
INTERNATIONAL PROGRAMME ON CHEMICAL SAFETY
CONCISE INTERNATIONAL CHEMICAL ASSESSMENT DOCUMENT NO. 26
BENZOIC ACID AND SODIUM BENZOATE
This report contains the collective views of an international group of
experts and does not necessarily represent the decisions or the stated
policy of the United Nations Environment Programme, the International
Labour Organization, or the World Health Organization.
First draft prepared by Dr A. Wibbertmann, Dr J. Kielhorn, Dr G.
Koennecker, Dr I. Mangelsdorf, and Dr C. Melber, Fraunhofer Institute
for Toxicology and Aerosol Research, Hanover, Germany
Published under the joint sponsorship of the United Nations
Environment Programme, the International Labour Organization, and the
World Health Organization, and produced within the framework of the
Inter-Organization Programme for the Sound Management of Chemicals.
World Health Organization
Geneva, 2000
The International Programme on Chemical Safety (IPCS),
established in 1980, is a joint venture of the United Nations
Environment Programme (UNEP), the International Labour Organization
(ILO), and the World Health Organization (WHO). The overall objectives
of the IPCS are to establish the scientific basis for assessment of
the risk to human health and the environment from exposure to
chemicals, through international peer review processes, as a
prerequisite for the promotion of chemical safety, and to provide
technical assistance in strengthening national capacities for the
sound management of chemicals.
The Inter-Organization Programme for the Sound Management of
Chemicals (IOMC) was established in 1995 by UNEP, ILO, the Food and
Agriculture Organization of the United Nations, WHO, the United
Nations Industrial Development Organization, the United Nations
Institute for Training and Research, and the Organisation for Economic
Co-operation and Development (Participating Organizations), following
recommendations made by the 1992 UN Conference on Environment and
Development to strengthen cooperation and increase coordination in the
field of chemical safety. The purpose of the IOMC is to promote
coordination of the policies and activities pursued by the
Participating Organizations, jointly or separately, to achieve the
sound management of chemicals in relation to human health and the
environment.
WHO Library Cataloguing-in-Publication Data
Benzoic acid and sodium benzoate.
(Concise international chemical assessment document ; 26)
1.Benzoic acid - toxicity 2.Sodium benzoate - toxicity
3.Risk assessment 4.Environmental exposure
I.International Programme on Chemical Safety II.Series
ISBN 92 4 153026 X (NLM Classification: QD 341.A2)
ISSN 1020-6167
The World Health Organization welcomes requests for permission to
reproduce or translate its publications, in part or in full.
Applications and enquiries should be addressed to the Office of
Publications, World Health Organization, Geneva, Switzerland, which
will be glad to provide the latest information on any changes made to
the text, plans for new editions, and reprints and translations
already available.
(c) World Health Organization 2000
Publications of the World Health Organization enjoy copyright
protection in accordance with the provisions of Protocol 2 of the
Universal Copyright Convention. All rights reserved.
The designations employed and the presentation of the material in
this publication do not imply the expression of any opinion whatsoever
on the part of the Secretariat of the World Health Organization
concerning the legal status of any country, territory, city, or area
or of its authorities, or concerning the delimitation of its frontiers
or boundaries.
The mention of specific companies or of certain manufacturers'
products does not imply that they are endorsed or recommended by the
World Health Organization in preference to others of a similar nature
that are not mentioned. Errors and omissions excepted, the names of
proprietary products are distinguished by initial capital letters.
The Federal Ministry for the Environment, Nature Conservation and
Nuclear Safety, Germany, provided financial support for the printing
of this publication.
TABLE OF CONTENTS
FOREWORD
1. EXECUTIVE SUMMARY
2. IDENTITY AND PHYSICAL/CHEMICAL PROPERTIES
3. ANALYTICAL METHODS
4. SOURCES OF HUMAN AND ENVIRONMENTAL EXPOSURE
4.1. Natural sources of benzoic acid
4.2. Anthropogenic sources
4.2.1. Benzoic acid
4.2.2. Sodium benzoate
4.3. Uses
4.3.1. Benzoic acid
4.3.2. Sodium benzoate
4.4. Estimated global release
5. ENVIRONMENTAL TRANSPORT, DISTRIBUTION, TRANSFORMATION, AND ACCUMULATION
5.1. Transport and distribution between media
5.1.1. Benzoic acid
5.1.2. Sodium benzoate
5.2. Transformation
5.2.1. Benzoic acid
5.2.2. Sodium benzoate
5.3. Accumulation
5.3.1. Benzoic acid
5.3.2. Sodium benzoate
6. ENVIRONMENTAL LEVELS AND HUMAN EXPOSURE
6.1. Environmental levels
6.2. Human exposure
7. COMPARATIVE KINETICS AND METABOLISM IN LABORATORY ANIMALS AND HUMANS
7.1. Precursors of benzoic acid
8. EFFECTS ON LABORATORY MAMMALS AND IN VITRO TEST SYSTEMS
8.1. Single exposure
8.2. Irritation and sensitization
8.2.1. Benzoic acid
8.2.2. Sodium benzoate
8.3. Short-term exposure
8.3.1. Oral exposure
8.3.2. Inhalation exposure
8.3.3. Dermal exposure
8.4. Long-term exposure
8.4.1. Subchronic exposure
8.4.2. Chronic exposure and carcinogenicity
8.4.3. Carcinogenicity of benzyl acetate, benzyl alcohol, and benzaldehyde
8.5. Genotoxicity and related end-points
8.5.1. Benzoic acid
8.5.2. Sodium benzoate
8.6. Reproductive and developmental toxicity
8.6.1. Fertility
8.6.2. Developmental toxicity
8.6.3. Reproductive toxicity of benzyl acetate, benzyl alcohol, and benzaldehyde
9. EFFECTS ON HUMANS
10. EFFECTS ON OTHER ORGANISMS IN THE LABORATORY AND FIELD
10.1. Aquatic environment
10.2. Terrestrial environment
11. EFFECTS EVALUATION
11.1. Evaluation of health effects
11.1.1. Hazard identification and dose-response assessment
11.1.2. Criteria for setting tolerable intakes or guidance values for benzoic acid and sodium benzoate
11.1.3. Sample risk characterization
11.2. Evaluation of environmental effects
12. PREVIOUS EVALUATIONS BY INTERNATIONAL BODIES
REFERENCES
APPENDIX 1 -- SOURCE DOCUMENTS
APPENDIX 2 -- CICAD PEER REVIEW
APPENDIX 3 -- CICAD FINAL REVIEW BOARD
APPENDIX 4 -- INTERNATIONAL CHEMICAL SAFETY CARD
RÉSUMÉ D'ORIENTATION
RESUMEN DE ORIENTACI²N
FOREWORD
Concise International Chemical Assessment Documents (CICADs) are
the latest in a family of publications from the International
Programme on Chemical Safety (IPCS) -- a cooperative programme of the
World Health Organization (WHO), the International Labour Organization
(ILO), and the United Nations Environment Programme (UNEP). CICADs
join the Environmental Health Criteria documents (EHCs) as
authoritative documents on the risk assessment of chemicals.
CICADs are concise documents that provide summaries of the
relevant scientific information concerning the potential effects of
chemicals upon human health and/or the environment. They are based on
selected national or regional evaluation documents or on existing
EHCs. Before acceptance for publication as CICADs by IPCS, these
documents undergo extensive peer review by internationally selected
experts to ensure their completeness, accuracy in the way in which the
original data are represented, and the validity of the conclusions
drawn.
The primary objective of CICADs is characterization of hazard and
dose-response from exposure to a chemical. CICADs are not a summary of
all available data on a particular chemical; rather, they include only
that information considered critical for characterization of the risk
posed by the chemical. The critical studies are, however, presented in
sufficient detail to support the conclusions drawn. For additional
information, the reader should consult the identified source documents
upon which the CICAD has been based.
Risks to human health and the environment will vary considerably
depending upon the type and extent of exposure. Responsible
authorities are strongly encouraged to characterize risk on the basis
of locally measured or predicted exposure scenarios. To assist the
reader, examples of exposure estimation and risk characterization are
provided in CICADs, whenever possible. These examples cannot be
considered as representing all possible exposure situations, but are
provided as guidance only. The reader is referred to EHC 1701 for
advice on the derivation of health-based tolerable intakes and
guidance values.
1 International Programme on Chemical Safety (1994)
Assessing human health risks of chemicals: deriviation of
guidance values for health-based exposure limits. Geneva, World
Health Organization (Environmental Health Criteria 170).
While every effort is made to ensure that CICADs represent the
current status of knowledge, new information is being developed
constantly. Unless otherwise stated, CICADs are based on a search of
the scientific literature to the date shown in the executive summary.
In the event that a reader becomes aware of new information that would
change the conclusions drawn in a CICAD, the reader is requested to
contact IPCS to inform it of the new information.
Procedures
The flow chart shows the procedures followed to produce a CICAD.
These procedures are designed to take advantage of the expertise that
exists around the world -- expertise that is required to produce the
high-quality evaluations of toxicological, exposure, and other data
that are necessary for assessing risks to human health and/or the
environment.
The first draft is based on an existing national, regional, or
international review. Authors of the first draft are usually, but not
necessarily, from the institution that developed the original review.
A standard outline has been developed to encourage consistency in
form. The first draft undergoes primary review by IPCS to ensure that
it meets the specified criteria for CICADs.
The second stage involves international peer review by scientists
known for their particular expertise and by scientists selected from
an international roster compiled by IPCS through recommendations from
IPCS national Contact Points and from IPCS Participating Institutions.
Adequate time is allowed for the selected experts to undertake a
thorough review. Authors are required to take reviewers' comments into
account and revise their draft, if necessary. The resulting second
draft is submitted to a Final Review Board together with the
reviewers' comments.
The CICAD Final Review Board has several important functions:
- to ensure that each CICAD has been subjected to an appropriate
and thorough peer review;
- to verify that the peer reviewers' comments have been addressed
appropriately;
- to provide guidance to those responsible for the preparation of
CICADs on how to resolve any remaining issues if, in the opinion
of the Board, the author has not adequately addressed all
comments of the reviewers; and
- to approve CICADs as international assessments.
Board members serve in their personal capacity, not as representatives
of any organization, government, or industry. They are selected
because of their expertise in human and environmental toxicology or
because of their experience in the regulation of chemicals. Boards are
chosen according to the range of expertise required for a meeting and
the need for balanced geographic representation.
Board members, authors, reviewers, consultants, and advisers who
participate in the preparation of a CICAD are required to declare any
real or potential conflict of interest in relation to the subjects
under discussion at any stage of the process. Representatives of
nongovernmental organizations may be invited to observe the
proceedings of the Final Review Board. Observers may participate in
Board discussions only at the invitation of the Chairperson, and they
may not participate in the final decision-making process.
 1. EXECUTIVE SUMMARY
This CICAD on benzoic acid and sodium benzoate was prepared by
the Fraunhofer Institute for Toxicology and Aerosol Research, Hanover,
Germany. The two compounds are being considered together because it is
undissociated benzoic acid that is responsible for its antimicrobial
activity. As benzoic acid itself is only slightly soluble in water,
sodium benzoate -- which, under acid conditions, converts to
undissociated benzoic acid -- is often used instead.
This CICAD was based on reviews compiled by the German Advisory
Committee on Existing Chemicals of Environmental Relevance (BUA,
1995), the US Food and Drug Administration (US FDA, 1972a), and the
Joint FAO/WHO Expert Committee on Food Additives (JECFA) (WHO, 1996)
to assess potential effects of benzoic acid and sodium benzoate on the
environment and on humans. A comprehensive literature search of
relevant databases was conducted in September 1999 to identify any
relevant references published subsequent to those incorporated in
these reports. Information on the preparation and peer review of the
source documents is presented in Appendix 1. Information on the peer
review of this CICAD is presented in Appendix 2. This CICAD was
approved as an international assessment at a meeting of the Final
Review Board, held in Sydney, Australia, on 21-24 November 1999.
Participants at the Final Review Board meeting are listed in Appendix
3. The International Chemical Safety Card (ICSC 0103) for benzoic
acid, produced by the International Programme on Chemical Safety
(IPCS, 1993), has also been reproduced in this document (Appendix 4).
Benzyl acetate, its hydrolysis product, benzyl alcohol, and the
oxidation product of this alcohol, benzaldehyde, are extensively
metabolized to benzoic acid in experimental animals and humans.
Therefore, toxicological data on these precursors were also utilized
in the assessment of the potential health effects of benzoic acid.
Benzoic acid (CAS No. 65-85-0) is a white solid that is slightly
soluble in water. Sodium benzoate (CAS No. 532-32-1) is about 200
times more soluble in water. Benzoic acid is used as an intermediate
in the synthesis of different compounds, primarily phenol (>50% of
the amount produced worldwide) and caprolactam. Other end products
include sodium and other benzoates, benzoyl chloride, and diethylene
and dipropylene glycol dibenzoate plasticizers. Sodium benzoate is
primarily used as a preservative and corrosion inhibitor (e.g., in
technical systems as an additive to automotive engine antifreeze
coolants). Benzoic acid and sodium benzoate are used as food
preservatives and are most suitable for foods, fruit juices, and soft
drinks that are naturally in an acidic pH range. Their use as
preservatives in food, beverages, toothpastes, mouthwashes,
dentifrices, cosmetics, and pharmaceuticals is regulated. The
estimated global production capacity for benzoic acid is about
600 000 tonnes per year. Worldwide sodium benzoate production in 1997
can be estimated at about 55 000-60 000 tonnes. Benzoic acid occurs
naturally in many plants and in animals. It is therefore a natural
constituent of many foods, including milk products. Anthropogenic
releases of benzoic acid and sodium benzoate into the environment are
primarily emissions into water and soil from their uses as
preservatives. Concentrations of naturally occurring benzoic acid in
several foods did not exceed average values of 40 mg/kg of food.
Maximum concentrations reported for benzoic acid or sodium benzoate
added to food for preservation purposes were in the range of 2000
mg/kg of food.
After oral uptake, benzoic acid and sodium benzoate are rapidly
absorbed from the gastrointestinal tract and metabolized in the liver
by conjugation with glycine, resulting in the formation of hippuric
acid, which is rapidly excreted via the urine. To a lesser extent,
benzoates applied dermally can penetrate through the skin. Owing to
rapid metabolism and excretion, an accumulation of the benzoates or
their metabolites is not to be expected.
In rodents, the acute oral toxicity of benzoic acid and sodium
benzoate is low (oral LD50 values of >1940 mg/kg body weight). In
cats, which seem to be more sensitive than rodents, toxic effects and
mortality were reported at much lower doses (about 450 mg/kg body
weight).
Benzoic acid is slightly irritating to the skin and irritating to
the eye, while sodium benzoate is not irritating to the skin and is
only a slight eye irritant. For benzoic acid, the available studies
gave no indication of a sensitizing effect; for sodium benzoate, no
data were identified in the literature.
In short-term studies with rats, disorders of the central nervous
system (benzoic acid/sodium benzoate) as well as histopathological
changes in the brain (benzoic acid) were seen after feeding high doses
(>1800 mg/kg body weight) over 5-10 days. Other effects included
reduced weight gain, changes in organ weights, changes in serum
parameters, or histopathological changes in the liver. The information
concerning long-term oral exposure of experimental animals to benzoic
acid is very limited, and there is no study available dealing
specifically with possible carcinogenic effects. From a limited
four-generation study, only a preliminary no-observed-(adverse-)effect
level (NO(A)EL) of about 500 mg/kg body weight per day can be derived.
With sodium benzoate, two long-term studies with rats and mice gave no
indication of a carcinogenic effect. However, the documentation of
effects is inadequate in most of these studies; therefore, no reliable
NO(A)EL values can be derived. Data on its precursors support the
notion that benzoic acid is unlikely to be carcinogenic.
Benzoic acid tested negative in several bacterial assays and in
tests with mammalian cells, while in vivo studies were not
identified. Sodium benzoate was also inactive in Ames tests, whereas
tests with mammalian cells gave consistently positive results. In one
in vivo study (dominant lethal assay with rats), a positive result
was obtained. At present, a genotoxic activity of sodium benzoate
cannot be ruled out entirely.
For benzoic acid, two limited studies gave no indication of
adverse reproductive or developmental effects. With sodium benzoate,
several studies on different species have been performed, and
embryotoxic and fetotoxic effects as well as malformations were seen
only at doses that induced severe maternal toxicity. In a dietary
study in rats, a NO(A)EL of about 1310 mg/kg body weight was
established. Data on its precursors support the notion that benzoic
acid is unlikely to have adverse reproductive effects at dose levels
not toxic to the mother.
In humans, the acute toxicity of benzoic acid and sodium benzoate
is low. However, both substances are known to cause non-immunological
contact reactions (pseudoallergy). This effect is scarce in healthy
subjects; in patients with frequent urticaria or asthma, symptoms or
exacerbation of symptoms was observed. A provisional tolerable intake
of 5 mg/kg body weight per day can be derived, although benzoates at
lower doses can cause non-immunological contact reactions
(pseudoallergy) in sensitive persons. As there are no adequate studies
available on inhalation exposure, a tolerable concentration for
exposure by inhalation cannot be calculated.
From their physical/chemical properties, benzoic acid and sodium
benzoate emitted to water and soil are not expected to volatilize to
the atmosphere or to adsorb to sediment or soil particles. From the
results of numerous removal experiments, the main elimination pathway
for both chemicals should be biotic mineralization. Data from
laboratory tests showed ready biodegradability for both substances
under aerobic conditions. Several isolated microorganisms (bacteria,
fungi) have been shown to utilize benzoic acid under aerobic or
anaerobic conditions. From the experimental data on bioconcentration,
a low to moderate potential for bioaccumulation is to be expected.
From valid test results available on the toxicity of benzoic acid
and sodium benzoate to various aquatic organisms, these compounds
appear to exhibit low to moderate toxicity in the aquatic compartment.
The lowest EC50 value of 9 mg/litre (cell multiplication inhibition)
reported in a chronic study was observed in the cyanobacterium
Anabaena inaequalis. EC50/LC50 values for the other aquatic
species tested were in the range of 60-1291 mg/litre. Immobilization
of Daphnia magna has been demonstrated to be pH dependent, with a
lower 24-h EC50 (102 mg/litre) at acidic pH. For the freshwater fish
golden ide (Leuciscus idus), a 48-h LC50 of 460 mg/litre has been
determined. Developmental effects have been found in frog (Xenopus)
embryos at a concentration of 433 mg/litre (96-h EC50 for
malformation). For sodium benzoate, exposure of juvenile stages of
aquatic organisms in a multispecies test (including Daphnia magna,
Gammarus fasciatus, Asellus intermedius, Dugesia tigrina,
Helisoma trivolvis, and Lumbriculus variegatus) resulted in 96-h
LC50 values of greater than100 mg/litre. A 96-h LC50 of 484
mg/litre has been determined in the freshwater fish fathead minnow
(Pimephales promelas). Owing to the limited available data on
exposure levels in water, a quantitative risk characterization with
respect to aquatic organisms in surface waters could not be performed.
Taking into account the rapid biodegradability, the low to moderate
bioaccumulation potential, the low toxicity to most aquatic species,
and the rapid metabolism of these substances, benzoic acid and sodium
benzoate will -- with the exception of accidental spills -- pose only
a minimal risk to aquatic organisms.
The few available data indicate that benzoic acid and sodium
benzoate have only a low toxicity potential in the terrestrial
environment. Except for the antimicrobial action of benzoic acid,
characterized by minimum microbiocidal concentrations ranging from 20
to 1200 mg/litre, no data on toxic effects of benzoic acid on
terrestrial organisms were available. For sodium benzoate, bacterial
and fungal growth were inhibited in a pH-dependent manner by
concentrations ranging from 100 to 60 000 mg/litre. Owing to the lack
of measured exposure levels, a sample risk characterization with
respect to terrestrial organisms could not be performed.
2. IDENTITY AND PHYSICAL/CHEMICAL PROPERTIES
Benzoic acid (CAS No. 65-85-0; C7H6O2; C6H5COOH;
benzenecarboxylic acid, phenyl carboxylic acid [E 210
(EU No. Regulation on Labelling of Foodstuffs)]; molecular weight
122.13) is a white solid that starts to sublime at 100°C, with a
melting point of 122°C and a boiling point of 249°C. Its solubility
in water is low (2.9 g/litre at 20°C), and its solution in water
is weakly acid (dissociation constant at 25°C = 6.335 × 10-5;
Maki & Suzuki, 1985; p Ka 4.19). It is soluble in ethanol
and very slightly soluble in benzene and acetone. It has an
octanol/water partition coefficient (log Kow) of 1.9. Its
vapour pressure at 20°C ranges from 0.11 to 0.53 Pa. Its calculated
Henry's law constant at 20°C was given as 0.0046-0.022 Pa.m3/mol
(BUA, 1995). Additional physical and chemical properties are
presented in the International Chemical Safety Card reproduced in
this document (Appendix 4).
Sodium benzoate (CAS No. 532-32-1; C7H5O2Na; benzoic acid,
sodium salt [E 211 (EU No. Regulation on Labelling of Foodstuffs)];
molecular weight 144.11) has a melting point above 300°C. It is very
soluble in water (550-630 g/litre at 20°C) and is hygroscopic at a
relative humidity above 50%. Its pH is about 7.5 at a concentration of
10 g/litre water. It is soluble in ethanol, methanol, and ethylene
glycol. Dry sodium benzoate is electrically charged by friction and
forms an explosive mixture when its dust is dispersed in air (Maki &
Suzuki, 1985).
1. EXECUTIVE SUMMARY
This CICAD on benzoic acid and sodium benzoate was prepared by
the Fraunhofer Institute for Toxicology and Aerosol Research, Hanover,
Germany. The two compounds are being considered together because it is
undissociated benzoic acid that is responsible for its antimicrobial
activity. As benzoic acid itself is only slightly soluble in water,
sodium benzoate -- which, under acid conditions, converts to
undissociated benzoic acid -- is often used instead.
This CICAD was based on reviews compiled by the German Advisory
Committee on Existing Chemicals of Environmental Relevance (BUA,
1995), the US Food and Drug Administration (US FDA, 1972a), and the
Joint FAO/WHO Expert Committee on Food Additives (JECFA) (WHO, 1996)
to assess potential effects of benzoic acid and sodium benzoate on the
environment and on humans. A comprehensive literature search of
relevant databases was conducted in September 1999 to identify any
relevant references published subsequent to those incorporated in
these reports. Information on the preparation and peer review of the
source documents is presented in Appendix 1. Information on the peer
review of this CICAD is presented in Appendix 2. This CICAD was
approved as an international assessment at a meeting of the Final
Review Board, held in Sydney, Australia, on 21-24 November 1999.
Participants at the Final Review Board meeting are listed in Appendix
3. The International Chemical Safety Card (ICSC 0103) for benzoic
acid, produced by the International Programme on Chemical Safety
(IPCS, 1993), has also been reproduced in this document (Appendix 4).
Benzyl acetate, its hydrolysis product, benzyl alcohol, and the
oxidation product of this alcohol, benzaldehyde, are extensively
metabolized to benzoic acid in experimental animals and humans.
Therefore, toxicological data on these precursors were also utilized
in the assessment of the potential health effects of benzoic acid.
Benzoic acid (CAS No. 65-85-0) is a white solid that is slightly
soluble in water. Sodium benzoate (CAS No. 532-32-1) is about 200
times more soluble in water. Benzoic acid is used as an intermediate
in the synthesis of different compounds, primarily phenol (>50% of
the amount produced worldwide) and caprolactam. Other end products
include sodium and other benzoates, benzoyl chloride, and diethylene
and dipropylene glycol dibenzoate plasticizers. Sodium benzoate is
primarily used as a preservative and corrosion inhibitor (e.g., in
technical systems as an additive to automotive engine antifreeze
coolants). Benzoic acid and sodium benzoate are used as food
preservatives and are most suitable for foods, fruit juices, and soft
drinks that are naturally in an acidic pH range. Their use as
preservatives in food, beverages, toothpastes, mouthwashes,
dentifrices, cosmetics, and pharmaceuticals is regulated. The
estimated global production capacity for benzoic acid is about
600 000 tonnes per year. Worldwide sodium benzoate production in 1997
can be estimated at about 55 000-60 000 tonnes. Benzoic acid occurs
naturally in many plants and in animals. It is therefore a natural
constituent of many foods, including milk products. Anthropogenic
releases of benzoic acid and sodium benzoate into the environment are
primarily emissions into water and soil from their uses as
preservatives. Concentrations of naturally occurring benzoic acid in
several foods did not exceed average values of 40 mg/kg of food.
Maximum concentrations reported for benzoic acid or sodium benzoate
added to food for preservation purposes were in the range of 2000
mg/kg of food.
After oral uptake, benzoic acid and sodium benzoate are rapidly
absorbed from the gastrointestinal tract and metabolized in the liver
by conjugation with glycine, resulting in the formation of hippuric
acid, which is rapidly excreted via the urine. To a lesser extent,
benzoates applied dermally can penetrate through the skin. Owing to
rapid metabolism and excretion, an accumulation of the benzoates or
their metabolites is not to be expected.
In rodents, the acute oral toxicity of benzoic acid and sodium
benzoate is low (oral LD50 values of >1940 mg/kg body weight). In
cats, which seem to be more sensitive than rodents, toxic effects and
mortality were reported at much lower doses (about 450 mg/kg body
weight).
Benzoic acid is slightly irritating to the skin and irritating to
the eye, while sodium benzoate is not irritating to the skin and is
only a slight eye irritant. For benzoic acid, the available studies
gave no indication of a sensitizing effect; for sodium benzoate, no
data were identified in the literature.
In short-term studies with rats, disorders of the central nervous
system (benzoic acid/sodium benzoate) as well as histopathological
changes in the brain (benzoic acid) were seen after feeding high doses
(>1800 mg/kg body weight) over 5-10 days. Other effects included
reduced weight gain, changes in organ weights, changes in serum
parameters, or histopathological changes in the liver. The information
concerning long-term oral exposure of experimental animals to benzoic
acid is very limited, and there is no study available dealing
specifically with possible carcinogenic effects. From a limited
four-generation study, only a preliminary no-observed-(adverse-)effect
level (NO(A)EL) of about 500 mg/kg body weight per day can be derived.
With sodium benzoate, two long-term studies with rats and mice gave no
indication of a carcinogenic effect. However, the documentation of
effects is inadequate in most of these studies; therefore, no reliable
NO(A)EL values can be derived. Data on its precursors support the
notion that benzoic acid is unlikely to be carcinogenic.
Benzoic acid tested negative in several bacterial assays and in
tests with mammalian cells, while in vivo studies were not
identified. Sodium benzoate was also inactive in Ames tests, whereas
tests with mammalian cells gave consistently positive results. In one
in vivo study (dominant lethal assay with rats), a positive result
was obtained. At present, a genotoxic activity of sodium benzoate
cannot be ruled out entirely.
For benzoic acid, two limited studies gave no indication of
adverse reproductive or developmental effects. With sodium benzoate,
several studies on different species have been performed, and
embryotoxic and fetotoxic effects as well as malformations were seen
only at doses that induced severe maternal toxicity. In a dietary
study in rats, a NO(A)EL of about 1310 mg/kg body weight was
established. Data on its precursors support the notion that benzoic
acid is unlikely to have adverse reproductive effects at dose levels
not toxic to the mother.
In humans, the acute toxicity of benzoic acid and sodium benzoate
is low. However, both substances are known to cause non-immunological
contact reactions (pseudoallergy). This effect is scarce in healthy
subjects; in patients with frequent urticaria or asthma, symptoms or
exacerbation of symptoms was observed. A provisional tolerable intake
of 5 mg/kg body weight per day can be derived, although benzoates at
lower doses can cause non-immunological contact reactions
(pseudoallergy) in sensitive persons. As there are no adequate studies
available on inhalation exposure, a tolerable concentration for
exposure by inhalation cannot be calculated.
From their physical/chemical properties, benzoic acid and sodium
benzoate emitted to water and soil are not expected to volatilize to
the atmosphere or to adsorb to sediment or soil particles. From the
results of numerous removal experiments, the main elimination pathway
for both chemicals should be biotic mineralization. Data from
laboratory tests showed ready biodegradability for both substances
under aerobic conditions. Several isolated microorganisms (bacteria,
fungi) have been shown to utilize benzoic acid under aerobic or
anaerobic conditions. From the experimental data on bioconcentration,
a low to moderate potential for bioaccumulation is to be expected.
From valid test results available on the toxicity of benzoic acid
and sodium benzoate to various aquatic organisms, these compounds
appear to exhibit low to moderate toxicity in the aquatic compartment.
The lowest EC50 value of 9 mg/litre (cell multiplication inhibition)
reported in a chronic study was observed in the cyanobacterium
Anabaena inaequalis. EC50/LC50 values for the other aquatic
species tested were in the range of 60-1291 mg/litre. Immobilization
of Daphnia magna has been demonstrated to be pH dependent, with a
lower 24-h EC50 (102 mg/litre) at acidic pH. For the freshwater fish
golden ide (Leuciscus idus), a 48-h LC50 of 460 mg/litre has been
determined. Developmental effects have been found in frog (Xenopus)
embryos at a concentration of 433 mg/litre (96-h EC50 for
malformation). For sodium benzoate, exposure of juvenile stages of
aquatic organisms in a multispecies test (including Daphnia magna,
Gammarus fasciatus, Asellus intermedius, Dugesia tigrina,
Helisoma trivolvis, and Lumbriculus variegatus) resulted in 96-h
LC50 values of greater than100 mg/litre. A 96-h LC50 of 484
mg/litre has been determined in the freshwater fish fathead minnow
(Pimephales promelas). Owing to the limited available data on
exposure levels in water, a quantitative risk characterization with
respect to aquatic organisms in surface waters could not be performed.
Taking into account the rapid biodegradability, the low to moderate
bioaccumulation potential, the low toxicity to most aquatic species,
and the rapid metabolism of these substances, benzoic acid and sodium
benzoate will -- with the exception of accidental spills -- pose only
a minimal risk to aquatic organisms.
The few available data indicate that benzoic acid and sodium
benzoate have only a low toxicity potential in the terrestrial
environment. Except for the antimicrobial action of benzoic acid,
characterized by minimum microbiocidal concentrations ranging from 20
to 1200 mg/litre, no data on toxic effects of benzoic acid on
terrestrial organisms were available. For sodium benzoate, bacterial
and fungal growth were inhibited in a pH-dependent manner by
concentrations ranging from 100 to 60 000 mg/litre. Owing to the lack
of measured exposure levels, a sample risk characterization with
respect to terrestrial organisms could not be performed.
2. IDENTITY AND PHYSICAL/CHEMICAL PROPERTIES
Benzoic acid (CAS No. 65-85-0; C7H6O2; C6H5COOH;
benzenecarboxylic acid, phenyl carboxylic acid [E 210
(EU No. Regulation on Labelling of Foodstuffs)]; molecular weight
122.13) is a white solid that starts to sublime at 100°C, with a
melting point of 122°C and a boiling point of 249°C. Its solubility
in water is low (2.9 g/litre at 20°C), and its solution in water
is weakly acid (dissociation constant at 25°C = 6.335 × 10-5;
Maki & Suzuki, 1985; p Ka 4.19). It is soluble in ethanol
and very slightly soluble in benzene and acetone. It has an
octanol/water partition coefficient (log Kow) of 1.9. Its
vapour pressure at 20°C ranges from 0.11 to 0.53 Pa. Its calculated
Henry's law constant at 20°C was given as 0.0046-0.022 Pa.m3/mol
(BUA, 1995). Additional physical and chemical properties are
presented in the International Chemical Safety Card reproduced in
this document (Appendix 4).
Sodium benzoate (CAS No. 532-32-1; C7H5O2Na; benzoic acid,
sodium salt [E 211 (EU No. Regulation on Labelling of Foodstuffs)];
molecular weight 144.11) has a melting point above 300°C. It is very
soluble in water (550-630 g/litre at 20°C) and is hygroscopic at a
relative humidity above 50%. Its pH is about 7.5 at a concentration of
10 g/litre water. It is soluble in ethanol, methanol, and ethylene
glycol. Dry sodium benzoate is electrically charged by friction and
forms an explosive mixture when its dust is dispersed in air (Maki &
Suzuki, 1985).
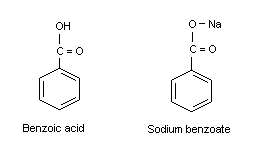 3. ANALYTICAL METHODS
Analytical methods for the determination of benzoic acid include
spectrophotometric methods, which need extensive extraction procedures
and are not very specific; gas chromatographic (GC) methods, which are
more sensitive and specific but need lengthy sample preparation and
derivatization prior to determination; and high-performance liquid
chromatography (HPLC), which has a high specificity and minimum sample
preparation and does not require derivatization.
A direct determination of benzoic acid in air by flash desorption
at 240°C with helium into capillary-GC gave a detection limit of
0.1 ppm (0.5 mg/m3) in a 20-litre sample (=10 µg benzoic acid).
This method has been developed and used for monitoring occupational
exposure (Halvorson, 1984).
A method for the determination of benzoic acid in solid food at
0.5-2 g/kg levels involves extraction with ether into aqueous sodium
hydroxide and methylene chloride, conversion to trimethylsilyl esters,
and detection by GC and flame ionization (Larsson, 1983; AOAC, 1990).
For margarine, a method using HPLC and ultraviolet (UV) detection has
been described with prior extraction with ammonium acetate/acetic
acid/methanol (Arens & Gertz, 1990).
When benzoic acid is used as a preservative in soft drinks and
fruit drinks, other additives, colouring agents, and other acids
(e.g., sorbate) may interfere with its analysis. Liquid
chromatographic methods were developed to overcome this (e.g., Bennett
& Petrus, 1977; Puttemans et al., 1984; Tyler, 1984). For the
sensitive determination of benzoic acid in fruit-derived products, a
clean-up pretreatment with solid-phase extraction followed by liquid
chromatography with UV absorbance detection is described (Mandrou et
al., 1998). The detection limit is 0.6 mg/kg, with a range of
quantification of 2-5 mg/kg. For soft drinks, a simultaneous
second-order derivative spectrophotometric determination has been
developed (detection limit 1 mg/litre) (Castro et al., 1992). Sodium
benzoate was measured in soya sauce, fruit juice, and soft drinks
using HPLC with a UV spectrophotometric detector. Before injection,
all samples were filtered (Villanueva et al., 1994).
GC determination of low concentrations (down to 10 ng/ml) of
benzoic acid in plasma and urine was preceded by diethyl ether
extraction and derivatization with pentafluorobenzyl bromide (Sioufi &
Pommier, 1980). Detection was by 63Ni electron capture. HPLC methods
have been developed for the simultaneous determination of benzoic acid
and hippuric acid -- the metabolite of sodium benzoate that is
eliminated in the urine -- that require no extraction step (detection
limit for both, 1 µg/ml; Kubota et al., 1988). Hippuric acid and
creatinine levels have been determined simultaneously by HPLC, and
measured hippuric acid levels corrected for urinary creatinine
excretion (Villanueva et al., 1994).
4. SOURCES OF HUMAN AND ENVIRONMENTAL EXPOSURE
4.1 Natural sources of benzoic acid
Benzoic acid is produced by many plants as an intermediate in the
formation of other compounds (Goodwin, 1976). High concentrations are
found in certain berries (see section 6.1). Benzoic acid has also been
detected in animals (see section 6.1). Benzoic acid therefore occurs
naturally in many foods, including milk products (Sieber et al., 1989,
1990).
4.2 Anthropogenic sources
4.2.1 Benzoic acid
Benzoic acid is produced exclusively by the liquid-phase
oxidation of toluene (Srour, 1998).
According to Srour (1998), the estimated global production
capacity of benzoic acid is 638 000 tonnes per year, although over
half of this is converted directly to phenol. The major producers of
benzoic acid are the Netherlands (220 000 tonnes per year) and Japan
(140 000 tonnes per year), followed by the USA (125 000 tonnes per
year). Another reference gives the total European capacity as less
than 153 000 tonnes (SRI, 1998).
Benzoic acid is detected in car exhaust gases, presumably as an
oxidation product of toluene (Kawamura et al., 1985), and in Japanese
cigarettes (12 and 28 µg per cigarette in mainstream and sidestream
smoke, respectively; Sakuma et al., 1983). It can also be produced
through the photochemical degradation of benzoic acid esters used as
fragrance ingredients (Shibamoto & Umano, 1985; Shibamoto, 1986).
Benzoic acid has been detected in wastewater from the wood production
industry in Norway and Sweden (Carlberg et al., 1986; Lindström &
Österberg, 1986) and in foundry waste leachates (Ham et al., 1989), as
well as in extracts of fly ash from municipal incinerators
(Tong et al., 1984).
4.2.2 Sodium benzoate
Sodium benzoate is produced by the neutralization of benzoic acid
with sodium hydroxide. Worldwide sodium benzoate production in 1997
can be estimated at about 55 000-60 000 tonnes (Srour, 1998). The
largest producers are the Netherlands, Estonia, the USA, and China.
4.3 Uses
4.3.1 Benzoic acid
In 1988, of the benzoic acid produced in Europe, about 60% was
further processed to phenol and 30% to caprolactam (for nylon fibres).
Five per cent was used for the production of sodium and other
benzoates, 3% for benzoyl chloride, and the rest for alkyd resins,
benzoate esters, such as methyl benzoate, and various other products
(Srour, 1989). These percentages are still approximately correct today
(Srour, 1998). Caprolactam seems to be produced only by European
companies (Srour, 1998).
Benzoic acid is increasingly used in the production of diethylene
and dipropylene glycol dibenzoate plasticizers in adhesive
formulations (about 40 000 tonnes in 1997). It is also used to improve
the properties of alkyd resins for paints and coatings and as a "down
hole" drilling mud additive in secondary oil production. Its use as a
rubber polymerization retarder is diminishing (Srour, 1998).
Benzoic acid and sodium benzoate (see section 4.3.2) are used as
preservatives in beverages, fruit products, chemically leavened baked
goods, and condiments, preferably in a pH range below 4.5.
A disadvantage is the off-flavour they may impart to foods (Chipley,
1983). Owing to their inhibitory effect on yeast, they cannot be used
in yeast-leavened products (Friedman & Greenwald, 1994). Examples of
upper concentrations allowed in food are up to 0.1% benzoic acid (USA)
and between 0.15% and 0.25% (other countries) (Chipley, 1983). The
European Commission limits for benzoic acid and sodium benzoate are
0.015-0.5% (EC, 1995).
Benzoic acid and its salts and esters are found in 11 of 48 (23%)
toothpastes (Sainio & Kanerva, 1995) to a maximum of 0.5% (Ishida,
1996) and in mouthwashes and dentifrices. Benzoic acid is also used in
cosmetics (in creams and lotions with pH values under 4, up to 0.5%)
(Wallhäusser, 1984). Sixteen out of 71 deodorants tested contained
benzoic acid (Rastogi et al., 1998).
Benzoic acid is a breakdown product of benzoyl peroxide, which is
used as an additive at levels of between 0.015% and 0.075% to bleach
flour (Friedman & Greenwald, 1994) and in dermatological antifungal
preparations (BMA, 1998). Benzoic acid is reported to leach from
denture-base acrylic resins, where benzoyl peroxide is added as a
polymerization initiator (Koda et al., 1989, 1990).
Benzoic acid can be used in combination with salicylic acid
(Whitfield's ointment) as a fungicidal treatment for ringworm (BMA,
1998).
4.3.2 Sodium benzoate
Although undissociated benzoic acid is the more effective
antimicrobial agent for preservation purposes, sodium benzoate is used
preferably, as it is about 200 times more soluble than benzoic acid.
About 0.1% is usually sufficient to preserve a product that has been
properly prepared and adjusted to pH 4.5 or below (Chipley, 1983).
A major market for sodium benzoate is as a preservative in the
soft drink industry, as a result of the demand for high-fructose corn
syrup in carbonated beverages. Sodium benzoate is also widely used as
a preservative in pickles, sauces, and fruit juices (Srour, 1998).
Benzoic acid and sodium benzoate are used as antimicrobial agents in
edible coatings (Baldwin et al., 1995).
Sodium benzoate is also used in pharmaceuticals for preservation
purposes (up to 1.0% in liquid medicines) and for therapeutic regimens
in the treatment of patients with urea cycle enzymopathies
(see section 9).
Possibly the largest use of sodium benzoate, accounting for
30-35% of the total demand (about 15 000 tonnes of benzoic acid), is
as an anticorrosive, particularly as an additive to automotive engine
antifreeze coolants and in other waterborne systems (Scholz &
Kortmann, 1991; Srour, 1998). A new use is the formulation of sodium
benzoate into plastics such as polypropylene, to improve strength and
clarity (BFGoodrich Kalama Inc., 1999). Sodium benzoate is used as a
stabilizer in photographic baths/processing (BUA, 1995).
4.4 Estimated global release
From data provided by the German producers, emissions of benzoic
acid from industrial processes were less than 525 kg per year into the
atmosphere, less than 3 tonnes per year into the River Rhine, and
8 tonnes per year into sewage or water purification plants (BUA,
1995). No data were available from other countries.
Other anthropogenic releases of benzoic acid and sodium benzoate
into the environment are emissions into water and soil from their uses
as preservatives in food, toothpastes, mouthwashes, dentifrices, and
cosmetics. There were no data available on the emission of benzoic
acid from the disposal of antifreeze mixtures and waterborne cooling
systems and other miscellaneous industrial uses.
The amount of benzoic acid emitted to air from car exhaust gases
as an oxidation product is not quantifiable from the available data.
5. ENVIRONMENTAL TRANSPORT, DISTRIBUTION, TRANSFORMATION,
AND ACCUMULATION
5.1 Transport and distribution between media
5.1.1 Benzoic acid
From its use pattern (see section 4), it can be expected that
benzoic acid is released to surface waters and (from dumping sites) to
leaching water (and groundwater). Minor amounts are expected to be
emitted to the atmosphere. From its physicochemical properties (vapour
pressure, Henry's law constant; see section 2), a significant
volatilization of benzoic acid from water or soil is not expected.
Owing to its solubility in water (see section 2), wet deposition from
air may occur. Experimental data on wet and dry deposition from air
are not available.
5.1.2 Sodium benzoate
No information on the environmental transport and distribution of
sodium benzoate could be identified. Owing to its use pattern, which
is similar to that of benzoic acid, most of the amounts released to
the environment are also expected to be emitted to aquatic
compartments (e.g., surface waters).
5.2 Transformation
5.2.1 Benzoic acid
The experimental determination of the photodegradation of benzoic
acid in aqueous solution (25°C; lambda = 240-300 nm) in terms of
quantum yield (average number of photons absorbed) resulted in very
low values -- in the order of 6 × 10-2 mol/einstein1 (Oussi et
al., 1998). However, benzoic acid adsorbed on silica gel (SiO2) and
irradiated with UV light (lambda > 290 nm) for 17 h showed 10.2%
photodegradation (Freitag et al., 1985). This may be due to a
photocatalytic effect, which was also observed with other oxides,
notably zinc oxide (ZnO) and titanium dioxide (TiO2). When benzoic
acid was irradiated with sunlight in aqueous suspensions of zinc or
titanium dioxide, 67% (after 2-3 h) or 90% (after 24 h) of the applied
amount was mineralized (Kinney & Ivanuski, 1969; Matthews, 1990).
1 An einstein is a unit of light energy used in photochemistry,
equal to Avogadro's number times the energy of one photon of light
of the frequency in question.
Indirect photolysis by reaction with hydroxyl radicals is
expected to be low. Hydroxyl radical rate constants (kOH) for
benzoic acid and its anion have been estimated to be approximately
0.5 × 10-12 and 2 × 10-12 cm3/s, respectively (Palm et al., 1998).
Standardized tests on ready (MITI, 1992) or inherent (Zahn &
Wellens, 1980) biodegradation showed benzoic acid to be readily
biodegraded. The degrees of aerobic degradation were as follows:
MITI I 85% (100 mg/litre; (MITI, 1992)
test 2 weeks; OECD
No. 301C)
Zahn-Wellens >90% (508 mg/litre; (Zahn & Wellens,
test 2 days) 1980)
Easy degradation of benzoic acid to methane and carbon dioxide
was also observed in different non-standardized experiments using
sewage sludge as inoculum (BUA, 1995). Benzoic acid was found to be
degraded by adapted anaerobic sewage sludge at 86-93% after 14 days
(Nottingham & Hungate, 1969), by aerobic activated sludge (adapted) at
>95% after 5-20 days (Pitter, 1976; Lund & Rodriguez, 1984), and by
unadapted aerobic activated sludge at 61-69% after 2-3 days with a
preceding lag time of 2-20 h (Urano & Kato, 1986). The use of a
synthetic sewage inoculated with laboratory bacterial cultures led to
complete degradation of benzoic acid after 14 days under anaerobic
conditions (Kameya et al., 1995).
A greater variability in degradation (0-100%) was seen in tests
using environmental matrices (e.g., rain, lake water, seawater, soil,
etc.). It depended mainly on substance concentration and time for
acclimation (see Table 1). Test durations exceeding 2 days resulted in
removal of >40% when initial concentrations were below 20 mg/litre.
A rapid mineralization occurred in groundwater and subsurface soil
samples. In groundwater, a half-life of 41 h has been found for
benzoic acid (initial concentration 1-100 µg/litre; metabolized to
14CO2) under aerobic conditions (Ventullo & Larson, 1985).
Half-lives of 7.3 h and 18.2 h, respectively, have been observed for
aerobic and anaerobic degradation of benzoic acid (initial
concentration 1 mg/kg dry weight; metabolized to 14CO2) in
subsurface soils of septic tank tile fields (Ward, 1985). Anaerobic
degradation of benzoic acid (initial concentration 250 mg
carbon/litre) in a methanogenic microcosm (consisting of aquifer
solids and groundwater) required 4 weeks of adaptation, followed by
nearly complete depletion after 8 weeks of incubation (Suflita &
Concannon, 1995).
Several isolated microorganisms have been shown to utilize (and
therefore probably degrade) benzoic acid under aerobic or anaerobic
conditions. They include, among others, fungal species such as
Rhodotorula glutinis and other yeast-like fungi (Kocwa-Haluch &
Lemek, 1995), the mould Penicillium frequentans (Hofrichter &
Fritsche, 1996), and bacteria, such as Alcaligenes denitrificans
(Miguez et al., 1995), Rhodopseudomonas palustris, several strains
of denitrifying pseudomonads (Fuchs et al., 1993; Elder & Kelly, 1994;
Harwood & Gibson, 1997), and Desulfomicrobium escambiense
(Sharak Genthner et al., 1997).
Although benzoic acid is primarily metabolized to hippuric acid
in rats (see section 7), some other species do excrete other
metabolites, such as dibenzoylornithine (hen), benzoylglutamic acid
(Indian fruit bat), benzoylarginine (tick, insects), or benzoyltaurine
(southern flounder, Paralichthys lethostigma) (Parke, 1968; Goodwin,
1976; James & Pritchard, 1987).
5.2.2 Sodium benzoate
Experimental data on photodegradation of sodium benzoate are not
available. As with benzoic acid, photolysis in aqueous solution is
assumed to be unlikely with regard to its known UV spectra
(Palm et al., 1998). Indirect photolysis by reaction with hydroxyl
radicals plays only a minor role, with estimated and measured hydroxyl
rate constants of about 0.33 × 10-11 cm3/s
(Palm et al., 1998).
Sodium benzoate was readily biodegradable under aerobic
conditions in several standard test systems:
Modified 84% (100 mg/litre; (King & Painter,
MITI test 10 days) 1983)
Modified 80-90% (50 mg/litre; (Salanitro et al.,
Sturm test 7 days) 1988)
Closed bottle 75-111% (5 mg/litre; (Richterich &
test 30 days) Steber, 1989)
Degradation assays using seawater as test medium ("natural
water") or as inoculum (marine filter material given into a synthetic
marine medium) according to an adapted Organisation for Economic
Co-operation and Development (OECD) guideline (301B) resulted in a
degradation of 85% and 97%, respectively (10 mg/litre; carbon dioxide
measurement; 28 days) (Courtes et al., 1995).
Anaerobic mineralization of sodium benzoate (50-90 mg/litre) by
domestic sewage sludge varied from 50% to 96.5% (measurement of carbon
dioxide and methane; 28-61 days) (Birch et al., 1989). In another
study using anaerobic sludge from sewage works receiving a mixture of
domestic and industrial wastewaters, 93% mineralization was observed
after 1 week of incubation (measurement of carbon dioxide and methane;
initial concentration 50 mg carbon/litre) (Battersby & Wilson, 1989).
Table 1: Removal of benzoic acid in freshwater, marine, and soil matrices.
Matrix Initial Conditions Duration Removal Measured Reference
concentration (days) (%) parameter
(mg/litre
or mg/kg)
Rainwater 0.001 22°C; shaking 2 0 benzoic acid Kawamura &
once per day; dark 7 40 Kaplan (1990)
45 100
Lake water 0.059 29°C; 7 98.7 14C (in CO2, Rubin et al.
(eutrophic/ no shaking; dark biomass) (1982)
mesotrophic)
Seawater 20°C; dark; 14C (in Shimp &
(estuary) rotary shaking CO2, biomass) Young (1987)
USA 20 30 <10
0.005 8 70-80
Canada 20 16 60
0.005 10 >70
Seawater 2 5 75 BODa Takemoto
et al. (1981)
Soil 20 2 mg benzoic acid 70 63 14CO2 Haider et al.
(grey soil, in 0.1 ml acetone (1974)
alkaline) + 100 g soil
+ 10 ml H2O
Soil 0.05 24°C; 20-25% 15 40 14CO2 Federle (1988)
(sand; moisture content
18.9 m depth)
a BOD = biological oxygen demand.
Benzoate-acclimated sludges were reported to be capable of completely
degrading benzoate concentrations of 3000 mg/litre within 5-7 days
(Kobayashi et al., 1989).
5.3 Accumulation
5.3.1 Benzoic acid
The n-octanol/water partition coefficient (log Kow) of 1.9
(see section 2) indicates a low potential for bioaccumulation.
Consistently, measured bioconcentration factors (BCFs) found in
aquatic biota were low. BCFs of <10 (based on wet weight) have been
determined for fish (golden ide, Leuciscus idus melanotus) and green
algae (Chlorella fusca) after 3 and 1 days, respectively
(Freitag et al., 1985). A 6-day BCF of 7.6 has been reported for
another green alga (Selenastrum capricornutum) (Mailhot, 1987),
and a 5-day BCF of 1300 (based on dry weight) in activated sludge
(Freitag et al., 1985). The following 24-h bioaccumulation factors
(focusing on uptake via medium plus feed within food chain members)
have been obtained in aquatic model ecosystems operated with
0.01-0.1 mg of radiolabelled benzoic acid per litre: 21 (mosquitofish,
Gambusia affinis), 102 (green alga, Oedogonium cardiacum),
138 (mosquito larvae, Culex quinquifasciatus), 1772 (water flea,
Daphnia magna), and 2786 (snail, Physa sp.). Except for
Daphnia and snail, the values were low (Lu & Metcalf, 1975).
However, the very low exposure concentrations could likely have
resulted in the calculation of the high BCF values, even with moderate
uptake. Moreover, because this was a radiolabel study, it remains
unclear if the label was still the parent compound.
Geoaccumulation of benzoic acid has also been found to be low.
Depending on soil depth, sorption coefficients (Kd) of 0.62 (18.9
m) to 1.92 (0.4 m) have been measured (Federle, 1988). Mobility
determinations of 14C-labelled benzoic acid in different soils by
means of thin-layer chromatography showed benzoic acid to be
moderately mobile. Its mobility was positively correlated with soil pH
and negatively correlated with aluminium and iron contents and
effective anion exchange capacity (Stolpe et al., 1993).
5.3.2 Sodium benzoate
No experimental data on bioaccumulation or geoaccumulation of
sodium benzoate have been identified. From the information on benzoic
acid, a significant potential for accumulation is not to be expected.
6. ENVIRONMENTAL LEVELS AND HUMAN EXPOSURE
6.1 Environmental levels
Generally, benzoic acid can occur in almost all environmental
compartments. Whether it exists in the undissociated or dissociated
form depends on the specific physicochemical conditions. Above pH 6,
the benzoate anion prevails (Chipley, 1983).
There is a series of reports on positive qualitative analyses of
benzoic acid in various environmental media, such as air (Belgium:
Cautreels & van Cauwenberghe, 1978; Germany: Helmig et al., 1989),
rain or snow (Norway: Lunde et al., 1977; Germany: Winkeler et al.,
1988), surface waters (Norway, river: Schou & Krane, 1981), and soils
(United Kingdom, heathland soil: Jalal & Read, 1983; Germany, river
terrace soil: Cordt & Kußmaul, 1990), but these do not provide
quantitative measurements.
Semiquantitative measurements of concentrations of benzoic acid
in urban air in Pasadena, California (USA) were in the range of
0.09-0.38 µg/m3 (Schuetzle et al., 1975). This was comparable to
quantitative measurements performed in 1984 in Los Angeles, California
(USA), which resulted in atmospheric concentrations of 0.005-0.13
µg/m3 (n = 8) (Kawamura et al., 1985). Most of the quantitative
data compiled in Table 2 with respect to water samples refer to
concentrations of benzoic acid in groundwater, with a maximum of 27.5
mg/litre measured in the vicinity of a point source.
Benzoic acid occurs naturally in free and bound form in many
plant and animal species. It is a common metabolite in plants and
organisms (Hegnauer, 1992). Appreciable amounts have been found in gum
benzoin (around 20%) and most berries (around 0.05%) (Budavari et al.,
1996). For example, ripe fruits of several Vaccinium species (e.g.,
cranberry, V. vitis idaea; bilberry, V. macrocarpon) contain as
much as 300-1300 mg free benzoic acid per kg fruit (Hegnauer, 1966).
Benzoic acid is also formed in apples after infection with the fungus
Nectria galligena (Harborne, 1983) or in Pinus thunbergii callus
inoculated with a pathogenic pine wood nematode (Bursaphelenchus
xylophilus) (Zhang et al., 1997). Among animals, benzoic acid has
been identified primarily in omnivorous or phytophageous species,
e.g., in viscera and muscles of the ptarmigan (Lagopus mutus)
(Hegnauer, 1989) as well as in gland secretions of male muskoxen
(Ovibos moschatus) (Flood et al., 1989) or Asian bull elephants
(Elephas maximus) (Rasmussen et al., 1990).
Owing to its occurrence in many organisms, benzoic acid is
naturally present in foods (review in Sieber et al., 1989, 1990). Some
typical examples specifying reported ranges of means in selected foods
have been compiled from Sieber et al. (1989) as follows:
Milk traces - 6 mg/kg
Yoghurt 12-40 mg/kg
Cheese traces - 40 mg/kg
Fruits (excluding traces - 14 mg/kg
Vaccinium species)
Potatoes, beans, cereals traces - 0.2 mg/kg
Soya flour, nuts 1.2-11 mg/kg
Honeys from different floral sources (n = 7) were found to
contain free benzoic acid at concentrations of <10 mg/kg (n = 5)
and of <100 mg/kg (n = 2) (Steeg & Montag, 1987).
Because benzoic acid and its compounds are used as food
preservatives (see section 4), some processed foods contain
artificially elevated concentrations of these substances (see section
6.2).
6.2 Human exposure
The main route of exposure of the general population to benzoic
acid or sodium benzoate is likely via foodstuffs that contain the
substances naturally or added as antimicrobial agents. There are a few
analyses of processed foodstuffs available. They refer to different
types of food items (juice, soft drinks, soya sauce varieties) from
the Philippines (a total of 44 samples) and from Japan (a total of 31
samples) and to orange drinks sampled in England. The concentrations
of sodium benzoate in the Philippine dietary samples ranged from 20 to
>2000 mg/litre. The range in the Japanese products was 50-200
mg/litre, thus reflecting the lower maximum level of sodium benzoate
allowed to be added to food in Japan as compared with the Philippines
(Villanueva et al., 1994). Orange drinks from England contained sodium
benzoate at concentrations ranging from 54 to 100 mg/litre (mean 76.7
mg/litre; n = 6) (Freedman, 1977).
Generally, the actual uptake depends on the individual's choice
of food to be consumed and the different limit values in different
countries. Several intake estimations have been published. Three
Japanese studies reported average daily intakes of benzoic acid from
processed foodstuffs to be 10.9 mg per person (Toyoda et al., 1983a)
and 1.4 mg per person (Toyoda et al., 1983b; Yomota et al., 1988),
corresponding to 0.02-0.2 mg/kg body weight (for persons with a body
weight of 50-70 kg). Both of the latter studies used the market basket
method for intake calculations, whereas the first-mentioned study
calculated intakes using the results of a national nutrition survey.
The concentrations of benzoic acid in 3319 food samples analysed for
this study (Toyoda et al., 1983a) ranged from not detected to 2100
mg/kg food. The maximum was found in salted fish (n = 7; mean 754
mg/kg). Another survey refers to the United Kingdom, where analyses of
benzoic acid in foods and drinks in which it is permitted as well as
intake estimates have been performed (UK MAFF, 1995). Sixty-five out
of 122 samples tested contained detectable benzoic acid. The highest
Table 2: Concentrations of benzoic acid in rain, snow, groundwater, and leachate samples.
Medium Location; sampling date Concentration Reference
(µg/litre)
Los Angeles area, California, Sum concentrationsa Kawamura & Kaplan
USA; 1982-1983 (1986)
Rain: urban 0.06-10.2 (n = 6)
Rain: semirural 0.02 (n = 1)
Snow: rural 0.04-0.1 (n = 3)
Groundwater Wyoming, USA (near underground 16-860 (n = 3) Stuermer et al.
coal gasification site; 15 months (1982)
after the end of gasification)
Groundwater Florida, USA (near wood treatment 10-27 500 (n = 3) Goerlitz et al.
facility); 1984 (1985)
Groundwater Ontario, Canada (near traces (n = 2) Barker et al.
landfillb); 1983 (1988)
Groundwater Barcelona area, Spain up to 0.21 (n = 3) Guardiola et al.
(near landfillb) (1989)
Leachate Ontario, Canada; 1981 <0.1->1000 (n = 5) Reinhard & Goodman
(from landfillb) (1984)
Leachate Ontario, Canada; 1983 traces (n = 2) Barker et al.
(from landfillb) (1988)
Leachate USA; 1986-1988 200-400c (n = 3) Ham et al.
(from foundry (1989)
wastes)
a Including benzoic acid, 3-methyl benzoic acid, and 4-methyl benzoic acid.
b Receiving rural, municipal (domestic), and industrial wastes.
c Concentrations estimated from gas chromatography/mass spectrometry data.
concentrations were found in sauces (mean 388 mg/kg; n = 20; range
71-948 mg/kg), reduced sugar jam (mean 216 mg/kg; n = 4; range
<20-333 mg/kg), non-alcoholic drinks (mean 162 mg/kg; n = 20; range
55-251 mg/kg), and semipreserved fish product (653 mg/kg; n = 1).
The survey found that the concentrations of benzoic acid detected
would lead to a dietary intake below 5 mg/kg body weight per day, even
for adults with an above-average consumption.
A frequent contributor to dietary exposure is soft drinks. A
rough estimation based on the average daily consumption in Germany of
such drinks (372 ml non-alcoholic beverages, excluding bottled water;
BAGS, 1995) by 19- to 24-year-old men, assuming the concentration of
benzoic acid present corresponds to a maximum allowable level of
150 mg/litre (EC, 1995), would result in a mean daily intake of
55.8 mg benzoic acid per person (or 0.80 mg/kg body weight, assuming
a 70-kg body weight). For comparison, a similar calculation with
sugar-free marmalade, jam, and similar spreads, which are allowed to
contain higher levels of benzoic acid (500 mg/kg; EC, 1995), would
result in a possible intake of 4.1 mg per person per day, or
0.06 mg/kg body weight per day (assumes a daily consumption of 8.2 g,
according to BAGS, 1995). This was more than a possible intake via
fruits containing natural benzoic acid. For example, a daily
consumption of 40.4 g of fruits (BAGS, 1995) would lead to a possible
intake of 0.57 mg benzoic acid per person per day (or 0.008 mg/kg body
weight for a 70-kg person), if the reported maximum of 14 mg benzoic
acid/kg (see section 6.1) were present.
The Joint FAO/WHO Expert Committee on Food Additives (JECFA)
assessed the intake of benzoates from information provided by nine
countries (Australia, China, Finland, France, Japan, New Zealand,
Spain, United Kingdom, and USA) (WHO, 1999). Because diets differ
among countries, the foods that contribute to benzoate intake would be
expected to vary. The food category that contributed most to benzoate
intake was soft drinks (carbonated, water-based, flavoured drinks) for
Australia/New Zealand, France, the United Kingdom, and the USA. In
Finland, 40% was in soft drinks. Soya sauce was the main source of
benzoate in China and the second most important in Japan. The best
estimates of national mean intakes of benzoates by consumers ranged
from 0.18 mg/kg body weight per day in Japan to 2.3 mg/kg body weight
per day in the USA. These estimates were based on analyses involving
either model diets or individual dietary records and maximum limits
specified by national governments or the European Union. The estimated
intake by high consumers of benzoates, based on food additive levels
in national standards, was 7.3 mg/kg body weight per day in the USA
and 14 mg/kg body weight per day in China.
Benzoates have been detected in groundwater, but not in
drinking-water.
Quantitative information on (oral or dermal/mucosal) exposure via
cosmetic, hygienic, or medical products is rare, but the data
available indicate a remarkable contribution to exposure. There are
reports on leaching of benzoic acid from denture-base acrylic resins.
After 10 days of immersion in artificial saliva, concentrations of up
to about 3 mg/litre have been observed for benzoic acid, which is
formed as a degradation product of the benzoyl peroxide that is added
as a polymerization initiator (Koda et al., 1989, 1990). In Japan,
commercial toothpastes have been found to contain benzoic acid at
concentrations ranging from 800 to 4450 mg/kg (n = 18). Use of the
toothpaste with the highest concentration (by 40 20-year-old female
students) would result in a calculated daily intake of about 2.23 mg
per person. This was about the same amount as their estimated intake
from diet (Ishida, 1996). Benzoic acid is also used in dermatology as
a fungicidal topical treatment for ringworm (Tinea spp.). The
emulsifying ointment preparation contains benzoic acid at 6% and is
applied twice daily (Goodman et al., 1990; BMA, 1998).
Recent quantitative monitoring data on concentrations of benzoic
acid or salts in ambient or indoor air are not available. Considering
the few (low) levels of benzoic acid measured in urban air in the
past, with a maximum of 0.38 µg/m3 (see section 6.1), inhalation may
contribute only marginally to exposure of the general population.
Using this maximum, a daily inhalative dose of 8.74 µg per person (or
0.12 µg/kg body weight) is obtained (assuming a daily inhalation
volume of 23 m3 for a 70-kg adult male; WHO, 1994).
Few quantitative data on occupational exposure have been
identified. Nevertheless, there is a potential for inhalation or
dermal contact in the chemical and allied product industries as well
as in workplaces where these products are used. Air samples (n = 50)
collected in an industrial environment (no further details given) over
a year's time showed benzoic acid concentrations ranging from not
detected to 1.5 mg/m3 (Halvorson, 1984). On the basis of the latter
value, an inhalative dose of 14.4 mg per person per 8-h working time
(or 0.2 mg/kg body weight) would result (assuming an inhalation volume
of 9.6 m3 for an 8-h exposure with light activity; WHO, 1994).
However, because of the lack of information on specific working
operations and conditions involved (e.g., duration of exposure, use of
protective clothes, etc.), it is impossible to derive a realistic
estimate of occupational exposure.
7. COMPARATIVE KINETICS AND METABOLISM IN LABORATORY ANIMALS
AND HUMANS
After oral ingestion of benzoic acid and sodium benzoate, there
is a rapid absorption (of undissociated benzoic acid) from the
gastrointestinal tract in experimental animals or humans (US FDA,
1972a, 1973). From the figures on excretion given below, 100%
absorption can be assumed. In humans, the peak plasma concentration is
reached within 1-2 h (Kubota et al., 1988; Kubota & Ishizaki, 1991).
Benzoic acid is not completely absorbed by the dermal route. In a
study with six human subjects, Feldmann & Maibach (1970) found an
uptake of 36% of the applied dose (14C-labelled benzoic acid
dissolved in acetone; 4 µg/cm2; circular area of 13 cm2; ventral
surface of the forearm; non-occlusive) within 12 h. The total uptake
within 5 days was 43%. In a second study with 6-7 subjects (comparable
method; application of 3, 400 or 2000 µg/cm2), the percent
absorption decreased from 35% to 14% within 24 h. However, the total
uptake per cm2 increased from 1 to 288 µg (Wester & Maibach, 1976).
For sodium benzoate, no data concerning dermal uptake were identified
in the literature.
In vivo dermal studies with benzoic acid in experimental
animals (e.g., guinea-pigs, mice, rats, pigs, dogs, rhesus monkeys)
confirm the results with humans (Hunziker et al., 1978;
Andersen et al., 1980; Wester & Noonan, 1980; Bronaugh et al., 1982a;
Reifenrath et al., 1984; Carver & Riviere, 1989; Maibach & Wester,
1989; Bucks et al., 1990). Absorption ranged from 25% in pigs
(Reifenrath et al., 1984; Carver & Riviere, 1989) to 89% in rhesus
monkeys (Wester & Noonan, 1980; Maibach & Wester, 1989; Bucks et al.,
1990). Due to the good database on humans and animals in vivo,
in vitro studies performed with animal or human skin are not
considered further (Franz, 1975; Bronaugh et al., 1982b;
Hotchkiss et al., 1992; MacPherson et al., 1996).
No information is available on absorption via inhalation.
After oral and dermal uptake, benzoate is metabolized in the
liver by conjugation with glycine, resulting in the formation of
hippuric acid (Feldmann & Maibach, 1970; US FDA, 1972a; WHO, 1996;
Feillet & Leonard, 1998). The rate of biotransformation in humans is
high: after oral doses of 40, 80 or 160 mg sodium benzoate/kg body
weight, the transformation to hippuric acid was independent of the
dose -- about 17-29 mg/kg body weight per hour, corresponding to about
500 mg/kg body weight per day (Kubota & Ishizaki, 1991). Other authors
obtained higher values of 0.8-2 g/kg body weight per day (US FDA,
1972a, 1973; WHO, 1996). Hippuric acid is rapidly excreted in urine.
In humans, after oral doses of up to 160 mg/kg body weight, 75-100% of
the applied dose is excreted as hippuric acid within 6 h after
administration, and the rest within 2-3 days (Kubota et al., 1988;
Fujii et al., 1991; Kubota & Ishizaki, 1991).
The limiting factor in the biosynthesis of hippuric acid is the
availability of glycine. The utilization of glycine in the
detoxification of benzoate results in a reduction in the glycine level
of the body. Therefore, the ingestion of benzoic acid or its salts
affects any body function or metabolic process in which glycine is
involved; for example, it leads to a reduction in creatinine,
glutamine, urea, and uric acid levels (US FDA, 1972a, 1973; Kubota &
Ishizaki, 1991; WHO, 1996).
Another metabolite of benzoate is the benzoyl glucuronide. For
example, the dog excretes considerable amounts of this metabolite in
the urine (20% after a single dose of 50 mg/kg body weight; Bridges et
al., 1970). In other species, this metabolite appears only after
higher doses of about 500 mg/kg body weight (see above) of benzoic
acid or sodium benzoate, resulting in a depletion of the glycine pool
(Bridges et al., 1970; US FDA, 1972a; Kubota et al., 1988). In cats,
glucuronidation is generally very low (Williams, 1967).
In some species, including humans, minor amounts of benzoic acid
itself are also excreted in the urine (Bridges et al., 1970; Kubota &
Ishizaki, 1991).
Experiments on the distribution and elimination of 14C-benzoate
in the rat have shown no accumulation of sodium benzoate or benzoic
acid in the body (US FDA, 1972a, 1973).
In the acid conditions of the stomach, the equilibrium moves to
the undissociated benzoic acid molecule, which should be absorbed
rapidly. Benzoate from sodium benzoate would change from the ionized
form to the undissociated benzoic acid molecule. As a result, the
metabolism and systemic effects of benzoic acid and sodium benzoate
can be evaluated together.
7.1 Precursors of benzoic acid
Benzyl acetate, its hydrolysis product, benzyl alcohol, and the
oxidation product of this alcohol, benzaldehyde, are precursors of
benzoic acid in experimental animals and humans. Benzyl acetate is
metabolized to benzoic acid and further to hippuric acid and benzoyl
glucuronide to an extent of >90% both in mice and in rats of
different strains. Benzyl alcohol was metabolized to benzoic acid and
its conjugates in preterm infants. Benzaldehyde is metabolized to
benzoic acid and its conjugates in rabbits to an extent of
approximately 90% (WHO, 1996).
8. EFFECTS ON LABORATORY MAMMALS AND IN VITRO TEST SYSTEMS
8.1 Single exposure
With oral LD50 values (administration by gavage) of 3040 mg
benzoic acid/kg body weight in rats (Bio-Fax, 1973) and 1940-2263 mg
benzoic acid/kg body weight in mice (McCormick, 1974; Abe et al.,
1984), the acute toxicity of benzoic acid is low. Clinical signs of
intoxication (reported for rats only) included diarrhoea, muscular
weakness, tremors, hypoactivity, and emaciation (Bio-Fax, 1973). With
oral LD50 values of 2100-4070 mg sodium benzoate/kg body weight in
rats, the acute toxicity of sodium benzoate is similar to that of
benzoic acid, as are the symptoms (Smyth & Carpenter, 1948;
Deuel et al., 1954; Bayer AG, 1977).
In four cats given diets containing 0 or 1% benzoic acid
(approximately 0 or 450-890 mg/kg body weight), aggression,
hyperaesthesia, and collapse starting 14-16 h after feed uptake were
seen at a dose level equal to 630 mg/kg body weight. The duration of
the syndrome was about 18-176 h, and the mortality rate was 50%. The
histopathological examination of the two cats that died revealed
degenerative changes in liver, kidneys, and lung, but no pathological
findings in brain or spinal cord (Bedford & Clarke, 1972). The authors
attributed the higher toxicity of benzoic acid in cats compared with
other species to the low capacity of cats for glucuronidation
(see section 7).
In rats, exposure by inhalation to 26 mg/m3 over 1 h caused no
mortality, but generalized inactivity and lacrimation were noted. The
gross autopsy gave no significant findings (no further information
available; Bio-Fax, 1973).
In a limit test with rabbits, no mortality or signs of
intoxication were seen after dermal application of 10 000 mg/kg body
weight. The gross autopsy gave no significant findings (no further
information available; Bio-Fax, 1973).
8.2 Irritation and sensitization
8.2.1 Benzoic acid
Although there is a wide range of results from mostly
non-standardized tests using various scoring systems, it can be
concluded that benzoic acid is slightly irritating to the skin and
irritating to the eyes.
In different experiments with rabbits, which have not been
performed according to current guidelines, benzoic acid applied as dry
powder or in the form of a paste was not irritating to slightly
irritating to the skin (score 1.66/8: Bio-Fax, 1973; no score given:
Bayer AG, 1978; primary skin irritation index 0.5 [no further
information available]: RCC Notox, 1988a).
In an acute eye irritation/corrosion study with rabbits conducted
according to OECD Guideline 405, some eye irritation was reported
after application of benzoic acid in the form of a paste. Within 72 h,
the scores for chemosis, reddening of the conjunctivae, iritis, and
keratitis always remained at <2 (Bayer AG, 1986).
In different non-standardized experiments with the solid
substance, moderately irritating to severely irritating effects on the
eye were noted (score 65/110: Bio-Fax, 1973; no score given: Bayer AG,
1978; score up to 108/110 [eyes rinsed after instillation] or up to
50/100 [eyes not rinsed]: Monsanto Co., 1983; score 35 according to
the scheme of Kay & Calandra, 1962: RCC Notox, 1988b).
In a maximization test, none of 15 guinea-pigs reacted positively
after induction and challenge with a 10-20% solution of benzoic acid
in water (Gad et al., 1986). In addition, the substance also tested
negative in a Buehler test with guinea-pigs and in an ear swelling
test and local lymph node assay with mice (Gad et al., 1986; Gerberick
et al., 1992). The concentrations used for induction and challenge
were 10-20% in acetone or water.
However, a dose-dependent positive result was obtained in an ear
swelling test with five guinea-pigs (induction with 0.2, 1, 5, or 20%
in absolute ethyl alcohol; no challenge) used as a model for detecting
agents causing non-immunological contact urticaria in humans. At
several other regions (back, abdomen, flank site), a concentration of
20% failed to produce any reactions (Lahti & Maibach, 1984).
8.2.2 Sodium benzoate
An acute dermal irritation/corrosion study with rabbits conducted
according to OECD Guideline 404 (no data about physical state; score
0: RCC Notox, n.d., a) as well as a non-standardized experiment with
the solid substance (score not given: Bayer AG, 1977) gave no
indication for skin irritating effects.
In a study performed according to OECD Guideline 405 (no data
about physical state; RCC Notox, n.d., b), sodium benzoate was only
slightly irritating to the eye (score 9.3, according to the scheme of
Kay & Calandra, 1962). The application of the solid substance in a
non-standardized experiment caused no irritation (score not given:
Bayer AG, 1977).
For sodium benzoate, no data on sensitizing effects were
identified in the available literature.
8.3 Short-term exposure
8.3.1 Oral exposure
In general, the database for benzoic acid and sodium benzoate is
limited, and there are no studies available performed according to
current guidelines. In addition, the documentation of these studies in
most cases is insufficient. Detailed information is given in Table 3.
From the available studies, it can be assumed that the toxicity
of benzoic acid after short-term oral exposure is low. In high-dosed
rats given approximately 2250 mg/kg body weight per day via diet over
5 days, excitation, ataxia, convulsions, and histopathological changes
in the brain were seen. The mortality was about 50%; in some cases,
bleeding into the gut was noted (Kreis et al., 1967). In two other
studies with rats dosed with approximately 825 mg/kg body weight per
day over 7-35 days (Kreis et al., 1967) or with 65-647 mg/kg body
weight per day over 28 days (Bio-Fax, 1973), no clear
treatment-related effects occurred. The reduced weight gain at 2250
and 825 mg/kg body weight per day may be attributed to reduced food
intake in the study by Kreis et al. (1967). The relevance of the
reduced relative kidney weight at 324 mg/kg body weight per day, which
was not dose-related and not accompanied by changes in
histopathological examinations, is unclear (Bio-Fax, 1973). As given
in Table 3, both studies have several limitations (i.e., missing
haematological and clinical chemical investigations, incomplete
histopathological examinations); therefore, both of these studies were
inadequate for derivation of a NO(A)EL.
More information on dose-response can be gained from the study of
Fujitani (1993), in which rats received sodium benzoate for 10 days in
feed. At the lowest tested concentration of 1358 mg/kg body weight per
day, changes in serum cholesterol levels occurred in females. At doses
of 1568 mg/kg body weight per day and above, changes in further serum
parameters and an increased relative liver weight were described.
Histopathological changes of the liver, increased relative kidney
weights, and disorders of the central nervous system (convulsions)
were seen after dosing via diet with approximately 1800 mg/kg body
weight per day. In several other studies listed in Table 3, adverse
effects were seen only at higher doses after feeding sodium benzoate
over periods from 10 to 42 days, so that a
lowest-observed-(adverse-)effect level (LO(A)EL) of 1358 mg sodium
benzoate/kg body weight per day for short-term exposure can be
derived.
With cats (Bedford & Clarke, 1972), also described in Table 3,
the effect levels with benzoic acid were lower. However, due to the
differences in the metabolism of benzoic acid in cats compared with
other experimental animals and humans, this study was not taken into
further consideration (see section 7).
Table 3: Toxicity of benzoic acid and sodium benzoate after short-term oral exposure.
Species; strain; Treatment Duration Organs examined in Resultsa Reference
number of animals (days) histopathology, clinical
per dosea chemistry, haematology
Benzoic acid
cat; 4 m 0 or 0.5% 3-4 liver, kidney, heart, mild hyperaesthesia, Bedford & Clarke
in diet stomach, lung, brain, apprehension, and (1972)
(approx. 0 spinal cord (only depression starting 48-92
or 300-420 mg/kg animals that died were h after uptake; duration of
body weight) examined); blood samples the syndrome: about 20-48 h;
were taken from surviving mortality rate: 50%;
cats degenerative changes in
liver, kidneys, and lung,
but no pathological findings
in brain or spinal cord;
surviving cats: urea and
serum alanine
aminotransferase (S-ALAT) *,
indicating liver and kidney
damage
cat; 4 m a) 100 or 200 a) 15 only blood samples no adverse effects Bedford & Clarke
mg/kg body weight were taken were reported (1972)
via diet
b) 0 or 0.25% in b) 23
diet (approx. 0
or 130-160 mg/kg
body weight)
Table 3 (cont'd)
Species; strain; Treatment Duration Organs examined in Resultsa Reference
number of animals (days) histopathology, clinical
per dosea chemistry, haematology
rat; Wistar; 0 or 3% in diet 1-5 heart, liver, spleen, body weight gain **; Kreis et al.
5-15 m (approx. 0 or kidney, brain in rats dosed over 5 days, (1967)
2250 mg/kg body disorders of the central
weight) nervous system (excitation,
ataxia, tonoclonic
convulsions); mortality
rate approx. 50%; in some
cases, bleeding into the
gut; brain damage (necrosis
of parenchymal cells of the
stratum granulosum of the
fascia dentata and the
cortex of the lobus
piriformis) in most animals
dosed over 3-5 days (still
present after 35 days)
rat; Wistar; 0 or 1.1% in 7-35 heart, liver, spleen, body weight gain **; Kreis et al.
5-10 m diet kidney, brain no clinical signs of (1967)
(approx. 0 or intoxication
825 mg/kg body
weight)
rat; albino; 10 m 0, 760, 3800, or 28 liver, kidney, no deaths or signs of Bio-Fax (1973)
7600 ppm via diet adrenals, testes intoxication
(approx. 0, 65, 324 mg/kg body
324, or 647 mg/kg weight: relative kidney
body weight) weights **; no further
information available
Table 3 (cont'd)
Species; strain; Treatment Duration Organs examined in Resultsa Reference
number of animals (days) histopathology, clinical
per dosea chemistry, haematology
Sodium benzoate
rat; F344/Ducrj; 0, 1.81, 2.09, or 10 liver, kidney; >1358 mg/kg body weight: Fujitani (1993)
6 m/f 2.4% in diet standard clinical changes in serum levels
(approx. 0, 1358, chemistry (cholesterol ** (f))
1568, or 1800 >1568 mg/kg body weight:
mg/kg body relative liver weight * (m);
weight) changes in serum levels
(albumin * (m), total
protein * (m))
1800 mg/kg body weight:
1/6 males died
(hypersensitivity,
convulsions); body
weight ** (m/f); relative
liver weight * (f); relative
kidney weights * (m/f);
absolute weights of spleen
and thymus ** (m);
absolute/relative weights
of thymus ** (f); changes
in serum levels
(gamma-glutamyltranspeptidase
(GGT) * (m), albumin * (f),
cholinesterase ** (f));
eosinophilic foci around
periportal vein and
enlargement of hepatocytes
with glassy cytoplasm in
the periportal area of the
liver (m); no changes in
the kidney (m)
Table 3 (cont'd)
Species; strain; Treatment Duration Organs examined in Resultsa Reference
number of animals (days) histopathology, clinical
per dosea chemistry, haematology
rat; Sherman; 0, 2, or 5% in 28 no data available 2200 mg/kg body weight: Fanelli & Halliday (1963)
6 m/f diet (approx. 0, slight depression of
2200, or 6700 body weight gain (m)
mg/kg body 6700 mg/kg body weight:
weight) mortality 100% within
11 days; signs of
intoxication included
hyperexcitability, urinary
incontinence, and
convulsions
no further information
available
rat; 28 (no 0 or 5% in diet 28 no data available mortality about 100% within Kieckebusch & Lang
further data) (approx. 0 or 3 weeks; decreased feed (1960)
3750 mg/kg body intake, diarrhoea,
weight) intestinal haemorrhage and
crusted blood in the nose;
no further information
available
rat; 5 (no 0 or 5% in diet >28 no data available mortality 80% within Kieckebusch & Lang
further data) (approx. 0 or 4-5 weeks; decreased (1960)
3750 mg/kg body weight; no further
body weight) information available
Table 3 (cont'd)
Species; strain; Treatment Duration Organs examined in Resultsa Reference
number of animals (days) histopathology, clinical
per dosea chemistry, haematology
rat; F344; 0, 0.5, 1, 2, 4, 42 histopathology performed, >375 mg/kg body weight: Sodemoto & Enomoto
10-11 m/f or 8% in diet but not further specified hypersensitivity after (1980)
(approx. 0, 375, dosing
750, 1500, 3000, >3000 mg/kg body weight:
or 6000 mg/kg mortality about 100% within
body weight) 4 weeks; apart from atrophy
of the spleen and lymph
nodes, no other
morphological changes were
noted
rat; Sherman; 0 or 16-1090 30 adrenals, upper intestine, no adverse effects were Smyth & Carpenter
5 m/f mg/kg body kidney, liver, spleen reported; no further (1948)
weight via diet information available
mouse; B6C3F1; 0, 2.08, 2.5, or 10 liver, kidney; standard „3750 mg/kg body weight: Fujitani (1993)
4-5 m/f 3% in diet clinical chemistry changes in serum levels
(approx. 0, 3000, (cholinesterase * (m))
3750, or 4500 4500 mg/kg body weight:
mg/kg body weight) hypersensitivity in all
animals; convulsions
1/5 males and 2/5 females
(both females died);
absolute/relative liver
weight * (m/f); relative
kidney weight * (f);
changes in serum levels
(cholesterol * (m),
phospholipids * (m));
Table 3 (cont'd)
Species; strain; Treatment Duration Organs examined in Resultsa Reference
number of animals (days) histopathology, clinical
per dosea chemistry, haematology
enlarged hepatocytes,
single cell necrosis
and vacuolation of
hepatocytes in all
livers (m); no changes
in the kidney (m/f)
mouse; albino 0, 0.5, 1, 2, 4, 35 survival, chemical 3000 mg/kg body weight: Toth (1984)
Swiss; 4 m/f or 8% via consumption, histological "suitable for lifelong
drinking-water changes (not further treatment" based on
(approx. specified) (prestudy four parameters: survival,
0-12 000 mg/kg for carcinogenicity study) body weight, chemical
body weight) consumption, and histology
6000 mg/kg body weight:
mortality 75% in m/f;
body weight of surviving
mice ** (m/f)
12 000 mg/kg body weight:
mortality 100% within
3 weeks
a m = male; f = female.
