
INTERNATIONAL PROGRAMME ON CHEMICAL SAFETY
CONCISE INTERNATIONAL CHEMICAL ASSESSMENT DOCUMENT NO. 23
2,2-DICHLORO-1,1,1-TRIFLUOROETHANE (HCFC-123)
INTER-ORGANIZATION PROGRAMME FOR THE SOUND MANAGEMENT OF CHEMICALS
A cooperative agreement among UNEP, ILO, FAO, WHO, UNIDO, UNITAR and
OECD
This report contains the collective views of an international group of
experts and does not necessarily represent the decisions or the stated
policy of the United Nations Environment Programme, the International
Labour Organization, or the World Health Organization.
First draft prepared by
Dr S. Kristensen, Mr S. Batt, and Ms D. Willcocks, Existing Chemicals
Section, National Industrial Chemicals Notification and Assessment
Scheme, Australia, and Mr C. Lee-Steere, Environment Australia
Published under the joint sponsorship of the United Nations
Environment Programme, the International Labour Organization, and the
World Health Organization, and produced within the framework of the
Inter-Organization Programme for the Sound Management of Chemicals.
World Health Organization
Geneva, 2000
The International Programme on Chemical Safety (IPCS),
established in 1980, is a joint venture of the United Nations
Environment Programme (UNEP), the International Labour Organization
(ILO), and the World Health Organization (WHO). The overall objectives
of the IPCS are to establish the scientific basis for assessment of
the risk to human health and the environment from exposure to
chemicals, through international peer review processes, as a
prerequisite for the promotion of chemical safety, and to provide
technical assistance in strengthening national capacities for the
sound management of chemicals.
The Inter-Organization Programme for the Sound Management of
Chemicals (IOMC) was established in 1995 by UNEP, ILO, the Food and
Agriculture Organization of the United Nations, WHO, the United
Nations Industrial Development Organization, the United Nations
Institute for Training and Research, and the Organisation for Economic
Co-operation and Development (Participating Organizations), following
recommendations made by the 1992 UN Conference on Environment and
Development to strengthen cooperation and increase coordination in the
field of chemical safety. The purpose of the IOMC is to promote
coordination of the policies and activities pursued by the
Participating Organizations, jointly or separately, to achieve the
sound management of chemicals in relation to human health and the
environment.
WHO Library Cataloguing-in-Publication Data
2,2-Dichloro-1,1,1-trifluoroethane (HCFC 123).
(Concise international chemical assessment document ; 23)
1.Chlorofluorocarbons - toxicity 2.Risk assessment
3.Occupational exposure 4.Environmental exposure
I.Programme on Chemical Safety II.Series
ISBN 92 4 153023 5 (NLM Classification: QV 633)
ISSN 1020-6167
The World Health Organization welcomes requests for permission to
reproduce or translate its publications, in part or in full.
Applications and enquiries should be addressed to the Office of
Publications, World Health Organization, Geneva, Switzerland, which
will be glad to provide the latest information on any changes made to
the text, plans for new editions, and reprints and translations
already available.
(c) World Health Organization 2000
Publications of the World Health Organization enjoy copyright
protection in accordance with the provisions of Protocol 2 of the
Universal Copyright Convention. All rights reserved.
The designations employed and the presentation of the material in
this publication do not imply the expression of any opinion whatsoever
on the part of the Secretariat of the World Health Organization
concerning the legal status of any country, territory, city, or area
or of its authorities, or concerning the delimitation of its frontiers
or boundaries.
The mention of specific companies or of certain manufacturers'
products does not imply that they are endorsed or recommended by the
World Health Organization in preference to others of a similar nature
that are not mentioned. Errors and omissions excepted, the names of
proprietary products are distinguished by initial capital letters.
The Federal Ministry for the Environment, Nature Conservation and
Nuclear Safety, Germany, provided financial support for the printing
of this publication.
TABLE OF CONTENTS
FOREWORD
1. EXECUTIVE SUMMARY
2. IDENTITY AND PHYSICAL/CHEMICAL PROPERTIES
3. ANALYTICAL METHODS
4. SOURCES OF HUMAN AND ENVIRONMENTAL EXPOSURE
5. ENVIRONMENTAL TRANSPORT, DISTRIBUTION, AND TRANSFORMATION
6. ENVIRONMENTAL LEVELS AND HUMAN EXPOSURE
6.1. Environmental levels
6.2. Human exposure
7. COMPARATIVE KINETICS AND METABOLISM IN LABORATORY ANIMALS AND HUMANS
8. EFFECTS ON LABORATORY MAMMALS AND IN VITRO TEST SYSTEMS
8.1. Single exposure
8.2. Irritation and sensitization
8.3. Short-term exposure
8.4. Long-term exposure
8.4.1. Subchronic exposure
8.4.2. Chronic exposure and carcinogenicity
8.5. Genotoxicity and related end-points
8.6. Reproductive and developmental toxicity
9. EFFECTS ON HUMANS
10. EFFECTS ON OTHER ORGANISMS IN THE LABORATORY AND FIELD
11. EFFECTS EVALUATION
11.1. Evaluation of health effects
11.1.1. Hazard identification and dose-response assessment
11.1.2. Criteria for setting tolerable intakes or guidance values for HCFC-123
11.1.3. Sample risk characterization
11.2. Evaluation of environmental effects
12. PREVIOUS EVALUATIONS BY INTERNATIONAL BODIES
REFERENCES
APPENDIX 1 -- SOURCE DOCUMENTS
APPENDIX 2 -- CICAD PEER REVIEW
APPENDIX 3 -- CICAD FINAL REVIEW BOARD
APPENDIX 4 -- INTERNATIONAL CHEMICAL SAFETY CARD
RÉSUMÉ D'ORIENTATION
RESUMEN DE ORIENTACI²N
FOREWORD
Concise International Chemical Assessment Documents (CICADs) are
the latest in a family of publications from the International
Programme on Chemical Safety (IPCS) -- a cooperative programme of the
World Health Organization (WHO), the International Labour Organization
(ILO), and the United Nations Environment Programme (UNEP). CICADs
join the Environmental Health Criteria documents (EHCs) as
authoritative documents on the risk assessment of chemicals.
CICADs are concise documents that provide summaries of the
relevant scientific information concerning the potential effects of
chemicals upon human health and/or the environment. They are based on
selected national or regional evaluation documents or on existing
EHCs. Before acceptance for publication as CICADs by IPCS, these
documents undergo extensive peer review by internationally selected
experts to ensure their completeness, accuracy in the way in which the
original data are represented, and the validity of the conclusions
drawn.
The primary objective of CICADs is characterization of hazard and
dose-response from exposure to a chemical. CICADs are not a summary of
all available data on a particular chemical; rather, they include only
that information considered critical for characterization of the risk
posed by the chemical. The critical studies are, however, presented in
sufficient detail to support the conclusions drawn. For additional
information, the reader should consult the identified source documents
upon which the CICAD has been based.
Risks to human health and the environment will vary considerably
depending upon the type and extent of exposure. Responsible
authorities are strongly encouraged to characterize risk on the basis
of locally measured or predicted exposure scenarios. To assist the
reader, examples of exposure estimation and risk characterization are
provided in CICADs, whenever possible. These examples cannot be
considered as representing all possible exposure situations, but are
provided as guidance only. The reader is referred to EHC 1701 for
advice on the derivation of health-based tolerable intakes and
guidance values.
While every effort is made to ensure that CICADs represent the
current status of knowledge, new information is being developed
constantly. Unless otherwise stated, CICADs are based on a search of
the scientific literature to the date shown in the executive summary.
In the event that a reader becomes aware of new information that would
change the conclusions drawn in a CICAD, the reader is requested to
contact IPCS to inform it of the new information.
1 International Programme on Chemical Safety (1994) Assessing
human health risks of chemicals: derivation of guidance values for
health-based exposure limits. Geneva, World Health Organization
(Environmental Health Criteria 170).
Procedures
The flow chart shows the procedures followed to produce a CICAD.
These procedures are designed to take advantage of the expertise that
exists around the world -- expertise that is required to produce the
high-quality evaluations of toxicological, exposure, and other data
that are necessary for assessing risks to human health and/or the
environment.
The first draft is based on an existing national, regional, or
international review. Authors of the first draft are usually, but not
necessarily, from the institution that developed the original review.
A standard outline has been developed to encourage consistency in
form. The first draft undergoes primary review by IPCS to ensure that
it meets the specified criteria for CICADs.
The second stage involves international peer review by scientists
known for their particular expertise and by scientists selected from
an international roster compiled by IPCS through recommendations from
IPCS national Contact Points and from IPCS Participating Institutions.
Adequate time is allowed for the selected experts to undertake a
thorough review. Authors are required to take reviewers' comments into
account and revise their draft, if necessary. The resulting second
draft is submitted to a Final Review Board together with the
reviewers' comments.
The CICAD Final Review Board has several important functions:
- to ensure that each CICAD has been subjected to an appropriate
and thorough peer review;
- to verify that the peer reviewers' comments have been addressed
appropriately;
- to provide guidance to those responsible for the preparation of
CICADs on how to resolve any remaining issues if, in the opinion
of the Board, the author has not adequately addressed all
comments of the reviewers; and
- to approve CICADs as international assessments.
Board members serve in their personal capacity, not as representatives
of any organization, government, or industry. They are selected
because of their expertise in human and environmental toxicology or
because of their experience in the regulation of chemicals. Boards are
chosen according to the range of expertise required for a meeting and
the need for balanced geographic representation.
 Board members, authors, reviewers, consultants, and advisers who
participate in the preparation of a CICAD are required to declare any
real or potential conflict of interest in relation to the subjects
under discussion at any stage of the process. Representatives of
nongovernmental organizations may be invited to observe the
proceedings of the Final Review Board. Observers may participate in
Board discussions only at the invitation of the Chairperson, and they
may not participate in the final decision-making process.
1. EXECUTIVE SUMMARY
This CICAD was based principally on the assessments of the
occupational health and environmental effects of
2,2-dichloro-1,1,1-trifluoroethane (HCFC-123) completed under the
Australian National Industrial Chemicals Notification and Assessment
Scheme (NICNAS) and published in March 1996 (NICNAS, 1996) and July
1999 (NICNAS, 1999). Relevant information that has become available
since completion of the NICNAS reports or that was identified in a
comprehensive search of several on-line databases up to August 1999
has also been assessed and included in this CICAD. This CICAD is an
update of the review of HCFC-123 in the monograph Environmental Health
Criteria 139 (IPCS, 1992), prompted by the advent of new and
significant data. Information on the nature of the peer review and the
availability of the source documents is presented in Appendix 1.
Information on the peer review of this CICAD is presented in Appendix
2. The CICAD was approved for publication at a meeting of the Final
Review Board, held in Sydney, Australia, on 21-24 November 1999.
Participants at the Final Review Board meeting are listed in Appendix
3. The International Chemical Safety Card (ICSC 1343) for
2,2-dichloro-1,1,1-trifluoroethane, produced by the International
Programme on Chemical Safety, has been reproduced in Appendix 4 (IPCS,
1998).
HCFC-123 (CAS No. 306-83-2) is a synthetic, non-combustible,
volatile liquid that is used as a refrigerant in commercial and
industrial air-conditioning installations, in gaseous fire
extinguishants, as a foam-blowing agent, and in metal and electronics
cleaning. Its ozone-depleting potential is only 2% of that of CFC-11
(trichlorofluoromethane). It has a global warming potential of 300
over a 20-year time horizon relative to carbon dioxide. As such,
HCFC-123 is currently used as a transitional replacement for
chlorofluorocarbons and bromofluorocarbons phased out pursuant to the
1987 Montreal Protocol on Substances that Deplete the Ozone Layer. The
1992 Copenhagen Amendment to the Montreal Protocol requires that
HCFC-123 and other hydrochlorofluorocarbons be phased out by 2020.
Releases of HCFC-123 to the environment are primarily to ambient
air. Although slightly toxic to fish, Daphnia, and algae, HCFC-123
is unlikely to pose a significant hazard to the aquatic environment,
as it is not persistent in water, even at concentrations below the
solubility limit. In the atmosphere, HCFC-123 has an estimated
lifetime of less than 2 years. The main atmospheric breakdown product
of HCFC-123 (and other, more widely used fluorocarbons) is
trifluoroacetic acid, which will partition into aqueous phases in the
environment. Although trifluoroacetic acid is resistant to degradation
and may accumulate in certain closed aquatic systems, current and
predicted concentrations from HCFC-123 emissions are below toxic
thresholds.
Exposure of the general public to HCFC-123 is expected to be
minimal. However, there is the potential for occupational exposure
during the manufacture of HCFC-123 and the manufacture and use of
products containing the chemical.
Limited information is available on the effects of HCFC-123 on
humans. Cases of dizziness, headache, and nausea following a single
exposure to unknown levels of airborne HCFC-123 have been reported, as
well as cases of manifest or subclinical liver disease associated with
repeated occupational exposures to HCFC-123 vapours at 5-1125 ppm
(31.3-7030 mg/m3) for 1-4 months.
The acute toxicity of HCFC-123 in laboratory animals is low.
Inhalation for a few minutes to a few hours causes liver lesions in
guinea-pigs at 1000 ppm (6.25 g/m3), central nervous system (CNS)
depression in all species examined at 5000 ppm (31.3 g/m3), and
adrenaline-induced cardiac arrhythmia in dogs at 20 000 ppm (125
g/m3). In the rat and hamster, inhalation of more than 30 000 ppm
(188 g/m3) for 4 h causes severe CNS depression and death. HCFC-123
is not a skin irritant or sensitizer, but it can cause eye irritation
in liquid form. In repeated-exposure inhalation toxicity studies
lasting 2-39 weeks in rats, guinea-pigs, dogs, and monkeys, the main
target organs were the liver, the hypothalamic-pituitary-gonadal
endocrine system, and the CNS. The lowest-observed-adverse-effect
level (LOAEL) based on liver effects was 30 ppm (188 mg/m3). The
no-observed-adverse-effect level (NOAEL) was 100 ppm (625 mg/m3)
based on endocrine effects and 300 ppm (1880 mg/m3) based on CNS
effects. There was no evidence that HCFC-123 is teratogenic in
laboratory animals or induces reproductive or fetal toxicity at levels
of exposure lower than those that cause other systemic effects. Growth
was retarded in neonatal rats and monkeys reared by dams exposed to
HCFC-123, with a LOAEL of 30 ppm (188 mg/m3). The main metabolite of
HCFC-123, trifluoroacetic acid, was found in the milk of the dams.
Although there was evidence of clastogenic activity in human
lymphocytes exposed to HCFC-123 at high, cytotoxic concentrations
in vitro, all other in vitro and in vivo tests for genetic
toxicity were negative. Therefore, the evidence suggests that the
chemical is unlikely to be genotoxic in vivo.
In a 2-year inhalation study in rats, there was an increased
incidence of pre-cancerous lesions and benign tumours in the liver,
pancreas, and testes, but no exposure-related increase in the
incidence of malignant tumours. It is likely that these tumours
involve one or more non-genotoxic mechanisms, including peroxisome
proliferation, hepatocellular damage, necrosis and regenerative
proliferation, and disturbance of the
hypothalamic-pituitary-testicular axis. Although humans may be less
sensitive to tumours arising from some of these mechanisms, overall it
is not possible to discount the tumours in an evaluation of the
potential risk for humans.
The most relevant critical effects for a single, brief exposure
to HCFC-123, such as from the discharge of a fire extinguishant, are
CNS depression and an increased likelihood of adrenaline-induced
cardiac arrhythmia. The most relevant critical effect from repeated
exposure is liver lesions, which have been reported in workers exposed
to air levels above 5 ppm (31.3 mg/m3) for 1-4 months.
2. IDENTITY AND PHYSICAL/CHEMICAL PROPERTIES
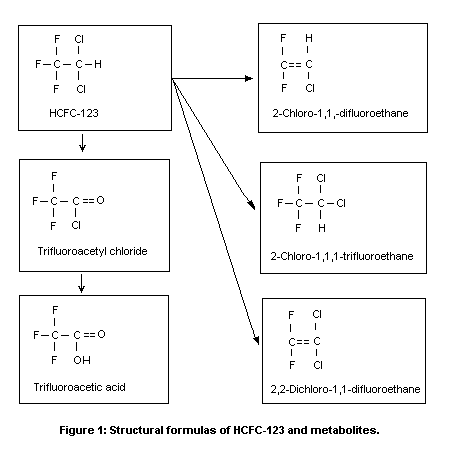
HCFC-123 (CAS No. 306-83-2; C2HCl2F3;
2,2-dichloro-1,1,1-trifluoroethane,
1,1,1-trifluoro-2,2-dichloroethane; see structural diagram in Figure
1) is a synthetic chemical that is a clear, colourless,
non-combustible liquid with a slight ethereal odour. Other common
names or abbreviations are FC 123, Fluorocarbon 123, Forane-123, Freon
123, Frigen, G 123, Genetron 123, R-123, and SUVA 123. HCFC-123 boils
at 27.6°C and is highly volatile, with a vapour pressure of 89.3 kPa
at 25°C. Its molecular weight is 152.93 g/mol. The solubility of
HCFC-123 in water is 2.1 g/litre at 25°C. The estimated log
octanol/water partition coefficient is 2.3-2.9 (NICNAS, 1996). Its
Henry's law constant has been measured at 2.6 m3.kPa/mol at 22°C
(Chang & Criddle, 1995), corresponding to a dimensionless constant of
1.057. Additional physical/chemical properties are presented in the
International Chemical Safety Card, reproduced in this document
(Appendix 4).
The conversion factors for airborne HCFC-123 at 101.3 kPa and
25°C are 1 ppm = 6.25 mg/m3 and 1 mg/m3 = 0.16 ppm.
3. ANALYTICAL METHODS
Methods of automated vapour detection include infrared
absorption, infrared photo-acoustic, halide ion, and metallic oxide
resistance sensors, with most systems having a detection limit of 1-2
ppm (6.25-12.5 mg/m3) (Trane Company, 1991). Analysis for HCFC-123
in environmental media is usually by gas chromatography with flame
ionization detection (Du Pont, 1993). This method has a detection
limit of less than 0.94 ppm (5.88 mg/m3).
There are no validated methods for biological monitoring of
HCFC-123, although urinary excretion of trifluoroacetic acid has been
used as an indicator of exposure (Tanaka et al., 1998).
4. SOURCES OF HUMAN AND ENVIRONMENTAL EXPOSURE
There are no known natural sources of HCFC-123. The principal use
for HCFC-123 is as a refrigerant in commercial and industrial
air-conditioning installations, in gaseous fire extinguishants, as a
foam-blowing agent, and in metal and electronics cleaning. These uses
are primarily as a temporary replacement for chlorofluorocarbons and
bromofluorocarbons phased out pursuant to the 1987 Montreal Protocol
on Substances that Deplete the Ozone Layer. Worldwide, commercially
available volumes of the chemical may reach 10 000 tonnes per year
(AIHA, 1998). In countries that have ratified the Copenhagen Amendment
to the Montreal Protocol, the manufacture, import, and export of
HCFC-123 and other hydrochlorofluorocarbons will be phased out by
2020, although very small amounts will continue to be available until
2030 to service existing equipment. For information on the Montreal
Protocol and subsequent amendments, see UNEP (1999).
5. ENVIRONMENTAL TRANSPORT, DISTRIBUTION, AND TRANSFORMATION
The majority of HCFC-123 released to the environment is in
emissions to air -- for example, from loss during normal running or
maintenance of air-conditioning installations, from the discharge of
fire extinguishants containing HCFC-123, or from the evaporation of
solvents used in metal or electronics cleaning. Because of the limited
solubility and high volatility of HCFC-123, only small amounts will
enter aquatic environments. In a 28-day closed bottle test conducted
according to Organisation for Economic Co-operation and Development
(OECD) guidelines, oxygen consumption at a concentration of 12.5 mg
HCFC-123/litre amounted to 24% of theoretical (Jenkins, 1992a); that
is, HCFC-123 is not readily biodegradable, and spills to water would
largely evaporate. HCFC-123 has been shown to undergo biodegradation
in the presence of methanotrophic bacteria (Chang & Criddle, 1995).
Microbial transformation involving reductive dechlorination to
2-chloro-1,1,1-trifluoroethane was observed in anoxic freshwater and
salt-marsh sediments, whereas no degradation was observed in aerobic
soils (Oremland et al., 1996). Although methanotrophic and anaerobic
biodegradation may occur, they are unlikely to be effective removal
mechanisms for this highly volatile chemical.
The estimated atmospheric lifetime of HCFC-123 is 1.4 years (WMO,
1995). HCFC-123 has an ozone-depleting potential of 0.02 relative to
CFC-11 (trichlorofluoromethane). The global warming potential relative
to carbon dioxide is 300, 93, and 29 over time horizons of 20, 100,
and 500 years, respectively (WMO, 1995).
In the troposphere, HCFC-123 is attacked by hydroxyl radicals to
form hydrogen chloride and trifluoroacetyl chloride (Hayman et al.,
1994). The latter may undergo photolysis to carbon monoxide, carbon
dioxide, hydrogen fluoride, and hydrogen chloride, but the major loss
process is hydrolysis to trifluoroacetic acid by cloud water and
precipitation in rain. Trifluoroacetic acid is also an atmospheric
degradation product of other, more widely used fluorocarbons
(Kotamarthi et al., 1998). It is very stable and may accumulate in
certain closed aquatic systems. Reported environmental levels range
from 30 to 3800 ng/litre in rain, snow, and fog and from 40 to 5400
ng/litre in most surface waters, with maximum concentrations of 6400
and 40 000 ng/litre in two desert lakes (Frank et al., 1996; Wujcik et
al., 1998). The environmental fate of trifluoroacetic acid was
reviewed by Boutonnet et al. (1999). The available evidence indicated
that soil retention of trifluoroacetic acid is poor, particularly in
soils with low levels of organic matter. Although biodegradation was
observed under specific anaerobic conditions, the relevance of these
findings was considered to be doubtful. Trifluoroacetic acid did not
accumulate in lower aquatic life forms, such as bacteria, small
invertebrates, oligochaete worms, and some aquatic plants, including
duckweed. In terrestrial higher plants, trifluoroacetic acid appeared
to be taken up with water and concentrated due to transpiration water
loss. The highest measured bioconcentration factor of 43 based on
fresh weight was found in shoot/leaf from hydroponic wheat.
Board members, authors, reviewers, consultants, and advisers who
participate in the preparation of a CICAD are required to declare any
real or potential conflict of interest in relation to the subjects
under discussion at any stage of the process. Representatives of
nongovernmental organizations may be invited to observe the
proceedings of the Final Review Board. Observers may participate in
Board discussions only at the invitation of the Chairperson, and they
may not participate in the final decision-making process.
1. EXECUTIVE SUMMARY
This CICAD was based principally on the assessments of the
occupational health and environmental effects of
2,2-dichloro-1,1,1-trifluoroethane (HCFC-123) completed under the
Australian National Industrial Chemicals Notification and Assessment
Scheme (NICNAS) and published in March 1996 (NICNAS, 1996) and July
1999 (NICNAS, 1999). Relevant information that has become available
since completion of the NICNAS reports or that was identified in a
comprehensive search of several on-line databases up to August 1999
has also been assessed and included in this CICAD. This CICAD is an
update of the review of HCFC-123 in the monograph Environmental Health
Criteria 139 (IPCS, 1992), prompted by the advent of new and
significant data. Information on the nature of the peer review and the
availability of the source documents is presented in Appendix 1.
Information on the peer review of this CICAD is presented in Appendix
2. The CICAD was approved for publication at a meeting of the Final
Review Board, held in Sydney, Australia, on 21-24 November 1999.
Participants at the Final Review Board meeting are listed in Appendix
3. The International Chemical Safety Card (ICSC 1343) for
2,2-dichloro-1,1,1-trifluoroethane, produced by the International
Programme on Chemical Safety, has been reproduced in Appendix 4 (IPCS,
1998).
HCFC-123 (CAS No. 306-83-2) is a synthetic, non-combustible,
volatile liquid that is used as a refrigerant in commercial and
industrial air-conditioning installations, in gaseous fire
extinguishants, as a foam-blowing agent, and in metal and electronics
cleaning. Its ozone-depleting potential is only 2% of that of CFC-11
(trichlorofluoromethane). It has a global warming potential of 300
over a 20-year time horizon relative to carbon dioxide. As such,
HCFC-123 is currently used as a transitional replacement for
chlorofluorocarbons and bromofluorocarbons phased out pursuant to the
1987 Montreal Protocol on Substances that Deplete the Ozone Layer. The
1992 Copenhagen Amendment to the Montreal Protocol requires that
HCFC-123 and other hydrochlorofluorocarbons be phased out by 2020.
Releases of HCFC-123 to the environment are primarily to ambient
air. Although slightly toxic to fish, Daphnia, and algae, HCFC-123
is unlikely to pose a significant hazard to the aquatic environment,
as it is not persistent in water, even at concentrations below the
solubility limit. In the atmosphere, HCFC-123 has an estimated
lifetime of less than 2 years. The main atmospheric breakdown product
of HCFC-123 (and other, more widely used fluorocarbons) is
trifluoroacetic acid, which will partition into aqueous phases in the
environment. Although trifluoroacetic acid is resistant to degradation
and may accumulate in certain closed aquatic systems, current and
predicted concentrations from HCFC-123 emissions are below toxic
thresholds.
Exposure of the general public to HCFC-123 is expected to be
minimal. However, there is the potential for occupational exposure
during the manufacture of HCFC-123 and the manufacture and use of
products containing the chemical.
Limited information is available on the effects of HCFC-123 on
humans. Cases of dizziness, headache, and nausea following a single
exposure to unknown levels of airborne HCFC-123 have been reported, as
well as cases of manifest or subclinical liver disease associated with
repeated occupational exposures to HCFC-123 vapours at 5-1125 ppm
(31.3-7030 mg/m3) for 1-4 months.
The acute toxicity of HCFC-123 in laboratory animals is low.
Inhalation for a few minutes to a few hours causes liver lesions in
guinea-pigs at 1000 ppm (6.25 g/m3), central nervous system (CNS)
depression in all species examined at 5000 ppm (31.3 g/m3), and
adrenaline-induced cardiac arrhythmia in dogs at 20 000 ppm (125
g/m3). In the rat and hamster, inhalation of more than 30 000 ppm
(188 g/m3) for 4 h causes severe CNS depression and death. HCFC-123
is not a skin irritant or sensitizer, but it can cause eye irritation
in liquid form. In repeated-exposure inhalation toxicity studies
lasting 2-39 weeks in rats, guinea-pigs, dogs, and monkeys, the main
target organs were the liver, the hypothalamic-pituitary-gonadal
endocrine system, and the CNS. The lowest-observed-adverse-effect
level (LOAEL) based on liver effects was 30 ppm (188 mg/m3). The
no-observed-adverse-effect level (NOAEL) was 100 ppm (625 mg/m3)
based on endocrine effects and 300 ppm (1880 mg/m3) based on CNS
effects. There was no evidence that HCFC-123 is teratogenic in
laboratory animals or induces reproductive or fetal toxicity at levels
of exposure lower than those that cause other systemic effects. Growth
was retarded in neonatal rats and monkeys reared by dams exposed to
HCFC-123, with a LOAEL of 30 ppm (188 mg/m3). The main metabolite of
HCFC-123, trifluoroacetic acid, was found in the milk of the dams.
Although there was evidence of clastogenic activity in human
lymphocytes exposed to HCFC-123 at high, cytotoxic concentrations
in vitro, all other in vitro and in vivo tests for genetic
toxicity were negative. Therefore, the evidence suggests that the
chemical is unlikely to be genotoxic in vivo.
In a 2-year inhalation study in rats, there was an increased
incidence of pre-cancerous lesions and benign tumours in the liver,
pancreas, and testes, but no exposure-related increase in the
incidence of malignant tumours. It is likely that these tumours
involve one or more non-genotoxic mechanisms, including peroxisome
proliferation, hepatocellular damage, necrosis and regenerative
proliferation, and disturbance of the
hypothalamic-pituitary-testicular axis. Although humans may be less
sensitive to tumours arising from some of these mechanisms, overall it
is not possible to discount the tumours in an evaluation of the
potential risk for humans.
The most relevant critical effects for a single, brief exposure
to HCFC-123, such as from the discharge of a fire extinguishant, are
CNS depression and an increased likelihood of adrenaline-induced
cardiac arrhythmia. The most relevant critical effect from repeated
exposure is liver lesions, which have been reported in workers exposed
to air levels above 5 ppm (31.3 mg/m3) for 1-4 months.
2. IDENTITY AND PHYSICAL/CHEMICAL PROPERTIES
HCFC-123 (CAS No. 306-83-2; C2HCl2F3;
2,2-dichloro-1,1,1-trifluoroethane,
1,1,1-trifluoro-2,2-dichloroethane; see structural diagram in Figure
1) is a synthetic chemical that is a clear, colourless,
non-combustible liquid with a slight ethereal odour. Other common
names or abbreviations are FC 123, Fluorocarbon 123, Forane-123, Freon
123, Frigen, G 123, Genetron 123, R-123, and SUVA 123. HCFC-123 boils
at 27.6°C and is highly volatile, with a vapour pressure of 89.3 kPa
at 25°C. Its molecular weight is 152.93 g/mol. The solubility of
HCFC-123 in water is 2.1 g/litre at 25°C. The estimated log
octanol/water partition coefficient is 2.3-2.9 (NICNAS, 1996). Its
Henry's law constant has been measured at 2.6 m3.kPa/mol at 22°C
(Chang & Criddle, 1995), corresponding to a dimensionless constant of
1.057. Additional physical/chemical properties are presented in the
International Chemical Safety Card, reproduced in this document
(Appendix 4).
The conversion factors for airborne HCFC-123 at 101.3 kPa and
25°C are 1 ppm = 6.25 mg/m3 and 1 mg/m3 = 0.16 ppm.
3. ANALYTICAL METHODS
Methods of automated vapour detection include infrared
absorption, infrared photo-acoustic, halide ion, and metallic oxide
resistance sensors, with most systems having a detection limit of 1-2
ppm (6.25-12.5 mg/m3) (Trane Company, 1991). Analysis for HCFC-123
in environmental media is usually by gas chromatography with flame
ionization detection (Du Pont, 1993). This method has a detection
limit of less than 0.94 ppm (5.88 mg/m3).
There are no validated methods for biological monitoring of
HCFC-123, although urinary excretion of trifluoroacetic acid has been
used as an indicator of exposure (Tanaka et al., 1998).
4. SOURCES OF HUMAN AND ENVIRONMENTAL EXPOSURE
There are no known natural sources of HCFC-123. The principal use
for HCFC-123 is as a refrigerant in commercial and industrial
air-conditioning installations, in gaseous fire extinguishants, as a
foam-blowing agent, and in metal and electronics cleaning. These uses
are primarily as a temporary replacement for chlorofluorocarbons and
bromofluorocarbons phased out pursuant to the 1987 Montreal Protocol
on Substances that Deplete the Ozone Layer. Worldwide, commercially
available volumes of the chemical may reach 10 000 tonnes per year
(AIHA, 1998). In countries that have ratified the Copenhagen Amendment
to the Montreal Protocol, the manufacture, import, and export of
HCFC-123 and other hydrochlorofluorocarbons will be phased out by
2020, although very small amounts will continue to be available until
2030 to service existing equipment. For information on the Montreal
Protocol and subsequent amendments, see UNEP (1999).
5. ENVIRONMENTAL TRANSPORT, DISTRIBUTION, AND TRANSFORMATION
The majority of HCFC-123 released to the environment is in
emissions to air -- for example, from loss during normal running or
maintenance of air-conditioning installations, from the discharge of
fire extinguishants containing HCFC-123, or from the evaporation of
solvents used in metal or electronics cleaning. Because of the limited
solubility and high volatility of HCFC-123, only small amounts will
enter aquatic environments. In a 28-day closed bottle test conducted
according to Organisation for Economic Co-operation and Development
(OECD) guidelines, oxygen consumption at a concentration of 12.5 mg
HCFC-123/litre amounted to 24% of theoretical (Jenkins, 1992a); that
is, HCFC-123 is not readily biodegradable, and spills to water would
largely evaporate. HCFC-123 has been shown to undergo biodegradation
in the presence of methanotrophic bacteria (Chang & Criddle, 1995).
Microbial transformation involving reductive dechlorination to
2-chloro-1,1,1-trifluoroethane was observed in anoxic freshwater and
salt-marsh sediments, whereas no degradation was observed in aerobic
soils (Oremland et al., 1996). Although methanotrophic and anaerobic
biodegradation may occur, they are unlikely to be effective removal
mechanisms for this highly volatile chemical.
The estimated atmospheric lifetime of HCFC-123 is 1.4 years (WMO,
1995). HCFC-123 has an ozone-depleting potential of 0.02 relative to
CFC-11 (trichlorofluoromethane). The global warming potential relative
to carbon dioxide is 300, 93, and 29 over time horizons of 20, 100,
and 500 years, respectively (WMO, 1995).
In the troposphere, HCFC-123 is attacked by hydroxyl radicals to
form hydrogen chloride and trifluoroacetyl chloride (Hayman et al.,
1994). The latter may undergo photolysis to carbon monoxide, carbon
dioxide, hydrogen fluoride, and hydrogen chloride, but the major loss
process is hydrolysis to trifluoroacetic acid by cloud water and
precipitation in rain. Trifluoroacetic acid is also an atmospheric
degradation product of other, more widely used fluorocarbons
(Kotamarthi et al., 1998). It is very stable and may accumulate in
certain closed aquatic systems. Reported environmental levels range
from 30 to 3800 ng/litre in rain, snow, and fog and from 40 to 5400
ng/litre in most surface waters, with maximum concentrations of 6400
and 40 000 ng/litre in two desert lakes (Frank et al., 1996; Wujcik et
al., 1998). The environmental fate of trifluoroacetic acid was
reviewed by Boutonnet et al. (1999). The available evidence indicated
that soil retention of trifluoroacetic acid is poor, particularly in
soils with low levels of organic matter. Although biodegradation was
observed under specific anaerobic conditions, the relevance of these
findings was considered to be doubtful. Trifluoroacetic acid did not
accumulate in lower aquatic life forms, such as bacteria, small
invertebrates, oligochaete worms, and some aquatic plants, including
duckweed. In terrestrial higher plants, trifluoroacetic acid appeared
to be taken up with water and concentrated due to transpiration water
loss. The highest measured bioconcentration factor of 43 based on
fresh weight was found in shoot/leaf from hydroponic wheat.
 6. ENVIRONMENTAL LEVELS AND HUMAN EXPOSURE
6.1 Environmental levels
HCFC-123 was detected in non-urban ambient air in Australia at
levels less than 0.01 ppt (62.5 pg/m3) (Fraser, 1994). Assuming that
worldwide emissions will increase to 45 000 tonnes in 2010, the global
average concentration of airborne HCFC-123 is projected to reach 1.1
ppt (7 ng/m3) in that year, with levels being 2-4 times higher than
the average near major sources of emissions in eastern North America
and central Europe (Kotamarthi et al., 1998).
Information on levels of HCFC-123 in water, wildlife or food was
not available.
6.2 Human exposure
HCFC-123 is not used in consumer products. Indirect exposure via
the environment would be low, as the concentration in ambient air is
less than 0.01 ppt (62.5 pg/m3) and HCFC-123 is unlikely to persist
in other media because of its limited solubility and high volatility.
Therefore, exposure of the general population to HCFC-123 is expected
to be minimal.
There is potential for occupational exposure, predominantly via
inhalation, during the manufacture of HCFC-123 and the manufacture and
use of products containing HCFC-123, such as the operation and
maintenance of air-conditioning installations running on HCFC-123, the
discharge of HCFC-123 from fire protection systems, and the use of
liquid HCFC-123 in metal and electronics cleaning.
In an HCFC-123 manufacturing plant in Canada, two operators
monitored over 165-480 min on 4 separate days had time-weighted
breathing-zone levels of HCFC-123 ranging from 1.16 to 8.94 ppm (7.25
to 55.9 mg/m3). On one occasion, a drum-filling lance failure
resulted in a level in excess of 33 ppm (206 mg/m3) (Du Pont,
personal communication, 1999). No monitoring data were available for
the manufacture of products containing HCFC-123.
Several studies have measured HCFC-123 levels in chiller
machinery rooms during normal operations as well as maintenance and
repair activities. In 4 of 12 unmanned machinery rooms containing
air-conditioning equipment running on HCFC-123, air levels were below
1 ppm (6.25 mg/m3) (4-h time-weighted average) at three sites (Trane
Company, 1991). At one site, where samples were taken near a leak and
a half-empty HCFC-123 drum, levels of 5.9-13.6 ppm (36.9-85.0 mg/m3)
(20-min time-weighted average) were recorded. At the rest of the
sites, machinery room air levels were below the limit of detection
(0.2-0.4 ppm [1.25-2.50 mg/m3]). Breathing-zone levels of HCFC-123
during routine chiller maintenance operations, including refrigerant
transfer, were measured at nine US installations. Two-hour to 12-h
time-weighted average concentrations were less than 1 ppm (6.25
mg/m3) in five cases, less than 2 ppm (12.5 mg/m3) in three cases,
and in the range of 2-5 ppm (12.5-31.3 mg/m3) in one case (MRI,
1991; Sibley, 1992; Trane Company, 1992). In Australia, 4- to 6-h
time-weighted average concentrations were less than 1 ppm (6.25
mg/m3) during repair work at a single installation (NICNAS, 1996).
In these studies, continuous area monitoring showed time-weighted
average air concentrations below 1 ppm (6.25 mg/m3), with
activity-related instantaneous peaks ranging from 30 to 500 ppm (188
to 3130 mg/m3).
Air levels resulting from the use of a fire extinguishant
containing 93% HCFC-123 were measured during fire control exercises in
which the firefighters wore a self-contained breathing apparatus (MRI,
1993a,b). Outdoor discharge resulted in maximum breathing-zone levels
ranging from 7 to 870 ppm (43.8 to 5440 mg/m3), depending on the
type of fire hazard. Inside an aircraft hangar, the discharge of
hand-held extinguishers resulted in a breathing-zone concentration of
20 ppm (125 mg/m3) during discharge, with average static air levels
ranging from 29 to 141 ppm (181 to 881 mg/m3) over the next 30 min.
With a large semi-portable fire extinguisher, breathing-zone
concentrations during discharge reached 180-300 ppm (1130-1880
mg/m3), whereas average static air levels ranged from 165 to 557 ppm
(1030 to 3480 mg/m3) over the next 30 min.
In a US factory converted to using HCFC-123 in its degreaser,
personal air monitoring during normal degreaser operations showed
5.5-h time-weighted average levels of HCFC-123 that ranged from 5.3 to
12.0 ppm (33.1 to 75.0 mg/m3) throughout the facility.1 Charging
and unloading the degreaser resulted in short-term breathing-zone
levels ranging from 160 to 460 ppm (1000 to 2880 mg/m3).
1 AlliedSignal Inc., personal communication, 1998 [cited in
NICNAS, 1999].
7. COMPARATIVE KINETICS AND METABOLISM IN LABORATORY ANIMALS
AND HUMANS
HCFC-123 is readily absorbed by inhalation and distributed
throughout the body, where it reaches levels in body fat up to 25
times higher than those in blood (Vinegar et al., 1994). Following
exposure to an initial concentration of 2000 ppm (12.5 g/m3)
radiolabelled HCFC-123 over two consecutive 3-h periods in a
closed-chamber system, total uptake was 50-60% in rats and 90-100% in
guinea-pigs (Urban & Dekant, 1994). In rats exposed to initial
concentrations of 120-11 000 ppm (0.75-68.8 g/m3) HCFC-123 for 6 h,
the inhalation uptake showed a rapid distribution phase lasting
30-45 min followed by a slow linear uptake phase (Vinegar et al.,
1994). Blood levels declined rapidly once exposure was terminated. In
rats exposed to 1000 ppm (6.25 g/m3), blood concentrations fell
from 15.0 mg/litre at the end of a 4-h exposure period to 4.5
mg/litre at 4 min post-exposure and 1.5 mg/litre at 1 h post-exposure
(Vinegar et al., 1994). After the initial decline, HCFC-123
concentrations in both blood and fat decreased log-linearly with
a half-life of approximately 80 min, indicating fat as the main
depository for unmetabolized HCFC-123. Experimentally determined
tissue to air partition coefficients were 2-3 for gut and muscle,
3-4 for blood, 3-5 for liver, and 60-70 for fat (Dekant, 1993;
Vinegar et al., 1994). Data on oral or dermal absorption were not
available.
In the rat, 26-32% of the uptake of HCFC-123 is metabolized,
predominantly by oxidation to trifluoroacetyl chloride, which is
hydrolysed to trifluoroacetic acid or reacts with lysine residues in
proteins or with low-molecular-weight amines to form
N-trifluoroacetyl amides (Harris et al., 1992; Dodd et al., 1993;
Urban & Dekant, 1994). Minor, reductive pathways lead to the formation
of very small amounts of 2-chloro-1,1,1-trifluoroethane,
2-chloro-1,1-difluoroethene, and 2,2-dichloro-1,1-difluoroethene. The
latter reacts with glutathione to form
N-acetyl- S-(2,2-dichloro-1,1-difluoroethyl)-L-cysteine. The
structures of these metabolites are shown in Figure 1. Both oxidation
and reduction of HCFC-123 are catalysed by cytochrome P450 2E1
(CYP2E1). In human liver microsomes, the major biotransformation
product is trifluoroacetic acid (Urban et al., 1994). Its rate of
formation was directly related to the amount of CYP2E1 present and
1.5-16 times faster than the rate in rat microsomes.
In experimental animals, the major metabolite in blood, urine,
and milk is trifluoroacetic acid. In rats exposed by inhalation to
1000 ppm (6.25 g/m3) HCFC-123 for 4 h, blood levels of parent
compound and trifluoroacetic acid amounted to 15.0 and 93.1 mg/litre,
respectively (Vinegar et al., 1994). At 10 000 ppm (62.5 g/m3), the
corresponding concentrations were 93.5 and 37.8 mg/litre,
respectively. Trifluoroacetic acid blood levels rebounded and peaked
12-26 h post-exposure, indicating that the metabolism of HCFC-123
is subject to substrate inhibition at exposures above 1000 ppm (6.25
g/m3). For exposure levels below 2000 ppm (12.5 g/m3), the
metabolic rate constants developed for HCFC-123 were Km = 1.2
mg/litre and Vmax = 7.20 mg/kg body weight per hour for male rats
and Km = 1.2 mg/litre and Vmax = 7.97 mg/kg body weight per
hour for female rats (Loizou et al., 1994). Generally, HCFC-123 was
not detected in blood samples collected within 1 h post-exposure from
lactating rhesus monkeys exposed by inhalation to 1000 ppm (6.25
g/m3) for 6 h per day, whereas trifluoroacetic acid concentrations
reached 150-190 µg/ml after 2-3 weeks of exposure. Based on data from
a single monkey, the half-life of trifluoroacetic acid in blood was
approximately 24 h (Slauter, 1997). In rats and guinea-pigs exposed to
14C-labelled HCFC-123 vapours for 6 h and sacrificed 48 h
post-exposure, only low amounts of radioactivity remained in the
organs examined (Urban & Dekant, 1994). The liver contained most of
the radiolabel, followed by testes and kidneys, lungs, brain,
pancreas, and spleen. Covalent binding of labelled material was
highest in liver tissue (0.4-0.7 nmol/mg protein), followed by lungs,
kidneys, and plasma (0.1-0.3 nmol/mg protein). Trifluoroacetylated
tissue proteins have been detected by immunological techniques in the
liver and at 20- to 200-fold lower levels in the kidney and heart of
rats 6-12 h after exposure to HCFC-123 by inhalation or
intraperitoneal injection (Harris et al., 1992; Huwyler & Gut, 1992;
Huwyler et al., 1992).
The available data indicate that the predominant routes of
HCFC-123 elimination are exhalation of the parent compound and urinary
excretion of trifluoroacetic acid. In rats exposed to an initial
concentration of 2000 ppm (12.5 g/m3) radiolabelled HCFC-123 for two
consecutive 3-h periods and sacrificed 48 h post-exposure, 23-28% of
the radioactive uptake was eliminated in the urine, predominantly as
trifluoroacetic acid, whereas 3-4% was recovered from the body (Urban
& Dekant, 1994). Small amounts of minor metabolites, such as
N-acetyl- S-(2,2-dichloro-1,1-difluoroethyl)-L-cysteine,
N-trifluoroacetyl-2-aminoethanol, and fluoride ion, were recovered
from urine, and trace amounts of 2-chloro-1,1,1-trifluoroethane were
detected in expired air (Urban & Dekant, 1994; Vinegar et al., 1994).
In lactating rats and monkeys exposed to 1000 ppm (6.25 g/m3)
HCFC-123 for 6 h per day for 3 weeks, trifluoroacetic acid was found
in milk at a maximum concentration of 65 and 30 µg/ml, respectively
(Buschman, 1996; Slauter, 1997). In monkeys, the milk also contained
small amounts (up to 5 µg/ml) of HCFC-123. Rat milk was not analysed
for HCFC-123, and neither monkey nor rat milk was analysed for
metabolites other than trifluoroacetic acid.
An analogue of HCFC-123, the common inhalation anaesthetic
halothane (2-bromo-2-chloro-1,1,1-trifluoroethane), is also
metabolized by hepatic CYP2E1 to trifluoroacetyl chloride, causing
trifluoroacetylation of liver proteins (Harris et al., 1992; Urban et
al., 1994). These include cytochrome P450 itself and other enzymes,
many of which have been identified as residing in the lumen of the
endoplasmic reticulum and involved in the maturation of newly
synthesized proteins (Cohen et al., 1997). Both halothane and HCFC-123
induce peroxisome proliferation and increased ß-oxidation in rat liver
cells (Keller et al., 1998). They are also highly effective in
inducing excess uncoupled cytochrome P450 activity in rabbit liver
microsomes, thus increasing hepatic oxygen consumption and
facilitating the oxidation of other cytochrome P450 substrates (Wang
et al., 1993).
Only limited information was available on the kinetics and
metabolism of HCFC-123 in humans in vivo. In four volunteers exposed
by inhalation to 60-73 ppm (375-460 mg/m3) HCFC-123 for 6 h, the
concentration of trifluoroacetic acid in the urine peaked at 10-27
mg/litre by 20-30 h and returned to zero by 96 h post-exposure,
indicating an elimination half-life of 25 h (Tanaka et al., 1998).
Physiologically based pharmacokinetic models for halothane in humans
and for halothane and HCFC-123 in rats have been used to deduce a
human model for HCFC-123 and its main metabolite, trifluoroacetic acid
(Williams et al., 1996). As the model has not been validated, its
usefulness as a predictive tool is unknown at this time.
8. EFFECTS ON LABORATORY MAMMALS AND IN VITRO TEST SYSTEMS
Unless otherwise indicated, only effects that were statistically
different ( P < 0.05) from controls have been considered. All
inhalation studies were performed by whole-body exposure, unless
otherwise mentioned.
8.1 Single exposure
When HCFC-123 was administered in corn oil by oral gavage to rats
at doses ranging from 2.25 to 11 g/kg body weight, rapid respiration
and prostration were recorded at and above 3.4 g/kg body weight. An
LD50 was not determined. The lowest dose causing mortality was 9
g/kg body weight (Henry, 1975).
In the rat and the hamster, inhalation of more than 30 000 ppm
(188 g/m3) caused severe CNS depression and death (Clayton, 1966;
Hall & Moore, 1975; Coate, 1976; Darr, 1981). The 4-h LC50 ranged
from 28 400 to 52 600 ppm (178-329 g/m3). Clinical signs of toxicity
included sedation, loss of muscle coordination and balance,
prostration, and dyspnoea. Gross pathological findings were either
negative or limited to congestion or discoloration of the lungs,
kidneys, liver, thymus, or small intestine. The lowest concentration
causing reversible CNS depression (failures in unconditioned reflexes)
in rats was 5000 ppm (31.3 g/m3) (Mullin, 1976). In a study of
cardiac sensitization to adrenaline, life-threatening or fatal
arrhythmias occurred in 0 of 3, 4 of 6, and 3 of 3 dogs exposed
nose-only to 10 000, 20 000, or 40 000 ppm (62.5, 125, or 250 g/m3).
The dogs were pretreated with intravenous adrenaline (8 µg/kg body
weight) prior to exposure and challenged with an identical adrenaline
dose after breathing the compound for 5 min. Based on these findings,
an EC50 (5 min) of 19 000 ppm (119 g/m3) and a NOAEL of 10 000 ppm
(62.5 g/m3) were determined (Trochimowicz & Mullin, 1973). Exposure
of guinea-pigs to 1000-30 000 ppm (6.25-188 g/m3) HCFC-123 for 4 h
produced non-fatal liver damage in all exposure groups (Marit et al.,
1994). At 48 h post-exposure, the liver effects included centrilobular
vacuolar (fatty) change, multifocal random and centrilobular
hepatocellular degeneration and necrosis, and increased levels of
plasma isocitrate dehydrogenase, alanine transaminase (ALT), and
aspartate transaminase (AST). In another study in guinea-pigs exposed
to 10 000 ppm (62.5 g/m3) for 4 h, liver injury was minimal unless
the animals had been glutathione depleted prior to exposure (Lind et
al., 1995).
In a rat and rabbit test for dermal toxicity carried out
according to OECD guidelines, the only effect observed after a dose of
2000 mg liquid HCFC-123/kg body weight was applied under occlusion for
24 h was a slight to moderate erythema in 6 of 10 rabbits up to 5 days
post-treatment. As such, the dermal LD50 for rats and rabbits was
greater than 2000 mg/kg body weight (Brock, 1988a,b).
8.2 Irritation and sensitization
Application under occlusion of 0.5 ml liquid HCFC-123 for 4 h
caused no erythema or oedema in a test for skin irritation potential
in rabbits conducted according to OECD guidelines (Brock, 1988c). In
rabbits, 0.1 ml undiluted HCFC-123 or 0.2 ml of a 50% solution in
propylene glycol caused mild to moderate, reversible eye damage,
including conjunctival irritation and corneal opacity (scoring not
reported) (Britelli, 1975). HCFC-123 did not produce skin
sensitization in guinea-pigs when 0.1 ml of a 1% solution in dimethyl
phthalate was administered intradermally once a week for 3 weeks,
followed by challenge 2 weeks later with 7 or 35 mg HCFC-123 dissolved
in propylene glycol (Goodman, 1975).
8.3 Short-term exposure
In rats exposed to 1000, 5000, 10 000, or 20 000 ppm (6.25, 31.3,
62.5, or 125 g/m3) HCFC-123 for 6 h per day, 5 days per week, in a
28-day inhalation toxicity study conducted in accordance with OECD
guidelines, exposures to 5000 ppm (31.3 g/m3) and above resulted in
dose-related narcotic effects, which were reversible overnight (Kelly,
1989; Rusch et al., 1994). Body weight was reduced at all dose levels
in females (8-10%) and at the two highest dose levels in males
(14-15%). There was a dose-related increase in relative liver weight
at all exposure levels in females (14-27%) and at the highest level in
males (18%). Plasma levels of ALT and AST were increased by 35% and
71%, respectively, at the highest level in males. Except for isolated
cases of fatty change at all dose levels, gross or microscopic liver
lesions were not observed. A NOAEL was not established in this study.
In a study in male rats exposed to approximately 1000, 5000, or
20 000 ppm (6.25, 31.3, or 125 g/m3) HCFC-123 for 6 h per day, 5
days per week, for 28 days, there was a decrease in body weight
(6-11%) and an increase in relative testis weight (12-30%) in all dose
groups and a 25% increase in relative liver weight in the highest dose
group (Lewis, 1990). Microscopic liver lesions including dose-related
hepatocyte hypertrophy and centrilobular fatty change were seen at all
exposure levels, with proliferation of peroxisomes and mitochondria in
liver cells of animals exposed to 5000 ppm (31.3 g/m3) and above.
Plasma levels of ALT, AST, and alkaline phosphatase (ALP) were
increased by up to 79%, whereas plasma triglycerides and cholesterol
were decreased by up to 70%, with AST and triglycerides being the most
sensitive markers of hepatic injury.
Similar findings accompanied by a 3-fold increase in hepatocyte
mitotic activity were reported from a study in which male rats were
exposed to 18 200 ppm (114 g/m3) HCFC-123 for 6 h per day, 5 days
per week, for 28 days (Warheit, 1993). This study also found
exposure-related testicular lesions, including germinal cell necrosis
and atrophy of the seminiferous tubules. In male guinea-pigs exposed
to 9400 ppm (58.8 g/m3) HCFC-123 for 6 h per day, 5 days per week,
for 28 days, there was evidence of microscopic liver lesions,
including centrilobular vacuolar (fatty) change and hepatocellular
necrosis, but no testicular effects were observed (Warheit, 1993).
8.4 Long-term exposure
8.4.1 Subchronic exposure
A number of subchronic inhalation studies have been carried out
in rats and dogs and are summarized in Table 1. In each case, HCFC-123
was administered for 6 h per day, 5 days per week. The main effects
were liver injury, with changes in liver-related clinical chemistry
parameters occurring at 300 ppm (1.88 g/m3) in rats and at 1000 ppm
(6.25 g/m3) in dogs, and CNS depression, with a reduction in arousal
occurring at 1000 ppm (6.25 g/m3) in rats.
8.4.2 Chronic exposure and carcinogenicity
In a 2-year inhalation study, groups of 80 male and female
Sprague-Dawley rats were exposed for 6 h per day for 5 days per week
to 300, 1000, or 5000 ppm (1.88, 6.25, or 31.3 g/m3) HCFC-123
(Malley, 1992; Malley et al., 1995).
During the first year, rats exposed to 5000 ppm (31.3 g/m3)
appeared sedated, but quickly recovered after the daily exposures
ended. Female rats exposed to 1000 ppm (6.25 g/m3) and males and
females exposed to 5000 ppm (31.3 g/m3) had lower body weight and
body weight gain. At the 12-month sacrifice, the relative liver weight
in male and female rats exposed to 5000 ppm (31.3 g/m3) was
increased by 12% and 24%, respectively. No exposure-related gross or
histopathological changes were observed. In both sexes, serum
triglycerides and glucose were decreased at all exposure levels in a
dose-related manner, by 65-100% and 15-31% for males and females,
respectively. Serum cholesterol was decreased by approximately 30% in
all exposed females and by 43% in males exposed to 5000 ppm
(31.3 g/m3).
