
Acetylsalicylic acid
| 1. NAME |
| 1.1 Substance |
| 1.2 Group |
| 1.3 Synonyms |
| 1.4 Identification numbers |
| 1.4.1 CAS number |
| 1.4.2 Other numbers |
| 1.5 Brand names, Trade names |
| 1.6 Manufacturers, Importers |
| 2. SUMMARY |
| 2.1 Main risks and target organs |
| 2.2 Summary of clinical effects |
| 2.3 Diagnosis |
| 2.4 First aid measures and management principles |
| 3. PHYSICO-CHEMICAL PROPERTIES |
| 3.1 Origin of the substance |
| 3.2 Chemical structure |
| 3.3 Physical properties |
| 3.3.1 Properties of the substance |
| 3.3.2 Properties of the locally available formulation |
| 3.4 Other characteristics |
| 3.4.1 Shelf-life of the substance |
| 3.4.2 Shelf-life of the locally available formulation |
| 3.4.3 Storage conditions |
| 3.4.4 Bioavailability |
| 3.4.5 Specific properties and composition |
| 4. USES |
| 4.1 Indications |
| 4.2 Therapeutic dosage |
| 4.2.1 Adults |
| 4.2.2 Children |
| 4.3 Contraindications |
| 5. ROUTES OF ENTRY |
| 5.1 Oral |
| 5.2 Inhalation |
| 5.3 Dermal |
| 5.4 Eye |
| 5.5 Parenteral |
| 5.6 Other |
| 6. KINETICS |
| 6.1 Absorption by route of exposure |
| 6.2 Distribution by route of exposure |
| 6.3 Biological half-life by route of exposure |
| 6.4 Metabolism |
| 6.5 Elimination by route of exposure |
| 7. PHARMACOLOGY AND TOXICOLOGY |
| 7.1 Mode of action |
| 7.1.1 Toxicodynamics |
| 7.1.2 Pharmacodynamics |
| 7.2 Toxicity |
| 7.2.1 Human data |
| 7.2.1.1 Adults |
| 7.2.1.2 Children |
| 7.2.2 Relevant animal data |
| 7.2.3 Relevant in vitro data |
| 7.3 Carcinogenicity |
| 7.4 Teratogenicity |
| 7.5 Mutagenicity |
| 7.6 Interactions |
| 7.7 Main adverse effects |
| 8. TOXICOLOGICAL ANALYSES AND BIOMEDICAL INVESTIGATIONS |
| 8.1 Material sampling plan |
| 8.1.1 Sampling and specimen collection |
| 8.1.1.1 Toxicological analyses |
| 8.1.1.2 Biomedical analyses |
| 8.1.1.3 Arterial blood gas analysis |
| 8.1.1.4 Haematological analyses |
| 8.1.1.5 Other (unspecified) analyses |
| 8.1.2 Storage of laboratory samples and specimens |
| 8.1.2.1 Toxicological analyses |
| 8.1.2.2 Biomedical analyses |
| 8.1.2.3 Arterial blood gas analysis |
| 8.1.2.4 Haematological analyses |
| 8.1.2.5 Other (unspecified) analyses |
| 8.1.3 Transport of laboratory samples and specimens |
| 8.1.3.1 Toxicological analyses |
| 8.1.3.2 Biomedical analyses |
| 8.1.3.3 Arterial blood gas analysis |
| 8.1.3.4 Haematological analyses |
| 8.1.3.5 Other (unspecified) analyses |
| 8.2 Toxicological Analyses and Their Interpretation |
| 8.2.1 Tests on toxic ingredient(s) of material |
| 8.2.1.1 Simple Qualitative Test(s) |
| 8.2.1.2 Advanced Qualitative Confirmation Test(s) |
| 8.2.1.3 Simple Quantitative Method(s) |
| 8.2.1.4 Advanced Quantitative Method(s) |
| 8.2.2 Tests for biological specimens |
| 8.2.2.1 Simple Qualitative Test(s) |
| 8.2.2.2 Advanced Qualitative Confirmation Test(s) |
| 8.2.2.3 Simple Quantitative Method(s) |
| 8.2.2.4 Advanced Quantitative Method(s) |
| 8.2.2.5 Other Dedicated Method(s) |
| 8.2.3 Interpretation of toxicological analyses |
| 8.3 Biomedical investigations and their interpretation |
| 8.3.1 Biochemical analysis |
| 8.3.1.1 Blood, plasma or serum |
| 8.3.1.2 Urine |
| 8.3.1.3 Other fluids |
| 8.3.2 Arterial blood gas analyses |
| 8.3.3 Haematological analyses |
| 8.3.4 Interpretation of biomedical investigations |
| 8.4 Other biomedical (diagnostic) investigations and their interpretation |
| 8.5 Overall Interpretation of all toxicological analyses and toxicological investigations |
| 8.6 References |
| 9. CLINICAL EFFECTS |
| 9.1 Acute poisoning |
| 9.1.1 Ingestion |
| 9.1.2 Inhalation |
| 9.1.3 Skin exposure |
| 9.1.4 Eye contact |
| 9.1.5 Parenteral exposure |
| 9.1.6 Other |
| 9.2 Chronic poisoning |
| 9.2.1 Ingestion |
| 9.2.2 Inhalation |
| 9.2.3 Skin exposure |
| 9.2.4 Eye contact |
| 9.2.5 Parenteral exposure |
| 9.2.6 Other |
| 9.3 Course, prognosis, cause of death |
| 9.4 Systematic description of clinical effects |
| 9.4.1 Cardiovascular |
| 9.4.2 Respiratory |
| 9.4.3 Neurological |
| 9.4.3.1 CNS |
| 9.4.3.2 Peripheral nervous system |
| 9.4.3.3 Autonomic nervous system |
| 9.4.3.4 Skeletal and smooth muscle |
| 9.4.4 Gastrointestinal |
| 9.4.5 Hepatic |
| 9.4.6 Urinary |
| 9.4.6.1 Renal |
| 9.4.6.2 Other |
| 9.4.7 Endocrine and reproductive systems |
| 9.4.8 Dermatological |
| 9.4.9 Eye, ear, nose, throat: local effects |
| 9.4.10 Haematological |
| 9.4.11 Immunological |
| 9.4.12 Metabolic |
| 9.4.12.1 Acid-base disturbances |
| 9.4.12.2 Fluid and electrolyte disturbances |
| 9.4.12.3 Others |
| 9.4.13 Allergic reactions |
| 9.4.14 Other clinical effects |
| 9.4.15 Special risks |
| 9.5 Other |
| 9.6 Summary |
| 10. MANAGEMENT |
| 10.1 General principles |
| 10.2 Relevant laboratory analyses |
| 10.2.1 Sample collection |
| 10.2.2 Biomedical analysis |
| 10.2.3 Toxicological analysis |
| 10.2.4 Other investigations |
| 10.3 Life supportive procedures and symptomatic/specific treatment |
| 10.4 Decontamination |
| 10.5 Elimination |
| 10.6 Antidote treatment |
| 10.6.1 Adults |
| 10.6.2 Children |
| 10.7 Management discussion |
| 11. ILLUSTRATIVE CASES |
| 11.1 Case reports from literature |
| 11.2 Internally extracted data on cases |
| 11.3 Internal cases |
| 12. Additional information |
| 12.1 Availability of antidotes |
| 12.2 Specific preventive measures |
| 12.3 Other |
| 13. REFERENCES |
| 14. AUTHOR(S), REVIEWER(S), DATE(S) (INCLUDING UPDATES), COMPLETE ADDRESS(ES) |
PHARMACEUTICALS
1. NAME
1.1 Substance
Acetylsalicylic acid
1.2 Group
Analgesics
1.3 Synonyms
2-Acetoxybenzoic acid
Acidum salicylicum
Aspirin (BP, EurP, BPVET, USP)
O-Acetylsalicylic acid
Polypiryna
Salicylic Acid Acetate
1.4 Identification numbers
1.4.1 CAS number
50-78.2
1.4.2 Other numbers
1.5 Brand names, Trade names
Argentina: A.A.S., Adiro, Asperinetas, Bayaspiraria, Enteretas,
Rhonal
Australia: Bi-prin, Codral Junior, Ecotrin, Elsprin, Novosprin,
Prodol, Provoprin, Rhusal, Sedalgin, Solusal, SRA, Winsprin
Belgium: Acentrine, Adiro, Aspegic (lysine acetylsalicylate),
Dispril, Dolean pH 8, Enterosarin, Primaspan, Rhodine, Rhonal,
Soparine
Canada: Acetophen, Asadrine C-200, Astrin, Coryphen,
Ecotrin, Entrophen, Neoprine-25, Nova-Phase, Novasen, Rhonal,
Salt Adult, Sal-Infant, Supasa, Triaphen-10
Denmark: Acetard, Albyl, Albyl-Selters, Globentyl, Idotyl,
Kalcatyl, Niaguyl, Reumyl
France: Aspegic (lysine acetylsalicylate), Aspirisucre,
Aspisol (lysine acetylsalicylate), Catalgene, Claragine,
Iv_pirine, Juv_pirine, Rhonal
Germany: Acetylin, Colfarit, Contreuma retard, Delgesic
(lysine acetylsalicylate), Godamet, Halgon, Monobeltin (with
aluminium acetylsalicylate), Pyracyl (magnesium
acetylsalicylate), Trineral 600
Hungary: Istopirine
Italy: Asatard, Aspegic (lysine acetylsalicylate), Cemerit,
Dolean pH 8, Domupirina, Endydol, Flectadol (lysine
acetylsalicylate), Kilios, Longasa, Rectosalyl
Japan: Rhonal, Salitison
Netherlands: Acenterine, Acetyl Acidum Acetylsalicylicum Daro,
Alka-Seltzer, Asp_gic (lysine acetylsalicylate), Asperine,
Aspro Darosal, Dispid, Durasil, Enterosarine, Rhonal,
Sinaspril
Norway: Albyl, Dispril, Globentyl, Licyl, Magnyl, Novid
South Africa: Aquapin, Aspasol, Aspegic (lysine
acetylsalicylate)
Spain: AAS, Adiro, Calmo Ver Analgesio, Casprium Retard,
Codalgina Retard, Dolomega (lysine acetylsalicylate), Lafena,
Mejoral, Infantil, Rhonal, Riane (arginine acetylsalicylate),
Salicilina, Soluspril (lysine acetylsalicylate)
Sweden: Acetard, Albyl, Albyl-Selters, Apernyl, Bamyl,
Bamyl S, Dispril, Magnecyl, Premaspin, Rheumyl
Switzerland: Acenterine, Acetylo, Aspegic (lysine
acetylsalicylate), Asrivo, Bebesan, Dispril, Dolean pH 8,
Enterosarine, Rhonal
USA: Aluprin, Ecotrin, Empirin
(Reynolds, 1989)
1.6 Manufacturers, Importers
To be completed by the PCC.
2. SUMMARY
2.1 Main risks and target organs
The toxic effects of salicylate are complex. The following
appear to be the principal primary effects of salicylate in
overdose.
stimulation of the respiratory centre
inhibition of citric acid cycle (carbohydrate metabolism)
stimulation of lipid metabolism
inhibition of amino acid metabolism
uncoupling of oxidative phosphorylation
Respiratory alkalosis, metabolic acidosis, water and
electrolyte loss occur as the principal secondary consequences
of salicylate intoxication. Central nervous system toxicity
(including tinnitus, hearing-loss, convulsions and coma),
hypoprothrombinaemia and non-cardiogenic pulmonary oedema may
also occur, though for some the mechanism remains uncertain
(see Section 7 for further details).
Target organs: all tissues (whose cellular metabolism is
affected), but in particular the liver, kidneys, lungs and the
VIIIth cranial nerve.
2.2 Summary of clinical effects
Nausea, vomiting, epigastric discomfort, gastrointestinal
bleeding (typically with chronic and rarely with acute
intoxication).
Tachypnoea and hyperpnoea.
Tinnitus, deafness, sweating, vasodilatation, hyperpyrexia
(rare), dehydration.
Irritability, tremor, blurring of vision, subconjunctival
haemorrhages.
Non-cardiogenic pulmonary oedema.
Confusion, delirium, stupor, asterixis, coma, cerebral oedema
(with severe intoxication only).
Acute renal failure.
Cardio-respiratory arrest (with severe intoxication only).
(Meredith & Vale, 1986)
2.3 Diagnosis
Symptoms of mild poisoning include tinnitus, dizziness,
sweating and vomiting. Severe poisoning is characterised by
hyperventilation, fever and restlessness. Metabolic acidosis
and respiratory alkalosis occur.
Effects on blood glucose: hyper- or hypoglycaemia
Effects on blood: hypoprothrombinaemia
Effects on liver: increased serum aminotransferase activities
(SGOT and SGPT).
Urine is very suitable for rapid screening for the presence of
a salicylate. Plasma (or serum) is the specimen of choice for
determination of total salicylates using Trinder's reagent
(8.2.2.2) to be repeated on at least one occasion, 4 - 6 hours
after the initial measurement. HPLC methods are only useful
when toxicokinetic analyses are required; they do not
otherwise offer any advantage over the simple and very rapid
Trinder's method.
2.4 First aid measures and management principles
Prevention of further absorption by inducingesis and/or
gastric lavage. Salicylates delay gastric emptying, and
gastric lavage may therefore be useful up to 12, and possibly
24, hours after the ingestion of a large salicylate overdose.
Enhancement of elimination using repeat-dose activated
charcoal.
Correction of dehydration, hypokalaemia and acidosis.
Urinary alkalinization and, when indicated, haemodialysis or
charcoal haemoperfusion.
3. PHYSICO-CHEMICAL PROPERTIES
3.1 Origin of the substance
Usually prepared by acetylation of salicylic acid with acetic
anhydride, using a small amount of sulphuric acid as catalyst.
3.2 Chemical structure
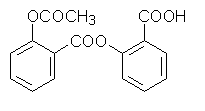 COOH
O-CO-CH3
2-acetoxy-benzoic acid
C9H8O4
Molecular weight 180.15
3.3 Physical properties
3.3.1 Properties of the substance
Colourless or white crystals or white crystaline
powder or granules; odourless or almost
odourless with a slight acid taste. Melting
point about 143o. Soluble 1 in 300 of water, 1
in 5 - 7 in alcohol, 1 in 17 of chloroform and 1
in 20 of ether; soluble in solutions of acetates
and citrates and, with decomposition, in
solutions of alkali hydroxides and carbonates. A
solution in water is acid to methyl red.
Incompatible with free acids, acetanilide,
aminopyrine, phenazone, hexamine, iron salts,
phenobarbitone sodium, quinine salts, potassium
and sodium iodides, and alkali hydroxides,
carbonates, and stearates.
Acetylsalicylic acid is stable in dry air, but
gradually hydrolyses in contact with moisture to
acetic and salicylic acids. In solution with
alkalis, the hydrolysis proceeds rapidly and the
clear solutions formed may consist entirely of
acetate and salicylate.
Acetylsalicylic acid decomposes rapidly in
solutions of ammonium acetate or of the acetates,
carbonates, citrates or hydroxides of the
alkali metals (Reynolds, 1989).
3.3.2 Properties of the locally available formulation
To be completed by the PCC.
3.4 Other characteristics
3.4.1 Shelf-life of the substance
Shelf-life is highly dependent on the manner of storage.
It is recommended that the active ingredient is tested
on a yearly basis using the principles outlined in the
relevant monograph of the European Pharmacopoeia. When
all the appropriate tests have been undertaken with
satisfactory results and when the active ingredient is
stored in the manner recommended by monograph, extension
of the period of storage for a further year may be
considered.
3.4.2 Shelf-life of the locally available formulation
To be completed by the PCCTo be completed by the PCC
3.4.3 Storage conditions
Store in air-tight containers.
3.4.4 Bioavailability
After oral administration, 80 - 100% will be absorbed in
the stomach and in the small intestine. However,
bioavailability is lower because partialhydrolysis
occurs during absorption and there is a "first-pass"
effect in the liver.
The non-protein bound fraction of salicylate increases
with the total plasma concentration, and the binding
capacity of albumin is partially saturated at
therapeutic concentrations of salicylate (Borga et al.,
1976). The greater proportion of unbound drug found at
high concentrations will mean that greater toxicity will
result than would be expected from the total salicylate
concentration (Alvan et al., 1981).
Absorption after rectal administration is slow and
unpredictable. Timed-release preparations are
therapeutically of limited value because of the
prolonged half-life of elimination of salicylate.
Absorption of enteric-coated tablets is sometimes
incomplete.
3.4.5 Specific properties and composition
To be completed by the PCC.
4. USES
4.1 Indications
As an analgesic for the treatment of mild to moderate pain,
as an anti-inflammatory agent for the treatment of soft tissue and
joint inflammation, and as an antipyretic drug.
In low doses salicylate is used for the prevention of
thrombosis.
4.2 Therapeutic dosage
4.2.1 Adults
(Informatorium Medicamentorum, 1990)
Oral (Na- or Ca- or lysine acetylsalicylate):
Indication: pain and fever: 300-1000 mg every 4 h; max 4
g a day.