8.3.2 Inhalation exposure
Ten CD rats per sex per group were exposed to 0, 25, 250, or
1200 mg benzoic acid dust aerosol/m3 (analytical concentration; mass
aerodynamic diameter [MAD]/sigma g (standard deviation): 0, 4.6/3.1,
4.4/2.1, 5.2/2.1; mass median aerodynamic diameter [MMAD]: 4.7 µm) for
6 h per day and 5 days per week over 4 weeks. After this time, various
serum biochemical, haematological, organ weight, and histopathological
examinations were conducted. At >25 mg/m3, an increased incidence
of interstitial inflammatory cell infiltrate and interstitial fibrosis
in the trachea and lungs in treated animals compared with controls was
seen. Although the number of these microscopic lesions was higher in
treated animals than in controls, there was no clear dose dependency
for this effect. A concentration of >250 mg/m3 resulted in upper
respiratory tract irritation, as indicated by inflammatory exudate
around the nares, and significantly decreased absolute kidney weights
in females. In the highest dose group, one rat per sex died, and the
body weight gain was significantly decreased in males and females
compared with controls. In addition, a significant decrease in
platelets (males/females), absolute/relative liver weights (males),
and trachea/lung weights (females) was noted (Velsicol Chemical
Corp., 1981).
Studies concerning repeated exposure by inhalation to sodium
benzoate were not identified in the available literature.
8.3.3 Dermal exposure
Studies concerning repeated dermal exposure to benzoic acid or
sodium benzoate were not identified in the available literature.
8.4 Long-term exposure
In general, the database for benzoic acid and sodium benzoate is
limited, and there are no studies available performed according to
current guidelines. In addition, the documentation in most cases is
limited. Detailed information is given in Table 4.
8.4.1 Subchronic exposure
In a 90-day study with rats dosed with 0, 1, 2, 4, or 8% sodium
benzoate via diet, the mortality in the highest dose group (approx.
6290 mg/kg body weight per day) was about 50%. Other effects in this
group included a reduced weight gain, increased relative weights of
liver and kidneys, and pathological changes (not further specified) in
these organs (Deuel et al., 1954).
Table 4: Results of studies concerning long-term oral exposure to benzoic acid and sodium benzoate.
Species; strain; Treatment Duration Examinations; Resultsa Reference
number of animals organs in
per dosea histopathology,
clinical chemistry,
haematology
Benzoic acid
rat; Wistar; 0 or 1.5% in diet 18 months no data available reduced weight gain with Marquardt (1960)
dose group: (approx. 0 or decreased feed intake;
30 m/20 f; 750 mg/kg body increased mortality rate
controls: weight) (15/50 vs. 3/25 in
13 m/12 f controls); no further
information available
(only provisional results
are given)
rat; Wistar or 0 or 1.5% in diet 18 months no data available reduced weight gain with Marquardt (1960)
Osborne-Mendel; (approx. 0 or decreased feed intake;
dose group: 750 mg/kg body no further information
20 m; controls: weight) available (only
10 m provisional results are
given)
rat; not given; 0, 0.5, or 1% in generation 1 histopathology in no effects on growth and Kieckebusch & Lang
20 m/f diet and 2: animals of organ weights; feeding of (1960)
(approx. 0, 250, lifelong generation 3 0.5% led to prolongation
or 500 mg/kg body generation 3: (not further specified) of survival compared with
weight) 16 weeks controls; no further
generation 4: information available
until breeding
Table 4 (cont'd)
Species; strain; Treatment Duration Examinations; Resultsa Reference
number of animals organs in
per dosea histopathology,
clinical chemistry,
haematology
Sodium benzoate
rat; Sherman; 0, 1, 2, 4, or 8% 90 days histopathology 6290 mg/kg body weight: Deuel et al.
5 m/f in diet (approx. 0, performed, but mortality about 50%; (1954)
640, 1320, 2620, not further specified weight gain **;
or 6290 mg/kg body relative weights of
weight) liver and kidneys *;
pathological lesions
(not further specified)
in liver and kidneys
rat; F344; 0, 1, or 2% in 18-24 months histopathology average mortality rate of Sodemoto & Enomoto
dose group: diet performed, but not all animals during the (1980)
50 m/52 f; (m: approx. 0, 700, further specified first 16 months: 14.5%
controls: or 1400 mg/kg (all dead rats showed
body weight; f: pneumonia with abscess);
25 m/43 f about 100 rats including
approx. 0, 290, controls died after
or 580 mg/kg 16 months due to
body weight) haemorrhagic pneumonia
(infection); no adverse
clinical signs and no
differences in average
body weight and mortality
in dosed animals compared
with controls;
non-carcinogenic effects
not reported
Table 4 (cont'd)
Species; strain; Treatment Duration Examinations; Resultsa Reference
number of animals organs in
per dosea histopathology,
clinical chemistry,
haematology
mouse; 0 or 2% via lifelong liver, spleen, kidney, no difference in survival Toth (1984)
albino Swiss; drinking-water bladder, thyroid, rates in treated animals
dose group: (approx. 0 or heart, pancreas, compared with controls;
50 m/f; 5960-6200 mg/kg testes, ovaries, brain, no pathological or
controls: 99 m/f body weight) nasal turbinates, lung statistical evidence of
tumour induction
a m = male; f = female.
8.4.2 Chronic exposure and carcinogenicity
In two studies with rats given 1.5% benzoic acid via diet
(approximately 750 mg/kg body weight per day), the animals showed a
reduced weight gain with decreased feed intake after dosing over
18 months. In one of these studies, mortality was increased
(15/50 rats of both sexes versus 3/25 in controls) (Marquardt, 1960).
No further information on these studies is available, as only
provisional results were published. In a four-generation study with
rats, no effects on life span, growth rate, or organ weights were
reported after dosing with up to 1% in the diet (approximately
500 mg/kg body weight per day) (Kieckebusch & Lang, 1960). Only
animals of the third generation were autopsied after 16 weeks, but
it is not clear if a complete histopathological investigation was
performed.
With sodium benzoate, two long-term studies with rats
(administration of up to 1400 mg/kg body weight per day via diet over
18-24 months; Sodemoto & Enomoto, 1980) or mice (lifelong application
of up to 6200 mg/kg body weight per day via drinking-water; Toth,
1984) are available. The results gave no indication of a carcinogenic
effect in the tested animals. Although the study with mice was not
performed according to current guidelines, the results seem to be
reliable, due to a sufficient number of animals and detailed
histopathological examinations. However, the results from the study
with rats are uncertain, due to a very high mortality in animals of
all dose groups, including controls (from an "infection" after
16 months), no detailed information about dosing regimen (only mean
values given), and the considerable differences in the body weight of
male and female rats (the body weight of females was about twice that
of males).
8.4.3 Carcinogenicity of benzyl acetate, benzyl alcohol, and
benzaldehyde
As benzyl acetate, benzyl alcohol, and benzaldehyde are
practically quantitatively metabolized via benzoic acid (see section
7.1), data on their carcinogenicity from 2-year studies may be used as
supportive evidence in the assessment of the hazards associated with
benzoic acid.
Benzyl acetate was administered in corn oil via gavage to F344/N
rats (0, 250, or 500 mg/kg body weight per day) or B6C3F1 mice
(0, 500, or 1000 mg/kg body weight per day). In high-dose male rats,
the incidence of acinar cell adenomas of the exocrine pancreas was
increased, whereas there was no evidence of carcinogenicity in female
rats. In high-dose male and female mice, benzyl acetate caused
increased incidences of hepatocellular adenomas and squamous cell
neoplasms of the forestomach (US NTP, 1986). In contrast to these
findings, no such tumours were observed in another study with the same
strain of rats and mice when benzyl acetate was administered via diet
(rats: <575 mg/kg body weight per day; mice: <375 mg/kg body
weight per day) (US NTP, 1993).
With benzyl alcohol, no treatment-related increase in tumours was
observed in F344/N rats or B6C3F1 mice after administration of
<400 mg/kg body weight per day in rats or <200 mg/kg body weight
per day in mice by gavage in corn oil (US NTP, 1989).
In B6C3F1 mice dosed with benzaldehyde in corn oil by gavage
(males: 0, 200, or 400 mg/kg body weight per day; females: 0, 300, or
600 mg/kg body weight per day), the incidences of squamous cell
papillomas of the forestomach were significantly greater in both
exposure groups than in controls. A dose-related increase in the
incidence of forestomach hyperplasia was also observed. In F344/N rats
dosed with <400 mg/kg body weight per day, there was no evidence of
carcinogenic activity (US NTP, 1990).
8.5 Genotoxicity and related end-points
8.5.1 Benzoic acid
Benzoic acid tested negative in several Ames tests and in one DNA
damage assay with different Salmonella typhimurium strains in the
presence or absence of metabolic activation (McCann et al., 1975;
Ishidate et al., 1984; Nakamura et al., 1987; Zeiger et al., 1988).
Only in one recombination assay with Bacillus subtilis H17 and M45
was a positive result obtained (Nonaka, 1989). However, due to missing
experimental details (only results given), the validity of this study
cannot be judged. There was no indication of genotoxic activity
(chromosome aberrations, sister chromatid exchange) in tests with
mammalian cells (Chinese hamster CHL and CHO cells, human
lymphoblastoid cells, human lymphocytes) without metabolic activation
(Oikawa et al., 1980; Tohda et al., 1980; Ishidate et al., 1984;
Jansson et al., 1988).
In vivo studies with benzoic acid were not identified in the
literature.
8.5.2 Sodium benzoate
Sodium benzoate also gave negative results in some Ames tests and
in Escherichia coli in the presence or absence of metabolic
activation (Ishidate et al., 1984; Prival et al., 1991). As with
benzoic acid in recombination assays with Bacillus subtilis H17 and
M45, positive results were obtained (Ishizaki & Ueno, 1989; Nonaka,
1989). Although sodium benzoate tested negative in a cytogenetic assay
with WI-38 cells in the absence of metabolic activation (US FDA,
1974), consistently positive results (in contrast to the negative
results of benzoic acid) were obtained in tests on sister chromatid
exchange and chromosome aberrations with CHL/CHO and DON cells or
human lymphocytes without metabolic activation (Abe & Sasaki, 1977;
Ishidate & Odashima, 1977; Ishidate et al., 1984, 1988; Xing & Zhang,
1990). However, from the limited information given in the publications
(i.e., only results given), it cannot be judged if these positive
results may have been attributable to cytotoxic effects.
In a valid in vivo study performed by the US FDA (1974), sodium
benzoate tested negative in a cytogenetic assay (bone marrow) in rats
after single or multiple oral application of doses up to 5000 mg/kg
body weight. In a study with mice (comparable dosing scheme), there
was also no indication of mutagenic activity in a host-mediated assay
(US FDA, 1974).
However, in a dominant lethal assay with rats (comparable dosing
scheme; males were mated with untreated females following 7 or 8 weeks
of dosing), some statistically significant and dose-related findings
were reported in week 7: decreased fertility index for both treatment
regimens and an increased number of preimplantation losses after
single dosing (US FDA, 1974).
In summary, the in vitro studies with benzoic acid gave no
indications for genotoxic effects, whereas in vivo studies were not
identified. Sodium benzoate was also inactive in bacterial test
systems, whereas tests with mammalian cells gave consistently positive
results. In addition, in an in vivo study with sodium benzoate
(dominant lethal assay in rats), a positive result was obtained. As a
result, a genotoxic activity of sodium benzoate cannot be ruled out
entirely at present.
Detailed information concerning the genotoxicity of benzoic acid
and sodium benzoate in vitro is given in Table 5.
8.6 Reproductive and developmental toxicity
8.6.1 Fertility
There are no studies available dealing specifically with the
effects of benzoic acid or sodium benzoate on fertility that have been
conducted according to current protocols.
In a four-generation study with male and female rats, no adverse
effects on fertility or lactation (only investigated parameters) were
seen after dosing with benzoic acid at up to 1% in the diet
(approximately 500 mg/kg body weight per day) (see also section 8.4.2;
Kieckebusch & Lang, 1960).
In studies with repeated oral application, no effects on the
testes were observed in rats after dosing with benzoic acid at up to
647 mg/kg body weight per day in the diet for 4 weeks (see also Table
3; Bio-Fax, 1973) or in mice after lifelong application of 6200 mg
sodium benzoate/kg body weight per day via drinking-water (see also
Table 4; Toth, 1984).
In summary, no clear statement can be given as to the possible
effects of benzoic acid or sodium benzoate on fertility.
8.6.2 Developmental toxicity
In a study with pregnant rats given only one oral dose of benzoic
acid (510 mg/kg body weight on gestation day 9), there was no
indication of an increase in resorption rates or malformations
(Kimmel et al., 1971).
For sodium benzoate, several teratogenicity studies are available
that have been performed with different species. As given in Table 6,
no effects were seen in dams or offspring of rats, mice, rabbits, or
hamsters given oral doses of up to 300 mg/kg body weight per day
(highest dose tested) during gestation (US FDA, 1972b). In a study
with rats by Onodera et al. (1978), doses of 4% or 8% via diet (uptake
of 1875 or 965 mg/kg body weight per day) induced severe maternal
toxicity (no weight gain/loss in body weight, increased mortality) and
were associated with embryotoxic and fetotoxic effects as well as
malformations. However, the authors suggested that the effects on the
dams and fetuses at >4% dietary levels were caused by reduced
maternal feed intake, leading to malnutrition. The intake of sodium
benzoate in the highest dose group (8%) was lower than that at 2%,
where no adverse effects were seen. From this study, a NO(A)EL of
about 1310 mg/kg body weight per day can be derived. In a study with
rats by Minor & Becker (1971), however, fetotoxic and teratogenic
effects occurred at 1000 mg/kg body weight per day. In this study,
sodium benzoate was applied by intraperitoneal injection. Therefore,
differences in pharmacokinetics between oral and intraperitoneal
administration may be the reason for the higher sensitivity.
Studies performed with eggs of leghorn hens (single injection of
<5 mg per egg), chick embryo neural retina cells
(lowest-observed-effect concentration [LOEC] of 34.7 mmol/litre), and
a chick embryotoxicity screening test (single injection of <0.1 mg
per embryo) gave no indication of embryotoxic or teratogenic effects
(Verrett et al., 1980; Jelinek et al., 1985; Daston et al., 1995).
8.6.3 Reproductive toxicity of benzyl acetate, benzyl alcohol,
and benzaldehyde
As benzyl acetate and benzyl alcohol are practically
quantitatively metabolized via benzoic acid (see section 7.1), data on
their reproductive toxicity may be used as supportive evidence in the
assessment of the hazards associated with benzoic acid.
Dietary benzyl acetate (up to 5% in the diet for 13 weeks) had no
effect on the weights of the epididymis, cauda epididymis, or testis,
on sperm motility or density, or on the percentage of abnormal sperm
in mice or rats (US NTP, 1993).
Benzyl acetate (0, 10, 100, 500, or 1000 mg/kg body weight per
day by gavage on days 6-15) had no significant effects on maternal
health in rats and did not induce changes in the numbers of corpora
lutea, implantations, live or dead fetuses, or resorptions,
implantation ratio, sex ratio, external or internal malformations, or
placental weight. Fetal weights were significantly reduced at the
highest dose (Ishiguro et al., 1993).
Benzyl alcohol at 550 mg/kg body weight per day by gavage on days
6-15 of pregnancy had no effect on gestation index, average number of
live pups per litter, postnatal survival, or pup body weight on days
0 and 3 in CD-1 mice (York et al., 1986), while 750 mg/kg body weight
per day (days 7-14) induced a reduction in the pup weight and maternal
weight gain, but no pup mortality or changes in mating or gestation
indices, the total number of resorptions, or the number of live pups
per litter (Hardin et al., 1987).
Table 5: Genotoxicity of benzoic acid and sodium benzoate in vitro.
Resultsa
Species End-point Concentration Without With Remarks Reference
(test system) range metabolic metabolic
activation activation
Benzoic acid
Salmonella Reverse 10-1000 - - McCann et al.
typhimurium mutations µg/plate (1975)
TA 98, TA 100,
TA 1535, TA 1537
Salmonella Reverse 33-10 000 - - cytotoxic effects Zeiger et al.
typhimurium mutations µg/plate at >5000 (1988)
TA 97, TA 98, µg/plate
TA 100, TA 1535,
TA 1537
Salmonella Reverse up to 10 000 - - 10 000 µg/plate was Ishidate et al.
typhimurium mutations µg/plate the highest (1984)
TA 92, TA 94, non-cytotoxic
TA 98, TA 100, concentration tested
TA 1535, TA 1537
Salmonella DNA damage up to - - no further Nakamura et al.
typhimurium (umu test) 1670 µg/ml information available (1987)
TA 1535/pSK 1002 (only results given)
Bacillus Recombination not given tested positive (no Nonaka
subtilis assay further information (1989)
H17, M45 available, only
summary given)
Table 5 (cont'd)
Resultsa
Species End-point Concentration Without With Remarks Reference
(test system) range metabolic metabolic
activation activation
Chinese hamster Chromosome up to ? 0 1500 µg/ml was given Ishidate et al.
cells (CHL) aberration 1500 µg/ml as maximum effective (1984)
concentration; result
given as negative in
Ishidate et al.
(1988)
Human Sister 1-30 - 0 cytotoxic effects Tohda et al.
lymphoblastoid chromatid mmol/litre at 30 mmol/litre (1980)
cells exchange
(transformed
by Epstein-Barr
virus)
Human Sister up to 2 - 0 Jansson et al.
lymphocytes chromatid mmol/litre (1988)
exchange
Chinese Sister up to - 0 Oikawa et al.
hamster chromatid 10 mmol/litre (1980)
cells (CHO) exchange
Table 5 (cont'd)
Resultsa
Species End-point Concentration Without With Remarks Reference
(test system) range metabolic metabolic
activation activation
Sodium benzoate
Salmonella Reverse up to - - 3000 µg/plate was Ishidate et al.
typhimurium mutations 3000 µg/plate the highest (1984)
TA 92, TA 94, non-cytotoxic
TA 98, TA 100, concentration tested
TA 1535, TA 1537
Salmonella Reverse 33-10 000 - - Prival et al.
typhimurium mutations µg/plate (1991)
TA 98, TA 100,
TA 1535, TA 1537,
TA 1538
Escherichia coli Reverse 33-10 000 - - Prival et al.
WP2 mutation µg/plate (1991)
assay
Bacillus Recombination not given tested positive Nonaka (1989)
subtilis assay information
H17, M45 available, only
summary given)
Bacillus Recombination -S9: (+) (+) Ishizaki & Ueno
subtilis assay 20 mg/disc (1989)
H17, M45 +S9:
16 mg/disc
Table 5 (cont'd)
Resultsa
Species End-point Concentration Without With Remarks Reference
(test system) range metabolic metabolic
activation activation
WI-38 cells Cytogenetic 10-1000 µg/ml - 0 examination of US FDA (1974)
assay anaphase preparations
cytotoxic effects
at >500 µg/ml
Chinese hamster Chromosome up to + 0 2000 µg/ml was Ishidate et al.
cells (CHL) aberration 2000 µg/ml given as maximum (1984, 1988)
effective
concentration
Chinese hamster Chromosome 139 mg/ml + 0 only maximum Ishidate &
cells (CHL) aberration effective dose given Odashima (1977)
Chinese hamster Chromosome 290 µg/ml 0 0 only minimum Ishidate et al.
cells (DON) aberration effective dose given (1988)
Chinese hamster Chromosome 1-10 + 0 Abe & Sasaki
cells (DON) aberration mmol/litre (1977)
Chinese hamster Sister 1-10 (+) 0 slight increase Abe & Sasaki
cells (DON) chromatid mmol/litre without dosage (1977)
exchange effect
Human Sister 10 + 0 Xing & Zhang
lymphocytes chromatid mmol/litre (1990)
exchange
a -, negative; +, positive; (+) weakly positive; ?, equivocal; 0, not tested.
Table 6: Results of studies concerning reproductive and developmental toxicity of benzoic acid and sodium benzoate.
Species; strain; Application Durationb Parameters Results NO(A)EL Reference
number of animals investigated (mg/kg
per dosea body
weight)
Benzoic acid
rat; Wistar; 0 or 510 gd 9 F0: implantation F0: resorption rates 510 Kimmel et al.
dose group: mg/kg body and resorption sites were given as (1971)
7 f; controls: weight F1: malformations "comparable with
not given via gavage controls"
F1: malformations
(not further specified)
were given as
"comparable with controls"
no further information
available
rat; not given; 0, 0.5, or 1% F0 and F1: fertility and F0-F3: no adverse 500 Kieckebusch &
20 f in diet lifelong lactation effects compared with Lang (1960)
(approx. 0, F2: 16 controls were reported
250, or 500 weeks no further information
mg/kg body F3: until available
weight) breeding
Sodium benzoate
rat; Wistar; 0, 1.75, 8, gd 6-15 F0: numbers of corpora F0 and F1: no adverse 175 US FDA
20 f 38, or 175 lutea, implantation effects compared with (1972b)
mg/kg body and resorption sites, controls were reported
weight via examination of the
gavage urogenital tract
Table 6 (cont'd)
Species; strain; Application Durationb Parameters Results NO(A)EL Reference
number of animals investigated (mg/kg
per dosea body
weight)
F1: numbers of live
and dead fetuses, body
weights, gross
examination for
external
malformations,
microscopic visceral
and skeletal
examination
mouse; 0, 1.75, 8, gd 6-15 F0: numbers of corpora F0 and F1: no adverse 175 US FDA
CD-1; 38, or 175 lutea, implantation effects compared with (1972b)
25-31 f mg/kg body and resorption sites, controls were reported
weight via examination of the
gavage urogenital tract
F1: numbers of live
and dead fetuses, body
weights, gross
examination for
external
malformations,
microscopic visceral
and skeletal
examination
rabbit; Dutch 0, 2.5, 12, gd 6-18 F0: numbers of corpora F0 and F1: no 250 US FDA
belted; 14-32 f 54, or 250 lutea, implantation adverse effects (1972b)
mg/kg body and resorption sites, compared with
weight via examination of the controls were
gavage urogenital tract reported
Table 6 (cont'd)
Species; strain; Application Durationb Parameters Results NO(A)EL Reference
number of animals investigated (mg/kg
per dosea body
weight)
F1: numbers of live
and dead fetuses, body
weights, gross
examination for
external
malformations,
microscopic visceral
and skeletal
examination
hamster; 0, 3, 14, 65, gd 6-10 F0: numbers of corpora F0 and F1: no adverse 300 US FDA
golden; or 300 mg/kg lutea, implantation effects compared with (1972b)
22 f body weight and resorption sites, controls were reported
via gavage examination of the
urogenital tract
F1: numbers of live
and dead fetuses,
body weights, gross
examination for
external
malformations,
microscopic visceral
and skeletal
examination
Table 6 (cont'd)
Species; strain; Application Durationb Parameters Results NO(A)EL Reference
number of animals investigated (mg/kg
per dosea body
weight)
rat; 0, 100, 315, a) gd 9-11 F0: not specified a) F0: no data given 315 Minor & Becker (1971)
Sprague-Dawley or 1000 mg/kg b) gd 12-14 F1: body weights, in F1: 1000 mg/kg body
(no further data) body weight utero deaths, weight: body weights **;
intraperitoneally gross anomalies in utero deaths * (16%);
gross anomalies * (not
further specified)
b) F0: no data given
F1: 1000 mg/kg body
weight: body weights **;
in utero deaths * (12%);
gross anomalies <--> (not
further specified)
no further information
available
rat; Wistar; 0, 1, 2, 4, gd 1-20 a) all but five a) >4% (1875 or 965 mg/kg 1310 Onodera et al. (1978)
27-30 f or 8% via diet animals in each group body weight):
(approx. 0, 700, were sacrificed on F0: weight gain <-->
1310, 1875, or gd 20 (numbers of feed intake **;
965 mg/kg body viable/dead fetuses, mortality * (convulsions,
weight) early/late depressed motor activity)
resorptions, fetal, F1: number of
placental, and ovarian dead/resorbed fetuses *;
weights, and body weight of viable
abnormalities of fetuses **; mild
maternal organs and systemic oedema,
fetal appearance were anophthalmia,
recorded) microphthalmia,
Table 6 (cont'd)
Species; strain; Application Durationb Parameters Results NO(A)EL Reference
number of animals investigated (mg/kg
per dosea body
weight)
b) the remaining five hydrocephalus,
dams delivered pyelectasis, hydroplasia,
naturally (number of cerebral hypoplasia;
offspring, survival, delayed ossification,
body weight, and lumbar or cervical ribs,
abnormalities were and varied sternebrae
recorded); 3 weeks 8%: F0: body weight **
after birth, all b) F1:
surviving pups were <2% (1310 mg/kg body
weaned and examined weight): no adverse
for gross effects compared with
abnormalities controls
(one-half of the pups >4% (1875 or 965 mg/kg
and all dams were body weight): delivery
necropsied); the rates ** (50 and 8.2%,
remaining pups were respectively); complete
necropsied at 8 weeks loss of litters after
of age (body weight parturition
and food intake were
measured weekly until
necropsy)
a m = male; f = female.
b gd = gestation day.
9. EFFECTS ON HUMANS
Cases of urticaria, asthma, rhinitis, or anaphylactic shock have
been reported following oral, dermal, or inhalation exposure to
benzoic acid and sodium benzoate. The symptoms appear shortly after
exposure and disappear within a few hours, even at low doses (Maibach
& Johnson, 1975; Clemmensen & Hjorth, 1982; Larmi et al., 1988; Ring,
1989; Gailhofer et al., 1990; Aberer et al., 1992; Lahti et al., 1995;
Anderson, 1996; Bindslev-Jensen, 1998; Coverly et al., 1998).
In the literature, several studies (e.g., oral provocation tests
or patch tests) are available, which have been performed with small
groups of patients suffering from urticaria, dermatitis, asthma, and
Melkersson-Rosenthal syndrome (Juhlin et al., 1972; Freedman, 1977;
Osterballe et al., 1979; Lahti & Hannuksela, 1981; Clemmensen &
Hjorth, 1982; Ibero et al., 1982; Moneret-Vautrin et al., 1982; Veien
et al., 1987; Aguirre et al., 1993; McKenna et al., 1994; BUA, 1995;
Munoz et al., 1996; Petrus et al., 1996; Vogt et al., 1999). In most
of these studies, atopic individuals have demonstrated reactions to
oral and dermal challenge with benzoic acid or sodium benzoate.
The information concerning skin reactions caused by benzoic acid
or sodium benzoate in the general population is limited. In a study
with 2045 patients of dermatological clinics, only 5 persons
(approximately 0.2%) showed a positive reaction in patch tests
(Brasch et al., 1993), while 34 of 5202 patients (approximately 0.7%)
with contact urticaria reacted positively (Broeckx et al., 1987). From
these data, it can be concluded that skin reactions caused by benzoic
acid or sodium benzoate in the healthy general population are rare.
In US FDA (1972a) and WHO (1996), several older studies
concerning oral exposure to benzoic acid or sodium benzoate are
described. However, owing to the limited number of individuals (mostly
single case studies), the validity of these studies is limited. No
adverse effects were reported after a single oral dose of 10 000 mg
benzoic acid or up to 1000 mg per day over a period of up to 92 days
(Gerlach, 1909). In another study with volunteers given 1000, 1500,
2000, or 2500 mg/day for 5 days each, marked symptoms, signs of
discomfort, and malaise (nausea, headache, weakness, burning and
irritation of oesophagus) were reported (Wiley & Bigelow, 1908).
Chittenden et al. (1909) found no abnormalities in blood picture,
urine composition, nitrogen balance, or well-being in six men given
300-400 mg per day via diet for up to 62 days. In nine patients on
penicillin treatment given 12 000 mg benzoic acid divided into eight
doses over 5 days in eight subjects and over 14 days in one subject,
no adverse effects on blood urea nitrogen or creatinine clearance were
reported (Waldo et al., 1949). A single dose of 2000-3000 mg sodium
benzoate caused signs of intoxication similar to those described for
benzoic acid by Wiley & Bigelow (1908).
Sodium benzoate is used in the treatment of patients with urea
cycle enzymopathies (i.e., hyperammonaemia due to inborn errors of
urea synthesis) in order to facilitate an alternative pathway of
nitrogen excretion. The therapeutic dose given over several years is
in the range of 250-500 mg/kg body weight per day (Batshaw & Brusilow,
1981; Green et al., 1983; Batshaw & Monahan, 1987; O'Connor et al.,
1987; Kubota & Ishizaki, 1991; Tremblay & Qureshi, 1993; Feillet &
Leonard, 1998). At this dose level, clinical signs of toxicity are
rare and in most cases limited to anorexia and vomiting, especially
after intravenous bolus infusions.
10. EFFECTS ON OTHER ORGANISMS IN THE LABORATORY
AND FIELD
10.1 Aquatic environment
For the toxicity data mentioned in this section, it is not always
stated whether the cited effect values are based on nominal or
measured concentrations of benzoic acid or sodium benzoate. However,
because of their water solubility, their insignificant volatility, and
their low adsorption potential (see sections 2 and 5), all nominal
concentrations of the test substances are expected to correspond to
effective concentrations, even in tests with open systems and longer
exposure durations.
In Table 7, several valid toxicity test results for the most
sensitive aquatic species of various taxonomic groups -- bacteria,
cyanobacteria, green algae, protozoa, invertebrates, and vertebrates
-- with benzoic acid have been compiled. From the aquatic organisms
tested so far, cyanobacteria (Anabaena inaequalis) proved to be most
sensitive, showing a 14-day EC50 of 9 mg/litre in the cell
multiplication inhibition test (Stratton & Corke, 1982). EC50/LC50
values (24-96 h) for most of the other aquatic species tested
(protozoa, molluscs, crustaceans, fish, amphibians) were in the range
of 100-1291 mg/litre. As seen with daphnids, the pH value of the test
medium influences the toxicity of benzoic acid, which proved to be
more toxic at lower pH levels (Bringmann & Kuehn, 1980). Developmental
toxicity effects seen in frog (Xenopus) embryos were craniofacial
defects, especially microcephaly, and abnormal gut coiling
(Dawson et al., 1996). A recently developed cytotoxicity assay with
cultured fathead minnow (Pimephales promelas) cells resulted in a
PI50 (the concentration required to induce a 50% reduction in total
protein content) of 1450 mg/litre (Dierickx, 1998).
Ninety-six-hour LC50 values of >100 mg sodium benzoate/litre
have been found for Daphnia magna (first and second larval instar)
and Gammarus fasciatus (juvenile: 7 mg in size) under static test
conditions (multispecies test; pH 6.5-8; 20°C) (Ewell et al., 1986).
The same was true for juveniles of other invertebrates tested
simultaneously: Asellus intermedius (Arthropoda; 12 mg body weight),
Dugesia tigrina (Platyhelminthes; 6 mg body weight), Helisoma
trivolvis (Mollusca; 180 mg body weight), and Lumbriculus variegatus
(Annelida; 6 mg body weight) (Ewell et al., 1986).
Two different tests with the freshwater fathead minnow
(P. promelas; juvenile stages) resulted in 96-h LC50 values of 484
mg sodium benzoate/litre (measured concentration; flow-through system;
pH 7.4; 24°C) (Geiger et al., 1985) and >100 mg/litre (nominal
concentration; static system; pH 6.5-8.5; 20°C) (Ewell et al., 1986).
Table 7: Aquatic toxicity of benzoic acid.
Most sensitive species Special Effective Reference
(test method/end-point) features concentration
(mg/litre)
Mixed microbial inoculum
Activated sludge pH 7.5 3-h EC50 >1000 Klecka et al.
(respiration inhibition (1985)
test; OECD Guideline
209)
Bacteria
Pseudomonas putida pH neutral 16-h MICa 480 Bringmann &
(cell multiplication Kuehn (1977)
inhibition test)
(static)
Photobacterium - 30-min EC50 16.85 Kaiser et al.
phosphoreum (1987)
(Microtox test:
bioluminescence
reduction)
Cyanobacteria
Anabaena inaequalis Stratton &
(cell multiplication - 14-day EC50 9 Corke (1982)
inhibition test)
(static)
(photosynthesis - 3-h EC50 5
reduction)
Algae
Scenedesmus quadricauda
(cell multiplication pH neutral 8-day MIC 1630 Bringmann &
inhibition test) Kuehn (1977)
(static)
(photosynthesis
reduction) - 3-h EC50 75 Stratton &
Corke (1982)
Table 7 (cont'd)
Most sensitive species Special Effective Reference
(test method/end-point) features concentration
(mg/litre)
Chlorella pyrenoides
(photosynthesis
reduction) - 3-h EC50 60 Stratton &
Corke (1982)
Protozoa
Uronema parduczi
(cell multiplication pH 6.9 20-h MIC 31 Bringmann &
inhibition test) Kuehn (1980)
Tetrahymena pyriformis
(cell multiplication - 2-day EC50 252 Schultz et al.
inhibition test) (1996)
Invertebrata: Mollusca
Teredo digensis larvae 72-h LC50 100 Vind &
(marine) (static) Hochman
(1960)
Invertebrata: Crustacea
Daphnia magna pH neutral 24-h EC50 500 Bringmann &
(immobilization) Kuehn (1982)
pH acid 24-h EC50 102
Vertebrata: Fish
Leuciscus idus
(lethality, DEV L15) pH 7-8 48-h LC50 460 Juhnke &
Luedemann
(1978)
Table 7 (cont'd)
Most sensitive species Special Effective Reference
(test method/end-point) features concentration
(mg/litre)
Vertebrata: Amphibia
Xenopus laevis
(lethality) embryos Dawson et al.
(malformation) pH 7.2-7.4 96-h LC50 1291 (1996)
96-h EC50 433
a MIC = minimum inhibitory concentration.
10.2 Terrestrial environment
It is undissociated benzoic acid that is responsible for its
antimicrobial activity. As benzoic acid itself is only slightly
soluble in water, sodium benzoate -- which, under acidic conditions,
converts to undissociated benzoic acid -- is often used instead. Their
antimicrobial properties are used for different applications, such as
food preservation (Chipley, 1983; see section 4), optimally under
acidic conditions.
Minimum microbiocidal concentrations ranged from 20 to 1200 mg
benzoic acid/litre in suspension tests (pH 6) with different bacterial
or fungal species (Wallhäusser, 1984; Russell & Furr, 1996). Minimum
inhibitory concentrations (serial dilution technique) were in the
range of 50-1000 mg/litre (Wallhäusser, 1984; Russell & Furr, 1996).
The pH dependence of benzoic acid's antimicrobial activity is
shown in several studies. Growth inhibition of the fungus Fusarium
oxysporum (related to dry weight) measured 5 days after incubation
with 610 mg benzoic acid/litre was 23.7% at pH 7.2 and 83.5% at pH 4
(Soni & Bhatia, 1980). There was no visible growth of yeast
(Saccharomyces cerevisiae, Willia anomala) or mould (Penicillium
glaucum) fungi at sodium benzoate concentrations of 120-600 mg/litre
at pH 2.6, 1000-4000 mg/litre at pH 5, or 20 000-60 000 mg/litre at pH
7 (Schelhorn, 1951). Minimum inhibitory concentrations preventing
growth of Talaromyces flavus on agar plates after 35 days of
incubation were 100 mg/litre at pH 3.5 and >600 mg/litre at pH 5.4
(King & Halbrook, 1987).
The minimum inhibitory concentrations of benzoic acid on the
growth of several species of yeasts ranged from 170 to 1250 mg/litre
in cultures preadapted to benzoic acid and from 100 to 600 mg/litre in
unadapted cultures (pH 3.5; 25°C; 6 weeks of incubation) (Warth,
1988).
No information on the toxic effects of benzoic acid or sodium
benzoate on plants, earthworms, or other terrestrial organisms or on
ecosystems was identified.
11. EFFECTS EVALUATION
11.1 Evaluation of health effects
11.1.1 Hazard identification and dose-response assessment
After oral ingestion of benzoic acid and sodium benzoate in
experimental animals or humans, there is rapid absorption of the
undissociated benzoic acid from the gastrointestinal tract. The
substances are metabolized in the liver mainly by conjugation with
glycine, resulting in the formation of hippuric acid, which is rapidly
excreted via the urine. Benzoates applied dermally can penetrate
through the skin. Owing to their rapid metabolism and excretion, an
accumulation of the benzoates or their metabolites is not to be
expected.
With oral LD50 values of >1940 mg/kg body weight, the acute
toxicity of benzoic acid and sodium benzoate in rodents is low.
Benzoic acid is slightly irritating to the skin and irritating to
the eye, whereas sodium benzoate is not irritating to the skin and is
only a slight eye irritant. Benzoic acid was not skin sensitizing in
several animal models. For sodium benzoate, no data were identified
covering this specific end-point.
Studies concerning short-term, subchronic, or chronic oral
exposure conducted according to current guidelines are not available
for benzoic acid or sodium benzoate. Effects on the central nervous
system, weight gain (in several cases without reduced food intake),
and liver and kidney were recorded at high concentrations of both
compounds. As expected, and as far as it is possible to conclude with
the limited database, toxic effects and effect levels seem to be
similar for both compounds. A preliminary NO(A)EL of about 500 mg/kg
body weight per day (the highest dose tested) may be derived based on
a limited four-generation study (Kieckebusch & Lang, 1960; see section
8.4.2 and Table 4). This is supported by two short-term studies in
which no adverse effects were observed at the highest tested dose
levels of 647-825 mg/kg body weight per day (Kreis et al., 1967;
Bio-Fax, 1973) and by the fact that no serious side-effects have been
reported after therapeutic use of sodium benzoate at a dose level of
250-500 mg/kg body weight per day in humans, although occasionally
anorexia and vomiting were observed.
In a short-term inhalation study with rats exposed to benzoic
acid (0, 25, 250, or 1200 mg dust aerosol/m3; 6 h per day, 5 days
per week, over 4 weeks), indications of fibrosis in the lung were seen
even at the lowest concentration. The number of these microscopic
lesions was higher in treated animals than in controls, but there was
no clear dose dependency for this effect. Therefore, a
no-observed-(adverse-)effect concentration (NO(A)EC) value cannot be
derived. Long-term inhalation studies with benzoic acid or sodium
benzoate were not identified.
Two long-term studies with rats (application of up to 1400 mg/kg
body weight per day via diet over 18-24 months; quality of the study
questionable) or mice (lifelong application of up to 6200 mg/kg body
weight per day via drinking-water) gave no indication of a
carcinogenic effect in either species. Studies on the precursors of
benzoic acid -- benzyl acetate, benzyl alcohol, and benzaldehyde --
support the notion that it is unlikely that benzoic acid is
carcinogenic.
In several in vitro tests on genotoxicity, benzoic acid and
sodium benzoate tested negative. For sodium benzoate, in contrast to
benzoic acid, consistently positive results were obtained in tests on
sister chromatid exchange and chromosome aberrations without metabolic
activation. In vivo studies for benzoic acid were not identified.
For sodium benzoate, negative results were obtained in vivo in a
cytogenetic assay with rats and a host-mediated assay with single or
multiple oral application. However, a dominant lethal assay with rats
gave a positive result. Therefore, a possible genotoxic activity of
sodium benzoate cannot be ruled out entirely at present.
For benzoic acid, two limited studies gave no indication of
adverse reproductive or developmental effects. With sodium benzoate,
several studies on different species have been performed. Embryotoxic
and fetotoxic effects as well as malformations were seen only at doses
that induced severe maternal toxicity. In a dietary study in rats, a
NO(A)EL of about 1310 mg/kg body weight per day was established.
Studies on the precursors of benzoic acid support the notion that
benzoic acid is unlikely to have adverse reproductive effects at dose
levels not toxic to the mother.
The acute toxicity of benzoic acid and sodium benzoate in humans
is low. However, both substances are known to cause contact dermatitis
(pseudoallergy). In patients with urticaria or asthma, an exacerbation
of the symptoms was observed after testing (oral provocation test or
patch tests), whereas this effect is unusual in healthy subjects.
11.1.2 Criteria for setting tolerable intakes or guidance values for
benzoic acid and sodium benzoate
As given in section 11.1.1, the database is insufficient for
deriving NO(A)EL values for oral uptake. If the provisional NO(A)EL of
about 500 mg/kg body weight per day is applied, and by incorporating
an uncertainty factor of 100 (10 for uncertainty of the database, 10
for interspecies variation), a provisional tolerable intake would be
5 mg/kg body weight per day.
Applying this tolerable intake, one has to keep in mind that
benzoates at lower doses can cause non-immunological contact reactions
(pseudoallergy) in sensitive persons.
There are also no studies available concerning longer-term
exposure by inhalation, and the only short-term inhalation toxicity
study is not adequate for confidently establishing a NO(A)EC.
Therefore, a tolerable concentration for exposure by inhalation cannot
be calculated.
11.1.3 Sample risk characterization
As given in section 6.2, workers may be exposed to benzoic acid
or sodium benzoate via inhalation or skin contact during production
and processing. However, owing to the lack of information on specific
working operations and conditions (e.g., duration of exposure)
involved, it is impossible to derive a realistic estimate of
occupational exposure.
For the general population, the main route of exposure to benzoic
acid and sodium benzoate is likely via foodstuffs, which contain the
substances naturally or added as antimicrobial agents. As given in
section 6.2, the uptake depends on the individual's choice of food to
be consumed and the limit values for benzoates in different countries.
Therefore, considerable deviations may occur. Recent intake
estimations from surveys from several countries gave mean values in
the range of 0.18-2.3 mg/kg body weight. Only in high consumers was an
intake of up to 14 mg/kg body weight calculated. Benzoates have not
been detected in drinking-water. As given in section 6.1, the
inhalative uptake via ambient or indoor air may contribute only
marginally to exposure of the general population.
For normal consumers, the uptake of benzoates is about 2-28 times
lower than the provisional tolerable intake of 5 mg/kg body weight
day, and only in high consumers would this value be exceeded 3 times.
Additional information is required in order to evaluate whether
sodium benzoate has a possible genotoxic activity.
11.2 Evaluation of environmental effects
Significant releases of benzoic acid and sodium benzoate into the
environment are primarily into water and soil from their uses as
preservatives in food, mouthwashes, dentifrices, and cosmetics.
Benzoic acid occurs naturally in many plants.
From their physical/chemical properties, benzoic acid and sodium
benzoate are not expected to volatilize from water and soil to the
atmosphere or to adsorb to sediment or soil particles. The main
elimination pathway for both chemicals should be biotic
mineralization. Because of their ready biodegradability and their low
volatility, both substances are not considered to contribute directly
to the depletion of the stratospheric ozone layer or to global
warming. From experimental data on bioconcentration, a low to moderate
potential for bioaccumulation is to be expected.
Benzoic acid and sodium benzoate exhibited low to moderate
toxicity to aquatic organisms. The lowest reported EC50 value of
9 mg/litre was determined in a chronic study (14 days) for cell
multiplication inhibition by benzoic acid in the cyanobacterium
Anabaena inaequalis. EC50/LC50 values for the other aquatic
species tested were in the range of 17-1291 mg/litre. Exposure levels
of benzoic acid and benzoate in water have been determined only in
rain and snow, groundwater, and leachate in the vicinity of point
sources. Thus, a quantitative risk characterization with respect to
aquatic organisms in surface waters could not be performed. Taking
into account the rapid biodegradability, the low to moderate
bioaccumulation potential, the low toxicity to most aquatic species,
and the rapid metabolism of these substances, benzoic acid and sodium
benzoate will -- with the exception of accidental spills -- pose only
a minimal risk to aquatic organisms.
The few available data from the antimicrobial action of benzoic
acid and sodium benzoate indicate only a low toxicity potential of
both substances in the terrestrial compartment. Due to the lack of
measured exposure levels, a sample risk characterization with respect
to terrestrial organisms could not be performed.
12. PREVIOUS EVALUATIONS BY INTERNATIONAL BODIES
JECFA (WHO, 1996) has allocated an acceptable daily intake (ADI)
for benzoic acid and sodium benzoate of 0-5 mg/kg body weight.
REFERENCES
Abe S, Sasaki M (1977) Chromosome aberrations and sister chromatid
exchanges in Chinese hamster cells exposed to various chemicals.
Journal of the National Cancer Institute, 58(6):1635-1641.
Abe S, Tsutsui Y, Tasrumoto Y, Nakane S (1984) Studies on the toxicity
of oxaprozin. 1. Acute toxicity of oxaprozin, its metabolites and
contaminants. Iyakuhin Kenkyu, 15(3):359-370.
Aberer W, Kager B, Ziegler V, Horak F (1992) Schnupfen durch
Schneiderkreide -- Allergie, Pseudoallergie, Rhinopathie oder
Einbildung? Dermatosen, 40(6):231-234.
Aguirre A, Izu R, Gardeazabal J, Diaz-Perez JL (1993) Edematous
allergic contact cheilitis from a toothpaste. Contact dermatitis,
28:42.
Andersen KE, Maibach HI, Anjo MD (1980) The guinea-pig: an animal
model for human skin absorption of hydrocortisone, testosterone and
benzoic acid? British journal of dermatology, 102:447-453.
Anderson JA (1996) Allergic reactions to food. Critical reviews in
food science and nutrition, 36:S19-S38.
AOAC (1990) Official methods of analysis, 15th ed. Arlington, VA,
Association of Official Analytical Chemists, pp. 1142-1145.
Arens M, Gertz C (1990) Bestimmung der Konservierungsstoffe -
Gemeinsschaftsarbeit der DGF, 110. Mitteilung: Deutsche
Einheitsmethoden zur Untersuchung von Fetten, Fettprodukten, Tensiden
und verwandten Stoffen, 83.ü Mitt.: Analyse von fettreichen
Lebensmitteln IV. Fat science and technology, 92(3):107-109.
BAGS (1995) Standards zur Expositionsabschätzung. Bericht des
Ausschusses für Umwelthygiene (Arbeitsgemeinschaft der leitenden
Medizinalbeamtinnen und -beamten der Länder). Hamburg, Behörde für
Arbeit, Gesundheit und Soziales.
Baldwin EA, Nisperos-Carriedo MO, Baker RA (1995) Use of edible
coatings to preserve quality of lightly (and slightly) processed
products. Critical reviews in food science and nutrition,
35(6):509-524.
Barker JF, Barbash JE, Labonte M (1988) Groundwater contamination at a
landfill sited on fractured carbonate and shale. Journal of
contaminant hydrology, 3:1-25.
Batshaw ML, Brusilow SW (1981) Evidence of lack of toxicity of sodium
phenylacetate and sodium benzoate in treating urea cycle
enzymopathies. Journal of inherited metabolic disease, 4:231.
Batshaw ML, Monahan PS (1987) Treatment of urea cycle disorders.
Enzyme, 38:242-250.
Battersby NS, Wilson V (1989) Survey of the anaerobic biodegradation
potential of organic chemicals in digesting sludge. Applied and
environmental microbiology, 55(2):433-439.
Bayer AG (1977) Akute orale Toxizität / Untersuchung zur Haut- und
Schleimhautverträglichkeit. Wuppertal (unpublished report).
Bayer AG (1978) Untersuchung zur Haut- und
Schleimhautverträglichkeit. Wuppertal (unpublished report).
Bayer AG (1986) Benzoesäure DAB 8. Prüfung auf primär reizende/
ätzende Wirkung am Kaninchenauge. Wuppertal (unpublished report).
Bedford PGC, Clarke EGC (1972) Experimental benzoic acid poisoning in
the cat. Veterinary record, 90:53-58.
Bennett MC, Petrus DR (1977) Quantitative determination of sorbic acid
and sodium benzoate in citrus juice. Journal of food science,
42:1220-1221.
BFGoodrich Kalama Inc. (1999) Benzoic acid. Product information
bulletin. Kalama, WA.
Bindslev-Jensen C (1998) ABC of allergies. Food allergy. British
medical journal, 316:1299-1302.
Bio-Fax (1973) Benzoic acid. Northbrook, IL, Industrial Bio-Test
Laboratories, Inc.
Birch RR, Biver C, Campagna R, Gledhill WE, Pagga U, Steber J, Reust
H, Bontinck WJ (1989) Screening of chemicals for anaerobic
biodegradability. Chemosphere, 19(10-11):1527-1550.
BMA (British Medical Association) (1998) Antifungal preparations --
Benzoic acid. Wallingford, Pharmaceutical Press (British National
Formulary 507).
Brasch J, Henseler T, Frosch P (1993) Patch test reactions to a
preliminary preservative series. Dermatosen, 41(2):71-76.
Bridges JW, French MR, Smith RL, Williams RT (1970) The fate of
benzoic acid in various species. Biochemical journal, 118:47-51.
Bringmann G, Kuehn R (1977) [Threshold values for the damaging action
of water pollutants to bacteria (Pseudomonas putida) and green algae
(Scenedesmus quadricauda) in the cell multiplication inhibition
test.] Zeitschrift für Wasser und Abwasser Forschung, 10:87-98 (in
German).
Bringmann G, Kuehn R (1980) Bestimmung der biologischen Schadwirkung
wassergefährdender Stoffe gegen Protozoen. II. Bakterienfressende
Ciliaten. Zeitschrift für Wasser und Abwasser Forschung, 1:26-31.
Bringmann G, Kuehn R (1982) [Results of toxic action of water
pollutants on Daphnia magna Straus tested by an improved
standardized procedure.] Zeitschrift für Wasser und Abwasser
Forschung, 15:1-6 (in German).
Broeckx W, Blondeel A, Do-Go A, Achten G (1987) Cosmetic intolerance.
Contact dermatitis, 16:189-194.
Bronaugh RL, Stewart RF, Congdon ER, Giles AL (1982a) Methods for in
vitro percutaneous absorption studies. I. Comparison with in vivo
results. Toxicology and applied pharmacology, 62:474-480.
Bronaugh RL, Stewart RF, Congdon ER (1982b) Methods for in vitro
percutaneous absorption studies. II. Animal methods for human skin.
Toxicology and applied pharmacology, 62:481-488.
BUA (1995) BUA-Stoffbericht Benzoesaeure, Natriumbenzoat.
Beratergremium für Umweltrelevante Altstoffe. Stuttgart, S. Hirzel
Verlag (Stoffbericht Nr. 145).
Bucks DA, Hinz RS, Sarason R, Maibach HI, Guy RH (1990) In vivo
percutaneous absorption of chemicals: a multiple dose study in rhesus
monkeys. Food and chemical toxicology, 28:129-132.
Budavari S, O'Neil MJ, Smith A, Heckelman PE, Kinneary JF, eds. (1996)
The Merck index -- an encyclopedia of chemicals, drugs, and
biologicals, 12th ed. Whitehouse Station, NJ, Merck & Co., Inc.
p. 183.
Carlberg GE, Drangsholt H, Gjoes N (1986) Identification of
chlorinated compounds in the spent chlorination liquor from
differently treated sulphite pulps with special emphasis on mutagenic
compounds. Science of the total environment, 48:157-167.
Carver MP, Riviere JE (1989) Percutaneous absorption and excretion of
xenobiotics after topical and intravenous administration to pigs.
Fundamental and applied toxicology, 13:714-722.
Castro JC, Delgado MA, Sánchez MJ, Montelongo FG (1992) Simultaneous
2nd order derivative spectrophotometric determination of sorbic and
benzoic acids in soft drinks. Analytical letters, 25(12):2357-2376.
Cautreels W, van Cauwenberghe K (1978) Experiments on the distribution
of organic pollutants between airborne particulate matter and the
corresponding gas phase. Atmospheric environment, 12:1133-1141.
Chipley JR (1983) Sodium benzoate and benzoic acid. In: Branen AL,
Davidson PM, eds. Antimicrobials in foods. New York, NY, M. Decker,
pp. 11-35.
Chittenden RH, Long JH, Herter CA (1909) Chemical bulletin, 88. US
Department of Agriculture [cited in WHO, 1996].
Clemmensen O, Hjorth N (1982) Perioral contact urticaria from sorbic
and benzoic acid in salad dressings. Contact dermatitis, 8:1-6.
Cordt T, Kußmaul H (1990) Niedermolekulare Carbonsäuren imBoden, in
der ungesättigten Zone und im Grundwasser. Vom wasser, 74:287-298.
Courtes R, Bahlaoui A, Rambaud A, Deschamps F, Sunde E, Dutrieux E
(1995) Ready biodegradability test in seawater: a new methodological
approach. Ecotoxicology and environmental safety, 31:142-148.
Coverly J, Peters L, Whittle E, Basketter DA (1998) Susceptibility to
skin stinging, non-immunologic contact urticaria and acute skin
irritation; is there a relationship? Contact dermatitis,
38(2):90-95.
Daston GP, Baines D, Elmore E, Fitzgerald MP, Sharma S (1995)
Evaluation of chick embryo neural retina cell culture as a screen for
developmental toxicants. Fundamental and applied toxicology,
26:203-210.
Dawson DA, Schultz TW, Hunter RS (1996) Developmental toxicity of
carboxylic acids to Xenopus embryos: A quantitative
structure-activity relationship and computer-automated structure
evaluation. Teratogenesis, carcinogenesis, and mutagenesis,
16:109-124.
Deuel HJJ, Alfin-Slater R, Weil CS, Smyth HF Jr (1954) Sorbic acid as
a fungistatic agent for foods. 1. Harmlessness of sorbic acid as a
dietary component. Food research, 19:1-12.
Dierickx PJ (1998) Increased cytotoxic sensitivity of cultured FHM
fish cells by simultaneous treatment with sodium dodecyl sulfate and
buthionine sulfoximine. Chemosphere, 36(6):1263-1274.
EC (1995) European Union Directive 95/2/CE from 20.02.1995 on food
additives, colourants and sweeteners. European Commission.
Elder DJE, Kelly DJ (1994) The bacterial degradation of benzoic acid
and benzenoid compounds under anaerobic conditions: Unifying trends
and new perspectives. FEMS microbiology reviews, 13:441-468.
Ewell WS, Gorsuch JW, Kringle RO, Robillard KA, Spiegel RC (1986)
Simultaneous evaluation of the acute effects of chemicals on seven
aquatic species. Environmental toxicology and chemistry, 5:831-840.
Fanelli GM, Halliday SL (1963) Relative toxicity of chlortetracycline
and sodium benzoate after oral administration to rats. Archives
internationales de pharmacodynamie et de thérapie, 144(1-2):120-125.
Federle TW (1988) Mineralization of monosubstituted aromatic compounds
in unsaturated and saturated subsurface soils. Canadian journal of
microbiology, 34:1037-1042.
Feillet F, Leonard JV (1998) Alternative pathway therapy for urea
cycle disorders. Journal of inherited metabolic disease, Suppl.
21(1):101-111.
Feldmann RJ, Maibach HI (1970) Absorption of some organic compounds
through the skin in man. Journal of investigative dermatology,
54:399-404.
Flood PF, Abrams SR, Muir GD, Rowell JE (1989) Odor of the muskox. A
preliminary investigation. Journal of chemical ecology,
15:2207-2217.
Franz TJ (1975) Percutaneous absorption. On the relevance of in vitro
data. Journal of investigative dermatology, 64:190-195.
Freedman BJ (1977) Asthma induced by sulphur dioxide, benzoate and
tartrazine contained in orange drinks. Clinical allergy, 7:407-415.
Freitag D, Ballhorn L, Geyer H, Korte F (1985) Environmental hazard of
organic chemicals. An experimental method for the assessment of the
behaviour of organic chemicals in the ecosphere by means of simple
laboratory tests with 14C labelled chemicals. Chemosphere,
14:1589-1616.
Friedman LJ, Greenwald CG (1994) Food additives. In: Kroschwitz J,
Howe-Grant M, eds. Kirk-Othmer: Encyclopedia of chemical technology.
Vol. 11. New York, NY, Wiley and Sons, pp. 805-833.
Fuchs G, el Said Mohammed M, Altenschmidt U, Koch J, Lack A, Brackmann
R, Lochmeyer C, Oswald B (1993) Biochemistry of anaerobic
biodegradation of aromatic compounds. In: Ratledge C, ed.
Biochemistry of microbial degradation. Dordrecht, Kluwer Academic
Publishers, pp. 513-553.
Fujii T, Omori T, Tagucji T, Ogata M (1991) Urinary excretion of
hippuric acid after administration of sodium benzoate (biological
monitoring 1). Shokuhin Eiseigaku Zasshi (Journal of the Food Hygiene
Society of Japan), 32(3):177-182.
Fujitani T (1993) Short-term effect of sodium benzoate in F344 rats
and B6C3F1 mice. Toxicology letters, 69:171-179.
Gad SC, Dunn BJ, Dobbs DW, Reilly C, Walsh RD (1986) Development and
validation of an alternative dermal sensitization test: the mouse ear
swelling test (MEST). Toxicology and applied pharmacology,
84:93-114.
Gailhofer G, Soyer HP, Ludvan M (1990) Nahrungsmittelallergien und
Pseudoallergien -- Mechanismen, Klinik und Diagnostik. Wiener
Medizinische Wochenschrift, 140:227-232.
Geiger DL, Northcott CE, Call DJ, Brooke LT (1985) Acute toxicities
of organic chemicals to fathead minnows (Pimephales promelas ).
Vol. II. Superior, WI, University of Wisconsin, pp. 139-140.
Gerberick GF, House RV, Fletcher ER, Ryan CA (1992) Examination of the
local lymph node assay for use in contact sensitization risk
assessment. Fundamental and applied toxicology, 19:438-445.
Gerlach V (1909) VII. Summary of the results. In: Physiological
activity of benzoic acid and sodium benzoate. Wiesbaden, Verlag von
Heinrich Staadt [cited in US FDA, 1972a].
Goerlitz DF, Troutman DE, Godsy EM, Franks BJ (1985) Migration of
wood-preserving chemicals in contaminated groundwater in a sand
aquifer at Pensacola, Florida. Environmental science and technology,
19(10):955-961.
Goodman Gilman A, Rall TW, Nies AS, Taylor P, eds. (1990) Goodman and
Gilman's the pharmacological basis of therapeutics, 8th ed. New York,
NY, Pergamon Press, p. 1179.
Goodwin BL (1976) Handbook of intermediary metabolism of aromatic
compounds. New York, NY, Wiley & Sons, pp. B6-B9.
Green TP, Marchessault RP, Freese DK (1983) Disposition of sodium
benzoate in newborn infants with hyperammonemia. Journal of
pediatrics, 102:785-790.
Guardiola J, Ventura J, Rivera J (1989) Occurrence of industrial
organic pollution in a groundwater supply: screening, monitoring and
evaluation of treatment processes. Water supply, 7:11-16.
Haider K, Jagnow G, Kohnen R, Lim SU (1974) Abbau chlorierter Benzole,
Phenole und Cyclohexan- Derivate durch Benzol und Phenol verwertende
Bakterien unter anaeroben Bedingungen. Archives of microbiology,
96:183-200.
Halvorson DO (1984) Determination of benzoic acid in air. American
Industrial Hygiene Association journal, 45(10):727-730.
Ham RK, Boyle WC, Engroff EC, Fero RL (1989) Determining the presence
of organic compounds in foundry waste leachates. Modern casting,
79:27-31.
Harborne JB (1983) Toxins of plant-fungal interactions. In: Keeler RF,
Tu AT, eds. Handbook of natural toxins. Vol. 1. Plant and fungal
toxins. New York, NY, Marcel Dekker, pp. 755-758.
Hardin BD, Schuler RL, Burg JR, Booth GM, Hazelden KP, MacKenzie KM,
Piccirillo VJ, Smith KN (1987) Evaluation of 60 chemicals in a
preliminary developmental toxicity test. Teratogenesis,
carcinogenesis, and mutagenesis, 7:29-48 [cited in WHO, 1996].
Harwood CS, Gibson J (1997) Shedding light on anaerobic benzene ring
degradation: a process unique to prokaryotes? Journal of
bacteriology, 179:301-309.
Hegnauer R, ed. (1966) Chemotaxonomie der Pflanzen. Basel,
Birkhäuser Verlag.
Hegnauer R, ed. (1989) Chemotaxonomie der Pflanzen. Basel,
Birkhäuser Verlag, pp. 415-416.
Hegnauer R (1992) Benzoesäure. In: Hegnauer R, ed. Chemotaxonomie der
Pflanzen. Basel, Birkhäuser Verlag.
Helmig D, Müller J, Klein W (1989) Volatile organic substances in a
forest atmosphere. Chemosphere, 19(8-9):1399-1412.
Hofrichter M, Fritsche W (1996) Abbau aromatischer Kohlenwasserstoffe
durch den Schimmelpilz Penicillium frequentans Bi 7/2. Gas- und
Wasserfach; Wasser-Abwasser, 137:199-204.
Hotchkiss SA, Hewitt P, Caldwell J, Chen WL, Rowe RR (1992)
Percutaneous absorption of nicotinic acid, phenol, benzoic acid and
triclopyr butoxyethyl ester through rat and human skin in vitro:
Further validation of an in vitro model by comparison with in vivo
data. Food and chemical toxicology, 30:891-899.
Hunziker N, Feldmann RJ, Maibach H (1978) Animal models of
percutaneous penetration: comparison between Mexican hairless dogs and
man. Dermatologica, 156:79-88.
Ibero M, Eseverri JL, Barroso C, Botey J (1982) Dyes, preservatives
and salicylates in the induction of food intolerance and/or
hypersensitivity. Allergologia et Immunopathologia, 10(4):263-268.
IPCS (1993) International Chemical Safety Card -- Benzoic acid.
Geneva, World Health Organization, International Programme on
Chemical Safety (ICSC 0103).
Ishida H (1996) Levels of preservatives in toothpastes and possibility
of their intake during brushing of teeth. Shokuhin Eiseigaku Zasshi
(Journal of the Food Hygiene Society of Japan), 37:234-239.
Ishidate M, Odashima S (1977) Chromosome tests with 134 compounds on
Chinese hamster cells in vitro -- a screening for chemical
carcinogenesis. Mutation research, 48:337-354.
Ishidate MJ, Sofuni T, Yoshikawa K, Hayashi M, Nohmi T, Sawada M,
Matsuoka A (1984) Primary mutagenicity screening of food additives
currently used in Japan. Food and chemical toxicology,
22(8):623-636.
Ishidate MJ, Harnois MC, Sofuni T (1988) A comparative analysis of
data on the clastogenicity of 951 chemical substances tested in
mammalian cell cultures. Mutation research, 195:151-213.
Ishiguro S, Miyamoto A, Obi T, Nishio A (1993) Teratological studies
on benzyl acetate in pregnant rats. Kadnau (Bulletin of the Faculty
of Agriculture, Kagoshima University), 43:25-31 [cited in WHO, 1996].
Ishizaki M, Ueno S (1989) The DNA damaging activity of natural and
synthetic food additives. Shokuhin Eiseigaku Zasshi (Journal of the
Food Hygiene Society of Japan), 30:447-451.
Jalal MAF, Read DJ (1983) The organic acid composition of Calluna
heathland soil with special reference to phyto- and fungitoxicity. I.
Isolation and identification of organic acids. Plant and soil,
70:257-272.
James MO, Pritchard JB (1987) In vivo and in vitro metabolism and
excretion of benzoic acid by a marine teleost, the southern flounder.
Drug metabolism and disposition, 15:665-670.
Jansson T, Curvall M, Hedin A, Enzell CR (1988) In vitro studies of
the biological effects of cigarette smoke condensate. III. Induction
of SCE by some phenolic and related constituents derived from
cigarette smoke. Mutation research, 206:17-24.
Jelinek R, Peterka M, Rychter Z (1985) Chick embryotoxicity screening
test -- 130 substances tested. Indian journal of experimental
biology, 23:588-595.
Juhlin L, Michaelsson G, Zetterström O (1972) Urticaria and asthma
induced by food and drug additives in patients with aspirin
hypersensitivity. Journal of allergy and clinical immunology,
50(2):92-98.
Juhnke I, Luedemann D (1978) Ergebnisse der Untersuchung von
chemischen Verbindungen auf akute Fischtoxizität mit dem
Goldorfentest. Zeitschrift für Wasser und Abwasser Forschung,
11(5):161-164.
Kaiser KLE, Palabrica VS, Ribo JM (1987) QSAR of acute toxicity of
mono-substituted benzene derivatives to Photobacterium phosphoreum.
QSAR environmental toxicology, 11:153-168.
Kameya T, Murayama T, Urano K, Kitano M (1995) Biodegradation ranks of
priority organic compounds under anaerobic conditions. Science of the
total environment, 170(1-2):43-51.
Kawamura K, Kaplan IR (1986) Biogenic and anthropogenic organic
compounds in rain and snow samples collected in Southern California.
Atmospheric environment, 20:115-124.
Kawamura K, Kaplan IR (1990) Stabilities of carboxylic acids and
phenols in Los Angeles rainwaters during storage. Water research,
24(11):1419-1423.
Kawamura K, Ng LL, Kaplan IR (1985) Determination of organic acids
(C1-C10) in the atmosphere, motor exhausts, and engine oils. IPCS
guidelines for the monitoring of genotoxic effects of carcinogens in
humans. Environmental science and technology, 19:1082-1086.
Kay JH, Calandra JC (1962) Interpretation of eye irritation tests.
Journal of the Society of Cosmetic Chemists, 13:281-289.
Kieckebusch W, Lang K (1960) Die Verträglichkeit der Benzoesäure im
chronischen Fütterungsversuch. Arzneimittel-Forschung, 10:1001-1003.
Kimmel CA, Wilson JG, Schumacher HJ (1971) Studies on metabolism and
identification of the causative agent in aspirin teratogenesis in
rats. Teratology, 4:15-24.
King ADJ, Halbrook WU (1987) Ascospore heat resistance and control
measures for Talaromyces flavus isolated from fruit juice
concentrate. Journal of food science, 52:1252-1254.
King EF, Painter HA (1983) Ring-test programme 1981-82. Assessment of
biodegradability of chemicals in water by manometric respirometry.
Luxemburg, Commission of the European Communities (EUR 8631 EN 31 S).
Kinney IC, Ivanuski VR (1969) Photolysis mechanisms for pollution
abatement. Cincinnati, OH, US Department of the Interior, Federal
Water Pollution Control Administration, Robert A. Taft Water Research
Center (TWRC-13).
Klecka GM, Landi LP, Bo KM (1985) Evaluation of the OECD activated
sludge, respiration inhibition test. Chemosphere, 14(9):1239-1251.
Kobayashi T, Hashinaga T, Mikami E, Suzuki T (1989) Methanogenic
degradation of phenol and benzoate in acclimated sludges. Water
science and technology, 21:55-65.
Kocwa-Haluch R, Lemek M (1995) Easy and inexpensive diffusion tests
for detecting the assimilation of aromatic compounds by yeast-like
fungi. Part II. Assimilation of aromatic acids. Chemosphere,
31(11/12):4333-4339.
Koda T, Tsuchiya H, Yamauchi M, Hoshino Y, Takagi N, Kawano J (1989)
High-performance liquid chromatographic estimation of eluates from
denture base polymers. Journal of dentistry, 17:84-89.
Koda T, Tsuchiya H, Yamaguchi M, Ohtani S, Takagi N, Kawano J (1990)
Leachability of denture-base acrylic resins in artificial saliva.
Dental materials, 6:13-16.
Kreis H, Frese F, Wilmes G (1967) Physiologische und morphologische
Veränderungen an Ratten nach peroraler Verabreichung von Benzoesäure.
Food and cosmetics toxicology, 5:505-511.
Kubota K, Ishizaki T (1991) Dose-dependent pharmacokinetics of benzoic
acid following oral administration of sodium benzoate to humans.
European journal of clinical pharmacology,41(4):363-368.
Kubota K, Horai Y, Kushida K, Ishizaki T (1988) Determination of
benzoic acid and hippuric acid in human plasma and urine by high
performance liquid chromatography. Journal of chromatography,
425(1):67-75.
Lahti A, Hannuksela M (1981) Is benzoic acid really harmful in cases
of atopy and urticaria? Lancet, 2:1055.
Lahti A, Maibach HI (1984) An animal model for nonimmunologic contact
urticaria. Toxicology and applied pharmacology, 76:219-224.
Lahti A, Pylvanen V, Hannuksela M (1995) Immediate irritant reactions
of benzoic acid are enhanced in washed skin areas. Contact
dermatitis, 33:177-182.
Larmi E, Lahti A, Hannuksela M (1988) Effects of sorbitan-sesquioleate
on non-immunologic immediate contact reactions to benzoic acid.
Contact dermatitis, 19:368-371.
Larsson B (1983) Gas-liquid chromatographic determination of benzoic
acid and sorbic acid in foods: MNKL collaborative study (1983).
Journal of the Association of Official Analytical Chemists,
66:775-780.
Lindström K, Österberg F (1986) Chlorinated carboxylic acids in
softwood kraft pulp spent bleach liquors. Environmental science and
technology, 20(2):133-138.
Lu PY, Metcalf RL (1975) Environmental fate and biodegradability of
benzene derivatives as studied in a model aquatic ecosystem.
Environmental health perspectives, 10:269-284.
Lund FA, Rodriguez DS (1984) Acclimation of activated sludge to
mono-substituted derivatives of phenol and benzoic acid. Journal of
general applied microbiology, 30:53-61.
Lunde G, Gehter J, Gjos N, Lande MBS (1977) Organic micropollutants in
precipitation in Norway. Atmospheric environment, 11:1007-1014.
MacPherson SE, Barton CN, Bronaugh RL (1996) Use of in vitro skin
penetration data and a physiologically based model to predict in vivo
blood levels of benzoic acid. Toxicology and applied pharmacology,
140:436-443.
Maibach HI, Johnson HL (1975) Contact urticaria syndrome. Archives of
dermatology, 111:726-730.
Maibach HI, Wester RC (1989) Percutaneous absorption: in vivo
methods in humans and animals. Journal of the American College of
Toxicology, 8:803-813.
Mailhot H (1987) Prediction of algal bioaccumulation and uptake rate
of nine organic compounds by ten physicochemical properties.
Environmental science and technology, 21:1009-1013.
Maki T, Suzuki Y (1985) Benzoic acid and derivatives. In: Ullmann's
encyclopedia of industrial chemistry. Vol. A3. Weinheim, VCH
Verlagsgesellschaft mbH, pp. 555-568.
Mandrou B, Nolleau V, Gastaldi E, Fabre H (1998) Solid-phase
extraction as a clean-up procedure for the liquid chromatographic
determination of benzoic acid and sorbic acid in fruit-derived
products. Journal of liquid chromatography and related technology,
21:829-842.
Marquardt P (1960) Zur Verträglichkeit der Benzoesäure.
Arzneimittel-Forschung, 10:1033.
Matthews RW (1990) Purification of water with near-U.V. illuminated
suspensions of titanium dioxide. Water research, 24:653-660.
McCann J, Choi E, Yamasaki E, Ames BN (1975) Detection of carcinogens
as mutagens in the Salmonella/microsome test: assay of
300 chemicals. Proceedings of the National Academy of Sciences
of the United States of America, 72(12):5135-5139.
McCormick GC (1974) A comparison of the acute toxicity, distribution,
fate, and some pharmacologic properties of the non-benzenoid aromatic
compound azuloic acid with those of benzoic and naphthoic acids in
mice. Dissertation abstracts international, B35(10):5029B-5030B.
McKenna KE, Walsh MY, Burrows D (1994) The Melkersson-Rosenthal
syndrome and food additive hypersensitivity. British journal of
dermatology, 131:921-922.
Miguez CB, Greer CW, Ingram JM, MacLeod RA (1995) Uptake of benzoic
acid and chloro-substituted benzoic acids by Alcaligenes
denitrificans BRI 3010 and BRI 6011. Applied and environmental
microbiology, 61:4152-4159.
Minor JL, Becker BA (1971) A comparison of the teratogenic properties
of sodium salicylate, sodium benzoate, and phenol. Toxicology and
applied pharmacology, 19:373.
MITI (1992) Biodegradation and bioaccumulation. Data of existing
chemicals based on the CSCL Japan. Tokyo, Ministry of International
Trade and Industry, Chemicals Inspection & Testing Institute.
Moneret-Vautrin DA, Moeller R, Malingrey L, Laxenaire MC (1982)
Anaphylactoid reaction to general anaesthesia: A case of intolerance
to sodium benzoate. Anaesthesia and intensive care, 10:156-157.
Monsanto Co. (1983) Primary eye irritation of benzoic acid to
rabbits. St. Louis, MO, Monsanto Company, Environmental Health
Laboratory.
Munoz FJ, Bellido J, Moyano JC, Alvarez M, Fonseca JL (1996) Perioral
contact urticaria from sodium benzoate in a toothpaste. Contact
dermatitis, 35:51.
Nakamura SI, Oda Y, Shimada T, Oki I, Sugimoto K (1987) SOS-inducing
activity of chemical carcinogens and mutagens in Salmonella
typhimurium TA1535/pSK1002: examination with 151 chemicals. Mutation
research, 192:239-246.
Nonaka M (1989) DNA repair tests on food additives. Environmental and
molecular mutagenesis, 14 (Suppl. 15):143.
Nottingham PM, Hungate RE (1969) Methanogenic fermentation of
benzoate. Journal of bacteriology, 98:1170-1172.
O'Connor JE, Costell M, Grisolia S (1987) The potentiation of ammonia
toxicity by sodium benzoate is prevented by L-carnithine. Biochemical
and biophysical research communications, 145:817-824.
Oikawa A, Tohda H, Kanai M, Miwa M, Sugimura T (1980) Inhibitors of
poly(adenosine diphosphate ribose) induced sister chromatid exchanges.
Biochemical and biophysical research communications,
97(4):1311-1316.
Onodera H, Ogiu T, Matsuoka C, Furuta K, Takeuchi M, Oono Y, Kubota T,
Miyahara M, Maekawa A, Odashima S (1978) [Studies on effects of sodium
benzoate on fetuses and offspring of Wistar rats.] Eisei Shikensho
Hokoku, 96:47-55 (in Japanese) [cited in WHO, 1996].
Osterballe O, Taudorf E, Haahr J (1979) Intolerance to aspirin,
food-colouring agents and food preservatives in childhood asthma.
Ugeskrift for Laeger, 141:1908-1910.
Oussi D, Mokrini A, Chamarro E, Esplugas S (1998) Photodegradation of
benzoic acid in aqueous solutions. Environmental technology,
19(9):955-960.
Palm WU, Millet M, Zetsch C (1998) OH radical reactivity of pesticides
adsorbed on aerosol materials: first results of experiments with
filter samples. Ecotoxicology and environmental safety, 41(1):36-43.
Parke DV (1968) The biochemistry of foreign compounds. Oxford,
Pergamon Press, pp. 1-261.
Petrus M, Bonaz S, Causse E, Rhabbour M, Moulie N, Netter JC,
Bildstein G (1996) [Asthma induced by benzoate contained in some foods
and anti-allergic drugs.] Archives de pédiatrie, 3(10):984-987 (in
French).
Pitter P (1976) Determination of biological degradability of organic
substances. Water research, 10:231-235.
Prival MJ, Simmon VF, Mortelmans KE (1991) Bacterial mutagenicity
testing of 49 food ingredients gives very few positive results.
Mutation research, 260:321-329.
Puttemans ML, Dryon L, Massart DL (1984) Extraction of organic acids
by iron-pair formation with tri- n-octylamine. Part V. Simultaneous
determination of synthetic dyes, benzoic acid, sorbic acid and
saccharin in soft drinks and lemonade syrups. Journal of the
Association of Official Analytical Chemists, 67:880-885.
Rasmussen LEL, Hess DL, Haight JD (1990) Chemical analysis of temporal
gland secretions collected from an Asian bull elephant during a
four-month musth episode. Journal of chemical ecology,
16(7):2167-2181.
Rastogi SC, Lepoittevin JP, Johansen JD, Frosch PJ, Menné T, Bruze M,
Dreier B, Andersen KE, White IR (1998) Fragrances and other materials
in deodorants: Search for potentially sensitizing molecules using
combined GC-MS and structure activity relationship (SAR) analysis.
Contact dermatitis, 39(6):293-303.
RCC Notox (1988a) Primary skin irritation/corrosion study of benzoic
acid in the rabbit. DD's-Hertogenbosch, RCC Notox B.V. (unpublished
report).
RCC Notox (1988b) Eye irritation/corrosion study of benzoic acid in
the rabbit. DD's-Hertogenbosch, RCC Notox B.V. (unpublished report).
RCC Notox (n.d., a) Primary skin irritation/corrosion study with
natrium benzoate in rabbits. DD's-Hertogenbosch, RCC Notox B.V.
(unpublished report).
RCC Notox (n.d., b) Acute eye irritation/corrosion study with natrium
benzoate in rabbits. DD's-Hertogenbosch, RCC Notox B.V. (unpublished
report).
Reifenrath WG, Chellquist EM, Shipwash EA, Jederberg WW, Krueger GG
(1984) Percutaneous penetration in the hairless dog, weanling pig and
grafted athymic nude mouse: Evaluation of models for predicting skin
penetration in man. British journal of dermatology,
Suppl. 111(S27):123-135.
Reinhard M, Goodman NL (1984) Occurrence and distribution of organic
chemicals in two landfill leachate plumes. Environmental science and
technology, 18:953-961.
Richterich K, Steber J (1989) Prevention of nitrification-caused
erroneous biodegradability data in the closed bottle test.
Chemosphere, 19(10-11):1643-1654.
Ring J (1989) Arzneimittelunverträglichkeit durch pseudo-allergische
Reaktionen. Wiener Medizinische Wochenschrift, 139:130-134.
Rubin HE, Subba-Rao RV, Alexander M (1982) Rates of mineralization of
trace concentrations of aromatic compounds in lake water and sewage
samples. Applied and environmental microbiology, 43(5):1133-1138.
Russell AD, Furr JR (1996) Biocides mechanisms of antifungal action
and fungal resistance. Science progress, 79(1):27-48.
Sainio E, Kanerva L (1995) Contact allergens in toothpastes and a
review of their hypersensitivity. Contact dermatitis, 33:100-105.
Sakuma H, Kusama M, Munakata S, Ohsumi T, Sugawara S (1983) The
distribution of cigarette smoke components between mainstream and
sidestream smoke. Beitraege zur Tabakforschung, 12:63-71.
Salanitro JP, Langston GC, Dorn PB, Kravetz L (1988) Activated sludge
treatment of ethoxylate surfactants at high industrial use
concentrations. Water science and technology, 20(11-12):125-130.
Schelhorn M (1951) Untersuchungen über Konservierungsmittel. VI.
Wirksamkeit und Anwendungsbereich von Benzoesäure, Estern der
Paraoxybenzoesäure und Kombination dieser beiden Arten von
Verbindungen. Deutsche Lebensmittel-Rundschau, 47:128-134.
Scholz W, Kortmann W (1991) Paint additives. In: Elvers B, Hawkins S,
Schulz G, eds. Ullmann's encyclopedia of industrial chemistry.
Weinheim, VCH Verlagsgesellschaft, pp. 465-472.
Schou L, Krane JE (1981) Organic micropollutants in a Norwegian
water-course. Science of the total environment, 20:277-286.
Schuetzle D, Cronn D, Crittenden AL (1975) Molecular composition of
secondary aerosol and its possible origin. Environmental science and
technology, 9:838-845.
Schultz TW, Bryant SE, Kissel ST (1996) Toxicological assessment in
Tetrahymena of intermediates in aerobic microbial transformation of
toluene and p-xylene. Bulletin of environmental contamination and
toxicology, 56:129-134.
Sharak Genthner BR, Townsend GT, Blattman BO (1997) Reduction of
3-chlorobenzoate, 3-bromobenzoate, and benzoate to corresponding
alcohols by Desulfomicrobium escambiense, isolated from a
3-chlorobenzoate-dechlorinating coculture. Applied and environmental
microbiology, 63:4698-4703.
Shibamoto T (1986) Photochemistry of a major essential oil
constituent, benzyl benzoate. Developments in food science,
12:745-754.
Shibamoto T, Umano K (1985) Photochemical products of benzyl benzoate:
possible formation of skin allergens. Journal of toxicology,
cutaneous and ocular toxicology, 4(2):97-104.
Shimp RJ, Young RL (1987) Comparison of OECD and radiolabeled
substrate methods for measuring biodegradation in marine environments.
Ecotoxicology and environmental safety, 14:223-230.
Sieber R, Bütikofer U, Bosset JO, Rüegg M (1989) Benzoesäure als
natürlicher Bestandteil von Lebensmitteln- eine Übersicht.
Mitteilungen aus dem Gebiete der Lebensmitteluntersuchung und
Hygiene, 80:345-362.
Sieber R, Bütikofer U, Baumann E, Bosset JO (1990) Über das Vorkommen
der Benzoesäure in Sauermilchprodukten und Käse. Mitteilungen aus dem
Gebiete der Lebensmitteluntersuchung und Hygiene, 84:484-493.
Sioufi A, Pommier F (1980) Gas chromatographic determination of low
concentrations of benzoic acid in human plasma and urine. Journal of
chromatography, biomedical applications, 181:161-168.
Smyth HF, Carpenter CP (1948) Further experience with the range
finding test in the industrial toxicology laboratory. Journal of
industrial hygiene and toxicology, 30:63-68.
Sodemoto Y, Enomoto M (1980) Report of carcinogenesis bioassay of
sodium benzoate in rats: absence of carcinogenicity of sodium benzoate
in rats. Journal of environmental pathology and toxicology, 4:87-95.
Soni GL, Bhatia IS (1980) Toxicity of phenolics and related compounds
to Fusarium oxasporium Schlecht. and effect of pH on their
toxicity. Indian journal of agricultural science, 50(10):772-777.
SRI (1998) 1997/98 directory of chemical producers Europe. Menlo
Park, CA, SRI International (807-808/1613).
Srour R (1989) Benzoic acid. In: Srour R, ed. Aromatic intermediates
and derivatives. Paris, pp. A.IV.1-A.IV.14 (unpublished report)
[cited in BUA, 1995].
Srour R (1998) Benzoic acid and derivatives. In: Srour R, ed.
Aromatic intermediates and derivatives. Paris, pp. A.IV.1- A.IV.17
(unpublished report).
Steeg E, Montag A (1987) Nachweis aromatischer Carbonsäuren in Honig.
Zeitschrift für Lebensmittel-Untersuchung und -Forschung, 184:17-19.
Stolpe NB, McCallister DL, Shea PJ, Lewis DT, Dam R (1993) Mobility of
aniline, benzoic acid, and toluene in four soils and correlation with
soil properties. Environmental pollution, 81:287-295.
Stratton GW, Corke CT (1982) Toxicity of the insecticide permethrin
and some degradation products towards algave and cyanobacteria.
Environmental pollution, 29:71-80.
Stuermer DH, Ng DJ, Morris CJ (1982) Organic contaminants in
groundwater near underground coal gasification site in northeastern
Wyoming. Environmental science and technology, 16:582-587.
Suflita JM, Concannon F (1995) Screening tests for assessing the
anaerobic biodegradation of pollutant chemicals in subsurface
environments. Journal of microbiological methods, 21:267-281.
Takemoto S, Kuge Y, Nakamoto M (1981) The measurement of BOD in sea
water. Japanese journal of water pollution research, 4:80-90.
Tohda H, Horaguchi K, Takahashi K, Oikawa A, Matsushima T (1980)
Epstein-Barr virus-transformed human lymphoblastoid cells for study of
sister chromatid exchange and their evaluation as a test system.
