cat, rabbit, and mouse, have been
observed at ozone concentrations of about 400 µg/m3 (0.2 ppm) and
higher (section 5.2.1). Short-term exposures (up to 24 h) produce
oedema, degeneration and destruction of type I alveolar cells, loss of
ciliated epithelium, and breakdown of capillary endothelium. When the
exposure is repeated or its length extended, the biological changes
become more severe and include emphysema, atelectasis, vascular
lesions, bronchopneumonia, and fibrosis.
Various functional changes in the respiratory tract begin at
levels of about 520 µg/m3 (0.26 ppm) (section 5.2.2). The activities
of several enzymes in the lung tissue are also influenced at exposure
levels of about 500 µg/m3 (0.25 ppm) (section 5.2.3).
After pre-exposure to ozone, animals appear to become tolerant to
ozone concentrations that would otherwise cause pulmonary oedema
(section 5.2.5). The development of tolerance in small rodents is
related to pre-exposure levels of at least 600 µ/m3 (0.3 ppm). This
tolerance does not protect animals from such effects of ozone as
inflammation, alterations in alveolar macrophage functions, and
impairment of respiratory functions, that can occur at concentrations
lower than those that produce oedema.
Although the primary target for ozone is the respiratory system, a
number of studies have indicated that exposure to ozone may also
result in some extrapulmonary effects, but the mechanisms of such
action are not clear (section 5.3). For example, ozone exposure at
400-500 µg/m3 (0.2-0.25 ppm) for less than 2 h produced changes in
the circulating lymphocytes (increasing the number of binucleated
cells) and increased the number of spherocytes (section 5.3.2.1). It
has also been shown that exposure of pregnant mice to ozone at
200-400 µg/m3 (0.1-0.2 ppm) for 7 h per day, 5 days per week, for 3
weeks significantly increased neonatal mortality (section 5.3.3).
The available information concerning the carcinogenicity and
mutagenicity of ozone is inadequate for the definite evaluation of
such effects (sections 5.2.4 and 5.4).
Biological effects produced by the exposure of experimental
animals to a combination of ozone and nitrogen dioxide or by exposure
to complex pollutant mixtures containing oxidants, such as irradiated
automobile exhaust, are generally similar to those produced by
exposure to pure ozone. However, one study in which mice were exposed
to a mixture of ozone and nitrogen dioxide indicated that the effect
(reduction in resistance to respiratory infection) of a single
exposure to this mixture was additive, and that repeated exposure to
this mixture might result in a synergistic action.
7.2.2 Controlled human exposures
Although limited in number, human volunteer studies with
short-term, controlled exposure to oxidants have proved useful for
establishing exposure-effect relationships for ozone at levels ranging
from about 200-700 µg/m3 (section 6.1). Some of these studies provide
evidence of changes in the respiratory function of healthy subjects
that are related to exposure. Physical exercise tends to enhance the
respiratory effects of ozone (section 6.1.3).
Three investigators found a significant increase in airway
resistance with exposure to an ozone level of 740 µg/m3 (0.37 ppm)
for 2 h. One of the investigators did not find any effect with a 2-h
exposure to a level of 500 µg/m3 (0.25 ppm). However, this particular
study was conducted on subjects from southern California who were
later found to be less sensitive to ozone. Another investigator, using
similar test conditions, found a significant increase in airway
resistance in 7 out of 11 subjects at an ozone level of 200 µg/m3
(0.1 ppm) for 2 h (section 6.1.3.1).
In two studies, an improvement in lung function was noted when
patients with chronic pulmonary disease breathed filtered air for 40 h
or more, as compared with unfiltered ambient air. The ambient air
concentrations of oxidant in the two studies ranged from
400-1400 µg/m3 (0.2-0.7 ppm) and up to 400 µg/m3 (0.2 ppm)
respectively (section 6.1.3.5).
Result of a 7-month study in which female employees were exposed
to unfiltered ambient air during office hours suggested that the
lowest 1-h oxidant level that could be associated with eye irritation
was about 200 µg/m3 (section 6.1.2).
Although in vitro studies using human cells have shown some
evidence of a joint action of ozone and nitrogen dioxide, this has not
been clearly demonstrated in in vivo studies. For example, whereas a
potentiated increase in airway resistance was observed with exposure
to a combination of ozone at 740 µg/m3 (0.37 ppm) and sulfur dioxide
at 960 µg/m3 (0.37 ppm) for a period of 2 h, it was not observed with
exposure to a combination of ozone at 500 µg/m3 (0.25 ppm) and
nitrogen dioxide at 560 µg/m3 (0.30 ppm). Exposure to ozone at
50 µg/m3 (0.025 ppm) combined with nitrogen dioxide at 100 µg/m3
(0.05 ppm) and sulfur dioxide at 260 µg/m3 (0.1 ppm) did not have any
effect on airway resistance; however, this combined exposure resulted
in enhancement of the bronchoconstrictor effect of acetylcholine
(section 6.1.3.2).
7.2.3 Industrial exposure
Acute symptoms of chest tightness, irritation of the throat, and
coughing have been documented in welders exposed to 1-h ozone levels
of 600-1600 µg/m3 (0.3-0.8 ppm). These symptoms disappeared when
ozone concentrations fell to 500 µg/m3 (0.25 ppm) or less. Possible
chronic effects of repeated occupational exposure to ozone are not
well documented, although one investigator reported that workers
exposed to ozone levels in the range of 500-800 µg/m3 (0.25-0.40 ppm)
for 7-10 years had an increased frequency of headaches and weakness,
increased muscle excitability, and impaired memory (section 6.2).
7.2.4 Community Exposure
Several studies have shown more frequent eye irritation, reduced
athletic performance, changes in lung function of children, and
increased frequency of asthma attacks, all of which have been
associated with changes in hourly oxidant levels (sections 6.3.2,
6.3.3, 6.3.4, 6.3.7). As in all studies of the effects of community
exposures, it is difficult to determine precisely the lowest level at
which adverse effects become manifest. However, most of these effects
were observed when 1-h oxidant levels were in the range of about
200-500 µg/m3 (0.1-0.25 ppm). Although other pollutants such as
nitrogen dioxide, particulate matter, and sulfur dioxide were
simultaneously present, the strongest correlation of the observed
effects was with hourly levels of photochemical oxidants.
On the other hand, there is no evidence, so far, that long-term
exposure to photochemical oxidants at levels currently present in
urban air is associated with increased mortality (section 6.3.1), and
there is no evidence that chronic respiratory diseases such as
bronchitis, emphysema, and lung cancer are more prevalent in
communities with high oxidant exposures (section 6.3.6). However, it
should be pointed out that the number of epidemiological studies
concerned with such associations is small.
7.3 Guidelines on Exposure Limits
The exposure-effect relationships discussed in section 7.2 make it
possible to draw the following conclusions concerning the exposures to
oxidants and ozone at which the effects in man begin to appear:
(a) There is presumptive evidence from one controlled exposure
study that some effects on the lung function of healthy human subjects
might occur with exposure to an ozone level of 200 µg/m3 (0.1 ppm)
for 2 h.
(b) There is also evidence from general population studies that
suggests that 1-h ambient oxidant levels in the range of about
200-500 µg/m3 (0.1-0.25 ppm) may affect lung function in children,
increase the frequency of asthma attacks, cause more frequent eye
irritation, and reduce athletic performance.
(c) There is limited evidence from controlled exposure studies
that living in an environment with 1-h oxidant levels within the range
of 400-1400 µg/m3 (0.2-0.7 ppm) may exert additional stress on
patients with chronic pulmonary disease.
(d) There is convincing evidence from controlled human exposure
studies that airway resistance may be increased in healthy human
subjects following exposure to ozone levels of 700-800 µg/m3
(0.35-0.40 ppm) for 2h.
Animal data generally support the results of human studies.
However, some effects have been observed in animals at an ozone level
of about 200 µg/m3 (0.1 ppm) or even less, which have not yet been
demonstrated in man. For example, in animals, short-term exposures to
such concentrations appear to reduce resistance to pulmonary
infections.
The role of ozone and other photochemical oxidants in the etiology
of cancer is not clear. The only available epidemiological study did
not indicate any association between exposure to oxidants and the risk
of lung cancer, and experimental studies on the carcinogenicity and
mutagenicity of ozone in animals are not adequate for evaluation.
Nevertheless, the Task Group felt that there may be reason for concern
about the possible carcinogenicity of ozone (based primarily on some
biochemical considerations regarding the mechanism of the biological
effects of ozone). This aspect of its toxicity should be kept under
continual surveillance.
On the basis of all these considerations, the Task Group agreed
that 1-h levels of ozone of 100-200 µg/m3 (0.05-0.1 ppm) (measured by
the chemiluminescence method) could be used as a guideline for the
protection of public health. The relatively high natural
concentrations of ozone precluded the use of any safety factor.
The Task Group also agreed that a 1-h maximum level of 120 µg/m3
(0.06 ppm), which is approximately the highest natural background
concentration of oxidants, would be the best single value estimate of
the exposure limit for oxidants in the ambient air. This level is in
agreement with the long-term goal for photochemical oxidants (as
measured by the NBKI method) proposed by a WHO Expert Committee (World
Health Organization, 1972).
The issue was raised as to whether the proposed guideline was
realistic in view of natural exposure levels and the long-distance
transport of ozone. In response to this question, the Group expressed
the view that every effort should, nevertheless, be made to develop
control strategies for achieving the proposed guideline or at least,
for not exceeding it more than once a month.
REFERENCES
ADACHI, T. & NAKAJIMA, T. (1974) [Symptoms and clinical examinations
of the patients seriously injured by photochemical smog.] Rinsho
Kagaku (Clin. Chem.), 3:257-267 (in Japanese).
ALPERT, S. M. & LEWIS, T. T. (1971) Ozone tolerance studies utilizing
unilateral lung exposure. J. appl. Physiol., 31: 243-246.
ALPERT, S. M., SCHWARTZ, B. B., LEE, S. D., & LEWIS, T. R. (1971a)
Alveolar protein accumulation: a sensitive indicator of low level
oxidant toxicity. Arch. intern. Med., 128: 69-73.
ALPERT, S. M., GARDNER, D. E., HURST, D. J., LEWIS, T. R., & COFFIN,
D. L. (1971b) Effects of exposure to ozone on defensive
mechanisms of the lung. J. appl. Physiol., 31: 247-252.
AMERICAN CONFERENCE OF GOVERNMENT INDUSTRIAL HYGIENISTS (1975)
Threshold limit values for chemical substances in workroom air
adopted by ACGIH for 1975, Cincinnati, ACGIH.
ATWAL, O. S., & WILSON, T. (1974) Parathyroid gland changes following
ozone inhalation. Arch. environ. Health, 28: 91-100.
ATWAL, O. S., SAMAGH, B. S., & BHATNAGAR, M. K. (1975) A possible
autoimmune parathyroiditis following ozone inhalation: IIA
histopathologic, ultrastructural, and immunofluorescent study.
Am. J. Pathol., 80: 53-68.
BALCHUM, O. J. (1973) Toxicological effects of ozone, oxidant and
hydrocarbons. Proceedings of the Conference on Health Effects of
Air Pollution, Assembly of Life Sciences, National Academy of
Sciences-National Research Council, Washington, DC, Oct. 3-5. In:
Committee Print, Committee on Public Works, United States
Senate, Washington, DC, US Government Printing Office, pp.
489-503.
BALL, D. J. (1976) Photochemical ozone in the atmosphere of Greater
London. Nature (Lond.), 263: 580-582.
BARSKY, R. M. & BIRAKOS, J. N., ed. (1971) Profile of air pollution
control, Los Angeles, CA, County of Los Angeles Air Pollution
Control District, p. 73.
BARTH, D. S., ROMANOVSKY, J. C., KNELSON, J. H., ALTSHULLER, A. P., &
HORTON, R. J. M. (1971) Discussion (of national ambient air
quality standards). J. Air Pollut. Control Assoc., 21: 544-548.
BARTLETT, D. JR, FAULKNER, C. S., & COOK, K. (1975) Effect of chronic
ozone exposure on lung elasticity in young rats. J. appl.
Physiol., 37: 92-96.
BATES, D. V. & HAZUCHA, M. (1973) The short-term effects of ozone on
the human lung. Proceedings of the Conference on the Health
Effects of Air Pollution, Assembly of Life Sciences, National
Academy of Sciences-National Research Council, Wasington, DC,
Oct. 3-5. In: Committee Print, Committee on Public Works, United
States Senate, Washington DC, US Government Printing Office,
pp. 507-540.
BATES, D. V., BELL, G. M., BURNHAM, C. D., HAZUCHA, M., MANTHA, J.,
PENGELLY, L. D., & SILVERMAN, F. (1972) Short-term effects of
ozone on the lung. J. appl. Physiol., 32: 176-181.
BECKER, K. H. & SCHURATH, U. (1975) [The influence of oxides of
nitrogen on atmospheric oxidation processes.] Staub-Reinhall,
Luft, 35(4): 156-161 (in German).
BENNETT, G. (1962) Ozone contamination of high altitude aircraft
cabins. Aerospace Med., 33: 969-973.
BERRY, C. R. (1964) Differences in concentration of surface oxidant
between valley and mountain top conditions in the southern
Appalachians. J. Air Pollut. Control Assoc., 14(6): 238-239.
BILS, R. F. (1966) Ultrastructural alterations of alveolar tissue of
mice: I. Due to heavy Los Angeles smog. Arch. environ. Health,
12: 689-697.
BILS, R. F. (1970) Ultrastructural alterations of alveolar tissue of
mice: III. Ozone. Arch. environ. Health, 20: 468-480.
BILS, R. F. & ROMANOVSKY, J. C. (1967) Ultrastructural alterations of
alveolar tissue of mice: II. Synthetic photochemical smog. Arch
environ. Health, 14: 844-858.
BLOCH, W. N. JR, MILLER, F. J., & LEWIS, T. R. (1971) Pulmonary
hypertension in dogs exposed to ozone. Cincinnati, OH, Air
Pollution Control Office, US Environmental Protection Agency.
BOATMAN, E. S., SATO, S., & FRANK, R. (1974) Acute effects of ozone on
cat lungs. II: Structural. Am. Rev. respir. Dis., 110: 157-169.
BOBB, G. A. & FAIRCHILD, E. J. (1967) Neutrophil-to-lymphocyte ratio
as indicator of O3 exposure. Toxicol. appl. Pharmacol.,
11: 558-564.
BOOTH, S., DEGROOT, I., MARKUSH, R., & HORTON, R. J. M. (1965)
Detection of asthma epidemics in seven cities. Arch. environ.
Health, 10: 152-155.
BRANT, J. W. A. (1965) Human cardiovascular diseases and atmospheric
air pollution in Los Angeles, California. Int. J. Air Water
Pollut., 9: 219-231.
BRANT, J. W. A. & HILL, S. R. G. (1964) Human respiratory diseases and
atmospheric air pollution in Los Angeles, California. Int. J.
Air Water Pollut., 8: 259-277.
BRINKMAN, R., LAMBERTS, H. B., & VENINGA, T. S. (1964) Radiomimetic
toxicity of ozonized air. Lancet, 1: 133-136.
BUELL, G. C., TOKIWA, Y., & MUELLER, P. K. (1965) Potential
crosslinking agents in lung tissue; formation and isolation after
in vivo exposure to ozone. Arch. environ. Health,
10: 213-219.
BUELL, P., DUNN, J. E., JR, & BRESLOW, L. (1967) Cancer of the lung
and Los Angeles type air pollution: prospective study. Cancer,
20: 2139-2147.
BUTCHER, S.S. & RUFF, R. E. (1971) Effect of inlet residence time on
analysis of atmospheric nitrogen oxides and ozone. Anal. Chem.,
43(13): 1890-1892.
BYERS, D. H. & SALTZMAN, B. E. (1958) Determination of ozone in air by
neutral and alkaline iodide procedures. J. Am. Ind. Hyg. Assoc.,
19: 251-257.
CALIFORNIA STATE DEPARTMENT OF PUBLIC HEALTH (1955) Clean air for
California. Initial Report of the Air Pollution Study Project,
March 1955, 37 pp.
CALIFORNIA STATE DEPARTMENT OF PUBLIC HEALTH (1956) Clean air for
California. Second Report, March 1956, 10 pp.
CALVERT, J. G. (1976) Hydrocarbon involvement in photochemical smog
formation in Los Angeles atmosphere. Environ. Sci. Technol.,
10(3): 256-262.
CARSON, S. & GOLDHAMER, R. E. (1968) Biochemical defense mechanisms
against pulmonary irritants. Ohio, USA, Aerospace Medical
Research Laboratories, Aerospace Medical Division, Air Force
Systems Command, Wright-Patterson Air Force Base, pp. 129.
CASTLEMAN, W. L., DUNGWORTH, D. L., & TYLER, W. S. (1973a)
Histochemically detected enzymatic alterations in rat lung
exposed to ozone. Exp. mol. Pathol., 19: 402-421.
CASTLEMAN, W. L., DUNGWORTH, D. L., & TYLER, W. S. (1973b)
Cytochemically detected alterations of lung acid phosphatase
reactivity following ozone exposure. Lab. Invest., 29: 310-319.
CHALLEN, P. J. R., HICKISH, D. E., & BEDFORD, J. (1958) An
investigation of some health hazards in an inert-gas tungsten-arc
welding shop. Br. J. ind. ed., 15: 276-282.
CHOW, C. K. & TAPPEL, A. L. (1972) An enzyme protective mechanism
against lipid peroxidation damage to lungs of ozone-exposed rats.
Lipids, 7: 518-524.
CHOW, C. K. & TAPPEL, A. L. (1973) Activites of pentose shunt and
glycolytic enzymes in lungs of ozone-exposed rats. Arch.
environ. Health, 26: 205-208.
CHOW, C. K., DILLARD, C. J., & TAPPEL, A. L. (1974) Glutathione
peroxidase system and lysozyme in rats exposed to ozone and
nitrogen dioxide. Environ. Res., 7: 311-319.
CHRISTENSEN, E. & GIESE, A. C. (1954) Changes in absorption spectra of
nucleic acids and their derivatives following exposure to ozone
and ultraviolet radiation. Arch. Biochem. Biophys., 51:
208-216.
COFFIN, D. L. & BLOMMER, E. J. (1965) The influence of cold on
mortality from streptococci following ozone exposure. J. Air
Pollut. Control Assoc., 19: 523-524.
COFFIN, D. L. & BLOMMER, E. J. (1967) Acute toxicity of irradiated
auto exhaust. Its indication by enhancement of mortality from
streptococcal pneumonia. Arch. environ. Health, 15: 36-38.
COFFIN, D. L. & BLOMMER, E. J. (1970) Alteration of the pathogenic
role of streptococci group C in mice conferred by previous
exposure to ozone. In: Silver, I. H., ed. Aerobiology,
Proceedings of the 3rd International Symposium on Aerobiology,
University of Sussex, England, 1969. New York, Academic Press,
pp. 54-61.
COFFIN, D. L. & GARDNER, D. E. (1972a) Interaction of biological
agents and chemical air pollutants. Ann. occup. Hyg.,
15: 219-234.
COFFIN, D. L. & GARDNER, D. E. (1972b) The role of tolerance in
protection of the lung against secondary insults. In:
Proceedings of the International Conference of Occupational
Physicians of the Chemical Industry, Ludwigshafen, Germany,
April 1972, Research Triangle Park, NC, Environmental
Protection Agency, pp. 344-364.
COFFIN, D. L. & GARDNER, D. E. (1975) [Ozone Toxicology: Correlation
between tolerance and protective mechanisms of the lung.] Gig. i
Sanit., 1: 86-92 (in Russian).
COFFIN, D. L., BLOMMER, E. J., GARDNER, D. E., & HOLZMAN, R. S. (1968)
Effect of air pollution on alteration of susceptibility to
pulmonary infection. In: Proceedings of the 3rd Annual
Conference on Atmospheric Contamination in Confined Spaces,
Aerospace Medical Research Laboratory, pp. 71-80.
COFFIN, D. L., GARDNER, D. E., HOLZMAN R. S., & WOLOCK, F. J. (1968)
Influence of ozone on pulmonary cells. Arch. environ. Health,
16: 633-636.
COHEN, C. A., HUDSON, A. R., CLAUSEN, J. L., & KNELSON, J. H. (1972)
Respiratory symptoms, spirometry, and oxidant air pollution in
non-smoking adults. Am. Rev. resp. Dis., 105: 251-261.
CORTESI, R. & PRIVETT, O. S. (1972) Toxicity of fatty ozonides and
peroxides. Lipids, 7: 715-721.
COX, R. A., EGGLETON, A. E. J., DERWENT, R. G., LOVELOCK, J. E., &
PACK, D. H. (1975) Long-range transport of photochemical ozone in
North-Western Europe. Nature (Lond.), 255(5504): 118-121.
CRIEGEE, R. (1957) The course of ozonization of unsaturated compounds.
Rec. chem. Prog, 18: 111-120.
CRONIN, S. R. & GIRI, S. N. (1974) Effects of pulmonary irritants on
DNA, ATPase activity, and histamine on rat lung. Proc. Soc. Exp.
Biol. Med., 146: 120-125.
DARNALL, K. R., LLOYD, A. C., WINER, A. C., & PITTS, J. N., JR (1976)
Reactivity scale for atmospheric hydrocarbons based on reaction
with hydroxyl radical. Environ. sci. Technol. 10(7): 692-296.
DAVIS, I. (1961) Microbiologic studies with ozone. Mutagenesis of
ozone for Escherichia coli, Texas, USAF Aerospace Medical
Center, School of Aerospace Medicine, 9 pp. (Rep. No. 61-60).
DEANE, M., GOLDSMITH, J. R., & TUMA, D. (1965) Respiratory conditions
in outside workers: report on outside plant telephone workers in
San Francisco and Los Angeles. Arch. environ. Health,
10: 323-331.
DELUCIA, A. J., HOQUE, P.M., MUSTAFA, M. G., & CROSS, C. E. (1972)
Ozone interaction with rodent lung: effect on sulfhydryls and
sulfhydryl-containing enzyme activities. J. lab. clin. Med.,
80: 559-566.
DELUCIA, A. J., MUSTAFA, M. G., HUSSAIN, M. Z., & CROSS, C. E. (1975)
Ozone interaction with rodent lung: III. Oxidation of reduced
glutathione and formation of mixed disulfides between protein and
non-protein sulfhydrils. J. clin. Invest., 55: 794-802.
DEMERJIAN, K. L., KERR, J. A., & CALVERT, J. G. (1974) The mechanism
of photochemical smog formation. Adv. environ. Sci. Technol.,
4: 1-262.
DEMORE, W. B. & PATAPOFF, M. (1976) Comparison of ozone determinations
by ultraviolet photometry and gas-phase titoation. Environ. Sci.
Technol., 10(9): 897-899.
DERWENT, R. G. & STEWART, H. N. M (1973) Elevated ozone levels in the
air of Central London. Nature (Lond.), 241(5388): 342-343.
DILLARD, C. J., URRIBARRI, N., REDDY, K., FLETCHER, B., TAYLOR, S., DE
LUMEN, B., LANGBERG, S., & TAPPEL, A. L. (1972) Increased
lysosomal enzymes in lungs of ozone-exposed rats. Arch. environ.
Health, 25: 426-431.
DIMITRIADES, B. (1976) Photochemical oxidants in the ambient air of
the United States. Research Triangle Park, NC, US Environmental
Protection Agency, 192 pp. (EPA-600/3-76-017).
DIXON, J. R. & MOUNTAIN, J. T. (1965) Role of histamine and related
substances in development of tolerance to edemagenic gases.
Toxicol. appl. Pharmacol., 7: 756-766.
DOWELL, A. R., LOHRBAUER, L. A., HURST, D., & LEE, S. D. (1970) Rabbit
alveolar macrophage damage caused by in vivo O3 inhalation.
Arch. environ. Health, 21: 121-127.
DUNGWORTH, D. L. (1976) Short-term effects of ozone on lungs of rats,
mice, and monkeys. Environ. Health Perspect., 16: 179.
DUNGWORTH, D. L., CASTLEMAN, W. L., CHOW, C. K., MELLICK, P. W.,
MUSTAFA, M. G., TARKINGTON, B., & TYLER, W. S. (1975) Effect of
ambient levels of ozone on monkeys. Fed. Proc., 34: 1670-1674.
DURHAM, W. (1974) Air pollution and student health. Arch. environ.
Health, 28: 241-254.
EASTON, R. E. & MURPHY, S. D. (1967) Experimental ozone preexposure
and histamine. Arch. environ. Health, 15: 160-166.
EGLITE, M. (1968)[The problem of hygienic assessment of atmospheric
ozone.] Gig. i Sanit., 33: 17-22 (in Russian).
EMIK, L. O., PLATA, R. L., CAMPBELL, K. I., & CLARKE, G. L. (1971)
Biological effects of urban air pollution. Arch. environ.
Health, 23: 335-342.
ENVIRONMENT AGENCY (1975a) [Proceedings of the Second US Japan
Conference on Photochemical Air Pollution.] Tokyo, Japan (in
Japanese).
ENVIRONMENT AGENCY (1975b) [Air Pollution in Japan. Air monitoring
data in fiscal year 1974.] Tokyo, Japan, Air Pollution Control
Division, Air Quality Bureau, pp. 1200-1240 (in Japanese).
ENVIRONMENT AGENCY (1976) [Quality of the environment in Japan.]
Tokyo, Japan, Environment Agency, pp. 152-153 (in Japanese).
ERLICH, R., FINDLAY, J. C., FENTERS, J. D., & GARDNER, D. E. (1977)
Health effects of short-term inhalation of nitrogen dioxide and
ozone mixtures. Environ. Res., 14: 223-231.
EVANS, M. J., MAYR, W., BILS, R. F., & LOOSLI, C. G. (1971) Effects of
ozone on cell renewal in pulmonary alveoli of ageing mice. Arch.
environ. Health, 22: 450-453.
FABIAN, P. & PRUCHNIEWlCZ, P. G. (1973) Meridional distribution of
tropospheric ozone from ground-based registrations between Norway
and South Africa. Pure appl. Geophys., 106-108 (V-VII):
1027-1035.
FAIRCHILD, E. J., II (1963) Neurohumoral factors in injury from
inhaled irritants. Arch. environ. Health, 6: 79-86.
FAIRCHILD, E. J., II (1967) Tolerance mechanisms. Determinants of lung
responses to injurious agents. Arch. environ. Health,
14: 111-125.
FAIRCHILD, E. J., II & BOBB, G. A. (1965) The effect of spinal cord
lesions on the pathophysiology of a lung irritant ozone.
Toxicol. appl. Pharmacol., 7: 483 (Abstract).
FARWELL, S. O. & RASMUSSEN, R. A. (1976) Limitations of the FPD and
ECD in atmospheric analysis: A review. J. Chromatogr. Sci.,
14: 224-234.
FEDERAL REPUBLIC OF GERMANY, Ministry of Internal Affairs (1974)
Nox CnH volatile fluoride and chlorine compounds.
FETNER, R. H. (1958) Chromosome breakage in Victa faba by ozone.
Nature (Lond.), 181: 504-505.
FETNER, R. H. (1962) Ozone-induced chromosome breakage in human cell
cultures. Nature (Lond.), 194: 793-794.
FETNER, R. H. (1963) Mitotic inhibition induced in grasshopper
neuroblasts by exposure to ozone. USAF School of Aerospace
Medicine (Technical Documentary Report SAM-TDR 63-39).
FLETCHER, B. L. & TAPPEL, A. L. (1973) Protective effects of dietary
alphatocopherol in rats exposed to toxic levels ozone and
nitrogen dioxide. Environ. Res., 6: 165-175.
FOLINSBEE, L. J., SILVERMAN, F., & SHEPHARD, R. J. (1975) Exercise
responses following ozone exposure. J. appl. Physiol.,
38: 996-1001.
FOURNIER, E. (1973) Radicaux libres et toxicologie. Eur. J. Toxicol.,
6: 109-122.
FRANK, H. R., BRAIN, J. D., & SHERRY, D. E. (1970a) Unilateral
tolerance and altered elastic behaviour in rabbits. Am. Ind.
Hyg. Assoc. J., 31: 31 (Abstract section).
FRANK, N. R., YOKOYAMA, E., WARANABE, S., SHERRY, D. E., & BRAIN, J.
D. (1970b) An ozone Journal presented at the American Medical
Association, Air Pollution Medical Research Conference New
Orleans, 5-7 Oct., 19 pp.
FRANK, R., FLESCH, J.P., & BRAIN, J. D. (1971) Effect of ozone on
elastic behaviour of excised lungs of dogs. Environ. Res.,
4: 343-354.
FREEMAN, G., STEPHENS, R. J., CRANE, S.C., & FURIOSI, N.J. (1968)
Lesion of the lung in rats continuously exposed to two parts per
million of nitrogen dioxide. Arch. environ. Health,
17: 181-192.
FREEMAN, G., STEPHENS, R. J., COFFIN, D. L., & STARA, J. F. (1973)
Changes in dogs' lungs after long-term exposure to ozone. Arch.
environ. Health, 26: 209-216.
FREEMAN, G., JULIOS, L. T., FURIOSI, N. J., MUSSENDEN, R., STEPHENS,
R. J., & EVANS, M. J. (1974) Pathology of pulmonary disease from
exposure to interdependent ambient gases (NO2 & O3). Arch.
environ. Health, 29: 203-210.
FRIBERG, L., HOLMA, B., & RYLANDER, R. (1972) Animal lung reactions
after inhalation of lead and ozone. Environ. Physiol. Biochem.,
2: 170-178.
FUKASE, O., ISOMURA, K., & WATANABE, H. (1975a) Effect of ozone on
glutathione in vivo. J. Jpn. Soc. Air Pollut., 10: 58-62 (in
Japanese).
FUKASE, O., ISOMURA, K., & WATANABE, H. (1975b) Effect of ozone on
vitamin C in vivo. J. Jpn. Soc. Air Pollut., 10: 63-66.
FUJII, T. (1972) Studies on air pollution by photochemical reaction.
1. The results of field research of photochemical smog in Osaka,
1971. In: Proceedings of the Research Section, Osaka
Environmental Pollution Control Centre, No. 3, pp. 21-34 (in
Japanese).
GARDNER, D. E. (1971) Environmental influences on living alveolar
macrophages. A dissertation submitted to the Division of
Graduate Studies of the University of Cincinnati, Ann Arbor,
Michigan, University Microfilms International, pp. 191.
GARDNER, D. E., PFITZER, E. A., CHRISTIAN, R. T., & COFFIN, D. L.
(1971) Loss of protective factor for alveolar macrophages when
exposed to ozone. Arch. intern. Med., 127: 1078-1084.
GARDNER, D. E., LEWIS, T. R., ALPERT, S. M., HURST, D. J., & COFFIN,
D. L. (1972) The role of tolerance in pulmonary defense
mechanisms. Arch. environ. Health, 25: 432-438.
GARDNER, D. E., ILLING, J. W., MILLER, F. J., & COFFIN, D. L. (1974a)
The effect of ozone on pentobarbital sleeping time in mice.
Chem. Pathol. Pharmacol., 9: 689-700.
GARDNER, D. E., ILLING, J. W., & COFFIN, D. L. (1974b) Enhancement of
effect of exposure to O3 and NO2 by exercise. Toxicol. appl.
Pharmacol., 29: 129-130.
GARDNER, M. B. (1966) Biological effects of urban air pollution. III.
Lung tumors in mice. Arch. environ. Health, 12: 305-513.
GARDNER, M. B., LOOSLI, C. G., HAINES, B., BLACKMORE, W., & TEEBKEN,
D. (1969) Histopathologic findings in rats exposed to ambient and
filtered air. Arch. environ. Health, 19: 637-647.
GOLDSMITH, J. R. & NADEL, J. A. (1969) Experimental exposure of human
subjects to ozone. J. Air Pollut. Control Assoc., 19: 329-330.
GOLDSTEIN B. D. (1973) Hydrogen peroxide in erythrocytes. Detection in
rats and mice inhaling ozone. Arch. environ. Health,
26: 279-280.
GOLDSTEIN B. D. (1976) Combined exposure to ozone and nitrogen
dioxide. Env. Health Perspect., 13: 107-110.
GOLDSTEIN B. D., PEARSON, B., LODI, C., BUCKLEY, R. D., & BALCHUM, O.
J. (1968) The effect of ozone on mouse blood in vivo. Arch.
environ. Health, 16: 648-650.
GOLDSTEIN B. D., LODI, C., COLLINSON, C., & BALCHUM, O. J. (1969)
Ozone and lipid peroxidation. Arch. environ. Health,
18: 631-635.
GOLDSTEIN B. D., BUCKLEY, R. D., CARDENAS, R., & BALCHUM, O. J. (1970)
Ozone and vitamin E. Science, 169: 605.
GOLDSTEIN B. D., LAI, L. Y., ROSS, S. R., & CUZZI-SPADA, R. (1973)
Susceptibility of inbred mouse strains to ozone. Arch. environ.
Health, 27: 412-413.
GOLDSTEIN B. D., SOLOMON, S., PASTERNACK, B. S., & BICKERS, D. R.
(1975) Decrease in rabbit lung microsomal cytochrome P-450 levels
following ozone exposure. Chem. Pathol. Pharmacol.,
10: 759-762.
GOLDSTEIN, E., TYLER, W. S., HOEPRICH, P. D., & EAGLE, C. (1971a)
Ozone and the antibacterial defense mechanisms of the murine
lung. Arch. intern. Med., 127: 1099-1102.
GOLDSTEIN, E., TYLER, W. S., HOEPRICH, P. D., & EAGLE, C. (197lb)
Adverse influence of ozone on pulmonary bactericidal activity of
murine lung. Nature (Lond.), 229: 262-263.
GOLDSTEIN, E., EAGLE, M. C., & HOEPRICH, P. D. (1972) Influence of
ozone on pulmonary defense mechanisms of silicotic mice. Arch.
environ. Health, 24: 444-448.
GOLDSTEIN, E., WARSHAUER, D., LIPPERT, W., & TARKINGTON, B. (1974)
Ozone and nitrogen dioxide exposure. Arch. environ. Health,
28: 85-90.
GREGORY, A. R., RIPPERTON, L. A., & MILLER, B. (1967) Effect of
neonatal thymectomy on the development of ozone tolerance in
mice. Am. Ind. Hyg. Assoc. J., 28: 278-282.
GRIMSRUD, E. P., WESTBERG, H. H., RASMUSSEN, R. A. (1975) Atmospheric
reactivity of monoterpene hydrocarbons, NOx photooxidation and
ozonolysis. Int. J. Chem. Kin. Symp., 1: 183-195.
GUICHERIT, R. (1975) [Photochemical smog formation in the
Netherlands.] Delft, Netherlands, TNO Institute for
Environmental Hygiene, p. 27 (in Dutch).
HAAGEN-SMIT, A. J. (1952) Chemistry and physiology of Los Angeles
smog. Ind. Eng. Chem., 44: 1342-1346.
HACKNEY, J. D., LINN, W. S., LAW, D.C., KARUZA, S. K., GREENBERG, H.,
BUCKLEY, R. D., & PEDERSEN, E. E. (1975) Experimental studies on
human health effects of air pollutants. III. Two-hour exposure to
ozone alone and in combination with other pollutant gases. Arch.
environ. Health, 30: 385-390.
HACKNEY, J. D., LINN, W. S., KARUZA, S. K., BUCKLEY, R. D., LAW, D.C.,
BATES, D. V., HAZUCHA, M., PENGELLY, L. D., & SILVERMAN, F.
(1977) Effects of ozone exposure in Canadians and Southern
Californians. Arch. environ. Health, 34: 110-116.
HALLET, W. Y. (1965) Effect of ozone and cigarette smoke on lung
function. Arch. environ. Health, 10: 295-302.
HAMMER, D. I., HASSELBLAD, V., PORTNOY, B., & WEHRLE, P. F. (1974) Los
Angeles student nurse study: Daily symptom reporting and
photochemical oxidants. Arch. environ. Health., 28: 255-260.
HASSELBLAD, V., CREASON, J.P., & NELSON, W. C. (1976) Regression
using "hockey stick" functions. Research-Triangle Park, NC, US
Environmental Protection Agency, Health Effects Research
Laboratory, 12 pp. (EPA-600/1-76-024).
HATTORI, K., KATO, N., KINOSHITA, S., & SUNADA, T. (1963) Protective
effect of ozone in mice against whole-body x-irradiation. Nature
(Lond.), 198: 1220.
HAUSKNECHT, R. (1960) Air pollution effects reported by California
residents from the California Health Survey, CA, USA, State of
California, Department of Public Health, pp. 1-55.
HAZUCHA, M., SILVERMAN, F., PARENT, C., FIELD, S., & BATES, D. V.
(1973) Pulmonary function in man after short-term exposure to
ozone. Arch. environ. Health, 27: 183-188.
HECHTER, H. H. & GOLDSMITH, J. R. (1961) Air pollution and daily
mortality. Am. J. med. Sci., 241: 581-588.
HENSCHLER, D. (1960) [Protective effect of pre-treatment with low gas
concentrations against lethal pulmonary edema caused by irritant
gas.] Arch. exp. Pathol. Pharmacol., 238: 66-67 (in German).
HENSCHLER, D., STIEN, A., BECK, H., & NEUMAN, W. (1960) [Olfactory
threshold of some important irritant gases and manifestations in
man by low concentrations.] Arch. Gewerbepathol. Gewerbehyg.,
17: 547-570 (in German).
HENSCHLER, D., HAHN, E., & ASSMANN, W. (1964) [Conditions for
increasing resistance by repeated inhalation of irritating
oedema- forming gases.] Naunyn-Schmiedebergs Arch. exp. Pathol.
Pharmakol., 249: 325-342 (in German).
HEUSS, J. M. & GLASSON, W. A. (1968) Hydrocarbon reactivity and eye
irritation. Environ. Sci. Technol., 3: 1109-1116.
HODGESON, J. A. (1972) Review of analytical methods for atmospheric
oxidants measurements. Int. J. environ. anal. Chem.,
2: 113-132.
HOLLAND, G. J., BENSON, D., BUSH, A., RICH, G. Q., & HOLLAND, R. P.
(1968) Air pollution simulation and human performance. Am. J.
public Health, 58: 1684-1691.
HOLZMAN, R. S., GARDNER, D. E., & COFFIN, D. L. (1968) In vivo
inactivation of lysosyme by ozone. J. Bacteriol.,
96: 1562-1566.
HUBER, G. L., MASON, R. J., LAFORCE, M., SPENCER, N.J., GARDNER, D.
E., & COFFIN, D. L. (1971) Alteration in the lung following the
administration of ozone. Arch. intern. Med., 128: 81-87.
HUETER, F. G., CONTNER, G. L., BUSCH, K. A., & HINNERS, R. G. (1966)
Biological effects of atmospheres contaminated by auto exhaust.
Arch. environ. Health, 12: 553-560.
HURST, D. J. & COFFIN, D. L. (1971) Ozone effect on lysosomal
hydrolases of alveolar macrophages in vitro. Arch. intern. Med.,
127: 1059-1063.
HURST, D. J., GARDNER, D. E., & COFFIN, D. L. (1970) Effect of O3 on
acid hydrolases of the pulmonary alveolar macrophage.
J. Reticuloendoth. Soc., 8: 288-300.
IKEMATSU, T., YAMAUCHI, Y., SAITO, H., NAGAOKA, S., & ENDO, R. (1976)
Tonsils and environmental pollution (second report) Influence of
ozone upon rabbit tonsils. Jpn. J. Tonsil., 15: 5-8.
ILO (1977) Occupational exposure limits for airborne toxic
substances. Geneva, International Labour Office, pp. 164-165
(Occupational Safety and Health Series No. 37).
ISOMURA, K., FUKASE, O., & WATANABE, H. (1976) [Cytoxicities and
mutagenicities of gaseous air pollutants.] J. Jpn. Soc. Air
Pollut., 11: 59-64 (in Japanese).
JAFFE, L. S. (1962) The biological effects of ozone on man and
animals. Am. J. public Health, 57: 1269-1277.
JAPAN PUBLIC HEALTH ASSOCIATION (1976) The relationship between
photochemical airpollution and adverse health effects-1975
Report, Tokyo, JPHA, pp. 24-27 (in Japanese).
JEGIER, Z. & P'AN, A. Y. S. (1973) Ozone as an air pollutant. Can. J.
public Health, 64: 161-166.
KAGAWA, J. & TOYAMA, T. (1975a) Effects of ozone and brief exercise on
specific airway conductance in man. Arch. environ. Health,
30: 36-39.
KAGAWA, J. & TOYAMA, T. (1975b) Photochemical air pollution; its
effects on respiratory function of elementary school children.
Arch. environ. Health, 30: 117-122.
KAGAWA, J., TOYAMA, T., & NAKAZA, M. (1976) Pulmonary function test in
children exposed to air pollution. In: Finkel, A. J. & Duel, W.
C., ed. Clinical implications of air pollution research. Acton,
MA, USA, Publishing Sciences Group Inc., pp. 305-320.
KERR, H. D., KULLE, T. J., MCILHANEY, M. L., & SWIDERSKY, P. (1975)
Effects of ozone on pulmonary function in normal subjects. An
environmental-chamber study. Am. Rev. resp. Dis., 111: 763-773.
KING, M. E. (1961) Biochemical effects of ozone, doctoral
dissertation, Illinois Institute of Technology, May 1961, pp.
1-85.
KLEINFELD, M., GIEL, C., & TABERSHAW, I. R. (1957) Health hazards
associated with inert-gas shielded metal arc welding. Arch. ind.
Health, 15: 27-31.
KONIGSBERG, A. S. & BACHMAN, C. H. (1970) Ozonized atmosphere and
gross motor activity of rats. Int. J. Biometeor, 14: 261-266.
KONNO, S. & OKITA, T. (1974) Determination of peroxacetyl (PAN)
produced in the atmosphere or in a photochemical reaction chamber
(1). Bull. Inst. Public Health, Japan, 23: 50-58.
KOTIN, P. & FALK, H. L. (1956) The experimental induction of pulmonary
tumors in strain-A mice after their exposure to an atmosphere of
ozonized gasoline. Cancer, 9: 910-917.
KOTIN, P., FALK, H. L., & MCCAMMON, C. J. (1958) III. The experimental
induction of pulmonary tumors and changes in the respiratory
epithelium in C 57 BL mice following their exposure to an
atmosphere of ozonized gasoline. Cancer, 11: 473-481.
KUDRJAVCEVA, O. F. (1963) [On the possibility of chronic effects of
ozone in working conditions.] Gig. truda i Prof. Zabol., 6: 52
(in Russian).
KULLE, T. J. (1972) The effects of ozone and formaldehyde on the
trigeminal nasal sensory system. Thesis, University of
Cincinnati.
KUSOMOTO, S., NARUYAMA, Y., YONEKAWA, E., ODA, H., & NAKAJIMA, T.
(1976) Acute effects of photochemical oxidants and ozone exposure
on mice. J. Jpn. Soc. Air Pollut., 11: 70-80 (in Japanese).
KYEI-ABOAGYE, K., HAZUCHA, M., WYSZOGRODSKI, I., RUBINSTEIN, D., &
AVERY, M. E. (1973) The effect of ozone exposure in vivo on the
appearance of lung tissue lipids in the endobronchial lavage of
rabbits. Biochem. Biophys. Res. Commun., 54: 907-913.
LAGERWERFF, J. M. (1963) Prolonged ozone inhalation and its effects on
visual parameters. Aerospace Med., 34: 479-486.
LARSEN, R. I. (1969) A new mathematical model of air pollution
concentration averaging time and frequency. J. Air Pollut.
Control Assoc., 19: 24-30.
LARSEN, R. I. (1974) An air quality data analysis system for
interrelating effects, standards, and needed source
reductions-part 2. J. Air Pollut. Control Assoc., 24(6):
551-558.
LEWIS, T. R., HUETER, F. G., & BUSCH, K. A. (1967) Irradiated
automobile exhaust: its effects on reproduction in mice. Arch.
environ. Health, 15: 26-35.
LITT, R. S., VAUGHAN, S., BIRKINSHAW, P., COIT, H., & SANDERS, B.
(1968) Effects of low concentrations of ozone on temporal
discrimination. In: Air pollution project: An educational
experiment in self-directed research, 1968, Pasadena,
Associated Students of the California Institute of Technology,
pp. 51-64.
LOOSLI, C. G., BUCKLEY, R. D., HERTWECK, M. S., HARDY, J. D., RYAN, D.
P., STINSON, S., & SEREBRIN, R. (1972) Pulmonary response of mice
exposed to synthetic smog. Ann. oecup. Hyg., 15: 251-260.
MACLEAN, S. A., LONOWELL, A. C., & BLOGOSLAWSKI, W. J. (1973) Effects
of ozone-treated sea water on the spawned, fertilized, meiotic,
and cleaving eggs of the commercial American oyster. Mutat.
Res., 21: 283-285.
MCJILTON, C., THIELKE, J., & FRANK, R. (1972) Ozone uptake model for
the respiratory system. Am. Ind. Hyg. Assoc. J., 33: 20.
MCMILLAN, R. S., WISEMAN, D. H., HANES, B., & WEHRLE, P. F. (1969)
Effects of oxidant air pollution on peak expiratory flow rates in
Los Angeles school children. Arch. environ. Health, 18:
941-949.
MAHONEY, L. E. J. (1971) Wind flow and respiratory mortality in Los
Angeles. Arch. environ. Health, 22: 344-347.
MATSUMURA, Y. (1970a) The effects of ozone, nitrogen dioxide, and
sulfur dioxide on experimentally induced allergic respiratory
disorder in guinea pigs. I. The effect on sensitization with
albumin through the airway. Am. Rev. respir. Dis.,
102: 430-437.
MATSUMURA, Y. (1970b) The effects of ozone, nitrogen dioxide and
sulfur dioxide on experimentally induced allergic respiratory
disorder in guinea pigs. II. The effects of ozone on the
absorption and the retention of antigen in the lung. Am. Rev.
respir. Dis., 102: 438-443.
MATSUMURA, Y., MIZUNO, K., MIYAMOTO, T., SUZUKI, T., & OSHIMA, Y.
(1972) The effects of ozone, nitrogen dioxide, and sulfur dioxide
on experimentally induced allergic respiratory disorder in guinea
pigs, IV effects on respiratory sensitivity to inhaled
acetylcholine. Am. Rev. respir. Dis., 105: 262-267.
MATZEN, R. N. (1957) Development of tolerance to ozone in reference to
pulmonary edema. Am. J. Physiol., 190: 84-88.
MENDENHALL, R. M. & STOKINGER, H. E. (1959) Tolerance and
cross-tolerance development to atmospheric pollutants, ketene and
ozone. J. appl. Physiol., 14: 923-926.
MENDENHALL, R. M. & STOKINGER, H. E. (1962) Films from lung washings
as a mechanism model for lung injury by ozone. J. appl.
Physiol., 17: 28-32.
MENZEL, D. B. (1970) Toxicity of ozone, oxygen and radiation. Ann.
Rev. Pharmacol., 10: 379-394.
MENZEL, D. B. (1971) Oxidation of biologically active reducing
substances by ozone. Arch. environ. Health, 23: 149-153.
MENZEL, D. B., ROEHM, J. N., & LEE, S. D. (1972) Vitamin E: the
biological and environmental antioxidant. J. agric. food Chem.,
20: 481-486.
MENZEL, D. B., SLAUGHTER, R. J., BRYANT, A.M., & JAUREGUI, H. O.
(1975) Heinz bodies formed in erythrocytes by fatty acid ozonides
and ozone. Arch. environ. Health, 30: 296-301.
MERZ, T., BENDER, M. A., KERR, H. D., & KULLE, T. J. (1975)
Observations of aberrations in chromosomes of lymphocytes from
human subjects exposed to ozone at a concentration of 0.5 ppm for
6 and 10 hours. Mutat. Res., 31: 299-302.
MIKAMI, R. & KUDO (1973) Air pollution -- especially in terms of
oxidants and respiratory organs. Intern. Med. (Japan),
32: 837-844.
MILLER, F. J. (1977) A mathematical model of transport and removal of
ozone in mammalian lungs. Thesis submitted to the Graduate
Faculty of North Carolina State University, Raleigh, NC, USA, pp.
66.
MILLER, P. R., MCCUTCHAN, M. H., & MILLIGAN, H. P. (1972) Oxidant air
pollution in the Central Valley, Sierra Nevada foothills and
Mineral King Valley of California. Atmos. Environ., 6: 623-633.
MILLER, S. & EHRLICH, R. (1958) Susceptibility to respiratory
infections of animals exposed to ozone. J. infect. Dis.,
103: 145-149.
MILLS, C. A. (1957) Respiratory and cardiac deaths in Los Angeles
smogs. Am. J. med. Sci., 233: 379-386.
MIZOGOUCHI, I., OSAWA, M., SATO, Y., MAKINO, K., & YAGYU, H. (1973) A
study on erythrocyte and photochemical smog. I: Effects of air
pollutants on erythrocyte resistance. J. Jpn. Soc. Air Pollut.,
8: 414.
MOORMAN, W. J., CHMIEL, J. J., STARA, J. F., & LEWIS, T. R. (1973)
Comparative decomposition of ozone in the nasopharynx of beagles.
Acute vs chronic exposure. Arch. environ. Health, 26: 153-155.
MOTLEY, H. L., SMART, R. H., & LEFTWICH, C. I. (1959) Effect of
polluted Los Angeles air (smog) on lung volume measurements.
J. Am. Med. Assoc., 171: 1469-1477.
MOUNTAIN, J. T. (1963) Detecting hypersusceptibility to toxic
substances: an appraisal of simple blood tests. Arch. environ.
Health, 6: 357-365.
MOUNTAIN, J. T., WAGNER, W. D., FAIRCHILD, E. J., STOCKELL, F. R., JR,
& STOKINGER, H. E. (1960) Biochemical effects of ozone and
nitrogen dioxide on laboratory animals. In: 138th National
Meeting of The American Chemical Society, New York, September
1960, Cincinnati, OH, US Dept of Health, Education and Welfare,
32 pp.
MUDD, J. B., LEAVITT, R., ONGUN, A., & MCMANUS, T. T. (1969) Reaction
of ozone with amino acids and proteins. Atmos. Environ.,
3: 669-682.
MUELLER, P. X., LOEB, L., & MAPES, W. H. (1973) Decomposition rates of
ozone in living areas. Environ. Sci. Technol., 7: 342-346.
MURPHY, S. D., LENA, J. K., ULRICH, C. E., & DAVIS, H. V. (1963)
Effects on animals of exposure to auto exhaust. Arch. environ.
Health, 7: 60-70.
MURPHY, S. D., ULRICH, C. E., FRANKOWITZ, & XINTARAS, C. (1964a)
Altered function in animals inhaling low concentrations of ozone
and nitrogen dioxide. Am. Ind. Hyg. Assoc. J., 25: 246-253.
MURPHY, S. D., DAVIS, H. V., & ZARATZIAN (1964b) Biochemical effects
in rats from irritating air contaminants. Toxicol. appl.
Pharmacol., 6: 520-528.
MUSTAFA, M. G. (1975) Influence of dietary vitamin E on lung cellular
sensitivity to ozone in rats. Nutr. Rep. Int., 11: 473-476.
MUSTAFA, M. G., DELUCIA, A. J., YORK, G. K., ARTH, C., & CROSS, C. E.
(1973) Ozone interaction with rodent lung. II. Effects on oxygen
consumption of mitochondria. J. lab. clin. Med., 82: 357-365.
NAKAJIMA, T., KUSUMOTO, S., TSUBOTA, Y., YONEKAWA, E., YOSHIDA, R.,
MOTOMIYA, K., ITO, K., IDE, G., & OTSU, H. (1972)
Histopathological studies on the respiratory organs of mice
exposed to photochemical oxidants and auto exhaust. Proc. Osaka
Prefect. Inst. Public Health, 10: 35-42 (in Japanese).
NATIONAL ACADEMY OF SCIENCES (1977) Ozone and other photochemical
oxidants, Washington DC, Printing and Publishing Office,
National Academy of Sciences, 719 pp.
NEDERBRAGT, G. W., VAN DER HORST, N., & VAN DUIJN, J. (1965) Rapid
ozone determination near an accelerator. Nature (Lond.),
206: 4979.
NEVSKAJA, A. I. & DITERIHS, D. D. (1957) Problems of hygiene of labor
in H2O2 production by electrodynamic method. Gig. truda i prof
Zabol., 4: 16.
NEVSKAJA, A. I. & KOCETKOVA, T. A. (1961 ) The toxicology of ozone and
sulfuric acid aerosol in their combined action. Gig. truda i
prof Zabol., 1: 20-29.
NIEBOER, H. & VAN HAM, J. (1976) Peroxyacetyl nitrate (PAN) in
relation to ozone and some meteorological parameters at Delft in
the Netherlands. Atmos. Environ., 10: 115-120.
NIEDING, VON, G. WAGNER, H. M., LOLLGEN, H., & KREKELER, H. (1977)
[Acute effects of ozone on lung function in man. VDI Colloquium
on ozone and other substances in photochemical smog.]
VDI-Berichte, 270: 123-129 (in German).
NORTH ATLANTIC TREATY ORGANIZATION (1974) Air Quality criteria for
photochemical oxidants and related hydrocarbons. A report by the
Expert Panel on Air Quality Criteria, Brussels, Belgium, NATO
Committee on the Challenges of Modern Society, 366 pp.
OHMORI, K. (1974) [The effect of photochemical smog on humans,
especially biological effects by low concentrations of ozone.]
Environ. Pollut. Control. 10: 1042-1046 (in Japanese).
PACE, D. M., LANDOLT, P. A., & AFTONOMOS, B. T. (1969) Effects of
ozone on cells in vitro. Arch. environ. Health, 18: 165-170.
PALMER, M. S., SWANSON, D. H., & COFFIN, D. L. (1971) Effect of O3 on
benzpyrene hydroxylase activity in the Syrian golden hamster.
Cancer Res., 31: 730-733.
PALMER, M. S., EXLEY, R. W., & COFFIN, D. L. (1972) Influence of
pollutant gases on benzpyrene hydroxylase activity. Arch.
environ. Health, 25: 439-442.
P'AN, A. Y. S. & JEGIER, Z. (1970) The effect of sulfur dioxide and
ozone on acetylcholinesterase. Arch. environ. Health,
21: 498-501.
P'AN, A. Y. S. & JEGIER, Z. (1972) Trypsin protein esterase in
relation to ozone-induced vascular damage. Arch. environ.
Health, 24: 233-236.
P'AN, A. Y. S., BELAND, J., & JEGIER, Z. (1972) Ozone-induced arterial
lesions. Arch. environ. Health, 24: 229-232.
PEARLMAN, M. E., FINKLEA, J. F., SHY, C. M., VAN BRUGGEN, J., &
NEWILL, V. A. (1971) Chronic oxidant exposure and epidemic
influenza. Environ. Res., 4: 129-140.
PENHA, P. D., AMARAL, L., & WERTHAMER, S. (1972) Ozone air pollutants
and lung damage. Ind. Med., 41: 17-20.
PLOPPER, C. G., DUNGWORTH, D. L., & TYLER, W. S. (1973a) Pulmonary
lesions in rats exposed to ozone. Am. J. Pathol., 71: 375-394.
PLOPPER, C. G., DUNGWORTH, D. L., & TYLER, W. S. (1973b)
Ultrastructure of pulmonary alveolar macrophages in situ in
lungs from rats exposed to ozone. Am. Rev. respir. Dis.,
108: 532-638.
PRAT, R., NOFRE, C., & CIER, A. (1968) Effets de l'hypochlorite de
sodium, de l'ozone et des radiations ionisantes sur les
constituants pyrimidiques d'Escherichia coli. Ann. Inst.
Pasteur, 114: 595-607.
PURVIS, M. R., MILLER, S., & EHRLICH, R. (1961) Effect of atmospheric
pollutants on susceptibility to respiratory infection. I. Effect
of ozone. J. infect. Dis., 109: 238-242.
RASMUSSEN, R. A. (1970) Isoprene: Identified as a forest-type emission
to the atmosphere. Environ. Sci. Technol., 4(8): 667-671.
RASMUSSEN, R. A. (1972) What do the hydrocarbons from trees contribute
to air pollution? J. Air Pollut. Control Assoc., 22: 537-543.
RAVEN, P. B., DRINKWATER, B. L., RUHLING, R. O., BOLDUAN, N., TAGUCHI,
S., GLINER, J., & HORVATH, S. M. (1974) Effect of carbon monoxide
and peroxyacetyl nitrate on man's maximal aerobic capacity.
J. appl. Physiol., 36: 288-293.
REGENER, V. H. (1964) Measurement of atmospheric ozone with the
chemiluminescent method. J. geophys. Res., 69: 3795-3800.
REGENER, V. H. & ALDAZ, L. (1969) Turbulent transport near the ground
as determined from measurements of the ozone flux and the ozone
gradient. J. geophys. Res., 74: 6935-6942.
RENZETTI, N. A. & GOBRAN, V. (1957) Studies of eye irritation due to
Los Angeles smog 1954-1956. San Marino, CA, Air Pollution
Foundation, pp. 170.
REYNOLDS, R. W. & CHAFFEE, R. R. J. (1970) Studies on the combined
effects of ozone and a hot environment on reaction time in
subhuman primates. In: Project clean air, Santa Barbara,
California University, pp. 1-8 (California University Res. Proj.
S-6.).
RICHARDSON, N. A. & MIDDLETON, W. C. (1957) Evaluation of filters for
removing irritants from polluted air. Los Angeles, University
of California, Dept of Engineering, 31 pp. (Report No. 57-43).
RICHARDSON, N. A. & MIDDLETON, W. C. (1958) Evaluation of filters for
removing irritants from polluted air. Heat. Piping Air Cond.,
30: 147-154.
RIPPERTON, L. A., JEFFRIES, H. E., & WORTH, J. B. (1971) Relationship
of measurements in nonurban air to air pollution: Ozone and
oxides of nitrogen, CP 13B, In: England, H. M. & Berry, W. T.,
ed. Proceedings of the Second International Clean Air Congress.
New York, Academic Press, pp. 386-390.
ROEHM, J. N., HADLEY, J. G., & MENZEL, D. B. (1971a) Oxidation of
unsaturated fatty acids by ozone and nitrogen dioxide. Arch.
environ. Health, 23: 142-148.
ROEHM, J. N., HADLEY, J. G., & MENZEL, D. B. (1971b) Antioxidants vs
lung disease. Arch. intern. Med., 128: 88-93.
ROEHM, J. N., HADLEY, J. G., & MENZEL, D. B. (1972) The influence of
vitamin E on the lung fatty acids of rats exposed to O3. Arch.
environ. Health, 24: 237-242.
ROKAW, S. N. & MASSEY, F. (1962) Air pollution and chronic respiratory
disease. Am. Rev. respir. Dis., 86: 703-704.
ROTH, R. P. & TANSY, M. F. (1972) Effects of gaseous air pollutants on
gastric secreto-motor activities in the rat. J. Air Pollut.
Control Assoc., 22: 796-709.
RUDOLF, W. (1974) [Investigation of oxidants in the city centre of
Frankfurt.] Schriftenr. Ver. wasser-, Boden- Lufthyg.,
42: 65-75 (in German).
SABERSKY, R. H., SINEMA, D. A., & SHAIR, F. H. (1973) Concentrations,
decay rates, and removal of ozone and their relation to
establishing clean indoor air. Env. Sci. Technol., 7: 347-353.
SACHSENMAIER, W., SIEBS, W., & TAN, T. (1965) [Effects of ozone upon
mouse ascites tumor cells and upon chick fibroblasts in tissue
culture.] Z. Krebsforsch., 67: 113-126 (in German).
SANDALLS, F. J., PENKETT, S. A., & JONES, B. M. R. (1974) Preparation
of peroxyacetyl nitrate (PAN) and its determination in the
atmosphere. London, England, Her Majesty's Stationery Office
(Atomic Energy Research Establishment Report AERE R7807).
SCHEEL, L. D., DOBROGORSKI, O. J., MOUNTAIN, J. T., SVIRBELY, J. L., &
STOKINGER, H. E. (1959) Physiologic biochemical, immunologic, and
pathologic changes following ozone exposure. J. appl. Physiol.,
14: 67-80.
SCHLIPKOETER, H. W. & BRUCH, J. (1973) [Functional and morphological
alterations caused by exposure to ozone.] Zentralb. Bakteriol.,
Parasitenk. Infektionskr. Hyg., Reihe B, 156: 486-499 (in
German).
SCHLIPKOETER, H. W., FODOR, G. C., GHELERTER, L., & DOLGNER, R. (1973)
[Findings from animal tests concerning synergism.] In:
Proceedings of the 3rd International Clean Air Congress,
Diisseldorf, Federal Republic of Germany, pp. A26-A30 (in
German).
SCHOETTLIN, C. E. (1962) The health effect of air pollution on elderly
males. Am. Rev. respir. Dis., 86: 878-897.
SCHOETTLIN, C. E. & LANDAU, E. (1961) Air pollution and asthmatic
attacks in the Los Angeles area. Public Health Rep.,
76: 545-548.
SCHUCK, E. A. & DOYLE, G. J. (1959) Photooxidation of hydrocarbons in
mixtures containing oxides of nitrogen and sulfur dioxide. San
Marina, CA, Air Pollution Foundation (Report No. 29).
SCHUCK, E. A. & STEPHENS, E. R. (1969) Oxides of nitrogen. Adv.
environ. Sci., 1: 73-118.
SHINGU, H., TANAKA, I., KUSUMOTO, S., NAKAJIMA, T., WATANABE, M., &
SUGIYAMA, M. (1972) [Effect of ozone on defense mechanisms to
infection.] J. Jpn. Soc. Air Pollut, 9: 332 (in Japanese).
SKILLEN, R. G., THIENES, C. H., CANGELOSI, J., & STRAIN, L. (1961)
Brain 5-hydroxytryptamine in ozone-exposed rats. Proc. Soc. Exp.
Biol. Med., 108: 121-122.
SMITH, L. E. (1965) Inhalation of the photochemical smog compound
peroxyacetyl nitrate. Am. J. public Health, 55(9): 1460-1468.
STASIUK, W. N. & COFFEY, P. E. (1974) Rural and urban ozone
relationships in New York State. J. Air Pollut. Control Assoc.,
24(6): 564-568.
STEPHENS, E. R. (1964) Absorptivities for infra-red determination of
peroxyacetyl nitrates. Anal. Chem., 36: 928-929.
STEPHENS, E. R. (1969) The formation, reactions and properties of
peroxyacetyl nitrates (PANs) in photochemical air pollution.
Adv. environ. Sci. Technol., 1: 119-146.
STEPHENS, E. R. (1976) Peroxyacetyl nitrate (PAN) in relation to ozone
and some meteorological parameters at Delft in the Netherlands.
Atmos. Environ., 10(7): 566.
STEPHENS, E. R., DARLEY, E. F., TAYLOR, O. C., & SCOTT, W. E. (1961)
Photochemical reaction products in air pollution. Int. J. Air
Water Pollut., 4: 79-100.
STEPHENS, R. J., FREEMAN, G., CRANE, S.C., & FURIOSI, N.J. (1971)
Ultrastructural changes in the terminal bronchiole of the rat
during continuous low level exposure to nitrogen dioxide. Exp.
molec. Pathol., 14: 1-19.
STEPHENS, R. J., FREEMAN, G., & EVANS, M. J. (1972) Early response of
lungs to low levels of nitrogen dioxide. Arch. environ. Health,
24: 160-179.
STEPHENS, R. J., FREEMAN, G., STARA, J. F., & COFFIN, D. L. (1973)
Cytologic changes in dog lungs induced by chronic exposure to
ozone. Am. J. Pathol., 73: 711-726.
STEPHENS, R. J., SLOAN, M. F., EVANS, M. J., & FREEMAN, G. (1974)
Early response of lung to low levels of O3. Am. J. Pathol.,
74:31-58.
STERLING, T. D., PHAIN, J. J., POLLACK, S. V., SCHUMSKY, D. A., &
DEGROOT, I. (1966) Urban morbidity and air pollution: a first
report. Arch. environ. Health, 13: 158-170.
STERLING, T. D., POLLACK, S. V., & PHAIR, J. J. (1967) Urban hospital
morbidity and air pollution: a second report. Arch. environ.
Health, 15: 362-374.
STOKINGER, H. E. (1957) Evaluation of the hazards of ozone and oxides
of nitrogen. Am. Med. Assoc. Arch. ind. Health, 15: 181-190.
STOKINGER, H. E. (1959) Factors modifying toxicity of ozone. Adv.
Chem. Ser., 21: 360-369.
STOKINGER, H. E. (1965) Ozone toxicology: a review of research and
industrial experience 1954-1964. Arch. environ. Health,
10: 719-731.
STOKINGER, H. E. & SCHEEL, L. D. (1962) Ozone toxicity: immunochemical
and tolerance-producing aspects. Arch. environ. Health,
4: 327-334.
STOKINGER, H. E., WAGNER, W. D., & WRIGHT, P. G. (1956) Studies of
ozone toxicity: I. potentiating effects of exercise and tolerance
development. Am. Med. Assoc. Arch. ind. Health, 14: 158-162.
STOKINGER, H. E., WAGNER, W. D., & DORBROGORSKI, O. J. (1957) Ozone
toxicity studies. III. chronic injury to lungs of animals
following exposure at a low level. Am. Med. Assoc. Arch. Ind.
Health, 16: 514-522.
SUZUKI, T. & NAGAOKA, S. (1973) [The susceptibility to serotonin and
exposure to ozone.] Ann. Rep. Tokyo Metrop. Res. Inst. Environ.
Prot., No. 4, pp. 262-263 (in Japanese).
SUZUKI, T., NAKAZAWA, K., UMEDA, M., GOTO, H., & NAGAOKA, S. (1975)
[Effect on the blood in rats exposed to ozone. I. Effect on
osmotic fragility of orythrocytes.] Ann. Rep. Tokyo Metrop. Res.
Inst. Environ. Prot., 6: 270-272 (in Japanese).
SWANN, H. E. & BALCHUM, O. J. (1966) Biological effects of urban air
pollution. Arch. environ. Health, 12: 698-704.
TAN, W. C., CORTESI, R., & PRIVETT, O. S. (1974) Lipid peroxide and
lung prostaglandins. Arch. environ. Health, 28: 82-84.
THOMPSON, G. E. (1971) Cardiovascular considerations of rat pulmonary
edema. Mil. Med., 136: 50-65.
TOMINGAS, V. R., POTT, F., & DEHNEN, W. (1973) Biological test for
carcinogenicity of polycyclic aromatic hydrocarbons. Arch.
Geschwilstforsch., 42: 298-306.
TOYAMA, T., KAGAWA, J., TSUNODA, T., & NAKAZA, M. (1977) [An
epidemiological estimation of dose-response relationship to the
mixture of atmospheric pollutants.] Jpn. J. Hyg., 32:70 (in
Japanese).
TRAMS, E., LAUTER, C. J., BRANDENBURGER-BROWN, E. A., & YOUNG, O.
(1972) Cerebral cortical metabolisms after chronic exposure to
O3. Arch. environ. Health, 24: 153-159.
URY, H. K. (1968) Photochemical air pollution and automobile accidents
in Los Angeles. Arch. environ. Health, 17: 334-342.
US DEPARTMENT OF HEALTH EDUCATION AND WELFARE (1970) Air Quality
criteria for photochemical oxidants. Washington, DC, US DHEW
(National Air Pollution Control Administration Publication No. AP
63).
US ENVIRONMENTAL PROTECTION AGENCY (1964-1973) Camp data. Research
Triangle Park, NC, National Aerometric data bank, US EPA.
US ENVIRONMENTAL PROTECTION AGENCY (1973) Investigation of high ozone
concentration in the vicinity of Gomet County, Maryland and
Preston County, West Virginia. Research Triangle Park, NC, EPA
(Report No. EPA-R4-73-019).
US ENVIRONMENTAL PROTECTION AGENCY (1976) Monitoring and air quality
trends report, 1974, pp. 115-123 (Report No. EPA-450/1-76).
VAUGHAN, T. R., JR, JENNELLE, L. F., & LEWIS, T. R. (1969) Long-term
exposure to low levels of air pollutants. Effects on pulmonary
function in the beagle. Arch. environ. Health, 19: 45-50.
VENINGA, T. S. (1967) Toxicity of ozone in comparison with ionizing
radiation. Strahlentherapie (Munich), 134: 469-477.
WARREN, G. J. & BABCOCK, G. (1970) Rev. Sci. Instrum., 41: 280-282.
WATANABE, M. TANAKA, I., SHINGU, H., ASAKA, J., KUSUMOTO, S.,
NAKAJIMA, T., & SUGIYAMA, M. (1973a) [The cytotoxicity of ozone.]
Jpn. J. public Health, 20: 554 (in Japanese).
WATANABE, S., FRANK, R., & YOKOYAMA, E. (1973b) Acute effects of ozone
on lungs of cats. I. Functional. Am. Rev. respir. Dis.,
108: 1141-1151.
WAYNE, W. S., WEHRLE, P. F., & CARROLL, R. E. (1967) Oxidant air
pollution and athletic performance. J. Am. Med. Assoc.,
199: 901-904.
WENT, F. W. (1966) On the nature of Aitken condensation nuclei.
Tellus, 28(2): 549-556.
WERTHAMER, S., SCHWARTZ, L. H., & SOSKIND, L. (1970) Bronchial
epithelial alterations and pulmonary neoplasia induced by ozone.
Pathol. Microbiol., 35: 224-230.
WERTHAMER, S., PENHA, P. D., & AMARAL, L. (1974) Pulmonary lesions
induced by chronic exposure to ozone. I. Biochemical alterations.
Arch. environ. Health, 28: 164-166.
WHITE, W. H., ANDERSON, J. A., BLUMENTHAL, D. L., HUSAR, R. B.,
GILLANI, N. V., HUSAR, J. D., & WILSON, W. E., JR (1976)
Formation and transport of secondary air pollutants: Ozone and
aerosols in the St Louis urban plume. Science, 194: 187-189.
WORLD HEALTH ORGANIZATION (1972) WHO Tech. Rep. Series No. 506. (Air
quality criteria and guides for urban air pollutants: Report of a
WHO Expert Committee.) 35 pp.
WORLD HEALTH ORGANIZATION (1976) Selected methods of measuring air
pollutants, Geneva WHO (WHO Offset Publ. No. 24).
WORLD HEALTH ORGANIZATION (1977) Oxides of nitrogen: Environmental
health criteria 4, Geneva, WHO, 79 pp.
XINTARAS, C., JOHNSON, B. L., ULRICH, C. E., TERRILL, R. E., &
SOBECKI, M. F. (1966) Application of the evoked response
technique in air pollution toxicology. Toxicol. appl.
Pharmacol., 8: 77-87.
YOKOYAMA, E. (1972a) [The effects of O3 on lung function. In:
Proceedings of a Conference on Photochemical Air Pollution.]
Tokyo, Jpn. Soc. Air Pollution, pp. 109-129 (in Japanese).
YOKOYAMA, E. (1972b) Effect of ventilation with ozone on
pressure-volume relationships of excised dogs' lungs. Am. Rev.
Respir. Dis., 105: 594-604.
YOKOYAMA, E. (1973) The effects of low-concentrations of ozone on the
lung pressure of rabbits and rats. Presented at: The Japan
Society of Industrial Hygiene Annual Meeting, 46th, April, pp.
134-135 (paper 223) (in Japanese).
YOKOYAMA, E. (1974) [Maximal expiratory flow volume curve of rabbit
lung exposed to ozone.] Jpn. J. thorac. Dis., 12: 556-561 (in
Japanese).
YOKOYAMA, E. & FRANK, R. (1972) Respiratory uptake of ozone in dogs.
Arch. environ. Health, 25: 132-138.
YOUNG, W. A., SHAW, D. B., & BATES, D. V. (1963) Pulmonary function in
welders exposed to ozone. Arch. environ. Health, 7: 337-340.
YOUNG, W. A., SHAW, D. B., & BATES, D. V. (1964) Effect of low
concentrations of ozone on pulmonary function in man. J. appl.
Physiol., 19: 765-768.
ZELAC, R. E., CROMROY, H. L., BOLCH, W. E., JR, DUNAVANT, B. G., &
BEVlS, H. A. (1971a) Inhaled ozone as a mutagen I. Chromosome
aberrations induced in chinese hamster lymphocytes. Environ.
Res., 4: 262-282.
ZELAC, R. E., CROMROY, H. L., BOLCH, W. E., JR, DUNAVANT, B. G., &
BEVIS, H. A. (1971b) Inhaled ozone as a mutagen II. Effect on the
frequency of chromosome aberrations observed in irradiated
chinese hamsters. Environ. Res., 4: 325-342.
6.3.4 Effects on children
Ventilatory performance, measured twice a month for 11 months by
the Wright Peak Flow Meter, in 78 elementary school children in two
cities in the Los Angeles Basin was unaffected by differences in the
daily mean oxidant levels which were 320 µg/m3 (0.16 ppm) and
220 µg/m3 (0.11 ppm) respectively (McMillan et al., 1969).
Kagawa & Toyama (1975b) and Kagawa et al. (1976) studied the
weekly variations in lung function (airway conductance and ventilatory
performance) of 20, normal, 11-year-old, school children in Tokyo in
relation to variations in temperature and ambient concentrations of
ozone, nitrogen dioxide, nitric oxide, sulfur dioxide, hydrocarbons,
and particulate matter. Students were tested from June 1972 to October
1973. Ozone levels were determined by the ethylene-chemiluminescence
method. Temperature was the factor most highly correlated with
variations in specific airway conductance (negative correlation) and
maximum expiratory flow rate (Vmax) at 25 and 50% FVC (positive
correlation). Significant negative correlations between ozone and
specific airway conductance, and between nitrogen dioxide, nitric
oxide, sulfur dioxide, and particulate matter and Vmax at 25 or 50%
FVC were observed in some children. The ranges of hourly pollutant
levels, at the time of the lung function test (13h00), that were used
for correlation during the study period were: 0-560 µg/m3
(0-0.28 ppm) for ozone, 40-550 µg/m3 (0.02-0.29 ppm) for nitrogen
dioxide, 30-340 µg/m3 (0.01-0.13 ppm) for sulfur dioxide, and
50-490 µg/m3 for particulate matter (Toyama et al., 1977).
The effect of oxidant air pollution exposure on the incidence and
duration of A2 influenza in an epidemic that occurred in 1968-69 was
studied retrospectively by Pearlman et al. (1971) in 3500 elementary
school children from 5 southern California communities. These areas
were selected to represent an exposure gradient for photochemical
oxidants, although no difference in community oxidant exposure was
present at the time (December 1968-January 1969) of the epidemic.
Information from school absenteeism, questionnaires on illness, and
haemagglutination-inhibition titres did not reveal any statistically
significant morbidity differences corresponding to the pollution
gradient that existed during the season of peak oxidant levels.
Several Japanese investigators have reported acute reactions among
school children exposed to moderately elevated concentrations of
oxidants of 200-400 µg/m3 (0.10-0.20 ppm), on smoggy days in a number
of urban areas. Not only eye and respiratory irritation, but systemic
symptoms such as paraesthesia, prostration, and convulsions were
observed. In reviewing these studies the WHO Task Group was aided by
several investigators and observers from Japan. In the opinion of the
Task Group, eye and respiratory effects observed during these episodes
may well have been caused by reported oxidant concentrations. However,
systemic symptoms could not be explained only by the observed oxidant
concentrations, but were more likely to be attributable to individual
psychosomatic reactions among the students. This judgement is
supported by the observation that acute systemic reactions were
observed in only some of the students, most of whom were exercising at
the time of peak oxidant levels. Thus, the students may well have
experienced acute irritation of the pharynx and trachea, enhanced by
their active status. However, the general population in the same
neighbourhood, including young children in primary grades and school
teachers, did not appear to have had these systemic symptoms. These
comments are based on the following studies.
Fujii (1972) reported the acute effects of an episode of
photochemical smog in Osaka, Japan, on 27 August 1971. The oxidant
concentration measured by the NBKI method rose to an hourly value of
360 µg/m3 (0.18 ppm) by 14h00. At this time, the peak ozone
concentration, determined by the chemiluminescent method was
480 µg/m3 (0.24 ppm) and the maximum sulfur dioxide concentration was
180 µg/m3 (0.07 ppm). A total of 249 school children reported
symptoms including pain in the throat, headache, coughing, breathing
difficulties, eye irritation, and numbness in the limbs. On 14
September 1971, maximum hourly ozone concentrations reached 480 µg/m3
(0.24 ppm) and a total of 290 persons complained of similar symptoms.
These data do not provide a basis for estimating symptom frequency in
the general population, because the total population at risk was not
reported.
In an evaluation of the clinical status of 15 high school children
who were hospitalized after the onset of acute symptoms that began
during photochemical smog episodes in Osaka, Japan, Adachi & Nakajima
(1974) stated that "they were astonished by the seriousness of the
conditions which surpassed by far the symptoms previously known in
connection with the relation between photochemical oxidant
concentration and its effect on man in the case of Los Angeles".
Affected students were not only found to have irritation of the eyes
and respiratory tract, but, systemic symptoms such as paraesthesia,
muscle spasms as well as an increased leukocyte count and borderline
elevation of serum alkaline phosphatase (3.1.3.1).
