
This report contains the collective views of an international group of experts and does not necessarily represent the decisions or the stated policy of the United Nations Environment Programme, the International Labour Organization, or the World Health Organization.
Concise International Chemical Assessment Document 52
First draft prepared by Dr Jun Sekizawa, National Institute of Health Sciences, Tokyo, Japan, and Dr Stuart Dobson, Centre for Ecology and Hydrology, Huntingdon, United Kingdom, with the assistance of Dr Ralph J. Touch III, Agency for Toxic Substances and Disease Registry, Atlanta, Georgia, USA
Published under the joint sponsorship of the United Nations Environment Programme, the International Labour Organization, and the World Health Organization, and produced within the framework of the Inter-Organization Programme for the Sound Management of Chemicals.
World Health Organization
Geneva, 2003
The International Programme on Chemical Safety (IPCS), established in 1980, is a joint venture of the United Nations Environment Programme (UNEP), the International Labour Organization (ILO), and the World Health Organization (WHO). The overall objectives of the IPCS are to establish the scientific basis for assessment of the risk to human health and the environment from exposure to chemicals, through international peer review processes, as a prerequisite for the promotion of chemical safety, and to provide technical assistance in strengthening national capacities for the sound management of chemicals.
The Inter-Organization Programme for the Sound Management of Chemicals (IOMC) was established in 1995 by UNEP, ILO, the Food and Agriculture Organization of the United Nations, WHO, the United Nations Industrial Development Organization, the United Nations Institute for Training and Research, and the Organisation for Economic Co-operation and Development (Participating Organizations), following recommendations made by the 1992 UN Conference on Environment and Development to strengthen cooperation and increase coordination in the field of chemical safety. The purpose of the IOMC is to promote coordination of the policies and activities pursued by the Participating Organizations, jointly or separately, to achieve the sound management of chemicals in relation to human health and the environment.
WHO Library Cataloguing-in-Publication Data
Diethyl phthalate.
(Concise international chemical assessment document ; 52)
1.Phthalic acids - adverse effects 2.Phthalic acids - toxicity 3.Risk assessment
4.Environmental exposure I.International Programme on Chemical Safety II.Series
ISBN 92 4 153052 9 (LC/NLM Classification: QV 612)
ISSN 1020-6167
©World Health Organization 2003
All rights reserved. Publications of the World Health Organization can be obtained from Marketing and Dissemination, World Health Organization, 20 Avenue Appia, 1211 Geneva 27, Switzerland (tel: +41 22 791 2476; fax: +41 22 791 4857; email: ). Requests for permission to reproduce or translate WHO publications — whether for sale or for noncommercial distribution — should be addressed to Publications, at the above address (fax: +41 22 791 4806; email: permissions@who.int).
The designations employed and the presentation of the material in this publication do not imply the expression of any opinion whatsoever on the part of the World Health Organization concerning the legal status of any country, territory, city or area or of its authorities, or concerning the delimitation of its frontiers or boundaries. Dotted lines on maps represent approximate border lines for which there may not yet be full agreement.
The mention of specific companies or of certain manufacturers’ products does not imply that they are endorsed or recommended by the World Health Organization in preference to others of a similar nature that are not mentioned. Errors and omissions excepted, the names of proprietary products are distinguished by initial capital letters.
The World Health Organization does not warrant that the information contained in this publication is complete and correct and shall not be liable for any damages incurred as a result of its use.
The Federal Ministry for the Environment, Nature Conservation and Nuclear Safety, Germany, provided financial support for the printing of this publication.
Concise International Chemical Assessment Documents (CICADs) are the latest in a family of publications from the International Programme on Chemical Safety (IPCS) — a cooperative programme of the World Health Organization (WHO), the International Labour Organization (ILO), and the United Nations Environment Programme (UNEP). CICADs join the Environmental Health Criteria documents (EHCs) as authoritative documents on the risk assessment of chemicals.
International Chemical Safety Cards on the relevant chemical(s) are attached at the end of the CICAD, to provide the reader with concise information on the protection of human health and on emergency action. They are produced in a separate peer-reviewed procedure at IPCS. They may be complemented by information from IPCS Poison Information Monographs (PIM), similarly produced separately from the CICAD process.
CICADs are concise documents that provide summaries of the relevant scientific information concerning the potential effects of chemicals upon human health and/or the environment. They are based on selected national or regional evaluation documents or on existing EHCs. Before acceptance for publication as CICADs by IPCS, these documents undergo extensive peer review by internationally selected experts to ensure their completeness, accuracy in the way in which the original data are represented, and the validity of the conclusions drawn.
The primary objective of CICADs is characterization of hazard and dose–response from exposure to a chemical. CICADs are not a summary of all available data on a particular chemical; rather, they include only that information considered critical for characterization of the risk posed by the chemical. The critical studies are, however, presented in sufficient detail to support the conclusions drawn. For additional information, the reader should consult the identified source documents upon which the CICAD has been based.
Risks to human health and the environment will vary considerably depending upon the type and extent of exposure. Responsible authorities are strongly encouraged to characterize risk on the basis of locally measured or predicted exposure scenarios. To assist the reader, examples of exposure estimation and risk characterization are provided in CICADs, whenever possible. These examples cannot be considered as representing all possible exposure situations, but are provided as guidance only. The reader is referred to EHC 170.1
While every effort is made to ensure that CICADs represent the current status of knowledge, new information is being developed constantly. Unless otherwise stated, CICADs are based on a search of the scientific literature to the date shown in the executive summary. In the event that a reader becomes aware of new information that would change the conclusions drawn in a CICAD, the reader is requested to contact IPCS to inform it of the new information.
Procedures
The flow chart on page 2 shows the procedures followed to produce a CICAD. These procedures are designed to take advantage of the expertise that exists around the world — expertise that is required to produce the high-quality evaluations of toxicological, exposure, and other data that are necessary for assessing risks to human health and/or the environment. The IPCS Risk Assessment Steering Group advises the Coordinator, IPCS, on the selection of chemicals for an IPCS risk assessment based on the following criteria:
Thus, it is typical of a priority chemical that
The Steering Group will also advise IPCS on the appropriate form of the document (i.e., EHC or CICAD) and which institution bears the responsibility of the document production, as well as on the type and extent of the international peer review.
The first draft is based on an existing national, regional, or international review. Authors of the first draft are usually, but not necessarily, from the institution that developed the original review. A standard outline has been developed to encourage consistency in form. The first draft undergoes primary review by IPCS to ensure that it meets the specified criteria for CICADs.

|
Advice from Risk Assessment Steering Group Criteria of priority:
Thus, it is typical of a priority chemical that
Special emphasis is placed on avoiding duplication of effort by WHO and other international organizations. A prerequisite of the production of a CICAD is the availability of a recent high-quality national/regional risk assessment document = source document. The source document and the CICAD may be produced in parallel. If the source document does not contain an environmental section, this may be produced de novo, provided it is not controversial. If no source document is available, IPCS may produce a de novo risk assessment document if the cost is justified. Depending on the complexity and extent of controversy of the issues involved, the steering group may advise on different levels of peer review:
|
The second stage involves international peer review by scientists known for their particular expertise and by scientists selected from an international roster compiled by IPCS through recommendations from IPCS national Contact Points and from IPCS Participating Institutions. Adequate time is allowed for the selected experts to undertake a thorough review. Authors are required to take reviewers’ comments into account and revise their draft, if necessary. The resulting second draft is submitted to a Final Review Board together with the reviewers’ comments. At any stage in the international review process, a consultative group may be necessary to address specific areas of the science.
The CICAD Final Review Board has several important functions:
Board members serve in their personal capacity, not as representatives of any organization, government, or industry. They are selected because of their expertise in human and environmental toxicology or because of their experience in the regulation of chemicals. Boards are chosen according to the range of expertise required for a meeting and the need for balanced geographic representation.
Board members, authors, reviewers, consultants, and advisers who participate in the preparation of a CICAD are required to declare any real or potential conflict of interest in relation to the subjects under discussion at any stage of the process. Representatives of nongovernmental organizations may be invited to observe the proceedings of the Final Review Board. Observers may participate in Board discussions only at the invitation of the Chairperson, and they may not participate in the final decision-making process.
This CICAD on diethyl phthalate was developed primarily based on the evaluation available in the report Toxicological profile for diethylphthalate (ATSDR, 1995). Data identified up to the end of 1994 were covered in the review. A BUA (1994) report on diethyl phthalate was also available to the authors as reference material. A further literature search was performed in October 2001 to identify any relevant information published after the original review. Information on the preparation and peer review of the source document is presented in Appendix 1. Information on the peer review of this CICAD is presented in Appendix 2. This CICAD was approved as an international assessment at a meeting of the Final Review Board, held in Ottawa, Canada, on 29 October – 1 November 2001. Participants at the Final Review Board meeting are listed in Appendix 3. The species sensitivity distribution method used to characterize the environmental risks is described in Appendix 4. The International Chemical Safety Card on diethyl phthalate (ICSC 0258), produced by the International Programme on Chemical Safety (IPCS, 2001), has also been reproduced in this document.
Diethyl phthalate (CAS No.
Diethyl phthalate is likely to undergo biodegradation in the environment. Compared with other phthalates, it has a much lower capacity for binding to aquatic sediments, with between 70% and 90% of diethyl phthalate estimated to be found in the water column. Diethyl phthalate was detected in surface water at concentrations ranging from <1 to 10 µg/litre and in drinking-water at concentrations ranging from 0.01 to 1.0 µg/litre. Fish collected from the Great Lakes area in the USA contained diethyl phthalate at concentrations up to 1.7 mg/kg. Diethyl phthalate is not likely to biomagnify through the food-chain.
In a recent duplicate-portion study in Japan, the average intake of diethyl phthalate in hospital diet was estimated to be 0.35 μg/day per person, which probably was a result of contact between plastic packaging or gloves and the food. General population exposure in the USA, as estimated from urinary concentrations of the monoester, was estimated to be 12 µg/kg body weight per day (median value). Leaching of diethyl phthalate from plastic tubing used in medical treatments reached 20 ng/litre with 1 h of perfusion with aqueous electrolyte solution, levels decreasing with extended perfusion time.
Dermally applied diethyl phthalate penetrates the skin and can be widely distributed in the body, but it does not accumulate in tissue. Diethyl phthalate is hydrolysed in the body to the monoester derivative. Hydrolytic metabolism of diethyl phthalate is qualitatively similar in rodents and humans.
LD50s for diethyl phthalate were 8600 mg/kg body weight and above following oral administration. Diethyl phthalate was a minimal to mild skin and eye irritant in experimental animals. Few cases of dermal irritation in humans after patch testing have been described; dermal sensitization has been described in humans, but seems to be rare. Slight increases in liver and kidney weights in rodents were observed following oral administration for up to 16 weeks. However, no adverse clinical chemical or histopathological changes were detected in the liver, kidney, or other organs in most studies. One 3-week study in rats showed an increase in liver weight at 1753 mg/kg body weight per day, which might be related to peroxisome proliferation.
No carcinogenic effect was detected after dermal exposure in rats, and an equivocal response was observed in mice exposed dermally. No initiation or promotion activity of diethyl phthalate was detected in mice in a 1-year initiation/promotion study. Results of in vitro mutagenicity and clastogenicity studies were equivocal.
No malformations but skeletal (rib) number variations were caused by an oral dose of 3215 mg/kg body weight per day in rats and a percutaneous dose of 5600 mg/kg body weight per day in mice — dose levels that also induced toxicity in the dams. No-observed-adverse-effect levels (NOAELs) of 1600 and 1900 mg/kg body weight per day were identified for mice and rats, respectively. A perinatal exposure to diethyl phthalate at 750 mg/kg body weight per day by gavage did not induce adverse effects in mothers or offspring and did not induce the malformations in male reproductive organs or the decreases in testis weights that were observed after exposure to other phthalates in the same study.
In a continuous-breeding study, no adverse effects were detected in the F0 generation of mice following dietary administration of 3640 mg/kg body weight per day. However, decreased epididymal sperm concentration of the F1 generation and decreased number of live F2 pups per litter were caused by the administration of 3640 mg/kg body weight per day, together with mild inhibition of body weight gain and moderate increases in liver and prostate weights. Ultrastructural changes in the Leydig cells of rats were observed at an oral dose of 2000 mg/kg body weight per day administered for 2 days.
No adverse immunological or neurological effects were reported in general toxicity studies.
A tolerable intake of 5 mg/kg body weight was estimated from a NOAEL of 1600 mg/kg body weight per day for developmental effects to which an uncertainty factor of 300 was applied. The average daily intake of 0.35 µg/person (0.007 µg/kg body weight per day for a 50-kg person) derived in a hospital diet study in Japan is about 6 orders of magnitude lower than the tolerable intake. Exposure of the general population in the USA, estimated at 12 µg/kg body weight per day from monoethyl phthalate concentrations in urine, corresponds to 0.3% of the tolerable intake. The 95th-percentile value derived from the same study (110 µg/kg body weight per day) corresponds to 2% of the tolerable intake.
Available data suggest that organisms in the freshwater aquatic environment are not likely to be at significant risk from exposure to diethyl phthalate, with measured concentrations in wastewater and surface water at least 1 order of magnitude lower than the predicted no-effect concentration (PNEC) of 0.9 mg/litre. There are insufficient data available to estimate risk to marine organisms. Risk to soil organisms is also expected to be low, but data are inadequate to make a quantitative estimate.
Diethyl phthalate (C12H14O4; relative molecular mass 222.3; CAS No.
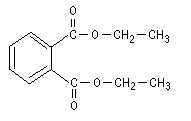
Figure 1: Structure of diethyl phthalate.
Table 1: Physical and chemical properties of diethyl phthalate.a
|
Property |
Value |
Reference |
|
Water solubility at 25 °C |
1000 mg/litre |
Yalkowsky & Dannenfelser, 1992 |
|
Solubility in organic solvents |
Soluble in alcohol, acetone, ether, benzene, ketones, esters, aromatic hydrocarbons, aliphatic solvents, and vegetable oils |
Lewis (1993) |
|
Partition coefficients |
||
|
Log Kocb |
2.65 |
Wolfe et al., 1980 |
|
Log Kow |
2.47, 2.51 |
Veith et al., 1980; Hansch et al., 1995 |
|
Vapour pressure |
||
|
At 20 °C |
4.59 × 10–2 Pa |
Grayson & Fosbraey, 1982 |
|
At 25 °C |
2.19 × 10–1 Pa |
Hinckley et al., 1990 |
|
Henry’s law constantb |
7.9 × 10–5 kPa |
US EPA, 1989 |
|
Dimensionless Henry’s law constant (air/water partition coefficient)c |
4.3 × 10–8 |
|
a |
From HSDB (1994); ATSDR (1995). |
|
b |
Temperature not specified. |
|
c |
Assuming a temperature for the dimensioned value at around 20 °C. |
Diethyl phthalate is produced industrially by the reaction of phthalic anhydride with ethanol in the presence of concentrated sulfuric acid catalyst (HSDB, 1994). The purity of manufactured phthalate esters is reportedly between 99.70% and 99.97%, with the main impurities being isophthalic acid, terephthalic acid, and maleic anhydride (Peakall, 1975).
Because phthalates are so pervasive in plastics and in the laboratory environment, rigorous control measures are needed to prevent contamination of the sample and to maintain a low background concentration. These procedures include prewashing columns, use of equipment with purified solvents, and baking at high temperatures to remove organic materials. Contamination from laboratory glassware limits the analysis of phthalate esters in the micrograms per litre to nanograms per litre range (Lopez-Avila et al., 1990). Organochlorine pesticides and polychlorinated biphenyls (PCBs) may cause interference in diethyl phthalate analysis by electron capture detector (ECD), requiring their removal.
Diethyl phthalate can be collected by pumping an air sample through ethylene glycol (Thomas, 1973) or directly through an activated Florisil column, with a detection limit of 10 ng per injection by gas chromatography (GC) with ECD and 90% recovery (Giam & Chan, 1976). Measurements in air can also be done by passive sampling on charcoal, which is less expensive than active sampling but requires much longer sampling times, with a detection limit of 200 ng/m3.
Solid-phase extraction methods using reverse-phase columns are particularly desirable for analysing liquid samples, because they eliminate the need for large solvent volumes and the resulting potential for contamination (Ritsema et al., 1989; Burkhard et al., 1991). US EPA (1981a) achieved over 100% recovery using Florisil or alumina columns and GC/ECD, with a sensitivity of 0.13 ng/injection, but found the method to be inappropriate for certain wastewaters because of high interference.
Sludge, sediment, and soil samples are extracted with moderately non-polar solvents and cleaned up by liquid chromatography, with detection by GC with ECD (Russell & McDuffie, 1983; Ritsema et al., 1989). Soxhlet extraction or extraction using ultrasonication was sometimes used to improve efficiency (Zurmuhl, 1990).
Preparation steps for the determination of diethyl phthalate in biological samples include extraction with petroleum ether followed by Florisil column chromatography. The detection limit for semen was 0.04 mg/kg, and recovery was excellent (95%) (Waliszewski & Szymczymski, 1990); the detection limit for liver and muscle was 30 ng per injection (Giam & Chan, 1976). Food samples can be extracted with acetonitrile followed by purification using Florisil and Bondasil columns, with detection limits as low as 0.1 ng/g and 93–100% recovery by GC/mass spectrometry (MS) (Tsumura et al., 2001).
Diethyl phthalate is most commonly measured using GC with detection by MS. MS is less prone to interference than HPLC. Other detection methods include high-performance liquid chromatography (HPLC) or liquid chromatography with ultraviolet (UV) detection.
Monoethyl phthalate, the main metabolite of diethyl phthalate, has been analysed in the urine using triple quadrupole tandem MS with chemical ionization after β-glucuronidase hydrolysis and HPLC separation (Blount et al., 2000a).