Cancer research, 40:4775-4780.
Tong HY, Shore DL, Karasek FW, Helland P, Jellum E (1984)
Identification of organic compounds obtained from incineration of
municipal waste by high performance liquid chromatographic
fractionation and gas chromatography-mass spectrometry. Journal of
chromatography, 285:423-441.
Toth B (1984) Lack of tumorigenicity of sodium benzoate in mice.
Fundamental and applied toxicology, 4:494-496.
Toyoda M, Ito Y, Isshiki K, Onishi K, Kato T, Kamikura M, Shiroishi Y,
Harada Y, Hukasawa Y, Yokoyama T, Yoneda M, Iwaida M (1983a) Daily
intake of preservatives, benzoic acid, dehydroacetic acid, propionic
acid and their salts, and esters of p-hydrobenzoic acid in Japan.
Journal of the Japanese Society of Nutrition and Food Science,
96:467-480.
Toyoda M, Ito Y, Isshiki K, Onishi K, Kato T, Kamikura M, Shiroishi Y,
Harada Y, Hukasawa Y, Yokoyama Y, Yamamoto Y, Fujii M, Iwaida M
(1983b) Estimation of daily intake of many kinds of food additives
according to the market basket studies in Japan. Journal of the
Japanese Society of Nutrition and Food Science, 36:489-497.
Tremblay GC, Qureshi IA (1993) The biochemistry and toxicology of
benzoic acid metabolism and its relationship to the elimination of
waste nitrogen. Pharmacology and therapeutics, 60:63-90.
Tyler TA (1984) Liquid chromatographic determination of sodium
saccharin, caffeine, aspartame, and sodium benzoate in cola beverages.
Journal of the Association of Official Analytical Chemists,
67(4):745-747.
UK MAFF (1995) Survey of sulphur dioxide and benzoic acid in foods
and drinks. London, Ministry of Agriculture, Fisheries and Food,
Joint Food Safety and Standards Group (Food Surveillance Information
Sheet No. 65; http://maff.gov.uk/food/infsheet/1995/
no65/65sulben.htm).
Urano K, Kato Z (1986) Evaluation of biodegradation ranks of priority
organic compounds. Journal of hazardous materials,13:147-159.
US FDA (1972a) GRAS (Generally Recognized As Safe) food ingredients:
benzoic acid and sodium benzoate. Washington, DC, US Food and Drug
Administration.
US FDA (1972b) Teratologic evaluation of FDA 71-37 (sodium benzoate).
East Orange, NJ, US Food and Drug Administration, Food and Drug
Research Laboratory.
US FDA (1973) Evaluation of the health aspects of benzoic acid and
sodium benzoate as food ingredients. Bethesda, MD, US Food and Drug
Administration, Life Sciences Research Office (PB-223 837).
US FDA (1974) Mutagenic evaluation of compound FDA 71-37, sodium
benzoate. Prepared by Litton Bionetics, Inc., Kensington, MD, for
the US Food and Drug Administration (Unpublished report PB-245-453/6).
US NTP (1986) Toxicology and carcinogenesis studies of benzyl acetate
(CAS No. 140-11-4) in F344/N rats and B6C3F1 mice (gavage studies).
Research Triangle Park, NC, US Department of Health, Education and
Welfare, National Institutes of Health, National Toxicology Program
(Technical Report Series No. 250).
US NTP (1989) Toxicology and carcinogenesis studies of benzyl alcohol
(CAS No. 100-51-6) in F344/N rats and B6C3F1 mice (gavage studies).
Research Triangle Park, NC, US Department of Health, Education and
Welfare, National Institutes of Health, National Toxicology Program
(Technical Report Series No. 343).
US NTP (1990) Toxicology and carcinogenesis studies of benzaldehyde
(CAS No. 100-52-7) in F344/N rats and B6C3F1 mice (gavage studies).
Research Triangle Park, NC, US Department of Health, Education and
Welfare, National Institutes of Health, National Toxicology Program
(Technical Report Series No. 378).
US NTP (1993) Toxicology and carcinogenesis studies of benzyl acetate
(CAS No. 140-11-4) in F344/N rats and B6C3F1 mice (feed studies).
Research Triangle Park, NC, US Department of Health, Education and
Welfare, National Institutes of Health, National Toxicology Program
(Technical Report Series No. 431).
Veien NK, Hattel T, Justesen O, Noerholm A (1987) Oral challenge with
food additives. Contact dermatitis, 17:100-103.
Velsicol Chemical Corp. (1981) Four week subacute inhalation toxicity
study of benzoic acid in rats. Report prepared by International
Research and Development Corporation, Mattawan, MI, for Velsicol
Chemical Corporation, Chicago, IL (FYI-OTS-1281-0147).
Ventullo RM, Larson RJ (1985) Metabolic diversity and activity of
heterotrophic bacteria in ground water. Environmental toxicology and
chemistry, 4:759-771.
Verrett MJ, Scott WF, Reynaldo EF, Alterman EK, Thomas CA (1980)
Toxicity and teratogenicity of food additive chemicals in the
developing chicken embryo. Toxicology and applied pharmacology,
56:265-273.
Villanueva MBG, Jonai H, Kanno S, Takeuchi Y (1994) Dietary sources
and background levels of hippuric acid in urine: Comparison of
Philippine and Japanese levels. Industrial health, 32:239-249.
Vind HP, Hochman H (1960) Toxicity of chemicals to marine borers --
1. Interim report. Port Hueneme, CA, US Naval Civil Engineering
Laboratory, pp. 1-134 (TR 048) [cited in Environmental Chemicals Data
and Information Network (ECDIN) Database, 1998, compiled by Commission
of the European Communities, Joint Research Centre, Ispra
Establishment, Ispra (Varese)].
Vogt T, Landthaler M, Stolz W (1999) Sodium benzoate-induced acute
leukocytoclastic vasculitis with unusual clinical appearance.
Archives of dermatology, 135:726-727.
Waldo JF, Masson JM, Lu WC, Tollstrup J (1949) The effect of benzoic
acid and caronamide on blood penicillin levels and on renal function.
American journal of medical sciences, 217:563-568.
Wallhäusser KH (1984) Praxis der Sterilisation,
Desinfektion-Konservierung; Keimidentifizierung- Betriebshygiene;
3. Auflage. Stuttgart, Georg Thieme Verlag, pp. 399-400.
Ward TE (1985) Characterizing the aerobic and anaerobic microbial
activities in surface and subsurface soils. Environmental toxicology
and chemistry, 4:727-737.
Warth AD (1988) Effect of benzoic acid on growth yield of yeasts
differing in their resistance to preservatives. Applied and
environmental microbiology, 54:2091-2095.
Wester RC, Maibach HI (1976) Relationship of topical dose and
percutaneous absorption in rhesus monkey and man. Journal of
investigative dermatology, 67:518-520.
Wester RC, Noonan PK (1980) Relevance of animal models for
percutaneous absorption. International journal of pharmaceutics,
7:99-110.
WHO (1994) Assessing human health risks of chemicals: derivation of
guidance values for health-based exposure limits. Geneva, World
Health Organization (Environmental Health Criteria 170).
WHO (1996) Toxicological evaluation of certain food additives.
Prepared by the 46th meeting of the Joint FAO/WHO Expert Committee on
Food Additives (JECFA). Geneva, World Health Organization (WHO Food
Additives Series 37).
WHO (1999) Safety evaluation of certain food additives. Prepared by
the 51st meeting of the Joint FAO/WHO Expert Committee on Food
Additives (JECFA). Geneva, World Health Organization, pp. 403-414 (WHO
Food Additives Series 42).
Wiley HM, Bigelow WD (1908) Influence of benzoic acid and benzoates
on digestion and health. Bulletin 84, Part IV. Bureau of Chemistry,
US Department of Agriculture [cited in US FDA, 1972a].
Williams RT (1967) Comparative patterns of drug metabolism.
Federation proceedings, 26:1029-1039.
Winkeler HD, Puttins U, Levsen K (1988) [Organic compounds in
rainwater.] Vom Wasser, 70:107-117 (in German).
Xing W, Zhang Z (1990) A comparison of SCE test in human lymphocytes
and Vicia faba: a hopeful technique using plants to detect mutagens
and carcinogens. Mutation research, 241:109-113.
Yomota C, Isshiki K, Kato T, Kamikura M, Shiroishi Y, Nishijima M,
Hayashi H, Hukasawa Y, Yokojama T, Yoneda M, Moriguchi H, Uchiyama H,
Shiro T, Ito Y (1988) [Estimation of daily intake of 8 kinds of
organic acids, 4 kinds of nucleic acids, ortho-phosphate, benzoic
acid, glycerol monostearate, sodium alginate, sulfur dioxide, nitrate,
nitrite, mannitol, sorbitol, glycerol and ammonium hydroxide from
fresh foods purchased in Japan according to the market basket method.]
Journal of the Japanese Society of Nutrition and Food Science,
41:11-16 (in Japanese).
York RG, Barnwell P, Bailes W (1986) Screening of priority chemicals
for reproductive hazards. Unpublished report (ETOX-85-1002)
submitted to Experimental Toxicology Branch, Division of Biomedical
and Behavioral Science, National Institute for Occupational Safety and
Health, Cincinnati, OH, by Environmental Health Research and Testing,
Inc., Cincinnati, OH. Submitted to WHO by ILSI Europe, Brussels [cited
in WHO, 1996].
Zahn R, Wellens H (1980) [Examination of biological degradability
through the batch method -- further experience and new possibilities
of usage.] Zeitschrift für Wasser und Abwasser Forschung, 13:1-7
(in German).
Zeiger E, Anderson B, Haworth S, Lawlor T, Mortelmans K (1988)
Salmonella mutagenicity tests: IV. Results from the testing of 300
chemicals. Environmental and molecular mutagenesis, Suppl.
11(12):1-158.
Zhang H, Kanzaki H, Kawazu K (1997) Benzoic acid accumulation in the
Pinus thunbergii callus inoculated with the pine wood nematode,
Bursaphelenchus xylophilus. Zeitschrift für Naturforschung,
52:329-332.
APPENDIX 1 -- SOURCE DOCUMENTS
US FDA (1972a) GRAS (Generally Recognized As Safe) food ingredients:
benzoic acid and sodium benzoate. Washington, DC, US Food and Drug
Administration
This report was prepared by Informatics Inc., Rockville, MD, for
the US Food and Drug Administration.
BUA (1995) BUA-Stoffbericht Benzoesaeure, Natriumbenzoat.
Beratergremium fuer Umweltrelevante Altstoffe. Stuttgart, S. Hirzel
Verlag (Stoffbericht Nr. 145)
For the BUA review process, the company that is in charge of
writing the report (usually the largest producer in Germany) prepares
a draft report using literature from an extensive literature search as
well as internal company studies. This draft is subject to a peer
review in several readings of a working group consisting of
representatives from government agencies, the scientific community,
and industry.
WHO (1996) Toxicological evaluation of certain food additives.
Prepared by the 46th meeting of the Joint FAO/WHO Expert Committee on
Food Additives (JECFA). Geneva, World Health Organization (WHO Food
Additives Series 37)
The first draft on benzyl acetate, benzyl alcohol, benzaldehyde,
and benzoic acid and its salts was prepared by E. Vavasour, Chemical
Health Hazard Assessment Division, Bureau of Chemical Safety, Food
Directorate, Health Protection Branch, Health Canada, Ottawa, Ontario.
The meeting of the Joint FAO/WHO Expert Committee on Food Additives
was held from 6 to 15 February 1996 in Geneva.
APPENDIX 2 -- CICAD PEER REVIEW
The draft CICAD on benzoic acid and sodium benzoate was sent for
review to institutions and organizations identified by IPCS after
contact with IPCS National Contact Points and Participating
Institutions, as well as to identified experts. Comments were received
from:
A. Aitio, International Programme on Chemical Safety, World
Health Organization, Switzerland
M. Baril, Institut de Recherche en Santé et en Sécurité du
Travail du Québec (IRSST), Canada
R. Benson, Drinking Water Program, US Environmental Protection
Agency, USA
W.F. ten Berge, WXS, Netherlands
R. Cary, Health and Safety Executive, United Kingdom
R.S. Chhabra, National Institute for Environmental and Health
Sciences/National Institutes of Health (NIEHS/NIH), USA
S. Dobson, Institute of Terrestrial Ecology, United Kingdom
P. Edwards, Department of Health, United Kingdom
R. Hertel, Federal Institute for Health Protection of Consumers
and Veterinary Medicine (BgVV), Germany
C. Hiremath, US Environmental Protection Agency, USA
P. Schulte, National Institute for Occupational Safety and
Health, USA
D. Willcocks, National Industrial Chemicals Notification and
Assessment Scheme (NICNAS), Australia
P. Yao, Chinese Academy of Preventive Medicine, People's Republic
of China
K. Ziegler-Skylakakis, Beratergremium für Umweltrelevante
Altstoffe (BUA), Germany
APPENDIX 3 -- CICAD FINAL REVIEW BOARD
Sydney, Australia, 21-24 November 1999
Members
Dr R. Benson, Drinking Water Program, US Environmental Protection
Agency, Region VIII, Denver, CO, USA
Dr T. Berzins, National Chemicals Inspectorate (KEMI), Solna, Sweden
Dr R.M. Bruce, National Center for Environmental Assessment,
US Environmental Protection Agency, Cincinnati, OH, USA
Mr R. Cary, Health and Safety Executive, Merseyside, United Kingdom
Dr R.S. Chhabra, National Institute of Environmental Health Sciences,
National Institutes of Health, Research Triangle Park, NC, USA
Dr S. Chou, Agency for Toxic Substances and Disease Registry,
Atlanta, GA, USA
Dr S. Dobson, Institute of Terrestrial Ecology, Monks Wood,
Cambridgeshire, United Kingdom
Dr H. Gibb, National Center for Environmental Assessment,
US Environmental Protection Agency, Washington, DC, USA
Dr R.F. Hertel, Federal Institute for Health Protection of Consumers
and Veterinary Medicine, Berlin, Germany
Dr J. Kielhorn, Fraunhofer Institute for Toxicology and Aerosol
Research, Hanover, Germany
Dr S. Kristensen, National Occupational Health and Safety Commission
(Worksafe), Sydney, NSW, Australia
Mr C. Lee-Steere, Environment Australia, Canberra, ACT, Australia
Ms M. Meek, Environmental Health Directorate, Health Canada, Ottawa,
Ontario, Canada
Ms F. Rice, National Institute for Occupational Safety and Health,
Cincinnati, OH, USA
Dr J. Sekizawa, National Institute of Health Sciences, Tokyo, Japan
Dr D. Willcocks, National Industrial Chemicals Notification and
Assessment Scheme (NICNAS), Sydney, NSW, Australia (Chairperson)
Professor P. Yao, Institute of Occupational Medicine, Chinese Academy
of Preventive Medicine, Beijing, People's Republic of China
Observers
Mr P. Howe, Institute of Terrestrial Ecology, Huntingdon,
Cambridgeshire, United Kingdom
Dr K. Ziegler-Skylakakis, GSF-Forschungszentrum für Umwelt und
Gesundheit, GmbH, Oberschleissheim, Germany
Secretariat
Dr A. Aitio, International Programme on Chemical Safety, World Health
Organization, Geneva, Switzerland
Ms M. Godden, Health and Safety Executive, Bootle, Merseyside, United
Kingdom
Dr M. Younes, International Programme on Chemical Safety, World Health
Organization, Geneva, Switzerland
APPENDIX 4 -- INTERNATIONAL CHEMICAL SAFETY CARD
BENZOIC ACID ICSC: 0103
October 1999
CAS# 65-85-0 Benzenecarboxylic acid
RTECS# DG0875000 Phenyl carboxylic acid
C7H6O2/C6H5COOH
Molecular mass: 122.1
TYPES OF HAZARD ACUTE HAZARDS/ PREVENTION FIRST AID / FIRE
/ EXPOSURE SYMPTOMS FIGHTING
FIRE Combustible. NO open flames. Powder, water spray,
foam, carbon dioxide.
EXPLOSION In case of fire: keep drums,
etc., cool by spraying with
water.
EXPOSURE
Inhalation Cough. Sore throat. Local exhaust Fresh air, rest.
or breathing
protection.
Skin Redness. Burning Protective gloves. Remove contaminated clothes.
sensation. Itching. Rinse and then wash skin with
water and soap.
Eyes Redness. Pain. Safety goggles. First rinse with plenty
of water for several minutes
(remove contact lenses if
easily possible), then take
to a doctor.
Ingestion Abdominal pain. Do not eat, drink, or Rinse mouth. Induce vomiting
Nausea. Vomiting smoke during work. (ONLY IN CONSCIOUS PERSONS!)
Wash hands before Refer for medical
eating. attention.
SPILLAGE DISPOSAL PACKAGING & LABELLING
Sweep spilled substance into plastic EU Classification
containers; if appropriate, moisten UN Classification
first to prevent dusting.
Use face shield and (extra personal
protection: protective clothing).
Wash away remainder with plenty of water.
EMERGENCY RESPONSE STORAGE
NFPA Code: H 2; F 1; R -;
IMPORTANT DATA
PHYSICAL STATE; APPEARANCE: ROUTES OF EXPOSURE:
WHITE CRYSTALS OR POWDER. The substance can be absorbed
into the body by inhalation and
by ingestion.
PHYSICAL DANGERS:
Dust explosion possible if in powder
or granular form, mixed with air. INHALATION RISK:
No indication can be given about the
rate in which a harmful concentration
CHEMICAL DANGERS: in the air is reached on evaporation
The solution in water is a weak acid. of this substance at 20°C.
Reacts with oxidants.
OCCUPATIONAL EXPOSURE LIMITS: EFFECTS OF LONG-TERM OR REPEATED EXPOSURE:
TLV not established. The substance irritates the eyes, the skin
and the respiratory tract. The substance
may cause a non-allergic rash on contact.
PHYSICAL PROPERTIES
Boiling Point: 249°C Flash point: 121°C c.c
Melting Point: 122°C (see Notes) Auto-ignition temperature: 570°C
Density: 1.3 g/cm3 Octanol/water partition
coefficient as log Pow: 1.87
Solubility in water, g/100 ml at 20°C: 0.29
Vapour pressure, Pa at 25°C: 0.1
Relative vapour density (air = 1): 4.2
Relative density of the vapour/air-mixture at 20°C (air = 1): 1
ENVIRONMENTAL DATA
NOTES
The substance begins to sublime at 100°C.
ADDITIONAL INFORMATION
LEGAL NOTICE Neither the CEC nor the IPCS nor any person acting on
behalf of the CEC or the IPCS is responsible for the use
which might be made of this information.
RÉSUMÉ D'ORIENTATION
Ce CICAD consacré à l'acide benzoïque et au benzoate de sodium
a été préparé par l'Institut Fraunhofer de Toxicologie et d'étude
des Aérosols de Hanovre (Allemagne). Ces deux composés sont
examinés ensemble car c'est l'acide benzoïque non dissocié qui est
responsable de l'activité anti-infectieuse de ces produits. Comme
l'acide benzoïque lui-même n'est que peu soluble dans l'eau, c'est
le benzoate de sodium - qui en milieu acide redonne l'acide non
dissocié - que l'on utilise à sa place.
Ce CICAD est basé sur des études bibliographiques effectuées
par le Comité consultatif allemand sur les produits chimiques qui
posent des problèmes écologiques (BUA, 1995), la Food and Drug
Administration des États-Unis (US FDA, 1972a) et le Comité mixte
FAO/OMS d'experts des Additifs alimentaires (JECFA) (WHO/OMS, 1996)
dans le but d'évaluer les effets possibles de l'acide benzoïque et
du benzoate de sodium sur l'environnement et sur l'organisme
humain. Une étude bibliographique exhaustive sur les bases de
données appropriées a été effectuée en septembre 1999 afin de
rechercher toute référence intéressante qui aurait été publiée
postérieurement à celles qui figurent dans ces différents rapports.
On trouvera à l'appendice 1 des indications sur la préparation de
l'examen par des pairs et sur les sources documentaires utilisées
pour cet examen. Les renseignements concernant l'examen du présent
CICAD font l'objet de l'appendice 2. Ce CICAD a été approuvé en
tant qu'évaluation internationale lors de la réunion du Comité
d'évaluation finale qui s'est tenue à Sydney (Australie) du 21 au
24 novembre 1999. La liste des participants à cette réunion figure
à l'appendice 3. La fiche d'information internationale sur la
sécurité chimique (ICSC 0103) relative à l'acide benzoïque, établie
par le Programme international sur la sécurité chimique (IPCS,
1993) est également reproduite dans ce document (appendice 4).
Chez les animaux de laboratoire et chez l'Homme, l'acétate de
benzyle, l'alcool benzylique, son produit d'hydrolyse, et le
produit qui en résulte par oxydation, c'est-à-dire le benzaldéhyde,
sont largement métabolisés en acide benzoïque. On a donc utilisé
les données toxicologiques relatives à ces précurseurs dans
l'évaluation des effets sanitaires potentiels de l'acide benzoïque.
L'acide benzoïque (No CAS 65-85-0) se présente sous la forme
d'un solide blanc légèrement soluble dans l'eau. La solubilité dans
l'eau du benzoate de sodium (No CAS 532-32-1) est environ 200 fois
plus élevée. On utilise l'acide benzoïque comme intermédiaire dans
la synthèse de divers composés, principalement le phénol (plus de
50 % de la production mondiale) et la caprolactame. Il sert
également à la préparation de divers sels, dont le sel de sodium,
du chlorure de benzoyle ainsi que de plastifiants comme les
dibenzoates de diéthylène- et de dipropylène-glycol. Le benzoate de
sodium est principalement utilisé comme conservateur et inhibiteur
de corrosion (par ex. comme additif à l'antigel des moteurs à
explosion). L'acide benzoïque et le benzoate de sodium sont
employés comme conservateurs dans l'alimentation et ils conviennent
particulièrement bien pour les produits comme les jus de fruits et
les boissons non alcoolisées, qui ont un pH acide. Leur utilisation
comme conservateurs dans les denrées alimentaires, les boissons,
les pâtes dentifrices, les bains de bouche, les cosmétiques et les
produits pharmaceutiques est réglementée. On estime que la capacité
mondiale de production d'acide benzoïque est d'environ 600 000
tonnes par an. On estime en outre qu'en 1997 la production mondiale
de benzoate de sodium a été comprise entre 55 000 et 60 000 tonnes.
L'acide benzoïque est naturellement présent dans beaucoup de
végétaux et chez un grand nombre d'animaux. C'est donc un
constituant normal de nombreux aliments, notamment du lait et des
produits laitiers. Les rejets d'acide benzoïque et de benzoate de
sodium dans l'environnement qui sont imputables aux activités
humaines aboutissent essentiellement dans les eaux et dans le sol
et proviennent de leur utilisation comme conservateurs. On constate
que dans un certain nombre de denrées alimentaires, la
concentration de l'acide benzoïque d'origine naturelle ne dépasse
pas 40 mg/kg en moyenne. Les concentrations maximales d'acide
benzoïque ou de benzoate de sodium relevées dans des produits
alimentaires auxquels ils avaient été ajoutés comme conservateurs
sont de l'ordre de 2000 mg/kg.
Après ingestion, l'acide benzoïque et le benzoate de sodium
sont rapidement résorbés dans les voies digestives et métabolisés
dans le foie par conjugaison avec la glycine pour donner de l'acide
hippurique, rapidement excrété dans les urines. Les benzoates
appliqués sur la peau sont résorbés dans une moindre proportion par
voie transcutanée. En raison de la rapidité du métabolisme et de
l'excrétion, il n'y a vraisemblablement pas d'accumulation des
benzoates ou de leurs métabolites.
Chez les rongeurs, la toxicité aiguë par voie orale de l'acide
benzoïque et du benzoate de sodium est faible (la DL50 par voie
orale est supérieure à 1940 mg/kg de poids corporel). Chez le chat,
qui semble plus sensible que les rongeurs, on a signalé des effets
toxiques et une mortalité à des doses beaucoup plus faibles
(environ 450 mg/kg p.c.).
L'acide benzoïque est légèrement irritant pour la peau et
irritant pour la muqueuse oculaire, tandis que le benzoate de
sodium n'irrite pas la peau est n'est que légèrement irritant pour
l'oeil. En ce qui concerne l'acide benzoïque, les données
disponibles n'indiquent aucun effet sensibilisateur; dans le cas du
benzoate de sodium, aucune donnée n'a été relevée dans la
littérature à ce sujet.
Les études à court terme effectuées sur des rats ont révélé la
présence de troubles du système nerveux central (acide benzoïque /
benzoate de sodium) ainsi que des anomalies histopathologiques dans
l'encéphale (acide benzoïque) après administration de doses élevées
dans l'alimentation (>1800 mg/kg p.c.) pendant 5 à 10 jours. Les
autres effets constatés étaient les suivants : réduction du gain de
poids, modification du poids des organes, modification des
paramètres sériques ou encore anomalies histopathologiques au
niveau du foie. On ne dispose que de données très limitées sur
l'exposition de longue durée des animaux de laboratoire à l'acide
benzoïque par voie orale et il n'existe pas d'étude qui soit
spécialement consacrée à la recherche d'effets cancérogènes
éventuels. Une étude limitée, portant sur quatre générations, n'a
permis d'obtenir qu'une estimation préliminaire de la dose sans
effet (nocif) observable (NO(A)EL), estimation qui est d'environ
500 mg/kg p.c. par jour. En ce qui concerne le benzoate de sodium,
les deux études à long terme effectuées sur des rats et des souris
n'ont pas mis en évidence d'effet cancérogène. Il faut dire
cependant que dans la plupart de ces études, les effets ne sont pas
parfaitement attestés, d'où l'impossibilité d'en tirer des valeurs
fiables pour la dose sans effet observable et la dose sans effet
nocif observable. Les données concernant leurs divers précurseurs
corroborent l'hypothèse selon laquelle l'acide benzoïque ne serait
pas vraisemblablement pas cancérogène.
L'acide benzoïque a donné des résultats négatifs dans un
certain nombre de tests sur bactéries ou cellules mammaliennes,
mais on n'a pas trouvé de comptes rendus de tests in vivo. Le
benzoate de sodium s'est également révélé inactif dans le test
d'Ames mais il a donné des résultats systématiquement positifs sur
cellules mammaliennes. Dans une étude in vivo (test de létalité
dominante chez le rat) on a également obtenu un résultat positif.
Dans ces conditions, on ne peut pour l'instant exclure que le
benzoate de sodium ait une activité génotoxique.
Dans le cas de l'acide benzoïque, on dispose de deux études
limitées qui n'indiquent aucun effet indésirable sur la
reproduction ou le développement. En ce qui concerne le benzoate de
sodium, plusieurs études ont été menées sur un certain nombre
d'espèces et si des effets embryotoxiques et foetotoxiques ou même
des malformations ont été observés, c'est uniquement à des doses
déjà toxiques pour les mères. Une étude d'alimentation sur des rats
a permis de fixer à environ 1310 mg/kg p.c. la dose sans effet
(nocif) observable. Les données concernant ses divers précurseurs
corroborent l'hypothèse selon laquelle l'acide benzoïque n'a
vraisemblablement pas d'effets indésirables sur la reproduction aux
doses qui ne sont pas toxiques pour la mère.
Chez l'Homme, l'acide benzoïque et le benzoate de sodium sont
peu toxiques. On sait cependant que ces deux composés peuvent
produire des réactions de contact non immunologiques
(pseudoallergie). Cet effet est rare chez les sujets en bonne
santé. En revanche, chez les personnes qui souffrent fréquemment
d'asthme ou d'urticaire, on a observé une exacerbation des
symptômes. On peut établir une dose journalière tolérable
provisoire par ingestion de 5 mg/kg de poids corporel, encore que
les benzoates soient susceptibles de provoquer chez les sujets
sensibles des pseudoallergies de contact à des doses plus faibles.
Comme on ne dispose pas d'étude appropriée sur l'exposition par
inhalation, on ne peut déterminer la concentration tolérable en cas
d'exposition par cette voie.
Compte tenu de leurs propriétés physiques et chimiques, il est
exclu que l'acide benzoïque et le benzoate de sodium rejetés dans
le sol ou dans l'eau soient à même de s'évaporer dans l'atmosphère
ou de s'adsorber aux sédiments ou aux particules du sol. De
nombreuses expériences on permis de constater que la principale
voie d'élimination de ces deux composés était la minéralisation
biologique. Des essais en laboratoire ont montré qu'en aérobiose,
les deux composés sont facilement biodégradables. Un certain nombre
de microorganismes isolés (bactéries, champignons) se sont révélés
capable d'utiliser l'acide benzoïque en aérobiose comme en
anaérobiose. Les données fournies par les expériences de
bioconcentration montrent que ces produits ont un potentiel de
bioaccumulation faible à modéré.
Ú en juger d'après les tests effectués sur des organismes
aquatiques dans de bonnes conditions de validité, l'acide benzoïque
et le benzoate de sodium se révèlent peu à modérément toxiques dans
ce milieu. La valeur la plus faible de la CE50 qui ait été
mesurée, soit 9 mg/litre (critère : inhibition de la multiplication
cellulaire), a été obtenue dans une étude de toxicité chronique sur
une corynébactérie, Anabaena inaequalis. Les valeurs de la CE50
et de la CL50 obtenues sur d'autres espèces aquatiques, se
situaient aux alentours de 60-1291 mg/litre. On a montré que
l'immobilisation de la daphnie dépendait du pH, la CE50 à 24 h
étant plus basse (102 mg/litre) pour les pH acides. Dans le cas de
l'ide rouge (Leuciscus idus), on a trouvé une CL50 à 48 h de
460 mg/litre. Des effets ont été constatés sur le développement des
embryons de grenouille (Xenopus) à la concentration de 433
mg/litre (CE50 à 96 h; critère : malformation). Dans le cas du
benzoate de sodium, l'exposition des stades juvéniles d'organismes
aquatiques appartenant à plusieurs espèces (Daphnia magna,
Gammarus fasciatus, Asellus intermedius, Dugesia tigrina,
Helisoma trivolvis et Lumbriculus variegatus) a permis de
constater que la CL50 à 96 h était supérieure à 100 mg/litre.
On a mesuré une CL50 à 96 h de 484 mg/litre pour le vairon à
grosse tête (Pimephales promelas). Compte tenu du caractère
limité des données concernant le niveau d'exposition dans l'eau, il
n'a pas été possible de quantifier le risque couru par les
organismes qui peuplent les eaux de surface. En s'appuyant sur la
biodégradation rapide des composés, la valeur faible à modérée de
leur potentiel de bioaccumulation, la faible toxicité qu'ils
présentent pour la plupart des espèces aquatiques et leur
métabolisation rapide, il apparaît que l'acide benzoïque et le
benzoate de sodium ne représentent qu'un risque minimum pour les
organismes aquatiques, mis à part le cas de déversements
accidentels.
Les quelques données disponibles indiquent que l'acide
benzoïque et le benzoate de sodium n'ont qu'un faible potentiel
toxique dans l'environnement terrestre. Ú l'exception de l'action
antimicrobienne de l'acide benzoïque, qui se caractérise par une
concentration microbicide comprise entre 20 et 1200 mg/litre, on ne
possède aucune donnée sur les effets toxiques de ce composé sur les
organismes terrestres. Dans le cas du benzoate de sodium, il y a
inhibition de la croissance bactérienne et fongique pour des
concentrations comprises entre 100 et 60 000 mg/litre et cette
inhibition dépend du pH. Faute de connaître la valeur des niveaux
d'exposition, il n'a pas été possible de caractériser le risque
pour les organismes terrestres.
RESUMEN DE ORIENTACI²N
El presente CICAD sobre el ácido benzoico y el benzoato de
sodio se preparó en el Instituto Fraunhofer de Toxicología y de
Investigación sobre los Aerosoles de Hannover, Alemania. Se
examinan los dos compuestos juntos porque es el ácido benzoico no
disociado el responsable de su actividad antimicrobiana. Debido a
que el propio ácido benzoico es sólo ligeramente soluble en agua,
con frecuencia se utiliza en su lugar el benzoato de sodio, que en
condiciones ácidas se convierte en ácido benzoico no disociado.
El presente CICAD se basa en exámenes compilados por el Comité
Consultivo Alemán sobre las Sustancias Químicas Importantes para el
Medio Ambiente (BUA, 1995), la Administración de Alimentos y
Medicamentos de los Estados Unidos (US FDA, 1972a) y el Comité
Mixto FAO/OMS de Expertos en Aditivos Alimentarios (JECFA)
(WHO/OMS, 1996) para evaluar los efectos potenciales del ácido
benzoico y el benzoato de sodio en el medio ambiente y en el ser
humano. En septiembre de 1999 se realizó una búsqueda bibliográfica
amplia de las bases de datos pertinentes para localizar cualquier
referencia de interés publicada después de las incorporadas a estos
informes. La información relativa a la preparación de los
documentos originales y su examen colegiado figura en el apéndice
1. La información sobre el examen colegiado de este CICAD aparece
en el apéndice 2. Este CICAD se aprobó como evaluación
internacional en una reunión de la Junta de Evaluación Final
celebrada en Sidney, Australia, los días 21-24 de noviembre de
1999. En el apéndice 3 figura la lista de los participantes en esta
reunión. La Ficha internacional de seguridad química (ICSC 0103)
para el ácido benzoico, preparada por el Programa Internacional de
Seguridad de las Sustancias Químicas (IPCS, 1993), también se
reproduce en el presente documento (apéndice 4).
El acetato de bencilo, su producto de hidrólisis, el alcohol
de bencilo, y el producto de la oxidación de este alcohol, el
benzaldehído, se metabolizan ampliamente a ácido benzoico en
animales experimentales y en el ser humano. Por consiguiente,
también se utilizaron datos toxicológicos sobre estos precursores
en la evaluación de los efectos potenciales del ácido benzoico para
la salud.
El ácido benzoico (CAS No 65-85-0) es una sustancia sólida
blanca ligeramente soluble en agua. El benzoato de sodio
(CAS No 532-32-1) es alrededor de 200 veces más soluble en
agua. El ácido benzoico se utiliza como producto intermedio en la
síntesis de distintos compuestos, fundamentalmente el fenol (>50
por ciento de la cantidad producida en todo el mundo) y la
caprolactama. Otros productos finales son el sodio y otros
benzoatos, el cloruro de benzoílo y los agentes plastificantes de
dibenzoato de dietilenglicol y dipropilenglicol. El benzoato de
sodio se utiliza sobre todo como conservante e inhibidor de la
corrosión (por ejemplo, en sistemas técnicos como aditivo de los
refrigerantes anticongelantes de los motores de automóviles).
El ácido benzoico y el benzoato de sodio se utilizan como
conservantes de los alimentos y son los más idóneos para los
productos alimenticios, los jugos de frutas y las bebidas no
alcohólicas, que por su naturaleza tienen un pH ácido. Su
utilización como conservantes en alimentos, bebidas, pastas de
dientes, colutorios, dentífricos, cosméticos y productos
farmacéuticos está reglamentada. La capacidad de producción mundial
estimada de ácido benzoico es de alrededor de 600 000 toneladas al
año. La producción mundial de benzoato de sodio en 1997 puede
estimarse en unas 55 000-60 000 toneladas. El ácido benzoico está
presente de manera natural en muchas plantas y en los animales. Por
consiguiente, es un elemento constitutivo natural de numerosos
alimentos, entre ellos los productos lácteos. Las liberaciones
antropogénicas de ácido benzoico y benzoato de sodio en el medio
ambiente son primordialmente emisiones al agua y al suelo a partir
de su uso como conservantes. Las concentraciones del ácido benzoico
presente de manera natural en varios productos alimenticios no
superaron el valor medio de 40 mg/kg de alimentos. Las
concentraciones máximas notificadas de ácido benzoico o benzoato de
sodio añadido a los productos alimenticios con fines de
conservación fueron del orden de 2000 mg/kg de alimentos.
Tras la ingesta oral, el ácido benzoico y el benzoato de sodio
se absorben con rapidez del tracto gastrointestinal y se
metabolizan en el hígado por conjugación con la glicina, dando
lugar a la formación de ácido hipúrico, que se excreta rápidamente
a través de la orina. Los benzoatos aplicados por vía cutánea
pueden penetrar en menor medida a través de la piel. Debido a la
rapidez del metabolismo y de la excreción, no cabe prever una
acumulación de benzoatos o sus metabolitos.
En roedores, la toxicidad oral aguda del ácido benzoico y el
benzoato de sodio es baja (valores de la DL50 por vía oral >1940
mg/kg de peso corporal). En gatos, que parecen ser más sensibles
que los roedores, se notificaron efectos tóxicos y mortalidad con
dosis mucho menores (unos 450 mg/kg de peso corporal).
El ácido benzoico es ligeramente irritante de la piel e
irritante de los ojos, mientras que el benzoato de sodio no irrita
la piel y es sólo ligeramente irritante de los ojos. En cuanto al
ácido benzoico, en los estudios disponibles no apareció ningún
indicio de efecto sensibilizante; para el benzoato de sodio no se
encontraron datos en la bibliografía.
En estudios de corta duración con ratas, se observaron
trastornos del sistema nervioso central (ácido benzoico/benzoato de
sodio), así como cambios histopatológicos en el cerebro (ácido
benzoico) después de administrar dosis elevadas (>1800 mg/kg de
peso corporal) durante 5-10 días. Otros efectos fueron una
reducción del aumento del peso corporal, cambios en el peso de los
órganos, cambios en los parámetros del suero o cambios
histopatológicos en el hígado. La información relativa a la
exposición oral prolongada de animales experimentales al ácido
benzoico es muy limitada y no hay ningún estudio disponible que
trate expresamente de los posibles efectos carcinogénicos. De un
estudio limitado de cuatro generaciones sólo puede derivarse una
concentración sin efectos (adversos) observados (NO(A)EL) de
carácter preliminar de alrededor de 500 mg/kg de peso corporal al
día. Con el benzoato de sodio, en dos estudios de larga duración
con ratas y ratones no se obtuvo ningún indicio de efecto
carcinogénico. Sin embargo, la documentación de los efectos es
insuficiente en la mayoría de estos estudios; por consiguiente, no
pueden derivarse valores de la NO(A)EL fidedignos. Los datos sobre
sus precursores respaldan la idea de que probablemente el ácido
benzoico no es carcinogénico.
El ácido benzoico dio resultados negativos en varias
valoraciones bacterianas y en pruebas con células de mamífero, pero
no se localizaron estudios in vivo. El benzoato de sodio también
fue inactivo en pruebas Ames, mientras que las pruebas con células
de mamífero dieron sistemáticamente resultados positivos. En un
estudio in vivo (valoración letal dominante con ratas) se obtuvo
un resultado positivo. En la actualidad no se puede excluir
totalmente la actividad genotóxica del benzoato de sodio.
En cuanto al ácido benzoico, en dos estudios limitados no se
obtuvieron indicios de efectos adversos reproductivos o en el
desarrollo. Con el benzoato de sodio se han realizado varios
estudios en distintas especies y solamente se observaron efectos
embriotóxicos y fetotóxicos, así como malformaciones, con dosis que
inducían una toxicidad materna grave. En un estudio de alimentación
en ratas se estableció una NO(A)EL de alrededor de 1310 mg/kg de
peso corporal. Los datos sobre sus precursores respaldan la idea de
que no es probable que el ácido benzoico tenga efectos
reproductivos adversos con los niveles de dosis que no son tóxicos
para la madre.
En el ser humano, la toxicidad aguda del ácido benzoico y el
benzoato de sodio es baja. Sin embargo, se sabe que ambas
sustancias provocan reacciones de contacto no inmunológicas
(pseudoalergia). Este efecto no es frecuente en personas sanas; en
pacientes con ataques frecuentes de urticaria o asma se observaron
síntomas o su exacerbación. Puede derivarse una ingesta tolerable
provisional de 5 mg/kg de peso corporal al día, aunque dosis
menores de benzoatos pueden producir reacciones de contacto no
inmunológicas (pseudoalergia) en personas sensibles. Debido a que
no hay estudios adecuados disponibles sobre la exposición por
inhalación, no se puede calcular una concentración tolerable para
este tipo de exposición.
Dadas sus propiedades físicas/químicas, no cabe prever que el
ácido benzoico y el benzoato de sodio que pasan al agua y al suelo
se volatilicen a la atmósfera o se adsorban sobre los sedimentos o
las partículas del suelo. Según los resultados de numerosos
experimentos de eliminación, la principal vía que siguen ambos
productos químicos debe ser la mineralización biótica. Los datos
obtenidos en pruebas de laboratorio pusieron de manifiesto una
biodegradabilidad rápida de ambas sustancias en condiciones
aerobias. Se ha comprobado que varios microorganismos aislados
(bacterias, hongos) utilizaron el ácido benzoico en condiciones
aerobias o anaerobias. De acuerdo con los datos experimentales
sobre la bioconcentración, cabe prever un potencial de bajo a
moderado para la bioacumulación.
Según los resultados de las pruebas válidas disponibles sobre
la toxicidad del ácido benzoico y el benzoato de sodio para
diversos organismos acuáticos, estos compuestos parecen mostrar una
toxicidad de baja a moderada en el compartimento acuático. El valor
más bajo de la CE50, de 9 mg/litro (inhibición de la
multiplicación celular), notificado en un estudio crónico se
observó en la cianobacteria Anabaena inaequalis. Los valores de
la CE50/CL50 para otras especies acuáticas estudiadas fueron
del orden de 60-1291 mg/litro. Se ha demostrado que la
inmovilización de Daphnia magna es dependiente del pH, con una
CE50 más baja en 24 horas (102 mg/litro) cuando el pH es ácido.
Para un pez de agua dulce, el cacho (Leuciscus idus), se ha
determinado una CL50 en 48 horas de 460 mg/litro. Se han
encontrado efectos en el desarrollo en embriones de rana (Xenopus)
con una concentración de 433 mg/litro (CE50 en 96 horas para la
malformación). Para el benzoato de sodio, en una exposición de
estadios juveniles de organismos acuáticos en una prueba
multiespecífica (con inclusión de Daphnia magna, Gammarus
fasciatus, Asellus intermedius, Dugesia tigrina, Helisoma
trivolvis, y Lumbriculus variegatus) se obtuvieron valores de la
CL50 en 96 horas superiores a 100 mg/litro. En el pez de agua
dulce Pimephales promelas se ha determinado una CL50 en 96
horas de 484 mg/litro. Debido a los limitados datos disponibles
sobre los niveles de exposición en agua, no se pudo realizar una
caracterización cuantitativa del riesgo con respecto a los
organismos acuáticos de aguas superficiales. Teniendo en cuenta la
rápida biodegradabilidad, el potencial de bioacumulación entre
moderado y bajo, la escasa toxicidad para la mayoría de las
especies acuáticas y el rápido metabolismo de estas sustancias, el
ácido benzoico y el benzoato de sodio - con la excepción de
vertidos accidentales - representan un riesgo sólo mínimo para los
organismos acuáticos.
Los escasos datos disponibles indican que el ácido benzoico y
el benzoato de sodio tienen solamente un potencial de toxicidad
bajo en el medio terrestre. Salvo la acción antimicrobiana del
ácido benzoico, que se caracteriza por concentraciones microbicidas
mínimas que oscilan entre 20 y 1200 mg/litro, no se encontraron
datos sobre los efectos tóxicos del ácido benzoico en los
organismos terrestres. En cuanto al benzoato de sodio, inhibió el
crecimiento bacteriano y fúngico de manera dependiente del pH en
concentraciones que oscilaban entre 100 y 60 000 mg/litro. Debido a
la falta de mediciones de los niveles de exposición, no se pudo
realizar un muestreo de la caracterización del riesgo con respecto
a los organismos terrestres.
3. ANALYTICAL METHODS
Analytical methods for the determination of benzoic acid include
spectrophotometric methods, which need extensive extraction procedures
and are not very specific; gas chromatographic (GC) methods, which are
more sensitive and specific but need lengthy sample preparation and
derivatization prior to determination; and high-performance liquid
chromatography (HPLC), which has a high specificity and minimum sample
preparation and does not require derivatization.
A direct determination of benzoic acid in air by flash desorption
at 240°C with helium into capillary-GC gave a detection limit of
0.1 ppm (0.5 mg/m3) in a 20-litre sample (=10 µg benzoic acid).
This method has been developed and used for monitoring occupational
exposure (Halvorson, 1984).
A method for the determination of benzoic acid in solid food at
0.5-2 g/kg levels involves extraction with ether into aqueous sodium
hydroxide and methylene chloride, conversion to trimethylsilyl esters,
and detection by GC and flame ionization (Larsson, 1983; AOAC, 1990).
For margarine, a method using HPLC and ultraviolet (UV) detection has
been described with prior extraction with ammonium acetate/acetic
acid/methanol (Arens & Gertz, 1990).
When benzoic acid is used as a preservative in soft drinks and
fruit drinks, other additives, colouring agents, and other acids
(e.g., sorbate) may interfere with its analysis. Liquid
chromatographic methods were developed to overcome this (e.g., Bennett
& Petrus, 1977; Puttemans et al., 1984; Tyler, 1984). For the
sensitive determination of benzoic acid in fruit-derived products, a
clean-up pretreatment with solid-phase extraction followed by liquid
chromatography with UV absorbance detection is described (Mandrou et
al., 1998). The detection limit is 0.6 mg/kg, with a range of
quantification of 2-5 mg/kg. For soft drinks, a simultaneous
second-order derivative spectrophotometric determination has been
developed (detection limit 1 mg/litre) (Castro et al., 1992). Sodium
benzoate was measured in soya sauce, fruit juice, and soft drinks
using HPLC with a UV spectrophotometric detector. Before injection,
all samples were filtered (Villanueva et al., 1994).
GC determination of low concentrations (down to 10 ng/ml) of
benzoic acid in plasma and urine was preceded by diethyl ether
extraction and derivatization with pentafluorobenzyl bromide (Sioufi &
Pommier, 1980). Detection was by 63Ni electron capture. HPLC methods
have been developed for the simultaneous determination of benzoic acid
and hippuric acid -- the metabolite of sodium benzoate that is
eliminated in the urine -- that require no extraction step (detection
limit for both, 1 µg/ml; Kubota et al., 1988). Hippuric acid and
creatinine levels have been determined simultaneously by HPLC, and
measured hippuric acid levels corrected for urinary creatinine
excretion (Villanueva et al., 1994).
4. SOURCES OF HUMAN AND ENVIRONMENTAL EXPOSURE
4.1 Natural sources of benzoic acid
Benzoic acid is produced by many plants as an intermediate in the
formation of other compounds (Goodwin, 1976). High concentrations are
found in certain berries (see section 6.1). Benzoic acid has also been
detected in animals (see section 6.1). Benzoic acid therefore occurs
naturally in many foods, including milk products (Sieber et al., 1989,
1990).
4.2 Anthropogenic sources
4.2.1 Benzoic acid
Benzoic acid is produced exclusively by the liquid-phase
oxidation of toluene (Srour, 1998).
According to Srour (1998), the estimated global production
capacity of benzoic acid is 638 000 tonnes per year, although over
half of this is converted directly to phenol. The major producers of
benzoic acid are the Netherlands (220 000 tonnes per year) and Japan
(140 000 tonnes per year), followed by the USA (125 000 tonnes per
year). Another reference gives the total European capacity as less
than 153 000 tonnes (SRI, 1998).
Benzoic acid is detected in car exhaust gases, presumably as an
oxidation product of toluene (Kawamura et al., 1985), and in Japanese
cigarettes (12 and 28 µg per cigarette in mainstream and sidestream
smoke, respectively; Sakuma et al., 1983). It can also be produced
through the photochemical degradation of benzoic acid esters used as
fragrance ingredients (Shibamoto & Umano, 1985; Shibamoto, 1986).
Benzoic acid has been detected in wastewater from the wood production
industry in Norway and Sweden (Carlberg et al., 1986; Lindström &
Österberg, 1986) and in foundry waste leachates (Ham et al., 1989), as
well as in extracts of fly ash from municipal incinerators
(Tong et al., 1984).
4.2.2 Sodium benzoate
Sodium benzoate is produced by the neutralization of benzoic acid
with sodium hydroxide. Worldwide sodium benzoate production in 1997
can be estimated at about 55 000-60 000 tonnes (Srour, 1998). The
largest producers are the Netherlands, Estonia, the USA, and China.
4.3 Uses
4.3.1 Benzoic acid
In 1988, of the benzoic acid produced in Europe, about 60% was
further processed to phenol and 30% to caprolactam (for nylon fibres).
Five per cent was used for the production of sodium and other
benzoates, 3% for benzoyl chloride, and the rest for alkyd resins,
benzoate esters, such as methyl benzoate, and various other products
(Srour, 1989). These percentages are still approximately correct today
(Srour, 1998). Caprolactam seems to be produced only by European
companies (Srour, 1998).
Benzoic acid is increasingly used in the production of diethylene
and dipropylene glycol dibenzoate plasticizers in adhesive
formulations (about 40 000 tonnes in 1997). It is also used to improve
the properties of alkyd resins for paints and coatings and as a "down
hole" drilling mud additive in secondary oil production. Its use as a
rubber polymerization retarder is diminishing (Srour, 1998).
Benzoic acid and sodium benzoate (see section 4.3.2) are used as
preservatives in beverages, fruit products, chemically leavened baked
goods, and condiments, preferably in a pH range below 4.5.
A disadvantage is the off-flavour they may impart to foods (Chipley,
1983). Owing to their inhibitory effect on yeast, they cannot be used
in yeast-leavened products (Friedman & Greenwald, 1994). Examples of
upper concentrations allowed in food are up to 0.1% benzoic acid (USA)
and between 0.15% and 0.25% (other countries) (Chipley, 1983). The
European Commission limits for benzoic acid and sodium benzoate are
0.015-0.5% (EC, 1995).
Benzoic acid and its salts and esters are found in 11 of 48 (23%)
toothpastes (Sainio & Kanerva, 1995) to a maximum of 0.5% (Ishida,
1996) and in mouthwashes and dentifrices. Benzoic acid is also used in
cosmetics (in creams and lotions with pH values under 4, up to 0.5%)
(Wallhäusser, 1984). Sixteen out of 71 deodorants tested contained
benzoic acid (Rastogi et al., 1998).
Benzoic acid is a breakdown product of benzoyl peroxide, which is
used as an additive at levels of between 0.015% and 0.075% to bleach
flour (Friedman & Greenwald, 1994) and in dermatological antifungal
preparations (BMA, 1998). Benzoic acid is reported to leach from
denture-base acrylic resins, where benzoyl peroxide is added as a
polymerization initiator (Koda et al., 1989, 1990).
Benzoic acid can be used in combination with salicylic acid
(Whitfield's ointment) as a fungicidal treatment for ringworm (BMA,
1998).
4.3.2 Sodium benzoate
Although undissociated benzoic acid is the more effective
antimicrobial agent for preservation purposes, sodium benzoate is used
preferably, as it is about 200 times more soluble than benzoic acid.
About 0.1% is usually sufficient to preserve a product that has been
properly prepared and adjusted to pH 4.5 or below (Chipley, 1983).
A major market for sodium benzoate is as a preservative in the
soft drink industry, as a result of the demand for high-fructose corn
syrup in carbonated beverages. Sodium benzoate is also widely used as
a preservative in pickles, sauces, and fruit juices (Srour, 1998).
Benzoic acid and sodium benzoate are used as antimicrobial agents in
edible coatings (Baldwin et al., 1995).
Sodium benzoate is also used in pharmaceuticals for preservation
purposes (up to 1.0% in liquid medicines) and for therapeutic regimens
in the treatment of patients with urea cycle enzymopathies
(see section 9).
Possibly the largest use of sodium benzoate, accounting for
30-35% of the total demand (about 15 000 tonnes of benzoic acid), is
as an anticorrosive, particularly as an additive to automotive engine
antifreeze coolants and in other waterborne systems (Scholz &
Kortmann, 1991; Srour, 1998). A new use is the formulation of sodium
benzoate into plastics such as polypropylene, to improve strength and
clarity (BFGoodrich Kalama Inc., 1999). Sodium benzoate is used as a
stabilizer in photographic baths/processing (BUA, 1995).
4.4 Estimated global release
From data provided by the German producers, emissions of benzoic
acid from industrial processes were less than 525 kg per year into the
atmosphere, less than 3 tonnes per year into the River Rhine, and
8 tonnes per year into sewage or water purification plants (BUA,
1995). No data were available from other countries.
Other anthropogenic releases of benzoic acid and sodium benzoate
into the environment are emissions into water and soil from their uses
as preservatives in food, toothpastes, mouthwashes, dentifrices, and
cosmetics. There were no data available on the emission of benzoic
acid from the disposal of antifreeze mixtures and waterborne cooling
systems and other miscellaneous industrial uses.
The amount of benzoic acid emitted to air from car exhaust gases
as an oxidation product is not quantifiable from the available data.
5. ENVIRONMENTAL TRANSPORT, DISTRIBUTION, TRANSFORMATION,
AND ACCUMULATION
5.1 Transport and distribution between media
5.1.1 Benzoic acid
From its use pattern (see section 4), it can be expected that
benzoic acid is released to surface waters and (from dumping sites) to
leaching water (and groundwater). Minor amounts are expected to be
emitted to the atmosphere. From its physicochemical properties (vapour
pressure, Henry's law constant; see section 2), a significant
volatilization of benzoic acid from water or soil is not expected.
Owing to its solubility in water (see section 2), wet deposition from
air may occur. Experimental data on wet and dry deposition from air
are not available.
5.1.2 Sodium benzoate
No information on the environmental transport and distribution of
sodium benzoate could be identified. Owing to its use pattern, which
is similar to that of benzoic acid, most of the amounts released to
the environment are also expected to be emitted to aquatic
compartments (e.g., surface waters).
5.2 Transformation
5.2.1 Benzoic acid
The experimental determination of the photodegradation of benzoic
acid in aqueous solution (25°C; lambda = 240-300 nm) in terms of
quantum yield (average number of photons absorbed) resulted in very
low values -- in the order of 6 × 10-2 mol/einstein1 (Oussi et
al., 1998). However, benzoic acid adsorbed on silica gel (SiO2) and
irradiated with UV light (lambda > 290 nm) for 17 h showed 10.2%
photodegradation (Freitag et al., 1985). This may be due to a
photocatalytic effect, which was also observed with other oxides,
notably zinc oxide (ZnO) and titanium dioxide (TiO2). When benzoic
acid was irradiated with sunlight in aqueous suspensions of zinc or
titanium dioxide, 67% (after 2-3 h) or 90% (after 24 h) of the applied
amount was mineralized (Kinney & Ivanuski, 1969; Matthews, 1990).
1 An einstein is a unit of light energy used in photochemistry,
equal to Avogadro's number times the energy of one photon of light
of the frequency in question.
Indirect photolysis by reaction with hydroxyl radicals is
expected to be low. Hydroxyl radical rate constants (kOH) for
benzoic acid and its anion have been estimated to be approximately
0.5 × 10-12 and 2 × 10-12 cm3/s, respectively (Palm et al., 1998).
Standardized tests on ready (MITI, 1992) or inherent (Zahn &
Wellens, 1980) biodegradation showed benzoic acid to be readily
biodegraded. The degrees of aerobic degradation were as follows:
MITI I 85% (100 mg/litre; (MITI, 1992)
test 2 weeks; OECD
No. 301C)
Zahn-Wellens >90% (508 mg/litre; (Zahn & Wellens,
test 2 days) 1980)
Easy degradation of benzoic acid to methane and carbon dioxide
was also observed in different non-standardized experiments using
sewage sludge as inoculum (BUA, 1995). Benzoic acid was found to be
degraded by adapted anaerobic sewage sludge at 86-93% after 14 days
(Nottingham & Hungate, 1969), by aerobic activated sludge (adapted) at
>95% after 5-20 days (Pitter, 1976; Lund & Rodriguez, 1984), and by
unadapted aerobic activated sludge at 61-69% after 2-3 days with a
preceding lag time of 2-20 h (Urano & Kato, 1986). The use of a
synthetic sewage inoculated with laboratory bacterial cultures led to
complete degradation of benzoic acid after 14 days under anaerobic
conditions (Kameya et al., 1995).
A greater variability in degradation (0-100%) was seen in tests
using environmental matrices (e.g., rain, lake water, seawater, soil,
etc.). It depended mainly on substance concentration and time for
acclimation (see Table 1). Test durations exceeding 2 days resulted in
removal of >40% when initial concentrations were below 20 mg/litre.
A rapid mineralization occurred in groundwater and subsurface soil
samples. In groundwater, a half-life of 41 h has been found for
benzoic acid (initial concentration 1-100 µg/litre; metabolized to
14CO2) under aerobic conditions (Ventullo & Larson, 1985).
Half-lives of 7.3 h and 18.2 h, respectively, have been observed for
aerobic and anaerobic degradation of benzoic acid (initial
concentration 1 mg/kg dry weight; metabolized to 14CO2) in
subsurface soils of septic tank tile fields (Ward, 1985). Anaerobic
degradation of benzoic acid (initial concentration 250 mg
carbon/litre) in a methanogenic microcosm (consisting of aquifer
solids and groundwater) required 4 weeks of adaptation, followed by
nearly complete depletion after 8 weeks of incubation (Suflita &
Concannon, 1995).
Several isolated microorganisms have been shown to utilize (and
therefore probably degrade) benzoic acid under aerobic or anaerobic
conditions. They include, among others, fungal species such as
Rhodotorula glutinis and other yeast-like fungi (Kocwa-Haluch &
Lemek, 1995), the mould Penicillium frequentans (Hofrichter &
Fritsche, 1996), and bacteria, such as Alcaligenes denitrificans
(Miguez et al., 1995), Rhodopseudomonas palustris, several strains
of denitrifying pseudomonads (Fuchs et al., 1993; Elder & Kelly, 1994;
Harwood & Gibson, 1997), and Desulfomicrobium escambiense
(Sharak Genthner et al., 1997).
Although benzoic acid is primarily metabolized to hippuric acid
in rats (see section 7), some other species do excrete other
metabolites, such as dibenzoylornithine (hen), benzoylglutamic acid
(Indian fruit bat), benzoylarginine (tick, insects), or benzoyltaurine
(southern flounder, Paralichthys lethostigma) (Parke, 1968; Goodwin,
1976; James & Pritchard, 1987).
5.2.2 Sodium benzoate
Experimental data on photodegradation of sodium benzoate are not
available. As with benzoic acid, photolysis in aqueous solution is
assumed to be unlikely with regard to its known UV spectra
(Palm et al., 1998). Indirect photolysis by reaction with hydroxyl
radicals plays only a minor role, with estimated and measured hydroxyl
rate constants of about 0.33 × 10-11 cm3/s
(Palm et al., 1998).
Sodium benzoate was readily biodegradable under aerobic
conditions in several standard test systems:
Modified 84% (100 mg/litre; (King & Painter,
MITI test 10 days) 1983)
Modified 80-90% (50 mg/litre; (Salanitro et al.,
Sturm test 7 days) 1988)
Closed bottle 75-111% (5 mg/litre; (Richterich &
test 30 days) Steber, 1989)
Degradation assays using seawater as test medium ("natural
water") or as inoculum (marine filter material given into a synthetic
marine medium) according to an adapted Organisation for Economic
Co-operation and Development (OECD) guideline (301B) resulted in a
degradation of 85% and 97%, respectively (10 mg/litre; carbon dioxide
measurement; 28 days) (Courtes et al., 1995).
Anaerobic mineralization of sodium benzoate (50-90 mg/litre) by
domestic sewage sludge varied from 50% to 96.5% (measurement of carbon
dioxide and methane; 28-61 days) (Birch et al., 1989). In another
study using anaerobic sludge from sewage works receiving a mixture of
domestic and industrial wastewaters, 93% mineralization was observed
after 1 week of incubation (measurement of carbon dioxide and methane;
initial concentration 50 mg carbon/litre) (Battersby & Wilson, 1989).
Table 1: Removal of benzoic acid in freshwater, marine, and soil matrices.
Matrix Initial Conditions Duration Removal Measured Reference
concentration (days) (%) parameter
(mg/litre
or mg/kg)
Rainwater 0.001 22°C; shaking 2 0 benzoic acid Kawamura &
once per day; dark 7 40 Kaplan (1990)
45 100
Lake water 0.059 29°C; 7 98.7 14C (in CO2, Rubin et al.
(eutrophic/ no shaking; dark biomass) (1982)
mesotrophic)
Seawater 20°C; dark; 14C (in Shimp &
(estuary) rotary shaking CO2, biomass) Young (1987)
USA 20 30 <10
0.005 8 70-80
Canada 20 16 60
0.005 10 >70
Seawater 2 5 75 BODa Takemoto
et al. (1981)
Soil 20 2 mg benzoic acid 70 63 14CO2 Haider et al.
(grey soil, in 0.1 ml acetone (1974)
alkaline) + 100 g soil
+ 10 ml H2O
Soil 0.05 24°C; 20-25% 15 40 14CO2 Federle (1988)
(sand; moisture content
18.9 m depth)
a BOD = biological oxygen demand.
Benzoate-acclimated sludges were reported to be capable of completely
degrading benzoate concentrations of 3000 mg/litre within 5-7 days
(Kobayashi et al., 1989).
5.3 Accumulation
5.3.1 Benzoic acid
The n-octanol/water partition coefficient (log Kow) of 1.9
(see section 2) indicates a low potential for bioaccumulation.
Consistently, measured bioconcentration factors (BCFs) found in
aquatic biota were low. BCFs of <10 (based on wet weight) have been
determined for fish (golden ide, Leuciscus idus melanotus) and green
algae (Chlorella fusca) after 3 and 1 days, respectively
(Freitag et al., 1985). A 6-day BCF of 7.6 has been reported for
another green alga (Selenastrum capricornutum) (Mailhot, 1987),
and a 5-day BCF of 1300 (based on dry weight) in activated sludge
(Freitag et al., 1985). The following 24-h bioaccumulation factors
(focusing on uptake via medium plus feed within food chain members)
have been obtained in aquatic model ecosystems operated with
0.01-0.1 mg of radiolabelled benzoic acid per litre: 21 (mosquitofish,
Gambusia affinis), 102 (green alga, Oedogonium cardiacum),
138 (mosquito larvae, Culex quinquifasciatus), 1772 (water flea,
Daphnia magna), and 2786 (snail, Physa sp.). Except for
Daphnia and snail, the values were low (Lu & Metcalf, 1975).
However, the very low exposure concentrations could likely have
resulted in the calculation of the high BCF values, even with moderate
uptake. Moreover, because this was a radiolabel study, it remains
unclear if the label was still the parent compound.
Geoaccumulation of benzoic acid has also been found to be low.
Depending on soil depth, sorption coefficients (Kd) of 0.62 (18.9
m) to 1.92 (0.4 m) have been measured (Federle, 1988). Mobility
determinations of 14C-labelled benzoic acid in different soils by
means of thin-layer chromatography showed benzoic acid to be
moderately mobile. Its mobility was positively correlated with soil pH
and negatively correlated with aluminium and iron contents and
effective anion exchange capacity (Stolpe et al., 1993).
5.3.2 Sodium benzoate
No experimental data on bioaccumulation or geoaccumulation of
sodium benzoate have been identified. From the information on benzoic
acid, a significant potential for accumulation is not to be expected.
6. ENVIRONMENTAL LEVELS AND HUMAN EXPOSURE
6.1 Environmental levels
Generally, benzoic acid can occur in almost all environmental
compartments. Whether it exists in the undissociated or dissociated
form depends on the specific physicochemical conditions. Above pH 6,
the benzoate anion prevails (Chipley, 1983).
There is a series of reports on positive qualitative analyses of
benzoic acid in various environmental media, such as air (Belgium:
Cautreels & van Cauwenberghe, 1978; Germany: Helmig et al., 1989),
rain or snow (Norway: Lunde et al., 1977; Germany: Winkeler et al.,
1988), surface waters (Norway, river: Schou & Krane, 1981), and soils
(United Kingdom, heathland soil: Jalal & Read, 1983; Germany, river
terrace soil: Cordt & Kußmaul, 1990), but these do not provide
quantitative measurements.
Semiquantitative measurements of concentrations of benzoic acid
in urban air in Pasadena, California (USA) were in the range of
0.09-0.38 µg/m3 (Schuetzle et al., 1975). This was comparable to
quantitative measurements performed in 1984 in Los Angeles, California
(USA), which resulted in atmospheric concentrations of 0.005-0.13
µg/m3 (n = 8) (Kawamura et al., 1985). Most of the quantitative
data compiled in Table 2 with respect to water samples refer to
concentrations of benzoic acid in groundwater, with a maximum of 27.5
mg/litre measured in the vicinity of a point source.
Benzoic acid occurs naturally in free and bound form in many
plant and animal species. It is a common metabolite in plants and
organisms (Hegnauer, 1992). Appreciable amounts have been found in gum
benzoin (around 20%) and most berries (around 0.05%) (Budavari et al.,
1996). For example, ripe fruits of several Vaccinium species (e.g.,
cranberry, V. vitis idaea; bilberry, V. macrocarpon) contain as
much as 300-1300 mg free benzoic acid per kg fruit (Hegnauer, 1966).
Benzoic acid is also formed in apples after infection with the fungus
Nectria galligena (Harborne, 1983) or in Pinus thunbergii callus
inoculated with a pathogenic pine wood nematode (Bursaphelenchus
xylophilus) (Zhang et al., 1997). Among animals, benzoic acid has
been identified primarily in omnivorous or phytophageous species,
e.g., in viscera and muscles of the ptarmigan (Lagopus mutus)
(Hegnauer, 1989) as well as in gland secretions of male muskoxen
(Ovibos moschatus) (Flood et al., 1989) or Asian bull elephants
(Elephas maximus) (Rasmussen et al., 1990).
Owing to its occurrence in many organisms, benzoic acid is
naturally present in foods (review in Sieber et al., 1989, 1990). Some
typical examples specifying reported ranges of means in selected foods
have been compiled from Sieber et al. (1989) as follows:
Milk traces - 6 mg/kg
Yoghurt 12-40 mg/kg
Cheese traces - 40 mg/kg
Fruits (excluding traces - 14 mg/kg
Vaccinium species)
Potatoes, beans, cereals traces - 0.2 mg/kg
Soya flour, nuts 1.2-11 mg/kg
Honeys from different floral sources (n = 7) were found to
contain free benzoic acid at concentrations of <10 mg/kg (n = 5)
and of <100 mg/kg (n = 2) (Steeg & Montag, 1987).
Because benzoic acid and its compounds are used as food
preservatives (see section 4), some processed foods contain
artificially elevated concentrations of these substances (see section
6.2).
6.2 Human exposure
The main route of exposure of the general population to benzoic
acid or sodium benzoate is likely via foodstuffs that contain the
substances naturally or added as antimicrobial agents. There are a few
analyses of processed foodstuffs available. They refer to different
types of food items (juice, soft drinks, soya sauce varieties) from
the Philippines (a total of 44 samples) and from Japan (a total of 31
samples) and to orange drinks sampled in England. The concentrations
of sodium benzoate in the Philippine dietary samples ranged from 20 to
>2000 mg/litre. The range in the Japanese products was 50-200
mg/litre, thus reflecting the lower maximum level of sodium benzoate
allowed to be added to food in Japan as compared with the Philippines
(Villanueva et al., 1994). Orange drinks from England contained sodium
benzoate at concentrations ranging from 54 to 100 mg/litre (mean 76.7
mg/litre; n = 6) (Freedman, 1977).
Generally, the actual uptake depends on the individual's choice
of food to be consumed and the different limit values in different
countries. Several intake estimations have been published. Three
Japanese studies reported average daily intakes of benzoic acid from
processed foodstuffs to be 10.9 mg per person (Toyoda et al., 1983a)
and 1.4 mg per person (Toyoda et al., 1983b; Yomota et al., 1988),
corresponding to 0.02-0.2 mg/kg body weight (for persons with a body
weight of 50-70 kg). Both of the latter studies used the market basket
method for intake calculations, whereas the first-mentioned study
calculated intakes using the results of a national nutrition survey.
The concentrations of benzoic acid in 3319 food samples analysed for
this study (Toyoda et al., 1983a) ranged from not detected to 2100
mg/kg food. The maximum was found in salted fish (n = 7; mean 754
mg/kg). Another survey refers to the United Kingdom, where analyses of
benzoic acid in foods and drinks in which it is permitted as well as
intake estimates have been performed (UK MAFF, 1995). Sixty-five out
of 122 samples tested contained detectable benzoic acid. The highest
Table 2: Concentrations of benzoic acid in rain, snow, groundwater, and leachate samples.
Medium Location; sampling date Concentration Reference
(µg/litre)
Los Angeles area, California, Sum concentrationsa Kawamura & Kaplan
USA; 1982-1983 (1986)
Rain: urban 0.06-10.2 (n = 6)
Rain: semirural 0.02 (n = 1)
Snow: rural 0.04-0.1 (n = 3)
Groundwater Wyoming, USA (near underground 16-860 (n = 3) Stuermer et al.
coal gasification site; 15 months (1982)
after the end of gasification)
Groundwater Florida, USA (near wood treatment 10-27 500 (n = 3) Goerlitz et al.
facility); 1984 (1985)
Groundwater Ontario, Canada (near traces (n = 2) Barker et al.
landfillb); 1983 (1988)
Groundwater Barcelona area, Spain up to 0.21 (n = 3) Guardiola et al.
(near landfillb) (1989)
Leachate Ontario, Canada; 1981 <0.1->1000 (n = 5) Reinhard & Goodman
(from landfillb) (1984)
Leachate Ontario, Canada; 1983 traces (n = 2) Barker et al.
(from landfillb) (1988)
Leachate USA; 1986-1988 200-400c (n = 3) Ham et al.
(from foundry (1989)
wastes)
a Including benzoic acid, 3-methyl benzoic acid, and 4-methyl benzoic acid.
b Receiving rural, municipal (domestic), and industrial wastes.
c Concentrations estimated from gas chromatography/mass spectrometry data.
concentrations were found in sauces (mean 388 mg/kg; n = 20; range
71-948 mg/kg), reduced sugar jam (mean 216 mg/kg; n = 4; range
<20-333 mg/kg), non-alcoholic drinks (mean 162 mg/kg; n = 20; range
55-251 mg/kg), and semipreserved fish product (653 mg/kg; n = 1).
The survey found that the concentrations of benzoic acid detected
would lead to a dietary intake below 5 mg/kg body weight per day, even
for adults with an above-average consumption.
A frequent contributor to dietary exposure is soft drinks. A
rough estimation based on the average daily consumption in Germany of
such drinks (372 ml non-alcoholic beverages, excluding bottled water;
BAGS, 1995) by 19- to 24-year-old men, assuming the concentration of
benzoic acid present corresponds to a maximum allowable level of
150 mg/litre (EC, 1995), would result in a mean daily intake of
55.8 mg benzoic acid per person (or 0.80 mg/kg body weight, assuming
a 70-kg body weight). For comparison, a similar calculation with
sugar-free marmalade, jam, and similar spreads, which are allowed to
contain higher levels of benzoic acid (500 mg/kg; EC, 1995), would
result in a possible intake of 4.1 mg per person per day, or
0.06 mg/kg body weight per day (assumes a daily consumption of 8.2 g,
according to BAGS, 1995). This was more than a possible intake via
fruits containing natural benzoic acid. For example, a daily
consumption of 40.4 g of fruits (BAGS, 1995) would lead to a possible
intake of 0.57 mg benzoic acid per person per day (or 0.008 mg/kg body
weight for a 70-kg person), if the reported maximum of 14 mg benzoic
acid/kg (see section 6.1) were present.
The Joint FAO/WHO Expert Committee on Food Additives (JECFA)
assessed the intake of benzoates from information provided by nine
countries (Australia, China, Finland, France, Japan, New Zealand,
Spain, United Kingdom, and USA) (WHO, 1999). Because diets differ
among countries, the foods that contribute to benzoate intake would be
expected to vary. The food category that contributed most to benzoate
intake was soft drinks (carbonated, water-based, flavoured drinks) for
Australia/New Zealand, France, the United Kingdom, and the USA. In
Finland, 40% was in soft drinks. Soya sauce was the main source of
benzoate in China and the second most important in Japan. The best
estimates of national mean intakes of benzoates by consumers ranged
from 0.18 mg/kg body weight per day in Japan to 2.3 mg/kg body weight
per day in the USA. These estimates were based on analyses involving
either model diets or individual dietary records and maximum limits
specified by national governments or the European Union. The estimated
intake by high consumers of benzoates, based on food additive levels
in national standards, was 7.3 mg/kg body weight per day in the USA
and 14 mg/kg body weight per day in China.
Benzoates have been detected in groundwater, but not in
drinking-water.
Quantitative information on (oral or dermal/mucosal) exposure via
cosmetic, hygienic, or medical products is rare, but the data
available indicate a remarkable contribution to exposure. There are
reports on leaching of benzoic acid from denture-base acrylic resins.
After 10 days of immersion in artificial saliva, concentrations of up
to about 3 mg/litre have been observed for benzoic acid, which is
formed as a degradation product of the benzoyl peroxide that is added
as a polymerization initiator (Koda et al., 1989, 1990). In Japan,
commercial toothpastes have been found to contain benzoic acid at
concentrations ranging from 800 to 4450 mg/kg (n = 18). Use of the
toothpaste with the highest concentration (by 40 20-year-old female
students) would result in a calculated daily intake of about 2.23 mg
per person. This was about the same amount as their estimated intake
from diet (Ishida, 1996). Benzoic acid is also used in dermatology as
a fungicidal topical treatment for ringworm (Tinea spp.). The
emulsifying ointment preparation contains benzoic acid at 6% and is
applied twice daily (Goodman et al., 1990; BMA, 1998).
Recent quantitative monitoring data on concentrations of benzoic
acid or salts in ambient or indoor air are not available. Considering
the few (low) levels of benzoic acid measured in urban air in the
past, with a maximum of 0.38 µg/m3 (see section 6.1), inhalation may
contribute only marginally to exposure of the general population.
Using this maximum, a daily inhalative dose of 8.74 µg per person (or
0.12 µg/kg body weight) is obtained (assuming a daily inhalation
volume of 23 m3 for a 70-kg adult male; WHO, 1994).
Few quantitative data on occupational exposure have been
identified. Nevertheless, there is a potential for inhalation or
dermal contact in the chemical and allied product industries as well
as in workplaces where these products are used. Air samples (n = 50)
collected in an industrial environment (no further details given) over
a year's time showed benzoic acid concentrations ranging from not
detected to 1.5 mg/m3 (Halvorson, 1984). On the basis of the latter
value, an inhalative dose of 14.4 mg per person per 8-h working time
(or 0.2 mg/kg body weight) would result (assuming an inhalation volume
of 9.6 m3 for an 8-h exposure with light activity; WHO, 1994).
However, because of the lack of information on specific working
operations and conditions involved (e.g., duration of exposure, use of
protective clothes, etc.), it is impossible to derive a realistic
estimate of occupational exposure.
7. COMPARATIVE KINETICS AND METABOLISM IN LABORATORY ANIMALS
AND HUMANS
After oral ingestion of benzoic acid and sodium benzoate, there
is a rapid absorption (of undissociated benzoic acid) from the
gastrointestinal tract in experimental animals or humans (US FDA,
1972a, 1973). From the figures on excretion given below, 100%
absorption can be assumed. In humans, the peak plasma concentration is
reached within 1-2 h (Kubota et al., 1988; Kubota & Ishizaki, 1991).
Benzoic acid is not completely absorbed by the dermal route. In a
study with six human subjects, Feldmann & Maibach (1970) found an
uptake of 36% of the applied dose (14C-labelled benzoic acid
dissolved in acetone; 4 µg/cm2; circular area of 13 cm2; ventral
surface of the forearm; non-occlusive) within 12 h. The total uptake
within 5 days was 43%. In a second study with 6-7 subjects (comparable
method; application of 3, 400 or 2000 µg/cm2), the percent
absorption decreased from 35% to 14% within 24 h. However, the total
uptake per cm2 increased from 1 to 288 µg (Wester & Maibach, 1976).
For sodium benzoate, no data concerning dermal uptake were identified
in the literature.
In vivo dermal studies with benzoic acid in experimental
animals (e.g., guinea-pigs, mice, rats, pigs, dogs, rhesus monkeys)
confirm the results with humans (Hunziker et al., 1978;
Andersen et al., 1980; Wester & Noonan, 1980; Bronaugh et al., 1982a;
Reifenrath et al., 1984; Carver & Riviere, 1989; Maibach & Wester,
1989; Bucks et al., 1990). Absorption ranged from 25% in pigs
(Reifenrath et al., 1984; Carver & Riviere, 1989) to 89% in rhesus
monkeys (Wester & Noonan, 1980; Maibach & Wester, 1989; Bucks et al.,
1990). Due to the good database on humans and animals in vivo,
in vitro studies performed with animal or human skin are not
considered further (Franz, 1975; Bronaugh et al., 1982b;
Hotchkiss et al., 1992; MacPherson et al., 1996).
No information is available on absorption via inhalation.
After oral and dermal uptake, benzoate is metabolized in the
liver by conjugation with glycine, resulting in the formation of
hippuric acid (Feldmann & Maibach, 1970; US FDA, 1972a; WHO, 1996;
Feillet & Leonard, 1998). The rate of biotransformation in humans is
high: after oral doses of 40, 80 or 160 mg sodium benzoate/kg body
weight, the transformation to hippuric acid was independent of the
dose -- about 17-29 mg/kg body weight per hour, corresponding to about
500 mg/kg body weight per day (Kubota & Ishizaki, 1991). Other authors
obtained higher values of 0.8-2 g/kg body weight per day (US FDA,
1972a, 1973; WHO, 1996). Hippuric acid is rapidly excreted in urine.
In humans, after oral doses of up to 160 mg/kg body weight, 75-100% of
the applied dose is excreted as hippuric acid within 6 h after
administration, and the rest within 2-3 days (Kubota et al., 1988;
Fujii et al., 1991; Kubota & Ishizaki, 1991).
The limiting factor in the biosynthesis of hippuric acid is the
availability of glycine. The utilization of glycine in the
detoxification of benzoate results in a reduction in the glycine level
of the body. Therefore, the ingestion of benzoic acid or its salts
affects any body function or metabolic process in which glycine is
involved; for example, it leads to a reduction in creatinine,
glutamine, urea, and uric acid levels (US FDA, 1972a, 1973; Kubota &
Ishizaki, 1991; WHO, 1996).
Another metabolite of benzoate is the benzoyl glucuronide. For
example, the dog excretes considerable amounts of this metabolite in
the urine (20% after a single dose of 50 mg/kg body weight; Bridges et
al., 1970). In other species, this metabolite appears only after
higher doses of about 500 mg/kg body weight (see above) of benzoic
acid or sodium benzoate, resulting in a depletion of the glycine pool
(Bridges et al., 1970; US FDA, 1972a; Kubota et al., 1988). In cats,
glucuronidation is generally very low (Williams, 1967).
In some species, including humans, minor amounts of benzoic acid
itself are also excreted in the urine (Bridges et al., 1970; Kubota &
Ishizaki, 1991).
Experiments on the distribution and elimination of 14C-benzoate
in the rat have shown no accumulation of sodium benzoate or benzoic
acid in the body (US FDA, 1972a, 1973).
In the acid conditions of the stomach, the equilibrium moves to
the undissociated benzoic acid molecule, which should be absorbed
rapidly. Benzoate from sodium benzoate would change from the ionized
form to the undissociated benzoic acid molecule. As a result, the
metabolism and systemic effects of benzoic acid and sodium benzoate
can be evaluated together.
7.1 Precursors of benzoic acid
Benzyl acetate, its hydrolysis product, benzyl alcohol, and the
oxidation product of this alcohol, benzaldehyde, are precursors of
benzoic acid in experimental animals and humans. Benzyl acetate is
metabolized to benzoic acid and further to hippuric acid and benzoyl
glucuronide to an extent of >90% both in mice and in rats of
different strains. Benzyl alcohol was metabolized to benzoic acid and
its conjugates in preterm infants. Benzaldehyde is metabolized to
benzoic acid and its conjugates in rabbits to an extent of
approximately 90% (WHO, 1996).
8. EFFECTS ON LABORATORY MAMMALS AND IN VITRO TEST SYSTEMS
8.1 Single exposure
With oral LD50 values (administration by gavage) of 3040 mg
benzoic acid/kg body weight in rats (Bio-Fax, 1973) and 1940-2263 mg
benzoic acid/kg body weight in mice (McCormick, 1974; Abe et al.,
1984), the acute toxicity of benzoic acid is low. Clinical signs of
intoxication (reported for rats only) included diarrhoea, muscular
weakness, tremors, hypoactivity, and emaciation (Bio-Fax, 1973). With
oral LD50 values of 2100-4070 mg sodium benzoate/kg body weight in
rats, the acute toxicity of sodium benzoate is similar to that of
benzoic acid, as are the symptoms (Smyth & Carpenter, 1948;
Deuel et al., 1954; Bayer AG, 1977).
In four cats given diets containing 0 or 1% benzoic acid
(approximately 0 or 450-890 mg/kg body weight), aggression,
hyperaesthesia, and collapse starting 14-16 h after feed uptake were
seen at a dose level equal to 630 mg/kg body weight. The duration of
the syndrome was about 18-176 h, and the mortality rate was 50%. The
histopathological examination of the two cats that died revealed
degenerative changes in liver, kidneys, and lung, but no pathological
findings in brain or spinal cord (Bedford & Clarke, 1972). The authors
attributed the higher toxicity of benzoic acid in cats compared with
other species to the low capacity of cats for glucuronidation
(see section 7).
In rats, exposure by inhalation to 26 mg/m3 over 1 h caused no
mortality, but generalized inactivity and lacrimation were noted. The
gross autopsy gave no significant findings (no further information
available; Bio-Fax, 1973).
In a limit test with rabbits, no mortality or signs of
intoxication were seen after dermal application of 10 000 mg/kg body
weight. The gross autopsy gave no significant findings (no further
information available; Bio-Fax, 1973).
8.2 Irritation and sensitization
8.2.1 Benzoic acid
Although there is a wide range of results from mostly
non-standardized tests using various scoring systems, it can be
concluded that benzoic acid is slightly irritating to the skin and
irritating to the eyes.
In different experiments with rabbits, which have not been
performed according to current guidelines, benzoic acid applied as dry
powder or in the form of a paste was not irritating to slightly
irritating to the skin (score 1.66/8: Bio-Fax, 1973; no score given:
Bayer AG, 1978; primary skin irritation index 0.5 [no further
information available]: RCC Notox, 1988a).
In an acute eye irritation/corrosion study with rabbits conducted
according to OECD Guideline 405, some eye irritation was reported
after application of benzoic acid in the form of a paste. Within 72 h,
the scores for chemosis, reddening of the conjunctivae, iritis, and
keratitis always remained at <2 (Bayer AG, 1986).
