
INTERNATIONAL PROGRAMME ON CHEMICAL SAFETY
CONCISE INTERNATIONAL CHEMICAL ASSESSMENT DOCUMENT NO. 7
o-TOLUIDINE
INTER-ORGANIZATION PROGRAMME FOR THE SOUND MANAGEMENT OF CHEMICALS
A cooperative agreement among UNEP, ILO, FAO, WHO, UNIDO, UNITAR and
OECD
This report contains the collective views of an international group of
experts and does not necessarily represent the decisions or the stated
policy of the United Nations Environment Programme, the International
Labour Organisation, or the World Health Organization.
First draft prepared by
Dr N. Gregg, Health & Safety Executive, Liverpool, United Kingdom,
Dr S. Dobson, Institute of Terrestrial Ecology, Cambridgeshire, United
Kingdom, and
Mr R. Cary, Health & Safety Executive, Liverpool, United Kingdom
Published under the joint sponsorship of the United Nations
Environment Programme, the International Labour Organisation, and the
World Health Organization, and produced within the framework of the
Inter-Organization Programme for the Sound Management of Chemicals.
World Health Organization Geneva, 1998
The International Programme on Chemical Safety (IPCS),
established in 1980, is a joint venture of the United Nations
Environment Programme (UNEP), the International Labour Organisation
(ILO), and the World Health Organization (WHO). The overall
objectives of the IPCS are to establish the scientific basis for
assessment of the risk to human health and the environment from
exposure to chemicals, through international peer review processes, as
a prerequisite for the promotion of chemical safety, and to provide
technical assistance in strengthening national capacities for the
sound management of chemicals.
The Inter-Organization Programme for the Sound Management of
Chemicals (IOMC) was established in 1995 by UNEP, ILO, the Food and
Agriculture Organization of the United Nations, WHO, the United
Nations Industrial Development Organization, and the Organisation for
Economic Co-operation and Development (Participating Organizations),
following recommendations made by the 1992 UN Conference on
Environment and Development to strengthen cooperation and increase
coordination in the field of chemical safety. The purpose of the IOMC
is to promote coordination of the policies and activities pursued by
the Participating Organizations, jointly or separately, to achieve the
sound management of chemicals in relation to human health and the
environment.
WHO Library Cataloguing in Publication Data
o-Toluidine.
(Concise international chemical assessment document ; 7)
1.Toluidines - toxicity 2.Toluidines - adverse effects
3.Occupational exposure I.International Programme on Chemical
Safety II.Series
ISBN 92 4 153007 3 (NLM Classification: QV 235)
ISSN 1020-6167
The World Health Organization welcomes requests for permission to
reproduce or translate its publications, in part or in full.
Applications and enquiries should be addressed to the Office of
Publications, World Health Organization, Geneva, Switzerland, which
will be glad to provide the latest information on any changes made to
the text, plans for new editions, and reprints and translations
already available.
(c) World Health Organization 1998
Publications of the World Health Organization enjoy copyright
protection in accordance with the provisions of Protocol 2 of the
Universal Copyright Convention. All rights reserved.
The designations employed and the presentation of the material in
this publication do not imply the expression of any opinion whatsoever
on the part of the Secretariat of the World Health Organization
concerning the legal status of any country, territory, city, or area
or of its authorities, or concerning the delimitation of its frontiers
or boundaries.
The mention of specific companies or of certain manufacturers'
products does not imply that they are endorsed or recommended by the
World Health Organization in preference to others of a similar nature
that are not mentioned. Errors and omissions excepted, the names of
proprietary products are distinguished by initial capital letters.
TABLE OF CONTENTS
FOREWORD
1. EXECUTIVE SUMMARY
2. IDENTITY AND PHYSICAL/CHEMICAL PROPERTIES
3. ANALYTICAL METHODS
4. SOURCES OF HUMAN AND ENVIRONMENTAL EXPOSURE
5. ENVIRONMENTAL TRANSPORT, DISTRIBUTION, AND TRANSFORMATION
6. ENVIRONMENTAL LEVELS AND HUMAN EXPOSURE
6.1. Environmental levels
6.2. Human exposure
7. COMPARATIVE KINETICS AND METABOLISM IN LABORATORY ANIMALS AND HUMANS
8. EFFECTS ON LABORATORY MAMMALS AND IN VITRO TEST SYSTEMS
8.1. Single exposure
8.2. Irritation and sensitization
8.3. Short-term exposure
8.4. Chronic exposure and carcinogenicity
8.5. Genotoxicity and related end-points
8.6. Reproductive and developmental toxicity
8.7. Immunological and neurological effects
9. EFFECTS ON HUMANS
10. EFFECTS ON OTHER ORGANISMS IN THE LABORATORY AND FIELD
11. EFFECTS EVALUATION
11.1. Evaluation of health effects
11.1.1. Hazard identification and dose-response assessment
11.1.2. Criteria for setting guidance values for o-toluidine
11.1.3. Sample risk characterization
11.2. Evaluation of environmental effects
12. PREVIOUS EVALUATIONS BY INTERNATIONAL BODIES
13. HUMAN HEALTH PROTECTION AND EMERGENCY ACTION
13.1. Human health hazards
13.2. Advice to physicians
13.3. Health surveillance advice
13.4. Spillage
14. CURRENT REGULATIONS, GUIDELINES, AND STANDARDS
INTERNATIONAL CHEMICAL SAFETY CARD
REFERENCES
APPENDIX 1 - SOURCE DOCUMENT
APPENDIX 2 - CICAD PEER REVIEW
APPENDIX 3 - CICAD FINAL REVIEW BOARD
RÉSUMÉ D'ORIENTATION
RESUMEN DE ORIENTACION
FOREWORD
Concise International Chemical Assessment Documents (CICADs) are
the latest in a family of publications from the International
Programme on Chemical Safety (IPCS) - a cooperative programme of the
World Health Organization (WHO), the International Labour Organisation
(ILO), and the United Nations Environment Programme (UNEP). CICADs
join the Environmental Health Criteria documents (EHCs) as
authoritative documents on the risk assessment of chemicals.
CICADs are concise documents that provide summaries of the
relevant scientific information concerning the potential effects of
chemicals upon human health and/or the environment. They are based on
selected national or regional evaluation documents or on existing
EHCs. Before acceptance for publication as CICADs by IPCS, these
documents have undergone extensive peer review by internationally
selected experts to ensure their completeness, accuracy in the way in
which the original data are represented, and the validity of the
conclusions drawn.
The primary objective of CICADs is characterization of hazard and
dose-response from exposure to a chemical. CICADs are not a summary
of all available data on a particular chemical; rather, they include
only that information considered critical for characterization of the
risk posed by the chemical. The critical studies are, however,
presented in sufficient detail to support the conclusions drawn. For
additional information, the reader should consult the identified
source documents upon which the CICAD has been based.
Risks to human health and the environment will vary considerably
depending upon the type and extent of exposure. Responsible
authorities are strongly encouraged to characterize risk on the basis
of locally measured or predicted exposure scenarios. To assist the
reader, examples of exposure estimation and risk characterization are
provided in CICADs, whenever possible. These examples cannot be
considered as representing all possible exposure situations, but are
provided as guidance only. The reader is referred to EHC 1701 for
advice on the derivation of health-based guidance values.
1 International Programme on Chemical Safety (1994) Assessing
human health risks of chemicals: derivation of guidance values
for health-based exposure limits. Geneva, World Health Organization
(Environmental Health Criteria 170).
While every effort is made to ensure that CICADs represent the
current status of knowledge, new information is being developed
constantly. Unless otherwise stated, CICADs are based on a search of
the scientific literature to the date shown in the executive summary.
In the event that a reader becomes aware of new information that would
change the conclusions drawn in a CICAD, the reader is requested to
contact the IPCS to inform it of the new information.
Procedures
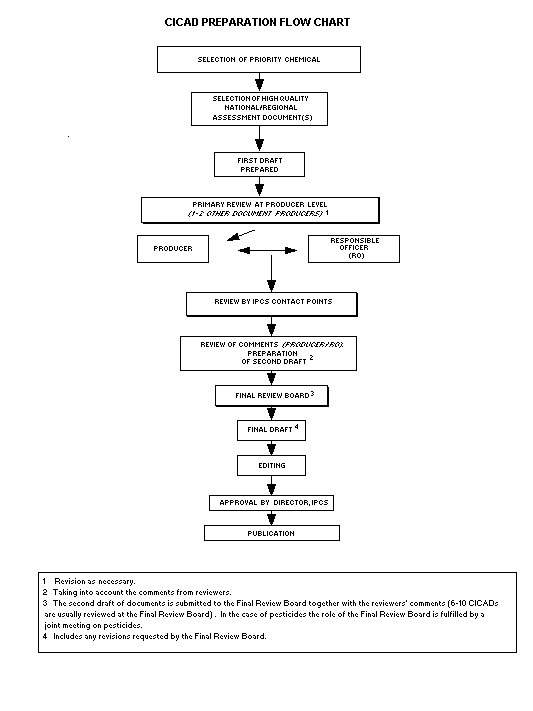
The flow chart shows the procedures followed to produce a CICAD.
These procedures are designed to take advantage of the expertise that
exists around the world - expertise that is required to produce the
high-quality evaluations of toxicological, exposure, and other data
that are necessary for assessing risks to human health and/or the
environment.
The first draft is based on an existing national, regional, or
international review. Authors of the first draft are usually, but not
necessarily, from the institution that developed the original review.
A standard outline has been developed to encourage consistency in
form. The first draft undergoes primary review by IPCS and one or
more experienced authors of criteria documents to ensure that it meets
the specified criteria for CICADs.
The second stage involves international peer review by scientists
known for their particular expertise and by scientists selected from
an international roster compiled by IPCS through recommendations from
IPCS national Contact Points and from IPCS Participating Institutions.
Adequate time is allowed for the selected experts to undertake a
thorough review. Authors are required to take reviewers' comments
into account and revise their draft, if necessary. The resulting
second draft is submitted to a Final Review Board together with the
reviewers' comments.
The CICAD Final Review Board has several important functions:
- to ensure that each CICAD has been subjected to an appropriate
and thorough peer review;
- to verify that the peer reviewers' comments have been addressed
appropriately;
- to provide guidance to those responsible for the preparation of
CICADs on how to resolve any remaining issues if, in the opinion
of the Board, the author has not adequately addressed all
comments of the reviewers; and
- to approve CICADs as international assessments.
Board members serve in their personal capacity, not as representatives
of any organization, government, or industry. They are selected
because of their expertise in human and environmental toxicology or
because of their experience in the regulation of chemicals. Boards
are chosen according to the range of expertise required for a meeting
and the need for balanced geographic representation.
Board members, authors, reviewers, consultants, and advisers who
participate in the preparation of a CICAD are required to declare any
real or potential conflict of interest in relation to the subjects
under discussion at any stage of the process. Representatives of
nongovernmental organizations may be invited to observe the
proceedings of the Final Review Board. Observers may participate in
Board discussions only at the invitation of the Chairperson, and they
may not participate in the final decision-making process.
 1. EXECUTIVE SUMMARY
This CICAD on ortho-toluidine (o-toluidine) was based on a
review of primarily occupational human health concerns, prepared by
the United Kingdom Health & Safety Executive (Gregg et al., 1996) and
covering data identified up to March 1992. Additional information
identified during the international peer review of this CICAD and
following consideration by the Final Review Board has been
incorporated as appropriate. Information on the preparation and peer
review of the source document is presented in Appendix 1. Information
on the peer review of this CICAD is presented in Appendix 2. This
CICAD was approved for publication at a meeting of the Final Review
Board, held in Brussels, Belgium, on 18-20 November 1996.
Participants at the Final Review Board meeting are listed in Appendix
3. The International Chemical Safety Card (ICSC 0341) for
o-toluidine, produced by the International Programme on Chemical
Safety (IPCS, 1993), has also been reproduced in this document.
o-Toluidine (CAS no. 95-53-4) is a synthetic chemical that is a
light yellow liquid at ambient temperature. It is used primarily in
the manufacture of dyestuffs, although it is also used in the
production of rubber, chemicals, and pesticides and as a curing agent
for epoxy resin systems.
o-Toluidine is of moderate to low acute toxicity and has the
potential to produce minimal skin irritation and mild eye irritation.
Information is not available on the skin or respiratory sensitization
potential of o-toluidine. The principal signs of toxicity following
acute and short-term exposure to this chemical are methaemoglobinaemia
and related effects in the spleen. These effects have been observed
in rats administered o-toluidine at 225 mg/kg body weight per day
for 5 days; a no-observed-adverse-effect level has not been
identified.
In several carcinogenicity studies in which o-toluidine was
administered orally to rats and mice, there was a significant increase
in the incidence of benign and malignant tumours in various tissues.
o-Toluidine is generally not mutagenic in standard bacterial
mutagenicity tests, but it is clastogenic in mammalian cells
in vitro. There is uncertainty concerning the genotoxicity of
o-toluidine in vivo; however, some positive results have been
reported. Based upon the wide distribution of tumours observed in
o-toluidine-exposed animals, as well as the clastogenic activity
observed in mammalian in vitro assays, o-toluidine may be acting
as a genotoxic carcinogen. Information relevant to assessing the
risks of reproductive or developmental effects of o-toluidine was
not identified.
Owing to the lack of relevant data on exposure, it was not
possible to assess risks to human health associated with indirect
exposure to o-toluidine present in the general environment. In the
occupational environment, there is the potential for significant risks
of carcinogenic and genotoxic effects. Useful data on concentrations
of o-toluidine in various environmental media and on its effects on
aquatic and terrestrial organisms were not identified, and therefore
it was not possible to assess the risks of exposure of environmental
organisms to o-toluidine.
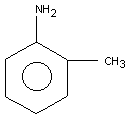
2. IDENTITY AND PHYSICAL/CHEMICAL PROPERTIES
o-Toluidine (CAS no. 95-53-4; C7H9N; 1-amino-2-methylbenzene,
2-aminotoluene, o-methylaniline), a synthetic chemical described as
having an "aromatic" odour, exists at ambient temperature as a light
yellow liquid that rapidly darkens on exposure to air and light.
o-Toluidine has a boiling point of 200°C, a melting point of -16°C,
and a vapour pressure of 0.2 kPa at 20°C. o-Toluidine is completely
miscible with ethanol and diethyl ether; its solubility in water is
poor. Additional physical/chemical properties are presented in the
International Chemical Safety Card reproduced in this document. The
structural formula for o-toluidine is:
1. EXECUTIVE SUMMARY
This CICAD on ortho-toluidine (o-toluidine) was based on a
review of primarily occupational human health concerns, prepared by
the United Kingdom Health & Safety Executive (Gregg et al., 1996) and
covering data identified up to March 1992. Additional information
identified during the international peer review of this CICAD and
following consideration by the Final Review Board has been
incorporated as appropriate. Information on the preparation and peer
review of the source document is presented in Appendix 1. Information
on the peer review of this CICAD is presented in Appendix 2. This
CICAD was approved for publication at a meeting of the Final Review
Board, held in Brussels, Belgium, on 18-20 November 1996.
Participants at the Final Review Board meeting are listed in Appendix
3. The International Chemical Safety Card (ICSC 0341) for
o-toluidine, produced by the International Programme on Chemical
Safety (IPCS, 1993), has also been reproduced in this document.
o-Toluidine (CAS no. 95-53-4) is a synthetic chemical that is a
light yellow liquid at ambient temperature. It is used primarily in
the manufacture of dyestuffs, although it is also used in the
production of rubber, chemicals, and pesticides and as a curing agent
for epoxy resin systems.
o-Toluidine is of moderate to low acute toxicity and has the
potential to produce minimal skin irritation and mild eye irritation.
Information is not available on the skin or respiratory sensitization
potential of o-toluidine. The principal signs of toxicity following
acute and short-term exposure to this chemical are methaemoglobinaemia
and related effects in the spleen. These effects have been observed
in rats administered o-toluidine at 225 mg/kg body weight per day
for 5 days; a no-observed-adverse-effect level has not been
identified.
In several carcinogenicity studies in which o-toluidine was
administered orally to rats and mice, there was a significant increase
in the incidence of benign and malignant tumours in various tissues.
o-Toluidine is generally not mutagenic in standard bacterial
mutagenicity tests, but it is clastogenic in mammalian cells
in vitro. There is uncertainty concerning the genotoxicity of
o-toluidine in vivo; however, some positive results have been
reported. Based upon the wide distribution of tumours observed in
o-toluidine-exposed animals, as well as the clastogenic activity
observed in mammalian in vitro assays, o-toluidine may be acting
as a genotoxic carcinogen. Information relevant to assessing the
risks of reproductive or developmental effects of o-toluidine was
not identified.
Owing to the lack of relevant data on exposure, it was not
possible to assess risks to human health associated with indirect
exposure to o-toluidine present in the general environment. In the
occupational environment, there is the potential for significant risks
of carcinogenic and genotoxic effects. Useful data on concentrations
of o-toluidine in various environmental media and on its effects on
aquatic and terrestrial organisms were not identified, and therefore
it was not possible to assess the risks of exposure of environmental
organisms to o-toluidine.
2. IDENTITY AND PHYSICAL/CHEMICAL PROPERTIES
o-Toluidine (CAS no. 95-53-4; C7H9N; 1-amino-2-methylbenzene,
2-aminotoluene, o-methylaniline), a synthetic chemical described as
having an "aromatic" odour, exists at ambient temperature as a light
yellow liquid that rapidly darkens on exposure to air and light.
o-Toluidine has a boiling point of 200°C, a melting point of -16°C,
and a vapour pressure of 0.2 kPa at 20°C. o-Toluidine is completely
miscible with ethanol and diethyl ether; its solubility in water is
poor. Additional physical/chemical properties are presented in the
International Chemical Safety Card reproduced in this document. The
structural formula for o-toluidine is:
 Although some toxicological studies on o-toluidine have employed its
hydrochloride salt (o-toluidine hydrochloride), this is unlikely to
significantly alter the observed health effects of the parent
chemical.
3. ANALYTICAL METHODS
Short- and long-term personal monitoring can be undertaken by
pumped sampling either through acid-coated filters or through
NIOSH-type silica gel tubes (NIOSH, 1987; HSE, 1993). The filters
are desorbed with a neutralizing solution and analysed by
high-performance liquid chromatography; the tubes are desorbed with
solvent and analysed by gas chromatography. Screening measurements
may be conducted using a colorimetric detector tube.
The analytical monitoring of urine for o-toluidine and its
metabolites may be a useful means of assessing occupational exposure,
especially where there is potential for skin absorption. Methods for
biological monitoring of occupationally exposed individuals have been
reported (Brown et al., 1995; Ward et al., 1996). These techniques
employ urine sampling for the determination of o-toluidine and its
N-acetyl metabolites and blood sampling for the detection of
o-toluidine-haemoglobin adducts.
The determination of o-toluidine in water samples may involve
extraction under acidic and alkali conditions, followed by brominated
ether extraction and subsequent analysis using gas chromatography with
electron capture detection (detection limit 0.1-0.6 µg/litre). The
analysis of o-toluidine in sediment may involve steam distillation
under alkali conditions with quantitation by gas chromatography
coupled with electron capture detection (detection limit 0.002-0.012
µg/g dry matter).
4. SOURCES OF HUMAN AND ENVIRONMENTAL EXPOSURE
The principal use of o-toluidine worldwide is in the
manufacture of dyestuffs, although it is also used in the production
of rubber, chemicals, and pesticides and as a curing agent for epoxy
resin systems. o-Toluidine is also used as a corrosion inhibitor in
paint formulations and possibly has limited uses in analytical
laboratory procedures. There are no known domestic or household uses
for o-toluidine.
Global production data for o-toluidine were not identified. In
the USA in 1975, more than 900 t of the chemical were produced, and
another 1000 t were imported. Total production of o-toluidine in
Great Britain is approximately 6000 t per year, 90% of which is
exported. Approximately 610 and 545 t of o-toluidine were imported
into Japan in 1992 and 1993, respectively.
5. ENVIRONMENTAL TRANSPORT, DISTRIBUTION, AND TRANSFORMATION
Using an equilibrium model to assess partitioning between
environmental media, Yoshida et al. (1983) estimated the distribution
of o-toluidine to be 14.5% to air, 83.3% to water, 0.4% to soil,
1.9% to sediment, 2.3 × 10-5 % to biota (fish), and 0.21% to suspended
sediment. The half-life for o-toluidine in Rhine River water was
estimated to be about 1 day (Zoeteman et al., 1980).
6. ENVIRONMENTAL LEVELS AND HUMAN EXPOSURE
6.1 Environmental levels
o-Toluidine was detected in 3/46 samples of Rhine River water
collected at the Germany-Netherlands border in 1979; the mean and
maximum concentrations were 0.03 and 1.8 µg/litre, respectively
(Wegman & de Korte, 1981). o-Toluidine was detected in water
collected from a shallow aquifer contaminated by coal tar wastes in
the USA; however, concentrations were not reported (Pereira et al.,
1983).
In Japan, 8/68 samples of surface water collected in 1976
contained o-toluidine (detection limit 0.1-0.6 µg/litre) at levels
ranging from 0.14 to 20 µg/litre; 27 samples of sediment collected in
the same year contained o-toluidine (detection limit 0.002-0.012
mg/kg) at concentrations ranging from 0.002 to 0.013 mg/kg dry weight
(J. Sekizawa, personal communication, 1996). o-Toluidine was not
detected (detection limit 0.05-150 ng/m3) in 72 samples of air
collected in Japan in 1985 (J. Sekizawa, personal communication,
1996).
6.2 Human exposure
Exposure of the general population to o-toluidine present in
the environment could not be estimated, owing to the lack of relevant
data on levels of this chemical in air, drinking-water, and
foodstuffs. Information on human exposure to o-toluidine is limited
to occupational settings.
In the United Kingdom in 1992, approximately 120 individuals were
potentially exposed to o-toluidine during activities involving its
manufacture and use; about a quarter of these were maintenance rather
than process workers. Eight-hour time-weighted-average exposures to
o-toluidine in seven different industries in the United Kingdom
ranged from 0.007 to 2.7 ppm (0.03-11.8 mg/m3); all but one of these
exposures were <0.3 ppm (<1.3 mg/m3). A concentration of 2.7
ppm (11.8 mg/m3) o-toluidine was measured at a site involving
centrifugation at high temperature. If it is assumed that a worker
weighing 70 kg breathes 10 m3 of air during a typical working day and
that inhaled o-toluidine is completely absorbed, exposure to
o-toluidine at a concentration of 1.3 mg/m3 in workplace air can be
estimated to result in a daily o-toluidine intake of approximately
0.2 mg/kg body weight. Exposure to o-toluidine associated with some
processes conducted at elevated temperatures (i.e. exposure to
o-toluidine at 11.8 mg/m3) can be estimated to result in a daily
o-toluidine intake of approximately 1.7 mg/kg body weight. Dermal
exposure to o-toluidine may also occur in the occupational
environment; however, quantitative data were not identified.
7. COMPARATIVE KINETICS AND METABOLISM IN LABORATORY ANIMALS
AND HUMANS
Biological monitoring to assess human exposure to o-toluidine
indicates that absorption may occur through inhalation and dermal
contact; however, quantitative information was not identified.
o-Toluidine binds to haemoglobin (Ward et al., 1996).
N-acetylated metabolites of o-toluidine are eliminated in the
urine (Brown et al., 1995).
In laboratory animals, studies on the kinetics and metabolism of
o-toluidine have involved rats exposed orally or via dermal contact.
There is extensive absorption (at least 92% of the administered oral
dose) of o-toluidine from the gastrointestinal tract (Cheever et
al., 1980). Limited dermal absorption was reported in a poor-quality
study (Senczuk et al., 1984), although the structure of o-toluidine
suggests that, like other aromatic amines, it is lipid soluble and
therefore likely to be readily absorbed through the skin. Oral and
subcutaneous studies have revealed that o-[14C]toluidine and its
metabolites are excreted mainly in the urine, with at least 90% of the
administered radioactivity appearing in the urine within 72 hours
after exposure (Son et al., 1977; Cheever et al., 1980). Small
amounts of radioactivity were also detected in the faeces and exhaled
carbon dioxide. Up to one-third of the o-toluidine administered was
recovered unchanged in the urine. The metabolism of o-toluidine is
characterized principally by ring hydroxylation and N-acetylation,
the major metabolites being 4-amino- m-cresol and, to a lesser
extent, N-acetyl-4-amino- m-cresol (Son et al., 1977; Cheever et
al., 1980). Sulfate conjugates of o-toluidine predominate over
glucuronide conjugates. Binding of o-toluidine metabolites to
haemoglobin has also been observed in rats (Birnier & Neumann, 1988).