During the second year of exposure, slight, reversible CNS
depression continued to be observed at 5000 ppm (31.3 g/m3). At the
end of the 2-year period, there was a dose-related increase in
survival rate, which reached a statistically significant level of 47%
and 59% in females exposed to 1000 or 5000 ppm (6.25 or 31.3 g/m3),
respectively. This is an expected effect of chemicals that reduce body
fat and blood lipids. Compared with the controls, body weight was
decreased by 8% in females exposed to 1000 ppm (6.25 g/m3) and by
12% in males and 21% in females exposed to 5000 ppm (31.3 g/m3). At
5000 ppm (31.3 g/m3), relative liver weight and the incidence of
enlarged and discoloured livers were increased in males, as were
grossly observed liver masses in females.
There were no exposure-related effects on the incidence of
malignant tumours. There was an increase in hepatocellular adenomas in
females and males, an increase in cholangiofibromas in high-dose
females, a dose-related increase in pancreatic acinar cell adenomas in
males, and an increase in Leydig (interstitial) cell adenomas in males
at all dose levels (Table 2). Except for hepatocellular adenomas in
males, the increase in the incidence of these tumours remained
statistically significant when corrected for mortality. Historical
data on tumour incidence in the strain used for this study were not
available. Other exposure-related lesions included hepatic foci of
cellular alteration and focal pancreatic acinar cell hyperplasia
(lesions less than 3 mm in diameter) in males and females at 1000 and
5000 ppm (6.25 and 31.3 g/m3), in addition to hepatic focal necrosis
in males, cholangiofibrosis in females, and hepatic centrilobular
fatty change in both sexes at 5000 ppm (31.3 g/m3). Dose-related
focal Leydig cell hyperplasia (lesions less than the diameter of three
adjacent tubules) was observed in male rats exposed to 1000 and
5000 ppm (6.25 and 31.3 g/m3). The incidence of diffuse retinal
atrophy was increased in both sexes at all exposure levels. Serum
triglycerides and cholesterol continued to be decreased in both sexes
by 46-75% and 31-48%, respectively.
As there were changes in clinical chemistry parameters and an
increased incidence of hepatocellular and Leydig cell adenomas at the
lowest dose tested (300 ppm [1.88 g/m3]), a NOAEL was not
established in this study.
8.5 Genotoxicity and related end-points
HCFC-123 was not mutagenic in Salmonella typhimurium strain
TA98, TA100, TA1525, TA1537, or TA1538 with or without metabolic
activation, even at concentrations of 750 mg per vessel or 150 000 ppm
(938 g/m3), which were clearly toxic (Callander, 1989). HCFC-123 was
found to be clastogenic in two separate studies in human lymphocytes
in vitro, both with and without metabolic activation, at relatively
high concentrations that also reduced the mitotic rate (Table 3). It
was also noted to be clastogenic in the absence, but not in the
presence, of a metabolic activation system in human lymphocytes
exposed to the chemical at 500 µg/ml; further details were not
available (ICI, 1992). No transformation to anchorage-independent
cells was observed when HCFC-123 was tested in baby hamster kidney
fibroblasts (BHK21 cells) with and without metabolic activation
(Longstaff et al., 1984).
Table 1: Summary of effect levels in subchronic inhalation toxicity studies.
Species Study design Effects Effect levels Reference
Rats, albino, Exposed to 0, 500, Body weight marginally LOAEL = 500 ppm Industrial Bio-Test
35 males and 1000, or 5000 ppm decreased in females at (3.13 g/m3) Laboratories, 1977;
25 females (0, 3.13, 6.25, or 1000 ppm and in both sexes Rusch et al., 1994
per group 31.3 g/m3) HCFC-123 at 5000 ppm. Kidney weight
for 90 days, with a increased in all male test
30-day recovery groups and in females at
period. 5000 ppm (% change not
reported). Relative liver
weight increased in females
at all exposure levels and
in males at 5000 ppm
(% change not reported).
Microscopic liver lesions
included mild focal necrosis
in males from all test groups
and minimal bile duct
proliferation in males at
5000 ppm. At end of recovery
period, there were no
exposure-related body or organ
weight changes or
histopathological findings.
Rats, Exposed to 0, 1000, Reversible motor LOAEL = 1000 ppm Doleba-Crowe, 1978;
Sprague-Dawley, or 10 000 ppm (0, incoordination and (6.25 g/m3) Rusch et al., 1994
27 per sex 6.25, or 62.5 g/m3) unresponsiveness to noise
per group HCFC-123 for 90 days. at 10 000 ppm. Reduced body
Histopathological weight (8-17%) and increased
examination performed relative liver weight
on 6 animals per (% change not reported) in
group. both test groups.
Table 1 (cont'd)
Species Study design Effects Effect levels Reference
No exposure-related gross or
histopathological findings.
Elevated levels of AST in
males at both exposure levels,
ALT in males at 1000 ppm, and
blood urea nitrogen (BUN) in
males at both exposure levels
and in females at 1000 ppm;
glucose decreased in females
in both test groups and in
males at 10 000 ppm (% change
not reported).
Rats, Exposed to 0, 300, Reduced responsiveness to LOAEL = 300 ppm Malley, 1990;
Sprague-Dawley, 1000, or 5000 ppm auditory stimuli at 1000 and (1.88 g/m3) Rusch et al.,
10 per sex (0, 1.88, 6.25, or 5000 ppm. Relative liver 1994
per group 31.3 g/m3) HCFC-123 weight increased by 12-17%
for 90 days. and 19-22%, respectively, at
1000 and 5000 ppm. No
exposure-related gross or
histopathological findings.
Dose-dependent elevations of
AST, ALT, lactate
dehydrogenase (LDH) in males
at 1000 and 5000 ppm and BUN
in females in all test groups
and in males at 1000 and
Table 1 (cont'd)
Species Study design Effects Effect levels Reference
5000 ppm. Triglycerides and
glucose markedly decreased
and hepatic ß-oxidation
activity increased 2- to
4-fold in all test groups.
Dose-dependent decrease in
cholesterol in females at
1000 and 5000 ppm.
Rats, Exposed to 0, 300, Reversible reduction in NOAEL = 300 ppm Coombs, 1994
Sprague-Dawley, 1000, or 5000 ppm arousal at 1000 and 5000 (1.88 g/m3)
10 per sex (0, 1.88, 6.25, or ppm. No exposure-related
per group 31.3 g/m3) HCFC-123 gross or histopathological
for 90 days, with a findings in cerebrum,
28-day recovery medulla/pons, cerebellar
period. Histological cortex, spinal cord, ganglia,
examinations limited dorsal and ventral root
to nervous tissues. fibres, or peripheral nerves.
Dogs, beagles, Exposed to 0, 1000, Reversible motor LOAEL = 1000 ppm Doleba-Crowe, 1978;
4 males per group or 10 000 ppm incoordination and (6.25 g/m3) Rusch et al., 1994
(0, 6.25, or 62.5 unresponsiveness at
g/m3) HCFC-123 for 10 000 ppm. At 10 000 ppm,
90 days. discoloured livers with
hepatocyte hypertrophy and
necrosis with inflammatory
cell infiltration. Elevated
levels of ALP in both test
groups and of BUN at 10 000
ppm (% change not reported).
Table 2: Incidence of selected non-neoplastic and neoplastic lesions in the liver, pancreas, and testes
in the 2-year rat inhalation study.a,b
Incidence of lesions
0 ppm 300 ppm 1000 ppm 5000 ppm
Male
Liver
Hepatocellular adenoma 3/67 2/66 2/66 8/66c
Basophilic foci of alteration 8/67 10/66 20/66d 30/66d
Clear cell foci of alteration 8/67 9/66 30/66d 19/66d
Mixed foci of alteration 3/67 6/66 6/66 12/66d
Eosinophilic foci of alteration 8/67 16/66 18/66d 13/66
Cholangiofibroma 0/67 0/66 0/66 0/66
Cholangiofibrosis 0/67 0/66 0/66 0/66
Pancreas
Acinar cell adenoma 1/67 4/66 12/64e 14/66e
Focal acinar cell hyperplasia 5/67 6/66 13/64d 19/66d
Testes
Leydig cell adenoma 4/67 12/66f 9/66f 14/66f
Leydig cell hyperplasia 8/67 15/66 23/66d 30/66d
Table 2 (cont'd)
Incidence of lesions
0 ppm 300 ppm 1000 ppm 5000 ppm
Female
Liver
Hepatocellular adenoma 0/65 5/67e 2/67 7/69e
Basophilic foci of alteration 17/65 26/67 32/67d 46/69d
Clear cell foci of alteration 14/65 7/67 16/67 15/69
Mixed foci of alteration 2/65 3/67 13/67d 22/69d
Eosinophilic foci of alteration 8/65 11/67 22/67 30/69d
Cholangiofibroma 0/65 0/67 0/67 6/69e
Cholangiofibrosis 0/65 0/67 0/67 9/69d
Pancreas
Acinar cell adenoma 0/65 2/66 0/67 2/69
Focal acinar cell hyperplasia 0/65 4/66 6/67d 8/69d
a From Malley (1992); Malley et al. (1995).
b Only lesions whose incidence attained statistical significance in at least one male or female dose
group are included in the table. Other non-statistically significant lesions are discussed in the
text. The figures give the number of lesions per number of tissues available for histological
examination. A few animals and tissues were lost due to autolysis.
c P < 0.05 (Cochran-Armitage test for trend).
d P < 0.05 (Fisher's exact test compared with controls).
e P < 0.05 (Cochran-Armitage test for trend and 2/2 tests for mortality-adjusted statistical analysis).
f P < 0.05 (Cochran-Armitage test for trend and 1/2 tests for mortality-adjusted statistical analysis).
In vivo, a test for chromosome aberrations in the lymphocytes
of rats exposed by inhalation to up to 5000 ppm (31.3 g/m3) HCFC-123
for 6 h per day, 5 days a week, for 2 weeks was negative (Marshal,
1992), although the failure to induce signs of cytotoxicity cast doubt
on the validity of this finding. No increase was found in the
incidence of micronuclei or in the ratio of polychromatic to
normochromatic erythrocytes in a micronucleus test in mice exposed
nose-only to up to 18 000 ppm (113 g/m3) HCFC-123 for 6 h (Muller &
Hofmann, 1988). In the livers of rats exposed to 12 500 or 20 000 ppm
(78.1 or 125 g/m3) HCFC-123 for 6 h, neither net nuclear grain count
nor percentage cells in repair showed any evidence of unscheduled DNA
synthesis (Kennelly, 1993).
Although HCFC-123 was clastogenic in vitro at high
concentrations, all other in vitro and in vivo tests for genetic
toxicity were negative. Overall, the available studies suggest that
HCFC-123 is unlikely to be genotoxic in vivo.
8.6 Reproductive and developmental toxicity
In studies conducted according to OECD guidelines, HCFC-123 was
neither embryotoxic nor teratogenic in pregnant rats exposed for 6 h
per day on days 6-15 of gestation by inhalation to 0, 5000, or 10 000
ppm (0, 31.3, or 62.5 g/m3) HCFC-123, although maternal toxicity in
the form of reduced weight gain and CNS depression were observed at
both exposure levels (Culik & Kelly, 1976; Brewer & Smith, 1977). In a
range-finding study in which fetal examinations were limited to
external structural abnormalities, HCFC-123 was not embryotoxic or
teratogenic in pregnant rabbits exposed for 6 h per day on days 6-18
of gestation by inhalation to 0, 500, 1500, or 5000 ppm (0, 3.13,
9.38, or 31.3 g/m3) HCFC-123, although dose-related maternal
toxicity characterized by reduced weight gain and food consumption was
evident at all exposure levels (Malinverno et al., 1996).
In a two-generation reproductive toxicity study in Sprague-Dawley
rats conducted in accordance with OECD guidelines, male and female
animals were exposed for 6 h per day, 7 days a week, by inhalation to
0, 30, 100, 300, or 1000 ppm (0, 0.188, 0.625, 1.88, or 6.25 g/m3)
HCFC-123 (Hughes, 1994; Malinverno et al., 1996). The F0 (parental
generation) animals were exposed from 6 weeks of age for 23-39 weeks,
including a 2-week mating period, the gestation period, and, except
for maternal animals on postpartum days 0-4, until the offspring were
weaned. The F1 generation was exposed from 4 weeks of age through to
weaning of their litters (F2 generation), for a total of
approximately 28 weeks.
Table 3: Chromosome aberrations in human lymphocytes in vitro.
Physical form of Exposure Concentrationb Mean mitotic Number of Reference
HCFC-123 protocola index cells with
aberrations
(excluding gaps)
Liquid 3-h exposure 0 µg/ml 25.2 1 Dance, 1991
(without S9) 73 µg/ml 19.6 2
146 µg/ml 21.7 3
292 µg/ml 21.1 3
CBC (2 µg/ml) 14.0 91
3-h exposure 0 µg/ml 23.8 1
(with S9) 146 µg/ml 21.8 2
292 µg/ml 14.7 6
584 µg/ml 6.6 5
CP (6 µg/ml) 8.4 106
24-h exposure 0 µg/ml 30.7 2
(without S9) 36 µg/ml 27.4 3
73 µg/ml 21.5 10c
292 µg/ml 9.7 31d
CBC (2 µg/ml) 22.9 70
Vapour 3-h exposure 0 ppm 24.9 1 Edwards, 1991
(without S9) 75 000 ppm 23.9 3
150 000 ppm 17.9 0e
300 000 ppm 10.3 5e
CBC (2 µg/ml) 18.5 60
Table 3 (cont'd)
Physical form of Exposure Concentrationb Mean mitotic Number of Reference
HCFC-123 protocola index cells with
aberrations
(excluding gaps)
3-h exposure 0 ppm 22.2 4
(with S9) 75 000 ppm 24.1 3
150 000 ppm 21.0 4
300 000 ppm 9.5 23e,f
CP (6 µg/ml) 15.2 107
24-h exposure 0 ppm 16.6 1
(without S9) 25 000 ppm 19.1 9d
50 000 ppm 13.2 18f
100 000 ppm 5.9 24f
CBC (2 µg/ml) 13.5 118
a S9 = metabolic activation system.
b Positive controls: CBC = chlorambucil; CP = cyclophosphamide.
c P < 0.05.
d P < 0.01.
e Increase in number of polyploid cells.
f P < 0.001.
The only adverse reproductive effect was a 17% decrease in
implantation count among F1 females at the highest exposure level.
In terms of development, pup growth was impaired during the
pre-weaning period when exposure was confined to the lactating parent
female. In the F1 generation, mean pup weight was decreased by
approximately 10% at exposures at and above 100 ppm (0.625 g/m3),
whereas mean pup weight in F1 offspring (F2 generation) was
decreased by approximately 20% at all exposure levels. In adult rats,
retarded weight gain was observed at 100 ppm (0.625 g/m3) and above
in F0 animals and at 300 ppm (1.88 g/m3) and above in the F1
generation. There was a dose-dependent, 8-39% increase in liver weight
in all exposed F0 groups and an 8-10% increase at 100 ppm (0.625
g/m3) and above in F1 animals. In F1 animals exposed to HCFC-123
for approximately 28 weeks, exposure-related histopathological changes
were confined to a dose-related increase in the incidence of
centrilobular hepatocyte enlargement and in the incidence and degree
of hepatocyte vacuolation at 300 ppm (1.88 g/m3) and above. No
microscopic changes were detected in the livers of F2 weanling rats
from the 1000 ppm (6.25 g/m3) exposure group. In both generations,
there was a decrease in serum triglycerides in both sexes, whereas
serum cholesterol was increased in males and decreased in females.
Levels of ALT, AST, or other liver enzymes were not determined. Male
rats exposed at or above 300 ppm (1.88 g/m3) had increased plasma
levels of luteinizing hormone (LH) after 10 weeks of exposure, which
had reverted to normal at week 38. In this study, the NOAEL, based on
effects on fertility, was 300 ppm (1.88 g/m3). The LOAEL, based on
developmental effects (retarded neonatal growth during lactation) and
on increased liver weight and changes in liver-related clinical
chemistry parameters, was 30 ppm (0.188 g/m3).
After 22 weeks of exposure, a sample of F1 male rats was drawn
from the two-generation reproductive toxicity study for
endocrinological investigations (Sandow et al., 1995b). In 10 males
from each exposure group, serum levels of LH and testosterone were
determined before and after an injection of LH releasing hormone. The
testes of another eight males per group were incubated in vitro with
human chorionic gonadotropin (which stimulates steroid hormone
biosynthesis). The incubation medium and testis tissue were analysed
for content of testosterone, progesterone, estradiol-17-ß, 17
alpha-OH-progesterone, and delta-4-androstenedione. Basal serum LH and
testosterone levels were similar to those of controls. However, after
stimulation with LH releasing hormone, the LH in rats exposed to 300
ppm (1.88 g/m3) was 32% lower than in controls; at 1000 ppm (6.25
g/m3), LH was 39% and testosterone 46% lower than in the control
group. In the ex vivo test, HCFC-123 inhalation did not affect the
secretory capacity for steroid hormones or alter the content of these
hormones in the testes at the end of the incubation period, except for
a slight reduction of delta-4-androstenedione at 1000 ppm (6.25
g/m3).
In an endocrinological study in rats of both sexes, exposure to
5200 ppm (32.5 g/m3) HCFC-123 for 6 h per day (similar to the
highest dose level in the 2-year bioassay) for 14 consecutive days was
associated with sedation, decreased body, kidney, ovary, and pituitary
weights in females, and increased relative liver weight in males
(Hofmann, 1995; Sandow et al., 1995a). In males, the prolactin
response after monoiodotyrosine stimulation, the testosterone response
after stimulation with buserelin (a synthetic gonadotropin releasing
hormone), and the testicular testosterone content were all reduced by
approximately 50%. In females, the gonadotropin response to buserelin
stimulation was enhanced and the pituitary content of follicle
stimulating hormone and prolactin was reduced, in both cases by
approximately 50%.
These endocrinological investigations indicate that HCFC-123 has
little, if any, effect on steroid production in rat testes, but
impairs the prolactin, LH, and testosterone response to pituitary
stimulants. The NOAEL based on this effect was 100 ppm (0.625 g/m3).
A lactation study was conducted in groups of pregnant and
lactating Sprague-Dawley (Crl:CD BR) rats exposed to 0 or 1000 ppm (0
or 6.25 g/m3) HCFC-123 for 6 h per day on days 5-19 of gestation and
on days 5-21 postpartum (Buschman, 1996). Within 2 days of birth,
litters were crossed over between dams to create four groups
comprising exposed or control dams rearing litters from different
exposed or control mothers. Absolute and relative liver weights were
increased and serum triglycerides, cholesterol, and glucose decreased
in dams exposed to HCFC-123. The milk of dams exposed to HCFC-123 was
of normal quantity and quality (with regard to content of protein,
lactose, and fat) but contained trifluoroacetic acid at an average
concentration of 50 µg/ml. Trifluoroacetic acid was also found in the
urine of pups reared by dams exposed to HCFC-123. There were no
differences in absolute or relative liver weight or any abnormal
clinical signs or gross findings in any of the groups of pups, but
pups reared by dams exposed to HCFC-123 had a 10% lower growth rate
and decreased serum triglycerides compared with pups reared by
non-exposed dams. Before crossover, there was no difference between
groups with respect to mean pup and litter weight. As such, these
findings indicate that the retarded neonatal growth observed in the
two-generation reproductive toxicity study was due to factors in the
milk of exposed mothers, probably trifluoroacetic acid, rather than to
exposure in utero.
When groups of four lactating rhesus monkeys and their neonates
were exposed to either 0 or 1000 ppm (0 or 6.25 g/m3) HCFC-123 for 6
h per day for 21-22 consecutive days, there were no effects on
maternal body weight, serum triglycerides, cholesterol, and glucose,
or milk composition (Slauter, 1997). Liver biopsy specimens taken from
the mothers at the end of the study revealed exposure-related lesions,
including mild to moderate centrilobular hepatocyte vacuolation, trace
to moderate centrilobular hepatocyte necrosis, and trace to mild
subacute inflammation. As a rule, HCFC-123 was not detected in the
blood of mothers or neonates, whereas trifluoroacetic acid was present
at concentrations of 9-70 µg/ml in exposed mothers and of 17-190 µg/ml
in neonates, with individual blood levels being 2-6 times higher in
the neonates than in their corresponding mothers. Milk from exposed
mothers contained HCFC-123 and trifluoroacetic acid at concentrations
of 1-5 µg/ml and 17-30 µg/ml, respectively. Although no statistical
analysis was attempted because of the small number of observations,
the average growth rate was 10% lower in exposed neonates than in
unexposed controls.
9. EFFECTS ON HUMANS
The available data on the human health effects of HCFC-123 were
limited to a single case report of dizziness, headache, and nausea in
workers exposed to unknown levels of the chemical following the
rupture of an industrial chiller1 and three case reports of hepatic
effects involving 26 workers following repeated exposure to HCFC-123
vapours.
Nine cases of liver effects were reported in gantry drivers at a
smelting depot in Belgium (Hoet et al., 1997). They occurred 1-4
months after the refrigerant utilized in the crane cabin
air-conditioning system had been replaced by a blend containing 57%
HCFC-123, 40% HCFC-124 (1-chloro-1,2,2,2-tetrafluoroethane), and 3%
propane.2 One driver admitted to hospital was found to have
increased levels of AST, ALT, ALP, gamma-glutamyl transferase, and
total and conjugated bilirubin and decreased prothrombin activity,
with AST and ALT levels being 15-23 times above the upper limit of the
normal range. Autoimmune, viral, and drug- or alcohol-induced
hepatitis were ruled out. A liver biopsy showed focal liver cell
necrosis, plugging of bile ducts, and the presence of
trifluoroacetylated proteins. The symptoms regressed during the period
of non-exposure but recurred when the driver returned to work 2 months
later. Eight other drivers showed signs of varying degrees of liver
abnormalities. Serum antibodies to human liver enzymes (CYP2E1 and/or
protein disulfide isomerase isoform P58) were detected in five of six
cases examined. A workplace inspection revealed that the plastic pipes
of the air-conditioning system were perforated and that refrigerant
was leaking into the crane cabin. No further cases occurred after the
system was repaired. Although the workers were exposed to both
HCFC-123 and HCFC-124, the latter is unlikely to have contributed to
the observed effects, as the NOAEL for HCFC-124 was 50 000 ppm (280
g/m3) in a 90-day inhalation toxicity study in rats (Malley et al.,
1996), whereas the LOAEL for HCFC-123 in a similar study in the same
strain and laboratory was 300 ppm (1.88 g/m3) (Table 1).
Eight cases of liver effects were reported in workers exposed to
the vapours of a solvent degreaser containing HCFC-123.3 Two months
after a US factory converted to using HCFC-123 in its degreaser, two
employees who worked closely with the degreaser were found to have
liver disease. They had elevated blood levels of liver enzymes,
particularly ALT (32-56 times above the upper limit of the normal
1 Carrier Canada Ltd, personal communication, 1993 [cited in
NICNAS, 1996].
2 N. Verlinden, personal communication, 1997 [cited in
NICNAS, 1999].
3 AlliedSignal Inc., personal communication, 1998 [cited in
NICNAS, 1999].
range) and AST (14-33 times above the upper limit of the normal
range), had elevated total and conjugated bilirubin, and tested
negative for viral hepatitis. Subsequent testing of all 27 factory
employees revealed four additional cases of elevated liver enzymes.
When retested 1 month later but before the use of HCFC-123 was
discontinued, five of the affected employees had improved markedly,
whereas one had deteriorated, and there was one new case with slightly
elevated ALT and AST levels. All in all, liver enzymes were elevated
in 3 of 4 employees who worked with the degreaser and in 4 of 23
workers who did not. Air monitoring was conducted when HCFC-123 use
began and again shortly after the first cases were diagnosed. Personal
air monitoring during normal degreaser operations showed 5.5-h
time-weighted average levels of HCFC-123 that ranged from 5.3 to
12.0 ppm (33.1 to 75.0 mg/m3) throughout the facility, whereas
short-term breathing-zone levels ranging from 160 to 460 ppm (1000 to
2880 mg/m3) were measured in workers charging and unloading the
degreaser. There was also one case of elevated liver enzymes with
negative tests for viral hepatitis in a technician employed in the
manufacturer's research laboratory where the degreaser was tested and
evaluated. Static air levels in the laboratory were reported to be
generally below 50 ppm (313 mg/m3) HCFC-123.
In a factory in Japan where miniature heat exchangers were filled
with HCFC-123 in a poorly ventilated room, 9 out of 14 workers were
found to have elevated levels of liver enzymes 4-5 weeks after
production had commenced (Takebayashi et al., 1998a,b).1 Four of
them were clinically ill, and two had jaundice. In these workers, AST
and ALT were up to 20-30 times above the upper limit of the normal
range. After an exhaust system was installed that maintains the
concentration of airborne HCFC-123 at about 1 ppm (6.25 mg/m3),
trifluoroacetic acid was not detected in the workers' urine; at
follow-up 1 year later, there were no further cases of clinical
illness or elevated liver enzymes.1 Exposure levels were not
measured, but a simulation of the original working conditions
indicated static air levels ranging from 5 to 1125 ppm (31.3 to
7030 mg/m3) (6-h time-weighted average), depending on the distance
from the filling area.
1 Also T. Takebayashi, personal communication, 1999 [cited in
NICNAS, 1999].
Table 4: Summary of effects of HCFC-123 in aquatic organisms.
Test Species Effects and effect Comments Reference
levels
Acute toxicity (96 h), Fathead minnow Lethargy Rapid degassing Pierson,
flow-through conditions (Pimephales LC50 > 76 mg/litre of HCFC-123 1990a
promelas) (measured) from test
solutions
Acute toxicity (96 h), Rainbow trout Lethargy, darkened Jenkins, 1992b
static conditions (Oncorhynchus pigmentation at
mykiss) 15 mg/litre and
above
LC50 = 56 mg/litre
(measured)
Immobilization (48 h), Daphnia magna Lethargy Jenkins, 1992c
static conditions EC50 = 17 mg/litre
(measured)
Immobilization (48 h), Daphnia magna Lethargy Measured Pierson, 1990b
static conditions EC50 = 45.8 mg/litre concentrations
(nominal) <75% of nominal
Algal growth inhibition Selenastrum EC50 = 68 Based on biomass Jenkins, 1992d
(96 h), static conditions capricornutum mg/litre (measured) integral
10. EFFECTS ON OTHER ORGANISMS IN THE LABORATORY AND FIELD
The available ecotoxicological data for HCFC-123 are summarized
in Table 4. The data indicate that the chemical is, at most, slightly
toxic to aquatic organisms under conditions of acute exposure. Chronic
effects would not be expected because of limited aquatic persistence.
Trifluoroacetic acid, which is formed by atmospheric breakdown of
HCFC-123, has been found to be of low toxicity in stream mesocosms,
algae, higher plants, fish, and mammals (Boutonnet et al., 1999). The
lowest threshold for any effect was 0.12 mg sodium
trifluoroacetate/litre, above which the chemical had reversible
effects on the growth of the alga Selenastrum capricornutum. In the
most sensitive terrestrial species tested (sunflower), sodium
trifluoroacetate at 1 mg/kg dry soil had clear effects on vegetative
growth, whereas long-term root exposure of wheat and soya to sodium
trifluoroacetate at 1 mg/litre had no effect.
11. EFFECTS EVALUATION
11.1 Evaluation of health effects
11.1.1 Hazard identification and dose-response assessment
There was inadequate information on the human health effects of
short-term exposure to HCFC-123. In laboratory animals, HCFC-123
exhibits low acute toxicity, with an approximate oral lethal dose of
9 g/kg body weight, a 4-h LC50 by inhalation in the range of
28 400-52 600 ppm (178-329 g/m3), and a dermal LD50 in excess of
2000 mg/kg body weight. It is not a skin irritant or sensitizer, but
liquid HCFC-123 produces mild to moderate eye irritation. The critical
effects associated with acute exposure are non-fatal liver damage, CNS
depression, and cardiac sensitization to adrenaline. When inhaled in
lethal concentrations, death was caused by severe CNS depression. For
a single, 4-h exposure by inhalation, the LOAEL was 1000 ppm
(6.25 g/m3) for liver damage, based on liver cell necrosis and
increased levels of circulating liver enzymes in guinea-pigs, and 5000
ppm (31.3 g/m3) for CNS depression, based on failures in
unconditioned reflexes in rats. The NOAEL for cardiac sensitization to
adrenaline in dogs was 10 000 ppm (62.5 g/m3). Although the dog is
a very sensitive species, cardiac sensitization to adrenaline-induced
arrhythmia is likely to be a relevant critical effect resulting from
short-term exposure in humans, such as the sudden discharge of fire
extinguishants in occupied rooms (NAS, 1996). Under these
circumstances, CNS depression may also be a critical effect. A large
number of short-chain halogenated hydrocarbons with different
metabolic patterns have similar CNS and cardiac effects, which likely
result from a direct anaesthetic action on neurons and myocardial
cells (IPCS, 1990, 1991, 1992).
The critical effects associated with repeated exposure to
HCFC-123 vapours are liver damage in humans as well as experimental
animals, in addition to neonatal growth retardation, an increased
incidence of benign tumours, and CNS depression, which have been
recorded only in animals.
Based on a limited number of case reports, biochemical
abnormalities associated with liver injury have been observed in
humans at exposure levels in the order of 5-1125 ppm (31.3-7030
mg/m3). The available data are insufficient to define the
dose-response relationship in humans. Liver damage was also seen in
rats, guinea-pigs, dogs, and monkeys. The lesions generally involved
increased liver weight accompanied by hepatocyte enlargement and
vacuolation at the lowest exposure levels, with necrosis, fatty
change, and mild subacute inflammation at higher concentrations. They
were associated with or preceded by increased levels of circulating
liver enzymes and decreased serum triglycerides, glucose, and
cholesterol. The LOAEL for liver effects in animals recorded in a
well-conducted two-generation reproductive toxicity study in rats
equalled 30 ppm (188 mg/m3).
The cytotoxicity of HCFC-123 is probably due to the reactive
metabolite trifluoroacetyl chloride, which can bind covalently to
proteins and interfere with their function and/or alter their
antigenicity. The 200 : 20 : 1 ratio of trifluoroacetylation of liver,
kidney, and heart tissue proteins reported by Huwyler et al. (1992)
and Huwyler & Gut (1992) correlates well with the observed effect
levels for these organs in subchronic toxicity studies (Table 1) and
probably reflects tissue differences in metabolic capacity.
There is no evidence that HCFC-123 is teratogenic in laboratory
animals or induces reproductive or fetal toxicity at levels of
exposure lower than those that cause other systemic effects in adults.
Growth was retarded in neonatal rats and monkeys reared by dams
exposed by inhalation to HCFC-123, with a LOAEL of 30 ppm
(188 mg/m3). The main metabolite of HCFC-123, trifluoroacetic acid,
was found in the milk of the dams. As such, breastfed babies may
represent a subpopulation that is uniquely sensitive to HCFC-123.
Reversible CNS depression was seen consistently in repeated-dose
inhalation studies, but did not change in severity or duration with
the number of exposures and was not associated with morphological
changes in nervous tissues. As such, the mechanism of action is
probably the same for both acute and chronic CNS depression. The
lowest NOAEL for CNS effects from repeated administration was recorded
in a neurotoxicity study in rats and equalled 300 ppm (1880 mg/m3),
based on a reduction in arousal.
Although there was evidence of clastogenic activity in human
lymphocytes in vitro at high, cytotoxic concentrations, all other
in vitro and in vivo tests for genetic toxicity were negative.
Therefore, the evidence suggests that HCFC-123 is unlikely to be
genotoxic in vivo.
In a 2-year inhalation study in rats, there was no
exposure-related effect on the incidence of malignant tumours, but
there was an increased incidence of hepatocellular adenomas,
cholangiofibromas, pancreatic acinar cell adenomas, and Leydig
adenomas. There was also an increase in the pre-cancerous lesions of
hepatic foci of alteration, cholangiofibrosis, focal pancreatic acinar
cell hyperplasia, and Leydig cell hyperplasia (Table 2). As stated
above, HCFC-123 is unlikely to be genotoxic in vivo. The minor,
non-mutagenic metabolite 2-chloro-1,1,1-trifluoroethane caused an
increased incidence of uterine carcinomas and Leydig cell adenomas in
rats when given by oral gavage at 300 mg/kg body weight for 52 weeks
(IPCS, 1992). However, only trace quantities would be formed from the
metabolism of HCFC-123. Therefore, it is necessary to examine the
mechanisms of tumour formation to establish if the modes of induction
can be ruled out as relevant for humans:
* Hepatocellular adenomas. Several repeated-exposure studies have
shown that HCFC-123 and its main metabolite, trifluoroacetic
acid, like several other structurally diverse chemicals, induce
peroxisome proliferation in rat hepatocytes (Warheit, 1993; Rusch
et al., 1994; Malley et al., 1995; Keller et al., 1998).
Peroxisome proliferators are generally not genotoxic but induce
hepatocellular proliferation in rats and mice through a mechanism
that appears to involve the expression of growth factors by
hepatic macrophages, thus leading to liver tumour formation
(Chevalier & Roberts, 1998). The hepatocellular adenomas seen in
rats exposed to HCFC-123 may be related to the induction of
peroxisome proliferation, which is a mechanism of questionable
relevance for humans (Ashby et al., 1994). However, since the
chemical is also hepatotoxic, which may have a role in the tumour
formation, it is not possible to discount the hepatocellular
adenomas as being of no concern to humans. It is, however,
reasonable to adopt a threshold approach, based on adverse
effects on the liver in subchronic exposure studies.
* Cholangiofibromas. Cholangiofibromas in the rat are atypical
glandular structures lined by intestinal-like epithelium
surrounded by dense connective tissue. Limited evidence from
animal studies of various non-genotoxic chemicals, including
chloroform and furan, suggests that this tumour type is
associated with significant hepatocyte necrosis and regenerative
cell proliferation that are relevant only at high dose/exposure
levels (Elmore & Sirica, 1993; Jamison et al., 1996). In the
2-year rat study, cholangiofibromas and cholangiofibrosis
occurred only in females exposed to HCFC-123 at 5000 ppm (31.3
g/m3). The incidence of basophilic and eosinophilic foci of
hepatocellular proliferation was also higher in females than in
males (Table 2). Although the threshold level for the induction
of cholangiofibromas in female rats was high, there is no
mechanistic evidence that this tumour type can be dismissed with
regard to its relevance to humans.
* Pancreatic acinar cell adenomas. Some hepatocarcinogenic
peroxisome proliferators have been reported to induce tumours in
other organs, including pancreatic acinar cell adenomas and
Leydig cell adenomas, although these extrahepatic tumours appear
not to be associated with peroxisome proliferation in the target
organ (IARC, 1995). Although pancreatic acinar cell adenomas were
found only in males at 1000 and 5000 ppm (6.25 and 31.3 g/m3),
the incidence was dose-related. Moreover, pancreatic acinar cell
hyperplasia occurred in both sexes, likewise in a dose-dependent
manner (Table 2). As such, until more is known about the
mechanism for acinar cell tumour induction in animals and humans,
the possibility that the pancreatic adenomas found in rats
exposed to HCFC-123 may have some relevance to humans cannot be
discounted.
* Leydig cell adenomas. The available studies indicate that
exposure to HCFC-123 may be associated with endocrine
disturbances in male rats, particularly in relation to prolactin
release and serum LH concentrations. In rats, but not in humans,
a decrease in serum prolactin causes a decrease in the number of
LH receptors on Leydig cells and thus a decrease in testosterone
production, which results in increased LH levels that in turn may
induce Leydig cell hyperplasia and adenomas (Clegg et al., 1997).
As such, it is conceivable that intermittent exposure to HCFC-123
could lead to fluctuations in prolactin and testosterone that in
rats could induce transient increases in LH and, with time,
Leydig cell growth and tumours. Although prolactin fluctuations
would not be of concern in men, the effects of HCFC-123 on the
sex hormone system are complex. Thus, in the absence of data from
studies in primates, the increased incidence of Leydig cell
adenomas in the 2-year rat study cannot be dismissed with respect
to its relevance for humans.
In summary, it is likely that the benign tumours in the 2-year
rat bioassay involve one or more non-genotoxic mechanisms, including
peroxisome proliferation, hepatocellular damage, necrosis and
regenerative proliferation, and disturbance of the
hypothalamic-pituitary-testicular axis. Although humans may be less
sensitive to tumours arising from some of these actions, overall it is
not possible to discount the tumours in an evaluation of the potential
risk for humans. Therefore, the increased incidence of benign tumours
in the rat raises some concern with respect to the potential
carcinogenicity in humans. The tumours probably arise from
non-genotoxic mechanisms. In the 2-year bioassay, the tumour incidence
was increased at 300 ppm (1.88 g/m3), the lowest level tested, and a
NOAEL was not established. In subchronic toxicity studies, the LOAEL
based on any adverse effect on the liver was 30 ppm (0.188 g/m3)
(Hughes, 1994; Malinverno et al., 1996).
11.1.2 Criteria for setting tolerable intakes or guidance values
for HCFC-123
Derivation of a guidance value for HCFC-123 was outside the scope
of the source documents (NICNAS, 1996, 1999). General advice about the
derivation of tolerable intakes and guidance values for health-based
exposure limits is set out in Environmental Health Criteria 170 (IPCS,
1994).
Exposure of the general public to HCFC-123 is likely to be
minimal. The main risk to human health is through repeated
occupational exposure via inhalation.
The critical effects of repeated low-level exposure to HCFC-123
are liver damage, which has been observed in all species investigated,
including monkeys and humans, and retarded neonatal growth during
lactation, which has been observed in rats and monkeys. In the absence
of sufficient data to establish a dose-response relationship in
humans, a guidance value for exposure to HCFC-123 must be based on the
observed effect levels for liver lesions and retarded neonatal growth
during lactation in pivotal animal studies. For both of these critical
effects, a LOAEL of 30 ppm (188 mg/m3) was established in a
well-conducted two-generation reproductive toxicity study in rats,
whereas a NOAEL was not achieved (Hughes, 1994; Malinverno et al.,
1996).
The uncertainty factor applied to extrapolate from the LOAEL to a
NOAEL must allow for the fact that whereas the recorded liver effects
at the LOAEL were mild, hepatotoxicity may have a role in the
induction of benign liver tumours in rats. Moreover, the growth
retardation in neonates during lactation was in the order of 10-20%.
It is likely that the liver effects are related to the formation of
protein adducts with trifluoroacetyl chloride and that growth
retardation in neonates is related to exposure to trifluoroacetic acid
in maternal milk. Therefore, the uncertainty factor used to
extrapolate the NOAEL from rats to humans must allow for the lack of
in vivo data on the metabolic rate and other toxicokinetic
properties of HCFC-123 in humans. Finally, an additional uncertainty
factor must be applied to allow for human variability in
toxicokinetics, as the metabolism of HCFC-123 to trifluoroacetyl
chloride and trifluoroacetic acid is catalysed by CYP2E1
(Urban et al., 1994), whose activity is known to be influenced by
genetic polymorphism, body weight, and dietary factors (Le Marchand
et al., 1999).
11.1.3 Sample risk characterization
Adverse effects of HCFC-123 in animals and humans have been
observed only at concentrations that were several orders of magnitude
higher than those in the only known medium of exposure (air) in the
general environment. The likelihood of public exposure as a
consequence of catastrophic accidents or fire extinguishant discharges
is very small, and the scale and duration of such exposures are
expected to be low.
With respect to repeated occupational exposures, 3- to 8-h
time-weighted average personal exposure levels in an HCFC-123
manufacturing plant were reported to be below 10 ppm (62.5 mg/m3).
Reported 2- to 12-h time-weighted average breathing-zone levels in
machinery rooms containing air-conditioning equipment generally ranged
from below 1 to 5 ppm (below 6.25 to 31.3 mg/m3), whereas the use of
a liquid HCFC-123 degreaser was associated with concentrations in the
range of 5.3-12 ppm (33.1-75.0 mg/m3) HCFC-123. The available case
reports indicate that humans may develop biochemical signs of liver
disease, such as elevated AST and ALT, after 1-4 months of repeated
exposure to HCFC-123 at levels above 5 ppm (31.3 mg/m3). Because the
effects of low levels of HCFC-123 are due to toxic metabolites formed
through CYP2E1, genetic, lifestyle, and dietary factors are expected
to cause considerable variability in human susceptibility to the
chemical.
11.2 Evaluation of environmental effects
Because of its high volatility, HCFC-123 released to the
environment will partition almost entirely to the atmosphere. It is
removed predominantly in the troposphere by reaction with hydroxyl
radicals to form trifluoroacetic acid, and only a small fraction is
transported to the stratosphere, where it may undergo photolysis and
release chlorine radicals that catalyse the destruction of ozone.
Because of its short atmospheric lifetime, estimated at 1.4 years, its
ozone-depleting potential is low (0.02 relative to CFC-11). The global
warming potential of HCFC-123 relative to carbon dioxide is 300, 93,
and 29 over a time horizon of 20, 100, and 500 years, respectively
(WMO, 1995).
The aquatic EC50/LC50 values were below 100 mg/litre but
above 10 mg/litre. As such, the chemical meets the European Community
criteria for classification as harmful to the environment (Berends et
al., 1999) and the globally harmonized criteria for classification as
hazardous to the aquatic environment (Class: Acute III) (OECD, 1998).
However, while HCFC-123 may be released to surface waters or soil, it
is unlikely to persist in these media because of its high volatility.
As such, it is considered that HCFC-123 does not constitute a
long-term or delayed danger to the aquatic environment.
Trifluoroacetic acid formed by degradation of HCFC-123 will
precipitate in rain and may accumulate in closed aquatic systems such
as salt lakes and seasonal wetlands. The maximum total contemporary
deposition rate of trifluoroacetic acid from fluorocarbons has been
estimated at 2800 tonnes a year, with 27% derived from HCFC-123 and
the remainder from HCFC-124, HFC-134a, HFC-227ea, and the anaesthetic
gases halothane and isoflurane (Boutonnet et al., 1999). In 2020, the
maximum deposition from fluorocarbons is predicted to reach 160 000
tonnes a year, yielding a maximum average concentration of
trifluoroacetic acid in rainwater of 0.1 µg/litre, which is several
orders of magnitude lower than the no-effect level in both surface and
soil water. By that year, HCFC-123 emissions will have declined, as
the chemical will have been phased out in accordance with the Montreal
Protocol. As such, it can be concluded that environmental levels of
trifluoroacetic acid resulting from the breakdown of HCFC-123 do not
pose a threat to the environment.
12. PREVIOUS EVALUATIONS BY INTERNATIONAL BODIES
A previous evaluation of HCFC-123 has been carried out by the
International Programme on Chemical Safety (IPCS, 1992).
Information on international hazard classification and labelling
is included in the International Chemical Safety Card reproduced in
this document (Appendix 4).
REFERENCES
AIHA (1998) Workplace environmental exposure level guide series:
1,1,1-Trifluoro-2,2-dichloroethane. Fairfax, VA, American Industrial
Hygiene Association Press.
Ashby J, Brady A, Elcombe CR, Elliott BM, Ishmael J, Odum J, Tugwood
JD, Kettle S, Purchase IF (1994) Mechanistically-based human hazard
assessment of peroxisome proliferator-induced hepatocarcinogenesis.
Human and experimental toxicology, 13 (Suppl. 2):S1-S117.
Berends AG, de Rooij CG, Shin-ya S, Thompson RS (1999) Biodegradation
and ecotoxicity of HFCs and HCFCs. Archives of environmental
contamination and toxicology, 36(2):146-151.
Boutonnet JC, Bingham P, Calamari D, de Rooij C, Franklin J, Kawano T,
Libre JM, McCulloch A, Malinverno G, Odom JM, Rusch GM, Smythe K,
Sobolev I, Thompson R, Tiedje JM (1999) Environmental risk assessment
of trifluoroacetic acid. Human and ecological risk assessment,
5(1):59-124.
Brewer WE, Smith S (1977) Teratogenic study via inhalation with
Genetron 123 in albino rats. Morristown, NJ, Allied Chemical
Corporation.
Britelli MR (1975) Eye irritation test in rabbits. Newark, DE, Du
Pont de Nemours and Co.
Brock WJ (1988a) Acute dermal toxicity study of HCFC-123 in rabbits.
Newark, DE, Du Pont de Nemours and Co.
Brock WJ (1988b) Acute dermal toxicity study of HCFC-123 in rats.
Newark, DE, Du Pont de Nemours and Co.
Brock WJ (1988c) Primary dermal irritation study with HCFC-123 in
rabbits. Newark, DE, Du Pont de Nemours and Co.
Buschman J (1996) Crossover study with HCFC 123 in lactating
Sprague-Dawley rats including additional studies on milk production
and metabolites in offspring urine. Hannover, Fraunhofer Institute
for Toxicology and Aerosol Research.
Callander RD (1989) HCFC-123 -- an evaluation using the Salmonella
mutagenicity assay. Macclesfield, Cheshire, Imperial Chemical
Industries Ltd.
Chang W-K, Criddle CS (1995) Biotransformation of HCFC-22, HCFC-142b,
HCFC-123 and HFC-134a by methanotrophic mixed culture MM1.
Biodegradation, 6(1):1-9.
Chevalier S, Roberts RA (1998) Perturbation of rodent hepatocyte
growth control by nongenotoxic hepatocarcinogens -- mechanisms and
lack of relevance for human health. Oncology reports, 5:1319-1327.
Clayton JW (1966) Acute inhalation toxicity. Newark, DE, Du Pont de
Nemours and Co.
Clegg ED, Cook JC, Chapin RE, Foster PMD, Daston GR (1997) Leydig cell
hyperplasia and adenoma formation: Mechanisms and relevance to humans.
Reproductive toxicology, 11:107-121.
Coate WB (1976) LC50 of G123 in rats. Vienna, VI, Hazleton
Laboratories America Inc.
Cohen SD, Pumford NR, Khairallah EA, Boekelheide K, Pohl LR, Amouzadeh
HR, Hinson JA (1997) Selective protein covalent binding and target
organ toxicity. Toxicology and applied pharmacology, 143:1-12.
Coombs DW (1994) HCFC-123 -- 13-week inhalation neurotoxicity study
in the rat. Cambridgeshire, Huntingdon Research Centre Ltd.
Culik R, Kelly DP (1976) Embryotoxic and teratogenic studies in rats
with inhaled dichlorofluoromethane (Freon 21) and
2,2-dichloro-1,1,1-trifluoroethane (FC-123). Newark, DE, Du Pont de
Nemours and Co.
Dance CA (1991) In vitro assessment of the clastogenic activity of
HCFC-123 in cultured human lymphocytes. Eye, Suffolk, Life Science
Research Ltd. (No. 91/PFE003/0093).
Darr RW (1981) An acute inhalation toxicity study of
Fluorocarbon 123 in the Chinese hamster. Morristown, NJ, Allied
Corporation.
Dekant W (1993) Metabolism of 1,1-dichloro-2,2,2-trifluoroethane
(HCFC-123). Würzburg, Institute of Toxicology, University of
Würzburg (Report No. MA-250B-82-207).
Dodd DE, Brashear WT, Vinegar A (1993) Metabolism and pharmacokinetics
of selected halon replacement candidates. Toxicology letters,
68:37-47.
Doleba-Crowe C (1978) 90-day inhalation exposure of rats and dogs to
vapours of 2,2-dichloro-1,1,1-trifluoroethane (FC-123) (summary).
Newark, DE, Du Pont de Nemours and Co. [cited in NICNAS, 1996].
Du Pont (1993) Workplace guidelines for Suva(R) Centri-LP
(HCFC-123) in refrigeration and air conditioning applications.
Wilmington, DE, Du Pont de Nemours and Co.
Edwards CN (1991) HCFC-123 (vapour phase): In vitro assessment of
the clastogenic activity in cultured human lymphocytes. Eye,
Suffolk, Life Science Research Ltd. (No. 91/PFE002/0125).
Elmore LW, Sirica AE (1993) "Intestinal-type" of adenocarcinoma
preferentially induced in right/caudate liver lobes of rats treated
with furan. Cancer research, 53:254-259.
Frank H, Klein A, Renschen D (1996) Environmental trifluoroacetate.
Nature, 382:34.
Fraser P (1994) CSIRO report to SPA-AFEAS. Canberra, Commonwealth
Scientific and Industrial Research Organisation.
Goodman NC (1975) Primary skin irritation and sensitization tests on
guinea pigs. Newark, DE, Du Pont de Nemours and Co.
Hall GT, Moore BL (1975) Acute inhalation toxicity on Freon 123.
Newark, DE, Du Pont de Nemours and Co.
Harris JW, Jones JP, Martin JL, LaRosa AC, Olson MJ, Pohl LR, Anders
MW (1992) Pentahaloethane-based chlorofluorocarbon substitutes and
halothane: correlation of in vivo hepatic protein
trifluoroacetylation and urinary trifluoroacetic acid excretion with
calculated enthalpies of activation. Chemical research
in toxicology, 5:720-725.
Hayman GD, Jenkin ME, Murrells TP, Johnson CE (1994) Tropospheric
degradation chemistry of HCFC-123 (CF3CHCl2): a proposed
replacement chlorofluorocarbon. Atmospheric environment, 28:421-437.
Henry JE (1975) Acute oral test on FC-123. Newark, DE, Du Pont de
Nemours and Co. [cited in NICNAS, 1996].
Hoet P, Graf MLM, Bourdi M, Pohl LR, Duray PH, Chen W, Peter RM,
Nelson SD, Verlinden N, Lison D (1997) Epidemic of liver disease
caused by hydrochlorofluorocarbons used as ozone-sparing substitutes
of chlorofluorocarbons. Lancet, 350:556-559.
Hofmann T (1995) HCFC 123, HCFC 141B and HCF 134a, testing for
subacute (2 weeks) inhalation toxicity in male and female Sprague
Dawley rats. Frankfurt am Main, Hoechst Aktiengesellschaft.
Hughes EW (1994) HCFC 123: A study of the effect on reproductive
function of two generations in the rat. Huntingdon, Cambridgeshire,
Huntingdon Research Centre Ltd.
Huwyler J, Gut J (1992) Exposure to the chlorofluorocarbon substitute
2,2-dichloro-1,1,1-trifluoroethane and the anaesthetic agent halothane
is associated with transient adduct formation in the heart.
Biochemical and biophysical research communications, 184:1344-1349.
Huwyler J, Aeschlimann D, Christen U, Gut J (1992) The kidney as a
novel target tissue for protein adduct formation associated with
metabolism of halothane and the candidate chlorofluorocarbon
replacement 2,2-dichloro-1,1,1-trifluoroethane. European journal of
biochemistry, 207:229-238.
IARC (1995) Peroxisome proliferation and its role in carcinogenesis:
views and expert opinions of an IARC working group, Lyon, 7-11
December 1994. Lyon, International Agency for Research on Cancer.
ICI (1992) An evaluation in the in vitro cytogenetic assay using
human lymphocytes. Macclesfield, Cheshire, Imperial Chemical
Industries Ltd. [cited in AIHA, 1998].
Industrial Bio-Test Laboratories (1977) 90-day subacute inhalation
toxicity study with Genetron 123 in albino rats. Northbrook, IL,
Industrial Bio-Test Laboratories, Inc.
IPCS (1990) Fully halogenated chlorofluorocarbons. Geneva, World
Health Organization, International Programme on Chemical Safety
(Environmental Health Criteria 113).
IPCS (1991) Partially halogenated chlorofluorocarbons (methane
derivatives). Geneva, World Health Organization, International
Programme on Chemical Safety (Environmental Health Criteria 126).
IPCS (1992) Partially halogenated chlorofluorocarbons (ethane
derivatives). Geneva, World Health Organization, International
Programme on Chemical Safety (Environmental Health Criteria 139).
IPCS (1998) International Chemical Safety Card
-- 2,2-Dichloro-1,1,1-trifluoroethane. Geneva, World Health
Organization, International Programme on Chemical Safety (ICSC 1343).
IPCS (1994) Assessing human health risks of chemicals: Derivation of
guidance values for health-based exposure limits. Geneva, World
Health Organization, International Programme on Chemical Safety
(Environmental Health Criteria 170).
Jamison KC, Larson JL, Butterworth BE, Harden R, Skinner BL, Wolf DC
(1996) A non-bile duct origin for intestinal crypt-like ducts with
peritubular fibrosis induced in livers of F344 rats by chloroform
inhalation. Carcinogenesis, 17:675-682.
Jenkins CA (1992a) HCFC-123 (liquid): Biotic degradation closed
bottle test. Eye, Suffolk, Life Sciences Research Ltd. (No.
91/PFE008/0477).
Jenkins CA (1992b) HCFC-123: Acute toxicity to rainbow trout. Eye,
Suffolk, Life Sciences Research Ltd. (No. 91/PFE004/0939).
Jenkins CA (1992c) HCFC-123: Acute toxicity to Daphnia magna. Eye,
Suffolk, Life Sciences Research Ltd. (No. 91/PFE006/0972).
Jenkins CA (1992d) HCFC-123: Determination of its EC50 to
Selenastrum capricornutum. Eye, Suffolk, Life Sciences Research Ltd
(No. 91/PFE007/0935).
Keller DA, Lieder PH, Brock WJ, Cook JC (1998)
1,1,1-Trifluoro-2,2-dichloroethane (HCFC-123) and
1,1,1-trifluoro-2-bromo-2-chloroethane (halothane) cause similar
biochemical effects in rats exposed by inhalation for five days.
Drug and chemical toxicology, 21:405-415.
Kelly DP (1989) Four-week inhalation toxicity study with HCFC-123
in rats. Newark, DE, Du Pont de Nemours and Co.
Kennelly JC (1993) HCFC 123: Assessment for the introduction of
unscheduled DNA synthesis in rat liver after inhalation exposure.
Macclesfield, Cheshire, Zeneca Ltd.
Kotamarthi VR, Rodriguez JM, Ko MKW, Tromp TK, Sze ND, Prather MJ
(1998) Trifluoroacetic acid from degradation of HCFCs and HFCs -- a
three-dimensional modelling study. Journal of geophysical research,
103:5747-5758.
Le Marchand L, Wilkinson GR, Wilkens LR (1999) Genetic and dietary
predictors of CYP2E1 activity: a phenotyping study in Hawaii Japanese
using chlorzoxazone. Cancer epidemiology, biomarkers and prevention,
8:495-500.
Lewis RW (1990) 28-day inhalation study to assess changes in rat
liver and plasma. Macclesfield, Cheshire, Imperial Chemical
Industries Ltd.
Lind RC, Gandolfi AJ, Hall PM (1995) Biotransformation and
hepatotoxicity of HCFC-123 in the guinea pig: Potentiation of hepatic
injury by prior glutathione depletion. Toxicology and applied
pharmacology, 134:175-181.