In different non-standardized experiments with the solid
substance, moderately irritating to severely irritating effects on the
eye were noted (score 65/110: Bio-Fax, 1973; no score given: Bayer AG,
1978; score up to 108/110 [eyes rinsed after instillation] or up to
50/100 [eyes not rinsed]: Monsanto Co., 1983; score 35 according to
the scheme of Kay & Calandra, 1962: RCC Notox, 1988b).
In a maximization test, none of 15 guinea-pigs reacted positively
after induction and challenge with a 10-20% solution of benzoic acid
in water (Gad et al., 1986). In addition, the substance also tested
negative in a Buehler test with guinea-pigs and in an ear swelling
test and local lymph node assay with mice (Gad et al., 1986; Gerberick
et al., 1992). The concentrations used for induction and challenge
were 10-20% in acetone or water.
However, a dose-dependent positive result was obtained in an ear
swelling test with five guinea-pigs (induction with 0.2, 1, 5, or 20%
in absolute ethyl alcohol; no challenge) used as a model for detecting
agents causing non-immunological contact urticaria in humans. At
several other regions (back, abdomen, flank site), a concentration of
20% failed to produce any reactions (Lahti & Maibach, 1984).
8.2.2 Sodium benzoate
An acute dermal irritation/corrosion study with rabbits conducted
according to OECD Guideline 404 (no data about physical state; score
0: RCC Notox, n.d., a) as well as a non-standardized experiment with
the solid substance (score not given: Bayer AG, 1977) gave no
indication for skin irritating effects.
In a study performed according to OECD Guideline 405 (no data
about physical state; RCC Notox, n.d., b), sodium benzoate was only
slightly irritating to the eye (score 9.3, according to the scheme of
Kay & Calandra, 1962). The application of the solid substance in a
non-standardized experiment caused no irritation (score not given:
Bayer AG, 1977).
For sodium benzoate, no data on sensitizing effects were
identified in the available literature.
8.3 Short-term exposure
8.3.1 Oral exposure
In general, the database for benzoic acid and sodium benzoate is
limited, and there are no studies available performed according to
current guidelines. In addition, the documentation of these studies in
most cases is insufficient. Detailed information is given in Table 3.
From the available studies, it can be assumed that the toxicity
of benzoic acid after short-term oral exposure is low. In high-dosed
rats given approximately 2250 mg/kg body weight per day via diet over
5 days, excitation, ataxia, convulsions, and histopathological changes
in the brain were seen. The mortality was about 50%; in some cases,
bleeding into the gut was noted (Kreis et al., 1967). In two other
studies with rats dosed with approximately 825 mg/kg body weight per
day over 7-35 days (Kreis et al., 1967) or with 65-647 mg/kg body
weight per day over 28 days (Bio-Fax, 1973), no clear
treatment-related effects occurred. The reduced weight gain at 2250
and 825 mg/kg body weight per day may be attributed to reduced food
intake in the study by Kreis et al. (1967). The relevance of the
reduced relative kidney weight at 324 mg/kg body weight per day, which
was not dose-related and not accompanied by changes in
histopathological examinations, is unclear (Bio-Fax, 1973). As given
in Table 3, both studies have several limitations (i.e., missing
haematological and clinical chemical investigations, incomplete
histopathological examinations); therefore, both of these studies were
inadequate for derivation of a NO(A)EL.
More information on dose-response can be gained from the study of
Fujitani (1993), in which rats received sodium benzoate for 10 days in
feed. At the lowest tested concentration of 1358 mg/kg body weight per
day, changes in serum cholesterol levels occurred in females. At doses
of 1568 mg/kg body weight per day and above, changes in further serum
parameters and an increased relative liver weight were described.
Histopathological changes of the liver, increased relative kidney
weights, and disorders of the central nervous system (convulsions)
were seen after dosing via diet with approximately 1800 mg/kg body
weight per day. In several other studies listed in Table 3, adverse
effects were seen only at higher doses after feeding sodium benzoate
over periods from 10 to 42 days, so that a
lowest-observed-(adverse-)effect level (LO(A)EL) of 1358 mg sodium
benzoate/kg body weight per day for short-term exposure can be
derived.
With cats (Bedford & Clarke, 1972), also described in Table 3,
the effect levels with benzoic acid were lower. However, due to the
differences in the metabolism of benzoic acid in cats compared with
other experimental animals and humans, this study was not taken into
further consideration (see section 7).
Table 3: Toxicity of benzoic acid and sodium benzoate after short-term oral exposure.
Species; strain; Treatment Duration Organs examined in Resultsa Reference
number of animals (days) histopathology, clinical
per dosea chemistry, haematology
Benzoic acid
cat; 4 m 0 or 0.5% 3-4 liver, kidney, heart, mild hyperaesthesia, Bedford & Clarke
in diet stomach, lung, brain, apprehension, and (1972)
(approx. 0 spinal cord (only depression starting 48-92
or 300-420 mg/kg animals that died were h after uptake; duration of
body weight) examined); blood samples the syndrome: about 20-48 h;
were taken from surviving mortality rate: 50%;
cats degenerative changes in
liver, kidneys, and lung,
but no pathological findings
in brain or spinal cord;
surviving cats: urea and
serum alanine
aminotransferase (S-ALAT) *,
indicating liver and kidney
damage
cat; 4 m a) 100 or 200 a) 15 only blood samples no adverse effects Bedford & Clarke
mg/kg body weight were taken were reported (1972)
via diet
b) 0 or 0.25% in b) 23
diet (approx. 0
or 130-160 mg/kg
body weight)
Table 3 (cont'd)
Species; strain; Treatment Duration Organs examined in Resultsa Reference
number of animals (days) histopathology, clinical
per dosea chemistry, haematology
rat; Wistar; 0 or 3% in diet 1-5 heart, liver, spleen, body weight gain **; Kreis et al.
5-15 m (approx. 0 or kidney, brain in rats dosed over 5 days, (1967)
2250 mg/kg body disorders of the central
weight) nervous system (excitation,
ataxia, tonoclonic
convulsions); mortality
rate approx. 50%; in some
cases, bleeding into the
gut; brain damage (necrosis
of parenchymal cells of the
stratum granulosum of the
fascia dentata and the
cortex of the lobus
piriformis) in most animals
dosed over 3-5 days (still
present after 35 days)
rat; Wistar; 0 or 1.1% in 7-35 heart, liver, spleen, body weight gain **; Kreis et al.
5-10 m diet kidney, brain no clinical signs of (1967)
(approx. 0 or intoxication
825 mg/kg body
weight)
rat; albino; 10 m 0, 760, 3800, or 28 liver, kidney, no deaths or signs of Bio-Fax (1973)
7600 ppm via diet adrenals, testes intoxication
(approx. 0, 65, 324 mg/kg body
324, or 647 mg/kg weight: relative kidney
body weight) weights **; no further
information available
Table 3 (cont'd)
Species; strain; Treatment Duration Organs examined in Resultsa Reference
number of animals (days) histopathology, clinical
per dosea chemistry, haematology
Sodium benzoate
rat; F344/Ducrj; 0, 1.81, 2.09, or 10 liver, kidney; >1358 mg/kg body weight: Fujitani (1993)
6 m/f 2.4% in diet standard clinical changes in serum levels
(approx. 0, 1358, chemistry (cholesterol ** (f))
1568, or 1800 >1568 mg/kg body weight:
mg/kg body relative liver weight * (m);
weight) changes in serum levels
(albumin * (m), total
protein * (m))
1800 mg/kg body weight:
1/6 males died
(hypersensitivity,
convulsions); body
weight ** (m/f); relative
liver weight * (f); relative
kidney weights * (m/f);
absolute weights of spleen
and thymus ** (m);
absolute/relative weights
of thymus ** (f); changes
in serum levels
(gamma-glutamyltranspeptidase
(GGT) * (m), albumin * (f),
cholinesterase ** (f));
eosinophilic foci around
periportal vein and
enlargement of hepatocytes
with glassy cytoplasm in
the periportal area of the
liver (m); no changes in
the kidney (m)
Table 3 (cont'd)
Species; strain; Treatment Duration Organs examined in Resultsa Reference
number of animals (days) histopathology, clinical
per dosea chemistry, haematology
rat; Sherman; 0, 2, or 5% in 28 no data available 2200 mg/kg body weight: Fanelli & Halliday (1963)
6 m/f diet (approx. 0, slight depression of
2200, or 6700 body weight gain (m)
mg/kg body 6700 mg/kg body weight:
weight) mortality 100% within
11 days; signs of
intoxication included
hyperexcitability, urinary
incontinence, and
convulsions
no further information
available
rat; 28 (no 0 or 5% in diet 28 no data available mortality about 100% within Kieckebusch & Lang
further data) (approx. 0 or 3 weeks; decreased feed (1960)
3750 mg/kg body intake, diarrhoea,
weight) intestinal haemorrhage and
crusted blood in the nose;
no further information
available
rat; 5 (no 0 or 5% in diet >28 no data available mortality 80% within Kieckebusch & Lang
further data) (approx. 0 or 4-5 weeks; decreased (1960)
3750 mg/kg body weight; no further
body weight) information available
Table 3 (cont'd)
Species; strain; Treatment Duration Organs examined in Resultsa Reference
number of animals (days) histopathology, clinical
per dosea chemistry, haematology
rat; F344; 0, 0.5, 1, 2, 4, 42 histopathology performed, >375 mg/kg body weight: Sodemoto & Enomoto
10-11 m/f or 8% in diet but not further specified hypersensitivity after (1980)
(approx. 0, 375, dosing
750, 1500, 3000, >3000 mg/kg body weight:
or 6000 mg/kg mortality about 100% within
body weight) 4 weeks; apart from atrophy
of the spleen and lymph
nodes, no other
morphological changes were
noted
rat; Sherman; 0 or 16-1090 30 adrenals, upper intestine, no adverse effects were Smyth & Carpenter
5 m/f mg/kg body kidney, liver, spleen reported; no further (1948)
weight via diet information available
mouse; B6C3F1; 0, 2.08, 2.5, or 10 liver, kidney; standard „3750 mg/kg body weight: Fujitani (1993)
4-5 m/f 3% in diet clinical chemistry changes in serum levels
(approx. 0, 3000, (cholinesterase * (m))
3750, or 4500 4500 mg/kg body weight:
mg/kg body weight) hypersensitivity in all
animals; convulsions
1/5 males and 2/5 females
(both females died);
absolute/relative liver
weight * (m/f); relative
kidney weight * (f);
changes in serum levels
(cholesterol * (m),
phospholipids * (m));
Table 3 (cont'd)
Species; strain; Treatment Duration Organs examined in Resultsa Reference
number of animals (days) histopathology, clinical
per dosea chemistry, haematology
enlarged hepatocytes,
single cell necrosis
and vacuolation of
hepatocytes in all
livers (m); no changes
in the kidney (m/f)
mouse; albino 0, 0.5, 1, 2, 4, 35 survival, chemical 3000 mg/kg body weight: Toth (1984)
Swiss; 4 m/f or 8% via consumption, histological "suitable for lifelong
drinking-water changes (not further treatment" based on
(approx. specified) (prestudy four parameters: survival,
0-12 000 mg/kg for carcinogenicity study) body weight, chemical
body weight) consumption, and histology
6000 mg/kg body weight:
mortality 75% in m/f;
body weight of surviving
mice ** (m/f)
12 000 mg/kg body weight:
mortality 100% within
3 weeks
a m = male; f = female.
8.3.2 Inhalation exposure
Ten CD rats per sex per group were exposed to 0, 25, 250, or
1200 mg benzoic acid dust aerosol/m3 (analytical concentration; mass
aerodynamic diameter [MAD]/sigma g (standard deviation): 0, 4.6/3.1,
4.4/2.1, 5.2/2.1; mass median aerodynamic diameter [MMAD]: 4.7 µm) for
6 h per day and 5 days per week over 4 weeks. After this time, various
serum biochemical, haematological, organ weight, and histopathological
examinations were conducted. At >25 mg/m3, an increased incidence
of interstitial inflammatory cell infiltrate and interstitial fibrosis
in the trachea and lungs in treated animals compared with controls was
seen. Although the number of these microscopic lesions was higher in
treated animals than in controls, there was no clear dose dependency
for this effect. A concentration of >250 mg/m3 resulted in upper
respiratory tract irritation, as indicated by inflammatory exudate
around the nares, and significantly decreased absolute kidney weights
in females. In the highest dose group, one rat per sex died, and the
body weight gain was significantly decreased in males and females
compared with controls. In addition, a significant decrease in
platelets (males/females), absolute/relative liver weights (males),
and trachea/lung weights (females) was noted (Velsicol Chemical
Corp., 1981).
Studies concerning repeated exposure by inhalation to sodium
benzoate were not identified in the available literature.
8.3.3 Dermal exposure
Studies concerning repeated dermal exposure to benzoic acid or
sodium benzoate were not identified in the available literature.
8.4 Long-term exposure
In general, the database for benzoic acid and sodium benzoate is
limited, and there are no studies available performed according to
current guidelines. In addition, the documentation in most cases is
limited. Detailed information is given in Table 4.
8.4.1 Subchronic exposure
In a 90-day study with rats dosed with 0, 1, 2, 4, or 8% sodium
benzoate via diet, the mortality in the highest dose group (approx.
6290 mg/kg body weight per day) was about 50%. Other effects in this
group included a reduced weight gain, increased relative weights of
liver and kidneys, and pathological changes (not further specified) in
these organs (Deuel et al., 1954).
Table 4: Results of studies concerning long-term oral exposure to benzoic acid and sodium benzoate.
Species; strain; Treatment Duration Examinations; Resultsa Reference
number of animals organs in
per dosea histopathology,
clinical chemistry,
haematology
Benzoic acid
rat; Wistar; 0 or 1.5% in diet 18 months no data available reduced weight gain with Marquardt (1960)
dose group: (approx. 0 or decreased feed intake;
30 m/20 f; 750 mg/kg body increased mortality rate
controls: weight) (15/50 vs. 3/25 in
13 m/12 f controls); no further
information available
(only provisional results
are given)
rat; Wistar or 0 or 1.5% in diet 18 months no data available reduced weight gain with Marquardt (1960)
Osborne-Mendel; (approx. 0 or decreased feed intake;
dose group: 750 mg/kg body no further information
20 m; controls: weight) available (only
10 m provisional results are
given)
rat; not given; 0, 0.5, or 1% in generation 1 histopathology in no effects on growth and Kieckebusch & Lang
20 m/f diet and 2: animals of organ weights; feeding of (1960)
(approx. 0, 250, lifelong generation 3 0.5% led to prolongation
or 500 mg/kg body generation 3: (not further specified) of survival compared with
weight) 16 weeks controls; no further
generation 4: information available
until breeding
Table 4 (cont'd)
Species; strain; Treatment Duration Examinations; Resultsa Reference
number of animals organs in
per dosea histopathology,
clinical chemistry,
haematology
Sodium benzoate
rat; Sherman; 0, 1, 2, 4, or 8% 90 days histopathology 6290 mg/kg body weight: Deuel et al.
5 m/f in diet (approx. 0, performed, but mortality about 50%; (1954)
640, 1320, 2620, not further specified weight gain **;
or 6290 mg/kg body relative weights of
weight) liver and kidneys *;
pathological lesions
(not further specified)
in liver and kidneys
rat; F344; 0, 1, or 2% in 18-24 months histopathology average mortality rate of Sodemoto & Enomoto
dose group: diet performed, but not all animals during the (1980)
50 m/52 f; (m: approx. 0, 700, further specified first 16 months: 14.5%
controls: or 1400 mg/kg (all dead rats showed
body weight; f: pneumonia with abscess);
25 m/43 f about 100 rats including
approx. 0, 290, controls died after
or 580 mg/kg 16 months due to
body weight) haemorrhagic pneumonia
(infection); no adverse
clinical signs and no
differences in average
body weight and mortality
in dosed animals compared
with controls;
non-carcinogenic effects
not reported
Table 4 (cont'd)
Species; strain; Treatment Duration Examinations; Resultsa Reference
number of animals organs in
per dosea histopathology,
clinical chemistry,
haematology
mouse; 0 or 2% via lifelong liver, spleen, kidney, no difference in survival Toth (1984)
albino Swiss; drinking-water bladder, thyroid, rates in treated animals
dose group: (approx. 0 or heart, pancreas, compared with controls;
50 m/f; 5960-6200 mg/kg testes, ovaries, brain, no pathological or
controls: 99 m/f body weight) nasal turbinates, lung statistical evidence of
tumour induction
a m = male; f = female.
8.4.2 Chronic exposure and carcinogenicity
In two studies with rats given 1.5% benzoic acid via diet
(approximately 750 mg/kg body weight per day), the animals showed a
reduced weight gain with decreased feed intake after dosing over
18 months. In one of these studies, mortality was increased
(15/50 rats of both sexes versus 3/25 in controls) (Marquardt, 1960).
No further information on these studies is available, as only
provisional results were published. In a four-generation study with
rats, no effects on life span, growth rate, or organ weights were
reported after dosing with up to 1% in the diet (approximately
500 mg/kg body weight per day) (Kieckebusch & Lang, 1960). Only
animals of the third generation were autopsied after 16 weeks, but
it is not clear if a complete histopathological investigation was
performed.
With sodium benzoate, two long-term studies with rats
(administration of up to 1400 mg/kg body weight per day via diet over
18-24 months; Sodemoto & Enomoto, 1980) or mice (lifelong application
of up to 6200 mg/kg body weight per day via drinking-water; Toth,
1984) are available. The results gave no indication of a carcinogenic
effect in the tested animals. Although the study with mice was not
performed according to current guidelines, the results seem to be
reliable, due to a sufficient number of animals and detailed
histopathological examinations. However, the results from the study
with rats are uncertain, due to a very high mortality in animals of
all dose groups, including controls (from an "infection" after
16 months), no detailed information about dosing regimen (only mean
values given), and the considerable differences in the body weight of
male and female rats (the body weight of females was about twice that
of males).
8.4.3 Carcinogenicity of benzyl acetate, benzyl alcohol, and
benzaldehyde
As benzyl acetate, benzyl alcohol, and benzaldehyde are
practically quantitatively metabolized via benzoic acid (see section
7.1), data on their carcinogenicity from 2-year studies may be used as
supportive evidence in the assessment of the hazards associated with
benzoic acid.
Benzyl acetate was administered in corn oil via gavage to F344/N
rats (0, 250, or 500 mg/kg body weight per day) or B6C3F1 mice
(0, 500, or 1000 mg/kg body weight per day). In high-dose male rats,
the incidence of acinar cell adenomas of the exocrine pancreas was
increased, whereas there was no evidence of carcinogenicity in female
rats. In high-dose male and female mice, benzyl acetate caused
increased incidences of hepatocellular adenomas and squamous cell
neoplasms of the forestomach (US NTP, 1986). In contrast to these
findings, no such tumours were observed in another study with the same
strain of rats and mice when benzyl acetate was administered via diet
(rats: <575 mg/kg body weight per day; mice: <375 mg/kg body
weight per day) (US NTP, 1993).
With benzyl alcohol, no treatment-related increase in tumours was
observed in F344/N rats or B6C3F1 mice after administration of
<400 mg/kg body weight per day in rats or <200 mg/kg body weight
per day in mice by gavage in corn oil (US NTP, 1989).
In B6C3F1 mice dosed with benzaldehyde in corn oil by gavage
(males: 0, 200, or 400 mg/kg body weight per day; females: 0, 300, or
600 mg/kg body weight per day), the incidences of squamous cell
papillomas of the forestomach were significantly greater in both
exposure groups than in controls. A dose-related increase in the
incidence of forestomach hyperplasia was also observed. In F344/N rats
dosed with <400 mg/kg body weight per day, there was no evidence of
carcinogenic activity (US NTP, 1990).
8.5 Genotoxicity and related end-points
8.5.1 Benzoic acid
Benzoic acid tested negative in several Ames tests and in one DNA
damage assay with different Salmonella typhimurium strains in the
presence or absence of metabolic activation (McCann et al., 1975;
Ishidate et al., 1984; Nakamura et al., 1987; Zeiger et al., 1988).
Only in one recombination assay with Bacillus subtilis H17 and M45
was a positive result obtained (Nonaka, 1989). However, due to missing
experimental details (only results given), the validity of this study
cannot be judged. There was no indication of genotoxic activity
(chromosome aberrations, sister chromatid exchange) in tests with
mammalian cells (Chinese hamster CHL and CHO cells, human
lymphoblastoid cells, human lymphocytes) without metabolic activation
(Oikawa et al., 1980; Tohda et al., 1980; Ishidate et al., 1984;
Jansson et al., 1988).
In vivo studies with benzoic acid were not identified in the
literature.
8.5.2 Sodium benzoate
Sodium benzoate also gave negative results in some Ames tests and
in Escherichia coli in the presence or absence of metabolic
activation (Ishidate et al., 1984; Prival et al., 1991). As with
benzoic acid in recombination assays with Bacillus subtilis H17 and
M45, positive results were obtained (Ishizaki & Ueno, 1989; Nonaka,
1989). Although sodium benzoate tested negative in a cytogenetic assay
with WI-38 cells in the absence of metabolic activation (US FDA,
1974), consistently positive results (in contrast to the negative
results of benzoic acid) were obtained in tests on sister chromatid
exchange and chromosome aberrations with CHL/CHO and DON cells or
human lymphocytes without metabolic activation (Abe & Sasaki, 1977;
Ishidate & Odashima, 1977; Ishidate et al., 1984, 1988; Xing & Zhang,
1990). However, from the limited information given in the publications
(i.e., only results given), it cannot be judged if these positive
results may have been attributable to cytotoxic effects.
In a valid in vivo study performed by the US FDA (1974), sodium
benzoate tested negative in a cytogenetic assay (bone marrow) in rats
after single or multiple oral application of doses up to 5000 mg/kg
body weight. In a study with mice (comparable dosing scheme), there
was also no indication of mutagenic activity in a host-mediated assay
(US FDA, 1974).
However, in a dominant lethal assay with rats (comparable dosing
scheme; males were mated with untreated females following 7 or 8 weeks
of dosing), some statistically significant and dose-related findings
were reported in week 7: decreased fertility index for both treatment
regimens and an increased number of preimplantation losses after
single dosing (US FDA, 1974).
In summary, the in vitro studies with benzoic acid gave no
indications for genotoxic effects, whereas in vivo studies were not
identified. Sodium benzoate was also inactive in bacterial test
systems, whereas tests with mammalian cells gave consistently positive
results. In addition, in an in vivo study with sodium benzoate
(dominant lethal assay in rats), a positive result was obtained. As a
result, a genotoxic activity of sodium benzoate cannot be ruled out
entirely at present.
Detailed information concerning the genotoxicity of benzoic acid
and sodium benzoate in vitro is given in Table 5.
8.6 Reproductive and developmental toxicity
8.6.1 Fertility
There are no studies available dealing specifically with the
effects of benzoic acid or sodium benzoate on fertility that have been
conducted according to current protocols.
In a four-generation study with male and female rats, no adverse
effects on fertility or lactation (only investigated parameters) were
seen after dosing with benzoic acid at up to 1% in the diet
(approximately 500 mg/kg body weight per day) (see also section 8.4.2;
Kieckebusch & Lang, 1960).
In studies with repeated oral application, no effects on the
testes were observed in rats after dosing with benzoic acid at up to
647 mg/kg body weight per day in the diet for 4 weeks (see also Table
3; Bio-Fax, 1973) or in mice after lifelong application of 6200 mg
sodium benzoate/kg body weight per day via drinking-water (see also
Table 4; Toth, 1984).
In summary, no clear statement can be given as to the possible
effects of benzoic acid or sodium benzoate on fertility.
8.6.2 Developmental toxicity
In a study with pregnant rats given only one oral dose of benzoic
acid (510 mg/kg body weight on gestation day 9), there was no
indication of an increase in resorption rates or malformations
(Kimmel et al., 1971).
For sodium benzoate, several teratogenicity studies are available
that have been performed with different species. As given in Table 6,
no effects were seen in dams or offspring of rats, mice, rabbits, or
hamsters given oral doses of up to 300 mg/kg body weight per day
(highest dose tested) during gestation (US FDA, 1972b). In a study
with rats by Onodera et al. (1978), doses of 4% or 8% via diet (uptake
of 1875 or 965 mg/kg body weight per day) induced severe maternal
toxicity (no weight gain/loss in body weight, increased mortality) and
were associated with embryotoxic and fetotoxic effects as well as
malformations. However, the authors suggested that the effects on the
dams and fetuses at >4% dietary levels were caused by reduced
maternal feed intake, leading to malnutrition. The intake of sodium
benzoate in the highest dose group (8%) was lower than that at 2%,
where no adverse effects were seen. From this study, a NO(A)EL of
about 1310 mg/kg body weight per day can be derived. In a study with
rats by Minor & Becker (1971), however, fetotoxic and teratogenic
effects occurred at 1000 mg/kg body weight per day. In this study,
sodium benzoate was applied by intraperitoneal injection. Therefore,
differences in pharmacokinetics between oral and intraperitoneal
administration may be the reason for the higher sensitivity.
Studies performed with eggs of leghorn hens (single injection of
<5 mg per egg), chick embryo neural retina cells
(lowest-observed-effect concentration [LOEC] of 34.7 mmol/litre), and
a chick embryotoxicity screening test (single injection of <0.1 mg
per embryo) gave no indication of embryotoxic or teratogenic effects
(Verrett et al., 1980; Jelinek et al., 1985; Daston et al., 1995).
8.6.3 Reproductive toxicity of benzyl acetate, benzyl alcohol,
and benzaldehyde
As benzyl acetate and benzyl alcohol are practically
quantitatively metabolized via benzoic acid (see section 7.1), data on
their reproductive toxicity may be used as supportive evidence in the
assessment of the hazards associated with benzoic acid.
Dietary benzyl acetate (up to 5% in the diet for 13 weeks) had no
effect on the weights of the epididymis, cauda epididymis, or testis,
on sperm motility or density, or on the percentage of abnormal sperm
in mice or rats (US NTP, 1993).
Benzyl acetate (0, 10, 100, 500, or 1000 mg/kg body weight per
day by gavage on days 6-15) had no significant effects on maternal
health in rats and did not induce changes in the numbers of corpora
lutea, implantations, live or dead fetuses, or resorptions,
implantation ratio, sex ratio, external or internal malformations, or
placental weight. Fetal weights were significantly reduced at the
highest dose (Ishiguro et al., 1993).
Benzyl alcohol at 550 mg/kg body weight per day by gavage on days
6-15 of pregnancy had no effect on gestation index, average number of
live pups per litter, postnatal survival, or pup body weight on days
0 and 3 in CD-1 mice (York et al., 1986), while 750 mg/kg body weight
per day (days 7-14) induced a reduction in the pup weight and maternal
weight gain, but no pup mortality or changes in mating or gestation
indices, the total number of resorptions, or the number of live pups
per litter (Hardin et al., 1987).
Table 5: Genotoxicity of benzoic acid and sodium benzoate in vitro.
Resultsa
Species End-point Concentration Without With Remarks Reference
(test system) range metabolic metabolic
activation activation
Benzoic acid
Salmonella Reverse 10-1000 - - McCann et al.
typhimurium mutations µg/plate (1975)
TA 98, TA 100,
TA 1535, TA 1537
Salmonella Reverse 33-10 000 - - cytotoxic effects Zeiger et al.
typhimurium mutations µg/plate at >5000 (1988)
TA 97, TA 98, µg/plate
TA 100, TA 1535,
TA 1537
Salmonella Reverse up to 10 000 - - 10 000 µg/plate was Ishidate et al.
typhimurium mutations µg/plate the highest (1984)
TA 92, TA 94, non-cytotoxic
TA 98, TA 100, concentration tested
TA 1535, TA 1537
Salmonella DNA damage up to - - no further Nakamura et al.
typhimurium (umu test) 1670 µg/ml information available (1987)
TA 1535/pSK 1002 (only results given)
Bacillus Recombination not given tested positive (no Nonaka
subtilis assay further information (1989)
H17, M45 available, only
summary given)
Table 5 (cont'd)
Resultsa
Species End-point Concentration Without With Remarks Reference
(test system) range metabolic metabolic
activation activation
Chinese hamster Chromosome up to ? 0 1500 µg/ml was given Ishidate et al.
cells (CHL) aberration 1500 µg/ml as maximum effective (1984)
concentration; result
given as negative in
Ishidate et al.
(1988)
Human Sister 1-30 - 0 cytotoxic effects Tohda et al.
lymphoblastoid chromatid mmol/litre at 30 mmol/litre (1980)
cells exchange
(transformed
by Epstein-Barr
virus)
Human Sister up to 2 - 0 Jansson et al.
lymphocytes chromatid mmol/litre (1988)
exchange
Chinese Sister up to - 0 Oikawa et al.
hamster chromatid 10 mmol/litre (1980)
cells (CHO) exchange
Table 5 (cont'd)
Resultsa
Species End-point Concentration Without With Remarks Reference
(test system) range metabolic metabolic
activation activation
Sodium benzoate
Salmonella Reverse up to - - 3000 µg/plate was Ishidate et al.
typhimurium mutations 3000 µg/plate the highest (1984)
TA 92, TA 94, non-cytotoxic
TA 98, TA 100, concentration tested
TA 1535, TA 1537
Salmonella Reverse 33-10 000 - - Prival et al.
typhimurium mutations µg/plate (1991)
TA 98, TA 100,
TA 1535, TA 1537,
TA 1538
Escherichia coli Reverse 33-10 000 - - Prival et al.
WP2 mutation µg/plate (1991)
assay
Bacillus Recombination not given tested positive Nonaka (1989)
subtilis assay information
H17, M45 available, only
summary given)
Bacillus Recombination -S9: (+) (+) Ishizaki & Ueno
subtilis assay 20 mg/disc (1989)
H17, M45 +S9:
16 mg/disc
Table 5 (cont'd)
Resultsa
Species End-point Concentration Without With Remarks Reference
(test system) range metabolic metabolic
activation activation
WI-38 cells Cytogenetic 10-1000 µg/ml - 0 examination of US FDA (1974)
assay anaphase preparations
cytotoxic effects
at >500 µg/ml
Chinese hamster Chromosome up to + 0 2000 µg/ml was Ishidate et al.
cells (CHL) aberration 2000 µg/ml given as maximum (1984, 1988)
effective
concentration
Chinese hamster Chromosome 139 mg/ml + 0 only maximum Ishidate &
cells (CHL) aberration effective dose given Odashima (1977)
Chinese hamster Chromosome 290 µg/ml 0 0 only minimum Ishidate et al.
cells (DON) aberration effective dose given (1988)
Chinese hamster Chromosome 1-10 + 0 Abe & Sasaki
cells (DON) aberration mmol/litre (1977)
Chinese hamster Sister 1-10 (+) 0 slight increase Abe & Sasaki
cells (DON) chromatid mmol/litre without dosage (1977)
exchange effect
Human Sister 10 + 0 Xing & Zhang
lymphocytes chromatid mmol/litre (1990)
exchange
a -, negative; +, positive; (+) weakly positive; ?, equivocal; 0, not tested.
Table 6: Results of studies concerning reproductive and developmental toxicity of benzoic acid and sodium benzoate.
Species; strain; Application Durationb Parameters Results NO(A)EL Reference
number of animals investigated (mg/kg
per dosea body
weight)
Benzoic acid
rat; Wistar; 0 or 510 gd 9 F0: implantation F0: resorption rates 510 Kimmel et al.
dose group: mg/kg body and resorption sites were given as (1971)
7 f; controls: weight F1: malformations "comparable with
not given via gavage controls"
F1: malformations
(not further specified)
were given as
"comparable with controls"
no further information
available
rat; not given; 0, 0.5, or 1% F0 and F1: fertility and F0-F3: no adverse 500 Kieckebusch &
20 f in diet lifelong lactation effects compared with Lang (1960)
(approx. 0, F2: 16 controls were reported
250, or 500 weeks no further information
mg/kg body F3: until available
weight) breeding
Sodium benzoate
rat; Wistar; 0, 1.75, 8, gd 6-15 F0: numbers of corpora F0 and F1: no adverse 175 US FDA
20 f 38, or 175 lutea, implantation effects compared with (1972b)
mg/kg body and resorption sites, controls were reported
weight via examination of the
gavage urogenital tract
Table 6 (cont'd)
Species; strain; Application Durationb Parameters Results NO(A)EL Reference
number of animals investigated (mg/kg
per dosea body
weight)
F1: numbers of live
and dead fetuses, body
weights, gross
examination for
external
malformations,
microscopic visceral
and skeletal
examination
mouse; 0, 1.75, 8, gd 6-15 F0: numbers of corpora F0 and F1: no adverse 175 US FDA
CD-1; 38, or 175 lutea, implantation effects compared with (1972b)
25-31 f mg/kg body and resorption sites, controls were reported
weight via examination of the
gavage urogenital tract
F1: numbers of live
and dead fetuses, body
weights, gross
examination for
external
malformations,
microscopic visceral
and skeletal
examination
rabbit; Dutch 0, 2.5, 12, gd 6-18 F0: numbers of corpora F0 and F1: no 250 US FDA
belted; 14-32 f 54, or 250 lutea, implantation adverse effects (1972b)
mg/kg body and resorption sites, compared with
weight via examination of the controls were
gavage urogenital tract reported
Table 6 (cont'd)
Species; strain; Application Durationb Parameters Results NO(A)EL Reference
number of animals investigated (mg/kg
per dosea body
weight)
F1: numbers of live
and dead fetuses, body
weights, gross
examination for
external
malformations,
microscopic visceral
and skeletal
examination
hamster; 0, 3, 14, 65, gd 6-10 F0: numbers of corpora F0 and F1: no adverse 300 US FDA
golden; or 300 mg/kg lutea, implantation effects compared with (1972b)
22 f body weight and resorption sites, controls were reported
via gavage examination of the
urogenital tract
F1: numbers of live
and dead fetuses,
body weights, gross
examination for
external
malformations,
microscopic visceral
and skeletal
examination
Table 6 (cont'd)
Species; strain; Application Durationb Parameters Results NO(A)EL Reference
number of animals investigated (mg/kg
per dosea body
weight)
rat; 0, 100, 315, a) gd 9-11 F0: not specified a) F0: no data given 315 Minor & Becker (1971)
Sprague-Dawley or 1000 mg/kg b) gd 12-14 F1: body weights, in F1: 1000 mg/kg body
(no further data) body weight utero deaths, weight: body weights **;
intraperitoneally gross anomalies in utero deaths * (16%);
gross anomalies * (not
further specified)
b) F0: no data given
F1: 1000 mg/kg body
weight: body weights **;
in utero deaths * (12%);
gross anomalies <--> (not
further specified)
no further information
available
rat; Wistar; 0, 1, 2, 4, gd 1-20 a) all but five a) >4% (1875 or 965 mg/kg 1310 Onodera et al. (1978)
27-30 f or 8% via diet animals in each group body weight):
(approx. 0, 700, were sacrificed on F0: weight gain <-->
1310, 1875, or gd 20 (numbers of feed intake **;
965 mg/kg body viable/dead fetuses, mortality * (convulsions,
weight) early/late depressed motor activity)
resorptions, fetal, F1: number of
placental, and ovarian dead/resorbed fetuses *;
weights, and body weight of viable
abnormalities of fetuses **; mild
maternal organs and systemic oedema,
fetal appearance were anophthalmia,
recorded) microphthalmia,
Table 6 (cont'd)
Species; strain; Application Durationb Parameters Results NO(A)EL Reference
number of animals investigated (mg/kg
per dosea body
weight)
b) the remaining five hydrocephalus,
dams delivered pyelectasis, hydroplasia,
naturally (number of cerebral hypoplasia;
offspring, survival, delayed ossification,
body weight, and lumbar or cervical ribs,
abnormalities were and varied sternebrae
recorded); 3 weeks 8%: F0: body weight **
after birth, all b) F1:
surviving pups were <2% (1310 mg/kg body
weaned and examined weight): no adverse
for gross effects compared with
abnormalities controls
(one-half of the pups >4% (1875 or 965 mg/kg
and all dams were body weight): delivery
necropsied); the rates ** (50 and 8.2%,
remaining pups were respectively); complete
necropsied at 8 weeks loss of litters after
of age (body weight parturition
and food intake were
measured weekly until
necropsy)
a m = male; f = female.
b gd = gestation day.
9. EFFECTS ON HUMANS
Cases of urticaria, asthma, rhinitis, or anaphylactic shock have
been reported following oral, dermal, or inhalation exposure to
benzoic acid and sodium benzoate. The symptoms appear shortly after
exposure and disappear within a few hours, even at low doses (Maibach
& Johnson, 1975; Clemmensen & Hjorth, 1982; Larmi et al., 1988; Ring,
1989; Gailhofer et al., 1990; Aberer et al., 1992; Lahti et al., 1995;
Anderson, 1996; Bindslev-Jensen, 1998; Coverly et al., 1998).
In the literature, several studies (e.g., oral provocation tests
or patch tests) are available, which have been performed with small
groups of patients suffering from urticaria, dermatitis, asthma, and
Melkersson-Rosenthal syndrome (Juhlin et al., 1972; Freedman, 1977;
Osterballe et al., 1979; Lahti & Hannuksela, 1981; Clemmensen &
Hjorth, 1982; Ibero et al., 1982; Moneret-Vautrin et al., 1982; Veien
et al., 1987; Aguirre et al., 1993; McKenna et al., 1994; BUA, 1995;
Munoz et al., 1996; Petrus et al., 1996; Vogt et al., 1999). In most
of these studies, atopic individuals have demonstrated reactions to
oral and dermal challenge with benzoic acid or sodium benzoate.
The information concerning skin reactions caused by benzoic acid
or sodium benzoate in the general population is limited. In a study
with 2045 patients of dermatological clinics, only 5 persons
(approximately 0.2%) showed a positive reaction in patch tests
(Brasch et al., 1993), while 34 of 5202 patients (approximately 0.7%)
with contact urticaria reacted positively (Broeckx et al., 1987). From
these data, it can be concluded that skin reactions caused by benzoic
acid or sodium benzoate in the healthy general population are rare.
In US FDA (1972a) and WHO (1996), several older studies
concerning oral exposure to benzoic acid or sodium benzoate are
described. However, owing to the limited number of individuals (mostly
single case studies), the validity of these studies is limited. No
adverse effects were reported after a single oral dose of 10 000 mg
benzoic acid or up to 1000 mg per day over a period of up to 92 days
(Gerlach, 1909). In another study with volunteers given 1000, 1500,
2000, or 2500 mg/day for 5 days each, marked symptoms, signs of
discomfort, and malaise (nausea, headache, weakness, burning and
irritation of oesophagus) were reported (Wiley & Bigelow, 1908).
Chittenden et al. (1909) found no abnormalities in blood picture,
urine composition, nitrogen balance, or well-being in six men given
300-400 mg per day via diet for up to 62 days. In nine patients on
penicillin treatment given 12 000 mg benzoic acid divided into eight
doses over 5 days in eight subjects and over 14 days in one subject,
no adverse effects on blood urea nitrogen or creatinine clearance were
reported (Waldo et al., 1949). A single dose of 2000-3000 mg sodium
benzoate caused signs of intoxication similar to those described for
benzoic acid by Wiley & Bigelow (1908).
Sodium benzoate is used in the treatment of patients with urea
cycle enzymopathies (i.e., hyperammonaemia due to inborn errors of
urea synthesis) in order to facilitate an alternative pathway of
nitrogen excretion. The therapeutic dose given over several years is
in the range of 250-500 mg/kg body weight per day (Batshaw & Brusilow,
1981; Green et al., 1983; Batshaw & Monahan, 1987; O'Connor et al.,
1987; Kubota & Ishizaki, 1991; Tremblay & Qureshi, 1993; Feillet &
Leonard, 1998). At this dose level, clinical signs of toxicity are
rare and in most cases limited to anorexia and vomiting, especially
after intravenous bolus infusions.
10. EFFECTS ON OTHER ORGANISMS IN THE LABORATORY
AND FIELD
10.1 Aquatic environment
For the toxicity data mentioned in this section, it is not always
stated whether the cited effect values are based on nominal or
measured concentrations of benzoic acid or sodium benzoate. However,
because of their water solubility, their insignificant volatility, and
their low adsorption potential (see sections 2 and 5), all nominal
concentrations of the test substances are expected to correspond to
effective concentrations, even in tests with open systems and longer
exposure durations.
In Table 7, several valid toxicity test results for the most
sensitive aquatic species of various taxonomic groups -- bacteria,
cyanobacteria, green algae, protozoa, invertebrates, and vertebrates
-- with benzoic acid have been compiled. From the aquatic organisms
tested so far, cyanobacteria (Anabaena inaequalis) proved to be most
sensitive, showing a 14-day EC50 of 9 mg/litre in the cell
multiplication inhibition test (Stratton & Corke, 1982). EC50/LC50
values (24-96 h) for most of the other aquatic species tested
(protozoa, molluscs, crustaceans, fish, amphibians) were in the range
of 100-1291 mg/litre. As seen with daphnids, the pH value of the test
medium influences the toxicity of benzoic acid, which proved to be
more toxic at lower pH levels (Bringmann & Kuehn, 1980). Developmental
toxicity effects seen in frog (Xenopus) embryos were craniofacial
defects, especially microcephaly, and abnormal gut coiling
(Dawson et al., 1996). A recently developed cytotoxicity assay with
cultured fathead minnow (Pimephales promelas) cells resulted in a
PI50 (the concentration required to induce a 50% reduction in total
protein content) of 1450 mg/litre (Dierickx, 1998).
Ninety-six-hour LC50 values of >100 mg sodium benzoate/litre
have been found for Daphnia magna (first and second larval instar)
and Gammarus fasciatus (juvenile: 7 mg in size) under static test
conditions (multispecies test; pH 6.5-8; 20°C) (Ewell et al., 1986).
The same was true for juveniles of other invertebrates tested
simultaneously: Asellus intermedius (Arthropoda; 12 mg body weight),
Dugesia tigrina (Platyhelminthes; 6 mg body weight), Helisoma
trivolvis (Mollusca; 180 mg body weight), and Lumbriculus variegatus
(Annelida; 6 mg body weight) (Ewell et al., 1986).
Two different tests with the freshwater fathead minnow
(P. promelas; juvenile stages) resulted in 96-h LC50 values of 484
mg sodium benzoate/litre (measured concentration; flow-through system;
pH 7.4; 24°C) (Geiger et al., 1985) and >100 mg/litre (nominal
concentration; static system; pH 6.5-8.5; 20°C) (Ewell et al., 1986).
Table 7: Aquatic toxicity of benzoic acid.
Most sensitive species Special Effective Reference
(test method/end-point) features concentration
(mg/litre)
Mixed microbial inoculum
Activated sludge pH 7.5 3-h EC50 >1000 Klecka et al.
(respiration inhibition (1985)
test; OECD Guideline
209)
Bacteria
Pseudomonas putida pH neutral 16-h MICa 480 Bringmann &
(cell multiplication Kuehn (1977)
inhibition test)
(static)
Photobacterium - 30-min EC50 16.85 Kaiser et al.
phosphoreum (1987)
(Microtox test:
bioluminescence
reduction)
Cyanobacteria
Anabaena inaequalis Stratton &
(cell multiplication - 14-day EC50 9 Corke (1982)
inhibition test)
(static)
(photosynthesis - 3-h EC50 5
reduction)
Algae
Scenedesmus quadricauda
(cell multiplication pH neutral 8-day MIC 1630 Bringmann &
inhibition test) Kuehn (1977)
(static)
(photosynthesis
reduction) - 3-h EC50 75 Stratton &
Corke (1982)
Table 7 (cont'd)
Most sensitive species Special Effective Reference
(test method/end-point) features concentration
(mg/litre)
Chlorella pyrenoides
(photosynthesis
reduction) - 3-h EC50 60 Stratton &
Corke (1982)
Protozoa
Uronema parduczi
(cell multiplication pH 6.9 20-h MIC 31 Bringmann &
inhibition test) Kuehn (1980)
Tetrahymena pyriformis
(cell multiplication - 2-day EC50 252 Schultz et al.
inhibition test) (1996)
Invertebrata: Mollusca
Teredo digensis larvae 72-h LC50 100 Vind &
(marine) (static) Hochman
(1960)
Invertebrata: Crustacea
Daphnia magna pH neutral 24-h EC50 500 Bringmann &
(immobilization) Kuehn (1982)
pH acid 24-h EC50 102
Vertebrata: Fish
Leuciscus idus
(lethality, DEV L15) pH 7-8 48-h LC50 460 Juhnke &
Luedemann
(1978)
Table 7 (cont'd)
Most sensitive species Special Effective Reference
(test method/end-point) features concentration
(mg/litre)
Vertebrata: Amphibia
Xenopus laevis
(lethality) embryos Dawson et al.
(malformation) pH 7.2-7.4 96-h LC50 1291 (1996)
96-h EC50 433
a MIC = minimum inhibitory concentration.
10.2 Terrestrial environment
It is undissociated benzoic acid that is responsible for its
antimicrobial activity. As benzoic acid itself is only slightly
soluble in water, sodium benzoate -- which, under acidic conditions,
converts to undissociated benzoic acid -- is often used instead. Their
antimicrobial properties are used for different applications, such as
food preservation (Chipley, 1983; see section 4), optimally under
acidic conditions.
Minimum microbiocidal concentrations ranged from 20 to 1200 mg
benzoic acid/litre in suspension tests (pH 6) with different bacterial
or fungal species (Wallhäusser, 1984; Russell & Furr, 1996). Minimum
inhibitory concentrations (serial dilution technique) were in the
range of 50-1000 mg/litre (Wallhäusser, 1984; Russell & Furr, 1996).
The pH dependence of benzoic acid's antimicrobial activity is
shown in several studies. Growth inhibition of the fungus Fusarium
oxysporum (related to dry weight) measured 5 days after incubation
with 610 mg benzoic acid/litre was 23.7% at pH 7.2 and 83.5% at pH 4
(Soni & Bhatia, 1980). There was no visible growth of yeast
(Saccharomyces cerevisiae, Willia anomala) or mould (Penicillium
glaucum) fungi at sodium benzoate concentrations of 120-600 mg/litre
at pH 2.6, 1000-4000 mg/litre at pH 5, or 20 000-60 000 mg/litre at pH
7 (Schelhorn, 1951). Minimum inhibitory concentrations preventing
growth of Talaromyces flavus on agar plates after 35 days of
incubation were 100 mg/litre at pH 3.5 and >600 mg/litre at pH 5.4
(King & Halbrook, 1987).
The minimum inhibitory concentrations of benzoic acid on the
growth of several species of yeasts ranged from 170 to 1250 mg/litre
in cultures preadapted to benzoic acid and from 100 to 600 mg/litre in
unadapted cultures (pH 3.5; 25°C; 6 weeks of incubation) (Warth,
1988).
No information on the toxic effects of benzoic acid or sodium
benzoate on plants, earthworms, or other terrestrial organisms or on
ecosystems was identified.
11. EFFECTS EVALUATION
11.1 Evaluation of health effects
11.1.1 Hazard identification and dose-response assessment
After oral ingestion of benzoic acid and sodium benzoate in
experimental animals or humans, there is rapid absorption of the
undissociated benzoic acid from the gastrointestinal tract. The
substances are metabolized in the liver mainly by conjugation with
glycine, resulting in the formation of hippuric acid, which is rapidly
excreted via the urine. Benzoates applied dermally can penetrate
through the skin. Owing to their rapid metabolism and excretion, an
accumulation of the benzoates or their metabolites is not to be
expected.
With oral LD50 values of >1940 mg/kg body weight, the acute
toxicity of benzoic acid and sodium benzoate in rodents is low.
Benzoic acid is slightly irritating to the skin and irritating to
the eye, whereas sodium benzoate is not irritating to the skin and is
only a slight eye irritant. Benzoic acid was not skin sensitizing in
several animal models. For sodium benzoate, no data were identified
covering this specific end-point.
Studies concerning short-term, subchronic, or chronic oral
exposure conducted according to current guidelines are not available
for benzoic acid or sodium benzoate. Effects on the central nervous
system, weight gain (in several cases without reduced food intake),
and liver and kidney were recorded at high concentrations of both
compounds. As expected, and as far as it is possible to conclude with
the limited database, toxic effects and effect levels seem to be
similar for both compounds. A preliminary NO(A)EL of about 500 mg/kg
body weight per day (the highest dose tested) may be derived based on
a limited four-generation study (Kieckebusch & Lang, 1960; see section
8.4.2 and Table 4). This is supported by two short-term studies in
which no adverse effects were observed at the highest tested dose
levels of 647-825 mg/kg body weight per day (Kreis et al., 1967;
Bio-Fax, 1973) and by the fact that no serious side-effects have been
reported after therapeutic use of sodium benzoate at a dose level of
250-500 mg/kg body weight per day in humans, although occasionally
anorexia and vomiting were observed.
In a short-term inhalation study with rats exposed to benzoic
acid (0, 25, 250, or 1200 mg dust aerosol/m3; 6 h per day, 5 days
per week, over 4 weeks), indications of fibrosis in the lung were seen
even at the lowest concentration. The number of these microscopic
lesions was higher in treated animals than in controls, but there was
no clear dose dependency for this effect. Therefore, a
no-observed-(adverse-)effect concentration (NO(A)EC) value cannot be
derived. Long-term inhalation studies with benzoic acid or sodium
benzoate were not identified.
Two long-term studies with rats (application of up to 1400 mg/kg
body weight per day via diet over 18-24 months; quality of the study
questionable) or mice (lifelong application of up to 6200 mg/kg body
weight per day via drinking-water) gave no indication of a
carcinogenic effect in either species. Studies on the precursors of
benzoic acid -- benzyl acetate, benzyl alcohol, and benzaldehyde --
support the notion that it is unlikely that benzoic acid is
carcinogenic.
In several in vitro tests on genotoxicity, benzoic acid and
sodium benzoate tested negative. For sodium benzoate, in contrast to
benzoic acid, consistently positive results were obtained in tests on
sister chromatid exchange and chromosome aberrations without metabolic
activation. In vivo studies for benzoic acid were not identified.
For sodium benzoate, negative results were obtained in vivo in a
cytogenetic assay with rats and a host-mediated assay with single or
multiple oral application. However, a dominant lethal assay with rats
gave a positive result. Therefore, a possible genotoxic activity of
sodium benzoate cannot be ruled out entirely at present.
For benzoic acid, two limited studies gave no indication of
adverse reproductive or developmental effects. With sodium benzoate,
several studies on different species have been performed. Embryotoxic
and fetotoxic effects as well as malformations were seen only at doses
that induced severe maternal toxicity. In a dietary study in rats, a
NO(A)EL of about 1310 mg/kg body weight per day was established.
Studies on the precursors of benzoic acid support the notion that
benzoic acid is unlikely to have adverse reproductive effects at dose
levels not toxic to the mother.
The acute toxicity of benzoic acid and sodium benzoate in humans
is low. However, both substances are known to cause contact dermatitis
(pseudoallergy). In patients with urticaria or asthma, an exacerbation
of the symptoms was observed after testing (oral provocation test or
patch tests), whereas this effect is unusual in healthy subjects.
11.1.2 Criteria for setting tolerable intakes or guidance values for
benzoic acid and sodium benzoate
As given in section 11.1.1, the database is insufficient for
deriving NO(A)EL values for oral uptake. If the provisional NO(A)EL of
about 500 mg/kg body weight per day is applied, and by incorporating
an uncertainty factor of 100 (10 for uncertainty of the database, 10
for interspecies variation), a provisional tolerable intake would be
5 mg/kg body weight per day.
Applying this tolerable intake, one has to keep in mind that
benzoates at lower doses can cause non-immunological contact reactions
(pseudoallergy) in sensitive persons.
There are also no studies available concerning longer-term
exposure by inhalation, and the only short-term inhalation toxicity
study is not adequate for confidently establishing a NO(A)EC.
Therefore, a tolerable concentration for exposure by inhalation cannot
be calculated.
11.1.3 Sample risk characterization
As given in section 6.2, workers may be exposed to benzoic acid
or sodium benzoate via inhalation or skin contact during production
and processing. However, owing to the lack of information on specific
working operations and conditions (e.g., duration of exposure)
involved, it is impossible to derive a realistic estimate of
occupational exposure.
For the general population, the main route of exposure to benzoic
acid and sodium benzoate is likely via foodstuffs, which contain the
substances naturally or added as antimicrobial agents. As given in
section 6.2, the uptake depends on the individual's choice of food to
be consumed and the limit values for benzoates in different countries.
Therefore, considerable deviations may occur. Recent intake
estimations from surveys from several countries gave mean values in
the range of 0.18-2.3 mg/kg body weight. Only in high consumers was an
intake of up to 14 mg/kg body weight calculated. Benzoates have not
been detected in drinking-water. As given in section 6.1, the
inhalative uptake via ambient or indoor air may contribute only
marginally to exposure of the general population.
For normal consumers, the uptake of benzoates is about 2-28 times
lower than the provisional tolerable intake of 5 mg/kg body weight
day, and only in high consumers would this value be exceeded 3 times.
Additional information is required in order to evaluate whether
sodium benzoate has a possible genotoxic activity.
11.2 Evaluation of environmental effects
Significant releases of benzoic acid and sodium benzoate into the
environment are primarily into water and soil from their uses as
preservatives in food, mouthwashes, dentifrices, and cosmetics.
Benzoic acid occurs naturally in many plants.
From their physical/chemical properties, benzoic acid and sodium
benzoate are not expected to volatilize from water and soil to the
atmosphere or to adsorb to sediment or soil particles. The main
elimination pathway for both chemicals should be biotic
mineralization. Because of their ready biodegradability and their low
volatility, both substances are not considered to contribute directly
to the depletion of the stratospheric ozone layer or to global
warming. From experimental data on bioconcentration, a low to moderate
potential for bioaccumulation is to be expected.
Benzoic acid and sodium benzoate exhibited low to moderate
toxicity to aquatic organisms. The lowest reported EC50 value of
9 mg/litre was determined in a chronic study (14 days) for cell
multiplication inhibition by benzoic acid in the cyanobacterium
Anabaena inaequalis. EC50/LC50 values for the other aquatic
species tested were in the range of 17-1291 mg/litre. Exposure levels
of benzoic acid and benzoate in water have been determined only in
rain and snow, groundwater, and leachate in the vicinity of point
sources. Thus, a quantitative risk characterization with respect to
aquatic organisms in surface waters could not be performed. Taking
into account the rapid biodegradability, the low to moderate
bioaccumulation potential, the low toxicity to most aquatic species,
and the rapid metabolism of these substances, benzoic acid and sodium
benzoate will -- with the exception of accidental spills -- pose only
a minimal risk to aquatic organisms.
The few available data from the antimicrobial action of benzoic
acid and sodium benzoate indicate only a low toxicity potential of
both substances in the terrestrial compartment. Due to the lack of
measured exposure levels, a sample risk characterization with respect
to terrestrial organisms could not be performed.
12. PREVIOUS EVALUATIONS BY INTERNATIONAL BODIES
JECFA (WHO, 1996) has allocated an acceptable daily intake (ADI)
for benzoic acid and sodium benzoate of 0-5 mg/kg body weight.
REFERENCES
Abe S, Sasaki M (1977) Chromosome aberrations and sister chromatid
exchanges in Chinese hamster cells exposed to various chemicals.
Journal of the National Cancer Institute, 58(6):1635-1641.
Abe S, Tsutsui Y, Tasrumoto Y, Nakane S (1984) Studies on the toxicity
of oxaprozin. 1. Acute toxicity of oxaprozin, its metabolites and
contaminants. Iyakuhin Kenkyu, 15(3):359-370.
Aberer W, Kager B, Ziegler V, Horak F (1992) Schnupfen durch
Schneiderkreide -- Allergie, Pseudoallergie, Rhinopathie oder
Einbildung? Dermatosen, 40(6):231-234.
Aguirre A, Izu R, Gardeazabal J, Diaz-Perez JL (1993) Edematous
allergic contact cheilitis from a toothpaste. Contact dermatitis,
28:42.
Andersen KE, Maibach HI, Anjo MD (1980) The guinea-pig: an animal
model for human skin absorption of hydrocortisone, testosterone and
benzoic acid? British journal of dermatology, 102:447-453.
Anderson JA (1996) Allergic reactions to food. Critical reviews in
food science and nutrition, 36:S19-S38.
AOAC (1990) Official methods of analysis, 15th ed. Arlington, VA,
Association of Official Analytical Chemists, pp. 1142-1145.
Arens M, Gertz C (1990) Bestimmung der Konservierungsstoffe -
Gemeinsschaftsarbeit der DGF, 110. Mitteilung: Deutsche
Einheitsmethoden zur Untersuchung von Fetten, Fettprodukten, Tensiden
und verwandten Stoffen, 83.ü Mitt.: Analyse von fettreichen
Lebensmitteln IV. Fat science and technology, 92(3):107-109.
BAGS (1995) Standards zur Expositionsabschätzung. Bericht des
Ausschusses für Umwelthygiene (Arbeitsgemeinschaft der leitenden
Medizinalbeamtinnen und -beamten der Länder). Hamburg, Behörde für
Arbeit, Gesundheit und Soziales.
Baldwin EA, Nisperos-Carriedo MO, Baker RA (1995) Use of edible
coatings to preserve quality of lightly (and slightly) processed
products. Critical reviews in food science and nutrition,
35(6):509-524.
Barker JF, Barbash JE, Labonte M (1988) Groundwater contamination at a
landfill sited on fractured carbonate and shale. Journal of
contaminant hydrology, 3:1-25.
Batshaw ML, Brusilow SW (1981) Evidence of lack of toxicity of sodium
phenylacetate and sodium benzoate in treating urea cycle
enzymopathies. Journal of inherited metabolic disease, 4:231.
Batshaw ML, Monahan PS (1987) Treatment of urea cycle disorders.
Enzyme, 38:242-250.
Battersby NS, Wilson V (1989) Survey of the anaerobic biodegradation
potential of organic chemicals in digesting sludge. Applied and
environmental microbiology, 55(2):433-439.
Bayer AG (1977) Akute orale Toxizität / Untersuchung zur Haut- und
Schleimhautverträglichkeit. Wuppertal (unpublished report).
Bayer AG (1978) Untersuchung zur Haut- und
Schleimhautverträglichkeit. Wuppertal (unpublished report).
Bayer AG (1986) Benzoesäure DAB 8. Prüfung auf primär reizende/
ätzende Wirkung am Kaninchenauge. Wuppertal (unpublished report).
Bedford PGC, Clarke EGC (1972) Experimental benzoic acid poisoning in
the cat. Veterinary record, 90:53-58.
Bennett MC, Petrus DR (1977) Quantitative determination of sorbic acid
and sodium benzoate in citrus juice. Journal of food science,
42:1220-1221.
BFGoodrich Kalama Inc. (1999) Benzoic acid. Product information
bulletin. Kalama, WA.
Bindslev-Jensen C (1998) ABC of allergies. Food allergy. British
medical journal, 316:1299-1302.
Bio-Fax (1973) Benzoic acid. Northbrook, IL, Industrial Bio-Test
Laboratories, Inc.
Birch RR, Biver C, Campagna R, Gledhill WE, Pagga U, Steber J, Reust
H, Bontinck WJ (1989) Screening of chemicals for anaerobic
biodegradability. Chemosphere, 19(10-11):1527-1550.
BMA (British Medical Association) (1998) Antifungal preparations --
Benzoic acid. Wallingford, Pharmaceutical Press (British National
Formulary 507).
Brasch J, Henseler T, Frosch P (1993) Patch test reactions to a
preliminary preservative series. Dermatosen, 41(2):71-76.
Bridges JW, French MR, Smith RL, Williams RT (1970) The fate of
benzoic acid in various species. Biochemical journal, 118:47-51.
Bringmann G, Kuehn R (1977) [Threshold values for the damaging action
of water pollutants to bacteria (Pseudomonas putida) and green algae
(Scenedesmus quadricauda) in the cell multiplication inhibition
test.] Zeitschrift für Wasser und Abwasser Forschung, 10:87-98 (in
German).
Bringmann G, Kuehn R (1980) Bestimmung der biologischen Schadwirkung
wassergefährdender Stoffe gegen Protozoen. II. Bakterienfressende
Ciliaten. Zeitschrift für Wasser und Abwasser Forschung, 1:26-31.
Bringmann G, Kuehn R (1982) [Results of toxic action of water
pollutants on Daphnia magna Straus tested by an improved
standardized procedure.] Zeitschrift für Wasser und Abwasser
Forschung, 15:1-6 (in German).
Broeckx W, Blondeel A, Do-Go A, Achten G (1987) Cosmetic intolerance.
Contact dermatitis, 16:189-194.
Bronaugh RL, Stewart RF, Congdon ER, Giles AL (1982a) Methods for in
vitro percutaneous absorption studies. I. Comparison with in vivo
results. Toxicology and applied pharmacology, 62:474-480.
Bronaugh RL, Stewart RF, Congdon ER (1982b) Methods for in vitro
percutaneous absorption studies. II. Animal methods for human skin.
Toxicology and applied pharmacology, 62:481-488.
BUA (1995) BUA-Stoffbericht Benzoesaeure, Natriumbenzoat.
Beratergremium für Umweltrelevante Altstoffe. Stuttgart, S. Hirzel
Verlag (Stoffbericht Nr. 145).
Bucks DA, Hinz RS, Sarason R, Maibach HI, Guy RH (1990) In vivo
percutaneous absorption of chemicals: a multiple dose study in rhesus
monkeys. Food and chemical toxicology, 28:129-132.
Budavari S, O'Neil MJ, Smith A, Heckelman PE, Kinneary JF, eds. (1996)
The Merck index -- an encyclopedia of chemicals, drugs, and
biologicals, 12th ed. Whitehouse Station, NJ, Merck & Co., Inc.
p. 183.
Carlberg GE, Drangsholt H, Gjoes N (1986) Identification of
chlorinated compounds in the spent chlorination liquor from
differently treated sulphite pulps with special emphasis on mutagenic
compounds. Science of the total environment, 48:157-167.
Carver MP, Riviere JE (1989) Percutaneous absorption and excretion of
xenobiotics after topical and intravenous administration to pigs.
Fundamental and applied toxicology, 13:714-722.
Castro JC, Delgado MA, Sánchez MJ, Montelongo FG (1992) Simultaneous
2nd order derivative spectrophotometric determination of sorbic and
benzoic acids in soft drinks. Analytical letters, 25(12):2357-2376.
Cautreels W, van Cauwenberghe K (1978) Experiments on the distribution
of organic pollutants between airborne particulate matter and the
corresponding gas phase. Atmospheric environment, 12:1133-1141.
Chipley JR (1983) Sodium benzoate and benzoic acid. In: Branen AL,
Davidson PM, eds. Antimicrobials in foods. New York, NY, M. Decker,
pp. 11-35.
Chittenden RH, Long JH, Herter CA (1909) Chemical bulletin, 88. US
Department of Agriculture [cited in WHO, 1996].
Clemmensen O, Hjorth N (1982) Perioral contact urticaria from sorbic
and benzoic acid in salad dressings. Contact dermatitis, 8:1-6.
Cordt T, Kußmaul H (1990) Niedermolekulare Carbonsäuren imBoden, in
der ungesättigten Zone und im Grundwasser. Vom wasser, 74:287-298.
Courtes R, Bahlaoui A, Rambaud A, Deschamps F, Sunde E, Dutrieux E
(1995) Ready biodegradability test in seawater: a new methodological
approach. Ecotoxicology and environmental safety, 31:142-148.
Coverly J, Peters L, Whittle E, Basketter DA (1998) Susceptibility to
skin stinging, non-immunologic contact urticaria and acute skin
irritation; is there a relationship? Contact dermatitis,
38(2):90-95.
Daston GP, Baines D, Elmore E, Fitzgerald MP, Sharma S (1995)
Evaluation of chick embryo neural retina cell culture as a screen for
developmental toxicants. Fundamental and applied toxicology,
26:203-210.
Dawson DA, Schultz TW, Hunter RS (1996) Developmental toxicity of
carboxylic acids to Xenopus embryos: A quantitative
structure-activity relationship and computer-automated structure
evaluation. Teratogenesis, carcinogenesis, and mutagenesis,
16:109-124.
Deuel HJJ, Alfin-Slater R, Weil CS, Smyth HF Jr (1954) Sorbic acid as
a fungistatic agent for foods. 1. Harmlessness of sorbic acid as a
dietary component. Food research, 19:1-12.
Dierickx PJ (1998) Increased cytotoxic sensitivity of cultured FHM
fish cells by simultaneous treatment with sodium dodecyl sulfate and
buthionine sulfoximine. Chemosphere, 36(6):1263-1274.
EC (1995) European Union Directive 95/2/CE from 20.02.1995 on food
additives, colourants and sweeteners. European Commission.
Elder DJE, Kelly DJ (1994) The bacterial degradation of benzoic acid
and benzenoid compounds under anaerobic conditions: Unifying trends
and new perspectives. FEMS microbiology reviews, 13:441-468.
Ewell WS, Gorsuch JW, Kringle RO, Robillard KA, Spiegel RC (1986)
Simultaneous evaluation of the acute effects of chemicals on seven
aquatic species. Environmental toxicology and chemistry, 5:831-840.
Fanelli GM, Halliday SL (1963) Relative toxicity of chlortetracycline
and sodium benzoate after oral administration to rats. Archives
internationales de pharmacodynamie et de thérapie, 144(1-2):120-125.
Federle TW (1988) Mineralization of monosubstituted aromatic compounds
in unsaturated and saturated subsurface soils. Canadian journal of
microbiology, 34:1037-1042.
Feillet F, Leonard JV (1998) Alternative pathway therapy for urea
cycle disorders. Journal of inherited metabolic disease, Suppl.
21(1):101-111.
Feldmann RJ, Maibach HI (1970) Absorption of some organic compounds
through the skin in man. Journal of investigative dermatology,
54:399-404.
Flood PF, Abrams SR, Muir GD, Rowell JE (1989) Odor of the muskox. A
preliminary investigation. Journal of chemical ecology,
15:2207-2217.
Franz TJ (1975) Percutaneous absorption. On the relevance of in vitro
data. Journal of investigative dermatology, 64:190-195.
Freedman BJ (1977) Asthma induced by sulphur dioxide, benzoate and
tartrazine contained in orange drinks. Clinical allergy, 7:407-415.
Freitag D, Ballhorn L, Geyer H, Korte F (1985) Environmental hazard of
organic chemicals. An experimental method for the assessment of the
behaviour of organic chemicals in the ecosphere by means of simple
laboratory tests with 14C labelled chemicals. Chemosphere,
14:1589-1616.
Friedman LJ, Greenwald CG (1994) Food additives. In: Kroschwitz J,
Howe-Grant M, eds. Kirk-Othmer: Encyclopedia of chemical technology.
Vol. 11. New York, NY, Wiley and Sons, pp. 805-833.
Fuchs G, el Said Mohammed M, Altenschmidt U, Koch J, Lack A, Brackmann
R, Lochmeyer C, Oswald B (1993) Biochemistry of anaerobic
biodegradation of aromatic compounds. In: Ratledge C, ed.
Biochemistry of microbial degradation. Dordrecht, Kluwer Academic
Publishers, pp. 513-553.
Fujii T, Omori T, Tagucji T, Ogata M (1991) Urinary excretion of
hippuric acid after administration of sodium benzoate (biological
monitoring 1). Shokuhin Eiseigaku Zasshi (Journal of the Food Hygiene
Society of Japan), 32(3):177-182.
Fujitani T (1993) Short-term effect of sodium benzoate in F344 rats
and B6C3F1 mice. Toxicology letters, 69:171-179.
Gad SC, Dunn BJ, Dobbs DW, Reilly C, Walsh RD (1986) Development and
validation of an alternative dermal sensitization test: the mouse ear
swelling test (MEST). Toxicology and applied pharmacology,
84:93-114.
Gailhofer G, Soyer HP, Ludvan M (1990) Nahrungsmittelallergien und
Pseudoallergien -- Mechanismen, Klinik und Diagnostik. Wiener
Medizinische Wochenschrift, 140:227-232.
Geiger DL, Northcott CE, Call DJ, Brooke LT (1985) Acute toxicities
of organic chemicals to fathead minnows (Pimephales promelas ).
Vol. II. Superior, WI, University of Wisconsin, pp. 139-140.
Gerberick GF, House RV, Fletcher ER, Ryan CA (1992) Examination of the
local lymph node assay for use in contact sensitization risk
assessment. Fundamental and applied toxicology, 19:438-445.
Gerlach V (1909) VII. Summary of the results. In: Physiological
activity of benzoic acid and sodium benzoate. Wiesbaden, Verlag von
Heinrich Staadt [cited in US FDA, 1972a].
Goerlitz DF, Troutman DE, Godsy EM, Franks BJ (1985) Migration of
wood-preserving chemicals in contaminated groundwater in a sand
aquifer at Pensacola, Florida. Environmental science and technology,
19(10):955-961.
Goodman Gilman A, Rall TW, Nies AS, Taylor P, eds. (1990) Goodman and
Gilman's the pharmacological basis of therapeutics, 8th ed. New York,
NY, Pergamon Press, p. 1179.
Goodwin BL (1976) Handbook of intermediary metabolism of aromatic
compounds. New York, NY, Wiley & Sons, pp. B6-B9.
Green TP, Marchessault RP, Freese DK (1983) Disposition of sodium
benzoate in newborn infants with hyperammonemia. Journal of
pediatrics, 102:785-790.
Guardiola J, Ventura J, Rivera J (1989) Occurrence of industrial
organic pollution in a groundwater supply: screening, monitoring and
evaluation of treatment processes. Water supply, 7:11-16.
Haider K, Jagnow G, Kohnen R, Lim SU (1974) Abbau chlorierter Benzole,
Phenole und Cyclohexan- Derivate durch Benzol und Phenol verwertende
Bakterien unter anaeroben Bedingungen. Archives of microbiology,
96:183-200.
Halvorson DO (1984) Determination of benzoic acid in air. American
Industrial Hygiene Association journal, 45(10):727-730.
Ham RK, Boyle WC, Engroff EC, Fero RL (1989) Determining the presence
of organic compounds in foundry waste leachates. Modern casting,
79:27-31.
Harborne JB (1983) Toxins of plant-fungal interactions. In: Keeler RF,
Tu AT, eds. Handbook of natural toxins. Vol. 1. Plant and fungal
toxins. New York, NY, Marcel Dekker, pp. 755-758.
Hardin BD, Schuler RL, Burg JR, Booth GM, Hazelden KP, MacKenzie KM,
Piccirillo VJ, Smith KN (1987) Evaluation of 60 chemicals in a
preliminary developmental toxicity test. Teratogenesis,
carcinogenesis, and mutagenesis, 7:29-48 [cited in WHO, 1996].
Harwood CS, Gibson J (1997) Shedding light on anaerobic benzene ring
degradation: a process unique to prokaryotes? Journal of
bacteriology, 179:301-309.
Hegnauer R, ed. (1966) Chemotaxonomie der Pflanzen. Basel,
Birkhäuser Verlag.
Hegnauer R, ed. (1989) Chemotaxonomie der Pflanzen. Basel,
Birkhäuser Verlag, pp. 415-416.
Hegnauer R (1992) Benzoesäure. In: Hegnauer R, ed. Chemotaxonomie der
Pflanzen. Basel, Birkhäuser Verlag.
Helmig D, Müller J, Klein W (1989) Volatile organic substances in a
forest atmosphere. Chemosphere, 19(8-9):1399-1412.
Hofrichter M, Fritsche W (1996) Abbau aromatischer Kohlenwasserstoffe
durch den Schimmelpilz Penicillium frequentans Bi 7/2. Gas- und
Wasserfach; Wasser-Abwasser, 137:199-204.
Hotchkiss SA, Hewitt P, Caldwell J, Chen WL, Rowe RR (1992)
Percutaneous absorption of nicotinic acid, phenol, benzoic acid and
triclopyr butoxyethyl ester through rat and human skin in vitro:
Further validation of an in vitro model by comparison with in vivo
data. Food and chemical toxicology, 30:891-899.
Hunziker N, Feldmann RJ, Maibach H (1978) Animal models of
percutaneous penetration: comparison between Mexican hairless dogs and
man. Dermatologica, 156:79-88.
Ibero M, Eseverri JL, Barroso C, Botey J (1982) Dyes, preservatives
and salicylates in the induction of food intolerance and/or
hypersensitivity. Allergologia et Immunopathologia, 10(4):263-268.
IPCS (1993) International Chemical Safety Card -- Benzoic acid.
Geneva, World Health Organization, International Programme on
Chemical Safety (ICSC 0103).
Ishida H (1996) Levels of preservatives in toothpastes and possibility
of their intake during brushing of teeth. Shokuhin Eiseigaku Zasshi
(Journal of the Food Hygiene Society of Japan), 37:234-239.
Ishidate M, Odashima S (1977) Chromosome tests with 134 compounds on
Chinese hamster cells in vitro -- a screening for chemical
carcinogenesis. Mutation research, 48:337-354.
Ishidate MJ, Sofuni T, Yoshikawa K, Hayashi M, Nohmi T, Sawada M,
Matsuoka A (1984) Primary mutagenicity screening of food additives
currently used in Japan. Food and chemical toxicology,
22(8):623-636.
Ishidate MJ, Harnois MC, Sofuni T (1988) A comparative analysis of
data on the clastogenicity of 951 chemical substances tested in
mammalian cell cultures. Mutation research, 195:151-213.
Ishiguro S, Miyamoto A, Obi T, Nishio A (1993) Teratological studies
on benzyl acetate in pregnant rats. Kadnau (Bulletin of the Faculty
of Agriculture, Kagoshima University), 43:25-31 [cited in WHO, 1996].
Ishizaki M, Ueno S (1989) The DNA damaging activity of natural and
synthetic food additives. Shokuhin Eiseigaku Zasshi (Journal of the
Food Hygiene Society of Japan), 30:447-451.
Jalal MAF, Read DJ (1983) The organic acid composition of Calluna
heathland soil with special reference to phyto- and fungitoxicity. I.
Isolation and identification of organic acids. Plant and soil,
70:257-272.
James MO, Pritchard JB (1987) In vivo and in vitro metabolism and
excretion of benzoic acid by a marine teleost, the southern flounder.
Drug metabolism and disposition, 15:665-670.
Jansson T, Curvall M, Hedin A, Enzell CR (1988) In vitro studies of
the biological effects of cigarette smoke condensate. III. Induction
of SCE by some phenolic and related constituents derived from
cigarette smoke. Mutation research, 206:17-24.
Jelinek R, Peterka M, Rychter Z (1985) Chick embryotoxicity screening
test -- 130 substances tested. Indian journal of experimental
biology, 23:588-595.
Juhlin L, Michaelsson G, Zetterström O (1972) Urticaria and asthma
induced by food and drug additives in patients with aspirin
hypersensitivity. Journal of allergy and clinical immunology,
50(2):92-98.
Juhnke I, Luedemann D (1978) Ergebnisse der Untersuchung von
chemischen Verbindungen auf akute Fischtoxizität mit dem
Goldorfentest. Zeitschrift für Wasser und Abwasser Forschung,
11(5):161-164.
Kaiser KLE, Palabrica VS, Ribo JM (1987) QSAR of acute toxicity of
mono-substituted benzene derivatives to Photobacterium phosphoreum.
QSAR environmental toxicology, 11:153-168.
Kameya T, Murayama T, Urano K, Kitano M (1995) Biodegradation ranks of
priority organic compounds under anaerobic conditions. Science of the
total environment, 170(1-2):43-51.
Kawamura K, Kaplan IR (1986) Biogenic and anthropogenic organic
compounds in rain and snow samples collected in Southern California.
Atmospheric environment, 20:115-124.
Kawamura K, Kaplan IR (1990) Stabilities of carboxylic acids and
phenols in Los Angeles rainwaters during storage. Water research,
24(11):1419-1423.
Kawamura K, Ng LL, Kaplan IR (1985) Determination of organic acids
(C1-C10) in the atmosphere, motor exhausts, and engine oils. IPCS
guidelines for the monitoring of genotoxic effects of carcinogens in
humans. Environmental science and technology, 19:1082-1086.
Kay JH, Calandra JC (1962) Interpretation of eye irritation tests.
Journal of the Society of Cosmetic Chemists, 13:281-289.
Kieckebusch W, Lang K (1960) Die Verträglichkeit der Benzoesäure im
chronischen Fütterungsversuch. Arzneimittel-Forschung, 10:1001-1003.
Kimmel CA, Wilson JG, Schumacher HJ (1971) Studies on metabolism and
identification of the causative agent in aspirin teratogenesis in
rats. Teratology, 4:15-24.
King ADJ, Halbrook WU (1987) Ascospore heat resistance and control
measures for Talaromyces flavus isolated from fruit juice
concentrate. Journal of food science, 52:1252-1254.
King EF, Painter HA (1983) Ring-test programme 1981-82. Assessment of
biodegradability of chemicals in water by manometric respirometry.
Luxemburg, Commission of the European Communities (EUR 8631 EN 31 S).
Kinney IC, Ivanuski VR (1969) Photolysis mechanisms for pollution
abatement. Cincinnati, OH, US Department of the Interior, Federal
Water Pollution Control Administration, Robert A. Taft Water Research
Center (TWRC-13).
Klecka GM, Landi LP, Bo KM (1985) Evaluation of the OECD activated
sludge, respiration inhibition test. Chemosphere, 14(9):1239-1251.
Kobayashi T, Hashinaga T, Mikami E, Suzuki T (1989) Methanogenic
degradation of phenol and benzoate in acclimated sludges. Water
science and technology, 21:55-65.
Kocwa-Haluch R, Lemek M (1995) Easy and inexpensive diffusion tests
for detecting the assimilation of aromatic compounds by yeast-like
fungi. Part II. Assimilation of aromatic acids. Chemosphere,
31(11/12):4333-4339.
Koda T, Tsuchiya H, Yamauchi M, Hoshino Y, Takagi N, Kawano J (1989)
High-performance liquid chromatographic estimation of eluates from
denture base polymers. Journal of dentistry, 17:84-89.
Koda T, Tsuchiya H, Yamaguchi M, Ohtani S, Takagi N, Kawano J (1990)
Leachability of denture-base acrylic resins in artificial saliva.
Dental materials, 6:13-16.
Kreis H, Frese F, Wilmes G (1967) Physiologische und morphologische
Veränderungen an Ratten nach peroraler Verabreichung von Benzoesäure.
Food and cosmetics toxicology, 5:505-511.
Kubota K, Ishizaki T (1991) Dose-dependent pharmacokinetics of benzoic
acid following oral administration of sodium benzoate to humans.
European journal of clinical pharmacology,41(4):363-368.
Kubota K, Horai Y, Kushida K, Ishizaki T (1988) Determination of
benzoic acid and hippuric acid in human plasma and urine by high
performance liquid chromatography. Journal of chromatography,
425(1):67-75.
Lahti A, Hannuksela M (1981) Is benzoic acid really harmful in cases
of atopy and urticaria? Lancet, 2:1055.
Lahti A, Maibach HI (1984) An animal model for nonimmunologic contact
urticaria. Toxicology and applied pharmacology, 76:219-224.
Lahti A, Pylvanen V, Hannuksela M (1995) Immediate irritant reactions
of benzoic acid are enhanced in washed skin areas. Contact
dermatitis, 33:177-182.
Larmi E, Lahti A, Hannuksela M (1988) Effects of sorbitan-sesquioleate
on non-immunologic immediate contact reactions to benzoic acid.
Contact dermatitis, 19:368-371.
Larsson B (1983) Gas-liquid chromatographic determination of benzoic
acid and sorbic acid in foods: MNKL collaborative study (1983).
Journal of the Association of Official Analytical Chemists,
66:775-780.
Lindström K, Österberg F (1986) Chlorinated carboxylic acids in
softwood kraft pulp spent bleach liquors. Environmental science and
technology, 20(2):133-138.
Lu PY, Metcalf RL (1975) Environmental fate and biodegradability of
benzene derivatives as studied in a model aquatic ecosystem.
Environmental health perspectives, 10:269-284.
Lund FA, Rodriguez DS (1984) Acclimation of activated sludge to
mono-substituted derivatives of phenol and benzoic acid. Journal of
general applied microbiology, 30:53-61.
Lunde G, Gehter J, Gjos N, Lande MBS (1977) Organic micropollutants in
precipitation in Norway. Atmospheric environment, 11:1007-1014.
MacPherson SE, Barton CN, Bronaugh RL (1996) Use of in vitro skin
penetration data and a physiologically based model to predict in vivo
blood levels of benzoic acid. Toxicology and applied pharmacology,
140:436-443.
Maibach HI, Johnson HL (1975) Contact urticaria syndrome. Archives of
dermatology, 111:726-730.
Maibach HI, Wester RC (1989) Percutaneous absorption: in vivo
methods in humans and animals. Journal of the American College of
Toxicology, 8:803-813.
Mailhot H (1987) Prediction of algal bioaccumulation and uptake rate
of nine organic compounds by ten physicochemical properties.
Environmental science and technology, 21:1009-1013.
Maki T, Suzuki Y (1985) Benzoic acid and derivatives. In: Ullmann's
encyclopedia of industrial chemistry. Vol. A3. Weinheim, VCH
Verlagsgesellschaft mbH, pp. 555-568.
Mandrou B, Nolleau V, Gastaldi E, Fabre H (1998) Solid-phase
extraction as a clean-up procedure for the liquid chromatographic
determination of benzoic acid and sorbic acid in fruit-derived
products. Journal of liquid chromatography and related technology,
21:829-842.
Marquardt P (1960) Zur Verträglichkeit der Benzoesäure.
Arzneimittel-Forschung, 10:1033.
Matthews RW (1990) Purification of water with near-U.V. illuminated
suspensions of titanium dioxide. Water research, 24:653-660.
McCann J, Choi E, Yamasaki E, Ames BN (1975) Detection of carcinogens
as mutagens in the Salmonella/microsome test: assay of
300 chemicals. Proceedings of the National Academy of Sciences
of the United States of America, 72(12):5135-5139.
McCormick GC (1974) A comparison of the acute toxicity, distribution,
fate, and some pharmacologic properties of the non-benzenoid aromatic
compound azuloic acid with those of benzoic and naphthoic acids in
mice. Dissertation abstracts international, B35(10):5029B-5030B.
McKenna KE, Walsh MY, Burrows D (1994) The Melkersson-Rosenthal
syndrome and food additive hypersensitivity. British journal of
dermatology, 131:921-922.
Miguez CB, Greer CW, Ingram JM, MacLeod RA (1995) Uptake of benzoic
acid and chloro-substituted benzoic acids by Alcaligenes
denitrificans BRI 3010 and BRI 6011. Applied and environmental
microbiology, 61:4152-4159.
Minor JL, Becker BA (1971) A comparison of the teratogenic properties
of sodium salicylate, sodium benzoate, and phenol. Toxicology and
applied pharmacology, 19:373.
MITI (1992) Biodegradation and bioaccumulation. Data of existing
chemicals based on the CSCL Japan. Tokyo, Ministry of International
Trade and Industry, Chemicals Inspection & Testing Institute.