Indication: acute polyarthritis rheumatica: 1 g 6 times
a day; max 8 g a day.
Indication: rheumatoid arthritis: 0.5-1 g 6 times a day;
max 8 g a day.
Compounds with controlled release: 1 g 2-3 times a day,
if necessary 6 times a day.
Indication: to prevent transient ischaemic attacks and
to prevent arterial thrombosis: 300-1200 mg a day in 2-3
doses.
Rectal:
Indication: pain and fever: 500-1000 mg every 6 h; max 4
g a day.
Indication: rheumatoid arthritis: 0.5-1 g, 6 times a
day; max 8 g a day.
Intramuscular or intravenous (lysine
acetylsalicylate):500 mg, 1-4 times a day.
4.2.2 Children
CAUTION see 9.4.14
Oral (Na- or Ca- or lysine acetylsalicylate):
Indication: pain and fever: the use of acetylsalicylic
acid in young children for these indications is no
longer advocated because of the risk of Reyes Syndrome.
Indication: acute polyarthritis rheumatica: to start
with 100-15 mg/kg a day; after one week 60 mg/kg a day.
Indication juvenile arthritis: children up to 25 kg, 60-
90 mg/kg a day; children over 25 kg, 2.4-3.6 g a day.
Rectal:
Indication: pain and fever:
Age < 2 years, 20 mg/kg, max 2 times a day
Age > 2 years, 20 mg/kg, max 3 times a day
Intramuscular, intravenous (lysine acetylsalicylate): 5-
25 mg/kg a day.
4.3 Contraindications
Active peptic ulcer
Febrile/post-febrile illness in children
Haemostatic disorders, including anticoagulant and
thrombolytic treatment
Hypoproteinemia
Hypersensitivity
Asthma induced by acetylsalicylic acid or other non-steroidal
anti-inflammatory drugs.
Caution is indicated in patients with:
a history of peptic ulceration or gastro-intestinal
haemorrhage
hepatic or renal insufficiency
asthma
children < 2 years, especially in those who are
dehydrated
(Informatorium Medicamentorum, 1990)
5. ROUTES OF ENTRY
5.1 Oral
Ingestion of acetylsalicylic acid tablets is the most frequent
cause of salicylate poisoning; in neonates, infants and
children other less common causes include application of
teething gels to gums (Paynter & Alexander, 1979), placental
transfer (Ahlfors et al, 1982; Lynd et al, 1974) and breast-
milk (Clark & Wilson, 1981).
Methyl salicylate is particularly toxic because of rapid
absorption and 1 teaspoonful (5 ml) contains the equivalent of
6.9 g acetylsalicylic acid (Johnson & Welch, 1984); methyl
salicylate intoxication has also been reported following its
use as candy flavouring (Howrie et al, 1985).
5.2 Inhalation
Maximum permissible atmospheric concentration 5 mg per m3
(Reynolds, 1989).
5.3 Dermal
The quantity absorbed after 10 hours dermal application of
salicylated vaseline under occlusive dressing was more than
60% (Taylor & Halprin, 1975). Salicylate poisoning has been
reported after application of salicylate ointment to burns
(Pluskwa et al, 1984) and other dermatological disturbances
(Editorial, 1964; Treguerer et al, 1980).
Percutaneous absorption of methyl salicylate ('oil of
wintergreen') may also cause toxicity (Davies et al, 1979).
5.4 Eye
Unknown.
5.5 Parenteral
Lysine acetylsalicylate is the conventional means of
administering salicylate by this route; acetylsalicylic acid
has also been used as an admixture with other drugs given
intravenously.
5.6 Other
Rectal administration of salicylic acid suppositories may be
necessary in infants or when oral dosing is either not
possible or is contraindicated.
6. KINETICS
6.1 Absorption by route of exposure
Salicylic acid is a weak acid (pKa 3); following oral
administration, almost all salicylate is found in the
unionized form in the stomach. Acetylsalicylic acid is poorly
soluble in the acid media of the stomach and precipitates may
coalesce to form concretions, thereby delaying absorption for
8 to 24 hours. Despite the higher pH of the small bowel, the
larger surface area allows absorption of salicylate, and this
occurs rapidly at therapeutic doses. However, absorption
following overdose commonly occurs more slowly, and blood
concentrations can continue to rise for up to 24 h after
ingestion (Ferguson & Boutros, 1970; Kaufman & Dubanksy, 1972;
Levy, 1978). Absorption of salicylate will be further delayed
if an enteric-coated preparation has been ingested (Wortzmann
& Grunfeld, 1987).
6.2 Distribution by route of exposure
About 50 - 80% of salicylate in the blood is bound by protein
while the rest remain in the active, ionized state; protein
binding is concentration-dependent. Saturation of binding
sites leads to more free salicylate and increased toxicity.
The volume of distribution is 0.1-0.2 l/kg. Acidosis increases
the volume of distribution because of enhancement of tissue
penetration of salicylates (Levy & Tsuchiya, 1972).
6.3 Biological half-life by route of exposure
Acetylsalicylic acid is hydrolyzed in the stomach and in blood
to salicylic acid and acetic acid; the biological half-life is
therefore only 20 minutes.
The plasma salicylate half-life following therapeutic doses is
2 to 4.5 h, but in overdose increases to 18 to 36 h (Done,
1960).
6.4 Metabolism
Approximately 80% of small doses of salicylic acid is
metabolised in the liver. Conjugation with glycine forms
salicyluric acid and with glucuronic acid forms salicyl acyl
and phenolic glucuronide. These metabolic pathways have only a
limited capacity. Small amounts of salicylic acid are also
hydroxylated to gentisic acid. With large salicylate doses
the kinetics switch from first order to zero order (Michaelis-
Menten kinetics) (Levy & Tsuchiya, 1972).
6.5 Elimination by route of exposure
Salicylates are excreted mainly by the kidney as salicyluric
acid (75%), free salicylic acid (10%), salicylic phenol (10%)
and acyl (5%) glucuronides, and gentisic acid (< 1%). When
small doses (less than 250 mg in an adult) are ingested, all
pathways proceed by first order kinetics, with an elimination
half-life of about 2-3 hours (Hartwig-Otto, 1983). When higher
doses of salicylate are ingested (more than 4 g), the half-
life becomes longer (15-30 hours) because the
biotransformation pathways concerned with the formation of
salicyluric acid and salicyl phenolic glucuronide become
saturated.
Renal excretion of salicylic acid becomes increasingly
important as the metabolic pathways become saturated, because
it is extremely sensitive to changes in urinary pH above pH 6.
The use of urinary alkalinization exploits this particular
aspect of salicylate elimination.
7. PHARMACOLOGY AND TOXICOLOGY
7.1 Mode of action
7.1.1 Toxicodynamics
Nausea and vomiting occur as a result of stimulation of
mucosal receptors by gastric irritation and stimulation
of receptors accessible from the cerebrospinal fluid,
probably in the medullary chemoreceptor ("chemoreceptor
trigger zone").
Marked hyperventilation occurs as a result of direct
stimulation of the respiratory centre. Indirect
stimulation of respiration is caused by increased
production of CO2 as a result of salicylate-induced
uncoupling of oxidative phosphorylation. A respiratory
alkalosis develops as a result of the direct and
indirect stimulation of the respiratory centre. In an
attempt to compensate, bicarbonate, accompanied by
sodium, potassium and water, is excreted in the urine.
Dehydration and hypokalcaemia result but, more
importantly, the loss of bicarbonate diminishes the
buffering capacity of the body and allows the
development of a metabolic acidosis (see below).
The pyretic effect of toxic doses of salicylate is a
direct result of the uncoupling of oxidative
phosphorylation, and the sweating that results further
contributes to dehydration.
High doses of salicylates have additional toxic effects
on the central nervous system consisting of stimulation
(including convulsions) followed by depression,
confusion, dizziness, asterixis, delirium, psychosis,
stupor and coma (Anderson et al. 1976; Anderson et al,
1981).
Very high doses of salicylate have a depressant effect
on the medulla and may cause central respiratory
paralysis as well as sudden circulatory collapse
secondary to vasomotor depression.
The loss of buffering capacity (see above), and the
effects of salicylate on carbohydrate, lipid and protein
metabolism lead to the development of a metabolic
acidosis, or more commonly in practice, a mixed acid-
base disturbance. Competitive inhibition of NAD+-
dependent dehydrogenases in the citric acid cycle will
lead to the accumulation of acid intermediates.
(Grisolia et al, 1969). Salicylate enhances the entry
and oxidation of fatty acids in liver cells, leading to
increased ketogenesis, and will also inhibit amino acid
incorporation into protein (Smith & Dawkins, 1971)
causing amino-acidaemia. In the presence of an acidosis,
entry of salicylate ion into cells is promoted, and the
metabolic effects exacerbated.
Both hypo-and hyperglycaemia occur in salicylate
poisoning, the former most probably being due to
increased tissue demand for glucose oxidation due to
uncoupling of oxidative phosphorylation; neuroglycopenia
can occur in the presence of normal blood sugar
concentrations (Thurston et al, 1970). If hepatic
glycogen stores are adequate, catecholamine production
stimulates glycogenolysis leading to hyperglycaemia
which may persist for several days (Cotton & Fahlberg,
1964; Mortimer & Lepow, 1962); raised plasma
corticosteroid concentrations probably augment this
effect.
Salicylate intoxication is often accompanied by
hypoprothrombinaemia due to a warfarin-like action of
salicylate on the vitamin K1-epoxide cycle, though this
rarely causes clinical problems (Bell, 1978).
7.1.2 Pharmacodynamics
The salicylates alleviate pain by virtue of both a
peripheral and a central nervous system effect.
Salicylates, by inhibiting the synthesis
ofprostaglandins that occur in inflamed tissues, prevent
the sensitization of pain receptors to mechanical
stimulation or to chemicals, such as bradykinin, that
appear to mediate the pain response. Direct effects on
the central nervous system have been described and
suggest a hypothalamic site for the analgesic as well as
the antipyretic effects (Woodbury & Fingl, 1975).
Acetylsalicylate decreases platelet adhesiveness for the
life-time of the platelet (Brantmark et al. 1981).
7.2 Toxicity
7.2.1 Human data
7.2.1.1 Adults
Mild to moderate toxicity 150-300 mg/kg
Serious toxicity 300-500 mg/kg
Potentially lethal > 500 mg/kg
(Temple, 1981)
7.2.1.2 Children
In a child, ingestion of 240 mg/kg will cause
moderately severe poisoning, but deaths rarely
occur when less than 480 mg/kg has been taken
(Done, 1978).
Salicylate poisoning in small children (< 4
years) is often more serious than in older
children because of the early development of a
metabolic acidosis rather than a respiratory
alkalosis (Winters et al. 1959).
7.2.2 Relevant animal data
None relevant.
7.2.3 Relevant in vitro data
None relevant.
7.3 Carcinogenicity
No data available.
7.4 Teratogenicity
In an embryo culture system, malformations were observed at
plasma salicylate concentrations approaching those after
single doses of salicylate (Greenaway et al., 1984).
Reputedly, the rat is sensitive to the teratogenic effects of
salicylates, whereas humans and non-human primates are
regarded as being resistant (Shepard, 1983; Wilson et al.,
1977).
7.5 Mutagenicity
No data available.
7.6 Interactions
Potentiating effects
Aspirin potentiates the ulcerogenic effects of caffeine,
indomethacin and phenybutazone (Goodman & Gilman, 1990).
Acetylsalicylic acid is also reported to potentiate the
effects of warfarin and other anticoagulants of the coumarin
type. Phenothiazines, and chlorpromazine in particular, are
potentiated by salicylates (Huang & Hirano, 1967).
Acetylsalicylic acid is highly protein-bound and may increase
the unbound or free drug concentrations of other drugs, for
instance hypoglycaemic drugs will be displaced from the bound
state (Hecht & Goldner, 1959) and cases of hypoglycaemic coma
have been reported (Bergman, 1965; Peaston & finnegan, 1965).
Aspirin displaces methotrexate from protein binding sites and
increases tissue concentrations, reaching toxic levels rapidly
because the therapeutic index of methorexate is very low
(Baker, 1970).
Antagonistic effects
The uricosuric activity of phenylbutazone, probenecid and
sulfinpyrazone is strongly antagonized and may be completely
abolished by aspirin even after small doses (Yu et al., 1963;
Pascale et al., 1955; and Oyer et al., 1966).
The mineralocorticoid blocking properties of spironolactone
are inhibited (Tweedale & Oglivie, 1971).
In a report of two cases of severe salicylate poisoning,
asystole occured shortly after the intravenous administration
of diazepam (Berk & Andersen, 1989).
7.7 Main adverse effects
The most common adverse effects seen following therapeutic
doses of acetylsalicylic acid are gastrointestinal in origin,
including nausea, epigastric discomfort and vomiting.
Irritation of the gastric mucosa with erosion, ulceration,
haematemesis and melaena may occur; occult blood loss may
occur in about 70% of patients unaccompanied by dyspepsia.
Slight blood loss whilst not usually of clinical significance,
may result in iron deficiency anaemia.
Some individuals, especially asthmatics, exhibit sensitivity
to acetylsalicylic acid. Urticaria, angioneurotic oedema,
rhinitis and severe, even fatal, paroxysmal bronchospasm and
dyspnoea may occur (Reynolds, 1989).
8. TOXICOLOGICAL ANALYSES AND BIOMEDICAL INVESTIGATIONS
8.1 Material sampling plan
8.1.1 Sampling and specimen collection
8.1.1.1 Toxicological analyses
Screen residues or suspected materials, plasma,
urine and stomach contents. Avoid sodium azide
preservation.
8.1.1.2 Biomedical analyses
8.1.1.3 Arterial blood gas analysis
8.1.1.4 Haematological analyses
8.1.1.5 Other (unspecified) analyses
8.1.2 Storage of laboratory samples and specimens
8.1.2.1 Toxicological analyses
Keep biological samples in a refrigerator prior
to analysis.
8.1.2.2 Biomedical analyses
8.1.2.3 Arterial blood gas analysis
8.1.2.4 Haematological analyses
8.1.2.5 Other (unspecified) analyses
8.1.3 Transport of laboratory samples and specimens
8.1.3.1 Toxicological analyses
No special conditions.
8.1.3.2 Biomedical analyses
8.1.3.3 Arterial blood gas analysis
8.1.3.4 Haematological analyses
8.1.3.5 Other (unspecified) analyses
8.2 Toxicological Analyses and Their Interpretation
8.2.1 Tests on toxic ingredient(s) of material
8.2.1.1 Simple Qualitative Test(s)
For detection of salicylate ion:
Acetylsalicylate ion is not present in
plasma/serum and urine; in plasma,
acetylsalicylate ion is only present when
fluoride is added to the blood sample.
(1) Principle of test: Direct colour reaction
on urine. Serum residues and stomach contents
require preliminary acid hydrolysis to
salicylate.
(2) Sampling: 2 ml of urine and small portion
of suspected material (+/-200 mg).
(3) Chemicals and reagents:
Chemicals
Mercuric chloride (Hg(II)Cl2)
Ferric nitrate 9 aq (Fe(NO3)3).9 H2O)
Hydrochloric acid 1 mol/L and 0.1 mol/L
Sodium hydroxide 0.1 mol/L
Sodium salicylate p.a.
Acetylsalicylic acid p.a.
Reagents
Trinder's Reagent: Dissolve 40 g of
Hg(II)Cl2 in 850 ml of hot water. After
cooling down add 120 ml of HCl 1 mol/L and 40 g
of hydrated ferric nitrate. When the ferric
nitrate has been dissolved, add water to 1000
ml.
(4) Equipment:
No special equipment needed
(5) Sample preparation:
Stomach contents and serum residues
1. Boil a portion of the specimen with 2
ml of hydrochloric acid 0.1 mol/L for 10
minutes.
2. Cool and filter if necessary.
3. Neutralise with sodium hydroxide 0.1
mol/L.
(6) Procedure
Urine
1. Add 100 uL Trinder's Reagent to 1 ml of
urine and mix.
2. A violet colour indicates the presence
of salicylates.
Stomach contents and serum residues
1. Add 100 uL of Trinder's Reagent to the
clear neutralized solution obtained under (5)
"Sample preparation".
2. A violet colour indicates the presence
of salicylate.
(7) Calibration procedure
Not applicable
(8) Quality control
Quality control can be achieved by running
spiked samples as a comparison.
(9) Specificity
Azide preservatives interfere strongly.
High concentrations of urinary ketone bodies may
give weak false positives.
Salicylamide, p-aminosalicylic acid and 4-
aminoantipyrin also react.
Acetylsalicylic acid and methylsalicylic
acid only react after preliminary acid
hydrolysis.
(10) Detection limit
Approximately 20 mg salicylate/L urine.
(11) Analytical assessment
A positive result may indicate the presence
of a salicylate.
(12) Medical interpretation
A positive result indicates the possibility
of salicylate poisoning.