Loizou GD, Urban G, Dekant W, Anders MW (1994) Gas-uptake
pharmacokinetics of 2,2-dichloro-1,1,1-trifluoroethane (HCFC-123).
Drug metabolism and disposition, 22:511-517.
Longstaff E, Robinson M, Bradbrook C, Styles JA, Purchase IF (1984)
Genotoxicity and carcinogenicity of fluorocarbons: Assessment by
short-term in vitro tests and chronic exposure in rats. Toxicology
and applied pharmacology, 72:15-31.
Malinverno G, Rusch GM, Millischer RJ, Hughes EW, Schroeder RE, Coombs
DW (1996) Inhalation teratology and reproduction studies with
1,1-dichloro-2,2,2-trifluoroethane (HCFC-123). Fundamental and
applied toxicology, 23:276-287.
Malley LA (1990) Subchronic inhalation toxicity: 90-day study with
HCFC-123 in rats. Newark, DE, Du Pont de Nemours and Co.
Malley LA (1992) Combined chronic toxicity/oncogenicity study with
HCFC-123: Two-year inhalation toxicity study in rats. Newark, DE, Du
Pont de Nemours and Co.
Malley LA, Carakostas M, Hansen JF, Rusch GM, Kelly DP, Trochimowicz
HJ (1995) Two-year inhalation toxicity study in rats with
hydrochlorofluorocarbon 123. Fundamental and applied toxicology,
25:101-114.
Malley LA, Carakostas M, Elliott GS, Alvarez L, Schroeder RE, Frame
SR, Van Pelt C, Trochimowicz HJ, Rusch GM (1996) Subchronic toxicity
and teratogenicity of 2-chloro-1,1,1,2-tetrafluoroethane (HCFC-124).
Fundamental and applied toxicology, 32:11-22.
Marit GB, Dodd DE, George ME, Vinegar A (1994) Hepatotoxicity in
guinea pigs following acute exposure to
1,1-dichloro-2,2,2-trifluoroethane. Toxicologic pathology,
22(4):404-414.
Marshal RR (1992) Evaluation of chromosome aberration frequencies in
cultured peripheral blood lymphocytes from rats treated with
HCFC-123. Harrogate, Yorkshire, Hazleton Microtest.
MRI (1991) Results of employee exposure monitoring for HCFC-123 at
centrifugal chiller installations. Final report submitted to the US
Environmental Protection Agency. Silver Spring, MD, Meridian Research,
Inc.
MRI (1993a) Assessment of firefighter exposure to HCFC-123 during
extinguishant efficiency tests conducted at the United States Naval
Air Station in Beaufort, South Carolina. Draft report submitted to
the US Environmental Protection Agency. Silver Spring, MD, Meridian
Research, Inc.
MRI (1993b) Assessment of firefighter exposure to HCFC-123 during
fire extinguisher use in aircraft hangars. Draft report submitted to
American Pacific Corporation. Silver Spring, MD, Meridian Research,
Inc.
Muller W, Hofmann T (1988) HCFC-123 -- micronucleus test in male and
female NMRI mice after inhalation. Frankfurt am Main, Hoechst
Aktiengesellschaft.
Mullin LS (1976) Behavioural toxicity testing of Fluorocarbon 123
in rats. Newark, DE, Du Pont de Nemours and Co.
NAS (1996) Toxicity of alternatives to chlorofluorocarbons: HFC-134a
and HCFC-123. Washington, DC, National Academy of Sciences, National
Academy Press.
NICNAS (1996) Priority Existing Chemical No. 4 --
2,2-Dichloro-1,1,1-trifluoroethane (HCFC-123), full public report,
National Industrial Chemicals Notification and Assessment Scheme.
Canberra, Australian Government Publishing Service.
NICNAS (1999) 2,2-Dichloro-1,1,1-trifluoroethane (HCFC-123):
Secondary Notification No. 4S, full public report. National
Industrial Chemicals Notification and Assessment Scheme. Sydney,
National Occupational Health and Safety Commission.
OECD (1998) Agreed harmonized integrated hazard classification
system for human health and environmental effects of chemical
substances. Paris, Organisation for Economic Co-operation and
Development, Environmental Health and Safety Division.
Oremland RS, Lonergan DJ, Culbertson CW, Lovley DR (1996) Microbial
degradation of hydrofluorocarbons (CHCl2F and CHCl2CF3) in soils
and sediments. Applied and environmental microbiology,
62(5):1818-1821.
Pierson K (1990a) Flow-through acute 96 hour LC50 of HCFC-123 in
fathead minnows (Pimephales promelas ). Newark, DE, Du Pont de
Nemours and Co.
Pierson K (1990b) Static acute 48 hour LC50 of HCFC-123 in Daphnia
magna . Newark, DE, Du Pont de Nemours and Co.
Rusch GM, Trochimowitz HJ, Malley LJ, Kelly DP, Peckham J, Hansen J,
Charm JB (1994) Subchronic inhalation toxicity studies with
hydrochlorofluorocarbon 123 (HCFC 123). Fundamental and applied
toxicology, 23:169-178.
Sandow J, Jerabek-Sandow G, Fenner-Nau D (1995a) Effect of
fluorocarbons on pituitary-gonadal function in a 14-day inhalation
toxicity study: HCFC 123 (Frigen), HCFC 141b (difluorchlorethane)
and HFC 134a (tetrafluorethane). Frankfurt am Main, Hoechst
Aktiengesellschaft.
Sandow J, Rechenberg W, Jerabek-Sandow G (1995b) Effect of HCFC-123
on androgen biosynthesis and gonadotropin secretion in rats.
Frankfurt am Main, Hoechst Aktiengesellschaft.
Sibley H (1992) A study for determining refrigerant exposure levels
while servicing an HCFC-123 centrifugal chiller. Syracuse, NY,
Carrier Corporation.
Slauter RW (1997) HCFC 123: Inhalation study in pregnant monkeys to
assess milk transfer and composition following postpartum exposure.
Mattawan, MI, MPI Research.
Takebayashi T, Kabe I, Endo Y, Tanaka S, Miyauchi H, Nozi K, Takahashi
K, Omae K (1998a) Acute liver dysfunction among workers exposed to
2,2-dichloro-1,1,1-trifluoroethane (HCFC-123): A case report.
Journal of occupational health, 40:169-170.
Takebayashi T, Kabe I, Endo Y, Tanaka S, Miyauchi H, Nozi K, Imamiya
S, Takahashi K, Omae K (1998b) Exposure to
2,2-dichloro-1,1,1-trifluoroethane (HCFC-123): A causal inference.
Journal of occupational health, 40:334-338.
Tanaka S, Kabe I, Takebayashi T, Endo Y, Miyauchi H, Nozi K, Takahashi
K, Seki Y, Omae K (1998) Environmental and biological monitoring of
2,2-dichloro-1,1,1-trifluoroethane (HCFC-123). Journal of
occupational health, 40:348-349.
Trane Company (1991) Report on testing and analysis of the
concentration of HCFC-123 in field installations with general
machinery rooms containing hermetic centrifugal chillers. La Crosse,
WI, The Trane Company.
Trane Company (1992) Report of worker exposure to HCFC-123 during
servicing of hermetic centrifugal chillers. La Crosse, WI, The Trane
Company.
Trochimowicz HJ, Mullin LS (1973) Cardiac sensitization potential
(EC50) of trifluoro-dichloroethane. Newark, DE, Du Pont de Nemours
and Co.
UNEP (1999) Ozone treaties. <http://www.unep.org/ozone/
treaties.htm>. United Nations Environment Programme.
Urban G, Dekant W (1994) Metabolism of
1,1-dichloro-2,2,2-trifluoroethane in rats. Xenobiotica, 24:881-892.
Urban G, Speerschneider P, Dekant W (1994) Metabolism of the
chlorofluorocarbon substitute 1,1-dichloro-2,2,2-trifluoroethane by
rat and human liver microsomes: the role of cytochrome P450 2E1.
Chemical research in toxicology, 7:170-176.
Vinegar A, Williams RJ, Fisher JW, McDougal JN (1994) Dose-dependent
metabolism of 2,2-dichloro-1,1,1-trifluoroethane: A physiologically
based pharmacokinetic model in the male Fischer 344 rat. Toxicology
and applied pharmacology, 129:103-113.
Wang Y, Olson MJ, Baker MT (1993) Interaction of fluoroethane
chlorofluorocarbon (CFC) substitutes with microsomal cytochrome P450.
Biochemical pharmacology, 46:87-94.
Warheit DB (1993) Mechanistic studies with HCFC-123. Newark, DE, Du
Pont de Nemours and Co.
Williams RJ, Vinegar A, McDougal JN, Jarabek AM, Fisher JW (1996) Rat
to human extrapolation of HCFC-123 kinetics deduced from halothane
kinetics -- a corollary approach to physiologically based
pharmacokinetic modeling. Fundamental and applied toxicology,
30:55-66.
WMO (1995) Scientific assessment of ozone depletion. Geneva, World
Meteorological Organization (Global Ozone Research and Monitoring
Project Report No. 37).
Wujcik CE, Zehavi D, Seiber JN (1998) Trifluoroacetic acid levels in
1994-1996 fog, rain, snow and surface waters from California and
Nevada. Chemosphere, 36:1233-1245.
APPENDIX 1 -- SOURCE DOCUMENTS
NICNAS (1996): Priority Existing Chemical No. 4 --
2,2-Dichloro-1,1,1-trifluoroethane (HCFC-123), full public report,
National Industrial Chemicals Notification and Assessment Scheme
Copies of the NICNAS (1996) report on HCFC-123 (prepared by S.
Batt, L. Onyon, L. Slosu, and D. Willcocks) may be obtained from:
NICNAS
Existing Chemicals
GPO Box 58
Sydney NSW 2001
Australia
NICNAS reports are prepared to meet the requirements of the
Industrial Chemicals Notification and Assessment Act, 1989, as
amended. In the preparation of the assessment report, both internal
and external peer reviews are undertaken. Under the NICNAS
legislation, applicants for the assessment of a chemical (i.e.,
importers and manufacturers of a chemical) may apply for variations to
the draft report. The following companies and industry associations
participated in the review of the assessment at this stage:
Association of Fluorocarbon Consumers and Manufacturers, Elf Atochem
(Australia) Pty Ltd, Lovelock Luke Pty Ltd, and North American Fire
Guardian Technology (Australia) Pty Ltd. The report was also open for
public comment.
NICNAS (1999): 2,2-Dichloro-1,1,1-trifluoroethane (HCFC-123):
Secondary Notification No. 4S, full public report. National
Industrial Chemicals Notification and Assessment Scheme
Copies of the NICNAS (1999) report on HCFC-123 (prepared by S.
Batt, S. Kristensen, and C. Lee-Steere) may be obtained from:
NICNAS
Existing Chemicals
GPO Box 58
Sydney NSW 2001
Australia
NICNAS reports are prepared to meet the requirements of the
Industrial Chemicals Notification and Assessment Act, 1989, as
amended. In the preparation of the assessment report, both internal
and external peer reviews are undertaken. Under the NICNAS
legislation, applicants for the reassessment of a chemical (i.e.,
importers and manufacturers of a chemical) may apply for variations to
the draft report. The following companies participated in the review
of the assessment at this stage: Du Pont (Australia) Pty Ltd, Elf
Atochem (Australia) Pty Ltd, GSA Industries (Australia) Pty Ltd, MSA
(Australia) Pty Ltd, North American Fire Guardian Technology
(Australia) Pty Ltd, and Solvents Australia Pty Ltd. The report was
also open for public comment.
APPENDIX 2 -- CICAD PEER REVIEW
The draft CICAD on HCFC-123 was sent for review to institutions
and organizations identified by IPCS after contact with IPCS National
Contact Points and Participating Institutions, as well as to
identified experts. Comments were received from:
Alexandria University, Faculty of Agriculture, Department of
Pesticide Chemistry, Egypt
AlliedSignal, Department of Toxicology and Risk Assessment,
Health, Safety, Environment and Remediation, USA
Department of Health, Protection of Health Division,
United Kingdom
DuPont Fluoroproducts, Haskell Laboratory for Toxicology and
Industrial Medicine, USA
Federal Institute for Health Protection of Consumers and
Veterinary Medicine, Germany
Glaxo Wellcome Research and Development, Medicines Safety
Evaluation Division, United Kingdom
Health and Safety Executive, United Kingdom
Institut de Recherche en Santé et en Sécurité du Travail du
Québec, Canada
Institute of Terrestrial Ecology, United Kingdom
National Chemicals Inspectorate (KEMI), Sweden
National Institute for Occupational Safety and Health, USA
National Institute of Environmental Health Sciences, National
Institutes of Health, USA
National Institute of Public Health, Centre of Industrial Hygiene
and Occupational Diseases, Czech Republic
Université Catholique de Louvain, Faculté de Médecine, Belgique
US Environmental Protection Agency, Drinking Water Program,
Region VIII, USA
World Health Organization, International Programme on Chemical
Safety, Switzerland
APPENDIX 3 -- CICAD FINAL REVIEW BOARD
Sydney, Australia, 21-24 November 1999
Members
Dr R. Benson, Drinking Water Program, US Environmental Protection
Agency, Region VIII, Denver, CO, USA
Dr T. Berzins, National Chemicals Inspectorate (KEMI), Solna, Sweden
Dr R.M. Bruce, National Center for Environmental Assessment, US
Environmental Protection Agency, Cincinnati, OH, USA
Mr R. Cary, Health and Safety Executive, Merseyside, United Kingdom
Dr R.S. Chhabra, National Institute of Environmental Health Sciences,
National Institutes of Health, Research Triangle Park, NC, USA
Dr S. Chou, Agency for Toxic Substances and Disease Registry, Atlanta,
GA, USA
Dr S. Dobson, Institute of Terrestrial Ecology, Monks Wood,
Cambridgeshire, United Kingdom
Dr H. Gibb, National Center for Environmental Assessment, US
Environmental Protection Agency, Washington, DC, USA
Dr R.F. Hertel, Federal Institute for Health Protection of Consumers
and Veterinary Medicine, Berlin, Germany
Dr J. Kielhorn, Fraunhofer Institute for Toxicology and Aerosol
Research, Hannover, Germany
Dr S. Kristensen, National Occupational Health and Safety Commission
(Worksafe), Sydney, NSW, Australia
Mr C. Lee-Steere, Environment Australia, Canberra, ACT, Australia
Ms M. Meek, Environmental Health Directorate, Health Canada, Ottawa,
Ontario, Canada
Ms F. Rice, National Institute for Occupational Safety and Health,
Cincinnati, OH, USA
Dr J. Sekizawa, National Institute of Health Sciences, Tokyo, Japan
Dr D. Willcocks, National Industrial Chemicals Notification and
Assessment Scheme (NICNAS), Sydney, NSW, Australia (Chairperson)
Professor P. Yao, Institute of Occupational Medicine, Chinese Academy
of Preventive Medicine, Beijing, People's Republic of China
Observers
Mr P. Howe, Institute of Terrestrial Ecology, Huntingdon,
Cambridgeshire, United Kingdom
Dr K. Ziegler-Skylakakis, GSF-Forschungszentrum für Umwelt und
Gesundheit, GmbH, Oberschleissheim, Germany
Secretariat
Dr A. Aitio, International Programme on Chemical Safety, World Health
Organization, Geneva, Switzerland
Ms M. Godden, Health and Safety Executive, Bootle, Merseyside, United
Kingdom
Dr M. Younes, International Programme on Chemical Safety, World Health
Organization, Geneva, Switzerland
APPENDIX 4 -- INTERNATIONAL CHEMICAL SAFETY CARD
2,2-DICHLORO-1,1,1-TRIFLUOROETHANE ICSC: 1343
November 1998
CAS# 306-83-2 HCFC 123
RTECS# KI1108000 C2HCI2F3/CHCI2CF3
Molecular mass: 152.9
TYPES OF HAZARD ACUTE HAZARDS/ PREVENTION FIRST AID / FIRE
/ EXPOSURE SYMPTOMS FIGHTING
FIRE Not combustible. NO open flames. In case of fire in the
surroundings: all
extinguishing
agents allowed.
EXPLOSION In case of fire: keep
drums, etc., cool by
spraying with water.
EXPOSURE
Inhalation Confusion. Dizziness. Local exhaust Fresh air, rest.
Drowsiness. or breathing Artificial respiration
Unconsciousness. protection. if indicated. Refer for
medical attention.
Skin Protective gloves. Rinse skin with plenty
of water or shower.
Eyes Redness. Pain. Safety spectacles. First rinse with plenty
of water for several
minutes (remove contact
lenses if easily possible),
then take to a doctor.
Ingestion (See Inhalation). Rest.
SPILLAGE DISPOSAL PACKAGING & LABELLING
Collect leaking liquid in sealable containers. EU Classification
Absorb remaining liquid in sand or inert UN Classification
absorbent and remove to safe place. Do NOT
let this chemical enter the environment.
Chemical protection suit including
self-contained breathing apparatus.
EMERGENCY RESPONSE STORAGE
Keep in a well-ventilated room.
IMPORTANT DATA
PHYSICAL STATE; APPEARANCE: ROUTES OF EXPOSURE:
COLOURLESS LIQUID, WITH CHARACTERISTIC ODOUR. The substance can be absorbed
into the body by inhalation.
PHYSICAL DANGERS: INHALATION RISK:
The vapour is heavier than air and may No indication can be given
accumulate in low ceiling spaces causing about the rate in which a
deficiency of oxygen. harmful concentration in the
air is reached on evaporation
of this substance at 20°C.
CHEMICAL DANGERS: EFFECTS OF SHORT-TERM EXPOSURE:
The substance decomposes on heating The substance irritates the eyes.
producing phosgene, hydrogen fluoride and The substance may cause effects on
hydrogen chloride. the central nervous system and
cardiovascular system, resulting in
narcosis and cardiac disorders.
OCCUPATIONAL EXPOSURE LIMITS: EFFECTS OF LONG-TERM OR REPEATED EXPOSURE:
TLV not established. The substance may have effects on the liver.
PHYSICAL PROPERTIES
Boiling Point: 28°C
Melting Point: -107°C
Relative density (water = 1): 1.5
Solubility in water, g/100 ml at 25°C: 0.21
Vapour pressure, Pa at 25°C: 14
Relative vapour density (air = 1): 6.4
ENVIRONMENTAL DATA
This substance may be hazardous to the environment; special attention should be given to the
ozone layer. It is strongly advised not to let the chemical enter into the environment because
it persists in the environment. Avoid release to the environment in circumstances different
to normal use.
NOTES
High concentrations in the air cause a deficiency of oxygen with the risk of
unconsciousness or death. Check oxygen content before entering area.
ADDITIONAL INFORMATION
LEGAL NOTICE Neither the CEC nor the IPCS nor any person acting on
behalf of the CEC or the IPCS is responsible for the use
which might be made of this information.
RÉSUMÉ D'ORIENTATION
Ce CICAD repose principalement sur un certain nombre
d'évaluations relatives aux effets du
2,2-dichloro-1,1,1-trifluoréthane (HCFC-123), évaluations qui relèvent
soit de la protection de l'environnement, soit de la médecine du
travail. Ces travaux ont été effectués dans le cadre de l'Australian
National Industrial Chemicals Notification and Assessment Scheme
(NICNAS) et publiés en mars 1996 (NICNAS, 1996) et en juillet 1999
(NICNAS, 1999). Les données intéressantes publiées depuis la parution
des rapports du NICNAS ou obtenues par une recherche approfondie
portant sur plusieurs bases de données jusqu'à août 1999 ont également
fait l'objet d'une étude critique et incluses dans le présent CICAD.
Ce document constitue une mise à jour de l'étude du HCFC-123 qui
figure dans la monographie consacrée à ce type de composé (Critère
d'Hygiène de l'Environnement No 139) (IPCS, 1992). Cette mise à jour à
été suscitée par la publication de données nouvelles et importantes
sur le composé. On trouvera à l'appendice 1 des indications sur la
nature des examens par des pairs ainsi que sur les sources
documentaires utilisées. L'appendice 2 donne des renseignements sur
l'examen de ce CICAD par des pairs. La publication de ce CICAD a été
approuvée lors d'une réunion du Comité d'évaluation finale qui s'est
tenue à Sydney (Australie) du 21 au 24 novembre 1999. La liste des
participants à cette réunion figure à l'appendice 3. La fiche
d'information internationale sur la sécurité chimique (ICSC 1343)
relative au 2,2-dichloro-1,1,1-trifluoréthane, établie par le
Programme international sur la sécurité chimique, est également
reproduite dans l'appendice 4 (IPCS, 1998).
Le HCFC-123 (No CAS 306-83-2) est un composé de synthèse qui se
présente sous la forme d'un liquide volatil incombustible. Il est
utilisé comme réfrigérant dans les installations de climatisation
commerciales et industrielles; il entre dans la composition de certain
produits gazeux anti-feu et sert également d'agent d'expansion pour
mousses. On l'emploie aussi pour le nettoyage des métaux et du
matériel électronique. Son agressivité vis-à-vis de la couche d'ozone
ne représente que le 2 % de celle du CFC-11 (trichlorofluorométhane).
Par rapport au dioxyde de carbone, on estime que son potentiel de
réchauffement du climat est de 300 sur une vingtaine d'années. C'est
pourquoi on l'utilise provisoirement pour remplacer les chloro- et
bromofluorocarbures auxquels il a été décidé de renoncer aux termes du
Protocole de Montréal sur les substances qui détruisent la couche
d'ozone. Selon l'Amendement de Copenhague au Protocole de Montréal, le
HCFC-123 et les autres hydrocarbures fluorés devront être éliminés
d'ici 2020.
Les émissions de HCFC-123 se font principalement dans l'air
ambiant. Bien que légèrement toxique pour les poissons, les daphnies
et les algues, ce composé ne devrait pas constituer de réel danger
pour le milieu aquatique car il ne persiste pas dans l'eau, même à des
concentrations inférieures à sa limite de solubilité. On estime que sa
demi-vie dans l'atmosphère est inférieure à 2 ans. Comme pour d'autres
fluorocarbures plus courants, son principal produit de décomposition
dans l'atmosphère est l'acide trifluoracétique, qui se répartit dans
les diverses phases aqueuses de l'environnement. L'acide
trifluoracétique est difficilement dégradable et il est susceptible de
s'accumuler dans certains systèmes aquatiques fermés, mais sa
concentration actuelle ou prévisible compte tenu des émissions de
HCFC-123 est inférieure au seuil de toxicité.
On pense que l'exposition de la population générale au HCFC-123
est minime. Il existe cependant une possibilité d'exposition
professionnelle lors de la production du composé ou de la préparation
et de l'utilisation de produits qui en contiennent.
On sait peu de chose concernant les effets du HCFC-123 sur
l'organisme humain. On a fait état de cas d'étourdissements, de
céphalées et de nausées à la suite d'une seule et unique exposition à
une concentration inconnue de ce composé dans l'air ambiant. Par
ailleurs, des cas d'atteinte hépatique manifeste ou infraclinique ont
été également observés après exposition professionnelle pendant 1 à 4
mois à des vapeurs de HCFC-123 dont la concentration était comprise
entre 5 et 1125 ppm (31,3-7030 mg/m3).
Le HCFC-123 présente une faible toxicité aiguë pour les animaux
de laboratoire. En le faisant inhaler pendant quelques minutes à
plusieurs heures par divers animaux d'expérience on a constaté à la
dose de 1000 ppm (6,25 g/m3) des lésions au niveau du foie chez les
cobayes, une dépression du système nerveux central (SNC) chez toutes
les espèces à la dose de 5000 ppm (31,3 g/m3) et une arythmie
cardiaque induite par l'adrénaline à la dose de 20 000 ppm (125
g/m3) chez des chiens. Chez le rat et le hamster, l'inhalation du
composé à des doses supérieures à 30 000 ppm (188 g/m3) pendant 4
heures provoque une grave dépression du SNC conduisant à la mort. Le
HCFC-123 n'est pas irritant pour la peau et il ne produit pas de
sensibilisation, mais il peut irriter la muqueuse oculaire lorsqu'il
est à l'état liquide. Lors d'études toxicologiques de 2 à 39 semaines
consistant à faire inhaler de manière répétée le produit par des
animaux de laboratoire (rats, cobayes, chiens et singes), on a
constaté que les principaux organes cibles étaient le foie, le système
endocrine hypothalamo-hypophyso-gonadique et le SNC. La concentration
minimale produisant un effet nocif observable (LOAEL) avec comme
critère les effets hépatiques a été trouvée égale à 30 ppm (188
mg/m3). En prenant comme critère les effets sur le système
endocrine, la concentration sans effet nocif observable (NOAEL) était
égale à 100 ppm (625 mg/m3) et dans le cas des effets sur le SNC,
elle était égale à 300 ppm (1880 mg/m3). Rien n'indique que le
HCFC-123 ait des effets tératogènes chez les animaux de laboratoire,
ni qu'il présente une toxicité génésique ou foetale à des doses
inférieures à celles qui ont d'autres effets toxiques généraux. Des
ratons et des singes nouveau-nés dont les mères allaitantes avaient
été exposées à du HCFC-123 à une concentration égale à la LOAEL (30
ppm ou 188 mg/m3), ont présenté un retard de croissance. Le
métabolite principal (acide trifluoracétique) a été retrouvé dans le
lait des mères allaitantes.
Des signes d'activité clastogène ont été relevés dans des
lymphocytes humains mis en présence de HCFC-123 in vitro à des doses
suffisamment élevées pour être cytotoxiques, mais tous les autres
tests de génotoxicité effectués in vitro ou in vivo se sont
révélés négatifs. On voit donc que ce composé est vraisemblablement
dénué de génotoxicité in vivo.
Lors d'une étude de 2 ans au cours de laquelle on a fait inhaler
le produit à des rats, on a constaté une augmentation de l'incidence
des lésions précancéreuses et des tumeurs bénignes du foie, du
pancréas et des testicules, mais aucun accroissement de l'incidence
des tumeurs malignes qui soit attribuable à ce traitement. Il est
probable que la formation de ces tumeurs implique un ou plusieurs
mécanismes non génotoxiques, notamment une prolifération des
peroxysomes, des lésions au niveau des hépatocytes, une nécrose et une
prolifération dégénérative ainsi qu'une perturbation de l'axe
hypothalamo-hypophyso-gonadique. Il est possible que l'organisme
humain soit moins sensible à la formation de tumeurs par l'un ou
l'autre de ces mécanismes, mais on ne peut cependant ne pas tenir
compte de ces tumeurs dans une évaluation du risque pour l'Homme.
L'effet le plus significatif dans le cas d'une seule et unique
exposition au HCFC-123, comme cela peut se produire lors de son
utilisation pour éteindre un feu, consiste dans son action dépressive
sur le système nerveux central à laquelle s'ajoute la possibilité d'un
accroissement des arythmies cardiaques induites par l'adrénaline. En
cas d'exposition répétée, l'effet le plus significatif est la
possibilité de lésions hépatiques, effet qui a été observé chez des
ouvriers exposés à des concentrations atmosphériques supérieures à 5
ppm (31,3 mg/m3) pendant 1 à 4 mois.
RESUMEN DE ORIENTACI²N
Este CICAD se basa principalmente en las evaluaciones de la salud
ocupacional y los efectos en el medio ambiente del
2,2-dicloro-1,1,1-trifluoroetano (HCFC-123) realizadas en el marco del
Plan Nacional Australiano de Notificación y Evaluación de Sustancias
Químicas Industriales (NICNAS) y publicadas en marzo de 1996 (NICNAS,
1996) y julio de 1999 (NICNAS, 1999). También se ha evaluado e
incorporado a este CICAD la información aparecida desde la terminación
de los informes del NICNAS y la obtenida en una búsqueda amplia
efectuada en varias bases de datos en línea hasta agosto de 1999. Este
CICAD es una actualización del examen del HCFC-123 de la monografía
Criterios de Salud Ambiental 139 (IPCS, 1992), necesaria tras la
aparición de nuevos datos significativos. La información relativa al
carácter del examen colegiado y a la disponibilidad de los documentos
originales figura en el apéndice 1. La información sobre el examen
colegiado de este CICAD se presenta en el apéndice 2. Su publicación
se aprobó en una reunión de la Junta de Evaluación Final celebrada en
Sydney, Australia, los días 21-24 de noviembre de 1999. En el apéndice
3 figura la lista de participantes en la Junta de Evaluación Final. La
Ficha internacional de seguridad química (ICSC 1343) para el
2,2-dicloro-1,1,1-trifluoroetano, preparada por el Programa
Internacional de Seguridad de las Sustancias Químicas (IPCS, 1998),
también se reproduce en el apéndice 4.
El HCFC-123 (CAS No 306-83-2) es un líquido sintético, no
combustible, volátil, que se utiliza como refrigerante en
instalaciones de aire acondicionado comerciales e industriales, en
extintores de incendios gaseosos, como agente espumante y en la
limpieza de metales y de componentes electrónicos. Su capacidad de
agotamiento del ozono es solamente el 2% de la que tiene el CFC-11
(triclorofluorometano). Su potencial de calentamiento de la Tierra es
de 300 en una perspectiva cronológica de 20 años con respecto al
anhídrido carbónico. El HCFC-123 como tal se utiliza en la actualidad
de manera transitoria para sustituir los clorofluorocarburos y los
bromofluorocarburos, que son objeto de supresión progresiva en
aplicación del Protocolo de Montreal relativo a las sustancias que
agotan la capa de ozono. La Enmienda de Copenhague al Protocolo de
Montreal de 1992 exige la eliminación progresiva del HCFC-123 y de
otros hidroclorofluorocarburos para el año 2020.
El HCFC-123 se libera en el medio ambiente fundamentalmente en el
aire atmosférico. Si bien es ligeramente tóxico para los peces,
Daphnia y las algas, no es probable que represente un peligro
importante para el medio acuático, dada su escasa persistencia en el
agua, incluso en concentraciones inferiores al límite de solubilidad.
En la atmósfera, el HCFC-123 tiene una vida estimada de menos de dos
años. El principal producto de degradación atmosférica del HCFC-123 (y
de otros fluorocarburos más ampliamente utilizados) es el ácido
trifluoroacético, que se distribuye en las fases acuosas del medio
ambiente. Aunque este ácido es resistente a la degradación y puede
acumularse en determinados sistemas acuáticos cerrados, las
concentraciones actuales y previstas a partir de las emisiones de
HCFC-123 son inferiores a los umbrales tóxicos.
Se prevé una exposición mínima del público general al HCFC-123.
Sin embargo, es posible la exposición ocupacional durante la
fabricación del HCFC-123 y la fabricación y el uso de productos que lo
contienen.
Se dispone de una información limitada sobre los efectos del
HCFC-123 en el ser humano. Se han notificado casos de vértigo, dolor
de cabeza y náuseas tras una exposición aislada a concentraciones
desconocidas de HCFC-123 en el aire, así como casos de enfermedad
hepática manifiesta o subclínica asociada con exposiciones
ocupacionales repetidas a vapores de HCFC-123 en concentraciones de
5-1125 ppm (31,3-7030 mg/m3) durante 1-4 meses.
La toxicidad aguda del HCFC-123 en animales de laboratorio es
baja. La inhalación durante un período comprendido entre unos minutos
y unas horas provoca lesiones hepáticas en los cobayas con 1000 ppm
(6,25 g/m3), depresión del sistema nervioso central en todas las
especies examinadas con 5000 ppm (31,3 g/m3) y arritmia cardíaca
inducida por la adrenalina en perros con 20 000 ppm (125 g/m3). En
la rata y el hámster, la inhalación de más de 30 000 ppm (188 g/m3)
durante cuatro horas provoca una fuerte depresión del sistema nervioso
central y la muerte. El HCFC-123 no es irritante o sensibilizador
cutáneo, pero en forma líquida puede causar irritación ocular. En
estudios de toxicidad por inhalación con exposiciones repetidas
durante un período de 2 a 39 semanas en ratas, cobayas, perros y
monos, los órganos más afectados fueron el hígado, el sistema
endocrino del hipotálamo, la hipófisis y las gónadas y el sistema
nervioso central. La concentración más baja con efectos adversos
observados (LOAEL) basada en los efectos hepáticos fue de 30 ppm
(188 mg/m3). La concentración sin efectos adversos observados
(NOAEL) fue de 100 ppm (625 mg/m3) basada en los efectos endocrinos
y de 300 ppm (1880 mg/m3) basada en los efectos en el sistema
nervioso central. No hay pruebas de que el HCFC-123 sea teratogénico o
induzca toxicidad reproductiva o fetal con niveles de exposición
inferiores a los que provocan otros efectos sistémicos. Se observó un
crecimiento retardado en ratas y monos recién nacidos de madres
expuestas al HCFC-123, con una LOAEL de 30 ppm (188 mg/m3). En la
leche de las madres se detectó ácido trifluoroacético, principal
metabolito del HCFC-123.
Aunque se obtuvieron pruebas de actividad clastogénica en los
linfocitos humanos expuestos a concentraciones altas citotóxicas de
HCFC-123 in vitro, todas las demás pruebas de toxicidad genética
in vitro e in vivo dieron resultados negativos. Por consiguiente,
las pruebas parecen indicar que no es probable que este producto
químico tenga actividad genotóxica in vivo.
En un estudio de inhalación de dos años en ratas se observó una
mayor incidencia de lesiones precancerosas y tumores benignos en el
hígado, el páncreas y los testículos, pero no se detectó un aumento de
la incidencia de tumores malignos relacionado con la exposición.
Probablemente se debe a que en estos tumores intervienen uno o más
mecanismos no genotóxicos por ejemplo, la proliferación de
peroxisomas, los daños hepatocelulares, la necrosis y la proliferación
regenerativa y el trastorno del eje hipotálamo-hipófisis-testículos.
Aunque el ser humano puede ser menos sensible a los tumores derivados
de algunos de estos mecanismos, en conjunto en una evaluación del
riesgo potencial para las personas no es posible descatar los tumores.
Los efectos críticos más importantes de una exposición breve
aislada al HCFC-123, por ejemplo debido a la descarga de un extintor
de incendios, son la depresión del sistema nervioso central y la mayor
probabilidad de arritmia cardíaca inducida por la adrenalina. Los
principales efectos derivados de una exposición repetida son las
lesiones hepáticas, notificadas en trabajadores expuestos a
concentraciones en el aire superiores a 5 ppm (31,1 mg/m3) durante
un período de 1 a 4 meses.
6. ENVIRONMENTAL LEVELS AND HUMAN EXPOSURE
6.1 Environmental levels
HCFC-123 was detected in non-urban ambient air in Australia at
levels less than 0.01 ppt (62.5 pg/m3) (Fraser, 1994). Assuming that
worldwide emissions will increase to 45 000 tonnes in 2010, the global
average concentration of airborne HCFC-123 is projected to reach 1.1
ppt (7 ng/m3) in that year, with levels being 2-4 times higher than
the average near major sources of emissions in eastern North America
and central Europe (Kotamarthi et al., 1998).
Information on levels of HCFC-123 in water, wildlife or food was
not available.
6.2 Human exposure
HCFC-123 is not used in consumer products. Indirect exposure via
the environment would be low, as the concentration in ambient air is
less than 0.01 ppt (62.5 pg/m3) and HCFC-123 is unlikely to persist
in other media because of its limited solubility and high volatility.
Therefore, exposure of the general population to HCFC-123 is expected
to be minimal.
There is potential for occupational exposure, predominantly via
inhalation, during the manufacture of HCFC-123 and the manufacture and
use of products containing HCFC-123, such as the operation and
maintenance of air-conditioning installations running on HCFC-123, the
discharge of HCFC-123 from fire protection systems, and the use of
liquid HCFC-123 in metal and electronics cleaning.
In an HCFC-123 manufacturing plant in Canada, two operators
monitored over 165-480 min on 4 separate days had time-weighted
breathing-zone levels of HCFC-123 ranging from 1.16 to 8.94 ppm (7.25
to 55.9 mg/m3). On one occasion, a drum-filling lance failure
resulted in a level in excess of 33 ppm (206 mg/m3) (Du Pont,
personal communication, 1999). No monitoring data were available for
the manufacture of products containing HCFC-123.
Several studies have measured HCFC-123 levels in chiller
machinery rooms during normal operations as well as maintenance and
repair activities. In 4 of 12 unmanned machinery rooms containing
air-conditioning equipment running on HCFC-123, air levels were below
1 ppm (6.25 mg/m3) (4-h time-weighted average) at three sites (Trane
Company, 1991). At one site, where samples were taken near a leak and
a half-empty HCFC-123 drum, levels of 5.9-13.6 ppm (36.9-85.0 mg/m3)
(20-min time-weighted average) were recorded. At the rest of the
sites, machinery room air levels were below the limit of detection
(0.2-0.4 ppm [1.25-2.50 mg/m3]). Breathing-zone levels of HCFC-123
during routine chiller maintenance operations, including refrigerant
transfer, were measured at nine US installations. Two-hour to 12-h
time-weighted average concentrations were less than 1 ppm (6.25
mg/m3) in five cases, less than 2 ppm (12.5 mg/m3) in three cases,
and in the range of 2-5 ppm (12.5-31.3 mg/m3) in one case (MRI,
1991; Sibley, 1992; Trane Company, 1992). In Australia, 4- to 6-h
time-weighted average concentrations were less than 1 ppm (6.25
mg/m3) during repair work at a single installation (NICNAS, 1996).
In these studies, continuous area monitoring showed time-weighted
average air concentrations below 1 ppm (6.25 mg/m3), with
activity-related instantaneous peaks ranging from 30 to 500 ppm (188
to 3130 mg/m3).
Air levels resulting from the use of a fire extinguishant
containing 93% HCFC-123 were measured during fire control exercises in
which the firefighters wore a self-contained breathing apparatus (MRI,
1993a,b). Outdoor discharge resulted in maximum breathing-zone levels
ranging from 7 to 870 ppm (43.8 to 5440 mg/m3), depending on the
type of fire hazard. Inside an aircraft hangar, the discharge of
hand-held extinguishers resulted in a breathing-zone concentration of
20 ppm (125 mg/m3) during discharge, with average static air levels
ranging from 29 to 141 ppm (181 to 881 mg/m3) over the next 30 min.
With a large semi-portable fire extinguisher, breathing-zone
concentrations during discharge reached 180-300 ppm (1130-1880
mg/m3), whereas average static air levels ranged from 165 to 557 ppm
(1030 to 3480 mg/m3) over the next 30 min.
In a US factory converted to using HCFC-123 in its degreaser,
personal air monitoring during normal degreaser operations showed
5.5-h time-weighted average levels of HCFC-123 that ranged from 5.3 to
12.0 ppm (33.1 to 75.0 mg/m3) throughout the facility.1 Charging
and unloading the degreaser resulted in short-term breathing-zone
levels ranging from 160 to 460 ppm (1000 to 2880 mg/m3).
1 AlliedSignal Inc., personal communication, 1998 [cited in
NICNAS, 1999].
7. COMPARATIVE KINETICS AND METABOLISM IN LABORATORY ANIMALS
AND HUMANS
HCFC-123 is readily absorbed by inhalation and distributed
throughout the body, where it reaches levels in body fat up to 25
times higher than those in blood (Vinegar et al., 1994). Following
exposure to an initial concentration of 2000 ppm (12.5 g/m3)
radiolabelled HCFC-123 over two consecutive 3-h periods in a
closed-chamber system, total uptake was 50-60% in rats and 90-100% in
guinea-pigs (Urban & Dekant, 1994). In rats exposed to initial
concentrations of 120-11 000 ppm (0.75-68.8 g/m3) HCFC-123 for 6 h,
the inhalation uptake showed a rapid distribution phase lasting
30-45 min followed by a slow linear uptake phase (Vinegar et al.,
1994). Blood levels declined rapidly once exposure was terminated. In
rats exposed to 1000 ppm (6.25 g/m3), blood concentrations fell
from 15.0 mg/litre at the end of a 4-h exposure period to 4.5
mg/litre at 4 min post-exposure and 1.5 mg/litre at 1 h post-exposure
(Vinegar et al., 1994). After the initial decline, HCFC-123
concentrations in both blood and fat decreased log-linearly with
a half-life of approximately 80 min, indicating fat as the main
depository for unmetabolized HCFC-123. Experimentally determined
tissue to air partition coefficients were 2-3 for gut and muscle,
3-4 for blood, 3-5 for liver, and 60-70 for fat (Dekant, 1993;
Vinegar et al., 1994). Data on oral or dermal absorption were not
available.
In the rat, 26-32% of the uptake of HCFC-123 is metabolized,
predominantly by oxidation to trifluoroacetyl chloride, which is
hydrolysed to trifluoroacetic acid or reacts with lysine residues in
proteins or with low-molecular-weight amines to form
N-trifluoroacetyl amides (Harris et al., 1992; Dodd et al., 1993;
Urban & Dekant, 1994). Minor, reductive pathways lead to the formation
of very small amounts of 2-chloro-1,1,1-trifluoroethane,
2-chloro-1,1-difluoroethene, and 2,2-dichloro-1,1-difluoroethene. The
latter reacts with glutathione to form
N-acetyl- S-(2,2-dichloro-1,1-difluoroethyl)-L-cysteine. The
structures of these metabolites are shown in Figure 1. Both oxidation
and reduction of HCFC-123 are catalysed by cytochrome P450 2E1
(CYP2E1). In human liver microsomes, the major biotransformation
product is trifluoroacetic acid (Urban et al., 1994). Its rate of
formation was directly related to the amount of CYP2E1 present and
1.5-16 times faster than the rate in rat microsomes.
In experimental animals, the major metabolite in blood, urine,
and milk is trifluoroacetic acid. In rats exposed by inhalation to
1000 ppm (6.25 g/m3) HCFC-123 for 4 h, blood levels of parent
compound and trifluoroacetic acid amounted to 15.0 and 93.1 mg/litre,
respectively (Vinegar et al., 1994). At 10 000 ppm (62.5 g/m3), the
corresponding concentrations were 93.5 and 37.8 mg/litre,
respectively. Trifluoroacetic acid blood levels rebounded and peaked
12-26 h post-exposure, indicating that the metabolism of HCFC-123
is subject to substrate inhibition at exposures above 1000 ppm (6.25
g/m3). For exposure levels below 2000 ppm (12.5 g/m3), the
metabolic rate constants developed for HCFC-123 were Km = 1.2
mg/litre and Vmax = 7.20 mg/kg body weight per hour for male rats
and Km = 1.2 mg/litre and Vmax = 7.97 mg/kg body weight per
hour for female rats (Loizou et al., 1994). Generally, HCFC-123 was
not detected in blood samples collected within 1 h post-exposure from
lactating rhesus monkeys exposed by inhalation to 1000 ppm (6.25
g/m3) for 6 h per day, whereas trifluoroacetic acid concentrations
reached 150-190 µg/ml after 2-3 weeks of exposure. Based on data from
a single monkey, the half-life of trifluoroacetic acid in blood was
approximately 24 h (Slauter, 1997). In rats and guinea-pigs exposed to
14C-labelled HCFC-123 vapours for 6 h and sacrificed 48 h
post-exposure, only low amounts of radioactivity remained in the
organs examined (Urban & Dekant, 1994). The liver contained most of
the radiolabel, followed by testes and kidneys, lungs, brain,
pancreas, and spleen. Covalent binding of labelled material was
highest in liver tissue (0.4-0.7 nmol/mg protein), followed by lungs,
kidneys, and plasma (0.1-0.3 nmol/mg protein). Trifluoroacetylated
tissue proteins have been detected by immunological techniques in the
liver and at 20- to 200-fold lower levels in the kidney and heart of
rats 6-12 h after exposure to HCFC-123 by inhalation or
intraperitoneal injection (Harris et al., 1992; Huwyler & Gut, 1992;
Huwyler et al., 1992).
The available data indicate that the predominant routes of
HCFC-123 elimination are exhalation of the parent compound and urinary
excretion of trifluoroacetic acid. In rats exposed to an initial
concentration of 2000 ppm (12.5 g/m3) radiolabelled HCFC-123 for two
consecutive 3-h periods and sacrificed 48 h post-exposure, 23-28% of
the radioactive uptake was eliminated in the urine, predominantly as
trifluoroacetic acid, whereas 3-4% was recovered from the body (Urban
& Dekant, 1994). Small amounts of minor metabolites, such as
N-acetyl- S-(2,2-dichloro-1,1-difluoroethyl)-L-cysteine,
N-trifluoroacetyl-2-aminoethanol, and fluoride ion, were recovered
from urine, and trace amounts of 2-chloro-1,1,1-trifluoroethane were
detected in expired air (Urban & Dekant, 1994; Vinegar et al., 1994).
In lactating rats and monkeys exposed to 1000 ppm (6.25 g/m3)
HCFC-123 for 6 h per day for 3 weeks, trifluoroacetic acid was found
in milk at a maximum concentration of 65 and 30 µg/ml, respectively
(Buschman, 1996; Slauter, 1997). In monkeys, the milk also contained
small amounts (up to 5 µg/ml) of HCFC-123. Rat milk was not analysed
for HCFC-123, and neither monkey nor rat milk was analysed for
metabolites other than trifluoroacetic acid.
An analogue of HCFC-123, the common inhalation anaesthetic
halothane (2-bromo-2-chloro-1,1,1-trifluoroethane), is also
metabolized by hepatic CYP2E1 to trifluoroacetyl chloride, causing
trifluoroacetylation of liver proteins (Harris et al., 1992; Urban et
al., 1994). These include cytochrome P450 itself and other enzymes,
many of which have been identified as residing in the lumen of the
endoplasmic reticulum and involved in the maturation of newly
synthesized proteins (Cohen et al., 1997). Both halothane and HCFC-123
induce peroxisome proliferation and increased ß-oxidation in rat liver
cells (Keller et al., 1998). They are also highly effective in
inducing excess uncoupled cytochrome P450 activity in rabbit liver
microsomes, thus increasing hepatic oxygen consumption and
facilitating the oxidation of other cytochrome P450 substrates (Wang
et al., 1993).
Only limited information was available on the kinetics and
metabolism of HCFC-123 in humans in vivo. In four volunteers exposed
by inhalation to 60-73 ppm (375-460 mg/m3) HCFC-123 for 6 h, the
concentration of trifluoroacetic acid in the urine peaked at 10-27
mg/litre by 20-30 h and returned to zero by 96 h post-exposure,
indicating an elimination half-life of 25 h (Tanaka et al., 1998).
Physiologically based pharmacokinetic models for halothane in humans
and for halothane and HCFC-123 in rats have been used to deduce a
human model for HCFC-123 and its main metabolite, trifluoroacetic acid
(Williams et al., 1996). As the model has not been validated, its
usefulness as a predictive tool is unknown at this time.
8. EFFECTS ON LABORATORY MAMMALS AND IN VITRO TEST SYSTEMS
Unless otherwise indicated, only effects that were statistically
different ( P < 0.05) from controls have been considered. All
inhalation studies were performed by whole-body exposure, unless
otherwise mentioned.
8.1 Single exposure
When HCFC-123 was administered in corn oil by oral gavage to rats
at doses ranging from 2.25 to 11 g/kg body weight, rapid respiration
and prostration were recorded at and above 3.4 g/kg body weight. An
LD50 was not determined. The lowest dose causing mortality was 9
g/kg body weight (Henry, 1975).
In the rat and the hamster, inhalation of more than 30 000 ppm
(188 g/m3) caused severe CNS depression and death (Clayton, 1966;
Hall & Moore, 1975; Coate, 1976; Darr, 1981). The 4-h LC50 ranged
from 28 400 to 52 600 ppm (178-329 g/m3). Clinical signs of toxicity
included sedation, loss of muscle coordination and balance,
prostration, and dyspnoea. Gross pathological findings were either
negative or limited to congestion or discoloration of the lungs,
kidneys, liver, thymus, or small intestine. The lowest concentration
causing reversible CNS depression (failures in unconditioned reflexes)
in rats was 5000 ppm (31.3 g/m3) (Mullin, 1976). In a study of
cardiac sensitization to adrenaline, life-threatening or fatal
arrhythmias occurred in 0 of 3, 4 of 6, and 3 of 3 dogs exposed
nose-only to 10 000, 20 000, or 40 000 ppm (62.5, 125, or 250 g/m3).
The dogs were pretreated with intravenous adrenaline (8 µg/kg body
weight) prior to exposure and challenged with an identical adrenaline
dose after breathing the compound for 5 min. Based on these findings,
an EC50 (5 min) of 19 000 ppm (119 g/m3) and a NOAEL of 10 000 ppm
(62.5 g/m3) were determined (Trochimowicz & Mullin, 1973). Exposure
of guinea-pigs to 1000-30 000 ppm (6.25-188 g/m3) HCFC-123 for 4 h
produced non-fatal liver damage in all exposure groups (Marit et al.,
1994). At 48 h post-exposure, the liver effects included centrilobular
vacuolar (fatty) change, multifocal random and centrilobular
hepatocellular degeneration and necrosis, and increased levels of
plasma isocitrate dehydrogenase, alanine transaminase (ALT), and
aspartate transaminase (AST). In another study in guinea-pigs exposed
to 10 000 ppm (62.5 g/m3) for 4 h, liver injury was minimal unless
the animals had been glutathione depleted prior to exposure (Lind et
al., 1995).
In a rat and rabbit test for dermal toxicity carried out
according to OECD guidelines, the only effect observed after a dose of
2000 mg liquid HCFC-123/kg body weight was applied under occlusion for
24 h was a slight to moderate erythema in 6 of 10 rabbits up to 5 days
post-treatment. As such, the dermal LD50 for rats and rabbits was
greater than 2000 mg/kg body weight (Brock, 1988a,b).
8.2 Irritation and sensitization
Application under occlusion of 0.5 ml liquid HCFC-123 for 4 h
caused no erythema or oedema in a test for skin irritation potential
in rabbits conducted according to OECD guidelines (Brock, 1988c). In
rabbits, 0.1 ml undiluted HCFC-123 or 0.2 ml of a 50% solution in
propylene glycol caused mild to moderate, reversible eye damage,
including conjunctival irritation and corneal opacity (scoring not
reported) (Britelli, 1975). HCFC-123 did not produce skin
sensitization in guinea-pigs when 0.1 ml of a 1% solution in dimethyl
phthalate was administered intradermally once a week for 3 weeks,
followed by challenge 2 weeks later with 7 or 35 mg HCFC-123 dissolved
in propylene glycol (Goodman, 1975).
8.3 Short-term exposure
In rats exposed to 1000, 5000, 10 000, or 20 000 ppm (6.25, 31.3,
62.5, or 125 g/m3) HCFC-123 for 6 h per day, 5 days per week, in a
28-day inhalation toxicity study conducted in accordance with OECD
guidelines, exposures to 5000 ppm (31.3 g/m3) and above resulted in
dose-related narcotic effects, which were reversible overnight (Kelly,
1989; Rusch et al., 1994). Body weight was reduced at all dose levels
in females (8-10%) and at the two highest dose levels in males
(14-15%). There was a dose-related increase in relative liver weight
at all exposure levels in females (14-27%) and at the highest level in
males (18%). Plasma levels of ALT and AST were increased by 35% and
71%, respectively, at the highest level in males. Except for isolated
cases of fatty change at all dose levels, gross or microscopic liver
lesions were not observed. A NOAEL was not established in this study.
In a study in male rats exposed to approximately 1000, 5000, or
20 000 ppm (6.25, 31.3, or 125 g/m3) HCFC-123 for 6 h per day, 5
days per week, for 28 days, there was a decrease in body weight
(6-11%) and an increase in relative testis weight (12-30%) in all dose
groups and a 25% increase in relative liver weight in the highest dose
group (Lewis, 1990). Microscopic liver lesions including dose-related
hepatocyte hypertrophy and centrilobular fatty change were seen at all
exposure levels, with proliferation of peroxisomes and mitochondria in
liver cells of animals exposed to 5000 ppm (31.3 g/m3) and above.
Plasma levels of ALT, AST, and alkaline phosphatase (ALP) were
increased by up to 79%, whereas plasma triglycerides and cholesterol
were decreased by up to 70%, with AST and triglycerides being the most
sensitive markers of hepatic injury.
Similar findings accompanied by a 3-fold increase in hepatocyte
mitotic activity were reported from a study in which male rats were
exposed to 18 200 ppm (114 g/m3) HCFC-123 for 6 h per day, 5 days
per week, for 28 days (Warheit, 1993). This study also found
exposure-related testicular lesions, including germinal cell necrosis
and atrophy of the seminiferous tubules. In male guinea-pigs exposed
to 9400 ppm (58.8 g/m3) HCFC-123 for 6 h per day, 5 days per week,
for 28 days, there was evidence of microscopic liver lesions,
including centrilobular vacuolar (fatty) change and hepatocellular
necrosis, but no testicular effects were observed (Warheit, 1993).
8.4 Long-term exposure
8.4.1 Subchronic exposure
A number of subchronic inhalation studies have been carried out
in rats and dogs and are summarized in Table 1. In each case, HCFC-123
was administered for 6 h per day, 5 days per week. The main effects
were liver injury, with changes in liver-related clinical chemistry
parameters occurring at 300 ppm (1.88 g/m3) in rats and at 1000 ppm
(6.25 g/m3) in dogs, and CNS depression, with a reduction in arousal
occurring at 1000 ppm (6.25 g/m3) in rats.
8.4.2 Chronic exposure and carcinogenicity
In a 2-year inhalation study, groups of 80 male and female
Sprague-Dawley rats were exposed for 6 h per day for 5 days per week
to 300, 1000, or 5000 ppm (1.88, 6.25, or 31.3 g/m3) HCFC-123
(Malley, 1992; Malley et al., 1995).
During the first year, rats exposed to 5000 ppm (31.3 g/m3)
appeared sedated, but quickly recovered after the daily exposures
ended. Female rats exposed to 1000 ppm (6.25 g/m3) and males and
females exposed to 5000 ppm (31.3 g/m3) had lower body weight and
body weight gain. At the 12-month sacrifice, the relative liver weight
in male and female rats exposed to 5000 ppm (31.3 g/m3) was
increased by 12% and 24%, respectively. No exposure-related gross or
histopathological changes were observed. In both sexes, serum
triglycerides and glucose were decreased at all exposure levels in a
dose-related manner, by 65-100% and 15-31% for males and females,
respectively. Serum cholesterol was decreased by approximately 30% in
all exposed females and by 43% in males exposed to 5000 ppm
(31.3 g/m3).
