
FERBAM
First draft prepared by
J.-J. Larsen,
Institute of Toxicology, National Food Agency,
Ministry of Health, Soborg, Denmark
Explanation
Evaluation for acceptable daily intake
Biochemical aspects
Absorption, distribution, and excretion
Biotransformation
Toxicological studies
Acute toxicity
Short-term toxicity
Long-term toxicity and carcinogenicity
Reproductive toxicity
Genotoxicity
Special studies: Dermal and ocular irritation and dermal
sensitization
Comments
Toxicological evaluation
References
Explanation
Ferbam was evaluated for toxicological effects by the Joint
Meeting in 1965, 1967, 1970, 1974, 1977, and 1980 (Annex 1, references
4, 8, 14, 22, 28, and 34). A temporary ADI of 0-0.025 mg/kg bw for
ferbam or ferbam in combination with other dimethyldithiocarbamates
was allocated in 1967, on the basis of a one-year study in dogs. This
temporary ADI was lowered to 0-0.005 mg/kg bw in 1974, and a full
group ADI of 0-0.02 mg/kg bw for ferbam and ziram was allocated in
1977, which was confirmed in 1980. Since 1980, new studies have become
available. This monograph summarizes the data received since the
previous evaluation and includes relevant summaries from the previous
monograph and monograph addenda on ziram (Annex I, references 4, 9,
15, 23, 29, and 35).
Evaluation for acceptable daily intake
1. Biochemical aspects
(a) Absorption, distribution, and excretion
Ferbam does not appear to be stored in the tissues of rats or
dogs, but feeding of ferbam to rats increased the skeletal stores of
iron (Annex 1, reference 4).
The disposition and metabolism of ferbam were studied in rats.
Male rats weighing 125-275 g, pregnant rats on day 16 of gestation,
and lactating rats six days post partum were given single oral doses
of 500 mg/kg bw spiked with 24 µCi of either 35S- or 14C-labelled
compound. The ferbam was suspended in 0.5% carboxymethyl cellulose.
After dosing, each male rat was placed in a metabolism cage for
examination of faeces, urine, and expired air. At termination of the
experiment, the rat was sacrificed for analysis of radiolabel. Bile
was collected from some of the male rats. Five pups per lactating
mother were left for nursing, and the dams and pups were sacrificed
24 h after dosing. About 40-70% of the dose of labelled ferbam was
absorbed through the gastrointestinal tract within the first 24 h. In
rats receiving 35S-ferbam, 23% of the radiolabel was found in urine,
18% in expired air, and 1% in bile; only small amounts were found
in tissues, including blood, kidneys, muscle, and brain. In rats
receiving 14C-ferbam, 43% of the radiolabel was found in urine and
1.4% in bile, whereas only 0.6% was recovered in expired air. The
other tissues contained only small amounts of radiolabel. In the
pregnant rats, a small but significant amount of radiolabel readily
crossed the placenta into the fetus. In lactating rats, radiolabel was
secreted into the milk, absorbed by the pups, and excreted in the
pups' urine (Lee et al., 1975).
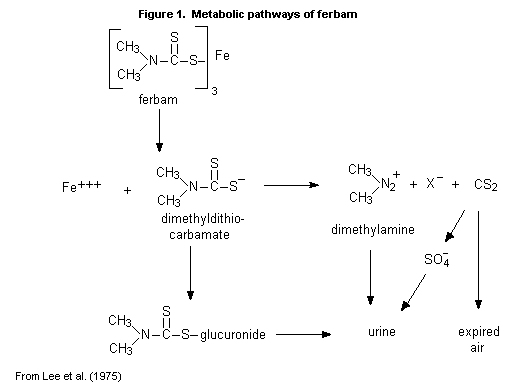
(b) Biotransformation
In rats given ferbam orally at a dose of 500 mg/kg bw, the main
product in the expired air was carbon disulfide; the main products in
the urine were inorganic sulfate, a salt of dimethylamine, and the
glucuronide conjugate of dimethyldithiocarbamate (Figure 1). No
unchanged ferbam was found in the urine (Lee et al., 1975).
2. Toxicological studies
Ferbam and ziram share the same core molecule, dimethyldithio-
carbamate, and differ in the metal ion and the number of dimethyl-
dithiocarbamate molecules. Ferbam contains three dimethyldithio-
carbamates and a ferric ion, while ziram contains two dimethyl-
dithiocarbamates and a zinc ion. The metabolism of ziram and ferbam in
mammalian systems is similar. In aqueous acid media, such as found in
the stomach, ziram and ferbam dissociate to dimethyldithiocarbamic
acid or carbon disulfide and dimethyl amine. The difference between
the two compounds is that ziram yields zinc ion while ferbam yields
ferric ion. Zinc and iron are both essential nutrients in mammals;
however, the amount of iron required for normal homeostasis and the
body's ability to handle excess iron are much greater than for zinc.
The greater toxicity of ziram, in comparison with ferbam, is thought
to result from the inability of organisms to handle excess amounts of
zinc. Extrapolation of data on the toxicity of ferbam to relate to
ziram is therefore justified.
 (a) Acute toxicity
The acute toxicity of ferbam is summarized in Table 1.
(b) Short-term toxicity
Rats
Groups of 10 male and 10 female weanling NR strain rats were
fed ferbam (purity unspecified) at concentrations of 0, 100, 500,
2500, or 5000 ppm (equivalent to 0, 10, 50, 250, or 500 mg/kg bw per
day) for one month. At doses > 500 ppm, growth depression was
found, and at 5000 ppm the mortality rate was increased. No significant
histopathological changes were seen in any of the animals. The NOAEL
was 100 ppm, equivalent to 10 mg/kg bw per day, on the basis of growth
depression at doses > 500 ppm (Annex 1, reference 4).
Groups of 10 male and 10 female NR strain rats were fed ferbam
(purity unspecified) at concentrations of 0 or 2500 ppm (equivalent to
0 or 250 mg/kg bw per day) for one month. Post-mortem examination
revealed no thyroid abnormalities (Annex I, reference 4).
Dogs
One dog (strain unspecified) was given ferbam (purity unspecified)
and ziram together for one month, each at a dose of 5 mg/kg bw per day.
The only adverse effect was slight anaemia. Another dog remained
healthy, except for slight anaemia, when given ferbam alone at a dose
of 25 mg/kg bw per day for one month or at 50 mg/kg bw per day for one
week. An attempt to raise the dose to 100 mg/kg bw per day immediately
provoked severe vomiting and malaise (Annex I, reference 4).
Groups of two adult dogs (strain unspecified) received ferbam
(purity unspecified) at doses of 0.5, 5, or 25 mg/kg bw per day for
one year. Convulsions occurred in animals at 25 mg/kg bw per day.
Urine analysis, blood picture, organ weights, and histological
examination (including the thyroid) showed no abnormalities. The NOAEL
was 5 mg/kg bw per day, on the basis of convulsions at 25 mg/kg bw per
day (Annex I, reference 4).
Table 1. Acute toxicity of ferbam in experimental animals
Species Sex Route Purity LD50 (mg/kg bw) Reference
(%) or LC50 (mg/litre)
Mouse Male Oral NR 1000 Annex 1, reference 4
Mouse Female Intraperitoneal NR 3000 Annex 1, reference 4
Rat Male Oral NR 11 000 Annex 1, reference 4
Rat Female Intraperitoneal NR 2700 Annex 1, reference 4
Rat Male, female Inhalation 91.8 0.40 Hardy & Jackson (1988)
Rat Male, female Inhalation 91.8 0.28 McDonald (1988)
Rabbit Male, female Dermal 91.8 > 4000 Reijnders (1987b)
NR, not reported
(c) Long-term toxicity and carcinogenicity
Rats
Groups of 25 male and 25 female rats (strain unspecified) were
fed ferbam (purity unspecified) at concentrations of 0, 25, 250, or
2500 ppm (equivalent to 1.3, 12.5, or 125 mg/kg bw per day) for two
years. In animals at the highest dose, the growth rate was depressed,
the life-span was shortened, neurological changes and cystic brain
lesions were seen, and the testes were atrophied. The thyroid glands
were normal in all groups, and there was no increase in tumour
incidence. The NOAEL was 250 ppm, equivalent to 12.5 mg/kg bw per day,
on the basis of the changes at 125 mg/kg bw per day (Annex I,
reference 4).
(d) Reproductive toxicity
Mice
Groups of six (C3H × C57B1/6)F1 mice, 11-15 weeks old, received
ferbam (purity, 99.8%) at doses of 0, 250, 500, or 1000 mg/kg bw per
day orally or 0, 125, 250, or 500 mg/kg bw per day intraperitoneally
for five days. After 35 days, toxicity to the testis was evaluated by
measuring the testicular weight, sperm counts, and the percentage of
abnormal sperm. Neither testicular weights nor sperm counts were
affected. The frequency of abnormal sperm in the control mice ranged
from 1.6 to 2.4%. The NOAEL was 500 mg/kg bw per day, on the basis of
a statistically significant increase in sperm abnormalities (6.3%)
after oral administration of ferbam at 1000 mg/kg bw per day. No
effects were seen after intraperitoneal administration, indicating
that active metabolites were responsible for the effects (Quinto
et al., 1989).
