
FLUMETHRIN
First draft prepared by
D.B. McGregor,
International Agency for Research on Cancer, Lyon, France
Explanation
Evaluation for acceptable daily intake
Biochemical aspects
Absorption, distribution, and excretion
Biotransformation
Effects on enzymes
Toxicological studies
Acute toxicity
Short-term toxicity
Long-term toxicity and carcinogenicity
Reproductive toxicity
Developmental toxicity
Genotoxicity
Special studies
Dermal and ocular irritation and dermal sensitization
Neurotoxicity
Anti-allergic and pseudi-allergic activity
Bronchial activity
Effect on concentration of glucose and triglycerides in blood
Effects on gastrointestinal tract of rats
Haematological and cardiovascular effects
Diuretic effects
Toxicity of metabolites: Flumethrin acid
Observations in humans
Comments
Toxicological evaluation
References
Explanation
Flumethrin is an alpha-cyano-3-phenoxyphenyl pyrethroid
insecticide used in the control of ectoparasites on cattle, sheep,
goats, horses, and dogs. It is formulated as a 6% solution for use as
a spray or dip and as a 1% solution for the pour-on treatment of
cattle. In addition, flumethrin is marketed as strips for the
diagnosis and control of varroatosis in bee hives. Flumethrin as
currently produced and used is the result of optimization of the
manufacturing process and consists of > 90% trans-Z-1 and trans-Z-2
isomers (with < 2% cis-Z and < 1% trans-E isomers as by-products). The
development of flumethrin first led to a substance that was a mixture
of 30-45% trans Z-1 and trans Z-2 isomers and 45-63% trans E-1 and trans
E-2 isomers, the corresponding cis isomers occurring as by-products at
< 6%. This material was used in a long-term study of toxicity and
carcinogenicity in rats and is referred to as flumethrin with a low
trans-Z content.
Flumethrin was evaluated for the first time by the present
Meeting.
Evaluation for acceptable daily intake
1. Biochemical aspects
(a) Absorption, distribution, and excretion
After a single oral dose of [F-phenyl-U-14C]-flumethrin was
given to rats, about 30% of the dose was absorbed (Steinke et al.,
1983).
The kinetics and metabolism of flumethrin were investigated in
Wistar BOR: WISW rats with [Cl-phenyl-U-14C]-labelled compound
administered in physiological saline solution containing 5% Cremophor
EL (Klein, 1993a). The dosing regimes were as follows: 1 mg/kg bw
administered orally as a single dose to male and female rats, 5 mg/kg
bw administered orally as a single dose to male rats, 1 mg/kg bw
administered orally to male rats on seven consecutive days, or 1 mg/kg
bw administered intraduodenally to bile-fistulated male rats.
Absorption was rapid but incomplete. In rats with a fistulated bile
duct, about 75% of the dose was absorbed and 77-88% was eliminated in
the faeces, mostly after absorption and excretion in the bile; only
about 2% was excreted in the urine. The difference in absorption found
in these studies appears to be dependent upon the position of the
label and may be due to ester hydrolysis in the stomach. The highest
concentrations were found in the plasma. The time for a rise in the
plasma concentration from 25 to 75% of the maximal value varied
between 2 and 3.5 h, the maximum being achieved in about 8 h. The
elimination half-lives were 130-160 h, demonstrating the slow release
of radiolabel from the plasma, also reflected in the low clearance
values (< 12 ml/kg bw per h) and very low renal clearance
(< 1.2 ml/kg bw per h). The concentrations of radiolabel found in
the organs examined 48 h after dosing were 3- to 50-fold lower than in
plasma. Particularly low concentrations were found in spleen, fat,
brain, and bone. The distribution volume under steady-state conditions
was 25-44% of the body volume, indicating either slow or limited
distribution to peripheral compartments from plasma (considered as the
central compartment). Redistribution into plasma before biliary
excretion was also slow, as indicated by the relatively large mean
residence times (190-235 h). Radiolabel accumulated in plasma after
multiple dosing, so that the relative plasma concentration had
increased almost 10-fold after seven days. Once dosing had stopped,
the plasma concentration of radiolabel fell very slowly, the
elimination half-life being about 155 h; seven days after
administration, 9-20% of the dose was still present in the body
(excluding the gastrointestinal tract).
The distribution of [Cl-phenyl-U-14C]-flumethrin and its
metabolites were investigated by whole-body autoradiography in rats
for 1-48 h after a single oral dose of 5 mg/kg bw. The distribution
pattern was established within 1 h after dosing, the concentrations
decreasing only slowly thereafter. The highest concentration was found
in the liver, but high concentrations were also found in the spleen,
kidney, lung, adrenal cortex, cartilage, bone marrow, pineal gland,
pituitary, and subcutaneous adipose tissue; the lowest concentrations
were found in the central nervous system (Klein, 1993b).
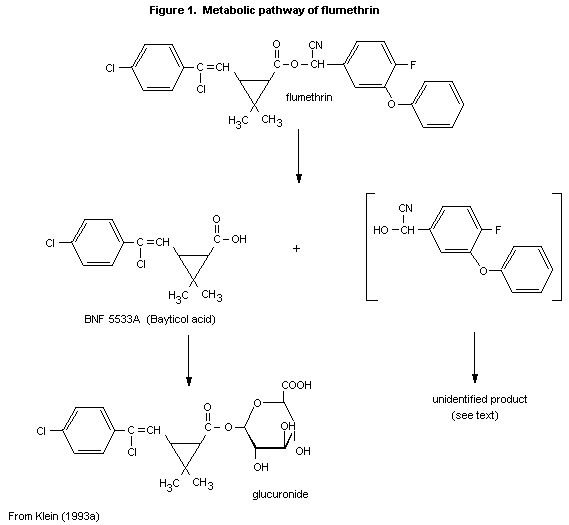
(b) Biotransformation
In the experiment of Klein (1993a), the main radioactive
compounds found in the faeces were unchanged flumethrin (which
accounted for about 50% of the radiolabel recovered from male rats and
about 25% of that from female rats) and the metabolite, 3-[2-chloro-
2-(4-chlorophenyl)ethenyl]-2,2-dimethylcyclopropanecarboxylic acid,
flumethrin acid (BNF 5533A), which accounted for 15-18% of the
radiolabel recovered from male rats and about 30% of that from female
rats. No other biotransformation products were found in the faeces.
With [fluorophenyl-U-14C]-flumethrin (low trans Z) given orally to
rats, two primary metabolites were identified in the urine (Ecker,
1983). These were 3-(4'-hydroxy-phenoxy)-4-fluorobenzoic acid and
3-phenoxy-4-fluorobenzoic acid, which accounted for 50 and 35%,
respectively, of the urinary radiolabel over 0-24 h and 80 and 10% of
the urinary activity over 24-48 h. Glycine conjugates of the primary
metabolites were also identified, but each accounted for no more than
4 and 7.4%, respectively, of the urinary radiolabel. In the metabolism
of other compounds containing the alpha-cyano-3-phenoxybenzyl moiety
(e.g. fenvalerate, Kaneko et al., 1981), the phenyl ring may be
hydroxylated and, following ester bond hydrolysis, the cyano group is
converted to SCN- and carbon dioxide and 3-phenoxybenzaldehyde is
oxidized to the carboxylic acid. The resultant acids and phenols can
then conjugate with glucuronic acid, sulfate, and/or amino acids. The
metabolic pathway of flumethrin is shown in Figure 1.
Kinetics and tissue residues were investigated in a 530-kg
lactating cow after application of [F-phenyl-U-14C]-flumethrin at
1.77 mg/kg bw to the skin of the back. The plasma concentration of
total radiolabel rose to a maximum of 6.3 ng/ml 23 h after application.
The highest concentration in the milk (3 ng/ml) was found 31 h after
application. By 48 h after dosing, when the cow was slaughtered, the
flumethrin-equivalent concentrations of radiolabel were 70 ng/ml in
bile in the gall-bladder and 281 ng/ml in urine in the urinary bladder.
Of the tissues examined, the liver (9 ng/g tissue) and kidneys (10 ng/g
tissue) contained the highest concentrations of residues; 71.7% of the
applied dose remained at the application site (Cameron, 1986).
 In a study with [Cl-phenyl-U-14C]-flumethrin, a dose of
1 mg/kg bw was administered intravenously to one 545-kg dairy cow
and one 340-kg steer. Both animals were slaughtered 8 h after
administration, and the quantities of radiolabel present in all
excreta and in liver, kidneys, muscle, fat, and milk were measured.
The highest concentrations were found in the liver (cow, 13 µg/g;
steer, 3.5 µg/g), followed by the kidney (cow, 0.88 µg/g; steer,
1.4 µg/g). The metabolite flumethrin acid was also found in all the
materials examined, except the milk, and was present as the glucuronide
in the liver and kidneys. In milk, an additional degradation product
was found but not identified, which constituted 11.5% of the total
residues in milk (Klein, 1995).
(c) Effects on enzymes
Pyrethroids can interact with liver drug-metabolizing enzymes,
but there appears to be a difference between some Type II pyrethroids
that contain an alpha-cyano function and inhibit such enzymes, e.g.
deltamethrin (Anadón et al., 1990), and the Type I pyrethroids that
do not contain this function and may induce drug-metabolizing enzymes,
e.g. permethrin (Carlson & Shoening, 1980; Anadón et al., 1988). In
experiments in which groups of 12 male Wistar rats received flumethrin
by intraperitoneal injection for six days, the Type II pyrethroid
response was demonstrated, in that there were reductions in cytochrome
P450 protein content (36%), NADPH-cytochrome c reductase activity
(38%), aniline hydroxylase activity (52%), aminopyrine N-demethylase
activity (54%), and UDP-glucuronosyl transferase activity (34%)
(Anadón et al., 1995).
2. Toxicological studies
(a) Acute toxicity
The results of studies of the acute toxicity of flumethrin are
listed in Table 1. The acute toxicity is slightly greater in female
than male rats and depends on the vehicle used. The sex difference
may reflect the greater metabolic conversion in female rats,
and differences between the solvents in respect of polarity,
which determines the proportion of the dose adsorbed from the
gastrointestinal tract, may explain the differences in oral toxicity.
The formulation containing Cremophor EL, which is known to enhance
absorption, was markedly more toxic; however, this formulation is used
only for administration in toxicological studies. The acute toxicity
of flumethrin in the other vehicles was moderate to low.
Table 1. Acute toxicity of flumethrin and products
Formulation Route Species Sex LD50 Reference
(mg/kg bw)
Flumethrin in Oral Rat Male > 100 Bomann (1994a)
water/Cremophor EL Female > 100
Oral Rat Male 56 Bomann (1992a)
Female 41
Flumethrin in arachis oil Oral Rat Male
911 Renhof (1983a)
Female 662
Flumethrin in miglyol Oral Rat Male 3849 Renhof (1983a)
Female 2248
Flumethrin in 1:10 Oral Rat Male 302 Renhof (1983b)
acetone:arachis oil Female 138
Flumethrin in corn oil Dermal Rat Male > 2000 Bomann (1994b)
Female > 2000
1% pour-on formulation Oral Rat Male > 20 Schmidt (1984a)
Female > 20
Oral Mouse Male > 20 Schmidt (1984a)
Female > 20
Dermal Rat Male > 5 Schmidt (1984a)
Female > 5
Dermala Rat Male > 5 Schmidt (1984a)
Female > 5
Intraperitoneal Mouse Male approx. .1 Schmidt (1984b)
Female approx. 5
Bayticol EC 6% in Oral Rat Male > 500-< 2000 Bomann (1992b)
Solvesso 200 Female > 500-< 2000
Dermal Rat Male > 5000 Bomann (1992c)
Female > 5000
Table 1. (Cont'd)
Formulation Route Species Sex LD50 Reference
(mg/kg bw)
Bayvarol strips Oral Rat Male > 2000 Bomann (1992d)
(0.55 g Bayticol/100g) Female > 2000
Dermal Rat Male > 5000 Bomann (1992e)
Female > 5000
Bayticol EC 7.5% Inhalation Rat Male approx. 3000 Thyssen (1982)
(4 h) Female > 2934
a On scarified skin
After acute administration of flumethrin, the most prominent
clinical signs were manifestations of central nervous system toxicity,
such as reduced motor activity, respiratory disorders, altered
gait, and salivation. The onset of action occurred 1-15 min after
administration, and the effects were comparatively long-lasting. The
reported manifestations of toxicity are largely consistent with those
known collectively as the choreoathetosis (sinuous writhing) with
salivation syndrome, which is produced by other insecticidal
pyrethroids, classified on this basis as Type II pyrethroids, which
contain an alpha-cyano-2-phenoxybenzyl alcohol group (Vijversberg &
van den Bercken, 1982).
In the studies of acute toxicity, particular attention was paid
to effects on the central nervous system. In animals investigated for
behaviour on an inclined plane, flumethrin was administered orally
in a vehicle which enhances toxicity, i.e. as an emulsion in
water/Cremophor EL, and, for comparison, in milk, a vehicle of
relevance as regards the consumer. Doses of 5 mg/kg bw in both
formulations had a slight effect, i.e. the inclination of the plane at
which the dosed animals slipped off was lower than that at which the
control animals slipped off. A dose of 1 mg/kg bw, administered as an
emulsion in water/Cremophor, also had a very slight effect, but no
effect was seen with 0.3 mg/kg bw or with 1 mg/kg bw administered as
an emulsion in milk (Bomann, 1994c).
After dermal application, the LD50 was > 2000 mg/kg bw. The
clinical signs were comparable to those observed after oral
administration. Evidence of skin reactions, such as scaling and
incrustation and sometimes scratches, were found at the application
site (Bomann, 1994b).
Studies of flumethrin products (Table 1) did not indicate
potentiation by the other constituents of commercially available
formulations. The results obtained were within the respective ranges
calculated on the basis of the toxicity of the active ingredient.
Dermal application of a dose of 5 ml/kg bw of a pour-on formulation
had no systemic or local effects (Schmidt, 1984a), and intraperitoneal
administration of the 1% pour-on formulation to mice also showed
little toxicity (Schmidt, 1984b).
Deaths occurred after inhalation of the product Flumethrin EC
7.5% at a nominal concentration > 10 000 mg/m3 (Table 1). At
500 mg/m3, inhalation was tolerated with no adverse effects (Thyssen,
1982).
(b) Short-term toxicity
Rats
Groups of 15 male and 15 female Wistar BOR: WISW rats received
diets containing flumethrin (purity, 98.7%) to provide concentrations
of 0, 10, 50, or 250/150 ppm (150 ppm from the third week onwards) for
13 weeks. An additional 10 rats of each sex per group were killed
after four weeks. At concentrations > 50 ppm, skin lesions were
seen on the head, neck, shoulder girdle, and front extremities.
Although treatment was continued, these changes had cleared up in
about half of the animals by the end of the study. During the first
two weeks of the study, both food intake and water consumption of the
group at 250 ppm were depressed by about 40%; these effects were
accompanied by body-weight losses in animals of each sex. With the
change to a dose of 150 ppm, food and water consumption became
indistinguishable from the control values and there were no further
weight losses. Body weights remained slightly reduced throughout the
study in this group (terminal differences in comparison with controls
being about 9% in males and 8% in females). Unscheduled deaths
occurred only in animals at 250/150 ppm, four females and one male
dying during the first two weeks of treatment and another male during
week 5. The NOAEL was 10 ppm, equal to 0.7 mg/kg bw per day in males
and 0.8 mg/kg bw per day in females (Hahnemann & Rühl, 1985).
Groups of 20 male and 20 female Wistar BOR: WISW rats received
flumethrin (purity, 94.6%) in the diet at levels providing
concentrations of 0, 10, 40, or 160 ppm for 15 weeks. Dosing at 160
ppm resulted in a reduction in food intake, retardation of body-weight
development, and clinical signs, but none of the animals died. The
body weights of animals at 160 ppm were 24% lower than those of
controls for males and 8% for females, but there were no significant
differences in the other groups. The principal clinical signs in
animals at 160 ppm were piloerection, increases or decreases in motor
activity, and spastic or staggering gait. Immediately after the start
of dosing, the animals at this dose were also seen to groom their fur
intensively and in particular to make frequent scratching movements.
This produced skin lesions, some of which were several centimeters in
diameter and bled after being scratched repeatedly. A small proportion
of these skin lesions, and similar ones found on a few animals at
40 ppm, healed as the study progressed. Alpha-cyano pyrethroids are
known to have a paraesthetic effect, which is regarded as the most
probable cause of the skin lesions. Correlated with the presence of
skin lesions on particular rats at this dose at the end of the study
were reductions in erythrocyte count (approx. 16%), haematocrit
(approx. 12%), and haemoglobin concentration (approx. 14%) and an
increase in the leukocyte count (approx. 50%). The differential blood
count showed a reduction in the proportion of lymphocytes (approx.
11%), with a corresponding increase in neutrophils (approx. 145%), a
usual reaction during inflammation. The compound did not appear to
affect blood chemistry, and the changes seen, such as a reduction in
protein (approx. 10%) and albumin (approx. 18%) concentrations in
animals at the highest dose, are regarded as consequences of the poor
condition and of the skin lesions. Male animals at 160 ppm also had a
reduced cholesterol concentration (24%) and a reduction in the protein
content of the urine, but females had an increased protein content
accompanied by a reduction in the volume of urine with a corresponding
increase in the density of the urine.
At necropsy, only skin lesions were found. In animals at the
highest dose, the marked differences in body weight resulted in
reductions in the weights of some organs and increases in the relative
weights. Animals at 160 ppm showed evidence of stimulation of
extramedullary haematopoiesis in the spleen and a reduction in stored
haemosiderin, which were considered to have been the result of the
blood losses described above. The reduction in the neutral fat content
of the liver and in the size of the seminal vesicles of animals at the
highest dose were considered to have been due to the poor condition of
the animals and not to be related directly to flumethrin. The NOAEL
was thus 10 ppm, equal to 0.7 mg/kg bw per day (Bomann & Sander,
1995).
Dogs
Groups of four male and four female beagle dogs, about eight
months old, received diets containing flumethrin (purity, 98.7%) to
provide concentrations of 0, 50, 100, or 200 ppm for 13 weeks. Animals
at doses > 50 ppm showed thinned hair or hairlessness, and in some
instances weeping, ulcerative, scabbed patches were seen on the neck,
back, tall, ears, and limbs. These lesions had partially healed by the
end of the study. The group at 200 ppm had reduced food intake and
body-weight gains. Those at 100 and 200 ppm had slightly raised blood
urea values, which were statistically significant in week 13 in
animals at the highest dose (6.4 versus 7.9 mmol/litre); however,
there was no evidence of gross pathological changes in the kidneys
(Hoffmann & Kaliner, 1984). There was no NOAEL in this study.
In a study to achieve a no-effect level that was not found in
the earlier study because of the presence of skin lesions, groups of
four male and four female beagle dogs about six months old received
diets containing flumethrin (same batch as used in the previous
study) providing concentrations of 0 or 25 ppm for 13 weeks. No
differences were observed between the two groups of dogs, although
histopathological examination was not performed since no histological
differences were observed in the previous study. In particular, no
skin lesions were detected. The NOAEL was 25 ppm, equal to 0.88 mg/kg
bw per day in males and 0.94 mg/kg bw per day in females (Hoffmann,
1985).
Cattle
A single dose of 50 ml of a 1% flumethrin formulation was
administered by gavage to two six-week-old calves, and one calf
received a placebo formulation. All three animals were observed for
five days. The two animals given the flumethrin formulation voided
watery faeces for a short time within the first 24 h, and their
food intake was lower in the first 48 h after administration. There
were no changes in body-weight development or in haematological,
clinicochemical, or urinary parameters, and no changes were seen in
the calf give the placebo. Thus, if an animal were to lick the product
off treated skin, the only effects likely to occur are mild and
reversible (Iida et al., 1988).
A dose of 4 mg/kg bw, i.e. twice the therapeutic dose, was
applied to the backs of 18 calves, which were then observed for the
next 2 h and daily for three weeks. The treatment was well tolerated,
and there were no clinical signs or local effects on the skin (Dorn,
1989a). In a similar study in which 4 mg/kg bw was applied to the
dorsal midline of 15 young bovines, there was no evidence of local or
systemic effects during daily observation for three weeks (Dorn,
1989b). When the same dose was applied to the backs of 13 pregnant
cows, the treatment had no effect on the animals' behaviour or general
health. There was also no evidence of an effect on labour or on the
calves, which were given a clinical examination at delivery and
thereafter at weekly intervals (Dorn, 1989c).
(c) Long-term toxicity and carcinogenicity
No studies of the long-term toxicity or carcinogenicity of
flumethrin have been conducted, but a two-year study was carried out
with flumethrin with a low trans-Z isomer content (purity, 91.3%) in
which groups of 50 male and 50 female Wistar BOR:WISW (SPF Cpb) rats
received diets containing providing concentrations of 0, 2, 10, 50,
or 250 ppm. An additional 10 rats of each sex per group were killed
after 12 months of treatment. No significant effects were seen on
growth, mortality, food or water consumption, or behaviour at doses
< 50 ppm. At 250 ppm, both male and female rats showed retarded
growth development, and their mean body weights were > 10% lower than
those of the controls at 50 weeks. At 103 weeks, the numbers of deaths
in the groups given 0, 2, 10, 50, and 250 ppm were 7, 5, 8, 7 and 3
males and 10, 11, 7, 9, and 19 females. Much of the increased
mortality among the female rats at 250 ppm was due to the fact that
several were killed in a moribund state or died from severe skin
lesions and the related poor general condition. These lesions
consisted of ulcerative dermatitis occurring at a dose-dependent
incidence and severity, which first appeared in the second week of
treatment. The maximal incidences were seen after four weeks. These
lesions later healed in females at 50 ppm and in males at 50 or
250 ppm. Some inconsistent, statistically significant changes were
observed on periodic haematological examination: the number of
polymorphonuclear neutrophils increased, mainly in males at 250 ppm,
and inconsistent increases were also seen in other groups. Slight
decreases in lymphocyte counts were observed almost exclusively in
males at 50 and 250 ppm after 26, 52, and 78 weeks, but the decreases
were not significant after 104 weeks. These may be nonspecific
reactions to the inflammatory changes in the skin and were similar to
those reported in the 15-week dietary study described above (Bomann &
Sander, 1995). Sporadic differences were observed in erythrocyte and
haemoglobin parameters, but these were inconsistent with respect to
dose and time and are not considered to be treatment-related.
While statistically significant differences between groups did
occur, studies of blood chemistry did not indicate any consistent,
dose-related changes in albumin, bilirubin, urea, creatinine,
cholesterol, glucose, or the activities of the plasma enzymes alkaline
phosphatase, alanine transaminase, aspartate transaminase, creatine
kinase, and lactate dehydrogenase. No biologically important changes
were seen in chloride (deviation from control, < 4%), and the
larger changes in phosphate ion concentrations did not show a
dose-response relationship. The sodium ion concentration was unaltered
and that of potassium showed dose-related increases in males only at
52 and 104 weeks, but not at 26 or 78 weeks. Similarly, decreases in
calcium ion in both males and females occurred at 52 and 104 weeks,
but not at 26 or 78 weeks. This erratic pattern of changes does not
indicate that they were the result of treatment. Urine analysis showed
no variations attributable to treatment in either male or female rats
at 26, 52, 78, or 104 weeks. At autopsy, no treatment-related changes
were observed, but one female at 250 ppm killed at 52 weeks had the
skin lesions described above.
The relative weights of the lungs and kidneys were increased in
both male and female rats at 250 ppm at the end of the study: lung,
368 mg/100 g bw in controls vs 407 in males at 250 ppm and 426 mg/
100 g bw vs 482 in females; kidney, 605 mg/100 g bw vs 658 at 250 ppm
in males and 665 mg/100 g bw vs 717 in females. The absolute weights
of these organs were not changed; the weights relative to brain weight
were not available because the brain was not weighed. Most of the
effects appeared to be due to reductions in total body weight in
animals at 250 ppm.
Histological examinations were restricted to the groups at 0 and
250 ppm groups. Few non-neoplastic histopathological findings were
considered to be treatment-related. Two male and five female rats had
skin ulcerations, the etiology of which was not established. Male
rats, but not females, also had slight proliferation of the bile duct,
the incidences after 104 weeks being 12/50 in controls, 17/50 animals
at 2 ppm, 12/50 of those at 10 ppm, 16/50 of those at 50 ppm, and
23/50 of those at 250 ppm. The incidences of neoplastic lesions were
not affected by treatment, the numbers of tumour-bearing rats per
group of 50 being 25 male controls and 11 at 2 ppm, five at 10 ppm,
eight at 50 ppm, and 15 at 250 ppm, and 31 female controls, 18 at
2 ppm, 19 at 10 ppm, 23 at 50 ppm, and 23 at 250 ppm. The incidences
of specific, more frequent tumours were similar in the controls and
animals at 250 ppm, with mammary carcinomas in 6/44 control females,
2/49 at 2 ppm, 0/47 at 10 ppm, 5/47 at 50 ppm, and 0/45 at 250 ppm;
mammary fibroadenomas were found in 2/44 controls, 5/49 at 2 ppm, 3/47
at 10 ppm, 4/47 at 50 ppm, and 4/45 at 250 ppm. Pituitary adenomas
were found in 14/39 controls, in 4/39 animals at 2 ppm (in 4/4 masses
subjected to histological examination), in 12/43 animals at 10 ppm
(12/12 masses), in 11/41 animals at 50 ppm (11/11 masses), and 6/30
animals at 250 ppm. The NOAEL was 10 ppm, equal to 0.5 mg/kg bw per
day in males and 0.6 mg/kg bw per day in females (Bomhard et al.,
1987, 1991).
(d) Reproductive toxicity
Rats
A two-generation study was conducted on groups of 30 male and 30
female Wistar/HAN rats [strain: Kfm: WIST (SPF)] fed diets containing
flumethrin (45.6% in Aerosil 200) at concentrations providing
concentrations of 0, 1, 5, or 50 ppm. The F0 generation received the
test diet for 84 days before mating and during the mating period,
gestation, and lactation until day 21 after littering. The F1
parental generation received the test diet from the age of four to
seven weeks for 105 days before mating and thereafter over the same
periods as the F0 generation. No effects of treatment with 1 or 5 ppm
were discernible in any generation. After treatment with 50 ppm, skin
lesions developed on male and female animals of the F0 generation and
on females of the F1 generation. Male rats at this dose showed a
reduction in food consumption before mating in the F0 generation and
in all phases of the study in the F1 generation; females had a
reduced food consumption in the F0 generation in all phases and in
the F1 generation during the two lactation phases. The body-weight
development of males of the F0 generation and of males and females of
the F1 generation was retarded in all phases. The reductions in food
consumption and body-weight gain were seen as early as the first week
of dosing before mating.
Survival in all F1 and F2 generations was lower during the
first four days after delivery, but the losses up to day 21 after
delivery were greatest in the F1b, F2a, and F2b generations. In
addition, the weight gain of the pups of the F1 and F2 generations
was retarded. A higher incidence of pups with a cramped or bent
posture, stiff limbs in caudal posture, and/or pectus carinatum was
seen, with hypothermia, and vocalization was more frequent. These
observations are probably secondary to the toxic effects on
the parents. No malformations were found. Investigations of the
haematological status of the F1 parental animals gave no indication
of treatment-related changes. The NOAEL was 5 ppm, equal to 0.36 mg/kg
bw per day for males and 0.40 mg/kg bw per day for females (Dotti et
al., 1992).
(e) Developmental toxicity
Rats
Groups of 28 female Charles River Crl:CD Br rats, 11 weeks old
when mated, were given flumethrin (purity, 93.5%) formulated as a
0.4 mg/ml solution in distilled water containing 5% Emulphor EL-719
and 5% ethanol, orally at doses of 0, 0.5, 1, or 2 mg/kg bw per day on
days 6-15 of gestation. Day 0 of gestation was defined as the day on
which spermatozoa were found in the vagina. The rats tolerated the
dose of 0.5 mg/kg bw per day, but doses > 1 mg/kg bw per day were
toxic to the dams, the effects including increased salivation and
lacrimation, reduced activity, ataxia, and ptosis. At 2 mg/kg bw per
day, there was a reduction in food intake during treatment and a
reduction in body-weight gain. There was no evidence of teratogenicity
at any dose and no embryotoxic or fetotoxic effects in animals at 0.5
or 1 mg/kg bw per day. Animals at 2 mg/kg bw per day had significant
reductions in placental weights (0.53 g vs 0.48 g in controls) and
fetal weights (3.8 g vs 3.4 g, sexes combined); they also had an
increased number of fetuses with reduced ossification of the skull
bones (42% vs 67%) and cervical vertebral arches (1% vs 16%). The
NOAEL was 0.5 mg/kg bw per day for maternal toxicity and 1 mg/kg bw
per day for developmental toxicity (Kowalski et al., 1987).
Rabbits
Groups of 17 American-Dutch rabbits, at least 4.5 months old when
they were artificially inseminated with semen from proven males,
received flumethrin (purity, 93.5%) formulated as a 0.4 mg/ml solution
in distilled water containing 5% Emulphor EL-719 and 5% ethanol orally
at doses of 0, 0.5, 1.7, or 6 mg/kg bw per day on days 7-19 of
gestation. The high dose was selected on the basis of a range-finding
study in pregnant rabbits (source not identified). Dosing at 0.5 or
1.7 mg/kg bw per day was tolerated with no adverse effects. Animals at
6 mg/kg bw per day had reduced food intake during treatment and
reduced body weights; reproductive function was not affected. At
6 mg/kg bw per day, a slight trend for a reduction in the weights of
the fetuses, particularly the females, was seen, but this was not
significant. There was no indication of teratogenic potential. The
NOAEL was 1.7 mg/kg bw per day (Clemens & Hartnagel, 1987).
(f) Genotoxicity
Flumethrin has been adequately tested for its ability to induce
point mutations, DNA damage, and clastogenicity (Table 2). An early
bacterial mutagenesis assay using four strains of Salmonella
typhimurium gave equivocal results in several strains and weakly
positive results in strain TA98, but the latter were not confirmed in
later experiments. Tests with the isolated trans-Z-1 and trans-Z-2
isomers in S. typhimurium also gave negative results. A small
increase in the frequency of chromosomal aberrations was observed in
Chinese hamster V79 cells 18 h after treatment with flumethrin in
the presence of an exogenous metabolic system. This effect was not
observed in an earlier experiment with human lymphocytes, and there
was no indication of chromosomal aberration induction in vivo, as
might be indicated by the results of tests for micronucleus formation
in bone-marrow cells of mice. In these and all other tests with
flumethrin, the results were clearly negative. The Meeting concluded
that flumethrin is not genotoxic.
(g) Special studies
(i) Dermal and ocular irritation and dermal sensitization
In tests for ocular and dermal (occluded patch) irritation in New
Zealand white rabbits, a 1% pour-on formulation of flumethrin produced
slight erythema of the conjunctivae for up to 48 h after treatment and
slight oedema of the conjunctivae for up to 24 h after treatment.