Moneret-Vautrin DA, Moeller R, Malingrey L, Laxenaire MC (1982)
Anaphylactoid reaction to general anaesthesia: A case of intolerance
to sodium benzoate. Anaesthesia and intensive care, 10:156-157.
Monsanto Co. (1983) Primary eye irritation of benzoic acid to
rabbits. St. Louis, MO, Monsanto Company, Environmental Health
Laboratory.
Munoz FJ, Bellido J, Moyano JC, Alvarez M, Fonseca JL (1996) Perioral
contact urticaria from sodium benzoate in a toothpaste. Contact
dermatitis, 35:51.
Nakamura SI, Oda Y, Shimada T, Oki I, Sugimoto K (1987) SOS-inducing
activity of chemical carcinogens and mutagens in Salmonella
typhimurium TA1535/pSK1002: examination with 151 chemicals. Mutation
research, 192:239-246.
Nonaka M (1989) DNA repair tests on food additives. Environmental and
molecular mutagenesis, 14 (Suppl. 15):143.
Nottingham PM, Hungate RE (1969) Methanogenic fermentation of
benzoate. Journal of bacteriology, 98:1170-1172.
O'Connor JE, Costell M, Grisolia S (1987) The potentiation of ammonia
toxicity by sodium benzoate is prevented by L-carnithine. Biochemical
and biophysical research communications, 145:817-824.
Oikawa A, Tohda H, Kanai M, Miwa M, Sugimura T (1980) Inhibitors of
poly(adenosine diphosphate ribose) induced sister chromatid exchanges.
Biochemical and biophysical research communications,
97(4):1311-1316.
Onodera H, Ogiu T, Matsuoka C, Furuta K, Takeuchi M, Oono Y, Kubota T,
Miyahara M, Maekawa A, Odashima S (1978) [Studies on effects of sodium
benzoate on fetuses and offspring of Wistar rats.] Eisei Shikensho
Hokoku, 96:47-55 (in Japanese) [cited in WHO, 1996].
Osterballe O, Taudorf E, Haahr J (1979) Intolerance to aspirin,
food-colouring agents and food preservatives in childhood asthma.
Ugeskrift for Laeger, 141:1908-1910.
Oussi D, Mokrini A, Chamarro E, Esplugas S (1998) Photodegradation of
benzoic acid in aqueous solutions. Environmental technology,
19(9):955-960.
Palm WU, Millet M, Zetsch C (1998) OH radical reactivity of pesticides
adsorbed on aerosol materials: first results of experiments with
filter samples. Ecotoxicology and environmental safety, 41(1):36-43.
Parke DV (1968) The biochemistry of foreign compounds. Oxford,
Pergamon Press, pp. 1-261.
Petrus M, Bonaz S, Causse E, Rhabbour M, Moulie N, Netter JC,
Bildstein G (1996) [Asthma induced by benzoate contained in some foods
and anti-allergic drugs.] Archives de pédiatrie, 3(10):984-987 (in
French).
Pitter P (1976) Determination of biological degradability of organic
substances. Water research, 10:231-235.
Prival MJ, Simmon VF, Mortelmans KE (1991) Bacterial mutagenicity
testing of 49 food ingredients gives very few positive results.
Mutation research, 260:321-329.
Puttemans ML, Dryon L, Massart DL (1984) Extraction of organic acids
by iron-pair formation with tri- n-octylamine. Part V. Simultaneous
determination of synthetic dyes, benzoic acid, sorbic acid and
saccharin in soft drinks and lemonade syrups. Journal of the
Association of Official Analytical Chemists, 67:880-885.
Rasmussen LEL, Hess DL, Haight JD (1990) Chemical analysis of temporal
gland secretions collected from an Asian bull elephant during a
four-month musth episode. Journal of chemical ecology,
16(7):2167-2181.
Rastogi SC, Lepoittevin JP, Johansen JD, Frosch PJ, Menné T, Bruze M,
Dreier B, Andersen KE, White IR (1998) Fragrances and other materials
in deodorants: Search for potentially sensitizing molecules using
combined GC-MS and structure activity relationship (SAR) analysis.
Contact dermatitis, 39(6):293-303.
RCC Notox (1988a) Primary skin irritation/corrosion study of benzoic
acid in the rabbit. DD's-Hertogenbosch, RCC Notox B.V. (unpublished
report).
RCC Notox (1988b) Eye irritation/corrosion study of benzoic acid in
the rabbit. DD's-Hertogenbosch, RCC Notox B.V. (unpublished report).
RCC Notox (n.d., a) Primary skin irritation/corrosion study with
natrium benzoate in rabbits. DD's-Hertogenbosch, RCC Notox B.V.
(unpublished report).
RCC Notox (n.d., b) Acute eye irritation/corrosion study with natrium
benzoate in rabbits. DD's-Hertogenbosch, RCC Notox B.V. (unpublished
report).
Reifenrath WG, Chellquist EM, Shipwash EA, Jederberg WW, Krueger GG
(1984) Percutaneous penetration in the hairless dog, weanling pig and
grafted athymic nude mouse: Evaluation of models for predicting skin
penetration in man. British journal of dermatology,
Suppl. 111(S27):123-135.
Reinhard M, Goodman NL (1984) Occurrence and distribution of organic
chemicals in two landfill leachate plumes. Environmental science and
technology, 18:953-961.
Richterich K, Steber J (1989) Prevention of nitrification-caused
erroneous biodegradability data in the closed bottle test.
Chemosphere, 19(10-11):1643-1654.
Ring J (1989) Arzneimittelunverträglichkeit durch pseudo-allergische
Reaktionen. Wiener Medizinische Wochenschrift, 139:130-134.
Rubin HE, Subba-Rao RV, Alexander M (1982) Rates of mineralization of
trace concentrations of aromatic compounds in lake water and sewage
samples. Applied and environmental microbiology, 43(5):1133-1138.
Russell AD, Furr JR (1996) Biocides mechanisms of antifungal action
and fungal resistance. Science progress, 79(1):27-48.
Sainio E, Kanerva L (1995) Contact allergens in toothpastes and a
review of their hypersensitivity. Contact dermatitis, 33:100-105.
Sakuma H, Kusama M, Munakata S, Ohsumi T, Sugawara S (1983) The
distribution of cigarette smoke components between mainstream and
sidestream smoke. Beitraege zur Tabakforschung, 12:63-71.
Salanitro JP, Langston GC, Dorn PB, Kravetz L (1988) Activated sludge
treatment of ethoxylate surfactants at high industrial use
concentrations. Water science and technology, 20(11-12):125-130.
Schelhorn M (1951) Untersuchungen über Konservierungsmittel. VI.
Wirksamkeit und Anwendungsbereich von Benzoesäure, Estern der
Paraoxybenzoesäure und Kombination dieser beiden Arten von
Verbindungen. Deutsche Lebensmittel-Rundschau, 47:128-134.
Scholz W, Kortmann W (1991) Paint additives. In: Elvers B, Hawkins S,
Schulz G, eds. Ullmann's encyclopedia of industrial chemistry.
Weinheim, VCH Verlagsgesellschaft, pp. 465-472.
Schou L, Krane JE (1981) Organic micropollutants in a Norwegian
water-course. Science of the total environment, 20:277-286.
Schuetzle D, Cronn D, Crittenden AL (1975) Molecular composition of
secondary aerosol and its possible origin. Environmental science and
technology, 9:838-845.
Schultz TW, Bryant SE, Kissel ST (1996) Toxicological assessment in
Tetrahymena of intermediates in aerobic microbial transformation of
toluene and p-xylene. Bulletin of environmental contamination and
toxicology, 56:129-134.
Sharak Genthner BR, Townsend GT, Blattman BO (1997) Reduction of
3-chlorobenzoate, 3-bromobenzoate, and benzoate to corresponding
alcohols by Desulfomicrobium escambiense, isolated from a
3-chlorobenzoate-dechlorinating coculture. Applied and environmental
microbiology, 63:4698-4703.
Shibamoto T (1986) Photochemistry of a major essential oil
constituent, benzyl benzoate. Developments in food science,
12:745-754.
Shibamoto T, Umano K (1985) Photochemical products of benzyl benzoate:
possible formation of skin allergens. Journal of toxicology,
cutaneous and ocular toxicology, 4(2):97-104.
Shimp RJ, Young RL (1987) Comparison of OECD and radiolabeled
substrate methods for measuring biodegradation in marine environments.
Ecotoxicology and environmental safety, 14:223-230.
Sieber R, Bütikofer U, Bosset JO, Rüegg M (1989) Benzoesäure als
natürlicher Bestandteil von Lebensmitteln- eine Übersicht.
Mitteilungen aus dem Gebiete der Lebensmitteluntersuchung und
Hygiene, 80:345-362.
Sieber R, Bütikofer U, Baumann E, Bosset JO (1990) Über das Vorkommen
der Benzoesäure in Sauermilchprodukten und Käse. Mitteilungen aus dem
Gebiete der Lebensmitteluntersuchung und Hygiene, 84:484-493.
Sioufi A, Pommier F (1980) Gas chromatographic determination of low
concentrations of benzoic acid in human plasma and urine. Journal of
chromatography, biomedical applications, 181:161-168.
Smyth HF, Carpenter CP (1948) Further experience with the range
finding test in the industrial toxicology laboratory. Journal of
industrial hygiene and toxicology, 30:63-68.
Sodemoto Y, Enomoto M (1980) Report of carcinogenesis bioassay of
sodium benzoate in rats: absence of carcinogenicity of sodium benzoate
in rats. Journal of environmental pathology and toxicology, 4:87-95.
Soni GL, Bhatia IS (1980) Toxicity of phenolics and related compounds
to Fusarium oxasporium Schlecht. and effect of pH on their
toxicity. Indian journal of agricultural science, 50(10):772-777.
SRI (1998) 1997/98 directory of chemical producers Europe. Menlo
Park, CA, SRI International (807-808/1613).
Srour R (1989) Benzoic acid. In: Srour R, ed. Aromatic intermediates
and derivatives. Paris, pp. A.IV.1-A.IV.14 (unpublished report)
[cited in BUA, 1995].
Srour R (1998) Benzoic acid and derivatives. In: Srour R, ed.
Aromatic intermediates and derivatives. Paris, pp. A.IV.1- A.IV.17
(unpublished report).
Steeg E, Montag A (1987) Nachweis aromatischer Carbonsäuren in Honig.
Zeitschrift für Lebensmittel-Untersuchung und -Forschung, 184:17-19.
Stolpe NB, McCallister DL, Shea PJ, Lewis DT, Dam R (1993) Mobility of
aniline, benzoic acid, and toluene in four soils and correlation with
soil properties. Environmental pollution, 81:287-295.
Stratton GW, Corke CT (1982) Toxicity of the insecticide permethrin
and some degradation products towards algave and cyanobacteria.
Environmental pollution, 29:71-80.
Stuermer DH, Ng DJ, Morris CJ (1982) Organic contaminants in
groundwater near underground coal gasification site in northeastern
Wyoming. Environmental science and technology, 16:582-587.
Suflita JM, Concannon F (1995) Screening tests for assessing the
anaerobic biodegradation of pollutant chemicals in subsurface
environments. Journal of microbiological methods, 21:267-281.
Takemoto S, Kuge Y, Nakamoto M (1981) The measurement of BOD in sea
water. Japanese journal of water pollution research, 4:80-90.
Tohda H, Horaguchi K, Takahashi K, Oikawa A, Matsushima T (1980)
Epstein-Barr virus-transformed human lymphoblastoid cells for study of
sister chromatid exchange and their evaluation as a test system.
Cancer research, 40:4775-4780.
Tong HY, Shore DL, Karasek FW, Helland P, Jellum E (1984)
Identification of organic compounds obtained from incineration of
municipal waste by high performance liquid chromatographic
fractionation and gas chromatography-mass spectrometry. Journal of
chromatography, 285:423-441.
Toth B (1984) Lack of tumorigenicity of sodium benzoate in mice.
Fundamental and applied toxicology, 4:494-496.
Toyoda M, Ito Y, Isshiki K, Onishi K, Kato T, Kamikura M, Shiroishi Y,
Harada Y, Hukasawa Y, Yokoyama T, Yoneda M, Iwaida M (1983a) Daily
intake of preservatives, benzoic acid, dehydroacetic acid, propionic
acid and their salts, and esters of p-hydrobenzoic acid in Japan.
Journal of the Japanese Society of Nutrition and Food Science,
96:467-480.
Toyoda M, Ito Y, Isshiki K, Onishi K, Kato T, Kamikura M, Shiroishi Y,
Harada Y, Hukasawa Y, Yokoyama Y, Yamamoto Y, Fujii M, Iwaida M
(1983b) Estimation of daily intake of many kinds of food additives
according to the market basket studies in Japan. Journal of the
Japanese Society of Nutrition and Food Science, 36:489-497.
Tremblay GC, Qureshi IA (1993) The biochemistry and toxicology of
benzoic acid metabolism and its relationship to the elimination of
waste nitrogen. Pharmacology and therapeutics, 60:63-90.
Tyler TA (1984) Liquid chromatographic determination of sodium
saccharin, caffeine, aspartame, and sodium benzoate in cola beverages.
Journal of the Association of Official Analytical Chemists,
67(4):745-747.
UK MAFF (1995) Survey of sulphur dioxide and benzoic acid in foods
and drinks. London, Ministry of Agriculture, Fisheries and Food,
Joint Food Safety and Standards Group (Food Surveillance Information
Sheet No. 65; http://maff.gov.uk/food/infsheet/1995/
no65/65sulben.htm).
Urano K, Kato Z (1986) Evaluation of biodegradation ranks of priority
organic compounds. Journal of hazardous materials,13:147-159.
US FDA (1972a) GRAS (Generally Recognized As Safe) food ingredients:
benzoic acid and sodium benzoate. Washington, DC, US Food and Drug
Administration.
US FDA (1972b) Teratologic evaluation of FDA 71-37 (sodium benzoate).
East Orange, NJ, US Food and Drug Administration, Food and Drug
Research Laboratory.
US FDA (1973) Evaluation of the health aspects of benzoic acid and
sodium benzoate as food ingredients. Bethesda, MD, US Food and Drug
Administration, Life Sciences Research Office (PB-223 837).
US FDA (1974) Mutagenic evaluation of compound FDA 71-37, sodium
benzoate. Prepared by Litton Bionetics, Inc., Kensington, MD, for
the US Food and Drug Administration (Unpublished report PB-245-453/6).
US NTP (1986) Toxicology and carcinogenesis studies of benzyl acetate
(CAS No. 140-11-4) in F344/N rats and B6C3F1 mice (gavage studies).
Research Triangle Park, NC, US Department of Health, Education and
Welfare, National Institutes of Health, National Toxicology Program
(Technical Report Series No. 250).
US NTP (1989) Toxicology and carcinogenesis studies of benzyl alcohol
(CAS No. 100-51-6) in F344/N rats and B6C3F1 mice (gavage studies).
Research Triangle Park, NC, US Department of Health, Education and
Welfare, National Institutes of Health, National Toxicology Program
(Technical Report Series No. 343).
US NTP (1990) Toxicology and carcinogenesis studies of benzaldehyde
(CAS No. 100-52-7) in F344/N rats and B6C3F1 mice (gavage studies).
Research Triangle Park, NC, US Department of Health, Education and
Welfare, National Institutes of Health, National Toxicology Program
(Technical Report Series No. 378).
US NTP (1993) Toxicology and carcinogenesis studies of benzyl acetate
(CAS No. 140-11-4) in F344/N rats and B6C3F1 mice (feed studies).
Research Triangle Park, NC, US Department of Health, Education and
Welfare, National Institutes of Health, National Toxicology Program
(Technical Report Series No. 431).
Veien NK, Hattel T, Justesen O, Noerholm A (1987) Oral challenge with
food additives. Contact dermatitis, 17:100-103.
Velsicol Chemical Corp. (1981) Four week subacute inhalation toxicity
study of benzoic acid in rats. Report prepared by International
Research and Development Corporation, Mattawan, MI, for Velsicol
Chemical Corporation, Chicago, IL (FYI-OTS-1281-0147).
Ventullo RM, Larson RJ (1985) Metabolic diversity and activity of
heterotrophic bacteria in ground water. Environmental toxicology and
chemistry, 4:759-771.
Verrett MJ, Scott WF, Reynaldo EF, Alterman EK, Thomas CA (1980)
Toxicity and teratogenicity of food additive chemicals in the
developing chicken embryo. Toxicology and applied pharmacology,
56:265-273.
Villanueva MBG, Jonai H, Kanno S, Takeuchi Y (1994) Dietary sources
and background levels of hippuric acid in urine: Comparison of
Philippine and Japanese levels. Industrial health, 32:239-249.
Vind HP, Hochman H (1960) Toxicity of chemicals to marine borers --
1. Interim report. Port Hueneme, CA, US Naval Civil Engineering
Laboratory, pp. 1-134 (TR 048) [cited in Environmental Chemicals Data
and Information Network (ECDIN) Database, 1998, compiled by Commission
of the European Communities, Joint Research Centre, Ispra
Establishment, Ispra (Varese)].
Vogt T, Landthaler M, Stolz W (1999) Sodium benzoate-induced acute
leukocytoclastic vasculitis with unusual clinical appearance.
Archives of dermatology, 135:726-727.
Waldo JF, Masson JM, Lu WC, Tollstrup J (1949) The effect of benzoic
acid and caronamide on blood penicillin levels and on renal function.
American journal of medical sciences, 217:563-568.
Wallhäusser KH (1984) Praxis der Sterilisation,
Desinfektion-Konservierung; Keimidentifizierung- Betriebshygiene;
3. Auflage. Stuttgart, Georg Thieme Verlag, pp. 399-400.
Ward TE (1985) Characterizing the aerobic and anaerobic microbial
activities in surface and subsurface soils. Environmental toxicology
and chemistry, 4:727-737.
Warth AD (1988) Effect of benzoic acid on growth yield of yeasts
differing in their resistance to preservatives. Applied and
environmental microbiology, 54:2091-2095.
Wester RC, Maibach HI (1976) Relationship of topical dose and
percutaneous absorption in rhesus monkey and man. Journal of
investigative dermatology, 67:518-520.
Wester RC, Noonan PK (1980) Relevance of animal models for
percutaneous absorption. International journal of pharmaceutics,
7:99-110.
WHO (1994) Assessing human health risks of chemicals: derivation of
guidance values for health-based exposure limits. Geneva, World
Health Organization (Environmental Health Criteria 170).
WHO (1996) Toxicological evaluation of certain food additives.
Prepared by the 46th meeting of the Joint FAO/WHO Expert Committee on
Food Additives (JECFA). Geneva, World Health Organization (WHO Food
Additives Series 37).
WHO (1999) Safety evaluation of certain food additives. Prepared by
the 51st meeting of the Joint FAO/WHO Expert Committee on Food
Additives (JECFA). Geneva, World Health Organization, pp. 403-414 (WHO
Food Additives Series 42).
Wiley HM, Bigelow WD (1908) Influence of benzoic acid and benzoates
on digestion and health. Bulletin 84, Part IV. Bureau of Chemistry,
US Department of Agriculture [cited in US FDA, 1972a].
Williams RT (1967) Comparative patterns of drug metabolism.
Federation proceedings, 26:1029-1039.
Winkeler HD, Puttins U, Levsen K (1988) [Organic compounds in
rainwater.] Vom Wasser, 70:107-117 (in German).
Xing W, Zhang Z (1990) A comparison of SCE test in human lymphocytes
and Vicia faba: a hopeful technique using plants to detect mutagens
and carcinogens. Mutation research, 241:109-113.
Yomota C, Isshiki K, Kato T, Kamikura M, Shiroishi Y, Nishijima M,
Hayashi H, Hukasawa Y, Yokojama T, Yoneda M, Moriguchi H, Uchiyama H,
Shiro T, Ito Y (1988) [Estimation of daily intake of 8 kinds of
organic acids, 4 kinds of nucleic acids, ortho-phosphate, benzoic
acid, glycerol monostearate, sodium alginate, sulfur dioxide, nitrate,
nitrite, mannitol, sorbitol, glycerol and ammonium hydroxide from
fresh foods purchased in Japan according to the market basket method.]
Journal of the Japanese Society of Nutrition and Food Science,
41:11-16 (in Japanese).
York RG, Barnwell P, Bailes W (1986) Screening of priority chemicals
for reproductive hazards. Unpublished report (ETOX-85-1002)
submitted to Experimental Toxicology Branch, Division of Biomedical
and Behavioral Science, National Institute for Occupational Safety and
Health, Cincinnati, OH, by Environmental Health Research and Testing,
Inc., Cincinnati, OH. Submitted to WHO by ILSI Europe, Brussels [cited
in WHO, 1996].
Zahn R, Wellens H (1980) [Examination of biological degradability
through the batch method -- further experience and new possibilities
of usage.] Zeitschrift für Wasser und Abwasser Forschung, 13:1-7
(in German).
Zeiger E, Anderson B, Haworth S, Lawlor T, Mortelmans K (1988)
Salmonella mutagenicity tests: IV. Results from the testing of 300
chemicals. Environmental and molecular mutagenesis, Suppl.
11(12):1-158.
Zhang H, Kanzaki H, Kawazu K (1997) Benzoic acid accumulation in the
Pinus thunbergii callus inoculated with the pine wood nematode,
Bursaphelenchus xylophilus. Zeitschrift für Naturforschung,
52:329-332.
APPENDIX 1 -- SOURCE DOCUMENTS
US FDA (1972a) GRAS (Generally Recognized As Safe) food ingredients:
benzoic acid and sodium benzoate. Washington, DC, US Food and Drug
Administration
This report was prepared by Informatics Inc., Rockville, MD, for
the US Food and Drug Administration.
BUA (1995) BUA-Stoffbericht Benzoesaeure, Natriumbenzoat.
Beratergremium fuer Umweltrelevante Altstoffe. Stuttgart, S. Hirzel
Verlag (Stoffbericht Nr. 145)
For the BUA review process, the company that is in charge of
writing the report (usually the largest producer in Germany) prepares
a draft report using literature from an extensive literature search as
well as internal company studies. This draft is subject to a peer
review in several readings of a working group consisting of
representatives from government agencies, the scientific community,
and industry.
WHO (1996) Toxicological evaluation of certain food additives.
Prepared by the 46th meeting of the Joint FAO/WHO Expert Committee on
Food Additives (JECFA). Geneva, World Health Organization (WHO Food
Additives Series 37)
The first draft on benzyl acetate, benzyl alcohol, benzaldehyde,
and benzoic acid and its salts was prepared by E. Vavasour, Chemical
Health Hazard Assessment Division, Bureau of Chemical Safety, Food
Directorate, Health Protection Branch, Health Canada, Ottawa, Ontario.
The meeting of the Joint FAO/WHO Expert Committee on Food Additives
was held from 6 to 15 February 1996 in Geneva.
APPENDIX 2 -- CICAD PEER REVIEW
The draft CICAD on benzoic acid and sodium benzoate was sent for
review to institutions and organizations identified by IPCS after
contact with IPCS National Contact Points and Participating
Institutions, as well as to identified experts. Comments were received
from:
A. Aitio, International Programme on Chemical Safety, World
Health Organization, Switzerland
M. Baril, Institut de Recherche en Santé et en Sécurité du
Travail du Québec (IRSST), Canada
R. Benson, Drinking Water Program, US Environmental Protection
Agency, USA
W.F. ten Berge, WXS, Netherlands
R. Cary, Health and Safety Executive, United Kingdom
R.S. Chhabra, National Institute for Environmental and Health
Sciences/National Institutes of Health (NIEHS/NIH), USA
S. Dobson, Institute of Terrestrial Ecology, United Kingdom
P. Edwards, Department of Health, United Kingdom
R. Hertel, Federal Institute for Health Protection of Consumers
and Veterinary Medicine (BgVV), Germany
C. Hiremath, US Environmental Protection Agency, USA
P. Schulte, National Institute for Occupational Safety and
Health, USA
D. Willcocks, National Industrial Chemicals Notification and
Assessment Scheme (NICNAS), Australia
P. Yao, Chinese Academy of Preventive Medicine, People's Republic
of China
K. Ziegler-Skylakakis, Beratergremium für Umweltrelevante
Altstoffe (BUA), Germany
APPENDIX 3 -- CICAD FINAL REVIEW BOARD
Sydney, Australia, 21-24 November 1999
Members
Dr R. Benson, Drinking Water Program, US Environmental Protection
Agency, Region VIII, Denver, CO, USA
Dr T. Berzins, National Chemicals Inspectorate (KEMI), Solna, Sweden
Dr R.M. Bruce, National Center for Environmental Assessment,
US Environmental Protection Agency, Cincinnati, OH, USA
Mr R. Cary, Health and Safety Executive, Merseyside, United Kingdom
Dr R.S. Chhabra, National Institute of Environmental Health Sciences,
National Institutes of Health, Research Triangle Park, NC, USA
Dr S. Chou, Agency for Toxic Substances and Disease Registry,
Atlanta, GA, USA
Dr S. Dobson, Institute of Terrestrial Ecology, Monks Wood,
Cambridgeshire, United Kingdom
Dr H. Gibb, National Center for Environmental Assessment,
US Environmental Protection Agency, Washington, DC, USA
Dr R.F. Hertel, Federal Institute for Health Protection of Consumers
and Veterinary Medicine, Berlin, Germany
Dr J. Kielhorn, Fraunhofer Institute for Toxicology and Aerosol
Research, Hanover, Germany
Dr S. Kristensen, National Occupational Health and Safety Commission
(Worksafe), Sydney, NSW, Australia
Mr C. Lee-Steere, Environment Australia, Canberra, ACT, Australia
Ms M. Meek, Environmental Health Directorate, Health Canada, Ottawa,
Ontario, Canada
Ms F. Rice, National Institute for Occupational Safety and Health,
Cincinnati, OH, USA
Dr J. Sekizawa, National Institute of Health Sciences, Tokyo, Japan
Dr D. Willcocks, National Industrial Chemicals Notification and
Assessment Scheme (NICNAS), Sydney, NSW, Australia (Chairperson)
Professor P. Yao, Institute of Occupational Medicine, Chinese Academy
of Preventive Medicine, Beijing, People's Republic of China
Observers
Mr P. Howe, Institute of Terrestrial Ecology, Huntingdon,
Cambridgeshire, United Kingdom
Dr K. Ziegler-Skylakakis, GSF-Forschungszentrum für Umwelt und
Gesundheit, GmbH, Oberschleissheim, Germany
Secretariat
Dr A. Aitio, International Programme on Chemical Safety, World Health
Organization, Geneva, Switzerland
Ms M. Godden, Health and Safety Executive, Bootle, Merseyside, United
Kingdom
Dr M. Younes, International Programme on Chemical Safety, World Health
Organization, Geneva, Switzerland
APPENDIX 4 -- INTERNATIONAL CHEMICAL SAFETY CARD
BENZOIC ACID ICSC: 0103
October 1999
CAS# 65-85-0 Benzenecarboxylic acid
RTECS# DG0875000 Phenyl carboxylic acid
C7H6O2/C6H5COOH
Molecular mass: 122.1
TYPES OF HAZARD ACUTE HAZARDS/ PREVENTION FIRST AID / FIRE
/ EXPOSURE SYMPTOMS FIGHTING
FIRE Combustible. NO open flames. Powder, water spray,
foam, carbon dioxide.
EXPLOSION In case of fire: keep drums,
etc., cool by spraying with
water.
EXPOSURE
Inhalation Cough. Sore throat. Local exhaust Fresh air, rest.
or breathing
protection.
Skin Redness. Burning Protective gloves. Remove contaminated clothes.
sensation. Itching. Rinse and then wash skin with
water and soap.
Eyes Redness. Pain. Safety goggles. First rinse with plenty
of water for several minutes
(remove contact lenses if
easily possible), then take
to a doctor.
Ingestion Abdominal pain. Do not eat, drink, or Rinse mouth. Induce vomiting
Nausea. Vomiting smoke during work. (ONLY IN CONSCIOUS PERSONS!)
Wash hands before Refer for medical
eating. attention.
SPILLAGE DISPOSAL PACKAGING & LABELLING
Sweep spilled substance into plastic EU Classification
containers; if appropriate, moisten UN Classification
first to prevent dusting.
Use face shield and (extra personal
protection: protective clothing).
Wash away remainder with plenty of water.
EMERGENCY RESPONSE STORAGE
NFPA Code: H 2; F 1; R -;
IMPORTANT DATA
PHYSICAL STATE; APPEARANCE: ROUTES OF EXPOSURE:
WHITE CRYSTALS OR POWDER. The substance can be absorbed
into the body by inhalation and
by ingestion.
PHYSICAL DANGERS:
Dust explosion possible if in powder
or granular form, mixed with air. INHALATION RISK:
No indication can be given about the
rate in which a harmful concentration
CHEMICAL DANGERS: in the air is reached on evaporation
The solution in water is a weak acid. of this substance at 20°C.
Reacts with oxidants.
OCCUPATIONAL EXPOSURE LIMITS: EFFECTS OF LONG-TERM OR REPEATED EXPOSURE:
TLV not established. The substance irritates the eyes, the skin
and the respiratory tract. The substance
may cause a non-allergic rash on contact.
PHYSICAL PROPERTIES
Boiling Point: 249°C Flash point: 121°C c.c
Melting Point: 122°C (see Notes) Auto-ignition temperature: 570°C
Density: 1.3 g/cm3 Octanol/water partition
coefficient as log Pow: 1.87
Solubility in water, g/100 ml at 20°C: 0.29
Vapour pressure, Pa at 25°C: 0.1
Relative vapour density (air = 1): 4.2
Relative density of the vapour/air-mixture at 20°C (air = 1): 1
ENVIRONMENTAL DATA
NOTES
The substance begins to sublime at 100°C.
ADDITIONAL INFORMATION
LEGAL NOTICE Neither the CEC nor the IPCS nor any person acting on
behalf of the CEC or the IPCS is responsible for the use
which might be made of this information.
RÉSUMÉ D'ORIENTATION
Ce CICAD consacré à l'acide benzoïque et au benzoate de sodium
a été préparé par l'Institut Fraunhofer de Toxicologie et d'étude
des Aérosols de Hanovre (Allemagne). Ces deux composés sont
examinés ensemble car c'est l'acide benzoïque non dissocié qui est
responsable de l'activité anti-infectieuse de ces produits. Comme
l'acide benzoïque lui-même n'est que peu soluble dans l'eau, c'est
le benzoate de sodium - qui en milieu acide redonne l'acide non
dissocié - que l'on utilise à sa place.
Ce CICAD est basé sur des études bibliographiques effectuées
par le Comité consultatif allemand sur les produits chimiques qui
posent des problèmes écologiques (BUA, 1995), la Food and Drug
Administration des États-Unis (US FDA, 1972a) et le Comité mixte
FAO/OMS d'experts des Additifs alimentaires (JECFA) (WHO/OMS, 1996)
dans le but d'évaluer les effets possibles de l'acide benzoïque et
du benzoate de sodium sur l'environnement et sur l'organisme
humain. Une étude bibliographique exhaustive sur les bases de
données appropriées a été effectuée en septembre 1999 afin de
rechercher toute référence intéressante qui aurait été publiée
postérieurement à celles qui figurent dans ces différents rapports.
On trouvera à l'appendice 1 des indications sur la préparation de
l'examen par des pairs et sur les sources documentaires utilisées
pour cet examen. Les renseignements concernant l'examen du présent
CICAD font l'objet de l'appendice 2. Ce CICAD a été approuvé en
tant qu'évaluation internationale lors de la réunion du Comité
d'évaluation finale qui s'est tenue à Sydney (Australie) du 21 au
24 novembre 1999. La liste des participants à cette réunion figure
à l'appendice 3. La fiche d'information internationale sur la
sécurité chimique (ICSC 0103) relative à l'acide benzoïque, établie
par le Programme international sur la sécurité chimique (IPCS,
1993) est également reproduite dans ce document (appendice 4).
Chez les animaux de laboratoire et chez l'Homme, l'acétate de
benzyle, l'alcool benzylique, son produit d'hydrolyse, et le
produit qui en résulte par oxydation, c'est-à-dire le benzaldéhyde,
sont largement métabolisés en acide benzoïque. On a donc utilisé
les données toxicologiques relatives à ces précurseurs dans
l'évaluation des effets sanitaires potentiels de l'acide benzoïque.
L'acide benzoïque (No CAS 65-85-0) se présente sous la forme
d'un solide blanc légèrement soluble dans l'eau. La solubilité dans
l'eau du benzoate de sodium (No CAS 532-32-1) est environ 200 fois
plus élevée. On utilise l'acide benzoïque comme intermédiaire dans
la synthèse de divers composés, principalement le phénol (plus de
50 % de la production mondiale) et la caprolactame. Il sert
également à la préparation de divers sels, dont le sel de sodium,
du chlorure de benzoyle ainsi que de plastifiants comme les
dibenzoates de diéthylène- et de dipropylène-glycol. Le benzoate de
sodium est principalement utilisé comme conservateur et inhibiteur
de corrosion (par ex. comme additif à l'antigel des moteurs à
explosion). L'acide benzoïque et le benzoate de sodium sont
employés comme conservateurs dans l'alimentation et ils conviennent
particulièrement bien pour les produits comme les jus de fruits et
les boissons non alcoolisées, qui ont un pH acide. Leur utilisation
comme conservateurs dans les denrées alimentaires, les boissons,
les pâtes dentifrices, les bains de bouche, les cosmétiques et les
produits pharmaceutiques est réglementée. On estime que la capacité
mondiale de production d'acide benzoïque est d'environ 600 000
tonnes par an. On estime en outre qu'en 1997 la production mondiale
de benzoate de sodium a été comprise entre 55 000 et 60 000 tonnes.
L'acide benzoïque est naturellement présent dans beaucoup de
végétaux et chez un grand nombre d'animaux. C'est donc un
constituant normal de nombreux aliments, notamment du lait et des
produits laitiers. Les rejets d'acide benzoïque et de benzoate de
sodium dans l'environnement qui sont imputables aux activités
humaines aboutissent essentiellement dans les eaux et dans le sol
et proviennent de leur utilisation comme conservateurs. On constate
que dans un certain nombre de denrées alimentaires, la
concentration de l'acide benzoïque d'origine naturelle ne dépasse
pas 40 mg/kg en moyenne. Les concentrations maximales d'acide
benzoïque ou de benzoate de sodium relevées dans des produits
alimentaires auxquels ils avaient été ajoutés comme conservateurs
sont de l'ordre de 2000 mg/kg.
Après ingestion, l'acide benzoïque et le benzoate de sodium
sont rapidement résorbés dans les voies digestives et métabolisés
dans le foie par conjugaison avec la glycine pour donner de l'acide
hippurique, rapidement excrété dans les urines. Les benzoates
appliqués sur la peau sont résorbés dans une moindre proportion par
voie transcutanée. En raison de la rapidité du métabolisme et de
l'excrétion, il n'y a vraisemblablement pas d'accumulation des
benzoates ou de leurs métabolites.
Chez les rongeurs, la toxicité aiguë par voie orale de l'acide
benzoïque et du benzoate de sodium est faible (la DL50 par voie
orale est supérieure à 1940 mg/kg de poids corporel). Chez le chat,
qui semble plus sensible que les rongeurs, on a signalé des effets
toxiques et une mortalité à des doses beaucoup plus faibles
(environ 450 mg/kg p.c.).
L'acide benzoïque est légèrement irritant pour la peau et
irritant pour la muqueuse oculaire, tandis que le benzoate de
sodium n'irrite pas la peau est n'est que légèrement irritant pour
l'oeil. En ce qui concerne l'acide benzoïque, les données
disponibles n'indiquent aucun effet sensibilisateur; dans le cas du
benzoate de sodium, aucune donnée n'a été relevée dans la
littérature à ce sujet.
Les études à court terme effectuées sur des rats ont révélé la
présence de troubles du système nerveux central (acide benzoïque /
benzoate de sodium) ainsi que des anomalies histopathologiques dans
l'encéphale (acide benzoïque) après administration de doses élevées
dans l'alimentation (>1800 mg/kg p.c.) pendant 5 à 10 jours. Les
autres effets constatés étaient les suivants : réduction du gain de
poids, modification du poids des organes, modification des
paramètres sériques ou encore anomalies histopathologiques au
niveau du foie. On ne dispose que de données très limitées sur
l'exposition de longue durée des animaux de laboratoire à l'acide
benzoïque par voie orale et il n'existe pas d'étude qui soit
spécialement consacrée à la recherche d'effets cancérogènes
éventuels. Une étude limitée, portant sur quatre générations, n'a
permis d'obtenir qu'une estimation préliminaire de la dose sans
effet (nocif) observable (NO(A)EL), estimation qui est d'environ
500 mg/kg p.c. par jour. En ce qui concerne le benzoate de sodium,
les deux études à long terme effectuées sur des rats et des souris
n'ont pas mis en évidence d'effet cancérogène. Il faut dire
cependant que dans la plupart de ces études, les effets ne sont pas
parfaitement attestés, d'où l'impossibilité d'en tirer des valeurs
fiables pour la dose sans effet observable et la dose sans effet
nocif observable. Les données concernant leurs divers précurseurs
corroborent l'hypothèse selon laquelle l'acide benzoïque ne serait
pas vraisemblablement pas cancérogène.
L'acide benzoïque a donné des résultats négatifs dans un
certain nombre de tests sur bactéries ou cellules mammaliennes,
mais on n'a pas trouvé de comptes rendus de tests in vivo. Le
benzoate de sodium s'est également révélé inactif dans le test
d'Ames mais il a donné des résultats systématiquement positifs sur
cellules mammaliennes. Dans une étude in vivo (test de létalité
dominante chez le rat) on a également obtenu un résultat positif.
Dans ces conditions, on ne peut pour l'instant exclure que le
benzoate de sodium ait une activité génotoxique.
Dans le cas de l'acide benzoïque, on dispose de deux études
limitées qui n'indiquent aucun effet indésirable sur la
reproduction ou le développement. En ce qui concerne le benzoate de
sodium, plusieurs études ont été menées sur un certain nombre
d'espèces et si des effets embryotoxiques et foetotoxiques ou même
des malformations ont été observés, c'est uniquement à des doses
déjà toxiques pour les mères. Une étude d'alimentation sur des rats
a permis de fixer à environ 1310 mg/kg p.c. la dose sans effet
(nocif) observable. Les données concernant ses divers précurseurs
corroborent l'hypothèse selon laquelle l'acide benzoïque n'a
vraisemblablement pas d'effets indésirables sur la reproduction aux
doses qui ne sont pas toxiques pour la mère.
Chez l'Homme, l'acide benzoïque et le benzoate de sodium sont
peu toxiques. On sait cependant que ces deux composés peuvent
produire des réactions de contact non immunologiques
(pseudoallergie). Cet effet est rare chez les sujets en bonne
santé. En revanche, chez les personnes qui souffrent fréquemment
d'asthme ou d'urticaire, on a observé une exacerbation des
symptômes. On peut établir une dose journalière tolérable
provisoire par ingestion de 5 mg/kg de poids corporel, encore que
les benzoates soient susceptibles de provoquer chez les sujets
sensibles des pseudoallergies de contact à des doses plus faibles.
Comme on ne dispose pas d'étude appropriée sur l'exposition par
inhalation, on ne peut déterminer la concentration tolérable en cas
d'exposition par cette voie.
Compte tenu de leurs propriétés physiques et chimiques, il est
exclu que l'acide benzoïque et le benzoate de sodium rejetés dans
le sol ou dans l'eau soient à même de s'évaporer dans l'atmosphère
ou de s'adsorber aux sédiments ou aux particules du sol. De
nombreuses expériences on permis de constater que la principale
voie d'élimination de ces deux composés était la minéralisation
biologique. Des essais en laboratoire ont montré qu'en aérobiose,
les deux composés sont facilement biodégradables. Un certain nombre
de microorganismes isolés (bactéries, champignons) se sont révélés
capable d'utiliser l'acide benzoïque en aérobiose comme en
anaérobiose. Les données fournies par les expériences de
bioconcentration montrent que ces produits ont un potentiel de
bioaccumulation faible à modéré.
Ú en juger d'après les tests effectués sur des organismes
aquatiques dans de bonnes conditions de validité, l'acide benzoïque
et le benzoate de sodium se révèlent peu à modérément toxiques dans
ce milieu. La valeur la plus faible de la CE50 qui ait été
mesurée, soit 9 mg/litre (critère : inhibition de la multiplication
cellulaire), a été obtenue dans une étude de toxicité chronique sur
une corynébactérie, Anabaena inaequalis. Les valeurs de la CE50
et de la CL50 obtenues sur d'autres espèces aquatiques, se
situaient aux alentours de 60-1291 mg/litre. On a montré que
l'immobilisation de la daphnie dépendait du pH, la CE50 à 24 h
étant plus basse (102 mg/litre) pour les pH acides. Dans le cas de
l'ide rouge (Leuciscus idus), on a trouvé une CL50 à 48 h de
460 mg/litre. Des effets ont été constatés sur le développement des
embryons de grenouille (Xenopus) à la concentration de 433
mg/litre (CE50 à 96 h; critère : malformation). Dans le cas du
benzoate de sodium, l'exposition des stades juvéniles d'organismes
aquatiques appartenant à plusieurs espèces (Daphnia magna,
Gammarus fasciatus, Asellus intermedius, Dugesia tigrina,
Helisoma trivolvis et Lumbriculus variegatus) a permis de
constater que la CL50 à 96 h était supérieure à 100 mg/litre.
On a mesuré une CL50 à 96 h de 484 mg/litre pour le vairon à
grosse tête (Pimephales promelas). Compte tenu du caractère
limité des données concernant le niveau d'exposition dans l'eau, il
n'a pas été possible de quantifier le risque couru par les
organismes qui peuplent les eaux de surface. En s'appuyant sur la
biodégradation rapide des composés, la valeur faible à modérée de
leur potentiel de bioaccumulation, la faible toxicité qu'ils
présentent pour la plupart des espèces aquatiques et leur
métabolisation rapide, il apparaît que l'acide benzoïque et le
benzoate de sodium ne représentent qu'un risque minimum pour les
organismes aquatiques, mis à part le cas de déversements
accidentels.
Les quelques données disponibles indiquent que l'acide
benzoïque et le benzoate de sodium n'ont qu'un faible potentiel
toxique dans l'environnement terrestre. Ú l'exception de l'action
antimicrobienne de l'acide benzoïque, qui se caractérise par une
concentration microbicide comprise entre 20 et 1200 mg/litre, on ne
possède aucune donnée sur les effets toxiques de ce composé sur les
organismes terrestres. Dans le cas du benzoate de sodium, il y a
inhibition de la croissance bactérienne et fongique pour des
concentrations comprises entre 100 et 60 000 mg/litre et cette
inhibition dépend du pH. Faute de connaître la valeur des niveaux
d'exposition, il n'a pas été possible de caractériser le risque
pour les organismes terrestres.
RESUMEN DE ORIENTACI²N
El presente CICAD sobre el ácido benzoico y el benzoato de
sodio se preparó en el Instituto Fraunhofer de Toxicología y de
Investigación sobre los Aerosoles de Hannover, Alemania. Se
examinan los dos compuestos juntos porque es el ácido benzoico no
disociado el responsable de su actividad antimicrobiana. Debido a
que el propio ácido benzoico es sólo ligeramente soluble en agua,
con frecuencia se utiliza en su lugar el benzoato de sodio, que en
condiciones ácidas se convierte en ácido benzoico no disociado.
El presente CICAD se basa en exámenes compilados por el Comité
Consultivo Alemán sobre las Sustancias Químicas Importantes para el
Medio Ambiente (BUA, 1995), la Administración de Alimentos y
Medicamentos de los Estados Unidos (US FDA, 1972a) y el Comité
Mixto FAO/OMS de Expertos en Aditivos Alimentarios (JECFA)
(WHO/OMS, 1996) para evaluar los efectos potenciales del ácido
benzoico y el benzoato de sodio en el medio ambiente y en el ser
humano. En septiembre de 1999 se realizó una búsqueda bibliográfica
amplia de las bases de datos pertinentes para localizar cualquier
referencia de interés publicada después de las incorporadas a estos
informes. La información relativa a la preparación de los
documentos originales y su examen colegiado figura en el apéndice
1. La información sobre el examen colegiado de este CICAD aparece
en el apéndice 2. Este CICAD se aprobó como evaluación
internacional en una reunión de la Junta de Evaluación Final
celebrada en Sidney, Australia, los días 21-24 de noviembre de
1999. En el apéndice 3 figura la lista de los participantes en esta
reunión. La Ficha internacional de seguridad química (ICSC 0103)
para el ácido benzoico, preparada por el Programa Internacional de
Seguridad de las Sustancias Químicas (IPCS, 1993), también se
reproduce en el presente documento (apéndice 4).
El acetato de bencilo, su producto de hidrólisis, el alcohol
de bencilo, y el producto de la oxidación de este alcohol, el
benzaldehído, se metabolizan ampliamente a ácido benzoico en
animales experimentales y en el ser humano. Por consiguiente,
también se utilizaron datos toxicológicos sobre estos precursores
en la evaluación de los efectos potenciales del ácido benzoico para
la salud.
El ácido benzoico (CAS No 65-85-0) es una sustancia sólida
blanca ligeramente soluble en agua. El benzoato de sodio
(CAS No 532-32-1) es alrededor de 200 veces más soluble en
agua. El ácido benzoico se utiliza como producto intermedio en la
síntesis de distintos compuestos, fundamentalmente el fenol (>50
por ciento de la cantidad producida en todo el mundo) y la
caprolactama. Otros productos finales son el sodio y otros
benzoatos, el cloruro de benzoílo y los agentes plastificantes de
dibenzoato de dietilenglicol y dipropilenglicol. El benzoato de
sodio se utiliza sobre todo como conservante e inhibidor de la
corrosión (por ejemplo, en sistemas técnicos como aditivo de los
refrigerantes anticongelantes de los motores de automóviles).
El ácido benzoico y el benzoato de sodio se utilizan como
conservantes de los alimentos y son los más idóneos para los
productos alimenticios, los jugos de frutas y las bebidas no
alcohólicas, que por su naturaleza tienen un pH ácido. Su
utilización como conservantes en alimentos, bebidas, pastas de
dientes, colutorios, dentífricos, cosméticos y productos
farmacéuticos está reglamentada. La capacidad de producción mundial
estimada de ácido benzoico es de alrededor de 600 000 toneladas al
año. La producción mundial de benzoato de sodio en 1997 puede
estimarse en unas 55 000-60 000 toneladas. El ácido benzoico está
presente de manera natural en muchas plantas y en los animales. Por
consiguiente, es un elemento constitutivo natural de numerosos
alimentos, entre ellos los productos lácteos. Las liberaciones
antropogénicas de ácido benzoico y benzoato de sodio en el medio
ambiente son primordialmente emisiones al agua y al suelo a partir
de su uso como conservantes. Las concentraciones del ácido benzoico
presente de manera natural en varios productos alimenticios no
superaron el valor medio de 40 mg/kg de alimentos. Las
concentraciones máximas notificadas de ácido benzoico o benzoato de
sodio añadido a los productos alimenticios con fines de
conservación fueron del orden de 2000 mg/kg de alimentos.
Tras la ingesta oral, el ácido benzoico y el benzoato de sodio
se absorben con rapidez del tracto gastrointestinal y se
metabolizan en el hígado por conjugación con la glicina, dando
lugar a la formación de ácido hipúrico, que se excreta rápidamente
a través de la orina. Los benzoatos aplicados por vía cutánea
pueden penetrar en menor medida a través de la piel. Debido a la
rapidez del metabolismo y de la excreción, no cabe prever una
acumulación de benzoatos o sus metabolitos.
En roedores, la toxicidad oral aguda del ácido benzoico y el
benzoato de sodio es baja (valores de la DL50 por vía oral >1940
mg/kg de peso corporal). En gatos, que parecen ser más sensibles
que los roedores, se notificaron efectos tóxicos y mortalidad con
dosis mucho menores (unos 450 mg/kg de peso corporal).
El ácido benzoico es ligeramente irritante de la piel e
irritante de los ojos, mientras que el benzoato de sodio no irrita
la piel y es sólo ligeramente irritante de los ojos. En cuanto al
ácido benzoico, en los estudios disponibles no apareció ningún
indicio de efecto sensibilizante; para el benzoato de sodio no se
encontraron datos en la bibliografía.
En estudios de corta duración con ratas, se observaron
trastornos del sistema nervioso central (ácido benzoico/benzoato de
sodio), así como cambios histopatológicos en el cerebro (ácido
benzoico) después de administrar dosis elevadas (>1800 mg/kg de
peso corporal) durante 5-10 días. Otros efectos fueron una
reducción del aumento del peso corporal, cambios en el peso de los
órganos, cambios en los parámetros del suero o cambios
histopatológicos en el hígado. La información relativa a la
exposición oral prolongada de animales experimentales al ácido
benzoico es muy limitada y no hay ningún estudio disponible que
trate expresamente de los posibles efectos carcinogénicos. De un
estudio limitado de cuatro generaciones sólo puede derivarse una
concentración sin efectos (adversos) observados (NO(A)EL) de
carácter preliminar de alrededor de 500 mg/kg de peso corporal al
día. Con el benzoato de sodio, en dos estudios de larga duración
con ratas y ratones no se obtuvo ningún indicio de efecto
carcinogénico. Sin embargo, la documentación de los efectos es
insuficiente en la mayoría de estos estudios; por consiguiente, no
pueden derivarse valores de la NO(A)EL fidedignos. Los datos sobre
sus precursores respaldan la idea de que probablemente el ácido
benzoico no es carcinogénico.
El ácido benzoico dio resultados negativos en varias
valoraciones bacterianas y en pruebas con células de mamífero, pero
no se localizaron estudios in vivo. El benzoato de sodio también
fue inactivo en pruebas Ames, mientras que las pruebas con células
de mamífero dieron sistemáticamente resultados positivos. En un
estudio in vivo (valoración letal dominante con ratas) se obtuvo
un resultado positivo. En la actualidad no se puede excluir
totalmente la actividad genotóxica del benzoato de sodio.
En cuanto al ácido benzoico, en dos estudios limitados no se
obtuvieron indicios de efectos adversos reproductivos o en el
desarrollo. Con el benzoato de sodio se han realizado varios
estudios en distintas especies y solamente se observaron efectos
embriotóxicos y fetotóxicos, así como malformaciones, con dosis que
inducían una toxicidad materna grave. En un estudio de alimentación
en ratas se estableció una NO(A)EL de alrededor de 1310 mg/kg de
peso corporal. Los datos sobre sus precursores respaldan la idea de
que no es probable que el ácido benzoico tenga efectos
reproductivos adversos con los niveles de dosis que no son tóxicos
para la madre.
En el ser humano, la toxicidad aguda del ácido benzoico y el
benzoato de sodio es baja. Sin embargo, se sabe que ambas
sustancias provocan reacciones de contacto no inmunológicas
(pseudoalergia). Este efecto no es frecuente en personas sanas; en
pacientes con ataques frecuentes de urticaria o asma se observaron
síntomas o su exacerbación. Puede derivarse una ingesta tolerable
provisional de 5 mg/kg de peso corporal al día, aunque dosis
menores de benzoatos pueden producir reacciones de contacto no
inmunológicas (pseudoalergia) en personas sensibles. Debido a que
no hay estudios adecuados disponibles sobre la exposición por
inhalación, no se puede calcular una concentración tolerable para
este tipo de exposición.
Dadas sus propiedades físicas/químicas, no cabe prever que el
ácido benzoico y el benzoato de sodio que pasan al agua y al suelo
se volatilicen a la atmósfera o se adsorban sobre los sedimentos o
las partículas del suelo. Según los resultados de numerosos
experimentos de eliminación, la principal vía que siguen ambos
productos químicos debe ser la mineralización biótica. Los datos
obtenidos en pruebas de laboratorio pusieron de manifiesto una
biodegradabilidad rápida de ambas sustancias en condiciones
aerobias. Se ha comprobado que varios microorganismos aislados
(bacterias, hongos) utilizaron el ácido benzoico en condiciones
aerobias o anaerobias. De acuerdo con los datos experimentales
sobre la bioconcentración, cabe prever un potencial de bajo a
moderado para la bioacumulación.
Según los resultados de las pruebas válidas disponibles sobre
la toxicidad del ácido benzoico y el benzoato de sodio para
diversos organismos acuáticos, estos compuestos parecen mostrar una
toxicidad de baja a moderada en el compartimento acuático. El valor
más bajo de la CE50, de 9 mg/litro (inhibición de la
multiplicación celular), notificado en un estudio crónico se
observó en la cianobacteria Anabaena inaequalis. Los valores de
la CE50/CL50 para otras especies acuáticas estudiadas fueron
del orden de 60-1291 mg/litro. Se ha demostrado que la
inmovilización de Daphnia magna es dependiente del pH, con una
CE50 más baja en 24 horas (102 mg/litro) cuando el pH es ácido.
Para un pez de agua dulce, el cacho (Leuciscus idus), se ha
determinado una CL50 en 48 horas de 460 mg/litro. Se han
encontrado efectos en el desarrollo en embriones de rana (Xenopus)
con una concentración de 433 mg/litro (CE50 en 96 horas para la
malformación). Para el benzoato de sodio, en una exposición de
estadios juveniles de organismos acuáticos en una prueba
multiespecífica (con inclusión de Daphnia magna, Gammarus
fasciatus, Asellus intermedius, Dugesia tigrina, Helisoma
trivolvis, y Lumbriculus variegatus) se obtuvieron valores de la
CL50 en 96 horas superiores a 100 mg/litro. En el pez de agua
dulce Pimephales promelas se ha determinado una CL50 en 96
horas de 484 mg/litro. Debido a los limitados datos disponibles
sobre los niveles de exposición en agua, no se pudo realizar una
caracterización cuantitativa del riesgo con respecto a los
organismos acuáticos de aguas superficiales. Teniendo en cuenta la
rápida biodegradabilidad, el potencial de bioacumulación entre
moderado y bajo, la escasa toxicidad para la mayoría de las
especies acuáticas y el rápido metabolismo de estas sustancias, el
ácido benzoico y el benzoato de sodio - con la excepción de
vertidos accidentales - representan un riesgo sólo mínimo para los
organismos acuáticos.
Los escasos datos disponibles indican que el ácido benzoico y
el benzoato de sodio tienen solamente un potencial de toxicidad
bajo en el medio terrestre. Salvo la acción antimicrobiana del
ácido benzoico, que se caracteriza por concentraciones microbicidas
mínimas que oscilan entre 20 y 1200 mg/litro, no se encontraron
datos sobre los efectos tóxicos del ácido benzoico en los
organismos terrestres. En cuanto al benzoato de sodio, inhibió el
crecimiento bacteriano y fúngico de manera dependiente del pH en
concentraciones que oscilaban entre 100 y 60 000 mg/litro. Debido a
la falta de mediciones de los niveles de exposición, no se pudo
realizar un muestreo de la caracterización del riesgo con respecto
a los organismos terrestres.
 1. EXECUTIVE SUMMARY
This CICAD on benzoic acid and sodium benzoate was prepared by
the Fraunhofer Institute for Toxicology and Aerosol Research, Hanover,
Germany. The two compounds are being considered together because it is
undissociated benzoic acid that is responsible for its antimicrobial
activity. As benzoic acid itself is only slightly soluble in water,
sodium benzoate -- which, under acid conditions, converts to
undissociated benzoic acid -- is often used instead.
This CICAD was based on reviews compiled by the German Advisory
Committee on Existing Chemicals of Environmental Relevance (BUA,
1995), the US Food and Drug Administration (US FDA, 1972a), and the
Joint FAO/WHO Expert Committee on Food Additives (JECFA) (WHO, 1996)
to assess potential effects of benzoic acid and sodium benzoate on the
environment and on humans. A comprehensive literature search of
relevant databases was conducted in September 1999 to identify any
relevant references published subsequent to those incorporated in
these reports. Information on the preparation and peer review of the
source documents is presented in Appendix 1. Information on the peer
review of this CICAD is presented in Appendix 2. This CICAD was
approved as an international assessment at a meeting of the Final
Review Board, held in Sydney, Australia, on 21-24 November 1999.
Participants at the Final Review Board meeting are listed in Appendix
3. The International Chemical Safety Card (ICSC 0103) for benzoic
acid, produced by the International Programme on Chemical Safety
(IPCS, 1993), has also been reproduced in this document (Appendix 4).
Benzyl acetate, its hydrolysis product, benzyl alcohol, and the
oxidation product of this alcohol, benzaldehyde, are extensively
metabolized to benzoic acid in experimental animals and humans.
Therefore, toxicological data on these precursors were also utilized
in the assessment of the potential health effects of benzoic acid.
Benzoic acid (CAS No.
1. EXECUTIVE SUMMARY
This CICAD on benzoic acid and sodium benzoate was prepared by
the Fraunhofer Institute for Toxicology and Aerosol Research, Hanover,
Germany. The two compounds are being considered together because it is
undissociated benzoic acid that is responsible for its antimicrobial
activity. As benzoic acid itself is only slightly soluble in water,
sodium benzoate -- which, under acid conditions, converts to
undissociated benzoic acid -- is often used instead.
This CICAD was based on reviews compiled by the German Advisory
Committee on Existing Chemicals of Environmental Relevance (BUA,
1995), the US Food and Drug Administration (US FDA, 1972a), and the
Joint FAO/WHO Expert Committee on Food Additives (JECFA) (WHO, 1996)
to assess potential effects of benzoic acid and sodium benzoate on the
environment and on humans. A comprehensive literature search of
relevant databases was conducted in September 1999 to identify any
relevant references published subsequent to those incorporated in
these reports. Information on the preparation and peer review of the
source documents is presented in Appendix 1. Information on the peer
review of this CICAD is presented in Appendix 2. This CICAD was
approved as an international assessment at a meeting of the Final
Review Board, held in Sydney, Australia, on 21-24 November 1999.
Participants at the Final Review Board meeting are listed in Appendix
3. The International Chemical Safety Card (ICSC 0103) for benzoic
acid, produced by the International Programme on Chemical Safety
(IPCS, 1993), has also been reproduced in this document (Appendix 4).
Benzyl acetate, its hydrolysis product, benzyl alcohol, and the
oxidation product of this alcohol, benzaldehyde, are extensively
metabolized to benzoic acid in experimental animals and humans.
Therefore, toxicological data on these precursors were also utilized
in the assessment of the potential health effects of benzoic acid.
Benzoic acid (CAS No.  3. ANALYTICAL METHODS
Analytical methods for the determination of benzoic acid include
spectrophotometric methods, which need extensive extraction procedures
and are not very specific; gas chromatographic (GC) methods, which are
more sensitive and specific but need lengthy sample preparation and
derivatization prior to determination; and high-performance liquid
chromatography (HPLC), which has a high specificity and minimum sample
preparation and does not require derivatization.
A direct determination of benzoic acid in air by flash desorption
at 240°C with helium into capillary-GC gave a detection limit of
0.1 ppm (0.5 mg/m3) in a 20-litre sample (=10 µg benzoic acid).
This method has been developed and used for monitoring occupational
exposure (Halvorson, 1984).
A method for the determination of benzoic acid in solid food at
0.5-2 g/kg levels involves extraction with ether into aqueous sodium
hydroxide and methylene chloride, conversion to trimethylsilyl esters,
and detection by GC and flame ionization (Larsson, 1983; AOAC, 1990).
For margarine, a method using HPLC and ultraviolet (UV) detection has
been described with prior extraction with ammonium acetate/acetic
acid/methanol (Arens & Gertz, 1990).
When benzoic acid is used as a preservative in soft drinks and
fruit drinks, other additives, colouring agents, and other acids
(e.g., sorbate) may interfere with its analysis. Liquid
chromatographic methods were developed to overcome this (e.g., Bennett
& Petrus, 1977; Puttemans et al., 1984; Tyler, 1984). For the
sensitive determination of benzoic acid in fruit-derived products, a
clean-up pretreatment with solid-phase extraction followed by liquid
chromatography with UV absorbance detection is described (Mandrou et
al., 1998). The detection limit is 0.6 mg/kg, with a range of
quantification of 2-5 mg/kg. For soft drinks, a simultaneous
second-order derivative spectrophotometric determination has been
developed (detection limit 1 mg/litre) (Castro et al., 1992). Sodium
benzoate was measured in soya sauce, fruit juice, and soft drinks
using HPLC with a UV spectrophotometric detector. Before injection,
all samples were filtered (Villanueva et al., 1994).
GC determination of low concentrations (down to 10 ng/ml) of
benzoic acid in plasma and urine was preceded by diethyl ether
extraction and derivatization with pentafluorobenzyl bromide (Sioufi &
Pommier, 1980). Detection was by 63Ni electron capture. HPLC methods
have been developed for the simultaneous determination of benzoic acid
and hippuric acid -- the metabolite of sodium benzoate that is
eliminated in the urine -- that require no extraction step (detection
limit for both, 1 µg/ml; Kubota et al., 1988). Hippuric acid and
creatinine levels have been determined simultaneously by HPLC, and
measured hippuric acid levels corrected for urinary creatinine
excretion (Villanueva et al., 1994).
4. SOURCES OF HUMAN AND ENVIRONMENTAL EXPOSURE
4.1 Natural sources of benzoic acid
Benzoic acid is produced by many plants as an intermediate in the
formation of other compounds (Goodwin, 1976). High concentrations are
found in certain berries (see section 6.1). Benzoic acid has also been
detected in animals (see section 6.1). Benzoic acid therefore occurs
naturally in many foods, including milk products (Sieber et al., 1989,
1990).
4.2 Anthropogenic sources
4.2.1 Benzoic acid
Benzoic acid is produced exclusively by the liquid-phase
oxidation of toluene (Srour, 1998).
According to Srour (1998), the estimated global production
capacity of benzoic acid is 638 000 tonnes per year, although over
half of this is converted directly to phenol. The major producers of
benzoic acid are the Netherlands (220 000 tonnes per year) and Japan
(140 000 tonnes per year), followed by the USA (125 000 tonnes per
year). Another reference gives the total European capacity as less
than 153 000 tonnes (SRI, 1998).
Benzoic acid is detected in car exhaust gases, presumably as an
oxidation product of toluene (Kawamura et al., 1985), and in Japanese
cigarettes (12 and 28 µg per cigarette in mainstream and sidestream
smoke, respectively; Sakuma et al., 1983). It can also be produced
through the photochemical degradation of benzoic acid esters used as
fragrance ingredients (Shibamoto & Umano, 1985; Shibamoto, 1986).
Benzoic acid has been detected in wastewater from the wood production
industry in Norway and Sweden (Carlberg et al., 1986; Lindström &
Österberg, 1986) and in foundry waste leachates (Ham et al., 1989), as
well as in extracts of fly ash from municipal incinerators
(Tong et al., 1984).
4.2.2 Sodium benzoate
Sodium benzoate is produced by the neutralization of benzoic acid
with sodium hydroxide. Worldwide sodium benzoate production in 1997
can be estimated at about 55 000-60 000 tonnes (Srour, 1998). The
largest producers are the Netherlands, Estonia, the USA, and China.
4.3 Uses
4.3.1 Benzoic acid
In 1988, of the benzoic acid produced in Europe, about 60% was
further processed to phenol and 30% to caprolactam (for nylon fibres).
Five per cent was used for the production of sodium and other
benzoates, 3% for benzoyl chloride, and the rest for alkyd resins,
benzoate esters, such as methyl benzoate, and various other products
(Srour, 1989). These percentages are still approximately correct today
(Srour, 1998). Caprolactam seems to be produced only by European
companies (Srour, 1998).
Benzoic acid is increasingly used in the production of diethylene
and dipropylene glycol dibenzoate plasticizers in adhesive
formulations (about 40 000 tonnes in 1997). It is also used to improve
the properties of alkyd resins for paints and coatings and as a "down
hole" drilling mud additive in secondary oil production. Its use as a
rubber polymerization retarder is diminishing (Srour, 1998).
Benzoic acid and sodium benzoate (see section 4.3.2) are used as
preservatives in beverages, fruit products, chemically leavened baked
goods, and condiments, preferably in a pH range below 4.5.
A disadvantage is the off-flavour they may impart to foods (Chipley,
1983). Owing to their inhibitory effect on yeast, they cannot be used
in yeast-leavened products (Friedman & Greenwald, 1994). Examples of
upper concentrations allowed in food are up to 0.1% benzoic acid (USA)
and between 0.15% and 0.25% (other countries) (Chipley, 1983). The
European Commission limits for benzoic acid and sodium benzoate are
0.015-0.5% (EC, 1995).
Benzoic acid and its salts and esters are found in 11 of 48 (23%)
toothpastes (Sainio & Kanerva, 1995) to a maximum of 0.5% (Ishida,
1996) and in mouthwashes and dentifrices. Benzoic acid is also used in
cosmetics (in creams and lotions with pH values under 4, up to 0.5%)
(Wallhäusser, 1984). Sixteen out of 71 deodorants tested contained
benzoic acid (Rastogi et al., 1998).
Benzoic acid is a breakdown product of benzoyl peroxide, which is
used as an additive at levels of between 0.015% and 0.075% to bleach
flour (Friedman & Greenwald, 1994) and in dermatological antifungal
preparations (BMA, 1998). Benzoic acid is reported to leach from
denture-base acrylic resins, where benzoyl peroxide is added as a
polymerization initiator (Koda et al., 1989, 1990).
Benzoic acid can be used in combination with salicylic acid
(Whitfield's ointment) as a fungicidal treatment for ringworm (BMA,
1998).
4.3.2 Sodium benzoate
Although undissociated benzoic acid is the more effective
antimicrobial agent for preservation purposes, sodium benzoate is used
preferably, as it is about 200 times more soluble than benzoic acid.
About 0.1% is usually sufficient to preserve a product that has been
properly prepared and adjusted to pH 4.5 or below (Chipley, 1983).
A major market for sodium benzoate is as a preservative in the
soft drink industry, as a result of the demand for high-fructose corn
syrup in carbonated beverages. Sodium benzoate is also widely used as
a preservative in pickles, sauces, and fruit juices (Srour, 1998).
Benzoic acid and sodium benzoate are used as antimicrobial agents in
edible coatings (Baldwin et al., 1995).
Sodium benzoate is also used in pharmaceuticals for preservation
purposes (up to 1.0% in liquid medicines) and for therapeutic regimens
in the treatment of patients with urea cycle enzymopathies
(see section 9).
Possibly the largest use of sodium benzoate, accounting for
30-35% of the total demand (about 15 000 tonnes of benzoic acid), is
as an anticorrosive, particularly as an additive to automotive engine
antifreeze coolants and in other waterborne systems (Scholz &
Kortmann, 1991; Srour, 1998). A new use is the formulation of sodium
benzoate into plastics such as polypropylene, to improve strength and
clarity (BFGoodrich Kalama Inc., 1999). Sodium benzoate is used as a
stabilizer in photographic baths/processing (BUA, 1995).
4.4 Estimated global release
From data provided by the German producers, emissions of benzoic
acid from industrial processes were less than 525 kg per year into the
atmosphere, less than 3 tonnes per year into the River Rhine, and
8 tonnes per year into sewage or water purification plants (BUA,
1995). No data were available from other countries.
Other anthropogenic releases of benzoic acid and sodium benzoate
into the environment are emissions into water and soil from their uses
as preservatives in food, toothpastes, mouthwashes, dentifrices, and
cosmetics. There were no data available on the emission of benzoic
acid from the disposal of antifreeze mixtures and waterborne cooling
systems and other miscellaneous industrial uses.
The amount of benzoic acid emitted to air from car exhaust gases
as an oxidation product is not quantifiable from the available data.
5. ENVIRONMENTAL TRANSPORT, DISTRIBUTION, TRANSFORMATION,
AND ACCUMULATION
5.1 Transport and distribution between media
5.1.1 Benzoic acid
From its use pattern (see section 4), it can be expected that
benzoic acid is released to surface waters and (from dumping sites) to
leaching water (and groundwater). Minor amounts are expected to be
emitted to the atmosphere. From its physicochemical properties (vapour
pressure, Henry's law constant; see section 2), a significant
volatilization of benzoic acid from water or soil is not expected.
Owing to its solubility in water (see section 2), wet deposition from
air may occur. Experimental data on wet and dry deposition from air
are not available.
5.1.2 Sodium benzoate
No information on the environmental transport and distribution of
sodium benzoate could be identified. Owing to its use pattern, which
is similar to that of benzoic acid, most of the amounts released to
the environment are also expected to be emitted to aquatic
compartments (e.g., surface waters).
5.2 Transformation
5.2.1 Benzoic acid
The experimental determination of the photodegradation of benzoic
acid in aqueous solution (25°C; lambda = 240-300 nm) in terms of
quantum yield (average number of photons absorbed) resulted in very
low values -- in the order of 6 × 10-2 mol/einstein1 (Oussi et
al., 1998). However, benzoic acid adsorbed on silica gel (SiO2) and
irradiated with UV light (lambda > 290 nm) for 17 h showed 10.2%
photodegradation (Freitag et al., 1985). This may be due to a
photocatalytic effect, which was also observed with other oxides,
notably zinc oxide (ZnO) and titanium dioxide (TiO2). When benzoic
acid was irradiated with sunlight in aqueous suspensions of zinc or
titanium dioxide, 67% (after 2-3 h) or 90% (after 24 h) of the applied
amount was mineralized (Kinney & Ivanuski, 1969; Matthews, 1990).
1 An einstein is a unit of light energy used in photochemistry,
equal to Avogadro's number times the energy of one photon of light
of the frequency in question.
Indirect photolysis by reaction with hydroxyl radicals is
expected to be low. Hydroxyl radical rate constants (kOH) for
benzoic acid and its anion have been estimated to be approximately
0.5 × 10-12 and 2 × 10-12 cm3/s, respectively (Palm et al., 1998).
Standardized tests on ready (MITI, 1992) or inherent (Zahn &
Wellens, 1980) biodegradation showed benzoic acid to be readily
biodegraded. The degrees of aerobic degradation were as follows:
MITI I 85% (100 mg/litre; (MITI, 1992)
test 2 weeks; OECD
No. 301C)
Zahn-Wellens >90% (508 mg/litre; (Zahn & Wellens,
test 2 days) 1980)
Easy degradation of benzoic acid to methane and carbon dioxide
was also observed in different non-standardized experiments using
sewage sludge as inoculum (BUA, 1995). Benzoic acid was found to be
degraded by adapted anaerobic sewage sludge at 86-93% after 14 days
(Nottingham & Hungate, 1969), by aerobic activated sludge (adapted) at
>95% after 5-20 days (Pitter, 1976; Lund & Rodriguez, 1984), and by
unadapted aerobic activated sludge at 61-69% after 2-3 days with a
preceding lag time of 2-20 h (Urano & Kato, 1986). The use of a
synthetic sewage inoculated with laboratory bacterial cultures led to
complete degradation of benzoic acid after 14 days under anaerobic
conditions (Kameya et al., 1995).
A greater variability in degradation (0-100%) was seen in tests
using environmental matrices (e.g., rain, lake water, seawater, soil,
etc.). It depended mainly on substance concentration and time for
acclimation (see Table 1). Test durations exceeding 2 days resulted in
removal of >40% when initial concentrations were below 20 mg/litre.
A rapid mineralization occurred in groundwater and subsurface soil
samples. In groundwater, a half-life of 41 h has been found for
benzoic acid (initial concentration 1-100 µg/litre; metabolized to
14CO2) under aerobic conditions (Ventullo & Larson, 1985).
Half-lives of 7.3 h and 18.2 h, respectively, have been observed for
aerobic and anaerobic degradation of benzoic acid (initial
concentration 1 mg/kg dry weight; metabolized to 14CO2) in
subsurface soils of septic tank tile fields (Ward, 1985). Anaerobic
degradation of benzoic acid (initial concentration 250 mg
carbon/litre) in a methanogenic microcosm (consisting of aquifer
solids and groundwater) required 4 weeks of adaptation, followed by
nearly complete depletion after 8 weeks of incubation (Suflita &
Concannon, 1995).
Several isolated microorganisms have been shown to utilize (and
therefore probably degrade) benzoic acid under aerobic or anaerobic
conditions. They include, among others, fungal species such as
Rhodotorula glutinis and other yeast-like fungi (Kocwa-Haluch &
Lemek, 1995), the mould Penicillium frequentans (Hofrichter &
Fritsche, 1996), and bacteria, such as Alcaligenes denitrificans
(Miguez et al., 1995), Rhodopseudomonas palustris, several strains
of denitrifying pseudomonads (Fuchs et al., 1993; Elder & Kelly, 1994;
Harwood & Gibson, 1997), and Desulfomicrobium escambiense
(Sharak Genthner et al., 1997).
Although benzoic acid is primarily metabolized to hippuric acid
in rats (see section 7), some other species do excrete other
metabolites, such as dibenzoylornithine (hen), benzoylglutamic acid
(Indian fruit bat), benzoylarginine (tick, insects), or benzoyltaurine
(southern flounder, Paralichthys lethostigma) (Parke, 1968; Goodwin,
1976; James & Pritchard, 1987).
5.2.2 Sodium benzoate
Experimental data on photodegradation of sodium benzoate are not
available. As with benzoic acid, photolysis in aqueous solution is
assumed to be unlikely with regard to its known UV spectra
(Palm et al., 1998). Indirect photolysis by reaction with hydroxyl
radicals plays only a minor role, with estimated and measured hydroxyl
rate constants of about 0.33 × 10-11 cm3/s
(Palm et al., 1998).
Sodium benzoate was readily biodegradable under aerobic
conditions in several standard test systems:
Modified 84% (100 mg/litre; (King & Painter,
MITI test 10 days) 1983)
Modified 80-90% (50 mg/litre; (Salanitro et al.,
Sturm test 7 days) 1988)
Closed bottle 75-111% (5 mg/litre; (Richterich &
test 30 days) Steber, 1989)
Degradation assays using seawater as test medium ("natural
water") or as inoculum (marine filter material given into a synthetic
marine medium) according to an adapted Organisation for Economic
Co-operation and Development (OECD) guideline (301B) resulted in a
degradation of 85% and 97%, respectively (10 mg/litre; carbon dioxide
measurement; 28 days) (Courtes et al., 1995).
Anaerobic mineralization of sodium benzoate (50-90 mg/litre) by
domestic sewage sludge varied from 50% to 96.5% (measurement of carbon
dioxide and methane; 28-61 days) (Birch et al., 1989). In another
study using anaerobic sludge from sewage works receiving a mixture of
domestic and industrial wastewaters, 93% mineralization was observed
after 1 week of incubation (measurement of carbon dioxide and methane;
initial concentration 50 mg carbon/litre) (Battersby & Wilson, 1989).
Table 1: Removal of benzoic acid in freshwater, marine, and soil matrices.
Matrix Initial Conditions Duration Removal Measured Reference
concentration (days) (%) parameter
(mg/litre
or mg/kg)
Rainwater 0.001 22°C; shaking 2 0 benzoic acid Kawamura &
once per day; dark 7 40 Kaplan (1990)
45 100
Lake water 0.059 29°C; 7 98.7 14C (in CO2, Rubin et al.
(eutrophic/ no shaking; dark biomass) (1982)
mesotrophic)
Seawater 20°C; dark; 14C (in Shimp &
(estuary) rotary shaking CO2, biomass) Young (1987)
USA 20 30 <10
0.005 8 70-80
Canada 20 16 60
0.005 10 >70
Seawater 2 5 75 BODa Takemoto
et al. (1981)
Soil 20 2 mg benzoic acid 70 63 14CO2 Haider et al.
(grey soil, in 0.1 ml acetone (1974)
alkaline) + 100 g soil
+ 10 ml H2O
Soil 0.05 24°C; 20-25% 15 40 14CO2 Federle (1988)
(sand; moisture content
18.9 m depth)
a BOD = biological oxygen demand.
Benzoate-acclimated sludges were reported to be capable of completely
degrading benzoate concentrations of 3000 mg/litre within 5-7 days
(Kobayashi et al., 1989).
5.3 Accumulation
5.3.1 Benzoic acid
The n-octanol/water partition coefficient (log Kow) of 1.9
(see section 2) indicates a low potential for bioaccumulation.
Consistently, measured bioconcentration factors (BCFs) found in
aquatic biota were low. BCFs of <10 (based on wet weight) have been
determined for fish (golden ide, Leuciscus idus melanotus) and green
algae (Chlorella fusca) after 3 and 1 days, respectively
(Freitag et al., 1985). A 6-day BCF of 7.6 has been reported for
another green alga (Selenastrum capricornutum) (Mailhot, 1987),
and a 5-day BCF of 1300 (based on dry weight) in activated sludge
(Freitag et al., 1985). The following 24-h bioaccumulation factors
(focusing on uptake via medium plus feed within food chain members)
have been obtained in aquatic model ecosystems operated with
0.01-0.1 mg of radiolabelled benzoic acid per litre: 21 (mosquitofish,
Gambusia affinis), 102 (green alga, Oedogonium cardiacum),
138 (mosquito larvae, Culex quinquifasciatus), 1772 (water flea,
Daphnia magna), and 2786 (snail, Physa sp.). Except for
Daphnia and snail, the values were low (Lu & Metcalf, 1975).
However, the very low exposure concentrations could likely have
resulted in the calculation of the high BCF values, even with moderate
uptake. Moreover, because this was a radiolabel study, it remains
unclear if the label was still the parent compound.
Geoaccumulation of benzoic acid has also been found to be low.
Depending on soil depth, sorption coefficients (Kd) of 0.62 (18.9
m) to 1.92 (0.4 m) have been measured (Federle, 1988). Mobility
determinations of 14C-labelled benzoic acid in different soils by
means of thin-layer chromatography showed benzoic acid to be
moderately mobile. Its mobility was positively correlated with soil pH
and negatively correlated with aluminium and iron contents and
effective anion exchange capacity (Stolpe et al., 1993).
5.3.2 Sodium benzoate
No experimental data on bioaccumulation or geoaccumulation of
sodium benzoate have been identified. From the information on benzoic
acid, a significant potential for accumulation is not to be expected.
6. ENVIRONMENTAL LEVELS AND HUMAN EXPOSURE
6.1 Environmental levels
Generally, benzoic acid can occur in almost all environmental
compartments. Whether it exists in the undissociated or dissociated
form depends on the specific physicochemical conditions. Above pH 6,
the benzoate anion prevails (Chipley, 1983).
There is a series of reports on positive qualitative analyses of
benzoic acid in various environmental media, such as air (Belgium:
Cautreels & van Cauwenberghe, 1978; Germany: Helmig et al., 1989),
rain or snow (Norway: Lunde et al., 1977; Germany: Winkeler et al.,
1988), surface waters (Norway, river: Schou & Krane, 1981), and soils
(United Kingdom, heathland soil: Jalal & Read, 1983; Germany, river
terrace soil: Cordt & Kußmaul, 1990), but these do not provide
quantitative measurements.
Semiquantitative measurements of concentrations of benzoic acid
in urban air in Pasadena, California (USA) were in the range of
0.09-0.38 µg/m3 (Schuetzle et al., 1975). This was comparable to
quantitative measurements performed in 1984 in Los Angeles, California
(USA), which resulted in atmospheric concentrations of 0.005-0.13
µg/m3 (n = 8) (Kawamura et al., 1985). Most of the quantitative
data compiled in Table 2 with respect to water samples refer to
concentrations of benzoic acid in groundwater, with a maximum of 27.5
mg/litre measured in the vicinity of a point source.
Benzoic acid occurs naturally in free and bound form in many
plant and animal species. It is a common metabolite in plants and
organisms (Hegnauer, 1992). Appreciable amounts have been found in gum
benzoin (around 20%) and most berries (around 0.05%) (Budavari et al.,
1996). For example, ripe fruits of several Vaccinium species (e.g.,
cranberry, V. vitis idaea; bilberry, V. macrocarpon) contain as
much as 300-1300 mg free benzoic acid per kg fruit (Hegnauer, 1966).
Benzoic acid is also formed in apples after infection with the fungus
Nectria galligena (Harborne, 1983) or in Pinus thunbergii callus
inoculated with a pathogenic pine wood nematode (Bursaphelenchus
xylophilus) (Zhang et al., 1997). Among animals, benzoic acid has
been identified primarily in omnivorous or phytophageous species,
e.g., in viscera and muscles of the ptarmigan (Lagopus mutus)
(Hegnauer, 1989) as well as in gland secretions of male muskoxen
(Ovibos moschatus) (Flood et al., 1989) or Asian bull elephants
(Elephas maximus) (Rasmussen et al., 1990).
Owing to its occurrence in many organisms, benzoic acid is
naturally present in foods (review in Sieber et al., 1989, 1990). Some
typical examples specifying reported ranges of means in selected foods
have been compiled from Sieber et al. (1989) as follows:
Milk traces - 6 mg/kg
Yoghurt 12-40 mg/kg
Cheese traces - 40 mg/kg
Fruits (excluding traces - 14 mg/kg
Vaccinium species)
Potatoes, beans, cereals traces - 0.2 mg/kg
Soya flour, nuts 1.2-11 mg/kg
Honeys from different floral sources (n = 7) were found to
contain free benzoic acid at concentrations of <10 mg/kg (n = 5)
and of <100 mg/kg (n = 2) (Steeg & Montag, 1987).
Because benzoic acid and its compounds are used as food
preservatives (see section 4), some processed foods contain
artificially elevated concentrations of these substances (see section
6.2).
6.2 Human exposure
The main route of exposure of the general population to benzoic
acid or sodium benzoate is likely via foodstuffs that contain the
substances naturally or added as antimicrobial agents. There are a few
analyses of processed foodstuffs available. They refer to different
types of food items (juice, soft drinks, soya sauce varieties) from
the Philippines (a total of 44 samples) and from Japan (a total of 31
samples) and to orange drinks sampled in England. The concentrations
of sodium benzoate in the Philippine dietary samples ranged from 20 to
>2000 mg/litre. The range in the Japanese products was 50-200
mg/litre, thus reflecting the lower maximum level of sodium benzoate
allowed to be added to food in Japan as compared with the Philippines
(Villanueva et al., 1994). Orange drinks from England contained sodium
benzoate at concentrations ranging from 54 to 100 mg/litre (mean 76.7
mg/litre; n = 6) (Freedman, 1977).
Generally, the actual uptake depends on the individual's choice
of food to be consumed and the different limit values in different
countries. Several intake estimations have been published. Three
Japanese studies reported average daily intakes of benzoic acid from
processed foodstuffs to be 10.9 mg per person (Toyoda et al., 1983a)
and 1.4 mg per person (Toyoda et al., 1983b; Yomota et al., 1988),
corresponding to 0.02-0.2 mg/kg body weight (for persons with a body
weight of 50-70 kg). Both of the latter studies used the market basket
method for intake calculations, whereas the first-mentioned study
calculated intakes using the results of a national nutrition survey.