8. EFFECTS ON LABORATORY MAMMALS AND IN VITRO TEST SYSTEMS
8.1 Single exposure
o-Toluidine is harmful following acute oral exposure (LD50s of
900 and 940 mg/kg body weight in rats) and is of low acute toxicity
following dermal exposure (LD50 of 3235 mg/kg body weight in rabbits)
(Smyth et al., 1962; Jacobson, 1972). Acute effects include cyanosis,
increased methaemoglobin levels, and related effects in the spleen.
Useful data on effects associated with inhalation exposure were not
identified.
8.2 Irritation and sensitization
Minimal skin irritation and ill-defined eye irritation have been
observed in o-toluidine-exposed rabbits (Smyth et al., 1962). There
is no information available on the skin or respiratory sensitization
potential of o-toluidine in animals.
8.3 Short-term exposure
A 13% decrease in body weight, a 1.5- to 3.0-fold increase in
spleen weight, increased methaemoglobin levels, and congestion,
haemosiderosis, and haematopoiesis in the spleen (all likely
associated with methaemoglobinaemia) were observed in rats orally
administered o-toluidine for 5 days at a dose of 225 mg/kg body
weight per day (Short et al., 1983); no other doses were tested, and a
no-effect level was not identified. Relevant toxicity studies
involving short-term inhalation or dermal exposure to o-toluidine
were not identified.
8.4 Chronic exposure and carcinogenicity
Increased incidences of benign and malignant tumours have been
observed in rats and mice administered o-toluidine hydrochloride in
the diet.
In one study, groups of 50 male and 50 female F344 rats were
given diets containing 3000 or 6000 ppm (mg/kg) o-toluidine
hydrochloride for 101-104 weeks (equivalent to intakes of
approximately 150 and 300 mg/kg body weight per day, based on a body
weight of 400 g and the consumption of 20 g of food per day) (NCI,
1979; Goodman et al., 1984). Controls consisted of 20 animals of each
sex. In addition to routine clinical observations, gross and
microscopic examinations were conducted on all major tissues and
organs and on all gross lesions. Exposure to o-toluidine
hydrochloride was associated with a dose-related decrease in body
weight gain and survival. In the control, low-dose, and high-dose
groups, the combined incidence of sarcomas, angiosarcomas, and
osteosarcomas of the spleen was 0/20, 9/49 (p = 0.36), and 12/49
(p = 0.01), respectively, in females and 0/20, 8/49, and 4/42,
respectively, in males. The combined incidence of sarcomas,
fibrosarcomas, angiosarcomas, and osteosarcomas in multiple
(unspecified) organs was, among females, 0/20, 3/50, and 21/49
(p < 0.001) and, among males, 0/20, 15/50 (p = 0.003), and
37/49 (p < 0.001), for animals in the control, low-dose, and
high-dose groups, respectively. In females, the incidence of
transitional cell carcinomas of the urinary bladder in the control,
low-dose, and high-dose groups was 0/20, 9/45 (p = 0.028), and
22/47 (p < 0.001), respectively; the incidence of this tumour
was not significantly increased in the male rats. The incidence of
malignant mesothelioma of the serous covering of the testes (tunica
vaginalis) was 0/20, 10/50, and 6/49 in the control, low-dose, and
high-dose groups, respectively. Although not observed among control
animals, splenic fibromas were noted in the exposed animals; however,
the incidence was significantly increased only for males in the
low-dose group (10/49; p = 0.024). For animals in the control,
low-dose, and high-dose groups, the incidence of skin fibromas among
males was 0/20, 28/50 (p < 0.001), and 27/49 (p < 0.001),
respectively, and the incidence of mammary gland fibroadenomas among
females was 6/20, 20/50, and 35/49 (p = 0.002), respectively.
Non-neoplastic effects that were not observed in control animals
included splenic fibrosis (in 12-27% and 6-12% of exposed males and
females, respectively), splenic mesothelial hyperplasia (in 12-37% and
24-65% of exposed males and females, respectively), hyperplasia of the
urinary bladder epithelium (in 16-18% and 28-47% of exposed males and
females, respectively), and myocardial fibrosis (in 17-34% of exposed
males). Liver necrosis was noted in 24-35% of exposed males (10% in
controls) and in 2-31% of exposed females (0% in controls).
In a study in which groups of 30 male F344 rats received 0 or 62
mg o-toluidine hydrochloride in the diet each day for 72 weeks
(approximately 470 and 130 mg/kg body weight per day at the beginning
and end of the study, respectively), followed by a 16-week recovery
period, exposure to o-toluidine reduced survival (6/30 and 18/30
survivors in the exposed and control groups, respectively) (Hecht et
al., 1982). Incidences of the following tumours (in the control and
exposed groups, respectively) were: bladder epithelial cell tumours,
0/30 and 4/30; skin fibromas, 1/30 and 25/30; splenic fibromas, 0/30
and 10/30; mammary tumours, 0/30 and 13/30; and peritoneal tumours,
2/30 and 14/30. Although neither the statistical significance of
these results nor the incidence of non-neoplastic effects was
discussed in this report, the results reveal an increased occurrence
of a variety of tumour types in animals administered o-toluidine
hydrochloride for 72 weeks.
In another study in which several chemicals were examined, groups
of 25 male Charles River CD rats were given diets containing
o-toluidine hydrochloride at concentrations of 8000 or 16 000 ppm
(mg/kg) for 3 months (estimated intakes of approximately 800 and 1600
mg/kg body weight per day, assuming 200 g for the body weight of rats
consuming 20 g of food per day) (Weisburger et al., 1978). Excessive
toxicity, based upon increased mortality and reductions in body
weight, resulted in the concentrations being reduced to 4000 and 8000
ppm (mg/kg) (with estimated intakes of 400 and 800 mg/kg body weight
per day) for a further 15 months, followed by a 6-month observation
and recovery period. Only animals that survived 6 months or more were
necropsied. Data on survival or general toxicity were not provided.
Controls included one matched group (n = 16 animals) used for this
portion of the overall study, as well as the pooled controls
(n = 111) from the entire investigation. There was a statistically
significant increase in the incidence (0/16, 18/111, 18/23, and 21/24
in the matched control, pooled control, low-dose, and high-dose
groups, respectively) of subcutaneous fibroma and fibrosarcoma in
o-toluidine-exposed animals. The incidence of bladder transitional
cell carcinoma was 0/16, 5/111, 3/23, and 4/24 in the matched control,
pooled control, low-dose, and high-dose groups, respectively.
Statistically significant increases in hepatocellular carcinomas
and adenomas, as well as haemangiosarcomas, were observed in a study
in which groups of 50 male and 50 female B6C3F1 mice were given diets
containing 1000 or 3000 ppm (mg/kg) o-toluidine hydrochloride
(estimated intakes of 110 and 340 mg/kg body weight per day, assuming
30 g for the body weight of mice consuming 3.4 g of food per day) for
101-104 weeks (NCI, 1979). Twenty animals of each sex served as
unexposed controls. In the control, low-dose, and high-dose groups,
the incidences of hepatocellular carcinoma (in females),
hepatocellular adenoma (in females), and haemangiosarcoma (in males)
were 0/20, 2/49, and 7/50; 0/20, 2/49, and 6/50; and 1/19, 1/50, and
10/50, respectively.
Significantly increased incidences of haemangiosarcomas and
haemangiomas were observed in a study in which groups of 25 male and
25 female CD-1 mice were given diets containing o-toluidine
hydrochloride at concentrations of 16 000 or 32 000 ppm (mg/kg)
(estimated intakes of 1800 and 3600 mg/kg body weight per day,
assuming 30 g for the body weight of mice consuming 3.4 g of food per
day) for 3 months (Weisburger et al., 1978). Excessive toxicity,
based upon increased mortality and reductions in body weight, resulted
in the concentrations being reduced to 8000 and 16 000 ppm (mg/kg) for
a further 15 months, followed by a 3-month observation and recovery
period. Only animals that survived 6 months or more were necropsied.
Controls included one matched group used for this portion of the
overall study, as well as the pooled controls from the entire
investigation. In the matched control, pooled control, low-dose, and
high-dose groups, the incidence of abdominal haemangiosarcomas and
haemangiomas was 0/14, 5/99, 5/14, and 9/11 (in males) and 0/15,
9/102, 5/18, and 9/21 (in females), respectively.
The results from limited (i.e. poorly conducted and/or reported)
dermal and parenteral studies conducted in various species and from an
oral toxicity study in dogs (Morigami & Nisimura, 1940; Steinhoff,
1981; Hecht et al., 1983) do not contribute meaningfully to the
assessment of o-toluidine.
8.5 Genotoxicity and related end-points
Based upon the results of assays conducted in Salmonella
typhimurium and Escherichia coli, o-toluidine was not considered
to be mutagenic in standard bacterial tests (McCann et al., 1975;
Ferretti et al., 1977; Garner & Nutman, 1977; Rosenkranz & Poirier,
1979; Simmon, 1979; Zimmer et al., 1980; Baker & Bonin, 1981, 1985;
Garner et al., 1981; MacDonald, 1981; Martire et al., 1981; Matsushima
et al., 1981; Richold & Jones, 1981; Rowland & Severn, 1981; Simmon &
Shepherd, 1981; Trueman, 1981; Venitt & Crofton-Sleigh, 1981; Rexroat
& Probst, 1985). However, positive results in the Ames test have been
observed when norharman was added to the test system (Nagao et al.,
1978; Nagao & Takahashi, 1981; Sugimura & Nagao, 1981). Results from
several cytogenetic studies have indicated that o-toluidine is
clastogenic in mammalian cells in vitro (Danford, 1985; Gulati et
al., 1985; Ishidate & Sofuni, 1985; Priston & Dean, 1985).
The in vivo genotoxicity of o-toluidine has been adequately
tested only in mice. No evidence of clastogenicity was observed in
several high-quality studies (a bone marrow cytogenetic assay and
three bone marrow micronucleus tests) in which the chemical was
injected intraperitoneally (Salamone et al., 1981; Tsuchimoto &
Matler, 1981; McFee et al., 1989). Although there was no reporting of
bone marrow toxicity in these studies, use of an intraperitoneal
injection with elevated dose levels would suggest that o-toluidine
probably reached the target tissue (bone marrow). In contrast, in the
best conducted bone marrow sister chromatid exchange assay, high doses
of o-toluidine apparently produced positive results in mice (McFee
et al., 1989). A positive result has also been reported for the
induction of DNA single strand breaks in mice; however, the poor
description of the study precludes the drawing of definitive
conclusions (Cesarone et al., 1982). Despite some suggestions of
clastogenicity, the genotoxic potential of o-toluidine in vivo
remains uncertain.
8.6 Reproductive and developmental toxicity
Relevant information on the reproductive and developmental
toxicity of o-toluidine in laboratory animals was not identified.
8.7 Immunological and neurological effects
Relevant toxicological studies in which immunological or
neurological effects were assessed were not available. However,
evidence of specific adverse immunological and neurological effects
has not been reported in general toxicity studies.
9. EFFECTS ON HUMANS
Relevant case reports on adverse health effects associated with
exposure to o-toluidine were not available.
Other than information on potential carcinogenic effects, there
are few useful data available on other health-related effects
associated with exposure to o-toluidine.
Exposure to chemicals including o-toluidine in the dyestuffs
industry and more recently in the rubber industry has been reported to
be associated with an increased incidence of bladder cancer. For
example, expected and observed cases of bladder cancer were recorded
in a thorough, well conducted retrospective cohort study at a rubber
chemical plant in upstate New York (Ward et al., 1991). The cohort
consisted of all 1749 male and female workers employed at the plant
between 1973 and 1988. The work-force was relatively young; 72% were
born after 1 January 1939. It was estimated that cohort members were
"slightly more likely" than the general population of the USA to be
current or former smokers. These workers were exposed primarily to
o-toluidine and aniline. In 1988, airborne o-toluidine and
aniline levels were <1 ppm (<4.4 mg/m3 for o-toluidine).
Based upon 13 identified cases of bladder cancer among all 1749
employees, compared with 3.61 cases expected (estimated from the rate
in the population of the state of New York, excluding New York City),
the standardized incidence ratio (SIR) was 3.6 (90% confidence
interval [CI] = 2.13-5.73). The SIRs for bladder cancer among workers
"definitely exposed" (n = 708), "possibly exposed" (n = 288), and
"probably unexposed" (n = 753) to o-toluidine and aniline were
6.48 (90% CI = 3.04-12.2; 7 observed cases), 3.66 (90% CI = 1.25-8.37;
4 observed cases), and 1.39 (90% CI = 0.25-4.39; 2 observed cases),
respectively. The risk of bladder cancer increased with duration of
exposure and time since first exposure. The SIRs for bladder cancer
among the "definitely exposed" workers with <5, 5-9.99, and >10
years of exposure to these chemicals were 0 (0 observed cases), 8.8
(90% CI = 0.45-41.7; 1 observed case), and 27.2 (90% CI = 11.8-53.7;
6 observed cases), respectively. The SIRs for bladder cancer among
workers with <10, 10-20, and >20 years since their first employment
in the "definitely exposed" department of the plant were 0 (0 observed
cases), 2.03 (90% CI = 0.10-9.64; 1 observed case), and 16.4 (90% CI =
7.13-32.3; 6 observed cases), respectively. It was calculated that
smoking was unlikely to account for the increased incidence of bladder
cancer in this group of workers. The mean latency period for the
group of seven "definitely exposed" workers with bladder cancer was 23
years. Although the carcinogenic potential of o-toluidine
specifically cannot be definitively determined from this study, the
findings merit considerable concern.
Other studies of workers employed in the dyestuffs industry
include those by Vigliani & Barsotti (1961), Khlebnikova et al.
(1970), Zavon et al. (1973), Conso & Pontal (1982), and Rubino et al.
(1982). However, as in the study of rubber chemical workers, it was
not possible to definitively link the increased incidence of bladder
cancer specifically to o-toluidine because of concurrent exposure to
other chemicals.
10. EFFECTS ON OTHER ORGANISMS IN THE LABORATORY AND FIELD
Relevant information on the effects of o-toluidine on aquatic
or terrestrial organisms was not identified. However, toxicity
thresholds for the inhibition of algal growth (Microcystis
aeruginosa, 0.31 mg/litre; Scenedesmus quadricauda, 6.3 mg/litre)
have been reported.1
1 Source: EnviChem Data Bank of Environmental Properties of
Chemicals. Helsinki, Finland, Finnish Environment Agency, version 1.0,
1995.
11. EFFECTS EVALUATION
11.1 Evaluation of health effects
11.1.1 Hazard identification and dose-response assessment
Available data are inadequate to allow the potential risks of
reproductive or developmental effects on human health to be assessed.
Carcinogenicity is considered, however, to be the critical effect
associated with potential exposure to o-toluidine. In several
experimental studies, the oral administration of o-toluidine
hydrochloride increased the incidence of benign and/or malignant
tumours in various tissues in rats (spleen sarcomas and fibromas in
males and females, mesotheliomas of the scrotum in males, transitional
cell carcinomas of the urinary bladder in females, skin fibromas and
mammary gland fibroadenomas in males and females, respectively).
Clear evidence of carcinogenicity has also been observed in oral
studies in mice (hepatocellular carcinomas and adenomas,
haemangiosarcomas, and haemangiomas). Even where the increased tumour
incidences were not statistically significant, they were considered to
be of biological significance in view of the low incidences of such
tumours in historical controls (Haseman et al., 1990). o-Toluidine
is genotoxic in vitro; although the genotoxicity studies conducted
in vivo do not allow definite conclusions to be drawn, there is some
evidence of genotoxic potential. Carcinogenicity studies in rats and
mice have yielded tumours in both sexes and in multiple organs;
therefore, the possibility of involvement of a genotoxic mechanism
cannot be eliminated.
Several studies of workers employed in the dyestuffs and rubber
industries found that exposure to chemicals, including o-toluidine,
appears to be associated with an increased incidence of bladder
cancer. Although it is difficult to draw definite conclusions
regarding the carcinogenic potential of o-toluidine in humans from
these studies of workers exposed to multiple chemicals, this evidence,
together with data from experimental carcinogenicity bioassays, raises
concern about the risk of cancer in exposed humans. It would
therefore be prudent to consider that o-toluidine is probably
carcinogenic in humans, possibly through involvement of a genotoxic
mechanism.
11.1.2 Criteria for setting guidance values for o-toluidine
On the basis that the carcinogenicity of o-toluidine may
involve a genotoxic mechanism, it is not possible to reliably identify
a threshold at which exposure to o-toluidine would not result in
some risk to human health.
11.1.3 Sample risk characterization
It is recognized that there are a number of different approaches
to assessing the risks to human health posed by genotoxic and
carcinogenic substances. In some jurisdictions, there are models for
characterizing potency, which may be of some benefit in
priority-setting schemes.
The example here is from the occupational environment, as the
lack of available data precludes the characterization of potential
cancer risks for the general population. In the United Kingdom, a
Maximum Exposure Limit for o-toluidine (which is not a health-based
standard) of 0.2 ppm (0.9 mg/m3; 8-hour time-weighted average) has
been proposed. The Maximum Exposure Limit was based on a level of
control that was deemed (by tripartite agreement) to be reasonably
practicable under workplace conditions within the United Kingdom. In
the United Kingdom, there is a continuing remit to reduce exposure
levels as much as reasonably practicable with the technology that is
currently available.
11.2 Evaluation of environmental effects
The lack of available information precludes adequate assessment
of potential risks to environmental organisms.
12. PREVIOUS EVALUATIONS BY INTERNATIONAL BODIES
The International Agency for Research on Cancer (IARC, 1987) has
classified o-toluidine in Group 2B (possibly carcinogenic to
humans), based upon sufficient evidence for carcinogenicity in animals
and inadequate evidence for carcinogenicity in humans.
Information on international hazard classification and labelling
is included in the International Chemical Safety Card reproduced in
this document.
13. HUMAN HEALTH PROTECTION AND EMERGENCY ACTION
Human health hazards, together with preventative and protective
measures and first aid recommendations, are presented in the
International Chemical Safety Card (ICSC 0341) reproduced in this
document.
13.1 Human health hazards
Following short-term exposure, o-toluidine could induce
methaemoglobinaemia. After long-term or repeated exposure,
o-toluidine is considered possibly carcinogenic to humans.
13.2 Advice to physicians
If splashed with o-toluidine, it is crucial to remove all wet
or contaminated clothing and wash the entire body with soap and water.
Following such an incident, the degree of methaemoglobinaemia needs to
be determined hourly until a decrease is well established. Above 30%
methaemoglobinaemia, administer oxygen under continuous observation;
above 50% methaemoglobinaemia, further administer intravenously 1000
cc of a 5% glucose solution containing ascorbic acid; above 60%
methaemoglobinaemia, further administer intravenously 10-20 cc of a 1%
solution of methylene blue. If there is no response to treatment with
methylene blue, then haemodialysis or exchange transfusion is useful.
13.3 Health surveillance advice
For workers exposed to o-toluidine, a health surveillance
programme should include regular urinary cytology, with more specific
procedures in the case of positive results.
13.4 Spillage
In the event of spillage, measures should be undertaken to
prevent this chemical from reaching drains and watercourses.
14. CURRENT REGULATIONS, GUIDELINES, AND STANDARDS
Information on national regulations, guidelines, and standards is
available from the International Register of Potentially Toxic
Chemicals (IRPTC) legal file.
The reader should be aware that regulatory decisions about
chemicals taken in a certain country can be fully understood only in
the framework of the legislation of that country. The regulations and
guidelines of all countries are subject to change and should always be
verified with appropriate regulatory authorities before application.
INTERNATIONAL CHEMICAL SAFETY CARD
ortho-TOLUIDINE ICSC: 0341
ortho-TOLUIDINE
1-Amino-2-methylbenzene
2-Aminotoluene
o-Methylaniline
C7H9N/C6H4CH3NH2
Molecular mass: 107.2
CAS # 95-53-4
RTECS # XU2975000
ICSC # 0341
UN # 1708
TYPES OF ACUTE HAZARDS/ PREVENTION FIRST AID/
HAZARD/ SYMPTOMS FIRE FIGHTING
EXPOSURE
FIRE Combustinble. Gives off NO open flames. NO contact Powder, AFFF, foam, carbon
irritating or toxic fumes (or with nitric acid. dioxide.
gases) in a fire.
EXPLOSION Above 85°C explosive Above 85°C use a closed In case of fire: keep drums,
vapour/air mixtures may be system, ventilation. etc., cool by spraying with
formed. water.
EXPOSURE AVOID ALL CONTACT! IN ALL CASES CONSULT A
DOCTOR!
* INHALATION Blue lipsor finger nails. Ventilation, local exhaust, Fresh air, rest. Artificial
Blue skin. Confusion. or breathing protection. respiration if indicated.
Dizziness. Headache. Refer for medical attention.
Shortness of breath.
Weakness.
INTERNATIONAL CHEMICAL SAFETY CARD (continued)
TYPES OF ACUTE HAZARDS/ PREVENTION FIRST AID/
HAZARD/ SYMPTOMS FIRE FIGHTING
EXPOSURE
* SKIN MAY BE ABSORBED! Redness. Protective gloves. Protective Remove contaminated clothes.
Blue lips or fingernails. clothing. Rinse and then wash skin with
Blue skin (Further see water and soap. Refer for
inhalation). medical attention.
* EYES Redness. Pain. Safety goggles. First rinse with plenty of
water for several minutes
(remove contact lenses if
easily possible), then take
to a doctor.
* INGESTION Blue lips or fingernails. Do not eat, drink, or smoke Rinse mouth. Induce vomiting
Blue skin. Dizziness. during work. (ONLY IN CONSCIOUS PERSONS!).
Headache. Laboured breathing Refer for medical attention.
(further see inhalation).
INTERNATIONAL CHEMICAL SAFETY CARD (continued)
SPILLAGE DISPOSAL STORAGE PACKAGING & LABELLING
Collect leaking and spilled liquid in Separated from strong oxidants, strong Do not transport with food and
sealable containers as far as acids, food and feedstuffs. Cool. Dry. feedstuffs.
possible. Absorb remaining liquid in Well closed. Ventilation along the
sand or inert absorbent and remove to floor. UN Hazard Class: 6.1
safe place (extra personal protection: N Packing Group: II
complete protective clothing including
self-contained breathing apparatus).
IMPORTANT DATA PHYSICAL STATE; APPEARANCE: EFFECTS OF SHORT-TERM EXPOSURE:
COLOURLESS TO YELLOW LIQUID, TURNS The substance irritates the eyes and the skin.
REDDISH-BROWN ON EXPOSURE TO AIR AND LIGHT. The substance may cause effects on the blood,
bladder and kidneys, resulting in tissue
CHEMICAL DANGERS: lesions, impaired functions and formation of
The substance decomposes on heating or on methaemoglobin. Exposure to high concentrations
burning producing toxic fumes including may result in damage to kidneys and bladder.
nitrogen oxides. Reacts with strong oxidants, The effects may be delayed. Medical observation
expecially nitric acid. is indicated. See Notes.
OCCUPATIONAL EXPOSURE LIMITS (OELs): EFFECTS OF LONG-TERM OR REPEATED EXPOSURE:
TLV: 2 ppm; 8.8 mg/m3 (as TWA) A2 (skin) The substance may have effects on the blood,
(ACGIH 1994-1995. resulting in the formation of methaemoglobin
(see Notes). This substance is possibly
ROUTES OF EXPOSURE: carcinogenic to humans.
The substanec can be absorbed into the body by
inhalation and through the skin, and by
ingestion.
INHALATION RISK:
Evaporation at 20°C is negligible; a harmful
concentration of airborne particles can,
however, be reached quickly on spraying.
INTERNATIONAL CHEMICAL SAFETY CARD (continued)
PHYSICAL Boiling point: 200°C Flash point: 85°C c.c.
PROPERTIES Melting -16°C Auto-ignition temperature: 482°C
Relative density (water = 1): 1.01 Explosive limits, vol% in air: 1.5-?