Rats
Groups of 16 male and 16 female rats (strain unspecified) were
fed ferbam (purity unspecified) at concentrations of 0 or 250 ppm
(equivalent to 0 or 25 mg/kg bw per day) in a three-generation study
with two litters per generation. The treated animals were maintained
for three months after weaning before the first mating. No effect was
seen on fertility, gestation, viability, lactation, or litter size,
and no gross or histological abnormalities in comparison with controls
were found in animals from the second litter of the third filial
generation selected for examination (Annex I, reference 9).
(e) Genotoxicity
The only results for genotoxicity that have been reported are
those of an Ames test for reverse mutation in Salmonella typhimurium
strains TA98, TA100, TA1535, TA1537, and TA1538 with a preparation
of ferbam of unspecified purity at doses up to 1500 µg/plate. No
mutagenicity was seen (De Lorenzo et al., 1978).
(f) Special studies: Dermal and ocular irritation and dermal
sensitization
The dermal irritancy of ferbam was tested in New Zealand white
rabbits by removing the hair from the dorsal flanks and 24 h later
applying a 6-cm2 gauze patch spread evenly with 0.5 g ferbam (purity,
91.8%) moistened with 0.5 ml water to the left flank of each of six
animals; the right flank was covered with the same dressing without
the test substance. The test sites were covered with permeable tape
and wrapped in flexible bandage. The sites were exposed for 4 h, after
which the remaining test substance was removed. The treated skin was
examined for erythema and oedema after 1 h and on days 1, 2, and 3.
The only dermal reaction seen was very slight erythema in one animal
1 h after removal of the dressings, which disappeared within 24 h. The
primary dermal irritation index was 0.02, indicating that ferbam is
slightly irritating to the skin; however, the occurrence of only a
brief reaction in one animal led the authors to conclude that it is
practically non-irritating (Weterings & Daamen, 1987a).
To test the ocular irritancy of ferbam, the eyes of six female
New Zealand white rabbits were examined, and then the conjunctival sac
of the left eye was installed with 21 mg of ferbam (purity, 91.8%),
equivalent to 0.1 ml, using a spatula. The ocular lesions were graded
and scored 1, 24, 48, and 72 h and 7 and 10 days after instillation.
Immediately after scoring, 24 and 72 h after treatment, a solution of
2% fluorescein in water was applied to both eyes to examine the
potential for corneal injury. Ferbam affected only the conjunctivae:
very slight conjunctival redness was seen in all six animals, with a
short increase to diffuse redness in two animals on day 3. The redness
had disappeared by day 10. Five of the six animals showed obvious
chemosis, and the sixth slight chemosis, which had dosappeared by day
7 in all animals. On the basis of a Draize score of 6.3 (24 h), the
authors concluded that ferbam is mildly irritating to the eye
(Weterings & Daamen, 1987b).
The dermal sensitizing potential of ferbam (purity, 91.8%) was
examined in 35 adult female Dunkin-Hartley guinea-pigs. After
induction by epicutaneous applications of the test substance (25% w/w
in 1% aqueous methylcellulose) and challenge with a series of
concentrations of ferbam (25, 10, and 5% w/w in 1% aqueous methyl-
cellulose), one of the experimental animals showed a positive reaction
to all three concentrations, indicating a sensitization rate of 5%
(grade 2). Ferbam was thus considered to have weak sensitizing
properties (Weterings & Daamen, 1987c).
Comments
Ferbam is well absorbed after oral administration to rats and is
extensively metabolized. Most of the administered radiolabel was found
in the urine, expired air, and bile. In pregnant rats, a small but
significant amount crossed the placenta into the fetus. In lactating
rats, the radiolabel was secreted into the milk, absorbed by the pups,
and excreted in the pups' urine. In expired air, the main product was
carbon disulfide; in the urine, the main products were inorganic
sulfate, a salt of dimethylamine, and the glucuronide conjugate of
dimethyldithiocarbamic acid.
Ferbam has low acute toxicity and has been classified by WHO as
unlikely to present an acute hazard in normal use (WHO, 1996).
In two four-week studies, rats were fed diets providing ferbam at
concentrations of 0, 100, 500, 2500, or 5000 ppm or 0 or 2500 ppm. The
NOAEL was 100 ppm, equivalent to 10 mg/kg bw per day, on the basis
of growth depression at doses > 500 ppm. Post-mortem examination
revealed no thyroid abnormalities. In another four-week study in which
one dog was given ferbam and ziram together, each at a dose of 5 mg/kg
bw per day; the only adverse effect was slight anaemia. Another dog
remained healthy, except for slight anaemia, when given ferbam alone
at a dose of 25 mg/kg bw per day for one month or 50 mg/kg bw per day
for one week. An attempt to raise the dose to 100 mg/kg bw per day
immediately provoked severe vomiting and malaise.
In a study in which dogs were treated with ferbam at doses of
0.5, 5, or 25 mg/kg bw per day for one year, the NOAEL was 5 mg/kg bw
per day, on the basis of convulsions at 25 mg/kg bw per day.
In a two-year study of toxicity and carcinogenicity in rats
treated at dietary concentrations of 0, 25, 250, or 2500 ppm, the
NOAEL was 250 ppm, equivalent to 12 mg/kg bw per day, on the basis
of depressed growth rate, shortened life span, neurological
changes, cystic brain lesions, and testicular atrophy at 2500 ppm.
Carcinogenicity was not demonstrated.
Sperm quality was investigated in mice given oral doses of
0, 250, 500, or 1000 mg/kg bw per day for five consecutive days. The
NOAEL was 500 mg/kg bw per day, on the basis of an increased frequency
of sperm abnormalities at 1000 mg/kg bw per day.
In a three-generation study of reproductive toxicity in rats fed
dietary concentrations of 0 or 250 ppm, the NOAEL was 250 ppm,
equivalent to 12 mg/kg bw per day.
Few data were available on genotoxicity. Ferbam did not induce
reverse mutation in bacteria.
Ferbam was slightly irritating to the skin and mildly irritating
to the eyes of rabbits. It has weak skin sensitizing properties in
guinea-pigs.
The Meeting concluded that the toxicological data specifically
generated for ferbam were inadequate to estimate an ADI; however,
because of the similarity of the chemical structure of ferbam to
that of ziram and the comparable toxicological profile of the two
compounds, ferbam was included in the group ADI of 0-0.003 mg/kg bw
for ferbam and ziram, which is derived from the information available
on ziram.
Toxicological evaluation
Levels that cause no toxicological effect
Mouse: 500 mg/kg bw per day (study of sperm quality)
Rat: 100 ppm, equivalent to 10 mg/kg bw per day (one-month
study of toxicity)
250 ppm, equivalent to 12 mg/kg bw per day (two-year
study of toxicity and carcinogenicity)
250 ppm, equivalent to 12 mg/kg bw per day (study of
reproductive toxicity)
Dog: 5 mg/kg bw per day (one-year study of toxicity)
Estimate of acceptable daily intake for humans
0-0.003 mg/kg bw (fgroup ADI for ferbam and ziram)
Studies that would provide information useful for continued evaluation
of the compound
1. Studies on dissociation in aqueous solutions
2. Observations in humans
Toxicological criteria for estimating guidance values for dietary and non-dietary exposure to ferbam
Exposure Relevant route, study type, species Results, remarks
Short-term (1-7 days) Oral, toxicity, mouse LD50 = 1000 mg/kg bw
Oral, toxicity, rat LD50 = 11 000 mg/kg bw
Inhalation, toxicity, rat LC50 = 0.3 mg/litre
Dermal, irritation, rabbit Slightly irritating
Ocular, irritation, rabbit Mildly irritating
Dermal, sensitization, guinea-pig Weakly sensitizing
Repeated oral, 5 days, testicular toxicity, mouse NOAEL = 500 mg/kg bw per day, increased sperm
abnormalities
Medium-term (1-26 weeks) Repeated oral, 4 weeks, toxicity, rat NOAEL = 10 mg/kg bw per day, reduced body
weight
Repeated oral, reproductive toxicity, rat NOAEL = 12 mg/kg bw per day, reproductive
toxicity
Long-term (> 1 year) Repeated oral, 104 weeks, toxicity and NOAEL = 12 mg/kg bw per day, reduced body
carcinogenicity, rat weight, shortened life-span, neurological
changes, cystic brain lesions, and atrophied
testis. No carcinogenicity
Repeated oral, 1 year, toxicity, dog NOAEL = 5 mg/kg bw per day, convulsions
References
DeLorenzo, F.D., Staiano, N., Lorenzo, S. & Cortese, R. (1978)
Mutagenicity of diallate, sulfallate, and triallate and relationship
between structure and mutagenic effects of carbamates used widely in
agriculture. Cancer Res., 38, 13-15.
