
FOLPET
First draft prepared by
J-J. Larsen
Institute of Toxicology, National Food Agency of Denmark, Ministry of
Health, Soborg, Denmark
Explanation
Evaluation for acceptable daily intake
Biochemical aspects
Absorption, distribution, and excretion
Biotransformation
Effects on enzymes and other biochemical parameters
Toxicological studies
Acute toxicity
Short-term toxicity
Long-term toxicity and carcinogenicity
Reproductive toxicity
Developmental toxicity
Genotoxicity
Special studies
Delayed cutaneous hypersensitivity
Observations in humans
Comments
Toxicological evaluation
References
Explanation
Folpet was evaluated toxicologically by the Joint Meeting in
1969, 1973, 1982, 1984, 1986, 1990, and 1993 (Annex I, references 12,
20, 38, 42, 47, 59, and 68). A toxicological monograph was prepared in
1969 (Annex I, reference 13), and monograph addenda were prepared in
1973, 1984, 1986, and 1990 (Annex I, references 21, 43, 49, and 61).
Additional information on the effects on enzymes and other biochemical
parameters, acute toxicity, long-term toxicity and carcinogenicity,
and delayed cutaneous hypersensitivity are reviewed in the present
monograph, together with a compilation of the relevant papers reviewed
by previous meetings.
Evaluation for acceptable daily intake
1. Biochemical aspects
(a) Absorption, distribution, and excretion
Mice (108) and rats (36) received 0, 50, or 5000 ppm folpet in
their diet for 21 days. Two, four or six hours after an oral pulse
dose of 14C-folpet (labelled in the trichloromethylthiol moiety), the
animals were killed and the gastrointestinal tract was removed.
Quantification of the radiolabel present as a percentage of the
administered dose showed that mice removed a greater proportion of
label from the gastrointestinal tract than did rats and at a faster
rate. Less unchanged folpet was found in mice than in rats at each
interval at both doses. The proportion of radiolabel in the tissues
of the gastrointestinal tract was 1-3% in both species. The
gastrointestinal transit time (from stomach to caecum) was less than
2 h in mice and 4-6 h in rats. At each interval after the low dose,
most of the parent compound was recovered from the stomach; however,
the smaller amounts found in the mouse stomach indicate a faster rate
of emptying in this species. At the high dose, most of the unchanged
folpet was located in the mouse caecum and the rat stomach. The
portion of residual activity (considered to be 'covalently' bound to
tissues), presented as the ratio of bound radiolabel at the high and
low dose and expressed in terms of microgram equivalents, varied from
14:1 and 30:1 in the stomach to 115:1 and 180:1 in the ileum of
rats and mice, respectively. Folpet is rapidly degraded in the
gastrointestinal tract, to a greater extent at lower than at higher
doses and in mice more than in rats. The greater amounts of
radioactivity 'covalently' bound after the high dose indicate lack of
sufficient glutathione for removal of thiophosgene (FAO/WHO, 1990).
A study of excretion and radioactive balance over five days in 36
mice and 12 rats treated as in the previous study showed that after a
pulse dose of 14C-folpet, 14C was excreted in expired air, urine, and
faeces. In the first 24 h, urinary excretion of the label was lower in
rats treated with 50 ppm (41.8%) than in those treated with 5000 ppm
(51.5%). More excretion was observed at the low dose in mice (50 ppm,
59.1%; 5000 ppm, 44.3%). Faecal excretion of radiolabel indicated that
the transit times in rats were slower (48 h) than those in mice
(24 h), and at 5000 ppm mice excreted five times more label than rats.
Biliary excretion of 14C-folpet was about 2% in rats and < 0.1% in
mice (FAO/WHO, 1990).
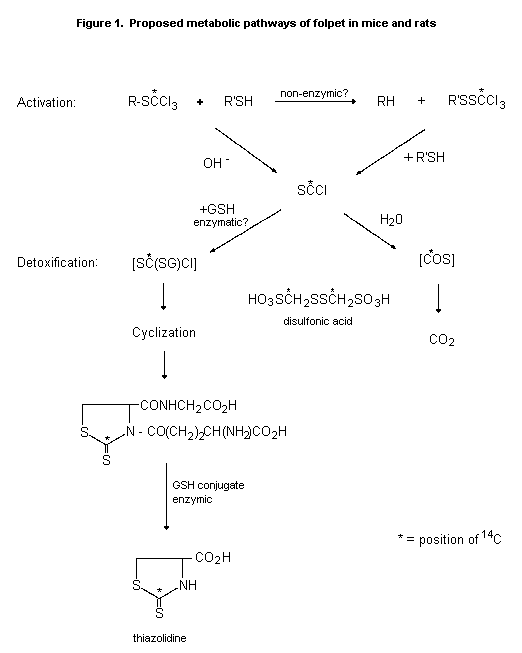
(b) Biotransformation
Hydrolysis of folpet yields phthalimide, which is further
hydrolysed to phthalic acid, chloride ions, and various organic
sulfurs. Similar rapid degradation occurs in the presence of
sulfhydryl compounds. The half-life of the sulfonamide bond in blood
is about 1 min. Data from studies of the trichloromethylthiol group in
captan indicate that the three chlorine atoms are liberated as
chloride ions, four sulfhydride groups being used in the reactions:
two thiol groups to give the tetrahydrophthalimide, thiophosgene, and
one chloride ion, and an additional two thiol groups to react with the
thiophosgene, giving two more chloride ions, carbon disulfide, and the
sulfide derived from the thiol groups (FAO/WHO, 1969). The proposed
metabolic pathways of folpet in mice and rats are shown in Figure 1.
Mice (108) and rats (36) received 0, 50, or 5000 ppm folpet in
the diet for 21 days. Two, four, or six hours after an oral pulse dose
of 14C-folpet (labelled in the trichloromethylthiol moiety), the
animals were killed and the gastrointestinal tract was removed. At
2 h, the disulfonic acid was the major metabolite in rat duodenum,
both the thiazolidine and the glutathione conjugate of thiophosgene
being present. At 4 h, the disulfonic acid predominated and the
thiazolidine metabolite was not seen. The same metabolites were seen
in mice, but the thiazolidine metabolite predominated in the later
samples, indicating that mice rely more than rats on glutathione
conjugation for detoxification of the 'active metabolite' of folpet
(FAO/WHO, 1990).
A study of excretion and radioactive balance over five days in 36
mice and 12 rats treated as in the previous study showed quantitative
differences in the urinary excretion of metabolites. The sulfonic acid
metabolite predominated in rats, while the thiazolidine metabolite
predominated in mice after the high dose. This again indicated a
possibly greater utilization of, and requirement for glutathione in
mice (FAO/WHO, 1990).
(c) Effects on enzymes and other biochemical parameters
Incubation of folpet with rat liver microsomes, with and without
NADPH, showed that folpet may not require metabolism to inhibit
microsomal enzymes. The inhibition of hepatic microsomal cytochrome
P450 by folpet in vitro could be prevented by prior addition of
reduced glutathione to the incubation medium (FAO/WHO, 1990)
Folpet at 5 µmol/litre inhibited the activity of the Ca2+
transport ATPase in human erythrocytes in vitro (FAO/WHO, 1990).
Groups of 150 CD-1 male mice, 30-35 g, and 75 Sprague-Dawley male
rats, 230-300 g, received a single dose of folpet at 0, 7.7, 72, or
668 mg/kg bw by gavage and were killed at various times thereafter,
when the glutathione concentrations were measured in their livers and
in different regions of the gastrointestinal tract. Depletion of the
hepatic and gastrointestinal concentrations of glutathione was
observed in both rats and mice, the latter species showing the most
pronounced effect. The depletion was evident in duodenum, ileum, and
jejunum within 0.5 h after treatment, with a clear dose-response
 relationship in mice. By 2 h after treatment, the glutathione
depletion in rat and mouse duodenum was similar, and at 6 h the
glutathione levels were higher than those in controls. More
glutathione was re-bound in the duodenum and jejunum of mice than of
rats, indicating that mice have a stronger requirement for and greater
utilization of glutathione, this perhaps being the major route of
metabolism in this species (FAO/WHO, 1990).
Mice (72) and rats (24) were fed dietary concentrations of 0, 50,
or 5000 ppm folpet for 21 days. The mean concentration of glutathione
in rat liver at both doses was similar to that in controls, whereas a
small decline was observed in mice at 5000 ppm. In both species, the
glutathione concentrations were significantly increased after 50 ppm
and 5000 ppm in both duodenum and jejunum. A similar effect in the
ileum was more pronounced in mice. These results suggest an initial
depletion of glutathione followed by increasing de novo synthesis of
glutathione, resulting in a 'rebound' elevation in tissue glutathione
concentrations in the gastrointestinal tract. The tissue weights of
the stomach, duodenum, and jejunum were increased in both species. The
mean cytosolic protein concentrations (total milligrams per tissue) in
duodenum and jejunum were also increased in both species, the greater
increase being found in rats. Total cytosolic protein in mouse liver
declined to 86% of control values. Glutathione- S-transferase
levels (with 1-chloro-2,4-dinitrobenzene as substrate) increased
significantly in the duodenum, jejunum, and ileum of both species,
in the liver of rats, and in the stomach of mice after treatment
with 5000 ppm. This led to a greater capacity to enzymatically
conjugate thiophosgene with glutathione. A marked reduction in the
concentrations of lipid peroxides (malonaldehyde was used as an
indicator of the overall lipid peroxidation state of the mucosal
cells) was noted in the duodenum of both species receiving 5000 ppm
folpet in the diet, but the reduction was statistically significant
only in the stomachs of mice. No alteration was found in the
intracellular level of conjugated dienes in either species in
comparison with controls. The non-selenium-dependent glutathione
peroxidase activity (i.e. that due to the activity of glutathione-
S-transferase) was increased in the duodenum, jejunum, and ileum in
both species receiving 5000 ppm folpet in the diet and in the stomachs
of rats at either dose (FAO/WHO, 1990).
In 240 mice and 48 rats treated as in the previous study, the
mean microsomal protein (total milligrams per tissue) was
significantly increased in rat duodenum, jejunum, and ileum. Although
increases were also observed in these tissues in mice, they were not
statistically significant. Total hepatic microsomal protein declined
significantly in rats but remained unchanged in mice. Cytochrome P450
was reduced in the livers of both species, but the reduction was
statistically significant only in mice receiving 5000 ppm folpet. Both
aniline hydroxylase and 7-ethyloxycoumarin O-deethylase activities
were reduced in hepatic microsomes in both species at both doses, the
reduction in aniline hydroxylase being statistically significant at
5000 ppm (FAO/WHO, 1990).
In an experiment with 90 mice and 54 rats treated as in the
previous study, a statistically significant decrease in pH was
observed in the duodenum and jejunum of mice fed 5000 ppm folpet.
Incorporation of 3H-thymidine into the mucosal DNA was reduced in
most tissues of both species. In neither species was their evidence of
a dose-related increase in DNA synthesis (FAO/WHO, 1990).
Male Sprague-Dawley rats (200-250 g) were treated with folpet
suspended in 0.5 ml corn oil by intraperitoneal injection at
doses up to 100 mg/kg bw or orally at doses up to 1000 mg/kg bw.
Intraperitoneal injection of 50 mg/kg bw significantly decreased the
activities of benzphetamine N-demethylase and cytochrome P450, while
that of serum aspartate transaminase was significantly increased. Oral
doses up to 10 times the intraperitoneal dose did not have similar
effects (FAO/WHO, 1990).
In a preliminary study, groups of 313 male Crl:CD-1 (ICR) BR
mice, about 48 days old, were fed 0 or 5000 ppm folpet for 21 days.
Clinical signs and food consumption were monitored weekly. At the
end of treatment, the animals were sacrificed and used for either
histopathological investigations or biochemical measurement of cyclin-
dependent kinases (CKD) and proliferating cell nuclear antigen (PCNA).
Treatment-related findings included glandular hyperplasia of the
crypts, hypertrophy of the villous epithelium of the duodenum,
hypertrophy of the villous epithelium of the jejunum, and a twofold
increase in CKD and PCNA levels in 9000 × g (S9) fractions from the
duodenum. It was concluded that the upper gastrointestinal tract
(especially the duodenum) could be used to assess the effect of
folpet. Sampling of the first 3 cm of duodenum for histopathological
examination and biochemical analysis of sonicated fractions was
recommended (Waterson, 1994a).
Subsequently, the mechanism of the effect of folpet on the
duodenum was studied in groups of 51 Crl:CD-1 (ICR) BR mice fed 0 or
5000 ppm folpet, equal to 692 mg/kg bw per day, or 11 or 111 ppm
perchlormethyl mercaptan, equal to 2 or 16 mg/kg bw per day, for 28
days. The latter compound was included because 5000 ppm technical-
grade folpet contains about 11 ppm. Clinical signs and food
consumption were monitored daily, and body weight was determined
weekly. At the end of treatment, the animals were sacrificed and the
duodenum used for either histopathological investigations or
measurement of CDK and PCNA in S9 fractions. There was no treatment-
related mortality or change in clinical signs. In week 1, the body-
weight gain and the food intake of the animals receiving folpet or
111 ppm perchlormethyl captan was lower than that of controls.
Haematoxylin staining revealed glandular hyperplasia of the crypts in
the first 2.5 cm of the duodenum of most animals receiving folpet and
hypertrophy of the villous epithelium in the entire duodenum of all
animals given folpet. The crypts in the entire duodenum of all animals
in all groups stained for PCNA; more animals receiving folpet showing
a greater degree of staining. An approximately twofold increase over
that in controls was seen in CDK concentrations in fractions from the
entire length of the duodenum of animals receiving folpet; there was
no significant difference from controls in CDK concentrations in mice
fed 11 or 111 ppm perchlormethyl mercaptan. The PCNA concentrations
were significantly higher than control values in S9 fractions from the
entire length of the duodenum in the groups receiving folpet or
11 ppm perchlormethyl mercaptan. It was concluded that folpet acts
significantly differently from perchlormethyl mercaptan in the
duodenum, when CDK is used as the index, and these findings were
supported by the findings of both the biochemical PCNA assay and
histopathological immunochemistry. Since both CDK and PCNA are
increased in proliferating cells, the findings of the two studies were
considered to demonstrate proliferative stimulation as early as three
to four weeks after initiation of treatment with a tumour-inducing
dose of folpet (Waterson, 1994b).
The mechanism of the effect of folpet on the duodenum was further
studied in five groups of 132 male Crl:CD-1 (ICR) BR mice, about 48
days old, which were fed 0 or 5000 ppm folpet (purity, 99.4%), equal
to 717 mg/kg bw; 5000 ppm folpet, equal to 679 mg/kg bw, plus 11 ppm
perchlormethyl mercaptan (purity, 95%), equal to 1.5 mg/kg bw;
11 ppm perchlormethyl mercaptan alone, equal to 1.6 mg/kg bw; or
0.4% hydrogen peroxide, equal to 527 mg/kg bw (positive control,
administered in the drinking-water) for 28 days followed by a 28-day
recovery period for selected mice. An acclimatization period of 11
days was allowed before commencement of treatment, at which time the
mice were nine weeks old and weighed 26-45 g. The mice were housed
three to a cage. The identity of the test compound was confirmed by
chemical analysis, and the accuracy of the preparations and the
homogeneity and stability of the dietary formulations were found to be
satisfactory. Clinical signs and food consumption were monitored
daily. At the end of treatment and recovery, specified animals were
sacrificed and allocated for either pathological investigation of the
duodenum or biochemical measurement of total protein, non-protein
thiol, CDK, and PCNA in duodenal fractions by means of commercially
available kits.
Animals receiving hydrogen peroxide had a statistically
significant reduction in body-weight gain and a lowered efficiency of
food use in comparison with controls. At termination, a marginal
increase in glandular hyperplasia of the crypts and hypertrophy of the
villous epithelium in the duodenum were seen in mice treated with
folpet, folpet plus perchlormethyl mercaptan, or hydrogen peroxide in
comparison with controls. No such changes were seen after the recovery
period.
Treatment of mice with folpet (with or without perchlormethyl
mercaptan) resulted in increased protein (per milligram of tissue) in
total duodenal mucosal epithelium. The effect of folpet was greater in
the first 2.5 cm of the duodenum than in the next 3.5 cm and was
completely reversed after 28 days of withdrawal of animals from the
test diet. Treatment with perchlormethyl mercaptan alone or with
hydrogen peroxide had no such effect. The non-protein thiol
concentration was also increased after administration of folpet (with
or without perchlormethyl mercaptan) and with hydrogen peroxide.
Again, the effects were greatest in the first 2.5 cm of the duodenum
and were fully reversible after the recovery period. Treatment with
perchlormethyl mercaptan alone had no such effect. Folpet (with or
without perchlormethyl mercaptan) and hydrogen peroxide, but not
perchlormethyl mercaptan alone, increased the concentrations of CDK in
the first 2.5 cm of the duodenum; after the recovery period, no
significant difference in CDK concentrations was detected between
treated and untreated animals. The PCNA concentrations in duodenal S9
fractions were close to the limit of detection of the assay system,
but a significant increase in PCNA concentrations was detected after
treatment with folpet (with or without perchlormethyl mercaptan) in
the first 2.5 cm, confirming the CDK response. In contrast, the PCNA
concentrations also indicated cell proliferation in the next 3.5 cm of
the duodenum, although the effect was not seen after the recovery
period. Treatment with perchlormethyl mercaptan or with hydrogen
peroxide had no clear effect on PCNA concentrations in the duodenum.
In none of the investigations was there evidence that perchlormethyl
mercaptan and folpet have interactive effects.
Thus, administration of folpet for 28 days (with or without
perchlormethyl mercaptan) increased protein and non-protein thiol
concentrations in both the first 2.5 cm and the next 3.5 cm of the
duodenum, while CDK concentrations were increased only in the first
2.5 cm. The latter finding was supported by the results of the PCNA
assay but not by histopathological immunocytochemistry. The results
also showed that the biochemical effects of folpet on the mouse
duodenum are reversible after a 28-day recovery period (Waterson,
1995).
2. Toxicological studies
(a) Acute toxicity
The LD50 in adult male and female rats and in female wealings
treated orally with folpet was > 5000 mg/kg bw (FAO/WHO, 1990),
and the LD50 in rabbits treated percutaneously with folpet was
> 23 000 mg/kg bw (FAO/WHO, 1969).
Exposure of rats to folpet (purity unspecified) by inhalation for
4 h showed an LC50 of 0.39 mg/litre for males and 0.43 mg/litre for
females (Blagden, 1991).
(b) Short-term toxicity
Rats
Groups of 10 rats of each sex were fed dietary levels of 0, 0.1,
0.32, or 1% folpet for 12 weeks. Growth was normal, except in male
rats fed the 1% level which showed a significant decrease There were
no gross abnormalities, and histopathological examination of the
liver, kidneys, adrenals, intestines, lungs, and gonads of two males
and two females from each group revealed no abnormalities (FAO/WHO,
1969).
Groups of 20 Fischer 344 rats of each sex were fed dietary
concentrations of 0, 0.2, 0.4 or 0.8% folpet (purity, 89%) for 13
weeks. During treatment, the body weights and food consumption of
males at the middle and high doses and of females at the high dose
were significantly reduced. After 10 weeks, there were no significant
differences between treated and control groups, but there were dose-
related decreases in the activities of alkaline phosphatase and
alanine aminotransferase in all treated groups, of aspartate
aminotransferase in all treated males and in females it the high dose,
and of lactate dehydrogenase in all treated males. Blood urea and
chloride levels were increased, but total serum proteins were reduced
in males at the middle and high doses. Blood urea was reduced in
treated females, total protein was reduced in the high-dose groups,
and albumin was reduced in the mid- and high-dose groups. Treatment-
related irritation of the proximal gastrointestinal tract was seen at
necropsy, as was hyperkeratosis of the non-glandular gastric mucosa.
Slight acanthosis of the stomach occurred in one female rat in each
group. The kidneys of males at the middle and high doses showed slight
but dose-related increases in the number of foci of atrophic
basophilic renal tubules, which were considered to be unrelated to
treatment (FAO/WHO, 1986).
Groups of 20 Sprague-Dawley rats of each sex received folpet
(purity unspecified) at dietary levels of 0, 0.03, 0.1, 0.3, or 1.0%
for 13 weeks. Dietary analyses showed that the achieved doses were
85-106% of the nominal values. At the end of treatment, half of the
rats in each group were sacrificed; the remainder were given the basal
diet for a further two weeks and were then sacrificed. No clinical
signs or mortality occurred during the study period. The growth of
rats at the high dose was significantly reduced during treatment, and
this retardation was not made up during the recovery period. Food
consumption, haematological parameters, serum hepatic enzyme levels,
and renal function were unaffected by treatment. At necropsy, animals
at the high dose had reduced mean body weights; relative brain weight
was also reduced, but kidney weight was increased. There were no
significant differences in organ weights of animals sacrificed two
weeks after treatment. No treatment-related gross pathological changes
were observed, but histopathological examination of animals sacrificed
immediately after treatment showed acanthosis, hyperkeratosis,
submucosal oedema, and pleocellular inflammatory infiltration, with
occasional focal gastric erosion and ulceration in the non-glandular
stomach of rats at the high dose. No such lesions were seen after the
recovery period. The NOAEL was 300 mg/kg bw per day, based on reduced
body weight and other effects at the high dose (FAO/WHO, 1986).
Dogs
In a pilot study, four beagle dogs of each sex were given folpet
(purity, 89.8-91.1%) at 0, 790, 1800 or 4000 mg/kg bw per day by
capsule for 13 weeks. Food intake was generally lower in treated dogs
than in controls. Body weight gain was reduced in all treated groups,
significantly so in animals at the middle and high doses. All treated
dogs showed vomiting and diarrhoea, the symptoms being especially
marked in those at the middle and high doses. Treatment-related
changes included poor condition, abdominal distension, excessive
salivation, and a progressive decrease in testicular size,
particularly in animals at the middle and high doses. All males and
one female at the high dose died or were killed in extremis.
Neurological examination of these dogs and of those males which
survived 12 weeks of treatment and ophthalmoscopic examination of
survivors showed no remarkable changes. Most dogs killed in extremis
had leukocytosis, and some had decreased serum phosphate levels. One
dog had normochromic normocytic anaemia. Surviving dogs had decreased
serum calcium and total plasma protein concentrations, but increased
serum chloride levels. At necropsy, most treated dogs had decreased
brain, liver, kidney, spleen, and testicular weights. Pathological
examination showed atrophy, depletion, and fibrosis of the lymphatic
and haematopoietic systems, gonadal degeneration with prostatic
atrophy and fibrosis, thyroid degeneration, and muscular dystrophy
(FAO/WHO, 1986).
Groups of six beagle dogs of each sex were given folpet (purity,
89.5%) in gelatine capsules at 0, 10, 60, or 140 mg/kg bw per day. The
latter dose was reduced to 120 mg/kg bw per day after 50 days because
of low food consumption and reduced body-weight gain. All animals were
sacrificed and necropsied after one year. All males at the high dose
and three at the middle dose initially lost weight, and the mean body
weights of animals at the middle and high doses showed a dose-related
reduction (which was not significant) throughout the rest of the
study. Dose-related decreases in food consumption were seen in animals
at the middle and high doses, in males during the first three months,
and in females during the first month. Although the food consumption
of males was subsequently comparable to that of controls, that
of females tended to be reduced in a non-dose-related manner.
Ophthalmoscopy at six and 12 months revealed no effect. There was a
tendency for reduced leukocyte counts in males at one, two, three, and
six months, but not at nine or 12 months; however, the counts were not
significantly different from control values. A significant decrease in
mean serum cholesterol and in total protein, albumin, and globulin
levels was seen in males at the middle and high doses; females at the
high dose had significantly reduced mean serum protein, albumin,
and cholesterol levels. Urinalysis, necropsy, and subsequent
histopathology showed no effect of treatment. The NOAEL was 10 mg/kg
bw per day, on the basis of decreased body weight and food consumption
and serum biochemical changes (FAO/WHO, 1986).
Groups of five beagle dogs of each sex received technical-grade
folpet in capsules at 0, 325, 650, or 1300 mg/kg bw per day for 52
weeks. None died. Animals of each sex at the high and intermediate
doses had decreased body weight during treatment; a concomitant
decrease in food intake was found in animals of each sex at the high
dose. Clinical signs seen in all treated groups included vomiting,
diarrhoea, and salivation; the latter was seen only during the first
eight weeks in animals at the low dose but was more pronounced at the
high and intermediate doses. Dogs at these doses were in poorer
condition than controls. Haematological parameters were affected in
all treated females during the first third of the study and included
decreased packed cell volume and haemoglobin and erythrocyte counts.
Clinical chemical changes (e.g. reduced total protein, cholesterol,
glucose, and urea) observed during treatment were related to the poor
physical condition of the dogs. Chloride levels were increased in
males, mainly those at the high dose, which also had decreased calcium
levels; the latter effect was also observed in females at the high and
intermediate doses up to week 25 of treatment. A reduced urine volume
was noted in females at the high dose after 13 weeks of treatment, and
both males and females had acid urine; this effect persisted in males
at the high and intermediate doses throughout the study. Tubular
testicular degeneration associated with an absence of spermatozoa in
the epididymides was found in two male dogs at the high dose; one also
had moderate prostatic gland atrophy. The absolute testicular weights
of males at the high dose were decreased. Changes in relative organs
weights were recorded in the adrenals (all males and females at the
intermediate dose), brain and kidney (males at the high dose and
females at the intermediate dose), liver, and thyroid (males at the
intermediate dose and females at the high dose). The NOAEL was
325 mg/kg bw per day (FAO/WHO, 1990).
(c) Long-term toxicity and carcinogenicity
Mice
Goups of 80 CD-1 (ICR derived) mice of each sex were fed dietary
concentrations of 1000, 5000, or 12 000 ppm folpet (purity, 93%) for
112-113 weeks. A group of 104 mice of each sex served as controls.
Survival was not affected, but body-weight gain was reduced in animals
at 5000 and 12 000 ppm. Although sporadic changes in food consumption
were noted, no dose-related effects were apparent. Haematological
parameters were normal at one year, but at the end of treatment,
possible macrocytic anaemia was seen in animals of each sex at
12 000 ppm. The only changes seen at gross pathological examination
were duodenal lesions and related gastrointestinal abnormalities. A
dose-related increase in the incidence of duodenal adenomas and
adenocarcinomas was observed in mice at 5000 and 12 000 ppm, with
incidences in males and females of 0 and 1% in controls, 2 and 3% at
1000 ppm, 10 and 12% at 5000 ppm, and 52 and 54% at 12 000 ppm,
respectively. Males at the high dose also had an increased incidence
of jejunal adenocarcinomas. There was no NOAEL (FAO/WHO, 1984).
Four groups of 52 B6C3F1 mice of each sex were given dietary
concentrations of folpet (purity, 89.0%) at 0, 0.1, 0.5, or 1.0% for
21 weeks and then 0, 0.1, 0.35, or 0.7% for 83 weeks. Dietary analyses
at weeks 0, 1, 13, and 26 showed the levels to be 88-91% of the
nominal value. Food consumption was depressed during the first few
weeks of treatment in mice at the middle and high doses, and body-
weight gain was reduced in these animals throughout the study.
Clinical signs, observed principally in animals at the high dose,
comprised erythema, dry flaking skin, reddish fur discolouration, and
weeping skin, particularly during the first 21 weeks. Leukocyte counts
in survivors at 52, 78, and 104 weeks were not affected by treatment.
The longevity of animals at the two highest doses was reduced. The
increased relative weights of the brain, heart, lungs, liver, kidneys,
and testes reflected the reduced body weight of treated animals.
Macroscopically, a dose-related increase in non-glandular gastric
mucosal ulceration and thickening of the gastric and duodenal walls
were observed. The jejunal wall was thickened in females at the middle
dose and in all mice at the high dose. Dose-related distension of the
duodenal lumen was also seen. After week 79, there was a dose-related
increase in the incidence of nodules or masses on the luminal surface
of the stomach and duodenum and on the duodenal serosa. Animals at the
middle and high doses had dose-related epidermal hyperkeratosis and
acanthosis and oesophageal hyperkeratosis. Increased areas of marked
acanthosis and hyperkeratosis of non-glandular gastric mucosa were
seen in animals at the middle dose and males at the high dose.
Microscopic gastric ulceration occurred, with no apparent
relation to dose. The dose-related appearance of areas of atypical
duodenal glandular hyperplasia and mucosal gland proliferation were
seen in all treated animals, but especially in males. This atypical
hyperplasia was often associated with duodenal adenomas and
adenocarcinomas. Atypical glandular proliferation was seen only
occasionally in the jejunum of treated females. Gastric papillomas and
squamous-cell carcinomas, which may have been secondary to mechanical
obstruction of the duodenal lumen, were found in all treated males but
not in control males. A dose-related increase in the incidence of
gastric papillomas was also found in all treated females, but
similar lesions occurred in 2/51 controls. Duodenal adenomas and
adenocarcinomas were found in all treated animals of each sex, with
a significant dose-response relationship. A single jejunal
adenocarcinoma was seen in mice at the high dose. Primary or
metastatic tumours of uncertain etiology were found in all treated
males, but the incidences of bronchioalveolar adenomas and malignant
lymphomas were lower in these animals, suggesting high incidences in
control males. There was no NOAEL (FAO/WHO, 1986).
Four groups of 52 six-week old B6C3F1 mice of each sex were given
dietary concentrations of 0, 1000, 5000, or 10 000 ppm folpet (purity,
89%) for 104 weeks. The formulated diets were analysed after one and
two weeks and were found to be stable (no data). During the first few
weeks of the study, marked irritation of the integumentary system was
observed. Typical lesions, usually on the neck, included flaking,
weeping, and eroded skin, erythema, and red discolouration of the fur.