Based upon complaints found in schools that were registered with
local health centres located in Japanese cities, the Japan Public
Health Association (1976) reported a significant increase in
subjective complaints, particularly eye irritation, when photochemical
oxidant concentrations exceeded hourly values of 300 µg/m3
(0.15 ppm), as determined by the 10% NBKI method.
Mikami & Kudo (1973) described various local and systemic symptoms
in 82 school children that were attributed to 3 photochemical air
pollution episodes in Tokyo and Osaka in 1970, 1971, and 1972 with
maximum 1-h concentrations ranging from 300 to 580 µg/m3 (0.15 to
0.29 ppm). A large proportion of cases were reported to have eye
irritation, throat irritation, cough, breathlessness, headache, and
chills. The authors pointed out that many of these symptoms had not
been reported under Los Angeles smog conditions.
6.3.5 Effects on the incidence of acute respiratory and
cardiovascular diseases
In studies on college students in the Los Angeles Basin, Durham
(1974) showed that acute episodes of pharyngitis, bronchitis, and
upper respiratory infections were associated with peak concentrations
of oxidants and mean concentrations of sulfur dioxide and nitrogen
dioxide. Oxidants, sulfur dioxide, and nitrogen dioxide
(concentrations not given) were, in this order, consistently
associated with the various episodes of illness. A comparison of
selected high and low pollution days indicated that photochemical air
pollution might have been responsible for a 16.7% increase in acute
respiratory symptoms seen in Los Angeles schools situated in areas of
highest concentration compared with those in areas of lowest
concentration. The author's method of data presentation did not permit
the estimation of dose-response relationships or of threshold
concentrations.
Brant & Hill (1964) and Brant (1965) did not find any correlation
between admissions for cardiovascular conditions to Los Angeles County
Hospital and oxidant levels (170 µg/m3; 0.08 ppm as a mean value of
daily 7-h measurements for the study periods) either on the day of
admission or for 2 weeks prior to admission, for the period between 8
August and 25 December 1954. However, Sterling et al. (1966, 1967)
found statistically significant but very low correlations between
admissions to Los Angeles hospitals for respiratory episodes during
the period 17 March-26 October 1961 and daily mean levels of oxidants
(74 µg/m3; 0.037 ppm), ozone (84 µg/m3; 0.042 ppm), and carbon
monoxide (11 mg/m3; 9.5 ppm). Because of the limited time period and
the extremely low correlation coefficients, it is difficult to
conclude that a real relationship was demonstrated by these studies.
6.3.6 Effects on the prevalence of chronic respiratory diseases and
on pulmonary function
Deane et al. (1965) conducted a survey on the prevalence of
chronic respiratory disease in outdoor male telephone workers in San
Francisco and Los Angeles. Symptoms of persistent cough and phlegm
were slightly less prevalent in Los Angeles than in San Francisco in
the 40-49-year group but more prevalent in the 50-59-year group (31.4%
compared with 16.3% in San Francisco). These data were adjusted for
cigarette smoking. No differences in pulmonary function test results
were found.
The prevalence of chronic respiratory symptoms and the results of
pulmonary function tests were compared in 2 similar groups of
nonsmoking, adult, male and female Seventh Day Adventists aged 45-64
years residing in Los Angeles and San Diego, respectively. Annual mean
oxidant values (94 µg/m3; 0.047 ppm and 76 µg/m3; 0.038 ppm), were
essentially the same in the 2 cities, but a mean of maximum daily
concentrations in Los Angeles (290 µg/m3; 0.144 ppm) was twice as
high as that in San Diego (148 µg/m3; 0.074 ppm). The survey was
performed when oxidant levels were low and similar in both cities, to
minimize effects attributable to acute oxidant exposures. No
differences were found in symptom prevalence or in several measures of
ventilatory function. Prevalence rates for chronic respiratory disease
were uniformly low (less than 4%) in both groups (Cohen et al., 1972).
6.3.7 Effects on patients with pre-existing diseases
6.3.7.1 Asthma
The association between reported asthma attacks in 137 patients
from Pasadena, California, and oxidant levels during the period 3
September-9 December 1956 was studied by Schoettlin & Landau (1961).
Daily records of the time of onset of asthma attacks were kept by each
patient and collected weekly. The daily number of patients afflicted
with asthma was moderately well correlated (r = 0.37) with concurrent
maximum hourly oxidant readings. Asthma attacks were more weakly
correlated with temperature, relative humidity, and water vapour
pressure than with oxidants. The number of patients having attacks on
days when maximum 1-h oxidant levels were higher than 500 µg/m3
(0.25 ppm) was significantly greater (p = 0.05) than the number of
those having attacks on days with lower oxidant levels. Unfortunately,
the analysis failed to isolate the pronounced seasonal variation of
asthma. In the northern hemisphere, asthma attack rates tend to reach
a peak in October and November (Booth et al., 1965), and decline
sharply in late November and early December. This pattern corresponds
closely to seasonal declines in peak oxidant levels. Hence, it is
possible that observed asthma-oxidant correlations have been secondary
to simultaneous seasonal changes in asthma frequency and oxidant
levels.
6.3.7.2 Chronic respiratory diseases
Studies over 18 months on 25 patients with severe, chronic,
obstructive lung disease at a chronic disease centre in Los Angeles
County showed that the prevailing levels of oxidants, oxidant
precursors, temperature, and relative humidity did not produce any
effects on lung function (Rokaw & Massey, 1962).
In studies on the effects of ambient air pollution exposure on
armed forces veterans with chronic respiratory disease living in the
Domiciliary Unit and Chronic Disease Annex of the Los Angeles Veterans
Administration Center, subjects were evaluated once a week by
pulmonary function tests and respiratory symptom questionnaires
(Schoettlin, 1962). Analysis of variance did not show any
statistically significant effects of air pollution (concentrations not
reported) on respiratory symptoms or function, although maximum
concentrations of oxidant and oxidant precursors consistently
accounted for more of the variation in frequency of symptoms and
clinical signs of disease than maximum temperature, relative humidity,
or pollen counts.
6.3.8 Cancer
A 5-year prospective study of lung cancer from 1958 to 1963 was
conducted by Buell et al. (1967) among 69 160 members of the
California American Legion. Long-term residents of Los Angeles County
had slightly lower age-smoking adjusted lung cancer rates (95.4 per
100 000) than residents of the San Francisco Bay area counties and San
Diego County (102 per 100 000). These urban groups, in turn, had
higher rates than all other California counties (75.5 per 100 000).
Nonsmokers followed the same geographical pattern: 28.1 per 100 000
for Los Angeles, 43.9 per 100 000 for San Francisco and San Diego,
11.2 per 100 000 for all others. Smokers of more than one packet of 20
cigarettes a day in Los Angeles had the highest lung cancer rates,
241.3 per 100 000, compared with San Francisco-San Diego, 226 per
100 000, and with other counties, 137.5 per 100 000. The duration of
exposure necessary to induce lung cancer may have been longer than the
actual ozone exposures in this population study. Thus, it would appear
worthwhile to extend these observations to subsequent years.
6.3.9 Motor vehicle accidents
The association between automobile accidents and days of elevated
oxidant levels (120-480 µg/m3; 0.06-0.24 ppm) in Los Angeles was
studied from August to October in 1963 and 1965. Applying a sign-test
and nonparametric correlation analysis to the data, Ury (1968) found a
statistically significant relationship between oxidant levels and
automobile accidents. Concentrations of carbon monoxide, oxides of
nitrogen, and other pollutants would also be relatively elevated when
oxidants were high and may have contributed to the results reported.
Furthermore, the association of accidents with oxidant levels may be
confounded by the fact that traffic jams produce both more accidents
and a greater output of oxidant precursors.
6.4. Summary Tables
Studies on the health effects of controlled, industrial, and
community exposures that provide quantitative information useful for
establishing guidelines for the protection of public health with
respect to photochemical oxidants are summarized in Tables 12, 13 and
14.
Table 12. Controlled human studies
I. Sensory effects
Ozone concentration Length of Effects Responsea Subjects Reference
exposure
µg/m3 (ppm) days h/day
400 (0.2) 1 3 and 6 Diminution in various measures of Not applicable 22 healthy males, 6 Lagerwerff (1963)
700 (0.35) 1 3 and 6 visual perception; noneffect dose healthy females
1000 (0.5) 1 3 and 6 not determined; no mention of
dose-response relationship.
> 200 (> 0.1) 1 working Increasing eye irritation with Not applicable 20 women office Richardson &
hours (study increase in oxidant exposures; workers Middleton
repeated for no apparent effects below (1957, 1958)
123 days) 200 µg/m3 (0.1 ppm).
40-100 (0.02- Immediate odour perception after 9/10 at 10-14 healthy males Henschler et al.
0.05) beginning of exposure; odour 40 µg/m3 (1960)
perception disappeared in ´-12 13/14 at
min; odour considerably stronger at 100 µg/m3
higher dose.
15-40 (0.008 Odour perception immediately after Not available 20 healthy subjects Eglite (1968)
-0.02) beginning of exposure; the most
sensitive person perceived odour
at the level of 15 µg/m3
(0.008 ppm).
II. Effects on respiratory function
A. Exposure to ozone
Ozone concentration Length of Effects Subjects Reference
exposure
µg/m3 (ppm) days h/day
2000 (1.0) 1 1 Consistent increase in airway 4 healthy males Goldsmith et Nadel
resistance; exposures at 200, 800, (1969)
and 1200 µg/m3 (0.1, 0.4, 0.6 ppm)
caused effect in some subjects,
but no dose-response pattern.
1800 (0.9) 1 5 min Highly significant decrease of airway 4 healthy males Kagawa & Toyama
conductance after inhalation with exercise. (1975a)
1200- (0.6-0.8) 1 2 Significant reduction in diffusing capacity of 10 healthy men and Young et al.
1600 lung and in FEV0.75b; substernal soreness one healthy woman (1964)
present in all subjects.
1000 (0.5) 6/wk 3 Significant decrease in FEV1.0c; no effect at 12 healthy males Bennet (1962)
x 12 400 µg/m3 (0.2 ppm).
wk5
1000 (0.5) 1 6 Significant change in airway conductance 20 healthy subjects Kerr et al. (1975)
and pulmonary resistance (dry cough and (19 men and 1
chest discomfort). woman)
1000 (0.5) 1 2 Decrease in pulmonary function 7 healthy males Hackney et al.
measurements. (1975a)
a Response = number of subjects showing effect described
total number of subjects
Table 12. Controlled human studies--continued
II. Effects on respiratory function
A. Exposure to ozone (cont'd)
Ozone concentration Length of Effects Subjects Reference
exposure
µg/m3 (ppm) days h/day
800 (0.4) 1 2 and 4 After 2-h exposure: Rawd increase, FVCe 22 healthy male Rummo et al.
decrease MMFRf decrease; after 4-h subjects (1975.
exposure: Rawd increase, FVCe decrease. unpublished
MMFRf decrease and additionally FEV1.0c data)
decrease; RVg, FRCh, and TCLi did not
change.
740 & (0.37 & 1 2 Significant decrease in ventilatory function 6-10 healthy males Bates et al.
1500 0.75) and in closing volume under intermittent (1972).
exercise; effect more pronounced at higher Hazucha et al.
dose. (1973)
740, (0.37, 1 2 Dose dependent change of ventilatory 28 healthy subjects Folinsbee et al.
1000 & 0.50 & pattern; significant reduction in MEFRj at (20 males and 8 (1975)
1500 0.75) 50% of vital capacity at 740 µg/m3 females)
(0.37 ppm) or more, under exercise.
740 (0.37) 1 2 Significant increase in total respiratory 2 healthy and 3 Hackney et al.
resistance under intermittent light exercise sensitively males. (1975a)
500 (0.25) 1 2 No consistent changes in lung function. 3 healthy and 3 Hackney et al.
sensitively males (1975a)
200- (0.1- 1 30 min Tendency towards increase in breathing 4 healthy male Ohmori (1974)
500 0.25) frequency and volume under exercise. subjects
200 (0.1) 1 2 Increase of Rawd and AaDO2k in 7 of 11 test 11 healthy male von Neiding et al.
subjects under intermittent light exercise. subjects (1977)
B. Exposure to mixtures of ozone and other air pollutants
Ozone concentration Length of Effects Subjects Reference
exposure
µg/m3 (ppm) days h/day
740 + 960 (0.37 + 0.37 1 2 More pronounced decrease in ventilatory 4 healthy males Bates & Hazucha
sulfur sulfur function end in closing volume than in (1973)
dioxide dioxide single exposure to ozone at 740 µg/m.3
(0.37 ppm).
200 + 9400 (0.1 + 5 1 2 Increases in Rawd and AaDO2k similar 11 healthy male von Nieding et al.
nitrogen nitrogen to those observed with ozone alone at subjects (1977)
dioxide dioxide 200 µg/m3 (0.1 ppm).
200 + 9400 (0.1 + 5 1 2 Increases in Rawd and AaDO2k similar 11 healthy male von Nieding et al.
nitrogen nitrogen to those observed with ozone at subjects (1977)
dioxide + dioxide + 5 200 µg/m3 (0.1 ppm) + nitrogen dioxide
sulfur sulfur at 9400 µg/m3 (5 ppm); recovery time
dioxide dioxide) delayed.
50 + 100 (0.025 + 0.05 1 2 No effect on Rawd and AaDO2k; 11 healthy male von Nieding et al.
dioxide +260 nitrogen sensitivity of the respiratory tract subjects (1977)
sulfur dioxide dioxide + 0.1 to acetylcholine increased.
sulfur dioxide)
C. Exposure to peroxyacetylnitrate
Ozone concentration Length of Effects Subjects Reference
exposure
µg/m3 (ppm) days h/day
1500 (0.3) 1 5 min Increase in oxygen uptake with exercise; 32 college males Smith (1965)
no effect at rest; significant decrease
in MEFRj during the recovery phase
following exercise.
1350 (0.27) 1 42 min Minor changes in cardiorespiratory and 20 healthy male Raven et al.
temperature regulation parameters. subjects (1974)
II. Effects on respiratory function
D. Exposure to irradiated automobile exhaust
Oxidants (0.22 Short term No significant changes in reaction 14 healthy Holland et al.
440-540 -0.27) (no details time, vital capacity, and submaximum subjects(1968)
given) work performance on the bicycle
ergometer.
Carbon (15-29)
monoxide
17-
33 mg/m3
Table 12. Controlled human studies--continued
II. Effects on respiratory function
E. Exposure to ambient air with an elevated concentration of oxidants
Ozone concentration Length of Effects Subjects Reference
exposure
µg/m3 (ppm) days h/day
Carbon (800-1400)
dioxide
1520-
2660 mg/m3
Nitric (0.38-0.58)
oxide
470-710
Nitrogen (0.7-1.0)
dioxide
1300-1900
Hydrocarbons
traces
Aldehydes (0.2-0.7)
Formaldehyde (0.2-0.24)
250-300
400-1400 (0.2-0.7) 2-90 h Improvement in lung function upon 46 patients with Motley et al.
residence in clean filtered room chronic lung (1959)
for 40 h or longer; threshold disease
concentration not determined.
100-460 (0.05-0.23) 21 24 Decrease in airway resistance and 15 patients with Balchum (1973)
increase in arterial partial oxygen moderately severe
pressure during week of residence chronic lung
in clean filtered air; threshold disease
concentration not determined.
Table 12. Controlled human studies
III. Changes in the electroencephalogram
Ozone concentration Length of Effects Responsea Subjects Reference
exposure
µg/m3 (ppm) days h/day
10 (0.005) 1 3 min Decrease in alpha rhythm. 1/3 3 healthy subjects Eglite (1968)
15 (0.008) 1 3 min Decrease in alpha rhythm. 2/3 3 healthy subjects Eglite (1968)
20 (0.01) 1 3 min Decrease in alpha rhythm. 3/3 3 healthy subjects Eglite (1968)
a Response = number of subjects showing effect described
total number of subjects
b FEV0.75 = 0.75 second forced expiratory volume
c FEV1.0 = one second forced expiratory volume
d Raw = airway resistance
e FVC = forced vital capacity
f MMFR = mid-maximal expiratory flow rate
g RV = residual volume
h FRC = functional residual capacity
i TLC = total lung capacity
j MEFR = maximum expiratory flow rate
k AaDO2 = alveolar to arterial oxygen pressure difference
l sensitive (subjects) = those with a prestudy history of cough, chest discomfort, or wheezing associated with
allergy or air pollution exposure, but with normal base-line pulmonary function studies
Table 13. Studies on the effects of industrial exposures
Ozone concentration Averaging Effects Reference
time
µg/m3 (ppm)
1600-3400 (0.8-1.7) 1 h 11 of 14 welders complained of respiratory symptoms; symptoms Challen et al. (1958)
disappeared when ozone levels were reduced to
400 µg/m3 (0.2 ppm); nitrogen dioxide probably present.
600-1600 (0.3-0.8) 1 h Increased frequency of chest tightness and throat irritation among Kleinfeld et al. (1957)
welders; no complaints at 500 µg/m3 (0.25 ppm); welders also
exposed to nitrogen dioxide and particulates but concentration
levels not given.
500-800 (0.25-0.40) long-term Increased frequency of headache, weakness, change in neuromuscular Kudrjavceva (1963)
sensitivity, and decrease in memory among workers manufacturing
hydrogen peroxide and exposed to ozone for 7-10 years; threshold
concentration not determined.
400-600 (0.2-0.3) 1 h No evidence for changes in vital capacity or functional residual Young et al. (1963)
capacity in welders; nitrogen dioxide probably present.
80-1000 (0.04-0.50) long-term Increased prevalence of bronchitis and emphysema in workers Nevskaja & Diterihs
engaged in manufacture of hydrogen peroxide for many years; (1957)
sulfuric acid aerosols also present; threshold concentration not
determined.
Table 14. Studies on the effects of community exposures
Hourly oxidant concentration Effects Subjects Reference
µg/m3 (ppm)
500 and above (0.25 and above) Increased frequency of asthma; possible confounding of 137 patients with Schoettlin
asthma-oxidant association with seasonal effects; other asthma & Landau (1961)
pollutants present but not reported.
approx. 1000 (approx. 0.50) Increased symptoms began at hourly concentrations of 102 student Hammer et al.
(headache) 100 µg/m3 (0.05 ppm), (eye irritation) nurses (1974)
300 µg/m3 (0.15 ppm), (cough) 530 µg/m3 (0.265 ppm).
(chest discomfort) 580 µg/m3 (0.29 ppm); threshold
levels determined by "hockey stick" functions.
240 and above (0.12 and above) Impaired performance determined by failure to improve 116 high school Wayne et al.
running times; threshold determined by "hockey stick" cross-country (1967)
functions. runners
> 300 (0.15) Increased frequency of complaints, particularly eye 7440 school Japan Public
irritation. children Health
Association (1976)
0-560 (0-0.28) Specific airway conductance of sensitive subjects was 20 healthy Kagawa et al.
(range at time significantly decreased with increasing hourly oxidant 11-year-old et al. (1977)
of lung concentrations; temperature, nitric oxide, nitrogen school children
function test) dioxide, sulfur dioxide, and particulate matter also
showed significant correlations with various
respiratory function tests of highly reactive children;
threshold concentration not determined.
7. EVALUATION OF HEALTH RISKS FROM EXPOSURE TO PHOTOCHEMICAL OXIDANTS
There appears to be sufficient information from experimental and
epidemiological studies to justify an attempt to establish guidelines
on the exposure limits for ozone and to review those for "oxidants"
(as measured by the neutral-buffered potassium iodide method (NBKI)),
proposed by a WHO Expert Committee in 1972. The Task Group appreciated
the fact that photochemical air pollution contains other substances
besides ozone, such as nitrogen dioxide, peroxyacetylnitrate, and
possibly many other gaseous and particulate products of atmospheric
photochemical reactions. However, present knowledge about the
composition of photochemical pollution, the concentrations of
individual components, and their possible impact on human health is so
limited that no attempt can be made to estimate exposure limits for
any single compound other than ozone. The Task Group was, of course,
aware that some sensory effects of photochemical air pollution (such
as eye irritation) might be due to a large extent, to these poorly
defined components of the photochemical oxidant mixture. As nitrogen
dioxide is an important air pollutant in its own right, it has been
discussed in a separate criteria document (World Health Organization,
1977).
7.1 Exposure Conditions
Exposure of man to ozone must have occurred for millions of years,
as ozone, present naturally in higher tropospheric layers, is also
found regularly in the lower atmosphere even in completely uninhabited
regions like the Antarctic. These natural concentrations have been
reported to have values ranging from 10 to 100 µg/m3
(0.005-0.05 ppm). It is difficult to determine the proportions of
natural to man-made oxidants (including ozone) that occur in rural
areas in most countries. In general, ozone concentrations greater than
120 µg/m3 (0.06 ppm) are considered to be related to man-made
activities. Ozone may be transported over hundreds of kilometres and
rural populations may be exposed to the pollutant, which earlier was
considered to exist only in Urban areas. A characteristic of such
exposures is that the precursors have vanished and ozone can therefore
persist for days in succession, since it does not come into contact
with other pollutants that act as its scavengers.
In large urban areas with strong sunshine and dense traffic or
other sources of precursors, photochemical air pollution is a daylight
phenomenon with maximum 1-h ozone concentrations sometimes as high as
300-800 µg/m3 (0.15-0.4 ppm), occurring around noon or somewhat
later. Such peak concentrations are preceded by nitrogen dioxide peaks
and accompanied by concurrent rises in peroxyacetylnitrate
concentrations. In contrast to oxides of sulfur and smoke, ozone
exposures are always intermittent, the peak concentrations rarely
lasting for more than 2-3 h. In the low temperature season,
photochemical reactions are much less likely to occur at rates
sufficient to produce large quantities of ozone.
Unless certain industrial technological processes (e.g., welding)
are in operation, ozone concentrations indoors tend to be considerably
lower than those outdoors due to the presence of reactive surfaces,
air conditioning, and indoor smoking.
7.2 Exposure-effect Relationships
Information presented in sections 5 and 6 is sufficient to
evaluate the relationship between exposure and the associated effects,
at least for some biological changes observed in man and experimental
animals.
7.2.1 Animal data
There is a considerable amount of evidence that short-term,
prolonged, or repeated exposure to ozone concentrations ranging from
200 to 400 µg/m3 (0.1-0.2 ppm) can cause a variety of biological
changes in several animal species and that these effects become more
pronounced with higher concentrations and increased exposure time (see
Table 10). These effects are, of course, also influenced by other
factors such as the animal species, the length of the interval between
exposures, the presence of other pollutants, low temperature, and
physical activity.
The host's pulmonary defence mechanisms against infectious
microorganisms are affected in several animal species by exposure to
ozone (section 5.2.6). This may result in rapid multiplication of
infectious microorganisms in situ, causing disease and eventually
death. An increase in mortality, resulting from the joint action of
infectious microorganisms and ozone, has been demonstrated in
artificially infected mice after a 3-h exposure to ozone at 160 µg/m3
(0.08 ppm). These effects were dose-related.
Pathomorphological changes in the respiratory tract of various
animal species, such as the rat, cat, rabbit, and mouse, have been
observed at ozone concentrations of about 400 µg/m3 (0.2 ppm) and
higher (section 5.2.1). Short-term exposures (up to 24 h) produce
oedema, degeneration and destruction of type I alveolar cells, loss of
ciliated epithelium, and breakdown of capillary endothelium. When the
exposure is repeated or its length extended, the biological changes
become more severe and include emphysema, atelectasis, vascular
lesions, bronchopneumonia, and fibrosis.
Various functional changes in the respiratory tract begin at
levels of about 520 µg/m3 (0.26 ppm) (section 5.2.2). The activities
of several enzymes in the lung tissue are also influenced at exposure
levels of about 500 µg/m3 (0.25 ppm) (section 5.2.3).
After pre-exposure to ozone, animals appear to become tolerant to
ozone concentrations that would otherwise cause pulmonary oedema
(section 5.2.5). The development of tolerance in small rodents is
related to pre-exposure levels of at least 600 µ/m3 (0.3 ppm). This
tolerance does not protect animals from such effects of ozone as
inflammation, alterations in alveolar macrophage functions, and
impairment of respiratory functions, that can occur at concentrations
lower than those that produce oedema.
Although the primary target for ozone is the respiratory system, a
number of studies have indicated that exposure to ozone may also
result in some extrapulmonary effects, but the mechanisms of such
action are not clear (section 5.3). For example, ozone exposure at
400-500 µg/m3 (0.2-0.25 ppm) for less than 2 h produced changes in
the circulating lymphocytes (increasing the number of binucleated
cells) and increased the number of spherocytes (section 5.3.2.1). It
has also been shown that exposure of pregnant mice to ozone at
200-400 µg/m3 (0.1-0.2 ppm) for 7 h per day, 5 days per week, for 3
weeks significantly increased neonatal mortality (section 5.3.3).
The available information concerning the carcinogenicity and
mutagenicity of ozone is inadequate for the definite evaluation of
such effects (sections 5.2.4 and 5.4).
Biological effects produced by the exposure of experimental
animals to a combination of ozone and nitrogen dioxide or by exposure
to complex pollutant mixtures containing oxidants, such as irradiated
automobile exhaust, are generally similar to those produced by
exposure to pure ozone. However, one study in which mice were exposed
to a mixture of ozone and nitrogen dioxide indicated that the effect
(reduction in resistance to respiratory infection) of a single
exposure to this mixture was additive, and that repeated exposure to
this mixture might result in a synergistic action.
7.2.2 Controlled human exposures
Although limited in number, human volunteer studies with
short-term, controlled exposure to oxidants have proved useful for
establishing exposure-effect relationships for ozone at levels ranging
from about 200-700 µg/m3 (section 6.1). Some of these studies provide
evidence of changes in the respiratory function of healthy subjects
that are related to exposure. Physical exercise tends to enhance the
respiratory effects of ozone (section 6.1.3).
Three investigators found a significant increase in airway
resistance with exposure to an ozone level of 740 µg/m3 (0.37 ppm)
for 2 h. One of the investigators did not find any effect with a 2-h
exposure to a level of 500 µg/m3 (0.25 ppm). However, this particular
study was conducted on subjects from southern California who were
later found to be less sensitive to ozone. Another investigator, using
similar test conditions, found a significant increase in airway
resistance in 7 out of 11 subjects at an ozone level of 200 µg/m3
(0.1 ppm) for 2 h (section 6.1.3.1).
In two studies, an improvement in lung function was noted when
patients with chronic pulmonary disease breathed filtered air for 40 h
or more, as compared with unfiltered ambient air. The ambient air
concentrations of oxidant in the two studies ranged from
400-1400 µg/m3 (0.2-0.7 ppm) and up to 400 µg/m3 (0.2 ppm)
respectively (section 6.1.3.5).
Result of a 7-month study in which female employees were exposed
to unfiltered ambient air during office hours suggested that the
lowest 1-h oxidant level that could be associated with eye irritation
was about 200 µg/m3 (section 6.1.2).
Although in vitro studies using human cells have shown some
evidence of a joint action of ozone and nitrogen dioxide, this has not
been clearly demonstrated in in vivo studies. For example, whereas a
potentiated increase in airway resistance was observed with exposure
to a combination of ozone at 740 µg/m3 (0.37 ppm) and sulfur dioxide
at 960 µg/m3 (0.37 ppm) for a period of 2 h, it was not observed with
exposure to a combination of ozone at 500 µg/m3 (0.25 ppm) and
nitrogen dioxide at 560 µg/m3 (0.30 ppm). Exposure to ozone at
50 µg/m3 (0.025 ppm) combined with nitrogen dioxide at 100 µg/m3
(0.05 ppm) and sulfur dioxide at 260 µg/m3 (0.1 ppm) did not have any
effect on airway resistance; however, this combined exposure resulted
in enhancement of the bronchoconstrictor effect of acetylcholine
(section 6.1.3.2).
7.2.3 Industrial exposure
Acute symptoms of chest tightness, irritation of the throat, and
coughing have been documented in welders exposed to 1-h ozone levels
of 600-1600 µg/m3 (0.3-0.8 ppm). These symptoms disappeared when
ozone concentrations fell to 500 µg/m3 (0.25 ppm) or less. Possible
chronic effects of repeated occupational exposure to ozone are not
well documented, although one investigator reported that workers
exposed to ozone levels in the range of 500-800 µg/m3 (0.25-0.40 ppm)
for 7-10 years had an increased frequency of headaches and weakness,
increased muscle excitability, and impaired memory (section 6.2).
7.2.4 Community Exposure
Several studies have shown more frequent eye irritation, reduced
athletic performance, changes in lung function of children, and
increased frequency of asthma attacks, all of which have been
associated with changes in hourly oxidant levels (sections 6.3.2,
6.3.3, 6.3.4, 6.3.7). As in all studies of the effects of community
exposures, it is difficult to determine precisely the lowest level at
which adverse effects become manifest. However, most of these effects
were observed when 1-h oxidant levels were in the range of about
200-500 µg/m3 (0.1-0.25 ppm). Although other pollutants such as
nitrogen dioxide, particulate matter, and sulfur dioxide were
simultaneously present, the strongest correlation of the observed
effects was with hourly levels of photochemical oxidants.
On the other hand, there is no evidence, so far, that long-term
exposure to photochemical oxidants at levels currently present in
urban air is associated with increased mortality (section 6.3.1), and
there is no evidence that chronic respiratory diseases such as
bronchitis, emphysema, and lung cancer are more prevalent in
communities with high oxidant exposures (section 6.3.6). However, it
should be pointed out that the number of epidemiological studies
concerned with such associations is small.
7.3 Guidelines on Exposure Limits
The exposure-effect relationships discussed in section 7.2 make it
possible to draw the following conclusions concerning the exposures to
oxidants and ozone at which the effects in man begin to appear:
(a) There is presumptive evidence from one controlled exposure
study that some effects on the lung function of healthy human subjects
might occur with exposure to an ozone level of 200 µg/m3 (0.1 ppm)
for 2 h.
(b) There is also evidence from general population studies that
suggests that 1-h ambient oxidant levels in the range of about
200-500 µg/m3 (0.1-0.25 ppm) may affect lung function in children,
increase the frequency of asthma attacks, cause more frequent eye
irritation, and reduce athletic performance.
(c) There is limited evidence from controlled exposure studies
that living in an environment with 1-h oxidant levels within the range
of 400-1400 µg/m3 (0.2-0.7 ppm) may exert additional stress on
patients with chronic pulmonary disease.
(d) There is convincing evidence from controlled human exposure
studies that airway resistance may be increased in healthy human
subjects following exposure to ozone levels of 700-800 µg/m3
(0.35-0.40 ppm) for 2h.
Animal data generally support the results of human studies.
However, some effects have been observed in animals at an ozone level
of about 200 µg/m3 (0.1 ppm) or even less, which have not yet been
demonstrated in man. For example, in animals, short-term exposures to
such concentrations appear to reduce resistance to pulmonary
infections.
The role of ozone and other photochemical oxidants in the etiology
of cancer is not clear. The only available epidemiological study did
not indicate any association between exposure to oxidants and the risk
of lung cancer, and experimental studies on the carcinogenicity and
mutagenicity of ozone in animals are not adequate for evaluation.
Nevertheless, the Task Group felt that there may be reason for concern
about the possible carcinogenicity of ozone (based primarily on some
biochemical considerations regarding the mechanism of the biological
effects of ozone). This aspect of its toxicity should be kept under
continual surveillance.
On the basis of all these considerations, the Task Group agreed
that 1-h levels of ozone of 100-200 µg/m3 (0.05-0.1 ppm) (measured by
the chemiluminescence method) could be used as a guideline for the
protection of public health. The relatively high natural
concentrations of ozone precluded the use of any safety factor.
The Task Group also agreed that a 1-h maximum level of 120 µg/m3
(0.06 ppm), which is approximately the highest natural background
concentration of oxidants, would be the best single value estimate of
the exposure limit for oxidants in the ambient air. This level is in
agreement with the long-term goal for photochemical oxidants (as
measured by the NBKI method) proposed by a WHO Expert Committee (World
Health Organization, 1972).
The issue was raised as to whether the proposed guideline was
realistic in view of natural exposure levels and the long-distance
transport of ozone. In response to this question, the Group expressed
the view that every effort should, nevertheless, be made to develop
control strategies for achieving the proposed guideline or at least,
for not exceeding it more than once a month.
REFERENCES
ADACHI, T. & NAKAJIMA, T. (1974) [Symptoms and clinical examinations
of the patients seriously injured by photochemical smog.] Rinsho
Kagaku (Clin. Chem.), 3:257-267 (in Japanese).
ALPERT, S. M. & LEWIS, T. T. (1971) Ozone tolerance studies utilizing
unilateral lung exposure. J. appl. Physiol., 31: 243-246.
ALPERT, S. M., SCHWARTZ, B. B., LEE, S. D., & LEWIS, T. R. (1971a)
Alveolar protein accumulation: a sensitive indicator of low level
oxidant toxicity. Arch. intern. Med., 128: 69-73.
ALPERT, S. M., GARDNER, D. E., HURST, D. J., LEWIS, T. R., & COFFIN,
D. L. (1971b) Effects of exposure to ozone on defensive
mechanisms of the lung. J. appl. Physiol., 31: 247-252.
AMERICAN CONFERENCE OF GOVERNMENT INDUSTRIAL HYGIENISTS (1975)
Threshold limit values for chemical substances in workroom air
adopted by ACGIH for 1975, Cincinnati, ACGIH.
ATWAL, O. S., & WILSON, T. (1974) Parathyroid gland changes following
ozone inhalation. Arch. environ. Health, 28: 91-100.
ATWAL, O. S., SAMAGH, B. S., & BHATNAGAR, M. K. (1975) A possible
autoimmune parathyroiditis following ozone inhalation: IIA
histopathologic, ultrastructural, and immunofluorescent study.
Am. J. Pathol., 80: 53-68.
BALCHUM, O. J. (1973) Toxicological effects of ozone, oxidant and
hydrocarbons. Proceedings of the Conference on Health Effects of
Air Pollution, Assembly of Life Sciences, National Academy of
Sciences-National Research Council, Washington, DC, Oct. 3-5. In:
Committee Print, Committee on Public Works, United States
Senate, Washington, DC, US Government Printing Office, pp.
489-503.
BALL, D. J. (1976) Photochemical ozone in the atmosphere of Greater
London. Nature (Lond.), 263: 580-582.
BARSKY, R. M. & BIRAKOS, J. N., ed. (1971) Profile of air pollution
control, Los Angeles, CA, County of Los Angeles Air Pollution
Control District, p. 73.
BARTH, D. S., ROMANOVSKY, J. C., KNELSON, J. H., ALTSHULLER, A. P., &
HORTON, R. J. M. (1971) Discussion (of national ambient air
quality standards). J. Air Pollut. Control Assoc., 21: 544-548.
BARTLETT, D. JR, FAULKNER, C. S., & COOK, K. (1975) Effect of chronic
ozone exposure on lung elasticity in young rats. J. appl.
Physiol., 37: 92-96.
BATES, D. V. & HAZUCHA, M. (1973) The short-term effects of ozone on
the human lung. Proceedings of the Conference on the Health
Effects of Air Pollution, Assembly of Life Sciences, National
Academy of Sciences-National Research Council, Wasington, DC,
Oct. 3-5. In: Committee Print, Committee on Public Works, United
States Senate, Washington DC, US Government Printing Office,
pp. 507-540.
BATES, D. V., BELL, G. M., BURNHAM, C. D., HAZUCHA, M., MANTHA, J.,
PENGELLY, L. D., & SILVERMAN, F. (1972) Short-term effects of
ozone on the lung. J. appl. Physiol., 32: 176-181.
BECKER, K. H. & SCHURATH, U. (1975) [The influence of oxides of
nitrogen on atmospheric oxidation processes.] Staub-Reinhall,
Luft, 35(4): 156-161 (in German).
BENNETT, G. (1962) Ozone contamination of high altitude aircraft
cabins. Aerospace Med., 33: 969-973.
BERRY, C. R. (1964) Differences in concentration of surface oxidant
between valley and mountain top conditions in the southern
Appalachians. J. Air Pollut. Control Assoc., 14(6): 238-239.
BILS, R. F. (1966) Ultrastructural alterations of alveolar tissue of
mice: I. Due to heavy Los Angeles smog. Arch. environ. Health,
12: 689-697.
BILS, R. F. (1970) Ultrastructural alterations of alveolar tissue of
mice: III. Ozone. Arch. environ. Health, 20: 468-480.
BILS, R. F. & ROMANOVSKY, J. C. (1967) Ultrastructural alterations of
alveolar tissue of mice: II. Synthetic photochemical smog. Arch
environ. Health, 14: 844-858.
BLOCH, W. N. JR, MILLER, F. J., & LEWIS, T. R. (1971) Pulmonary
hypertension in dogs exposed to ozone. Cincinnati, OH, Air
Pollution Control Office, US Environmental Protection Agency.
BOATMAN, E. S., SATO, S., & FRANK, R. (1974) Acute effects of ozone on
cat lungs. II: Structural. Am. Rev. respir. Dis., 110: 157-169.
BOBB, G. A. & FAIRCHILD, E. J. (1967) Neutrophil-to-lymphocyte ratio
as indicator of O3 exposure. Toxicol. appl. Pharmacol.,
11: 558-564.
BOOTH, S., DEGROOT, I., MARKUSH, R., & HORTON, R. J. M. (1965)
Detection of asthma epidemics in seven cities. Arch. environ.
Health, 10: 152-155.
BRANT, J. W. A. (1965) Human cardiovascular diseases and atmospheric
air pollution in Los Angeles, California. Int. J. Air Water
Pollut., 9: 219-231.
BRANT, J. W. A. & HILL, S. R. G. (1964) Human respiratory diseases and
atmospheric air pollution in Los Angeles, California. Int. J.
Air Water Pollut., 8: 259-277.
BRINKMAN, R., LAMBERTS, H. B., & VENINGA, T. S. (1964) Radiomimetic
toxicity of ozonized air. Lancet, 1: 133-136.
BUELL, G. C., TOKIWA, Y., & MUELLER, P. K. (1965) Potential
crosslinking agents in lung tissue; formation and isolation after
in vivo exposure to ozone. Arch. environ. Health,
10: 213-219.
BUELL, P., DUNN, J. E., JR, & BRESLOW, L. (1967) Cancer of the lung
and Los Angeles type air pollution: prospective study. Cancer,
20: 2139-2147.
BUTCHER, S.S. & RUFF, R. E. (1971) Effect of inlet residence time on
analysis of atmospheric nitrogen oxides and ozone. Anal. Chem.,
43(13): 1890-1892.
BYERS, D. H. & SALTZMAN, B. E. (1958) Determination of ozone in air by
neutral and alkaline iodide procedures. J. Am. Ind. Hyg. Assoc.,
19: 251-257.
CALIFORNIA STATE DEPARTMENT OF PUBLIC HEALTH (1955) Clean air for
California. Initial Report of the Air Pollution Study Project,
March 1955, 37 pp.
CALIFORNIA STATE DEPARTMENT OF PUBLIC HEALTH (1956) Clean air for
California. Second Report, March 1956, 10 pp.
CALVERT, J. G. (1976) Hydrocarbon involvement in photochemical smog
formation in Los Angeles atmosphere. Environ. Sci. Technol.,
10(3): 256-262.
CARSON, S. & GOLDHAMER, R. E. (1968) Biochemical defense mechanisms
against pulmonary irritants. Ohio, USA, Aerospace Medical
Research Laboratories, Aerospace Medical Division, Air Force
Systems Command, Wright-Patterson Air Force Base, pp. 129.
CASTLEMAN, W. L., DUNGWORTH, D. L., & TYLER, W. S. (1973a)
Histochemically detected enzymatic alterations in rat lung
exposed to ozone. Exp. mol. Pathol., 19: 402-421.
CASTLEMAN, W. L., DUNGWORTH, D. L., & TYLER, W. S. (1973b)
Cytochemically detected alterations of lung acid phosphatase
reactivity following ozone exposure. Lab. Invest., 29: 310-319.
CHALLEN, P. J. R., HICKISH, D. E., & BEDFORD, J. (1958) An
investigation of some health hazards in an inert-gas tungsten-arc
welding shop. Br. J. ind. ed., 15: 276-282.
CHOW, C. K. & TAPPEL, A. L. (1972) An enzyme protective mechanism
against lipid peroxidation damage to lungs of ozone-exposed rats.
Lipids, 7: 518-524.
CHOW, C. K. & TAPPEL, A. L. (1973) Activites of pentose shunt and
glycolytic enzymes in lungs of ozone-exposed rats. Arch.
environ. Health, 26: 205-208.
CHOW, C. K., DILLARD, C. J., & TAPPEL, A. L. (1974) Glutathione
peroxidase system and lysozyme in rats exposed to ozone and
nitrogen dioxide. Environ. Res., 7: 311-319.
CHRISTENSEN, E. & GIESE, A. C. (1954) Changes in absorption spectra of
nucleic acids and their derivatives following exposure to ozone
and ultraviolet radiation. Arch. Biochem. Biophys., 51:
208-216.
COFFIN, D. L. & BLOMMER, E. J. (1965) The influence of cold on
mortality from streptococci following ozone exposure. J. Air
Pollut. Control Assoc., 19: 523-524.
COFFIN, D. L. & BLOMMER, E. J. (1967) Acute toxicity of irradiated
auto exhaust. Its indication by enhancement of mortality from
streptococcal pneumonia. Arch. environ. Health, 15: 36-38.
COFFIN, D. L. & BLOMMER, E. J. (1970) Alteration of the pathogenic
role of streptococci group C in mice conferred by previous
exposure to ozone. In: Silver, I. H., ed. Aerobiology,
Proceedings of the 3rd International Symposium on Aerobiology,
University of Sussex, England, 1969. New York, Academic Press,
pp. 54-61.
COFFIN, D. L. & GARDNER, D. E. (1972a) Interaction of biological
agents and chemical air pollutants. Ann. occup. Hyg.,
15: 219-234.
COFFIN, D. L. & GARDNER, D. E. (1972b) The role of tolerance in
protection of the lung against secondary insults. In:
Proceedings of the International Conference of Occupational
Physicians of the Chemical Industry, Ludwigshafen, Germany,
April 1972, Research Triangle Park, NC, Environmental
Protection Agency, pp. 344-364.
COFFIN, D. L. & GARDNER, D. E. (1975) [Ozone Toxicology: Correlation
between tolerance and protective mechanisms of the lung.] Gig. i
Sanit., 1: 86-92 (in Russian).
COFFIN, D. L., BLOMMER, E. J., GARDNER, D. E., & HOLZMAN, R. S. (1968)
Effect of air pollution on alteration of susceptibility to
pulmonary infection. In: Proceedings of the 3rd Annual
Conference on Atmospheric Contamination in Confined Spaces,
Aerospace Medical Research Laboratory, pp. 71-80.
COFFIN, D. L., GARDNER, D. E., HOLZMAN R. S., & WOLOCK, F. J. (1968)
Influence of ozone on pulmonary cells. Arch. environ. Health,
16: 633-636.
COHEN, C. A., HUDSON, A. R., CLAUSEN, J. L., & KNELSON, J. H. (1972)
Respiratory symptoms, spirometry, and oxidant air pollution in
non-smoking adults. Am. Rev. resp. Dis., 105: 251-261.
CORTESI, R. & PRIVETT, O. S. (1972) Toxicity of fatty ozonides and
peroxides. Lipids, 7: 715-721.
COX, R. A., EGGLETON, A. E. J., DERWENT, R. G., LOVELOCK, J. E., &
PACK, D. H. (1975) Long-range transport of photochemical ozone in
North-Western Europe. Nature (Lond.), 255(5504): 118-121.
CRIEGEE, R. (1957) The course of ozonization of unsaturated compounds.
Rec. chem. Prog, 18: 111-120.
CRONIN, S. R. & GIRI, S. N. (1974) Effects of pulmonary irritants on
DNA, ATPase activity, and histamine on rat lung. Proc. Soc. Exp.
Biol. Med., 146: 120-125.
DARNALL, K. R., LLOYD, A. C., WINER, A. C., & PITTS, J. N., JR (1976)
Reactivity scale for atmospheric hydrocarbons based on reaction
with hydroxyl radical. Environ. sci. Technol. 10(7): 692-296.
DAVIS, I. (1961) Microbiologic studies with ozone. Mutagenesis of
ozone for Escherichia coli, Texas, USAF Aerospace Medical
Center, School of Aerospace Medicine, 9 pp. (Rep. No. 61-60).
DEANE, M., GOLDSMITH, J. R., & TUMA, D. (1965) Respiratory conditions
in outside workers: report on outside plant telephone workers in
San Francisco and Los Angeles. Arch. environ. Health,
10: 323-331.
DELUCIA, A. J., HOQUE, P.M., MUSTAFA, M. G., & CROSS, C. E. (1972)
Ozone interaction with rodent lung: effect on sulfhydryls and
sulfhydryl-containing enzyme activities. J. lab. clin. Med.,
80: 559-566.
DELUCIA, A. J., MUSTAFA, M. G., HUSSAIN, M. Z., & CROSS, C. E. (1975)
Ozone interaction with rodent lung: III. Oxidation of reduced
glutathione and formation of mixed disulfides between protein and
non-protein sulfhydrils. J. clin. Invest., 55: 794-802.
DEMERJIAN, K. L., KERR, J. A., & CALVERT, J. G. (1974) The mechanism
of photochemical smog formation. Adv. environ. Sci. Technol.,
4: 1-262.
DEMORE, W. B. & PATAPOFF, M. (1976) Comparison of ozone determinations
by ultraviolet photometry and gas-phase titoation. Environ. Sci.
Technol., 10(9): 897-899.
DERWENT, R. G. & STEWART, H. N. M (1973) Elevated ozone levels in the
air of Central London. Nature (Lond.), 241(5388): 342-343.
DILLARD, C. J., URRIBARRI, N., REDDY, K., FLETCHER, B., TAYLOR, S., DE
LUMEN, B., LANGBERG, S., & TAPPEL, A. L. (1972) Increased
lysosomal enzymes in lungs of ozone-exposed rats. Arch. environ.
Health, 25: 426-431.
DIMITRIADES, B. (1976) Photochemical oxidants in the ambient air of
the United States. Research Triangle Park, NC, US Environmental
Protection Agency, 192 pp. (EPA-600/3-76-017).
DIXON, J. R. & MOUNTAIN, J. T. (1965) Role of histamine and related
substances in development of tolerance to edemagenic gases.
Toxicol. appl. Pharmacol., 7: 756-766.
DOWELL, A. R., LOHRBAUER, L. A., HURST, D., & LEE, S. D. (1970) Rabbit
alveolar macrophage damage caused by in vivo O3 inhalation.
Arch. environ. Health, 21: 121-127.
DUNGWORTH, D. L. (1976) Short-term effects of ozone on lungs of rats,
mice, and monkeys. Environ. Health Perspect., 16: 179.
DUNGWORTH, D. L., CASTLEMAN, W. L., CHOW, C. K., MELLICK, P. W.,
MUSTAFA, M. G., TARKINGTON, B., & TYLER, W. S. (1975) Effect of
ambient levels of ozone on monkeys. Fed. Proc., 34: 1670-1674.
DURHAM, W. (1974) Air pollution and student health. Arch. environ.
Health, 28: 241-254.
EASTON, R. E. & MURPHY, S. D. (1967) Experimental ozone preexposure
and histamine. Arch. environ. Health, 15: 160-166.
EGLITE, M. (1968)[The problem of hygienic assessment of atmospheric
ozone.] Gig. i Sanit., 33: 17-22 (in Russian).
EMIK, L. O., PLATA, R. L., CAMPBELL, K. I., & CLARKE, G. L. (1971)
Biological effects of urban air pollution. Arch. environ.
Health, 23: 335-342.
ENVIRONMENT AGENCY (1975a) [Proceedings of the Second US Japan
Conference on Photochemical Air Pollution.] Tokyo, Japan (in
Japanese).
ENVIRONMENT AGENCY (1975b) [Air Pollution in Japan. Air monitoring
data in fiscal year 1974.] Tokyo, Japan, Air Pollution Control
Division, Air Quality Bureau, pp. 1200-1240 (in Japanese).
ENVIRONMENT AGENCY (1976) [Quality of the environment in Japan.]
Tokyo, Japan, Environment Agency, pp. 152-153 (in Japanese).
ERLICH, R., FINDLAY, J. C., FENTERS, J. D., & GARDNER, D. E. (1977)
Health effects of short-term inhalation of nitrogen dioxide and
ozone mixtures. Environ. Res., 14: 223-231.
EVANS, M. J., MAYR, W., BILS, R. F., & LOOSLI, C. G. (1971) Effects of
ozone on cell renewal in pulmonary alveoli of ageing mice. Arch.
environ. Health, 22: 450-453.
FABIAN, P. & PRUCHNIEWlCZ, P. G. (1973) Meridional distribution of
tropospheric ozone from ground-based registrations between Norway
and South Africa. Pure appl. Geophys., 106-108 (V-VII):
1027-1035.
FAIRCHILD, E. J., II (1963) Neurohumoral factors in injury from
inhaled irritants. Arch. environ. Health, 6: 79-86.
FAIRCHILD, E. J., II (1967) Tolerance mechanisms. Determinants of lung
responses to injurious agents. Arch. environ. Health,
14: 111-125.
FAIRCHILD, E. J., II & BOBB, G. A. (1965) The effect of spinal cord
lesions on the pathophysiology of a lung irritant ozone.
Toxicol. appl. Pharmacol., 7: 483 (Abstract).
FARWELL, S. O. & RASMUSSEN, R. A. (1976) Limitations of the FPD and
ECD in atmospheric analysis: A review. J. Chromatogr. Sci.,
14: 224-234.
FEDERAL REPUBLIC OF GERMANY, Ministry of Internal Affairs (1974)
Nox CnH volatile fluoride and chlorine compounds.
FETNER, R. H. (1958) Chromosome breakage in Victa faba by ozone.
Nature (Lond.), 181: 504-505.
FETNER, R. H. (1962) Ozone-induced chromosome breakage in human cell
cultures. Nature (Lond.), 194: 793-794.
FETNER, R. H. (1963) Mitotic inhibition induced in grasshopper
neuroblasts by exposure to ozone. USAF School of Aerospace
Medicine (Technical Documentary Report SAM-TDR 63-39).
FLETCHER, B. L. & TAPPEL, A. L. (1973) Protective effects of dietary
alphatocopherol in rats exposed to toxic levels ozone and
nitrogen dioxide. Environ. Res., 6: 165-175.
FOLINSBEE, L. J., SILVERMAN, F., & SHEPHARD, R. J. (1975) Exercise
responses following ozone exposure. J. appl. Physiol.,
38: 996-1001.
FOURNIER, E. (1973) Radicaux libres et toxicologie. Eur. J. Toxicol.,
6: 109-122.
FRANK, H. R., BRAIN, J. D., & SHERRY, D. E. (1970a) Unilateral
tolerance and altered elastic behaviour in rabbits. Am. Ind.
Hyg. Assoc. J., 31: 31 (Abstract section).
FRANK, N. R., YOKOYAMA, E., WARANABE, S., SHERRY, D. E., & BRAIN, J.
D. (1970b) An ozone Journal presented at the American Medical
Association, Air Pollution Medical Research Conference New
Orleans, 5-7 Oct., 19 pp.
FRANK, R., FLESCH, J.P., & BRAIN, J. D. (1971) Effect of ozone on
elastic behaviour of excised lungs of dogs. Environ. Res.,
4: 343-354.
FREEMAN, G., STEPHENS, R. J., CRANE, S.C., & FURIOSI, N.J. (1968)
Lesion of the lung in rats continuously exposed to two parts per
million of nitrogen dioxide. Arch. environ. Health,
17: 181-192.
FREEMAN, G., STEPHENS, R. J., COFFIN, D. L., & STARA, J. F. (1973)
Changes in dogs' lungs after long-term exposure to ozone. Arch.
environ. Health, 26: 209-216.
FREEMAN, G., JULIOS, L. T., FURIOSI, N. J., MUSSENDEN, R., STEPHENS,
R. J., & EVANS, M. J. (1974) Pathology of pulmonary disease from
exposure to interdependent ambient gases (NO2 & O3). Arch.
environ. Health, 29: 203-210.
FRIBERG, L., HOLMA, B., & RYLANDER, R. (1972) Animal lung reactions
after inhalation of lead and ozone. Environ. Physiol. Biochem.,
2: 170-178.
FUKASE, O., ISOMURA, K., & WATANABE, H. (1975a) Effect of ozone on
glutathione in vivo. J. Jpn. Soc. Air Pollut., 10: 58-62 (in
Japanese).
FUKASE, O., ISOMURA, K., & WATANABE, H. (1975b) Effect of ozone on
vitamin C in vivo. J. Jpn. Soc. Air Pollut., 10: 63-66.
FUJII, T. (1972) Studies on air pollution by photochemical reaction.
1. The results of field research of photochemical smog in Osaka,
1971. In: Proceedings of the Research Section, Osaka
Environmental Pollution Control Centre, No. 3, pp. 21-34 (in
Japanese).
GARDNER, D. E. (1971) Environmental influences on living alveolar
macrophages. A dissertation submitted to the Division of
Graduate Studies of the University of Cincinnati, Ann Arbor,
Michigan, University Microfilms International, pp. 191.
GARDNER, D. E., PFITZER, E. A., CHRISTIAN, R. T., & COFFIN, D. L.
(1971) Loss of protective factor for alveolar macrophages when
exposed to ozone. Arch. intern. Med., 127: 1078-1084.
GARDNER, D. E., LEWIS, T. R., ALPERT, S. M., HURST, D. J., & COFFIN,
D. L. (1972) The role of tolerance in pulmonary defense
mechanisms. Arch. environ. Health, 25: 432-438.
GARDNER, D. E., ILLING, J. W., MILLER, F. J., & COFFIN, D. L. (1974a)
The effect of ozone on pentobarbital sleeping time in mice.
Chem. Pathol. Pharmacol., 9: 689-700.
GARDNER, D. E., ILLING, J. W., & COFFIN, D. L. (1974b) Enhancement of
effect of exposure to O3 and NO2 by exercise. Toxicol. appl.
Pharmacol., 29: 129-130.
GARDNER, M. B. (1966) Biological effects of urban air pollution. III.
Lung tumors in mice. Arch. environ. Health, 12: 305-513.
GARDNER, M. B., LOOSLI, C. G., HAINES, B., BLACKMORE, W., & TEEBKEN,
D. (1969) Histopathologic findings in rats exposed to ambient and
filtered air. Arch. environ. Health, 19: 637-647.
GOLDSMITH, J. R. & NADEL, J. A. (1969) Experimental exposure of human
subjects to ozone. J. Air Pollut. Control Assoc., 19: 329-330.
GOLDSTEIN B. D. (1973) Hydrogen peroxide in erythrocytes. Detection in
rats and mice inhaling ozone. Arch. environ. Health,
26: 279-280.
GOLDSTEIN B. D. (1976) Combined exposure to ozone and nitrogen
dioxide. Env. Health Perspect., 13: 107-110.
GOLDSTEIN B. D., PEARSON, B., LODI, C., BUCKLEY, R. D., & BALCHUM, O.
J. (1968) The effect of ozone on mouse blood in vivo. Arch.
environ. Health, 16: 648-650.
GOLDSTEIN B. D., LODI, C., COLLINSON, C., & BALCHUM, O. J. (1969)
Ozone and lipid peroxidation. Arch. environ. Health,
18: 631-635.
GOLDSTEIN B. D., BUCKLEY, R. D., CARDENAS, R., & BALCHUM, O. J. (1970)
Ozone and vitamin E. Science, 169: 605.
GOLDSTEIN B. D., LAI, L. Y., ROSS, S. R., & CUZZI-SPADA, R. (1973)
Susceptibility of inbred mouse strains to ozone. Arch. environ.
Health, 27: 412-413.
GOLDSTEIN B. D., SOLOMON, S., PASTERNACK, B. S., & BICKERS, D. R.
(1975) Decrease in rabbit lung microsomal cytochrome P-450 levels
following ozone exposure. Chem. Pathol. Pharmacol.,
10: 759-762.
GOLDSTEIN, E., TYLER, W. S., HOEPRICH, P. D., & EAGLE, C. (1971a)
Ozone and the antibacterial defense mechanisms of the murine
lung. Arch. intern. Med., 127: 1099-1102.
GOLDSTEIN, E., TYLER, W. S., HOEPRICH, P. D., & EAGLE, C. (197lb)
Adverse influence of ozone on pulmonary bactericidal activity of
murine lung. Nature (Lond.), 229: 262-263.
GOLDSTEIN, E., EAGLE, M. C., & HOEPRICH, P. D. (1972) Influence of
ozone on pulmonary defense mechanisms of silicotic mice. Arch.
environ. Health, 24: 444-448.
GOLDSTEIN, E., WARSHAUER, D., LIPPERT, W., & TARKINGTON, B. (1974)
Ozone and nitrogen dioxide exposure. Arch. environ. Health,
28: 85-90.
GREGORY, A. R., RIPPERTON, L. A., & MILLER, B. (1967) Effect of
neonatal thymectomy on the development of ozone tolerance in
mice. Am. Ind. Hyg. Assoc. J., 28: 278-282.
GRIMSRUD, E. P., WESTBERG, H. H., RASMUSSEN, R. A. (1975) Atmospheric
reactivity of monoterpene hydrocarbons, NOx photooxidation and
ozonolysis. Int. J. Chem. Kin. Symp., 1: 183-195.
GUICHERIT, R. (1975) [Photochemical smog formation in the
Netherlands.] Delft, Netherlands, TNO Institute for
Environmental Hygiene, p. 27 (in Dutch).
HAAGEN-SMIT, A. J. (1952) Chemistry and physiology of Los Angeles
smog. Ind. Eng. Chem., 44: 1342-1346.
HACKNEY, J. D., LINN, W. S., LAW, D.C., KARUZA, S. K., GREENBERG, H.,
BUCKLEY, R. D., & PEDERSEN, E. E. (1975) Experimental studies on
human health effects of air pollutants. III. Two-hour exposure to
ozone alone and in combination with other pollutant gases. Arch.
environ. Health, 30: 385-390.
HACKNEY, J. D., LINN, W. S., KARUZA, S. K., BUCKLEY, R. D., LAW, D.C.,
BATES, D. V., HAZUCHA, M., PENGELLY, L. D., & SILVERMAN, F.
(1977) Effects of ozone exposure in Canadians and Southern
Californians. Arch. environ. Health, 34: 110-116.
HALLET, W. Y. (1965) Effect of ozone and cigarette smoke on lung
function. Arch. environ. Health, 10: 295-302.
HAMMER, D. I., HASSELBLAD, V., PORTNOY, B., & WEHRLE, P. F. (1974) Los
Angeles student nurse study: Daily symptom reporting and
photochemical oxidants. Arch. environ. Health., 28: 255-260.
HASSELBLAD, V., CREASON, J.P., & NELSON, W. C. (1976) Regression
using "hockey stick" functions. Research-Triangle Park, NC, US
Environmental Protection Agency, Health Effects Research
Laboratory, 12 pp. (EPA-600/1-76-024).
HATTORI, K., KATO, N., KINOSHITA, S., & SUNADA, T. (1963) Protective
effect of ozone in mice against whole-body x-irradiation. Nature
(Lond.), 198: 1220.
HAUSKNECHT, R. (1960) Air pollution effects reported by California
residents from the California Health Survey, CA, USA, State of
California, Department of Public Health, pp. 1-55.
HAZUCHA, M., SILVERMAN, F., PARENT, C., FIELD, S., & BATES, D. V.
(1973) Pulmonary function in man after short-term exposure to
ozone. Arch. environ. Health, 27: 183-188.
HECHTER, H. H. & GOLDSMITH, J. R. (1961) Air pollution and daily
mortality. Am. J. med. Sci., 241: 581-588.
HENSCHLER, D. (1960) [Protective effect of pre-treatment with low gas
concentrations against lethal pulmonary edema caused by irritant
gas.] Arch. exp. Pathol. Pharmacol., 238: 66-67 (in German).
HENSCHLER, D., STIEN, A., BECK, H., & NEUMAN, W. (1960) [Olfactory
threshold of some important irritant gases and manifestations in
man by low concentrations.] Arch. Gewerbepathol. Gewerbehyg.,
17: 547-570 (in German).
HENSCHLER, D., HAHN, E., & ASSMANN, W. (1964) [Conditions for
increasing resistance by repeated inhalation of irritating
oedema- forming gases.] Naunyn-Schmiedebergs Arch. exp. Pathol.
Pharmakol., 249: 325-342 (in German).
HEUSS, J. M. & GLASSON, W. A. (1968) Hydrocarbon reactivity and eye
irritation. Environ. Sci. Technol., 3: 1109-1116.
HODGESON, J. A. (1972) Review of analytical methods for atmospheric
oxidants measurements. Int. J. environ. anal. Chem.,
2: 113-132.
HOLLAND, G. J., BENSON, D., BUSH, A., RICH, G. Q., & HOLLAND, R. P.
(1968) Air pollution simulation and human performance. Am. J.
public Health, 58: 1684-1691.
HOLZMAN, R. S., GARDNER, D. E., & COFFIN, D. L. (1968) In vivo
inactivation of lysosyme by ozone. J. Bacteriol.,
96: 1562-1566.
HUBER, G. L., MASON, R. J., LAFORCE, M., SPENCER, N.J., GARDNER, D.
E., & COFFIN, D. L. (1971) Alteration in the lung following the
administration of ozone. Arch. intern. Med., 128: 81-87.
HUETER, F. G., CONTNER, G. L., BUSCH, K. A., & HINNERS, R. G. (1966)
Biological effects of atmospheres contaminated by auto exhaust.
Arch. environ. Health, 12: 553-560.
HURST, D. J. & COFFIN, D. L. (1971) Ozone effect on lysosomal
hydrolases of alveolar macrophages in vitro. Arch. intern. Med.,
127: 1059-1063.
HURST, D. J., GARDNER, D. E., & COFFIN, D. L. (1970) Effect of O3 on
acid hydrolases of the pulmonary alveolar macrophage.
J. Reticuloendoth. Soc., 8: 288-300.
IKEMATSU, T., YAMAUCHI, Y., SAITO, H., NAGAOKA, S., & ENDO, R. (1976)
Tonsils and environmental pollution (second report) Influence of
ozone upon rabbit tonsils. Jpn. J. Tonsil., 15: 5-8.
ILO (1977) Occupational exposure limits for airborne toxic
substances. Geneva, International Labour Office, pp. 164-165
(Occupational Safety and Health Series No. 37).
ISOMURA, K., FUKASE, O., & WATANABE, H. (1976) [Cytoxicities and
mutagenicities of gaseous air pollutants.] J. Jpn. Soc. Air
Pollut., 11: 59-64 (in Japanese).
JAFFE, L. S. (1962) The biological effects of ozone on man and
animals. Am. J. public Health, 57: 1269-1277.
JAPAN PUBLIC HEALTH ASSOCIATION (1976) The relationship between
photochemical airpollution and adverse health effects-1975
Report, Tokyo, JPHA, pp. 24-27 (in Japanese).
JEGIER, Z. & P'AN, A. Y. S. (1973) Ozone as an air pollutant. Can. J.
public Health, 64: 161-166.
KAGAWA, J. & TOYAMA, T. (1975a) Effects of ozone and brief exercise on
specific airway conductance in man. Arch. environ. Health,
30: 36-39.
KAGAWA, J. & TOYAMA, T. (1975b) Photochemical air pollution; its
effects on respiratory function of elementary school children.
Arch. environ. Health, 30: 117-122.
KAGAWA, J., TOYAMA, T., & NAKAZA, M. (1976) Pulmonary function test in
children exposed to air pollution. In: Finkel, A. J. & Duel, W.
C., ed. Clinical implications of air pollution research. Acton,
MA, USA, Publishing Sciences Group Inc., pp. 305-320.
KERR, H. D., KULLE, T. J., MCILHANEY, M. L., & SWIDERSKY, P. (1975)
Effects of ozone on pulmonary function in normal subjects. An
environmental-chamber study. Am. Rev. resp. Dis., 111: 763-773.
KING, M. E. (1961) Biochemical effects of ozone, doctoral
dissertation, Illinois Institute of Technology, May 1961, pp.
1-85.
KLEINFELD, M., GIEL, C., & TABERSHAW, I. R. (1957) Health hazards
associated with inert-gas shielded metal arc welding. Arch. ind.
Health, 15: 27-31.
KONIGSBERG, A. S. & BACHMAN, C. H. (1970) Ozonized atmosphere and
gross motor activity of rats. Int. J. Biometeor, 14: 261-266.
KONNO, S. & OKITA, T. (1974) Determination of peroxacetyl (PAN)
produced in the atmosphere or in a photochemical reaction chamber
(1). Bull. Inst. Public Health, Japan, 23: 50-58.
KOTIN, P. & FALK, H. L. (1956) The experimental induction of pulmonary
tumors in strain-A mice after their exposure to an atmosphere of
ozonized gasoline. Cancer, 9: 910-917.
KOTIN, P., FALK, H. L., & MCCAMMON, C. J. (1958) III. The experimental
induction of pulmonary tumors and changes in the respiratory
epithelium in C 57 BL mice following their exposure to an
atmosphere of ozonized gasoline. Cancer, 11: 473-481.
KUDRJAVCEVA, O. F. (1963) [On the possibility of chronic effects of
ozone in working conditions.] Gig. truda i Prof. Zabol., 6: 52
(in Russian).
KULLE, T. J. (1972) The effects of ozone and formaldehyde on the
trigeminal nasal sensory system. Thesis, University of
Cincinnati.
KUSOMOTO, S., NARUYAMA, Y., YONEKAWA, E., ODA, H., & NAKAJIMA, T.
(1976) Acute effects of photochemical oxidants and ozone exposure
on mice. J. Jpn. Soc. Air Pollut., 11: 70-80 (in Japanese).
KYEI-ABOAGYE, K., HAZUCHA, M., WYSZOGRODSKI, I., RUBINSTEIN, D., &
AVERY, M. E. (1973) The effect of ozone exposure in vivo on the
appearance of lung tissue lipids in the endobronchial lavage of
rabbits. Biochem. Biophys. Res. Commun., 54: 907-913.
LAGERWERFF, J. M. (1963) Prolonged ozone inhalation and its effects on
visual parameters. Aerospace Med., 34: 479-486.
LARSEN, R. I. (1969) A new mathematical model of air pollution
concentration averaging time and frequency. J. Air Pollut.
Control Assoc., 19: 24-30.
LARSEN, R. I. (1974) An air quality data analysis system for
interrelating effects, standards, and needed source
reductions-part 2. J. Air Pollut. Control Assoc., 24(6):
551-558.
LEWIS, T. R., HUETER, F. G., & BUSCH, K. A. (1967) Irradiated
automobile exhaust: its effects on reproduction in mice. Arch.
environ. Health, 15: 26-35.
LITT, R. S., VAUGHAN, S., BIRKINSHAW, P., COIT, H., & SANDERS, B.
(1968) Effects of low concentrations of ozone on temporal
discrimination. In: Air pollution project: An educational
experiment in self-directed research, 1968, Pasadena,
Associated Students of the California Institute of Technology,
pp. 51-64.
LOOSLI, C. G., BUCKLEY, R. D., HERTWECK, M. S., HARDY, J. D., RYAN, D.
P., STINSON, S., & SEREBRIN, R. (1972) Pulmonary response of mice
exposed to synthetic smog. Ann. oecup. Hyg., 15: 251-260.
MACLEAN, S. A., LONOWELL, A. C., & BLOGOSLAWSKI, W. J. (1973) Effects
of ozone-treated sea water on the spawned, fertilized, meiotic,
and cleaving eggs of the commercial American oyster. Mutat.
Res., 21: 283-285.
MCJILTON, C., THIELKE, J., & FRANK, R. (1972) Ozone uptake model for
the respiratory system. Am. Ind. Hyg. Assoc. J., 33: 20.
MCMILLAN, R. S., WISEMAN, D. H., HANES, B., & WEHRLE, P. F. (1969)
Effects of oxidant air pollution on peak expiratory flow rates in
Los Angeles school children. Arch. environ. Health, 18:
941-949.
MAHONEY, L. E. J. (1971) Wind flow and respiratory mortality in Los
Angeles. Arch. environ. Health, 22: 344-347.
MATSUMURA, Y. (1970a) The effects of ozone, nitrogen dioxide, and
sulfur dioxide on experimentally induced allergic respiratory
disorder in guinea pigs. I. The effect on sensitization with
albumin through the airway. Am. Rev. respir. Dis.,
102: 430-437.
MATSUMURA, Y. (1970b) The effects of ozone, nitrogen dioxide and
sulfur dioxide on experimentally induced allergic respiratory
disorder in guinea pigs. II. The effects of ozone on the
absorption and the retention of antigen in the lung. Am. Rev.
respir. Dis., 102: 438-443.
MATSUMURA, Y., MIZUNO, K., MIYAMOTO, T., SUZUKI, T., & OSHIMA, Y.
(1972) The effects of ozone, nitrogen dioxide, and sulfur dioxide
on experimentally induced allergic respiratory disorder in guinea
pigs, IV effects on respiratory sensitivity to inhaled
acetylcholine. Am. Rev. respir. Dis., 105: 262-267.
MATZEN, R. N. (1957) Development of tolerance to ozone in reference to
pulmonary edema. Am. J. Physiol., 190: 84-88.
MENDENHALL, R. M. & STOKINGER, H. E. (1959) Tolerance and
cross-tolerance development to atmospheric pollutants, ketene and
ozone. J. appl. Physiol., 14: 923-926.
MENDENHALL, R. M. & STOKINGER, H. E. (1962) Films from lung washings
as a mechanism model for lung injury by ozone. J. appl.
Physiol., 17: 28-32.
MENZEL, D. B. (1970) Toxicity of ozone, oxygen and radiation. Ann.
Rev. Pharmacol., 10: 379-394.
MENZEL, D. B. (1971) Oxidation of biologically active reducing
substances by ozone. Arch. environ. Health, 23: 149-153.
MENZEL, D. B., ROEHM, J. N., & LEE, S. D. (1972) Vitamin E: the
biological and environmental antioxidant. J. agric. food Chem.,
20: 481-486.
MENZEL, D. B., SLAUGHTER, R. J., BRYANT, A.M., & JAUREGUI, H. O.
(1975) Heinz bodies formed in erythrocytes by fatty acid ozonides
and ozone. Arch. environ. Health, 30: 296-301.
MERZ, T., BENDER, M. A., KERR, H. D., & KULLE, T. J. (1975)
Observations of aberrations in chromosomes of lymphocytes from
human subjects exposed to ozone at a concentration of 0.5 ppm for
6 and 10 hours. Mutat. Res., 31: 299-302.
MIKAMI, R. & KUDO (1973) Air pollution -- especially in terms of
oxidants and respiratory organs. Intern. Med. (Japan),
32: 837-844.
MILLER, F. J. (1977) A mathematical model of transport and removal of
ozone in mammalian lungs. Thesis submitted to the Graduate
Faculty of North Carolina State University, Raleigh, NC, USA, pp.
66.
MILLER, P. R., MCCUTCHAN, M. H., & MILLIGAN, H. P. (1972) Oxidant air
pollution in the Central Valley, Sierra Nevada foothills and
Mineral King Valley of California. Atmos. Environ., 6: 623-633.
MILLER, S. & EHRLICH, R. (1958) Susceptibility to respiratory
infections of animals exposed to ozone. J. infect. Dis.,
103: 145-149.
MILLS, C. A. (1957) Respiratory and cardiac deaths in Los Angeles
smogs. Am. J. med. Sci., 233: 379-386.
MIZOGOUCHI, I., OSAWA, M., SATO, Y., MAKINO, K., & YAGYU, H. (1973) A
study on erythrocyte and photochemical smog. I: Effects of air
pollutants on erythrocyte resistance. J. Jpn. Soc. Air Pollut.,
8: 414.
MOORMAN, W. J., CHMIEL, J. J., STARA, J. F., & LEWIS, T. R. (1973)
Comparative decomposition of ozone in the nasopharynx of beagles.
Acute vs chronic exposure. Arch. environ. Health, 26: 153-155.
MOTLEY, H. L., SMART, R. H., & LEFTWICH, C. I. (1959) Effect of
polluted Los Angeles air (smog) on lung volume measurements.
J. Am. Med. Assoc., 171: 1469-1477.
MOUNTAIN, J. T. (1963) Detecting hypersusceptibility to toxic
substances: an appraisal of simple blood tests. Arch. environ.
Health, 6: 357-365.
MOUNTAIN, J. T., WAGNER, W. D., FAIRCHILD, E. J., STOCKELL, F. R., JR,
& STOKINGER, H. E. (1960) Biochemical effects of ozone and
nitrogen dioxide on laboratory animals. In: 138th National
Meeting of The American Chemical Society, New York, September
1960, Cincinnati, OH, US Dept of Health, Education and Welfare,
32 pp.
MUDD, J. B., LEAVITT, R., ONGUN, A., & MCMANUS, T. T. (1969) Reaction
of ozone with amino acids and proteins. Atmos. Environ.,
3: 669-682.
MUELLER, P. X., LOEB, L., & MAPES, W. H. (1973) Decomposition rates of
ozone in living areas. Environ. Sci. Technol., 7: 342-346.
MURPHY, S. D., LENA, J. K., ULRICH, C. E., & DAVIS, H. V. (1963)
Effects on animals of exposure to auto exhaust. Arch. environ.
Health, 7: 60-70.
MURPHY, S. D., ULRICH, C. E., FRANKOWITZ, & XINTARAS, C. (1964a)
Altered function in animals inhaling low concentrations of ozone
and nitrogen dioxide. Am. Ind. Hyg. Assoc. J., 25: 246-253.
MURPHY, S. D., DAVIS, H. V., & ZARATZIAN (1964b) Biochemical effects
in rats from irritating air contaminants. Toxicol. appl.
Pharmacol., 6: 520-528.
MUSTAFA, M. G. (1975) Influence of dietary vitamin E on lung cellular
sensitivity to ozone in rats. Nutr. Rep. Int., 11: 473-476.
MUSTAFA, M. G., DELUCIA, A. J., YORK, G. K., ARTH, C., & CROSS, C. E.
(1973) Ozone interaction with rodent lung. II. Effects on oxygen
consumption of mitochondria. J. lab. clin. Med., 82: 357-365.
NAKAJIMA, T., KUSUMOTO, S., TSUBOTA, Y., YONEKAWA, E., YOSHIDA, R.,
MOTOMIYA, K., ITO, K., IDE, G., & OTSU, H. (1972)
Histopathological studies on the respiratory organs of mice
exposed to photochemical oxidants and auto exhaust. Proc. Osaka
Prefect. Inst. Public Health, 10: 35-42 (in Japanese).
NATIONAL ACADEMY OF SCIENCES (1977) Ozone and other photochemical
oxidants, Washington DC, Printing and Publishing Office,
National Academy of Sciences, 719 pp.
NEDERBRAGT, G. W., VAN DER HORST, N., & VAN DUIJN, J. (1965) Rapid
ozone determination near an accelerator. Nature (Lond.),
206: 4979.
NEVSKAJA, A. I. & DITERIHS, D. D. (1957) Problems of hygiene of labor
in H2O2 production by electrodynamic method. Gig. truda i prof
Zabol., 4: 16.
NEVSKAJA, A. I. & KOCETKOVA, T. A. (1961 ) The toxicology of ozone and
sulfuric acid aerosol in their combined action. Gig. truda i
prof Zabol., 1: 20-29.
NIEBOER, H. & VAN HAM, J. (1976) Peroxyacetyl nitrate (PAN) in
relation to ozone and some meteorological parameters at Delft in
the Netherlands. Atmos. Environ., 10: 115-120.
NIEDING, VON, G. WAGNER, H. M., LOLLGEN, H., & KREKELER, H. (1977)
[Acute effects of ozone on lung function in man. VDI Colloquium
on ozone and other substances in photochemical smog.]
VDI-Berichte, 270: 123-129 (in German).
NORTH ATLANTIC TREATY ORGANIZATION (1974) Air Quality criteria for
photochemical oxidants and related hydrocarbons. A report by the
Expert Panel on Air Quality Criteria, Brussels, Belgium, NATO
Committee on the Challenges of Modern Society, 366 pp.
OHMORI, K. (1974) [The effect of photochemical smog on humans,
especially biological effects by low concentrations of ozone.]
Environ. Pollut. Control. 10: 1042-1046 (in Japanese).
PACE, D. M., LANDOLT, P. A., & AFTONOMOS, B. T. (1969) Effects of
ozone on cells in vitro. Arch. environ. Health, 18: 165-170.
PALMER, M. S., SWANSON, D. H., & COFFIN, D. L. (1971) Effect of O3 on
benzpyrene hydroxylase activity in the Syrian golden hamster.
Cancer Res., 31: 730-733.
PALMER, M. S., EXLEY, R. W., & COFFIN, D. L. (1972) Influence of
pollutant gases on benzpyrene hydroxylase activity. Arch.
environ. Health, 25: 439-442.
P'AN, A. Y. S. & JEGIER, Z. (1970) The effect of sulfur dioxide and
ozone on acetylcholinesterase. Arch. environ. Health,
21: 498-501.