Hardy, C.J. & Jackson, G.C. (1988) Ferbam technical, acute inhalation
toxicity in rats, 4-hour exposure. Unpublished report No. UCB
285/88179 from Huntingdon Research Centre Ltd, Huntingdon, Cambs,
United Kingdom. Submitted to WHO by UCB Chemicals, Brussels, Belgium.
Lee, C.-C., Russell, J.Q., Minor, J.L., Kowalski, J.J., Sanyer, J.L.,
Kintner, L.D., Hodgson, J.R., Short, R.D., Peters, P.J., Dilley, J.V.,
Murrill, E.A., Holton, D.O. & Ellis, H.V. (1975) Toxicological
evaluation of ferric dimethyldithiocarbamate (ferbam) and dithio-
carbamate (thiram) with acute toxicity of manganese and zinc ethylene-
bisdithiocarbamates (maneb and zineb). Unpublished report No. 3612-B
from National Institute of Environmental Health Sciences, Research
Triangle Park, NC, USA. Submitted to WHO by UCB Chemicals, Brussels,
Belgium.
McDonald, P. (1988) Ferbam technical, acute inhalation toxicity
study in rats. Unpublished report No. 5115 from Inveresk Research
International, Musselburgh, Scotland. Submitted to WHO by UCB
Chemicals, Brussels, Belgium.
Reijnders, J.B.J. (1987a) Evaluation of the acute oral toxicity of
ferbam technical in the rat. Unpublished report No. Notox 0740/930
from Notox CV, 's-Hertogenbosch, Netherlands. Submitted to WHO by UCB
Chemicals, Brussels, Belgium.
Reijnders, J.B.J. (1987b) Evaluation of the acute dermal toxicity of
ferbam technical in the rabbit. Unpublished report No. Notox 0740/931
from Notox CV, 's-Hertogenbosch, Netherlands. Submitted to WHO by UCB
Chemicals, Brussels, Belgium.
Quinto, I., Marinis, E.D., Mallardo, M., Arcucci, A., Morte, R.D. &
Staiano, N. (1989) Effect of DNOC, ferbam and imidan exposure on mouse
sperm morphology. Mutat. Res., 224, 405-408.
Weterings, P.J.J.M. & Daamen, P.A.M. (1987a) Assessment of primary
skin irritation/corrosion by ferbam technical in the rabbit.
Unpublished report No. Notox 0740/932 from Notox CV, 's-Hertogenbosch,
Netherlands. Submitted to WHO by UCB Chemicals, Brussels, Belgium.
Weterings, P.J.J.M. & Daamen, P.A.M (1987b) Assessment of the
primary eye irritation/corrosion by ferbam technical in the rabbit.
Unpublished report No. Notox 0740/933 from Notox CV, 's-Hertogenbosch,
Netherlands. Submitted to WHO by UCB Chemicals, Brussels, Belgium.
Weterings, P.J.J.M. & Daamen, P.A.M. (1987c) Assessment of the skin
sensitization potential of ferbam technical in the guinea-pig (split
adjuvant test). Unpublished-report No. Notox 0740/934 from Notox CV,
's-Hertogenbosch, Netherlands. Submitted to WHO by UCB Chemicals,
Brussels, Belgium.
WHO (1996) The WHO Recommended Classification of Pesticides by Hazard
and Guidelines to Classification 1996-1997 (WHO/PCS/96.3), International
Programme on Chemical Safety, Geneva.
(a) Acute toxicity
The acute toxicity of ferbam is summarized in Table 1.
(b) Short-term toxicity
Rats
Groups of 10 male and 10 female weanling NR strain rats were
fed ferbam (purity unspecified) at concentrations of 0, 100, 500,
2500, or 5000 ppm (equivalent to 0, 10, 50, 250, or 500 mg/kg bw per
day) for one month. At doses > 500 ppm, growth depression was
found, and at 5000 ppm the mortality rate was increased. No significant
histopathological changes were seen in any of the animals. The NOAEL
was 100 ppm, equivalent to 10 mg/kg bw per day, on the basis of growth
depression at doses > 500 ppm (Annex 1, reference 4).
Groups of 10 male and 10 female NR strain rats were fed ferbam
(purity unspecified) at concentrations of 0 or 2500 ppm (equivalent to
0 or 250 mg/kg bw per day) for one month. Post-mortem examination
revealed no thyroid abnormalities (Annex I, reference 4).
Dogs
One dog (strain unspecified) was given ferbam (purity unspecified)
and ziram together for one month, each at a dose of 5 mg/kg bw per day.
The only adverse effect was slight anaemia. Another dog remained
healthy, except for slight anaemia, when given ferbam alone at a dose
of 25 mg/kg bw per day for one month or at 50 mg/kg bw per day for one
week. An attempt to raise the dose to 100 mg/kg bw per day immediately
provoked severe vomiting and malaise (Annex I, reference 4).
Groups of two adult dogs (strain unspecified) received ferbam
(purity unspecified) at doses of 0.5, 5, or 25 mg/kg bw per day for
one year. Convulsions occurred in animals at 25 mg/kg bw per day.
Urine analysis, blood picture, organ weights, and histological
examination (including the thyroid) showed no abnormalities. The NOAEL
was 5 mg/kg bw per day, on the basis of convulsions at 25 mg/kg bw per
day (Annex I, reference 4).
Table 1. Acute toxicity of ferbam in experimental animals
Species Sex Route Purity LD50 (mg/kg bw) Reference
(%) or LC50 (mg/litre)
Mouse Male Oral NR 1000 Annex 1, reference 4
Mouse Female Intraperitoneal NR 3000 Annex 1, reference 4
Rat Male Oral NR 11 000 Annex 1, reference 4
Rat Female Intraperitoneal NR 2700 Annex 1, reference 4
Rat Male, female Inhalation 91.8 0.40 Hardy & Jackson (1988)
Rat Male, female Inhalation 91.8 0.28 McDonald (1988)
Rabbit Male, female Dermal 91.8 > 4000 Reijnders (1987b)
NR, not reported
(c) Long-term toxicity and carcinogenicity
Rats
Groups of 25 male and 25 female rats (strain unspecified) were
fed ferbam (purity unspecified) at concentrations of 0, 25, 250, or
2500 ppm (equivalent to 1.3, 12.5, or 125 mg/kg bw per day) for two
years. In animals at the highest dose, the growth rate was depressed,
the life-span was shortened, neurological changes and cystic brain
lesions were seen, and the testes were atrophied. The thyroid glands
were normal in all groups, and there was no increase in tumour
incidence. The NOAEL was 250 ppm, equivalent to 12.5 mg/kg bw per day,
on the basis of the changes at 125 mg/kg bw per day (Annex I,
reference 4).
(d) Reproductive toxicity
Mice
Groups of six (C3H × C57B1/6)F1 mice, 11-15 weeks old, received
ferbam (purity, 99.8%) at doses of 0, 250, 500, or 1000 mg/kg bw per
day orally or 0, 125, 250, or 500 mg/kg bw per day intraperitoneally
for five days. After 35 days, toxicity to the testis was evaluated by
measuring the testicular weight, sperm counts, and the percentage of
abnormal sperm. Neither testicular weights nor sperm counts were
affected. The frequency of abnormal sperm in the control mice ranged
from 1.6 to 2.4%. The NOAEL was 500 mg/kg bw per day, on the basis of
a statistically significant increase in sperm abnormalities (6.3%)
after oral administration of ferbam at 1000 mg/kg bw per day. No
effects were seen after intraperitoneal administration, indicating
that active metabolites were responsible for the effects (Quinto
et al., 1989).
Rats
Groups of 16 male and 16 female rats (strain unspecified) were
fed ferbam (purity unspecified) at concentrations of 0 or 250 ppm
(equivalent to 0 or 25 mg/kg bw per day) in a three-generation study
with two litters per generation. The treated animals were maintained
for three months after weaning before the first mating. No effect was
seen on fertility, gestation, viability, lactation, or litter size,
and no gross or histological abnormalities in comparison with controls
were found in animals from the second litter of the third filial
generation selected for examination (Annex I, reference 9).
(e) Genotoxicity
The only results for genotoxicity that have been reported are
those of an Ames test for reverse mutation in Salmonella typhimurium
strains TA98, TA100, TA1535, TA1537, and TA1538 with a preparation
of ferbam of unspecified purity at doses up to 1500 µg/plate. No
mutagenicity was seen (De Lorenzo et al., 1978).