During the second year of exposure, slight, reversible CNS
depression continued to be observed at 5000 ppm (31.3 g/m3). At the
end of the 2-year period, there was a dose-related increase in
survival rate, which reached a statistically significant level of 47%
and 59% in females exposed to 1000 or 5000 ppm (6.25 or 31.3 g/m3),
respectively. This is an expected effect of chemicals that reduce body
fat and blood lipids. Compared with the controls, body weight was
decreased by 8% in females exposed to 1000 ppm (6.25 g/m3) and by
12% in males and 21% in females exposed to 5000 ppm (31.3 g/m3). At
5000 ppm (31.3 g/m3), relative liver weight and the incidence of
enlarged and discoloured livers were increased in males, as were
grossly observed liver masses in females.
There were no exposure-related effects on the incidence of
malignant tumours. There was an increase in hepatocellular adenomas in
females and males, an increase in cholangiofibromas in high-dose
females, a dose-related increase in pancreatic acinar cell adenomas in
males, and an increase in Leydig (interstitial) cell adenomas in males
at all dose levels (Table 2). Except for hepatocellular adenomas in
males, the increase in the incidence of these tumours remained
statistically significant when corrected for mortality. Historical
data on tumour incidence in the strain used for this study were not
available. Other exposure-related lesions included hepatic foci of
cellular alteration and focal pancreatic acinar cell hyperplasia
(lesions less than 3 mm in diameter) in males and females at 1000 and
5000 ppm (6.25 and 31.3 g/m3), in addition to hepatic focal necrosis
in males, cholangiofibrosis in females, and hepatic centrilobular
fatty change in both sexes at 5000 ppm (31.3 g/m3). Dose-related
focal Leydig cell hyperplasia (lesions less than the diameter of three
adjacent tubules) was observed in male rats exposed to 1000 and
5000 ppm (6.25 and 31.3 g/m3). The incidence of diffuse retinal
atrophy was increased in both sexes at all exposure levels. Serum
triglycerides and cholesterol continued to be decreased in both sexes
by 46-75% and 31-48%, respectively.
As there were changes in clinical chemistry parameters and an
increased incidence of hepatocellular and Leydig cell adenomas at the
lowest dose tested (300 ppm [1.88 g/m3]), a NOAEL was not
established in this study.
8.5 Genotoxicity and related end-points
HCFC-123 was not mutagenic in Salmonella typhimurium strain
TA98, TA100, TA1525, TA1537, or TA1538 with or without metabolic
activation, even at concentrations of 750 mg per vessel or 150 000 ppm
(938 g/m3), which were clearly toxic (Callander, 1989). HCFC-123 was
found to be clastogenic in two separate studies in human lymphocytes
in vitro, both with and without metabolic activation, at relatively
high concentrations that also reduced the mitotic rate (Table 3). It
was also noted to be clastogenic in the absence, but not in the
presence, of a metabolic activation system in human lymphocytes
exposed to the chemical at 500 µg/ml; further details were not
available (ICI, 1992). No transformation to anchorage-independent
cells was observed when HCFC-123 was tested in baby hamster kidney
fibroblasts (BHK21 cells) with and without metabolic activation
(Longstaff et al., 1984).
Table 1: Summary of effect levels in subchronic inhalation toxicity studies.
Species Study design Effects Effect levels Reference
Rats, albino, Exposed to 0, 500, Body weight marginally LOAEL = 500 ppm Industrial Bio-Test
35 males and 1000, or 5000 ppm decreased in females at (3.13 g/m3) Laboratories, 1977;
25 females (0, 3.13, 6.25, or 1000 ppm and in both sexes Rusch et al., 1994
per group 31.3 g/m3) HCFC-123 at 5000 ppm. Kidney weight
for 90 days, with a increased in all male test
30-day recovery groups and in females at
period. 5000 ppm (% change not
reported). Relative liver
weight increased in females
at all exposure levels and
in males at 5000 ppm
(% change not reported).
Microscopic liver lesions
included mild focal necrosis
in males from all test groups
and minimal bile duct
proliferation in males at
5000 ppm. At end of recovery
period, there were no
exposure-related body or organ
weight changes or
histopathological findings.
Rats, Exposed to 0, 1000, Reversible motor LOAEL = 1000 ppm Doleba-Crowe, 1978;
Sprague-Dawley, or 10 000 ppm (0, incoordination and (6.25 g/m3) Rusch et al., 1994
27 per sex 6.25, or 62.5 g/m3) unresponsiveness to noise
per group HCFC-123 for 90 days. at 10 000 ppm. Reduced body
Histopathological weight (8-17%) and increased
examination performed relative liver weight
on 6 animals per (% change not reported) in
group. both test groups.
Table 1 (cont'd)
Species Study design Effects Effect levels Reference
No exposure-related gross or
histopathological findings.
Elevated levels of AST in
males at both exposure levels,
ALT in males at 1000 ppm, and
blood urea nitrogen (BUN) in
males at both exposure levels
and in females at 1000 ppm;
glucose decreased in females
in both test groups and in
males at 10 000 ppm (% change
not reported).
Rats, Exposed to 0, 300, Reduced responsiveness to LOAEL = 300 ppm Malley, 1990;
Sprague-Dawley, 1000, or 5000 ppm auditory stimuli at 1000 and (1.88 g/m3) Rusch et al.,
10 per sex (0, 1.88, 6.25, or 5000 ppm. Relative liver 1994
per group 31.3 g/m3) HCFC-123 weight increased by 12-17%
for 90 days. and 19-22%, respectively, at
1000 and 5000 ppm. No
exposure-related gross or
histopathological findings.
Dose-dependent elevations of
AST, ALT, lactate
dehydrogenase (LDH) in males
at 1000 and 5000 ppm and BUN
in females in all test groups
and in males at 1000 and
Table 1 (cont'd)
Species Study design Effects Effect levels Reference
5000 ppm. Triglycerides and
glucose markedly decreased
and hepatic ß-oxidation
activity increased 2- to
4-fold in all test groups.
Dose-dependent decrease in
cholesterol in females at
1000 and 5000 ppm.
Rats, Exposed to 0, 300, Reversible reduction in NOAEL = 300 ppm Coombs, 1994
Sprague-Dawley, 1000, or 5000 ppm arousal at 1000 and 5000 (1.88 g/m3)
10 per sex (0, 1.88, 6.25, or ppm. No exposure-related
per group 31.3 g/m3) HCFC-123 gross or histopathological
for 90 days, with a findings in cerebrum,
28-day recovery medulla/pons, cerebellar
period. Histological cortex, spinal cord, ganglia,
examinations limited dorsal and ventral root
to nervous tissues. fibres, or peripheral nerves.
Dogs, beagles, Exposed to 0, 1000, Reversible motor LOAEL = 1000 ppm Doleba-Crowe, 1978;
4 males per group or 10 000 ppm incoordination and (6.25 g/m3) Rusch et al., 1994
(0, 6.25, or 62.5 unresponsiveness at
g/m3) HCFC-123 for 10 000 ppm. At 10 000 ppm,
90 days. discoloured livers with
hepatocyte hypertrophy and
necrosis with inflammatory
cell infiltration. Elevated
levels of ALP in both test
groups and of BUN at 10 000
ppm (% change not reported).
Table 2: Incidence of selected non-neoplastic and neoplastic lesions in the liver, pancreas, and testes
in the 2-year rat inhalation study.a,b
Incidence of lesions
0 ppm 300 ppm 1000 ppm 5000 ppm
Male
Liver
Hepatocellular adenoma 3/67 2/66 2/66 8/66c
Basophilic foci of alteration 8/67 10/66 20/66d 30/66d
Clear cell foci of alteration 8/67 9/66 30/66d 19/66d
Mixed foci of alteration 3/67 6/66 6/66 12/66d
Eosinophilic foci of alteration 8/67 16/66 18/66d 13/66
Cholangiofibroma 0/67 0/66 0/66 0/66
Cholangiofibrosis 0/67 0/66 0/66 0/66
Pancreas
Acinar cell adenoma 1/67 4/66 12/64e 14/66e
Focal acinar cell hyperplasia 5/67 6/66 13/64d 19/66d
Testes
Leydig cell adenoma 4/67 12/66f 9/66f 14/66f
Leydig cell hyperplasia 8/67 15/66 23/66d 30/66d
Table 2 (cont'd)
Incidence of lesions
0 ppm 300 ppm 1000 ppm 5000 ppm
Female
Liver
Hepatocellular adenoma 0/65 5/67e 2/67 7/69e
Basophilic foci of alteration 17/65 26/67 32/67d 46/69d
Clear cell foci of alteration 14/65 7/67 16/67 15/69
Mixed foci of alteration 2/65 3/67 13/67d 22/69d
Eosinophilic foci of alteration 8/65 11/67 22/67 30/69d
Cholangiofibroma 0/65 0/67 0/67 6/69e
Cholangiofibrosis 0/65 0/67 0/67 9/69d
Pancreas
Acinar cell adenoma 0/65 2/66 0/67 2/69
Focal acinar cell hyperplasia 0/65 4/66 6/67d 8/69d
a From Malley (1992); Malley et al. (1995).
b Only lesions whose incidence attained statistical significance in at least one male or female dose
group are included in the table. Other non-statistically significant lesions are discussed in the
text. The figures give the number of lesions per number of tissues available for histological
examination. A few animals and tissues were lost due to autolysis.
c P < 0.05 (Cochran-Armitage test for trend).
d P < 0.05 (Fisher's exact test compared with controls).
e P < 0.05 (Cochran-Armitage test for trend and 2/2 tests for mortality-adjusted statistical analysis).
f P < 0.05 (Cochran-Armitage test for trend and 1/2 tests for mortality-adjusted statistical analysis).
In vivo, a test for chromosome aberrations in the lymphocytes
of rats exposed by inhalation to up to 5000 ppm (31.3 g/m3) HCFC-123
for 6 h per day, 5 days a week, for 2 weeks was negative (Marshal,
1992), although the failure to induce signs of cytotoxicity cast doubt
on the validity of this finding. No increase was found in the
incidence of micronuclei or in the ratio of polychromatic to
normochromatic erythrocytes in a micronucleus test in mice exposed
nose-only to up to 18 000 ppm (113 g/m3) HCFC-123 for 6 h (Muller &
Hofmann, 1988). In the livers of rats exposed to 12 500 or 20 000 ppm
(78.1 or 125 g/m3) HCFC-123 for 6 h, neither net nuclear grain count
nor percentage cells in repair showed any evidence of unscheduled DNA
synthesis (Kennelly, 1993).
Although HCFC-123 was clastogenic in vitro at high
concentrations, all other in vitro and in vivo tests for genetic
toxicity were negative. Overall, the available studies suggest that
HCFC-123 is unlikely to be genotoxic in vivo.
8.6 Reproductive and developmental toxicity
In studies conducted according to OECD guidelines, HCFC-123 was
neither embryotoxic nor teratogenic in pregnant rats exposed for 6 h
per day on days 6-15 of gestation by inhalation to 0, 5000, or 10 000
ppm (0, 31.3, or 62.5 g/m3) HCFC-123, although maternal toxicity in
the form of reduced weight gain and CNS depression were observed at
both exposure levels (Culik & Kelly, 1976; Brewer & Smith, 1977). In a
range-finding study in which fetal examinations were limited to
external structural abnormalities, HCFC-123 was not embryotoxic or
teratogenic in pregnant rabbits exposed for 6 h per day on days 6-18
of gestation by inhalation to 0, 500, 1500, or 5000 ppm (0, 3.13,
9.38, or 31.3 g/m3) HCFC-123, although dose-related maternal
toxicity characterized by reduced weight gain and food consumption was
evident at all exposure levels (Malinverno et al., 1996).
In a two-generation reproductive toxicity study in Sprague-Dawley
rats conducted in accordance with OECD guidelines, male and female
animals were exposed for 6 h per day, 7 days a week, by inhalation to
0, 30, 100, 300, or 1000 ppm (0, 0.188, 0.625, 1.88, or 6.25 g/m3)
HCFC-123 (Hughes, 1994; Malinverno et al., 1996). The F0 (parental
generation) animals were exposed from 6 weeks of age for 23-39 weeks,
including a 2-week mating period, the gestation period, and, except
for maternal animals on postpartum days 0-4, until the offspring were
weaned. The F1 generation was exposed from 4 weeks of age through to
weaning of their litters (F2 generation), for a total of
approximately 28 weeks.
Table 3: Chromosome aberrations in human lymphocytes in vitro.
Physical form of Exposure Concentrationb Mean mitotic Number of Reference
HCFC-123 protocola index cells with
aberrations
(excluding gaps)
Liquid 3-h exposure 0 µg/ml 25.2 1 Dance, 1991
(without S9) 73 µg/ml 19.6 2
146 µg/ml 21.7 3
292 µg/ml 21.1 3
CBC (2 µg/ml) 14.0 91
3-h exposure 0 µg/ml 23.8 1
(with S9) 146 µg/ml 21.8 2
292 µg/ml 14.7 6
584 µg/ml 6.6 5
CP (6 µg/ml) 8.4 106
24-h exposure 0 µg/ml 30.7 2
(without S9) 36 µg/ml 27.4 3
73 µg/ml 21.5 10c
292 µg/ml 9.7 31d
CBC (2 µg/ml) 22.9 70
Vapour 3-h exposure 0 ppm 24.9 1 Edwards, 1991
(without S9) 75 000 ppm 23.9 3
150 000 ppm 17.9 0e
300 000 ppm 10.3 5e
CBC (2 µg/ml) 18.5 60
Table 3 (cont'd)
Physical form of Exposure Concentrationb Mean mitotic Number of Reference
HCFC-123 protocola index cells with
aberrations
(excluding gaps)
3-h exposure 0 ppm 22.2 4
(with S9) 75 000 ppm 24.1 3
150 000 ppm 21.0 4
300 000 ppm 9.5 23e,f
CP (6 µg/ml) 15.2 107
24-h exposure 0 ppm 16.6 1
(without S9) 25 000 ppm 19.1 9d
50 000 ppm 13.2 18f
100 000 ppm 5.9 24f
CBC (2 µg/ml) 13.5 118
a S9 = metabolic activation system.
b Positive controls: CBC = chlorambucil; CP = cyclophosphamide.
c P < 0.05.
d P < 0.01.
e Increase in number of polyploid cells.
f P < 0.001.
The only adverse reproductive effect was a 17% decrease in
implantation count among F1 females at the highest exposure level.
In terms of development, pup growth was impaired during the
pre-weaning period when exposure was confined to the lactating parent
female. In the F1 generation, mean pup weight was decreased by
approximately 10% at exposures at and above 100 ppm (0.625 g/m3),
whereas mean pup weight in F1 offspring (F2 generation) was
decreased by approximately 20% at all exposure levels. In adult rats,
retarded weight gain was observed at 100 ppm (0.625 g/m3) and above
in F0 animals and at 300 ppm (1.88 g/m3) and above in the F1
generation. There was a dose-dependent, 8-39% increase in liver weight
in all exposed F0 groups and an 8-10% increase at 100 ppm (0.625
g/m3) and above in F1 animals. In F1 animals exposed to HCFC-123
for approximately 28 weeks, exposure-related histopathological changes
were confined to a dose-related increase in the incidence of
centrilobular hepatocyte enlargement and in the incidence and degree
of hepatocyte vacuolation at 300 ppm (1.88 g/m3) and above. No
microscopic changes were detected in the livers of F2 weanling rats
from the 1000 ppm (6.25 g/m3) exposure group. In both generations,
there was a decrease in serum triglycerides in both sexes, whereas
serum cholesterol was increased in males and decreased in females.
Levels of ALT, AST, or other liver enzymes were not determined. Male
rats exposed at or above 300 ppm (1.88 g/m3) had increased plasma
levels of luteinizing hormone (LH) after 10 weeks of exposure, which
had reverted to normal at week 38. In this study, the NOAEL, based on
effects on fertility, was 300 ppm (1.88 g/m3). The LOAEL, based on
developmental effects (retarded neonatal growth during lactation) and
on increased liver weight and changes in liver-related clinical
chemistry parameters, was 30 ppm (0.188 g/m3).
After 22 weeks of exposure, a sample of F1 male rats was drawn
from the two-generation reproductive toxicity study for
endocrinological investigations (Sandow et al., 1995b). In 10 males
from each exposure group, serum levels of LH and testosterone were
determined before and after an injection of LH releasing hormone. The
testes of another eight males per group were incubated in vitro with
human chorionic gonadotropin (which stimulates steroid hormone
biosynthesis). The incubation medium and testis tissue were analysed
for content of testosterone, progesterone, estradiol-17-ß, 17
alpha-OH-progesterone, and delta-4-androstenedione. Basal serum LH and
testosterone levels were similar to those of controls. However, after
stimulation with LH releasing hormone, the LH in rats exposed to 300
ppm (1.88 g/m3) was 32% lower than in controls; at 1000 ppm (6.25
g/m3), LH was 39% and testosterone 46% lower than in the control
group. In the ex vivo test, HCFC-123 inhalation did not affect the
secretory capacity for steroid hormones or alter the content of these
hormones in the testes at the end of the incubation period, except for
a slight reduction of delta-4-androstenedione at 1000 ppm (6.25
g/m3).
In an endocrinological study in rats of both sexes, exposure to
5200 ppm (32.5 g/m3) HCFC-123 for 6 h per day (similar to the
highest dose level in the 2-year bioassay) for 14 consecutive days was
associated with sedation, decreased body, kidney, ovary, and pituitary
weights in females, and increased relative liver weight in males
(Hofmann, 1995; Sandow et al., 1995a). In males, the prolactin
response after monoiodotyrosine stimulation, the testosterone response
after stimulation with buserelin (a synthetic gonadotropin releasing
hormone), and the testicular testosterone content were all reduced by
approximately 50%. In females, the gonadotropin response to buserelin
stimulation was enhanced and the pituitary content of follicle
stimulating hormone and prolactin was reduced, in both cases by
approximately 50%.
These endocrinological investigations indicate that HCFC-123 has
little, if any, effect on steroid production in rat testes, but
impairs the prolactin, LH, and testosterone response to pituitary
stimulants. The NOAEL based on this effect was 100 ppm (0.625 g/m3).
A lactation study was conducted in groups of pregnant and
lactating Sprague-Dawley (Crl:CD BR) rats exposed to 0 or 1000 ppm (0
or 6.25 g/m3) HCFC-123 for 6 h per day on days 5-19 of gestation and
on days 5-21 postpartum (Buschman, 1996). Within 2 days of birth,
litters were crossed over between dams to create four groups
comprising exposed or control dams rearing litters from different
exposed or control mothers. Absolute and relative liver weights were
increased and serum triglycerides, cholesterol, and glucose decreased
in dams exposed to HCFC-123. The milk of dams exposed to HCFC-123 was
of normal quantity and quality (with regard to content of protein,
lactose, and fat) but contained trifluoroacetic acid at an average
concentration of 50 µg/ml. Trifluoroacetic acid was also found in the
urine of pups reared by dams exposed to HCFC-123. There were no
differences in absolute or relative liver weight or any abnormal
clinical signs or gross findings in any of the groups of pups, but
pups reared by dams exposed to HCFC-123 had a 10% lower growth rate
and decreased serum triglycerides compared with pups reared by
non-exposed dams. Before crossover, there was no difference between
groups with respect to mean pup and litter weight. As such, these
findings indicate that the retarded neonatal growth observed in the
two-generation reproductive toxicity study was due to factors in the
milk of exposed mothers, probably trifluoroacetic acid, rather than to
exposure in utero.
When groups of four lactating rhesus monkeys and their neonates
were exposed to either 0 or 1000 ppm (0 or 6.25 g/m3) HCFC-123 for 6
h per day for 21-22 consecutive days, there were no effects on
maternal body weight, serum triglycerides, cholesterol, and glucose,
or milk composition (Slauter, 1997). Liver biopsy specimens taken from
the mothers at the end of the study revealed exposure-related lesions,
including mild to moderate centrilobular hepatocyte vacuolation, trace
to moderate centrilobular hepatocyte necrosis, and trace to mild
subacute inflammation. As a rule, HCFC-123 was not detected in the
blood of mothers or neonates, whereas trifluoroacetic acid was present
at concentrations of 9-70 µg/ml in exposed mothers and of 17-190 µg/ml
in neonates, with individual blood levels being 2-6 times higher in
the neonates than in their corresponding mothers. Milk from exposed
mothers contained HCFC-123 and trifluoroacetic acid at concentrations
of 1-5 µg/ml and 17-30 µg/ml, respectively. Although no statistical
analysis was attempted because of the small number of observations,
the average growth rate was 10% lower in exposed neonates than in
unexposed controls.
9. EFFECTS ON HUMANS
The available data on the human health effects of HCFC-123 were
limited to a single case report of dizziness, headache, and nausea in
workers exposed to unknown levels of the chemical following the
rupture of an industrial chiller1 and three case reports of hepatic
effects involving 26 workers following repeated exposure to HCFC-123
vapours.
Nine cases of liver effects were reported in gantry drivers at a
smelting depot in Belgium (Hoet et al., 1997). They occurred 1-4
months after the refrigerant utilized in the crane cabin
air-conditioning system had been replaced by a blend containing 57%
HCFC-123, 40% HCFC-124 (1-chloro-1,2,2,2-tetrafluoroethane), and 3%
propane.2 One driver admitted to hospital was found to have
increased levels of AST, ALT, ALP, gamma-glutamyl transferase, and
total and conjugated bilirubin and decreased prothrombin activity,
with AST and ALT levels being 15-23 times above the upper limit of the
normal range. Autoimmune, viral, and drug- or alcohol-induced
hepatitis were ruled out. A liver biopsy showed focal liver cell
necrosis, plugging of bile ducts, and the presence of
trifluoroacetylated proteins. The symptoms regressed during the period
of non-exposure but recurred when the driver returned to work 2 months
later. Eight other drivers showed signs of varying degrees of liver
abnormalities. Serum antibodies to human liver enzymes (CYP2E1 and/or
protein disulfide isomerase isoform P58) were detected in five of six
cases examined. A workplace inspection revealed that the plastic pipes
of the air-conditioning system were perforated and that refrigerant
was leaking into the crane cabin. No further cases occurred after the
system was repaired. Although the workers were exposed to both
HCFC-123 and HCFC-124, the latter is unlikely to have contributed to
the observed effects, as the NOAEL for HCFC-124 was 50 000 ppm (280
g/m3) in a 90-day inhalation toxicity study in rats (Malley et al.,
1996), whereas the LOAEL for HCFC-123 in a similar study in the same
strain and laboratory was 300 ppm (1.88 g/m3) (Table 1).
Eight cases of liver effects were reported in workers exposed to
the vapours of a solvent degreaser containing HCFC-123.3 Two months
after a US factory converted to using HCFC-123 in its degreaser, two
employees who worked closely with the degreaser were found to have
liver disease. They had elevated blood levels of liver enzymes,
particularly ALT (32-56 times above the upper limit of the normal
1 Carrier Canada Ltd, personal communication, 1993 [cited in
NICNAS, 1996].
2 N. Verlinden, personal communication, 1997 [cited in
NICNAS, 1999].
3 AlliedSignal Inc., personal communication, 1998 [cited in
NICNAS, 1999].
range) and AST (14-33 times above the upper limit of the normal
range), had elevated total and conjugated bilirubin, and tested
negative for viral hepatitis. Subsequent testing of all 27 factory
employees revealed four additional cases of elevated liver enzymes.
When retested 1 month later but before the use of HCFC-123 was
discontinued, five of the affected employees had improved markedly,
whereas one had deteriorated, and there was one new case with slightly
elevated ALT and AST levels. All in all, liver enzymes were elevated
in 3 of 4 employees who worked with the degreaser and in 4 of 23
workers who did not. Air monitoring was conducted when HCFC-123 use
began and again shortly after the first cases were diagnosed. Personal
air monitoring during normal degreaser operations showed 5.5-h
time-weighted average levels of HCFC-123 that ranged from 5.3 to
12.0 ppm (33.1 to 75.0 mg/m3) throughout the facility, whereas
short-term breathing-zone levels ranging from 160 to 460 ppm (1000 to
2880 mg/m3) were measured in workers charging and unloading the
degreaser. There was also one case of elevated liver enzymes with
negative tests for viral hepatitis in a technician employed in the
manufacturer's research laboratory where the degreaser was tested and
evaluated. Static air levels in the laboratory were reported to be
generally below 50 ppm (313 mg/m3) HCFC-123.
In a factory in Japan where miniature heat exchangers were filled
with HCFC-123 in a poorly ventilated room, 9 out of 14 workers were
found to have elevated levels of liver enzymes 4-5 weeks after
production had commenced (Takebayashi et al., 1998a,b).1 Four of
them were clinically ill, and two had jaundice. In these workers, AST
and ALT were up to 20-30 times above the upper limit of the normal
range. After an exhaust system was installed that maintains the
concentration of airborne HCFC-123 at about 1 ppm (6.25 mg/m3),
trifluoroacetic acid was not detected in the workers' urine; at
follow-up 1 year later, there were no further cases of clinical
illness or elevated liver enzymes.1 Exposure levels were not
measured, but a simulation of the original working conditions
indicated static air levels ranging from 5 to 1125 ppm (31.3 to
7030 mg/m3) (6-h time-weighted average), depending on the distance
from the filling area.
1 Also T. Takebayashi, personal communication, 1999 [cited in
NICNAS, 1999].
Table 4: Summary of effects of HCFC-123 in aquatic organisms.
Test Species Effects and effect Comments Reference
levels
Acute toxicity (96 h), Fathead minnow Lethargy Rapid degassing Pierson,
flow-through conditions (Pimephales LC50 > 76 mg/litre of HCFC-123 1990a
promelas) (measured) from test
solutions
Acute toxicity (96 h), Rainbow trout Lethargy, darkened Jenkins, 1992b
static conditions (Oncorhynchus pigmentation at
mykiss) 15 mg/litre and
above
LC50 = 56 mg/litre
(measured)
Immobilization (48 h), Daphnia magna Lethargy Jenkins, 1992c
static conditions EC50 = 17 mg/litre
(measured)
Immobilization (48 h), Daphnia magna Lethargy Measured Pierson, 1990b
static conditions EC50 = 45.8 mg/litre concentrations
(nominal) <75% of nominal
Algal growth inhibition Selenastrum EC50 = 68 Based on biomass Jenkins, 1992d
(96 h), static conditions capricornutum mg/litre (measured) integral
10. EFFECTS ON OTHER ORGANISMS IN THE LABORATORY AND FIELD
The available ecotoxicological data for HCFC-123 are summarized
in Table 4. The data indicate that the chemical is, at most, slightly
toxic to aquatic organisms under conditions of acute exposure. Chronic
effects would not be expected because of limited aquatic persistence.
Trifluoroacetic acid, which is formed by atmospheric breakdown of
HCFC-123, has been found to be of low toxicity in stream mesocosms,
algae, higher plants, fish, and mammals (Boutonnet et al., 1999). The
lowest threshold for any effect was 0.12 mg sodium
trifluoroacetate/litre, above which the chemical had reversible
effects on the growth of the alga Selenastrum capricornutum. In the
most sensitive terrestrial species tested (sunflower), sodium
trifluoroacetate at 1 mg/kg dry soil had clear effects on vegetative
growth, whereas long-term root exposure of wheat and soya to sodium
trifluoroacetate at 1 mg/litre had no effect.
11. EFFECTS EVALUATION
11.1 Evaluation of health effects
11.1.1 Hazard identification and dose-response assessment
There was inadequate information on the human health effects of
short-term exposure to HCFC-123. In laboratory animals, HCFC-123
exhibits low acute toxicity, with an approximate oral lethal dose of
9 g/kg body weight, a 4-h LC50 by inhalation in the range of
28 400-52 600 ppm (178-329 g/m3), and a dermal LD50 in excess of
2000 mg/kg body weight. It is not a skin irritant or sensitizer, but
liquid HCFC-123 produces mild to moderate eye irritation. The critical
effects associated with acute exposure are non-fatal liver damage, CNS
depression, and cardiac sensitization to adrenaline. When inhaled in
lethal concentrations, death was caused by severe CNS depression. For
a single, 4-h exposure by inhalation, the LOAEL was 1000 ppm
(6.25 g/m3) for liver damage, based on liver cell necrosis and
increased levels of circulating liver enzymes in guinea-pigs, and 5000
ppm (31.3 g/m3) for CNS depression, based on failures in
unconditioned reflexes in rats. The NOAEL for cardiac sensitization to
adrenaline in dogs was 10 000 ppm (62.5 g/m3). Although the dog is
a very sensitive species, cardiac sensitization to adrenaline-induced
arrhythmia is likely to be a relevant critical effect resulting from
short-term exposure in humans, such as the sudden discharge of fire
extinguishants in occupied rooms (NAS, 1996). Under these
circumstances, CNS depression may also be a critical effect. A large
number of short-chain halogenated hydrocarbons with different
metabolic patterns have similar CNS and cardiac effects, which likely
result from a direct anaesthetic action on neurons and myocardial
cells (IPCS, 1990, 1991, 1992).
The critical effects associated with repeated exposure to
HCFC-123 vapours are liver damage in humans as well as experimental
animals, in addition to neonatal growth retardation, an increased
incidence of benign tumours, and CNS depression, which have been
recorded only in animals.
Based on a limited number of case reports, biochemical
abnormalities associated with liver injury have been observed in
humans at exposure levels in the order of 5-1125 ppm (31.3-7030
mg/m3). The available data are insufficient to define the
dose-response relationship in humans. Liver damage was also seen in
rats, guinea-pigs, dogs, and monkeys. The lesions generally involved
increased liver weight accompanied by hepatocyte enlargement and
vacuolation at the lowest exposure levels, with necrosis, fatty
change, and mild subacute inflammation at higher concentrations. They
were associated with or preceded by increased levels of circulating
liver enzymes and decreased serum triglycerides, glucose, and
cholesterol. The LOAEL for liver effects in animals recorded in a
well-conducted two-generation reproductive toxicity study in rats
equalled 30 ppm (188 mg/m3).
The cytotoxicity of HCFC-123 is probably due to the reactive
metabolite trifluoroacetyl chloride, which can bind covalently to
proteins and interfere with their function and/or alter their
antigenicity. The 200 : 20 : 1 ratio of trifluoroacetylation of liver,
kidney, and heart tissue proteins reported by Huwyler et al. (1992)
and Huwyler & Gut (1992) correlates well with the observed effect
levels for these organs in subchronic toxicity studies (Table 1) and
probably reflects tissue differences in metabolic capacity.
There is no evidence that HCFC-123 is teratogenic in laboratory
animals or induces reproductive or fetal toxicity at levels of
exposure lower than those that cause other systemic effects in adults.
Growth was retarded in neonatal rats and monkeys reared by dams
exposed by inhalation to HCFC-123, with a LOAEL of 30 ppm
(188 mg/m3). The main metabolite of HCFC-123, trifluoroacetic acid,
was found in the milk of the dams. As such, breastfed babies may
represent a subpopulation that is uniquely sensitive to HCFC-123.
Reversible CNS depression was seen consistently in repeated-dose
inhalation studies, but did not change in severity or duration with
the number of exposures and was not associated with morphological
changes in nervous tissues. As such, the mechanism of action is
probably the same for both acute and chronic CNS depression. The
lowest NOAEL for CNS effects from repeated administration was recorded
in a neurotoxicity study in rats and equalled 300 ppm (1880 mg/m3),
based on a reduction in arousal.
Although there was evidence of clastogenic activity in human
lymphocytes in vitro at high, cytotoxic concentrations, all other
in vitro and in vivo tests for genetic toxicity were negative.
Therefore, the evidence suggests that HCFC-123 is unlikely to be
genotoxic in vivo.
In a 2-year inhalation study in rats, there was no
exposure-related effect on the incidence of malignant tumours, but
there was an increased incidence of hepatocellular adenomas,
cholangiofibromas, pancreatic acinar cell adenomas, and Leydig
adenomas. There was also an increase in the pre-cancerous lesions of
hepatic foci of alteration, cholangiofibrosis, focal pancreatic acinar
cell hyperplasia, and Leydig cell hyperplasia (Table 2). As stated
above, HCFC-123 is unlikely to be genotoxic in vivo. The minor,
non-mutagenic metabolite 2-chloro-1,1,1-trifluoroethane caused an
increased incidence of uterine carcinomas and Leydig cell adenomas in
rats when given by oral gavage at 300 mg/kg body weight for 52 weeks
(IPCS, 1992). However, only trace quantities would be formed from the
metabolism of HCFC-123. Therefore, it is necessary to examine the
mechanisms of tumour formation to establish if the modes of induction
can be ruled out as relevant for humans:
* Hepatocellular adenomas. Several repeated-exposure studies have
shown that HCFC-123 and its main metabolite, trifluoroacetic
acid, like several other structurally diverse chemicals, induce
peroxisome proliferation in rat hepatocytes (Warheit, 1993; Rusch
et al., 1994; Malley et al., 1995; Keller et al., 1998).
Peroxisome proliferators are generally not genotoxic but induce
hepatocellular proliferation in rats and mice through a mechanism
that appears to involve the expression of growth factors by
hepatic macrophages, thus leading to liver tumour formation
(Chevalier & Roberts, 1998). The hepatocellular adenomas seen in
rats exposed to HCFC-123 may be related to the induction of
peroxisome proliferation, which is a mechanism of questionable
relevance for humans (Ashby et al., 1994). However, since the
chemical is also hepatotoxic, which may have a role in the tumour
formation, it is not possible to discount the hepatocellular
adenomas as being of no concern to humans. It is, however,
reasonable to adopt a threshold approach, based on adverse
effects on the liver in subchronic exposure studies.
* Cholangiofibromas. Cholangiofibromas in the rat are atypical
glandular structures lined by intestinal-like epithelium
surrounded by dense connective tissue. Limited evidence from
animal studies of various non-genotoxic chemicals, including
chloroform and furan, suggests that this tumour type is
associated with significant hepatocyte necrosis and regenerative
cell proliferation that are relevant only at high dose/exposure
levels (Elmore & Sirica, 1993; Jamison et al., 1996). In the
2-year rat study, cholangiofibromas and cholangiofibrosis
occurred only in females exposed to HCFC-123 at 5000 ppm (31.3
g/m3). The incidence of basophilic and eosinophilic foci of
hepatocellular proliferation was also higher in females than in
males (Table 2). Although the threshold level for the induction
of cholangiofibromas in female rats was high, there is no
mechanistic evidence that this tumour type can be dismissed with
regard to its relevance to humans.
* Pancreatic acinar cell adenomas. Some hepatocarcinogenic
peroxisome proliferators have been reported to induce tumours in
other organs, including pancreatic acinar cell adenomas and
Leydig cell adenomas, although these extrahepatic tumours appear
not to be associated with peroxisome proliferation in the target
organ (IARC, 1995). Although pancreatic acinar cell adenomas were
found only in males at 1000 and 5000 ppm (6.25 and 31.3 g/m3),
the incidence was dose-related. Moreover, pancreatic acinar cell
hyperplasia occurred in both sexes, likewise in a dose-dependent
manner (Table 2). As such, until more is known about the
mechanism for acinar cell tumour induction in animals and humans,
the possibility that the pancreatic adenomas found in rats
exposed to HCFC-123 may have some relevance to humans cannot be
discounted.
* Leydig cell adenomas. The available studies indicate that
exposure to HCFC-123 may be associated with endocrine
disturbances in male rats, particularly in relation to prolactin
release and serum LH concentrations. In rats, but not in humans,
a decrease in serum prolactin causes a decrease in the number of
LH receptors on Leydig cells and thus a decrease in testosterone
production, which results in increased LH levels that in turn may
induce Leydig cell hyperplasia and adenomas (Clegg et al., 1997).
As such, it is conceivable that intermittent exposure to HCFC-123
could lead to fluctuations in prolactin and testosterone that in
rats could induce transient increases in LH and, with time,
Leydig cell growth and tumours. Although prolactin fluctuations
would not be of concern in men, the effects of HCFC-123 on the
sex hormone system are complex. Thus, in the absence of data from
studies in primates, the increased incidence of Leydig cell
adenomas in the 2-year rat study cannot be dismissed with respect
to its relevance for humans.
In summary, it is likely that the benign tumours in the 2-year
rat bioassay involve one or more non-genotoxic mechanisms, including
peroxisome proliferation, hepatocellular damage, necrosis and
regenerative proliferation, and disturbance of the
hypothalamic-pituitary-testicular axis. Although humans may be less
sensitive to tumours arising from some of these actions, overall it is
not possible to discount the tumours in an evaluation of the potential
risk for humans. Therefore, the increased incidence of benign tumours
in the rat raises some concern with respect to the potential
carcinogenicity in humans. The tumours probably arise from
non-genotoxic mechanisms. In the 2-year bioassay, the tumour incidence
was increased at 300 ppm (1.88 g/m3), the lowest level tested, and a
NOAEL was not established. In subchronic toxicity studies, the LOAEL
based on any adverse effect on the liver was 30 ppm (0.188 g/m3)
(Hughes, 1994; Malinverno et al., 1996).
11.1.2 Criteria for setting tolerable intakes or guidance values
for HCFC-123
Derivation of a guidance value for HCFC-123 was outside the scope
of the source documents (NICNAS, 1996, 1999). General advice about the
derivation of tolerable intakes and guidance values for health-based
exposure limits is set out in Environmental Health Criteria 170 (IPCS,
1994).
Exposure of the general public to HCFC-123 is likely to be
minimal. The main risk to human health is through repeated
occupational exposure via inhalation.
The critical effects of repeated low-level exposure to HCFC-123
are liver damage, which has been observed in all species investigated,
including monkeys and humans, and retarded neonatal growth during
lactation, which has been observed in rats and monkeys. In the absence
of sufficient data to establish a dose-response relationship in
humans, a guidance value for exposure to HCFC-123 must be based on the
observed effect levels for liver lesions and retarded neonatal growth
during lactation in pivotal animal studies. For both of these critical
effects, a LOAEL of 30 ppm (188 mg/m3) was established in a
well-conducted two-generation reproductive toxicity study in rats,
whereas a NOAEL was not achieved (Hughes, 1994; Malinverno et al.,
1996).
The uncertainty factor applied to extrapolate from the LOAEL to a
NOAEL must allow for the fact that whereas the recorded liver effects
at the LOAEL were mild, hepatotoxicity may have a role in the
induction of benign liver tumours in rats. Moreover, the growth
retardation in neonates during lactation was in the order of 10-20%.
It is likely that the liver effects are related to the formation of
protein adducts with trifluoroacetyl chloride and that growth
retardation in neonates is related to exposure to trifluoroacetic acid
in maternal milk. Therefore, the uncertainty factor used to
extrapolate the NOAEL from rats to humans must allow for the lack of
in vivo data on the metabolic rate and other toxicokinetic
properties of HCFC-123 in humans. Finally, an additional uncertainty
factor must be applied to allow for human variability in
toxicokinetics, as the metabolism of HCFC-123 to trifluoroacetyl
chloride and trifluoroacetic acid is catalysed by CYP2E1
(Urban et al., 1994), whose activity is known to be influenced by
genetic polymorphism, body weight, and dietary factors (Le Marchand
et al., 1999).
11.1.3 Sample risk characterization
Adverse effects of HCFC-123 in animals and humans have been
observed only at concentrations that were several orders of magnitude
higher than those in the only known medium of exposure (air) in the
general environment. The likelihood of public exposure as a
consequence of catastrophic accidents or fire extinguishant discharges
is very small, and the scale and duration of such exposures are
expected to be low.
With respect to repeated occupational exposures, 3- to 8-h
time-weighted average personal exposure levels in an HCFC-123
manufacturing plant were reported to be below 10 ppm (62.5 mg/m3).
Reported 2- to 12-h time-weighted average breathing-zone levels in
machinery rooms containing air-conditioning equipment generally ranged
from below 1 to 5 ppm (below 6.25 to 31.3 mg/m3), whereas the use of
a liquid HCFC-123 degreaser was associated with concentrations in the
range of 5.3-12 ppm (33.1-75.0 mg/m3) HCFC-123. The available case
reports indicate that humans may develop biochemical signs of liver
disease, such as elevated AST and ALT, after 1-4 months of repeated
exposure to HCFC-123 at levels above 5 ppm (31.3 mg/m3). Because the
effects of low levels of HCFC-123 are due to toxic metabolites formed
through CYP2E1, genetic, lifestyle, and dietary factors are expected
to cause considerable variability in human susceptibility to the
chemical.
11.2 Evaluation of environmental effects
Because of its high volatility, HCFC-123 released to the
environment will partition almost entirely to the atmosphere. It is
removed predominantly in the troposphere by reaction with hydroxyl
radicals to form trifluoroacetic acid, and only a small fraction is
transported to the stratosphere, where it may undergo photolysis and
release chlorine radicals that catalyse the destruction of ozone.
Because of its short atmospheric lifetime, estimated at 1.4 years, its
ozone-depleting potential is low (0.02 relative to CFC-11). The global
warming potential of HCFC-123 relative to carbon dioxide is 300, 93,
and 29 over a time horizon of 20, 100, and 500 years, respectively
(WMO, 1995).
The aquatic EC50/LC50 values were below 100 mg/litre but
above 10 mg/litre. As such, the chemical meets the European Community
criteria for classification as harmful to the environment (Berends et
al., 1999) and the globally harmonized criteria for classification as
hazardous to the aquatic environment (Class: Acute III) (OECD, 1998).
However, while HCFC-123 may be released to surface waters or soil, it
is unlikely to persist in these media because of its high volatility.
As such, it is considered that HCFC-123 does not constitute a
long-term or delayed danger to the aquatic environment.
Trifluoroacetic acid formed by degradation of HCFC-123 will
precipitate in rain and may accumulate in closed aquatic systems such
as salt lakes and seasonal wetlands. The maximum total contemporary
deposition rate of trifluoroacetic acid from fluorocarbons has been
estimated at 2800 tonnes a year, with 27% derived from HCFC-123 and
the remainder from HCFC-124, HFC-134a, HFC-227ea, and the anaesthetic
gases halothane and isoflurane (Boutonnet et al., 1999). In 2020, the
maximum deposition from fluorocarbons is predicted to reach 160 000
tonnes a year, yielding a maximum average concentration of
trifluoroacetic acid in rainwater of 0.1 µg/litre, which is several
orders of magnitude lower than the no-effect level in both surface and
soil water. By that year, HCFC-123 emissions will have declined, as
the chemical will have been phased out in accordance with the Montreal
Protocol. As such, it can be concluded that environmental levels of
trifluoroacetic acid resulting from the breakdown of HCFC-123 do not
pose a threat to the environment.
12. PREVIOUS EVALUATIONS BY INTERNATIONAL BODIES
A previous evaluation of HCFC-123 has been carried out by the
International Programme on Chemical Safety (IPCS, 1992).
Information on international hazard classification and labelling
is included in the International Chemical Safety Card reproduced in
this document (Appendix 4).
REFERENCES
AIHA (1998) Workplace environmental exposure level guide series:
1,1,1-Trifluoro-2,2-dichloroethane. Fairfax, VA, American Industrial
Hygiene Association Press.
Ashby J, Brady A, Elcombe CR, Elliott BM, Ishmael J, Odum J, Tugwood
JD, Kettle S, Purchase IF (1994) Mechanistically-based human hazard
assessment of peroxisome proliferator-induced hepatocarcinogenesis.
Human and experimental toxicology, 13 (Suppl. 2):S1-S117.
Berends AG, de Rooij CG, Shin-ya S, Thompson RS (1999) Biodegradation
and ecotoxicity of HFCs and HCFCs. Archives of environmental
contamination and toxicology, 36(2):146-151.
Boutonnet JC, Bingham P, Calamari D, de Rooij C, Franklin J, Kawano T,
Libre JM, McCulloch A, Malinverno G, Odom JM, Rusch GM, Smythe K,
Sobolev I, Thompson R, Tiedje JM (1999) Environmental risk assessment
of trifluoroacetic acid. Human and ecological risk assessment,
5(1):59-124.
Brewer WE, Smith S (1977) Teratogenic study via inhalation with
Genetron 123 in albino rats. Morristown, NJ, Allied Chemical
Corporation.
Britelli MR (1975) Eye irritation test in rabbits. Newark, DE, Du
Pont de Nemours and Co.
Brock WJ (1988a) Acute dermal toxicity study of HCFC-123 in rabbits.
Newark, DE, Du Pont de Nemours and Co.
Brock WJ (1988b) Acute dermal toxicity study of HCFC-123 in rats.
Newark, DE, Du Pont de Nemours and Co.
Brock WJ (1988c) Primary dermal irritation study with HCFC-123 in
rabbits. Newark, DE, Du Pont de Nemours and Co.
Buschman J (1996) Crossover study with HCFC 123 in lactating
Sprague-Dawley rats including additional studies on milk production
and metabolites in offspring urine. Hannover, Fraunhofer Institute
for Toxicology and Aerosol Research.
Callander RD (1989) HCFC-123 -- an evaluation using the Salmonella
mutagenicity assay. Macclesfield, Cheshire, Imperial Chemical
Industries Ltd.
Chang W-K, Criddle CS (1995) Biotransformation of HCFC-22, HCFC-142b,
HCFC-123 and HFC-134a by methanotrophic mixed culture MM1.
Biodegradation, 6(1):1-9.
Chevalier S, Roberts RA (1998) Perturbation of rodent hepatocyte
growth control by nongenotoxic hepatocarcinogens -- mechanisms and
lack of relevance for human health. Oncology reports, 5:1319-1327.
Clayton JW (1966) Acute inhalation toxicity. Newark, DE, Du Pont de
Nemours and Co.
Clegg ED, Cook JC, Chapin RE, Foster PMD, Daston GR (1997) Leydig cell
hyperplasia and adenoma formation: Mechanisms and relevance to humans.
Reproductive toxicology, 11:107-121.
Coate WB (1976) LC50 of G123 in rats. Vienna, VI, Hazleton
Laboratories America Inc.
Cohen SD, Pumford NR, Khairallah EA, Boekelheide K, Pohl LR, Amouzadeh
HR, Hinson JA (1997) Selective protein covalent binding and target
organ toxicity. Toxicology and applied pharmacology, 143:1-12.
Coombs DW (1994) HCFC-123 -- 13-week inhalation neurotoxicity study
in the rat. Cambridgeshire, Huntingdon Research Centre Ltd.
Culik R, Kelly DP (1976) Embryotoxic and teratogenic studies in rats
with inhaled dichlorofluoromethane (Freon 21) and
2,2-dichloro-1,1,1-trifluoroethane (FC-123). Newark, DE, Du Pont de
Nemours and Co.
Dance CA (1991) In vitro assessment of the clastogenic activity of
HCFC-123 in cultured human lymphocytes. Eye, Suffolk, Life Science
Research Ltd. (No. 91/PFE003/0093).
Darr RW (1981) An acute inhalation toxicity study of
Fluorocarbon 123 in the Chinese hamster. Morristown, NJ, Allied
Corporation.
Dekant W (1993) Metabolism of 1,1-dichloro-2,2,2-trifluoroethane
(HCFC-123). Würzburg, Institute of Toxicology, University of
Würzburg (Report No. MA-250B-82-207).
Dodd DE, Brashear WT, Vinegar A (1993) Metabolism and pharmacokinetics
of selected halon replacement candidates. Toxicology letters,
68:37-47.
Doleba-Crowe C (1978) 90-day inhalation exposure of rats and dogs to
vapours of 2,2-dichloro-1,1,1-trifluoroethane (FC-123) (summary).
Newark, DE, Du Pont de Nemours and Co. [cited in NICNAS, 1996].
Du Pont (1993) Workplace guidelines for Suva(R) Centri-LP
(HCFC-123) in refrigeration and air conditioning applications.
Wilmington, DE, Du Pont de Nemours and Co.
Edwards CN (1991) HCFC-123 (vapour phase): In vitro assessment of
the clastogenic activity in cultured human lymphocytes. Eye,
Suffolk, Life Science Research Ltd. (No. 91/PFE002/0125).
Elmore LW, Sirica AE (1993) "Intestinal-type" of adenocarcinoma
preferentially induced in right/caudate liver lobes of rats treated
with furan. Cancer research, 53:254-259.
Frank H, Klein A, Renschen D (1996) Environmental trifluoroacetate.
Nature, 382:34.
Fraser P (1994) CSIRO report to SPA-AFEAS. Canberra, Commonwealth
Scientific and Industrial Research Organisation.
Goodman NC (1975) Primary skin irritation and sensitization tests on
guinea pigs. Newark, DE, Du Pont de Nemours and Co.
Hall GT, Moore BL (1975) Acute inhalation toxicity on Freon 123.
Newark, DE, Du Pont de Nemours and Co.
Harris JW, Jones JP, Martin JL, LaRosa AC, Olson MJ, Pohl LR, Anders
MW (1992) Pentahaloethane-based chlorofluorocarbon substitutes and
halothane: correlation of in vivo hepatic protein
trifluoroacetylation and urinary trifluoroacetic acid excretion with
calculated enthalpies of activation. Chemical research
in toxicology, 5:720-725.
Hayman GD, Jenkin ME, Murrells TP, Johnson CE (1994) Tropospheric
degradation chemistry of HCFC-123 (CF3CHCl2): a proposed
replacement chlorofluorocarbon. Atmospheric environment, 28:421-437.
Henry JE (1975) Acute oral test on FC-123. Newark, DE, Du Pont de
Nemours and Co. [cited in NICNAS, 1996].
Hoet P, Graf MLM, Bourdi M, Pohl LR, Duray PH, Chen W, Peter RM,
Nelson SD, Verlinden N, Lison D (1997) Epidemic of liver disease
caused by hydrochlorofluorocarbons used as ozone-sparing substitutes
of chlorofluorocarbons. Lancet, 350:556-559.
Hofmann T (1995) HCFC 123, HCFC 141B and HCF 134a, testing for
subacute (2 weeks) inhalation toxicity in male and female Sprague
Dawley rats. Frankfurt am Main, Hoechst Aktiengesellschaft.
Hughes EW (1994) HCFC 123: A study of the effect on reproductive
function of two generations in the rat. Huntingdon, Cambridgeshire,
Huntingdon Research Centre Ltd.
Huwyler J, Gut J (1992) Exposure to the chlorofluorocarbon substitute
2,2-dichloro-1,1,1-trifluoroethane and the anaesthetic agent halothane
is associated with transient adduct formation in the heart.
Biochemical and biophysical research communications, 184:1344-1349.
Huwyler J, Aeschlimann D, Christen U, Gut J (1992) The kidney as a
novel target tissue for protein adduct formation associated with
metabolism of halothane and the candidate chlorofluorocarbon
replacement 2,2-dichloro-1,1,1-trifluoroethane. European journal of
biochemistry, 207:229-238.
IARC (1995) Peroxisome proliferation and its role in carcinogenesis:
views and expert opinions of an IARC working group, Lyon, 7-11
December 1994. Lyon, International Agency for Research on Cancer.
ICI (1992) An evaluation in the in vitro cytogenetic assay using
human lymphocytes. Macclesfield, Cheshire, Imperial Chemical
Industries Ltd. [cited in AIHA, 1998].
Industrial Bio-Test Laboratories (1977) 90-day subacute inhalation
toxicity study with Genetron 123 in albino rats. Northbrook, IL,
Industrial Bio-Test Laboratories, Inc.
IPCS (1990) Fully halogenated chlorofluorocarbons. Geneva, World
Health Organization, International Programme on Chemical Safety
(Environmental Health Criteria 113).
IPCS (1991) Partially halogenated chlorofluorocarbons (methane
derivatives). Geneva, World Health Organization, International
Programme on Chemical Safety (Environmental Health Criteria 126).
IPCS (1992) Partially halogenated chlorofluorocarbons (ethane
derivatives). Geneva, World Health Organization, International
Programme on Chemical Safety (Environmental Health Criteria 139).
IPCS (1998) International Chemical Safety Card
-- 2,2-Dichloro-1,1,1-trifluoroethane. Geneva, World Health
Organization, International Programme on Chemical Safety (ICSC 1343).
IPCS (1994) Assessing human health risks of chemicals: Derivation of
guidance values for health-based exposure limits. Geneva, World
Health Organization, International Programme on Chemical Safety
(Environmental Health Criteria 170).
Jamison KC, Larson JL, Butterworth BE, Harden R, Skinner BL, Wolf DC
(1996) A non-bile duct origin for intestinal crypt-like ducts with
peritubular fibrosis induced in livers of F344 rats by chloroform
inhalation. Carcinogenesis, 17:675-682.
Jenkins CA (1992a) HCFC-123 (liquid): Biotic degradation closed
bottle test. Eye, Suffolk, Life Sciences Research Ltd. (No.
91/PFE008/0477).
Jenkins CA (1992b) HCFC-123: Acute toxicity to rainbow trout. Eye,
Suffolk, Life Sciences Research Ltd. (No. 91/PFE004/0939).
Jenkins CA (1992c) HCFC-123: Acute toxicity to Daphnia magna. Eye,
Suffolk, Life Sciences Research Ltd. (No. 91/PFE006/0972).
Jenkins CA (1992d) HCFC-123: Determination of its EC50 to
Selenastrum capricornutum. Eye, Suffolk, Life Sciences Research Ltd
(No. 91/PFE007/0935).
Keller DA, Lieder PH, Brock WJ, Cook JC (1998)
1,1,1-Trifluoro-2,2-dichloroethane (HCFC-123) and
1,1,1-trifluoro-2-bromo-2-chloroethane (halothane) cause similar
biochemical effects in rats exposed by inhalation for five days.
Drug and chemical toxicology, 21:405-415.
Kelly DP (1989) Four-week inhalation toxicity study with HCFC-123
in rats. Newark, DE, Du Pont de Nemours and Co.
Kennelly JC (1993) HCFC 123: Assessment for the introduction of
unscheduled DNA synthesis in rat liver after inhalation exposure.
Macclesfield, Cheshire, Zeneca Ltd.
Kotamarthi VR, Rodriguez JM, Ko MKW, Tromp TK, Sze ND, Prather MJ
(1998) Trifluoroacetic acid from degradation of HCFCs and HFCs -- a
three-dimensional modelling study. Journal of geophysical research,
103:5747-5758.
Le Marchand L, Wilkinson GR, Wilkens LR (1999) Genetic and dietary
predictors of CYP2E1 activity: a phenotyping study in Hawaii Japanese
using chlorzoxazone. Cancer epidemiology, biomarkers and prevention,
8:495-500.
Lewis RW (1990) 28-day inhalation study to assess changes in rat
liver and plasma. Macclesfield, Cheshire, Imperial Chemical
Industries Ltd.
Lind RC, Gandolfi AJ, Hall PM (1995) Biotransformation and
hepatotoxicity of HCFC-123 in the guinea pig: Potentiation of hepatic
injury by prior glutathione depletion. Toxicology and applied
pharmacology, 134:175-181.