P'AN, A. Y. S. & JEGIER, Z. (1972) Trypsin protein esterase in
relation to ozone-induced vascular damage. Arch. environ.
Health, 24: 233-236.
P'AN, A. Y. S., BELAND, J., & JEGIER, Z. (1972) Ozone-induced arterial
lesions. Arch. environ. Health, 24: 229-232.
PEARLMAN, M. E., FINKLEA, J. F., SHY, C. M., VAN BRUGGEN, J., &
NEWILL, V. A. (1971) Chronic oxidant exposure and epidemic
influenza. Environ. Res., 4: 129-140.
PENHA, P. D., AMARAL, L., & WERTHAMER, S. (1972) Ozone air pollutants
and lung damage. Ind. Med., 41: 17-20.
PLOPPER, C. G., DUNGWORTH, D. L., & TYLER, W. S. (1973a) Pulmonary
lesions in rats exposed to ozone. Am. J. Pathol., 71: 375-394.
PLOPPER, C. G., DUNGWORTH, D. L., & TYLER, W. S. (1973b)
Ultrastructure of pulmonary alveolar macrophages in situ in
lungs from rats exposed to ozone. Am. Rev. respir. Dis.,
108: 532-638.
PRAT, R., NOFRE, C., & CIER, A. (1968) Effets de l'hypochlorite de
sodium, de l'ozone et des radiations ionisantes sur les
constituants pyrimidiques d'Escherichia coli. Ann. Inst.
Pasteur, 114: 595-607.
PURVIS, M. R., MILLER, S., & EHRLICH, R. (1961) Effect of atmospheric
pollutants on susceptibility to respiratory infection. I. Effect
of ozone. J. infect. Dis., 109: 238-242.
RASMUSSEN, R. A. (1970) Isoprene: Identified as a forest-type emission
to the atmosphere. Environ. Sci. Technol., 4(8): 667-671.
RASMUSSEN, R. A. (1972) What do the hydrocarbons from trees contribute
to air pollution? J. Air Pollut. Control Assoc., 22: 537-543.
RAVEN, P. B., DRINKWATER, B. L., RUHLING, R. O., BOLDUAN, N., TAGUCHI,
S., GLINER, J., & HORVATH, S. M. (1974) Effect of carbon monoxide
and peroxyacetyl nitrate on man's maximal aerobic capacity.
J. appl. Physiol., 36: 288-293.
REGENER, V. H. (1964) Measurement of atmospheric ozone with the
chemiluminescent method. J. geophys. Res., 69: 3795-3800.
REGENER, V. H. & ALDAZ, L. (1969) Turbulent transport near the ground
as determined from measurements of the ozone flux and the ozone
gradient. J. geophys. Res., 74: 6935-6942.
RENZETTI, N. A. & GOBRAN, V. (1957) Studies of eye irritation due to
Los Angeles smog 1954-1956. San Marino, CA, Air Pollution
Foundation, pp. 170.
REYNOLDS, R. W. & CHAFFEE, R. R. J. (1970) Studies on the combined
effects of ozone and a hot environment on reaction time in
subhuman primates. In: Project clean air, Santa Barbara,
California University, pp. 1-8 (California University Res. Proj.
S-6.).
RICHARDSON, N. A. & MIDDLETON, W. C. (1957) Evaluation of filters for
removing irritants from polluted air. Los Angeles, University
of California, Dept of Engineering, 31 pp. (Report No. 57-43).
RICHARDSON, N. A. & MIDDLETON, W. C. (1958) Evaluation of filters for
removing irritants from polluted air. Heat. Piping Air Cond.,
30: 147-154.
RIPPERTON, L. A., JEFFRIES, H. E., & WORTH, J. B. (1971) Relationship
of measurements in nonurban air to air pollution: Ozone and
oxides of nitrogen, CP 13B, In: England, H. M. & Berry, W. T.,
ed. Proceedings of the Second International Clean Air Congress.
New York, Academic Press, pp. 386-390.
ROEHM, J. N., HADLEY, J. G., & MENZEL, D. B. (1971a) Oxidation of
unsaturated fatty acids by ozone and nitrogen dioxide. Arch.
environ. Health, 23: 142-148.
ROEHM, J. N., HADLEY, J. G., & MENZEL, D. B. (1971b) Antioxidants vs
lung disease. Arch. intern. Med., 128: 88-93.
ROEHM, J. N., HADLEY, J. G., & MENZEL, D. B. (1972) The influence of
vitamin E on the lung fatty acids of rats exposed to O3. Arch.
environ. Health, 24: 237-242.
ROKAW, S. N. & MASSEY, F. (1962) Air pollution and chronic respiratory
disease. Am. Rev. respir. Dis., 86: 703-704.
ROTH, R. P. & TANSY, M. F. (1972) Effects of gaseous air pollutants on
gastric secreto-motor activities in the rat. J. Air Pollut.
Control Assoc., 22: 796-709.
RUDOLF, W. (1974) [Investigation of oxidants in the city centre of
Frankfurt.] Schriftenr. Ver. wasser-, Boden- Lufthyg.,
42: 65-75 (in German).
SABERSKY, R. H., SINEMA, D. A., & SHAIR, F. H. (1973) Concentrations,
decay rates, and removal of ozone and their relation to
establishing clean indoor air. Env. Sci. Technol., 7: 347-353.
SACHSENMAIER, W., SIEBS, W., & TAN, T. (1965) [Effects of ozone upon
mouse ascites tumor cells and upon chick fibroblasts in tissue
culture.] Z. Krebsforsch., 67: 113-126 (in German).
SANDALLS, F. J., PENKETT, S. A., & JONES, B. M. R. (1974) Preparation
of peroxyacetyl nitrate (PAN) and its determination in the
atmosphere. London, England, Her Majesty's Stationery Office
(Atomic Energy Research Establishment Report AERE R7807).
SCHEEL, L. D., DOBROGORSKI, O. J., MOUNTAIN, J. T., SVIRBELY, J. L., &
STOKINGER, H. E. (1959) Physiologic biochemical, immunologic, and
pathologic changes following ozone exposure. J. appl. Physiol.,
14: 67-80.
SCHLIPKOETER, H. W. & BRUCH, J. (1973) [Functional and morphological
alterations caused by exposure to ozone.] Zentralb. Bakteriol.,
Parasitenk. Infektionskr. Hyg., Reihe B, 156: 486-499 (in
German).
SCHLIPKOETER, H. W., FODOR, G. C., GHELERTER, L., & DOLGNER, R. (1973)
[Findings from animal tests concerning synergism.] In:
Proceedings of the 3rd International Clean Air Congress,
Diisseldorf, Federal Republic of Germany, pp. A26-A30 (in
German).
SCHOETTLIN, C. E. (1962) The health effect of air pollution on elderly
males. Am. Rev. respir. Dis., 86: 878-897.
SCHOETTLIN, C. E. & LANDAU, E. (1961) Air pollution and asthmatic
attacks in the Los Angeles area. Public Health Rep.,
76: 545-548.
SCHUCK, E. A. & DOYLE, G. J. (1959) Photooxidation of hydrocarbons in
mixtures containing oxides of nitrogen and sulfur dioxide. San
Marina, CA, Air Pollution Foundation (Report No. 29).
SCHUCK, E. A. & STEPHENS, E. R. (1969) Oxides of nitrogen. Adv.
environ. Sci., 1: 73-118.
SHINGU, H., TANAKA, I., KUSUMOTO, S., NAKAJIMA, T., WATANABE, M., &
SUGIYAMA, M. (1972) [Effect of ozone on defense mechanisms to
infection.] J. Jpn. Soc. Air Pollut, 9: 332 (in Japanese).
SKILLEN, R. G., THIENES, C. H., CANGELOSI, J., & STRAIN, L. (1961)
Brain 5-hydroxytryptamine in ozone-exposed rats. Proc. Soc. Exp.
Biol. Med., 108: 121-122.
SMITH, L. E. (1965) Inhalation of the photochemical smog compound
peroxyacetyl nitrate. Am. J. public Health, 55(9): 1460-1468.
STASIUK, W. N. & COFFEY, P. E. (1974) Rural and urban ozone
relationships in New York State. J. Air Pollut. Control Assoc.,
24(6): 564-568.
STEPHENS, E. R. (1964) Absorptivities for infra-red determination of
peroxyacetyl nitrates. Anal. Chem., 36: 928-929.
STEPHENS, E. R. (1969) The formation, reactions and properties of
peroxyacetyl nitrates (PANs) in photochemical air pollution.
Adv. environ. Sci. Technol., 1: 119-146.
STEPHENS, E. R. (1976) Peroxyacetyl nitrate (PAN) in relation to ozone
and some meteorological parameters at Delft in the Netherlands.
Atmos. Environ., 10(7): 566.
STEPHENS, E. R., DARLEY, E. F., TAYLOR, O. C., & SCOTT, W. E. (1961)
Photochemical reaction products in air pollution. Int. J. Air
Water Pollut., 4: 79-100.
STEPHENS, R. J., FREEMAN, G., CRANE, S.C., & FURIOSI, N.J. (1971)
Ultrastructural changes in the terminal bronchiole of the rat
during continuous low level exposure to nitrogen dioxide. Exp.
molec. Pathol., 14: 1-19.
STEPHENS, R. J., FREEMAN, G., & EVANS, M. J. (1972) Early response of
lungs to low levels of nitrogen dioxide. Arch. environ. Health,
24: 160-179.
STEPHENS, R. J., FREEMAN, G., STARA, J. F., & COFFIN, D. L. (1973)
Cytologic changes in dog lungs induced by chronic exposure to
ozone. Am. J. Pathol., 73: 711-726.
STEPHENS, R. J., SLOAN, M. F., EVANS, M. J., & FREEMAN, G. (1974)
Early response of lung to low levels of O3. Am. J. Pathol.,
74:31-58.
STERLING, T. D., PHAIN, J. J., POLLACK, S. V., SCHUMSKY, D. A., &
DEGROOT, I. (1966) Urban morbidity and air pollution: a first
report. Arch. environ. Health, 13: 158-170.
STERLING, T. D., POLLACK, S. V., & PHAIR, J. J. (1967) Urban hospital
morbidity and air pollution: a second report. Arch. environ.
Health, 15: 362-374.
STOKINGER, H. E. (1957) Evaluation of the hazards of ozone and oxides
of nitrogen. Am. Med. Assoc. Arch. ind. Health, 15: 181-190.
STOKINGER, H. E. (1959) Factors modifying toxicity of ozone. Adv.
Chem. Ser., 21: 360-369.
STOKINGER, H. E. (1965) Ozone toxicology: a review of research and
industrial experience 1954-1964. Arch. environ. Health,
10: 719-731.
STOKINGER, H. E. & SCHEEL, L. D. (1962) Ozone toxicity: immunochemical
and tolerance-producing aspects. Arch. environ. Health,
4: 327-334.
STOKINGER, H. E., WAGNER, W. D., & WRIGHT, P. G. (1956) Studies of
ozone toxicity: I. potentiating effects of exercise and tolerance
development. Am. Med. Assoc. Arch. ind. Health, 14: 158-162.
STOKINGER, H. E., WAGNER, W. D., & DORBROGORSKI, O. J. (1957) Ozone
toxicity studies. III. chronic injury to lungs of animals
following exposure at a low level. Am. Med. Assoc. Arch. Ind.
Health, 16: 514-522.
SUZUKI, T. & NAGAOKA, S. (1973) [The susceptibility to serotonin and
exposure to ozone.] Ann. Rep. Tokyo Metrop. Res. Inst. Environ.
Prot., No. 4, pp. 262-263 (in Japanese).
SUZUKI, T., NAKAZAWA, K., UMEDA, M., GOTO, H., & NAGAOKA, S. (1975)
[Effect on the blood in rats exposed to ozone. I. Effect on
osmotic fragility of orythrocytes.] Ann. Rep. Tokyo Metrop. Res.
Inst. Environ. Prot., 6: 270-272 (in Japanese).
SWANN, H. E. & BALCHUM, O. J. (1966) Biological effects of urban air
pollution. Arch. environ. Health, 12: 698-704.
TAN, W. C., CORTESI, R., & PRIVETT, O. S. (1974) Lipid peroxide and
lung prostaglandins. Arch. environ. Health, 28: 82-84.
THOMPSON, G. E. (1971) Cardiovascular considerations of rat pulmonary
edema. Mil. Med., 136: 50-65.
TOMINGAS, V. R., POTT, F., & DEHNEN, W. (1973) Biological test for
carcinogenicity of polycyclic aromatic hydrocarbons. Arch.
Geschwilstforsch., 42: 298-306.
TOYAMA, T., KAGAWA, J., TSUNODA, T., & NAKAZA, M. (1977) [An
epidemiological estimation of dose-response relationship to the
mixture of atmospheric pollutants.] Jpn. J. Hyg., 32:70 (in
Japanese).
TRAMS, E., LAUTER, C. J., BRANDENBURGER-BROWN, E. A., & YOUNG, O.
(1972) Cerebral cortical metabolisms after chronic exposure to
O3. Arch. environ. Health, 24: 153-159.
URY, H. K. (1968) Photochemical air pollution and automobile accidents
in Los Angeles. Arch. environ. Health, 17: 334-342.
US DEPARTMENT OF HEALTH EDUCATION AND WELFARE (1970) Air Quality
criteria for photochemical oxidants. Washington, DC, US DHEW
(National Air Pollution Control Administration Publication No. AP
63).
US ENVIRONMENTAL PROTECTION AGENCY (1964-1973) Camp data. Research
Triangle Park, NC, National Aerometric data bank, US EPA.
US ENVIRONMENTAL PROTECTION AGENCY (1973) Investigation of high ozone
concentration in the vicinity of Gomet County, Maryland and
Preston County, West Virginia. Research Triangle Park, NC, EPA
(Report No. EPA-R4-73-019).
US ENVIRONMENTAL PROTECTION AGENCY (1976) Monitoring and air quality
trends report, 1974, pp. 115-123 (Report No. EPA-450/1-76).
VAUGHAN, T. R., JR, JENNELLE, L. F., & LEWIS, T. R. (1969) Long-term
exposure to low levels of air pollutants. Effects on pulmonary
function in the beagle. Arch. environ. Health, 19: 45-50.
VENINGA, T. S. (1967) Toxicity of ozone in comparison with ionizing
radiation. Strahlentherapie (Munich), 134: 469-477.
WARREN, G. J. & BABCOCK, G. (1970) Rev. Sci. Instrum., 41: 280-282.
WATANABE, M. TANAKA, I., SHINGU, H., ASAKA, J., KUSUMOTO, S.,
NAKAJIMA, T., & SUGIYAMA, M. (1973a) [The cytotoxicity of ozone.]
Jpn. J. public Health, 20: 554 (in Japanese).
WATANABE, S., FRANK, R., & YOKOYAMA, E. (1973b) Acute effects of ozone
on lungs of cats. I. Functional. Am. Rev. respir. Dis.,
108: 1141-1151.
WAYNE, W. S., WEHRLE, P. F., & CARROLL, R. E. (1967) Oxidant air
pollution and athletic performance. J. Am. Med. Assoc.,
199: 901-904.
WENT, F. W. (1966) On the nature of Aitken condensation nuclei.
Tellus, 28(2): 549-556.
WERTHAMER, S., SCHWARTZ, L. H., & SOSKIND, L. (1970) Bronchial
epithelial alterations and pulmonary neoplasia induced by ozone.
Pathol. Microbiol., 35: 224-230.
WERTHAMER, S., PENHA, P. D., & AMARAL, L. (1974) Pulmonary lesions
induced by chronic exposure to ozone. I. Biochemical alterations.
Arch. environ. Health, 28: 164-166.
WHITE, W. H., ANDERSON, J. A., BLUMENTHAL, D. L., HUSAR, R. B.,
GILLANI, N. V., HUSAR, J. D., & WILSON, W. E., JR (1976)
Formation and transport of secondary air pollutants: Ozone and
aerosols in the St Louis urban plume. Science, 194: 187-189.
WORLD HEALTH ORGANIZATION (1972) WHO Tech. Rep. Series No. 506. (Air
quality criteria and guides for urban air pollutants: Report of a
WHO Expert Committee.) 35 pp.
WORLD HEALTH ORGANIZATION (1976) Selected methods of measuring air
pollutants, Geneva WHO (WHO Offset Publ. No. 24).
WORLD HEALTH ORGANIZATION (1977) Oxides of nitrogen: Environmental
health criteria 4, Geneva, WHO, 79 pp.
XINTARAS, C., JOHNSON, B. L., ULRICH, C. E., TERRILL, R. E., &
SOBECKI, M. F. (1966) Application of the evoked response
technique in air pollution toxicology. Toxicol. appl.
Pharmacol., 8: 77-87.
YOKOYAMA, E. (1972a) [The effects of O3 on lung function. In:
Proceedings of a Conference on Photochemical Air Pollution.]
Tokyo, Jpn. Soc. Air Pollution, pp. 109-129 (in Japanese).
YOKOYAMA, E. (1972b) Effect of ventilation with ozone on
pressure-volume relationships of excised dogs' lungs. Am. Rev.
Respir. Dis., 105: 594-604.
YOKOYAMA, E. (1973) The effects of low-concentrations of ozone on the
lung pressure of rabbits and rats. Presented at: The Japan
Society of Industrial Hygiene Annual Meeting, 46th, April, pp.
134-135 (paper 223) (in Japanese).
YOKOYAMA, E. (1974) [Maximal expiratory flow volume curve of rabbit
lung exposed to ozone.] Jpn. J. thorac. Dis., 12: 556-561 (in
Japanese).
YOKOYAMA, E. & FRANK, R. (1972) Respiratory uptake of ozone in dogs.
Arch. environ. Health, 25: 132-138.
YOUNG, W. A., SHAW, D. B., & BATES, D. V. (1963) Pulmonary function in
welders exposed to ozone. Arch. environ. Health, 7: 337-340.
YOUNG, W. A., SHAW, D. B., & BATES, D. V. (1964) Effect of low
concentrations of ozone on pulmonary function in man. J. appl.
Physiol., 19: 765-768.
ZELAC, R. E., CROMROY, H. L., BOLCH, W. E., JR, DUNAVANT, B. G., &
BEVlS, H. A. (1971a) Inhaled ozone as a mutagen I. Chromosome
aberrations induced in chinese hamster lymphocytes. Environ.
Res., 4: 262-282.
ZELAC, R. E., CROMROY, H. L., BOLCH, W. E., JR, DUNAVANT, B. G., &
BEVIS, H. A. (1971b) Inhaled ozone as a mutagen II. Effect on the
frequency of chromosome aberrations observed in irradiated
chinese hamsters. Environ. Res., 4: 325-342.
 This class of compounds includes:
R = CH3: peroxyacetylnitrate (PAN)
R = C2H5: peroxyproprionylnitrate (PPN)
R = C6H5: peroxybenzoylnitrate (PBzN)
Although each of these species has received some attention, monitoring
data are available only for peroxyacetylnitrate. The physical
properties of this species are described in Table 2.
Table 2. Physical properties of peroxyacetylnitratea
O
"
"
Chemical formula CH3COONO2
Physical state at NTP colourless liquid
Relative molecular mass 121
Boiling point No true boiling point, compound
decomposes before boiling
Vapour pressure at room About 2 kPa (15 mmHg)
temperature
a From: US Department of Health, Education and Welfare (1970).
Peroxyacylnitrates have two characteristics that help in their
detection at low concentrations i.e., absorption in the infrared
region of the spectrum and electron-capturing ability (Stephens,
1969). The second of these characteristics is exploited in the
electron-capture detector which, when used in conjunction with gas
chromatography, provides the basis of an accepted method for the
measurement of peroxyacetylnitrate levels in air.
2.1.3 Other oxidants
Hydrogen peroxide has been identified as a potential photochemical
oxidant. However, it is an extremely difficult substance to detect
specifically in the atmosphere and, at present, it is not possible to
assess its significance as a photochemical air pollutant.
2.2 Atmospheric Chemistry
There are no significant primary emissions of ozone into the
atmosphere and all the ozone found has been formed by chemical
reactions that occur in the air.
In the upper atmosphere, ozone is mainly formed by the action of
solar radiation on molecular oxygena:
O2 + radiation (lambda < 175 nm) --> O(3P) + O(1D) (1)
O2 + radiation (lambda < 242 nm) --> 2 O(3P) (2)
O(3P) + O2 + M --> O3 + M (3)
In the lower atmosphere, ozone-producing processes involve
absorption of solar radiation by nitrogen dioxide:
NO2 + radiation (lambda < 430 nm) ka NO + O(3P) (4)
->
O(3P) + O2 + M --> O3 + M (3)
O3 + NO kb NO2 + O2 (5)
->
a O(3P) is the symbol for atomic oxygen in its lowest energy state
("triplet oxygen"); O(1D) represents the next higher energy state
of atomic oxygen ("singlet oxygen"); M is another molecule that
must be present for the reaction to take place ("third body"),
usually oxygen or nitrogen. Square brackets e.g. [NO] represent
the concentration of the chemical species inside the brackets.
Thus, the mechanism of ozone production during the sunlight
irradiation of polluted air is simple in outline (ozone formation by
the interaction of molecular oxygen with the photoproducts of nitrogen
dioxide) but complex in detail. It is based on reactions (3)-(5) which
give the following expression for the ozone concentration:
ka[NO2]
O3 approx. (6)
kb[NO]
In polluted atmospheres, the most readily observed sink for ozone
involves the emission of nitric oxide. At night-time, the equilibrium
expressed by equation (6) is displaced by the rapid reaction (5), and
continuous nitric oxide emissions rapidly reduce the ozone
concentration to undetectable levels. This atmospheric chemical
process is supplemented by the destruction of ozone at ground level by
contact with soil and vegetation surfaces (Regener & Aldaz, 1969).
In a hydrogen-free atmosphere, a fairly even balance between
oxidizing and reducing agents would be maintained. However, peroxy
radicals (RO2) produced by the photochemical degradation of
hydrocarbons have the important property of reacting with nitric oxide
thereby converting it to nitrogen dioxide. The significance of any
process resulting in the conversion of nitric oxide to nitrogen
dioxide is that during daylight the equilibrium expressed by equation
(6) shifts in favour of ozone production.
There are no significant primary emissions into the atmosphere of
peroxyacylnitrates all of which are formed by atmospheric chemical
reactions of the general type:
RCO(O2) + NO2 --> RCO(O2)NO2 (7)
2.3 Measurement of Photochemical Oxidant Concentrations
2.3.1 Sampling
Ozone is highly reactive with most materials including plastics,
metals, and fabrics. The most reproducible ambient concentration data
have been obtained using sampling manifolds made entirely of teflon or
glass.
As with oxides of nitrogen, residence time in these sampling
manifolds requires specific consideration when sampling air containing
nitric oxide, nitrogen dioxide, and ozone during daylight. Since the
equilibrium shown in equation (6) is disturbed inside the sampling
manifold, ozone concentrations can be underestimated if sampling times
exceed 10 seconds (Butcher & Ruff, 1971).
When measuring ozone, the site of sampling should be selected with
extreme care as exhaust gases from motor vehicles and central heating
appliances readily remove ozone.
2.3.2 Analytical methods
For measurement purposes, oxidants are generally divided into
three categories: ozone, total oxidants, and peroxyacylnitrates. Ozone
and peroxyacylnitrates can be measured specifically while total
oxidants are usually determined as a class of compounds.
The reagent employed to measure the oxidizing property of
photochemical oxidants is a solution of neutral-buffered potassium
iodide (NBKI). This reagent reacts with ozone, nitrogen dioxide, and
peroxyacylnitrates. Reducing agents such as sulfur dioxide have an
inhibiting effect on the reagent solution and must be removed, from
the inlet stream. To this extent, reaction with the potassium iodide
reagent is a measure of the net oxidizing capacity of a sample of
ambient air.
The terms "oxidant" or "total oxidant" are used to describe the
net oxidizing capacity of the sampled air as determined by reaction
with NBKI. The terms "corrected oxidant" or "adjusted oxidant"
indicate that measurements have been corrected for the presence of
reducing agents (sulfur dioxide) or other oxidizing agents (nitrogen
dioxide) in the air.
Long-term averaging values are usually meaningless for
photochemical oxidants and attention is directed to 1-h values
(section 4.3). Thus, the use of continuous instruments with automatic
data collection systems has an advantage. Manual methods are available
for the determination of photochemical oxidants and these may be
automated to a certain extent.
2.3.2.1 Ozone
Continuous ozone analysers are usually based on the
chemiluminescent reaction of ozone with ethylene (Nederbragt et al.,
1965; Warren & Babcock, 1970). A chemiluminescence method based on the
reaction of ozone with certain dyes (Regener, 1964) has been further
developed in the Netherlands (Guicherit, 1975). These techniques are
not subject to atmospheric interference, are highly sensitive
(2 µg/m3 or 0.001 ppm), and perform well under field conditions. The
methods are not absolute and hence some form of calibration involving
potassium iodide or UV absorption spectroscopy is necessary.
Specific determination of ozone may be accomplished by ultraviolet
absorption spectroscopy in the 200-300 nm wavelength range (Hodgeson,
1972). The advantages of this method are that it is highly sensitive,
does not require cylinders of explosive gases, and that it gives an
absolute measurement (De More & Patapoff, 1976). Very high
concentrations of certain hydrocarbons or mercury vapour may cause
interference.
The dihydroacridine method is a specific, inexpensive, manual
method for ozone determination in which samples are taken every
30 min. Interference effects can be eliminated by parallel sampling
with an identical system fitted with an ozone scrubber. This is the
only suitable method for short-term ozone measurements when a
chemiluminescent or UV absorption instrument is not available (World
Health Organization, 1976).
2.3.2.2 Total oxidants
Total oxidants are generally measured using acid- or neutral-
buffered solutions of potassium iodide (Byers & Saltzman, 1958; US
Department of Health, Education and Welfare, 1970; World Health
Organization, 1976). There are indications that the accuracy of the
method depends on the pH of the buffer solution, the concentration of
the reagent, and other variables. In addition to these drawbacks, the
method is basically unspecific. Any other oxidizing or reducing
species can cause interference. Such interference results when
sampling air containing sulfur dioxide, chlorine, nitrogen dioxide, or
peroxides. Nitrogen dioxide and sulfur dioxide interfere most by
giving erroneously high and low values, respectively.
Interference from sulfur dioxide can be eliminated by passing the
air sample through glass fibre filters impregnated with a suitable
material such as chromium trioxide. Humidity often renders these
scrubbers ineffective because under such conditions they oxidize
nitric oxide to nitrogen dioxide, thus increasing nitrogen dioxide
interference. As there is also ozone loss after extended use, these
scrubbers are not entirely satisfactory (World Health Organization,
1976).
Interference from nitrogen dioxide is more difficult to eliminate.
Some form of adjustment can be made to the total oxidant reading if
continuous nitrogen dioxide measurements are also available. This
correction depends to some extent on experimental conditions and, in
view of the problems previously noted with measurements of oxides of
nitrogen, may not be easy to make.
Other methods are available for the determination of oxidants but
none of them is commonly used. They include the ferrous ammonium
sulphate, alkali potassium iodide, and phenolphthalein methods (World
Health Organization, 1976).
2.3.2.3 Peroxyacetylnitrate
The infrared absorption and electron-capturing properties of
peroxyacetylnitrate have been used for its measurement in simulated
and real atmospheres (Stephens, 1969). Electron-capture detectors
preceded by gas chromatographic separation offer a limit of detection
of 0.5 µg/m3 (0.0001 ppm) for the automatic determination of ambient
concentrations of peroxyacetylnitrate. Water vapours may interfere
with the passage of peroxyacetylnitrate along the chromatographic
column (Farwell & Rasmussen, 1976) and trace contaminants in cylinder
gases can be a problem because of their slow accumulation on
chromatography columns used for continuous measurements.
Peroxyacetylnitrate can also be measured by the hydrolysis of
peroxyacetylnitrate solution to give nitrite ions that can be
determined colorimetrically. However, this method suffers from
interference by nitrogen dioxide (Konno & Okita, 1974; Stephens,
1969).
3. SOURCES OF PHOTOCHEMICAL OXIDANTS AND THEIR PRECURSORS
3.1 Natural Sources
As mentioned previously, ozone is a natural constituent of the
upper atmosphere. A small amount of ozone, which is formed by the
photolysis of molecular oxygen, is carried by atmospheric circulation
into the lower atmosphere (section 4). Natural sources of ozone are
associated with the passage of cold fronts (Ripperton et al., 1971)
and atmospheric electrical phenomena (US Department of Health,
Education & Welfare, 1970).
The photochemical oxidation of natural hydrocarbons including
terpenes from trees and other vegetation takes place during daylight
hours (Rasmussen, 1972; Went, 1966). Generally, these processes are
difficult to study because of the low concentrations of the
hydrocarbons and their short atmospheric residence. However, hazes
often associated with certain forest regions may well be explained by
photochemical aerosol production from natural airborne hydrocarbons
(Grimsrud et al., 1975).
3.2 Man-made Sources of Oxidant Precursors
Emissions of oxides of nitrogen from man-made activities include
important contributions from both stationary and mobile sources (World
Health Organization, 1977).
By comparison, the position with regard to hydrocarbon precursors
is much more complex. In this instance, the term "hydrocarbon" refers
to a class of pollutants that contain carbon atoms and produce
oxidants under irradiation in the presence of oxides of nitrogen. This
is a very wide definition that covers many hundreds of different
organic compounds emitted into the atmosphere by man-made processes.
Not all organic compounds play an equal role in oxidant formation.
The relative importance of each organic compound in this process
depends on its chemical structure and reactivity. The chemical
structure determines the number of nitric oxide --> nitrogen dioxide
conversions involved in the atmospheric degradation of each organic
compound and ozone may be produced at each of these steps (Calvert,
1976; Demerjian et al., 1974). Reactivity requires special attention
because the time scale for ozone or peroxyacetylnitrate production is
related to the time scale for hydrocarbon degradation. For the
so-called "highly reactive" hydrocarbons this may be 1 h or less. It
may take up to 3 h for the less reactive hydrocarbons and require
several days for the so-called unreactive hydrocarbons. Even in the
presence of reactive hydrocarbons, ozone production may only become
significant 10 km downwind from a source, and peak ozone
concentrations may be observed over 60 km downwind (White et al.,
1976). Thus, the relationship between hydrocarbon precursors and
observed oxidant concentrations in large urban areas may be obscured.
There may be marked differences in the nature of the oxidants in air
sampled during the early morning when peak concentrations of oxidant
precursors occur and in that sampled after midday when ozone
concentrations are elevated.
In view of this complexity, it is clearly necessary to have some
form of rational assessment of hydrocarbon reactivity (Darnall et al.,
1976). Complete inventories of individual substances are only
available for gasoline-engine exhaust emissions; for the storage,
distribution, and use of organic substances such as petroleum
products; and for specific industrial processes. An example of total
emissions of oxides of nitrogen and hydrocarbons is given in Table 3.
These figures are presented merely to show the wide diversity of the
processes responsible for oxidant precursor emissions; it is not
sufficient to consider motor vehicles as the only important source.
Table 3. Total emissions of hydrocarbons and oxides of nitrogen
in the Federal Republic of Germany in 1971 in thousands
of tonnesa
Source Total emission (103 tonnes)
Hydrocarbons Oxides of nitrogen
Domestic heating 173 117
Traffic exhaust 325 308
Power plants 14 373
Industrial combustion 53 470
Industrial processes 955 30
a From: Federal Republic of Germany, Ministry of Internal
Affairs (1974).
3.3 Indoor Sources
The use of ultraviolet lamps, electrostatic precipitators,
photocopying machines, and odour control equipment can lead to
increases in indoor concentrations of ozone. Welding and the
manufacture of hydrogen peroxide are important indoor sources of ozone
in industry and pose problems in occupational health (section 6.2).
However, other indoor activities such as smoking and cooking with
gas stoves tend to produce elevated nitric oxide concentrations that
destroy ozone and peroxyacylnitrates (Schuck & Stephens, 1969).
3.4 Oxidant-precursor Relationships
While potential synergistic health effects of oxidants and
nitrogen dioxide are recognized, programmes for the control of these
two pollutants are usually developed independently of one another.
From a control point of view, however, such an approach is not
justified because of the complexity of the photochemical reaction
system. The only predictable way to control the formation of
photochemical oxidants is to reduce the initial components in
incremental steps. Decreasing the primary emission of oxides of
nitrogen without reducing hydrocarbon emissions will lead to an
increase in oxidant levels.
A further example of the uncoordinated approach to air pollution
abatement can be illustrated by examination of the current methods
applied to hydrocarbon control. These methods have focused on
increasing combustion efficiency, an action which does reduce the
hydrocarbon concentrations in exhaust but has the side effect of
causing an increase in atmospheric concentrations of nitrogen dioxide.
As expected, changing the ratio of oxidant precursors has a complex
effect on the formation of photochemical oxidants. For example, the
effect of this change in ratio in the south coast air basin of
California has been to produce substantial reductions in the
concentrations of oxidants in Los Angeles town centre. However, the
reductions become smaller at downwind sites and at 80 kilometres
downwind, an increase in maximum daily values was observed
(Dimitriades, 1976).
4. ENVIRONMENTAL CONCENTRATIONS AND EXPOSURES
4.1 Background Concentrations
Ozone concentrations in places far removed from sources of
pollution show fairly constant values with some seasonal variations
interspersed with irregular maxima due to specific meteorological
events. Monthly mean concentrations of ozone, which vary considerably
with both latitude and the month of the year, are illustrated in
Fig. 1. The reported values range from 10 to 80 µg/m3
(0.005-0.04 ppm).
Many observations indicate that hourly values range from 10 to
100 µg/m3 (0.005-0.05 ppm) (Berry, 1964; Haagen-Smit, 1952; US
Department of Health, Education and Welfare, 1970). However, higher
values have been observed on isolated occasions. In a study at Chalk
River, Ontario, Canada, a maximum value was observed of 120 µg/m3
(0.06 ppm) for 4 h (US Department of Health, Education and Welfare,
1970).
4.2 Rural Areas
Polluted air masses from urban and industrial areas can affect
suburban and rural areas in the direction of the prevailing wind for
considerable distances. Elevated oxidant concentrations have been
measured in a number of downwind rural locations where local sources
of oxidant precursors were insignificant. It has been suggested that
long-distance atmospheric transport might be responsible for many
cases of high oxidant concentrations found over rural areas and some
specific examples are given in Table 4.
A Midwest study in the USA in 1974 showed elevated ozone levels
over an extensive rural area (radius of over 240 km) due to the
combined effects of a number of urban areas. High ozone levels from
one particular urban area extended as far as 48-80 kilometres downwind
(US Environmental Protection Agency, 1976).
Observations of a 1-h concentration of 120 µg/m3 (0.06 ppm) in
rural areas can generally be associated with the transport of man-made
oxidants from distant sources.
This class of compounds includes:
R = CH3: peroxyacetylnitrate (PAN)
R = C2H5: peroxyproprionylnitrate (PPN)
R = C6H5: peroxybenzoylnitrate (PBzN)
Although each of these species has received some attention, monitoring
data are available only for peroxyacetylnitrate. The physical
properties of this species are described in Table 2.
Table 2. Physical properties of peroxyacetylnitratea
O
"
"
Chemical formula CH3COONO2
Physical state at NTP colourless liquid
Relative molecular mass 121
Boiling point No true boiling point, compound
decomposes before boiling
Vapour pressure at room About 2 kPa (15 mmHg)
temperature
a From: US Department of Health, Education and Welfare (1970).
Peroxyacylnitrates have two characteristics that help in their
detection at low concentrations i.e., absorption in the infrared
region of the spectrum and electron-capturing ability (Stephens,
1969). The second of these characteristics is exploited in the
electron-capture detector which, when used in conjunction with gas
chromatography, provides the basis of an accepted method for the
measurement of peroxyacetylnitrate levels in air.
2.1.3 Other oxidants
Hydrogen peroxide has been identified as a potential photochemical
oxidant. However, it is an extremely difficult substance to detect
specifically in the atmosphere and, at present, it is not possible to
assess its significance as a photochemical air pollutant.
2.2 Atmospheric Chemistry
There are no significant primary emissions of ozone into the
atmosphere and all the ozone found has been formed by chemical
reactions that occur in the air.
In the upper atmosphere, ozone is mainly formed by the action of
solar radiation on molecular oxygena:
O2 + radiation (lambda < 175 nm) --> O(3P) + O(1D) (1)
O2 + radiation (lambda < 242 nm) --> 2 O(3P) (2)
O(3P) + O2 + M --> O3 + M (3)
In the lower atmosphere, ozone-producing processes involve
absorption of solar radiation by nitrogen dioxide:
NO2 + radiation (lambda < 430 nm) ka NO + O(3P) (4)
->
O(3P) + O2 + M --> O3 + M (3)
O3 + NO kb NO2 + O2 (5)
->
a O(3P) is the symbol for atomic oxygen in its lowest energy state
("triplet oxygen"); O(1D) represents the next higher energy state
of atomic oxygen ("singlet oxygen"); M is another molecule that
must be present for the reaction to take place ("third body"),
usually oxygen or nitrogen. Square brackets e.g. [NO] represent
the concentration of the chemical species inside the brackets.
Thus, the mechanism of ozone production during the sunlight
irradiation of polluted air is simple in outline (ozone formation by
the interaction of molecular oxygen with the photoproducts of nitrogen
dioxide) but complex in detail. It is based on reactions (3)-(5) which
give the following expression for the ozone concentration:
ka[NO2]
O3 approx. (6)
kb[NO]
In polluted atmospheres, the most readily observed sink for ozone
involves the emission of nitric oxide. At night-time, the equilibrium
expressed by equation (6) is displaced by the rapid reaction (5), and
continuous nitric oxide emissions rapidly reduce the ozone
concentration to undetectable levels. This atmospheric chemical
process is supplemented by the destruction of ozone at ground level by
contact with soil and vegetation surfaces (Regener & Aldaz, 1969).
In a hydrogen-free atmosphere, a fairly even balance between
oxidizing and reducing agents would be maintained. However, peroxy
radicals (RO2) produced by the photochemical degradation of
hydrocarbons have the important property of reacting with nitric oxide
thereby converting it to nitrogen dioxide. The significance of any
process resulting in the conversion of nitric oxide to nitrogen
dioxide is that during daylight the equilibrium expressed by equation
(6) shifts in favour of ozone production.
There are no significant primary emissions into the atmosphere of
peroxyacylnitrates all of which are formed by atmospheric chemical
reactions of the general type:
RCO(O2) + NO2 --> RCO(O2)NO2 (7)
2.3 Measurement of Photochemical Oxidant Concentrations
2.3.1 Sampling
Ozone is highly reactive with most materials including plastics,
metals, and fabrics. The most reproducible ambient concentration data
have been obtained using sampling manifolds made entirely of teflon or
glass.
As with oxides of nitrogen, residence time in these sampling
manifolds requires specific consideration when sampling air containing
nitric oxide, nitrogen dioxide, and ozone during daylight. Since the
equilibrium shown in equation (6) is disturbed inside the sampling
manifold, ozone concentrations can be underestimated if sampling times
exceed 10 seconds (Butcher & Ruff, 1971).
When measuring ozone, the site of sampling should be selected with
extreme care as exhaust gases from motor vehicles and central heating
appliances readily remove ozone.
2.3.2 Analytical methods
For measurement purposes, oxidants are generally divided into
three categories: ozone, total oxidants, and peroxyacylnitrates. Ozone
and peroxyacylnitrates can be measured specifically while total
oxidants are usually determined as a class of compounds.
The reagent employed to measure the oxidizing property of
photochemical oxidants is a solution of neutral-buffered potassium
iodide (NBKI). This reagent reacts with ozone, nitrogen dioxide, and
peroxyacylnitrates. Reducing agents such as sulfur dioxide have an
inhibiting effect on the reagent solution and must be removed, from
the inlet stream. To this extent, reaction with the potassium iodide
reagent is a measure of the net oxidizing capacity of a sample of
ambient air.
The terms "oxidant" or "total oxidant" are used to describe the
net oxidizing capacity of the sampled air as determined by reaction
with NBKI. The terms "corrected oxidant" or "adjusted oxidant"
indicate that measurements have been corrected for the presence of
reducing agents (sulfur dioxide) or other oxidizing agents (nitrogen
dioxide) in the air.
Long-term averaging values are usually meaningless for
photochemical oxidants and attention is directed to 1-h values
(section 4.3). Thus, the use of continuous instruments with automatic
data collection systems has an advantage. Manual methods are available
for the determination of photochemical oxidants and these may be
automated to a certain extent.
2.3.2.1 Ozone
Continuous ozone analysers are usually based on the
chemiluminescent reaction of ozone with ethylene (Nederbragt et al.,
1965; Warren & Babcock, 1970). A chemiluminescence method based on the
reaction of ozone with certain dyes (Regener, 1964) has been further
developed in the Netherlands (Guicherit, 1975). These techniques are
not subject to atmospheric interference, are highly sensitive
(2 µg/m3 or 0.001 ppm), and perform well under field conditions. The
methods are not absolute and hence some form of calibration involving
potassium iodide or UV absorption spectroscopy is necessary.
Specific determination of ozone may be accomplished by ultraviolet
absorption spectroscopy in the 200-300 nm wavelength range (Hodgeson,
1972). The advantages of this method are that it is highly sensitive,
does not require cylinders of explosive gases, and that it gives an
absolute measurement (De More & Patapoff, 1976). Very high
concentrations of certain hydrocarbons or mercury vapour may cause
interference.
The dihydroacridine method is a specific, inexpensive, manual
method for ozone determination in which samples are taken every
30 min. Interference effects can be eliminated by parallel sampling
with an identical system fitted with an ozone scrubber. This is the
only suitable method for short-term ozone measurements when a
chemiluminescent or UV absorption instrument is not available (World
Health Organization, 1976).
2.3.2.2 Total oxidants
Total oxidants are generally measured using acid- or neutral-
buffered solutions of potassium iodide (Byers & Saltzman, 1958; US
Department of Health, Education and Welfare, 1970; World Health
Organization, 1976). There are indications that the accuracy of the
method depends on the pH of the buffer solution, the concentration of
the reagent, and other variables. In addition to these drawbacks, the
method is basically unspecific. Any other oxidizing or reducing
species can cause interference. Such interference results when
sampling air containing sulfur dioxide, chlorine, nitrogen dioxide, or
peroxides. Nitrogen dioxide and sulfur dioxide interfere most by
giving erroneously high and low values, respectively.
Interference from sulfur dioxide can be eliminated by passing the
air sample through glass fibre filters impregnated with a suitable
material such as chromium trioxide. Humidity often renders these
scrubbers ineffective because under such conditions they oxidize
nitric oxide to nitrogen dioxide, thus increasing nitrogen dioxide
interference. As there is also ozone loss after extended use, these
scrubbers are not entirely satisfactory (World Health Organization,
1976).
Interference from nitrogen dioxide is more difficult to eliminate.
Some form of adjustment can be made to the total oxidant reading if
continuous nitrogen dioxide measurements are also available. This
correction depends to some extent on experimental conditions and, in
view of the problems previously noted with measurements of oxides of
nitrogen, may not be easy to make.
Other methods are available for the determination of oxidants but
none of them is commonly used. They include the ferrous ammonium
sulphate, alkali potassium iodide, and phenolphthalein methods (World
Health Organization, 1976).
2.3.2.3 Peroxyacetylnitrate
The infrared absorption and electron-capturing properties of
peroxyacetylnitrate have been used for its measurement in simulated
and real atmospheres (Stephens, 1969). Electron-capture detectors
preceded by gas chromatographic separation offer a limit of detection
of 0.5 µg/m3 (0.0001 ppm) for the automatic determination of ambient
concentrations of peroxyacetylnitrate. Water vapours may interfere
with the passage of peroxyacetylnitrate along the chromatographic
column (Farwell & Rasmussen, 1976) and trace contaminants in cylinder
gases can be a problem because of their slow accumulation on
chromatography columns used for continuous measurements.
Peroxyacetylnitrate can also be measured by the hydrolysis of
peroxyacetylnitrate solution to give nitrite ions that can be
determined colorimetrically. However, this method suffers from
interference by nitrogen dioxide (Konno & Okita, 1974; Stephens,
1969).
3. SOURCES OF PHOTOCHEMICAL OXIDANTS AND THEIR PRECURSORS
3.1 Natural Sources
As mentioned previously, ozone is a natural constituent of the
upper atmosphere. A small amount of ozone, which is formed by the
photolysis of molecular oxygen, is carried by atmospheric circulation
into the lower atmosphere (section 4). Natural sources of ozone are
associated with the passage of cold fronts (Ripperton et al., 1971)
and atmospheric electrical phenomena (US Department of Health,
Education & Welfare, 1970).
The photochemical oxidation of natural hydrocarbons including
terpenes from trees and other vegetation takes place during daylight
hours (Rasmussen, 1972; Went, 1966). Generally, these processes are
difficult to study because of the low concentrations of the
hydrocarbons and their short atmospheric residence. However, hazes
often associated with certain forest regions may well be explained by
photochemical aerosol production from natural airborne hydrocarbons
(Grimsrud et al., 1975).
3.2 Man-made Sources of Oxidant Precursors
Emissions of oxides of nitrogen from man-made activities include
important contributions from both stationary and mobile sources (World
Health Organization, 1977).
By comparison, the position with regard to hydrocarbon precursors
is much more complex. In this instance, the term "hydrocarbon" refers
to a class of pollutants that contain carbon atoms and produce
oxidants under irradiation in the presence of oxides of nitrogen. This
is a very wide definition that covers many hundreds of different
organic compounds emitted into the atmosphere by man-made processes.
Not all organic compounds play an equal role in oxidant formation.
The relative importance of each organic compound in this process
depends on its chemical structure and reactivity. The chemical
structure determines the number of nitric oxide --> nitrogen dioxide
conversions involved in the atmospheric degradation of each organic
compound and ozone may be produced at each of these steps (Calvert,
1976; Demerjian et al., 1974). Reactivity requires special attention
because the time scale for ozone or peroxyacetylnitrate production is
related to the time scale for hydrocarbon degradation. For the
so-called "highly reactive" hydrocarbons this may be 1 h or less. It
may take up to 3 h for the less reactive hydrocarbons and require
several days for the so-called unreactive hydrocarbons. Even in the
presence of reactive hydrocarbons, ozone production may only become
significant 10 km downwind from a source, and peak ozone
concentrations may be observed over 60 km downwind (White et al.,
1976). Thus, the relationship between hydrocarbon precursors and
observed oxidant concentrations in large urban areas may be obscured.
There may be marked differences in the nature of the oxidants in air
sampled during the early morning when peak concentrations of oxidant
precursors occur and in that sampled after midday when ozone
concentrations are elevated.
In view of this complexity, it is clearly necessary to have some
form of rational assessment of hydrocarbon reactivity (Darnall et al.,
1976). Complete inventories of individual substances are only
available for gasoline-engine exhaust emissions; for the storage,
distribution, and use of organic substances such as petroleum
products; and for specific industrial processes. An example of total
emissions of oxides of nitrogen and hydrocarbons is given in Table 3.
These figures are presented merely to show the wide diversity of the
processes responsible for oxidant precursor emissions; it is not
sufficient to consider motor vehicles as the only important source.
Table 3. Total emissions of hydrocarbons and oxides of nitrogen
in the Federal Republic of Germany in 1971 in thousands
of tonnesa
Source Total emission (103 tonnes)
Hydrocarbons Oxides of nitrogen
Domestic heating 173 117
Traffic exhaust 325 308
Power plants 14 373
Industrial combustion 53 470
Industrial processes 955 30
a From: Federal Republic of Germany, Ministry of Internal
Affairs (1974).
3.3 Indoor Sources
The use of ultraviolet lamps, electrostatic precipitators,
photocopying machines, and odour control equipment can lead to
increases in indoor concentrations of ozone. Welding and the
manufacture of hydrogen peroxide are important indoor sources of ozone
in industry and pose problems in occupational health (section 6.2).
However, other indoor activities such as smoking and cooking with
gas stoves tend to produce elevated nitric oxide concentrations that
destroy ozone and peroxyacylnitrates (Schuck & Stephens, 1969).
3.4 Oxidant-precursor Relationships
While potential synergistic health effects of oxidants and
nitrogen dioxide are recognized, programmes for the control of these
two pollutants are usually developed independently of one another.
From a control point of view, however, such an approach is not
justified because of the complexity of the photochemical reaction
system. The only predictable way to control the formation of
photochemical oxidants is to reduce the initial components in
incremental steps. Decreasing the primary emission of oxides of
nitrogen without reducing hydrocarbon emissions will lead to an
increase in oxidant levels.
A further example of the uncoordinated approach to air pollution
abatement can be illustrated by examination of the current methods
applied to hydrocarbon control. These methods have focused on
increasing combustion efficiency, an action which does reduce the
hydrocarbon concentrations in exhaust but has the side effect of
causing an increase in atmospheric concentrations of nitrogen dioxide.
As expected, changing the ratio of oxidant precursors has a complex
effect on the formation of photochemical oxidants. For example, the
effect of this change in ratio in the south coast air basin of
California has been to produce substantial reductions in the
concentrations of oxidants in Los Angeles town centre. However, the
reductions become smaller at downwind sites and at 80 kilometres
downwind, an increase in maximum daily values was observed
(Dimitriades, 1976).
4. ENVIRONMENTAL CONCENTRATIONS AND EXPOSURES
4.1 Background Concentrations
Ozone concentrations in places far removed from sources of
pollution show fairly constant values with some seasonal variations
interspersed with irregular maxima due to specific meteorological
events. Monthly mean concentrations of ozone, which vary considerably
with both latitude and the month of the year, are illustrated in
Fig. 1. The reported values range from 10 to 80 µg/m3
(0.005-0.04 ppm).
Many observations indicate that hourly values range from 10 to
100 µg/m3 (0.005-0.05 ppm) (Berry, 1964; Haagen-Smit, 1952; US
Department of Health, Education and Welfare, 1970). However, higher
values have been observed on isolated occasions. In a study at Chalk
River, Ontario, Canada, a maximum value was observed of 120 µg/m3
(0.06 ppm) for 4 h (US Department of Health, Education and Welfare,
1970).
4.2 Rural Areas
Polluted air masses from urban and industrial areas can affect
suburban and rural areas in the direction of the prevailing wind for
considerable distances. Elevated oxidant concentrations have been
measured in a number of downwind rural locations where local sources
of oxidant precursors were insignificant. It has been suggested that
long-distance atmospheric transport might be responsible for many
cases of high oxidant concentrations found over rural areas and some
specific examples are given in Table 4.
A Midwest study in the USA in 1974 showed elevated ozone levels
over an extensive rural area (radius of over 240 km) due to the
combined effects of a number of urban areas. High ozone levels from
one particular urban area extended as far as 48-80 kilometres downwind
(US Environmental Protection Agency, 1976).
Observations of a 1-h concentration of 120 µg/m3 (0.06 ppm) in
rural areas can generally be associated with the transport of man-made
oxidants from distant sources.
 Table 4. Long-distance transport of photochemical oxidants
Rural region Possible source Trajectory Reference
length
(km)
Mineral King Valley, Fresno, California < 100 Miller et al. (1972)
California, USA
Garrett County, New York, Philadelphia, > 100 US Environmental
Maryland, USA Baltimore, Washington DC Protection Agency
or Pittsburgh (1973)
Southern Eire Continental Europe 100-700 Cox et al. (1975)
& Southern UK
New York State, USA Buffalo, New York 100-300 Stasiuk & Coffey (1974)
Tochigi and Gunma Tokyo < 100 Environment Agency
Prefectures, Japan (1976)
Midwest, USA St. Louis > 150 White et al. (1976)
4.3 Urban Areas
Since photochemical oxidants are the products of sunlight-induced
photochemical reactions, elevated concentrations of oxidants in urban
areas are generally restricted to a 4- to 6-h period within a day,
representing only 15-25% of the 24-h interval. For this reason, the
reporting of oxidant or ozone data as daily, monthly, or yearly means
can be misleading when evaluating trends or comparing oxidant
concentrations in different cities. Thus, oxidant or ozone data are
usually reported in terms of highest 1-h concentrations or in terms of
the number of days with hourly concentrations exceeding a specified
value or the number of hours when a given range of concentrations
occurred within a year. However, they may also be given as
instantaneous or five minute peak concentrations or frequency
distributions.
As shown in Table 5, the highest 1-h concentrations at 8 locations
were of the order of 300-800 µg/m3 (0.15-0.40 ppm). It is important
to recognize that the data presented are only for one site in each of
the cities and do not necessarily represent the maximum levels
occurring in these urban areas or provide a good indication of human
exposure levels. For this reason, frequency distributions or reporting
of the number of days or hours when a given concentration was exceeded
are helpful. Such data are presented in Tables 6, 7, and 8 for
selected monitoring stations in Tokyo, Washington DC, and Delft,
respectively, to illustrate the distribution of the concentrations
recorded at these monitoring stations. These tables are not intended
to indicate long-term trends since meteorological patterns that
greatly influence the ambient concentrations of oxidants can vary
considerably from year to year.
The data for Tokyo show both the number of days when a given
concentration was exceeded and the total number of hours in which
concentrations falling within the specified ranges were observed. As
shown in Table 6, an hourly concentration of 200 µg/m3 (0.1 ppm) was
exceeded on 10-30 of days in these years, and in approximately 1-4% of
all the hours in the year.
In Washington, DC, a concentration of 200 µg/m3 (0.1 ppm) was
exceeded, on average, on about 5% of the days, with most of the days
having maximum 1-h concentrations of about 100 µg/m3 (0.05 ppm).
The data for Delft, Netherlands, show the number of hours in the
year when given concentrations were observed. More than 90% of the
hours exhibited concentrations of less than 100 µg/m3 (0.05 ppm).
However, in 1971 and 1973, there were 48 and 30 hours, respectively,
when concentrations exceeded 200 µg/m3 (0.1 ppm).
Table 5. Highest 1-h concentrations of ozone or total oxidants observed at
selected sites in 1974
City Concentration Method
µg/m3 (ppm)
Bonn, Federal Republic of Germany1 290 (0.145) chemiluminescence
Eindhoven, Netherlands2 420 (0.210) chemiluminescence
London, UK3a 294 (0.147) chemiluminescence
Los Angeles, USA4 548 (0.274) NBKI
Osaka, Japan5 320 (0.160) NBKI
Riverside, USA4 744 (0.372) NBKI
Tokyo, Japan5 380 (0.190) NBKI
Washington, USA4 312 (0.156) chemiluminescence
From: 1 Becker & Schurath (1975).
2 Guicherit (1975).
3 Ball (1976).
4 US Environmental Protection Agency (1976).
5 Environment Agency (1975b).
a Data for 1975.
Table 6. Number of days and hours when hourly oxidant concentrations were in the
range of indicated levels at a National Air Sampling Nelwork Station,
Tokyo, Japana
Concentrationb Number of days (hours)
µg/m3 (ppm) 1971 1972 1973 1974
0-100 (0 -0.05) 113 (7032) 185 (7863) 60 (7248) 184 (8122)
120-180 (0.06-0.09) 135 (798) 111 (379) 199 (1050) 134 (495)
200-280 (0.10-0.14) 60 (251) 31 (81) 73 (298) 40 (107)
300-380 (0.15-0.19) 18 (42) 8 (14) 21 (59) 5 (10)
400-480 (0.20-0.24) 6 (8) 1 (2) 7 (14) 0 (0)
>500 (>0.25) 0 (0) 0 (0) 1 (2) 0 (0)
Total 332 (8131) 336 (8339) 361 (8671) 363 (8734)
a From: Tokyo Metropolitan Government (1971-1974) (unpublished data).
b Measured by NBKI method.
Table 7. Number of days when at least one hourly oxidant
concentration was in the range of indicated levels in
Washington, DC, USAa
Concentrationb Number of days
µg/m3 (ppm) 1970 1971 1972
0-100 (0 -0.05) 72 155 134
120-200 (0.06-0.10) 85 127 39
220-300 (0.11-0.15) 8 17 6
>300 (>0.15) 2 0 0
Total 167 299 179
a From: US Environmental Protection Agency (1964-1973).
b Measured by the NBKI method.
Table 8. Number of hours when hourly ozone concentrations were in
the range of indicated levels in Delft, Netherlandsa
Concentrationb Number of hours
µg/m3 (ppm) 1971 1972 1973 1974
0-100 (0 -0.05) 7799 8370 8364 6907
120-150 (0.06 -0.075) 647 325 267 401
152-200 (0.076-0.10) 130 34 81 39
>200 (>0.10) 48 8 30 8
Total 8624 8737 8742 7355
a From: Guicherit (1975).
b Measured by the chemiluminescence method.
When the maximum concentration for a given averaging time, e.g.,
1 h, is known, maximum values for other averaging times can be
estimated from the averaging time-concentration model of Larsen
(1974). The concentrations for various averaging periods, calculated
from the base data of 1-h concentrations of 300 and 800 µg/m3, are
shown in Table 9.
Table 9. Estimated ozone concentrations for various
averaging periodsa
Averaging period Maximum concentration (µg/m3)
1-h (base data) 300 800
3-h 190-215 435-520
1 day 85-120 225-310
1 year 15-30 35-80
a Adapted from: Larsen (1969).
These calculations are based on geometric standard deviations
reported for several cities in the USA (Larsen, 1969).
Caution should be exercised when applying Larsen's model. Although
it is not applicable to averaging times of less than 1 h, the model is
generally accurate when using observed annual means to predict
concentrations between 1 h and 1 day. For periods longer than 1 day it
is applicable only if the observed data closely follow a log-normal
distribution.
Seasonal and diurnal variations in oxidant values are important
characteristics of the urban pattern of environmental concentrations
of this group of pollutants. These temporal variations result from:
(a) variations in oxidant precursors; (b) variations in atmospheric
transport and dilution of pollutants, and (c) variations in
meteorological conditions and other atmospheric variables involved in
the photochemical reaction process. Because of diurnal variations in
intensity of both solar ultraviolet radiation and of precursor
emission rates, maximum daily ozone concentrations frequently occur
around noon. However, such maxima have also been observed during the
morning or afternoon hours, mostly in suburban areas.
Examples of diurnal patterns of oxidants or ozone and nitrogen
dioxide concentrations are shown in Figs. 2, 3, and 4. The close
relationship between oxidants and nitrogen dioxide quite frequently
leads to oxidant peaks following nitrogen dioxide peaks. However, this
is not always, as shown in Fig. 3.
Table 4. Long-distance transport of photochemical oxidants
Rural region Possible source Trajectory Reference
length
(km)
Mineral King Valley, Fresno, California < 100 Miller et al. (1972)
California, USA
Garrett County, New York, Philadelphia, > 100 US Environmental
Maryland, USA Baltimore, Washington DC Protection Agency
or Pittsburgh (1973)
Southern Eire Continental Europe 100-700 Cox et al. (1975)
& Southern UK
New York State, USA Buffalo, New York 100-300 Stasiuk & Coffey (1974)
Tochigi and Gunma Tokyo < 100 Environment Agency
Prefectures, Japan (1976)
Midwest, USA St. Louis > 150 White et al. (1976)
4.3 Urban Areas
Since photochemical oxidants are the products of sunlight-induced
photochemical reactions, elevated concentrations of oxidants in urban
areas are generally restricted to a 4- to 6-h period within a day,
representing only 15-25% of the 24-h interval. For this reason, the
reporting of oxidant or ozone data as daily, monthly, or yearly means
can be misleading when evaluating trends or comparing oxidant
concentrations in different cities. Thus, oxidant or ozone data are
usually reported in terms of highest 1-h concentrations or in terms of
the number of days with hourly concentrations exceeding a specified
value or the number of hours when a given range of concentrations
occurred within a year. However, they may also be given as
instantaneous or five minute peak concentrations or frequency
distributions.
As shown in Table 5, the highest 1-h concentrations at 8 locations
were of the order of 300-800 µg/m3 (0.15-0.40 ppm). It is important
to recognize that the data presented are only for one site in each of
the cities and do not necessarily represent the maximum levels
occurring in these urban areas or provide a good indication of human
exposure levels. For this reason, frequency distributions or reporting
of the number of days or hours when a given concentration was exceeded
are helpful. Such data are presented in Tables 6, 7, and 8 for
selected monitoring stations in Tokyo, Washington DC, and Delft,
respectively, to illustrate the distribution of the concentrations
recorded at these monitoring stations. These tables are not intended
to indicate long-term trends since meteorological patterns that
greatly influence the ambient concentrations of oxidants can vary
considerably from year to year.
The data for Tokyo show both the number of days when a given
concentration was exceeded and the total number of hours in which
concentrations falling within the specified ranges were observed. As
shown in Table 6, an hourly concentration of 200 µg/m3 (0.1 ppm) was
exceeded on 10-30 of days in these years, and in approximately 1-4% of
all the hours in the year.
In Washington, DC, a concentration of 200 µg/m3 (0.1 ppm) was
exceeded, on average, on about 5% of the days, with most of the days
having maximum 1-h concentrations of about 100 µg/m3 (0.05 ppm).
The data for Delft, Netherlands, show the number of hours in the
year when given concentrations were observed. More than 90% of the
hours exhibited concentrations of less than 100 µg/m3 (0.05 ppm).
However, in 1971 and 1973, there were 48 and 30 hours, respectively,
when concentrations exceeded 200 µg/m3 (0.1 ppm).
Table 5. Highest 1-h concentrations of ozone or total oxidants observed at
selected sites in 1974
City Concentration Method
µg/m3 (ppm)
Bonn, Federal Republic of Germany1 290 (0.145) chemiluminescence
Eindhoven, Netherlands2 420 (0.210) chemiluminescence
London, UK3a 294 (0.147) chemiluminescence
Los Angeles, USA4 548 (0.274) NBKI
Osaka, Japan5 320 (0.160) NBKI
Riverside, USA4 744 (0.372) NBKI
Tokyo, Japan5 380 (0.190) NBKI
Washington, USA4 312 (0.156) chemiluminescence
From: 1 Becker & Schurath (1975).
2 Guicherit (1975).
3 Ball (1976).
4 US Environmental Protection Agency (1976).
5 Environment Agency (1975b).
a Data for 1975.
Table 6. Number of days and hours when hourly oxidant concentrations were in the
range of indicated levels at a National Air Sampling Nelwork Station,
Tokyo, Japana
Concentrationb Number of days (hours)
µg/m3 (ppm) 1971 1972 1973 1974
0-100 (0 -0.05) 113 (7032) 185 (7863) 60 (7248) 184 (8122)
120-180 (0.06-0.09) 135 (798) 111 (379) 199 (1050) 134 (495)
200-280 (0.10-0.14) 60 (251) 31 (81) 73 (298) 40 (107)
300-380 (0.15-0.19) 18 (42) 8 (14) 21 (59) 5 (10)
400-480 (0.20-0.24) 6 (8) 1 (2) 7 (14) 0 (0)
>500 (>0.25) 0 (0) 0 (0) 1 (2) 0 (0)
Total 332 (8131) 336 (8339) 361 (8671) 363 (8734)
a From: Tokyo Metropolitan Government (1971-1974) (unpublished data).
b Measured by NBKI method.
Table 7. Number of days when at least one hourly oxidant
concentration was in the range of indicated levels in
Washington, DC, USAa
Concentrationb Number of days
µg/m3 (ppm) 1970 1971 1972
0-100 (0 -0.05) 72 155 134
120-200 (0.06-0.10) 85 127 39
220-300 (0.11-0.15) 8 17 6
>300 (>0.15) 2 0 0
Total 167 299 179
a From: US Environmental Protection Agency (1964-1973).
b Measured by the NBKI method.
Table 8. Number of hours when hourly ozone concentrations were in
the range of indicated levels in Delft, Netherlandsa
Concentrationb Number of hours
µg/m3 (ppm) 1971 1972 1973 1974
0-100 (0 -0.05) 7799 8370 8364 6907
120-150 (0.06 -0.075) 647 325 267 401
152-200 (0.076-0.10) 130 34 81 39
>200 (>0.10) 48 8 30 8
Total 8624 8737 8742 7355
a From: Guicherit (1975).
b Measured by the chemiluminescence method.
When the maximum concentration for a given averaging time, e.g.,
1 h, is known, maximum values for other averaging times can be
estimated from the averaging time-concentration model of Larsen
(1974). The concentrations for various averaging periods, calculated
from the base data of 1-h concentrations of 300 and 800 µg/m3, are
shown in Table 9.
Table 9. Estimated ozone concentrations for various
averaging periodsa
Averaging period Maximum concentration (µg/m3)
1-h (base data) 300 800
3-h 190-215 435-520
1 day 85-120 225-310
1 year 15-30 35-80
a Adapted from: Larsen (1969).
These calculations are based on geometric standard deviations
reported for several cities in the USA (Larsen, 1969).
Caution should be exercised when applying Larsen's model. Although
it is not applicable to averaging times of less than 1 h, the model is
generally accurate when using observed annual means to predict
concentrations between 1 h and 1 day. For periods longer than 1 day it
is applicable only if the observed data closely follow a log-normal
distribution.
Seasonal and diurnal variations in oxidant values are important
characteristics of the urban pattern of environmental concentrations
of this group of pollutants. These temporal variations result from:
(a) variations in oxidant precursors; (b) variations in atmospheric
transport and dilution of pollutants, and (c) variations in
meteorological conditions and other atmospheric variables involved in
the photochemical reaction process. Because of diurnal variations in
intensity of both solar ultraviolet radiation and of precursor
emission rates, maximum daily ozone concentrations frequently occur
around noon. However, such maxima have also been observed during the
morning or afternoon hours, mostly in suburban areas.
Examples of diurnal patterns of oxidants or ozone and nitrogen
dioxide concentrations are shown in Figs. 2, 3, and 4. The close
relationship between oxidants and nitrogen dioxide quite frequently
leads to oxidant peaks following nitrogen dioxide peaks. However, this
is not always, as shown in Fig. 3.


 Seasonal variations in oxidant concentrations are manifested by
increases in the diurnal maxima and by increases in the number of days
per month that exhibit elevated oxidant values. For example, Fig. 5
shows the number of days per month in which the oxidant concentration
in Los Angeles, USA, equalled or exceeded 200 µg/m3 (0.1 ppm).
Similarly, Fig. 6 shows the number of days per month in Delft,
Netherlands, that also exhibited ozone concentrations equal to or
above 200 µg/m3 (0.1 ppm).
Peroxyacetylnitrate is generally formed simultaneously with ozone.
However, comparatively few measurements have been made of
peroxyacetylnitrate in the ambient atmosphere. The ratio of
peroxyacetylnitrate to ozone observed at a maximum concentration of
peroxyacetylnitrate was about 1:100 in rural England (Sandalls et al.,
1974) and about 1:50 in Delft, Netherlands (Nieboer & Van Ham, 1976).
Variations in concentrations of peroxyacetylnitrate often follow those
of ozone, as shown in Fig. 7 (Sandalls et al., 1974). However, this is
not always the case (Stephens, 1976).
4.4 Indoor Concentrations
Oxidant concentrations inside buildings tend to be lower than those
outdoors because of destructive reactions that occur on most surfaces
(Mueller et al., 1973; Sabersky et al., 1973). However, certain indoor
sources of ozone (section 3.3) may increase indoor concentrations.
Indoor concentrations at places of work are discussed in section 6.2.
Seasonal variations in oxidant concentrations are manifested by
increases in the diurnal maxima and by increases in the number of days
per month that exhibit elevated oxidant values. For example, Fig. 5
shows the number of days per month in which the oxidant concentration
in Los Angeles, USA, equalled or exceeded 200 µg/m3 (0.1 ppm).
Similarly, Fig. 6 shows the number of days per month in Delft,
Netherlands, that also exhibited ozone concentrations equal to or
above 200 µg/m3 (0.1 ppm).
Peroxyacetylnitrate is generally formed simultaneously with ozone.
However, comparatively few measurements have been made of
peroxyacetylnitrate in the ambient atmosphere. The ratio of
peroxyacetylnitrate to ozone observed at a maximum concentration of
peroxyacetylnitrate was about 1:100 in rural England (Sandalls et al.,
1974) and about 1:50 in Delft, Netherlands (Nieboer & Van Ham, 1976).
Variations in concentrations of peroxyacetylnitrate often follow those
of ozone, as shown in Fig. 7 (Sandalls et al., 1974). However, this is
not always the case (Stephens, 1976).
4.4 Indoor Concentrations
Oxidant concentrations inside buildings tend to be lower than those
outdoors because of destructive reactions that occur on most surfaces
(Mueller et al., 1973; Sabersky et al., 1973). However, certain indoor
sources of ozone (section 3.3) may increase indoor concentrations.
Indoor concentrations at places of work are discussed in section 6.2.


 5. EFFECTS ON EXPERIMENTAL ANIMALS
There is considerable evidence to show that even short exposure to
high concentrations of ozone may endanger the health of experimental
animals. In reviewing this evidence, emphasis has been placed on
studies in which animals were exposed to concentrations of oxidants of
2000 µg/m3 (1.0 ppm) or less, since these studies are more relevant
for predicting the health risk to man. However, some experiments
conducted at higher concentrations have also been discussed when it
was considered that they would contribute to a better understanding of
the mechanism of the biological action of oxidants.
5.1 Absorption of Ozone
A number of factors can influence the transport and removal of
ozone in the upper airways such as: (a) nasal morphology; (b) route,
rate, and depth of breathing; and (c) biochemical composition and
amount of mucus. The decomposition of ozone within the upper airways
may protect the lower part of the respiratory tract against the
irritant gas. Various attempts to determine or model the respiratory
absorption of ozone in the upper airways have been made (McJilton et
al., 1972; Vaughan et al., 1969; Yokoyama & Frank, 1972). This work
has recently been reviewed by Miller (1977) who developed a
mathematical model for the transport and removal of ozone in the
respiratory tract of guineapigs, rabbits, and man, and predicted that
whatever the initial concentration, the respiratory bronchioles would
receive the highest dose of ozone. This agrees well with various
experimental studies in animals. For all three species the
relationship between respiratory bronchiolar concentration and the
inhaled ozone concentration at the tracheal level is linear on a
log-log scale at concentrations greater than 100 µg/m3 (0.05 ppm),
the respiratory bronchiolar dose for rabbits being 80% of that for man
and twice that for guineapigs.
5.2 Effects on the Respiratory System
5.2.1 Morphological changes
5.2.1.1 Short-term exposure (24 h or less)
The primary target of ozone is the respiratory tract and
particularly the pulmonary parenchyma. In small laboratory animals,
exposure to ozone at acutely toxic concentrations results in pulmonary
oedema, haemorrhage, and death. The LD50 is about 12 mg/m3 (6.0 ppm).
At lower concentrations in the range of 400-2000 µg/m3 (0.2-1.0 ppm),
ozone causes numerous changes in both the epithelial and endothelial
cells of the lung and ultrastructural effect indicate that the primary
lesions are in the epithelial lining of the terminal bronchioles and
the proximal alveoli.
The sequence of degeneration, desquamation, and destruction of
type I alveolar cells in rats following exposure to an ozone
concentration of 400 µg/m3 (0.2 ppm) for 2 h was demonstrated in
electron microscopic studies by Stephens et al. (1974). The type II
epithelial cells appeared to be more resistant. Freeman et al. (1974)
also reported significant histological changes in type I epithelial
cells after 4 h exposure to a concentration of 1800 µg/m3. The loss
of ciliated epithelium throughout the upper respiratory tract,
swelling and denudation of type I cells, erythrocyte lysis within
alveolar capillaries, and breakdown of capillary endothelium has been
reported in cats exposed to ozone concentrations of 520, 1000, and
2000 µg/m3 (0.26, 0.5, and 1.0 ppm) for 4.7-6.6 h (Boatman et al.,
1974). These effects appeared to be dose-related. Similar effects were
noted by Bils (1970) in mice after a 7-h exposure to ozone at
concentrations of 1200 and 2600 µg/m3 (0.6 and 1.3 ppm).
Cell renewal rate within the alveoli was studied by Evans et al.
(1971) by injecting tritiated thymidine into 18 to 20-month-old mice
before exposing them for 6 h to ozone concentrations of 1000, 2400,
5000, or 7000 µg/m3 (0.5, 1.2, 2.5, and 3.5 ppm). Immediately
following exposure, the number of labelled alveolar cells (those
synthesizing DNA) was significantly lower in all exposed groups
compared with controls.
Similar morphological studies have also been performed using
complex mixtures containing oxidants. Ultrastructural alterations
consisting of disrupted cytoplasm and abnormal mitochondria were seen
in the alveolar tissue of mice exposed for 2-3 h to Los Angeles air
that had a total oxidant concentration of 800 µg/m3 (0.4 ppm) (Bils,
1966). Exposure of mice for 3 h to an irradiated synthetic atmosphere
that contained propylene, nitric oxide, carbon monoxide, and water
vapour and simulated a heavy smog, gave rise to a similar pattern of
ultrastructural changes (Bils & Romanovsky, 1967). Rats continuously
exposed for 24 h to a mixture of ozone at a concentration of
500 µg/m3 (0.25 ppm) and nitrogen dioxide at a concentration of
4700 µg/m3 (2.5 ppm) exhibited increases in the numbers of alveolar
macrophages and of free cells in the lung resembling desquamated type
I cells, and hypertrophy of the epithelium were noted in rats
continuously exposed for 24 h to a mixture of ozone at a concentration
of 500 µg/m3 (0.25 ppm) and nitrogen dioxide at a concentration of
4700 µg/m3 (2.5 ppm) (Freeman et al., 1974).
5.2.1.2 Prolonged and repeated exposures
Prolonged exposure to low levels of ozone causes more extensive
and irreparable damage to the lung than the oedematigenous and acute
inflammatory reactions observed following short exposure to high
concentrations. Emphysema, atelectasis, focal necrosis, broncho-
pneumonia, and fibrosis have been reported, often accompanied by a
variety of cellular alterations. The degree of morphological injury
appears to be proportional to the concentration and time of exposure.
Freeman et al. (1974) described a number of pathomorphological
changes in rats continuously exposed to ozone at concentrations of
1100 and 1800 µg/m3 (0.54 and 0.88 ppm) for as long as 6 months.
After 48 h of exposure to the lower concentration, an influx of
macrophages and an increase in mitotic figures were observed, and the
alveolar ducts became demarcated by hypertrophic alveolar epithelium.
Most of these changes became more obvious with increasing length of
exposure with the exception of the terminal bronchioles which appeared
to recover and return to normal. After exposure to the higher
concentration of 1800 µg/m3 (0.88 ppm) for 48 h, the bronchiolar
epithelium exhibited both metaplasia and fibrosis. Adenoma-like
structures containing large numbers of macrophages and fibrotic
lesions appeared after 6 days and after 3 weeks of exposure, half of
the rats had died and were found to have emphysema-like lesions.
Using scanning electron microscopy, Ikematsu et al. (1976) found
that continuous exposure to an ozone concentration of 2000 µg/m3
(1.0 ppm) for 10 days resulted not only in desquamation of epithelial
cells but also in the appearance of inflammatory cells in the tonsils
of rabbits. Similar pathological effects were observed with
intermittent exposure indicating that the animals were unable to
recover sufficiently during the periods of exposure to clean air.
Emphysematous and vascular lesions in the lung of the rabbit described
by P'an et al. (1972), were the result of repeated exposure to
800 µg/m3 (0.4 ppm) for 6 h per day, 5 days per week, for 10 months.
Stokinger et al. (1957) also reported fibrotic changes and chronic
bronchial and bronchiolar emphysema in the lungs of mice, rats,
guineapigs, and hamsters exposed to an ozone concentration of
2000 µg/m3 (1.0 ppm) for 6 h per day, 5 days per week, for 433 days.
However, under the same conditions of exposure, the effects in dogs
were limited to the trachea and large bronchi. When dogs were exposed
to ozone concentrations ranging from 2000 to 6000 µg/m3 (1-3 ppm) for
8, 16, or 24 h daily for 18 months, the morphological damage was
roughly proportional to the product of the concentration and the time
of exposure, the epithelial lining of the terminal airways and
proximal alveoli being most adversely affected (Freeman et al., 1973;
Stephens et al., 1973). Similarly, Castleman et al. (1973a) found that
the walls of the terminal airways and the interalveolar septa of rat
lung were most affected by continuous exposure to an ozone
concentration of 1600 µg/m3 for 7 days.
Intermittent exposure for 8 h per day, for 7 days to an ozone
concentration of 400 µg/m3 (0.2 ppm) produced damage to the
respiratory bronchioles in bonnet monkeys. In rats fed a normal diet,
similar effects were seen at the same concentration of ozone, but, in
vitamin E-deficient rats, an equivalent effect was caused by ozone at
200 µg/m3 (0.1 ppm) (Dungworth et al., 1975; Dungworth, 1976).
Loosli et al. (1972) measured the cell turnover rate in mice and
found it to be significantly higher in animals exposed to synthetic
photochemical air pollution for 8-12 months than in control animals
breathing filtered air. The synthetic air pollution contained ozone at
600-840 µg/m3 (0.30-42 ppm), carbon monoxide at 3.5-12 mg/m3
(3-10 ppm), nitrogen dioxide at 1300-1600 µg/m3 (0.70-0.85 ppm), and
sulfur dioxide at 5700-6000 µg/m3 (2.2-2.3 ppm). The effects were
similar to those seen with exposure to pure ozone.