Diethyl phthalate is used as a plasticizer for cellulose ester plastic films and sheets (photographic, blister packaging, and tape applications) and moulded and extruded articles (consumer articles such as toothbrushes, automotive components, tool handles, and toys). There is a wide variety of consumer products that contain diethyl phthalate or are covered with diethyl phthalate-containing plastic packaging (Kamrin & Mayor, 1991). Diethyl phthalate was reported as an ingredient in 67 cosmetic formulations, including bath preparations (oils, tablets, and salts), eye shadow, toilet waters, perfumes and other fragrance preparations, hair sprays, wave sets, nail polish and enamel removers, nail extenders, bath soaps, detergents, aftershave lotions, and skin care preparations (Anonymous, 1985; Kamrin & Mayor, 1991). More specifically, diethyl phthalate is used in nail polish as a solvent for nitrocellulose and cellulose acetate, in perfumes as a fixative and solvent, in toilet preparations as an alcohol denaturant, and in fingernail elongators as a plasticizer (Verschueren, 1983; Anonymous, 1985; Hawley, 1987; US EPA, 1989). In addition, diethyl phthalate is used as a component in insecticide sprays and mosquito repellents, as a camphor substitute, as a plasticizer in solid rocket propellants, as a wetting agent, as a dye application agent, as an ingredient in aspirin coatings, as a diluent in polysulfide dental impression materials, and in adhesives, plasticizers, and surface lubricants used in food and pharmaceutical packaging. In a limited study, the concentrations of diethyl phthalate in different medical devices, including dialysis tubing, were generally low (<1% of total volatiles), with the exception of a sample of intestinal tubing, in which the concentration of diethyl phthalate reached <20% of total volatiles (Wahl et al., 1999). Polyvinyl chloride (PVC) tubing may still be used for dialysis patients (Verschueren, 1983; Anonymous, 1985; Hawley, 1987; US EPA, 1989).
The US production volume of diethyl phthalate gradually declined from approximately 9500 tonnes in 1980 to 8600 tonnes in 1987 (USITC, 1981, 1988). Production volumes increased again in 1988 to 11 800 tonnes (Kamrin & Mayor, 1991). Production in European Union countries is around 10 000 tonnes based on 1999 data. The production volume in Japan in 1999 was 700 tonnes (Chemical Daily, 2001). A survey of fragrance manufacturers conducted in 1995–1996 by the Research Institute for Fragrance Materials reported an annual use of approximately 4000 tonnes in the preparation of fragrance mixtures (Api, 2001).
Releases to the environment occur primarily as a result of the production and manufacturing of diethyl phthalate itself and during the use and disposal of products containing diethyl phthalate (US EPA, 1981b).
As a result of its use as a plasticizer for cellulose ester films and extruded materials and in a variety of consumer products, human exposure to diethyl phthalate is expected to be significant. Releases are expected to be primarily to water or to soil as a result of leaching from landfills. Diethyl phthalate may enter the atmosphere through combustion of plastics and, to a lesser degree, by volatilization.
Based on 1994 Toxics Release Inventory data, US EPA (1995) estimated that 72 tonnes and 341 kg of diethyl phthalate would be released annually to the air and water, respectively, as a result of manufacturing, use, or disposal, and 364 kg of diethyl phthalate would be released annually to the environment as a result of landfilling activities. Total off-site releases were 1.26 tonnes annually.
Diethyl phthalate is likely to undergo biodegradation in the environment. Abiotic degradation processes such as hydrolysis, oxidation, and photolysis are unlikely to play significant roles in the environmental fate of diethyl phthalate. Diethyl phthalate is not likely to biomagnify through the food-chain.
Volatilization of diethyl phthalate is expected to be slow based on its low vapour pressure of 4.59 × 10–2 at 20 °C (Grayson & Fosbraey, 1982). Diethyl phthalate may be removed from the atmosphere by wet or dry deposition (US EPA, 1989).
Diethyl phthalate reacts photochemically with hydroxyl radicals in the air, with an estimated half-life of 22.2 h (HSDB, 1994). UV absorption spectra for diethyl phthalate suggest that although there is a potential for photodegradation in the atmosphere, this is not a significant removal process (US EPA, 1989). Diethyl phthalate may exist in the atmosphere in vapour form and adsorb to airborne particulates.
The distribution of diethyl phthalate between the gaseous and particulate phases in air was estimated by the Junge-Pankow model, which determined the fraction of diethyl phthalate in the particulate (aerosol) phase to be 0.000 39 (Staples et al., 1997a).
It has been estimated that approximately 1% of the phthalate ester content of plastic materials in direct contact with water or other liquids may be released to the aquatic environment (Peakall, 1975).
Diethyl phthalate can be biodegraded either aerobically or anaerobically; abiotic degradation processes are not significant. Diethyl phthalate may leach from soils with low organic matter content into the underlying groundwater (US EPA, 1979). Based on a Henry’s law constant of 4.3 × 10–8, volatilization from water is not expected to be a significant removal process for diethyl phthalate (US EPA, 1989).
A computer simulation of the transport of diethyl phthalate in four aquatic systems using EXAMS (the Exposure Analysis Modeling System) estimated that, based on an organic carbon partition coefficient (Koc) of 4.5 × 102 , >90% of the phthalate would be found in the water column in a river or eutrophic or oligotrophic lake ecosystem, with <10% in the bottom sediment. In a pond, 70% of the diethyl phthalate would be found in the water column, with 30% in the sediment (US EPA, 1989).
In a study of phthalate esters in surface sediment samples of the River Mersey in England, diethyl phthalate was enriched in the coarser sediment fraction with high lipid content in one sample (0.102 µg/g dry weight, background 0.050 µg/g), but was more concentrated in the finer particle fraction in another sample (0.060 µg/g, background 0.013 µg/g) (Preston & Al-Omran, 1989).
Diethyl phthalate can adsorb to suspended particles in marine waters, with the maximum adsorption occurring onto particles 353–698 µm in size (Al-Omran & Preston, 1987).
Based on its log octanol/water partition coefficient (log Kow 2.47), diethyl phthalate is considered to be moderately lipophilic and may be taken up by lipids in aquatic organisms. Diethyl phthalate has been detected in aquatic organisms and has been found to bioconcentrate modestly in these organisms (Camanzo et al., 1983; DeVault, 1985; McFall et al., 1985). However, diethyl phthalate may also be degraded by these organisms, suggesting that it is unlikely to biomagnify up the food-chain (US EPA, 1979). The bioconcentration factor for diethyl phthalate in bluegill (Lepomis macrochirus) in a 21-day study was 117 (mean diethyl phthalate concentration in water was 9.42 µg/litre), and the half-life in fish tissue was between 1 and 2 days (Barrows et al., 1980; Veith et al., 1980). A study of the uptake of diethyl phthalate through the gills of English sole (Parophrys vetulus) indicated that the uptake efficiency was inversely correlated with weight-specific ventilation volume and was not correlated with fish weight or with diethyl phthalate exposure concentration; the mean uptake was only 11.3% (Boese, 1984).
Diethyl phthalate did not adsorb to any aquatic surfaces in a simulated aquatic ecosystem consisting of microbial growth attached to submerged surfaces or suspended as mats or streamers in the water. It was virtually untransformed by photolysis (<1%), and only about 5% of an initial diethyl phthalate concentration of 191 µg/litre was lost by hydrolysis in 12 h at pH 10 (Lewis et al., 1984).
Degradation occurred as a result of bacterial transformation (95–99% of loss), which was dependent on the surface area colonized by the bacteria and unaffected by dissolved organic carbon, nitrogen, or phosphorus. Further studies using laboratory microcosms and field-collected microbiota found that while diethyl phthalate was degraded by all of the laboratory microcosms, it was degraded by only 2 of 10 field-collected microbiota (Lewis et al., 1985).
Aerobic degradation of diethyl phthalate by acclimated soil and activated sewage sludge microbes was studied using carbon dioxide evolution. Primary biodegradation (loss of parent ester) of diethyl phthalate was greater than 99%, with a lag phase of 2.3 days, and ultimate biodegradation (carbon dioxide evolution) was 95%. The half-life for the compound under these conditions was 2.21 days (Sugatt et al., 1984). More than 94% of diethyl phthalate, however, was biodegraded within 1.1 days using semicontinuous activated sludge treatment (O’Grady et al., 1985). Other studies of the aerobic biodegradation of diethyl phthalate indicated that degradation was complete within 1 week of incubation in the dark using settled domestic wastewater as the microbial inoculum in the static culture flask test and 5 or 10 mg diethyl phthalate/litre (Tabak et al., 1981).
A summary of data on aerobic and anaerobic biodegradation of diethyl phthalate under various conditions showed that degradation was mostly greater than 76%, except when the initial concentrations were very low (Staples et al., 1997a).
Under anaerobic conditions, diethyl phthalate was degraded to carbon dioxide and methane (>75% of theoretical methane production) by a 10% sludge solution from a primary digester and partially degraded (30–75% of theoretical methane production) by a 10% sludge solution from a secondary digester (Shelton & Tiedje, 1984). Diethyl phthalate removal was greater than 90% within 1 week with undiluted sludge (Shelton et al., 1984).
Degradation of diethyl phthalate applied to soil at an initial concentration of 1 mg/kg was 4% at 24 h, 11% at 48 h, 40% at 72 h, and 86% at 120 h. Addition of landfill leachate to the soil significantly increased the degradation rate, with all of the diethyl phthalate being degraded within 72 h (Russell et al., 1985).
A 2-year study of slow-rate land treatment using wastewaters containing diethyl phthalate found that diethyl phthalate was relatively non-volatile during spray application. Applied at a rate of 56 µg/litre to sandy loam and silty loam soils, diethyl phthalate accumulated in the top 5 cm of sandy loam soils to concentrations of 1000–6700 ng/g and on the surface of the silty soil from below the detection limit (1 ng/g) to 2200 ng/g dry soil. Although diethyl phthalate was detectable in each soil type down to a depth of 150 cm, it was not detected to any significant degree in the percolate from either soil (Parker & Jenkins, 1986).
Biodegradation of diethyl phthalate in soil has been shown to occur as a series of sequential steps common to the degradation of all phthalates. Primary degradation of diethyl phthalate to phthalic acid has been reported to involve the hydrolysis of each of the two diethyl chains of the phthalate to produce the monoester, monoethyl phthalate, and then phthalic acid (Cartwright et al., 2000a). Diethyl phthalate (0.1–100 mg/g) was biodegraded rapidly in soil with a half-life of 0.75 days at 20 °C and was not expected to persist in the environment (Cartwright et al., 2000b).
Analytical data on diethyl phthalate concentrations in environmental media must be interpreted with caution, because of the extensive contamination of laboratory glassware with this chemical agent (Lopez-Avila et al., 1990).
Diethyl phthalate has been detected in ambient indoor air, wastewaters from industrial facilities, surface waters and sediments, and marine waters. Fish and other aquatic biota living in contaminated waters have been shown to contain diethyl phthalate in their tissues, although depuration is relatively rapid when the organisms are placed in uncontaminated water.
Diethyl phthalate has been measured in the indoor air of a telephone switching office and in outdoor air in Newark, USA, at concentrations ranging from 1.60 to 2.03 µg/m3 and from 0.40 to 0.52 µg/m3, respectively, during a 43-day sampling period (Shields & Weschler, 1987).
Diethyl phthalate has been detected in the treated wastewaters from various manufacturing facilities: 3.2 µg/litre at textile manufacturing plants (Walsh et al., 1980), 60 µg/litre at a tire manufacturing plant (Jungclaus et al., 1976), and 50 µg/litre at a pulp and paper manufacturer (Brownlee & Strachan, 1977; Voss, 1984). Diethyl phthalate has been found at a median concentration of <10 µg/litre in 10% of the industrial effluent samples and in 3.0% of the ambient water samples in the Storage and Retrieval (STORET) database maintained by the US Environmental Protection Agency (EPA) (Staples et al., 1985).
River water samples from the lower Tennessee River, USA, were found to contain diethyl phthalate at a concentration of 11.2 µg/litre (Goodley & Gordon, 1976). Diethyl phthalate was detected at 21 ng/litre in tap water from the Kitakyushu area of Japan; sources were considered to be domestic sewage and industrial waste (Akiyama et al., 1980). River water samples and sewage effluent collected in 1984 from the Rivers Irwell and Etherow near Manchester, England, contained 0.4–0.6 µg diethyl phthalate/litre (Fatoki & Vernon, 1990). The Nationwide Urban Runoff Program, conducted in 1982 in the USA, detected diethyl phthalate in 4% (three locations) of 86 samples at concentrations of 0.5–11.0 µg/litre (Cole et al., 1984).
Diethyl phthalate levels in water from the Rhine River in the Netherlands ranged from <0.15 to approximately 0.45 µg/litre over a 12-day period; on days 7 through 11, concentrations in suspended particulate matter from the river stayed relatively constant at 0.1 mg/kg. Water samples and suspended particulate matter from Lake Yssel, also in the Netherlands, contained diethyl phthalate at 0.02–0.08 µg/litre and <0.1–0.8 mg/kg, respectively (Ritsema et al., 1989). River water samples and sewage effluent collected in 1984 from the Rivers Irwell and Etherow near Manchester, England, contained 0.4–0.6 µg diethyl phthalate/litre (Fatoki & Vernon, 1990).
In a compilation of concentrations (1984–1997) of diethyl phthalate in North American and western European surface waters (USA, Canada, United Kingdom, Germany, Netherlands, Sweden), geometric mean concentrations ranged from about 0.01 to 0.5 µg/litre (Staples et al., 2000).
Diethyl phthalate has been detected in sediment samples taken from Chesapeake Bay, USA, at concentrations ranging from 11 to 42 µg/kg. A sediment sample taken from the Chester River (which flows into Chesapeake Bay) contained 26 µg/kg, and a sediment sample from a wastewater holding pond adjacent to a plasticizer manufacturing plant outfall near the river had less than 100 µg diethyl phthalate/kg (Peterson & Freeman, 1982a).
Sediment core samples taken from Chesapeake Bay below Baltimore Harbor contained diethyl phthalate at levels that reflected increasing water concentrations as a result of industrial production of phthalates. The sample taken closest to Baltimore had diethyl phthalate concentrations of 19 µg/kg at a core depth corresponding to the years 1923–1929. These levels remained relatively constant until 1963–1968, when the diethyl phthalate level jumped to 35 µg/kg; diethyl phthalate was detected at the surface core level of 42 µg/kg from 1974 to 1979. A core sample taken farther down the bay at a core depth corresponding to the years 1884–1892 (110–120 cm in depth) had a diethyl phthalate concentration of 3.1 µg/kg. Sediment concentrations in the distant samples in this area increased chronologically until they reached a maximum of 22 µg/kg for the period 1972–1979. Production volumes were correlated (R = 0.83) for both the sediment nearest Baltimore and the more distant sample (R = 0.60) (Peterson & Freeman, 1982b).
Diethyl phthalate was detected in 10% of aquatic sediment samples at a median concentration of <500 µg/kg dry weight and in 6.0% of aquatic biota samples at a median concentration of <2.5 mg/kg wet weight (Staples et al., 1985).
Diethyl phthalate was detected in 4.26% of the soil samples taken from the National Priorities List hazardous waste sites, at a mean concentration of 39 mg/kg in the positive samples (CLPSD, 1989).
Fish collected from Great Lakes tributaries in Wisconsin and Ohio, USA, during 1981 contained diethyl phthalate in composite whole-body tissue samples at concentrations ranging from <0.02 mg/kg to <0.30 mg/kg (DeVault, 1985). Lake trout (Salvelinus namaycush) and whitefish (Coregonus clupeaformis) taken from Lake Superior near Isle Royale, Michigan, USA, had elevated levels of diethyl phthalate (0.5 and 2.2 µg/g, respectively) compared with lake trout and whitefish taken from other parts of Lake Superior (both values below the level of quantification of 0.001 µg/g wet weight). Fish taken from Siskiwit Lake on Isle Royale, Michigan, a pristine area supposedly unaffected by human activity, also had relatively high concentrations of diethyl phthalate in their tissue, 0.4 mg/kg for lake trout and 1.7 mg/kg for whitefish.
Human exposure to diethyl phthalate can result from eating foods into which diethyl phthalate has leached from packaging materials, eating contaminated seafood, drinking contaminated water, or breathing contaminated air, or as a result of medical treatment involving the use of PVC tubing (e.g., dialysis patients). The use of diethyl phthalate in consumer products and intake from contaminated foods, however, are likely to be the primary sources of human exposure. Diethyl phthalate has been detected in adipose tissue samples taken from people (including children) in the USA. Occupational exposure may occur in industrial facilities where diethyl phthalate is used in the manufacture of plastics or consumer products.
In a duplicate-portion study, Tsumura et al. (2001) estimated daily intake of 11 phthalate esters, including diethyl phthalate and di(2-ethylhexyl) adipate, in 1-week total diet samples provided in hospitals. Portions of meals of breakfast, lunch, and supper were obtained from three hospitals located in three areas in Japan in October or December 1999, for a period of 7 days. Recovery of the spiked samples and quality assurance of analysis were performed at three laboratories. Daily intakes of diethyl phthalate were 0.07–1.41 µg/person (samples in which diethyl phthalate was not detected were assumed to contain diethyl phthalate at 50% of the limit of detection, which was 0.1, 0.2, and 0.5 ng/g for the three participating laboratories after subtraction of the blank value). Average daily intakes in the three hospitals were estimated to be 0.10, 0.28, and 0.67 µg (overall average 0.35 µg) per day per person, respectively.
Baked foods in the United Kingdom packaged in cardboard boxes with cellulose acetate windows (containing 16–17% w/w diethyl phthalate) had diethyl phthalate concentrations of 1.7–4.5 mg/kg. It was suggested that diethyl phthalate may volatilize from the plastic window to the food without direct contact or be adsorbed in condensate on the window, which would then fall back onto the food (Castle et al., 1988). Diethyl phthalate was quantified from retort food at concentrations of 0–0.51 mg/kg (Giam & Wong, 1987). Based on the levels of diethyl phthalate found in food by Castle et al. (1988), Kamrin & Mayor (1991) estimated a total daily dietary exposure to diethyl phthalate of 4 mg, assuming daily ingestion of 1 kg of cellulose acetate-wrapped food containing 4 mg diethyl phthalate/kg. This represents a worst-case scenario, as it assumes that most foods are packed in cardboard boxes with cellulose acetate windows containing diethyl phthalate.
The occurrence of phthalate esters and di(2-ethylhexyl) adipate in selected foods and in packaging was analysed in the 1985–1989 Canadian Health Protection Branch Total Diet Programme (Page & Lacroix, 1995). Diethyl phthalate was detected in pies, crackers, and chocolate bars at 1.8 µg/g (average), 1.2 µg/g, and 5.3 µg/g as a migrant from the pie carton windows, paperboard box, and aluminium foil paper, respectively.