Loizou GD, Urban G, Dekant W, Anders MW (1994) Gas-uptake
pharmacokinetics of 2,2-dichloro-1,1,1-trifluoroethane (HCFC-123).
Drug metabolism and disposition, 22:511-517.
Longstaff E, Robinson M, Bradbrook C, Styles JA, Purchase IF (1984)
Genotoxicity and carcinogenicity of fluorocarbons: Assessment by
short-term in vitro tests and chronic exposure in rats. Toxicology
and applied pharmacology, 72:15-31.
Malinverno G, Rusch GM, Millischer RJ, Hughes EW, Schroeder RE, Coombs
DW (1996) Inhalation teratology and reproduction studies with
1,1-dichloro-2,2,2-trifluoroethane (HCFC-123). Fundamental and
applied toxicology, 23:276-287.
Malley LA (1990) Subchronic inhalation toxicity: 90-day study with
HCFC-123 in rats. Newark, DE, Du Pont de Nemours and Co.
Malley LA (1992) Combined chronic toxicity/oncogenicity study with
HCFC-123: Two-year inhalation toxicity study in rats. Newark, DE, Du
Pont de Nemours and Co.
Malley LA, Carakostas M, Hansen JF, Rusch GM, Kelly DP, Trochimowicz
HJ (1995) Two-year inhalation toxicity study in rats with
hydrochlorofluorocarbon 123. Fundamental and applied toxicology,
25:101-114.
Malley LA, Carakostas M, Elliott GS, Alvarez L, Schroeder RE, Frame
SR, Van Pelt C, Trochimowicz HJ, Rusch GM (1996) Subchronic toxicity
and teratogenicity of 2-chloro-1,1,1,2-tetrafluoroethane (HCFC-124).
Fundamental and applied toxicology, 32:11-22.
Marit GB, Dodd DE, George ME, Vinegar A (1994) Hepatotoxicity in
guinea pigs following acute exposure to
1,1-dichloro-2,2,2-trifluoroethane. Toxicologic pathology,
22(4):404-414.
Marshal RR (1992) Evaluation of chromosome aberration frequencies in
cultured peripheral blood lymphocytes from rats treated with
HCFC-123. Harrogate, Yorkshire, Hazleton Microtest.
MRI (1991) Results of employee exposure monitoring for HCFC-123 at
centrifugal chiller installations. Final report submitted to the US
Environmental Protection Agency. Silver Spring, MD, Meridian Research,
Inc.
MRI (1993a) Assessment of firefighter exposure to HCFC-123 during
extinguishant efficiency tests conducted at the United States Naval
Air Station in Beaufort, South Carolina. Draft report submitted to
the US Environmental Protection Agency. Silver Spring, MD, Meridian
Research, Inc.
MRI (1993b) Assessment of firefighter exposure to HCFC-123 during
fire extinguisher use in aircraft hangars. Draft report submitted to
American Pacific Corporation. Silver Spring, MD, Meridian Research,
Inc.
Muller W, Hofmann T (1988) HCFC-123 -- micronucleus test in male and
female NMRI mice after inhalation. Frankfurt am Main, Hoechst
Aktiengesellschaft.
Mullin LS (1976) Behavioural toxicity testing of Fluorocarbon 123
in rats. Newark, DE, Du Pont de Nemours and Co.
NAS (1996) Toxicity of alternatives to chlorofluorocarbons: HFC-134a
and HCFC-123. Washington, DC, National Academy of Sciences, National
Academy Press.
NICNAS (1996) Priority Existing Chemical No. 4 --
2,2-Dichloro-1,1,1-trifluoroethane (HCFC-123), full public report,
National Industrial Chemicals Notification and Assessment Scheme.
Canberra, Australian Government Publishing Service.
NICNAS (1999) 2,2-Dichloro-1,1,1-trifluoroethane (HCFC-123):
Secondary Notification No. 4S, full public report. National
Industrial Chemicals Notification and Assessment Scheme. Sydney,
National Occupational Health and Safety Commission.
OECD (1998) Agreed harmonized integrated hazard classification
system for human health and environmental effects of chemical
substances. Paris, Organisation for Economic Co-operation and
Development, Environmental Health and Safety Division.
Oremland RS, Lonergan DJ, Culbertson CW, Lovley DR (1996) Microbial
degradation of hydrofluorocarbons (CHCl2F and CHCl2CF3) in soils
and sediments. Applied and environmental microbiology,
62(5):1818-1821.
Pierson K (1990a) Flow-through acute 96 hour LC50 of HCFC-123 in
fathead minnows (Pimephales promelas ). Newark, DE, Du Pont de
Nemours and Co.
Pierson K (1990b) Static acute 48 hour LC50 of HCFC-123 in Daphnia
magna . Newark, DE, Du Pont de Nemours and Co.
Rusch GM, Trochimowitz HJ, Malley LJ, Kelly DP, Peckham J, Hansen J,
Charm JB (1994) Subchronic inhalation toxicity studies with
hydrochlorofluorocarbon 123 (HCFC 123). Fundamental and applied
toxicology, 23:169-178.
Sandow J, Jerabek-Sandow G, Fenner-Nau D (1995a) Effect of
fluorocarbons on pituitary-gonadal function in a 14-day inhalation
toxicity study: HCFC 123 (Frigen), HCFC 141b (difluorchlorethane)
and HFC 134a (tetrafluorethane). Frankfurt am Main, Hoechst
Aktiengesellschaft.
Sandow J, Rechenberg W, Jerabek-Sandow G (1995b) Effect of HCFC-123
on androgen biosynthesis and gonadotropin secretion in rats.
Frankfurt am Main, Hoechst Aktiengesellschaft.
Sibley H (1992) A study for determining refrigerant exposure levels
while servicing an HCFC-123 centrifugal chiller. Syracuse, NY,
Carrier Corporation.
Slauter RW (1997) HCFC 123: Inhalation study in pregnant monkeys to
assess milk transfer and composition following postpartum exposure.
Mattawan, MI, MPI Research.
Takebayashi T, Kabe I, Endo Y, Tanaka S, Miyauchi H, Nozi K, Takahashi
K, Omae K (1998a) Acute liver dysfunction among workers exposed to
2,2-dichloro-1,1,1-trifluoroethane (HCFC-123): A case report.
Journal of occupational health, 40:169-170.
Takebayashi T, Kabe I, Endo Y, Tanaka S, Miyauchi H, Nozi K, Imamiya
S, Takahashi K, Omae K (1998b) Exposure to
2,2-dichloro-1,1,1-trifluoroethane (HCFC-123): A causal inference.
Journal of occupational health, 40:334-338.
Tanaka S, Kabe I, Takebayashi T, Endo Y, Miyauchi H, Nozi K, Takahashi
K, Seki Y, Omae K (1998) Environmental and biological monitoring of
2,2-dichloro-1,1,1-trifluoroethane (HCFC-123). Journal of
occupational health, 40:348-349.
Trane Company (1991) Report on testing and analysis of the
concentration of HCFC-123 in field installations with general
machinery rooms containing hermetic centrifugal chillers. La Crosse,
WI, The Trane Company.
Trane Company (1992) Report of worker exposure to HCFC-123 during
servicing of hermetic centrifugal chillers. La Crosse, WI, The Trane
Company.
Trochimowicz HJ, Mullin LS (1973) Cardiac sensitization potential
(EC50) of trifluoro-dichloroethane. Newark, DE, Du Pont de Nemours
and Co.
UNEP (1999) Ozone treaties. <http://www.unep.org/ozone/
treaties.htm>. United Nations Environment Programme.
Urban G, Dekant W (1994) Metabolism of
1,1-dichloro-2,2,2-trifluoroethane in rats. Xenobiotica, 24:881-892.
Urban G, Speerschneider P, Dekant W (1994) Metabolism of the
chlorofluorocarbon substitute 1,1-dichloro-2,2,2-trifluoroethane by
rat and human liver microsomes: the role of cytochrome P450 2E1.
Chemical research in toxicology, 7:170-176.
Vinegar A, Williams RJ, Fisher JW, McDougal JN (1994) Dose-dependent
metabolism of 2,2-dichloro-1,1,1-trifluoroethane: A physiologically
based pharmacokinetic model in the male Fischer 344 rat. Toxicology
and applied pharmacology, 129:103-113.
Wang Y, Olson MJ, Baker MT (1993) Interaction of fluoroethane
chlorofluorocarbon (CFC) substitutes with microsomal cytochrome P450.
Biochemical pharmacology, 46:87-94.
Warheit DB (1993) Mechanistic studies with HCFC-123. Newark, DE, Du
Pont de Nemours and Co.
Williams RJ, Vinegar A, McDougal JN, Jarabek AM, Fisher JW (1996) Rat
to human extrapolation of HCFC-123 kinetics deduced from halothane
kinetics -- a corollary approach to physiologically based
pharmacokinetic modeling. Fundamental and applied toxicology,
30:55-66.
WMO (1995) Scientific assessment of ozone depletion. Geneva, World
Meteorological Organization (Global Ozone Research and Monitoring
Project Report No. 37).
Wujcik CE, Zehavi D, Seiber JN (1998) Trifluoroacetic acid levels in
1994-1996 fog, rain, snow and surface waters from California and
Nevada. Chemosphere, 36:1233-1245.
APPENDIX 1 -- SOURCE DOCUMENTS
NICNAS (1996): Priority Existing Chemical No. 4 --
2,2-Dichloro-1,1,1-trifluoroethane (HCFC-123), full public report,
National Industrial Chemicals Notification and Assessment Scheme
Copies of the NICNAS (1996) report on HCFC-123 (prepared by S.
Batt, L. Onyon, L. Slosu, and D. Willcocks) may be obtained from:
NICNAS
Existing Chemicals
GPO Box 58
Sydney NSW 2001
Australia
NICNAS reports are prepared to meet the requirements of the
Industrial Chemicals Notification and Assessment Act, 1989, as
amended. In the preparation of the assessment report, both internal
and external peer reviews are undertaken. Under the NICNAS
legislation, applicants for the assessment of a chemical (i.e.,
importers and manufacturers of a chemical) may apply for variations to
the draft report. The following companies and industry associations
participated in the review of the assessment at this stage:
Association of Fluorocarbon Consumers and Manufacturers, Elf Atochem
(Australia) Pty Ltd, Lovelock Luke Pty Ltd, and North American Fire
Guardian Technology (Australia) Pty Ltd. The report was also open for
public comment.
NICNAS (1999): 2,2-Dichloro-1,1,1-trifluoroethane (HCFC-123):
Secondary Notification No. 4S, full public report. National
Industrial Chemicals Notification and Assessment Scheme
Copies of the NICNAS (1999) report on HCFC-123 (prepared by S.
Batt, S. Kristensen, and C. Lee-Steere) may be obtained from:
NICNAS
Existing Chemicals
GPO Box 58
Sydney NSW 2001
Australia
NICNAS reports are prepared to meet the requirements of the
Industrial Chemicals Notification and Assessment Act, 1989, as
amended. In the preparation of the assessment report, both internal
and external peer reviews are undertaken. Under the NICNAS
legislation, applicants for the reassessment of a chemical (i.e.,
importers and manufacturers of a chemical) may apply for variations to
the draft report. The following companies participated in the review
of the assessment at this stage: Du Pont (Australia) Pty Ltd, Elf
Atochem (Australia) Pty Ltd, GSA Industries (Australia) Pty Ltd, MSA
(Australia) Pty Ltd, North American Fire Guardian Technology
(Australia) Pty Ltd, and Solvents Australia Pty Ltd. The report was
also open for public comment.
APPENDIX 2 -- CICAD PEER REVIEW
The draft CICAD on HCFC-123 was sent for review to institutions
and organizations identified by IPCS after contact with IPCS National
Contact Points and Participating Institutions, as well as to
identified experts. Comments were received from:
Alexandria University, Faculty of Agriculture, Department of
Pesticide Chemistry, Egypt
AlliedSignal, Department of Toxicology and Risk Assessment,
Health, Safety, Environment and Remediation, USA
Department of Health, Protection of Health Division,
United Kingdom
DuPont Fluoroproducts, Haskell Laboratory for Toxicology and
Industrial Medicine, USA
Federal Institute for Health Protection of Consumers and
Veterinary Medicine, Germany
Glaxo Wellcome Research and Development, Medicines Safety
Evaluation Division, United Kingdom
Health and Safety Executive, United Kingdom
Institut de Recherche en Santé et en Sécurité du Travail du
Québec, Canada
Institute of Terrestrial Ecology, United Kingdom
National Chemicals Inspectorate (KEMI), Sweden
National Institute for Occupational Safety and Health, USA
National Institute of Environmental Health Sciences, National
Institutes of Health, USA
National Institute of Public Health, Centre of Industrial Hygiene
and Occupational Diseases, Czech Republic
Université Catholique de Louvain, Faculté de Médecine, Belgique
US Environmental Protection Agency, Drinking Water Program,
Region VIII, USA
World Health Organization, International Programme on Chemical
Safety, Switzerland
APPENDIX 3 -- CICAD FINAL REVIEW BOARD
Sydney, Australia, 21-24 November 1999
Members
Dr R. Benson, Drinking Water Program, US Environmental Protection
Agency, Region VIII, Denver, CO, USA
Dr T. Berzins, National Chemicals Inspectorate (KEMI), Solna, Sweden
Dr R.M. Bruce, National Center for Environmental Assessment, US
Environmental Protection Agency, Cincinnati, OH, USA
Mr R. Cary, Health and Safety Executive, Merseyside, United Kingdom
Dr R.S. Chhabra, National Institute of Environmental Health Sciences,
National Institutes of Health, Research Triangle Park, NC, USA
Dr S. Chou, Agency for Toxic Substances and Disease Registry, Atlanta,
GA, USA
Dr S. Dobson, Institute of Terrestrial Ecology, Monks Wood,
Cambridgeshire, United Kingdom
Dr H. Gibb, National Center for Environmental Assessment, US
Environmental Protection Agency, Washington, DC, USA
Dr R.F. Hertel, Federal Institute for Health Protection of Consumers
and Veterinary Medicine, Berlin, Germany
Dr J. Kielhorn, Fraunhofer Institute for Toxicology and Aerosol
Research, Hannover, Germany
Dr S. Kristensen, National Occupational Health and Safety Commission
(Worksafe), Sydney, NSW, Australia
Mr C. Lee-Steere, Environment Australia, Canberra, ACT, Australia
Ms M. Meek, Environmental Health Directorate, Health Canada, Ottawa,
Ontario, Canada
Ms F. Rice, National Institute for Occupational Safety and Health,
Cincinnati, OH, USA
Dr J. Sekizawa, National Institute of Health Sciences, Tokyo, Japan
Dr D. Willcocks, National Industrial Chemicals Notification and
Assessment Scheme (NICNAS), Sydney, NSW, Australia (Chairperson)
Professor P. Yao, Institute of Occupational Medicine, Chinese Academy
of Preventive Medicine, Beijing, People's Republic of China
Observers
Mr P. Howe, Institute of Terrestrial Ecology, Huntingdon,
Cambridgeshire, United Kingdom
Dr K. Ziegler-Skylakakis, GSF-Forschungszentrum für Umwelt und
Gesundheit, GmbH, Oberschleissheim, Germany
Secretariat
Dr A. Aitio, International Programme on Chemical Safety, World Health
Organization, Geneva, Switzerland
Ms M. Godden, Health and Safety Executive, Bootle, Merseyside, United
Kingdom
Dr M. Younes, International Programme on Chemical Safety, World Health
Organization, Geneva, Switzerland
APPENDIX 4 -- INTERNATIONAL CHEMICAL SAFETY CARD
2,2-DICHLORO-1,1,1-TRIFLUOROETHANE ICSC: 1343
November 1998
CAS# 306-83-2 HCFC 123
RTECS# KI1108000 C2HCI2F3/CHCI2CF3
Molecular mass: 152.9
TYPES OF HAZARD ACUTE HAZARDS/ PREVENTION FIRST AID / FIRE
/ EXPOSURE SYMPTOMS FIGHTING
FIRE Not combustible. NO open flames. In case of fire in the
surroundings: all
extinguishing
agents allowed.
EXPLOSION In case of fire: keep
drums, etc., cool by
spraying with water.
EXPOSURE
Inhalation Confusion. Dizziness. Local exhaust Fresh air, rest.
Drowsiness. or breathing Artificial respiration
Unconsciousness. protection. if indicated. Refer for
medical attention.
Skin Protective gloves. Rinse skin with plenty
of water or shower.
Eyes Redness. Pain. Safety spectacles. First rinse with plenty
of water for several
minutes (remove contact
lenses if easily possible),
then take to a doctor.
Ingestion (See Inhalation). Rest.
SPILLAGE DISPOSAL PACKAGING & LABELLING
Collect leaking liquid in sealable containers. EU Classification
Absorb remaining liquid in sand or inert UN Classification
absorbent and remove to safe place. Do NOT
let this chemical enter the environment.
Chemical protection suit including
self-contained breathing apparatus.
EMERGENCY RESPONSE STORAGE
Keep in a well-ventilated room.
IMPORTANT DATA
PHYSICAL STATE; APPEARANCE: ROUTES OF EXPOSURE:
COLOURLESS LIQUID, WITH CHARACTERISTIC ODOUR. The substance can be absorbed
into the body by inhalation.
PHYSICAL DANGERS: INHALATION RISK:
The vapour is heavier than air and may No indication can be given
accumulate in low ceiling spaces causing about the rate in which a
deficiency of oxygen. harmful concentration in the
air is reached on evaporation
of this substance at 20°C.
CHEMICAL DANGERS: EFFECTS OF SHORT-TERM EXPOSURE:
The substance decomposes on heating The substance irritates the eyes.
producing phosgene, hydrogen fluoride and The substance may cause effects on
hydrogen chloride. the central nervous system and
cardiovascular system, resulting in
narcosis and cardiac disorders.
OCCUPATIONAL EXPOSURE LIMITS: EFFECTS OF LONG-TERM OR REPEATED EXPOSURE:
TLV not established. The substance may have effects on the liver.
PHYSICAL PROPERTIES
Boiling Point: 28°C
Melting Point: -107°C
Relative density (water = 1): 1.5
Solubility in water, g/100 ml at 25°C: 0.21
Vapour pressure, Pa at 25°C: 14
Relative vapour density (air = 1): 6.4
ENVIRONMENTAL DATA
This substance may be hazardous to the environment; special attention should be given to the
ozone layer. It is strongly advised not to let the chemical enter into the environment because
it persists in the environment. Avoid release to the environment in circumstances different
to normal use.
NOTES
High concentrations in the air cause a deficiency of oxygen with the risk of
unconsciousness or death. Check oxygen content before entering area.
ADDITIONAL INFORMATION
LEGAL NOTICE Neither the CEC nor the IPCS nor any person acting on
behalf of the CEC or the IPCS is responsible for the use
which might be made of this information.
RÉSUMÉ D'ORIENTATION
Ce CICAD repose principalement sur un certain nombre
d'évaluations relatives aux effets du
2,2-dichloro-1,1,1-trifluoréthane (HCFC-123), évaluations qui relèvent
soit de la protection de l'environnement, soit de la médecine du
travail. Ces travaux ont été effectués dans le cadre de l'Australian
National Industrial Chemicals Notification and Assessment Scheme
(NICNAS) et publiés en mars 1996 (NICNAS, 1996) et en juillet 1999
(NICNAS, 1999). Les données intéressantes publiées depuis la parution
des rapports du NICNAS ou obtenues par une recherche approfondie
portant sur plusieurs bases de données jusqu'à août 1999 ont également
fait l'objet d'une étude critique et incluses dans le présent CICAD.
Ce document constitue une mise à jour de l'étude du HCFC-123 qui
figure dans la monographie consacrée à ce type de composé (Critère
d'Hygiène de l'Environnement No 139) (IPCS, 1992). Cette mise à jour à
été suscitée par la publication de données nouvelles et importantes
sur le composé. On trouvera à l'appendice 1 des indications sur la
nature des examens par des pairs ainsi que sur les sources
documentaires utilisées. L'appendice 2 donne des renseignements sur
l'examen de ce CICAD par des pairs. La publication de ce CICAD a été
approuvée lors d'une réunion du Comité d'évaluation finale qui s'est
tenue à Sydney (Australie) du 21 au 24 novembre 1999. La liste des
participants à cette réunion figure à l'appendice 3. La fiche
d'information internationale sur la sécurité chimique (ICSC 1343)
relative au 2,2-dichloro-1,1,1-trifluoréthane, établie par le
Programme international sur la sécurité chimique, est également
reproduite dans l'appendice 4 (IPCS, 1998).
Le HCFC-123 (No CAS 306-83-2) est un composé de synthèse qui se
présente sous la forme d'un liquide volatil incombustible. Il est
utilisé comme réfrigérant dans les installations de climatisation
commerciales et industrielles; il entre dans la composition de certain
produits gazeux anti-feu et sert également d'agent d'expansion pour
mousses. On l'emploie aussi pour le nettoyage des métaux et du
matériel électronique. Son agressivité vis-à-vis de la couche d'ozone
ne représente que le 2 % de celle du CFC-11 (trichlorofluorométhane).
Par rapport au dioxyde de carbone, on estime que son potentiel de
réchauffement du climat est de 300 sur une vingtaine d'années. C'est
pourquoi on l'utilise provisoirement pour remplacer les chloro- et
bromofluorocarbures auxquels il a été décidé de renoncer aux termes du
Protocole de Montréal sur les substances qui détruisent la couche
d'ozone. Selon l'Amendement de Copenhague au Protocole de Montréal, le
HCFC-123 et les autres hydrocarbures fluorés devront être éliminés
d'ici 2020.
Les émissions de HCFC-123 se font principalement dans l'air
ambiant. Bien que légèrement toxique pour les poissons, les daphnies
et les algues, ce composé ne devrait pas constituer de réel danger
pour le milieu aquatique car il ne persiste pas dans l'eau, même à des
concentrations inférieures à sa limite de solubilité. On estime que sa
demi-vie dans l'atmosphère est inférieure à 2 ans. Comme pour d'autres
fluorocarbures plus courants, son principal produit de décomposition
dans l'atmosphère est l'acide trifluoracétique, qui se répartit dans
les diverses phases aqueuses de l'environnement. L'acide
trifluoracétique est difficilement dégradable et il est susceptible de
s'accumuler dans certains systèmes aquatiques fermés, mais sa
concentration actuelle ou prévisible compte tenu des émissions de
HCFC-123 est inférieure au seuil de toxicité.
On pense que l'exposition de la population générale au HCFC-123
est minime. Il existe cependant une possibilité d'exposition
professionnelle lors de la production du composé ou de la préparation
et de l'utilisation de produits qui en contiennent.
On sait peu de chose concernant les effets du HCFC-123 sur
l'organisme humain. On a fait état de cas d'étourdissements, de
céphalées et de nausées à la suite d'une seule et unique exposition à
une concentration inconnue de ce composé dans l'air ambiant. Par
ailleurs, des cas d'atteinte hépatique manifeste ou infraclinique ont
été également observés après exposition professionnelle pendant 1 à 4
mois à des vapeurs de HCFC-123 dont la concentration était comprise
entre 5 et 1125 ppm (31,3-7030 mg/m3).
Le HCFC-123 présente une faible toxicité aiguë pour les animaux
de laboratoire. En le faisant inhaler pendant quelques minutes à
plusieurs heures par divers animaux d'expérience on a constaté à la
dose de 1000 ppm (6,25 g/m3) des lésions au niveau du foie chez les
cobayes, une dépression du système nerveux central (SNC) chez toutes
les espèces à la dose de 5000 ppm (31,3 g/m3) et une arythmie
cardiaque induite par l'adrénaline à la dose de 20 000 ppm (125
g/m3) chez des chiens. Chez le rat et le hamster, l'inhalation du
composé à des doses supérieures à 30 000 ppm (188 g/m3) pendant 4
heures provoque une grave dépression du SNC conduisant à la mort. Le
HCFC-123 n'est pas irritant pour la peau et il ne produit pas de
sensibilisation, mais il peut irriter la muqueuse oculaire lorsqu'il
est à l'état liquide. Lors d'études toxicologiques de 2 à 39 semaines
consistant à faire inhaler de manière répétée le produit par des
animaux de laboratoire (rats, cobayes, chiens et singes), on a
constaté que les principaux organes cibles étaient le foie, le système
endocrine hypothalamo-hypophyso-gonadique et le SNC. La concentration
minimale produisant un effet nocif observable (LOAEL) avec comme
critère les effets hépatiques a été trouvée égale à 30 ppm (188
mg/m3). En prenant comme critère les effets sur le système
endocrine, la concentration sans effet nocif observable (NOAEL) était
égale à 100 ppm (625 mg/m3) et dans le cas des effets sur le SNC,
elle était égale à 300 ppm (1880 mg/m3). Rien n'indique que le
HCFC-123 ait des effets tératogènes chez les animaux de laboratoire,
ni qu'il présente une toxicité génésique ou foetale à des doses
inférieures à celles qui ont d'autres effets toxiques généraux. Des
ratons et des singes nouveau-nés dont les mères allaitantes avaient
été exposées à du HCFC-123 à une concentration égale à la LOAEL (30
ppm ou 188 mg/m3), ont présenté un retard de croissance. Le
métabolite principal (acide trifluoracétique) a été retrouvé dans le
lait des mères allaitantes.
Des signes d'activité clastogène ont été relevés dans des
lymphocytes humains mis en présence de HCFC-123 in vitro à des doses
suffisamment élevées pour être cytotoxiques, mais tous les autres
tests de génotoxicité effectués in vitro ou in vivo se sont
révélés négatifs. On voit donc que ce composé est vraisemblablement
dénué de génotoxicité in vivo.
Lors d'une étude de 2 ans au cours de laquelle on a fait inhaler
le produit à des rats, on a constaté une augmentation de l'incidence
des lésions précancéreuses et des tumeurs bénignes du foie, du
pancréas et des testicules, mais aucun accroissement de l'incidence
des tumeurs malignes qui soit attribuable à ce traitement. Il est
probable que la formation de ces tumeurs implique un ou plusieurs
mécanismes non génotoxiques, notamment une prolifération des
peroxysomes, des lésions au niveau des hépatocytes, une nécrose et une
prolifération dégénérative ainsi qu'une perturbation de l'axe
hypothalamo-hypophyso-gonadique. Il est possible que l'organisme
humain soit moins sensible à la formation de tumeurs par l'un ou
l'autre de ces mécanismes, mais on ne peut cependant ne pas tenir
compte de ces tumeurs dans une évaluation du risque pour l'Homme.
L'effet le plus significatif dans le cas d'une seule et unique
exposition au HCFC-123, comme cela peut se produire lors de son
utilisation pour éteindre un feu, consiste dans son action dépressive
sur le système nerveux central à laquelle s'ajoute la possibilité d'un
accroissement des arythmies cardiaques induites par l'adrénaline. En
cas d'exposition répétée, l'effet le plus significatif est la
possibilité de lésions hépatiques, effet qui a été observé chez des
ouvriers exposés à des concentrations atmosphériques supérieures à 5
ppm (31,3 mg/m3) pendant 1 à 4 mois.
RESUMEN DE ORIENTACI²N
Este CICAD se basa principalmente en las evaluaciones de la salud
ocupacional y los efectos en el medio ambiente del
2,2-dicloro-1,1,1-trifluoroetano (HCFC-123) realizadas en el marco del
Plan Nacional Australiano de Notificación y Evaluación de Sustancias
Químicas Industriales (NICNAS) y publicadas en marzo de 1996 (NICNAS,
1996) y julio de 1999 (NICNAS, 1999). También se ha evaluado e
incorporado a este CICAD la información aparecida desde la terminación
de los informes del NICNAS y la obtenida en una búsqueda amplia
efectuada en varias bases de datos en línea hasta agosto de 1999. Este
CICAD es una actualización del examen del HCFC-123 de la monografía
Criterios de Salud Ambiental 139 (IPCS, 1992), necesaria tras la
aparición de nuevos datos significativos. La información relativa al
carácter del examen colegiado y a la disponibilidad de los documentos
originales figura en el apéndice 1. La información sobre el examen
colegiado de este CICAD se presenta en el apéndice 2. Su publicación
se aprobó en una reunión de la Junta de Evaluación Final celebrada en
Sydney, Australia, los días 21-24 de noviembre de 1999. En el apéndice
3 figura la lista de participantes en la Junta de Evaluación Final. La
Ficha internacional de seguridad química (ICSC 1343) para el
2,2-dicloro-1,1,1-trifluoroetano, preparada por el Programa
Internacional de Seguridad de las Sustancias Químicas (IPCS, 1998),
también se reproduce en el apéndice 4.
El HCFC-123 (CAS No 306-83-2) es un líquido sintético, no
combustible, volátil, que se utiliza como refrigerante en
instalaciones de aire acondicionado comerciales e industriales, en
extintores de incendios gaseosos, como agente espumante y en la
limpieza de metales y de componentes electrónicos. Su capacidad de
agotamiento del ozono es solamente el 2% de la que tiene el CFC-11
(triclorofluorometano). Su potencial de calentamiento de la Tierra es
de 300 en una perspectiva cronológica de 20 años con respecto al
anhídrido carbónico. El HCFC-123 como tal se utiliza en la actualidad
de manera transitoria para sustituir los clorofluorocarburos y los
bromofluorocarburos, que son objeto de supresión progresiva en
aplicación del Protocolo de Montreal relativo a las sustancias que
agotan la capa de ozono. La Enmienda de Copenhague al Protocolo de
Montreal de 1992 exige la eliminación progresiva del HCFC-123 y de
otros hidroclorofluorocarburos para el año 2020.
El HCFC-123 se libera en el medio ambiente fundamentalmente en el
aire atmosférico. Si bien es ligeramente tóxico para los peces,
Daphnia y las algas, no es probable que represente un peligro
importante para el medio acuático, dada su escasa persistencia en el
agua, incluso en concentraciones inferiores al límite de solubilidad.
En la atmósfera, el HCFC-123 tiene una vida estimada de menos de dos
años. El principal producto de degradación atmosférica del HCFC-123 (y
de otros fluorocarburos más ampliamente utilizados) es el ácido
trifluoroacético, que se distribuye en las fases acuosas del medio
ambiente. Aunque este ácido es resistente a la degradación y puede
acumularse en determinados sistemas acuáticos cerrados, las
concentraciones actuales y previstas a partir de las emisiones de
HCFC-123 son inferiores a los umbrales tóxicos.
Se prevé una exposición mínima del público general al HCFC-123.
Sin embargo, es posible la exposición ocupacional durante la
fabricación del HCFC-123 y la fabricación y el uso de productos que lo
contienen.
Se dispone de una información limitada sobre los efectos del
HCFC-123 en el ser humano. Se han notificado casos de vértigo, dolor
de cabeza y náuseas tras una exposición aislada a concentraciones
desconocidas de HCFC-123 en el aire, así como casos de enfermedad
hepática manifiesta o subclínica asociada con exposiciones
ocupacionales repetidas a vapores de HCFC-123 en concentraciones de
5-1125 ppm (31,3-7030 mg/m3) durante 1-4 meses.
La toxicidad aguda del HCFC-123 en animales de laboratorio es
baja. La inhalación durante un período comprendido entre unos minutos
y unas horas provoca lesiones hepáticas en los cobayas con 1000 ppm
(6,25 g/m3), depresión del sistema nervioso central en todas las
especies examinadas con 5000 ppm (31,3 g/m3) y arritmia cardíaca
inducida por la adrenalina en perros con 20 000 ppm (125 g/m3). En
la rata y el hámster, la inhalación de más de 30 000 ppm (188 g/m3)
durante cuatro horas provoca una fuerte depresión del sistema nervioso
central y la muerte. El HCFC-123 no es irritante o sensibilizador
cutáneo, pero en forma líquida puede causar irritación ocular. En
estudios de toxicidad por inhalación con exposiciones repetidas
durante un período de 2 a 39 semanas en ratas, cobayas, perros y
monos, los órganos más afectados fueron el hígado, el sistema
endocrino del hipotálamo, la hipófisis y las gónadas y el sistema
nervioso central. La concentración más baja con efectos adversos
observados (LOAEL) basada en los efectos hepáticos fue de 30 ppm
(188 mg/m3). La concentración sin efectos adversos observados
(NOAEL) fue de 100 ppm (625 mg/m3) basada en los efectos endocrinos
y de 300 ppm (1880 mg/m3) basada en los efectos en el sistema
nervioso central. No hay pruebas de que el HCFC-123 sea teratogénico o
induzca toxicidad reproductiva o fetal con niveles de exposición
inferiores a los que provocan otros efectos sistémicos. Se observó un
crecimiento retardado en ratas y monos recién nacidos de madres
expuestas al HCFC-123, con una LOAEL de 30 ppm (188 mg/m3). En la
leche de las madres se detectó ácido trifluoroacético, principal
metabolito del HCFC-123.
Aunque se obtuvieron pruebas de actividad clastogénica en los
linfocitos humanos expuestos a concentraciones altas citotóxicas de
HCFC-123 in vitro, todas las demás pruebas de toxicidad genética
in vitro e in vivo dieron resultados negativos. Por consiguiente,
las pruebas parecen indicar que no es probable que este producto
químico tenga actividad genotóxica in vivo.
En un estudio de inhalación de dos años en ratas se observó una
mayor incidencia de lesiones precancerosas y tumores benignos en el
hígado, el páncreas y los testículos, pero no se detectó un aumento de
la incidencia de tumores malignos relacionado con la exposición.
Probablemente se debe a que en estos tumores intervienen uno o más
mecanismos no genotóxicos por ejemplo, la proliferación de
peroxisomas, los daños hepatocelulares, la necrosis y la proliferación
regenerativa y el trastorno del eje hipotálamo-hipófisis-testículos.
Aunque el ser humano puede ser menos sensible a los tumores derivados
de algunos de estos mecanismos, en conjunto en una evaluación del
riesgo potencial para las personas no es posible descatar los tumores.
Los efectos críticos más importantes de una exposición breve
aislada al HCFC-123, por ejemplo debido a la descarga de un extintor
de incendios, son la depresión del sistema nervioso central y la mayor
probabilidad de arritmia cardíaca inducida por la adrenalina. Los
principales efectos derivados de una exposición repetida son las
lesiones hepáticas, notificadas en trabajadores expuestos a
concentraciones en el aire superiores a 5 ppm (31,1 mg/m3) durante
un período de 1 a 4 meses.
 Board members, authors, reviewers, consultants, and advisers who
participate in the preparation of a CICAD are required to declare any
real or potential conflict of interest in relation to the subjects
under discussion at any stage of the process. Representatives of
nongovernmental organizations may be invited to observe the
proceedings of the Final Review Board. Observers may participate in
Board discussions only at the invitation of the Chairperson, and they
may not participate in the final decision-making process.
1. EXECUTIVE SUMMARY
This CICAD was based principally on the assessments of the
occupational health and environmental effects of
2,2-dichloro-1,1,1-trifluoroethane (HCFC-123) completed under the
Australian National Industrial Chemicals Notification and Assessment
Scheme (NICNAS) and published in March 1996 (NICNAS, 1996) and July
1999 (NICNAS, 1999). Relevant information that has become available
since completion of the NICNAS reports or that was identified in a
comprehensive search of several on-line databases up to August 1999
has also been assessed and included in this CICAD. This CICAD is an
update of the review of HCFC-123 in the monograph Environmental Health
Criteria 139 (IPCS, 1992), prompted by the advent of new and
significant data. Information on the nature of the peer review and the
availability of the source documents is presented in Appendix 1.
Information on the peer review of this CICAD is presented in Appendix
2. The CICAD was approved for publication at a meeting of the Final
Review Board, held in Sydney, Australia, on 21-24 November 1999.
Participants at the Final Review Board meeting are listed in Appendix
3. The International Chemical Safety Card (ICSC 1343) for
2,2-dichloro-1,1,1-trifluoroethane, produced by the International
Programme on Chemical Safety, has been reproduced in Appendix 4 (IPCS,
1998).
HCFC-123 (CAS No.
Board members, authors, reviewers, consultants, and advisers who
participate in the preparation of a CICAD are required to declare any
real or potential conflict of interest in relation to the subjects
under discussion at any stage of the process. Representatives of
nongovernmental organizations may be invited to observe the
proceedings of the Final Review Board. Observers may participate in
Board discussions only at the invitation of the Chairperson, and they
may not participate in the final decision-making process.
1. EXECUTIVE SUMMARY
This CICAD was based principally on the assessments of the
occupational health and environmental effects of
2,2-dichloro-1,1,1-trifluoroethane (HCFC-123) completed under the
Australian National Industrial Chemicals Notification and Assessment
Scheme (NICNAS) and published in March 1996 (NICNAS, 1996) and July
1999 (NICNAS, 1999). Relevant information that has become available
since completion of the NICNAS reports or that was identified in a
comprehensive search of several on-line databases up to August 1999
has also been assessed and included in this CICAD. This CICAD is an
update of the review of HCFC-123 in the monograph Environmental Health
Criteria 139 (IPCS, 1992), prompted by the advent of new and
significant data. Information on the nature of the peer review and the
availability of the source documents is presented in Appendix 1.
Information on the peer review of this CICAD is presented in Appendix
2. The CICAD was approved for publication at a meeting of the Final
Review Board, held in Sydney, Australia, on 21-24 November 1999.
Participants at the Final Review Board meeting are listed in Appendix
3. The International Chemical Safety Card (ICSC 1343) for
2,2-dichloro-1,1,1-trifluoroethane, produced by the International
Programme on Chemical Safety, has been reproduced in Appendix 4 (IPCS,
1998).
HCFC-123 (CAS No.  6. ENVIRONMENTAL LEVELS AND HUMAN EXPOSURE
6.1 Environmental levels
HCFC-123 was detected in non-urban ambient air in Australia at
levels less than 0.01 ppt (62.5 pg/m3) (Fraser, 1994). Assuming that
worldwide emissions will increase to 45 000 tonnes in 2010, the global
average concentration of airborne HCFC-123 is projected to reach 1.1
ppt (7 ng/m3) in that year, with levels being 2-4 times higher than
the average near major sources of emissions in eastern North America
and central Europe (Kotamarthi et al., 1998).
Information on levels of HCFC-123 in water, wildlife or food was
not available.
6.2 Human exposure
HCFC-123 is not used in consumer products. Indirect exposure via
the environment would be low, as the concentration in ambient air is
less than 0.01 ppt (62.5 pg/m3) and HCFC-123 is unlikely to persist
in other media because of its limited solubility and high volatility.
Therefore, exposure of the general population to HCFC-123 is expected
to be minimal.
There is potential for occupational exposure, predominantly via
inhalation, during the manufacture of HCFC-123 and the manufacture and
use of products containing HCFC-123, such as the operation and
maintenance of air-conditioning installations running on HCFC-123, the
discharge of HCFC-123 from fire protection systems, and the use of
liquid HCFC-123 in metal and electronics cleaning.
In an HCFC-123 manufacturing plant in Canada, two operators
monitored over 165-480 min on 4 separate days had time-weighted
breathing-zone levels of HCFC-123 ranging from 1.16 to 8.94 ppm (7.25
to 55.9 mg/m3). On one occasion, a drum-filling lance failure
resulted in a level in excess of 33 ppm (206 mg/m3) (Du Pont,
personal communication, 1999). No monitoring data were available for
the manufacture of products containing HCFC-123.
Several studies have measured HCFC-123 levels in chiller
machinery rooms during normal operations as well as maintenance and
repair activities. In 4 of 12 unmanned machinery rooms containing
air-conditioning equipment running on HCFC-123, air levels were below
1 ppm (6.25 mg/m3) (4-h time-weighted average) at three sites (Trane
Company, 1991). At one site, where samples were taken near a leak and
a half-empty HCFC-123 drum, levels of 5.9-13.6 ppm (36.9-85.0 mg/m3)
(20-min time-weighted average) were recorded. At the rest of the
sites, machinery room air levels were below the limit of detection
(0.2-0.4 ppm [1.25-2.50 mg/m3]). Breathing-zone levels of HCFC-123
during routine chiller maintenance operations, including refrigerant
transfer, were measured at nine US installations. Two-hour to 12-h
time-weighted average concentrations were less than 1 ppm (6.25
mg/m3) in five cases, less than 2 ppm (12.5 mg/m3) in three cases,
and in the range of 2-5 ppm (12.5-31.3 mg/m3) in one case (MRI,
1991; Sibley, 1992; Trane Company, 1992). In Australia, 4- to 6-h
time-weighted average concentrations were less than 1 ppm (6.25
mg/m3) during repair work at a single installation (NICNAS, 1996).
In these studies, continuous area monitoring showed time-weighted
average air concentrations below 1 ppm (6.25 mg/m3), with
activity-related instantaneous peaks ranging from 30 to 500 ppm (188
to 3130 mg/m3).
Air levels resulting from the use of a fire extinguishant
containing 93% HCFC-123 were measured during fire control exercises in
which the firefighters wore a self-contained breathing apparatus (MRI,
1993a,b). Outdoor discharge resulted in maximum breathing-zone levels
ranging from 7 to 870 ppm (43.8 to 5440 mg/m3), depending on the
type of fire hazard. Inside an aircraft hangar, the discharge of
hand-held extinguishers resulted in a breathing-zone concentration of
20 ppm (125 mg/m3) during discharge, with average static air levels
ranging from 29 to 141 ppm (181 to 881 mg/m3) over the next 30 min.
With a large semi-portable fire extinguisher, breathing-zone
concentrations during discharge reached 180-300 ppm (1130-1880
mg/m3), whereas average static air levels ranged from 165 to 557 ppm
(1030 to 3480 mg/m3) over the next 30 min.
In a US factory converted to using HCFC-123 in its degreaser,
personal air monitoring during normal degreaser operations showed
5.5-h time-weighted average levels of HCFC-123 that ranged from 5.3 to
12.0 ppm (33.1 to 75.0 mg/m3) throughout the facility.1 Charging
and unloading the degreaser resulted in short-term breathing-zone
levels ranging from 160 to 460 ppm (1000 to 2880 mg/m3).
1 AlliedSignal Inc., personal communication, 1998 [cited in
NICNAS, 1999].
7. COMPARATIVE KINETICS AND METABOLISM IN LABORATORY ANIMALS
AND HUMANS
HCFC-123 is readily absorbed by inhalation and distributed
throughout the body, where it reaches levels in body fat up to 25
times higher than those in blood (Vinegar et al., 1994). Following
exposure to an initial concentration of 2000 ppm (12.5 g/m3)
radiolabelled HCFC-123 over two consecutive 3-h periods in a
closed-chamber system, total uptake was 50-60% in rats and 90-100% in
guinea-pigs (Urban & Dekant, 1994). In rats exposed to initial
concentrations of 120-11 000 ppm (0.75-68.8 g/m3) HCFC-123 for 6 h,
the inhalation uptake showed a rapid distribution phase lasting
30-45 min followed by a slow linear uptake phase (Vinegar et al.,
1994). Blood levels declined rapidly once exposure was terminated. In
rats exposed to 1000 ppm (6.25 g/m3), blood concentrations fell
from 15.0 mg/litre at the end of a 4-h exposure period to 4.5
mg/litre at 4 min post-exposure and 1.5 mg/litre at 1 h post-exposure
(Vinegar et al., 1994). After the initial decline, HCFC-123
concentrations in both blood and fat decreased log-linearly with
a half-life of approximately 80 min, indicating fat as the main
depository for unmetabolized HCFC-123. Experimentally determined
tissue to air partition coefficients were 2-3 for gut and muscle,
3-4 for blood, 3-5 for liver, and 60-70 for fat (Dekant, 1993;
Vinegar et al., 1994). Data on oral or dermal absorption were not
available.
In the rat, 26-32% of the uptake of HCFC-123 is metabolized,
predominantly by oxidation to trifluoroacetyl chloride, which is
hydrolysed to trifluoroacetic acid or reacts with lysine residues in
proteins or with low-molecular-weight amines to form
N-trifluoroacetyl amides (Harris et al., 1992; Dodd et al., 1993;
Urban & Dekant, 1994). Minor, reductive pathways lead to the formation
of very small amounts of 2-chloro-1,1,1-trifluoroethane,
2-chloro-1,1-difluoroethene, and 2,2-dichloro-1,1-difluoroethene. The
latter reacts with glutathione to form
N-acetyl- S-(2,2-dichloro-1,1-difluoroethyl)-L-cysteine. The
structures of these metabolites are shown in Figure 1. Both oxidation
and reduction of HCFC-123 are catalysed by cytochrome P450 2E1
(CYP2E1). In human liver microsomes, the major biotransformation
product is trifluoroacetic acid (Urban et al., 1994). Its rate of
formation was directly related to the amount of CYP2E1 present and
1.5-16 times faster than the rate in rat microsomes.
In experimental animals, the major metabolite in blood, urine,
and milk is trifluoroacetic acid. In rats exposed by inhalation to
1000 ppm (6.25 g/m3) HCFC-123 for 4 h, blood levels of parent
compound and trifluoroacetic acid amounted to 15.0 and 93.1 mg/litre,
respectively (Vinegar et al., 1994). At 10 000 ppm (62.5 g/m3), the
corresponding concentrations were 93.5 and 37.8 mg/litre,
respectively. Trifluoroacetic acid blood levels rebounded and peaked
12-26 h post-exposure, indicating that the metabolism of HCFC-123
is subject to substrate inhibition at exposures above 1000 ppm (6.25
g/m3). For exposure levels below 2000 ppm (12.5 g/m3), the
metabolic rate constants developed for HCFC-123 were Km = 1.2
mg/litre and Vmax = 7.20 mg/kg body weight per hour for male rats
and Km = 1.2 mg/litre and Vmax = 7.97 mg/kg body weight per
hour for female rats (Loizou et al., 1994). Generally, HCFC-123 was
not detected in blood samples collected within 1 h post-exposure from
lactating rhesus monkeys exposed by inhalation to 1000 ppm (6.25
g/m3) for 6 h per day, whereas trifluoroacetic acid concentrations
reached 150-190 µg/ml after 2-3 weeks of exposure. Based on data from
a single monkey, the half-life of trifluoroacetic acid in blood was
approximately 24 h (Slauter, 1997). In rats and guinea-pigs exposed to
14C-labelled HCFC-123 vapours for 6 h and sacrificed 48 h
post-exposure, only low amounts of radioactivity remained in the
organs examined (Urban & Dekant, 1994). The liver contained most of
the radiolabel, followed by testes and kidneys, lungs, brain,
pancreas, and spleen. Covalent binding of labelled material was
highest in liver tissue (0.4-0.7 nmol/mg protein), followed by lungs,
kidneys, and plasma (0.1-0.3 nmol/mg protein). Trifluoroacetylated
tissue proteins have been detected by immunological techniques in the
liver and at 20- to 200-fold lower levels in the kidney and heart of
rats 6-12 h after exposure to HCFC-123 by inhalation or
intraperitoneal injection (Harris et al., 1992; Huwyler & Gut, 1992;
Huwyler et al., 1992).
The available data indicate that the predominant routes of
HCFC-123 elimination are exhalation of the parent compound and urinary
excretion of trifluoroacetic acid. In rats exposed to an initial
concentration of 2000 ppm (12.5 g/m3) radiolabelled HCFC-123 for two
consecutive 3-h periods and sacrificed 48 h post-exposure, 23-28% of
the radioactive uptake was eliminated in the urine, predominantly as
trifluoroacetic acid, whereas 3-4% was recovered from the body (Urban
& Dekant, 1994). Small amounts of minor metabolites, such as
N-acetyl- S-(2,2-dichloro-1,1-difluoroethyl)-L-cysteine,
N-trifluoroacetyl-2-aminoethanol, and fluoride ion, were recovered
from urine, and trace amounts of 2-chloro-1,1,1-trifluoroethane were
detected in expired air (Urban & Dekant, 1994; Vinegar et al., 1994).
In lactating rats and monkeys exposed to 1000 ppm (6.25 g/m3)
HCFC-123 for 6 h per day for 3 weeks, trifluoroacetic acid was found
in milk at a maximum concentration of 65 and 30 µg/ml, respectively
(Buschman, 1996; Slauter, 1997). In monkeys, the milk also contained
small amounts (up to 5 µg/ml) of HCFC-123. Rat milk was not analysed
for HCFC-123, and neither monkey nor rat milk was analysed for
metabolites other than trifluoroacetic acid.
An analogue of HCFC-123, the common inhalation anaesthetic
halothane (2-bromo-2-chloro-1,1,1-trifluoroethane), is also
metabolized by hepatic CYP2E1 to trifluoroacetyl chloride, causing
trifluoroacetylation of liver proteins (Harris et al., 1992; Urban et
al., 1994). These include cytochrome P450 itself and other enzymes,
many of which have been identified as residing in the lumen of the
endoplasmic reticulum and involved in the maturation of newly
synthesized proteins (Cohen et al., 1997). Both halothane and HCFC-123
induce peroxisome proliferation and increased ß-oxidation in rat liver
cells (Keller et al., 1998). They are also highly effective in
inducing excess uncoupled cytochrome P450 activity in rabbit liver
microsomes, thus increasing hepatic oxygen consumption and
facilitating the oxidation of other cytochrome P450 substrates (Wang
et al., 1993).
Only limited information was available on the kinetics and
metabolism of HCFC-123 in humans in vivo. In four volunteers exposed
by inhalation to 60-73 ppm (375-460 mg/m3) HCFC-123 for 6 h, the
concentration of trifluoroacetic acid in the urine peaked at 10-27
mg/litre by 20-30 h and returned to zero by 96 h post-exposure,
indicating an elimination half-life of 25 h (Tanaka et al., 1998).
Physiologically based pharmacokinetic models for halothane in humans
and for halothane and HCFC-123 in rats have been used to deduce a
human model for HCFC-123 and its main metabolite, trifluoroacetic acid
(Williams et al., 1996). As the model has not been validated, its
usefulness as a predictive tool is unknown at this time.
8. EFFECTS ON LABORATORY MAMMALS AND IN VITRO TEST SYSTEMS
Unless otherwise indicated, only effects that were statistically
different ( P < 0.05) from controls have been considered. All
inhalation studies were performed by whole-body exposure, unless
otherwise mentioned.
8.1 Single exposure
When HCFC-123 was administered in corn oil by oral gavage to rats
at doses ranging from 2.25 to 11 g/kg body weight, rapid respiration
and prostration were recorded at and above 3.4 g/kg body weight. An
LD50 was not determined. The lowest dose causing mortality was 9
g/kg body weight (Henry, 1975).
In the rat and the hamster, inhalation of more than 30 000 ppm
(188 g/m3) caused severe CNS depression and death (Clayton, 1966;
Hall & Moore, 1975; Coate, 1976; Darr, 1981). The 4-h LC50 ranged
from 28 400 to 52 600 ppm (178-329 g/m3). Clinical signs of toxicity
included sedation, loss of muscle coordination and balance,
prostration, and dyspnoea. Gross pathological findings were either
negative or limited to congestion or discoloration of the lungs,
kidneys, liver, thymus, or small intestine. The lowest concentration
causing reversible CNS depression (failures in unconditioned reflexes)
in rats was 5000 ppm (31.3 g/m3) (Mullin, 1976). In a study of
cardiac sensitization to adrenaline, life-threatening or fatal
arrhythmias occurred in 0 of 3, 4 of 6, and 3 of 3 dogs exposed
nose-only to 10 000, 20 000, or 40 000 ppm (62.5, 125, or 250 g/m3).
The dogs were pretreated with intravenous adrenaline (8 µg/kg body
weight) prior to exposure and challenged with an identical adrenaline
dose after breathing the compound for 5 min. Based on these findings,
an EC50 (5 min) of 19 000 ppm (119 g/m3) and a NOAEL of 10 000 ppm
(62.5 g/m3) were determined (Trochimowicz & Mullin, 1973). Exposure
of guinea-pigs to 1000-30 000 ppm (6.25-188 g/m3) HCFC-123 for 4 h
produced non-fatal liver damage in all exposure groups (Marit et al.,
1994). At 48 h post-exposure, the liver effects included centrilobular
vacuolar (fatty) change, multifocal random and centrilobular
hepatocellular degeneration and necrosis, and increased levels of
plasma isocitrate dehydrogenase, alanine transaminase (ALT), and
aspartate transaminase (AST). In another study in guinea-pigs exposed
to 10 000 ppm (62.5 g/m3) for 4 h, liver injury was minimal unless
the animals had been glutathione depleted prior to exposure (Lind et
al., 1995).
In a rat and rabbit test for dermal toxicity carried out
according to OECD guidelines, the only effect observed after a dose of
2000 mg liquid HCFC-123/kg body weight was applied under occlusion for
24 h was a slight to moderate erythema in 6 of 10 rabbits up to 5 days
post-treatment. As such, the dermal LD50 for rats and rabbits was
greater than 2000 mg/kg body weight (Brock, 1988a,b).
8.2 Irritation and sensitization
Application under occlusion of 0.5 ml liquid HCFC-123 for 4 h
caused no erythema or oedema in a test for skin irritation potential
in rabbits conducted according to OECD guidelines (Brock, 1988c). In
rabbits, 0.1 ml undiluted HCFC-123 or 0.2 ml of a 50% solution in
propylene glycol caused mild to moderate, reversible eye damage,
including conjunctival irritation and corneal opacity (scoring not
reported) (Britelli, 1975). HCFC-123 did not produce skin
sensitization in guinea-pigs when 0.1 ml of a 1% solution in dimethyl
phthalate was administered intradermally once a week for 3 weeks,
followed by challenge 2 weeks later with 7 or 35 mg HCFC-123 dissolved
in propylene glycol (Goodman, 1975).
8.3 Short-term exposure
In rats exposed to 1000, 5000, 10 000, or 20 000 ppm (6.25, 31.3,
62.5, or 125 g/m3) HCFC-123 for 6 h per day, 5 days per week, in a
28-day inhalation toxicity study conducted in accordance with OECD
guidelines, exposures to 5000 ppm (31.3 g/m3) and above resulted in
dose-related narcotic effects, which were reversible overnight (Kelly,
1989; Rusch et al., 1994). Body weight was reduced at all dose levels
in females (8-10%) and at the two highest dose levels in males
(14-15%). There was a dose-related increase in relative liver weight
at all exposure levels in females (14-27%) and at the highest level in
males (18%). Plasma levels of ALT and AST were increased by 35% and
71%, respectively, at the highest level in males. Except for isolated
cases of fatty change at all dose levels, gross or microscopic liver
lesions were not observed. A NOAEL was not established in this study.