Rats exposed for 1 month to a mixture of ozone at 1800 µg/m3
(0.9 ppm) and nitrogen dioxide at 1700 µg/m3 (0.9 ppm), developed
enlarged alveolar spaces (Freeman et al., 1974). In rats exposed for
2 weeks to a combination of ozone at 500 µg/m3 (0.25 ppm), and
nitrogen dioxide at 4700 µg/m3 (2.5 ppm), the bronchiolar epithelium
became cuboidal. However, after 6 months of exposure, the tissue
appeared to be normal (Freeman et al., 1974).
Slightly inflammatory and proliferative changes were observed by
Nakajima et al. (1972) in the bronchial membranes of mice exposed to
irradiated auto exhaust that contained oxidant concentrations of
200-300 µg/m3 (0.1-0.15 ppm), for 2-3 h per day, 5 days per week, for
30 days.
5.2.2 Functional changes
5.2.2.1 Short-term exposure (24 h or less)
The first abnormal sign observed in various animal species during
exposure to ozone is an irregular respiratory pattern. This usually
appears within the first few minutes of exposure and, in most cases,
the animal returns to normal, when allowed to recover in the clean
air.
Functional changes in the respiratory system have been observed in
several species of animals at ozone concentrations of less than
2000 µg/m3 (1.0 ppm). Yokoyama (1972a) studied the ventilatory
function of guinea-pigs before, during, and after a 2-h exposure to an
ozone concentration of 1000 µg/m3 (0.5 ppm). He reported increases in
the frequency of respiration and airway resistance, but a decrease in
tidal volume. In another investigation, Yokoyama (1973) exposed only
the right lung of rabbits to ozone at 2000 µg/m3 (1 ppm) for
3 h and used the left lung as a control. The exposed lung of rabbits
killed 1 and 3 days after exposure, had a reduced vital capacity.
However, the reduction in vital capacity in those killed 7 days after
exposure was only slight and was not significant. Other studies on
lung function in guineapigs exposed to ozone for 2 h indicated that
pulmonary flow resistance was not altered by exposure to
concentrations of 700 and 1400 µg/m3 (0.34 and 0.68 ppm), but that it
increased significantly at exposure levels of 2000 and 2700 µg/m3
(1.08 and 1.35 ppm) (Murphy et al., 1964a).
Scheel et al. (1959) exposed rats to an ozone concentration of
4000 µg/m3 (2 ppm) for 3 h. Decreases in minute ventilation, tidal
volume, and oxygen uptake, that occurred immediately after exposure,
reached minimum recorded values 8 h later. All measurements returned
to normal levels 20 h after exposure.
When cats were exposed for an average of 4.6 h to ozone
concentrations of 520, 1000, and 2000 µg/m3 (0.26, 0.5, and 1 ppm),
Watanabe et al. (1973b) found that pulmonary flow resistance increased
with increasing ozone concentrations. Dynamic compliance was reduced,
but to a lesser extent, and vital capacity was unaffected. The
proportion of animals that showed a reduction in diffusion capacity
appeared to increase with increasing ozone concentrations.
There are a few studies in which animals were exposed to ambient
air and irradiated auto exhaust containing high concentrations of
oxidants, including those of Swann & Balchum (1966) who measured the
total expiratory flow resistance in guineapigs on days of unusual
weather and smog conditions in Los Angeles. When the resistance was
compared with routine monthly measurements on the same animals,
significant increases were found at oxidant levels of approximately
600 µg/m3 (0.30 ppm) or more. Substantial increases in resistance
were also observed, when relatively high concentrations of nitrogen
dioxide (1700 µg/m3; 0.92 ppm), carbon monoxide (30 mg/m3;
26 ppm), and hydrocarbons (16 ppm) were present, but the oxidant level
(80 µg/m3; 0.04 ppm) was relatively low.
The effects on guineapigs of a 4-h exposure to diluted,
irradiated, or nonirradiated exhaust atmospheres were reported by
Murphy et al. (1963). Marked, rapid increases in total expiratory flow
resistance accompanied by a decrease in respiratory rate and a small
increase in tidal volume occurred during exposure to irradiated
exhaust. The reaction to nonirradiated exhaust was comparatively
slight. Ranges of concentrations of the main pollutants in the
exhaust-contaminated air used in this study were: for irradiated
exhaust gases: total oxidants, 660-1640 µg/m3 (0.33-0.82 ppm);
nitrogen dioxide, 800-10 000 µg/m3 (0.43-5.5 ppm); and carbon
monoxide, 39-360 mg/m3 (34-310 ppm): for nonirradiated exhaust gases:
total oxidants, less than 40 µg/m3 (0.02 ppm); nitrogen dioxide,
710-3000 µg/m3 (0.38-1.58 ppm); and carbon monoxide, 98-345 mg/m3
(85-300 ppm). In addition, increased levels of formaldehyde, acrolein,
and olefin were present.
5.2.2.2 Prolonged and repeated exposures
The few available pulmonary function studies on animals exposed to
ozone for extended periods of time include a study by Bartlett et al.
(1975) who reported that continuous exposure of rats to an ozone
concentration of 400 µg/m3 (0.2 ppm) for 30 days caused a 16%
increase in lung volume and an increase in alveolar dimension. A
reduction in lung elasticity that was also reported was possibly an
effect of ozone on collagen. Yokoyama (1974) exposed rabbits to an
ozone concentration of 4000 µg/ma (2 ppm), for 6 h per day, for 3-4
days, and found that the pulmonary flow resistance was greater, and
compliance lower, than in the controls. In animals exposed to
2000 µg/m3 (1 ppm), for 6 h per day, for 7-8 days, the values were
between those of the controls and of the group treated with
4000 µg/m3 (2 ppm).
5.2.3 Biochemical changes
5.2.3.1 Effects indicating possible mechanisms of action
The actual mechanism of ozone toxicity at the subcellular level is
still obscure. Studies of the biochemical effect of ozone have mainly
been based on two hypotheses: (a) that ozone interacts with readily
oxidizable substances thus altering the course of metabolism and
producing a toxic effect; and (b) that ozone interacts with
unsaturated lipids to produce lipid peroxidation and consequent cell
damage.
Sulfhydryl systems appear in the cell not only as reducing
substances but also as functional constituents of a variety of enzymes
and proteins. Ozone is capable of oxidizing these substances causing
inactivation of enzymes and alterations in the structure and function
of the cell membrane.
In studies on ozone oxidation of glutathione in vitro, Mudd et
al. (1969) showed that both fast and slow oxidation occurred. It has
also been shown that the oxidation of reduced glutathione (GSH)
results in oxidized glutathione (GSSG), though some of the sulfhydryl
groups form higher oxidation products that are not reduced by the
reductases available in the cells (Menzel, 1971). Mountain (1963)
demonstrated in vivo oxidation of reduced glutathione and a decrease
in the sulfur-containing enzyme succinic dehydrogenase (1.3.99.1)
activity in the lungs of mice. King (1961) also found a decrease in
sulfhydryl content and of enzymatic function of partially purified
glyceraldehyde-3-phosphate dehydrogenase (1.2.1.12) in rat lungs
following exposure to an ozone concentration of 2400 µg/m3 (1.2 ppm)
for 4 weeks.
When rats were exposed to an ozone concentration of 4000 µg/m3
(2.0 ppm) for 4-8h, the concentrations of both the protein and
nonprotein sulfhydryls in the lungs decreased. The activities of lung
enzymes containing sulfhydryl also decreased including those of
glucose-6-phosphate dehydrogenase (1.1.1.49), glutathione reductase
(1.6.4.2), and cytochrome c reductase related to succinate and
reduced nicotinamide adenine dinucleotide (NADH) (DeLucia et al.,
1972). Expanding these studies, DeLucia et al. (1975) showed that the
magnitude of the decrease in the nonprotein sulfhydryl groups in rats
was dependent on the duration of exposure and the concentration of
ozone. Significant decreases were not observed at an ozone
concentration of 1600 µg/m3 (0.8 ppm) for 24 h.
Further studies have shown that exposure to ozone tends to
increase the activity of enzymes that protect against intracellular
oxidation. When rats were continuously exposed to an ozone
concentration of 1500 µg/m3 (0.75 ppm) for 1, 3, 10, or 29 days, it
was noted that the activities of glutathione peroxidase (1.11.1.9),
glutathione reductase (1.6.4.2), glucose-6-phosphate dehydrogenase,
6-phosphogluconate dehydrogenase (1.1.1.43), and pyruvate kinase
(2.7.1.40) were lower than those in the controls after 1 day but
higher after, 3, 10, and 29 days of exposure (Chow & Tappel, 1973). In
another study by the same investigators, rats exposed to 400 µg/m3
(0.2 ppm) continuously for 8 days or intermittently (8 h per day) for
7 days showed significantly increased glutathione peroxidase activity
in the lung. Fukase et al. (1975a, 1975b) exposed mice to ozone
concentrations of 400, 1000, or 2000 µg/m3 (0.2, 0.5 or 1.0 ppm) for
4 h per day, for 30 days, and found progressive increases in the
levels of glutathione and vitamin C in the lung with increasing ozone
concentration. The authors also reported significant increases in the
activities of glutathione peroxidase, glutathione reductase, and
glucose-6-phosphate dehydrogenase in the lungs of mice. Exposure of
both rhesus and bonnet monkeys to levels of ozone ranging from 400 to
1600 µg/m3 (0.2-0.8 ppm) for 8 h per day for 7 days, resulted in
increased activities of succinate oxidase and glutathione peroxidase
in the lungs. Linear regression analysis showed a significant
correlation between ozone concentration and the augmentation in
activity of these enzymes (Dungworth et al., 1975).
Many investigators have attributed the biological effect of ozone
to lipid peroxidation (Fournier, 1973; Goldstein et al., 1969; Menzel,
1970; Roehm et al., 1971b). Oxidation of unsaturated fatty acids by
ozone has been demonstrated both in vivo and in vitro. The
mechanism of such action is based on the proclivity of ozone to react
with the ethylene groups of the acid to form peroxides. Their
decomposition results in the further formation of free radicals
capable of initiating peroxidation of other unsaturated fatty acids.
The breakdown products (peroxides, carbonyl compounds) may themselves
be cytotoxic.
Evidence of lung lipid peroxidation during ozone exposure was
suggested by Goldstein et al. (1969) who found conjugated diene bonds
in an extract of lungs of mice exposed to 800-1400 µg/m3
(0.4-0.7 ppm) for 4 h. Another index of lipid peroxidation is an
increase in malonaldehyde. In studies by Chow & Tappel (1972), the
malonaldehyde concentration increased in the lungs of rats exposed
continuously to 1400-1600 µg/m3 (0.7-0.8 ppm) for 5 and 7 days.
Further evidence of the role of peroxidation in ozone toxicity is
the fact that animals deficient in vitamin E are more susceptible to
ozone. This is discussed in detail in section 5.2.7.
5.2.3.2 Biochemical effects at the subcellular level
In morphological and ultrastructural studies, swelling and
degenerative changes in lung mitochondria have frequently been
reported following ozone exposure. Mitochondrial functions are
critical to the cellular terminal substrate oxidation and energy
production. These organelles in lung cells may be the target for ozone
since many mitochondrial enzyme activities are sulfhydryl-dependent
and mitochondrial membranes contain abundant unsaturated
phospholipids. It has therefore been suggested that the lung
mitochondria may be a sensitive test system for detecting and
evaluating ozone toxicity.
Mustafa et al. (1973) found a 45% increase in pulmonary
mitochondrial oxygen consumption in rats continuously exposed to
1600 µg/m3 (0.8 ppm) for 10-20 days. A 3-fold increase in the number
of type II cells was also reported. These cells are rich in
mitochondria and may have been responsible for the increase in oxygen
consumption. A 17% increase in oxygen consumption was noted with
continuous exposure to an ozone concentration of 400 µg/m3 (0.2 ppm)
for 7 days. This effect appeared to be dose-related.
Lysosomes are vitally important in the intra- and possibly
extracellular destruction of inhaled matter. The hydrolytic enzyme
system of lysosomes in the alveolar machrophage is crucial to maintain
the sterility of the lung against inhaled microbes. Any inactivation
of these enzymes would be expected to increase the risk of respiratory
disease.
Hurst et al. (1970) reported that, when rabbits were exposed for
3 h to an ozone concentration of 500 µg/m3 (0.25 ppm), there was a
reduction in the activity of lysosomal hydrolases, i.e., acid
phosphatase (3.1.3.2), lysozyme (3.2.1.17), and beta-glucuronidase
(3.2.1.31). In vitro exposure of rabbit alveolar macrophages
produced a similar decrease in lysosomal hydrolases (Hurst & Coffin,
1971).
Ozone may also induce increases in the concentrations of these
enzymes which may be related to eventual chronic lung disease.
When the specific activities of a number of lysosomal hydrolases
were measured in whole lung homogenates of rats, there was a
significant increase in activities after the animals had been
continuously exposed to an ozone concentration of 1400-1600 µg/m3
(0.7-0.8 ppm) for 5-7 days (Dillard et al., 1972). This increase could
be attributed to an inflammatory reaction induced by the ozone (Coffin
et al., 1968b).
Castleman et al. (1973b) exposed rats continuously for 7 days to
ozone concentrations of 1400-1600 µg/m3 (0.7-0.8 ppm). Using histo-
and cytochemical techniques, they observed increased acid phosphatase
activity but no change in beta-glucuronidase activity.
One of the structural alterations reported following exposure to
ozone is a change in the appearance of the endoplasmic reticulum.
Biochemical evidence of the effects of ozone on microsomal enzymes was
reported by Palmer et al. (1971, 1972). After a 3-h exposure to ozone
at 1500 µg/m3 (0.75 ppm), the lung tissue of hamsters and the
tracheobronchial mucosa of rabbits showed reductions of 33% and 53%,
respectively, in the activity of benzopyrene hydroxylase (1.14.14.2),
a mixed function oxidase that depends on cytochrome P-450 and is
located in the endoplasmic reticulum. Similar results were obtained by
Goldstein et al. (1975) who exposed rabbits for 90 rain to
2000 µg/m3 (1.0 ppm) and demonstrated a decrease in the rabbit lung
cytochrome P-450 concentration. It is of interest that the maximum
effect was observed a few days after exposure.
Very little information is available describing alterations in
nucleic acids related to ozone exposure. Most of the studies have been
conducted at much higher concentrations than those found in the
environment and have yielded conflicting results. Since DNA synthesis,
cell division, and growth are closely linked, further studies
clarifying the potential hazard would be of value.
In studies on mice exposed to an ozone concentration of
5000 µg/m3 (2.5 ppm) for 2 h per day, for 120 days, Werthamer et al.
(1974) noted reductions in both DNA and RNA syntheses and a
concomitant increase in protein synthesis. Evans et al. (1971) exposed
aging mice for 6 h to 1000-7000 µg/m3 (0.5-3.5 ppm) and found that,
regardless of the ozone concentration, there was inhibition of DNA
synthesis. The authors believed that this indicated a reduction in the
ability of the alveoli to act as a source of new cells and to maintain
the integrity of lung tissue during ozone exposure.
5.2.3.3 Extracellular effects
Since the primary cause of death from high concentrations of ozone
is pulmonary oedema, investigators have attempted to determine the
role of histamine in the pulmonary toxicity of ozone. The lung is rich
in histamine -- containing mast cells and among the many effects of
histamine are oedematigenous alterations in vascular capillaries.
Oedema, whatever the cause, reduces the number of alveoli
participating in gas exchange, and produces conditions favourable for
bacterial growth. The exact concentration of ozone required to produce
oedema depends on the animal species. It is generally believed that
gross oedema is probably not elicited in any species exposed to ozone
at the concentrations found in the ambient air.
Alpert et al. (1971a) used a radio labelled albumen technique to
detect the presence of pulmonary oedema in rats. A significant
increase in albumen levels in pulmonary lavage fluid appeared after
6 h exposure to 1000 µg/m3 (0.5 ppm).
The available data on lung histamine are conflicting. Dixon &
Mountain (1965) reported that, following a single exposure of mice to
an ozone concentration of 2000 µg/m3 (1.0 ppm) for 5 h, there was a
release of histamine from the lungs that persisted for at least 4
days. Pretreatment with an antihistamine (promethazine) reduced the
amount of oedema following exposure to a sublethal dose of ozone. It
should be noted that the antihistamine used is a phenothiazine
derivative the action of which might stabilize membranes and trap free
radicals. In contrast, Easton & Murphy (1967) were unable to
demonstrate any reduction of lung histamine in guineapigs exposed to
ozone concentrations of 10-12 mg/m3 (5-6 ppm) for 2 h. Cronin & Giri
(1974) also failed to observe any differences in the lung histamine
level in rats exposed to an ozone concentration of 8000 µg/m3
(4.0 ppm) for 4 h, although pulmonary oedema was evident.
Surface tension is an important contributor to the elastic
properties of the lung. Any alteration of the normal surface tension
in the alveoli may be implicated in the development of chronic lung
disease. Consequently, several investigators have examined the effect
of ozone on pulmonary surface activity. Gardner et al. (1971) exposed
rabbits to ozone levels as high as 20 mg/m3 (10 ppm) for 2.5 h and
then isolated the surface active material by pulmonary lavage. They
found that ozone did not alter the surface tension of this material
and that in vitro exposure did not affect the properties of
dipalmitoyl lecithin, a principal component of this surface active
substance. Similar results were reported by Huber et al. (1971) who
showed that a 3-h exposure of rabbits to ozone at 10 mg/m3 (5 ppm)
did not alter surface activity in the lavaged alveolar lining material
nor in extracts of the whole lung. On the other hand Yokoyama (1972a)
reported that in vitro exposure of guineapig lung extracts to ozone
concentrations of 1000 to 24000 µg/m3 (0.5-12 ppm) for 25-60 min
resulted in a rapid increase in surface tension. However, he, too, did
not find any change when dipalmitoyl lecithin was exposed to ozone.
The effect of ozone on the appearance of lung tissue lipids in
saline lavage fluid was studied by Kyei-Aboagye et al. (1973). They
proposed that ozone affected the lung by decreasing lecithin formation
while simultaneously stimulating the release of surfactant lecithins
(palmitoyl and oleyl).
In the lung, there is a ground substance between the basement
membrane of the alveolar epithelium and the capillary endothelium. Any
destruction of the integrity of this substance could affect the
elasticity of the lung. Buell et al. (1965) fractionated the lung
tissue of rabbits, after a 1-h exposure to an ozone concentration of
2000 µg/m3 (1.0 ppm), into soluble, lipid, and protein fractions. The
isolation of aldehydes and ketones from the protein fraction indicated
structural changes. It was suggested that, once these compounds were
formed, they might affect the intra-and intermolecular crosslinking of
elastic protein molecules which would in turn cause a reduction in the
elasticity of the lung.
5.2.4 Carcinogenicity
The possibility that ozone might be carcinogenic has been studied
in experimental animals.
Exposure to ozonized gasoline with ozone concentrations of
2000-7600 µg/m3 (1.0-3.8 ppm) for 52 weeks caused an increased
incidence of lung tumours in strain A mice (350 in each experimental
group). After 40 weeks, tumours were found in 21% of animals in the
control group and in 63% in the test group. After 52 weeks, the
incidence of tumour-bearing animals in the control group exposed to
washed air was 41% compared with 80% in the test group (Kotin & Falk,
1956). Additional studies using the same atmosphere of ozonized
gasoline were conducted on C57BL mice (405 in each experimental group)
(Kotin et al., 1958). After 92 weeks, the incidence of tumour-bearing
animals in the control group was 1.6% compared with 9.6% in the
exposed group.
Although these studies indicate that ozone may be tumorigenic
further work is necessary to confirm these results.
A number of studies including that of Penha et al. (1972) have not
been considered in this document because information concerning the
numbers of animals tested, control groups, etc. was inadequate.
5.2.5 Tolerance to ozone
The term "tolerance" refers to the fact that exposure to a
nonlethal dose of a specific toxic substance protects the host against
subsequent exposure to higher dose of the same chemical or of
different agents with similar toxicological properties (cross-
tolerance). Stokinger et al. (1956) reported such a protective effect
for ozone. Tolerance to ozone has been reviewed by Fairchild (1967)
and has also been reported by many other investigators (Henschler,
1960; Matzen, 1957; Mendenhall & Stokinger, 1959), In small rodents,
tolerance can be initiated by a concentration as low as 600 µg/m3
(0.3 ppm), maximum protection being obtained with concentrations
within the range of 2000-8000 µg/m3 (1-4 ppm) (Stokinger & Scheel,
1962). A single exposure to ozone can also induce a cross-tolerance
against subsequent lethal doses of X-ray irradiation (Hattori et al.,
1963), nitrogen dioxide, hydrogen peroxide, carbonic dichloride
(phosgene), ethanone (ketene), nitrosyl chloride, or hydrogen sulfide
(Fairchild, 1967).
Although the mechanism of tolerance is still not well understood,
several possibilities have been envisaged. Fairchild (1967) observed
that the level of reduced glutathione was maintained in ozone-tolerant
animals but not in the nontolerant group. This could be brought about
either by directly blocking the oxidation of reduced glutathione or
through some enzymatic pathway that would stimulate production of
reduced glutathione in response to a second exposure to ozone.
However, it is possible that the maintenance of these thiols in the
tolerant animal may be a result of the tolerance rather than the
cause. The studies of Chow & Tappel (1972, 1973) provide biochemical
support for this hypothesis. In addition, it has been shown (Mountain
et al., 1960) that the activities of serum alkaline phosphatase
(3.1.3.1), adrenal succinate dehydrogenase (1.3.99.1), and glucose-6-
phosphatase (3.1.3.9) either remain normal or are only slightly
altered in tolerant animals.
Fukase et al. (1975a) reported tolerance in mice to an ozone
concentration of 20-58 mg/m3 (10-29 ppm) after pre-exposure to
concentrations of 400-2000 [µg/m3 (0.2-1.0 ppm) and proposed that the
mechanism involved an increase in reduced glutathione and the
elevation of the activity of the peroxidative metabolic pathway.
Physical swelling of the intra-alveolar septa has also been proposed
by Henschler et al. (1964) as a mechanism of protection against the
oedematigenous effects of ozone.
Unilateral lung exposure models showed that, in order to induce
protection in a particular lung, the tissue must have actually come
into contact with ozone (Alpert & Lewis, 1971; Frank et al., 1970a).
There was no significant crossover effect from contralateral lung,
suggesting that there was no basis for assuming a circulating humoral
factor, and that the phenomenon was purely local. While reduction in
oedema development could be induced by pre-exposure to ozone, no
protection was obtained against the influx of polymorphonuclear
leukocytes into the lung (Gardner et al., 1972). It was also apparent
that tolerance did not influence the number of recoverable cells in
pulmonary lavage, their phagocytic capability, or the loss of
macrophage hydrolytic enzyme activity. This indicated the possibility
that tolerance protects only against pulmonary oedema and not against
other more subtle reactions.
As previously mentioned, tolerance is initiated in small rodents
at approximately 600 µg/m3 (0.3 ppm) (Stokinger & Scheel, 1962).
According to Alpert et al. (1971a) this approximates the lowest level
at which oedema can be demonstrated in rats by means of recovery of
labelled serum albumen via lung lavage. Thus, Coffin & Gardner (1972b)
postulated that it is probably necessary to produce a minimal
oedematigenous response before tolerance develops.
Experiments designed to test the susceptibility to bacterial
infection of "tolerant" animals have been conducted by Coffin &
Gardner (1972b). These studies illustrated that the "tolerant" animals
were only partially protected against the joint effects of ozone and a
viable microorganism. Partial protection was afforded by tolerance
only when the initiating dose of ozone was 600 µg/m3 (0.3 ppm) for
3 h (Coffin & Gardner, 1975). The authors considered that the
tolerance to infection seen at levels of ozone above 600 µg/m3
(0.3 ppm) could be due to the prevention of the formation of oedema
and that tolerance was only partial because tolerance to the action of
ozone on cellular and noncellular specific defence systems could not
be induced. This was shown by Gardner et al. (1972) who reported that
there was no tolerance to ozone damage in the alveolar macrophages,
which are the prime defence against infectious disease in the lower
part of the respiratory tract.
In their study on the effects of ozone on laboratory-induced
allergic respiratory disease in guineapigs, Matsumura et al. (1972)
found that animals that had received pretreatment with ozone at
2000 µg/m3 (1 ppm) for 1 h before a challenging exposure of
4000 µg/m3 (2 ppm) showed only slight respiratory reaction to
acetylcholine inhalation compared with those in which tolerance had
not been induced.
A variety of neurohumoral factors resulting from exposure to ozone
have been described by Fairchild (1963), who reported that
thyroidectomy, adrenalectomy, and hypophysectomy would increase the
resistance of rats to otherwise lethal doses of ozone by preventing
pulmonary oedema.
While the mechanism of tolerance in relation to ozone-induced
oedema is still not well understood, it has been suggested that the
thymus might play a role. Thymectomized mice were unable to develop
tolerance to ozone although the sham-operated animals exhibited
tolerance under the same experimental conditions (Gregory et al.,
1967).
5.2.6 Effects on the host defence system
Animals that are exposed to ozone have an increased susceptibility
to disease-producing biological agents, which can result in an
increased incidence of pulmonary infectious disease and death. Animal
models have been developed by several investigators to examine this
phenomenon experimentally by exposing animals to the pollutants and to
aerosolized viable microorganisms. The high sensitivity of this model
system is probably due to the fact that it reflects a summation of all
the subtle effects that ozone has on the lung. Any alteration in
cellular defence and ciliary activity, oedema, and immunosuppression
would allow the viable organism to multiply and cause disease. In mice
exposed to ozone at concentrations ranging from 2600 to 8800 µg/m3
(1.3 to 4.4 ppm) for 3 h, or 1700 µg/m3 (0.84 ppm) for 4 h, per day,
5 days per week, for 2 weeks, and subsequently challenged with
Klebsiella pneumoniae, resistance to respiratory infection was
significantly reduced (Miller & Ehrlich, 1958). Similar results were
obtained with hamsters. In a study to elucidate a relationship between
the time of exposure to ozone and the time of challenge with the
infectious agent, Purvis et al. (1961) found a decrease in resistance
to infection in mice treated with the bacterial aerosol within 19 h of
exposure to the gas or 27 h before exposure. A subsequent study by
Coffin and co-workers (1968a) showed that the lowest concentration of
ozone necessary to produce this effect in mice was 160 µg/m3
(0.08 ppm) for 3 h; Coffin & Gardner (1972) established a dose-
response relationship.
Further enhancement of toxicity could be demonstrated when a third
interactant such as cold or exercise was added to this infectivity
model. Housing mice at 6°-9°C for 3 h prior to exposure to ozone at
1400-1800 µg/m3 (0.7-0.9 ppm) for 2 h and to the microorganisms
increased the mortality rate compared with that in animals housed at
room temperature (Coffin & Blommer, 1965). Mice subjected to physical
activity while being exposed to ozone at 200-600 µg/m3 (0.1-0.3 ppm)
were less resistant to infectious agents than animals that were at
rest during exposure (Gardner et al., 1974b).
The effects of low concentrations of ozone on mice with induced
silicosis were investigated by Goldstein et al. (1972). Starting at an
ozone concentration of 800 µg/m3 (0.4 ppm) for 4 h, a progressive
decrease in pulmonary bactericidal activity occurred with exposure to
increasing concentrations of ozone. Silicosis itself did not inhibit
bactericidal activity.
Further research has sought to delineate the mechanisms by which
ozone reduces the resistance of animals to bacterial infection. It
appears that a number of factors may be responsible.
The number of organisms initially deposited within the lung does
not play a major role in the enhancement of mortality since the
pulmonary deposition of the microorganisms is less in ozone-treated
animals. However, regardless of the initial deposition, ozone-treated
animals subsequently have more organisms within the lungs, mainly
because of reduced bactericidal ability and the subsequent
multiplication of the inhaled organism (Coffin & Gardner, 1972a).
Goldstein et al. (1971b) found that the magnitude of the increase in
bacterial numbers was correlated with an increase in ozone
concentration up to 5200 µg/m3 (2.58 ppm). In rabbits exposed to
ozone at a concentration of 1000 µg/m3 (0.5 ppm), for 16 h per day,
for 7 months, Friberg et al. (1972) did not find any effect on the
physical removal of inhaled particles or on the number of macrophages
but, under the same conditions, a significant decrease was found in
the clearance of viable Escherichia coli in the lungs of guinea-
pigs.
The effect of ozone on antibacterial activity in the mouse lung
was determined in vivo by investigating the removal of bacteria by
mucociliary activity and by bactericidal activity simultaneously
(Goldstein, 1971a, 1971b). Mice were exposed to various concentrations
or ozone including 1200, 1400, 1600, and 2200 µg/m3 (0.62, 0.70,
0.80, and 1.1 ppm), for 17 h prior to, or 4 h after, infection with
aerosols of radiolabelled Staphylococcus aureus. Inhibition of
pulmonary bactericidal activity was shown at an ozone concentration as
low as 1200 µg/m3 (0.62 ppm) and activity decreased progressively
with increasing levels of ozone. The authors proposed that the
bactericidal defect was due to dysfunction of the alveolar macrophage.
Rabbits exposed to an ozone concentration of 600 µg/m3 (0.3 ppm)
for 3 h showed an impairment of the phagocytic properties of the
pulmonary alveolar macrophages (Coffin et al., 1968b). Furthermore,
exposure to ozone at 10 mg/m3 (5 ppm) for 3 h resulted in a reduction
in the total number of macrophages with a concomitant influx of
polymorphonuclear leukocytes into the lower respiratory tract (Coffin
& Gardner, 1972a). Enzyme activity was reduced in macrophages
recovered by lavage after exposure of rabbits to ozone at levels as
low as 500 µg/m3 (0.25 ppm) for 3 h (Hurst et al., 1970). The enzymes
studied were lysozyme (3.2.1.17), acid phosphatase, and beta-
glucuronidase and since these enzymes are involved in the
intracellular degradation of ingested bacteria, their reduction could
contribute to the poor bactericidal effect seen in the studies
mentioned previously.
Other effects on the alveolar macrophage have also been reported.
For example, alveolar macrophages from rabbits exposed to 4000 µg/m3
(2.0 ppm) for 8 h per day for 7 days exhibited an increased membrane
fragility (Dowell et al., 1970) and a dose-related reduction in
interferon was observed in the alveolar macrophages of rabbits exposed
to ozone concentrations of 2000, 6000, and 10 000 µg/m3 (1, 3, and
5 ppm) for 3 h (Shingu et al., 1972).
Morphological changes seen in rabbit alveolar macrophages after
exposure to ozone at a concentration of 10 mg/m3 (5 ppm) for 3 h
included dilatation of the endoplasmic reticulum and perinuclear
envelope, swelling of the mitochondria, intracellular vacuolization,
cell lysis, and the formation of myelin figures and autophagic
vacuoles (Huber et al., 1971). The ultrastructure of pulmonary
macrophages was also examined in situ in the lung tissue of rats
exposed to ozone at 6000 µg/m3 (3 ppm) for 4 h (Plopper et al.,
1973b). Immediately after exposure, the cells resembled those of
unexposed animals, but 12 h later there were twice as many
macrophages. Many of these had large granular cytoplasmic inclusions
that indicated increased phagocytic activity.
There is evidence that the effect seen on the alveolar macrophage
may be mediated through the noncellular milieu of the lung. Within the
lung, the macrophages are located close to the so-called pulmonary
surfactant contained in the extracellular lining of the epithelial
surface of the lung alveoli. It is possible that ozone might also
react with this lining film, which in turn, might be deleterious to
the cell. Gardner (1971) and Gardner et al. (1971) conducted
experiments on lavage fluid containing this surface-active substance
and found that, when isolated from rabbits exposed to ozone at
20 mg/m3 (10 ppm) for 2.5 h, it could adversely affect the stability
of the alveolar macrophage in vitro. A similar effect was noted when
ozone was bubbled through the lavage fluid in vitro. The effect on
the lavage fluid could be seen at levels as low as 200 µg/m3
(0.1 ppm) for a 2.5-h, in vivo exposure or after only 30-min in
vitro exposure. Since no significant alteration in the surface
tension of the lavage fluid was produced by ozone exposure, it is
suggested that the effect might be on a nonlipid component, possibly a
protein. The activity found in the cell-free pulmonary lavage fluid
has been called the "protective factor".
Ozone may also inactivate some opsinogenic factor within the lung
since Holzman et al. (1968) showed that it has a protein-degrading
property. Exposure of mice and rabbits to a concentration of
10 mg/m3 (5 ppm) for 3 h reduced the activity of active lysozyme,
obtained by bronchopulmonary lavage, by approximately 30%.
Various components of the endogenous defence mechanism of the lung
were studied through a unilateral lung exposure model (Alpert et al.,
1971b). Exposure to ozone at concentrations ranging from 1000 to
6000 µg/m3 (0.5 to 3 ppm) for 3 h decreased cellular viability,
depressed the intracellular hydrolytic enzymes and increased the
absolute number of polymorphonuclear leucocytes in the pulmonary
lavage fluid. All effects were dose-related and were found only in the
lung under treatment and not in the contralateral lung that breathed
ambient air.
Heuter et al. (1966) reported that exposure to an irradiated
automobile exhaust atmosphere for 15 months increased the
susceptibility of mice to pulmonary infection during the latter half
of the animal's lifetime. The oxidant concentrations in the irradiated
exposure, chamber ranged from 400 to 2000 µg/m3 (0.2-1.0 ppm). In a
similar study using irradiated automobile exhaust, enhancement of
mortality compared with that of control animals kept in filtered air
was noted with exposure to total oxidants at 300 µg/m3 (0.15 ppm) and
carbon monoxide at 29 mg/m3 (25 ppm) for 4 h. Coexistent
concentrations of nitrogen dioxide ranged from a trace to 1900 µg/m3
(1.0 ppm) (Coffin & Blommer, 1967).
Goldstein et al. (1974) studied the bactericidal effect when mice
were exposed to a combination of nitrogen dioxide and ozone, and
concluded that the combined pollutants caused bactericidal dysfunction
at concentrations that were approximately the same as the lowest
concentrations that caused similar dysfunction when the animals were
exposed to the gases individually.
Erlich et al. (1977) observed that a single joint exposure of mice
to nitrogen dioxide and ozone resulted in an addition of effects and
that a synergistic action might result from repeated exposures to the
mixture.
The animals were exposed for 3 h to 16 different combinations of
nitrogen dioxide at levels of 0, 2800, 3800, 6600, and 9400 µg/m3
(0, 1.5, 2.0, 3.5, and 5.0 ppm) and ozone at 0, 100, 200, and
1000 µg/m3 (0, 0.05, 0.1, and 0.5 ppm). Within 1 h of termination of
exposure to the pollutants, the mice were infected with Streptococcus
pyogenes. Excess mortality rates due to exposure to the mixture of
the two gases were approximately equivalent to the sum of those
induced by the inhalation of each individual pollutant. In mice
exposed repeatedly for 3 h per day, for 20 days, to a mixture of
nitrogen dioxide and ozone at concentrations of 3800 µg/ma (2.0 ppm)
and 100 µg/m3 (0.05 ppm), respectively, and challenged with
Streptococcus aerosol, the number of deaths was significantly higher
than in the control group. On the other hand, repeated daily exposure
to either nitrogen dioxide or ozone at the above-mentioned
concentrations did not show any major effect on the mortality rate.
The authors considered that this result might suggest a synergistic
action of the two pollutants making them more effective in reducing
resistance to respiratory infection.
5.2.7 Interaction of ozone with bronchoactive and other chemicals
The effects of pre-exposure to ozone on the sensitivity of
guineapigs to inhaled acetylcholine were studied by Matsumura et al.
(1972). Animals pre-exposed to an ozone concentration of 4000 µg/m3
(2 ppm) for 30 min manifested severe difficulty in breathing and many
died. This effect persisted for as long as 2 h after exposure. In a
similar study, Matsumura (1970a) sensitized guineapigs to albumen and
found that repeated 30 min pre-exposures to an ozone concentration of
10 mg/m3 (5 ppm) enhanced the sensitization. An ozone concentration
of 2000 µg/m3 (1 ppm) had no such effect.
Ozone-exposed guineapigs were also more susceptible to the toxic
action of injected histamine (Easton & Murphy, 1967). A 2-h exposure
to ozone at 10 mg/m3 (5 ppm) caused severe lung function changes and
increased mortality. This increased susceptibility to histamine was
detectable for as long as 12 h after the end of the exposure.
In order to study the effects of ozone on susceptibility to
serotonin, Suzuki & Nagaoka (1973) injected the compound into the
abdominal cavity of rats after exposing the animals to ozone at
concentrations ranging from 2000 to 12 000 µg/m3 (1-6 ppm) for 3 h.
Mortality increased with increasing levels of ozone, but fatalities
did not occur in a control group injected with serotonin and breathing
filtered air.
A report by Goldstein et al. (1970) that a deficiency of vitamin E
increased the toxicity of ozone in the rat has been supported by the
studies of Roehm et al. (1971b, 1972) and Menzel et al. (1972). These
investigators observed a significantly shorter 50% lethal time and
more pronounced signs of respiratory distress in ozone-exposed rats
(2000 µg/m3 (1.0 ppm) for 9 days continuously) that were fed vitamin
E-depleted diets in comparison with those fed vitamin E-supplemented
diets. When the ozone concentration was reduced to 1000 µg/m3
(0.5 ppm), pulmonary oedema became evident and mortality rates
increased in animals fed vitamin E-deficient diets after 6 weeks of
exposure. In rats continuously exposed to toxic levels of ozone
ranging from 1400 to 1600 µg/m3 (0.7-0.8 ppm), protection by dietary
vitamin E against lung lipid peroxidation was proportional to the
logarithm of the concentration of the vitamin in the diet (Fletcher &
Tappel, 1973). In further studies by Mustafa (1975), rats were fed a
basal diet containing vitamin E at either 66 mg/kg or 11 mg/kg for 5
weeks, and then exposed continuously to an ozone concentration of
200 µg/m3 (0.1 ppm), for 7 days. Oxygen consumption was measured in
lung homogenate using succinate as a substrate. Animals receiving the
higher concentration of vitamin E (66 mg/kg) were relatively
insensitive to this level of ozone, i.e., there was no significant
increase in oxygen consumption, but those receiving the lower
concentration of vitamin E (11 mg/kg) showed a significant increase in
oxygen consumption.
5.3 Systemic Reactions and other Effects
The number of studies on the effects of ozone on a wide range of
biological phenomena including growth, reproduction, and behaviour is
increasing but many of these studies need further confirmation.
Furthermore, even when a correlation is found between ozone exposure
and effects, it is difficult to decide whether the effects are due to
the direct oxidizing action of ozone or are secondary reactions to
pulmonary injury caused by ozone.
5.3.1 Effects on growth
There does not seem to be any convincing study which shows that
ozone, at the concentrations found in ambient air, has any detrimental
effect on body growth. Nevskaja & Kocetkova (1961) who exposed rats to
a mixture of ozone at 800 µg/m3 (0.4 ppm) and sulfuric acid at
7000 µg/m3 for 5 h per day, 6 days per week, for 100 days, and Loosli
et al. (1972) who exposed mice to synthetic photochemical air
pollution containing ozone at 600-840 µg/m3 (0.30-0.42 ppm) reported
that exposed animals weighed less than the controls. However, Emik et
al. (1971) did not find any significant differences between the growth
of guineapigs breathing ambient air containing oxidants at a mean
concentration of 110 µg/m3 (0.057 ppm) for over two years, and that
of the controls breathing filtered air.
5.3.2 Haematological effects
It is still questionable whether the haematological effects noted
in ozone exposure are due to the direct action of ozone on the
cellular and acellular components of the blood as it passes through
the lung capillaries or whether they are caused by oxidizing
intermediates, such as ozonides or peroxides, that might penetrate the
alveolar basement membrane and enter the pulmonary circulation. A
third possibility would be that these effects are secondary reactions
induced by ozone perhaps causing the release of some mediating
substance, yet to be identified.
5.3.2.1 Short-term exposure (24 h or less)
Short-term exposure to high concentrations of ozone ranging from
6000 to 16 000 µg/m3 (3.0-8.0 ppm) has been reported to cause
increases in neutrophil-lymphocyte ratios in rats (Bobb & Fairchild,
1967), and in the number of erythrocytes and leukocyte indices in mice
(Kusumoto et al., 1976). Within this range of concentrations,
Goldstein et al. (1968) and Goldstein (1973) found a reduction in
acetylcholinesterase (3.1.1.7) in the erythrocytes of mice and
induction of hydrogen peroxide formation in the circulating
erythrocytes of rats and mice. Veninga (1970, unpublished data)a
reported doubling in the number of binucleated lymphocytes and
increased levels of serum glutamic pyruvic transaminase (2.6.1.2) but
no changes in blood catalase (1.11.1.6) in mice exposed for 2 h to an
ozone concentration of 400 µg/m3 (0.2 ppm).
Increased resistance to haemolysis of erythrocytes was reported in
mice exposed to an ozone concentration of 2000 µg/m3 (1 ppm) for
30 min (Mizoguchi et al., 1973). Menzel et al. (1975) presented
evidence that fatty acid ozonides produced Heinz bodies in
erythrocytes in mice exposed to ozone at 1700 µg/m3 (0.85 ppm) for
4 h. Further exposure for 3 days resulted in a decline in the number
of Heinz body positive cells. It is of interest to note that these
bodies were not produced with ozone in the absence of serum containing
unsaturated lipids. The authors postulated that this indicated an
oxidation of the erythrocyte membrane and suggested that fatty acid
ozonides might be the toxic intermediaries. At lower concentrations,
Brinkman et al. (1964) noted increased sphering of erythrocytes of
mice, rabbits, and rats, after an in vitro exposure to ozone at
400 µg/m3 (0.2 ppm) for 1-2 h.
a Veninga, T. S. Ozone-induced alterations in murine blood and liver.
Paper presented at the 2nd International Clean Air Congress, Washington,
DC, 6-11 December 1970 (No. MB-15E).
A 3-h exposure to irradiated automobile exhaust containing an
oxidant concentration of 740-1200 µg/m3 (0.37-0.58 ppm) was found to
increase the number of leukocytes in the blood of mice and reduce the
level of serum alkaline phosphatase (3.1.3.1) (Kusumoto et al., 1976).
5.3.2.2 Prolonged and repeated exposures
Increasing the length of exposure provided further evidence for
the oxidizing effect of ozone on the blood of rabbits and rats. After
continuous exposure for 8 days to ozone at 1600 µg/m3 (0.8 ppm), the
lysozyme activity in the plasma and soluble fraction of lung of rats
significantly increased (Chow et al., 1974). Continuous exposure of
rats to an ozone concentration of 110 µg/m3 (0.06 ppm) for 93 days
resulted in a decrease in blood cholinesterase (3.1.1.8) activity
which returned to normal 12 days after exposure ceased (Eglite, 1968).
More recently, Jegier & P'an (1973) and P'an & Jegier (1972) reported
a rise in serum trypsin protein esterase in rabbits exposed to
800 µg/m3 (0.4 ppm) for 6 h per day, 5 days per week, for 10 months.
Long-term, combined exposure to ozone and carbon monoxide produced
a reduced level of serum glutamic oxaloacetic transaminase (2.6.1.1)
in rabbits. The animals were exposed for 1000 days to urban air
containing oxidants and carbon monoxide at mean concentrations over 2
years of 110 µg/m3 (0.057 ppm) and 2000 µg/m3 (1.7 ppm)
respectively, (Emik et al., 1971).
5.3.3 Effects on reproduction
A few studies have been reported concerning the possible effects
of ozone and photochemical oxidants on reproduction. The data indicate
that the newborn animals may be more susceptible to exposure to
oxidants than the parents.
Brinkman et al. (1964) and Veninga (1967) exposed pregnant mice
for 7 h per day, 5 days per week, for 3 weeks to ozone concentrations
of 200-400 µg/m3 (0.1-0.2 ppm). They found a 4-fold increase in
neonatal mortality. The second author also observed increases in the
incidence of both incisor growth and blepharophimosis in the new born.
Similar studies using irradiated automobile exhaust were performed
by Hueter et al. (1966) who found that mice exposed for 13 months
before, during, and after gestation showed a marked decrease in the
number and frequency of litters, the survival of infants, and the
total number of pups born. Nonirradiated automobile exhaust did not
produce any significant effects. The oxidant concentrations in the
irradiated exposure chamber ranged from 400 to 2000 µg/m3 (0.2 to
1.0 ppm). In a follow-up study, Lewis et al. (1967) found that the
number of female mice that did not become pregnant after mating with
pretreated males was twice that found in mice mated with untreated
controls.
5.3.4 Behavioural and related changes
5.3.4.1 Short-term exposure (24 h or less)
Behavioural changes in response to acute ozone exposures have not
been studied very extensively. The major effect that has been noted is
a reduction in spontaneous activity. Murphy et al. (1964a) and
Konigsberg & Bachman (1970) found significant losses in motor activity
in mice exposed to 400 µg/m3 (0.2 ppm) for 6 h and in rats exposed to
1000 µg/m3 (0.5 ppm) for 45 min, respectively.
Using an evoked response technique, Xintaras et al. (1966)
measured a reduction in the amplitude of response to a flash in the
specific visual cortex and in the superior colliculus in rats exposed
to ozone at 1000-2000 µg/m3 (0.5-1.0 ppm) for 1 h. In studies on
rhesus monkeys, Reynolds & Chaffee (1970) reported increases in both
simple and choice reaction times after a 30-min exposure to an ozone
concentration of 1000 µg/m3 (0.5 ppm).
Gardner et al. (1974) showed that the pentobarbital-induced
sleeping time of mice significantly increased after 2 and 3 exposures
each of 3 h, to an ozone concentration of 2000 µg/m3 (1 ppm). After
the third exposure, there was no change in the sleeping time compared
with the controls unless in subsequent exposure the ozone
concentration was raised to 10 mg/m3 (5 ppm), when the sleeping time
again differed significantly. The authors suggested that ozone might
be deactivating a liver microsomal enzyme that was responsible for the
detoxification of the drug.
5.3.4.2 Prolonged and repeated exposures
Additional studies on the effects of ozone on the behaviour of
animals have been conducted with longer exposures and complex mixtures
of air pollutants. It appears that the reduction of voluntary running
time may be an extremely sensitive indicator of ozone toxicity. The
mechanism of this change remains obscure.
Continuous exposure for 1 week to an ozone concentration of
2000 µg/m3 (1.0 ppm) caused an 84% reduction in voluntary activity in
rats (Fletcher & Tappel, 1973). Decreased running activity has also
been observed in mice exposed continuously to urban air with elevated
oxidant levels and to irradiated motor vehicle exhaust (Emik et al.,
1971; Heuter et al., 1966).
According to Litt et al. (1968), ozone-exposed rats required a
longer time to learn a specific task; learning was unstable and they
exhibited a reduced ability for temporal discrimination. The animals
were exposed intermittently for 2 months to ozone concentrations of
600-1000 µg/m3 (0.3-0.5 ppm). Nevskaja & Kocetkova (1961) exposed
rats to a mixture of ozone at 800 µg/m3 (0.4 ppm) and sulfuric acid
at 7000 µg/m3 for 5 h per day, 6 days per week, for 100 days, and
found that a conditioned reflex was retarded.
5.3.5 Miscellaneous systemic reactions to lung damage
Other changes have been reported which indicate biochemical,
physiological, and structural effects at sites distant from the lung
that are possibly related to the inhalation of ozone. In most of these
studies, the levels of ozone employed greatly exceeded those in the
ambient air. After exposing rabbits to an ozone concentration of
1500 µg/m3 (0.75 ppm) for 4-8 h, Atwal & Wilson (1974) found
histological and ultrastructural changes in the parathyroid glands.
They reported hyperplasia of the chief cells, large numbers of
secretion granules, proliferation and hypertrophy of the rough
endoplasmic reticulum, Golgi complex, mitochondria, lipid bodies, and
free ribosomes. These effects were seen up to 66 h after exposure.
Atwal et al. (1975) expanded their short-term exposure study by
exposing rabbits to 1500 µg/m3 (0.75 ppm) for 48 h and found
morphological changes in the parathyroid gland similar to those that
they had reported for 4-8 h of exposure. The data suggested that ozone
might trigger off an immune reaction which caused inflammatory injury
to the parathyroid gland.
It was demonstrated by Brinkman et al. (1964) that exposure to an
ozone concentration of 400 µg/m3 (0.2 ppm), for 5 h per day, for 3
weeks, resulted in the rupture of nuclear envelopes and the extrusion
of the contents of myocardial muscle fibres in rabbits and mice. This
effect became reversible one month after the exposure.
There are some data which suggest that ozone might accelerate the
aging process. Stokinger (1965) reported premature aging in rabbits
after 1 year of weekly 1-h exposures to ozone. The major changes found
in the exposed animals were premature calcification of the
sternocostal cartilage, severe depletion of body fat, dull cornea,
sagging conjunctivae, and a general appearance of not thriving.
5.4 Mutagenicity
Chromosome aberrations (anaphase bridges) were found in 42% of
root meristem cells of Vicia faba exposed to an ozone concentration
of 8000 mg/m3 (4000 ppm) for 1 h, but none were found in cells
exposed to clean air (Fetner, 1958). When chick embryonic fibroblasts
were exposed to ozone concentrations of 5000-10 000 mg/m3
(2500-5000 ppm) for 24 h, a small number of cells were found with
chromosome bridges in anaphase and telophase and with nuclear
fragments (Sachsenmaier et al., 1965). Using a much lower
concentration, Pace et al. (1969) exposed strain L cells continuously
to an ozone concentration of 8000 µg/m3 (4 ppm) for 30 h, and found a
significant reduction in cell survival compared with that in the
control group. The authors considered that this was due to ozone
interference with mitotic activity.
In vivo exposure studies on mammals by Zelac et al. (1971a,
1971b) are of special interest. Female Chinese hamsters were exposed
to an ozone concentration of 400 µg/m3 (0.2 ppm) for 5 h and the
number of circulating blood lymphocytes with chromosomal breaks was
measured immediately after exposure and 6, and 15.5 days later. A
significant increase in chromosomal breaks compared with pre-exposure
values was still observed 15 days after exposure.
Although further confirmation is required, these data and the
results of studies on chromosomal changes in human tissues (see
section 6.1.1) seem to indicate that ozone might be a mutagenic agent.
5.5 Summary Table
Table 10 is a summary of experimental animal studies that provide
quantitative information useful for the establishment of guidelines
for the protection of public health with respect to exposure to ozone
at concentrations of up to 2000 µg/m3 (1.0 ppm).
Table 10. Experimental animal studies
Local effects on the respiratory system
1. Morphological changes
Ozone Length of exposure
concentration Number
of
µg/m3 (ppm) number h/day Effects Responsea Species animals Reference
of days
1800 (0.88) 180 24 Epithelial injury seen as early as 4 h n.a.b rat n.a. Freeman et al.
after the beginning of exposure; after (1974)
3 weeks half of animals died and
emphysema-like lesions observed.
1600 (0.8) 7 24 Walls and interalveolar septa of terminal -- rat 8 (8) Castleman et al.
airways thickened and infiltrated by (1973a)
mononuclear cells.
1200 (0.6) 1 7 Swelling of epithelial alveolar lining -- mouse 32 (13) Bile (1970)
cells & endothelium cells with
occasional breaks in basement
membrane.
1100 (0.54) 180 24 Progressive changes in the airway -- rat n.a. Freeman et al.
epithelium after 6 days. (1974)
1000 (0.5) 1 6 Immediately after the exposure the -- mouse 16(12) Evans et al.
number of alveolar cells significantly (1971)
decreased.
800 (0.4) 5 per 6 Emphysematous & vascular-type -- rabbit 6 (6) P'an et al.
week x lesions. (1972)
10 month
a Number of animals showing effects/total number of animals; numbers in brackets refer to control groups.
b Not available.
Table 10. Experimental animal studies
Local effects on the respiratory system
1. Morphological changes cont'd.
Ozone Length of exposure
concentration Number
of
µg/m3 (ppm) number h/day Effects Responsea Species animals Reference
of days
520- (0.26 1 4.7-6.6 Dose-related loss of ciliated epithelium. -- cat 14 (3) Boatman et al.
2000 -1.0) (1974)
400 (0.2) 1 2 Degenerative changes in type I cells. -- rat n.a. Stephens et al.
(1974)
2. Functional changes
1400 (0.68) 1 2 No significant increase in flow -- guinea- 10 (10) Murphy et al.
resistance. pig (1964a)
1000 (0.5) 1 2 Increase in airways resistance and -- guinea- 10 (10) Yokoyame
breathing frequency with decrease in pig (1972a)
tidal volume.
520- (0.26 1 4.6 Increased flow resistance. -- cat 10 (4) Watanabe et al.
1000 -0.5) (1973b)
400 (0.2) 30 24 Reduction in lung elasticity; increase in -- rat 44 (44) Bartlett et al.
lung volume and in alveolar (1975)
dimensions.
a Number of animals showing effects/total number of animals; numbers in brackets refer to control groups.
b Not available.
Table 10. Experimental animal studies
Local effects on the respiratory system
3. Biochemical changes
Ozone Length of exposure
concentration Number
of
µg/m3 (ppm) number h/day Effects Responsea Species animals Reference
of days
1500 (0.75) 1 3 Reduction in activity of benzopyrene -- hamster 25 (95) Palmer et al.
hydroxylase (1.14.14.2). & 8 (15) (1971. 1972)
rabbit
1400- (0.7 7 24 Increased acid phosphatase activity. -- rat 14 (12) Castleman et al.
1600 -0.8) (1973b)
1400 (0.7) 5 24 Indication of lipid peroxidation; -- rat 33 (20) Chow & Tappel
increase in lysosomal hydrolase (1972); Dillard
activity. et al. (1972)
1000 (0.5) 1 6 Increased albumen recovery from -- rat 10 (18) Alpert et al.
alveolar spaces. (1971a)
800- (0.4 1 4 Evidence of formation of lipid peroxides n.a.b mouse n.a. Goldstein et al.
1400 -0.7) in the lung. (1969)
500 (0.25) 1 3 Reduced activity of several lysosomal -- rabbit 6 (6) Hurst et al.
hydrolases. (1970)
400 (0.2) 7 24 Increase in pulmonary mitochondrial -- rat 5-8 Mustafa et al.
oxygen consumption. (5-8) (1973)
a Number of animals showing effects/total number of animals; numbers in brackets refer to control groups.
b Not available.
Table 10. Experimental animal studies
Local effects on the respiratory system
4. Effects on the host defence system
Ozone Length of exposure
concentration Number
of
µg/m3 (ppm) number h/day Effects Responsea Species animals Reference
of days
1200- (0.62 1 17 Inhibition of pulmonary bactericidal -- mouse 20 (29) Goldstein et al.
1600 -0.80) activity. (1971b)
1000 (0.5) 210 16 No effect on physical clearance of -- rabbit 8 (8) Friberg et al.
inhaled particles or on the number of (1972)
macrophages.
1000 (0.5) 60 16 Decrease in the clearance of viable -- guinea- 18 (18) Friberg et al.
Escherichia coli. pig (1972)
800 (0.4) 1 4 Inhibition of bactericidal activity; no -- mouse 38 (37) Goldstein et al.
additive role of the induced silicosis. (1972)
600 (0.3) 1 3 Impairment of phagocytic properties of -- rabbit n.a.b Coffin et al.
pulmonary alveolar macrophages. (1968b)
500 (0.25) 1 3 Diminished enzyme activities of alveolar -- rabbit 6 (6) Hurst et al.
(1970)
macrophages.
160 (0.08) 1 3 Increased susceptibility to 15/40 mouse 40 (40) Coffin et al.
Streptococcus. (6/40) (1968a)
a Number of animals showing effects/total number of animals; numbers in brackets refer to control groups.
b Not available.
Table 10. Experimental animal studies--continued
II. Systemic reactions and other effects
1. Haematological effects
Ozone Length of exposure
concentration Number
of
µg/m3 (ppm) number h/day Effects Responsea Species animals Reference
of days
1700 (0.85) 1 4 Formation of Heinz bodies in red cells. n.a.b mouse n.a. Menzel et al.
(1975)
1600 (0.8) 8 24 Increase of lysozyme activity in plasma -- rat 8 (8) Chow et al.
and soluble fraction of lung. (1974)
800 (0.4) 5 per 6 increase in serum trypsin protein -- rabbit 6 (6) P'an & Jegier
week x esterase. (1972); Jegier
10 months & P'an (1973)
600 (0.3) 1 1 Inhibition of acetylcholine esterase -- ox (in -- P'an & Jegier
(3.1.1.7) activity. vitro) (1970)
400 (0.2) 1 1-2 Increased sphering of red blood cells. -- mouse, -- Brinkman et al.
rabbit, (1964)
rat (in
vitro)
400 (0.2) 1 2 Doubling in number of binucleated -- mouse n.a. Veninga (1970,
lymphocytes. unpublished)
110 (0.06) 93 24 Decrease in blood choline esterase -- rat 15 (15) Eglite (1968)
activity.
a Number of animals showing effects/total number of animals; numbers in brackets refer to control groups.
b Not available.
Table 10. Experimental animal studies--continued
II. Systemic reactions and other effects
2. Effects on reproduction
Ozone Length of exposure
concentration Number
of
µg/m3 (ppm) number h/day Effects Responsea Species animals Reference
of days
400 (0.2) 5 per 7 Increase in neonatal mortality. n.a. mouse n.a. Veninga (1967)
week x
gestation
period +
1st 3
weeks of
life
200- (0.1 5 per 7 Increase in neonatal mortality. n.a. mouse n.a. Brinkman et al.
400 -0.2) week x (1964)
3 weeks
1000- (0.5 1 1 Decrease in the amplitude of evoked -- rat 3 (3) Xintaras et al.
2000 -1.0) response to flash. (1966)
a Number of animals showing effects/total number of animals; numbers in brackets refer to control groups.
b Not available.
Table 10. Experimental animal studies--continued
II. Systemic reactions and other effects
3. Behavioural changes
Ozone Length of exposure
concentration Number
of
µg/m3 (ppm) number h/day Effects Responsea Species animals Reference
of days
1000 (0.5) 1 0.5 increase in simple and choice reaction -- rhesus 4 (4) Reynolds &
time. monkey Chaffee (1970)
1000 (0.5) 1 0.75 Significantly reduced motor activity. -- rat 12 (12) Konigsberg &
Bachman
(1970)
600- (0.3 60 intermittently Increase in time to learn specific tasks. -- rat 6 (6) Litt et al.
1000 -0.5) (variable (1966)
intervals)
400 (0.2) 1 6 Reduction in spontaneous running -- mouse 9 (9) Murphy et al.
activity. (1964a)
4. Miscellaneous extrapulmonary changes
1500 (0.75) 1-2 4-8, 24 Histological changes in parathyroid -- rabbit 16 (16) Atwal & Wilson
gland. (1974); Atwal
et al. (1975)
400 (0.2) 21 5 Structural changes in myocardial -- rabbit n.a. Brinkman et al.
muscle fibres. & mouse (1964)
400 (0.2) 1 5 Increase in chromosomal breaks in -- hamster 8 (4) Zelac et al.
circulating lymphocytes. (1971a,b)
a Number of animals showing effects/total number of animals; numbers in brackets refer to control groups.
b Not available.
6. EFFECTS ON MAN
6.1 Controlled Exposures
A considerable number of studies have been performed, under
controlled conditions, on the effects of ozone on both healthy
subjects and patients, with their consent. Some of these studies have
provided useful information for the evaluation of exposure-effect
relationships. A few in vitro studies using human tissues have also
helped to clarify the mechanisms of the biological actions of ozone.
There have been few human studies on other oxidants.
6.1.1 In vitro effects on human tissues
Brinkman et al. (1964) found that in vitro exposure of human red
blood cells to an ozone concentration of 0.5 mg/m3 (0.25 ppm) for
30 min accelerated sphering of these cells by X-ray irradiation,
compared with unexposed cells. Chromosome breakages in human cell
(epidermoid carcinoma cell) cultures exposed to an ozone concentration
of 16 mg/m3 (8 ppm) for 5 or 10 min were equivalent to those produced
by X-rays (200R, 250 kV) (Fetner, 1962).
The production of interferon was suppressed when human tonsil
lymphocytes were exposed in vitro to an ozone concentration of
10 mg/m3 (5 ppm) for 3 h (Watanabe et al., 1973a).
Goldstein (1976) measured the combined effects of ozone at
4-82 mg/m3 (2-41 ppm) and nitrogen dioxide at 6.8-190 mg/m3
(3.6-102 ppm) on human red cells, in vitro, and found that the
absolute and relative concentrations of the pollutants as well as the
sequence of administration could affect the interaction. In general,
the effects of the two pollutants on the variables measured (osmotic
fragility, acetylcholin-esterase (3.1.1.7) activity, lipid
peroxidation, reduced glutathione, and methaemoglobin levels) were
additive. At lower pollutant doses, a synergistic effect on the
increase in lipid peroxides was reported. Ozone also potentiated the
formation of methaemoglobin due to the action of nitrogen dioxide.
6.1.2 Sensory effects
The effects of ozone or oxidants on sensory organs have been
studied in terms of eye irritation, changes in visual parameters, and
olfactory thresholds.
Eye irritation in two groups of 20 female telephone company
employees working in identical adjacent rooms was evaluated by
Richardson & Middleton (1957, 1958) in relation to oxidant
concentrations from May to November 1956. Activated-carbon and dummy
air-filter media were switched periodically between the two rooms so
that the groups were alternately exposed to test and control
conditions. In all cases, differences in eye irritation between the
activated-carbon filtered and nonfiltered test conditions were highly
significant (p < 0.01). The scatter diagram (Fig. 8) suggests the
existence of a threshold for eye irritation at an oxidant
concentration of approximately 200 µg/m3 (0.10 ppm).
5. EFFECTS ON EXPERIMENTAL ANIMALS
There is considerable evidence to show that even short exposure to
high concentrations of ozone may endanger the health of experimental
animals. In reviewing this evidence, emphasis has been placed on
studies in which animals were exposed to concentrations of oxidants of
2000 µg/m3 (1.0 ppm) or less, since these studies are more relevant
for predicting the health risk to man. However, some experiments
conducted at higher concentrations have also been discussed when it
was considered that they would contribute to a better understanding of
the mechanism of the biological action of oxidants.
5.1 Absorption of Ozone
A number of factors can influence the transport and removal of
ozone in the upper airways such as: (a) nasal morphology; (b) route,
rate, and depth of breathing; and (c) biochemical composition and
amount of mucus. The decomposition of ozone within the upper airways
may protect the lower part of the respiratory tract against the
irritant gas. Various attempts to determine or model the respiratory
absorption of ozone in the upper airways have been made (McJilton et
al., 1972; Vaughan et al., 1969; Yokoyama & Frank, 1972). This work
has recently been reviewed by Miller (1977) who developed a
mathematical model for the transport and removal of ozone in the
respiratory tract of guineapigs, rabbits, and man, and predicted that
whatever the initial concentration, the respiratory bronchioles would
receive the highest dose of ozone. This agrees well with various
experimental studies in animals. For all three species the
relationship between respiratory bronchiolar concentration and the
inhaled ozone concentration at the tracheal level is linear on a
log-log scale at concentrations greater than 100 µg/m3 (0.05 ppm),
the respiratory bronchiolar dose for rabbits being 80% of that for man
and twice that for guineapigs.
5.2 Effects on the Respiratory System
5.2.1 Morphological changes
5.2.1.1 Short-term exposure (24 h or less)
The primary target of ozone is the respiratory tract and
particularly the pulmonary parenchyma. In small laboratory animals,
exposure to ozone at acutely toxic concentrations results in pulmonary
oedema, haemorrhage, and death. The LD50 is about 12 mg/m3 (6.0 ppm).
At lower concentrations in the range of 400-2000 µg/m3 (0.2-1.0 ppm),
ozone causes numerous changes in both the epithelial and endothelial
cells of the lung and ultrastructural effect indicate that the primary
lesions are in the epithelial lining of the terminal bronchioles and
the proximal alveoli.
The sequence of degeneration, desquamation, and destruction of
type I alveolar cells in rats following exposure to an ozone
concentration of 400 µg/m3 (0.2 ppm) for 2 h was demonstrated in
electron microscopic studies by Stephens et al. (1974). The type II
epithelial cells appeared to be more resistant. Freeman et al. (1974)
also reported significant histological changes in type I epithelial
cells after 4 h exposure to a concentration of 1800 µg/m3. The loss
of ciliated epithelium throughout the upper respiratory tract,
swelling and denudation of type I cells, erythrocyte lysis within
alveolar capillaries, and breakdown of capillary endothelium has been
reported in cats exposed to ozone concentrations of 520, 1000, and
2000 µg/m3 (0.26, 0.5, and 1.0 ppm) for 4.7-6.6 h (Boatman et al.,
1974). These effects appeared to be dose-related. Similar effects were
noted by Bils (1970) in mice after a 7-h exposure to ozone at
concentrations of 1200 and 2600 µg/m3 (0.6 and 1.3 ppm).
Cell renewal rate within the alveoli was studied by Evans et al.
(1971) by injecting tritiated thymidine into 18 to 20-month-old mice
before exposing them for 6 h to ozone concentrations of 1000, 2400,
5000, or 7000 µg/m3 (0.5, 1.2, 2.5, and 3.5 ppm). Immediately
following exposure, the number of labelled alveolar cells (those
synthesizing DNA) was significantly lower in all exposed groups
compared with controls.
Similar morphological studies have also been performed using
complex mixtures containing oxidants. Ultrastructural alterations
consisting of disrupted cytoplasm and abnormal mitochondria were seen
in the alveolar tissue of mice exposed for 2-3 h to Los Angeles air
that had a total oxidant concentration of 800 µg/m3 (0.4 ppm) (Bils,
1966). Exposure of mice for 3 h to an irradiated synthetic atmosphere
that contained propylene, nitric oxide, carbon monoxide, and water
vapour and simulated a heavy smog, gave rise to a similar pattern of
ultrastructural changes (Bils & Romanovsky, 1967). Rats continuously
exposed for 24 h to a mixture of ozone at a concentration of
500 µg/m3 (0.25 ppm) and nitrogen dioxide at a concentration of
4700 µg/m3 (2.5 ppm) exhibited increases in the numbers of alveolar
macrophages and of free cells in the lung resembling desquamated type
I cells, and hypertrophy of the epithelium were noted in rats
continuously exposed for 24 h to a mixture of ozone at a concentration
of 500 µg/m3 (0.25 ppm) and nitrogen dioxide at a concentration of
4700 µg/m3 (2.5 ppm) (Freeman et al., 1974).
5.2.1.2 Prolonged and repeated exposures
Prolonged exposure to low levels of ozone causes more extensive
and irreparable damage to the lung than the oedematigenous and acute
inflammatory reactions observed following short exposure to high
concentrations. Emphysema, atelectasis, focal necrosis, broncho-
pneumonia, and fibrosis have been reported, often accompanied by a
variety of cellular alterations. The degree of morphological injury
appears to be proportional to the concentration and time of exposure.
Freeman et al. (1974) described a number of pathomorphological
changes in rats continuously exposed to ozone at concentrations of
1100 and 1800 µg/m3 (0.54 and 0.88 ppm) for as long as 6 months.
After 48 h of exposure to the lower concentration, an influx of
macrophages and an increase in mitotic figures were observed, and the
alveolar ducts became demarcated by hypertrophic alveolar epithelium.
Most of these changes became more obvious with increasing length of
exposure with the exception of the terminal bronchioles which appeared
to recover and return to normal. After exposure to the higher
concentration of 1800 µg/m3 (0.88 ppm) for 48 h, the bronchiolar
epithelium exhibited both metaplasia and fibrosis. Adenoma-like
structures containing large numbers of macrophages and fibrotic
lesions appeared after 6 days and after 3 weeks of exposure, half of
the rats had died and were found to have emphysema-like lesions.
Using scanning electron microscopy, Ikematsu et al. (1976) found
that continuous exposure to an ozone concentration of 2000 µg/m3
(1.0 ppm) for 10 days resulted not only in desquamation of epithelial
cells but also in the appearance of inflammatory cells in the tonsils
of rabbits. Similar pathological effects were observed with
intermittent exposure indicating that the animals were unable to
recover sufficiently during the periods of exposure to clean air.
Emphysematous and vascular lesions in the lung of the rabbit described
by P'an et al. (1972), were the result of repeated exposure to
800 µg/m3 (0.4 ppm) for 6 h per day, 5 days per week, for 10 months.
Stokinger et al. (1957) also reported fibrotic changes and chronic
bronchial and bronchiolar emphysema in the lungs of mice, rats,
guineapigs, and hamsters exposed to an ozone concentration of
2000 µg/m3 (1.0 ppm) for 6 h per day, 5 days per week, for 433 days.
However, under the same conditions of exposure, the effects in dogs
were limited to the trachea and large bronchi. When dogs were exposed
to ozone concentrations ranging from 2000 to 6000 µg/m3 (1-3 ppm) for
8, 16, or 24 h daily for 18 months, the morphological damage was
roughly proportional to the product of the concentration and the time
of exposure, the epithelial lining of the terminal airways and
proximal alveoli being most adversely affected (Freeman et al., 1973;
Stephens et al., 1973). Similarly, Castleman et al. (1973a) found that
the walls of the terminal airways and the interalveolar septa of rat
lung were most affected by continuous exposure to an ozone
concentration of 1600 µg/m3 for 7 days.
Intermittent exposure for 8 h per day, for 7 days to an ozone
concentration of 400 µg/m3 (0.2 ppm) produced damage to the
respiratory bronchioles in bonnet monkeys. In rats fed a normal diet,
similar effects were seen at the same concentration of ozone, but, in
vitamin E-deficient rats, an equivalent effect was caused by ozone at
200 µg/m3 (0.1 ppm) (Dungworth et al., 1975; Dungworth, 1976).
Loosli et al. (1972) measured the cell turnover rate in mice and
found it to be significantly higher in animals exposed to synthetic
photochemical air pollution for 8-12 months than in control animals
breathing filtered air. The synthetic air pollution contained ozone at
600-840 µg/m3 (0.30-42 ppm), carbon monoxide at 3.5-12 mg/m3
(3-10 ppm), nitrogen dioxide at 1300-1600 µg/m3 (0.70-0.85 ppm), and
sulfur dioxide at 5700-6000 µg/m3 (2.2-2.3 ppm). The effects were
similar to those seen with exposure to pure ozone.
Rats exposed for 1 month to a mixture of ozone at 1800 µg/m3
(0.9 ppm) and nitrogen dioxide at 1700 µg/m3 (0.9 ppm), developed
enlarged alveolar spaces (Freeman et al., 1974). In rats exposed for
2 weeks to a combination of ozone at 500 µg/m3 (0.25 ppm), and
nitrogen dioxide at 4700 µg/m3 (2.5 ppm), the bronchiolar epithelium
became cuboidal. However, after 6 months of exposure, the tissue
appeared to be normal (Freeman et al., 1974).
Slightly inflammatory and proliferative changes were observed by
Nakajima et al. (1972) in the bronchial membranes of mice exposed to
irradiated auto exhaust that contained oxidant concentrations of
200-300 µg/m3 (0.1-0.15 ppm), for 2-3 h per day, 5 days per week, for
30 days.
5.2.2 Functional changes
5.2.2.1 Short-term exposure (24 h or less)
The first abnormal sign observed in various animal species during
exposure to ozone is an irregular respiratory pattern. This usually
appears within the first few minutes of exposure and, in most cases,
the animal returns to normal, when allowed to recover in the clean
air.
Functional changes in the respiratory system have been observed in
several species of animals at ozone concentrations of less than
2000 µg/m3 (1.0 ppm). Yokoyama (1972a) studied the ventilatory
function of guinea-pigs before, during, and after a 2-h exposure to an
ozone concentration of 1000 µg/m3 (0.5 ppm). He reported increases in
the frequency of respiration and airway resistance, but a decrease in
tidal volume. In another investigation, Yokoyama (1973) exposed only
the right lung of rabbits to ozone at 2000 µg/m3 (1 ppm) for
3 h and used the left lung as a control. The exposed lung of rabbits
killed 1 and 3 days after exposure, had a reduced vital capacity.
However, the reduction in vital capacity in those killed 7 days after
exposure was only slight and was not significant. Other studies on
lung function in guineapigs exposed to ozone for 2 h indicated that
pulmonary flow resistance was not altered by exposure to
concentrations of 700 and 1400 µg/m3 (0.34 and 0.68 ppm), but that it
increased significantly at exposure levels of 2000 and 2700 µg/m3
(1.08 and 1.35 ppm) (Murphy et al., 1964a).
Scheel et al. (1959) exposed rats to an ozone concentration of
4000 µg/m3 (2 ppm) for 3 h. Decreases in minute ventilation, tidal
volume, and oxygen uptake, that occurred immediately after exposure,
reached minimum recorded values 8 h later. All measurements returned
to normal levels 20 h after exposure.
When cats were exposed for an average of 4.6 h to ozone
concentrations of 520, 1000, and 2000 µg/m3 (0.26, 0.5, and 1 ppm),
Watanabe et al. (1973b) found that pulmonary flow resistance increased
with increasing ozone concentrations. Dynamic compliance was reduced,
but to a lesser extent, and vital capacity was unaffected. The
proportion of animals that showed a reduction in diffusion capacity
appeared to increase with increasing ozone concentrations.
There are a few studies in which animals were exposed to ambient
air and irradiated auto exhaust containing high concentrations of
oxidants, including those of Swann & Balchum (1966) who measured the
total expiratory flow resistance in guineapigs on days of unusual
weather and smog conditions in Los Angeles. When the resistance was
compared with routine monthly measurements on the same animals,
significant increases were found at oxidant levels of approximately
600 µg/m3 (0.30 ppm) or more. Substantial increases in resistance
were also observed, when relatively high concentrations of nitrogen
dioxide (1700 µg/m3; 0.92 ppm), carbon monoxide (30 mg/m3;
26 ppm), and hydrocarbons (16 ppm) were present, but the oxidant level
(80 µg/m3; 0.04 ppm) was relatively low.
The effects on guineapigs of a 4-h exposure to diluted,
irradiated, or nonirradiated exhaust atmospheres were reported by
Murphy et al. (1963). Marked, rapid increases in total expiratory flow
resistance accompanied by a decrease in respiratory rate and a small
increase in tidal volume occurred during exposure to irradiated
exhaust. The reaction to nonirradiated exhaust was comparatively
slight. Ranges of concentrations of the main pollutants in the
exhaust-contaminated air used in this study were: for irradiated
exhaust gases: total oxidants, 660-1640 µg/m3 (0.33-0.82 ppm);
nitrogen dioxide, 800-10 000 µg/m3 (0.43-5.5 ppm); and carbon
monoxide, 39-360 mg/m3 (34-310 ppm): for nonirradiated exhaust gases:
total oxidants, less than 40 µg/m3 (0.02 ppm); nitrogen dioxide,
710-3000 µg/m3 (0.38-1.58 ppm); and carbon monoxide, 98-345 mg/m3
(85-300 ppm). In addition, increased levels of formaldehyde, acrolein,
and olefin were present.
5.2.2.2 Prolonged and repeated exposures
The few available pulmonary function studies on animals exposed to
ozone for extended periods of time include a study by Bartlett et al.
(1975) who reported that continuous exposure of rats to an ozone
concentration of 400 µg/m3 (0.2 ppm) for 30 days caused a 16%
increase in lung volume and an increase in alveolar dimension. A
reduction in lung elasticity that was also reported was possibly an
effect of ozone on collagen. Yokoyama (1974) exposed rabbits to an
ozone concentration of 4000 µg/ma (2 ppm), for 6 h per day, for 3-4
days, and found that the pulmonary flow resistance was greater, and
compliance lower, than in the controls. In animals exposed to
2000 µg/m3 (1 ppm), for 6 h per day, for 7-8 days, the values were
between those of the controls and of the group treated with
4000 µg/m3 (2 ppm).
5.2.3 Biochemical changes
5.2.3.1 Effects indicating possible mechanisms of action
The actual mechanism of ozone toxicity at the subcellular level is
still obscure. Studies of the biochemical effect of ozone have mainly
been based on two hypotheses: (a) that ozone interacts with readily
oxidizable substances thus altering the course of metabolism and
producing a toxic effect; and (b) that ozone interacts with
unsaturated lipids to produce lipid peroxidation and consequent cell
damage.
Sulfhydryl systems appear in the cell not only as reducing
substances but also as functional constituents of a variety of enzymes
and proteins. Ozone is capable of oxidizing these substances causing
inactivation of enzymes and alterations in the structure and function
of the cell membrane.
In studies on ozone oxidation of glutathione in vitro, Mudd et
al. (1969) showed that both fast and slow oxidation occurred. It has
also been shown that the oxidation of reduced glutathione (GSH)
results in oxidized glutathione (GSSG), though some of the sulfhydryl
groups form higher oxidation products that are not reduced by the
reductases available in the cells (Menzel, 1971). Mountain (1963)
demonstrated in vivo oxidation of reduced glutathione and a decrease
in the sulfur-containing enzyme succinic dehydrogenase (1.3.99.1)
activity in the lungs of mice. King (1961) also found a decrease in
sulfhydryl content and of enzymatic function of partially purified
glyceraldehyde-3-phosphate dehydrogenase (1.2.1.12) in rat lungs
following exposure to an ozone concentration of 2400 µg/m3 (1.2 ppm)
for 4 weeks.