Early deaths occurred in all treated males and in females at the
middle and high doses and were attributed to the presence of fatal
tumours. Dose-related macroscopic lesions were found in the stomach
and duodenum. The non-glandular gastric mucosa of all treated animals
showed an increased incidence of nodules, masses, wall thickenings,
and ulceration, and an increased incidence of partial luminal duodenal
obstruction was seen 1-5 cm distal to the pylorus in the form of wall
thickenings and mucosal nodules. An increased incidence of these
lesions was noted in animals that died after 79 weeks of treatment.
Microscopic examination of the skin revealed hyperkeratosis, and a
dose-related in, crease in the incidence of oesophageal and non-
glandular gastric mucosal hyperkeratosis was seen in all treated
groups. Ulceration of the non-glandular stomach was observed in
animals at the low and intermediate doses. Atypical hyperplasia of the
duodenal mucosa was seen in all treated mice. Duodenal adenomas and
carcinomas were seen in all treated groups, and the incidence was
dose-related. Gastric squamous-cell papillomas and carcinomas were
seen more frequently in animals at 5000 and 10 000 ppm. There appeared
to be a correlation between the presence of macroscopically visible
gastric neoplasia and duodenal obstruction: the four males with
gastric carcinoma had partial duodenal nodular obstruction, and five
of seven females at the high dose with an increased incidence of
gastric papillomas had partially obstructed duodenal lumens. No other
toxic or carcinogenic effect was seen in any organ.
The authors suggested that the partial duodenal obstruction
disturbed the natural flow of the gastrointestinal contents and may
have exacerbated the mucosal changes in the stomach. The resulting
stagnation may have raised the effective concentration and residence
time of folpet, and the elevated concentration of this irritant, with
the possible presence of bile acids and pancreatic enzymes, may have
overwhelmed the defence mechanism of the mucosal cells, resulting
in the development of gastric neoplasms. In conclusion, dietary
administration of folpet to B6C3F1 mice induced a dose-related
appearance of atypical duodenal hyperplasia, adenomas, and
adenocarcinomas, which were suggested to have appeared subsequent
to, and as an indirect result of, the partial luminal duodenal
obstruction. There was no NOAEL (Nyska et al., 1990).
Groups of 52 male and 52 female CD-1 mice were fed diets
containing folpet (purity, 92.4%) at concentrations of 0, 150, 450, or
1350 ppm, equal to 0, 16, 47, and 151 mg/kg bw per day for 98 weeks
for males and 0, 16, 51, and 154 mg/kg bw per day for 104 weeks for
females. A control group consisting of 100 male and 100 female mice
received a normal diet. The mice were assigned to treatment groups on
the basis of computer-generated random numbers and were acclimatized
for 14 days before the beginning of treatment. The mice were 3542 days
of age and had mean body weights of 29.7, 29.6, 29.5, and 29.6 g
(males) and 23.4, 23.7, 23.0, and 23.7 g (females) at 0, 150, 450, and
1350 ppm, respectively, at the time of the first dose. The 150-ppm
preparation was found to contain 89-91% of the nominal concentration
and was stable after storage at -20°C for seven days and then at 20°C
for four days; samples taken from six places in the mix showed
satisfactory homogeneity: 89-95% of the nominal value with 150 ppm and
78-88% with 1350 ppm. Verification of the concentrations resulted in
analytical values of 89-96% of the nominal concentrations. Animals
were inspected daily for evidence of reactions to treatment. Body
weight and food and water consumption were determined weekly. All 187
animals found dead or killed during the treatment period were
subjected to gross pathological and histopathological examination.
The overall mortality rate was unaffected by treatment, and no
inter-group differences in mortality were seen by either pairwise or
trend analyses; therefore, none of the deaths was considered to be
related to treatment with folpet. The incidence of skin encrustations,
particularly on the dorsal surface, was generally higher in males
receiving a dietary concentration of 1350 ppm. This finding was
considered to be associated with a greater incidence of fighting among
these animals during the first 70 weeks of treatment. In the later
part of the treatment period, the incidence of skin encrustations
in males was similar to that of controls. The incidence, group
distribution, location, and mean onset time of palpable swellings were
not considered to be related to treatment. The body-weight gain of
males receiving 1350 ppm was slightly lower than that of controls for
about the first 70 weeks of treatment. Food consumption was unaffected
by treatment, but the efficiency of food use during the first 14 weeks
of treatment with 1350 ppm was slightly inferior to that of the
controls. The treatment-related effect on male body-weight gain and
the low food conversion efficiency seen at this dose during the first
14 weeks of treatment were considered to be nonspecific toxic effects.
The body-weight gain of females was unaffected by treatment.
Organ weight analysis revealed lower absolute and relative liver
weights in males that received 1350 ppm in comparison with controls.
High absolute and relative spleen weights in males at 150 ppm were due
to an unusually large spleen in one animal. Macroscopic examination of
animals killed after 104 weeks of treatment revealed a high incidence
of masses in the duodena of females at 1350 ppm. When all of the
animals were considered together, there was a higher incidence of
duodenal masses and thickening of the stomach wall in females at
1350 ppm than in controls. Microscopic examination revealed benign
papillomas in the keratinized region of the stomach in one male and
three females at 1350 ppm and in one female at 450 ppm. A benign
adenoma was found in the duodenal mucosa of one female at 1350 ppm.
Villous hyperplasia was seen in the duodenal mucosa of three females
at the highest dose and in one male at 450 ppm; a similar finding was
seen in the jejunum of one male at 450 ppm. Hyperplasia of the lamina
propria of the duodenum was seen in two males at 1350 ppm, and another
male at this dose had similar changes in the jejunum and ileum in
addition to villous fusion, mucosal dysplasia, and hyperplasia of
Paneth cells. The incidence of keratoachanthosis of the nonglandular
stomach was higher among females at 1350 ppm. than among controls.
Although one female at 450 ppm had a benign squamous-cell papilloma
and two males at this dose had gastrointestinal hyperplasia, these
findings were considered to have occurred by chance. There were no
other changes in the gastrointestinal tract, and there were no changes
in the liver that were considered to be related to treatment. As the
low liver weights of males at 1350 ppm were confined to one sex and
there was no histopathological correlate, the toxicological
significance of this finding is unclear. It was concluded that
administration of folpet in the diet at a concentration of 1350 ppm
produced tumours in the upper parts of the gastrointestinal tract of
CD-1 mice. The NOAEL was 16 mg/kg bw per day on the basis of duodenal
hyperplasia (East, 1994).
Rats
Groups of 60 Charles River Crl:CD(SD)BR rats of each sex were fed
dietary concentrations of 0, 200, 800, or 3200 ppm technical-grade
folpet (purity, 89.5%) for 104 weeks. An interim sacrifice of 10 rats
of each sex per dose was conducted at 52 weeks. Growth rates and
survival were unaffected by treatment, except for a slight tendency to
reduced body weights in females at the high dose during the first
year; food consumption was correspondingly reduced at this dose.
Conventional ophthalmoscopic, haematological, biochemical, and
urinary analyses indicated no effect of treatment. At necropsy, no
changes attributable to treatment were observed on organ weights.
Hyperkeratosis and/or acanthosis and some erosion and/or ulceration in
the non-glandular stomach were seen in animals at the high dose. These
lesions were occasionally accompanied by submucosal oedema and
submucosal inflammatory cellular infiltrates. The NOAEL was 800 ppm,
equivalent to 40 mg/kg bw per day (FAO/WHO, 1986).
Groups of 60 Fischer 344 rats were fed diets containing 0, 500,
1000, or 2000 ppm folpet (purity, 89%) for 104 weeks. The diets were
prepared weekly and analysed frequently for folpet content. Food
intake was reduced in all treated groups in comparison with controls,
but body weight was reduced only in treated males. There were no
clinical signs of toxicity. Necropsy indicated a tendency to an
increased incidence of gastric ulceration, which was significant
only in females. Treatment also induced an increased incidence
of ulceration in the forestomach in animals at the high dose.
Hyperkeratosis of the oesophagus was seen in rats at the high dose
and of the forestomach in animals at the middle and high doses. An
increased incidence of gastric ulceration was present in the
forestomach of animals of each sex at the high dose. The incidences of
C-cell adenoma, benign mammary fibroepithelioma and malignant lymphoma
showed positive significant trends with dose, but only that for the
latter neoplasms was statistically significant. All tumour incidences
were within the normal historical range for rats of this strain. The
NOAEL was 500 ppm, equivalent to 25 mg/kg bw per day (FAO/WHO, 1986).
Groups of 20 Fischer 344 rats of each sex were fed diets intended
to provide concentrations of 0, 250, 1500, or 5000 ppm folpet (purity,
91%) for 104 weeks. The actual dietary concentrations, calculated
on a weekly basis, were 0, 190, 1290, and 4530 ppm. Longevity was
unaffected. The mean body weight and food intake of animals at the
high dose were depressed, by up to 10% in males and 6% in females,
and water intake was depressed at the high dose, especially in
females. Serum alkaline phosphatase and alanine aminotransferase
activities were reduced in treated groups throughout the study, and
those of aspartate aminotransferase, creatinine phosphokinase, and
gamma-glutamyl transferase were decreased sporadically. A significant
reduction in blood cholesterol was seen in animals of each sex of the
high dose throughout treatment. The plasma protein level was reduced
in males at the high dose during the first year of treatment, and the
level of phosphate was elevated at most examinations. The urea
concentration was increased in females at the high dose at 18 months.
Most treated males excreted more concentrated urine in smaller volumes
at three and six months. Only non-neoplastic lesions were observed,
consisting of hyperkeratosis of the oesophageal and gastric squamous
epithelium. The NOAEL was 190 ppm, equivalent to 10 mg/kg bw per day
(FAO/WHO, 1990).
(d) Reproductive toxicity
Rats
Four groups of 25 CD rats of each sex were fed dietary
concentrations of 0, 250, 1500, or 5000 ppm folpet (purity, 91%) over
the course of two generations. Body weight gain and food consumption
were reduced in parental animals of the F0 and F1 generations at
the highest dose; a minor decrease in body weight was observed in F1
and F2 offspring. The principal histopathological effect was
hyperkeratosis of the non-glandular gastric mucosa in F0 and F1
animals at the intermediate and high doses, oesophageal hyperkeratosis
in F1 animals at the intermediate and high doses, and an increased
incidence of basophilic renal tubules in males of the F0 generation
at the high dose. Folpet was concluded not to be a specific
reproductive toxin in this test system (FAO/WHO, 1990).
In a two-generation study, groups of 30 Crl:COBS/CD(SD)/Charles
River rats of each sex were fed diets containing 0, 200, 800, or
3600 ppm folpet (purity, 89.5%) during growth, mating, gestation, and
lactation for two litters per generation. Initial mating was begun 62
days after the start of exposure. The test diets were fed to F0 males
until the end of mating to produce the F1b litters and to F0 females
until weaning of the F1b litters. Pups were sacrificed 21-23 days
after parturition, with the exception of those F1b pups selected to
produce the F2 generation. Mating of the F1b pups was begun after 12
weeks of exposure, and the above sequence was repeated. Gross necropsy
was performed on all parental rats and on the F1a, F1b, and F2b
litters. The diet was changed three times per week, and each batch was
analysed: the diets contained 79.9-101% of the nominal concentration.
The body weights of F0 and F1 males and F1 females at the high dose
and of their pups were depressed by treatment; the effect was most
marked in adult males, especially in the second generation. Food
consumption was correspondingly reduced. Treatment had no significant
effect on mating, fertility indices, pregnancy rates, litter sizes,
pup weights, growth, or litter survival rates. No treatment-related
effects were found at necropsy or on histopathological examination.
The NOAEL for maternal toxicity was 800 ppm, equivalent to 40 mg/kg bw
per day, based on the reduction in body weights; there was no evidence
of reproductive toxicity (FAO/WHO, 1986).
(e) Developmental toxicity
Rats
In a pilot study, groups of eight female mated CRL:COBS CD (SD)
BR rats were given 0, 20, 80, 320, or 640 mg folpet/kg bw by gavage on
days 6-19 of gestation. There were no deaths, but clinical signs
including rales, excess salivation, chromorhinorrhoea, gasping, soft
or liquid faeces, decreased motor activity, dyspnoea, and distended
gut were observed. Reduced maternal body weight was seen at
> 80 mg/kg bw, and food consumption was reduced at > 320 mg/kg
bw; the average fetal body weight was also reduced at the latter dose.
In the main study, groups of 25 rats were given folpet (purity, 89%)
at 0, 10, 60, or 360 mg/kg bw by gavage on days 6-19 of gestation.
Animals were killed on day 20. Clinical signs including excess
salivation, chromorhinorrhoea, decreased motor activity, soft or
liquid faeces, dyspnoea, and urine-stained fur were observed. Three
rats at the high dose died, two from intubation errors. No gross
lesions attributable to treatment were seen in surviving rats. Rats at
> 10 mg/kg bw gained less weight than controls during treatment.
Food consumption was reduced in animals at the high dose only. The
numbers of implantations, live and dead fetuses, fetal viability, and
resorptions, the average fetal body weight per litter, the fetal sex
ratio, and the number of corpora lutea were comparable in all groups.
Gross, visceral, and skeletal abnormalities were seen in all groups,
but there were no compound-related effects on ossification. The NOAEL
for maternal toxicity was 10 mg/kg bw per day (FAO/WHO, 1984).
Groups of six rats were treated daily on days 6-15 of gestation
with folpet (purity, 88.6%) at 10, 65, 420, or 2750 mg/kg bw by
gavage. Maternal toxicity, reduced body-weight gain, and reduced fetal
weight were seen only at 2750 mg/kg bw. Following this pilot study,
groups of 22 female Charles River CD rats received folpet (purity,
91.1%) in 0.5% acetic acid containing 0.5% carboxymethylcellulose at
0, 150, 550, or 2000 mg/kg bw on days 6-15 of gestation. The animals
were sacrificed on day 20. Clear signs of toxicity were observed in
animals at the high dose, including soft faeces (in 21/21 rats), fur
staining (in 4/21), and perianal staining (in 8/21); one animal died.
Food consumption was decreased during the first days of treatment with
the intermediate dose and was markedly decreased throughout treatment
with the high dose. Maternal body weight was also decreased at the
middle and high doses. Gravid uterine weights were depressed in dams
at the middle and high doses, but terminal maternal body weight
(without the gravid uterus) was significantly depressed only at the
high dose. Pre- and post-implantation losses were greater than in
controls in animals at the middle dose, and fetal weights were reduced
at the middle and high doses. Fetal crown-rump length was slightly
decreased after treatment with the middle and high doses. A single
fetus at the high dose (1/277) had multiple major malformations, and a
second pup had unilateral microphthalmia. The incidence of hepatic
discolouration was significant at the high dose. Skeletal anomalies
occurred in all treated groups, and the incidence of reduced
ossification of cranial and pubic bones, sternebrae, metacarpals, and
metatarsals was significant in animals at the middle and high doses.
There was also a dose-related reduction in ossification of the
interparietal bone in all treated groups, and the occurrence of
angulated ribs was dose-related. There was no NOAEL for teratogenicity
(FAO/WHO, 1986).
Hamsters
Groups of 2-13 pregnant hamsters were given a single dose of
400-1000 mg/kg bw folpet on day 7 or 8 of gestation or were treated
daily on days 6-10 with a total of 1000-2500 mg/kg bw. The animals
were killed and examined on day 15 of gestation. Maternal mortality
was increased and some abnormal fetuses were produced at the highest
doses, but the lower doses did not induce teratogenic effects
(FAO/WHO, 1973).
Rabbits
Groups of 20 artificially inseminated New Zealand white rabbits
were given folpet (purity, 89%) at 0, 10, 20, or 60 mg/kg bw by oral
intubation on days 6-28 of gestation and were killed on day 29. The
death of one doe at 60 mg/kg bw was considered to be related to
treatment. One doe at the low dose aborted on day 21 of gestation and
one at the high dose on day 22 of gestation; one doe in the control
group delivered a litter on day 28 and one at the high dose on day 29.
Significant inhibition of body-weight gain and food consumption was
seen in animals at the middle and high doses. The average numbers of
corpora lutea, implantations, resorptions, and fetuses per litter and
the sex ratio and numbers of dead and resorbed implantations per
litter were comparable in all groups. The mean body weights of all
fetuses were decreased at the middle and high doses. There was a
significant increase in the incidence of hydrocephaly, and four
fetuses in three litters of dams at the high dose also had skull,
gastric, and pulmonary abnormalities. The NOAEL for maternal and
fetoxicity was 10 mg/kg bw per day, and that tor teratogenicity was
29 mg/kg bw per day (FAO/WHO, 1984).
Groups of six mated HY/CR female New Zealand white rabbits were
given folpet (purity, 91.1%) at 0, 10, 60, or 150 mg/kg bw per day by
gavage on days 6-18 of gestation. Marked body-weight loss was seen at
the high dose. Although fetal size was unaffected by treatment, fetal
mortality was more marked at the high dose. Post-implantation losses
were increased in animals at the middle dose. Subsequently, groups of
14 mated HY/CR New Zealand white rabbits were given folpet (purity,
91.1%) at 0, 10, 40, or 160 mg/kg bw per day by gavage on days 7-19 of
gestation. Dams were sacrificed on day 29 of gestation. Body-weight
gain was decreased during the initial few days of treatment with the
middle dose and after initiation of treatment with the high dose;
there was a corresponding decrease in food consumption in animals at
the high dose. There were no deaths. Gravid uterine weight was
significantly reduced in dams at the middle and high doses. Fetal
death (post-implantation loss) occurred more frequently at the high
dose than in controls; the proportion of small fetuses was also
increased in this group and mean fetal weight was nonsignificantly
reduced. There was evidence of delayed skeletal maturation at the high
dose and, to a lesser degree, at the middle dose. The incidence of
bilateral lumbar ribs appeared to increase with dose in animals at the
middle and high doses. Ossification of caudal vertebrae, sternebrae,
and long-bone epiphyses was reduced at the high dose. Other, minor
skeletal malformations did not apper to be related to treatment. There
was no evidence of hydrocephalus in either treated or control rabbits.
The NOAEL for maternal toxicity, fetotoxicity, and teratogenicity was
10 mg/kg bw per day (FAO/WHO, 1986).
Groups of 20 artificially inserminated female Hazelton Dutchland
New Zealand white (D1A Hra: (NZW) specific pathogen-free) rabbits
were given folpet (purity, 89.5%) in Tween 80 (10.5% by weight) and
carboxymethylcellulose (0.7% by weight) at a volume of 5 ml/kg bw per
day by gavage, to give a dose of 60 mg/kg bw per day on days 7-9,
10-12, 13-15, or 16-18 of gestation. Analysis of the formulations
indicated a folpet content of 87.8-104% of the nominal concentration.
Dams were sacrificed on day 29 of gestation; those that aborted
or delivered, the single animal that died, and those terminally
sacrificed were subjected to necropsy and examination of the uterine
contents. Abortion by two rabbits that received folpet on days 7-9 and
10-12 of gestation may have been related to treatment; otherwise, no
clinical signs of toxicity were observed during the study, although
the incidence of soft or liquid faeces was increased in all treated
groups, usually after treatment. No gross lesions attributable
to treatment were seen at necropsy. Maternal body weight was
significantly reduced in all treated animals, although less so in
those treated on days 7-9 and 10-12 than in the others. Food
consumption was correspondingly reduced. Treatment had no apparent
effect on the rate of abortion or on fetal resorption. The average
litter sizes were unaffected, as were the average fetal weights, the
number of viable fetuses, and the sex ratio. A significantly increased
incidence of fetuses with an irregularly shaped fontanelle was
observed in the group treated on days 13-15; the control incidence was
4.5%, but this variation did not occur in groups treated on days 7-9
or 16-18. It was possibly related to treatment, but the significance
of the effect was not clear. There were no other significant
variations in fetal skull morphology, and the incidences of
hydrocephalus and of gastric or pulmonary anomalies were not increased
in any group. The NOAEL for teratogenicity was 60 mg/kg bw per day
(FAO/WHO, 1986).
Hens
The yolks or air sacs of 830 fresh, fertilized hens' eggs were
injected with folpet in dimethyl sulfoxide at 3-20 mg/kg egg weight.
After incubation, the incidence of malformations was 8.19%. The
metabolites of folpet, phthalimide and phthalic acid, were
administered under similar conditions, except that ethanol was used as
the solvent for phthalic acid. Gross abnormalities were seen in 3.93%
of 305 eggs injected with phthalimide and in 3.1% of 290 eggs treated
with phthalic acid. Controls -- 1500 eggs injected with dimethyl
sulfoxide and several thousand with ethanol -- had incidences of < 2%
gross abnormalities. Micromelia, amelia, and phocomelia accounted for
most of the deformities (FAO/WHO, 1969).
(f) Genotoxicity
Folpet has been adequately tested in a number of assays for
genotoxicity in vitro and in vivo. The positive controls gave the
expected positive responses. The results are summarized in Table 1.
(g) Special studies
Delayed cutaneous hypersensitivity
The skin sensitizing potential of folpet (purity and stability
not given) was evaluated in albino Dunkin-Hartley guinea-pigs, 8-11
weeks old, weighing 309-411 g, by the Magnusson-Kligman test. The
animals were obtained from David Hall Limited, Burton-on-Trent,
Staffordshire, United Kingdom, and were acclimatized for at least five
days before treatment. Twenty animals were allocated to the treatment
group (for induction and challenge with folpet) and 10 animals to the
control group (induction with vehicle and/or Freund's complete
adjuvant and challenge with folpet). The animals were induced by three
series of two 0.1-ml intradermal injections of a 1:1 mixture of
Freund's complete adjuvant and water, 0.1% folpet (w/v) in arachis
oil, and 0.1% folpet (w/v) in a 1:1 preparation of Freund's complete
adjuvant plus arachis oil; one week later, 0.2-0.3 ml of 50% folpet
(w/w) in arachis oil was applied topically under an occlusive bandage
for 48 h. Erythematous reactions were quantified after 1 and 24 h.
During challenge, 0.1-0.2 ml of 25% folpet (w/w) in arachis oil was
applied topically under an occlusive bandage for 24 h on the right
flank, and the vehicle alone was applied on the left flank.
Erythematous reactions were quantified after 24 and 48 h. Seven days
after the original challenge, all test and control animals were
rechallenged with 10% folpet (w/w) in arachis oil.
Moderate and diffuse redness was noted at 18 treatment sites 1 h
after removal of the patches. Scattered mild redness was noted at nine
treatment sites at 24 h. Skin reactions observed after the initial
topical challenge included moderate and diffuse redness (at three
sites at 24 h and one site at 48 h) and scattered mild redness (at 14
sites at 24 h and 13 sites at 48 h). The responses were considered to
be due to primary cutaneous irritation caused by folpet. The animals
were re-challenged with a lower concentration of folpet, as the
irritation might have precluded sensitization responses. Scattered
mild redness was elicited at six treatment sites at 24 h and at two
sites at 48 h, the reaction extending beyond the treatment sites in
some animals. Erythema could not be evaluated at some sites because of
other adverse reactions, including desquamation, oedema, scabs,
cracking of the epidermis, fur loss, fissuring, hyperkeratinization,
and loss of skin elasticity. Technical-grade folpet was thus a strong
sensitizer on guinea-pig skin (Dreher, 1990).
Table 1. Results of tests for the genotoxicity of folpet
End-point Test system Concentration Purity Results Reference
or dose (%)
In vitro
Reverse mutation E. coli PQ37 0.03, 0.10, 0.3, or 1.0 µg/ml 90 Positivea FAO/WHO (1986)
Reverse mutation E. coil PQ37 3.0, 10, or 30 µg/ml 90 Negativeb FAO/WHO (1986)
Reverse mutation S. typhimurium TA100, 0 or 50 µg/plate NR Positive only Tennekes (1995)
TA98, TA1535, TA1537, in TA100a
TA1538, E. coli WP2uvrA-
Reverse mutation S. typhimurium TA1535, 0 or 100 µg/plate NR Positivea Tennekes (1995)
TA1537, TA1538
Reverse mutation S. typhimurium TA100, 0, 25, or 45 µg/plate NR Positivea Tennekes (1995)
TA1535
Reverse mutation E. coli TKJ6321, NR Positivea Tennekes (1995)
TKJ5211 Weakly positiveb
Reverse mutation E. coli WP2hcr+ WP2hcr- 0 or 100 µg/plate NR Positivea Tennekes (1995)
Reverse mutation E. coli WP2hcr 0 or 45 µg/plate NR Positivea Tennekes (1995)
Chromosomal aberration Chinese hamster ovary NR NR Positivea Tennekes (1995)
cells Weakly positiveb
Chromosomal aberration Human lymphoid cell line NR NR Positivea Tennekes (1995)
Chromosomal aberration Human lymphocytes 0, 1, 2, or 3 µg/ml for 24 h 90.1 Negativec Tennekes (1995)
Gene mutation hprt locus Chinese hamster V79 0, 125, 0.25, 0.5, 1.0, or 2.0 90.1 Negativea FAO/WHO (1986)
lung fibroblasts µg/ml
Gene mutation hprt locus Chinese hamster V79 3.125, 6.25, 12.5, or 50 µg/ml 90.1 Negativeb FAO/WHO (1986)
lung fibroblasts
Gene mutation hprt locus Chinese hamster ovary NR NR Positivea Tennekes (1995)
cells
Gene mutation tk locus Mouse lymphoma NR Positivea Tennekes (1995)
L5178Y cells
Table 1. (con't)
End-point Test system Concentration Purity Results Reference
or dose (%)
In vivo
Somatic cell mutation T strain male mice Oral; 0, 100, 1500, or 5000 ppm 88.7 Negative FAO/WHO (1986)
C57Bl/6 female mice on days 8.5-12.5 of gestation
Micronucleus formation CD-1 mice 10, 50, or 250 mg/kg bw 91 Negative FAO/WHO (1986)
Micronucleus formation Groups of five male and Oral; 125, 625, or 1250 mg/kg 97 Negatived Ivett (1988)
five female Sprague-Dawley bwj; sacrifice at 24, 48, and
rats; bone-marrow cells 72h; vehicle, corn oil
Sex-linked recessive Drosophila melanogaster NR Positive Tennekes {1995)
lethal mutation
NR, not reported
a In the absence of metabolic activation
b With metabolic activation
c Authors concluded result was positive
3. Observations in humans
A retrospective study of mortality was conducted in a cohort of
134 workers occupationally exposed during the manufacture of captan
for up to nine months annually and to folpet for up to three months
annually. There was an apparent increase in the number of deaths from
all causes (18) in the cohort in comparison with the number expected
from US mortality rates (standardized mortality ratio, 164). The
excess mortality was due mainly to cardiovascular disease and
'external causes' unrelated to occupation. No statistically
significant increases were observed for any specific cause of death,
including neoplasia, but there were too few deaths to evaluate cause-
specific mortality. Lack of adequate industrial hygiene monitoring
precluded satisfactory estimation of previous exposure (FAO/WHO,
1986).
Comments
Excretion of orally administered folpet by mice and rats is about
45-55% in urine, 30-40% in expired air, and 11-17% in faeces; very
little biliary excretion occurs, particularly in mice.
Important degradation pathways of folpet in rodents result in the
formation of phthalimide and thiophosgene. The latter is detoxified,
at least in part, by three mechanisms: oxidation and/or hydrolysis to
carbon dioxide; reaction with the cysteine moiety of glutathione to
yield thiazolidine-2-thione-4-carboxylic acid; and reaction with
sulfite to produce dithiobis(methanesulfonic acid).
A study in which rats were exposed by acute inhalation indicated
an LC50 of 0.39 (male) to 0.43 (female) mg/litre. The WHO has
classified folpet as unlikely to present an acute hazard in normal
use.
A two-year study of carcinogenicity in mice with dietary
concentrations of 0, 1000, 5000, or 10 000 ppm showed a dose-related
increase in the incidence of atypical duodenal hyperplasia, adenomas,
and adenocarcinomas, leading to partial obstruction of the duodenal
lumen in animals of each sex. A no-effect level was not observed. In
another two-year study of carcinogenicity, mice were given dietary
concentrations of 0, 150, 450, or 1350 ppm. A treatment-related
decrease in male body-weight gain was seen. There was a higher
incidence of duodenal masses and thickening of the stomach wall in
females and reduced liver weight in males at 1350 ppm. Microscopic
changes at 1350 ppm and to a minor degree at 450 ppm included benign
papillomas in the keratinized region of the stomach, benign adenomas
in the duodenal mucosa, villous hyperplasia in the duodenal and
jejunal mucosa, and hyperplasia of the lamina propria of the duodenum.
Administration of folpet in the diet at a concentration of 1350 ppm
induced tumours in the upper parts of the gastrointestinal tract
(non-glandular stomach and duodenum) of mice of each sex. The NOAEL
was 150 ppm, equal to 16 mg/kg bw per day.
Folpet has been adequately tested for genotoxicity in a range of
assays, which demonstrate that it is mutagenic and clastogenic in
vitro but not in vivo. The in-vitro responses were reduced or
abolished by the presence of liver homogenates, serum, glutathione, or
cysteine whenever these experimental modifications were investigated.
The Meeting concluded that folpet does not present a significant
genotoxic risk, owing to the presence of an efficient detoxification
mechanism in vivo.
Oral administration of folpet causes time-dependent changes in
the glutathione content and glutathione S-transferase activities of
different regions of the gastrointestinal tract of mice and rats, with
consequent effects on the detoxification of folpet. As early as three
to four weeks after initiation of treatment with a tumour-inducing
dose of folpet, both protein and non-protein thiol concentrations were
increased throughout the duodenum of mice. Reaction with protein thiol
is important for toxicity, while reaction with glutathione is
important for detoxification. At the same time, some hyperplasia and
hypertrophy were observed and the concentration of cyclin-dependent
kinase was increased, but only in the proximal half of the duodenum, a
result supported by assaying for proliferating cell nuclear antigen.
These data indicate that sustained proliferative stimulation of the
proximal duodenum is a consequence of oral administration of folpet.
The Meeting concluded that this finding represents an important
element in the process by which folpet, which is not genotoxic
in vivo, induces tumours in the mouse gastrointestinal tract.