Oysters collected from the Inner Harbor Navigation Canal in Louisiana, USA, and clams from the Chef Menteur and Rigolets tributaries to Lake Pontchartrain, Louisiana, contained 1100, 450, and 340 µg diethyl phthalate/kg wet weight, respectively (McFall et al., 1985).
Diethyl phthalate is listed as an ingredient in a variety of cosmetic formulations at concentrations ranging from <0.1% to 28.6% (97.5th percentile of use based on data from the International Fragrance Association), although most products contain less than 1% diethyl phthalate (Api, 2001). A 2001 survey of fragrance manufacturers in the USA provided maximum concentrations of 1–11% diethyl phthalate in perfume and up to 1.0% in deodorants and other personal cleanliness products. The products may be applied to skin, eyes, hair, and nails, and they may come in contact with mucous membranes and the respiratory tract; contact may be frequent (several times a day) and of prolonged duration (years). Diethyl phthalate is also approved for use as a component of food manufacturing equipment and packaging at unlimited concentrations (Anonymous, 1985) and in drug product containers (Kamrin & Mayor, 1991).
In a methodological pilot study, exposure to 12 volatile organic compounds was assessed among 12 residents of New Jersey or North Carolina, USA (Wallace et al., 1984). Diethyl phthalate was detected in 1 of 8 ambient air samples, 2 of 12 exhaled breath samples, and 1 of 1 drinking-water sample.
Diethyl phthalate concentrations ranging from 0.01 µg/litre (in 6 of 10 US cities) to 1.0 µg/litre (in Miami, Florida) were found in drinking-water samples from water treatment plants in the USA (Keith et al., 1976). As details of the analytical procedure are not described, it cannot be ruled out that di(2-ethylhexyl) phthalate in the water originated from contamination during sampling and analysis. US EPA (1989) summarized various studies (originally reported in 1980–1982) in which diethyl phthalate was detected in the groundwater of 33% of 39 public water wells in New York state; other phthalate esters were also detected. Again, it is difficult to determine whether these phthalates originated from the waterworks systems or from sample contaminations.
Based on an average concentration of diethyl phthalate in Toronto, Canada, drinking-water of 0.0107 µg/litre, the mean drinking-water exposure for the years 1978–1984 was estimated to be approximately 6 µg/year, assuming an average consumption of 1.5 litres of water per day (Davies, 1990).
Diethyl phthalate was detected in 42% of the human adipose tissue samples taken from children and adults (cadavers and surgical patients) in various regions of the USA during 1982. Concentrations ranged from below the limit of detection (0.20 µg/sample) to a maximum of 0.65 µg/g tissue wet weight (US EPA, 1986).
People receiving medical treatments that involve the use of PVC tubing may be exposed to diethyl phthalate as a result of its leaching from the tubing. Diethyl phthalate was found to be leached from PVC dialysis tubing containing aqueous electrolyte solution, human blood, or bovine plasma perfusates. The tubing was perfused with the aqueous electrolyte solution for 22–96 h, resulting in a level of diethyl phthalate ranging from 18 to 26 mg/litre, as determined by UV spectrometry. Even with only 1 h of perfusion, diethyl phthalate levels reached 20 mg/litre, although the levels per unit time dropped with extended perfusion time. When the tubing was perfused with either human blood or bovine plasma for 8 h, infrared spectrometry showed diethyl phthalate levels 2–4 times greater than with water, suggesting that diethyl phthalate has greater solubility in lipid-containing fluids than in inorganic solutions (Christensen et al., 1976).
Monoester metabolites of seven phthalate esters (monoethyl, monobenzyl, monobutyl, monocyclohexyl, mono-2-ethylhexyl, monoisononyl, and monooctyl), analysed after glucuronidase treatment, were measured in urine samples of an adult population, which comprised a part of the National Health and Nutrition Examination Survey in the USA during 1988–1994 (Blount et al., 2000b). The population studied comprised 289 adults aged 20–60 years (mean ± standard deviation [SD]: 37.4 ± 10.6 years), with gender distribution (56% female) similar across age groups. Monoethyl phthalate was found at the highest concentration in urine among metabolites of phthalate esters assayed, with a geometric mean level of 345 µg/litre and a 95th percentile of 3750 µg/litre. Creatinine-adjusted monoethyl phthalate levels increased on average by 1.7% for every yearly increase in age. Using data from humans on the relationship between a single oral dose and urinary concentration of monoethyl phthalate (Anderson et al., 2001), it was estimated that these urinary concentrations corresponded to 12.3 µg/kg body weight per day (geometric mean) and 93 µg/kg body weight per day (95th percentile) (David, 2000). Using kinetic modelling from rat data and assuming similar metabolic rate and kinetics for diethylphthalate and di-n-butylphthalate, the median exposure for adults in the USA from the same Blount et al. (2000b) data was estimated at 12 µg/kg body weight per day, with a 95th percentile at 110 µg/kg body weight per day (Kohn et al., 2000). The latter study further estimated exposures of 97 women aged 20–40 years, in order to determine potential reproductive and developmental effects of phthalate esters. The median exposure to diethyl phthalate for these women was 13 µg/kg body weight per day, and the 95th percentile value was 90 µg/kg body weight per day (maximum 170 µg/kg body weight per day).
In an expansion of the Blount et al. (2000b) study, CDC (2001) analysed urine from a population sample of 1024 persons, representative of the US population 6 years of age and older, and found the 50th and 90th percentiles of the urinary monoethyl phthalate concentration to be 171 and 1160 µg/litre, respectively.
No studies were located on the distribution or excretion of diethyl phthalate in humans following inhalation, oral, dermal, or other routes of exposure. However, the main metabolite of diethyl phthalate, monoethyl phthalate, has been detected in the urine in the general population, indicating absorption and metabolism of diethyl phthalate (Blount et al., 2000b).
Human faeces (0.2 g/ml, not specified) hydrolysed only 3.0% of diethyl phthalate (1 mg/ml) in vitro within 16 h at 37 °C (Rowland et al., 1977).
Human small intestinal preparations obtained at surgery and stored frozen were used for an assay of esterase activity. The diethyl phthalate hydrolase activities were 31.2–153 nmol/h per milligram of protein in the duodenum and 129 nmol/h per milligram in the jejunum (Lake et al., 1977).
Absorption of diethyl phthalate and three other phthalates (dimethyl, dibutyl, and di(2-ethylhexyl)) was measured using human epidermal skin obtained from the abdominal skin of 11 cadavers (mostly females 55 years of age or older) and subcutaneous fat removed in vitro (Scott et al., 1987, 1989). Epidermal membranes were set up in glass diffusion cells, and their permeability to tritiated water was measured to establish the integrity of the skin. Lag time for absorption of diethyl phthalate was 6 h, and the steady-state absorption rate was 12.8 µg/cm2 per hour. An inverse relationship was observed between absorption rate and aqueous solubility of the various phthalates.
Percutaneous absorption of diethyl phthalate was evaluated in vitro in flow-through diffusion cells using human breast skin (Mint et al., 1994). Neat chemical (16–21 mg/cm2) was applied over 72 h to the epidermal surface of the skin, which was either uncovered or covered. The absorption of diethyl phthalate through skin was 3.9% and 4.8% of the applied doses for covered and uncovered conditions, respectively. The interindividual variation was 4-fold, ranging from 1.6% (SD 1.2) (n = 3) to 8.7% (SD 3.9) (n = 6) among skin donors.
Orally ingested di(2-ethylhexyl) phthalate is rapidly hydrolysed in the gut and absorbed as monoester from the digestive tract (NTP-CERHR, 2001); the extent of the hydrolysis of diethyl phthalate under in vivo conditions in humans, however, has not been established.
Diethyl phthalate (10 or 100 mg) was administered to each of three Wistar rats by stomach intubation. Daily urine collections were analysed for 10 days by GC-MS (Kawano, 1980). For both doses, 77–78% of the administered dose was excreted in urine within 24 h as monoester derivative (67–70% of the dose), phthalic acid (8–9% of the dose), or parent compound (0.1–0.4%), and about 85–93% was excreted within 1 week after administration.
Male rats exposed to a single dermal application of [14C]diethyl phthalate (5–8 mg/cm2) excreted 24% of the administered dose in the urine and 1% of the dose in faeces within 24 h (Elsisi et al., 1989). The radioactivity was widely distributed, but diethyl phthalate and its metabolites are not likely to accumulate to any great extent in tissues, because very little of the 14C radioactivity was found in the tissues 1 week after exposure to diethyl phthalate. The amounts of label found in the brain, lung, liver, spleen, small intestine, kidney, testis, spinal cord, and blood were each less than 0.5% of the administered dose. Adipose tissue, muscle, and skin accounted for 0.03%, 0.14%, and 0.06% of the administered 14C radioactivity, respectively. Thirty-four per cent remained in the area of application, and 4.8% remained in the plastic cap used to protect the application site. Total recovery of the radiolabel in the urine, faeces, tissues, and plastic cap after 7 days was 74 ± 21%. The exhaled amount was not determined. No attempt was made to characterize the metabolites found in the urine.
[14C]Carboxy-labelled diethyl phthalate (2850 mg/kg body weight) was administered intraperitoneally to a group of 13 pregnant rats on either day 5 or day 10 of gestation (Singh et al., 1975). The results showed that radioactivity in the maternal blood peaked during the first 24 h, then diminished quickly. A similar pattern was observed in amniotic fluid and fetal tissues. The reduction in concentration of 14C from these tissues as a function of time was found to fit a first-order excretion curve. From this model curve, the half-life was calculated to be 2.22 days for diethyl phthalate. Radioactivity from [14C]diethyl phthalate is transmitted across the placenta from mother to fetus for at least 15 days post-injection. 14C radioactivity was widely distributed and was detected (<1%) in maternal blood, placenta, amniotic fluid, and developing fetuses at all gestational stages investigated. Although the exact chemical nature of the radioactive compounds was not determined, the investigators reported that some of them were probably mixtures of parent compound, monoester, and phthalic acid.
The first step of metabolism involves hydrolysis to the monoester. This was seen in the in vitro metabolism of [14C]diethyl phthalate (5 nmol/litre solution) by hepatic and small intestinal preparations from a rodent (rat), a non-rodent (ferret), and a non-human primate (baboon) (Lake et al., 1977). Hepatic post-mitochondrial supernatant and intestinal preparations from the rat, baboon, and ferret were able to catalyse the hydrolysis of diethyl phthalate to its monoester derivative. Quantitative species differences were observed in the hepatic and intestinal studies. In the hepatic studies, diethyl phthalate hydrolase activity decreased in the following order: baboon (516 µmol/h per gram liver wet weight) > rat (231) > ferret (45.9). In the intestinal preparation, diethyl phthalate hydrolase activity decreased in the same order: baboon (4.33 µmol/h per milligram protein) > rat (0.648) > ferret (0.053). These results show a qualitative species similarity in the hydrolytic metabolism of diethyl phthalate in humans, a rodent, a non-rodent, and a non-human primate.
Of the three tissue contents (0.2 g/ml) from adult male rats studied in vitro, the small intestine contents hydrolysed the greatest amount (36.4%) of diethyl phthalate (1 mg/ml) in 16 h at 37 °C, followed by caecum (11.5%) and stomach (2.5%) (Rowland et al., 1977).
Once formed, the monoester derivative can be further hydrolysed in vivo to phthalic acid and excreted or conjugated to glucuronide and excreted; the terminal or next-to-last carbon atom in the monoester can be oxidized to an alcohol and excreted; or the alcohol can be successively oxidized to an aldehyde, ketone, or carboxylic acid and excreted (Albro et al., 1973; Albro & Moore, 1974; Kluwe, 1982; US EPA, 1989).
Absorption of diethyl phthalate and three other phthalates was measured using rat dorsal epidermal skin in vitro (Scott et al., 1987). Lag time for absorption was 1.1 h, and the steady-state absorption rate was 414 µg/cm2 per hour. The different percutaneous absorption rates between human and rat would suggest differences in bioavailability and subsequent differences in toxicity following dermal exposure.
Percutaneous absorption of diethyl phthalate was evaluated in vitro in flow-through diffusion cells using full-thickness male rat skin (Mint et al., 1994). Absorption of diethyl phthalate through rat skin into receptor fluid was relatively extensive, reaching 35.9% and 38.4% over 72 h for covered and uncovered conditions, respectively. Percutaneous absorption of rat skin in vitro compares well with rat in vivo data from the literature.
Blount and co-workers (2000b) measured the concentration of monoethyl phthalate in the urine as a measure of exposure to diethyl phthalate. However, no data from humans are available to quantitatively elucidate the relationship between the concentration of monoethyl phthalate in urine and exposure to diethyl phthalate. Such information is available, however, for other phthalates (Anderson et al., 2001). For dibutyl phthalate, for example, the urinary monoester represents on average 69% of an oral dose; practically all is excreted within 24 h after a single oral dose.
Various values of LD50 are presented in Table 2. Diethyl phthalate has low acute toxicity.
Table 2: Acute toxicity of diethyl phthalate.a
|
Species |
Route of administration |
LD50 (mg/kg body weight) |
|
Mouse |
Oral |
8600 |
|
Mouse |
Intraperitoneal |
2800 |
|
Mouse |
Intraperitoneal |
2830 (2420–3290) |
|
Mouse (ICR) |
Intraperitoneal |
3220 (2860–3620) |
|
Rat |
Oral |
9200–9500 |
|
Rat (Sprague-Dawley) |
Intraperitoneal |
5675 (4261–7559) |
a From BUA (1994).
Following oral and intravenous administration of diethyl phthalate to rats, rabbits, dogs, and leghorn chickens, stimulated respiration (initially), lethargy and imbalance, cramps, and respiratory arrest were observed (Blickensdorfer & Templeton, 1930).
Long-term dermal diethyl phthalate (99% pure, 100 or 300 µl) administration is associated with mild, dermal acanthosis in rats (NTP, 1995). One study reported that intradermal injection of diethyl phthalate (0.2 ml of 100 mg/ml emulsion) into cleanly shaven backs caused marked inflammatory reaction after 10–26 min, as measured by injection of 1% trypan blue in rabbits (Calley et al., 1966).
Standard irritation tests using diethyl phthalate were not identified. Ocular irritation tests conducted in rabbits indicate that diethyl phthalate (0.1 ml, undiluted) applied to the conjunctival sac is not an ocular irritant (Lawrence et al., 1975). The compound caused minimal irritation when applied to the eye without washing and was practically non-irritating when the eye was washed after instillation (Dear & Jassup, 1978). In a local lymph node assay, diethyl phthalate (25 µl of 25–100% diethyl phthalate in acetone–olive oil) did not induce significant stimulation of thymidine incorporation into lymph node cells (Ryan et al., 2000).
Several studies have reported increases in absolute and relative liver weights in animals after 1–16 weeks of exposure to diethyl phthalate (Brown et al., 1978; Moody & Reddy, 1978; Oishi & Hiraga, 1980).
Four male Fischer 344 rats (150–180 g) received 2% diethyl phthalate in the diet (corresponding to 1753 mg/kg body weight per day) for 3 weeks (Moody & Reddy, 1978). Thirteen animals served as controls. Diethyl phthalate treatment resulted in a significant reduction in the serum triglyceride level (69.2 ± 2.6 mg per 100 ml, compared with 114.8 ± 17.8 mg per 100 ml in the control), while no significant difference was observed in the concentration of serum cholesterol. Only slight, but statistically significant, increases (P < 0.01) in liver weight (4.4% of the body weight, compared with 3.8% in the control) and activities of peroxisomal enzymes, such as catalase (52 ± 5.5 U per mg protein, compared with 44 ± 2.7 U per mg protein in the control) and carnitine acetyltransferase (8.0 ± 0.6 U per mg protein, compared with 2.7 ± 0.5 U per mg protein in the control), occurred in the diethyl phthalate-treated rats. Moreover, the ratio of mitochondria to peroxisome changed slightly to 5:2 from 5:1 in the control group. Under the same test conditions, di(2-ethylhexyl) phthalate, a well known peroxisome proliferator, showed a mitochondria to peroxisome ratio of 5:4. These results suggested that diethyl phthalate showed weak potential for peroxisome proliferation. No histopathological examination or other investigation was performed with diethyl phthalate.
Ten male Wistar rats were administered 2% diethyl phthalate in the diet (corresponding to approximately 2000 mg/kg body weight per day) for 1 week (Oishi & Hiraga, 1980). A significant increase (12%) in relative liver weights was detected, with no changes in kidney and testis weights. No haematological or histopathological examinations or measurements of any other organ weight were reported.
In 4-week studies, diethyl phthalate was dermally applied to rats and mice. Groups of 10 male and 10 female rats were administered 0, 37.5, 75, 150, or 300 µl (corresponding to 0, 200, 400, 800, or 1600 mg/kg body weight per day for males and 0, 300, 600, 1200, or 2500 mg/kg body weight per day for females). In mice, 10 males or 10 females per group were administered 0, 12.5, 25, 50, or 100 µl (corresponding to 0, 560, 1090, 2100, or 4300 mg/kg body weight per day for males and 0, 630, 1250, 2500, or 5000 mg/kg body weight per day for females). Doses were applied to clipped interscapular skin 5 times per week. Increased relative liver weights were observed in 300 µl male (9%) and female rats (7%), 150 µl female rats (10%), and 100 µl female mice (10%). However, no adverse effects on clinical indices of liver or kidney function were noted (NTP, 1995). No adverse effects on histopathology of heart, lung, liver, kidney, oesophagus, gallbladder (mouse only), large intestine, small intestine, stomach, or bladder in rats or mice were observed.