When rats were exposed to an ozone concentration of 4000 µg/m3
(2.0 ppm) for 4-8h, the concentrations of both the protein and
nonprotein sulfhydryls in the lungs decreased. The activities of lung
enzymes containing sulfhydryl also decreased including those of
glucose-6-phosphate dehydrogenase (1.1.1.49), glutathione reductase
(1.6.4.2), and cytochrome c reductase related to succinate and
reduced nicotinamide adenine dinucleotide (NADH) (DeLucia et al.,
1972). Expanding these studies, DeLucia et al. (1975) showed that the
magnitude of the decrease in the nonprotein sulfhydryl groups in rats
was dependent on the duration of exposure and the concentration of
ozone. Significant decreases were not observed at an ozone
concentration of 1600 µg/m3 (0.8 ppm) for 24 h.
Further studies have shown that exposure to ozone tends to
increase the activity of enzymes that protect against intracellular
oxidation. When rats were continuously exposed to an ozone
concentration of 1500 µg/m3 (0.75 ppm) for 1, 3, 10, or 29 days, it
was noted that the activities of glutathione peroxidase (1.11.1.9),
glutathione reductase (1.6.4.2), glucose-6-phosphate dehydrogenase,
6-phosphogluconate dehydrogenase (1.1.1.43), and pyruvate kinase
(2.7.1.40) were lower than those in the controls after 1 day but
higher after, 3, 10, and 29 days of exposure (Chow & Tappel, 1973). In
another study by the same investigators, rats exposed to 400 µg/m3
(0.2 ppm) continuously for 8 days or intermittently (8 h per day) for
7 days showed significantly increased glutathione peroxidase activity
in the lung. Fukase et al. (1975a, 1975b) exposed mice to ozone
concentrations of 400, 1000, or 2000 µg/m3 (0.2, 0.5 or 1.0 ppm) for
4 h per day, for 30 days, and found progressive increases in the
levels of glutathione and vitamin C in the lung with increasing ozone
concentration. The authors also reported significant increases in the
activities of glutathione peroxidase, glutathione reductase, and
glucose-6-phosphate dehydrogenase in the lungs of mice. Exposure of
both rhesus and bonnet monkeys to levels of ozone ranging from 400 to
1600 µg/m3 (0.2-0.8 ppm) for 8 h per day for 7 days, resulted in
increased activities of succinate oxidase and glutathione peroxidase
in the lungs. Linear regression analysis showed a significant
correlation between ozone concentration and the augmentation in
activity of these enzymes (Dungworth et al., 1975).
Many investigators have attributed the biological effect of ozone
to lipid peroxidation (Fournier, 1973; Goldstein et al., 1969; Menzel,
1970; Roehm et al., 1971b). Oxidation of unsaturated fatty acids by
ozone has been demonstrated both in vivo and in vitro. The
mechanism of such action is based on the proclivity of ozone to react
with the ethylene groups of the acid to form peroxides. Their
decomposition results in the further formation of free radicals
capable of initiating peroxidation of other unsaturated fatty acids.
The breakdown products (peroxides, carbonyl compounds) may themselves
be cytotoxic.
Evidence of lung lipid peroxidation during ozone exposure was
suggested by Goldstein et al. (1969) who found conjugated diene bonds
in an extract of lungs of mice exposed to 800-1400 µg/m3
(0.4-0.7 ppm) for 4 h. Another index of lipid peroxidation is an
increase in malonaldehyde. In studies by Chow & Tappel (1972), the
malonaldehyde concentration increased in the lungs of rats exposed
continuously to 1400-1600 µg/m3 (0.7-0.8 ppm) for 5 and 7 days.
Further evidence of the role of peroxidation in ozone toxicity is
the fact that animals deficient in vitamin E are more susceptible to
ozone. This is discussed in detail in section 5.2.7.
5.2.3.2 Biochemical effects at the subcellular level
In morphological and ultrastructural studies, swelling and
degenerative changes in lung mitochondria have frequently been
reported following ozone exposure. Mitochondrial functions are
critical to the cellular terminal substrate oxidation and energy
production. These organelles in lung cells may be the target for ozone
since many mitochondrial enzyme activities are sulfhydryl-dependent
and mitochondrial membranes contain abundant unsaturated
phospholipids. It has therefore been suggested that the lung
mitochondria may be a sensitive test system for detecting and
evaluating ozone toxicity.
Mustafa et al. (1973) found a 45% increase in pulmonary
mitochondrial oxygen consumption in rats continuously exposed to
1600 µg/m3 (0.8 ppm) for 10-20 days. A 3-fold increase in the number
of type II cells was also reported. These cells are rich in
mitochondria and may have been responsible for the increase in oxygen
consumption. A 17% increase in oxygen consumption was noted with
continuous exposure to an ozone concentration of 400 µg/m3 (0.2 ppm)
for 7 days. This effect appeared to be dose-related.
Lysosomes are vitally important in the intra- and possibly
extracellular destruction of inhaled matter. The hydrolytic enzyme
system of lysosomes in the alveolar machrophage is crucial to maintain
the sterility of the lung against inhaled microbes. Any inactivation
of these enzymes would be expected to increase the risk of respiratory
disease.
Hurst et al. (1970) reported that, when rabbits were exposed for
3 h to an ozone concentration of 500 µg/m3 (0.25 ppm), there was a
reduction in the activity of lysosomal hydrolases, i.e., acid
phosphatase (3.1.3.2), lysozyme (3.2.1.17), and beta-glucuronidase
(3.2.1.31). In vitro exposure of rabbit alveolar macrophages
produced a similar decrease in lysosomal hydrolases (Hurst & Coffin,
1971).
Ozone may also induce increases in the concentrations of these
enzymes which may be related to eventual chronic lung disease.
When the specific activities of a number of lysosomal hydrolases
were measured in whole lung homogenates of rats, there was a
significant increase in activities after the animals had been
continuously exposed to an ozone concentration of 1400-1600 µg/m3
(0.7-0.8 ppm) for 5-7 days (Dillard et al., 1972). This increase could
be attributed to an inflammatory reaction induced by the ozone (Coffin
et al., 1968b).
Castleman et al. (1973b) exposed rats continuously for 7 days to
ozone concentrations of 1400-1600 µg/m3 (0.7-0.8 ppm). Using histo-
and cytochemical techniques, they observed increased acid phosphatase
activity but no change in beta-glucuronidase activity.
One of the structural alterations reported following exposure to
ozone is a change in the appearance of the endoplasmic reticulum.
Biochemical evidence of the effects of ozone on microsomal enzymes was
reported by Palmer et al. (1971, 1972). After a 3-h exposure to ozone
at 1500 µg/m3 (0.75 ppm), the lung tissue of hamsters and the
tracheobronchial mucosa of rabbits showed reductions of 33% and 53%,
respectively, in the activity of benzopyrene hydroxylase (1.14.14.2),
a mixed function oxidase that depends on cytochrome P-450 and is
located in the endoplasmic reticulum. Similar results were obtained by
Goldstein et al. (1975) who exposed rabbits for 90 rain to
2000 µg/m3 (1.0 ppm) and demonstrated a decrease in the rabbit lung
cytochrome P-450 concentration. It is of interest that the maximum
effect was observed a few days after exposure.
Very little information is available describing alterations in
nucleic acids related to ozone exposure. Most of the studies have been
conducted at much higher concentrations than those found in the
environment and have yielded conflicting results. Since DNA synthesis,
cell division, and growth are closely linked, further studies
clarifying the potential hazard would be of value.
In studies on mice exposed to an ozone concentration of
5000 µg/m3 (2.5 ppm) for 2 h per day, for 120 days, Werthamer et al.
(1974) noted reductions in both DNA and RNA syntheses and a
concomitant increase in protein synthesis. Evans et al. (1971) exposed
aging mice for 6 h to 1000-7000 µg/m3 (0.5-3.5 ppm) and found that,
regardless of the ozone concentration, there was inhibition of DNA
synthesis. The authors believed that this indicated a reduction in the
ability of the alveoli to act as a source of new cells and to maintain
the integrity of lung tissue during ozone exposure.
5.2.3.3 Extracellular effects
Since the primary cause of death from high concentrations of ozone
is pulmonary oedema, investigators have attempted to determine the
role of histamine in the pulmonary toxicity of ozone. The lung is rich
in histamine -- containing mast cells and among the many effects of
histamine are oedematigenous alterations in vascular capillaries.
Oedema, whatever the cause, reduces the number of alveoli
participating in gas exchange, and produces conditions favourable for
bacterial growth. The exact concentration of ozone required to produce
oedema depends on the animal species. It is generally believed that
gross oedema is probably not elicited in any species exposed to ozone
at the concentrations found in the ambient air.
Alpert et al. (1971a) used a radio labelled albumen technique to
detect the presence of pulmonary oedema in rats. A significant
increase in albumen levels in pulmonary lavage fluid appeared after
6 h exposure to 1000 µg/m3 (0.5 ppm).
The available data on lung histamine are conflicting. Dixon &
Mountain (1965) reported that, following a single exposure of mice to
an ozone concentration of 2000 µg/m3 (1.0 ppm) for 5 h, there was a
release of histamine from the lungs that persisted for at least 4
days. Pretreatment with an antihistamine (promethazine) reduced the
amount of oedema following exposure to a sublethal dose of ozone. It
should be noted that the antihistamine used is a phenothiazine
derivative the action of which might stabilize membranes and trap free
radicals. In contrast, Easton & Murphy (1967) were unable to
demonstrate any reduction of lung histamine in guineapigs exposed to
ozone concentrations of 10-12 mg/m3 (5-6 ppm) for 2 h. Cronin & Giri
(1974) also failed to observe any differences in the lung histamine
level in rats exposed to an ozone concentration of 8000 µg/m3
(4.0 ppm) for 4 h, although pulmonary oedema was evident.
Surface tension is an important contributor to the elastic
properties of the lung. Any alteration of the normal surface tension
in the alveoli may be implicated in the development of chronic lung
disease. Consequently, several investigators have examined the effect
of ozone on pulmonary surface activity. Gardner et al. (1971) exposed
rabbits to ozone levels as high as 20 mg/m3 (10 ppm) for 2.5 h and
then isolated the surface active material by pulmonary lavage. They
found that ozone did not alter the surface tension of this material
and that in vitro exposure did not affect the properties of
dipalmitoyl lecithin, a principal component of this surface active
substance. Similar results were reported by Huber et al. (1971) who
showed that a 3-h exposure of rabbits to ozone at 10 mg/m3 (5 ppm)
did not alter surface activity in the lavaged alveolar lining material
nor in extracts of the whole lung. On the other hand Yokoyama (1972a)
reported that in vitro exposure of guineapig lung extracts to ozone
concentrations of 1000 to 24000 µg/m3 (0.5-12 ppm) for 25-60 min
resulted in a rapid increase in surface tension. However, he, too, did
not find any change when dipalmitoyl lecithin was exposed to ozone.
The effect of ozone on the appearance of lung tissue lipids in
saline lavage fluid was studied by Kyei-Aboagye et al. (1973). They
proposed that ozone affected the lung by decreasing lecithin formation
while simultaneously stimulating the release of surfactant lecithins
(palmitoyl and oleyl).
In the lung, there is a ground substance between the basement
membrane of the alveolar epithelium and the capillary endothelium. Any
destruction of the integrity of this substance could affect the
elasticity of the lung. Buell et al. (1965) fractionated the lung
tissue of rabbits, after a 1-h exposure to an ozone concentration of
2000 µg/m3 (1.0 ppm), into soluble, lipid, and protein fractions. The
isolation of aldehydes and ketones from the protein fraction indicated
structural changes. It was suggested that, once these compounds were
formed, they might affect the intra-and intermolecular crosslinking of
elastic protein molecules which would in turn cause a reduction in the
elasticity of the lung.
5.2.4 Carcinogenicity
The possibility that ozone might be carcinogenic has been studied
in experimental animals.
Exposure to ozonized gasoline with ozone concentrations of
2000-7600 µg/m3 (1.0-3.8 ppm) for 52 weeks caused an increased
incidence of lung tumours in strain A mice (350 in each experimental
group). After 40 weeks, tumours were found in 21% of animals in the
control group and in 63% in the test group. After 52 weeks, the
incidence of tumour-bearing animals in the control group exposed to
washed air was 41% compared with 80% in the test group (Kotin & Falk,
1956). Additional studies using the same atmosphere of ozonized
gasoline were conducted on C57BL mice (405 in each experimental group)
(Kotin et al., 1958). After 92 weeks, the incidence of tumour-bearing
animals in the control group was 1.6% compared with 9.6% in the
exposed group.
Although these studies indicate that ozone may be tumorigenic
further work is necessary to confirm these results.
A number of studies including that of Penha et al. (1972) have not
been considered in this document because information concerning the
numbers of animals tested, control groups, etc. was inadequate.
5.2.5 Tolerance to ozone
The term "tolerance" refers to the fact that exposure to a
nonlethal dose of a specific toxic substance protects the host against
subsequent exposure to higher dose of the same chemical or of
different agents with similar toxicological properties (cross-
tolerance). Stokinger et al. (1956) reported such a protective effect
for ozone. Tolerance to ozone has been reviewed by Fairchild (1967)
and has also been reported by many other investigators (Henschler,
1960; Matzen, 1957; Mendenhall & Stokinger, 1959), In small rodents,
tolerance can be initiated by a concentration as low as 600 µg/m3
(0.3 ppm), maximum protection being obtained with concentrations
within the range of 2000-8000 µg/m3 (1-4 ppm) (Stokinger & Scheel,
1962). A single exposure to ozone can also induce a cross-tolerance
against subsequent lethal doses of X-ray irradiation (Hattori et al.,
1963), nitrogen dioxide, hydrogen peroxide, carbonic dichloride
(phosgene), ethanone (ketene), nitrosyl chloride, or hydrogen sulfide
(Fairchild, 1967).
Although the mechanism of tolerance is still not well understood,
several possibilities have been envisaged. Fairchild (1967) observed
that the level of reduced glutathione was maintained in ozone-tolerant
animals but not in the nontolerant group. This could be brought about
either by directly blocking the oxidation of reduced glutathione or
through some enzymatic pathway that would stimulate production of
reduced glutathione in response to a second exposure to ozone.
However, it is possible that the maintenance of these thiols in the
tolerant animal may be a result of the tolerance rather than the
cause. The studies of Chow & Tappel (1972, 1973) provide biochemical
support for this hypothesis. In addition, it has been shown (Mountain
et al., 1960) that the activities of serum alkaline phosphatase
(3.1.3.1), adrenal succinate dehydrogenase (1.3.99.1), and glucose-6-
phosphatase (3.1.3.9) either remain normal or are only slightly
altered in tolerant animals.
Fukase et al. (1975a) reported tolerance in mice to an ozone
concentration of 20-58 mg/m3 (10-29 ppm) after pre-exposure to
concentrations of 400-2000 [µg/m3 (0.2-1.0 ppm) and proposed that the
mechanism involved an increase in reduced glutathione and the
elevation of the activity of the peroxidative metabolic pathway.
Physical swelling of the intra-alveolar septa has also been proposed
by Henschler et al. (1964) as a mechanism of protection against the
oedematigenous effects of ozone.
Unilateral lung exposure models showed that, in order to induce
protection in a particular lung, the tissue must have actually come
into contact with ozone (Alpert & Lewis, 1971; Frank et al., 1970a).
There was no significant crossover effect from contralateral lung,
suggesting that there was no basis for assuming a circulating humoral
factor, and that the phenomenon was purely local. While reduction in
oedema development could be induced by pre-exposure to ozone, no
protection was obtained against the influx of polymorphonuclear
leukocytes into the lung (Gardner et al., 1972). It was also apparent
that tolerance did not influence the number of recoverable cells in
pulmonary lavage, their phagocytic capability, or the loss of
macrophage hydrolytic enzyme activity. This indicated the possibility
that tolerance protects only against pulmonary oedema and not against
other more subtle reactions.
As previously mentioned, tolerance is initiated in small rodents
at approximately 600 µg/m3 (0.3 ppm) (Stokinger & Scheel, 1962).
According to Alpert et al. (1971a) this approximates the lowest level
at which oedema can be demonstrated in rats by means of recovery of
labelled serum albumen via lung lavage. Thus, Coffin & Gardner (1972b)
postulated that it is probably necessary to produce a minimal
oedematigenous response before tolerance develops.
Experiments designed to test the susceptibility to bacterial
infection of "tolerant" animals have been conducted by Coffin &
Gardner (1972b). These studies illustrated that the "tolerant" animals
were only partially protected against the joint effects of ozone and a
viable microorganism. Partial protection was afforded by tolerance
only when the initiating dose of ozone was 600 µg/m3 (0.3 ppm) for
3 h (Coffin & Gardner, 1975). The authors considered that the
tolerance to infection seen at levels of ozone above 600 µg/m3
(0.3 ppm) could be due to the prevention of the formation of oedema
and that tolerance was only partial because tolerance to the action of
ozone on cellular and noncellular specific defence systems could not
be induced. This was shown by Gardner et al. (1972) who reported that
there was no tolerance to ozone damage in the alveolar macrophages,
which are the prime defence against infectious disease in the lower
part of the respiratory tract.
In their study on the effects of ozone on laboratory-induced
allergic respiratory disease in guineapigs, Matsumura et al. (1972)
found that animals that had received pretreatment with ozone at
2000 µg/m3 (1 ppm) for 1 h before a challenging exposure of
4000 µg/m3 (2 ppm) showed only slight respiratory reaction to
acetylcholine inhalation compared with those in which tolerance had
not been induced.
A variety of neurohumoral factors resulting from exposure to ozone
have been described by Fairchild (1963), who reported that
thyroidectomy, adrenalectomy, and hypophysectomy would increase the
resistance of rats to otherwise lethal doses of ozone by preventing
pulmonary oedema.
While the mechanism of tolerance in relation to ozone-induced
oedema is still not well understood, it has been suggested that the
thymus might play a role. Thymectomized mice were unable to develop
tolerance to ozone although the sham-operated animals exhibited
tolerance under the same experimental conditions (Gregory et al.,
1967).
5.2.6 Effects on the host defence system
Animals that are exposed to ozone have an increased susceptibility
to disease-producing biological agents, which can result in an
increased incidence of pulmonary infectious disease and death. Animal
models have been developed by several investigators to examine this
phenomenon experimentally by exposing animals to the pollutants and to
aerosolized viable microorganisms. The high sensitivity of this model
system is probably due to the fact that it reflects a summation of all
the subtle effects that ozone has on the lung. Any alteration in
cellular defence and ciliary activity, oedema, and immunosuppression
would allow the viable organism to multiply and cause disease. In mice
exposed to ozone at concentrations ranging from 2600 to 8800 µg/m3
(1.3 to 4.4 ppm) for 3 h, or 1700 µg/m3 (0.84 ppm) for 4 h, per day,
5 days per week, for 2 weeks, and subsequently challenged with
Klebsiella pneumoniae, resistance to respiratory infection was
significantly reduced (Miller & Ehrlich, 1958). Similar results were
obtained with hamsters. In a study to elucidate a relationship between
the time of exposure to ozone and the time of challenge with the
infectious agent, Purvis et al. (1961) found a decrease in resistance
to infection in mice treated with the bacterial aerosol within 19 h of
exposure to the gas or 27 h before exposure. A subsequent study by
Coffin and co-workers (1968a) showed that the lowest concentration of
ozone necessary to produce this effect in mice was 160 µg/m3
(0.08 ppm) for 3 h; Coffin & Gardner (1972) established a dose-
response relationship.
Further enhancement of toxicity could be demonstrated when a third
interactant such as cold or exercise was added to this infectivity
model. Housing mice at 6°-9°C for 3 h prior to exposure to ozone at
1400-1800 µg/m3 (0.7-0.9 ppm) for 2 h and to the microorganisms
increased the mortality rate compared with that in animals housed at
room temperature (Coffin & Blommer, 1965). Mice subjected to physical
activity while being exposed to ozone at 200-600 µg/m3 (0.1-0.3 ppm)
were less resistant to infectious agents than animals that were at
rest during exposure (Gardner et al., 1974b).
The effects of low concentrations of ozone on mice with induced
silicosis were investigated by Goldstein et al. (1972). Starting at an
ozone concentration of 800 µg/m3 (0.4 ppm) for 4 h, a progressive
decrease in pulmonary bactericidal activity occurred with exposure to
increasing concentrations of ozone. Silicosis itself did not inhibit
bactericidal activity.
Further research has sought to delineate the mechanisms by which
ozone reduces the resistance of animals to bacterial infection. It
appears that a number of factors may be responsible.
The number of organisms initially deposited within the lung does
not play a major role in the enhancement of mortality since the
pulmonary deposition of the microorganisms is less in ozone-treated
animals. However, regardless of the initial deposition, ozone-treated
animals subsequently have more organisms within the lungs, mainly
because of reduced bactericidal ability and the subsequent
multiplication of the inhaled organism (Coffin & Gardner, 1972a).
Goldstein et al. (1971b) found that the magnitude of the increase in
bacterial numbers was correlated with an increase in ozone
concentration up to 5200 µg/m3 (2.58 ppm). In rabbits exposed to
ozone at a concentration of 1000 µg/m3 (0.5 ppm), for 16 h per day,
for 7 months, Friberg et al. (1972) did not find any effect on the
physical removal of inhaled particles or on the number of macrophages
but, under the same conditions, a significant decrease was found in
the clearance of viable Escherichia coli in the lungs of guinea-
pigs.
The effect of ozone on antibacterial activity in the mouse lung
was determined in vivo by investigating the removal of bacteria by
mucociliary activity and by bactericidal activity simultaneously
(Goldstein, 1971a, 1971b). Mice were exposed to various concentrations
or ozone including 1200, 1400, 1600, and 2200 µg/m3 (0.62, 0.70,
0.80, and 1.1 ppm), for 17 h prior to, or 4 h after, infection with
aerosols of radiolabelled Staphylococcus aureus. Inhibition of
pulmonary bactericidal activity was shown at an ozone concentration as
low as 1200 µg/m3 (0.62 ppm) and activity decreased progressively
with increasing levels of ozone. The authors proposed that the
bactericidal defect was due to dysfunction of the alveolar macrophage.
Rabbits exposed to an ozone concentration of 600 µg/m3 (0.3 ppm)
for 3 h showed an impairment of the phagocytic properties of the
pulmonary alveolar macrophages (Coffin et al., 1968b). Furthermore,
exposure to ozone at 10 mg/m3 (5 ppm) for 3 h resulted in a reduction
in the total number of macrophages with a concomitant influx of
polymorphonuclear leukocytes into the lower respiratory tract (Coffin
& Gardner, 1972a). Enzyme activity was reduced in macrophages
recovered by lavage after exposure of rabbits to ozone at levels as
low as 500 µg/m3 (0.25 ppm) for 3 h (Hurst et al., 1970). The enzymes
studied were lysozyme (3.2.1.17), acid phosphatase, and beta-
glucuronidase and since these enzymes are involved in the
intracellular degradation of ingested bacteria, their reduction could
contribute to the poor bactericidal effect seen in the studies
mentioned previously.
Other effects on the alveolar macrophage have also been reported.
For example, alveolar macrophages from rabbits exposed to 4000 µg/m3
(2.0 ppm) for 8 h per day for 7 days exhibited an increased membrane
fragility (Dowell et al., 1970) and a dose-related reduction in
interferon was observed in the alveolar macrophages of rabbits exposed
to ozone concentrations of 2000, 6000, and 10 000 µg/m3 (1, 3, and
5 ppm) for 3 h (Shingu et al., 1972).
Morphological changes seen in rabbit alveolar macrophages after
exposure to ozone at a concentration of 10 mg/m3 (5 ppm) for 3 h
included dilatation of the endoplasmic reticulum and perinuclear
envelope, swelling of the mitochondria, intracellular vacuolization,
cell lysis, and the formation of myelin figures and autophagic
vacuoles (Huber et al., 1971). The ultrastructure of pulmonary
macrophages was also examined in situ in the lung tissue of rats
exposed to ozone at 6000 µg/m3 (3 ppm) for 4 h (Plopper et al.,
1973b). Immediately after exposure, the cells resembled those of
unexposed animals, but 12 h later there were twice as many
macrophages. Many of these had large granular cytoplasmic inclusions
that indicated increased phagocytic activity.
There is evidence that the effect seen on the alveolar macrophage
may be mediated through the noncellular milieu of the lung. Within the
lung, the macrophages are located close to the so-called pulmonary
surfactant contained in the extracellular lining of the epithelial
surface of the lung alveoli. It is possible that ozone might also
react with this lining film, which in turn, might be deleterious to
the cell. Gardner (1971) and Gardner et al. (1971) conducted
experiments on lavage fluid containing this surface-active substance
and found that, when isolated from rabbits exposed to ozone at
20 mg/m3 (10 ppm) for 2.5 h, it could adversely affect the stability
of the alveolar macrophage in vitro. A similar effect was noted when
ozone was bubbled through the lavage fluid in vitro. The effect on
the lavage fluid could be seen at levels as low as 200 µg/m3
(0.1 ppm) for a 2.5-h, in vivo exposure or after only 30-min in
vitro exposure. Since no significant alteration in the surface
tension of the lavage fluid was produced by ozone exposure, it is
suggested that the effect might be on a nonlipid component, possibly a
protein. The activity found in the cell-free pulmonary lavage fluid
has been called the "protective factor".
Ozone may also inactivate some opsinogenic factor within the lung
since Holzman et al. (1968) showed that it has a protein-degrading
property. Exposure of mice and rabbits to a concentration of
10 mg/m3 (5 ppm) for 3 h reduced the activity of active lysozyme,
obtained by bronchopulmonary lavage, by approximately 30%.
Various components of the endogenous defence mechanism of the lung
were studied through a unilateral lung exposure model (Alpert et al.,
1971b). Exposure to ozone at concentrations ranging from 1000 to
6000 µg/m3 (0.5 to 3 ppm) for 3 h decreased cellular viability,
depressed the intracellular hydrolytic enzymes and increased the
absolute number of polymorphonuclear leucocytes in the pulmonary
lavage fluid. All effects were dose-related and were found only in the
lung under treatment and not in the contralateral lung that breathed
ambient air.
Heuter et al. (1966) reported that exposure to an irradiated
automobile exhaust atmosphere for 15 months increased the
susceptibility of mice to pulmonary infection during the latter half
of the animal's lifetime. The oxidant concentrations in the irradiated
exposure, chamber ranged from 400 to 2000 µg/m3 (0.2-1.0 ppm). In a
similar study using irradiated automobile exhaust, enhancement of
mortality compared with that of control animals kept in filtered air
was noted with exposure to total oxidants at 300 µg/m3 (0.15 ppm) and
carbon monoxide at 29 mg/m3 (25 ppm) for 4 h. Coexistent
concentrations of nitrogen dioxide ranged from a trace to 1900 µg/m3
(1.0 ppm) (Coffin & Blommer, 1967).
Goldstein et al. (1974) studied the bactericidal effect when mice
were exposed to a combination of nitrogen dioxide and ozone, and
concluded that the combined pollutants caused bactericidal dysfunction
at concentrations that were approximately the same as the lowest
concentrations that caused similar dysfunction when the animals were
exposed to the gases individually.
Erlich et al. (1977) observed that a single joint exposure of mice
to nitrogen dioxide and ozone resulted in an addition of effects and
that a synergistic action might result from repeated exposures to the
mixture.
The animals were exposed for 3 h to 16 different combinations of
nitrogen dioxide at levels of 0, 2800, 3800, 6600, and 9400 µg/m3
(0, 1.5, 2.0, 3.5, and 5.0 ppm) and ozone at 0, 100, 200, and
1000 µg/m3 (0, 0.05, 0.1, and 0.5 ppm). Within 1 h of termination of
exposure to the pollutants, the mice were infected with Streptococcus
pyogenes. Excess mortality rates due to exposure to the mixture of
the two gases were approximately equivalent to the sum of those
induced by the inhalation of each individual pollutant. In mice
exposed repeatedly for 3 h per day, for 20 days, to a mixture of
nitrogen dioxide and ozone at concentrations of 3800 µg/ma (2.0 ppm)
and 100 µg/m3 (0.05 ppm), respectively, and challenged with
Streptococcus aerosol, the number of deaths was significantly higher
than in the control group. On the other hand, repeated daily exposure
to either nitrogen dioxide or ozone at the above-mentioned
concentrations did not show any major effect on the mortality rate.
The authors considered that this result might suggest a synergistic
action of the two pollutants making them more effective in reducing
resistance to respiratory infection.
5.2.7 Interaction of ozone with bronchoactive and other chemicals
The effects of pre-exposure to ozone on the sensitivity of
guineapigs to inhaled acetylcholine were studied by Matsumura et al.
(1972). Animals pre-exposed to an ozone concentration of 4000 µg/m3
(2 ppm) for 30 min manifested severe difficulty in breathing and many
died. This effect persisted for as long as 2 h after exposure. In a
similar study, Matsumura (1970a) sensitized guineapigs to albumen and
found that repeated 30 min pre-exposures to an ozone concentration of
10 mg/m3 (5 ppm) enhanced the sensitization. An ozone concentration
of 2000 µg/m3 (1 ppm) had no such effect.
Ozone-exposed guineapigs were also more susceptible to the toxic
action of injected histamine (Easton & Murphy, 1967). A 2-h exposure
to ozone at 10 mg/m3 (5 ppm) caused severe lung function changes and
increased mortality. This increased susceptibility to histamine was
detectable for as long as 12 h after the end of the exposure.
In order to study the effects of ozone on susceptibility to
serotonin, Suzuki & Nagaoka (1973) injected the compound into the
abdominal cavity of rats after exposing the animals to ozone at
concentrations ranging from 2000 to 12 000 µg/m3 (1-6 ppm) for 3 h.
Mortality increased with increasing levels of ozone, but fatalities
did not occur in a control group injected with serotonin and breathing
filtered air.
A report by Goldstein et al. (1970) that a deficiency of vitamin E
increased the toxicity of ozone in the rat has been supported by the
studies of Roehm et al. (1971b, 1972) and Menzel et al. (1972). These
investigators observed a significantly shorter 50% lethal time and
more pronounced signs of respiratory distress in ozone-exposed rats
(2000 µg/m3 (1.0 ppm) for 9 days continuously) that were fed vitamin
E-depleted diets in comparison with those fed vitamin E-supplemented
diets. When the ozone concentration was reduced to 1000 µg/m3
(0.5 ppm), pulmonary oedema became evident and mortality rates
increased in animals fed vitamin E-deficient diets after 6 weeks of
exposure. In rats continuously exposed to toxic levels of ozone
ranging from 1400 to 1600 µg/m3 (0.7-0.8 ppm), protection by dietary
vitamin E against lung lipid peroxidation was proportional to the
logarithm of the concentration of the vitamin in the diet (Fletcher &
Tappel, 1973). In further studies by Mustafa (1975), rats were fed a
basal diet containing vitamin E at either 66 mg/kg or 11 mg/kg for 5
weeks, and then exposed continuously to an ozone concentration of
200 µg/m3 (0.1 ppm), for 7 days. Oxygen consumption was measured in
lung homogenate using succinate as a substrate. Animals receiving the
higher concentration of vitamin E (66 mg/kg) were relatively
insensitive to this level of ozone, i.e., there was no significant
increase in oxygen consumption, but those receiving the lower
concentration of vitamin E (11 mg/kg) showed a significant increase in
oxygen consumption.
5.3 Systemic Reactions and other Effects
The number of studies on the effects of ozone on a wide range of
biological phenomena including growth, reproduction, and behaviour is
increasing but many of these studies need further confirmation.
Furthermore, even when a correlation is found between ozone exposure
and effects, it is difficult to decide whether the effects are due to
the direct oxidizing action of ozone or are secondary reactions to
pulmonary injury caused by ozone.
5.3.1 Effects on growth
There does not seem to be any convincing study which shows that
ozone, at the concentrations found in ambient air, has any detrimental
effect on body growth. Nevskaja & Kocetkova (1961) who exposed rats to
a mixture of ozone at 800 µg/m3 (0.4 ppm) and sulfuric acid at
7000 µg/m3 for 5 h per day, 6 days per week, for 100 days, and Loosli
et al. (1972) who exposed mice to synthetic photochemical air
pollution containing ozone at 600-840 µg/m3 (0.30-0.42 ppm) reported
that exposed animals weighed less than the controls. However, Emik et
al. (1971) did not find any significant differences between the growth
of guineapigs breathing ambient air containing oxidants at a mean
concentration of 110 µg/m3 (0.057 ppm) for over two years, and that
of the controls breathing filtered air.
5.3.2 Haematological effects
It is still questionable whether the haematological effects noted
in ozone exposure are due to the direct action of ozone on the
cellular and acellular components of the blood as it passes through
the lung capillaries or whether they are caused by oxidizing
intermediates, such as ozonides or peroxides, that might penetrate the
alveolar basement membrane and enter the pulmonary circulation. A
third possibility would be that these effects are secondary reactions
induced by ozone perhaps causing the release of some mediating
substance, yet to be identified.
5.3.2.1 Short-term exposure (24 h or less)
Short-term exposure to high concentrations of ozone ranging from
6000 to 16 000 µg/m3 (3.0-8.0 ppm) has been reported to cause
increases in neutrophil-lymphocyte ratios in rats (Bobb & Fairchild,
1967), and in the number of erythrocytes and leukocyte indices in mice
(Kusumoto et al., 1976). Within this range of concentrations,
Goldstein et al. (1968) and Goldstein (1973) found a reduction in
acetylcholinesterase (3.1.1.7) in the erythrocytes of mice and
induction of hydrogen peroxide formation in the circulating
erythrocytes of rats and mice. Veninga (1970, unpublished data)a
reported doubling in the number of binucleated lymphocytes and
increased levels of serum glutamic pyruvic transaminase (2.6.1.2) but
no changes in blood catalase (1.11.1.6) in mice exposed for 2 h to an
ozone concentration of 400 µg/m3 (0.2 ppm).
Increased resistance to haemolysis of erythrocytes was reported in
mice exposed to an ozone concentration of 2000 µg/m3 (1 ppm) for
30 min (Mizoguchi et al., 1973). Menzel et al. (1975) presented
evidence that fatty acid ozonides produced Heinz bodies in
erythrocytes in mice exposed to ozone at 1700 µg/m3 (0.85 ppm) for
4 h. Further exposure for 3 days resulted in a decline in the number
of Heinz body positive cells. It is of interest to note that these
bodies were not produced with ozone in the absence of serum containing
unsaturated lipids. The authors postulated that this indicated an
oxidation of the erythrocyte membrane and suggested that fatty acid
ozonides might be the toxic intermediaries. At lower concentrations,
Brinkman et al. (1964) noted increased sphering of erythrocytes of
mice, rabbits, and rats, after an in vitro exposure to ozone at
400 µg/m3 (0.2 ppm) for 1-2 h.
a Veninga, T. S. Ozone-induced alterations in murine blood and liver.
Paper presented at the 2nd International Clean Air Congress, Washington,
DC, 6-11 December 1970 (No. MB-15E).
A 3-h exposure to irradiated automobile exhaust containing an
oxidant concentration of 740-1200 µg/m3 (0.37-0.58 ppm) was found to
increase the number of leukocytes in the blood of mice and reduce the
level of serum alkaline phosphatase (3.1.3.1) (Kusumoto et al., 1976).
5.3.2.2 Prolonged and repeated exposures
Increasing the length of exposure provided further evidence for
the oxidizing effect of ozone on the blood of rabbits and rats. After
continuous exposure for 8 days to ozone at 1600 µg/m3 (0.8 ppm), the
lysozyme activity in the plasma and soluble fraction of lung of rats
significantly increased (Chow et al., 1974). Continuous exposure of
rats to an ozone concentration of 110 µg/m3 (0.06 ppm) for 93 days
resulted in a decrease in blood cholinesterase (3.1.1.8) activity
which returned to normal 12 days after exposure ceased (Eglite, 1968).
More recently, Jegier & P'an (1973) and P'an & Jegier (1972) reported
a rise in serum trypsin protein esterase in rabbits exposed to
800 µg/m3 (0.4 ppm) for 6 h per day, 5 days per week, for 10 months.
Long-term, combined exposure to ozone and carbon monoxide produced
a reduced level of serum glutamic oxaloacetic transaminase (2.6.1.1)
in rabbits. The animals were exposed for 1000 days to urban air
containing oxidants and carbon monoxide at mean concentrations over 2
years of 110 µg/m3 (0.057 ppm) and 2000 µg/m3 (1.7 ppm)
respectively, (Emik et al., 1971).
5.3.3 Effects on reproduction
A few studies have been reported concerning the possible effects
of ozone and photochemical oxidants on reproduction. The data indicate
that the newborn animals may be more susceptible to exposure to
oxidants than the parents.
Brinkman et al. (1964) and Veninga (1967) exposed pregnant mice
for 7 h per day, 5 days per week, for 3 weeks to ozone concentrations
of 200-400 µg/m3 (0.1-0.2 ppm). They found a 4-fold increase in
neonatal mortality. The second author also observed increases in the
incidence of both incisor growth and blepharophimosis in the new born.
Similar studies using irradiated automobile exhaust were performed
by Hueter et al. (1966) who found that mice exposed for 13 months
before, during, and after gestation showed a marked decrease in the
number and frequency of litters, the survival of infants, and the
total number of pups born. Nonirradiated automobile exhaust did not
produce any significant effects. The oxidant concentrations in the
irradiated exposure chamber ranged from 400 to 2000 µg/m3 (0.2 to
1.0 ppm). In a follow-up study, Lewis et al. (1967) found that the
number of female mice that did not become pregnant after mating with
pretreated males was twice that found in mice mated with untreated
controls.
5.3.4 Behavioural and related changes
5.3.4.1 Short-term exposure (24 h or less)
Behavioural changes in response to acute ozone exposures have not
been studied very extensively. The major effect that has been noted is
a reduction in spontaneous activity. Murphy et al. (1964a) and
Konigsberg & Bachman (1970) found significant losses in motor activity
in mice exposed to 400 µg/m3 (0.2 ppm) for 6 h and in rats exposed to
1000 µg/m3 (0.5 ppm) for 45 min, respectively.
Using an evoked response technique, Xintaras et al. (1966)
measured a reduction in the amplitude of response to a flash in the
specific visual cortex and in the superior colliculus in rats exposed
to ozone at 1000-2000 µg/m3 (0.5-1.0 ppm) for 1 h. In studies on
rhesus monkeys, Reynolds & Chaffee (1970) reported increases in both
simple and choice reaction times after a 30-min exposure to an ozone
concentration of 1000 µg/m3 (0.5 ppm).
Gardner et al. (1974) showed that the pentobarbital-induced
sleeping time of mice significantly increased after 2 and 3 exposures
each of 3 h, to an ozone concentration of 2000 µg/m3 (1 ppm). After
the third exposure, there was no change in the sleeping time compared
with the controls unless in subsequent exposure the ozone
concentration was raised to 10 mg/m3 (5 ppm), when the sleeping time
again differed significantly. The authors suggested that ozone might
be deactivating a liver microsomal enzyme that was responsible for the
detoxification of the drug.
5.3.4.2 Prolonged and repeated exposures
Additional studies on the effects of ozone on the behaviour of
animals have been conducted with longer exposures and complex mixtures
of air pollutants. It appears that the reduction of voluntary running
time may be an extremely sensitive indicator of ozone toxicity. The
mechanism of this change remains obscure.
Continuous exposure for 1 week to an ozone concentration of
2000 µg/m3 (1.0 ppm) caused an 84% reduction in voluntary activity in
rats (Fletcher & Tappel, 1973). Decreased running activity has also
been observed in mice exposed continuously to urban air with elevated
oxidant levels and to irradiated motor vehicle exhaust (Emik et al.,
1971; Heuter et al., 1966).
According to Litt et al. (1968), ozone-exposed rats required a
longer time to learn a specific task; learning was unstable and they
exhibited a reduced ability for temporal discrimination. The animals
were exposed intermittently for 2 months to ozone concentrations of
600-1000 µg/m3 (0.3-0.5 ppm). Nevskaja & Kocetkova (1961) exposed
rats to a mixture of ozone at 800 µg/m3 (0.4 ppm) and sulfuric acid
at 7000 µg/m3 for 5 h per day, 6 days per week, for 100 days, and
found that a conditioned reflex was retarded.
5.3.5 Miscellaneous systemic reactions to lung damage
Other changes have been reported which indicate biochemical,
physiological, and structural effects at sites distant from the lung
that are possibly related to the inhalation of ozone. In most of these
studies, the levels of ozone employed greatly exceeded those in the
ambient air. After exposing rabbits to an ozone concentration of
1500 µg/m3 (0.75 ppm) for 4-8 h, Atwal & Wilson (1974) found
histological and ultrastructural changes in the parathyroid glands.
They reported hyperplasia of the chief cells, large numbers of
secretion granules, proliferation and hypertrophy of the rough
endoplasmic reticulum, Golgi complex, mitochondria, lipid bodies, and
free ribosomes. These effects were seen up to 66 h after exposure.
Atwal et al. (1975) expanded their short-term exposure study by
exposing rabbits to 1500 µg/m3 (0.75 ppm) for 48 h and found
morphological changes in the parathyroid gland similar to those that
they had reported for 4-8 h of exposure. The data suggested that ozone
might trigger off an immune reaction which caused inflammatory injury
to the parathyroid gland.
It was demonstrated by Brinkman et al. (1964) that exposure to an
ozone concentration of 400 µg/m3 (0.2 ppm), for 5 h per day, for 3
weeks, resulted in the rupture of nuclear envelopes and the extrusion
of the contents of myocardial muscle fibres in rabbits and mice. This
effect became reversible one month after the exposure.
There are some data which suggest that ozone might accelerate the
aging process. Stokinger (1965) reported premature aging in rabbits
after 1 year of weekly 1-h exposures to ozone. The major changes found
in the exposed animals were premature calcification of the
sternocostal cartilage, severe depletion of body fat, dull cornea,
sagging conjunctivae, and a general appearance of not thriving.
5.4 Mutagenicity
Chromosome aberrations (anaphase bridges) were found in 42% of
root meristem cells of Vicia faba exposed to an ozone concentration
of 8000 mg/m3 (4000 ppm) for 1 h, but none were found in cells
exposed to clean air (Fetner, 1958). When chick embryonic fibroblasts
were exposed to ozone concentrations of 5000-10 000 mg/m3
(2500-5000 ppm) for 24 h, a small number of cells were found with
chromosome bridges in anaphase and telophase and with nuclear
fragments (Sachsenmaier et al., 1965). Using a much lower
concentration, Pace et al. (1969) exposed strain L cells continuously
to an ozone concentration of 8000 µg/m3 (4 ppm) for 30 h, and found a
significant reduction in cell survival compared with that in the
control group. The authors considered that this was due to ozone
interference with mitotic activity.
In vivo exposure studies on mammals by Zelac et al. (1971a,
1971b) are of special interest. Female Chinese hamsters were exposed
to an ozone concentration of 400 µg/m3 (0.2 ppm) for 5 h and the
number of circulating blood lymphocytes with chromosomal breaks was
measured immediately after exposure and 6, and 15.5 days later. A
significant increase in chromosomal breaks compared with pre-exposure
values was still observed 15 days after exposure.
Although further confirmation is required, these data and the
results of studies on chromosomal changes in human tissues (see
section 6.1.1) seem to indicate that ozone might be a mutagenic agent.
5.5 Summary Table
Table 10 is a summary of experimental animal studies that provide
quantitative information useful for the establishment of guidelines
for the protection of public health with respect to exposure to ozone
at concentrations of up to 2000 µg/m3 (1.0 ppm).
Table 10. Experimental animal studies
Local effects on the respiratory system
1. Morphological changes
Ozone Length of exposure
concentration Number
of
µg/m3 (ppm) number h/day Effects Responsea Species animals Reference
of days
1800 (0.88) 180 24 Epithelial injury seen as early as 4 h n.a.b rat n.a. Freeman et al.
after the beginning of exposure; after (1974)
3 weeks half of animals died and
emphysema-like lesions observed.
1600 (0.8) 7 24 Walls and interalveolar septa of terminal -- rat 8 (8) Castleman et al.
airways thickened and infiltrated by (1973a)
mononuclear cells.
1200 (0.6) 1 7 Swelling of epithelial alveolar lining -- mouse 32 (13) Bile (1970)
cells & endothelium cells with
occasional breaks in basement
membrane.
1100 (0.54) 180 24 Progressive changes in the airway -- rat n.a. Freeman et al.
epithelium after 6 days. (1974)
1000 (0.5) 1 6 Immediately after the exposure the -- mouse 16(12) Evans et al.
number of alveolar cells significantly (1971)
decreased.
800 (0.4) 5 per 6 Emphysematous & vascular-type -- rabbit 6 (6) P'an et al.
week x lesions. (1972)
10 month
a Number of animals showing effects/total number of animals; numbers in brackets refer to control groups.
b Not available.
Table 10. Experimental animal studies
Local effects on the respiratory system
1. Morphological changes cont'd.
Ozone Length of exposure
concentration Number
of
µg/m3 (ppm) number h/day Effects Responsea Species animals Reference
of days
520- (0.26 1 4.7-6.6 Dose-related loss of ciliated epithelium. -- cat 14 (3) Boatman et al.
2000 -1.0) (1974)
400 (0.2) 1 2 Degenerative changes in type I cells. -- rat n.a. Stephens et al.
(1974)
2. Functional changes
1400 (0.68) 1 2 No significant increase in flow -- guinea- 10 (10) Murphy et al.
resistance. pig (1964a)
1000 (0.5) 1 2 Increase in airways resistance and -- guinea- 10 (10) Yokoyame
breathing frequency with decrease in pig (1972a)
tidal volume.
520- (0.26 1 4.6 Increased flow resistance. -- cat 10 (4) Watanabe et al.
1000 -0.5) (1973b)
400 (0.2) 30 24 Reduction in lung elasticity; increase in -- rat 44 (44) Bartlett et al.
lung volume and in alveolar (1975)
dimensions.
a Number of animals showing effects/total number of animals; numbers in brackets refer to control groups.
b Not available.
Table 10. Experimental animal studies
Local effects on the respiratory system
3. Biochemical changes
Ozone Length of exposure
concentration Number
of
µg/m3 (ppm) number h/day Effects Responsea Species animals Reference
of days
1500 (0.75) 1 3 Reduction in activity of benzopyrene -- hamster 25 (95) Palmer et al.
hydroxylase (1.14.14.2). & 8 (15) (1971. 1972)
rabbit
1400- (0.7 7 24 Increased acid phosphatase activity. -- rat 14 (12) Castleman et al.
1600 -0.8) (1973b)
1400 (0.7) 5 24 Indication of lipid peroxidation; -- rat 33 (20) Chow & Tappel
increase in lysosomal hydrolase (1972); Dillard
activity. et al. (1972)
1000 (0.5) 1 6 Increased albumen recovery from -- rat 10 (18) Alpert et al.
alveolar spaces. (1971a)
800- (0.4 1 4 Evidence of formation of lipid peroxides n.a.b mouse n.a. Goldstein et al.
1400 -0.7) in the lung. (1969)
500 (0.25) 1 3 Reduced activity of several lysosomal -- rabbit 6 (6) Hurst et al.
hydrolases. (1970)
400 (0.2) 7 24 Increase in pulmonary mitochondrial -- rat 5-8 Mustafa et al.
oxygen consumption. (5-8) (1973)
a Number of animals showing effects/total number of animals; numbers in brackets refer to control groups.
b Not available.
Table 10. Experimental animal studies
Local effects on the respiratory system
4. Effects on the host defence system
Ozone Length of exposure
concentration Number
of
µg/m3 (ppm) number h/day Effects Responsea Species animals Reference
of days
1200- (0.62 1 17 Inhibition of pulmonary bactericidal -- mouse 20 (29) Goldstein et al.
1600 -0.80) activity. (1971b)
1000 (0.5) 210 16 No effect on physical clearance of -- rabbit 8 (8) Friberg et al.
inhaled particles or on the number of (1972)
macrophages.
1000 (0.5) 60 16 Decrease in the clearance of viable -- guinea- 18 (18) Friberg et al.
Escherichia coli. pig (1972)
800 (0.4) 1 4 Inhibition of bactericidal activity; no -- mouse 38 (37) Goldstein et al.
additive role of the induced silicosis. (1972)
600 (0.3) 1 3 Impairment of phagocytic properties of -- rabbit n.a.b Coffin et al.
pulmonary alveolar macrophages. (1968b)
500 (0.25) 1 3 Diminished enzyme activities of alveolar -- rabbit 6 (6) Hurst et al.
(1970)
macrophages.
160 (0.08) 1 3 Increased susceptibility to 15/40 mouse 40 (40) Coffin et al.
Streptococcus. (6/40) (1968a)
a Number of animals showing effects/total number of animals; numbers in brackets refer to control groups.
b Not available.
Table 10. Experimental animal studies--continued
II. Systemic reactions and other effects
1. Haematological effects
Ozone Length of exposure
concentration Number
of
µg/m3 (ppm) number h/day Effects Responsea Species animals Reference
of days
1700 (0.85) 1 4 Formation of Heinz bodies in red cells. n.a.b mouse n.a. Menzel et al.
(1975)
1600 (0.8) 8 24 Increase of lysozyme activity in plasma -- rat 8 (8) Chow et al.
and soluble fraction of lung. (1974)
800 (0.4) 5 per 6 increase in serum trypsin protein -- rabbit 6 (6) P'an & Jegier
week x esterase. (1972); Jegier
10 months & P'an (1973)
600 (0.3) 1 1 Inhibition of acetylcholine esterase -- ox (in -- P'an & Jegier
(3.1.1.7) activity. vitro) (1970)
400 (0.2) 1 1-2 Increased sphering of red blood cells. -- mouse, -- Brinkman et al.
rabbit, (1964)
rat (in
vitro)
400 (0.2) 1 2 Doubling in number of binucleated -- mouse n.a. Veninga (1970,
lymphocytes. unpublished)
110 (0.06) 93 24 Decrease in blood choline esterase -- rat 15 (15) Eglite (1968)
activity.
a Number of animals showing effects/total number of animals; numbers in brackets refer to control groups.
b Not available.
Table 10. Experimental animal studies--continued
II. Systemic reactions and other effects
2. Effects on reproduction
Ozone Length of exposure
concentration Number
of
µg/m3 (ppm) number h/day Effects Responsea Species animals Reference
of days
400 (0.2) 5 per 7 Increase in neonatal mortality. n.a. mouse n.a. Veninga (1967)
week x
gestation
period +
1st 3
weeks of
life
200- (0.1 5 per 7 Increase in neonatal mortality. n.a. mouse n.a. Brinkman et al.
400 -0.2) week x (1964)
3 weeks
1000- (0.5 1 1 Decrease in the amplitude of evoked -- rat 3 (3) Xintaras et al.
2000 -1.0) response to flash. (1966)
a Number of animals showing effects/total number of animals; numbers in brackets refer to control groups.
b Not available.
Table 10. Experimental animal studies--continued
II. Systemic reactions and other effects
3. Behavioural changes
Ozone Length of exposure
concentration Number
of
µg/m3 (ppm) number h/day Effects Responsea Species animals Reference
of days
1000 (0.5) 1 0.5 increase in simple and choice reaction -- rhesus 4 (4) Reynolds &
time. monkey Chaffee (1970)
1000 (0.5) 1 0.75 Significantly reduced motor activity. -- rat 12 (12) Konigsberg &
Bachman
(1970)
600- (0.3 60 intermittently Increase in time to learn specific tasks. -- rat 6 (6) Litt et al.
1000 -0.5) (variable (1966)
intervals)
400 (0.2) 1 6 Reduction in spontaneous running -- mouse 9 (9) Murphy et al.
activity. (1964a)
4. Miscellaneous extrapulmonary changes
1500 (0.75) 1-2 4-8, 24 Histological changes in parathyroid -- rabbit 16 (16) Atwal & Wilson
gland. (1974); Atwal
et al. (1975)
400 (0.2) 21 5 Structural changes in myocardial -- rabbit n.a. Brinkman et al.
muscle fibres. & mouse (1964)
400 (0.2) 1 5 Increase in chromosomal breaks in -- hamster 8 (4) Zelac et al.
circulating lymphocytes. (1971a,b)
a Number of animals showing effects/total number of animals; numbers in brackets refer to control groups.
b Not available.
6. EFFECTS ON MAN
6.1 Controlled Exposures
A considerable number of studies have been performed, under
controlled conditions, on the effects of ozone on both healthy
subjects and patients, with their consent. Some of these studies have
provided useful information for the evaluation of exposure-effect
relationships. A few in vitro studies using human tissues have also
helped to clarify the mechanisms of the biological actions of ozone.
There have been few human studies on other oxidants.
6.1.1 In vitro effects on human tissues
Brinkman et al. (1964) found that in vitro exposure of human red
blood cells to an ozone concentration of 0.5 mg/m3 (0.25 ppm) for
30 min accelerated sphering of these cells by X-ray irradiation,
compared with unexposed cells. Chromosome breakages in human cell
(epidermoid carcinoma cell) cultures exposed to an ozone concentration
of 16 mg/m3 (8 ppm) for 5 or 10 min were equivalent to those produced
by X-rays (200R, 250 kV) (Fetner, 1962).
The production of interferon was suppressed when human tonsil
lymphocytes were exposed in vitro to an ozone concentration of
10 mg/m3 (5 ppm) for 3 h (Watanabe et al., 1973a).
Goldstein (1976) measured the combined effects of ozone at
4-82 mg/m3 (2-41 ppm) and nitrogen dioxide at 6.8-190 mg/m3
(3.6-102 ppm) on human red cells, in vitro, and found that the
absolute and relative concentrations of the pollutants as well as the
sequence of administration could affect the interaction. In general,
the effects of the two pollutants on the variables measured (osmotic
fragility, acetylcholin-esterase (3.1.1.7) activity, lipid
peroxidation, reduced glutathione, and methaemoglobin levels) were
additive. At lower pollutant doses, a synergistic effect on the
increase in lipid peroxides was reported. Ozone also potentiated the
formation of methaemoglobin due to the action of nitrogen dioxide.
6.1.2 Sensory effects
The effects of ozone or oxidants on sensory organs have been
studied in terms of eye irritation, changes in visual parameters, and
olfactory thresholds.
Eye irritation in two groups of 20 female telephone company
employees working in identical adjacent rooms was evaluated by
Richardson & Middleton (1957, 1958) in relation to oxidant
concentrations from May to November 1956. Activated-carbon and dummy
air-filter media were switched periodically between the two rooms so
that the groups were alternately exposed to test and control
conditions. In all cases, differences in eye irritation between the
activated-carbon filtered and nonfiltered test conditions were highly
significant (p < 0.01). The scatter diagram (Fig. 8) suggests the
existence of a threshold for eye irritation at an oxidant
concentration of approximately 200 µg/m3 (0.10 ppm).
 The index of eye irritation increased progressively as oxidant
concentrations exceeded this value. No significant correlations
between eye irritation and concentrations of nitrogen dioxide or
suspended particulates were observed. However, in interpreting these
results, care must be exercised in drawing conclusions regarding
cause-effect relationships, as other controlled exposure studies have
shown that ozone is not an eye irritant. By comparison,
peroxyacylnitrates, acrolein, and peroxybenzoyl-nitrates have all been
shown to be strong eye irritants (Heuss & Glasson, 1968; Schuck &
Doyle, 1959; Stephens et al., 1961). Each of these compounds is a
product of the photochemical reaction system and thus is highly
correlated in time with measured levels of oxidants or ozone.
Lagerwerff (1963) measured the effect of exposure to ozone on
visual parameters in 22 male and 6 female volunteers. The subjects
were exposed to ozone at 400, 700, and 1000 µg/m3 (0.20, 0.35, and
0.50 ppm) for 3 h, and again for 6 h, with 10 days rest between
exposures. Visual acuity, depth perception, lateral and vertical
phoria, divergence and convergence, near vision, and peripheral,
colour, and night vision were tested. Considerable decreases in visual
acuity in scotopic and mesopic ranges, increases in peripheral vision,
changes in extra ocular muscle balance, and decrease in night vision
were observed in the majority of subjects. A few subjects also
developed a nonproductive cough at the highest ozone concentration,
and several complained of difficulties with mental concentration at
these levels.
Studies of the olfactory threshold in 10-14 male volunteer test
subjects exposed for 30 min to a series of different ozone
concentrations were performed by Henschler et al. (1960). The lowest
ozone concentration used, 40 µg/m3 (0.02 ppm), was recognized
immediately by 9 out of 10 subjects. The subjects reported that the
odour diminished rapidly, and that it was no longer perceptible within
´-12 min. When exposed to an ozone concentration of 100 µg/m3
(0.05 ppm), 13 out of 14 subjects indicated that the odour was
considerably stronger and that it lasted longer (2-30 min, with an
average duration of 13 min). In a study by Eglite (1968), it was found
that the threshold of smell for ozone was 15 µg/m3 (0.008 ppm) for
the most sensitive subject in a group of 20 persons.
6.1.3 Effects on respiratory function
6.1.3.1 Exposure to ozone
A considerable number of controlled studies on exposure to ozone
have been reported. However, with one exception, all these studies
were concerned with short-term exposures of less than 6 h.
A highly significant fall in steady state diffusion capacity and
0.75-second forced expiratory volume (FEVo.75) was noted in each of 16
test replicates on 10 men and 1 woman aged 20-45 years, exposed to
ozone concentrations of 1200-1600 µg/m3 (0.6 to 0.8 ppm) for 2 h
(Young et al., 1964).
Goldsmith & Nadel (1969) exposed 4 healthy males, for 1 h, to
ozone concentrations of 200, 800, 1200, and 2000 µg/m3 (0.1, 0.4,
0.6, and 1.0 ppm). Consistent increases in airway resistance (Raw)
were demonstrated only with exposure to 2000 µg/m3 (1 ppm). Lower
concentrations caused an increase in Raw in some subjects, but a
clear dose-effect relationship could not be established at levels
below 2000 µg/m3 (1 ppm). When exposed to an ozone concentration of
1500 µg/m3 (0.75 ppm) for 2 h, 10 healthy males exhibited significant
increases in Raw, reductions in maximum static elastic recoil
pressure of the lung, and a fall in maximum flow at 50% of vital
capacity (Bates et al., 1972). Exercise on a bicycle ergometer during
ozone exposure accentuated these pulmonary function changes, and most
subjects complained of substernal soreness and cough at the end of a
2-h period. These observations were extended by Hazucha et al. (1973)
to include an ozone concentration of 740 µg/m3 (0.37 ppm) for 2 h and
subjects were intermittently exercised during exposure. After exposure
to 740 and 1500 µg/m3 (0.37 and 0.75 ppm) for 2 h, both smokers and
non-smokers (6 subjects per group) revealed significant decreases in
forced vital capacity (FVC), one-second forced expiratory volume
(FEV1.0), mid-maximal expiratory flow rate (MMFR), and maximum
expiratory flow rate at 50% of vital capacity (MEFR 50%), but the
effects were greater at 1500 µg/m3 (0.75 ppm). These effects were
closely related to the changes measured in the closing volume and
indicated an early effect in the small airways.
Seven healthy males, exposed to an ozone concentration of
1000 µg/m3 (0.50 ppm) for 2 h while performing intermittent light
exercise, showed decreases in lung function measurements and oxidative
changes in erythrocytes; 3 subjects had symptoms such as cough,
substernal discomfort, and malaise. When 2 healthy and 3 sensitive
subjects (those with a prestudy history of cough, chest discomfort, or
wheezing associated with allergy or air pollution exposure, but with
normal base line pulmonary function studies) were exposed to an ozone
concentration of 740 µg/m3 (0.37 ppm) for 2 h under the same
conditions of exercise, there was a significant increase in the total
respiratory resistance compared with the pre-exposure resistance.
Oxidative biochemical changes were detected in the blood of this
group, but were not as severe as in those exposed to an ozone
concentration of 1000 µg/m3 (0.5 ppm). Three healthy and 3 sensitive
subjects exposed to 500 µg/m3 (0.25 ppm) for 2 h did not show any
consistent physiological changes attributable to exposure (Hackney et
al., 1975). All the subjects of the above study were southern
Californians. In order to study possible adaptation of these subjects
to long-term ambient ozone exposure, the same investigators compared
the effects of ozone exposure on southern Californians with those on
Canadians and found that the latter were more responsive (Hackney et
al., 1977).
Ohmori (1974) found increased breathing frequency and volume in 4
healthy males who were exposed under exercise to ozone concentrations
of 200-500 µg/m3 (0.1-0.25 ppm) for 30 min. Four normal male subjects
exposed for 5 min to a combination of ozone at 1800 µg/m3 (0.9 ppm)
and light exercise showed a highly significant decrease in specific
airway conductance (Kagawa & Toyama, 1975a). Folinsbee and co-workers
(1975) tested the reaction of 28 normal adults (20 males, 8 females)
exposed to ozone at concentrations of 740, 1000, or 1500 µg/m3 (0.37,
0.50, or 0.75 ppm) for 2h: the subjects were either at rest or
exercising intermittently with sufficient intensity to increase lung
ventilation by a factor of 2.5. There was a gradation of complaints
depending on the ozone concentration. Under exercise, increase in
respiratory frequency was closely correlated with the total dose of
ozone and, at any given concentration, the effect was greater with the
subject who had exercised. Statistically significant decreases were
found in FVC at ozone concentrations of 1000 µg/m3 (0.50 ppm) or
more. Decrease in MEFR occurred in 50% of subjects at concentrations
of 740 µg/m3 (0.37 ppm) or more.
The effects of ozone on pulmonary function were studied in 22
young, healthy, male, nonsmokers who were exposed to 800 µg/m3
(0.4 ppm) for 2 and 4 h. Subjects were seated during the exposure
except for 2 exercise periods of 15 min (bicycle), beginning after 1
and 3 h of exposure, respectively. Significant changes in FVC, MMFR
and Raw occurred after 2 h of exposure. Borderline effects were
reported for FEV1.0, V50 (expiratory flow rate at 50% FVC) and V25
(expiratory flow at 25% FVC). After 4 h of exposure, changes in all of
these lung function variables became statistically significant (Rummo
et al., 1975, unpublished data).a In studies by Kerr et al. (1975),
20 lightly exercising subjects (19 males and 1 female) were exposed to
an ozone concentration of 1000 µg/m3 (0.5 ppm) for 6 h. Subjects,
particularly nonsmokers, commonly reported dry cough and chest
discomfort. Significant changes were produced in airway conductance,
pulmonary resistance, and forced expiratory volumes, but not in
diffusing capacity.
a Rummo, N.J., Knelson, I. H., Lassiter, S., Cram, J. J., & House,
D. (1975). Effects of ozone on pulmonary function in healthy young
men. Research Triangle Park, NC, US Environmental Protection Agency,
17 pp. (In-house Technical Report).
Changes in the pulmonary function of 11 male subjects, aged 24-38
years, exposed to an ozone concentration of 200 µg/m3 (0.1 ppm) for
2 h, were compared with a 1-h pre- and post-control period without
ozone and with a control series without ozone. Under test conditions,
which included intermittent light exercise, a significant increase in
Raw and in the alveolar to arterial oxygen pressure difference
(AaDO2) was observed in 7 out of 11 subjects (yon Nieding et al.,
1977).
In an attempt to study the effects of long-term repeated exposures
to ozone, Bennet (1962) exposed 2 groups of 6 healthy males to
400 µg/m3 (0.2 ppm) or 1000 µg/m3 (0.5 ppm) respectively, for 3 h
per day, 6 days per week for 12 weeks. The 400 µg/m3 (0.2 ppm)
exposure group did not experience any symptoms or changes in the
forced expiratory volume. While the second group was also
asymptomatic, the FEV1.0 showed a significant decrease during the last
few weeks of exposure.
6.1.3.2 Exposure to mixtures of ozone and other air pollutants
Bates & Hazucha (1973) demonstrated a synergistic action of ozone
at 740 µg/m3 (0.37) and sulfur dioxide at 960 µg/m3 (0.37 ppm). With
4 normal subjects engaged intermittently in light exercise, a 2-h
exposure to sulfur dioxide, only, at 960 µg/m3 (0.37 ppm) did not
produce any changes in FVC, FEV1.0, MMFR, and MEFR 50%, while exposure
to an ozone concentration of 740 µg/m3 (0.37 ppm) caused a 10-15%
reduction in these lung function variables. However, exposure to a
combination of these gases at the same concentrations resulted in a
20-45% decline in lung function measurements and this effect was even
greater than the changes caused by a 2-h exposure to an ozone
concentration of 1500 µg/m3 (0.75 ppm). On the other hand, addition
of nitrogen dioxide at a concentration of 560 µg/m3 (0.30 ppm) to
ozone at 500 µg/m3 (0.25 ppm) did not result in any decrement in the
lung functions of 3 healthy and 3 sensitive (section 6.1.3.1) subjects
(Hackney et al., 1975a).
In a series of studies already mentioned in section 6.1.3.1, von
Nieding et al. (1977) found that the effects of exposure to an ozone
concentration of 200 µg/m3 (0.1 ppm) were not enhanced by combination
with nitrogen dioxide at 9400 µg/m3 (5 ppm), or by combination with
nitrogen dioxide at 9400 µg/m3 (5 ppm) and sulfur dioxide at
13 000 µg/m3 (5 ppm), though recovery time was delayed in the last
experiment. Exposure for 2 h to a combination of ozone at 50 µg/m3
(0.025 ppm), nitrogen dioxide at 100 µg/m3 (0.05 ppm), and sulfur
dioxide at 260 µg/m3 (0.1 ppm) did not have any effect on Raw, or on
AaDO2. However, there was a dose-dependent increase in the
sensitivity to acetylcholine of the bronchial tree compared with the
controls. This effect was observed by measuring the increase in Raw
after inhalation of 2% acetylcholine alone and in combination with the
previously mentioned mixture of gases at the same concentrations. The
effect became more pronounced when the acetylcholine was combined with
ozone at 200 µg/m3 (0.1 ppm), nitrogen dioxide at 9400 µg/m3
(5 ppm), and sulfur dioxide at 13 000 µg/m3 (5 ppm).
6.1.3.3 Exposure to peroxyacetylnitrate alone or in combination with
carbon monoxide
Thirty-two male college students were exposed to a peroxyacetyl-
nitrate concentration of 1500 µg/m3 (0.3 ppm) for 5 min while
exercising on a bicycle ergometer (Smith, 1965). A statistically
significant increase in oxygen uptake was observed during exercise, in
comparison with that observed when filtered air was breathed. MEFR was
significantly decreased by exposure to this gas during the recovery
phase following exercise. However, peroxyacetylnitrate did not have
any effect when the subjects were at rest.
The metabolic, temperature, and cardiorespiratory reactions of 20
healthy males (10 smokers and 10 nonsmokers) were monitored while
working to their maximum and breathing filtered air or 3 different gas
mixtures at 25 ± 0.5°C and a relative humidity of 20 ± 2% (Raven et
al., 1974). The mixtures were carbon monoxide in filtered air at
57 500 µg/m3 (50 ppm), peroxyacetylnitrate in filtered air at
1350 µg/m3 (0.27 ppm), and a combination of the two. Maximum aerobic
capacity did not decrease significantly in either group during
exercise and exposure to any of the pollutant gas mixtures compared
with filtered air. For both smokers and nonsmokers exposure to
peroxyacetylnitrate and carbon monoxide alone or in combination, while
exercising to maximum aerobic capacity, produced minor alterations in
cardiorespiratory and temperature regulating variables.
6.1.3.4 Exposure to irradiated automobile exhaust
Fourteen college students were exposed on 2 occasions to
irradiated automobile exhaust and measurements were made of reaction
time, vital capacity, and submaximum work performance on the bicycle
ergometer. The individuals were exposed to a mixture of the following
gases: carbon monoxide, carbon dioxide, nitric oxide, nitrogen
dioxide, hydrocarbons, aldehydes, formaldehyde, and oxidants (Table
12, section IID). It appeared that exposure to this mixture of
pollutants had little effect on the types of human motor performance
chosen for this study (Holland et al., 1968).
6.1.3.5 Exposure to ambient air containing elevated concentrations of
oxidants
The effect of oxidant air pollution on patients with chronic
obstructive lung disease was studied by Motley et al. (1959). Twenty
normal individuals and 46 patients with chronic lung disease were
evaluated by measuring lung volumes, forced expiratory volumes, and
nitrogen washout before and after removal from ambient Los Angeles air
into a room with clean filtered air. No significant changes were
detected in the pulmonary function of normal subjects or in patients
with chronic lung disease who entered the room on nonsmoggy days.
However an improvement in lung function, particularly a decrease in
residual lung volume and nitrogen washout time, occurred among
patients who entered the filtered room on smoggy days and remained for
40 or more hours. Simultaneous measurements of oxidants were not
obtained at the study site, but during the study, ambient hourly
oxidant concentrations at nearby monitoring stations ranged from
400-1400 µg/m3 (0.2-0.7 ppm). Subjects with chronic lung disease, who
remained in the filtered air for only 18-20 h, did not experience any
significant improvement in lung function.
In a similar study, Balchum (1973) observed the pulmonary function
of 15 patients with moderately severe chronic obstructive lung
disease, who spent one week in a room without air filtration and a
second week in clean filtered air. During the first week, when
unfiltered ambient air was drawn into the room, 1-h oxidant
concentrations averaged 220 µg/m3 (0.11 ppm) and ranged up to
400 µg/m3 (0.2 ppm). During the second week, when air filters were
activated, oxidant exposures ranged from 40 to 60 µg/m3 (0.02 to
0.03 ppm). Comparison of the effects of unfiltered and filtered air
revealed a decrease in airway resistance and an increase in the
arterial pO2 during the week of filtered air breathing. Changes were
observed both at rest and during exercise in about 75% of the
subjects. Decreases in airway resistance first became apparent after
breathing filtered air for 48 h.
6.1.4 Changes in electroencephalograms
Changes (decrease in the alpha rhythms) in electroencephalograms
were studied by Eglite (1968) in relation to exposure to ozone for
3 min. All 3 healthy subjects studied showed changes when exposed to
an ozone concentration of 20 µg/m3 (0.01 ppm) and 1 subject reacted
at a concentration at 10 µg/m3 (0.005 ppm).
6.1.5 Chromosomal effects
In a recent study by Metz et al (1975), 6 human volunteers were
exposed to an ozone concentration of 1 mg/m3 (0.5 ppm) for 6-10 h,
and the circulating lymphocytes were examined for chromosomal changes.
Although no chromosome aberrations were found, there was a significant
increase in the number of minor chromosomal abnormalities (chromatid
deletions) compared with pre-exposed lymphocytes.
6.2 Industrial Exposure
Several studies on the effects of industrial exposure have been
reported, but in most of them, the effects of ozone have been
confounded by the coexistence of other pollutants and a threshold
concentration has not been determined.
Kleinfield et al. (1957) reported several cases of severe ozone
intoxication in welders using an inert gas-shielded consumable
electrode which greatly increased the ultraviolet radiation. Ozone was
measured at the breathing zone in 3 plants using this welding
technique. No complaints or clinical findings were associated with
ozone concentrations of 500 µg/m3 (0.25 ppm) or less. At
concentrations of 600-1600 µg/m3 (0.3-0.8 ppm), an increasing number
of welders complained of chest constriction and irritation of the
throat.
Similar findings using this welding technique were reported by
Challen et al. (1958), when 11 out of 14 workers complained of
respiratory symptoms at ozone concentrations in the range of
1600-3400 µg/m3 (0.8-1.7 ppm). Symptoms disappeared when ozone levels
were reduced to 400 µg/m3 (0.2 ppm). Young et al. (1963) failed to
detect any significant changes in lung function tests (vital capacity,
functional residual capacity, MMFR, FEV0.75, or diffusing capacity) in
7 men engaged in argon-shielded electric arc welding when ozone
concentrations were 400-600 µg/m3 (0.2-0.3 ppm).
Kudrjavceva (1963) described a complex of symptoms that included
headache, weakness, increased muscular excitability, and decreased
memory among workers engaged in the manufacture of hydrogen peroxide
and exposed to ozone concentrations ranging from 500-800 µg/m3
(0.25-0.40 ppm) for 7-10 years. The investigator suggested that these
reactions might be due to prolonged exposure to ozone under working
conditions. Increased prevalence of bronchitis and emphysema,
accompanied by decreased expiratory flow rate, was also reported in
workers involved in the manufacture of hydrogen peroxide by Nevskaja &
Diterihs (1957). Ozone concentrations of 80-1000 µg/m3
(0.04-0.50 ppm) were measured; sulfuric acid aerosol was also present.
Control groups were not included in either of these studies.
In several countries, occupational exposure limits for ozone have
been set as maximum, time-weighted averages for an 8-h workday or 40-h
week (ILO, 1977). In some countries, the exposure limit is 100 µg/m3
(0.05 ppm), in others, 200 µg/m3 (0.1 ppm). In addition, a short-term
exposure limit of 600 µg/m3 (0.3 ppm), for periods up to 15 min, has
been tentatively proposed by the USA provided that there are not more
than 4 such periods per day with at least 60 min between each period,
and that the daily time-weighted average is not exceeded. The German
Democratic Republic has set a limit of 200 µg/m3 for exposure periods
not exceeding 30 min and the USSR has proposed an 8-h mean value of
100 µg/m3 (0.05 ppm).
6.3 Community Exposure
Epidemiological studies on the association between human health
effects and exposure to photochemical oxidants have largely been
carried out in the Los Angeles air basin. For most of these studies,
investigators made use of measurements of photochemical oxidants
obtained from a network of air monitoring stations operated by the Los
Angeles County Air Pollution Control District. Oxidants were measured
by the unbuffered potassium iodide method which yields oxidant values
15-25% lower than the values obtained with the 1 or 2% neutral-
buffered potassium iodide method more commonly used in regions outside
the Los Angeles air basin.
As in all studies on urban populations, the observed health
effects of photochemical oxidant exposure cannot be attributed only to
oxidants. Photochemical smog typically consists of ozone, nitrogen
dioxide, peroxyacylnitrates and other nitrate compounds, sulfates,
other particulate aerosols, and reducing agents. In combination, these
pollutants may have an independent, additive, or synergistic effect on
human health. In general, however, ozone appears to be the most
biologically active pollutant and the correlations between health
effects and pollutant exposure were found when results concerning
ozone, rather than other identified pollutants, were statistically
analysed. Since controlled exposure studies in man and animals confirm
the greater biological reactivity of ozone compared with other
components of photochemical smog, it seems reasonable to conclude, for
the purpose of developing health protection guidelines, that ozone is
the principal agent responsible for the exposure-response
relationships observed in epidemiological studies on photochemical
oxidants.
6.3.1 Mortality
Studies conducted by the California State Department of Public
Health (1955, 1956) over the periods July-November 1953 and 1954, and
July-December 1955 revealed that, during these months, the daily
mortality rate of Los Angeles residents, aged 65 or over, was strongly
influenced by a heat wave but was not consistently altered either by
variations in oxidant concentrations or by the occurrence of smog-
alert days (ozone concentrations of 600 µg/m3 (0.3 ppm) or more).
No significant correlations were found by Massey et al. (1961,
unpublished data) between daily mortality rates and daily oxidant
levels in an analysis of two areas of Los Angeles County, selected for
similarities in temperature and for differences in air pollution
levels (values not given). Hechter & Goldsmith (1961) also failed to
find a significant correlation between monthly mortality rates due to
cardiorespiratory diseases and pollutant levels (monthly means of
daily maxima that ranged from 80 to 400 µg/m3; 0.04-0.2 ppm) in Los
Angeles County for the years 1956-58. The authors made Fourier curve
analyses of the data in order to remove the major effect of season of
year on mortality rate and pollutant levels. An association between
respiratory and cardiac deaths and Los Angeles smog episodes with 1-h
concentrations exceeding 400 µg/m3 (0.2 ppm) was observed by Mills
(1957) but he failed to take into account seasonal fluctuations in
deaths and pollutants. However, it is possible that the method of
adjustment for seasonal effects might mask a real effect of pollutant
concentrations on mortality. Therefore no conclusive statement can be
made concerning the lack of an association between mortality rate and
short-term variations in oxidant levels.
A model of daytime wind flow over Los Angeles was constructed by
Mahoney (1971) to divide the city into 5 wind zones each about
10 kilometres in width and representing distance downwind along the
path of air flow. The mortality rate of the white population, adjusted
for age, sex, and income level, from noncancerous respiratory diseases
during 1961 increased in successive downwind zones affected by the Los
Angeles sea breeze. The adjusted mortality rate in the wind zone
immediately adjacent to the Pacific Ocean was 53 per 100 000, while
mortality in the afferent wind zone most remote from the ocean was
111 per 100 000. The author suggested that this geographical
difference in mortality might be consistent with an effect of
temperature, humidity, or oxidant air pollution.
6.3.2 Annoyance and irritation
In Los Angeles, 75% of the population complained that they were
"bothered" by air pollution either at home or at work compared with
24% in San Francisco and 22% in the "rest of the State". Eye
complaints, which were relatively high (33%) in Los Angeles County and
in the "rest of the State", were low (14%) in the San Francisco Bay
area. However, fewer cases of hayfever and sinus trouble were reported
in Los Angeles County compared with other areas (Hausknecht, 1960).