Solubility in water: poor Octanol/water partition coefficient
Vapour pressure, kPa at 20°C: 0.2 as log Pow: 1.32
Relative vapour density (air = 1): 3.7
Relative density of the vapour/
air-mixture at 20°C (air = 1): 1.00
ENVIRONMENTAL The substance is harmful to aquatic organisms.
DATA
NOTES Depending on the degree of exposure, periodic medical examination is indicated.
Specific treatment is necessary in case of poisoning with this substance; the
appropriate means with instructions must be available.
The odour warning when the exposure limit value is exceeded is insufficient.
Also consult ICSC #0342, meta-Toluidine and 0343, para-Toluidine.
ICSC: 0341 1.1 NFPA Code: H3; F2; R0
REFERENCES
Baker R, Bonin A (1981) Study of 42 coded compounds with
Salmonella/mammalian microsome assay. In: de Serres FJ, Ashby J,
eds. Progress in mutation research. Vol. 1. Evaluation of
short-term tests for carcinogens. Amsterdam, Elsevier Science
Publishers, pp. 249-260.
Baker R, Bonin A (1985) Tests with the Salmonella
plate-incorporation assay. In: Ashby J, de Serres FJ, Draper M,
Ishidate M Jr, Margolin BH, Matter BE, Shelby MD, eds. Progress
in mutation research. Vol. 5. Evaluation of short-term tests for
carcinogens. Amsterdam, Elsevier Science Publishers, pp. 177-180.
Birnier G, Neumann H (1988) Biomonitoring of aromatic amines. II:
Haemoglobin binding of some monocyclic aromatic amines. Archives
of toxicology, 62(2/3):110-115.
Brown K, Teass A, Simon S, Ward E (1995) A biological monitoring
method for o-toluidine in urine using high performance liquid
chromatography with electrochemical detection. Applied
occupational and environmental hygiene, 10(6):557-565.
Cesarone C, Bolognesi C, Santi L (1982) Evaluation of damage to DNA
after in vivo exposure to different classes of chemicals.
Archives of toxicology, Suppl. 5:355-359.
Cheever K, Richards D, Plotnick H (1980) Metabolism of o-, m- and
p-toluidine in the adult male rat. Toxicology and applied
pharmacology, 56:361-369.
Conso F, Pontal P (1982) Amino-tumours of the bladder. Possible part
of the industrial exposure to o-toluidine and o-aminoazotoluene.
Archives des maladies professionnelles de medecine du travail et de
securité sociale, 43(4):273-319 (HSE Translation No. 14195A).
Danford N (1985) Tests for chromosome aberrations and aneuploidy in
the Chinese hamster fibroblast cell line CH1-L. In: Ashby J, de Serres
FJ, Draper M, Ishidate M Jr, Margolin BH, Matter BE, Shelby MD, eds.
Progress in mutation research. Vol. 5. Evaluation of short-term
tests for carcinogens. Amsterdam, Elsevier Science Publishers, pp.
397-411.
Ferretti J, Lu W, Liu M (1977) Mutagenicity of benzidine and related
compounds employed in the detection of haemoglobin. American
journal of clinical pathology, 67(6):526-527.
Garner R, Nutman C (1977) Testing of some azo dyes and their reduction
products for mutagenicity using Salmonella typhimurium TA 1538.
Mutation research, 44:9-19.
Garner R, Welch A, Pickering C (1981) Mutagenic activity of 42 coded
compounds in the Salmonella/microsome assay. In: de Serres FJ, Ashby
J, eds. Progress in mutation research. Vol. 1. Evaluation of
short-term tests for carcinogens. Amsterdam, Elsevier Science
Publishers, pp. 280-284.
Goodman D, Ward J, Reichardt W (1984) Splenic fibrosis and sarcomas in
F344 rats fed diets containing aniline HCL, p-chloroaniline,
azobenzene, o-toluidine HCL, 4,4'-sulfonyldianiline or D+C red No.
9. Journal of the National Cancer Institute, 73(1):265-273.
Gregg N, South D, Brown R, Cocker J (1996) o-Toluidine; Criteria
document for an occupational exposure limit. London, HSE Books.
Gulati D, Sabharwal P, Shelby M (1985) Tests for the induction of
chromosomal aberrations and sister-chromatid exchanges in cultured
Chinese hamster ovary (CHO) cells. In: Ashby J, de Serres FJ, Draper
M, Ishidate M Jr, Margolin BH, Matter BE, Shelby MD, eds. Progress
in mutation research. Vol. 5. Evaluation of short-term tests for
carcinogens. Amsterdam, Elsevier Science Publishers, pp. 413-426.
Haseman JK, Arnold J, Eustis SL (1990) Tumour incidences in Fischer
344 rats: NTP historical data. In: Boorman GA, Eustis SL, Elwell MR,
Montgomery CA Jr, MacKenzie WF, eds. Pathology of the Fischer rat.
Reference and atlas. San Diego, CA, Academic Press, pp. 555-564.
Hecht S, El-Bayoumy K, Riverson A, Fiala E (1982) Comparative
carcinogenicity of o-toluidine hydrochloride and o-nitrosotoluene
in F-344 rats. Cancer letters, 16:103-108.
Hecht S, El-Bayoumy K, Riverson A, Fiala E (1983) Bioassay for
carcinogenicity of 3,2'-dimethyl-4-nitrosobiphenyl,
o-nitrosotoluene, nitrosobenzene and the corresponding amines in
Syrian golden hamsters. Cancer letters, 20(3):349-354.
HSE (1993) Aromatic amines in air and on surfaces. London, Health &
Safety Executive (Methods for the Determination of Hazardous
Substances, No. 75).
IARC (1987) Ortho-toluidine. In: Overall evaluations of
carcinogenicity: an updating of IARC Monographs Volumes 1-42. Lyon,
International Agency for Research on Cancer, pp. 362-363 (IARC
Monographs on the Evaluation of Carcinogenic Risks to Humans,
Supplement No. 7).
Ishidate M Jr, Sofuni T (1985) The in vitro chromosomal aberration
test using Chinese hamster lung (CHL) fibroblast cells in culture. In:
Ashby J, de Serres FJ, Draper M, Ishidate M Jr, Margolin BH, Matter
BE, Shelby MD, eds. Progress in mutation research. Vol. 5.
Evaluation of short-term tests for carcinogens. Amsterdam, Elsevier
Science Publishers, pp. 427-432.
Jacobson K (1972) Acute oral toxicity of mono- and di-alkyl
ring-substituted derivatives of aniline. Toxicology and applied
pharmacology, 22:153-154.
Khlebnikova M, Gladkova E, Kurenko L, Pshenitsyn A, Shalin B (1970)
[Problems of industrial hygiene and health status of workers engaged
in the production of o-toluidine.] Gigiena Truda i
Professional'nye Zabolevaniya, 14:7-10 (in Russian).
MacDonald D (1981) Salmonella/microsome tests on 42 coded compounds.
In: de Serres FJ, Ashby J, eds. Progress in mutation research.
Vol. 1. Evaluation of short-term tests for carcinogens. Amsterdam,
Elsevier Science Publishers, pp. 285-297.
Martire G, Vricella G, Perfumo A, de Lorenzo F (1981) Evaluation of
the mutagenic activity of coded compounds in the Salmonella test.
In: de Serres FJ, Ashby J, eds. Progress in mutation research.
Vol. 1. Evaluation of short-term tests for carcinogens. Amsterdam,
Elsevier Science Publishers, pp. 271-279.
Matsushima T, Takamoto Y, Shirai A, Sawamura M, Sugimura T (1981)
Reverse mutation test on 42 coded compounds with the E. coli WP2
system. In: de Serres FJ, Ashby J, eds. Progress in mutation
research. Vol. 1. Evaluation of short-term tests for carcinogens.
Amsterdam, Elsevier Science Publishers, pp. 387-395.
McCann J, Choi E, Yamasaki E, Ames B (1975) Detection of carcinogens
as mutagens in the Salmonella/microsome test: assay of 300
chemicals. Proceedings of the National Academy of Sciences of the
United States of America, 72:5135-5139.
McFee A, Jauhar P, Lowe K, MacGregor J, Wehr C (1989) Assays of three
carcinogen/noncarcinogen chemical pairs for in vivo induction of
chromosome aberrations, sister chromatid exchanges and micronuclei.
Environmental and molecular mutagenesis, 14(4):207-220.
Morigami S, Nisimura I (1940) Experimental studies on aniline bladder
tumours. Gann, 34:146-147.
Nagao M, Takahashi Y (1981) Mutagenic activity of 42 coded compounds
in the Salmonella/microsome assay. In: de Serres FJ, Ashby J, eds.
Progress in mutation research. Vol. 1. Evaluation of short-term
tests for carcinogens. Amsterdam, Elsevier Science Publishers,
pp. 361-370.
Nagao M, Yahagi T, Sugimura T (1978) Differences in effects of
norharman with various classes of chemical mutagens and amounts of S9.
Biochemical and biophysical research communications, 83(2):373-378.
NCI (1979) Bioassay of o-toluidine hydrochloride for possible
carcinogenicity (CAS No. 636-21-5). Bethesda, MD, US Department of
Health, Education and Welfare, Public Health Service, National
Institutes of Health, National Cancer Institute (Carcinogenesis
Technical Report Series No. 153; NIH Publication No. 79-1709).
NIOSH (1987) Method 2002. In: NIOSH manual of analytical methods,
3rd ed. Department of Health, Education and Welfare, National
Institute for Occupational Safety and Health (DHEW Publication No.
84-100; 1984, revised 1987).
Pereira W, Rostad C, Garbarino J, Hult M (1983) Groundwater
contamination by organic bases derived from coal tar wastes.
Toxicology and chemistry, 2:283-294.
Priston R, Dean B (1985) Tests for the induction of chromosome
aberrations, polyploidy and sister-chromatid exchanges in rat liver
(RL4) cells. In: Ashby J, de Serres FJ, Draper M, Ishidate M Jr,
Margolin BH, Matter BE, Shelby MD, eds. Progress in mutation
research. Vol. 5. Evaluation of short-term tests for carcinogens.
Amsterdam, Elsevier Science Publishers, pp. 387-395.
Rexroat M, Probst G (1985) Mutation tests with Salmonella using the
plate incorporation assay. In: Ashby J, de Serres FJ, Draper M,
Ishidate M Jr, Margolin BH, Matter BE, Shelby MD, eds. Progress in
mutation research. Vol. 5. Evaluation of short-term tests for
carcinogens. Amsterdam, Elsevier Science Publishers, pp. 201-212.
Richold M, Jones E (1981) Mutagenic activity of 42 coded compounds in
the Salmonella/microsome assay. In: de Serres FJ, Ashby J, eds.
Progress in mutation research. Vol. 1. Evaluation of short-term
tests for carcinogens. Amsterdam, Elsevier Science Publishers,
pp. 314-322.
Rosenkranz H, Poirier L (1979) Evaluation of the mutagenicity and DNA
modifying activity of carcinogens and non-carcinogens in microbial
systems. Journal of the National Cancer Institute, 62:873-892.
Rowland I, Severn B (1981) Mutagenicity of carcinogens and
noncarcinogens in the Salmonella/microsome test. In: de Serres FJ,
Ashby J, eds. Progress in mutation research. Vol. 1. Evaluation of
short-term tests for carcinogens. Amsterdam, Elsevier Science
Publishers, pp. 323-332.
Rubino G, Scansetti G, Piolatto G, Pira E (1982) The carcinogenic
effect of aromatic amines. An epidemiological study on the role of
o-toluidine and 4,4'-methylene bis (2-methylaniline) in inducing
bladder cancer in man. Environmental research, 27(2):241-254.
Salamone M, Heddle J, Katz M (1981) Mutagenic activity of 41 compounds
in the in vivo micronucleus assay. In: de Serres FJ, Ashby J, eds.
Progress in mutation research. Vol. 1. Evaluation of short-term
tests for carcinogens. Amsterdam, Elsevier Science Publishers, pp.
686-697.
Senczuk W, Rucinska H, Zak I (1984) Toxicodynamic properties of
toluidines. Part IV. Dermal absorption of toluidines. Bromatologia
i Chemia Toksykologiczna, 17(2):109-112 (HSE Translation No. 14309).
Short C, King C, Sistrunk P, Kerr K (1983) Subacute toxicity of
several ring-substituted dialkylanilines in the rat. Fundamental
and applied toxicology, 3(4):285-292.
Simmon V (1979) In vitro mutagenicity assays of chemical carcinogens
and related compounds with Salmonella typhimurium. Journal of the
National Cancer Institute, 62:893-899.
Simmon V, Shepherd G (1981) Mutagenic activity of 42 coded compounds
in the Salmonella/microsome assay. In: de Serres FJ, Ashby J, eds.
Progress in mutation research. Vol. 1. Evaluation of short-term
tests for carcinogens. Amsterdam, Elsevier Science Publishers,
pp. 333-342.
Smyth H, Carpenter C, Weil C (1962) Range-finding toxicity data - List
VI. American Industrial Hygiene Association journal, 23:95-107.
Son O, Weiss L, Fiala E, Weisburger E (1977) Metabolism of the
carcinogen o-toluidine. Proceedings of the American Association
for Cancer Research, 18:492.
Steinhoff D (1981) Possible methods of carcinogenicity testing for
substances used at the place of work. Cancer detection and
prevention, 4:41-46.
Sugimura T, Nagao M (1981) Carcinogenic, mutagenic and comutagenic
aromatic amines in human foods. National Cancer Institute
monographs, 58:27-33.
Trueman R (1981) Mutagenicity of 42 coded compounds in a bacterial
assay using Escherichia coli and Salmonella typhimurium. In: de
Serres FJ, Ashby J, eds. Progress in mutation research. Vol. 1.
Evaluation of short-term tests for carcinogens. Amsterdam, Elsevier
Science Publishers, pp. 343-350.
Tsuchimoto T, Matler B (1981) Activity of coded compounds in the
micronucleus test. In: de Serres FJ, Ashby J, eds. Progress in
mutation research. Vol. 1. Evaluation of short-term tests for
carcinogens. Amsterdam, Elsevier Science Publishers, pp. 705-711.
Venitt S, Crofton-Sleigh C (1981) Mutagenicity of 42 coded compounds
in a bacterial assay using E. coli and S. typhimurium. In: de
Serres FJ, Ashby J, eds. Progress in mutation research. Vol. 1.
Evaluation of short-term tests for carcinogens. Amsterdam, Elsevier
Science Publishers, pp. 351-361.
Vigliani E, Barsotti M (1961) Environmental tumours of the bladder in
some Italian dyestuff factories. Medicina del Lavoro, 52:241-250.
Ward E, Carpenter A, Markowitz S, Roberts D, Halperin W (1991) Excess
number of bladder cancers in workers exposed to o-toluidine and
aniline. Journal of the National Cancer Institute, 83(7):501-506.
Ward E, Sabbioni G, DeBord D, Teass A, Brown K, Talaska G, Roberts D,
Ruder A, Streicher R (1996) Monitoring of aromatic amine exposures in
workers at a chemical plant with a known bladder cancer excess.
Journal of the National Cancer Institute, 88(15):1046-1052.
Wegman R, de Korte G (1981) Aromatic amines in surface waters of the
Netherlands. Water research, 15:391-394.
Weisburger E, Russfield A, Homburger F, Weisburger J, Boger E, van
Dongen C, Chu K (1978) Testing of twenty-one environmental aromatic
amines or derivatives for long term toxicity or carcinogenicity.
Journal of environmental pathology and toxicology, 2(2):325-356.
Yoshida K, Shigeoka T, Yamauchi F (1983) Non-steady-state equilibrium
model for the preliminary prediction of the fate of chemicals in the
environment. Ecotoxicology and environmental safety, 7:179-190.
Zavon M, Hoegg V, Bingham E (1973) Benzidine exposure as a cause of
bladder tumours. Archives of environmental health, 27:1-7.
Zimmer D, Mazurek J, Petzold G, Bhuyan B (1980) Bacterial mutagenicity
and mammalian cell DNA damage by several substituted anilines.
Mutation research, 77:317-326.
Zoeteman B, Harmsen K, Linders J, Morra C, Slooff W (1980) Persistent
organic pollutants in river water and ground water of the Netherlands.
Chemosphere, 9:231-249.
APPENDIX 1 - SOURCE DOCUMENT
Gregg N, South D, Brown R, Cocker J (1996) o-Toluidine; Criteria
document for an occupational exposure limit. London, HSE Books (ISBN
0 7176 1057 8)
The authors' draft version was initially reviewed internally by a
group of approximately 10 Health & Safety Executive experts mainly
toxicologists, but also experts in other relevant disciplines (e.g.
epidemiology, occupational hygiene). The toxicology section of the
amended draft was then reviewed by toxicologists from the United
Kingdom Department of Health. Subsequently, the entire Criteria
Document was reviewed by a tripartite advisory committee to the United
Kingdom Health & Safety Commission, the Working Group for the
Assessment of Toxic Chemicals (WATCH). This committee is composed of
experts in toxicology and occupational health and hygiene from
industry, trade unions, and academia.
The members of the WATCH committee at the time of the peer review
were:
Professor J. Bridges (University of Surrey)
Dr A. Hay (Trade Unions Congress)
Dr L. Levy (Institute of Occupational Hygiene, Birmingham)
Dr M. Molyneux (Chemical Industries Association)
Mr A. Moses (Chemical Industries Association)
Dr R. Owen (Trade Unions Congress)
Mr J. Sanderson (independent consultant)
Dr M. Sharratt (University of Surrey)
Dr A. Smith (independent consultant)
APPENDIX 2 - CICAD PEER REVIEW
The draft CICAD on o-toluidine was sent for review to
institutions and organizations identified by IPCS after contact with
IPCS national Contact Points and Participating Institutions, as well
as to identified experts. Comments were received from:
BASF Aktiengesellschaft, Ludwigshafen, Germany
Bayer AG, Leverkusen, Germany
Department of Health, London, United Kingdom
Department of Public Health, Albert Szent-Gyorgyi University
Medical School, Szeged, Hungary
Environmental Health Directorate, Health Canada, Ottawa, Canada
International Agency for Research on Cancer, Lyon, France
Ministry of Health and Welfare, International Affairs Division,
Government of Japan, Tokyo, Japan
National Food Agency of Denmark, Institute of Toxicology,
Ministry of Health, Soborg, Denmark
National Institute for Working Life, Solna, Sweden
National Institute of Occupational Health, Budapest, Hungary
United States Department of Health and Human Services (National
Institute of Environmental Health Sciences)
United States Environmental Protection Agency (Office of
Pollution Prevention and Toxics; National Center for
Environmental Assessment, Office of Research and Development;
Office of Drinking Water)
APPENDIX 3 - CICAD FINAL REVIEW BOARD
Brussels, Belgium, 18-20 November 1996
Members
Dr A. Aitio, Institute of Occupational Health, Helsinki, Finland
Dr K. Bentley, Director, Environment Policy Section, Commonwealth
Department of Human Services and Health, Canberra, Australia
Mr R. Cary, Toxicology and Existing Substances Regulation Unit, Health
& Safety Executive, Merseyside, United Kingdom
Dr J. de Fouw, National Institute of Public Health and Environmental
Protection, Bilthoven, The Netherlands
Dr C. DeRosa, Director, Division of Toxicology, Agency for Toxic
Substances and Disease Registry, Atlanta, GA, USA
Dr S. Dobson, Institute of Terrestrial Ecology, Monks Wood, Abbots
Ripton, Huntingdon, Cambridgeshire, United Kingdom
Dr W. Farland, Director, National Center for Environmental Assessment,
Office of Research and Development, US Environmental Protection
Agency, Washington, DC, USA (Chairperson)
Dr T.I. Fortoul, Depto. Biologia Celular y Tisular, National
University of Mexico and Environmental Health Directorate of the
Health Ministry, Mexico D.F., Mexico
Dr H. Gibb, National Center for Environmental Assessment, US
Environmental Protection Agency, Washington, DC, USA
Dr R.F. Hertel, Federal Institute for Health Protection of Consumers &
Veterinary Medicine, Berlin, Germany
Mr J.R. Hickman, Environmental Health Directorate, Health Canada,
Ottawa, Ontario, Canada
Dr T. Lakhanisky, Head, Division of Toxicology, Institute of Hygiene
and Epidemiology, Brussels, Belgium (Vice-Chairperson)
Dr I. Mangelsdorf, Documentation and Assessment of Chemicals,
Fraunhofer Institute for Toxicology and Aerosol Sciences, Hanover,
Germany
Ms E. Meek, Head, Priority Substances Section, Environmental Health
Directorate, Health Canada, Ottawa, Ontario, Canada
Dr K. Paksy, National Institute of Occupational Health, Budapest,
Hungary
Mr D. Renshaw, Department of Health, London, United Kingdom
Dr J. Sekizawa, Division of Chemo-Bio Informatics, National Institute
of Hygienic Sciences, Tokyo, Japan
Dr H. Sterzl-Eckert, GSF-Forschungszentrum für Umwelt und Gesundheit
GmbH, Institut für Toxikologie, Oberschleissheim, Germany
Professor S. Tarkowski, Department of Environmental Health Hazards,
The Nofer Institute of Occupational Medicine, Lodz, Poland
Dr M. Wallen, National Chemicals Inspectorate (KEMI), Solna, Sweden
Observers
Professor F.M.C. Carpanini,1 Director, Centre for Ecotoxicology and
Toxicology of Chemicals (ECETOC), Brussels, Belgium
Mr R. Haigh,1 Head of Unit, Health and Safety Directorate, European
Commission, Luxembourg
Mr B.U. Hildebrandt, Federal Ministry for the Environment, Nature
Conservation and Nuclear Safety, Bonn, Germany
Mr P. Hurst,1 Chemical and Consumer Policy Officer, Conservation
Policy Division, World Wide Fund for Nature, Gland, Switzerland
Dr A. Lombard (Representative of CEFIC), ELF-ATOCHEM, Paris, France
Dr P. McCutcheon,1 Environment, Consumer Protection and Nuclear
Safety, European Commission, Brussels, Belgium
Dr R. Montaigne, Counsellor, Technical Affairs Department, European
Chemical Industry Council (CEFIC), Brussels, Belgium
Dr M. Pemberton, ICI Acrylics, Lancashire, United Kingdom
Dr A. Smith, Organisation for Economic Co-operation and Development,
Environment Division, Paris, France
1 Invited but unable to attend.
Secretariat
Dr M. Baril, International Programme on Chemical Safety, World Health
Organization, Geneva, Switzerland
Dr L. Harrison, International Programme on Chemical Safety, World
Health Organization, Geneva, Switzerland
Dr M. Mercier, Director, International Programme on Chemical Safety,
World Health Organization, Geneva, Switzerland
Dr P. Toft, Associate Director, International Programme on Chemical
Safety, World Health Organization, Geneva, Switzerland
RÉSUMÉ D'ORIENTATION
Ce CICAD (document international succinct sur l'évaluation des
risques chimiques) relatif à l' ortho-toluidine (o-toluidine) est
fondé sur une étude, menée par le United Kingdom Health & Safety
Executive (Gregg et al., 1996), des principaux problèmes posés par
cette substance du point de vue de l'hygiène industrielle, étude qui
prenait en compte les données connues en mars 1992. Des informations
complémentaires ont été incorporées à ce CICAD lors de l'examen par
les pairs, puis par le Comité d'Évaluation finale. Les informations
relatives à la préparation du document initial et à son examen par les
pairs figurent à l'appendice 1. Les renseignements concernant
l'examen du CICAD par les pairs font l'objet de l'appendice 2. La
publication de ce CICAD a été approuvée à une réunion du Comité
d'Évaluation finale qui s'est tenue à Bruxelles (Belgique) du 18 au
20 novembre 1996. La liste des participants à cette réunion figure à
l'appendice 3. La fiche d'information sur la sécurité chimique de
l' o-toluidine (ICSC 0341), préparée par le Programme international
sur la sécurité chimique (IPCS, 1993), est également reproduite dans
le présent document.
L' o-toluidine (CAS N° 95-53-4) est un produit chimique de
synthèse qui se présente à la température ambiante sous la forme d'un
liquide jaune pâle. Elle est utilisée principalement dans la
fabrication de colorants, mais aussi de caoutchouc, de produits
chimiques et de pesticides. C'est également un agent de
polymérisation des résines époxy.