The concentrations of benzoic acid in 3319 food samples analysed for
this study (Toyoda et al., 1983a) ranged from not detected to 2100
mg/kg food. The maximum was found in salted fish (n = 7; mean 754
mg/kg). Another survey refers to the United Kingdom, where analyses of
benzoic acid in foods and drinks in which it is permitted as well as
intake estimates have been performed (UK MAFF, 1995). Sixty-five out
of 122 samples tested contained detectable benzoic acid. The highest
Table 2: Concentrations of benzoic acid in rain, snow, groundwater, and leachate samples.
Medium Location; sampling date Concentration Reference
(µg/litre)
Los Angeles area, California, Sum concentrationsa Kawamura & Kaplan
USA; 1982-1983 (1986)
Rain: urban 0.06-10.2 (n = 6)
Rain: semirural 0.02 (n = 1)
Snow: rural 0.04-0.1 (n = 3)
Groundwater Wyoming, USA (near underground 16-860 (n = 3) Stuermer et al.
coal gasification site; 15 months (1982)
after the end of gasification)
Groundwater Florida, USA (near wood treatment 10-27 500 (n = 3) Goerlitz et al.
facility); 1984 (1985)
Groundwater Ontario, Canada (near traces (n = 2) Barker et al.
landfillb); 1983 (1988)
Groundwater Barcelona area, Spain up to 0.21 (n = 3) Guardiola et al.
(near landfillb) (1989)
Leachate Ontario, Canada; 1981 <0.1->1000 (n = 5) Reinhard & Goodman
(from landfillb) (1984)
Leachate Ontario, Canada; 1983 traces (n = 2) Barker et al.
(from landfillb) (1988)
Leachate USA; 1986-1988 200-400c (n = 3) Ham et al.
(from foundry (1989)
wastes)
a Including benzoic acid, 3-methyl benzoic acid, and 4-methyl benzoic acid.
b Receiving rural, municipal (domestic), and industrial wastes.
c Concentrations estimated from gas chromatography/mass spectrometry data.
concentrations were found in sauces (mean 388 mg/kg; n = 20; range
71-948 mg/kg), reduced sugar jam (mean 216 mg/kg; n = 4; range
<20-333 mg/kg), non-alcoholic drinks (mean 162 mg/kg; n = 20; range
55-251 mg/kg), and semipreserved fish product (653 mg/kg; n = 1).
The survey found that the concentrations of benzoic acid detected
would lead to a dietary intake below 5 mg/kg body weight per day, even
for adults with an above-average consumption.
A frequent contributor to dietary exposure is soft drinks. A
rough estimation based on the average daily consumption in Germany of
such drinks (372 ml non-alcoholic beverages, excluding bottled water;
BAGS, 1995) by 19- to 24-year-old men, assuming the concentration of
benzoic acid present corresponds to a maximum allowable level of
150 mg/litre (EC, 1995), would result in a mean daily intake of
55.8 mg benzoic acid per person (or 0.80 mg/kg body weight, assuming
a 70-kg body weight). For comparison, a similar calculation with
sugar-free marmalade, jam, and similar spreads, which are allowed to
contain higher levels of benzoic acid (500 mg/kg; EC, 1995), would
result in a possible intake of 4.1 mg per person per day, or
0.06 mg/kg body weight per day (assumes a daily consumption of 8.2 g,
according to BAGS, 1995). This was more than a possible intake via
fruits containing natural benzoic acid. For example, a daily
consumption of 40.4 g of fruits (BAGS, 1995) would lead to a possible
intake of 0.57 mg benzoic acid per person per day (or 0.008 mg/kg body
weight for a 70-kg person), if the reported maximum of 14 mg benzoic
acid/kg (see section 6.1) were present.
The Joint FAO/WHO Expert Committee on Food Additives (JECFA)
assessed the intake of benzoates from information provided by nine
countries (Australia, China, Finland, France, Japan, New Zealand,
Spain, United Kingdom, and USA) (WHO, 1999). Because diets differ
among countries, the foods that contribute to benzoate intake would be
expected to vary. The food category that contributed most to benzoate
intake was soft drinks (carbonated, water-based, flavoured drinks) for
Australia/New Zealand, France, the United Kingdom, and the USA. In
Finland, 40% was in soft drinks. Soya sauce was the main source of
benzoate in China and the second most important in Japan. The best
estimates of national mean intakes of benzoates by consumers ranged
from 0.18 mg/kg body weight per day in Japan to 2.3 mg/kg body weight
per day in the USA. These estimates were based on analyses involving
either model diets or individual dietary records and maximum limits
specified by national governments or the European Union. The estimated
intake by high consumers of benzoates, based on food additive levels
in national standards, was 7.3 mg/kg body weight per day in the USA
and 14 mg/kg body weight per day in China.
Benzoates have been detected in groundwater, but not in
drinking-water.
Quantitative information on (oral or dermal/mucosal) exposure via
cosmetic, hygienic, or medical products is rare, but the data
available indicate a remarkable contribution to exposure. There are
reports on leaching of benzoic acid from denture-base acrylic resins.
After 10 days of immersion in artificial saliva, concentrations of up
to about 3 mg/litre have been observed for benzoic acid, which is
formed as a degradation product of the benzoyl peroxide that is added
as a polymerization initiator (Koda et al., 1989, 1990). In Japan,
commercial toothpastes have been found to contain benzoic acid at
concentrations ranging from 800 to 4450 mg/kg (n = 18). Use of the
toothpaste with the highest concentration (by 40 20-year-old female
students) would result in a calculated daily intake of about 2.23 mg
per person. This was about the same amount as their estimated intake
from diet (Ishida, 1996). Benzoic acid is also used in dermatology as
a fungicidal topical treatment for ringworm (Tinea spp.). The
emulsifying ointment preparation contains benzoic acid at 6% and is
applied twice daily (Goodman et al., 1990; BMA, 1998).
Recent quantitative monitoring data on concentrations of benzoic
acid or salts in ambient or indoor air are not available. Considering
the few (low) levels of benzoic acid measured in urban air in the
past, with a maximum of 0.38 µg/m3 (see section 6.1), inhalation may
contribute only marginally to exposure of the general population.
Using this maximum, a daily inhalative dose of 8.74 µg per person (or
0.12 µg/kg body weight) is obtained (assuming a daily inhalation
volume of 23 m3 for a 70-kg adult male; WHO, 1994).
Few quantitative data on occupational exposure have been
identified. Nevertheless, there is a potential for inhalation or
dermal contact in the chemical and allied product industries as well
as in workplaces where these products are used. Air samples (n = 50)
collected in an industrial environment (no further details given) over
a year's time showed benzoic acid concentrations ranging from not
detected to 1.5 mg/m3 (Halvorson, 1984). On the basis of the latter
value, an inhalative dose of 14.4 mg per person per 8-h working time
(or 0.2 mg/kg body weight) would result (assuming an inhalation volume
of 9.6 m3 for an 8-h exposure with light activity; WHO, 1994).
However, because of the lack of information on specific working
operations and conditions involved (e.g., duration of exposure, use of
protective clothes, etc.), it is impossible to derive a realistic
estimate of occupational exposure.
7. COMPARATIVE KINETICS AND METABOLISM IN LABORATORY ANIMALS
AND HUMANS
After oral ingestion of benzoic acid and sodium benzoate, there
is a rapid absorption (of undissociated benzoic acid) from the
gastrointestinal tract in experimental animals or humans (US FDA,
1972a, 1973). From the figures on excretion given below, 100%
absorption can be assumed. In humans, the peak plasma concentration is
reached within 1-2 h (Kubota et al., 1988; Kubota & Ishizaki, 1991).
Benzoic acid is not completely absorbed by the dermal route. In a
study with six human subjects, Feldmann & Maibach (1970) found an
uptake of 36% of the applied dose (14C-labelled benzoic acid
dissolved in acetone; 4 µg/cm2; circular area of 13 cm2; ventral
surface of the forearm; non-occlusive) within 12 h. The total uptake
within 5 days was 43%. In a second study with 6-7 subjects (comparable
method; application of 3, 400 or 2000 µg/cm2), the percent
absorption decreased from 35% to 14% within 24 h. However, the total
uptake per cm2 increased from 1 to 288 µg (Wester & Maibach, 1976).
For sodium benzoate, no data concerning dermal uptake were identified
in the literature.
In vivo dermal studies with benzoic acid in experimental
animals (e.g., guinea-pigs, mice, rats, pigs, dogs, rhesus monkeys)
confirm the results with humans (Hunziker et al., 1978;
Andersen et al., 1980; Wester & Noonan, 1980; Bronaugh et al., 1982a;
Reifenrath et al., 1984; Carver & Riviere, 1989; Maibach & Wester,
1989; Bucks et al., 1990). Absorption ranged from 25% in pigs
(Reifenrath et al., 1984; Carver & Riviere, 1989) to 89% in rhesus
monkeys (Wester & Noonan, 1980; Maibach & Wester, 1989; Bucks et al.,
1990). Due to the good database on humans and animals in vivo,
in vitro studies performed with animal or human skin are not
considered further (Franz, 1975; Bronaugh et al., 1982b;
Hotchkiss et al., 1992; MacPherson et al., 1996).
No information is available on absorption via inhalation.
After oral and dermal uptake, benzoate is metabolized in the
liver by conjugation with glycine, resulting in the formation of
hippuric acid (Feldmann & Maibach, 1970; US FDA, 1972a; WHO, 1996;
Feillet & Leonard, 1998). The rate of biotransformation in humans is
high: after oral doses of 40, 80 or 160 mg sodium benzoate/kg body
weight, the transformation to hippuric acid was independent of the
dose -- about 17-29 mg/kg body weight per hour, corresponding to about
500 mg/kg body weight per day (Kubota & Ishizaki, 1991). Other authors
obtained higher values of 0.8-2 g/kg body weight per day (US FDA,
1972a, 1973; WHO, 1996). Hippuric acid is rapidly excreted in urine.
In humans, after oral doses of up to 160 mg/kg body weight, 75-100% of
the applied dose is excreted as hippuric acid within 6 h after
administration, and the rest within 2-3 days (Kubota et al., 1988;
Fujii et al., 1991; Kubota & Ishizaki, 1991).
The limiting factor in the biosynthesis of hippuric acid is the
availability of glycine. The utilization of glycine in the
detoxification of benzoate results in a reduction in the glycine level
of the body. Therefore, the ingestion of benzoic acid or its salts
affects any body function or metabolic process in which glycine is
involved; for example, it leads to a reduction in creatinine,
glutamine, urea, and uric acid levels (US FDA, 1972a, 1973; Kubota &
Ishizaki, 1991; WHO, 1996).
Another metabolite of benzoate is the benzoyl glucuronide. For
example, the dog excretes considerable amounts of this metabolite in
the urine (20% after a single dose of 50 mg/kg body weight; Bridges et
al., 1970). In other species, this metabolite appears only after
higher doses of about 500 mg/kg body weight (see above) of benzoic
acid or sodium benzoate, resulting in a depletion of the glycine pool
(Bridges et al., 1970; US FDA, 1972a; Kubota et al., 1988). In cats,
glucuronidation is generally very low (Williams, 1967).
In some species, including humans, minor amounts of benzoic acid
itself are also excreted in the urine (Bridges et al., 1970; Kubota &
Ishizaki, 1991).
Experiments on the distribution and elimination of 14C-benzoate
in the rat have shown no accumulation of sodium benzoate or benzoic
acid in the body (US FDA, 1972a, 1973).
In the acid conditions of the stomach, the equilibrium moves to
the undissociated benzoic acid molecule, which should be absorbed
rapidly. Benzoate from sodium benzoate would change from the ionized
form to the undissociated benzoic acid molecule. As a result, the
metabolism and systemic effects of benzoic acid and sodium benzoate
can be evaluated together.
7.1 Precursors of benzoic acid
Benzyl acetate, its hydrolysis product, benzyl alcohol, and the
oxidation product of this alcohol, benzaldehyde, are precursors of
benzoic acid in experimental animals and humans. Benzyl acetate is
metabolized to benzoic acid and further to hippuric acid and benzoyl
glucuronide to an extent of >90% both in mice and in rats of
different strains. Benzyl alcohol was metabolized to benzoic acid and
its conjugates in preterm infants. Benzaldehyde is metabolized to
benzoic acid and its conjugates in rabbits to an extent of
approximately 90% (WHO, 1996).
8. EFFECTS ON LABORATORY MAMMALS AND IN VITRO TEST SYSTEMS
8.1 Single exposure
With oral LD50 values (administration by gavage) of 3040 mg
benzoic acid/kg body weight in rats (Bio-Fax, 1973) and 1940-2263 mg
benzoic acid/kg body weight in mice (McCormick, 1974; Abe et al.,
1984), the acute toxicity of benzoic acid is low. Clinical signs of
intoxication (reported for rats only) included diarrhoea, muscular
weakness, tremors, hypoactivity, and emaciation (Bio-Fax, 1973). With
oral LD50 values of 2100-4070 mg sodium benzoate/kg body weight in
rats, the acute toxicity of sodium benzoate is similar to that of
benzoic acid, as are the symptoms (Smyth & Carpenter, 1948;
Deuel et al., 1954; Bayer AG, 1977).
In four cats given diets containing 0 or 1% benzoic acid
(approximately 0 or 450-890 mg/kg body weight), aggression,
hyperaesthesia, and collapse starting 14-16 h after feed uptake were
seen at a dose level equal to 630 mg/kg body weight. The duration of
the syndrome was about 18-176 h, and the mortality rate was 50%. The
histopathological examination of the two cats that died revealed
degenerative changes in liver, kidneys, and lung, but no pathological
findings in brain or spinal cord (Bedford & Clarke, 1972). The authors
attributed the higher toxicity of benzoic acid in cats compared with
other species to the low capacity of cats for glucuronidation
(see section 7).
In rats, exposure by inhalation to 26 mg/m3 over 1 h caused no
mortality, but generalized inactivity and lacrimation were noted. The
gross autopsy gave no significant findings (no further information
available; Bio-Fax, 1973).
In a limit test with rabbits, no mortality or signs of
intoxication were seen after dermal application of 10 000 mg/kg body
weight. The gross autopsy gave no significant findings (no further
information available; Bio-Fax, 1973).
8.2 Irritation and sensitization
8.2.1 Benzoic acid
Although there is a wide range of results from mostly
non-standardized tests using various scoring systems, it can be
concluded that benzoic acid is slightly irritating to the skin and
irritating to the eyes.
In different experiments with rabbits, which have not been
performed according to current guidelines, benzoic acid applied as dry
powder or in the form of a paste was not irritating to slightly
irritating to the skin (score 1.66/8: Bio-Fax, 1973; no score given:
Bayer AG, 1978; primary skin irritation index 0.5 [no further
information available]: RCC Notox, 1988a).
In an acute eye irritation/corrosion study with rabbits conducted
according to OECD Guideline 405, some eye irritation was reported
after application of benzoic acid in the form of a paste. Within 72 h,
the scores for chemosis, reddening of the conjunctivae, iritis, and
keratitis always remained at <2 (Bayer AG, 1986).
In different non-standardized experiments with the solid
substance, moderately irritating to severely irritating effects on the
eye were noted (score 65/110: Bio-Fax, 1973; no score given: Bayer AG,
1978; score up to 108/110 [eyes rinsed after instillation] or up to
50/100 [eyes not rinsed]: Monsanto Co., 1983; score 35 according to
the scheme of Kay & Calandra, 1962: RCC Notox, 1988b).
In a maximization test, none of 15 guinea-pigs reacted positively
after induction and challenge with a 10-20% solution of benzoic acid
in water (Gad et al., 1986). In addition, the substance also tested
negative in a Buehler test with guinea-pigs and in an ear swelling
test and local lymph node assay with mice (Gad et al., 1986; Gerberick
et al., 1992). The concentrations used for induction and challenge
were 10-20% in acetone or water.
However, a dose-dependent positive result was obtained in an ear
swelling test with five guinea-pigs (induction with 0.2, 1, 5, or 20%
in absolute ethyl alcohol; no challenge) used as a model for detecting
agents causing non-immunological contact urticaria in humans. At
several other regions (back, abdomen, flank site), a concentration of
20% failed to produce any reactions (Lahti & Maibach, 1984).
8.2.2 Sodium benzoate
An acute dermal irritation/corrosion study with rabbits conducted
according to OECD Guideline 404 (no data about physical state; score
0: RCC Notox, n.d., a) as well as a non-standardized experiment with
the solid substance (score not given: Bayer AG, 1977) gave no
indication for skin irritating effects.
In a study performed according to OECD Guideline 405 (no data
about physical state; RCC Notox, n.d., b), sodium benzoate was only
slightly irritating to the eye (score 9.3, according to the scheme of
Kay & Calandra, 1962). The application of the solid substance in a
non-standardized experiment caused no irritation (score not given:
Bayer AG, 1977).
For sodium benzoate, no data on sensitizing effects were
identified in the available literature.
8.3 Short-term exposure
8.3.1 Oral exposure
In general, the database for benzoic acid and sodium benzoate is
limited, and there are no studies available performed according to
current guidelines. In addition, the documentation of these studies in
most cases is insufficient. Detailed information is given in Table 3.
From the available studies, it can be assumed that the toxicity
of benzoic acid after short-term oral exposure is low. In high-dosed
rats given approximately 2250 mg/kg body weight per day via diet over
5 days, excitation, ataxia, convulsions, and histopathological changes
in the brain were seen. The mortality was about 50%; in some cases,
bleeding into the gut was noted (Kreis et al., 1967). In two other
studies with rats dosed with approximately 825 mg/kg body weight per
day over 7-35 days (Kreis et al., 1967) or with 65-647 mg/kg body
weight per day over 28 days (Bio-Fax, 1973), no clear
treatment-related effects occurred. The reduced weight gain at 2250
and 825 mg/kg body weight per day may be attributed to reduced food
intake in the study by Kreis et al. (1967). The relevance of the
reduced relative kidney weight at 324 mg/kg body weight per day, which
was not dose-related and not accompanied by changes in
histopathological examinations, is unclear (Bio-Fax, 1973). As given
in Table 3, both studies have several limitations (i.e., missing
haematological and clinical chemical investigations, incomplete
histopathological examinations); therefore, both of these studies were
inadequate for derivation of a NO(A)EL.
More information on dose-response can be gained from the study of
Fujitani (1993), in which rats received sodium benzoate for 10 days in
feed. At the lowest tested concentration of 1358 mg/kg body weight per
day, changes in serum cholesterol levels occurred in females. At doses
of 1568 mg/kg body weight per day and above, changes in further serum
parameters and an increased relative liver weight were described.
Histopathological changes of the liver, increased relative kidney
weights, and disorders of the central nervous system (convulsions)
were seen after dosing via diet with approximately 1800 mg/kg body
weight per day. In several other studies listed in Table 3, adverse
effects were seen only at higher doses after feeding sodium benzoate
over periods from 10 to 42 days, so that a
lowest-observed-(adverse-)effect level (LO(A)EL) of 1358 mg sodium
benzoate/kg body weight per day for short-term exposure can be
derived.
With cats (Bedford & Clarke, 1972), also described in Table 3,
the effect levels with benzoic acid were lower. However, due to the
differences in the metabolism of benzoic acid in cats compared with
other experimental animals and humans, this study was not taken into
further consideration (see section 7).
Table 3: Toxicity of benzoic acid and sodium benzoate after short-term oral exposure.
Species; strain; Treatment Duration Organs examined in Resultsa Reference
number of animals (days) histopathology, clinical
per dosea chemistry, haematology
Benzoic acid
cat; 4 m 0 or 0.5% 3-4 liver, kidney, heart, mild hyperaesthesia, Bedford & Clarke
in diet stomach, lung, brain, apprehension, and (1972)
(approx. 0 spinal cord (only depression starting 48-92
or 300-420 mg/kg animals that died were h after uptake; duration of
body weight) examined); blood samples the syndrome: about 20-48 h;
were taken from surviving mortality rate: 50%;
cats degenerative changes in
liver, kidneys, and lung,
but no pathological findings
in brain or spinal cord;
surviving cats: urea and
serum alanine
aminotransferase (S-ALAT) *,
indicating liver and kidney
damage
cat; 4 m a) 100 or 200 a) 15 only blood samples no adverse effects Bedford & Clarke
mg/kg body weight were taken were reported (1972)
via diet
b) 0 or 0.25% in b) 23
diet (approx. 0
or 130-160 mg/kg
body weight)
Table 3 (cont'd)
Species; strain; Treatment Duration Organs examined in Resultsa Reference
number of animals (days) histopathology, clinical
per dosea chemistry, haematology
rat; Wistar; 0 or 3% in diet 1-5 heart, liver, spleen, body weight gain **; Kreis et al.
5-15 m (approx. 0 or kidney, brain in rats dosed over 5 days, (1967)
2250 mg/kg body disorders of the central
weight) nervous system (excitation,
ataxia, tonoclonic
convulsions); mortality
rate approx. 50%; in some
cases, bleeding into the
gut; brain damage (necrosis
of parenchymal cells of the
stratum granulosum of the
fascia dentata and the
cortex of the lobus
piriformis) in most animals
dosed over 3-5 days (still
present after 35 days)
rat; Wistar; 0 or 1.1% in 7-35 heart, liver, spleen, body weight gain **; Kreis et al.
5-10 m diet kidney, brain no clinical signs of (1967)
(approx. 0 or intoxication
825 mg/kg body
weight)
rat; albino; 10 m 0, 760, 3800, or 28 liver, kidney, no deaths or signs of Bio-Fax (1973)
7600 ppm via diet adrenals, testes intoxication
(approx. 0, 65, 324 mg/kg body
324, or 647 mg/kg weight: relative kidney
body weight) weights **; no further
information available
Table 3 (cont'd)
Species; strain; Treatment Duration Organs examined in Resultsa Reference
number of animals (days) histopathology, clinical
per dosea chemistry, haematology
Sodium benzoate
rat; F344/Ducrj; 0, 1.81, 2.09, or 10 liver, kidney; >1358 mg/kg body weight: Fujitani (1993)
6 m/f 2.4% in diet standard clinical changes in serum levels
(approx. 0, 1358, chemistry (cholesterol ** (f))
1568, or 1800 >1568 mg/kg body weight:
mg/kg body relative liver weight * (m);
weight) changes in serum levels
(albumin * (m), total
protein * (m))
1800 mg/kg body weight:
1/6 males died
(hypersensitivity,
convulsions); body
weight ** (m/f); relative
liver weight * (f); relative
kidney weights * (m/f);
absolute weights of spleen
and thymus ** (m);
absolute/relative weights
of thymus ** (f); changes
in serum levels
(gamma-glutamyltranspeptidase
(GGT) * (m), albumin * (f),
cholinesterase ** (f));
eosinophilic foci around
periportal vein and
enlargement of hepatocytes
with glassy cytoplasm in
the periportal area of the
liver (m); no changes in
the kidney (m)
Table 3 (cont'd)
Species; strain; Treatment Duration Organs examined in Resultsa Reference
number of animals (days) histopathology, clinical
per dosea chemistry, haematology
rat; Sherman; 0, 2, or 5% in 28 no data available 2200 mg/kg body weight: Fanelli & Halliday (1963)
6 m/f diet (approx. 0, slight depression of
2200, or 6700 body weight gain (m)
mg/kg body 6700 mg/kg body weight:
weight) mortality 100% within
11 days; signs of
intoxication included
hyperexcitability, urinary
incontinence, and
convulsions
no further information
available
rat; 28 (no 0 or 5% in diet 28 no data available mortality about 100% within Kieckebusch & Lang
further data) (approx. 0 or 3 weeks; decreased feed (1960)
3750 mg/kg body intake, diarrhoea,
weight) intestinal haemorrhage and
crusted blood in the nose;
no further information
available
rat; 5 (no 0 or 5% in diet >28 no data available mortality 80% within Kieckebusch & Lang
further data) (approx. 0 or 4-5 weeks; decreased (1960)
3750 mg/kg body weight; no further
body weight) information available
Table 3 (cont'd)
Species; strain; Treatment Duration Organs examined in Resultsa Reference
number of animals (days) histopathology, clinical
per dosea chemistry, haematology
rat; F344; 0, 0.5, 1, 2, 4, 42 histopathology performed, >375 mg/kg body weight: Sodemoto & Enomoto
10-11 m/f or 8% in diet but not further specified hypersensitivity after (1980)
(approx. 0, 375, dosing
750, 1500, 3000, >3000 mg/kg body weight:
or 6000 mg/kg mortality about 100% within
body weight) 4 weeks; apart from atrophy
of the spleen and lymph
nodes, no other
morphological changes were
noted
rat; Sherman; 0 or 16-1090 30 adrenals, upper intestine, no adverse effects were Smyth & Carpenter
5 m/f mg/kg body kidney, liver, spleen reported; no further (1948)
weight via diet information available
mouse; B6C3F1; 0, 2.08, 2.5, or 10 liver, kidney; standard „3750 mg/kg body weight: Fujitani (1993)
4-5 m/f 3% in diet clinical chemistry changes in serum levels
(approx. 0, 3000, (cholinesterase * (m))
3750, or 4500 4500 mg/kg body weight:
mg/kg body weight) hypersensitivity in all
animals; convulsions
1/5 males and 2/5 females
(both females died);
absolute/relative liver
weight * (m/f); relative
kidney weight * (f);
changes in serum levels
(cholesterol * (m),
phospholipids * (m));
Table 3 (cont'd)
Species; strain; Treatment Duration Organs examined in Resultsa Reference
number of animals (days) histopathology, clinical
per dosea chemistry, haematology
enlarged hepatocytes,
single cell necrosis
and vacuolation of
hepatocytes in all
livers (m); no changes
in the kidney (m/f)
mouse; albino 0, 0.5, 1, 2, 4, 35 survival, chemical 3000 mg/kg body weight: Toth (1984)
Swiss; 4 m/f or 8% via consumption, histological "suitable for lifelong
drinking-water changes (not further treatment" based on
(approx. specified) (prestudy four parameters: survival,
0-12 000 mg/kg for carcinogenicity study) body weight, chemical
body weight) consumption, and histology
6000 mg/kg body weight:
mortality 75% in m/f;
body weight of surviving
mice ** (m/f)
12 000 mg/kg body weight:
mortality 100% within
3 weeks
a m = male; f = female.
8.3.2 Inhalation exposure
Ten CD rats per sex per group were exposed to 0, 25, 250, or
1200 mg benzoic acid dust aerosol/m3 (analytical concentration; mass
aerodynamic diameter [MAD]/sigma g (standard deviation): 0, 4.6/3.1,
4.4/2.1, 5.2/2.1; mass median aerodynamic diameter [MMAD]: 4.7 µm) for
6 h per day and 5 days per week over 4 weeks. After this time, various
serum biochemical, haematological, organ weight, and histopathological
examinations were conducted. At >25 mg/m3, an increased incidence
of interstitial inflammatory cell infiltrate and interstitial fibrosis
in the trachea and lungs in treated animals compared with controls was
seen. Although the number of these microscopic lesions was higher in
treated animals than in controls, there was no clear dose dependency
for this effect. A concentration of >250 mg/m3 resulted in upper
respiratory tract irritation, as indicated by inflammatory exudate
around the nares, and significantly decreased absolute kidney weights
in females. In the highest dose group, one rat per sex died, and the
body weight gain was significantly decreased in males and females
compared with controls. In addition, a significant decrease in
platelets (males/females), absolute/relative liver weights (males),
and trachea/lung weights (females) was noted (Velsicol Chemical
Corp., 1981).
Studies concerning repeated exposure by inhalation to sodium
benzoate were not identified in the available literature.
8.3.3 Dermal exposure
Studies concerning repeated dermal exposure to benzoic acid or
sodium benzoate were not identified in the available literature.
8.4 Long-term exposure
In general, the database for benzoic acid and sodium benzoate is
limited, and there are no studies available performed according to
current guidelines. In addition, the documentation in most cases is
limited. Detailed information is given in Table 4.
8.4.1 Subchronic exposure
In a 90-day study with rats dosed with 0, 1, 2, 4, or 8% sodium
benzoate via diet, the mortality in the highest dose group (approx.
6290 mg/kg body weight per day) was about 50%. Other effects in this
group included a reduced weight gain, increased relative weights of
liver and kidneys, and pathological changes (not further specified) in
these organs (Deuel et al., 1954).
Table 4: Results of studies concerning long-term oral exposure to benzoic acid and sodium benzoate.
Species; strain; Treatment Duration Examinations; Resultsa Reference
number of animals organs in
per dosea histopathology,
clinical chemistry,
haematology
Benzoic acid
rat; Wistar; 0 or 1.5% in diet 18 months no data available reduced weight gain with Marquardt (1960)
dose group: (approx. 0 or decreased feed intake;
30 m/20 f; 750 mg/kg body increased mortality rate
controls: weight) (15/50 vs. 3/25 in
13 m/12 f controls); no further
information available
(only provisional results
are given)
rat; Wistar or 0 or 1.5% in diet 18 months no data available reduced weight gain with Marquardt (1960)
Osborne-Mendel; (approx. 0 or decreased feed intake;
dose group: 750 mg/kg body no further information
20 m; controls: weight) available (only
10 m provisional results are
given)
rat; not given; 0, 0.5, or 1% in generation 1 histopathology in no effects on growth and Kieckebusch & Lang
20 m/f diet and 2: animals of organ weights; feeding of (1960)
(approx. 0, 250, lifelong generation 3 0.5% led to prolongation
or 500 mg/kg body generation 3: (not further specified) of survival compared with
weight) 16 weeks controls; no further
generation 4: information available
until breeding
Table 4 (cont'd)
Species; strain; Treatment Duration Examinations; Resultsa Reference
number of animals organs in
per dosea histopathology,
clinical chemistry,
haematology
Sodium benzoate
rat; Sherman; 0, 1, 2, 4, or 8% 90 days histopathology 6290 mg/kg body weight: Deuel et al.
5 m/f in diet (approx. 0, performed, but mortality about 50%; (1954)
640, 1320, 2620, not further specified weight gain **;
or 6290 mg/kg body relative weights of
weight) liver and kidneys *;
pathological lesions
(not further specified)
in liver and kidneys
rat; F344; 0, 1, or 2% in 18-24 months histopathology average mortality rate of Sodemoto & Enomoto
dose group: diet performed, but not all animals during the (1980)
50 m/52 f; (m: approx. 0, 700, further specified first 16 months: 14.5%
controls: or 1400 mg/kg (all dead rats showed
body weight; f: pneumonia with abscess);
25 m/43 f about 100 rats including
approx. 0, 290, controls died after
or 580 mg/kg 16 months due to
body weight) haemorrhagic pneumonia
(infection); no adverse
clinical signs and no
differences in average
body weight and mortality
in dosed animals compared
with controls;
non-carcinogenic effects
not reported
Table 4 (cont'd)
Species; strain; Treatment Duration Examinations; Resultsa Reference
number of animals organs in
per dosea histopathology,
clinical chemistry,
haematology
mouse; 0 or 2% via lifelong liver, spleen, kidney, no difference in survival Toth (1984)
albino Swiss; drinking-water bladder, thyroid, rates in treated animals
dose group: (approx. 0 or heart, pancreas, compared with controls;
50 m/f; 5960-6200 mg/kg testes, ovaries, brain, no pathological or
controls: 99 m/f body weight) nasal turbinates, lung statistical evidence of
tumour induction
a m = male; f = female.
8.4.2 Chronic exposure and carcinogenicity
In two studies with rats given 1.5% benzoic acid via diet
(approximately 750 mg/kg body weight per day), the animals showed a
reduced weight gain with decreased feed intake after dosing over
18 months. In one of these studies, mortality was increased
(15/50 rats of both sexes versus 3/25 in controls) (Marquardt, 1960).
No further information on these studies is available, as only
provisional results were published. In a four-generation study with
rats, no effects on life span, growth rate, or organ weights were
reported after dosing with up to 1% in the diet (approximately
500 mg/kg body weight per day) (Kieckebusch & Lang, 1960). Only
animals of the third generation were autopsied after 16 weeks, but
it is not clear if a complete histopathological investigation was
performed.
With sodium benzoate, two long-term studies with rats
(administration of up to 1400 mg/kg body weight per day via diet over
18-24 months; Sodemoto & Enomoto, 1980) or mice (lifelong application
of up to 6200 mg/kg body weight per day via drinking-water; Toth,
1984) are available. The results gave no indication of a carcinogenic
effect in the tested animals. Although the study with mice was not
performed according to current guidelines, the results seem to be
reliable, due to a sufficient number of animals and detailed
histopathological examinations. However, the results from the study
with rats are uncertain, due to a very high mortality in animals of
all dose groups, including controls (from an "infection" after
16 months), no detailed information about dosing regimen (only mean
values given), and the considerable differences in the body weight of
male and female rats (the body weight of females was about twice that
of males).
8.4.3 Carcinogenicity of benzyl acetate, benzyl alcohol, and
benzaldehyde
As benzyl acetate, benzyl alcohol, and benzaldehyde are
practically quantitatively metabolized via benzoic acid (see section
7.1), data on their carcinogenicity from 2-year studies may be used as
supportive evidence in the assessment of the hazards associated with
benzoic acid.
Benzyl acetate was administered in corn oil via gavage to F344/N
rats (0, 250, or 500 mg/kg body weight per day) or B6C3F1 mice
(0, 500, or 1000 mg/kg body weight per day). In high-dose male rats,
the incidence of acinar cell adenomas of the exocrine pancreas was
increased, whereas there was no evidence of carcinogenicity in female
rats. In high-dose male and female mice, benzyl acetate caused
increased incidences of hepatocellular adenomas and squamous cell
neoplasms of the forestomach (US NTP, 1986). In contrast to these
findings, no such tumours were observed in another study with the same
strain of rats and mice when benzyl acetate was administered via diet
(rats: <575 mg/kg body weight per day; mice: <375 mg/kg body
weight per day) (US NTP, 1993).
With benzyl alcohol, no treatment-related increase in tumours was
observed in F344/N rats or B6C3F1 mice after administration of
<400 mg/kg body weight per day in rats or <200 mg/kg body weight
per day in mice by gavage in corn oil (US NTP, 1989).
In B6C3F1 mice dosed with benzaldehyde in corn oil by gavage
(males: 0, 200, or 400 mg/kg body weight per day; females: 0, 300, or
600 mg/kg body weight per day), the incidences of squamous cell
papillomas of the forestomach were significantly greater in both
exposure groups than in controls. A dose-related increase in the
incidence of forestomach hyperplasia was also observed. In F344/N rats
dosed with <400 mg/kg body weight per day, there was no evidence of
carcinogenic activity (US NTP, 1990).
8.5 Genotoxicity and related end-points
8.5.1 Benzoic acid
Benzoic acid tested negative in several Ames tests and in one DNA
damage assay with different Salmonella typhimurium strains in the
presence or absence of metabolic activation (McCann et al., 1975;
Ishidate et al., 1984; Nakamura et al., 1987; Zeiger et al., 1988).
Only in one recombination assay with Bacillus subtilis H17 and M45
was a positive result obtained (Nonaka, 1989). However, due to missing
experimental details (only results given), the validity of this study
cannot be judged. There was no indication of genotoxic activity
(chromosome aberrations, sister chromatid exchange) in tests with
mammalian cells (Chinese hamster CHL and CHO cells, human
lymphoblastoid cells, human lymphocytes) without metabolic activation
(Oikawa et al., 1980; Tohda et al., 1980; Ishidate et al., 1984;
Jansson et al., 1988).
In vivo studies with benzoic acid were not identified in the
literature.
8.5.2 Sodium benzoate
Sodium benzoate also gave negative results in some Ames tests and
in Escherichia coli in the presence or absence of metabolic
activation (Ishidate et al., 1984; Prival et al., 1991). As with
benzoic acid in recombination assays with Bacillus subtilis H17 and
M45, positive results were obtained (Ishizaki & Ueno, 1989; Nonaka,
1989). Although sodium benzoate tested negative in a cytogenetic assay
with WI-38 cells in the absence of metabolic activation (US FDA,
1974), consistently positive results (in contrast to the negative
results of benzoic acid) were obtained in tests on sister chromatid
exchange and chromosome aberrations with CHL/CHO and DON cells or
human lymphocytes without metabolic activation (Abe & Sasaki, 1977;
Ishidate & Odashima, 1977; Ishidate et al., 1984, 1988; Xing & Zhang,
1990). However, from the limited information given in the publications
(i.e., only results given), it cannot be judged if these positive
results may have been attributable to cytotoxic effects.
In a valid in vivo study performed by the US FDA (1974), sodium
benzoate tested negative in a cytogenetic assay (bone marrow) in rats
after single or multiple oral application of doses up to 5000 mg/kg
body weight. In a study with mice (comparable dosing scheme), there
was also no indication of mutagenic activity in a host-mediated assay
(US FDA, 1974).
However, in a dominant lethal assay with rats (comparable dosing
scheme; males were mated with untreated females following 7 or 8 weeks
of dosing), some statistically significant and dose-related findings
were reported in week 7: decreased fertility index for both treatment
regimens and an increased number of preimplantation losses after
single dosing (US FDA, 1974).
In summary, the in vitro studies with benzoic acid gave no
indications for genotoxic effects, whereas in vivo studies were not
identified. Sodium benzoate was also inactive in bacterial test
systems, whereas tests with mammalian cells gave consistently positive
results. In addition, in an in vivo study with sodium benzoate
(dominant lethal assay in rats), a positive result was obtained. As a
result, a genotoxic activity of sodium benzoate cannot be ruled out
entirely at present.
Detailed information concerning the genotoxicity of benzoic acid
and sodium benzoate in vitro is given in Table 5.
8.6 Reproductive and developmental toxicity
8.6.1 Fertility
There are no studies available dealing specifically with the
effects of benzoic acid or sodium benzoate on fertility that have been
conducted according to current protocols.
In a four-generation study with male and female rats, no adverse
effects on fertility or lactation (only investigated parameters) were
seen after dosing with benzoic acid at up to 1% in the diet
(approximately 500 mg/kg body weight per day) (see also section 8.4.2;
Kieckebusch & Lang, 1960).
In studies with repeated oral application, no effects on the
testes were observed in rats after dosing with benzoic acid at up to
647 mg/kg body weight per day in the diet for 4 weeks (see also Table
3; Bio-Fax, 1973) or in mice after lifelong application of 6200 mg
sodium benzoate/kg body weight per day via drinking-water (see also
Table 4; Toth, 1984).
In summary, no clear statement can be given as to the possible
effects of benzoic acid or sodium benzoate on fertility.
8.6.2 Developmental toxicity
In a study with pregnant rats given only one oral dose of benzoic
acid (510 mg/kg body weight on gestation day 9), there was no
indication of an increase in resorption rates or malformations
(Kimmel et al., 1971).
For sodium benzoate, several teratogenicity studies are available
that have been performed with different species. As given in Table 6,
no effects were seen in dams or offspring of rats, mice, rabbits, or
hamsters given oral doses of up to 300 mg/kg body weight per day
(highest dose tested) during gestation (US FDA, 1972b). In a study
with rats by Onodera et al. (1978), doses of 4% or 8% via diet (uptake
of 1875 or 965 mg/kg body weight per day) induced severe maternal
toxicity (no weight gain/loss in body weight, increased mortality) and
were associated with embryotoxic and fetotoxic effects as well as
malformations. However, the authors suggested that the effects on the
dams and fetuses at >4% dietary levels were caused by reduced
maternal feed intake, leading to malnutrition. The intake of sodium
benzoate in the highest dose group (8%) was lower than that at 2%,
where no adverse effects were seen. From this study, a NO(A)EL of
about 1310 mg/kg body weight per day can be derived. In a study with
rats by Minor & Becker (1971), however, fetotoxic and teratogenic
effects occurred at 1000 mg/kg body weight per day. In this study,
sodium benzoate was applied by intraperitoneal injection. Therefore,
differences in pharmacokinetics between oral and intraperitoneal
administration may be the reason for the higher sensitivity.
Studies performed with eggs of leghorn hens (single injection of
<5 mg per egg), chick embryo neural retina cells
(lowest-observed-effect concentration [LOEC] of 34.7 mmol/litre), and
a chick embryotoxicity screening test (single injection of <0.1 mg
per embryo) gave no indication of embryotoxic or teratogenic effects
(Verrett et al., 1980; Jelinek et al., 1985; Daston et al., 1995).
8.6.3 Reproductive toxicity of benzyl acetate, benzyl alcohol,
and benzaldehyde
As benzyl acetate and benzyl alcohol are practically
quantitatively metabolized via benzoic acid (see section 7.1), data on
their reproductive toxicity may be used as supportive evidence in the
assessment of the hazards associated with benzoic acid.
Dietary benzyl acetate (up to 5% in the diet for 13 weeks) had no
effect on the weights of the epididymis, cauda epididymis, or testis,
on sperm motility or density, or on the percentage of abnormal sperm
in mice or rats (US NTP, 1993).
Benzyl acetate (0, 10, 100, 500, or 1000 mg/kg body weight per
day by gavage on days 6-15) had no significant effects on maternal
health in rats and did not induce changes in the numbers of corpora
lutea, implantations, live or dead fetuses, or resorptions,
implantation ratio, sex ratio, external or internal malformations, or
placental weight. Fetal weights were significantly reduced at the
highest dose (Ishiguro et al., 1993).
Benzyl alcohol at 550 mg/kg body weight per day by gavage on days
6-15 of pregnancy had no effect on gestation index, average number of
live pups per litter, postnatal survival, or pup body weight on days
0 and 3 in CD-1 mice (York et al., 1986), while 750 mg/kg body weight
per day (days 7-14) induced a reduction in the pup weight and maternal
weight gain, but no pup mortality or changes in mating or gestation
indices, the total number of resorptions, or the number of live pups
per litter (Hardin et al., 1987).
Table 5: Genotoxicity of benzoic acid and sodium benzoate in vitro.
Resultsa
Species End-point Concentration Without With Remarks Reference
(test system) range metabolic metabolic
activation activation
Benzoic acid
Salmonella Reverse 10-1000 - - McCann et al.
typhimurium mutations µg/plate (1975)
TA 98, TA 100,
TA 1535, TA 1537
Salmonella Reverse 33-10 000 - - cytotoxic effects Zeiger et al.
typhimurium mutations µg/plate at >5000 (1988)
TA 97, TA 98, µg/plate
TA 100, TA 1535,
TA 1537
Salmonella Reverse up to 10 000 - - 10 000 µg/plate was Ishidate et al.
typhimurium mutations µg/plate the highest (1984)
TA 92, TA 94, non-cytotoxic
TA 98, TA 100, concentration tested
TA 1535, TA 1537
Salmonella DNA damage up to - - no further Nakamura et al.
typhimurium (umu test) 1670 µg/ml information available (1987)
TA 1535/pSK 1002 (only results given)
Bacillus Recombination not given tested positive (no Nonaka
subtilis assay further information (1989)
H17, M45 available, only
summary given)
Table 5 (cont'd)
Resultsa
Species End-point Concentration Without With Remarks Reference
(test system) range metabolic metabolic
activation activation
Chinese hamster Chromosome up to ? 0 1500 µg/ml was given Ishidate et al.
cells (CHL) aberration 1500 µg/ml as maximum effective (1984)
concentration; result
given as negative in
Ishidate et al.
(1988)
Human Sister 1-30 - 0 cytotoxic effects Tohda et al.
lymphoblastoid chromatid mmol/litre at 30 mmol/litre (1980)
cells exchange
(transformed
by Epstein-Barr
virus)
Human Sister up to 2 - 0 Jansson et al.
lymphocytes chromatid mmol/litre (1988)
exchange
Chinese Sister up to - 0 Oikawa et al.
hamster chromatid 10 mmol/litre (1980)
cells (CHO) exchange
Table 5 (cont'd)
Resultsa
Species End-point Concentration Without With Remarks Reference
(test system) range metabolic metabolic
activation activation
Sodium benzoate
Salmonella Reverse up to - - 3000 µg/plate was Ishidate et al.
typhimurium mutations 3000 µg/plate the highest (1984)
TA 92, TA 94, non-cytotoxic
TA 98, TA 100, concentration tested
TA 1535, TA 1537
Salmonella Reverse 33-10 000 - - Prival et al.
typhimurium mutations µg/plate (1991)
TA 98, TA 100,
TA 1535, TA 1537,
TA 1538
Escherichia coli Reverse 33-10 000 - - Prival et al.
WP2 mutation µg/plate (1991)
assay
Bacillus Recombination not given tested positive Nonaka (1989)
subtilis assay information
H17, M45 available, only
summary given)
Bacillus Recombination -S9: (+) (+) Ishizaki & Ueno
subtilis assay 20 mg/disc (1989)
H17, M45 +S9:
16 mg/disc
Table 5 (cont'd)
Resultsa
Species End-point Concentration Without With Remarks Reference
(test system) range metabolic metabolic
activation activation
WI-38 cells Cytogenetic 10-1000 µg/ml - 0 examination of US FDA (1974)
assay anaphase preparations
cytotoxic effects
at >500 µg/ml
Chinese hamster Chromosome up to + 0 2000 µg/ml was Ishidate et al.
cells (CHL) aberration 2000 µg/ml given as maximum (1984, 1988)
effective
concentration
Chinese hamster Chromosome 139 mg/ml + 0 only maximum Ishidate &
cells (CHL) aberration effective dose given Odashima (1977)
Chinese hamster Chromosome 290 µg/ml 0 0 only minimum Ishidate et al.
cells (DON) aberration effective dose given (1988)
Chinese hamster Chromosome 1-10 + 0 Abe & Sasaki
cells (DON) aberration mmol/litre (1977)
Chinese hamster Sister 1-10 (+) 0 slight increase Abe & Sasaki
cells (DON) chromatid mmol/litre without dosage (1977)
exchange effect
Human Sister 10 + 0 Xing & Zhang
lymphocytes chromatid mmol/litre (1990)
exchange
a -, negative; +, positive; (+) weakly positive; ?, equivocal; 0, not tested.
Table 6: Results of studies concerning reproductive and developmental toxicity of benzoic acid and sodium benzoate.
Species; strain; Application Durationb Parameters Results NO(A)EL Reference
number of animals investigated (mg/kg
per dosea body
weight)
Benzoic acid
rat; Wistar; 0 or 510 gd 9 F0: implantation F0: resorption rates 510 Kimmel et al.
dose group: mg/kg body and resorption sites were given as (1971)
7 f; controls: weight F1: malformations "comparable with
not given via gavage controls"
F1: malformations
(not further specified)
were given as
"comparable with controls"
no further information
available
rat; not given; 0, 0.5, or 1% F0 and F1: fertility and F0-F3: no adverse 500 Kieckebusch &
20 f in diet lifelong lactation effects compared with Lang (1960)
(approx. 0, F2: 16 controls were reported
250, or 500 weeks no further information
mg/kg body F3: until available
weight) breeding
Sodium benzoate
rat; Wistar; 0, 1.75, 8, gd 6-15 F0: numbers of corpora F0 and F1: no adverse 175 US FDA
20 f 38, or 175 lutea, implantation effects compared with (1972b)
mg/kg body and resorption sites, controls were reported
weight via examination of the
gavage urogenital tract
Table 6 (cont'd)
Species; strain; Application Durationb Parameters Results NO(A)EL Reference
number of animals investigated (mg/kg
per dosea body
weight)
F1: numbers of live
and dead fetuses, body
weights, gross
examination for
external
malformations,
microscopic visceral
and skeletal
examination
mouse; 0, 1.75, 8, gd 6-15 F0: numbers of corpora F0 and F1: no adverse 175 US FDA
CD-1; 38, or 175 lutea, implantation effects compared with (1972b)
25-31 f mg/kg body and resorption sites, controls were reported
weight via examination of the
gavage urogenital tract
F1: numbers of live
and dead fetuses, body
weights, gross
examination for
external
malformations,
microscopic visceral
and skeletal
examination
rabbit; Dutch 0, 2.5, 12, gd 6-18 F0: numbers of corpora F0 and F1: no 250 US FDA
belted; 14-32 f 54, or 250 lutea, implantation adverse effects (1972b)
mg/kg body and resorption sites, compared with
weight via examination of the controls were
gavage urogenital tract reported
Table 6 (cont'd)
Species; strain; Application Durationb Parameters Results NO(A)EL Reference
number of animals investigated (mg/kg
per dosea body
weight)
F1: numbers of live
and dead fetuses, body
weights, gross
examination for
external
malformations,
microscopic visceral
and skeletal
examination
hamster; 0, 3, 14, 65, gd 6-10 F0: numbers of corpora F0 and F1: no adverse 300 US FDA
golden; or 300 mg/kg lutea, implantation effects compared with (1972b)
22 f body weight and resorption sites, controls were reported
via gavage examination of the
urogenital tract
F1: numbers of live
and dead fetuses,
body weights, gross
examination for
external
malformations,
microscopic visceral
and skeletal
examination
Table 6 (cont'd)
Species; strain; Application Durationb Parameters Results NO(A)EL Reference
number of animals investigated (mg/kg
per dosea body
weight)
rat; 0, 100, 315, a) gd 9-11 F0: not specified a) F0: no data given 315 Minor & Becker (1971)
Sprague-Dawley or 1000 mg/kg b) gd 12-14 F1: body weights, in F1: 1000 mg/kg body
(no further data) body weight utero deaths, weight: body weights **;
intraperitoneally gross anomalies in utero deaths * (16%);
gross anomalies * (not
further specified)
b) F0: no data given
F1: 1000 mg/kg body
weight: body weights **;
in utero deaths * (12%);
gross anomalies <--> (not
further specified)
no further information
available
rat; Wistar; 0, 1, 2, 4, gd 1-20 a) all but five a) >4% (1875 or 965 mg/kg 1310 Onodera et al. (1978)
27-30 f or 8% via diet animals in each group body weight):
(approx. 0, 700, were sacrificed on F0: weight gain <-->
1310, 1875, or gd 20 (numbers of feed intake **;
965 mg/kg body viable/dead fetuses, mortality * (convulsions,
weight) early/late depressed motor activity)
resorptions, fetal, F1: number of
placental, and ovarian dead/resorbed fetuses *;
weights, and body weight of viable
abnormalities of fetuses **; mild
maternal organs and systemic oedema,
fetal appearance were anophthalmia,
recorded) microphthalmia,
Table 6 (cont'd)
Species; strain; Application Durationb Parameters Results NO(A)EL Reference
number of animals investigated (mg/kg
per dosea body
weight)
b) the remaining five hydrocephalus,
dams delivered pyelectasis, hydroplasia,
naturally (number of cerebral hypoplasia;
offspring, survival, delayed ossification,
body weight, and lumbar or cervical ribs,
abnormalities were and varied sternebrae
recorded); 3 weeks 8%: F0: body weight **
after birth, all b) F1:
surviving pups were <2% (1310 mg/kg body
weaned and examined weight): no adverse
for gross effects compared with
abnormalities controls
(one-half of the pups >4% (1875 or 965 mg/kg
and all dams were body weight): delivery
necropsied); the rates ** (50 and 8.2%,
remaining pups were respectively); complete
necropsied at 8 weeks loss of litters after
of age (body weight parturition
and food intake were
measured weekly until
necropsy)
a m = male; f = female.
b gd = gestation day.
9. EFFECTS ON HUMANS
Cases of urticaria, asthma, rhinitis, or anaphylactic shock have
been reported following oral, dermal, or inhalation exposure to
benzoic acid and sodium benzoate. The symptoms appear shortly after
exposure and disappear within a few hours, even at low doses (Maibach
& Johnson, 1975; Clemmensen & Hjorth, 1982; Larmi et al., 1988; Ring,
1989; Gailhofer et al., 1990; Aberer et al., 1992; Lahti et al., 1995;
Anderson, 1996; Bindslev-Jensen, 1998; Coverly et al., 1998).
In the literature, several studies (e.g., oral provocation tests
or patch tests) are available, which have been performed with small
groups of patients suffering from urticaria, dermatitis, asthma, and
Melkersson-Rosenthal syndrome (Juhlin et al., 1972; Freedman, 1977;
Osterballe et al., 1979; Lahti & Hannuksela, 1981; Clemmensen &
Hjorth, 1982; Ibero et al., 1982; Moneret-Vautrin et al., 1982; Veien
et al., 1987; Aguirre et al., 1993; McKenna et al., 1994; BUA, 1995;
Munoz et al., 1996; Petrus et al., 1996; Vogt et al., 1999). In most
of these studies, atopic individuals have demonstrated reactions to
oral and dermal challenge with benzoic acid or sodium benzoate.
The information concerning skin reactions caused by benzoic acid
or sodium benzoate in the general population is limited. In a study
with 2045 patients of dermatological clinics, only 5 persons
(approximately 0.2%) showed a positive reaction in patch tests
(Brasch et al., 1993), while 34 of 5202 patients (approximately 0.7%)
with contact urticaria reacted positively (Broeckx et al., 1987). From
these data, it can be concluded that skin reactions caused by benzoic
acid or sodium benzoate in the healthy general population are rare.
In US FDA (1972a) and WHO (1996), several older studies
concerning oral exposure to benzoic acid or sodium benzoate are
described. However, owing to the limited number of individuals (mostly
single case studies), the validity of these studies is limited. No
adverse effects were reported after a single oral dose of 10 000 mg
benzoic acid or up to 1000 mg per day over a period of up to 92 days
(Gerlach, 1909). In another study with volunteers given 1000, 1500,
2000, or 2500 mg/day for 5 days each, marked symptoms, signs of
discomfort, and malaise (nausea, headache, weakness, burning and
irritation of oesophagus) were reported (Wiley & Bigelow, 1908).
Chittenden et al. (1909) found no abnormalities in blood picture,
urine composition, nitrogen balance, or well-being in six men given
300-400 mg per day via diet for up to 62 days. In nine patients on
penicillin treatment given 12 000 mg benzoic acid divided into eight
doses over 5 days in eight subjects and over 14 days in one subject,
no adverse effects on blood urea nitrogen or creatinine clearance were
reported (Waldo et al., 1949). A single dose of 2000-3000 mg sodium
benzoate caused signs of intoxication similar to those described for
benzoic acid by Wiley & Bigelow (1908).
Sodium benzoate is used in the treatment of patients with urea
cycle enzymopathies (i.e., hyperammonaemia due to inborn errors of
urea synthesis) in order to facilitate an alternative pathway of
nitrogen excretion. The therapeutic dose given over several years is
in the range of 250-500 mg/kg body weight per day (Batshaw & Brusilow,
1981; Green et al., 1983; Batshaw & Monahan, 1987; O'Connor et al.,
1987; Kubota & Ishizaki, 1991; Tremblay & Qureshi, 1993; Feillet &
Leonard, 1998). At this dose level, clinical signs of toxicity are
rare and in most cases limited to anorexia and vomiting, especially
after intravenous bolus infusions.
10. EFFECTS ON OTHER ORGANISMS IN THE LABORATORY
AND FIELD
10.1 Aquatic environment
For the toxicity data mentioned in this section, it is not always
stated whether the cited effect values are based on nominal or
measured concentrations of benzoic acid or sodium benzoate. However,
because of their water solubility, their insignificant volatility, and
their low adsorption potential (see sections 2 and 5), all nominal
concentrations of the test substances are expected to correspond to
effective concentrations, even in tests with open systems and longer
exposure durations.
In Table 7, several valid toxicity test results for the most
sensitive aquatic species of various taxonomic groups -- bacteria,
cyanobacteria, green algae, protozoa, invertebrates, and vertebrates
-- with benzoic acid have been compiled. From the aquatic organisms
tested so far, cyanobacteria (Anabaena inaequalis) proved to be most
sensitive, showing a 14-day EC50 of 9 mg/litre in the cell
multiplication inhibition test (Stratton & Corke, 1982). EC50/LC50
values (24-96 h) for most of the other aquatic species tested
(protozoa, molluscs, crustaceans, fish, amphibians) were in the range
of 100-1291 mg/litre. As seen with daphnids, the pH value of the test
medium influences the toxicity of benzoic acid, which proved to be
more toxic at lower pH levels (Bringmann & Kuehn, 1980). Developmental
toxicity effects seen in frog (Xenopus) embryos were craniofacial
defects, especially microcephaly, and abnormal gut coiling
(Dawson et al., 1996). A recently developed cytotoxicity assay with
cultured fathead minnow (Pimephales promelas) cells resulted in a
PI50 (the concentration required to induce a 50% reduction in total
protein content) of 1450 mg/litre (Dierickx, 1998).
Ninety-six-hour LC50 values of >100 mg sodium benzoate/litre
have been found for Daphnia magna (first and second larval instar)
and Gammarus fasciatus (juvenile: 7 mg in size) under static test
conditions (multispecies test; pH 6.5-8; 20°C) (Ewell et al., 1986).
The same was true for juveniles of other invertebrates tested
simultaneously: Asellus intermedius (Arthropoda; 12 mg body weight),
Dugesia tigrina (Platyhelminthes; 6 mg body weight), Helisoma
trivolvis (Mollusca; 180 mg body weight), and Lumbriculus variegatus
(Annelida; 6 mg body weight) (Ewell et al., 1986).
Two different tests with the freshwater fathead minnow
(P. promelas; juvenile stages) resulted in 96-h LC50 values of 484
mg sodium benzoate/litre (measured concentration; flow-through system;
pH 7.4; 24°C) (Geiger et al., 1985) and >100 mg/litre (nominal
concentration; static system; pH 6.5-8.5; 20°C) (Ewell et al., 1986).
Table 7: Aquatic toxicity of benzoic acid.
Most sensitive species Special Effective Reference
(test method/end-point) features concentration
(mg/litre)
Mixed microbial inoculum
Activated sludge pH 7.5 3-h EC50 >1000 Klecka et al.
(respiration inhibition (1985)
test; OECD Guideline
209)
Bacteria
Pseudomonas putida pH neutral 16-h MICa 480 Bringmann &
(cell multiplication Kuehn (1977)
inhibition test)
(static)
Photobacterium - 30-min EC50 16.85 Kaiser et al.
phosphoreum (1987)
(Microtox test:
bioluminescence
reduction)
Cyanobacteria
Anabaena inaequalis Stratton &
(cell multiplication - 14-day EC50 9 Corke (1982)
inhibition test)
(static)
(photosynthesis - 3-h EC50 5
reduction)
Algae
Scenedesmus quadricauda
(cell multiplication pH neutral 8-day MIC 1630 Bringmann &
inhibition test) Kuehn (1977)
(static)
(photosynthesis
reduction) - 3-h EC50 75 Stratton &
Corke (1982)
Table 7 (cont'd)
Most sensitive species Special Effective Reference
(test method/end-point) features concentration
(mg/litre)
Chlorella pyrenoides
(photosynthesis
reduction) - 3-h EC50 60 Stratton &
Corke (1982)
Protozoa
Uronema parduczi
(cell multiplication pH 6.9 20-h MIC 31 Bringmann &
inhibition test) Kuehn (1980)
Tetrahymena pyriformis
(cell multiplication - 2-day EC50 252 Schultz et al.
inhibition test) (1996)
Invertebrata: Mollusca
Teredo digensis larvae 72-h LC50 100 Vind &
(marine) (static) Hochman
(1960)
Invertebrata: Crustacea
Daphnia magna pH neutral 24-h EC50 500 Bringmann &
(immobilization) Kuehn (1982)
pH acid 24-h EC50 102
Vertebrata: Fish
Leuciscus idus
(lethality, DEV L15) pH 7-8 48-h LC50 460 Juhnke &
Luedemann
(1978)
Table 7 (cont'd)
Most sensitive species Special Effective Reference
(test method/end-point) features concentration
(mg/litre)
Vertebrata: Amphibia
Xenopus laevis
(lethality) embryos Dawson et al.
(malformation) pH 7.2-7.4 96-h LC50 1291 (1996)
96-h EC50 433
a MIC = minimum inhibitory concentration.
10.2 Terrestrial environment
It is undissociated benzoic acid that is responsible for its
antimicrobial activity. As benzoic acid itself is only slightly
soluble in water, sodium benzoate -- which, under acidic conditions,
converts to undissociated benzoic acid -- is often used instead. Their
antimicrobial properties are used for different applications, such as
food preservation (Chipley, 1983; see section 4), optimally under
acidic conditions.
Minimum microbiocidal concentrations ranged from 20 to 1200 mg
benzoic acid/litre in suspension tests (pH 6) with different bacterial
or fungal species (Wallhäusser, 1984; Russell & Furr, 1996). Minimum
inhibitory concentrations (serial dilution technique) were in the
range of 50-1000 mg/litre (Wallhäusser, 1984; Russell & Furr, 1996).
The pH dependence of benzoic acid's antimicrobial activity is
shown in several studies. Growth inhibition of the fungus Fusarium
oxysporum (related to dry weight) measured 5 days after incubation
with 610 mg benzoic acid/litre was 23.7% at pH 7.2 and 83.5% at pH 4
(Soni & Bhatia, 1980). There was no visible growth of yeast
(Saccharomyces cerevisiae, Willia anomala) or mould (Penicillium
glaucum) fungi at sodium benzoate concentrations of 120-600 mg/litre
at pH 2.6, 1000-4000 mg/litre at pH 5, or 20 000-60 000 mg/litre at pH
7 (Schelhorn, 1951). Minimum inhibitory concentrations preventing
growth of Talaromyces flavus on agar plates after 35 days of
incubation were 100 mg/litre at pH 3.5 and >600 mg/litre at pH 5.4
(King & Halbrook, 1987).
The minimum inhibitory concentrations of benzoic acid on the
growth of several species of yeasts ranged from 170 to 1250 mg/litre
in cultures preadapted to benzoic acid and from 100 to 600 mg/litre in
unadapted cultures (pH 3.5; 25°C; 6 weeks of incubation) (Warth,
1988).
No information on the toxic effects of benzoic acid or sodium
benzoate on plants, earthworms, or other terrestrial organisms or on
ecosystems was identified.
11. EFFECTS EVALUATION
11.1 Evaluation of health effects
11.1.1 Hazard identification and dose-response assessment
After oral ingestion of benzoic acid and sodium benzoate in
experimental animals or humans, there is rapid absorption of the
undissociated benzoic acid from the gastrointestinal tract. The
substances are metabolized in the liver mainly by conjugation with
glycine, resulting in the formation of hippuric acid, which is rapidly
excreted via the urine. Benzoates applied dermally can penetrate
through the skin. Owing to their rapid metabolism and excretion, an
accumulation of the benzoates or their metabolites is not to be
expected.
With oral LD50 values of >1940 mg/kg body weight, the acute
toxicity of benzoic acid and sodium benzoate in rodents is low.
Benzoic acid is slightly irritating to the skin and irritating to
the eye, whereas sodium benzoate is not irritating to the skin and is
only a slight eye irritant. Benzoic acid was not skin sensitizing in
several animal models. For sodium benzoate, no data were identified
covering this specific end-point.
Studies concerning short-term, subchronic, or chronic oral
exposure conducted according to current guidelines are not available
for benzoic acid or sodium benzoate. Effects on the central nervous
system, weight gain (in several cases without reduced food intake),
and liver and kidney were recorded at high concentrations of both
compounds. As expected, and as far as it is possible to conclude with
the limited database, toxic effects and effect levels seem to be
similar for both compounds. A preliminary NO(A)EL of about 500 mg/kg
body weight per day (the highest dose tested) may be derived based on
a limited four-generation study (Kieckebusch & Lang, 1960; see section
8.4.2 and Table 4). This is supported by two short-term studies in
which no adverse effects were observed at the highest tested dose
levels of 647-825 mg/kg body weight per day (Kreis et al., 1967;
Bio-Fax, 1973) and by the fact that no serious side-effects have been
reported after therapeutic use of sodium benzoate at a dose level of
250-500 mg/kg body weight per day in humans, although occasionally
anorexia and vomiting were observed.
In a short-term inhalation study with rats exposed to benzoic
acid (0, 25, 250, or 1200 mg dust aerosol/m3; 6 h per day, 5 days
per week, over 4 weeks), indications of fibrosis in the lung were seen
even at the lowest concentration. The number of these microscopic
lesions was higher in treated animals than in controls, but there was
no clear dose dependency for this effect. Therefore, a
no-observed-(adverse-)effect concentration (NO(A)EC) value cannot be
derived. Long-term inhalation studies with benzoic acid or sodium
benzoate were not identified.
Two long-term studies with rats (application of up to 1400 mg/kg
body weight per day via diet over 18-24 months; quality of the study
questionable) or mice (lifelong application of up to 6200 mg/kg body
weight per day via drinking-water) gave no indication of a
carcinogenic effect in either species. Studies on the precursors of
benzoic acid -- benzyl acetate, benzyl alcohol, and benzaldehyde --
support the notion that it is unlikely that benzoic acid is
carcinogenic.
In several in vitro tests on genotoxicity, benzoic acid and
sodium benzoate tested negative. For sodium benzoate, in contrast to
benzoic acid, consistently positive results were obtained in tests on
sister chromatid exchange and chromosome aberrations without metabolic
activation. In vivo studies for benzoic acid were not identified.
For sodium benzoate, negative results were obtained in vivo in a
cytogenetic assay with rats and a host-mediated assay with single or
multiple oral application. However, a dominant lethal assay with rats
gave a positive result. Therefore, a possible genotoxic activity of
sodium benzoate cannot be ruled out entirely at present.
For benzoic acid, two limited studies gave no indication of
adverse reproductive or developmental effects. With sodium benzoate,
several studies on different species have been performed. Embryotoxic
and fetotoxic effects as well as malformations were seen only at doses
that induced severe maternal toxicity. In a dietary study in rats, a
NO(A)EL of about 1310 mg/kg body weight per day was established.
Studies on the precursors of benzoic acid support the notion that
benzoic acid is unlikely to have adverse reproductive effects at dose
levels not toxic to the mother.
The acute toxicity of benzoic acid and sodium benzoate in humans
is low. However, both substances are known to cause contact dermatitis
(pseudoallergy). In patients with urticaria or asthma, an exacerbation
of the symptoms was observed after testing (oral provocation test or
patch tests), whereas this effect is unusual in healthy subjects.
11.1.2 Criteria for setting tolerable intakes or guidance values for
benzoic acid and sodium benzoate
As given in section 11.1.1, the database is insufficient for
deriving NO(A)EL values for oral uptake. If the provisional NO(A)EL of
about 500 mg/kg body weight per day is applied, and by incorporating
an uncertainty factor of 100 (10 for uncertainty of the database, 10
for interspecies variation), a provisional tolerable intake would be
5 mg/kg body weight per day.
Applying this tolerable intake, one has to keep in mind that
benzoates at lower doses can cause non-immunological contact reactions
(pseudoallergy) in sensitive persons.
There are also no studies available concerning longer-term
exposure by inhalation, and the only short-term inhalation toxicity
study is not adequate for confidently establishing a NO(A)EC.
Therefore, a tolerable concentration for exposure by inhalation cannot
be calculated.
11.1.3 Sample risk characterization
As given in section 6.2, workers may be exposed to benzoic acid
or sodium benzoate via inhalation or skin contact during production
and processing. However, owing to the lack of information on specific
working operations and conditions (e.g., duration of exposure)
involved, it is impossible to derive a realistic estimate of
occupational exposure.
For the general population, the main route of exposure to benzoic
acid and sodium benzoate is likely via foodstuffs, which contain the
substances naturally or added as antimicrobial agents. As given in
section 6.2, the uptake depends on the individual's choice of food to
be consumed and the limit values for benzoates in different countries.
Therefore, considerable deviations may occur. Recent intake
estimations from surveys from several countries gave mean values in
the range of 0.18-2.3 mg/kg body weight. Only in high consumers was an
intake of up to 14 mg/kg body weight calculated. Benzoates have not
been detected in drinking-water. As given in section 6.1, the
inhalative uptake via ambient or indoor air may contribute only
marginally to exposure of the general population.
For normal consumers, the uptake of benzoates is about 2-28 times
lower than the provisional tolerable intake of 5 mg/kg body weight
day, and only in high consumers would this value be exceeded 3 times.
Additional information is required in order to evaluate whether
sodium benzoate has a possible genotoxic activity.
11.2 Evaluation of environmental effects
Significant releases of benzoic acid and sodium benzoate into the
environment are primarily into water and soil from their uses as
preservatives in food, mouthwashes, dentifrices, and cosmetics.
Benzoic acid occurs naturally in many plants.
From their physical/chemical properties, benzoic acid and sodium
benzoate are not expected to volatilize from water and soil to the
atmosphere or to adsorb to sediment or soil particles. The main
elimination pathway for both chemicals should be biotic
mineralization. Because of their ready biodegradability and their low
volatility, both substances are not considered to contribute directly
to the depletion of the stratospheric ozone layer or to global
warming. From experimental data on bioconcentration, a low to moderate
potential for bioaccumulation is to be expected.
Benzoic acid and sodium benzoate exhibited low to moderate
toxicity to aquatic organisms. The lowest reported EC50 value of
9 mg/litre was determined in a chronic study (14 days) for cell
multiplication inhibition by benzoic acid in the cyanobacterium
Anabaena inaequalis. EC50/LC50 values for the other aquatic
species tested were in the range of 17-1291 mg/litre. Exposure levels
of benzoic acid and benzoate in water have been determined only in
rain and snow, groundwater, and leachate in the vicinity of point
sources. Thus, a quantitative risk characterization with respect to
aquatic organisms in surface waters could not be performed. Taking
into account the rapid biodegradability, the low to moderate
bioaccumulation potential, the low toxicity to most aquatic species,
and the rapid metabolism of these substances, benzoic acid and sodium
benzoate will -- with the exception of accidental spills -- pose only
a minimal risk to aquatic organisms.
The few available data from the antimicrobial action of benzoic
acid and sodium benzoate indicate only a low toxicity potential of
both substances in the terrestrial compartment. Due to the lack of
measured exposure levels, a sample risk characterization with respect
to terrestrial organisms could not be performed.
12. PREVIOUS EVALUATIONS BY INTERNATIONAL BODIES
JECFA (WHO, 1996) has allocated an acceptable daily intake (ADI)
for benzoic acid and sodium benzoate of 0-5 mg/kg body weight.
REFERENCES
Abe S, Sasaki M (1977) Chromosome aberrations and sister chromatid
exchanges in Chinese hamster cells exposed to various chemicals.
Journal of the National Cancer Institute, 58(6):1635-1641.
Abe S, Tsutsui Y, Tasrumoto Y, Nakane S (1984) Studies on the toxicity
of oxaprozin. 1. Acute toxicity of oxaprozin, its metabolites and
contaminants. Iyakuhin Kenkyu, 15(3):359-370.
Aberer W, Kager B, Ziegler V, Horak F (1992) Schnupfen durch
Schneiderkreide -- Allergie, Pseudoallergie, Rhinopathie oder
Einbildung? Dermatosen, 40(6):231-234.
Aguirre A, Izu R, Gardeazabal J, Diaz-Perez JL (1993) Edematous
allergic contact cheilitis from a toothpaste. Contact dermatitis,
28:42.
Andersen KE, Maibach HI, Anjo MD (1980) The guinea-pig: an animal
model for human skin absorption of hydrocortisone, testosterone and
benzoic acid? British journal of dermatology, 102:447-453.
Anderson JA (1996) Allergic reactions to food. Critical reviews in
food science and nutrition, 36:S19-S38.
AOAC (1990) Official methods of analysis, 15th ed. Arlington, VA,
Association of Official Analytical Chemists, pp. 1142-1145.
Arens M, Gertz C (1990) Bestimmung der Konservierungsstoffe -
Gemeinsschaftsarbeit der DGF, 110. Mitteilung: Deutsche
Einheitsmethoden zur Untersuchung von Fetten, Fettprodukten, Tensiden
und verwandten Stoffen, 83.ü Mitt.: Analyse von fettreichen
Lebensmitteln IV. Fat science and technology, 92(3):107-109.
BAGS (1995) Standards zur Expositionsabschätzung. Bericht des
Ausschusses für Umwelthygiene (Arbeitsgemeinschaft der leitenden
Medizinalbeamtinnen und -beamten der Länder). Hamburg, Behörde für
Arbeit, Gesundheit und Soziales.
Baldwin EA, Nisperos-Carriedo MO, Baker RA (1995) Use of edible
coatings to preserve quality of lightly (and slightly) processed
products. Critical reviews in food science and nutrition,
35(6):509-524.
Barker JF, Barbash JE, Labonte M (1988) Groundwater contamination at a
landfill sited on fractured carbonate and shale. Journal of
contaminant hydrology, 3:1-25.
Batshaw ML, Brusilow SW (1981) Evidence of lack of toxicity of sodium
phenylacetate and sodium benzoate in treating urea cycle
enzymopathies. Journal of inherited metabolic disease, 4:231.
Batshaw ML, Monahan PS (1987) Treatment of urea cycle disorders.
Enzyme, 38:242-250.
Battersby NS, Wilson V (1989) Survey of the anaerobic biodegradation
potential of organic chemicals in digesting sludge. Applied and
environmental microbiology, 55(2):433-439.
Bayer AG (1977) Akute orale Toxizität / Untersuchung zur Haut- und
Schleimhautverträglichkeit. Wuppertal (unpublished report).
Bayer AG (1978) Untersuchung zur Haut- und
Schleimhautverträglichkeit. Wuppertal (unpublished report).
Bayer AG (1986) Benzoesäure DAB 8. Prüfung auf primär reizende/
ätzende Wirkung am Kaninchenauge. Wuppertal (unpublished report).
Bedford PGC, Clarke EGC (1972) Experimental benzoic acid poisoning in
the cat. Veterinary record, 90:53-58.
Bennett MC, Petrus DR (1977) Quantitative determination of sorbic acid
and sodium benzoate in citrus juice. Journal of food science,
42:1220-1221.
BFGoodrich Kalama Inc. (1999) Benzoic acid. Product information
bulletin. Kalama, WA.
Bindslev-Jensen C (1998) ABC of allergies. Food allergy. British
medical journal, 316:1299-1302.
Bio-Fax (1973) Benzoic acid. Northbrook, IL, Industrial Bio-Test
Laboratories, Inc.
Birch RR, Biver C, Campagna R, Gledhill WE, Pagga U, Steber J, Reust
H, Bontinck WJ (1989) Screening of chemicals for anaerobic
biodegradability. Chemosphere, 19(10-11):1527-1550.
BMA (British Medical Association) (1998) Antifungal preparations --
Benzoic acid. Wallingford, Pharmaceutical Press (British National
Formulary 507).
Brasch J, Henseler T, Frosch P (1993) Patch test reactions to a
preliminary preservative series. Dermatosen, 41(2):71-76.
Bridges JW, French MR, Smith RL, Williams RT (1970) The fate of
benzoic acid in various species. Biochemical journal, 118:47-51.
Bringmann G, Kuehn R (1977) [Threshold values for the damaging action
of water pollutants to bacteria (Pseudomonas putida) and green algae
(Scenedesmus quadricauda) in the cell multiplication inhibition
test.] Zeitschrift für Wasser und Abwasser Forschung, 10:87-98 (in
German).
Bringmann G, Kuehn R (1980) Bestimmung der biologischen Schadwirkung
wassergefährdender Stoffe gegen Protozoen. II. Bakterienfressende
Ciliaten. Zeitschrift für Wasser und Abwasser Forschung, 1:26-31.
Bringmann G, Kuehn R (1982) [Results of toxic action of water
pollutants on Daphnia magna Straus tested by an improved
standardized procedure.] Zeitschrift für Wasser und Abwasser
Forschung, 15:1-6 (in German).
Broeckx W, Blondeel A, Do-Go A, Achten G (1987) Cosmetic intolerance.
Contact dermatitis, 16:189-194.
Bronaugh RL, Stewart RF, Congdon ER, Giles AL (1982a) Methods for in
vitro percutaneous absorption studies. I. Comparison with in vivo
results. Toxicology and applied pharmacology, 62:474-480.
Bronaugh RL, Stewart RF, Congdon ER (1982b) Methods for in vitro
percutaneous absorption studies. II. Animal methods for human skin.
Toxicology and applied pharmacology, 62:481-488.
BUA (1995) BUA-Stoffbericht Benzoesaeure, Natriumbenzoat.
Beratergremium für Umweltrelevante Altstoffe. Stuttgart, S. Hirzel
Verlag (Stoffbericht Nr. 145).
Bucks DA, Hinz RS, Sarason R, Maibach HI, Guy RH (1990) In vivo
percutaneous absorption of chemicals: a multiple dose study in rhesus
monkeys. Food and chemical toxicology, 28:129-132.
Budavari S, O'Neil MJ, Smith A, Heckelman PE, Kinneary JF, eds. (1996)
The Merck index -- an encyclopedia of chemicals, drugs, and
biologicals, 12th ed. Whitehouse Station, NJ, Merck & Co., Inc.
p. 183.
Carlberg GE, Drangsholt H, Gjoes N (1986) Identification of
chlorinated compounds in the spent chlorination liquor from
differently treated sulphite pulps with special emphasis on mutagenic
compounds. Science of the total environment, 48:157-167.
Carver MP, Riviere JE (1989) Percutaneous absorption and excretion of
xenobiotics after topical and intravenous administration to pigs.
Fundamental and applied toxicology, 13:714-722.
Castro JC, Delgado MA, Sánchez MJ, Montelongo FG (1992) Simultaneous
2nd order derivative spectrophotometric determination of sorbic and
benzoic acids in soft drinks. Analytical letters, 25(12):2357-2376.
Cautreels W, van Cauwenberghe K (1978) Experiments on the distribution
of organic pollutants between airborne particulate matter and the
corresponding gas phase. Atmospheric environment, 12:1133-1141.
Chipley JR (1983) Sodium benzoate and benzoic acid. In: Branen AL,
Davidson PM, eds. Antimicrobials in foods. New York, NY, M. Decker,
pp. 11-35.
Chittenden RH, Long JH, Herter CA (1909) Chemical bulletin, 88. US
Department of Agriculture [cited in WHO, 1996].
Clemmensen O, Hjorth N (1982) Perioral contact urticaria from sorbic
and benzoic acid in salad dressings. Contact dermatitis, 8:1-6.
Cordt T, Kußmaul H (1990) Niedermolekulare Carbonsäuren imBoden, in
der ungesättigten Zone und im Grundwasser. Vom wasser, 74:287-298.
Courtes R, Bahlaoui A, Rambaud A, Deschamps F, Sunde E, Dutrieux E
(1995) Ready biodegradability test in seawater: a new methodological
approach. Ecotoxicology and environmental safety, 31:142-148.
Coverly J, Peters L, Whittle E, Basketter DA (1998) Susceptibility to
skin stinging, non-immunologic contact urticaria and acute skin
irritation; is there a relationship? Contact dermatitis,
38(2):90-95.
Daston GP, Baines D, Elmore E, Fitzgerald MP, Sharma S (1995)
Evaluation of chick embryo neural retina cell culture as a screen for
developmental toxicants. Fundamental and applied toxicology,
26:203-210.
Dawson DA, Schultz TW, Hunter RS (1996) Developmental toxicity of
carboxylic acids to Xenopus embryos: A quantitative
structure-activity relationship and computer-automated structure
evaluation. Teratogenesis, carcinogenesis, and mutagenesis,
16:109-124.
Deuel HJJ, Alfin-Slater R, Weil CS, Smyth HF Jr (1954) Sorbic acid as
a fungistatic agent for foods. 1. Harmlessness of sorbic acid as a
dietary component. Food research, 19:1-12.
Dierickx PJ (1998) Increased cytotoxic sensitivity of cultured FHM
fish cells by simultaneous treatment with sodium dodecyl sulfate and
buthionine sulfoximine. Chemosphere, 36(6):1263-1274.
EC (1995) European Union Directive 95/2/CE from 20.02.1995 on food
additives, colourants and sweeteners. European Commission.
Elder DJE, Kelly DJ (1994) The bacterial degradation of benzoic acid
and benzenoid compounds under anaerobic conditions: Unifying trends
and new perspectives. FEMS microbiology reviews, 13:441-468.
Ewell WS, Gorsuch JW, Kringle RO, Robillard KA, Spiegel RC (1986)
Simultaneous evaluation of the acute effects of chemicals on seven
aquatic species. Environmental toxicology and chemistry, 5:831-840.
Fanelli GM, Halliday SL (1963) Relative toxicity of chlortetracycline
and sodium benzoate after oral administration to rats. Archives
internationales de pharmacodynamie et de thérapie, 144(1-2):120-125.
Federle TW (1988) Mineralization of monosubstituted aromatic compounds
in unsaturated and saturated subsurface soils. Canadian journal of
microbiology, 34:1037-1042.
Feillet F, Leonard JV (1998) Alternative pathway therapy for urea
cycle disorders. Journal of inherited metabolic disease, Suppl.
21(1):101-111.
Feldmann RJ, Maibach HI (1970) Absorption of some organic compounds
through the skin in man. Journal of investigative dermatology,
54:399-404.
Flood PF, Abrams SR, Muir GD, Rowell JE (1989) Odor of the muskox. A
preliminary investigation. Journal of chemical ecology,
15:2207-2217.
Franz TJ (1975) Percutaneous absorption. On the relevance of in vitro
data. Journal of investigative dermatology, 64:190-195.
Freedman BJ (1977) Asthma induced by sulphur dioxide, benzoate and
tartrazine contained in orange drinks. Clinical allergy, 7:407-415.
Freitag D, Ballhorn L, Geyer H, Korte F (1985) Environmental hazard of
organic chemicals. An experimental method for the assessment of the
behaviour of organic chemicals in the ecosphere by means of simple
laboratory tests with 14C labelled chemicals. Chemosphere,
14:1589-1616.
Friedman LJ, Greenwald CG (1994) Food additives. In: Kroschwitz J,
Howe-Grant M, eds. Kirk-Othmer: Encyclopedia of chemical technology.
Vol. 11. New York, NY, Wiley and Sons, pp. 805-833.
Fuchs G, el Said Mohammed M, Altenschmidt U, Koch J, Lack A, Brackmann
R, Lochmeyer C, Oswald B (1993) Biochemistry of anaerobic
biodegradation of aromatic compounds. In: Ratledge C, ed.
Biochemistry of microbial degradation. Dordrecht, Kluwer Academic
Publishers, pp. 513-553.
Fujii T, Omori T, Tagucji T, Ogata M (1991) Urinary excretion of
hippuric acid after administration of sodium benzoate (biological
monitoring 1). Shokuhin Eiseigaku Zasshi (Journal of the Food Hygiene
Society of Japan), 32(3):177-182.
Fujitani T (1993) Short-term effect of sodium benzoate in F344 rats
and B6C3F1 mice. Toxicology letters, 69:171-179.
Gad SC, Dunn BJ, Dobbs DW, Reilly C, Walsh RD (1986) Development and
validation of an alternative dermal sensitization test: the mouse ear
swelling test (MEST). Toxicology and applied pharmacology,
84:93-114.
Gailhofer G, Soyer HP, Ludvan M (1990) Nahrungsmittelallergien und
Pseudoallergien -- Mechanismen, Klinik und Diagnostik. Wiener
Medizinische Wochenschrift, 140:227-232.
Geiger DL, Northcott CE, Call DJ, Brooke LT (1985) Acute toxicities
of organic chemicals to fathead minnows (Pimephales promelas ).