A maximization test indicated that folpet is a sensitizer and
irritant in guinea-pig skin.
An ADI of 0-0.1 mg/kg bw was allocated on the basis of the NOAEL
of 10 mg/kg bw per day in the two-year study of toxicity and
carcinogenicity in rats, the one-year study of toxicity in dogs, and
studies of reproductive toxicity in rats and rabbits, and a safety
factor of 100.
Toxicological evaluation
Levels that cause no toxic effect
Mouse: 150 ppm, equal to 16 mg/kg bw per day (104-week study of
toxicity and carcinogenicity)
Rat: 190 ppm, equivalent to 10 mg/kg bw per day (104-week study
of toxicity and carcinogenicity)
800 ppm, equivalent to 40 mg/kg bw per day (two-generation
study of reproductive toxicity)
10 mg/kg bw per day (maternal toxicity in study of
developmental toxicity)
Rabbit: 10 mg/kg bw per day (maternal and fetotoxicity in study of
developmental toxicity)
Dog: 10 mg/kg bw per day (one-year study of toxicity)
Estimate of acceptable daily intake for humans
0-0.1 mg/kg bw
Studies that would provide information useful for continued
evaluation of the compound
Further observations in humans
Toxicological criteria for setting guidance values for dietary and non-dietary exposure to folpet
Exposure Relevant route, study type, species Results, Remarks
Short-term (1-7 days) Skin, sensitization, guinea-pig Sensitizer and irritant in maximization test
Oral, toxicity, rat LD50 > 5000 mg/kg bw
Inhalation, 4 h lethality, rat LC50 = 0.4 mg/litre
Dermal, toxicity, rabbit LD50 > 23 000 mg/kg bw
Medium-term (1-26 weeks) Repeated oral, 13-week toxicity, rat NOAEL = 300 mg/kg bw per day based on reduced
body weight, irritation of proximal gastrointestinal
tract, and other effects
Repeated oral, reproductive toxicity, NOAEL = 40 mg/kg bw per day based on reduced
rat body weight
Oral, developmental toxicity, rabbit NOAEL = 10 mg/kg bw per day based on reduced
body weight and food consumption; no fetotoxicity
or teratogenic effect
Long-term (> one year) Repeated oral one year, toxicity, dog NOAEL = 10 mg/kg bw per day based on reduced
body weight and food consumption and serum
biochemical changes
References
Blagden, S.M. (1991) Folpet technical: Acute inhalation toxicity
study, four-hour exposure (nose only) in the rat. Unpublished and
unnumbered report (Project No. 306/51) from Safepharm
Laboratories Ltd, Derby, United Kingdom. Submitted to WHO by
Makhteshim Chemical Works Ltd, Beer-Sheva, Israel.
Dreher, D.M. (1990) Folpet technical: Magnusson & Kligman maximization
study in the guinea pig. Unpublished and unnumbered report
(Project No. 8/81) from Safepharm Laboratories Ltd, Derby, United
Kingdom. Submitted to WHO by Makhteshim Chemical Works Ltd,
Beer-Sheva, Israel.
East, P.W. (1994) Folpet: Oncogenicity study by dietary administration
to CD-1 mice for 104 weeks. Unpublished report No. 94/0403,
Pharmaco LSR No. MAK/117, from Pharmaco LSR Ltd, Eye, Suffolk,
United Kingdom. Submitted to WHO by Makhteshim Chemical Works
Ltd, Beer-Sheva, Israel.
FAO/WHO (1970) 1969 Evaluations of some pesticide residues in food.
The monographs. FAO/PL: 1969/M/17/1, WHO/Food Add./70.38, Rome,
FAO.
FAO/WHO (1974) 1973 Evaluations of some pesticide residues in food.
WHO Pesticide Residue Series No. 3, Geneva, WHO.
FAO/WHO (1985) FAO Plant Production and Protection Paper 67.
Evaluations, 1984. Rome, FAO. FAO/WHO (1987) Pesticide Residues
in Food -- 1986. Evaluations, Part II -- Toxicology. FAO
Production and Protection Paper 78/2. Rome, FAO.
FAO/WHO (1991) Pesticide Residues in Food -- 1990. Toxicology
Evaluations. WHO/PCS/91.47, Geneva, WHO.
Nyska, A., Waner, T., Paster, Z., Bracha, P., Gordon E.B. & Klein, B.
(1990) Induction of gastrointestinal tumors in mice fed the
fungicide folpet: Possible mechanisms. Jpn. J. Cancer Res., 81,
545-549.
Tennekes, H. (1995) The genetic toxicology of folpet. Position paper
commissioned by Makhteshim Chemical Works Ltd, Beer-Sheva,
Israel. Submitted to WHO by Makhteshim Chemical Works Ltd,
Beer-Sheva, Israel
Waterson, L.A. (1994a) Folpet, feasibility study by dietary
administration to male mice for 21 days. Report No. MBS
43/942221, from Huntingdon Research Centre Ltd, Huntingdon,
Cambs, United Kingdom. Submitted to WHO by Makhteshim Chemical
Works Ltd, Beer-Sheva, Israel.
Waterson, L.A. (1994b) Folpet, extended feasibility/preliminary study
by dietary administration to male mice for 28 days. Report number
MBS 44/942343, from Huntingdon Research Centre Ltd, Huntingdon,
Cambs, United Kingdom. Submitted to WHO by Makhteshim Chemical
Works Ltd, Beer-Sheva, Israel.
Waterson, L.A. (1995) Folpet, investigation of the effect on the
duodenum of male mice after dietary administration for 28 days
with recovery. Report No. MBS 45/9423003, from Huntingdon
Research Centre Ltd, Huntingdon, Cambs, United Kingdom Submitted
to WHO by Makhteshim Chemical Works Ltd, Beer-Sheva, Israel.
relationship in mice. By 2 h after treatment, the glutathione
depletion in rat and mouse duodenum was similar, and at 6 h the
glutathione levels were higher than those in controls. More
glutathione was re-bound in the duodenum and jejunum of mice than of
rats, indicating that mice have a stronger requirement for and greater
utilization of glutathione, this perhaps being the major route of
metabolism in this species (FAO/WHO, 1990).
Mice (72) and rats (24) were fed dietary concentrations of 0, 50,
or 5000 ppm folpet for 21 days. The mean concentration of glutathione
in rat liver at both doses was similar to that in controls, whereas a
small decline was observed in mice at 5000 ppm. In both species, the
glutathione concentrations were significantly increased after 50 ppm
and 5000 ppm in both duodenum and jejunum. A similar effect in the
ileum was more pronounced in mice. These results suggest an initial
depletion of glutathione followed by increasing de novo synthesis of
glutathione, resulting in a 'rebound' elevation in tissue glutathione
concentrations in the gastrointestinal tract. The tissue weights of
the stomach, duodenum, and jejunum were increased in both species. The
mean cytosolic protein concentrations (total milligrams per tissue) in
duodenum and jejunum were also increased in both species, the greater
increase being found in rats. Total cytosolic protein in mouse liver
declined to 86% of control values. Glutathione- S-transferase
levels (with 1-chloro-2,4-dinitrobenzene as substrate) increased
significantly in the duodenum, jejunum, and ileum of both species,
in the liver of rats, and in the stomach of mice after treatment
with 5000 ppm. This led to a greater capacity to enzymatically
conjugate thiophosgene with glutathione. A marked reduction in the
concentrations of lipid peroxides (malonaldehyde was used as an
indicator of the overall lipid peroxidation state of the mucosal
cells) was noted in the duodenum of both species receiving 5000 ppm
folpet in the diet, but the reduction was statistically significant
only in the stomachs of mice. No alteration was found in the
intracellular level of conjugated dienes in either species in
comparison with controls. The non-selenium-dependent glutathione
peroxidase activity (i.e. that due to the activity of glutathione-
S-transferase) was increased in the duodenum, jejunum, and ileum in
both species receiving 5000 ppm folpet in the diet and in the stomachs
of rats at either dose (FAO/WHO, 1990).
In 240 mice and 48 rats treated as in the previous study, the
mean microsomal protein (total milligrams per tissue) was
significantly increased in rat duodenum, jejunum, and ileum. Although
increases were also observed in these tissues in mice, they were not
statistically significant. Total hepatic microsomal protein declined
significantly in rats but remained unchanged in mice. Cytochrome P450
was reduced in the livers of both species, but the reduction was
statistically significant only in mice receiving 5000 ppm folpet. Both
aniline hydroxylase and 7-ethyloxycoumarin O-deethylase activities
were reduced in hepatic microsomes in both species at both doses, the
reduction in aniline hydroxylase being statistically significant at
5000 ppm (FAO/WHO, 1990).
In an experiment with 90 mice and 54 rats treated as in the
previous study, a statistically significant decrease in pH was
observed in the duodenum and jejunum of mice fed 5000 ppm folpet.
Incorporation of 3H-thymidine into the mucosal DNA was reduced in
most tissues of both species. In neither species was their evidence of
a dose-related increase in DNA synthesis (FAO/WHO, 1990).
Male Sprague-Dawley rats (200-250 g) were treated with folpet
suspended in 0.5 ml corn oil by intraperitoneal injection at
doses up to 100 mg/kg bw or orally at doses up to 1000 mg/kg bw.
Intraperitoneal injection of 50 mg/kg bw significantly decreased the
activities of benzphetamine N-demethylase and cytochrome P450, while
that of serum aspartate transaminase was significantly increased. Oral
doses up to 10 times the intraperitoneal dose did not have similar
effects (FAO/WHO, 1990).
In a preliminary study, groups of 313 male Crl:CD-1 (ICR) BR
mice, about 48 days old, were fed 0 or 5000 ppm folpet for 21 days.
Clinical signs and food consumption were monitored weekly. At the
end of treatment, the animals were sacrificed and used for either
histopathological investigations or biochemical measurement of cyclin-
dependent kinases (CKD) and proliferating cell nuclear antigen (PCNA).
Treatment-related findings included glandular hyperplasia of the
crypts, hypertrophy of the villous epithelium of the duodenum,
hypertrophy of the villous epithelium of the jejunum, and a twofold
increase in CKD and PCNA levels in 9000 × g (S9) fractions from the
duodenum. It was concluded that the upper gastrointestinal tract
(especially the duodenum) could be used to assess the effect of
folpet. Sampling of the first 3 cm of duodenum for histopathological
examination and biochemical analysis of sonicated fractions was
recommended (Waterson, 1994a).
Subsequently, the mechanism of the effect of folpet on the
duodenum was studied in groups of 51 Crl:CD-1 (ICR) BR mice fed 0 or
5000 ppm folpet, equal to 692 mg/kg bw per day, or 11 or 111 ppm
perchlormethyl mercaptan, equal to 2 or 16 mg/kg bw per day, for 28
days. The latter compound was included because 5000 ppm technical-
grade folpet contains about 11 ppm. Clinical signs and food
consumption were monitored daily, and body weight was determined
weekly. At the end of treatment, the animals were sacrificed and the
duodenum used for either histopathological investigations or
measurement of CDK and PCNA in S9 fractions. There was no treatment-
related mortality or change in clinical signs. In week 1, the body-
weight gain and the food intake of the animals receiving folpet or
111 ppm perchlormethyl captan was lower than that of controls.
Haematoxylin staining revealed glandular hyperplasia of the crypts in
the first 2.5 cm of the duodenum of most animals receiving folpet and
hypertrophy of the villous epithelium in the entire duodenum of all
animals given folpet. The crypts in the entire duodenum of all animals
in all groups stained for PCNA; more animals receiving folpet showing
a greater degree of staining. An approximately twofold increase over
that in controls was seen in CDK concentrations in fractions from the
entire length of the duodenum of animals receiving folpet; there was
no significant difference from controls in CDK concentrations in mice
fed 11 or 111 ppm perchlormethyl mercaptan. The PCNA concentrations
were significantly higher than control values in S9 fractions from the
entire length of the duodenum in the groups receiving folpet or
11 ppm perchlormethyl mercaptan. It was concluded that folpet acts
significantly differently from perchlormethyl mercaptan in the
duodenum, when CDK is used as the index, and these findings were
supported by the findings of both the biochemical PCNA assay and
histopathological immunochemistry. Since both CDK and PCNA are
increased in proliferating cells, the findings of the two studies were
considered to demonstrate proliferative stimulation as early as three
to four weeks after initiation of treatment with a tumour-inducing
dose of folpet (Waterson, 1994b).
The mechanism of the effect of folpet on the duodenum was further
studied in five groups of 132 male Crl:CD-1 (ICR) BR mice, about 48
days old, which were fed 0 or 5000 ppm folpet (purity, 99.4%), equal
to 717 mg/kg bw; 5000 ppm folpet, equal to 679 mg/kg bw, plus 11 ppm
perchlormethyl mercaptan (purity, 95%), equal to 1.5 mg/kg bw;
11 ppm perchlormethyl mercaptan alone, equal to 1.6 mg/kg bw; or
0.4% hydrogen peroxide, equal to 527 mg/kg bw (positive control,
administered in the drinking-water) for 28 days followed by a 28-day
recovery period for selected mice. An acclimatization period of 11
days was allowed before commencement of treatment, at which time the
mice were nine weeks old and weighed 26-45 g. The mice were housed
three to a cage. The identity of the test compound was confirmed by
chemical analysis, and the accuracy of the preparations and the
homogeneity and stability of the dietary formulations were found to be
satisfactory. Clinical signs and food consumption were monitored
daily. At the end of treatment and recovery, specified animals were
sacrificed and allocated for either pathological investigation of the
duodenum or biochemical measurement of total protein, non-protein
thiol, CDK, and PCNA in duodenal fractions by means of commercially
available kits.
Animals receiving hydrogen peroxide had a statistically
significant reduction in body-weight gain and a lowered efficiency of
food use in comparison with controls. At termination, a marginal
increase in glandular hyperplasia of the crypts and hypertrophy of the
villous epithelium in the duodenum were seen in mice treated with
folpet, folpet plus perchlormethyl mercaptan, or hydrogen peroxide in
comparison with controls. No such changes were seen after the recovery
period.
Treatment of mice with folpet (with or without perchlormethyl
mercaptan) resulted in increased protein (per milligram of tissue) in
total duodenal mucosal epithelium. The effect of folpet was greater in
the first 2.5 cm of the duodenum than in the next 3.5 cm and was
completely reversed after 28 days of withdrawal of animals from the
test diet. Treatment with perchlormethyl mercaptan alone or with
hydrogen peroxide had no such effect. The non-protein thiol
concentration was also increased after administration of folpet (with
or without perchlormethyl mercaptan) and with hydrogen peroxide.
Again, the effects were greatest in the first 2.5 cm of the duodenum
and were fully reversible after the recovery period. Treatment with
perchlormethyl mercaptan alone had no such effect. Folpet (with or
without perchlormethyl mercaptan) and hydrogen peroxide, but not
perchlormethyl mercaptan alone, increased the concentrations of CDK in
the first 2.5 cm of the duodenum; after the recovery period, no
significant difference in CDK concentrations was detected between
treated and untreated animals. The PCNA concentrations in duodenal S9
fractions were close to the limit of detection of the assay system,
but a significant increase in PCNA concentrations was detected after
treatment with folpet (with or without perchlormethyl mercaptan) in
the first 2.5 cm, confirming the CDK response. In contrast, the PCNA
concentrations also indicated cell proliferation in the next 3.5 cm of
the duodenum, although the effect was not seen after the recovery
period. Treatment with perchlormethyl mercaptan or with hydrogen
peroxide had no clear effect on PCNA concentrations in the duodenum.
In none of the investigations was there evidence that perchlormethyl
mercaptan and folpet have interactive effects.
Thus, administration of folpet for 28 days (with or without
perchlormethyl mercaptan) increased protein and non-protein thiol
concentrations in both the first 2.5 cm and the next 3.5 cm of the
duodenum, while CDK concentrations were increased only in the first
2.5 cm. The latter finding was supported by the results of the PCNA
assay but not by histopathological immunocytochemistry. The results
also showed that the biochemical effects of folpet on the mouse
duodenum are reversible after a 28-day recovery period (Waterson,
1995).
2. Toxicological studies
(a) Acute toxicity
The LD50 in adult male and female rats and in female wealings
treated orally with folpet was > 5000 mg/kg bw (FAO/WHO, 1990),
and the LD50 in rabbits treated percutaneously with folpet was
> 23 000 mg/kg bw (FAO/WHO, 1969).
Exposure of rats to folpet (purity unspecified) by inhalation for
4 h showed an LC50 of 0.39 mg/litre for males and 0.43 mg/litre for
females (Blagden, 1991).
(b) Short-term toxicity
Rats
Groups of 10 rats of each sex were fed dietary levels of 0, 0.1,
0.32, or 1% folpet for 12 weeks. Growth was normal, except in male
rats fed the 1% level which showed a significant decrease There were
no gross abnormalities, and histopathological examination of the
liver, kidneys, adrenals, intestines, lungs, and gonads of two males
and two females from each group revealed no abnormalities (FAO/WHO,
1969).
Groups of 20 Fischer 344 rats of each sex were fed dietary
concentrations of 0, 0.2, 0.4 or 0.8% folpet (purity, 89%) for 13
weeks. During treatment, the body weights and food consumption of
males at the middle and high doses and of females at the high dose
were significantly reduced. After 10 weeks, there were no significant
differences between treated and control groups, but there were dose-
related decreases in the activities of alkaline phosphatase and
alanine aminotransferase in all treated groups, of aspartate
aminotransferase in all treated males and in females it the high dose,
and of lactate dehydrogenase in all treated males. Blood urea and
chloride levels were increased, but total serum proteins were reduced
in males at the middle and high doses. Blood urea was reduced in
treated females, total protein was reduced in the high-dose groups,
and albumin was reduced in the mid- and high-dose groups. Treatment-
related irritation of the proximal gastrointestinal tract was seen at
necropsy, as was hyperkeratosis of the non-glandular gastric mucosa.
Slight acanthosis of the stomach occurred in one female rat in each
group. The kidneys of males at the middle and high doses showed slight
but dose-related increases in the number of foci of atrophic
basophilic renal tubules, which were considered to be unrelated to
treatment (FAO/WHO, 1986).
Groups of 20 Sprague-Dawley rats of each sex received folpet
(purity unspecified) at dietary levels of 0, 0.03, 0.1, 0.3, or 1.0%
for 13 weeks. Dietary analyses showed that the achieved doses were
85-106% of the nominal values. At the end of treatment, half of the
rats in each group were sacrificed; the remainder were given the basal
diet for a further two weeks and were then sacrificed. No clinical
signs or mortality occurred during the study period. The growth of
rats at the high dose was significantly reduced during treatment, and
this retardation was not made up during the recovery period. Food
consumption, haematological parameters, serum hepatic enzyme levels,
and renal function were unaffected by treatment. At necropsy, animals
at the high dose had reduced mean body weights; relative brain weight
was also reduced, but kidney weight was increased. There were no
significant differences in organ weights of animals sacrificed two
weeks after treatment. No treatment-related gross pathological changes
were observed, but histopathological examination of animals sacrificed
immediately after treatment showed acanthosis, hyperkeratosis,
submucosal oedema, and pleocellular inflammatory infiltration, with
occasional focal gastric erosion and ulceration in the non-glandular
stomach of rats at the high dose. No such lesions were seen after the
recovery period. The NOAEL was 300 mg/kg bw per day, based on reduced
body weight and other effects at the high dose (FAO/WHO, 1986).
Dogs
In a pilot study, four beagle dogs of each sex were given folpet
(purity, 89.8-91.1%) at 0, 790, 1800 or 4000 mg/kg bw per day by
capsule for 13 weeks. Food intake was generally lower in treated dogs
than in controls. Body weight gain was reduced in all treated groups,
significantly so in animals at the middle and high doses. All treated
dogs showed vomiting and diarrhoea, the symptoms being especially
marked in those at the middle and high doses. Treatment-related
changes included poor condition, abdominal distension, excessive
salivation, and a progressive decrease in testicular size,
particularly in animals at the middle and high doses. All males and
one female at the high dose died or were killed in extremis.
Neurological examination of these dogs and of those males which
survived 12 weeks of treatment and ophthalmoscopic examination of
survivors showed no remarkable changes. Most dogs killed in extremis
had leukocytosis, and some had decreased serum phosphate levels. One
dog had normochromic normocytic anaemia. Surviving dogs had decreased
serum calcium and total plasma protein concentrations, but increased
serum chloride levels. At necropsy, most treated dogs had decreased
brain, liver, kidney, spleen, and testicular weights. Pathological
examination showed atrophy, depletion, and fibrosis of the lymphatic
and haematopoietic systems, gonadal degeneration with prostatic
atrophy and fibrosis, thyroid degeneration, and muscular dystrophy
(FAO/WHO, 1986).
Groups of six beagle dogs of each sex were given folpet (purity,
89.5%) in gelatine capsules at 0, 10, 60, or 140 mg/kg bw per day. The
latter dose was reduced to 120 mg/kg bw per day after 50 days because
of low food consumption and reduced body-weight gain. All animals were
sacrificed and necropsied after one year. All males at the high dose
and three at the middle dose initially lost weight, and the mean body
weights of animals at the middle and high doses showed a dose-related
reduction (which was not significant) throughout the rest of the
study. Dose-related decreases in food consumption were seen in animals
at the middle and high doses, in males during the first three months,
and in females during the first month. Although the food consumption
of males was subsequently comparable to that of controls, that
of females tended to be reduced in a non-dose-related manner.
Ophthalmoscopy at six and 12 months revealed no effect. There was a
tendency for reduced leukocyte counts in males at one, two, three, and
six months, but not at nine or 12 months; however, the counts were not
significantly different from control values. A significant decrease in
mean serum cholesterol and in total protein, albumin, and globulin
levels was seen in males at the middle and high doses; females at the
high dose had significantly reduced mean serum protein, albumin,
and cholesterol levels. Urinalysis, necropsy, and subsequent
histopathology showed no effect of treatment. The NOAEL was 10 mg/kg
bw per day, on the basis of decreased body weight and food consumption
and serum biochemical changes (FAO/WHO, 1986).
Groups of five beagle dogs of each sex received technical-grade
folpet in capsules at 0, 325, 650, or 1300 mg/kg bw per day for 52
weeks. None died. Animals of each sex at the high and intermediate
doses had decreased body weight during treatment; a concomitant
decrease in food intake was found in animals of each sex at the high
dose. Clinical signs seen in all treated groups included vomiting,
diarrhoea, and salivation; the latter was seen only during the first
eight weeks in animals at the low dose but was more pronounced at the
high and intermediate doses. Dogs at these doses were in poorer
condition than controls. Haematological parameters were affected in
all treated females during the first third of the study and included
decreased packed cell volume and haemoglobin and erythrocyte counts.
Clinical chemical changes (e.g. reduced total protein, cholesterol,
glucose, and urea) observed during treatment were related to the poor
physical condition of the dogs. Chloride levels were increased in
males, mainly those at the high dose, which also had decreased calcium
levels; the latter effect was also observed in females at the high and
intermediate doses up to week 25 of treatment. A reduced urine volume
was noted in females at the high dose after 13 weeks of treatment, and
both males and females had acid urine; this effect persisted in males
at the high and intermediate doses throughout the study. Tubular
testicular degeneration associated with an absence of spermatozoa in
the epididymides was found in two male dogs at the high dose; one also
had moderate prostatic gland atrophy. The absolute testicular weights
of males at the high dose were decreased. Changes in relative organs
weights were recorded in the adrenals (all males and females at the
intermediate dose), brain and kidney (males at the high dose and
females at the intermediate dose), liver, and thyroid (males at the
intermediate dose and females at the high dose). The NOAEL was
325 mg/kg bw per day (FAO/WHO, 1990).
(c) Long-term toxicity and carcinogenicity
Mice
Goups of 80 CD-1 (ICR derived) mice of each sex were fed dietary
concentrations of 1000, 5000, or 12 000 ppm folpet (purity, 93%) for
112-113 weeks. A group of 104 mice of each sex served as controls.
Survival was not affected, but body-weight gain was reduced in animals
at 5000 and 12 000 ppm. Although sporadic changes in food consumption
were noted, no dose-related effects were apparent. Haematological
parameters were normal at one year, but at the end of treatment,
possible macrocytic anaemia was seen in animals of each sex at
12 000 ppm. The only changes seen at gross pathological examination
were duodenal lesions and related gastrointestinal abnormalities. A
dose-related increase in the incidence of duodenal adenomas and
adenocarcinomas was observed in mice at 5000 and 12 000 ppm, with
incidences in males and females of 0 and 1% in controls, 2 and 3% at
1000 ppm, 10 and 12% at 5000 ppm, and 52 and 54% at 12 000 ppm,
respectively. Males at the high dose also had an increased incidence
of jejunal adenocarcinomas. There was no NOAEL (FAO/WHO, 1984).
Four groups of 52 B6C3F1 mice of each sex were given dietary
concentrations of folpet (purity, 89.0%) at 0, 0.1, 0.5, or 1.0% for
21 weeks and then 0, 0.1, 0.35, or 0.7% for 83 weeks. Dietary analyses
at weeks 0, 1, 13, and 26 showed the levels to be 88-91% of the
nominal value. Food consumption was depressed during the first few
weeks of treatment in mice at the middle and high doses, and body-
weight gain was reduced in these animals throughout the study.
Clinical signs, observed principally in animals at the high dose,
comprised erythema, dry flaking skin, reddish fur discolouration, and
weeping skin, particularly during the first 21 weeks. Leukocyte counts
in survivors at 52, 78, and 104 weeks were not affected by treatment.
The longevity of animals at the two highest doses was reduced. The
increased relative weights of the brain, heart, lungs, liver, kidneys,
and testes reflected the reduced body weight of treated animals.
Macroscopically, a dose-related increase in non-glandular gastric
mucosal ulceration and thickening of the gastric and duodenal walls
were observed. The jejunal wall was thickened in females at the middle
dose and in all mice at the high dose. Dose-related distension of the
duodenal lumen was also seen. After week 79, there was a dose-related
increase in the incidence of nodules or masses on the luminal surface
of the stomach and duodenum and on the duodenal serosa. Animals at the
middle and high doses had dose-related epidermal hyperkeratosis and
acanthosis and oesophageal hyperkeratosis. Increased areas of marked
acanthosis and hyperkeratosis of non-glandular gastric mucosa were
seen in animals at the middle dose and males at the high dose.
Microscopic gastric ulceration occurred, with no apparent
relation to dose. The dose-related appearance of areas of atypical
duodenal glandular hyperplasia and mucosal gland proliferation were
seen in all treated animals, but especially in males. This atypical
hyperplasia was often associated with duodenal adenomas and
adenocarcinomas. Atypical glandular proliferation was seen only
occasionally in the jejunum of treated females. Gastric papillomas and
squamous-cell carcinomas, which may have been secondary to mechanical
obstruction of the duodenal lumen, were found in all treated males but
not in control males. A dose-related increase in the incidence of
gastric papillomas was also found in all treated females, but
similar lesions occurred in 2/51 controls. Duodenal adenomas and
adenocarcinomas were found in all treated animals of each sex, with
a significant dose-response relationship. A single jejunal
adenocarcinoma was seen in mice at the high dose. Primary or
metastatic tumours of uncertain etiology were found in all treated
males, but the incidences of bronchioalveolar adenomas and malignant
lymphomas were lower in these animals, suggesting high incidences in
control males. There was no NOAEL (FAO/WHO, 1986).
Four groups of 52 six-week old B6C3F1 mice of each sex were given
dietary concentrations of 0, 1000, 5000, or 10 000 ppm folpet (purity,
89%) for 104 weeks. The formulated diets were analysed after one and
two weeks and were found to be stable (no data). During the first few
weeks of the study, marked irritation of the integumentary system was
observed. Typical lesions, usually on the neck, included flaking,
weeping, and eroded skin, erythema, and red discolouration of the fur.
Early deaths occurred in all treated males and in females at the
middle and high doses and were attributed to the presence of fatal
tumours. Dose-related macroscopic lesions were found in the stomach
and duodenum. The non-glandular gastric mucosa of all treated animals
showed an increased incidence of nodules, masses, wall thickenings,
and ulceration, and an increased incidence of partial luminal duodenal
obstruction was seen 1-5 cm distal to the pylorus in the form of wall
thickenings and mucosal nodules. An increased incidence of these
lesions was noted in animals that died after 79 weeks of treatment.
Microscopic examination of the skin revealed hyperkeratosis, and a
dose-related in, crease in the incidence of oesophageal and non-
glandular gastric mucosal hyperkeratosis was seen in all treated
groups. Ulceration of the non-glandular stomach was observed in
animals at the low and intermediate doses. Atypical hyperplasia of the
duodenal mucosa was seen in all treated mice. Duodenal adenomas and
carcinomas were seen in all treated groups, and the incidence was
dose-related. Gastric squamous-cell papillomas and carcinomas were
seen more frequently in animals at 5000 and 10 000 ppm. There appeared
to be a correlation between the presence of macroscopically visible
gastric neoplasia and duodenal obstruction: the four males with
gastric carcinoma had partial duodenal nodular obstruction, and five
of seven females at the high dose with an increased incidence of
gastric papillomas had partially obstructed duodenal lumens. No other
toxic or carcinogenic effect was seen in any organ.
The authors suggested that the partial duodenal obstruction
disturbed the natural flow of the gastrointestinal contents and may
have exacerbated the mucosal changes in the stomach. The resulting
stagnation may have raised the effective concentration and residence
time of folpet, and the elevated concentration of this irritant, with
the possible presence of bile acids and pancreatic enzymes, may have
overwhelmed the defence mechanism of the mucosal cells, resulting
in the development of gastric neoplasms. In conclusion, dietary
administration of folpet to B6C3F1 mice induced a dose-related
appearance of atypical duodenal hyperplasia, adenomas, and
adenocarcinomas, which were suggested to have appeared subsequent
to, and as an indirect result of, the partial luminal duodenal
obstruction. There was no NOAEL (Nyska et al., 1990).