Groups of 15 rats of each sex were given diets containing 0, 0.2, 1.0, or 5.0% of diethyl phthalate (corresponding to 0, 150, 770, or 3160 mg/kg body weight per day for males and 0, 150, 750, or 3710 mg/kg body weight per day for females) for 16 weeks. Additional groups of five rats of each sex were fed similar diets for 2 or 6 weeks (Brown et al., 1978). No significant effects on haematology, serum enzyme levels, or urinary examinations were detected. Significant decreases in body weight gain were observed in the 5.0% groups of both sexes at 2, 6, or 16 weeks (23–32% for males, 15–20% for females) and in the 1.0% group of females at 16 weeks (8%). A concurrent paired-feeding experiment indicated that the decrease in body weight gain was primarily attributable to lower food consumption and/or poorer food utilization, rather than to a direct toxic action of diethyl phthalate. There were over 30% increases in relative liver weight at the highest dose groups of both sexes in all treatment periods (2, 6, and 16 weeks). The increases in relative liver weight of females at all doses in the 16-week study were significant and dose-dependent. Similar effects were detected in relative organ weights of stomach and small intestine. Relative weights of kidney were also significantly increased at the highest dose only in the 16-week study (18% for males and 11% for females). However, there were no abnormal histopathological findings in the liver, kidney, digestive organs, or any other organs. Although the authors postulate the 1.0% dose as a lowest-observed-adverse-effect level (LOAEL), based on the decrease in body weight, the magnitude of the body weight change at the 1.0% dose was much smaller than that at the 5.0% dose, and the change was primarily due to a decrease in food consumption, as described above. Therefore, the dose of 1.0% (750 mg/kg body weight per day) is considered to be the NOAEL.
Sprague-Dawley rats (six per group) received 50 mg diethyl phthalate/litre, 5% ethyl alcohol, or a combination of both in the drinking-water for 120 days (Sonde et al., 2000). There was no significant difference in body weight, liver weight, or water consumption between control and treated groups. However, serum aspartate and alanine aminotransferase levels were significantly increased, while those of liver were decreased in the diethyl phthalate and combined treatment groups. Significant increases in liver glycogen levels and liver cholesterol levels were also found in those two treated groups. These findings indicate liver damage due to toxic injury and enhancement of glycogen and cholesterol storage and uptake. Moreover, lipid peroxidation as measured by diene conjugation was enhanced in the livers of diethyl phthalate-treated groups. Alteration in membrane properties due to enhanced lipid peroxidation could be the reason for increased glycogen, triglyceride, and cholesterol storage in diethyl phthalate-treated groups.
US EPA (1993) reviewed an unpublished study in which groups of 15 male and 15 female rats were administered 0, 0.5, 2.5, or 5.0% diethyl phthalate (corresponding to approximately 0, 250, 1250, or 2500 mg/kg body weight per day, respectively) in the diet for 2 years. Decreased body weight gain without depression of food intake was detected in the high-dose groups (males and females) only throughout the study. No other effects related to diethyl phthalate exposure were observed in the following examinations: haematology, blood sugar and nitrogen, urinalysis, and gross pathological observation or histopathology. Due to the small study size, the study is inadequate for the evaluation of carcinogenicity.
Male and female F344/N rats (60 per sex per dose) dermally administered 100 or 300 µl diethyl phthalate/day (approximately 320 or 1010 mg/kg body weight per day for males and 510 or 1560 mg/kg body weight per day for females), 5 days/week for 2 years, exhibited a slight decrease in body weight gain (NTP, 1995). NTP (1995) considered that there was no evidence of carcinogenic activity. Survival rates of all treated animals were similar to control. The mean body weights of 300 µl males were slightly less (4–9%) than those of the controls throughout the study. No effects on haematological or blood clinical chemistry parameters were detected. No morphological evidence (including neoplasms and non-neoplastic lesions) of dermal or systemic toxicity was observed in male or female rats, except for a dose-related increase of minimal to mild epidermal acanthosis at the site of application in both sexes, which was considered to be a subtle adaptive response to local irritation.
Groups of B6C3F1 mice (60 per sex per dose) received dermally 0, 7.5, 15, or 30 µl diethyl phthalate/day (corresponding to approximately 0, 280, 520, or 1020 mg/kg body weight per day for males and 0, 280, 550, or 1140 mg/kg body weight per day for females) in 100 µl acetone, 5 days/week for 103 weeks (NTP, 1995). Survival and mean body weights of the dosed animals were similar to those of controls throughout the study. No effects on haematological or blood clinical chemistry parameters and no dermatotoxicological lesions (including neoplasms and non-neoplastic lesions) were observed in both sexes. An increased incidence of non-neoplastic proliferative lesions (basophilic foci) in the liver was statistically significant at the 15 µl dose in males, but not females. Dose-related trends (incidence in order from low to high dose: 0/50, 1/50, 9/50, 3/50 in males and 2/50, 3/50, 6/50, 2/50 in females) were not apparent. The incidences of combined hepatocellular adenomas/carcinomas in the male mice dosed with 0, 7.5, 15, and 30 µl/day were 9/50, 14/50, 14/50, and 18/50, respectively; the corresponding incidences in the female mice were 7/50, 16/51, 19/50, and 12/50, respectively. The combined tumour incidence was dose-related in males only (the dose-related trend by the logistic regression test had a P value of 0.040 in males and 0.231 in females). The authors considered that there was equivocal evidence of carcinogenicity in both sexes of mice, because there was no dose-related response in females and an unusually low control incidence compared with historical data. However, considering that oral administration of di(2-ethylhexyl) phthalate induced the incidences of hepatocellular carcinoma and adenoma in mice (NTP, 1982) and diethyl phthalate showed a weak potential for peroxisome proliferation (Moody & Reddy, 1978), this positive trend of the combined incidence of hepatocellular adenomas/carcinomas in male mice by diethyl phthalate may be related to the peroxisome proliferation activity.
Groups of 50 male mice (Swiss CD-1) were applied 0.1 ml of diethyl phthalate (neat) as an initiator once during the first week of treatment by means of a toe clip, followed by 0.1 ml of 12-O-tetradecanoylphorbol-13-acetate (TPA; 0.05 mg/ml solution for initial 8 weeks, then 0.025 mg/ml solution) as a promoter starting from week 2 for 1 year. The promotion potential of diethyl phthalate was also tested similarly using 7,12-dimethylbenz(a)anthracene (DMBA) as an initiator. DMBA and TPA were used as positive controls of an initiator and a promoter, respectively. Diethyl phthalate had no tumour initiation or promotion capability in this study (NTP, 1995).
A comparison of the results of in vitro mutagenic assays of diethyl phthalate in various strains of Salmonella typhimurium shows contradictory findings. Diethyl phthalate has been shown to be mutagenic for S. typhimurium strains TA100 and TA1535 only without metabolic activation (Kozumbo et al., 1982; Agarwal et al., 1985). The maximum ratios of induced revertants to control were about 2–3 (Kozumbo et al., 1982; Agarwal et al., 1985) and about 2 (Agarwal et al., 1985) for TA100 and TA1535, respectively. No induced revertants were observed for TA98 and TA1537 with or without metabolic activation (Rubin et al., 1979; Agarwal et al., 1985).
Contrary to positive findings, diethyl phthalate has been found to be non-mutagenic in S. typhimurium strains TA98, TA100, TA1535, and TA1537 with or without metabolic activation (Zeiger et al., 1982, 1985; NTP, 1995).
Two chromosomal aberration assays with Chinese hamster fibroblasts and ovaries, respectively, produced negative results for diethyl phthalate at concentrations up to 0.324 mg/ml (Ishidate & Odashima, 1977; NTP, 1995). However, at culture concentrations of 0.05, 0.167, and 0.5 µg/litre, diethyl phthalate produced a concentration-related increase in the number of relative sister chromatid exchanges per chromosome. This effect occurred only in the presence of the S9 fraction from rat liver homogenates (NTP, 1995).
In summary, the results of in vitro mutagenicity tests in microbial assays are equivocal. No in vivo studies were located.
Several investigators have studied the effects of diethyl phthalate on male reproductive function in rats, since other phthalic acid esters have been shown to be toxic to the male reproductive system (Foster et al., 1980, 1983; Gray & Butterworth, 1980; Oishi & Hiraga, 1980; ATSDR, 1989). Testicular and accessory gland weight and histopathology were unaffected by treatment of male rats with diethyl phthalate at doses up to 1600 mg/kg body weight per day (Foster et al., 1980; Gray & Butterworth, 1980; Oishi & Hiraga, 1980). In addition, diethyl phthalate had no effect on progesterone binding to testes microsomes, testicular cytochrome P-450 content, or testicular steroidogenic enzyme activity, whereas other phthalates known to cause testicular toxicity have induced changes in these parameters (Foster et al., 1983). Although treatment with 2% diethyl phthalate (corresponding to approximately 2000 mg/kg body weight) in diet for 1 week in male Wistar rats (5 weeks old) decreased testosterone concentrations in testes and serum (approximately 40% in both), other phthalate esters (di-n-butyl phthalate, diisobutyl phthalate, di(2-ethylhexyl) phthalate) increased testosterone levels (Oishi & Hiraga, 1980). The toxicological significance of the decreased intratesticular testosterone levels is unknown.
In an investigation of ultrastructural changes of Leydig cells caused by treatment with four phthalate esters (di(2-ethylhexyl) phthalate, di-n-pentyl phthalate, di-n-octyl phthalate, diethyl phthalate) (Jones et al., 1993), male Wistar rats were dosed by gavage with 2000 mg/kg body weight per day for 2 days. Diethyl phthalate produced mitochondrial swelling, smooth endoplasmic reticulum focal dilation and vesiculation, and increased interstitial macrophage activity associated with the surface of the Leydig cells of rats. The same dose of di(2-ethylhexyl) phthalate also induced these ultrastructural alterations, but the potencies of the two esters were not compared (Jones et al., 1993).
In a continuous-breeding study, Swiss CD-1 mice (10–12 weeks old) were administered dietary concentrations of 0, 0.25, 1.25, or 2.5% diethyl phthalate (>99% pure) (corresponding to 0, 340, 1770, and 3640 mg/kg body weight per day) for 14 weeks beginning 1 week before cohabitation (NTP, 1984; Lamb et al., 1987; Chapin & Sloane, 1997). No adverse effects on the physiology, fertility, or reproductive performance (e.g., mean number of litters per pair, the numbers of live pups per litter, the viability of the pups, or pup body weight adjusted for litter size) of the F0 generation were observed. The second generation was tested using the F1 mice from the control and high-dose groups only. All 20 pairs of mice mated in both groups, and fertility indices were the same for both groups (95%). The diethyl phthalate F1 litters had 14% fewer pups (11.53 ± 0.54 for control, 9.95 ± 0.67 for the dosed group); viability and pup weight adjusted for litter size were unchanged. Treated F1 males weighed 12% less than controls, while their liver weight and prostate weight, both adjusted for body weight, were increased statistically significantly by 18% and 32%, respectively. Epididymal sperm concentration in F1 males at the 2.5% dose was reduced by 30%, while the percentage of motile sperm and the proportion of abnormal forms were unaffected by diethyl phthalate. In summary, diethyl phthalate had no effect on F0 reproductive performance, but induced moderate reproductive effects in the second generation, together with mild inhibition of body weight gain and a moderate increase in liver and prostate weights, at 3640 mg/kg body weight per day. As only one dose was used in the second-generation study, the NOAEL could not be established. The LOAEL in this study is estimated to be 3640 mg/kg body weight per day.
In a teratogenicity study performed in accordance with today’s standards within the framework of the National Toxicology Program (NTP) (Price et al., 1988, 1989; Field et al., 1993), CD rats (27–32 per dose) were given diethyl phthalate at concentrations of 0.25, 2.5, or 5.0% in their feed (corresponding to 200, 1900, and 3200 mg/kg body weight per day) on days 6–15 of pregnancy. The laparotomy took place on day 20 of pregnancy. One-half of the fetuses were examined for skeletal malformations, and the other half for organ malformations. The dams’ body weights in the 2.5 and 5.0% groups were significantly lower (P < 0.05) on day 9 and from day 9 to day 18, respectively, but lay within those of the control on the day of postmortem examination. The dams in the 0.25% group had significantly higher body weights (P < 0.05). Uterus weights as well as absolute and relative liver and kidney weights were unaffected. Further symptoms of incompatibility were not observed. The fertility indices in the control group and in the dose groups (0.25–5.0%) were 87.1, 93.5, 93.8, and 100%, respectively. Not affected were the numbers of corpora lutea per dam, numbers of implants and absorptions per litter, numbers of dead and living fetuses per litter, the body weights of the fetuses, or the ratio of male to female fetuses. There were no externally visible visceral or skeletal malformations. The incidence of fetuses with an extra rib (variation) was significantly higher in the high-dose group (P < 0.05; 21% compared with 8.8% in the control). The significance of finding an increased incidence of fetal lumbar ribs at the high dose, however, was obscured by the high incidence of skeletal variations in the controls and by maternal toxicity due to reduced food and water consumption of the high-dose dams early in gestation. Although the decreased body weight in dams was observed at the 2.5% dose only on day 9, the change could be due to transient decreased food consumption. Other adverse effects were seen only at the highest dose. The dose of 1900 mg/kg body weight per day (2.5% in the diet) was identified as the NOAEL both for the mother and for the offspring.
Similar skeletal malformations as with CD rats in the NTP study were observed when diethyl phthalate at 500, 1600, or 5600 mg/kg body weight per day was administered percutaneously to pregnant ICR mice (18–20 per dose) from day 0 to day 17 of gestation (Tanaka et al., 1987). The body weights of the dams in all dose groups were in the same range as those of the controls. A significant reduction in thymus weight and a non-significant 7% reduction in spleen weight of the dams relative to the controls were observed in all dose groups. Additionally, the weights of the adrenal glands and kidneys of dams were increased in the highest dose group. Brain, lung, and liver weights of dams were unaffected. Fetal weight was significantly lower in the high-dose group (P < 0.01). Fertility index, number of corpora lutea, number of implantations, number of living fetuses, and the ratio of male to female fetuses lay in the same range as those of the control. The number of malformations in the dose groups did not differ from that of the corresponding controls. The number of variations/retardations in the area of the cervical and lumbar ribs was significantly higher in the high-dose group (P < 0.05), but this finding is probably linked to the maternal toxicity associated with the high dose. A dose of 1600 mg/kg body weight per day was identified as the NOAEL for effects on both the mother and the offspring.
In a preliminary developmental toxicity study, 50 CD-1 mice received diethyl phthalate at a dose of 4500 mg/kg body weight by gavage, once daily on gestation days 6–13, and were allowed normal delivery. No effects on body weight of dams, numbers of viable litters, neonatal survival, or neonatal body weights were observed (Hardin et al., 1987).
Pregnant SD rats (3–16 animals per phthalate ester) were administered phthalate esters (di(2-ethylhexyl) phthalate, benzyl butyl phthalate, diisononyl phthalate, dioctyl terephthalate, dimethyl phthalate, diethyl phthalate) at 750 mg/kg body weight per day by gavage from gestation day 14 to postnatal day 3 (Gray et al., 2000). Three dams were used for diethyl phthalate (two others died in an unexplained manner). Twelve male offspring were examined for the incidence of malformation, changes in body weights of dams or pups, and effects on genital organs (testis, seminal vesicle, prostate or epididymis, penis), liver, pituitary, or adrenal gland weights, and pubertal development. No effects on the above were observed following treatment with diethyl phthalate, whereas treatments with di(2-ethylhexyl) phthalate and benzyl butyl phthalate induced shortened anogenital distances and decreased testis weights or weights of other genital organs in male offspring. In this study, 750 mg/kg body weight per day was identified as the NOAEL for diethyl phthalate (but is limited by the design of the study: only one dose level).
Two- to 16-week dietary administration of diethyl phthalate at concentrations up to 3710 mg/kg body weight per day had no effect on the gross or microscopic pathology of lymph nodes or the thymus (Brown et al., 1978).
Repeated dermal administration of diethyl phthalate had no adverse effects on the histopathology of the spleen, thymus, or lymph nodes or on thyroid/brain weights in rats (up to 855 mg/kg body weight) or mice (up to 772 mg/kg body weight) after exposure for 2 years (NTP, 1995).
Two- to 16-week dietary administration of diethyl phthalate at concentrations up to 3710 mg/kg body weight per day had no effect on the gross or microscopic pathology of the brain or sciatic nerve (Brown et al., 1978). Exposure to 3160 mg/kg body weight per day (males) or 3710 mg/kg body weight per day (females) resulted in increased relative brain weights (Brown et al., 1978).
The ultrastructural and functional effects on the Leydig cell culture by treatment with four mono phthalate esters (2-ethylhexyl, n-pentyl, n-octyl, ethyl) were studied in vitro (Jones et al., 1993). The effects (mitochondrial swelling and smooth endoplasmic reticulum focal dilation or vesiculation) produced by in vivo treatment with di(2-ethylhexyl) or diethyl phthalate, described in the same report, were also observed by treatment with 1000 µmol/litre of monoethylhexyl phthalate incubated in vitro, but not with 1000 µmol/litre of monoethyl phthalate. The luteinizing hormone-stimulated secretion of testosterone from Leydig cells incubated in vitro with monoethylhexyl phthalate was inhibited significantly (to 25% level of control), but not when cells were incubated with monoethyl phthalate.
In a factory that produces shoes from PVC granulate, 30 workers with dermatitis and 30 others without dermatitis were patch-tested with diethyl phthalate (concentration and purity not specified) and compared with 30 controls who had no known exposure to PVC or phthalates. One worker of the 30 with dermatitis and 1 of the 30 without dermatitis responded positively with an allergic contact response. None of the controls had a positive response. Similar results were obtained with dibutyl phthalate, with more positive responses among exposed workers. Criteria and severity of positive reaction were not described. Since some of the workers exposed and sensitive to dioctyl phthalate were also sensitive to diethyl phthalate, the possibility of cross-sensitization was suggested (Vidovic & Kansky, 1985).
In a skin patch test, none of 25 healthy adult volunteers showed a positive reaction to 10% diethyl phthalate (purity, details of method, and results not specified) (Greif, 1967). Patients of the dermatology section in the Finnish Institute of Occupational Health were tested using modified European standard series for allergic and irritant patch test reactions to plastic and glue components. Diethyl phthalate (5%) caused no allergic reactions in 143 patients, but was irritating to 2 patients. There was no description of the irritation level or intensity of response (Kanerva et al., 1997).
Two cases of potential contact dermatitis of women from the plastic of their computer mice, which is known to contain phthalates, were reported (Capon et al., 1996). One woman showed a positive reaction in a patch test with 5% diethyl phthalate, and the other showed sensitization with 5% dimethyl phthalate. When the women used a cover on their computer mice that did not contain diethyl phthalate, the lesions cleared.