(f) Special studies: Dermal and ocular irritation and dermal
sensitization
The dermal irritancy of ferbam was tested in New Zealand white
rabbits by removing the hair from the dorsal flanks and 24 h later
applying a 6-cm2 gauze patch spread evenly with 0.5 g ferbam (purity,
91.8%) moistened with 0.5 ml water to the left flank of each of six
animals; the right flank was covered with the same dressing without
the test substance. The test sites were covered with permeable tape
and wrapped in flexible bandage. The sites were exposed for 4 h, after
which the remaining test substance was removed. The treated skin was
examined for erythema and oedema after 1 h and on days 1, 2, and 3.
The only dermal reaction seen was very slight erythema in one animal
1 h after removal of the dressings, which disappeared within 24 h. The
primary dermal irritation index was 0.02, indicating that ferbam is
slightly irritating to the skin; however, the occurrence of only a
brief reaction in one animal led the authors to conclude that it is
practically non-irritating (Weterings & Daamen, 1987a).
To test the ocular irritancy of ferbam, the eyes of six female
New Zealand white rabbits were examined, and then the conjunctival sac
of the left eye was installed with 21 mg of ferbam (purity, 91.8%),
equivalent to 0.1 ml, using a spatula. The ocular lesions were graded
and scored 1, 24, 48, and 72 h and 7 and 10 days after instillation.
Immediately after scoring, 24 and 72 h after treatment, a solution of
2% fluorescein in water was applied to both eyes to examine the
potential for corneal injury. Ferbam affected only the conjunctivae:
very slight conjunctival redness was seen in all six animals, with a
short increase to diffuse redness in two animals on day 3. The redness
had disappeared by day 10. Five of the six animals showed obvious
chemosis, and the sixth slight chemosis, which had dosappeared by day
7 in all animals. On the basis of a Draize score of 6.3 (24 h), the
authors concluded that ferbam is mildly irritating to the eye
(Weterings & Daamen, 1987b).
The dermal sensitizing potential of ferbam (purity, 91.8%) was
examined in 35 adult female Dunkin-Hartley guinea-pigs. After
induction by epicutaneous applications of the test substance (25% w/w
in 1% aqueous methylcellulose) and challenge with a series of
concentrations of ferbam (25, 10, and 5% w/w in 1% aqueous methyl-
cellulose), one of the experimental animals showed a positive reaction
to all three concentrations, indicating a sensitization rate of 5%
(grade 2). Ferbam was thus considered to have weak sensitizing
properties (Weterings & Daamen, 1987c).
Comments
Ferbam is well absorbed after oral administration to rats and is
extensively metabolized. Most of the administered radiolabel was found
in the urine, expired air, and bile. In pregnant rats, a small but
significant amount crossed the placenta into the fetus. In lactating
rats, the radiolabel was secreted into the milk, absorbed by the pups,
and excreted in the pups' urine. In expired air, the main product was
carbon disulfide; in the urine, the main products were inorganic
sulfate, a salt of dimethylamine, and the glucuronide conjugate of
dimethyldithiocarbamic acid.
Ferbam has low acute toxicity and has been classified by WHO as
unlikely to present an acute hazard in normal use (WHO, 1996).
In two four-week studies, rats were fed diets providing ferbam at
concentrations of 0, 100, 500, 2500, or 5000 ppm or 0 or 2500 ppm. The
NOAEL was 100 ppm, equivalent to 10 mg/kg bw per day, on the basis
of growth depression at doses > 500 ppm. Post-mortem examination
revealed no thyroid abnormalities. In another four-week study in which
one dog was given ferbam and ziram together, each at a dose of 5 mg/kg
bw per day; the only adverse effect was slight anaemia. Another dog
remained healthy, except for slight anaemia, when given ferbam alone
at a dose of 25 mg/kg bw per day for one month or 50 mg/kg bw per day
for one week. An attempt to raise the dose to 100 mg/kg bw per day
immediately provoked severe vomiting and malaise.
In a study in which dogs were treated with ferbam at doses of
0.5, 5, or 25 mg/kg bw per day for one year, the NOAEL was 5 mg/kg bw
per day, on the basis of convulsions at 25 mg/kg bw per day.
In a two-year study of toxicity and carcinogenicity in rats
treated at dietary concentrations of 0, 25, 250, or 2500 ppm, the
NOAEL was 250 ppm, equivalent to 12 mg/kg bw per day, on the basis
of depressed growth rate, shortened life span, neurological
changes, cystic brain lesions, and testicular atrophy at 2500 ppm.
Carcinogenicity was not demonstrated.
Sperm quality was investigated in mice given oral doses of
0, 250, 500, or 1000 mg/kg bw per day for five consecutive days. The
NOAEL was 500 mg/kg bw per day, on the basis of an increased frequency
of sperm abnormalities at 1000 mg/kg bw per day.
In a three-generation study of reproductive toxicity in rats fed
dietary concentrations of 0 or 250 ppm, the NOAEL was 250 ppm,
equivalent to 12 mg/kg bw per day.
Few data were available on genotoxicity. Ferbam did not induce
reverse mutation in bacteria.
Ferbam was slightly irritating to the skin and mildly irritating
to the eyes of rabbits. It has weak skin sensitizing properties in
guinea-pigs.
The Meeting concluded that the toxicological data specifically
generated for ferbam were inadequate to estimate an ADI; however,
because of the similarity of the chemical structure of ferbam to
that of ziram and the comparable toxicological profile of the two
compounds, ferbam was included in the group ADI of 0-0.003 mg/kg bw
for ferbam and ziram, which is derived from the information available
on ziram.
Toxicological evaluation
Levels that cause no toxicological effect
Mouse: 500 mg/kg bw per day (study of sperm quality)
Rat: 100 ppm, equivalent to 10 mg/kg bw per day (one-month
study of toxicity)
250 ppm, equivalent to 12 mg/kg bw per day (two-year
study of toxicity and carcinogenicity)
250 ppm, equivalent to 12 mg/kg bw per day (study of
reproductive toxicity)
Dog: 5 mg/kg bw per day (one-year study of toxicity)
Estimate of acceptable daily intake for humans
0-0.003 mg/kg bw (fgroup ADI for ferbam and ziram)
Studies that would provide information useful for continued evaluation
of the compound
1. Studies on dissociation in aqueous solutions
2. Observations in humans
Toxicological criteria for estimating guidance values for dietary and non-dietary exposure to ferbam
Exposure Relevant route, study type, species Results, remarks
Short-term (1-7 days) Oral, toxicity, mouse LD50 = 1000 mg/kg bw
Oral, toxicity, rat LD50 = 11 000 mg/kg bw
Inhalation, toxicity, rat LC50 = 0.3 mg/litre
Dermal, irritation, rabbit Slightly irritating
Ocular, irritation, rabbit Mildly irritating
Dermal, sensitization, guinea-pig Weakly sensitizing
Repeated oral, 5 days, testicular toxicity, mouse NOAEL = 500 mg/kg bw per day, increased sperm
abnormalities
Medium-term (1-26 weeks) Repeated oral, 4 weeks, toxicity, rat NOAEL = 10 mg/kg bw per day, reduced body
weight
Repeated oral, reproductive toxicity, rat NOAEL = 12 mg/kg bw per day, reproductive
toxicity
Long-term (> 1 year) Repeated oral, 104 weeks, toxicity and NOAEL = 12 mg/kg bw per day, reduced body
carcinogenicity, rat weight, shortened life-span, neurological
changes, cystic brain lesions, and atrophied
testis. No carcinogenicity
Repeated oral, 1 year, toxicity, dog NOAEL = 5 mg/kg bw per day, convulsions
References
DeLorenzo, F.D., Staiano, N., Lorenzo, S. & Cortese, R. (1978)
Mutagenicity of diallate, sulfallate, and triallate and relationship
between structure and mutagenic effects of carbamates used widely in
agriculture. Cancer Res., 38, 13-15.
Hardy, C.J. & Jackson, G.C. (1988) Ferbam technical, acute inhalation
toxicity in rats, 4-hour exposure. Unpublished report No. UCB
285/88179 from Huntingdon Research Centre Ltd, Huntingdon, Cambs,
United Kingdom. Submitted to WHO by UCB Chemicals, Brussels, Belgium.
Lee, C.-C., Russell, J.Q., Minor, J.L., Kowalski, J.J., Sanyer, J.L.,
Kintner, L.D., Hodgson, J.R., Short, R.D., Peters, P.J., Dilley, J.V.,
Murrill, E.A., Holton, D.O. & Ellis, H.V. (1975) Toxicological
evaluation of ferric dimethyldithiocarbamate (ferbam) and dithio-
carbamate (thiram) with acute toxicity of manganese and zinc ethylene-
bisdithiocarbamates (maneb and zineb). Unpublished report No. 3612-B
from National Institute of Environmental Health Sciences, Research
Triangle Park, NC, USA. Submitted to WHO by UCB Chemicals, Brussels,
Belgium.