Vol. II. Superior, WI, University of Wisconsin, pp. 139-140.
Gerberick GF, House RV, Fletcher ER, Ryan CA (1992) Examination of the
local lymph node assay for use in contact sensitization risk
assessment. Fundamental and applied toxicology, 19:438-445.
Gerlach V (1909) VII. Summary of the results. In: Physiological
activity of benzoic acid and sodium benzoate. Wiesbaden, Verlag von
Heinrich Staadt [cited in US FDA, 1972a].
Goerlitz DF, Troutman DE, Godsy EM, Franks BJ (1985) Migration of
wood-preserving chemicals in contaminated groundwater in a sand
aquifer at Pensacola, Florida. Environmental science and technology,
19(10):955-961.
Goodman Gilman A, Rall TW, Nies AS, Taylor P, eds. (1990) Goodman and
Gilman's the pharmacological basis of therapeutics, 8th ed. New York,
NY, Pergamon Press, p. 1179.
Goodwin BL (1976) Handbook of intermediary metabolism of aromatic
compounds. New York, NY, Wiley & Sons, pp. B6-B9.
Green TP, Marchessault RP, Freese DK (1983) Disposition of sodium
benzoate in newborn infants with hyperammonemia. Journal of
pediatrics, 102:785-790.
Guardiola J, Ventura J, Rivera J (1989) Occurrence of industrial
organic pollution in a groundwater supply: screening, monitoring and
evaluation of treatment processes. Water supply, 7:11-16.
Haider K, Jagnow G, Kohnen R, Lim SU (1974) Abbau chlorierter Benzole,
Phenole und Cyclohexan- Derivate durch Benzol und Phenol verwertende
Bakterien unter anaeroben Bedingungen. Archives of microbiology,
96:183-200.
Halvorson DO (1984) Determination of benzoic acid in air. American
Industrial Hygiene Association journal, 45(10):727-730.
Ham RK, Boyle WC, Engroff EC, Fero RL (1989) Determining the presence
of organic compounds in foundry waste leachates. Modern casting,
79:27-31.
Harborne JB (1983) Toxins of plant-fungal interactions. In: Keeler RF,
Tu AT, eds. Handbook of natural toxins. Vol. 1. Plant and fungal
toxins. New York, NY, Marcel Dekker, pp. 755-758.
Hardin BD, Schuler RL, Burg JR, Booth GM, Hazelden KP, MacKenzie KM,
Piccirillo VJ, Smith KN (1987) Evaluation of 60 chemicals in a
preliminary developmental toxicity test. Teratogenesis,
carcinogenesis, and mutagenesis, 7:29-48 [cited in WHO, 1996].
Harwood CS, Gibson J (1997) Shedding light on anaerobic benzene ring
degradation: a process unique to prokaryotes? Journal of
bacteriology, 179:301-309.
Hegnauer R, ed. (1966) Chemotaxonomie der Pflanzen. Basel,
Birkhäuser Verlag.
Hegnauer R, ed. (1989) Chemotaxonomie der Pflanzen. Basel,
Birkhäuser Verlag, pp. 415-416.
Hegnauer R (1992) Benzoesäure. In: Hegnauer R, ed. Chemotaxonomie der
Pflanzen. Basel, Birkhäuser Verlag.
Helmig D, Müller J, Klein W (1989) Volatile organic substances in a
forest atmosphere. Chemosphere, 19(8-9):1399-1412.
Hofrichter M, Fritsche W (1996) Abbau aromatischer Kohlenwasserstoffe
durch den Schimmelpilz Penicillium frequentans Bi 7/2. Gas- und
Wasserfach; Wasser-Abwasser, 137:199-204.
Hotchkiss SA, Hewitt P, Caldwell J, Chen WL, Rowe RR (1992)
Percutaneous absorption of nicotinic acid, phenol, benzoic acid and
triclopyr butoxyethyl ester through rat and human skin in vitro:
Further validation of an in vitro model by comparison with in vivo
data. Food and chemical toxicology, 30:891-899.
Hunziker N, Feldmann RJ, Maibach H (1978) Animal models of
percutaneous penetration: comparison between Mexican hairless dogs and
man. Dermatologica, 156:79-88.
Ibero M, Eseverri JL, Barroso C, Botey J (1982) Dyes, preservatives
and salicylates in the induction of food intolerance and/or
hypersensitivity. Allergologia et Immunopathologia, 10(4):263-268.
IPCS (1993) International Chemical Safety Card -- Benzoic acid.
Geneva, World Health Organization, International Programme on
Chemical Safety (ICSC 0103).
Ishida H (1996) Levels of preservatives in toothpastes and possibility
of their intake during brushing of teeth. Shokuhin Eiseigaku Zasshi
(Journal of the Food Hygiene Society of Japan), 37:234-239.
Ishidate M, Odashima S (1977) Chromosome tests with 134 compounds on
Chinese hamster cells in vitro -- a screening for chemical
carcinogenesis. Mutation research, 48:337-354.
Ishidate MJ, Sofuni T, Yoshikawa K, Hayashi M, Nohmi T, Sawada M,
Matsuoka A (1984) Primary mutagenicity screening of food additives
currently used in Japan. Food and chemical toxicology,
22(8):623-636.
Ishidate MJ, Harnois MC, Sofuni T (1988) A comparative analysis of
data on the clastogenicity of 951 chemical substances tested in
mammalian cell cultures. Mutation research, 195:151-213.
Ishiguro S, Miyamoto A, Obi T, Nishio A (1993) Teratological studies
on benzyl acetate in pregnant rats. Kadnau (Bulletin of the Faculty
of Agriculture, Kagoshima University), 43:25-31 [cited in WHO, 1996].
Ishizaki M, Ueno S (1989) The DNA damaging activity of natural and
synthetic food additives. Shokuhin Eiseigaku Zasshi (Journal of the
Food Hygiene Society of Japan), 30:447-451.
Jalal MAF, Read DJ (1983) The organic acid composition of Calluna
heathland soil with special reference to phyto- and fungitoxicity. I.
Isolation and identification of organic acids. Plant and soil,
70:257-272.
James MO, Pritchard JB (1987) In vivo and in vitro metabolism and
excretion of benzoic acid by a marine teleost, the southern flounder.
Drug metabolism and disposition, 15:665-670.
Jansson T, Curvall M, Hedin A, Enzell CR (1988) In vitro studies of
the biological effects of cigarette smoke condensate. III. Induction
of SCE by some phenolic and related constituents derived from
cigarette smoke. Mutation research, 206:17-24.
Jelinek R, Peterka M, Rychter Z (1985) Chick embryotoxicity screening
test -- 130 substances tested. Indian journal of experimental
biology, 23:588-595.
Juhlin L, Michaelsson G, Zetterström O (1972) Urticaria and asthma
induced by food and drug additives in patients with aspirin
hypersensitivity. Journal of allergy and clinical immunology,
50(2):92-98.
Juhnke I, Luedemann D (1978) Ergebnisse der Untersuchung von
chemischen Verbindungen auf akute Fischtoxizität mit dem
Goldorfentest. Zeitschrift für Wasser und Abwasser Forschung,
11(5):161-164.
Kaiser KLE, Palabrica VS, Ribo JM (1987) QSAR of acute toxicity of
mono-substituted benzene derivatives to Photobacterium phosphoreum.
QSAR environmental toxicology, 11:153-168.
Kameya T, Murayama T, Urano K, Kitano M (1995) Biodegradation ranks of
priority organic compounds under anaerobic conditions. Science of the
total environment, 170(1-2):43-51.
Kawamura K, Kaplan IR (1986) Biogenic and anthropogenic organic
compounds in rain and snow samples collected in Southern California.
Atmospheric environment, 20:115-124.
Kawamura K, Kaplan IR (1990) Stabilities of carboxylic acids and
phenols in Los Angeles rainwaters during storage. Water research,
24(11):1419-1423.
Kawamura K, Ng LL, Kaplan IR (1985) Determination of organic acids
(C1-C10) in the atmosphere, motor exhausts, and engine oils. IPCS
guidelines for the monitoring of genotoxic effects of carcinogens in
humans. Environmental science and technology, 19:1082-1086.
Kay JH, Calandra JC (1962) Interpretation of eye irritation tests.
Journal of the Society of Cosmetic Chemists, 13:281-289.
Kieckebusch W, Lang K (1960) Die Verträglichkeit der Benzoesäure im
chronischen Fütterungsversuch. Arzneimittel-Forschung, 10:1001-1003.
Kimmel CA, Wilson JG, Schumacher HJ (1971) Studies on metabolism and
identification of the causative agent in aspirin teratogenesis in
rats. Teratology, 4:15-24.
King ADJ, Halbrook WU (1987) Ascospore heat resistance and control
measures for Talaromyces flavus isolated from fruit juice
concentrate. Journal of food science, 52:1252-1254.
King EF, Painter HA (1983) Ring-test programme 1981-82. Assessment of
biodegradability of chemicals in water by manometric respirometry.
Luxemburg, Commission of the European Communities (EUR 8631 EN 31 S).
Kinney IC, Ivanuski VR (1969) Photolysis mechanisms for pollution
abatement. Cincinnati, OH, US Department of the Interior, Federal
Water Pollution Control Administration, Robert A. Taft Water Research
Center (TWRC-13).
Klecka GM, Landi LP, Bo KM (1985) Evaluation of the OECD activated
sludge, respiration inhibition test. Chemosphere, 14(9):1239-1251.
Kobayashi T, Hashinaga T, Mikami E, Suzuki T (1989) Methanogenic
degradation of phenol and benzoate in acclimated sludges. Water
science and technology, 21:55-65.
Kocwa-Haluch R, Lemek M (1995) Easy and inexpensive diffusion tests
for detecting the assimilation of aromatic compounds by yeast-like
fungi. Part II. Assimilation of aromatic acids. Chemosphere,
31(11/12):4333-4339.
Koda T, Tsuchiya H, Yamauchi M, Hoshino Y, Takagi N, Kawano J (1989)
High-performance liquid chromatographic estimation of eluates from
denture base polymers. Journal of dentistry, 17:84-89.
Koda T, Tsuchiya H, Yamaguchi M, Ohtani S, Takagi N, Kawano J (1990)
Leachability of denture-base acrylic resins in artificial saliva.
Dental materials, 6:13-16.
Kreis H, Frese F, Wilmes G (1967) Physiologische und morphologische
Veränderungen an Ratten nach peroraler Verabreichung von Benzoesäure.
Food and cosmetics toxicology, 5:505-511.
Kubota K, Ishizaki T (1991) Dose-dependent pharmacokinetics of benzoic
acid following oral administration of sodium benzoate to humans.
European journal of clinical pharmacology,41(4):363-368.
Kubota K, Horai Y, Kushida K, Ishizaki T (1988) Determination of
benzoic acid and hippuric acid in human plasma and urine by high
performance liquid chromatography. Journal of chromatography,
425(1):67-75.
Lahti A, Hannuksela M (1981) Is benzoic acid really harmful in cases
of atopy and urticaria? Lancet, 2:1055.
Lahti A, Maibach HI (1984) An animal model for nonimmunologic contact
urticaria. Toxicology and applied pharmacology, 76:219-224.
Lahti A, Pylvanen V, Hannuksela M (1995) Immediate irritant reactions
of benzoic acid are enhanced in washed skin areas. Contact
dermatitis, 33:177-182.
Larmi E, Lahti A, Hannuksela M (1988) Effects of sorbitan-sesquioleate
on non-immunologic immediate contact reactions to benzoic acid.
Contact dermatitis, 19:368-371.
Larsson B (1983) Gas-liquid chromatographic determination of benzoic
acid and sorbic acid in foods: MNKL collaborative study (1983).
Journal of the Association of Official Analytical Chemists,
66:775-780.
Lindström K, Österberg F (1986) Chlorinated carboxylic acids in
softwood kraft pulp spent bleach liquors. Environmental science and
technology, 20(2):133-138.
Lu PY, Metcalf RL (1975) Environmental fate and biodegradability of
benzene derivatives as studied in a model aquatic ecosystem.
Environmental health perspectives, 10:269-284.
Lund FA, Rodriguez DS (1984) Acclimation of activated sludge to
mono-substituted derivatives of phenol and benzoic acid. Journal of
general applied microbiology, 30:53-61.
Lunde G, Gehter J, Gjos N, Lande MBS (1977) Organic micropollutants in
precipitation in Norway. Atmospheric environment, 11:1007-1014.
MacPherson SE, Barton CN, Bronaugh RL (1996) Use of in vitro skin
penetration data and a physiologically based model to predict in vivo
blood levels of benzoic acid. Toxicology and applied pharmacology,
140:436-443.
Maibach HI, Johnson HL (1975) Contact urticaria syndrome. Archives of
dermatology, 111:726-730.
Maibach HI, Wester RC (1989) Percutaneous absorption: in vivo
methods in humans and animals. Journal of the American College of
Toxicology, 8:803-813.
Mailhot H (1987) Prediction of algal bioaccumulation and uptake rate
of nine organic compounds by ten physicochemical properties.
Environmental science and technology, 21:1009-1013.
Maki T, Suzuki Y (1985) Benzoic acid and derivatives. In: Ullmann's
encyclopedia of industrial chemistry. Vol. A3. Weinheim, VCH
Verlagsgesellschaft mbH, pp. 555-568.
Mandrou B, Nolleau V, Gastaldi E, Fabre H (1998) Solid-phase
extraction as a clean-up procedure for the liquid chromatographic
determination of benzoic acid and sorbic acid in fruit-derived
products. Journal of liquid chromatography and related technology,
21:829-842.
Marquardt P (1960) Zur Verträglichkeit der Benzoesäure.
Arzneimittel-Forschung, 10:1033.
Matthews RW (1990) Purification of water with near-U.V. illuminated
suspensions of titanium dioxide. Water research, 24:653-660.
McCann J, Choi E, Yamasaki E, Ames BN (1975) Detection of carcinogens
as mutagens in the Salmonella/microsome test: assay of
300 chemicals. Proceedings of the National Academy of Sciences
of the United States of America, 72(12):5135-5139.
McCormick GC (1974) A comparison of the acute toxicity, distribution,
fate, and some pharmacologic properties of the non-benzenoid aromatic
compound azuloic acid with those of benzoic and naphthoic acids in
mice. Dissertation abstracts international, B35(10):5029B-5030B.
McKenna KE, Walsh MY, Burrows D (1994) The Melkersson-Rosenthal
syndrome and food additive hypersensitivity. British journal of
dermatology, 131:921-922.
Miguez CB, Greer CW, Ingram JM, MacLeod RA (1995) Uptake of benzoic
acid and chloro-substituted benzoic acids by Alcaligenes
denitrificans BRI 3010 and BRI 6011. Applied and environmental
microbiology, 61:4152-4159.
Minor JL, Becker BA (1971) A comparison of the teratogenic properties
of sodium salicylate, sodium benzoate, and phenol. Toxicology and
applied pharmacology, 19:373.
MITI (1992) Biodegradation and bioaccumulation. Data of existing
chemicals based on the CSCL Japan. Tokyo, Ministry of International
Trade and Industry, Chemicals Inspection & Testing Institute.
Moneret-Vautrin DA, Moeller R, Malingrey L, Laxenaire MC (1982)
Anaphylactoid reaction to general anaesthesia: A case of intolerance
to sodium benzoate. Anaesthesia and intensive care, 10:156-157.
Monsanto Co. (1983) Primary eye irritation of benzoic acid to
rabbits. St. Louis, MO, Monsanto Company, Environmental Health
Laboratory.
Munoz FJ, Bellido J, Moyano JC, Alvarez M, Fonseca JL (1996) Perioral
contact urticaria from sodium benzoate in a toothpaste. Contact
dermatitis, 35:51.
Nakamura SI, Oda Y, Shimada T, Oki I, Sugimoto K (1987) SOS-inducing
activity of chemical carcinogens and mutagens in Salmonella
typhimurium TA1535/pSK1002: examination with 151 chemicals. Mutation
research, 192:239-246.
Nonaka M (1989) DNA repair tests on food additives. Environmental and
molecular mutagenesis, 14 (Suppl. 15):143.
Nottingham PM, Hungate RE (1969) Methanogenic fermentation of
benzoate. Journal of bacteriology, 98:1170-1172.
O'Connor JE, Costell M, Grisolia S (1987) The potentiation of ammonia
toxicity by sodium benzoate is prevented by L-carnithine. Biochemical
and biophysical research communications, 145:817-824.
Oikawa A, Tohda H, Kanai M, Miwa M, Sugimura T (1980) Inhibitors of
poly(adenosine diphosphate ribose) induced sister chromatid exchanges.
Biochemical and biophysical research communications,
97(4):1311-1316.
Onodera H, Ogiu T, Matsuoka C, Furuta K, Takeuchi M, Oono Y, Kubota T,
Miyahara M, Maekawa A, Odashima S (1978) [Studies on effects of sodium
benzoate on fetuses and offspring of Wistar rats.] Eisei Shikensho
Hokoku, 96:47-55 (in Japanese) [cited in WHO, 1996].
Osterballe O, Taudorf E, Haahr J (1979) Intolerance to aspirin,
food-colouring agents and food preservatives in childhood asthma.
Ugeskrift for Laeger, 141:1908-1910.
Oussi D, Mokrini A, Chamarro E, Esplugas S (1998) Photodegradation of
benzoic acid in aqueous solutions. Environmental technology,
19(9):955-960.
Palm WU, Millet M, Zetsch C (1998) OH radical reactivity of pesticides
adsorbed on aerosol materials: first results of experiments with
filter samples. Ecotoxicology and environmental safety, 41(1):36-43.
Parke DV (1968) The biochemistry of foreign compounds. Oxford,
Pergamon Press, pp. 1-261.
Petrus M, Bonaz S, Causse E, Rhabbour M, Moulie N, Netter JC,
Bildstein G (1996) [Asthma induced by benzoate contained in some foods
and anti-allergic drugs.] Archives de pédiatrie, 3(10):984-987 (in
French).
Pitter P (1976) Determination of biological degradability of organic
substances. Water research, 10:231-235.
Prival MJ, Simmon VF, Mortelmans KE (1991) Bacterial mutagenicity
testing of 49 food ingredients gives very few positive results.
Mutation research, 260:321-329.
Puttemans ML, Dryon L, Massart DL (1984) Extraction of organic acids
by iron-pair formation with tri- n-octylamine. Part V. Simultaneous
determination of synthetic dyes, benzoic acid, sorbic acid and
saccharin in soft drinks and lemonade syrups. Journal of the
Association of Official Analytical Chemists, 67:880-885.
Rasmussen LEL, Hess DL, Haight JD (1990) Chemical analysis of temporal
gland secretions collected from an Asian bull elephant during a
four-month musth episode. Journal of chemical ecology,
16(7):2167-2181.
Rastogi SC, Lepoittevin JP, Johansen JD, Frosch PJ, Menné T, Bruze M,
Dreier B, Andersen KE, White IR (1998) Fragrances and other materials
in deodorants: Search for potentially sensitizing molecules using
combined GC-MS and structure activity relationship (SAR) analysis.
Contact dermatitis, 39(6):293-303.
RCC Notox (1988a) Primary skin irritation/corrosion study of benzoic
acid in the rabbit. DD's-Hertogenbosch, RCC Notox B.V. (unpublished
report).
RCC Notox (1988b) Eye irritation/corrosion study of benzoic acid in
the rabbit. DD's-Hertogenbosch, RCC Notox B.V. (unpublished report).
RCC Notox (n.d., a) Primary skin irritation/corrosion study with
natrium benzoate in rabbits. DD's-Hertogenbosch, RCC Notox B.V.
(unpublished report).
RCC Notox (n.d., b) Acute eye irritation/corrosion study with natrium
benzoate in rabbits. DD's-Hertogenbosch, RCC Notox B.V. (unpublished
report).
Reifenrath WG, Chellquist EM, Shipwash EA, Jederberg WW, Krueger GG
(1984) Percutaneous penetration in the hairless dog, weanling pig and
grafted athymic nude mouse: Evaluation of models for predicting skin
penetration in man. British journal of dermatology,
Suppl. 111(S27):123-135.
Reinhard M, Goodman NL (1984) Occurrence and distribution of organic
chemicals in two landfill leachate plumes. Environmental science and
technology, 18:953-961.
Richterich K, Steber J (1989) Prevention of nitrification-caused
erroneous biodegradability data in the closed bottle test.
Chemosphere, 19(10-11):1643-1654.
Ring J (1989) Arzneimittelunverträglichkeit durch pseudo-allergische
Reaktionen. Wiener Medizinische Wochenschrift, 139:130-134.
Rubin HE, Subba-Rao RV, Alexander M (1982) Rates of mineralization of
trace concentrations of aromatic compounds in lake water and sewage
samples. Applied and environmental microbiology, 43(5):1133-1138.
Russell AD, Furr JR (1996) Biocides mechanisms of antifungal action
and fungal resistance. Science progress, 79(1):27-48.
Sainio E, Kanerva L (1995) Contact allergens in toothpastes and a
review of their hypersensitivity. Contact dermatitis, 33:100-105.
Sakuma H, Kusama M, Munakata S, Ohsumi T, Sugawara S (1983) The
distribution of cigarette smoke components between mainstream and
sidestream smoke. Beitraege zur Tabakforschung, 12:63-71.
Salanitro JP, Langston GC, Dorn PB, Kravetz L (1988) Activated sludge
treatment of ethoxylate surfactants at high industrial use
concentrations. Water science and technology, 20(11-12):125-130.
Schelhorn M (1951) Untersuchungen über Konservierungsmittel. VI.
Wirksamkeit und Anwendungsbereich von Benzoesäure, Estern der
Paraoxybenzoesäure und Kombination dieser beiden Arten von
Verbindungen. Deutsche Lebensmittel-Rundschau, 47:128-134.
Scholz W, Kortmann W (1991) Paint additives. In: Elvers B, Hawkins S,
Schulz G, eds. Ullmann's encyclopedia of industrial chemistry.
Weinheim, VCH Verlagsgesellschaft, pp. 465-472.
Schou L, Krane JE (1981) Organic micropollutants in a Norwegian
water-course. Science of the total environment, 20:277-286.
Schuetzle D, Cronn D, Crittenden AL (1975) Molecular composition of
secondary aerosol and its possible origin. Environmental science and
technology, 9:838-845.
Schultz TW, Bryant SE, Kissel ST (1996) Toxicological assessment in
Tetrahymena of intermediates in aerobic microbial transformation of
toluene and p-xylene. Bulletin of environmental contamination and
toxicology, 56:129-134.
Sharak Genthner BR, Townsend GT, Blattman BO (1997) Reduction of
3-chlorobenzoate, 3-bromobenzoate, and benzoate to corresponding
alcohols by Desulfomicrobium escambiense, isolated from a
3-chlorobenzoate-dechlorinating coculture. Applied and environmental
microbiology, 63:4698-4703.
Shibamoto T (1986) Photochemistry of a major essential oil
constituent, benzyl benzoate. Developments in food science,
12:745-754.
Shibamoto T, Umano K (1985) Photochemical products of benzyl benzoate:
possible formation of skin allergens. Journal of toxicology,
cutaneous and ocular toxicology, 4(2):97-104.
Shimp RJ, Young RL (1987) Comparison of OECD and radiolabeled
substrate methods for measuring biodegradation in marine environments.
Ecotoxicology and environmental safety, 14:223-230.
Sieber R, Bütikofer U, Bosset JO, Rüegg M (1989) Benzoesäure als
natürlicher Bestandteil von Lebensmitteln- eine Übersicht.
Mitteilungen aus dem Gebiete der Lebensmitteluntersuchung und
Hygiene, 80:345-362.
Sieber R, Bütikofer U, Baumann E, Bosset JO (1990) Über das Vorkommen
der Benzoesäure in Sauermilchprodukten und Käse. Mitteilungen aus dem
Gebiete der Lebensmitteluntersuchung und Hygiene, 84:484-493.
Sioufi A, Pommier F (1980) Gas chromatographic determination of low
concentrations of benzoic acid in human plasma and urine. Journal of
chromatography, biomedical applications, 181:161-168.
Smyth HF, Carpenter CP (1948) Further experience with the range
finding test in the industrial toxicology laboratory. Journal of
industrial hygiene and toxicology, 30:63-68.
Sodemoto Y, Enomoto M (1980) Report of carcinogenesis bioassay of
sodium benzoate in rats: absence of carcinogenicity of sodium benzoate
in rats. Journal of environmental pathology and toxicology, 4:87-95.
Soni GL, Bhatia IS (1980) Toxicity of phenolics and related compounds
to Fusarium oxasporium Schlecht. and effect of pH on their
toxicity. Indian journal of agricultural science, 50(10):772-777.
SRI (1998) 1997/98 directory of chemical producers Europe. Menlo
Park, CA, SRI International (807-808/1613).
Srour R (1989) Benzoic acid. In: Srour R, ed. Aromatic intermediates
and derivatives. Paris, pp. A.IV.1-A.IV.14 (unpublished report)
[cited in BUA, 1995].
Srour R (1998) Benzoic acid and derivatives. In: Srour R, ed.
Aromatic intermediates and derivatives. Paris, pp. A.IV.1- A.IV.17
(unpublished report).
Steeg E, Montag A (1987) Nachweis aromatischer Carbonsäuren in Honig.
Zeitschrift für Lebensmittel-Untersuchung und -Forschung, 184:17-19.
Stolpe NB, McCallister DL, Shea PJ, Lewis DT, Dam R (1993) Mobility of
aniline, benzoic acid, and toluene in four soils and correlation with
soil properties. Environmental pollution, 81:287-295.
Stratton GW, Corke CT (1982) Toxicity of the insecticide permethrin
and some degradation products towards algave and cyanobacteria.
Environmental pollution, 29:71-80.
Stuermer DH, Ng DJ, Morris CJ (1982) Organic contaminants in
groundwater near underground coal gasification site in northeastern
Wyoming. Environmental science and technology, 16:582-587.
Suflita JM, Concannon F (1995) Screening tests for assessing the
anaerobic biodegradation of pollutant chemicals in subsurface
environments. Journal of microbiological methods, 21:267-281.
Takemoto S, Kuge Y, Nakamoto M (1981) The measurement of BOD in sea
water. Japanese journal of water pollution research, 4:80-90.
Tohda H, Horaguchi K, Takahashi K, Oikawa A, Matsushima T (1980)
Epstein-Barr virus-transformed human lymphoblastoid cells for study of
sister chromatid exchange and their evaluation as a test system.
Cancer research, 40:4775-4780.
Tong HY, Shore DL, Karasek FW, Helland P, Jellum E (1984)
Identification of organic compounds obtained from incineration of
municipal waste by high performance liquid chromatographic
fractionation and gas chromatography-mass spectrometry. Journal of
chromatography, 285:423-441.
Toth B (1984) Lack of tumorigenicity of sodium benzoate in mice.
Fundamental and applied toxicology, 4:494-496.
Toyoda M, Ito Y, Isshiki K, Onishi K, Kato T, Kamikura M, Shiroishi Y,
Harada Y, Hukasawa Y, Yokoyama T, Yoneda M, Iwaida M (1983a) Daily
intake of preservatives, benzoic acid, dehydroacetic acid, propionic
acid and their salts, and esters of p-hydrobenzoic acid in Japan.
Journal of the Japanese Society of Nutrition and Food Science,
96:467-480.
Toyoda M, Ito Y, Isshiki K, Onishi K, Kato T, Kamikura M, Shiroishi Y,
Harada Y, Hukasawa Y, Yokoyama Y, Yamamoto Y, Fujii M, Iwaida M
(1983b) Estimation of daily intake of many kinds of food additives
according to the market basket studies in Japan. Journal of the
Japanese Society of Nutrition and Food Science, 36:489-497.
Tremblay GC, Qureshi IA (1993) The biochemistry and toxicology of
benzoic acid metabolism and its relationship to the elimination of
waste nitrogen. Pharmacology and therapeutics, 60:63-90.
Tyler TA (1984) Liquid chromatographic determination of sodium
saccharin, caffeine, aspartame, and sodium benzoate in cola beverages.
Journal of the Association of Official Analytical Chemists,
67(4):745-747.
UK MAFF (1995) Survey of sulphur dioxide and benzoic acid in foods
and drinks. London, Ministry of Agriculture, Fisheries and Food,
Joint Food Safety and Standards Group (Food Surveillance Information
Sheet No. 65; http://maff.gov.uk/food/infsheet/1995/
no65/65sulben.htm).
Urano K, Kato Z (1986) Evaluation of biodegradation ranks of priority
organic compounds. Journal of hazardous materials,13:147-159.
US FDA (1972a) GRAS (Generally Recognized As Safe) food ingredients:
benzoic acid and sodium benzoate. Washington, DC, US Food and Drug
Administration.
US FDA (1972b) Teratologic evaluation of FDA 71-37 (sodium benzoate).
East Orange, NJ, US Food and Drug Administration, Food and Drug
Research Laboratory.
US FDA (1973) Evaluation of the health aspects of benzoic acid and
sodium benzoate as food ingredients. Bethesda, MD, US Food and Drug
Administration, Life Sciences Research Office (PB-223 837).
US FDA (1974) Mutagenic evaluation of compound FDA 71-37, sodium
benzoate. Prepared by Litton Bionetics, Inc., Kensington, MD, for
the US Food and Drug Administration (Unpublished report PB-245-453/6).
US NTP (1986) Toxicology and carcinogenesis studies of benzyl acetate
(CAS No.
3. ANALYTICAL METHODS
Analytical methods for the determination of benzoic acid include
spectrophotometric methods, which need extensive extraction procedures
and are not very specific; gas chromatographic (GC) methods, which are
more sensitive and specific but need lengthy sample preparation and
derivatization prior to determination; and high-performance liquid
chromatography (HPLC), which has a high specificity and minimum sample
preparation and does not require derivatization.
A direct determination of benzoic acid in air by flash desorption
at 240°C with helium into capillary-GC gave a detection limit of
0.1 ppm (0.5 mg/m3) in a 20-litre sample (=10 µg benzoic acid).
This method has been developed and used for monitoring occupational
exposure (Halvorson, 1984).
A method for the determination of benzoic acid in solid food at
0.5-2 g/kg levels involves extraction with ether into aqueous sodium
hydroxide and methylene chloride, conversion to trimethylsilyl esters,
and detection by GC and flame ionization (Larsson, 1983; AOAC, 1990).
For margarine, a method using HPLC and ultraviolet (UV) detection has
been described with prior extraction with ammonium acetate/acetic
acid/methanol (Arens & Gertz, 1990).
When benzoic acid is used as a preservative in soft drinks and
fruit drinks, other additives, colouring agents, and other acids
(e.g., sorbate) may interfere with its analysis. Liquid
chromatographic methods were developed to overcome this (e.g., Bennett
& Petrus, 1977; Puttemans et al., 1984; Tyler, 1984). For the
sensitive determination of benzoic acid in fruit-derived products, a
clean-up pretreatment with solid-phase extraction followed by liquid
chromatography with UV absorbance detection is described (Mandrou et
al., 1998). The detection limit is 0.6 mg/kg, with a range of
quantification of 2-5 mg/kg. For soft drinks, a simultaneous
second-order derivative spectrophotometric determination has been
developed (detection limit 1 mg/litre) (Castro et al., 1992). Sodium
benzoate was measured in soya sauce, fruit juice, and soft drinks
using HPLC with a UV spectrophotometric detector. Before injection,
all samples were filtered (Villanueva et al., 1994).
GC determination of low concentrations (down to 10 ng/ml) of
benzoic acid in plasma and urine was preceded by diethyl ether
extraction and derivatization with pentafluorobenzyl bromide (Sioufi &
Pommier, 1980). Detection was by 63Ni electron capture. HPLC methods
have been developed for the simultaneous determination of benzoic acid
and hippuric acid -- the metabolite of sodium benzoate that is
eliminated in the urine -- that require no extraction step (detection
limit for both, 1 µg/ml; Kubota et al., 1988). Hippuric acid and
creatinine levels have been determined simultaneously by HPLC, and
measured hippuric acid levels corrected for urinary creatinine
excretion (Villanueva et al., 1994).
4. SOURCES OF HUMAN AND ENVIRONMENTAL EXPOSURE
4.1 Natural sources of benzoic acid
Benzoic acid is produced by many plants as an intermediate in the
formation of other compounds (Goodwin, 1976). High concentrations are
found in certain berries (see section 6.1). Benzoic acid has also been
detected in animals (see section 6.1). Benzoic acid therefore occurs
naturally in many foods, including milk products (Sieber et al., 1989,
1990).
4.2 Anthropogenic sources
4.2.1 Benzoic acid
Benzoic acid is produced exclusively by the liquid-phase
oxidation of toluene (Srour, 1998).
According to Srour (1998), the estimated global production
capacity of benzoic acid is 638 000 tonnes per year, although over
half of this is converted directly to phenol. The major producers of
benzoic acid are the Netherlands (220 000 tonnes per year) and Japan
(140 000 tonnes per year), followed by the USA (125 000 tonnes per
year). Another reference gives the total European capacity as less
than 153 000 tonnes (SRI, 1998).
Benzoic acid is detected in car exhaust gases, presumably as an
oxidation product of toluene (Kawamura et al., 1985), and in Japanese
cigarettes (12 and 28 µg per cigarette in mainstream and sidestream
smoke, respectively; Sakuma et al., 1983). It can also be produced
through the photochemical degradation of benzoic acid esters used as
fragrance ingredients (Shibamoto & Umano, 1985; Shibamoto, 1986).
Benzoic acid has been detected in wastewater from the wood production
industry in Norway and Sweden (Carlberg et al., 1986; Lindström &
Österberg, 1986) and in foundry waste leachates (Ham et al., 1989), as
well as in extracts of fly ash from municipal incinerators
(Tong et al., 1984).
4.2.2 Sodium benzoate
Sodium benzoate is produced by the neutralization of benzoic acid
with sodium hydroxide. Worldwide sodium benzoate production in 1997
can be estimated at about 55 000-60 000 tonnes (Srour, 1998). The
largest producers are the Netherlands, Estonia, the USA, and China.
4.3 Uses
4.3.1 Benzoic acid
In 1988, of the benzoic acid produced in Europe, about 60% was
further processed to phenol and 30% to caprolactam (for nylon fibres).
Five per cent was used for the production of sodium and other
benzoates, 3% for benzoyl chloride, and the rest for alkyd resins,
benzoate esters, such as methyl benzoate, and various other products
(Srour, 1989). These percentages are still approximately correct today
(Srour, 1998). Caprolactam seems to be produced only by European
companies (Srour, 1998).
Benzoic acid is increasingly used in the production of diethylene
and dipropylene glycol dibenzoate plasticizers in adhesive
formulations (about 40 000 tonnes in 1997). It is also used to improve
the properties of alkyd resins for paints and coatings and as a "down
hole" drilling mud additive in secondary oil production. Its use as a
rubber polymerization retarder is diminishing (Srour, 1998).
Benzoic acid and sodium benzoate (see section 4.3.2) are used as
preservatives in beverages, fruit products, chemically leavened baked
goods, and condiments, preferably in a pH range below 4.5.
A disadvantage is the off-flavour they may impart to foods (Chipley,
1983). Owing to their inhibitory effect on yeast, they cannot be used
in yeast-leavened products (Friedman & Greenwald, 1994). Examples of
upper concentrations allowed in food are up to 0.1% benzoic acid (USA)
and between 0.15% and 0.25% (other countries) (Chipley, 1983). The
European Commission limits for benzoic acid and sodium benzoate are
0.015-0.5% (EC, 1995).
Benzoic acid and its salts and esters are found in 11 of 48 (23%)
toothpastes (Sainio & Kanerva, 1995) to a maximum of 0.5% (Ishida,
1996) and in mouthwashes and dentifrices. Benzoic acid is also used in
cosmetics (in creams and lotions with pH values under 4, up to 0.5%)
(Wallhäusser, 1984). Sixteen out of 71 deodorants tested contained
benzoic acid (Rastogi et al., 1998).
Benzoic acid is a breakdown product of benzoyl peroxide, which is
used as an additive at levels of between 0.015% and 0.075% to bleach
flour (Friedman & Greenwald, 1994) and in dermatological antifungal
preparations (BMA, 1998). Benzoic acid is reported to leach from
denture-base acrylic resins, where benzoyl peroxide is added as a
polymerization initiator (Koda et al., 1989, 1990).
Benzoic acid can be used in combination with salicylic acid
(Whitfield's ointment) as a fungicidal treatment for ringworm (BMA,
1998).
4.3.2 Sodium benzoate
Although undissociated benzoic acid is the more effective
antimicrobial agent for preservation purposes, sodium benzoate is used
preferably, as it is about 200 times more soluble than benzoic acid.
About 0.1% is usually sufficient to preserve a product that has been
properly prepared and adjusted to pH 4.5 or below (Chipley, 1983).
A major market for sodium benzoate is as a preservative in the
soft drink industry, as a result of the demand for high-fructose corn
syrup in carbonated beverages. Sodium benzoate is also widely used as
a preservative in pickles, sauces, and fruit juices (Srour, 1998).
Benzoic acid and sodium benzoate are used as antimicrobial agents in
edible coatings (Baldwin et al., 1995).
Sodium benzoate is also used in pharmaceuticals for preservation
purposes (up to 1.0% in liquid medicines) and for therapeutic regimens
in the treatment of patients with urea cycle enzymopathies
(see section 9).
Possibly the largest use of sodium benzoate, accounting for
30-35% of the total demand (about 15 000 tonnes of benzoic acid), is
as an anticorrosive, particularly as an additive to automotive engine
antifreeze coolants and in other waterborne systems (Scholz &
Kortmann, 1991; Srour, 1998). A new use is the formulation of sodium
benzoate into plastics such as polypropylene, to improve strength and
clarity (BFGoodrich Kalama Inc., 1999). Sodium benzoate is used as a
stabilizer in photographic baths/processing (BUA, 1995).
4.4 Estimated global release
From data provided by the German producers, emissions of benzoic
acid from industrial processes were less than 525 kg per year into the
atmosphere, less than 3 tonnes per year into the River Rhine, and
8 tonnes per year into sewage or water purification plants (BUA,
1995). No data were available from other countries.
Other anthropogenic releases of benzoic acid and sodium benzoate
into the environment are emissions into water and soil from their uses
as preservatives in food, toothpastes, mouthwashes, dentifrices, and
cosmetics. There were no data available on the emission of benzoic
acid from the disposal of antifreeze mixtures and waterborne cooling
systems and other miscellaneous industrial uses.
The amount of benzoic acid emitted to air from car exhaust gases
as an oxidation product is not quantifiable from the available data.
5. ENVIRONMENTAL TRANSPORT, DISTRIBUTION, TRANSFORMATION,
AND ACCUMULATION
5.1 Transport and distribution between media
5.1.1 Benzoic acid
From its use pattern (see section 4), it can be expected that
benzoic acid is released to surface waters and (from dumping sites) to
leaching water (and groundwater). Minor amounts are expected to be
emitted to the atmosphere. From its physicochemical properties (vapour
pressure, Henry's law constant; see section 2), a significant
volatilization of benzoic acid from water or soil is not expected.
Owing to its solubility in water (see section 2), wet deposition from
air may occur. Experimental data on wet and dry deposition from air
are not available.
5.1.2 Sodium benzoate
No information on the environmental transport and distribution of
sodium benzoate could be identified. Owing to its use pattern, which
is similar to that of benzoic acid, most of the amounts released to
the environment are also expected to be emitted to aquatic
compartments (e.g., surface waters).
5.2 Transformation
5.2.1 Benzoic acid
The experimental determination of the photodegradation of benzoic
acid in aqueous solution (25°C; lambda = 240-300 nm) in terms of
quantum yield (average number of photons absorbed) resulted in very
low values -- in the order of 6 × 10-2 mol/einstein1 (Oussi et
al., 1998). However, benzoic acid adsorbed on silica gel (SiO2) and
irradiated with UV light (lambda > 290 nm) for 17 h showed 10.2%
photodegradation (Freitag et al., 1985). This may be due to a
photocatalytic effect, which was also observed with other oxides,
notably zinc oxide (ZnO) and titanium dioxide (TiO2). When benzoic
acid was irradiated with sunlight in aqueous suspensions of zinc or
titanium dioxide, 67% (after 2-3 h) or 90% (after 24 h) of the applied
amount was mineralized (Kinney & Ivanuski, 1969; Matthews, 1990).
1 An einstein is a unit of light energy used in photochemistry,
equal to Avogadro's number times the energy of one photon of light
of the frequency in question.
Indirect photolysis by reaction with hydroxyl radicals is
expected to be low. Hydroxyl radical rate constants (kOH) for
benzoic acid and its anion have been estimated to be approximately
0.5 × 10-12 and 2 × 10-12 cm3/s, respectively (Palm et al., 1998).
Standardized tests on ready (MITI, 1992) or inherent (Zahn &
Wellens, 1980) biodegradation showed benzoic acid to be readily
biodegraded. The degrees of aerobic degradation were as follows:
MITI I 85% (100 mg/litre; (MITI, 1992)
test 2 weeks; OECD
No. 301C)
Zahn-Wellens >90% (508 mg/litre; (Zahn & Wellens,
test 2 days) 1980)
Easy degradation of benzoic acid to methane and carbon dioxide
was also observed in different non-standardized experiments using
sewage sludge as inoculum (BUA, 1995). Benzoic acid was found to be
degraded by adapted anaerobic sewage sludge at 86-93% after 14 days
(Nottingham & Hungate, 1969), by aerobic activated sludge (adapted) at
>95% after 5-20 days (Pitter, 1976; Lund & Rodriguez, 1984), and by
unadapted aerobic activated sludge at 61-69% after 2-3 days with a
preceding lag time of 2-20 h (Urano & Kato, 1986). The use of a
synthetic sewage inoculated with laboratory bacterial cultures led to
complete degradation of benzoic acid after 14 days under anaerobic
conditions (Kameya et al., 1995).
A greater variability in degradation (0-100%) was seen in tests
using environmental matrices (e.g., rain, lake water, seawater, soil,
etc.). It depended mainly on substance concentration and time for
acclimation (see Table 1). Test durations exceeding 2 days resulted in
removal of >40% when initial concentrations were below 20 mg/litre.
A rapid mineralization occurred in groundwater and subsurface soil
samples. In groundwater, a half-life of 41 h has been found for
benzoic acid (initial concentration 1-100 µg/litre; metabolized to
14CO2) under aerobic conditions (Ventullo & Larson, 1985).
Half-lives of 7.3 h and 18.2 h, respectively, have been observed for
aerobic and anaerobic degradation of benzoic acid (initial
concentration 1 mg/kg dry weight; metabolized to 14CO2) in
subsurface soils of septic tank tile fields (Ward, 1985). Anaerobic
degradation of benzoic acid (initial concentration 250 mg
carbon/litre) in a methanogenic microcosm (consisting of aquifer
solids and groundwater) required 4 weeks of adaptation, followed by
nearly complete depletion after 8 weeks of incubation (Suflita &
Concannon, 1995).
Several isolated microorganisms have been shown to utilize (and
therefore probably degrade) benzoic acid under aerobic or anaerobic
conditions. They include, among others, fungal species such as
Rhodotorula glutinis and other yeast-like fungi (Kocwa-Haluch &
Lemek, 1995), the mould Penicillium frequentans (Hofrichter &
Fritsche, 1996), and bacteria, such as Alcaligenes denitrificans
(Miguez et al., 1995), Rhodopseudomonas palustris, several strains
of denitrifying pseudomonads (Fuchs et al., 1993; Elder & Kelly, 1994;
Harwood & Gibson, 1997), and Desulfomicrobium escambiense
(Sharak Genthner et al., 1997).
Although benzoic acid is primarily metabolized to hippuric acid
in rats (see section 7), some other species do excrete other
metabolites, such as dibenzoylornithine (hen), benzoylglutamic acid
(Indian fruit bat), benzoylarginine (tick, insects), or benzoyltaurine
(southern flounder, Paralichthys lethostigma) (Parke, 1968; Goodwin,
1976; James & Pritchard, 1987).
5.2.2 Sodium benzoate
Experimental data on photodegradation of sodium benzoate are not
available. As with benzoic acid, photolysis in aqueous solution is
assumed to be unlikely with regard to its known UV spectra
(Palm et al., 1998). Indirect photolysis by reaction with hydroxyl
radicals plays only a minor role, with estimated and measured hydroxyl
rate constants of about 0.33 × 10-11 cm3/s
(Palm et al., 1998).
Sodium benzoate was readily biodegradable under aerobic
conditions in several standard test systems:
Modified 84% (100 mg/litre; (King & Painter,
MITI test 10 days) 1983)
Modified 80-90% (50 mg/litre; (Salanitro et al.,
Sturm test 7 days) 1988)
Closed bottle 75-111% (5 mg/litre; (Richterich &
test 30 days) Steber, 1989)
Degradation assays using seawater as test medium ("natural
water") or as inoculum (marine filter material given into a synthetic
marine medium) according to an adapted Organisation for Economic
Co-operation and Development (OECD) guideline (301B) resulted in a
degradation of 85% and 97%, respectively (10 mg/litre; carbon dioxide
measurement; 28 days) (Courtes et al., 1995).
Anaerobic mineralization of sodium benzoate (50-90 mg/litre) by
domestic sewage sludge varied from 50% to 96.5% (measurement of carbon
dioxide and methane; 28-61 days) (Birch et al., 1989). In another
study using anaerobic sludge from sewage works receiving a mixture of
domestic and industrial wastewaters, 93% mineralization was observed
after 1 week of incubation (measurement of carbon dioxide and methane;
initial concentration 50 mg carbon/litre) (Battersby & Wilson, 1989).
Table 1: Removal of benzoic acid in freshwater, marine, and soil matrices.
Matrix Initial Conditions Duration Removal Measured Reference
concentration (days) (%) parameter
(mg/litre
or mg/kg)
Rainwater 0.001 22°C; shaking 2 0 benzoic acid Kawamura &
once per day; dark 7 40 Kaplan (1990)
45 100
Lake water 0.059 29°C; 7 98.7 14C (in CO2, Rubin et al.
(eutrophic/ no shaking; dark biomass) (1982)
mesotrophic)
Seawater 20°C; dark; 14C (in Shimp &
(estuary) rotary shaking CO2, biomass) Young (1987)
USA 20 30 <10
0.005 8 70-80
Canada 20 16 60
0.005 10 >70
Seawater 2 5 75 BODa Takemoto
et al. (1981)
Soil 20 2 mg benzoic acid 70 63 14CO2 Haider et al.
(grey soil, in 0.1 ml acetone (1974)
alkaline) + 100 g soil
+ 10 ml H2O
Soil 0.05 24°C; 20-25% 15 40 14CO2 Federle (1988)
(sand; moisture content
18.9 m depth)
a BOD = biological oxygen demand.
Benzoate-acclimated sludges were reported to be capable of completely
degrading benzoate concentrations of 3000 mg/litre within 5-7 days
(Kobayashi et al., 1989).
5.3 Accumulation
5.3.1 Benzoic acid
The n-octanol/water partition coefficient (log Kow) of 1.9
(see section 2) indicates a low potential for bioaccumulation.
Consistently, measured bioconcentration factors (BCFs) found in
aquatic biota were low. BCFs of <10 (based on wet weight) have been
determined for fish (golden ide, Leuciscus idus melanotus) and green
algae (Chlorella fusca) after 3 and 1 days, respectively
(Freitag et al., 1985). A 6-day BCF of 7.6 has been reported for
another green alga (Selenastrum capricornutum) (Mailhot, 1987),
and a 5-day BCF of 1300 (based on dry weight) in activated sludge
(Freitag et al., 1985). The following 24-h bioaccumulation factors
(focusing on uptake via medium plus feed within food chain members)
have been obtained in aquatic model ecosystems operated with
0.01-0.1 mg of radiolabelled benzoic acid per litre: 21 (mosquitofish,
Gambusia affinis), 102 (green alga, Oedogonium cardiacum),
138 (mosquito larvae, Culex quinquifasciatus), 1772 (water flea,
Daphnia magna), and 2786 (snail, Physa sp.). Except for
Daphnia and snail, the values were low (Lu & Metcalf, 1975).
However, the very low exposure concentrations could likely have
resulted in the calculation of the high BCF values, even with moderate
uptake. Moreover, because this was a radiolabel study, it remains
unclear if the label was still the parent compound.
Geoaccumulation of benzoic acid has also been found to be low.
Depending on soil depth, sorption coefficients (Kd) of 0.62 (18.9
m) to 1.92 (0.4 m) have been measured (Federle, 1988). Mobility
determinations of 14C-labelled benzoic acid in different soils by
means of thin-layer chromatography showed benzoic acid to be
moderately mobile. Its mobility was positively correlated with soil pH
and negatively correlated with aluminium and iron contents and
effective anion exchange capacity (Stolpe et al., 1993).
5.3.2 Sodium benzoate
No experimental data on bioaccumulation or geoaccumulation of
sodium benzoate have been identified. From the information on benzoic
acid, a significant potential for accumulation is not to be expected.
6. ENVIRONMENTAL LEVELS AND HUMAN EXPOSURE
6.1 Environmental levels
Generally, benzoic acid can occur in almost all environmental
compartments. Whether it exists in the undissociated or dissociated
form depends on the specific physicochemical conditions. Above pH 6,
the benzoate anion prevails (Chipley, 1983).
There is a series of reports on positive qualitative analyses of
benzoic acid in various environmental media, such as air (Belgium:
Cautreels & van Cauwenberghe, 1978; Germany: Helmig et al., 1989),
rain or snow (Norway: Lunde et al., 1977; Germany: Winkeler et al.,
1988), surface waters (Norway, river: Schou & Krane, 1981), and soils
(United Kingdom, heathland soil: Jalal & Read, 1983; Germany, river
terrace soil: Cordt & Kußmaul, 1990), but these do not provide
quantitative measurements.
Semiquantitative measurements of concentrations of benzoic acid
in urban air in Pasadena, California (USA) were in the range of
0.09-0.38 µg/m3 (Schuetzle et al., 1975). This was comparable to
quantitative measurements performed in 1984 in Los Angeles, California
(USA), which resulted in atmospheric concentrations of 0.005-0.13
µg/m3 (n = 8) (Kawamura et al., 1985). Most of the quantitative
data compiled in Table 2 with respect to water samples refer to
concentrations of benzoic acid in groundwater, with a maximum of 27.5
mg/litre measured in the vicinity of a point source.
Benzoic acid occurs naturally in free and bound form in many
plant and animal species. It is a common metabolite in plants and
organisms (Hegnauer, 1992). Appreciable amounts have been found in gum
benzoin (around 20%) and most berries (around 0.05%) (Budavari et al.,
1996). For example, ripe fruits of several Vaccinium species (e.g.,
cranberry, V. vitis idaea; bilberry, V. macrocarpon) contain as
much as 300-1300 mg free benzoic acid per kg fruit (Hegnauer, 1966).
Benzoic acid is also formed in apples after infection with the fungus
Nectria galligena (Harborne, 1983) or in Pinus thunbergii callus
inoculated with a pathogenic pine wood nematode (Bursaphelenchus
xylophilus) (Zhang et al., 1997). Among animals, benzoic acid has
been identified primarily in omnivorous or phytophageous species,
e.g., in viscera and muscles of the ptarmigan (Lagopus mutus)
(Hegnauer, 1989) as well as in gland secretions of male muskoxen
(Ovibos moschatus) (Flood et al., 1989) or Asian bull elephants
(Elephas maximus) (Rasmussen et al., 1990).
Owing to its occurrence in many organisms, benzoic acid is
naturally present in foods (review in Sieber et al., 1989, 1990). Some
typical examples specifying reported ranges of means in selected foods
have been compiled from Sieber et al. (1989) as follows:
Milk traces - 6 mg/kg
Yoghurt 12-40 mg/kg
Cheese traces - 40 mg/kg
Fruits (excluding traces - 14 mg/kg
Vaccinium species)
Potatoes, beans, cereals traces - 0.2 mg/kg
Soya flour, nuts 1.2-11 mg/kg
Honeys from different floral sources (n = 7) were found to
contain free benzoic acid at concentrations of <10 mg/kg (n = 5)
and of <100 mg/kg (n = 2) (Steeg & Montag, 1987).
Because benzoic acid and its compounds are used as food
preservatives (see section 4), some processed foods contain
artificially elevated concentrations of these substances (see section
6.2).
6.2 Human exposure
The main route of exposure of the general population to benzoic
acid or sodium benzoate is likely via foodstuffs that contain the
substances naturally or added as antimicrobial agents. There are a few
analyses of processed foodstuffs available. They refer to different
types of food items (juice, soft drinks, soya sauce varieties) from
the Philippines (a total of 44 samples) and from Japan (a total of 31
samples) and to orange drinks sampled in England. The concentrations
of sodium benzoate in the Philippine dietary samples ranged from 20 to
>2000 mg/litre. The range in the Japanese products was 50-200
mg/litre, thus reflecting the lower maximum level of sodium benzoate
allowed to be added to food in Japan as compared with the Philippines
(Villanueva et al., 1994). Orange drinks from England contained sodium
benzoate at concentrations ranging from 54 to 100 mg/litre (mean 76.7
mg/litre; n = 6) (Freedman, 1977).
Generally, the actual uptake depends on the individual's choice
of food to be consumed and the different limit values in different
countries. Several intake estimations have been published. Three
Japanese studies reported average daily intakes of benzoic acid from
processed foodstuffs to be 10.9 mg per person (Toyoda et al., 1983a)
and 1.4 mg per person (Toyoda et al., 1983b; Yomota et al., 1988),
corresponding to 0.02-0.2 mg/kg body weight (for persons with a body
weight of 50-70 kg). Both of the latter studies used the market basket
method for intake calculations, whereas the first-mentioned study
calculated intakes using the results of a national nutrition survey.
The concentrations of benzoic acid in 3319 food samples analysed for
this study (Toyoda et al., 1983a) ranged from not detected to 2100
mg/kg food. The maximum was found in salted fish (n = 7; mean 754
mg/kg). Another survey refers to the United Kingdom, where analyses of
benzoic acid in foods and drinks in which it is permitted as well as
intake estimates have been performed (UK MAFF, 1995). Sixty-five out
of 122 samples tested contained detectable benzoic acid. The highest
Table 2: Concentrations of benzoic acid in rain, snow, groundwater, and leachate samples.
Medium Location; sampling date Concentration Reference
(µg/litre)
Los Angeles area, California, Sum concentrationsa Kawamura & Kaplan
USA; 1982-1983 (1986)
Rain: urban 0.06-10.2 (n = 6)
Rain: semirural 0.02 (n = 1)
Snow: rural 0.04-0.1 (n = 3)
Groundwater Wyoming, USA (near underground 16-860 (n = 3) Stuermer et al.
coal gasification site; 15 months (1982)
after the end of gasification)
Groundwater Florida, USA (near wood treatment 10-27 500 (n = 3) Goerlitz et al.
facility); 1984 (1985)
Groundwater Ontario, Canada (near traces (n = 2) Barker et al.
landfillb); 1983 (1988)
Groundwater Barcelona area, Spain up to 0.21 (n = 3) Guardiola et al.
(near landfillb) (1989)
Leachate Ontario, Canada; 1981 <0.1->1000 (n = 5) Reinhard & Goodman
(from landfillb) (1984)
Leachate Ontario, Canada; 1983 traces (n = 2) Barker et al.
(from landfillb) (1988)
Leachate USA; 1986-1988 200-400c (n = 3) Ham et al.
(from foundry (1989)
wastes)
a Including benzoic acid, 3-methyl benzoic acid, and 4-methyl benzoic acid.
b Receiving rural, municipal (domestic), and industrial wastes.
c Concentrations estimated from gas chromatography/mass spectrometry data.
concentrations were found in sauces (mean 388 mg/kg; n = 20; range
71-948 mg/kg), reduced sugar jam (mean 216 mg/kg; n = 4; range
<20-333 mg/kg), non-alcoholic drinks (mean 162 mg/kg; n = 20; range
55-251 mg/kg), and semipreserved fish product (653 mg/kg; n = 1).
The survey found that the concentrations of benzoic acid detected
would lead to a dietary intake below 5 mg/kg body weight per day, even
for adults with an above-average consumption.
A frequent contributor to dietary exposure is soft drinks. A
rough estimation based on the average daily consumption in Germany of
such drinks (372 ml non-alcoholic beverages, excluding bottled water;
BAGS, 1995) by 19- to 24-year-old men, assuming the concentration of
benzoic acid present corresponds to a maximum allowable level of
150 mg/litre (EC, 1995), would result in a mean daily intake of
55.8 mg benzoic acid per person (or 0.80 mg/kg body weight, assuming
a 70-kg body weight). For comparison, a similar calculation with
sugar-free marmalade, jam, and similar spreads, which are allowed to
contain higher levels of benzoic acid (500 mg/kg; EC, 1995), would
result in a possible intake of 4.1 mg per person per day, or
0.06 mg/kg body weight per day (assumes a daily consumption of 8.2 g,
according to BAGS, 1995). This was more than a possible intake via
fruits containing natural benzoic acid. For example, a daily
consumption of 40.4 g of fruits (BAGS, 1995) would lead to a possible
intake of 0.57 mg benzoic acid per person per day (or 0.008 mg/kg body
weight for a 70-kg person), if the reported maximum of 14 mg benzoic
acid/kg (see section 6.1) were present.
The Joint FAO/WHO Expert Committee on Food Additives (JECFA)
assessed the intake of benzoates from information provided by nine
countries (Australia, China, Finland, France, Japan, New Zealand,
Spain, United Kingdom, and USA) (WHO, 1999). Because diets differ
among countries, the foods that contribute to benzoate intake would be
expected to vary. The food category that contributed most to benzoate
intake was soft drinks (carbonated, water-based, flavoured drinks) for
Australia/New Zealand, France, the United Kingdom, and the USA. In
Finland, 40% was in soft drinks. Soya sauce was the main source of
benzoate in China and the second most important in Japan. The best
estimates of national mean intakes of benzoates by consumers ranged
from 0.18 mg/kg body weight per day in Japan to 2.3 mg/kg body weight
per day in the USA. These estimates were based on analyses involving
either model diets or individual dietary records and maximum limits
specified by national governments or the European Union. The estimated
intake by high consumers of benzoates, based on food additive levels
in national standards, was 7.3 mg/kg body weight per day in the USA
and 14 mg/kg body weight per day in China.
Benzoates have been detected in groundwater, but not in
drinking-water.
Quantitative information on (oral or dermal/mucosal) exposure via
cosmetic, hygienic, or medical products is rare, but the data
available indicate a remarkable contribution to exposure. There are
reports on leaching of benzoic acid from denture-base acrylic resins.
After 10 days of immersion in artificial saliva, concentrations of up
to about 3 mg/litre have been observed for benzoic acid, which is
formed as a degradation product of the benzoyl peroxide that is added
as a polymerization initiator (Koda et al., 1989, 1990). In Japan,
commercial toothpastes have been found to contain benzoic acid at
concentrations ranging from 800 to 4450 mg/kg (n = 18). Use of the
toothpaste with the highest concentration (by 40 20-year-old female
students) would result in a calculated daily intake of about 2.23 mg
per person. This was about the same amount as their estimated intake
from diet (Ishida, 1996). Benzoic acid is also used in dermatology as
a fungicidal topical treatment for ringworm (Tinea spp.). The
emulsifying ointment preparation contains benzoic acid at 6% and is
applied twice daily (Goodman et al., 1990; BMA, 1998).
Recent quantitative monitoring data on concentrations of benzoic
acid or salts in ambient or indoor air are not available. Considering
the few (low) levels of benzoic acid measured in urban air in the
past, with a maximum of 0.38 µg/m3 (see section 6.1), inhalation may
contribute only marginally to exposure of the general population.
Using this maximum, a daily inhalative dose of 8.74 µg per person (or
0.12 µg/kg body weight) is obtained (assuming a daily inhalation
volume of 23 m3 for a 70-kg adult male; WHO, 1994).
Few quantitative data on occupational exposure have been
identified. Nevertheless, there is a potential for inhalation or
dermal contact in the chemical and allied product industries as well
as in workplaces where these products are used. Air samples (n = 50)
collected in an industrial environment (no further details given) over
a year's time showed benzoic acid concentrations ranging from not
detected to 1.5 mg/m3 (Halvorson, 1984). On the basis of the latter
value, an inhalative dose of 14.4 mg per person per 8-h working time
(or 0.2 mg/kg body weight) would result (assuming an inhalation volume
of 9.6 m3 for an 8-h exposure with light activity; WHO, 1994).
However, because of the lack of information on specific working
operations and conditions involved (e.g., duration of exposure, use of
protective clothes, etc.), it is impossible to derive a realistic
estimate of occupational exposure.
7. COMPARATIVE KINETICS AND METABOLISM IN LABORATORY ANIMALS
AND HUMANS
After oral ingestion of benzoic acid and sodium benzoate, there
is a rapid absorption (of undissociated benzoic acid) from the
gastrointestinal tract in experimental animals or humans (US FDA,
1972a, 1973). From the figures on excretion given below, 100%
absorption can be assumed. In humans, the peak plasma concentration is
reached within 1-2 h (Kubota et al., 1988; Kubota & Ishizaki, 1991).
Benzoic acid is not completely absorbed by the dermal route. In a
study with six human subjects, Feldmann & Maibach (1970) found an
uptake of 36% of the applied dose (14C-labelled benzoic acid
dissolved in acetone; 4 µg/cm2; circular area of 13 cm2; ventral
surface of the forearm; non-occlusive) within 12 h. The total uptake
within 5 days was 43%. In a second study with 6-7 subjects (comparable
method; application of 3, 400 or 2000 µg/cm2), the percent
absorption decreased from 35% to 14% within 24 h. However, the total
uptake per cm2 increased from 1 to 288 µg (Wester & Maibach, 1976).
For sodium benzoate, no data concerning dermal uptake were identified
in the literature.
In vivo dermal studies with benzoic acid in experimental
animals (e.g., guinea-pigs, mice, rats, pigs, dogs, rhesus monkeys)
confirm the results with humans (Hunziker et al., 1978;
Andersen et al., 1980; Wester & Noonan, 1980; Bronaugh et al., 1982a;
Reifenrath et al., 1984; Carver & Riviere, 1989; Maibach & Wester,
1989; Bucks et al., 1990). Absorption ranged from 25% in pigs
(Reifenrath et al., 1984; Carver & Riviere, 1989) to 89% in rhesus
monkeys (Wester & Noonan, 1980; Maibach & Wester, 1989; Bucks et al.,
1990). Due to the good database on humans and animals in vivo,
in vitro studies performed with animal or human skin are not
considered further (Franz, 1975; Bronaugh et al., 1982b;
Hotchkiss et al., 1992; MacPherson et al., 1996).
No information is available on absorption via inhalation.
After oral and dermal uptake, benzoate is metabolized in the
liver by conjugation with glycine, resulting in the formation of
hippuric acid (Feldmann & Maibach, 1970; US FDA, 1972a; WHO, 1996;
Feillet & Leonard, 1998). The rate of biotransformation in humans is
high: after oral doses of 40, 80 or 160 mg sodium benzoate/kg body
weight, the transformation to hippuric acid was independent of the
dose -- about 17-29 mg/kg body weight per hour, corresponding to about
500 mg/kg body weight per day (Kubota & Ishizaki, 1991). Other authors
obtained higher values of 0.8-2 g/kg body weight per day (US FDA,
1972a, 1973; WHO, 1996). Hippuric acid is rapidly excreted in urine.
In humans, after oral doses of up to 160 mg/kg body weight, 75-100% of
the applied dose is excreted as hippuric acid within 6 h after
administration, and the rest within 2-3 days (Kubota et al., 1988;
Fujii et al., 1991; Kubota & Ishizaki, 1991).
The limiting factor in the biosynthesis of hippuric acid is the
availability of glycine. The utilization of glycine in the
detoxification of benzoate results in a reduction in the glycine level
of the body. Therefore, the ingestion of benzoic acid or its salts
affects any body function or metabolic process in which glycine is
involved; for example, it leads to a reduction in creatinine,
glutamine, urea, and uric acid levels (US FDA, 1972a, 1973; Kubota &
Ishizaki, 1991; WHO, 1996).
Another metabolite of benzoate is the benzoyl glucuronide. For
example, the dog excretes considerable amounts of this metabolite in
the urine (20% after a single dose of 50 mg/kg body weight; Bridges et
al., 1970). In other species, this metabolite appears only after
higher doses of about 500 mg/kg body weight (see above) of benzoic
acid or sodium benzoate, resulting in a depletion of the glycine pool
(Bridges et al., 1970; US FDA, 1972a; Kubota et al., 1988). In cats,
glucuronidation is generally very low (Williams, 1967).
In some species, including humans, minor amounts of benzoic acid
itself are also excreted in the urine (Bridges et al., 1970; Kubota &
Ishizaki, 1991).
Experiments on the distribution and elimination of 14C-benzoate
in the rat have shown no accumulation of sodium benzoate or benzoic
acid in the body (US FDA, 1972a, 1973).
In the acid conditions of the stomach, the equilibrium moves to
the undissociated benzoic acid molecule, which should be absorbed
rapidly. Benzoate from sodium benzoate would change from the ionized
form to the undissociated benzoic acid molecule. As a result, the
metabolism and systemic effects of benzoic acid and sodium benzoate
can be evaluated together.
7.1 Precursors of benzoic acid
Benzyl acetate, its hydrolysis product, benzyl alcohol, and the
oxidation product of this alcohol, benzaldehyde, are precursors of
benzoic acid in experimental animals and humans. Benzyl acetate is
metabolized to benzoic acid and further to hippuric acid and benzoyl
glucuronide to an extent of >90% both in mice and in rats of
different strains. Benzyl alcohol was metabolized to benzoic acid and
its conjugates in preterm infants. Benzaldehyde is metabolized to
benzoic acid and its conjugates in rabbits to an extent of
approximately 90% (WHO, 1996).
8. EFFECTS ON LABORATORY MAMMALS AND IN VITRO TEST SYSTEMS
8.1 Single exposure
With oral LD50 values (administration by gavage) of 3040 mg
benzoic acid/kg body weight in rats (Bio-Fax, 1973) and 1940-2263 mg
benzoic acid/kg body weight in mice (McCormick, 1974; Abe et al.,
1984), the acute toxicity of benzoic acid is low. Clinical signs of
intoxication (reported for rats only) included diarrhoea, muscular
weakness, tremors, hypoactivity, and emaciation (Bio-Fax, 1973). With
oral LD50 values of 2100-4070 mg sodium benzoate/kg body weight in
rats, the acute toxicity of sodium benzoate is similar to that of
benzoic acid, as are the symptoms (Smyth & Carpenter, 1948;
Deuel et al., 1954; Bayer AG, 1977).
In four cats given diets containing 0 or 1% benzoic acid
(approximately 0 or 450-890 mg/kg body weight), aggression,
hyperaesthesia, and collapse starting 14-16 h after feed uptake were
seen at a dose level equal to 630 mg/kg body weight. The duration of
the syndrome was about 18-176 h, and the mortality rate was 50%. The
histopathological examination of the two cats that died revealed
degenerative changes in liver, kidneys, and lung, but no pathological
findings in brain or spinal cord (Bedford & Clarke, 1972). The authors
attributed the higher toxicity of benzoic acid in cats compared with
other species to the low capacity of cats for glucuronidation
(see section 7).
In rats, exposure by inhalation to 26 mg/m3 over 1 h caused no
mortality, but generalized inactivity and lacrimation were noted. The
gross autopsy gave no significant findings (no further information
available; Bio-Fax, 1973).
In a limit test with rabbits, no mortality or signs of
intoxication were seen after dermal application of 10 000 mg/kg body
weight. The gross autopsy gave no significant findings (no further
information available; Bio-Fax, 1973).
8.2 Irritation and sensitization
8.2.1 Benzoic acid
Although there is a wide range of results from mostly
non-standardized tests using various scoring systems, it can be
concluded that benzoic acid is slightly irritating to the skin and
irritating to the eyes.
In different experiments with rabbits, which have not been
performed according to current guidelines, benzoic acid applied as dry
powder or in the form of a paste was not irritating to slightly
irritating to the skin (score 1.66/8: Bio-Fax, 1973; no score given:
Bayer AG, 1978; primary skin irritation index 0.5 [no further
information available]: RCC Notox, 1988a).
In an acute eye irritation/corrosion study with rabbits conducted
according to OECD Guideline 405, some eye irritation was reported
after application of benzoic acid in the form of a paste. Within 72 h,
the scores for chemosis, reddening of the conjunctivae, iritis, and
keratitis always remained at <2 (Bayer AG, 1986).
In different non-standardized experiments with the solid
substance, moderately irritating to severely irritating effects on the
eye were noted (score 65/110: Bio-Fax, 1973; no score given: Bayer AG,
1978; score up to 108/110 [eyes rinsed after instillation] or up to
50/100 [eyes not rinsed]: Monsanto Co., 1983; score 35 according to
the scheme of Kay & Calandra, 1962: RCC Notox, 1988b).
In a maximization test, none of 15 guinea-pigs reacted positively
after induction and challenge with a 10-20% solution of benzoic acid
in water (Gad et al., 1986). In addition, the substance also tested
negative in a Buehler test with guinea-pigs and in an ear swelling
test and local lymph node assay with mice (Gad et al., 1986; Gerberick
et al., 1992). The concentrations used for induction and challenge
were 10-20% in acetone or water.
However, a dose-dependent positive result was obtained in an ear
swelling test with five guinea-pigs (induction with 0.2, 1, 5, or 20%
in absolute ethyl alcohol; no challenge) used as a model for detecting
agents causing non-immunological contact urticaria in humans. At
several other regions (back, abdomen, flank site), a concentration of
20% failed to produce any reactions (Lahti & Maibach, 1984).
8.2.2 Sodium benzoate
An acute dermal irritation/corrosion study with rabbits conducted
according to OECD Guideline 404 (no data about physical state; score
0: RCC Notox, n.d., a) as well as a non-standardized experiment with
the solid substance (score not given: Bayer AG, 1977) gave no
indication for skin irritating effects.
In a study performed according to OECD Guideline 405 (no data
about physical state; RCC Notox, n.d., b), sodium benzoate was only
slightly irritating to the eye (score 9.3, according to the scheme of
Kay & Calandra, 1962). The application of the solid substance in a
non-standardized experiment caused no irritation (score not given:
Bayer AG, 1977).
For sodium benzoate, no data on sensitizing effects were
identified in the available literature.
8.3 Short-term exposure
8.3.1 Oral exposure
In general, the database for benzoic acid and sodium benzoate is
limited, and there are no studies available performed according to
current guidelines. In addition, the documentation of these studies in
most cases is insufficient. Detailed information is given in Table 3.
From the available studies, it can be assumed that the toxicity
of benzoic acid after short-term oral exposure is low. In high-dosed
rats given approximately 2250 mg/kg body weight per day via diet over
5 days, excitation, ataxia, convulsions, and histopathological changes
in the brain were seen. The mortality was about 50%; in some cases,
bleeding into the gut was noted (Kreis et al., 1967). In two other
studies with rats dosed with approximately 825 mg/kg body weight per
day over 7-35 days (Kreis et al., 1967) or with 65-647 mg/kg body
weight per day over 28 days (Bio-Fax, 1973), no clear
treatment-related effects occurred. The reduced weight gain at 2250
and 825 mg/kg body weight per day may be attributed to reduced food
intake in the study by Kreis et al. (1967). The relevance of the
reduced relative kidney weight at 324 mg/kg body weight per day, which
was not dose-related and not accompanied by changes in
histopathological examinations, is unclear (Bio-Fax, 1973). As given
in Table 3, both studies have several limitations (i.e., missing
haematological and clinical chemical investigations, incomplete
histopathological examinations); therefore, both of these studies were
inadequate for derivation of a NO(A)EL.
More information on dose-response can be gained from the study of
Fujitani (1993), in which rats received sodium benzoate for 10 days in
feed. At the lowest tested concentration of 1358 mg/kg body weight per
day, changes in serum cholesterol levels occurred in females. At doses
of 1568 mg/kg body weight per day and above, changes in further serum
parameters and an increased relative liver weight were described.
Histopathological changes of the liver, increased relative kidney
weights, and disorders of the central nervous system (convulsions)
were seen after dosing via diet with approximately 1800 mg/kg body
weight per day. In several other studies listed in Table 3, adverse
effects were seen only at higher doses after feeding sodium benzoate
over periods from 10 to 42 days, so that a
lowest-observed-(adverse-)effect level (LO(A)EL) of 1358 mg sodium
benzoate/kg body weight per day for short-term exposure can be
derived.
With cats (Bedford & Clarke, 1972), also described in Table 3,
the effect levels with benzoic acid were lower. However, due to the
differences in the metabolism of benzoic acid in cats compared with
other experimental animals and humans, this study was not taken into
further consideration (see section 7).
Table 3: Toxicity of benzoic acid and sodium benzoate after short-term oral exposure.
Species; strain; Treatment Duration Organs examined in Resultsa Reference
number of animals (days) histopathology, clinical
per dosea chemistry, haematology
Benzoic acid
cat; 4 m 0 or 0.5% 3-4 liver, kidney, heart, mild hyperaesthesia, Bedford & Clarke
in diet stomach, lung, brain, apprehension, and (1972)
(approx. 0 spinal cord (only depression starting 48-92
or 300-420 mg/kg animals that died were h after uptake; duration of
body weight) examined); blood samples the syndrome: about 20-48 h;
were taken from surviving mortality rate: 50%;
cats degenerative changes in
liver, kidneys, and lung,
but no pathological findings
in brain or spinal cord;
surviving cats: urea and
serum alanine
aminotransferase (S-ALAT) *,
indicating liver and kidney
damage
cat; 4 m a) 100 or 200 a) 15 only blood samples no adverse effects Bedford & Clarke
mg/kg body weight were taken were reported (1972)
via diet
b) 0 or 0.25% in b) 23
diet (approx. 0
or 130-160 mg/kg
body weight)
Table 3 (cont'd)
Species; strain; Treatment Duration Organs examined in Resultsa Reference
number of animals (days) histopathology, clinical
per dosea chemistry, haematology
rat; Wistar; 0 or 3% in diet 1-5 heart, liver, spleen, body weight gain **; Kreis et al.
5-15 m (approx. 0 or kidney, brain in rats dosed over 5 days, (1967)
2250 mg/kg body disorders of the central
weight) nervous system (excitation,
ataxia, tonoclonic
convulsions); mortality
rate approx. 50%; in some
cases, bleeding into the
gut; brain damage (necrosis
of parenchymal cells of the
stratum granulosum of the
fascia dentata and the
cortex of the lobus
piriformis) in most animals
dosed over 3-5 days (still
present after 35 days)
rat; Wistar; 0 or 1.1% in 7-35 heart, liver, spleen, body weight gain **; Kreis et al.
5-10 m diet kidney, brain no clinical signs of (1967)
(approx. 0 or intoxication
825 mg/kg body
weight)
rat; albino; 10 m 0, 760, 3800, or 28 liver, kidney, no deaths or signs of Bio-Fax (1973)
7600 ppm via diet adrenals, testes intoxication
(approx. 0, 65, 324 mg/kg body
324, or 647 mg/kg weight: relative kidney
body weight) weights **; no further
information available
Table 3 (cont'd)
Species; strain; Treatment Duration Organs examined in Resultsa Reference
number of animals (days) histopathology, clinical
per dosea chemistry, haematology
Sodium benzoate
rat; F344/Ducrj; 0, 1.81, 2.09, or 10 liver, kidney; >1358 mg/kg body weight: Fujitani (1993)
6 m/f 2.4% in diet standard clinical changes in serum levels
(approx. 0, 1358, chemistry (cholesterol ** (f))
1568, or 1800 >1568 mg/kg body weight:
mg/kg body relative liver weight * (m);
weight) changes in serum levels
(albumin * (m), total
protein * (m))
1800 mg/kg body weight:
1/6 males died
(hypersensitivity,
convulsions); body
weight ** (m/f); relative
liver weight * (f); relative
kidney weights * (m/f);
absolute weights of spleen
and thymus ** (m);
absolute/relative weights
of thymus ** (f); changes
in serum levels
(gamma-glutamyltranspeptidase
(GGT) * (m), albumin * (f),
cholinesterase ** (f));
eosinophilic foci around
periportal vein and
enlargement of hepatocytes
with glassy cytoplasm in
the periportal area of the
liver (m); no changes in
the kidney (m)
Table 3 (cont'd)
Species; strain; Treatment Duration Organs examined in Resultsa Reference
number of animals (days) histopathology, clinical
per dosea chemistry, haematology
rat; Sherman; 0, 2, or 5% in 28 no data available 2200 mg/kg body weight: Fanelli & Halliday (1963)
6 m/f diet (approx. 0, slight depression of
2200, or 6700 body weight gain (m)
mg/kg body 6700 mg/kg body weight:
weight) mortality 100% within
11 days; signs of
intoxication included
hyperexcitability, urinary
incontinence, and
convulsions
no further information
available
rat; 28 (no 0 or 5% in diet 28 no data available mortality about 100% within Kieckebusch & Lang
further data) (approx. 0 or 3 weeks; decreased feed (1960)
3750 mg/kg body intake, diarrhoea,
weight) intestinal haemorrhage and
crusted blood in the nose;
no further information
available
rat; 5 (no 0 or 5% in diet >28 no data available mortality 80% within Kieckebusch & Lang
further data) (approx. 0 or 4-5 weeks; decreased (1960)
3750 mg/kg body weight; no further
body weight) information available
Table 3 (cont'd)
Species; strain; Treatment Duration Organs examined in Resultsa Reference
number of animals (days) histopathology, clinical
per dosea chemistry, haematology
rat; F344; 0, 0.5, 1, 2, 4, 42 histopathology performed, >375 mg/kg body weight: Sodemoto & Enomoto
10-11 m/f or 8% in diet but not further specified hypersensitivity after (1980)
(approx. 0, 375, dosing
750, 1500, 3000, >3000 mg/kg body weight:
or 6000 mg/kg mortality about 100% within
body weight) 4 weeks; apart from atrophy
of the spleen and lymph
nodes, no other
morphological changes were
noted
rat; Sherman; 0 or 16-1090 30 adrenals, upper intestine, no adverse effects were Smyth & Carpenter
5 m/f mg/kg body kidney, liver, spleen reported; no further (1948)
weight via diet information available
mouse; B6C3F1; 0, 2.08, 2.5, or 10 liver, kidney; standard „3750 mg/kg body weight: Fujitani (1993)
4-5 m/f 3% in diet clinical chemistry changes in serum levels
(approx. 0, 3000, (cholinesterase * (m))
3750, or 4500 4500 mg/kg body weight:
mg/kg body weight) hypersensitivity in all
animals; convulsions
1/5 males and 2/5 females
(both females died);
absolute/relative liver
weight * (m/f); relative
kidney weight * (f);
changes in serum levels
(cholesterol * (m),
phospholipids * (m));
Table 3 (cont'd)
Species; strain; Treatment Duration Organs examined in Resultsa Reference
number of animals (days) histopathology, clinical
per dosea chemistry, haematology
enlarged hepatocytes,
single cell necrosis
and vacuolation of
hepatocytes in all
livers (m); no changes
in the kidney (m/f)
mouse; albino 0, 0.5, 1, 2, 4, 35 survival, chemical 3000 mg/kg body weight: Toth (1984)
Swiss; 4 m/f or 8% via consumption, histological "suitable for lifelong
drinking-water changes (not further treatment" based on
(approx. specified) (prestudy four parameters: survival,
0-12 000 mg/kg for carcinogenicity study) body weight, chemical
body weight) consumption, and histology
6000 mg/kg body weight:
mortality 75% in m/f;
body weight of surviving
mice ** (m/f)
12 000 mg/kg body weight:
mortality 100% within
3 weeks
a m = male; f = female.
8.3.2 Inhalation exposure
Ten CD rats per sex per group were exposed to 0, 25, 250, or
1200 mg benzoic acid dust aerosol/m3 (analytical concentration; mass
aerodynamic diameter [MAD]/sigma g (standard deviation): 0, 4.6/3.1,
4.4/2.1, 5.2/2.1; mass median aerodynamic diameter [MMAD]: 4.7 µm) for
6 h per day and 5 days per week over 4 weeks. After this time, various
serum biochemical, haematological, organ weight, and histopathological
examinations were conducted. At >25 mg/m3, an increased incidence
of interstitial inflammatory cell infiltrate and interstitial fibrosis
in the trachea and lungs in treated animals compared with controls was
seen. Although the number of these microscopic lesions was higher in
treated animals than in controls, there was no clear dose dependency
for this effect. A concentration of >250 mg/m3 resulted in upper
respiratory tract irritation, as indicated by inflammatory exudate
around the nares, and significantly decreased absolute kidney weights
in females. In the highest dose group, one rat per sex died, and the
body weight gain was significantly decreased in males and females
compared with controls. In addition, a significant decrease in
platelets (males/females), absolute/relative liver weights (males),
and trachea/lung weights (females) was noted (Velsicol Chemical
Corp., 1981).
Studies concerning repeated exposure by inhalation to sodium
benzoate were not identified in the available literature.
8.3.3 Dermal exposure
Studies concerning repeated dermal exposure to benzoic acid or
sodium benzoate were not identified in the available literature.
8.4 Long-term exposure
In general, the database for benzoic acid and sodium benzoate is
limited, and there are no studies available performed according to
current guidelines. In addition, the documentation in most cases is
limited. Detailed information is given in Table 4.
8.4.1 Subchronic exposure
In a 90-day study with rats dosed with 0, 1, 2, 4, or 8% sodium
benzoate via diet, the mortality in the highest dose group (approx.
6290 mg/kg body weight per day) was about 50%. Other effects in this
group included a reduced weight gain, increased relative weights of
liver and kidneys, and pathological changes (not further specified) in
these organs (Deuel et al., 1954).
Table 4: Results of studies concerning long-term oral exposure to benzoic acid and sodium benzoate.
Species; strain; Treatment Duration Examinations; Resultsa Reference
number of animals organs in
per dosea histopathology,
clinical chemistry,
haematology
Benzoic acid
rat; Wistar; 0 or 1.5% in diet 18 months no data available reduced weight gain with Marquardt (1960)
dose group: (approx. 0 or decreased feed intake;
30 m/20 f; 750 mg/kg body increased mortality rate
controls: weight) (15/50 vs. 3/25 in
13 m/12 f controls); no further
information available
(only provisional results
are given)
rat; Wistar or 0 or 1.5% in diet 18 months no data available reduced weight gain with Marquardt (1960)
Osborne-Mendel; (approx. 0 or decreased feed intake;
dose group: 750 mg/kg body no further information
20 m; controls: weight) available (only
10 m provisional results are
given)
rat; not given; 0, 0.5, or 1% in generation 1 histopathology in no effects on growth and Kieckebusch & Lang
20 m/f diet and 2: animals of organ weights; feeding of (1960)
(approx. 0, 250, lifelong generation 3 0.5% led to prolongation
or 500 mg/kg body generation 3: (not further specified) of survival compared with
weight) 16 weeks controls; no further
generation 4: information available
until breeding
Table 4 (cont'd)
Species; strain; Treatment Duration Examinations; Resultsa Reference
number of animals organs in
per dosea histopathology,
clinical chemistry,
haematology
Sodium benzoate
rat; Sherman; 0, 1, 2, 4, or 8% 90 days histopathology 6290 mg/kg body weight: Deuel et al.