Groups of 52 male and 52 female CD-1 mice were fed diets
containing folpet (purity, 92.4%) at concentrations of 0, 150, 450, or
1350 ppm, equal to 0, 16, 47, and 151 mg/kg bw per day for 98 weeks
for males and 0, 16, 51, and 154 mg/kg bw per day for 104 weeks for
females. A control group consisting of 100 male and 100 female mice
received a normal diet. The mice were assigned to treatment groups on
the basis of computer-generated random numbers and were acclimatized
for 14 days before the beginning of treatment. The mice were 3542 days
of age and had mean body weights of 29.7, 29.6, 29.5, and 29.6 g
(males) and 23.4, 23.7, 23.0, and 23.7 g (females) at 0, 150, 450, and
1350 ppm, respectively, at the time of the first dose. The 150-ppm
preparation was found to contain 89-91% of the nominal concentration
and was stable after storage at -20°C for seven days and then at 20°C
for four days; samples taken from six places in the mix showed
satisfactory homogeneity: 89-95% of the nominal value with 150 ppm and
78-88% with 1350 ppm. Verification of the concentrations resulted in
analytical values of 89-96% of the nominal concentrations. Animals
were inspected daily for evidence of reactions to treatment. Body
weight and food and water consumption were determined weekly. All 187
animals found dead or killed during the treatment period were
subjected to gross pathological and histopathological examination.
The overall mortality rate was unaffected by treatment, and no
inter-group differences in mortality were seen by either pairwise or
trend analyses; therefore, none of the deaths was considered to be
related to treatment with folpet. The incidence of skin encrustations,
particularly on the dorsal surface, was generally higher in males
receiving a dietary concentration of 1350 ppm. This finding was
considered to be associated with a greater incidence of fighting among
these animals during the first 70 weeks of treatment. In the later
part of the treatment period, the incidence of skin encrustations
in males was similar to that of controls. The incidence, group
distribution, location, and mean onset time of palpable swellings were
not considered to be related to treatment. The body-weight gain of
males receiving 1350 ppm was slightly lower than that of controls for
about the first 70 weeks of treatment. Food consumption was unaffected
by treatment, but the efficiency of food use during the first 14 weeks
of treatment with 1350 ppm was slightly inferior to that of the
controls. The treatment-related effect on male body-weight gain and
the low food conversion efficiency seen at this dose during the first
14 weeks of treatment were considered to be nonspecific toxic effects.
The body-weight gain of females was unaffected by treatment.
Organ weight analysis revealed lower absolute and relative liver
weights in males that received 1350 ppm in comparison with controls.
High absolute and relative spleen weights in males at 150 ppm were due
to an unusually large spleen in one animal. Macroscopic examination of
animals killed after 104 weeks of treatment revealed a high incidence
of masses in the duodena of females at 1350 ppm. When all of the
animals were considered together, there was a higher incidence of
duodenal masses and thickening of the stomach wall in females at
1350 ppm than in controls. Microscopic examination revealed benign
papillomas in the keratinized region of the stomach in one male and
three females at 1350 ppm and in one female at 450 ppm. A benign
adenoma was found in the duodenal mucosa of one female at 1350 ppm.
Villous hyperplasia was seen in the duodenal mucosa of three females
at the highest dose and in one male at 450 ppm; a similar finding was
seen in the jejunum of one male at 450 ppm. Hyperplasia of the lamina
propria of the duodenum was seen in two males at 1350 ppm, and another
male at this dose had similar changes in the jejunum and ileum in
addition to villous fusion, mucosal dysplasia, and hyperplasia of
Paneth cells. The incidence of keratoachanthosis of the nonglandular
stomach was higher among females at 1350 ppm. than among controls.
Although one female at 450 ppm had a benign squamous-cell papilloma
and two males at this dose had gastrointestinal hyperplasia, these
findings were considered to have occurred by chance. There were no
other changes in the gastrointestinal tract, and there were no changes
in the liver that were considered to be related to treatment. As the
low liver weights of males at 1350 ppm were confined to one sex and
there was no histopathological correlate, the toxicological
significance of this finding is unclear. It was concluded that
administration of folpet in the diet at a concentration of 1350 ppm
produced tumours in the upper parts of the gastrointestinal tract of
CD-1 mice. The NOAEL was 16 mg/kg bw per day on the basis of duodenal
hyperplasia (East, 1994).
Rats
Groups of 60 Charles River Crl:CD(SD)BR rats of each sex were fed
dietary concentrations of 0, 200, 800, or 3200 ppm technical-grade
folpet (purity, 89.5%) for 104 weeks. An interim sacrifice of 10 rats
of each sex per dose was conducted at 52 weeks. Growth rates and
survival were unaffected by treatment, except for a slight tendency to
reduced body weights in females at the high dose during the first
year; food consumption was correspondingly reduced at this dose.
Conventional ophthalmoscopic, haematological, biochemical, and
urinary analyses indicated no effect of treatment. At necropsy, no
changes attributable to treatment were observed on organ weights.
Hyperkeratosis and/or acanthosis and some erosion and/or ulceration in
the non-glandular stomach were seen in animals at the high dose. These
lesions were occasionally accompanied by submucosal oedema and
submucosal inflammatory cellular infiltrates. The NOAEL was 800 ppm,
equivalent to 40 mg/kg bw per day (FAO/WHO, 1986).
Groups of 60 Fischer 344 rats were fed diets containing 0, 500,
1000, or 2000 ppm folpet (purity, 89%) for 104 weeks. The diets were
prepared weekly and analysed frequently for folpet content. Food
intake was reduced in all treated groups in comparison with controls,
but body weight was reduced only in treated males. There were no
clinical signs of toxicity. Necropsy indicated a tendency to an
increased incidence of gastric ulceration, which was significant
only in females. Treatment also induced an increased incidence
of ulceration in the forestomach in animals at the high dose.
Hyperkeratosis of the oesophagus was seen in rats at the high dose
and of the forestomach in animals at the middle and high doses. An
increased incidence of gastric ulceration was present in the
forestomach of animals of each sex at the high dose. The incidences of
C-cell adenoma, benign mammary fibroepithelioma and malignant lymphoma
showed positive significant trends with dose, but only that for the
latter neoplasms was statistically significant. All tumour incidences
were within the normal historical range for rats of this strain. The
NOAEL was 500 ppm, equivalent to 25 mg/kg bw per day (FAO/WHO, 1986).
Groups of 20 Fischer 344 rats of each sex were fed diets intended
to provide concentrations of 0, 250, 1500, or 5000 ppm folpet (purity,
91%) for 104 weeks. The actual dietary concentrations, calculated
on a weekly basis, were 0, 190, 1290, and 4530 ppm. Longevity was
unaffected. The mean body weight and food intake of animals at the
high dose were depressed, by up to 10% in males and 6% in females,
and water intake was depressed at the high dose, especially in
females. Serum alkaline phosphatase and alanine aminotransferase
activities were reduced in treated groups throughout the study, and
those of aspartate aminotransferase, creatinine phosphokinase, and
gamma-glutamyl transferase were decreased sporadically. A significant
reduction in blood cholesterol was seen in animals of each sex of the
high dose throughout treatment. The plasma protein level was reduced
in males at the high dose during the first year of treatment, and the
level of phosphate was elevated at most examinations. The urea
concentration was increased in females at the high dose at 18 months.
Most treated males excreted more concentrated urine in smaller volumes
at three and six months. Only non-neoplastic lesions were observed,
consisting of hyperkeratosis of the oesophageal and gastric squamous
epithelium. The NOAEL was 190 ppm, equivalent to 10 mg/kg bw per day
(FAO/WHO, 1990).
(d) Reproductive toxicity
Rats
Four groups of 25 CD rats of each sex were fed dietary
concentrations of 0, 250, 1500, or 5000 ppm folpet (purity, 91%) over
the course of two generations. Body weight gain and food consumption
were reduced in parental animals of the F0 and F1 generations at
the highest dose; a minor decrease in body weight was observed in F1
and F2 offspring. The principal histopathological effect was
hyperkeratosis of the non-glandular gastric mucosa in F0 and F1
animals at the intermediate and high doses, oesophageal hyperkeratosis
in F1 animals at the intermediate and high doses, and an increased
incidence of basophilic renal tubules in males of the F0 generation
at the high dose. Folpet was concluded not to be a specific
reproductive toxin in this test system (FAO/WHO, 1990).
In a two-generation study, groups of 30 Crl:COBS/CD(SD)/Charles
River rats of each sex were fed diets containing 0, 200, 800, or
3600 ppm folpet (purity, 89.5%) during growth, mating, gestation, and
lactation for two litters per generation. Initial mating was begun 62
days after the start of exposure. The test diets were fed to F0 males
until the end of mating to produce the F1b litters and to F0 females
until weaning of the F1b litters. Pups were sacrificed 21-23 days
after parturition, with the exception of those F1b pups selected to
produce the F2 generation. Mating of the F1b pups was begun after 12
weeks of exposure, and the above sequence was repeated. Gross necropsy
was performed on all parental rats and on the F1a, F1b, and F2b
litters. The diet was changed three times per week, and each batch was
analysed: the diets contained 79.9-101% of the nominal concentration.
The body weights of F0 and F1 males and F1 females at the high dose
and of their pups were depressed by treatment; the effect was most
marked in adult males, especially in the second generation. Food
consumption was correspondingly reduced. Treatment had no significant
effect on mating, fertility indices, pregnancy rates, litter sizes,
pup weights, growth, or litter survival rates. No treatment-related
effects were found at necropsy or on histopathological examination.
The NOAEL for maternal toxicity was 800 ppm, equivalent to 40 mg/kg bw
per day, based on the reduction in body weights; there was no evidence
of reproductive toxicity (FAO/WHO, 1986).
(e) Developmental toxicity
Rats
In a pilot study, groups of eight female mated CRL:COBS CD (SD)
BR rats were given 0, 20, 80, 320, or 640 mg folpet/kg bw by gavage on
days 6-19 of gestation. There were no deaths, but clinical signs
including rales, excess salivation, chromorhinorrhoea, gasping, soft
or liquid faeces, decreased motor activity, dyspnoea, and distended
gut were observed. Reduced maternal body weight was seen at
> 80 mg/kg bw, and food consumption was reduced at > 320 mg/kg
bw; the average fetal body weight was also reduced at the latter dose.
In the main study, groups of 25 rats were given folpet (purity, 89%)
at 0, 10, 60, or 360 mg/kg bw by gavage on days 6-19 of gestation.
Animals were killed on day 20. Clinical signs including excess
salivation, chromorhinorrhoea, decreased motor activity, soft or
liquid faeces, dyspnoea, and urine-stained fur were observed. Three
rats at the high dose died, two from intubation errors. No gross
lesions attributable to treatment were seen in surviving rats. Rats at
> 10 mg/kg bw gained less weight than controls during treatment.
Food consumption was reduced in animals at the high dose only. The
numbers of implantations, live and dead fetuses, fetal viability, and
resorptions, the average fetal body weight per litter, the fetal sex
ratio, and the number of corpora lutea were comparable in all groups.
Gross, visceral, and skeletal abnormalities were seen in all groups,
but there were no compound-related effects on ossification. The NOAEL
for maternal toxicity was 10 mg/kg bw per day (FAO/WHO, 1984).
Groups of six rats were treated daily on days 6-15 of gestation
with folpet (purity, 88.6%) at 10, 65, 420, or 2750 mg/kg bw by
gavage. Maternal toxicity, reduced body-weight gain, and reduced fetal
weight were seen only at 2750 mg/kg bw. Following this pilot study,
groups of 22 female Charles River CD rats received folpet (purity,
91.1%) in 0.5% acetic acid containing 0.5% carboxymethylcellulose at
0, 150, 550, or 2000 mg/kg bw on days 6-15 of gestation. The animals
were sacrificed on day 20. Clear signs of toxicity were observed in
animals at the high dose, including soft faeces (in 21/21 rats), fur
staining (in 4/21), and perianal staining (in 8/21); one animal died.
Food consumption was decreased during the first days of treatment with
the intermediate dose and was markedly decreased throughout treatment
with the high dose. Maternal body weight was also decreased at the
middle and high doses. Gravid uterine weights were depressed in dams
at the middle and high doses, but terminal maternal body weight
(without the gravid uterus) was significantly depressed only at the
high dose. Pre- and post-implantation losses were greater than in
controls in animals at the middle dose, and fetal weights were reduced
at the middle and high doses. Fetal crown-rump length was slightly
decreased after treatment with the middle and high doses. A single
fetus at the high dose (1/277) had multiple major malformations, and a
second pup had unilateral microphthalmia. The incidence of hepatic
discolouration was significant at the high dose. Skeletal anomalies
occurred in all treated groups, and the incidence of reduced
ossification of cranial and pubic bones, sternebrae, metacarpals, and
metatarsals was significant in animals at the middle and high doses.
There was also a dose-related reduction in ossification of the
interparietal bone in all treated groups, and the occurrence of
angulated ribs was dose-related. There was no NOAEL for teratogenicity
(FAO/WHO, 1986).
Hamsters
Groups of 2-13 pregnant hamsters were given a single dose of
400-1000 mg/kg bw folpet on day 7 or 8 of gestation or were treated
daily on days 6-10 with a total of 1000-2500 mg/kg bw. The animals
were killed and examined on day 15 of gestation. Maternal mortality
was increased and some abnormal fetuses were produced at the highest
doses, but the lower doses did not induce teratogenic effects
(FAO/WHO, 1973).
Rabbits
Groups of 20 artificially inseminated New Zealand white rabbits
were given folpet (purity, 89%) at 0, 10, 20, or 60 mg/kg bw by oral
intubation on days 6-28 of gestation and were killed on day 29. The
death of one doe at 60 mg/kg bw was considered to be related to
treatment. One doe at the low dose aborted on day 21 of gestation and
one at the high dose on day 22 of gestation; one doe in the control
group delivered a litter on day 28 and one at the high dose on day 29.
Significant inhibition of body-weight gain and food consumption was
seen in animals at the middle and high doses. The average numbers of
corpora lutea, implantations, resorptions, and fetuses per litter and
the sex ratio and numbers of dead and resorbed implantations per
litter were comparable in all groups. The mean body weights of all
fetuses were decreased at the middle and high doses. There was a
significant increase in the incidence of hydrocephaly, and four
fetuses in three litters of dams at the high dose also had skull,
gastric, and pulmonary abnormalities. The NOAEL for maternal and
fetoxicity was 10 mg/kg bw per day, and that tor teratogenicity was
29 mg/kg bw per day (FAO/WHO, 1984).
Groups of six mated HY/CR female New Zealand white rabbits were
given folpet (purity, 91.1%) at 0, 10, 60, or 150 mg/kg bw per day by
gavage on days 6-18 of gestation. Marked body-weight loss was seen at
the high dose. Although fetal size was unaffected by treatment, fetal
mortality was more marked at the high dose. Post-implantation losses
were increased in animals at the middle dose. Subsequently, groups of
14 mated HY/CR New Zealand white rabbits were given folpet (purity,
91.1%) at 0, 10, 40, or 160 mg/kg bw per day by gavage on days 7-19 of
gestation. Dams were sacrificed on day 29 of gestation. Body-weight
gain was decreased during the initial few days of treatment with the
middle dose and after initiation of treatment with the high dose;
there was a corresponding decrease in food consumption in animals at
the high dose. There were no deaths. Gravid uterine weight was
significantly reduced in dams at the middle and high doses. Fetal
death (post-implantation loss) occurred more frequently at the high
dose than in controls; the proportion of small fetuses was also
increased in this group and mean fetal weight was nonsignificantly
reduced. There was evidence of delayed skeletal maturation at the high
dose and, to a lesser degree, at the middle dose. The incidence of
bilateral lumbar ribs appeared to increase with dose in animals at the
middle and high doses. Ossification of caudal vertebrae, sternebrae,
and long-bone epiphyses was reduced at the high dose. Other, minor
skeletal malformations did not apper to be related to treatment. There
was no evidence of hydrocephalus in either treated or control rabbits.
The NOAEL for maternal toxicity, fetotoxicity, and teratogenicity was
10 mg/kg bw per day (FAO/WHO, 1986).
Groups of 20 artificially inserminated female Hazelton Dutchland
New Zealand white (D1A Hra: (NZW) specific pathogen-free) rabbits
were given folpet (purity, 89.5%) in Tween 80 (10.5% by weight) and
carboxymethylcellulose (0.7% by weight) at a volume of 5 ml/kg bw per
day by gavage, to give a dose of 60 mg/kg bw per day on days 7-9,
10-12, 13-15, or 16-18 of gestation. Analysis of the formulations
indicated a folpet content of 87.8-104% of the nominal concentration.
Dams were sacrificed on day 29 of gestation; those that aborted
or delivered, the single animal that died, and those terminally
sacrificed were subjected to necropsy and examination of the uterine
contents. Abortion by two rabbits that received folpet on days 7-9 and
10-12 of gestation may have been related to treatment; otherwise, no
clinical signs of toxicity were observed during the study, although
the incidence of soft or liquid faeces was increased in all treated
groups, usually after treatment. No gross lesions attributable
to treatment were seen at necropsy. Maternal body weight was
significantly reduced in all treated animals, although less so in
those treated on days 7-9 and 10-12 than in the others. Food
consumption was correspondingly reduced. Treatment had no apparent
effect on the rate of abortion or on fetal resorption. The average
litter sizes were unaffected, as were the average fetal weights, the
number of viable fetuses, and the sex ratio. A significantly increased
incidence of fetuses with an irregularly shaped fontanelle was
observed in the group treated on days 13-15; the control incidence was
4.5%, but this variation did not occur in groups treated on days 7-9
or 16-18. It was possibly related to treatment, but the significance
of the effect was not clear. There were no other significant
variations in fetal skull morphology, and the incidences of
hydrocephalus and of gastric or pulmonary anomalies were not increased
in any group. The NOAEL for teratogenicity was 60 mg/kg bw per day
(FAO/WHO, 1986).
Hens
The yolks or air sacs of 830 fresh, fertilized hens' eggs were
injected with folpet in dimethyl sulfoxide at 3-20 mg/kg egg weight.
After incubation, the incidence of malformations was 8.19%. The
metabolites of folpet, phthalimide and phthalic acid, were
administered under similar conditions, except that ethanol was used as
the solvent for phthalic acid. Gross abnormalities were seen in 3.93%
of 305 eggs injected with phthalimide and in 3.1% of 290 eggs treated
with phthalic acid. Controls -- 1500 eggs injected with dimethyl
sulfoxide and several thousand with ethanol -- had incidences of < 2%
gross abnormalities. Micromelia, amelia, and phocomelia accounted for
most of the deformities (FAO/WHO, 1969).
(f) Genotoxicity
Folpet has been adequately tested in a number of assays for
genotoxicity in vitro and in vivo. The positive controls gave the
expected positive responses. The results are summarized in Table 1.
(g) Special studies
Delayed cutaneous hypersensitivity
The skin sensitizing potential of folpet (purity and stability
not given) was evaluated in albino Dunkin-Hartley guinea-pigs, 8-11
weeks old, weighing 309-411 g, by the Magnusson-Kligman test. The
animals were obtained from David Hall Limited, Burton-on-Trent,
Staffordshire, United Kingdom, and were acclimatized for at least five
days before treatment. Twenty animals were allocated to the treatment
group (for induction and challenge with folpet) and 10 animals to the
control group (induction with vehicle and/or Freund's complete
adjuvant and challenge with folpet). The animals were induced by three
series of two 0.1-ml intradermal injections of a 1:1 mixture of
Freund's complete adjuvant and water, 0.1% folpet (w/v) in arachis
oil, and 0.1% folpet (w/v) in a 1:1 preparation of Freund's complete
adjuvant plus arachis oil; one week later, 0.2-0.3 ml of 50% folpet
(w/w) in arachis oil was applied topically under an occlusive bandage
for 48 h. Erythematous reactions were quantified after 1 and 24 h.
During challenge, 0.1-0.2 ml of 25% folpet (w/w) in arachis oil was
applied topically under an occlusive bandage for 24 h on the right
flank, and the vehicle alone was applied on the left flank.
Erythematous reactions were quantified after 24 and 48 h. Seven days
after the original challenge, all test and control animals were
rechallenged with 10% folpet (w/w) in arachis oil.
Moderate and diffuse redness was noted at 18 treatment sites 1 h
after removal of the patches. Scattered mild redness was noted at nine
treatment sites at 24 h. Skin reactions observed after the initial
topical challenge included moderate and diffuse redness (at three
sites at 24 h and one site at 48 h) and scattered mild redness (at 14
sites at 24 h and 13 sites at 48 h). The responses were considered to
be due to primary cutaneous irritation caused by folpet. The animals
were re-challenged with a lower concentration of folpet, as the
irritation might have precluded sensitization responses. Scattered
mild redness was elicited at six treatment sites at 24 h and at two
sites at 48 h, the reaction extending beyond the treatment sites in
some animals. Erythema could not be evaluated at some sites because of
other adverse reactions, including desquamation, oedema, scabs,
cracking of the epidermis, fur loss, fissuring, hyperkeratinization,
and loss of skin elasticity. Technical-grade folpet was thus a strong
sensitizer on guinea-pig skin (Dreher, 1990).
Table 1. Results of tests for the genotoxicity of folpet
End-point Test system Concentration Purity Results Reference
or dose (%)
In vitro
Reverse mutation E. coli PQ37 0.03, 0.10, 0.3, or 1.0 µg/ml 90 Positivea FAO/WHO (1986)
Reverse mutation E. coil PQ37 3.0, 10, or 30 µg/ml 90 Negativeb FAO/WHO (1986)
Reverse mutation S. typhimurium TA100, 0 or 50 µg/plate NR Positive only Tennekes (1995)
TA98, TA1535, TA1537, in TA100a
TA1538, E. coli WP2uvrA-
Reverse mutation S. typhimurium TA1535, 0 or 100 µg/plate NR Positivea Tennekes (1995)
TA1537, TA1538
Reverse mutation S. typhimurium TA100, 0, 25, or 45 µg/plate NR Positivea Tennekes (1995)
TA1535
Reverse mutation E. coli TKJ6321, NR Positivea Tennekes (1995)
TKJ5211 Weakly positiveb
Reverse mutation E. coli WP2hcr+ WP2hcr- 0 or 100 µg/plate NR Positivea Tennekes (1995)
Reverse mutation E. coli WP2hcr 0 or 45 µg/plate NR Positivea Tennekes (1995)
Chromosomal aberration Chinese hamster ovary NR NR Positivea Tennekes (1995)
cells Weakly positiveb
Chromosomal aberration Human lymphoid cell line NR NR Positivea Tennekes (1995)
Chromosomal aberration Human lymphocytes 0, 1, 2, or 3 µg/ml for 24 h 90.1 Negativec Tennekes (1995)
Gene mutation hprt locus Chinese hamster V79 0, 125, 0.25, 0.5, 1.0, or 2.0 90.1 Negativea FAO/WHO (1986)
lung fibroblasts µg/ml
Gene mutation hprt locus Chinese hamster V79 3.125, 6.25, 12.5, or 50 µg/ml 90.1 Negativeb FAO/WHO (1986)
lung fibroblasts
Gene mutation hprt locus Chinese hamster ovary NR NR Positivea Tennekes (1995)
cells
Gene mutation tk locus Mouse lymphoma NR Positivea Tennekes (1995)
L5178Y cells
Table 1. (con't)
End-point Test system Concentration Purity Results Reference
or dose (%)
In vivo
Somatic cell mutation T strain male mice Oral; 0, 100, 1500, or 5000 ppm 88.7 Negative FAO/WHO (1986)
C57Bl/6 female mice on days 8.5-12.5 of gestation
Micronucleus formation CD-1 mice 10, 50, or 250 mg/kg bw 91 Negative FAO/WHO (1986)
Micronucleus formation Groups of five male and Oral; 125, 625, or 1250 mg/kg 97 Negatived Ivett (1988)
five female Sprague-Dawley bwj; sacrifice at 24, 48, and
rats; bone-marrow cells 72h; vehicle, corn oil
Sex-linked recessive Drosophila melanogaster NR Positive Tennekes {1995)
lethal mutation
NR, not reported
a In the absence of metabolic activation
b With metabolic activation
c Authors concluded result was positive
3. Observations in humans
A retrospective study of mortality was conducted in a cohort of
134 workers occupationally exposed during the manufacture of captan
for up to nine months annually and to folpet for up to three months
annually. There was an apparent increase in the number of deaths from
all causes (18) in the cohort in comparison with the number expected
from US mortality rates (standardized mortality ratio, 164). The
excess mortality was due mainly to cardiovascular disease and
'external causes' unrelated to occupation. No statistically
significant increases were observed for any specific cause of death,
including neoplasia, but there were too few deaths to evaluate cause-
specific mortality. Lack of adequate industrial hygiene monitoring
precluded satisfactory estimation of previous exposure (FAO/WHO,
1986).
Comments
Excretion of orally administered folpet by mice and rats is about
45-55% in urine, 30-40% in expired air, and 11-17% in faeces; very
little biliary excretion occurs, particularly in mice.
Important degradation pathways of folpet in rodents result in the
formation of phthalimide and thiophosgene. The latter is detoxified,
at least in part, by three mechanisms: oxidation and/or hydrolysis to
carbon dioxide; reaction with the cysteine moiety of glutathione to
yield thiazolidine-2-thione-4-carboxylic acid; and reaction with
sulfite to produce dithiobis(methanesulfonic acid).
A study in which rats were exposed by acute inhalation indicated
an LC50 of 0.39 (male) to 0.43 (female) mg/litre. The WHO has
classified folpet as unlikely to present an acute hazard in normal
use.
A two-year study of carcinogenicity in mice with dietary
concentrations of 0, 1000, 5000, or 10 000 ppm showed a dose-related
increase in the incidence of atypical duodenal hyperplasia, adenomas,
and adenocarcinomas, leading to partial obstruction of the duodenal
lumen in animals of each sex. A no-effect level was not observed. In
another two-year study of carcinogenicity, mice were given dietary
concentrations of 0, 150, 450, or 1350 ppm. A treatment-related
decrease in male body-weight gain was seen. There was a higher
incidence of duodenal masses and thickening of the stomach wall in
females and reduced liver weight in males at 1350 ppm. Microscopic
changes at 1350 ppm and to a minor degree at 450 ppm included benign
papillomas in the keratinized region of the stomach, benign adenomas
in the duodenal mucosa, villous hyperplasia in the duodenal and
jejunal mucosa, and hyperplasia of the lamina propria of the duodenum.
Administration of folpet in the diet at a concentration of 1350 ppm
induced tumours in the upper parts of the gastrointestinal tract
(non-glandular stomach and duodenum) of mice of each sex. The NOAEL
was 150 ppm, equal to 16 mg/kg bw per day.
Folpet has been adequately tested for genotoxicity in a range of
assays, which demonstrate that it is mutagenic and clastogenic in
vitro but not in vivo. The in-vitro responses were reduced or
abolished by the presence of liver homogenates, serum, glutathione, or
cysteine whenever these experimental modifications were investigated.
The Meeting concluded that folpet does not present a significant
genotoxic risk, owing to the presence of an efficient detoxification
mechanism in vivo.
Oral administration of folpet causes time-dependent changes in
the glutathione content and glutathione S-transferase activities of
different regions of the gastrointestinal tract of mice and rats, with
consequent effects on the detoxification of folpet. As early as three
to four weeks after initiation of treatment with a tumour-inducing
dose of folpet, both protein and non-protein thiol concentrations were
increased throughout the duodenum of mice. Reaction with protein thiol
is important for toxicity, while reaction with glutathione is
important for detoxification. At the same time, some hyperplasia and
hypertrophy were observed and the concentration of cyclin-dependent
kinase was increased, but only in the proximal half of the duodenum, a
result supported by assaying for proliferating cell nuclear antigen.
These data indicate that sustained proliferative stimulation of the
proximal duodenum is a consequence of oral administration of folpet.
The Meeting concluded that this finding represents an important
element in the process by which folpet, which is not genotoxic
in vivo, induces tumours in the mouse gastrointestinal tract.
A maximization test indicated that folpet is a sensitizer and
irritant in guinea-pig skin.
An ADI of 0-0.1 mg/kg bw was allocated on the basis of the NOAEL
of 10 mg/kg bw per day in the two-year study of toxicity and
carcinogenicity in rats, the one-year study of toxicity in dogs, and
studies of reproductive toxicity in rats and rabbits, and a safety
factor of 100.
Toxicological evaluation
Levels that cause no toxic effect
Mouse: 150 ppm, equal to 16 mg/kg bw per day (104-week study of
toxicity and carcinogenicity)
Rat: 190 ppm, equivalent to 10 mg/kg bw per day (104-week study
of toxicity and carcinogenicity)
800 ppm, equivalent to 40 mg/kg bw per day (two-generation
study of reproductive toxicity)
10 mg/kg bw per day (maternal toxicity in study of
developmental toxicity)
Rabbit: 10 mg/kg bw per day (maternal and fetotoxicity in study of
developmental toxicity)
Dog: 10 mg/kg bw per day (one-year study of toxicity)
Estimate of acceptable daily intake for humans
0-0.1 mg/kg bw
Studies that would provide information useful for continued
evaluation of the compound
Further observations in humans
Toxicological criteria for setting guidance values for dietary and non-dietary exposure to folpet
Exposure Relevant route, study type, species Results, Remarks
Short-term (1-7 days) Skin, sensitization, guinea-pig Sensitizer and irritant in maximization test
Oral, toxicity, rat LD50 > 5000 mg/kg bw
Inhalation, 4 h lethality, rat LC50 = 0.4 mg/litre
Dermal, toxicity, rabbit LD50 > 23 000 mg/kg bw
Medium-term (1-26 weeks) Repeated oral, 13-week toxicity, rat NOAEL = 300 mg/kg bw per day based on reduced
body weight, irritation of proximal gastrointestinal
tract, and other effects
Repeated oral, reproductive toxicity, NOAEL = 40 mg/kg bw per day based on reduced
rat body weight
Oral, developmental toxicity, rabbit NOAEL = 10 mg/kg bw per day based on reduced
body weight and food consumption; no fetotoxicity
or teratogenic effect
Long-term (> one year) Repeated oral one year, toxicity, dog NOAEL = 10 mg/kg bw per day based on reduced
body weight and food consumption and serum
biochemical changes
References
Blagden, S.M. (1991) Folpet technical: Acute inhalation toxicity
study, four-hour exposure (nose only) in the rat. Unpublished and
unnumbered report (Project No. 306/51) from Safepharm
Laboratories Ltd, Derby, United Kingdom. Submitted to WHO by
Makhteshim Chemical Works Ltd, Beer-Sheva, Israel.