McDonald, P. (1988) Ferbam technical, acute inhalation toxicity
study in rats. Unpublished report No. 5115 from Inveresk Research
International, Musselburgh, Scotland. Submitted to WHO by UCB
Chemicals, Brussels, Belgium.
Reijnders, J.B.J. (1987a) Evaluation of the acute oral toxicity of
ferbam technical in the rat. Unpublished report No. Notox 0740/930
from Notox CV, 's-Hertogenbosch, Netherlands. Submitted to WHO by UCB
Chemicals, Brussels, Belgium.
Reijnders, J.B.J. (1987b) Evaluation of the acute dermal toxicity of
ferbam technical in the rabbit. Unpublished report No. Notox 0740/931
from Notox CV, 's-Hertogenbosch, Netherlands. Submitted to WHO by UCB
Chemicals, Brussels, Belgium.
Quinto, I., Marinis, E.D., Mallardo, M., Arcucci, A., Morte, R.D. &
Staiano, N. (1989) Effect of DNOC, ferbam and imidan exposure on mouse
sperm morphology. Mutat. Res., 224, 405-408.
Weterings, P.J.J.M. & Daamen, P.A.M. (1987a) Assessment of primary
skin irritation/corrosion by ferbam technical in the rabbit.
Unpublished report No. Notox 0740/932 from Notox CV, 's-Hertogenbosch,
Netherlands. Submitted to WHO by UCB Chemicals, Brussels, Belgium.
Weterings, P.J.J.M. & Daamen, P.A.M (1987b) Assessment of the
primary eye irritation/corrosion by ferbam technical in the rabbit.
Unpublished report No. Notox 0740/933 from Notox CV, 's-Hertogenbosch,
Netherlands. Submitted to WHO by UCB Chemicals, Brussels, Belgium.
Weterings, P.J.J.M. & Daamen, P.A.M. (1987c) Assessment of the skin
sensitization potential of ferbam technical in the guinea-pig (split
adjuvant test). Unpublished-report No. Notox 0740/934 from Notox CV,
's-Hertogenbosch, Netherlands. Submitted to WHO by UCB Chemicals,
Brussels, Belgium.
WHO (1996) The WHO Recommended Classification of Pesticides by Hazard
and Guidelines to Classification 1996-1997 (WHO/PCS/96.3), International
Programme on Chemical Safety, Geneva.
 (a) Acute toxicity
The acute toxicity of ferbam is summarized in Table 1.
(b) Short-term toxicity
Rats
Groups of 10 male and 10 female weanling NR strain rats were
fed ferbam (purity unspecified) at concentrations of 0, 100, 500,
2500, or 5000 ppm (equivalent to 0, 10, 50, 250, or 500 mg/kg bw per
day) for one month. At doses > 500 ppm, growth depression was
found, and at 5000 ppm the mortality rate was increased. No significant
histopathological changes were seen in any of the animals. The NOAEL
was 100 ppm, equivalent to 10 mg/kg bw per day, on the basis of growth
depression at doses > 500 ppm (Annex 1, reference 4).
Groups of 10 male and 10 female NR strain rats were fed ferbam
(purity unspecified) at concentrations of 0 or 2500 ppm (equivalent to
0 or 250 mg/kg bw per day) for one month. Post-mortem examination
revealed no thyroid abnormalities (Annex I, reference 4).
Dogs
One dog (strain unspecified) was given ferbam (purity unspecified)
and ziram together for one month, each at a dose of 5 mg/kg bw per day.
The only adverse effect was slight anaemia. Another dog remained
healthy, except for slight anaemia, when given ferbam alone at a dose
of 25 mg/kg bw per day for one month or at 50 mg/kg bw per day for one
week. An attempt to raise the dose to 100 mg/kg bw per day immediately
provoked severe vomiting and malaise (Annex I, reference 4).
Groups of two adult dogs (strain unspecified) received ferbam
(purity unspecified) at doses of 0.5, 5, or 25 mg/kg bw per day for
one year. Convulsions occurred in animals at 25 mg/kg bw per day.
Urine analysis, blood picture, organ weights, and histological
examination (including the thyroid) showed no abnormalities. The NOAEL
was 5 mg/kg bw per day, on the basis of convulsions at 25 mg/kg bw per
day (Annex I, reference 4).
Table 1. Acute toxicity of ferbam in experimental animals
Species Sex Route Purity LD50 (mg/kg bw) Reference
(%) or LC50 (mg/litre)
Mouse Male Oral NR 1000 Annex 1, reference 4
Mouse Female Intraperitoneal NR 3000 Annex 1, reference 4
Rat Male Oral NR 11 000 Annex 1, reference 4
Rat Female Intraperitoneal NR 2700 Annex 1, reference 4
Rat Male, female Inhalation 91.8 0.40 Hardy & Jackson (1988)
Rat Male, female Inhalation 91.8 0.28 McDonald (1988)
Rabbit Male, female Dermal 91.8 > 4000 Reijnders (1987b)
NR, not reported
(c) Long-term toxicity and carcinogenicity
Rats
Groups of 25 male and 25 female rats (strain unspecified) were
fed ferbam (purity unspecified) at concentrations of 0, 25, 250, or
2500 ppm (equivalent to 1.3, 12.5, or 125 mg/kg bw per day) for two
years. In animals at the highest dose, the growth rate was depressed,
the life-span was shortened, neurological changes and cystic brain
lesions were seen, and the testes were atrophied. The thyroid glands
were normal in all groups, and there was no increase in tumour
incidence. The NOAEL was 250 ppm, equivalent to 12.5 mg/kg bw per day,
on the basis of the changes at 125 mg/kg bw per day (Annex I,
reference 4).
(d) Reproductive toxicity
Mice
Groups of six (C3H × C57B1/6)F1 mice, 11-15 weeks old, received
ferbam (purity, 99.8%) at doses of 0, 250, 500, or 1000 mg/kg bw per
day orally or 0, 125, 250, or 500 mg/kg bw per day intraperitoneally
for five days. After 35 days, toxicity to the testis was evaluated by
measuring the testicular weight, sperm counts, and the percentage of
abnormal sperm. Neither testicular weights nor sperm counts were
affected. The frequency of abnormal sperm in the control mice ranged
from 1.6 to 2.4%. The NOAEL was 500 mg/kg bw per day, on the basis of
a statistically significant increase in sperm abnormalities (6.3%)
after oral administration of ferbam at 1000 mg/kg bw per day. No
effects were seen after intraperitoneal administration, indicating
that active metabolites were responsible for the effects (Quinto
et al., 1989).
Rats
Groups of 16 male and 16 female rats (strain unspecified) were
fed ferbam (purity unspecified) at concentrations of 0 or 250 ppm
(equivalent to 0 or 25 mg/kg bw per day) in a three-generation study
with two litters per generation. The treated animals were maintained
for three months after weaning before the first mating. No effect was
seen on fertility, gestation, viability, lactation, or litter size,
and no gross or histological abnormalities in comparison with controls
were found in animals from the second litter of the third filial
generation selected for examination (Annex I, reference 9).
(e) Genotoxicity
The only results for genotoxicity that have been reported are
those of an Ames test for reverse mutation in Salmonella typhimurium
strains TA98, TA100, TA1535, TA1537, and TA1538 with a preparation
of ferbam of unspecified purity at doses up to 1500 µg/plate. No
mutagenicity was seen (De Lorenzo et al., 1978).
(f) Special studies: Dermal and ocular irritation and dermal
sensitization
The dermal irritancy of ferbam was tested in New Zealand white
rabbits by removing the hair from the dorsal flanks and 24 h later
applying a 6-cm2 gauze patch spread evenly with 0.5 g ferbam (purity,
91.8%) moistened with 0.5 ml water to the left flank of each of six
animals; the right flank was covered with the same dressing without
the test substance. The test sites were covered with permeable tape
and wrapped in flexible bandage. The sites were exposed for 4 h, after
which the remaining test substance was removed. The treated skin was
examined for erythema and oedema after 1 h and on days 1, 2, and 3.
The only dermal reaction seen was very slight erythema in one animal
1 h after removal of the dressings, which disappeared within 24 h. The
primary dermal irritation index was 0.02, indicating that ferbam is
slightly irritating to the skin; however, the occurrence of only a
brief reaction in one animal led the authors to conclude that it is
practically non-irritating (Weterings & Daamen, 1987a).
To test the ocular irritancy of ferbam, the eyes of six female
New Zealand white rabbits were examined, and then the conjunctival sac
of the left eye was installed with 21 mg of ferbam (purity, 91.8%),
equivalent to 0.1 ml, using a spatula. The ocular lesions were graded
and scored 1, 24, 48, and 72 h and 7 and 10 days after instillation.