When sperm suspensions from healthy donors or male partners in barren unions were incubated with diethyl phthalate (33, 330, 3300 µmol/litre), the mean motility was dose-dependently decreased at doses higher than 330 µmol/litre (about 10% inhibition at 3300 µmol/litre) (Fredricsson et al., 1993).
The aquatic toxicity of phthalate esters, including diethyl phthalate, was reviewed by Staples et al. (1997b). The toxicity of diethyl phthalate to aquatic organisms is summarized in Table 3. LC50/EC50 values range from 3 mg/litre (marine alga Gymnodinium breve) to 132 mg/litre (protozoan Tetrahymena pyriformis), with the lowest no-observed-effect concentrations (NOECs) for algae, invertebrates, and fish being in the range 1.7–4 mg/litre.
Addition of diethyl phthalate to soil at a concentration similar to that detected in non-industrial environments (0.1 mg/g) had no impact on the structural diversity (bacterial numbers, fatty acid methyl ester analysis) or functional diversity of the microbial community. At concentrations representative of a phthalate spill, diethyl phthalate (>1 mg/g) reduced numbers of both total culturable bacteria (by 47%) and pseudomonads (by 62%) within 1 day. The authors stated that these results were due to disruption of membrane fluidity by the lipophilic phthalate, a mechanism not previously attributed to phthalates (Cartwright et al., 2000b).
Hulzebos et al. (1993) grew lettuce (Lactuca sativa) in soil containing diethyl phthalate and found 7- and 14-day EC50s, based on growth, to be 106 and 134 mg/kg, respectively. In solution culture tests, the 16-day EC50 was 25 mg/litre.
In contact toxicity tests, red earthworms (Eisenia foetida) were exposed to diethyl phthalate via filter paper in glass vials. The authors classified diethyl phthalate as "moderately toxic" to earthworms based on a 48-h LC50 of 550 µg/cm2 (Neuhauser et al., 1985).
Diethyl phthalate is minimally irritating or non-irritating to the eyes and skin of rabbits. Negative results, with few exceptions, have been reported after patch testing in humans. The contribution of exposure by the dermal and oral routes to total exposure in humans is uncertain, but the rate of dermal absorption is probably low in humans. Once absorbed, diethyl phthalate is widely distributed in the body.
Very mild hepatic effects are observed only after administration of extremely high doses of diethyl phthalate. The effects reported in animals after short-term and medium-term oral exposure to this compound were decreases in body weight gain and organ weight changes that were not accompanied by any biochemical, functional, or histopathological evidence of organ injury.
Long-term studies by dermal administration in rats and mice did not demonstrate carcinogenic activity for diethyl phthalate, and in vitro genotoxicity studies gave equivocal results.
In a standard NTP teratogenicity study with rats, no malformations but rib number variation and a decrease in fetal weight were observed at an oral dose level of 3200 mg/kg body weight per day, which was also maternally toxic. In this study, the NOAEL for maternal and fetal toxicity was 1900 mg/kg body weight per day. In a dermal exposure study in mice, variation in rib numbers, but no fetotoxicity or teratogenicity, was observed at the highest dose tested, 5600 mg/kg body weight per day, which also was maternally toxic. The NOAEL for maternal and offspring effects in the mouse study was 1600 mg/kg body weight per day. This value, 1600 mg/kg body weight per day, is considered a NOAEL for reproductive toxicity. The NOAEL is supported by a single dose level study in which no adverse effects in dams or pups were observed (specifically, no malformations in male rat reproductive organs, which were observed after exposure to other phthalate esters) after perinatal exposure to diethyl phthalate at 750 mg/kg body weight per day in rats.
In a two-generation continuous-breeding dietary NTP study in mice, effects observed, other than a decrease in body weight gain, included a reduction in the number of live fetuses born to F1 parents, increased liver and prostate weights, and decreased epididymal sperm concentrations in F1 animals. Although these changes were very weak and found at a high dose (3640 mg/kg body weight per day) only, they can be regarded as critical effects from exposure to this compound, and the dose of 3640 mg/kg body weight per day can be considered to constitute the LOAEL.
Uncertainty factors of 3 for incompleteness of the database and another 10 each for intra- and interspecies variation were applied to the NOAEL of 1600 mg/kg body weight per day to derive a tolerable intake of 5 mg/kg body weight per day. This figure is close to the value (3.6 mg/kg body weight per day) that can be derived by applying a 1000-fold uncertainty factor (10 each for use of LOAEL, interspecies variation, and intraspecies variation) to the LOAEL of 3640 mg/kg body weight per day.
The estimated average daily intake of 0.35 µg for a person on a hospital diet in Japan is 6 orders of magnitude lower than the tolerable intake of 5 mg/kg body weight per day (corresponding to 250 mg for a 50-kg person).
Monoethyl phthalate data in urine in the general population in the USA demonstrate a markedly higher diethyl phthalate intake (the difference perhaps being partly due to discontinuation of use of diethyl phthalate in food packaging films in Japan). For women aged 20–40 years, the estimated median daily intake of diethyl phthalate was 13 µg/kg body weight per day, and the 95th percentile value was 90 µg/kg body weight per day (maximum 170 µg/kg body weight per day). The ratios of these intake estimates to the tolerable intake were 3 × 10–3 for the median value and 2 × 10–2 for the 95th percentile value.
No estimation for exposure to diethyl phthalate from its cosmetic or medical use is available.
Levels in drinking-water account for a minor portion of exposure. An average concentration of diethyl phthalate in drinking-water of 0.01 µg/litre (Davies, 1990) will correspond to an intake of 0.33 µg/kg body weight per day (corresponding to 0.007% of the tolerable intake), assuming consumption of 2 litres of water per day and a body weight of 60 kg (WHO, 1996).
The exposure estimate in the USA is based on extrapolation from urinary monoester concentrations, but the kinetic data in humans are very limited; even kinetic data in experimental animals are mainly extrapolated from other phthalate esters.
The contribution of diethyl phthalate from medical devices, which is unlikely to be of importance to the general population, may be significant for hospitalized patients, but the data are very limited.
Orally ingested diethyl phthalate is absorbed as a monoester from the digestive tract, and the reproductive and developmental effects of di(2-ethylhexyl) phthalate are considered to be due to the monoester rather than the original diester compound. The extent of hydrolysis of diethyl phthalate under in vivo conditions in humans, however, has not been established.
Diethyl phthalate has a water solubility of 1 g/litre, low volatility (vapour pressure 4.6 × 10–2 Pa at 20 °C), a low Henry’s law constant (4.3 × 10–8), and a moderate log octanol/water partition coefficient, at around 2.5. Release to water would not be expected to lead to volatilization to the atmosphere. The extent of partitioning within aquatic media is not entirely clear. Modelling suggests that a low to moderate proportion of the total diethyl phthalate will partition to sediment (between 10 and 30%); measurements have shown some sediment enrichment with diethyl phthalate. The overall conclusion has to be that there is moderate partitioning to particulates, with much of the diethyl phthalate remaining in the water column.
Abiotic degradation is not expected to be a significant component of breakdown of diethyl phthalate in the environment. Biotic degradation occurs in soil, surface waters, and sewage treatment plants. There is some field evidence to suggest that degradation is less in the field than would be predicted from laboratory experiments. Biodegradation occurs under both aerobic and anaerobic conditions. Given the uncertainties concerning the extent of biodegradation, diethyl phthalate would be expected to persist in the environment for a period ranging from a few days to a few weeks. Bioaccumulation is moderate experimentally, consistent with the reported log Kow.
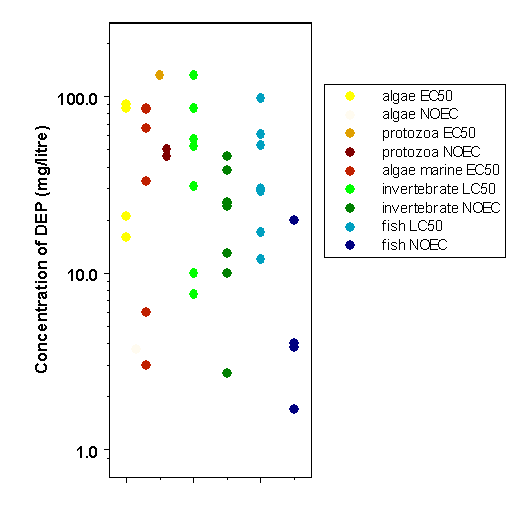
Figure 2: Plot of reported toxicity values for diethyl phthalate in aquatic organisms.
There are limited data on measured concentrations of diethyl phthalate in surface waters (rivers, lakes, and treated wastewater), but no data on field concentrations in soil.
Exposure of organisms in the environment is from production (release to surface waters) and leaching from consumer products in waste sites. The most likely organisms to be exposed are, therefore, free-living aquatic biota and soil organisms. Sewage effluent is a significant source of exposure for aquatic biota.
Toxicity data are available for a range of taxa and organisms. Almost all of the information relates to freshwater organisms. Three test results for marine species are available, and the tested marine invertebrate is the most sensitive in this group. Overall, the variation is limited, ranging across 2 orders of magnitude (1–100 mg/litre) for all taxa. No single group of organisms stands out as being most sensitive. Toxicity data for aquatic organisms (freshwater and marine) are plotted in Figure 2.
The eight acute NOEC values from Table 3 were divided by 2 (estimated from the cladoceran acute and chronic data) to give estimated chronic NOECs, combined with the one chronic NOEC (for the alga Selenastrum), and fitted to a log-logistic distribution (see Appendix 4 for details). From this chronic species sensitivity distribution curve, a concentration to protect 95% of freshwater aquatic species with 50% confidence is derived at 0.9 mg/litre, which is considered to be the PNEC. There are insufficient data to derive a marine PNEC, but the freshwater value may be used until more data are available on the toxicity of diethyl phthalate to marine biota.
Table 3: Toxicity of diethyl phthalate to aquatic organisms.
|
Organism |
End-pointa |
Concentration (mg/litre) |
Reference |
|
Microorganisms |
|||
|
Green alga (Selenastrum capricornutum) |
96-h EC50 (chlorophyll a) |
90 |
US EPA, 1980 |
|
96-h EC50 (cell yield) |
86 |
US EPA, 1980 |
|
|
96-h EC50 (growth rate) |
16 |
Adams et al., 1995 |
|
|
96-h NOEC |
3.7 |
Adams et al., 1995 |
|
|
Green alga (Scenedesmus subspicatus) |
96-h EC50 (cell yield) |
21 |
Kuhn & Pattard, 1990 |
|
Protozoa (Tetrahymena pyriformis) |
48-h EC50 (growth rate) |
132 |
Jaworska et al., 1995 |
|
48-h NOEC |
50 |
Jaworska et al., 1995 |
|
|
48-h LOEC |
100 |
Jaworska et al., 1995 |
|
|
96-h NOEC |
46 |
Adams et al., 1995 |
|
|
Marine alga (Skeletonema costatum) |
96-h EC50 (chlorophyll a) |
66 |
US EPA, 1980 |
|
96-h EC50 (cell yield) |
85 |
US EPA, 1980 |
|
|
Marine dinoflagellate (Gymnodinium breve) |
96-h EC50 (chlorophyll a) |
3-6 |
Wilson et al., 1978 |
|
96-h EC50 (cell yield) |
33 |
Wilson et al., 1978 |
|
|
Invertebrates |
|||
|
Oligochaete worm (Lumbriculus variegatus) |
10-day LC50 |
102 |
Call et al., 2001a |
|
Daphnid (Daphnia magna) |
48-h EC50 (immobilization) |
86 |
Adams et al., 1995 |
|
48-h EC50 (immobilization) |
52 |
LeBlanc, 1980 |
|
|
48-h LC50 |
57 |
Zou & Fingerman, 1997 |
|
|
48-h NOEC |
38 |
Adams et al., 1995 |
|
|
48-h NOEC |
10 |
LeBlanc, 1980 |
|
|
21-day NOEC (survival/reproduction) |
25 |
Rhodes et al., 1995 |
|
|
21-day NOEC (survival/reproduction) |
13 |
Kuhn et al., 1989 |
|
|
21-day LOEC (survival/reproduction) |
59 |
Rhodes et al., 1995 |
|
|
21-day MATC (survival/reproduction) |
38 |
Rhodes et al., 1995 |
|
|
LOEC (1st to 4th instar moulting) |
22 |
Zou & Fingerman, 1997 |
|
|
Midge (Chironomus tentans) |
10-day LC50 |
31 |
Call et al., 2001a |
|
10-day EC50 (biomass) |
28 |
Call et al., 2001a |
|
|
10-day NOEC (biomass) |
24 |
Call et al., 2001a |
|
|
10-day LC50 |
>3100 mg/kgb |
Call et al., 2001b |
|
|
Midge (Paratanytarsus parthenogenica) |
96-h LC50 |
131 |
Adams et al., 1995 |
|
96-h NOEC |
46 |
Adams et al., 1995 |
|
|
Mysid shrimp (Mysidopsis bahia) |
96-h LC50 |
7.6 |
US EPA, 1980 |
|
96-h LC50 |
10 |
Adams et al., 1995 |
|
|
96-h NOEC |
2.7 |
Adams et al., 1995 |
|
|
Fish |
|||
|
Bluegill (Lepomis macrochirus) |
96-h LC50 |
98 |
US EPA, 1980 |
|
96-h LC50 |
17 |
Adams et al., 1995 |
|
|
96-h NOEC |
1.7 |
Adams et al., 1995 |
|
|
Rainbow trout (Oncorhynchus mykiss) |
96-h LC50 |
12 |
Adams et al., 1995 |
|
96-h NOEC |
3.8 |
Adams et al., 1995 |
|
|
Fathead minnow (Pimephales promelas) |
96-h LC50 |
17 |
Adams et al., 1995 |
|
96-h NOEC |
4 |
Adams et al., 1995 |
|
|
Golden orfe (Leuciscus idus melanotus) |
48-h LC50 |
53–61 |
Juhnke & Ludemann, 1978 |
|
Sheepshead minnow (Cyprinodon variegatus) |
96-h LC50 |
30 |
US EPA, 1980 |
|
96-h LC50 |
29 |
Adams et al., 1995 |
|
|
96-h NOEC |
20 |
Adams et al., 1995 |
|
a |
LOEC = lowest-observed-effect concentration; NOEC = no-observed-effect concentration; MATC = maximum acceptable toxicant concentration. |
|
b |
Spiked sediment exposure (mg/kg dry weight). |
Compared with measured concentrations of diethyl phthalate in wastewater and river and lake waters, the risk factors are substantially lower than 1, with approximately 2 orders of magnitude difference between the highest reported concentration in the field and the PNEC. Risk for aquatic organisms, based largely on lethal end-points, is therefore considered low.
The only exposure data for soils come from National Priorities List sites in the USA, where 4% of samples contained diethyl phthalate at a mean concentration of 0.039 mg/kg soil. This compares with toxicity values of greater than 100 mg/kg for plant growth, suggesting very low risk. Effects were seen on soil microorganisms at greater than 1000 mg/kg soil, also suggesting low risk, except following spills. The toxicity value for earthworms (550 mg/cm2) is based on exposure on filter paper and cannot be used for risk estimation. Overall, the risks for terrestrial soil organisms appear to be low.
Previous evaluations of diethyl phthalate by international bodies were not identified.
Adams WJ, Biddinger GR, Robillard KA, Gorsuch JW (1995) A summary of the acute toxicity of 14 phthalate esters to representative aquatic organisms. Environmental Toxicology and Chemistry, 14:1569–1574.
Agarwal DK, Lawrence WH, Nunez LJ, Autian J (1985) Mutagenicity evaluation of phthalic acid esters and metabolites in Salmonella typhimurium cultures. Journal of Toxicology and Environmental Health, 16(1):61–69.
Akiyama T, Koga M, Shinohara R, Kido H, Gito S (1980) [Detection and identification of trace organic substances in the aquatic environment.] Journal of the University of Occupational and Environmental Health, 2:285–300 (in Japanese).
Albro PW, Moore B (1974) Identification of the metabolites of simple phthalate diesters in rat urine. Journal of Chromatography, 94:209–218.
Albro PW, Thomas R, Fishbein L (1973) Metabolism of diethylhexyl phthalate by rats: Isolation and characterization of the urinary metabolites. Journal of Chromatography, 76:321–330.
Aldenberg T (1993) ETX 1.3a. A program to calculate confidence limits for hazardous concentrations based on small samples of toxicity data. Bilthoven, National Institute of Public Health (#719102015).
Aldenberg T, Slob W (1993) Confidence limits for hazardous concentrations based on logistically distributed NOEC toxicity data. Ecotoxicology and Environmental Safety, 25:48–63.
Al-Omran LA, Preston MR (1987) The interactions of phthalate esters with suspended particulate material in fresh and marine waters. Environmental Pollution, 46:177–186.
Anderson WA, Castle L, Scotter MJ, Massey RC, Springall C (2001) A biomarker approach to measuring human dietary exposure to certain phthalate diesters. Food Additives and Contaminants, 18:1068–1074.
Anonymous (1985) Final report on the safety assessment of dibutylphthalate, dimethylphthalate, and diethylphthalate. Journal of the American College of Toxicology, 4(3):267–303.
ANZECC/ARMCANZ (2000) Australian and New Zealand guidelines for fresh and marine water quality. Canberra, Australian and New Zealand Environment Conservation Council and Agriculture and Resource Management Council of Australia and New Zealand, National Water Quality Management Strategy.
Api AM (2001) Toxicological profile of diethyl phthalate: a vehicle for fragrance and cosmetic ingredients. Food and Chemical Toxicology, 39:97–108.
ATSDR (1989) Toxicological profile for di-[2-ethylhexyl]phthalate. Atlanta, GA, US Department of Health and Human Services, Agency for Toxic Substances and Disease Registry.
ATSDR (1995) Toxicological profile for diethylphthalate. Atlanta, GA, US Department of Health and Human Services, Agency for Toxic Substances and Disease Registry.
Barrows ME, Petrocelli SR, Macek KJ, Carroll JJ (1980) Bioconcentration and elimination of selected water pollutants by bluegill sunfish (Lepomis macrochirus). In: Haque R, ed. Dynamics, exposure and hazard assessment of toxic chemicals. Ann Arbor, MI, Ann Arbor Science, pp. 379–392.