8.2.1.2 Advanced Qualitative Confirmation Test(s)
Acetylsalicylic acid is only present in stomach
contents and serum residues. Confirmation may
be obtained by TLC (DFG, Report VII, 1987) or
GLC (DFG, Report II, 1985); the same applies to
the salicylate ion.
8.2.1.3 Simple Quantitative Method(s)
..... to be completed
8.2.1.4 Advanced Quantitative Method(s)
..... to be completed
8.2.2 Tests for biological specimens
8.2.2.1 Simple Qualitative Test(s)
(1) Principle:
Measurement of red-violet complex of ferric
and salicylate ions at 530 or 546 nm
(2) Sampling:
Plasma or serum.
(3) Chemicals and reagents:
As for 8.2.1.1 -(3).
Salicylate stock solution 2000 mg/L:
Dissolve 580 mg of sodium salicylate in 250
ml of distilled water.
Salicylate calibration samples:
Dilute appropriate volumes of the stock
solution with distilled water to salicylate
concentrations of 100, 200, 300, 400 and 500
mg/L.
(4) Equipment:
Pipettes, glass tubes, cuvettes, centrifuge
and (spectro)photometer.
(5) Sample preparation:
Not applicable.
(6) Procedure:
1. Pipette into glass tube:
Blank Sample
Calibration points
Plasma (serum) - 0.5 ml -
Calibration points - - 0.5 ml
(100-500 mg/l)
Distilled water 0.5 ml - -
Trinder's Reagent 2 ml 2 ml 2 ml
2. Mix by vortexing for 30 seconds,
centrifuge and measure the extinction of the
clear supernatant at 530 or 546 nm against the
blank.
(7) Calibration procedure:
A calibration curve is constructed by
plotting the concentration of the calibration
samples (100-500 mg/L) against the measured
extinction. From this curve the concentration in
the sample is read.
(8) Quality control:
The use of a plasma (serum) sample with a
known concentration is advised. This specimen
can be obtained commercially or made by
appropriate spiking of pre-tested blank plasma
(serum).
(9) Specificity:
As for 8.2.1.1 -(9).
(10) Detection limit:
The limit of detection of the method is 70
mg salicylate/L plasma(serum) using a 0.5 ml
sample size.
(11) Analytical assessment:
If no interfering substances are present
and if the procedure has been followed, an
accurate measurement of total salicylates
content will be made.
(12) Medical assessment:
The total salicylate plasma (serum)
concentration and the expected severity of
intoxication should be related to the interval
that has elapsed between sampling and ingestion.
Generally:
(a) Time interval 6 hours: Salicylate
<400 mg/L: usually
asymptomatic
Salicylate 400-800 mg/L: mild to
moderate toxicity
Salicylate >800 mg/L: severe toxicity
(b) Time interval 12 hours:
Salicylate <300 mg/L: usually
asymptomatic
Salicylate 300-600 mg/L: mild to
moderate toxicity
Salicylate >600 mg/L: severe toxicity
(c) Time interval 24 hours:
Salicylate <200 mg/L: usually
asymptomatic
Salicylate 200-500 mg/L: mild to
moderate toxicity
Salicylate >500 mg/L: severe toxicity
8.2.2.2 Advanced Qualitative Confirmation Test(s)
8.2.2.3 Simple Quantitative Method(s)
In plasma (serum) or urine only salicylate can
be determined.
Acetylsalicylic acid can only be quantitated in
plasma when sufficient sodium fluoride has been
added to the blood collection tube. Any HPLC
method described in the literature is suitable.
8.2.2.4 Advanced Quantitative Method(s)
8.2.2.5 Other Dedicated Method(s)
8.2.3 Interpretation of toxicological analyses
8.3 Biomedical investigations and their interpretation
8.3.1 Biochemical analysis
8.3.1.1 Blood, plasma or serum
Tests of platelet function and coagulation to
determine/exclude presence of: inhibition of
platelet aggregation and hypoprothrombinaemia.
8.3.1.2 Urine
8.3.1.3 Other fluids
8.3.2 Arterial blood gas analyses
In adults an initial respiratory alkalosis is followed
in severe salicylate poisoning by metabolic acidosis;
typically the acid-base disturbance is mixed in nature.
Young children tend to develop a metabolic acidosis but,
by the age of 12 years, the usual adult picture of a
mixed respiratory alkalosis and metabolic acidosis is
seen.
8.3.3 Haematological analyses
Electrolytes and urea to determine/exclude presence of:
hypernatraemia due to water loss (sweating, vomiting,
osmotic diuresis) and hypokalaemia due to renal and
extrarenal factors.
Blood sugar level to determine/exclude presence of:
hypoglycaemia or hyperglycaemia.
Urinary pH (typically alkaline in the early stages of
salicylate overdose and then subsequently becomes acid).
8.3.4 Interpretation of biomedical investigations
Not relevant.
8.4 Other biomedical (diagnostic) investigations and their
interpretation
8.5 Overall Interpretation of all toxicological analyses and
toxicological investigations
The plasma salicylate concentration should be determined on
admission, provided that more than 4 to 6 hours have elapsed
from the time of ingestion of the overdose because
measurements made before this time are difficult to interpret.
In addition, it is important to repeat the measurement to
make sure that the salicylate concentration is not continuing
to rise because of continued absorption (Vale et al, 1985).
In adults, plasma concentrations 6 hours after an overdose:
300-500 mg/l: mild toxicity
500-750 mg/l: moderate toxicity
> 750 mg/l: severe toxicity (Proudfoot,
1983).
The presence or absence of symptoms and signs and the type of
acid-base disturbance should be considered when interpreting
the plasma salicylate concentration and deciding upon
management (Meredith & Vale, 1986).
The DONE-nomogram (fig.) categorizes the severity of poisoning
for single ingestions based on peak salicylate concentrations.
In patients with a significant acidosis and in patients who
ingest multiple doses or sustained release preparations, the
DONE-nomogram will tend to underestimate the severity of
intoxication (Todd et al., 1981).
The validity of the DONE-monograph was evaluated in 54 cases
by Dugandzic et al. (1989). The predictive index for the
moderate and severe classifications were poor. Development of
the nomogram was based on the assumption that salicylates are
eliminated by a first order process, whereas in fact they are
partially eliminated through saturable processes. This may
lead to overprediction of severity. For this reason the DONE-
monograph is not commonly used.
After ingestion of enteric coated tablets, plasma salicylate
concentrations on admission are unreliable guides to the
severity of poisoning. Salicylate levels may not peak until
more than 12 hours after such an overdose (Todd et al., 1981;
Springer & Groll, 1980).
8.6 References
9. CLINICAL EFFECTS
9.1 Acute poisoning
9.1.1 Ingestion
Early symptoms are nausea, vomiting, epigastric pain and,
sometimes, haematemesis. Additional characteristic
features include hyperventilation,elevated body
temperature, irritability and tinnitus. Less commonly,
cardiac dysrhythmias may occur because of hypokalaemia,
and tetany and paraesthesiae due to low ionised calcium
levels. In serious poisoning hallucinations, stupor,
convulsions, papilloedema and coma may be observed
together with metabolic acidosis; hepatotoxicity may
also occur and non-cardiogenic pulmonary oedema is well
described.
9.1.2 Inhalation
No data available.
9.1.3 Skin exposure
Severe poisoning has been reported as a result of use of
salicylic acid ointment for dermatological problems
(Editorial, 1964; Taylor & Halprin, 1975) and in the
treatment of skin burns (Pluskwa et al. 1984).
9.1.4 Eye contact
No data available.
9.1.5 Parenteral exposure
No data available.
9.1.6 Other
Acetylsalicylic acid suppositories can cause rectal
irritation; absorption is slow and unpredictable.
9.2 Chronic poisoning
9.2.1 Ingestion
Chronic salicylate poisoning occurs as a result of
excessive therapeutic administration over a period of 12
hours or more (Dove & Jones, 1982) because the metabolic
pathways become saturated and salicylic acid is
eliminated by zero order kinetics. Plasma salicylate
concentrations then increase, producing toxicity. Small
children are at particular risk of well-intentioned but
over-enthusiastic treatment by their parents. The risk
is further increased when the fever, sweating and
tachycardia of salicylate intoxication are attributed to
the underlying illness and are used as indications for
increasing the dose (Proudfoot, 1983). Children may
become intoxicated through breast milk (Clark & Wilson,
1981), and chronic salicylate poisoning has also been
described in the elderly (De Groen, 1989).
Neurological features in adults occur more frequently
with chronic salicylate poisoning than as a result of
acute overdose, and may lead to extensive investigations
before the correct diagnosis is reached, often late
after hospital admission (Anderson et al. 1976). Older
patients present with breathlessness, and pulmonary
oedema caused by salicylate poisoning can be mistakenly
attributed to cardiac or respiratory disease (and vice
versa).
The mortality from chronic salicylate poisoning in
adults is considerably greater than from acute overdose
(Anderson et al. 1976). In children, chronic salicylism
is accompanied by a greater morbidity than is acute
salicylate poisoning. Hyperventilation, dehydration and
severe central nervous system manifestations occur more
frequently in those chronically poisoned (Gaudreault et
al. 1982).
9.2.2 Inhalation
No data available.
9.2.3 Skin exposure
The application of teething gels to the gums (Paynter &
Alexander, 1979), or percutaneous absorption of
salicylic acid from ointments used for such skin
disorders as psoriasis or methyl salicylate may cause
salicylate poisoning.
9.2.4 Eye contact
No data available.
9.2.5 Parenteral exposure
No data available.
9.2.6 Other
No data available.
9.3 Course, prognosis, cause of death
Adults seldom lose conciousness and are typically miserable
and uncomfortable. If a sufficient quantity of a salicylate
has been taken, nausea, vomiting, tinnitus, deafness, sweating,
vasodilatation and hyperventilation develop.
Cause of death.
A review of 51 fatal cases of acute salicylate poisoning in
Ontario during 1983 and 1984 disclosed that salicylates was
the most common cause of death due to the ingestion of single
drugs. Autopsy results showed that 50% of the patients had
pulmonary abnormalities, 28% had lesions of the
gastrointestinal tract, 18% had nervous system abnormalities
and 25.6% had no pathologic changes (McGuigan, 1987).
Mortality from chronic salicylate intoxication is considerable
higher (25%) than from acute overdose (1-2%) (Anderson et al.
1976). Death is often due to sudden cardiac arrest or,
occasionally, to multiple complications following severe brain
damage (Proudfoot, 1983).
9.4 Systematic description of clinical effects
9.4.1 Cardiovascular
Abrupt cardiovascular collapse is a recognized
complication of salicylate poisoning (Anderson et al.
1976; Benowitz et al. 1979; Beveridge et al. 1964; Pei &
Thomson, 1987). Two patients with severe salicylate
intoxication developed asystole shortly after
intravenous diazepam administration (Berk & Andersen,
1989).
9.4.2 Respiratory
Non-cardiogenic pulmonary oedema can occur in salicylate-
intoxicated patients who are over 30 years of age.
Cigarette smoking, chronic salicylate ingestion,
metabolic acidosis and the presence of neurological
symptoms and signs on admission are strong risk factors
for the subsequent development of pulmonary oedema. In
the absence of these risk factors, salicylate-induced
pulmonary oedema is rare. The exact mechanism is
unknown. Three possible explanations include: a direct
toxic effect on pulmonary microvasculature by
salicylates, interaction with endogenous mediators such
as prostaglandins, and a central nervous system mediated
effect (Walters et al. 1983; Liebman & Katz, 1981).
9.4.3 Neurological
9.4.3.1 CNS
High doses of salicylates cause stimulation
(irritability, convulsions) followed by
depression of the CNS. Confusion, dizziness,
delirium, psychosis, asterixis, stupor and coma
occur usually when metabolic acidosis is the
dominant acid-base abnormality (Proudfoot &
Brown, 1969; Gabow et al. 1978; Anderson, 1981).
These features are thought to be due to reduced
ionisation of salicylic acid and a shift of
salicylate from plasma into the brain. Dominant
metabolic acidosis is common in young children
who are therefore more likely to experience
serious intoxication at relatively low plasma
salicylate concentrations (Proudfoot, 1983).
Very high doses of salicylate have a depressant
effect on the medulla and may cause central
respiratory paralysis as well as sudden
circulatory collapse secondary to vasomotor
depression.
9.4.3.2 Peripheral nervous system
No data available.
9.4.3.3 Autonomic nervous system
No data available.
9.4.3.4 Skeletal and smooth muscle
No data available.
9.4.4 Gastrointestinal
The ingestion of salicylate may result in epigastric
discomfort, nausea and vomiting. It may also cause
gastric ulceration. Perforated peptic ulcer occurs
extremely rarely (Robins et al. 1985; Christensen &
Schmidt, 1987).
9.4.5 Hepatic
Hepatotoxicity may occur both after therapeutic use of
salicylate or following salicylate overdose (Wolfe et
al. 1974). Liver biopsy in such cases reveals acute
hepatocellular necrosis with periportal inflammation and
fatty changes in hepatocytes.
9.4.6 Urinary
9.4.6.1 Renal
Oliguria is sometimes seen; the most common
cause is dehydration (Temple et al. 1976) but
renal failure may rarely occur in individuals
without pre-existing renal disease, systemic
disease, or volume depletion (Rupp et al. 1983).
9.4.6.2 Other
No data available.
9.4.7 Endocrine and reproductive systems
Influence on adrenal medulla:
High doses of salicylate cause release of adrenaline
from the adrenal medulla; this is thought to be partly
responsible for the observed hyperglycaemia due to
glycogenolysis that sometimes occurs.
Influence on the adrenal cortex:
Large doses of salicylate stimulate corticosteroid
secretion by the adrenal cortex.
9.4.8 Dermatological
Thirteen cases of toxic epidermal necrolysis have been
reported associated with the use of either
acetylsalicylic acid or methylsalicylate (Lowney et al.
1967).
9.4.9 Eye, ear, nose, throat: local effects
Ear: tinnitus and hearing loss caused by salicylate in
overdose are due to increased labyrinthine pressure
(Waltner, 1955) and/or an effect on the hair cells of
the cochlea. There is a relation between the hearing
loss and the plasma salicylate concentration (Myers et
al. 1965).
Eye: transient myopia occurred in a patient following
ingestion of 2.7 g acetylsalicylic acid (Sandford-Smith,
1974). Bilateral subconjunctival haemorrhages have been
described (Black & Bensinger, 1982).
9.4.10 Haematological
Ingestion of salicylic acid by normal individuals
causes prolongation of the bleeding time due to
inhibition of collagen glucosyltransferase present in
membranes of platelets. As a result, the adherence of
platelets to connective tissue or collagen fibres is
diminished.
Salicylate in large therapeutic doses (over 6 g per
day) and in overdose reduces the concentration of
vitamin K-dependent coagulation factors and in
particular that of prothrombin.
9.4.11 Immunological
In vitro, acetylsalicylic acid is an efficient
suppressor of the lymphocyte-transformation reaction.
An association with the mechanism by which it exerts
its anti-inflammatory, anti-rheumatic and anti-pyretic
effect could be postulated (Teraski et al., 1973;
Twomey et al., 1973). Similar results were observed in
rats (Loveday et al., 1973). In 19 normal volunteers,
marked and statistically significant suppression of
blastogenesis has been reported (Crout et al., 1975).
9.4.12 Metabolic
9.4.12.1 Acid-base disturbances
In adults there is an initial respiratory
alkalosis which is compensated for by
excretion of bicarbonate in urine. In infants
and children the respiratory alkalosis does
not occur and metabolic acidosis develops
(Proudfoot & Brown, 1969; Winters et al.,
1958). In severe salicylate poisoning in
adults, metabolic acidosis may also result
from a number of factors. Even when present
in high concentrations, salicylate will not
displace more than 2-3 mmol of bicarbonate;
the acidosis is not therefore due to the
presence of salicylic acid itself. The
principal cause is competitive inhibition of
NAD+-dependent dehydrogenases, including
lactate and oxoglutarate dehydrogenases and of
other oxidative enzymes such as succinate
dehydrogenase (Grisolia et al., 1969; Hines &
Smith, 1964; Koplan et al., 1954).
Consequent impairment of the oxidation of fuel
substrates leads to the accumulation of acid
intermediates, notably lactate and pyruvate.
Acidaemia caused by the effect of salicylate
on carbohydrate metabolism is compounded by
effects on lipid and amino-acid metabolism.
Salicylate enhances entry and oxidation of
fatty acids in liver cells, leading to
increased ketogenesis. Competitive inhibition
of amino-acyl-tRNA synthetases in pairs and
amino-acid incorporation (Smith & Dawkins,
1971); amino-acidaemia results.
Finally, dehydration and vasomotor depression
results in poor renal perfusion and
accumulation of sulphuric and phosphoric acids
(Tenney & Miller, 1955; Winters et al., 1959).
9.4.12.2 Fluid and electrolyte disturbances
Decreased renal tubular reabsorption of
bicarbonate occurs as a result of respiratory
alkalosis. Increased renal secretion of sodium,
potassium and water accompanies loss of
bicarbonate in the urine. Fluid loss also
results from vomiting, sweating and
hyperventilation; dehydration is commonly
associated with hypernatremia.