Immediately after scoring, 24 and 72 h after treatment, a solution of
2% fluorescein in water was applied to both eyes to examine the
potential for corneal injury. Ferbam affected only the conjunctivae:
very slight conjunctival redness was seen in all six animals, with a
short increase to diffuse redness in two animals on day 3. The redness
had disappeared by day 10. Five of the six animals showed obvious
chemosis, and the sixth slight chemosis, which had dosappeared by day
7 in all animals. On the basis of a Draize score of 6.3 (24 h), the
authors concluded that ferbam is mildly irritating to the eye
(Weterings & Daamen, 1987b).
The dermal sensitizing potential of ferbam (purity, 91.8%) was
examined in 35 adult female Dunkin-Hartley guinea-pigs. After
induction by epicutaneous applications of the test substance (25% w/w
in 1% aqueous methylcellulose) and challenge with a series of
concentrations of ferbam (25, 10, and 5% w/w in 1% aqueous methyl-
cellulose), one of the experimental animals showed a positive reaction
to all three concentrations, indicating a sensitization rate of 5%
(grade 2). Ferbam was thus considered to have weak sensitizing
properties (Weterings & Daamen, 1987c).
Comments
Ferbam is well absorbed after oral administration to rats and is
extensively metabolized. Most of the administered radiolabel was found
in the urine, expired air, and bile. In pregnant rats, a small but
significant amount crossed the placenta into the fetus. In lactating
rats, the radiolabel was secreted into the milk, absorbed by the pups,
and excreted in the pups' urine. In expired air, the main product was
carbon disulfide; in the urine, the main products were inorganic
sulfate, a salt of dimethylamine, and the glucuronide conjugate of
dimethyldithiocarbamic acid.
Ferbam has low acute toxicity and has been classified by WHO as
unlikely to present an acute hazard in normal use (WHO, 1996).
In two four-week studies, rats were fed diets providing ferbam at
concentrations of 0, 100, 500, 2500, or 5000 ppm or 0 or 2500 ppm. The
NOAEL was 100 ppm, equivalent to 10 mg/kg bw per day, on the basis
of growth depression at doses > 500 ppm. Post-mortem examination
revealed no thyroid abnormalities. In another four-week study in which
one dog was given ferbam and ziram together, each at a dose of 5 mg/kg
bw per day; the only adverse effect was slight anaemia. Another dog
remained healthy, except for slight anaemia, when given ferbam alone
at a dose of 25 mg/kg bw per day for one month or 50 mg/kg bw per day
for one week. An attempt to raise the dose to 100 mg/kg bw per day
immediately provoked severe vomiting and malaise.
In a study in which dogs were treated with ferbam at doses of
0.5, 5, or 25 mg/kg bw per day for one year, the NOAEL was 5 mg/kg bw
per day, on the basis of convulsions at 25 mg/kg bw per day.
In a two-year study of toxicity and carcinogenicity in rats
treated at dietary concentrations of 0, 25, 250, or 2500 ppm, the
NOAEL was 250 ppm, equivalent to 12 mg/kg bw per day, on the basis
of depressed growth rate, shortened life span, neurological
changes, cystic brain lesions, and testicular atrophy at 2500 ppm.
Carcinogenicity was not demonstrated.
Sperm quality was investigated in mice given oral doses of
0, 250, 500, or 1000 mg/kg bw per day for five consecutive days. The
NOAEL was 500 mg/kg bw per day, on the basis of an increased frequency
of sperm abnormalities at 1000 mg/kg bw per day.
In a three-generation study of reproductive toxicity in rats fed
dietary concentrations of 0 or 250 ppm, the NOAEL was 250 ppm,
equivalent to 12 mg/kg bw per day.
Few data were available on genotoxicity. Ferbam did not induce
reverse mutation in bacteria.
Ferbam was slightly irritating to the skin and mildly irritating
to the eyes of rabbits. It has weak skin sensitizing properties in
guinea-pigs.
The Meeting concluded that the toxicological data specifically
generated for ferbam were inadequate to estimate an ADI; however,
because of the similarity of the chemical structure of ferbam to
that of ziram and the comparable toxicological profile of the two
compounds, ferbam was included in the group ADI of 0-0.003 mg/kg bw
for ferbam and ziram, which is derived from the information available
on ziram.
Toxicological evaluation
Levels that cause no toxicological effect
Mouse: 500 mg/kg bw per day (study of sperm quality)
Rat: 100 ppm, equivalent to 10 mg/kg bw per day (one-month
study of toxicity)
250 ppm, equivalent to 12 mg/kg bw per day (two-year
study of toxicity and carcinogenicity)
250 ppm, equivalent to 12 mg/kg bw per day (study of
reproductive toxicity)
Dog: 5 mg/kg bw per day (one-year study of toxicity)
Estimate of acceptable daily intake for humans
0-0.003 mg/kg bw (fgroup ADI for ferbam and ziram)
Studies that would provide information useful for continued evaluation
of the compound
1. Studies on dissociation in aqueous solutions
2. Observations in humans
Toxicological criteria for estimating guidance values for dietary and non-dietary exposure to ferbam
Exposure Relevant route, study type, species Results, remarks
Short-term (1-7 days) Oral, toxicity, mouse LD50 = 1000 mg/kg bw
Oral, toxicity, rat LD50 = 11 000 mg/kg bw
Inhalation, toxicity, rat LC50 = 0.3 mg/litre
Dermal, irritation, rabbit Slightly irritating
Ocular, irritation, rabbit Mildly irritating
Dermal, sensitization, guinea-pig Weakly sensitizing
Repeated oral, 5 days, testicular toxicity, mouse NOAEL = 500 mg/kg bw per day, increased sperm
abnormalities
Medium-term (1-26 weeks) Repeated oral, 4 weeks, toxicity, rat NOAEL = 10 mg/kg bw per day, reduced body
weight
Repeated oral, reproductive toxicity, rat NOAEL = 12 mg/kg bw per day, reproductive
toxicity
Long-term (> 1 year) Repeated oral, 104 weeks, toxicity and NOAEL = 12 mg/kg bw per day, reduced body
carcinogenicity, rat weight, shortened life-span, neurological
changes, cystic brain lesions, and atrophied
testis. No carcinogenicity
Repeated oral, 1 year, toxicity, dog NOAEL = 5 mg/kg bw per day, convulsions
References
DeLorenzo, F.D., Staiano, N., Lorenzo, S. & Cortese, R. (1978)
Mutagenicity of diallate, sulfallate, and triallate and relationship
between structure and mutagenic effects of carbamates used widely in
agriculture. Cancer Res., 38, 13-15.
Hardy, C.J. & Jackson, G.C. (1988) Ferbam technical, acute inhalation
toxicity in rats, 4-hour exposure. Unpublished report No. UCB
285/88179 from Huntingdon Research Centre Ltd, Huntingdon, Cambs,
United Kingdom. Submitted to WHO by UCB Chemicals, Brussels, Belgium.
Lee, C.-C., Russell, J.Q., Minor, J.L., Kowalski, J.J., Sanyer, J.L.,
Kintner, L.D., Hodgson, J.R., Short, R.D., Peters, P.J., Dilley, J.V.,
Murrill, E.A., Holton, D.O. & Ellis, H.V. (1975) Toxicological
evaluation of ferric dimethyldithiocarbamate (ferbam) and dithio-
carbamate (thiram) with acute toxicity of manganese and zinc ethylene-
bisdithiocarbamates (maneb and zineb). Unpublished report No. 3612-B
from National Institute of Environmental Health Sciences, Research
Triangle Park, NC, USA. Submitted to WHO by UCB Chemicals, Brussels,
Belgium.
McDonald, P. (1988) Ferbam technical, acute inhalation toxicity
study in rats. Unpublished report No. 5115 from Inveresk Research
International, Musselburgh, Scotland. Submitted to WHO by UCB
Chemicals, Brussels, Belgium.
Reijnders, J.B.J. (1987a) Evaluation of the acute oral toxicity of
ferbam technical in the rat. Unpublished report No. Notox 0740/930
from Notox CV, 's-Hertogenbosch, Netherlands. Submitted to WHO by UCB
Chemicals, Brussels, Belgium.
Reijnders, J.B.J. (1987b) Evaluation of the acute dermal toxicity of
ferbam technical in the rabbit. Unpublished report No. Notox 0740/931
from Notox CV, 's-Hertogenbosch, Netherlands. Submitted to WHO by UCB
Chemicals, Brussels, Belgium.
Quinto, I., Marinis, E.D., Mallardo, M., Arcucci, A., Morte, R.D. &
Staiano, N. (1989) Effect of DNOC, ferbam and imidan exposure on mouse
sperm morphology. Mutat. Res., 224, 405-408.