Blickensdorfer P, Templeton L (1930) A study of the toxic properties of diethylphthalate. Journal of the American Pharmaceutical Association, Scientific Edition, 19:1179–1181.
Blount BC, Milgram KE, Silva MJ, Malek NA, Reidy JA, Needham LL, Brock JW (2000a) Quantitative detection of eight phthalate metabolites in human urine using HPLC-APCI-MS/MS. Analytical Chemistry, 72:4127–4134.
Blount BC, Silva MJ, Caudill SP, Needham LL, Pirkle JL, Sampson EJ, Lucier GW, Jackson RJ, Brock JW (2000b) Levels of seven urinary metabolites in a human reference population. Environmental Health Perspectives, 108(10):979–982.
Boese BL (1984) Uptake efficiency of the gills of English sole (Parophrys vetulus) for four phthalate esters. Canadian Journal of Fisheries and Aquatic Sciences, 41:1713–1718.
Brown D, Butterworth KR, Gaunt IF, Grasso P, Gangolli SD (1978) Short-term oral toxicity study of diethyl phthalate in the rat. Food and Cosmetics Toxicology, 16(5):415–422.
Brownlee B, Strachan WMJ (1977) Distribution of some organic compounds in the receiving waters of a kraft pulp and paper mill. Journal of the Fisheries Research Board of Canada, 34:830–837.
BUA (1994) Diethyl phthalate. German Chemical Society (GDCh) Advisory Committee on Existing Chemicals of Environmental Relevance (BUA). Stuttgart, S. Hirge Verlag (BUA Report 104; original German version published in 1992).
Burkhard LP, Durhan EJ, Lukasewycz MT (1991) Identification of nonpolar toxicants in effluents using toxicity-based fractionation with gas chromatography/mass spectrometry. Analytical Chemistry, 63(3):277–283.
Call DJ, Markee TP, Geiger DL, Brooke LT, VandeVenter FA, Cox DA, Genisot KI, Robillard KA, Gorsuch JW, Parkerton TF, Reiley MC, Ankley GT, Mount DR (2001a) An assessment of the toxicity of phthalate esters to freshwater benthos. 1. Aqueous exposures. Environmental Toxicology and Chemistry, 20(8):1798–1804.
Call DJ, Cox DA, Geiger DL, Genisot KI, Markee TP, Brooke LT, Polkinghorne CN, VandeVenter FA, Gorsuch JW, Robillard KA, Parkerton TF, Reiley MC, Ankley GT, Mount DR (2001b) An assessment of the toxicity of phthalate esters to freshwater benthos. 2. Sediment exposures. Environmental Toxicology and Chemistry, 20(8):1805–1815.
Calley D, Autian J, Guess WL (1966) Toxicology of a series of phthalate esters. Journal of Pharmaceutical Sciences, 55(2):158–162.
Camanzo J, Rice CP, Jude DJ, Rossmann R (1983) Organic priority pollutants in nearshore fish from 14 Lake Michigan tributaries and embayments. Journal of Great Lakes Research, 13:296–309.
Capon F, Cambie MP, Clinard F, Bernardeau K, Kailis B (1996) Occupational contact dermatitis caused by computer mice. Contact Dermatitis, 35:57–58.
Cartwright CD, Owen SA, Thompson IP, Burns RG (2000a) Biodegradation of diethyl phthalate in soil by a novel pathway. FEMS Microbiology Letters, 186(1):27–34.
Cartwright CD, Thompson IP, Burns RG (2000b) Degradation and impact of phthalate plasticizers on soil microbial communities. Environmental Toxicology and Chemistry, 19(5):1253–1261.
Castle L, Mercer AJ, Startin JR, Gilbert J (1988) Migration from plasticized films into foods: 3. Migration of phthalate, sebacate, citrate and phosphate esters from films used for retail food packaging. Food Additives and Contaminants, 5(1):9–20.
CDC (2001) National report on human exposure to environmental chemicals. Atlanta, GA, Centers for Disease Control and Prevention, 60 pp., at website http://www.cdc.gov/NCEH/dls/report/PDF/CompleteReport.PDF.
Chapin RE, Sloane RA (1997) Reproductive assessment by continuous breeding: evolving study design and summaries of ninety studies. Environmental Health Perspectives, 105(Suppl. 1):199–246.
Chapman PM, Fairbrother A, Brown D (1998) A critical evaluation of safety (uncertainty) factors for ecological risk assessment. Environmental Toxicology and Chemistry, 17:99–108.
Chemical Daily (2001) 13901 chemical products. Tokyo, The Chemical Daily Co., Ltd., pp. 1070–1071.
Christensen D, Neergaard J, Neilsen B, Faurby V, Nielsen OF (1976) The release of plasticizers from PVC tubing. In: Proceedings of the 6th International Congress on Pharmacology, pp. 191–197 [cited in ATSDR, 1995].
CLPSD (1989) Contract Laboratories Program Statistical Database. Washington, DC, US Environmental Protection Agency, July.
Cole RH, Frederick RE, Healy RP, Rolan RG (1984) Preliminary findings of the priority pollutant monitoring project of the nationwide urban runoff program. Journal of the Water Pollution Control Federation, 56:898–908.
David RM (2000) Exposure to phthalate esters. Environmental Health Perspectives, 108(10):A440.
Davies K (1990) Human exposure pathways to selected organochlorines and PCBs in Toronto and southern Ontario. Advances in Environmental Science and Technology, 23:525–540.
Dear WP, Jassup DC (1978) Primary eye irritation study in the albino rabbit. Mattawan, MI, International Research and Development Corp., pp. 1–22.
DeVault DS (1985) Contaminants in fish from Great Lakes harbors and tributary mouths. Archives of Environmental Contamination and Toxicology, 14:587–594.
Elsisi AE, Carter DE, Sipes IG (1989) Dermal absorption of phthalate diesters in rats. Fundamental and Applied Toxicology, 12(1):70–77.
Fatoki OS, Vernon F (1990) Phthalate esters in rivers of the greater Manchester area, England. The Science of the Total Environment, 95:227–232.
Field EA, Price CJ, Sleet RB, George JD, Marr MC, Myers CB, Schwetz BA, Morrissey RE (1993) Developmental toxicity evaluation of diethyl and dimethyl phthalate in rats. Teratology, 48:33–44.
Foster PMD, Thomas LV, Cook MW, Gangolli SD (1980) Study of the testicular effects and changes in zinc excretion produced by some n-alkyl phthalates in the rat. Toxicology and Applied Pharmacology, 54(3):392–398.
Foster PMD, Thomas LV, Cook MW, Walters DG (1983) Effect of di-n-pentyl phthalate treatment on testicular steroidogenic enzymes and cytochrome P-450 in the rat. Toxicology Letters, 15(2–3):265–271.
Fredricsson B, Moller L, Pousette A, Westerholm R (1993) Human sperm motility is affected by plasticizers and diesel particle extracts. Pharmacology and Toxicology, 72:128–133.
Giam CS, Chan HS (1976) Control of blanks in the analysis of phthalates in air and ocean biota samples. In: la Fleur P, ed. Accuracy in trace analysis. Gaithersburg, MD, National Bureau of Standards, pp. 701–708 (National Bureau of Standards Special Publication 422).
Giam CS, Wong MK (1987) Plasticizers in food. Journal of Food Protection, 50(9):769–782.
Goodley PC, Gordon M (1976) Characterization of industrial organic compounds in water. Transactions of the Kentucky Academy of Science, 37:11–15.
Gray TJ, Butterworth KR (1980) Testicular atrophy produced by phthalate esters. Archives of Toxicology, Supplement 4:452–455.
Gray LE Jr, Ostby J, Furr J, Price M, Veeramachaneni DNR, Parks L (2000) Perinatal exposure to the phthalates DEHP, BBP, and DINP, but not DEP, DMP, or DOTP, alters sexual differentiation of the male rat. Toxicological Sciences, 58:350–365.
Grayson BT, Fosbraey LA (1982) Determination of the vapor pressure of pesticides. Pesticide Science, 13:269–278.
Greif N (1967) Cutaneous safety of fragrance materials as measured by the Maximization Test. American Perfumer and Cosmetics, 82:54–57.
Hansch C, Leo A, Hoekman D (1995) Exploring QSAR — hydrophobic, electronic, and steric constants. Washington, DC, American Chemical Society, p. 101.
Hardin BD, Schuler RL, Burg JR, Booth GM, Hazelden KP, MacKenzie KM, Piccirillo VJ, Smith KN (1987) Evaluation of 60 chemicals in a preliminary developmental toxicity test. Teratogenesis, Carcinogenesis, and Mutagenesis, 7(1):29–48.
Hawley GG (1987) Hawley’s condensed chemical dictionary, 10th ed. New York, NY, Van Nostrand Reinhold Company, Inc., p. 394.
Hinckley DA, Bidleman TF, Foreman WT, Tuschall JR (1990) Determination of vapor pressures for nonpolar and semipolar organic compounds from gas chromatographic retention data. Journal of Chemical and Engineering Data, 35(3):232–237.
HSDB (1994) Hazardous Substances Data Bank. Bethesda, MD, National Library of Medicine, National Toxicology Information Program, 11 September 1994.
Hulzebos EM, Adema DMM, Dirven-van Breeman EM, Henzen L, van Dis WA, Herbold HA, Hoekstra JA, Baerselman R, van Gestel CAM (1993) Phytotoxicity studies with Lactuca sativa in soil and nutrient solution. Environmental Toxicology and Chemistry, 12:1079–1094.
IPCS (2001) International Chemical Safety Card — Diethyl phthalate. Geneva, World Health Organization, International Programme on Chemical Safety (ICSC 0258), at website http://www.ilo.org/public/english/protection/safework/cis/products/icsc/dtasht/_icsc02/icsc0258.htm.
Ishidate M Jr, Odashima S (1977) Chromosome tests with 134 compounds on Chinese hamster cells in vitro: A screening for chemical carcinogens. Mutation Research, 48:337–354.
Jaworska JS, Hunter RS, Schultz TW (1995) Quantitative structure–toxicity relationships and volume fraction analyses for selected esters. Archives of Environmental Contamination and Toxicology, 29:86–93.
Jones HB, Garside DA, Liu R, Roberts JC (1993) The influence of phthalate esters on Leydig cell structure and function in vitro and in vivo. Experimental and Molecular Pathology, 58:179–193.
Juhnke I, Ludemann D (1978) Research results in the acute toxicity of 200 chemical substances using golden orfe test. Zeitschrift für Wasser und Abwasser Forschung, 11:161–165.
Jungclaus GA, Games LM, Hites RA (1976) Identification of trace organic compounds in tire manufacturing plant wastewaters. Analytical Chemistry, 48:1894–1896.
Kamrin MA, Mayor GH (1991) Diethyl phthalate — a perspective. Journal of Clinical Pharmacology, 31(5):484–489.
Kanerva L, Jolanki R, Estlander T (1997) Allergic and irritant patch test reactions to plastic and glue allergens. Contact Dermatitis, 37:301–302.
Kawano M (1980) Toxicological studies on phthalate esters. 2. Metabolism, accumulation and excretion of phthalate esters in rats. Japanese Journal of Hygiene, 35:693–701.
Keith LH, Garrison AW, Allen FR, Carter MH, Floyd TL, Pope JD, Thruston AD (1976) Identification of organic compounds in drinking water from thirteen U.S. cities. In: Keith LH, ed. Identification and analysis of organic pollutants in water. Ann Arbor, MI, Ann Arbor Press, pp. 329–373.
Kluwe WM (1982) Overview of phthalate ester pharmacokinetics in mammalian species. Environmental Health Perspectives, 45:3–9.
Kohn MC, Parham F, Masten SA, Portier CJ, Shelby MD, Brock JW, Needham LL (2000) Human exposure estimates for phthalates. Environmental Health Perspectives, 108(10):A440–A442.
Kozumbo WJ, Kroll R, Rubin RJ (1982) Assessment of the mutagenicity of phthalate esters. Environmental Health Perspectives, 45:103–109.
Kuhn R, Pattard M (1990) Results of the harmful effects of water pollutants to green algae (Scenedesmus subspicatus) in the cell multiplication inhibition test. Water Research, 24:31–38.
Kuhn R, Pattard M, Pernak KD, Winter A (1989) Results of the harmful effects of water pollutants to Daphnia magna in the 21-day reproduction test. Water Research, 23:501–510.
Lake BG, Phillips JC, Linnell JC, Gangelli SD (1977) The in vitro hydrolysis of some phthalate diesters by hepatic and intestinal preparations from various species. Toxicology and Applied Pharmacology, 39(2):239–248.
Lamb JC, Chapin C IV, Teague RE, Lawton AD, Reel JR (1987) Reproductive effects of four phthalic acid esters in the mouse. Toxicology and Applied Pharmacology, 88:255–269.
Lawrence WH, Malik M, Turner JE, Singh AR, Autian J (1975) A toxicological investigation of some acute, short-term, and chronic effects of administering di-2-ethylhexyl phthalate (DEHP) and other phthalate esters. Environmental Research, 9:1–11.
LeBlanc GA (1980) Acute toxicity of priority pollutants to water flea (Daphnia magna). Bulletin of Environmental Contamination and Toxicology, 24:684–691.
Lewis DL, Holm HW, Kollig HP, Hall TL (1984) Transport and fate of diethyl phthalate in aquatic ecosystems. Environmental Toxicology and Chemistry, 3:223–232.
Lewis DL, Kellogg RB, Holm HW (1985) Comparison of microbial transformation rate coefficients of xenobiotic chemicals between field-collected and laboratory microcosm microbiota. In: Boyle TO, ed. Validation and predictability of laboratory methods for assessing the fate and effects of contaminants in aquatic ecosystems. Philadelphia, PA, American Society for Testing and Materials, pp. 3–13 (ASTM Special Technical Publication 865).
Lewis RJ Sr, ed. (1993) Hawley’s condensed chemical dictionary, 12th ed. New York, NY, van Nostrand Reinhold Co., p. 396.
Lopez-Avila V, Milanes J, Constantine F, Beckert WF (1990) Typical phthalate ester contamination incurred using EPA method 8060. Journal of the Association of Official Analytical Chemists, 73(5):709–720.
McFall JA, Antoine SR, DeLeon IR (1985) Base-neutral extractable organic pollutants in biota and sediments from Lake Pontchartrain. Chemosphere, 14(10):1561–1569.
Mint A, Hotchkiss SAM, Caldwell J (1994) Percutaneous absorption of diethyl phthalate through rat and human skin in vitro. Toxicology in Vitro, 8(2):251–256.
Moody DE, Reddy JK (1978) Hepatic peroxisome (microbody) proliferation in rats fed plasticizers and related compounds. Toxicology and Applied Pharmacology, 45(2):497–504.
Neuhauser EF, Loehr RC, Malecki MR, Milligan DL, Durkin PR (1985) The toxicity of selected organic chemicals to the earthworm Eisenia fetida. Journal of Environmental Quality, 14(3):383–388.
NTP (1982) Carcinogenesis bioassay of di(2-ethylhexyl) phthalate (CAS No.
NTP (1984) Diethyl phthalate: Reproduction and fertility assessment in CD-1 mice when administered in the feed. Research Triangle Park, NC, US Department of Health and Human Services, National Institutes of Health, National Toxicology Program (NTP-84-262).
NTP (1995) NTP technical report on the toxicology and carcinogenesis studies of diethylphthalate (CAS No.
NTP-CERHR (2001) Final NTP-CERHR Expert Panel report on di(2-ethylhexyl) phthalate, July 1, 2001. Research Triangle Park, NC, National Toxicology Program, Center for the Evaluation of Risks to Human Reproduction, at website http://cerhr.niehs.nih.gov/news/index.html.
OECD (1992) Report of the OECD workshop on extrapolation of laboratory aquatic toxicity data to the real environment. Paris, Organisation for Economic Co-operation and Development (OECD Environment Monograph No. 59).
OECD (1995) Guidance document for aquatic effects assessment. Paris, Organisation for Economic Co-operation and Development (OECD Environment Monograph No. 92).
O’Grady DP, Howard PH, Werner AF (1985) Activated sludge biodegradation of 12 commercial phthalate esters. Applied Environmental Microbiology, 49(2):443–445.
Oishi S, Hiraga K (1980) Testicular atrophy induced by phthalic acid esters: Effect on testosterone and zinc concentrations. Toxicology and Applied Pharmacology, 53(1):35–41.
Page BD, Lacroix GM (1995) The occurrence of phthalate ester and di-2-ethylhexyl adipate plasticizers in Canadian packaging and food sampled in 1985–1989: a survey. Food Additives and Contaminants, 12(1):129–151.
Parker LV, Jenkins TV (1986) Removal of trace-level organics by slow-rate land treatment. Water Research, 20:1417–1426.
Peakall DB (1975) Phthalate esters: Occurrence and biological effects. Residue Reviews, 54:1–41.
Peterson JC, Freeman DH (1982a) Method validation of gas chromatography–mass spectrometry-selected ion monitoring analysis of phthalate esters in sediment. International Journal of Environmental Analytical Chemistry, 12(3–4):277–292.
Peterson JC, Freeman DH (1982b) Phthalate ester concentration variations in dated sediment cores from the Chesapeake Bay. Environmental Science and Technology, 16:464–469.
Preston MR, Al-Omran LA (1989) Phthalate ester speciation in estuarine water, suspended particulates and sediments. Environmental Pollution, 62(2–3):183–194.
Price CJ, Sleet RB, George JD, Marr MC, Schwetz BA (1988) Developmental toxicity evaluation of diethyl phthalate (CAS No.
Price CJ, Sleet RB, George JD, Marr MC, Schwetz BA, Morrissey RE (1989) Developmental toxicity evaluation of diethyl phthalate (DEP) in CD rats. Teratology, 39:473–474.
Rhodes JE, Adams WJ, Biddinger GR, Robillard KA, Gorsuch JW (1995) Chronic toxicity of 14 phthalate esters to Daphnia magna and rainbow trout (Oncorhynchus mykiss). Environmental Toxicology and Chemistry, 14(11):1967–1976.
Ritsema R, Cofino WP, Frintrop PCM, Brinkman UA (1989) Trace-level analysis of phthalate esters in surface water and suspended particulate matter by means of capillary gas chromatography with electron-capture and mass-selective detection. Chemosphere, 18:2161–2175.