Dreher, D.M. (1990) Folpet technical: Magnusson & Kligman maximization
study in the guinea pig. Unpublished and unnumbered report
(Project No. 8/81) from Safepharm Laboratories Ltd, Derby, United
Kingdom. Submitted to WHO by Makhteshim Chemical Works Ltd,
Beer-Sheva, Israel.
East, P.W. (1994) Folpet: Oncogenicity study by dietary administration
to CD-1 mice for 104 weeks. Unpublished report No. 94/0403,
Pharmaco LSR No. MAK/117, from Pharmaco LSR Ltd, Eye, Suffolk,
United Kingdom. Submitted to WHO by Makhteshim Chemical Works
Ltd, Beer-Sheva, Israel.
FAO/WHO (1970) 1969 Evaluations of some pesticide residues in food.
The monographs. FAO/PL: 1969/M/17/1, WHO/Food Add./70.38, Rome,
FAO.
FAO/WHO (1974) 1973 Evaluations of some pesticide residues in food.
WHO Pesticide Residue Series No. 3, Geneva, WHO.
FAO/WHO (1985) FAO Plant Production and Protection Paper 67.
Evaluations, 1984. Rome, FAO. FAO/WHO (1987) Pesticide Residues
in Food -- 1986. Evaluations, Part II -- Toxicology. FAO
Production and Protection Paper 78/2. Rome, FAO.
FAO/WHO (1991) Pesticide Residues in Food -- 1990. Toxicology
Evaluations. WHO/PCS/91.47, Geneva, WHO.
Nyska, A., Waner, T., Paster, Z., Bracha, P., Gordon E.B. & Klein, B.
(1990) Induction of gastrointestinal tumors in mice fed the
fungicide folpet: Possible mechanisms. Jpn. J. Cancer Res., 81,
545-549.
Tennekes, H. (1995) The genetic toxicology of folpet. Position paper
commissioned by Makhteshim Chemical Works Ltd, Beer-Sheva,
Israel. Submitted to WHO by Makhteshim Chemical Works Ltd,
Beer-Sheva, Israel
Waterson, L.A. (1994a) Folpet, feasibility study by dietary
administration to male mice for 21 days. Report No. MBS
43/942221, from Huntingdon Research Centre Ltd, Huntingdon,
Cambs, United Kingdom. Submitted to WHO by Makhteshim Chemical
Works Ltd, Beer-Sheva, Israel.
Waterson, L.A. (1994b) Folpet, extended feasibility/preliminary study
by dietary administration to male mice for 28 days. Report number
MBS 44/942343, from Huntingdon Research Centre Ltd, Huntingdon,
Cambs, United Kingdom. Submitted to WHO by Makhteshim Chemical
Works Ltd, Beer-Sheva, Israel.
Waterson, L.A. (1995) Folpet, investigation of the effect on the
duodenum of male mice after dietary administration for 28 days
with recovery. Report No. MBS 45/9423003, from Huntingdon
Research Centre Ltd, Huntingdon, Cambs, United Kingdom Submitted
to WHO by Makhteshim Chemical Works Ltd, Beer-Sheva, Israel.
 relationship in mice. By 2 h after treatment, the glutathione
depletion in rat and mouse duodenum was similar, and at 6 h the
glutathione levels were higher than those in controls. More
glutathione was re-bound in the duodenum and jejunum of mice than of
rats, indicating that mice have a stronger requirement for and greater
utilization of glutathione, this perhaps being the major route of
metabolism in this species (FAO/WHO, 1990).
Mice (72) and rats (24) were fed dietary concentrations of 0, 50,
or 5000 ppm folpet for 21 days. The mean concentration of glutathione
in rat liver at both doses was similar to that in controls, whereas a
small decline was observed in mice at 5000 ppm. In both species, the
glutathione concentrations were significantly increased after 50 ppm
and 5000 ppm in both duodenum and jejunum. A similar effect in the
ileum was more pronounced in mice. These results suggest an initial
depletion of glutathione followed by increasing de novo synthesis of
glutathione, resulting in a 'rebound' elevation in tissue glutathione
concentrations in the gastrointestinal tract. The tissue weights of
the stomach, duodenum, and jejunum were increased in both species. The
mean cytosolic protein concentrations (total milligrams per tissue) in
duodenum and jejunum were also increased in both species, the greater
increase being found in rats. Total cytosolic protein in mouse liver
declined to 86% of control values. Glutathione- S-transferase
levels (with 1-chloro-2,4-dinitrobenzene as substrate) increased
significantly in the duodenum, jejunum, and ileum of both species,
in the liver of rats, and in the stomach of mice after treatment
with 5000 ppm. This led to a greater capacity to enzymatically
conjugate thiophosgene with glutathione. A marked reduction in the
concentrations of lipid peroxides (malonaldehyde was used as an
indicator of the overall lipid peroxidation state of the mucosal
cells) was noted in the duodenum of both species receiving 5000 ppm
folpet in the diet, but the reduction was statistically significant
only in the stomachs of mice. No alteration was found in the
intracellular level of conjugated dienes in either species in
comparison with controls. The non-selenium-dependent glutathione
peroxidase activity (i.e. that due to the activity of glutathione-
S-transferase) was increased in the duodenum, jejunum, and ileum in
both species receiving 5000 ppm folpet in the diet and in the stomachs
of rats at either dose (FAO/WHO, 1990).
In 240 mice and 48 rats treated as in the previous study, the
mean microsomal protein (total milligrams per tissue) was
significantly increased in rat duodenum, jejunum, and ileum. Although
increases were also observed in these tissues in mice, they were not
statistically significant. Total hepatic microsomal protein declined
significantly in rats but remained unchanged in mice. Cytochrome P450
was reduced in the livers of both species, but the reduction was
statistically significant only in mice receiving 5000 ppm folpet. Both
aniline hydroxylase and 7-ethyloxycoumarin O-deethylase activities
were reduced in hepatic microsomes in both species at both doses, the
reduction in aniline hydroxylase being statistically significant at
5000 ppm (FAO/WHO, 1990).
In an experiment with 90 mice and 54 rats treated as in the
previous study, a statistically significant decrease in pH was
observed in the duodenum and jejunum of mice fed 5000 ppm folpet.
Incorporation of 3H-thymidine into the mucosal DNA was reduced in
most tissues of both species. In neither species was their evidence of
a dose-related increase in DNA synthesis (FAO/WHO, 1990).
Male Sprague-Dawley rats (200-250 g) were treated with folpet
suspended in 0.5 ml corn oil by intraperitoneal injection at
doses up to 100 mg/kg bw or orally at doses up to 1000 mg/kg bw.
Intraperitoneal injection of 50 mg/kg bw significantly decreased the
activities of benzphetamine N-demethylase and cytochrome P450, while
that of serum aspartate transaminase was significantly increased. Oral
doses up to 10 times the intraperitoneal dose did not have similar
effects (FAO/WHO, 1990).
In a preliminary study, groups of 313 male Crl:CD-1 (ICR) BR
mice, about 48 days old, were fed 0 or 5000 ppm folpet for 21 days.
Clinical signs and food consumption were monitored weekly. At the
end of treatment, the animals were sacrificed and used for either
histopathological investigations or biochemical measurement of cyclin-
dependent kinases (CKD) and proliferating cell nuclear antigen (PCNA).
Treatment-related findings included glandular hyperplasia of the
crypts, hypertrophy of the villous epithelium of the duodenum,
hypertrophy of the villous epithelium of the jejunum, and a twofold
increase in CKD and PCNA levels in 9000 × g (S9) fractions from the
duodenum. It was concluded that the upper gastrointestinal tract
(especially the duodenum) could be used to assess the effect of
folpet. Sampling of the first 3 cm of duodenum for histopathological
examination and biochemical analysis of sonicated fractions was
recommended (Waterson, 1994a).
Subsequently, the mechanism of the effect of folpet on the
duodenum was studied in groups of 51 Crl:CD-1 (ICR) BR mice fed 0 or
5000 ppm folpet, equal to 692 mg/kg bw per day, or 11 or 111 ppm
perchlormethyl mercaptan, equal to 2 or 16 mg/kg bw per day, for 28
days. The latter compound was included because 5000 ppm technical-
grade folpet contains about 11 ppm. Clinical signs and food
consumption were monitored daily, and body weight was determined
weekly. At the end of treatment, the animals were sacrificed and the
duodenum used for either histopathological investigations or
measurement of CDK and PCNA in S9 fractions. There was no treatment-
related mortality or change in clinical signs. In week 1, the body-
weight gain and the food intake of the animals receiving folpet or
111 ppm perchlormethyl captan was lower than that of controls.
Haematoxylin staining revealed glandular hyperplasia of the crypts in
the first 2.5 cm of the duodenum of most animals receiving folpet and
hypertrophy of the villous epithelium in the entire duodenum of all
animals given folpet. The crypts in the entire duodenum of all animals
in all groups stained for PCNA; more animals receiving folpet showing
a greater degree of staining. An approximately twofold increase over
that in controls was seen in CDK concentrations in fractions from the
entire length of the duodenum of animals receiving folpet; there was
no significant difference from controls in CDK concentrations in mice
fed 11 or 111 ppm perchlormethyl mercaptan. The PCNA concentrations
were significantly higher than control values in S9 fractions from the
entire length of the duodenum in the groups receiving folpet or
11 ppm perchlormethyl mercaptan. It was concluded that folpet acts
significantly differently from perchlormethyl mercaptan in the
duodenum, when CDK is used as the index, and these findings were
supported by the findings of both the biochemical PCNA assay and
histopathological immunochemistry. Since both CDK and PCNA are
increased in proliferating cells, the findings of the two studies were
considered to demonstrate proliferative stimulation as early as three
to four weeks after initiation of treatment with a tumour-inducing
dose of folpet (Waterson, 1994b).
The mechanism of the effect of folpet on the duodenum was further
studied in five groups of 132 male Crl:CD-1 (ICR) BR mice, about 48
days old, which were fed 0 or 5000 ppm folpet (purity, 99.4%), equal
to 717 mg/kg bw; 5000 ppm folpet, equal to 679 mg/kg bw, plus 11 ppm
perchlormethyl mercaptan (purity, 95%), equal to 1.5 mg/kg bw;
11 ppm perchlormethyl mercaptan alone, equal to 1.6 mg/kg bw; or
0.4% hydrogen peroxide, equal to 527 mg/kg bw (positive control,
administered in the drinking-water) for 28 days followed by a 28-day
recovery period for selected mice. An acclimatization period of 11
days was allowed before commencement of treatment, at which time the
mice were nine weeks old and weighed 26-45 g. The mice were housed
three to a cage. The identity of the test compound was confirmed by
chemical analysis, and the accuracy of the preparations and the
homogeneity and stability of the dietary formulations were found to be
satisfactory. Clinical signs and food consumption were monitored
daily. At the end of treatment and recovery, specified animals were
sacrificed and allocated for either pathological investigation of the
duodenum or biochemical measurement of total protein, non-protein
thiol, CDK, and PCNA in duodenal fractions by means of commercially
available kits.
Animals receiving hydrogen peroxide had a statistically
significant reduction in body-weight gain and a lowered efficiency of
food use in comparison with controls. At termination, a marginal
increase in glandular hyperplasia of the crypts and hypertrophy of the
villous epithelium in the duodenum were seen in mice treated with
folpet, folpet plus perchlormethyl mercaptan, or hydrogen peroxide in
comparison with controls. No such changes were seen after the recovery
period.
Treatment of mice with folpet (with or without perchlormethyl
mercaptan) resulted in increased protein (per milligram of tissue) in
total duodenal mucosal epithelium. The effect of folpet was greater in
the first 2.5 cm of the duodenum than in the next 3.5 cm and was
completely reversed after 28 days of withdrawal of animals from the
test diet. Treatment with perchlormethyl mercaptan alone or with
hydrogen peroxide had no such effect. The non-protein thiol
concentration was also increased after administration of folpet (with
or without perchlormethyl mercaptan) and with hydrogen peroxide.
Again, the effects were greatest in the first 2.5 cm of the duodenum
and were fully reversible after the recovery period. Treatment with
perchlormethyl mercaptan alone had no such effect. Folpet (with or
without perchlormethyl mercaptan) and hydrogen peroxide, but not
perchlormethyl mercaptan alone, increased the concentrations of CDK in
the first 2.5 cm of the duodenum; after the recovery period, no
significant difference in CDK concentrations was detected between
treated and untreated animals. The PCNA concentrations in duodenal S9
fractions were close to the limit of detection of the assay system,
but a significant increase in PCNA concentrations was detected after
treatment with folpet (with or without perchlormethyl mercaptan) in
the first 2.5 cm, confirming the CDK response. In contrast, the PCNA
concentrations also indicated cell proliferation in the next 3.5 cm of
the duodenum, although the effect was not seen after the recovery
period. Treatment with perchlormethyl mercaptan or with hydrogen
peroxide had no clear effect on PCNA concentrations in the duodenum.
In none of the investigations was there evidence that perchlormethyl
mercaptan and folpet have interactive effects.
Thus, administration of folpet for 28 days (with or without
perchlormethyl mercaptan) increased protein and non-protein thiol
concentrations in both the first 2.5 cm and the next 3.5 cm of the
duodenum, while CDK concentrations were increased only in the first
2.5 cm. The latter finding was supported by the results of the PCNA
assay but not by histopathological immunocytochemistry. The results
also showed that the biochemical effects of folpet on the mouse
duodenum are reversible after a 28-day recovery period (Waterson,
1995).
2. Toxicological studies
(a) Acute toxicity
The LD50 in adult male and female rats and in female wealings
treated orally with folpet was > 5000 mg/kg bw (FAO/WHO, 1990),
and the LD50 in rabbits treated percutaneously with folpet was
> 23 000 mg/kg bw (FAO/WHO, 1969).
Exposure of rats to folpet (purity unspecified) by inhalation for
4 h showed an LC50 of 0.39 mg/litre for males and 0.43 mg/litre for
females (Blagden, 1991).
(b) Short-term toxicity
Rats
Groups of 10 rats of each sex were fed dietary levels of 0, 0.1,
0.32, or 1% folpet for 12 weeks. Growth was normal, except in male
rats fed the 1% level which showed a significant decrease There were
no gross abnormalities, and histopathological examination of the
liver, kidneys, adrenals, intestines, lungs, and gonads of two males
and two females from each group revealed no abnormalities (FAO/WHO,
1969).
Groups of 20 Fischer 344 rats of each sex were fed dietary
concentrations of 0, 0.2, 0.4 or 0.8% folpet (purity, 89%) for 13
weeks. During treatment, the body weights and food consumption of
males at the middle and high doses and of females at the high dose
were significantly reduced. After 10 weeks, there were no significant
differences between treated and control groups, but there were dose-
related decreases in the activities of alkaline phosphatase and
alanine aminotransferase in all treated groups, of aspartate
aminotransferase in all treated males and in females it the high dose,
and of lactate dehydrogenase in all treated males. Blood urea and
chloride levels were increased, but total serum proteins were reduced
in males at the middle and high doses. Blood urea was reduced in
treated females, total protein was reduced in the high-dose groups,
and albumin was reduced in the mid- and high-dose groups. Treatment-
related irritation of the proximal gastrointestinal tract was seen at
necropsy, as was hyperkeratosis of the non-glandular gastric mucosa.
Slight acanthosis of the stomach occurred in one female rat in each
group. The kidneys of males at the middle and high doses showed slight
but dose-related increases in the number of foci of atrophic
basophilic renal tubules, which were considered to be unrelated to
treatment (FAO/WHO, 1986).
Groups of 20 Sprague-Dawley rats of each sex received folpet
(purity unspecified) at dietary levels of 0, 0.03, 0.1, 0.3, or 1.0%
for 13 weeks. Dietary analyses showed that the achieved doses were
85-106% of the nominal values. At the end of treatment, half of the
rats in each group were sacrificed; the remainder were given the basal
diet for a further two weeks and were then sacrificed. No clinical
signs or mortality occurred during the study period. The growth of
rats at the high dose was significantly reduced during treatment, and
this retardation was not made up during the recovery period. Food
consumption, haematological parameters, serum hepatic enzyme levels,
and renal function were unaffected by treatment. At necropsy, animals
at the high dose had reduced mean body weights; relative brain weight
was also reduced, but kidney weight was increased. There were no
significant differences in organ weights of animals sacrificed two
weeks after treatment. No treatment-related gross pathological changes
were observed, but histopathological examination of animals sacrificed
immediately after treatment showed acanthosis, hyperkeratosis,
submucosal oedema, and pleocellular inflammatory infiltration, with
occasional focal gastric erosion and ulceration in the non-glandular
stomach of rats at the high dose. No such lesions were seen after the
recovery period. The NOAEL was 300 mg/kg bw per day, based on reduced
body weight and other effects at the high dose (FAO/WHO, 1986).
Dogs
In a pilot study, four beagle dogs of each sex were given folpet
(purity, 89.8-91.1%) at 0, 790, 1800 or 4000 mg/kg bw per day by
capsule for 13 weeks. Food intake was generally lower in treated dogs
than in controls. Body weight gain was reduced in all treated groups,
significantly so in animals at the middle and high doses. All treated
dogs showed vomiting and diarrhoea, the symptoms being especially
marked in those at the middle and high doses. Treatment-related
changes included poor condition, abdominal distension, excessive
salivation, and a progressive decrease in testicular size,
particularly in animals at the middle and high doses. All males and
one female at the high dose died or were killed in extremis.
Neurological examination of these dogs and of those males which
survived 12 weeks of treatment and ophthalmoscopic examination of
survivors showed no remarkable changes. Most dogs killed in extremis
had leukocytosis, and some had decreased serum phosphate levels. One
dog had normochromic normocytic anaemia. Surviving dogs had decreased
serum calcium and total plasma protein concentrations, but increased
serum chloride levels. At necropsy, most treated dogs had decreased
brain, liver, kidney, spleen, and testicular weights. Pathological
examination showed atrophy, depletion, and fibrosis of the lymphatic
and haematopoietic systems, gonadal degeneration with prostatic
atrophy and fibrosis, thyroid degeneration, and muscular dystrophy
(FAO/WHO, 1986).
Groups of six beagle dogs of each sex were given folpet (purity,
89.5%) in gelatine capsules at 0, 10, 60, or 140 mg/kg bw per day. The
latter dose was reduced to 120 mg/kg bw per day after 50 days because
of low food consumption and reduced body-weight gain. All animals were
sacrificed and necropsied after one year. All males at the high dose
and three at the middle dose initially lost weight, and the mean body
weights of animals at the middle and high doses showed a dose-related
reduction (which was not significant) throughout the rest of the
study. Dose-related decreases in food consumption were seen in animals
at the middle and high doses, in males during the first three months,
and in females during the first month. Although the food consumption
of males was subsequently comparable to that of controls, that
of females tended to be reduced in a non-dose-related manner.
Ophthalmoscopy at six and 12 months revealed no effect. There was a
tendency for reduced leukocyte counts in males at one, two, three, and
six months, but not at nine or 12 months; however, the counts were not
significantly different from control values. A significant decrease in
mean serum cholesterol and in total protein, albumin, and globulin
levels was seen in males at the middle and high doses; females at the
high dose had significantly reduced mean serum protein, albumin,
and cholesterol levels. Urinalysis, necropsy, and subsequent
histopathology showed no effect of treatment. The NOAEL was 10 mg/kg
bw per day, on the basis of decreased body weight and food consumption
and serum biochemical changes (FAO/WHO, 1986).
Groups of five beagle dogs of each sex received technical-grade
folpet in capsules at 0, 325, 650, or 1300 mg/kg bw per day for 52
weeks. None died. Animals of each sex at the high and intermediate
doses had decreased body weight during treatment; a concomitant
decrease in food intake was found in animals of each sex at the high
dose. Clinical signs seen in all treated groups included vomiting,
diarrhoea, and salivation; the latter was seen only during the first
eight weeks in animals at the low dose but was more pronounced at the
high and intermediate doses. Dogs at these doses were in poorer
condition than controls. Haematological parameters were affected in
all treated females during the first third of the study and included
decreased packed cell volume and haemoglobin and erythrocyte counts.
Clinical chemical changes (e.g. reduced total protein, cholesterol,
glucose, and urea) observed during treatment were related to the poor
physical condition of the dogs. Chloride levels were increased in
males, mainly those at the high dose, which also had decreased calcium
levels; the latter effect was also observed in females at the high and
intermediate doses up to week 25 of treatment. A reduced urine volume
was noted in females at the high dose after 13 weeks of treatment, and
both males and females had acid urine; this effect persisted in males
at the high and intermediate doses throughout the study. Tubular
testicular degeneration associated with an absence of spermatozoa in
the epididymides was found in two male dogs at the high dose; one also
had moderate prostatic gland atrophy. The absolute testicular weights
of males at the high dose were decreased. Changes in relative organs
weights were recorded in the adrenals (all males and females at the
intermediate dose), brain and kidney (males at the high dose and
females at the intermediate dose), liver, and thyroid (males at the
intermediate dose and females at the high dose). The NOAEL was
325 mg/kg bw per day (FAO/WHO, 1990).
(c) Long-term toxicity and carcinogenicity
Mice
Goups of 80 CD-1 (ICR derived) mice of each sex were fed dietary
concentrations of 1000, 5000, or 12 000 ppm folpet (purity, 93%) for
112-113 weeks. A group of 104 mice of each sex served as controls.
Survival was not affected, but body-weight gain was reduced in animals
at 5000 and 12 000 ppm. Although sporadic changes in food consumption
were noted, no dose-related effects were apparent. Haematological
parameters were normal at one year, but at the end of treatment,
possible macrocytic anaemia was seen in animals of each sex at
12 000 ppm. The only changes seen at gross pathological examination
were duodenal lesions and related gastrointestinal abnormalities. A
dose-related increase in the incidence of duodenal adenomas and
adenocarcinomas was observed in mice at 5000 and 12 000 ppm, with
incidences in males and females of 0 and 1% in controls, 2 and 3% at
1000 ppm, 10 and 12% at 5000 ppm, and 52 and 54% at 12 000 ppm,
respectively. Males at the high dose also had an increased incidence
of jejunal adenocarcinomas. There was no NOAEL (FAO/WHO, 1984).
Four groups of 52 B6C3F1 mice of each sex were given dietary
concentrations of folpet (purity, 89.0%) at 0, 0.1, 0.5, or 1.0% for
21 weeks and then 0, 0.1, 0.35, or 0.7% for 83 weeks. Dietary analyses
at weeks 0, 1, 13, and 26 showed the levels to be 88-91% of the
nominal value. Food consumption was depressed during the first few
weeks of treatment in mice at the middle and high doses, and body-
weight gain was reduced in these animals throughout the study.
Clinical signs, observed principally in animals at the high dose,
comprised erythema, dry flaking skin, reddish fur discolouration, and
weeping skin, particularly during the first 21 weeks. Leukocyte counts
in survivors at 52, 78, and 104 weeks were not affected by treatment.
The longevity of animals at the two highest doses was reduced. The
increased relative weights of the brain, heart, lungs, liver, kidneys,
and testes reflected the reduced body weight of treated animals.
Macroscopically, a dose-related increase in non-glandular gastric
mucosal ulceration and thickening of the gastric and duodenal walls
were observed. The jejunal wall was thickened in females at the middle
dose and in all mice at the high dose. Dose-related distension of the
duodenal lumen was also seen. After week 79, there was a dose-related
increase in the incidence of nodules or masses on the luminal surface
of the stomach and duodenum and on the duodenal serosa. Animals at the
middle and high doses had dose-related epidermal hyperkeratosis and
acanthosis and oesophageal hyperkeratosis. Increased areas of marked
acanthosis and hyperkeratosis of non-glandular gastric mucosa were
seen in animals at the middle dose and males at the high dose.
Microscopic gastric ulceration occurred, with no apparent
relation to dose. The dose-related appearance of areas of atypical
duodenal glandular hyperplasia and mucosal gland proliferation were
seen in all treated animals, but especially in males. This atypical
hyperplasia was often associated with duodenal adenomas and
adenocarcinomas. Atypical glandular proliferation was seen only
occasionally in the jejunum of treated females. Gastric papillomas and
squamous-cell carcinomas, which may have been secondary to mechanical
obstruction of the duodenal lumen, were found in all treated males but
not in control males. A dose-related increase in the incidence of
gastric papillomas was also found in all treated females, but
similar lesions occurred in 2/51 controls. Duodenal adenomas and
adenocarcinomas were found in all treated animals of each sex, with
a significant dose-response relationship. A single jejunal
adenocarcinoma was seen in mice at the high dose. Primary or
metastatic tumours of uncertain etiology were found in all treated
males, but the incidences of bronchioalveolar adenomas and malignant
lymphomas were lower in these animals, suggesting high incidences in
control males. There was no NOAEL (FAO/WHO, 1986).
Four groups of 52 six-week old B6C3F1 mice of each sex were given
dietary concentrations of 0, 1000, 5000, or 10 000 ppm folpet (purity,
89%) for 104 weeks. The formulated diets were analysed after one and
two weeks and were found to be stable (no data). During the first few
weeks of the study, marked irritation of the integumentary system was
observed. Typical lesions, usually on the neck, included flaking,
weeping, and eroded skin, erythema, and red discolouration of the fur.
Early deaths occurred in all treated males and in females at the
middle and high doses and were attributed to the presence of fatal
tumours. Dose-related macroscopic lesions were found in the stomach
and duodenum. The non-glandular gastric mucosa of all treated animals
showed an increased incidence of nodules, masses, wall thickenings,
and ulceration, and an increased incidence of partial luminal duodenal
obstruction was seen 1-5 cm distal to the pylorus in the form of wall
thickenings and mucosal nodules. An increased incidence of these
lesions was noted in animals that died after 79 weeks of treatment.
Microscopic examination of the skin revealed hyperkeratosis, and a
dose-related in, crease in the incidence of oesophageal and non-
glandular gastric mucosal hyperkeratosis was seen in all treated
groups. Ulceration of the non-glandular stomach was observed in
animals at the low and intermediate doses. Atypical hyperplasia of the
duodenal mucosa was seen in all treated mice. Duodenal adenomas and
carcinomas were seen in all treated groups, and the incidence was
dose-related. Gastric squamous-cell papillomas and carcinomas were
seen more frequently in animals at 5000 and 10 000 ppm. There appeared
to be a correlation between the presence of macroscopically visible
gastric neoplasia and duodenal obstruction: the four males with
gastric carcinoma had partial duodenal nodular obstruction, and five
of seven females at the high dose with an increased incidence of
gastric papillomas had partially obstructed duodenal lumens. No other
toxic or carcinogenic effect was seen in any organ.
The authors suggested that the partial duodenal obstruction
disturbed the natural flow of the gastrointestinal contents and may
have exacerbated the mucosal changes in the stomach. The resulting
stagnation may have raised the effective concentration and residence
time of folpet, and the elevated concentration of this irritant, with
the possible presence of bile acids and pancreatic enzymes, may have
overwhelmed the defence mechanism of the mucosal cells, resulting
in the development of gastric neoplasms. In conclusion, dietary
administration of folpet to B6C3F1 mice induced a dose-related
appearance of atypical duodenal hyperplasia, adenomas, and
adenocarcinomas, which were suggested to have appeared subsequent
to, and as an indirect result of, the partial luminal duodenal
obstruction. There was no NOAEL (Nyska et al., 1990).
Groups of 52 male and 52 female CD-1 mice were fed diets
containing folpet (purity, 92.4%) at concentrations of 0, 150, 450, or
1350 ppm, equal to 0, 16, 47, and 151 mg/kg bw per day for 98 weeks
for males and 0, 16, 51, and 154 mg/kg bw per day for 104 weeks for
females. A control group consisting of 100 male and 100 female mice
received a normal diet. The mice were assigned to treatment groups on
the basis of computer-generated random numbers and were acclimatized
for 14 days before the beginning of treatment. The mice were 3542 days
of age and had mean body weights of 29.7, 29.6, 29.5, and 29.6 g
(males) and 23.4, 23.7, 23.0, and 23.7 g (females) at 0, 150, 450, and
1350 ppm, respectively, at the time of the first dose. The 150-ppm
preparation was found to contain 89-91% of the nominal concentration
and was stable after storage at -20°C for seven days and then at 20°C
for four days; samples taken from six places in the mix showed
satisfactory homogeneity: 89-95% of the nominal value with 150 ppm and
78-88% with 1350 ppm. Verification of the concentrations resulted in
analytical values of 89-96% of the nominal concentrations. Animals
were inspected daily for evidence of reactions to treatment. Body
weight and food and water consumption were determined weekly. All 187
animals found dead or killed during the treatment period were
subjected to gross pathological and histopathological examination.
The overall mortality rate was unaffected by treatment, and no
inter-group differences in mortality were seen by either pairwise or
trend analyses; therefore, none of the deaths was considered to be
related to treatment with folpet. The incidence of skin encrustations,
particularly on the dorsal surface, was generally higher in males
receiving a dietary concentration of 1350 ppm. This finding was
considered to be associated with a greater incidence of fighting among
these animals during the first 70 weeks of treatment. In the later
part of the treatment period, the incidence of skin encrustations
in males was similar to that of controls. The incidence, group
distribution, location, and mean onset time of palpable swellings were
not considered to be related to treatment. The body-weight gain of
males receiving 1350 ppm was slightly lower than that of controls for
about the first 70 weeks of treatment. Food consumption was unaffected
by treatment, but the efficiency of food use during the first 14 weeks
of treatment with 1350 ppm was slightly inferior to that of the
controls. The treatment-related effect on male body-weight gain and
the low food conversion efficiency seen at this dose during the first
14 weeks of treatment were considered to be nonspecific toxic effects.