Dermal application induced pronounced reddening and some oedema after
24 h. These effects had largely resolved by 72 h (Schmidt, 1984a).
Two pour-on formulations of flumethrin were tested in New Zealand
white rabbits exposed for 4 h. One formulation was not irritating,
while the other was slightly irritating to the skin. Exposure for 24 h
resulted in irritation with both formulations (Pauluhn, 1985a).
In a test for irritation on the skin and the mucous membrane of
the eyes of New Zealand white rabbits, flumethrin was applied as a 10%
formulation in olive oil. No irritation of either skin (4-h exposure)
or eyes (24-h exposure) was observed (Krötlinger, 1994).
Flumethrin (purity, 88.3%) was tested for dermal sensitizing
activity in the Magnusson and Kligman maximization test on male
Bor:DHPW guinea-pigs. For intradermal induction, flumethrin was
administered as a 5% solution in PEG 400; for topical induction and
for challenge, it was administered as a 50% solution in PEG 400. There
were no post-challenge skin reactions (Diesing, 1991).
Table 2. Results of tests for the genotoxicity of flumethrin
End-point Test system Concentration Purity Results Reference
(%)
Without S9 With S9
Flumethrin
Reverse mutation S. typhimurium TA100 15 625 µg/plate 97.6 Inconclusive Negative Herbold (1984)
Reverse mutation S. typhimurium TA1535 15 625 µg/plate 97.6 Negative Inconclusive Herbold (1984)
Reverse mutation S. typhimurium TA1537 15 625 µg/plate 97.6 Inconclusive Negative Herbold (1984)
Reverse mutation S. typhimurium TA98 15 625 µg/plate 97.6 Inconclusive Weakly Herbold (1984)
positive
Reverse mutation S. typhimurium TA100 5000 µg/plate 94.6 Negative Negativea Gahlmann (1993a)
Reverse mutation S. typhimurium TA1535 5000 µg/plate 94.6 Negative Negativea Gahlmann (1993a)
Reverse mutation S. typhimurium TA1537 5000 µg/plate 94.6 Negative Negativea Gahlmann (1993a)
Reverse mutation S. typhimurium TA98 5000 µg/plate 94.6 Negative Negativea Gahlmann (1993a)
Reverse mutation S. typhimurium TA100 15 000 µg/plate 94.6 Negative Negative Gahlmann (1993a)
Reverse mutation S. typhimurium TA1535 15 000 µg/plate 94.6 Negative Negative Gahlmann (1993a)
Reverse mutation S. typhimurium TA1537 15 000 µg/plate 94.6 Negative Negative Gahlmann (1993a)
Reverse mutation S. typhimurium TA98 15 000 µg/plate 94.6 Negative Negative Gahlmann (1993a)
Reverse mutation S. cerevisiae D7 10 000 µg/ml 92.7 Negative Negative Herbold (1985a)
Cell mutation, tk Mouse lymphoma 1000 µg/ml 92.7 Negative Negative Cifone & Myhr
locus L5178Y cells (1985)
Cell mutation, hprt Chinese hamster lung 100 µg/ml 95.1 Inconclusive Negative Brendler-Schwaab
locus V79 cells (1995)
Unscheduled DNA Rat hepatocyte 300 µg/ml 94.6 Negative Not tested Brendler-Schwaab
synthesis primary culture (1994)
Chromosomal Chinese hamster lung 125 µg/ml 95.1 Negative Negative Herbold (1995a)
aberration V79 cells (18- and
30-h sampling)
Table 2. (Cont'd)
End-point Test system Concentration Purity Results Reference
(%)
Without S9 With S9
Chromosomal Human lymphocyte 1000 µg/ml 92.7 Negative Negative Herbold (1985b)
aberration primary culture (24-h
sampling)
Micronucleus Mouse bone marrow 50 mg/kg bw × 1 93.5 Negative Herbold (1986)
induction in vivo (24-, 48-, and orally
72-h sampling)
Micronucleus Mouse bone marrow 1000 mg/kg × 1 95.1 Negative Herbold (1995b)
induction in vivo (16-, 24-, and intraperitoneally
48-h sampling)
Flumethrin-trans-Z-1 isomer
Reverse mutation S. typhimurium TA98 15 000 µg/plate 98.3 Negative Negative Herbold (1990a)
Flumethrin-trans-Z-2-isomer
Reverse mutation S. typhimurium TA98 15 000 µg/plate 95.4 Negative Negative Herbold (1990b)
S9, 9000 × g supernatant of liver microsomes used as exogenous metabolic activation system
a An additional test in the presence of 30% S9; all bacterial studies include duplicate experiments with 10% S9
Repetition of this maximization test with another batch of
flumethrin (purity, 94.6), formulated in the same manner and at the
same concentrations as above but with an additional 25% concentration
for challenge, also gave no evidence of any potential for inducing
skin sensitization (Vohr, 1994).
(ii) Neurotoxicity
Male and female Wistar Bor: WISW rats received flumethrin by
gavage for 14 days at doses of 20 mg/kg bw for males and 10 mg/kg bw
for females for the first four days, but due to signs of severe
toxicity and one death, the doses for the remainder of the study were
reduced to 10 mg/kg bw for males and 5 mg/kg bw for females. The
treatment was followed by 31 days of observation. This study was
conducted because other pyrethroids such as cypermethrin, fenvalerate,
and permethrin cause slight axonal degeneration, mainly in peripheral
nerves, at highly toxic doses in rats (WHO, 1989, 1990a,b). Two to
three hours after the first administration, the rats showed apathy,
reduced motility, accelerated breathing, salivation, and head
twitching. Later in the study, spastic gait was also seen, and digging
and shaking movements replaced the head twitch. The intensity of these
symptoms had declined only marginally by 24 h. When the doses were
reduced, the symptoms moderated in some rats. Some symptoms persisted
for up to two days after the 14-day dosing period, but the surviving
rats had completely recovered by the end of the observation period.
Thus, the high doses used caused dysfunction of the nervous
system, the observable effects of which were fully reversible.
Histopathological examination of the central and peripheral nervous
system gave no evidence of neurotoxicity-related morphological damage
(Flucke & Schilde, 1988).
Single doses of 0, 10, 31.5, or 100 mg/kg bw flumethrin were
administered orally to male Bor: WISW rats and Bor: CF1 mice. No
muscle-relaxant, analgesic, anticonvulsant, or cataleptic effects were
seen in animals at any dose, using standard pharmacological tests.
There was no evidence of impairment of central coordination, function,
reflexes, or neuromuscular transmission in the rats. Flumethrin caused
moderate stimulation of spontaneous motor activity in mice at all
doses, and the degree of stimulation was statistically significant
among animals of 31.5 and 100 mg/kg bw. Orientational activity was
also inhibited in these animals. Those at 100 mg/kg bw showed slight
potentiation of the duration and depth of hexobarbital-induced
anaesthesia (Starke, 1985).
(iii) Anti-allergic and pseudo-allergic activity
Histamine release in rat peritoneal mast cells sensitized
passively by exposure to immunoglobulin E from mouse serum was not
affected by concentrations of 0, 10-7, 10-6, or 10-5 g/ml flumethrin
(Gardiner et al., 1985).
(iv) Bronchial activity
Flumethrin at concentrations of 10-9 to 10-5 g/ml had no effect
on leukotrine D4- or histamine-induced contraction in isolated
guinea-pig tracheas (Gardiner et al., 1985).
(v) Effect on concentration of glucose and triglycerides in blood
Single doses of 0, 10, 32, or 100 mg/kg bw flumethrin were
administered orally to fasting and fed rats. The concentrations of
glucose in the blood of fed animals at all doses and of fasted
animals at the lowest and highest dose were slightly (24-64%) but
significantly increased for dose-related times of 60-240 min. There
was no effect on the triglyceride concentration. As marked variations
in the blood glucose concentration are seen even under physiological
conditions, these increases are not considered to be of particular
relevance (Puls & Bischoff, 1985).
(vi) Effects on gastrointestinal tract of rats
Single doses of 0, 10, 30, or 100 mg/kg bw flumethrin were
administered orally to rats. At the highest dose, a statistically
significant increase in intestinal transit time was seen in the
charcoal propulsion test. No gastric lesions were observed at autopsy.
Intraduodenal administration of 10, 30, or 100 mg/kg bw caused a
slight, non-significant, not dose-related reduction in acid secretion
in the perfused stomach (to 79, 58, and 75%, respectively, of the
control level) (Bonabello et al., 1987).
(vii) Haematological and cardiovascular effects
Single doses of 0, 10, 32, or 100 mg/kg bw flumethrin were
administered orally to rats, and blood samples were taken 90 min
later. Neither coagulation, platelet aggregation, nor fibrinolysis was
affected (Seuter et al., 1985).
Flumethrin at doses of 10, 32, or 100 mg/kg bw administered
orally to anaesthetized dogs induced slight increases in heart rate,
unrelated to dose, in one of three animals at 10 and 100 mg/kg bw.
These increases were not considered to be related to treatment (Knorr,
1986).
(viii) Diuretic activity
Flumethrin at doses of 0, 10, 32, or 100 mg/kg bw was administered
orally to rats, and urine was collected over 6 h to determine sodium
and potassium concentrations. Clinical signs including increased
salivation and reduced motor activity were seen in a few treated
animals. None of the doses changed urine output or sodium excretion.
Potassium excretion was significantly increased by the 10 and 100 mg/kg
bw doses but not by 32 mg/kg bw. The variations were not considered to
be treatment-related (Hirth, 1985).
(ix) Toxicity of metabolites: Flumethrin acid
Flumethrin acid is the major metabolite of flumethrin in rats and
cattle. The oral LD50 of flumethrin acid in fasted rats was 935 mg/kg
bw (95% confidence interval [CI], 549-1594) in males and 620 mg/kg bw
(95% CI, 500-771) in females. The principal clinical signs were
piloerection, lethargy, reduced motor activity, staggering gait,
animals lying prone or on their side, atonia, and slow and laboured
breathing. After dermal application of 5000 mg/kg bw, none of the
animals died. The only clinical signs were lethargy and reduced motor
activity. Small lesions and slight hyperaemia were observed at the
application site. Exposure of male and female rats to 338 mg/m3 by
inhalation, the highest concentration of flumethrin acid that it was
technically feasible to generate, for 4 h was well tolerated, and no
clinical signs of toxicity were observed (Pauluhn, 1985b).
When rats were observed for possible effects on the central
nervous system, by measuring the angle at which they slip from a plane
before and at various times from 1 to 24 h after treatment with 0, 1,
3, or 10 mg/kg bw flumethrin, no treatment-related reduction in the
angle was observed (Bomann, 1995a). In tests for dermal and ocular
irritancy in rabbits, flumethrin acid had no effect (Pauluhn, 1985b).
In a test for reverse mutation in S. typhimurium TA98, flumethrin
acid showed no activity (Herbold, 1984a).
In a four-week study in which Wistar rats were given dietary
concentrations of 0, 30, 100, or 300 ppm flumethrin acid, no clinical
signs of toxicity or effects on food consumption or body-weight
development were seen. There was no evidence of treatment-related
changes in haematological, clinicochemical, or necroscopic findings.
No treatment-related histological changes were observed. The NOAEL was
thus 300 ppm, equivalent to 27 mg/kg bw, the highest dose tested
(Bomann, 1995b).
3. Observations in humans
No reported cases of systemic poisoning with flumethrin in humans
were available to the Meeting. There are, however, published reports
of cases of poisoning with other pyrethroids. In a review of 573
cases of poisoning with other alpha-cyano pyrethroids (delamethrin,
fenvalerate, and cypermethrin), poisoning was due to either incorrect
occupational use of the product, attempted suicide, or accidents. In
cases in which the product had come into contact with the skin, the
presentation comprised a burning sensation on the face, tingling,
papules, and dermatitis. In cases of mild poisoning, there was also
dizziness, headache, nausea, anorexia, and weakness. In cases of
moderate poisoning, the signs and symptoms were more intense, and
there were states of reduced consciousness and muscle twitching in
the extremities. Seven deaths are listed; two are attributable to
misdiagnosis and inappropriate treatment. Most patients recovered
within six days. In the more severe cases, recovery took up to 55
days. Treatment consisted of symptomatic and supportive measures.
There were no delayed complications (He et al., 1989). There is no
basis for considering that flumethrin would act differently from the
compounds studied.
Comments
Flumethrin was absorbed rapidly, but not completely, after oral
administration in all species investigated. The concentrations in the
tissues of rats two days after dosing were three- to 50-fold lower
than those in the blood; the lung contained higher concentrations
than other tissues, and the central nervous system had the lowest
concentrations. Excretion occurred primarily in the faeces. The main
metabolite was flumethrin acid, which was distinctly less toxic than
the parent substance in acute and four-week dietary studies in rats
and did not induce reverse mutation in bacteria.
The acute oral toxicity of flumethrin in laboratory animals is
moderate to low. The reported manifestations of its toxicity are
largely consistent with those known collectively as the choreoathetosis
with salivation (CS) syndrome, which is produced by other insecticidal
pyrethroids containing an alpha-cyano-3-phenoxybenzyl alcohol group.
After acute dermal application, the toxicity of flumethrin was low; the
clinical signs were the same as those seen after oral administration.
There was no evidence of acute toxicity after dermal application of
5 ml/kg bw of a 1% pour-on formulation. In tests for dermal and ocular
irritancy, the active substance proved not to be irritating. In tests
for local irritancy with the 1% pour-on formulation, slight, transient
skin changes (mainly barely perceptible erythema and/or swelling) were
seen, but no changes in the mucous membrane of the eye, were observed.
WHO has not classified flumethrin for acute toxicity.
After oral administration of flumethrin for three months to rats
at dietary concentrations of 0, 10, 40, or 160 ppm and to dogs at
dietary concentrations of 0, 25, 50, 100, or 200 ppm, the NOAELs were
10 ppm (equal to 0.7 mg/kg bw per day) for rats and 25 ppm (equal to
0.88 mg/kg bw per day) for dogs. In both species, the most obvious
findings were skin alterations, but these were not due to primary
dermatitis caused by flumethrin but to frequent scratching, with
attendant bleeding and, in some instances, inflammation. Alpha-cyano
pyrethroids are known to produce paraesthesia, which is considered
to be the most probable cause of the observed skin lesions. The
toxicological studies provided no evidence of immunotoxicity, e.g.
effects on leukocyte counts or on other relevant organs (thymus and
spleen).
The results of studies of developmental toxicity in rats at doses
of 0, 0.5, 1, or 2 mg/kg bw per day on days 6-15 of gestation and
rabbits at doses of 0, 0.5, 1.7, or 6 mg/kg bw per day on days 7-19 of
gestation provided no evidence that flumethrin is teratogenic at doses
extending into the range that is toxic to the dams. Some fetotoxicity
was observed at doses that also induced maternal toxicity in both
species. The NOAELs were 0.5 mg/kg bw per day for rats and 1.7 mg/kg
bw per day for rabbits.
A two-generation study of reproductive toxicity in rats exposed
to flumethrin at dietary concentrations of 0, 1, 5, or 50 ppm did not
indicate primary reproductive toxicity; the reduced pup survival and
body-weight gain and certain postural and behavioural changes in the
pups at the highest dose may have been secondary to maternal toxicity.
The NOAEL was 5 ppm, equal to 0.36 mg/kg bw per day.
No studies of long-term toxicity or carcinogenicity have been
conducted with the currently used isomeric mixture of flumethrin.
A two-year study was available, however, in which rats were fed
diets providing flumethrin with a low trans-Z content at dietary
concentrations of 0, 2, 10, 50, or 250 ppm. Skin lesions developed in
rats at 50 and 250 ppm, and there was slight proliferation of the bile
ducts in male rats at 250 ppm. Neither the number of tumour-bearing
rats nor the incidence of any specific neoplasm was increased.
The Meeting considered the following toxicological findings:
(i) Flumethrin with a low trans-Z content has no carcinogenic
potential. (ii) Other pyrethroids, such as cyhalothrin (WHO, 1990a),
cypermethrin (WHO, 1989a), fenvalerate (WHO, 1990d), and the related
resmethrins (WHO, 1989b) also have no carcinogenic potential.
(iii) Treatment with permethrin (WHO, 1990b) resulted in small
increases in the incidence of lung tumours in female mice in three
studies, but no increases were found in either rats or male mice.
(iv) Treatment with deltamethrin was associated with unspecified
thyroid adenomas in rats in one study, but no tumours were induced
in mice or in either species in other studies (WHO, 1990c).
(v) Flumethrin had no genotoxic potential in a number of well-conducted
tests covering a variety of end-points. (vi) Flumethrin showed no
sensitizing potential. (vii) No preneoplastic responses were seen in
studies of up to 13 weeks in duration. The Meeting considered that the
carcinogenic potential of the trans-Z isomers that are present in the
currently used isomeric mixture of flumethrin had been assessed in the
study in rats in which the low trans-Z product was tested.
Oral administration of highly toxic doses of flumethrin to rats
can cause dysfunction of the nervous system, but the effect is rapidly
reversible and is not accompanied by morphological damage to the
central or peripheral nervous system.
Pharmacological tests in experimental animals gave no evidence of
impairment of vital functions. Studies to establish the tolerance of
calves and cattle to flumethrin showed no significant effects, even
when animals licked the application site.
An ADI of 0-0.004 mg/kg bw was allocated, on the basis of the
NOAEL of 0.36 mg/kg bw per day in the two-generation study of
reproductive toxicity in rats, using a 100-fold safety factor.
Toxicological evaluation
Levels that cause no toxic effect
Rat: 10 ppm, equal to 0.7 mg/kg bw per day (13- and 15-week
studies of toxicity)
5 ppm, equal to 0.36 mg/kg bw per day (two-generation
study of reproductive toxicity)
0.5 mg/kg bw per day (maternal toxicity in a study of
developmental toxicity)
Rabbit: 1.7 mg/kg bw per day (maternal and fetal toxicity in a
study of developmental toxicity)
Dog: 25 ppm, equal to 0.88 mg/kg bw per day (13-week study
of toxicity)
Estimate of acceptable daily intake for humans
0-0.004 mg/kg bw
Studies that would provide information useful for continued evaluation
of the compound
Results of any studies that are planned or in progress in
rodents, dogs, or exposed human subjects
Toxicological criteria for estimating guidance values for dietary and non-dietary exposure to flumethrin
Exposure Relevant route, study type, species Results, remarks
Short-term (1-7 days) Oral, toxicity, rat LD50 = 41-3849 mg/kg bw, depending on the vehicle
Dermal, toxicity, rat LD50 > 2000 mg/kg bw
Inhalation, toxicity, rat LC50 = 225 mg/m3
Dermal, irritation, rabbit Not irritating
Ocular, irritation, rabbit Not irritating
Dermal, sensitization, guinea-pig Not sensitizing
Medium-term (1-26 weeks) Repeated oral, 15 weeks, toxicity, rat NOAEL = 0.7 mg/kg bw per day
Repeated oral, 13 weeks, toxicity, dog NOAEL = 0.88 mg/kg bw per day
Repeated oral, reproductive toxicity, rat NOAEL = 0.36 mg/kg bw per day, reduced body-weight
gain of adults
Repeated oral, developmental toxicity, rat NOAEL = 1 mg/kg bw per day, developmental toxicity
Repeated oral, developmental toxicity, rabbit NOAEL = 1.7 mg/kg bw per day, maternal and
developmental toxicity
Long-term (> 1 year) Repeated oral, 2 years, toxicity and NOAEL = 0.5 mg/kg bw per day, skin lesions;
carcinogenicity, rat no carcinogenicity
References
Anadón, A., Diez, M.J., Sierra, M., Sanchez, J.A. & Teran, M.T. (1988)
Microsomal enzyme induction by permethrin in rats. Vet. Hum. Toxicol.,
30, 309-312.
Anadón, A., Martinez-Larrańaga, M.R., Fernandez, M.C., Dķaz, M.J.
& Bringas, P. (1990) Effect of ciprofloxacin on antipyrine
pharmacokinetics and metabolism in rats. Antimicrob. Agents Chemother.
34, 2148-2151.
Anadón, A., Martinez-Larrańaga, M.R., Dķaz, M.J., Bringas, P.,
Fernandez, M.C., Martinez, M.A. & Fernandez-Cruz, M.L. (1995) Effects
of flumethrin on hepatic drug-metabolizing enzymes and antipyrine
disposition in rats. Toxicol. Appl. Pharmacol., 132, 14-18.
Bomann, W. (1992a) Bay Vq 1950 (Flumethrin). Investigations of acute
oral toxicity in rats. Report No. 20924, 7.1.1992. Submitted to WHO by
Bayer AG, Leverkusen, Germany.
Bomann, W. (1992b) Bayticol EC 6% (c.n.: Flumethrin). Investigations
of acute oral toxicity in rats. Report No. 20922, 7.1.1992. Submitted
to WHO by Bayer AG, Leverkusen, Germany.
Bomann, W. (1992c) Bayticol EC 6% Bayticol EC6% (c.n.: Flumethrin).
Investigations of acute oral toxicity in rats. Report No. 20923,
7.1.1992. Submitted to WHO by Bayer AG, Leverkusen, Germany.
Bomann, W. (1992d) Bayvarol-Strips (c.n.: Flumethrin). Untersuchungen
zur akuten oralen Toxizität an Ratten. Ber. No. 21093, 17.2.1992.
Submitted to WHO by Bayer AG, Leverkusen, Germany.
Bomann, W. (1992e) Bayvarol-Strips (c.n.: Flumethrin). Untersuch-
ungenzur akuten dermalen Toxizität an Ratten. Ber. No. 21094,
17.2.1992. Submitted to WHO by Bayer AG, Leverkusen, Germany.
Bomann, W. (1994a) Bayticol P (c.n.: Flumethrin). Study for acute oral
toxicity in rats. Report No. 23422, 19.10.1994. Submitted to WHO by
Bayer AG, Leverkusen, Germany.
Bomann, W. (1994b) Bayticol P (c.n.: Flumethrin). Study for acute
dermal toxicity in rats. Report No. 23421, 19.10.1994. Submitted to
WHO by Bayer AG, Leverkusen, Germany.
Bomann, W. (1994c) Bayticol P (c.n.: Flumethrin). Study for acute oral
toxicity of Bayticol P formulated in water/Cremophor EL and in milk in
female rats. Report No. 23493, 23.11.1994. Submitted to WHO by Bayer
AG, Leverkusen, Germany.
Bomann, W. (1995a) Bayticol acid (Bayticolsäure). Study for acute oral
toxicity in Wistar rats. Report No. 23863, 22.3.1995. Submitted to WHO
by Bayer AG, Leverkusen, Germany.
Bomann, W. (1995b) Bayticol acid (Bayticolsäure). Investigations of
subacute toxicity in Wistar rats (feeding study over 4 weeks). Report
No. 24057, 7.6.1995. Submitted to WHO by Bayer AG, Leverkusen,
Germany.
Bomann, W. & Sander, E. (1995) Bayticol P (c.n.: Flumethrin).
Investigations or subchronic toxicity in Wistar rats (feeding study
over 15 weeks). Report No. 23925, 11.4.1995. Submitted to WHO by Bayer
AG, Leverkusen, Germany.
Bomhard, E., Ramm, W. & Rühl-Fehlert, C. (1987) Flumethrin (BAY V1
6045) Studies on the chronic toxicity and carcinogenicity in Wistar
rats. Report No. 16245, 25.11.1987. Submitted to WHO by Bayer, AG,
Leverkusen, Germany.
Bomhard, E., Ramm, W. & Rühl-Fehlert, C. (1991) Flumethrin (BAY V1
6045) Studies on the chronic toxicity and carcinogenicity in Wistar
rats. Amendment. Report No. 16245a, 24.5.1991. Submitted to WHO by
Bayer, AG, Leverkusen, Germany.
Bonabello, A. & Grassi, A. (1987) Safety pharmacology of Bay Vq 1950
in the gastrointestinal tract: Its effect on intestinal charcoal
transit, on gastric tolerability and on basal gastric acid secretion
in rats. Berich No. 4137 (P), 23.7.1987. Submitted to WHO by Bayer,
AG, Leverkusen, Germany.
Brendler-Schwaab, S. (1994) Evaluation of Bayticol P in the rat
primary hepatocyte unscheduled DNA synthesis assay. Report No. 23461,
8.11.1994. Submitted to WHO by Bayer AG, Leverkusen, Germany.
Brendler-Schwaab, S. (1995) Flumethrin. Mutagenicity study for the
detection of induced forward mutation in the V79-HPRT assay in
vitro. Report No. 24162, 14.7.1995. Submitted to WHO by Bayer AG,
Leverkusen, Germany.
Cabral, J.R.P., Galendo, D., Laval, M. & Lyandrat, N. (1990)
Carcinogenicity studies with deltamethrin in mice and rats. Cancer
Lett., 49, 147-152.
Cameron, B. (1986) Bayticol, Bay Vq 1950. The pharmacokinetics and
tissue residues of [14C]-Bayticol in the lactating cow following
topical administration. Report No. 4108. Unpublished report from
Inveresk Research International Ltd, Musselburgh, Scotland. Submitted
to WHO by Bayer AG, Leverkusen, Germany.
Carlson, G.P. & Shoening, G.P. (1980) Induction of liver microsomal
NADPH cytochrome c reductase and cytochrome P450 by some new synthetic
pyrethroids. Toxicol. Appl. Pharmacol., 52, 507-512.
Cifone, M.A. & Myhr, B.C. (1985) Mutagenicity evaluation of Bay Vq
1950 in the mouse lymphoma forward mutation assay. Pharma-Bericht No.
3170 (P). Unpublished report from Litton Bionetics, Inc., Kensington,
MD, USA. Submitted to WHO by Bayer AG, Leverkusen, Germany.
Clemens, G.R. & Hartnagel, R.E. (1987) A teratology study in the
rabbit with Bay Vq 1950 (Bayticol-P). Report No. MTD 0021. Unpublished
report from Miles Laboratories, Inc., Elkhart, IN, USA. Submitted to
WHO by Bayer AG, Leverkusen, Germany.
Diesing, L. (1991) Bayticol. Investigations of the skin-sensitizing
action in the guinea pig (maximization test after Magnusson & Ligman).
Report No. 20542. Submitted to WHO by Bayer AG, Leverkusen, Germany.
Dorn, H. (1989a) To assess the safety of Bayticol pour on after dermal
application in cattle. Report No. 89/13401. Submitted to WHO by Bayer,
AG, Leverkusen, Germany.
Dorn, H. (1989b) To assess the safety of Bayticol pour on after dermal
application in cattle. Report No. 89/13395. Submitted to WHO by Bayer,
AG, Leverkusen, Germany.
Dorn, H. (1989c) To assess the tolerance of Bayticol pour on 1% in
pregnant cows. Report No. 89/13394. Submitted to WHO by Bayer, AG,
Leverkusen, Germany.
Dotti, A., Kinder, J., Biedermann, K., Luetkemeier, H., Wright, J. &
Terrier, C. (1992) Bay Vq 1950. Multiple generation reproduction study
in rats. Report No. R 5500. Unpublished report from Research and
Consulting Co., Itingen, Switzerland. Submitted to WHO by Bayer, AG,
Leverkusen, Germany.
Ecker, W. (1983) Biotransformation von [fluorbenzoiring-U-14C] Bay V1
6045 bei der Ratte. Unpublished Bayer AG Report No. 83/11072 (11545).
Submitted to WHO by Bayer, AG, Leverkusen, Germany.
Flucke, W. & Schilde, B. (1988) Bay Vq 1950 (c.n.: Flumethrin).
Studies of the neurotoxic effects on the peripheral and central
nervous system of rats following subacute oral administration. Report
No. 16983. Submitted to WHO by Bayer, AG, Leverkusen, Germany.
Gahlmann, R. (1993a) Flumethrin. Salmonella/Mikrosome test. Special
study. Report No. 22613. Submitted to WHO by Bayer AG, Leverkusen,
Germany.
Gahlmann, R. (1993b) Flumethrin. w/Mikrosome test. Special study.
Report No. 22614. Submitted to WHO by Bayer AG, Leverkusen, Germany.
Gardiner, P.J., Hammond, M.D. & Francis, D.L. (1985) BAY Vq 1950:
General/Safety respiratory pharmacology: I. Anti-allergic and
pseudo-allergic activity in rat peritoneal mast cells. II. Evaluation
of bronchoactivity in the guinea-pig isolated trachea. Report No. 3555
(P). Submitted to WHO by Bayer AG, Leverkusen, Germany.
Hahnemann, S. & Rühl, C.H. (1985) Bay Vq 1950 (BAY V1 6045p).
Subchronic toxicological investigations in rats. Feeding study over 13
weeks. Report No. 13658 (E). Submitted to WHO by Bayer AG, Leverkusen,
Germany.
He, F., Wang, S., Liu, L., Chen, S., Zhang, Z. & Sun, J. (1989)
Clinical manifestation and diagnosis of acute pyrethroid poisoning.
Arch. Toxicol., 63, 54-58.
Herbold, B. (1984) Bay Vq 1950. Salmonella/microsome test to evaluate
for point-mutagenic effects. Report No. 12904 (F), 30.8.1984. Submitted
to WHO by Bayer AG, Leverkusen, Germany.
Herbold, B. (1985a) Bay Vq 1950. Test for point-mutagenic effects on
S. cerevisiae D7. Report No. 13737 (P). Submitted to WHO by Bayer
AG, Leverkusen, Germany.
Herbold, B. (1985b) BAY Vq 1950. Cytogenetic investigations in human
lymphocyte cultures in vitro to check for chromosome damaging
activity (in vitro). Report No. 13167 (F). Submitted to WHO by Bayer
AG, Leverkusen, Germany.
Herbold, B. (1986) Bayticol P. Micronucleus test in mice to evaluate
for clastogenic activity. Report No. 14501. Submitted to WHO by Bayer
AG, Leverkusen, Germany.
Herbold, B. (1990a) Flumethrin- trans-Z-1-isomer. Salmonella/microsome
test using Salmonella TA98. Report No. 19573. Submitted to WHO by Bayer
AG, Leverkusen, Germany.
Herbold, B. (1990b) Flumethrin- trans-Z-2-isomer. Salmonella/microsome
test using Salmonella TA 98. Report No. 19572. Submitted to WHO by Bayer
AG, Leverkusen, Germany.
Herbold, B. (1995a) Flumethrin. In vitro mammalian chromosome
aberration test with Chinese hamster V79 cells. Report No. 24524.
Submitted to WHO by Bayer AG, Leverkusen, Germany.
Herbold, B. (1995b) Flumethrin. Micronucleus test on the mouse. Report
No. 23869. Submitted to WHO by Bayer AG, Leverkusen, Germany.
Hirth, C. (1985) Bay Vq 1950. Study of diuretic activity in rats.
Report No. 14092. Submitted to WHO by Bayer AG, Leverkusen, Germany.
Hoffmann, K. & Kaliner, G. (1984) Bay Vq 1950. Subchronic toxicity
study in dogs with oral administration (13 weeks feeding study).