The association of oxidant levels with eye irritation was
investigated (Renzetti & Gobran, 1957) in a panel of office and
factory workers and a second panel of scientists residing in the Los
Angeles Basin. The data demonstrated increasing eye irritation with
increasing maximum instantaneous oxidant concentrations over a range
of values from 100 to 900 µg/m3 (0.05-0.45 ppm).
Daily symptoms associated with eye and respiratory irritation were
studied by Hammer et al. (1974) in a group of Los Angeles student
nurses in relation to daily oxidant levels. Headache, eye discomfort,
cough, and chest discomfort were all found to be related to daily
maximum hourly oxidant concentrations. On days when maximum hourly
concentrations were 800-1000 µg/m3 (0.40-0.50 ppm), students reported
48% more cough and 100% more chest discomfort compared with days when
oxidant levels were below the US air quality standard of 160 µg/m3
(0.80 ppm). Using "hockey stick" functions,a the threshold levels
have been determined as maximum 1-h concentrations of 100 µg/m3
(0.05 ppm) for headache, 300 µg/m3 (0.15 ppm) for eye irritation,
530 µg/m3 (0.27 ppm) for cough, and 580 µg/m3 (0.29 ppm) for chest
discomfort. Exposure-response relationships are shown in Table 11.
6.3.3 Athletic performance
The effect of oxidant concentrations on athletic performance was
studied in Los Angeles by Wayne et al. (1967) in 21 competitive team
events for High school cross-country runners from 1959-64. Since
running times tended to improve throughout the season, team
performance at a meeting was evaluated by determining the percentage
of individual athletes who failed to improve when their running time
was compared with that of their previous performance on the same
course. Oxidant levels in the hour before the meeting, which ranged
from below 100 µg/m3 (0.05 ppm) to 600 µg/m3 (0.3 ppm), were highly
correlated ( r=0.88) with decreased performance, as shown in Fig. 9.
Consistently high correlations were obtained when the observations
were divided into the periods 1959-1961 ( r=0.945) and 1962-64
( r=0.945). Correlations with other pollutants (carbon monoxide and
particulates) and with meteorological variables (temperature, relative
humidity, wind velocity, wind direction) were considerably lower and
usually not significant. The authors speculated that the observed
oxidant-athletic performance relationship could be due to a direct
effect on oxygen use or to respiratory discomfort associated with
exercise in an atmosphere containing a high concentration of oxidants.
A statistical test for threshold values, based on segmental regression
analysis ("hockey stick" functions) applied to these data (Barth et
al., 1971; Hasselblad et al., 1976) gave a threshold estimate of
240 µg/m3 (0.12 ppm) (1-h value) with a 95% confidence interval of
134 to 326 µg/m3 (0.067-0.163 ppm).
a The term hockey stick function has been used by Hammer et al.
(1974) and Hasselblad et al. (1976) to describe a function
consisting of a curve with zero slope up to a certain point and
increasing monotonically from that point.
Table 11. Relative increase in headache, eye discomfort, cough, and chest discomfort in relation to photochemical oxidant
exposure of student nurses in Los Angelesa
Relative increase in symptom
Daily maximum 1-h oxidant Headache Eye discomfort Cough Chest discomfort
level
µg/m3 (ppm) Simple Adjustedb Simple Adjustedb Simple Adjustedb Simple Adjustedb
<160 (<0.08) 1.00 (16.4)c 1.00 (10.6) 1.00 (8.9) 1.00 (5.2) 1.00 (13.0) 1.00 (9.5) 1.00 (3.5) 1.00 (1.8)
180 (0.09) 0.97 1.00 1.01 1.07 1.02 1.07 0.91 1.05
200-280 (0.10-0.14) 0.95 1.03 0.96 1.13 0.91 0.99 0.97 1.00
300-380 (0.15-0.19) 0.95 1.07 1.12 1.33 0.95 1.02 1.05 0.94
400-480 (0.20-0.24) 0.95 1.08 1.37 1.77 0.89 0.96 0.89 0.89
500-580 (0.25-0.29) 1.01 1.07 1.67 2.15 0.95 1.01 1.03 1.11
600-780 (0.30-0.39) 1.03 1.26 2.37 3.42 1.16 1.23 1.17 1.27
800-1000 (0.40-0.50) 1.02 1.41 3.93 6.11 1.48 1.77 2.00 3.22
a From: Hammer et al. (1974).
b Excludes all days when the symptom was reported in conjunction with either "feverish", "chilly", or "temperature".
c Bracketed figure gives baseline symptom rate as mean daily percentage of symptom reported.
The index of eye irritation increased progressively as oxidant
concentrations exceeded this value. No significant correlations
between eye irritation and concentrations of nitrogen dioxide or
suspended particulates were observed. However, in interpreting these
results, care must be exercised in drawing conclusions regarding
cause-effect relationships, as other controlled exposure studies have
shown that ozone is not an eye irritant. By comparison,
peroxyacylnitrates, acrolein, and peroxybenzoyl-nitrates have all been
shown to be strong eye irritants (Heuss & Glasson, 1968; Schuck &
Doyle, 1959; Stephens et al., 1961). Each of these compounds is a
product of the photochemical reaction system and thus is highly
correlated in time with measured levels of oxidants or ozone.
Lagerwerff (1963) measured the effect of exposure to ozone on
visual parameters in 22 male and 6 female volunteers. The subjects
were exposed to ozone at 400, 700, and 1000 µg/m3 (0.20, 0.35, and
0.50 ppm) for 3 h, and again for 6 h, with 10 days rest between
exposures. Visual acuity, depth perception, lateral and vertical
phoria, divergence and convergence, near vision, and peripheral,
colour, and night vision were tested. Considerable decreases in visual
acuity in scotopic and mesopic ranges, increases in peripheral vision,
changes in extra ocular muscle balance, and decrease in night vision
were observed in the majority of subjects. A few subjects also
developed a nonproductive cough at the highest ozone concentration,
and several complained of difficulties with mental concentration at
these levels.
Studies of the olfactory threshold in 10-14 male volunteer test
subjects exposed for 30 min to a series of different ozone
concentrations were performed by Henschler et al. (1960). The lowest
ozone concentration used, 40 µg/m3 (0.02 ppm), was recognized
immediately by 9 out of 10 subjects. The subjects reported that the
odour diminished rapidly, and that it was no longer perceptible within
´-12 min. When exposed to an ozone concentration of 100 µg/m3
(0.05 ppm), 13 out of 14 subjects indicated that the odour was
considerably stronger and that it lasted longer (2-30 min, with an
average duration of 13 min). In a study by Eglite (1968), it was found
that the threshold of smell for ozone was 15 µg/m3 (0.008 ppm) for
the most sensitive subject in a group of 20 persons.
6.1.3 Effects on respiratory function
6.1.3.1 Exposure to ozone
A considerable number of controlled studies on exposure to ozone
have been reported. However, with one exception, all these studies
were concerned with short-term exposures of less than 6 h.
A highly significant fall in steady state diffusion capacity and
0.75-second forced expiratory volume (FEVo.75) was noted in each of 16
test replicates on 10 men and 1 woman aged 20-45 years, exposed to
ozone concentrations of 1200-1600 µg/m3 (0.6 to 0.8 ppm) for 2 h
(Young et al., 1964).
Goldsmith & Nadel (1969) exposed 4 healthy males, for 1 h, to
ozone concentrations of 200, 800, 1200, and 2000 µg/m3 (0.1, 0.4,
0.6, and 1.0 ppm). Consistent increases in airway resistance (Raw)
were demonstrated only with exposure to 2000 µg/m3 (1 ppm). Lower
concentrations caused an increase in Raw in some subjects, but a
clear dose-effect relationship could not be established at levels
below 2000 µg/m3 (1 ppm). When exposed to an ozone concentration of
1500 µg/m3 (0.75 ppm) for 2 h, 10 healthy males exhibited significant
increases in Raw, reductions in maximum static elastic recoil
pressure of the lung, and a fall in maximum flow at 50% of vital
capacity (Bates et al., 1972). Exercise on a bicycle ergometer during
ozone exposure accentuated these pulmonary function changes, and most
subjects complained of substernal soreness and cough at the end of a
2-h period. These observations were extended by Hazucha et al. (1973)
to include an ozone concentration of 740 µg/m3 (0.37 ppm) for 2 h and
subjects were intermittently exercised during exposure. After exposure
to 740 and 1500 µg/m3 (0.37 and 0.75 ppm) for 2 h, both smokers and
non-smokers (6 subjects per group) revealed significant decreases in
forced vital capacity (FVC), one-second forced expiratory volume
(FEV1.0), mid-maximal expiratory flow rate (MMFR), and maximum
expiratory flow rate at 50% of vital capacity (MEFR 50%), but the
effects were greater at 1500 µg/m3 (0.75 ppm). These effects were
closely related to the changes measured in the closing volume and
indicated an early effect in the small airways.
Seven healthy males, exposed to an ozone concentration of
1000 µg/m3 (0.50 ppm) for 2 h while performing intermittent light
exercise, showed decreases in lung function measurements and oxidative
changes in erythrocytes; 3 subjects had symptoms such as cough,
substernal discomfort, and malaise. When 2 healthy and 3 sensitive
subjects (those with a prestudy history of cough, chest discomfort, or
wheezing associated with allergy or air pollution exposure, but with
normal base line pulmonary function studies) were exposed to an ozone
concentration of 740 µg/m3 (0.37 ppm) for 2 h under the same
conditions of exercise, there was a significant increase in the total
respiratory resistance compared with the pre-exposure resistance.
Oxidative biochemical changes were detected in the blood of this
group, but were not as severe as in those exposed to an ozone
concentration of 1000 µg/m3 (0.5 ppm). Three healthy and 3 sensitive
subjects exposed to 500 µg/m3 (0.25 ppm) for 2 h did not show any
consistent physiological changes attributable to exposure (Hackney et
al., 1975). All the subjects of the above study were southern
Californians. In order to study possible adaptation of these subjects
to long-term ambient ozone exposure, the same investigators compared
the effects of ozone exposure on southern Californians with those on
Canadians and found that the latter were more responsive (Hackney et
al., 1977).
Ohmori (1974) found increased breathing frequency and volume in 4
healthy males who were exposed under exercise to ozone concentrations
of 200-500 µg/m3 (0.1-0.25 ppm) for 30 min. Four normal male subjects
exposed for 5 min to a combination of ozone at 1800 µg/m3 (0.9 ppm)
and light exercise showed a highly significant decrease in specific
airway conductance (Kagawa & Toyama, 1975a). Folinsbee and co-workers
(1975) tested the reaction of 28 normal adults (20 males, 8 females)
exposed to ozone at concentrations of 740, 1000, or 1500 µg/m3 (0.37,
0.50, or 0.75 ppm) for 2h: the subjects were either at rest or
exercising intermittently with sufficient intensity to increase lung
ventilation by a factor of 2.5. There was a gradation of complaints
depending on the ozone concentration. Under exercise, increase in
respiratory frequency was closely correlated with the total dose of
ozone and, at any given concentration, the effect was greater with the
subject who had exercised. Statistically significant decreases were
found in FVC at ozone concentrations of 1000 µg/m3 (0.50 ppm) or
more. Decrease in MEFR occurred in 50% of subjects at concentrations
of 740 µg/m3 (0.37 ppm) or more.
The effects of ozone on pulmonary function were studied in 22
young, healthy, male, nonsmokers who were exposed to 800 µg/m3
(0.4 ppm) for 2 and 4 h. Subjects were seated during the exposure
except for 2 exercise periods of 15 min (bicycle), beginning after 1
and 3 h of exposure, respectively. Significant changes in FVC, MMFR
and Raw occurred after 2 h of exposure. Borderline effects were
reported for FEV1.0, V50 (expiratory flow rate at 50% FVC) and V25
(expiratory flow at 25% FVC). After 4 h of exposure, changes in all of
these lung function variables became statistically significant (Rummo
et al., 1975, unpublished data).a In studies by Kerr et al. (1975),
20 lightly exercising subjects (19 males and 1 female) were exposed to
an ozone concentration of 1000 µg/m3 (0.5 ppm) for 6 h. Subjects,
particularly nonsmokers, commonly reported dry cough and chest
discomfort. Significant changes were produced in airway conductance,
pulmonary resistance, and forced expiratory volumes, but not in
diffusing capacity.
a Rummo, N.J., Knelson, I. H., Lassiter, S., Cram, J. J., & House,
D. (1975). Effects of ozone on pulmonary function in healthy young
men. Research Triangle Park, NC, US Environmental Protection Agency,
17 pp. (In-house Technical Report).
Changes in the pulmonary function of 11 male subjects, aged 24-38
years, exposed to an ozone concentration of 200 µg/m3 (0.1 ppm) for
2 h, were compared with a 1-h pre- and post-control period without
ozone and with a control series without ozone. Under test conditions,
which included intermittent light exercise, a significant increase in
Raw and in the alveolar to arterial oxygen pressure difference
(AaDO2) was observed in 7 out of 11 subjects (yon Nieding et al.,
1977).
In an attempt to study the effects of long-term repeated exposures
to ozone, Bennet (1962) exposed 2 groups of 6 healthy males to
400 µg/m3 (0.2 ppm) or 1000 µg/m3 (0.5 ppm) respectively, for 3 h
per day, 6 days per week for 12 weeks. The 400 µg/m3 (0.2 ppm)
exposure group did not experience any symptoms or changes in the
forced expiratory volume. While the second group was also
asymptomatic, the FEV1.0 showed a significant decrease during the last
few weeks of exposure.
6.1.3.2 Exposure to mixtures of ozone and other air pollutants
Bates & Hazucha (1973) demonstrated a synergistic action of ozone
at 740 µg/m3 (0.37) and sulfur dioxide at 960 µg/m3 (0.37 ppm). With
4 normal subjects engaged intermittently in light exercise, a 2-h
exposure to sulfur dioxide, only, at 960 µg/m3 (0.37 ppm) did not
produce any changes in FVC, FEV1.0, MMFR, and MEFR 50%, while exposure
to an ozone concentration of 740 µg/m3 (0.37 ppm) caused a 10-15%
reduction in these lung function variables. However, exposure to a
combination of these gases at the same concentrations resulted in a
20-45% decline in lung function measurements and this effect was even
greater than the changes caused by a 2-h exposure to an ozone
concentration of 1500 µg/m3 (0.75 ppm). On the other hand, addition
of nitrogen dioxide at a concentration of 560 µg/m3 (0.30 ppm) to
ozone at 500 µg/m3 (0.25 ppm) did not result in any decrement in the
lung functions of 3 healthy and 3 sensitive (section 6.1.3.1) subjects
(Hackney et al., 1975a).
In a series of studies already mentioned in section 6.1.3.1, von
Nieding et al. (1977) found that the effects of exposure to an ozone
concentration of 200 µg/m3 (0.1 ppm) were not enhanced by combination
with nitrogen dioxide at 9400 µg/m3 (5 ppm), or by combination with
nitrogen dioxide at 9400 µg/m3 (5 ppm) and sulfur dioxide at
13 000 µg/m3 (5 ppm), though recovery time was delayed in the last
experiment. Exposure for 2 h to a combination of ozone at 50 µg/m3
(0.025 ppm), nitrogen dioxide at 100 µg/m3 (0.05 ppm), and sulfur
dioxide at 260 µg/m3 (0.1 ppm) did not have any effect on Raw, or on
AaDO2. However, there was a dose-dependent increase in the
sensitivity to acetylcholine of the bronchial tree compared with the
controls. This effect was observed by measuring the increase in Raw
after inhalation of 2% acetylcholine alone and in combination with the
previously mentioned mixture of gases at the same concentrations. The
effect became more pronounced when the acetylcholine was combined with
ozone at 200 µg/m3 (0.1 ppm), nitrogen dioxide at 9400 µg/m3
(5 ppm), and sulfur dioxide at 13 000 µg/m3 (5 ppm).
6.1.3.3 Exposure to peroxyacetylnitrate alone or in combination with
carbon monoxide
Thirty-two male college students were exposed to a peroxyacetyl-
nitrate concentration of 1500 µg/m3 (0.3 ppm) for 5 min while
exercising on a bicycle ergometer (Smith, 1965). A statistically
significant increase in oxygen uptake was observed during exercise, in
comparison with that observed when filtered air was breathed. MEFR was
significantly decreased by exposure to this gas during the recovery
phase following exercise. However, peroxyacetylnitrate did not have
any effect when the subjects were at rest.
The metabolic, temperature, and cardiorespiratory reactions of 20
healthy males (10 smokers and 10 nonsmokers) were monitored while
working to their maximum and breathing filtered air or 3 different gas
mixtures at 25 ± 0.5°C and a relative humidity of 20 ± 2% (Raven et
al., 1974). The mixtures were carbon monoxide in filtered air at
57 500 µg/m3 (50 ppm), peroxyacetylnitrate in filtered air at
1350 µg/m3 (0.27 ppm), and a combination of the two. Maximum aerobic
capacity did not decrease significantly in either group during
exercise and exposure to any of the pollutant gas mixtures compared
with filtered air. For both smokers and nonsmokers exposure to
peroxyacetylnitrate and carbon monoxide alone or in combination, while
exercising to maximum aerobic capacity, produced minor alterations in
cardiorespiratory and temperature regulating variables.
6.1.3.4 Exposure to irradiated automobile exhaust
Fourteen college students were exposed on 2 occasions to
irradiated automobile exhaust and measurements were made of reaction
time, vital capacity, and submaximum work performance on the bicycle
ergometer. The individuals were exposed to a mixture of the following
gases: carbon monoxide, carbon dioxide, nitric oxide, nitrogen
dioxide, hydrocarbons, aldehydes, formaldehyde, and oxidants (Table
12, section IID). It appeared that exposure to this mixture of
pollutants had little effect on the types of human motor performance
chosen for this study (Holland et al., 1968).
6.1.3.5 Exposure to ambient air containing elevated concentrations of
oxidants
The effect of oxidant air pollution on patients with chronic
obstructive lung disease was studied by Motley et al. (1959). Twenty
normal individuals and 46 patients with chronic lung disease were
evaluated by measuring lung volumes, forced expiratory volumes, and
nitrogen washout before and after removal from ambient Los Angeles air
into a room with clean filtered air. No significant changes were
detected in the pulmonary function of normal subjects or in patients
with chronic lung disease who entered the room on nonsmoggy days.
However an improvement in lung function, particularly a decrease in
residual lung volume and nitrogen washout time, occurred among
patients who entered the filtered room on smoggy days and remained for
40 or more hours. Simultaneous measurements of oxidants were not
obtained at the study site, but during the study, ambient hourly
oxidant concentrations at nearby monitoring stations ranged from
400-1400 µg/m3 (0.2-0.7 ppm). Subjects with chronic lung disease, who
remained in the filtered air for only 18-20 h, did not experience any
significant improvement in lung function.
In a similar study, Balchum (1973) observed the pulmonary function
of 15 patients with moderately severe chronic obstructive lung
disease, who spent one week in a room without air filtration and a
second week in clean filtered air. During the first week, when
unfiltered ambient air was drawn into the room, 1-h oxidant
concentrations averaged 220 µg/m3 (0.11 ppm) and ranged up to
400 µg/m3 (0.2 ppm). During the second week, when air filters were
activated, oxidant exposures ranged from 40 to 60 µg/m3 (0.02 to
0.03 ppm). Comparison of the effects of unfiltered and filtered air
revealed a decrease in airway resistance and an increase in the
arterial pO2 during the week of filtered air breathing. Changes were
observed both at rest and during exercise in about 75% of the
subjects. Decreases in airway resistance first became apparent after
breathing filtered air for 48 h.
6.1.4 Changes in electroencephalograms
Changes (decrease in the alpha rhythms) in electroencephalograms
were studied by Eglite (1968) in relation to exposure to ozone for
3 min. All 3 healthy subjects studied showed changes when exposed to
an ozone concentration of 20 µg/m3 (0.01 ppm) and 1 subject reacted
at a concentration at 10 µg/m3 (0.005 ppm).
6.1.5 Chromosomal effects
In a recent study by Metz et al (1975), 6 human volunteers were
exposed to an ozone concentration of 1 mg/m3 (0.5 ppm) for 6-10 h,
and the circulating lymphocytes were examined for chromosomal changes.
Although no chromosome aberrations were found, there was a significant
increase in the number of minor chromosomal abnormalities (chromatid
deletions) compared with pre-exposed lymphocytes.
6.2 Industrial Exposure
Several studies on the effects of industrial exposure have been
reported, but in most of them, the effects of ozone have been
confounded by the coexistence of other pollutants and a threshold
concentration has not been determined.
Kleinfield et al. (1957) reported several cases of severe ozone
intoxication in welders using an inert gas-shielded consumable
electrode which greatly increased the ultraviolet radiation. Ozone was
measured at the breathing zone in 3 plants using this welding
technique. No complaints or clinical findings were associated with
ozone concentrations of 500 µg/m3 (0.25 ppm) or less. At
concentrations of 600-1600 µg/m3 (0.3-0.8 ppm), an increasing number
of welders complained of chest constriction and irritation of the
throat.
Similar findings using this welding technique were reported by
Challen et al. (1958), when 11 out of 14 workers complained of
respiratory symptoms at ozone concentrations in the range of
1600-3400 µg/m3 (0.8-1.7 ppm). Symptoms disappeared when ozone levels
were reduced to 400 µg/m3 (0.2 ppm). Young et al. (1963) failed to
detect any significant changes in lung function tests (vital capacity,
functional residual capacity, MMFR, FEV0.75, or diffusing capacity) in
7 men engaged in argon-shielded electric arc welding when ozone
concentrations were 400-600 µg/m3 (0.2-0.3 ppm).
Kudrjavceva (1963) described a complex of symptoms that included
headache, weakness, increased muscular excitability, and decreased
memory among workers engaged in the manufacture of hydrogen peroxide
and exposed to ozone concentrations ranging from 500-800 µg/m3
(0.25-0.40 ppm) for 7-10 years. The investigator suggested that these
reactions might be due to prolonged exposure to ozone under working
conditions. Increased prevalence of bronchitis and emphysema,
accompanied by decreased expiratory flow rate, was also reported in
workers involved in the manufacture of hydrogen peroxide by Nevskaja &
Diterihs (1957). Ozone concentrations of 80-1000 µg/m3
(0.04-0.50 ppm) were measured; sulfuric acid aerosol was also present.
Control groups were not included in either of these studies.
In several countries, occupational exposure limits for ozone have
been set as maximum, time-weighted averages for an 8-h workday or 40-h
week (ILO, 1977). In some countries, the exposure limit is 100 µg/m3
(0.05 ppm), in others, 200 µg/m3 (0.1 ppm). In addition, a short-term
exposure limit of 600 µg/m3 (0.3 ppm), for periods up to 15 min, has
been tentatively proposed by the USA provided that there are not more
than 4 such periods per day with at least 60 min between each period,
and that the daily time-weighted average is not exceeded. The German
Democratic Republic has set a limit of 200 µg/m3 for exposure periods
not exceeding 30 min and the USSR has proposed an 8-h mean value of
100 µg/m3 (0.05 ppm).
6.3 Community Exposure
Epidemiological studies on the association between human health
effects and exposure to photochemical oxidants have largely been
carried out in the Los Angeles air basin. For most of these studies,
investigators made use of measurements of photochemical oxidants
obtained from a network of air monitoring stations operated by the Los
Angeles County Air Pollution Control District. Oxidants were measured
by the unbuffered potassium iodide method which yields oxidant values
15-25% lower than the values obtained with the 1 or 2% neutral-
buffered potassium iodide method more commonly used in regions outside
the Los Angeles air basin.
As in all studies on urban populations, the observed health
effects of photochemical oxidant exposure cannot be attributed only to
oxidants. Photochemical smog typically consists of ozone, nitrogen
dioxide, peroxyacylnitrates and other nitrate compounds, sulfates,
other particulate aerosols, and reducing agents. In combination, these
pollutants may have an independent, additive, or synergistic effect on
human health. In general, however, ozone appears to be the most
biologically active pollutant and the correlations between health
effects and pollutant exposure were found when results concerning
ozone, rather than other identified pollutants, were statistically
analysed. Since controlled exposure studies in man and animals confirm
the greater biological reactivity of ozone compared with other
components of photochemical smog, it seems reasonable to conclude, for
the purpose of developing health protection guidelines, that ozone is
the principal agent responsible for the exposure-response
relationships observed in epidemiological studies on photochemical
oxidants.
6.3.1 Mortality
Studies conducted by the California State Department of Public
Health (1955, 1956) over the periods July-November 1953 and 1954, and
July-December 1955 revealed that, during these months, the daily
mortality rate of Los Angeles residents, aged 65 or over, was strongly
influenced by a heat wave but was not consistently altered either by
variations in oxidant concentrations or by the occurrence of smog-
alert days (ozone concentrations of 600 µg/m3 (0.3 ppm) or more).
No significant correlations were found by Massey et al. (1961,
unpublished data) between daily mortality rates and daily oxidant
levels in an analysis of two areas of Los Angeles County, selected for
similarities in temperature and for differences in air pollution
levels (values not given). Hechter & Goldsmith (1961) also failed to
find a significant correlation between monthly mortality rates due to
cardiorespiratory diseases and pollutant levels (monthly means of
daily maxima that ranged from 80 to 400 µg/m3; 0.04-0.2 ppm) in Los
Angeles County for the years 1956-58. The authors made Fourier curve
analyses of the data in order to remove the major effect of season of
year on mortality rate and pollutant levels. An association between
respiratory and cardiac deaths and Los Angeles smog episodes with 1-h
concentrations exceeding 400 µg/m3 (0.2 ppm) was observed by Mills
(1957) but he failed to take into account seasonal fluctuations in
deaths and pollutants. However, it is possible that the method of
adjustment for seasonal effects might mask a real effect of pollutant
concentrations on mortality. Therefore no conclusive statement can be
made concerning the lack of an association between mortality rate and
short-term variations in oxidant levels.
A model of daytime wind flow over Los Angeles was constructed by
Mahoney (1971) to divide the city into 5 wind zones each about
10 kilometres in width and representing distance downwind along the
path of air flow. The mortality rate of the white population, adjusted
for age, sex, and income level, from noncancerous respiratory diseases
during 1961 increased in successive downwind zones affected by the Los
Angeles sea breeze. The adjusted mortality rate in the wind zone
immediately adjacent to the Pacific Ocean was 53 per 100 000, while
mortality in the afferent wind zone most remote from the ocean was
111 per 100 000. The author suggested that this geographical
difference in mortality might be consistent with an effect of
temperature, humidity, or oxidant air pollution.
6.3.2 Annoyance and irritation
In Los Angeles, 75% of the population complained that they were
"bothered" by air pollution either at home or at work compared with
24% in San Francisco and 22% in the "rest of the State". Eye
complaints, which were relatively high (33%) in Los Angeles County and
in the "rest of the State", were low (14%) in the San Francisco Bay
area. However, fewer cases of hayfever and sinus trouble were reported
in Los Angeles County compared with other areas (Hausknecht, 1960).
The association of oxidant levels with eye irritation was
investigated (Renzetti & Gobran, 1957) in a panel of office and
factory workers and a second panel of scientists residing in the Los
Angeles Basin. The data demonstrated increasing eye irritation with
increasing maximum instantaneous oxidant concentrations over a range
of values from 100 to 900 µg/m3 (0.05-0.45 ppm).
Daily symptoms associated with eye and respiratory irritation were
studied by Hammer et al. (1974) in a group of Los Angeles student
nurses in relation to daily oxidant levels. Headache, eye discomfort,
cough, and chest discomfort were all found to be related to daily
maximum hourly oxidant concentrations. On days when maximum hourly
concentrations were 800-1000 µg/m3 (0.40-0.50 ppm), students reported
48% more cough and 100% more chest discomfort compared with days when
oxidant levels were below the US air quality standard of 160 µg/m3
(0.80 ppm). Using "hockey stick" functions,a the threshold levels
have been determined as maximum 1-h concentrations of 100 µg/m3
(0.05 ppm) for headache, 300 µg/m3 (0.15 ppm) for eye irritation,
530 µg/m3 (0.27 ppm) for cough, and 580 µg/m3 (0.29 ppm) for chest
discomfort. Exposure-response relationships are shown in Table 11.
6.3.3 Athletic performance
The effect of oxidant concentrations on athletic performance was
studied in Los Angeles by Wayne et al. (1967) in 21 competitive team
events for High school cross-country runners from 1959-64. Since
running times tended to improve throughout the season, team
performance at a meeting was evaluated by determining the percentage
of individual athletes who failed to improve when their running time
was compared with that of their previous performance on the same
course. Oxidant levels in the hour before the meeting, which ranged
from below 100 µg/m3 (0.05 ppm) to 600 µg/m3 (0.3 ppm), were highly
correlated ( r=0.88) with decreased performance, as shown in Fig. 9.
Consistently high correlations were obtained when the observations
were divided into the periods 1959-1961 ( r=0.945) and 1962-64
( r=0.945). Correlations with other pollutants (carbon monoxide and
particulates) and with meteorological variables (temperature, relative
humidity, wind velocity, wind direction) were considerably lower and
usually not significant. The authors speculated that the observed
oxidant-athletic performance relationship could be due to a direct
effect on oxygen use or to respiratory discomfort associated with
exercise in an atmosphere containing a high concentration of oxidants.
A statistical test for threshold values, based on segmental regression
analysis ("hockey stick" functions) applied to these data (Barth et
al., 1971; Hasselblad et al., 1976) gave a threshold estimate of
240 µg/m3 (0.12 ppm) (1-h value) with a 95% confidence interval of
134 to 326 µg/m3 (0.067-0.163 ppm).
a The term hockey stick function has been used by Hammer et al.
(1974) and Hasselblad et al. (1976) to describe a function
consisting of a curve with zero slope up to a certain point and
increasing monotonically from that point.
Table 11. Relative increase in headache, eye discomfort, cough, and chest discomfort in relation to photochemical oxidant
exposure of student nurses in Los Angelesa
Relative increase in symptom
Daily maximum 1-h oxidant Headache Eye discomfort Cough Chest discomfort
level
µg/m3 (ppm) Simple Adjustedb Simple Adjustedb Simple Adjustedb Simple Adjustedb
<160 (<0.08) 1.00 (16.4)c 1.00 (10.6) 1.00 (8.9) 1.00 (5.2) 1.00 (13.0) 1.00 (9.5) 1.00 (3.5) 1.00 (1.8)
180 (0.09) 0.97 1.00 1.01 1.07 1.02 1.07 0.91 1.05
200-280 (0.10-0.14) 0.95 1.03 0.96 1.13 0.91 0.99 0.97 1.00
300-380 (0.15-0.19) 0.95 1.07 1.12 1.33 0.95 1.02 1.05 0.94
400-480 (0.20-0.24) 0.95 1.08 1.37 1.77 0.89 0.96 0.89 0.89
500-580 (0.25-0.29) 1.01 1.07 1.67 2.15 0.95 1.01 1.03 1.11
600-780 (0.30-0.39) 1.03 1.26 2.37 3.42 1.16 1.23 1.17 1.27
800-1000 (0.40-0.50) 1.02 1.41 3.93 6.11 1.48 1.77 2.00 3.22
a From: Hammer et al. (1974).
b Excludes all days when the symptom was reported in conjunction with either "feverish", "chilly", or "temperature".
c Bracketed figure gives baseline symptom rate as mean daily percentage of symptom reported.
 6.3.4 Effects on children
Ventilatory performance, measured twice a month for 11 months by
the Wright Peak Flow Meter, in 78 elementary school children in two
cities in the Los Angeles Basin was unaffected by differences in the
daily mean oxidant levels which were 320 µg/m3 (0.16 ppm) and
220 µg/m3 (0.11 ppm) respectively (McMillan et al., 1969).
Kagawa & Toyama (1975b) and Kagawa et al. (1976) studied the
weekly variations in lung function (airway conductance and ventilatory
performance) of 20, normal, 11-year-old, school children in Tokyo in
relation to variations in temperature and ambient concentrations of
ozone, nitrogen dioxide, nitric oxide, sulfur dioxide, hydrocarbons,
and particulate matter. Students were tested from June 1972 to October
1973. Ozone levels were determined by the ethylene-chemiluminescence
method. Temperature was the factor most highly correlated with
variations in specific airway conductance (negative correlation) and
maximum expiratory flow rate (Vmax) at 25 and 50% FVC (positive
correlation). Significant negative correlations between ozone and
specific airway conductance, and between nitrogen dioxide, nitric
oxide, sulfur dioxide, and particulate matter and Vmax at 25 or 50%
FVC were observed in some children. The ranges of hourly pollutant
levels, at the time of the lung function test (13h00), that were used
for correlation during the study period were: 0-560 µg/m3
(0-0.28 ppm) for ozone, 40-550 µg/m3 (0.02-0.29 ppm) for nitrogen
dioxide, 30-340 µg/m3 (0.01-0.13 ppm) for sulfur dioxide, and
50-490 µg/m3 for particulate matter (Toyama et al., 1977).
The effect of oxidant air pollution exposure on the incidence and
duration of A2 influenza in an epidemic that occurred in 1968-69 was
studied retrospectively by Pearlman et al. (1971) in 3500 elementary
school children from 5 southern California communities. These areas
were selected to represent an exposure gradient for photochemical
oxidants, although no difference in community oxidant exposure was
present at the time (December 1968-January 1969) of the epidemic.
Information from school absenteeism, questionnaires on illness, and
haemagglutination-inhibition titres did not reveal any statistically
significant morbidity differences corresponding to the pollution
gradient that existed during the season of peak oxidant levels.
Several Japanese investigators have reported acute reactions among
school children exposed to moderately elevated concentrations of
oxidants of 200-400 µg/m3 (0.10-0.20 ppm), on smoggy days in a number
of urban areas. Not only eye and respiratory irritation, but systemic
symptoms such as paraesthesia, prostration, and convulsions were
observed. In reviewing these studies the WHO Task Group was aided by
several investigators and observers from Japan. In the opinion of the
Task Group, eye and respiratory effects observed during these episodes
may well have been caused by reported oxidant concentrations. However,
systemic symptoms could not be explained only by the observed oxidant
concentrations, but were more likely to be attributable to individual
psychosomatic reactions among the students. This judgement is
supported by the observation that acute systemic reactions were
observed in only some of the students, most of whom were exercising at
the time of peak oxidant levels. Thus, the students may well have
experienced acute irritation of the pharynx and trachea, enhanced by
their active status. However, the general population in the same
neighbourhood, including young children in primary grades and school
teachers, did not appear to have had these systemic symptoms. These
comments are based on the following studies.
Fujii (1972) reported the acute effects of an episode of
photochemical smog in Osaka, Japan, on 27 August 1971. The oxidant
concentration measured by the NBKI method rose to an hourly value of
360 µg/m3 (0.18 ppm) by 14h00. At this time, the peak ozone
concentration, determined by the chemiluminescent method was
480 µg/m3 (0.24 ppm) and the maximum sulfur dioxide concentration was
180 µg/m3 (0.07 ppm). A total of 249 school children reported
symptoms including pain in the throat, headache, coughing, breathing
difficulties, eye irritation, and numbness in the limbs. On 14
September 1971, maximum hourly ozone concentrations reached 480 µg/m3
(0.24 ppm) and a total of 290 persons complained of similar symptoms.
These data do not provide a basis for estimating symptom frequency in
the general population, because the total population at risk was not
reported.
In an evaluation of the clinical status of 15 high school children
who were hospitalized after the onset of acute symptoms that began
during photochemical smog episodes in Osaka, Japan, Adachi & Nakajima
(1974) stated that "they were astonished by the seriousness of the
conditions which surpassed by far the symptoms previously known in
connection with the relation between photochemical oxidant
concentration and its effect on man in the case of Los Angeles".
Affected students were not only found to have irritation of the eyes
and respiratory tract, but, systemic symptoms such as paraesthesia,
muscle spasms as well as an increased leukocyte count and borderline
elevation of serum alkaline phosphatase (3.1.3.1).
Based upon complaints found in schools that were registered with
local health centres located in Japanese cities, the Japan Public
Health Association (1976) reported a significant increase in
subjective complaints, particularly eye irritation, when photochemical
oxidant concentrations exceeded hourly values of 300 µg/m3
(0.15 ppm), as determined by the 10% NBKI method.
Mikami & Kudo (1973) described various local and systemic symptoms
in 82 school children that were attributed to 3 photochemical air
pollution episodes in Tokyo and Osaka in 1970, 1971, and 1972 with
maximum 1-h concentrations ranging from 300 to 580 µg/m3 (0.15 to
0.29 ppm). A large proportion of cases were reported to have eye
irritation, throat irritation, cough, breathlessness, headache, and
chills. The authors pointed out that many of these symptoms had not
been reported under Los Angeles smog conditions.
6.3.5 Effects on the incidence of acute respiratory and
cardiovascular diseases
In studies on college students in the Los Angeles Basin, Durham
(1974) showed that acute episodes of pharyngitis, bronchitis, and
upper respiratory infections were associated with peak concentrations
of oxidants and mean concentrations of sulfur dioxide and nitrogen
dioxide. Oxidants, sulfur dioxide, and nitrogen dioxide
(concentrations not given) were, in this order, consistently
associated with the various episodes of illness. A comparison of
selected high and low pollution days indicated that photochemical air
pollution might have been responsible for a 16.7% increase in acute
respiratory symptoms seen in Los Angeles schools situated in areas of
highest concentration compared with those in areas of lowest
concentration. The author's method of data presentation did not permit
the estimation of dose-response relationships or of threshold
concentrations.
Brant & Hill (1964) and Brant (1965) did not find any correlation
between admissions for cardiovascular conditions to Los Angeles County
Hospital and oxidant levels (170 µg/m3; 0.08 ppm as a mean value of
daily 7-h measurements for the study periods) either on the day of
admission or for 2 weeks prior to admission, for the period between 8
August and 25 December 1954. However, Sterling et al. (1966, 1967)
found statistically significant but very low correlations between
admissions to Los Angeles hospitals for respiratory episodes during
the period 17 March-26 October 1961 and daily mean levels of oxidants
(74 µg/m3; 0.037 ppm), ozone (84 µg/m3; 0.042 ppm), and carbon
monoxide (11 mg/m3; 9.5 ppm). Because of the limited time period and
the extremely low correlation coefficients, it is difficult to
conclude that a real relationship was demonstrated by these studies.
6.3.6 Effects on the prevalence of chronic respiratory diseases and
on pulmonary function
Deane et al. (1965) conducted a survey on the prevalence of
chronic respiratory disease in outdoor male telephone workers in San
Francisco and Los Angeles. Symptoms of persistent cough and phlegm
were slightly less prevalent in Los Angeles than in San Francisco in
the 40-49-year group but more prevalent in the 50-59-year group (31.4%
compared with 16.3% in San Francisco). These data were adjusted for
cigarette smoking. No differences in pulmonary function test results
were found.
The prevalence of chronic respiratory symptoms and the results of
pulmonary function tests were compared in 2 similar groups of
nonsmoking, adult, male and female Seventh Day Adventists aged 45-64
years residing in Los Angeles and San Diego, respectively. Annual mean
oxidant values (94 µg/m3; 0.047 ppm and 76 µg/m3; 0.038 ppm), were
essentially the same in the 2 cities, but a mean of maximum daily
concentrations in Los Angeles (290 µg/m3; 0.144 ppm) was twice as
high as that in San Diego (148 µg/m3; 0.074 ppm). The survey was
performed when oxidant levels were low and similar in both cities, to
minimize effects attributable to acute oxidant exposures. No
differences were found in symptom prevalence or in several measures of
ventilatory function. Prevalence rates for chronic respiratory disease
were uniformly low (less than 4%) in both groups (Cohen et al., 1972).
6.3.7 Effects on patients with pre-existing diseases
6.3.7.1 Asthma
The association between reported asthma attacks in 137 patients
from Pasadena, California, and oxidant levels during the period 3
September-9 December 1956 was studied by Schoettlin & Landau (1961).
Daily records of the time of onset of asthma attacks were kept by each
patient and collected weekly. The daily number of patients afflicted
with asthma was moderately well correlated (r = 0.37) with concurrent
maximum hourly oxidant readings. Asthma attacks were more weakly
correlated with temperature, relative humidity, and water vapour
pressure than with oxidants. The number of patients having attacks on
days when maximum 1-h oxidant levels were higher than 500 µg/m3
(0.25 ppm) was significantly greater (p = 0.05) than the number of
those having attacks on days with lower oxidant levels. Unfortunately,
the analysis failed to isolate the pronounced seasonal variation of
asthma. In the northern hemisphere, asthma attack rates tend to reach
a peak in October and November (Booth et al., 1965), and decline
sharply in late November and early December. This pattern corresponds
closely to seasonal declines in peak oxidant levels. Hence, it is
possible that observed asthma-oxidant correlations have been secondary
to simultaneous seasonal changes in asthma frequency and oxidant
levels.
6.3.7.2 Chronic respiratory diseases
Studies over 18 months on 25 patients with severe, chronic,
obstructive lung disease at a chronic disease centre in Los Angeles
County showed that the prevailing levels of oxidants, oxidant
precursors, temperature, and relative humidity did not produce any
effects on lung function (Rokaw & Massey, 1962).
In studies on the effects of ambient air pollution exposure on
armed forces veterans with chronic respiratory disease living in the
Domiciliary Unit and Chronic Disease Annex of the Los Angeles Veterans
Administration Center, subjects were evaluated once a week by
pulmonary function tests and respiratory symptom questionnaires
(Schoettlin, 1962). Analysis of variance did not show any
statistically significant effects of air pollution (concentrations not
reported) on respiratory symptoms or function, although maximum
concentrations of oxidant and oxidant precursors consistently
accounted for more of the variation in frequency of symptoms and
clinical signs of disease than maximum temperature, relative humidity,
or pollen counts.
6.3.8 Cancer
A 5-year prospective study of lung cancer from 1958 to 1963 was
conducted by Buell et al. (1967) among 69 160 members of the
California American Legion. Long-term residents of Los Angeles County
had slightly lower age-smoking adjusted lung cancer rates (95.4 per
100 000) than residents of the San Francisco Bay area counties and San
Diego County (102 per 100 000). These urban groups, in turn, had
higher rates than all other California counties (75.5 per 100 000).
Nonsmokers followed the same geographical pattern: 28.1 per 100 000
for Los Angeles, 43.9 per 100 000 for San Francisco and San Diego,
11.2 per 100 000 for all others. Smokers of more than one packet of 20
cigarettes a day in Los Angeles had the highest lung cancer rates,
241.3 per 100 000, compared with San Francisco-San Diego, 226 per
100 000, and with other counties, 137.5 per 100 000. The duration of
exposure necessary to induce lung cancer may have been longer than the
actual ozone exposures in this population study. Thus, it would appear
worthwhile to extend these observations to subsequent years.
6.3.9 Motor vehicle accidents
The association between automobile accidents and days of elevated
oxidant levels (120-480 µg/m3; 0.06-0.24 ppm) in Los Angeles was
studied from August to October in 1963 and 1965. Applying a sign-test
and nonparametric correlation analysis to the data, Ury (1968) found a
statistically significant relationship between oxidant levels and
automobile accidents. Concentrations of carbon monoxide, oxides of
nitrogen, and other pollutants would also be relatively elevated when
oxidants were high and may have contributed to the results reported.
Furthermore, the association of accidents with oxidant levels may be
confounded by the fact that traffic jams produce both more accidents
and a greater output of oxidant precursors.
6.4. Summary Tables
Studies on the health effects of controlled, industrial, and
community exposures that provide quantitative information useful for
establishing guidelines for the protection of public health with
respect to photochemical oxidants are summarized in Tables 12, 13 and
14.
Table 12. Controlled human studies
I. Sensory effects
Ozone concentration Length of Effects Responsea Subjects Reference
exposure
µg/m3 (ppm) days h/day
400 (0.2) 1 3 and 6 Diminution in various measures of Not applicable 22 healthy males, 6 Lagerwerff (1963)
700 (0.35) 1 3 and 6 visual perception; noneffect dose healthy females
1000 (0.5) 1 3 and 6 not determined; no mention of
dose-response relationship.
> 200 (> 0.1) 1 working Increasing eye irritation with Not applicable 20 women office Richardson &
hours (study increase in oxidant exposures; workers Middleton
repeated for no apparent effects below (1957, 1958)
123 days) 200 µg/m3 (0.1 ppm).
40-100 (0.02- Immediate odour perception after 9/10 at 10-14 healthy males Henschler et al.
0.05) beginning of exposure; odour 40 µg/m3 (1960)
perception disappeared in ´-12 13/14 at
min; odour considerably stronger at 100 µg/m3
higher dose.
15-40 (0.008 Odour perception immediately after Not available 20 healthy subjects Eglite (1968)
-0.02) beginning of exposure; the most
sensitive person perceived odour
at the level of 15 µg/m3
(0.008 ppm).
II. Effects on respiratory function
A. Exposure to ozone
Ozone concentration Length of Effects Subjects Reference
exposure
µg/m3 (ppm) days h/day
2000 (1.0) 1 1 Consistent increase in airway 4 healthy males Goldsmith et Nadel
resistance; exposures at 200, 800, (1969)
and 1200 µg/m3 (0.1, 0.4, 0.6 ppm)
caused effect in some subjects,
but no dose-response pattern.
1800 (0.9) 1 5 min Highly significant decrease of airway 4 healthy males Kagawa & Toyama
conductance after inhalation with exercise. (1975a)
1200- (0.6-0.8) 1 2 Significant reduction in diffusing capacity of 10 healthy men and Young et al.
1600 lung and in FEV0.75b; substernal soreness one healthy woman (1964)
present in all subjects.
1000 (0.5) 6/wk 3 Significant decrease in FEV1.0c; no effect at 12 healthy males Bennet (1962)
x 12 400 µg/m3 (0.2 ppm).
wk5
1000 (0.5) 1 6 Significant change in airway conductance 20 healthy subjects Kerr et al. (1975)
and pulmonary resistance (dry cough and (19 men and 1
chest discomfort). woman)
1000 (0.5) 1 2 Decrease in pulmonary function 7 healthy males Hackney et al.
measurements. (1975a)
a Response = number of subjects showing effect described
total number of subjects
Table 12. Controlled human studies--continued
II. Effects on respiratory function
A. Exposure to ozone (cont'd)
Ozone concentration Length of Effects Subjects Reference
exposure
µg/m3 (ppm) days h/day
800 (0.4) 1 2 and 4 After 2-h exposure: Rawd increase, FVCe 22 healthy male Rummo et al.
decrease MMFRf decrease; after 4-h subjects (1975.
exposure: Rawd increase, FVCe decrease. unpublished
MMFRf decrease and additionally FEV1.0c data)
decrease; RVg, FRCh, and TCLi did not
change.
740 & (0.37 & 1 2 Significant decrease in ventilatory function 6-10 healthy males Bates et al.
1500 0.75) and in closing volume under intermittent (1972).
exercise; effect more pronounced at higher Hazucha et al.
dose. (1973)
740, (0.37, 1 2 Dose dependent change of ventilatory 28 healthy subjects Folinsbee et al.
1000 & 0.50 & pattern; significant reduction in MEFRj at (20 males and 8 (1975)
1500 0.75) 50% of vital capacity at 740 µg/m3 females)
(0.37 ppm) or more, under exercise.
740 (0.37) 1 2 Significant increase in total respiratory 2 healthy and 3 Hackney et al.
resistance under intermittent light exercise sensitively males. (1975a)
500 (0.25) 1 2 No consistent changes in lung function. 3 healthy and 3 Hackney et al.
sensitively males (1975a)
200- (0.1- 1 30 min Tendency towards increase in breathing 4 healthy male Ohmori (1974)
500 0.25) frequency and volume under exercise. subjects
200 (0.1) 1 2 Increase of Rawd and AaDO2k in 7 of 11 test 11 healthy male von Neiding et al.
subjects under intermittent light exercise. subjects (1977)
B. Exposure to mixtures of ozone and other air pollutants
Ozone concentration Length of Effects Subjects Reference
exposure
µg/m3 (ppm) days h/day
740 + 960 (0.37 + 0.37 1 2 More pronounced decrease in ventilatory 4 healthy males Bates & Hazucha
sulfur sulfur function end in closing volume than in (1973)
dioxide dioxide single exposure to ozone at 740 µg/m.3
(0.37 ppm).
200 + 9400 (0.1 + 5 1 2 Increases in Rawd and AaDO2k similar 11 healthy male von Nieding et al.
nitrogen nitrogen to those observed with ozone alone at subjects (1977)
dioxide dioxide 200 µg/m3 (0.1 ppm).
200 + 9400 (0.1 + 5 1 2 Increases in Rawd and AaDO2k similar 11 healthy male von Nieding et al.
nitrogen nitrogen to those observed with ozone at subjects (1977)
dioxide + dioxide + 5 200 µg/m3 (0.1 ppm) + nitrogen dioxide
sulfur sulfur at 9400 µg/m3 (5 ppm); recovery time
dioxide dioxide) delayed.
50 + 100 (0.025 + 0.05 1 2 No effect on Rawd and AaDO2k; 11 healthy male von Nieding et al.
dioxide +260 nitrogen sensitivity of the respiratory tract subjects (1977)
sulfur dioxide dioxide + 0.1 to acetylcholine increased.
sulfur dioxide)
C. Exposure to peroxyacetylnitrate
Ozone concentration Length of Effects Subjects Reference
exposure
µg/m3 (ppm) days h/day
1500 (0.3) 1 5 min Increase in oxygen uptake with exercise; 32 college males Smith (1965)
no effect at rest; significant decrease
in MEFRj during the recovery phase
following exercise.
1350 (0.27) 1 42 min Minor changes in cardiorespiratory and 20 healthy male Raven et al.
temperature regulation parameters. subjects (1974)
II. Effects on respiratory function
D. Exposure to irradiated automobile exhaust
Oxidants (0.22 Short term No significant changes in reaction 14 healthy Holland et al.
440-540 -0.27) (no details time, vital capacity, and submaximum subjects(1968)
given) work performance on the bicycle
ergometer.
Carbon (15-29)
monoxide
17-
33 mg/m3
Table 12. Controlled human studies--continued
II. Effects on respiratory function
E. Exposure to ambient air with an elevated concentration of oxidants
Ozone concentration Length of Effects Subjects Reference
exposure
µg/m3 (ppm) days h/day
Carbon (800-1400)
dioxide
1520-
2660 mg/m3
Nitric (0.38-0.58)
oxide
470-710
Nitrogen (0.7-1.0)
dioxide
1300-1900
Hydrocarbons
traces
Aldehydes (0.2-0.7)
Formaldehyde (0.2-0.24)
250-300
400-1400 (0.2-0.7) 2-90 h Improvement in lung function upon 46 patients with Motley et al.
residence in clean filtered room chronic lung (1959)
for 40 h or longer; threshold disease
concentration not determined.
100-460 (0.05-0.23) 21 24 Decrease in airway resistance and 15 patients with Balchum (1973)
increase in arterial partial oxygen moderately severe
pressure during week of residence chronic lung
in clean filtered air; threshold disease
concentration not determined.
Table 12. Controlled human studies
III. Changes in the electroencephalogram
Ozone concentration Length of Effects Responsea Subjects Reference
exposure
µg/m3 (ppm) days h/day
10 (0.005) 1 3 min Decrease in alpha rhythm. 1/3 3 healthy subjects Eglite (1968)
15 (0.008) 1 3 min Decrease in alpha rhythm. 2/3 3 healthy subjects Eglite (1968)
20 (0.01) 1 3 min Decrease in alpha rhythm. 3/3 3 healthy subjects Eglite (1968)
a Response = number of subjects showing effect described
total number of subjects
b FEV0.75 = 0.75 second forced expiratory volume
c FEV1.0 = one second forced expiratory volume
d Raw = airway resistance
e FVC = forced vital capacity
f MMFR = mid-maximal expiratory flow rate
g RV = residual volume
h FRC = functional residual capacity
i TLC = total lung capacity
j MEFR = maximum expiratory flow rate
k AaDO2 = alveolar to arterial oxygen pressure difference
l sensitive (subjects) = those with a prestudy history of cough, chest discomfort, or wheezing associated with
allergy or air pollution exposure, but with normal base-line pulmonary function studies
Table 13. Studies on the effects of industrial exposures
Ozone concentration Averaging Effects Reference
time
µg/m3 (ppm)
1600-3400 (0.8-1.7) 1 h 11 of 14 welders complained of respiratory symptoms; symptoms Challen et al. (1958)
disappeared when ozone levels were reduced to
400 µg/m3 (0.2 ppm); nitrogen dioxide probably present.
600-1600 (0.3-0.8) 1 h Increased frequency of chest tightness and throat irritation among Kleinfeld et al. (1957)
welders; no complaints at 500 µg/m3 (0.25 ppm); welders also
exposed to nitrogen dioxide and particulates but concentration
levels not given.
500-800 (0.25-0.40) long-term Increased frequency of headache, weakness, change in neuromuscular Kudrjavceva (1963)
sensitivity, and decrease in memory among workers manufacturing
hydrogen peroxide and exposed to ozone for 7-10 years; threshold
concentration not determined.
400-600 (0.2-0.3) 1 h No evidence for changes in vital capacity or functional residual Young et al. (1963)
capacity in welders; nitrogen dioxide probably present.
80-1000 (0.04-0.50) long-term Increased prevalence of bronchitis and emphysema in workers Nevskaja & Diterihs
engaged in manufacture of hydrogen peroxide for many years; (1957)
sulfuric acid aerosols also present; threshold concentration not
determined.
Table 14. Studies on the effects of community exposures
Hourly oxidant concentration Effects Subjects Reference
µg/m3 (ppm)
500 and above (0.25 and above) Increased frequency of asthma; possible confounding of 137 patients with Schoettlin
asthma-oxidant association with seasonal effects; other asthma & Landau (1961)
pollutants present but not reported.
approx. 1000 (approx. 0.50) Increased symptoms began at hourly concentrations of 102 student Hammer et al.
(headache) 100 µg/m3 (0.05 ppm), (eye irritation) nurses (1974)
300 µg/m3 (0.15 ppm), (cough) 530 µg/m3 (0.265 ppm).
(chest discomfort) 580 µg/m3 (0.29 ppm); threshold
levels determined by "hockey stick" functions.
240 and above (0.12 and above) Impaired performance determined by failure to improve 116 high school Wayne et al.
running times; threshold determined by "hockey stick" cross-country (1967)
functions. runners
> 300 (0.15) Increased frequency of complaints, particularly eye 7440 school Japan Public
irritation. children Health
Association (1976)
0-560 (0-0.28) Specific airway conductance of sensitive subjects was 20 healthy Kagawa et al.
(range at time significantly decreased with increasing hourly oxidant 11-year-old et al. (1977)
of lung concentrations; temperature, nitric oxide, nitrogen school children
function test) dioxide, sulfur dioxide, and particulate matter also
showed significant correlations with various
respiratory function tests of highly reactive children;
threshold concentration not determined.
7. EVALUATION OF HEALTH RISKS FROM EXPOSURE TO PHOTOCHEMICAL OXIDANTS
There appears to be sufficient information from experimental and
epidemiological studies to justify an attempt to establish guidelines
on the exposure limits for ozone and to review those for "oxidants"
(as measured by the neutral-buffered potassium iodide method (NBKI)),
proposed by a WHO Expert Committee in 1972. The Task Group appreciated
the fact that photochemical air pollution contains other substances
besides ozone, such as nitrogen dioxide, peroxyacetylnitrate, and
possibly many other gaseous and particulate products of atmospheric
photochemical reactions. However, present knowledge about the
composition of photochemical pollution, the concentrations of
individual components, and their possible impact on human health is so
limited that no attempt can be made to estimate exposure limits for
any single compound other than ozone. The Task Group was, of course,
aware that some sensory effects of photochemical air pollution (such
as eye irritation) might be due to a large extent, to these poorly
defined components of the photochemical oxidant mixture. As nitrogen
dioxide is an important air pollutant in its own right, it has been
discussed in a separate criteria document (World Health Organization,
1977).
7.1 Exposure Conditions
Exposure of man to ozone must have occurred for millions of years,
as ozone, present naturally in higher tropospheric layers, is also
found regularly in the lower atmosphere even in completely uninhabited
regions like the Antarctic. These natural concentrations have been
reported to have values ranging from 10 to 100 µg/m3
(0.005-0.05 ppm). It is difficult to determine the proportions of
natural to man-made oxidants (including ozone) that occur in rural
areas in most countries. In general, ozone concentrations greater than
120 µg/m3 (0.06 ppm) are considered to be related to man-made
activities. Ozone may be transported over hundreds of kilometres and
rural populations may be exposed to the pollutant, which earlier was
considered to exist only in Urban areas. A characteristic of such
exposures is that the precursors have vanished and ozone can therefore
persist for days in succession, since it does not come into contact
with other pollutants that act as its scavengers.
In large urban areas with strong sunshine and dense traffic or
other sources of precursors, photochemical air pollution is a daylight
phenomenon with maximum 1-h ozone concentrations sometimes as high as
300-800 µg/m3 (0.15-0.4 ppm), occurring around noon or somewhat
later. Such peak concentrations are preceded by nitrogen dioxide peaks
and accompanied by concurrent rises in peroxyacetylnitrate
concentrations. In contrast to oxides of sulfur and smoke, ozone
exposures are always intermittent, the peak concentrations rarely
lasting for more than 2-3 h. In the low temperature season,
photochemical reactions are much less likely to occur at rates
sufficient to produce large quantities of ozone.
Unless certain industrial technological processes (e.g., welding)
are in operation, ozone concentrations indoors tend to be considerably
lower than those outdoors due to the presence of reactive surfaces,
air conditioning, and indoor smoking.
7.2 Exposure-effect Relationships
Information presented in sections 5 and 6 is sufficient to
evaluate the relationship between exposure and the associated effects,
at least for some biological changes observed in man and experimental
animals.
7.2.1 Animal data
There is a considerable amount of evidence that short-term,
prolonged, or repeated exposure to ozone concentrations ranging from
200 to 400 µg/m3 (0.1-0.2 ppm) can cause a variety of biological
changes in several animal species and that these effects become more
pronounced with higher concentrations and increased exposure time (see
Table 10). These effects are, of course, also influenced by other
factors such as the animal species, the length of the interval between
exposures, the presence of other pollutants, low temperature, and
physical activity.
The host's pulmonary defence mechanisms against infectious
microorganisms are affected in several animal species by exposure to
ozone (section 5.2.6). This may result in rapid multiplication of
infectious microorganisms in situ, causing disease and eventually
death. An increase in mortality, resulting from the joint action of
infectious microorganisms and ozone, has been demonstrated in
artificially infected mice after a 3-h exposure to ozone at 160 µg/m3
(0.08 ppm). These effects were dose-related.
Pathomorphological changes in the respiratory tract of various
animal species, such as the rat, cat, rabbit, and mouse, have been
observed at ozone concentrations of about 400 µg/m3 (0.2 ppm) and
higher (section 5.2.1). Short-term exposures (up to 24 h) produce
oedema, degeneration and destruction of type I alveolar cells, loss of
ciliated epithelium, and breakdown of capillary endothelium. When the
exposure is repeated or its length extended, the biological changes
become more severe and include emphysema, atelectasis, vascular
lesions, bronchopneumonia, and fibrosis.
Various functional changes in the respiratory tract begin at
levels of about 520 µg/m3 (0.26 ppm) (section 5.2.2). The activities
of several enzymes in the lung tissue are also influenced at exposure
levels of about 500 µg/m3 (0.25 ppm) (section 5.2.3).
After pre-exposure to ozone, animals appear to become tolerant to
ozone concentrations that would otherwise cause pulmonary oedema
(section 5.2.5). The development of tolerance in small rodents is
related to pre-exposure levels of at least 600 µ/m3 (0.3 ppm). This
tolerance does not protect animals from such effects of ozone as
inflammation, alterations in alveolar macrophage functions, and
impairment of respiratory functions, that can occur at concentrations
lower than those that produce oedema.
Although the primary target for ozone is the respiratory system, a
number of studies have indicated that exposure to ozone may also
result in some extrapulmonary effects, but the mechanisms of such
action are not clear (section 5.3). For example, ozone exposure at
400-500 µg/m3 (0.2-0.25 ppm) for less than 2 h produced changes in
the circulating lymphocytes (increasing the number of binucleated
cells) and increased the number of spherocytes (section 5.3.2.1). It
has also been shown that exposure of pregnant mice to ozone at
200-400 µg/m3 (0.1-0.2 ppm) for 7 h per day, 5 days per week, for 3
weeks significantly increased neonatal mortality (section 5.3.3).
The available information concerning the carcinogenicity and
mutagenicity of ozone is inadequate for the definite evaluation of
such effects (sections 5.2.4 and 5.4).
Biological effects produced by the exposure of experimental
animals to a combination of ozone and nitrogen dioxide or by exposure
to complex pollutant mixtures containing oxidants, such as irradiated
automobile exhaust, are generally similar to those produced by
exposure to pure ozone. However, one study in which mice were exposed
to a mixture of ozone and nitrogen dioxide indicated that the effect
(reduction in resistance to respiratory infection) of a single
exposure to this mixture was additive, and that repeated exposure to
this mixture might result in a synergistic action.
7.2.2 Controlled human exposures
Although limited in number, human volunteer studies with
short-term, controlled exposure to oxidants have proved useful for
establishing exposure-effect relationships for ozone at levels ranging
from about 200-700 µg/m3 (section 6.1). Some of these studies provide
evidence of changes in the respiratory function of healthy subjects
that are related to exposure. Physical exercise tends to enhance the
respiratory effects of ozone (section 6.1.3).
Three investigators found a significant increase in airway
resistance with exposure to an ozone level of 740 µg/m3 (0.37 ppm)
for 2 h. One of the investigators did not find any effect with a 2-h
exposure to a level of 500 µg/m3 (0.25 ppm). However, this particular
study was conducted on subjects from southern California who were
later found to be less sensitive to ozone. Another investigator, using
similar test conditions, found a significant increase in airway
resistance in 7 out of 11 subjects at an ozone level of 200 µg/m3
(0.1 ppm) for 2 h (section 6.1.3.1).
In two studies, an improvement in lung function was noted when
patients with chronic pulmonary disease breathed filtered air for 40 h
or more, as compared with unfiltered ambient air. The ambient air
concentrations of oxidant in the two studies ranged from
400-1400 µg/m3 (0.2-0.7 ppm) and up to 400 µg/m3 (0.2 ppm)
respectively (section 6.1.3.5).
Result of a 7-month study in which female employees were exposed
to unfiltered ambient air during office hours suggested that the
lowest 1-h oxidant level that could be associated with eye irritation
was about 200 µg/m3 (section 6.1.2).
Although in vitro studies using human cells have shown some
evidence of a joint action of ozone and nitrogen dioxide, this has not
been clearly demonstrated in in vivo studies. For example, whereas a
potentiated increase in airway resistance was observed with exposure
to a combination of ozone at 740 µg/m3 (0.37 ppm) and sulfur dioxide
at 960 µg/m3 (0.37 ppm) for a period of 2 h, it was not observed with
exposure to a combination of ozone at 500 µg/m3 (0.25 ppm) and
nitrogen dioxide at 560 µg/m3 (0.30 ppm). Exposure to ozone at
50 µg/m3 (0.025 ppm) combined with nitrogen dioxide at 100 µg/m3
(0.05 ppm) and sulfur dioxide at 260 µg/m3 (0.1 ppm) did not have any
effect on airway resistance; however, this combined exposure resulted
in enhancement of the bronchoconstrictor effect of acetylcholine
(section 6.1.3.2).
7.2.3 Industrial exposure
Acute symptoms of chest tightness, irritation of the throat, and
coughing have been documented in welders exposed to 1-h ozone levels
of 600-1600 µg/m3 (0.3-0.8 ppm). These symptoms disappeared when
ozone concentrations fell to 500 µg/m3 (0.25 ppm) or less. Possible
chronic effects of repeated occupational exposure to ozone are not
well documented, although one investigator reported that workers
exposed to ozone levels in the range of 500-800 µg/m3 (0.25-0.40 ppm)
for 7-10 years had an increased frequency of headaches and weakness,
increased muscle excitability, and impaired memory (section 6.2).
7.2.4 Community Exposure
Several studies have shown more frequent eye irritation, reduced
athletic performance, changes in lung function of children, and
increased frequency of asthma attacks, all of which have been
associated with changes in hourly oxidant levels (sections 6.3.2,
6.3.3, 6.3.4, 6.3.7). As in all studies of the effects of community
exposures, it is difficult to determine precisely the lowest level at
which adverse effects become manifest. However, most of these effects
were observed when 1-h oxidant levels were in the range of about
200-500 µg/m3 (0.1-0.25 ppm). Although other pollutants such as
nitrogen dioxide, particulate matter, and sulfur dioxide were
simultaneously present, the strongest correlation of the observed
effects was with hourly levels of photochemical oxidants.
On the other hand, there is no evidence, so far, that long-term
exposure to photochemical oxidants at levels currently present in
urban air is associated with increased mortality (section 6.3.1), and
there is no evidence that chronic respiratory diseases such as
bronchitis, emphysema, and lung cancer are more prevalent in
communities with high oxidant exposures (section 6.3.6). However, it
should be pointed out that the number of epidemiological studies
concerned with such associations is small.
7.3 Guidelines on Exposure Limits
The exposure-effect relationships discussed in section 7.2 make it
possible to draw the following conclusions concerning the exposures to
oxidants and ozone at which the effects in man begin to appear:
(a) There is presumptive evidence from one controlled exposure
study that some effects on the lung function of healthy human subjects
might occur with exposure to an ozone level of 200 µg/m3 (0.1 ppm)
for 2 h.
(b) There is also evidence from general population studies that
suggests that 1-h ambient oxidant levels in the range of about
200-500 µg/m3 (0.1-0.25 ppm) may affect lung function in children,
increase the frequency of asthma attacks, cause more frequent eye
irritation, and reduce athletic performance.
(c) There is limited evidence from controlled exposure studies
that living in an environment with 1-h oxidant levels within the range
of 400-1400 µg/m3 (0.2-0.7 ppm) may exert additional stress on
patients with chronic pulmonary disease.
(d) There is convincing evidence from controlled human exposure
studies that airway resistance may be increased in healthy human
subjects following exposure to ozone levels of 700-800 µg/m3
(0.35-0.40 ppm) for 2h.
Animal data generally support the results of human studies.
However, some effects have been observed in animals at an ozone level
of about 200 µg/m3 (0.1 ppm) or even less, which have not yet been
demonstrated in man. For example, in animals, short-term exposures to
such concentrations appear to reduce resistance to pulmonary
infections.
The role of ozone and other photochemical oxidants in the etiology
of cancer is not clear. The only available epidemiological study did
not indicate any association between exposure to oxidants and the risk
of lung cancer, and experimental studies on the carcinogenicity and
mutagenicity of ozone in animals are not adequate for evaluation.
Nevertheless, the Task Group felt that there may be reason for concern
about the possible carcinogenicity of ozone (based primarily on some
biochemical considerations regarding the mechanism of the biological
effects of ozone). This aspect of its toxicity should be kept under
continual surveillance.
On the basis of all these considerations, the Task Group agreed
that 1-h levels of ozone of 100-200 µg/m3 (0.05-0.1 ppm) (measured by
the chemiluminescence method) could be used as a guideline for the
protection of public health. The relatively high natural
concentrations of ozone precluded the use of any safety factor.
The Task Group also agreed that a 1-h maximum level of 120 µg/m3
(0.06 ppm), which is approximately the highest natural background
concentration of oxidants, would be the best single value estimate of
the exposure limit for oxidants in the ambient air. This level is in
agreement with the long-term goal for photochemical oxidants (as
measured by the NBKI method) proposed by a WHO Expert Committee (World
Health Organization, 1972).
The issue was raised as to whether the proposed guideline was
realistic in view of natural exposure levels and the long-distance
transport of ozone. In response to this question, the Group expressed
the view that every effort should, nevertheless, be made to develop
control strategies for achieving the proposed guideline or at least,
for not exceeding it more than once a month.
REFERENCES
ADACHI, T. & NAKAJIMA, T. (1974) [Symptoms and clinical examinations
of the patients seriously injured by photochemical smog.] Rinsho
Kagaku (Clin. Chem.), 3:257-267 (in Japanese).
ALPERT, S. M. & LEWIS, T. T. (1971) Ozone tolerance studies utilizing
unilateral lung exposure. J. appl. Physiol., 31: 243-246.
ALPERT, S. M., SCHWARTZ, B. B., LEE, S. D., & LEWIS, T. R. (1971a)
Alveolar protein accumulation: a sensitive indicator of low level
oxidant toxicity. Arch. intern. Med., 128: 69-73.
ALPERT, S. M., GARDNER, D. E., HURST, D. J., LEWIS, T. R., & COFFIN,
D. L. (1971b) Effects of exposure to ozone on defensive
mechanisms of the lung. J. appl. Physiol., 31: 247-252.
AMERICAN CONFERENCE OF GOVERNMENT INDUSTRIAL HYGIENISTS (1975)
Threshold limit values for chemical substances in workroom air
adopted by ACGIH for 1975, Cincinnati, ACGIH.
ATWAL, O. S., & WILSON, T. (1974) Parathyroid gland changes following
ozone inhalation. Arch. environ. Health, 28: 91-100.
ATWAL, O. S., SAMAGH, B. S., & BHATNAGAR, M. K. (1975) A possible
autoimmune parathyroiditis following ozone inhalation: IIA
histopathologic, ultrastructural, and immunofluorescent study.
Am. J. Pathol., 80: 53-68.
BALCHUM, O. J. (1973) Toxicological effects of ozone, oxidant and
hydrocarbons. Proceedings of the Conference on Health Effects of
Air Pollution, Assembly of Life Sciences, National Academy of
Sciences-National Research Council, Washington, DC, Oct. 3-5. In:
Committee Print, Committee on Public Works, United States
Senate, Washington, DC, US Government Printing Office, pp.
489-503.
BALL, D. J. (1976) Photochemical ozone in the atmosphere of Greater
London. Nature (Lond.), 263: 580-582.
BARSKY, R. M. & BIRAKOS, J. N., ed. (1971) Profile of air pollution
control, Los Angeles, CA, County of Los Angeles Air Pollution
Control District, p. 73.
BARTH, D. S., ROMANOVSKY, J. C., KNELSON, J. H., ALTSHULLER, A. P., &
HORTON, R. J. M. (1971) Discussion (of national ambient air
quality standards). J. Air Pollut. Control Assoc., 21: 544-548.
BARTLETT, D. JR, FAULKNER, C. S., & COOK, K. (1975) Effect of chronic
ozone exposure on lung elasticity in young rats. J. appl.
Physiol., 37: 92-96.
BATES, D. V. & HAZUCHA, M. (1973) The short-term effects of ozone on
the human lung. Proceedings of the Conference on the Health
Effects of Air Pollution, Assembly of Life Sciences, National
Academy of Sciences-National Research Council, Wasington, DC,
Oct. 3-5. In: Committee Print, Committee on Public Works, United
States Senate, Washington DC, US Government Printing Office,
pp. 507-540.
BATES, D. V., BELL, G. M., BURNHAM, C. D., HAZUCHA, M., MANTHA, J.,
PENGELLY, L. D., & SILVERMAN, F. (1972) Short-term effects of
ozone on the lung. J. appl. Physiol., 32: 176-181.
BECKER, K. H. & SCHURATH, U. (1975) [The influence of oxides of
nitrogen on atmospheric oxidation processes.] Staub-Reinhall,
Luft, 35(4): 156-161 (in German).
BENNETT, G. (1962) Ozone contamination of high altitude aircraft
cabins. Aerospace Med., 33: 969-973.
BERRY, C. R. (1964) Differences in concentration of surface oxidant
between valley and mountain top conditions in the southern
Appalachians. J. Air Pollut. Control Assoc., 14(6): 238-239.
BILS, R. F. (1966) Ultrastructural alterations of alveolar tissue of
mice: I. Due to heavy Los Angeles smog. Arch. environ. Health,
12: 689-697.
BILS, R. F. (1970) Ultrastructural alterations of alveolar tissue of
mice: III. Ozone. Arch. environ. Health, 20: 468-480.
BILS, R. F. & ROMANOVSKY, J. C. (1967) Ultrastructural alterations of
alveolar tissue of mice: II. Synthetic photochemical smog. Arch
environ. Health, 14: 844-858.
BLOCH, W. N. JR, MILLER, F. J., & LEWIS, T. R. (1971) Pulmonary
hypertension in dogs exposed to ozone. Cincinnati, OH, Air
Pollution Control Office, US Environmental Protection Agency.
BOATMAN, E. S., SATO, S., & FRANK, R. (1974) Acute effects of ozone on
cat lungs. II: Structural. Am. Rev. respir. Dis., 110: 157-169.
BOBB, G. A. & FAIRCHILD, E. J. (1967) Neutrophil-to-lymphocyte ratio
as indicator of O3 exposure. Toxicol. appl. Pharmacol.,
11: 558-564.
BOOTH, S., DEGROOT, I., MARKUSH, R., & HORTON, R. J. M. (1965)
Detection of asthma epidemics in seven cities. Arch. environ.
Health, 10: 152-155.
BRANT, J. W. A. (1965) Human cardiovascular diseases and atmospheric
air pollution in Los Angeles, California. Int. J. Air Water
Pollut., 9: 219-231.
BRANT, J. W. A. & HILL, S. R. G. (1964) Human respiratory diseases and
atmospheric air pollution in Los Angeles, California. Int. J.
Air Water Pollut., 8: 259-277.
BRINKMAN, R., LAMBERTS, H. B., & VENINGA, T. S. (1964) Radiomimetic
toxicity of ozonized air. Lancet, 1: 133-136.
BUELL, G. C., TOKIWA, Y., & MUELLER, P. K. (1965) Potential
crosslinking agents in lung tissue; formation and isolation after
in vivo exposure to ozone. Arch. environ. Health,
10: 213-219.
BUELL, P., DUNN, J. E., JR, & BRESLOW, L. (1967) Cancer of the lung
and Los Angeles type air pollution: prospective study. Cancer,
20: 2139-2147.
BUTCHER, S.S. & RUFF, R. E. (1971) Effect of inlet residence time on
analysis of atmospheric nitrogen oxides and ozone. Anal. Chem.,
43(13): 1890-1892.
BYERS, D. H. & SALTZMAN, B. E. (1958) Determination of ozone in air by
neutral and alkaline iodide procedures. J. Am. Ind. Hyg. Assoc.,
19: 251-257.
CALIFORNIA STATE DEPARTMENT OF PUBLIC HEALTH (1955) Clean air for
California. Initial Report of the Air Pollution Study Project,
March 1955, 37 pp.
CALIFORNIA STATE DEPARTMENT OF PUBLIC HEALTH (1956) Clean air for
California. Second Report, March 1956, 10 pp.
CALVERT, J. G. (1976) Hydrocarbon involvement in photochemical smog
formation in Los Angeles atmosphere. Environ. Sci. Technol.,
10(3): 256-262.
CARSON, S. & GOLDHAMER, R. E. (1968) Biochemical defense mechanisms
against pulmonary irritants. Ohio, USA, Aerospace Medical
Research Laboratories, Aerospace Medical Division, Air Force
Systems Command, Wright-Patterson Air Force Base, pp. 129.
CASTLEMAN, W. L., DUNGWORTH, D. L., & TYLER, W. S. (1973a)
Histochemically detected enzymatic alterations in rat lung
exposed to ozone. Exp. mol. Pathol., 19: 402-421.
CASTLEMAN, W. L., DUNGWORTH, D. L., & TYLER, W. S. (1973b)
Cytochemically detected alterations of lung acid phosphatase
reactivity following ozone exposure. Lab. Invest., 29: 310-319.
CHALLEN, P. J. R., HICKISH, D. E., & BEDFORD, J. (1958) An
investigation of some health hazards in an inert-gas tungsten-arc
welding shop. Br. J. ind. ed., 15: 276-282.
CHOW, C. K. & TAPPEL, A. L. (1972) An enzyme protective mechanism
against lipid peroxidation damage to lungs of ozone-exposed rats.
Lipids, 7: 518-524.
CHOW, C. K. & TAPPEL, A. L. (1973) Activites of pentose shunt and
glycolytic enzymes in lungs of ozone-exposed rats. Arch.
environ. Health, 26: 205-208.
CHOW, C. K., DILLARD, C. J., & TAPPEL, A. L. (1974) Glutathione
peroxidase system and lysozyme in rats exposed to ozone and
nitrogen dioxide. Environ. Res., 7: 311-319.
CHRISTENSEN, E. & GIESE, A. C. (1954) Changes in absorption spectra of
nucleic acids and their derivatives following exposure to ozone
and ultraviolet radiation. Arch. Biochem. Biophys., 51:
208-216.
COFFIN, D. L. & BLOMMER, E. J. (1965) The influence of cold on
mortality from streptococci following ozone exposure. J. Air
Pollut. Control Assoc., 19: 523-524.
COFFIN, D. L. & BLOMMER, E. J. (1967) Acute toxicity of irradiated
auto exhaust. Its indication by enhancement of mortality from
streptococcal pneumonia. Arch. environ. Health, 15: 36-38.