In a study in male rats exposed to approximately 1000, 5000, or
20 000 ppm (6.25, 31.3, or 125 g/m3) HCFC-123 for 6 h per day, 5
days per week, for 28 days, there was a decrease in body weight
(6-11%) and an increase in relative testis weight (12-30%) in all dose
groups and a 25% increase in relative liver weight in the highest dose
group (Lewis, 1990). Microscopic liver lesions including dose-related
hepatocyte hypertrophy and centrilobular fatty change were seen at all
exposure levels, with proliferation of peroxisomes and mitochondria in
liver cells of animals exposed to 5000 ppm (31.3 g/m3) and above.
Plasma levels of ALT, AST, and alkaline phosphatase (ALP) were
increased by up to 79%, whereas plasma triglycerides and cholesterol
were decreased by up to 70%, with AST and triglycerides being the most
sensitive markers of hepatic injury.
Similar findings accompanied by a 3-fold increase in hepatocyte
mitotic activity were reported from a study in which male rats were
exposed to 18 200 ppm (114 g/m3) HCFC-123 for 6 h per day, 5 days
per week, for 28 days (Warheit, 1993). This study also found
exposure-related testicular lesions, including germinal cell necrosis
and atrophy of the seminiferous tubules. In male guinea-pigs exposed
to 9400 ppm (58.8 g/m3) HCFC-123 for 6 h per day, 5 days per week,
for 28 days, there was evidence of microscopic liver lesions,
including centrilobular vacuolar (fatty) change and hepatocellular
necrosis, but no testicular effects were observed (Warheit, 1993).
8.4 Long-term exposure
8.4.1 Subchronic exposure
A number of subchronic inhalation studies have been carried out
in rats and dogs and are summarized in Table 1. In each case, HCFC-123
was administered for 6 h per day, 5 days per week. The main effects
were liver injury, with changes in liver-related clinical chemistry
parameters occurring at 300 ppm (1.88 g/m3) in rats and at 1000 ppm
(6.25 g/m3) in dogs, and CNS depression, with a reduction in arousal
occurring at 1000 ppm (6.25 g/m3) in rats.
8.4.2 Chronic exposure and carcinogenicity
In a 2-year inhalation study, groups of 80 male and female
Sprague-Dawley rats were exposed for 6 h per day for 5 days per week
to 300, 1000, or 5000 ppm (1.88, 6.25, or 31.3 g/m3) HCFC-123
(Malley, 1992; Malley et al., 1995).
During the first year, rats exposed to 5000 ppm (31.3 g/m3)
appeared sedated, but quickly recovered after the daily exposures
ended. Female rats exposed to 1000 ppm (6.25 g/m3) and males and
females exposed to 5000 ppm (31.3 g/m3) had lower body weight and
body weight gain. At the 12-month sacrifice, the relative liver weight
in male and female rats exposed to 5000 ppm (31.3 g/m3) was
increased by 12% and 24%, respectively. No exposure-related gross or
histopathological changes were observed. In both sexes, serum
triglycerides and glucose were decreased at all exposure levels in a
dose-related manner, by 65-100% and 15-31% for males and females,
respectively. Serum cholesterol was decreased by approximately 30% in
all exposed females and by 43% in males exposed to 5000 ppm
(31.3 g/m3).
During the second year of exposure, slight, reversible CNS
depression continued to be observed at 5000 ppm (31.3 g/m3). At the
end of the 2-year period, there was a dose-related increase in
survival rate, which reached a statistically significant level of 47%
and 59% in females exposed to 1000 or 5000 ppm (6.25 or 31.3 g/m3),
respectively. This is an expected effect of chemicals that reduce body
fat and blood lipids. Compared with the controls, body weight was
decreased by 8% in females exposed to 1000 ppm (6.25 g/m3) and by
12% in males and 21% in females exposed to 5000 ppm (31.3 g/m3). At
5000 ppm (31.3 g/m3), relative liver weight and the incidence of
enlarged and discoloured livers were increased in males, as were
grossly observed liver masses in females.
There were no exposure-related effects on the incidence of
malignant tumours. There was an increase in hepatocellular adenomas in
females and males, an increase in cholangiofibromas in high-dose
females, a dose-related increase in pancreatic acinar cell adenomas in
males, and an increase in Leydig (interstitial) cell adenomas in males
at all dose levels (Table 2). Except for hepatocellular adenomas in
males, the increase in the incidence of these tumours remained
statistically significant when corrected for mortality. Historical
data on tumour incidence in the strain used for this study were not
available. Other exposure-related lesions included hepatic foci of
cellular alteration and focal pancreatic acinar cell hyperplasia
(lesions less than 3 mm in diameter) in males and females at 1000 and
5000 ppm (6.25 and 31.3 g/m3), in addition to hepatic focal necrosis
in males, cholangiofibrosis in females, and hepatic centrilobular
fatty change in both sexes at 5000 ppm (31.3 g/m3). Dose-related
focal Leydig cell hyperplasia (lesions less than the diameter of three
adjacent tubules) was observed in male rats exposed to 1000 and
5000 ppm (6.25 and 31.3 g/m3). The incidence of diffuse retinal
atrophy was increased in both sexes at all exposure levels. Serum
triglycerides and cholesterol continued to be decreased in both sexes
by 46-75% and 31-48%, respectively.
As there were changes in clinical chemistry parameters and an
increased incidence of hepatocellular and Leydig cell adenomas at the
lowest dose tested (300 ppm [1.88 g/m3]), a NOAEL was not
established in this study.
8.5 Genotoxicity and related end-points
HCFC-123 was not mutagenic in Salmonella typhimurium strain
TA98, TA100, TA1525, TA1537, or TA1538 with or without metabolic
activation, even at concentrations of 750 mg per vessel or 150 000 ppm
(938 g/m3), which were clearly toxic (Callander, 1989). HCFC-123 was
found to be clastogenic in two separate studies in human lymphocytes
in vitro, both with and without metabolic activation, at relatively
high concentrations that also reduced the mitotic rate (Table 3). It
was also noted to be clastogenic in the absence, but not in the
presence, of a metabolic activation system in human lymphocytes
exposed to the chemical at 500 µg/ml; further details were not
available (ICI, 1992). No transformation to anchorage-independent
cells was observed when HCFC-123 was tested in baby hamster kidney
fibroblasts (BHK21 cells) with and without metabolic activation
(Longstaff et al., 1984).
Table 1: Summary of effect levels in subchronic inhalation toxicity studies.
Species Study design Effects Effect levels Reference
Rats, albino, Exposed to 0, 500, Body weight marginally LOAEL = 500 ppm Industrial Bio-Test
35 males and 1000, or 5000 ppm decreased in females at (3.13 g/m3) Laboratories, 1977;
25 females (0, 3.13, 6.25, or 1000 ppm and in both sexes Rusch et al., 1994
per group 31.3 g/m3) HCFC-123 at 5000 ppm. Kidney weight
for 90 days, with a increased in all male test
30-day recovery groups and in females at
period. 5000 ppm (% change not
reported). Relative liver
weight increased in females
at all exposure levels and
in males at 5000 ppm
(% change not reported).
Microscopic liver lesions
included mild focal necrosis
in males from all test groups
and minimal bile duct
proliferation in males at
5000 ppm. At end of recovery
period, there were no
exposure-related body or organ
weight changes or
histopathological findings.
Rats, Exposed to 0, 1000, Reversible motor LOAEL = 1000 ppm Doleba-Crowe, 1978;
Sprague-Dawley, or 10 000 ppm (0, incoordination and (6.25 g/m3) Rusch et al., 1994
27 per sex 6.25, or 62.5 g/m3) unresponsiveness to noise
per group HCFC-123 for 90 days. at 10 000 ppm. Reduced body
Histopathological weight (8-17%) and increased
examination performed relative liver weight
on 6 animals per (% change not reported) in
group. both test groups.
Table 1 (cont'd)
Species Study design Effects Effect levels Reference
No exposure-related gross or
histopathological findings.
Elevated levels of AST in
males at both exposure levels,
ALT in males at 1000 ppm, and
blood urea nitrogen (BUN) in
males at both exposure levels
and in females at 1000 ppm;
glucose decreased in females
in both test groups and in
males at 10 000 ppm (% change
not reported).
Rats, Exposed to 0, 300, Reduced responsiveness to LOAEL = 300 ppm Malley, 1990;
Sprague-Dawley, 1000, or 5000 ppm auditory stimuli at 1000 and (1.88 g/m3) Rusch et al.,
10 per sex (0, 1.88, 6.25, or 5000 ppm. Relative liver 1994
per group 31.3 g/m3) HCFC-123 weight increased by 12-17%
for 90 days. and 19-22%, respectively, at
1000 and 5000 ppm. No
exposure-related gross or
histopathological findings.
Dose-dependent elevations of
AST, ALT, lactate
dehydrogenase (LDH) in males
at 1000 and 5000 ppm and BUN
in females in all test groups
and in males at 1000 and
Table 1 (cont'd)
Species Study design Effects Effect levels Reference
5000 ppm. Triglycerides and
glucose markedly decreased
and hepatic ß-oxidation
activity increased 2- to
4-fold in all test groups.
Dose-dependent decrease in
cholesterol in females at
1000 and 5000 ppm.
Rats, Exposed to 0, 300, Reversible reduction in NOAEL = 300 ppm Coombs, 1994
Sprague-Dawley, 1000, or 5000 ppm arousal at 1000 and 5000 (1.88 g/m3)
10 per sex (0, 1.88, 6.25, or ppm. No exposure-related
per group 31.3 g/m3) HCFC-123 gross or histopathological
for 90 days, with a findings in cerebrum,
28-day recovery medulla/pons, cerebellar
period. Histological cortex, spinal cord, ganglia,
examinations limited dorsal and ventral root
to nervous tissues. fibres, or peripheral nerves.
Dogs, beagles, Exposed to 0, 1000, Reversible motor LOAEL = 1000 ppm Doleba-Crowe, 1978;
4 males per group or 10 000 ppm incoordination and (6.25 g/m3) Rusch et al., 1994
(0, 6.25, or 62.5 unresponsiveness at
g/m3) HCFC-123 for 10 000 ppm. At 10 000 ppm,
90 days. discoloured livers with
hepatocyte hypertrophy and
necrosis with inflammatory
cell infiltration. Elevated
levels of ALP in both test
groups and of BUN at 10 000
ppm (% change not reported).
Table 2: Incidence of selected non-neoplastic and neoplastic lesions in the liver, pancreas, and testes
in the 2-year rat inhalation study.a,b
Incidence of lesions
0 ppm 300 ppm 1000 ppm 5000 ppm
Male
Liver
Hepatocellular adenoma 3/67 2/66 2/66 8/66c
Basophilic foci of alteration 8/67 10/66 20/66d 30/66d
Clear cell foci of alteration 8/67 9/66 30/66d 19/66d
Mixed foci of alteration 3/67 6/66 6/66 12/66d
Eosinophilic foci of alteration 8/67 16/66 18/66d 13/66
Cholangiofibroma 0/67 0/66 0/66 0/66
Cholangiofibrosis 0/67 0/66 0/66 0/66
Pancreas
Acinar cell adenoma 1/67 4/66 12/64e 14/66e
Focal acinar cell hyperplasia 5/67 6/66 13/64d 19/66d
Testes
Leydig cell adenoma 4/67 12/66f 9/66f 14/66f
Leydig cell hyperplasia 8/67 15/66 23/66d 30/66d
Table 2 (cont'd)
Incidence of lesions
0 ppm 300 ppm 1000 ppm 5000 ppm
Female
Liver
Hepatocellular adenoma 0/65 5/67e 2/67 7/69e
Basophilic foci of alteration 17/65 26/67 32/67d 46/69d
Clear cell foci of alteration 14/65 7/67 16/67 15/69
Mixed foci of alteration 2/65 3/67 13/67d 22/69d
Eosinophilic foci of alteration 8/65 11/67 22/67 30/69d
Cholangiofibroma 0/65 0/67 0/67 6/69e
Cholangiofibrosis 0/65 0/67 0/67 9/69d
Pancreas
Acinar cell adenoma 0/65 2/66 0/67 2/69
Focal acinar cell hyperplasia 0/65 4/66 6/67d 8/69d
a From Malley (1992); Malley et al. (1995).
b Only lesions whose incidence attained statistical significance in at least one male or female dose
group are included in the table. Other non-statistically significant lesions are discussed in the
text. The figures give the number of lesions per number of tissues available for histological
examination. A few animals and tissues were lost due to autolysis.
c P < 0.05 (Cochran-Armitage test for trend).
d P < 0.05 (Fisher's exact test compared with controls).
e P < 0.05 (Cochran-Armitage test for trend and 2/2 tests for mortality-adjusted statistical analysis).
f P < 0.05 (Cochran-Armitage test for trend and 1/2 tests for mortality-adjusted statistical analysis).
In vivo, a test for chromosome aberrations in the lymphocytes
of rats exposed by inhalation to up to 5000 ppm (31.3 g/m3) HCFC-123
for 6 h per day, 5 days a week, for 2 weeks was negative (Marshal,
1992), although the failure to induce signs of cytotoxicity cast doubt
on the validity of this finding. No increase was found in the
incidence of micronuclei or in the ratio of polychromatic to
normochromatic erythrocytes in a micronucleus test in mice exposed
nose-only to up to 18 000 ppm (113 g/m3) HCFC-123 for 6 h (Muller &
Hofmann, 1988). In the livers of rats exposed to 12 500 or 20 000 ppm
(78.1 or 125 g/m3) HCFC-123 for 6 h, neither net nuclear grain count
nor percentage cells in repair showed any evidence of unscheduled DNA
synthesis (Kennelly, 1993).
Although HCFC-123 was clastogenic in vitro at high
concentrations, all other in vitro and in vivo tests for genetic
toxicity were negative. Overall, the available studies suggest that
HCFC-123 is unlikely to be genotoxic in vivo.
8.6 Reproductive and developmental toxicity
In studies conducted according to OECD guidelines, HCFC-123 was
neither embryotoxic nor teratogenic in pregnant rats exposed for 6 h
per day on days 6-15 of gestation by inhalation to 0, 5000, or 10 000
ppm (0, 31.3, or 62.5 g/m3) HCFC-123, although maternal toxicity in
the form of reduced weight gain and CNS depression were observed at
both exposure levels (Culik & Kelly, 1976; Brewer & Smith, 1977). In a
range-finding study in which fetal examinations were limited to
external structural abnormalities, HCFC-123 was not embryotoxic or
teratogenic in pregnant rabbits exposed for 6 h per day on days 6-18
of gestation by inhalation to 0, 500, 1500, or 5000 ppm (0, 3.13,
9.38, or 31.3 g/m3) HCFC-123, although dose-related maternal
toxicity characterized by reduced weight gain and food consumption was
evident at all exposure levels (Malinverno et al., 1996).
In a two-generation reproductive toxicity study in Sprague-Dawley
rats conducted in accordance with OECD guidelines, male and female
animals were exposed for 6 h per day, 7 days a week, by inhalation to
0, 30, 100, 300, or 1000 ppm (0, 0.188, 0.625, 1.88, or 6.25 g/m3)
HCFC-123 (Hughes, 1994; Malinverno et al., 1996). The F0 (parental
generation) animals were exposed from 6 weeks of age for 23-39 weeks,
including a 2-week mating period, the gestation period, and, except
for maternal animals on postpartum days 0-4, until the offspring were
weaned. The F1 generation was exposed from 4 weeks of age through to
weaning of their litters (F2 generation), for a total of
approximately 28 weeks.
Table 3: Chromosome aberrations in human lymphocytes in vitro.
Physical form of Exposure Concentrationb Mean mitotic Number of Reference
HCFC-123 protocola index cells with
aberrations
(excluding gaps)
Liquid 3-h exposure 0 µg/ml 25.2 1 Dance, 1991
(without S9) 73 µg/ml 19.6 2
146 µg/ml 21.7 3
292 µg/ml 21.1 3
CBC (2 µg/ml) 14.0 91
3-h exposure 0 µg/ml 23.8 1
(with S9) 146 µg/ml 21.8 2
292 µg/ml 14.7 6
584 µg/ml 6.6 5
CP (6 µg/ml) 8.4 106
24-h exposure 0 µg/ml 30.7 2
(without S9) 36 µg/ml 27.4 3
73 µg/ml 21.5 10c
292 µg/ml 9.7 31d
CBC (2 µg/ml) 22.9 70
Vapour 3-h exposure 0 ppm 24.9 1 Edwards, 1991
(without S9) 75 000 ppm 23.9 3
150 000 ppm 17.9 0e
300 000 ppm 10.3 5e
CBC (2 µg/ml) 18.5 60
Table 3 (cont'd)
Physical form of Exposure Concentrationb Mean mitotic Number of Reference
HCFC-123 protocola index cells with
aberrations
(excluding gaps)
3-h exposure 0 ppm 22.2 4
(with S9) 75 000 ppm 24.1 3
150 000 ppm 21.0 4
300 000 ppm 9.5 23e,f
CP (6 µg/ml) 15.2 107
24-h exposure 0 ppm 16.6 1
(without S9) 25 000 ppm 19.1 9d
50 000 ppm 13.2 18f
100 000 ppm 5.9 24f
CBC (2 µg/ml) 13.5 118
a S9 = metabolic activation system.
b Positive controls: CBC = chlorambucil; CP = cyclophosphamide.
c P < 0.05.
d P < 0.01.
e Increase in number of polyploid cells.
f P < 0.001.
The only adverse reproductive effect was a 17% decrease in
implantation count among F1 females at the highest exposure level.
In terms of development, pup growth was impaired during the
pre-weaning period when exposure was confined to the lactating parent
female. In the F1 generation, mean pup weight was decreased by
approximately 10% at exposures at and above 100 ppm (0.625 g/m3),
whereas mean pup weight in F1 offspring (F2 generation) was
decreased by approximately 20% at all exposure levels. In adult rats,
retarded weight gain was observed at 100 ppm (0.625 g/m3) and above
in F0 animals and at 300 ppm (1.88 g/m3) and above in the F1
generation. There was a dose-dependent, 8-39% increase in liver weight
in all exposed F0 groups and an 8-10% increase at 100 ppm (0.625
g/m3) and above in F1 animals. In F1 animals exposed to HCFC-123
for approximately 28 weeks, exposure-related histopathological changes
were confined to a dose-related increase in the incidence of
centrilobular hepatocyte enlargement and in the incidence and degree
of hepatocyte vacuolation at 300 ppm (1.88 g/m3) and above. No
microscopic changes were detected in the livers of F2 weanling rats
from the 1000 ppm (6.25 g/m3) exposure group. In both generations,
there was a decrease in serum triglycerides in both sexes, whereas
serum cholesterol was increased in males and decreased in females.
Levels of ALT, AST, or other liver enzymes were not determined. Male
rats exposed at or above 300 ppm (1.88 g/m3) had increased plasma
levels of luteinizing hormone (LH) after 10 weeks of exposure, which
had reverted to normal at week 38. In this study, the NOAEL, based on
effects on fertility, was 300 ppm (1.88 g/m3). The LOAEL, based on
developmental effects (retarded neonatal growth during lactation) and
on increased liver weight and changes in liver-related clinical
chemistry parameters, was 30 ppm (0.188 g/m3).
After 22 weeks of exposure, a sample of F1 male rats was drawn
from the two-generation reproductive toxicity study for
endocrinological investigations (Sandow et al., 1995b). In 10 males
from each exposure group, serum levels of LH and testosterone were
determined before and after an injection of LH releasing hormone. The
testes of another eight males per group were incubated in vitro with
human chorionic gonadotropin (which stimulates steroid hormone
biosynthesis). The incubation medium and testis tissue were analysed
for content of testosterone, progesterone, estradiol-17-ß, 17
alpha-OH-progesterone, and delta-4-androstenedione. Basal serum LH and
testosterone levels were similar to those of controls. However, after
stimulation with LH releasing hormone, the LH in rats exposed to 300
ppm (1.88 g/m3) was 32% lower than in controls; at 1000 ppm (6.25
g/m3), LH was 39% and testosterone 46% lower than in the control
group. In the ex vivo test, HCFC-123 inhalation did not affect the
secretory capacity for steroid hormones or alter the content of these
hormones in the testes at the end of the incubation period, except for
a slight reduction of delta-4-androstenedione at 1000 ppm (6.25
g/m3).
In an endocrinological study in rats of both sexes, exposure to
5200 ppm (32.5 g/m3) HCFC-123 for 6 h per day (similar to the
highest dose level in the 2-year bioassay) for 14 consecutive days was
associated with sedation, decreased body, kidney, ovary, and pituitary
weights in females, and increased relative liver weight in males
(Hofmann, 1995; Sandow et al., 1995a). In males, the prolactin
response after monoiodotyrosine stimulation, the testosterone response
after stimulation with buserelin (a synthetic gonadotropin releasing
hormone), and the testicular testosterone content were all reduced by
approximately 50%. In females, the gonadotropin response to buserelin
stimulation was enhanced and the pituitary content of follicle
stimulating hormone and prolactin was reduced, in both cases by
approximately 50%.
These endocrinological investigations indicate that HCFC-123 has
little, if any, effect on steroid production in rat testes, but
impairs the prolactin, LH, and testosterone response to pituitary
stimulants. The NOAEL based on this effect was 100 ppm (0.625 g/m3).
A lactation study was conducted in groups of pregnant and
lactating Sprague-Dawley (Crl:CD BR) rats exposed to 0 or 1000 ppm (0
or 6.25 g/m3) HCFC-123 for 6 h per day on days 5-19 of gestation and
on days 5-21 postpartum (Buschman, 1996). Within 2 days of birth,
litters were crossed over between dams to create four groups
comprising exposed or control dams rearing litters from different
exposed or control mothers. Absolute and relative liver weights were
increased and serum triglycerides, cholesterol, and glucose decreased
in dams exposed to HCFC-123. The milk of dams exposed to HCFC-123 was
of normal quantity and quality (with regard to content of protein,
lactose, and fat) but contained trifluoroacetic acid at an average
concentration of 50 µg/ml. Trifluoroacetic acid was also found in the
urine of pups reared by dams exposed to HCFC-123. There were no
differences in absolute or relative liver weight or any abnormal
clinical signs or gross findings in any of the groups of pups, but
pups reared by dams exposed to HCFC-123 had a 10% lower growth rate
and decreased serum triglycerides compared with pups reared by
non-exposed dams. Before crossover, there was no difference between
groups with respect to mean pup and litter weight. As such, these
findings indicate that the retarded neonatal growth observed in the
two-generation reproductive toxicity study was due to factors in the
milk of exposed mothers, probably trifluoroacetic acid, rather than to
exposure in utero.
When groups of four lactating rhesus monkeys and their neonates
were exposed to either 0 or 1000 ppm (0 or 6.25 g/m3) HCFC-123 for 6
h per day for 21-22 consecutive days, there were no effects on
maternal body weight, serum triglycerides, cholesterol, and glucose,
or milk composition (Slauter, 1997). Liver biopsy specimens taken from
the mothers at the end of the study revealed exposure-related lesions,
including mild to moderate centrilobular hepatocyte vacuolation, trace
to moderate centrilobular hepatocyte necrosis, and trace to mild
subacute inflammation. As a rule, HCFC-123 was not detected in the
blood of mothers or neonates, whereas trifluoroacetic acid was present
at concentrations of 9-70 µg/ml in exposed mothers and of 17-190 µg/ml
in neonates, with individual blood levels being 2-6 times higher in
the neonates than in their corresponding mothers. Milk from exposed
mothers contained HCFC-123 and trifluoroacetic acid at concentrations
of 1-5 µg/ml and 17-30 µg/ml, respectively. Although no statistical
analysis was attempted because of the small number of observations,
the average growth rate was 10% lower in exposed neonates than in
unexposed controls.
9. EFFECTS ON HUMANS
The available data on the human health effects of HCFC-123 were
limited to a single case report of dizziness, headache, and nausea in
workers exposed to unknown levels of the chemical following the
rupture of an industrial chiller1 and three case reports of hepatic
effects involving 26 workers following repeated exposure to HCFC-123
vapours.
Nine cases of liver effects were reported in gantry drivers at a
smelting depot in Belgium (Hoet et al., 1997). They occurred 1-4
months after the refrigerant utilized in the crane cabin
air-conditioning system had been replaced by a blend containing 57%
HCFC-123, 40% HCFC-124 (1-chloro-1,2,2,2-tetrafluoroethane), and 3%
propane.2 One driver admitted to hospital was found to have
increased levels of AST, ALT, ALP, gamma-glutamyl transferase, and
total and conjugated bilirubin and decreased prothrombin activity,
with AST and ALT levels being 15-23 times above the upper limit of the
normal range. Autoimmune, viral, and drug- or alcohol-induced
hepatitis were ruled out. A liver biopsy showed focal liver cell
necrosis, plugging of bile ducts, and the presence of
trifluoroacetylated proteins. The symptoms regressed during the period
of non-exposure but recurred when the driver returned to work 2 months
later. Eight other drivers showed signs of varying degrees of liver
abnormalities. Serum antibodies to human liver enzymes (CYP2E1 and/or
protein disulfide isomerase isoform P58) were detected in five of six
cases examined. A workplace inspection revealed that the plastic pipes
of the air-conditioning system were perforated and that refrigerant
was leaking into the crane cabin. No further cases occurred after the
system was repaired. Although the workers were exposed to both
HCFC-123 and HCFC-124, the latter is unlikely to have contributed to
the observed effects, as the NOAEL for HCFC-124 was 50 000 ppm (280
g/m3) in a 90-day inhalation toxicity study in rats (Malley et al.,
1996), whereas the LOAEL for HCFC-123 in a similar study in the same
strain and laboratory was 300 ppm (1.88 g/m3) (Table 1).
Eight cases of liver effects were reported in workers exposed to
the vapours of a solvent degreaser containing HCFC-123.3 Two months
after a US factory converted to using HCFC-123 in its degreaser, two
employees who worked closely with the degreaser were found to have
liver disease. They had elevated blood levels of liver enzymes,
particularly ALT (32-56 times above the upper limit of the normal
1 Carrier Canada Ltd, personal communication, 1993 [cited in
NICNAS, 1996].
2 N. Verlinden, personal communication, 1997 [cited in
NICNAS, 1999].
3 AlliedSignal Inc., personal communication, 1998 [cited in
NICNAS, 1999].
range) and AST (14-33 times above the upper limit of the normal
range), had elevated total and conjugated bilirubin, and tested
negative for viral hepatitis. Subsequent testing of all 27 factory
employees revealed four additional cases of elevated liver enzymes.
When retested 1 month later but before the use of HCFC-123 was
discontinued, five of the affected employees had improved markedly,
whereas one had deteriorated, and there was one new case with slightly
elevated ALT and AST levels. All in all, liver enzymes were elevated
in 3 of 4 employees who worked with the degreaser and in 4 of 23
workers who did not. Air monitoring was conducted when HCFC-123 use
began and again shortly after the first cases were diagnosed. Personal
air monitoring during normal degreaser operations showed 5.5-h
time-weighted average levels of HCFC-123 that ranged from 5.3 to
12.0 ppm (33.1 to 75.0 mg/m3) throughout the facility, whereas
short-term breathing-zone levels ranging from 160 to 460 ppm (1000 to
2880 mg/m3) were measured in workers charging and unloading the
degreaser. There was also one case of elevated liver enzymes with
negative tests for viral hepatitis in a technician employed in the
manufacturer's research laboratory where the degreaser was tested and
evaluated. Static air levels in the laboratory were reported to be
generally below 50 ppm (313 mg/m3) HCFC-123.
In a factory in Japan where miniature heat exchangers were filled
with HCFC-123 in a poorly ventilated room, 9 out of 14 workers were
found to have elevated levels of liver enzymes 4-5 weeks after
production had commenced (Takebayashi et al., 1998a,b).1 Four of
them were clinically ill, and two had jaundice. In these workers, AST
and ALT were up to 20-30 times above the upper limit of the normal
range. After an exhaust system was installed that maintains the
concentration of airborne HCFC-123 at about 1 ppm (6.25 mg/m3),
trifluoroacetic acid was not detected in the workers' urine; at
follow-up 1 year later, there were no further cases of clinical
illness or elevated liver enzymes.1 Exposure levels were not
measured, but a simulation of the original working conditions
indicated static air levels ranging from 5 to 1125 ppm (31.3 to
7030 mg/m3) (6-h time-weighted average), depending on the distance
from the filling area.
1 Also T. Takebayashi, personal communication, 1999 [cited in
NICNAS, 1999].
Table 4: Summary of effects of HCFC-123 in aquatic organisms.
Test Species Effects and effect Comments Reference
levels
Acute toxicity (96 h), Fathead minnow Lethargy Rapid degassing Pierson,
flow-through conditions (Pimephales LC50 > 76 mg/litre of HCFC-123 1990a
promelas) (measured) from test
solutions
Acute toxicity (96 h), Rainbow trout Lethargy, darkened Jenkins, 1992b
static conditions (Oncorhynchus pigmentation at
mykiss) 15 mg/litre and
above
LC50 = 56 mg/litre
(measured)
Immobilization (48 h), Daphnia magna Lethargy Jenkins, 1992c
static conditions EC50 = 17 mg/litre
(measured)
Immobilization (48 h), Daphnia magna Lethargy Measured Pierson, 1990b
static conditions EC50 = 45.8 mg/litre concentrations
(nominal) <75% of nominal
Algal growth inhibition Selenastrum EC50 = 68 Based on biomass Jenkins, 1992d
(96 h), static conditions capricornutum mg/litre (measured) integral
10. EFFECTS ON OTHER ORGANISMS IN THE LABORATORY AND FIELD
The available ecotoxicological data for HCFC-123 are summarized
in Table 4. The data indicate that the chemical is, at most, slightly
toxic to aquatic organisms under conditions of acute exposure. Chronic
effects would not be expected because of limited aquatic persistence.
Trifluoroacetic acid, which is formed by atmospheric breakdown of
HCFC-123, has been found to be of low toxicity in stream mesocosms,
algae, higher plants, fish, and mammals (Boutonnet et al., 1999). The
lowest threshold for any effect was 0.12 mg sodium
trifluoroacetate/litre, above which the chemical had reversible
effects on the growth of the alga Selenastrum capricornutum. In the
most sensitive terrestrial species tested (sunflower), sodium
trifluoroacetate at 1 mg/kg dry soil had clear effects on vegetative
growth, whereas long-term root exposure of wheat and soya to sodium
trifluoroacetate at 1 mg/litre had no effect.
11. EFFECTS EVALUATION
11.1 Evaluation of health effects
11.1.1 Hazard identification and dose-response assessment
There was inadequate information on the human health effects of
short-term exposure to HCFC-123. In laboratory animals, HCFC-123
exhibits low acute toxicity, with an approximate oral lethal dose of
9 g/kg body weight, a 4-h LC50 by inhalation in the range of
28 400-52 600 ppm (178-329 g/m3), and a dermal LD50 in excess of
2000 mg/kg body weight. It is not a skin irritant or sensitizer, but
liquid HCFC-123 produces mild to moderate eye irritation. The critical
effects associated with acute exposure are non-fatal liver damage, CNS
depression, and cardiac sensitization to adrenaline. When inhaled in
lethal concentrations, death was caused by severe CNS depression. For
a single, 4-h exposure by inhalation, the LOAEL was 1000 ppm
(6.25 g/m3) for liver damage, based on liver cell necrosis and
increased levels of circulating liver enzymes in guinea-pigs, and 5000
ppm (31.3 g/m3) for CNS depression, based on failures in
unconditioned reflexes in rats. The NOAEL for cardiac sensitization to
adrenaline in dogs was 10 000 ppm (62.5 g/m3). Although the dog is
a very sensitive species, cardiac sensitization to adrenaline-induced
arrhythmia is likely to be a relevant critical effect resulting from
short-term exposure in humans, such as the sudden discharge of fire
extinguishants in occupied rooms (NAS, 1996). Under these
circumstances, CNS depression may also be a critical effect. A large
number of short-chain halogenated hydrocarbons with different
metabolic patterns have similar CNS and cardiac effects, which likely
result from a direct anaesthetic action on neurons and myocardial
cells (IPCS, 1990, 1991, 1992).
The critical effects associated with repeated exposure to
HCFC-123 vapours are liver damage in humans as well as experimental
animals, in addition to neonatal growth retardation, an increased
incidence of benign tumours, and CNS depression, which have been
recorded only in animals.
Based on a limited number of case reports, biochemical
abnormalities associated with liver injury have been observed in
humans at exposure levels in the order of 5-1125 ppm (31.3-7030
mg/m3). The available data are insufficient to define the
dose-response relationship in humans. Liver damage was also seen in
rats, guinea-pigs, dogs, and monkeys. The lesions generally involved
increased liver weight accompanied by hepatocyte enlargement and
vacuolation at the lowest exposure levels, with necrosis, fatty
change, and mild subacute inflammation at higher concentrations. They
were associated with or preceded by increased levels of circulating
liver enzymes and decreased serum triglycerides, glucose, and
cholesterol. The LOAEL for liver effects in animals recorded in a
well-conducted two-generation reproductive toxicity study in rats
equalled 30 ppm (188 mg/m3).
The cytotoxicity of HCFC-123 is probably due to the reactive
metabolite trifluoroacetyl chloride, which can bind covalently to
proteins and interfere with their function and/or alter their
antigenicity. The 200 : 20 : 1 ratio of trifluoroacetylation of liver,
kidney, and heart tissue proteins reported by Huwyler et al. (1992)
and Huwyler & Gut (1992) correlates well with the observed effect
levels for these organs in subchronic toxicity studies (Table 1) and
probably reflects tissue differences in metabolic capacity.
There is no evidence that HCFC-123 is teratogenic in laboratory
animals or induces reproductive or fetal toxicity at levels of
exposure lower than those that cause other systemic effects in adults.
Growth was retarded in neonatal rats and monkeys reared by dams
exposed by inhalation to HCFC-123, with a LOAEL of 30 ppm
(188 mg/m3). The main metabolite of HCFC-123, trifluoroacetic acid,
was found in the milk of the dams. As such, breastfed babies may
represent a subpopulation that is uniquely sensitive to HCFC-123.
Reversible CNS depression was seen consistently in repeated-dose
inhalation studies, but did not change in severity or duration with
the number of exposures and was not associated with morphological
changes in nervous tissues. As such, the mechanism of action is
probably the same for both acute and chronic CNS depression. The
lowest NOAEL for CNS effects from repeated administration was recorded
in a neurotoxicity study in rats and equalled 300 ppm (1880 mg/m3),
based on a reduction in arousal.
Although there was evidence of clastogenic activity in human
lymphocytes in vitro at high, cytotoxic concentrations, all other
in vitro and in vivo tests for genetic toxicity were negative.
Therefore, the evidence suggests that HCFC-123 is unlikely to be
genotoxic in vivo.
In a 2-year inhalation study in rats, there was no
exposure-related effect on the incidence of malignant tumours, but
there was an increased incidence of hepatocellular adenomas,
cholangiofibromas, pancreatic acinar cell adenomas, and Leydig
adenomas. There was also an increase in the pre-cancerous lesions of
hepatic foci of alteration, cholangiofibrosis, focal pancreatic acinar
cell hyperplasia, and Leydig cell hyperplasia (Table 2). As stated
above, HCFC-123 is unlikely to be genotoxic in vivo. The minor,
non-mutagenic metabolite 2-chloro-1,1,1-trifluoroethane caused an
increased incidence of uterine carcinomas and Leydig cell adenomas in
rats when given by oral gavage at 300 mg/kg body weight for 52 weeks
(IPCS, 1992). However, only trace quantities would be formed from the
metabolism of HCFC-123. Therefore, it is necessary to examine the
mechanisms of tumour formation to establish if the modes of induction
can be ruled out as relevant for humans:
* Hepatocellular adenomas. Several repeated-exposure studies have
shown that HCFC-123 and its main metabolite, trifluoroacetic
acid, like several other structurally diverse chemicals, induce
peroxisome proliferation in rat hepatocytes (Warheit, 1993; Rusch
et al., 1994; Malley et al., 1995; Keller et al., 1998).
Peroxisome proliferators are generally not genotoxic but induce
hepatocellular proliferation in rats and mice through a mechanism
that appears to involve the expression of growth factors by
hepatic macrophages, thus leading to liver tumour formation
(Chevalier & Roberts, 1998). The hepatocellular adenomas seen in
rats exposed to HCFC-123 may be related to the induction of
peroxisome proliferation, which is a mechanism of questionable
relevance for humans (Ashby et al., 1994). However, since the
chemical is also hepatotoxic, which may have a role in the tumour
formation, it is not possible to discount the hepatocellular
adenomas as being of no concern to humans. It is, however,
reasonable to adopt a threshold approach, based on adverse
effects on the liver in subchronic exposure studies.
* Cholangiofibromas. Cholangiofibromas in the rat are atypical
glandular structures lined by intestinal-like epithelium
surrounded by dense connective tissue. Limited evidence from
animal studies of various non-genotoxic chemicals, including
chloroform and furan, suggests that this tumour type is
associated with significant hepatocyte necrosis and regenerative
cell proliferation that are relevant only at high dose/exposure
levels (Elmore & Sirica, 1993; Jamison et al., 1996). In the
2-year rat study, cholangiofibromas and cholangiofibrosis
occurred only in females exposed to HCFC-123 at 5000 ppm (31.3
g/m3). The incidence of basophilic and eosinophilic foci of
hepatocellular proliferation was also higher in females than in
males (Table 2). Although the threshold level for the induction
of cholangiofibromas in female rats was high, there is no
mechanistic evidence that this tumour type can be dismissed with
regard to its relevance to humans.
* Pancreatic acinar cell adenomas. Some hepatocarcinogenic
peroxisome proliferators have been reported to induce tumours in
other organs, including pancreatic acinar cell adenomas and
Leydig cell adenomas, although these extrahepatic tumours appear
not to be associated with peroxisome proliferation in the target
organ (IARC, 1995). Although pancreatic acinar cell adenomas were
found only in males at 1000 and 5000 ppm (6.25 and 31.3 g/m3),
the incidence was dose-related. Moreover, pancreatic acinar cell
hyperplasia occurred in both sexes, likewise in a dose-dependent
manner (Table 2). As such, until more is known about the
mechanism for acinar cell tumour induction in animals and humans,
the possibility that the pancreatic adenomas found in rats
exposed to HCFC-123 may have some relevance to humans cannot be
discounted.
* Leydig cell adenomas. The available studies indicate that
exposure to HCFC-123 may be associated with endocrine
disturbances in male rats, particularly in relation to prolactin
release and serum LH concentrations. In rats, but not in humans,
a decrease in serum prolactin causes a decrease in the number of
LH receptors on Leydig cells and thus a decrease in testosterone
production, which results in increased LH levels that in turn may
induce Leydig cell hyperplasia and adenomas (Clegg et al., 1997).
As such, it is conceivable that intermittent exposure to HCFC-123
could lead to fluctuations in prolactin and testosterone that in
rats could induce transient increases in LH and, with time,
Leydig cell growth and tumours. Although prolactin fluctuations
would not be of concern in men, the effects of HCFC-123 on the
sex hormone system are complex. Thus, in the absence of data from
studies in primates, the increased incidence of Leydig cell
adenomas in the 2-year rat study cannot be dismissed with respect
to its relevance for humans.
In summary, it is likely that the benign tumours in the 2-year
rat bioassay involve one or more non-genotoxic mechanisms, including
peroxisome proliferation, hepatocellular damage, necrosis and
regenerative proliferation, and disturbance of the
hypothalamic-pituitary-testicular axis. Although humans may be less
sensitive to tumours arising from some of these actions, overall it is
not possible to discount the tumours in an evaluation of the potential
risk for humans. Therefore, the increased incidence of benign tumours
in the rat raises some concern with respect to the potential
carcinogenicity in humans. The tumours probably arise from
non-genotoxic mechanisms. In the 2-year bioassay, the tumour incidence
was increased at 300 ppm (1.88 g/m3), the lowest level tested, and a
NOAEL was not established. In subchronic toxicity studies, the LOAEL
based on any adverse effect on the liver was 30 ppm (0.188 g/m3)
(Hughes, 1994; Malinverno et al., 1996).
11.1.2 Criteria for setting tolerable intakes or guidance values
for HCFC-123
Derivation of a guidance value for HCFC-123 was outside the scope
of the source documents (NICNAS, 1996, 1999). General advice about the
derivation of tolerable intakes and guidance values for health-based
exposure limits is set out in Environmental Health Criteria 170 (IPCS,
1994).
Exposure of the general public to HCFC-123 is likely to be
minimal. The main risk to human health is through repeated
occupational exposure via inhalation.
The critical effects of repeated low-level exposure to HCFC-123
are liver damage, which has been observed in all species investigated,
including monkeys and humans, and retarded neonatal growth during
lactation, which has been observed in rats and monkeys. In the absence
of sufficient data to establish a dose-response relationship in
humans, a guidance value for exposure to HCFC-123 must be based on the
observed effect levels for liver lesions and retarded neonatal growth
during lactation in pivotal animal studies. For both of these critical
effects, a LOAEL of 30 ppm (188 mg/m3) was established in a
well-conducted two-generation reproductive toxicity study in rats,
whereas a NOAEL was not achieved (Hughes, 1994; Malinverno et al.,
1996).
The uncertainty factor applied to extrapolate from the LOAEL to a
NOAEL must allow for the fact that whereas the recorded liver effects
at the LOAEL were mild, hepatotoxicity may have a role in the
induction of benign liver tumours in rats. Moreover, the growth
retardation in neonates during lactation was in the order of 10-20%.
It is likely that the liver effects are related to the formation of
protein adducts with trifluoroacetyl chloride and that growth
retardation in neonates is related to exposure to trifluoroacetic acid
in maternal milk. Therefore, the uncertainty factor used to
extrapolate the NOAEL from rats to humans must allow for the lack of
in vivo data on the metabolic rate and other toxicokinetic
properties of HCFC-123 in humans. Finally, an additional uncertainty
factor must be applied to allow for human variability in
toxicokinetics, as the metabolism of HCFC-123 to trifluoroacetyl
chloride and trifluoroacetic acid is catalysed by CYP2E1
(Urban et al., 1994), whose activity is known to be influenced by
genetic polymorphism, body weight, and dietary factors (Le Marchand
et al., 1999).
11.1.3 Sample risk characterization
Adverse effects of HCFC-123 in animals and humans have been
observed only at concentrations that were several orders of magnitude
higher than those in the only known medium of exposure (air) in the
general environment. The likelihood of public exposure as a
consequence of catastrophic accidents or fire extinguishant discharges
is very small, and the scale and duration of such exposures are
expected to be low.
With respect to repeated occupational exposures, 3- to 8-h
time-weighted average personal exposure levels in an HCFC-123
manufacturing plant were reported to be below 10 ppm (62.5 mg/m3).
Reported 2- to 12-h time-weighted average breathing-zone levels in
machinery rooms containing air-conditioning equipment generally ranged
from below 1 to 5 ppm (below 6.25 to 31.3 mg/m3), whereas the use of
a liquid HCFC-123 degreaser was associated with concentrations in the
range of 5.3-12 ppm (33.1-75.0 mg/m3) HCFC-123. The available case
reports indicate that humans may develop biochemical signs of liver
disease, such as elevated AST and ALT, after 1-4 months of repeated
exposure to HCFC-123 at levels above 5 ppm (31.3 mg/m3). Because the
effects of low levels of HCFC-123 are due to toxic metabolites formed
through CYP2E1, genetic, lifestyle, and dietary factors are expected
to cause considerable variability in human susceptibility to the
chemical.
11.2 Evaluation of environmental effects
Because of its high volatility, HCFC-123 released to the
environment will partition almost entirely to the atmosphere. It is
removed predominantly in the troposphere by reaction with hydroxyl
radicals to form trifluoroacetic acid, and only a small fraction is
transported to the stratosphere, where it may undergo photolysis and
release chlorine radicals that catalyse the destruction of ozone.
Because of its short atmospheric lifetime, estimated at 1.4 years, its
ozone-depleting potential is low (0.02 relative to CFC-11). The global
warming potential of HCFC-123 relative to carbon dioxide is 300, 93,
and 29 over a time horizon of 20, 100, and 500 years, respectively
(WMO, 1995).
The aquatic EC50/LC50 values were below 100 mg/litre but
above 10 mg/litre. As such, the chemical meets the European Community
criteria for classification as harmful to the environment (Berends et
al., 1999) and the globally harmonized criteria for classification as
hazardous to the aquatic environment (Class: Acute III) (OECD, 1998).
However, while HCFC-123 may be released to surface waters or soil, it
is unlikely to persist in these media because of its high volatility.
As such, it is considered that HCFC-123 does not constitute a
long-term or delayed danger to the aquatic environment.
Trifluoroacetic acid formed by degradation of HCFC-123 will
precipitate in rain and may accumulate in closed aquatic systems such
as salt lakes and seasonal wetlands. The maximum total contemporary
deposition rate of trifluoroacetic acid from fluorocarbons has been
estimated at 2800 tonnes a year, with 27% derived from HCFC-123 and
the remainder from HCFC-124, HFC-134a, HFC-227ea, and the anaesthetic
gases halothane and isoflurane (Boutonnet et al., 1999). In 2020, the
maximum deposition from fluorocarbons is predicted to reach 160 000
tonnes a year, yielding a maximum average concentration of
trifluoroacetic acid in rainwater of 0.1 µg/litre, which is several
orders of magnitude lower than the no-effect level in both surface and
soil water. By that year, HCFC-123 emissions will have declined, as
the chemical will have been phased out in accordance with the Montreal
Protocol. As such, it can be concluded that environmental levels of
trifluoroacetic acid resulting from the breakdown of HCFC-123 do not
pose a threat to the environment.
12. PREVIOUS EVALUATIONS BY INTERNATIONAL BODIES
A previous evaluation of HCFC-123 has been carried out by the
International Programme on Chemical Safety (IPCS, 1992).
Information on international hazard classification and labelling
is included in the International Chemical Safety Card reproduced in
this document (Appendix 4).
REFERENCES
AIHA (1998) Workplace environmental exposure level guide series:
1,1,1-Trifluoro-2,2-dichloroethane. Fairfax, VA, American Industrial
Hygiene Association Press.
Ashby J, Brady A, Elcombe CR, Elliott BM, Ishmael J, Odum J, Tugwood
JD, Kettle S, Purchase IF (1994) Mechanistically-based human hazard
assessment of peroxisome proliferator-induced hepatocarcinogenesis.
Human and experimental toxicology, 13 (Suppl. 2):S1-S117.
Berends AG, de Rooij CG, Shin-ya S, Thompson RS (1999) Biodegradation
and ecotoxicity of HFCs and HCFCs. Archives of environmental
contamination and toxicology, 36(2):146-151.
Boutonnet JC, Bingham P, Calamari D, de Rooij C, Franklin J, Kawano T,
Libre JM, McCulloch A, Malinverno G, Odom JM, Rusch GM, Smythe K,
Sobolev I, Thompson R, Tiedje JM (1999) Environmental risk assessment
of trifluoroacetic acid. Human and ecological risk assessment,
5(1):59-124.
Brewer WE, Smith S (1977) Teratogenic study via inhalation with
Genetron 123 in albino rats. Morristown, NJ, Allied Chemical
Corporation.
Britelli MR (1975) Eye irritation test in rabbits. Newark, DE, Du
Pont de Nemours and Co.
Brock WJ (1988a) Acute dermal toxicity study of HCFC-123 in rabbits.
Newark, DE, Du Pont de Nemours and Co.
Brock WJ (1988b) Acute dermal toxicity study of HCFC-123 in rats.
Newark, DE, Du Pont de Nemours and Co.
Brock WJ (1988c) Primary dermal irritation study with HCFC-123 in
rabbits. Newark, DE, Du Pont de Nemours and Co.
Buschman J (1996) Crossover study with HCFC 123 in lactating
Sprague-Dawley rats including additional studies on milk production
and metabolites in offspring urine. Hannover, Fraunhofer Institute
for Toxicology and Aerosol Research.
Callander RD (1989) HCFC-123 -- an evaluation using the Salmonella
mutagenicity assay. Macclesfield, Cheshire, Imperial Chemical
Industries Ltd.
Chang W-K, Criddle CS (1995) Biotransformation of HCFC-22, HCFC-142b,
HCFC-123 and HFC-134a by methanotrophic mixed culture MM1.
Biodegradation, 6(1):1-9.
Chevalier S, Roberts RA (1998) Perturbation of rodent hepatocyte
growth control by nongenotoxic hepatocarcinogens -- mechanisms and
lack of relevance for human health. Oncology reports, 5:1319-1327.
Clayton JW (1966) Acute inhalation toxicity. Newark, DE, Du Pont de
Nemours and Co.
Clegg ED, Cook JC, Chapin RE, Foster PMD, Daston GR (1997) Leydig cell
hyperplasia and adenoma formation: Mechanisms and relevance to humans.
Reproductive toxicology, 11:107-121.
Coate WB (1976) LC50 of G123 in rats. Vienna, VI, Hazleton
Laboratories America Inc.
Cohen SD, Pumford NR, Khairallah EA, Boekelheide K, Pohl LR, Amouzadeh
HR, Hinson JA (1997) Selective protein covalent binding and target
organ toxicity. Toxicology and applied pharmacology, 143:1-12.
Coombs DW (1994) HCFC-123 -- 13-week inhalation neurotoxicity study
in the rat. Cambridgeshire, Huntingdon Research Centre Ltd.
Culik R, Kelly DP (1976) Embryotoxic and teratogenic studies in rats
with inhaled dichlorofluoromethane (Freon 21) and
2,2-dichloro-1,1,1-trifluoroethane (FC-123). Newark, DE, Du Pont de
Nemours and Co.
Dance CA (1991) In vitro assessment of the clastogenic activity of
HCFC-123 in cultured human lymphocytes. Eye, Suffolk, Life Science
Research Ltd. (No. 91/PFE003/0093).
Darr RW (1981) An acute inhalation toxicity study of
Fluorocarbon 123 in the Chinese hamster. Morristown, NJ, Allied
Corporation.
Dekant W (1993) Metabolism of 1,1-dichloro-2,2,2-trifluoroethane
(HCFC-123). Würzburg, Institute of Toxicology, University of
Würzburg (Report No. MA-250B-82-207).
Dodd DE, Brashear WT, Vinegar A (1993) Metabolism and pharmacokinetics
of selected halon replacement candidates. Toxicology letters,
68:37-47.
Doleba-Crowe C (1978) 90-day inhalation exposure of rats and dogs to
vapours of 2,2-dichloro-1,1,1-trifluoroethane (FC-123) (summary).
Newark, DE, Du Pont de Nemours and Co. [cited in NICNAS, 1996].
Du Pont (1993) Workplace guidelines for Suva(R) Centri-LP
(HCFC-123) in refrigeration and air conditioning applications.
Wilmington, DE, Du Pont de Nemours and Co.
Edwards CN (1991) HCFC-123 (vapour phase): In vitro assessment of
the clastogenic activity in cultured human lymphocytes. Eye,
Suffolk, Life Science Research Ltd. (No. 91/PFE002/0125).
Elmore LW, Sirica AE (1993) "Intestinal-type" of adenocarcinoma
preferentially induced in right/caudate liver lobes of rats treated
with furan. Cancer research, 53:254-259.
Frank H, Klein A, Renschen D (1996) Environmental trifluoroacetate.
Nature, 382:34.
Fraser P (1994) CSIRO report to SPA-AFEAS. Canberra, Commonwealth
Scientific and Industrial Research Organisation.
Goodman NC (1975) Primary skin irritation and sensitization tests on
guinea pigs. Newark, DE, Du Pont de Nemours and Co.
Hall GT, Moore BL (1975) Acute inhalation toxicity on Freon 123.
Newark, DE, Du Pont de Nemours and Co.
Harris JW, Jones JP, Martin JL, LaRosa AC, Olson MJ, Pohl LR, Anders
MW (1992) Pentahaloethane-based chlorofluorocarbon substitutes and
halothane: correlation of in vivo hepatic protein
trifluoroacetylation and urinary trifluoroacetic acid excretion with
calculated enthalpies of activation. Chemical research
in toxicology, 5:720-725.
Hayman GD, Jenkin ME, Murrells TP, Johnson CE (1994) Tropospheric
degradation chemistry of HCFC-123 (CF3CHCl2): a proposed
replacement chlorofluorocarbon. Atmospheric environment, 28:421-437.
Henry JE (1975) Acute oral test on FC-123. Newark, DE, Du Pont de
Nemours and Co. [cited in NICNAS, 1996].
Hoet P, Graf MLM, Bourdi M, Pohl LR, Duray PH, Chen W, Peter RM,
Nelson SD, Verlinden N, Lison D (1997) Epidemic of liver disease
caused by hydrochlorofluorocarbons used as ozone-sparing substitutes
of chlorofluorocarbons. Lancet, 350:556-559.
Hofmann T (1995) HCFC 123, HCFC 141B and HCF 134a, testing for
subacute (2 weeks) inhalation toxicity in male and female Sprague
Dawley rats. Frankfurt am Main, Hoechst Aktiengesellschaft.
Hughes EW (1994) HCFC 123: A study of the effect on reproductive
function of two generations in the rat. Huntingdon, Cambridgeshire,
Huntingdon Research Centre Ltd.
Huwyler J, Gut J (1992) Exposure to the chlorofluorocarbon substitute
2,2-dichloro-1,1,1-trifluoroethane and the anaesthetic agent halothane
is associated with transient adduct formation in the heart.
Biochemical and biophysical research communications, 184:1344-1349.
Huwyler J, Aeschlimann D, Christen U, Gut J (1992) The kidney as a
novel target tissue for protein adduct formation associated with
metabolism of halothane and the candidate chlorofluorocarbon
replacement 2,2-dichloro-1,1,1-trifluoroethane. European journal of
biochemistry, 207:229-238.
IARC (1995) Peroxisome proliferation and its role in carcinogenesis:
views and expert opinions of an IARC working group, Lyon, 7-11
December 1994. Lyon, International Agency for Research on Cancer.
ICI (1992) An evaluation in the in vitro cytogenetic assay using
human lymphocytes. Macclesfield, Cheshire, Imperial Chemical
Industries Ltd. [cited in AIHA, 1998].
Industrial Bio-Test Laboratories (1977) 90-day subacute inhalation
toxicity study with Genetron 123 in albino rats. Northbrook, IL,
Industrial Bio-Test Laboratories, Inc.
IPCS (1990) Fully halogenated chlorofluorocarbons. Geneva, World
Health Organization, International Programme on Chemical Safety
(Environmental Health Criteria 113).
IPCS (1991) Partially halogenated chlorofluorocarbons (methane
derivatives). Geneva, World Health Organization, International
Programme on Chemical Safety (Environmental Health Criteria 126).