Rowland IR, Davies MJ, Meffin PJ (1977) Hydrolysis of phthalate esters by the gastro-intestinal contents of the rat. Food and Cosmetics Toxicology, 15(1):17–21.
Rubin RJ, Kozumbo W, Kroll R (1979) Ames mutagenic assay of a series of phthalate esters: Positive response of dimethyl and diethyl esters. Toxicology and Applied Pharmacology, 48:A133.
Russell DJ, McDuffie B (1983) Analysis for phthalate esters in environmental samples: Separation from polychlorinated biphenyls and pesticides using dual column liquid chromatography. International Journal of Environmental Analytical Chemistry, 15(3):165–184.
Russell DJ, McDuffie B, Fineberg S (1985) The effect of biodegradation on the determination of some chemodynamic properties of phthalate esters. Journal of Environmental Science and Health, Part A, 20:927–941.
Ryan CA, Gerberick GF, Cruse LW, Basketter DA, Lea L, Blaikie L, Dearman RJ, Warbrick EV, Kimber I (2000) Activity of human contact allergens in the murine local lymph node assay. Contact Dermatitis, 43:95–102.
Scott RC, Dugard PH, Ramsey JD, Rhodes C (1987) In vitro absorption of some o-phthalate diesters through human and rat skin. Environmental Health Perspectives, 74:223–227.
Scott RC, Dugard PH, Ramsey JD, Rhodes C (1989) Errata: In vitro absorption of some o-phthalate diesters through human and rat skin. Environmental Health Perspectives, 79:323.
Shelton DR, Tiedje JM (1984) General method for determining anaerobic biodegradation potential. Applied and Environmental Microbiology, 47:850–857.
Shelton DR, Boyd SA, Tiedje JM (1984) Anaerobic biodegradation of phthalic acid esters in sludge. Environmental Science and Technology, 18:93–97.
Shields HC, Weschler CJ (1987) Analysis of ambient concentrations of organic vapors with a passive sampler. Journal of the Air Pollution Control Association, 37(9):1039–1045.
Singh AR, Lawrence WH, Autian J (1975) Maternal–fetal transfer of 14C-di-2-ethylhexyl phthalate and 14C-diethyl phthalate in rats. Journal of Pharmaceutical Science, 64(8):1347–1350.
Sonde V, D’Souza A, Tarapore R, Khare MP, Sinkar P, Krishnan S, Rao CV (2000) Simultaneous administration of diethylphthalate and ethyl alcohol and its toxicity in male Sprague-Dawley rats. Toxicology, 147:23–31.
Staples CA, Werner A, Hoogheem T (1985) Assessment of priority pollutant concentrations in the United States using STORET database. Environmental Toxicology and Chemistry, 4:131–142.
Staples CA, Peterson DR, Parkerton TF, Adams WJ (1997a) The environmental fate of phthalate esters: a literature review. Chemosphere, 35:667–749.
Staples CA, Adams WJ, Parkerton TF, Gorsuch W (1997b) Aquatic toxicity of eighteen phthalate esters. Environmental Toxicology and Chemistry, 16:875–891.
Staples CA, Parkerton TF, Peterson DR (2000) A risk assessment of selected phthalate esters in North American and Western European surface waters. Chemosphere, 40:885–891.
Sugatt RH, O’Grady DP, Banerjee S, Howard PH, Gliedhill WE (1984) Shake flask biodegradation of 14 commercial phthalate esters. Applied Environmental Microbiology, 47:601–606.
Tabak HH, Quave SA, Mashni CI, Barth EF (1981) Biodegradability studies with organic priority pollutant compounds. Journal of the Water Pollution Control Federation, 53:1503–1508.
Tanaka C, Siratori K, Ikegami K, Wakisaka Y (1987) A teratological evaluation following dermal application of diethyl phthalate to pregnant mice. Oyo Yakuri, 33(2):387–392.
Thomas GH (1973) Quantitative determination and confirmation of identity of trace amounts of dialkyl phthalates. Environmental Health Perspectives, 3:23–28.
Tsumura Y, Ishimitsu S, Saito I, Sakai H, Kobayashi Y, Tonogai Y (2001) Eleven phthalate esters and di(2-ethylhexyl)adipate in one-week duplicate diet samples obtained from hospitals and their estimates of daily intake. Food Additives and Contaminants, 18(5):449–460.
US EPA (1979) Water-related environmental fate of 129 pollutants. Volume II. Washington, DC, US Environmental Protection Agency (EPA-440/4-79-029B).
US EPA (1980) Ambient water quality criteria for phthalate esters. Washington, DC, US Environmental Protection Agency (EPA 440/5-80-067).
US EPA (1981a) Determination of phthalates in industrial and municipal waste waters. Technical report. Cincinnati, OH, US Environmental Protection Agency (EPA-600/4-81-063).
US EPA (1981b) Exposure and risk assessment for phthalate esters: Di(2-ethylhexyl) phthalate, di-n-butyl phthalate, dimethyl phthalate, diethyl phthalate, di-n-octyl phthalate, butyl benzyl phthalate. Final report. Washington, DC, US Environmental Protection Agency (EPA-440/4-81-020).
US EPA (1986) Broad scan analysis of the FY82 national human adipose tissue survey specimens. Volume III. Semi-volatile organic compounds. Washington, DC, US Environmental Protection Agency (EPA-560/5-860-037).
US EPA (1989) Health and environmental effects profile for phthalic acid esters. Cincinnati, OH, US Environmental Protection Agency, Office of Research and Development, Environmental Criteria and Assessment Office (EPA/600/22).
US EPA (1993) Integrated Risk Information System: Diethyl phthalate (CASRN
US EPA (1995) Toxics Release Inventory. Washington, DC, US Environmental Protection Agency, at website http://www.epa.gov/TRI.
USITC (1981) Synthetic organic chemicals: United States production and sales, 1980. Washington, DC, US International Trade Commission, pp. 187–188 (USITC Publication 1183).
USITC (1988) Synthetic organic chemicals: United States production and sales, 1987. Washington, DC, US International Trade Commission, pp. 11-2–11-3 (USITC Publication 2118).
Veith GD, Macek KJ, Petrocelli SR, Carroll J (1980) An evaluation of using partition coefficients and water solubility to estimate bioconcentration factors for organic chemicals in fish. In: Eaton JG, Parrish PR, Hendricks AC, eds. Aquatic toxicology. Philadelphia, PA, American Society for Testing and Materials, pp. 116–129 (ASTM Special Technical Publication 707).
Verschueren K (1983) Handbook of environmental data on organic chemicals. New York, NY, Van Nostrand Reinhold, pp. 530–531.
Vidovic R, Kansky A (1985) Contact dermatitis in workers processing polyvinyl chloride plastics. Dermatosen in Beruf und Umwelt, 33(3):104–105.
Voss RH (1984) Neutral organic compounds in biologically treated bleached kraft mill effluents. Environmental Science and Technology, 18(12):938–946.
Wahl HG, Hoffmann A, Häring H-U, Liebich HM (1999) Identification of plasticizers in medical products by a combined direct thermodesorption-cooled injection system and gas chromatography–mass spectrometry. Journal of Chromatography A, 847:1–7.
Waliszewski SM, Szymczymski GA (1990) Determination of phthalate esters in human semen. Andrologia, 22(1):69–73.
Wallace LA, Pellizzari E, Hartwell T, Rosenzweig M, Erickson M, Sparacino C, Zelon H (1984) Personal exposure to volatile organic compounds: I. Direct measurements in breathing-zone air, drinking water, food, and exhaled breath. Environmental Research, 35:293–319.
Walsh GE, Bahner LH, Horning WB (1980) Toxicity of textile mill effluents to freshwater and estuarine algae, crustaceans and fishes. Environmental Pollution, Series A, 21:169–179.
Warne MS (1998) Critical review of methods to derive water quality guidelines for toxicants and a proposal for a new framework. Canberra, Environment Australia (Supervising Scientist Report 135).
Wilson WB, Giam CS, Goodwin TE, Aldrich A, Carpenter V, Hrung YC (1978) The toxicity of phthalates to the marine dinoflagellate Gymnodinium breve. Bulletin of Environmental Contamination and Toxicology, 20:149–154.
Wolfe NL, Steen WC, Burns LA (1980) Phthalate ester hydrolysis: Linear free relationships. Chemosphere, 9:403–408.
Yalkowsky SH, Dannenfelser RM (1992) The AWUASOL database of aqueous solubility, 5th ed. Tucson, AZ, University of Aruiziba, College of Pharmacology.
Zeiger E, Haworth E, Speck S, Mortelmans K (1982) Phthalate ester testing in the National Toxicology Program’s environmental mutagenesis test development program. Environmental Health Perspectives, 45:99–101.
Zeiger E, Haworth S, Mortelmans K, Speck W (1985) Mutagenicity testing of di(2-ethylhexyl) phthalate and related chemicals in Salmonella. Environmental Mutagenesis, 7:213–232.
Zou EM, Fingerman M (1997) Effects of estrogenic xenobiotics on molting of the water flea, Daphnia magna. Ecotoxicology and Environmental Safety, 38(3):281–285.
Zurmuhl T (1990) Development of a method for the determination of phthalate esters in sewage sludge including chromatographic separation from polychlorinated biphenyls, pesticides and polyaromatic hydrocarbons. Analyst (London), 115(9):1171–1175.
ATSDR (1995): Toxicological profile for diethylphthalate
The Agency for Toxic Substances and Disease Registry (ATSDR) toxicological profile is prepared in accordance with guidelines developed by the ATSDR and the US Environmental Protection Agency (EPA) and in support of US Department of Defense information needs. The original guidelines were published in the Federal Register on 17 April 1987. The ATSDR toxicological profile succinctly characterizes the toxicological and adverse health effects information for the hazardous substance being described. It has been peer reviewed by scientists from ATSDR, the Centers for Disease Control and Prevention (CDC), and other federal agencies. It has also been reviewed by a panel of nongovernmental peer reviewers (below) and was made available for public review.
The following people contributed as either chemical manager or author of the toxicological profile on diethyl phthalate: Malcolm Williams, PhD, Division of Toxicology, ATSDR, Atlanta, GA; and
Charles Shore, PhD, Sciences International, Inc., Alexandria, VA.
The profile has undergone the following ATSDR internal reviews:
Green Border Review: Green Border Review assures consistency with ATSDR policy.
Health Effects Review: The Health Effects Review Committee examines the health effects chapter of each profile for consistency and accuracy in interpreting health effects and classifying end-points.
Minimal Risk Level Review: The Minimal Risk Level Workgroup considers issues relevant to substance-specific minimal risk levels (MRLs), reviews the health effects database of each profile, and makes recommendations for derivation of MRLs.
Quality Assurance Review: The Quality Assurance Branch assures that consistency across profiles is maintained, identifies any significant problem in format or content, and establishes that Guidance has been followed.
A peer review panel assembled for diethyl phthalate consisted of the following members:
Dr Martin Alexander, Cornell University, Department of Agronomy, Ithaca, NY
Dr John Lech, Medical College of Wisconsin, Department of Pharmacology and Toxicology, Milwaukee, WI
Dr Fumio Matsumura, University of California, Davis, CA
These experts collectively have knowledge of diethyl phthalate’s physical and chemical properties, toxicokinetics, key health end-points, mechanisms of action, human and animal exposure, and quantification of risk to humans. All reviewers were selected in conformity with the conditions for peer review in Section 104(i)(13) of the Comprehensive Environmental Response, Compensation, and Liability Act, as amended.
The draft CICAD on diethyl phthalate was sent for review to institutions and organizations identified by IPCS after contact with IPCS national Contact Points and Participating Institutions, as well as to identified experts. Comments were received from:
M. Baril, International Programme on Chemical Safety/Institut de Recherche en Santé et en Sécurité du Travail du Québec, Canada
R.P. Beliles, National Center for Environmental Assessment, US Environmental Protection Agency, USA
R. Benson, Drinking Water Program, US Environmental Protection Agency, USA
R. Cary, Health and Safety Executive, United Kingdom
R. Chhabra, National Institute of Environmental Health Sciences, National Institutes of Health, USA
H. Conacher, Bureau of Chemical Safety, Health Canada, Canada
A. Cummings, Office of Research and Development, US Environmental Protection Agency, USA
S. Dobson, Centre for Ecology and Hydrology, United Kingdom
A. Filipsson, Institute of Environmental Medicine, Karolinska Institute, Sweden
H. Gibb, National Center for Environmental Assessment, US Environmental Protection Agency, USA
R. Hertel, Federal Institute for Health Protection of Consumers and Veterinary Medicine, Germany
C. Hiremath, National Center for Environmental Assessment, US Environmental Protection Agency, USA
K. Igarashi, Japanese Chemical Industry Association, Japan
J. Kielhorn, Fraunhofer Institute of Toxicology and Aerosol Research, Germany
J. Reid, National Center for Environmental Assessment, US Environmental Protection Agency, USA
M.K. Stanley, Phthalate Expert Panel of the American Chemistry Council, USA
J. Stauber, CSIRO Energy Technology, Australia
K. Svensson, Toxicology Division, Research and Development Department, National Food Administration, Sweden
S.H. Tao, Center for Food Safety and Applied Nutrition, Food and Drug Administration, USA
J. Temmink, Wageningen University, The Netherlands
K. Ziegler-Skylakakis, European Commission, DG Employment and Social Affairs, Luxembourg
Ottawa, Canada,
29 October – 1 November 2001
Members
Mr R. Cary, Health and Safety Executive, Merseyside, United Kingdom
Dr T. Chakrabarti, National Environmental Engineering Research Institute, Nehru Marg, India
Dr B.-H. Chen, School of Public Health, Fudan University (formerly Shanghai Medical University), Shanghai, China
Dr R. Chhabra, National Institute of Environmental Health Sciences, National Institutes of Health, Research Triangle Park, NC, USA (teleconference participant)
Dr C. De Rosa, Agency for Toxic Substances and Disease Registry, Department of Health and Human Services, Atlanta, GA, USA (Chairman)
Dr S. Dobson, Centre for Ecology and Hydrology, Huntingdon, Cambridgeshire, United Kingdom (Vice-Chairman)
Dr O. Faroon, Agency for Toxic Substances and Disease Registry, Department of Health and Human Services, Atlanta, GA, USA
Dr H. Gibb, National Center for Environmental Assessment, US Environmental Protection Agency, Washington, DC, USA
Ms R. Gomes, Healthy Environments and Consumer Safety Branch, Health Canada, Ottawa, Ontario, Canada
Dr M. Gulumian, National Centre for Occupational Health, Johannesburg, South Africa
Dr R.F. Hertel, Federal Institute for Health Protection of Consumers and Veterinary Medicine, Berlin, Germany
Dr A. Hirose, National Institute of Health Sciences, Tokyo, Japan
Mr P. Howe, Centre for Ecology and Hydrology, Huntingdon, Cambridgeshire, United Kingdom (Co-Rapporteur)
Dr J. Kielhorn, Fraunhofer Institute of Toxicology and Aerosol Research, Hanover, Germany (Co-Rapporteur)
Dr S.-H. Lee, College of Medicine, The Catholic University of Korea, Seoul, Korea
Ms B. Meek, Healthy Environments and Consumer Safety Branch, Health Canada, Ottawa, Ontario, Canada
Dr J.A. Menezes Filho, Faculty of Pharmacy, Federal University of Bahia, Salvador, Bahia, Brazil
Dr R. Rolecki, Nofer Institute of Occupational Medicine, Lodz, Poland
Dr J. Sekizawa, Division of Chem-Bio Informatics, National Institute of Health Sciences, Tokyo, Japan
Dr S.A. Soliman, Faculty of Agriculture, Alexandria University, Alexandria, Egypt
Dr M.H. Sweeney, Document Development Branch, Education and Information Division, National Institute for Occupational Safety and Health, Cincinnati, OH, USA
Dr J. Temmink, Department of Agrotechnology & Food Sciences, Wageningen University, Wageningen, The Netherlands
Ms D. Willcocks, National Industrial Chemicals Notification and Assessment Scheme (NICNAS), Sydney, Australia
Representative of the European Union
Dr K. Ziegler-Skylakakis, European Commission, DG Employment and Social Affairs, Luxembourg
Observers
Dr R.M. David, Eastman Kodak Company, Rochester, NY, USA
Dr R.J. Golden, ToxLogic LC, Potomac, MD, USA
Mr J.W. Gorsuch, Eastman Kodak Company, Rochester, NY, USA
Mr W. Gulledge, American Chemistry Council, Arlington, VA, USA
Mr S.B. Hamilton, General Electric Company, Fairfield, CN, USA
Dr J.B. Silkworth, GE Corporate Research and Development, Schenectady, NY, USA
Dr W.M. Snellings, Union Carbide Corporation, Danbury, CN, USA
Dr E. Watson, American Chemistry Council, Arlington, VA, USA
Secretariat
Dr A. Aitio, International Programme on Chemical Safety, World Health Organization, Geneva, Switzerland
Mr T. Ehara, International Programme on Chemical Safety, World Health Organization, Geneva, Switzerland
Dr P. Jenkins, International Programme on Chemical Safety, World Health Organization, Geneva, Switzerl
Introduction
The traditional approach to using single-species toxicity data to protect field ecosystems has been to apply arbitrary assessment factors — safety factors or application factors — to the lowest toxicity figure for a particular chemical. The magnitude of these safety factors depends on whether acute or chronic toxicity figures are available and the degree of confidence that one has in whether the figures reflect the field situation. Most of the factors are multiples of 10, and larger factors are applied where there is less certainty in the data. For example, a factor of 1000 is generally used for acute data, except for essential elements, where a factor of 200 is applied. This factor of 200 includes a factor of 10 for extrapolating from laboratory to field, a further factor of 10 for a limited data set, and a factor of 2 for conversion of an acute end-point to a chronic end-point (e.g., for an essential metal).
Concerns have often been raised as to the arbitrary nature of assessment factors (Chapman et al., 1998) and the fact that they do not conform to risk assessment principles. OECD (1992) recommended that assessment factors be used only when there are inadequate data for statistical extrapolation methods to be used.
The following sections briefly outline the statistical extrapolation method used to derive the diethyl phthalate guideline for the protection of aquatic organisms. Much of the text is taken directly from the Australian and New Zealand Guidelines for Fresh and Marine Water Quality (ANZECC/ARMCANZ, 2000).