5 m/f in diet (approx. 0, performed, but mortality about 50%; (1954)
640, 1320, 2620, not further specified weight gain **;
or 6290 mg/kg body relative weights of
weight) liver and kidneys *;
pathological lesions
(not further specified)
in liver and kidneys
rat; F344; 0, 1, or 2% in 18-24 months histopathology average mortality rate of Sodemoto & Enomoto
dose group: diet performed, but not all animals during the (1980)
50 m/52 f; (m: approx. 0, 700, further specified first 16 months: 14.5%
controls: or 1400 mg/kg (all dead rats showed
body weight; f: pneumonia with abscess);
25 m/43 f about 100 rats including
approx. 0, 290, controls died after
or 580 mg/kg 16 months due to
body weight) haemorrhagic pneumonia
(infection); no adverse
clinical signs and no
differences in average
body weight and mortality
in dosed animals compared
with controls;
non-carcinogenic effects
not reported
Table 4 (cont'd)
Species; strain; Treatment Duration Examinations; Resultsa Reference
number of animals organs in
per dosea histopathology,
clinical chemistry,
haematology
mouse; 0 or 2% via lifelong liver, spleen, kidney, no difference in survival Toth (1984)
albino Swiss; drinking-water bladder, thyroid, rates in treated animals
dose group: (approx. 0 or heart, pancreas, compared with controls;
50 m/f; 5960-6200 mg/kg testes, ovaries, brain, no pathological or
controls: 99 m/f body weight) nasal turbinates, lung statistical evidence of
tumour induction
a m = male; f = female.
8.4.2 Chronic exposure and carcinogenicity
In two studies with rats given 1.5% benzoic acid via diet
(approximately 750 mg/kg body weight per day), the animals showed a
reduced weight gain with decreased feed intake after dosing over
18 months. In one of these studies, mortality was increased
(15/50 rats of both sexes versus 3/25 in controls) (Marquardt, 1960).
No further information on these studies is available, as only
provisional results were published. In a four-generation study with
rats, no effects on life span, growth rate, or organ weights were
reported after dosing with up to 1% in the diet (approximately
500 mg/kg body weight per day) (Kieckebusch & Lang, 1960). Only
animals of the third generation were autopsied after 16 weeks, but
it is not clear if a complete histopathological investigation was
performed.
With sodium benzoate, two long-term studies with rats
(administration of up to 1400 mg/kg body weight per day via diet over
18-24 months; Sodemoto & Enomoto, 1980) or mice (lifelong application
of up to 6200 mg/kg body weight per day via drinking-water; Toth,
1984) are available. The results gave no indication of a carcinogenic
effect in the tested animals. Although the study with mice was not
performed according to current guidelines, the results seem to be
reliable, due to a sufficient number of animals and detailed
histopathological examinations. However, the results from the study
with rats are uncertain, due to a very high mortality in animals of
all dose groups, including controls (from an "infection" after
16 months), no detailed information about dosing regimen (only mean
values given), and the considerable differences in the body weight of
male and female rats (the body weight of females was about twice that
of males).
8.4.3 Carcinogenicity of benzyl acetate, benzyl alcohol, and
benzaldehyde
As benzyl acetate, benzyl alcohol, and benzaldehyde are
practically quantitatively metabolized via benzoic acid (see section
7.1), data on their carcinogenicity from 2-year studies may be used as
supportive evidence in the assessment of the hazards associated with
benzoic acid.
Benzyl acetate was administered in corn oil via gavage to F344/N
rats (0, 250, or 500 mg/kg body weight per day) or B6C3F1 mice
(0, 500, or 1000 mg/kg body weight per day). In high-dose male rats,
the incidence of acinar cell adenomas of the exocrine pancreas was
increased, whereas there was no evidence of carcinogenicity in female
rats. In high-dose male and female mice, benzyl acetate caused
increased incidences of hepatocellular adenomas and squamous cell
neoplasms of the forestomach (US NTP, 1986). In contrast to these
findings, no such tumours were observed in another study with the same
strain of rats and mice when benzyl acetate was administered via diet
(rats: <575 mg/kg body weight per day; mice: <375 mg/kg body
weight per day) (US NTP, 1993).
With benzyl alcohol, no treatment-related increase in tumours was
observed in F344/N rats or B6C3F1 mice after administration of
<400 mg/kg body weight per day in rats or <200 mg/kg body weight
per day in mice by gavage in corn oil (US NTP, 1989).
In B6C3F1 mice dosed with benzaldehyde in corn oil by gavage
(males: 0, 200, or 400 mg/kg body weight per day; females: 0, 300, or
600 mg/kg body weight per day), the incidences of squamous cell
papillomas of the forestomach were significantly greater in both
exposure groups than in controls. A dose-related increase in the
incidence of forestomach hyperplasia was also observed. In F344/N rats
dosed with <400 mg/kg body weight per day, there was no evidence of
carcinogenic activity (US NTP, 1990).
8.5 Genotoxicity and related end-points
8.5.1 Benzoic acid
Benzoic acid tested negative in several Ames tests and in one DNA
damage assay with different Salmonella typhimurium strains in the
presence or absence of metabolic activation (McCann et al., 1975;
Ishidate et al., 1984; Nakamura et al., 1987; Zeiger et al., 1988).
Only in one recombination assay with Bacillus subtilis H17 and M45
was a positive result obtained (Nonaka, 1989). However, due to missing
experimental details (only results given), the validity of this study
cannot be judged. There was no indication of genotoxic activity
(chromosome aberrations, sister chromatid exchange) in tests with
mammalian cells (Chinese hamster CHL and CHO cells, human
lymphoblastoid cells, human lymphocytes) without metabolic activation
(Oikawa et al., 1980; Tohda et al., 1980; Ishidate et al., 1984;
Jansson et al., 1988).
In vivo studies with benzoic acid were not identified in the
literature.
8.5.2 Sodium benzoate
Sodium benzoate also gave negative results in some Ames tests and
in Escherichia coli in the presence or absence of metabolic
activation (Ishidate et al., 1984; Prival et al., 1991). As with
benzoic acid in recombination assays with Bacillus subtilis H17 and
M45, positive results were obtained (Ishizaki & Ueno, 1989; Nonaka,
1989). Although sodium benzoate tested negative in a cytogenetic assay
with WI-38 cells in the absence of metabolic activation (US FDA,
1974), consistently positive results (in contrast to the negative
results of benzoic acid) were obtained in tests on sister chromatid
exchange and chromosome aberrations with CHL/CHO and DON cells or
human lymphocytes without metabolic activation (Abe & Sasaki, 1977;
Ishidate & Odashima, 1977; Ishidate et al., 1984, 1988; Xing & Zhang,
1990). However, from the limited information given in the publications
(i.e., only results given), it cannot be judged if these positive
results may have been attributable to cytotoxic effects.
In a valid in vivo study performed by the US FDA (1974), sodium
benzoate tested negative in a cytogenetic assay (bone marrow) in rats
after single or multiple oral application of doses up to 5000 mg/kg
body weight. In a study with mice (comparable dosing scheme), there
was also no indication of mutagenic activity in a host-mediated assay
(US FDA, 1974).
However, in a dominant lethal assay with rats (comparable dosing
scheme; males were mated with untreated females following 7 or 8 weeks
of dosing), some statistically significant and dose-related findings
were reported in week 7: decreased fertility index for both treatment
regimens and an increased number of preimplantation losses after
single dosing (US FDA, 1974).
In summary, the in vitro studies with benzoic acid gave no
indications for genotoxic effects, whereas in vivo studies were not
identified. Sodium benzoate was also inactive in bacterial test
systems, whereas tests with mammalian cells gave consistently positive
results. In addition, in an in vivo study with sodium benzoate
(dominant lethal assay in rats), a positive result was obtained. As a
result, a genotoxic activity of sodium benzoate cannot be ruled out
entirely at present.
Detailed information concerning the genotoxicity of benzoic acid
and sodium benzoate in vitro is given in Table 5.
8.6 Reproductive and developmental toxicity
8.6.1 Fertility
There are no studies available dealing specifically with the
effects of benzoic acid or sodium benzoate on fertility that have been
conducted according to current protocols.
In a four-generation study with male and female rats, no adverse
effects on fertility or lactation (only investigated parameters) were
seen after dosing with benzoic acid at up to 1% in the diet
(approximately 500 mg/kg body weight per day) (see also section 8.4.2;
Kieckebusch & Lang, 1960).
In studies with repeated oral application, no effects on the
testes were observed in rats after dosing with benzoic acid at up to
647 mg/kg body weight per day in the diet for 4 weeks (see also Table
3; Bio-Fax, 1973) or in mice after lifelong application of 6200 mg
sodium benzoate/kg body weight per day via drinking-water (see also
Table 4; Toth, 1984).
In summary, no clear statement can be given as to the possible
effects of benzoic acid or sodium benzoate on fertility.
8.6.2 Developmental toxicity
In a study with pregnant rats given only one oral dose of benzoic
acid (510 mg/kg body weight on gestation day 9), there was no
indication of an increase in resorption rates or malformations
(Kimmel et al., 1971).
For sodium benzoate, several teratogenicity studies are available
that have been performed with different species. As given in Table 6,
no effects were seen in dams or offspring of rats, mice, rabbits, or
hamsters given oral doses of up to 300 mg/kg body weight per day
(highest dose tested) during gestation (US FDA, 1972b). In a study
with rats by Onodera et al. (1978), doses of 4% or 8% via diet (uptake
of 1875 or 965 mg/kg body weight per day) induced severe maternal
toxicity (no weight gain/loss in body weight, increased mortality) and
were associated with embryotoxic and fetotoxic effects as well as
malformations. However, the authors suggested that the effects on the
dams and fetuses at >4% dietary levels were caused by reduced
maternal feed intake, leading to malnutrition. The intake of sodium
benzoate in the highest dose group (8%) was lower than that at 2%,
where no adverse effects were seen. From this study, a NO(A)EL of
about 1310 mg/kg body weight per day can be derived. In a study with
rats by Minor & Becker (1971), however, fetotoxic and teratogenic
effects occurred at 1000 mg/kg body weight per day. In this study,
sodium benzoate was applied by intraperitoneal injection. Therefore,
differences in pharmacokinetics between oral and intraperitoneal
administration may be the reason for the higher sensitivity.
Studies performed with eggs of leghorn hens (single injection of
<5 mg per egg), chick embryo neural retina cells
(lowest-observed-effect concentration [LOEC] of 34.7 mmol/litre), and
a chick embryotoxicity screening test (single injection of <0.1 mg
per embryo) gave no indication of embryotoxic or teratogenic effects
(Verrett et al., 1980; Jelinek et al., 1985; Daston et al., 1995).
8.6.3 Reproductive toxicity of benzyl acetate, benzyl alcohol,
and benzaldehyde
As benzyl acetate and benzyl alcohol are practically
quantitatively metabolized via benzoic acid (see section 7.1), data on
their reproductive toxicity may be used as supportive evidence in the
assessment of the hazards associated with benzoic acid.
Dietary benzyl acetate (up to 5% in the diet for 13 weeks) had no
effect on the weights of the epididymis, cauda epididymis, or testis,
on sperm motility or density, or on the percentage of abnormal sperm
in mice or rats (US NTP, 1993).
Benzyl acetate (0, 10, 100, 500, or 1000 mg/kg body weight per
day by gavage on days 6-15) had no significant effects on maternal
health in rats and did not induce changes in the numbers of corpora
lutea, implantations, live or dead fetuses, or resorptions,
implantation ratio, sex ratio, external or internal malformations, or
placental weight. Fetal weights were significantly reduced at the
highest dose (Ishiguro et al., 1993).
Benzyl alcohol at 550 mg/kg body weight per day by gavage on days
6-15 of pregnancy had no effect on gestation index, average number of
live pups per litter, postnatal survival, or pup body weight on days
0 and 3 in CD-1 mice (York et al., 1986), while 750 mg/kg body weight
per day (days 7-14) induced a reduction in the pup weight and maternal
weight gain, but no pup mortality or changes in mating or gestation
indices, the total number of resorptions, or the number of live pups
per litter (Hardin et al., 1987).
Table 5: Genotoxicity of benzoic acid and sodium benzoate in vitro.
Resultsa
Species End-point Concentration Without With Remarks Reference
(test system) range metabolic metabolic
activation activation
Benzoic acid
Salmonella Reverse 10-1000 - - McCann et al.
typhimurium mutations µg/plate (1975)
TA 98, TA 100,
TA 1535, TA 1537
Salmonella Reverse 33-10 000 - - cytotoxic effects Zeiger et al.
typhimurium mutations µg/plate at >5000 (1988)
TA 97, TA 98, µg/plate
TA 100, TA 1535,
TA 1537
Salmonella Reverse up to 10 000 - - 10 000 µg/plate was Ishidate et al.
typhimurium mutations µg/plate the highest (1984)
TA 92, TA 94, non-cytotoxic
TA 98, TA 100, concentration tested
TA 1535, TA 1537
Salmonella DNA damage up to - - no further Nakamura et al.
typhimurium (umu test) 1670 µg/ml information available (1987)
TA 1535/pSK 1002 (only results given)
Bacillus Recombination not given tested positive (no Nonaka
subtilis assay further information (1989)
H17, M45 available, only
summary given)
Table 5 (cont'd)
Resultsa
Species End-point Concentration Without With Remarks Reference
(test system) range metabolic metabolic
activation activation
Chinese hamster Chromosome up to ? 0 1500 µg/ml was given Ishidate et al.
cells (CHL) aberration 1500 µg/ml as maximum effective (1984)
concentration; result
given as negative in
Ishidate et al.
(1988)
Human Sister 1-30 - 0 cytotoxic effects Tohda et al.
lymphoblastoid chromatid mmol/litre at 30 mmol/litre (1980)
cells exchange
(transformed
by Epstein-Barr
virus)
Human Sister up to 2 - 0 Jansson et al.
lymphocytes chromatid mmol/litre (1988)
exchange
Chinese Sister up to - 0 Oikawa et al.
hamster chromatid 10 mmol/litre (1980)
cells (CHO) exchange
Table 5 (cont'd)
Resultsa
Species End-point Concentration Without With Remarks Reference
(test system) range metabolic metabolic
activation activation
Sodium benzoate
Salmonella Reverse up to - - 3000 µg/plate was Ishidate et al.
typhimurium mutations 3000 µg/plate the highest (1984)
TA 92, TA 94, non-cytotoxic
TA 98, TA 100, concentration tested
TA 1535, TA 1537
Salmonella Reverse 33-10 000 - - Prival et al.
typhimurium mutations µg/plate (1991)
TA 98, TA 100,
TA 1535, TA 1537,
TA 1538
Escherichia coli Reverse 33-10 000 - - Prival et al.
WP2 mutation µg/plate (1991)
assay
Bacillus Recombination not given tested positive Nonaka (1989)
subtilis assay information
H17, M45 available, only
summary given)
Bacillus Recombination -S9: (+) (+) Ishizaki & Ueno
subtilis assay 20 mg/disc (1989)
H17, M45 +S9:
16 mg/disc
Table 5 (cont'd)
Resultsa
Species End-point Concentration Without With Remarks Reference
(test system) range metabolic metabolic
activation activation
WI-38 cells Cytogenetic 10-1000 µg/ml - 0 examination of US FDA (1974)
assay anaphase preparations
cytotoxic effects
at >500 µg/ml
Chinese hamster Chromosome up to + 0 2000 µg/ml was Ishidate et al.
cells (CHL) aberration 2000 µg/ml given as maximum (1984, 1988)
effective
concentration
Chinese hamster Chromosome 139 mg/ml + 0 only maximum Ishidate &
cells (CHL) aberration effective dose given Odashima (1977)
Chinese hamster Chromosome 290 µg/ml 0 0 only minimum Ishidate et al.
cells (DON) aberration effective dose given (1988)
Chinese hamster Chromosome 1-10 + 0 Abe & Sasaki
cells (DON) aberration mmol/litre (1977)
Chinese hamster Sister 1-10 (+) 0 slight increase Abe & Sasaki
cells (DON) chromatid mmol/litre without dosage (1977)
exchange effect
Human Sister 10 + 0 Xing & Zhang
lymphocytes chromatid mmol/litre (1990)
exchange
a -, negative; +, positive; (+) weakly positive; ?, equivocal; 0, not tested.
Table 6: Results of studies concerning reproductive and developmental toxicity of benzoic acid and sodium benzoate.
Species; strain; Application Durationb Parameters Results NO(A)EL Reference
number of animals investigated (mg/kg
per dosea body
weight)
Benzoic acid
rat; Wistar; 0 or 510 gd 9 F0: implantation F0: resorption rates 510 Kimmel et al.
dose group: mg/kg body and resorption sites were given as (1971)
7 f; controls: weight F1: malformations "comparable with
not given via gavage controls"
F1: malformations
(not further specified)
were given as
"comparable with controls"
no further information
available
rat; not given; 0, 0.5, or 1% F0 and F1: fertility and F0-F3: no adverse 500 Kieckebusch &
20 f in diet lifelong lactation effects compared with Lang (1960)
(approx. 0, F2: 16 controls were reported
250, or 500 weeks no further information
mg/kg body F3: until available
weight) breeding
Sodium benzoate
rat; Wistar; 0, 1.75, 8, gd 6-15 F0: numbers of corpora F0 and F1: no adverse 175 US FDA
20 f 38, or 175 lutea, implantation effects compared with (1972b)
mg/kg body and resorption sites, controls were reported
weight via examination of the
gavage urogenital tract
Table 6 (cont'd)
Species; strain; Application Durationb Parameters Results NO(A)EL Reference
number of animals investigated (mg/kg
per dosea body
weight)
F1: numbers of live
and dead fetuses, body
weights, gross
examination for
external
malformations,
microscopic visceral
and skeletal
examination
mouse; 0, 1.75, 8, gd 6-15 F0: numbers of corpora F0 and F1: no adverse 175 US FDA
CD-1; 38, or 175 lutea, implantation effects compared with (1972b)
25-31 f mg/kg body and resorption sites, controls were reported
weight via examination of the
gavage urogenital tract
F1: numbers of live
and dead fetuses, body
weights, gross
examination for
external
malformations,
microscopic visceral
and skeletal
examination
rabbit; Dutch 0, 2.5, 12, gd 6-18 F0: numbers of corpora F0 and F1: no 250 US FDA
belted; 14-32 f 54, or 250 lutea, implantation adverse effects (1972b)
mg/kg body and resorption sites, compared with
weight via examination of the controls were
gavage urogenital tract reported
Table 6 (cont'd)
Species; strain; Application Durationb Parameters Results NO(A)EL Reference
number of animals investigated (mg/kg
per dosea body
weight)
F1: numbers of live
and dead fetuses, body
weights, gross
examination for
external
malformations,
microscopic visceral
and skeletal
examination
hamster; 0, 3, 14, 65, gd 6-10 F0: numbers of corpora F0 and F1: no adverse 300 US FDA
golden; or 300 mg/kg lutea, implantation effects compared with (1972b)
22 f body weight and resorption sites, controls were reported
via gavage examination of the
urogenital tract
F1: numbers of live
and dead fetuses,
body weights, gross
examination for
external
malformations,
microscopic visceral
and skeletal
examination
Table 6 (cont'd)
Species; strain; Application Durationb Parameters Results NO(A)EL Reference
number of animals investigated (mg/kg
per dosea body
weight)
rat; 0, 100, 315, a) gd 9-11 F0: not specified a) F0: no data given 315 Minor & Becker (1971)
Sprague-Dawley or 1000 mg/kg b) gd 12-14 F1: body weights, in F1: 1000 mg/kg body
(no further data) body weight utero deaths, weight: body weights **;
intraperitoneally gross anomalies in utero deaths * (16%);
gross anomalies * (not
further specified)
b) F0: no data given
F1: 1000 mg/kg body
weight: body weights **;
in utero deaths * (12%);
gross anomalies <--> (not
further specified)
no further information
available
rat; Wistar; 0, 1, 2, 4, gd 1-20 a) all but five a) >4% (1875 or 965 mg/kg 1310 Onodera et al. (1978)
27-30 f or 8% via diet animals in each group body weight):
(approx. 0, 700, were sacrificed on F0: weight gain <-->
1310, 1875, or gd 20 (numbers of feed intake **;
965 mg/kg body viable/dead fetuses, mortality * (convulsions,
weight) early/late depressed motor activity)
resorptions, fetal, F1: number of
placental, and ovarian dead/resorbed fetuses *;
weights, and body weight of viable
abnormalities of fetuses **; mild
maternal organs and systemic oedema,
fetal appearance were anophthalmia,
recorded) microphthalmia,
Table 6 (cont'd)
Species; strain; Application Durationb Parameters Results NO(A)EL Reference
number of animals investigated (mg/kg
per dosea body
weight)
b) the remaining five hydrocephalus,
dams delivered pyelectasis, hydroplasia,
naturally (number of cerebral hypoplasia;
offspring, survival, delayed ossification,
body weight, and lumbar or cervical ribs,
abnormalities were and varied sternebrae
recorded); 3 weeks 8%: F0: body weight **
after birth, all b) F1:
surviving pups were <2% (1310 mg/kg body
weaned and examined weight): no adverse
for gross effects compared with
abnormalities controls
(one-half of the pups >4% (1875 or 965 mg/kg
and all dams were body weight): delivery
necropsied); the rates ** (50 and 8.2%,
remaining pups were respectively); complete
necropsied at 8 weeks loss of litters after
of age (body weight parturition
and food intake were
measured weekly until
necropsy)
a m = male; f = female.
b gd = gestation day.
9. EFFECTS ON HUMANS
Cases of urticaria, asthma, rhinitis, or anaphylactic shock have
been reported following oral, dermal, or inhalation exposure to
benzoic acid and sodium benzoate. The symptoms appear shortly after
exposure and disappear within a few hours, even at low doses (Maibach
& Johnson, 1975; Clemmensen & Hjorth, 1982; Larmi et al., 1988; Ring,
1989; Gailhofer et al., 1990; Aberer et al., 1992; Lahti et al., 1995;
Anderson, 1996; Bindslev-Jensen, 1998; Coverly et al., 1998).
In the literature, several studies (e.g., oral provocation tests
or patch tests) are available, which have been performed with small
groups of patients suffering from urticaria, dermatitis, asthma, and
Melkersson-Rosenthal syndrome (Juhlin et al., 1972; Freedman, 1977;
Osterballe et al., 1979; Lahti & Hannuksela, 1981; Clemmensen &
Hjorth, 1982; Ibero et al., 1982; Moneret-Vautrin et al., 1982; Veien
et al., 1987; Aguirre et al., 1993; McKenna et al., 1994; BUA, 1995;
Munoz et al., 1996; Petrus et al., 1996; Vogt et al., 1999). In most
of these studies, atopic individuals have demonstrated reactions to
oral and dermal challenge with benzoic acid or sodium benzoate.
The information concerning skin reactions caused by benzoic acid
or sodium benzoate in the general population is limited. In a study
with 2045 patients of dermatological clinics, only 5 persons
(approximately 0.2%) showed a positive reaction in patch tests
(Brasch et al., 1993), while 34 of 5202 patients (approximately 0.7%)
with contact urticaria reacted positively (Broeckx et al., 1987). From
these data, it can be concluded that skin reactions caused by benzoic
acid or sodium benzoate in the healthy general population are rare.
In US FDA (1972a) and WHO (1996), several older studies
concerning oral exposure to benzoic acid or sodium benzoate are
described. However, owing to the limited number of individuals (mostly
single case studies), the validity of these studies is limited. No
adverse effects were reported after a single oral dose of 10 000 mg
benzoic acid or up to 1000 mg per day over a period of up to 92 days
(Gerlach, 1909). In another study with volunteers given 1000, 1500,
2000, or 2500 mg/day for 5 days each, marked symptoms, signs of
discomfort, and malaise (nausea, headache, weakness, burning and
irritation of oesophagus) were reported (Wiley & Bigelow, 1908).
Chittenden et al. (1909) found no abnormalities in blood picture,
urine composition, nitrogen balance, or well-being in six men given
300-400 mg per day via diet for up to 62 days. In nine patients on
penicillin treatment given 12 000 mg benzoic acid divided into eight
doses over 5 days in eight subjects and over 14 days in one subject,
no adverse effects on blood urea nitrogen or creatinine clearance were
reported (Waldo et al., 1949). A single dose of 2000-3000 mg sodium
benzoate caused signs of intoxication similar to those described for
benzoic acid by Wiley & Bigelow (1908).
Sodium benzoate is used in the treatment of patients with urea
cycle enzymopathies (i.e., hyperammonaemia due to inborn errors of
urea synthesis) in order to facilitate an alternative pathway of
nitrogen excretion. The therapeutic dose given over several years is
in the range of 250-500 mg/kg body weight per day (Batshaw & Brusilow,
1981; Green et al., 1983; Batshaw & Monahan, 1987; O'Connor et al.,
1987; Kubota & Ishizaki, 1991; Tremblay & Qureshi, 1993; Feillet &
Leonard, 1998). At this dose level, clinical signs of toxicity are
rare and in most cases limited to anorexia and vomiting, especially
after intravenous bolus infusions.
10. EFFECTS ON OTHER ORGANISMS IN THE LABORATORY
AND FIELD
10.1 Aquatic environment
For the toxicity data mentioned in this section, it is not always
stated whether the cited effect values are based on nominal or
measured concentrations of benzoic acid or sodium benzoate. However,
because of their water solubility, their insignificant volatility, and
their low adsorption potential (see sections 2 and 5), all nominal
concentrations of the test substances are expected to correspond to
effective concentrations, even in tests with open systems and longer
exposure durations.
In Table 7, several valid toxicity test results for the most
sensitive aquatic species of various taxonomic groups -- bacteria,
cyanobacteria, green algae, protozoa, invertebrates, and vertebrates
-- with benzoic acid have been compiled. From the aquatic organisms
tested so far, cyanobacteria (Anabaena inaequalis) proved to be most
sensitive, showing a 14-day EC50 of 9 mg/litre in the cell
multiplication inhibition test (Stratton & Corke, 1982). EC50/LC50
values (24-96 h) for most of the other aquatic species tested
(protozoa, molluscs, crustaceans, fish, amphibians) were in the range
of 100-1291 mg/litre. As seen with daphnids, the pH value of the test
medium influences the toxicity of benzoic acid, which proved to be
more toxic at lower pH levels (Bringmann & Kuehn, 1980). Developmental
toxicity effects seen in frog (Xenopus) embryos were craniofacial
defects, especially microcephaly, and abnormal gut coiling
(Dawson et al., 1996). A recently developed cytotoxicity assay with
cultured fathead minnow (Pimephales promelas) cells resulted in a
PI50 (the concentration required to induce a 50% reduction in total
protein content) of 1450 mg/litre (Dierickx, 1998).
Ninety-six-hour LC50 values of >100 mg sodium benzoate/litre
have been found for Daphnia magna (first and second larval instar)
and Gammarus fasciatus (juvenile: 7 mg in size) under static test
conditions (multispecies test; pH 6.5-8; 20°C) (Ewell et al., 1986).
The same was true for juveniles of other invertebrates tested
simultaneously: Asellus intermedius (Arthropoda; 12 mg body weight),
Dugesia tigrina (Platyhelminthes; 6 mg body weight), Helisoma
trivolvis (Mollusca; 180 mg body weight), and Lumbriculus variegatus
(Annelida; 6 mg body weight) (Ewell et al., 1986).
Two different tests with the freshwater fathead minnow
(P. promelas; juvenile stages) resulted in 96-h LC50 values of 484
mg sodium benzoate/litre (measured concentration; flow-through system;
pH 7.4; 24°C) (Geiger et al., 1985) and >100 mg/litre (nominal
concentration; static system; pH 6.5-8.5; 20°C) (Ewell et al., 1986).
Table 7: Aquatic toxicity of benzoic acid.
Most sensitive species Special Effective Reference
(test method/end-point) features concentration
(mg/litre)
Mixed microbial inoculum
Activated sludge pH 7.5 3-h EC50 >1000 Klecka et al.
(respiration inhibition (1985)
test; OECD Guideline
209)
Bacteria
Pseudomonas putida pH neutral 16-h MICa 480 Bringmann &
(cell multiplication Kuehn (1977)
inhibition test)
(static)
Photobacterium - 30-min EC50 16.85 Kaiser et al.
phosphoreum (1987)
(Microtox test:
bioluminescence
reduction)
Cyanobacteria
Anabaena inaequalis Stratton &
(cell multiplication - 14-day EC50 9 Corke (1982)
inhibition test)
(static)
(photosynthesis - 3-h EC50 5
reduction)
Algae
Scenedesmus quadricauda
(cell multiplication pH neutral 8-day MIC 1630 Bringmann &
inhibition test) Kuehn (1977)
(static)
(photosynthesis
reduction) - 3-h EC50 75 Stratton &
Corke (1982)
Table 7 (cont'd)
Most sensitive species Special Effective Reference
(test method/end-point) features concentration
(mg/litre)
Chlorella pyrenoides
(photosynthesis
reduction) - 3-h EC50 60 Stratton &
Corke (1982)
Protozoa
Uronema parduczi
(cell multiplication pH 6.9 20-h MIC 31 Bringmann &
inhibition test) Kuehn (1980)
Tetrahymena pyriformis
(cell multiplication - 2-day EC50 252 Schultz et al.
inhibition test) (1996)
Invertebrata: Mollusca
Teredo digensis larvae 72-h LC50 100 Vind &
(marine) (static) Hochman
(1960)
Invertebrata: Crustacea
Daphnia magna pH neutral 24-h EC50 500 Bringmann &
(immobilization) Kuehn (1982)
pH acid 24-h EC50 102
Vertebrata: Fish
Leuciscus idus
(lethality, DEV L15) pH 7-8 48-h LC50 460 Juhnke &
Luedemann
(1978)
Table 7 (cont'd)
Most sensitive species Special Effective Reference
(test method/end-point) features concentration
(mg/litre)
Vertebrata: Amphibia
Xenopus laevis
(lethality) embryos Dawson et al.
(malformation) pH 7.2-7.4 96-h LC50 1291 (1996)
96-h EC50 433
a MIC = minimum inhibitory concentration.
10.2 Terrestrial environment
It is undissociated benzoic acid that is responsible for its
antimicrobial activity. As benzoic acid itself is only slightly
soluble in water, sodium benzoate -- which, under acidic conditions,
converts to undissociated benzoic acid -- is often used instead. Their
antimicrobial properties are used for different applications, such as
food preservation (Chipley, 1983; see section 4), optimally under
acidic conditions.
Minimum microbiocidal concentrations ranged from 20 to 1200 mg
benzoic acid/litre in suspension tests (pH 6) with different bacterial
or fungal species (Wallhäusser, 1984; Russell & Furr, 1996). Minimum
inhibitory concentrations (serial dilution technique) were in the
range of 50-1000 mg/litre (Wallhäusser, 1984; Russell & Furr, 1996).
The pH dependence of benzoic acid's antimicrobial activity is
shown in several studies. Growth inhibition of the fungus Fusarium
oxysporum (related to dry weight) measured 5 days after incubation
with 610 mg benzoic acid/litre was 23.7% at pH 7.2 and 83.5% at pH 4
(Soni & Bhatia, 1980). There was no visible growth of yeast
(Saccharomyces cerevisiae, Willia anomala) or mould (Penicillium
glaucum) fungi at sodium benzoate concentrations of 120-600 mg/litre
at pH 2.6, 1000-4000 mg/litre at pH 5, or 20 000-60 000 mg/litre at pH
7 (Schelhorn, 1951). Minimum inhibitory concentrations preventing
growth of Talaromyces flavus on agar plates after 35 days of
incubation were 100 mg/litre at pH 3.5 and >600 mg/litre at pH 5.4
(King & Halbrook, 1987).
The minimum inhibitory concentrations of benzoic acid on the
growth of several species of yeasts ranged from 170 to 1250 mg/litre
in cultures preadapted to benzoic acid and from 100 to 600 mg/litre in
unadapted cultures (pH 3.5; 25°C; 6 weeks of incubation) (Warth,
1988).
No information on the toxic effects of benzoic acid or sodium
benzoate on plants, earthworms, or other terrestrial organisms or on
ecosystems was identified.
11. EFFECTS EVALUATION
11.1 Evaluation of health effects
11.1.1 Hazard identification and dose-response assessment
After oral ingestion of benzoic acid and sodium benzoate in
experimental animals or humans, there is rapid absorption of the
undissociated benzoic acid from the gastrointestinal tract. The
substances are metabolized in the liver mainly by conjugation with
glycine, resulting in the formation of hippuric acid, which is rapidly
excreted via the urine. Benzoates applied dermally can penetrate
through the skin. Owing to their rapid metabolism and excretion, an
accumulation of the benzoates or their metabolites is not to be
expected.
With oral LD50 values of >1940 mg/kg body weight, the acute
toxicity of benzoic acid and sodium benzoate in rodents is low.
Benzoic acid is slightly irritating to the skin and irritating to
the eye, whereas sodium benzoate is not irritating to the skin and is
only a slight eye irritant. Benzoic acid was not skin sensitizing in
several animal models. For sodium benzoate, no data were identified
covering this specific end-point.
Studies concerning short-term, subchronic, or chronic oral
exposure conducted according to current guidelines are not available
for benzoic acid or sodium benzoate. Effects on the central nervous
system, weight gain (in several cases without reduced food intake),
and liver and kidney were recorded at high concentrations of both
compounds. As expected, and as far as it is possible to conclude with
the limited database, toxic effects and effect levels seem to be
similar for both compounds. A preliminary NO(A)EL of about 500 mg/kg
body weight per day (the highest dose tested) may be derived based on
a limited four-generation study (Kieckebusch & Lang, 1960; see section
8.4.2 and Table 4). This is supported by two short-term studies in
which no adverse effects were observed at the highest tested dose
levels of 647-825 mg/kg body weight per day (Kreis et al., 1967;
Bio-Fax, 1973) and by the fact that no serious side-effects have been
reported after therapeutic use of sodium benzoate at a dose level of
250-500 mg/kg body weight per day in humans, although occasionally
anorexia and vomiting were observed.
In a short-term inhalation study with rats exposed to benzoic
acid (0, 25, 250, or 1200 mg dust aerosol/m3; 6 h per day, 5 days
per week, over 4 weeks), indications of fibrosis in the lung were seen
even at the lowest concentration. The number of these microscopic
lesions was higher in treated animals than in controls, but there was
no clear dose dependency for this effect. Therefore, a
no-observed-(adverse-)effect concentration (NO(A)EC) value cannot be
derived. Long-term inhalation studies with benzoic acid or sodium
benzoate were not identified.
Two long-term studies with rats (application of up to 1400 mg/kg
body weight per day via diet over 18-24 months; quality of the study
questionable) or mice (lifelong application of up to 6200 mg/kg body
weight per day via drinking-water) gave no indication of a
carcinogenic effect in either species. Studies on the precursors of
benzoic acid -- benzyl acetate, benzyl alcohol, and benzaldehyde --
support the notion that it is unlikely that benzoic acid is
carcinogenic.
In several in vitro tests on genotoxicity, benzoic acid and
sodium benzoate tested negative. For sodium benzoate, in contrast to
benzoic acid, consistently positive results were obtained in tests on
sister chromatid exchange and chromosome aberrations without metabolic
activation. In vivo studies for benzoic acid were not identified.
For sodium benzoate, negative results were obtained in vivo in a
cytogenetic assay with rats and a host-mediated assay with single or
multiple oral application. However, a dominant lethal assay with rats
gave a positive result. Therefore, a possible genotoxic activity of
sodium benzoate cannot be ruled out entirely at present.
For benzoic acid, two limited studies gave no indication of
adverse reproductive or developmental effects. With sodium benzoate,
several studies on different species have been performed. Embryotoxic
and fetotoxic effects as well as malformations were seen only at doses
that induced severe maternal toxicity. In a dietary study in rats, a
NO(A)EL of about 1310 mg/kg body weight per day was established.
Studies on the precursors of benzoic acid support the notion that
benzoic acid is unlikely to have adverse reproductive effects at dose
levels not toxic to the mother.
The acute toxicity of benzoic acid and sodium benzoate in humans
is low. However, both substances are known to cause contact dermatitis
(pseudoallergy). In patients with urticaria or asthma, an exacerbation
of the symptoms was observed after testing (oral provocation test or
patch tests), whereas this effect is unusual in healthy subjects.
11.1.2 Criteria for setting tolerable intakes or guidance values for
benzoic acid and sodium benzoate
As given in section 11.1.1, the database is insufficient for
deriving NO(A)EL values for oral uptake. If the provisional NO(A)EL of
about 500 mg/kg body weight per day is applied, and by incorporating
an uncertainty factor of 100 (10 for uncertainty of the database, 10
for interspecies variation), a provisional tolerable intake would be
5 mg/kg body weight per day.
Applying this tolerable intake, one has to keep in mind that
benzoates at lower doses can cause non-immunological contact reactions
(pseudoallergy) in sensitive persons.
There are also no studies available concerning longer-term
exposure by inhalation, and the only short-term inhalation toxicity
study is not adequate for confidently establishing a NO(A)EC.
Therefore, a tolerable concentration for exposure by inhalation cannot
be calculated.
11.1.3 Sample risk characterization
As given in section 6.2, workers may be exposed to benzoic acid
or sodium benzoate via inhalation or skin contact during production
and processing. However, owing to the lack of information on specific
working operations and conditions (e.g., duration of exposure)
involved, it is impossible to derive a realistic estimate of
occupational exposure.
For the general population, the main route of exposure to benzoic
acid and sodium benzoate is likely via foodstuffs, which contain the
substances naturally or added as antimicrobial agents. As given in
section 6.2, the uptake depends on the individual's choice of food to
be consumed and the limit values for benzoates in different countries.
Therefore, considerable deviations may occur. Recent intake
estimations from surveys from several countries gave mean values in
the range of 0.18-2.3 mg/kg body weight. Only in high consumers was an
intake of up to 14 mg/kg body weight calculated. Benzoates have not
been detected in drinking-water. As given in section 6.1, the
inhalative uptake via ambient or indoor air may contribute only
marginally to exposure of the general population.
For normal consumers, the uptake of benzoates is about 2-28 times
lower than the provisional tolerable intake of 5 mg/kg body weight
day, and only in high consumers would this value be exceeded 3 times.
Additional information is required in order to evaluate whether
sodium benzoate has a possible genotoxic activity.
11.2 Evaluation of environmental effects
Significant releases of benzoic acid and sodium benzoate into the
environment are primarily into water and soil from their uses as
preservatives in food, mouthwashes, dentifrices, and cosmetics.
Benzoic acid occurs naturally in many plants.
From their physical/chemical properties, benzoic acid and sodium
benzoate are not expected to volatilize from water and soil to the
atmosphere or to adsorb to sediment or soil particles. The main
elimination pathway for both chemicals should be biotic
mineralization. Because of their ready biodegradability and their low
volatility, both substances are not considered to contribute directly
to the depletion of the stratospheric ozone layer or to global
warming. From experimental data on bioconcentration, a low to moderate
potential for bioaccumulation is to be expected.
Benzoic acid and sodium benzoate exhibited low to moderate
toxicity to aquatic organisms. The lowest reported EC50 value of
9 mg/litre was determined in a chronic study (14 days) for cell
multiplication inhibition by benzoic acid in the cyanobacterium
Anabaena inaequalis. EC50/LC50 values for the other aquatic
species tested were in the range of 17-1291 mg/litre. Exposure levels
of benzoic acid and benzoate in water have been determined only in
rain and snow, groundwater, and leachate in the vicinity of point
sources. Thus, a quantitative risk characterization with respect to
aquatic organisms in surface waters could not be performed. Taking
into account the rapid biodegradability, the low to moderate
bioaccumulation potential, the low toxicity to most aquatic species,
and the rapid metabolism of these substances, benzoic acid and sodium
benzoate will -- with the exception of accidental spills -- pose only
a minimal risk to aquatic organisms.
The few available data from the antimicrobial action of benzoic
acid and sodium benzoate indicate only a low toxicity potential of
both substances in the terrestrial compartment. Due to the lack of
measured exposure levels, a sample risk characterization with respect
to terrestrial organisms could not be performed.
12. PREVIOUS EVALUATIONS BY INTERNATIONAL BODIES
JECFA (WHO, 1996) has allocated an acceptable daily intake (ADI)
for benzoic acid and sodium benzoate of 0-5 mg/kg body weight.
REFERENCES
Abe S, Sasaki M (1977) Chromosome aberrations and sister chromatid
exchanges in Chinese hamster cells exposed to various chemicals.
Journal of the National Cancer Institute, 58(6):1635-1641.
Abe S, Tsutsui Y, Tasrumoto Y, Nakane S (1984) Studies on the toxicity
of oxaprozin. 1. Acute toxicity of oxaprozin, its metabolites and
contaminants. Iyakuhin Kenkyu, 15(3):359-370.
Aberer W, Kager B, Ziegler V, Horak F (1992) Schnupfen durch
Schneiderkreide -- Allergie, Pseudoallergie, Rhinopathie oder
Einbildung? Dermatosen, 40(6):231-234.
Aguirre A, Izu R, Gardeazabal J, Diaz-Perez JL (1993) Edematous
allergic contact cheilitis from a toothpaste. Contact dermatitis,
28:42.
Andersen KE, Maibach HI, Anjo MD (1980) The guinea-pig: an animal
model for human skin absorption of hydrocortisone, testosterone and
benzoic acid? British journal of dermatology, 102:447-453.
Anderson JA (1996) Allergic reactions to food. Critical reviews in
food science and nutrition, 36:S19-S38.
AOAC (1990) Official methods of analysis, 15th ed. Arlington, VA,
Association of Official Analytical Chemists, pp. 1142-1145.
Arens M, Gertz C (1990) Bestimmung der Konservierungsstoffe -
Gemeinsschaftsarbeit der DGF, 110. Mitteilung: Deutsche
Einheitsmethoden zur Untersuchung von Fetten, Fettprodukten, Tensiden
und verwandten Stoffen, 83.ü Mitt.: Analyse von fettreichen
Lebensmitteln IV. Fat science and technology, 92(3):107-109.
BAGS (1995) Standards zur Expositionsabschätzung. Bericht des
Ausschusses für Umwelthygiene (Arbeitsgemeinschaft der leitenden
Medizinalbeamtinnen und -beamten der Länder). Hamburg, Behörde für
Arbeit, Gesundheit und Soziales.
Baldwin EA, Nisperos-Carriedo MO, Baker RA (1995) Use of edible
coatings to preserve quality of lightly (and slightly) processed
products. Critical reviews in food science and nutrition,
35(6):509-524.
Barker JF, Barbash JE, Labonte M (1988) Groundwater contamination at a
landfill sited on fractured carbonate and shale. Journal of
contaminant hydrology, 3:1-25.
Batshaw ML, Brusilow SW (1981) Evidence of lack of toxicity of sodium
phenylacetate and sodium benzoate in treating urea cycle
enzymopathies. Journal of inherited metabolic disease, 4:231.
Batshaw ML, Monahan PS (1987) Treatment of urea cycle disorders.
Enzyme, 38:242-250.
Battersby NS, Wilson V (1989) Survey of the anaerobic biodegradation
potential of organic chemicals in digesting sludge. Applied and
environmental microbiology, 55(2):433-439.
Bayer AG (1977) Akute orale Toxizität / Untersuchung zur Haut- und
Schleimhautverträglichkeit. Wuppertal (unpublished report).
Bayer AG (1978) Untersuchung zur Haut- und
Schleimhautverträglichkeit. Wuppertal (unpublished report).
Bayer AG (1986) Benzoesäure DAB 8. Prüfung auf primär reizende/
ätzende Wirkung am Kaninchenauge. Wuppertal (unpublished report).
Bedford PGC, Clarke EGC (1972) Experimental benzoic acid poisoning in
the cat. Veterinary record, 90:53-58.
Bennett MC, Petrus DR (1977) Quantitative determination of sorbic acid
and sodium benzoate in citrus juice. Journal of food science,
42:1220-1221.
BFGoodrich Kalama Inc. (1999) Benzoic acid. Product information
bulletin. Kalama, WA.
Bindslev-Jensen C (1998) ABC of allergies. Food allergy. British
medical journal, 316:1299-1302.
Bio-Fax (1973) Benzoic acid. Northbrook, IL, Industrial Bio-Test
Laboratories, Inc.
Birch RR, Biver C, Campagna R, Gledhill WE, Pagga U, Steber J, Reust
H, Bontinck WJ (1989) Screening of chemicals for anaerobic
biodegradability. Chemosphere, 19(10-11):1527-1550.
BMA (British Medical Association) (1998) Antifungal preparations --
Benzoic acid. Wallingford, Pharmaceutical Press (British National
Formulary 507).
Brasch J, Henseler T, Frosch P (1993) Patch test reactions to a
preliminary preservative series. Dermatosen, 41(2):71-76.
Bridges JW, French MR, Smith RL, Williams RT (1970) The fate of
benzoic acid in various species. Biochemical journal, 118:47-51.
Bringmann G, Kuehn R (1977) [Threshold values for the damaging action
of water pollutants to bacteria (Pseudomonas putida) and green algae
(Scenedesmus quadricauda) in the cell multiplication inhibition
test.] Zeitschrift für Wasser und Abwasser Forschung, 10:87-98 (in
German).
Bringmann G, Kuehn R (1980) Bestimmung der biologischen Schadwirkung
wassergefährdender Stoffe gegen Protozoen. II. Bakterienfressende
Ciliaten. Zeitschrift für Wasser und Abwasser Forschung, 1:26-31.
Bringmann G, Kuehn R (1982) [Results of toxic action of water
pollutants on Daphnia magna Straus tested by an improved
standardized procedure.] Zeitschrift für Wasser und Abwasser
Forschung, 15:1-6 (in German).
Broeckx W, Blondeel A, Do-Go A, Achten G (1987) Cosmetic intolerance.
Contact dermatitis, 16:189-194.
Bronaugh RL, Stewart RF, Congdon ER, Giles AL (1982a) Methods for in
vitro percutaneous absorption studies. I. Comparison with in vivo
results. Toxicology and applied pharmacology, 62:474-480.
Bronaugh RL, Stewart RF, Congdon ER (1982b) Methods for in vitro
percutaneous absorption studies. II. Animal methods for human skin.
Toxicology and applied pharmacology, 62:481-488.
BUA (1995) BUA-Stoffbericht Benzoesaeure, Natriumbenzoat.
Beratergremium für Umweltrelevante Altstoffe. Stuttgart, S. Hirzel
Verlag (Stoffbericht Nr. 145).
Bucks DA, Hinz RS, Sarason R, Maibach HI, Guy RH (1990) In vivo
percutaneous absorption of chemicals: a multiple dose study in rhesus
monkeys. Food and chemical toxicology, 28:129-132.
Budavari S, O'Neil MJ, Smith A, Heckelman PE, Kinneary JF, eds. (1996)
The Merck index -- an encyclopedia of chemicals, drugs, and
biologicals, 12th ed. Whitehouse Station, NJ, Merck & Co., Inc.
p. 183.
Carlberg GE, Drangsholt H, Gjoes N (1986) Identification of
chlorinated compounds in the spent chlorination liquor from
differently treated sulphite pulps with special emphasis on mutagenic
compounds. Science of the total environment, 48:157-167.
Carver MP, Riviere JE (1989) Percutaneous absorption and excretion of
xenobiotics after topical and intravenous administration to pigs.
Fundamental and applied toxicology, 13:714-722.
Castro JC, Delgado MA, Sánchez MJ, Montelongo FG (1992) Simultaneous
2nd order derivative spectrophotometric determination of sorbic and
benzoic acids in soft drinks. Analytical letters, 25(12):2357-2376.
Cautreels W, van Cauwenberghe K (1978) Experiments on the distribution
of organic pollutants between airborne particulate matter and the
corresponding gas phase. Atmospheric environment, 12:1133-1141.
Chipley JR (1983) Sodium benzoate and benzoic acid. In: Branen AL,
Davidson PM, eds. Antimicrobials in foods. New York, NY, M. Decker,
pp. 11-35.
Chittenden RH, Long JH, Herter CA (1909) Chemical bulletin, 88. US
Department of Agriculture [cited in WHO, 1996].
Clemmensen O, Hjorth N (1982) Perioral contact urticaria from sorbic
and benzoic acid in salad dressings. Contact dermatitis, 8:1-6.
Cordt T, Kußmaul H (1990) Niedermolekulare Carbonsäuren imBoden, in
der ungesättigten Zone und im Grundwasser. Vom wasser, 74:287-298.
Courtes R, Bahlaoui A, Rambaud A, Deschamps F, Sunde E, Dutrieux E
(1995) Ready biodegradability test in seawater: a new methodological
approach. Ecotoxicology and environmental safety, 31:142-148.
Coverly J, Peters L, Whittle E, Basketter DA (1998) Susceptibility to
skin stinging, non-immunologic contact urticaria and acute skin
irritation; is there a relationship? Contact dermatitis,
38(2):90-95.
Daston GP, Baines D, Elmore E, Fitzgerald MP, Sharma S (1995)
Evaluation of chick embryo neural retina cell culture as a screen for
developmental toxicants. Fundamental and applied toxicology,
26:203-210.
Dawson DA, Schultz TW, Hunter RS (1996) Developmental toxicity of
carboxylic acids to Xenopus embryos: A quantitative
structure-activity relationship and computer-automated structure
evaluation. Teratogenesis, carcinogenesis, and mutagenesis,
16:109-124.
Deuel HJJ, Alfin-Slater R, Weil CS, Smyth HF Jr (1954) Sorbic acid as
a fungistatic agent for foods. 1. Harmlessness of sorbic acid as a
dietary component. Food research, 19:1-12.
Dierickx PJ (1998) Increased cytotoxic sensitivity of cultured FHM
fish cells by simultaneous treatment with sodium dodecyl sulfate and
buthionine sulfoximine. Chemosphere, 36(6):1263-1274.
EC (1995) European Union Directive 95/2/CE from 20.02.1995 on food
additives, colourants and sweeteners. European Commission.
Elder DJE, Kelly DJ (1994) The bacterial degradation of benzoic acid
and benzenoid compounds under anaerobic conditions: Unifying trends
and new perspectives. FEMS microbiology reviews, 13:441-468.
Ewell WS, Gorsuch JW, Kringle RO, Robillard KA, Spiegel RC (1986)
Simultaneous evaluation of the acute effects of chemicals on seven
aquatic species. Environmental toxicology and chemistry, 5:831-840.
Fanelli GM, Halliday SL (1963) Relative toxicity of chlortetracycline
and sodium benzoate after oral administration to rats. Archives
internationales de pharmacodynamie et de thérapie, 144(1-2):120-125.
Federle TW (1988) Mineralization of monosubstituted aromatic compounds
in unsaturated and saturated subsurface soils. Canadian journal of
microbiology, 34:1037-1042.
Feillet F, Leonard JV (1998) Alternative pathway therapy for urea
cycle disorders. Journal of inherited metabolic disease, Suppl.
21(1):101-111.
Feldmann RJ, Maibach HI (1970) Absorption of some organic compounds
through the skin in man. Journal of investigative dermatology,
54:399-404.
Flood PF, Abrams SR, Muir GD, Rowell JE (1989) Odor of the muskox. A
preliminary investigation. Journal of chemical ecology,
15:2207-2217.
Franz TJ (1975) Percutaneous absorption. On the relevance of in vitro
data. Journal of investigative dermatology, 64:190-195.
Freedman BJ (1977) Asthma induced by sulphur dioxide, benzoate and
tartrazine contained in orange drinks. Clinical allergy, 7:407-415.
Freitag D, Ballhorn L, Geyer H, Korte F (1985) Environmental hazard of
organic chemicals. An experimental method for the assessment of the
behaviour of organic chemicals in the ecosphere by means of simple
laboratory tests with 14C labelled chemicals. Chemosphere,
14:1589-1616.
Friedman LJ, Greenwald CG (1994) Food additives. In: Kroschwitz J,
Howe-Grant M, eds. Kirk-Othmer: Encyclopedia of chemical technology.
Vol. 11. New York, NY, Wiley and Sons, pp. 805-833.
Fuchs G, el Said Mohammed M, Altenschmidt U, Koch J, Lack A, Brackmann
R, Lochmeyer C, Oswald B (1993) Biochemistry of anaerobic
biodegradation of aromatic compounds. In: Ratledge C, ed.
Biochemistry of microbial degradation. Dordrecht, Kluwer Academic
Publishers, pp. 513-553.
Fujii T, Omori T, Tagucji T, Ogata M (1991) Urinary excretion of
hippuric acid after administration of sodium benzoate (biological
monitoring 1). Shokuhin Eiseigaku Zasshi (Journal of the Food Hygiene
Society of Japan), 32(3):177-182.
Fujitani T (1993) Short-term effect of sodium benzoate in F344 rats
and B6C3F1 mice. Toxicology letters, 69:171-179.
Gad SC, Dunn BJ, Dobbs DW, Reilly C, Walsh RD (1986) Development and
validation of an alternative dermal sensitization test: the mouse ear
swelling test (MEST). Toxicology and applied pharmacology,
84:93-114.
Gailhofer G, Soyer HP, Ludvan M (1990) Nahrungsmittelallergien und
Pseudoallergien -- Mechanismen, Klinik und Diagnostik. Wiener
Medizinische Wochenschrift, 140:227-232.
Geiger DL, Northcott CE, Call DJ, Brooke LT (1985) Acute toxicities
of organic chemicals to fathead minnows (Pimephales promelas ).
Vol. II. Superior, WI, University of Wisconsin, pp. 139-140.
Gerberick GF, House RV, Fletcher ER, Ryan CA (1992) Examination of the
local lymph node assay for use in contact sensitization risk
assessment. Fundamental and applied toxicology, 19:438-445.
Gerlach V (1909) VII. Summary of the results. In: Physiological
activity of benzoic acid and sodium benzoate. Wiesbaden, Verlag von
Heinrich Staadt [cited in US FDA, 1972a].
Goerlitz DF, Troutman DE, Godsy EM, Franks BJ (1985) Migration of
wood-preserving chemicals in contaminated groundwater in a sand
aquifer at Pensacola, Florida. Environmental science and technology,
19(10):955-961.
Goodman Gilman A, Rall TW, Nies AS, Taylor P, eds. (1990) Goodman and
Gilman's the pharmacological basis of therapeutics, 8th ed. New York,
NY, Pergamon Press, p. 1179.
Goodwin BL (1976) Handbook of intermediary metabolism of aromatic
compounds. New York, NY, Wiley & Sons, pp. B6-B9.
Green TP, Marchessault RP, Freese DK (1983) Disposition of sodium
benzoate in newborn infants with hyperammonemia. Journal of
pediatrics, 102:785-790.
Guardiola J, Ventura J, Rivera J (1989) Occurrence of industrial
organic pollution in a groundwater supply: screening, monitoring and
evaluation of treatment processes. Water supply, 7:11-16.
Haider K, Jagnow G, Kohnen R, Lim SU (1974) Abbau chlorierter Benzole,
Phenole und Cyclohexan- Derivate durch Benzol und Phenol verwertende
Bakterien unter anaeroben Bedingungen. Archives of microbiology,
96:183-200.
Halvorson DO (1984) Determination of benzoic acid in air. American
Industrial Hygiene Association journal, 45(10):727-730.
Ham RK, Boyle WC, Engroff EC, Fero RL (1989) Determining the presence
of organic compounds in foundry waste leachates. Modern casting,
79:27-31.
Harborne JB (1983) Toxins of plant-fungal interactions. In: Keeler RF,
Tu AT, eds. Handbook of natural toxins. Vol. 1. Plant and fungal
toxins. New York, NY, Marcel Dekker, pp. 755-758.
Hardin BD, Schuler RL, Burg JR, Booth GM, Hazelden KP, MacKenzie KM,
Piccirillo VJ, Smith KN (1987) Evaluation of 60 chemicals in a
preliminary developmental toxicity test. Teratogenesis,
carcinogenesis, and mutagenesis, 7:29-48 [cited in WHO, 1996].
Harwood CS, Gibson J (1997) Shedding light on anaerobic benzene ring
degradation: a process unique to prokaryotes? Journal of
bacteriology, 179:301-309.
Hegnauer R, ed. (1966) Chemotaxonomie der Pflanzen. Basel,
Birkhäuser Verlag.
Hegnauer R, ed. (1989) Chemotaxonomie der Pflanzen. Basel,
Birkhäuser Verlag, pp. 415-416.
Hegnauer R (1992) Benzoesäure. In: Hegnauer R, ed. Chemotaxonomie der
Pflanzen. Basel, Birkhäuser Verlag.
Helmig D, Müller J, Klein W (1989) Volatile organic substances in a
forest atmosphere. Chemosphere, 19(8-9):1399-1412.
Hofrichter M, Fritsche W (1996) Abbau aromatischer Kohlenwasserstoffe
durch den Schimmelpilz Penicillium frequentans Bi 7/2. Gas- und
Wasserfach; Wasser-Abwasser, 137:199-204.
Hotchkiss SA, Hewitt P, Caldwell J, Chen WL, Rowe RR (1992)
Percutaneous absorption of nicotinic acid, phenol, benzoic acid and
triclopyr butoxyethyl ester through rat and human skin in vitro:
Further validation of an in vitro model by comparison with in vivo
data. Food and chemical toxicology, 30:891-899.
Hunziker N, Feldmann RJ, Maibach H (1978) Animal models of
percutaneous penetration: comparison between Mexican hairless dogs and
man. Dermatologica, 156:79-88.
Ibero M, Eseverri JL, Barroso C, Botey J (1982) Dyes, preservatives
and salicylates in the induction of food intolerance and/or
hypersensitivity. Allergologia et Immunopathologia, 10(4):263-268.
IPCS (1993) International Chemical Safety Card -- Benzoic acid.
Geneva, World Health Organization, International Programme on
Chemical Safety (ICSC 0103).
Ishida H (1996) Levels of preservatives in toothpastes and possibility
of their intake during brushing of teeth. Shokuhin Eiseigaku Zasshi
(Journal of the Food Hygiene Society of Japan), 37:234-239.
Ishidate M, Odashima S (1977) Chromosome tests with 134 compounds on
Chinese hamster cells in vitro -- a screening for chemical
carcinogenesis. Mutation research, 48:337-354.
Ishidate MJ, Sofuni T, Yoshikawa K, Hayashi M, Nohmi T, Sawada M,
Matsuoka A (1984) Primary mutagenicity screening of food additives
currently used in Japan. Food and chemical toxicology,
22(8):623-636.
Ishidate MJ, Harnois MC, Sofuni T (1988) A comparative analysis of
data on the clastogenicity of 951 chemical substances tested in
mammalian cell cultures. Mutation research, 195:151-213.
Ishiguro S, Miyamoto A, Obi T, Nishio A (1993) Teratological studies
on benzyl acetate in pregnant rats. Kadnau (Bulletin of the Faculty
of Agriculture, Kagoshima University), 43:25-31 [cited in WHO, 1996].
Ishizaki M, Ueno S (1989) The DNA damaging activity of natural and
synthetic food additives. Shokuhin Eiseigaku Zasshi (Journal of the
Food Hygiene Society of Japan), 30:447-451.
Jalal MAF, Read DJ (1983) The organic acid composition of Calluna
heathland soil with special reference to phyto- and fungitoxicity. I.
Isolation and identification of organic acids. Plant and soil,
70:257-272.
James MO, Pritchard JB (1987) In vivo and in vitro metabolism and
excretion of benzoic acid by a marine teleost, the southern flounder.
Drug metabolism and disposition, 15:665-670.
Jansson T, Curvall M, Hedin A, Enzell CR (1988) In vitro studies of
the biological effects of cigarette smoke condensate. III. Induction
of SCE by some phenolic and related constituents derived from
cigarette smoke. Mutation research, 206:17-24.
Jelinek R, Peterka M, Rychter Z (1985) Chick embryotoxicity screening
test -- 130 substances tested. Indian journal of experimental
biology, 23:588-595.
Juhlin L, Michaelsson G, Zetterström O (1972) Urticaria and asthma
induced by food and drug additives in patients with aspirin
hypersensitivity. Journal of allergy and clinical immunology,
50(2):92-98.
Juhnke I, Luedemann D (1978) Ergebnisse der Untersuchung von
chemischen Verbindungen auf akute Fischtoxizität mit dem
Goldorfentest. Zeitschrift für Wasser und Abwasser Forschung,
11(5):161-164.
Kaiser KLE, Palabrica VS, Ribo JM (1987) QSAR of acute toxicity of
mono-substituted benzene derivatives to Photobacterium phosphoreum.
QSAR environmental toxicology, 11:153-168.
Kameya T, Murayama T, Urano K, Kitano M (1995) Biodegradation ranks of
priority organic compounds under anaerobic conditions. Science of the
total environment, 170(1-2):43-51.
Kawamura K, Kaplan IR (1986) Biogenic and anthropogenic organic
compounds in rain and snow samples collected in Southern California.
Atmospheric environment, 20:115-124.
Kawamura K, Kaplan IR (1990) Stabilities of carboxylic acids and
phenols in Los Angeles rainwaters during storage. Water research,
24(11):1419-1423.
Kawamura K, Ng LL, Kaplan IR (1985) Determination of organic acids
(C1-C10) in the atmosphere, motor exhausts, and engine oils. IPCS
guidelines for the monitoring of genotoxic effects of carcinogens in
humans. Environmental science and technology, 19:1082-1086.
Kay JH, Calandra JC (1962) Interpretation of eye irritation tests.
Journal of the Society of Cosmetic Chemists, 13:281-289.
Kieckebusch W, Lang K (1960) Die Verträglichkeit der Benzoesäure im
chronischen Fütterungsversuch. Arzneimittel-Forschung, 10:1001-1003.
Kimmel CA, Wilson JG, Schumacher HJ (1971) Studies on metabolism and
identification of the causative agent in aspirin teratogenesis in
rats. Teratology, 4:15-24.
King ADJ, Halbrook WU (1987) Ascospore heat resistance and control
measures for Talaromyces flavus isolated from fruit juice
concentrate. Journal of food science, 52:1252-1254.
King EF, Painter HA (1983) Ring-test programme 1981-82. Assessment of
biodegradability of chemicals in water by manometric respirometry.
Luxemburg, Commission of the European Communities (EUR 8631 EN 31 S).
Kinney IC, Ivanuski VR (1969) Photolysis mechanisms for pollution
abatement. Cincinnati, OH, US Department of the Interior, Federal
Water Pollution Control Administration, Robert A. Taft Water Research
Center (TWRC-13).
Klecka GM, Landi LP, Bo KM (1985) Evaluation of the OECD activated
sludge, respiration inhibition test. Chemosphere, 14(9):1239-1251.
Kobayashi T, Hashinaga T, Mikami E, Suzuki T (1989) Methanogenic
degradation of phenol and benzoate in acclimated sludges. Water
science and technology, 21:55-65.
Kocwa-Haluch R, Lemek M (1995) Easy and inexpensive diffusion tests
for detecting the assimilation of aromatic compounds by yeast-like
fungi. Part II. Assimilation of aromatic acids. Chemosphere,
31(11/12):4333-4339.
Koda T, Tsuchiya H, Yamauchi M, Hoshino Y, Takagi N, Kawano J (1989)
High-performance liquid chromatographic estimation of eluates from
denture base polymers. Journal of dentistry, 17:84-89.
Koda T, Tsuchiya H, Yamaguchi M, Ohtani S, Takagi N, Kawano J (1990)
Leachability of denture-base acrylic resins in artificial saliva.
Dental materials, 6:13-16.
Kreis H, Frese F, Wilmes G (1967) Physiologische und morphologische
Veränderungen an Ratten nach peroraler Verabreichung von Benzoesäure.
Food and cosmetics toxicology, 5:505-511.
Kubota K, Ishizaki T (1991) Dose-dependent pharmacokinetics of benzoic
acid following oral administration of sodium benzoate to humans.
European journal of clinical pharmacology,41(4):363-368.
Kubota K, Horai Y, Kushida K, Ishizaki T (1988) Determination of
benzoic acid and hippuric acid in human plasma and urine by high
performance liquid chromatography. Journal of chromatography,
425(1):67-75.
Lahti A, Hannuksela M (1981) Is benzoic acid really harmful in cases
of atopy and urticaria? Lancet, 2:1055.
Lahti A, Maibach HI (1984) An animal model for nonimmunologic contact
urticaria. Toxicology and applied pharmacology, 76:219-224.
Lahti A, Pylvanen V, Hannuksela M (1995) Immediate irritant reactions
of benzoic acid are enhanced in washed skin areas. Contact
dermatitis, 33:177-182.
Larmi E, Lahti A, Hannuksela M (1988) Effects of sorbitan-sesquioleate
on non-immunologic immediate contact reactions to benzoic acid.
Contact dermatitis, 19:368-371.
Larsson B (1983) Gas-liquid chromatographic determination of benzoic
acid and sorbic acid in foods: MNKL collaborative study (1983).
Journal of the Association of Official Analytical Chemists,
66:775-780.
Lindström K, Österberg F (1986) Chlorinated carboxylic acids in
softwood kraft pulp spent bleach liquors. Environmental science and
technology, 20(2):133-138.
Lu PY, Metcalf RL (1975) Environmental fate and biodegradability of
benzene derivatives as studied in a model aquatic ecosystem.
Environmental health perspectives, 10:269-284.
Lund FA, Rodriguez DS (1984) Acclimation of activated sludge to
mono-substituted derivatives of phenol and benzoic acid. Journal of
general applied microbiology, 30:53-61.
Lunde G, Gehter J, Gjos N, Lande MBS (1977) Organic micropollutants in
precipitation in Norway. Atmospheric environment, 11:1007-1014.
MacPherson SE, Barton CN, Bronaugh RL (1996) Use of in vitro skin
penetration data and a physiologically based model to predict in vivo
blood levels of benzoic acid. Toxicology and applied pharmacology,
140:436-443.
Maibach HI, Johnson HL (1975) Contact urticaria syndrome. Archives of
dermatology, 111:726-730.
Maibach HI, Wester RC (1989) Percutaneous absorption: in vivo
methods in humans and animals. Journal of the American College of
Toxicology, 8:803-813.
Mailhot H (1987) Prediction of algal bioaccumulation and uptake rate
of nine organic compounds by ten physicochemical properties.
Environmental science and technology, 21:1009-1013.
Maki T, Suzuki Y (1985) Benzoic acid and derivatives. In: Ullmann's
encyclopedia of industrial chemistry. Vol. A3. Weinheim, VCH
Verlagsgesellschaft mbH, pp. 555-568.
Mandrou B, Nolleau V, Gastaldi E, Fabre H (1998) Solid-phase
extraction as a clean-up procedure for the liquid chromatographic
determination of benzoic acid and sorbic acid in fruit-derived
products. Journal of liquid chromatography and related technology,
21:829-842.
Marquardt P (1960) Zur Verträglichkeit der Benzoesäure.
Arzneimittel-Forschung, 10:1033.
Matthews RW (1990) Purification of water with near-U.V. illuminated
suspensions of titanium dioxide. Water research, 24:653-660.
McCann J, Choi E, Yamasaki E, Ames BN (1975) Detection of carcinogens
as mutagens in the Salmonella/microsome test: assay of
300 chemicals. Proceedings of the National Academy of Sciences
of the United States of America, 72(12):5135-5139.
McCormick GC (1974) A comparison of the acute toxicity, distribution,
fate, and some pharmacologic properties of the non-benzenoid aromatic
compound azuloic acid with those of benzoic and naphthoic acids in
mice. Dissertation abstracts international, B35(10):5029B-5030B.
McKenna KE, Walsh MY, Burrows D (1994) The Melkersson-Rosenthal
syndrome and food additive hypersensitivity. British journal of
dermatology, 131:921-922.
Miguez CB, Greer CW, Ingram JM, MacLeod RA (1995) Uptake of benzoic
acid and chloro-substituted benzoic acids by Alcaligenes
denitrificans BRI 3010 and BRI 6011. Applied and environmental
microbiology, 61:4152-4159.
Minor JL, Becker BA (1971) A comparison of the teratogenic properties
of sodium salicylate, sodium benzoate, and phenol. Toxicology and
applied pharmacology, 19:373.
MITI (1992) Biodegradation and bioaccumulation. Data of existing
chemicals based on the CSCL Japan. Tokyo, Ministry of International
Trade and Industry, Chemicals Inspection & Testing Institute.
Moneret-Vautrin DA, Moeller R, Malingrey L, Laxenaire MC (1982)
Anaphylactoid reaction to general anaesthesia: A case of intolerance
to sodium benzoate. Anaesthesia and intensive care, 10:156-157.
Monsanto Co. (1983) Primary eye irritation of benzoic acid to
rabbits. St. Louis, MO, Monsanto Company, Environmental Health
Laboratory.
Munoz FJ, Bellido J, Moyano JC, Alvarez M, Fonseca JL (1996) Perioral
contact urticaria from sodium benzoate in a toothpaste. Contact
dermatitis, 35:51.
Nakamura SI, Oda Y, Shimada T, Oki I, Sugimoto K (1987) SOS-inducing
activity of chemical carcinogens and mutagens in Salmonella
typhimurium TA1535/pSK1002: examination with 151 chemicals. Mutation
research, 192:239-246.
Nonaka M (1989) DNA repair tests on food additives. Environmental and
molecular mutagenesis, 14 (Suppl. 15):143.
Nottingham PM, Hungate RE (1969) Methanogenic fermentation of
benzoate. Journal of bacteriology, 98:1170-1172.
O'Connor JE, Costell M, Grisolia S (1987) The potentiation of ammonia
toxicity by sodium benzoate is prevented by L-carnithine. Biochemical
and biophysical research communications, 145:817-824.
Oikawa A, Tohda H, Kanai M, Miwa M, Sugimura T (1980) Inhibitors of
poly(adenosine diphosphate ribose) induced sister chromatid exchanges.
Biochemical and biophysical research communications,
97(4):1311-1316.
Onodera H, Ogiu T, Matsuoka C, Furuta K, Takeuchi M, Oono Y, Kubota T,
Miyahara M, Maekawa A, Odashima S (1978) [Studies on effects of sodium
benzoate on fetuses and offspring of Wistar rats.] Eisei Shikensho
Hokoku, 96:47-55 (in Japanese) [cited in WHO, 1996].
Osterballe O, Taudorf E, Haahr J (1979) Intolerance to aspirin,
food-colouring agents and food preservatives in childhood asthma.
Ugeskrift for Laeger, 141:1908-1910.
Oussi D, Mokrini A, Chamarro E, Esplugas S (1998) Photodegradation of
benzoic acid in aqueous solutions. Environmental technology,
19(9):955-960.
Palm WU, Millet M, Zetsch C (1998) OH radical reactivity of pesticides
adsorbed on aerosol materials: first results of experiments with
filter samples. Ecotoxicology and environmental safety, 41(1):36-43.
Parke DV (1968) The biochemistry of foreign compounds. Oxford,
Pergamon Press, pp. 1-261.
Petrus M, Bonaz S, Causse E, Rhabbour M, Moulie N, Netter JC,
Bildstein G (1996) [Asthma induced by benzoate contained in some foods
and anti-allergic drugs.] Archives de pédiatrie, 3(10):984-987 (in
French).
Pitter P (1976) Determination of biological degradability of organic
substances. Water research, 10:231-235.
Prival MJ, Simmon VF, Mortelmans KE (1991) Bacterial mutagenicity
testing of 49 food ingredients gives very few positive results.
Mutation research, 260:321-329.
Puttemans ML, Dryon L, Massart DL (1984) Extraction of organic acids
by iron-pair formation with tri- n-octylamine. Part V. Simultaneous
determination of synthetic dyes, benzoic acid, sorbic acid and
saccharin in soft drinks and lemonade syrups. Journal of the
Association of Official Analytical Chemists, 67:880-885.
Rasmussen LEL, Hess DL, Haight JD (1990) Chemical analysis of temporal
gland secretions collected from an Asian bull elephant during a
four-month musth episode. Journal of chemical ecology,
16(7):2167-2181.
Rastogi SC, Lepoittevin JP, Johansen JD, Frosch PJ, Menné T, Bruze M,
Dreier B, Andersen KE, White IR (1998) Fragrances and other materials
in deodorants: Search for potentially sensitizing molecules using
combined GC-MS and structure activity relationship (SAR) analysis.
Contact dermatitis, 39(6):293-303.
RCC Notox (1988a) Primary skin irritation/corrosion study of benzoic
acid in the rabbit. DD's-Hertogenbosch, RCC Notox B.V. (unpublished
report).
RCC Notox (1988b) Eye irritation/corrosion study of benzoic acid in
the rabbit. DD's-Hertogenbosch, RCC Notox B.V. (unpublished report).
RCC Notox (n.d., a) Primary skin irritation/corrosion study with
natrium benzoate in rabbits. DD's-Hertogenbosch, RCC Notox B.V.
(unpublished report).
RCC Notox (n.d., b) Acute eye irritation/corrosion study with natrium
benzoate in rabbits. DD's-Hertogenbosch, RCC Notox B.V. (unpublished
report).
Reifenrath WG, Chellquist EM, Shipwash EA, Jederberg WW, Krueger GG
(1984) Percutaneous penetration in the hairless dog, weanling pig and
grafted athymic nude mouse: Evaluation of models for predicting skin
penetration in man. British journal of dermatology,
Suppl. 111(S27):123-135.
Reinhard M, Goodman NL (1984) Occurrence and distribution of organic
chemicals in two landfill leachate plumes. Environmental science and
technology, 18:953-961.
Richterich K, Steber J (1989) Prevention of nitrification-caused
erroneous biodegradability data in the closed bottle test.
Chemosphere, 19(10-11):1643-1654.
Ring J (1989) Arzneimittelunverträglichkeit durch pseudo-allergische
Reaktionen. Wiener Medizinische Wochenschrift, 139:130-134.
Rubin HE, Subba-Rao RV, Alexander M (1982) Rates of mineralization of
trace concentrations of aromatic compounds in lake water and sewage
samples. Applied and environmental microbiology, 43(5):1133-1138.
Russell AD, Furr JR (1996) Biocides mechanisms of antifungal action
and fungal resistance. Science progress, 79(1):27-48.
Sainio E, Kanerva L (1995) Contact allergens in toothpastes and a
review of their hypersensitivity. Contact dermatitis, 33:100-105.
Sakuma H, Kusama M, Munakata S, Ohsumi T, Sugawara S (1983) The
distribution of cigarette smoke components between mainstream and
sidestream smoke. Beitraege zur Tabakforschung, 12:63-71.
Salanitro JP, Langston GC, Dorn PB, Kravetz L (1988) Activated sludge
treatment of ethoxylate surfactants at high industrial use
concentrations. Water science and technology, 20(11-12):125-130.
Schelhorn M (1951) Untersuchungen über Konservierungsmittel. VI.
Wirksamkeit und Anwendungsbereich von Benzoesäure, Estern der
Paraoxybenzoesäure und Kombination dieser beiden Arten von
Verbindungen. Deutsche Lebensmittel-Rundschau, 47:128-134.
Scholz W, Kortmann W (1991) Paint additives. In: Elvers B, Hawkins S,
Schulz G, eds. Ullmann's encyclopedia of industrial chemistry.
Weinheim, VCH Verlagsgesellschaft, pp. 465-472.
Schou L, Krane JE (1981) Organic micropollutants in a Norwegian
water-course. Science of the total environment, 20:277-286.
Schuetzle D, Cronn D, Crittenden AL (1975) Molecular composition of
secondary aerosol and its possible origin. Environmental science and
technology, 9:838-845.
Schultz TW, Bryant SE, Kissel ST (1996) Toxicological assessment in
Tetrahymena of intermediates in aerobic microbial transformation of
toluene and p-xylene. Bulletin of environmental contamination and
toxicology, 56:129-134.
Sharak Genthner BR, Townsend GT, Blattman BO (1997) Reduction of
3-chlorobenzoate, 3-bromobenzoate, and benzoate to corresponding
alcohols by Desulfomicrobium escambiense, isolated from a
3-chlorobenzoate-dechlorinating coculture. Applied and environmental
microbiology, 63:4698-4703.
Shibamoto T (1986) Photochemistry of a major essential oil
constituent, benzyl benzoate. Developments in food science,
12:745-754.
Shibamoto T, Umano K (1985) Photochemical products of benzyl benzoate:
possible formation of skin allergens. Journal of toxicology,
cutaneous and ocular toxicology, 4(2):97-104.
Shimp RJ, Young RL (1987) Comparison of OECD and radiolabeled
substrate methods for measuring biodegradation in marine environments.
Ecotoxicology and environmental safety, 14:223-230.
Sieber R, Bütikofer U, Bosset JO, Rüegg M (1989) Benzoesäure als
natürlicher Bestandteil von Lebensmitteln- eine Übersicht.
Mitteilungen aus dem Gebiete der Lebensmitteluntersuchung und
Hygiene, 80:345-362.
Sieber R, Bütikofer U, Baumann E, Bosset JO (1990) Über das Vorkommen
der Benzoesäure in Sauermilchprodukten und Käse. Mitteilungen aus dem
Gebiete der Lebensmitteluntersuchung und Hygiene, 84:484-493.
Sioufi A, Pommier F (1980) Gas chromatographic determination of low
concentrations of benzoic acid in human plasma and urine. Journal of
chromatography, biomedical applications, 181:161-168.
Smyth HF, Carpenter CP (1948) Further experience with the range
finding test in the industrial toxicology laboratory. Journal of
industrial hygiene and toxicology, 30:63-68.
Sodemoto Y, Enomoto M (1980) Report of carcinogenesis bioassay of
sodium benzoate in rats: absence of carcinogenicity of sodium benzoate
in rats. Journal of environmental pathology and toxicology, 4:87-95.
Soni GL, Bhatia IS (1980) Toxicity of phenolics and related compounds
to Fusarium oxasporium Schlecht. and effect of pH on their
toxicity. Indian journal of agricultural science, 50(10):772-777.
SRI (1998) 1997/98 directory of chemical producers Europe. Menlo
Park, CA, SRI International (807-808/1613).
Srour R (1989) Benzoic acid. In: Srour R, ed. Aromatic intermediates
and derivatives. Paris, pp. A.IV.1-A.IV.14 (unpublished report)
[cited in BUA, 1995].
Srour R (1998) Benzoic acid and derivatives. In: Srour R, ed.
Aromatic intermediates and derivatives. Paris, pp. A.IV.1- A.IV.17
(unpublished report).
Steeg E, Montag A (1987) Nachweis aromatischer Carbonsäuren in Honig.
Zeitschrift für Lebensmittel-Untersuchung und -Forschung, 184:17-19.
Stolpe NB, McCallister DL, Shea PJ, Lewis DT, Dam R (1993) Mobility of
aniline, benzoic acid, and toluene in four soils and correlation with
soil properties. Environmental pollution, 81:287-295.
Stratton GW, Corke CT (1982) Toxicity of the insecticide permethrin
and some degradation products towards algave and cyanobacteria.
Environmental pollution, 29:71-80.
Stuermer DH, Ng DJ, Morris CJ (1982) Organic contaminants in
groundwater near underground coal gasification site in northeastern
Wyoming. Environmental science and technology, 16:582-587.
Suflita JM, Concannon F (1995) Screening tests for assessing the
anaerobic biodegradation of pollutant chemicals in subsurface
environments. Journal of microbiological methods, 21:267-281.
Takemoto S, Kuge Y, Nakamoto M (1981) The measurement of BOD in sea
water. Japanese journal of water pollution research, 4:80-90.
Tohda H, Horaguchi K, Takahashi K, Oikawa A, Matsushima T (1980)
Epstein-Barr virus-transformed human lymphoblastoid cells for study of
sister chromatid exchange and their evaluation as a test system.
Cancer research, 40:4775-4780.
Tong HY, Shore DL, Karasek FW, Helland P, Jellum E (1984)
Identification of organic compounds obtained from incineration of
municipal waste by high performance liquid chromatographic
fractionation and gas chromatography-mass spectrometry. Journal of
chromatography, 285:423-441.
Toth B (1984) Lack of tumorigenicity of sodium benzoate in mice.
Fundamental and applied toxicology, 4:494-496.
Toyoda M, Ito Y, Isshiki K, Onishi K, Kato T, Kamikura M, Shiroishi Y,
Harada Y, Hukasawa Y, Yokoyama T, Yoneda M, Iwaida M (1983a) Daily
intake of preservatives, benzoic acid, dehydroacetic acid, propionic
acid and their salts, and esters of p-hydrobenzoic acid in Japan.
Journal of the Japanese Society of Nutrition and Food Science,
96:467-480.
Toyoda M, Ito Y, Isshiki K, Onishi K, Kato T, Kamikura M, Shiroishi Y,
Harada Y, Hukasawa Y, Yokoyama Y, Yamamoto Y, Fujii M, Iwaida M
(1983b) Estimation of daily intake of many kinds of food additives
according to the market basket studies in Japan. Journal of the
Japanese Society of Nutrition and Food Science, 36:489-497.
Tremblay GC, Qureshi IA (1993) The biochemistry and toxicology of
benzoic acid metabolism and its relationship to the elimination of
waste nitrogen. Pharmacology and therapeutics, 60:63-90.
Tyler TA (1984) Liquid chromatographic determination of sodium
saccharin, caffeine, aspartame, and sodium benzoate in cola beverages.
Journal of the Association of Official Analytical Chemists,
67(4):745-747.
UK MAFF (1995) Survey of sulphur dioxide and benzoic acid in foods
and drinks. London, Ministry of Agriculture, Fisheries and Food,
Joint Food Safety and Standards Group (Food Surveillance Information
Sheet No. 65; http://maff.gov.uk/food/infsheet/1995/
no65/65sulben.htm).
Urano K, Kato Z (1986) Evaluation of biodegradation ranks of priority
organic compounds. Journal of hazardous materials,13:147-159.
US FDA (1972a) GRAS (Generally Recognized As Safe) food ingredients:
benzoic acid and sodium benzoate. Washington, DC, US Food and Drug
Administration.
US FDA (1972b) Teratologic evaluation of FDA 71-37 (sodium benzoate).
East Orange, NJ, US Food and Drug Administration, Food and Drug
Research Laboratory.
US FDA (1973) Evaluation of the health aspects of benzoic acid and
sodium benzoate as food ingredients. Bethesda, MD, US Food and Drug
Administration, Life Sciences Research Office (PB-223 837).
US FDA (1974) Mutagenic evaluation of compound FDA 71-37, sodium
benzoate. Prepared by Litton Bionetics, Inc., Kensington, MD, for
the US Food and Drug Administration (Unpublished report PB-245-453/6).
US NTP (1986) Toxicology and carcinogenesis studies of benzyl acetate
(CAS No. 