La toxicité aigue de l' o-toluidine est modérée à faible.
L' o-toluidine est très légèrement irritante pour la peau et
légèrement irritante pour les yeux. On ne possède pas d'information
sur le potentiel de sensibilisation cutanée ou respiratoire. Les
principaux signes de toxicité à la suite d'une exposition massive et
de courte durée sont une méthémoglobinémie et des effets connexes sur
la rate. Ces effets ont été observés chez des rats soumis à une dose
de 225 mg/kg de poids corporel par jour pendant cinq jours. Il n'a
pas été établi de dose sans effet observé.
Dans plusieurs études de cancérogénicité, au cours desquelles de
l' o-toluidine avait été administrée par voie orale à des rats et à
des souris, on a noté une augmentation significative de l'incidence
des tumeurs bénignes et malignes dans divers tissus. L' o-toluidine
n'est généralement pas mutagène dans les épreuves classiques de
mutagénicité bactérienne, mais elle est clastogène dans les cellules
mammaliennes in vitro. Sa génotoxicité n'a pas été établie avec
certitude in vivo, encore que des résultats positifs aient été
signalés. Compte tenu de la large distribution des tumeurs observées
chez les animaux exposés, ainsi que de l'activité clastogène constatée
dans les épreuves in vitro sur des systèmes mammaliens, on peut
considérer que l' o-toluidine se comporte comme un cancérogène
génotoxique. On ne dispose pas de données permettant d'évaluer le
risque d'effets indésirables sur la reproduction ou le développement.
En raison de l'absence de données pertinentes relatives à
l'exposition, il n'a pas été possible d'évaluer les risques que
l'exposition indirecte à l' o-toluidine présente dans l'environnement
général pourraient poser pour la santé de l'homme. Dans
l'environnement professionnel, les risques d'effets cancérogènes et
génotoxiques pourraient être significatifs. Il n'a pas été possible
de recueillir de données utiles sur les concentrations d' o-toluidine
dans différents milieux et sur ses effets sur les organismes
aquatiques et terrestres, de sorte que les risques pour les organismes
en question n'ont pu être évalués.
RESUMEN DE ORIENTACION
Esta reseña de la evaluación química internacional de la
orto-toluidina (o-toluidina) está basada en un análisis del Comité
Ejecutivo sobre Salud y Seguridad del Reino Unido acerca de los
aspectos de interés de ese compuesto en relación con la salud humana,
sobre todo la salud ocupacional (Gregg et al., 1996); el análisis
abarca los datos obtenidos hasta marzo de 1992. Posteriormente se ha
incorporado la información adicional pertinente reunida durante el
examen colegiado internacional de la presente reseña, previo examen
por el Comité de Revisión Final. En el apéndice 1 se informa sobre la
preparación y el examen colegiado del documento de partida, y en el
apéndice 2 sobre el examen colegiado de la presenta reseña. La
publicación de ésta fue aprobada en una reunión del Comité de Revisión
Final, celebrada en Bruselas (Bélgica) del 18 al 20 de noviembre de
1996. El apéndice 3 contiene la lista de los participantes en esa
reunión. Se ha reproducido asimismo la ficha internacional de
seguridad quimica (ICSC 0341) de la o-toluidina, elaborada por el
Programa Internacional de Seguridad de las Sustancias Químicas (IPCS,
1993).
La o-toluidina (CAS No.95-53-4) es una sustancia química
sintética que a temperatura ambiente es un liquido amarillo claro. Se
utiliza principalmente en la elaboración de pigmentos, aunque también
para producir caucho, productos químicos y plaguicidas, y como agente
polimerizante para los sistemas de resinas epoxidicas.
La o-toluidina tiene una toxicidad entre moderada y baja y puede
producir una muy ligera irritación cutánea e irritación moderada de
los ojos. No se dispone de información sobre su potencial de
sensibilización de la piel o de las vías respiratorias. Los
principales signos de toxicidad tras la exposición aguda y de corta
duración a esa sustancia quimica son la metahemoglobinemia y los
efectos asociados en el bazo. Tales efectos se han observado en ratas
a las que se había administrado o-toluidina a dosis diarias de 225
mg/kg de peso corporal durante 5 días; no se ha establecido un nivel
sin efectos adversos observados.
En diversos estudios de carcinogenicidad en que se administró
o-toluidina por vía oral a ratas y ratones se observó un aumento
significativo de la incidencia de tumores benignos y malignos en
distintos tejidos. La o-toluidina no suele tener efectos mutagénicos
en las pruebas habituales de mutagenicidad bacteriana, pero es
clastogénica en células de mamífero in vitro. No se conoce con certeza
la genotoxicidad de la o-toluidina in vivo, pero se ha informado de
algunos resultados positivos. Teniendo en cuenta la amplia
distribución de los rumores que aparecen en los animales expuestos a
esa sustancia, así como la actividad clastogénica detectada en ensayos
in vitro con células de mamífero, es probable que la o-toluidina actúe
como carcinógeno genotóxico. No se hallaron datos de interés para
evaluar el riesgo de efectos para la reproducción o el desarrollo.
Debido a la falta de datos pertinentes sobre la exposición, no se
han podido evaluar los riesgos para la salud humana asociados a la
exposición indirecta a la o-toluidina presente en el medio ambiente
general. En el entorno ocupacional, es posible que haya riesgos
significativos de efectos carcinogénicos y genotóxicos. No se han
hallado datos útiles sobre las concentraciones de o-toluidina en
diversos ambientes y sobre sus efectos en organismos acuáticos y
terrestres, por lo que no ha sido posible evaluar los riesgos de la
exposición al compuesto entre los organismos presentes en el medio.
Although some toxicological studies on o-toluidine have employed its
hydrochloride salt (o-toluidine hydrochloride), this is unlikely to
significantly alter the observed health effects of the parent
chemical.
3. ANALYTICAL METHODS
Short- and long-term personal monitoring can be undertaken by
pumped sampling either through acid-coated filters or through
NIOSH-type silica gel tubes (NIOSH, 1987; HSE, 1993). The filters
are desorbed with a neutralizing solution and analysed by
high-performance liquid chromatography; the tubes are desorbed with
solvent and analysed by gas chromatography. Screening measurements
may be conducted using a colorimetric detector tube.
The analytical monitoring of urine for o-toluidine and its
metabolites may be a useful means of assessing occupational exposure,
especially where there is potential for skin absorption. Methods for
biological monitoring of occupationally exposed individuals have been
reported (Brown et al., 1995; Ward et al., 1996). These techniques
employ urine sampling for the determination of o-toluidine and its
N-acetyl metabolites and blood sampling for the detection of
o-toluidine-haemoglobin adducts.
The determination of o-toluidine in water samples may involve
extraction under acidic and alkali conditions, followed by brominated
ether extraction and subsequent analysis using gas chromatography with
electron capture detection (detection limit 0.1-0.6 µg/litre). The
analysis of o-toluidine in sediment may involve steam distillation
under alkali conditions with quantitation by gas chromatography
coupled with electron capture detection (detection limit 0.002-0.012
µg/g dry matter).
4. SOURCES OF HUMAN AND ENVIRONMENTAL EXPOSURE
The principal use of o-toluidine worldwide is in the
manufacture of dyestuffs, although it is also used in the production
of rubber, chemicals, and pesticides and as a curing agent for epoxy
resin systems. o-Toluidine is also used as a corrosion inhibitor in
paint formulations and possibly has limited uses in analytical
laboratory procedures. There are no known domestic or household uses
for o-toluidine.
Global production data for o-toluidine were not identified. In
the USA in 1975, more than 900 t of the chemical were produced, and
another 1000 t were imported. Total production of o-toluidine in
Great Britain is approximately 6000 t per year, 90% of which is
exported. Approximately 610 and 545 t of o-toluidine were imported
into Japan in 1992 and 1993, respectively.
5. ENVIRONMENTAL TRANSPORT, DISTRIBUTION, AND TRANSFORMATION
Using an equilibrium model to assess partitioning between
environmental media, Yoshida et al. (1983) estimated the distribution
of o-toluidine to be 14.5% to air, 83.3% to water, 0.4% to soil,
1.9% to sediment, 2.3 × 10-5 % to biota (fish), and 0.21% to suspended
sediment. The half-life for o-toluidine in Rhine River water was
estimated to be about 1 day (Zoeteman et al., 1980).
6. ENVIRONMENTAL LEVELS AND HUMAN EXPOSURE
6.1 Environmental levels
o-Toluidine was detected in 3/46 samples of Rhine River water
collected at the Germany-Netherlands border in 1979; the mean and
maximum concentrations were 0.03 and 1.8 µg/litre, respectively
(Wegman & de Korte, 1981). o-Toluidine was detected in water
collected from a shallow aquifer contaminated by coal tar wastes in
the USA; however, concentrations were not reported (Pereira et al.,
1983).
In Japan, 8/68 samples of surface water collected in 1976
contained o-toluidine (detection limit 0.1-0.6 µg/litre) at levels
ranging from 0.14 to 20 µg/litre; 27 samples of sediment collected in
the same year contained o-toluidine (detection limit 0.002-0.012
mg/kg) at concentrations ranging from 0.002 to 0.013 mg/kg dry weight
(J. Sekizawa, personal communication, 1996). o-Toluidine was not
detected (detection limit 0.05-150 ng/m3) in 72 samples of air
collected in Japan in 1985 (J. Sekizawa, personal communication,
1996).
6.2 Human exposure
Exposure of the general population to o-toluidine present in
the environment could not be estimated, owing to the lack of relevant
data on levels of this chemical in air, drinking-water, and
foodstuffs. Information on human exposure to o-toluidine is limited
to occupational settings.
In the United Kingdom in 1992, approximately 120 individuals were
potentially exposed to o-toluidine during activities involving its
manufacture and use; about a quarter of these were maintenance rather
than process workers. Eight-hour time-weighted-average exposures to
o-toluidine in seven different industries in the United Kingdom
ranged from 0.007 to 2.7 ppm (0.03-11.8 mg/m3); all but one of these
exposures were <0.3 ppm (<1.3 mg/m3). A concentration of 2.7
ppm (11.8 mg/m3) o-toluidine was measured at a site involving
centrifugation at high temperature. If it is assumed that a worker
weighing 70 kg breathes 10 m3 of air during a typical working day and
that inhaled o-toluidine is completely absorbed, exposure to
o-toluidine at a concentration of 1.3 mg/m3 in workplace air can be
estimated to result in a daily o-toluidine intake of approximately
0.2 mg/kg body weight. Exposure to o-toluidine associated with some
processes conducted at elevated temperatures (i.e. exposure to
o-toluidine at 11.8 mg/m3) can be estimated to result in a daily
o-toluidine intake of approximately 1.7 mg/kg body weight. Dermal
exposure to o-toluidine may also occur in the occupational
environment; however, quantitative data were not identified.
7. COMPARATIVE KINETICS AND METABOLISM IN LABORATORY ANIMALS
AND HUMANS
Biological monitoring to assess human exposure to o-toluidine
indicates that absorption may occur through inhalation and dermal
contact; however, quantitative information was not identified.
o-Toluidine binds to haemoglobin (Ward et al., 1996).
N-acetylated metabolites of o-toluidine are eliminated in the
urine (Brown et al., 1995).
In laboratory animals, studies on the kinetics and metabolism of
o-toluidine have involved rats exposed orally or via dermal contact.
There is extensive absorption (at least 92% of the administered oral
dose) of o-toluidine from the gastrointestinal tract (Cheever et
al., 1980). Limited dermal absorption was reported in a poor-quality
study (Senczuk et al., 1984), although the structure of o-toluidine
suggests that, like other aromatic amines, it is lipid soluble and
therefore likely to be readily absorbed through the skin. Oral and
subcutaneous studies have revealed that o-[14C]toluidine and its
metabolites are excreted mainly in the urine, with at least 90% of the
administered radioactivity appearing in the urine within 72 hours
after exposure (Son et al., 1977; Cheever et al., 1980). Small
amounts of radioactivity were also detected in the faeces and exhaled
carbon dioxide. Up to one-third of the o-toluidine administered was
recovered unchanged in the urine. The metabolism of o-toluidine is
characterized principally by ring hydroxylation and N-acetylation,
the major metabolites being 4-amino- m-cresol and, to a lesser
extent, N-acetyl-4-amino- m-cresol (Son et al., 1977; Cheever et
al., 1980). Sulfate conjugates of o-toluidine predominate over
glucuronide conjugates. Binding of o-toluidine metabolites to
haemoglobin has also been observed in rats (Birnier & Neumann, 1988).
8. EFFECTS ON LABORATORY MAMMALS AND IN VITRO TEST SYSTEMS
8.1 Single exposure
o-Toluidine is harmful following acute oral exposure (LD50s of
900 and 940 mg/kg body weight in rats) and is of low acute toxicity
following dermal exposure (LD50 of 3235 mg/kg body weight in rabbits)
(Smyth et al., 1962; Jacobson, 1972). Acute effects include cyanosis,
increased methaemoglobin levels, and related effects in the spleen.
Useful data on effects associated with inhalation exposure were not
identified.
8.2 Irritation and sensitization
Minimal skin irritation and ill-defined eye irritation have been
observed in o-toluidine-exposed rabbits (Smyth et al., 1962). There
is no information available on the skin or respiratory sensitization
potential of o-toluidine in animals.
8.3 Short-term exposure
A 13% decrease in body weight, a 1.5- to 3.0-fold increase in
spleen weight, increased methaemoglobin levels, and congestion,
haemosiderosis, and haematopoiesis in the spleen (all likely
associated with methaemoglobinaemia) were observed in rats orally
administered o-toluidine for 5 days at a dose of 225 mg/kg body
weight per day (Short et al., 1983); no other doses were tested, and a
no-effect level was not identified. Relevant toxicity studies
involving short-term inhalation or dermal exposure to o-toluidine
were not identified.
8.4 Chronic exposure and carcinogenicity
Increased incidences of benign and malignant tumours have been
observed in rats and mice administered o-toluidine hydrochloride in
the diet.
In one study, groups of 50 male and 50 female F344 rats were
given diets containing 3000 or 6000 ppm (mg/kg) o-toluidine
hydrochloride for 101-104 weeks (equivalent to intakes of
approximately 150 and 300 mg/kg body weight per day, based on a body
weight of 400 g and the consumption of 20 g of food per day) (NCI,
1979; Goodman et al., 1984). Controls consisted of 20 animals of each
sex. In addition to routine clinical observations, gross and
microscopic examinations were conducted on all major tissues and
organs and on all gross lesions. Exposure to o-toluidine
hydrochloride was associated with a dose-related decrease in body
weight gain and survival. In the control, low-dose, and high-dose
groups, the combined incidence of sarcomas, angiosarcomas, and
osteosarcomas of the spleen was 0/20, 9/49 (p = 0.36), and 12/49
(p = 0.01), respectively, in females and 0/20, 8/49, and 4/42,
respectively, in males. The combined incidence of sarcomas,
fibrosarcomas, angiosarcomas, and osteosarcomas in multiple
(unspecified) organs was, among females, 0/20, 3/50, and 21/49
(p < 0.001) and, among males, 0/20, 15/50 (p = 0.003), and
37/49 (p < 0.001), for animals in the control, low-dose, and
high-dose groups, respectively. In females, the incidence of
transitional cell carcinomas of the urinary bladder in the control,
low-dose, and high-dose groups was 0/20, 9/45 (p = 0.028), and
22/47 (p < 0.001), respectively; the incidence of this tumour
was not significantly increased in the male rats. The incidence of
malignant mesothelioma of the serous covering of the testes (tunica
vaginalis) was 0/20, 10/50, and 6/49 in the control, low-dose, and
high-dose groups, respectively. Although not observed among control
animals, splenic fibromas were noted in the exposed animals; however,
the incidence was significantly increased only for males in the
low-dose group (10/49; p = 0.024). For animals in the control,
low-dose, and high-dose groups, the incidence of skin fibromas among
males was 0/20, 28/50 (p < 0.001), and 27/49 (p < 0.001),
respectively, and the incidence of mammary gland fibroadenomas among
females was 6/20, 20/50, and 35/49 (p = 0.002), respectively.
Non-neoplastic effects that were not observed in control animals
included splenic fibrosis (in 12-27% and 6-12% of exposed males and
females, respectively), splenic mesothelial hyperplasia (in 12-37% and
24-65% of exposed males and females, respectively), hyperplasia of the
urinary bladder epithelium (in 16-18% and 28-47% of exposed males and
females, respectively), and myocardial fibrosis (in 17-34% of exposed
males). Liver necrosis was noted in 24-35% of exposed males (10% in
controls) and in 2-31% of exposed females (0% in controls).
In a study in which groups of 30 male F344 rats received 0 or 62
mg o-toluidine hydrochloride in the diet each day for 72 weeks
(approximately 470 and 130 mg/kg body weight per day at the beginning
and end of the study, respectively), followed by a 16-week recovery
period, exposure to o-toluidine reduced survival (6/30 and 18/30
survivors in the exposed and control groups, respectively) (Hecht et
al., 1982). Incidences of the following tumours (in the control and
exposed groups, respectively) were: bladder epithelial cell tumours,
0/30 and 4/30; skin fibromas, 1/30 and 25/30; splenic fibromas, 0/30
and 10/30; mammary tumours, 0/30 and 13/30; and peritoneal tumours,
2/30 and 14/30. Although neither the statistical significance of
these results nor the incidence of non-neoplastic effects was
discussed in this report, the results reveal an increased occurrence
of a variety of tumour types in animals administered o-toluidine
hydrochloride for 72 weeks.
In another study in which several chemicals were examined, groups
of 25 male Charles River CD rats were given diets containing
o-toluidine hydrochloride at concentrations of 8000 or 16 000 ppm
(mg/kg) for 3 months (estimated intakes of approximately 800 and 1600
mg/kg body weight per day, assuming 200 g for the body weight of rats
consuming 20 g of food per day) (Weisburger et al., 1978). Excessive
toxicity, based upon increased mortality and reductions in body
weight, resulted in the concentrations being reduced to 4000 and 8000
ppm (mg/kg) (with estimated intakes of 400 and 800 mg/kg body weight
per day) for a further 15 months, followed by a 6-month observation
and recovery period. Only animals that survived 6 months or more were
necropsied. Data on survival or general toxicity were not provided.
Controls included one matched group (n = 16 animals) used for this
portion of the overall study, as well as the pooled controls
(n = 111) from the entire investigation. There was a statistically
significant increase in the incidence (0/16, 18/111, 18/23, and 21/24
in the matched control, pooled control, low-dose, and high-dose
groups, respectively) of subcutaneous fibroma and fibrosarcoma in
o-toluidine-exposed animals. The incidence of bladder transitional
cell carcinoma was 0/16, 5/111, 3/23, and 4/24 in the matched control,
pooled control, low-dose, and high-dose groups, respectively.
Statistically significant increases in hepatocellular carcinomas
and adenomas, as well as haemangiosarcomas, were observed in a study
in which groups of 50 male and 50 female B6C3F1 mice were given diets
containing 1000 or 3000 ppm (mg/kg) o-toluidine hydrochloride
(estimated intakes of 110 and 340 mg/kg body weight per day, assuming
30 g for the body weight of mice consuming 3.4 g of food per day) for
101-104 weeks (NCI, 1979). Twenty animals of each sex served as
unexposed controls. In the control, low-dose, and high-dose groups,
the incidences of hepatocellular carcinoma (in females),
hepatocellular adenoma (in females), and haemangiosarcoma (in males)
were 0/20, 2/49, and 7/50; 0/20, 2/49, and 6/50; and 1/19, 1/50, and
10/50, respectively.
Significantly increased incidences of haemangiosarcomas and
haemangiomas were observed in a study in which groups of 25 male and
25 female CD-1 mice were given diets containing o-toluidine
hydrochloride at concentrations of 16 000 or 32 000 ppm (mg/kg)
(estimated intakes of 1800 and 3600 mg/kg body weight per day,
assuming 30 g for the body weight of mice consuming 3.4 g of food per
day) for 3 months (Weisburger et al., 1978). Excessive toxicity,
based upon increased mortality and reductions in body weight, resulted
in the concentrations being reduced to 8000 and 16 000 ppm (mg/kg) for
a further 15 months, followed by a 3-month observation and recovery
period. Only animals that survived 6 months or more were necropsied.
Controls included one matched group used for this portion of the
overall study, as well as the pooled controls from the entire
investigation. In the matched control, pooled control, low-dose, and
high-dose groups, the incidence of abdominal haemangiosarcomas and
haemangiomas was 0/14, 5/99, 5/14, and 9/11 (in males) and 0/15,
9/102, 5/18, and 9/21 (in females), respectively.
The results from limited (i.e. poorly conducted and/or reported)
dermal and parenteral studies conducted in various species and from an
oral toxicity study in dogs (Morigami & Nisimura, 1940; Steinhoff,
1981; Hecht et al., 1983) do not contribute meaningfully to the
assessment of o-toluidine.
8.5 Genotoxicity and related end-points
Based upon the results of assays conducted in Salmonella
typhimurium and Escherichia coli, o-toluidine was not considered
to be mutagenic in standard bacterial tests (McCann et al., 1975;
Ferretti et al., 1977; Garner & Nutman, 1977; Rosenkranz & Poirier,
1979; Simmon, 1979; Zimmer et al., 1980; Baker & Bonin, 1981, 1985;
Garner et al., 1981; MacDonald, 1981; Martire et al., 1981; Matsushima
et al., 1981; Richold & Jones, 1981; Rowland & Severn, 1981; Simmon &
Shepherd, 1981; Trueman, 1981; Venitt & Crofton-Sleigh, 1981; Rexroat
& Probst, 1985). However, positive results in the Ames test have been
observed when norharman was added to the test system (Nagao et al.,
1978; Nagao & Takahashi, 1981; Sugimura & Nagao, 1981). Results from
several cytogenetic studies have indicated that o-toluidine is
clastogenic in mammalian cells in vitro (Danford, 1985; Gulati et
al., 1985; Ishidate & Sofuni, 1985; Priston & Dean, 1985).
The in vivo genotoxicity of o-toluidine has been adequately
tested only in mice. No evidence of clastogenicity was observed in
several high-quality studies (a bone marrow cytogenetic assay and
three bone marrow micronucleus tests) in which the chemical was
injected intraperitoneally (Salamone et al., 1981; Tsuchimoto &
Matler, 1981; McFee et al., 1989). Although there was no reporting of
bone marrow toxicity in these studies, use of an intraperitoneal
injection with elevated dose levels would suggest that o-toluidine
probably reached the target tissue (bone marrow). In contrast, in the
best conducted bone marrow sister chromatid exchange assay, high doses
of o-toluidine apparently produced positive results in mice (McFee
et al., 1989). A positive result has also been reported for the
induction of DNA single strand breaks in mice; however, the poor
description of the study precludes the drawing of definitive
conclusions (Cesarone et al., 1982). Despite some suggestions of
clastogenicity, the genotoxic potential of o-toluidine in vivo
remains uncertain.
8.6 Reproductive and developmental toxicity
Relevant information on the reproductive and developmental
toxicity of o-toluidine in laboratory animals was not identified.
8.7 Immunological and neurological effects
Relevant toxicological studies in which immunological or
neurological effects were assessed were not available. However,
evidence of specific adverse immunological and neurological effects
has not been reported in general toxicity studies.
9. EFFECTS ON HUMANS
Relevant case reports on adverse health effects associated with
exposure to o-toluidine were not available.
Other than information on potential carcinogenic effects, there
are few useful data available on other health-related effects
associated with exposure to o-toluidine.
Exposure to chemicals including o-toluidine in the dyestuffs
industry and more recently in the rubber industry has been reported to
be associated with an increased incidence of bladder cancer. For
example, expected and observed cases of bladder cancer were recorded
in a thorough, well conducted retrospective cohort study at a rubber
chemical plant in upstate New York (Ward et al., 1991). The cohort
consisted of all 1749 male and female workers employed at the plant
between 1973 and 1988. The work-force was relatively young; 72% were
born after 1 January 1939. It was estimated that cohort members were
"slightly more likely" than the general population of the USA to be
current or former smokers. These workers were exposed primarily to
o-toluidine and aniline. In 1988, airborne o-toluidine and
aniline levels were <1 ppm (<4.4 mg/m3 for o-toluidine).