Water losses may be considerable: from 2 - 3
l/m2 surface area in moderate severe poisoning,
up to 6 l/m2 in severely poisoned patients
(Temple, 1978).
9.4.12.3 Others
Influence on oxidative phosphorylation.
The uncoupling of oxidative phosphorylation by
salicylate results in the inhibition of a
number of ATP-dependent reactions and:
an increase in O2 uptake and CO2
production;
depletion of hepatic glycogen; with
the energy normally used for the
conversion of inorganic phosphate to ATP being
dissipated as heat, hence the pyretic effect
of toxic doses of salicylate.
Influence on carbohydrate metabolism.
Multiple factors appear to be involved. Hyper-
or hypoglycaemia may result from the
mechanisms listed above at A. (see 9.4.,
12.1).
Influence on nitrogen metabolism.
Salicylate in toxic doses causes a significant
negative nitrogen balance, characterized by
amino-aciduria, though this is due in part to
inhibition of active tubular absorption
because of reduced ATP formation.
Influence on fat metabolism.
Increased entry and enhanced oxidation of
fatty acids in muscle, liver and other tissues
occur, together with a lowering of
concentrations of plasma free fatty acids,
phospholipid and cholesterol (Woodbury & Fingl,
1975).
9.4.13 Allergic reactions
Some persons, particularly asthmatics, exhibit marked
sensitivity to acetylsalicylic acid which provokes
various reactions including urticaria and other skin
eruptions, angioneurotic oedema, rhinitis and severe,
even fatal, paroxysmal bronchospasm and dyspnoea,
hypotension, shock and syncope (Reynolds, 1989).
9.4.14 Other clinical effects
The relationship between the use of acetylsalicylic
acid and Reye's syndrome (Reye et al., 1963) remains
controversial. Reye's syndrome is a disease with a high
mortality characterized by encephalopathy and fatty
degeneration of the viscera, especially the liver.
Similar findings were noted (Starko & Mullick, 1983) in
the histology and necropsy records of 13 children with
accidental or iatrogenic salicylate intoxication.
The clinical features most often observed are severe
vomiting followed by increasing drowsiness and coma.
Transaminase activities and ammonia levels in the blood
become elevated but jaundice does not occur. In severe
cases, hypoglycaemia and hypoprothrombinaemia develop.
Reye's syndrome may be seen in children and adolescents,
the peak incidence being between 5-15 years. Often
varicella or influenza precede the development of the
syndrome (Sullivan-Bolyai & Corey, 1981), but some
cases reported have been in children with chronic
inflammatory disorders such as juvenile rheumatoid
arthritis or lupus erythematosis (Young et al., 1984;
Hansen et al., 1985).
A possible pointer to a causal relationship between
acetylsalicylic acid use and Reye's syndrome is the
declining incidence in the USA that has occurred
following a reduction in use of acetylsalicylic acid
during the period 1980-1987 (Remington et al., 1986;
Banco, 1987); for this reason, the use of the drug in
young children in the UK is now restricted (Notes and
News, 1986).
9.4.15 Special risks
Salicylate intoxication may occur through placental
transfer (Ahlfors et al., 1982; Lynd et al., 1976) and
breast milk (Clarke & Wilson, 1981).
9.5 Other
No data available.
9.6 Summary
10. MANAGEMENT
10.1 General principles
Treatment of acute salicylate poisoning is directed
primarily towards prevention of absorption, correction of
acid-base and fluid and electrolyte balance; and in patients
with features of moderate or severe intoxication, towards
enhancing elimination of the drug. Respiratory alkalosis
needs no specific treatment, but severe acidosis requires at
least partial correction with bicarbonate. Bicarbonate
should be administered carefully because hypokalaemia may be
aggravated and, if large quantities are administered, the
sodium and water load may precipitate pulmonary oedema.
Sedatives and respiratory depressant drugs must be avoided.
Tetany may be corrected with the use of calcium gluconate
(10 ml of a 10% solution, intravenously) (Merdith & Vale,
1986).
If it is thought that an enteric-coated or other slow-
release salicylic acid preparation has been taken, then the
patient must be kept under observation for at least twenty-
four hours; abdominal ultrasonoscopy may help to identify
concentrations or retained enteric-coated tablets.
10.2 Relevant laboratory analyses
10.2.1 Sample collection
See section 8.
10.2.2 Biomedical analysis
Hypokalaemia is usual. Hypoglycaemia can be a feature
of salicylate intoxication, it is more common in
children than in adults and is often severe.
Hyperglycaemia may also occur and persist for several
days (Cotton & Fahlberg, 1964; Mortimer & Lepow,
1962).
10.2.3 Toxicological analysis
See section 8.
10.2.4 Other investigations
No data available.
10.3 Life supportive procedures and symptomatic/specific
treatment
If non-cardiogenic pulmonary oedema is present, mechanical
ventilation with positive end-expiratory pressure (PEEP) may
be indicated
Fluid and electrolyte replacement is important with special
attention being paid to potassium depletion;
ECG monitoring may be indicated
Correction of metabolic acidosis by sodium bicarbonate
intravenously
Sponging for hyperpyrexia.
10.4 Decontamination
In those cases where vomiting has not already occurred
further absorption of the drug may be prevented by inducing
emesis and/or undertaking gastric lavage, as appropriate.
Acetylsalicylic acid is poorly soluble in an acid
environment and may coalesce to form a mass or coating in
the stomach, from which absorption may continue slowly over
many hours. Thus gastric lavage may be indicated more than
12 hours following ingestion of the overdose.
Although activated charcoal proved to be equally effective
as emesis and gastric lavage in volunteers (Danel & Henry,
1988), it may only be exploited therapeutically if the
patient presents soon after the overdose and is not vomiting
- which is unlikely in those who are at least moderately
severely poisoned. Moreover substantial quantities of
activated charcoal (50-100 g) need to be administered if
significant absorption is to be prevented. Repeated doses
of activated charcoal (50-75 g immediately and 50 g 4-
hourly) will increase the non-renal elimination of
salicylate and will greatly diminish the plasma half-life
(Hillman & Prescott, 1985; Boldy & Vale, 1986).
The administration of activated charcoal may be of
particular value in those adults who have ingested
substantial quantities of an enteric-coated or sustained-
release preparation of acetylsalicylic acid, and it has been
employed in repeated doses to increase the non-renal
elimination of acetylsalicylic acid (Meredith & Vale, 1986).
The addition of sodium sulphate (as a saline cathartic
agent) to activated charcoal was not found to have any
additional effect on the prevention of acetylsalicylic acid
absorption in six healthy volunteers (Sketris et al. 1982).
10.5 Elimination
Whilst (forced) alkaline diuresis has been employed in the
management of salicylate poisoning for the last two decades
(Berg, 1977; Cumming et al. 1964; Dukes et al., 1963; Lawson
et al., 1969) fluid retention may occur during forced
diuresis, and increase the risk of pulmonary oedema in
severely intoxicated patients (Proudfoot & Brown, 1969;
Heffner & Sahn, 1981). It is now recognized that the urine
pH is of far greater importance than the volume of urine
excreted (Meredith & Vale, 1981; Prescott et al., 1982). To
achieve maximum excretion of salicylate a urine pH of 7.5 or
higher is indicated (careful monitoring of urine pH is
necessary).
Urinary alkalinization requires close supervision in an
intensive care area. It is sometimes difficult to
alkalinize the urine so that maximum excretion is achieved
without creating a potentially dangerous alkalaemia (Done,
1978), but in these cases adequate potassium repletion will
normally allow urinary alkalinization. In patients with
cardiac and/or renal impairment, and in those who are in
shock, dialysis should be considered (Meredith & Vale,
1986).
Haemodialysis should be considered in severely poisoned
patients with features of central nervous system toxicity,
pulmonary oedema, renal failure, cerebral oedema and in
cases with plasma levels higher than 800 mg/l, haemodialysis
should be considered.
Haemodialysis is preferred to haemoperfusion because it more
rapidly corrects acid-base and electrolyte abnormalities and
may avoid the need for the administration of large amounts
of sodium bicarbonate (Winchester et al. 1981).
Peritoneal dialysis is less effective than alkaline diuresis,
is 2 - 3 times less effective than haemodialysis, and its
use is not recommended (Winchester et al. 1977). Similarly,
charcoal haemoperfusion is less effective than haemodialysis
and again its use is not advocated (Meredith & Vale, 1986).
10.6 Antidote treatment
10.6.1 Adults
There is no specific antidote.
10.6.2 Children
There is no specific antidote.
10.7 Management discussion
Alkalinization of the urine is considerably more effective
in promoting salicylate excretion than induced diuresis and
the need for the latter requires reappraisal (Prescott et
al. 1982).
The use of gastroscopic and other measures to break up
enteric-coated tablets requires further evaluation.
11. ILLUSTRATIVE CASES
11.1 Case reports from literature
11.2 Internally extracted data on cases
To be added by the PC.
11.3 Internal cases
To be added by the PC using the monograph.
12. Additional information
12.1 Availability of antidotes
No antidotes available.
12.2 Specific preventive measures
Flavoured tablets are attractive to children and, to avoid
temptation, the use of this type of medication should be
avoided.
The use of child resistant closures, smaller pack sizes and
the elimination of attractive coloured products will help to
reduce the problem of salicylate poisoning.
Parents need to be warned of the potential risks of chronic
administration of salicylates.
Physicians should be made aware that chronic salicylate
poisoning in hospitalized patients occurs almost as
frequently as acute poisoning, and may result in more severe
intoxication.
12.3 Other
No data available.
13. REFERENCES
Ahlfors CE, Shwer ML, Ford KB, (1982). Bilirubin-albumin binding
in neonatal salicylate intoxication. Developmental Pharmacol Ther,
4:47-60.
Alvan G, Bergman U, Gustafsson LL, (1981). High unbound fraction
of salicylate in plasma during intoxication. Br J Clin Pharmacol,
11:625-6.
Anderson RJ, Potts DE, Gabow PA, Rumack BH, Schrier RW, (1976).
Unrecognised adult salicylate intoxication. Ann Intern Med,
85:745-8.
Anderson RJ, (1981). Asterixis as manifestation of salicylate
toxicity. Ann Intern Med, 95:188-9.
Banco L. Use of aspirin and Reye's syndrome, (1987). Am J Dis
Child, 141:240-1.
Bell RG, (1978). Metabolism of vitamin K and prothrombin
synthesis: anticoagulants and the vitamin K-epoxide cycle. Fed
Proc, 37: 2599.
Benowitz NL, Rosenberg J, Becker CE, (1979). Cardiopulmonary
catastrophes in drug overdose patients. Med Clin North Am, 63:267-
95.
Berg KJ, (1977). Acute acetylsalicylic acid poisoning. Treatment
with forced alkaline diuresis and diuretics. Eur J Clin Pharmacol,
12:111-6.
Berk WA, Andersen JC, (1989). Salicylate-associated asystole:
report of two cases. Am J Med, 86:505-6.
Beveridge GW, Forshall W, Munro JF, Owen JA, Weston IG, (1964).
Acute salicylate poisoning in adults. Lancet, 1:1406-9.
Black RA, Bensinger RE, (1982). Bilateral subconjunctival
hemorrhage after acetylsalicylic acid overdose. Ann Opthalmol,
14:1024-5.
Boldy DAR, Vale JA, (1986). Treatment of salicylate poisoning
with repeated oral charcoal. Br Med J, 292:136.
Borga O, Odar-Cederlof I, Ringberger VA, Norlin A, (1976).
Protein binding of salicylate in uremic and normal plasma. Clin
Pharm Ther, 20:464-75.
Brantmark B, Hedner U, Melander A et al, (1981). Salicylate
inhibition of antiplatelet effect of aspirin. Lancet, 2:1349.
Christensen LA, Schmidt EB, (1987). Perforated peptic ulcer. A
complication in acute salicylate intoxication. Acta Med Scand,
222:191-2.
Clarke JH, Wilson WG, (1981). A 16-day-old breast-fed infant with
metabolic acidosis caused by salicylate. Clin Pediatr, 20:53-4.
Cotton EK, Fahlberg VI, (1964). Hypoglycaemia with salicylate
poisoning. A report of two cases. Am J Dis Children, 108:171-3.
Cumming G, Dukes DC, Widdowson G, (1964). Alkaline diuresis in
treatment of aspirin poisoning. Br Med J, 2:1033-6.
Danel V, Henry JA, (1988). Activated charcoal, emesis, and
gastric lavage in aspirin overdose. Br Med J, 296:1507.
Davies MG, Vella Briffa D, Greaves MW, (1979). Systemic toxicity
from topically applied salicylic acid. Br Med J, 1:661.
De Groen PC, (1989). Chronische intoxicatie door salicylzuur
verbindingen: vaak niet herkend of verkeerd behandeld. Ned
Tijdschr Geneeskd, 133:1481-5.
Done AK, (1960). Salicylate intoxication. Significance of
measurements of salicylate in blood in cases of acute ingestion.
Pediatrics, 26:800-7.
Done AK, (1978). Aspirin overdosage: incidence, diagnosis, and
management. Pediatrics, 62 (suppl):890-7.
Dove DJ, Jones T, (1982). Delayed coma associated with salicylate
intoxication. J Pediatr, 100:493-6.
Dukes DC, Blainey JD, Cumming G, Widdowson G, (1963). The
treatment of severe aspirin poisoning. Lancet, 1:329-31.
Editorial, (1964). Percutaneous salicylic acid intoxication. JAMA,
190:1064.
Ferguson RK, Boutros AR, (1970). Death following self-poisoning
with aspirin. JAMA, 213:1186-7.
Gabow PA, Anderson RJ, Potts DE, Schrier RW, (1978). Acid-base
disturbances in the salicylate-intoxicated adult. Arch Intern Med,
138:1481-4.
Gaudrealt P, Temple AR, Lovejoy FH, (1982). The relative severity
of acute versus chronic salicylate poisoning in children. A
clinical comparison. Pediatrics, 70:566-9.
Grisolia S, Mendelson J, Diederich D, (1969). Inactivation of
enzymes by therapeutic concentrations of aspirin and salicylate.
Nature, 223:79.
Hansen JR, McCray PB, Bale JF et al, (1985). Reye syndrome
associated with aspirin therapy for systemic lupus erythematosus.
Pediatrics, 76:202-5.
Hartwig-Otto H, (1983). Pharmacokinetic considerations of common
analgesics and antipyretics. Am J Med, 75 (suppl):30-7.
Heffner JE, Sahn SA, (1981). Salicylate-induced pulmonary edema.
Ann Intern Med, 95:405-9.
Hillman RJ, Prescott LF, (1985). Treatment of salicylate
poisoning with repeated oral charcoal. Br Med J, 291:1472.
Hines WJW, Smith MJH, (1964). Inhibition of dehydrogenases by
salicylate, Nature, 201:192.
Howrie DL, Moriarty R, Breit R, (1985). Candy flavouring as a
source of salicylate poisoning. Pediatrics, 75:869.
Informatorium Medicamentorum, (1986). Ed Kon Mij Pharmacie's,
Gravenhage.
Johnson PN, Welch DW, (1984). Methylsalicylate/aspirin
(salicylate) equivalence: Who do you trust? Vet Human Toxicol,
26:317-8.
Kaplan EH, Kennedy J, Davies J, (1954). Effects of salicylate and
other benzoates on oxidative enzymes of the tricarboxylic acid
cycle in rat tissue homogenates. Arch Biochem Biophys, 51:47
Kaufman FL, Dubanksy AS, (1972). Darvon poisoning with delayed
salicylism: a case report. Pediatrics, 49:610-1.
Lawson AAH, Proudfoot AT, Brown SS et al, (1969). Forced diuresis
in the treatment of acute salicylate poisoning in adults. Quart J
Med, 38:31-48.
Levy G, Tsuchiya T, (1972). Salicylate accumulation kinetics in
man. N Engl J Med, 287:430-2.
Levy G, (1978). Clinical pharmacokinetics of aspirin. Pediatrics,
62 (suppl):867-72.
Liebman RM, Katz HM, (1981). Pulmonary edema in a 52-year-old
woman ingesting large amounts of aspirin. JAMA, 246:2227-8.
Lowney ED, Baublis JV, Kreye GM, Harrell ER, McKenzie AR, (1967).
The scalded skin syndrome in small children. Arch Dermatol,
95:359-69.
Lynd PA, Andreasen AC, Wyatt RJ, (1976). Intrauterine salicylate
intoxication in a newborn. Clin Pediatrics, 15:912-3.
McGuigan MA, (1987). A two-year review of salicylate deaths in
Ontario. Arch Intern Med, 147:510-2.
Meredith TJ, Vale JA, (1981). Salicylate poisoning. Poisoning:
Diagnosis and Treatment, Vale and Meredith Eds, (Update Books,
London): 97-103.
Meredith TJ, Vale JA, (1986). Non-narcotic analgesics. Drugs, 32
(suppl 4).