Report No. 13155. Submitted to WHO by Bayer AG, Leverkusen, Germany.
Hoffmann, K. (1985) Bay Vq 1950. Subchronic toxicity study in dogs
with oral administration/supplementary study (13 weeks feeding study).
Report No. 13169. Submitted to WHO by Bayer AG, Leverkusen, Germany.
Iida, M., Akagi, H. & Hashizume, M. (1988) Study on safety of Bayticol
pour-on in calves. Rep. No. 89/13245. Submitted to WHO by Bayer AG,
Leverkusen, Germany.
Kaneko, H., Ohkawa, H. & Miyamoto, J. (1981) Comparative metabolism of
fenvalerate and the [2S, alpha S]-isomer in rats and mice. J. Pestic.
Sci., 6, 317-326.
Klein, O. (1993a) [Cl-Phenyl-U-14C] Flumethrin: Investigation of the
biokinetic behaviour and the metabolism in the rat. Bericht No.,
PF-3823, ME-16/93, KNO 58. Submitted to WHO by Bayer AG, Leverkusen,
Germany.
Klein, O. (1993b) [Cl-Phenyl-U-14C] Flumethrin: Investigations on the
distribution of the total radioactivity in the rat. Projekt-No.
M1840667. Report in preparation, 1995. Submitted to WHO by Bayer AG,
Leverkusen, Germany.
Klein, O. (1995) Metabolism of [14C]-Bayticol in the dairy cow and
male beef cattle. Project No. M1840667-2. Report No. MR 323/95 KNO68.
Submitted to WHO by Bayer AG, Leverkusen, Germany.
Knorr, A. (1986) Influence on hemodynamics and cardiac contractility
of anesthesized dogs after oral administration. Pharma-Bericht No.
Submitted to WHO by Bayer AG, Leverkusen, Germany.
Kowalski, R.L., Clemens, G.R. & Hartnagel, R.E. (1987) A teratology
study with Bayticol-P (Flumethrin = Bay Vq 1950) in the rat. R-Bericht
No. 3960. Unpublished report from Miles Laboratories, Inc., Elkhart,
IN, USA. Submitted to WHO by Bayer, AG, Leverkusen, Germany.
Krötlinger, F. (1994) Bayticol P. Study for skin and eye irritation/
corrosion in rabbits. Ber-No. 23559. Submitted to WHO by Bayer AG,
Leverkusen, Germany.
Pauluhn, J. (1985a) Bayticol P pour on 1%. Study on the irritancy/
corrosion potential on rabbit skin. Report No. 14033. Submitted to WHO
by Bayer AG, Leverkusen, Germany.
Pauluhn, J. (1985b) Bayticol-P-Säure. Untersuchungen zur Gewer-
betoxikologie. Ber-No. 13643. Submitted to WHO by Bayer AG, Leverkusen,
Germany.
Puls, W. & Bischoff, H. (1985) Bay Vq 1950. Influence of orally
administered Bay Vq 1950 on the blood glucose and serum triglyceride
concentrations in fed rats and fasted rats. Report No. 14366.
Submitted to WHO by Bayer AG, Leverkusen, Germany.
Renhof, M. (1983a) Bay Vq 1950 in peanut oil and miglyol. Acute oral
toxicity in the rat. Report No. 12047 (P). Submitted to WHO by Bayer
AG, Leverkusen, Germany.
Renhof, M. (1983b) Bay Vq 1950 in acetone/peanut oil 1:10. Acute oral
toxicity in the rat. Report No. 12048 (P). Submitted to WHO by Bayer
AG, Leverkusen, Germany.
Schmidt, M. (1984a) Bay Vq 1950 pour on 1%. Acute toxicity in the rat
and the mouse. Study of primary irritant/corrosive activity on rabbit
skin and eye. Report No. 12761 (P). Submitted to WHO by Bayer AG,
Leverkusen, Germany.
Schmidt, M. (1984b) Bay Vq 1950 pour on 1%. Acute toxicity in the rat
and mouse after intraperitoneal application. Report No. 12760 (P).
Submitted to WHO by Bayer AG, Leverkusen, Germany.
Seuter, F. & Perzborn, E. (1985) Blood-pharmacological investigations.
Report No. 14213. Submitted to WHO by Bayer AG, Leverkusen, Germany.
Starke, B. (1985) CNS safety pharmacology study with Bay Vq 1950
(Bayticol P) on oral administration. Pharma-Bericht No. 3541.
Submitted to WHO by Bayer AG, Leverkusen, Germany.
Steinke, W., Weber, H. & Suwelack, D. (1983) [14C] BAY V1 6045:
Pharmakokinetik in Ratten. PH-Report No. 11941. Submitted to WHO by
Bayer AG, Leverkusen, Germany.
Thyssen, J. (1982) Bayticol EC 7.5%. Investigation into the acute
toxicity on inhalation. Report No. 10946. Submitted to WHO by Bayer
AG, Leverkusen, Germany.
Vijversberg, H.P.M. & Van den Bercken, J. (1982) Action of pyrethroid
insecticides on the vertebrae nervous system. Neuropathol. Appl.
Neurobiol., 8, 421-440
Vohr, H.-W. (1994) Bayticol P. Investigations of skin sensitization in
guinea pigs (Magnusson & Kligman test). Report No. 23026. Submitted to
WHO by Bayer AG, Leverkusen, Germany.
WHO (1989a) Cypermethrin (Environmental Health Criteria 82),
International Programme on Chemical Safety, Geneva, 154 pp.
WHO (1989b) Resmethrins (Environmental Health Criteria 92),
International Programme on Chemical Safety, Geneva, 79 pp.
WHO (1990a) Cyhalothrin (Environmental Health Criteria 99),
International Programme on Chemical Safety, Geneva, 106 pp.
WHO (1990b) Deltamethrin (Environmental Health Criteria 97),
International Programme on Chemical Safety, Geneva, 133 pp.
WHO (1990c) Fenvalerate (Environmental Health Criteria 95),
International Programme on Chemical Safety, Geneva, 121 pp.
WHO (1990d) Permethrin (Environmental Health Criteria 94),
International Programme on Chemical Safety, Geneva, 125 pp.
In a study with [Cl-phenyl-U-14C]-flumethrin, a dose of
1 mg/kg bw was administered intravenously to one 545-kg dairy cow
and one 340-kg steer. Both animals were slaughtered 8 h after
administration, and the quantities of radiolabel present in all
excreta and in liver, kidneys, muscle, fat, and milk were measured.
The highest concentrations were found in the liver (cow, 13 µg/g;
steer, 3.5 µg/g), followed by the kidney (cow, 0.88 µg/g; steer,
1.4 µg/g). The metabolite flumethrin acid was also found in all the
materials examined, except the milk, and was present as the glucuronide
in the liver and kidneys. In milk, an additional degradation product
was found but not identified, which constituted 11.5% of the total
residues in milk (Klein, 1995).
(c) Effects on enzymes
Pyrethroids can interact with liver drug-metabolizing enzymes,
but there appears to be a difference between some Type II pyrethroids
that contain an alpha-cyano function and inhibit such enzymes, e.g.
deltamethrin (Anadón et al., 1990), and the Type I pyrethroids that
do not contain this function and may induce drug-metabolizing enzymes,
e.g. permethrin (Carlson & Shoening, 1980; Anadón et al., 1988). In
experiments in which groups of 12 male Wistar rats received flumethrin
by intraperitoneal injection for six days, the Type II pyrethroid
response was demonstrated, in that there were reductions in cytochrome
P450 protein content (36%), NADPH-cytochrome c reductase activity
(38%), aniline hydroxylase activity (52%), aminopyrine N-demethylase
activity (54%), and UDP-glucuronosyl transferase activity (34%)
(Anadón et al., 1995).
2. Toxicological studies
(a) Acute toxicity
The results of studies of the acute toxicity of flumethrin are
listed in Table 1. The acute toxicity is slightly greater in female
than male rats and depends on the vehicle used. The sex difference
may reflect the greater metabolic conversion in female rats,
and differences between the solvents in respect of polarity,
which determines the proportion of the dose adsorbed from the
gastrointestinal tract, may explain the differences in oral toxicity.
The formulation containing Cremophor EL, which is known to enhance
absorption, was markedly more toxic; however, this formulation is used
only for administration in toxicological studies. The acute toxicity
of flumethrin in the other vehicles was moderate to low.
Table 1. Acute toxicity of flumethrin and products
Formulation Route Species Sex LD50 Reference
(mg/kg bw)
Flumethrin in Oral Rat Male > 100 Bomann (1994a)
water/Cremophor EL Female > 100
Oral Rat Male 56 Bomann (1992a)
Female 41
Flumethrin in arachis oil Oral Rat Male
911 Renhof (1983a)
Female 662
Flumethrin in miglyol Oral Rat Male 3849 Renhof (1983a)
Female 2248
Flumethrin in 1:10 Oral Rat Male 302 Renhof (1983b)
acetone:arachis oil Female 138
Flumethrin in corn oil Dermal Rat Male > 2000 Bomann (1994b)
Female > 2000
1% pour-on formulation Oral Rat Male > 20 Schmidt (1984a)
Female > 20
Oral Mouse Male > 20 Schmidt (1984a)
Female > 20
Dermal Rat Male > 5 Schmidt (1984a)
Female > 5
Dermala Rat Male > 5 Schmidt (1984a)
Female > 5
Intraperitoneal Mouse Male approx. .1 Schmidt (1984b)
Female approx. 5
Bayticol EC 6% in Oral Rat Male > 500-< 2000 Bomann (1992b)
Solvesso 200 Female > 500-< 2000
Dermal Rat Male > 5000 Bomann (1992c)
Female > 5000
Table 1. (Cont'd)
Formulation Route Species Sex LD50 Reference
(mg/kg bw)
Bayvarol strips Oral Rat Male > 2000 Bomann (1992d)
(0.55 g Bayticol/100g) Female > 2000
Dermal Rat Male > 5000 Bomann (1992e)
Female > 5000
Bayticol EC 7.5% Inhalation Rat Male approx. 3000 Thyssen (1982)
(4 h) Female > 2934
a On scarified skin
After acute administration of flumethrin, the most prominent
clinical signs were manifestations of central nervous system toxicity,
such as reduced motor activity, respiratory disorders, altered
gait, and salivation. The onset of action occurred 1-15 min after
administration, and the effects were comparatively long-lasting. The
reported manifestations of toxicity are largely consistent with those
known collectively as the choreoathetosis (sinuous writhing) with
salivation syndrome, which is produced by other insecticidal
pyrethroids, classified on this basis as Type II pyrethroids, which
contain an alpha-cyano-2-phenoxybenzyl alcohol group (Vijversberg &
van den Bercken, 1982).
In the studies of acute toxicity, particular attention was paid
to effects on the central nervous system. In animals investigated for
behaviour on an inclined plane, flumethrin was administered orally
in a vehicle which enhances toxicity, i.e. as an emulsion in
water/Cremophor EL, and, for comparison, in milk, a vehicle of
relevance as regards the consumer. Doses of 5 mg/kg bw in both
formulations had a slight effect, i.e. the inclination of the plane at
which the dosed animals slipped off was lower than that at which the
control animals slipped off. A dose of 1 mg/kg bw, administered as an
emulsion in water/Cremophor, also had a very slight effect, but no
effect was seen with 0.3 mg/kg bw or with 1 mg/kg bw administered as
an emulsion in milk (Bomann, 1994c).
After dermal application, the LD50 was > 2000 mg/kg bw. The
clinical signs were comparable to those observed after oral
administration. Evidence of skin reactions, such as scaling and
incrustation and sometimes scratches, were found at the application
site (Bomann, 1994b).
Studies of flumethrin products (Table 1) did not indicate
potentiation by the other constituents of commercially available
formulations. The results obtained were within the respective ranges
calculated on the basis of the toxicity of the active ingredient.
Dermal application of a dose of 5 ml/kg bw of a pour-on formulation
had no systemic or local effects (Schmidt, 1984a), and intraperitoneal
administration of the 1% pour-on formulation to mice also showed
little toxicity (Schmidt, 1984b).
Deaths occurred after inhalation of the product Flumethrin EC
7.5% at a nominal concentration > 10 000 mg/m3 (Table 1). At
500 mg/m3, inhalation was tolerated with no adverse effects (Thyssen,
1982).
(b) Short-term toxicity
Rats
Groups of 15 male and 15 female Wistar BOR: WISW rats received
diets containing flumethrin (purity, 98.7%) to provide concentrations
of 0, 10, 50, or 250/150 ppm (150 ppm from the third week onwards) for
13 weeks. An additional 10 rats of each sex per group were killed
after four weeks. At concentrations > 50 ppm, skin lesions were
seen on the head, neck, shoulder girdle, and front extremities.
Although treatment was continued, these changes had cleared up in
about half of the animals by the end of the study. During the first
two weeks of the study, both food intake and water consumption of the
group at 250 ppm were depressed by about 40%; these effects were
accompanied by body-weight losses in animals of each sex. With the
change to a dose of 150 ppm, food and water consumption became
indistinguishable from the control values and there were no further
weight losses. Body weights remained slightly reduced throughout the
study in this group (terminal differences in comparison with controls
being about 9% in males and 8% in females). Unscheduled deaths
occurred only in animals at 250/150 ppm, four females and one male
dying during the first two weeks of treatment and another male during
week 5. The NOAEL was 10 ppm, equal to 0.7 mg/kg bw per day in males
and 0.8 mg/kg bw per day in females (Hahnemann & Rühl, 1985).
Groups of 20 male and 20 female Wistar BOR: WISW rats received
flumethrin (purity, 94.6%) in the diet at levels providing
concentrations of 0, 10, 40, or 160 ppm for 15 weeks. Dosing at 160
ppm resulted in a reduction in food intake, retardation of body-weight
development, and clinical signs, but none of the animals died. The
body weights of animals at 160 ppm were 24% lower than those of
controls for males and 8% for females, but there were no significant
differences in the other groups. The principal clinical signs in
animals at 160 ppm were piloerection, increases or decreases in motor
activity, and spastic or staggering gait. Immediately after the start
of dosing, the animals at this dose were also seen to groom their fur
intensively and in particular to make frequent scratching movements.
This produced skin lesions, some of which were several centimeters in
diameter and bled after being scratched repeatedly. A small proportion
of these skin lesions, and similar ones found on a few animals at
40 ppm, healed as the study progressed. Alpha-cyano pyrethroids are
known to have a paraesthetic effect, which is regarded as the most
probable cause of the skin lesions. Correlated with the presence of
skin lesions on particular rats at this dose at the end of the study
were reductions in erythrocyte count (approx. 16%), haematocrit
(approx. 12%), and haemoglobin concentration (approx. 14%) and an
increase in the leukocyte count (approx. 50%). The differential blood
count showed a reduction in the proportion of lymphocytes (approx.
11%), with a corresponding increase in neutrophils (approx. 145%), a
usual reaction during inflammation. The compound did not appear to
affect blood chemistry, and the changes seen, such as a reduction in
protein (approx. 10%) and albumin (approx. 18%) concentrations in
animals at the highest dose, are regarded as consequences of the poor
condition and of the skin lesions. Male animals at 160 ppm also had a
reduced cholesterol concentration (24%) and a reduction in the protein
content of the urine, but females had an increased protein content
accompanied by a reduction in the volume of urine with a corresponding
increase in the density of the urine.
At necropsy, only skin lesions were found. In animals at the
highest dose, the marked differences in body weight resulted in
reductions in the weights of some organs and increases in the relative
weights. Animals at 160 ppm showed evidence of stimulation of
extramedullary haematopoiesis in the spleen and a reduction in stored
haemosiderin, which were considered to have been the result of the
blood losses described above. The reduction in the neutral fat content
of the liver and in the size of the seminal vesicles of animals at the
highest dose were considered to have been due to the poor condition of
the animals and not to be related directly to flumethrin. The NOAEL
was thus 10 ppm, equal to 0.7 mg/kg bw per day (Bomann & Sander,
1995).
Dogs
Groups of four male and four female beagle dogs, about eight
months old, received diets containing flumethrin (purity, 98.7%) to
provide concentrations of 0, 50, 100, or 200 ppm for 13 weeks. Animals
at doses > 50 ppm showed thinned hair or hairlessness, and in some
instances weeping, ulcerative, scabbed patches were seen on the neck,
back, tall, ears, and limbs. These lesions had partially healed by the
end of the study. The group at 200 ppm had reduced food intake and
body-weight gains. Those at 100 and 200 ppm had slightly raised blood
urea values, which were statistically significant in week 13 in
animals at the highest dose (6.4 versus 7.9 mmol/litre); however,
there was no evidence of gross pathological changes in the kidneys
(Hoffmann & Kaliner, 1984). There was no NOAEL in this study.
In a study to achieve a no-effect level that was not found in
the earlier study because of the presence of skin lesions, groups of
four male and four female beagle dogs about six months old received
diets containing flumethrin (same batch as used in the previous
study) providing concentrations of 0 or 25 ppm for 13 weeks. No
differences were observed between the two groups of dogs, although
histopathological examination was not performed since no histological
differences were observed in the previous study. In particular, no
skin lesions were detected. The NOAEL was 25 ppm, equal to 0.88 mg/kg
bw per day in males and 0.94 mg/kg bw per day in females (Hoffmann,
1985).
Cattle
A single dose of 50 ml of a 1% flumethrin formulation was
administered by gavage to two six-week-old calves, and one calf
received a placebo formulation. All three animals were observed for
five days. The two animals given the flumethrin formulation voided
watery faeces for a short time within the first 24 h, and their
food intake was lower in the first 48 h after administration. There
were no changes in body-weight development or in haematological,
clinicochemical, or urinary parameters, and no changes were seen in
the calf give the placebo. Thus, if an animal were to lick the product
off treated skin, the only effects likely to occur are mild and
reversible (Iida et al., 1988).
A dose of 4 mg/kg bw, i.e. twice the therapeutic dose, was
applied to the backs of 18 calves, which were then observed for the
next 2 h and daily for three weeks. The treatment was well tolerated,
and there were no clinical signs or local effects on the skin (Dorn,
1989a). In a similar study in which 4 mg/kg bw was applied to the
dorsal midline of 15 young bovines, there was no evidence of local or
systemic effects during daily observation for three weeks (Dorn,
1989b). When the same dose was applied to the backs of 13 pregnant
cows, the treatment had no effect on the animals' behaviour or general
health. There was also no evidence of an effect on labour or on the
calves, which were given a clinical examination at delivery and
thereafter at weekly intervals (Dorn, 1989c).
(c) Long-term toxicity and carcinogenicity
No studies of the long-term toxicity or carcinogenicity of
flumethrin have been conducted, but a two-year study was carried out
with flumethrin with a low trans-Z isomer content (purity, 91.3%) in
which groups of 50 male and 50 female Wistar BOR:WISW (SPF Cpb) rats
received diets containing providing concentrations of 0, 2, 10, 50,
or 250 ppm. An additional 10 rats of each sex per group were killed
after 12 months of treatment. No significant effects were seen on
growth, mortality, food or water consumption, or behaviour at doses
< 50 ppm. At 250 ppm, both male and female rats showed retarded
growth development, and their mean body weights were > 10% lower than
those of the controls at 50 weeks. At 103 weeks, the numbers of deaths
in the groups given 0, 2, 10, 50, and 250 ppm were 7, 5, 8, 7 and 3
males and 10, 11, 7, 9, and 19 females. Much of the increased
mortality among the female rats at 250 ppm was due to the fact that
several were killed in a moribund state or died from severe skin
lesions and the related poor general condition. These lesions
consisted of ulcerative dermatitis occurring at a dose-dependent
incidence and severity, which first appeared in the second week of
treatment. The maximal incidences were seen after four weeks. These
lesions later healed in females at 50 ppm and in males at 50 or
250 ppm. Some inconsistent, statistically significant changes were
observed on periodic haematological examination: the number of
polymorphonuclear neutrophils increased, mainly in males at 250 ppm,
and inconsistent increases were also seen in other groups. Slight
decreases in lymphocyte counts were observed almost exclusively in
males at 50 and 250 ppm after 26, 52, and 78 weeks, but the decreases
were not significant after 104 weeks. These may be nonspecific
reactions to the inflammatory changes in the skin and were similar to
those reported in the 15-week dietary study described above (Bomann &
Sander, 1995). Sporadic differences were observed in erythrocyte and
haemoglobin parameters, but these were inconsistent with respect to
dose and time and are not considered to be treatment-related.
While statistically significant differences between groups did
occur, studies of blood chemistry did not indicate any consistent,
dose-related changes in albumin, bilirubin, urea, creatinine,
cholesterol, glucose, or the activities of the plasma enzymes alkaline
phosphatase, alanine transaminase, aspartate transaminase, creatine
kinase, and lactate dehydrogenase. No biologically important changes
were seen in chloride (deviation from control, < 4%), and the
larger changes in phosphate ion concentrations did not show a
dose-response relationship. The sodium ion concentration was unaltered
and that of potassium showed dose-related increases in males only at
52 and 104 weeks, but not at 26 or 78 weeks. Similarly, decreases in
calcium ion in both males and females occurred at 52 and 104 weeks,
but not at 26 or 78 weeks. This erratic pattern of changes does not
indicate that they were the result of treatment. Urine analysis showed
no variations attributable to treatment in either male or female rats
at 26, 52, 78, or 104 weeks. At autopsy, no treatment-related changes
were observed, but one female at 250 ppm killed at 52 weeks had the
skin lesions described above.
The relative weights of the lungs and kidneys were increased in
both male and female rats at 250 ppm at the end of the study: lung,
368 mg/100 g bw in controls vs 407 in males at 250 ppm and 426 mg/
100 g bw vs 482 in females; kidney, 605 mg/100 g bw vs 658 at 250 ppm
in males and 665 mg/100 g bw vs 717 in females. The absolute weights
of these organs were not changed; the weights relative to brain weight
were not available because the brain was not weighed. Most of the
effects appeared to be due to reductions in total body weight in
animals at 250 ppm.
Histological examinations were restricted to the groups at 0 and
250 ppm groups. Few non-neoplastic histopathological findings were
considered to be treatment-related. Two male and five female rats had
skin ulcerations, the etiology of which was not established. Male
rats, but not females, also had slight proliferation of the bile duct,
the incidences after 104 weeks being 12/50 in controls, 17/50 animals
at 2 ppm, 12/50 of those at 10 ppm, 16/50 of those at 50 ppm, and
23/50 of those at 250 ppm. The incidences of neoplastic lesions were
not affected by treatment, the numbers of tumour-bearing rats per
group of 50 being 25 male controls and 11 at 2 ppm, five at 10 ppm,
eight at 50 ppm, and 15 at 250 ppm, and 31 female controls, 18 at
2 ppm, 19 at 10 ppm, 23 at 50 ppm, and 23 at 250 ppm. The incidences
of specific, more frequent tumours were similar in the controls and
animals at 250 ppm, with mammary carcinomas in 6/44 control females,
2/49 at 2 ppm, 0/47 at 10 ppm, 5/47 at 50 ppm, and 0/45 at 250 ppm;
mammary fibroadenomas were found in 2/44 controls, 5/49 at 2 ppm, 3/47
at 10 ppm, 4/47 at 50 ppm, and 4/45 at 250 ppm. Pituitary adenomas
were found in 14/39 controls, in 4/39 animals at 2 ppm (in 4/4 masses
subjected to histological examination), in 12/43 animals at 10 ppm
(12/12 masses), in 11/41 animals at 50 ppm (11/11 masses), and 6/30
animals at 250 ppm. The NOAEL was 10 ppm, equal to 0.5 mg/kg bw per
day in males and 0.6 mg/kg bw per day in females (Bomhard et al.,
1987, 1991).
(d) Reproductive toxicity
Rats
A two-generation study was conducted on groups of 30 male and 30
female Wistar/HAN rats [strain: Kfm: WIST (SPF)] fed diets containing
flumethrin (45.6% in Aerosil 200) at concentrations providing
concentrations of 0, 1, 5, or 50 ppm. The F0 generation received the
test diet for 84 days before mating and during the mating period,
gestation, and lactation until day 21 after littering. The F1
parental generation received the test diet from the age of four to
seven weeks for 105 days before mating and thereafter over the same
periods as the F0 generation. No effects of treatment with 1 or 5 ppm
were discernible in any generation. After treatment with 50 ppm, skin
lesions developed on male and female animals of the F0 generation and
on females of the F1 generation. Male rats at this dose showed a
reduction in food consumption before mating in the F0 generation and
in all phases of the study in the F1 generation; females had a
reduced food consumption in the F0 generation in all phases and in
the F1 generation during the two lactation phases. The body-weight
development of males of the F0 generation and of males and females of
the F1 generation was retarded in all phases. The reductions in food
consumption and body-weight gain were seen as early as the first week
of dosing before mating.
Survival in all F1 and F2 generations was lower during the
first four days after delivery, but the losses up to day 21 after
delivery were greatest in the F1b, F2a, and F2b generations. In
addition, the weight gain of the pups of the F1 and F2 generations
was retarded. A higher incidence of pups with a cramped or bent
posture, stiff limbs in caudal posture, and/or pectus carinatum was
seen, with hypothermia, and vocalization was more frequent. These
observations are probably secondary to the toxic effects on
the parents. No malformations were found. Investigations of the
haematological status of the F1 parental animals gave no indication
of treatment-related changes. The NOAEL was 5 ppm, equal to 0.36 mg/kg
bw per day for males and 0.40 mg/kg bw per day for females (Dotti et
al., 1992).
(e) Developmental toxicity
Rats
Groups of 28 female Charles River Crl:CD Br rats, 11 weeks old
when mated, were given flumethrin (purity, 93.5%) formulated as a
0.4 mg/ml solution in distilled water containing 5% Emulphor EL-719
and 5% ethanol, orally at doses of 0, 0.5, 1, or 2 mg/kg bw per day on
days 6-15 of gestation. Day 0 of gestation was defined as the day on
which spermatozoa were found in the vagina. The rats tolerated the
dose of 0.5 mg/kg bw per day, but doses > 1 mg/kg bw per day were
toxic to the dams, the effects including increased salivation and
lacrimation, reduced activity, ataxia, and ptosis. At 2 mg/kg bw per
day, there was a reduction in food intake during treatment and a
reduction in body-weight gain. There was no evidence of teratogenicity
at any dose and no embryotoxic or fetotoxic effects in animals at 0.5
or 1 mg/kg bw per day. Animals at 2 mg/kg bw per day had significant
reductions in placental weights (0.53 g vs 0.48 g in controls) and
fetal weights (3.8 g vs 3.4 g, sexes combined); they also had an
increased number of fetuses with reduced ossification of the skull
bones (42% vs 67%) and cervical vertebral arches (1% vs 16%). The
NOAEL was 0.5 mg/kg bw per day for maternal toxicity and 1 mg/kg bw
per day for developmental toxicity (Kowalski et al., 1987).
Rabbits
Groups of 17 American-Dutch rabbits, at least 4.5 months old when
they were artificially inseminated with semen from proven males,
received flumethrin (purity, 93.5%) formulated as a 0.4 mg/ml solution
in distilled water containing 5% Emulphor EL-719 and 5% ethanol orally
at doses of 0, 0.5, 1.7, or 6 mg/kg bw per day on days 7-19 of
gestation. The high dose was selected on the basis of a range-finding
study in pregnant rabbits (source not identified). Dosing at 0.5 or
1.7 mg/kg bw per day was tolerated with no adverse effects. Animals at
6 mg/kg bw per day had reduced food intake during treatment and
reduced body weights; reproductive function was not affected. At
6 mg/kg bw per day, a slight trend for a reduction in the weights of
the fetuses, particularly the females, was seen, but this was not
significant. There was no indication of teratogenic potential. The
NOAEL was 1.7 mg/kg bw per day (Clemens & Hartnagel, 1987).
(f) Genotoxicity
Flumethrin has been adequately tested for its ability to induce
point mutations, DNA damage, and clastogenicity (Table 2). An early
bacterial mutagenesis assay using four strains of Salmonella
typhimurium gave equivocal results in several strains and weakly
positive results in strain TA98, but the latter were not confirmed in
later experiments. Tests with the isolated trans-Z-1 and trans-Z-2
isomers in S. typhimurium also gave negative results. A small
increase in the frequency of chromosomal aberrations was observed in
Chinese hamster V79 cells 18 h after treatment with flumethrin in
the presence of an exogenous metabolic system. This effect was not
observed in an earlier experiment with human lymphocytes, and there
was no indication of chromosomal aberration induction in vivo, as
might be indicated by the results of tests for micronucleus formation
in bone-marrow cells of mice. In these and all other tests with
flumethrin, the results were clearly negative. The Meeting concluded
that flumethrin is not genotoxic.
(g) Special studies
(i) Dermal and ocular irritation and dermal sensitization
In tests for ocular and dermal (occluded patch) irritation in New
Zealand white rabbits, a 1% pour-on formulation of flumethrin produced
slight erythema of the conjunctivae for up to 48 h after treatment and
slight oedema of the conjunctivae for up to 24 h after treatment.
Dermal application induced pronounced reddening and some oedema after
24 h. These effects had largely resolved by 72 h (Schmidt, 1984a).
Two pour-on formulations of flumethrin were tested in New Zealand
white rabbits exposed for 4 h. One formulation was not irritating,
while the other was slightly irritating to the skin. Exposure for 24 h
resulted in irritation with both formulations (Pauluhn, 1985a).
In a test for irritation on the skin and the mucous membrane of
the eyes of New Zealand white rabbits, flumethrin was applied as a 10%
formulation in olive oil. No irritation of either skin (4-h exposure)
or eyes (24-h exposure) was observed (Krötlinger, 1994).
Flumethrin (purity, 88.3%) was tested for dermal sensitizing
activity in the Magnusson and Kligman maximization test on male
Bor:DHPW guinea-pigs. For intradermal induction, flumethrin was
administered as a 5% solution in PEG 400; for topical induction and
for challenge, it was administered as a 50% solution in PEG 400. There
were no post-challenge skin reactions (Diesing, 1991).