Based upon 13 identified cases of bladder cancer among all 1749
employees, compared with 3.61 cases expected (estimated from the rate
in the population of the state of New York, excluding New York City),
the standardized incidence ratio (SIR) was 3.6 (90% confidence
interval [CI] = 2.13-5.73). The SIRs for bladder cancer among workers
"definitely exposed" (n = 708), "possibly exposed" (n = 288), and
"probably unexposed" (n = 753) to o-toluidine and aniline were
6.48 (90% CI = 3.04-12.2; 7 observed cases), 3.66 (90% CI = 1.25-8.37;
4 observed cases), and 1.39 (90% CI = 0.25-4.39; 2 observed cases),
respectively. The risk of bladder cancer increased with duration of
exposure and time since first exposure. The SIRs for bladder cancer
among the "definitely exposed" workers with <5, 5-9.99, and >10
years of exposure to these chemicals were 0 (0 observed cases), 8.8
(90% CI = 0.45-41.7; 1 observed case), and 27.2 (90% CI = 11.8-53.7;
6 observed cases), respectively. The SIRs for bladder cancer among
workers with <10, 10-20, and >20 years since their first employment
in the "definitely exposed" department of the plant were 0 (0 observed
cases), 2.03 (90% CI = 0.10-9.64; 1 observed case), and 16.4 (90% CI =
7.13-32.3; 6 observed cases), respectively. It was calculated that
smoking was unlikely to account for the increased incidence of bladder
cancer in this group of workers. The mean latency period for the
group of seven "definitely exposed" workers with bladder cancer was 23
years. Although the carcinogenic potential of o-toluidine
specifically cannot be definitively determined from this study, the
findings merit considerable concern.
Other studies of workers employed in the dyestuffs industry
include those by Vigliani & Barsotti (1961), Khlebnikova et al.
(1970), Zavon et al. (1973), Conso & Pontal (1982), and Rubino et al.
(1982). However, as in the study of rubber chemical workers, it was
not possible to definitively link the increased incidence of bladder
cancer specifically to o-toluidine because of concurrent exposure to
other chemicals.
10. EFFECTS ON OTHER ORGANISMS IN THE LABORATORY AND FIELD
Relevant information on the effects of o-toluidine on aquatic
or terrestrial organisms was not identified. However, toxicity
thresholds for the inhibition of algal growth (Microcystis
aeruginosa, 0.31 mg/litre; Scenedesmus quadricauda, 6.3 mg/litre)
have been reported.1
1 Source: EnviChem Data Bank of Environmental Properties of
Chemicals. Helsinki, Finland, Finnish Environment Agency, version 1.0,
1995.
11. EFFECTS EVALUATION
11.1 Evaluation of health effects
11.1.1 Hazard identification and dose-response assessment
Available data are inadequate to allow the potential risks of
reproductive or developmental effects on human health to be assessed.
Carcinogenicity is considered, however, to be the critical effect
associated with potential exposure to o-toluidine. In several
experimental studies, the oral administration of o-toluidine
hydrochloride increased the incidence of benign and/or malignant
tumours in various tissues in rats (spleen sarcomas and fibromas in
males and females, mesotheliomas of the scrotum in males, transitional
cell carcinomas of the urinary bladder in females, skin fibromas and
mammary gland fibroadenomas in males and females, respectively).
Clear evidence of carcinogenicity has also been observed in oral
studies in mice (hepatocellular carcinomas and adenomas,
haemangiosarcomas, and haemangiomas). Even where the increased tumour
incidences were not statistically significant, they were considered to
be of biological significance in view of the low incidences of such
tumours in historical controls (Haseman et al., 1990). o-Toluidine
is genotoxic in vitro; although the genotoxicity studies conducted
in vivo do not allow definite conclusions to be drawn, there is some
evidence of genotoxic potential. Carcinogenicity studies in rats and
mice have yielded tumours in both sexes and in multiple organs;
therefore, the possibility of involvement of a genotoxic mechanism
cannot be eliminated.
Several studies of workers employed in the dyestuffs and rubber
industries found that exposure to chemicals, including o-toluidine,
appears to be associated with an increased incidence of bladder
cancer. Although it is difficult to draw definite conclusions
regarding the carcinogenic potential of o-toluidine in humans from
these studies of workers exposed to multiple chemicals, this evidence,
together with data from experimental carcinogenicity bioassays, raises
concern about the risk of cancer in exposed humans. It would
therefore be prudent to consider that o-toluidine is probably
carcinogenic in humans, possibly through involvement of a genotoxic
mechanism.
11.1.2 Criteria for setting guidance values for o-toluidine
On the basis that the carcinogenicity of o-toluidine may
involve a genotoxic mechanism, it is not possible to reliably identify
a threshold at which exposure to o-toluidine would not result in
some risk to human health.
11.1.3 Sample risk characterization
It is recognized that there are a number of different approaches
to assessing the risks to human health posed by genotoxic and
carcinogenic substances. In some jurisdictions, there are models for
characterizing potency, which may be of some benefit in
priority-setting schemes.
The example here is from the occupational environment, as the
lack of available data precludes the characterization of potential
cancer risks for the general population. In the United Kingdom, a
Maximum Exposure Limit for o-toluidine (which is not a health-based
standard) of 0.2 ppm (0.9 mg/m3; 8-hour time-weighted average) has
been proposed. The Maximum Exposure Limit was based on a level of
control that was deemed (by tripartite agreement) to be reasonably
practicable under workplace conditions within the United Kingdom. In
the United Kingdom, there is a continuing remit to reduce exposure
levels as much as reasonably practicable with the technology that is
currently available.
11.2 Evaluation of environmental effects
The lack of available information precludes adequate assessment
of potential risks to environmental organisms.
12. PREVIOUS EVALUATIONS BY INTERNATIONAL BODIES
The International Agency for Research on Cancer (IARC, 1987) has
classified o-toluidine in Group 2B (possibly carcinogenic to
humans), based upon sufficient evidence for carcinogenicity in animals
and inadequate evidence for carcinogenicity in humans.
Information on international hazard classification and labelling
is included in the International Chemical Safety Card reproduced in
this document.
13. HUMAN HEALTH PROTECTION AND EMERGENCY ACTION
Human health hazards, together with preventative and protective
measures and first aid recommendations, are presented in the
International Chemical Safety Card (ICSC 0341) reproduced in this
document.
13.1 Human health hazards
Following short-term exposure, o-toluidine could induce
methaemoglobinaemia. After long-term or repeated exposure,
o-toluidine is considered possibly carcinogenic to humans.
13.2 Advice to physicians
If splashed with o-toluidine, it is crucial to remove all wet
or contaminated clothing and wash the entire body with soap and water.
Following such an incident, the degree of methaemoglobinaemia needs to
be determined hourly until a decrease is well established. Above 30%
methaemoglobinaemia, administer oxygen under continuous observation;
above 50% methaemoglobinaemia, further administer intravenously 1000
cc of a 5% glucose solution containing ascorbic acid; above 60%
methaemoglobinaemia, further administer intravenously 10-20 cc of a 1%
solution of methylene blue. If there is no response to treatment with
methylene blue, then haemodialysis or exchange transfusion is useful.
13.3 Health surveillance advice
For workers exposed to o-toluidine, a health surveillance
programme should include regular urinary cytology, with more specific
procedures in the case of positive results.
13.4 Spillage
In the event of spillage, measures should be undertaken to
prevent this chemical from reaching drains and watercourses.
14. CURRENT REGULATIONS, GUIDELINES, AND STANDARDS
Information on national regulations, guidelines, and standards is
available from the International Register of Potentially Toxic
Chemicals (IRPTC) legal file.
The reader should be aware that regulatory decisions about
chemicals taken in a certain country can be fully understood only in
the framework of the legislation of that country. The regulations and
guidelines of all countries are subject to change and should always be
verified with appropriate regulatory authorities before application.
INTERNATIONAL CHEMICAL SAFETY CARD
ortho-TOLUIDINE ICSC: 0341
ortho-TOLUIDINE
1-Amino-2-methylbenzene
2-Aminotoluene
o-Methylaniline
C7H9N/C6H4CH3NH2
Molecular mass: 107.2
CAS # 95-53-4
RTECS # XU2975000
ICSC # 0341
UN # 1708
TYPES OF ACUTE HAZARDS/ PREVENTION FIRST AID/
HAZARD/ SYMPTOMS FIRE FIGHTING
EXPOSURE
FIRE Combustinble. Gives off NO open flames. NO contact Powder, AFFF, foam, carbon
irritating or toxic fumes (or with nitric acid. dioxide.
gases) in a fire.
EXPLOSION Above 85°C explosive Above 85°C use a closed In case of fire: keep drums,
vapour/air mixtures may be system, ventilation. etc., cool by spraying with
formed. water.
EXPOSURE AVOID ALL CONTACT! IN ALL CASES CONSULT A
DOCTOR!
* INHALATION Blue lipsor finger nails. Ventilation, local exhaust, Fresh air, rest. Artificial
Blue skin. Confusion. or breathing protection. respiration if indicated.
Dizziness. Headache. Refer for medical attention.
Shortness of breath.
Weakness.
INTERNATIONAL CHEMICAL SAFETY CARD (continued)
TYPES OF ACUTE HAZARDS/ PREVENTION FIRST AID/
HAZARD/ SYMPTOMS FIRE FIGHTING
EXPOSURE
* SKIN MAY BE ABSORBED! Redness. Protective gloves. Protective Remove contaminated clothes.
Blue lips or fingernails. clothing. Rinse and then wash skin with
Blue skin (Further see water and soap. Refer for
inhalation). medical attention.
* EYES Redness. Pain. Safety goggles. First rinse with plenty of
water for several minutes
(remove contact lenses if
easily possible), then take
to a doctor.
* INGESTION Blue lips or fingernails. Do not eat, drink, or smoke Rinse mouth. Induce vomiting
Blue skin. Dizziness. during work. (ONLY IN CONSCIOUS PERSONS!).
Headache. Laboured breathing Refer for medical attention.
(further see inhalation).
INTERNATIONAL CHEMICAL SAFETY CARD (continued)
SPILLAGE DISPOSAL STORAGE PACKAGING & LABELLING
Collect leaking and spilled liquid in Separated from strong oxidants, strong Do not transport with food and
sealable containers as far as acids, food and feedstuffs. Cool. Dry. feedstuffs.
possible. Absorb remaining liquid in Well closed. Ventilation along the
sand or inert absorbent and remove to floor. UN Hazard Class: 6.1
safe place (extra personal protection: N Packing Group: II
complete protective clothing including
self-contained breathing apparatus).
IMPORTANT DATA PHYSICAL STATE; APPEARANCE: EFFECTS OF SHORT-TERM EXPOSURE:
COLOURLESS TO YELLOW LIQUID, TURNS The substance irritates the eyes and the skin.
REDDISH-BROWN ON EXPOSURE TO AIR AND LIGHT. The substance may cause effects on the blood,
bladder and kidneys, resulting in tissue
CHEMICAL DANGERS: lesions, impaired functions and formation of
The substance decomposes on heating or on methaemoglobin. Exposure to high concentrations
burning producing toxic fumes including may result in damage to kidneys and bladder.
nitrogen oxides. Reacts with strong oxidants, The effects may be delayed. Medical observation
expecially nitric acid. is indicated. See Notes.
OCCUPATIONAL EXPOSURE LIMITS (OELs): EFFECTS OF LONG-TERM OR REPEATED EXPOSURE:
TLV: 2 ppm; 8.8 mg/m3 (as TWA) A2 (skin) The substance may have effects on the blood,
(ACGIH 1994-1995. resulting in the formation of methaemoglobin
(see Notes). This substance is possibly
ROUTES OF EXPOSURE: carcinogenic to humans.
The substanec can be absorbed into the body by
inhalation and through the skin, and by
ingestion.
INHALATION RISK:
Evaporation at 20°C is negligible; a harmful
concentration of airborne particles can,
however, be reached quickly on spraying.
INTERNATIONAL CHEMICAL SAFETY CARD (continued)
PHYSICAL Boiling point: 200°C Flash point: 85°C c.c.
PROPERTIES Melting -16°C Auto-ignition temperature: 482°C
Relative density (water = 1): 1.01 Explosive limits, vol% in air: 1.5-?
Solubility in water: poor Octanol/water partition coefficient
Vapour pressure, kPa at 20°C: 0.2 as log Pow: 1.32
Relative vapour density (air = 1): 3.7
Relative density of the vapour/
air-mixture at 20°C (air = 1): 1.00
ENVIRONMENTAL The substance is harmful to aquatic organisms.
DATA
NOTES Depending on the degree of exposure, periodic medical examination is indicated.
Specific treatment is necessary in case of poisoning with this substance; the
appropriate means with instructions must be available.
The odour warning when the exposure limit value is exceeded is insufficient.
Also consult ICSC #0342, meta-Toluidine and 0343, para-Toluidine.
ICSC: 0341 1.1 NFPA Code: H3; F2; R0
REFERENCES
Baker R, Bonin A (1981) Study of 42 coded compounds with
Salmonella/mammalian microsome assay. In: de Serres FJ, Ashby J,
eds. Progress in mutation research. Vol. 1. Evaluation of
short-term tests for carcinogens. Amsterdam, Elsevier Science
Publishers, pp. 249-260.
Baker R, Bonin A (1985) Tests with the Salmonella
plate-incorporation assay. In: Ashby J, de Serres FJ, Draper M,
Ishidate M Jr, Margolin BH, Matter BE, Shelby MD, eds. Progress
in mutation research. Vol. 5. Evaluation of short-term tests for
carcinogens. Amsterdam, Elsevier Science Publishers, pp. 177-180.
Birnier G, Neumann H (1988) Biomonitoring of aromatic amines. II:
Haemoglobin binding of some monocyclic aromatic amines. Archives
of toxicology, 62(2/3):110-115.
Brown K, Teass A, Simon S, Ward E (1995) A biological monitoring
method for o-toluidine in urine using high performance liquid
chromatography with electrochemical detection. Applied
occupational and environmental hygiene, 10(6):557-565.
Cesarone C, Bolognesi C, Santi L (1982) Evaluation of damage to DNA
after in vivo exposure to different classes of chemicals.
Archives of toxicology, Suppl. 5:355-359.
Cheever K, Richards D, Plotnick H (1980) Metabolism of o-, m- and
p-toluidine in the adult male rat. Toxicology and applied
pharmacology, 56:361-369.
Conso F, Pontal P (1982) Amino-tumours of the bladder. Possible part
of the industrial exposure to o-toluidine and o-aminoazotoluene.
Archives des maladies professionnelles de medecine du travail et de
securité sociale, 43(4):273-319 (HSE Translation No. 14195A).
Danford N (1985) Tests for chromosome aberrations and aneuploidy in
the Chinese hamster fibroblast cell line CH1-L. In: Ashby J, de Serres
FJ, Draper M, Ishidate M Jr, Margolin BH, Matter BE, Shelby MD, eds.
Progress in mutation research. Vol. 5. Evaluation of short-term
tests for carcinogens. Amsterdam, Elsevier Science Publishers, pp.
397-411.
Ferretti J, Lu W, Liu M (1977) Mutagenicity of benzidine and related
compounds employed in the detection of haemoglobin. American
journal of clinical pathology, 67(6):526-527.
Garner R, Nutman C (1977) Testing of some azo dyes and their reduction
products for mutagenicity using Salmonella typhimurium TA 1538.
Mutation research, 44:9-19.
Garner R, Welch A, Pickering C (1981) Mutagenic activity of 42 coded
compounds in the Salmonella/microsome assay. In: de Serres FJ, Ashby
J, eds. Progress in mutation research. Vol. 1. Evaluation of
short-term tests for carcinogens. Amsterdam, Elsevier Science
Publishers, pp. 280-284.
Goodman D, Ward J, Reichardt W (1984) Splenic fibrosis and sarcomas in
F344 rats fed diets containing aniline HCL, p-chloroaniline,
azobenzene, o-toluidine HCL, 4,4'-sulfonyldianiline or D+C red No.
9. Journal of the National Cancer Institute, 73(1):265-273.
Gregg N, South D, Brown R, Cocker J (1996) o-Toluidine; Criteria
document for an occupational exposure limit. London, HSE Books.
Gulati D, Sabharwal P, Shelby M (1985) Tests for the induction of
chromosomal aberrations and sister-chromatid exchanges in cultured
Chinese hamster ovary (CHO) cells. In: Ashby J, de Serres FJ, Draper
M, Ishidate M Jr, Margolin BH, Matter BE, Shelby MD, eds. Progress
in mutation research. Vol. 5. Evaluation of short-term tests for
carcinogens. Amsterdam, Elsevier Science Publishers, pp. 413-426.
Haseman JK, Arnold J, Eustis SL (1990) Tumour incidences in Fischer
344 rats: NTP historical data. In: Boorman GA, Eustis SL, Elwell MR,
Montgomery CA Jr, MacKenzie WF, eds. Pathology of the Fischer rat.
Reference and atlas. San Diego, CA, Academic Press, pp. 555-564.
Hecht S, El-Bayoumy K, Riverson A, Fiala E (1982) Comparative
carcinogenicity of o-toluidine hydrochloride and o-nitrosotoluene
in F-344 rats. Cancer letters, 16:103-108.
Hecht S, El-Bayoumy K, Riverson A, Fiala E (1983) Bioassay for
carcinogenicity of 3,2'-dimethyl-4-nitrosobiphenyl,
o-nitrosotoluene, nitrosobenzene and the corresponding amines in
Syrian golden hamsters. Cancer letters, 20(3):349-354.
HSE (1993) Aromatic amines in air and on surfaces. London, Health &
Safety Executive (Methods for the Determination of Hazardous
Substances, No. 75).
IARC (1987) Ortho-toluidine. In: Overall evaluations of
carcinogenicity: an updating of IARC Monographs Volumes 1-42. Lyon,
International Agency for Research on Cancer, pp. 362-363 (IARC
Monographs on the Evaluation of Carcinogenic Risks to Humans,
Supplement No. 7).
Ishidate M Jr, Sofuni T (1985) The in vitro chromosomal aberration
test using Chinese hamster lung (CHL) fibroblast cells in culture. In:
Ashby J, de Serres FJ, Draper M, Ishidate M Jr, Margolin BH, Matter
BE, Shelby MD, eds. Progress in mutation research. Vol. 5.
Evaluation of short-term tests for carcinogens. Amsterdam, Elsevier
Science Publishers, pp. 427-432.
Jacobson K (1972) Acute oral toxicity of mono- and di-alkyl
ring-substituted derivatives of aniline. Toxicology and applied
pharmacology, 22:153-154.
Khlebnikova M, Gladkova E, Kurenko L, Pshenitsyn A, Shalin B (1970)
[Problems of industrial hygiene and health status of workers engaged
in the production of o-toluidine.] Gigiena Truda i
Professional'nye Zabolevaniya, 14:7-10 (in Russian).
MacDonald D (1981) Salmonella/microsome tests on 42 coded compounds.
In: de Serres FJ, Ashby J, eds. Progress in mutation research.
Vol. 1. Evaluation of short-term tests for carcinogens. Amsterdam,
Elsevier Science Publishers, pp. 285-297.
Martire G, Vricella G, Perfumo A, de Lorenzo F (1981) Evaluation of
the mutagenic activity of coded compounds in the Salmonella test.
In: de Serres FJ, Ashby J, eds. Progress in mutation research.
Vol. 1. Evaluation of short-term tests for carcinogens. Amsterdam,
Elsevier Science Publishers, pp. 271-279.
Matsushima T, Takamoto Y, Shirai A, Sawamura M, Sugimura T (1981)
Reverse mutation test on 42 coded compounds with the E. coli WP2
system. In: de Serres FJ, Ashby J, eds. Progress in mutation
research. Vol. 1. Evaluation of short-term tests for carcinogens.
Amsterdam, Elsevier Science Publishers, pp. 387-395.
McCann J, Choi E, Yamasaki E, Ames B (1975) Detection of carcinogens
as mutagens in the Salmonella/microsome test: assay of 300
chemicals. Proceedings of the National Academy of Sciences of the
United States of America, 72:5135-5139.
McFee A, Jauhar P, Lowe K, MacGregor J, Wehr C (1989) Assays of three
carcinogen/noncarcinogen chemical pairs for in vivo induction of
chromosome aberrations, sister chromatid exchanges and micronuclei.
Environmental and molecular mutagenesis, 14(4):207-220.
Morigami S, Nisimura I (1940) Experimental studies on aniline bladder
tumours. Gann, 34:146-147.
Nagao M, Takahashi Y (1981) Mutagenic activity of 42 coded compounds
in the Salmonella/microsome assay. In: de Serres FJ, Ashby J, eds.
Progress in mutation research. Vol. 1. Evaluation of short-term
tests for carcinogens. Amsterdam, Elsevier Science Publishers,
pp. 361-370.
Nagao M, Yahagi T, Sugimura T (1978) Differences in effects of
norharman with various classes of chemical mutagens and amounts of S9.
Biochemical and biophysical research communications, 83(2):373-378.
NCI (1979) Bioassay of o-toluidine hydrochloride for possible
carcinogenicity (CAS No. 636-21-5). Bethesda, MD, US Department of
Health, Education and Welfare, Public Health Service, National
Institutes of Health, National Cancer Institute (Carcinogenesis
Technical Report Series No. 153; NIH Publication No. 79-1709).
NIOSH (1987) Method 2002. In: NIOSH manual of analytical methods,
3rd ed. Department of Health, Education and Welfare, National
Institute for Occupational Safety and Health (DHEW Publication No.
84-100; 1984, revised 1987).
Pereira W, Rostad C, Garbarino J, Hult M (1983) Groundwater
contamination by organic bases derived from coal tar wastes.
Toxicology and chemistry, 2:283-294.
Priston R, Dean B (1985) Tests for the induction of chromosome
aberrations, polyploidy and sister-chromatid exchanges in rat liver
(RL4) cells. In: Ashby J, de Serres FJ, Draper M, Ishidate M Jr,
Margolin BH, Matter BE, Shelby MD, eds. Progress in mutation
research. Vol. 5. Evaluation of short-term tests for carcinogens.
Amsterdam, Elsevier Science Publishers, pp. 387-395.
Rexroat M, Probst G (1985) Mutation tests with Salmonella using the
plate incorporation assay. In: Ashby J, de Serres FJ, Draper M,
Ishidate M Jr, Margolin BH, Matter BE, Shelby MD, eds. Progress in
mutation research. Vol. 5. Evaluation of short-term tests for
carcinogens. Amsterdam, Elsevier Science Publishers, pp. 201-212.
Richold M, Jones E (1981) Mutagenic activity of 42 coded compounds in
the Salmonella/microsome assay. In: de Serres FJ, Ashby J, eds.
Progress in mutation research. Vol. 1. Evaluation of short-term
tests for carcinogens. Amsterdam, Elsevier Science Publishers,
pp. 314-322.
Rosenkranz H, Poirier L (1979) Evaluation of the mutagenicity and DNA
modifying activity of carcinogens and non-carcinogens in microbial
systems. Journal of the National Cancer Institute, 62:873-892.
Rowland I, Severn B (1981) Mutagenicity of carcinogens and
noncarcinogens in the Salmonella/microsome test. In: de Serres FJ,
Ashby J, eds. Progress in mutation research. Vol. 1. Evaluation of
short-term tests for carcinogens. Amsterdam, Elsevier Science
Publishers, pp. 323-332.
Rubino G, Scansetti G, Piolatto G, Pira E (1982) The carcinogenic
effect of aromatic amines. An epidemiological study on the role of
o-toluidine and 4,4'-methylene bis (2-methylaniline) in inducing
bladder cancer in man. Environmental research, 27(2):241-254.
Salamone M, Heddle J, Katz M (1981) Mutagenic activity of 41 compounds
in the in vivo micronucleus assay. In: de Serres FJ, Ashby J, eds.
Progress in mutation research. Vol. 1. Evaluation of short-term
tests for carcinogens. Amsterdam, Elsevier Science Publishers, pp.
686-697.
Senczuk W, Rucinska H, Zak I (1984) Toxicodynamic properties of
toluidines. Part IV. Dermal absorption of toluidines. Bromatologia
i Chemia Toksykologiczna, 17(2):109-112 (HSE Translation No. 14309).
Short C, King C, Sistrunk P, Kerr K (1983) Subacute toxicity of
several ring-substituted dialkylanilines in the rat. Fundamental
and applied toxicology, 3(4):285-292.