Mortimer EA, Lepow ML, (1962). Varicella with hypoglycaemia
possibly due to salicylates. Am J Dis Child, 103:583-90.
Myers EN, Bernstein JM, Fostiropolous G, (1965). Salicylate
Tototoxicity: a clinical study. N Engl J Med, 273:587-90.
Notes and News, (1986). Reye's syndrome and the giving of aspirin
to children. Lancet, 1:1396.
Paynter AS, Alexander FW, (1979). Salicylate intoxication caused
by teething ointment. Lancet, 2:1132.
Pei YP, Thomson DA, (1987). Severe salicylate intoxication
mimicking septic shock (letter). Am J Med, 82:581-2.
Pluskwa F, Couturier M, Kirsh JM, Fourgeaux B, Campinos L, Duval
G, (1984). Intoxication, salicyl_e, grave par vou transcutan_e
chez un br_l_. La Presse M_d, 13:1391.
Prescott LF, Balali-Mood M, Critchley JAJH, Johnstone AF,
Proudfoot AT, (1982). Diuresis or urinary alkalinisation for
salicylate poisoning. Br Med J, 285:1383-6.
Proudfoot AT, Brown SS, (1969). Acidaemia and salicylate
poisoning in adults. Br Med J, 2:547-50.
Proudfoot AT, (1983). Toxicity of salicylates. Am J Med, 14:99-
103.
Remington PL, Rowley D, McGee H. et al, (1986). Decreasing trends
in Reye syndrome and aspirin use in Michigan, 1979-1984.
Pediatrics, 77:93-8.
Reynolds EF (ed) (1982). Aspirin and similar analgesic and anti-
inflammatory agents. Martindale, The Extra Pharmacopoeia 28 Ed,
234-82.
Reye RDK, Morgan G, Baral J, (1963). Encephalopathy and fatty
degeneration of the viscera. A disease entity in childhood.
Lancet, 11:749-53.
Robins JB, Turnbull JA, Robertson C, (1985). Gastric perforation
after acute aspirin overdose. Hum Toxicol, 4:527-8.
Rupp DJ, Seaton RD, Wiegmann TB, (1983). Acute polyuric renal
failure after aspirin intoxication. Arch Int Med, 143:1237-8.
Sandford-Smith JH, (1974). Transient myopia occured in a patient
following the ingestion of 2.7 g of aspirin. Br J Ophthal,
58:698.
Sketris IS, Mowry JB, Czajka PA, Anderson WH, Stafford DT,
(1982). Saline catharsis: effect on aspirin bioavailability in
combination with activated charcoal. J Clin Pharmacol, 22:59-64.
Smith MJH, Dawkins, PD, (1971). Salicylate and enzymes. J Pharm
Pharmacol, 23:729.
Springer DJ, Groll A, (1980). Poisoning with enteric coated
acetylsalicylic acid complicating gastric outlet obstruction. Can
Med Assoc J, 122:1032-3.
Starko KM, Mullick FG, (1983). Hepatic and cerebral pathology
findings in children with fatal salicylate intoxication: futher
evidence for a causal relation between salicylate and Reye's
Syndrome. Lancet, 1:326-9.
Sullivan-Bolyai JZ, Corey L, (1981). Epidemiology of Reye
syndrome. Epidemiologic Rev, 3:1-26.
Taylor JR, Halprin KM, (1975). Percutaneous absorption of
salicylic acid. Arch Dermatol, 111:740-3.
Temple AR, (1978). Pathophysiology of aspirin overdosage toxicity,
with implications for management. Pediatrics, 62 (suppl):873-6.
Temple AR, (1981). Acute and chronic effects of aspirin toxicity
and their treatment. Arch Intern Med, 141:364-9.
Temple AR, George DJ, Done AK, Thompson JA, (1976). Salicylate
poisoning complicated by fluid retention. Clin Toxicol, 9:61-8.
Tenney SM, Miller RM, (1955). The respiratory and circulatory
actions of salicylate. Am J Med, 19:495-508.
Thurston JH, Pollock PG, Warren SK, Jones EM, (1970). Reduced
brain glucose with normal plasma glucose in salicylate poisoning.
J Clin Invest, 49:2139.
Todd PJ, Sills JA, Harris F, Cowen JM, (1981). Problems with
overdose of sustained-release aspirin. Lancet, 1:777.
Treguerer H, Le Bihan G, Coloignier M, Le Roux P, Bernard JP,
(1980). Intoxication salicyl_e par application locale de
vaseline salicyl_e a 20% chez un psoriasique. Nouv Presse Med,
9:192-3.
Vale JA, Buckley GM, Meredith TJ, (1985). Algorithm for modified
alkaline diuresis in salicylate poisoning. Br Med J, 290:155.
Walters JS, Woodring JH, Stelling CB, Rosenbaum HD, (1983).
Salicylate-induced pulmonary edema. Radiology, 146:289-93.
Waltner JG, (1955). The effect of salicylates on the inner ear.
Ann Otol Rhino Lar, 64:617-22.
Winchester JF, Gelfland MC, Knepshield JH, Schreiner GE, (1977).
Dialysis and hemoperfusion of poisons and drugs. Transactions of
the American Society of Artificial and Internal Organs, 23:762-
842.
Winchester JF, Gelfland MC, Helliwell M, Vale JA, Goulding R,
Schreiner GE, (1981). Extracorporeal treatment of salicylate or
acetaminophen poisoning. Is there a role? Arch Intern Med,
141:370-4.
Winters RW, White JS, Ordway NK, (1959). Disturbances of acid-
base equilibrium in salicylate intoxication. Pediatrics, 23:260-
85.
Wolfe JD, Metger AL, Goldstein RC, (1974). Aspirin hepatitis. Ann
Intern Med, 80:74-6.
Woodbury DM, Fingl E, (1975). Analgesic-antipyretics, anti-
inflammatory agents, and drugs employed in the therapy of gout.
In: The pharmacological basis of therapeutics, 325-43. Eds
Goodman and Gilman.
Wortzmann DJ, Grunfeld D, (1987). Delayed absorption following
enteric-coated aspirin overdose. Ann Emerg Med, 16:434.
Young RSK, Torretti D, Williams RH et al, (1984). Reye's syndrome
associated with long-term aspirin therapy. JAMA, 251:754-6.
14. AUTHOR(S), REVIEWER(S), DATE(S) (INCLUDING UPDATES), COMPLETE
ADDRESS(ES)
Authors: Professor A.N.P. van Heijst
Baarnseweg 42a
3735 MJ Bosch en Duin
Netherlands
Tel: 31-30-287178
Dr A. van Dijk
Central Hospital Pharmacy
University Hospital Utrecht
P.O. Box 85500
3508 GA Utrecht
Netherlands
Tel: 31-30-507190
Fax: 31-30-516756
Date: 22 June 1990
Reviewer: Dr T. Meredith
Department of Health
Hannibal House Room 913
Elephant & Castle
London SE1 6TE
United Kingdom
Tel: 44-71-9722449
Fax: 44-71-7039565
Date: 12 January 1991
Peer Review: Newcastle-upon-Tyne, United Kingdom, January 1991
COOH
O-CO-CH3
2-acetoxy-benzoic acid
C9H8O4
Molecular weight 180.15
3.3 Physical properties
3.3.1 Properties of the substance
Colourless or white crystals or white crystaline
powder or granules; odourless or almost
odourless with a slight acid taste. Melting
point about 143o. Soluble 1 in 300 of water, 1
in 5 - 7 in alcohol, 1 in 17 of chloroform and 1
in 20 of ether; soluble in solutions of acetates
and citrates and, with decomposition, in
solutions of alkali hydroxides and carbonates. A
solution in water is acid to methyl red.
Incompatible with free acids, acetanilide,
aminopyrine, phenazone, hexamine, iron salts,
phenobarbitone sodium, quinine salts, potassium
and sodium iodides, and alkali hydroxides,
carbonates, and stearates.
Acetylsalicylic acid is stable in dry air, but
gradually hydrolyses in contact with moisture to
acetic and salicylic acids. In solution with
alkalis, the hydrolysis proceeds rapidly and the
clear solutions formed may consist entirely of
acetate and salicylate.
Acetylsalicylic acid decomposes rapidly in
solutions of ammonium acetate or of the acetates,
carbonates, citrates or hydroxides of the
alkali metals (Reynolds, 1989).
3.3.2 Properties of the locally available formulation
To be completed by the PCC.
3.4 Other characteristics
3.4.1 Shelf-life of the substance
Shelf-life is highly dependent on the manner of storage.
It is recommended that the active ingredient is tested
on a yearly basis using the principles outlined in the
relevant monograph of the European Pharmacopoeia. When
all the appropriate tests have been undertaken with
satisfactory results and when the active ingredient is
stored in the manner recommended by monograph, extension
of the period of storage for a further year may be
considered.
3.4.2 Shelf-life of the locally available formulation
To be completed by the PCCTo be completed by the PCC
3.4.3 Storage conditions
Store in air-tight containers.
3.4.4 Bioavailability
After oral administration, 80 - 100% will be absorbed in
the stomach and in the small intestine. However,
bioavailability is lower because partialhydrolysis
occurs during absorption and there is a "first-pass"
effect in the liver.
The non-protein bound fraction of salicylate increases
with the total plasma concentration, and the binding
capacity of albumin is partially saturated at
therapeutic concentrations of salicylate (Borga et al.,
1976). The greater proportion of unbound drug found at
high concentrations will mean that greater toxicity will
result than would be expected from the total salicylate
concentration (Alvan et al., 1981).
Absorption after rectal administration is slow and
unpredictable. Timed-release preparations are
therapeutically of limited value because of the
prolonged half-life of elimination of salicylate.
Absorption of enteric-coated tablets is sometimes
incomplete.
3.4.5 Specific properties and composition
To be completed by the PCC.
4. USES
4.1 Indications
As an analgesic for the treatment of mild to moderate pain,
as an anti-inflammatory agent for the treatment of soft tissue and
joint inflammation, and as an antipyretic drug.
In low doses salicylate is used for the prevention of
thrombosis.
4.2 Therapeutic dosage
4.2.1 Adults
(Informatorium Medicamentorum, 1990)
Oral (Na- or Ca- or lysine acetylsalicylate):
Indication: pain and fever: 300-1000 mg every 4 h; max 4
g a day.
Indication: acute polyarthritis rheumatica: 1 g 6 times
a day; max 8 g a day.
Indication: rheumatoid arthritis: 0.5-1 g 6 times a day;
max 8 g a day.
Compounds with controlled release: 1 g 2-3 times a day,
if necessary 6 times a day.
Indication: to prevent transient ischaemic attacks and
to prevent arterial thrombosis: 300-1200 mg a day in 2-3
doses.
Rectal:
Indication: pain and fever: 500-1000 mg every 6 h; max 4
g a day.
Indication: rheumatoid arthritis: 0.5-1 g, 6 times a
day; max 8 g a day.
Intramuscular or intravenous (lysine
acetylsalicylate):500 mg, 1-4 times a day.
4.2.2 Children
CAUTION see 9.4.14
Oral (Na- or Ca- or lysine acetylsalicylate):
Indication: pain and fever: the use of acetylsalicylic
acid in young children for these indications is no
longer advocated because of the risk of Reyes Syndrome.
Indication: acute polyarthritis rheumatica: to start
with 100-15 mg/kg a day; after one week 60 mg/kg a day.
Indication juvenile arthritis: children up to 25 kg, 60-
90 mg/kg a day; children over 25 kg, 2.4-3.6 g a day.
Rectal:
Indication: pain and fever:
Age < 2 years, 20 mg/kg, max 2 times a day
Age > 2 years, 20 mg/kg, max 3 times a day
Intramuscular, intravenous (lysine acetylsalicylate): 5-
25 mg/kg a day.
4.3 Contraindications
Active peptic ulcer
Febrile/post-febrile illness in children
Haemostatic disorders, including anticoagulant and
thrombolytic treatment
Hypoproteinemia
Hypersensitivity
Asthma induced by acetylsalicylic acid or other non-steroidal
anti-inflammatory drugs.
Caution is indicated in patients with:
a history of peptic ulceration or gastro-intestinal
haemorrhage
hepatic or renal insufficiency
asthma
children < 2 years, especially in those who are
dehydrated
(Informatorium Medicamentorum, 1990)
5. ROUTES OF ENTRY
5.1 Oral
Ingestion of acetylsalicylic acid tablets is the most frequent
cause of salicylate poisoning; in neonates, infants and
children other less common causes include application of
teething gels to gums (Paynter & Alexander, 1979), placental
transfer (Ahlfors et al, 1982; Lynd et al, 1974) and breast-
milk (Clark & Wilson, 1981).
Methyl salicylate is particularly toxic because of rapid
absorption and 1 teaspoonful (5 ml) contains the equivalent of
6.9 g acetylsalicylic acid (Johnson & Welch, 1984); methyl
salicylate intoxication has also been reported following its
use as candy flavouring (Howrie et al, 1985).
5.2 Inhalation
Maximum permissible atmospheric concentration 5 mg per m3
(Reynolds, 1989).
5.3 Dermal
The quantity absorbed after 10 hours dermal application of
salicylated vaseline under occlusive dressing was more than
60% (Taylor & Halprin, 1975). Salicylate poisoning has been
reported after application of salicylate ointment to burns
(Pluskwa et al, 1984) and other dermatological disturbances
(Editorial, 1964; Treguerer et al, 1980).
Percutaneous absorption of methyl salicylate ('oil of
wintergreen') may also cause toxicity (Davies et al, 1979).
5.4 Eye
Unknown.
5.5 Parenteral
Lysine acetylsalicylate is the conventional means of
administering salicylate by this route; acetylsalicylic acid
has also been used as an admixture with other drugs given
intravenously.
5.6 Other
Rectal administration of salicylic acid suppositories may be
necessary in infants or when oral dosing is either not
possible or is contraindicated.
6. KINETICS
6.1 Absorption by route of exposure
Salicylic acid is a weak acid (pKa 3); following oral
administration, almost all salicylate is found in the
unionized form in the stomach. Acetylsalicylic acid is poorly
soluble in the acid media of the stomach and precipitates may
coalesce to form concretions, thereby delaying absorption for
8 to 24 hours. Despite the higher pH of the small bowel, the
larger surface area allows absorption of salicylate, and this
occurs rapidly at therapeutic doses. However, absorption
following overdose commonly occurs more slowly, and blood
concentrations can continue to rise for up to 24 h after
ingestion (Ferguson & Boutros, 1970; Kaufman & Dubanksy, 1972;
Levy, 1978). Absorption of salicylate will be further delayed
if an enteric-coated preparation has been ingested (Wortzmann
& Grunfeld, 1987).
6.2 Distribution by route of exposure
About 50 - 80% of salicylate in the blood is bound by protein
while the rest remain in the active, ionized state; protein
binding is concentration-dependent. Saturation of binding
sites leads to more free salicylate and increased toxicity.
The volume of distribution is 0.1-0.2 l/kg. Acidosis increases
the volume of distribution because of enhancement of tissue
penetration of salicylates (Levy & Tsuchiya, 1972).
6.3 Biological half-life by route of exposure
Acetylsalicylic acid is hydrolyzed in the stomach and in blood
to salicylic acid and acetic acid; the biological half-life is
therefore only 20 minutes.
The plasma salicylate half-life following therapeutic doses is
2 to 4.5 h, but in overdose increases to 18 to 36 h (Done,
1960).
6.4 Metabolism
Approximately 80% of small doses of salicylic acid is
metabolised in the liver. Conjugation with glycine forms
salicyluric acid and with glucuronic acid forms salicyl acyl
and phenolic glucuronide. These metabolic pathways have only a
limited capacity. Small amounts of salicylic acid are also
hydroxylated to gentisic acid. With large salicylate doses
the kinetics switch from first order to zero order (Michaelis-
Menten kinetics) (Levy & Tsuchiya, 1972).
6.5 Elimination by route of exposure
Salicylates are excreted mainly by the kidney as salicyluric
acid (75%), free salicylic acid (10%), salicylic phenol (10%)
and acyl (5%) glucuronides, and gentisic acid (< 1%). When
small doses (less than 250 mg in an adult) are ingested, all
pathways proceed by first order kinetics, with an elimination
half-life of about 2-3 hours (Hartwig-Otto, 1983). When higher
doses of salicylate are ingested (more than 4 g), the half-
life becomes longer (15-30 hours) because the
biotransformation pathways concerned with the formation of
salicyluric acid and salicyl phenolic glucuronide become
saturated.
Renal excretion of salicylic acid becomes increasingly
important as the metabolic pathways become saturated, because
it is extremely sensitive to changes in urinary pH above pH 6.
The use of urinary alkalinization exploits this particular
aspect of salicylate elimination.
7. PHARMACOLOGY AND TOXICOLOGY
7.1 Mode of action
7.1.1 Toxicodynamics
Nausea and vomiting occur as a result of stimulation of
mucosal receptors by gastric irritation and stimulation
of receptors accessible from the cerebrospinal fluid,
probably in the medullary chemoreceptor ("chemoreceptor
trigger zone").