Table 2. Results of tests for the genotoxicity of flumethrin
End-point Test system Concentration Purity Results Reference
(%)
Without S9 With S9
Flumethrin
Reverse mutation S. typhimurium TA100 15 625 µg/plate 97.6 Inconclusive Negative Herbold (1984)
Reverse mutation S. typhimurium TA1535 15 625 µg/plate 97.6 Negative Inconclusive Herbold (1984)
Reverse mutation S. typhimurium TA1537 15 625 µg/plate 97.6 Inconclusive Negative Herbold (1984)
Reverse mutation S. typhimurium TA98 15 625 µg/plate 97.6 Inconclusive Weakly Herbold (1984)
positive
Reverse mutation S. typhimurium TA100 5000 µg/plate 94.6 Negative Negativea Gahlmann (1993a)
Reverse mutation S. typhimurium TA1535 5000 µg/plate 94.6 Negative Negativea Gahlmann (1993a)
Reverse mutation S. typhimurium TA1537 5000 µg/plate 94.6 Negative Negativea Gahlmann (1993a)
Reverse mutation S. typhimurium TA98 5000 µg/plate 94.6 Negative Negativea Gahlmann (1993a)
Reverse mutation S. typhimurium TA100 15 000 µg/plate 94.6 Negative Negative Gahlmann (1993a)
Reverse mutation S. typhimurium TA1535 15 000 µg/plate 94.6 Negative Negative Gahlmann (1993a)
Reverse mutation S. typhimurium TA1537 15 000 µg/plate 94.6 Negative Negative Gahlmann (1993a)
Reverse mutation S. typhimurium TA98 15 000 µg/plate 94.6 Negative Negative Gahlmann (1993a)
Reverse mutation S. cerevisiae D7 10 000 µg/ml 92.7 Negative Negative Herbold (1985a)
Cell mutation, tk Mouse lymphoma 1000 µg/ml 92.7 Negative Negative Cifone & Myhr
locus L5178Y cells (1985)
Cell mutation, hprt Chinese hamster lung 100 µg/ml 95.1 Inconclusive Negative Brendler-Schwaab
locus V79 cells (1995)
Unscheduled DNA Rat hepatocyte 300 µg/ml 94.6 Negative Not tested Brendler-Schwaab
synthesis primary culture (1994)
Chromosomal Chinese hamster lung 125 µg/ml 95.1 Negative Negative Herbold (1995a)
aberration V79 cells (18- and
30-h sampling)
Table 2. (Cont'd)
End-point Test system Concentration Purity Results Reference
(%)
Without S9 With S9
Chromosomal Human lymphocyte 1000 µg/ml 92.7 Negative Negative Herbold (1985b)
aberration primary culture (24-h
sampling)
Micronucleus Mouse bone marrow 50 mg/kg bw × 1 93.5 Negative Herbold (1986)
induction in vivo (24-, 48-, and orally
72-h sampling)
Micronucleus Mouse bone marrow 1000 mg/kg × 1 95.1 Negative Herbold (1995b)
induction in vivo (16-, 24-, and intraperitoneally
48-h sampling)
Flumethrin-trans-Z-1 isomer
Reverse mutation S. typhimurium TA98 15 000 µg/plate 98.3 Negative Negative Herbold (1990a)
Flumethrin-trans-Z-2-isomer
Reverse mutation S. typhimurium TA98 15 000 µg/plate 95.4 Negative Negative Herbold (1990b)
S9, 9000 × g supernatant of liver microsomes used as exogenous metabolic activation system
a An additional test in the presence of 30% S9; all bacterial studies include duplicate experiments with 10% S9
Repetition of this maximization test with another batch of
flumethrin (purity, 94.6), formulated in the same manner and at the
same concentrations as above but with an additional 25% concentration
for challenge, also gave no evidence of any potential for inducing
skin sensitization (Vohr, 1994).
(ii) Neurotoxicity
Male and female Wistar Bor: WISW rats received flumethrin by
gavage for 14 days at doses of 20 mg/kg bw for males and 10 mg/kg bw
for females for the first four days, but due to signs of severe
toxicity and one death, the doses for the remainder of the study were
reduced to 10 mg/kg bw for males and 5 mg/kg bw for females. The
treatment was followed by 31 days of observation. This study was
conducted because other pyrethroids such as cypermethrin, fenvalerate,
and permethrin cause slight axonal degeneration, mainly in peripheral
nerves, at highly toxic doses in rats (WHO, 1989, 1990a,b). Two to
three hours after the first administration, the rats showed apathy,
reduced motility, accelerated breathing, salivation, and head
twitching. Later in the study, spastic gait was also seen, and digging
and shaking movements replaced the head twitch. The intensity of these
symptoms had declined only marginally by 24 h. When the doses were
reduced, the symptoms moderated in some rats. Some symptoms persisted
for up to two days after the 14-day dosing period, but the surviving
rats had completely recovered by the end of the observation period.
Thus, the high doses used caused dysfunction of the nervous
system, the observable effects of which were fully reversible.
Histopathological examination of the central and peripheral nervous
system gave no evidence of neurotoxicity-related morphological damage
(Flucke & Schilde, 1988).
Single doses of 0, 10, 31.5, or 100 mg/kg bw flumethrin were
administered orally to male Bor: WISW rats and Bor: CF1 mice. No
muscle-relaxant, analgesic, anticonvulsant, or cataleptic effects were
seen in animals at any dose, using standard pharmacological tests.
There was no evidence of impairment of central coordination, function,
reflexes, or neuromuscular transmission in the rats. Flumethrin caused
moderate stimulation of spontaneous motor activity in mice at all
doses, and the degree of stimulation was statistically significant
among animals of 31.5 and 100 mg/kg bw. Orientational activity was
also inhibited in these animals. Those at 100 mg/kg bw showed slight
potentiation of the duration and depth of hexobarbital-induced
anaesthesia (Starke, 1985).
(iii) Anti-allergic and pseudo-allergic activity
Histamine release in rat peritoneal mast cells sensitized
passively by exposure to immunoglobulin E from mouse serum was not
affected by concentrations of 0, 10-7, 10-6, or 10-5 g/ml flumethrin
(Gardiner et al., 1985).
(iv) Bronchial activity
Flumethrin at concentrations of 10-9 to 10-5 g/ml had no effect
on leukotrine D4- or histamine-induced contraction in isolated
guinea-pig tracheas (Gardiner et al., 1985).
(v) Effect on concentration of glucose and triglycerides in blood
Single doses of 0, 10, 32, or 100 mg/kg bw flumethrin were
administered orally to fasting and fed rats. The concentrations of
glucose in the blood of fed animals at all doses and of fasted
animals at the lowest and highest dose were slightly (24-64%) but
significantly increased for dose-related times of 60-240 min. There
was no effect on the triglyceride concentration. As marked variations
in the blood glucose concentration are seen even under physiological
conditions, these increases are not considered to be of particular
relevance (Puls & Bischoff, 1985).
(vi) Effects on gastrointestinal tract of rats
Single doses of 0, 10, 30, or 100 mg/kg bw flumethrin were
administered orally to rats. At the highest dose, a statistically
significant increase in intestinal transit time was seen in the
charcoal propulsion test. No gastric lesions were observed at autopsy.
Intraduodenal administration of 10, 30, or 100 mg/kg bw caused a
slight, non-significant, not dose-related reduction in acid secretion
in the perfused stomach (to 79, 58, and 75%, respectively, of the
control level) (Bonabello et al., 1987).
(vii) Haematological and cardiovascular effects
Single doses of 0, 10, 32, or 100 mg/kg bw flumethrin were
administered orally to rats, and blood samples were taken 90 min
later. Neither coagulation, platelet aggregation, nor fibrinolysis was
affected (Seuter et al., 1985).
Flumethrin at doses of 10, 32, or 100 mg/kg bw administered
orally to anaesthetized dogs induced slight increases in heart rate,
unrelated to dose, in one of three animals at 10 and 100 mg/kg bw.
These increases were not considered to be related to treatment (Knorr,
1986).
(viii) Diuretic activity
Flumethrin at doses of 0, 10, 32, or 100 mg/kg bw was administered
orally to rats, and urine was collected over 6 h to determine sodium
and potassium concentrations. Clinical signs including increased
salivation and reduced motor activity were seen in a few treated
animals. None of the doses changed urine output or sodium excretion.
Potassium excretion was significantly increased by the 10 and 100 mg/kg
bw doses but not by 32 mg/kg bw. The variations were not considered to
be treatment-related (Hirth, 1985).
(ix) Toxicity of metabolites: Flumethrin acid
Flumethrin acid is the major metabolite of flumethrin in rats and
cattle. The oral LD50 of flumethrin acid in fasted rats was 935 mg/kg
bw (95% confidence interval [CI], 549-1594) in males and 620 mg/kg bw
(95% CI, 500-771) in females. The principal clinical signs were
piloerection, lethargy, reduced motor activity, staggering gait,
animals lying prone or on their side, atonia, and slow and laboured
breathing. After dermal application of 5000 mg/kg bw, none of the
animals died. The only clinical signs were lethargy and reduced motor
activity. Small lesions and slight hyperaemia were observed at the
application site. Exposure of male and female rats to 338 mg/m3 by
inhalation, the highest concentration of flumethrin acid that it was
technically feasible to generate, for 4 h was well tolerated, and no
clinical signs of toxicity were observed (Pauluhn, 1985b).
When rats were observed for possible effects on the central
nervous system, by measuring the angle at which they slip from a plane
before and at various times from 1 to 24 h after treatment with 0, 1,
3, or 10 mg/kg bw flumethrin, no treatment-related reduction in the
angle was observed (Bomann, 1995a). In tests for dermal and ocular
irritancy in rabbits, flumethrin acid had no effect (Pauluhn, 1985b).
In a test for reverse mutation in S. typhimurium TA98, flumethrin
acid showed no activity (Herbold, 1984a).
In a four-week study in which Wistar rats were given dietary
concentrations of 0, 30, 100, or 300 ppm flumethrin acid, no clinical
signs of toxicity or effects on food consumption or body-weight
development were seen. There was no evidence of treatment-related
changes in haematological, clinicochemical, or necroscopic findings.
No treatment-related histological changes were observed. The NOAEL was
thus 300 ppm, equivalent to 27 mg/kg bw, the highest dose tested
(Bomann, 1995b).
3. Observations in humans
No reported cases of systemic poisoning with flumethrin in humans
were available to the Meeting. There are, however, published reports
of cases of poisoning with other pyrethroids. In a review of 573
cases of poisoning with other alpha-cyano pyrethroids (delamethrin,
fenvalerate, and cypermethrin), poisoning was due to either incorrect
occupational use of the product, attempted suicide, or accidents. In
cases in which the product had come into contact with the skin, the
presentation comprised a burning sensation on the face, tingling,
papules, and dermatitis. In cases of mild poisoning, there was also
dizziness, headache, nausea, anorexia, and weakness. In cases of
moderate poisoning, the signs and symptoms were more intense, and
there were states of reduced consciousness and muscle twitching in
the extremities. Seven deaths are listed; two are attributable to
misdiagnosis and inappropriate treatment. Most patients recovered
within six days. In the more severe cases, recovery took up to 55
days. Treatment consisted of symptomatic and supportive measures.
There were no delayed complications (He et al., 1989). There is no
basis for considering that flumethrin would act differently from the
compounds studied.
Comments
Flumethrin was absorbed rapidly, but not completely, after oral
administration in all species investigated. The concentrations in the
tissues of rats two days after dosing were three- to 50-fold lower
than those in the blood; the lung contained higher concentrations
than other tissues, and the central nervous system had the lowest
concentrations. Excretion occurred primarily in the faeces. The main
metabolite was flumethrin acid, which was distinctly less toxic than
the parent substance in acute and four-week dietary studies in rats
and did not induce reverse mutation in bacteria.
The acute oral toxicity of flumethrin in laboratory animals is
moderate to low. The reported manifestations of its toxicity are
largely consistent with those known collectively as the choreoathetosis
with salivation (CS) syndrome, which is produced by other insecticidal
pyrethroids containing an alpha-cyano-3-phenoxybenzyl alcohol group.
After acute dermal application, the toxicity of flumethrin was low; the
clinical signs were the same as those seen after oral administration.
There was no evidence of acute toxicity after dermal application of
5 ml/kg bw of a 1% pour-on formulation. In tests for dermal and ocular
irritancy, the active substance proved not to be irritating. In tests
for local irritancy with the 1% pour-on formulation, slight, transient
skin changes (mainly barely perceptible erythema and/or swelling) were
seen, but no changes in the mucous membrane of the eye, were observed.
WHO has not classified flumethrin for acute toxicity.
After oral administration of flumethrin for three months to rats
at dietary concentrations of 0, 10, 40, or 160 ppm and to dogs at
dietary concentrations of 0, 25, 50, 100, or 200 ppm, the NOAELs were
10 ppm (equal to 0.7 mg/kg bw per day) for rats and 25 ppm (equal to
0.88 mg/kg bw per day) for dogs. In both species, the most obvious
findings were skin alterations, but these were not due to primary
dermatitis caused by flumethrin but to frequent scratching, with
attendant bleeding and, in some instances, inflammation. Alpha-cyano
pyrethroids are known to produce paraesthesia, which is considered
to be the most probable cause of the observed skin lesions. The
toxicological studies provided no evidence of immunotoxicity, e.g.
effects on leukocyte counts or on other relevant organs (thymus and
spleen).
The results of studies of developmental toxicity in rats at doses
of 0, 0.5, 1, or 2 mg/kg bw per day on days 6-15 of gestation and
rabbits at doses of 0, 0.5, 1.7, or 6 mg/kg bw per day on days 7-19 of
gestation provided no evidence that flumethrin is teratogenic at doses
extending into the range that is toxic to the dams. Some fetotoxicity
was observed at doses that also induced maternal toxicity in both
species. The NOAELs were 0.5 mg/kg bw per day for rats and 1.7 mg/kg
bw per day for rabbits.
A two-generation study of reproductive toxicity in rats exposed
to flumethrin at dietary concentrations of 0, 1, 5, or 50 ppm did not
indicate primary reproductive toxicity; the reduced pup survival and
body-weight gain and certain postural and behavioural changes in the
pups at the highest dose may have been secondary to maternal toxicity.
The NOAEL was 5 ppm, equal to 0.36 mg/kg bw per day.
No studies of long-term toxicity or carcinogenicity have been
conducted with the currently used isomeric mixture of flumethrin.
A two-year study was available, however, in which rats were fed
diets providing flumethrin with a low trans-Z content at dietary
concentrations of 0, 2, 10, 50, or 250 ppm. Skin lesions developed in
rats at 50 and 250 ppm, and there was slight proliferation of the bile
ducts in male rats at 250 ppm. Neither the number of tumour-bearing
rats nor the incidence of any specific neoplasm was increased.
The Meeting considered the following toxicological findings:
(i) Flumethrin with a low trans-Z content has no carcinogenic
potential. (ii) Other pyrethroids, such as cyhalothrin (WHO, 1990a),
cypermethrin (WHO, 1989a), fenvalerate (WHO, 1990d), and the related
resmethrins (WHO, 1989b) also have no carcinogenic potential.
(iii) Treatment with permethrin (WHO, 1990b) resulted in small
increases in the incidence of lung tumours in female mice in three
studies, but no increases were found in either rats or male mice.
(iv) Treatment with deltamethrin was associated with unspecified
thyroid adenomas in rats in one study, but no tumours were induced
in mice or in either species in other studies (WHO, 1990c).
(v) Flumethrin had no genotoxic potential in a number of well-conducted
tests covering a variety of end-points. (vi) Flumethrin showed no
sensitizing potential. (vii) No preneoplastic responses were seen in
studies of up to 13 weeks in duration. The Meeting considered that the
carcinogenic potential of the trans-Z isomers that are present in the
currently used isomeric mixture of flumethrin had been assessed in the
study in rats in which the low trans-Z product was tested.
Oral administration of highly toxic doses of flumethrin to rats
can cause dysfunction of the nervous system, but the effect is rapidly
reversible and is not accompanied by morphological damage to the
central or peripheral nervous system.
Pharmacological tests in experimental animals gave no evidence of
impairment of vital functions. Studies to establish the tolerance of
calves and cattle to flumethrin showed no significant effects, even
when animals licked the application site.
An ADI of 0-0.004 mg/kg bw was allocated, on the basis of the
NOAEL of 0.36 mg/kg bw per day in the two-generation study of
reproductive toxicity in rats, using a 100-fold safety factor.
Toxicological evaluation
Levels that cause no toxic effect
Rat: 10 ppm, equal to 0.7 mg/kg bw per day (13- and 15-week
studies of toxicity)
5 ppm, equal to 0.36 mg/kg bw per day (two-generation
study of reproductive toxicity)
0.5 mg/kg bw per day (maternal toxicity in a study of
developmental toxicity)
Rabbit: 1.7 mg/kg bw per day (maternal and fetal toxicity in a
study of developmental toxicity)
Dog: 25 ppm, equal to 0.88 mg/kg bw per day (13-week study
of toxicity)
Estimate of acceptable daily intake for humans
0-0.004 mg/kg bw
Studies that would provide information useful for continued evaluation
of the compound
Results of any studies that are planned or in progress in
rodents, dogs, or exposed human subjects
Toxicological criteria for estimating guidance values for dietary and non-dietary exposure to flumethrin
Exposure Relevant route, study type, species Results, remarks
Short-term (1-7 days) Oral, toxicity, rat LD50 = 41-3849 mg/kg bw, depending on the vehicle
Dermal, toxicity, rat LD50 > 2000 mg/kg bw
Inhalation, toxicity, rat LC50 = 225 mg/m3
Dermal, irritation, rabbit Not irritating
Ocular, irritation, rabbit Not irritating
Dermal, sensitization, guinea-pig Not sensitizing
Medium-term (1-26 weeks) Repeated oral, 15 weeks, toxicity, rat NOAEL = 0.7 mg/kg bw per day
Repeated oral, 13 weeks, toxicity, dog NOAEL = 0.88 mg/kg bw per day
Repeated oral, reproductive toxicity, rat NOAEL = 0.36 mg/kg bw per day, reduced body-weight
gain of adults
Repeated oral, developmental toxicity, rat NOAEL = 1 mg/kg bw per day, developmental toxicity
Repeated oral, developmental toxicity, rabbit NOAEL = 1.7 mg/kg bw per day, maternal and
developmental toxicity
Long-term (> 1 year) Repeated oral, 2 years, toxicity and NOAEL = 0.5 mg/kg bw per day, skin lesions;
carcinogenicity, rat no carcinogenicity
References
Anadón, A., Diez, M.J., Sierra, M., Sanchez, J.A. & Teran, M.T. (1988)
Microsomal enzyme induction by permethrin in rats. Vet. Hum. Toxicol.,
30, 309-312.
Anadón, A., Martinez-Larrańaga, M.R., Fernandez, M.C., Dķaz, M.J.
& Bringas, P. (1990) Effect of ciprofloxacin on antipyrine
pharmacokinetics and metabolism in rats. Antimicrob. Agents Chemother.
34, 2148-2151.
Anadón, A., Martinez-Larrańaga, M.R., Dķaz, M.J., Bringas, P.,
Fernandez, M.C., Martinez, M.A. & Fernandez-Cruz, M.L. (1995) Effects
of flumethrin on hepatic drug-metabolizing enzymes and antipyrine
disposition in rats. Toxicol. Appl. Pharmacol., 132, 14-18.
Bomann, W. (1992a) Bay Vq 1950 (Flumethrin). Investigations of acute
oral toxicity in rats. Report No. 20924, 7.1.1992. Submitted to WHO by
Bayer AG, Leverkusen, Germany.
Bomann, W. (1992b) Bayticol EC 6% (c.n.: Flumethrin). Investigations
of acute oral toxicity in rats. Report No. 20922, 7.1.1992. Submitted
to WHO by Bayer AG, Leverkusen, Germany.
Bomann, W. (1992c) Bayticol EC 6% Bayticol EC6% (c.n.: Flumethrin).
Investigations of acute oral toxicity in rats. Report No. 20923,
7.1.1992. Submitted to WHO by Bayer AG, Leverkusen, Germany.
Bomann, W. (1992d) Bayvarol-Strips (c.n.: Flumethrin). Untersuchungen
zur akuten oralen Toxizität an Ratten. Ber. No. 21093, 17.2.1992.
Submitted to WHO by Bayer AG, Leverkusen, Germany.
Bomann, W. (1992e) Bayvarol-Strips (c.n.: Flumethrin). Untersuch-
ungenzur akuten dermalen Toxizität an Ratten. Ber. No. 21094,
17.2.1992. Submitted to WHO by Bayer AG, Leverkusen, Germany.
Bomann, W. (1994a) Bayticol P (c.n.: Flumethrin). Study for acute oral
toxicity in rats. Report No. 23422, 19.10.1994. Submitted to WHO by
Bayer AG, Leverkusen, Germany.
Bomann, W. (1994b) Bayticol P (c.n.: Flumethrin). Study for acute
dermal toxicity in rats. Report No. 23421, 19.10.1994. Submitted to
WHO by Bayer AG, Leverkusen, Germany.
Bomann, W. (1994c) Bayticol P (c.n.: Flumethrin). Study for acute oral
toxicity of Bayticol P formulated in water/Cremophor EL and in milk in
female rats. Report No. 23493, 23.11.1994. Submitted to WHO by Bayer
AG, Leverkusen, Germany.
Bomann, W. (1995a) Bayticol acid (Bayticolsäure). Study for acute oral
toxicity in Wistar rats. Report No. 23863, 22.3.1995. Submitted to WHO
by Bayer AG, Leverkusen, Germany.
Bomann, W. (1995b) Bayticol acid (Bayticolsäure). Investigations of
subacute toxicity in Wistar rats (feeding study over 4 weeks). Report
No. 24057, 7.6.1995. Submitted to WHO by Bayer AG, Leverkusen,
Germany.
Bomann, W. & Sander, E. (1995) Bayticol P (c.n.: Flumethrin).
Investigations or subchronic toxicity in Wistar rats (feeding study
over 15 weeks). Report No. 23925, 11.4.1995. Submitted to WHO by Bayer
AG, Leverkusen, Germany.
Bomhard, E., Ramm, W. & Rühl-Fehlert, C. (1987) Flumethrin (BAY V1
6045) Studies on the chronic toxicity and carcinogenicity in Wistar
rats. Report No. 16245, 25.11.1987. Submitted to WHO by Bayer, AG,
Leverkusen, Germany.
Bomhard, E., Ramm, W. & Rühl-Fehlert, C. (1991) Flumethrin (BAY V1
6045) Studies on the chronic toxicity and carcinogenicity in Wistar
rats. Amendment. Report No. 16245a, 24.5.1991. Submitted to WHO by
Bayer, AG, Leverkusen, Germany.
Bonabello, A. & Grassi, A. (1987) Safety pharmacology of Bay Vq 1950
in the gastrointestinal tract: Its effect on intestinal charcoal
transit, on gastric tolerability and on basal gastric acid secretion
in rats. Berich No. 4137 (P), 23.7.1987. Submitted to WHO by Bayer,
AG, Leverkusen, Germany.
Brendler-Schwaab, S. (1994) Evaluation of Bayticol P in the rat
primary hepatocyte unscheduled DNA synthesis assay. Report No. 23461,
8.11.1994. Submitted to WHO by Bayer AG, Leverkusen, Germany.
Brendler-Schwaab, S. (1995) Flumethrin. Mutagenicity study for the
detection of induced forward mutation in the V79-HPRT assay in
vitro. Report No. 24162, 14.7.1995. Submitted to WHO by Bayer AG,
Leverkusen, Germany.
Cabral, J.R.P., Galendo, D., Laval, M. & Lyandrat, N. (1990)
Carcinogenicity studies with deltamethrin in mice and rats. Cancer
Lett., 49, 147-152.
Cameron, B. (1986) Bayticol, Bay Vq 1950. The pharmacokinetics and
tissue residues of [14C]-Bayticol in the lactating cow following
topical administration. Report No. 4108. Unpublished report from
Inveresk Research International Ltd, Musselburgh, Scotland. Submitted
to WHO by Bayer AG, Leverkusen, Germany.
Carlson, G.P. & Shoening, G.P. (1980) Induction of liver microsomal
NADPH cytochrome c reductase and cytochrome P450 by some new synthetic
pyrethroids. Toxicol. Appl. Pharmacol., 52, 507-512.
Cifone, M.A. & Myhr, B.C. (1985) Mutagenicity evaluation of Bay Vq
1950 in the mouse lymphoma forward mutation assay. Pharma-Bericht No.
3170 (P). Unpublished report from Litton Bionetics, Inc., Kensington,
MD, USA. Submitted to WHO by Bayer AG, Leverkusen, Germany.
Clemens, G.R. & Hartnagel, R.E. (1987) A teratology study in the
rabbit with Bay Vq 1950 (Bayticol-P). Report No. MTD 0021. Unpublished
report from Miles Laboratories, Inc., Elkhart, IN, USA. Submitted to
WHO by Bayer AG, Leverkusen, Germany.
Diesing, L. (1991) Bayticol. Investigations of the skin-sensitizing
action in the guinea pig (maximization test after Magnusson & Ligman).
Report No. 20542. Submitted to WHO by Bayer AG, Leverkusen, Germany.
Dorn, H. (1989a) To assess the safety of Bayticol pour on after dermal
application in cattle. Report No. 89/13401. Submitted to WHO by Bayer,
AG, Leverkusen, Germany.
Dorn, H. (1989b) To assess the safety of Bayticol pour on after dermal
application in cattle. Report No. 89/13395. Submitted to WHO by Bayer,
AG, Leverkusen, Germany.
Dorn, H. (1989c) To assess the tolerance of Bayticol pour on 1% in
pregnant cows. Report No. 89/13394. Submitted to WHO by Bayer, AG,
Leverkusen, Germany.
Dotti, A., Kinder, J., Biedermann, K., Luetkemeier, H., Wright, J. &
Terrier, C. (1992) Bay Vq 1950. Multiple generation reproduction study
in rats. Report No. R 5500. Unpublished report from Research and
Consulting Co., Itingen, Switzerland. Submitted to WHO by Bayer, AG,
Leverkusen, Germany.
Ecker, W. (1983) Biotransformation von [fluorbenzoiring-U-14C] Bay V1
6045 bei der Ratte. Unpublished Bayer AG Report No. 83/11072 (11545).
Submitted to WHO by Bayer, AG, Leverkusen, Germany.
Flucke, W. & Schilde, B. (1988) Bay Vq 1950 (c.n.: Flumethrin).
Studies of the neurotoxic effects on the peripheral and central
nervous system of rats following subacute oral administration. Report
No. 16983. Submitted to WHO by Bayer, AG, Leverkusen, Germany.
Gahlmann, R. (1993a) Flumethrin. Salmonella/Mikrosome test. Special
study. Report No. 22613. Submitted to WHO by Bayer AG, Leverkusen,
Germany.
Gahlmann, R. (1993b) Flumethrin. w/Mikrosome test. Special study.
Report No. 22614. Submitted to WHO by Bayer AG, Leverkusen, Germany.
Gardiner, P.J., Hammond, M.D. & Francis, D.L. (1985) BAY Vq 1950:
General/Safety respiratory pharmacology: I. Anti-allergic and
pseudo-allergic activity in rat peritoneal mast cells. II. Evaluation
of bronchoactivity in the guinea-pig isolated trachea. Report No. 3555
(P). Submitted to WHO by Bayer AG, Leverkusen, Germany.
Hahnemann, S. & Rühl, C.H. (1985) Bay Vq 1950 (BAY V1 6045p).
Subchronic toxicological investigations in rats. Feeding study over 13
weeks. Report No. 13658 (E). Submitted to WHO by Bayer AG, Leverkusen,
Germany.
He, F., Wang, S., Liu, L., Chen, S., Zhang, Z. & Sun, J. (1989)
Clinical manifestation and diagnosis of acute pyrethroid poisoning.
Arch. Toxicol., 63, 54-58.
Herbold, B. (1984) Bay Vq 1950. Salmonella/microsome test to evaluate
for point-mutagenic effects. Report No. 12904 (F), 30.8.1984. Submitted
to WHO by Bayer AG, Leverkusen, Germany.
Herbold, B. (1985a) Bay Vq 1950. Test for point-mutagenic effects on
S. cerevisiae D7. Report No. 13737 (P). Submitted to WHO by Bayer
AG, Leverkusen, Germany.
Herbold, B. (1985b) BAY Vq 1950. Cytogenetic investigations in human
lymphocyte cultures in vitro to check for chromosome damaging
activity (in vitro). Report No. 13167 (F). Submitted to WHO by Bayer
AG, Leverkusen, Germany.
Herbold, B. (1986) Bayticol P. Micronucleus test in mice to evaluate
for clastogenic activity. Report No. 14501. Submitted to WHO by Bayer
AG, Leverkusen, Germany.
Herbold, B. (1990a) Flumethrin- trans-Z-1-isomer. Salmonella/microsome
test using Salmonella TA98. Report No. 19573. Submitted to WHO by Bayer
AG, Leverkusen, Germany.
Herbold, B. (1990b) Flumethrin- trans-Z-2-isomer. Salmonella/microsome
test using Salmonella TA 98. Report No. 19572. Submitted to WHO by Bayer
AG, Leverkusen, Germany.
Herbold, B. (1995a) Flumethrin. In vitro mammalian chromosome
aberration test with Chinese hamster V79 cells. Report No. 24524.
Submitted to WHO by Bayer AG, Leverkusen, Germany.
Herbold, B. (1995b) Flumethrin. Micronucleus test on the mouse. Report
No. 23869. Submitted to WHO by Bayer AG, Leverkusen, Germany.
Hirth, C. (1985) Bay Vq 1950. Study of diuretic activity in rats.
Report No. 14092. Submitted to WHO by Bayer AG, Leverkusen, Germany.
Hoffmann, K. & Kaliner, G. (1984) Bay Vq 1950. Subchronic toxicity
study in dogs with oral administration (13 weeks feeding study).
Report No. 13155. Submitted to WHO by Bayer AG, Leverkusen, Germany.
Hoffmann, K. (1985) Bay Vq 1950. Subchronic toxicity study in dogs
with oral administration/supplementary study (13 weeks feeding study).
Report No. 13169. Submitted to WHO by Bayer AG, Leverkusen, Germany.
Iida, M., Akagi, H. & Hashizume, M. (1988) Study on safety of Bayticol
pour-on in calves. Rep. No. 89/13245. Submitted to WHO by Bayer AG,
Leverkusen, Germany.
Kaneko, H., Ohkawa, H. & Miyamoto, J. (1981) Comparative metabolism of
fenvalerate and the [2S, alpha S]-isomer in rats and mice. J. Pestic.
Sci., 6, 317-326.
Klein, O. (1993a) [Cl-Phenyl-U-14C] Flumethrin: Investigation of the
biokinetic behaviour and the metabolism in the rat. Bericht No.,
PF-3823, ME-16/93, KNO 58. Submitted to WHO by Bayer AG, Leverkusen,
Germany.
Klein, O. (1993b) [Cl-Phenyl-U-14C] Flumethrin: Investigations on the
distribution of the total radioactivity in the rat. Projekt-No.
M1840667. Report in preparation, 1995. Submitted to WHO by Bayer AG,
Leverkusen, Germany.
Klein, O. (1995) Metabolism of [14C]-Bayticol in the dairy cow and
male beef cattle. Project No. M1840667-2. Report No. MR 323/95 KNO68.
Submitted to WHO by Bayer AG, Leverkusen, Germany.
Knorr, A. (1986) Influence on hemodynamics and cardiac contractility
of anesthesized dogs after oral administration. Pharma-Bericht No.
Submitted to WHO by Bayer AG, Leverkusen, Germany.
Kowalski, R.L., Clemens, G.R. & Hartnagel, R.E. (1987) A teratology
study with Bayticol-P (Flumethrin = Bay Vq 1950) in the rat. R-Bericht
No. 3960. Unpublished report from Miles Laboratories, Inc., Elkhart,
IN, USA. Submitted to WHO by Bayer, AG, Leverkusen, Germany.