Simmon V (1979) In vitro mutagenicity assays of chemical carcinogens
and related compounds with Salmonella typhimurium. Journal of the
National Cancer Institute, 62:893-899.
Simmon V, Shepherd G (1981) Mutagenic activity of 42 coded compounds
in the Salmonella/microsome assay. In: de Serres FJ, Ashby J, eds.
Progress in mutation research. Vol. 1. Evaluation of short-term
tests for carcinogens. Amsterdam, Elsevier Science Publishers,
pp. 333-342.
Smyth H, Carpenter C, Weil C (1962) Range-finding toxicity data - List
VI. American Industrial Hygiene Association journal, 23:95-107.
Son O, Weiss L, Fiala E, Weisburger E (1977) Metabolism of the
carcinogen o-toluidine. Proceedings of the American Association
for Cancer Research, 18:492.
Steinhoff D (1981) Possible methods of carcinogenicity testing for
substances used at the place of work. Cancer detection and
prevention, 4:41-46.
Sugimura T, Nagao M (1981) Carcinogenic, mutagenic and comutagenic
aromatic amines in human foods. National Cancer Institute
monographs, 58:27-33.
Trueman R (1981) Mutagenicity of 42 coded compounds in a bacterial
assay using Escherichia coli and Salmonella typhimurium. In: de
Serres FJ, Ashby J, eds. Progress in mutation research. Vol. 1.
Evaluation of short-term tests for carcinogens. Amsterdam, Elsevier
Science Publishers, pp. 343-350.
Tsuchimoto T, Matler B (1981) Activity of coded compounds in the
micronucleus test. In: de Serres FJ, Ashby J, eds. Progress in
mutation research. Vol. 1. Evaluation of short-term tests for
carcinogens. Amsterdam, Elsevier Science Publishers, pp. 705-711.
Venitt S, Crofton-Sleigh C (1981) Mutagenicity of 42 coded compounds
in a bacterial assay using E. coli and S. typhimurium. In: de
Serres FJ, Ashby J, eds. Progress in mutation research. Vol. 1.
Evaluation of short-term tests for carcinogens. Amsterdam, Elsevier
Science Publishers, pp. 351-361.
Vigliani E, Barsotti M (1961) Environmental tumours of the bladder in
some Italian dyestuff factories. Medicina del Lavoro, 52:241-250.
Ward E, Carpenter A, Markowitz S, Roberts D, Halperin W (1991) Excess
number of bladder cancers in workers exposed to o-toluidine and
aniline. Journal of the National Cancer Institute, 83(7):501-506.
Ward E, Sabbioni G, DeBord D, Teass A, Brown K, Talaska G, Roberts D,
Ruder A, Streicher R (1996) Monitoring of aromatic amine exposures in
workers at a chemical plant with a known bladder cancer excess.
Journal of the National Cancer Institute, 88(15):1046-1052.
Wegman R, de Korte G (1981) Aromatic amines in surface waters of the
Netherlands. Water research, 15:391-394.
Weisburger E, Russfield A, Homburger F, Weisburger J, Boger E, van
Dongen C, Chu K (1978) Testing of twenty-one environmental aromatic
amines or derivatives for long term toxicity or carcinogenicity.
Journal of environmental pathology and toxicology, 2(2):325-356.
Yoshida K, Shigeoka T, Yamauchi F (1983) Non-steady-state equilibrium
model for the preliminary prediction of the fate of chemicals in the
environment. Ecotoxicology and environmental safety, 7:179-190.
Zavon M, Hoegg V, Bingham E (1973) Benzidine exposure as a cause of
bladder tumours. Archives of environmental health, 27:1-7.
Zimmer D, Mazurek J, Petzold G, Bhuyan B (1980) Bacterial mutagenicity
and mammalian cell DNA damage by several substituted anilines.
Mutation research, 77:317-326.
Zoeteman B, Harmsen K, Linders J, Morra C, Slooff W (1980) Persistent
organic pollutants in river water and ground water of the Netherlands.
Chemosphere, 9:231-249.
APPENDIX 1 - SOURCE DOCUMENT
Gregg N, South D, Brown R, Cocker J (1996) o-Toluidine; Criteria
document for an occupational exposure limit. London, HSE Books (ISBN
0 7176 1057 8)
The authors' draft version was initially reviewed internally by a
group of approximately 10 Health & Safety Executive experts mainly
toxicologists, but also experts in other relevant disciplines (e.g.
epidemiology, occupational hygiene). The toxicology section of the
amended draft was then reviewed by toxicologists from the United
Kingdom Department of Health. Subsequently, the entire Criteria
Document was reviewed by a tripartite advisory committee to the United
Kingdom Health & Safety Commission, the Working Group for the
Assessment of Toxic Chemicals (WATCH). This committee is composed of
experts in toxicology and occupational health and hygiene from
industry, trade unions, and academia.
The members of the WATCH committee at the time of the peer review
were:
Professor J. Bridges (University of Surrey)
Dr A. Hay (Trade Unions Congress)
Dr L. Levy (Institute of Occupational Hygiene, Birmingham)
Dr M. Molyneux (Chemical Industries Association)
Mr A. Moses (Chemical Industries Association)
Dr R. Owen (Trade Unions Congress)
Mr J. Sanderson (independent consultant)
Dr M. Sharratt (University of Surrey)
Dr A. Smith (independent consultant)
APPENDIX 2 - CICAD PEER REVIEW
The draft CICAD on o-toluidine was sent for review to
institutions and organizations identified by IPCS after contact with
IPCS national Contact Points and Participating Institutions, as well
as to identified experts. Comments were received from:
BASF Aktiengesellschaft, Ludwigshafen, Germany
Bayer AG, Leverkusen, Germany
Department of Health, London, United Kingdom
Department of Public Health, Albert Szent-Gyorgyi University
Medical School, Szeged, Hungary
Environmental Health Directorate, Health Canada, Ottawa, Canada
International Agency for Research on Cancer, Lyon, France
Ministry of Health and Welfare, International Affairs Division,
Government of Japan, Tokyo, Japan
National Food Agency of Denmark, Institute of Toxicology,
Ministry of Health, Soborg, Denmark
National Institute for Working Life, Solna, Sweden
National Institute of Occupational Health, Budapest, Hungary
United States Department of Health and Human Services (National
Institute of Environmental Health Sciences)
United States Environmental Protection Agency (Office of
Pollution Prevention and Toxics; National Center for
Environmental Assessment, Office of Research and Development;
Office of Drinking Water)
APPENDIX 3 - CICAD FINAL REVIEW BOARD
Brussels, Belgium, 18-20 November 1996
Members
Dr A. Aitio, Institute of Occupational Health, Helsinki, Finland
Dr K. Bentley, Director, Environment Policy Section, Commonwealth
Department of Human Services and Health, Canberra, Australia
Mr R. Cary, Toxicology and Existing Substances Regulation Unit, Health
& Safety Executive, Merseyside, United Kingdom
Dr J. de Fouw, National Institute of Public Health and Environmental
Protection, Bilthoven, The Netherlands
Dr C. DeRosa, Director, Division of Toxicology, Agency for Toxic
Substances and Disease Registry, Atlanta, GA, USA
Dr S. Dobson, Institute of Terrestrial Ecology, Monks Wood, Abbots
Ripton, Huntingdon, Cambridgeshire, United Kingdom
Dr W. Farland, Director, National Center for Environmental Assessment,
Office of Research and Development, US Environmental Protection
Agency, Washington, DC, USA (Chairperson)
Dr T.I. Fortoul, Depto. Biologia Celular y Tisular, National
University of Mexico and Environmental Health Directorate of the
Health Ministry, Mexico D.F., Mexico
Dr H. Gibb, National Center for Environmental Assessment, US
Environmental Protection Agency, Washington, DC, USA
Dr R.F. Hertel, Federal Institute for Health Protection of Consumers &
Veterinary Medicine, Berlin, Germany
Mr J.R. Hickman, Environmental Health Directorate, Health Canada,
Ottawa, Ontario, Canada
Dr T. Lakhanisky, Head, Division of Toxicology, Institute of Hygiene
and Epidemiology, Brussels, Belgium (Vice-Chairperson)
Dr I. Mangelsdorf, Documentation and Assessment of Chemicals,
Fraunhofer Institute for Toxicology and Aerosol Sciences, Hanover,
Germany
Ms E. Meek, Head, Priority Substances Section, Environmental Health
Directorate, Health Canada, Ottawa, Ontario, Canada
Dr K. Paksy, National Institute of Occupational Health, Budapest,
Hungary
Mr D. Renshaw, Department of Health, London, United Kingdom
Dr J. Sekizawa, Division of Chemo-Bio Informatics, National Institute
of Hygienic Sciences, Tokyo, Japan
Dr H. Sterzl-Eckert, GSF-Forschungszentrum für Umwelt und Gesundheit
GmbH, Institut für Toxikologie, Oberschleissheim, Germany
Professor S. Tarkowski, Department of Environmental Health Hazards,
The Nofer Institute of Occupational Medicine, Lodz, Poland
Dr M. Wallen, National Chemicals Inspectorate (KEMI), Solna, Sweden
Observers
Professor F.M.C. Carpanini,1 Director, Centre for Ecotoxicology and
Toxicology of Chemicals (ECETOC), Brussels, Belgium
Mr R. Haigh,1 Head of Unit, Health and Safety Directorate, European
Commission, Luxembourg
Mr B.U. Hildebrandt, Federal Ministry for the Environment, Nature
Conservation and Nuclear Safety, Bonn, Germany
Mr P. Hurst,1 Chemical and Consumer Policy Officer, Conservation
Policy Division, World Wide Fund for Nature, Gland, Switzerland
Dr A. Lombard (Representative of CEFIC), ELF-ATOCHEM, Paris, France
Dr P. McCutcheon,1 Environment, Consumer Protection and Nuclear
Safety, European Commission, Brussels, Belgium
Dr R. Montaigne, Counsellor, Technical Affairs Department, European
Chemical Industry Council (CEFIC), Brussels, Belgium
Dr M. Pemberton, ICI Acrylics, Lancashire, United Kingdom
Dr A. Smith, Organisation for Economic Co-operation and Development,
Environment Division, Paris, France
1 Invited but unable to attend.
Secretariat
Dr M. Baril, International Programme on Chemical Safety, World Health
Organization, Geneva, Switzerland
Dr L. Harrison, International Programme on Chemical Safety, World
Health Organization, Geneva, Switzerland
Dr M. Mercier, Director, International Programme on Chemical Safety,
World Health Organization, Geneva, Switzerland
Dr P. Toft, Associate Director, International Programme on Chemical
Safety, World Health Organization, Geneva, Switzerland
RÉSUMÉ D'ORIENTATION
Ce CICAD (document international succinct sur l'évaluation des
risques chimiques) relatif à l' ortho-toluidine (o-toluidine) est
fondé sur une étude, menée par le United Kingdom Health & Safety
Executive (Gregg et al., 1996), des principaux problèmes posés par
cette substance du point de vue de l'hygiène industrielle, étude qui
prenait en compte les données connues en mars 1992. Des informations
complémentaires ont été incorporées à ce CICAD lors de l'examen par
les pairs, puis par le Comité d'Évaluation finale. Les informations
relatives à la préparation du document initial et à son examen par les
pairs figurent à l'appendice 1. Les renseignements concernant
l'examen du CICAD par les pairs font l'objet de l'appendice 2. La
publication de ce CICAD a été approuvée à une réunion du Comité
d'Évaluation finale qui s'est tenue à Bruxelles (Belgique) du 18 au
20 novembre 1996. La liste des participants à cette réunion figure à
l'appendice 3. La fiche d'information sur la sécurité chimique de
l' o-toluidine (ICSC 0341), préparée par le Programme international
sur la sécurité chimique (IPCS, 1993), est également reproduite dans
le présent document.
L' o-toluidine (CAS N° 95-53-4) est un produit chimique de
synthèse qui se présente à la température ambiante sous la forme d'un
liquide jaune pâle. Elle est utilisée principalement dans la
fabrication de colorants, mais aussi de caoutchouc, de produits
chimiques et de pesticides. C'est également un agent de
polymérisation des résines époxy.
La toxicité aigue de l' o-toluidine est modérée à faible.
L' o-toluidine est très légèrement irritante pour la peau et
légèrement irritante pour les yeux. On ne possède pas d'information
sur le potentiel de sensibilisation cutanée ou respiratoire. Les
principaux signes de toxicité à la suite d'une exposition massive et
de courte durée sont une méthémoglobinémie et des effets connexes sur
la rate. Ces effets ont été observés chez des rats soumis à une dose
de 225 mg/kg de poids corporel par jour pendant cinq jours. Il n'a
pas été établi de dose sans effet observé.
Dans plusieurs études de cancérogénicité, au cours desquelles de
l' o-toluidine avait été administrée par voie orale à des rats et à
des souris, on a noté une augmentation significative de l'incidence
des tumeurs bénignes et malignes dans divers tissus. L' o-toluidine
n'est généralement pas mutagène dans les épreuves classiques de
mutagénicité bactérienne, mais elle est clastogène dans les cellules
mammaliennes in vitro. Sa génotoxicité n'a pas été établie avec
certitude in vivo, encore que des résultats positifs aient été
signalés. Compte tenu de la large distribution des tumeurs observées
chez les animaux exposés, ainsi que de l'activité clastogène constatée
dans les épreuves in vitro sur des systèmes mammaliens, on peut
considérer que l' o-toluidine se comporte comme un cancérogène
génotoxique. On ne dispose pas de données permettant d'évaluer le
risque d'effets indésirables sur la reproduction ou le développement.
En raison de l'absence de données pertinentes relatives à
l'exposition, il n'a pas été possible d'évaluer les risques que
l'exposition indirecte à l' o-toluidine présente dans l'environnement
général pourraient poser pour la santé de l'homme. Dans
l'environnement professionnel, les risques d'effets cancérogènes et
génotoxiques pourraient être significatifs. Il n'a pas été possible
de recueillir de données utiles sur les concentrations d' o-toluidine
dans différents milieux et sur ses effets sur les organismes
aquatiques et terrestres, de sorte que les risques pour les organismes
en question n'ont pu être évalués.
RESUMEN DE ORIENTACION
Esta reseña de la evaluación química internacional de la
orto-toluidina (o-toluidina) está basada en un análisis del Comité
Ejecutivo sobre Salud y Seguridad del Reino Unido acerca de los
aspectos de interés de ese compuesto en relación con la salud humana,
sobre todo la salud ocupacional (Gregg et al., 1996); el análisis
abarca los datos obtenidos hasta marzo de 1992. Posteriormente se ha
incorporado la información adicional pertinente reunida durante el
examen colegiado internacional de la presente reseña, previo examen
por el Comité de Revisión Final. En el apéndice 1 se informa sobre la
preparación y el examen colegiado del documento de partida, y en el
apéndice 2 sobre el examen colegiado de la presenta reseña. La
publicación de ésta fue aprobada en una reunión del Comité de Revisión
Final, celebrada en Bruselas (Bélgica) del 18 al 20 de noviembre de
1996. El apéndice 3 contiene la lista de los participantes en esa
reunión. Se ha reproducido asimismo la ficha internacional de
seguridad quimica (ICSC 0341) de la o-toluidina, elaborada por el
Programa Internacional de Seguridad de las Sustancias Químicas (IPCS,
1993).
La o-toluidina (CAS No.95-53-4) es una sustancia química
sintética que a temperatura ambiente es un liquido amarillo claro. Se
utiliza principalmente en la elaboración de pigmentos, aunque también
para producir caucho, productos químicos y plaguicidas, y como agente
polimerizante para los sistemas de resinas epoxidicas.
La o-toluidina tiene una toxicidad entre moderada y baja y puede
producir una muy ligera irritación cutánea e irritación moderada de
los ojos. No se dispone de información sobre su potencial de
sensibilización de la piel o de las vías respiratorias. Los
principales signos de toxicidad tras la exposición aguda y de corta
duración a esa sustancia quimica son la metahemoglobinemia y los
efectos asociados en el bazo. Tales efectos se han observado en ratas
a las que se había administrado o-toluidina a dosis diarias de 225
mg/kg de peso corporal durante 5 días; no se ha establecido un nivel
sin efectos adversos observados.
En diversos estudios de carcinogenicidad en que se administró
o-toluidina por vía oral a ratas y ratones se observó un aumento
significativo de la incidencia de tumores benignos y malignos en
distintos tejidos. La o-toluidina no suele tener efectos mutagénicos
en las pruebas habituales de mutagenicidad bacteriana, pero es
clastogénica en células de mamífero in vitro. No se conoce con certeza
la genotoxicidad de la o-toluidina in vivo, pero se ha informado de
algunos resultados positivos. Teniendo en cuenta la amplia
distribución de los rumores que aparecen en los animales expuestos a
esa sustancia, así como la actividad clastogénica detectada en ensayos
in vitro con células de mamífero, es probable que la o-toluidina actúe
como carcinógeno genotóxico. No se hallaron datos de interés para
evaluar el riesgo de efectos para la reproducción o el desarrollo.
Debido a la falta de datos pertinentes sobre la exposición, no se
han podido evaluar los riesgos para la salud humana asociados a la
exposición indirecta a la o-toluidina presente en el medio ambiente
general. En el entorno ocupacional, es posible que haya riesgos
significativos de efectos carcinogénicos y genotóxicos. No se han
hallado datos útiles sobre las concentraciones de o-toluidina en
diversos ambientes y sobre sus efectos en organismos acuáticos y
terrestres, por lo que no ha sido posible evaluar los riesgos de la
exposición al compuesto entre los organismos presentes en el medio.
 1. EXECUTIVE SUMMARY
This CICAD on ortho-toluidine (o-toluidine) was based on a
review of primarily occupational human health concerns, prepared by
the United Kingdom Health & Safety Executive (Gregg et al., 1996) and
covering data identified up to March 1992. Additional information
identified during the international peer review of this CICAD and
following consideration by the Final Review Board has been
incorporated as appropriate. Information on the preparation and peer
review of the source document is presented in Appendix 1. Information
on the peer review of this CICAD is presented in Appendix 2. This
CICAD was approved for publication at a meeting of the Final Review
Board, held in Brussels, Belgium, on 18-20 November 1996.
Participants at the Final Review Board meeting are listed in Appendix
3. The International Chemical Safety Card (ICSC 0341) for
o-toluidine, produced by the International Programme on Chemical
Safety (IPCS, 1993), has also been reproduced in this document.
o-Toluidine (CAS no.
1. EXECUTIVE SUMMARY
This CICAD on ortho-toluidine (o-toluidine) was based on a
review of primarily occupational human health concerns, prepared by
the United Kingdom Health & Safety Executive (Gregg et al., 1996) and
covering data identified up to March 1992. Additional information
identified during the international peer review of this CICAD and
following consideration by the Final Review Board has been
incorporated as appropriate. Information on the preparation and peer
review of the source document is presented in Appendix 1. Information
on the peer review of this CICAD is presented in Appendix 2. This
CICAD was approved for publication at a meeting of the Final Review
Board, held in Brussels, Belgium, on 18-20 November 1996.
Participants at the Final Review Board meeting are listed in Appendix
3. The International Chemical Safety Card (ICSC 0341) for
o-toluidine, produced by the International Programme on Chemical
Safety (IPCS, 1993), has also been reproduced in this document.
o-Toluidine (CAS no.  Although some toxicological studies on o-toluidine have employed its
hydrochloride salt (o-toluidine hydrochloride), this is unlikely to
significantly alter the observed health effects of the parent
chemical.
3. ANALYTICAL METHODS
Short- and long-term personal monitoring can be undertaken by
pumped sampling either through acid-coated filters or through
NIOSH-type silica gel tubes (NIOSH, 1987; HSE, 1993). The filters
are desorbed with a neutralizing solution and analysed by
high-performance liquid chromatography; the tubes are desorbed with
solvent and analysed by gas chromatography. Screening measurements
may be conducted using a colorimetric detector tube.
The analytical monitoring of urine for o-toluidine and its
metabolites may be a useful means of assessing occupational exposure,
especially where there is potential for skin absorption. Methods for
biological monitoring of occupationally exposed individuals have been
reported (Brown et al., 1995; Ward et al., 1996). These techniques
employ urine sampling for the determination of o-toluidine and its
N-acetyl metabolites and blood sampling for the detection of
o-toluidine-haemoglobin adducts.
The determination of o-toluidine in water samples may involve
extraction under acidic and alkali conditions, followed by brominated
ether extraction and subsequent analysis using gas chromatography with
electron capture detection (detection limit 0.1-0.6 µg/litre). The
analysis of o-toluidine in sediment may involve steam distillation
under alkali conditions with quantitation by gas chromatography
coupled with electron capture detection (detection limit 0.002-0.012
µg/g dry matter).
4. SOURCES OF HUMAN AND ENVIRONMENTAL EXPOSURE
The principal use of o-toluidine worldwide is in the
manufacture of dyestuffs, although it is also used in the production
of rubber, chemicals, and pesticides and as a curing agent for epoxy
resin systems. o-Toluidine is also used as a corrosion inhibitor in
paint formulations and possibly has limited uses in analytical
laboratory procedures. There are no known domestic or household uses
for o-toluidine.
Global production data for o-toluidine were not identified. In
the USA in 1975, more than 900 t of the chemical were produced, and
another 1000 t were imported. Total production of o-toluidine in
Great Britain is approximately 6000 t per year, 90% of which is
exported. Approximately 610 and 545 t of o-toluidine were imported
into Japan in 1992 and 1993, respectively.
5. ENVIRONMENTAL TRANSPORT, DISTRIBUTION, AND TRANSFORMATION
Using an equilibrium model to assess partitioning between
environmental media, Yoshida et al. (1983) estimated the distribution
of o-toluidine to be 14.5% to air, 83.3% to water, 0.4% to soil,
1.9% to sediment, 2.3 × 10-5 % to biota (fish), and 0.21% to suspended
sediment. The half-life for o-toluidine in Rhine River water was
estimated to be about 1 day (Zoeteman et al., 1980).
6. ENVIRONMENTAL LEVELS AND HUMAN EXPOSURE
6.1 Environmental levels
o-Toluidine was detected in 3/46 samples of Rhine River water
collected at the Germany-Netherlands border in 1979; the mean and
maximum concentrations were 0.03 and 1.8 µg/litre, respectively
(Wegman & de Korte, 1981). o-Toluidine was detected in water
collected from a shallow aquifer contaminated by coal tar wastes in
the USA; however, concentrations were not reported (Pereira et al.,
1983).
In Japan, 8/68 samples of surface water collected in 1976
contained o-toluidine (detection limit 0.1-0.6 µg/litre) at levels
ranging from 0.14 to 20 µg/litre; 27 samples of sediment collected in
the same year contained o-toluidine (detection limit 0.002-0.012
mg/kg) at concentrations ranging from 0.002 to 0.013 mg/kg dry weight
(J. Sekizawa, personal communication, 1996). o-Toluidine was not
detected (detection limit 0.05-150 ng/m3) in 72 samples of air
collected in Japan in 1985 (J. Sekizawa, personal communication,
1996).
6.2 Human exposure
Exposure of the general population to o-toluidine present in
the environment could not be estimated, owing to the lack of relevant
data on levels of this chemical in air, drinking-water, and
foodstuffs. Information on human exposure to o-toluidine is limited
to occupational settings.
In the United Kingdom in 1992, approximately 120 individuals were
potentially exposed to o-toluidine during activities involving its
manufacture and use; about a quarter of these were maintenance rather
than process workers. Eight-hour time-weighted-average exposures to
o-toluidine in seven different industries in the United Kingdom
ranged from 0.007 to 2.7 ppm (0.03-11.8 mg/m3); all but one of these
exposures were <0.3 ppm (<1.3 mg/m3). A concentration of 2.7
ppm (11.8 mg/m3) o-toluidine was measured at a site involving
centrifugation at high temperature. If it is assumed that a worker
weighing 70 kg breathes 10 m3 of air during a typical working day and
that inhaled o-toluidine is completely absorbed, exposure to
o-toluidine at a concentration of 1.3 mg/m3 in workplace air can be
estimated to result in a daily o-toluidine intake of approximately
0.2 mg/kg body weight. Exposure to o-toluidine associated with some
processes conducted at elevated temperatures (i.e. exposure to
o-toluidine at 11.8 mg/m3) can be estimated to result in a daily
o-toluidine intake of approximately 1.7 mg/kg body weight. Dermal
exposure to o-toluidine may also occur in the occupational
environment; however, quantitative data were not identified.