Marked hyperventilation occurs as a result of direct
stimulation of the respiratory centre. Indirect
stimulation of respiration is caused by increased
production of CO2 as a result of salicylate-induced
uncoupling of oxidative phosphorylation. A respiratory
alkalosis develops as a result of the direct and
indirect stimulation of the respiratory centre. In an
attempt to compensate, bicarbonate, accompanied by
sodium, potassium and water, is excreted in the urine.
Dehydration and hypokalcaemia result but, more
importantly, the loss of bicarbonate diminishes the
buffering capacity of the body and allows the
development of a metabolic acidosis (see below).
The pyretic effect of toxic doses of salicylate is a
direct result of the uncoupling of oxidative
phosphorylation, and the sweating that results further
contributes to dehydration.
High doses of salicylates have additional toxic effects
on the central nervous system consisting of stimulation
(including convulsions) followed by depression,
confusion, dizziness, asterixis, delirium, psychosis,
stupor and coma (Anderson et al. 1976; Anderson et al,
1981).
Very high doses of salicylate have a depressant effect
on the medulla and may cause central respiratory
paralysis as well as sudden circulatory collapse
secondary to vasomotor depression.
The loss of buffering capacity (see above), and the
effects of salicylate on carbohydrate, lipid and protein
metabolism lead to the development of a metabolic
acidosis, or more commonly in practice, a mixed acid-
base disturbance. Competitive inhibition of NAD+-
dependent dehydrogenases in the citric acid cycle will
lead to the accumulation of acid intermediates.
(Grisolia et al, 1969). Salicylate enhances the entry
and oxidation of fatty acids in liver cells, leading to
increased ketogenesis, and will also inhibit amino acid
incorporation into protein (Smith & Dawkins, 1971)
causing amino-acidaemia. In the presence of an acidosis,
entry of salicylate ion into cells is promoted, and the
metabolic effects exacerbated.
Both hypo-and hyperglycaemia occur in salicylate
poisoning, the former most probably being due to
increased tissue demand for glucose oxidation due to
uncoupling of oxidative phosphorylation; neuroglycopenia
can occur in the presence of normal blood sugar
concentrations (Thurston et al, 1970). If hepatic
glycogen stores are adequate, catecholamine production
stimulates glycogenolysis leading to hyperglycaemia
which may persist for several days (Cotton & Fahlberg,
1964; Mortimer & Lepow, 1962); raised plasma
corticosteroid concentrations probably augment this
effect.
Salicylate intoxication is often accompanied by
hypoprothrombinaemia due to a warfarin-like action of
salicylate on the vitamin K1-epoxide cycle, though this
rarely causes clinical problems (Bell, 1978).
7.1.2 Pharmacodynamics
The salicylates alleviate pain by virtue of both a
peripheral and a central nervous system effect.
Salicylates, by inhibiting the synthesis
ofprostaglandins that occur in inflamed tissues, prevent
the sensitization of pain receptors to mechanical
stimulation or to chemicals, such as bradykinin, that
appear to mediate the pain response. Direct effects on
the central nervous system have been described and
suggest a hypothalamic site for the analgesic as well as
the antipyretic effects (Woodbury & Fingl, 1975).
Acetylsalicylate decreases platelet adhesiveness for the
life-time of the platelet (Brantmark et al. 1981).
7.2 Toxicity
7.2.1 Human data
7.2.1.1 Adults
Mild to moderate toxicity 150-300 mg/kg
Serious toxicity 300-500 mg/kg
Potentially lethal > 500 mg/kg
(Temple, 1981)
7.2.1.2 Children
In a child, ingestion of 240 mg/kg will cause
moderately severe poisoning, but deaths rarely
occur when less than 480 mg/kg has been taken
(Done, 1978).
Salicylate poisoning in small children (< 4
years) is often more serious than in older
children because of the early development of a
metabolic acidosis rather than a respiratory
alkalosis (Winters et al. 1959).
7.2.2 Relevant animal data
None relevant.
7.2.3 Relevant in vitro data
None relevant.
7.3 Carcinogenicity
No data available.
7.4 Teratogenicity
In an embryo culture system, malformations were observed at
plasma salicylate concentrations approaching those after
single doses of salicylate (Greenaway et al., 1984).
Reputedly, the rat is sensitive to the teratogenic effects of
salicylates, whereas humans and non-human primates are
regarded as being resistant (Shepard, 1983; Wilson et al.,
1977).
7.5 Mutagenicity
No data available.
7.6 Interactions
Potentiating effects
Aspirin potentiates the ulcerogenic effects of caffeine,
indomethacin and phenybutazone (Goodman & Gilman, 1990).
Acetylsalicylic acid is also reported to potentiate the
effects of warfarin and other anticoagulants of the coumarin
type. Phenothiazines, and chlorpromazine in particular, are
potentiated by salicylates (Huang & Hirano, 1967).
Acetylsalicylic acid is highly protein-bound and may increase
the unbound or free drug concentrations of other drugs, for
instance hypoglycaemic drugs will be displaced from the bound
state (Hecht & Goldner, 1959) and cases of hypoglycaemic coma
have been reported (Bergman, 1965; Peaston & finnegan, 1965).
Aspirin displaces methotrexate from protein binding sites and
increases tissue concentrations, reaching toxic levels rapidly
because the therapeutic index of methorexate is very low
(Baker, 1970).
Antagonistic effects
The uricosuric activity of phenylbutazone, probenecid and
sulfinpyrazone is strongly antagonized and may be completely
abolished by aspirin even after small doses (Yu et al., 1963;
Pascale et al., 1955; and Oyer et al., 1966).
The mineralocorticoid blocking properties of spironolactone
are inhibited (Tweedale & Oglivie, 1971).
In a report of two cases of severe salicylate poisoning,
asystole occured shortly after the intravenous administration
of diazepam (Berk & Andersen, 1989).
7.7 Main adverse effects
The most common adverse effects seen following therapeutic
doses of acetylsalicylic acid are gastrointestinal in origin,
including nausea, epigastric discomfort and vomiting.
Irritation of the gastric mucosa with erosion, ulceration,
haematemesis and melaena may occur; occult blood loss may
occur in about 70% of patients unaccompanied by dyspepsia.
Slight blood loss whilst not usually of clinical significance,
may result in iron deficiency anaemia.
Some individuals, especially asthmatics, exhibit sensitivity
to acetylsalicylic acid. Urticaria, angioneurotic oedema,
rhinitis and severe, even fatal, paroxysmal bronchospasm and
dyspnoea may occur (Reynolds, 1989).
8. TOXICOLOGICAL ANALYSES AND BIOMEDICAL INVESTIGATIONS
8.1 Material sampling plan
8.1.1 Sampling and specimen collection
8.1.1.1 Toxicological analyses
Screen residues or suspected materials, plasma,
urine and stomach contents. Avoid sodium azide
preservation.
8.1.1.2 Biomedical analyses
8.1.1.3 Arterial blood gas analysis
8.1.1.4 Haematological analyses
8.1.1.5 Other (unspecified) analyses
8.1.2 Storage of laboratory samples and specimens
8.1.2.1 Toxicological analyses
Keep biological samples in a refrigerator prior
to analysis.
8.1.2.2 Biomedical analyses
8.1.2.3 Arterial blood gas analysis
8.1.2.4 Haematological analyses
8.1.2.5 Other (unspecified) analyses
8.1.3 Transport of laboratory samples and specimens
8.1.3.1 Toxicological analyses
No special conditions.
8.1.3.2 Biomedical analyses
8.1.3.3 Arterial blood gas analysis
8.1.3.4 Haematological analyses
8.1.3.5 Other (unspecified) analyses
8.2 Toxicological Analyses and Their Interpretation
8.2.1 Tests on toxic ingredient(s) of material
8.2.1.1 Simple Qualitative Test(s)
For detection of salicylate ion:
Acetylsalicylate ion is not present in
plasma/serum and urine; in plasma,
acetylsalicylate ion is only present when
fluoride is added to the blood sample.
(1) Principle of test: Direct colour reaction
on urine. Serum residues and stomach contents
require preliminary acid hydrolysis to
salicylate.
(2) Sampling: 2 ml of urine and small portion
of suspected material (+/-200 mg).
(3) Chemicals and reagents:
Chemicals
Mercuric chloride (Hg(II)Cl2)
Ferric nitrate 9 aq (Fe(NO3)3).9 H2O)
Hydrochloric acid 1 mol/L and 0.1 mol/L
Sodium hydroxide 0.1 mol/L
Sodium salicylate p.a.
Acetylsalicylic acid p.a.
Reagents
Trinder's Reagent: Dissolve 40 g of
Hg(II)Cl2 in 850 ml of hot water. After
cooling down add 120 ml of HCl 1 mol/L and 40 g
of hydrated ferric nitrate. When the ferric
nitrate has been dissolved, add water to 1000
ml.
(4) Equipment:
No special equipment needed
(5) Sample preparation:
Stomach contents and serum residues
1. Boil a portion of the specimen with 2
ml of hydrochloric acid 0.1 mol/L for 10
minutes.
2. Cool and filter if necessary.
3. Neutralise with sodium hydroxide 0.1
mol/L.
(6) Procedure
Urine
1. Add 100 uL Trinder's Reagent to 1 ml of
urine and mix.
2. A violet colour indicates the presence
of salicylates.
Stomach contents and serum residues
1. Add 100 uL of Trinder's Reagent to the
clear neutralized solution obtained under (5)
"Sample preparation".
2. A violet colour indicates the presence
of salicylate.
(7) Calibration procedure
Not applicable
(8) Quality control
Quality control can be achieved by running
spiked samples as a comparison.
(9) Specificity
Azide preservatives interfere strongly.
High concentrations of urinary ketone bodies may
give weak false positives.
Salicylamide, p-aminosalicylic acid and 4-
aminoantipyrin also react.
Acetylsalicylic acid and methylsalicylic
acid only react after preliminary acid
hydrolysis.
(10) Detection limit
Approximately 20 mg salicylate/L urine.
(11) Analytical assessment
A positive result may indicate the presence
of a salicylate.
(12) Medical interpretation
A positive result indicates the possibility
of salicylate poisoning.
8.2.1.2 Advanced Qualitative Confirmation Test(s)
Acetylsalicylic acid is only present in stomach
contents and serum residues. Confirmation may
be obtained by TLC (DFG, Report VII, 1987) or
GLC (DFG, Report II, 1985); the same applies to
the salicylate ion.
8.2.1.3 Simple Quantitative Method(s)
..... to be completed
8.2.1.4 Advanced Quantitative Method(s)
..... to be completed
8.2.2 Tests for biological specimens
8.2.2.1 Simple Qualitative Test(s)
(1) Principle:
Measurement of red-violet complex of ferric
and salicylate ions at 530 or 546 nm
(2) Sampling:
Plasma or serum.
(3) Chemicals and reagents:
As for 8.2.1.1 -(3).
Salicylate stock solution 2000 mg/L:
Dissolve 580 mg of sodium salicylate in 250
ml of distilled water.
Salicylate calibration samples:
Dilute appropriate volumes of the stock
solution with distilled water to salicylate
concentrations of 100, 200, 300, 400 and 500
mg/L.
(4) Equipment:
Pipettes, glass tubes, cuvettes, centrifuge
and (spectro)photometer.
(5) Sample preparation:
Not applicable.
(6) Procedure:
1. Pipette into glass tube:
Blank Sample
Calibration points
Plasma (serum) - 0.5 ml -
Calibration points - - 0.5 ml
(100-500 mg/l)
Distilled water 0.5 ml - -
Trinder's Reagent 2 ml 2 ml 2 ml
2. Mix by vortexing for 30 seconds,
centrifuge and measure the extinction of the
clear supernatant at 530 or 546 nm against the
blank.
(7) Calibration procedure:
A calibration curve is constructed by
plotting the concentration of the calibration
samples (100-500 mg/L) against the measured
extinction. From this curve the concentration in
the sample is read.
(8) Quality control:
The use of a plasma (serum) sample with a
known concentration is advised. This specimen
can be obtained commercially or made by
appropriate spiking of pre-tested blank plasma
(serum).
(9) Specificity:
As for 8.2.1.1 -(9).
(10) Detection limit:
The limit of detection of the method is 70
mg salicylate/L plasma(serum) using a 0.5 ml
sample size.
(11) Analytical assessment:
If no interfering substances are present
and if the procedure has been followed, an
accurate measurement of total salicylates
content will be made.
(12) Medical assessment:
The total salicylate plasma (serum)
concentration and the expected severity of
intoxication should be related to the interval
that has elapsed between sampling and ingestion.
Generally:
(a) Time interval 6 hours: Salicylate
<400 mg/L: usually
asymptomatic
Salicylate 400-800 mg/L: mild to
moderate toxicity
Salicylate >800 mg/L: severe toxicity
(b) Time interval 12 hours:
Salicylate <300 mg/L: usually
asymptomatic
Salicylate 300-600 mg/L: mild to
moderate toxicity
Salicylate >600 mg/L: severe toxicity
(c) Time interval 24 hours:
Salicylate <200 mg/L: usually
asymptomatic
Salicylate 200-500 mg/L: mild to
moderate toxicity
Salicylate >500 mg/L: severe toxicity
8.2.2.2 Advanced Qualitative Confirmation Test(s)
8.2.2.3 Simple Quantitative Method(s)
In plasma (serum) or urine only salicylate can
be determined.
Acetylsalicylic acid can only be quantitated in
plasma when sufficient sodium fluoride has been
added to the blood collection tube. Any HPLC
method described in the literature is suitable.
8.2.2.4 Advanced Quantitative Method(s)
8.2.2.5 Other Dedicated Method(s)
8.2.3 Interpretation of toxicological analyses
8.3 Biomedical investigations and their interpretation
8.3.1 Biochemical analysis
8.3.1.1 Blood, plasma or serum
Tests of platelet function and coagulation to
determine/exclude presence of: inhibition of
platelet aggregation and hypoprothrombinaemia.
8.3.1.2 Urine
8.3.1.3 Other fluids
8.3.2 Arterial blood gas analyses
In adults an initial respiratory alkalosis is followed
in severe salicylate poisoning by metabolic acidosis;
typically the acid-base disturbance is mixed in nature.
Young children tend to develop a metabolic acidosis but,
by the age of 12 years, the usual adult picture of a
mixed respiratory alkalosis and metabolic acidosis is
seen.
8.3.3 Haematological analyses
Electrolytes and urea to determine/exclude presence of:
hypernatraemia due to water loss (sweating, vomiting,
osmotic diuresis) and hypokalaemia due to renal and
extrarenal factors.
Blood sugar level to determine/exclude presence of:
hypoglycaemia or hyperglycaemia.
Urinary pH (typically alkaline in the early stages of
salicylate overdose and then subsequently becomes acid).
8.3.4 Interpretation of biomedical investigations
Not relevant.
8.4 Other biomedical (diagnostic) investigations and their
interpretation
8.5 Overall Interpretation of all toxicological analyses and
toxicological investigations
The plasma salicylate concentration should be determined on
admission, provided that more than 4 to 6 hours have elapsed
from the time of ingestion of the overdose because
measurements made before this time are difficult to interpret.
In addition, it is important to repeat the measurement to
make sure that the salicylate concentration is not continuing
to rise because of continued absorption (Vale et al, 1985).
In adults, plasma concentrations 6 hours after an overdose:
300-500 mg/l: mild toxicity
500-750 mg/l: moderate toxicity
> 750 mg/l: severe toxicity (Proudfoot,
1983).
The presence or absence of symptoms and signs and the type of
acid-base disturbance should be considered when interpreting
the plasma salicylate concentration and deciding upon
management (Meredith & Vale, 1986).
The DONE-nomogram (fig.) categorizes the severity of poisoning
for single ingestions based on peak salicylate concentrations.
In patients with a significant acidosis and in patients who
ingest multiple doses or sustained release preparations, the
DONE-nomogram will tend to underestimate the severity of
intoxication (Todd et al., 1981).
The validity of the DONE-monograph was evaluated in 54 cases
by Dugandzic et al. (1989). The predictive index for the
moderate and severe classifications were poor. Development of
the nomogram was based on the assumption that salicylates are
eliminated by a first order process, whereas in fact they are
partially eliminated through saturable processes. This may
lead to overprediction of severity. For this reason the DONE-
monograph is not commonly used.
After ingestion of enteric coated tablets, plasma salicylate
concentrations on admission are unreliable guides to the
severity of poisoning. Salicylate levels may not peak until
more than 12 hours after such an overdose (Todd et al., 1981;
Springer & Groll, 1980).
8.6 References
9. CLINICAL EFFECTS
9.1 Acute poisoning
9.1.1 Ingestion
Early symptoms are nausea, vomiting, epigastric pain and,
sometimes, haematemesis. Additional characteristic
features include hyperventilation,elevated body
temperature, irritability and tinnitus. Less commonly,
cardiac dysrhythmias may occur because of hypokalaemia,
and tetany and paraesthesiae due to low ionised calcium
levels. In serious poisoning hallucinations, stupor,
convulsions, papilloedema and coma may be observed
together with metabolic acidosis; hepatotoxicity may
also occur and non-cardiogenic pulmonary oedema is well
described.