Krötlinger, F. (1994) Bayticol P. Study for skin and eye irritation/
corrosion in rabbits. Ber-No. 23559. Submitted to WHO by Bayer AG,
Leverkusen, Germany.
Pauluhn, J. (1985a) Bayticol P pour on 1%. Study on the irritancy/
corrosion potential on rabbit skin. Report No. 14033. Submitted to WHO
by Bayer AG, Leverkusen, Germany.
Pauluhn, J. (1985b) Bayticol-P-Säure. Untersuchungen zur Gewer-
betoxikologie. Ber-No. 13643. Submitted to WHO by Bayer AG, Leverkusen,
Germany.
Puls, W. & Bischoff, H. (1985) Bay Vq 1950. Influence of orally
administered Bay Vq 1950 on the blood glucose and serum triglyceride
concentrations in fed rats and fasted rats. Report No. 14366.
Submitted to WHO by Bayer AG, Leverkusen, Germany.
Renhof, M. (1983a) Bay Vq 1950 in peanut oil and miglyol. Acute oral
toxicity in the rat. Report No. 12047 (P). Submitted to WHO by Bayer
AG, Leverkusen, Germany.
Renhof, M. (1983b) Bay Vq 1950 in acetone/peanut oil 1:10. Acute oral
toxicity in the rat. Report No. 12048 (P). Submitted to WHO by Bayer
AG, Leverkusen, Germany.
Schmidt, M. (1984a) Bay Vq 1950 pour on 1%. Acute toxicity in the rat
and the mouse. Study of primary irritant/corrosive activity on rabbit
skin and eye. Report No. 12761 (P). Submitted to WHO by Bayer AG,
Leverkusen, Germany.
Schmidt, M. (1984b) Bay Vq 1950 pour on 1%. Acute toxicity in the rat
and mouse after intraperitoneal application. Report No. 12760 (P).
Submitted to WHO by Bayer AG, Leverkusen, Germany.
Seuter, F. & Perzborn, E. (1985) Blood-pharmacological investigations.
Report No. 14213. Submitted to WHO by Bayer AG, Leverkusen, Germany.
Starke, B. (1985) CNS safety pharmacology study with Bay Vq 1950
(Bayticol P) on oral administration. Pharma-Bericht No. 3541.
Submitted to WHO by Bayer AG, Leverkusen, Germany.
Steinke, W., Weber, H. & Suwelack, D. (1983) [14C] BAY V1 6045:
Pharmakokinetik in Ratten. PH-Report No. 11941. Submitted to WHO by
Bayer AG, Leverkusen, Germany.
Thyssen, J. (1982) Bayticol EC 7.5%. Investigation into the acute
toxicity on inhalation. Report No. 10946. Submitted to WHO by Bayer
AG, Leverkusen, Germany.
Vijversberg, H.P.M. & Van den Bercken, J. (1982) Action of pyrethroid
insecticides on the vertebrae nervous system. Neuropathol. Appl.
Neurobiol., 8, 421-440
Vohr, H.-W. (1994) Bayticol P. Investigations of skin sensitization in
guinea pigs (Magnusson & Kligman test). Report No. 23026. Submitted to
WHO by Bayer AG, Leverkusen, Germany.
WHO (1989a) Cypermethrin (Environmental Health Criteria 82),
International Programme on Chemical Safety, Geneva, 154 pp.
WHO (1989b) Resmethrins (Environmental Health Criteria 92),
International Programme on Chemical Safety, Geneva, 79 pp.
WHO (1990a) Cyhalothrin (Environmental Health Criteria 99),
International Programme on Chemical Safety, Geneva, 106 pp.
WHO (1990b) Deltamethrin (Environmental Health Criteria 97),
International Programme on Chemical Safety, Geneva, 133 pp.
WHO (1990c) Fenvalerate (Environmental Health Criteria 95),
International Programme on Chemical Safety, Geneva, 121 pp.
WHO (1990d) Permethrin (Environmental Health Criteria 94),
International Programme on Chemical Safety, Geneva, 125 pp.
 In a study with [Cl-phenyl-U-14C]-flumethrin, a dose of
1 mg/kg bw was administered intravenously to one 545-kg dairy cow
and one 340-kg steer. Both animals were slaughtered 8 h after
administration, and the quantities of radiolabel present in all
excreta and in liver, kidneys, muscle, fat, and milk were measured.
The highest concentrations were found in the liver (cow, 13 µg/g;
steer, 3.5 µg/g), followed by the kidney (cow, 0.88 µg/g; steer,
1.4 µg/g). The metabolite flumethrin acid was also found in all the
materials examined, except the milk, and was present as the glucuronide
in the liver and kidneys. In milk, an additional degradation product
was found but not identified, which constituted 11.5% of the total
residues in milk (Klein, 1995).
(c) Effects on enzymes
Pyrethroids can interact with liver drug-metabolizing enzymes,
but there appears to be a difference between some Type II pyrethroids
that contain an alpha-cyano function and inhibit such enzymes, e.g.
deltamethrin (Anadón et al., 1990), and the Type I pyrethroids that
do not contain this function and may induce drug-metabolizing enzymes,
e.g. permethrin (Carlson & Shoening, 1980; Anadón et al., 1988). In
experiments in which groups of 12 male Wistar rats received flumethrin
by intraperitoneal injection for six days, the Type II pyrethroid
response was demonstrated, in that there were reductions in cytochrome
P450 protein content (36%), NADPH-cytochrome c reductase activity
(38%), aniline hydroxylase activity (52%), aminopyrine N-demethylase
activity (54%), and UDP-glucuronosyl transferase activity (34%)
(Anadón et al., 1995).
2. Toxicological studies
(a) Acute toxicity
The results of studies of the acute toxicity of flumethrin are
listed in Table 1. The acute toxicity is slightly greater in female
than male rats and depends on the vehicle used. The sex difference
may reflect the greater metabolic conversion in female rats,
and differences between the solvents in respect of polarity,
which determines the proportion of the dose adsorbed from the
gastrointestinal tract, may explain the differences in oral toxicity.
The formulation containing Cremophor EL, which is known to enhance
absorption, was markedly more toxic; however, this formulation is used
only for administration in toxicological studies. The acute toxicity
of flumethrin in the other vehicles was moderate to low.
Table 1. Acute toxicity of flumethrin and products
Formulation Route Species Sex LD50 Reference
(mg/kg bw)
Flumethrin in Oral Rat Male > 100 Bomann (1994a)
water/Cremophor EL Female > 100
Oral Rat Male 56 Bomann (1992a)
Female 41
Flumethrin in arachis oil Oral Rat Male
911 Renhof (1983a)
Female 662
Flumethrin in miglyol Oral Rat Male 3849 Renhof (1983a)
Female 2248
Flumethrin in 1:10 Oral Rat Male 302 Renhof (1983b)
acetone:arachis oil Female 138
Flumethrin in corn oil Dermal Rat Male > 2000 Bomann (1994b)
Female > 2000
1% pour-on formulation Oral Rat Male > 20 Schmidt (1984a)
Female > 20
Oral Mouse Male > 20 Schmidt (1984a)
Female > 20
Dermal Rat Male > 5 Schmidt (1984a)
Female > 5
Dermala Rat Male > 5 Schmidt (1984a)
Female > 5
Intraperitoneal Mouse Male approx. .1 Schmidt (1984b)
Female approx. 5
Bayticol EC 6% in Oral Rat Male > 500-< 2000 Bomann (1992b)
Solvesso 200 Female > 500-< 2000
Dermal Rat Male > 5000 Bomann (1992c)
Female > 5000
Table 1. (Cont'd)
Formulation Route Species Sex LD50 Reference
(mg/kg bw)
Bayvarol strips Oral Rat Male > 2000 Bomann (1992d)
(0.55 g Bayticol/100g) Female > 2000
Dermal Rat Male > 5000 Bomann (1992e)
Female > 5000
Bayticol EC 7.5% Inhalation Rat Male approx. 3000 Thyssen (1982)
(4 h) Female > 2934
a On scarified skin
After acute administration of flumethrin, the most prominent
clinical signs were manifestations of central nervous system toxicity,
such as reduced motor activity, respiratory disorders, altered
gait, and salivation. The onset of action occurred 1-15 min after
administration, and the effects were comparatively long-lasting. The
reported manifestations of toxicity are largely consistent with those
known collectively as the choreoathetosis (sinuous writhing) with
salivation syndrome, which is produced by other insecticidal
pyrethroids, classified on this basis as Type II pyrethroids, which
contain an alpha-cyano-2-phenoxybenzyl alcohol group (Vijversberg &
van den Bercken, 1982).
In the studies of acute toxicity, particular attention was paid
to effects on the central nervous system. In animals investigated for
behaviour on an inclined plane, flumethrin was administered orally
in a vehicle which enhances toxicity, i.e. as an emulsion in
water/Cremophor EL, and, for comparison, in milk, a vehicle of
relevance as regards the consumer. Doses of 5 mg/kg bw in both
formulations had a slight effect, i.e. the inclination of the plane at
which the dosed animals slipped off was lower than that at which the
control animals slipped off. A dose of 1 mg/kg bw, administered as an
emulsion in water/Cremophor, also had a very slight effect, but no
effect was seen with 0.3 mg/kg bw or with 1 mg/kg bw administered as
an emulsion in milk (Bomann, 1994c).
After dermal application, the LD50 was > 2000 mg/kg bw. The
clinical signs were comparable to those observed after oral
administration. Evidence of skin reactions, such as scaling and
incrustation and sometimes scratches, were found at the application
site (Bomann, 1994b).
Studies of flumethrin products (Table 1) did not indicate
potentiation by the other constituents of commercially available
formulations. The results obtained were within the respective ranges
calculated on the basis of the toxicity of the active ingredient.
Dermal application of a dose of 5 ml/kg bw of a pour-on formulation
had no systemic or local effects (Schmidt, 1984a), and intraperitoneal
administration of the 1% pour-on formulation to mice also showed
little toxicity (Schmidt, 1984b).
Deaths occurred after inhalation of the product Flumethrin EC
7.5% at a nominal concentration > 10 000 mg/m3 (Table 1). At
500 mg/m3, inhalation was tolerated with no adverse effects (Thyssen,
1982).
(b) Short-term toxicity
Rats
Groups of 15 male and 15 female Wistar BOR: WISW rats received
diets containing flumethrin (purity, 98.7%) to provide concentrations
of 0, 10, 50, or 250/150 ppm (150 ppm from the third week onwards) for
13 weeks. An additional 10 rats of each sex per group were killed
after four weeks. At concentrations > 50 ppm, skin lesions were
seen on the head, neck, shoulder girdle, and front extremities.
Although treatment was continued, these changes had cleared up in
about half of the animals by the end of the study. During the first
two weeks of the study, both food intake and water consumption of the
group at 250 ppm were depressed by about 40%; these effects were
accompanied by body-weight losses in animals of each sex. With the
change to a dose of 150 ppm, food and water consumption became
indistinguishable from the control values and there were no further
weight losses. Body weights remained slightly reduced throughout the
study in this group (terminal differences in comparison with controls
being about 9% in males and 8% in females). Unscheduled deaths
occurred only in animals at 250/150 ppm, four females and one male
dying during the first two weeks of treatment and another male during
week 5. The NOAEL was 10 ppm, equal to 0.7 mg/kg bw per day in males
and 0.8 mg/kg bw per day in females (Hahnemann & Rühl, 1985).
Groups of 20 male and 20 female Wistar BOR: WISW rats received
flumethrin (purity, 94.6%) in the diet at levels providing
concentrations of 0, 10, 40, or 160 ppm for 15 weeks. Dosing at 160
ppm resulted in a reduction in food intake, retardation of body-weight
development, and clinical signs, but none of the animals died. The
body weights of animals at 160 ppm were 24% lower than those of
controls for males and 8% for females, but there were no significant
differences in the other groups. The principal clinical signs in
animals at 160 ppm were piloerection, increases or decreases in motor
activity, and spastic or staggering gait. Immediately after the start
of dosing, the animals at this dose were also seen to groom their fur
intensively and in particular to make frequent scratching movements.
This produced skin lesions, some of which were several centimeters in
diameter and bled after being scratched repeatedly. A small proportion
of these skin lesions, and similar ones found on a few animals at
40 ppm, healed as the study progressed. Alpha-cyano pyrethroids are
known to have a paraesthetic effect, which is regarded as the most
probable cause of the skin lesions. Correlated with the presence of
skin lesions on particular rats at this dose at the end of the study
were reductions in erythrocyte count (approx. 16%), haematocrit
(approx. 12%), and haemoglobin concentration (approx. 14%) and an
increase in the leukocyte count (approx. 50%). The differential blood
count showed a reduction in the proportion of lymphocytes (approx.
11%), with a corresponding increase in neutrophils (approx. 145%), a
usual reaction during inflammation. The compound did not appear to
affect blood chemistry, and the changes seen, such as a reduction in
protein (approx. 10%) and albumin (approx. 18%) concentrations in
animals at the highest dose, are regarded as consequences of the poor
condition and of the skin lesions. Male animals at 160 ppm also had a
reduced cholesterol concentration (24%) and a reduction in the protein
content of the urine, but females had an increased protein content
accompanied by a reduction in the volume of urine with a corresponding
increase in the density of the urine.
At necropsy, only skin lesions were found. In animals at the
highest dose, the marked differences in body weight resulted in
reductions in the weights of some organs and increases in the relative
weights. Animals at 160 ppm showed evidence of stimulation of
extramedullary haematopoiesis in the spleen and a reduction in stored
haemosiderin, which were considered to have been the result of the
blood losses described above. The reduction in the neutral fat content
of the liver and in the size of the seminal vesicles of animals at the
highest dose were considered to have been due to the poor condition of
the animals and not to be related directly to flumethrin. The NOAEL
was thus 10 ppm, equal to 0.7 mg/kg bw per day (Bomann & Sander,
1995).
Dogs
Groups of four male and four female beagle dogs, about eight
months old, received diets containing flumethrin (purity, 98.7%) to
provide concentrations of 0, 50, 100, or 200 ppm for 13 weeks. Animals
at doses > 50 ppm showed thinned hair or hairlessness, and in some
instances weeping, ulcerative, scabbed patches were seen on the neck,
back, tall, ears, and limbs. These lesions had partially healed by the
end of the study. The group at 200 ppm had reduced food intake and
body-weight gains. Those at 100 and 200 ppm had slightly raised blood
urea values, which were statistically significant in week 13 in
animals at the highest dose (6.4 versus 7.9 mmol/litre); however,
there was no evidence of gross pathological changes in the kidneys
(Hoffmann & Kaliner, 1984). There was no NOAEL in this study.
In a study to achieve a no-effect level that was not found in
the earlier study because of the presence of skin lesions, groups of
four male and four female beagle dogs about six months old received
diets containing flumethrin (same batch as used in the previous
study) providing concentrations of 0 or 25 ppm for 13 weeks. No
differences were observed between the two groups of dogs, although
histopathological examination was not performed since no histological
differences were observed in the previous study. In particular, no
skin lesions were detected. The NOAEL was 25 ppm, equal to 0.88 mg/kg
bw per day in males and 0.94 mg/kg bw per day in females (Hoffmann,
1985).
Cattle
A single dose of 50 ml of a 1% flumethrin formulation was
administered by gavage to two six-week-old calves, and one calf
received a placebo formulation. All three animals were observed for
five days. The two animals given the flumethrin formulation voided
watery faeces for a short time within the first 24 h, and their
food intake was lower in the first 48 h after administration. There
were no changes in body-weight development or in haematological,
clinicochemical, or urinary parameters, and no changes were seen in
the calf give the placebo. Thus, if an animal were to lick the product
off treated skin, the only effects likely to occur are mild and
reversible (Iida et al., 1988).
A dose of 4 mg/kg bw, i.e. twice the therapeutic dose, was
applied to the backs of 18 calves, which were then observed for the
next 2 h and daily for three weeks. The treatment was well tolerated,
and there were no clinical signs or local effects on the skin (Dorn,
1989a). In a similar study in which 4 mg/kg bw was applied to the
dorsal midline of 15 young bovines, there was no evidence of local or
systemic effects during daily observation for three weeks (Dorn,
1989b). When the same dose was applied to the backs of 13 pregnant
cows, the treatment had no effect on the animals' behaviour or general
health. There was also no evidence of an effect on labour or on the
calves, which were given a clinical examination at delivery and
thereafter at weekly intervals (Dorn, 1989c).
(c) Long-term toxicity and carcinogenicity
No studies of the long-term toxicity or carcinogenicity of
flumethrin have been conducted, but a two-year study was carried out
with flumethrin with a low trans-Z isomer content (purity, 91.3%) in
which groups of 50 male and 50 female Wistar BOR:WISW (SPF Cpb) rats
received diets containing providing concentrations of 0, 2, 10, 50,
or 250 ppm. An additional 10 rats of each sex per group were killed
after 12 months of treatment. No significant effects were seen on
growth, mortality, food or water consumption, or behaviour at doses
< 50 ppm. At 250 ppm, both male and female rats showed retarded
growth development, and their mean body weights were > 10% lower than
those of the controls at 50 weeks. At 103 weeks, the numbers of deaths
in the groups given 0, 2, 10, 50, and 250 ppm were 7, 5, 8, 7 and 3
males and 10, 11, 7, 9, and 19 females. Much of the increased
mortality among the female rats at 250 ppm was due to the fact that
several were killed in a moribund state or died from severe skin
lesions and the related poor general condition. These lesions
consisted of ulcerative dermatitis occurring at a dose-dependent
incidence and severity, which first appeared in the second week of
treatment. The maximal incidences were seen after four weeks. These
lesions later healed in females at 50 ppm and in males at 50 or
250 ppm. Some inconsistent, statistically significant changes were
observed on periodic haematological examination: the number of
polymorphonuclear neutrophils increased, mainly in males at 250 ppm,
and inconsistent increases were also seen in other groups. Slight
decreases in lymphocyte counts were observed almost exclusively in
males at 50 and 250 ppm after 26, 52, and 78 weeks, but the decreases
were not significant after 104 weeks. These may be nonspecific
reactions to the inflammatory changes in the skin and were similar to
those reported in the 15-week dietary study described above (Bomann &
Sander, 1995). Sporadic differences were observed in erythrocyte and
haemoglobin parameters, but these were inconsistent with respect to
dose and time and are not considered to be treatment-related.
While statistically significant differences between groups did
occur, studies of blood chemistry did not indicate any consistent,
dose-related changes in albumin, bilirubin, urea, creatinine,
cholesterol, glucose, or the activities of the plasma enzymes alkaline
phosphatase, alanine transaminase, aspartate transaminase, creatine
kinase, and lactate dehydrogenase. No biologically important changes
were seen in chloride (deviation from control, < 4%), and the
larger changes in phosphate ion concentrations did not show a
dose-response relationship. The sodium ion concentration was unaltered
and that of potassium showed dose-related increases in males only at
52 and 104 weeks, but not at 26 or 78 weeks. Similarly, decreases in
calcium ion in both males and females occurred at 52 and 104 weeks,
but not at 26 or 78 weeks. This erratic pattern of changes does not
indicate that they were the result of treatment. Urine analysis showed
no variations attributable to treatment in either male or female rats
at 26, 52, 78, or 104 weeks. At autopsy, no treatment-related changes
were observed, but one female at 250 ppm killed at 52 weeks had the
skin lesions described above.
The relative weights of the lungs and kidneys were increased in
both male and female rats at 250 ppm at the end of the study: lung,
368 mg/100 g bw in controls vs 407 in males at 250 ppm and 426 mg/
100 g bw vs 482 in females; kidney, 605 mg/100 g bw vs 658 at 250 ppm
in males and 665 mg/100 g bw vs 717 in females. The absolute weights
of these organs were not changed; the weights relative to brain weight
were not available because the brain was not weighed. Most of the
effects appeared to be due to reductions in total body weight in
animals at 250 ppm.
Histological examinations were restricted to the groups at 0 and
250 ppm groups. Few non-neoplastic histopathological findings were
considered to be treatment-related. Two male and five female rats had
skin ulcerations, the etiology of which was not established. Male
rats, but not females, also had slight proliferation of the bile duct,
the incidences after 104 weeks being 12/50 in controls, 17/50 animals
at 2 ppm, 12/50 of those at 10 ppm, 16/50 of those at 50 ppm, and
23/50 of those at 250 ppm. The incidences of neoplastic lesions were
not affected by treatment, the numbers of tumour-bearing rats per
group of 50 being 25 male controls and 11 at 2 ppm, five at 10 ppm,
eight at 50 ppm, and 15 at 250 ppm, and 31 female controls, 18 at
2 ppm, 19 at 10 ppm, 23 at 50 ppm, and 23 at 250 ppm. The incidences
of specific, more frequent tumours were similar in the controls and
animals at 250 ppm, with mammary carcinomas in 6/44 control females,
2/49 at 2 ppm, 0/47 at 10 ppm, 5/47 at 50 ppm, and 0/45 at 250 ppm;
mammary fibroadenomas were found in 2/44 controls, 5/49 at 2 ppm, 3/47
at 10 ppm, 4/47 at 50 ppm, and 4/45 at 250 ppm. Pituitary adenomas
were found in 14/39 controls, in 4/39 animals at 2 ppm (in 4/4 masses
subjected to histological examination), in 12/43 animals at 10 ppm
(12/12 masses), in 11/41 animals at 50 ppm (11/11 masses), and 6/30
animals at 250 ppm. The NOAEL was 10 ppm, equal to 0.5 mg/kg bw per
day in males and 0.6 mg/kg bw per day in females (Bomhard et al.,
1987, 1991).
(d) Reproductive toxicity
Rats
A two-generation study was conducted on groups of 30 male and 30
female Wistar/HAN rats [strain: Kfm: WIST (SPF)] fed diets containing
flumethrin (45.6% in Aerosil 200) at concentrations providing
concentrations of 0, 1, 5, or 50 ppm. The F0 generation received the
test diet for 84 days before mating and during the mating period,
gestation, and lactation until day 21 after littering. The F1
parental generation received the test diet from the age of four to
seven weeks for 105 days before mating and thereafter over the same
periods as the F0 generation. No effects of treatment with 1 or 5 ppm
were discernible in any generation. After treatment with 50 ppm, skin
lesions developed on male and female animals of the F0 generation and
on females of the F1 generation. Male rats at this dose showed a
reduction in food consumption before mating in the F0 generation and
in all phases of the study in the F1 generation; females had a
reduced food consumption in the F0 generation in all phases and in
the F1 generation during the two lactation phases. The body-weight
development of males of the F0 generation and of males and females of
the F1 generation was retarded in all phases. The reductions in food
consumption and body-weight gain were seen as early as the first week
of dosing before mating.
Survival in all F1 and F2 generations was lower during the
first four days after delivery, but the losses up to day 21 after
delivery were greatest in the F1b, F2a, and F2b generations. In
addition, the weight gain of the pups of the F1 and F2 generations
was retarded. A higher incidence of pups with a cramped or bent
posture, stiff limbs in caudal posture, and/or pectus carinatum was
seen, with hypothermia, and vocalization was more frequent. These
observations are probably secondary to the toxic effects on
the parents. No malformations were found. Investigations of the
haematological status of the F1 parental animals gave no indication
of treatment-related changes. The NOAEL was 5 ppm, equal to 0.36 mg/kg
bw per day for males and 0.40 mg/kg bw per day for females (Dotti et
al., 1992).
(e) Developmental toxicity
Rats
Groups of 28 female Charles River Crl:CD Br rats, 11 weeks old
when mated, were given flumethrin (purity, 93.5%) formulated as a
0.4 mg/ml solution in distilled water containing 5% Emulphor EL-719
and 5% ethanol, orally at doses of 0, 0.5, 1, or 2 mg/kg bw per day on
days 6-15 of gestation. Day 0 of gestation was defined as the day on
which spermatozoa were found in the vagina. The rats tolerated the
dose of 0.5 mg/kg bw per day, but doses > 1 mg/kg bw per day were
toxic to the dams, the effects including increased salivation and
lacrimation, reduced activity, ataxia, and ptosis. At 2 mg/kg bw per
day, there was a reduction in food intake during treatment and a
reduction in body-weight gain. There was no evidence of teratogenicity
at any dose and no embryotoxic or fetotoxic effects in animals at 0.5
or 1 mg/kg bw per day. Animals at 2 mg/kg bw per day had significant
reductions in placental weights (0.53 g vs 0.48 g in controls) and
fetal weights (3.8 g vs 3.4 g, sexes combined); they also had an
increased number of fetuses with reduced ossification of the skull
bones (42% vs 67%) and cervical vertebral arches (1% vs 16%). The
NOAEL was 0.5 mg/kg bw per day for maternal toxicity and 1 mg/kg bw
per day for developmental toxicity (Kowalski et al., 1987).
Rabbits
Groups of 17 American-Dutch rabbits, at least 4.5 months old when
they were artificially inseminated with semen from proven males,
received flumethrin (purity, 93.5%) formulated as a 0.4 mg/ml solution
in distilled water containing 5% Emulphor EL-719 and 5% ethanol orally
at doses of 0, 0.5, 1.7, or 6 mg/kg bw per day on days 7-19 of
gestation. The high dose was selected on the basis of a range-finding
study in pregnant rabbits (source not identified). Dosing at 0.5 or
1.7 mg/kg bw per day was tolerated with no adverse effects. Animals at
6 mg/kg bw per day had reduced food intake during treatment and
reduced body weights; reproductive function was not affected. At
6 mg/kg bw per day, a slight trend for a reduction in the weights of
the fetuses, particularly the females, was seen, but this was not
significant. There was no indication of teratogenic potential. The
NOAEL was 1.7 mg/kg bw per day (Clemens & Hartnagel, 1987).
(f) Genotoxicity
Flumethrin has been adequately tested for its ability to induce
point mutations, DNA damage, and clastogenicity (Table 2). An early
bacterial mutagenesis assay using four strains of Salmonella
typhimurium gave equivocal results in several strains and weakly
positive results in strain TA98, but the latter were not confirmed in
later experiments. Tests with the isolated trans-Z-1 and trans-Z-2
isomers in S. typhimurium also gave negative results. A small
increase in the frequency of chromosomal aberrations was observed in
Chinese hamster V79 cells 18 h after treatment with flumethrin in
the presence of an exogenous metabolic system. This effect was not
observed in an earlier experiment with human lymphocytes, and there
was no indication of chromosomal aberration induction in vivo, as
might be indicated by the results of tests for micronucleus formation
in bone-marrow cells of mice. In these and all other tests with
flumethrin, the results were clearly negative. The Meeting concluded
that flumethrin is not genotoxic.
(g) Special studies
(i) Dermal and ocular irritation and dermal sensitization
In tests for ocular and dermal (occluded patch) irritation in New
Zealand white rabbits, a 1% pour-on formulation of flumethrin produced
slight erythema of the conjunctivae for up to 48 h after treatment and
slight oedema of the conjunctivae for up to 24 h after treatment.
Dermal application induced pronounced reddening and some oedema after
24 h. These effects had largely resolved by 72 h (Schmidt, 1984a).
Two pour-on formulations of flumethrin were tested in New Zealand
white rabbits exposed for 4 h. One formulation was not irritating,
while the other was slightly irritating to the skin. Exposure for 24 h
resulted in irritation with both formulations (Pauluhn, 1985a).
In a test for irritation on the skin and the mucous membrane of
the eyes of New Zealand white rabbits, flumethrin was applied as a 10%
formulation in olive oil. No irritation of either skin (4-h exposure)
or eyes (24-h exposure) was observed (Krötlinger, 1994).
Flumethrin (purity, 88.3%) was tested for dermal sensitizing
activity in the Magnusson and Kligman maximization test on male
Bor:DHPW guinea-pigs. For intradermal induction, flumethrin was
administered as a 5% solution in PEG 400; for topical induction and
for challenge, it was administered as a 50% solution in PEG 400. There
were no post-challenge skin reactions (Diesing, 1991).
Table 2. Results of tests for the genotoxicity of flumethrin
End-point Test system Concentration Purity Results Reference
(%)
Without S9 With S9
Flumethrin
Reverse mutation S. typhimurium TA100 15 625 µg/plate 97.6 Inconclusive Negative Herbold (1984)
Reverse mutation S. typhimurium TA1535 15 625 µg/plate 97.6 Negative Inconclusive Herbold (1984)
Reverse mutation S. typhimurium TA1537 15 625 µg/plate 97.6 Inconclusive Negative Herbold (1984)
Reverse mutation S. typhimurium TA98 15 625 µg/plate 97.6 Inconclusive Weakly Herbold (1984)
positive
Reverse mutation S. typhimurium TA100 5000 µg/plate 94.6 Negative Negativea Gahlmann (1993a)
Reverse mutation S. typhimurium TA1535 5000 µg/plate 94.6 Negative Negativea Gahlmann (1993a)
Reverse mutation S. typhimurium TA1537 5000 µg/plate 94.6 Negative Negativea Gahlmann (1993a)
Reverse mutation S. typhimurium TA98 5000 µg/plate 94.6 Negative Negativea Gahlmann (1993a)
Reverse mutation S. typhimurium TA100 15 000 µg/plate 94.6 Negative Negative Gahlmann (1993a)
Reverse mutation S. typhimurium TA1535 15 000 µg/plate 94.6 Negative Negative Gahlmann (1993a)
Reverse mutation S. typhimurium TA1537 15 000 µg/plate 94.6 Negative Negative Gahlmann (1993a)
Reverse mutation S. typhimurium TA98 15 000 µg/plate 94.6 Negative Negative Gahlmann (1993a)
Reverse mutation S. cerevisiae D7 10 000 µg/ml 92.7 Negative Negative Herbold (1985a)
Cell mutation, tk Mouse lymphoma 1000 µg/ml 92.7 Negative Negative Cifone & Myhr
locus L5178Y cells (1985)
Cell mutation, hprt Chinese hamster lung 100 µg/ml 95.1 Inconclusive Negative Brendler-Schwaab
locus V79 cells (1995)
Unscheduled DNA Rat hepatocyte 300 µg/ml 94.6 Negative Not tested Brendler-Schwaab
synthesis primary culture (1994)
Chromosomal Chinese hamster lung 125 µg/ml 95.1 Negative Negative Herbold (1995a)
aberration V79 cells (18- and
30-h sampling)
Table 2. (Cont'd)
End-point Test system Concentration Purity Results Reference
(%)
Without S9 With S9
Chromosomal Human lymphocyte 1000 µg/ml 92.7 Negative Negative Herbold (1985b)
aberration primary culture (24-h
sampling)
Micronucleus Mouse bone marrow 50 mg/kg bw × 1 93.5 Negative Herbold (1986)
induction in vivo (24-, 48-, and orally
72-h sampling)
Micronucleus Mouse bone marrow 1000 mg/kg × 1 95.1 Negative Herbold (1995b)
induction in vivo (16-, 24-, and intraperitoneally
48-h sampling)
Flumethrin-trans-Z-1 isomer
Reverse mutation S. typhimurium TA98 15 000 µg/plate 98.3 Negative Negative Herbold (1990a)
Flumethrin-trans-Z-2-isomer
Reverse mutation S. typhimurium TA98 15 000 µg/plate 95.4 Negative Negative Herbold (1990b)
S9, 9000 × g supernatant of liver microsomes used as exogenous metabolic activation system
a An additional test in the presence of 30% S9; all bacterial studies include duplicate experiments with 10% S9
Repetition of this maximization test with another batch of
flumethrin (purity, 94.6), formulated in the same manner and at the
same concentrations as above but with an additional 25% concentration
for challenge, also gave no evidence of any potential for inducing
skin sensitization (Vohr, 1994).