7. COMPARATIVE KINETICS AND METABOLISM IN LABORATORY ANIMALS
AND HUMANS
Biological monitoring to assess human exposure to o-toluidine
indicates that absorption may occur through inhalation and dermal
contact; however, quantitative information was not identified.
o-Toluidine binds to haemoglobin (Ward et al., 1996).
N-acetylated metabolites of o-toluidine are eliminated in the
urine (Brown et al., 1995).
In laboratory animals, studies on the kinetics and metabolism of
o-toluidine have involved rats exposed orally or via dermal contact.
There is extensive absorption (at least 92% of the administered oral
dose) of o-toluidine from the gastrointestinal tract (Cheever et
al., 1980). Limited dermal absorption was reported in a poor-quality
study (Senczuk et al., 1984), although the structure of o-toluidine
suggests that, like other aromatic amines, it is lipid soluble and
therefore likely to be readily absorbed through the skin. Oral and
subcutaneous studies have revealed that o-[14C]toluidine and its
metabolites are excreted mainly in the urine, with at least 90% of the
administered radioactivity appearing in the urine within 72 hours
after exposure (Son et al., 1977; Cheever et al., 1980). Small
amounts of radioactivity were also detected in the faeces and exhaled
carbon dioxide. Up to one-third of the o-toluidine administered was
recovered unchanged in the urine. The metabolism of o-toluidine is
characterized principally by ring hydroxylation and N-acetylation,
the major metabolites being 4-amino- m-cresol and, to a lesser
extent, N-acetyl-4-amino- m-cresol (Son et al., 1977; Cheever et
al., 1980). Sulfate conjugates of o-toluidine predominate over
glucuronide conjugates. Binding of o-toluidine metabolites to
haemoglobin has also been observed in rats (Birnier & Neumann, 1988).
8. EFFECTS ON LABORATORY MAMMALS AND IN VITRO TEST SYSTEMS
8.1 Single exposure
o-Toluidine is harmful following acute oral exposure (LD50s of
900 and 940 mg/kg body weight in rats) and is of low acute toxicity
following dermal exposure (LD50 of 3235 mg/kg body weight in rabbits)
(Smyth et al., 1962; Jacobson, 1972). Acute effects include cyanosis,
increased methaemoglobin levels, and related effects in the spleen.
Useful data on effects associated with inhalation exposure were not
identified.
8.2 Irritation and sensitization
Minimal skin irritation and ill-defined eye irritation have been
observed in o-toluidine-exposed rabbits (Smyth et al., 1962). There
is no information available on the skin or respiratory sensitization
potential of o-toluidine in animals.
8.3 Short-term exposure
A 13% decrease in body weight, a 1.5- to 3.0-fold increase in
spleen weight, increased methaemoglobin levels, and congestion,
haemosiderosis, and haematopoiesis in the spleen (all likely
associated with methaemoglobinaemia) were observed in rats orally
administered o-toluidine for 5 days at a dose of 225 mg/kg body
weight per day (Short et al., 1983); no other doses were tested, and a
no-effect level was not identified. Relevant toxicity studies
involving short-term inhalation or dermal exposure to o-toluidine
were not identified.
8.4 Chronic exposure and carcinogenicity
Increased incidences of benign and malignant tumours have been
observed in rats and mice administered o-toluidine hydrochloride in
the diet.
In one study, groups of 50 male and 50 female F344 rats were
given diets containing 3000 or 6000 ppm (mg/kg) o-toluidine
hydrochloride for 101-104 weeks (equivalent to intakes of
approximately 150 and 300 mg/kg body weight per day, based on a body
weight of 400 g and the consumption of 20 g of food per day) (NCI,
1979; Goodman et al., 1984). Controls consisted of 20 animals of each
sex. In addition to routine clinical observations, gross and
microscopic examinations were conducted on all major tissues and
organs and on all gross lesions. Exposure to o-toluidine
hydrochloride was associated with a dose-related decrease in body
weight gain and survival. In the control, low-dose, and high-dose
groups, the combined incidence of sarcomas, angiosarcomas, and
osteosarcomas of the spleen was 0/20, 9/49 (p = 0.36), and 12/49
(p = 0.01), respectively, in females and 0/20, 8/49, and 4/42,
respectively, in males. The combined incidence of sarcomas,
fibrosarcomas, angiosarcomas, and osteosarcomas in multiple
(unspecified) organs was, among females, 0/20, 3/50, and 21/49
(p < 0.001) and, among males, 0/20, 15/50 (p = 0.003), and
37/49 (p < 0.001), for animals in the control, low-dose, and
high-dose groups, respectively. In females, the incidence of
transitional cell carcinomas of the urinary bladder in the control,
low-dose, and high-dose groups was 0/20, 9/45 (p = 0.028), and
22/47 (p < 0.001), respectively; the incidence of this tumour
was not significantly increased in the male rats. The incidence of
malignant mesothelioma of the serous covering of the testes (tunica
vaginalis) was 0/20, 10/50, and 6/49 in the control, low-dose, and
high-dose groups, respectively. Although not observed among control
animals, splenic fibromas were noted in the exposed animals; however,
the incidence was significantly increased only for males in the
low-dose group (10/49; p = 0.024). For animals in the control,
low-dose, and high-dose groups, the incidence of skin fibromas among
males was 0/20, 28/50 (p < 0.001), and 27/49 (p < 0.001),
respectively, and the incidence of mammary gland fibroadenomas among
females was 6/20, 20/50, and 35/49 (p = 0.002), respectively.
Non-neoplastic effects that were not observed in control animals
included splenic fibrosis (in 12-27% and 6-12% of exposed males and
females, respectively), splenic mesothelial hyperplasia (in 12-37% and
24-65% of exposed males and females, respectively), hyperplasia of the
urinary bladder epithelium (in 16-18% and 28-47% of exposed males and
females, respectively), and myocardial fibrosis (in 17-34% of exposed
males). Liver necrosis was noted in 24-35% of exposed males (10% in
controls) and in 2-31% of exposed females (0% in controls).
In a study in which groups of 30 male F344 rats received 0 or 62
mg o-toluidine hydrochloride in the diet each day for 72 weeks
(approximately 470 and 130 mg/kg body weight per day at the beginning
and end of the study, respectively), followed by a 16-week recovery
period, exposure to o-toluidine reduced survival (6/30 and 18/30
survivors in the exposed and control groups, respectively) (Hecht et
al., 1982). Incidences of the following tumours (in the control and
exposed groups, respectively) were: bladder epithelial cell tumours,
0/30 and 4/30; skin fibromas, 1/30 and 25/30; splenic fibromas, 0/30
and 10/30; mammary tumours, 0/30 and 13/30; and peritoneal tumours,
2/30 and 14/30. Although neither the statistical significance of
these results nor the incidence of non-neoplastic effects was
discussed in this report, the results reveal an increased occurrence
of a variety of tumour types in animals administered o-toluidine
hydrochloride for 72 weeks.
In another study in which several chemicals were examined, groups
of 25 male Charles River CD rats were given diets containing
o-toluidine hydrochloride at concentrations of 8000 or 16 000 ppm
(mg/kg) for 3 months (estimated intakes of approximately 800 and 1600
mg/kg body weight per day, assuming 200 g for the body weight of rats
consuming 20 g of food per day) (Weisburger et al., 1978). Excessive
toxicity, based upon increased mortality and reductions in body
weight, resulted in the concentrations being reduced to 4000 and 8000
ppm (mg/kg) (with estimated intakes of 400 and 800 mg/kg body weight
per day) for a further 15 months, followed by a 6-month observation
and recovery period. Only animals that survived 6 months or more were
necropsied. Data on survival or general toxicity were not provided.
Controls included one matched group (n = 16 animals) used for this
portion of the overall study, as well as the pooled controls
(n = 111) from the entire investigation. There was a statistically
significant increase in the incidence (0/16, 18/111, 18/23, and 21/24
in the matched control, pooled control, low-dose, and high-dose
groups, respectively) of subcutaneous fibroma and fibrosarcoma in
o-toluidine-exposed animals. The incidence of bladder transitional
cell carcinoma was 0/16, 5/111, 3/23, and 4/24 in the matched control,
pooled control, low-dose, and high-dose groups, respectively.
Statistically significant increases in hepatocellular carcinomas
and adenomas, as well as haemangiosarcomas, were observed in a study
in which groups of 50 male and 50 female B6C3F1 mice were given diets
containing 1000 or 3000 ppm (mg/kg) o-toluidine hydrochloride
(estimated intakes of 110 and 340 mg/kg body weight per day, assuming
30 g for the body weight of mice consuming 3.4 g of food per day) for
101-104 weeks (NCI, 1979). Twenty animals of each sex served as
unexposed controls. In the control, low-dose, and high-dose groups,
the incidences of hepatocellular carcinoma (in females),
hepatocellular adenoma (in females), and haemangiosarcoma (in males)
were 0/20, 2/49, and 7/50; 0/20, 2/49, and 6/50; and 1/19, 1/50, and
10/50, respectively.
Significantly increased incidences of haemangiosarcomas and
haemangiomas were observed in a study in which groups of 25 male and
25 female CD-1 mice were given diets containing o-toluidine
hydrochloride at concentrations of 16 000 or 32 000 ppm (mg/kg)
(estimated intakes of 1800 and 3600 mg/kg body weight per day,
assuming 30 g for the body weight of mice consuming 3.4 g of food per
day) for 3 months (Weisburger et al., 1978). Excessive toxicity,
based upon increased mortality and reductions in body weight, resulted
in the concentrations being reduced to 8000 and 16 000 ppm (mg/kg) for
a further 15 months, followed by a 3-month observation and recovery
period. Only animals that survived 6 months or more were necropsied.
Controls included one matched group used for this portion of the
overall study, as well as the pooled controls from the entire
investigation. In the matched control, pooled control, low-dose, and
high-dose groups, the incidence of abdominal haemangiosarcomas and
haemangiomas was 0/14, 5/99, 5/14, and 9/11 (in males) and 0/15,
9/102, 5/18, and 9/21 (in females), respectively.
The results from limited (i.e. poorly conducted and/or reported)
dermal and parenteral studies conducted in various species and from an
oral toxicity study in dogs (Morigami & Nisimura, 1940; Steinhoff,
1981; Hecht et al., 1983) do not contribute meaningfully to the
assessment of o-toluidine.
8.5 Genotoxicity and related end-points
Based upon the results of assays conducted in Salmonella
typhimurium and Escherichia coli, o-toluidine was not considered
to be mutagenic in standard bacterial tests (McCann et al., 1975;
Ferretti et al., 1977; Garner & Nutman, 1977; Rosenkranz & Poirier,
1979; Simmon, 1979; Zimmer et al., 1980; Baker & Bonin, 1981, 1985;
Garner et al., 1981; MacDonald, 1981; Martire et al., 1981; Matsushima
et al., 1981; Richold & Jones, 1981; Rowland & Severn, 1981; Simmon &
Shepherd, 1981; Trueman, 1981; Venitt & Crofton-Sleigh, 1981; Rexroat
& Probst, 1985). However, positive results in the Ames test have been
observed when norharman was added to the test system (Nagao et al.,
1978; Nagao & Takahashi, 1981; Sugimura & Nagao, 1981). Results from
several cytogenetic studies have indicated that o-toluidine is
clastogenic in mammalian cells in vitro (Danford, 1985; Gulati et
al., 1985; Ishidate & Sofuni, 1985; Priston & Dean, 1985).
The in vivo genotoxicity of o-toluidine has been adequately
tested only in mice. No evidence of clastogenicity was observed in
several high-quality studies (a bone marrow cytogenetic assay and
three bone marrow micronucleus tests) in which the chemical was
injected intraperitoneally (Salamone et al., 1981; Tsuchimoto &
Matler, 1981; McFee et al., 1989). Although there was no reporting of
bone marrow toxicity in these studies, use of an intraperitoneal
injection with elevated dose levels would suggest that o-toluidine
probably reached the target tissue (bone marrow). In contrast, in the
best conducted bone marrow sister chromatid exchange assay, high doses
of o-toluidine apparently produced positive results in mice (McFee
et al., 1989). A positive result has also been reported for the
induction of DNA single strand breaks in mice; however, the poor
description of the study precludes the drawing of definitive
conclusions (Cesarone et al., 1982). Despite some suggestions of
clastogenicity, the genotoxic potential of o-toluidine in vivo
remains uncertain.
8.6 Reproductive and developmental toxicity
Relevant information on the reproductive and developmental
toxicity of o-toluidine in laboratory animals was not identified.
8.7 Immunological and neurological effects
Relevant toxicological studies in which immunological or
neurological effects were assessed were not available. However,
evidence of specific adverse immunological and neurological effects
has not been reported in general toxicity studies.
9. EFFECTS ON HUMANS
Relevant case reports on adverse health effects associated with
exposure to o-toluidine were not available.
Other than information on potential carcinogenic effects, there
are few useful data available on other health-related effects
associated with exposure to o-toluidine.
Exposure to chemicals including o-toluidine in the dyestuffs
industry and more recently in the rubber industry has been reported to
be associated with an increased incidence of bladder cancer. For
example, expected and observed cases of bladder cancer were recorded
in a thorough, well conducted retrospective cohort study at a rubber
chemical plant in upstate New York (Ward et al., 1991). The cohort
consisted of all 1749 male and female workers employed at the plant
between 1973 and 1988. The work-force was relatively young; 72% were
born after 1 January 1939. It was estimated that cohort members were
"slightly more likely" than the general population of the USA to be
current or former smokers. These workers were exposed primarily to
o-toluidine and aniline. In 1988, airborne o-toluidine and
aniline levels were <1 ppm (<4.4 mg/m3 for o-toluidine).
Based upon 13 identified cases of bladder cancer among all 1749
employees, compared with 3.61 cases expected (estimated from the rate
in the population of the state of New York, excluding New York City),
the standardized incidence ratio (SIR) was 3.6 (90% confidence
interval [CI] = 2.13-5.73). The SIRs for bladder cancer among workers
"definitely exposed" (n = 708), "possibly exposed" (n = 288), and
"probably unexposed" (n = 753) to o-toluidine and aniline were
6.48 (90% CI = 3.04-12.2; 7 observed cases), 3.66 (90% CI = 1.25-8.37;
4 observed cases), and 1.39 (90% CI = 0.25-4.39; 2 observed cases),
respectively. The risk of bladder cancer increased with duration of
exposure and time since first exposure. The SIRs for bladder cancer
among the "definitely exposed" workers with <5, 5-9.99, and >10
years of exposure to these chemicals were 0 (0 observed cases), 8.8
(90% CI = 0.45-41.7; 1 observed case), and 27.2 (90% CI = 11.8-53.7;
6 observed cases), respectively. The SIRs for bladder cancer among
workers with <10, 10-20, and >20 years since their first employment
in the "definitely exposed" department of the plant were 0 (0 observed
cases), 2.03 (90% CI = 0.10-9.64; 1 observed case), and 16.4 (90% CI =
7.13-32.3; 6 observed cases), respectively. It was calculated that
smoking was unlikely to account for the increased incidence of bladder
cancer in this group of workers. The mean latency period for the
group of seven "definitely exposed" workers with bladder cancer was 23
years. Although the carcinogenic potential of o-toluidine
specifically cannot be definitively determined from this study, the
findings merit considerable concern.
Other studies of workers employed in the dyestuffs industry
include those by Vigliani & Barsotti (1961), Khlebnikova et al.
(1970), Zavon et al. (1973), Conso & Pontal (1982), and Rubino et al.
(1982). However, as in the study of rubber chemical workers, it was
not possible to definitively link the increased incidence of bladder
cancer specifically to o-toluidine because of concurrent exposure to
other chemicals.
10. EFFECTS ON OTHER ORGANISMS IN THE LABORATORY AND FIELD
Relevant information on the effects of o-toluidine on aquatic
or terrestrial organisms was not identified. However, toxicity
thresholds for the inhibition of algal growth (Microcystis
aeruginosa, 0.31 mg/litre; Scenedesmus quadricauda, 6.3 mg/litre)
have been reported.1
1 Source: EnviChem Data Bank of Environmental Properties of
Chemicals. Helsinki, Finland, Finnish Environment Agency, version 1.0,
1995.
11. EFFECTS EVALUATION
11.1 Evaluation of health effects
11.1.1 Hazard identification and dose-response assessment
Available data are inadequate to allow the potential risks of
reproductive or developmental effects on human health to be assessed.
Carcinogenicity is considered, however, to be the critical effect
associated with potential exposure to o-toluidine. In several
experimental studies, the oral administration of o-toluidine
hydrochloride increased the incidence of benign and/or malignant
tumours in various tissues in rats (spleen sarcomas and fibromas in
males and females, mesotheliomas of the scrotum in males, transitional
cell carcinomas of the urinary bladder in females, skin fibromas and
mammary gland fibroadenomas in males and females, respectively).
Clear evidence of carcinogenicity has also been observed in oral
studies in mice (hepatocellular carcinomas and adenomas,
haemangiosarcomas, and haemangiomas). Even where the increased tumour
incidences were not statistically significant, they were considered to
be of biological significance in view of the low incidences of such
tumours in historical controls (Haseman et al., 1990). o-Toluidine
is genotoxic in vitro; although the genotoxicity studies conducted
in vivo do not allow definite conclusions to be drawn, there is some
evidence of genotoxic potential. Carcinogenicity studies in rats and
mice have yielded tumours in both sexes and in multiple organs;
therefore, the possibility of involvement of a genotoxic mechanism
cannot be eliminated.
Several studies of workers employed in the dyestuffs and rubber
industries found that exposure to chemicals, including o-toluidine,
appears to be associated with an increased incidence of bladder
cancer. Although it is difficult to draw definite conclusions
regarding the carcinogenic potential of o-toluidine in humans from
these studies of workers exposed to multiple chemicals, this evidence,
together with data from experimental carcinogenicity bioassays, raises
concern about the risk of cancer in exposed humans. It would
therefore be prudent to consider that o-toluidine is probably
carcinogenic in humans, possibly through involvement of a genotoxic
mechanism.
11.1.2 Criteria for setting guidance values for o-toluidine
On the basis that the carcinogenicity of o-toluidine may
involve a genotoxic mechanism, it is not possible to reliably identify
a threshold at which exposure to o-toluidine would not result in
some risk to human health.
11.1.3 Sample risk characterization
It is recognized that there are a number of different approaches
to assessing the risks to human health posed by genotoxic and
carcinogenic substances. In some jurisdictions, there are models for
characterizing potency, which may be of some benefit in
priority-setting schemes.
The example here is from the occupational environment, as the
lack of available data precludes the characterization of potential
cancer risks for the general population. In the United Kingdom, a
Maximum Exposure Limit for o-toluidine (which is not a health-based
standard) of 0.2 ppm (0.9 mg/m3; 8-hour time-weighted average) has
been proposed. The Maximum Exposure Limit was based on a level of
control that was deemed (by tripartite agreement) to be reasonably
practicable under workplace conditions within the United Kingdom. In
the United Kingdom, there is a continuing remit to reduce exposure
levels as much as reasonably practicable with the technology that is
currently available.
11.2 Evaluation of environmental effects
The lack of available information precludes adequate assessment
of potential risks to environmental organisms.
12. PREVIOUS EVALUATIONS BY INTERNATIONAL BODIES
The International Agency for Research on Cancer (IARC, 1987) has
classified o-toluidine in Group 2B (possibly carcinogenic to
humans), based upon sufficient evidence for carcinogenicity in animals
and inadequate evidence for carcinogenicity in humans.
Information on international hazard classification and labelling
is included in the International Chemical Safety Card reproduced in
this document.
13. HUMAN HEALTH PROTECTION AND EMERGENCY ACTION
Human health hazards, together with preventative and protective
measures and first aid recommendations, are presented in the
International Chemical Safety Card (ICSC 0341) reproduced in this
document.
13.1 Human health hazards
Following short-term exposure, o-toluidine could induce
methaemoglobinaemia. After long-term or repeated exposure,
o-toluidine is considered possibly carcinogenic to humans.
13.2 Advice to physicians
If splashed with o-toluidine, it is crucial to remove all wet
or contaminated clothing and wash the entire body with soap and water.
Following such an incident, the degree of methaemoglobinaemia needs to
be determined hourly until a decrease is well established. Above 30%
methaemoglobinaemia, administer oxygen under continuous observation;
above 50% methaemoglobinaemia, further administer intravenously 1000
cc of a 5% glucose solution containing ascorbic acid; above 60%
methaemoglobinaemia, further administer intravenously 10-20 cc of a 1%
solution of methylene blue. If there is no response to treatment with
methylene blue, then haemodialysis or exchange transfusion is useful.
13.3 Health surveillance advice
For workers exposed to o-toluidine, a health surveillance
programme should include regular urinary cytology, with more specific
procedures in the case of positive results.
13.4 Spillage
In the event of spillage, measures should be undertaken to
prevent this chemical from reaching drains and watercourses.
14. CURRENT REGULATIONS, GUIDELINES, AND STANDARDS
Information on national regulations, guidelines, and standards is
available from the International Register of Potentially Toxic
Chemicals (IRPTC) legal file.
The reader should be aware that regulatory decisions about
chemicals taken in a certain country can be fully understood only in
the framework of the legislation of that country. The regulations and
guidelines of all countries are subject to change and should always be
verified with appropriate regulatory authorities before application.
INTERNATIONAL CHEMICAL SAFETY CARD
ortho-TOLUIDINE ICSC: 0341
ortho-TOLUIDINE
1-Amino-2-methylbenzene
2-Aminotoluene
o-Methylaniline
C7H9N/C6H4CH3NH2
Molecular mass: 107.2
CAS #
Although some toxicological studies on o-toluidine have employed its
hydrochloride salt (o-toluidine hydrochloride), this is unlikely to
significantly alter the observed health effects of the parent
chemical.
3. ANALYTICAL METHODS
Short- and long-term personal monitoring can be undertaken by
pumped sampling either through acid-coated filters or through
NIOSH-type silica gel tubes (NIOSH, 1987; HSE, 1993). The filters
are desorbed with a neutralizing solution and analysed by
high-performance liquid chromatography; the tubes are desorbed with
solvent and analysed by gas chromatography. Screening measurements
may be conducted using a colorimetric detector tube.
The analytical monitoring of urine for o-toluidine and its
metabolites may be a useful means of assessing occupational exposure,
especially where there is potential for skin absorption. Methods for
biological monitoring of occupationally exposed individuals have been
reported (Brown et al., 1995; Ward et al., 1996). These techniques
employ urine sampling for the determination of o-toluidine and its
N-acetyl metabolites and blood sampling for the detection of
o-toluidine-haemoglobin adducts.
The determination of o-toluidine in water samples may involve
extraction under acidic and alkali conditions, followed by brominated
ether extraction and subsequent analysis using gas chromatography with
electron capture detection (detection limit 0.1-0.6 µg/litre). The
analysis of o-toluidine in sediment may involve steam distillation
under alkali conditions with quantitation by gas chromatography
coupled with electron capture detection (detection limit 0.002-0.012
µg/g dry matter).
4. SOURCES OF HUMAN AND ENVIRONMENTAL EXPOSURE
The principal use of o-toluidine worldwide is in the
manufacture of dyestuffs, although it is also used in the production
of rubber, chemicals, and pesticides and as a curing agent for epoxy
resin systems. o-Toluidine is also used as a corrosion inhibitor in
paint formulations and possibly has limited uses in analytical
laboratory procedures. There are no known domestic or household uses
for o-toluidine.
Global production data for o-toluidine were not identified. In
the USA in 1975, more than 900 t of the chemical were produced, and
another 1000 t were imported. Total production of o-toluidine in
Great Britain is approximately 6000 t per year, 90% of which is
exported. Approximately 610 and 545 t of o-toluidine were imported
into Japan in 1992 and 1993, respectively.
5. ENVIRONMENTAL TRANSPORT, DISTRIBUTION, AND TRANSFORMATION
Using an equilibrium model to assess partitioning between
environmental media, Yoshida et al. (1983) estimated the distribution
of o-toluidine to be 14.5% to air, 83.3% to water, 0.4% to soil,
1.9% to sediment, 2.3 × 10-5 % to biota (fish), and 0.21% to suspended
sediment. The half-life for o-toluidine in Rhine River water was
estimated to be about 1 day (Zoeteman et al., 1980).