Use of statistical extrapolation methods
New methods using statistical risk-based approaches have been developed over the last decade for deriving guideline (trigger) values. These are based on calculations of a statistical distribution of laboratory ecotoxicity data and attempt to offer a predetermined level of protection, usually 95%. The approach of Aldenberg & Slob (1993) has been adopted in the Netherlands, Australia, and New Zealand for guideline derivation and is recommended for use by the Organisation for Economic Co-operation and Development. It was chosen because of its theoretical basis, its ease of use, and the fact that it has been extensively evaluated. Warne (1998) compared in detail the risk-based and assessment factor approaches used in various countries.
The Aldenberg & Slob (1993) method uses a statistical approach to protect 95% of species with a predetermined level of confidence, provided there is an adequate data set. This approach uses available data from all tested species (not just the most sensitive species) and considers these data to be a subsample of the range of concentrations at which effects would occur in all species in the environment. The method may be applied if toxicity data, usually chronic NOEC values, are available for at least five different species from at least four taxonomic groups. Data are entered into a computer program EcoToX (ETX) (Aldenberg, 1993) and generally fitted to a log-logistic distribution. A hazardous concentration for p per cent of the species (HCp) is derived. HCp is a value such that the probability of selecting a species from the community with a NOEC smaller than HCp is equal to p (e.g., 5%, HC5). HC5 is the estimated concentration that should protect 95% of species. A level of uncertainty is associated with this derived value, and so values with a given confidence level (e.g., 50% or 95%) are computed in the ETX program by attaching a distribution to the error in the tail (Figure A-1). The ANZECC/ARMCANZ (2000) guidelines use the median of 50% confidence.
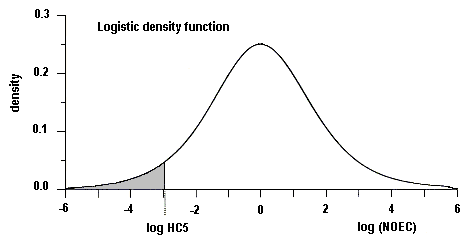
Figure A-1: The Dutch statistical approach for the derivation of trigger values (from Aldenberg & Slob, 1993)
HC5 (or the 95% protection level) is estimated using the ETX approach by dividing the geometric mean of the NOEC values for m species by an extrapolation factor k (OECD, 1995):
K = exp(sm x k)
where:
|
sm |
= |
sample standard deviation of natural logarithm of the NOEC values for m species |
|
k |
= |
one-sided tolerance limit factor for a logistic or normal distribution (from computer simulations) |
Where acute LC50 data are used to derive a trigger value, the figure resulting from the statistical distribution model is converted to a chronic trigger value using an acute-to-chronic (LC50 to NOEC) conversion (ACR). When acute and chronic data are available, an ACR is first applied to each of the species’ acute data, which are then combined with the chronic data prior to using the statistical distribution.
The Aldenberg & Slob (1993) extrapolation method is based on several critical assumptions, outlined below. Many of these are common to other statistical distribution methods:
Modification of the Aldenberg and Slob Approach
The Aldenberg & Slob (1993) approach assumes that the data are best fitted to a log-logistic distribution. For some data sets, however, a better fit is obtained with other models. By using a program developed by CSIRO Biometrics, the data are compared with a range of statistical distributions called the Burr family of distributions, of which the log-logistic distribution is one case. The program determines the distribution that best fits the available toxicity data and calculates the 95% protection level with 50% confidence (ANZECC/ARMCANZ, 2000). This method has been used to calculate the HC5 for diethyl phthalate.
Ce CICAD sur le phtalate de diéthyle a, pour l’essentiel, été établi à partir de l’évaluation qui figure dans le document Profil toxicologique du phtalate de diéthyle (ATSDR, 1995), document qui prend en compte les données répertoriées jusqu’à fin 1994. Les auteurs se sont également référés à un rapport de la BUA (1994) sur ce composé. Le dépouillement de la littérature a été poursuivi jusqu’à octobre 2001 à la recherche de toute information intéressante qui aurait été publiée après la mise au point originale. L’appendice 1 donne des informations sur la préparation du document de base et sur son examen par des pairs. Des renseignements sur l’examen par des pairs du présent CICAD sont également donnés à l’appendice 2. Ce CICAD a été approuvé en tant qu’évaluation internationale lors de la réunion du Comité d’évaluation finale qui s’est tenue à Ottawa (Canada) du 29 octobre au 1er novembre 2001. La liste des participants à cette réunion figure à l’appendice 3. La méthode de distribution de la sensibilité par espèce utilisée pour caractériser le risque écologique est décrite à l’appendice 4. La fiche internationale sur la sécurité chimique du phtalate de diéthyle (ICSC 0258) établie par le Programme international sur la sécurité chimique (IPCS, 2001), est également reproduite dans le présent document.
Le phtalate de diéthyle (No CAS
Le phtalate de diéthyle subit vraisemblablement une biodégradation dans l’environnement. Comparativement à d’autres phtalates, sa capacité de fixation aux sédiments aquatiques est beaucoup plus faible, puisqu’on estime qu’il se retrouve à 70-90 % dans la colonne d’eau. On a mis sa présence en évidence dans des eaux de surface à des concentrations allant de < 1 à 10 µg/litre ou encore dans de l’eau de boisson à des concentrations comprises entre 0,01 et 1,0 µg/litre. Des poissons capturés aux Etats-Unis dans la région des Grands Lacs contenaient jusqu’à 1,7 mg/kg de phtalate de diéthyle. Il est peu probable que le phtalate de diéthyle subisse une bioamplification le long de la chaîne alimentaire.
Une étude comportant à chaque fois l’analyse de deux portions en parallèle a été récemment effectuée au Japon. Elle a montré que les repas servis aux malades dans les hôpitaux apportaient en moyenne 0,35 µg de phtalate de diéthyle par personne et par jour, ce qui s’explique probablement par un contact entre l’emballage plastique ou les gants et la nourriture. Aux Etats-Unis, l’exposition de la population dans son ensemble, estimée d’après la concentration urinaire en monoester, a été évaluée à 12 µg/kg de poids corporel par jour (valeur médiane). La lixiviation du phtalate de diéthyle à partir des tubulures utilisées pour certains traitements médicaux a pu atteindre 20 ng/litre au bout de 1 h de perfusion avec une solution aqueuse d’électrolytes, cette valeur s’abaissant ensuite avec l’allongement de la durée de perfusion.
Appliqué sur l’épiderme, le phtalate de diéthyle traverse le tégument et peut se répartir ensuite largement dans l’organisme, sans toutefois s’accumuler dans les tissus. Dans l’organisme, le phtalate de diéthyle est hydrolysé en monoester. L’hydrolyse métabolique du phtalate de diéthyle est qualitativement analogue chez l’Homme et les rongeurs.
Après administration par voie orale, la DL50 du phtalate de diéthyle est égale ou supérieure à 8600 mg/kg de poids corporel. L’expérimentation animale montre que ce composé n’est que très peu à légèrement irritant pour la peau et la muqueuse oculaire. On a décrit quelques cas d’irritation après pose d’un timbre cutané chez l’Homme ou encore des cas de sensibilisation cutanée, mais il semble que le phénomène soit rare. Après administration par voie orale pendant des durées allant jusqu’à 16 semaines, on a observé une légère augmentation du poids du foie et des reins chez des rongeurs. Toutefois la plupart des études n’ont révélé aucune anomalie histopathologique ou concernant les constantes biochimiques au niveau du foie, du rein ou des autres organes. Lors d’une étude de 3 semaines sur des rats, on a constaté une augmentation journalière du poids du foie de 1753 mg/kg de poids corporel, qui pourrait être en rapport avec une prolifération des peroxysomes.
Aucun effet cancérogène n’a été observé après exposition cutanée chez le rat, en revanche une réaction douteuse a été notée chez la souris dans les mêmes circonstances. Lors d’une étude de 1 an sur des souris destinée à évaluer le caractère promoteur/initiateur du phtalate de diéthyle, on n’a décelé aucune activité de ce genre attribuable au composé. Les études de mutagénicité et de clastogénicité in vitro ont donné des résultats douteux.
Aucune malformation, si ce n’est des variations dans le nombre de côtes, n’a été constatée après l’administration par voie orale d’une dose journalière de 3215 mg/kg de phtalate de diéthyle à des rats, ni par l’administration percutanée d’une dose journalière de 5600 mg/kg de ce composé à des souris - toutes doses qui ont produit des effets toxiques chez les mères. La dose sans effet nocif observable (NOAEL) était respectivement égale à 1600 et à 1900 mg/kg de poids corporel par jour pour la souris et le rat. L’exposition pendant la période périnatale à une dose journalière de 750 mg/kg p.c. de phtalate de diéthyle par gavage, n’a produit d’effets indésirables ni chez les mères ni dans leur descendance et elle n’a pas non plus causé les malformations des organes reproducteurs mâles ni la diminution du poids des testicules qui ont été observées au cours de la même étude après exposition à d’autres phtalates.
Lors d’une étude sur des générations successives de souris, on n’a mis en évidence aucun effet indésirable dans la génération F0 après administration par voie alimentaire d’une dose journalière de phtalate de diéthyle égale à 3640 mg/kg p.c. Toutefois, cette même dose a provoqué une diminution de la concentration épididymaire des spermatozoïdes dans la génération F1 ainsi qu’une réduction du nombre de souriceaux vivants par portée dans la génération F2, avec en outre un léger infléchissement du gain de poids et une augmentation modérée du poids du foie et de la prostate. Après administration par voie orale pendant 2 jours d’une dose journalière égale à 2000 mg/kg p.c. à des rats, on a observé des modifications ultrastructurales dans les cellules de Leydig.
Les études toxicologiques générales ne font ressortir aucun effet immunologique ou neurologique indésirable.
A partir de la NOAEL journalière de 1600 mg/kg p.c. relative aux effets sur le développement, on a établi une dose tolérable par ingestion de 5 mg/kg p.c. en appliquant un facteur d’incertitude de 300. L’apport alimentaire journalier moyen de 0,35 µg/personne (0,007 µg/kg p.c. par jour pour une personne de 50 kg) obtenu au Japon en se basant sur les repas servis aux malades hospitalisés, est inférieur d’environ 6 ordres de grandeur (millionième) à la dose tolérable. Aux Etats- Unis, l’exposition de la population générale, que l’on estime égale à 12 µg/kg p.c. par jour d’après la concentration de phtalate de monoéthyle mesurée dans les urines, correspond à 0,3 % de la dose tolérable. La valeur correspondant au 95ième percentile et tirée de la même étude, à savoir 110 µg/kg p.c. par jour, représente 2 % de la dose tolérable.
Les données disponibles incitent à penser que les organismes dulçaquicoles ne courent pas de risque important d’exposition au phtalate de diéthyle, les concentrations mesurées dans les eaux usées et les eaux de surface étant d’au moins 1 ordre de grandeur inférieures (le dixième) à la concentration prédite sans effet (PNEC) de 0,9 mg/litre. On ne possède pas suffisamment de données pour estimer le risque dans le cas des organismes marins. Pour les organismes terricoles, le risque devrait également être faible, mais les données sont insuffisantes pour que l’on puisse l’évaluer quantitativement.
Este CICAD sobre el dietilftalato se basa fundamentalmente en la evaluación disponible en el informe titulado Perfil toxicológico del dietilftalato (ATSDR, 1995). En el examen figuran los datos identificados hasta el final de 1994. Los autores dispusieron también de un informe del BUA (1994) sobre el dietilftalato como material de referencia. En octubre de 2001 se realizó una nueva búsqueda bibliográfica para localizar cualquier información pertinente publicada después del examen original. La información sobre la preparación y el examen colegiado del documento original figura en el apéndice 1. La información sobre el examen colegiado de este CICAD aparece en el apéndice 2. Este CICAD se aprobó como evaluación internacional en una reunión de la Junta de Evaluación Final, celebrada en Ottawa (Canadá) del 29 de octubre al 1ş de noviembre de 2001. La lista de participantes en esta reunión figura en el apéndice 3. El método de distribución de la sensibilidad por especies utilizado en la caracterización del riesgo para el medio ambiente se describe en el apéndice 4. También se reproduce en este documento la Ficha internacional de seguridad química (ICSC 0258) para el dietilftalato, preparada por el Programa Internacional de Seguridad de las Sustancias Químicas (IPCS, 2001).
El dietilftalato (CAS Nş
El dietilftalato es probable que sufra biodegradación en el medio ambiente. En comparación con otros ftalatos tiene una capacidad mucho menor para unirse a los sedimentos acuáticos, dado que se estima que entre el 70% y el 90% del dietilftalato se encuentra en la columna de agua. Se ha detectado dietilftalato en el agua superficial en concentraciones que oscilan entre <1 y 10 µg/l y en el agua de bebida en concentraciones que varían de 0,01 a 1,0 µg/litro. Los peces recogidos en la zona de los Grandes Lagos de los Estados Unidos contenían dietilftalato en concentraciones de hasta 1,7 mg/kg. No es probable una bioamplificación de este producto a través de la cadena trófica.
En un estudio reciente de porciones duplicadas realizado en el Japón, la ingesta media de dietilftalato en los alimentos en un hospital se estimó en 0,35 µg/día por persona, probablemente debido al contacto del plástico de los envases o los guantes con los alimentos. La exposición de la población general de los Estados Unidos, estimada a partir de las concentraciones urinarias del monoéster, se estimó en 12 µg/kg de peso corporal al día (valor medio). La lixiviación de dietilftalato a partir de los tubos de plástico utilizados en los tratamientos médicos ascendió a 20 ng/l en una hora de perfusión con una solución electrolítica acuosa, decreciendo los niveles al aumentar el tiempo de perfusión.
El dietilftalato de aplicación cutánea atraviesa la piel y se puede distribuir ampliamente por el organismo, pero no se acumula en los tejidos. Se hidroliza en el cuerpo para formar el derivado monoéster. El metabolismo hidrolítico del dietilftalato es cualitativamente semejante en los roedores y las personas.
La DL50 para el dietilftalato tras la administración oral fue de 8600 mg/kg de peso corporal y superior. El dietilftalato tuvo un efecto irritante entre mínimo y ligero de la piel y los ojos en animales de experimentación. Se ha descrito un pequeño número de casos de irritación cutánea en las personas tras las pruebas con parches; se ha descrito sensibilización cutánea en personas, pero parece ser un caso raro. Se observó un ligero aumento del peso del hígado y el riñón de roedores tras la administración oral durante 16 semanas. Sin embargo, en la mayor parte de los estudios no se detectaron cambios químicos o histopatológicos adversos de carácter clínico del hígado, el riñón u otros órganos. En un estudio de tres semanas realizado en ratas se puso de manifiesto un aumento de peso del hígado de 1753 mg/kg de peso corporal al día, que podría estar relacionado con la proliferación de peroxisomas.
No se detectaron efectos carcinogénicos tras la exposición cutánea de ratas y se observó una respuesta equívoca en ratones expuestos por esta misma vía. En un estudio de iniciación/inducción de un año en ratones no se detectó actividad alguna de este tipo. Los resultados de los estudios in vitro de mutagenicidad y clastogenicidad fueron equívocos.
No se observaron malformaciones, pero sí variaciones esqueléticas (número de costillas) tras una dosis oral de 3215 mg/kg de peso corporal al día en ratas y una dosis percutánea de 5600 mg/kg de peso corporal al día en ratones, dosis que también indujeron toxicidad materna. Se identificaron concentraciones sin efectos adversos observados (NOAEL) de 1600 y 1900 mg/kg de peso corporal al día en ratones y ratas, respectivamente. Una exposición perinatal a 750 mg de dietilftalato/ kg de peso corporal al día mediante sonda no indujo efectos adversos en las madres o la progenie ni provocó las malformaciones en los órganos reproductores masculinos o la disminución del peso de los testículos que se observaron tras la exposición a otros ftalatos en el mismo estudio.
En un estudio de reproducción continua, no se detectaron efectos en la generación F0 de ratones tras la administración de 3640 mg/kg de peso corporal al día con los alimentos. Sin embargo, la administración de la misma dosis provocó una disminución de la concentración de esperma en el epidídimo en la generación F1 y un número de crías vivas menor por camada en la F2, junto con una inhibición ligera del aumento de peso corporal y un aumento moderado del peso del hígado y la próstata. Se observaron cambios ultraestructurales en las células de Leydig de ratas con una dosis oral de 2000 mg/kg de peso corporal al día administrada durante dos días.
No se notificaron efectos inmunitarios o neurológicos adversos en estudios de toxicidad general.
Se estimó una ingesta tolerable de 5 mg/kg de peso corporal al día a partir de una NOAEL de 1600 mg/kg de peso corporal al día para los efectos en el desarrollo a la que se aplicó un factor de incertidumbre de 300. La ingesta diaria media de 0,35 µg/persona (0,007 µg/kg de peso corporal al día para una persona de 50 kg) obtenida en un estudio de la alimentación en los hospitales en el Japón es alrededor de seis órdenes de magnitud inferior a la ingesta tolerable. La exposición de la población general en los Estados Unidos, estimada en 12 µg/kg de peso corporal al día a partir de las concentraciones de monoetilftalato en orina, corresponde al 0,3% de la ingesta tolerable. El valor del percentil 95 obtenido del mismo estudio (110 µg/kg de peso corporal al día) corresponde al 2% de la ingesta tolerable.
Los datos disponibles parecen indicar que no es probable que los organismos de agua dulce corran un riesgo significativo a partir de la exposición al dietilftalato, con concentraciones medidas en las aguas residuales y el agua superficial por lo menos un orden de magnitud inferiores a la concentración prevista sin efectos (PNEC) de 0,9 mg/litro. No se dispone de datos suficientes que permitan estimar el riesgo para los organismos marinos. Se prevé que el riesgo para los organismos del suelo también será bajo, pero con los datos disponibles no es posible hacer una estimación cuantitativa.
ENDNOTES
|
International Programme on Chemical Safety (1994) Assessing human health risks of chemicals: derivation of guidance values for health-based exposure limits. Geneva, World Health Organization (Environmental Health Criteria 170) (also available at http://www.who.int/pcs/). |
|
|
Text provided by Dr Jenny Stauber, CSIRO. |
See Also:
Toxicological Abbreviations
Diethyl phthalate (ICSC)