COFFIN, D. L. & BLOMMER, E. J. (1970) Alteration of the pathogenic
role of streptococci group C in mice conferred by previous
exposure to ozone. In: Silver, I. H., ed. Aerobiology,
Proceedings of the 3rd International Symposium on Aerobiology,
University of Sussex, England, 1969. New York, Academic Press,
pp. 54-61.
COFFIN, D. L. & GARDNER, D. E. (1972a) Interaction of biological
agents and chemical air pollutants. Ann. occup. Hyg.,
15: 219-234.
COFFIN, D. L. & GARDNER, D. E. (1972b) The role of tolerance in
protection of the lung against secondary insults. In:
Proceedings of the International Conference of Occupational
Physicians of the Chemical Industry, Ludwigshafen, Germany,
April 1972, Research Triangle Park, NC, Environmental
Protection Agency, pp. 344-364.
COFFIN, D. L. & GARDNER, D. E. (1975) [Ozone Toxicology: Correlation
between tolerance and protective mechanisms of the lung.] Gig. i
Sanit., 1: 86-92 (in Russian).
COFFIN, D. L., BLOMMER, E. J., GARDNER, D. E., & HOLZMAN, R. S. (1968)
Effect of air pollution on alteration of susceptibility to
pulmonary infection. In: Proceedings of the 3rd Annual
Conference on Atmospheric Contamination in Confined Spaces,
Aerospace Medical Research Laboratory, pp. 71-80.
COFFIN, D. L., GARDNER, D. E., HOLZMAN R. S., & WOLOCK, F. J. (1968)
Influence of ozone on pulmonary cells. Arch. environ. Health,
16: 633-636.
COHEN, C. A., HUDSON, A. R., CLAUSEN, J. L., & KNELSON, J. H. (1972)
Respiratory symptoms, spirometry, and oxidant air pollution in
non-smoking adults. Am. Rev. resp. Dis., 105: 251-261.
CORTESI, R. & PRIVETT, O. S. (1972) Toxicity of fatty ozonides and
peroxides. Lipids, 7: 715-721.
COX, R. A., EGGLETON, A. E. J., DERWENT, R. G., LOVELOCK, J. E., &
PACK, D. H. (1975) Long-range transport of photochemical ozone in
North-Western Europe. Nature (Lond.), 255(5504): 118-121.
CRIEGEE, R. (1957) The course of ozonization of unsaturated compounds.
Rec. chem. Prog, 18: 111-120.
CRONIN, S. R. & GIRI, S. N. (1974) Effects of pulmonary irritants on
DNA, ATPase activity, and histamine on rat lung. Proc. Soc. Exp.
Biol. Med., 146: 120-125.
DARNALL, K. R., LLOYD, A. C., WINER, A. C., & PITTS, J. N., JR (1976)
Reactivity scale for atmospheric hydrocarbons based on reaction
with hydroxyl radical. Environ. sci. Technol. 10(7): 692-296.
DAVIS, I. (1961) Microbiologic studies with ozone. Mutagenesis of
ozone for Escherichia coli, Texas, USAF Aerospace Medical
Center, School of Aerospace Medicine, 9 pp. (Rep. No. 61-60).
DEANE, M., GOLDSMITH, J. R., & TUMA, D. (1965) Respiratory conditions
in outside workers: report on outside plant telephone workers in
San Francisco and Los Angeles. Arch. environ. Health,
10: 323-331.
DELUCIA, A. J., HOQUE, P.M., MUSTAFA, M. G., & CROSS, C. E. (1972)
Ozone interaction with rodent lung: effect on sulfhydryls and
sulfhydryl-containing enzyme activities. J. lab. clin. Med.,
80: 559-566.
DELUCIA, A. J., MUSTAFA, M. G., HUSSAIN, M. Z., & CROSS, C. E. (1975)
Ozone interaction with rodent lung: III. Oxidation of reduced
glutathione and formation of mixed disulfides between protein and
non-protein sulfhydrils. J. clin. Invest., 55: 794-802.
DEMERJIAN, K. L., KERR, J. A., & CALVERT, J. G. (1974) The mechanism
of photochemical smog formation. Adv. environ. Sci. Technol.,
4: 1-262.
DEMORE, W. B. & PATAPOFF, M. (1976) Comparison of ozone determinations
by ultraviolet photometry and gas-phase titoation. Environ. Sci.
Technol., 10(9): 897-899.
DERWENT, R. G. & STEWART, H. N. M (1973) Elevated ozone levels in the
air of Central London. Nature (Lond.), 241(5388): 342-343.
DILLARD, C. J., URRIBARRI, N., REDDY, K., FLETCHER, B., TAYLOR, S., DE
LUMEN, B., LANGBERG, S., & TAPPEL, A. L. (1972) Increased
lysosomal enzymes in lungs of ozone-exposed rats. Arch. environ.
Health, 25: 426-431.
DIMITRIADES, B. (1976) Photochemical oxidants in the ambient air of
the United States. Research Triangle Park, NC, US Environmental
Protection Agency, 192 pp. (EPA-600/3-76-017).
DIXON, J. R. & MOUNTAIN, J. T. (1965) Role of histamine and related
substances in development of tolerance to edemagenic gases.
Toxicol. appl. Pharmacol., 7: 756-766.
DOWELL, A. R., LOHRBAUER, L. A., HURST, D., & LEE, S. D. (1970) Rabbit
alveolar macrophage damage caused by in vivo O3 inhalation.
Arch. environ. Health, 21: 121-127.
DUNGWORTH, D. L. (1976) Short-term effects of ozone on lungs of rats,
mice, and monkeys. Environ. Health Perspect., 16: 179.
DUNGWORTH, D. L., CASTLEMAN, W. L., CHOW, C. K., MELLICK, P. W.,
MUSTAFA, M. G., TARKINGTON, B., & TYLER, W. S. (1975) Effect of
ambient levels of ozone on monkeys. Fed. Proc., 34: 1670-1674.
DURHAM, W. (1974) Air pollution and student health. Arch. environ.
Health, 28: 241-254.
EASTON, R. E. & MURPHY, S. D. (1967) Experimental ozone preexposure
and histamine. Arch. environ. Health, 15: 160-166.
EGLITE, M. (1968)[The problem of hygienic assessment of atmospheric
ozone.] Gig. i Sanit., 33: 17-22 (in Russian).
EMIK, L. O., PLATA, R. L., CAMPBELL, K. I., & CLARKE, G. L. (1971)
Biological effects of urban air pollution. Arch. environ.
Health, 23: 335-342.
ENVIRONMENT AGENCY (1975a) [Proceedings of the Second US Japan
Conference on Photochemical Air Pollution.] Tokyo, Japan (in
Japanese).
ENVIRONMENT AGENCY (1975b) [Air Pollution in Japan. Air monitoring
data in fiscal year 1974.] Tokyo, Japan, Air Pollution Control
Division, Air Quality Bureau, pp. 1200-1240 (in Japanese).
ENVIRONMENT AGENCY (1976) [Quality of the environment in Japan.]
Tokyo, Japan, Environment Agency, pp. 152-153 (in Japanese).
ERLICH, R., FINDLAY, J. C., FENTERS, J. D., & GARDNER, D. E. (1977)
Health effects of short-term inhalation of nitrogen dioxide and
ozone mixtures. Environ. Res., 14: 223-231.
EVANS, M. J., MAYR, W., BILS, R. F., & LOOSLI, C. G. (1971) Effects of
ozone on cell renewal in pulmonary alveoli of ageing mice. Arch.
environ. Health, 22: 450-453.
FABIAN, P. & PRUCHNIEWlCZ, P. G. (1973) Meridional distribution of
tropospheric ozone from ground-based registrations between Norway
and South Africa. Pure appl. Geophys., 106-108 (V-VII):
1027-1035.
FAIRCHILD, E. J., II (1963) Neurohumoral factors in injury from
inhaled irritants. Arch. environ. Health, 6: 79-86.
FAIRCHILD, E. J., II (1967) Tolerance mechanisms. Determinants of lung
responses to injurious agents. Arch. environ. Health,
14: 111-125.
FAIRCHILD, E. J., II & BOBB, G. A. (1965) The effect of spinal cord
lesions on the pathophysiology of a lung irritant ozone.
Toxicol. appl. Pharmacol., 7: 483 (Abstract).
FARWELL, S. O. & RASMUSSEN, R. A. (1976) Limitations of the FPD and
ECD in atmospheric analysis: A review. J. Chromatogr. Sci.,
14: 224-234.
FEDERAL REPUBLIC OF GERMANY, Ministry of Internal Affairs (1974)
Nox CnH volatile fluoride and chlorine compounds.
FETNER, R. H. (1958) Chromosome breakage in Victa faba by ozone.
Nature (Lond.), 181: 504-505.
FETNER, R. H. (1962) Ozone-induced chromosome breakage in human cell
cultures. Nature (Lond.), 194: 793-794.
FETNER, R. H. (1963) Mitotic inhibition induced in grasshopper
neuroblasts by exposure to ozone. USAF School of Aerospace
Medicine (Technical Documentary Report SAM-TDR 63-39).
FLETCHER, B. L. & TAPPEL, A. L. (1973) Protective effects of dietary
alphatocopherol in rats exposed to toxic levels ozone and
nitrogen dioxide. Environ. Res., 6: 165-175.
FOLINSBEE, L. J., SILVERMAN, F., & SHEPHARD, R. J. (1975) Exercise
responses following ozone exposure. J. appl. Physiol.,
38: 996-1001.
FOURNIER, E. (1973) Radicaux libres et toxicologie. Eur. J. Toxicol.,
6: 109-122.
FRANK, H. R., BRAIN, J. D., & SHERRY, D. E. (1970a) Unilateral
tolerance and altered elastic behaviour in rabbits. Am. Ind.
Hyg. Assoc. J., 31: 31 (Abstract section).
FRANK, N. R., YOKOYAMA, E., WARANABE, S., SHERRY, D. E., & BRAIN, J.
D. (1970b) An ozone Journal presented at the American Medical
Association, Air Pollution Medical Research Conference New
Orleans, 5-7 Oct., 19 pp.
FRANK, R., FLESCH, J.P., & BRAIN, J. D. (1971) Effect of ozone on
elastic behaviour of excised lungs of dogs. Environ. Res.,
4: 343-354.
FREEMAN, G., STEPHENS, R. J., CRANE, S.C., & FURIOSI, N.J. (1968)
Lesion of the lung in rats continuously exposed to two parts per
million of nitrogen dioxide. Arch. environ. Health,
17: 181-192.
FREEMAN, G., STEPHENS, R. J., COFFIN, D. L., & STARA, J. F. (1973)
Changes in dogs' lungs after long-term exposure to ozone. Arch.
environ. Health, 26: 209-216.
FREEMAN, G., JULIOS, L. T., FURIOSI, N. J., MUSSENDEN, R., STEPHENS,
R. J., & EVANS, M. J. (1974) Pathology of pulmonary disease from
exposure to interdependent ambient gases (NO2 & O3). Arch.
environ. Health, 29: 203-210.
FRIBERG, L., HOLMA, B., & RYLANDER, R. (1972) Animal lung reactions
after inhalation of lead and ozone. Environ. Physiol. Biochem.,
2: 170-178.
FUKASE, O., ISOMURA, K., & WATANABE, H. (1975a) Effect of ozone on
glutathione in vivo. J. Jpn. Soc. Air Pollut., 10: 58-62 (in
Japanese).
FUKASE, O., ISOMURA, K., & WATANABE, H. (1975b) Effect of ozone on
vitamin C in vivo. J. Jpn. Soc. Air Pollut., 10: 63-66.
FUJII, T. (1972) Studies on air pollution by photochemical reaction.
1. The results of field research of photochemical smog in Osaka,
1971. In: Proceedings of the Research Section, Osaka
Environmental Pollution Control Centre, No. 3, pp. 21-34 (in
Japanese).
GARDNER, D. E. (1971) Environmental influences on living alveolar
macrophages. A dissertation submitted to the Division of
Graduate Studies of the University of Cincinnati, Ann Arbor,
Michigan, University Microfilms International, pp. 191.
GARDNER, D. E., PFITZER, E. A., CHRISTIAN, R. T., & COFFIN, D. L.
(1971) Loss of protective factor for alveolar macrophages when
exposed to ozone. Arch. intern. Med., 127: 1078-1084.
GARDNER, D. E., LEWIS, T. R., ALPERT, S. M., HURST, D. J., & COFFIN,
D. L. (1972) The role of tolerance in pulmonary defense
mechanisms. Arch. environ. Health, 25: 432-438.
GARDNER, D. E., ILLING, J. W., MILLER, F. J., & COFFIN, D. L. (1974a)
The effect of ozone on pentobarbital sleeping time in mice.
Chem. Pathol. Pharmacol., 9: 689-700.
GARDNER, D. E., ILLING, J. W., & COFFIN, D. L. (1974b) Enhancement of
effect of exposure to O3 and NO2 by exercise. Toxicol. appl.
Pharmacol., 29: 129-130.
GARDNER, M. B. (1966) Biological effects of urban air pollution. III.
Lung tumors in mice. Arch. environ. Health, 12: 305-513.
GARDNER, M. B., LOOSLI, C. G., HAINES, B., BLACKMORE, W., & TEEBKEN,
D. (1969) Histopathologic findings in rats exposed to ambient and
filtered air. Arch. environ. Health, 19: 637-647.
GOLDSMITH, J. R. & NADEL, J. A. (1969) Experimental exposure of human
subjects to ozone. J. Air Pollut. Control Assoc., 19: 329-330.
GOLDSTEIN B. D. (1973) Hydrogen peroxide in erythrocytes. Detection in
rats and mice inhaling ozone. Arch. environ. Health,
26: 279-280.
GOLDSTEIN B. D. (1976) Combined exposure to ozone and nitrogen
dioxide. Env. Health Perspect., 13: 107-110.
GOLDSTEIN B. D., PEARSON, B., LODI, C., BUCKLEY, R. D., & BALCHUM, O.
J. (1968) The effect of ozone on mouse blood in vivo. Arch.
environ. Health, 16: 648-650.
GOLDSTEIN B. D., LODI, C., COLLINSON, C., & BALCHUM, O. J. (1969)
Ozone and lipid peroxidation. Arch. environ. Health,
18: 631-635.
GOLDSTEIN B. D., BUCKLEY, R. D., CARDENAS, R., & BALCHUM, O. J. (1970)
Ozone and vitamin E. Science, 169: 605.
GOLDSTEIN B. D., LAI, L. Y., ROSS, S. R., & CUZZI-SPADA, R. (1973)
Susceptibility of inbred mouse strains to ozone. Arch. environ.
Health, 27: 412-413.
GOLDSTEIN B. D., SOLOMON, S., PASTERNACK, B. S., & BICKERS, D. R.
(1975) Decrease in rabbit lung microsomal cytochrome P-450 levels
following ozone exposure. Chem. Pathol. Pharmacol.,
10: 759-762.
GOLDSTEIN, E., TYLER, W. S., HOEPRICH, P. D., & EAGLE, C. (1971a)
Ozone and the antibacterial defense mechanisms of the murine
lung. Arch. intern. Med., 127: 1099-1102.
GOLDSTEIN, E., TYLER, W. S., HOEPRICH, P. D., & EAGLE, C. (197lb)
Adverse influence of ozone on pulmonary bactericidal activity of
murine lung. Nature (Lond.), 229: 262-263.
GOLDSTEIN, E., EAGLE, M. C., & HOEPRICH, P. D. (1972) Influence of
ozone on pulmonary defense mechanisms of silicotic mice. Arch.
environ. Health, 24: 444-448.
GOLDSTEIN, E., WARSHAUER, D., LIPPERT, W., & TARKINGTON, B. (1974)
Ozone and nitrogen dioxide exposure. Arch. environ. Health,
28: 85-90.
GREGORY, A. R., RIPPERTON, L. A., & MILLER, B. (1967) Effect of
neonatal thymectomy on the development of ozone tolerance in
mice. Am. Ind. Hyg. Assoc. J., 28: 278-282.
GRIMSRUD, E. P., WESTBERG, H. H., RASMUSSEN, R. A. (1975) Atmospheric
reactivity of monoterpene hydrocarbons, NOx photooxidation and
ozonolysis. Int. J. Chem. Kin. Symp., 1: 183-195.
GUICHERIT, R. (1975) [Photochemical smog formation in the
Netherlands.] Delft, Netherlands, TNO Institute for
Environmental Hygiene, p. 27 (in Dutch).
HAAGEN-SMIT, A. J. (1952) Chemistry and physiology of Los Angeles
smog. Ind. Eng. Chem., 44: 1342-1346.
HACKNEY, J. D., LINN, W. S., LAW, D.C., KARUZA, S. K., GREENBERG, H.,
BUCKLEY, R. D., & PEDERSEN, E. E. (1975) Experimental studies on
human health effects of air pollutants. III. Two-hour exposure to
ozone alone and in combination with other pollutant gases. Arch.
environ. Health, 30: 385-390.
HACKNEY, J. D., LINN, W. S., KARUZA, S. K., BUCKLEY, R. D., LAW, D.C.,
BATES, D. V., HAZUCHA, M., PENGELLY, L. D., & SILVERMAN, F.
(1977) Effects of ozone exposure in Canadians and Southern
Californians. Arch. environ. Health, 34: 110-116.
HALLET, W. Y. (1965) Effect of ozone and cigarette smoke on lung
function. Arch. environ. Health, 10: 295-302.
HAMMER, D. I., HASSELBLAD, V., PORTNOY, B., & WEHRLE, P. F. (1974) Los
Angeles student nurse study: Daily symptom reporting and
photochemical oxidants. Arch. environ. Health., 28: 255-260.
HASSELBLAD, V., CREASON, J.P., & NELSON, W. C. (1976) Regression
using "hockey stick" functions. Research-Triangle Park, NC, US
Environmental Protection Agency, Health Effects Research
Laboratory, 12 pp. (EPA-600/1-76-024).
HATTORI, K., KATO, N., KINOSHITA, S., & SUNADA, T. (1963) Protective
effect of ozone in mice against whole-body x-irradiation. Nature
(Lond.), 198: 1220.
HAUSKNECHT, R. (1960) Air pollution effects reported by California
residents from the California Health Survey, CA, USA, State of
California, Department of Public Health, pp. 1-55.
HAZUCHA, M., SILVERMAN, F., PARENT, C., FIELD, S., & BATES, D. V.
(1973) Pulmonary function in man after short-term exposure to
ozone. Arch. environ. Health, 27: 183-188.
HECHTER, H. H. & GOLDSMITH, J. R. (1961) Air pollution and daily
mortality. Am. J. med. Sci., 241: 581-588.
HENSCHLER, D. (1960) [Protective effect of pre-treatment with low gas
concentrations against lethal pulmonary edema caused by irritant
gas.] Arch. exp. Pathol. Pharmacol., 238: 66-67 (in German).
HENSCHLER, D., STIEN, A., BECK, H., & NEUMAN, W. (1960) [Olfactory
threshold of some important irritant gases and manifestations in
man by low concentrations.] Arch. Gewerbepathol. Gewerbehyg.,
17: 547-570 (in German).
HENSCHLER, D., HAHN, E., & ASSMANN, W. (1964) [Conditions for
increasing resistance by repeated inhalation of irritating
oedema- forming gases.] Naunyn-Schmiedebergs Arch. exp. Pathol.
Pharmakol., 249: 325-342 (in German).
HEUSS, J. M. & GLASSON, W. A. (1968) Hydrocarbon reactivity and eye
irritation. Environ. Sci. Technol., 3: 1109-1116.
HODGESON, J. A. (1972) Review of analytical methods for atmospheric
oxidants measurements. Int. J. environ. anal. Chem.,
2: 113-132.
HOLLAND, G. J., BENSON, D., BUSH, A., RICH, G. Q., & HOLLAND, R. P.
(1968) Air pollution simulation and human performance. Am. J.
public Health, 58: 1684-1691.
HOLZMAN, R. S., GARDNER, D. E., & COFFIN, D. L. (1968) In vivo
inactivation of lysosyme by ozone. J. Bacteriol.,
96: 1562-1566.
HUBER, G. L., MASON, R. J., LAFORCE, M., SPENCER, N.J., GARDNER, D.
E., & COFFIN, D. L. (1971) Alteration in the lung following the
administration of ozone. Arch. intern. Med., 128: 81-87.
HUETER, F. G., CONTNER, G. L., BUSCH, K. A., & HINNERS, R. G. (1966)
Biological effects of atmospheres contaminated by auto exhaust.
Arch. environ. Health, 12: 553-560.
HURST, D. J. & COFFIN, D. L. (1971) Ozone effect on lysosomal
hydrolases of alveolar macrophages in vitro. Arch. intern. Med.,
127: 1059-1063.
HURST, D. J., GARDNER, D. E., & COFFIN, D. L. (1970) Effect of O3 on
acid hydrolases of the pulmonary alveolar macrophage.
J. Reticuloendoth. Soc., 8: 288-300.
IKEMATSU, T., YAMAUCHI, Y., SAITO, H., NAGAOKA, S., & ENDO, R. (1976)
Tonsils and environmental pollution (second report) Influence of
ozone upon rabbit tonsils. Jpn. J. Tonsil., 15: 5-8.
ILO (1977) Occupational exposure limits for airborne toxic
substances. Geneva, International Labour Office, pp. 164-165
(Occupational Safety and Health Series No. 37).
ISOMURA, K., FUKASE, O., & WATANABE, H. (1976) [Cytoxicities and
mutagenicities of gaseous air pollutants.] J. Jpn. Soc. Air
Pollut., 11: 59-64 (in Japanese).
JAFFE, L. S. (1962) The biological effects of ozone on man and
animals. Am. J. public Health, 57: 1269-1277.
JAPAN PUBLIC HEALTH ASSOCIATION (1976) The relationship between
photochemical airpollution and adverse health effects-1975
Report, Tokyo, JPHA, pp. 24-27 (in Japanese).
JEGIER, Z. & P'AN, A. Y. S. (1973) Ozone as an air pollutant. Can. J.
public Health, 64: 161-166.
KAGAWA, J. & TOYAMA, T. (1975a) Effects of ozone and brief exercise on
specific airway conductance in man. Arch. environ. Health,
30: 36-39.
KAGAWA, J. & TOYAMA, T. (1975b) Photochemical air pollution; its
effects on respiratory function of elementary school children.
Arch. environ. Health, 30: 117-122.
KAGAWA, J., TOYAMA, T., & NAKAZA, M. (1976) Pulmonary function test in
children exposed to air pollution. In: Finkel, A. J. & Duel, W.
C., ed. Clinical implications of air pollution research. Acton,
MA, USA, Publishing Sciences Group Inc., pp. 305-320.
KERR, H. D., KULLE, T. J., MCILHANEY, M. L., & SWIDERSKY, P. (1975)
Effects of ozone on pulmonary function in normal subjects. An
environmental-chamber study. Am. Rev. resp. Dis., 111: 763-773.
KING, M. E. (1961) Biochemical effects of ozone, doctoral
dissertation, Illinois Institute of Technology, May 1961, pp.
1-85.
KLEINFELD, M., GIEL, C., & TABERSHAW, I. R. (1957) Health hazards
associated with inert-gas shielded metal arc welding. Arch. ind.
Health, 15: 27-31.
KONIGSBERG, A. S. & BACHMAN, C. H. (1970) Ozonized atmosphere and
gross motor activity of rats. Int. J. Biometeor, 14: 261-266.
KONNO, S. & OKITA, T. (1974) Determination of peroxacetyl (PAN)
produced in the atmosphere or in a photochemical reaction chamber
(1). Bull. Inst. Public Health, Japan, 23: 50-58.
KOTIN, P. & FALK, H. L. (1956) The experimental induction of pulmonary
tumors in strain-A mice after their exposure to an atmosphere of
ozonized gasoline. Cancer, 9: 910-917.
KOTIN, P., FALK, H. L., & MCCAMMON, C. J. (1958) III. The experimental
induction of pulmonary tumors and changes in the respiratory
epithelium in C 57 BL mice following their exposure to an
atmosphere of ozonized gasoline. Cancer, 11: 473-481.
KUDRJAVCEVA, O. F. (1963) [On the possibility of chronic effects of
ozone in working conditions.] Gig. truda i Prof. Zabol., 6: 52
(in Russian).
KULLE, T. J. (1972) The effects of ozone and formaldehyde on the
trigeminal nasal sensory system. Thesis, University of
Cincinnati.
KUSOMOTO, S., NARUYAMA, Y., YONEKAWA, E., ODA, H., & NAKAJIMA, T.
(1976) Acute effects of photochemical oxidants and ozone exposure
on mice. J. Jpn. Soc. Air Pollut., 11: 70-80 (in Japanese).
KYEI-ABOAGYE, K., HAZUCHA, M., WYSZOGRODSKI, I., RUBINSTEIN, D., &
AVERY, M. E. (1973) The effect of ozone exposure in vivo on the
appearance of lung tissue lipids in the endobronchial lavage of
rabbits. Biochem. Biophys. Res. Commun., 54: 907-913.
LAGERWERFF, J. M. (1963) Prolonged ozone inhalation and its effects on
visual parameters. Aerospace Med., 34: 479-486.
LARSEN, R. I. (1969) A new mathematical model of air pollution
concentration averaging time and frequency. J. Air Pollut.
Control Assoc., 19: 24-30.
LARSEN, R. I. (1974) An air quality data analysis system for
interrelating effects, standards, and needed source
reductions-part 2. J. Air Pollut. Control Assoc., 24(6):
551-558.
LEWIS, T. R., HUETER, F. G., & BUSCH, K. A. (1967) Irradiated
automobile exhaust: its effects on reproduction in mice. Arch.
environ. Health, 15: 26-35.
LITT, R. S., VAUGHAN, S., BIRKINSHAW, P., COIT, H., & SANDERS, B.
(1968) Effects of low concentrations of ozone on temporal
discrimination. In: Air pollution project: An educational
experiment in self-directed research, 1968, Pasadena,
Associated Students of the California Institute of Technology,
pp. 51-64.
LOOSLI, C. G., BUCKLEY, R. D., HERTWECK, M. S., HARDY, J. D., RYAN, D.
P., STINSON, S., & SEREBRIN, R. (1972) Pulmonary response of mice
exposed to synthetic smog. Ann. oecup. Hyg., 15: 251-260.
MACLEAN, S. A., LONOWELL, A. C., & BLOGOSLAWSKI, W. J. (1973) Effects
of ozone-treated sea water on the spawned, fertilized, meiotic,
and cleaving eggs of the commercial American oyster. Mutat.
Res., 21: 283-285.
MCJILTON, C., THIELKE, J., & FRANK, R. (1972) Ozone uptake model for
the respiratory system. Am. Ind. Hyg. Assoc. J., 33: 20.
MCMILLAN, R. S., WISEMAN, D. H., HANES, B., & WEHRLE, P. F. (1969)
Effects of oxidant air pollution on peak expiratory flow rates in
Los Angeles school children. Arch. environ. Health, 18:
941-949.
MAHONEY, L. E. J. (1971) Wind flow and respiratory mortality in Los
Angeles. Arch. environ. Health, 22: 344-347.
MATSUMURA, Y. (1970a) The effects of ozone, nitrogen dioxide, and
sulfur dioxide on experimentally induced allergic respiratory
disorder in guinea pigs. I. The effect on sensitization with
albumin through the airway. Am. Rev. respir. Dis.,
102: 430-437.
MATSUMURA, Y. (1970b) The effects of ozone, nitrogen dioxide and
sulfur dioxide on experimentally induced allergic respiratory
disorder in guinea pigs. II. The effects of ozone on the
absorption and the retention of antigen in the lung. Am. Rev.
respir. Dis., 102: 438-443.
MATSUMURA, Y., MIZUNO, K., MIYAMOTO, T., SUZUKI, T., & OSHIMA, Y.
(1972) The effects of ozone, nitrogen dioxide, and sulfur dioxide
on experimentally induced allergic respiratory disorder in guinea
pigs, IV effects on respiratory sensitivity to inhaled
acetylcholine. Am. Rev. respir. Dis., 105: 262-267.
MATZEN, R. N. (1957) Development of tolerance to ozone in reference to
pulmonary edema. Am. J. Physiol., 190: 84-88.
MENDENHALL, R. M. & STOKINGER, H. E. (1959) Tolerance and
cross-tolerance development to atmospheric pollutants, ketene and
ozone. J. appl. Physiol., 14: 923-926.
MENDENHALL, R. M. & STOKINGER, H. E. (1962) Films from lung washings
as a mechanism model for lung injury by ozone. J. appl.
Physiol., 17: 28-32.
MENZEL, D. B. (1970) Toxicity of ozone, oxygen and radiation. Ann.
Rev. Pharmacol., 10: 379-394.
MENZEL, D. B. (1971) Oxidation of biologically active reducing
substances by ozone. Arch. environ. Health, 23: 149-153.
MENZEL, D. B., ROEHM, J. N., & LEE, S. D. (1972) Vitamin E: the
biological and environmental antioxidant. J. agric. food Chem.,
20: 481-486.
MENZEL, D. B., SLAUGHTER, R. J., BRYANT, A.M., & JAUREGUI, H. O.
(1975) Heinz bodies formed in erythrocytes by fatty acid ozonides
and ozone. Arch. environ. Health, 30: 296-301.
MERZ, T., BENDER, M. A., KERR, H. D., & KULLE, T. J. (1975)
Observations of aberrations in chromosomes of lymphocytes from
human subjects exposed to ozone at a concentration of 0.5 ppm for
6 and 10 hours. Mutat. Res., 31: 299-302.
MIKAMI, R. & KUDO (1973) Air pollution -- especially in terms of
oxidants and respiratory organs. Intern. Med. (Japan),
32: 837-844.
MILLER, F. J. (1977) A mathematical model of transport and removal of
ozone in mammalian lungs. Thesis submitted to the Graduate
Faculty of North Carolina State University, Raleigh, NC, USA, pp.
66.
MILLER, P. R., MCCUTCHAN, M. H., & MILLIGAN, H. P. (1972) Oxidant air
pollution in the Central Valley, Sierra Nevada foothills and
Mineral King Valley of California. Atmos. Environ., 6: 623-633.
MILLER, S. & EHRLICH, R. (1958) Susceptibility to respiratory
infections of animals exposed to ozone. J. infect. Dis.,
103: 145-149.
MILLS, C. A. (1957) Respiratory and cardiac deaths in Los Angeles
smogs. Am. J. med. Sci., 233: 379-386.
MIZOGOUCHI, I., OSAWA, M., SATO, Y., MAKINO, K., & YAGYU, H. (1973) A
study on erythrocyte and photochemical smog. I: Effects of air
pollutants on erythrocyte resistance. J. Jpn. Soc. Air Pollut.,
8: 414.
MOORMAN, W. J., CHMIEL, J. J., STARA, J. F., & LEWIS, T. R. (1973)
Comparative decomposition of ozone in the nasopharynx of beagles.
Acute vs chronic exposure. Arch. environ. Health, 26: 153-155.
MOTLEY, H. L., SMART, R. H., & LEFTWICH, C. I. (1959) Effect of
polluted Los Angeles air (smog) on lung volume measurements.
J. Am. Med. Assoc., 171: 1469-1477.
MOUNTAIN, J. T. (1963) Detecting hypersusceptibility to toxic
substances: an appraisal of simple blood tests. Arch. environ.
Health, 6: 357-365.
MOUNTAIN, J. T., WAGNER, W. D., FAIRCHILD, E. J., STOCKELL, F. R., JR,
& STOKINGER, H. E. (1960) Biochemical effects of ozone and
nitrogen dioxide on laboratory animals. In: 138th National
Meeting of The American Chemical Society, New York, September
1960, Cincinnati, OH, US Dept of Health, Education and Welfare,
32 pp.
MUDD, J. B., LEAVITT, R., ONGUN, A., & MCMANUS, T. T. (1969) Reaction
of ozone with amino acids and proteins. Atmos. Environ.,
3: 669-682.
MUELLER, P. X., LOEB, L., & MAPES, W. H. (1973) Decomposition rates of
ozone in living areas. Environ. Sci. Technol., 7: 342-346.
MURPHY, S. D., LENA, J. K., ULRICH, C. E., & DAVIS, H. V. (1963)
Effects on animals of exposure to auto exhaust. Arch. environ.
Health, 7: 60-70.
MURPHY, S. D., ULRICH, C. E., FRANKOWITZ, & XINTARAS, C. (1964a)
Altered function in animals inhaling low concentrations of ozone
and nitrogen dioxide. Am. Ind. Hyg. Assoc. J., 25: 246-253.
MURPHY, S. D., DAVIS, H. V., & ZARATZIAN (1964b) Biochemical effects
in rats from irritating air contaminants. Toxicol. appl.
Pharmacol., 6: 520-528.
MUSTAFA, M. G. (1975) Influence of dietary vitamin E on lung cellular
sensitivity to ozone in rats. Nutr. Rep. Int., 11: 473-476.
MUSTAFA, M. G., DELUCIA, A. J., YORK, G. K., ARTH, C., & CROSS, C. E.
(1973) Ozone interaction with rodent lung. II. Effects on oxygen
consumption of mitochondria. J. lab. clin. Med., 82: 357-365.
NAKAJIMA, T., KUSUMOTO, S., TSUBOTA, Y., YONEKAWA, E., YOSHIDA, R.,
MOTOMIYA, K., ITO, K., IDE, G., & OTSU, H. (1972)
Histopathological studies on the respiratory organs of mice
exposed to photochemical oxidants and auto exhaust. Proc. Osaka
Prefect. Inst. Public Health, 10: 35-42 (in Japanese).
NATIONAL ACADEMY OF SCIENCES (1977) Ozone and other photochemical
oxidants, Washington DC, Printing and Publishing Office,
National Academy of Sciences, 719 pp.
NEDERBRAGT, G. W., VAN DER HORST, N., & VAN DUIJN, J. (1965) Rapid
ozone determination near an accelerator. Nature (Lond.),
206: 4979.
NEVSKAJA, A. I. & DITERIHS, D. D. (1957) Problems of hygiene of labor
in H2O2 production by electrodynamic method. Gig. truda i prof
Zabol., 4: 16.
NEVSKAJA, A. I. & KOCETKOVA, T. A. (1961 ) The toxicology of ozone and
sulfuric acid aerosol in their combined action. Gig. truda i
prof Zabol., 1: 20-29.
NIEBOER, H. & VAN HAM, J. (1976) Peroxyacetyl nitrate (PAN) in
relation to ozone and some meteorological parameters at Delft in
the Netherlands. Atmos. Environ., 10: 115-120.
NIEDING, VON, G. WAGNER, H. M., LOLLGEN, H., & KREKELER, H. (1977)
[Acute effects of ozone on lung function in man. VDI Colloquium
on ozone and other substances in photochemical smog.]
VDI-Berichte, 270: 123-129 (in German).
NORTH ATLANTIC TREATY ORGANIZATION (1974) Air Quality criteria for
photochemical oxidants and related hydrocarbons. A report by the
Expert Panel on Air Quality Criteria, Brussels, Belgium, NATO
Committee on the Challenges of Modern Society, 366 pp.
OHMORI, K. (1974) [The effect of photochemical smog on humans,
especially biological effects by low concentrations of ozone.]
Environ. Pollut. Control. 10: 1042-1046 (in Japanese).
PACE, D. M., LANDOLT, P. A., & AFTONOMOS, B. T. (1969) Effects of
ozone on cells in vitro. Arch. environ. Health, 18: 165-170.
PALMER, M. S., SWANSON, D. H., & COFFIN, D. L. (1971) Effect of O3 on
benzpyrene hydroxylase activity in the Syrian golden hamster.
Cancer Res., 31: 730-733.
PALMER, M. S., EXLEY, R. W., & COFFIN, D. L. (1972) Influence of
pollutant gases on benzpyrene hydroxylase activity. Arch.
environ. Health, 25: 439-442.
P'AN, A. Y. S. & JEGIER, Z. (1970) The effect of sulfur dioxide and
ozone on acetylcholinesterase. Arch. environ. Health,
21: 498-501.
P'AN, A. Y. S. & JEGIER, Z. (1972) Trypsin protein esterase in
relation to ozone-induced vascular damage. Arch. environ.
Health, 24: 233-236.
P'AN, A. Y. S., BELAND, J., & JEGIER, Z. (1972) Ozone-induced arterial
lesions. Arch. environ. Health, 24: 229-232.
PEARLMAN, M. E., FINKLEA, J. F., SHY, C. M., VAN BRUGGEN, J., &
NEWILL, V. A. (1971) Chronic oxidant exposure and epidemic
influenza. Environ. Res., 4: 129-140.
PENHA, P. D., AMARAL, L., & WERTHAMER, S. (1972) Ozone air pollutants
and lung damage. Ind. Med., 41: 17-20.
PLOPPER, C. G., DUNGWORTH, D. L., & TYLER, W. S. (1973a) Pulmonary
lesions in rats exposed to ozone. Am. J. Pathol., 71: 375-394.
PLOPPER, C. G., DUNGWORTH, D. L., & TYLER, W. S. (1973b)
Ultrastructure of pulmonary alveolar macrophages in situ in
lungs from rats exposed to ozone. Am. Rev. respir. Dis.,
108: 532-638.
PRAT, R., NOFRE, C., & CIER, A. (1968) Effets de l'hypochlorite de
sodium, de l'ozone et des radiations ionisantes sur les
constituants pyrimidiques d'Escherichia coli. Ann. Inst.
Pasteur, 114: 595-607.
PURVIS, M. R., MILLER, S., & EHRLICH, R. (1961) Effect of atmospheric
pollutants on susceptibility to respiratory infection. I. Effect
of ozone. J. infect. Dis., 109: 238-242.
RASMUSSEN, R. A. (1970) Isoprene: Identified as a forest-type emission
to the atmosphere. Environ. Sci. Technol., 4(8): 667-671.
RASMUSSEN, R. A. (1972) What do the hydrocarbons from trees contribute
to air pollution? J. Air Pollut. Control Assoc., 22: 537-543.
RAVEN, P. B., DRINKWATER, B. L., RUHLING, R. O., BOLDUAN, N., TAGUCHI,
S., GLINER, J., & HORVATH, S. M. (1974) Effect of carbon monoxide
and peroxyacetyl nitrate on man's maximal aerobic capacity.
J. appl. Physiol., 36: 288-293.
REGENER, V. H. (1964) Measurement of atmospheric ozone with the
chemiluminescent method. J. geophys. Res., 69: 3795-3800.
REGENER, V. H. & ALDAZ, L. (1969) Turbulent transport near the ground
as determined from measurements of the ozone flux and the ozone
gradient. J. geophys. Res., 74: 6935-6942.
RENZETTI, N. A. & GOBRAN, V. (1957) Studies of eye irritation due to
Los Angeles smog 1954-1956. San Marino, CA, Air Pollution
Foundation, pp. 170.
REYNOLDS, R. W. & CHAFFEE, R. R. J. (1970) Studies on the combined
effects of ozone and a hot environment on reaction time in
subhuman primates. In: Project clean air, Santa Barbara,
California University, pp. 1-8 (California University Res. Proj.
S-6.).
RICHARDSON, N. A. & MIDDLETON, W. C. (1957) Evaluation of filters for
removing irritants from polluted air. Los Angeles, University
of California, Dept of Engineering, 31 pp. (Report No. 57-43).
RICHARDSON, N. A. & MIDDLETON, W. C. (1958) Evaluation of filters for
removing irritants from polluted air. Heat. Piping Air Cond.,
30: 147-154.
RIPPERTON, L. A., JEFFRIES, H. E., & WORTH, J. B. (1971) Relationship
of measurements in nonurban air to air pollution: Ozone and
oxides of nitrogen, CP 13B, In: England, H. M. & Berry, W. T.,
ed. Proceedings of the Second International Clean Air Congress.
New York, Academic Press, pp. 386-390.
ROEHM, J. N., HADLEY, J. G., & MENZEL, D. B. (1971a) Oxidation of
unsaturated fatty acids by ozone and nitrogen dioxide. Arch.
environ. Health, 23: 142-148.
ROEHM, J. N., HADLEY, J. G., & MENZEL, D. B. (1971b) Antioxidants vs
lung disease. Arch. intern. Med., 128: 88-93.
ROEHM, J. N., HADLEY, J. G., & MENZEL, D. B. (1972) The influence of
vitamin E on the lung fatty acids of rats exposed to O3. Arch.
environ. Health, 24: 237-242.
ROKAW, S. N. & MASSEY, F. (1962) Air pollution and chronic respiratory
disease. Am. Rev. respir. Dis., 86: 703-704.
ROTH, R. P. & TANSY, M. F. (1972) Effects of gaseous air pollutants on
gastric secreto-motor activities in the rat. J. Air Pollut.
Control Assoc., 22: 796-709.
RUDOLF, W. (1974) [Investigation of oxidants in the city centre of
Frankfurt.] Schriftenr. Ver. wasser-, Boden- Lufthyg.,
42: 65-75 (in German).
SABERSKY, R. H., SINEMA, D. A., & SHAIR, F. H. (1973) Concentrations,
decay rates, and removal of ozone and their relation to
establishing clean indoor air. Env. Sci. Technol., 7: 347-353.
SACHSENMAIER, W., SIEBS, W., & TAN, T. (1965) [Effects of ozone upon
mouse ascites tumor cells and upon chick fibroblasts in tissue
culture.] Z. Krebsforsch., 67: 113-126 (in German).
SANDALLS, F. J., PENKETT, S. A., & JONES, B. M. R. (1974) Preparation
of peroxyacetyl nitrate (PAN) and its determination in the
atmosphere. London, England, Her Majesty's Stationery Office
(Atomic Energy Research Establishment Report AERE R7807).
SCHEEL, L. D., DOBROGORSKI, O. J., MOUNTAIN, J. T., SVIRBELY, J. L., &
STOKINGER, H. E. (1959) Physiologic biochemical, immunologic, and
pathologic changes following ozone exposure. J. appl. Physiol.,
14: 67-80.
SCHLIPKOETER, H. W. & BRUCH, J. (1973) [Functional and morphological
alterations caused by exposure to ozone.] Zentralb. Bakteriol.,
Parasitenk. Infektionskr. Hyg., Reihe B, 156: 486-499 (in
German).
SCHLIPKOETER, H. W., FODOR, G. C., GHELERTER, L., & DOLGNER, R. (1973)
[Findings from animal tests concerning synergism.] In:
Proceedings of the 3rd International Clean Air Congress,
Diisseldorf, Federal Republic of Germany, pp. A26-A30 (in
German).
SCHOETTLIN, C. E. (1962) The health effect of air pollution on elderly
males. Am. Rev. respir. Dis., 86: 878-897.
SCHOETTLIN, C. E. & LANDAU, E. (1961) Air pollution and asthmatic
attacks in the Los Angeles area. Public Health Rep.,
76: 545-548.
SCHUCK, E. A. & DOYLE, G. J. (1959) Photooxidation of hydrocarbons in
mixtures containing oxides of nitrogen and sulfur dioxide. San
Marina, CA, Air Pollution Foundation (Report No. 29).
SCHUCK, E. A. & STEPHENS, E. R. (1969) Oxides of nitrogen. Adv.
environ. Sci., 1: 73-118.
SHINGU, H., TANAKA, I., KUSUMOTO, S., NAKAJIMA, T., WATANABE, M., &
SUGIYAMA, M. (1972) [Effect of ozone on defense mechanisms to
infection.] J. Jpn. Soc. Air Pollut, 9: 332 (in Japanese).
SKILLEN, R. G., THIENES, C. H., CANGELOSI, J., & STRAIN, L. (1961)
Brain 5-hydroxytryptamine in ozone-exposed rats. Proc. Soc. Exp.
Biol. Med., 108: 121-122.
SMITH, L. E. (1965) Inhalation of the photochemical smog compound
peroxyacetyl nitrate. Am. J. public Health, 55(9): 1460-1468.
STASIUK, W. N. & COFFEY, P. E. (1974) Rural and urban ozone
relationships in New York State. J. Air Pollut. Control Assoc.,
24(6): 564-568.
STEPHENS, E. R. (1964) Absorptivities for infra-red determination of
peroxyacetyl nitrates. Anal. Chem., 36: 928-929.
STEPHENS, E. R. (1969) The formation, reactions and properties of
peroxyacetyl nitrates (PANs) in photochemical air pollution.
Adv. environ. Sci. Technol., 1: 119-146.
STEPHENS, E. R. (1976) Peroxyacetyl nitrate (PAN) in relation to ozone
and some meteorological parameters at Delft in the Netherlands.
Atmos. Environ., 10(7): 566.
STEPHENS, E. R., DARLEY, E. F., TAYLOR, O. C., & SCOTT, W. E. (1961)
Photochemical reaction products in air pollution. Int. J. Air
Water Pollut., 4: 79-100.
STEPHENS, R. J., FREEMAN, G., CRANE, S.C., & FURIOSI, N.J. (1971)
Ultrastructural changes in the terminal bronchiole of the rat
during continuous low level exposure to nitrogen dioxide. Exp.
molec. Pathol., 14: 1-19.
STEPHENS, R. J., FREEMAN, G., & EVANS, M. J. (1972) Early response of
lungs to low levels of nitrogen dioxide. Arch. environ. Health,
24: 160-179.
STEPHENS, R. J., FREEMAN, G., STARA, J. F., & COFFIN, D. L. (1973)
Cytologic changes in dog lungs induced by chronic exposure to
ozone. Am. J. Pathol., 73: 711-726.
STEPHENS, R. J., SLOAN, M. F., EVANS, M. J., & FREEMAN, G. (1974)
Early response of lung to low levels of O3. Am. J. Pathol.,
74:31-58.
STERLING, T. D., PHAIN, J. J., POLLACK, S. V., SCHUMSKY, D. A., &
DEGROOT, I. (1966) Urban morbidity and air pollution: a first
report. Arch. environ. Health, 13: 158-170.
STERLING, T. D., POLLACK, S. V., & PHAIR, J. J. (1967) Urban hospital
morbidity and air pollution: a second report. Arch. environ.
Health, 15: 362-374.
STOKINGER, H. E. (1957) Evaluation of the hazards of ozone and oxides
of nitrogen. Am. Med. Assoc. Arch. ind. Health, 15: 181-190.
STOKINGER, H. E. (1959) Factors modifying toxicity of ozone. Adv.
Chem. Ser., 21: 360-369.
STOKINGER, H. E. (1965) Ozone toxicology: a review of research and
industrial experience 1954-1964. Arch. environ. Health,
10: 719-731.
STOKINGER, H. E. & SCHEEL, L. D. (1962) Ozone toxicity: immunochemical
and tolerance-producing aspects. Arch. environ. Health,
4: 327-334.
STOKINGER, H. E., WAGNER, W. D., & WRIGHT, P. G. (1956) Studies of
ozone toxicity: I. potentiating effects of exercise and tolerance
development. Am. Med. Assoc. Arch. ind. Health, 14: 158-162.
STOKINGER, H. E., WAGNER, W. D., & DORBROGORSKI, O. J. (1957) Ozone
toxicity studies. III. chronic injury to lungs of animals
following exposure at a low level. Am. Med. Assoc. Arch. Ind.
Health, 16: 514-522.
SUZUKI, T. & NAGAOKA, S. (1973) [The susceptibility to serotonin and
exposure to ozone.] Ann. Rep. Tokyo Metrop. Res. Inst. Environ.
Prot., No. 4, pp. 262-263 (in Japanese).
SUZUKI, T., NAKAZAWA, K., UMEDA, M., GOTO, H., & NAGAOKA, S. (1975)
[Effect on the blood in rats exposed to ozone. I. Effect on
osmotic fragility of orythrocytes.] Ann. Rep. Tokyo Metrop. Res.
Inst. Environ. Prot., 6: 270-272 (in Japanese).
SWANN, H. E. & BALCHUM, O. J. (1966) Biological effects of urban air
pollution. Arch. environ. Health, 12: 698-704.
TAN, W. C., CORTESI, R., & PRIVETT, O. S. (1974) Lipid peroxide and
lung prostaglandins. Arch. environ. Health, 28: 82-84.
THOMPSON, G. E. (1971) Cardiovascular considerations of rat pulmonary
edema. Mil. Med., 136: 50-65.
TOMINGAS, V. R., POTT, F., & DEHNEN, W. (1973) Biological test for
carcinogenicity of polycyclic aromatic hydrocarbons. Arch.
Geschwilstforsch., 42: 298-306.
TOYAMA, T., KAGAWA, J., TSUNODA, T., & NAKAZA, M. (1977) [An
epidemiological estimation of dose-response relationship to the
mixture of atmospheric pollutants.] Jpn. J. Hyg., 32:70 (in
Japanese).
TRAMS, E., LAUTER, C. J., BRANDENBURGER-BROWN, E. A., & YOUNG, O.
(1972) Cerebral cortical metabolisms after chronic exposure to
O3. Arch. environ. Health, 24: 153-159.
URY, H. K. (1968) Photochemical air pollution and automobile accidents
in Los Angeles. Arch. environ. Health, 17: 334-342.
US DEPARTMENT OF HEALTH EDUCATION AND WELFARE (1970) Air Quality
criteria for photochemical oxidants. Washington, DC, US DHEW
(National Air Pollution Control Administration Publication No. AP
63).
US ENVIRONMENTAL PROTECTION AGENCY (1964-1973) Camp data. Research
Triangle Park, NC, National Aerometric data bank, US EPA.
US ENVIRONMENTAL PROTECTION AGENCY (1973) Investigation of high ozone
concentration in the vicinity of Gomet County, Maryland and
Preston County, West Virginia. Research Triangle Park, NC, EPA
(Report No. EPA-R4-73-019).
US ENVIRONMENTAL PROTECTION AGENCY (1976) Monitoring and air quality
trends report, 1974, pp. 115-123 (Report No. EPA-450/1-76).
VAUGHAN, T. R., JR, JENNELLE, L. F., & LEWIS, T. R. (1969) Long-term
exposure to low levels of air pollutants. Effects on pulmonary
function in the beagle. Arch. environ. Health, 19: 45-50.
VENINGA, T. S. (1967) Toxicity of ozone in comparison with ionizing
radiation. Strahlentherapie (Munich), 134: 469-477.
WARREN, G. J. & BABCOCK, G. (1970) Rev. Sci. Instrum., 41: 280-282.
WATANABE, M. TANAKA, I., SHINGU, H., ASAKA, J., KUSUMOTO, S.,
NAKAJIMA, T., & SUGIYAMA, M. (1973a) [The cytotoxicity of ozone.]
Jpn. J. public Health, 20: 554 (in Japanese).
WATANABE, S., FRANK, R., & YOKOYAMA, E. (1973b) Acute effects of ozone
on lungs of cats. I. Functional. Am. Rev. respir. Dis.,
108: 1141-1151.
WAYNE, W. S., WEHRLE, P. F., & CARROLL, R. E. (1967) Oxidant air
pollution and athletic performance. J. Am. Med. Assoc.,
199: 901-904.
WENT, F. W. (1966) On the nature of Aitken condensation nuclei.
Tellus, 28(2): 549-556.
WERTHAMER, S., SCHWARTZ, L. H., & SOSKIND, L. (1970) Bronchial
epithelial alterations and pulmonary neoplasia induced by ozone.
Pathol. Microbiol., 35: 224-230.
WERTHAMER, S., PENHA, P. D., & AMARAL, L. (1974) Pulmonary lesions
induced by chronic exposure to ozone. I. Biochemical alterations.
Arch. environ. Health, 28: 164-166.
WHITE, W. H., ANDERSON, J. A., BLUMENTHAL, D. L., HUSAR, R. B.,
GILLANI, N. V., HUSAR, J. D., & WILSON, W. E., JR (1976)
Formation and transport of secondary air pollutants: Ozone and
aerosols in the St Louis urban plume. Science, 194: 187-189.
WORLD HEALTH ORGANIZATION (1972) WHO Tech. Rep. Series No. 506. (Air
quality criteria and guides for urban air pollutants: Report of a
WHO Expert Committee.) 35 pp.
WORLD HEALTH ORGANIZATION (1976) Selected methods of measuring air
pollutants, Geneva WHO (WHO Offset Publ. No. 24).
WORLD HEALTH ORGANIZATION (1977) Oxides of nitrogen: Environmental
health criteria 4, Geneva, WHO, 79 pp.
XINTARAS, C., JOHNSON, B. L., ULRICH, C. E., TERRILL, R. E., &
SOBECKI, M. F. (1966) Application of the evoked response
technique in air pollution toxicology. Toxicol. appl.
Pharmacol., 8: 77-87.
YOKOYAMA, E. (1972a) [The effects of O3 on lung function. In:
Proceedings of a Conference on Photochemical Air Pollution.]
Tokyo, Jpn. Soc. Air Pollution, pp. 109-129 (in Japanese).
YOKOYAMA, E. (1972b) Effect of ventilation with ozone on
pressure-volume relationships of excised dogs' lungs. Am. Rev.
Respir. Dis., 105: 594-604.
YOKOYAMA, E. (1973) The effects of low-concentrations of ozone on the
lung pressure of rabbits and rats. Presented at: The Japan
Society of Industrial Hygiene Annual Meeting, 46th, April, pp.
134-135 (paper 223) (in Japanese).
YOKOYAMA, E. (1974) [Maximal expiratory flow volume curve of rabbit
lung exposed to ozone.] Jpn. J. thorac. Dis., 12: 556-561 (in
Japanese).
YOKOYAMA, E. & FRANK, R. (1972) Respiratory uptake of ozone in dogs.
Arch. environ. Health, 25: 132-138.
YOUNG, W. A., SHAW, D. B., & BATES, D. V. (1963) Pulmonary function in
welders exposed to ozone. Arch. environ. Health, 7: 337-340.
YOUNG, W. A., SHAW, D. B., & BATES, D. V. (1964) Effect of low
concentrations of ozone on pulmonary function in man. J. appl.
Physiol., 19: 765-768.
ZELAC, R. E., CROMROY, H. L., BOLCH, W. E., JR, DUNAVANT, B. G., &
BEVlS, H. A. (1971a) Inhaled ozone as a mutagen I. Chromosome
aberrations induced in chinese hamster lymphocytes. Environ.
Res., 4: 262-282.
ZELAC, R. E., CROMROY, H. L., BOLCH, W. E., JR, DUNAVANT, B. G., &
BEVIS, H. A. (1971b) Inhaled ozone as a mutagen II. Effect on the
frequency of chromosome aberrations observed in irradiated
chinese hamsters. Environ. Res., 4: 325-342.
6.3.4 Effects on children
Ventilatory performance, measured twice a month for 11 months by
the Wright Peak Flow Meter, in 78 elementary school children in two
cities in the Los Angeles Basin was unaffected by differences in the
daily mean oxidant levels which were 320 µg/m3 (0.16 ppm) and
220 µg/m3 (0.11 ppm) respectively (McMillan et al., 1969).
Kagawa & Toyama (1975b) and Kagawa et al. (1976) studied the
weekly variations in lung function (airway conductance and ventilatory
performance) of 20, normal, 11-year-old, school children in Tokyo in
relation to variations in temperature and ambient concentrations of
ozone, nitrogen dioxide, nitric oxide, sulfur dioxide, hydrocarbons,
and particulate matter. Students were tested from June 1972 to October
1973. Ozone levels were determined by the ethylene-chemiluminescence
method. Temperature was the factor most highly correlated with
variations in specific airway conductance (negative correlation) and
maximum expiratory flow rate (Vmax) at 25 and 50% FVC (positive
correlation). Significant negative correlations between ozone and
specific airway conductance, and between nitrogen dioxide, nitric
oxide, sulfur dioxide, and particulate matter and Vmax at 25 or 50%
FVC were observed in some children. The ranges of hourly pollutant
levels, at the time of the lung function test (13h00), that were used
for correlation during the study period were: 0-560 µg/m3
(0-0.28 ppm) for ozone, 40-550 µg/m3 (0.02-0.29 ppm) for nitrogen
dioxide, 30-340 µg/m3 (0.01-0.13 ppm) for sulfur dioxide, and
50-490 µg/m3 for particulate matter (Toyama et al., 1977).
The effect of oxidant air pollution exposure on the incidence and
duration of A2 influenza in an epidemic that occurred in 1968-69 was
studied retrospectively by Pearlman et al. (1971) in 3500 elementary
school children from 5 southern California communities. These areas
were selected to represent an exposure gradient for photochemical
oxidants, although no difference in community oxidant exposure was
present at the time (December 1968-January 1969) of the epidemic.
Information from school absenteeism, questionnaires on illness, and
haemagglutination-inhibition titres did not reveal any statistically
significant morbidity differences corresponding to the pollution
gradient that existed during the season of peak oxidant levels.
Several Japanese investigators have reported acute reactions among
school children exposed to moderately elevated concentrations of
oxidants of 200-400 µg/m3 (0.10-0.20 ppm), on smoggy days in a number
of urban areas. Not only eye and respiratory irritation, but systemic
symptoms such as paraesthesia, prostration, and convulsions were
observed. In reviewing these studies the WHO Task Group was aided by
several investigators and observers from Japan. In the opinion of the
Task Group, eye and respiratory effects observed during these episodes
may well have been caused by reported oxidant concentrations. However,
systemic symptoms could not be explained only by the observed oxidant
concentrations, but were more likely to be attributable to individual
psychosomatic reactions among the students. This judgement is
supported by the observation that acute systemic reactions were
observed in only some of the students, most of whom were exercising at
the time of peak oxidant levels. Thus, the students may well have
experienced acute irritation of the pharynx and trachea, enhanced by
their active status. However, the general population in the same
neighbourhood, including young children in primary grades and school
teachers, did not appear to have had these systemic symptoms. These
comments are based on the following studies.
Fujii (1972) reported the acute effects of an episode of
photochemical smog in Osaka, Japan, on 27 August 1971. The oxidant
concentration measured by the NBKI method rose to an hourly value of
360 µg/m3 (0.18 ppm) by 14h00. At this time, the peak ozone
concentration, determined by the chemiluminescent method was
480 µg/m3 (0.24 ppm) and the maximum sulfur dioxide concentration was
180 µg/m3 (0.07 ppm). A total of 249 school children reported
symptoms including pain in the throat, headache, coughing, breathing
difficulties, eye irritation, and numbness in the limbs. On 14
September 1971, maximum hourly ozone concentrations reached 480 µg/m3
(0.24 ppm) and a total of 290 persons complained of similar symptoms.
These data do not provide a basis for estimating symptom frequency in
the general population, because the total population at risk was not
reported.
In an evaluation of the clinical status of 15 high school children
who were hospitalized after the onset of acute symptoms that began
during photochemical smog episodes in Osaka, Japan, Adachi & Nakajima
(1974) stated that "they were astonished by the seriousness of the
conditions which surpassed by far the symptoms previously known in
connection with the relation between photochemical oxidant
concentration and its effect on man in the case of Los Angeles".
Affected students were not only found to have irritation of the eyes
and respiratory tract, but, systemic symptoms such as paraesthesia,
muscle spasms as well as an increased leukocyte count and borderline
elevation of serum alkaline phosphatase (3.1.3.1).
Based upon complaints found in schools that were registered with
local health centres located in Japanese cities, the Japan Public
Health Association (1976) reported a significant increase in
subjective complaints, particularly eye irritation, when photochemical
oxidant concentrations exceeded hourly values of 300 µg/m3
(0.15 ppm), as determined by the 10% NBKI method.
Mikami & Kudo (1973) described various local and systemic symptoms
in 82 school children that were attributed to 3 photochemical air
pollution episodes in Tokyo and Osaka in 1970, 1971, and 1972 with
maximum 1-h concentrations ranging from 300 to 580 µg/m3 (0.15 to
0.29 ppm). A large proportion of cases were reported to have eye
irritation, throat irritation, cough, breathlessness, headache, and
chills. The authors pointed out that many of these symptoms had not
been reported under Los Angeles smog conditions.
6.3.5 Effects on the incidence of acute respiratory and
cardiovascular diseases
In studies on college students in the Los Angeles Basin, Durham
(1974) showed that acute episodes of pharyngitis, bronchitis, and
upper respiratory infections were associated with peak concentrations
of oxidants and mean concentrations of sulfur dioxide and nitrogen
dioxide. Oxidants, sulfur dioxide, and nitrogen dioxide
(concentrations not given) were, in this order, consistently
associated with the various episodes of illness. A comparison of
selected high and low pollution days indicated that photochemical air
pollution might have been responsible for a 16.7% increase in acute
respiratory symptoms seen in Los Angeles schools situated in areas of
highest concentration compared with those in areas of lowest
concentration. The author's method of data presentation did not permit
the estimation of dose-response relationships or of threshold
concentrations.
Brant & Hill (1964) and Brant (1965) did not find any correlation
between admissions for cardiovascular conditions to Los Angeles County
Hospital and oxidant levels (170 µg/m3; 0.08 ppm as a mean value of
daily 7-h measurements for the study periods) either on the day of
admission or for 2 weeks prior to admission, for the period between 8
August and 25 December 1954. However, Sterling et al. (1966, 1967)
found statistically significant but very low correlations between
admissions to Los Angeles hospitals for respiratory episodes during
the period 17 March-26 October 1961 and daily mean levels of oxidants
(74 µg/m3; 0.037 ppm), ozone (84 µg/m3; 0.042 ppm), and carbon
monoxide (11 mg/m3; 9.5 ppm). Because of the limited time period and
the extremely low correlation coefficients, it is difficult to
conclude that a real relationship was demonstrated by these studies.
6.3.6 Effects on the prevalence of chronic respiratory diseases and
on pulmonary function
Deane et al. (1965) conducted a survey on the prevalence of
chronic respiratory disease in outdoor male telephone workers in San
Francisco and Los Angeles. Symptoms of persistent cough and phlegm
were slightly less prevalent in Los Angeles than in San Francisco in
the 40-49-year group but more prevalent in the 50-59-year group (31.4%
compared with 16.3% in San Francisco). These data were adjusted for
cigarette smoking. No differences in pulmonary function test results
were found.
The prevalence of chronic respiratory symptoms and the results of
pulmonary function tests were compared in 2 similar groups of
nonsmoking, adult, male and female Seventh Day Adventists aged 45-64
years residing in Los Angeles and San Diego, respectively. Annual mean
oxidant values (94 µg/m3; 0.047 ppm and 76 µg/m3; 0.038 ppm), were
essentially the same in the 2 cities, but a mean of maximum daily
concentrations in Los Angeles (290 µg/m3; 0.144 ppm) was twice as
high as that in San Diego (148 µg/m3; 0.074 ppm). The survey was
performed when oxidant levels were low and similar in both cities, to
minimize effects attributable to acute oxidant exposures. No
differences were found in symptom prevalence or in several measures of
ventilatory function. Prevalence rates for chronic respiratory disease
were uniformly low (less than 4%) in both groups (Cohen et al., 1972).
6.3.7 Effects on patients with pre-existing diseases
6.3.7.1 Asthma
The association between reported asthma attacks in 137 patients
from Pasadena, California, and oxidant levels during the period 3
September-9 December 1956 was studied by Schoettlin & Landau (1961).
Daily records of the time of onset of asthma attacks were kept by each
patient and collected weekly. The daily number of patients afflicted
with asthma was moderately well correlated (r = 0.37) with concurrent
maximum hourly oxidant readings. Asthma attacks were more weakly
correlated with temperature, relative humidity, and water vapour
pressure than with oxidants. The number of patients having attacks on
days when maximum 1-h oxidant levels were higher than 500 µg/m3
(0.25 ppm) was significantly greater (p = 0.05) than the number of
those having attacks on days with lower oxidant levels. Unfortunately,
the analysis failed to isolate the pronounced seasonal variation of
asthma. In the northern hemisphere, asthma attack rates tend to reach
a peak in October and November (Booth et al., 1965), and decline
sharply in late November and early December. This pattern corresponds
closely to seasonal declines in peak oxidant levels. Hence, it is
possible that observed asthma-oxidant correlations have been secondary
to simultaneous seasonal changes in asthma frequency and oxidant
levels.
6.3.7.2 Chronic respiratory diseases
Studies over 18 months on 25 patients with severe, chronic,
obstructive lung disease at a chronic disease centre in Los Angeles
County showed that the prevailing levels of oxidants, oxidant
precursors, temperature, and relative humidity did not produce any
effects on lung function (Rokaw & Massey, 1962).
In studies on the effects of ambient air pollution exposure on
armed forces veterans with chronic respiratory disease living in the
Domiciliary Unit and Chronic Disease Annex of the Los Angeles Veterans
Administration Center, subjects were evaluated once a week by
pulmonary function tests and respiratory symptom questionnaires
(Schoettlin, 1962). Analysis of variance did not show any
statistically significant effects of air pollution (concentrations not
reported) on respiratory symptoms or function, although maximum
concentrations of oxidant and oxidant precursors consistently
accounted for more of the variation in frequency of symptoms and
clinical signs of disease than maximum temperature, relative humidity,
or pollen counts.
6.3.8 Cancer
A 5-year prospective study of lung cancer from 1958 to 1963 was
conducted by Buell et al. (1967) among 69 160 members of the
California American Legion. Long-term residents of Los Angeles County
had slightly lower age-smoking adjusted lung cancer rates (95.4 per
100 000) than residents of the San Francisco Bay area counties and San
Diego County (102 per 100 000). These urban groups, in turn, had
higher rates than all other California counties (75.5 per 100 000).
Nonsmokers followed the same geographical pattern: 28.1 per 100 000
for Los Angeles, 43.9 per 100 000 for San Francisco and San Diego,
11.2 per 100 000 for all others. Smokers of more than one packet of 20
cigarettes a day in Los Angeles had the highest lung cancer rates,
241.3 per 100 000, compared with San Francisco-San Diego, 226 per
100 000, and with other counties, 137.5 per 100 000. The duration of
exposure necessary to induce lung cancer may have been longer than the
actual ozone exposures in this population study. Thus, it would appear
worthwhile to extend these observations to subsequent years.
6.3.9 Motor vehicle accidents
The association between automobile accidents and days of elevated
oxidant levels (120-480 µg/m3; 0.06-0.24 ppm) in Los Angeles was
studied from August to October in 1963 and 1965. Applying a sign-test
and nonparametric correlation analysis to the data, Ury (1968) found a
statistically significant relationship between oxidant levels and
automobile accidents. Concentrations of carbon monoxide, oxides of
nitrogen, and other pollutants would also be relatively elevated when
oxidants were high and may have contributed to the results reported.
Furthermore, the association of accidents with oxidant levels may be
confounded by the fact that traffic jams produce both more accidents
and a greater output of oxidant precursors.
6.4. Summary Tables
Studies on the health effects of controlled, industrial, and
community exposures that provide quantitative information useful for
establishing guidelines for the protection of public health with
respect to photochemical oxidants are summarized in Tables 12, 13 and
14.
Table 12. Controlled human studies
I. Sensory effects
Ozone concentration Length of Effects Responsea Subjects Reference
exposure
µg/m3 (ppm) days h/day
400 (0.2) 1 3 and 6 Diminution in various measures of Not applicable 22 healthy males, 6 Lagerwerff (1963)
700 (0.35) 1 3 and 6 visual perception; noneffect dose healthy females
1000 (0.5) 1 3 and 6 not determined; no mention of
dose-response relationship.
> 200 (> 0.1) 1 working Increasing eye irritation with Not applicable 20 women office Richardson &
hours (study increase in oxidant exposures; workers Middleton
repeated for no apparent effects below (1957, 1958)
123 days) 200 µg/m3 (0.1 ppm).
40-100 (0.02- Immediate odour perception after 9/10 at 10-14 healthy males Henschler et al.
0.05) beginning of exposure; odour 40 µg/m3 (1960)
perception disappeared in ´-12 13/14 at
min; odour considerably stronger at 100 µg/m3
higher dose.
15-40 (0.008 Odour perception immediately after Not available 20 healthy subjects Eglite (1968)
-0.02) beginning of exposure; the most
sensitive person perceived odour
at the level of 15 µg/m3
(0.008 ppm).
II. Effects on respiratory function
A. Exposure to ozone
Ozone concentration Length of Effects Subjects Reference
exposure
µg/m3 (ppm) days h/day
2000 (1.0) 1 1 Consistent increase in airway 4 healthy males Goldsmith et Nadel
resistance; exposures at 200, 800, (1969)
and 1200 µg/m3 (0.1, 0.4, 0.6 ppm)
caused effect in some subjects,
but no dose-response pattern.
1800 (0.9) 1 5 min Highly significant decrease of airway 4 healthy males Kagawa & Toyama
conductance after inhalation with exercise. (1975a)
1200- (0.6-0.8) 1 2 Significant reduction in diffusing capacity of 10 healthy men and Young et al.
1600 lung and in FEV0.75b; substernal soreness one healthy woman (1964)
present in all subjects.
1000 (0.5) 6/wk 3 Significant decrease in FEV1.0c; no effect at 12 healthy males Bennet (1962)
x 12 400 µg/m3 (0.2 ppm).
wk5
1000 (0.5) 1 6 Significant change in airway conductance 20 healthy subjects Kerr et al. (1975)
and pulmonary resistance (dry cough and (19 men and 1
chest discomfort). woman)
1000 (0.5) 1 2 Decrease in pulmonary function 7 healthy males Hackney et al.
measurements. (1975a)
a Response = number of subjects showing effect described
total number of subjects
Table 12. Controlled human studies--continued
II. Effects on respiratory function
A. Exposure to ozone (cont'd)
Ozone concentration Length of Effects Subjects Reference
exposure
µg/m3 (ppm) days h/day
800 (0.4) 1 2 and 4 After 2-h exposure: Rawd increase, FVCe 22 healthy male Rummo et al.
decrease MMFRf decrease; after 4-h subjects (1975.
exposure: Rawd increase, FVCe decrease. unpublished
MMFRf decrease and additionally FEV1.0c data)
decrease; RVg, FRCh, and TCLi did not
change.
740 & (0.37 & 1 2 Significant decrease in ventilatory function 6-10 healthy males Bates et al.
1500 0.75) and in closing volume under intermittent (1972).
exercise; effect more pronounced at higher Hazucha et al.
dose. (1973)
740, (0.37, 1 2 Dose dependent change of ventilatory 28 healthy subjects Folinsbee et al.
1000 & 0.50 & pattern; significant reduction in MEFRj at (20 males and 8 (1975)
1500 0.75) 50% of vital capacity at 740 µg/m3 females)
(0.37 ppm) or more, under exercise.
740 (0.37) 1 2 Significant increase in total respiratory 2 healthy and 3 Hackney et al.
resistance under intermittent light exercise sensitively males. (1975a)
500 (0.25) 1 2 No consistent changes in lung function. 3 healthy and 3 Hackney et al.
sensitively males (1975a)
200- (0.1- 1 30 min Tendency towards increase in breathing 4 healthy male Ohmori (1974)
500 0.25) frequency and volume under exercise. subjects
200 (0.1) 1 2 Increase of Rawd and AaDO2k in 7 of 11 test 11 healthy male von Neiding et al.
subjects under intermittent light exercise. subjects (1977)
B. Exposure to mixtures of ozone and other air pollutants
Ozone concentration Length of Effects Subjects Reference
exposure
µg/m3 (ppm) days h/day
740 + 960 (0.37 + 0.37 1 2 More pronounced decrease in ventilatory 4 healthy males Bates & Hazucha
sulfur sulfur function end in closing volume than in (1973)
dioxide dioxide single exposure to ozone at 740 µg/m.3
(0.37 ppm).
200 + 9400 (0.1 + 5 1 2 Increases in Rawd and AaDO2k similar 11 healthy male von Nieding et al.
nitrogen nitrogen to those observed with ozone alone at subjects (1977)
dioxide dioxide 200 µg/m3 (0.1 ppm).
200 + 9400 (0.1 + 5 1 2 Increases in Rawd and AaDO2k similar 11 healthy male von Nieding et al.
nitrogen nitrogen to those observed with ozone at subjects (1977)
dioxide + dioxide + 5 200 µg/m3 (0.1 ppm) + nitrogen dioxide
sulfur sulfur at 9400 µg/m3 (5 ppm); recovery time
dioxide dioxide) delayed.
50 + 100 (0.025 + 0.05 1 2 No effect on Rawd and AaDO2k; 11 healthy male von Nieding et al.
dioxide +260 nitrogen sensitivity of the respiratory tract subjects (1977)
sulfur dioxide dioxide + 0.1 to acetylcholine increased.
sulfur dioxide)
C. Exposure to peroxyacetylnitrate
Ozone concentration Length of Effects Subjects Reference
exposure
µg/m3 (ppm) days h/day
1500 (0.3) 1 5 min Increase in oxygen uptake with exercise; 32 college males Smith (1965)
no effect at rest; significant decrease
in MEFRj during the recovery phase
following exercise.
1350 (0.27) 1 42 min Minor changes in cardiorespiratory and 20 healthy male Raven et al.
temperature regulation parameters. subjects (1974)
II. Effects on respiratory function
D. Exposure to irradiated automobile exhaust
Oxidants (0.22 Short term No significant changes in reaction 14 healthy Holland et al.
440-540 -0.27) (no details time, vital capacity, and submaximum subjects(1968)
given) work performance on the bicycle
ergometer.
Carbon (15-29)
monoxide
17-
33 mg/m3
Table 12. Controlled human studies--continued
II. Effects on respiratory function
E. Exposure to ambient air with an elevated concentration of oxidants
Ozone concentration Length of Effects Subjects Reference
exposure
µg/m3 (ppm) days h/day
Carbon (800-1400)
dioxide
1520-
2660 mg/m3
Nitric (0.38-0.58)
oxide
470-710
Nitrogen (0.7-1.0)
dioxide
1300-1900
Hydrocarbons
traces
Aldehydes (0.2-0.7)
Formaldehyde (0.2-0.24)
250-300
400-1400 (0.2-0.7) 2-90 h Improvement in lung function upon 46 patients with Motley et al.
residence in clean filtered room chronic lung (1959)
for 40 h or longer; threshold disease
concentration not determined.
100-460 (0.05-0.23) 21 24 Decrease in airway resistance and 15 patients with Balchum (1973)
increase in arterial partial oxygen moderately severe
pressure during week of residence chronic lung
in clean filtered air; threshold disease
concentration not determined.
Table 12. Controlled human studies
III. Changes in the electroencephalogram
Ozone concentration Length of Effects Responsea Subjects Reference
exposure
µg/m3 (ppm) days h/day
10 (0.005) 1 3 min Decrease in alpha rhythm. 1/3 3 healthy subjects Eglite (1968)
15 (0.008) 1 3 min Decrease in alpha rhythm. 2/3 3 healthy subjects Eglite (1968)
20 (0.01) 1 3 min Decrease in alpha rhythm. 3/3 3 healthy subjects Eglite (1968)
a Response = number of subjects showing effect described
total number of subjects
b FEV0.75 = 0.75 second forced expiratory volume
c FEV1.0 = one second forced expiratory volume
d Raw = airway resistance
e FVC = forced vital capacity
f MMFR = mid-maximal expiratory flow rate
g RV = residual volume
h FRC = functional residual capacity
i TLC = total lung capacity
j MEFR = maximum expiratory flow rate
k AaDO2 = alveolar to arterial oxygen pressure difference
l sensitive (subjects) = those with a prestudy history of cough, chest discomfort, or wheezing associated with
allergy or air pollution exposure, but with normal base-line pulmonary function studies
Table 13. Studies on the effects of industrial exposures
Ozone concentration Averaging Effects Reference
time
µg/m3 (ppm)
1600-3400 (0.8-1.7) 1 h 11 of 14 welders complained of respiratory symptoms; symptoms Challen et al. (1958)
disappeared when ozone levels were reduced to
400 µg/m3 (0.2 ppm); nitrogen dioxide probably present.
600-1600 (0.3-0.8) 1 h Increased frequency of chest tightness and throat irritation among Kleinfeld et al. (1957)
welders; no complaints at 500 µg/m3 (0.25 ppm); welders also
exposed to nitrogen dioxide and particulates but concentration
levels not given.
500-800 (0.25-0.40) long-term Increased frequency of headache, weakness, change in neuromuscular Kudrjavceva (1963)
sensitivity, and decrease in memory among workers manufacturing
hydrogen peroxide and exposed to ozone for 7-10 years; threshold
concentration not determined.
400-600 (0.2-0.3) 1 h No evidence for changes in vital capacity or functional residual Young et al. (1963)
capacity in welders; nitrogen dioxide probably present.
80-1000 (0.04-0.50) long-term Increased prevalence of bronchitis and emphysema in workers Nevskaja & Diterihs
engaged in manufacture of hydrogen peroxide for many years; (1957)
sulfuric acid aerosols also present; threshold concentration not
determined.
Table 14. Studies on the effects of community exposures
Hourly oxidant concentration Effects Subjects Reference
µg/m3 (ppm)
500 and above (0.25 and above) Increased frequency of asthma; possible confounding of 137 patients with Schoettlin
asthma-oxidant association with seasonal effects; other asthma & Landau (1961)
pollutants present but not reported.
approx. 1000 (approx. 0.50) Increased symptoms began at hourly concentrations of 102 student Hammer et al.
(headache) 100 µg/m3 (0.05 ppm), (eye irritation) nurses (1974)
300 µg/m3 (0.15 ppm), (cough) 530 µg/m3 (0.265 ppm).
(chest discomfort) 580 µg/m3 (0.29 ppm); threshold
levels determined by "hockey stick" functions.