IPCS (1992) Partially halogenated chlorofluorocarbons (ethane
derivatives). Geneva, World Health Organization, International
Programme on Chemical Safety (Environmental Health Criteria 139).
IPCS (1998) International Chemical Safety Card
-- 2,2-Dichloro-1,1,1-trifluoroethane. Geneva, World Health
Organization, International Programme on Chemical Safety (ICSC 1343).
IPCS (1994) Assessing human health risks of chemicals: Derivation of
guidance values for health-based exposure limits. Geneva, World
Health Organization, International Programme on Chemical Safety
(Environmental Health Criteria 170).
Jamison KC, Larson JL, Butterworth BE, Harden R, Skinner BL, Wolf DC
(1996) A non-bile duct origin for intestinal crypt-like ducts with
peritubular fibrosis induced in livers of F344 rats by chloroform
inhalation. Carcinogenesis, 17:675-682.
Jenkins CA (1992a) HCFC-123 (liquid): Biotic degradation closed
bottle test. Eye, Suffolk, Life Sciences Research Ltd. (No.
91/PFE008/0477).
Jenkins CA (1992b) HCFC-123: Acute toxicity to rainbow trout. Eye,
Suffolk, Life Sciences Research Ltd. (No. 91/PFE004/0939).
Jenkins CA (1992c) HCFC-123: Acute toxicity to Daphnia magna. Eye,
Suffolk, Life Sciences Research Ltd. (No. 91/PFE006/0972).
Jenkins CA (1992d) HCFC-123: Determination of its EC50 to
Selenastrum capricornutum. Eye, Suffolk, Life Sciences Research Ltd
(No. 91/PFE007/0935).
Keller DA, Lieder PH, Brock WJ, Cook JC (1998)
1,1,1-Trifluoro-2,2-dichloroethane (HCFC-123) and
1,1,1-trifluoro-2-bromo-2-chloroethane (halothane) cause similar
biochemical effects in rats exposed by inhalation for five days.
Drug and chemical toxicology, 21:405-415.
Kelly DP (1989) Four-week inhalation toxicity study with HCFC-123
in rats. Newark, DE, Du Pont de Nemours and Co.
Kennelly JC (1993) HCFC 123: Assessment for the introduction of
unscheduled DNA synthesis in rat liver after inhalation exposure.
Macclesfield, Cheshire, Zeneca Ltd.
Kotamarthi VR, Rodriguez JM, Ko MKW, Tromp TK, Sze ND, Prather MJ
(1998) Trifluoroacetic acid from degradation of HCFCs and HFCs -- a
three-dimensional modelling study. Journal of geophysical research,
103:5747-5758.
Le Marchand L, Wilkinson GR, Wilkens LR (1999) Genetic and dietary
predictors of CYP2E1 activity: a phenotyping study in Hawaii Japanese
using chlorzoxazone. Cancer epidemiology, biomarkers and prevention,
8:495-500.
Lewis RW (1990) 28-day inhalation study to assess changes in rat
liver and plasma. Macclesfield, Cheshire, Imperial Chemical
Industries Ltd.
Lind RC, Gandolfi AJ, Hall PM (1995) Biotransformation and
hepatotoxicity of HCFC-123 in the guinea pig: Potentiation of hepatic
injury by prior glutathione depletion. Toxicology and applied
pharmacology, 134:175-181.
Loizou GD, Urban G, Dekant W, Anders MW (1994) Gas-uptake
pharmacokinetics of 2,2-dichloro-1,1,1-trifluoroethane (HCFC-123).
Drug metabolism and disposition, 22:511-517.
Longstaff E, Robinson M, Bradbrook C, Styles JA, Purchase IF (1984)
Genotoxicity and carcinogenicity of fluorocarbons: Assessment by
short-term in vitro tests and chronic exposure in rats. Toxicology
and applied pharmacology, 72:15-31.
Malinverno G, Rusch GM, Millischer RJ, Hughes EW, Schroeder RE, Coombs
DW (1996) Inhalation teratology and reproduction studies with
1,1-dichloro-2,2,2-trifluoroethane (HCFC-123). Fundamental and
applied toxicology, 23:276-287.
Malley LA (1990) Subchronic inhalation toxicity: 90-day study with
HCFC-123 in rats. Newark, DE, Du Pont de Nemours and Co.
Malley LA (1992) Combined chronic toxicity/oncogenicity study with
HCFC-123: Two-year inhalation toxicity study in rats. Newark, DE, Du
Pont de Nemours and Co.
Malley LA, Carakostas M, Hansen JF, Rusch GM, Kelly DP, Trochimowicz
HJ (1995) Two-year inhalation toxicity study in rats with
hydrochlorofluorocarbon 123. Fundamental and applied toxicology,
25:101-114.
Malley LA, Carakostas M, Elliott GS, Alvarez L, Schroeder RE, Frame
SR, Van Pelt C, Trochimowicz HJ, Rusch GM (1996) Subchronic toxicity
and teratogenicity of 2-chloro-1,1,1,2-tetrafluoroethane (HCFC-124).
Fundamental and applied toxicology, 32:11-22.
Marit GB, Dodd DE, George ME, Vinegar A (1994) Hepatotoxicity in
guinea pigs following acute exposure to
1,1-dichloro-2,2,2-trifluoroethane. Toxicologic pathology,
22(4):404-414.
Marshal RR (1992) Evaluation of chromosome aberration frequencies in
cultured peripheral blood lymphocytes from rats treated with
HCFC-123. Harrogate, Yorkshire, Hazleton Microtest.
MRI (1991) Results of employee exposure monitoring for HCFC-123 at
centrifugal chiller installations. Final report submitted to the US
Environmental Protection Agency. Silver Spring, MD, Meridian Research,
Inc.
MRI (1993a) Assessment of firefighter exposure to HCFC-123 during
extinguishant efficiency tests conducted at the United States Naval
Air Station in Beaufort, South Carolina. Draft report submitted to
the US Environmental Protection Agency. Silver Spring, MD, Meridian
Research, Inc.
MRI (1993b) Assessment of firefighter exposure to HCFC-123 during
fire extinguisher use in aircraft hangars. Draft report submitted to
American Pacific Corporation. Silver Spring, MD, Meridian Research,
Inc.
Muller W, Hofmann T (1988) HCFC-123 -- micronucleus test in male and
female NMRI mice after inhalation. Frankfurt am Main, Hoechst
Aktiengesellschaft.
Mullin LS (1976) Behavioural toxicity testing of Fluorocarbon 123
in rats. Newark, DE, Du Pont de Nemours and Co.
NAS (1996) Toxicity of alternatives to chlorofluorocarbons: HFC-134a
and HCFC-123. Washington, DC, National Academy of Sciences, National
Academy Press.
NICNAS (1996) Priority Existing Chemical No. 4 --
2,2-Dichloro-1,1,1-trifluoroethane (HCFC-123), full public report,
National Industrial Chemicals Notification and Assessment Scheme.
Canberra, Australian Government Publishing Service.
NICNAS (1999) 2,2-Dichloro-1,1,1-trifluoroethane (HCFC-123):
Secondary Notification No. 4S, full public report. National
Industrial Chemicals Notification and Assessment Scheme. Sydney,
National Occupational Health and Safety Commission.
OECD (1998) Agreed harmonized integrated hazard classification
system for human health and environmental effects of chemical
substances. Paris, Organisation for Economic Co-operation and
Development, Environmental Health and Safety Division.
Oremland RS, Lonergan DJ, Culbertson CW, Lovley DR (1996) Microbial
degradation of hydrofluorocarbons (CHCl2F and CHCl2CF3) in soils
and sediments. Applied and environmental microbiology,
62(5):1818-1821.
Pierson K (1990a) Flow-through acute 96 hour LC50 of HCFC-123 in
fathead minnows (Pimephales promelas ). Newark, DE, Du Pont de
Nemours and Co.
Pierson K (1990b) Static acute 48 hour LC50 of HCFC-123 in Daphnia
magna . Newark, DE, Du Pont de Nemours and Co.
Rusch GM, Trochimowitz HJ, Malley LJ, Kelly DP, Peckham J, Hansen J,
Charm JB (1994) Subchronic inhalation toxicity studies with
hydrochlorofluorocarbon 123 (HCFC 123). Fundamental and applied
toxicology, 23:169-178.
Sandow J, Jerabek-Sandow G, Fenner-Nau D (1995a) Effect of
fluorocarbons on pituitary-gonadal function in a 14-day inhalation
toxicity study: HCFC 123 (Frigen), HCFC 141b (difluorchlorethane)
and HFC 134a (tetrafluorethane). Frankfurt am Main, Hoechst
Aktiengesellschaft.
Sandow J, Rechenberg W, Jerabek-Sandow G (1995b) Effect of HCFC-123
on androgen biosynthesis and gonadotropin secretion in rats.
Frankfurt am Main, Hoechst Aktiengesellschaft.
Sibley H (1992) A study for determining refrigerant exposure levels
while servicing an HCFC-123 centrifugal chiller. Syracuse, NY,
Carrier Corporation.
Slauter RW (1997) HCFC 123: Inhalation study in pregnant monkeys to
assess milk transfer and composition following postpartum exposure.
Mattawan, MI, MPI Research.
Takebayashi T, Kabe I, Endo Y, Tanaka S, Miyauchi H, Nozi K, Takahashi
K, Omae K (1998a) Acute liver dysfunction among workers exposed to
2,2-dichloro-1,1,1-trifluoroethane (HCFC-123): A case report.
Journal of occupational health, 40:169-170.
Takebayashi T, Kabe I, Endo Y, Tanaka S, Miyauchi H, Nozi K, Imamiya
S, Takahashi K, Omae K (1998b) Exposure to
2,2-dichloro-1,1,1-trifluoroethane (HCFC-123): A causal inference.
Journal of occupational health, 40:334-338.
Tanaka S, Kabe I, Takebayashi T, Endo Y, Miyauchi H, Nozi K, Takahashi
K, Seki Y, Omae K (1998) Environmental and biological monitoring of
2,2-dichloro-1,1,1-trifluoroethane (HCFC-123). Journal of
occupational health, 40:348-349.
Trane Company (1991) Report on testing and analysis of the
concentration of HCFC-123 in field installations with general
machinery rooms containing hermetic centrifugal chillers. La Crosse,
WI, The Trane Company.
Trane Company (1992) Report of worker exposure to HCFC-123 during
servicing of hermetic centrifugal chillers. La Crosse, WI, The Trane
Company.
Trochimowicz HJ, Mullin LS (1973) Cardiac sensitization potential
(EC50) of trifluoro-dichloroethane. Newark, DE, Du Pont de Nemours
and Co.
UNEP (1999) Ozone treaties. <http://www.unep.org/ozone/
treaties.htm>. United Nations Environment Programme.
Urban G, Dekant W (1994) Metabolism of
1,1-dichloro-2,2,2-trifluoroethane in rats. Xenobiotica, 24:881-892.
Urban G, Speerschneider P, Dekant W (1994) Metabolism of the
chlorofluorocarbon substitute 1,1-dichloro-2,2,2-trifluoroethane by
rat and human liver microsomes: the role of cytochrome P450 2E1.
Chemical research in toxicology, 7:170-176.
Vinegar A, Williams RJ, Fisher JW, McDougal JN (1994) Dose-dependent
metabolism of 2,2-dichloro-1,1,1-trifluoroethane: A physiologically
based pharmacokinetic model in the male Fischer 344 rat. Toxicology
and applied pharmacology, 129:103-113.
Wang Y, Olson MJ, Baker MT (1993) Interaction of fluoroethane
chlorofluorocarbon (CFC) substitutes with microsomal cytochrome P450.
Biochemical pharmacology, 46:87-94.
Warheit DB (1993) Mechanistic studies with HCFC-123. Newark, DE, Du
Pont de Nemours and Co.
Williams RJ, Vinegar A, McDougal JN, Jarabek AM, Fisher JW (1996) Rat
to human extrapolation of HCFC-123 kinetics deduced from halothane
kinetics -- a corollary approach to physiologically based
pharmacokinetic modeling. Fundamental and applied toxicology,
30:55-66.
WMO (1995) Scientific assessment of ozone depletion. Geneva, World
Meteorological Organization (Global Ozone Research and Monitoring
Project Report No. 37).
Wujcik CE, Zehavi D, Seiber JN (1998) Trifluoroacetic acid levels in
1994-1996 fog, rain, snow and surface waters from California and
Nevada. Chemosphere, 36:1233-1245.
APPENDIX 1 -- SOURCE DOCUMENTS
NICNAS (1996): Priority Existing Chemical No. 4 --
2,2-Dichloro-1,1,1-trifluoroethane (HCFC-123), full public report,
National Industrial Chemicals Notification and Assessment Scheme
Copies of the NICNAS (1996) report on HCFC-123 (prepared by S.
Batt, L. Onyon, L. Slosu, and D. Willcocks) may be obtained from:
NICNAS
Existing Chemicals
GPO Box 58
Sydney NSW 2001
Australia
NICNAS reports are prepared to meet the requirements of the
Industrial Chemicals Notification and Assessment Act, 1989, as
amended. In the preparation of the assessment report, both internal
and external peer reviews are undertaken. Under the NICNAS
legislation, applicants for the assessment of a chemical (i.e.,
importers and manufacturers of a chemical) may apply for variations to
the draft report. The following companies and industry associations
participated in the review of the assessment at this stage:
Association of Fluorocarbon Consumers and Manufacturers, Elf Atochem
(Australia) Pty Ltd, Lovelock Luke Pty Ltd, and North American Fire
Guardian Technology (Australia) Pty Ltd. The report was also open for
public comment.
NICNAS (1999): 2,2-Dichloro-1,1,1-trifluoroethane (HCFC-123):
Secondary Notification No. 4S, full public report. National
Industrial Chemicals Notification and Assessment Scheme
Copies of the NICNAS (1999) report on HCFC-123 (prepared by S.
Batt, S. Kristensen, and C. Lee-Steere) may be obtained from:
NICNAS
Existing Chemicals
GPO Box 58
Sydney NSW 2001
Australia
NICNAS reports are prepared to meet the requirements of the
Industrial Chemicals Notification and Assessment Act, 1989, as
amended. In the preparation of the assessment report, both internal
and external peer reviews are undertaken. Under the NICNAS
legislation, applicants for the reassessment of a chemical (i.e.,
importers and manufacturers of a chemical) may apply for variations to
the draft report. The following companies participated in the review
of the assessment at this stage: Du Pont (Australia) Pty Ltd, Elf
Atochem (Australia) Pty Ltd, GSA Industries (Australia) Pty Ltd, MSA
(Australia) Pty Ltd, North American Fire Guardian Technology
(Australia) Pty Ltd, and Solvents Australia Pty Ltd. The report was
also open for public comment.
APPENDIX 2 -- CICAD PEER REVIEW
The draft CICAD on HCFC-123 was sent for review to institutions
and organizations identified by IPCS after contact with IPCS National
Contact Points and Participating Institutions, as well as to
identified experts. Comments were received from:
Alexandria University, Faculty of Agriculture, Department of
Pesticide Chemistry, Egypt
AlliedSignal, Department of Toxicology and Risk Assessment,
Health, Safety, Environment and Remediation, USA
Department of Health, Protection of Health Division,
United Kingdom
DuPont Fluoroproducts, Haskell Laboratory for Toxicology and
Industrial Medicine, USA
Federal Institute for Health Protection of Consumers and
Veterinary Medicine, Germany
Glaxo Wellcome Research and Development, Medicines Safety
Evaluation Division, United Kingdom
Health and Safety Executive, United Kingdom
Institut de Recherche en Santé et en Sécurité du Travail du
Québec, Canada
Institute of Terrestrial Ecology, United Kingdom
National Chemicals Inspectorate (KEMI), Sweden
National Institute for Occupational Safety and Health, USA
National Institute of Environmental Health Sciences, National
Institutes of Health, USA
National Institute of Public Health, Centre of Industrial Hygiene
and Occupational Diseases, Czech Republic
Université Catholique de Louvain, Faculté de Médecine, Belgique
US Environmental Protection Agency, Drinking Water Program,
Region VIII, USA
World Health Organization, International Programme on Chemical
Safety, Switzerland
APPENDIX 3 -- CICAD FINAL REVIEW BOARD
Sydney, Australia, 21-24 November 1999
Members
Dr R. Benson, Drinking Water Program, US Environmental Protection
Agency, Region VIII, Denver, CO, USA
Dr T. Berzins, National Chemicals Inspectorate (KEMI), Solna, Sweden
Dr R.M. Bruce, National Center for Environmental Assessment, US
Environmental Protection Agency, Cincinnati, OH, USA
Mr R. Cary, Health and Safety Executive, Merseyside, United Kingdom
Dr R.S. Chhabra, National Institute of Environmental Health Sciences,
National Institutes of Health, Research Triangle Park, NC, USA
Dr S. Chou, Agency for Toxic Substances and Disease Registry, Atlanta,
GA, USA
Dr S. Dobson, Institute of Terrestrial Ecology, Monks Wood,
Cambridgeshire, United Kingdom
Dr H. Gibb, National Center for Environmental Assessment, US
Environmental Protection Agency, Washington, DC, USA
Dr R.F. Hertel, Federal Institute for Health Protection of Consumers
and Veterinary Medicine, Berlin, Germany
Dr J. Kielhorn, Fraunhofer Institute for Toxicology and Aerosol
Research, Hannover, Germany
Dr S. Kristensen, National Occupational Health and Safety Commission
(Worksafe), Sydney, NSW, Australia
Mr C. Lee-Steere, Environment Australia, Canberra, ACT, Australia
Ms M. Meek, Environmental Health Directorate, Health Canada, Ottawa,
Ontario, Canada
Ms F. Rice, National Institute for Occupational Safety and Health,
Cincinnati, OH, USA
Dr J. Sekizawa, National Institute of Health Sciences, Tokyo, Japan
Dr D. Willcocks, National Industrial Chemicals Notification and
Assessment Scheme (NICNAS), Sydney, NSW, Australia (Chairperson)
Professor P. Yao, Institute of Occupational Medicine, Chinese Academy
of Preventive Medicine, Beijing, People's Republic of China
Observers
Mr P. Howe, Institute of Terrestrial Ecology, Huntingdon,
Cambridgeshire, United Kingdom
Dr K. Ziegler-Skylakakis, GSF-Forschungszentrum für Umwelt und
Gesundheit, GmbH, Oberschleissheim, Germany
Secretariat
Dr A. Aitio, International Programme on Chemical Safety, World Health
Organization, Geneva, Switzerland
Ms M. Godden, Health and Safety Executive, Bootle, Merseyside, United
Kingdom
Dr M. Younes, International Programme on Chemical Safety, World Health
Organization, Geneva, Switzerland
APPENDIX 4 -- INTERNATIONAL CHEMICAL SAFETY CARD
2,2-DICHLORO-1,1,1-TRIFLUOROETHANE ICSC: 1343
November 1998
CAS#
6. ENVIRONMENTAL LEVELS AND HUMAN EXPOSURE
6.1 Environmental levels
HCFC-123 was detected in non-urban ambient air in Australia at
levels less than 0.01 ppt (62.5 pg/m3) (Fraser, 1994). Assuming that
worldwide emissions will increase to 45 000 tonnes in 2010, the global
average concentration of airborne HCFC-123 is projected to reach 1.1
ppt (7 ng/m3) in that year, with levels being 2-4 times higher than
the average near major sources of emissions in eastern North America
and central Europe (Kotamarthi et al., 1998).
Information on levels of HCFC-123 in water, wildlife or food was
not available.
6.2 Human exposure
HCFC-123 is not used in consumer products. Indirect exposure via
the environment would be low, as the concentration in ambient air is
less than 0.01 ppt (62.5 pg/m3) and HCFC-123 is unlikely to persist
in other media because of its limited solubility and high volatility.
Therefore, exposure of the general population to HCFC-123 is expected
to be minimal.
There is potential for occupational exposure, predominantly via
inhalation, during the manufacture of HCFC-123 and the manufacture and
use of products containing HCFC-123, such as the operation and
maintenance of air-conditioning installations running on HCFC-123, the
discharge of HCFC-123 from fire protection systems, and the use of
liquid HCFC-123 in metal and electronics cleaning.
In an HCFC-123 manufacturing plant in Canada, two operators
monitored over 165-480 min on 4 separate days had time-weighted
breathing-zone levels of HCFC-123 ranging from 1.16 to 8.94 ppm (7.25
to 55.9 mg/m3). On one occasion, a drum-filling lance failure
resulted in a level in excess of 33 ppm (206 mg/m3) (Du Pont,
personal communication, 1999). No monitoring data were available for
the manufacture of products containing HCFC-123.
Several studies have measured HCFC-123 levels in chiller
machinery rooms during normal operations as well as maintenance and
repair activities. In 4 of 12 unmanned machinery rooms containing
air-conditioning equipment running on HCFC-123, air levels were below
1 ppm (6.25 mg/m3) (4-h time-weighted average) at three sites (Trane
Company, 1991). At one site, where samples were taken near a leak and
a half-empty HCFC-123 drum, levels of 5.9-13.6 ppm (36.9-85.0 mg/m3)
(20-min time-weighted average) were recorded. At the rest of the
sites, machinery room air levels were below the limit of detection
(0.2-0.4 ppm [1.25-2.50 mg/m3]). Breathing-zone levels of HCFC-123
during routine chiller maintenance operations, including refrigerant
transfer, were measured at nine US installations. Two-hour to 12-h
time-weighted average concentrations were less than 1 ppm (6.25
mg/m3) in five cases, less than 2 ppm (12.5 mg/m3) in three cases,
and in the range of 2-5 ppm (12.5-31.3 mg/m3) in one case (MRI,
1991; Sibley, 1992; Trane Company, 1992). In Australia, 4- to 6-h
time-weighted average concentrations were less than 1 ppm (6.25
mg/m3) during repair work at a single installation (NICNAS, 1996).
In these studies, continuous area monitoring showed time-weighted
average air concentrations below 1 ppm (6.25 mg/m3), with
activity-related instantaneous peaks ranging from 30 to 500 ppm (188
to 3130 mg/m3).
Air levels resulting from the use of a fire extinguishant
containing 93% HCFC-123 were measured during fire control exercises in
which the firefighters wore a self-contained breathing apparatus (MRI,
1993a,b). Outdoor discharge resulted in maximum breathing-zone levels
ranging from 7 to 870 ppm (43.8 to 5440 mg/m3), depending on the
type of fire hazard. Inside an aircraft hangar, the discharge of
hand-held extinguishers resulted in a breathing-zone concentration of
20 ppm (125 mg/m3) during discharge, with average static air levels
ranging from 29 to 141 ppm (181 to 881 mg/m3) over the next 30 min.
With a large semi-portable fire extinguisher, breathing-zone
concentrations during discharge reached 180-300 ppm (1130-1880
mg/m3), whereas average static air levels ranged from 165 to 557 ppm
(1030 to 3480 mg/m3) over the next 30 min.
In a US factory converted to using HCFC-123 in its degreaser,
personal air monitoring during normal degreaser operations showed
5.5-h time-weighted average levels of HCFC-123 that ranged from 5.3 to
12.0 ppm (33.1 to 75.0 mg/m3) throughout the facility.1 Charging
and unloading the degreaser resulted in short-term breathing-zone
levels ranging from 160 to 460 ppm (1000 to 2880 mg/m3).
1 AlliedSignal Inc., personal communication, 1998 [cited in
NICNAS, 1999].
7. COMPARATIVE KINETICS AND METABOLISM IN LABORATORY ANIMALS
AND HUMANS
HCFC-123 is readily absorbed by inhalation and distributed
throughout the body, where it reaches levels in body fat up to 25
times higher than those in blood (Vinegar et al., 1994). Following
exposure to an initial concentration of 2000 ppm (12.5 g/m3)
radiolabelled HCFC-123 over two consecutive 3-h periods in a
closed-chamber system, total uptake was 50-60% in rats and 90-100% in
guinea-pigs (Urban & Dekant, 1994). In rats exposed to initial
concentrations of 120-11 000 ppm (0.75-68.8 g/m3) HCFC-123 for 6 h,
the inhalation uptake showed a rapid distribution phase lasting
30-45 min followed by a slow linear uptake phase (Vinegar et al.,
1994). Blood levels declined rapidly once exposure was terminated. In
rats exposed to 1000 ppm (6.25 g/m3), blood concentrations fell
from 15.0 mg/litre at the end of a 4-h exposure period to 4.5
mg/litre at 4 min post-exposure and 1.5 mg/litre at 1 h post-exposure
(Vinegar et al., 1994). After the initial decline, HCFC-123
concentrations in both blood and fat decreased log-linearly with
a half-life of approximately 80 min, indicating fat as the main
depository for unmetabolized HCFC-123. Experimentally determined
tissue to air partition coefficients were 2-3 for gut and muscle,
3-4 for blood, 3-5 for liver, and 60-70 for fat (Dekant, 1993;
Vinegar et al., 1994). Data on oral or dermal absorption were not
available.
In the rat, 26-32% of the uptake of HCFC-123 is metabolized,
predominantly by oxidation to trifluoroacetyl chloride, which is
hydrolysed to trifluoroacetic acid or reacts with lysine residues in
proteins or with low-molecular-weight amines to form
N-trifluoroacetyl amides (Harris et al., 1992; Dodd et al., 1993;
Urban & Dekant, 1994). Minor, reductive pathways lead to the formation
of very small amounts of 2-chloro-1,1,1-trifluoroethane,
2-chloro-1,1-difluoroethene, and 2,2-dichloro-1,1-difluoroethene. The
latter reacts with glutathione to form
N-acetyl- S-(2,2-dichloro-1,1-difluoroethyl)-L-cysteine. The
structures of these metabolites are shown in Figure 1. Both oxidation
and reduction of HCFC-123 are catalysed by cytochrome P450 2E1
(CYP2E1). In human liver microsomes, the major biotransformation
product is trifluoroacetic acid (Urban et al., 1994). Its rate of
formation was directly related to the amount of CYP2E1 present and
1.5-16 times faster than the rate in rat microsomes.
In experimental animals, the major metabolite in blood, urine,
and milk is trifluoroacetic acid. In rats exposed by inhalation to
1000 ppm (6.25 g/m3) HCFC-123 for 4 h, blood levels of parent
compound and trifluoroacetic acid amounted to 15.0 and 93.1 mg/litre,
respectively (Vinegar et al., 1994). At 10 000 ppm (62.5 g/m3), the
corresponding concentrations were 93.5 and 37.8 mg/litre,
respectively. Trifluoroacetic acid blood levels rebounded and peaked
12-26 h post-exposure, indicating that the metabolism of HCFC-123
is subject to substrate inhibition at exposures above 1000 ppm (6.25
g/m3). For exposure levels below 2000 ppm (12.5 g/m3), the
metabolic rate constants developed for HCFC-123 were Km = 1.2
mg/litre and Vmax = 7.20 mg/kg body weight per hour for male rats
and Km = 1.2 mg/litre and Vmax = 7.97 mg/kg body weight per
hour for female rats (Loizou et al., 1994). Generally, HCFC-123 was
not detected in blood samples collected within 1 h post-exposure from
lactating rhesus monkeys exposed by inhalation to 1000 ppm (6.25
g/m3) for 6 h per day, whereas trifluoroacetic acid concentrations
reached 150-190 µg/ml after 2-3 weeks of exposure. Based on data from
a single monkey, the half-life of trifluoroacetic acid in blood was
approximately 24 h (Slauter, 1997). In rats and guinea-pigs exposed to
14C-labelled HCFC-123 vapours for 6 h and sacrificed 48 h
post-exposure, only low amounts of radioactivity remained in the
organs examined (Urban & Dekant, 1994). The liver contained most of
the radiolabel, followed by testes and kidneys, lungs, brain,
pancreas, and spleen. Covalent binding of labelled material was
highest in liver tissue (0.4-0.7 nmol/mg protein), followed by lungs,
kidneys, and plasma (0.1-0.3 nmol/mg protein). Trifluoroacetylated
tissue proteins have been detected by immunological techniques in the
liver and at 20- to 200-fold lower levels in the kidney and heart of
rats 6-12 h after exposure to HCFC-123 by inhalation or
intraperitoneal injection (Harris et al., 1992; Huwyler & Gut, 1992;
Huwyler et al., 1992).
The available data indicate that the predominant routes of
HCFC-123 elimination are exhalation of the parent compound and urinary
excretion of trifluoroacetic acid. In rats exposed to an initial
concentration of 2000 ppm (12.5 g/m3) radiolabelled HCFC-123 for two
consecutive 3-h periods and sacrificed 48 h post-exposure, 23-28% of
the radioactive uptake was eliminated in the urine, predominantly as
trifluoroacetic acid, whereas 3-4% was recovered from the body (Urban
& Dekant, 1994). Small amounts of minor metabolites, such as
N-acetyl- S-(2,2-dichloro-1,1-difluoroethyl)-L-cysteine,
N-trifluoroacetyl-2-aminoethanol, and fluoride ion, were recovered
from urine, and trace amounts of 2-chloro-1,1,1-trifluoroethane were
detected in expired air (Urban & Dekant, 1994; Vinegar et al., 1994).
In lactating rats and monkeys exposed to 1000 ppm (6.25 g/m3)
HCFC-123 for 6 h per day for 3 weeks, trifluoroacetic acid was found
in milk at a maximum concentration of 65 and 30 µg/ml, respectively
(Buschman, 1996; Slauter, 1997). In monkeys, the milk also contained
small amounts (up to 5 µg/ml) of HCFC-123. Rat milk was not analysed
for HCFC-123, and neither monkey nor rat milk was analysed for
metabolites other than trifluoroacetic acid.
An analogue of HCFC-123, the common inhalation anaesthetic
halothane (2-bromo-2-chloro-1,1,1-trifluoroethane), is also
metabolized by hepatic CYP2E1 to trifluoroacetyl chloride, causing
trifluoroacetylation of liver proteins (Harris et al., 1992; Urban et
al., 1994). These include cytochrome P450 itself and other enzymes,
many of which have been identified as residing in the lumen of the
endoplasmic reticulum and involved in the maturation of newly
synthesized proteins (Cohen et al., 1997). Both halothane and HCFC-123
induce peroxisome proliferation and increased ß-oxidation in rat liver
cells (Keller et al., 1998). They are also highly effective in
inducing excess uncoupled cytochrome P450 activity in rabbit liver
microsomes, thus increasing hepatic oxygen consumption and
facilitating the oxidation of other cytochrome P450 substrates (Wang
et al., 1993).
Only limited information was available on the kinetics and
metabolism of HCFC-123 in humans in vivo. In four volunteers exposed
by inhalation to 60-73 ppm (375-460 mg/m3) HCFC-123 for 6 h, the
concentration of trifluoroacetic acid in the urine peaked at 10-27
mg/litre by 20-30 h and returned to zero by 96 h post-exposure,
indicating an elimination half-life of 25 h (Tanaka et al., 1998).
Physiologically based pharmacokinetic models for halothane in humans
and for halothane and HCFC-123 in rats have been used to deduce a
human model for HCFC-123 and its main metabolite, trifluoroacetic acid
(Williams et al., 1996). As the model has not been validated, its
usefulness as a predictive tool is unknown at this time.
8. EFFECTS ON LABORATORY MAMMALS AND IN VITRO TEST SYSTEMS
Unless otherwise indicated, only effects that were statistically
different ( P < 0.05) from controls have been considered. All
inhalation studies were performed by whole-body exposure, unless
otherwise mentioned.
8.1 Single exposure
When HCFC-123 was administered in corn oil by oral gavage to rats
at doses ranging from 2.25 to 11 g/kg body weight, rapid respiration
and prostration were recorded at and above 3.4 g/kg body weight. An
LD50 was not determined. The lowest dose causing mortality was 9
g/kg body weight (Henry, 1975).
In the rat and the hamster, inhalation of more than 30 000 ppm
(188 g/m3) caused severe CNS depression and death (Clayton, 1966;
Hall & Moore, 1975; Coate, 1976; Darr, 1981). The 4-h LC50 ranged
from 28 400 to 52 600 ppm (178-329 g/m3). Clinical signs of toxicity
included sedation, loss of muscle coordination and balance,
prostration, and dyspnoea. Gross pathological findings were either
negative or limited to congestion or discoloration of the lungs,
kidneys, liver, thymus, or small intestine. The lowest concentration
causing reversible CNS depression (failures in unconditioned reflexes)
in rats was 5000 ppm (31.3 g/m3) (Mullin, 1976). In a study of
cardiac sensitization to adrenaline, life-threatening or fatal
arrhythmias occurred in 0 of 3, 4 of 6, and 3 of 3 dogs exposed
nose-only to 10 000, 20 000, or 40 000 ppm (62.5, 125, or 250 g/m3).
The dogs were pretreated with intravenous adrenaline (8 µg/kg body
weight) prior to exposure and challenged with an identical adrenaline
dose after breathing the compound for 5 min. Based on these findings,
an EC50 (5 min) of 19 000 ppm (119 g/m3) and a NOAEL of 10 000 ppm
(62.5 g/m3) were determined (Trochimowicz & Mullin, 1973). Exposure
of guinea-pigs to 1000-30 000 ppm (6.25-188 g/m3) HCFC-123 for 4 h
produced non-fatal liver damage in all exposure groups (Marit et al.,
1994). At 48 h post-exposure, the liver effects included centrilobular
vacuolar (fatty) change, multifocal random and centrilobular
hepatocellular degeneration and necrosis, and increased levels of
plasma isocitrate dehydrogenase, alanine transaminase (ALT), and
aspartate transaminase (AST). In another study in guinea-pigs exposed
to 10 000 ppm (62.5 g/m3) for 4 h, liver injury was minimal unless
the animals had been glutathione depleted prior to exposure (Lind et
al., 1995).
In a rat and rabbit test for dermal toxicity carried out
according to OECD guidelines, the only effect observed after a dose of
2000 mg liquid HCFC-123/kg body weight was applied under occlusion for
24 h was a slight to moderate erythema in 6 of 10 rabbits up to 5 days
post-treatment. As such, the dermal LD50 for rats and rabbits was
greater than 2000 mg/kg body weight (Brock, 1988a,b).
8.2 Irritation and sensitization
Application under occlusion of 0.5 ml liquid HCFC-123 for 4 h
caused no erythema or oedema in a test for skin irritation potential
in rabbits conducted according to OECD guidelines (Brock, 1988c). In
rabbits, 0.1 ml undiluted HCFC-123 or 0.2 ml of a 50% solution in
propylene glycol caused mild to moderate, reversible eye damage,
including conjunctival irritation and corneal opacity (scoring not
reported) (Britelli, 1975). HCFC-123 did not produce skin
sensitization in guinea-pigs when 0.1 ml of a 1% solution in dimethyl
phthalate was administered intradermally once a week for 3 weeks,
followed by challenge 2 weeks later with 7 or 35 mg HCFC-123 dissolved
in propylene glycol (Goodman, 1975).
8.3 Short-term exposure
In rats exposed to 1000, 5000, 10 000, or 20 000 ppm (6.25, 31.3,
62.5, or 125 g/m3) HCFC-123 for 6 h per day, 5 days per week, in a
28-day inhalation toxicity study conducted in accordance with OECD
guidelines, exposures to 5000 ppm (31.3 g/m3) and above resulted in
dose-related narcotic effects, which were reversible overnight (Kelly,
1989; Rusch et al., 1994). Body weight was reduced at all dose levels
in females (8-10%) and at the two highest dose levels in males
(14-15%). There was a dose-related increase in relative liver weight
at all exposure levels in females (14-27%) and at the highest level in
males (18%). Plasma levels of ALT and AST were increased by 35% and
71%, respectively, at the highest level in males. Except for isolated
cases of fatty change at all dose levels, gross or microscopic liver
lesions were not observed. A NOAEL was not established in this study.
In a study in male rats exposed to approximately 1000, 5000, or
20 000 ppm (6.25, 31.3, or 125 g/m3) HCFC-123 for 6 h per day, 5
days per week, for 28 days, there was a decrease in body weight
(6-11%) and an increase in relative testis weight (12-30%) in all dose
groups and a 25% increase in relative liver weight in the highest dose
group (Lewis, 1990). Microscopic liver lesions including dose-related
hepatocyte hypertrophy and centrilobular fatty change were seen at all
exposure levels, with proliferation of peroxisomes and mitochondria in
liver cells of animals exposed to 5000 ppm (31.3 g/m3) and above.
Plasma levels of ALT, AST, and alkaline phosphatase (ALP) were
increased by up to 79%, whereas plasma triglycerides and cholesterol
were decreased by up to 70%, with AST and triglycerides being the most
sensitive markers of hepatic injury.
Similar findings accompanied by a 3-fold increase in hepatocyte
mitotic activity were reported from a study in which male rats were
exposed to 18 200 ppm (114 g/m3) HCFC-123 for 6 h per day, 5 days
per week, for 28 days (Warheit, 1993). This study also found
exposure-related testicular lesions, including germinal cell necrosis
and atrophy of the seminiferous tubules. In male guinea-pigs exposed
to 9400 ppm (58.8 g/m3) HCFC-123 for 6 h per day, 5 days per week,
for 28 days, there was evidence of microscopic liver lesions,
including centrilobular vacuolar (fatty) change and hepatocellular
necrosis, but no testicular effects were observed (Warheit, 1993).
8.4 Long-term exposure
8.4.1 Subchronic exposure
A number of subchronic inhalation studies have been carried out
in rats and dogs and are summarized in Table 1. In each case, HCFC-123
was administered for 6 h per day, 5 days per week. The main effects
were liver injury, with changes in liver-related clinical chemistry
parameters occurring at 300 ppm (1.88 g/m3) in rats and at 1000 ppm
(6.25 g/m3) in dogs, and CNS depression, with a reduction in arousal
occurring at 1000 ppm (6.25 g/m3) in rats.
8.4.2 Chronic exposure and carcinogenicity
In a 2-year inhalation study, groups of 80 male and female
Sprague-Dawley rats were exposed for 6 h per day for 5 days per week
to 300, 1000, or 5000 ppm (1.88, 6.25, or 31.3 g/m3) HCFC-123
(Malley, 1992; Malley et al., 1995).
During the first year, rats exposed to 5000 ppm (31.3 g/m3)
appeared sedated, but quickly recovered after the daily exposures
ended. Female rats exposed to 1000 ppm (6.25 g/m3) and males and
females exposed to 5000 ppm (31.3 g/m3) had lower body weight and
body weight gain. At the 12-month sacrifice, the relative liver weight
in male and female rats exposed to 5000 ppm (31.3 g/m3) was
increased by 12% and 24%, respectively. No exposure-related gross or
histopathological changes were observed. In both sexes, serum
triglycerides and glucose were decreased at all exposure levels in a
dose-related manner, by 65-100% and 15-31% for males and females,
respectively. Serum cholesterol was decreased by approximately 30% in
all exposed females and by 43% in males exposed to 5000 ppm
(31.3 g/m3).
During the second year of exposure, slight, reversible CNS
depression continued to be observed at 5000 ppm (31.3 g/m3). At the
end of the 2-year period, there was a dose-related increase in
survival rate, which reached a statistically significant level of 47%
and 59% in females exposed to 1000 or 5000 ppm (6.25 or 31.3 g/m3),
respectively. This is an expected effect of chemicals that reduce body
fat and blood lipids. Compared with the controls, body weight was
decreased by 8% in females exposed to 1000 ppm (6.25 g/m3) and by
12% in males and 21% in females exposed to 5000 ppm (31.3 g/m3). At
5000 ppm (31.3 g/m3), relative liver weight and the incidence of
enlarged and discoloured livers were increased in males, as were
grossly observed liver masses in females.
There were no exposure-related effects on the incidence of
malignant tumours. There was an increase in hepatocellular adenomas in
females and males, an increase in cholangiofibromas in high-dose
females, a dose-related increase in pancreatic acinar cell adenomas in
males, and an increase in Leydig (interstitial) cell adenomas in males
at all dose levels (Table 2). Except for hepatocellular adenomas in
males, the increase in the incidence of these tumours remained
statistically significant when corrected for mortality. Historical
data on tumour incidence in the strain used for this study were not
available. Other exposure-related lesions included hepatic foci of
cellular alteration and focal pancreatic acinar cell hyperplasia
(lesions less than 3 mm in diameter) in males and females at 1000 and
5000 ppm (6.25 and 31.3 g/m3), in addition to hepatic focal necrosis
in males, cholangiofibrosis in females, and hepatic centrilobular
fatty change in both sexes at 5000 ppm (31.3 g/m3). Dose-related
focal Leydig cell hyperplasia (lesions less than the diameter of three
adjacent tubules) was observed in male rats exposed to 1000 and
5000 ppm (6.25 and 31.3 g/m3). The incidence of diffuse retinal
atrophy was increased in both sexes at all exposure levels. Serum
triglycerides and cholesterol continued to be decreased in both sexes
by 46-75% and 31-48%, respectively.
As there were changes in clinical chemistry parameters and an
increased incidence of hepatocellular and Leydig cell adenomas at the
lowest dose tested (300 ppm [1.88 g/m3]), a NOAEL was not
established in this study.
8.5 Genotoxicity and related end-points
HCFC-123 was not mutagenic in Salmonella typhimurium strain
TA98, TA100, TA1525, TA1537, or TA1538 with or without metabolic
activation, even at concentrations of 750 mg per vessel or 150 000 ppm
(938 g/m3), which were clearly toxic (Callander, 1989). HCFC-123 was
found to be clastogenic in two separate studies in human lymphocytes
in vitro, both with and without metabolic activation, at relatively
high concentrations that also reduced the mitotic rate (Table 3). It
was also noted to be clastogenic in the absence, but not in the
presence, of a metabolic activation system in human lymphocytes
exposed to the chemical at 500 µg/ml; further details were not
available (ICI, 1992). No transformation to anchorage-independent
cells was observed when HCFC-123 was tested in baby hamster kidney
fibroblasts (BHK21 cells) with and without metabolic activation
(Longstaff et al., 1984).
Table 1: Summary of effect levels in subchronic inhalation toxicity studies.
Species Study design Effects Effect levels Reference
Rats, albino, Exposed to 0, 500, Body weight marginally LOAEL = 500 ppm Industrial Bio-Test
35 males and 1000, or 5000 ppm decreased in females at (3.13 g/m3) Laboratories, 1977;
25 females (0, 3.13, 6.25, or 1000 ppm and in both sexes Rusch et al., 1994
per group 31.3 g/m3) HCFC-123 at 5000 ppm. Kidney weight
for 90 days, with a increased in all male test
30-day recovery groups and in females at
period. 5000 ppm (% change not
reported). Relative liver
weight increased in females
at all exposure levels and
in males at 5000 ppm
(% change not reported).
Microscopic liver lesions
included mild focal necrosis
in males from all test groups
and minimal bile duct
proliferation in males at
5000 ppm. At end of recovery
period, there were no
exposure-related body or organ
weight changes or
histopathological findings.
Rats, Exposed to 0, 1000, Reversible motor LOAEL = 1000 ppm Doleba-Crowe, 1978;
Sprague-Dawley, or 10 000 ppm (0, incoordination and (6.25 g/m3) Rusch et al., 1994
27 per sex 6.25, or 62.5 g/m3) unresponsiveness to noise
per group HCFC-123 for 90 days. at 10 000 ppm. Reduced body
Histopathological weight (8-17%) and increased
examination performed relative liver weight
on 6 animals per (% change not reported) in
group. both test groups.
Table 1 (cont'd)
Species Study design Effects Effect levels Reference
No exposure-related gross or
histopathological findings.
Elevated levels of AST in
males at both exposure levels,
ALT in males at 1000 ppm, and
blood urea nitrogen (BUN) in
males at both exposure levels
and in females at 1000 ppm;
glucose decreased in females
in both test groups and in
males at 10 000 ppm (% change
not reported).
Rats, Exposed to 0, 300, Reduced responsiveness to LOAEL = 300 ppm Malley, 1990;
Sprague-Dawley, 1000, or 5000 ppm auditory stimuli at 1000 and (1.88 g/m3) Rusch et al.,
10 per sex (0, 1.88, 6.25, or 5000 ppm. Relative liver 1994
per group 31.3 g/m3) HCFC-123 weight increased by 12-17%
for 90 days. and 19-22%, respectively, at
1000 and 5000 ppm. No
exposure-related gross or
histopathological findings.
Dose-dependent elevations of
AST, ALT, lactate
dehydrogenase (LDH) in males
at 1000 and 5000 ppm and BUN
in females in all test groups
and in males at 1000 and
Table 1 (cont'd)
Species Study design Effects Effect levels Reference
5000 ppm. Triglycerides and
glucose markedly decreased
and hepatic ß-oxidation
activity increased 2- to
4-fold in all test groups.
Dose-dependent decrease in
cholesterol in females at
1000 and 5000 ppm.
Rats, Exposed to 0, 300, Reversible reduction in NOAEL = 300 ppm Coombs, 1994
Sprague-Dawley, 1000, or 5000 ppm arousal at 1000 and 5000 (1.88 g/m3)
10 per sex (0, 1.88, 6.25, or ppm. No exposure-related
per group 31.3 g/m3) HCFC-123 gross or histopathological
for 90 days, with a findings in cerebrum,
28-day recovery medulla/pons, cerebellar
period. Histological cortex, spinal cord, ganglia,
examinations limited dorsal and ventral root
to nervous tissues. fibres, or peripheral nerves.
Dogs, beagles, Exposed to 0, 1000, Reversible motor LOAEL = 1000 ppm Doleba-Crowe, 1978;
4 males per group or 10 000 ppm incoordination and (6.25 g/m3) Rusch et al., 1994
(0, 6.25, or 62.5 unresponsiveness at
g/m3) HCFC-123 for 10 000 ppm. At 10 000 ppm,
90 days. discoloured livers with
hepatocyte hypertrophy and
necrosis with inflammatory
cell infiltration. Elevated
levels of ALP in both test
groups and of BUN at 10 000
ppm (% change not reported).
Table 2: Incidence of selected non-neoplastic and neoplastic lesions in the liver, pancreas, and testes
in the 2-year rat inhalation study.a,b
Incidence of lesions
0 ppm 300 ppm 1000 ppm 5000 ppm
Male
Liver
Hepatocellular adenoma 3/67 2/66 2/66 8/66c
Basophilic foci of alteration 8/67 10/66 20/66d 30/66d
Clear cell foci of alteration 8/67 9/66 30/66d 19/66d
Mixed foci of alteration 3/67 6/66 6/66 12/66d
Eosinophilic foci of alteration 8/67 16/66 18/66d 13/66
Cholangiofibroma 0/67 0/66 0/66 0/66
Cholangiofibrosis 0/67 0/66 0/66 0/66
Pancreas
Acinar cell adenoma 1/67 4/66 12/64e 14/66e
Focal acinar cell hyperplasia 5/67 6/66 13/64d 19/66d
Testes
Leydig cell adenoma 4/67 12/66f 9/66f 14/66f
Leydig cell hyperplasia 8/67 15/66 23/66d 30/66d
Table 2 (cont'd)
Incidence of lesions
0 ppm 300 ppm 1000 ppm 5000 ppm
Female
Liver
Hepatocellular adenoma 0/65 5/67e 2/67 7/69e
Basophilic foci of alteration 17/65 26/67 32/67d 46/69d
Clear cell foci of alteration 14/65 7/67 16/67 15/69
Mixed foci of alteration 2/65 3/67 13/67d 22/69d
Eosinophilic foci of alteration 8/65 11/67 22/67 30/69d
Cholangiofibroma 0/65 0/67 0/67 6/69e
Cholangiofibrosis 0/65 0/67 0/67 9/69d
Pancreas
Acinar cell adenoma 0/65 2/66 0/67 2/69
Focal acinar cell hyperplasia 0/65 4/66 6/67d 8/69d
a From Malley (1992); Malley et al. (1995).
b Only lesions whose incidence attained statistical significance in at least one male or female dose
group are included in the table. Other non-statistically significant lesions are discussed in the
text. The figures give the number of lesions per number of tissues available for histological
examination. A few animals and tissues were lost due to autolysis.
c P < 0.05 (Cochran-Armitage test for trend).
d P < 0.05 (Fisher's exact test compared with controls).
e P < 0.05 (Cochran-Armitage test for trend and 2/2 tests for mortality-adjusted statistical analysis).
f P < 0.05 (Cochran-Armitage test for trend and 1/2 tests for mortality-adjusted statistical analysis).
In vivo, a test for chromosome aberrations in the lymphocytes
of rats exposed by inhalation to up to 5000 ppm (31.3 g/m3) HCFC-123
for 6 h per day, 5 days a week, for 2 weeks was negative (Marshal,
1992), although the failure to induce signs of cytotoxicity cast doubt
on the validity of this finding. No increase was found in the
incidence of micronuclei or in the ratio of polychromatic to
normochromatic erythrocytes in a micronucleus test in mice exposed
nose-only to up to 18 000 ppm (113 g/m3) HCFC-123 for 6 h (Muller &
Hofmann, 1988). In the livers of rats exposed to 12 500 or 20 000 ppm
(78.1 or 125 g/m3) HCFC-123 for 6 h, neither net nuclear grain count
nor percentage cells in repair showed any evidence of unscheduled DNA
synthesis (Kennelly, 1993).
Although HCFC-123 was clastogenic in vitro at high
concentrations, all other in vitro and in vivo tests for genetic
toxicity were negative. Overall, the available studies suggest that
HCFC-123 is unlikely to be genotoxic in vivo.
8.6 Reproductive and developmental toxicity
In studies conducted according to OECD guidelines, HCFC-123 was
neither embryotoxic nor teratogenic in pregnant rats exposed for 6 h
per day on days 6-15 of gestation by inhalation to 0, 5000, or 10 000
ppm (0, 31.3, or 62.5 g/m3) HCFC-123, although maternal toxicity in
the form of reduced weight gain and CNS depression were observed at
both exposure levels (Culik & Kelly, 1976; Brewer & Smith, 1977). In a
range-finding study in which fetal examinations were limited to
external structural abnormalities, HCFC-123 was not embryotoxic or
teratogenic in pregnant rabbits exposed for 6 h per day on days 6-18
of gestation by inhalation to 0, 500, 1500, or 5000 ppm (0, 3.13,
9.38, or 31.3 g/m3) HCFC-123, although dose-related maternal
toxicity characterized by reduced weight gain and food consumption was
evident at all exposure levels (Malinverno et al., 1996).
In a two-generation reproductive toxicity study in Sprague-Dawley
rats conducted in accordance with OECD guidelines, male and female
animals were exposed for 6 h per day, 7 days a week, by inhalation to
0, 30, 100, 300, or 1000 ppm (0, 0.188, 0.625, 1.88, or 6.25 g/m3)
HCFC-123 (Hughes, 1994; Malinverno et al., 1996). The F0 (parental
generation) animals were exposed from 6 weeks of age for 23-39 weeks,
including a 2-week mating period, the gestation period, and, except
for maternal animals on postpartum days 0-4, until the offspring were
weaned. The F1 generation was exposed from 4 weeks of age through to
weaning of their litters (F2 generation), for a total of
approximately 28 weeks.
Table 3: Chromosome aberrations in human lymphocytes in vitro.
Physical form of Exposure Concentrationb Mean mitotic Number of Reference
HCFC-123 protocola index cells with
aberrations
(excluding gaps)
Liquid 3-h exposure 0 µg/ml 25.2 1 Dance, 1991
(without S9) 73 µg/ml 19.6 2
146 µg/ml 21.7 3
292 µg/ml 21.1 3
CBC (2 µg/ml) 14.0 91
3-h exposure 0 µg/ml 23.8 1
(with S9) 146 µg/ml 21.8 2
292 µg/ml 14.7 6
584 µg/ml 6.6 5
CP (6 µg/ml) 8.4 106
24-h exposure 0 µg/ml 30.7 2
(without S9) 36 µg/ml 27.4 3
73 µg/ml 21.5 10c
292 µg/ml 9.7 31d
CBC (2 µg/ml) 22.9 70
Vapour 3-h exposure 0 ppm 24.9 1 Edwards, 1991
(without S9) 75 000 ppm 23.9 3
150 000 ppm 17.9 0e
300 000 ppm 10.3 5e
CBC (2 µg/ml) 18.5 60
Table 3 (cont'd)
Physical form of Exposure Concentrationb Mean mitotic Number of Reference
HCFC-123 protocola index cells with
aberrations
(excluding gaps)
3-h exposure 0 ppm 22.2 4
(with S9) 75 000 ppm 24.1 3
150 000 ppm 21.0 4
300 000 ppm 9.5 23e,f
CP (6 µg/ml) 15.2 107
24-h exposure 0 ppm 16.6 1
(without S9) 25 000 ppm 19.1 9d
50 000 ppm 13.2 18f
100 000 ppm 5.9 24f
CBC (2 µg/ml) 13.5 118
a S9 = metabolic activation system.
b Positive controls: CBC = chlorambucil; CP = cyclophosphamide.
c P < 0.05.
d P < 0.01.
e Increase in number of polyploid cells.
f P < 0.001.
The only adverse reproductive effect was a 17% decrease in
implantation count among F1 females at the highest exposure level.
In terms of development, pup growth was impaired during the
pre-weaning period when exposure was confined to the lactating parent
female. In the F1 generation, mean pup weight was decreased by
approximately 10% at exposures at and above 100 ppm (0.625 g/m3),
whereas mean pup weight in F1 offspring (F2 generation) was
decreased by approximately 20% at all exposure levels. In adult rats,
retarded weight gain was observed at 100 ppm (0.625 g/m3) and above
in F0 animals and at 300 ppm (1.88 g/m3) and above in the F1
generation. There was a dose-dependent, 8-39% increase in liver weight
in all exposed F0 groups and an 8-10% increase at 100 ppm (0.625
g/m3) and above in F1 animals. In F1 animals exposed to HCFC-123
for approximately 28 weeks, exposure-related histopathological changes
were confined to a dose-related increase in the incidence of
centrilobular hepatocyte enlargement and in the incidence and degree
of hepatocyte vacuolation at 300 ppm (1.88 g/m3) and above. No
microscopic changes were detected in the livers of F2 weanling rats
from the 1000 ppm (6.25 g/m3) exposure group. In both generations,
there was a decrease in serum triglycerides in both sexes, whereas
serum cholesterol was increased in males and decreased in females.
Levels of ALT, AST, or other liver enzymes were not determined. Male
rats exposed at or above 300 ppm (1.88 g/m3) had increased plasma
levels of luteinizing hormone (LH) after 10 weeks of exposure, which
had reverted to normal at week 38. In this study, the NOAEL, based on
effects on fertility, was 300 ppm (1.88 g/m3). The LOAEL, based on
developmental effects (retarded neonatal growth during lactation) and
on increased liver weight and changes in liver-related clinical
chemistry parameters, was 30 ppm (0.188 g/m3).