(ii) Neurotoxicity
Male and female Wistar Bor: WISW rats received flumethrin by
gavage for 14 days at doses of 20 mg/kg bw for males and 10 mg/kg bw
for females for the first four days, but due to signs of severe
toxicity and one death, the doses for the remainder of the study were
reduced to 10 mg/kg bw for males and 5 mg/kg bw for females. The
treatment was followed by 31 days of observation. This study was
conducted because other pyrethroids such as cypermethrin, fenvalerate,
and permethrin cause slight axonal degeneration, mainly in peripheral
nerves, at highly toxic doses in rats (WHO, 1989, 1990a,b). Two to
three hours after the first administration, the rats showed apathy,
reduced motility, accelerated breathing, salivation, and head
twitching. Later in the study, spastic gait was also seen, and digging
and shaking movements replaced the head twitch. The intensity of these
symptoms had declined only marginally by 24 h. When the doses were
reduced, the symptoms moderated in some rats. Some symptoms persisted
for up to two days after the 14-day dosing period, but the surviving
rats had completely recovered by the end of the observation period.
Thus, the high doses used caused dysfunction of the nervous
system, the observable effects of which were fully reversible.
Histopathological examination of the central and peripheral nervous
system gave no evidence of neurotoxicity-related morphological damage
(Flucke & Schilde, 1988).
Single doses of 0, 10, 31.5, or 100 mg/kg bw flumethrin were
administered orally to male Bor: WISW rats and Bor: CF1 mice. No
muscle-relaxant, analgesic, anticonvulsant, or cataleptic effects were
seen in animals at any dose, using standard pharmacological tests.
There was no evidence of impairment of central coordination, function,
reflexes, or neuromuscular transmission in the rats. Flumethrin caused
moderate stimulation of spontaneous motor activity in mice at all
doses, and the degree of stimulation was statistically significant
among animals of 31.5 and 100 mg/kg bw. Orientational activity was
also inhibited in these animals. Those at 100 mg/kg bw showed slight
potentiation of the duration and depth of hexobarbital-induced
anaesthesia (Starke, 1985).
(iii) Anti-allergic and pseudo-allergic activity
Histamine release in rat peritoneal mast cells sensitized
passively by exposure to immunoglobulin E from mouse serum was not
affected by concentrations of 0, 10-7, 10-6, or 10-5 g/ml flumethrin
(Gardiner et al., 1985).
(iv) Bronchial activity
Flumethrin at concentrations of 10-9 to 10-5 g/ml had no effect
on leukotrine D4- or histamine-induced contraction in isolated
guinea-pig tracheas (Gardiner et al., 1985).
(v) Effect on concentration of glucose and triglycerides in blood
Single doses of 0, 10, 32, or 100 mg/kg bw flumethrin were
administered orally to fasting and fed rats. The concentrations of
glucose in the blood of fed animals at all doses and of fasted
animals at the lowest and highest dose were slightly (24-64%) but
significantly increased for dose-related times of 60-240 min. There
was no effect on the triglyceride concentration. As marked variations
in the blood glucose concentration are seen even under physiological
conditions, these increases are not considered to be of particular
relevance (Puls & Bischoff, 1985).
(vi) Effects on gastrointestinal tract of rats
Single doses of 0, 10, 30, or 100 mg/kg bw flumethrin were
administered orally to rats. At the highest dose, a statistically
significant increase in intestinal transit time was seen in the
charcoal propulsion test. No gastric lesions were observed at autopsy.
Intraduodenal administration of 10, 30, or 100 mg/kg bw caused a
slight, non-significant, not dose-related reduction in acid secretion
in the perfused stomach (to 79, 58, and 75%, respectively, of the
control level) (Bonabello et al., 1987).
(vii) Haematological and cardiovascular effects
Single doses of 0, 10, 32, or 100 mg/kg bw flumethrin were
administered orally to rats, and blood samples were taken 90 min
later. Neither coagulation, platelet aggregation, nor fibrinolysis was
affected (Seuter et al., 1985).
Flumethrin at doses of 10, 32, or 100 mg/kg bw administered
orally to anaesthetized dogs induced slight increases in heart rate,
unrelated to dose, in one of three animals at 10 and 100 mg/kg bw.
These increases were not considered to be related to treatment (Knorr,
1986).
(viii) Diuretic activity
Flumethrin at doses of 0, 10, 32, or 100 mg/kg bw was administered
orally to rats, and urine was collected over 6 h to determine sodium
and potassium concentrations. Clinical signs including increased
salivation and reduced motor activity were seen in a few treated
animals. None of the doses changed urine output or sodium excretion.
Potassium excretion was significantly increased by the 10 and 100 mg/kg
bw doses but not by 32 mg/kg bw. The variations were not considered to
be treatment-related (Hirth, 1985).
(ix) Toxicity of metabolites: Flumethrin acid
Flumethrin acid is the major metabolite of flumethrin in rats and
cattle. The oral LD50 of flumethrin acid in fasted rats was 935 mg/kg
bw (95% confidence interval [CI], 549-1594) in males and 620 mg/kg bw
(95% CI, 500-771) in females. The principal clinical signs were
piloerection, lethargy, reduced motor activity, staggering gait,
animals lying prone or on their side, atonia, and slow and laboured
breathing. After dermal application of 5000 mg/kg bw, none of the
animals died. The only clinical signs were lethargy and reduced motor
activity. Small lesions and slight hyperaemia were observed at the
application site. Exposure of male and female rats to 338 mg/m3 by
inhalation, the highest concentration of flumethrin acid that it was
technically feasible to generate, for 4 h was well tolerated, and no
clinical signs of toxicity were observed (Pauluhn, 1985b).
When rats were observed for possible effects on the central
nervous system, by measuring the angle at which they slip from a plane
before and at various times from 1 to 24 h after treatment with 0, 1,
3, or 10 mg/kg bw flumethrin, no treatment-related reduction in the
angle was observed (Bomann, 1995a). In tests for dermal and ocular
irritancy in rabbits, flumethrin acid had no effect (Pauluhn, 1985b).
In a test for reverse mutation in S. typhimurium TA98, flumethrin
acid showed no activity (Herbold, 1984a).
In a four-week study in which Wistar rats were given dietary
concentrations of 0, 30, 100, or 300 ppm flumethrin acid, no clinical
signs of toxicity or effects on food consumption or body-weight
development were seen. There was no evidence of treatment-related
changes in haematological, clinicochemical, or necroscopic findings.
No treatment-related histological changes were observed. The NOAEL was
thus 300 ppm, equivalent to 27 mg/kg bw, the highest dose tested
(Bomann, 1995b).
3. Observations in humans
No reported cases of systemic poisoning with flumethrin in humans
were available to the Meeting. There are, however, published reports
of cases of poisoning with other pyrethroids. In a review of 573
cases of poisoning with other alpha-cyano pyrethroids (delamethrin,
fenvalerate, and cypermethrin), poisoning was due to either incorrect
occupational use of the product, attempted suicide, or accidents. In
cases in which the product had come into contact with the skin, the
presentation comprised a burning sensation on the face, tingling,
papules, and dermatitis. In cases of mild poisoning, there was also
dizziness, headache, nausea, anorexia, and weakness. In cases of
moderate poisoning, the signs and symptoms were more intense, and
there were states of reduced consciousness and muscle twitching in
the extremities. Seven deaths are listed; two are attributable to
misdiagnosis and inappropriate treatment. Most patients recovered
within six days. In the more severe cases, recovery took up to 55
days. Treatment consisted of symptomatic and supportive measures.
There were no delayed complications (He et al., 1989). There is no
basis for considering that flumethrin would act differently from the
compounds studied.
Comments
Flumethrin was absorbed rapidly, but not completely, after oral
administration in all species investigated. The concentrations in the
tissues of rats two days after dosing were three- to 50-fold lower
than those in the blood; the lung contained higher concentrations
than other tissues, and the central nervous system had the lowest
concentrations. Excretion occurred primarily in the faeces. The main
metabolite was flumethrin acid, which was distinctly less toxic than
the parent substance in acute and four-week dietary studies in rats
and did not induce reverse mutation in bacteria.
The acute oral toxicity of flumethrin in laboratory animals is
moderate to low. The reported manifestations of its toxicity are
largely consistent with those known collectively as the choreoathetosis
with salivation (CS) syndrome, which is produced by other insecticidal
pyrethroids containing an alpha-cyano-3-phenoxybenzyl alcohol group.
After acute dermal application, the toxicity of flumethrin was low; the
clinical signs were the same as those seen after oral administration.
There was no evidence of acute toxicity after dermal application of
5 ml/kg bw of a 1% pour-on formulation. In tests for dermal and ocular
irritancy, the active substance proved not to be irritating. In tests
for local irritancy with the 1% pour-on formulation, slight, transient
skin changes (mainly barely perceptible erythema and/or swelling) were
seen, but no changes in the mucous membrane of the eye, were observed.
WHO has not classified flumethrin for acute toxicity.
After oral administration of flumethrin for three months to rats
at dietary concentrations of 0, 10, 40, or 160 ppm and to dogs at
dietary concentrations of 0, 25, 50, 100, or 200 ppm, the NOAELs were
10 ppm (equal to 0.7 mg/kg bw per day) for rats and 25 ppm (equal to
0.88 mg/kg bw per day) for dogs. In both species, the most obvious
findings were skin alterations, but these were not due to primary
dermatitis caused by flumethrin but to frequent scratching, with
attendant bleeding and, in some instances, inflammation. Alpha-cyano
pyrethroids are known to produce paraesthesia, which is considered
to be the most probable cause of the observed skin lesions. The
toxicological studies provided no evidence of immunotoxicity, e.g.
effects on leukocyte counts or on other relevant organs (thymus and
spleen).
The results of studies of developmental toxicity in rats at doses
of 0, 0.5, 1, or 2 mg/kg bw per day on days 6-15 of gestation and
rabbits at doses of 0, 0.5, 1.7, or 6 mg/kg bw per day on days 7-19 of
gestation provided no evidence that flumethrin is teratogenic at doses
extending into the range that is toxic to the dams. Some fetotoxicity
was observed at doses that also induced maternal toxicity in both
species. The NOAELs were 0.5 mg/kg bw per day for rats and 1.7 mg/kg
bw per day for rabbits.
A two-generation study of reproductive toxicity in rats exposed
to flumethrin at dietary concentrations of 0, 1, 5, or 50 ppm did not
indicate primary reproductive toxicity; the reduced pup survival and
body-weight gain and certain postural and behavioural changes in the
pups at the highest dose may have been secondary to maternal toxicity.
The NOAEL was 5 ppm, equal to 0.36 mg/kg bw per day.
No studies of long-term toxicity or carcinogenicity have been
conducted with the currently used isomeric mixture of flumethrin.
A two-year study was available, however, in which rats were fed
diets providing flumethrin with a low trans-Z content at dietary
concentrations of 0, 2, 10, 50, or 250 ppm. Skin lesions developed in
rats at 50 and 250 ppm, and there was slight proliferation of the bile
ducts in male rats at 250 ppm. Neither the number of tumour-bearing
rats nor the incidence of any specific neoplasm was increased.
The Meeting considered the following toxicological findings:
(i) Flumethrin with a low trans-Z content has no carcinogenic
potential. (ii) Other pyrethroids, such as cyhalothrin (WHO, 1990a),
cypermethrin (WHO, 1989a), fenvalerate (WHO, 1990d), and the related
resmethrins (WHO, 1989b) also have no carcinogenic potential.
(iii) Treatment with permethrin (WHO, 1990b) resulted in small
increases in the incidence of lung tumours in female mice in three
studies, but no increases were found in either rats or male mice.
(iv) Treatment with deltamethrin was associated with unspecified
thyroid adenomas in rats in one study, but no tumours were induced
in mice or in either species in other studies (WHO, 1990c).
(v) Flumethrin had no genotoxic potential in a number of well-conducted
tests covering a variety of end-points. (vi) Flumethrin showed no
sensitizing potential. (vii) No preneoplastic responses were seen in
studies of up to 13 weeks in duration. The Meeting considered that the
carcinogenic potential of the trans-Z isomers that are present in the
currently used isomeric mixture of flumethrin had been assessed in the
study in rats in which the low trans-Z product was tested.
Oral administration of highly toxic doses of flumethrin to rats
can cause dysfunction of the nervous system, but the effect is rapidly
reversible and is not accompanied by morphological damage to the
central or peripheral nervous system.
Pharmacological tests in experimental animals gave no evidence of
impairment of vital functions. Studies to establish the tolerance of
calves and cattle to flumethrin showed no significant effects, even
when animals licked the application site.
An ADI of 0-0.004 mg/kg bw was allocated, on the basis of the
NOAEL of 0.36 mg/kg bw per day in the two-generation study of
reproductive toxicity in rats, using a 100-fold safety factor.
Toxicological evaluation
Levels that cause no toxic effect
Rat: 10 ppm, equal to 0.7 mg/kg bw per day (13- and 15-week
studies of toxicity)
5 ppm, equal to 0.36 mg/kg bw per day (two-generation
study of reproductive toxicity)
0.5 mg/kg bw per day (maternal toxicity in a study of
developmental toxicity)
Rabbit: 1.7 mg/kg bw per day (maternal and fetal toxicity in a
study of developmental toxicity)
Dog: 25 ppm, equal to 0.88 mg/kg bw per day (13-week study
of toxicity)
Estimate of acceptable daily intake for humans
0-0.004 mg/kg bw
Studies that would provide information useful for continued evaluation
of the compound
Results of any studies that are planned or in progress in
rodents, dogs, or exposed human subjects
Toxicological criteria for estimating guidance values for dietary and non-dietary exposure to flumethrin
Exposure Relevant route, study type, species Results, remarks
Short-term (1-7 days) Oral, toxicity, rat LD50 = 41-3849 mg/kg bw, depending on the vehicle
Dermal, toxicity, rat LD50 > 2000 mg/kg bw
Inhalation, toxicity, rat LC50 = 225 mg/m3
Dermal, irritation, rabbit Not irritating
Ocular, irritation, rabbit Not irritating
Dermal, sensitization, guinea-pig Not sensitizing
Medium-term (1-26 weeks) Repeated oral, 15 weeks, toxicity, rat NOAEL = 0.7 mg/kg bw per day
Repeated oral, 13 weeks, toxicity, dog NOAEL = 0.88 mg/kg bw per day
Repeated oral, reproductive toxicity, rat NOAEL = 0.36 mg/kg bw per day, reduced body-weight
gain of adults
Repeated oral, developmental toxicity, rat NOAEL = 1 mg/kg bw per day, developmental toxicity
Repeated oral, developmental toxicity, rabbit NOAEL = 1.7 mg/kg bw per day, maternal and
developmental toxicity
Long-term (> 1 year) Repeated oral, 2 years, toxicity and NOAEL = 0.5 mg/kg bw per day, skin lesions;
carcinogenicity, rat no carcinogenicity
References
Anadón, A., Diez, M.J., Sierra, M., Sanchez, J.A. & Teran, M.T. (1988)
Microsomal enzyme induction by permethrin in rats. Vet. Hum. Toxicol.,
30, 309-312.
Anadón, A., Martinez-Larrańaga, M.R., Fernandez, M.C., Dķaz, M.J.
& Bringas, P. (1990) Effect of ciprofloxacin on antipyrine
pharmacokinetics and metabolism in rats. Antimicrob. Agents Chemother.
34, 2148-2151.
Anadón, A., Martinez-Larrańaga, M.R., Dķaz, M.J., Bringas, P.,
Fernandez, M.C., Martinez, M.A. & Fernandez-Cruz, M.L. (1995) Effects
of flumethrin on hepatic drug-metabolizing enzymes and antipyrine
disposition in rats. Toxicol. Appl. Pharmacol., 132, 14-18.
Bomann, W. (1992a) Bay Vq 1950 (Flumethrin). Investigations of acute
oral toxicity in rats. Report No. 20924, 7.1.1992. Submitted to WHO by
Bayer AG, Leverkusen, Germany.
Bomann, W. (1992b) Bayticol EC 6% (c.n.: Flumethrin). Investigations
of acute oral toxicity in rats. Report No. 20922, 7.1.1992. Submitted
to WHO by Bayer AG, Leverkusen, Germany.
Bomann, W. (1992c) Bayticol EC 6% Bayticol EC6% (c.n.: Flumethrin).
Investigations of acute oral toxicity in rats. Report No. 20923,
7.1.1992. Submitted to WHO by Bayer AG, Leverkusen, Germany.
Bomann, W. (1992d) Bayvarol-Strips (c.n.: Flumethrin). Untersuchungen
zur akuten oralen Toxizität an Ratten. Ber. No. 21093, 17.2.1992.
Submitted to WHO by Bayer AG, Leverkusen, Germany.
Bomann, W. (1992e) Bayvarol-Strips (c.n.: Flumethrin). Untersuch-
ungenzur akuten dermalen Toxizität an Ratten. Ber. No. 21094,
17.2.1992. Submitted to WHO by Bayer AG, Leverkusen, Germany.
Bomann, W. (1994a) Bayticol P (c.n.: Flumethrin). Study for acute oral
toxicity in rats. Report No. 23422, 19.10.1994. Submitted to WHO by
Bayer AG, Leverkusen, Germany.
Bomann, W. (1994b) Bayticol P (c.n.: Flumethrin). Study for acute
dermal toxicity in rats. Report No. 23421, 19.10.1994. Submitted to
WHO by Bayer AG, Leverkusen, Germany.
Bomann, W. (1994c) Bayticol P (c.n.: Flumethrin). Study for acute oral
toxicity of Bayticol P formulated in water/Cremophor EL and in milk in
female rats. Report No. 23493, 23.11.1994. Submitted to WHO by Bayer
AG, Leverkusen, Germany.
Bomann, W. (1995a) Bayticol acid (Bayticolsäure). Study for acute oral
toxicity in Wistar rats. Report No. 23863, 22.3.1995. Submitted to WHO
by Bayer AG, Leverkusen, Germany.
Bomann, W. (1995b) Bayticol acid (Bayticolsäure). Investigations of
subacute toxicity in Wistar rats (feeding study over 4 weeks). Report
No. 24057, 7.6.1995. Submitted to WHO by Bayer AG, Leverkusen,
Germany.
Bomann, W. & Sander, E. (1995) Bayticol P (c.n.: Flumethrin).
Investigations or subchronic toxicity in Wistar rats (feeding study
over 15 weeks). Report No. 23925, 11.4.1995. Submitted to WHO by Bayer
AG, Leverkusen, Germany.
Bomhard, E., Ramm, W. & Rühl-Fehlert, C. (1987) Flumethrin (BAY V1
6045) Studies on the chronic toxicity and carcinogenicity in Wistar
rats. Report No. 16245, 25.11.1987. Submitted to WHO by Bayer, AG,
Leverkusen, Germany.
Bomhard, E., Ramm, W. & Rühl-Fehlert, C. (1991) Flumethrin (BAY V1
6045) Studies on the chronic toxicity and carcinogenicity in Wistar
rats. Amendment. Report No. 16245a, 24.5.1991. Submitted to WHO by
Bayer, AG, Leverkusen, Germany.
Bonabello, A. & Grassi, A. (1987) Safety pharmacology of Bay Vq 1950
in the gastrointestinal tract: Its effect on intestinal charcoal
transit, on gastric tolerability and on basal gastric acid secretion
in rats. Berich No. 4137 (P), 23.7.1987. Submitted to WHO by Bayer,
AG, Leverkusen, Germany.
Brendler-Schwaab, S. (1994) Evaluation of Bayticol P in the rat
primary hepatocyte unscheduled DNA synthesis assay. Report No. 23461,
8.11.1994. Submitted to WHO by Bayer AG, Leverkusen, Germany.
Brendler-Schwaab, S. (1995) Flumethrin. Mutagenicity study for the
detection of induced forward mutation in the V79-HPRT assay in
vitro. Report No. 24162, 14.7.1995. Submitted to WHO by Bayer AG,
Leverkusen, Germany.
Cabral, J.R.P., Galendo, D., Laval, M. & Lyandrat, N. (1990)
Carcinogenicity studies with deltamethrin in mice and rats. Cancer
Lett., 49, 147-152.
Cameron, B. (1986) Bayticol, Bay Vq 1950. The pharmacokinetics and
tissue residues of [14C]-Bayticol in the lactating cow following
topical administration. Report No. 4108. Unpublished report from
Inveresk Research International Ltd, Musselburgh, Scotland. Submitted
to WHO by Bayer AG, Leverkusen, Germany.
Carlson, G.P. & Shoening, G.P. (1980) Induction of liver microsomal
NADPH cytochrome c reductase and cytochrome P450 by some new synthetic
pyrethroids. Toxicol. Appl. Pharmacol., 52, 507-512.
Cifone, M.A. & Myhr, B.C. (1985) Mutagenicity evaluation of Bay Vq
1950 in the mouse lymphoma forward mutation assay. Pharma-Bericht No.
3170 (P). Unpublished report from Litton Bionetics, Inc., Kensington,
MD, USA. Submitted to WHO by Bayer AG, Leverkusen, Germany.
Clemens, G.R. & Hartnagel, R.E. (1987) A teratology study in the
rabbit with Bay Vq 1950 (Bayticol-P). Report No. MTD 0021. Unpublished
report from Miles Laboratories, Inc., Elkhart, IN, USA. Submitted to
WHO by Bayer AG, Leverkusen, Germany.
Diesing, L. (1991) Bayticol. Investigations of the skin-sensitizing
action in the guinea pig (maximization test after Magnusson & Ligman).
Report No. 20542. Submitted to WHO by Bayer AG, Leverkusen, Germany.
Dorn, H. (1989a) To assess the safety of Bayticol pour on after dermal
application in cattle. Report No. 89/13401. Submitted to WHO by Bayer,
AG, Leverkusen, Germany.
Dorn, H. (1989b) To assess the safety of Bayticol pour on after dermal
application in cattle. Report No. 89/13395. Submitted to WHO by Bayer,
AG, Leverkusen, Germany.
Dorn, H. (1989c) To assess the tolerance of Bayticol pour on 1% in
pregnant cows. Report No. 89/13394. Submitted to WHO by Bayer, AG,
Leverkusen, Germany.
Dotti, A., Kinder, J., Biedermann, K., Luetkemeier, H., Wright, J. &
Terrier, C. (1992) Bay Vq 1950. Multiple generation reproduction study
in rats. Report No. R 5500. Unpublished report from Research and
Consulting Co., Itingen, Switzerland. Submitted to WHO by Bayer, AG,
Leverkusen, Germany.
Ecker, W. (1983) Biotransformation von [fluorbenzoiring-U-14C] Bay V1
6045 bei der Ratte. Unpublished Bayer AG Report No. 83/11072 (11545).
Submitted to WHO by Bayer, AG, Leverkusen, Germany.
Flucke, W. & Schilde, B. (1988) Bay Vq 1950 (c.n.: Flumethrin).
Studies of the neurotoxic effects on the peripheral and central
nervous system of rats following subacute oral administration. Report
No. 16983. Submitted to WHO by Bayer, AG, Leverkusen, Germany.
Gahlmann, R. (1993a) Flumethrin. Salmonella/Mikrosome test. Special
study. Report No. 22613. Submitted to WHO by Bayer AG, Leverkusen,
Germany.
Gahlmann, R. (1993b) Flumethrin. w/Mikrosome test. Special study.
Report No. 22614. Submitted to WHO by Bayer AG, Leverkusen, Germany.
Gardiner, P.J., Hammond, M.D. & Francis, D.L. (1985) BAY Vq 1950:
General/Safety respiratory pharmacology: I. Anti-allergic and
pseudo-allergic activity in rat peritoneal mast cells. II. Evaluation
of bronchoactivity in the guinea-pig isolated trachea. Report No. 3555
(P). Submitted to WHO by Bayer AG, Leverkusen, Germany.
Hahnemann, S. & Rühl, C.H. (1985) Bay Vq 1950 (BAY V1 6045p).
Subchronic toxicological investigations in rats. Feeding study over 13
weeks. Report No. 13658 (E). Submitted to WHO by Bayer AG, Leverkusen,
Germany.
He, F., Wang, S., Liu, L., Chen, S., Zhang, Z. & Sun, J. (1989)
Clinical manifestation and diagnosis of acute pyrethroid poisoning.
Arch. Toxicol., 63, 54-58.
Herbold, B. (1984) Bay Vq 1950. Salmonella/microsome test to evaluate
for point-mutagenic effects. Report No. 12904 (F), 30.8.1984. Submitted
to WHO by Bayer AG, Leverkusen, Germany.
Herbold, B. (1985a) Bay Vq 1950. Test for point-mutagenic effects on
S. cerevisiae D7. Report No. 13737 (P). Submitted to WHO by Bayer
AG, Leverkusen, Germany.
Herbold, B. (1985b) BAY Vq 1950. Cytogenetic investigations in human
lymphocyte cultures in vitro to check for chromosome damaging
activity (in vitro). Report No. 13167 (F). Submitted to WHO by Bayer
AG, Leverkusen, Germany.
Herbold, B. (1986) Bayticol P. Micronucleus test in mice to evaluate
for clastogenic activity. Report No. 14501. Submitted to WHO by Bayer
AG, Leverkusen, Germany.
Herbold, B. (1990a) Flumethrin- trans-Z-1-isomer. Salmonella/microsome
test using Salmonella TA98. Report No. 19573. Submitted to WHO by Bayer
AG, Leverkusen, Germany.
Herbold, B. (1990b) Flumethrin- trans-Z-2-isomer. Salmonella/microsome
test using Salmonella TA 98. Report No. 19572. Submitted to WHO by Bayer
AG, Leverkusen, Germany.
Herbold, B. (1995a) Flumethrin. In vitro mammalian chromosome
aberration test with Chinese hamster V79 cells. Report No. 24524.
Submitted to WHO by Bayer AG, Leverkusen, Germany.
Herbold, B. (1995b) Flumethrin. Micronucleus test on the mouse. Report
No. 23869. Submitted to WHO by Bayer AG, Leverkusen, Germany.
Hirth, C. (1985) Bay Vq 1950. Study of diuretic activity in rats.
Report No. 14092. Submitted to WHO by Bayer AG, Leverkusen, Germany.
Hoffmann, K. & Kaliner, G. (1984) Bay Vq 1950. Subchronic toxicity
study in dogs with oral administration (13 weeks feeding study).
Report No. 13155. Submitted to WHO by Bayer AG, Leverkusen, Germany.
Hoffmann, K. (1985) Bay Vq 1950. Subchronic toxicity study in dogs
with oral administration/supplementary study (13 weeks feeding study).
Report No. 13169. Submitted to WHO by Bayer AG, Leverkusen, Germany.
Iida, M., Akagi, H. & Hashizume, M. (1988) Study on safety of Bayticol
pour-on in calves. Rep. No. 89/13245. Submitted to WHO by Bayer AG,
Leverkusen, Germany.
Kaneko, H., Ohkawa, H. & Miyamoto, J. (1981) Comparative metabolism of
fenvalerate and the [2S, alpha S]-isomer in rats and mice. J. Pestic.
Sci., 6, 317-326.
Klein, O. (1993a) [Cl-Phenyl-U-14C] Flumethrin: Investigation of the
biokinetic behaviour and the metabolism in the rat. Bericht No.,
PF-3823, ME-16/93, KNO 58. Submitted to WHO by Bayer AG, Leverkusen,
Germany.
Klein, O. (1993b) [Cl-Phenyl-U-14C] Flumethrin: Investigations on the
distribution of the total radioactivity in the rat. Projekt-No.
M1840667. Report in preparation, 1995. Submitted to WHO by Bayer AG,
Leverkusen, Germany.
Klein, O. (1995) Metabolism of [14C]-Bayticol in the dairy cow and
male beef cattle. Project No. M1840667-2. Report No. MR 323/95 KNO68.
Submitted to WHO by Bayer AG, Leverkusen, Germany.
Knorr, A. (1986) Influence on hemodynamics and cardiac contractility
of anesthesized dogs after oral administration. Pharma-Bericht No.
Submitted to WHO by Bayer AG, Leverkusen, Germany.
Kowalski, R.L., Clemens, G.R. & Hartnagel, R.E. (1987) A teratology
study with Bayticol-P (Flumethrin = Bay Vq 1950) in the rat. R-Bericht
No. 3960. Unpublished report from Miles Laboratories, Inc., Elkhart,
IN, USA. Submitted to WHO by Bayer, AG, Leverkusen, Germany.
Krötlinger, F. (1994) Bayticol P. Study for skin and eye irritation/
corrosion in rabbits. Ber-No. 23559. Submitted to WHO by Bayer AG,
Leverkusen, Germany.
Pauluhn, J. (1985a) Bayticol P pour on 1%. Study on the irritancy/
corrosion potential on rabbit skin. Report No. 14033. Submitted to WHO
by Bayer AG, Leverkusen, Germany.
Pauluhn, J. (1985b) Bayticol-P-Säure. Untersuchungen zur Gewer-
betoxikologie. Ber-No. 13643. Submitted to WHO by Bayer AG, Leverkusen,
Germany.
Puls, W. & Bischoff, H. (1985) Bay Vq 1950. Influence of orally
administered Bay Vq 1950 on the blood glucose and serum triglyceride
concentrations in fed rats and fasted rats. Report No. 14366.
Submitted to WHO by Bayer AG, Leverkusen, Germany.
Renhof, M. (1983a) Bay Vq 1950 in peanut oil and miglyol. Acute oral
toxicity in the rat. Report No. 12047 (P). Submitted to WHO by Bayer
AG, Leverkusen, Germany.
Renhof, M. (1983b) Bay Vq 1950 in acetone/peanut oil 1:10. Acute oral
toxicity in the rat. Report No. 12048 (P). Submitted to WHO by Bayer
AG, Leverkusen, Germany.
Schmidt, M. (1984a) Bay Vq 1950 pour on 1%. Acute toxicity in the rat
and the mouse. Study of primary irritant/corrosive activity on rabbit
skin and eye. Report No. 12761 (P). Submitted to WHO by Bayer AG,
Leverkusen, Germany.
Schmidt, M. (1984b) Bay Vq 1950 pour on 1%. Acute toxicity in the rat
and mouse after intraperitoneal application. Report No. 12760 (P).
Submitted to WHO by Bayer AG, Leverkusen, Germany.