9.1.2 Inhalation
No data available.
9.1.3 Skin exposure
Severe poisoning has been reported as a result of use of
salicylic acid ointment for dermatological problems
(Editorial, 1964; Taylor & Halprin, 1975) and in the
treatment of skin burns (Pluskwa et al. 1984).
9.1.4 Eye contact
No data available.
9.1.5 Parenteral exposure
No data available.
9.1.6 Other
Acetylsalicylic acid suppositories can cause rectal
irritation; absorption is slow and unpredictable.
9.2 Chronic poisoning
9.2.1 Ingestion
Chronic salicylate poisoning occurs as a result of
excessive therapeutic administration over a period of 12
hours or more (Dove & Jones, 1982) because the metabolic
pathways become saturated and salicylic acid is
eliminated by zero order kinetics. Plasma salicylate
concentrations then increase, producing toxicity. Small
children are at particular risk of well-intentioned but
over-enthusiastic treatment by their parents. The risk
is further increased when the fever, sweating and
tachycardia of salicylate intoxication are attributed to
the underlying illness and are used as indications for
increasing the dose (Proudfoot, 1983). Children may
become intoxicated through breast milk (Clark & Wilson,
1981), and chronic salicylate poisoning has also been
described in the elderly (De Groen, 1989).
Neurological features in adults occur more frequently
with chronic salicylate poisoning than as a result of
acute overdose, and may lead to extensive investigations
before the correct diagnosis is reached, often late
after hospital admission (Anderson et al. 1976). Older
patients present with breathlessness, and pulmonary
oedema caused by salicylate poisoning can be mistakenly
attributed to cardiac or respiratory disease (and vice
versa).
The mortality from chronic salicylate poisoning in
adults is considerably greater than from acute overdose
(Anderson et al. 1976). In children, chronic salicylism
is accompanied by a greater morbidity than is acute
salicylate poisoning. Hyperventilation, dehydration and
severe central nervous system manifestations occur more
frequently in those chronically poisoned (Gaudreault et
al. 1982).
9.2.2 Inhalation
No data available.
9.2.3 Skin exposure
The application of teething gels to the gums (Paynter &
Alexander, 1979), or percutaneous absorption of
salicylic acid from ointments used for such skin
disorders as psoriasis or methyl salicylate may cause
salicylate poisoning.
9.2.4 Eye contact
No data available.
9.2.5 Parenteral exposure
No data available.
9.2.6 Other
No data available.
9.3 Course, prognosis, cause of death
Adults seldom lose conciousness and are typically miserable
and uncomfortable. If a sufficient quantity of a salicylate
has been taken, nausea, vomiting, tinnitus, deafness, sweating,
vasodilatation and hyperventilation develop.
Cause of death.
A review of 51 fatal cases of acute salicylate poisoning in
Ontario during 1983 and 1984 disclosed that salicylates was
the most common cause of death due to the ingestion of single
drugs. Autopsy results showed that 50% of the patients had
pulmonary abnormalities, 28% had lesions of the
gastrointestinal tract, 18% had nervous system abnormalities
and 25.6% had no pathologic changes (McGuigan, 1987).
Mortality from chronic salicylate intoxication is considerable
higher (25%) than from acute overdose (1-2%) (Anderson et al.
1976). Death is often due to sudden cardiac arrest or,
occasionally, to multiple complications following severe brain
damage (Proudfoot, 1983).
9.4 Systematic description of clinical effects
9.4.1 Cardiovascular
Abrupt cardiovascular collapse is a recognized
complication of salicylate poisoning (Anderson et al.
1976; Benowitz et al. 1979; Beveridge et al. 1964; Pei &
Thomson, 1987). Two patients with severe salicylate
intoxication developed asystole shortly after
intravenous diazepam administration (Berk & Andersen,
1989).
9.4.2 Respiratory
Non-cardiogenic pulmonary oedema can occur in salicylate-
intoxicated patients who are over 30 years of age.
Cigarette smoking, chronic salicylate ingestion,
metabolic acidosis and the presence of neurological
symptoms and signs on admission are strong risk factors
for the subsequent development of pulmonary oedema. In
the absence of these risk factors, salicylate-induced
pulmonary oedema is rare. The exact mechanism is
unknown. Three possible explanations include: a direct
toxic effect on pulmonary microvasculature by
salicylates, interaction with endogenous mediators such
as prostaglandins, and a central nervous system mediated
effect (Walters et al. 1983; Liebman & Katz, 1981).
9.4.3 Neurological
9.4.3.1 CNS
High doses of salicylates cause stimulation
(irritability, convulsions) followed by
depression of the CNS. Confusion, dizziness,
delirium, psychosis, asterixis, stupor and coma
occur usually when metabolic acidosis is the
dominant acid-base abnormality (Proudfoot &
Brown, 1969; Gabow et al. 1978; Anderson, 1981).
These features are thought to be due to reduced
ionisation of salicylic acid and a shift of
salicylate from plasma into the brain. Dominant
metabolic acidosis is common in young children
who are therefore more likely to experience
serious intoxication at relatively low plasma
salicylate concentrations (Proudfoot, 1983).
Very high doses of salicylate have a depressant
effect on the medulla and may cause central
respiratory paralysis as well as sudden
circulatory collapse secondary to vasomotor
depression.
9.4.3.2 Peripheral nervous system
No data available.
9.4.3.3 Autonomic nervous system
No data available.
9.4.3.4 Skeletal and smooth muscle
No data available.
9.4.4 Gastrointestinal
The ingestion of salicylate may result in epigastric
discomfort, nausea and vomiting. It may also cause
gastric ulceration. Perforated peptic ulcer occurs
extremely rarely (Robins et al. 1985; Christensen &
Schmidt, 1987).
9.4.5 Hepatic
Hepatotoxicity may occur both after therapeutic use of
salicylate or following salicylate overdose (Wolfe et
al. 1974). Liver biopsy in such cases reveals acute
hepatocellular necrosis with periportal inflammation and
fatty changes in hepatocytes.
9.4.6 Urinary
9.4.6.1 Renal
Oliguria is sometimes seen; the most common
cause is dehydration (Temple et al. 1976) but
renal failure may rarely occur in individuals
without pre-existing renal disease, systemic
disease, or volume depletion (Rupp et al. 1983).
9.4.6.2 Other
No data available.
9.4.7 Endocrine and reproductive systems
Influence on adrenal medulla:
High doses of salicylate cause release of adrenaline
from the adrenal medulla; this is thought to be partly
responsible for the observed hyperglycaemia due to
glycogenolysis that sometimes occurs.
Influence on the adrenal cortex:
Large doses of salicylate stimulate corticosteroid
secretion by the adrenal cortex.
9.4.8 Dermatological
Thirteen cases of toxic epidermal necrolysis have been
reported associated with the use of either
acetylsalicylic acid or methylsalicylate (Lowney et al.
1967).
9.4.9 Eye, ear, nose, throat: local effects
Ear: tinnitus and hearing loss caused by salicylate in
overdose are due to increased labyrinthine pressure
(Waltner, 1955) and/or an effect on the hair cells of
the cochlea. There is a relation between the hearing
loss and the plasma salicylate concentration (Myers et
al. 1965).
Eye: transient myopia occurred in a patient following
ingestion of 2.7 g acetylsalicylic acid (Sandford-Smith,
1974). Bilateral subconjunctival haemorrhages have been
described (Black & Bensinger, 1982).
9.4.10 Haematological
Ingestion of salicylic acid by normal individuals
causes prolongation of the bleeding time due to
inhibition of collagen glucosyltransferase present in
membranes of platelets. As a result, the adherence of
platelets to connective tissue or collagen fibres is
diminished.
Salicylate in large therapeutic doses (over 6 g per
day) and in overdose reduces the concentration of
vitamin K-dependent coagulation factors and in
particular that of prothrombin.
9.4.11 Immunological
In vitro, acetylsalicylic acid is an efficient
suppressor of the lymphocyte-transformation reaction.
An association with the mechanism by which it exerts
its anti-inflammatory, anti-rheumatic and anti-pyretic
effect could be postulated (Teraski et al., 1973;
Twomey et al., 1973). Similar results were observed in
rats (Loveday et al., 1973). In 19 normal volunteers,
marked and statistically significant suppression of
blastogenesis has been reported (Crout et al., 1975).
9.4.12 Metabolic
9.4.12.1 Acid-base disturbances
In adults there is an initial respiratory
alkalosis which is compensated for by
excretion of bicarbonate in urine. In infants
and children the respiratory alkalosis does
not occur and metabolic acidosis develops
(Proudfoot & Brown, 1969; Winters et al.,
1958). In severe salicylate poisoning in
adults, metabolic acidosis may also result
from a number of factors. Even when present
in high concentrations, salicylate will not
displace more than 2-3 mmol of bicarbonate;
the acidosis is not therefore due to the
presence of salicylic acid itself. The
principal cause is competitive inhibition of
NAD+-dependent dehydrogenases, including
lactate and oxoglutarate dehydrogenases and of
other oxidative enzymes such as succinate
dehydrogenase (Grisolia et al., 1969; Hines &
Smith, 1964; Koplan et al., 1954).
Consequent impairment of the oxidation of fuel
substrates leads to the accumulation of acid
intermediates, notably lactate and pyruvate.
Acidaemia caused by the effect of salicylate
on carbohydrate metabolism is compounded by
effects on lipid and amino-acid metabolism.
Salicylate enhances entry and oxidation of
fatty acids in liver cells, leading to
increased ketogenesis. Competitive inhibition
of amino-acyl-tRNA synthetases in pairs and
amino-acid incorporation (Smith & Dawkins,
1971); amino-acidaemia results.
Finally, dehydration and vasomotor depression
results in poor renal perfusion and
accumulation of sulphuric and phosphoric acids
(Tenney & Miller, 1955; Winters et al., 1959).
9.4.12.2 Fluid and electrolyte disturbances
Decreased renal tubular reabsorption of
bicarbonate occurs as a result of respiratory
alkalosis. Increased renal secretion of sodium,
potassium and water accompanies loss of
bicarbonate in the urine. Fluid loss also
results from vomiting, sweating and
hyperventilation; dehydration is commonly
associated with hypernatremia.
Water losses may be considerable: from 2 - 3
l/m2 surface area in moderate severe poisoning,
up to 6 l/m2 in severely poisoned patients
(Temple, 1978).
9.4.12.3 Others
Influence on oxidative phosphorylation.
The uncoupling of oxidative phosphorylation by
salicylate results in the inhibition of a
number of ATP-dependent reactions and:
an increase in O2 uptake and CO2
production;
depletion of hepatic glycogen; with
the energy normally used for the
conversion of inorganic phosphate to ATP being
dissipated as heat, hence the pyretic effect
of toxic doses of salicylate.
Influence on carbohydrate metabolism.
Multiple factors appear to be involved. Hyper-
or hypoglycaemia may result from the
mechanisms listed above at A. (see 9.4.,
12.1).
Influence on nitrogen metabolism.
Salicylate in toxic doses causes a significant
negative nitrogen balance, characterized by
amino-aciduria, though this is due in part to
inhibition of active tubular absorption
because of reduced ATP formation.
Influence on fat metabolism.
Increased entry and enhanced oxidation of
fatty acids in muscle, liver and other tissues
occur, together with a lowering of
concentrations of plasma free fatty acids,
phospholipid and cholesterol (Woodbury & Fingl,
1975).
9.4.13 Allergic reactions
Some persons, particularly asthmatics, exhibit marked
sensitivity to acetylsalicylic acid which provokes
various reactions including urticaria and other skin
eruptions, angioneurotic oedema, rhinitis and severe,
even fatal, paroxysmal bronchospasm and dyspnoea,
hypotension, shock and syncope (Reynolds, 1989).
9.4.14 Other clinical effects
The relationship between the use of acetylsalicylic
acid and Reye's syndrome (Reye et al., 1963) remains
controversial. Reye's syndrome is a disease with a high
mortality characterized by encephalopathy and fatty
degeneration of the viscera, especially the liver.
Similar findings were noted (Starko & Mullick, 1983) in
the histology and necropsy records of 13 children with
accidental or iatrogenic salicylate intoxication.
The clinical features most often observed are severe
vomiting followed by increasing drowsiness and coma.
Transaminase activities and ammonia levels in the blood
become elevated but jaundice does not occur. In severe
cases, hypoglycaemia and hypoprothrombinaemia develop.
Reye's syndrome may be seen in children and adolescents,
the peak incidence being between 5-15 years. Often
varicella or influenza precede the development of the
syndrome (Sullivan-Bolyai & Corey, 1981), but some
cases reported have been in children with chronic
inflammatory disorders such as juvenile rheumatoid
arthritis or lupus erythematosis (Young et al., 1984;
Hansen et al., 1985).
A possible pointer to a causal relationship between
acetylsalicylic acid use and Reye's syndrome is the
declining incidence in the USA that has occurred
following a reduction in use of acetylsalicylic acid
during the period 1980-1987 (Remington et al., 1986;
Banco, 1987); for this reason, the use of the drug in
young children in the UK is now restricted (Notes and
News, 1986).
9.4.15 Special risks
Salicylate intoxication may occur through placental
transfer (Ahlfors et al., 1982; Lynd et al., 1976) and
breast milk (Clarke & Wilson, 1981).
9.5 Other
No data available.
9.6 Summary
10. MANAGEMENT
10.1 General principles
Treatment of acute salicylate poisoning is directed
primarily towards prevention of absorption, correction of
acid-base and fluid and electrolyte balance; and in patients
with features of moderate or severe intoxication, towards
enhancing elimination of the drug. Respiratory alkalosis
needs no specific treatment, but severe acidosis requires at
least partial correction with bicarbonate. Bicarbonate
should be administered carefully because hypokalaemia may be
aggravated and, if large quantities are administered, the
sodium and water load may precipitate pulmonary oedema.
Sedatives and respiratory depressant drugs must be avoided.
Tetany may be corrected with the use of calcium gluconate
(10 ml of a 10% solution, intravenously) (Merdith & Vale,
1986).
If it is thought that an enteric-coated or other slow-
release salicylic acid preparation has been taken, then the
patient must be kept under observation for at least twenty-
four hours; abdominal ultrasonoscopy may help to identify
concentrations or retained enteric-coated tablets.
10.2 Relevant laboratory analyses
10.2.1 Sample collection
See section 8.
10.2.2 Biomedical analysis
Hypokalaemia is usual. Hypoglycaemia can be a feature
of salicylate intoxication, it is more common in
children than in adults and is often severe.
Hyperglycaemia may also occur and persist for several
days (Cotton & Fahlberg, 1964; Mortimer & Lepow,
1962).
10.2.3 Toxicological analysis
See section 8.
10.2.4 Other investigations
No data available.
10.3 Life supportive procedures and symptomatic/specific
treatment
If non-cardiogenic pulmonary oedema is present, mechanical
ventilation with positive end-expiratory pressure (PEEP) may
be indicated
Fluid and electrolyte replacement is important with special
attention being paid to potassium depletion;
ECG monitoring may be indicated
Correction of metabolic acidosis by sodium bicarbonate
intravenously
Sponging for hyperpyrexia.
10.4 Decontamination
In those cases where vomiting has not already occurred
further absorption of the drug may be prevented by inducing
emesis and/or undertaking gastric lavage, as appropriate.
Acetylsalicylic acid is poorly soluble in an acid
environment and may coalesce to form a mass or coating in
the stomach, from which absorption may continue slowly over
many hours. Thus gastric lavage may be indicated more than
12 hours following ingestion of the overdose.
Although activated charcoal proved to be equally effective
as emesis and gastric lavage in volunteers (Danel & Henry,
1988), it may only be exploited therapeutically if the
patient presents soon after the overdose and is not vomiting
- which is unlikely in those who are at least moderately
severely poisoned. Moreover substantial quantities of
activated charcoal (50-100 g) need to be administered if
significant absorption is to be prevented. Repeated doses
of activated charcoal (50-75 g immediately and 50 g 4-
hourly) will increase the non-renal elimination of
salicylate and will greatly diminish the plasma half-life
(Hillman & Prescott, 1985; Boldy & Vale, 1986).
The administration of activated charcoal may be of
particular value in those adults who have ingested
substantial quantities of an enteric-coated or sustained-
release preparation of acetylsalicylic acid, and it has been
employed in repeated doses to increase the non-renal
elimination of acetylsalicylic acid (Meredith & Vale, 1986).
The addition of sodium sulphate (as a saline cathartic
agent) to activated charcoal was not found to have any
additional effect on the prevention of acetylsalicylic acid
absorption in six healthy volunteers (Sketris et al. 1982).
10.5 Elimination
Whilst (forced) alkaline diuresis has been employed in the
management of salicylate poisoning for the last two decades
(Berg, 1977; Cumming et al. 1964; Dukes et al., 1963; Lawson
et al., 1969) fluid retention may occur during forced
diuresis, and increase the risk of pulmonary oedema in
severely intoxicated patients (Proudfoot & Brown, 1969;
Heffner & Sahn, 1981). It is now recognized that the urine
pH is of far greater importance than the volume of urine
excreted (Meredith & Vale, 1981; Prescott et al., 1982). To
achieve maximum excretion of salicylate a urine pH of 7.5 or
higher is indicated (careful monitoring of urine pH is
necessary).