The body-weight gain of females was unaffected by treatment.
Organ weight analysis revealed lower absolute and relative liver
weights in males that received 1350 ppm in comparison with controls.
High absolute and relative spleen weights in males at 150 ppm were due
to an unusually large spleen in one animal. Macroscopic examination of
animals killed after 104 weeks of treatment revealed a high incidence
of masses in the duodena of females at 1350 ppm. When all of the
animals were considered together, there was a higher incidence of
duodenal masses and thickening of the stomach wall in females at
1350 ppm than in controls. Microscopic examination revealed benign
papillomas in the keratinized region of the stomach in one male and
three females at 1350 ppm and in one female at 450 ppm. A benign
adenoma was found in the duodenal mucosa of one female at 1350 ppm.
Villous hyperplasia was seen in the duodenal mucosa of three females
at the highest dose and in one male at 450 ppm; a similar finding was
seen in the jejunum of one male at 450 ppm. Hyperplasia of the lamina
propria of the duodenum was seen in two males at 1350 ppm, and another
male at this dose had similar changes in the jejunum and ileum in
addition to villous fusion, mucosal dysplasia, and hyperplasia of
Paneth cells. The incidence of keratoachanthosis of the nonglandular
stomach was higher among females at 1350 ppm. than among controls.
Although one female at 450 ppm had a benign squamous-cell papilloma
and two males at this dose had gastrointestinal hyperplasia, these
findings were considered to have occurred by chance. There were no
other changes in the gastrointestinal tract, and there were no changes
in the liver that were considered to be related to treatment. As the
low liver weights of males at 1350 ppm were confined to one sex and
there was no histopathological correlate, the toxicological
significance of this finding is unclear. It was concluded that
administration of folpet in the diet at a concentration of 1350 ppm
produced tumours in the upper parts of the gastrointestinal tract of
CD-1 mice. The NOAEL was 16 mg/kg bw per day on the basis of duodenal
hyperplasia (East, 1994).
Rats
Groups of 60 Charles River Crl:CD(SD)BR rats of each sex were fed
dietary concentrations of 0, 200, 800, or 3200 ppm technical-grade
folpet (purity, 89.5%) for 104 weeks. An interim sacrifice of 10 rats
of each sex per dose was conducted at 52 weeks. Growth rates and
survival were unaffected by treatment, except for a slight tendency to
reduced body weights in females at the high dose during the first
year; food consumption was correspondingly reduced at this dose.
Conventional ophthalmoscopic, haematological, biochemical, and
urinary analyses indicated no effect of treatment. At necropsy, no
changes attributable to treatment were observed on organ weights.
Hyperkeratosis and/or acanthosis and some erosion and/or ulceration in
the non-glandular stomach were seen in animals at the high dose. These
lesions were occasionally accompanied by submucosal oedema and
submucosal inflammatory cellular infiltrates. The NOAEL was 800 ppm,
equivalent to 40 mg/kg bw per day (FAO/WHO, 1986).
Groups of 60 Fischer 344 rats were fed diets containing 0, 500,
1000, or 2000 ppm folpet (purity, 89%) for 104 weeks. The diets were
prepared weekly and analysed frequently for folpet content. Food
intake was reduced in all treated groups in comparison with controls,
but body weight was reduced only in treated males. There were no
clinical signs of toxicity. Necropsy indicated a tendency to an
increased incidence of gastric ulceration, which was significant
only in females. Treatment also induced an increased incidence
of ulceration in the forestomach in animals at the high dose.
Hyperkeratosis of the oesophagus was seen in rats at the high dose
and of the forestomach in animals at the middle and high doses. An
increased incidence of gastric ulceration was present in the
forestomach of animals of each sex at the high dose. The incidences of
C-cell adenoma, benign mammary fibroepithelioma and malignant lymphoma
showed positive significant trends with dose, but only that for the
latter neoplasms was statistically significant. All tumour incidences
were within the normal historical range for rats of this strain. The
NOAEL was 500 ppm, equivalent to 25 mg/kg bw per day (FAO/WHO, 1986).
Groups of 20 Fischer 344 rats of each sex were fed diets intended
to provide concentrations of 0, 250, 1500, or 5000 ppm folpet (purity,
91%) for 104 weeks. The actual dietary concentrations, calculated
on a weekly basis, were 0, 190, 1290, and 4530 ppm. Longevity was
unaffected. The mean body weight and food intake of animals at the
high dose were depressed, by up to 10% in males and 6% in females,
and water intake was depressed at the high dose, especially in
females. Serum alkaline phosphatase and alanine aminotransferase
activities were reduced in treated groups throughout the study, and
those of aspartate aminotransferase, creatinine phosphokinase, and
gamma-glutamyl transferase were decreased sporadically. A significant
reduction in blood cholesterol was seen in animals of each sex of the
high dose throughout treatment. The plasma protein level was reduced
in males at the high dose during the first year of treatment, and the
level of phosphate was elevated at most examinations. The urea
concentration was increased in females at the high dose at 18 months.
Most treated males excreted more concentrated urine in smaller volumes
at three and six months. Only non-neoplastic lesions were observed,
consisting of hyperkeratosis of the oesophageal and gastric squamous
epithelium. The NOAEL was 190 ppm, equivalent to 10 mg/kg bw per day
(FAO/WHO, 1990).
(d) Reproductive toxicity
Rats
Four groups of 25 CD rats of each sex were fed dietary
concentrations of 0, 250, 1500, or 5000 ppm folpet (purity, 91%) over
the course of two generations. Body weight gain and food consumption
were reduced in parental animals of the F0 and F1 generations at
the highest dose; a minor decrease in body weight was observed in F1
and F2 offspring. The principal histopathological effect was
hyperkeratosis of the non-glandular gastric mucosa in F0 and F1
animals at the intermediate and high doses, oesophageal hyperkeratosis
in F1 animals at the intermediate and high doses, and an increased
incidence of basophilic renal tubules in males of the F0 generation
at the high dose. Folpet was concluded not to be a specific
reproductive toxin in this test system (FAO/WHO, 1990).
In a two-generation study, groups of 30 Crl:COBS/CD(SD)/Charles
River rats of each sex were fed diets containing 0, 200, 800, or
3600 ppm folpet (purity, 89.5%) during growth, mating, gestation, and
lactation for two litters per generation. Initial mating was begun 62
days after the start of exposure. The test diets were fed to F0 males
until the end of mating to produce the F1b litters and to F0 females
until weaning of the F1b litters. Pups were sacrificed 21-23 days
after parturition, with the exception of those F1b pups selected to
produce the F2 generation. Mating of the F1b pups was begun after 12
weeks of exposure, and the above sequence was repeated. Gross necropsy
was performed on all parental rats and on the F1a, F1b, and F2b
litters. The diet was changed three times per week, and each batch was
analysed: the diets contained 79.9-101% of the nominal concentration.
The body weights of F0 and F1 males and F1 females at the high dose
and of their pups were depressed by treatment; the effect was most
marked in adult males, especially in the second generation. Food
consumption was correspondingly reduced. Treatment had no significant
effect on mating, fertility indices, pregnancy rates, litter sizes,
pup weights, growth, or litter survival rates. No treatment-related
effects were found at necropsy or on histopathological examination.
The NOAEL for maternal toxicity was 800 ppm, equivalent to 40 mg/kg bw
per day, based on the reduction in body weights; there was no evidence
of reproductive toxicity (FAO/WHO, 1986).
(e) Developmental toxicity
Rats
In a pilot study, groups of eight female mated CRL:COBS CD (SD)
BR rats were given 0, 20, 80, 320, or 640 mg folpet/kg bw by gavage on
days 6-19 of gestation. There were no deaths, but clinical signs
including rales, excess salivation, chromorhinorrhoea, gasping, soft
or liquid faeces, decreased motor activity, dyspnoea, and distended
gut were observed. Reduced maternal body weight was seen at
> 80 mg/kg bw, and food consumption was reduced at > 320 mg/kg
bw; the average fetal body weight was also reduced at the latter dose.
In the main study, groups of 25 rats were given folpet (purity, 89%)
at 0, 10, 60, or 360 mg/kg bw by gavage on days 6-19 of gestation.
Animals were killed on day 20. Clinical signs including excess
salivation, chromorhinorrhoea, decreased motor activity, soft or
liquid faeces, dyspnoea, and urine-stained fur were observed. Three
rats at the high dose died, two from intubation errors. No gross
lesions attributable to treatment were seen in surviving rats. Rats at
> 10 mg/kg bw gained less weight than controls during treatment.
Food consumption was reduced in animals at the high dose only. The
numbers of implantations, live and dead fetuses, fetal viability, and
resorptions, the average fetal body weight per litter, the fetal sex
ratio, and the number of corpora lutea were comparable in all groups.
Gross, visceral, and skeletal abnormalities were seen in all groups,
but there were no compound-related effects on ossification. The NOAEL
for maternal toxicity was 10 mg/kg bw per day (FAO/WHO, 1984).
Groups of six rats were treated daily on days 6-15 of gestation
with folpet (purity, 88.6%) at 10, 65, 420, or 2750 mg/kg bw by
gavage. Maternal toxicity, reduced body-weight gain, and reduced fetal
weight were seen only at 2750 mg/kg bw. Following this pilot study,
groups of 22 female Charles River CD rats received folpet (purity,
91.1%) in 0.5% acetic acid containing 0.5% carboxymethylcellulose at
0, 150, 550, or 2000 mg/kg bw on days 6-15 of gestation. The animals
were sacrificed on day 20. Clear signs of toxicity were observed in
animals at the high dose, including soft faeces (in 21/21 rats), fur
staining (in 4/21), and perianal staining (in 8/21); one animal died.
Food consumption was decreased during the first days of treatment with
the intermediate dose and was markedly decreased throughout treatment
with the high dose. Maternal body weight was also decreased at the
middle and high doses. Gravid uterine weights were depressed in dams
at the middle and high doses, but terminal maternal body weight
(without the gravid uterus) was significantly depressed only at the
high dose. Pre- and post-implantation losses were greater than in
controls in animals at the middle dose, and fetal weights were reduced
at the middle and high doses. Fetal crown-rump length was slightly
decreased after treatment with the middle and high doses. A single
fetus at the high dose (1/277) had multiple major malformations, and a
second pup had unilateral microphthalmia. The incidence of hepatic
discolouration was significant at the high dose. Skeletal anomalies
occurred in all treated groups, and the incidence of reduced
ossification of cranial and pubic bones, sternebrae, metacarpals, and
metatarsals was significant in animals at the middle and high doses.
There was also a dose-related reduction in ossification of the
interparietal bone in all treated groups, and the occurrence of
angulated ribs was dose-related. There was no NOAEL for teratogenicity
(FAO/WHO, 1986).
Hamsters
Groups of 2-13 pregnant hamsters were given a single dose of
400-1000 mg/kg bw folpet on day 7 or 8 of gestation or were treated
daily on days 6-10 with a total of 1000-2500 mg/kg bw. The animals
were killed and examined on day 15 of gestation. Maternal mortality
was increased and some abnormal fetuses were produced at the highest
doses, but the lower doses did not induce teratogenic effects
(FAO/WHO, 1973).
Rabbits
Groups of 20 artificially inseminated New Zealand white rabbits
were given folpet (purity, 89%) at 0, 10, 20, or 60 mg/kg bw by oral
intubation on days 6-28 of gestation and were killed on day 29. The
death of one doe at 60 mg/kg bw was considered to be related to
treatment. One doe at the low dose aborted on day 21 of gestation and
one at the high dose on day 22 of gestation; one doe in the control
group delivered a litter on day 28 and one at the high dose on day 29.
Significant inhibition of body-weight gain and food consumption was
seen in animals at the middle and high doses. The average numbers of
corpora lutea, implantations, resorptions, and fetuses per litter and
the sex ratio and numbers of dead and resorbed implantations per
litter were comparable in all groups. The mean body weights of all
fetuses were decreased at the middle and high doses. There was a
significant increase in the incidence of hydrocephaly, and four
fetuses in three litters of dams at the high dose also had skull,
gastric, and pulmonary abnormalities. The NOAEL for maternal and
fetoxicity was 10 mg/kg bw per day, and that tor teratogenicity was
29 mg/kg bw per day (FAO/WHO, 1984).
Groups of six mated HY/CR female New Zealand white rabbits were
given folpet (purity, 91.1%) at 0, 10, 60, or 150 mg/kg bw per day by
gavage on days 6-18 of gestation. Marked body-weight loss was seen at
the high dose. Although fetal size was unaffected by treatment, fetal
mortality was more marked at the high dose. Post-implantation losses
were increased in animals at the middle dose. Subsequently, groups of
14 mated HY/CR New Zealand white rabbits were given folpet (purity,
91.1%) at 0, 10, 40, or 160 mg/kg bw per day by gavage on days 7-19 of
gestation. Dams were sacrificed on day 29 of gestation. Body-weight
gain was decreased during the initial few days of treatment with the
middle dose and after initiation of treatment with the high dose;
there was a corresponding decrease in food consumption in animals at
the high dose. There were no deaths. Gravid uterine weight was
significantly reduced in dams at the middle and high doses. Fetal
death (post-implantation loss) occurred more frequently at the high
dose than in controls; the proportion of small fetuses was also
increased in this group and mean fetal weight was nonsignificantly
reduced. There was evidence of delayed skeletal maturation at the high
dose and, to a lesser degree, at the middle dose. The incidence of
bilateral lumbar ribs appeared to increase with dose in animals at the
middle and high doses. Ossification of caudal vertebrae, sternebrae,
and long-bone epiphyses was reduced at the high dose. Other, minor
skeletal malformations did not apper to be related to treatment. There
was no evidence of hydrocephalus in either treated or control rabbits.
The NOAEL for maternal toxicity, fetotoxicity, and teratogenicity was
10 mg/kg bw per day (FAO/WHO, 1986).
Groups of 20 artificially inserminated female Hazelton Dutchland
New Zealand white (D1A Hra: (NZW) specific pathogen-free) rabbits
were given folpet (purity, 89.5%) in Tween 80 (10.5% by weight) and
carboxymethylcellulose (0.7% by weight) at a volume of 5 ml/kg bw per
day by gavage, to give a dose of 60 mg/kg bw per day on days 7-9,
10-12, 13-15, or 16-18 of gestation. Analysis of the formulations
indicated a folpet content of 87.8-104% of the nominal concentration.
Dams were sacrificed on day 29 of gestation; those that aborted
or delivered, the single animal that died, and those terminally
sacrificed were subjected to necropsy and examination of the uterine
contents. Abortion by two rabbits that received folpet on days 7-9 and
10-12 of gestation may have been related to treatment; otherwise, no
clinical signs of toxicity were observed during the study, although
the incidence of soft or liquid faeces was increased in all treated
groups, usually after treatment. No gross lesions attributable
to treatment were seen at necropsy. Maternal body weight was
significantly reduced in all treated animals, although less so in
those treated on days 7-9 and 10-12 than in the others. Food
consumption was correspondingly reduced. Treatment had no apparent
effect on the rate of abortion or on fetal resorption. The average
litter sizes were unaffected, as were the average fetal weights, the
number of viable fetuses, and the sex ratio. A significantly increased
incidence of fetuses with an irregularly shaped fontanelle was
observed in the group treated on days 13-15; the control incidence was
4.5%, but this variation did not occur in groups treated on days 7-9
or 16-18. It was possibly related to treatment, but the significance
of the effect was not clear. There were no other significant
variations in fetal skull morphology, and the incidences of
hydrocephalus and of gastric or pulmonary anomalies were not increased
in any group. The NOAEL for teratogenicity was 60 mg/kg bw per day
(FAO/WHO, 1986).
Hens
The yolks or air sacs of 830 fresh, fertilized hens' eggs were
injected with folpet in dimethyl sulfoxide at 3-20 mg/kg egg weight.
After incubation, the incidence of malformations was 8.19%. The
metabolites of folpet, phthalimide and phthalic acid, were
administered under similar conditions, except that ethanol was used as
the solvent for phthalic acid. Gross abnormalities were seen in 3.93%
of 305 eggs injected with phthalimide and in 3.1% of 290 eggs treated
with phthalic acid. Controls -- 1500 eggs injected with dimethyl
sulfoxide and several thousand with ethanol -- had incidences of < 2%
gross abnormalities. Micromelia, amelia, and phocomelia accounted for
most of the deformities (FAO/WHO, 1969).
(f) Genotoxicity
Folpet has been adequately tested in a number of assays for
genotoxicity in vitro and in vivo. The positive controls gave the
expected positive responses. The results are summarized in Table 1.
(g) Special studies
Delayed cutaneous hypersensitivity
The skin sensitizing potential of folpet (purity and stability
not given) was evaluated in albino Dunkin-Hartley guinea-pigs, 8-11
weeks old, weighing 309-411 g, by the Magnusson-Kligman test. The
animals were obtained from David Hall Limited, Burton-on-Trent,
Staffordshire, United Kingdom, and were acclimatized for at least five
days before treatment. Twenty animals were allocated to the treatment
group (for induction and challenge with folpet) and 10 animals to the
control group (induction with vehicle and/or Freund's complete
adjuvant and challenge with folpet). The animals were induced by three
series of two 0.1-ml intradermal injections of a 1:1 mixture of
Freund's complete adjuvant and water, 0.1% folpet (w/v) in arachis
oil, and 0.1% folpet (w/v) in a 1:1 preparation of Freund's complete
adjuvant plus arachis oil; one week later, 0.2-0.3 ml of 50% folpet
(w/w) in arachis oil was applied topically under an occlusive bandage
for 48 h. Erythematous reactions were quantified after 1 and 24 h.
During challenge, 0.1-0.2 ml of 25% folpet (w/w) in arachis oil was
applied topically under an occlusive bandage for 24 h on the right
flank, and the vehicle alone was applied on the left flank.
Erythematous reactions were quantified after 24 and 48 h. Seven days
after the original challenge, all test and control animals were
rechallenged with 10% folpet (w/w) in arachis oil.
Moderate and diffuse redness was noted at 18 treatment sites 1 h
after removal of the patches. Scattered mild redness was noted at nine
treatment sites at 24 h. Skin reactions observed after the initial
topical challenge included moderate and diffuse redness (at three
sites at 24 h and one site at 48 h) and scattered mild redness (at 14
sites at 24 h and 13 sites at 48 h). The responses were considered to
be due to primary cutaneous irritation caused by folpet. The animals
were re-challenged with a lower concentration of folpet, as the
irritation might have precluded sensitization responses. Scattered
mild redness was elicited at six treatment sites at 24 h and at two
sites at 48 h, the reaction extending beyond the treatment sites in
some animals. Erythema could not be evaluated at some sites because of
other adverse reactions, including desquamation, oedema, scabs,
cracking of the epidermis, fur loss, fissuring, hyperkeratinization,
and loss of skin elasticity. Technical-grade folpet was thus a strong
sensitizer on guinea-pig skin (Dreher, 1990).
Table 1. Results of tests for the genotoxicity of folpet
End-point Test system Concentration Purity Results Reference
or dose (%)
In vitro
Reverse mutation E. coli PQ37 0.03, 0.10, 0.3, or 1.0 µg/ml 90 Positivea FAO/WHO (1986)
Reverse mutation E. coil PQ37 3.0, 10, or 30 µg/ml 90 Negativeb FAO/WHO (1986)
Reverse mutation S. typhimurium TA100, 0 or 50 µg/plate NR Positive only Tennekes (1995)
TA98, TA1535, TA1537, in TA100a
TA1538, E. coli WP2uvrA-
Reverse mutation S. typhimurium TA1535, 0 or 100 µg/plate NR Positivea Tennekes (1995)
TA1537, TA1538
Reverse mutation S. typhimurium TA100, 0, 25, or 45 µg/plate NR Positivea Tennekes (1995)
TA1535
Reverse mutation E. coli TKJ6321, NR Positivea Tennekes (1995)
TKJ5211 Weakly positiveb
Reverse mutation E. coli WP2hcr+ WP2hcr- 0 or 100 µg/plate NR Positivea Tennekes (1995)
Reverse mutation E. coli WP2hcr 0 or 45 µg/plate NR Positivea Tennekes (1995)
Chromosomal aberration Chinese hamster ovary NR NR Positivea Tennekes (1995)
cells Weakly positiveb
Chromosomal aberration Human lymphoid cell line NR NR Positivea Tennekes (1995)
Chromosomal aberration Human lymphocytes 0, 1, 2, or 3 µg/ml for 24 h 90.1 Negativec Tennekes (1995)
Gene mutation hprt locus Chinese hamster V79 0, 125, 0.25, 0.5, 1.0, or 2.0 90.1 Negativea FAO/WHO (1986)
lung fibroblasts µg/ml
Gene mutation hprt locus Chinese hamster V79 3.125, 6.25, 12.5, or 50 µg/ml 90.1 Negativeb FAO/WHO (1986)
lung fibroblasts
Gene mutation hprt locus Chinese hamster ovary NR NR Positivea Tennekes (1995)
cells
Gene mutation tk locus Mouse lymphoma NR Positivea Tennekes (1995)
L5178Y cells
Table 1. (con't)
End-point Test system Concentration Purity Results Reference
or dose (%)
In vivo
Somatic cell mutation T strain male mice Oral; 0, 100, 1500, or 5000 ppm 88.7 Negative FAO/WHO (1986)
C57Bl/6 female mice on days 8.5-12.5 of gestation
Micronucleus formation CD-1 mice 10, 50, or 250 mg/kg bw 91 Negative FAO/WHO (1986)
Micronucleus formation Groups of five male and Oral; 125, 625, or 1250 mg/kg 97 Negatived Ivett (1988)
five female Sprague-Dawley bwj; sacrifice at 24, 48, and
rats; bone-marrow cells 72h; vehicle, corn oil
Sex-linked recessive Drosophila melanogaster NR Positive Tennekes {1995)
lethal mutation
NR, not reported
a In the absence of metabolic activation
b With metabolic activation
c Authors concluded result was positive
3. Observations in humans
A retrospective study of mortality was conducted in a cohort of
134 workers occupationally exposed during the manufacture of captan
for up to nine months annually and to folpet for up to three months
annually. There was an apparent increase in the number of deaths from
all causes (18) in the cohort in comparison with the number expected
from US mortality rates (standardized mortality ratio, 164). The
excess mortality was due mainly to cardiovascular disease and
'external causes' unrelated to occupation. No statistically
significant increases were observed for any specific cause of death,
including neoplasia, but there were too few deaths to evaluate cause-
specific mortality. Lack of adequate industrial hygiene monitoring
precluded satisfactory estimation of previous exposure (FAO/WHO,
1986).
Comments
Excretion of orally administered folpet by mice and rats is about
45-55% in urine, 30-40% in expired air, and 11-17% in faeces; very
little biliary excretion occurs, particularly in mice.
Important degradation pathways of folpet in rodents result in the
formation of phthalimide and thiophosgene. The latter is detoxified,
at least in part, by three mechanisms: oxidation and/or hydrolysis to
carbon dioxide; reaction with the cysteine moiety of glutathione to
yield thiazolidine-2-thione-4-carboxylic acid; and reaction with
sulfite to produce dithiobis(methanesulfonic acid).
A study in which rats were exposed by acute inhalation indicated
an LC50 of 0.39 (male) to 0.43 (female) mg/litre. The WHO has
classified folpet as unlikely to present an acute hazard in normal
use.
A two-year study of carcinogenicity in mice with dietary
concentrations of 0, 1000, 5000, or 10 000 ppm showed a dose-related
increase in the incidence of atypical duodenal hyperplasia, adenomas,
and adenocarcinomas, leading to partial obstruction of the duodenal
lumen in animals of each sex. A no-effect level was not observed. In
another two-year study of carcinogenicity, mice were given dietary
concentrations of 0, 150, 450, or 1350 ppm. A treatment-related
decrease in male body-weight gain was seen. There was a higher
incidence of duodenal masses and thickening of the stomach wall in
females and reduced liver weight in males at 1350 ppm. Microscopic
changes at 1350 ppm and to a minor degree at 450 ppm included benign
papillomas in the keratinized region of the stomach, benign adenomas
in the duodenal mucosa, villous hyperplasia in the duodenal and
jejunal mucosa, and hyperplasia of the lamina propria of the duodenum.
Administration of folpet in the diet at a concentration of 1350 ppm
induced tumours in the upper parts of the gastrointestinal tract
(non-glandular stomach and duodenum) of mice of each sex. The NOAEL
was 150 ppm, equal to 16 mg/kg bw per day.
Folpet has been adequately tested for genotoxicity in a range of
assays, which demonstrate that it is mutagenic and clastogenic in
vitro but not in vivo. The in-vitro responses were reduced or
abolished by the presence of liver homogenates, serum, glutathione, or
cysteine whenever these experimental modifications were investigated.
The Meeting concluded that folpet does not present a significant
genotoxic risk, owing to the presence of an efficient detoxification
mechanism in vivo.
Oral administration of folpet causes time-dependent changes in
the glutathione content and glutathione S-transferase activities of
different regions of the gastrointestinal tract of mice and rats, with
consequent effects on the detoxification of folpet. As early as three
to four weeks after initiation of treatment with a tumour-inducing
dose of folpet, both protein and non-protein thiol concentrations were
increased throughout the duodenum of mice. Reaction with protein thiol
is important for toxicity, while reaction with glutathione is
important for detoxification. At the same time, some hyperplasia and
hypertrophy were observed and the concentration of cyclin-dependent
kinase was increased, but only in the proximal half of the duodenum, a
result supported by assaying for proliferating cell nuclear antigen.
These data indicate that sustained proliferative stimulation of the
proximal duodenum is a consequence of oral administration of folpet.
The Meeting concluded that this finding represents an important
element in the process by which folpet, which is not genotoxic
in vivo, induces tumours in the mouse gastrointestinal tract.
A maximization test indicated that folpet is a sensitizer and
irritant in guinea-pig skin.
An ADI of 0-0.1 mg/kg bw was allocated on the basis of the NOAEL
of 10 mg/kg bw per day in the two-year study of toxicity and
carcinogenicity in rats, the one-year study of toxicity in dogs, and
studies of reproductive toxicity in rats and rabbits, and a safety
factor of 100.
Toxicological evaluation
Levels that cause no toxic effect
Mouse: 150 ppm, equal to 16 mg/kg bw per day (104-week study of
toxicity and carcinogenicity)
Rat: 190 ppm, equivalent to 10 mg/kg bw per day (104-week study
of toxicity and carcinogenicity)
800 ppm, equivalent to 40 mg/kg bw per day (two-generation
study of reproductive toxicity)
10 mg/kg bw per day (maternal toxicity in study of
developmental toxicity)
Rabbit: 10 mg/kg bw per day (maternal and fetotoxicity in study of
developmental toxicity)
Dog: 10 mg/kg bw per day (one-year study of toxicity)
Estimate of acceptable daily intake for humans
0-0.1 mg/kg bw
Studies that would provide information useful for continued
evaluation of the compound
Further observations in humans
Toxicological criteria for setting guidance values for dietary and non-dietary exposure to folpet
Exposure Relevant route, study type, species Results, Remarks
Short-term (1-7 days) Skin, sensitization, guinea-pig Sensitizer and irritant in maximization test
Oral, toxicity, rat LD50 > 5000 mg/kg bw
Inhalation, 4 h lethality, rat LC50 = 0.4 mg/litre
Dermal, toxicity, rabbit LD50 > 23 000 mg/kg bw
Medium-term (1-26 weeks) Repeated oral, 13-week toxicity, rat NOAEL = 300 mg/kg bw per day based on reduced
body weight, irritation of proximal gastrointestinal
tract, and other effects
Repeated oral, reproductive toxicity, NOAEL = 40 mg/kg bw per day based on reduced
rat body weight
Oral, developmental toxicity, rabbit NOAEL = 10 mg/kg bw per day based on reduced
body weight and food consumption; no fetotoxicity
or teratogenic effect
Long-term (> one year) Repeated oral one year, toxicity, dog NOAEL = 10 mg/kg bw per day based on reduced
body weight and food consumption and serum
biochemical changes
References
Blagden, S.M. (1991) Folpet technical: Acute inhalation toxicity
study, four-hour exposure (nose only) in the rat. Unpublished and
unnumbered report (Project No. 306/51) from Safepharm
Laboratories Ltd, Derby, United Kingdom. Submitted to WHO by
Makhteshim Chemical Works Ltd, Beer-Sheva, Israel.
Dreher, D.M. (1990) Folpet technical: Magnusson & Kligman maximization
study in the guinea pig. Unpublished and unnumbered report
(Project No. 8/81) from Safepharm Laboratories Ltd, Derby, United
Kingdom. Submitted to WHO by Makhteshim Chemical Works Ltd,
Beer-Sheva, Israel.
East, P.W. (1994) Folpet: Oncogenicity study by dietary administration
to CD-1 mice for 104 weeks. Unpublished report No. 94/0403,
Pharmaco LSR No. MAK/117, from Pharmaco LSR Ltd, Eye, Suffolk,
United Kingdom. Submitted to WHO by Makhteshim Chemical Works
Ltd, Beer-Sheva, Israel.
FAO/WHO (1970) 1969 Evaluations of some pesticide residues in food.
The monographs. FAO/PL: 1969/M/17/1, WHO/Food Add./70.38, Rome,
FAO.
FAO/WHO (1974) 1973 Evaluations of some pesticide residues in food.
WHO Pesticide Residue Series No. 3, Geneva, WHO.
FAO/WHO (1985) FAO Plant Production and Protection Paper 67.