Weterings, P.J.J.M. & Daamen, P.A.M. (1987a) Assessment of primary
skin irritation/corrosion by ferbam technical in the rabbit.
Unpublished report No. Notox 0740/932 from Notox CV, 's-Hertogenbosch,
Netherlands. Submitted to WHO by UCB Chemicals, Brussels, Belgium.
Weterings, P.J.J.M. & Daamen, P.A.M (1987b) Assessment of the
primary eye irritation/corrosion by ferbam technical in the rabbit.
Unpublished report No. Notox 0740/933 from Notox CV, 's-Hertogenbosch,
Netherlands. Submitted to WHO by UCB Chemicals, Brussels, Belgium.
Weterings, P.J.J.M. & Daamen, P.A.M. (1987c) Assessment of the skin
sensitization potential of ferbam technical in the guinea-pig (split
adjuvant test). Unpublished-report No. Notox 0740/934 from Notox CV,
's-Hertogenbosch, Netherlands. Submitted to WHO by UCB Chemicals,
Brussels, Belgium.
WHO (1996) The WHO Recommended Classification of Pesticides by Hazard
and Guidelines to Classification 1996-1997 (WHO/PCS/96.3), International
Programme on Chemical Safety, Geneva.
(a) Acute toxicity
The acute toxicity of ferbam is summarized in Table 1.
(b) Short-term toxicity
Rats
Groups of 10 male and 10 female weanling NR strain rats were
fed ferbam (purity unspecified) at concentrations of 0, 100, 500,
2500, or 5000 ppm (equivalent to 0, 10, 50, 250, or 500 mg/kg bw per
day) for one month. At doses > 500 ppm, growth depression was
found, and at 5000 ppm the mortality rate was increased. No significant
histopathological changes were seen in any of the animals. The NOAEL
was 100 ppm, equivalent to 10 mg/kg bw per day, on the basis of growth
depression at doses > 500 ppm (Annex 1, reference 4).
Groups of 10 male and 10 female NR strain rats were fed ferbam
(purity unspecified) at concentrations of 0 or 2500 ppm (equivalent to
0 or 250 mg/kg bw per day) for one month. Post-mortem examination
revealed no thyroid abnormalities (Annex I, reference 4).
Dogs
One dog (strain unspecified) was given ferbam (purity unspecified)
and ziram together for one month, each at a dose of 5 mg/kg bw per day.
The only adverse effect was slight anaemia. Another dog remained
healthy, except for slight anaemia, when given ferbam alone at a dose
of 25 mg/kg bw per day for one month or at 50 mg/kg bw per day for one
week. An attempt to raise the dose to 100 mg/kg bw per day immediately
provoked severe vomiting and malaise (Annex I, reference 4).
Groups of two adult dogs (strain unspecified) received ferbam
(purity unspecified) at doses of 0.5, 5, or 25 mg/kg bw per day for
one year. Convulsions occurred in animals at 25 mg/kg bw per day.
Urine analysis, blood picture, organ weights, and histological
examination (including the thyroid) showed no abnormalities. The NOAEL
was 5 mg/kg bw per day, on the basis of convulsions at 25 mg/kg bw per
day (Annex I, reference 4).
Table 1. Acute toxicity of ferbam in experimental animals
Species Sex Route Purity LD50 (mg/kg bw) Reference
(%) or LC50 (mg/litre)
Mouse Male Oral NR 1000 Annex 1, reference 4
Mouse Female Intraperitoneal NR 3000 Annex 1, reference 4
Rat Male Oral NR 11 000 Annex 1, reference 4
Rat Female Intraperitoneal NR 2700 Annex 1, reference 4
Rat Male, female Inhalation 91.8 0.40 Hardy & Jackson (1988)
Rat Male, female Inhalation 91.8 0.28 McDonald (1988)
Rabbit Male, female Dermal 91.8 > 4000 Reijnders (1987b)
NR, not reported
(c) Long-term toxicity and carcinogenicity
Rats
Groups of 25 male and 25 female rats (strain unspecified) were
fed ferbam (purity unspecified) at concentrations of 0, 25, 250, or
2500 ppm (equivalent to 1.3, 12.5, or 125 mg/kg bw per day) for two
years. In animals at the highest dose, the growth rate was depressed,
the life-span was shortened, neurological changes and cystic brain
lesions were seen, and the testes were atrophied. The thyroid glands
were normal in all groups, and there was no increase in tumour
incidence. The NOAEL was 250 ppm, equivalent to 12.5 mg/kg bw per day,
on the basis of the changes at 125 mg/kg bw per day (Annex I,
reference 4).
(d) Reproductive toxicity
Mice
Groups of six (C3H × C57B1/6)F1 mice, 11-15 weeks old, received
ferbam (purity, 99.8%) at doses of 0, 250, 500, or 1000 mg/kg bw per
day orally or 0, 125, 250, or 500 mg/kg bw per day intraperitoneally
for five days. After 35 days, toxicity to the testis was evaluated by
measuring the testicular weight, sperm counts, and the percentage of
abnormal sperm. Neither testicular weights nor sperm counts were
affected. The frequency of abnormal sperm in the control mice ranged
from 1.6 to 2.4%. The NOAEL was 500 mg/kg bw per day, on the basis of
a statistically significant increase in sperm abnormalities (6.3%)
after oral administration of ferbam at 1000 mg/kg bw per day. No
effects were seen after intraperitoneal administration, indicating
that active metabolites were responsible for the effects (Quinto
et al., 1989).
Rats
Groups of 16 male and 16 female rats (strain unspecified) were
fed ferbam (purity unspecified) at concentrations of 0 or 250 ppm
(equivalent to 0 or 25 mg/kg bw per day) in a three-generation study
with two litters per generation. The treated animals were maintained
for three months after weaning before the first mating. No effect was
seen on fertility, gestation, viability, lactation, or litter size,
and no gross or histological abnormalities in comparison with controls
were found in animals from the second litter of the third filial
generation selected for examination (Annex I, reference 9).
(e) Genotoxicity
The only results for genotoxicity that have been reported are
those of an Ames test for reverse mutation in Salmonella typhimurium
strains TA98, TA100, TA1535, TA1537, and TA1538 with a preparation
of ferbam of unspecified purity at doses up to 1500 µg/plate. No
mutagenicity was seen (De Lorenzo et al., 1978).
(f) Special studies: Dermal and ocular irritation and dermal
sensitization
The dermal irritancy of ferbam was tested in New Zealand white
rabbits by removing the hair from the dorsal flanks and 24 h later
applying a 6-cm2 gauze patch spread evenly with 0.5 g ferbam (purity,
91.8%) moistened with 0.5 ml water to the left flank of each of six
animals; the right flank was covered with the same dressing without
the test substance. The test sites were covered with permeable tape
and wrapped in flexible bandage. The sites were exposed for 4 h, after
which the remaining test substance was removed. The treated skin was
examined for erythema and oedema after 1 h and on days 1, 2, and 3.
The only dermal reaction seen was very slight erythema in one animal
1 h after removal of the dressings, which disappeared within 24 h. The
primary dermal irritation index was 0.02, indicating that ferbam is
slightly irritating to the skin; however, the occurrence of only a
brief reaction in one animal led the authors to conclude that it is
practically non-irritating (Weterings & Daamen, 1987a).
To test the ocular irritancy of ferbam, the eyes of six female
New Zealand white rabbits were examined, and then the conjunctival sac
of the left eye was installed with 21 mg of ferbam (purity, 91.8%),
equivalent to 0.1 ml, using a spatula. The ocular lesions were graded
and scored 1, 24, 48, and 72 h and 7 and 10 days after instillation.
Immediately after scoring, 24 and 72 h after treatment, a solution of
2% fluorescein in water was applied to both eyes to examine the
potential for corneal injury. Ferbam affected only the conjunctivae:
very slight conjunctival redness was seen in all six animals, with a
short increase to diffuse redness in two animals on day 3. The redness
had disappeared by day 10. Five of the six animals showed obvious
chemosis, and the sixth slight chemosis, which had dosappeared by day
7 in all animals. On the basis of a Draize score of 6.3 (24 h), the
authors concluded that ferbam is mildly irritating to the eye
(Weterings & Daamen, 1987b).
The dermal sensitizing potential of ferbam (purity, 91.8%) was
examined in 35 adult female Dunkin-Hartley guinea-pigs. After
induction by epicutaneous applications of the test substance (25% w/w
in 1% aqueous methylcellulose) and challenge with a series of
concentrations of ferbam (25, 10, and 5% w/w in 1% aqueous methyl-
cellulose), one of the experimental animals showed a positive reaction
to all three concentrations, indicating a sensitization rate of 5%
(grade 2). Ferbam was thus considered to have weak sensitizing
properties (Weterings & Daamen, 1987c).