Seuter, F. & Perzborn, E. (1985) Blood-pharmacological investigations.
Report No. 14213. Submitted to WHO by Bayer AG, Leverkusen, Germany.
Starke, B. (1985) CNS safety pharmacology study with Bay Vq 1950
(Bayticol P) on oral administration. Pharma-Bericht No. 3541.
Submitted to WHO by Bayer AG, Leverkusen, Germany.
Steinke, W., Weber, H. & Suwelack, D. (1983) [14C] BAY V1 6045:
Pharmakokinetik in Ratten. PH-Report No. 11941. Submitted to WHO by
Bayer AG, Leverkusen, Germany.
Thyssen, J. (1982) Bayticol EC 7.5%. Investigation into the acute
toxicity on inhalation. Report No. 10946. Submitted to WHO by Bayer
AG, Leverkusen, Germany.
Vijversberg, H.P.M. & Van den Bercken, J. (1982) Action of pyrethroid
insecticides on the vertebrae nervous system. Neuropathol. Appl.
Neurobiol., 8, 421-440
Vohr, H.-W. (1994) Bayticol P. Investigations of skin sensitization in
guinea pigs (Magnusson & Kligman test). Report No. 23026. Submitted to
WHO by Bayer AG, Leverkusen, Germany.
WHO (1989a) Cypermethrin (Environmental Health Criteria 82),
International Programme on Chemical Safety, Geneva, 154 pp.
WHO (1989b) Resmethrins (Environmental Health Criteria 92),
International Programme on Chemical Safety, Geneva, 79 pp.
WHO (1990a) Cyhalothrin (Environmental Health Criteria 99),
International Programme on Chemical Safety, Geneva, 106 pp.
WHO (1990b) Deltamethrin (Environmental Health Criteria 97),
International Programme on Chemical Safety, Geneva, 133 pp.
WHO (1990c) Fenvalerate (Environmental Health Criteria 95),
International Programme on Chemical Safety, Geneva, 121 pp.
WHO (1990d) Permethrin (Environmental Health Criteria 94),
International Programme on Chemical Safety, Geneva, 125 pp.
In a study with [Cl-phenyl-U-14C]-flumethrin, a dose of
1 mg/kg bw was administered intravenously to one 545-kg dairy cow
and one 340-kg steer. Both animals were slaughtered 8 h after
administration, and the quantities of radiolabel present in all
excreta and in liver, kidneys, muscle, fat, and milk were measured.
The highest concentrations were found in the liver (cow, 13 µg/g;
steer, 3.5 µg/g), followed by the kidney (cow, 0.88 µg/g; steer,
1.4 µg/g). The metabolite flumethrin acid was also found in all the
materials examined, except the milk, and was present as the glucuronide
in the liver and kidneys. In milk, an additional degradation product
was found but not identified, which constituted 11.5% of the total
residues in milk (Klein, 1995).
(c) Effects on enzymes
Pyrethroids can interact with liver drug-metabolizing enzymes,
but there appears to be a difference between some Type II pyrethroids
that contain an alpha-cyano function and inhibit such enzymes, e.g.
deltamethrin (Anadón et al., 1990), and the Type I pyrethroids that
do not contain this function and may induce drug-metabolizing enzymes,
e.g. permethrin (Carlson & Shoening, 1980; Anadón et al., 1988). In
experiments in which groups of 12 male Wistar rats received flumethrin
by intraperitoneal injection for six days, the Type II pyrethroid
response was demonstrated, in that there were reductions in cytochrome
P450 protein content (36%), NADPH-cytochrome c reductase activity
(38%), aniline hydroxylase activity (52%), aminopyrine N-demethylase
activity (54%), and UDP-glucuronosyl transferase activity (34%)
(Anadón et al., 1995).
2. Toxicological studies
(a) Acute toxicity
The results of studies of the acute toxicity of flumethrin are
listed in Table 1. The acute toxicity is slightly greater in female
than male rats and depends on the vehicle used. The sex difference
may reflect the greater metabolic conversion in female rats,
and differences between the solvents in respect of polarity,
which determines the proportion of the dose adsorbed from the
gastrointestinal tract, may explain the differences in oral toxicity.
The formulation containing Cremophor EL, which is known to enhance
absorption, was markedly more toxic; however, this formulation is used
only for administration in toxicological studies. The acute toxicity
of flumethrin in the other vehicles was moderate to low.
Table 1. Acute toxicity of flumethrin and products
Formulation Route Species Sex LD50 Reference
(mg/kg bw)
Flumethrin in Oral Rat Male > 100 Bomann (1994a)
water/Cremophor EL Female > 100
Oral Rat Male 56 Bomann (1992a)
Female 41
Flumethrin in arachis oil Oral Rat Male
911 Renhof (1983a)
Female 662
Flumethrin in miglyol Oral Rat Male 3849 Renhof (1983a)
Female 2248
Flumethrin in 1:10 Oral Rat Male 302 Renhof (1983b)
acetone:arachis oil Female 138
Flumethrin in corn oil Dermal Rat Male > 2000 Bomann (1994b)
Female > 2000
1% pour-on formulation Oral Rat Male > 20 Schmidt (1984a)
Female > 20
Oral Mouse Male > 20 Schmidt (1984a)
Female > 20
Dermal Rat Male > 5 Schmidt (1984a)
Female > 5
Dermala Rat Male > 5 Schmidt (1984a)
Female > 5
Intraperitoneal Mouse Male approx. .1 Schmidt (1984b)
Female approx. 5
Bayticol EC 6% in Oral Rat Male > 500-< 2000 Bomann (1992b)
Solvesso 200 Female > 500-< 2000
Dermal Rat Male > 5000 Bomann (1992c)
Female > 5000
Table 1. (Cont'd)
Formulation Route Species Sex LD50 Reference
(mg/kg bw)
Bayvarol strips Oral Rat Male > 2000 Bomann (1992d)
(0.55 g Bayticol/100g) Female > 2000
Dermal Rat Male > 5000 Bomann (1992e)
Female > 5000
Bayticol EC 7.5% Inhalation Rat Male approx. 3000 Thyssen (1982)
(4 h) Female > 2934
a On scarified skin
After acute administration of flumethrin, the most prominent
clinical signs were manifestations of central nervous system toxicity,
such as reduced motor activity, respiratory disorders, altered
gait, and salivation. The onset of action occurred 1-15 min after
administration, and the effects were comparatively long-lasting. The
reported manifestations of toxicity are largely consistent with those
known collectively as the choreoathetosis (sinuous writhing) with
salivation syndrome, which is produced by other insecticidal
pyrethroids, classified on this basis as Type II pyrethroids, which
contain an alpha-cyano-2-phenoxybenzyl alcohol group (Vijversberg &
van den Bercken, 1982).
In the studies of acute toxicity, particular attention was paid
to effects on the central nervous system. In animals investigated for
behaviour on an inclined plane, flumethrin was administered orally
in a vehicle which enhances toxicity, i.e. as an emulsion in
water/Cremophor EL, and, for comparison, in milk, a vehicle of
relevance as regards the consumer. Doses of 5 mg/kg bw in both
formulations had a slight effect, i.e. the inclination of the plane at
which the dosed animals slipped off was lower than that at which the
control animals slipped off. A dose of 1 mg/kg bw, administered as an
emulsion in water/Cremophor, also had a very slight effect, but no
effect was seen with 0.3 mg/kg bw or with 1 mg/kg bw administered as
an emulsion in milk (Bomann, 1994c).
After dermal application, the LD50 was > 2000 mg/kg bw. The
clinical signs were comparable to those observed after oral
administration. Evidence of skin reactions, such as scaling and
incrustation and sometimes scratches, were found at the application
site (Bomann, 1994b).
Studies of flumethrin products (Table 1) did not indicate
potentiation by the other constituents of commercially available
formulations. The results obtained were within the respective ranges
calculated on the basis of the toxicity of the active ingredient.
Dermal application of a dose of 5 ml/kg bw of a pour-on formulation
had no systemic or local effects (Schmidt, 1984a), and intraperitoneal
administration of the 1% pour-on formulation to mice also showed
little toxicity (Schmidt, 1984b).
Deaths occurred after inhalation of the product Flumethrin EC
7.5% at a nominal concentration > 10 000 mg/m3 (Table 1). At
500 mg/m3, inhalation was tolerated with no adverse effects (Thyssen,
1982).
(b) Short-term toxicity
Rats
Groups of 15 male and 15 female Wistar BOR: WISW rats received
diets containing flumethrin (purity, 98.7%) to provide concentrations
of 0, 10, 50, or 250/150 ppm (150 ppm from the third week onwards) for
13 weeks. An additional 10 rats of each sex per group were killed
after four weeks. At concentrations > 50 ppm, skin lesions were
seen on the head, neck, shoulder girdle, and front extremities.
Although treatment was continued, these changes had cleared up in
about half of the animals by the end of the study. During the first
two weeks of the study, both food intake and water consumption of the
group at 250 ppm were depressed by about 40%; these effects were
accompanied by body-weight losses in animals of each sex. With the
change to a dose of 150 ppm, food and water consumption became
indistinguishable from the control values and there were no further
weight losses. Body weights remained slightly reduced throughout the
study in this group (terminal differences in comparison with controls
being about 9% in males and 8% in females). Unscheduled deaths
occurred only in animals at 250/150 ppm, four females and one male
dying during the first two weeks of treatment and another male during
week 5. The NOAEL was 10 ppm, equal to 0.7 mg/kg bw per day in males
and 0.8 mg/kg bw per day in females (Hahnemann & Rühl, 1985).
Groups of 20 male and 20 female Wistar BOR: WISW rats received
flumethrin (purity, 94.6%) in the diet at levels providing
concentrations of 0, 10, 40, or 160 ppm for 15 weeks. Dosing at 160
ppm resulted in a reduction in food intake, retardation of body-weight
development, and clinical signs, but none of the animals died. The
body weights of animals at 160 ppm were 24% lower than those of
controls for males and 8% for females, but there were no significant
differences in the other groups. The principal clinical signs in
animals at 160 ppm were piloerection, increases or decreases in motor
activity, and spastic or staggering gait. Immediately after the start
of dosing, the animals at this dose were also seen to groom their fur
intensively and in particular to make frequent scratching movements.
This produced skin lesions, some of which were several centimeters in
diameter and bled after being scratched repeatedly. A small proportion
of these skin lesions, and similar ones found on a few animals at
40 ppm, healed as the study progressed. Alpha-cyano pyrethroids are
known to have a paraesthetic effect, which is regarded as the most
probable cause of the skin lesions. Correlated with the presence of
skin lesions on particular rats at this dose at the end of the study
were reductions in erythrocyte count (approx. 16%), haematocrit
(approx. 12%), and haemoglobin concentration (approx. 14%) and an
increase in the leukocyte count (approx. 50%). The differential blood
count showed a reduction in the proportion of lymphocytes (approx.
11%), with a corresponding increase in neutrophils (approx. 145%), a
usual reaction during inflammation. The compound did not appear to
affect blood chemistry, and the changes seen, such as a reduction in
protein (approx. 10%) and albumin (approx. 18%) concentrations in
animals at the highest dose, are regarded as consequences of the poor
condition and of the skin lesions. Male animals at 160 ppm also had a
reduced cholesterol concentration (24%) and a reduction in the protein
content of the urine, but females had an increased protein content
accompanied by a reduction in the volume of urine with a corresponding
increase in the density of the urine.
At necropsy, only skin lesions were found. In animals at the
highest dose, the marked differences in body weight resulted in
reductions in the weights of some organs and increases in the relative
weights. Animals at 160 ppm showed evidence of stimulation of
extramedullary haematopoiesis in the spleen and a reduction in stored
haemosiderin, which were considered to have been the result of the
blood losses described above. The reduction in the neutral fat content
of the liver and in the size of the seminal vesicles of animals at the
highest dose were considered to have been due to the poor condition of
the animals and not to be related directly to flumethrin. The NOAEL
was thus 10 ppm, equal to 0.7 mg/kg bw per day (Bomann & Sander,
1995).
Dogs
Groups of four male and four female beagle dogs, about eight
months old, received diets containing flumethrin (purity, 98.7%) to
provide concentrations of 0, 50, 100, or 200 ppm for 13 weeks. Animals
at doses > 50 ppm showed thinned hair or hairlessness, and in some
instances weeping, ulcerative, scabbed patches were seen on the neck,
back, tall, ears, and limbs. These lesions had partially healed by the
end of the study. The group at 200 ppm had reduced food intake and
body-weight gains. Those at 100 and 200 ppm had slightly raised blood
urea values, which were statistically significant in week 13 in
animals at the highest dose (6.4 versus 7.9 mmol/litre); however,
there was no evidence of gross pathological changes in the kidneys
(Hoffmann & Kaliner, 1984). There was no NOAEL in this study.
In a study to achieve a no-effect level that was not found in
the earlier study because of the presence of skin lesions, groups of
four male and four female beagle dogs about six months old received
diets containing flumethrin (same batch as used in the previous
study) providing concentrations of 0 or 25 ppm for 13 weeks. No
differences were observed between the two groups of dogs, although
histopathological examination was not performed since no histological
differences were observed in the previous study. In particular, no
skin lesions were detected. The NOAEL was 25 ppm, equal to 0.88 mg/kg
bw per day in males and 0.94 mg/kg bw per day in females (Hoffmann,
1985).
Cattle
A single dose of 50 ml of a 1% flumethrin formulation was
administered by gavage to two six-week-old calves, and one calf
received a placebo formulation. All three animals were observed for
five days. The two animals given the flumethrin formulation voided
watery faeces for a short time within the first 24 h, and their
food intake was lower in the first 48 h after administration. There
were no changes in body-weight development or in haematological,
clinicochemical, or urinary parameters, and no changes were seen in
the calf give the placebo. Thus, if an animal were to lick the product
off treated skin, the only effects likely to occur are mild and
reversible (Iida et al., 1988).
A dose of 4 mg/kg bw, i.e. twice the therapeutic dose, was
applied to the backs of 18 calves, which were then observed for the
next 2 h and daily for three weeks. The treatment was well tolerated,
and there were no clinical signs or local effects on the skin (Dorn,
1989a). In a similar study in which 4 mg/kg bw was applied to the
dorsal midline of 15 young bovines, there was no evidence of local or
systemic effects during daily observation for three weeks (Dorn,
1989b). When the same dose was applied to the backs of 13 pregnant
cows, the treatment had no effect on the animals' behaviour or general
health. There was also no evidence of an effect on labour or on the
calves, which were given a clinical examination at delivery and
thereafter at weekly intervals (Dorn, 1989c).
(c) Long-term toxicity and carcinogenicity
No studies of the long-term toxicity or carcinogenicity of
flumethrin have been conducted, but a two-year study was carried out
with flumethrin with a low trans-Z isomer content (purity, 91.3%) in
which groups of 50 male and 50 female Wistar BOR:WISW (SPF Cpb) rats
received diets containing providing concentrations of 0, 2, 10, 50,
or 250 ppm. An additional 10 rats of each sex per group were killed
after 12 months of treatment. No significant effects were seen on
growth, mortality, food or water consumption, or behaviour at doses
< 50 ppm. At 250 ppm, both male and female rats showed retarded
growth development, and their mean body weights were > 10% lower than
those of the controls at 50 weeks. At 103 weeks, the numbers of deaths
in the groups given 0, 2, 10, 50, and 250 ppm were 7, 5, 8, 7 and 3
males and 10, 11, 7, 9, and 19 females. Much of the increased
mortality among the female rats at 250 ppm was due to the fact that
several were killed in a moribund state or died from severe skin
lesions and the related poor general condition. These lesions
consisted of ulcerative dermatitis occurring at a dose-dependent
incidence and severity, which first appeared in the second week of
treatment. The maximal incidences were seen after four weeks. These
lesions later healed in females at 50 ppm and in males at 50 or
250 ppm. Some inconsistent, statistically significant changes were
observed on periodic haematological examination: the number of
polymorphonuclear neutrophils increased, mainly in males at 250 ppm,
and inconsistent increases were also seen in other groups. Slight
decreases in lymphocyte counts were observed almost exclusively in
males at 50 and 250 ppm after 26, 52, and 78 weeks, but the decreases
were not significant after 104 weeks. These may be nonspecific
reactions to the inflammatory changes in the skin and were similar to
those reported in the 15-week dietary study described above (Bomann &
Sander, 1995). Sporadic differences were observed in erythrocyte and
haemoglobin parameters, but these were inconsistent with respect to
dose and time and are not considered to be treatment-related.
While statistically significant differences between groups did
occur, studies of blood chemistry did not indicate any consistent,
dose-related changes in albumin, bilirubin, urea, creatinine,
cholesterol, glucose, or the activities of the plasma enzymes alkaline
phosphatase, alanine transaminase, aspartate transaminase, creatine
kinase, and lactate dehydrogenase. No biologically important changes
were seen in chloride (deviation from control, < 4%), and the
larger changes in phosphate ion concentrations did not show a
dose-response relationship. The sodium ion concentration was unaltered
and that of potassium showed dose-related increases in males only at
52 and 104 weeks, but not at 26 or 78 weeks. Similarly, decreases in
calcium ion in both males and females occurred at 52 and 104 weeks,
but not at 26 or 78 weeks. This erratic pattern of changes does not
indicate that they were the result of treatment. Urine analysis showed
no variations attributable to treatment in either male or female rats
at 26, 52, 78, or 104 weeks. At autopsy, no treatment-related changes
were observed, but one female at 250 ppm killed at 52 weeks had the
skin lesions described above.
The relative weights of the lungs and kidneys were increased in
both male and female rats at 250 ppm at the end of the study: lung,
368 mg/100 g bw in controls vs 407 in males at 250 ppm and 426 mg/
100 g bw vs 482 in females; kidney, 605 mg/100 g bw vs 658 at 250 ppm
in males and 665 mg/100 g bw vs 717 in females. The absolute weights
of these organs were not changed; the weights relative to brain weight
were not available because the brain was not weighed. Most of the
effects appeared to be due to reductions in total body weight in
animals at 250 ppm.
Histological examinations were restricted to the groups at 0 and
250 ppm groups. Few non-neoplastic histopathological findings were
considered to be treatment-related. Two male and five female rats had
skin ulcerations, the etiology of which was not established. Male
rats, but not females, also had slight proliferation of the bile duct,
the incidences after 104 weeks being 12/50 in controls, 17/50 animals
at 2 ppm, 12/50 of those at 10 ppm, 16/50 of those at 50 ppm, and
23/50 of those at 250 ppm. The incidences of neoplastic lesions were
not affected by treatment, the numbers of tumour-bearing rats per
group of 50 being 25 male controls and 11 at 2 ppm, five at 10 ppm,
eight at 50 ppm, and 15 at 250 ppm, and 31 female controls, 18 at
2 ppm, 19 at 10 ppm, 23 at 50 ppm, and 23 at 250 ppm. The incidences
of specific, more frequent tumours were similar in the controls and
animals at 250 ppm, with mammary carcinomas in 6/44 control females,
2/49 at 2 ppm, 0/47 at 10 ppm, 5/47 at 50 ppm, and 0/45 at 250 ppm;
mammary fibroadenomas were found in 2/44 controls, 5/49 at 2 ppm, 3/47
at 10 ppm, 4/47 at 50 ppm, and 4/45 at 250 ppm. Pituitary adenomas
were found in 14/39 controls, in 4/39 animals at 2 ppm (in 4/4 masses
subjected to histological examination), in 12/43 animals at 10 ppm
(12/12 masses), in 11/41 animals at 50 ppm (11/11 masses), and 6/30
animals at 250 ppm. The NOAEL was 10 ppm, equal to 0.5 mg/kg bw per
day in males and 0.6 mg/kg bw per day in females (Bomhard et al.,
1987, 1991).
(d) Reproductive toxicity
Rats
A two-generation study was conducted on groups of 30 male and 30
female Wistar/HAN rats [strain: Kfm: WIST (SPF)] fed diets containing
flumethrin (45.6% in Aerosil 200) at concentrations providing
concentrations of 0, 1, 5, or 50 ppm. The F0 generation received the
test diet for 84 days before mating and during the mating period,
gestation, and lactation until day 21 after littering. The F1
parental generation received the test diet from the age of four to
seven weeks for 105 days before mating and thereafter over the same
periods as the F0 generation. No effects of treatment with 1 or 5 ppm
were discernible in any generation. After treatment with 50 ppm, skin
lesions developed on male and female animals of the F0 generation and
on females of the F1 generation. Male rats at this dose showed a
reduction in food consumption before mating in the F0 generation and
in all phases of the study in the F1 generation; females had a
reduced food consumption in the F0 generation in all phases and in
the F1 generation during the two lactation phases. The body-weight
development of males of the F0 generation and of males and females of
the F1 generation was retarded in all phases. The reductions in food
consumption and body-weight gain were seen as early as the first week
of dosing before mating.
Survival in all F1 and F2 generations was lower during the
first four days after delivery, but the losses up to day 21 after
delivery were greatest in the F1b, F2a, and F2b generations. In
addition, the weight gain of the pups of the F1 and F2 generations
was retarded. A higher incidence of pups with a cramped or bent
posture, stiff limbs in caudal posture, and/or pectus carinatum was
seen, with hypothermia, and vocalization was more frequent. These
observations are probably secondary to the toxic effects on
the parents. No malformations were found. Investigations of the
haematological status of the F1 parental animals gave no indication
of treatment-related changes. The NOAEL was 5 ppm, equal to 0.36 mg/kg
bw per day for males and 0.40 mg/kg bw per day for females (Dotti et
al., 1992).
(e) Developmental toxicity
Rats
Groups of 28 female Charles River Crl:CD Br rats, 11 weeks old
when mated, were given flumethrin (purity, 93.5%) formulated as a
0.4 mg/ml solution in distilled water containing 5% Emulphor EL-719
and 5% ethanol, orally at doses of 0, 0.5, 1, or 2 mg/kg bw per day on
days 6-15 of gestation. Day 0 of gestation was defined as the day on
which spermatozoa were found in the vagina. The rats tolerated the
dose of 0.5 mg/kg bw per day, but doses > 1 mg/kg bw per day were
toxic to the dams, the effects including increased salivation and
lacrimation, reduced activity, ataxia, and ptosis. At 2 mg/kg bw per
day, there was a reduction in food intake during treatment and a
reduction in body-weight gain. There was no evidence of teratogenicity
at any dose and no embryotoxic or fetotoxic effects in animals at 0.5
or 1 mg/kg bw per day. Animals at 2 mg/kg bw per day had significant
reductions in placental weights (0.53 g vs 0.48 g in controls) and
fetal weights (3.8 g vs 3.4 g, sexes combined); they also had an
increased number of fetuses with reduced ossification of the skull
bones (42% vs 67%) and cervical vertebral arches (1% vs 16%). The
NOAEL was 0.5 mg/kg bw per day for maternal toxicity and 1 mg/kg bw
per day for developmental toxicity (Kowalski et al., 1987).
Rabbits
Groups of 17 American-Dutch rabbits, at least 4.5 months old when
they were artificially inseminated with semen from proven males,
received flumethrin (purity, 93.5%) formulated as a 0.4 mg/ml solution
in distilled water containing 5% Emulphor EL-719 and 5% ethanol orally
at doses of 0, 0.5, 1.7, or 6 mg/kg bw per day on days 7-19 of
gestation. The high dose was selected on the basis of a range-finding
study in pregnant rabbits (source not identified). Dosing at 0.5 or
1.7 mg/kg bw per day was tolerated with no adverse effects. Animals at
6 mg/kg bw per day had reduced food intake during treatment and
reduced body weights; reproductive function was not affected. At
6 mg/kg bw per day, a slight trend for a reduction in the weights of
the fetuses, particularly the females, was seen, but this was not
significant. There was no indication of teratogenic potential. The
NOAEL was 1.7 mg/kg bw per day (Clemens & Hartnagel, 1987).
(f) Genotoxicity
Flumethrin has been adequately tested for its ability to induce
point mutations, DNA damage, and clastogenicity (Table 2). An early
bacterial mutagenesis assay using four strains of Salmonella
typhimurium gave equivocal results in several strains and weakly
positive results in strain TA98, but the latter were not confirmed in
later experiments. Tests with the isolated trans-Z-1 and trans-Z-2
isomers in S. typhimurium also gave negative results. A small
increase in the frequency of chromosomal aberrations was observed in
Chinese hamster V79 cells 18 h after treatment with flumethrin in
the presence of an exogenous metabolic system. This effect was not
observed in an earlier experiment with human lymphocytes, and there
was no indication of chromosomal aberration induction in vivo, as
might be indicated by the results of tests for micronucleus formation
in bone-marrow cells of mice. In these and all other tests with
flumethrin, the results were clearly negative. The Meeting concluded
that flumethrin is not genotoxic.
(g) Special studies
(i) Dermal and ocular irritation and dermal sensitization
In tests for ocular and dermal (occluded patch) irritation in New
Zealand white rabbits, a 1% pour-on formulation of flumethrin produced
slight erythema of the conjunctivae for up to 48 h after treatment and
slight oedema of the conjunctivae for up to 24 h after treatment.
Dermal application induced pronounced reddening and some oedema after
24 h. These effects had largely resolved by 72 h (Schmidt, 1984a).
Two pour-on formulations of flumethrin were tested in New Zealand
white rabbits exposed for 4 h. One formulation was not irritating,
while the other was slightly irritating to the skin. Exposure for 24 h
resulted in irritation with both formulations (Pauluhn, 1985a).
In a test for irritation on the skin and the mucous membrane of
the eyes of New Zealand white rabbits, flumethrin was applied as a 10%
formulation in olive oil. No irritation of either skin (4-h exposure)
or eyes (24-h exposure) was observed (Krötlinger, 1994).
Flumethrin (purity, 88.3%) was tested for dermal sensitizing
activity in the Magnusson and Kligman maximization test on male
Bor:DHPW guinea-pigs. For intradermal induction, flumethrin was
administered as a 5% solution in PEG 400; for topical induction and
for challenge, it was administered as a 50% solution in PEG 400. There
were no post-challenge skin reactions (Diesing, 1991).
Table 2. Results of tests for the genotoxicity of flumethrin
End-point Test system Concentration Purity Results Reference
(%)
Without S9 With S9
Flumethrin
Reverse mutation S. typhimurium TA100 15 625 µg/plate 97.6 Inconclusive Negative Herbold (1984)
Reverse mutation S. typhimurium TA1535 15 625 µg/plate 97.6 Negative Inconclusive Herbold (1984)
Reverse mutation S. typhimurium TA1537 15 625 µg/plate 97.6 Inconclusive Negative Herbold (1984)
Reverse mutation S. typhimurium TA98 15 625 µg/plate 97.6 Inconclusive Weakly Herbold (1984)
positive
Reverse mutation S. typhimurium TA100 5000 µg/plate 94.6 Negative Negativea Gahlmann (1993a)
Reverse mutation S. typhimurium TA1535 5000 µg/plate 94.6 Negative Negativea Gahlmann (1993a)
Reverse mutation S. typhimurium TA1537 5000 µg/plate 94.6 Negative Negativea Gahlmann (1993a)
Reverse mutation S. typhimurium TA98 5000 µg/plate 94.6 Negative Negativea Gahlmann (1993a)
Reverse mutation S. typhimurium TA100 15 000 µg/plate 94.6 Negative Negative Gahlmann (1993a)
Reverse mutation S. typhimurium TA1535 15 000 µg/plate 94.6 Negative Negative Gahlmann (1993a)
Reverse mutation S. typhimurium TA1537 15 000 µg/plate 94.6 Negative Negative Gahlmann (1993a)
Reverse mutation S. typhimurium TA98 15 000 µg/plate 94.6 Negative Negative Gahlmann (1993a)
Reverse mutation S. cerevisiae D7 10 000 µg/ml 92.7 Negative Negative Herbold (1985a)
Cell mutation, tk Mouse lymphoma 1000 µg/ml 92.7 Negative Negative Cifone & Myhr
locus L5178Y cells (1985)
Cell mutation, hprt Chinese hamster lung 100 µg/ml 95.1 Inconclusive Negative Brendler-Schwaab
locus V79 cells (1995)
Unscheduled DNA Rat hepatocyte 300 µg/ml 94.6 Negative Not tested Brendler-Schwaab
synthesis primary culture (1994)
Chromosomal Chinese hamster lung 125 µg/ml 95.1 Negative Negative Herbold (1995a)
aberration V79 cells (18- and
30-h sampling)
Table 2. (Cont'd)
End-point Test system Concentration Purity Results Reference
(%)
Without S9 With S9
Chromosomal Human lymphocyte 1000 µg/ml 92.7 Negative Negative Herbold (1985b)
aberration primary culture (24-h
sampling)
Micronucleus Mouse bone marrow 50 mg/kg bw × 1 93.5 Negative Herbold (1986)
induction in vivo (24-, 48-, and orally
72-h sampling)
Micronucleus Mouse bone marrow 1000 mg/kg × 1 95.1 Negative Herbold (1995b)
induction in vivo (16-, 24-, and intraperitoneally
48-h sampling)
Flumethrin-trans-Z-1 isomer
Reverse mutation S. typhimurium TA98 15 000 µg/plate 98.3 Negative Negative Herbold (1990a)
Flumethrin-trans-Z-2-isomer
Reverse mutation S. typhimurium TA98 15 000 µg/plate 95.4 Negative Negative Herbold (1990b)
S9, 9000 × g supernatant of liver microsomes used as exogenous metabolic activation system
a An additional test in the presence of 30% S9; all bacterial studies include duplicate experiments with 10% S9
Repetition of this maximization test with another batch of
flumethrin (purity, 94.6), formulated in the same manner and at the
same concentrations as above but with an additional 25% concentration
for challenge, also gave no evidence of any potential for inducing
skin sensitization (Vohr, 1994).
(ii) Neurotoxicity
Male and female Wistar Bor: WISW rats received flumethrin by
gavage for 14 days at doses of 20 mg/kg bw for males and 10 mg/kg bw
for females for the first four days, but due to signs of severe
toxicity and one death, the doses for the remainder of the study were
reduced to 10 mg/kg bw for males and 5 mg/kg bw for females. The
treatment was followed by 31 days of observation. This study was
conducted because other pyrethroids such as cypermethrin, fenvalerate,
and permethrin cause slight axonal degeneration, mainly in peripheral
nerves, at highly toxic doses in rats (WHO, 1989, 1990a,b). Two to
three hours after the first administration, the rats showed apathy,
reduced motility, accelerated breathing, salivation, and head
twitching. Later in the study, spastic gait was also seen, and digging
and shaking movements replaced the head twitch. The intensity of these
symptoms had declined only marginally by 24 h. When the doses were
reduced, the symptoms moderated in some rats. Some symptoms persisted
for up to two days after the 14-day dosing period, but the surviving
rats had completely recovered by the end of the observation period.
Thus, the high doses used caused dysfunction of the nervous
system, the observable effects of which were fully reversible.
Histopathological examination of the central and peripheral nervous
system gave no evidence of neurotoxicity-related morphological damage
(Flucke & Schilde, 1988).