Urinary alkalinization requires close supervision in an
intensive care area. It is sometimes difficult to
alkalinize the urine so that maximum excretion is achieved
without creating a potentially dangerous alkalaemia (Done,
1978), but in these cases adequate potassium repletion will
normally allow urinary alkalinization. In patients with
cardiac and/or renal impairment, and in those who are in
shock, dialysis should be considered (Meredith & Vale,
1986).
Haemodialysis should be considered in severely poisoned
patients with features of central nervous system toxicity,
pulmonary oedema, renal failure, cerebral oedema and in
cases with plasma levels higher than 800 mg/l, haemodialysis
should be considered.
Haemodialysis is preferred to haemoperfusion because it more
rapidly corrects acid-base and electrolyte abnormalities and
may avoid the need for the administration of large amounts
of sodium bicarbonate (Winchester et al. 1981).
Peritoneal dialysis is less effective than alkaline diuresis,
is 2 - 3 times less effective than haemodialysis, and its
use is not recommended (Winchester et al. 1977). Similarly,
charcoal haemoperfusion is less effective than haemodialysis
and again its use is not advocated (Meredith & Vale, 1986).
10.6 Antidote treatment
10.6.1 Adults
There is no specific antidote.
10.6.2 Children
There is no specific antidote.
10.7 Management discussion
Alkalinization of the urine is considerably more effective
in promoting salicylate excretion than induced diuresis and
the need for the latter requires reappraisal (Prescott et
al. 1982).
The use of gastroscopic and other measures to break up
enteric-coated tablets requires further evaluation.
11. ILLUSTRATIVE CASES
11.1 Case reports from literature
11.2 Internally extracted data on cases
To be added by the PC.
11.3 Internal cases
To be added by the PC using the monograph.
12. Additional information
12.1 Availability of antidotes
No antidotes available.
12.2 Specific preventive measures
Flavoured tablets are attractive to children and, to avoid
temptation, the use of this type of medication should be
avoided.
The use of child resistant closures, smaller pack sizes and
the elimination of attractive coloured products will help to
reduce the problem of salicylate poisoning.
Parents need to be warned of the potential risks of chronic
administration of salicylates.
Physicians should be made aware that chronic salicylate
poisoning in hospitalized patients occurs almost as
frequently as acute poisoning, and may result in more severe
intoxication.
12.3 Other
No data available.
13. REFERENCES
Ahlfors CE, Shwer ML, Ford KB, (1982). Bilirubin-albumin binding
in neonatal salicylate intoxication. Developmental Pharmacol Ther,
4:47-60.
Alvan G, Bergman U, Gustafsson LL, (1981). High unbound fraction
of salicylate in plasma during intoxication. Br J Clin Pharmacol,
11:625-6.
Anderson RJ, Potts DE, Gabow PA, Rumack BH, Schrier RW, (1976).
Unrecognised adult salicylate intoxication. Ann Intern Med,
85:745-8.
Anderson RJ, (1981). Asterixis as manifestation of salicylate
toxicity. Ann Intern Med, 95:188-9.
Banco L. Use of aspirin and Reye's syndrome, (1987). Am J Dis
Child, 141:240-1.
Bell RG, (1978). Metabolism of vitamin K and prothrombin
synthesis: anticoagulants and the vitamin K-epoxide cycle. Fed
Proc, 37: 2599.
Benowitz NL, Rosenberg J, Becker CE, (1979). Cardiopulmonary
catastrophes in drug overdose patients. Med Clin North Am, 63:267-
95.
Berg KJ, (1977). Acute acetylsalicylic acid poisoning. Treatment
with forced alkaline diuresis and diuretics. Eur J Clin Pharmacol,
12:111-6.
Berk WA, Andersen JC, (1989). Salicylate-associated asystole:
report of two cases. Am J Med, 86:505-6.
Beveridge GW, Forshall W, Munro JF, Owen JA, Weston IG, (1964).
Acute salicylate poisoning in adults. Lancet, 1:1406-9.
Black RA, Bensinger RE, (1982). Bilateral subconjunctival
hemorrhage after acetylsalicylic acid overdose. Ann Opthalmol,
14:1024-5.
Boldy DAR, Vale JA, (1986). Treatment of salicylate poisoning
with repeated oral charcoal. Br Med J, 292:136.
Borga O, Odar-Cederlof I, Ringberger VA, Norlin A, (1976).
Protein binding of salicylate in uremic and normal plasma. Clin
Pharm Ther, 20:464-75.
Brantmark B, Hedner U, Melander A et al, (1981). Salicylate
inhibition of antiplatelet effect of aspirin. Lancet, 2:1349.
Christensen LA, Schmidt EB, (1987). Perforated peptic ulcer. A
complication in acute salicylate intoxication. Acta Med Scand,
222:191-2.
Clarke JH, Wilson WG, (1981). A 16-day-old breast-fed infant with
metabolic acidosis caused by salicylate. Clin Pediatr, 20:53-4.
Cotton EK, Fahlberg VI, (1964). Hypoglycaemia with salicylate
poisoning. A report of two cases. Am J Dis Children, 108:171-3.
Cumming G, Dukes DC, Widdowson G, (1964). Alkaline diuresis in
treatment of aspirin poisoning. Br Med J, 2:1033-6.
Danel V, Henry JA, (1988). Activated charcoal, emesis, and
gastric lavage in aspirin overdose. Br Med J, 296:1507.
Davies MG, Vella Briffa D, Greaves MW, (1979). Systemic toxicity
from topically applied salicylic acid. Br Med J, 1:661.
De Groen PC, (1989). Chronische intoxicatie door salicylzuur
verbindingen: vaak niet herkend of verkeerd behandeld. Ned
Tijdschr Geneeskd, 133:1481-5.
Done AK, (1960). Salicylate intoxication. Significance of
measurements of salicylate in blood in cases of acute ingestion.
Pediatrics, 26:800-7.
Done AK, (1978). Aspirin overdosage: incidence, diagnosis, and
management. Pediatrics, 62 (suppl):890-7.
Dove DJ, Jones T, (1982). Delayed coma associated with salicylate
intoxication. J Pediatr, 100:493-6.
Dukes DC, Blainey JD, Cumming G, Widdowson G, (1963). The
treatment of severe aspirin poisoning. Lancet, 1:329-31.
Editorial, (1964). Percutaneous salicylic acid intoxication. JAMA,
190:1064.
Ferguson RK, Boutros AR, (1970). Death following self-poisoning
with aspirin. JAMA, 213:1186-7.
Gabow PA, Anderson RJ, Potts DE, Schrier RW, (1978). Acid-base
disturbances in the salicylate-intoxicated adult. Arch Intern Med,
138:1481-4.
Gaudrealt P, Temple AR, Lovejoy FH, (1982). The relative severity
of acute versus chronic salicylate poisoning in children. A
clinical comparison. Pediatrics, 70:566-9.
Grisolia S, Mendelson J, Diederich D, (1969). Inactivation of
enzymes by therapeutic concentrations of aspirin and salicylate.
Nature, 223:79.
Hansen JR, McCray PB, Bale JF et al, (1985). Reye syndrome
associated with aspirin therapy for systemic lupus erythematosus.
Pediatrics, 76:202-5.
Hartwig-Otto H, (1983). Pharmacokinetic considerations of common
analgesics and antipyretics. Am J Med, 75 (suppl):30-7.
Heffner JE, Sahn SA, (1981). Salicylate-induced pulmonary edema.
Ann Intern Med, 95:405-9.
Hillman RJ, Prescott LF, (1985). Treatment of salicylate
poisoning with repeated oral charcoal. Br Med J, 291:1472.
Hines WJW, Smith MJH, (1964). Inhibition of dehydrogenases by
salicylate, Nature, 201:192.
Howrie DL, Moriarty R, Breit R, (1985). Candy flavouring as a
source of salicylate poisoning. Pediatrics, 75:869.
Informatorium Medicamentorum, (1986). Ed Kon Mij Pharmacie's,
Gravenhage.
Johnson PN, Welch DW, (1984). Methylsalicylate/aspirin
(salicylate) equivalence: Who do you trust? Vet Human Toxicol,
26:317-8.
Kaplan EH, Kennedy J, Davies J, (1954). Effects of salicylate and
other benzoates on oxidative enzymes of the tricarboxylic acid
cycle in rat tissue homogenates. Arch Biochem Biophys, 51:47
Kaufman FL, Dubanksy AS, (1972). Darvon poisoning with delayed
salicylism: a case report. Pediatrics, 49:610-1.
Lawson AAH, Proudfoot AT, Brown SS et al, (1969). Forced diuresis
in the treatment of acute salicylate poisoning in adults. Quart J
Med, 38:31-48.
Levy G, Tsuchiya T, (1972). Salicylate accumulation kinetics in
man. N Engl J Med, 287:430-2.
Levy G, (1978). Clinical pharmacokinetics of aspirin. Pediatrics,
62 (suppl):867-72.
Liebman RM, Katz HM, (1981). Pulmonary edema in a 52-year-old
woman ingesting large amounts of aspirin. JAMA, 246:2227-8.
Lowney ED, Baublis JV, Kreye GM, Harrell ER, McKenzie AR, (1967).
The scalded skin syndrome in small children. Arch Dermatol,
95:359-69.
Lynd PA, Andreasen AC, Wyatt RJ, (1976). Intrauterine salicylate
intoxication in a newborn. Clin Pediatrics, 15:912-3.
McGuigan MA, (1987). A two-year review of salicylate deaths in
Ontario. Arch Intern Med, 147:510-2.
Meredith TJ, Vale JA, (1981). Salicylate poisoning. Poisoning:
Diagnosis and Treatment, Vale and Meredith Eds, (Update Books,
London): 97-103.
Meredith TJ, Vale JA, (1986). Non-narcotic analgesics. Drugs, 32
(suppl 4).
Mortimer EA, Lepow ML, (1962). Varicella with hypoglycaemia
possibly due to salicylates. Am J Dis Child, 103:583-90.
Myers EN, Bernstein JM, Fostiropolous G, (1965). Salicylate
Tototoxicity: a clinical study. N Engl J Med, 273:587-90.
Notes and News, (1986). Reye's syndrome and the giving of aspirin
to children. Lancet, 1:1396.
Paynter AS, Alexander FW, (1979). Salicylate intoxication caused
by teething ointment. Lancet, 2:1132.
Pei YP, Thomson DA, (1987). Severe salicylate intoxication
mimicking septic shock (letter). Am J Med, 82:581-2.
Pluskwa F, Couturier M, Kirsh JM, Fourgeaux B, Campinos L, Duval
G, (1984). Intoxication, salicyl_e, grave par vou transcutan_e
chez un br_l_. La Presse M_d, 13:1391.
Prescott LF, Balali-Mood M, Critchley JAJH, Johnstone AF,
Proudfoot AT, (1982). Diuresis or urinary alkalinisation for
salicylate poisoning. Br Med J, 285:1383-6.
Proudfoot AT, Brown SS, (1969). Acidaemia and salicylate
poisoning in adults. Br Med J, 2:547-50.
Proudfoot AT, (1983). Toxicity of salicylates. Am J Med, 14:99-
103.
Remington PL, Rowley D, McGee H. et al, (1986). Decreasing trends
in Reye syndrome and aspirin use in Michigan, 1979-1984.
Pediatrics, 77:93-8.
Reynolds EF (ed) (1982). Aspirin and similar analgesic and anti-
inflammatory agents. Martindale, The Extra Pharmacopoeia 28 Ed,
234-82.
Reye RDK, Morgan G, Baral J, (1963). Encephalopathy and fatty
degeneration of the viscera. A disease entity in childhood.
Lancet, 11:749-53.
Robins JB, Turnbull JA, Robertson C, (1985). Gastric perforation
after acute aspirin overdose. Hum Toxicol, 4:527-8.
Rupp DJ, Seaton RD, Wiegmann TB, (1983). Acute polyuric renal
failure after aspirin intoxication. Arch Int Med, 143:1237-8.
Sandford-Smith JH, (1974). Transient myopia occured in a patient
following the ingestion of 2.7 g of aspirin. Br J Ophthal,
58:698.
Sketris IS, Mowry JB, Czajka PA, Anderson WH, Stafford DT,
(1982). Saline catharsis: effect on aspirin bioavailability in
combination with activated charcoal. J Clin Pharmacol, 22:59-64.
Smith MJH, Dawkins, PD, (1971). Salicylate and enzymes. J Pharm
Pharmacol, 23:729.
Springer DJ, Groll A, (1980). Poisoning with enteric coated
acetylsalicylic acid complicating gastric outlet obstruction. Can
Med Assoc J, 122:1032-3.
Starko KM, Mullick FG, (1983). Hepatic and cerebral pathology
findings in children with fatal salicylate intoxication: futher
evidence for a causal relation between salicylate and Reye's
Syndrome. Lancet, 1:326-9.
Sullivan-Bolyai JZ, Corey L, (1981). Epidemiology of Reye
syndrome. Epidemiologic Rev, 3:1-26.
Taylor JR, Halprin KM, (1975). Percutaneous absorption of
salicylic acid. Arch Dermatol, 111:740-3.
Temple AR, (1978). Pathophysiology of aspirin overdosage toxicity,
with implications for management. Pediatrics, 62 (suppl):873-6.
Temple AR, (1981). Acute and chronic effects of aspirin toxicity
and their treatment. Arch Intern Med, 141:364-9.
Temple AR, George DJ, Done AK, Thompson JA, (1976). Salicylate
poisoning complicated by fluid retention. Clin Toxicol, 9:61-8.
Tenney SM, Miller RM, (1955). The respiratory and circulatory
actions of salicylate. Am J Med, 19:495-508.
Thurston JH, Pollock PG, Warren SK, Jones EM, (1970). Reduced
brain glucose with normal plasma glucose in salicylate poisoning.
J Clin Invest, 49:2139.
Todd PJ, Sills JA, Harris F, Cowen JM, (1981). Problems with
overdose of sustained-release aspirin. Lancet, 1:777.
Treguerer H, Le Bihan G, Coloignier M, Le Roux P, Bernard JP,
(1980). Intoxication salicyl_e par application locale de
vaseline salicyl_e a 20% chez un psoriasique. Nouv Presse Med,
9:192-3.
Vale JA, Buckley GM, Meredith TJ, (1985). Algorithm for modified
alkaline diuresis in salicylate poisoning. Br Med J, 290:155.
Walters JS, Woodring JH, Stelling CB, Rosenbaum HD, (1983).
Salicylate-induced pulmonary edema. Radiology, 146:289-93.
Waltner JG, (1955). The effect of salicylates on the inner ear.
Ann Otol Rhino Lar, 64:617-22.
Winchester JF, Gelfland MC, Knepshield JH, Schreiner GE, (1977).
Dialysis and hemoperfusion of poisons and drugs. Transactions of
the American Society of Artificial and Internal Organs, 23:762-
842.
Winchester JF, Gelfland MC, Helliwell M, Vale JA, Goulding R,
Schreiner GE, (1981). Extracorporeal treatment of salicylate or
acetaminophen poisoning. Is there a role? Arch Intern Med,
141:370-4.
Winters RW, White JS, Ordway NK, (1959). Disturbances of acid-
base equilibrium in salicylate intoxication. Pediatrics, 23:260-
85.
Wolfe JD, Metger AL, Goldstein RC, (1974). Aspirin hepatitis. Ann
Intern Med, 80:74-6.
Woodbury DM, Fingl E, (1975). Analgesic-antipyretics, anti-
inflammatory agents, and drugs employed in the therapy of gout.
In: The pharmacological basis of therapeutics, 325-43. Eds
Goodman and Gilman.
Wortzmann DJ, Grunfeld D, (1987). Delayed absorption following
enteric-coated aspirin overdose. Ann Emerg Med, 16:434.
Young RSK, Torretti D, Williams RH et al, (1984). Reye's syndrome
associated with long-term aspirin therapy. JAMA, 251:754-6.
14. AUTHOR(S), REVIEWER(S), DATE(S) (INCLUDING UPDATES), COMPLETE
ADDRESS(ES)
Authors: Professor A.N.P. van Heijst
Baarnseweg 42a
3735 MJ Bosch en Duin
Netherlands
Tel: 31-30-287178
Dr A. van Dijk
Central Hospital Pharmacy
University Hospital Utrecht
P.O. Box 85500
3508 GA Utrecht
Netherlands
Tel: 31-30-507190
Fax: 31-30-516756
Date: 22 June 1990
Reviewer: Dr T. Meredith
Department of Health
Hannibal House Room 913
Elephant & Castle
London SE1 6TE
United Kingdom
Tel: 44-71-9722449
Fax: 44-71-7039565
Date: 12 January 1991
Peer Review: Newcastle-upon-Tyne, United Kingdom, January 1991