Comments
Ferbam is well absorbed after oral administration to rats and is
extensively metabolized. Most of the administered radiolabel was found
in the urine, expired air, and bile. In pregnant rats, a small but
significant amount crossed the placenta into the fetus. In lactating
rats, the radiolabel was secreted into the milk, absorbed by the pups,
and excreted in the pups' urine. In expired air, the main product was
carbon disulfide; in the urine, the main products were inorganic
sulfate, a salt of dimethylamine, and the glucuronide conjugate of
dimethyldithiocarbamic acid.
Ferbam has low acute toxicity and has been classified by WHO as
unlikely to present an acute hazard in normal use (WHO, 1996).
In two four-week studies, rats were fed diets providing ferbam at
concentrations of 0, 100, 500, 2500, or 5000 ppm or 0 or 2500 ppm. The
NOAEL was 100 ppm, equivalent to 10 mg/kg bw per day, on the basis
of growth depression at doses > 500 ppm. Post-mortem examination
revealed no thyroid abnormalities. In another four-week study in which
one dog was given ferbam and ziram together, each at a dose of 5 mg/kg
bw per day; the only adverse effect was slight anaemia. Another dog
remained healthy, except for slight anaemia, when given ferbam alone
at a dose of 25 mg/kg bw per day for one month or 50 mg/kg bw per day
for one week. An attempt to raise the dose to 100 mg/kg bw per day
immediately provoked severe vomiting and malaise.
In a study in which dogs were treated with ferbam at doses of
0.5, 5, or 25 mg/kg bw per day for one year, the NOAEL was 5 mg/kg bw
per day, on the basis of convulsions at 25 mg/kg bw per day.
In a two-year study of toxicity and carcinogenicity in rats
treated at dietary concentrations of 0, 25, 250, or 2500 ppm, the
NOAEL was 250 ppm, equivalent to 12 mg/kg bw per day, on the basis
of depressed growth rate, shortened life span, neurological
changes, cystic brain lesions, and testicular atrophy at 2500 ppm.
Carcinogenicity was not demonstrated.
Sperm quality was investigated in mice given oral doses of
0, 250, 500, or 1000 mg/kg bw per day for five consecutive days. The
NOAEL was 500 mg/kg bw per day, on the basis of an increased frequency
of sperm abnormalities at 1000 mg/kg bw per day.
In a three-generation study of reproductive toxicity in rats fed
dietary concentrations of 0 or 250 ppm, the NOAEL was 250 ppm,
equivalent to 12 mg/kg bw per day.
Few data were available on genotoxicity. Ferbam did not induce
reverse mutation in bacteria.
Ferbam was slightly irritating to the skin and mildly irritating
to the eyes of rabbits. It has weak skin sensitizing properties in
guinea-pigs.
The Meeting concluded that the toxicological data specifically
generated for ferbam were inadequate to estimate an ADI; however,
because of the similarity of the chemical structure of ferbam to
that of ziram and the comparable toxicological profile of the two
compounds, ferbam was included in the group ADI of 0-0.003 mg/kg bw
for ferbam and ziram, which is derived from the information available
on ziram.
Toxicological evaluation
Levels that cause no toxicological effect
Mouse: 500 mg/kg bw per day (study of sperm quality)
Rat: 100 ppm, equivalent to 10 mg/kg bw per day (one-month
study of toxicity)
250 ppm, equivalent to 12 mg/kg bw per day (two-year
study of toxicity and carcinogenicity)
250 ppm, equivalent to 12 mg/kg bw per day (study of
reproductive toxicity)
Dog: 5 mg/kg bw per day (one-year study of toxicity)
Estimate of acceptable daily intake for humans
0-0.003 mg/kg bw (fgroup ADI for ferbam and ziram)
Studies that would provide information useful for continued evaluation
of the compound
1. Studies on dissociation in aqueous solutions
2. Observations in humans
Toxicological criteria for estimating guidance values for dietary and non-dietary exposure to ferbam
Exposure Relevant route, study type, species Results, remarks
Short-term (1-7 days) Oral, toxicity, mouse LD50 = 1000 mg/kg bw
Oral, toxicity, rat LD50 = 11 000 mg/kg bw
Inhalation, toxicity, rat LC50 = 0.3 mg/litre
Dermal, irritation, rabbit Slightly irritating
Ocular, irritation, rabbit Mildly irritating
Dermal, sensitization, guinea-pig Weakly sensitizing
Repeated oral, 5 days, testicular toxicity, mouse NOAEL = 500 mg/kg bw per day, increased sperm
abnormalities
Medium-term (1-26 weeks) Repeated oral, 4 weeks, toxicity, rat NOAEL = 10 mg/kg bw per day, reduced body
weight
Repeated oral, reproductive toxicity, rat NOAEL = 12 mg/kg bw per day, reproductive
toxicity
Long-term (> 1 year) Repeated oral, 104 weeks, toxicity and NOAEL = 12 mg/kg bw per day, reduced body
carcinogenicity, rat weight, shortened life-span, neurological
changes, cystic brain lesions, and atrophied
testis. No carcinogenicity
Repeated oral, 1 year, toxicity, dog NOAEL = 5 mg/kg bw per day, convulsions
References
DeLorenzo, F.D., Staiano, N., Lorenzo, S. & Cortese, R. (1978)
Mutagenicity of diallate, sulfallate, and triallate and relationship
between structure and mutagenic effects of carbamates used widely in
agriculture. Cancer Res., 38, 13-15.
Hardy, C.J. & Jackson, G.C. (1988) Ferbam technical, acute inhalation
toxicity in rats, 4-hour exposure. Unpublished report No. UCB
285/88179 from Huntingdon Research Centre Ltd, Huntingdon, Cambs,
United Kingdom. Submitted to WHO by UCB Chemicals, Brussels, Belgium.
Lee, C.-C., Russell, J.Q., Minor, J.L., Kowalski, J.J., Sanyer, J.L.,
Kintner, L.D., Hodgson, J.R., Short, R.D., Peters, P.J., Dilley, J.V.,
Murrill, E.A., Holton, D.O. & Ellis, H.V. (1975) Toxicological
evaluation of ferric dimethyldithiocarbamate (ferbam) and dithio-
carbamate (thiram) with acute toxicity of manganese and zinc ethylene-
bisdithiocarbamates (maneb and zineb). Unpublished report No. 3612-B
from National Institute of Environmental Health Sciences, Research
Triangle Park, NC, USA. Submitted to WHO by UCB Chemicals, Brussels,
Belgium.
McDonald, P. (1988) Ferbam technical, acute inhalation toxicity
study in rats. Unpublished report No. 5115 from Inveresk Research
International, Musselburgh, Scotland. Submitted to WHO by UCB
Chemicals, Brussels, Belgium.
Reijnders, J.B.J. (1987a) Evaluation of the acute oral toxicity of
ferbam technical in the rat. Unpublished report No. Notox 0740/930
from Notox CV, 's-Hertogenbosch, Netherlands. Submitted to WHO by UCB
Chemicals, Brussels, Belgium.
Reijnders, J.B.J. (1987b) Evaluation of the acute dermal toxicity of
ferbam technical in the rabbit. Unpublished report No. Notox 0740/931
from Notox CV, 's-Hertogenbosch, Netherlands. Submitted to WHO by UCB
Chemicals, Brussels, Belgium.
Quinto, I., Marinis, E.D., Mallardo, M., Arcucci, A., Morte, R.D. &
Staiano, N. (1989) Effect of DNOC, ferbam and imidan exposure on mouse
sperm morphology. Mutat. Res., 224, 405-408.
Weterings, P.J.J.M. & Daamen, P.A.M. (1987a) Assessment of primary
skin irritation/corrosion by ferbam technical in the rabbit.
Unpublished report No. Notox 0740/932 from Notox CV, 's-Hertogenbosch,
Netherlands. Submitted to WHO by UCB Chemicals, Brussels, Belgium.
Weterings, P.J.J.M. & Daamen, P.A.M (1987b) Assessment of the
primary eye irritation/corrosion by ferbam technical in the rabbit.
Unpublished report No. Notox 0740/933 from Notox CV, 's-Hertogenbosch,
Netherlands. Submitted to WHO by UCB Chemicals, Brussels, Belgium.
Weterings, P.J.J.M. & Daamen, P.A.M. (1987c) Assessment of the skin
sensitization potential of ferbam technical in the guinea-pig (split
adjuvant test). Unpublished-report No. Notox 0740/934 from Notox CV,
's-Hertogenbosch, Netherlands. Submitted to WHO by UCB Chemicals,
Brussels, Belgium.
WHO (1996) The WHO Recommended Classification of Pesticides by Hazard
and Guidelines to Classification 1996-1997 (WHO/PCS/96.3), International
Programme on Chemical Safety, Geneva.