Evaluations, 1984. Rome, FAO. FAO/WHO (1987) Pesticide Residues
in Food -- 1986. Evaluations, Part II -- Toxicology. FAO
Production and Protection Paper 78/2. Rome, FAO.
FAO/WHO (1991) Pesticide Residues in Food -- 1990. Toxicology
Evaluations. WHO/PCS/91.47, Geneva, WHO.
Nyska, A., Waner, T., Paster, Z., Bracha, P., Gordon E.B. & Klein, B.
(1990) Induction of gastrointestinal tumors in mice fed the
fungicide folpet: Possible mechanisms. Jpn. J. Cancer Res., 81,
545-549.
Tennekes, H. (1995) The genetic toxicology of folpet. Position paper
commissioned by Makhteshim Chemical Works Ltd, Beer-Sheva,
Israel. Submitted to WHO by Makhteshim Chemical Works Ltd,
Beer-Sheva, Israel
Waterson, L.A. (1994a) Folpet, feasibility study by dietary
administration to male mice for 21 days. Report No. MBS
43/942221, from Huntingdon Research Centre Ltd, Huntingdon,
Cambs, United Kingdom. Submitted to WHO by Makhteshim Chemical
Works Ltd, Beer-Sheva, Israel.
Waterson, L.A. (1994b) Folpet, extended feasibility/preliminary study
by dietary administration to male mice for 28 days. Report number
MBS 44/942343, from Huntingdon Research Centre Ltd, Huntingdon,
Cambs, United Kingdom. Submitted to WHO by Makhteshim Chemical
Works Ltd, Beer-Sheva, Israel.
Waterson, L.A. (1995) Folpet, investigation of the effect on the
duodenum of male mice after dietary administration for 28 days
with recovery. Report No. MBS 45/9423003, from Huntingdon
Research Centre Ltd, Huntingdon, Cambs, United Kingdom Submitted
to WHO by Makhteshim Chemical Works Ltd, Beer-Sheva, Israel.
relationship in mice. By 2 h after treatment, the glutathione
depletion in rat and mouse duodenum was similar, and at 6 h the
glutathione levels were higher than those in controls. More
glutathione was re-bound in the duodenum and jejunum of mice than of
rats, indicating that mice have a stronger requirement for and greater
utilization of glutathione, this perhaps being the major route of
metabolism in this species (FAO/WHO, 1990).
Mice (72) and rats (24) were fed dietary concentrations of 0, 50,
or 5000 ppm folpet for 21 days. The mean concentration of glutathione
in rat liver at both doses was similar to that in controls, whereas a
small decline was observed in mice at 5000 ppm. In both species, the
glutathione concentrations were significantly increased after 50 ppm
and 5000 ppm in both duodenum and jejunum. A similar effect in the
ileum was more pronounced in mice. These results suggest an initial
depletion of glutathione followed by increasing de novo synthesis of
glutathione, resulting in a 'rebound' elevation in tissue glutathione
concentrations in the gastrointestinal tract. The tissue weights of
the stomach, duodenum, and jejunum were increased in both species. The
mean cytosolic protein concentrations (total milligrams per tissue) in
duodenum and jejunum were also increased in both species, the greater
increase being found in rats. Total cytosolic protein in mouse liver
declined to 86% of control values. Glutathione- S-transferase
levels (with 1-chloro-2,4-dinitrobenzene as substrate) increased
significantly in the duodenum, jejunum, and ileum of both species,
in the liver of rats, and in the stomach of mice after treatment
with 5000 ppm. This led to a greater capacity to enzymatically
conjugate thiophosgene with glutathione. A marked reduction in the
concentrations of lipid peroxides (malonaldehyde was used as an
indicator of the overall lipid peroxidation state of the mucosal
cells) was noted in the duodenum of both species receiving 5000 ppm
folpet in the diet, but the reduction was statistically significant
only in the stomachs of mice. No alteration was found in the
intracellular level of conjugated dienes in either species in
comparison with controls. The non-selenium-dependent glutathione
peroxidase activity (i.e. that due to the activity of glutathione-
S-transferase) was increased in the duodenum, jejunum, and ileum in
both species receiving 5000 ppm folpet in the diet and in the stomachs
of rats at either dose (FAO/WHO, 1990).
In 240 mice and 48 rats treated as in the previous study, the
mean microsomal protein (total milligrams per tissue) was
significantly increased in rat duodenum, jejunum, and ileum. Although
increases were also observed in these tissues in mice, they were not
statistically significant. Total hepatic microsomal protein declined
significantly in rats but remained unchanged in mice. Cytochrome P450
was reduced in the livers of both species, but the reduction was
statistically significant only in mice receiving 5000 ppm folpet. Both
aniline hydroxylase and 7-ethyloxycoumarin O-deethylase activities
were reduced in hepatic microsomes in both species at both doses, the
reduction in aniline hydroxylase being statistically significant at
5000 ppm (FAO/WHO, 1990).
In an experiment with 90 mice and 54 rats treated as in the
previous study, a statistically significant decrease in pH was
observed in the duodenum and jejunum of mice fed 5000 ppm folpet.
Incorporation of 3H-thymidine into the mucosal DNA was reduced in
most tissues of both species. In neither species was their evidence of
a dose-related increase in DNA synthesis (FAO/WHO, 1990).
Male Sprague-Dawley rats (200-250 g) were treated with folpet
suspended in 0.5 ml corn oil by intraperitoneal injection at
doses up to 100 mg/kg bw or orally at doses up to 1000 mg/kg bw.
Intraperitoneal injection of 50 mg/kg bw significantly decreased the
activities of benzphetamine N-demethylase and cytochrome P450, while
that of serum aspartate transaminase was significantly increased. Oral
doses up to 10 times the intraperitoneal dose did not have similar
effects (FAO/WHO, 1990).
In a preliminary study, groups of 313 male Crl:CD-1 (ICR) BR
mice, about 48 days old, were fed 0 or 5000 ppm folpet for 21 days.
Clinical signs and food consumption were monitored weekly. At the
end of treatment, the animals were sacrificed and used for either
histopathological investigations or biochemical measurement of cyclin-
dependent kinases (CKD) and proliferating cell nuclear antigen (PCNA).
Treatment-related findings included glandular hyperplasia of the
crypts, hypertrophy of the villous epithelium of the duodenum,
hypertrophy of the villous epithelium of the jejunum, and a twofold
increase in CKD and PCNA levels in 9000 × g (S9) fractions from the
duodenum. It was concluded that the upper gastrointestinal tract
(especially the duodenum) could be used to assess the effect of
folpet. Sampling of the first 3 cm of duodenum for histopathological
examination and biochemical analysis of sonicated fractions was
recommended (Waterson, 1994a).
Subsequently, the mechanism of the effect of folpet on the
duodenum was studied in groups of 51 Crl:CD-1 (ICR) BR mice fed 0 or
5000 ppm folpet, equal to 692 mg/kg bw per day, or 11 or 111 ppm
perchlormethyl mercaptan, equal to 2 or 16 mg/kg bw per day, for 28
days. The latter compound was included because 5000 ppm technical-
grade folpet contains about 11 ppm. Clinical signs and food
consumption were monitored daily, and body weight was determined
weekly. At the end of treatment, the animals were sacrificed and the
duodenum used for either histopathological investigations or
measurement of CDK and PCNA in S9 fractions. There was no treatment-
related mortality or change in clinical signs. In week 1, the body-
weight gain and the food intake of the animals receiving folpet or
111 ppm perchlormethyl captan was lower than that of controls.
Haematoxylin staining revealed glandular hyperplasia of the crypts in
the first 2.5 cm of the duodenum of most animals receiving folpet and
hypertrophy of the villous epithelium in the entire duodenum of all
animals given folpet. The crypts in the entire duodenum of all animals
in all groups stained for PCNA; more animals receiving folpet showing
a greater degree of staining. An approximately twofold increase over
that in controls was seen in CDK concentrations in fractions from the
entire length of the duodenum of animals receiving folpet; there was
no significant difference from controls in CDK concentrations in mice
fed 11 or 111 ppm perchlormethyl mercaptan. The PCNA concentrations
were significantly higher than control values in S9 fractions from the
entire length of the duodenum in the groups receiving folpet or
11 ppm perchlormethyl mercaptan. It was concluded that folpet acts
significantly differently from perchlormethyl mercaptan in the
duodenum, when CDK is used as the index, and these findings were
supported by the findings of both the biochemical PCNA assay and
histopathological immunochemistry. Since both CDK and PCNA are
increased in proliferating cells, the findings of the two studies were
considered to demonstrate proliferative stimulation as early as three
to four weeks after initiation of treatment with a tumour-inducing
dose of folpet (Waterson, 1994b).
The mechanism of the effect of folpet on the duodenum was further
studied in five groups of 132 male Crl:CD-1 (ICR) BR mice, about 48
days old, which were fed 0 or 5000 ppm folpet (purity, 99.4%), equal
to 717 mg/kg bw; 5000 ppm folpet, equal to 679 mg/kg bw, plus 11 ppm
perchlormethyl mercaptan (purity, 95%), equal to 1.5 mg/kg bw;
11 ppm perchlormethyl mercaptan alone, equal to 1.6 mg/kg bw; or
0.4% hydrogen peroxide, equal to 527 mg/kg bw (positive control,
administered in the drinking-water) for 28 days followed by a 28-day
recovery period for selected mice. An acclimatization period of 11
days was allowed before commencement of treatment, at which time the
mice were nine weeks old and weighed 26-45 g. The mice were housed
three to a cage. The identity of the test compound was confirmed by
chemical analysis, and the accuracy of the preparations and the
homogeneity and stability of the dietary formulations were found to be
satisfactory. Clinical signs and food consumption were monitored
daily. At the end of treatment and recovery, specified animals were
sacrificed and allocated for either pathological investigation of the
duodenum or biochemical measurement of total protein, non-protein
thiol, CDK, and PCNA in duodenal fractions by means of commercially
available kits.
Animals receiving hydrogen peroxide had a statistically
significant reduction in body-weight gain and a lowered efficiency of
food use in comparison with controls. At termination, a marginal
increase in glandular hyperplasia of the crypts and hypertrophy of the
villous epithelium in the duodenum were seen in mice treated with
folpet, folpet plus perchlormethyl mercaptan, or hydrogen peroxide in
comparison with controls. No such changes were seen after the recovery
period.
Treatment of mice with folpet (with or without perchlormethyl
mercaptan) resulted in increased protein (per milligram of tissue) in
total duodenal mucosal epithelium. The effect of folpet was greater in
the first 2.5 cm of the duodenum than in the next 3.5 cm and was
completely reversed after 28 days of withdrawal of animals from the
test diet. Treatment with perchlormethyl mercaptan alone or with
hydrogen peroxide had no such effect. The non-protein thiol
concentration was also increased after administration of folpet (with
or without perchlormethyl mercaptan) and with hydrogen peroxide.
Again, the effects were greatest in the first 2.5 cm of the duodenum
and were fully reversible after the recovery period. Treatment with
perchlormethyl mercaptan alone had no such effect. Folpet (with or
without perchlormethyl mercaptan) and hydrogen peroxide, but not
perchlormethyl mercaptan alone, increased the concentrations of CDK in
the first 2.5 cm of the duodenum; after the recovery period, no
significant difference in CDK concentrations was detected between
treated and untreated animals. The PCNA concentrations in duodenal S9
fractions were close to the limit of detection of the assay system,
but a significant increase in PCNA concentrations was detected after
treatment with folpet (with or without perchlormethyl mercaptan) in
the first 2.5 cm, confirming the CDK response. In contrast, the PCNA
concentrations also indicated cell proliferation in the next 3.5 cm of
the duodenum, although the effect was not seen after the recovery
period. Treatment with perchlormethyl mercaptan or with hydrogen
peroxide had no clear effect on PCNA concentrations in the duodenum.
In none of the investigations was there evidence that perchlormethyl
mercaptan and folpet have interactive effects.
Thus, administration of folpet for 28 days (with or without
perchlormethyl mercaptan) increased protein and non-protein thiol
concentrations in both the first 2.5 cm and the next 3.5 cm of the
duodenum, while CDK concentrations were increased only in the first
2.5 cm. The latter finding was supported by the results of the PCNA
assay but not by histopathological immunocytochemistry. The results
also showed that the biochemical effects of folpet on the mouse
duodenum are reversible after a 28-day recovery period (Waterson,
1995).
2. Toxicological studies
(a) Acute toxicity
The LD50 in adult male and female rats and in female wealings
treated orally with folpet was > 5000 mg/kg bw (FAO/WHO, 1990),
and the LD50 in rabbits treated percutaneously with folpet was
> 23 000 mg/kg bw (FAO/WHO, 1969).
Exposure of rats to folpet (purity unspecified) by inhalation for
4 h showed an LC50 of 0.39 mg/litre for males and 0.43 mg/litre for
females (Blagden, 1991).
(b) Short-term toxicity
Rats
Groups of 10 rats of each sex were fed dietary levels of 0, 0.1,
0.32, or 1% folpet for 12 weeks. Growth was normal, except in male
rats fed the 1% level which showed a significant decrease There were
no gross abnormalities, and histopathological examination of the
liver, kidneys, adrenals, intestines, lungs, and gonads of two males
and two females from each group revealed no abnormalities (FAO/WHO,
1969).
Groups of 20 Fischer 344 rats of each sex were fed dietary
concentrations of 0, 0.2, 0.4 or 0.8% folpet (purity, 89%) for 13
weeks. During treatment, the body weights and food consumption of
males at the middle and high doses and of females at the high dose
were significantly reduced. After 10 weeks, there were no significant
differences between treated and control groups, but there were dose-
related decreases in the activities of alkaline phosphatase and
alanine aminotransferase in all treated groups, of aspartate
aminotransferase in all treated males and in females it the high dose,
and of lactate dehydrogenase in all treated males. Blood urea and
chloride levels were increased, but total serum proteins were reduced
in males at the middle and high doses. Blood urea was reduced in
treated females, total protein was reduced in the high-dose groups,
and albumin was reduced in the mid- and high-dose groups. Treatment-
related irritation of the proximal gastrointestinal tract was seen at
necropsy, as was hyperkeratosis of the non-glandular gastric mucosa.
Slight acanthosis of the stomach occurred in one female rat in each
group. The kidneys of males at the middle and high doses showed slight
but dose-related increases in the number of foci of atrophic
basophilic renal tubules, which were considered to be unrelated to
treatment (FAO/WHO, 1986).
Groups of 20 Sprague-Dawley rats of each sex received folpet
(purity unspecified) at dietary levels of 0, 0.03, 0.1, 0.3, or 1.0%
for 13 weeks. Dietary analyses showed that the achieved doses were
85-106% of the nominal values. At the end of treatment, half of the
rats in each group were sacrificed; the remainder were given the basal
diet for a further two weeks and were then sacrificed. No clinical
signs or mortality occurred during the study period. The growth of
rats at the high dose was significantly reduced during treatment, and
this retardation was not made up during the recovery period. Food
consumption, haematological parameters, serum hepatic enzyme levels,
and renal function were unaffected by treatment. At necropsy, animals
at the high dose had reduced mean body weights; relative brain weight
was also reduced, but kidney weight was increased. There were no
significant differences in organ weights of animals sacrificed two
weeks after treatment. No treatment-related gross pathological changes
were observed, but histopathological examination of animals sacrificed
immediately after treatment showed acanthosis, hyperkeratosis,
submucosal oedema, and pleocellular inflammatory infiltration, with
occasional focal gastric erosion and ulceration in the non-glandular
stomach of rats at the high dose. No such lesions were seen after the
recovery period. The NOAEL was 300 mg/kg bw per day, based on reduced
body weight and other effects at the high dose (FAO/WHO, 1986).
Dogs
In a pilot study, four beagle dogs of each sex were given folpet
(purity, 89.8-91.1%) at 0, 790, 1800 or 4000 mg/kg bw per day by
capsule for 13 weeks. Food intake was generally lower in treated dogs
than in controls. Body weight gain was reduced in all treated groups,
significantly so in animals at the middle and high doses. All treated
dogs showed vomiting and diarrhoea, the symptoms being especially
marked in those at the middle and high doses. Treatment-related
changes included poor condition, abdominal distension, excessive
salivation, and a progressive decrease in testicular size,
particularly in animals at the middle and high doses. All males and
one female at the high dose died or were killed in extremis.
Neurological examination of these dogs and of those males which
survived 12 weeks of treatment and ophthalmoscopic examination of
survivors showed no remarkable changes. Most dogs killed in extremis
had leukocytosis, and some had decreased serum phosphate levels. One
dog had normochromic normocytic anaemia. Surviving dogs had decreased
serum calcium and total plasma protein concentrations, but increased
serum chloride levels. At necropsy, most treated dogs had decreased
brain, liver, kidney, spleen, and testicular weights. Pathological
examination showed atrophy, depletion, and fibrosis of the lymphatic
and haematopoietic systems, gonadal degeneration with prostatic
atrophy and fibrosis, thyroid degeneration, and muscular dystrophy
(FAO/WHO, 1986).
Groups of six beagle dogs of each sex were given folpet (purity,
89.5%) in gelatine capsules at 0, 10, 60, or 140 mg/kg bw per day. The
latter dose was reduced to 120 mg/kg bw per day after 50 days because
of low food consumption and reduced body-weight gain. All animals were
sacrificed and necropsied after one year. All males at the high dose
and three at the middle dose initially lost weight, and the mean body
weights of animals at the middle and high doses showed a dose-related
reduction (which was not significant) throughout the rest of the
study. Dose-related decreases in food consumption were seen in animals
at the middle and high doses, in males during the first three months,
and in females during the first month. Although the food consumption
of males was subsequently comparable to that of controls, that
of females tended to be reduced in a non-dose-related manner.
Ophthalmoscopy at six and 12 months revealed no effect. There was a
tendency for reduced leukocyte counts in males at one, two, three, and
six months, but not at nine or 12 months; however, the counts were not
significantly different from control values. A significant decrease in
mean serum cholesterol and in total protein, albumin, and globulin
levels was seen in males at the middle and high doses; females at the
high dose had significantly reduced mean serum protein, albumin,
and cholesterol levels. Urinalysis, necropsy, and subsequent
histopathology showed no effect of treatment. The NOAEL was 10 mg/kg
bw per day, on the basis of decreased body weight and food consumption
and serum biochemical changes (FAO/WHO, 1986).
Groups of five beagle dogs of each sex received technical-grade
folpet in capsules at 0, 325, 650, or 1300 mg/kg bw per day for 52
weeks. None died. Animals of each sex at the high and intermediate
doses had decreased body weight during treatment; a concomitant
decrease in food intake was found in animals of each sex at the high
dose. Clinical signs seen in all treated groups included vomiting,
diarrhoea, and salivation; the latter was seen only during the first
eight weeks in animals at the low dose but was more pronounced at the
high and intermediate doses. Dogs at these doses were in poorer
condition than controls. Haematological parameters were affected in
all treated females during the first third of the study and included
decreased packed cell volume and haemoglobin and erythrocyte counts.
Clinical chemical changes (e.g. reduced total protein, cholesterol,
glucose, and urea) observed during treatment were related to the poor
physical condition of the dogs. Chloride levels were increased in
males, mainly those at the high dose, which also had decreased calcium
levels; the latter effect was also observed in females at the high and
intermediate doses up to week 25 of treatment. A reduced urine volume
was noted in females at the high dose after 13 weeks of treatment, and
both males and females had acid urine; this effect persisted in males
at the high and intermediate doses throughout the study. Tubular
testicular degeneration associated with an absence of spermatozoa in
the epididymides was found in two male dogs at the high dose; one also
had moderate prostatic gland atrophy. The absolute testicular weights
of males at the high dose were decreased. Changes in relative organs
weights were recorded in the adrenals (all males and females at the
intermediate dose), brain and kidney (males at the high dose and
females at the intermediate dose), liver, and thyroid (males at the
intermediate dose and females at the high dose). The NOAEL was
325 mg/kg bw per day (FAO/WHO, 1990).
(c) Long-term toxicity and carcinogenicity
Mice
Goups of 80 CD-1 (ICR derived) mice of each sex were fed dietary
concentrations of 1000, 5000, or 12 000 ppm folpet (purity, 93%) for
112-113 weeks. A group of 104 mice of each sex served as controls.
Survival was not affected, but body-weight gain was reduced in animals
at 5000 and 12 000 ppm. Although sporadic changes in food consumption
were noted, no dose-related effects were apparent. Haematological
parameters were normal at one year, but at the end of treatment,
possible macrocytic anaemia was seen in animals of each sex at
12 000 ppm. The only changes seen at gross pathological examination
were duodenal lesions and related gastrointestinal abnormalities. A
dose-related increase in the incidence of duodenal adenomas and
adenocarcinomas was observed in mice at 5000 and 12 000 ppm, with
incidences in males and females of 0 and 1% in controls, 2 and 3% at
1000 ppm, 10 and 12% at 5000 ppm, and 52 and 54% at 12 000 ppm,
respectively. Males at the high dose also had an increased incidence
of jejunal adenocarcinomas. There was no NOAEL (FAO/WHO, 1984).
Four groups of 52 B6C3F1 mice of each sex were given dietary
concentrations of folpet (purity, 89.0%) at 0, 0.1, 0.5, or 1.0% for
21 weeks and then 0, 0.1, 0.35, or 0.7% for 83 weeks. Dietary analyses
at weeks 0, 1, 13, and 26 showed the levels to be 88-91% of the
nominal value. Food consumption was depressed during the first few
weeks of treatment in mice at the middle and high doses, and body-
weight gain was reduced in these animals throughout the study.
Clinical signs, observed principally in animals at the high dose,
comprised erythema, dry flaking skin, reddish fur discolouration, and
weeping skin, particularly during the first 21 weeks. Leukocyte counts
in survivors at 52, 78, and 104 weeks were not affected by treatment.
The longevity of animals at the two highest doses was reduced. The
increased relative weights of the brain, heart, lungs, liver, kidneys,
and testes reflected the reduced body weight of treated animals.
Macroscopically, a dose-related increase in non-glandular gastric
mucosal ulceration and thickening of the gastric and duodenal walls
were observed. The jejunal wall was thickened in females at the middle
dose and in all mice at the high dose. Dose-related distension of the
duodenal lumen was also seen. After week 79, there was a dose-related
increase in the incidence of nodules or masses on the luminal surface
of the stomach and duodenum and on the duodenal serosa. Animals at the
middle and high doses had dose-related epidermal hyperkeratosis and
acanthosis and oesophageal hyperkeratosis. Increased areas of marked
acanthosis and hyperkeratosis of non-glandular gastric mucosa were
seen in animals at the middle dose and males at the high dose.
Microscopic gastric ulceration occurred, with no apparent
relation to dose. The dose-related appearance of areas of atypical
duodenal glandular hyperplasia and mucosal gland proliferation were
seen in all treated animals, but especially in males. This atypical
hyperplasia was often associated with duodenal adenomas and
adenocarcinomas. Atypical glandular proliferation was seen only
occasionally in the jejunum of treated females. Gastric papillomas and
squamous-cell carcinomas, which may have been secondary to mechanical
obstruction of the duodenal lumen, were found in all treated males but
not in control males. A dose-related increase in the incidence of
gastric papillomas was also found in all treated females, but
similar lesions occurred in 2/51 controls. Duodenal adenomas and
adenocarcinomas were found in all treated animals of each sex, with
a significant dose-response relationship. A single jejunal
adenocarcinoma was seen in mice at the high dose. Primary or
metastatic tumours of uncertain etiology were found in all treated
males, but the incidences of bronchioalveolar adenomas and malignant
lymphomas were lower in these animals, suggesting high incidences in
control males. There was no NOAEL (FAO/WHO, 1986).
Four groups of 52 six-week old B6C3F1 mice of each sex were given
dietary concentrations of 0, 1000, 5000, or 10 000 ppm folpet (purity,
89%) for 104 weeks. The formulated diets were analysed after one and
two weeks and were found to be stable (no data). During the first few
weeks of the study, marked irritation of the integumentary system was
observed. Typical lesions, usually on the neck, included flaking,
weeping, and eroded skin, erythema, and red discolouration of the fur.
Early deaths occurred in all treated males and in females at the
middle and high doses and were attributed to the presence of fatal
tumours. Dose-related macroscopic lesions were found in the stomach
and duodenum. The non-glandular gastric mucosa of all treated animals
showed an increased incidence of nodules, masses, wall thickenings,
and ulceration, and an increased incidence of partial luminal duodenal
obstruction was seen 1-5 cm distal to the pylorus in the form of wall
thickenings and mucosal nodules. An increased incidence of these
lesions was noted in animals that died after 79 weeks of treatment.
Microscopic examination of the skin revealed hyperkeratosis, and a
dose-related in, crease in the incidence of oesophageal and non-
glandular gastric mucosal hyperkeratosis was seen in all treated
groups. Ulceration of the non-glandular stomach was observed in
animals at the low and intermediate doses. Atypical hyperplasia of the
duodenal mucosa was seen in all treated mice. Duodenal adenomas and
carcinomas were seen in all treated groups, and the incidence was
dose-related. Gastric squamous-cell papillomas and carcinomas were
seen more frequently in animals at 5000 and 10 000 ppm. There appeared
to be a correlation between the presence of macroscopically visible
gastric neoplasia and duodenal obstruction: the four males with
gastric carcinoma had partial duodenal nodular obstruction, and five
of seven females at the high dose with an increased incidence of
gastric papillomas had partially obstructed duodenal lumens. No other
toxic or carcinogenic effect was seen in any organ.
The authors suggested that the partial duodenal obstruction
disturbed the natural flow of the gastrointestinal contents and may
have exacerbated the mucosal changes in the stomach. The resulting
stagnation may have raised the effective concentration and residence
time of folpet, and the elevated concentration of this irritant, with
the possible presence of bile acids and pancreatic enzymes, may have
overwhelmed the defence mechanism of the mucosal cells, resulting
in the development of gastric neoplasms. In conclusion, dietary
administration of folpet to B6C3F1 mice induced a dose-related
appearance of atypical duodenal hyperplasia, adenomas, and
adenocarcinomas, which were suggested to have appeared subsequent
to, and as an indirect result of, the partial luminal duodenal
obstruction. There was no NOAEL (Nyska et al., 1990).
Groups of 52 male and 52 female CD-1 mice were fed diets
containing folpet (purity, 92.4%) at concentrations of 0, 150, 450, or
1350 ppm, equal to 0, 16, 47, and 151 mg/kg bw per day for 98 weeks
for males and 0, 16, 51, and 154 mg/kg bw per day for 104 weeks for
females. A control group consisting of 100 male and 100 female mice
received a normal diet. The mice were assigned to treatment groups on
the basis of computer-generated random numbers and were acclimatized
for 14 days before the beginning of treatment. The mice were 3542 days
of age and had mean body weights of 29.7, 29.6, 29.5, and 29.6 g
(males) and 23.4, 23.7, 23.0, and 23.7 g (females) at 0, 150, 450, and
1350 ppm, respectively, at the time of the first dose. The 150-ppm
preparation was found to contain 89-91% of the nominal concentration
and was stable after storage at -20°C for seven days and then at 20°C
for four days; samples taken from six places in the mix showed
satisfactory homogeneity: 89-95% of the nominal value with 150 ppm and
78-88% with 1350 ppm. Verification of the concentrations resulted in
analytical values of 89-96% of the nominal concentrations. Animals
were inspected daily for evidence of reactions to treatment. Body
weight and food and water consumption were determined weekly. All 187
animals found dead or killed during the treatment period were
subjected to gross pathological and histopathological examination.
The overall mortality rate was unaffected by treatment, and no
inter-group differences in mortality were seen by either pairwise or
trend analyses; therefore, none of the deaths was considered to be
related to treatment with folpet. The incidence of skin encrustations,
particularly on the dorsal surface, was generally higher in males
receiving a dietary concentration of 1350 ppm. This finding was
considered to be associated with a greater incidence of fighting among
these animals during the first 70 weeks of treatment. In the later
part of the treatment period, the incidence of skin encrustations
in males was similar to that of controls. The incidence, group
distribution, location, and mean onset time of palpable swellings were
not considered to be related to treatment. The body-weight gain of
males receiving 1350 ppm was slightly lower than that of controls for
about the first 70 weeks of treatment. Food consumption was unaffected
by treatment, but the efficiency of food use during the first 14 weeks
of treatment with 1350 ppm was slightly inferior to that of the
controls. The treatment-related effect on male body-weight gain and
the low food conversion efficiency seen at this dose during the first
14 weeks of treatment were considered to be nonspecific toxic effects.
The body-weight gain of females was unaffected by treatment.
Organ weight analysis revealed lower absolute and relative liver
weights in males that received 1350 ppm in comparison with controls.
High absolute and relative spleen weights in males at 150 ppm were due
to an unusually large spleen in one animal. Macroscopic examination of
animals killed after 104 weeks of treatment revealed a high incidence
of masses in the duodena of females at 1350 ppm. When all of the
animals were considered together, there was a higher incidence of
duodenal masses and thickening of the stomach wall in females at
1350 ppm than in controls. Microscopic examination revealed benign
papillomas in the keratinized region of the stomach in one male and
three females at 1350 ppm and in one female at 450 ppm. A benign
adenoma was found in the duodenal mucosa of one female at 1350 ppm.
Villous hyperplasia was seen in the duodenal mucosa of three females
at the highest dose and in one male at 450 ppm; a similar finding was
seen in the jejunum of one male at 450 ppm. Hyperplasia of the lamina
propria of the duodenum was seen in two males at 1350 ppm, and another
male at this dose had similar changes in the jejunum and ileum in
addition to villous fusion, mucosal dysplasia, and hyperplasia of
Paneth cells. The incidence of keratoachanthosis of the nonglandular
stomach was higher among females at 1350 ppm. than among controls.