6. ENVIRONMENTAL LEVELS AND HUMAN EXPOSURE
6.1 Environmental levels
o-Toluidine was detected in 3/46 samples of Rhine River water
collected at the Germany-Netherlands border in 1979; the mean and
maximum concentrations were 0.03 and 1.8 µg/litre, respectively
(Wegman & de Korte, 1981). o-Toluidine was detected in water
collected from a shallow aquifer contaminated by coal tar wastes in
the USA; however, concentrations were not reported (Pereira et al.,
1983).
In Japan, 8/68 samples of surface water collected in 1976
contained o-toluidine (detection limit 0.1-0.6 µg/litre) at levels
ranging from 0.14 to 20 µg/litre; 27 samples of sediment collected in
the same year contained o-toluidine (detection limit 0.002-0.012
mg/kg) at concentrations ranging from 0.002 to 0.013 mg/kg dry weight
(J. Sekizawa, personal communication, 1996). o-Toluidine was not
detected (detection limit 0.05-150 ng/m3) in 72 samples of air
collected in Japan in 1985 (J. Sekizawa, personal communication,
1996).
6.2 Human exposure
Exposure of the general population to o-toluidine present in
the environment could not be estimated, owing to the lack of relevant
data on levels of this chemical in air, drinking-water, and
foodstuffs. Information on human exposure to o-toluidine is limited
to occupational settings.
In the United Kingdom in 1992, approximately 120 individuals were
potentially exposed to o-toluidine during activities involving its
manufacture and use; about a quarter of these were maintenance rather
than process workers. Eight-hour time-weighted-average exposures to
o-toluidine in seven different industries in the United Kingdom
ranged from 0.007 to 2.7 ppm (0.03-11.8 mg/m3); all but one of these
exposures were <0.3 ppm (<1.3 mg/m3). A concentration of 2.7
ppm (11.8 mg/m3) o-toluidine was measured at a site involving
centrifugation at high temperature. If it is assumed that a worker
weighing 70 kg breathes 10 m3 of air during a typical working day and
that inhaled o-toluidine is completely absorbed, exposure to
o-toluidine at a concentration of 1.3 mg/m3 in workplace air can be
estimated to result in a daily o-toluidine intake of approximately
0.2 mg/kg body weight. Exposure to o-toluidine associated with some
processes conducted at elevated temperatures (i.e. exposure to
o-toluidine at 11.8 mg/m3) can be estimated to result in a daily
o-toluidine intake of approximately 1.7 mg/kg body weight. Dermal
exposure to o-toluidine may also occur in the occupational
environment; however, quantitative data were not identified.
7. COMPARATIVE KINETICS AND METABOLISM IN LABORATORY ANIMALS
AND HUMANS
Biological monitoring to assess human exposure to o-toluidine
indicates that absorption may occur through inhalation and dermal
contact; however, quantitative information was not identified.
o-Toluidine binds to haemoglobin (Ward et al., 1996).
N-acetylated metabolites of o-toluidine are eliminated in the
urine (Brown et al., 1995).
In laboratory animals, studies on the kinetics and metabolism of
o-toluidine have involved rats exposed orally or via dermal contact.
There is extensive absorption (at least 92% of the administered oral
dose) of o-toluidine from the gastrointestinal tract (Cheever et
al., 1980). Limited dermal absorption was reported in a poor-quality
study (Senczuk et al., 1984), although the structure of o-toluidine
suggests that, like other aromatic amines, it is lipid soluble and
therefore likely to be readily absorbed through the skin. Oral and
subcutaneous studies have revealed that o-[14C]toluidine and its
metabolites are excreted mainly in the urine, with at least 90% of the
administered radioactivity appearing in the urine within 72 hours
after exposure (Son et al., 1977; Cheever et al., 1980). Small
amounts of radioactivity were also detected in the faeces and exhaled
carbon dioxide. Up to one-third of the o-toluidine administered was
recovered unchanged in the urine. The metabolism of o-toluidine is
characterized principally by ring hydroxylation and N-acetylation,
the major metabolites being 4-amino- m-cresol and, to a lesser
extent, N-acetyl-4-amino- m-cresol (Son et al., 1977; Cheever et
al., 1980). Sulfate conjugates of o-toluidine predominate over
glucuronide conjugates. Binding of o-toluidine metabolites to
haemoglobin has also been observed in rats (Birnier & Neumann, 1988).
8. EFFECTS ON LABORATORY MAMMALS AND IN VITRO TEST SYSTEMS
8.1 Single exposure
o-Toluidine is harmful following acute oral exposure (LD50s of
900 and 940 mg/kg body weight in rats) and is of low acute toxicity
following dermal exposure (LD50 of 3235 mg/kg body weight in rabbits)
(Smyth et al., 1962; Jacobson, 1972). Acute effects include cyanosis,
increased methaemoglobin levels, and related effects in the spleen.
Useful data on effects associated with inhalation exposure were not
identified.
8.2 Irritation and sensitization
Minimal skin irritation and ill-defined eye irritation have been
observed in o-toluidine-exposed rabbits (Smyth et al., 1962). There
is no information available on the skin or respiratory sensitization
potential of o-toluidine in animals.
8.3 Short-term exposure
A 13% decrease in body weight, a 1.5- to 3.0-fold increase in
spleen weight, increased methaemoglobin levels, and congestion,
haemosiderosis, and haematopoiesis in the spleen (all likely
associated with methaemoglobinaemia) were observed in rats orally
administered o-toluidine for 5 days at a dose of 225 mg/kg body
weight per day (Short et al., 1983); no other doses were tested, and a
no-effect level was not identified. Relevant toxicity studies
involving short-term inhalation or dermal exposure to o-toluidine
were not identified.
8.4 Chronic exposure and carcinogenicity
Increased incidences of benign and malignant tumours have been
observed in rats and mice administered o-toluidine hydrochloride in
the diet.
In one study, groups of 50 male and 50 female F344 rats were
given diets containing 3000 or 6000 ppm (mg/kg) o-toluidine
hydrochloride for 101-104 weeks (equivalent to intakes of
approximately 150 and 300 mg/kg body weight per day, based on a body
weight of 400 g and the consumption of 20 g of food per day) (NCI,
1979; Goodman et al., 1984). Controls consisted of 20 animals of each
sex. In addition to routine clinical observations, gross and
microscopic examinations were conducted on all major tissues and
organs and on all gross lesions. Exposure to o-toluidine
hydrochloride was associated with a dose-related decrease in body
weight gain and survival. In the control, low-dose, and high-dose
groups, the combined incidence of sarcomas, angiosarcomas, and
osteosarcomas of the spleen was 0/20, 9/49 (p = 0.36), and 12/49
(p = 0.01), respectively, in females and 0/20, 8/49, and 4/42,
respectively, in males. The combined incidence of sarcomas,
fibrosarcomas, angiosarcomas, and osteosarcomas in multiple
(unspecified) organs was, among females, 0/20, 3/50, and 21/49
(p < 0.001) and, among males, 0/20, 15/50 (p = 0.003), and
37/49 (p < 0.001), for animals in the control, low-dose, and
high-dose groups, respectively. In females, the incidence of
transitional cell carcinomas of the urinary bladder in the control,
low-dose, and high-dose groups was 0/20, 9/45 (p = 0.028), and
22/47 (p < 0.001), respectively; the incidence of this tumour
was not significantly increased in the male rats. The incidence of
malignant mesothelioma of the serous covering of the testes (tunica
vaginalis) was 0/20, 10/50, and 6/49 in the control, low-dose, and
high-dose groups, respectively. Although not observed among control
animals, splenic fibromas were noted in the exposed animals; however,
the incidence was significantly increased only for males in the
low-dose group (10/49; p = 0.024). For animals in the control,
low-dose, and high-dose groups, the incidence of skin fibromas among
males was 0/20, 28/50 (p < 0.001), and 27/49 (p < 0.001),
respectively, and the incidence of mammary gland fibroadenomas among
females was 6/20, 20/50, and 35/49 (p = 0.002), respectively.
Non-neoplastic effects that were not observed in control animals
included splenic fibrosis (in 12-27% and 6-12% of exposed males and
females, respectively), splenic mesothelial hyperplasia (in 12-37% and
24-65% of exposed males and females, respectively), hyperplasia of the
urinary bladder epithelium (in 16-18% and 28-47% of exposed males and
females, respectively), and myocardial fibrosis (in 17-34% of exposed
males). Liver necrosis was noted in 24-35% of exposed males (10% in
controls) and in 2-31% of exposed females (0% in controls).
In a study in which groups of 30 male F344 rats received 0 or 62
mg o-toluidine hydrochloride in the diet each day for 72 weeks
(approximately 470 and 130 mg/kg body weight per day at the beginning
and end of the study, respectively), followed by a 16-week recovery
period, exposure to o-toluidine reduced survival (6/30 and 18/30
survivors in the exposed and control groups, respectively) (Hecht et
al., 1982). Incidences of the following tumours (in the control and
exposed groups, respectively) were: bladder epithelial cell tumours,
0/30 and 4/30; skin fibromas, 1/30 and 25/30; splenic fibromas, 0/30
and 10/30; mammary tumours, 0/30 and 13/30; and peritoneal tumours,
2/30 and 14/30. Although neither the statistical significance of
these results nor the incidence of non-neoplastic effects was
discussed in this report, the results reveal an increased occurrence
of a variety of tumour types in animals administered o-toluidine
hydrochloride for 72 weeks.
In another study in which several chemicals were examined, groups
of 25 male Charles River CD rats were given diets containing
o-toluidine hydrochloride at concentrations of 8000 or 16 000 ppm
(mg/kg) for 3 months (estimated intakes of approximately 800 and 1600
mg/kg body weight per day, assuming 200 g for the body weight of rats
consuming 20 g of food per day) (Weisburger et al., 1978). Excessive
toxicity, based upon increased mortality and reductions in body
weight, resulted in the concentrations being reduced to 4000 and 8000
ppm (mg/kg) (with estimated intakes of 400 and 800 mg/kg body weight
per day) for a further 15 months, followed by a 6-month observation
and recovery period. Only animals that survived 6 months or more were
necropsied. Data on survival or general toxicity were not provided.
Controls included one matched group (n = 16 animals) used for this
portion of the overall study, as well as the pooled controls
(n = 111) from the entire investigation. There was a statistically
significant increase in the incidence (0/16, 18/111, 18/23, and 21/24
in the matched control, pooled control, low-dose, and high-dose
groups, respectively) of subcutaneous fibroma and fibrosarcoma in
o-toluidine-exposed animals. The incidence of bladder transitional
cell carcinoma was 0/16, 5/111, 3/23, and 4/24 in the matched control,
pooled control, low-dose, and high-dose groups, respectively.
Statistically significant increases in hepatocellular carcinomas
and adenomas, as well as haemangiosarcomas, were observed in a study
in which groups of 50 male and 50 female B6C3F1 mice were given diets
containing 1000 or 3000 ppm (mg/kg) o-toluidine hydrochloride
(estimated intakes of 110 and 340 mg/kg body weight per day, assuming
30 g for the body weight of mice consuming 3.4 g of food per day) for
101-104 weeks (NCI, 1979). Twenty animals of each sex served as
unexposed controls. In the control, low-dose, and high-dose groups,
the incidences of hepatocellular carcinoma (in females),
hepatocellular adenoma (in females), and haemangiosarcoma (in males)
were 0/20, 2/49, and 7/50; 0/20, 2/49, and 6/50; and 1/19, 1/50, and
10/50, respectively.
Significantly increased incidences of haemangiosarcomas and
haemangiomas were observed in a study in which groups of 25 male and
25 female CD-1 mice were given diets containing o-toluidine
hydrochloride at concentrations of 16 000 or 32 000 ppm (mg/kg)
(estimated intakes of 1800 and 3600 mg/kg body weight per day,
assuming 30 g for the body weight of mice consuming 3.4 g of food per
day) for 3 months (Weisburger et al., 1978). Excessive toxicity,
based upon increased mortality and reductions in body weight, resulted
in the concentrations being reduced to 8000 and 16 000 ppm (mg/kg) for
a further 15 months, followed by a 3-month observation and recovery
period. Only animals that survived 6 months or more were necropsied.
Controls included one matched group used for this portion of the
overall study, as well as the pooled controls from the entire
investigation. In the matched control, pooled control, low-dose, and
high-dose groups, the incidence of abdominal haemangiosarcomas and
haemangiomas was 0/14, 5/99, 5/14, and 9/11 (in males) and 0/15,
9/102, 5/18, and 9/21 (in females), respectively.
The results from limited (i.e. poorly conducted and/or reported)
dermal and parenteral studies conducted in various species and from an
oral toxicity study in dogs (Morigami & Nisimura, 1940; Steinhoff,
1981; Hecht et al., 1983) do not contribute meaningfully to the
assessment of o-toluidine.
8.5 Genotoxicity and related end-points
Based upon the results of assays conducted in Salmonella
typhimurium and Escherichia coli, o-toluidine was not considered
to be mutagenic in standard bacterial tests (McCann et al., 1975;
Ferretti et al., 1977; Garner & Nutman, 1977; Rosenkranz & Poirier,
1979; Simmon, 1979; Zimmer et al., 1980; Baker & Bonin, 1981, 1985;
Garner et al., 1981; MacDonald, 1981; Martire et al., 1981; Matsushima
et al., 1981; Richold & Jones, 1981; Rowland & Severn, 1981; Simmon &
Shepherd, 1981; Trueman, 1981; Venitt & Crofton-Sleigh, 1981; Rexroat
& Probst, 1985). However, positive results in the Ames test have been
observed when norharman was added to the test system (Nagao et al.,
1978; Nagao & Takahashi, 1981; Sugimura & Nagao, 1981). Results from
several cytogenetic studies have indicated that o-toluidine is
clastogenic in mammalian cells in vitro (Danford, 1985; Gulati et
al., 1985; Ishidate & Sofuni, 1985; Priston & Dean, 1985).
The in vivo genotoxicity of o-toluidine has been adequately
tested only in mice. No evidence of clastogenicity was observed in
several high-quality studies (a bone marrow cytogenetic assay and
three bone marrow micronucleus tests) in which the chemical was
injected intraperitoneally (Salamone et al., 1981; Tsuchimoto &
Matler, 1981; McFee et al., 1989). Although there was no reporting of
bone marrow toxicity in these studies, use of an intraperitoneal
injection with elevated dose levels would suggest that o-toluidine
probably reached the target tissue (bone marrow). In contrast, in the
best conducted bone marrow sister chromatid exchange assay, high doses
of o-toluidine apparently produced positive results in mice (McFee
et al., 1989). A positive result has also been reported for the
induction of DNA single strand breaks in mice; however, the poor
description of the study precludes the drawing of definitive
conclusions (Cesarone et al., 1982). Despite some suggestions of
clastogenicity, the genotoxic potential of o-toluidine in vivo
remains uncertain.
8.6 Reproductive and developmental toxicity
Relevant information on the reproductive and developmental
toxicity of o-toluidine in laboratory animals was not identified.
8.7 Immunological and neurological effects
Relevant toxicological studies in which immunological or
neurological effects were assessed were not available. However,
evidence of specific adverse immunological and neurological effects
has not been reported in general toxicity studies.
9. EFFECTS ON HUMANS
Relevant case reports on adverse health effects associated with
exposure to o-toluidine were not available.
Other than information on potential carcinogenic effects, there
are few useful data available on other health-related effects
associated with exposure to o-toluidine.
Exposure to chemicals including o-toluidine in the dyestuffs
industry and more recently in the rubber industry has been reported to
be associated with an increased incidence of bladder cancer. For
example, expected and observed cases of bladder cancer were recorded
in a thorough, well conducted retrospective cohort study at a rubber
chemical plant in upstate New York (Ward et al., 1991). The cohort
consisted of all 1749 male and female workers employed at the plant
between 1973 and 1988. The work-force was relatively young; 72% were
born after 1 January 1939. It was estimated that cohort members were
"slightly more likely" than the general population of the USA to be
current or former smokers. These workers were exposed primarily to
o-toluidine and aniline. In 1988, airborne o-toluidine and
aniline levels were <1 ppm (<4.4 mg/m3 for o-toluidine).
Based upon 13 identified cases of bladder cancer among all 1749
employees, compared with 3.61 cases expected (estimated from the rate
in the population of the state of New York, excluding New York City),
the standardized incidence ratio (SIR) was 3.6 (90% confidence
interval [CI] = 2.13-5.73). The SIRs for bladder cancer among workers
"definitely exposed" (n = 708), "possibly exposed" (n = 288), and
"probably unexposed" (n = 753) to o-toluidine and aniline were
6.48 (90% CI = 3.04-12.2; 7 observed cases), 3.66 (90% CI = 1.25-8.37;
4 observed cases), and 1.39 (90% CI = 0.25-4.39; 2 observed cases),
respectively. The risk of bladder cancer increased with duration of
exposure and time since first exposure. The SIRs for bladder cancer
among the "definitely exposed" workers with <5, 5-9.99, and >10
years of exposure to these chemicals were 0 (0 observed cases), 8.8
(90% CI = 0.45-41.7; 1 observed case), and 27.2 (90% CI = 11.8-53.7;
6 observed cases), respectively. The SIRs for bladder cancer among
workers with <10, 10-20, and >20 years since their first employment
in the "definitely exposed" department of the plant were 0 (0 observed
cases), 2.03 (90% CI = 0.10-9.64; 1 observed case), and 16.4 (90% CI =
7.13-32.3; 6 observed cases), respectively. It was calculated that
smoking was unlikely to account for the increased incidence of bladder
cancer in this group of workers. The mean latency period for the
group of seven "definitely exposed" workers with bladder cancer was 23
years. Although the carcinogenic potential of o-toluidine
specifically cannot be definitively determined from this study, the
findings merit considerable concern.
Other studies of workers employed in the dyestuffs industry
include those by Vigliani & Barsotti (1961), Khlebnikova et al.
(1970), Zavon et al. (1973), Conso & Pontal (1982), and Rubino et al.
(1982). However, as in the study of rubber chemical workers, it was
not possible to definitively link the increased incidence of bladder
cancer specifically to o-toluidine because of concurrent exposure to
other chemicals.
10. EFFECTS ON OTHER ORGANISMS IN THE LABORATORY AND FIELD
Relevant information on the effects of o-toluidine on aquatic
or terrestrial organisms was not identified. However, toxicity
thresholds for the inhibition of algal growth (Microcystis
aeruginosa, 0.31 mg/litre; Scenedesmus quadricauda, 6.3 mg/litre)
have been reported.1
1 Source: EnviChem Data Bank of Environmental Properties of
Chemicals. Helsinki, Finland, Finnish Environment Agency, version 1.0,
1995.
11. EFFECTS EVALUATION
11.1 Evaluation of health effects
11.1.1 Hazard identification and dose-response assessment
Available data are inadequate to allow the potential risks of
reproductive or developmental effects on human health to be assessed.
Carcinogenicity is considered, however, to be the critical effect
associated with potential exposure to o-toluidine. In several
experimental studies, the oral administration of o-toluidine
hydrochloride increased the incidence of benign and/or malignant
tumours in various tissues in rats (spleen sarcomas and fibromas in
males and females, mesotheliomas of the scrotum in males, transitional
cell carcinomas of the urinary bladder in females, skin fibromas and
mammary gland fibroadenomas in males and females, respectively).
Clear evidence of carcinogenicity has also been observed in oral
studies in mice (hepatocellular carcinomas and adenomas,
haemangiosarcomas, and haemangiomas). Even where the increased tumour
incidences were not statistically significant, they were considered to
be of biological significance in view of the low incidences of such
tumours in historical controls (Haseman et al., 1990). o-Toluidine
is genotoxic in vitro; although the genotoxicity studies conducted
in vivo do not allow definite conclusions to be drawn, there is some
evidence of genotoxic potential. Carcinogenicity studies in rats and
mice have yielded tumours in both sexes and in multiple organs;
therefore, the possibility of involvement of a genotoxic mechanism
cannot be eliminated.
Several studies of workers employed in the dyestuffs and rubber
industries found that exposure to chemicals, including o-toluidine,
appears to be associated with an increased incidence of bladder
cancer. Although it is difficult to draw definite conclusions
regarding the carcinogenic potential of o-toluidine in humans from
these studies of workers exposed to multiple chemicals, this evidence,
together with data from experimental carcinogenicity bioassays, raises
concern about the risk of cancer in exposed humans. It would
therefore be prudent to consider that o-toluidine is probably
carcinogenic in humans, possibly through involvement of a genotoxic
mechanism.
11.1.2 Criteria for setting guidance values for o-toluidine
On the basis that the carcinogenicity of o-toluidine may
involve a genotoxic mechanism, it is not possible to reliably identify
a threshold at which exposure to o-toluidine would not result in
some risk to human health.
11.1.3 Sample risk characterization
It is recognized that there are a number of different approaches
to assessing the risks to human health posed by genotoxic and
carcinogenic substances. In some jurisdictions, there are models for
characterizing potency, which may be of some benefit in
priority-setting schemes.
The example here is from the occupational environment, as the
lack of available data precludes the characterization of potential
cancer risks for the general population. In the United Kingdom, a
Maximum Exposure Limit for o-toluidine (which is not a health-based
standard) of 0.2 ppm (0.9 mg/m3; 8-hour time-weighted average) has
been proposed. The Maximum Exposure Limit was based on a level of
control that was deemed (by tripartite agreement) to be reasonably
practicable under workplace conditions within the United Kingdom. In
the United Kingdom, there is a continuing remit to reduce exposure
levels as much as reasonably practicable with the technology that is
currently available.
11.2 Evaluation of environmental effects
The lack of available information precludes adequate assessment
of potential risks to environmental organisms.
12. PREVIOUS EVALUATIONS BY INTERNATIONAL BODIES
The International Agency for Research on Cancer (IARC, 1987) has
classified o-toluidine in Group 2B (possibly carcinogenic to
humans), based upon sufficient evidence for carcinogenicity in animals
and inadequate evidence for carcinogenicity in humans.
Information on international hazard classification and labelling
is included in the International Chemical Safety Card reproduced in
this document.
13. HUMAN HEALTH PROTECTION AND EMERGENCY ACTION
Human health hazards, together with preventative and protective
measures and first aid recommendations, are presented in the
International Chemical Safety Card (ICSC 0341) reproduced in this
document.
13.1 Human health hazards
Following short-term exposure, o-toluidine could induce
methaemoglobinaemia. After long-term or repeated exposure,
o-toluidine is considered possibly carcinogenic to humans.
13.2 Advice to physicians
If splashed with o-toluidine, it is crucial to remove all wet
or contaminated clothing and wash the entire body with soap and water.
Following such an incident, the degree of methaemoglobinaemia needs to
be determined hourly until a decrease is well established. Above 30%
methaemoglobinaemia, administer oxygen under continuous observation;
above 50% methaemoglobinaemia, further administer intravenously 1000
cc of a 5% glucose solution containing ascorbic acid; above 60%
methaemoglobinaemia, further administer intravenously 10-20 cc of a 1%
solution of methylene blue. If there is no response to treatment with
methylene blue, then haemodialysis or exchange transfusion is useful.
13.3 Health surveillance advice
For workers exposed to o-toluidine, a health surveillance
programme should include regular urinary cytology, with more specific
procedures in the case of positive results.
13.4 Spillage
In the event of spillage, measures should be undertaken to
prevent this chemical from reaching drains and watercourses.
14. CURRENT REGULATIONS, GUIDELINES, AND STANDARDS
Information on national regulations, guidelines, and standards is
available from the International Register of Potentially Toxic
Chemicals (IRPTC) legal file.
The reader should be aware that regulatory decisions about
chemicals taken in a certain country can be fully understood only in
the framework of the legislation of that country. The regulations and
guidelines of all countries are subject to change and should always be
verified with appropriate regulatory authorities before application.
INTERNATIONAL CHEMICAL SAFETY CARD
ortho-TOLUIDINE ICSC: 0341
ortho-TOLUIDINE
1-Amino-2-methylbenzene
2-Aminotoluene
o-Methylaniline
C7H9N/C6H4CH3NH2
Molecular mass: 107.2
CAS # 