240 and above (0.12 and above) Impaired performance determined by failure to improve 116 high school Wayne et al.
running times; threshold determined by "hockey stick" cross-country (1967)
functions. runners
> 300 (0.15) Increased frequency of complaints, particularly eye 7440 school Japan Public
irritation. children Health
Association (1976)
0-560 (0-0.28) Specific airway conductance of sensitive subjects was 20 healthy Kagawa et al.
(range at time significantly decreased with increasing hourly oxidant 11-year-old et al. (1977)
of lung concentrations; temperature, nitric oxide, nitrogen school children
function test) dioxide, sulfur dioxide, and particulate matter also
showed significant correlations with various
respiratory function tests of highly reactive children;
threshold concentration not determined.
7. EVALUATION OF HEALTH RISKS FROM EXPOSURE TO PHOTOCHEMICAL OXIDANTS
There appears to be sufficient information from experimental and
epidemiological studies to justify an attempt to establish guidelines
on the exposure limits for ozone and to review those for "oxidants"
(as measured by the neutral-buffered potassium iodide method (NBKI)),
proposed by a WHO Expert Committee in 1972. The Task Group appreciated
the fact that photochemical air pollution contains other substances
besides ozone, such as nitrogen dioxide, peroxyacetylnitrate, and
possibly many other gaseous and particulate products of atmospheric
photochemical reactions. However, present knowledge about the
composition of photochemical pollution, the concentrations of
individual components, and their possible impact on human health is so
limited that no attempt can be made to estimate exposure limits for
any single compound other than ozone. The Task Group was, of course,
aware that some sensory effects of photochemical air pollution (such
as eye irritation) might be due to a large extent, to these poorly
defined components of the photochemical oxidant mixture. As nitrogen
dioxide is an important air pollutant in its own right, it has been
discussed in a separate criteria document (World Health Organization,
1977).
7.1 Exposure Conditions
Exposure of man to ozone must have occurred for millions of years,
as ozone, present naturally in higher tropospheric layers, is also
found regularly in the lower atmosphere even in completely uninhabited
regions like the Antarctic. These natural concentrations have been
reported to have values ranging from 10 to 100 µg/m3
(0.005-0.05 ppm). It is difficult to determine the proportions of
natural to man-made oxidants (including ozone) that occur in rural
areas in most countries. In general, ozone concentrations greater than
120 µg/m3 (0.06 ppm) are considered to be related to man-made
activities. Ozone may be transported over hundreds of kilometres and
rural populations may be exposed to the pollutant, which earlier was
considered to exist only in Urban areas. A characteristic of such
exposures is that the precursors have vanished and ozone can therefore
persist for days in succession, since it does not come into contact
with other pollutants that act as its scavengers.
In large urban areas with strong sunshine and dense traffic or
other sources of precursors, photochemical air pollution is a daylight
phenomenon with maximum 1-h ozone concentrations sometimes as high as
300-800 µg/m3 (0.15-0.4 ppm), occurring around noon or somewhat
later. Such peak concentrations are preceded by nitrogen dioxide peaks
and accompanied by concurrent rises in peroxyacetylnitrate
concentrations. In contrast to oxides of sulfur and smoke, ozone
exposures are always intermittent, the peak concentrations rarely
lasting for more than 2-3 h. In the low temperature season,
photochemical reactions are much less likely to occur at rates
sufficient to produce large quantities of ozone.
Unless certain industrial technological processes (e.g., welding)
are in operation, ozone concentrations indoors tend to be considerably
lower than those outdoors due to the presence of reactive surfaces,
air conditioning, and indoor smoking.
7.2 Exposure-effect Relationships
Information presented in sections 5 and 6 is sufficient to
evaluate the relationship between exposure and the associated effects,
at least for some biological changes observed in man and experimental
animals.
7.2.1 Animal data
There is a considerable amount of evidence that short-term,
prolonged, or repeated exposure to ozone concentrations ranging from
200 to 400 µg/m3 (0.1-0.2 ppm) can cause a variety of biological
changes in several animal species and that these effects become more
pronounced with higher concentrations and increased exposure time (see
Table 10). These effects are, of course, also influenced by other
factors such as the animal species, the length of the interval between
exposures, the presence of other pollutants, low temperature, and
physical activity.
The host's pulmonary defence mechanisms against infectious
microorganisms are affected in several animal species by exposure to
ozone (section 5.2.6). This may result in rapid multiplication of
infectious microorganisms in situ, causing disease and eventually
death. An increase in mortality, resulting from the joint action of
infectious microorganisms and ozone, has been demonstrated in
artificially infected mice after a 3-h exposure to ozone at 160 µg/m3
(0.08 ppm). These effects were dose-related.
Pathomorphological changes in the respiratory tract of various
animal species, such as the rat, cat, rabbit, and mouse, have been
observed at ozone concentrations of about 400 µg/m3 (0.2 ppm) and
higher (section 5.2.1). Short-term exposures (up to 24 h) produce
oedema, degeneration and destruction of type I alveolar cells, loss of
ciliated epithelium, and breakdown of capillary endothelium. When the
exposure is repeated or its length extended, the biological changes
become more severe and include emphysema, atelectasis, vascular
lesions, bronchopneumonia, and fibrosis.
Various functional changes in the respiratory tract begin at
levels of about 520 µg/m3 (0.26 ppm) (section 5.2.2). The activities
of several enzymes in the lung tissue are also influenced at exposure
levels of about 500 µg/m3 (0.25 ppm) (section 5.2.3).
After pre-exposure to ozone, animals appear to become tolerant to
ozone concentrations that would otherwise cause pulmonary oedema
(section 5.2.5). The development of tolerance in small rodents is
related to pre-exposure levels of at least 600 µ/m3 (0.3 ppm). This
tolerance does not protect animals from such effects of ozone as
inflammation, alterations in alveolar macrophage functions, and
impairment of respiratory functions, that can occur at concentrations
lower than those that produce oedema.
Although the primary target for ozone is the respiratory system, a
number of studies have indicated that exposure to ozone may also
result in some extrapulmonary effects, but the mechanisms of such
action are not clear (section 5.3). For example, ozone exposure at
400-500 µg/m3 (0.2-0.25 ppm) for less than 2 h produced changes in
the circulating lymphocytes (increasing the number of binucleated
cells) and increased the number of spherocytes (section 5.3.2.1). It
has also been shown that exposure of pregnant mice to ozone at
200-400 µg/m3 (0.1-0.2 ppm) for 7 h per day, 5 days per week, for 3
weeks significantly increased neonatal mortality (section 5.3.3).
The available information concerning the carcinogenicity and
mutagenicity of ozone is inadequate for the definite evaluation of
such effects (sections 5.2.4 and 5.4).
Biological effects produced by the exposure of experimental
animals to a combination of ozone and nitrogen dioxide or by exposure
to complex pollutant mixtures containing oxidants, such as irradiated
automobile exhaust, are generally similar to those produced by
exposure to pure ozone. However, one study in which mice were exposed
to a mixture of ozone and nitrogen dioxide indicated that the effect
(reduction in resistance to respiratory infection) of a single
exposure to this mixture was additive, and that repeated exposure to
this mixture might result in a synergistic action.
7.2.2 Controlled human exposures
Although limited in number, human volunteer studies with
short-term, controlled exposure to oxidants have proved useful for
establishing exposure-effect relationships for ozone at levels ranging
from about 200-700 µg/m3 (section 6.1). Some of these studies provide
evidence of changes in the respiratory function of healthy subjects
that are related to exposure. Physical exercise tends to enhance the
respiratory effects of ozone (section 6.1.3).
Three investigators found a significant increase in airway
resistance with exposure to an ozone level of 740 µg/m3 (0.37 ppm)
for 2 h. One of the investigators did not find any effect with a 2-h
exposure to a level of 500 µg/m3 (0.25 ppm). However, this particular
study was conducted on subjects from southern California who were
later found to be less sensitive to ozone. Another investigator, using
similar test conditions, found a significant increase in airway
resistance in 7 out of 11 subjects at an ozone level of 200 µg/m3
(0.1 ppm) for 2 h (section 6.1.3.1).
In two studies, an improvement in lung function was noted when
patients with chronic pulmonary disease breathed filtered air for 40 h
or more, as compared with unfiltered ambient air. The ambient air
concentrations of oxidant in the two studies ranged from
400-1400 µg/m3 (0.2-0.7 ppm) and up to 400 µg/m3 (0.2 ppm)
respectively (section 6.1.3.5).
Result of a 7-month study in which female employees were exposed
to unfiltered ambient air during office hours suggested that the
lowest 1-h oxidant level that could be associated with eye irritation
was about 200 µg/m3 (section 6.1.2).
Although in vitro studies using human cells have shown some
evidence of a joint action of ozone and nitrogen dioxide, this has not
been clearly demonstrated in in vivo studies. For example, whereas a
potentiated increase in airway resistance was observed with exposure
to a combination of ozone at 740 µg/m3 (0.37 ppm) and sulfur dioxide
at 960 µg/m3 (0.37 ppm) for a period of 2 h, it was not observed with
exposure to a combination of ozone at 500 µg/m3 (0.25 ppm) and
nitrogen dioxide at 560 µg/m3 (0.30 ppm). Exposure to ozone at
50 µg/m3 (0.025 ppm) combined with nitrogen dioxide at 100 µg/m3
(0.05 ppm) and sulfur dioxide at 260 µg/m3 (0.1 ppm) did not have any
effect on airway resistance; however, this combined exposure resulted
in enhancement of the bronchoconstrictor effect of acetylcholine
(section 6.1.3.2).
7.2.3 Industrial exposure
Acute symptoms of chest tightness, irritation of the throat, and
coughing have been documented in welders exposed to 1-h ozone levels
of 600-1600 µg/m3 (0.3-0.8 ppm). These symptoms disappeared when
ozone concentrations fell to 500 µg/m3 (0.25 ppm) or less. Possible
chronic effects of repeated occupational exposure to ozone are not
well documented, although one investigator reported that workers
exposed to ozone levels in the range of 500-800 µg/m3 (0.25-0.40 ppm)
for 7-10 years had an increased frequency of headaches and weakness,
increased muscle excitability, and impaired memory (section 6.2).
7.2.4 Community Exposure
Several studies have shown more frequent eye irritation, reduced
athletic performance, changes in lung function of children, and
increased frequency of asthma attacks, all of which have been
associated with changes in hourly oxidant levels (sections 6.3.2,
6.3.3, 6.3.4, 6.3.7). As in all studies of the effects of community
exposures, it is difficult to determine precisely the lowest level at
which adverse effects become manifest. However, most of these effects
were observed when 1-h oxidant levels were in the range of about
200-500 µg/m3 (0.1-0.25 ppm). Although other pollutants such as
nitrogen dioxide, particulate matter, and sulfur dioxide were
simultaneously present, the strongest correlation of the observed
effects was with hourly levels of photochemical oxidants.
On the other hand, there is no evidence, so far, that long-term
exposure to photochemical oxidants at levels currently present in
urban air is associated with increased mortality (section 6.3.1), and
there is no evidence that chronic respiratory diseases such as
bronchitis, emphysema, and lung cancer are more prevalent in
communities with high oxidant exposures (section 6.3.6). However, it
should be pointed out that the number of epidemiological studies
concerned with such associations is small.
7.3 Guidelines on Exposure Limits
The exposure-effect relationships discussed in section 7.2 make it
possible to draw the following conclusions concerning the exposures to
oxidants and ozone at which the effects in man begin to appear:
(a) There is presumptive evidence from one controlled exposure
study that some effects on the lung function of healthy human subjects
might occur with exposure to an ozone level of 200 µg/m3 (0.1 ppm)
for 2 h.
(b) There is also evidence from general population studies that
suggests that 1-h ambient oxidant levels in the range of about
200-500 µg/m3 (0.1-0.25 ppm) may affect lung function in children,
increase the frequency of asthma attacks, cause more frequent eye
irritation, and reduce athletic performance.
(c) There is limited evidence from controlled exposure studies
that living in an environment with 1-h oxidant levels within the range
of 400-1400 µg/m3 (0.2-0.7 ppm) may exert additional stress on
patients with chronic pulmonary disease.
(d) There is convincing evidence from controlled human exposure
studies that airway resistance may be increased in healthy human
subjects following exposure to ozone levels of 700-800 µg/m3
(0.35-0.40 ppm) for 2h.
Animal data generally support the results of human studies.
However, some effects have been observed in animals at an ozone level
of about 200 µg/m3 (0.1 ppm) or even less, which have not yet been
demonstrated in man. For example, in animals, short-term exposures to
such concentrations appear to reduce resistance to pulmonary
infections.
The role of ozone and other photochemical oxidants in the etiology
of cancer is not clear. The only available epidemiological study did
not indicate any association between exposure to oxidants and the risk
of lung cancer, and experimental studies on the carcinogenicity and
mutagenicity of ozone in animals are not adequate for evaluation.
Nevertheless, the Task Group felt that there may be reason for concern
about the possible carcinogenicity of ozone (based primarily on some
biochemical considerations regarding the mechanism of the biological
effects of ozone). This aspect of its toxicity should be kept under
continual surveillance.
On the basis of all these considerations, the Task Group agreed
that 1-h levels of ozone of 100-200 µg/m3 (0.05-0.1 ppm) (measured by
the chemiluminescence method) could be used as a guideline for the
protection of public health. The relatively high natural
concentrations of ozone precluded the use of any safety factor.
The Task Group also agreed that a 1-h maximum level of 120 µg/m3
(0.06 ppm), which is approximately the highest natural background
concentration of oxidants, would be the best single value estimate of
the exposure limit for oxidants in the ambient air. This level is in
agreement with the long-term goal for photochemical oxidants (as
measured by the NBKI method) proposed by a WHO Expert Committee (World
Health Organization, 1972).
The issue was raised as to whether the proposed guideline was
realistic in view of natural exposure levels and the long-distance
transport of ozone. In response to this question, the Group expressed
the view that every effort should, nevertheless, be made to develop
control strategies for achieving the proposed guideline or at least,
for not exceeding it more than once a month.
REFERENCES
ADACHI, T. & NAKAJIMA, T. (1974) [Symptoms and clinical examinations
of the patients seriously injured by photochemical smog.] Rinsho
Kagaku (Clin. Chem.), 3:257-267 (in Japanese).
ALPERT, S. M. & LEWIS, T. T. (1971) Ozone tolerance studies utilizing
unilateral lung exposure. J. appl. Physiol., 31: 243-246.
ALPERT, S. M., SCHWARTZ, B. B., LEE, S. D., & LEWIS, T. R. (1971a)
Alveolar protein accumulation: a sensitive indicator of low level
oxidant toxicity. Arch. intern. Med., 128: 69-73.
ALPERT, S. M., GARDNER, D. E., HURST, D. J., LEWIS, T. R., & COFFIN,
D. L. (1971b) Effects of exposure to ozone on defensive
mechanisms of the lung. J. appl. Physiol., 31: 247-252.
AMERICAN CONFERENCE OF GOVERNMENT INDUSTRIAL HYGIENISTS (1975)
Threshold limit values for chemical substances in workroom air
adopted by ACGIH for 1975, Cincinnati, ACGIH.
ATWAL, O. S., & WILSON, T. (1974) Parathyroid gland changes following
ozone inhalation. Arch. environ. Health, 28: 91-100.
ATWAL, O. S., SAMAGH, B. S., & BHATNAGAR, M. K. (1975) A possible
autoimmune parathyroiditis following ozone inhalation: IIA
histopathologic, ultrastructural, and immunofluorescent study.
Am. J. Pathol., 80: 53-68.
BALCHUM, O. J. (1973) Toxicological effects of ozone, oxidant and
hydrocarbons. Proceedings of the Conference on Health Effects of
Air Pollution, Assembly of Life Sciences, National Academy of
Sciences-National Research Council, Washington, DC, Oct. 3-5. In:
Committee Print, Committee on Public Works, United States
Senate, Washington, DC, US Government Printing Office, pp.
489-503.
BALL, D. J. (1976) Photochemical ozone in the atmosphere of Greater
London. Nature (Lond.), 263: 580-582.
BARSKY, R. M. & BIRAKOS, J. N., ed. (1971) Profile of air pollution
control, Los Angeles, CA, County of Los Angeles Air Pollution
Control District, p. 73.
BARTH, D. S., ROMANOVSKY, J. C., KNELSON, J. H., ALTSHULLER, A. P., &
HORTON, R. J. M. (1971) Discussion (of national ambient air
quality standards). J. Air Pollut. Control Assoc., 21: 544-548.
BARTLETT, D. JR, FAULKNER, C. S., & COOK, K. (1975) Effect of chronic
ozone exposure on lung elasticity in young rats. J. appl.
Physiol., 37: 92-96.
BATES, D. V. & HAZUCHA, M. (1973) The short-term effects of ozone on
the human lung. Proceedings of the Conference on the Health
Effects of Air Pollution, Assembly of Life Sciences, National
Academy of Sciences-National Research Council, Wasington, DC,
Oct. 3-5. In: Committee Print, Committee on Public Works, United
States Senate, Washington DC, US Government Printing Office,
pp. 507-540.
BATES, D. V., BELL, G. M., BURNHAM, C. D., HAZUCHA, M., MANTHA, J.,
PENGELLY, L. D., & SILVERMAN, F. (1972) Short-term effects of
ozone on the lung. J. appl. Physiol., 32: 176-181.
BECKER, K. H. & SCHURATH, U. (1975) [The influence of oxides of
nitrogen on atmospheric oxidation processes.] Staub-Reinhall,
Luft, 35(4): 156-161 (in German).
BENNETT, G. (1962) Ozone contamination of high altitude aircraft
cabins. Aerospace Med., 33: 969-973.
BERRY, C. R. (1964) Differences in concentration of surface oxidant
between valley and mountain top conditions in the southern
Appalachians. J. Air Pollut. Control Assoc., 14(6): 238-239.
BILS, R. F. (1966) Ultrastructural alterations of alveolar tissue of
mice: I. Due to heavy Los Angeles smog. Arch. environ. Health,
12: 689-697.
BILS, R. F. (1970) Ultrastructural alterations of alveolar tissue of
mice: III. Ozone. Arch. environ. Health, 20: 468-480.
BILS, R. F. & ROMANOVSKY, J. C. (1967) Ultrastructural alterations of
alveolar tissue of mice: II. Synthetic photochemical smog. Arch
environ. Health, 14: 844-858.
BLOCH, W. N. JR, MILLER, F. J., & LEWIS, T. R. (1971) Pulmonary
hypertension in dogs exposed to ozone. Cincinnati, OH, Air
Pollution Control Office, US Environmental Protection Agency.
BOATMAN, E. S., SATO, S., & FRANK, R. (1974) Acute effects of ozone on
cat lungs. II: Structural. Am. Rev. respir. Dis., 110: 157-169.
BOBB, G. A. & FAIRCHILD, E. J. (1967) Neutrophil-to-lymphocyte ratio
as indicator of O3 exposure. Toxicol. appl. Pharmacol.,
11: 558-564.
BOOTH, S., DEGROOT, I., MARKUSH, R., & HORTON, R. J. M. (1965)
Detection of asthma epidemics in seven cities. Arch. environ.
Health, 10: 152-155.
BRANT, J. W. A. (1965) Human cardiovascular diseases and atmospheric
air pollution in Los Angeles, California. Int. J. Air Water
Pollut., 9: 219-231.
BRANT, J. W. A. & HILL, S. R. G. (1964) Human respiratory diseases and
atmospheric air pollution in Los Angeles, California. Int. J.
Air Water Pollut., 8: 259-277.
BRINKMAN, R., LAMBERTS, H. B., & VENINGA, T. S. (1964) Radiomimetic
toxicity of ozonized air. Lancet, 1: 133-136.
BUELL, G. C., TOKIWA, Y., & MUELLER, P. K. (1965) Potential
crosslinking agents in lung tissue; formation and isolation after
in vivo exposure to ozone. Arch. environ. Health,
10: 213-219.
BUELL, P., DUNN, J. E., JR, & BRESLOW, L. (1967) Cancer of the lung
and Los Angeles type air pollution: prospective study. Cancer,
20: 2139-2147.
BUTCHER, S.S. & RUFF, R. E. (1971) Effect of inlet residence time on
analysis of atmospheric nitrogen oxides and ozone. Anal. Chem.,
43(13): 1890-1892.
BYERS, D. H. & SALTZMAN, B. E. (1958) Determination of ozone in air by
neutral and alkaline iodide procedures. J. Am. Ind. Hyg. Assoc.,
19: 251-257.
CALIFORNIA STATE DEPARTMENT OF PUBLIC HEALTH (1955) Clean air for
California. Initial Report of the Air Pollution Study Project,
March 1955, 37 pp.
CALIFORNIA STATE DEPARTMENT OF PUBLIC HEALTH (1956) Clean air for
California. Second Report, March 1956, 10 pp.
CALVERT, J. G. (1976) Hydrocarbon involvement in photochemical smog
formation in Los Angeles atmosphere. Environ. Sci. Technol.,
10(3): 256-262.
CARSON, S. & GOLDHAMER, R. E. (1968) Biochemical defense mechanisms
against pulmonary irritants. Ohio, USA, Aerospace Medical
Research Laboratories, Aerospace Medical Division, Air Force
Systems Command, Wright-Patterson Air Force Base, pp. 129.
CASTLEMAN, W. L., DUNGWORTH, D. L., & TYLER, W. S. (1973a)
Histochemically detected enzymatic alterations in rat lung
exposed to ozone. Exp. mol. Pathol., 19: 402-421.
CASTLEMAN, W. L., DUNGWORTH, D. L., & TYLER, W. S. (1973b)
Cytochemically detected alterations of lung acid phosphatase
reactivity following ozone exposure. Lab. Invest., 29: 310-319.
CHALLEN, P. J. R., HICKISH, D. E., & BEDFORD, J. (1958) An
investigation of some health hazards in an inert-gas tungsten-arc
welding shop. Br. J. ind. ed., 15: 276-282.
CHOW, C. K. & TAPPEL, A. L. (1972) An enzyme protective mechanism
against lipid peroxidation damage to lungs of ozone-exposed rats.
Lipids, 7: 518-524.
CHOW, C. K. & TAPPEL, A. L. (1973) Activites of pentose shunt and
glycolytic enzymes in lungs of ozone-exposed rats. Arch.
environ. Health, 26: 205-208.
CHOW, C. K., DILLARD, C. J., & TAPPEL, A. L. (1974) Glutathione
peroxidase system and lysozyme in rats exposed to ozone and
nitrogen dioxide. Environ. Res., 7: 311-319.
CHRISTENSEN, E. & GIESE, A. C. (1954) Changes in absorption spectra of
nucleic acids and their derivatives following exposure to ozone
and ultraviolet radiation. Arch. Biochem. Biophys., 51:
208-216.
COFFIN, D. L. & BLOMMER, E. J. (1965) The influence of cold on
mortality from streptococci following ozone exposure. J. Air
Pollut. Control Assoc., 19: 523-524.
COFFIN, D. L. & BLOMMER, E. J. (1967) Acute toxicity of irradiated
auto exhaust. Its indication by enhancement of mortality from
streptococcal pneumonia. Arch. environ. Health, 15: 36-38.
COFFIN, D. L. & BLOMMER, E. J. (1970) Alteration of the pathogenic
role of streptococci group C in mice conferred by previous
exposure to ozone. In: Silver, I. H., ed. Aerobiology,
Proceedings of the 3rd International Symposium on Aerobiology,
University of Sussex, England, 1969. New York, Academic Press,
pp. 54-61.
COFFIN, D. L. & GARDNER, D. E. (1972a) Interaction of biological
agents and chemical air pollutants. Ann. occup. Hyg.,
15: 219-234.
COFFIN, D. L. & GARDNER, D. E. (1972b) The role of tolerance in
protection of the lung against secondary insults. In:
Proceedings of the International Conference of Occupational
Physicians of the Chemical Industry, Ludwigshafen, Germany,
April 1972, Research Triangle Park, NC, Environmental
Protection Agency, pp. 344-364.
COFFIN, D. L. & GARDNER, D. E. (1975) [Ozone Toxicology: Correlation
between tolerance and protective mechanisms of the lung.] Gig. i
Sanit., 1: 86-92 (in Russian).
COFFIN, D. L., BLOMMER, E. J., GARDNER, D. E., & HOLZMAN, R. S. (1968)
Effect of air pollution on alteration of susceptibility to
pulmonary infection. In: Proceedings of the 3rd Annual
Conference on Atmospheric Contamination in Confined Spaces,
Aerospace Medical Research Laboratory, pp. 71-80.
COFFIN, D. L., GARDNER, D. E., HOLZMAN R. S., & WOLOCK, F. J. (1968)
Influence of ozone on pulmonary cells. Arch. environ. Health,
16: 633-636.
COHEN, C. A., HUDSON, A. R., CLAUSEN, J. L., & KNELSON, J. H. (1972)
Respiratory symptoms, spirometry, and oxidant air pollution in
non-smoking adults. Am. Rev. resp. Dis., 105: 251-261.
CORTESI, R. & PRIVETT, O. S. (1972) Toxicity of fatty ozonides and
peroxides. Lipids, 7: 715-721.
COX, R. A., EGGLETON, A. E. J., DERWENT, R. G., LOVELOCK, J. E., &
PACK, D. H. (1975) Long-range transport of photochemical ozone in
North-Western Europe. Nature (Lond.), 255(5504): 118-121.
CRIEGEE, R. (1957) The course of ozonization of unsaturated compounds.
Rec. chem. Prog, 18: 111-120.
CRONIN, S. R. & GIRI, S. N. (1974) Effects of pulmonary irritants on
DNA, ATPase activity, and histamine on rat lung. Proc. Soc. Exp.
Biol. Med., 146: 120-125.
DARNALL, K. R., LLOYD, A. C., WINER, A. C., & PITTS, J. N., JR (1976)
Reactivity scale for atmospheric hydrocarbons based on reaction
with hydroxyl radical. Environ. sci. Technol. 10(7): 692-296.
DAVIS, I. (1961) Microbiologic studies with ozone. Mutagenesis of
ozone for Escherichia coli, Texas, USAF Aerospace Medical
Center, School of Aerospace Medicine, 9 pp. (Rep. No. 61-60).
DEANE, M., GOLDSMITH, J. R., & TUMA, D. (1965) Respiratory conditions
in outside workers: report on outside plant telephone workers in
San Francisco and Los Angeles. Arch. environ. Health,
10: 323-331.
DELUCIA, A. J., HOQUE, P.M., MUSTAFA, M. G., & CROSS, C. E. (1972)
Ozone interaction with rodent lung: effect on sulfhydryls and
sulfhydryl-containing enzyme activities. J. lab. clin. Med.,
80: 559-566.
DELUCIA, A. J., MUSTAFA, M. G., HUSSAIN, M. Z., & CROSS, C. E. (1975)
Ozone interaction with rodent lung: III. Oxidation of reduced
glutathione and formation of mixed disulfides between protein and
non-protein sulfhydrils. J. clin. Invest., 55: 794-802.
DEMERJIAN, K. L., KERR, J. A., & CALVERT, J. G. (1974) The mechanism
of photochemical smog formation. Adv. environ. Sci. Technol.,
4: 1-262.
DEMORE, W. B. & PATAPOFF, M. (1976) Comparison of ozone determinations
by ultraviolet photometry and gas-phase titoation. Environ. Sci.
Technol., 10(9): 897-899.
DERWENT, R. G. & STEWART, H. N. M (1973) Elevated ozone levels in the
air of Central London. Nature (Lond.), 241(5388): 342-343.
DILLARD, C. J., URRIBARRI, N., REDDY, K., FLETCHER, B., TAYLOR, S., DE
LUMEN, B., LANGBERG, S., & TAPPEL, A. L. (1972) Increased
lysosomal enzymes in lungs of ozone-exposed rats. Arch. environ.
Health, 25: 426-431.
DIMITRIADES, B. (1976) Photochemical oxidants in the ambient air of
the United States. Research Triangle Park, NC, US Environmental
Protection Agency, 192 pp. (EPA-600/3-76-017).
DIXON, J. R. & MOUNTAIN, J. T. (1965) Role of histamine and related
substances in development of tolerance to edemagenic gases.
Toxicol. appl. Pharmacol., 7: 756-766.
DOWELL, A. R., LOHRBAUER, L. A., HURST, D., & LEE, S. D. (1970) Rabbit
alveolar macrophage damage caused by in vivo O3 inhalation.
Arch. environ. Health, 21: 121-127.
DUNGWORTH, D. L. (1976) Short-term effects of ozone on lungs of rats,
mice, and monkeys. Environ. Health Perspect., 16: 179.
DUNGWORTH, D. L., CASTLEMAN, W. L., CHOW, C. K., MELLICK, P. W.,
MUSTAFA, M. G., TARKINGTON, B., & TYLER, W. S. (1975) Effect of
ambient levels of ozone on monkeys. Fed. Proc., 34: 1670-1674.
DURHAM, W. (1974) Air pollution and student health. Arch. environ.
Health, 28: 241-254.
EASTON, R. E. & MURPHY, S. D. (1967) Experimental ozone preexposure
and histamine. Arch. environ. Health, 15: 160-166.
EGLITE, M. (1968)[The problem of hygienic assessment of atmospheric
ozone.] Gig. i Sanit., 33: 17-22 (in Russian).
EMIK, L. O., PLATA, R. L., CAMPBELL, K. I., & CLARKE, G. L. (1971)
Biological effects of urban air pollution. Arch. environ.
Health, 23: 335-342.
ENVIRONMENT AGENCY (1975a) [Proceedings of the Second US Japan
Conference on Photochemical Air Pollution.] Tokyo, Japan (in
Japanese).
ENVIRONMENT AGENCY (1975b) [Air Pollution in Japan. Air monitoring
data in fiscal year 1974.] Tokyo, Japan, Air Pollution Control
Division, Air Quality Bureau, pp. 1200-1240 (in Japanese).
ENVIRONMENT AGENCY (1976) [Quality of the environment in Japan.]
Tokyo, Japan, Environment Agency, pp. 152-153 (in Japanese).
ERLICH, R., FINDLAY, J. C., FENTERS, J. D., & GARDNER, D. E. (1977)
Health effects of short-term inhalation of nitrogen dioxide and
ozone mixtures. Environ. Res., 14: 223-231.
EVANS, M. J., MAYR, W., BILS, R. F., & LOOSLI, C. G. (1971) Effects of
ozone on cell renewal in pulmonary alveoli of ageing mice. Arch.
environ. Health, 22: 450-453.
FABIAN, P. & PRUCHNIEWlCZ, P. G. (1973) Meridional distribution of
tropospheric ozone from ground-based registrations between Norway
and South Africa. Pure appl. Geophys., 106-108 (V-VII):
1027-1035.
FAIRCHILD, E. J., II (1963) Neurohumoral factors in injury from
inhaled irritants. Arch. environ. Health, 6: 79-86.
FAIRCHILD, E. J., II (1967) Tolerance mechanisms. Determinants of lung
responses to injurious agents. Arch. environ. Health,
14: 111-125.
FAIRCHILD, E. J., II & BOBB, G. A. (1965) The effect of spinal cord
lesions on the pathophysiology of a lung irritant ozone.
Toxicol. appl. Pharmacol., 7: 483 (Abstract).
FARWELL, S. O. & RASMUSSEN, R. A. (1976) Limitations of the FPD and
ECD in atmospheric analysis: A review. J. Chromatogr. Sci.,
14: 224-234.
FEDERAL REPUBLIC OF GERMANY, Ministry of Internal Affairs (1974)
Nox CnH volatile fluoride and chlorine compounds.
FETNER, R. H. (1958) Chromosome breakage in Victa faba by ozone.
Nature (Lond.), 181: 504-505.
FETNER, R. H. (1962) Ozone-induced chromosome breakage in human cell
cultures. Nature (Lond.), 194: 793-794.
FETNER, R. H. (1963) Mitotic inhibition induced in grasshopper
neuroblasts by exposure to ozone. USAF School of Aerospace
Medicine (Technical Documentary Report SAM-TDR 63-39).
FLETCHER, B. L. & TAPPEL, A. L. (1973) Protective effects of dietary
alphatocopherol in rats exposed to toxic levels ozone and
nitrogen dioxide. Environ. Res., 6: 165-175.
FOLINSBEE, L. J., SILVERMAN, F., & SHEPHARD, R. J. (1975) Exercise
responses following ozone exposure. J. appl. Physiol.,
38: 996-1001.
FOURNIER, E. (1973) Radicaux libres et toxicologie. Eur. J. Toxicol.,
6: 109-122.
FRANK, H. R., BRAIN, J. D., & SHERRY, D. E. (1970a) Unilateral
tolerance and altered elastic behaviour in rabbits. Am. Ind.
Hyg. Assoc. J., 31: 31 (Abstract section).
FRANK, N. R., YOKOYAMA, E., WARANABE, S., SHERRY, D. E., & BRAIN, J.
D. (1970b) An ozone Journal presented at the American Medical
Association, Air Pollution Medical Research Conference New
Orleans, 5-7 Oct., 19 pp.
FRANK, R., FLESCH, J.P., & BRAIN, J. D. (1971) Effect of ozone on
elastic behaviour of excised lungs of dogs. Environ. Res.,
4: 343-354.
FREEMAN, G., STEPHENS, R. J., CRANE, S.C., & FURIOSI, N.J. (1968)
Lesion of the lung in rats continuously exposed to two parts per
million of nitrogen dioxide. Arch. environ. Health,
17: 181-192.
FREEMAN, G., STEPHENS, R. J., COFFIN, D. L., & STARA, J. F. (1973)
Changes in dogs' lungs after long-term exposure to ozone. Arch.
environ. Health, 26: 209-216.
FREEMAN, G., JULIOS, L. T., FURIOSI, N. J., MUSSENDEN, R., STEPHENS,
R. J., & EVANS, M. J. (1974) Pathology of pulmonary disease from
exposure to interdependent ambient gases (NO2 & O3). Arch.
environ. Health, 29: 203-210.
FRIBERG, L., HOLMA, B., & RYLANDER, R. (1972) Animal lung reactions
after inhalation of lead and ozone. Environ. Physiol. Biochem.,
2: 170-178.
FUKASE, O., ISOMURA, K., & WATANABE, H. (1975a) Effect of ozone on
glutathione in vivo. J. Jpn. Soc. Air Pollut., 10: 58-62 (in
Japanese).
FUKASE, O., ISOMURA, K., & WATANABE, H. (1975b) Effect of ozone on
vitamin C in vivo. J. Jpn. Soc. Air Pollut., 10: 63-66.
FUJII, T. (1972) Studies on air pollution by photochemical reaction.
1. The results of field research of photochemical smog in Osaka,
1971. In: Proceedings of the Research Section, Osaka
Environmental Pollution Control Centre, No. 3, pp. 21-34 (in
Japanese).
GARDNER, D. E. (1971) Environmental influences on living alveolar
macrophages. A dissertation submitted to the Division of
Graduate Studies of the University of Cincinnati, Ann Arbor,
Michigan, University Microfilms International, pp. 191.
GARDNER, D. E., PFITZER, E. A., CHRISTIAN, R. T., & COFFIN, D. L.
(1971) Loss of protective factor for alveolar macrophages when
exposed to ozone. Arch. intern. Med., 127: 1078-1084.
GARDNER, D. E., LEWIS, T. R., ALPERT, S. M., HURST, D. J., & COFFIN,
D. L. (1972) The role of tolerance in pulmonary defense
mechanisms. Arch. environ. Health, 25: 432-438.
GARDNER, D. E., ILLING, J. W., MILLER, F. J., & COFFIN, D. L. (1974a)
The effect of ozone on pentobarbital sleeping time in mice.
Chem. Pathol. Pharmacol., 9: 689-700.
GARDNER, D. E., ILLING, J. W., & COFFIN, D. L. (1974b) Enhancement of
effect of exposure to O3 and NO2 by exercise. Toxicol. appl.
Pharmacol., 29: 129-130.
GARDNER, M. B. (1966) Biological effects of urban air pollution. III.
Lung tumors in mice. Arch. environ. Health, 12: 305-513.
GARDNER, M. B., LOOSLI, C. G., HAINES, B., BLACKMORE, W., & TEEBKEN,
D. (1969) Histopathologic findings in rats exposed to ambient and
filtered air. Arch. environ. Health, 19: 637-647.
GOLDSMITH, J. R. & NADEL, J. A. (1969) Experimental exposure of human
subjects to ozone. J. Air Pollut. Control Assoc., 19: 329-330.
GOLDSTEIN B. D. (1973) Hydrogen peroxide in erythrocytes. Detection in
rats and mice inhaling ozone. Arch. environ. Health,
26: 279-280.
GOLDSTEIN B. D. (1976) Combined exposure to ozone and nitrogen
dioxide. Env. Health Perspect., 13: 107-110.
GOLDSTEIN B. D., PEARSON, B., LODI, C., BUCKLEY, R. D., & BALCHUM, O.
J. (1968) The effect of ozone on mouse blood in vivo. Arch.
environ. Health, 16: 648-650.
GOLDSTEIN B. D., LODI, C., COLLINSON, C., & BALCHUM, O. J. (1969)
Ozone and lipid peroxidation. Arch. environ. Health,
18: 631-635.
GOLDSTEIN B. D., BUCKLEY, R. D., CARDENAS, R., & BALCHUM, O. J. (1970)
Ozone and vitamin E. Science, 169: 605.
GOLDSTEIN B. D., LAI, L. Y., ROSS, S. R., & CUZZI-SPADA, R. (1973)
Susceptibility of inbred mouse strains to ozone. Arch. environ.
Health, 27: 412-413.
GOLDSTEIN B. D., SOLOMON, S., PASTERNACK, B. S., & BICKERS, D. R.
(1975) Decrease in rabbit lung microsomal cytochrome P-450 levels
following ozone exposure. Chem. Pathol. Pharmacol.,
10: 759-762.
GOLDSTEIN, E., TYLER, W. S., HOEPRICH, P. D., & EAGLE, C. (1971a)
Ozone and the antibacterial defense mechanisms of the murine
lung. Arch. intern. Med., 127: 1099-1102.
GOLDSTEIN, E., TYLER, W. S., HOEPRICH, P. D., & EAGLE, C. (197lb)
Adverse influence of ozone on pulmonary bactericidal activity of
murine lung. Nature (Lond.), 229: 262-263.
GOLDSTEIN, E., EAGLE, M. C., & HOEPRICH, P. D. (1972) Influence of
ozone on pulmonary defense mechanisms of silicotic mice. Arch.
environ. Health, 24: 444-448.
GOLDSTEIN, E., WARSHAUER, D., LIPPERT, W., & TARKINGTON, B. (1974)
Ozone and nitrogen dioxide exposure. Arch. environ. Health,
28: 85-90.
GREGORY, A. R., RIPPERTON, L. A., & MILLER, B. (1967) Effect of
neonatal thymectomy on the development of ozone tolerance in
mice. Am. Ind. Hyg. Assoc. J., 28: 278-282.
GRIMSRUD, E. P., WESTBERG, H. H., RASMUSSEN, R. A. (1975) Atmospheric
reactivity of monoterpene hydrocarbons, NOx photooxidation and
ozonolysis. Int. J. Chem. Kin. Symp., 1: 183-195.
GUICHERIT, R. (1975) [Photochemical smog formation in the
Netherlands.] Delft, Netherlands, TNO Institute for
Environmental Hygiene, p. 27 (in Dutch).
HAAGEN-SMIT, A. J. (1952) Chemistry and physiology of Los Angeles
smog. Ind. Eng. Chem., 44: 1342-1346.
HACKNEY, J. D., LINN, W. S., LAW, D.C., KARUZA, S. K., GREENBERG, H.,
BUCKLEY, R. D., & PEDERSEN, E. E. (1975) Experimental studies on
human health effects of air pollutants. III. Two-hour exposure to
ozone alone and in combination with other pollutant gases. Arch.
environ. Health, 30: 385-390.
HACKNEY, J. D., LINN, W. S., KARUZA, S. K., BUCKLEY, R. D., LAW, D.C.,
BATES, D. V., HAZUCHA, M., PENGELLY, L. D., & SILVERMAN, F.
(1977) Effects of ozone exposure in Canadians and Southern
Californians. Arch. environ. Health, 34: 110-116.
HALLET, W. Y. (1965) Effect of ozone and cigarette smoke on lung
function. Arch. environ. Health, 10: 295-302.
HAMMER, D. I., HASSELBLAD, V., PORTNOY, B., & WEHRLE, P. F. (1974) Los
Angeles student nurse study: Daily symptom reporting and
photochemical oxidants. Arch. environ. Health., 28: 255-260.
HASSELBLAD, V., CREASON, J.P., & NELSON, W. C. (1976) Regression
using "hockey stick" functions. Research-Triangle Park, NC, US
Environmental Protection Agency, Health Effects Research
Laboratory, 12 pp. (EPA-600/1-76-024).
HATTORI, K., KATO, N., KINOSHITA, S., & SUNADA, T. (1963) Protective
effect of ozone in mice against whole-body x-irradiation. Nature
(Lond.), 198: 1220.
HAUSKNECHT, R. (1960) Air pollution effects reported by California
residents from the California Health Survey, CA, USA, State of
California, Department of Public Health, pp. 1-55.
HAZUCHA, M., SILVERMAN, F., PARENT, C., FIELD, S., & BATES, D. V.
(1973) Pulmonary function in man after short-term exposure to
ozone. Arch. environ. Health, 27: 183-188.
HECHTER, H. H. & GOLDSMITH, J. R. (1961) Air pollution and daily
mortality. Am. J. med. Sci., 241: 581-588.
HENSCHLER, D. (1960) [Protective effect of pre-treatment with low gas
concentrations against lethal pulmonary edema caused by irritant
gas.] Arch. exp. Pathol. Pharmacol., 238: 66-67 (in German).
HENSCHLER, D., STIEN, A., BECK, H., & NEUMAN, W. (1960) [Olfactory
threshold of some important irritant gases and manifestations in
man by low concentrations.] Arch. Gewerbepathol. Gewerbehyg.,
17: 547-570 (in German).
HENSCHLER, D., HAHN, E., & ASSMANN, W. (1964) [Conditions for
increasing resistance by repeated inhalation of irritating
oedema- forming gases.] Naunyn-Schmiedebergs Arch. exp. Pathol.
Pharmakol., 249: 325-342 (in German).
HEUSS, J. M. & GLASSON, W. A. (1968) Hydrocarbon reactivity and eye
irritation. Environ. Sci. Technol., 3: 1109-1116.
HODGESON, J. A. (1972) Review of analytical methods for atmospheric
oxidants measurements. Int. J. environ. anal. Chem.,
2: 113-132.
HOLLAND, G. J., BENSON, D., BUSH, A., RICH, G. Q., & HOLLAND, R. P.
(1968) Air pollution simulation and human performance. Am. J.
public Health, 58: 1684-1691.
HOLZMAN, R. S., GARDNER, D. E., & COFFIN, D. L. (1968) In vivo
inactivation of lysosyme by ozone. J. Bacteriol.,
96: 1562-1566.
HUBER, G. L., MASON, R. J., LAFORCE, M., SPENCER, N.J., GARDNER, D.
E., & COFFIN, D. L. (1971) Alteration in the lung following the
administration of ozone. Arch. intern. Med., 128: 81-87.
HUETER, F. G., CONTNER, G. L., BUSCH, K. A., & HINNERS, R. G. (1966)
Biological effects of atmospheres contaminated by auto exhaust.
Arch. environ. Health, 12: 553-560.
HURST, D. J. & COFFIN, D. L. (1971) Ozone effect on lysosomal
hydrolases of alveolar macrophages in vitro. Arch. intern. Med.,
127: 1059-1063.
HURST, D. J., GARDNER, D. E., & COFFIN, D. L. (1970) Effect of O3 on
acid hydrolases of the pulmonary alveolar macrophage.
J. Reticuloendoth. Soc., 8: 288-300.
IKEMATSU, T., YAMAUCHI, Y., SAITO, H., NAGAOKA, S., & ENDO, R. (1976)
Tonsils and environmental pollution (second report) Influence of
ozone upon rabbit tonsils. Jpn. J. Tonsil., 15: 5-8.
ILO (1977) Occupational exposure limits for airborne toxic
substances. Geneva, International Labour Office, pp. 164-165
(Occupational Safety and Health Series No. 37).
ISOMURA, K., FUKASE, O., & WATANABE, H. (1976) [Cytoxicities and
mutagenicities of gaseous air pollutants.] J. Jpn. Soc. Air
Pollut., 11: 59-64 (in Japanese).
JAFFE, L. S. (1962) The biological effects of ozone on man and
animals. Am. J. public Health, 57: 1269-1277.
JAPAN PUBLIC HEALTH ASSOCIATION (1976) The relationship between
photochemical airpollution and adverse health effects-1975
Report, Tokyo, JPHA, pp. 24-27 (in Japanese).
JEGIER, Z. & P'AN, A. Y. S. (1973) Ozone as an air pollutant. Can. J.
public Health, 64: 161-166.
KAGAWA, J. & TOYAMA, T. (1975a) Effects of ozone and brief exercise on
specific airway conductance in man. Arch. environ. Health,
30: 36-39.
KAGAWA, J. & TOYAMA, T. (1975b) Photochemical air pollution; its
effects on respiratory function of elementary school children.
Arch. environ. Health, 30: 117-122.
KAGAWA, J., TOYAMA, T., & NAKAZA, M. (1976) Pulmonary function test in
children exposed to air pollution. In: Finkel, A. J. & Duel, W.
C., ed. Clinical implications of air pollution research. Acton,
MA, USA, Publishing Sciences Group Inc., pp. 305-320.
KERR, H. D., KULLE, T. J., MCILHANEY, M. L., & SWIDERSKY, P. (1975)
Effects of ozone on pulmonary function in normal subjects. An
environmental-chamber study. Am. Rev. resp. Dis., 111: 763-773.
KING, M. E. (1961) Biochemical effects of ozone, doctoral
dissertation, Illinois Institute of Technology, May 1961, pp.
1-85.
KLEINFELD, M., GIEL, C., & TABERSHAW, I. R. (1957) Health hazards
associated with inert-gas shielded metal arc welding. Arch. ind.
Health, 15: 27-31.
KONIGSBERG, A. S. & BACHMAN, C. H. (1970) Ozonized atmosphere and
gross motor activity of rats. Int. J. Biometeor, 14: 261-266.
KONNO, S. & OKITA, T. (1974) Determination of peroxacetyl (PAN)
produced in the atmosphere or in a photochemical reaction chamber
(1). Bull. Inst. Public Health, Japan, 23: 50-58.
KOTIN, P. & FALK, H. L. (1956) The experimental induction of pulmonary
tumors in strain-A mice after their exposure to an atmosphere of
ozonized gasoline. Cancer, 9: 910-917.
KOTIN, P., FALK, H. L., & MCCAMMON, C. J. (1958) III. The experimental
induction of pulmonary tumors and changes in the respiratory
epithelium in C 57 BL mice following their exposure to an
atmosphere of ozonized gasoline. Cancer, 11: 473-481.
KUDRJAVCEVA, O. F. (1963) [On the possibility of chronic effects of
ozone in working conditions.] Gig. truda i Prof. Zabol., 6: 52
(in Russian).
KULLE, T. J. (1972) The effects of ozone and formaldehyde on the
trigeminal nasal sensory system. Thesis, University of
Cincinnati.
KUSOMOTO, S., NARUYAMA, Y., YONEKAWA, E., ODA, H., & NAKAJIMA, T.
(1976) Acute effects of photochemical oxidants and ozone exposure
on mice. J. Jpn. Soc. Air Pollut., 11: 70-80 (in Japanese).
KYEI-ABOAGYE, K., HAZUCHA, M., WYSZOGRODSKI, I., RUBINSTEIN, D., &
AVERY, M. E. (1973) The effect of ozone exposure in vivo on the
appearance of lung tissue lipids in the endobronchial lavage of
rabbits. Biochem. Biophys. Res. Commun., 54: 907-913.
LAGERWERFF, J. M. (1963) Prolonged ozone inhalation and its effects on
visual parameters. Aerospace Med., 34: 479-486.
LARSEN, R. I. (1969) A new mathematical model of air pollution
concentration averaging time and frequency. J. Air Pollut.
Control Assoc., 19: 24-30.
LARSEN, R. I. (1974) An air quality data analysis system for
interrelating effects, standards, and needed source
reductions-part 2. J. Air Pollut. Control Assoc., 24(6):
551-558.
LEWIS, T. R., HUETER, F. G., & BUSCH, K. A. (1967) Irradiated
automobile exhaust: its effects on reproduction in mice. Arch.
environ. Health, 15: 26-35.
LITT, R. S., VAUGHAN, S., BIRKINSHAW, P., COIT, H., & SANDERS, B.
(1968) Effects of low concentrations of ozone on temporal
discrimination. In: Air pollution project: An educational
experiment in self-directed research, 1968, Pasadena,
Associated Students of the California Institute of Technology,
pp. 51-64.
LOOSLI, C. G., BUCKLEY, R. D., HERTWECK, M. S., HARDY, J. D., RYAN, D.
P., STINSON, S., & SEREBRIN, R. (1972) Pulmonary response of mice
exposed to synthetic smog. Ann. oecup. Hyg., 15: 251-260.
MACLEAN, S. A., LONOWELL, A. C., & BLOGOSLAWSKI, W. J. (1973) Effects
of ozone-treated sea water on the spawned, fertilized, meiotic,
and cleaving eggs of the commercial American oyster. Mutat.
Res., 21: 283-285.
MCJILTON, C., THIELKE, J., & FRANK, R. (1972) Ozone uptake model for
the respiratory system. Am. Ind. Hyg. Assoc. J., 33: 20.
MCMILLAN, R. S., WISEMAN, D. H., HANES, B., & WEHRLE, P. F. (1969)
Effects of oxidant air pollution on peak expiratory flow rates in
Los Angeles school children. Arch. environ. Health, 18:
941-949.
MAHONEY, L. E. J. (1971) Wind flow and respiratory mortality in Los
Angeles. Arch. environ. Health, 22: 344-347.
MATSUMURA, Y. (1970a) The effects of ozone, nitrogen dioxide, and
sulfur dioxide on experimentally induced allergic respiratory
disorder in guinea pigs. I. The effect on sensitization with
albumin through the airway. Am. Rev. respir. Dis.,
102: 430-437.
MATSUMURA, Y. (1970b) The effects of ozone, nitrogen dioxide and
sulfur dioxide on experimentally induced allergic respiratory
disorder in guinea pigs. II. The effects of ozone on the
absorption and the retention of antigen in the lung. Am. Rev.
respir. Dis., 102: 438-443.
MATSUMURA, Y., MIZUNO, K., MIYAMOTO, T., SUZUKI, T., & OSHIMA, Y.
(1972) The effects of ozone, nitrogen dioxide, and sulfur dioxide
on experimentally induced allergic respiratory disorder in guinea
pigs, IV effects on respiratory sensitivity to inhaled
acetylcholine. Am. Rev. respir. Dis., 105: 262-267.
MATZEN, R. N. (1957) Development of tolerance to ozone in reference to
pulmonary edema. Am. J. Physiol., 190: 84-88.
MENDENHALL, R. M. & STOKINGER, H. E. (1959) Tolerance and
cross-tolerance development to atmospheric pollutants, ketene and
ozone. J. appl. Physiol., 14: 923-926.
MENDENHALL, R. M. & STOKINGER, H. E. (1962) Films from lung washings
as a mechanism model for lung injury by ozone. J. appl.
Physiol., 17: 28-32.
MENZEL, D. B. (1970) Toxicity of ozone, oxygen and radiation. Ann.
Rev. Pharmacol., 10: 379-394.
MENZEL, D. B. (1971) Oxidation of biologically active reducing
substances by ozone. Arch. environ. Health, 23: 149-153.
MENZEL, D. B., ROEHM, J. N., & LEE, S. D. (1972) Vitamin E: the
biological and environmental antioxidant. J. agric. food Chem.,
20: 481-486.
MENZEL, D. B., SLAUGHTER, R. J., BRYANT, A.M., & JAUREGUI, H. O.
(1975) Heinz bodies formed in erythrocytes by fatty acid ozonides
and ozone. Arch. environ. Health, 30: 296-301.
MERZ, T., BENDER, M. A., KERR, H. D., & KULLE, T. J. (1975)
Observations of aberrations in chromosomes of lymphocytes from
human subjects exposed to ozone at a concentration of 0.5 ppm for
6 and 10 hours. Mutat. Res., 31: 299-302.
MIKAMI, R. & KUDO (1973) Air pollution -- especially in terms of
oxidants and respiratory organs. Intern. Med. (Japan),
32: 837-844.
MILLER, F. J. (1977) A mathematical model of transport and removal of
ozone in mammalian lungs. Thesis submitted to the Graduate
Faculty of North Carolina State University, Raleigh, NC, USA, pp.
66.
MILLER, P. R., MCCUTCHAN, M. H., & MILLIGAN, H. P. (1972) Oxidant air
pollution in the Central Valley, Sierra Nevada foothills and
Mineral King Valley of California. Atmos. Environ., 6: 623-633.
MILLER, S. & EHRLICH, R. (1958) Susceptibility to respiratory
infections of animals exposed to ozone. J. infect. Dis.,
103: 145-149.
MILLS, C. A. (1957) Respiratory and cardiac deaths in Los Angeles
smogs. Am. J. med. Sci., 233: 379-386.
MIZOGOUCHI, I., OSAWA, M., SATO, Y., MAKINO, K., & YAGYU, H. (1973) A
study on erythrocyte and photochemical smog. I: Effects of air
pollutants on erythrocyte resistance. J. Jpn. Soc. Air Pollut.,
8: 414.
MOORMAN, W. J., CHMIEL, J. J., STARA, J. F., & LEWIS, T. R. (1973)
Comparative decomposition of ozone in the nasopharynx of beagles.
Acute vs chronic exposure. Arch. environ. Health, 26: 153-155.
MOTLEY, H. L., SMART, R. H., & LEFTWICH, C. I. (1959) Effect of
polluted Los Angeles air (smog) on lung volume measurements.
J. Am. Med. Assoc., 171: 1469-1477.
MOUNTAIN, J. T. (1963) Detecting hypersusceptibility to toxic
substances: an appraisal of simple blood tests. Arch. environ.
Health, 6: 357-365.
MOUNTAIN, J. T., WAGNER, W. D., FAIRCHILD, E. J., STOCKELL, F. R., JR,
& STOKINGER, H. E. (1960) Biochemical effects of ozone and
nitrogen dioxide on laboratory animals. In: 138th National
Meeting of The American Chemical Society, New York, September
1960, Cincinnati, OH, US Dept of Health, Education and Welfare,
32 pp.
MUDD, J. B., LEAVITT, R., ONGUN, A., & MCMANUS, T. T. (1969) Reaction
of ozone with amino acids and proteins. Atmos. Environ.,
3: 669-682.
MUELLER, P. X., LOEB, L., & MAPES, W. H. (1973) Decomposition rates of
ozone in living areas. Environ. Sci. Technol., 7: 342-346.
MURPHY, S. D., LENA, J. K., ULRICH, C. E., & DAVIS, H. V. (1963)
Effects on animals of exposure to auto exhaust. Arch. environ.
Health, 7: 60-70.
MURPHY, S. D., ULRICH, C. E., FRANKOWITZ, & XINTARAS, C. (1964a)
Altered function in animals inhaling low concentrations of ozone
and nitrogen dioxide. Am. Ind. Hyg. Assoc. J., 25: 246-253.
MURPHY, S. D., DAVIS, H. V., & ZARATZIAN (1964b) Biochemical effects
in rats from irritating air contaminants. Toxicol. appl.
Pharmacol., 6: 520-528.
MUSTAFA, M. G. (1975) Influence of dietary vitamin E on lung cellular
sensitivity to ozone in rats. Nutr. Rep. Int., 11: 473-476.
MUSTAFA, M. G., DELUCIA, A. J., YORK, G. K., ARTH, C., & CROSS, C. E.
(1973) Ozone interaction with rodent lung. II. Effects on oxygen
consumption of mitochondria. J. lab. clin. Med., 82: 357-365.
NAKAJIMA, T., KUSUMOTO, S., TSUBOTA, Y., YONEKAWA, E., YOSHIDA, R.,
MOTOMIYA, K., ITO, K., IDE, G., & OTSU, H. (1972)
Histopathological studies on the respiratory organs of mice
exposed to photochemical oxidants and auto exhaust. Proc. Osaka
Prefect. Inst. Public Health, 10: 35-42 (in Japanese).
NATIONAL ACADEMY OF SCIENCES (1977) Ozone and other photochemical
oxidants, Washington DC, Printing and Publishing Office,
National Academy of Sciences, 719 pp.
NEDERBRAGT, G. W., VAN DER HORST, N., & VAN DUIJN, J. (1965) Rapid
ozone determination near an accelerator. Nature (Lond.),
206: 4979.
NEVSKAJA, A. I. & DITERIHS, D. D. (1957) Problems of hygiene of labor
in H2O2 production by electrodynamic method. Gig. truda i prof
Zabol., 4: 16.
NEVSKAJA, A. I. & KOCETKOVA, T. A. (1961 ) The toxicology of ozone and
sulfuric acid aerosol in their combined action. Gig. truda i
prof Zabol., 1: 20-29.
NIEBOER, H. & VAN HAM, J. (1976) Peroxyacetyl nitrate (PAN) in
relation to ozone and some meteorological parameters at Delft in
the Netherlands. Atmos. Environ., 10: 115-120.
NIEDING, VON, G. WAGNER, H. M., LOLLGEN, H., & KREKELER, H. (1977)
[Acute effects of ozone on lung function in man. VDI Colloquium
on ozone and other substances in photochemical smog.]
VDI-Berichte, 270: 123-129 (in German).
NORTH ATLANTIC TREATY ORGANIZATION (1974) Air Quality criteria for
photochemical oxidants and related hydrocarbons. A report by the
Expert Panel on Air Quality Criteria, Brussels, Belgium, NATO
Committee on the Challenges of Modern Society, 366 pp.
OHMORI, K. (1974) [The effect of photochemical smog on humans,
especially biological effects by low concentrations of ozone.]
Environ. Pollut. Control. 10: 1042-1046 (in Japanese).
PACE, D. M., LANDOLT, P. A., & AFTONOMOS, B. T. (1969) Effects of
ozone on cells in vitro. Arch. environ. Health, 18: 165-170.
PALMER, M. S., SWANSON, D. H., & COFFIN, D. L. (1971) Effect of O3 on
benzpyrene hydroxylase activity in the Syrian golden hamster.
Cancer Res., 31: 730-733.
PALMER, M. S., EXLEY, R. W., & COFFIN, D. L. (1972) Influence of
pollutant gases on benzpyrene hydroxylase activity. Arch.
environ. Health, 25: 439-442.
P'AN, A. Y. S. & JEGIER, Z. (1970) The effect of sulfur dioxide and
ozone on acetylcholinesterase. Arch. environ. Health,
21: 498-501.
P'AN, A. Y. S. & JEGIER, Z. (1972) Trypsin protein esterase in
relation to ozone-induced vascular damage. Arch. environ.
Health, 24: 233-236.
P'AN, A. Y. S., BELAND, J., & JEGIER, Z. (1972) Ozone-induced arterial
lesions. Arch. environ. Health, 24: 229-232.
PEARLMAN, M. E., FINKLEA, J. F., SHY, C. M., VAN BRUGGEN, J., &
NEWILL, V. A. (1971) Chronic oxidant exposure and epidemic
influenza. Environ. Res., 4: 129-140.
PENHA, P. D., AMARAL, L., & WERTHAMER, S. (1972) Ozone air pollutants
and lung damage. Ind. Med., 41: 17-20.
PLOPPER, C. G., DUNGWORTH, D. L., & TYLER, W. S. (1973a) Pulmonary
lesions in rats exposed to ozone. Am. J. Pathol., 71: 375-394.
PLOPPER, C. G., DUNGWORTH, D. L., & TYLER, W. S. (1973b)
Ultrastructure of pulmonary alveolar macrophages in situ in
lungs from rats exposed to ozone. Am. Rev. respir. Dis.,
108: 532-638.
PRAT, R., NOFRE, C., & CIER, A. (1968) Effets de l'hypochlorite de
sodium, de l'ozone et des radiations ionisantes sur les
constituants pyrimidiques d'Escherichia coli. Ann. Inst.
Pasteur, 114: 595-607.
PURVIS, M. R., MILLER, S., & EHRLICH, R. (1961) Effect of atmospheric
pollutants on susceptibility to respiratory infection. I. Effect
of ozone. J. infect. Dis., 109: 238-242.
RASMUSSEN, R. A. (1970) Isoprene: Identified as a forest-type emission
to the atmosphere. Environ. Sci. Technol., 4(8): 667-671.
RASMUSSEN, R. A. (1972) What do the hydrocarbons from trees contribute
to air pollution? J. Air Pollut. Control Assoc., 22: 537-543.
RAVEN, P. B., DRINKWATER, B. L., RUHLING, R. O., BOLDUAN, N., TAGUCHI,
S., GLINER, J., & HORVATH, S. M. (1974) Effect of carbon monoxide
and peroxyacetyl nitrate on man's maximal aerobic capacity.
J. appl. Physiol., 36: 288-293.
REGENER, V. H. (1964) Measurement of atmospheric ozone with the
chemiluminescent method. J. geophys. Res., 69: 3795-3800.
REGENER, V. H. & ALDAZ, L. (1969) Turbulent transport near the ground
as determined from measurements of the ozone flux and the ozone
gradient. J. geophys. Res., 74: 6935-6942.
RENZETTI, N. A. & GOBRAN, V. (1957) Studies of eye irritation due to
Los Angeles smog 1954-1956. San Marino, CA, Air Pollution
Foundation, pp. 170.
REYNOLDS, R. W. & CHAFFEE, R. R. J. (1970) Studies on the combined
effects of ozone and a hot environment on reaction time in
subhuman primates. In: Project clean air, Santa Barbara,
California University, pp. 1-8 (California University Res. Proj.
S-6.).
RICHARDSON, N. A. & MIDDLETON, W. C. (1957) Evaluation of filters for
removing irritants from polluted air. Los Angeles, University
of California, Dept of Engineering, 31 pp. (Report No. 57-43).
RICHARDSON, N. A. & MIDDLETON, W. C. (1958) Evaluation of filters for
removing irritants from polluted air. Heat. Piping Air Cond.,
30: 147-154.
RIPPERTON, L. A., JEFFRIES, H. E., & WORTH, J. B. (1971) Relationship
of measurements in nonurban air to air pollution: Ozone and
oxides of nitrogen, CP 13B, In: England, H. M. & Berry, W. T.,
ed. Proceedings of the Second International Clean Air Congress.
New York, Academic Press, pp. 386-390.
ROEHM, J. N., HADLEY, J. G., & MENZEL, D. B. (1971a) Oxidation of
unsaturated fatty acids by ozone and nitrogen dioxide. Arch.
environ. Health, 23: 142-148.
ROEHM, J. N., HADLEY, J. G., & MENZEL, D. B. (1971b) Antioxidants vs
lung disease. Arch. intern. Med., 128: 88-93.
ROEHM, J. N., HADLEY, J. G., & MENZEL, D. B. (1972) The influence of
vitamin E on the lung fatty acids of rats exposed to O3. Arch.
environ. Health, 24: 237-242.
ROKAW, S. N. & MASSEY, F. (1962) Air pollution and chronic respiratory
disease. Am. Rev. respir. Dis., 86: 703-704.
ROTH, R. P. & TANSY, M. F. (1972) Effects of gaseous air pollutants on
gastric secreto-motor activities in the rat. J. Air Pollut.
Control Assoc., 22: 796-709.
RUDOLF, W. (1974) [Investigation of oxidants in the city centre of
Frankfurt.] Schriftenr. Ver. wasser-, Boden- Lufthyg.,
42: 65-75 (in German).
SABERSKY, R. H., SINEMA, D. A., & SHAIR, F. H. (1973) Concentrations,
decay rates, and removal of ozone and their relation to
establishing clean indoor air. Env. Sci. Technol., 7: 347-353.
SACHSENMAIER, W., SIEBS, W., & TAN, T. (1965) [Effects of ozone upon
mouse ascites tumor cells and upon chick fibroblasts in tissue
culture.] Z. Krebsforsch., 67: 113-126 (in German).
SANDALLS, F. J., PENKETT, S. A., & JONES, B. M. R. (1974) Preparation
of peroxyacetyl nitrate (PAN) and its determination in the
atmosphere. London, England, Her Majesty's Stationery Office
(Atomic Energy Research Establishment Report AERE R7807).
SCHEEL, L. D., DOBROGORSKI, O. J., MOUNTAIN, J. T., SVIRBELY, J. L., &
STOKINGER, H. E. (1959) Physiologic biochemical, immunologic, and
pathologic changes following ozone exposure. J. appl. Physiol.,
14: 67-80.
SCHLIPKOETER, H. W. & BRUCH, J. (1973) [Functional and morphological
alterations caused by exposure to ozone.] Zentralb. Bakteriol.,
Parasitenk. Infektionskr. Hyg., Reihe B, 156: 486-499 (in
German).
SCHLIPKOETER, H. W., FODOR, G. C., GHELERTER, L., & DOLGNER, R. (1973)
[Findings from animal tests concerning synergism.] In:
Proceedings of the 3rd International Clean Air Congress,
Diisseldorf, Federal Republic of Germany, pp. A26-A30 (in
German).
SCHOETTLIN, C. E. (1962) The health effect of air pollution on elderly
males. Am. Rev. respir. Dis., 86: 878-897.
SCHOETTLIN, C. E. & LANDAU, E. (1961) Air pollution and asthmatic
attacks in the Los Angeles area. Public Health Rep.,
76: 545-548.
SCHUCK, E. A. & DOYLE, G. J. (1959) Photooxidation of hydrocarbons in
mixtures containing oxides of nitrogen and sulfur dioxide. San
Marina, CA, Air Pollution Foundation (Report No. 29).
SCHUCK, E. A. & STEPHENS, E. R. (1969) Oxides of nitrogen. Adv.
environ. Sci., 1: 73-118.
SHINGU, H., TANAKA, I., KUSUMOTO, S., NAKAJIMA, T., WATANABE, M., &
SUGIYAMA, M. (1972) [Effect of ozone on defense mechanisms to
infection.] J. Jpn. Soc. Air Pollut, 9: 332 (in Japanese).
SKILLEN, R. G., THIENES, C. H., CANGELOSI, J., & STRAIN, L. (1961)
Brain 5-hydroxytryptamine in ozone-exposed rats. Proc. Soc. Exp.
Biol. Med., 108: 121-122.
SMITH, L. E. (1965) Inhalation of the photochemical smog compound
peroxyacetyl nitrate. Am. J. public Health, 55(9): 1460-1468.
STASIUK, W. N. & COFFEY, P. E. (1974) Rural and urban ozone
relationships in New York State. J. Air Pollut. Control Assoc.,
24(6): 564-568.
STEPHENS, E. R. (1964) Absorptivities for infra-red determination of
peroxyacetyl nitrates. Anal. Chem., 36: 928-929.
STEPHENS, E. R. (1969) The formation, reactions and properties of
peroxyacetyl nitrates (PANs) in photochemical air pollution.
Adv. environ. Sci. Technol., 1: 119-146.
STEPHENS, E. R. (1976) Peroxyacetyl nitrate (PAN) in relation to ozone
and some meteorological parameters at Delft in the Netherlands.
Atmos. Environ., 10(7): 566.
STEPHENS, E. R., DARLEY, E. F., TAYLOR, O. C., & SCOTT, W. E. (1961)
Photochemical reaction products in air pollution. Int. J. Air
Water Pollut., 4: 79-100.
STEPHENS, R. J., FREEMAN, G., CRANE, S.C., & FURIOSI, N.J. (1971)
Ultrastructural changes in the terminal bronchiole of the rat
during continuous low level exposure to nitrogen dioxide. Exp.
molec. Pathol., 14: 1-19.
STEPHENS, R. J., FREEMAN, G., & EVANS, M. J. (1972) Early response of
lungs to low levels of nitrogen dioxide. Arch. environ. Health,
24: 160-179.
STEPHENS, R. J., FREEMAN, G., STARA, J. F., & COFFIN, D. L. (1973)
Cytologic changes in dog lungs induced by chronic exposure to
ozone. Am. J. Pathol., 73: 711-726.
STEPHENS, R. J., SLOAN, M. F., EVANS, M. J., & FREEMAN, G. (1974)
Early response of lung to low levels of O3. Am. J. Pathol.,
74:31-58.
STERLING, T. D., PHAIN, J. J., POLLACK, S. V., SCHUMSKY, D. A., &
DEGROOT, I. (1966) Urban morbidity and air pollution: a first
report. Arch. environ. Health, 13: 158-170.
STERLING, T. D., POLLACK, S. V., & PHAIR, J. J. (1967) Urban hospital
morbidity and air pollution: a second report. Arch. environ.
Health, 15: 362-374.
STOKINGER, H. E. (1957) Evaluation of the hazards of ozone and oxides
of nitrogen. Am. Med. Assoc. Arch. ind. Health, 15: 181-190.
STOKINGER, H. E. (1959) Factors modifying toxicity of ozone. Adv.
Chem. Ser., 21: 360-369.
STOKINGER, H. E. (1965) Ozone toxicology: a review of research and
industrial experience 1954-1964. Arch. environ. Health,
10: 719-731.
STOKINGER, H. E. & SCHEEL, L. D. (1962) Ozone toxicity: immunochemical
and tolerance-producing aspects. Arch. environ. Health,
4: 327-334.
STOKINGER, H. E., WAGNER, W. D., & WRIGHT, P. G. (1956) Studies of
ozone toxicity: I. potentiating effects of exercise and tolerance
development. Am. Med. Assoc. Arch. ind. Health, 14: 158-162.
STOKINGER, H. E., WAGNER, W. D., & DORBROGORSKI, O. J. (1957) Ozone
toxicity studies. III. chronic injury to lungs of animals
following exposure at a low level. Am. Med. Assoc. Arch. Ind.
Health, 16: 514-522.
SUZUKI, T. & NAGAOKA, S. (1973) [The susceptibility to serotonin and
exposure to ozone.] Ann. Rep. Tokyo Metrop. Res. Inst. Environ.
Prot., No. 4, pp. 262-263 (in Japanese).
SUZUKI, T., NAKAZAWA, K., UMEDA, M., GOTO, H., & NAGAOKA, S. (1975)
[Effect on the blood in rats exposed to ozone. I. Effect on
osmotic fragility of orythrocytes.] Ann. Rep. Tokyo Metrop. Res.
Inst. Environ. Prot., 6: 270-272 (in Japanese).
SWANN, H. E. & BALCHUM, O. J. (1966) Biological effects of urban air
pollution. Arch. environ. Health, 12: 698-704.
TAN, W. C., CORTESI, R., & PRIVETT, O. S. (1974) Lipid peroxide and
lung prostaglandins. Arch. environ. Health, 28: 82-84.
THOMPSON, G. E. (1971) Cardiovascular considerations of rat pulmonary
edema. Mil. Med., 136: 50-65.
TOMINGAS, V. R., POTT, F., & DEHNEN, W. (1973) Biological test for
carcinogenicity of polycyclic aromatic hydrocarbons. Arch.
Geschwilstforsch., 42: 298-306.
TOYAMA, T., KAGAWA, J., TSUNODA, T., & NAKAZA, M. (1977) [An
epidemiological estimation of dose-response relationship to the
mixture of atmospheric pollutants.] Jpn. J. Hyg., 32:70 (in
Japanese).
TRAMS, E., LAUTER, C. J., BRANDENBURGER-BROWN, E. A., & YOUNG, O.
(1972) Cerebral cortical metabolisms after chronic exposure to
O3. Arch. environ. Health, 24: 153-159.
URY, H. K. (1968) Photochemical air pollution and automobile accidents
in Los Angeles. Arch. environ. Health, 17: 334-342.
US DEPARTMENT OF HEALTH EDUCATION AND WELFARE (1970) Air Quality
criteria for photochemical oxidants. Washington, DC, US DHEW
(National Air Pollution Control Administration Publication No. AP
63).
US ENVIRONMENTAL PROTECTION AGENCY (1964-1973) Camp data. Research
Triangle Park, NC, National Aerometric data bank, US EPA.
US ENVIRONMENTAL PROTECTION AGENCY (1973) Investigation of high ozone
concentration in the vicinity of Gomet County, Maryland and
Preston County, West Virginia. Research Triangle Park, NC, EPA
(Report No. EPA-R4-73-019).
US ENVIRONMENTAL PROTECTION AGENCY (1976) Monitoring and air quality
trends report, 1974, pp. 115-123 (Report No. EPA-450/1-76).
VAUGHAN, T. R., JR, JENNELLE, L. F., & LEWIS, T. R. (1969) Long-term
exposure to low levels of air pollutants. Effects on pulmonary
function in the beagle. Arch. environ. Health, 19: 45-50.
VENINGA, T. S. (1967) Toxicity of ozone in comparison with ionizing
radiation. Strahlentherapie (Munich), 134: 469-477.
WARREN, G. J. & BABCOCK, G. (1970) Rev. Sci. Instrum., 41: 280-282.
WATANABE, M. TANAKA, I., SHINGU, H., ASAKA, J., KUSUMOTO, S.,
NAKAJIMA, T., & SUGIYAMA, M. (1973a) [The cytotoxicity of ozone.]
Jpn. J. public Health, 20: 554 (in Japanese).
WATANABE, S., FRANK, R., & YOKOYAMA, E. (1973b) Acute effects of ozone
on lungs of cats. I. Functional. Am. Rev. respir. Dis.,
108: 1141-1151.
WAYNE, W. S., WEHRLE, P. F., & CARROLL, R. E. (1967) Oxidant air
pollution and athletic performance. J. Am. Med. Assoc.,
199: 901-904.
WENT, F. W. (1966) On the nature of Aitken condensation nuclei.
Tellus, 28(2): 549-556.
WERTHAMER, S., SCHWARTZ, L. H., & SOSKIND, L. (1970) Bronchial
epithelial alterations and pulmonary neoplasia induced by ozone.
Pathol. Microbiol., 35: 224-230.
WERTHAMER, S., PENHA, P. D., & AMARAL, L. (1974) Pulmonary lesions
induced by chronic exposure to ozone. I. Biochemical alterations.
Arch. environ. Health, 28: 164-166.
WHITE, W. H., ANDERSON, J. A., BLUMENTHAL, D. L., HUSAR, R. B.,
GILLANI, N. V., HUSAR, J. D., & WILSON, W. E., JR (1976)
Formation and transport of secondary air pollutants: Ozone and
aerosols in the St Louis urban plume. Science, 194: 187-189.
WORLD HEALTH ORGANIZATION (1972) WHO Tech. Rep. Series No. 506. (Air
quality criteria and guides for urban air pollutants: Report of a
WHO Expert Committee.) 35 pp.
WORLD HEALTH ORGANIZATION (1976) Selected methods of measuring air
pollutants, Geneva WHO (WHO Offset Publ. No. 24).
WORLD HEALTH ORGANIZATION (1977) Oxides of nitrogen: Environmental
health criteria 4, Geneva, WHO, 79 pp.
XINTARAS, C., JOHNSON, B. L., ULRICH, C. E., TERRILL, R. E., &
SOBECKI, M. F. (1966) Application of the evoked response
technique in air pollution toxicology. Toxicol. appl.
Pharmacol., 8: 77-87.
YOKOYAMA, E. (1972a) [The effects of O3 on lung function. In:
Proceedings of a Conference on Photochemical Air Pollution.]
Tokyo, Jpn. Soc. Air Pollution, pp. 109-129 (in Japanese).
YOKOYAMA, E. (1972b) Effect of ventilation with ozone on
pressure-volume relationships of excised dogs' lungs. Am. Rev.
Respir. Dis., 105: 594-604.
YOKOYAMA, E. (1973) The effects of low-concentrations of ozone on the
lung pressure of rabbits and rats. Presented at: The Japan
Society of Industrial Hygiene Annual Meeting, 46th, April, pp.
134-135 (paper 223) (in Japanese).
YOKOYAMA, E. (1974) [Maximal expiratory flow volume curve of rabbit
lung exposed to ozone.] Jpn. J. thorac. Dis., 12: 556-561 (in
Japanese).
YOKOYAMA, E. & FRANK, R. (1972) Respiratory uptake of ozone in dogs.
Arch. environ. Health, 25: 132-138.
YOUNG, W. A., SHAW, D. B., & BATES, D. V. (1963) Pulmonary function in
welders exposed to ozone. Arch. environ. Health, 7: 337-340.
YOUNG, W. A., SHAW, D. B., & BATES, D. V. (1964) Effect of low
concentrations of ozone on pulmonary function in man. J. appl.
Physiol., 19: 765-768.
ZELAC, R. E., CROMROY, H. L., BOLCH, W. E., JR, DUNAVANT, B. G., &
BEVlS, H. A. (1971a) Inhaled ozone as a mutagen I. Chromosome
aberrations induced in chinese hamster lymphocytes. Environ.
Res., 4: 262-282.
ZELAC, R. E., CROMROY, H. L., BOLCH, W. E., JR, DUNAVANT, B. G., &
BEVIS, H. A. (1971b) Inhaled ozone as a mutagen II. Effect on the
frequency of chromosome aberrations observed in irradiated
chinese hamsters. Environ. Res., 4: 325-342.