Single doses of 0, 10, 31.5, or 100 mg/kg bw flumethrin were
administered orally to male Bor: WISW rats and Bor: CF1 mice. No
muscle-relaxant, analgesic, anticonvulsant, or cataleptic effects were
seen in animals at any dose, using standard pharmacological tests.
There was no evidence of impairment of central coordination, function,
reflexes, or neuromuscular transmission in the rats. Flumethrin caused
moderate stimulation of spontaneous motor activity in mice at all
doses, and the degree of stimulation was statistically significant
among animals of 31.5 and 100 mg/kg bw. Orientational activity was
also inhibited in these animals. Those at 100 mg/kg bw showed slight
potentiation of the duration and depth of hexobarbital-induced
anaesthesia (Starke, 1985).
(iii) Anti-allergic and pseudo-allergic activity
Histamine release in rat peritoneal mast cells sensitized
passively by exposure to immunoglobulin E from mouse serum was not
affected by concentrations of 0, 10-7, 10-6, or 10-5 g/ml flumethrin
(Gardiner et al., 1985).
(iv) Bronchial activity
Flumethrin at concentrations of 10-9 to 10-5 g/ml had no effect
on leukotrine D4- or histamine-induced contraction in isolated
guinea-pig tracheas (Gardiner et al., 1985).
(v) Effect on concentration of glucose and triglycerides in blood
Single doses of 0, 10, 32, or 100 mg/kg bw flumethrin were
administered orally to fasting and fed rats. The concentrations of
glucose in the blood of fed animals at all doses and of fasted
animals at the lowest and highest dose were slightly (24-64%) but
significantly increased for dose-related times of 60-240 min. There
was no effect on the triglyceride concentration. As marked variations
in the blood glucose concentration are seen even under physiological
conditions, these increases are not considered to be of particular
relevance (Puls & Bischoff, 1985).
(vi) Effects on gastrointestinal tract of rats
Single doses of 0, 10, 30, or 100 mg/kg bw flumethrin were
administered orally to rats. At the highest dose, a statistically
significant increase in intestinal transit time was seen in the
charcoal propulsion test. No gastric lesions were observed at autopsy.
Intraduodenal administration of 10, 30, or 100 mg/kg bw caused a
slight, non-significant, not dose-related reduction in acid secretion
in the perfused stomach (to 79, 58, and 75%, respectively, of the
control level) (Bonabello et al., 1987).
(vii) Haematological and cardiovascular effects
Single doses of 0, 10, 32, or 100 mg/kg bw flumethrin were
administered orally to rats, and blood samples were taken 90 min
later. Neither coagulation, platelet aggregation, nor fibrinolysis was
affected (Seuter et al., 1985).
Flumethrin at doses of 10, 32, or 100 mg/kg bw administered
orally to anaesthetized dogs induced slight increases in heart rate,
unrelated to dose, in one of three animals at 10 and 100 mg/kg bw.
These increases were not considered to be related to treatment (Knorr,
1986).
(viii) Diuretic activity
Flumethrin at doses of 0, 10, 32, or 100 mg/kg bw was administered
orally to rats, and urine was collected over 6 h to determine sodium
and potassium concentrations. Clinical signs including increased
salivation and reduced motor activity were seen in a few treated
animals. None of the doses changed urine output or sodium excretion.
Potassium excretion was significantly increased by the 10 and 100 mg/kg
bw doses but not by 32 mg/kg bw. The variations were not considered to
be treatment-related (Hirth, 1985).
(ix) Toxicity of metabolites: Flumethrin acid
Flumethrin acid is the major metabolite of flumethrin in rats and
cattle. The oral LD50 of flumethrin acid in fasted rats was 935 mg/kg
bw (95% confidence interval [CI], 549-1594) in males and 620 mg/kg bw
(95% CI, 500-771) in females. The principal clinical signs were
piloerection, lethargy, reduced motor activity, staggering gait,
animals lying prone or on their side, atonia, and slow and laboured
breathing. After dermal application of 5000 mg/kg bw, none of the
animals died. The only clinical signs were lethargy and reduced motor
activity. Small lesions and slight hyperaemia were observed at the
application site. Exposure of male and female rats to 338 mg/m3 by
inhalation, the highest concentration of flumethrin acid that it was
technically feasible to generate, for 4 h was well tolerated, and no
clinical signs of toxicity were observed (Pauluhn, 1985b).
When rats were observed for possible effects on the central
nervous system, by measuring the angle at which they slip from a plane
before and at various times from 1 to 24 h after treatment with 0, 1,
3, or 10 mg/kg bw flumethrin, no treatment-related reduction in the
angle was observed (Bomann, 1995a). In tests for dermal and ocular
irritancy in rabbits, flumethrin acid had no effect (Pauluhn, 1985b).
In a test for reverse mutation in S. typhimurium TA98, flumethrin
acid showed no activity (Herbold, 1984a).
In a four-week study in which Wistar rats were given dietary
concentrations of 0, 30, 100, or 300 ppm flumethrin acid, no clinical
signs of toxicity or effects on food consumption or body-weight
development were seen. There was no evidence of treatment-related
changes in haematological, clinicochemical, or necroscopic findings.
No treatment-related histological changes were observed. The NOAEL was
thus 300 ppm, equivalent to 27 mg/kg bw, the highest dose tested
(Bomann, 1995b).
3. Observations in humans
No reported cases of systemic poisoning with flumethrin in humans
were available to the Meeting. There are, however, published reports
of cases of poisoning with other pyrethroids. In a review of 573
cases of poisoning with other alpha-cyano pyrethroids (delamethrin,
fenvalerate, and cypermethrin), poisoning was due to either incorrect
occupational use of the product, attempted suicide, or accidents. In
cases in which the product had come into contact with the skin, the
presentation comprised a burning sensation on the face, tingling,
papules, and dermatitis. In cases of mild poisoning, there was also
dizziness, headache, nausea, anorexia, and weakness. In cases of
moderate poisoning, the signs and symptoms were more intense, and
there were states of reduced consciousness and muscle twitching in
the extremities. Seven deaths are listed; two are attributable to
misdiagnosis and inappropriate treatment. Most patients recovered
within six days. In the more severe cases, recovery took up to 55
days. Treatment consisted of symptomatic and supportive measures.
There were no delayed complications (He et al., 1989). There is no
basis for considering that flumethrin would act differently from the
compounds studied.
Comments
Flumethrin was absorbed rapidly, but not completely, after oral
administration in all species investigated. The concentrations in the
tissues of rats two days after dosing were three- to 50-fold lower
than those in the blood; the lung contained higher concentrations
than other tissues, and the central nervous system had the lowest
concentrations. Excretion occurred primarily in the faeces. The main
metabolite was flumethrin acid, which was distinctly less toxic than
the parent substance in acute and four-week dietary studies in rats
and did not induce reverse mutation in bacteria.
The acute oral toxicity of flumethrin in laboratory animals is
moderate to low. The reported manifestations of its toxicity are
largely consistent with those known collectively as the choreoathetosis
with salivation (CS) syndrome, which is produced by other insecticidal
pyrethroids containing an alpha-cyano-3-phenoxybenzyl alcohol group.
After acute dermal application, the toxicity of flumethrin was low; the
clinical signs were the same as those seen after oral administration.
There was no evidence of acute toxicity after dermal application of
5 ml/kg bw of a 1% pour-on formulation. In tests for dermal and ocular
irritancy, the active substance proved not to be irritating. In tests
for local irritancy with the 1% pour-on formulation, slight, transient
skin changes (mainly barely perceptible erythema and/or swelling) were
seen, but no changes in the mucous membrane of the eye, were observed.
WHO has not classified flumethrin for acute toxicity.
After oral administration of flumethrin for three months to rats
at dietary concentrations of 0, 10, 40, or 160 ppm and to dogs at
dietary concentrations of 0, 25, 50, 100, or 200 ppm, the NOAELs were
10 ppm (equal to 0.7 mg/kg bw per day) for rats and 25 ppm (equal to
0.88 mg/kg bw per day) for dogs. In both species, the most obvious
findings were skin alterations, but these were not due to primary
dermatitis caused by flumethrin but to frequent scratching, with
attendant bleeding and, in some instances, inflammation. Alpha-cyano
pyrethroids are known to produce paraesthesia, which is considered
to be the most probable cause of the observed skin lesions. The
toxicological studies provided no evidence of immunotoxicity, e.g.
effects on leukocyte counts or on other relevant organs (thymus and
spleen).
The results of studies of developmental toxicity in rats at doses
of 0, 0.5, 1, or 2 mg/kg bw per day on days 6-15 of gestation and
rabbits at doses of 0, 0.5, 1.7, or 6 mg/kg bw per day on days 7-19 of
gestation provided no evidence that flumethrin is teratogenic at doses
extending into the range that is toxic to the dams. Some fetotoxicity
was observed at doses that also induced maternal toxicity in both
species. The NOAELs were 0.5 mg/kg bw per day for rats and 1.7 mg/kg
bw per day for rabbits.
A two-generation study of reproductive toxicity in rats exposed
to flumethrin at dietary concentrations of 0, 1, 5, or 50 ppm did not
indicate primary reproductive toxicity; the reduced pup survival and
body-weight gain and certain postural and behavioural changes in the
pups at the highest dose may have been secondary to maternal toxicity.
The NOAEL was 5 ppm, equal to 0.36 mg/kg bw per day.
No studies of long-term toxicity or carcinogenicity have been
conducted with the currently used isomeric mixture of flumethrin.
A two-year study was available, however, in which rats were fed
diets providing flumethrin with a low trans-Z content at dietary
concentrations of 0, 2, 10, 50, or 250 ppm. Skin lesions developed in
rats at 50 and 250 ppm, and there was slight proliferation of the bile
ducts in male rats at 250 ppm. Neither the number of tumour-bearing
rats nor the incidence of any specific neoplasm was increased.
The Meeting considered the following toxicological findings:
(i) Flumethrin with a low trans-Z content has no carcinogenic
potential. (ii) Other pyrethroids, such as cyhalothrin (WHO, 1990a),
cypermethrin (WHO, 1989a), fenvalerate (WHO, 1990d), and the related
resmethrins (WHO, 1989b) also have no carcinogenic potential.
(iii) Treatment with permethrin (WHO, 1990b) resulted in small
increases in the incidence of lung tumours in female mice in three
studies, but no increases were found in either rats or male mice.
(iv) Treatment with deltamethrin was associated with unspecified
thyroid adenomas in rats in one study, but no tumours were induced
in mice or in either species in other studies (WHO, 1990c).
(v) Flumethrin had no genotoxic potential in a number of well-conducted
tests covering a variety of end-points. (vi) Flumethrin showed no
sensitizing potential. (vii) No preneoplastic responses were seen in
studies of up to 13 weeks in duration. The Meeting considered that the
carcinogenic potential of the trans-Z isomers that are present in the
currently used isomeric mixture of flumethrin had been assessed in the
study in rats in which the low trans-Z product was tested.
Oral administration of highly toxic doses of flumethrin to rats
can cause dysfunction of the nervous system, but the effect is rapidly
reversible and is not accompanied by morphological damage to the
central or peripheral nervous system.
Pharmacological tests in experimental animals gave no evidence of
impairment of vital functions. Studies to establish the tolerance of
calves and cattle to flumethrin showed no significant effects, even
when animals licked the application site.
An ADI of 0-0.004 mg/kg bw was allocated, on the basis of the
NOAEL of 0.36 mg/kg bw per day in the two-generation study of
reproductive toxicity in rats, using a 100-fold safety factor.
Toxicological evaluation
Levels that cause no toxic effect
Rat: 10 ppm, equal to 0.7 mg/kg bw per day (13- and 15-week
studies of toxicity)
5 ppm, equal to 0.36 mg/kg bw per day (two-generation
study of reproductive toxicity)
0.5 mg/kg bw per day (maternal toxicity in a study of
developmental toxicity)
Rabbit: 1.7 mg/kg bw per day (maternal and fetal toxicity in a
study of developmental toxicity)
Dog: 25 ppm, equal to 0.88 mg/kg bw per day (13-week study
of toxicity)
Estimate of acceptable daily intake for humans
0-0.004 mg/kg bw
Studies that would provide information useful for continued evaluation
of the compound
Results of any studies that are planned or in progress in
rodents, dogs, or exposed human subjects
Toxicological criteria for estimating guidance values for dietary and non-dietary exposure to flumethrin
Exposure Relevant route, study type, species Results, remarks
Short-term (1-7 days) Oral, toxicity, rat LD50 = 41-3849 mg/kg bw, depending on the vehicle
Dermal, toxicity, rat LD50 > 2000 mg/kg bw
Inhalation, toxicity, rat LC50 = 225 mg/m3
Dermal, irritation, rabbit Not irritating
Ocular, irritation, rabbit Not irritating
Dermal, sensitization, guinea-pig Not sensitizing
Medium-term (1-26 weeks) Repeated oral, 15 weeks, toxicity, rat NOAEL = 0.7 mg/kg bw per day
Repeated oral, 13 weeks, toxicity, dog NOAEL = 0.88 mg/kg bw per day
Repeated oral, reproductive toxicity, rat NOAEL = 0.36 mg/kg bw per day, reduced body-weight
gain of adults
Repeated oral, developmental toxicity, rat NOAEL = 1 mg/kg bw per day, developmental toxicity
Repeated oral, developmental toxicity, rabbit NOAEL = 1.7 mg/kg bw per day, maternal and
developmental toxicity
Long-term (> 1 year) Repeated oral, 2 years, toxicity and NOAEL = 0.5 mg/kg bw per day, skin lesions;
carcinogenicity, rat no carcinogenicity
References
Anadón, A., Diez, M.J., Sierra, M., Sanchez, J.A. & Teran, M.T. (1988)
Microsomal enzyme induction by permethrin in rats. Vet. Hum. Toxicol.,
30, 309-312.
Anadón, A., Martinez-Larrańaga, M.R., Fernandez, M.C., Dķaz, M.J.
& Bringas, P. (1990) Effect of ciprofloxacin on antipyrine
pharmacokinetics and metabolism in rats. Antimicrob. Agents Chemother.
34, 2148-2151.
Anadón, A., Martinez-Larrańaga, M.R., Dķaz, M.J., Bringas, P.,
Fernandez, M.C., Martinez, M.A. & Fernandez-Cruz, M.L. (1995) Effects
of flumethrin on hepatic drug-metabolizing enzymes and antipyrine
disposition in rats. Toxicol. Appl. Pharmacol., 132, 14-18.
Bomann, W. (1992a) Bay Vq 1950 (Flumethrin). Investigations of acute
oral toxicity in rats. Report No. 20924, 7.1.1992. Submitted to WHO by
Bayer AG, Leverkusen, Germany.
Bomann, W. (1992b) Bayticol EC 6% (c.n.: Flumethrin). Investigations
of acute oral toxicity in rats. Report No. 20922, 7.1.1992. Submitted
to WHO by Bayer AG, Leverkusen, Germany.
Bomann, W. (1992c) Bayticol EC 6% Bayticol EC6% (c.n.: Flumethrin).
Investigations of acute oral toxicity in rats. Report No. 20923,
7.1.1992. Submitted to WHO by Bayer AG, Leverkusen, Germany.
Bomann, W. (1992d) Bayvarol-Strips (c.n.: Flumethrin). Untersuchungen
zur akuten oralen Toxizität an Ratten. Ber. No. 21093, 17.2.1992.
Submitted to WHO by Bayer AG, Leverkusen, Germany.
Bomann, W. (1992e) Bayvarol-Strips (c.n.: Flumethrin). Untersuch-
ungenzur akuten dermalen Toxizität an Ratten. Ber. No. 21094,
17.2.1992. Submitted to WHO by Bayer AG, Leverkusen, Germany.
Bomann, W. (1994a) Bayticol P (c.n.: Flumethrin). Study for acute oral
toxicity in rats. Report No. 23422, 19.10.1994. Submitted to WHO by
Bayer AG, Leverkusen, Germany.
Bomann, W. (1994b) Bayticol P (c.n.: Flumethrin). Study for acute
dermal toxicity in rats. Report No. 23421, 19.10.1994. Submitted to
WHO by Bayer AG, Leverkusen, Germany.
Bomann, W. (1994c) Bayticol P (c.n.: Flumethrin). Study for acute oral
toxicity of Bayticol P formulated in water/Cremophor EL and in milk in
female rats. Report No. 23493, 23.11.1994. Submitted to WHO by Bayer
AG, Leverkusen, Germany.
Bomann, W. (1995a) Bayticol acid (Bayticolsäure). Study for acute oral
toxicity in Wistar rats. Report No. 23863, 22.3.1995. Submitted to WHO
by Bayer AG, Leverkusen, Germany.
Bomann, W. (1995b) Bayticol acid (Bayticolsäure). Investigations of
subacute toxicity in Wistar rats (feeding study over 4 weeks). Report
No. 24057, 7.6.1995. Submitted to WHO by Bayer AG, Leverkusen,
Germany.
Bomann, W. & Sander, E. (1995) Bayticol P (c.n.: Flumethrin).
Investigations or subchronic toxicity in Wistar rats (feeding study
over 15 weeks). Report No. 23925, 11.4.1995. Submitted to WHO by Bayer
AG, Leverkusen, Germany.
Bomhard, E., Ramm, W. & Rühl-Fehlert, C. (1987) Flumethrin (BAY V1
6045) Studies on the chronic toxicity and carcinogenicity in Wistar
rats. Report No. 16245, 25.11.1987. Submitted to WHO by Bayer, AG,
Leverkusen, Germany.
Bomhard, E., Ramm, W. & Rühl-Fehlert, C. (1991) Flumethrin (BAY V1
6045) Studies on the chronic toxicity and carcinogenicity in Wistar
rats. Amendment. Report No. 16245a, 24.5.1991. Submitted to WHO by
Bayer, AG, Leverkusen, Germany.
Bonabello, A. & Grassi, A. (1987) Safety pharmacology of Bay Vq 1950
in the gastrointestinal tract: Its effect on intestinal charcoal
transit, on gastric tolerability and on basal gastric acid secretion
in rats. Berich No. 4137 (P), 23.7.1987. Submitted to WHO by Bayer,
AG, Leverkusen, Germany.
Brendler-Schwaab, S. (1994) Evaluation of Bayticol P in the rat
primary hepatocyte unscheduled DNA synthesis assay. Report No. 23461,
8.11.1994. Submitted to WHO by Bayer AG, Leverkusen, Germany.
Brendler-Schwaab, S. (1995) Flumethrin. Mutagenicity study for the
detection of induced forward mutation in the V79-HPRT assay in
vitro. Report No. 24162, 14.7.1995. Submitted to WHO by Bayer AG,
Leverkusen, Germany.
Cabral, J.R.P., Galendo, D., Laval, M. & Lyandrat, N. (1990)
Carcinogenicity studies with deltamethrin in mice and rats. Cancer
Lett., 49, 147-152.
Cameron, B. (1986) Bayticol, Bay Vq 1950. The pharmacokinetics and
tissue residues of [14C]-Bayticol in the lactating cow following
topical administration. Report No. 4108. Unpublished report from
Inveresk Research International Ltd, Musselburgh, Scotland. Submitted
to WHO by Bayer AG, Leverkusen, Germany.
Carlson, G.P. & Shoening, G.P. (1980) Induction of liver microsomal
NADPH cytochrome c reductase and cytochrome P450 by some new synthetic
pyrethroids. Toxicol. Appl. Pharmacol., 52, 507-512.
Cifone, M.A. & Myhr, B.C. (1985) Mutagenicity evaluation of Bay Vq
1950 in the mouse lymphoma forward mutation assay. Pharma-Bericht No.
3170 (P). Unpublished report from Litton Bionetics, Inc., Kensington,
MD, USA. Submitted to WHO by Bayer AG, Leverkusen, Germany.
Clemens, G.R. & Hartnagel, R.E. (1987) A teratology study in the
rabbit with Bay Vq 1950 (Bayticol-P). Report No. MTD 0021. Unpublished
report from Miles Laboratories, Inc., Elkhart, IN, USA. Submitted to
WHO by Bayer AG, Leverkusen, Germany.
Diesing, L. (1991) Bayticol. Investigations of the skin-sensitizing
action in the guinea pig (maximization test after Magnusson & Ligman).
Report No. 20542. Submitted to WHO by Bayer AG, Leverkusen, Germany.
Dorn, H. (1989a) To assess the safety of Bayticol pour on after dermal
application in cattle. Report No. 89/13401. Submitted to WHO by Bayer,
AG, Leverkusen, Germany.
Dorn, H. (1989b) To assess the safety of Bayticol pour on after dermal
application in cattle. Report No. 89/13395. Submitted to WHO by Bayer,
AG, Leverkusen, Germany.
Dorn, H. (1989c) To assess the tolerance of Bayticol pour on 1% in
pregnant cows. Report No. 89/13394. Submitted to WHO by Bayer, AG,
Leverkusen, Germany.
Dotti, A., Kinder, J., Biedermann, K., Luetkemeier, H., Wright, J. &
Terrier, C. (1992) Bay Vq 1950. Multiple generation reproduction study
in rats. Report No. R 5500. Unpublished report from Research and
Consulting Co., Itingen, Switzerland. Submitted to WHO by Bayer, AG,
Leverkusen, Germany.
Ecker, W. (1983) Biotransformation von [fluorbenzoiring-U-14C] Bay V1
6045 bei der Ratte. Unpublished Bayer AG Report No. 83/11072 (11545).
Submitted to WHO by Bayer, AG, Leverkusen, Germany.
Flucke, W. & Schilde, B. (1988) Bay Vq 1950 (c.n.: Flumethrin).
Studies of the neurotoxic effects on the peripheral and central
nervous system of rats following subacute oral administration. Report
No. 16983. Submitted to WHO by Bayer, AG, Leverkusen, Germany.
Gahlmann, R. (1993a) Flumethrin. Salmonella/Mikrosome test. Special
study. Report No. 22613. Submitted to WHO by Bayer AG, Leverkusen,
Germany.
Gahlmann, R. (1993b) Flumethrin. w/Mikrosome test. Special study.
Report No. 22614. Submitted to WHO by Bayer AG, Leverkusen, Germany.
Gardiner, P.J., Hammond, M.D. & Francis, D.L. (1985) BAY Vq 1950:
General/Safety respiratory pharmacology: I. Anti-allergic and
pseudo-allergic activity in rat peritoneal mast cells. II. Evaluation
of bronchoactivity in the guinea-pig isolated trachea. Report No. 3555
(P). Submitted to WHO by Bayer AG, Leverkusen, Germany.
Hahnemann, S. & Rühl, C.H. (1985) Bay Vq 1950 (BAY V1 6045p).
Subchronic toxicological investigations in rats. Feeding study over 13
weeks. Report No. 13658 (E). Submitted to WHO by Bayer AG, Leverkusen,
Germany.
He, F., Wang, S., Liu, L., Chen, S., Zhang, Z. & Sun, J. (1989)
Clinical manifestation and diagnosis of acute pyrethroid poisoning.
Arch. Toxicol., 63, 54-58.
Herbold, B. (1984) Bay Vq 1950. Salmonella/microsome test to evaluate
for point-mutagenic effects. Report No. 12904 (F), 30.8.1984. Submitted
to WHO by Bayer AG, Leverkusen, Germany.
Herbold, B. (1985a) Bay Vq 1950. Test for point-mutagenic effects on
S. cerevisiae D7. Report No. 13737 (P). Submitted to WHO by Bayer
AG, Leverkusen, Germany.
Herbold, B. (1985b) BAY Vq 1950. Cytogenetic investigations in human
lymphocyte cultures in vitro to check for chromosome damaging
activity (in vitro). Report No. 13167 (F). Submitted to WHO by Bayer
AG, Leverkusen, Germany.
Herbold, B. (1986) Bayticol P. Micronucleus test in mice to evaluate
for clastogenic activity. Report No. 14501. Submitted to WHO by Bayer
AG, Leverkusen, Germany.
Herbold, B. (1990a) Flumethrin- trans-Z-1-isomer. Salmonella/microsome
test using Salmonella TA98. Report No. 19573. Submitted to WHO by Bayer
AG, Leverkusen, Germany.
Herbold, B. (1990b) Flumethrin- trans-Z-2-isomer. Salmonella/microsome
test using Salmonella TA 98. Report No. 19572. Submitted to WHO by Bayer
AG, Leverkusen, Germany.
Herbold, B. (1995a) Flumethrin. In vitro mammalian chromosome
aberration test with Chinese hamster V79 cells. Report No. 24524.
Submitted to WHO by Bayer AG, Leverkusen, Germany.
Herbold, B. (1995b) Flumethrin. Micronucleus test on the mouse. Report
No. 23869. Submitted to WHO by Bayer AG, Leverkusen, Germany.
Hirth, C. (1985) Bay Vq 1950. Study of diuretic activity in rats.
Report No. 14092. Submitted to WHO by Bayer AG, Leverkusen, Germany.
Hoffmann, K. & Kaliner, G. (1984) Bay Vq 1950. Subchronic toxicity
study in dogs with oral administration (13 weeks feeding study).
Report No. 13155. Submitted to WHO by Bayer AG, Leverkusen, Germany.
Hoffmann, K. (1985) Bay Vq 1950. Subchronic toxicity study in dogs
with oral administration/supplementary study (13 weeks feeding study).
Report No. 13169. Submitted to WHO by Bayer AG, Leverkusen, Germany.
Iida, M., Akagi, H. & Hashizume, M. (1988) Study on safety of Bayticol
pour-on in calves. Rep. No. 89/13245. Submitted to WHO by Bayer AG,
Leverkusen, Germany.
Kaneko, H., Ohkawa, H. & Miyamoto, J. (1981) Comparative metabolism of
fenvalerate and the [2S, alpha S]-isomer in rats and mice. J. Pestic.
Sci., 6, 317-326.
Klein, O. (1993a) [Cl-Phenyl-U-14C] Flumethrin: Investigation of the
biokinetic behaviour and the metabolism in the rat. Bericht No.,
PF-3823, ME-16/93, KNO 58. Submitted to WHO by Bayer AG, Leverkusen,
Germany.
Klein, O. (1993b) [Cl-Phenyl-U-14C] Flumethrin: Investigations on the
distribution of the total radioactivity in the rat. Projekt-No.
M1840667. Report in preparation, 1995. Submitted to WHO by Bayer AG,
Leverkusen, Germany.
Klein, O. (1995) Metabolism of [14C]-Bayticol in the dairy cow and
male beef cattle. Project No. M1840667-2. Report No. MR 323/95 KNO68.
Submitted to WHO by Bayer AG, Leverkusen, Germany.
Knorr, A. (1986) Influence on hemodynamics and cardiac contractility
of anesthesized dogs after oral administration. Pharma-Bericht No.
Submitted to WHO by Bayer AG, Leverkusen, Germany.
Kowalski, R.L., Clemens, G.R. & Hartnagel, R.E. (1987) A teratology
study with Bayticol-P (Flumethrin = Bay Vq 1950) in the rat. R-Bericht
No. 3960. Unpublished report from Miles Laboratories, Inc., Elkhart,
IN, USA. Submitted to WHO by Bayer, AG, Leverkusen, Germany.
Krötlinger, F. (1994) Bayticol P. Study for skin and eye irritation/
corrosion in rabbits. Ber-No. 23559. Submitted to WHO by Bayer AG,
Leverkusen, Germany.
Pauluhn, J. (1985a) Bayticol P pour on 1%. Study on the irritancy/
corrosion potential on rabbit skin. Report No. 14033. Submitted to WHO
by Bayer AG, Leverkusen, Germany.
Pauluhn, J. (1985b) Bayticol-P-Säure. Untersuchungen zur Gewer-
betoxikologie. Ber-No. 13643. Submitted to WHO by Bayer AG, Leverkusen,
Germany.
Puls, W. & Bischoff, H. (1985) Bay Vq 1950. Influence of orally
administered Bay Vq 1950 on the blood glucose and serum triglyceride
concentrations in fed rats and fasted rats. Report No. 14366.
Submitted to WHO by Bayer AG, Leverkusen, Germany.
Renhof, M. (1983a) Bay Vq 1950 in peanut oil and miglyol. Acute oral
toxicity in the rat. Report No. 12047 (P). Submitted to WHO by Bayer
AG, Leverkusen, Germany.
Renhof, M. (1983b) Bay Vq 1950 in acetone/peanut oil 1:10. Acute oral
toxicity in the rat. Report No. 12048 (P). Submitted to WHO by Bayer
AG, Leverkusen, Germany.
Schmidt, M. (1984a) Bay Vq 1950 pour on 1%. Acute toxicity in the rat
and the mouse. Study of primary irritant/corrosive activity on rabbit
skin and eye. Report No. 12761 (P). Submitted to WHO by Bayer AG,
Leverkusen, Germany.
Schmidt, M. (1984b) Bay Vq 1950 pour on 1%. Acute toxicity in the rat
and mouse after intraperitoneal application. Report No. 12760 (P).
Submitted to WHO by Bayer AG, Leverkusen, Germany.
Seuter, F. & Perzborn, E. (1985) Blood-pharmacological investigations.
Report No. 14213. Submitted to WHO by Bayer AG, Leverkusen, Germany.
Starke, B. (1985) CNS safety pharmacology study with Bay Vq 1950
(Bayticol P) on oral administration. Pharma-Bericht No. 3541.
Submitted to WHO by Bayer AG, Leverkusen, Germany.
Steinke, W., Weber, H. & Suwelack, D. (1983) [14C] BAY V1 6045:
Pharmakokinetik in Ratten. PH-Report No. 11941. Submitted to WHO by
Bayer AG, Leverkusen, Germany.
Thyssen, J. (1982) Bayticol EC 7.5%. Investigation into the acute
toxicity on inhalation. Report No. 10946. Submitted to WHO by Bayer
AG, Leverkusen, Germany.
Vijversberg, H.P.M. & Van den Bercken, J. (1982) Action of pyrethroid
insecticides on the vertebrae nervous system. Neuropathol. Appl.
Neurobiol., 8, 421-440
Vohr, H.-W. (1994) Bayticol P. Investigations of skin sensitization in
guinea pigs (Magnusson & Kligman test). Report No. 23026. Submitted to
WHO by Bayer AG, Leverkusen, Germany.
WHO (1989a) Cypermethrin (Environmental Health Criteria 82),
International Programme on Chemical Safety, Geneva, 154 pp.
WHO (1989b) Resmethrins (Environmental Health Criteria 92),
International Programme on Chemical Safety, Geneva, 79 pp.
WHO (1990a) Cyhalothrin (Environmental Health Criteria 99),
International Programme on Chemical Safety, Geneva, 106 pp.
WHO (1990b) Deltamethrin (Environmental Health Criteria 97),
International Programme on Chemical Safety, Geneva, 133 pp.
WHO (1990c) Fenvalerate (Environmental Health Criteria 95),
International Programme on Chemical Safety, Geneva, 121 pp.
WHO (1990d) Permethrin (Environmental Health Criteria 94),
International Programme on Chemical Safety, Geneva, 125 pp.