Although one female at 450 ppm had a benign squamous-cell papilloma
and two males at this dose had gastrointestinal hyperplasia, these
findings were considered to have occurred by chance. There were no
other changes in the gastrointestinal tract, and there were no changes
in the liver that were considered to be related to treatment. As the
low liver weights of males at 1350 ppm were confined to one sex and
there was no histopathological correlate, the toxicological
significance of this finding is unclear. It was concluded that
administration of folpet in the diet at a concentration of 1350 ppm
produced tumours in the upper parts of the gastrointestinal tract of
CD-1 mice. The NOAEL was 16 mg/kg bw per day on the basis of duodenal
hyperplasia (East, 1994).
Rats
Groups of 60 Charles River Crl:CD(SD)BR rats of each sex were fed
dietary concentrations of 0, 200, 800, or 3200 ppm technical-grade
folpet (purity, 89.5%) for 104 weeks. An interim sacrifice of 10 rats
of each sex per dose was conducted at 52 weeks. Growth rates and
survival were unaffected by treatment, except for a slight tendency to
reduced body weights in females at the high dose during the first
year; food consumption was correspondingly reduced at this dose.
Conventional ophthalmoscopic, haematological, biochemical, and
urinary analyses indicated no effect of treatment. At necropsy, no
changes attributable to treatment were observed on organ weights.
Hyperkeratosis and/or acanthosis and some erosion and/or ulceration in
the non-glandular stomach were seen in animals at the high dose. These
lesions were occasionally accompanied by submucosal oedema and
submucosal inflammatory cellular infiltrates. The NOAEL was 800 ppm,
equivalent to 40 mg/kg bw per day (FAO/WHO, 1986).
Groups of 60 Fischer 344 rats were fed diets containing 0, 500,
1000, or 2000 ppm folpet (purity, 89%) for 104 weeks. The diets were
prepared weekly and analysed frequently for folpet content. Food
intake was reduced in all treated groups in comparison with controls,
but body weight was reduced only in treated males. There were no
clinical signs of toxicity. Necropsy indicated a tendency to an
increased incidence of gastric ulceration, which was significant
only in females. Treatment also induced an increased incidence
of ulceration in the forestomach in animals at the high dose.
Hyperkeratosis of the oesophagus was seen in rats at the high dose
and of the forestomach in animals at the middle and high doses. An
increased incidence of gastric ulceration was present in the
forestomach of animals of each sex at the high dose. The incidences of
C-cell adenoma, benign mammary fibroepithelioma and malignant lymphoma
showed positive significant trends with dose, but only that for the
latter neoplasms was statistically significant. All tumour incidences
were within the normal historical range for rats of this strain. The
NOAEL was 500 ppm, equivalent to 25 mg/kg bw per day (FAO/WHO, 1986).
Groups of 20 Fischer 344 rats of each sex were fed diets intended
to provide concentrations of 0, 250, 1500, or 5000 ppm folpet (purity,
91%) for 104 weeks. The actual dietary concentrations, calculated
on a weekly basis, were 0, 190, 1290, and 4530 ppm. Longevity was
unaffected. The mean body weight and food intake of animals at the
high dose were depressed, by up to 10% in males and 6% in females,
and water intake was depressed at the high dose, especially in
females. Serum alkaline phosphatase and alanine aminotransferase
activities were reduced in treated groups throughout the study, and
those of aspartate aminotransferase, creatinine phosphokinase, and
gamma-glutamyl transferase were decreased sporadically. A significant
reduction in blood cholesterol was seen in animals of each sex of the
high dose throughout treatment. The plasma protein level was reduced
in males at the high dose during the first year of treatment, and the
level of phosphate was elevated at most examinations. The urea
concentration was increased in females at the high dose at 18 months.
Most treated males excreted more concentrated urine in smaller volumes
at three and six months. Only non-neoplastic lesions were observed,
consisting of hyperkeratosis of the oesophageal and gastric squamous
epithelium. The NOAEL was 190 ppm, equivalent to 10 mg/kg bw per day
(FAO/WHO, 1990).
(d) Reproductive toxicity
Rats
Four groups of 25 CD rats of each sex were fed dietary
concentrations of 0, 250, 1500, or 5000 ppm folpet (purity, 91%) over
the course of two generations. Body weight gain and food consumption
were reduced in parental animals of the F0 and F1 generations at
the highest dose; a minor decrease in body weight was observed in F1
and F2 offspring. The principal histopathological effect was
hyperkeratosis of the non-glandular gastric mucosa in F0 and F1
animals at the intermediate and high doses, oesophageal hyperkeratosis
in F1 animals at the intermediate and high doses, and an increased
incidence of basophilic renal tubules in males of the F0 generation
at the high dose. Folpet was concluded not to be a specific
reproductive toxin in this test system (FAO/WHO, 1990).
In a two-generation study, groups of 30 Crl:COBS/CD(SD)/Charles
River rats of each sex were fed diets containing 0, 200, 800, or
3600 ppm folpet (purity, 89.5%) during growth, mating, gestation, and
lactation for two litters per generation. Initial mating was begun 62
days after the start of exposure. The test diets were fed to F0 males
until the end of mating to produce the F1b litters and to F0 females
until weaning of the F1b litters. Pups were sacrificed 21-23 days
after parturition, with the exception of those F1b pups selected to
produce the F2 generation. Mating of the F1b pups was begun after 12
weeks of exposure, and the above sequence was repeated. Gross necropsy
was performed on all parental rats and on the F1a, F1b, and F2b
litters. The diet was changed three times per week, and each batch was
analysed: the diets contained 79.9-101% of the nominal concentration.
The body weights of F0 and F1 males and F1 females at the high dose
and of their pups were depressed by treatment; the effect was most
marked in adult males, especially in the second generation. Food
consumption was correspondingly reduced. Treatment had no significant
effect on mating, fertility indices, pregnancy rates, litter sizes,
pup weights, growth, or litter survival rates. No treatment-related
effects were found at necropsy or on histopathological examination.
The NOAEL for maternal toxicity was 800 ppm, equivalent to 40 mg/kg bw
per day, based on the reduction in body weights; there was no evidence
of reproductive toxicity (FAO/WHO, 1986).
(e) Developmental toxicity
Rats
In a pilot study, groups of eight female mated CRL:COBS CD (SD)
BR rats were given 0, 20, 80, 320, or 640 mg folpet/kg bw by gavage on
days 6-19 of gestation. There were no deaths, but clinical signs
including rales, excess salivation, chromorhinorrhoea, gasping, soft
or liquid faeces, decreased motor activity, dyspnoea, and distended
gut were observed. Reduced maternal body weight was seen at
> 80 mg/kg bw, and food consumption was reduced at > 320 mg/kg
bw; the average fetal body weight was also reduced at the latter dose.
In the main study, groups of 25 rats were given folpet (purity, 89%)
at 0, 10, 60, or 360 mg/kg bw by gavage on days 6-19 of gestation.
Animals were killed on day 20. Clinical signs including excess
salivation, chromorhinorrhoea, decreased motor activity, soft or
liquid faeces, dyspnoea, and urine-stained fur were observed. Three
rats at the high dose died, two from intubation errors. No gross
lesions attributable to treatment were seen in surviving rats. Rats at
> 10 mg/kg bw gained less weight than controls during treatment.
Food consumption was reduced in animals at the high dose only. The
numbers of implantations, live and dead fetuses, fetal viability, and
resorptions, the average fetal body weight per litter, the fetal sex
ratio, and the number of corpora lutea were comparable in all groups.
Gross, visceral, and skeletal abnormalities were seen in all groups,
but there were no compound-related effects on ossification. The NOAEL
for maternal toxicity was 10 mg/kg bw per day (FAO/WHO, 1984).
Groups of six rats were treated daily on days 6-15 of gestation
with folpet (purity, 88.6%) at 10, 65, 420, or 2750 mg/kg bw by
gavage. Maternal toxicity, reduced body-weight gain, and reduced fetal
weight were seen only at 2750 mg/kg bw. Following this pilot study,
groups of 22 female Charles River CD rats received folpet (purity,
91.1%) in 0.5% acetic acid containing 0.5% carboxymethylcellulose at
0, 150, 550, or 2000 mg/kg bw on days 6-15 of gestation. The animals
were sacrificed on day 20. Clear signs of toxicity were observed in
animals at the high dose, including soft faeces (in 21/21 rats), fur
staining (in 4/21), and perianal staining (in 8/21); one animal died.
Food consumption was decreased during the first days of treatment with
the intermediate dose and was markedly decreased throughout treatment
with the high dose. Maternal body weight was also decreased at the
middle and high doses. Gravid uterine weights were depressed in dams
at the middle and high doses, but terminal maternal body weight
(without the gravid uterus) was significantly depressed only at the
high dose. Pre- and post-implantation losses were greater than in
controls in animals at the middle dose, and fetal weights were reduced
at the middle and high doses. Fetal crown-rump length was slightly
decreased after treatment with the middle and high doses. A single
fetus at the high dose (1/277) had multiple major malformations, and a
second pup had unilateral microphthalmia. The incidence of hepatic
discolouration was significant at the high dose. Skeletal anomalies
occurred in all treated groups, and the incidence of reduced
ossification of cranial and pubic bones, sternebrae, metacarpals, and
metatarsals was significant in animals at the middle and high doses.
There was also a dose-related reduction in ossification of the
interparietal bone in all treated groups, and the occurrence of
angulated ribs was dose-related. There was no NOAEL for teratogenicity
(FAO/WHO, 1986).
Hamsters
Groups of 2-13 pregnant hamsters were given a single dose of
400-1000 mg/kg bw folpet on day 7 or 8 of gestation or were treated
daily on days 6-10 with a total of 1000-2500 mg/kg bw. The animals
were killed and examined on day 15 of gestation. Maternal mortality
was increased and some abnormal fetuses were produced at the highest
doses, but the lower doses did not induce teratogenic effects
(FAO/WHO, 1973).
Rabbits
Groups of 20 artificially inseminated New Zealand white rabbits
were given folpet (purity, 89%) at 0, 10, 20, or 60 mg/kg bw by oral
intubation on days 6-28 of gestation and were killed on day 29. The
death of one doe at 60 mg/kg bw was considered to be related to
treatment. One doe at the low dose aborted on day 21 of gestation and
one at the high dose on day 22 of gestation; one doe in the control
group delivered a litter on day 28 and one at the high dose on day 29.
Significant inhibition of body-weight gain and food consumption was
seen in animals at the middle and high doses. The average numbers of
corpora lutea, implantations, resorptions, and fetuses per litter and
the sex ratio and numbers of dead and resorbed implantations per
litter were comparable in all groups. The mean body weights of all
fetuses were decreased at the middle and high doses. There was a
significant increase in the incidence of hydrocephaly, and four
fetuses in three litters of dams at the high dose also had skull,
gastric, and pulmonary abnormalities. The NOAEL for maternal and
fetoxicity was 10 mg/kg bw per day, and that tor teratogenicity was
29 mg/kg bw per day (FAO/WHO, 1984).
Groups of six mated HY/CR female New Zealand white rabbits were
given folpet (purity, 91.1%) at 0, 10, 60, or 150 mg/kg bw per day by
gavage on days 6-18 of gestation. Marked body-weight loss was seen at
the high dose. Although fetal size was unaffected by treatment, fetal
mortality was more marked at the high dose. Post-implantation losses
were increased in animals at the middle dose. Subsequently, groups of
14 mated HY/CR New Zealand white rabbits were given folpet (purity,
91.1%) at 0, 10, 40, or 160 mg/kg bw per day by gavage on days 7-19 of
gestation. Dams were sacrificed on day 29 of gestation. Body-weight
gain was decreased during the initial few days of treatment with the
middle dose and after initiation of treatment with the high dose;
there was a corresponding decrease in food consumption in animals at
the high dose. There were no deaths. Gravid uterine weight was
significantly reduced in dams at the middle and high doses. Fetal
death (post-implantation loss) occurred more frequently at the high
dose than in controls; the proportion of small fetuses was also
increased in this group and mean fetal weight was nonsignificantly
reduced. There was evidence of delayed skeletal maturation at the high
dose and, to a lesser degree, at the middle dose. The incidence of
bilateral lumbar ribs appeared to increase with dose in animals at the
middle and high doses. Ossification of caudal vertebrae, sternebrae,
and long-bone epiphyses was reduced at the high dose. Other, minor
skeletal malformations did not apper to be related to treatment. There
was no evidence of hydrocephalus in either treated or control rabbits.
The NOAEL for maternal toxicity, fetotoxicity, and teratogenicity was
10 mg/kg bw per day (FAO/WHO, 1986).
Groups of 20 artificially inserminated female Hazelton Dutchland
New Zealand white (D1A Hra: (NZW) specific pathogen-free) rabbits
were given folpet (purity, 89.5%) in Tween 80 (10.5% by weight) and
carboxymethylcellulose (0.7% by weight) at a volume of 5 ml/kg bw per
day by gavage, to give a dose of 60 mg/kg bw per day on days 7-9,
10-12, 13-15, or 16-18 of gestation. Analysis of the formulations
indicated a folpet content of 87.8-104% of the nominal concentration.
Dams were sacrificed on day 29 of gestation; those that aborted
or delivered, the single animal that died, and those terminally
sacrificed were subjected to necropsy and examination of the uterine
contents. Abortion by two rabbits that received folpet on days 7-9 and
10-12 of gestation may have been related to treatment; otherwise, no
clinical signs of toxicity were observed during the study, although
the incidence of soft or liquid faeces was increased in all treated
groups, usually after treatment. No gross lesions attributable
to treatment were seen at necropsy. Maternal body weight was
significantly reduced in all treated animals, although less so in
those treated on days 7-9 and 10-12 than in the others. Food
consumption was correspondingly reduced. Treatment had no apparent
effect on the rate of abortion or on fetal resorption. The average
litter sizes were unaffected, as were the average fetal weights, the
number of viable fetuses, and the sex ratio. A significantly increased
incidence of fetuses with an irregularly shaped fontanelle was
observed in the group treated on days 13-15; the control incidence was
4.5%, but this variation did not occur in groups treated on days 7-9
or 16-18. It was possibly related to treatment, but the significance
of the effect was not clear. There were no other significant
variations in fetal skull morphology, and the incidences of
hydrocephalus and of gastric or pulmonary anomalies were not increased
in any group. The NOAEL for teratogenicity was 60 mg/kg bw per day
(FAO/WHO, 1986).
Hens
The yolks or air sacs of 830 fresh, fertilized hens' eggs were
injected with folpet in dimethyl sulfoxide at 3-20 mg/kg egg weight.
After incubation, the incidence of malformations was 8.19%. The
metabolites of folpet, phthalimide and phthalic acid, were
administered under similar conditions, except that ethanol was used as
the solvent for phthalic acid. Gross abnormalities were seen in 3.93%
of 305 eggs injected with phthalimide and in 3.1% of 290 eggs treated
with phthalic acid. Controls -- 1500 eggs injected with dimethyl
sulfoxide and several thousand with ethanol -- had incidences of < 2%
gross abnormalities. Micromelia, amelia, and phocomelia accounted for
most of the deformities (FAO/WHO, 1969).
(f) Genotoxicity
Folpet has been adequately tested in a number of assays for
genotoxicity in vitro and in vivo. The positive controls gave the
expected positive responses. The results are summarized in Table 1.
(g) Special studies
Delayed cutaneous hypersensitivity
The skin sensitizing potential of folpet (purity and stability
not given) was evaluated in albino Dunkin-Hartley guinea-pigs, 8-11
weeks old, weighing 309-411 g, by the Magnusson-Kligman test. The
animals were obtained from David Hall Limited, Burton-on-Trent,
Staffordshire, United Kingdom, and were acclimatized for at least five
days before treatment. Twenty animals were allocated to the treatment
group (for induction and challenge with folpet) and 10 animals to the
control group (induction with vehicle and/or Freund's complete
adjuvant and challenge with folpet). The animals were induced by three
series of two 0.1-ml intradermal injections of a 1:1 mixture of
Freund's complete adjuvant and water, 0.1% folpet (w/v) in arachis
oil, and 0.1% folpet (w/v) in a 1:1 preparation of Freund's complete
adjuvant plus arachis oil; one week later, 0.2-0.3 ml of 50% folpet
(w/w) in arachis oil was applied topically under an occlusive bandage
for 48 h. Erythematous reactions were quantified after 1 and 24 h.
During challenge, 0.1-0.2 ml of 25% folpet (w/w) in arachis oil was
applied topically under an occlusive bandage for 24 h on the right
flank, and the vehicle alone was applied on the left flank.
Erythematous reactions were quantified after 24 and 48 h. Seven days
after the original challenge, all test and control animals were
rechallenged with 10% folpet (w/w) in arachis oil.
Moderate and diffuse redness was noted at 18 treatment sites 1 h
after removal of the patches. Scattered mild redness was noted at nine
treatment sites at 24 h. Skin reactions observed after the initial
topical challenge included moderate and diffuse redness (at three
sites at 24 h and one site at 48 h) and scattered mild redness (at 14
sites at 24 h and 13 sites at 48 h). The responses were considered to
be due to primary cutaneous irritation caused by folpet. The animals
were re-challenged with a lower concentration of folpet, as the
irritation might have precluded sensitization responses. Scattered
mild redness was elicited at six treatment sites at 24 h and at two
sites at 48 h, the reaction extending beyond the treatment sites in
some animals. Erythema could not be evaluated at some sites because of
other adverse reactions, including desquamation, oedema, scabs,
cracking of the epidermis, fur loss, fissuring, hyperkeratinization,
and loss of skin elasticity. Technical-grade folpet was thus a strong
sensitizer on guinea-pig skin (Dreher, 1990).
Table 1. Results of tests for the genotoxicity of folpet
End-point Test system Concentration Purity Results Reference
or dose (%)
In vitro
Reverse mutation E. coli PQ37 0.03, 0.10, 0.3, or 1.0 µg/ml 90 Positivea FAO/WHO (1986)
Reverse mutation E. coil PQ37 3.0, 10, or 30 µg/ml 90 Negativeb FAO/WHO (1986)
Reverse mutation S. typhimurium TA100, 0 or 50 µg/plate NR Positive only Tennekes (1995)
TA98, TA1535, TA1537, in TA100a
TA1538, E. coli WP2uvrA-
Reverse mutation S. typhimurium TA1535, 0 or 100 µg/plate NR Positivea Tennekes (1995)
TA1537, TA1538
Reverse mutation S. typhimurium TA100, 0, 25, or 45 µg/plate NR Positivea Tennekes (1995)
TA1535
Reverse mutation E. coli TKJ6321, NR Positivea Tennekes (1995)
TKJ5211 Weakly positiveb
Reverse mutation E. coli WP2hcr+ WP2hcr- 0 or 100 µg/plate NR Positivea Tennekes (1995)
Reverse mutation E. coli WP2hcr 0 or 45 µg/plate NR Positivea Tennekes (1995)
Chromosomal aberration Chinese hamster ovary NR NR Positivea Tennekes (1995)
cells Weakly positiveb
Chromosomal aberration Human lymphoid cell line NR NR Positivea Tennekes (1995)
Chromosomal aberration Human lymphocytes 0, 1, 2, or 3 µg/ml for 24 h 90.1 Negativec Tennekes (1995)
Gene mutation hprt locus Chinese hamster V79 0, 125, 0.25, 0.5, 1.0, or 2.0 90.1 Negativea FAO/WHO (1986)
lung fibroblasts µg/ml
Gene mutation hprt locus Chinese hamster V79 3.125, 6.25, 12.5, or 50 µg/ml 90.1 Negativeb FAO/WHO (1986)
lung fibroblasts
Gene mutation hprt locus Chinese hamster ovary NR NR Positivea Tennekes (1995)
cells
Gene mutation tk locus Mouse lymphoma NR Positivea Tennekes (1995)
L5178Y cells
Table 1. (con't)
End-point Test system Concentration Purity Results Reference
or dose (%)
In vivo
Somatic cell mutation T strain male mice Oral; 0, 100, 1500, or 5000 ppm 88.7 Negative FAO/WHO (1986)
C57Bl/6 female mice on days 8.5-12.5 of gestation
Micronucleus formation CD-1 mice 10, 50, or 250 mg/kg bw 91 Negative FAO/WHO (1986)
Micronucleus formation Groups of five male and Oral; 125, 625, or 1250 mg/kg 97 Negatived Ivett (1988)
five female Sprague-Dawley bwj; sacrifice at 24, 48, and
rats; bone-marrow cells 72h; vehicle, corn oil
Sex-linked recessive Drosophila melanogaster NR Positive Tennekes {1995)
lethal mutation
NR, not reported
a In the absence of metabolic activation
b With metabolic activation
c Authors concluded result was positive
3. Observations in humans
A retrospective study of mortality was conducted in a cohort of
134 workers occupationally exposed during the manufacture of captan
for up to nine months annually and to folpet for up to three months
annually. There was an apparent increase in the number of deaths from
all causes (18) in the cohort in comparison with the number expected
from US mortality rates (standardized mortality ratio, 164). The
excess mortality was due mainly to cardiovascular disease and
'external causes' unrelated to occupation. No statistically
significant increases were observed for any specific cause of death,
including neoplasia, but there were too few deaths to evaluate cause-
specific mortality. Lack of adequate industrial hygiene monitoring
precluded satisfactory estimation of previous exposure (FAO/WHO,
1986).
Comments
Excretion of orally administered folpet by mice and rats is about
45-55% in urine, 30-40% in expired air, and 11-17% in faeces; very
little biliary excretion occurs, particularly in mice.
Important degradation pathways of folpet in rodents result in the
formation of phthalimide and thiophosgene. The latter is detoxified,
at least in part, by three mechanisms: oxidation and/or hydrolysis to
carbon dioxide; reaction with the cysteine moiety of glutathione to
yield thiazolidine-2-thione-4-carboxylic acid; and reaction with
sulfite to produce dithiobis(methanesulfonic acid).
A study in which rats were exposed by acute inhalation indicated
an LC50 of 0.39 (male) to 0.43 (female) mg/litre. The WHO has
classified folpet as unlikely to present an acute hazard in normal
use.
A two-year study of carcinogenicity in mice with dietary
concentrations of 0, 1000, 5000, or 10 000 ppm showed a dose-related
increase in the incidence of atypical duodenal hyperplasia, adenomas,
and adenocarcinomas, leading to partial obstruction of the duodenal
lumen in animals of each sex. A no-effect level was not observed. In
another two-year study of carcinogenicity, mice were given dietary
concentrations of 0, 150, 450, or 1350 ppm. A treatment-related
decrease in male body-weight gain was seen. There was a higher
incidence of duodenal masses and thickening of the stomach wall in
females and reduced liver weight in males at 1350 ppm. Microscopic
changes at 1350 ppm and to a minor degree at 450 ppm included benign
papillomas in the keratinized region of the stomach, benign adenomas
in the duodenal mucosa, villous hyperplasia in the duodenal and
jejunal mucosa, and hyperplasia of the lamina propria of the duodenum.
Administration of folpet in the diet at a concentration of 1350 ppm
induced tumours in the upper parts of the gastrointestinal tract
(non-glandular stomach and duodenum) of mice of each sex. The NOAEL
was 150 ppm, equal to 16 mg/kg bw per day.
Folpet has been adequately tested for genotoxicity in a range of
assays, which demonstrate that it is mutagenic and clastogenic in
vitro but not in vivo. The in-vitro responses were reduced or
abolished by the presence of liver homogenates, serum, glutathione, or
cysteine whenever these experimental modifications were investigated.
The Meeting concluded that folpet does not present a significant
genotoxic risk, owing to the presence of an efficient detoxification
mechanism in vivo.
Oral administration of folpet causes time-dependent changes in
the glutathione content and glutathione S-transferase activities of
different regions of the gastrointestinal tract of mice and rats, with
consequent effects on the detoxification of folpet. As early as three
to four weeks after initiation of treatment with a tumour-inducing
dose of folpet, both protein and non-protein thiol concentrations were
increased throughout the duodenum of mice. Reaction with protein thiol
is important for toxicity, while reaction with glutathione is
important for detoxification. At the same time, some hyperplasia and
hypertrophy were observed and the concentration of cyclin-dependent
kinase was increased, but only in the proximal half of the duodenum, a
result supported by assaying for proliferating cell nuclear antigen.
These data indicate that sustained proliferative stimulation of the
proximal duodenum is a consequence of oral administration of folpet.
The Meeting concluded that this finding represents an important
element in the process by which folpet, which is not genotoxic
in vivo, induces tumours in the mouse gastrointestinal tract.
A maximization test indicated that folpet is a sensitizer and
irritant in guinea-pig skin.
An ADI of 0-0.1 mg/kg bw was allocated on the basis of the NOAEL
of 10 mg/kg bw per day in the two-year study of toxicity and
carcinogenicity in rats, the one-year study of toxicity in dogs, and
studies of reproductive toxicity in rats and rabbits, and a safety
factor of 100.
Toxicological evaluation
Levels that cause no toxic effect
Mouse: 150 ppm, equal to 16 mg/kg bw per day (104-week study of
toxicity and carcinogenicity)
Rat: 190 ppm, equivalent to 10 mg/kg bw per day (104-week study
of toxicity and carcinogenicity)
800 ppm, equivalent to 40 mg/kg bw per day (two-generation
study of reproductive toxicity)
10 mg/kg bw per day (maternal toxicity in study of
developmental toxicity)
Rabbit: 10 mg/kg bw per day (maternal and fetotoxicity in study of
developmental toxicity)
Dog: 10 mg/kg bw per day (one-year study of toxicity)
Estimate of acceptable daily intake for humans
0-0.1 mg/kg bw
Studies that would provide information useful for continued
evaluation of the compound
Further observations in humans
Toxicological criteria for setting guidance values for dietary and non-dietary exposure to folpet
Exposure Relevant route, study type, species Results, Remarks
Short-term (1-7 days) Skin, sensitization, guinea-pig Sensitizer and irritant in maximization test
Oral, toxicity, rat LD50 > 5000 mg/kg bw
Inhalation, 4 h lethality, rat LC50 = 0.4 mg/litre
Dermal, toxicity, rabbit LD50 > 23 000 mg/kg bw
Medium-term (1-26 weeks) Repeated oral, 13-week toxicity, rat NOAEL = 300 mg/kg bw per day based on reduced
body weight, irritation of proximal gastrointestinal
tract, and other effects
Repeated oral, reproductive toxicity, NOAEL = 40 mg/kg bw per day based on reduced
rat body weight
Oral, developmental toxicity, rabbit NOAEL = 10 mg/kg bw per day based on reduced
body weight and food consumption; no fetotoxicity
or teratogenic effect
Long-term (> one year) Repeated oral one year, toxicity, dog NOAEL = 10 mg/kg bw per day based on reduced
body weight and food consumption and serum
biochemical changes
References
Blagden, S.M. (1991) Folpet technical: Acute inhalation toxicity
study, four-hour exposure (nose only) in the rat. Unpublished and
unnumbered report (Project No. 306/51) from Safepharm
Laboratories Ltd, Derby, United Kingdom. Submitted to WHO by
Makhteshim Chemical Works Ltd, Beer-Sheva, Israel.
Dreher, D.M. (1990) Folpet technical: Magnusson & Kligman maximization
study in the guinea pig. Unpublished and unnumbered report
(Project No. 8/81) from Safepharm Laboratories Ltd, Derby, United
Kingdom. Submitted to WHO by Makhteshim Chemical Works Ltd,
Beer-Sheva, Israel.
East, P.W. (1994) Folpet: Oncogenicity study by dietary administration
to CD-1 mice for 104 weeks. Unpublished report No. 94/0403,
Pharmaco LSR No. MAK/117, from Pharmaco LSR Ltd, Eye, Suffolk,
United Kingdom. Submitted to WHO by Makhteshim Chemical Works
Ltd, Beer-Sheva, Israel.
FAO/WHO (1970) 1969 Evaluations of some pesticide residues in food.
The monographs. FAO/PL: 1969/M/17/1, WHO/Food Add./70.38, Rome,
FAO.
FAO/WHO (1974) 1973 Evaluations of some pesticide residues in food.
WHO Pesticide Residue Series No. 3, Geneva, WHO.
FAO/WHO (1985) FAO Plant Production and Protection Paper 67.
Evaluations, 1984. Rome, FAO. FAO/WHO (1987) Pesticide Residues
in Food -- 1986. Evaluations, Part II -- Toxicology. FAO
Production and Protection Paper 78/2. Rome, FAO.
FAO/WHO (1991) Pesticide Residues in Food -- 1990. Toxicology
Evaluations. WHO/PCS/91.47, Geneva, WHO.
Nyska, A., Waner, T., Paster, Z., Bracha, P., Gordon E.B. & Klein, B.
(1990) Induction of gastrointestinal tumors in mice fed the
fungicide folpet: Possible mechanisms. Jpn. J. Cancer Res., 81,
545-549.
Tennekes, H. (1995) The genetic toxicology of folpet. Position paper
commissioned by Makhteshim Chemical Works Ltd, Beer-Sheva,
Israel. Submitted to WHO by Makhteshim Chemical Works Ltd,
Beer-Sheva, Israel
Waterson, L.A. (1994a) Folpet, feasibility study by dietary
administration to male mice for 21 days. Report No. MBS
43/942221, from Huntingdon Research Centre Ltd, Huntingdon,
Cambs, United Kingdom. Submitted to WHO by Makhteshim Chemical
Works Ltd, Beer-Sheva, Israel.
Waterson, L.A. (1994b) Folpet, extended feasibility/preliminary study
by dietary administration to male mice for 28 days. Report number
MBS 44/942343, from Huntingdon Research Centre Ltd, Huntingdon,
Cambs, United Kingdom. Submitted to WHO by Makhteshim Chemical
Works Ltd, Beer-Sheva, Israel.
Waterson, L.A. (1995) Folpet, investigation of the effect on the
duodenum of male mice after dietary administration for 28 days
with recovery. Report No. MBS 45/9423003, from Huntingdon
Research Centre Ltd, Huntingdon, Cambs, United Kingdom Submitted
to WHO by Makhteshim Chemical Works Ltd, Beer-Sheva, Israel.