After 22 weeks of exposure, a sample of F1 male rats was drawn
from the two-generation reproductive toxicity study for
endocrinological investigations (Sandow et al., 1995b). In 10 males
from each exposure group, serum levels of LH and testosterone were
determined before and after an injection of LH releasing hormone. The
testes of another eight males per group were incubated in vitro with
human chorionic gonadotropin (which stimulates steroid hormone
biosynthesis). The incubation medium and testis tissue were analysed
for content of testosterone, progesterone, estradiol-17-ß, 17
alpha-OH-progesterone, and delta-4-androstenedione. Basal serum LH and
testosterone levels were similar to those of controls. However, after
stimulation with LH releasing hormone, the LH in rats exposed to 300
ppm (1.88 g/m3) was 32% lower than in controls; at 1000 ppm (6.25
g/m3), LH was 39% and testosterone 46% lower than in the control
group. In the ex vivo test, HCFC-123 inhalation did not affect the
secretory capacity for steroid hormones or alter the content of these
hormones in the testes at the end of the incubation period, except for
a slight reduction of delta-4-androstenedione at 1000 ppm (6.25
g/m3).
In an endocrinological study in rats of both sexes, exposure to
5200 ppm (32.5 g/m3) HCFC-123 for 6 h per day (similar to the
highest dose level in the 2-year bioassay) for 14 consecutive days was
associated with sedation, decreased body, kidney, ovary, and pituitary
weights in females, and increased relative liver weight in males
(Hofmann, 1995; Sandow et al., 1995a). In males, the prolactin
response after monoiodotyrosine stimulation, the testosterone response
after stimulation with buserelin (a synthetic gonadotropin releasing
hormone), and the testicular testosterone content were all reduced by
approximately 50%. In females, the gonadotropin response to buserelin
stimulation was enhanced and the pituitary content of follicle
stimulating hormone and prolactin was reduced, in both cases by
approximately 50%.
These endocrinological investigations indicate that HCFC-123 has
little, if any, effect on steroid production in rat testes, but
impairs the prolactin, LH, and testosterone response to pituitary
stimulants. The NOAEL based on this effect was 100 ppm (0.625 g/m3).
A lactation study was conducted in groups of pregnant and
lactating Sprague-Dawley (Crl:CD BR) rats exposed to 0 or 1000 ppm (0
or 6.25 g/m3) HCFC-123 for 6 h per day on days 5-19 of gestation and
on days 5-21 postpartum (Buschman, 1996). Within 2 days of birth,
litters were crossed over between dams to create four groups
comprising exposed or control dams rearing litters from different
exposed or control mothers. Absolute and relative liver weights were
increased and serum triglycerides, cholesterol, and glucose decreased
in dams exposed to HCFC-123. The milk of dams exposed to HCFC-123 was
of normal quantity and quality (with regard to content of protein,
lactose, and fat) but contained trifluoroacetic acid at an average
concentration of 50 µg/ml. Trifluoroacetic acid was also found in the
urine of pups reared by dams exposed to HCFC-123. There were no
differences in absolute or relative liver weight or any abnormal
clinical signs or gross findings in any of the groups of pups, but
pups reared by dams exposed to HCFC-123 had a 10% lower growth rate
and decreased serum triglycerides compared with pups reared by
non-exposed dams. Before crossover, there was no difference between
groups with respect to mean pup and litter weight. As such, these
findings indicate that the retarded neonatal growth observed in the
two-generation reproductive toxicity study was due to factors in the
milk of exposed mothers, probably trifluoroacetic acid, rather than to
exposure in utero.
When groups of four lactating rhesus monkeys and their neonates
were exposed to either 0 or 1000 ppm (0 or 6.25 g/m3) HCFC-123 for 6
h per day for 21-22 consecutive days, there were no effects on
maternal body weight, serum triglycerides, cholesterol, and glucose,
or milk composition (Slauter, 1997). Liver biopsy specimens taken from
the mothers at the end of the study revealed exposure-related lesions,
including mild to moderate centrilobular hepatocyte vacuolation, trace
to moderate centrilobular hepatocyte necrosis, and trace to mild
subacute inflammation. As a rule, HCFC-123 was not detected in the
blood of mothers or neonates, whereas trifluoroacetic acid was present
at concentrations of 9-70 µg/ml in exposed mothers and of 17-190 µg/ml
in neonates, with individual blood levels being 2-6 times higher in
the neonates than in their corresponding mothers. Milk from exposed
mothers contained HCFC-123 and trifluoroacetic acid at concentrations
of 1-5 µg/ml and 17-30 µg/ml, respectively. Although no statistical
analysis was attempted because of the small number of observations,
the average growth rate was 10% lower in exposed neonates than in
unexposed controls.
9. EFFECTS ON HUMANS
The available data on the human health effects of HCFC-123 were
limited to a single case report of dizziness, headache, and nausea in
workers exposed to unknown levels of the chemical following the
rupture of an industrial chiller1 and three case reports of hepatic
effects involving 26 workers following repeated exposure to HCFC-123
vapours.
Nine cases of liver effects were reported in gantry drivers at a
smelting depot in Belgium (Hoet et al., 1997). They occurred 1-4
months after the refrigerant utilized in the crane cabin
air-conditioning system had been replaced by a blend containing 57%
HCFC-123, 40% HCFC-124 (1-chloro-1,2,2,2-tetrafluoroethane), and 3%
propane.2 One driver admitted to hospital was found to have
increased levels of AST, ALT, ALP, gamma-glutamyl transferase, and
total and conjugated bilirubin and decreased prothrombin activity,
with AST and ALT levels being 15-23 times above the upper limit of the
normal range. Autoimmune, viral, and drug- or alcohol-induced
hepatitis were ruled out. A liver biopsy showed focal liver cell
necrosis, plugging of bile ducts, and the presence of
trifluoroacetylated proteins. The symptoms regressed during the period
of non-exposure but recurred when the driver returned to work 2 months
later. Eight other drivers showed signs of varying degrees of liver
abnormalities. Serum antibodies to human liver enzymes (CYP2E1 and/or
protein disulfide isomerase isoform P58) were detected in five of six
cases examined. A workplace inspection revealed that the plastic pipes
of the air-conditioning system were perforated and that refrigerant
was leaking into the crane cabin. No further cases occurred after the
system was repaired. Although the workers were exposed to both
HCFC-123 and HCFC-124, the latter is unlikely to have contributed to
the observed effects, as the NOAEL for HCFC-124 was 50 000 ppm (280
g/m3) in a 90-day inhalation toxicity study in rats (Malley et al.,
1996), whereas the LOAEL for HCFC-123 in a similar study in the same
strain and laboratory was 300 ppm (1.88 g/m3) (Table 1).
Eight cases of liver effects were reported in workers exposed to
the vapours of a solvent degreaser containing HCFC-123.3 Two months
after a US factory converted to using HCFC-123 in its degreaser, two
employees who worked closely with the degreaser were found to have
liver disease. They had elevated blood levels of liver enzymes,
particularly ALT (32-56 times above the upper limit of the normal
1 Carrier Canada Ltd, personal communication, 1993 [cited in
NICNAS, 1996].
2 N. Verlinden, personal communication, 1997 [cited in
NICNAS, 1999].
3 AlliedSignal Inc., personal communication, 1998 [cited in
NICNAS, 1999].
range) and AST (14-33 times above the upper limit of the normal
range), had elevated total and conjugated bilirubin, and tested
negative for viral hepatitis. Subsequent testing of all 27 factory
employees revealed four additional cases of elevated liver enzymes.
When retested 1 month later but before the use of HCFC-123 was
discontinued, five of the affected employees had improved markedly,
whereas one had deteriorated, and there was one new case with slightly
elevated ALT and AST levels. All in all, liver enzymes were elevated
in 3 of 4 employees who worked with the degreaser and in 4 of 23
workers who did not. Air monitoring was conducted when HCFC-123 use
began and again shortly after the first cases were diagnosed. Personal
air monitoring during normal degreaser operations showed 5.5-h
time-weighted average levels of HCFC-123 that ranged from 5.3 to
12.0 ppm (33.1 to 75.0 mg/m3) throughout the facility, whereas
short-term breathing-zone levels ranging from 160 to 460 ppm (1000 to
2880 mg/m3) were measured in workers charging and unloading the
degreaser. There was also one case of elevated liver enzymes with
negative tests for viral hepatitis in a technician employed in the
manufacturer's research laboratory where the degreaser was tested and
evaluated. Static air levels in the laboratory were reported to be
generally below 50 ppm (313 mg/m3) HCFC-123.
In a factory in Japan where miniature heat exchangers were filled
with HCFC-123 in a poorly ventilated room, 9 out of 14 workers were
found to have elevated levels of liver enzymes 4-5 weeks after
production had commenced (Takebayashi et al., 1998a,b).1 Four of
them were clinically ill, and two had jaundice. In these workers, AST
and ALT were up to 20-30 times above the upper limit of the normal
range. After an exhaust system was installed that maintains the
concentration of airborne HCFC-123 at about 1 ppm (6.25 mg/m3),
trifluoroacetic acid was not detected in the workers' urine; at
follow-up 1 year later, there were no further cases of clinical
illness or elevated liver enzymes.1 Exposure levels were not
measured, but a simulation of the original working conditions
indicated static air levels ranging from 5 to 1125 ppm (31.3 to
7030 mg/m3) (6-h time-weighted average), depending on the distance
from the filling area.
1 Also T. Takebayashi, personal communication, 1999 [cited in
NICNAS, 1999].
Table 4: Summary of effects of HCFC-123 in aquatic organisms.
Test Species Effects and effect Comments Reference
levels
Acute toxicity (96 h), Fathead minnow Lethargy Rapid degassing Pierson,
flow-through conditions (Pimephales LC50 > 76 mg/litre of HCFC-123 1990a
promelas) (measured) from test
solutions
Acute toxicity (96 h), Rainbow trout Lethargy, darkened Jenkins, 1992b
static conditions (Oncorhynchus pigmentation at
mykiss) 15 mg/litre and
above
LC50 = 56 mg/litre
(measured)
Immobilization (48 h), Daphnia magna Lethargy Jenkins, 1992c
static conditions EC50 = 17 mg/litre
(measured)
Immobilization (48 h), Daphnia magna Lethargy Measured Pierson, 1990b
static conditions EC50 = 45.8 mg/litre concentrations
(nominal) <75% of nominal
Algal growth inhibition Selenastrum EC50 = 68 Based on biomass Jenkins, 1992d
(96 h), static conditions capricornutum mg/litre (measured) integral
10. EFFECTS ON OTHER ORGANISMS IN THE LABORATORY AND FIELD
The available ecotoxicological data for HCFC-123 are summarized
in Table 4. The data indicate that the chemical is, at most, slightly
toxic to aquatic organisms under conditions of acute exposure. Chronic
effects would not be expected because of limited aquatic persistence.
Trifluoroacetic acid, which is formed by atmospheric breakdown of
HCFC-123, has been found to be of low toxicity in stream mesocosms,
algae, higher plants, fish, and mammals (Boutonnet et al., 1999). The
lowest threshold for any effect was 0.12 mg sodium
trifluoroacetate/litre, above which the chemical had reversible
effects on the growth of the alga Selenastrum capricornutum. In the
most sensitive terrestrial species tested (sunflower), sodium
trifluoroacetate at 1 mg/kg dry soil had clear effects on vegetative
growth, whereas long-term root exposure of wheat and soya to sodium
trifluoroacetate at 1 mg/litre had no effect.
11. EFFECTS EVALUATION
11.1 Evaluation of health effects
11.1.1 Hazard identification and dose-response assessment
There was inadequate information on the human health effects of
short-term exposure to HCFC-123. In laboratory animals, HCFC-123
exhibits low acute toxicity, with an approximate oral lethal dose of
9 g/kg body weight, a 4-h LC50 by inhalation in the range of
28 400-52 600 ppm (178-329 g/m3), and a dermal LD50 in excess of
2000 mg/kg body weight. It is not a skin irritant or sensitizer, but
liquid HCFC-123 produces mild to moderate eye irritation. The critical
effects associated with acute exposure are non-fatal liver damage, CNS
depression, and cardiac sensitization to adrenaline. When inhaled in
lethal concentrations, death was caused by severe CNS depression. For
a single, 4-h exposure by inhalation, the LOAEL was 1000 ppm
(6.25 g/m3) for liver damage, based on liver cell necrosis and
increased levels of circulating liver enzymes in guinea-pigs, and 5000
ppm (31.3 g/m3) for CNS depression, based on failures in
unconditioned reflexes in rats. The NOAEL for cardiac sensitization to
adrenaline in dogs was 10 000 ppm (62.5 g/m3). Although the dog is
a very sensitive species, cardiac sensitization to adrenaline-induced
arrhythmia is likely to be a relevant critical effect resulting from
short-term exposure in humans, such as the sudden discharge of fire
extinguishants in occupied rooms (NAS, 1996). Under these
circumstances, CNS depression may also be a critical effect. A large
number of short-chain halogenated hydrocarbons with different
metabolic patterns have similar CNS and cardiac effects, which likely
result from a direct anaesthetic action on neurons and myocardial
cells (IPCS, 1990, 1991, 1992).
The critical effects associated with repeated exposure to
HCFC-123 vapours are liver damage in humans as well as experimental
animals, in addition to neonatal growth retardation, an increased
incidence of benign tumours, and CNS depression, which have been
recorded only in animals.
Based on a limited number of case reports, biochemical
abnormalities associated with liver injury have been observed in
humans at exposure levels in the order of 5-1125 ppm (31.3-7030
mg/m3). The available data are insufficient to define the
dose-response relationship in humans. Liver damage was also seen in
rats, guinea-pigs, dogs, and monkeys. The lesions generally involved
increased liver weight accompanied by hepatocyte enlargement and
vacuolation at the lowest exposure levels, with necrosis, fatty
change, and mild subacute inflammation at higher concentrations. They
were associated with or preceded by increased levels of circulating
liver enzymes and decreased serum triglycerides, glucose, and
cholesterol. The LOAEL for liver effects in animals recorded in a
well-conducted two-generation reproductive toxicity study in rats
equalled 30 ppm (188 mg/m3).
The cytotoxicity of HCFC-123 is probably due to the reactive
metabolite trifluoroacetyl chloride, which can bind covalently to
proteins and interfere with their function and/or alter their
antigenicity. The 200 : 20 : 1 ratio of trifluoroacetylation of liver,
kidney, and heart tissue proteins reported by Huwyler et al. (1992)
and Huwyler & Gut (1992) correlates well with the observed effect
levels for these organs in subchronic toxicity studies (Table 1) and
probably reflects tissue differences in metabolic capacity.
There is no evidence that HCFC-123 is teratogenic in laboratory
animals or induces reproductive or fetal toxicity at levels of
exposure lower than those that cause other systemic effects in adults.
Growth was retarded in neonatal rats and monkeys reared by dams
exposed by inhalation to HCFC-123, with a LOAEL of 30 ppm
(188 mg/m3). The main metabolite of HCFC-123, trifluoroacetic acid,
was found in the milk of the dams. As such, breastfed babies may
represent a subpopulation that is uniquely sensitive to HCFC-123.
Reversible CNS depression was seen consistently in repeated-dose
inhalation studies, but did not change in severity or duration with
the number of exposures and was not associated with morphological
changes in nervous tissues. As such, the mechanism of action is
probably the same for both acute and chronic CNS depression. The
lowest NOAEL for CNS effects from repeated administration was recorded
in a neurotoxicity study in rats and equalled 300 ppm (1880 mg/m3),
based on a reduction in arousal.
Although there was evidence of clastogenic activity in human
lymphocytes in vitro at high, cytotoxic concentrations, all other
in vitro and in vivo tests for genetic toxicity were negative.
Therefore, the evidence suggests that HCFC-123 is unlikely to be
genotoxic in vivo.
In a 2-year inhalation study in rats, there was no
exposure-related effect on the incidence of malignant tumours, but
there was an increased incidence of hepatocellular adenomas,
cholangiofibromas, pancreatic acinar cell adenomas, and Leydig
adenomas. There was also an increase in the pre-cancerous lesions of
hepatic foci of alteration, cholangiofibrosis, focal pancreatic acinar
cell hyperplasia, and Leydig cell hyperplasia (Table 2). As stated
above, HCFC-123 is unlikely to be genotoxic in vivo. The minor,
non-mutagenic metabolite 2-chloro-1,1,1-trifluoroethane caused an
increased incidence of uterine carcinomas and Leydig cell adenomas in
rats when given by oral gavage at 300 mg/kg body weight for 52 weeks
(IPCS, 1992). However, only trace quantities would be formed from the
metabolism of HCFC-123. Therefore, it is necessary to examine the
mechanisms of tumour formation to establish if the modes of induction
can be ruled out as relevant for humans:
* Hepatocellular adenomas. Several repeated-exposure studies have
shown that HCFC-123 and its main metabolite, trifluoroacetic
acid, like several other structurally diverse chemicals, induce
peroxisome proliferation in rat hepatocytes (Warheit, 1993; Rusch
et al., 1994; Malley et al., 1995; Keller et al., 1998).
Peroxisome proliferators are generally not genotoxic but induce
hepatocellular proliferation in rats and mice through a mechanism
that appears to involve the expression of growth factors by
hepatic macrophages, thus leading to liver tumour formation
(Chevalier & Roberts, 1998). The hepatocellular adenomas seen in
rats exposed to HCFC-123 may be related to the induction of
peroxisome proliferation, which is a mechanism of questionable
relevance for humans (Ashby et al., 1994). However, since the
chemical is also hepatotoxic, which may have a role in the tumour
formation, it is not possible to discount the hepatocellular
adenomas as being of no concern to humans. It is, however,
reasonable to adopt a threshold approach, based on adverse
effects on the liver in subchronic exposure studies.
* Cholangiofibromas. Cholangiofibromas in the rat are atypical
glandular structures lined by intestinal-like epithelium
surrounded by dense connective tissue. Limited evidence from
animal studies of various non-genotoxic chemicals, including
chloroform and furan, suggests that this tumour type is
associated with significant hepatocyte necrosis and regenerative
cell proliferation that are relevant only at high dose/exposure
levels (Elmore & Sirica, 1993; Jamison et al., 1996). In the
2-year rat study, cholangiofibromas and cholangiofibrosis
occurred only in females exposed to HCFC-123 at 5000 ppm (31.3
g/m3). The incidence of basophilic and eosinophilic foci of
hepatocellular proliferation was also higher in females than in
males (Table 2). Although the threshold level for the induction
of cholangiofibromas in female rats was high, there is no
mechanistic evidence that this tumour type can be dismissed with
regard to its relevance to humans.
* Pancreatic acinar cell adenomas. Some hepatocarcinogenic
peroxisome proliferators have been reported to induce tumours in
other organs, including pancreatic acinar cell adenomas and
Leydig cell adenomas, although these extrahepatic tumours appear
not to be associated with peroxisome proliferation in the target
organ (IARC, 1995). Although pancreatic acinar cell adenomas were
found only in males at 1000 and 5000 ppm (6.25 and 31.3 g/m3),
the incidence was dose-related. Moreover, pancreatic acinar cell
hyperplasia occurred in both sexes, likewise in a dose-dependent
manner (Table 2). As such, until more is known about the
mechanism for acinar cell tumour induction in animals and humans,
the possibility that the pancreatic adenomas found in rats
exposed to HCFC-123 may have some relevance to humans cannot be
discounted.
* Leydig cell adenomas. The available studies indicate that
exposure to HCFC-123 may be associated with endocrine
disturbances in male rats, particularly in relation to prolactin
release and serum LH concentrations. In rats, but not in humans,
a decrease in serum prolactin causes a decrease in the number of
LH receptors on Leydig cells and thus a decrease in testosterone
production, which results in increased LH levels that in turn may
induce Leydig cell hyperplasia and adenomas (Clegg et al., 1997).
As such, it is conceivable that intermittent exposure to HCFC-123
could lead to fluctuations in prolactin and testosterone that in
rats could induce transient increases in LH and, with time,
Leydig cell growth and tumours. Although prolactin fluctuations
would not be of concern in men, the effects of HCFC-123 on the
sex hormone system are complex. Thus, in the absence of data from
studies in primates, the increased incidence of Leydig cell
adenomas in the 2-year rat study cannot be dismissed with respect
to its relevance for humans.
In summary, it is likely that the benign tumours in the 2-year
rat bioassay involve one or more non-genotoxic mechanisms, including
peroxisome proliferation, hepatocellular damage, necrosis and
regenerative proliferation, and disturbance of the
hypothalamic-pituitary-testicular axis. Although humans may be less
sensitive to tumours arising from some of these actions, overall it is
not possible to discount the tumours in an evaluation of the potential
risk for humans. Therefore, the increased incidence of benign tumours
in the rat raises some concern with respect to the potential
carcinogenicity in humans. The tumours probably arise from
non-genotoxic mechanisms. In the 2-year bioassay, the tumour incidence
was increased at 300 ppm (1.88 g/m3), the lowest level tested, and a
NOAEL was not established. In subchronic toxicity studies, the LOAEL
based on any adverse effect on the liver was 30 ppm (0.188 g/m3)
(Hughes, 1994; Malinverno et al., 1996).
11.1.2 Criteria for setting tolerable intakes or guidance values
for HCFC-123
Derivation of a guidance value for HCFC-123 was outside the scope
of the source documents (NICNAS, 1996, 1999). General advice about the
derivation of tolerable intakes and guidance values for health-based
exposure limits is set out in Environmental Health Criteria 170 (IPCS,
1994).
Exposure of the general public to HCFC-123 is likely to be
minimal. The main risk to human health is through repeated
occupational exposure via inhalation.
The critical effects of repeated low-level exposure to HCFC-123
are liver damage, which has been observed in all species investigated,
including monkeys and humans, and retarded neonatal growth during
lactation, which has been observed in rats and monkeys. In the absence
of sufficient data to establish a dose-response relationship in
humans, a guidance value for exposure to HCFC-123 must be based on the
observed effect levels for liver lesions and retarded neonatal growth
during lactation in pivotal animal studies. For both of these critical
effects, a LOAEL of 30 ppm (188 mg/m3) was established in a
well-conducted two-generation reproductive toxicity study in rats,
whereas a NOAEL was not achieved (Hughes, 1994; Malinverno et al.,
1996).
The uncertainty factor applied to extrapolate from the LOAEL to a
NOAEL must allow for the fact that whereas the recorded liver effects
at the LOAEL were mild, hepatotoxicity may have a role in the
induction of benign liver tumours in rats. Moreover, the growth
retardation in neonates during lactation was in the order of 10-20%.
It is likely that the liver effects are related to the formation of
protein adducts with trifluoroacetyl chloride and that growth
retardation in neonates is related to exposure to trifluoroacetic acid
in maternal milk. Therefore, the uncertainty factor used to
extrapolate the NOAEL from rats to humans must allow for the lack of
in vivo data on the metabolic rate and other toxicokinetic
properties of HCFC-123 in humans. Finally, an additional uncertainty
factor must be applied to allow for human variability in
toxicokinetics, as the metabolism of HCFC-123 to trifluoroacetyl
chloride and trifluoroacetic acid is catalysed by CYP2E1
(Urban et al., 1994), whose activity is known to be influenced by
genetic polymorphism, body weight, and dietary factors (Le Marchand
et al., 1999).
11.1.3 Sample risk characterization
Adverse effects of HCFC-123 in animals and humans have been
observed only at concentrations that were several orders of magnitude
higher than those in the only known medium of exposure (air) in the
general environment. The likelihood of public exposure as a
consequence of catastrophic accidents or fire extinguishant discharges
is very small, and the scale and duration of such exposures are
expected to be low.
With respect to repeated occupational exposures, 3- to 8-h
time-weighted average personal exposure levels in an HCFC-123
manufacturing plant were reported to be below 10 ppm (62.5 mg/m3).
Reported 2- to 12-h time-weighted average breathing-zone levels in
machinery rooms containing air-conditioning equipment generally ranged
from below 1 to 5 ppm (below 6.25 to 31.3 mg/m3), whereas the use of
a liquid HCFC-123 degreaser was associated with concentrations in the
range of 5.3-12 ppm (33.1-75.0 mg/m3) HCFC-123. The available case
reports indicate that humans may develop biochemical signs of liver
disease, such as elevated AST and ALT, after 1-4 months of repeated
exposure to HCFC-123 at levels above 5 ppm (31.3 mg/m3). Because the
effects of low levels of HCFC-123 are due to toxic metabolites formed
through CYP2E1, genetic, lifestyle, and dietary factors are expected
to cause considerable variability in human susceptibility to the
chemical.
11.2 Evaluation of environmental effects
Because of its high volatility, HCFC-123 released to the
environment will partition almost entirely to the atmosphere. It is
removed predominantly in the troposphere by reaction with hydroxyl
radicals to form trifluoroacetic acid, and only a small fraction is
transported to the stratosphere, where it may undergo photolysis and
release chlorine radicals that catalyse the destruction of ozone.
Because of its short atmospheric lifetime, estimated at 1.4 years, its
ozone-depleting potential is low (0.02 relative to CFC-11). The global
warming potential of HCFC-123 relative to carbon dioxide is 300, 93,
and 29 over a time horizon of 20, 100, and 500 years, respectively
(WMO, 1995).
The aquatic EC50/LC50 values were below 100 mg/litre but
above 10 mg/litre. As such, the chemical meets the European Community
criteria for classification as harmful to the environment (Berends et
al., 1999) and the globally harmonized criteria for classification as
hazardous to the aquatic environment (Class: Acute III) (OECD, 1998).
However, while HCFC-123 may be released to surface waters or soil, it
is unlikely to persist in these media because of its high volatility.
As such, it is considered that HCFC-123 does not constitute a
long-term or delayed danger to the aquatic environment.
Trifluoroacetic acid formed by degradation of HCFC-123 will
precipitate in rain and may accumulate in closed aquatic systems such
as salt lakes and seasonal wetlands. The maximum total contemporary
deposition rate of trifluoroacetic acid from fluorocarbons has been
estimated at 2800 tonnes a year, with 27% derived from HCFC-123 and
the remainder from HCFC-124, HFC-134a, HFC-227ea, and the anaesthetic
gases halothane and isoflurane (Boutonnet et al., 1999). In 2020, the
maximum deposition from fluorocarbons is predicted to reach 160 000
tonnes a year, yielding a maximum average concentration of
trifluoroacetic acid in rainwater of 0.1 µg/litre, which is several
orders of magnitude lower than the no-effect level in both surface and
soil water. By that year, HCFC-123 emissions will have declined, as
the chemical will have been phased out in accordance with the Montreal
Protocol. As such, it can be concluded that environmental levels of
trifluoroacetic acid resulting from the breakdown of HCFC-123 do not
pose a threat to the environment.
12. PREVIOUS EVALUATIONS BY INTERNATIONAL BODIES
A previous evaluation of HCFC-123 has been carried out by the
International Programme on Chemical Safety (IPCS, 1992).
Information on international hazard classification and labelling
is included in the International Chemical Safety Card reproduced in
this document (Appendix 4).
REFERENCES
AIHA (1998) Workplace environmental exposure level guide series:
1,1,1-Trifluoro-2,2-dichloroethane. Fairfax, VA, American Industrial
Hygiene Association Press.
Ashby J, Brady A, Elcombe CR, Elliott BM, Ishmael J, Odum J, Tugwood
JD, Kettle S, Purchase IF (1994) Mechanistically-based human hazard
assessment of peroxisome proliferator-induced hepatocarcinogenesis.
Human and experimental toxicology, 13 (Suppl. 2):S1-S117.
Berends AG, de Rooij CG, Shin-ya S, Thompson RS (1999) Biodegradation
and ecotoxicity of HFCs and HCFCs. Archives of environmental
contamination and toxicology, 36(2):146-151.
Boutonnet JC, Bingham P, Calamari D, de Rooij C, Franklin J, Kawano T,
Libre JM, McCulloch A, Malinverno G, Odom JM, Rusch GM, Smythe K,
Sobolev I, Thompson R, Tiedje JM (1999) Environmental risk assessment
of trifluoroacetic acid. Human and ecological risk assessment,
5(1):59-124.
Brewer WE, Smith S (1977) Teratogenic study via inhalation with
Genetron 123 in albino rats. Morristown, NJ, Allied Chemical
Corporation.
Britelli MR (1975) Eye irritation test in rabbits. Newark, DE, Du
Pont de Nemours and Co.
Brock WJ (1988a) Acute dermal toxicity study of HCFC-123 in rabbits.
Newark, DE, Du Pont de Nemours and Co.
Brock WJ (1988b) Acute dermal toxicity study of HCFC-123 in rats.
Newark, DE, Du Pont de Nemours and Co.
Brock WJ (1988c) Primary dermal irritation study with HCFC-123 in
rabbits. Newark, DE, Du Pont de Nemours and Co.
Buschman J (1996) Crossover study with HCFC 123 in lactating
Sprague-Dawley rats including additional studies on milk production
and metabolites in offspring urine. Hannover, Fraunhofer Institute
for Toxicology and Aerosol Research.
Callander RD (1989) HCFC-123 -- an evaluation using the Salmonella
mutagenicity assay. Macclesfield, Cheshire, Imperial Chemical
Industries Ltd.
Chang W-K, Criddle CS (1995) Biotransformation of HCFC-22, HCFC-142b,
HCFC-123 and HFC-134a by methanotrophic mixed culture MM1.
Biodegradation, 6(1):1-9.
Chevalier S, Roberts RA (1998) Perturbation of rodent hepatocyte
growth control by nongenotoxic hepatocarcinogens -- mechanisms and
lack of relevance for human health. Oncology reports, 5:1319-1327.
Clayton JW (1966) Acute inhalation toxicity. Newark, DE, Du Pont de
Nemours and Co.
Clegg ED, Cook JC, Chapin RE, Foster PMD, Daston GR (1997) Leydig cell
hyperplasia and adenoma formation: Mechanisms and relevance to humans.
Reproductive toxicology, 11:107-121.
Coate WB (1976) LC50 of G123 in rats. Vienna, VI, Hazleton
Laboratories America Inc.
Cohen SD, Pumford NR, Khairallah EA, Boekelheide K, Pohl LR, Amouzadeh
HR, Hinson JA (1997) Selective protein covalent binding and target
organ toxicity. Toxicology and applied pharmacology, 143:1-12.
Coombs DW (1994) HCFC-123 -- 13-week inhalation neurotoxicity study
in the rat. Cambridgeshire, Huntingdon Research Centre Ltd.
Culik R, Kelly DP (1976) Embryotoxic and teratogenic studies in rats
with inhaled dichlorofluoromethane (Freon 21) and
2,2-dichloro-1,1,1-trifluoroethane (FC-123). Newark, DE, Du Pont de
Nemours and Co.
Dance CA (1991) In vitro assessment of the clastogenic activity of
HCFC-123 in cultured human lymphocytes. Eye, Suffolk, Life Science
Research Ltd. (No. 91/PFE003/0093).
Darr RW (1981) An acute inhalation toxicity study of
Fluorocarbon 123 in the Chinese hamster. Morristown, NJ, Allied
Corporation.
Dekant W (1993) Metabolism of 1,1-dichloro-2,2,2-trifluoroethane
(HCFC-123). Würzburg, Institute of Toxicology, University of
Würzburg (Report No. MA-250B-82-207).
Dodd DE, Brashear WT, Vinegar A (1993) Metabolism and pharmacokinetics
of selected halon replacement candidates. Toxicology letters,
68:37-47.
Doleba-Crowe C (1978) 90-day inhalation exposure of rats and dogs to
vapours of 2,2-dichloro-1,1,1-trifluoroethane (FC-123) (summary).
Newark, DE, Du Pont de Nemours and Co. [cited in NICNAS, 1996].
Du Pont (1993) Workplace guidelines for Suva(R) Centri-LP
(HCFC-123) in refrigeration and air conditioning applications.
Wilmington, DE, Du Pont de Nemours and Co.
Edwards CN (1991) HCFC-123 (vapour phase): In vitro assessment of
the clastogenic activity in cultured human lymphocytes. Eye,
Suffolk, Life Science Research Ltd. (No. 91/PFE002/0125).
Elmore LW, Sirica AE (1993) "Intestinal-type" of adenocarcinoma
preferentially induced in right/caudate liver lobes of rats treated
with furan. Cancer research, 53:254-259.
Frank H, Klein A, Renschen D (1996) Environmental trifluoroacetate.
Nature, 382:34.
Fraser P (1994) CSIRO report to SPA-AFEAS. Canberra, Commonwealth
Scientific and Industrial Research Organisation.
Goodman NC (1975) Primary skin irritation and sensitization tests on
guinea pigs. Newark, DE, Du Pont de Nemours and Co.
Hall GT, Moore BL (1975) Acute inhalation toxicity on Freon 123.
Newark, DE, Du Pont de Nemours and Co.
Harris JW, Jones JP, Martin JL, LaRosa AC, Olson MJ, Pohl LR, Anders
MW (1992) Pentahaloethane-based chlorofluorocarbon substitutes and
halothane: correlation of in vivo hepatic protein
trifluoroacetylation and urinary trifluoroacetic acid excretion with
calculated enthalpies of activation. Chemical research
in toxicology, 5:720-725.
Hayman GD, Jenkin ME, Murrells TP, Johnson CE (1994) Tropospheric
degradation chemistry of HCFC-123 (CF3CHCl2): a proposed
replacement chlorofluorocarbon. Atmospheric environment, 28:421-437.
Henry JE (1975) Acute oral test on FC-123. Newark, DE, Du Pont de
Nemours and Co. [cited in NICNAS, 1996].
Hoet P, Graf MLM, Bourdi M, Pohl LR, Duray PH, Chen W, Peter RM,
Nelson SD, Verlinden N, Lison D (1997) Epidemic of liver disease
caused by hydrochlorofluorocarbons used as ozone-sparing substitutes
of chlorofluorocarbons. Lancet, 350:556-559.
Hofmann T (1995) HCFC 123, HCFC 141B and HCF 134a, testing for
subacute (2 weeks) inhalation toxicity in male and female Sprague
Dawley rats. Frankfurt am Main, Hoechst Aktiengesellschaft.
Hughes EW (1994) HCFC 123: A study of the effect on reproductive
function of two generations in the rat. Huntingdon, Cambridgeshire,
Huntingdon Research Centre Ltd.
Huwyler J, Gut J (1992) Exposure to the chlorofluorocarbon substitute
2,2-dichloro-1,1,1-trifluoroethane and the anaesthetic agent halothane
is associated with transient adduct formation in the heart.
Biochemical and biophysical research communications, 184:1344-1349.
Huwyler J, Aeschlimann D, Christen U, Gut J (1992) The kidney as a
novel target tissue for protein adduct formation associated with
metabolism of halothane and the candidate chlorofluorocarbon
replacement 2,2-dichloro-1,1,1-trifluoroethane. European journal of
biochemistry, 207:229-238.
IARC (1995) Peroxisome proliferation and its role in carcinogenesis:
views and expert opinions of an IARC working group, Lyon, 7-11
December 1994. Lyon, International Agency for Research on Cancer.
ICI (1992) An evaluation in the in vitro cytogenetic assay using
human lymphocytes. Macclesfield, Cheshire, Imperial Chemical
Industries Ltd. [cited in AIHA, 1998].
Industrial Bio-Test Laboratories (1977) 90-day subacute inhalation
toxicity study with Genetron 123 in albino rats. Northbrook, IL,
Industrial Bio-Test Laboratories, Inc.
IPCS (1990) Fully halogenated chlorofluorocarbons. Geneva, World
Health Organization, International Programme on Chemical Safety
(Environmental Health Criteria 113).
IPCS (1991) Partially halogenated chlorofluorocarbons (methane
derivatives). Geneva, World Health Organization, International
Programme on Chemical Safety (Environmental Health Criteria 126).
IPCS (1992) Partially halogenated chlorofluorocarbons (ethane
derivatives). Geneva, World Health Organization, International
Programme on Chemical Safety (Environmental Health Criteria 139).
IPCS (1998) International Chemical Safety Card
-- 2,2-Dichloro-1,1,1-trifluoroethane. Geneva, World Health
Organization, International Programme on Chemical Safety (ICSC 1343).
IPCS (1994) Assessing human health risks of chemicals: Derivation of
guidance values for health-based exposure limits. Geneva, World
Health Organization, International Programme on Chemical Safety
(Environmental Health Criteria 170).
Jamison KC, Larson JL, Butterworth BE, Harden R, Skinner BL, Wolf DC
(1996) A non-bile duct origin for intestinal crypt-like ducts with
peritubular fibrosis induced in livers of F344 rats by chloroform
inhalation. Carcinogenesis, 17:675-682.
Jenkins CA (1992a) HCFC-123 (liquid): Biotic degradation closed
bottle test. Eye, Suffolk, Life Sciences Research Ltd. (No.
91/PFE008/0477).
Jenkins CA (1992b) HCFC-123: Acute toxicity to rainbow trout. Eye,
Suffolk, Life Sciences Research Ltd. (No. 91/PFE004/0939).
Jenkins CA (1992c) HCFC-123: Acute toxicity to Daphnia magna. Eye,
Suffolk, Life Sciences Research Ltd. (No. 91/PFE006/0972).
Jenkins CA (1992d) HCFC-123: Determination of its EC50 to
Selenastrum capricornutum. Eye, Suffolk, Life Sciences Research Ltd
(No. 91/PFE007/0935).
Keller DA, Lieder PH, Brock WJ, Cook JC (1998)
1,1,1-Trifluoro-2,2-dichloroethane (HCFC-123) and
1,1,1-trifluoro-2-bromo-2-chloroethane (halothane) cause similar
biochemical effects in rats exposed by inhalation for five days.
Drug and chemical toxicology, 21:405-415.
Kelly DP (1989) Four-week inhalation toxicity study with HCFC-123
in rats. Newark, DE, Du Pont de Nemours and Co.
Kennelly JC (1993) HCFC 123: Assessment for the introduction of
unscheduled DNA synthesis in rat liver after inhalation exposure.
Macclesfield, Cheshire, Zeneca Ltd.
Kotamarthi VR, Rodriguez JM, Ko MKW, Tromp TK, Sze ND, Prather MJ
(1998) Trifluoroacetic acid from degradation of HCFCs and HFCs -- a
three-dimensional modelling study. Journal of geophysical research,
103:5747-5758.
Le Marchand L, Wilkinson GR, Wilkens LR (1999) Genetic and dietary
predictors of CYP2E1 activity: a phenotyping study in Hawaii Japanese
using chlorzoxazone. Cancer epidemiology, biomarkers and prevention,
8:495-500.
Lewis RW (1990) 28-day inhalation study to assess changes in rat
liver and plasma. Macclesfield, Cheshire, Imperial Chemical
Industries Ltd.
Lind RC, Gandolfi AJ, Hall PM (1995) Biotransformation and
hepatotoxicity of HCFC-123 in the guinea pig: Potentiation of hepatic
injury by prior glutathione depletion. Toxicology and applied
pharmacology, 134:175-181.
Loizou GD, Urban G, Dekant W, Anders MW (1994) Gas-uptake
pharmacokinetics of 2,2-dichloro-1,1,1-trifluoroethane (HCFC-123).
Drug metabolism and disposition, 22:511-517.
Longstaff E, Robinson M, Bradbrook C, Styles JA, Purchase IF (1984)
Genotoxicity and carcinogenicity of fluorocarbons: Assessment by
short-term in vitro tests and chronic exposure in rats. Toxicology
and applied pharmacology, 72:15-31.
Malinverno G, Rusch GM, Millischer RJ, Hughes EW, Schroeder RE, Coombs
DW (1996) Inhalation teratology and reproduction studies with
1,1-dichloro-2,2,2-trifluoroethane (HCFC-123). Fundamental and
applied toxicology, 23:276-287.
Malley LA (1990) Subchronic inhalation toxicity: 90-day study with
HCFC-123 in rats. Newark, DE, Du Pont de Nemours and Co.
Malley LA (1992) Combined chronic toxicity/oncogenicity study with
HCFC-123: Two-year inhalation toxicity study in rats. Newark, DE, Du
Pont de Nemours and Co.
Malley LA, Carakostas M, Hansen JF, Rusch GM, Kelly DP, Trochimowicz
HJ (1995) Two-year inhalation toxicity study in rats with
hydrochlorofluorocarbon 123. Fundamental and applied toxicology,
25:101-114.
Malley LA, Carakostas M, Elliott GS, Alvarez L, Schroeder RE, Frame
SR, Van Pelt C, Trochimowicz HJ, Rusch GM (1996) Subchronic toxicity
and teratogenicity of 2-chloro-1,1,1,2-tetrafluoroethane (HCFC-124).
Fundamental and applied toxicology, 32:11-22.
Marit GB, Dodd DE, George ME, Vinegar A (1994) Hepatotoxicity in
guinea pigs following acute exposure to
1,1-dichloro-2,2,2-trifluoroethane. Toxicologic pathology,
22(4):404-414.
Marshal RR (1992) Evaluation of chromosome aberration frequencies in
cultured peripheral blood lymphocytes from rats treated with
HCFC-123. Harrogate, Yorkshire, Hazleton Microtest.
MRI (1991) Results of employee exposure monitoring for HCFC-123 at
centrifugal chiller installations. Final report submitted to the US
Environmental Protection Agency. Silver Spring, MD, Meridian Research,
Inc.
MRI (1993a) Assessment of firefighter exposure to HCFC-123 during
extinguishant efficiency tests conducted at the United States Naval
Air Station in Beaufort, South Carolina. Draft report submitted to
the US Environmental Protection Agency. Silver Spring, MD, Meridian
Research, Inc.
MRI (1993b) Assessment of firefighter exposure to HCFC-123 during
fire extinguisher use in aircraft hangars. Draft report submitted to
American Pacific Corporation. Silver Spring, MD, Meridian Research,
Inc.
Muller W, Hofmann T (1988) HCFC-123 -- micronucleus test in male and
female NMRI mice after inhalation. Frankfurt am Main, Hoechst
Aktiengesellschaft.
Mullin LS (1976) Behavioural toxicity testing of Fluorocarbon 123
in rats. Newark, DE, Du Pont de Nemours and Co.
NAS (1996) Toxicity of alternatives to chlorofluorocarbons: HFC-134a
and HCFC-123. Washington, DC, National Academy of Sciences, National
Academy Press.
NICNAS (1996) Priority Existing Chemical No. 4 --
2,2-Dichloro-1,1,1-trifluoroethane (HCFC-123), full public report,
National Industrial Chemicals Notification and Assessment Scheme.
Canberra, Australian Government Publishing Service.
NICNAS (1999) 2,2-Dichloro-1,1,1-trifluoroethane (HCFC-123):
Secondary Notification No. 4S, full public report. National
Industrial Chemicals Notification and Assessment Scheme. Sydney,
National Occupational Health and Safety Commission.
OECD (1998) Agreed harmonized integrated hazard classification
system for human health and environmental effects of chemical
substances. Paris, Organisation for Economic Co-operation and
Development, Environmental Health and Safety Division.
Oremland RS, Lonergan DJ, Culbertson CW, Lovley DR (1996) Microbial
degradation of hydrofluorocarbons (CHCl2F and CHCl2CF3) in soils
and sediments. Applied and environmental microbiology,
62(5):1818-1821.
Pierson K (1990a) Flow-through acute 96 hour LC50 of HCFC-123 in
fathead minnows (Pimephales promelas ). Newark, DE, Du Pont de
Nemours and Co.
Pierson K (1990b) Static acute 48 hour LC50 of HCFC-123 in Daphnia
magna . Newark, DE, Du Pont de Nemours and Co.
Rusch GM, Trochimowitz HJ, Malley LJ, Kelly DP, Peckham J, Hansen J,
Charm JB (1994) Subchronic inhalation toxicity studies with
hydrochlorofluorocarbon 123 (HCFC 123). Fundamental and applied
toxicology, 23:169-178.
Sandow J, Jerabek-Sandow G, Fenner-Nau D (1995a) Effect of
fluorocarbons on pituitary-gonadal function in a 14-day inhalation
toxicity study: HCFC 123 (Frigen), HCFC 141b (difluorchlorethane)
and HFC 134a (tetrafluorethane). Frankfurt am Main, Hoechst
Aktiengesellschaft.
Sandow J, Rechenberg W, Jerabek-Sandow G (1995b) Effect of HCFC-123
on androgen biosynthesis and gonadotropin secretion in rats.
Frankfurt am Main, Hoechst Aktiengesellschaft.
Sibley H (1992) A study for determining refrigerant exposure levels
while servicing an HCFC-123 centrifugal chiller. Syracuse, NY,
Carrier Corporation.
Slauter RW (1997) HCFC 123: Inhalation study in pregnant monkeys to
assess milk transfer and composition following postpartum exposure.
Mattawan, MI, MPI Research.
Takebayashi T, Kabe I, Endo Y, Tanaka S, Miyauchi H, Nozi K, Takahashi
K, Omae K (1998a) Acute liver dysfunction among workers exposed to
2,2-dichloro-1,1,1-trifluoroethane (HCFC-123): A case report.
Journal of occupational health, 40:169-170.
Takebayashi T, Kabe I, Endo Y, Tanaka S, Miyauchi H, Nozi K, Imamiya
S, Takahashi K, Omae K (1998b) Exposure to
2,2-dichloro-1,1,1-trifluoroethane (HCFC-123): A causal inference.
Journal of occupational health, 40:334-338.
Tanaka S, Kabe I, Takebayashi T, Endo Y, Miyauchi H, Nozi K, Takahashi
K, Seki Y, Omae K (1998) Environmental and biological monitoring of
2,2-dichloro-1,1,1-trifluoroethane (HCFC-123). Journal of
occupational health, 40:348-349.
Trane Company (1991) Report on testing and analysis of the
concentration of HCFC-123 in field installations with general
machinery rooms containing hermetic centrifugal chillers. La Crosse,
WI, The Trane Company.
Trane Company (1992) Report of worker exposure to HCFC-123 during
servicing of hermetic centrifugal chillers. La Crosse, WI, The Trane
Company.
Trochimowicz HJ, Mullin LS (1973) Cardiac sensitization potential
(EC50) of trifluoro-dichloroethane. Newark, DE, Du Pont de Nemours
and Co.
UNEP (1999) Ozone treaties. <http://www.unep.org/ozone/
treaties.htm>. United Nations Environment Programme.
Urban G, Dekant W (1994) Metabolism of
1,1-dichloro-2,2,2-trifluoroethane in rats. Xenobiotica, 24:881-892.
Urban G, Speerschneider P, Dekant W (1994) Metabolism of the
chlorofluorocarbon substitute 1,1-dichloro-2,2,2-trifluoroethane by
rat and human liver microsomes: the role of cytochrome P450 2E1.
Chemical research in toxicology, 7:170-176.
Vinegar A, Williams RJ, Fisher JW, McDougal JN (1994) Dose-dependent
metabolism of 2,2-dichloro-1,1,1-trifluoroethane: A physiologically
based pharmacokinetic model in the male Fischer 344 rat. Toxicology
and applied pharmacology, 129:103-113.
Wang Y, Olson MJ, Baker MT (1993) Interaction of fluoroethane
chlorofluorocarbon (CFC) substitutes with microsomal cytochrome P450.
Biochemical pharmacology, 46:87-94.
Warheit DB (1993) Mechanistic studies with HCFC-123. Newark, DE, Du
Pont de Nemours and Co.
Williams RJ, Vinegar A, McDougal JN, Jarabek AM, Fisher JW (1996) Rat
to human extrapolation of HCFC-123 kinetics deduced from halothane
kinetics -- a corollary approach to physiologically based
pharmacokinetic modeling. Fundamental and applied toxicology,
30:55-66.
WMO (1995) Scientific assessment of ozone depletion. Geneva, World
Meteorological Organization (Global Ozone Research and Monitoring
Project Report No. 37).
Wujcik CE, Zehavi D, Seiber JN (1998) Trifluoroacetic acid levels in
1994-1996 fog, rain, snow and surface waters from California and
Nevada. Chemosphere, 36:1233-1245.
APPENDIX 1 -- SOURCE DOCUMENTS
NICNAS (1996): Priority Existing Chemical No. 4 --
2,2-Dichloro-1,1,1-trifluoroethane (HCFC-123), full public report,
National Industrial Chemicals Notification and Assessment Scheme
Copies of the NICNAS (1996) report on HCFC-123 (prepared by S.
Batt, L. Onyon, L. Slosu, and D. Willcocks) may be obtained from:
NICNAS
Existing Chemicals
GPO Box 58
Sydney NSW 2001
Australia
NICNAS reports are prepared to meet the requirements of the
Industrial Chemicals Notification and Assessment Act, 1989, as
amended. In the preparation of the assessment report, both internal
and external peer reviews are undertaken. Under the NICNAS
legislation, applicants for the assessment of a chemical (i.e.,
importers and manufacturers of a chemical) may apply for variations to
the draft report. The following companies and industry associations
participated in the review of the assessment at this stage:
Association of Fluorocarbon Consumers and Manufacturers, Elf Atochem
(Australia) Pty Ltd, Lovelock Luke Pty Ltd, and North American Fire
Guardian Technology (Australia) Pty Ltd. The report was also open for
public comment.
NICNAS (1999): 2,2-Dichloro-1,1,1-trifluoroethane (HCFC-123):
Secondary Notification No. 4S, full public report. National
Industrial Chemicals Notification and Assessment Scheme
Copies of the NICNAS (1999) report on HCFC-123 (prepared by S.
Batt, S. Kristensen, and C. Lee-Steere) may be obtained from:
NICNAS
Existing Chemicals
GPO Box 58
Sydney NSW 2001
Australia
NICNAS reports are prepared to meet the requirements of the
Industrial Chemicals Notification and Assessment Act, 1989, as
amended. In the preparation of the assessment report, both internal
and external peer reviews are undertaken. Under the NICNAS
legislation, applicants for the reassessment of a chemical (i.e.,
importers and manufacturers of a chemical) may apply for variations to
the draft report. The following companies participated in the review
of the assessment at this stage: Du Pont (Australia) Pty Ltd, Elf
Atochem (Australia) Pty Ltd, GSA Industries (Australia) Pty Ltd, MSA
(Australia) Pty Ltd, North American Fire Guardian Technology
(Australia) Pty Ltd, and Solvents Australia Pty Ltd. The report was
also open for public comment.
APPENDIX 2 -- CICAD PEER REVIEW
The draft CICAD on HCFC-123 was sent for review to institutions
and organizations identified by IPCS after contact with IPCS National
Contact Points and Participating Institutions, as well as to
identified experts. Comments were received from:
Alexandria University, Faculty of Agriculture, Department of
Pesticide Chemistry, Egypt
AlliedSignal, Department of Toxicology and Risk Assessment,
Health, Safety, Environment and Remediation, USA
Department of Health, Protection of Health Division,
United Kingdom
DuPont Fluoroproducts, Haskell Laboratory for Toxicology and
Industrial Medicine, USA
Federal Institute for Health Protection of Consumers and
Veterinary Medicine, Germany
Glaxo Wellcome Research and Development, Medicines Safety
Evaluation Division, United Kingdom
Health and Safety Executive, United Kingdom
Institut de Recherche en Santé et en Sécurité du Travail du
Québec, Canada
Institute of Terrestrial Ecology, United Kingdom
National Chemicals Inspectorate (KEMI), Sweden
National Institute for Occupational Safety and Health, USA
National Institute of Environmental Health Sciences, National
Institutes of Health, USA
National Institute of Public Health, Centre of Industrial Hygiene
and Occupational Diseases, Czech Republic
Université Catholique de Louvain, Faculté de Médecine, Belgique
US Environmental Protection Agency, Drinking Water Program,
Region VIII, USA
World Health Organization, International Programme on Chemical
Safety, Switzerland
APPENDIX 3 -- CICAD FINAL REVIEW BOARD
Sydney, Australia, 21-24 November 1999
Members
Dr R. Benson, Drinking Water Program, US Environmental Protection
Agency, Region VIII, Denver, CO, USA
Dr T. Berzins, National Chemicals Inspectorate (KEMI), Solna, Sweden
Dr R.M. Bruce, National Center for Environmental Assessment, US
Environmental Protection Agency, Cincinnati, OH, USA
Mr R. Cary, Health and Safety Executive, Merseyside, United Kingdom
Dr R.S. Chhabra, National Institute of Environmental Health Sciences,
National Institutes of Health, Research Triangle Park, NC, USA
Dr S. Chou, Agency for Toxic Substances and Disease Registry, Atlanta,
GA, USA
Dr S. Dobson, Institute of Terrestrial Ecology, Monks Wood,
Cambridgeshire, United Kingdom
Dr H. Gibb, National Center for Environmental Assessment, US
Environmental Protection Agency, Washington, DC, USA
Dr R.F. Hertel, Federal Institute for Health Protection of Consumers
and Veterinary Medicine, Berlin, Germany
Dr J. Kielhorn, Fraunhofer Institute for Toxicology and Aerosol
Research, Hannover, Germany
Dr S. Kristensen, National Occupational Health and Safety Commission
(Worksafe), Sydney, NSW, Australia
Mr C. Lee-Steere, Environment Australia, Canberra, ACT, Australia
Ms M. Meek, Environmental Health Directorate, Health Canada, Ottawa,
Ontario, Canada
Ms F. Rice, National Institute for Occupational Safety and Health,
Cincinnati, OH, USA
Dr J. Sekizawa, National Institute of Health Sciences, Tokyo, Japan
Dr D. Willcocks, National Industrial Chemicals Notification and
Assessment Scheme (NICNAS), Sydney, NSW, Australia (Chairperson)
Professor P. Yao, Institute of Occupational Medicine, Chinese Academy
of Preventive Medicine, Beijing, People's Republic of China
Observers
Mr P. Howe, Institute of Terrestrial Ecology, Huntingdon,
Cambridgeshire, United Kingdom
Dr K. Ziegler-Skylakakis, GSF-Forschungszentrum für Umwelt und
Gesundheit, GmbH, Oberschleissheim, Germany
Secretariat
Dr A. Aitio, International Programme on Chemical Safety, World Health
Organization, Geneva, Switzerland
Ms M. Godden, Health and Safety Executive, Bootle, Merseyside, United
Kingdom
Dr M. Younes, International Programme on Chemical Safety, World Health
Organization, Geneva, Switzerland
APPENDIX 4 -- INTERNATIONAL CHEMICAL SAFETY CARD
2,2-DICHLORO-1,1,1-TRIFLUOROETHANE ICSC: 1343
November 1998
CAS# 